User login
Testifying for civil commitment
Testifying in civil commitment proceedings sometimes is the only way to make sure dangerous patients get the hospital care they need. But for many psychiatrists, providing courtroom testimony can be a nerve-wracking experience because they:
- lack formal training about how to testify
- lack familiarity with laws and court procedures
- fear cross-examination.
Training programs are required to teach psychiatry residents about civil commitment but not about how to testify.1,2 Residents who get to take the stand during training usually do not receive any instruction.2 Knowing some fundamentals of testifying can reduce your anxiety and reluctance to take the stand3 and help you to perform better in court.
Court procedures
A doctor may not force a patient to stay in a hospital, no matter how much the patient needs treatment. Only courts have legal authority to order involuntary psychiatric hospitalization, and courts may do this only after receiving proof that civil commitment is legally justified. Statutory criteria for civil commitment vary across jurisdictions, but typically, the court must hear evidence proving that a person:
- exhibits clear signs of a mental illness
- and because of the mental illness recently did something that placed himself or others in physical danger.
Courts usually rely on testimony from patients’ caregivers for this evidence. Thus, testifying is a skill psychiatrists must exercise to care for seriously ill patients who need treatment but don’t want it.
Testifying and playing basketball have a lot in common. To score points in basketball, a player must put the ball through the hoop and stay in bounds.
To be effective in a civil commitment hearing, a psychiatrist needs a similar game plan. The ball is your testimony, the hoop is the law’s exact wording in your state, and the bounds are recent dangerous behavior.
Completing the Civil Commitment Checklist ( Figure ) will help you determine if you are ready to go to court. Download a PDF of this checklist and a worksheet to compile information you will need to provide accurate and relevant testimony.
Figure: Are you ready to testify in a civil commitment hearing?
*If you answer “yes” to all questions, you are ready to testify about the need for civil commitment. If you answer “no” to any bulleted items, civil commitment may be inappropriate. If you answer “no” to any of the other questions, you’re not ready to go to court
Shoot the ball through the hoop
As early as the mid-19th century, attorneys and physicians realized that “no physician or surgeon could be a satisfactory expert witness without some knowledge of the law.”4 You may have the best basketball shooting technique in the world, but it won’t help if you don’t know where the hoop is. Likewise, you’ll be shooting blind if you come to court without knowing your state’s requirements for civil commitment—which many psychiatrists don’t know.5
Your skills at diagnosis and verbal persuasiveness are critical to good testimony, but if you don’t directly address the requirements for involuntary hospitalization in your state, your testimony may be irrelevant. A court cannot authorize civil commitment unless your testimony clearly and convincingly shows that a patient is mentally ill and dangerous—as defined by the law in your state.
In most states, you can look up your state’s commitment statute on the Internet, and you can take a printed copy of the statute to the witness stand if you wish. Using the law’s actual wording, you can give the court examples of behavior that show why your patient needs hospitalization.
For example, Ohio law defines a “mental disorder” for purposes of involuntary hospitalization as “a substantial disorder of thought, mood, perception, orientation, or memory that grossly impairs judgment, behavior, capacity to recognize reality, or ability to meet the ordinary demands of life.”6 In Ohio and many other states, an official psychiatric diagnosis is neither necessary nor sufficient for civil commitment. Of course, psychiatrists should formulate diagnostic opinions using well-established criteria. But in court, the diagnosis is like the backboard—it is not the hoop that the ball must pass through. The court needs to know whether a patient’s recent actions are manifestations of impairments listed in the statute.
Here’s an example of testimony that makes the basketball hit the backboard but doesn’t put the ball through the hoop:
Doctor: “Your honor, my patient has schizophrenia, paranoid type, which he’s had for quite a number of years. Patients with paranoid schizophrenia have a hard time because they think people are after them. Based on my experience, I don’t see how my patient can survive outside the hospital right now. He’s too paranoid, and his thinking is messed up.”
Though this testimony may be medically sound, the psychiatrist has not told the court specifically how the patient’s illness impairs his present functioning. Here’s an example of a ball going through the hoop after bouncing off the backboard:
Doctor: “Your honor, my patient has schizophrenia, paranoid type. He hears voices saying that the government is trying to assassinate him by poisoning his food and medications. As a result, he stopped taking medications 5 days ago and left the safety of his group home. He has been living under an overpass and refusing to eat. He has become malnourished and dehydrated. This shows he has a substantial disorder of thought and judgment that keeps him from recognizing reality and meeting the ordinary demands of life.”
Staying in bounds
As a witness, your role is to tell the court the truth about your patient’s situation so that justice can be served—if necessary, by allowing the state to override your patient’s liberty interests through involuntary hospitalization.7 To justify involuntary hospitalization in most states, the court must find that your patient is both mentally ill and has recently done something dangerous.
In basketball, the referee stops the game when the ball goes out of bounds. Similarly, the court may stop you if your testimony goes out of bounds by invoking non-recent evidence to demonstrate current dangerousness. For example:
Doctor: “Your honor, this patient has been hospitalized twice in the last 10 months after intentionally overdosing on medications. He told me that last month he tried to kill himself with pills. I know this patient well, and I fear he’s going to overdose again.”
Patient’s attorney: “Objection, your honor. What my client said 1 month ago has nothing to do with his alleged dangerousness today.”
The judge may sustain the objection and disregard your testimony because the events you have recounted fall outside your jurisdiction’s time frame for a “recent” event. What your patient said last month may well make you think your patient is at risk now, but the law establishes sometimes-arbitrary boundaries to protect patients’ liberty. The legal justification is that placing time limits on dangerous behavior minimizes the risk of an erroneous civil commitment; evidence of actual, recent behavior increases the likelihood of real, current danger and reduces the risk of involuntarily hospitalizing someone who would not do harm.8
The definition of “recent” varies from state to state. Pennsylvania looks at behavior within the last 30 days;9 Utah uses 7 days.10 Often, the law is not clear; rather than set firm time restrictions, some states consider whether the actions in question are “material and relevant to the person’s present condition.”11
Know what your jurisdiction considers “recent” behavior. If your state’s statute is not clear, ask a judge or attorney. As you think about your testimony, make sure that information describes dangerous behavior within the time frame that the court will accept as “recent.”
Cross-examination
Civil commitment hearings are supposed to be adversarial. The “proponent” of involuntary hospitalization (the State) puts on its best case, hoping to convince the judge (or occasionally, a jury) that commitment is justified. The “respondent” (the patient facing potential commitment) has many of the rights that criminal defendants have, including the right to an attorney and the right to challenge witnesses—including psychiatrists—through cross-examination.12
Psychiatrists aren’t accustomed to having their clinical reasoning forcefully challenged. When they disagree, psychiatrists usually seek to persuade each other and reach consensus rather than openly criticize colleagues and point out flaws in their opinions about patients. Even when insurance companies and patients disagree with you, they usually don’t try to discredit you.
Testifying is different.13 Expect to have your conclusions bluntly challenged in civil commitment hearings. But remember: as in a basketball game, attorneys’ cross-examination challenges are not personal—they’re intended only to win the game. Also, you can practice. Just as practicing against opponents improves skills needed to win basketball games, practicing against real or anticipated cross-examination can help you respond when you’re testifying. Be prepared to answer commonly asked questions ( Table 1 ), such as:
“Doctor, you’ve testified that Mr. Jones has bipolar disorder. Aren’t all psychiatric diagnoses just a guess?”
“Doctor, how can you be certain that Mr. Smith’s psychosis, as you call it, was the result of schizophrenia, not alcohol intoxication?”
“Well, doctor, if you’re saying that Mrs. Clark’s psychosis was caused by a urinary tract infection, isn’t that a medical problem and not a psychiatric problem that we can lock her up for?”
Other ways to prepare:
- Have a colleague play the part of an opposing attorney who is trying to find fault with your clinical reasoning.
- Imagine you are retained by an attorney who wants to find holes in your own testimony.
- Watch other psychiatrists testify, and learn from their triumphs or mistakes.
Table 1
Questions you’re likely to face when testifying
| Question | Importance |
|---|---|
| What is your diagnosis? | In all states, a mental illness leading to dangerous behavior is required for involuntary hospitalization. Courts are less interested in the name of the disorder than in knowing how its symptoms affect the respondent and lead to danger |
| Why is the hospital the least restrictive environment? | The “least restrictive” legal standard14 is a safeguard against unwarranted hospitalization. Be ready to explain why other treatment options (outpatient, day hospitals, etc.) are not appropriate |
| What medications is the patient taking? | Be prepared to tell the court whether the patient was taking medications before and since admission. Know which medications are being prescribed for the patient in the hospital |
| Why does your diagnosis differ from the diagnosis of another clinician in the chart? | Cross-examining attorneys will often try to discredit your opinion by pointing out that other clinicians diagnosed something different. You can explain that different disorders belong within common diagnostic categories (for example, schizophrenia and schizoaffective disorder are both psychoses). Explaining your answer this way enhances its credibility by demonstrating agreement with the other clinician |
| What is the patient’s response to the allegations of dangerousness listed in the chart? | Don’t neglect patients’ views. Their answers allow you to testify about the patient from direct knowledge and often provide evidence of thought, mood, or judgment problems |
Good and bad shots
Good basketball players avoid fouls and taking shots that opponents can block easily. A good psychiatric witness knows how to avoid committing legal fouls and having testimony blocked.
The hearsay block. In clinical practice, psychiatrists should use and often rely on information about their patients that they obtain from other persons. But in court, testifying about such information could be disallowed on grounds that it is hearsay—testimony about what someone else saw or heard.
In civil commitment hearings, rules against allowing hearsay protect patients from accusations that may be false or misleading and that they cannot challenge through cross-examination. Although many states have a “hearsay exception” for civil commitment hearings—meaning that doctors may testify about what others have told them—not all do. If you practice in a state without this exception, you’ll need to gather information and plan your testimony carefully to avoid having the basis for your opinion excluded.
To avoid this “block,” testify only about events you saw or heard. To be fair to patients, always ask them about their side of the story. You can then testify about your clinical findings—what you saw and heard—instead of what someone else said. For example:
Doctor: “Your wife told me you wanted to kill yourself. Is that true?”
Patient: “It wasn’t my wife. I told my brother I wanted to kill myself.”
Doctor: “How about now? Do you still want to kill yourself?”
Patient: “Yes, I do.”
The doctor now can testify about first-hand experience with the patient:
Doctor: “Your honor, I asked the patient whether he told anyone that he wanted to kill himself. He told me he had told his brother he wanted to kill himself, and that he still felt that way.”
Play only your position
Basketball teams get into trouble if players try to do things others are supposed to do. Players are not supposed to give orders to the coach. In court, your role is to provide expert testimony about your patient and psychiatry. Stay in that role:
- Don’t address the ultimate legal question, as in saying, “My patient meets this state’s commitment criteria.” That’s for the judge to decide.
- Don’t opine about the moral virtues or shortcomings of the courts or hearings: “My patient desperately needs treatment, but you’re just asking about whether he fits narrow legal rules.”
- Don’t testify about topics on which you’re not an expert: “I think the police used too much force when they handcuffed my patient.”
Table 2
Dos and don’ts of testifying in a civil commitment hearing
| Do… | Don’t… |
|---|---|
| Wear conservative business attire, which shows that you take your work seriously | Dress casually. Though casual dress is OK in many workplaces, lawyers wear suits in court |
| Remain calm, professional, and respectful | Make jokes or sarcastic remarks; a cross-examining attorney will easily discredit you by pointing out that this is a ‘serious matter’ |
| Use recent examples of the patient’s dangerousness | Testify about your patient’s childhood or remote events |
| Describe behaviors or statements you witnessed or obtained from the patient | Testify about information obtained only from other people |
| Pause before answering each question. Doing this allows time for you to think and for an attorney to object to the question | Lose your cool or argue with the attorneys or judge |
| Source: Adapted from Knoll JL, Resnick PJ. Deposition dos and don’ts: how to answer 8 tricky questions. Current Psychiatry. 2007;7(3):25-40 | |
Related resources
- Melton GB, Petrila J, Poythress NG, et al. Psychological evaluations for the courts. New York, NY: Guilford Press; 2007.
- Perlin ML. Mental disability law—civil and criminal. 2nd ed. Charlottesville, VA: Lexis Law Publishing; 1998.
Disclosure
The authors report no financial relationship with any company whose products are mentioned in the article or with manufacturers of competing products.
1. Barr NI, Suarez JM. The teaching of forensic psychiatry in law schools, medical schools and psychiatric residencies in the United States. Am J Psychiatry. 1965;122(6):612-616.
2. Lewis CF. Teaching forensic psychiatry to general psychiatry residents. Acad Psychiatry. 2004;28(1):40-46.
3. Sata LS, Goldenberg EE. A study of involuntary patients in Seattle. Hosp Comm Psychiatry. 1977;28(11):834-837.
4. Curran WJ. Titles in the medicolegal field: a proposal for reform. Am J Law Med. 1975;1:1-11.
5. Brooks RA. Psychiatrist’s opinions about involuntary civil commitment: results of a national survey. J Am Acad Psychiatry Law. 2007;35:219-228.
6. Ohio Rev Code § 5122.01(A).
7. Rotter M, Preven D. Commentary: general residency training—the first forensic stage. J Am Acad Psychiatry Law. 2005;33:324-327.
8. Melton GB, Petrila J, Poythress NG, et al. Psychological evaluations for the courts. New York, NY: Guilford Press; 2007:337.
9. 50 PS § 7301(b).
10. Utah Code Ann § 62A-15-631(a).
11. Matter of D.D., 920 P2d 973, 975 (Mont 1996).
12. Perlin ML. Mental disability law—civil and criminal. 2nd ed, vol. 1, § 2B-3.1. Charlottesville, VA: Lexis Law Publishing; 1998.
13. Gutheil T. The psychiatrist as expert witness. Arlington, VA: American Psychiatric Publishing, Inc.; 1998:11–18.
14. Lake v Cameron, 364 F2d 657 (DC Cir 1967).
Testifying in civil commitment proceedings sometimes is the only way to make sure dangerous patients get the hospital care they need. But for many psychiatrists, providing courtroom testimony can be a nerve-wracking experience because they:
- lack formal training about how to testify
- lack familiarity with laws and court procedures
- fear cross-examination.
Training programs are required to teach psychiatry residents about civil commitment but not about how to testify.1,2 Residents who get to take the stand during training usually do not receive any instruction.2 Knowing some fundamentals of testifying can reduce your anxiety and reluctance to take the stand3 and help you to perform better in court.
Court procedures
A doctor may not force a patient to stay in a hospital, no matter how much the patient needs treatment. Only courts have legal authority to order involuntary psychiatric hospitalization, and courts may do this only after receiving proof that civil commitment is legally justified. Statutory criteria for civil commitment vary across jurisdictions, but typically, the court must hear evidence proving that a person:
- exhibits clear signs of a mental illness
- and because of the mental illness recently did something that placed himself or others in physical danger.
Courts usually rely on testimony from patients’ caregivers for this evidence. Thus, testifying is a skill psychiatrists must exercise to care for seriously ill patients who need treatment but don’t want it.
Testifying and playing basketball have a lot in common. To score points in basketball, a player must put the ball through the hoop and stay in bounds.
To be effective in a civil commitment hearing, a psychiatrist needs a similar game plan. The ball is your testimony, the hoop is the law’s exact wording in your state, and the bounds are recent dangerous behavior.
Completing the Civil Commitment Checklist ( Figure ) will help you determine if you are ready to go to court. Download a PDF of this checklist and a worksheet to compile information you will need to provide accurate and relevant testimony.
Figure: Are you ready to testify in a civil commitment hearing?
*If you answer “yes” to all questions, you are ready to testify about the need for civil commitment. If you answer “no” to any bulleted items, civil commitment may be inappropriate. If you answer “no” to any of the other questions, you’re not ready to go to court
Shoot the ball through the hoop
As early as the mid-19th century, attorneys and physicians realized that “no physician or surgeon could be a satisfactory expert witness without some knowledge of the law.”4 You may have the best basketball shooting technique in the world, but it won’t help if you don’t know where the hoop is. Likewise, you’ll be shooting blind if you come to court without knowing your state’s requirements for civil commitment—which many psychiatrists don’t know.5
Your skills at diagnosis and verbal persuasiveness are critical to good testimony, but if you don’t directly address the requirements for involuntary hospitalization in your state, your testimony may be irrelevant. A court cannot authorize civil commitment unless your testimony clearly and convincingly shows that a patient is mentally ill and dangerous—as defined by the law in your state.
In most states, you can look up your state’s commitment statute on the Internet, and you can take a printed copy of the statute to the witness stand if you wish. Using the law’s actual wording, you can give the court examples of behavior that show why your patient needs hospitalization.
For example, Ohio law defines a “mental disorder” for purposes of involuntary hospitalization as “a substantial disorder of thought, mood, perception, orientation, or memory that grossly impairs judgment, behavior, capacity to recognize reality, or ability to meet the ordinary demands of life.”6 In Ohio and many other states, an official psychiatric diagnosis is neither necessary nor sufficient for civil commitment. Of course, psychiatrists should formulate diagnostic opinions using well-established criteria. But in court, the diagnosis is like the backboard—it is not the hoop that the ball must pass through. The court needs to know whether a patient’s recent actions are manifestations of impairments listed in the statute.
Here’s an example of testimony that makes the basketball hit the backboard but doesn’t put the ball through the hoop:
Doctor: “Your honor, my patient has schizophrenia, paranoid type, which he’s had for quite a number of years. Patients with paranoid schizophrenia have a hard time because they think people are after them. Based on my experience, I don’t see how my patient can survive outside the hospital right now. He’s too paranoid, and his thinking is messed up.”
Though this testimony may be medically sound, the psychiatrist has not told the court specifically how the patient’s illness impairs his present functioning. Here’s an example of a ball going through the hoop after bouncing off the backboard:
Doctor: “Your honor, my patient has schizophrenia, paranoid type. He hears voices saying that the government is trying to assassinate him by poisoning his food and medications. As a result, he stopped taking medications 5 days ago and left the safety of his group home. He has been living under an overpass and refusing to eat. He has become malnourished and dehydrated. This shows he has a substantial disorder of thought and judgment that keeps him from recognizing reality and meeting the ordinary demands of life.”
Staying in bounds
As a witness, your role is to tell the court the truth about your patient’s situation so that justice can be served—if necessary, by allowing the state to override your patient’s liberty interests through involuntary hospitalization.7 To justify involuntary hospitalization in most states, the court must find that your patient is both mentally ill and has recently done something dangerous.
In basketball, the referee stops the game when the ball goes out of bounds. Similarly, the court may stop you if your testimony goes out of bounds by invoking non-recent evidence to demonstrate current dangerousness. For example:
Doctor: “Your honor, this patient has been hospitalized twice in the last 10 months after intentionally overdosing on medications. He told me that last month he tried to kill himself with pills. I know this patient well, and I fear he’s going to overdose again.”
Patient’s attorney: “Objection, your honor. What my client said 1 month ago has nothing to do with his alleged dangerousness today.”
The judge may sustain the objection and disregard your testimony because the events you have recounted fall outside your jurisdiction’s time frame for a “recent” event. What your patient said last month may well make you think your patient is at risk now, but the law establishes sometimes-arbitrary boundaries to protect patients’ liberty. The legal justification is that placing time limits on dangerous behavior minimizes the risk of an erroneous civil commitment; evidence of actual, recent behavior increases the likelihood of real, current danger and reduces the risk of involuntarily hospitalizing someone who would not do harm.8
The definition of “recent” varies from state to state. Pennsylvania looks at behavior within the last 30 days;9 Utah uses 7 days.10 Often, the law is not clear; rather than set firm time restrictions, some states consider whether the actions in question are “material and relevant to the person’s present condition.”11
Know what your jurisdiction considers “recent” behavior. If your state’s statute is not clear, ask a judge or attorney. As you think about your testimony, make sure that information describes dangerous behavior within the time frame that the court will accept as “recent.”
Cross-examination
Civil commitment hearings are supposed to be adversarial. The “proponent” of involuntary hospitalization (the State) puts on its best case, hoping to convince the judge (or occasionally, a jury) that commitment is justified. The “respondent” (the patient facing potential commitment) has many of the rights that criminal defendants have, including the right to an attorney and the right to challenge witnesses—including psychiatrists—through cross-examination.12
Psychiatrists aren’t accustomed to having their clinical reasoning forcefully challenged. When they disagree, psychiatrists usually seek to persuade each other and reach consensus rather than openly criticize colleagues and point out flaws in their opinions about patients. Even when insurance companies and patients disagree with you, they usually don’t try to discredit you.
Testifying is different.13 Expect to have your conclusions bluntly challenged in civil commitment hearings. But remember: as in a basketball game, attorneys’ cross-examination challenges are not personal—they’re intended only to win the game. Also, you can practice. Just as practicing against opponents improves skills needed to win basketball games, practicing against real or anticipated cross-examination can help you respond when you’re testifying. Be prepared to answer commonly asked questions ( Table 1 ), such as:
“Doctor, you’ve testified that Mr. Jones has bipolar disorder. Aren’t all psychiatric diagnoses just a guess?”
“Doctor, how can you be certain that Mr. Smith’s psychosis, as you call it, was the result of schizophrenia, not alcohol intoxication?”
“Well, doctor, if you’re saying that Mrs. Clark’s psychosis was caused by a urinary tract infection, isn’t that a medical problem and not a psychiatric problem that we can lock her up for?”
Other ways to prepare:
- Have a colleague play the part of an opposing attorney who is trying to find fault with your clinical reasoning.
- Imagine you are retained by an attorney who wants to find holes in your own testimony.
- Watch other psychiatrists testify, and learn from their triumphs or mistakes.
Table 1
Questions you’re likely to face when testifying
| Question | Importance |
|---|---|
| What is your diagnosis? | In all states, a mental illness leading to dangerous behavior is required for involuntary hospitalization. Courts are less interested in the name of the disorder than in knowing how its symptoms affect the respondent and lead to danger |
| Why is the hospital the least restrictive environment? | The “least restrictive” legal standard14 is a safeguard against unwarranted hospitalization. Be ready to explain why other treatment options (outpatient, day hospitals, etc.) are not appropriate |
| What medications is the patient taking? | Be prepared to tell the court whether the patient was taking medications before and since admission. Know which medications are being prescribed for the patient in the hospital |
| Why does your diagnosis differ from the diagnosis of another clinician in the chart? | Cross-examining attorneys will often try to discredit your opinion by pointing out that other clinicians diagnosed something different. You can explain that different disorders belong within common diagnostic categories (for example, schizophrenia and schizoaffective disorder are both psychoses). Explaining your answer this way enhances its credibility by demonstrating agreement with the other clinician |
| What is the patient’s response to the allegations of dangerousness listed in the chart? | Don’t neglect patients’ views. Their answers allow you to testify about the patient from direct knowledge and often provide evidence of thought, mood, or judgment problems |
Good and bad shots
Good basketball players avoid fouls and taking shots that opponents can block easily. A good psychiatric witness knows how to avoid committing legal fouls and having testimony blocked.
The hearsay block. In clinical practice, psychiatrists should use and often rely on information about their patients that they obtain from other persons. But in court, testifying about such information could be disallowed on grounds that it is hearsay—testimony about what someone else saw or heard.
In civil commitment hearings, rules against allowing hearsay protect patients from accusations that may be false or misleading and that they cannot challenge through cross-examination. Although many states have a “hearsay exception” for civil commitment hearings—meaning that doctors may testify about what others have told them—not all do. If you practice in a state without this exception, you’ll need to gather information and plan your testimony carefully to avoid having the basis for your opinion excluded.
To avoid this “block,” testify only about events you saw or heard. To be fair to patients, always ask them about their side of the story. You can then testify about your clinical findings—what you saw and heard—instead of what someone else said. For example:
Doctor: “Your wife told me you wanted to kill yourself. Is that true?”
Patient: “It wasn’t my wife. I told my brother I wanted to kill myself.”
Doctor: “How about now? Do you still want to kill yourself?”
Patient: “Yes, I do.”
The doctor now can testify about first-hand experience with the patient:
Doctor: “Your honor, I asked the patient whether he told anyone that he wanted to kill himself. He told me he had told his brother he wanted to kill himself, and that he still felt that way.”
Play only your position
Basketball teams get into trouble if players try to do things others are supposed to do. Players are not supposed to give orders to the coach. In court, your role is to provide expert testimony about your patient and psychiatry. Stay in that role:
- Don’t address the ultimate legal question, as in saying, “My patient meets this state’s commitment criteria.” That’s for the judge to decide.
- Don’t opine about the moral virtues or shortcomings of the courts or hearings: “My patient desperately needs treatment, but you’re just asking about whether he fits narrow legal rules.”
- Don’t testify about topics on which you’re not an expert: “I think the police used too much force when they handcuffed my patient.”
Table 2
Dos and don’ts of testifying in a civil commitment hearing
| Do… | Don’t… |
|---|---|
| Wear conservative business attire, which shows that you take your work seriously | Dress casually. Though casual dress is OK in many workplaces, lawyers wear suits in court |
| Remain calm, professional, and respectful | Make jokes or sarcastic remarks; a cross-examining attorney will easily discredit you by pointing out that this is a ‘serious matter’ |
| Use recent examples of the patient’s dangerousness | Testify about your patient’s childhood or remote events |
| Describe behaviors or statements you witnessed or obtained from the patient | Testify about information obtained only from other people |
| Pause before answering each question. Doing this allows time for you to think and for an attorney to object to the question | Lose your cool or argue with the attorneys or judge |
| Source: Adapted from Knoll JL, Resnick PJ. Deposition dos and don’ts: how to answer 8 tricky questions. Current Psychiatry. 2007;7(3):25-40 | |
Related resources
- Melton GB, Petrila J, Poythress NG, et al. Psychological evaluations for the courts. New York, NY: Guilford Press; 2007.
- Perlin ML. Mental disability law—civil and criminal. 2nd ed. Charlottesville, VA: Lexis Law Publishing; 1998.
Disclosure
The authors report no financial relationship with any company whose products are mentioned in the article or with manufacturers of competing products.
Testifying in civil commitment proceedings sometimes is the only way to make sure dangerous patients get the hospital care they need. But for many psychiatrists, providing courtroom testimony can be a nerve-wracking experience because they:
- lack formal training about how to testify
- lack familiarity with laws and court procedures
- fear cross-examination.
Training programs are required to teach psychiatry residents about civil commitment but not about how to testify.1,2 Residents who get to take the stand during training usually do not receive any instruction.2 Knowing some fundamentals of testifying can reduce your anxiety and reluctance to take the stand3 and help you to perform better in court.
Court procedures
A doctor may not force a patient to stay in a hospital, no matter how much the patient needs treatment. Only courts have legal authority to order involuntary psychiatric hospitalization, and courts may do this only after receiving proof that civil commitment is legally justified. Statutory criteria for civil commitment vary across jurisdictions, but typically, the court must hear evidence proving that a person:
- exhibits clear signs of a mental illness
- and because of the mental illness recently did something that placed himself or others in physical danger.
Courts usually rely on testimony from patients’ caregivers for this evidence. Thus, testifying is a skill psychiatrists must exercise to care for seriously ill patients who need treatment but don’t want it.
Testifying and playing basketball have a lot in common. To score points in basketball, a player must put the ball through the hoop and stay in bounds.
To be effective in a civil commitment hearing, a psychiatrist needs a similar game plan. The ball is your testimony, the hoop is the law’s exact wording in your state, and the bounds are recent dangerous behavior.
Completing the Civil Commitment Checklist ( Figure ) will help you determine if you are ready to go to court. Download a PDF of this checklist and a worksheet to compile information you will need to provide accurate and relevant testimony.
Figure: Are you ready to testify in a civil commitment hearing?
*If you answer “yes” to all questions, you are ready to testify about the need for civil commitment. If you answer “no” to any bulleted items, civil commitment may be inappropriate. If you answer “no” to any of the other questions, you’re not ready to go to court
Shoot the ball through the hoop
As early as the mid-19th century, attorneys and physicians realized that “no physician or surgeon could be a satisfactory expert witness without some knowledge of the law.”4 You may have the best basketball shooting technique in the world, but it won’t help if you don’t know where the hoop is. Likewise, you’ll be shooting blind if you come to court without knowing your state’s requirements for civil commitment—which many psychiatrists don’t know.5
Your skills at diagnosis and verbal persuasiveness are critical to good testimony, but if you don’t directly address the requirements for involuntary hospitalization in your state, your testimony may be irrelevant. A court cannot authorize civil commitment unless your testimony clearly and convincingly shows that a patient is mentally ill and dangerous—as defined by the law in your state.
In most states, you can look up your state’s commitment statute on the Internet, and you can take a printed copy of the statute to the witness stand if you wish. Using the law’s actual wording, you can give the court examples of behavior that show why your patient needs hospitalization.
For example, Ohio law defines a “mental disorder” for purposes of involuntary hospitalization as “a substantial disorder of thought, mood, perception, orientation, or memory that grossly impairs judgment, behavior, capacity to recognize reality, or ability to meet the ordinary demands of life.”6 In Ohio and many other states, an official psychiatric diagnosis is neither necessary nor sufficient for civil commitment. Of course, psychiatrists should formulate diagnostic opinions using well-established criteria. But in court, the diagnosis is like the backboard—it is not the hoop that the ball must pass through. The court needs to know whether a patient’s recent actions are manifestations of impairments listed in the statute.
Here’s an example of testimony that makes the basketball hit the backboard but doesn’t put the ball through the hoop:
Doctor: “Your honor, my patient has schizophrenia, paranoid type, which he’s had for quite a number of years. Patients with paranoid schizophrenia have a hard time because they think people are after them. Based on my experience, I don’t see how my patient can survive outside the hospital right now. He’s too paranoid, and his thinking is messed up.”
Though this testimony may be medically sound, the psychiatrist has not told the court specifically how the patient’s illness impairs his present functioning. Here’s an example of a ball going through the hoop after bouncing off the backboard:
Doctor: “Your honor, my patient has schizophrenia, paranoid type. He hears voices saying that the government is trying to assassinate him by poisoning his food and medications. As a result, he stopped taking medications 5 days ago and left the safety of his group home. He has been living under an overpass and refusing to eat. He has become malnourished and dehydrated. This shows he has a substantial disorder of thought and judgment that keeps him from recognizing reality and meeting the ordinary demands of life.”
Staying in bounds
As a witness, your role is to tell the court the truth about your patient’s situation so that justice can be served—if necessary, by allowing the state to override your patient’s liberty interests through involuntary hospitalization.7 To justify involuntary hospitalization in most states, the court must find that your patient is both mentally ill and has recently done something dangerous.
In basketball, the referee stops the game when the ball goes out of bounds. Similarly, the court may stop you if your testimony goes out of bounds by invoking non-recent evidence to demonstrate current dangerousness. For example:
Doctor: “Your honor, this patient has been hospitalized twice in the last 10 months after intentionally overdosing on medications. He told me that last month he tried to kill himself with pills. I know this patient well, and I fear he’s going to overdose again.”
Patient’s attorney: “Objection, your honor. What my client said 1 month ago has nothing to do with his alleged dangerousness today.”
The judge may sustain the objection and disregard your testimony because the events you have recounted fall outside your jurisdiction’s time frame for a “recent” event. What your patient said last month may well make you think your patient is at risk now, but the law establishes sometimes-arbitrary boundaries to protect patients’ liberty. The legal justification is that placing time limits on dangerous behavior minimizes the risk of an erroneous civil commitment; evidence of actual, recent behavior increases the likelihood of real, current danger and reduces the risk of involuntarily hospitalizing someone who would not do harm.8
The definition of “recent” varies from state to state. Pennsylvania looks at behavior within the last 30 days;9 Utah uses 7 days.10 Often, the law is not clear; rather than set firm time restrictions, some states consider whether the actions in question are “material and relevant to the person’s present condition.”11
Know what your jurisdiction considers “recent” behavior. If your state’s statute is not clear, ask a judge or attorney. As you think about your testimony, make sure that information describes dangerous behavior within the time frame that the court will accept as “recent.”
Cross-examination
Civil commitment hearings are supposed to be adversarial. The “proponent” of involuntary hospitalization (the State) puts on its best case, hoping to convince the judge (or occasionally, a jury) that commitment is justified. The “respondent” (the patient facing potential commitment) has many of the rights that criminal defendants have, including the right to an attorney and the right to challenge witnesses—including psychiatrists—through cross-examination.12
Psychiatrists aren’t accustomed to having their clinical reasoning forcefully challenged. When they disagree, psychiatrists usually seek to persuade each other and reach consensus rather than openly criticize colleagues and point out flaws in their opinions about patients. Even when insurance companies and patients disagree with you, they usually don’t try to discredit you.
Testifying is different.13 Expect to have your conclusions bluntly challenged in civil commitment hearings. But remember: as in a basketball game, attorneys’ cross-examination challenges are not personal—they’re intended only to win the game. Also, you can practice. Just as practicing against opponents improves skills needed to win basketball games, practicing against real or anticipated cross-examination can help you respond when you’re testifying. Be prepared to answer commonly asked questions ( Table 1 ), such as:
“Doctor, you’ve testified that Mr. Jones has bipolar disorder. Aren’t all psychiatric diagnoses just a guess?”
“Doctor, how can you be certain that Mr. Smith’s psychosis, as you call it, was the result of schizophrenia, not alcohol intoxication?”
“Well, doctor, if you’re saying that Mrs. Clark’s psychosis was caused by a urinary tract infection, isn’t that a medical problem and not a psychiatric problem that we can lock her up for?”
Other ways to prepare:
- Have a colleague play the part of an opposing attorney who is trying to find fault with your clinical reasoning.
- Imagine you are retained by an attorney who wants to find holes in your own testimony.
- Watch other psychiatrists testify, and learn from their triumphs or mistakes.
Table 1
Questions you’re likely to face when testifying
| Question | Importance |
|---|---|
| What is your diagnosis? | In all states, a mental illness leading to dangerous behavior is required for involuntary hospitalization. Courts are less interested in the name of the disorder than in knowing how its symptoms affect the respondent and lead to danger |
| Why is the hospital the least restrictive environment? | The “least restrictive” legal standard14 is a safeguard against unwarranted hospitalization. Be ready to explain why other treatment options (outpatient, day hospitals, etc.) are not appropriate |
| What medications is the patient taking? | Be prepared to tell the court whether the patient was taking medications before and since admission. Know which medications are being prescribed for the patient in the hospital |
| Why does your diagnosis differ from the diagnosis of another clinician in the chart? | Cross-examining attorneys will often try to discredit your opinion by pointing out that other clinicians diagnosed something different. You can explain that different disorders belong within common diagnostic categories (for example, schizophrenia and schizoaffective disorder are both psychoses). Explaining your answer this way enhances its credibility by demonstrating agreement with the other clinician |
| What is the patient’s response to the allegations of dangerousness listed in the chart? | Don’t neglect patients’ views. Their answers allow you to testify about the patient from direct knowledge and often provide evidence of thought, mood, or judgment problems |
Good and bad shots
Good basketball players avoid fouls and taking shots that opponents can block easily. A good psychiatric witness knows how to avoid committing legal fouls and having testimony blocked.
The hearsay block. In clinical practice, psychiatrists should use and often rely on information about their patients that they obtain from other persons. But in court, testifying about such information could be disallowed on grounds that it is hearsay—testimony about what someone else saw or heard.
In civil commitment hearings, rules against allowing hearsay protect patients from accusations that may be false or misleading and that they cannot challenge through cross-examination. Although many states have a “hearsay exception” for civil commitment hearings—meaning that doctors may testify about what others have told them—not all do. If you practice in a state without this exception, you’ll need to gather information and plan your testimony carefully to avoid having the basis for your opinion excluded.
To avoid this “block,” testify only about events you saw or heard. To be fair to patients, always ask them about their side of the story. You can then testify about your clinical findings—what you saw and heard—instead of what someone else said. For example:
Doctor: “Your wife told me you wanted to kill yourself. Is that true?”
Patient: “It wasn’t my wife. I told my brother I wanted to kill myself.”
Doctor: “How about now? Do you still want to kill yourself?”
Patient: “Yes, I do.”
The doctor now can testify about first-hand experience with the patient:
Doctor: “Your honor, I asked the patient whether he told anyone that he wanted to kill himself. He told me he had told his brother he wanted to kill himself, and that he still felt that way.”
Play only your position
Basketball teams get into trouble if players try to do things others are supposed to do. Players are not supposed to give orders to the coach. In court, your role is to provide expert testimony about your patient and psychiatry. Stay in that role:
- Don’t address the ultimate legal question, as in saying, “My patient meets this state’s commitment criteria.” That’s for the judge to decide.
- Don’t opine about the moral virtues or shortcomings of the courts or hearings: “My patient desperately needs treatment, but you’re just asking about whether he fits narrow legal rules.”
- Don’t testify about topics on which you’re not an expert: “I think the police used too much force when they handcuffed my patient.”
Table 2
Dos and don’ts of testifying in a civil commitment hearing
| Do… | Don’t… |
|---|---|
| Wear conservative business attire, which shows that you take your work seriously | Dress casually. Though casual dress is OK in many workplaces, lawyers wear suits in court |
| Remain calm, professional, and respectful | Make jokes or sarcastic remarks; a cross-examining attorney will easily discredit you by pointing out that this is a ‘serious matter’ |
| Use recent examples of the patient’s dangerousness | Testify about your patient’s childhood or remote events |
| Describe behaviors or statements you witnessed or obtained from the patient | Testify about information obtained only from other people |
| Pause before answering each question. Doing this allows time for you to think and for an attorney to object to the question | Lose your cool or argue with the attorneys or judge |
| Source: Adapted from Knoll JL, Resnick PJ. Deposition dos and don’ts: how to answer 8 tricky questions. Current Psychiatry. 2007;7(3):25-40 | |
Related resources
- Melton GB, Petrila J, Poythress NG, et al. Psychological evaluations for the courts. New York, NY: Guilford Press; 2007.
- Perlin ML. Mental disability law—civil and criminal. 2nd ed. Charlottesville, VA: Lexis Law Publishing; 1998.
Disclosure
The authors report no financial relationship with any company whose products are mentioned in the article or with manufacturers of competing products.
1. Barr NI, Suarez JM. The teaching of forensic psychiatry in law schools, medical schools and psychiatric residencies in the United States. Am J Psychiatry. 1965;122(6):612-616.
2. Lewis CF. Teaching forensic psychiatry to general psychiatry residents. Acad Psychiatry. 2004;28(1):40-46.
3. Sata LS, Goldenberg EE. A study of involuntary patients in Seattle. Hosp Comm Psychiatry. 1977;28(11):834-837.
4. Curran WJ. Titles in the medicolegal field: a proposal for reform. Am J Law Med. 1975;1:1-11.
5. Brooks RA. Psychiatrist’s opinions about involuntary civil commitment: results of a national survey. J Am Acad Psychiatry Law. 2007;35:219-228.
6. Ohio Rev Code § 5122.01(A).
7. Rotter M, Preven D. Commentary: general residency training—the first forensic stage. J Am Acad Psychiatry Law. 2005;33:324-327.
8. Melton GB, Petrila J, Poythress NG, et al. Psychological evaluations for the courts. New York, NY: Guilford Press; 2007:337.
9. 50 PS § 7301(b).
10. Utah Code Ann § 62A-15-631(a).
11. Matter of D.D., 920 P2d 973, 975 (Mont 1996).
12. Perlin ML. Mental disability law—civil and criminal. 2nd ed, vol. 1, § 2B-3.1. Charlottesville, VA: Lexis Law Publishing; 1998.
13. Gutheil T. The psychiatrist as expert witness. Arlington, VA: American Psychiatric Publishing, Inc.; 1998:11–18.
14. Lake v Cameron, 364 F2d 657 (DC Cir 1967).
1. Barr NI, Suarez JM. The teaching of forensic psychiatry in law schools, medical schools and psychiatric residencies in the United States. Am J Psychiatry. 1965;122(6):612-616.
2. Lewis CF. Teaching forensic psychiatry to general psychiatry residents. Acad Psychiatry. 2004;28(1):40-46.
3. Sata LS, Goldenberg EE. A study of involuntary patients in Seattle. Hosp Comm Psychiatry. 1977;28(11):834-837.
4. Curran WJ. Titles in the medicolegal field: a proposal for reform. Am J Law Med. 1975;1:1-11.
5. Brooks RA. Psychiatrist’s opinions about involuntary civil commitment: results of a national survey. J Am Acad Psychiatry Law. 2007;35:219-228.
6. Ohio Rev Code § 5122.01(A).
7. Rotter M, Preven D. Commentary: general residency training—the first forensic stage. J Am Acad Psychiatry Law. 2005;33:324-327.
8. Melton GB, Petrila J, Poythress NG, et al. Psychological evaluations for the courts. New York, NY: Guilford Press; 2007:337.
9. 50 PS § 7301(b).
10. Utah Code Ann § 62A-15-631(a).
11. Matter of D.D., 920 P2d 973, 975 (Mont 1996).
12. Perlin ML. Mental disability law—civil and criminal. 2nd ed, vol. 1, § 2B-3.1. Charlottesville, VA: Lexis Law Publishing; 1998.
13. Gutheil T. The psychiatrist as expert witness. Arlington, VA: American Psychiatric Publishing, Inc.; 1998:11–18.
14. Lake v Cameron, 364 F2d 657 (DC Cir 1967).
Clinical trials support new algorithm for treating pediatric bipolar mania
Five recent randomized controlled trials (RCTs) have demonstrated the efficacy of atypical antipsychotics for treating bipolar disorder in children and adolescents, but 4 of these 5 trials remain unpublished. The lag time between the completion of these trials and publication of their results—typically 4 to 5 years1—leaves psychiatrists without important evidence to explain to families and critics2 why they might recommend using these powerful medications in children with mental illness.
This article previews the preliminary results of these 5 RCTs of atypical antipsychotics, offers a treatment algorithm supported by this evidence, and discusses how to manage potentially serious risks when using antipsychotics to treat children and adolescents with bipolar disorder (BPD).
Where do atypical antipsychotics fit in?
Details of the 5 industry-sponsored RCTs of atypical antipsychotics in children and adolescents with bipolar I manic or mixed episodes are summarized in Table 1.3-7 Only the olanzapine study4 has been published; data from the other 4 trials were presented at medical meetings in 2007 and 2008.
Change in Young Mania Rating Scale (YMRS) score was the primary outcome measure in these 5 trials, and each compound was more effective than placebo. The trials demonstrated statistically significant and clinically relevant differences between each antipsychotic and placebo. The number needed to treat (NNT)—how many patients need to be treated for 1 to benefit in a controlled clinical trial—ranged from 2 to 4. For comparison, the NNT for statins in the prevention of coronary events is 12 to 22,8 and the NNT in an analysis of trials of selective serotonin reuptake inhibitors for pediatric major depressive disorder was 9.9 Thus, an NNT of ≤4 represents a clinically significant effect.
Risperidone is FDA-approved for short-term treatment of acute bipolar I manic or mixed episodes in patients age 10 to 17. Aripiprazole is approved for acute and maintenance treatment of bipolar I manic or mixed episodes (with or without psychosis) as monotherapy or with lithium or valproate in patients age 10 to 17. In June, an FDA advisory committee recommended pediatric bipolar indications for olanzapine, quetiapine, and ziprasidone.
‘Mood stabilizers’ such as lithium, valproate, and carbamazepine have been used for years to treat bipolar mania in adults, adolescents, and children, despite limited supporting evidence. Preliminary results of a National Institute of Mental Health-funded double-blind RCT provide insights on their efficacy.10
The 153 outpatients age 7 to 17 in a bipolar I manic or mixed episode were randomly assigned to lithium, divalproex, or placebo for 8 weeks. Response rates—based on a Clinical Global Impressions-Improvement score of 1 or 2 (very much or much improved)—were divalproex, 54%; lithium, 42%; and placebo, 29%. Lithium showed a trend toward efficacy but did not clearly separate from placebo on the primary outcome measures. Effect sizes for lithium and divalproex were moderate.10
Only 1 study has compared a mood stabilizer with an atypical antipsychotic for treating mania in adolescents. In a double-blind trial, DelBello et al11 randomly assigned 50 patients age 12 to 18 with a bipolar I manic or mixed episode to quetiapine, 400 to 600 mg/d, or divalproex, serum level 80 to 120 μg/mL, for 28 days. Manic symptoms resolved more rapidly, and remission rates measured by the YMRS were higher with quetiapine than with divalproex. Both medications were well tolerated.
Combination therapy. BPD as it presents in children and adolescents is often difficult to treat because of the disorder’s various phases (manic, depressed, mixed), frequent psychotic symptoms, and high rate of comorbidity. Pediatric BPD patients frequently require several psychotropics, including mood stabilizers and atypical antipsychotics.
In a double-blind, placebo-controlled study, 30 adolescents in a bipolar I manic or mixed episode initially received divalproex, 20 mg/kg/d, then were randomly assigned to 6 weeks of adjunctive quetiapine, titrated to 450 mg/d in 7 days (n=15), or placebo (n=15). Those receiving divalproex plus quetiapine showed a statistically significant greater reduction in manic symptoms (P=.03) and a higher response rate (87% vs 53%, P=.05), compared with those receiving divalproex and placebo. This suggests that a mood stabilizer plus an atypical antipsychotic is more effective than a mood stabilizer alone for adolescent mania. Quetiapine was well tolerated.12
Treatment. The American Psychiatric Association’s outdated 2002 practice guideline for acute bipolar I manic or mixed episodes in adults recommends lithium, valproate, and/or an antipsychotic.13 The more recent Texas Medication Algorithm Project (TMAP) guidelines recommend monotherapy with lithium, valproate, aripiprazole, quetiapine, risperidone, or ziprasidone for adults with euphoric or irritable manic or hypomanic symptoms.14
Based on the TMAP algorithm, recent clinical trial evidence, and our experience in treating pediatric BPD, we offer an approach for treating mania/hypomania in patients age 10 to 17 (see Proposed Algorithm). For dosing and precautions when using atypical antipsychotics in children and adolescents with BPD, see Table 2.15-17
Comorbid psychiatric illnesses (such as anxiety disorders) are prevalent in adolescents with BPD. Evidence in adults and adolescents suggests that some atypical antipsychotics may provide additional benefit for these conditions as well. Thus, consider comorbid conditions and symptoms when choosing antimanic agents.
Attention-deficit/hyperactivity disorder (ADHD) is a common comorbidity in children with BPD, and stimulant medications are most often prescribed to treat inattentiveness and hyperactivity. Caution is imperative when treating bipolar youth with stimulants, which can exacerbate manic symptoms. Treat the patient’s mania before adding or reintroducing stimulant medication. Research and clinical experience suggest that if you first stabilize these patients on a mood stabilizer or atypical antipsychotic, adding a stimulant can be very helpful in treating comorbid ADHD symptoms. Start with low stimulant doses, and increase slowly.
Table 1
RCTs of atypical antipsychotics in patients age 10 to 17
with bipolar I disorder*
| Antipsychotic and source | Bipolar I episode (# of subjects) | Trial duration (days) | Dosage (mg/d) | Response rate or YMRS score change | NNT | Mean weight gain (kg) |
|---|---|---|---|---|---|---|
| Risperidone Pandina et al3 AACAP 2007 | Manic, mixed (169) | 21 | 0.5 to 2.5 3 to 6 | 59% 63% | 3.3 3.5 | 1.9 1.4 |
| Olanzapine Tohen et al4 | Manic, mixed (161) | 21 | 10.4 ± 4.5 | 49% | 4.1 | 3.7 ± 2.2 |
| Quetiapine DelBello et al5 AACAP 2007 | Manic (284) | 21 | 400 600 | 64% 58% | 4.4 4.2 | 1.7 1.7 |
| Aripiprazole Wagner et al6 ACNP 2007 | Manic, mixed (296) | 28 | 10 30 | 45% 64% | 4.1 2.4 | 0.9 0.54 |
| Ziprasidone DelBello et al7 APA 2008 | Manic, mixed (238) | 28 | 80 to 160 | –13.83 with ziprasidone, –8.61 with placebo | 3.7 | None |
| *Each trial included a 6-month open extension phase; results are pending | ||||||
| AACAP: American Academy of Child and Adolescent Psychiatry; ACNP: American College of Neuropsychopharmacology; APA: American Psychiatric Association; NNT: number needed to treat; RCT: randomized controlled trial; YMRS: Young Mania Rating Scale | ||||||
Table 2
Recommended antipsychotic use in pediatric bipolar disorder
| Drug | Starting dosage (mg) | Target dosage (mg/d) | Precautions |
|---|---|---|---|
| Aripiprazole | 2.5 to 5 at bedtime | 10 to 30 | Monitor for CYP 3A4 and 2D6 interactions, weight, BMI, cholesterol, lipids, and glucose |
| Olanzapine | 2.5 bid | 10 to 20 | Monitor for CYP 2D6 interactions, weight, BMI, cholesterol, lipids, glucose, and prolactin levels |
| Quetiapine | 50 bid | 400 to 1,200 | Monitor for weight, BMI, cholesterol, lipids, and glucose |
| Risperidone | 0.25 bid | 1 to 2.5 | Monitor for EPS, hyperprolactinemia (and associated sexual side effects, including galactorrhea), weight, BMI, cholesterol, lipids, glucose, and prolactin levels |
| Ziprasidone | 20 bid | 80 to 160 | Check baseline ECG and as dose increases or with reason for high level of concern; monitor prolactin levels |
| BMI: body mass index; CYP: cytochrome P450; ECG: electrocardiography; EPS: extrapyramidal symptoms | |||
| Source: References 15-17 | |||
Proposed Algorithm: Treating a bipolar mixed/manic episode in patients age 10 to 17
Stage 1. Consider patient’s experience with antipsychotics, body weight, and family history when choosing first-line monotherapy (1A). Quetiapine poses low risk for extrapyramidal symptoms and tardive dyskinesia. Aripiprazole and ziprasidone pose relatively low risk of weight gain. Risperidone is potent at low doses but increases prolactin levels (long-term effect unknown).
Second-line choices (1B) are mood stabilizers lithium and valproate (because of lower potency than atypical antipsychotics), and olanzapine (which—although potent—causes substantial weight gain). In case of lack of response or intolerable side effects with initial agent, select an alternate from 1A or 1B. If this is not effective, move to Stage 2.
Stage 2. Consider augmentation for patients who show partial response to monotherapy (in your clinical judgment “mild to moderately improved” but not “much or very much improved”).
Stage 3. Combination therapy could include 2 mood stabilizers (such as lithium and valproate) plus an atypical antipsychotic; 2 atypical antipsychotics; or other combinations based on patient’s past responses. No research has shown these combinations to be efficacious in bipolar children and adolescents, but we find they sometimes help those with treatment-resistant symptoms.
Duration. Maintain psychotropics 12 to 18 months. When patient is euthymic, slowly taper 1 medication across several months. If symptoms recur, reintroduce the mood-stabilizing agent(s).
Source: Adapted and reprinted with permission from Kowatch RA, Fristad MA, Findling R, et al. Clinical manual for the management of bipolar disorder in children and adolescents. Arlington, VA: American Psychiatric Publishing, Inc.; 2008
Managing adverse effects
Although clinically effective, atypical antipsychotics may cause serious side effects that must be recognized and managed. These include extrapyramidal symptoms (EPS), tardive dyskinesia (TD), weight gain and obesity, hyperlipidemia, increased prolactin levels, and QTc changes. Counsel patients and families about the risks and benefits of antipsychotics when you consider them for children and adolescents with BPD (Table 3).
EPS. Drug-induced parkinsonism and akathisia are the most common EPS in children and adolescents with BPD treated with atypical antipsychotics.18
Correll et al19 reported a 10% rate of EPS in patients treated with aripiprazole. Treatment-emergent EPS also was observed in the RCT of risperidone.20 EPS-related adverse events were associated with higher doses of risperidone, although none of the akathisia/EPS measures were thought to be “clinically significant.”
EPS frequency was relatively low and similar to placebo in the 3-week quetiapine trial,21 and no changes in movement disorder scale scores were observed during the olanzapine or ziprasidone RCTs.4,7
Recommendations. If your pediatric patient develops EPS, first try an antipsychotic dose reduction. Because anticholinergics can contribute to antipsychotic-induced weight gain, reserve them until after a dosage reduction has been unsuccessful.
Benztropine (0.25 to 0.5 mg given 2 to 3 times daily, not to exceed 3 mg/d) or diphenhydramine (25 to 50 mg given 3 to 4 times daily; maximum dosage 5 mg/kg/d) can be effective in treating EPS. Avoid anticholinergics in children with narrow-angle glaucoma or age <3.
Akathisia may be managed with propranolol (20 to 120 mg/d in divided doses). Multiple doses (typically 3 times daily) are needed to prevent interdose withdrawal symptoms. Use this beta blocker with caution in children with asthma because of the possibility of bronchospasm.
TD. Short-term trials and a meta-analysis of atypical antipsychotic trials (>11 months’ duration, subject age <18) suggest a low annual risk for TD (0.4%).22 Large, prospective, long-term trials of atypical antipsychotics are necessary to more accurately define the risk of TD in the pediatric population, however. Retrospective analyses of adolescents treated with antipsychotics suggest 3 TD risk factors:
- early age of antipsychotic use
- medication nonadherence
- concomitant use of antiparkinsonian agents.23
Kumra et al24 identified lower premorbid functioning and greater positive symptoms at baseline as factors associated with “withdrawal dyskinesia/tardive dyskinesia” in children and adolescents with early-onset psychotic-spectrum disorders treated with typical or atypical antipsychotics.
Recommendations. To minimize TD risk, use the lowest effective antipsychotic dose, monitor for abnormal involuntary movements with standardized assessments (such as the Abnormal Involuntary Movement Scale), review risks and benefits with parents and patients, and regularly evaluate the indication and need for antipsychotic therapy. It is reasonable to attempt to lower the antipsychotic dose after the patient has attained remission and been stable for 1 year.
Neuroleptic malignant syndrome (NMS). This complication of dopamine-blocking medications:
- is among the most serious adverse effects of antipsychotic treatment
- continues to be associated with a mortality rate of 10%.25
Recommendation. At least 1 recent review of pediatric NMS cases suggests that essential features (hyperthermia and severe muscular rigidity) are retained in children.26 Nonetheless, monitor for variant presentations; hyper thermia or muscle rigidity may be absent or develop slowly over several days in patients treated with atypical antipsychotics.27
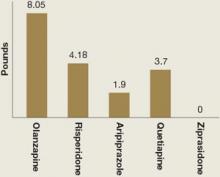
Weight gain and glucose metabolism. A major adverse effect of most atypical antipsychotics is increased appetite, weight gain, and possible obesity.28 In children, “obesity” refers to a body mass index (BMI) >95th percentile for age and sex; “over-weight” refers to BMI between the 85th and 95th percentile. Mean weight gain in the 5 atypical antipsychotic pediatric bipolar trials ranged from 0 to 8 lbs across 3 to 4 weeks of treatment (Figure).3-7
Recommendations. Emphasize diet and exercise, with restriction of high-carbohydrate food, “fast foods,” and soft drinks. Another option is a trial of metformin, which decreases hepatic glucose production, decreases intestinal absorption of glucose, and improves insulin sensitivity by increasing peripheral glucose uptake and utilization.
Klein et al29 studied 39 patients age 10 to 17 with mood and psychotic disorders whose weight increased by >10% during <1 year of olanzapine, risperidone, or quetiapine therapy. In this 16-week, double-blind, placebo-controlled trial, weight was stabilized in subjects receiving metformin, whereas those receiving placebo continued to gain weight (0.31 kg [0.68 lb]/week).
The usual starting metformin dose is 500 mg bid with meals. Increase in increments of 500 mg weekly, up to a maximum of 2,000 mg/d in divided doses. Potential side effects include diarrhea, nausea/vomiting, flatulence, and headache.
Hyperlipidemia. Patients who gain weight with atypical antipsychotics also may develop hyperlipidemia. Fasting serum triglycerides >150 mg/dL (1.70 mmol/L) in obese children are considered to be elevated and an early sign of metabolic syndrome.30 Fasting total cholesterol >200 mg/dL (5.18 mmol/L) or low-density lipoprotein cholesterol >130 mg/dL (3.38 mmol/L) is consistent with hyperlipidemia.
Recommendation. Monitor and treat hyperlipidemia, which increases the risk of atherosclerosis as obese children grow older.31
Prolactin. Elevated prolactin concentrations may have deleterious effects in the developing child or adolescent, including gynecomastia, oligomenorrhea, and amenorrhea.17 Long-term effects on growth and sexual maturation have not been fully evaluated.
The relative tendency of atypical antipsychotics to cause hyperprolactinemia is roughly: risperidone/paliperidone > olanzapine > ziprasidone > quetiapine > clozapine > aripiprazole.18 In the risperidone RCT, mean changes in baseline prolactin levels were 41 ng/mL for boys and 59 ng/mL in girls.3 Results of the olanzapine RCT suggest a high incidence of hyperprolactinemia (26% of girls, 63% of boys).4 Decreases in serum prolactin were observed in bipolar children and adolescents treated with aripiprazole for 30 weeks.19
Recommendations. For any pediatric patient treated with an atypical antipsychotic that increases prolactin levels:
- Obtain a baseline prolactin level.
- Repeat after 6 months of treatment or with the emergence of elevated prolactin symptoms, such as gynecomastia in boys. Ask about increases in breast size, galactorrhea, changes in menstruation, sexual functioning, and pubertal development.
Switch patients who develop any of these side effects to another atypical agent that does not increase serum prolactin.32
QTc interval prolongation. All atypical antipsychotics can cause QTc prolongation. Several cases of significant QTc prolongation have been reported in children and adolescents treated with ziprasidone.33,34 In the RCT of ziprasidone, QTc prolongation was not clinically significant in most of the patients in which it was reported, and it did not lead to adverse events.34 Mean QTc change was 8.1 msec at study termination.7
Patients enrolled in clinical trails are screened very carefully, however, and those with preexisting medical abnormalities typically are excluded. Thus, these findings may have limited usefulness for “real-world” patients.
Recommendations. Until additional information is known about the cardiac effects of atypical antipsychotics in children and adolescents:
- Perform a careful history, review of symptoms, and physical exam looking for any history of palpitations, shortness of breath, or syncope.
- Query specifically about any family history of sudden cardiac death.
- Perform a baseline resting ECG for patients starting ziprasidone or clozapine, or for other atypicals if indicated by history, review of systems, physical exam, etc.
- For patients treated with ziprasidone or clozapine, repeat ECG as the dose increases or if the patient has cardiac symptoms (unexplained shortness of breath, palpitations, skipped beats, etc.).
Table 3
Talking to families about using antipsychotics
in children with bipolar disorder
| Effectiveness. Large, placebo-controlled studies have shown that atypical antipsychotics can significantly reduce manic symptoms in children and adolescents with bipolar disorder |
| Safety data. Additional 6-month safety data indicate that atypical antipsychotics continue to be effective in children and adolescents, without dramatic changes in side effects |
| Precautions. Antipsychotics are powerful medications and must be used carefully in pediatric patients |
| Potential side effects. All antipsychotics have serious potential side effects that must be recognized, monitored, and managed |
| Potential benefits from using atypical antipsychotics include mood stabilization, treatment of psychotic symptoms, and lower risk of extrapyramidal symptoms compared with typical antipsychotics |
| Risk vs benefit. On balance, the potential benefit of these agents outweighs the potential risk for children and adolescents with bipolar disorder |
Figure: Mean weight gain with atypical antipsychotics in pediatric bipolar trials
Weight gain in children and adolescents with bipolar disorder varied among atypical antipsychotics used in 5 recent randomized controlled trials. Treatment duration was 3 weeks with olanzapine, risperidone, and quetiapine and 4 weeks with aripiprazole and ziprasidone. Dosages were olanzapine, 10.4 ± 4.5 mg/d; risperidone, 0.5 to 2.5 mg/d or 3 to 6 mg/d; aripiprazole, 10 or 30 mg/d; quetiapine, 400 or 600 mg/d; and ziprasidone, 80 to 160 mg/d.
Source: References 3-7Related resources
- Child and Adolescent Bipolar Foundation. www.bpkids.org.
- University of Illinois at Chicago Pediatric Mood Disorders Clinic. www.psych.uic.edu/pmdc.
- Ryan Licht Sang Bipolar Foundation. www.ryanlichtsangbipolarfoundation.org.
Drug brand names
- Aripiprazole • Abilify
- Benztropine • Cogentin
- Carbamazepine • Carbatrol
- Clozapine • Clozaril
- Diphenhydramine • Benadryl
- Divalproex sodium • Depakote
- Lithium • Lithobid, others
- Metformin • Glucophage
- Olanzapine • Zyprexa
- Paliperidone • Invega
- Propranolol • Inderal
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Valproate • Depacon
- Ziprasidone • Geodon
Disclosures
Dr. Kowatch is a consultant to and speaker for AstraZeneca and a consultant to Forest Pharmaceuticals. He receives research support from the National Alliance for Research on Schizophrenia and Depression, the National Institute of Child Health and Human Development, the National Institute of Mental Health, and the Stanley Foundation.
Dr. Strawn has received research support from the American Academy of Child and Adolescent Psychiatry (Lilly Pilot Research Award).
Dr. Sorter receives research support from the National Institute of Mental Health and the Health Foundation of Greater Cincinnati.
1. Hopewell S, Clarke M, Stewart L, et al. Time to publication for results of clinical trials. Cochrane Database Syst Rev. 2007;(2):MR000011.-
2. Carey B. Risks found for youths in new antipsychotics. The New York Times. September 15, 2008:A17.
3. Pandina G, DelBello M, Kushner S, et al. Risperidone for the treatment of acute mania in bipolar youth. Paper presented at: Annual Meeting of the American Academy of Child and Adolescent Psychiatry, October 23-28, 2007; Boston, MA.
4. Tohen M, Kryzhanovskaya L, Carlson G, et al. Olanzapine versus placebo in the treatment of adolescents with bipolar mania. Am J Psychiatry. 2007;164(10):1547-1556.
5. DelBello M, Findling RL, Earley W, et al. Efficacy of quetiapine in children and adolescents with bipolar mania: a 3-week, double-blind, randomized, placebo-controlled trial. Paper presented at: Annual Meeting of the American Academy of Child and Adolescent Psychiatry, October 23-28, 2007; Boston, MA.
6. Wagner K, Nyilas M, Forbes R, et al. Acute efficacy of aripiprazole for the treatment of bipolar I disorder, mixed or manic, in pediatric patients. Paper presented at: Annual Meeting of the American College of Neuropsychopharmacology, December 9-13, 2007; Boca Raton, FL.
7. DelBello M, Findling RL, Wang P, et al. Safety and efficacy of ziprasidone in pediatric bipolar disorder. Paper presented at: Annual Meeting of the American Psychiatric Association, May 3-8, 2008; Washington, DC.
8. McElduff P, Jaefarnezhad M, Durrington PN. American, British and European recommendations for statins in the primary prevention of cardiovascular disease applied to British men studied prospectively. Heart. 2006;92(9):1213-1218.
9. Tsapakis EM, Soldani F, Tondo L, et al. Efficacy of antidepressants in juvenile depression: meta-analysis. Br J Psychiatry. 2008;193(1):10-17.
10. Kowatch R, Findling R, Scheffer R, et al. Placebo controlled trial of divalproex versus lithium for bipolar disorder. Paper presented at: Annual Meeting of the American Academy of Child and Adolescent Psychiatry; October 23-28, 2007; Boston, MA.
11. DelBello MP, Kowatch RA, Adler CM, et al. A double-blind randomized pilot study comparing quetiapine and divalproex for adolescent mania. J Am Acad Child Adolesc Psychiatry. 2006;45(3):305-313.
12. DelBello MP, Schwiers ML, Rosenberg HL, et al. A double-blind, randomized, placebo-controlled study of quetiapine as adjunctive treatment for adolescent mania. J Am Acad Child Adolesc Psychiatry. 2002;41(10):1216-1223.
13. Practice guideline for the treatment of patients with bipolar disorder (revision). Am J Psychiatry. 2002;159(4 suppl):1-50.
14. Suppes T, Dennehy EB, Hirschfeld RM, et al. The Texas implementation of medication algorithms: update to the algorithms for treatment of bipolar I disorder. J Clin Psychiatry. 2005;66(7):870-886.
15. Becker AL, Epperson CN. Female puberty: clinical implications for the use of prolactin-modulating psychotropics. Child Adolesc Psychiatr Clin N Am. 2006;15(1):207-220.
16. Correll CU, Penzner JB, Parikh UH, et al. Recognizing and monitoring adverse events of second-generation antipsychotics in children and adolescents. Child Adolesc Psychiatr Clin N Am. 2006;15(1):177-206.
17. Correll CU. Effect of hyperprolactinemia during development in children and adolescents. J Clin Psychiatry. 2008;69(8):e24.-
18. Correll CU. Antipsychotic use in children and adolescents: minimizing adverse effects to maximize outcomes. J Am Acad Child Adolesc Psychiatry. 2008;47(1):9-20.
19. Correll CU, Nyilas M, Ashfaque S, et al. Long-term safety and tolerability of aripiprazole in children (10-17 years) with bipolar disorder. Paper presented at: Annual Meeting of the American College of Neuropsychopharmacology; December 9-13, 2007; Boca Raton, FL.
20. Pandina GJ, Bossie CA, Youssef E, et al. Risperidone improves behavioral symptoms in children with autism in a improves behavioral symptoms in children with autism in a randomized, double-blind, placebo-controlled trial. J Autism Dev Disord. 2007;37(2):367-373.
21. DelBello M, Findling RL, Earley W, et al. Efficacy of quetiapine in children and adolescent with bipolar mania; a 3-week, double-blind, randomized, placebo-controlled trial. Paper presented at: Annual Meeting of the American College of Neuropsychopharmacology; December 9-13, 2007; Boca Raton, FL.
22. Correll CU, Kane JM. One-year incidence rates of tardive dyskinesia in children and adolescents treated with second-generation antipsychotics: a systematic review. J Child Adolesc Psychopharmacol. 2007;17(5):647-656.
23. McDermid SA, Hood J, Bockus S, et al. Adolescents on neuroleptic medication: is this population at risk for tardive dyskinesia? Can J Psychiatry. 1998;43(6):629-631.
24. Kumra S, Jacobsen LK, Lenane M, et al. Case series: spectrum of neuroleptic-induced movement disorders and extrapyramidal side effects in childhood-onset schizophrenia. J Am Acad Child Adolesc Psychiatry. 1998;37(2):221-227.
25. Strawn JR, Keck PE, Jr, Caroff SN. Neuroleptic malignant syndrome. Am J Psychiatry. 2007;164(6):870-876.
26. Croarkin PE, Emslie GJ, Mayes TL. Neuroleptic malignant syndrome associated with atypical antipsychotics in pediatric patients: a review of published cases. J Clin Psychiatry. 2008;69(7):1157-1165.
27. Picard LS, Lindsay S, Strawn JR, et al. Atypical neuroleptic malignant syndrome: diagnostic controversies and considerations. Pharmacotherapy. 2008;28(4):530-535.
28. Correll CU. Metabolic side effects of second-generation antipsychotics in children and adolescents: a different story? J Clin Psychiatry. 2005;66(10):1331-1332.
29. Klein DJ, Cottingham EM, Sorter M, et al. A randomized, double-blind, placebo-controlled trial of metformin treatment of weight gain associated with initiation of atypical antipsychotic therapy in children and adolescents. Am J Psychiatry. 2006;163(12):2072-2079.
30. Kavey RE, Allada V, Daniels SR, et al. Cardiovascular risk reduction in high-risk pediatric patients: a scientific statement from the American Heart Association Expert Panel on Population and Prevention Science; the Councils on Cardiovascular Disease in the Young, Epidemiology and Prevention, Nutrition, Physical Activity and Metabolism, High Blood Pressure Research, Cardiovascular Nursing, and the Kidney in Heart Disease; and the Interdisciplinary Working Group on Quality of Care and Outcomes Research. J Cardiovasc Nurs. 2007;22(3):218-253.
31. O’Grady MJ, Brown AM, O’Neill MB. Cholesterol screening in an at-risk pediatric population. Pediatr Cardiol. 2008;29(3):609-613.
32. Ali J, Khemka M. Hyperprolactinemia: monitoring children on long-term risperidone. Current Psychiatry. 2008;7(11):64-72.
33. Blair J, Scahill L, State M, et al. Electrocardiographic changes in children and adolescents treated with ziprasidone: a prospective study. J Am Acad Child Adolesc Psychiatry. 2005;44(1):73-79.
34. Malone RP, Delaney MA, Hyman SB, et al. Ziprasidone in adolescents with autism: an open-label pilot study. J Child Adolesc Psychopharmacol. 2007;17(6):779-790.
Five recent randomized controlled trials (RCTs) have demonstrated the efficacy of atypical antipsychotics for treating bipolar disorder in children and adolescents, but 4 of these 5 trials remain unpublished. The lag time between the completion of these trials and publication of their results—typically 4 to 5 years1—leaves psychiatrists without important evidence to explain to families and critics2 why they might recommend using these powerful medications in children with mental illness.
This article previews the preliminary results of these 5 RCTs of atypical antipsychotics, offers a treatment algorithm supported by this evidence, and discusses how to manage potentially serious risks when using antipsychotics to treat children and adolescents with bipolar disorder (BPD).
Where do atypical antipsychotics fit in?
Details of the 5 industry-sponsored RCTs of atypical antipsychotics in children and adolescents with bipolar I manic or mixed episodes are summarized in Table 1.3-7 Only the olanzapine study4 has been published; data from the other 4 trials were presented at medical meetings in 2007 and 2008.
Change in Young Mania Rating Scale (YMRS) score was the primary outcome measure in these 5 trials, and each compound was more effective than placebo. The trials demonstrated statistically significant and clinically relevant differences between each antipsychotic and placebo. The number needed to treat (NNT)—how many patients need to be treated for 1 to benefit in a controlled clinical trial—ranged from 2 to 4. For comparison, the NNT for statins in the prevention of coronary events is 12 to 22,8 and the NNT in an analysis of trials of selective serotonin reuptake inhibitors for pediatric major depressive disorder was 9.9 Thus, an NNT of ≤4 represents a clinically significant effect.
Risperidone is FDA-approved for short-term treatment of acute bipolar I manic or mixed episodes in patients age 10 to 17. Aripiprazole is approved for acute and maintenance treatment of bipolar I manic or mixed episodes (with or without psychosis) as monotherapy or with lithium or valproate in patients age 10 to 17. In June, an FDA advisory committee recommended pediatric bipolar indications for olanzapine, quetiapine, and ziprasidone.
‘Mood stabilizers’ such as lithium, valproate, and carbamazepine have been used for years to treat bipolar mania in adults, adolescents, and children, despite limited supporting evidence. Preliminary results of a National Institute of Mental Health-funded double-blind RCT provide insights on their efficacy.10
The 153 outpatients age 7 to 17 in a bipolar I manic or mixed episode were randomly assigned to lithium, divalproex, or placebo for 8 weeks. Response rates—based on a Clinical Global Impressions-Improvement score of 1 or 2 (very much or much improved)—were divalproex, 54%; lithium, 42%; and placebo, 29%. Lithium showed a trend toward efficacy but did not clearly separate from placebo on the primary outcome measures. Effect sizes for lithium and divalproex were moderate.10
Only 1 study has compared a mood stabilizer with an atypical antipsychotic for treating mania in adolescents. In a double-blind trial, DelBello et al11 randomly assigned 50 patients age 12 to 18 with a bipolar I manic or mixed episode to quetiapine, 400 to 600 mg/d, or divalproex, serum level 80 to 120 μg/mL, for 28 days. Manic symptoms resolved more rapidly, and remission rates measured by the YMRS were higher with quetiapine than with divalproex. Both medications were well tolerated.
Combination therapy. BPD as it presents in children and adolescents is often difficult to treat because of the disorder’s various phases (manic, depressed, mixed), frequent psychotic symptoms, and high rate of comorbidity. Pediatric BPD patients frequently require several psychotropics, including mood stabilizers and atypical antipsychotics.
In a double-blind, placebo-controlled study, 30 adolescents in a bipolar I manic or mixed episode initially received divalproex, 20 mg/kg/d, then were randomly assigned to 6 weeks of adjunctive quetiapine, titrated to 450 mg/d in 7 days (n=15), or placebo (n=15). Those receiving divalproex plus quetiapine showed a statistically significant greater reduction in manic symptoms (P=.03) and a higher response rate (87% vs 53%, P=.05), compared with those receiving divalproex and placebo. This suggests that a mood stabilizer plus an atypical antipsychotic is more effective than a mood stabilizer alone for adolescent mania. Quetiapine was well tolerated.12
Treatment. The American Psychiatric Association’s outdated 2002 practice guideline for acute bipolar I manic or mixed episodes in adults recommends lithium, valproate, and/or an antipsychotic.13 The more recent Texas Medication Algorithm Project (TMAP) guidelines recommend monotherapy with lithium, valproate, aripiprazole, quetiapine, risperidone, or ziprasidone for adults with euphoric or irritable manic or hypomanic symptoms.14
Based on the TMAP algorithm, recent clinical trial evidence, and our experience in treating pediatric BPD, we offer an approach for treating mania/hypomania in patients age 10 to 17 (see Proposed Algorithm). For dosing and precautions when using atypical antipsychotics in children and adolescents with BPD, see Table 2.15-17
Comorbid psychiatric illnesses (such as anxiety disorders) are prevalent in adolescents with BPD. Evidence in adults and adolescents suggests that some atypical antipsychotics may provide additional benefit for these conditions as well. Thus, consider comorbid conditions and symptoms when choosing antimanic agents.
Attention-deficit/hyperactivity disorder (ADHD) is a common comorbidity in children with BPD, and stimulant medications are most often prescribed to treat inattentiveness and hyperactivity. Caution is imperative when treating bipolar youth with stimulants, which can exacerbate manic symptoms. Treat the patient’s mania before adding or reintroducing stimulant medication. Research and clinical experience suggest that if you first stabilize these patients on a mood stabilizer or atypical antipsychotic, adding a stimulant can be very helpful in treating comorbid ADHD symptoms. Start with low stimulant doses, and increase slowly.
Table 1
RCTs of atypical antipsychotics in patients age 10 to 17
with bipolar I disorder*
| Antipsychotic and source | Bipolar I episode (# of subjects) | Trial duration (days) | Dosage (mg/d) | Response rate or YMRS score change | NNT | Mean weight gain (kg) |
|---|---|---|---|---|---|---|
| Risperidone Pandina et al3 AACAP 2007 | Manic, mixed (169) | 21 | 0.5 to 2.5 3 to 6 | 59% 63% | 3.3 3.5 | 1.9 1.4 |
| Olanzapine Tohen et al4 | Manic, mixed (161) | 21 | 10.4 ± 4.5 | 49% | 4.1 | 3.7 ± 2.2 |
| Quetiapine DelBello et al5 AACAP 2007 | Manic (284) | 21 | 400 600 | 64% 58% | 4.4 4.2 | 1.7 1.7 |
| Aripiprazole Wagner et al6 ACNP 2007 | Manic, mixed (296) | 28 | 10 30 | 45% 64% | 4.1 2.4 | 0.9 0.54 |
| Ziprasidone DelBello et al7 APA 2008 | Manic, mixed (238) | 28 | 80 to 160 | –13.83 with ziprasidone, –8.61 with placebo | 3.7 | None |
| *Each trial included a 6-month open extension phase; results are pending | ||||||
| AACAP: American Academy of Child and Adolescent Psychiatry; ACNP: American College of Neuropsychopharmacology; APA: American Psychiatric Association; NNT: number needed to treat; RCT: randomized controlled trial; YMRS: Young Mania Rating Scale | ||||||
Table 2
Recommended antipsychotic use in pediatric bipolar disorder
| Drug | Starting dosage (mg) | Target dosage (mg/d) | Precautions |
|---|---|---|---|
| Aripiprazole | 2.5 to 5 at bedtime | 10 to 30 | Monitor for CYP 3A4 and 2D6 interactions, weight, BMI, cholesterol, lipids, and glucose |
| Olanzapine | 2.5 bid | 10 to 20 | Monitor for CYP 2D6 interactions, weight, BMI, cholesterol, lipids, glucose, and prolactin levels |
| Quetiapine | 50 bid | 400 to 1,200 | Monitor for weight, BMI, cholesterol, lipids, and glucose |
| Risperidone | 0.25 bid | 1 to 2.5 | Monitor for EPS, hyperprolactinemia (and associated sexual side effects, including galactorrhea), weight, BMI, cholesterol, lipids, glucose, and prolactin levels |
| Ziprasidone | 20 bid | 80 to 160 | Check baseline ECG and as dose increases or with reason for high level of concern; monitor prolactin levels |
| BMI: body mass index; CYP: cytochrome P450; ECG: electrocardiography; EPS: extrapyramidal symptoms | |||
| Source: References 15-17 | |||
Proposed Algorithm: Treating a bipolar mixed/manic episode in patients age 10 to 17
Stage 1. Consider patient’s experience with antipsychotics, body weight, and family history when choosing first-line monotherapy (1A). Quetiapine poses low risk for extrapyramidal symptoms and tardive dyskinesia. Aripiprazole and ziprasidone pose relatively low risk of weight gain. Risperidone is potent at low doses but increases prolactin levels (long-term effect unknown).
Second-line choices (1B) are mood stabilizers lithium and valproate (because of lower potency than atypical antipsychotics), and olanzapine (which—although potent—causes substantial weight gain). In case of lack of response or intolerable side effects with initial agent, select an alternate from 1A or 1B. If this is not effective, move to Stage 2.
Stage 2. Consider augmentation for patients who show partial response to monotherapy (in your clinical judgment “mild to moderately improved” but not “much or very much improved”).
Stage 3. Combination therapy could include 2 mood stabilizers (such as lithium and valproate) plus an atypical antipsychotic; 2 atypical antipsychotics; or other combinations based on patient’s past responses. No research has shown these combinations to be efficacious in bipolar children and adolescents, but we find they sometimes help those with treatment-resistant symptoms.
Duration. Maintain psychotropics 12 to 18 months. When patient is euthymic, slowly taper 1 medication across several months. If symptoms recur, reintroduce the mood-stabilizing agent(s).
Source: Adapted and reprinted with permission from Kowatch RA, Fristad MA, Findling R, et al. Clinical manual for the management of bipolar disorder in children and adolescents. Arlington, VA: American Psychiatric Publishing, Inc.; 2008
Managing adverse effects
Although clinically effective, atypical antipsychotics may cause serious side effects that must be recognized and managed. These include extrapyramidal symptoms (EPS), tardive dyskinesia (TD), weight gain and obesity, hyperlipidemia, increased prolactin levels, and QTc changes. Counsel patients and families about the risks and benefits of antipsychotics when you consider them for children and adolescents with BPD (Table 3).
EPS. Drug-induced parkinsonism and akathisia are the most common EPS in children and adolescents with BPD treated with atypical antipsychotics.18
Correll et al19 reported a 10% rate of EPS in patients treated with aripiprazole. Treatment-emergent EPS also was observed in the RCT of risperidone.20 EPS-related adverse events were associated with higher doses of risperidone, although none of the akathisia/EPS measures were thought to be “clinically significant.”
EPS frequency was relatively low and similar to placebo in the 3-week quetiapine trial,21 and no changes in movement disorder scale scores were observed during the olanzapine or ziprasidone RCTs.4,7
Recommendations. If your pediatric patient develops EPS, first try an antipsychotic dose reduction. Because anticholinergics can contribute to antipsychotic-induced weight gain, reserve them until after a dosage reduction has been unsuccessful.
Benztropine (0.25 to 0.5 mg given 2 to 3 times daily, not to exceed 3 mg/d) or diphenhydramine (25 to 50 mg given 3 to 4 times daily; maximum dosage 5 mg/kg/d) can be effective in treating EPS. Avoid anticholinergics in children with narrow-angle glaucoma or age <3.
Akathisia may be managed with propranolol (20 to 120 mg/d in divided doses). Multiple doses (typically 3 times daily) are needed to prevent interdose withdrawal symptoms. Use this beta blocker with caution in children with asthma because of the possibility of bronchospasm.
TD. Short-term trials and a meta-analysis of atypical antipsychotic trials (>11 months’ duration, subject age <18) suggest a low annual risk for TD (0.4%).22 Large, prospective, long-term trials of atypical antipsychotics are necessary to more accurately define the risk of TD in the pediatric population, however. Retrospective analyses of adolescents treated with antipsychotics suggest 3 TD risk factors:
- early age of antipsychotic use
- medication nonadherence
- concomitant use of antiparkinsonian agents.23
Kumra et al24 identified lower premorbid functioning and greater positive symptoms at baseline as factors associated with “withdrawal dyskinesia/tardive dyskinesia” in children and adolescents with early-onset psychotic-spectrum disorders treated with typical or atypical antipsychotics.
Recommendations. To minimize TD risk, use the lowest effective antipsychotic dose, monitor for abnormal involuntary movements with standardized assessments (such as the Abnormal Involuntary Movement Scale), review risks and benefits with parents and patients, and regularly evaluate the indication and need for antipsychotic therapy. It is reasonable to attempt to lower the antipsychotic dose after the patient has attained remission and been stable for 1 year.
Neuroleptic malignant syndrome (NMS). This complication of dopamine-blocking medications:
- is among the most serious adverse effects of antipsychotic treatment
- continues to be associated with a mortality rate of 10%.25
Recommendation. At least 1 recent review of pediatric NMS cases suggests that essential features (hyperthermia and severe muscular rigidity) are retained in children.26 Nonetheless, monitor for variant presentations; hyper thermia or muscle rigidity may be absent or develop slowly over several days in patients treated with atypical antipsychotics.27
Weight gain and glucose metabolism. A major adverse effect of most atypical antipsychotics is increased appetite, weight gain, and possible obesity.28 In children, “obesity” refers to a body mass index (BMI) >95th percentile for age and sex; “over-weight” refers to BMI between the 85th and 95th percentile. Mean weight gain in the 5 atypical antipsychotic pediatric bipolar trials ranged from 0 to 8 lbs across 3 to 4 weeks of treatment (Figure).3-7
Recommendations. Emphasize diet and exercise, with restriction of high-carbohydrate food, “fast foods,” and soft drinks. Another option is a trial of metformin, which decreases hepatic glucose production, decreases intestinal absorption of glucose, and improves insulin sensitivity by increasing peripheral glucose uptake and utilization.
Klein et al29 studied 39 patients age 10 to 17 with mood and psychotic disorders whose weight increased by >10% during <1 year of olanzapine, risperidone, or quetiapine therapy. In this 16-week, double-blind, placebo-controlled trial, weight was stabilized in subjects receiving metformin, whereas those receiving placebo continued to gain weight (0.31 kg [0.68 lb]/week).
The usual starting metformin dose is 500 mg bid with meals. Increase in increments of 500 mg weekly, up to a maximum of 2,000 mg/d in divided doses. Potential side effects include diarrhea, nausea/vomiting, flatulence, and headache.
Hyperlipidemia. Patients who gain weight with atypical antipsychotics also may develop hyperlipidemia. Fasting serum triglycerides >150 mg/dL (1.70 mmol/L) in obese children are considered to be elevated and an early sign of metabolic syndrome.30 Fasting total cholesterol >200 mg/dL (5.18 mmol/L) or low-density lipoprotein cholesterol >130 mg/dL (3.38 mmol/L) is consistent with hyperlipidemia.
Recommendation. Monitor and treat hyperlipidemia, which increases the risk of atherosclerosis as obese children grow older.31
Prolactin. Elevated prolactin concentrations may have deleterious effects in the developing child or adolescent, including gynecomastia, oligomenorrhea, and amenorrhea.17 Long-term effects on growth and sexual maturation have not been fully evaluated.
The relative tendency of atypical antipsychotics to cause hyperprolactinemia is roughly: risperidone/paliperidone > olanzapine > ziprasidone > quetiapine > clozapine > aripiprazole.18 In the risperidone RCT, mean changes in baseline prolactin levels were 41 ng/mL for boys and 59 ng/mL in girls.3 Results of the olanzapine RCT suggest a high incidence of hyperprolactinemia (26% of girls, 63% of boys).4 Decreases in serum prolactin were observed in bipolar children and adolescents treated with aripiprazole for 30 weeks.19
Recommendations. For any pediatric patient treated with an atypical antipsychotic that increases prolactin levels:
- Obtain a baseline prolactin level.
- Repeat after 6 months of treatment or with the emergence of elevated prolactin symptoms, such as gynecomastia in boys. Ask about increases in breast size, galactorrhea, changes in menstruation, sexual functioning, and pubertal development.
Switch patients who develop any of these side effects to another atypical agent that does not increase serum prolactin.32
QTc interval prolongation. All atypical antipsychotics can cause QTc prolongation. Several cases of significant QTc prolongation have been reported in children and adolescents treated with ziprasidone.33,34 In the RCT of ziprasidone, QTc prolongation was not clinically significant in most of the patients in which it was reported, and it did not lead to adverse events.34 Mean QTc change was 8.1 msec at study termination.7
Patients enrolled in clinical trails are screened very carefully, however, and those with preexisting medical abnormalities typically are excluded. Thus, these findings may have limited usefulness for “real-world” patients.
Recommendations. Until additional information is known about the cardiac effects of atypical antipsychotics in children and adolescents:
- Perform a careful history, review of symptoms, and physical exam looking for any history of palpitations, shortness of breath, or syncope.
- Query specifically about any family history of sudden cardiac death.
- Perform a baseline resting ECG for patients starting ziprasidone or clozapine, or for other atypicals if indicated by history, review of systems, physical exam, etc.
- For patients treated with ziprasidone or clozapine, repeat ECG as the dose increases or if the patient has cardiac symptoms (unexplained shortness of breath, palpitations, skipped beats, etc.).
Table 3
Talking to families about using antipsychotics
in children with bipolar disorder
| Effectiveness. Large, placebo-controlled studies have shown that atypical antipsychotics can significantly reduce manic symptoms in children and adolescents with bipolar disorder |
| Safety data. Additional 6-month safety data indicate that atypical antipsychotics continue to be effective in children and adolescents, without dramatic changes in side effects |
| Precautions. Antipsychotics are powerful medications and must be used carefully in pediatric patients |
| Potential side effects. All antipsychotics have serious potential side effects that must be recognized, monitored, and managed |
| Potential benefits from using atypical antipsychotics include mood stabilization, treatment of psychotic symptoms, and lower risk of extrapyramidal symptoms compared with typical antipsychotics |
| Risk vs benefit. On balance, the potential benefit of these agents outweighs the potential risk for children and adolescents with bipolar disorder |
Figure: Mean weight gain with atypical antipsychotics in pediatric bipolar trials
Weight gain in children and adolescents with bipolar disorder varied among atypical antipsychotics used in 5 recent randomized controlled trials. Treatment duration was 3 weeks with olanzapine, risperidone, and quetiapine and 4 weeks with aripiprazole and ziprasidone. Dosages were olanzapine, 10.4 ± 4.5 mg/d; risperidone, 0.5 to 2.5 mg/d or 3 to 6 mg/d; aripiprazole, 10 or 30 mg/d; quetiapine, 400 or 600 mg/d; and ziprasidone, 80 to 160 mg/d.
Source: References 3-7Related resources
- Child and Adolescent Bipolar Foundation. www.bpkids.org.
- University of Illinois at Chicago Pediatric Mood Disorders Clinic. www.psych.uic.edu/pmdc.
- Ryan Licht Sang Bipolar Foundation. www.ryanlichtsangbipolarfoundation.org.
Drug brand names
- Aripiprazole • Abilify
- Benztropine • Cogentin
- Carbamazepine • Carbatrol
- Clozapine • Clozaril
- Diphenhydramine • Benadryl
- Divalproex sodium • Depakote
- Lithium • Lithobid, others
- Metformin • Glucophage
- Olanzapine • Zyprexa
- Paliperidone • Invega
- Propranolol • Inderal
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Valproate • Depacon
- Ziprasidone • Geodon
Disclosures
Dr. Kowatch is a consultant to and speaker for AstraZeneca and a consultant to Forest Pharmaceuticals. He receives research support from the National Alliance for Research on Schizophrenia and Depression, the National Institute of Child Health and Human Development, the National Institute of Mental Health, and the Stanley Foundation.
Dr. Strawn has received research support from the American Academy of Child and Adolescent Psychiatry (Lilly Pilot Research Award).
Dr. Sorter receives research support from the National Institute of Mental Health and the Health Foundation of Greater Cincinnati.
Five recent randomized controlled trials (RCTs) have demonstrated the efficacy of atypical antipsychotics for treating bipolar disorder in children and adolescents, but 4 of these 5 trials remain unpublished. The lag time between the completion of these trials and publication of their results—typically 4 to 5 years1—leaves psychiatrists without important evidence to explain to families and critics2 why they might recommend using these powerful medications in children with mental illness.
This article previews the preliminary results of these 5 RCTs of atypical antipsychotics, offers a treatment algorithm supported by this evidence, and discusses how to manage potentially serious risks when using antipsychotics to treat children and adolescents with bipolar disorder (BPD).
Where do atypical antipsychotics fit in?
Details of the 5 industry-sponsored RCTs of atypical antipsychotics in children and adolescents with bipolar I manic or mixed episodes are summarized in Table 1.3-7 Only the olanzapine study4 has been published; data from the other 4 trials were presented at medical meetings in 2007 and 2008.
Change in Young Mania Rating Scale (YMRS) score was the primary outcome measure in these 5 trials, and each compound was more effective than placebo. The trials demonstrated statistically significant and clinically relevant differences between each antipsychotic and placebo. The number needed to treat (NNT)—how many patients need to be treated for 1 to benefit in a controlled clinical trial—ranged from 2 to 4. For comparison, the NNT for statins in the prevention of coronary events is 12 to 22,8 and the NNT in an analysis of trials of selective serotonin reuptake inhibitors for pediatric major depressive disorder was 9.9 Thus, an NNT of ≤4 represents a clinically significant effect.
Risperidone is FDA-approved for short-term treatment of acute bipolar I manic or mixed episodes in patients age 10 to 17. Aripiprazole is approved for acute and maintenance treatment of bipolar I manic or mixed episodes (with or without psychosis) as monotherapy or with lithium or valproate in patients age 10 to 17. In June, an FDA advisory committee recommended pediatric bipolar indications for olanzapine, quetiapine, and ziprasidone.
‘Mood stabilizers’ such as lithium, valproate, and carbamazepine have been used for years to treat bipolar mania in adults, adolescents, and children, despite limited supporting evidence. Preliminary results of a National Institute of Mental Health-funded double-blind RCT provide insights on their efficacy.10
The 153 outpatients age 7 to 17 in a bipolar I manic or mixed episode were randomly assigned to lithium, divalproex, or placebo for 8 weeks. Response rates—based on a Clinical Global Impressions-Improvement score of 1 or 2 (very much or much improved)—were divalproex, 54%; lithium, 42%; and placebo, 29%. Lithium showed a trend toward efficacy but did not clearly separate from placebo on the primary outcome measures. Effect sizes for lithium and divalproex were moderate.10
Only 1 study has compared a mood stabilizer with an atypical antipsychotic for treating mania in adolescents. In a double-blind trial, DelBello et al11 randomly assigned 50 patients age 12 to 18 with a bipolar I manic or mixed episode to quetiapine, 400 to 600 mg/d, or divalproex, serum level 80 to 120 μg/mL, for 28 days. Manic symptoms resolved more rapidly, and remission rates measured by the YMRS were higher with quetiapine than with divalproex. Both medications were well tolerated.
Combination therapy. BPD as it presents in children and adolescents is often difficult to treat because of the disorder’s various phases (manic, depressed, mixed), frequent psychotic symptoms, and high rate of comorbidity. Pediatric BPD patients frequently require several psychotropics, including mood stabilizers and atypical antipsychotics.
In a double-blind, placebo-controlled study, 30 adolescents in a bipolar I manic or mixed episode initially received divalproex, 20 mg/kg/d, then were randomly assigned to 6 weeks of adjunctive quetiapine, titrated to 450 mg/d in 7 days (n=15), or placebo (n=15). Those receiving divalproex plus quetiapine showed a statistically significant greater reduction in manic symptoms (P=.03) and a higher response rate (87% vs 53%, P=.05), compared with those receiving divalproex and placebo. This suggests that a mood stabilizer plus an atypical antipsychotic is more effective than a mood stabilizer alone for adolescent mania. Quetiapine was well tolerated.12
Treatment. The American Psychiatric Association’s outdated 2002 practice guideline for acute bipolar I manic or mixed episodes in adults recommends lithium, valproate, and/or an antipsychotic.13 The more recent Texas Medication Algorithm Project (TMAP) guidelines recommend monotherapy with lithium, valproate, aripiprazole, quetiapine, risperidone, or ziprasidone for adults with euphoric or irritable manic or hypomanic symptoms.14
Based on the TMAP algorithm, recent clinical trial evidence, and our experience in treating pediatric BPD, we offer an approach for treating mania/hypomania in patients age 10 to 17 (see Proposed Algorithm). For dosing and precautions when using atypical antipsychotics in children and adolescents with BPD, see Table 2.15-17
Comorbid psychiatric illnesses (such as anxiety disorders) are prevalent in adolescents with BPD. Evidence in adults and adolescents suggests that some atypical antipsychotics may provide additional benefit for these conditions as well. Thus, consider comorbid conditions and symptoms when choosing antimanic agents.
Attention-deficit/hyperactivity disorder (ADHD) is a common comorbidity in children with BPD, and stimulant medications are most often prescribed to treat inattentiveness and hyperactivity. Caution is imperative when treating bipolar youth with stimulants, which can exacerbate manic symptoms. Treat the patient’s mania before adding or reintroducing stimulant medication. Research and clinical experience suggest that if you first stabilize these patients on a mood stabilizer or atypical antipsychotic, adding a stimulant can be very helpful in treating comorbid ADHD symptoms. Start with low stimulant doses, and increase slowly.
Table 1
RCTs of atypical antipsychotics in patients age 10 to 17
with bipolar I disorder*
| Antipsychotic and source | Bipolar I episode (# of subjects) | Trial duration (days) | Dosage (mg/d) | Response rate or YMRS score change | NNT | Mean weight gain (kg) |
|---|---|---|---|---|---|---|
| Risperidone Pandina et al3 AACAP 2007 | Manic, mixed (169) | 21 | 0.5 to 2.5 3 to 6 | 59% 63% | 3.3 3.5 | 1.9 1.4 |
| Olanzapine Tohen et al4 | Manic, mixed (161) | 21 | 10.4 ± 4.5 | 49% | 4.1 | 3.7 ± 2.2 |
| Quetiapine DelBello et al5 AACAP 2007 | Manic (284) | 21 | 400 600 | 64% 58% | 4.4 4.2 | 1.7 1.7 |
| Aripiprazole Wagner et al6 ACNP 2007 | Manic, mixed (296) | 28 | 10 30 | 45% 64% | 4.1 2.4 | 0.9 0.54 |
| Ziprasidone DelBello et al7 APA 2008 | Manic, mixed (238) | 28 | 80 to 160 | –13.83 with ziprasidone, –8.61 with placebo | 3.7 | None |
| *Each trial included a 6-month open extension phase; results are pending | ||||||
| AACAP: American Academy of Child and Adolescent Psychiatry; ACNP: American College of Neuropsychopharmacology; APA: American Psychiatric Association; NNT: number needed to treat; RCT: randomized controlled trial; YMRS: Young Mania Rating Scale | ||||||
Table 2
Recommended antipsychotic use in pediatric bipolar disorder
| Drug | Starting dosage (mg) | Target dosage (mg/d) | Precautions |
|---|---|---|---|
| Aripiprazole | 2.5 to 5 at bedtime | 10 to 30 | Monitor for CYP 3A4 and 2D6 interactions, weight, BMI, cholesterol, lipids, and glucose |
| Olanzapine | 2.5 bid | 10 to 20 | Monitor for CYP 2D6 interactions, weight, BMI, cholesterol, lipids, glucose, and prolactin levels |
| Quetiapine | 50 bid | 400 to 1,200 | Monitor for weight, BMI, cholesterol, lipids, and glucose |
| Risperidone | 0.25 bid | 1 to 2.5 | Monitor for EPS, hyperprolactinemia (and associated sexual side effects, including galactorrhea), weight, BMI, cholesterol, lipids, glucose, and prolactin levels |
| Ziprasidone | 20 bid | 80 to 160 | Check baseline ECG and as dose increases or with reason for high level of concern; monitor prolactin levels |
| BMI: body mass index; CYP: cytochrome P450; ECG: electrocardiography; EPS: extrapyramidal symptoms | |||
| Source: References 15-17 | |||
Proposed Algorithm: Treating a bipolar mixed/manic episode in patients age 10 to 17
Stage 1. Consider patient’s experience with antipsychotics, body weight, and family history when choosing first-line monotherapy (1A). Quetiapine poses low risk for extrapyramidal symptoms and tardive dyskinesia. Aripiprazole and ziprasidone pose relatively low risk of weight gain. Risperidone is potent at low doses but increases prolactin levels (long-term effect unknown).
Second-line choices (1B) are mood stabilizers lithium and valproate (because of lower potency than atypical antipsychotics), and olanzapine (which—although potent—causes substantial weight gain). In case of lack of response or intolerable side effects with initial agent, select an alternate from 1A or 1B. If this is not effective, move to Stage 2.
Stage 2. Consider augmentation for patients who show partial response to monotherapy (in your clinical judgment “mild to moderately improved” but not “much or very much improved”).
Stage 3. Combination therapy could include 2 mood stabilizers (such as lithium and valproate) plus an atypical antipsychotic; 2 atypical antipsychotics; or other combinations based on patient’s past responses. No research has shown these combinations to be efficacious in bipolar children and adolescents, but we find they sometimes help those with treatment-resistant symptoms.
Duration. Maintain psychotropics 12 to 18 months. When patient is euthymic, slowly taper 1 medication across several months. If symptoms recur, reintroduce the mood-stabilizing agent(s).
Source: Adapted and reprinted with permission from Kowatch RA, Fristad MA, Findling R, et al. Clinical manual for the management of bipolar disorder in children and adolescents. Arlington, VA: American Psychiatric Publishing, Inc.; 2008
Managing adverse effects
Although clinically effective, atypical antipsychotics may cause serious side effects that must be recognized and managed. These include extrapyramidal symptoms (EPS), tardive dyskinesia (TD), weight gain and obesity, hyperlipidemia, increased prolactin levels, and QTc changes. Counsel patients and families about the risks and benefits of antipsychotics when you consider them for children and adolescents with BPD (Table 3).
EPS. Drug-induced parkinsonism and akathisia are the most common EPS in children and adolescents with BPD treated with atypical antipsychotics.18
Correll et al19 reported a 10% rate of EPS in patients treated with aripiprazole. Treatment-emergent EPS also was observed in the RCT of risperidone.20 EPS-related adverse events were associated with higher doses of risperidone, although none of the akathisia/EPS measures were thought to be “clinically significant.”
EPS frequency was relatively low and similar to placebo in the 3-week quetiapine trial,21 and no changes in movement disorder scale scores were observed during the olanzapine or ziprasidone RCTs.4,7
Recommendations. If your pediatric patient develops EPS, first try an antipsychotic dose reduction. Because anticholinergics can contribute to antipsychotic-induced weight gain, reserve them until after a dosage reduction has been unsuccessful.
Benztropine (0.25 to 0.5 mg given 2 to 3 times daily, not to exceed 3 mg/d) or diphenhydramine (25 to 50 mg given 3 to 4 times daily; maximum dosage 5 mg/kg/d) can be effective in treating EPS. Avoid anticholinergics in children with narrow-angle glaucoma or age <3.
Akathisia may be managed with propranolol (20 to 120 mg/d in divided doses). Multiple doses (typically 3 times daily) are needed to prevent interdose withdrawal symptoms. Use this beta blocker with caution in children with asthma because of the possibility of bronchospasm.
TD. Short-term trials and a meta-analysis of atypical antipsychotic trials (>11 months’ duration, subject age <18) suggest a low annual risk for TD (0.4%).22 Large, prospective, long-term trials of atypical antipsychotics are necessary to more accurately define the risk of TD in the pediatric population, however. Retrospective analyses of adolescents treated with antipsychotics suggest 3 TD risk factors:
- early age of antipsychotic use
- medication nonadherence
- concomitant use of antiparkinsonian agents.23
Kumra et al24 identified lower premorbid functioning and greater positive symptoms at baseline as factors associated with “withdrawal dyskinesia/tardive dyskinesia” in children and adolescents with early-onset psychotic-spectrum disorders treated with typical or atypical antipsychotics.
Recommendations. To minimize TD risk, use the lowest effective antipsychotic dose, monitor for abnormal involuntary movements with standardized assessments (such as the Abnormal Involuntary Movement Scale), review risks and benefits with parents and patients, and regularly evaluate the indication and need for antipsychotic therapy. It is reasonable to attempt to lower the antipsychotic dose after the patient has attained remission and been stable for 1 year.
Neuroleptic malignant syndrome (NMS). This complication of dopamine-blocking medications:
- is among the most serious adverse effects of antipsychotic treatment
- continues to be associated with a mortality rate of 10%.25
Recommendation. At least 1 recent review of pediatric NMS cases suggests that essential features (hyperthermia and severe muscular rigidity) are retained in children.26 Nonetheless, monitor for variant presentations; hyper thermia or muscle rigidity may be absent or develop slowly over several days in patients treated with atypical antipsychotics.27
Weight gain and glucose metabolism. A major adverse effect of most atypical antipsychotics is increased appetite, weight gain, and possible obesity.28 In children, “obesity” refers to a body mass index (BMI) >95th percentile for age and sex; “over-weight” refers to BMI between the 85th and 95th percentile. Mean weight gain in the 5 atypical antipsychotic pediatric bipolar trials ranged from 0 to 8 lbs across 3 to 4 weeks of treatment (Figure).3-7
Recommendations. Emphasize diet and exercise, with restriction of high-carbohydrate food, “fast foods,” and soft drinks. Another option is a trial of metformin, which decreases hepatic glucose production, decreases intestinal absorption of glucose, and improves insulin sensitivity by increasing peripheral glucose uptake and utilization.
Klein et al29 studied 39 patients age 10 to 17 with mood and psychotic disorders whose weight increased by >10% during <1 year of olanzapine, risperidone, or quetiapine therapy. In this 16-week, double-blind, placebo-controlled trial, weight was stabilized in subjects receiving metformin, whereas those receiving placebo continued to gain weight (0.31 kg [0.68 lb]/week).
The usual starting metformin dose is 500 mg bid with meals. Increase in increments of 500 mg weekly, up to a maximum of 2,000 mg/d in divided doses. Potential side effects include diarrhea, nausea/vomiting, flatulence, and headache.
Hyperlipidemia. Patients who gain weight with atypical antipsychotics also may develop hyperlipidemia. Fasting serum triglycerides >150 mg/dL (1.70 mmol/L) in obese children are considered to be elevated and an early sign of metabolic syndrome.30 Fasting total cholesterol >200 mg/dL (5.18 mmol/L) or low-density lipoprotein cholesterol >130 mg/dL (3.38 mmol/L) is consistent with hyperlipidemia.
Recommendation. Monitor and treat hyperlipidemia, which increases the risk of atherosclerosis as obese children grow older.31
Prolactin. Elevated prolactin concentrations may have deleterious effects in the developing child or adolescent, including gynecomastia, oligomenorrhea, and amenorrhea.17 Long-term effects on growth and sexual maturation have not been fully evaluated.
The relative tendency of atypical antipsychotics to cause hyperprolactinemia is roughly: risperidone/paliperidone > olanzapine > ziprasidone > quetiapine > clozapine > aripiprazole.18 In the risperidone RCT, mean changes in baseline prolactin levels were 41 ng/mL for boys and 59 ng/mL in girls.3 Results of the olanzapine RCT suggest a high incidence of hyperprolactinemia (26% of girls, 63% of boys).4 Decreases in serum prolactin were observed in bipolar children and adolescents treated with aripiprazole for 30 weeks.19
Recommendations. For any pediatric patient treated with an atypical antipsychotic that increases prolactin levels:
- Obtain a baseline prolactin level.
- Repeat after 6 months of treatment or with the emergence of elevated prolactin symptoms, such as gynecomastia in boys. Ask about increases in breast size, galactorrhea, changes in menstruation, sexual functioning, and pubertal development.
Switch patients who develop any of these side effects to another atypical agent that does not increase serum prolactin.32
QTc interval prolongation. All atypical antipsychotics can cause QTc prolongation. Several cases of significant QTc prolongation have been reported in children and adolescents treated with ziprasidone.33,34 In the RCT of ziprasidone, QTc prolongation was not clinically significant in most of the patients in which it was reported, and it did not lead to adverse events.34 Mean QTc change was 8.1 msec at study termination.7
Patients enrolled in clinical trails are screened very carefully, however, and those with preexisting medical abnormalities typically are excluded. Thus, these findings may have limited usefulness for “real-world” patients.
Recommendations. Until additional information is known about the cardiac effects of atypical antipsychotics in children and adolescents:
- Perform a careful history, review of symptoms, and physical exam looking for any history of palpitations, shortness of breath, or syncope.
- Query specifically about any family history of sudden cardiac death.
- Perform a baseline resting ECG for patients starting ziprasidone or clozapine, or for other atypicals if indicated by history, review of systems, physical exam, etc.
- For patients treated with ziprasidone or clozapine, repeat ECG as the dose increases or if the patient has cardiac symptoms (unexplained shortness of breath, palpitations, skipped beats, etc.).
Table 3
Talking to families about using antipsychotics
in children with bipolar disorder
| Effectiveness. Large, placebo-controlled studies have shown that atypical antipsychotics can significantly reduce manic symptoms in children and adolescents with bipolar disorder |
| Safety data. Additional 6-month safety data indicate that atypical antipsychotics continue to be effective in children and adolescents, without dramatic changes in side effects |
| Precautions. Antipsychotics are powerful medications and must be used carefully in pediatric patients |
| Potential side effects. All antipsychotics have serious potential side effects that must be recognized, monitored, and managed |
| Potential benefits from using atypical antipsychotics include mood stabilization, treatment of psychotic symptoms, and lower risk of extrapyramidal symptoms compared with typical antipsychotics |
| Risk vs benefit. On balance, the potential benefit of these agents outweighs the potential risk for children and adolescents with bipolar disorder |
Figure: Mean weight gain with atypical antipsychotics in pediatric bipolar trials
Weight gain in children and adolescents with bipolar disorder varied among atypical antipsychotics used in 5 recent randomized controlled trials. Treatment duration was 3 weeks with olanzapine, risperidone, and quetiapine and 4 weeks with aripiprazole and ziprasidone. Dosages were olanzapine, 10.4 ± 4.5 mg/d; risperidone, 0.5 to 2.5 mg/d or 3 to 6 mg/d; aripiprazole, 10 or 30 mg/d; quetiapine, 400 or 600 mg/d; and ziprasidone, 80 to 160 mg/d.
Source: References 3-7Related resources
- Child and Adolescent Bipolar Foundation. www.bpkids.org.
- University of Illinois at Chicago Pediatric Mood Disorders Clinic. www.psych.uic.edu/pmdc.
- Ryan Licht Sang Bipolar Foundation. www.ryanlichtsangbipolarfoundation.org.
Drug brand names
- Aripiprazole • Abilify
- Benztropine • Cogentin
- Carbamazepine • Carbatrol
- Clozapine • Clozaril
- Diphenhydramine • Benadryl
- Divalproex sodium • Depakote
- Lithium • Lithobid, others
- Metformin • Glucophage
- Olanzapine • Zyprexa
- Paliperidone • Invega
- Propranolol • Inderal
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Valproate • Depacon
- Ziprasidone • Geodon
Disclosures
Dr. Kowatch is a consultant to and speaker for AstraZeneca and a consultant to Forest Pharmaceuticals. He receives research support from the National Alliance for Research on Schizophrenia and Depression, the National Institute of Child Health and Human Development, the National Institute of Mental Health, and the Stanley Foundation.
Dr. Strawn has received research support from the American Academy of Child and Adolescent Psychiatry (Lilly Pilot Research Award).
Dr. Sorter receives research support from the National Institute of Mental Health and the Health Foundation of Greater Cincinnati.
1. Hopewell S, Clarke M, Stewart L, et al. Time to publication for results of clinical trials. Cochrane Database Syst Rev. 2007;(2):MR000011.-
2. Carey B. Risks found for youths in new antipsychotics. The New York Times. September 15, 2008:A17.
3. Pandina G, DelBello M, Kushner S, et al. Risperidone for the treatment of acute mania in bipolar youth. Paper presented at: Annual Meeting of the American Academy of Child and Adolescent Psychiatry, October 23-28, 2007; Boston, MA.
4. Tohen M, Kryzhanovskaya L, Carlson G, et al. Olanzapine versus placebo in the treatment of adolescents with bipolar mania. Am J Psychiatry. 2007;164(10):1547-1556.
5. DelBello M, Findling RL, Earley W, et al. Efficacy of quetiapine in children and adolescents with bipolar mania: a 3-week, double-blind, randomized, placebo-controlled trial. Paper presented at: Annual Meeting of the American Academy of Child and Adolescent Psychiatry, October 23-28, 2007; Boston, MA.
6. Wagner K, Nyilas M, Forbes R, et al. Acute efficacy of aripiprazole for the treatment of bipolar I disorder, mixed or manic, in pediatric patients. Paper presented at: Annual Meeting of the American College of Neuropsychopharmacology, December 9-13, 2007; Boca Raton, FL.
7. DelBello M, Findling RL, Wang P, et al. Safety and efficacy of ziprasidone in pediatric bipolar disorder. Paper presented at: Annual Meeting of the American Psychiatric Association, May 3-8, 2008; Washington, DC.
8. McElduff P, Jaefarnezhad M, Durrington PN. American, British and European recommendations for statins in the primary prevention of cardiovascular disease applied to British men studied prospectively. Heart. 2006;92(9):1213-1218.
9. Tsapakis EM, Soldani F, Tondo L, et al. Efficacy of antidepressants in juvenile depression: meta-analysis. Br J Psychiatry. 2008;193(1):10-17.
10. Kowatch R, Findling R, Scheffer R, et al. Placebo controlled trial of divalproex versus lithium for bipolar disorder. Paper presented at: Annual Meeting of the American Academy of Child and Adolescent Psychiatry; October 23-28, 2007; Boston, MA.
11. DelBello MP, Kowatch RA, Adler CM, et al. A double-blind randomized pilot study comparing quetiapine and divalproex for adolescent mania. J Am Acad Child Adolesc Psychiatry. 2006;45(3):305-313.
12. DelBello MP, Schwiers ML, Rosenberg HL, et al. A double-blind, randomized, placebo-controlled study of quetiapine as adjunctive treatment for adolescent mania. J Am Acad Child Adolesc Psychiatry. 2002;41(10):1216-1223.
13. Practice guideline for the treatment of patients with bipolar disorder (revision). Am J Psychiatry. 2002;159(4 suppl):1-50.
14. Suppes T, Dennehy EB, Hirschfeld RM, et al. The Texas implementation of medication algorithms: update to the algorithms for treatment of bipolar I disorder. J Clin Psychiatry. 2005;66(7):870-886.
15. Becker AL, Epperson CN. Female puberty: clinical implications for the use of prolactin-modulating psychotropics. Child Adolesc Psychiatr Clin N Am. 2006;15(1):207-220.
16. Correll CU, Penzner JB, Parikh UH, et al. Recognizing and monitoring adverse events of second-generation antipsychotics in children and adolescents. Child Adolesc Psychiatr Clin N Am. 2006;15(1):177-206.
17. Correll CU. Effect of hyperprolactinemia during development in children and adolescents. J Clin Psychiatry. 2008;69(8):e24.-
18. Correll CU. Antipsychotic use in children and adolescents: minimizing adverse effects to maximize outcomes. J Am Acad Child Adolesc Psychiatry. 2008;47(1):9-20.
19. Correll CU, Nyilas M, Ashfaque S, et al. Long-term safety and tolerability of aripiprazole in children (10-17 years) with bipolar disorder. Paper presented at: Annual Meeting of the American College of Neuropsychopharmacology; December 9-13, 2007; Boca Raton, FL.
20. Pandina GJ, Bossie CA, Youssef E, et al. Risperidone improves behavioral symptoms in children with autism in a improves behavioral symptoms in children with autism in a randomized, double-blind, placebo-controlled trial. J Autism Dev Disord. 2007;37(2):367-373.
21. DelBello M, Findling RL, Earley W, et al. Efficacy of quetiapine in children and adolescent with bipolar mania; a 3-week, double-blind, randomized, placebo-controlled trial. Paper presented at: Annual Meeting of the American College of Neuropsychopharmacology; December 9-13, 2007; Boca Raton, FL.
22. Correll CU, Kane JM. One-year incidence rates of tardive dyskinesia in children and adolescents treated with second-generation antipsychotics: a systematic review. J Child Adolesc Psychopharmacol. 2007;17(5):647-656.
23. McDermid SA, Hood J, Bockus S, et al. Adolescents on neuroleptic medication: is this population at risk for tardive dyskinesia? Can J Psychiatry. 1998;43(6):629-631.
24. Kumra S, Jacobsen LK, Lenane M, et al. Case series: spectrum of neuroleptic-induced movement disorders and extrapyramidal side effects in childhood-onset schizophrenia. J Am Acad Child Adolesc Psychiatry. 1998;37(2):221-227.
25. Strawn JR, Keck PE, Jr, Caroff SN. Neuroleptic malignant syndrome. Am J Psychiatry. 2007;164(6):870-876.
26. Croarkin PE, Emslie GJ, Mayes TL. Neuroleptic malignant syndrome associated with atypical antipsychotics in pediatric patients: a review of published cases. J Clin Psychiatry. 2008;69(7):1157-1165.
27. Picard LS, Lindsay S, Strawn JR, et al. Atypical neuroleptic malignant syndrome: diagnostic controversies and considerations. Pharmacotherapy. 2008;28(4):530-535.
28. Correll CU. Metabolic side effects of second-generation antipsychotics in children and adolescents: a different story? J Clin Psychiatry. 2005;66(10):1331-1332.
29. Klein DJ, Cottingham EM, Sorter M, et al. A randomized, double-blind, placebo-controlled trial of metformin treatment of weight gain associated with initiation of atypical antipsychotic therapy in children and adolescents. Am J Psychiatry. 2006;163(12):2072-2079.
30. Kavey RE, Allada V, Daniels SR, et al. Cardiovascular risk reduction in high-risk pediatric patients: a scientific statement from the American Heart Association Expert Panel on Population and Prevention Science; the Councils on Cardiovascular Disease in the Young, Epidemiology and Prevention, Nutrition, Physical Activity and Metabolism, High Blood Pressure Research, Cardiovascular Nursing, and the Kidney in Heart Disease; and the Interdisciplinary Working Group on Quality of Care and Outcomes Research. J Cardiovasc Nurs. 2007;22(3):218-253.
31. O’Grady MJ, Brown AM, O’Neill MB. Cholesterol screening in an at-risk pediatric population. Pediatr Cardiol. 2008;29(3):609-613.
32. Ali J, Khemka M. Hyperprolactinemia: monitoring children on long-term risperidone. Current Psychiatry. 2008;7(11):64-72.
33. Blair J, Scahill L, State M, et al. Electrocardiographic changes in children and adolescents treated with ziprasidone: a prospective study. J Am Acad Child Adolesc Psychiatry. 2005;44(1):73-79.
34. Malone RP, Delaney MA, Hyman SB, et al. Ziprasidone in adolescents with autism: an open-label pilot study. J Child Adolesc Psychopharmacol. 2007;17(6):779-790.
1. Hopewell S, Clarke M, Stewart L, et al. Time to publication for results of clinical trials. Cochrane Database Syst Rev. 2007;(2):MR000011.-
2. Carey B. Risks found for youths in new antipsychotics. The New York Times. September 15, 2008:A17.
3. Pandina G, DelBello M, Kushner S, et al. Risperidone for the treatment of acute mania in bipolar youth. Paper presented at: Annual Meeting of the American Academy of Child and Adolescent Psychiatry, October 23-28, 2007; Boston, MA.
4. Tohen M, Kryzhanovskaya L, Carlson G, et al. Olanzapine versus placebo in the treatment of adolescents with bipolar mania. Am J Psychiatry. 2007;164(10):1547-1556.
5. DelBello M, Findling RL, Earley W, et al. Efficacy of quetiapine in children and adolescents with bipolar mania: a 3-week, double-blind, randomized, placebo-controlled trial. Paper presented at: Annual Meeting of the American Academy of Child and Adolescent Psychiatry, October 23-28, 2007; Boston, MA.
6. Wagner K, Nyilas M, Forbes R, et al. Acute efficacy of aripiprazole for the treatment of bipolar I disorder, mixed or manic, in pediatric patients. Paper presented at: Annual Meeting of the American College of Neuropsychopharmacology, December 9-13, 2007; Boca Raton, FL.
7. DelBello M, Findling RL, Wang P, et al. Safety and efficacy of ziprasidone in pediatric bipolar disorder. Paper presented at: Annual Meeting of the American Psychiatric Association, May 3-8, 2008; Washington, DC.
8. McElduff P, Jaefarnezhad M, Durrington PN. American, British and European recommendations for statins in the primary prevention of cardiovascular disease applied to British men studied prospectively. Heart. 2006;92(9):1213-1218.
9. Tsapakis EM, Soldani F, Tondo L, et al. Efficacy of antidepressants in juvenile depression: meta-analysis. Br J Psychiatry. 2008;193(1):10-17.
10. Kowatch R, Findling R, Scheffer R, et al. Placebo controlled trial of divalproex versus lithium for bipolar disorder. Paper presented at: Annual Meeting of the American Academy of Child and Adolescent Psychiatry; October 23-28, 2007; Boston, MA.
11. DelBello MP, Kowatch RA, Adler CM, et al. A double-blind randomized pilot study comparing quetiapine and divalproex for adolescent mania. J Am Acad Child Adolesc Psychiatry. 2006;45(3):305-313.
12. DelBello MP, Schwiers ML, Rosenberg HL, et al. A double-blind, randomized, placebo-controlled study of quetiapine as adjunctive treatment for adolescent mania. J Am Acad Child Adolesc Psychiatry. 2002;41(10):1216-1223.
13. Practice guideline for the treatment of patients with bipolar disorder (revision). Am J Psychiatry. 2002;159(4 suppl):1-50.
14. Suppes T, Dennehy EB, Hirschfeld RM, et al. The Texas implementation of medication algorithms: update to the algorithms for treatment of bipolar I disorder. J Clin Psychiatry. 2005;66(7):870-886.
15. Becker AL, Epperson CN. Female puberty: clinical implications for the use of prolactin-modulating psychotropics. Child Adolesc Psychiatr Clin N Am. 2006;15(1):207-220.
16. Correll CU, Penzner JB, Parikh UH, et al. Recognizing and monitoring adverse events of second-generation antipsychotics in children and adolescents. Child Adolesc Psychiatr Clin N Am. 2006;15(1):177-206.
17. Correll CU. Effect of hyperprolactinemia during development in children and adolescents. J Clin Psychiatry. 2008;69(8):e24.-
18. Correll CU. Antipsychotic use in children and adolescents: minimizing adverse effects to maximize outcomes. J Am Acad Child Adolesc Psychiatry. 2008;47(1):9-20.
19. Correll CU, Nyilas M, Ashfaque S, et al. Long-term safety and tolerability of aripiprazole in children (10-17 years) with bipolar disorder. Paper presented at: Annual Meeting of the American College of Neuropsychopharmacology; December 9-13, 2007; Boca Raton, FL.
20. Pandina GJ, Bossie CA, Youssef E, et al. Risperidone improves behavioral symptoms in children with autism in a improves behavioral symptoms in children with autism in a randomized, double-blind, placebo-controlled trial. J Autism Dev Disord. 2007;37(2):367-373.
21. DelBello M, Findling RL, Earley W, et al. Efficacy of quetiapine in children and adolescent with bipolar mania; a 3-week, double-blind, randomized, placebo-controlled trial. Paper presented at: Annual Meeting of the American College of Neuropsychopharmacology; December 9-13, 2007; Boca Raton, FL.
22. Correll CU, Kane JM. One-year incidence rates of tardive dyskinesia in children and adolescents treated with second-generation antipsychotics: a systematic review. J Child Adolesc Psychopharmacol. 2007;17(5):647-656.
23. McDermid SA, Hood J, Bockus S, et al. Adolescents on neuroleptic medication: is this population at risk for tardive dyskinesia? Can J Psychiatry. 1998;43(6):629-631.
24. Kumra S, Jacobsen LK, Lenane M, et al. Case series: spectrum of neuroleptic-induced movement disorders and extrapyramidal side effects in childhood-onset schizophrenia. J Am Acad Child Adolesc Psychiatry. 1998;37(2):221-227.
25. Strawn JR, Keck PE, Jr, Caroff SN. Neuroleptic malignant syndrome. Am J Psychiatry. 2007;164(6):870-876.
26. Croarkin PE, Emslie GJ, Mayes TL. Neuroleptic malignant syndrome associated with atypical antipsychotics in pediatric patients: a review of published cases. J Clin Psychiatry. 2008;69(7):1157-1165.
27. Picard LS, Lindsay S, Strawn JR, et al. Atypical neuroleptic malignant syndrome: diagnostic controversies and considerations. Pharmacotherapy. 2008;28(4):530-535.
28. Correll CU. Metabolic side effects of second-generation antipsychotics in children and adolescents: a different story? J Clin Psychiatry. 2005;66(10):1331-1332.
29. Klein DJ, Cottingham EM, Sorter M, et al. A randomized, double-blind, placebo-controlled trial of metformin treatment of weight gain associated with initiation of atypical antipsychotic therapy in children and adolescents. Am J Psychiatry. 2006;163(12):2072-2079.
30. Kavey RE, Allada V, Daniels SR, et al. Cardiovascular risk reduction in high-risk pediatric patients: a scientific statement from the American Heart Association Expert Panel on Population and Prevention Science; the Councils on Cardiovascular Disease in the Young, Epidemiology and Prevention, Nutrition, Physical Activity and Metabolism, High Blood Pressure Research, Cardiovascular Nursing, and the Kidney in Heart Disease; and the Interdisciplinary Working Group on Quality of Care and Outcomes Research. J Cardiovasc Nurs. 2007;22(3):218-253.
31. O’Grady MJ, Brown AM, O’Neill MB. Cholesterol screening in an at-risk pediatric population. Pediatr Cardiol. 2008;29(3):609-613.
32. Ali J, Khemka M. Hyperprolactinemia: monitoring children on long-term risperidone. Current Psychiatry. 2008;7(11):64-72.
33. Blair J, Scahill L, State M, et al. Electrocardiographic changes in children and adolescents treated with ziprasidone: a prospective study. J Am Acad Child Adolesc Psychiatry. 2005;44(1):73-79.
34. Malone RP, Delaney MA, Hyman SB, et al. Ziprasidone in adolescents with autism: an open-label pilot study. J Child Adolesc Psychopharmacol. 2007;17(6):779-790.
CAM for your depressed patient: 6 recommended options
Americans with depression turn to complementary and alternative medicine (CAM) more often than conventional psychotherapy or FDA-approved medication. In a nationally representative sample, 54% of respondents with self-reported “severe depression”—including two-thirds of those receiving conventional therapies—reported using CAM during the previous 12 months.1
Unfortunately, popular acceptance of CAM for depression is disproportionate to the evidence base, which—although growing—remains limited. As a result, your patients may be self-medicating with poorly supported treatments that are unlikely to help them recover from depression.
In reviewing CAM treatments for depression, we found some with enough evidence of positive effect that we feel comfortable recommending them as evidence-based options. These promising, short-term treatments are supported by level 1a or 1b evidence and at least 1 study that demonstrates an ability to induce remission ( Table 1 ).2
For patients seeking “natural” or nonprescription treatments—or when you wish to augment standard treatments that are not working adequately—you might recommend fatty acids, St. John’s wort, or S-adenosyl-L-methionine (SAMe). Similar recommendations can be made for yoga, exercise, and bibliotherapy, as we discuss here.
Table 1
Evidence these authors required to recommend a CAM treatment
| Minimum requirements | Level of evidence | Recommendation |
|---|---|---|
| Systematic review showing superiority to placebo or standard treatment Plus 1 study showing CAM treatment can induce remission | 1a + | A |
| At least 2 RCTs showing superiority to placebo or standard treatment Plus 1 study showing CAM treatment can induce remission | 1b | A– |
| CAM: complementary and alternative medicine; RCT: randomized controlled trial | ||
| Source: Reference 2 | ||
Reviewing CAM evidence
This article refers to as “alternative” any treatment other than a form of psychotherapy or an FDA-approved medication that substitutes for a standard psychiatric treatment. When used to augment standard psychiatric treatments, these approaches are considered “complementary.”
Our search for evidence on CAM treatments for depressive disorders raised questions about what constitutes acceptable and convincing methodology:
- Studies often had problems with blinding and suitable placebos. Many were small, with short duration and no long-term follow-up.
- Comparisons with active treatments that showed no differences were assumed to imply comparability, even though the studies were powered to detect only large differences.
Applying the evidence. Because CAM use is widespread, be sure to ask psychiatric patients if they are using CAM treatments. If the answer is “yes,” a risk-benefit assessment is needed. Inform patients who are using poorly supported CAM approaches that they could consider better-supported CAM options as well as standard effective antidepressants.
Monitor patients for an adequately prompt positive response to an evidence-based CAM approach that has shown efficacy for their level of depression. As with any treatment, consider other evidence-based options when CAM treatments are inadequate or unsuccessful in achieving remission of depressive symptoms.
Sufficient evidence of efficacy
Yoga. In their systematic review of yoga’s effectiveness for depression, Pilkington et al3 analyzed 5 RCTs that met 3 criteria:
- participants had mild to severe depression or depressive disorders
- yoga or yoga-based exercises alone were used for treatment
- depression rating scales were used as outcome measures.
Conclusion. Yoga has been studied primarily as an alternative treatment, but its physical health and group participation benefits and lack of side effects make it a suitable complementary treatment as well.
- 45% with supervised exercise
- 40% with home-based exercise
- 47% with sertraline, 50 to 200 mg/d
- 31% with placebo.4
5 RCTs of yoga’s effectiveness in treating depression
| RCT | Interventions | Conclusion |
|---|---|---|
| Broota and Dhir, 1990 | Yoga and PMR vs control | Yoga and PMR were more effective than control, with yoga more effective than PMR |
| Khumar et al, 1993 | Shavasana yoga vs no intervention | College students with severe depression improved during and after yoga treatment |
| Janakiramaiah et al, 2000 | SKY vs ECT vs imipramine | Reductions in BDI scores for all 3 groups; ECT > SKY or imipramine, SKY=imipramine |
| Rohini et al, 2000 | Full SKY vs partial SKY | 30 individuals with MDD improved with either therapy, but results were not statistically significant |
| Woolery, 2004 | Iyengar yoga vs wait list | 28 mildly depressed individuals benefitted from yoga, as measured by BDI scores at midpoint and throughout treatment |
| BDI: Beck Depression Inventory; ECT: electroconvulsive therapy; MDD: major depressive disorder; PMR: progressive muscle relaxation; RCT: randomized controlled trial; SKY: Sudarshan Kriya yoga | ||
| Source: Broota A, Dhir R. Efficacy of two relaxation techniques in depression. Journal of Personality and Clinical Studies. 1990;6(1):83-90. Khumar SS, Kaur P, Kaur S. Effectiveness of Shavasana on depression among university students. Indian J Clin Psychol. 1993;20(2):82-87. Janakiramaiah N, Gangadhar BN, Naga Venkatesha Murthy PJ, et al. Antidepressant efficacy of Sudarshan Kriya yoga (SKY) in melancholia: a randomized comparison with electroconvulsive therapy (ECT) and imipramine. J Affect Disord. 2000;57(1-3):255-259. Rohini V, Pandey RS, Janakiramaiah N, et al. A comparative study of full and partial Sudarshan Kriya yoga (SKY) in major depressive disorder. NIMHANS Journal. 2000;18(1):53-57. Woolery A, Myers H, Sternlieb B, et al. A yoga intervention for young adults with elevated symptoms of depression. Altern Ther Health Med. 2004;10(2):60-63. | ||
Table 3
Evidence of the antidepressant effect of exercise
| Literature review | Methodology | Conclusion |
|---|---|---|
| Byrne and Byrne, 1993 | 13 studies, clinical samples, measured changes in depressed mood | 90% of studies reported beneficial effects, especially in clinical populations |
| Scully et al, 1998 | 4 literature reviews, 1 monograph, 1 study | Positive relationship of physical activity and depression in healthy and clinical samples, increased over time |
| Lawlor and Hopker, 2001 | 14 RCTs from 1966 to 1999 with depression as an outcome | Significant methodologic weaknesses, but exercise effect > no treatment and=cognitive therapy |
| Dunn et al, 2001 | Examined dose effect in 37 studies; subjects diagnosed with depressive disorders, mild-to-moderate symptoms, and no medical comorbidity | Only level B and C evidence; positive effects with exercise from light to heavy intensity; aerobic=nonaerobic; improvement may or may not be related to improved fitness |
| Brosse et al, 2002 | 12 RCTs from 1979 to 1999 | Significant methodologic limitations, but authors concluded evidence supports a positive effect of exercise in healthy and clinical populations |
| Larun et al, 2006 | 4 RCTs in children and youth age | Exercise effect same as no intervention, low-intensity relaxation, or psychosocial intervention |
| Barbour et al, 2007 | 2 meta-analyses, 1 RCT, 2 studies | Positive effect; high-energy was optimal dose; aerobic=nonaerobic; improvement may or may not be related to improved fitness |
| RCT: randomized controlled trial | ||
| Source: Byrne AE, Byrne DG. The effect of exercise on depression, anxiety and other mood states: A review. J Psychosom Res. 1993;37(6):565-574. Scully D, Kremer J, Meade MM, et al. Physical exercise and psychological well being: a critical review. Br J Sports Med. 1998;32(2):111-120. Lawlor DA, Hopker SW. The effectiveness of exercise as an intervention in the management of depression: systematic review and meta-regression analysis of randomised controlled trials. BMJ. 2001;322(7289):763-767. Dunn AL, Trivedi MH, O’Neal HA. Physical activity dose-response effects on outcomes of depression and anxiety. Med Sci Sports Exerc. 2001;33(6):S587. Brosse AL, Sheets ES, Lett HS, et al. Exercise and the treatment of clinical depression in adults: recent findings and future directions. Sports Med. 2002;32(12):741-760. Larun L, Nordheim LV, Ekeland E, et al. Exercise in prevention and treatment of anxiety and depression among children and young people. Cochrane Database Syst Rev. 2006;3:CD004691. Barbour KA, Edenfield TM, Blumenthal JA. Exercise as a treatment for depression and other psychiatric disorders: a review. J Cardiopulm Rehabil Prev. 2007;27(6):359-367. | ||
At least 2 studies suggest that high-energy exercise and aerobic or resistance training provide greater reductions in depressive symptoms than exercises such as walking.5,6 Yoga’s positive effects suggest, however, that an aerobic effect is not necessary for an antidepressant benefit.
Exercise has not been adequately tested as a complementary treatment but likely is safe for most psychiatric patients. Perspiration and dehydration might alter therapeutic blood levels of lithium or other medications. Advise patients to drink water before, during, and after exercise and to avoid outdoor exercise in extreme temperatures. More vigorous monitoring might be indicated in specific cases.
Tailor exercise programs to individual needs, considering the patient’s age and health status. Refer a patient with a known heart problem or increased cardiovascular risk to his or her physician for selective exercise testing.
Bibliotherapy—reading self-help books, usually about cognitive-behavioral approaches to depressive disorders—has been relatively well studied. A recent meta-analysis examined 29 studies with pre-post designs. Group differences in the 17 controlled studies yielded a large effect size of 0.77. Participants who read the materials benefitted similarly whether they met in groups or applied the information on their own. Older adults tended to be less depressed at baseline and made smaller treatment gains.7
Conclusion. Evidence supports bibliotherapy as an effective treatment for mild-to-moderate depression. No convincing data support its use as a complementary treatment, but it poses virtually no risk.
St. John’s wort (Hypericum perforatum) has been extensively studied for depressive disorders, with 29 RCTs in a meta-analysis of MDD trials through July 2008.10 Another meta-analysis compared St. John’s wort with selective serotonin reuptake inhibitors (SSRIs) in 13 studies through June 2008.11 These and most RCTs have found St. John’s wort significantly more effective than placebo in reducing depressive symptoms.
Data selected from double-blind RCTs totaling 217 patients with mild depression [Hamilton Depression Rating Scale (HDRS) scores 12 Studies routinely show that treating MDD with St. John’s wort is comparable to using tricyclic or SSRI antidepressants.
Side effects with St. John’s wort generally are no different than with placebo and significantly less than with comparison treatments. Even so, using St. John’s wort instead of SSRIs for MDD remains controversial.
Studies vs SSRIs. Many of the favorable St. John’s wort trials were conducted in Europe, particularly in Germany. Two large RCTs conducted in the United States reported that the St. John’s wort standardized extract LI-160 was not more effective than placebo, but neither could be clearly interpreted as negative for St. John’s wort:
- In an 8-week trial, St. John’s wort and placebo groups improved significantly but at unusually low rates. The remission rate with St. John’s wort was small but significantly higher than with placebo.13
- A study sponsored by the National Institute of Mental Health compared St. John’s wort, 900 to 1,500 mg/d; sertraline, 50 to 100 mg/d; and placebo in 340 adults with MDD. No positive effects were found for St. John’s wort or sertraline.14
Conclusion. Standardized extracts of St. John’s wort—particularly WS5570, 300 mg tid, and ZE117, 250 mg bid—appear to be effective treatments, especially for mild-to-moderate MDD. Because St. John’s wort is available without prescription and can interact with SSRIs or other antidepressants:
- care is required for its complementary use
- it is important to ask if patients are using St. John’s wort on their own.
- for first-line use only when you can adequately gauge its effects on your patient’s other medications
- especially for depressed patients who cannot tolerate SSRIs.
SAMe has become a popular alternative treatment for depression since its introduction to the United States in the late 1990s, but it has been studied in only 2 U.S. open trials. One showed SAMe to be very effective in reducing depressive symptoms in patients with HIV/AIDS.17 The other found a 50% response rate and 43% remission rate with adjunctive SAMe, 800 to 1,600 mg/d for 6 weeks, in 30 adults with MDD who failed to respond adequately to SSRIs or the serotonin-norepinephrine reuptake inhibitor (SNRI) venlafaxine. The most common side effects were gastrointestinal (GI) symptoms and headaches.18 This open trial led to an ongoing National Institutes of Health-sponsored RCT on the safety and efficacy of SAMe for patients with treatment-resistant depression.
Conclusion. SAMe appears to have a faster onset of antidepressant effect than standard SSRIs or SNRIs and a favorable side-effect profile, which make the lack of rigorous trials in the United States striking. We recommend that you consider SAMe:
- as an adjunct in patients with incomplete response to standard treatments
- as a complementary treatment to speed onset of antidepressant effects.
Four meta-analyses independently looked at largely the same dozen RCTs through 2006 and found that 1 to 2 grams daily of omega-3 PUFAs was significantly more effective at reducing depressive symptoms than placebo.19-22 Other data suggest that omega-3 PUFAs can induce depression remission in depressed Parkinson’s disease patients23 and depressed pregnant women.24 Since 2006, however, findings have been inconsistent. Several trials have found PUFAs no more effective than placebo.25-27
An 8-week double-blind study compared EPA, 1 gram daily; fluoxetine, 20 mg/d; or both agents in 60 outpatients with MDD. Response rates—as measured by ≥50% reduction in baseline HDRS scores—were 50% with fluoxetine, 56% with EPA, and 81% with combination therapy.28
Insufficient evidence
L-tryptophan. It seems reasonable to expect a serotonin precursor to increase serotonin in the CNS and improve depressive symptoms. Of 111 trials on L-tryptophan for depression, however, only 2 met the quality criteria for inclusion in a recent meta-analysis.29 Combining the 2 trials showed L-tryptophan alone and in combination with a tricyclic antidepressant was more effective than placebo for treating depressive disorders in adults.
Conclusion. Very little research continues to test L-tryptophan as a viable CAM for depressive disorder. Its serious side effect of eosinophilia-myalgia syndrome makes clinical use of this agent unlikely.
Acupuncture. Numerous small studies with questionable controls, different needling placements, and poor allocation concealment and blinding limit the ability to draw conclusions about acupuncture for treating depression ( Table 4 ). A recent meta-analysis by Wang et al30 added 2 Chinese trials not included in an earlier review31 and found acupuncture significantly reduced depressive symptoms. No consistent differences were detected in response or remission rates, however.
Conclusion. Evidence is methodologically weak, and the use of acupuncture as an alternative or complementary treatment of depression is questionable.
Table 4
Acupuncture: Insufficient evidence of antidepressant effect
| Literature review | Methodology | Conclusion |
|---|---|---|
| Mukaino et al, 2005 | Systematic review of 7 RCTs including 509 patients; compared either manual or electroacupuncture with any control procedure | Inconsistent evidence of manual acupuncture’s effectiveness vs sham; electroacupuncture’s effect may be similar to that of antidepressant medication and merits further study |
| Leo and Ligot, 2007 | Systematic review of 9 RCTs, 5 considered low quality; some focused on very specific populations (ie, hospitalized stroke patients or pregnant depressed patients) | Evidence inconclusive because of study designs and methodologies |
| Smith and Hay, 2005 | Meta-analysis of 7 trials including 517 adults with mild-to-moderate depression; 5 trials (409 participants) compared acupuncture with medication; 2 trials compared acupuncture with wait list or sham acupuncture | No difference between acupuncture and medication; study quality too poor to support acupuncture’s efficacy |
| Wang et al, 2008 | Meta-analysis of 8 small RCTs totalling 477 subjects (256 received active acupuncture, remainder received sham acupuncture); sham acupuncture design, number of acupuncture sessions, and duration varied among studies | Significant reduction in HRSD or BDI scores for acupuncture vs sham, but no significant effect of acupuncture on response or remission rates |
| BDI: Beck Depression Inventory; HRSD: Hamilton Rating Scale for Depression; RCT: randomized controlled trial | ||
| Source: Mukaino Y, Park J, White A, et al. The effectiveness of acupuncture for depression—a systematic review of randomised controlled trials. Acupunct Med. 2005;23(2):70-76. Leo RJ, Ligot JS Jr. A systematic review of randomized controlled trials of acupuncture in the treatment of depression. J Affect Disord. 2007;97(1-3):13-22. Smith CA, Hay PPJ. Acupuncture for depression. Cochrane Database Syst Rev. 2005;(2):CD004046. Wang H, Qi H, Wang BS, et al. Is acupuncture beneficial in depression? A meta-analysis of 8 randomized controlled trials. J Affect Disord. 2008;111(2-3):125-134. | ||
- National Center for Complementary and Alternative Medicine, National Institutes of Health. http://nccam.nih.gov.
- Journal of Alternative and Complementary Medicine. www.liebertpub.com/products/product.aspx?pid=26.
- Complementary and alternative medicine. www.nlm.nih.gov/medlineplus/complementaryandalternativemedicine.html.
- Fluoxetine • Prozac
- Imipramine • Tofranil
- Lithium • Eskalith, Lithobid
- Sertraline • Zoloft
- Venlafaxine • Effexor
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Kessler RC, Soukup J, Davis RB, et al. The use of complementary and alternative therapies to treat anxiety and depression in the United States. Am J Psychiatry. 2001;158(2):289-294.
2. Phillips B, Ball C, Sackett D, et al. Oxford University Centre for Evidence Based Medicine levels of evidence and grades of recommendation (March 2009). Available at: http://www.cebm.net/index.aspx?o=1025#levels. Accessed August 19, 2009.
3. Pilkington K, Kirkwood G, Rampes H, et al. Yoga for depression: the research evidence. J Affect Disord. 2005;89(1-3):13-24.
4. Blumenthal JA, Babyak MA, Doraiswamy PM, et al. Exercise and pharmacotherapy in the treatment of major depressive disorder. Psychosom Med. 2007;69(7):587-596.
5. Dunn AL, Trivedi MH, Kampert JB, et al. Exercise treatment for depression: efficacy and dose response. Am J Prev Med. 2005;28(1):1-8.
6. Legrand F, Heuze JP. Antidepressant effects associated with different exercise conditions in participants with depression: a pilot study. J Sport Exercise Psychol. 2007;29(3):348-364.
7. Gregory RJ, Schwer Canning S, Lee TW, et al. Cognitive bibliotherapy for depression: a meta-analysis. Professional Psychology: Research and Practice. 2004;35(3):275-280.
8. Floyd M, Scogin F, McKendree-Smith NL, et al. Cognitive therapy for depression: a comparison of individual psychotherapy and bibliotherapy for depressed older adults. Behav Modif. 2004;28(2):297-318.
9. Burns DD. Feeling good: the new mood therapy. New York, NY: HarperCollins; 1980.
10. Linde K, Berner MM, Kriston L. St John’s Wort for major depression. [update of Cochrane Database Syst Rev. 2005;(2):CD000448; PMID: 15846605]. Cochrane Database Syst Rev. 2008(4):000448.
11. Rahimi R, Nikfar S, Abdollahi M. Efficacy and tolerability of Hypericum perforatum in major depressive disorder in comparison with selective serotonin reuptake inhibitors: a meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33(1):118-127.
12. Kasper S, Gastpar M, Müller WE, et al. Efficacy of St. John’s wort extract WS 5570 in acute treatment of mild depression: a reanalysis of data from controlled clinical trials. Eur Arch Psychiatry Clin Neurosci. 2008;258(1):59-63.
13. Shelton RC, Keller MB, Gelenberg A, et al. Effectiveness of St John’s wort in major depression: a randomized controlled trial. JAMA. 2001;285(15):1978-1986.
14. Hypericum Depression Trial Study Group. Effect of Hypericum perforatum (St. John’s wort) in major depressive disorder: a randomized controlled trial. JAMA. 2002;287(14):1807-1814.
15. Mischoulon D, Fava M. Role of S-adenosyl-L-methionine in the treatment of depression: a review of the evidence. Am J Clin Nutr. 2002;76(suppl):1158S-1161S.
16. Hardy ML, Coulter I, Morton SC, et al. S-adenosyl-L-methionine for treatment of depression, osteoarthritis, and liver disease [comment in ACP J Club. 2003;139(1):20]. Evid Rep Technol Assess (Summ). 2003;Aug(64):1-3.
17. Shippy RA, Mendez D, Jones K, et al. S-adenosylmethionine (SAM-e) for the treatment of depression in people living with HIV/AIDS. BMC Psychiatry. 2004;4:38.-
18. Alpert JE, Papakostas G, Mischoulon D, et al. S-adenosyl-L-methionine (SAMe) as an adjunct for resistant major depressive disorder: an open trial following partial or nonresponse to selective serotonin reuptake inhibitors or venlafaxine. J Clin Psychopharmacol. 2004;24(6):661-664.
19. Lin PY, Su KP. A meta-analytic review of double-blind, placebo-controlled trials of antidepressant efficacy of omega-3 fatty acids. J Clin Psychiatry. 2007;68(7):1056-1061.
20. Freeman MP, Hibbeln JR, Wisner KL, et al. Omega-3 fatty acids: evidence basis for treatment and future research in psychiatry [published correction appears in J Clin Psychiatry. 2007;68(2):338]. J Clin Psychiatry. 2006;67(12):1954-1967.
21. Ross BM, Seguin J, Sieswerda LE. Omega-3 fatty acids as treatments for mental illness: which disorder and which fatty acid? Lipids Health Dis. 2007;6:21.-
22. Appleton KM, Hayward RC, Gunnell D, et al. Effects of n-3 long-chain polyunsaturated fatty acids on depressed mood: systematic review of published trials [comment in Am J Clin Nutr. 2007;85(6):1665-1666; author reply 1666]. Am J Clin Nutr. 2006;84(6):1308-1316.
23. da Silva TM, Munhoz RP, Alvarez C, et al. Depression in Parkinson’s disease: a double-blind, randomized, placebo-controlled pilot study of omega-3 fatty-acid supplementation. J Affect Disord. 2008;111(2-3):351-359.
24. Su KP, Huang SY, Chiu TH, et al. Omega-3 fatty acids for major depressive disorder during pregnancy: results from a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2008;69(4):644-651.
25. Grenyer BF, Crowe T, Meyer B, et al. Fish oil supplementation in the treatment of major depression: a randomised double-blind placebo-controlled trial. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(7):1393-1396.
26. Rees AM, Austin MP, Parker GB. Omega-3 fatty acids as a treatment for perinatal depression: randomized double-blind placebo-controlled trial. Aust N Z J Psychiatry. 2008;42(3):199-205.
27. Rogers PJ, Appleton KM, Kessler D, et al. No effect of n-3 long-chain polyunsaturated fatty acid (EPA and DHA) supplementation on depressed mood and cognitive function: a randomised controlled trial. Br J Nutr. 2008;99(2):421-431.
28. Jazayeri S, Tehrani-Doost M, Keshavarz SA, et al. Comparison of therapeutic effects of omega-3 fatty acid eicosapentaenoic acid and fluoxetine, separately and in combination, in major depressive disorder. Aust N Z J Psychiatry. 2008;42(3):192-198.
29. Shaw K, Turner J, Del Mar C. Tryptophan and 5-Hydroxytryptophan for depression. Cochrane Database Syst Rev. 2002;(1):CD003198.-
30. Wang H, Qi H, Wang BS, et al. Is acupuncture beneficial in depression: a meta-analysis of 8 randomized controlled trials? J Affect Disord. 2008;111(2-3):125-134.
31. Smith CA, Hay PPJ. Acupuncture for depression. Cochrane Database Syst Rev. 2005;(2):CD004046.-
Americans with depression turn to complementary and alternative medicine (CAM) more often than conventional psychotherapy or FDA-approved medication. In a nationally representative sample, 54% of respondents with self-reported “severe depression”—including two-thirds of those receiving conventional therapies—reported using CAM during the previous 12 months.1
Unfortunately, popular acceptance of CAM for depression is disproportionate to the evidence base, which—although growing—remains limited. As a result, your patients may be self-medicating with poorly supported treatments that are unlikely to help them recover from depression.
In reviewing CAM treatments for depression, we found some with enough evidence of positive effect that we feel comfortable recommending them as evidence-based options. These promising, short-term treatments are supported by level 1a or 1b evidence and at least 1 study that demonstrates an ability to induce remission ( Table 1 ).2
For patients seeking “natural” or nonprescription treatments—or when you wish to augment standard treatments that are not working adequately—you might recommend fatty acids, St. John’s wort, or S-adenosyl-L-methionine (SAMe). Similar recommendations can be made for yoga, exercise, and bibliotherapy, as we discuss here.
Table 1
Evidence these authors required to recommend a CAM treatment
| Minimum requirements | Level of evidence | Recommendation |
|---|---|---|
| Systematic review showing superiority to placebo or standard treatment Plus 1 study showing CAM treatment can induce remission | 1a + | A |
| At least 2 RCTs showing superiority to placebo or standard treatment Plus 1 study showing CAM treatment can induce remission | 1b | A– |
| CAM: complementary and alternative medicine; RCT: randomized controlled trial | ||
| Source: Reference 2 | ||
Reviewing CAM evidence
This article refers to as “alternative” any treatment other than a form of psychotherapy or an FDA-approved medication that substitutes for a standard psychiatric treatment. When used to augment standard psychiatric treatments, these approaches are considered “complementary.”
Our search for evidence on CAM treatments for depressive disorders raised questions about what constitutes acceptable and convincing methodology:
- Studies often had problems with blinding and suitable placebos. Many were small, with short duration and no long-term follow-up.
- Comparisons with active treatments that showed no differences were assumed to imply comparability, even though the studies were powered to detect only large differences.
Applying the evidence. Because CAM use is widespread, be sure to ask psychiatric patients if they are using CAM treatments. If the answer is “yes,” a risk-benefit assessment is needed. Inform patients who are using poorly supported CAM approaches that they could consider better-supported CAM options as well as standard effective antidepressants.
Monitor patients for an adequately prompt positive response to an evidence-based CAM approach that has shown efficacy for their level of depression. As with any treatment, consider other evidence-based options when CAM treatments are inadequate or unsuccessful in achieving remission of depressive symptoms.
Sufficient evidence of efficacy
Yoga. In their systematic review of yoga’s effectiveness for depression, Pilkington et al3 analyzed 5 RCTs that met 3 criteria:
- participants had mild to severe depression or depressive disorders
- yoga or yoga-based exercises alone were used for treatment
- depression rating scales were used as outcome measures.
Conclusion. Yoga has been studied primarily as an alternative treatment, but its physical health and group participation benefits and lack of side effects make it a suitable complementary treatment as well.
- 45% with supervised exercise
- 40% with home-based exercise
- 47% with sertraline, 50 to 200 mg/d
- 31% with placebo.4
5 RCTs of yoga’s effectiveness in treating depression
| RCT | Interventions | Conclusion |
|---|---|---|
| Broota and Dhir, 1990 | Yoga and PMR vs control | Yoga and PMR were more effective than control, with yoga more effective than PMR |
| Khumar et al, 1993 | Shavasana yoga vs no intervention | College students with severe depression improved during and after yoga treatment |
| Janakiramaiah et al, 2000 | SKY vs ECT vs imipramine | Reductions in BDI scores for all 3 groups; ECT > SKY or imipramine, SKY=imipramine |
| Rohini et al, 2000 | Full SKY vs partial SKY | 30 individuals with MDD improved with either therapy, but results were not statistically significant |
| Woolery, 2004 | Iyengar yoga vs wait list | 28 mildly depressed individuals benefitted from yoga, as measured by BDI scores at midpoint and throughout treatment |
| BDI: Beck Depression Inventory; ECT: electroconvulsive therapy; MDD: major depressive disorder; PMR: progressive muscle relaxation; RCT: randomized controlled trial; SKY: Sudarshan Kriya yoga | ||
| Source: Broota A, Dhir R. Efficacy of two relaxation techniques in depression. Journal of Personality and Clinical Studies. 1990;6(1):83-90. Khumar SS, Kaur P, Kaur S. Effectiveness of Shavasana on depression among university students. Indian J Clin Psychol. 1993;20(2):82-87. Janakiramaiah N, Gangadhar BN, Naga Venkatesha Murthy PJ, et al. Antidepressant efficacy of Sudarshan Kriya yoga (SKY) in melancholia: a randomized comparison with electroconvulsive therapy (ECT) and imipramine. J Affect Disord. 2000;57(1-3):255-259. Rohini V, Pandey RS, Janakiramaiah N, et al. A comparative study of full and partial Sudarshan Kriya yoga (SKY) in major depressive disorder. NIMHANS Journal. 2000;18(1):53-57. Woolery A, Myers H, Sternlieb B, et al. A yoga intervention for young adults with elevated symptoms of depression. Altern Ther Health Med. 2004;10(2):60-63. | ||
Table 3
Evidence of the antidepressant effect of exercise
| Literature review | Methodology | Conclusion |
|---|---|---|
| Byrne and Byrne, 1993 | 13 studies, clinical samples, measured changes in depressed mood | 90% of studies reported beneficial effects, especially in clinical populations |
| Scully et al, 1998 | 4 literature reviews, 1 monograph, 1 study | Positive relationship of physical activity and depression in healthy and clinical samples, increased over time |
| Lawlor and Hopker, 2001 | 14 RCTs from 1966 to 1999 with depression as an outcome | Significant methodologic weaknesses, but exercise effect > no treatment and=cognitive therapy |
| Dunn et al, 2001 | Examined dose effect in 37 studies; subjects diagnosed with depressive disorders, mild-to-moderate symptoms, and no medical comorbidity | Only level B and C evidence; positive effects with exercise from light to heavy intensity; aerobic=nonaerobic; improvement may or may not be related to improved fitness |
| Brosse et al, 2002 | 12 RCTs from 1979 to 1999 | Significant methodologic limitations, but authors concluded evidence supports a positive effect of exercise in healthy and clinical populations |
| Larun et al, 2006 | 4 RCTs in children and youth age | Exercise effect same as no intervention, low-intensity relaxation, or psychosocial intervention |
| Barbour et al, 2007 | 2 meta-analyses, 1 RCT, 2 studies | Positive effect; high-energy was optimal dose; aerobic=nonaerobic; improvement may or may not be related to improved fitness |
| RCT: randomized controlled trial | ||
| Source: Byrne AE, Byrne DG. The effect of exercise on depression, anxiety and other mood states: A review. J Psychosom Res. 1993;37(6):565-574. Scully D, Kremer J, Meade MM, et al. Physical exercise and psychological well being: a critical review. Br J Sports Med. 1998;32(2):111-120. Lawlor DA, Hopker SW. The effectiveness of exercise as an intervention in the management of depression: systematic review and meta-regression analysis of randomised controlled trials. BMJ. 2001;322(7289):763-767. Dunn AL, Trivedi MH, O’Neal HA. Physical activity dose-response effects on outcomes of depression and anxiety. Med Sci Sports Exerc. 2001;33(6):S587. Brosse AL, Sheets ES, Lett HS, et al. Exercise and the treatment of clinical depression in adults: recent findings and future directions. Sports Med. 2002;32(12):741-760. Larun L, Nordheim LV, Ekeland E, et al. Exercise in prevention and treatment of anxiety and depression among children and young people. Cochrane Database Syst Rev. 2006;3:CD004691. Barbour KA, Edenfield TM, Blumenthal JA. Exercise as a treatment for depression and other psychiatric disorders: a review. J Cardiopulm Rehabil Prev. 2007;27(6):359-367. | ||
At least 2 studies suggest that high-energy exercise and aerobic or resistance training provide greater reductions in depressive symptoms than exercises such as walking.5,6 Yoga’s positive effects suggest, however, that an aerobic effect is not necessary for an antidepressant benefit.
Exercise has not been adequately tested as a complementary treatment but likely is safe for most psychiatric patients. Perspiration and dehydration might alter therapeutic blood levels of lithium or other medications. Advise patients to drink water before, during, and after exercise and to avoid outdoor exercise in extreme temperatures. More vigorous monitoring might be indicated in specific cases.
Tailor exercise programs to individual needs, considering the patient’s age and health status. Refer a patient with a known heart problem or increased cardiovascular risk to his or her physician for selective exercise testing.
Bibliotherapy—reading self-help books, usually about cognitive-behavioral approaches to depressive disorders—has been relatively well studied. A recent meta-analysis examined 29 studies with pre-post designs. Group differences in the 17 controlled studies yielded a large effect size of 0.77. Participants who read the materials benefitted similarly whether they met in groups or applied the information on their own. Older adults tended to be less depressed at baseline and made smaller treatment gains.7
Conclusion. Evidence supports bibliotherapy as an effective treatment for mild-to-moderate depression. No convincing data support its use as a complementary treatment, but it poses virtually no risk.
St. John’s wort (Hypericum perforatum) has been extensively studied for depressive disorders, with 29 RCTs in a meta-analysis of MDD trials through July 2008.10 Another meta-analysis compared St. John’s wort with selective serotonin reuptake inhibitors (SSRIs) in 13 studies through June 2008.11 These and most RCTs have found St. John’s wort significantly more effective than placebo in reducing depressive symptoms.
Data selected from double-blind RCTs totaling 217 patients with mild depression [Hamilton Depression Rating Scale (HDRS) scores 12 Studies routinely show that treating MDD with St. John’s wort is comparable to using tricyclic or SSRI antidepressants.
Side effects with St. John’s wort generally are no different than with placebo and significantly less than with comparison treatments. Even so, using St. John’s wort instead of SSRIs for MDD remains controversial.
Studies vs SSRIs. Many of the favorable St. John’s wort trials were conducted in Europe, particularly in Germany. Two large RCTs conducted in the United States reported that the St. John’s wort standardized extract LI-160 was not more effective than placebo, but neither could be clearly interpreted as negative for St. John’s wort:
- In an 8-week trial, St. John’s wort and placebo groups improved significantly but at unusually low rates. The remission rate with St. John’s wort was small but significantly higher than with placebo.13
- A study sponsored by the National Institute of Mental Health compared St. John’s wort, 900 to 1,500 mg/d; sertraline, 50 to 100 mg/d; and placebo in 340 adults with MDD. No positive effects were found for St. John’s wort or sertraline.14
Conclusion. Standardized extracts of St. John’s wort—particularly WS5570, 300 mg tid, and ZE117, 250 mg bid—appear to be effective treatments, especially for mild-to-moderate MDD. Because St. John’s wort is available without prescription and can interact with SSRIs or other antidepressants:
- care is required for its complementary use
- it is important to ask if patients are using St. John’s wort on their own.
- for first-line use only when you can adequately gauge its effects on your patient’s other medications
- especially for depressed patients who cannot tolerate SSRIs.
SAMe has become a popular alternative treatment for depression since its introduction to the United States in the late 1990s, but it has been studied in only 2 U.S. open trials. One showed SAMe to be very effective in reducing depressive symptoms in patients with HIV/AIDS.17 The other found a 50% response rate and 43% remission rate with adjunctive SAMe, 800 to 1,600 mg/d for 6 weeks, in 30 adults with MDD who failed to respond adequately to SSRIs or the serotonin-norepinephrine reuptake inhibitor (SNRI) venlafaxine. The most common side effects were gastrointestinal (GI) symptoms and headaches.18 This open trial led to an ongoing National Institutes of Health-sponsored RCT on the safety and efficacy of SAMe for patients with treatment-resistant depression.
Conclusion. SAMe appears to have a faster onset of antidepressant effect than standard SSRIs or SNRIs and a favorable side-effect profile, which make the lack of rigorous trials in the United States striking. We recommend that you consider SAMe:
- as an adjunct in patients with incomplete response to standard treatments
- as a complementary treatment to speed onset of antidepressant effects.
Four meta-analyses independently looked at largely the same dozen RCTs through 2006 and found that 1 to 2 grams daily of omega-3 PUFAs was significantly more effective at reducing depressive symptoms than placebo.19-22 Other data suggest that omega-3 PUFAs can induce depression remission in depressed Parkinson’s disease patients23 and depressed pregnant women.24 Since 2006, however, findings have been inconsistent. Several trials have found PUFAs no more effective than placebo.25-27
An 8-week double-blind study compared EPA, 1 gram daily; fluoxetine, 20 mg/d; or both agents in 60 outpatients with MDD. Response rates—as measured by ≥50% reduction in baseline HDRS scores—were 50% with fluoxetine, 56% with EPA, and 81% with combination therapy.28
Insufficient evidence
L-tryptophan. It seems reasonable to expect a serotonin precursor to increase serotonin in the CNS and improve depressive symptoms. Of 111 trials on L-tryptophan for depression, however, only 2 met the quality criteria for inclusion in a recent meta-analysis.29 Combining the 2 trials showed L-tryptophan alone and in combination with a tricyclic antidepressant was more effective than placebo for treating depressive disorders in adults.
Conclusion. Very little research continues to test L-tryptophan as a viable CAM for depressive disorder. Its serious side effect of eosinophilia-myalgia syndrome makes clinical use of this agent unlikely.
Acupuncture. Numerous small studies with questionable controls, different needling placements, and poor allocation concealment and blinding limit the ability to draw conclusions about acupuncture for treating depression ( Table 4 ). A recent meta-analysis by Wang et al30 added 2 Chinese trials not included in an earlier review31 and found acupuncture significantly reduced depressive symptoms. No consistent differences were detected in response or remission rates, however.
Conclusion. Evidence is methodologically weak, and the use of acupuncture as an alternative or complementary treatment of depression is questionable.
Table 4
Acupuncture: Insufficient evidence of antidepressant effect
| Literature review | Methodology | Conclusion |
|---|---|---|
| Mukaino et al, 2005 | Systematic review of 7 RCTs including 509 patients; compared either manual or electroacupuncture with any control procedure | Inconsistent evidence of manual acupuncture’s effectiveness vs sham; electroacupuncture’s effect may be similar to that of antidepressant medication and merits further study |
| Leo and Ligot, 2007 | Systematic review of 9 RCTs, 5 considered low quality; some focused on very specific populations (ie, hospitalized stroke patients or pregnant depressed patients) | Evidence inconclusive because of study designs and methodologies |
| Smith and Hay, 2005 | Meta-analysis of 7 trials including 517 adults with mild-to-moderate depression; 5 trials (409 participants) compared acupuncture with medication; 2 trials compared acupuncture with wait list or sham acupuncture | No difference between acupuncture and medication; study quality too poor to support acupuncture’s efficacy |
| Wang et al, 2008 | Meta-analysis of 8 small RCTs totalling 477 subjects (256 received active acupuncture, remainder received sham acupuncture); sham acupuncture design, number of acupuncture sessions, and duration varied among studies | Significant reduction in HRSD or BDI scores for acupuncture vs sham, but no significant effect of acupuncture on response or remission rates |
| BDI: Beck Depression Inventory; HRSD: Hamilton Rating Scale for Depression; RCT: randomized controlled trial | ||
| Source: Mukaino Y, Park J, White A, et al. The effectiveness of acupuncture for depression—a systematic review of randomised controlled trials. Acupunct Med. 2005;23(2):70-76. Leo RJ, Ligot JS Jr. A systematic review of randomized controlled trials of acupuncture in the treatment of depression. J Affect Disord. 2007;97(1-3):13-22. Smith CA, Hay PPJ. Acupuncture for depression. Cochrane Database Syst Rev. 2005;(2):CD004046. Wang H, Qi H, Wang BS, et al. Is acupuncture beneficial in depression? A meta-analysis of 8 randomized controlled trials. J Affect Disord. 2008;111(2-3):125-134. | ||
- National Center for Complementary and Alternative Medicine, National Institutes of Health. http://nccam.nih.gov.
- Journal of Alternative and Complementary Medicine. www.liebertpub.com/products/product.aspx?pid=26.
- Complementary and alternative medicine. www.nlm.nih.gov/medlineplus/complementaryandalternativemedicine.html.
- Fluoxetine • Prozac
- Imipramine • Tofranil
- Lithium • Eskalith, Lithobid
- Sertraline • Zoloft
- Venlafaxine • Effexor
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Americans with depression turn to complementary and alternative medicine (CAM) more often than conventional psychotherapy or FDA-approved medication. In a nationally representative sample, 54% of respondents with self-reported “severe depression”—including two-thirds of those receiving conventional therapies—reported using CAM during the previous 12 months.1
Unfortunately, popular acceptance of CAM for depression is disproportionate to the evidence base, which—although growing—remains limited. As a result, your patients may be self-medicating with poorly supported treatments that are unlikely to help them recover from depression.
In reviewing CAM treatments for depression, we found some with enough evidence of positive effect that we feel comfortable recommending them as evidence-based options. These promising, short-term treatments are supported by level 1a or 1b evidence and at least 1 study that demonstrates an ability to induce remission ( Table 1 ).2
For patients seeking “natural” or nonprescription treatments—or when you wish to augment standard treatments that are not working adequately—you might recommend fatty acids, St. John’s wort, or S-adenosyl-L-methionine (SAMe). Similar recommendations can be made for yoga, exercise, and bibliotherapy, as we discuss here.
Table 1
Evidence these authors required to recommend a CAM treatment
| Minimum requirements | Level of evidence | Recommendation |
|---|---|---|
| Systematic review showing superiority to placebo or standard treatment Plus 1 study showing CAM treatment can induce remission | 1a + | A |
| At least 2 RCTs showing superiority to placebo or standard treatment Plus 1 study showing CAM treatment can induce remission | 1b | A– |
| CAM: complementary and alternative medicine; RCT: randomized controlled trial | ||
| Source: Reference 2 | ||
Reviewing CAM evidence
This article refers to as “alternative” any treatment other than a form of psychotherapy or an FDA-approved medication that substitutes for a standard psychiatric treatment. When used to augment standard psychiatric treatments, these approaches are considered “complementary.”
Our search for evidence on CAM treatments for depressive disorders raised questions about what constitutes acceptable and convincing methodology:
- Studies often had problems with blinding and suitable placebos. Many were small, with short duration and no long-term follow-up.
- Comparisons with active treatments that showed no differences were assumed to imply comparability, even though the studies were powered to detect only large differences.
Applying the evidence. Because CAM use is widespread, be sure to ask psychiatric patients if they are using CAM treatments. If the answer is “yes,” a risk-benefit assessment is needed. Inform patients who are using poorly supported CAM approaches that they could consider better-supported CAM options as well as standard effective antidepressants.
Monitor patients for an adequately prompt positive response to an evidence-based CAM approach that has shown efficacy for their level of depression. As with any treatment, consider other evidence-based options when CAM treatments are inadequate or unsuccessful in achieving remission of depressive symptoms.
Sufficient evidence of efficacy
Yoga. In their systematic review of yoga’s effectiveness for depression, Pilkington et al3 analyzed 5 RCTs that met 3 criteria:
- participants had mild to severe depression or depressive disorders
- yoga or yoga-based exercises alone were used for treatment
- depression rating scales were used as outcome measures.
Conclusion. Yoga has been studied primarily as an alternative treatment, but its physical health and group participation benefits and lack of side effects make it a suitable complementary treatment as well.
- 45% with supervised exercise
- 40% with home-based exercise
- 47% with sertraline, 50 to 200 mg/d
- 31% with placebo.4
5 RCTs of yoga’s effectiveness in treating depression
| RCT | Interventions | Conclusion |
|---|---|---|
| Broota and Dhir, 1990 | Yoga and PMR vs control | Yoga and PMR were more effective than control, with yoga more effective than PMR |
| Khumar et al, 1993 | Shavasana yoga vs no intervention | College students with severe depression improved during and after yoga treatment |
| Janakiramaiah et al, 2000 | SKY vs ECT vs imipramine | Reductions in BDI scores for all 3 groups; ECT > SKY or imipramine, SKY=imipramine |
| Rohini et al, 2000 | Full SKY vs partial SKY | 30 individuals with MDD improved with either therapy, but results were not statistically significant |
| Woolery, 2004 | Iyengar yoga vs wait list | 28 mildly depressed individuals benefitted from yoga, as measured by BDI scores at midpoint and throughout treatment |
| BDI: Beck Depression Inventory; ECT: electroconvulsive therapy; MDD: major depressive disorder; PMR: progressive muscle relaxation; RCT: randomized controlled trial; SKY: Sudarshan Kriya yoga | ||
| Source: Broota A, Dhir R. Efficacy of two relaxation techniques in depression. Journal of Personality and Clinical Studies. 1990;6(1):83-90. Khumar SS, Kaur P, Kaur S. Effectiveness of Shavasana on depression among university students. Indian J Clin Psychol. 1993;20(2):82-87. Janakiramaiah N, Gangadhar BN, Naga Venkatesha Murthy PJ, et al. Antidepressant efficacy of Sudarshan Kriya yoga (SKY) in melancholia: a randomized comparison with electroconvulsive therapy (ECT) and imipramine. J Affect Disord. 2000;57(1-3):255-259. Rohini V, Pandey RS, Janakiramaiah N, et al. A comparative study of full and partial Sudarshan Kriya yoga (SKY) in major depressive disorder. NIMHANS Journal. 2000;18(1):53-57. Woolery A, Myers H, Sternlieb B, et al. A yoga intervention for young adults with elevated symptoms of depression. Altern Ther Health Med. 2004;10(2):60-63. | ||
Table 3
Evidence of the antidepressant effect of exercise
| Literature review | Methodology | Conclusion |
|---|---|---|
| Byrne and Byrne, 1993 | 13 studies, clinical samples, measured changes in depressed mood | 90% of studies reported beneficial effects, especially in clinical populations |
| Scully et al, 1998 | 4 literature reviews, 1 monograph, 1 study | Positive relationship of physical activity and depression in healthy and clinical samples, increased over time |
| Lawlor and Hopker, 2001 | 14 RCTs from 1966 to 1999 with depression as an outcome | Significant methodologic weaknesses, but exercise effect > no treatment and=cognitive therapy |
| Dunn et al, 2001 | Examined dose effect in 37 studies; subjects diagnosed with depressive disorders, mild-to-moderate symptoms, and no medical comorbidity | Only level B and C evidence; positive effects with exercise from light to heavy intensity; aerobic=nonaerobic; improvement may or may not be related to improved fitness |
| Brosse et al, 2002 | 12 RCTs from 1979 to 1999 | Significant methodologic limitations, but authors concluded evidence supports a positive effect of exercise in healthy and clinical populations |
| Larun et al, 2006 | 4 RCTs in children and youth age | Exercise effect same as no intervention, low-intensity relaxation, or psychosocial intervention |
| Barbour et al, 2007 | 2 meta-analyses, 1 RCT, 2 studies | Positive effect; high-energy was optimal dose; aerobic=nonaerobic; improvement may or may not be related to improved fitness |
| RCT: randomized controlled trial | ||
| Source: Byrne AE, Byrne DG. The effect of exercise on depression, anxiety and other mood states: A review. J Psychosom Res. 1993;37(6):565-574. Scully D, Kremer J, Meade MM, et al. Physical exercise and psychological well being: a critical review. Br J Sports Med. 1998;32(2):111-120. Lawlor DA, Hopker SW. The effectiveness of exercise as an intervention in the management of depression: systematic review and meta-regression analysis of randomised controlled trials. BMJ. 2001;322(7289):763-767. Dunn AL, Trivedi MH, O’Neal HA. Physical activity dose-response effects on outcomes of depression and anxiety. Med Sci Sports Exerc. 2001;33(6):S587. Brosse AL, Sheets ES, Lett HS, et al. Exercise and the treatment of clinical depression in adults: recent findings and future directions. Sports Med. 2002;32(12):741-760. Larun L, Nordheim LV, Ekeland E, et al. Exercise in prevention and treatment of anxiety and depression among children and young people. Cochrane Database Syst Rev. 2006;3:CD004691. Barbour KA, Edenfield TM, Blumenthal JA. Exercise as a treatment for depression and other psychiatric disorders: a review. J Cardiopulm Rehabil Prev. 2007;27(6):359-367. | ||
At least 2 studies suggest that high-energy exercise and aerobic or resistance training provide greater reductions in depressive symptoms than exercises such as walking.5,6 Yoga’s positive effects suggest, however, that an aerobic effect is not necessary for an antidepressant benefit.
Exercise has not been adequately tested as a complementary treatment but likely is safe for most psychiatric patients. Perspiration and dehydration might alter therapeutic blood levels of lithium or other medications. Advise patients to drink water before, during, and after exercise and to avoid outdoor exercise in extreme temperatures. More vigorous monitoring might be indicated in specific cases.
Tailor exercise programs to individual needs, considering the patient’s age and health status. Refer a patient with a known heart problem or increased cardiovascular risk to his or her physician for selective exercise testing.
Bibliotherapy—reading self-help books, usually about cognitive-behavioral approaches to depressive disorders—has been relatively well studied. A recent meta-analysis examined 29 studies with pre-post designs. Group differences in the 17 controlled studies yielded a large effect size of 0.77. Participants who read the materials benefitted similarly whether they met in groups or applied the information on their own. Older adults tended to be less depressed at baseline and made smaller treatment gains.7
Conclusion. Evidence supports bibliotherapy as an effective treatment for mild-to-moderate depression. No convincing data support its use as a complementary treatment, but it poses virtually no risk.
St. John’s wort (Hypericum perforatum) has been extensively studied for depressive disorders, with 29 RCTs in a meta-analysis of MDD trials through July 2008.10 Another meta-analysis compared St. John’s wort with selective serotonin reuptake inhibitors (SSRIs) in 13 studies through June 2008.11 These and most RCTs have found St. John’s wort significantly more effective than placebo in reducing depressive symptoms.
Data selected from double-blind RCTs totaling 217 patients with mild depression [Hamilton Depression Rating Scale (HDRS) scores 12 Studies routinely show that treating MDD with St. John’s wort is comparable to using tricyclic or SSRI antidepressants.
Side effects with St. John’s wort generally are no different than with placebo and significantly less than with comparison treatments. Even so, using St. John’s wort instead of SSRIs for MDD remains controversial.
Studies vs SSRIs. Many of the favorable St. John’s wort trials were conducted in Europe, particularly in Germany. Two large RCTs conducted in the United States reported that the St. John’s wort standardized extract LI-160 was not more effective than placebo, but neither could be clearly interpreted as negative for St. John’s wort:
- In an 8-week trial, St. John’s wort and placebo groups improved significantly but at unusually low rates. The remission rate with St. John’s wort was small but significantly higher than with placebo.13
- A study sponsored by the National Institute of Mental Health compared St. John’s wort, 900 to 1,500 mg/d; sertraline, 50 to 100 mg/d; and placebo in 340 adults with MDD. No positive effects were found for St. John’s wort or sertraline.14
Conclusion. Standardized extracts of St. John’s wort—particularly WS5570, 300 mg tid, and ZE117, 250 mg bid—appear to be effective treatments, especially for mild-to-moderate MDD. Because St. John’s wort is available without prescription and can interact with SSRIs or other antidepressants:
- care is required for its complementary use
- it is important to ask if patients are using St. John’s wort on their own.
- for first-line use only when you can adequately gauge its effects on your patient’s other medications
- especially for depressed patients who cannot tolerate SSRIs.
SAMe has become a popular alternative treatment for depression since its introduction to the United States in the late 1990s, but it has been studied in only 2 U.S. open trials. One showed SAMe to be very effective in reducing depressive symptoms in patients with HIV/AIDS.17 The other found a 50% response rate and 43% remission rate with adjunctive SAMe, 800 to 1,600 mg/d for 6 weeks, in 30 adults with MDD who failed to respond adequately to SSRIs or the serotonin-norepinephrine reuptake inhibitor (SNRI) venlafaxine. The most common side effects were gastrointestinal (GI) symptoms and headaches.18 This open trial led to an ongoing National Institutes of Health-sponsored RCT on the safety and efficacy of SAMe for patients with treatment-resistant depression.
Conclusion. SAMe appears to have a faster onset of antidepressant effect than standard SSRIs or SNRIs and a favorable side-effect profile, which make the lack of rigorous trials in the United States striking. We recommend that you consider SAMe:
- as an adjunct in patients with incomplete response to standard treatments
- as a complementary treatment to speed onset of antidepressant effects.
Four meta-analyses independently looked at largely the same dozen RCTs through 2006 and found that 1 to 2 grams daily of omega-3 PUFAs was significantly more effective at reducing depressive symptoms than placebo.19-22 Other data suggest that omega-3 PUFAs can induce depression remission in depressed Parkinson’s disease patients23 and depressed pregnant women.24 Since 2006, however, findings have been inconsistent. Several trials have found PUFAs no more effective than placebo.25-27
An 8-week double-blind study compared EPA, 1 gram daily; fluoxetine, 20 mg/d; or both agents in 60 outpatients with MDD. Response rates—as measured by ≥50% reduction in baseline HDRS scores—were 50% with fluoxetine, 56% with EPA, and 81% with combination therapy.28
Insufficient evidence
L-tryptophan. It seems reasonable to expect a serotonin precursor to increase serotonin in the CNS and improve depressive symptoms. Of 111 trials on L-tryptophan for depression, however, only 2 met the quality criteria for inclusion in a recent meta-analysis.29 Combining the 2 trials showed L-tryptophan alone and in combination with a tricyclic antidepressant was more effective than placebo for treating depressive disorders in adults.
Conclusion. Very little research continues to test L-tryptophan as a viable CAM for depressive disorder. Its serious side effect of eosinophilia-myalgia syndrome makes clinical use of this agent unlikely.
Acupuncture. Numerous small studies with questionable controls, different needling placements, and poor allocation concealment and blinding limit the ability to draw conclusions about acupuncture for treating depression ( Table 4 ). A recent meta-analysis by Wang et al30 added 2 Chinese trials not included in an earlier review31 and found acupuncture significantly reduced depressive symptoms. No consistent differences were detected in response or remission rates, however.
Conclusion. Evidence is methodologically weak, and the use of acupuncture as an alternative or complementary treatment of depression is questionable.
Table 4
Acupuncture: Insufficient evidence of antidepressant effect
| Literature review | Methodology | Conclusion |
|---|---|---|
| Mukaino et al, 2005 | Systematic review of 7 RCTs including 509 patients; compared either manual or electroacupuncture with any control procedure | Inconsistent evidence of manual acupuncture’s effectiveness vs sham; electroacupuncture’s effect may be similar to that of antidepressant medication and merits further study |
| Leo and Ligot, 2007 | Systematic review of 9 RCTs, 5 considered low quality; some focused on very specific populations (ie, hospitalized stroke patients or pregnant depressed patients) | Evidence inconclusive because of study designs and methodologies |
| Smith and Hay, 2005 | Meta-analysis of 7 trials including 517 adults with mild-to-moderate depression; 5 trials (409 participants) compared acupuncture with medication; 2 trials compared acupuncture with wait list or sham acupuncture | No difference between acupuncture and medication; study quality too poor to support acupuncture’s efficacy |
| Wang et al, 2008 | Meta-analysis of 8 small RCTs totalling 477 subjects (256 received active acupuncture, remainder received sham acupuncture); sham acupuncture design, number of acupuncture sessions, and duration varied among studies | Significant reduction in HRSD or BDI scores for acupuncture vs sham, but no significant effect of acupuncture on response or remission rates |
| BDI: Beck Depression Inventory; HRSD: Hamilton Rating Scale for Depression; RCT: randomized controlled trial | ||
| Source: Mukaino Y, Park J, White A, et al. The effectiveness of acupuncture for depression—a systematic review of randomised controlled trials. Acupunct Med. 2005;23(2):70-76. Leo RJ, Ligot JS Jr. A systematic review of randomized controlled trials of acupuncture in the treatment of depression. J Affect Disord. 2007;97(1-3):13-22. Smith CA, Hay PPJ. Acupuncture for depression. Cochrane Database Syst Rev. 2005;(2):CD004046. Wang H, Qi H, Wang BS, et al. Is acupuncture beneficial in depression? A meta-analysis of 8 randomized controlled trials. J Affect Disord. 2008;111(2-3):125-134. | ||
- National Center for Complementary and Alternative Medicine, National Institutes of Health. http://nccam.nih.gov.
- Journal of Alternative and Complementary Medicine. www.liebertpub.com/products/product.aspx?pid=26.
- Complementary and alternative medicine. www.nlm.nih.gov/medlineplus/complementaryandalternativemedicine.html.
- Fluoxetine • Prozac
- Imipramine • Tofranil
- Lithium • Eskalith, Lithobid
- Sertraline • Zoloft
- Venlafaxine • Effexor
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Kessler RC, Soukup J, Davis RB, et al. The use of complementary and alternative therapies to treat anxiety and depression in the United States. Am J Psychiatry. 2001;158(2):289-294.
2. Phillips B, Ball C, Sackett D, et al. Oxford University Centre for Evidence Based Medicine levels of evidence and grades of recommendation (March 2009). Available at: http://www.cebm.net/index.aspx?o=1025#levels. Accessed August 19, 2009.
3. Pilkington K, Kirkwood G, Rampes H, et al. Yoga for depression: the research evidence. J Affect Disord. 2005;89(1-3):13-24.
4. Blumenthal JA, Babyak MA, Doraiswamy PM, et al. Exercise and pharmacotherapy in the treatment of major depressive disorder. Psychosom Med. 2007;69(7):587-596.
5. Dunn AL, Trivedi MH, Kampert JB, et al. Exercise treatment for depression: efficacy and dose response. Am J Prev Med. 2005;28(1):1-8.
6. Legrand F, Heuze JP. Antidepressant effects associated with different exercise conditions in participants with depression: a pilot study. J Sport Exercise Psychol. 2007;29(3):348-364.
7. Gregory RJ, Schwer Canning S, Lee TW, et al. Cognitive bibliotherapy for depression: a meta-analysis. Professional Psychology: Research and Practice. 2004;35(3):275-280.
8. Floyd M, Scogin F, McKendree-Smith NL, et al. Cognitive therapy for depression: a comparison of individual psychotherapy and bibliotherapy for depressed older adults. Behav Modif. 2004;28(2):297-318.
9. Burns DD. Feeling good: the new mood therapy. New York, NY: HarperCollins; 1980.
10. Linde K, Berner MM, Kriston L. St John’s Wort for major depression. [update of Cochrane Database Syst Rev. 2005;(2):CD000448; PMID: 15846605]. Cochrane Database Syst Rev. 2008(4):000448.
11. Rahimi R, Nikfar S, Abdollahi M. Efficacy and tolerability of Hypericum perforatum in major depressive disorder in comparison with selective serotonin reuptake inhibitors: a meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33(1):118-127.
12. Kasper S, Gastpar M, Müller WE, et al. Efficacy of St. John’s wort extract WS 5570 in acute treatment of mild depression: a reanalysis of data from controlled clinical trials. Eur Arch Psychiatry Clin Neurosci. 2008;258(1):59-63.
13. Shelton RC, Keller MB, Gelenberg A, et al. Effectiveness of St John’s wort in major depression: a randomized controlled trial. JAMA. 2001;285(15):1978-1986.
14. Hypericum Depression Trial Study Group. Effect of Hypericum perforatum (St. John’s wort) in major depressive disorder: a randomized controlled trial. JAMA. 2002;287(14):1807-1814.
15. Mischoulon D, Fava M. Role of S-adenosyl-L-methionine in the treatment of depression: a review of the evidence. Am J Clin Nutr. 2002;76(suppl):1158S-1161S.
16. Hardy ML, Coulter I, Morton SC, et al. S-adenosyl-L-methionine for treatment of depression, osteoarthritis, and liver disease [comment in ACP J Club. 2003;139(1):20]. Evid Rep Technol Assess (Summ). 2003;Aug(64):1-3.
17. Shippy RA, Mendez D, Jones K, et al. S-adenosylmethionine (SAM-e) for the treatment of depression in people living with HIV/AIDS. BMC Psychiatry. 2004;4:38.-
18. Alpert JE, Papakostas G, Mischoulon D, et al. S-adenosyl-L-methionine (SAMe) as an adjunct for resistant major depressive disorder: an open trial following partial or nonresponse to selective serotonin reuptake inhibitors or venlafaxine. J Clin Psychopharmacol. 2004;24(6):661-664.
19. Lin PY, Su KP. A meta-analytic review of double-blind, placebo-controlled trials of antidepressant efficacy of omega-3 fatty acids. J Clin Psychiatry. 2007;68(7):1056-1061.
20. Freeman MP, Hibbeln JR, Wisner KL, et al. Omega-3 fatty acids: evidence basis for treatment and future research in psychiatry [published correction appears in J Clin Psychiatry. 2007;68(2):338]. J Clin Psychiatry. 2006;67(12):1954-1967.
21. Ross BM, Seguin J, Sieswerda LE. Omega-3 fatty acids as treatments for mental illness: which disorder and which fatty acid? Lipids Health Dis. 2007;6:21.-
22. Appleton KM, Hayward RC, Gunnell D, et al. Effects of n-3 long-chain polyunsaturated fatty acids on depressed mood: systematic review of published trials [comment in Am J Clin Nutr. 2007;85(6):1665-1666; author reply 1666]. Am J Clin Nutr. 2006;84(6):1308-1316.
23. da Silva TM, Munhoz RP, Alvarez C, et al. Depression in Parkinson’s disease: a double-blind, randomized, placebo-controlled pilot study of omega-3 fatty-acid supplementation. J Affect Disord. 2008;111(2-3):351-359.
24. Su KP, Huang SY, Chiu TH, et al. Omega-3 fatty acids for major depressive disorder during pregnancy: results from a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2008;69(4):644-651.
25. Grenyer BF, Crowe T, Meyer B, et al. Fish oil supplementation in the treatment of major depression: a randomised double-blind placebo-controlled trial. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(7):1393-1396.
26. Rees AM, Austin MP, Parker GB. Omega-3 fatty acids as a treatment for perinatal depression: randomized double-blind placebo-controlled trial. Aust N Z J Psychiatry. 2008;42(3):199-205.
27. Rogers PJ, Appleton KM, Kessler D, et al. No effect of n-3 long-chain polyunsaturated fatty acid (EPA and DHA) supplementation on depressed mood and cognitive function: a randomised controlled trial. Br J Nutr. 2008;99(2):421-431.
28. Jazayeri S, Tehrani-Doost M, Keshavarz SA, et al. Comparison of therapeutic effects of omega-3 fatty acid eicosapentaenoic acid and fluoxetine, separately and in combination, in major depressive disorder. Aust N Z J Psychiatry. 2008;42(3):192-198.
29. Shaw K, Turner J, Del Mar C. Tryptophan and 5-Hydroxytryptophan for depression. Cochrane Database Syst Rev. 2002;(1):CD003198.-
30. Wang H, Qi H, Wang BS, et al. Is acupuncture beneficial in depression: a meta-analysis of 8 randomized controlled trials? J Affect Disord. 2008;111(2-3):125-134.
31. Smith CA, Hay PPJ. Acupuncture for depression. Cochrane Database Syst Rev. 2005;(2):CD004046.-
1. Kessler RC, Soukup J, Davis RB, et al. The use of complementary and alternative therapies to treat anxiety and depression in the United States. Am J Psychiatry. 2001;158(2):289-294.
2. Phillips B, Ball C, Sackett D, et al. Oxford University Centre for Evidence Based Medicine levels of evidence and grades of recommendation (March 2009). Available at: http://www.cebm.net/index.aspx?o=1025#levels. Accessed August 19, 2009.
3. Pilkington K, Kirkwood G, Rampes H, et al. Yoga for depression: the research evidence. J Affect Disord. 2005;89(1-3):13-24.
4. Blumenthal JA, Babyak MA, Doraiswamy PM, et al. Exercise and pharmacotherapy in the treatment of major depressive disorder. Psychosom Med. 2007;69(7):587-596.
5. Dunn AL, Trivedi MH, Kampert JB, et al. Exercise treatment for depression: efficacy and dose response. Am J Prev Med. 2005;28(1):1-8.
6. Legrand F, Heuze JP. Antidepressant effects associated with different exercise conditions in participants with depression: a pilot study. J Sport Exercise Psychol. 2007;29(3):348-364.
7. Gregory RJ, Schwer Canning S, Lee TW, et al. Cognitive bibliotherapy for depression: a meta-analysis. Professional Psychology: Research and Practice. 2004;35(3):275-280.
8. Floyd M, Scogin F, McKendree-Smith NL, et al. Cognitive therapy for depression: a comparison of individual psychotherapy and bibliotherapy for depressed older adults. Behav Modif. 2004;28(2):297-318.
9. Burns DD. Feeling good: the new mood therapy. New York, NY: HarperCollins; 1980.
10. Linde K, Berner MM, Kriston L. St John’s Wort for major depression. [update of Cochrane Database Syst Rev. 2005;(2):CD000448; PMID: 15846605]. Cochrane Database Syst Rev. 2008(4):000448.
11. Rahimi R, Nikfar S, Abdollahi M. Efficacy and tolerability of Hypericum perforatum in major depressive disorder in comparison with selective serotonin reuptake inhibitors: a meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33(1):118-127.
12. Kasper S, Gastpar M, Müller WE, et al. Efficacy of St. John’s wort extract WS 5570 in acute treatment of mild depression: a reanalysis of data from controlled clinical trials. Eur Arch Psychiatry Clin Neurosci. 2008;258(1):59-63.
13. Shelton RC, Keller MB, Gelenberg A, et al. Effectiveness of St John’s wort in major depression: a randomized controlled trial. JAMA. 2001;285(15):1978-1986.
14. Hypericum Depression Trial Study Group. Effect of Hypericum perforatum (St. John’s wort) in major depressive disorder: a randomized controlled trial. JAMA. 2002;287(14):1807-1814.
15. Mischoulon D, Fava M. Role of S-adenosyl-L-methionine in the treatment of depression: a review of the evidence. Am J Clin Nutr. 2002;76(suppl):1158S-1161S.
16. Hardy ML, Coulter I, Morton SC, et al. S-adenosyl-L-methionine for treatment of depression, osteoarthritis, and liver disease [comment in ACP J Club. 2003;139(1):20]. Evid Rep Technol Assess (Summ). 2003;Aug(64):1-3.
17. Shippy RA, Mendez D, Jones K, et al. S-adenosylmethionine (SAM-e) for the treatment of depression in people living with HIV/AIDS. BMC Psychiatry. 2004;4:38.-
18. Alpert JE, Papakostas G, Mischoulon D, et al. S-adenosyl-L-methionine (SAMe) as an adjunct for resistant major depressive disorder: an open trial following partial or nonresponse to selective serotonin reuptake inhibitors or venlafaxine. J Clin Psychopharmacol. 2004;24(6):661-664.
19. Lin PY, Su KP. A meta-analytic review of double-blind, placebo-controlled trials of antidepressant efficacy of omega-3 fatty acids. J Clin Psychiatry. 2007;68(7):1056-1061.
20. Freeman MP, Hibbeln JR, Wisner KL, et al. Omega-3 fatty acids: evidence basis for treatment and future research in psychiatry [published correction appears in J Clin Psychiatry. 2007;68(2):338]. J Clin Psychiatry. 2006;67(12):1954-1967.
21. Ross BM, Seguin J, Sieswerda LE. Omega-3 fatty acids as treatments for mental illness: which disorder and which fatty acid? Lipids Health Dis. 2007;6:21.-
22. Appleton KM, Hayward RC, Gunnell D, et al. Effects of n-3 long-chain polyunsaturated fatty acids on depressed mood: systematic review of published trials [comment in Am J Clin Nutr. 2007;85(6):1665-1666; author reply 1666]. Am J Clin Nutr. 2006;84(6):1308-1316.
23. da Silva TM, Munhoz RP, Alvarez C, et al. Depression in Parkinson’s disease: a double-blind, randomized, placebo-controlled pilot study of omega-3 fatty-acid supplementation. J Affect Disord. 2008;111(2-3):351-359.
24. Su KP, Huang SY, Chiu TH, et al. Omega-3 fatty acids for major depressive disorder during pregnancy: results from a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2008;69(4):644-651.
25. Grenyer BF, Crowe T, Meyer B, et al. Fish oil supplementation in the treatment of major depression: a randomised double-blind placebo-controlled trial. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(7):1393-1396.
26. Rees AM, Austin MP, Parker GB. Omega-3 fatty acids as a treatment for perinatal depression: randomized double-blind placebo-controlled trial. Aust N Z J Psychiatry. 2008;42(3):199-205.
27. Rogers PJ, Appleton KM, Kessler D, et al. No effect of n-3 long-chain polyunsaturated fatty acid (EPA and DHA) supplementation on depressed mood and cognitive function: a randomised controlled trial. Br J Nutr. 2008;99(2):421-431.
28. Jazayeri S, Tehrani-Doost M, Keshavarz SA, et al. Comparison of therapeutic effects of omega-3 fatty acid eicosapentaenoic acid and fluoxetine, separately and in combination, in major depressive disorder. Aust N Z J Psychiatry. 2008;42(3):192-198.
29. Shaw K, Turner J, Del Mar C. Tryptophan and 5-Hydroxytryptophan for depression. Cochrane Database Syst Rev. 2002;(1):CD003198.-
30. Wang H, Qi H, Wang BS, et al. Is acupuncture beneficial in depression: a meta-analysis of 8 randomized controlled trials? J Affect Disord. 2008;111(2-3):125-134.
31. Smith CA, Hay PPJ. Acupuncture for depression. Cochrane Database Syst Rev. 2005;(2):CD004046.-
How to select pharmacologic treatments to manage recidivism risk in sex offenders
Sex offenders traditionally are managed by the criminal justice system, but psychiatrists are frequently called on to assess and treat these individuals. Part of the reason is the overlap of paraphilias (disorders of sexual preference) and sexual offending. Many sexual offenders do not meet DSM criteria for paraphilias,1 however, and individuals with paraphilias do not necessarily commit offenses or come into contact with the legal system.
As clinicians, we may need to assess and treat a wide range of sexual issues, from persons with paraphilias who are self-referred and have no legal involvement, to recurrent sexual offenders who are at a high risk of repeat offending. Successfully managing sex offenders includes psychological and pharmacologic interventions and possibly incarceration and post-incarceration surveillance. This article focuses on pharmacologic interventions for male sexual offenders.
Reducing sexual drive
Sex offending likely is the result of a complex interplay of environment and psychological and biologic factors. The biology of sexual function provides numerous targets for pharmacologic intervention, including:2
- endocrine factors, such as testosterone
- neurotransmitters, such as serotonin.
The use of pharmacologic treatments for sex offenders is off-label, and evidence is limited. In general, pharmacologic treatments are geared toward reducing sexual drive through nonhormonal or hormonal means (Table 1).3-5
Table 1: Pharmacologic treatment of male sex offenders: A risk-based approach
*Not available in the United States
†Some authors suggest administering this dosage once every 2 weeks
CPA: cyproterone acetate; GNRH: gonadotropin-releasing hormone; IM: intramuscular; MPA: medroxyprogesterone acetate; SSRI: selective serotonin reuptake inhibitor
Source: References 3-5
Nonhormonal treatments
SSRIs. Selective serotonin reuptake inhibitors act by blocking serotonin reuptake in the synaptic cleft. Soon after the first SSRIs were approved in 1988, reports appeared of SSRIs interfering with sexual functioning.6 This side effect quickly was exploited to assist the treatment of sexual offenders.7
The mechanism of action may include:8
- direct effects, such as general inhibition of sexual activity, reduced impulsiveness, and an effect on the hypothesized “obsessive-compulsive” nature of paraphilias9
- indirect reduction of testosterone.
A growing body of literature supports SSRIs’ effectiveness in treating paraphilias and sexual offenders. Greenberg7 reviewed case studies and open drug trials of nearly 200 patients receiving fluoxetine, fluvoxamine, or sertraline. Most studies showed response rates of 50% to 90%.10 Positive effects included decreases in:
- paraphiliac fantasies, urges, and sexual acts
- masturbation
- hypersexual activity.
Some studies reported a preferential decrease in paraphiliac interests with an increase in conventional sexual interests, although this may be related to placebo or halo effects—patients may have reported an increase in conventional interests because they noticed a decrease in paraphiliac interests. Negative side effects included decreased sexual desires, delayed ejaculation, decreased libido, and anorgasmia.
Adi et al11 completed a more rigorous literature evaluation that included 9 studies with a total of 225 patients receiving fluoxetine, fluvoxamine, sertraline, or paroxetine. Eight studies showed benefits; however, Adi noted that this preliminary evidence was “far from conclusive.”
SSRIs generally are well tolerated and may be more appealing to patients than the “chemical castration” of hormonal treatments. Dosing is similar to that used in depression or obsessive-compulsive disorder. Although most patients notice beneficial effects in 2 to 4 weeks, some notice the effect nearly immediately.
Naltrexone. An opioid antagonist thought to affect the CNS processes of pleasure and pain, naltrexone has been used to treat alcohol dependence and pathologic gambling. A few case studies12-14 and 1 study of 21 adolescent sex offenders15 have shown benefits in treating sexual offenders or paraphiliacs. Benefits were seen at 50 mg/d, with suggested dosing of 100 to 200 mg/d. Because data are very limited, consider naltrexone only on an individual basis or as a possible adjunctive treatment.
Psychostimulants. Methylphenidate was added to augment SSRI treatment in a study of 26 men with paraphilias or paraphilia-related disorders.16 Results included further significant decreases in total sexual outlets (orgasms per week) and average time spent per day in paraphilia and paraphilia-related behavior. These gains appeared to be independent of the presence of attention-deficit/hyperactivity disorder.
Again, because data are very limited, consider this strategy only on an individual basis or as a possible adjunctive treatment. Because sexual offenders have high rates of substance abuse,17 consider the potential for stimulant abuse.
Hormonal treatments
Because testosterone is required for healthy bone metabolism, the antiandrogen medications used in hormonal treatment can cause osteoporosis.18,19 Therefore, long-term antiandrogen treatment should include bone scans to monitor for osteopenia and osteoporosis. Some authors have suggested that monthly doses of 25 to 50 mg of testosterone could minimize this risk.20 Bisphosphonates, vitamin D, and calcium supplements at osteoporosis treatment levels might be helpful.18 Other common side effects of antiandrogen medications are listed in Table 2.
Finasteride is approved for treating benign prostatic hyperplasia and androgenetic alopecia. It works by preventing conversion of testosterone to dihydrotestosterone (DHT) by the type II isoenzyme.21 Serum DHT contributes to male sexual behavior and predicts frequency of orgasms in healthy volunteers.22 Although there have been no studies of finasteride in sex offenders, it may be more acceptable to patients than other hormonal treatments and have a theoretical benefit in reducing sexual drive. Clinically, some patients describe increased control over urges without substantial side effects. Because there is no evidence supporting finasteride use in sex offenders, consider this medication only on an individual basis or as a possible adjunctive treatment.
Cyproterone acetate (CPA) is a synthetic steroid that blocks androgen receptors.3,23 Some evidence supports its use in treating sex offenders,24 although this agent is not available in the United States. For more information about CPA, see Box.
Medroxyprogesterone acetate (MPA), a derivative of progesterone, lowers serum testosterone by inhibiting its production through reducing pituitary luteinizing hormone (LH).24 The typical dose range for use in sex offenders is 100 to 600 mg/d orally or 100 to 700 mg IM every week,3 although some authors suggest giving similar doses every 2 weeks.4
Side effects of MPA include hypersomnia; neurasthenia; weight gain; hot flashes; gynecomastia; increased scalp hair; and decreased erections, ejaculate volume, spermatogenesis, and body and facial hair. The drug decreases testosterone levels by about 50%. Positive effects include reduced interest in and energy spent on pursuing sexual goals, but preservation of nondeviant sexual arousal.4
MPA has been shown to effectively decrease deviant sexual arousal and recidivism. In a study of 100 patients receiving MPA (average 250 mg IM every 2 weeks) for an average of 3 years, only 1 re-offended while taking MPA.4
In a 5-year follow-up study25 of 275 men, subjects were classified into high risk/treatment with MPA, 200 to 400 mg IM every 2 weeks, and low risk/nontreatment groups. A portion of the high risk/treatment group did not receive MPA. No sexual re-offenses occurred among high-risk subjects who received MPA, whereas the recidivism rate was 18% among high-risk subjects who did not receive MPA. Subjects in the low risk/nontreatment group had a recidivism rate of 15%, which suggests the need for more liberal use of antiandrogens. One major confounding factor was that subjects in the high risk/treatment group had to report every 2 weeks for injections; this may have resulted in closer follow-up, monitoring, and support, which may have contributed to lower recidivism.
Gonadotropin-releasing hormone (GNRH) agonists. The terms gonadotropin-releasing hormone and luteinizing-releasing hormone are used interchangeably. Most body testosterone is produced and released by Leydig cells in the testes in response to stimulation by LH released by the pituitary gland. LH release is controlled by the pulsatile release of GNRH from the hypothalamus. GNRH agonists are high-potency analogs of GNRH that work by causing an initial surge of LH followed by down-regulation of gonadotroph cells, a drop in LH, and a drop in testosterone to castration levels.
The GNRH analogs leuprolide, goserelin, and triptorelin are used to treat paraphiliacs and sexual offenders.20 Leuprolide typically is dosed at 7.5 mg IM every month, 22.5 mg IM every 3 months, or 30 mg IM every 4 months. Goserelin is provided as a subcutaneous implant/depot injected as 3.6 mg every month or 10.8 mg every 3 months.
Triptorelin is FDA-approved as treatment for advanced prostate cancer. Triptorelin is given in depot formulation as 3.75 mg IM every month or in a long-acting form as 11.25 mg IM every 3 months.
When starting these medications, an initial surge of LH and testosterone can last up to 3 weeks.26 Theoretically, this could worsen paraphiliac interests. Many practitioners will use a testosterone blocker such as flutamide, 250 mg tid, for the initial weeks of treatment.
Side effects of the GNRH agonists are similar. Most patients initially experience hot flashes. A systemic literature review27 reported:
- weight gain
- perspiration
- gynecomastia
- urinary incontinence
- hot flashes
- decreased growth of facial and body hair
- asthenia
- erectile failure
- muscle tenderness
- frequent bone demineralization.
Rare cases of pituitary apoplexy (a clinical syndrome secondary to infarction of the pituitary gland) have been reported, possibly related to an underlying pituitary adenoma.28
In a literature review that totalled 118 patients,27 GNRH agonists significantly decreased erections, ejaculations, paraphiliac fantasies, and paraphiliac behavior. Patients also reported feeling more relaxed, and recidivism rates were low. Some patients who failed to respond to CPA and MPA responded to GNRH agonists. Subsequent studies found similar results.29,30
Cyproterone acetate (CPA), which is available in Canada and Europe, is a synthetic steroid with structure similar to that of progesterone. CPA blocks androgen receptors, which makes it antiandrogenic, progestational, and antigonadotrophic.a
Dosages for paraphilias range from 100 to 500 mg/d orally or 100 to 600 mg intramuscularly every 1 or 2 weeks. b
Once stabilized, some individuals can be maintained on very low doses, such as 12.5 to 50 mg/d. a Lower doses may be appropriate for individuals who are self-motivated for treatment and who reliably report their sexual interests.
CPA reduces testosterone by approximately 50%. Side effects include decreases in:
- erections
- ejaculate volume
- spermatogenesis.
Some patients experience hypersomnia, neurasthenia, weight gain, hot flashes, decreased body and facial hair, and increased scalp hair. About 20% of patients may experience gynecomastia, particularly at higher doses.
Evidence shows CPA reduces sexual arousal, activity, fantasy, and masturbation.a In a systematic review of 7 studies that included 127 patients, the re-offense rate averaged 6%.c This is significantly lower than the expected recidivism of approximately 13.4%.d
References
a. Rösler A, Witztum E. Pharmacotherapy of paraphilias in the next millennium. Behav Sci Law. 2000;18:43-56.
b. Finn DA, Beadles-Bohling AS, Beckley EH, et al. A new look at the 5-alpha-reductase inhibitor finasteride. CNS Drug Rev. 2006;12(1):53-76.
c. Mantzoros CS, Georgiadis EI, Trichopoulos D. Contribution of dihydrotestosterone to male sexual behaviour. BMJ. 1995;310(6990):1289-1291.
d. Hanson R, Bussiere MT. Predicting relapse: A meta-analysis of sexual offender recidivism studies. J Consult Clin Psychology. 1998;66:348-362.
Table 2
Common side effects of antiandrogen therapy
| Depression |
| Erectile dysfunction |
| Fatigue |
| Gynecomastia |
| Hot flashes |
| Hypertension |
| Low libido |
| Myalgia |
| Osteopenia |
| Osteoporosis |
| Sweating |
| Thromboembolism |
| Weight gain |
Monitoring
Laboratory investigations are recommended to monitor for side effects of antiandrogen medications (Table 3).19,27,31 Medical contraindications to rule out before initiating antiandrogen medications include:
- thromboembolic diseases
- liver disease
- bone demineralization disorders
- hypersensitivity to the drug.
Measure prolactin to rule out pituitary adenomas. Monitor serum testosterone because some patients will not experience testosterone suppression from GNRH agonists or other antiandrogens. Noncompliant patients could potentially reverse the effects of MPA and GNRH agonists by taking exogenous testosterone.
Table 3
Monitoring patients receiving antiandrogen medications
| Pre-therapy workup | Periodic monitoring |
|---|---|
| Endocrinology or internist consultation Bone scan Weight Blood pressure Electrocardiogram CBC, renal function, liver function, fasting glucose, and lipids LH, FSH, testosterone, prolactin | Monthly: testosterone for the first 6 months Every 6 months: testosterone, LH, FSH, prolactin, CBC, renal function, liver function, fasting glucose and lipids, weight, blood pressure Yearly: bone scan |
| CBC: complete blood count; FSH: follicle-stimulating hormone; LH: luteinizing hormone | |
| Source: References 19,27,31 | |
Medication selection
The goals of pharmacologic treatment of sex offenders are to:
- reduce sexual offending and victimization
- suppress sexual drive to a controllable level
- possibly preferentially eliminate deviant arousal/thoughts
- allow normal sexual relationships.
Gauging risk. In determining which pharmacologic treatment to offer a patient, first evaluate the individual’s risk for recidivism. Actuarial scales32,33 suggest that recidivism risk can be categorized, based on clinical factors (Table 4).4,25,34
In addition to statistical risk factors, several other factors affect medication selection. Self-referred individuals may be more reliable in taking oral medications than those referred by the courts. A developmentally delayed individual may be a poor candidate for oral medication, unless he resides in a group home setting where compliance can be assured. Efficacy also guides medication choice. Finally, some patients will be legally required to provide proof of compliance, which only IM medications provide.
Treatment. Based on clinical experience and available literature, Bradford5 created an algorithm to help clinicians select appropriate pharmacologic interventions. Although it has not been validated, this algorithm provides a reasonable starting place.
In general, start treatment with an SSRI for low-risk individuals (Table 1).3-5 If this strategy is insufficient, consider augmentation with methylphenidate, naltrexone, or finasteride.
The next step would be to add oral MPA or CPA, 50 mg/d, which would partially inhibit testosterone and may allow some normal sexual functioning.4,23 Higher-dose oral MPA or CPA would be tried next. For higher-risk individuals or treatment failures, IM MPA or CPA would be offered next, followed by a GNRH agonist. For individuals at highest risk for re-offending, combinations of agents may be indicated.
This simple strategy is appealing, but in reality, treatment should be individualized. Choose medications based on the patient’s risk, wishes, and the previously mentioned clinical factors.
Table 4: Will my patient commit another sexual offense? Evaluating risk
Source: References 4,25,34Related resources
- Krueger RB, Kaplan MS. The paraphilic and hypersexual disorders: an overview. J Psychiatr Pract. 2001;7:391-403.
- Krueger RB, Kaplan MS. Behavioral and psychopharmacological treatment of the paraphilic and hypersexual disorders. J Psychiatr Pract. 2002;8:21-32.
- Association for the Treatment of Sexual Abusers. www.atsa.com.
Drug brand names
- Cyproterone acetate • Androcur
- Finasteride • Propecia, Proscar
- Fluoxetine • Prozac
- Flutamide • Eulexin
- Fluvoxamine • Luvox
- Goserelin • Zoladex
- Leuprolide • Eligard, Lupron
- Medroxyprogesterone acetate • Depo-Provera, Provera
- Methylphenidate • Ritalin
- Naltrexone • ReVia
- Paroxetine • Paxil
- Sertraline • Zoloft
- Triptorelin • Trelstar Depot
Disclosure
Dr. Booth reports no financial relationship with any company whose products are mentioned in the article or with manufacturers of competing products.
1. First MB, Halon RL. Use of DSM paraphilia diagnoses in sexually violent predator commitment cases. J Am Acad Psychiatry Law. 2008;36(4):443-454.
2. Meston CM, Frohlich PF. The neurobiology of sexual function. Arch Gen Psychiatry. 2000;57:1012-1030.
3. Bezchlibnyk-Butler KZ, Jeffries JJ, eds. Clinical handbook of psychotropic drugs. 16th ed. Seattle, WA: Hogrefe & Huber Publishers; 2006:222–225.
4. Maletzky B. The use of medroxyprogesterone acetate to assist in the treatment of sexual offenders. Annals of Sex Research. 1991;4:117-129.
5. Bradford JM. The neurobiology, neuropharmacology, and pharmacological treatment of the paraphilias and compulsive sexual behaviour. Can J Psychiatry. 2001;46(1):26-34.
6. Baldwin DS. Sexual dysfunction associated with antidepressant drugs. Expert Opin Drug Saf. 2004;3(5):457-470.
7. Greenberg DM, Bradford JMW. Treatment of the paraphilic disorders: a review of the role of the selective serotonin reuptake inhibitors. Sex Abuse. 1997;9(4):349-360.
8. Hill A, Briken P, Kraus C, et al. Differential pharmacological treatment of paraphilias and sex offenders. Int J Offender Ther Comp Criminol. 2003;47(4):407-421.
9. Bradford JMW. The paraphilias, obsessive compulsive spectrum disorder, and the treatment of sexually deviant behaviour. Psychiatr Q. 1999;70(3):209-219.
10. Krueger RB, Kaplan MS. Behavioral and psychopharmacological treatment of the paraphilic and hypersexual disorders. J Psychiatr Prac. 2002;8(1):21-32.
11. Adi Y, Ashcroft D, Browne K, et al. Clinical effectiveness and cost-consequences of selective serotonin reuptake inhibitors in the treatment of sex offenders. Health Technol Assess. 2002;6(28):1-66.
12. Raymond NC, Grant JE, Kim SW, et al. Treatment of compulsive sexual behaviour with naltrexone and serotonin reuptake inhibitors: two case studies. Int Clin Psychopharm. 2002;17(4):201-205.
13. Grant JE, Kim SW. A case of kleptomania and compulsive sexual behavior treated with naltrexone. Ann Clin Psychiatry. 2001;13(4):229-231.
14. Sandyk R. Naltrexone suppresses abnormal sexual behavior in Tourette’s syndrome. Int J Neurosci. 1988;43(1-2):107-110.
15. Ryback RS. Naltrexone in the treatment of adolescent sexual offenders. J Clin Psychiatry. 2004;65(7):982-986.
16. Kafka M, Hennen J. Psychostimulant augmentation during treatment with selective serotonin reuptake inhibitors in men with paraphilias and paraphilia-related disorders: a case series. J Clin Psychiatry. 2000;61(9):664-670.
17. Langstrom N, Sjostedt G, Grann M. Psychiatric disorders and recidivism in sexual offenders. Sex Abuse. 2004;16(2):139-150.
18. Smith MR. Osteoporosis during androgen deprivation therapy for prostate cancer. Urology. 2002;60(3 suppl 1):79-85.
19. Grasswick LJ, Bradford JM. Osteoporosis associated with the treatment of paraphilias: a clinical review of seven case reports. J Forensic Sci. 2003;48:849-855.
20. Rösler A, Witztum E. Pharmacotherapy of paraphilias in the next millennium. Behav Sci Law. 2000;18:43-56.
21. Finn DA, Beadles-Bohling AS, Beckley EH, et al. A new look at the 5-alpha-reductase inhibitor finasteride. CNS Drug Rev. 2006;12(1):53-76.
22. Mantzoros CS, Georgiadis EI, Trichopoulos D. Contribution of dihydrotestosterone to male sexual behaviour. BMJ. 1995;310(6990):1289-1291.
23. Bradford JMW, Pawlak A. Double-blind placebo crossover study of cyproterone acetate in the treatment of the paraphilias. Arch Sex Behav. 1993;22(5):383-402.
24. Meyer WJ, Cole CM. Physical and chemical castration of sex offenders: a review. J Offender Rehab. 1997;25(3/4):1-18.
25. Maletzky BM, Tolan A, McFarland B. The Oregon depoProvera program: a five-year follow-up. Sex Abuse. 2006;18:303-316.
26. van Poppel H, Nilsson S. Testosterone surge: rationale for gonadotropin-releasing hormone blockers? Urology. 2008;71:1001-1006.
27. Briken P, Hill A, Berner W. Pharmacotherapy of paraphilias with long-acting agonists of luteinizing hormone–releasing hormone: a systematic review. J Clin Psychiatry. 2003;64:890-897.
28. Blaut K, Winiewski P, Syrenicz A, et al. Apoplexy of clinically silent pituitary adenoma during prostate cancer treatment with LHRH analog. Neuro Endocrinol Lett. 2006;27(5):569-572.
29. Schober JM, Kuhn PJ, Kovacs P, et al. Leuprolide acetate suppresses pedophilic urges and arousability. Arch Sex Behav. 2005;34(6):691-705.
30. Ösler A, Witztum E. Treatment of men with paraphilia with a long-acting analogue of gonadotropin-releasing hormone. N Engl J Med. 1998;338:416-422.
31. Reilly DR, Delva NJ, Hudson RW. Protocols for the use of cyproterone, medroxyprogesterone, and leuprolide in the treatment of paraphilia. Can J Psychiatry. 2000;45:559-563.
32. Quinsey VL, Harris GT, Rice ME, et al. Violent offenders: appraising and managing risk. Washington, DC: American Psychological Association; 1998.
33. Epperson DL, Kaul JD, Huot SJ, et al. Minnesota Sex Offender Screening Tool–revised (MnSOST-R). St. Paul, MN: Minnesota Department of Corrections; 1998.
34. Bradford JMW. The treatment of sexual deviation using a pharmacological approach. J Sex Res. 2000;37(3):248-257.
Sex offenders traditionally are managed by the criminal justice system, but psychiatrists are frequently called on to assess and treat these individuals. Part of the reason is the overlap of paraphilias (disorders of sexual preference) and sexual offending. Many sexual offenders do not meet DSM criteria for paraphilias,1 however, and individuals with paraphilias do not necessarily commit offenses or come into contact with the legal system.
As clinicians, we may need to assess and treat a wide range of sexual issues, from persons with paraphilias who are self-referred and have no legal involvement, to recurrent sexual offenders who are at a high risk of repeat offending. Successfully managing sex offenders includes psychological and pharmacologic interventions and possibly incarceration and post-incarceration surveillance. This article focuses on pharmacologic interventions for male sexual offenders.
Reducing sexual drive
Sex offending likely is the result of a complex interplay of environment and psychological and biologic factors. The biology of sexual function provides numerous targets for pharmacologic intervention, including:2
- endocrine factors, such as testosterone
- neurotransmitters, such as serotonin.
The use of pharmacologic treatments for sex offenders is off-label, and evidence is limited. In general, pharmacologic treatments are geared toward reducing sexual drive through nonhormonal or hormonal means (Table 1).3-5
Table 1: Pharmacologic treatment of male sex offenders: A risk-based approach
*Not available in the United States
†Some authors suggest administering this dosage once every 2 weeks
CPA: cyproterone acetate; GNRH: gonadotropin-releasing hormone; IM: intramuscular; MPA: medroxyprogesterone acetate; SSRI: selective serotonin reuptake inhibitor
Source: References 3-5
Nonhormonal treatments
SSRIs. Selective serotonin reuptake inhibitors act by blocking serotonin reuptake in the synaptic cleft. Soon after the first SSRIs were approved in 1988, reports appeared of SSRIs interfering with sexual functioning.6 This side effect quickly was exploited to assist the treatment of sexual offenders.7
The mechanism of action may include:8
- direct effects, such as general inhibition of sexual activity, reduced impulsiveness, and an effect on the hypothesized “obsessive-compulsive” nature of paraphilias9
- indirect reduction of testosterone.
A growing body of literature supports SSRIs’ effectiveness in treating paraphilias and sexual offenders. Greenberg7 reviewed case studies and open drug trials of nearly 200 patients receiving fluoxetine, fluvoxamine, or sertraline. Most studies showed response rates of 50% to 90%.10 Positive effects included decreases in:
- paraphiliac fantasies, urges, and sexual acts
- masturbation
- hypersexual activity.
Some studies reported a preferential decrease in paraphiliac interests with an increase in conventional sexual interests, although this may be related to placebo or halo effects—patients may have reported an increase in conventional interests because they noticed a decrease in paraphiliac interests. Negative side effects included decreased sexual desires, delayed ejaculation, decreased libido, and anorgasmia.
Adi et al11 completed a more rigorous literature evaluation that included 9 studies with a total of 225 patients receiving fluoxetine, fluvoxamine, sertraline, or paroxetine. Eight studies showed benefits; however, Adi noted that this preliminary evidence was “far from conclusive.”
SSRIs generally are well tolerated and may be more appealing to patients than the “chemical castration” of hormonal treatments. Dosing is similar to that used in depression or obsessive-compulsive disorder. Although most patients notice beneficial effects in 2 to 4 weeks, some notice the effect nearly immediately.
Naltrexone. An opioid antagonist thought to affect the CNS processes of pleasure and pain, naltrexone has been used to treat alcohol dependence and pathologic gambling. A few case studies12-14 and 1 study of 21 adolescent sex offenders15 have shown benefits in treating sexual offenders or paraphiliacs. Benefits were seen at 50 mg/d, with suggested dosing of 100 to 200 mg/d. Because data are very limited, consider naltrexone only on an individual basis or as a possible adjunctive treatment.
Psychostimulants. Methylphenidate was added to augment SSRI treatment in a study of 26 men with paraphilias or paraphilia-related disorders.16 Results included further significant decreases in total sexual outlets (orgasms per week) and average time spent per day in paraphilia and paraphilia-related behavior. These gains appeared to be independent of the presence of attention-deficit/hyperactivity disorder.
Again, because data are very limited, consider this strategy only on an individual basis or as a possible adjunctive treatment. Because sexual offenders have high rates of substance abuse,17 consider the potential for stimulant abuse.
Hormonal treatments
Because testosterone is required for healthy bone metabolism, the antiandrogen medications used in hormonal treatment can cause osteoporosis.18,19 Therefore, long-term antiandrogen treatment should include bone scans to monitor for osteopenia and osteoporosis. Some authors have suggested that monthly doses of 25 to 50 mg of testosterone could minimize this risk.20 Bisphosphonates, vitamin D, and calcium supplements at osteoporosis treatment levels might be helpful.18 Other common side effects of antiandrogen medications are listed in Table 2.
Finasteride is approved for treating benign prostatic hyperplasia and androgenetic alopecia. It works by preventing conversion of testosterone to dihydrotestosterone (DHT) by the type II isoenzyme.21 Serum DHT contributes to male sexual behavior and predicts frequency of orgasms in healthy volunteers.22 Although there have been no studies of finasteride in sex offenders, it may be more acceptable to patients than other hormonal treatments and have a theoretical benefit in reducing sexual drive. Clinically, some patients describe increased control over urges without substantial side effects. Because there is no evidence supporting finasteride use in sex offenders, consider this medication only on an individual basis or as a possible adjunctive treatment.
Cyproterone acetate (CPA) is a synthetic steroid that blocks androgen receptors.3,23 Some evidence supports its use in treating sex offenders,24 although this agent is not available in the United States. For more information about CPA, see Box.
Medroxyprogesterone acetate (MPA), a derivative of progesterone, lowers serum testosterone by inhibiting its production through reducing pituitary luteinizing hormone (LH).24 The typical dose range for use in sex offenders is 100 to 600 mg/d orally or 100 to 700 mg IM every week,3 although some authors suggest giving similar doses every 2 weeks.4
Side effects of MPA include hypersomnia; neurasthenia; weight gain; hot flashes; gynecomastia; increased scalp hair; and decreased erections, ejaculate volume, spermatogenesis, and body and facial hair. The drug decreases testosterone levels by about 50%. Positive effects include reduced interest in and energy spent on pursuing sexual goals, but preservation of nondeviant sexual arousal.4
MPA has been shown to effectively decrease deviant sexual arousal and recidivism. In a study of 100 patients receiving MPA (average 250 mg IM every 2 weeks) for an average of 3 years, only 1 re-offended while taking MPA.4
In a 5-year follow-up study25 of 275 men, subjects were classified into high risk/treatment with MPA, 200 to 400 mg IM every 2 weeks, and low risk/nontreatment groups. A portion of the high risk/treatment group did not receive MPA. No sexual re-offenses occurred among high-risk subjects who received MPA, whereas the recidivism rate was 18% among high-risk subjects who did not receive MPA. Subjects in the low risk/nontreatment group had a recidivism rate of 15%, which suggests the need for more liberal use of antiandrogens. One major confounding factor was that subjects in the high risk/treatment group had to report every 2 weeks for injections; this may have resulted in closer follow-up, monitoring, and support, which may have contributed to lower recidivism.
Gonadotropin-releasing hormone (GNRH) agonists. The terms gonadotropin-releasing hormone and luteinizing-releasing hormone are used interchangeably. Most body testosterone is produced and released by Leydig cells in the testes in response to stimulation by LH released by the pituitary gland. LH release is controlled by the pulsatile release of GNRH from the hypothalamus. GNRH agonists are high-potency analogs of GNRH that work by causing an initial surge of LH followed by down-regulation of gonadotroph cells, a drop in LH, and a drop in testosterone to castration levels.
The GNRH analogs leuprolide, goserelin, and triptorelin are used to treat paraphiliacs and sexual offenders.20 Leuprolide typically is dosed at 7.5 mg IM every month, 22.5 mg IM every 3 months, or 30 mg IM every 4 months. Goserelin is provided as a subcutaneous implant/depot injected as 3.6 mg every month or 10.8 mg every 3 months.
Triptorelin is FDA-approved as treatment for advanced prostate cancer. Triptorelin is given in depot formulation as 3.75 mg IM every month or in a long-acting form as 11.25 mg IM every 3 months.
When starting these medications, an initial surge of LH and testosterone can last up to 3 weeks.26 Theoretically, this could worsen paraphiliac interests. Many practitioners will use a testosterone blocker such as flutamide, 250 mg tid, for the initial weeks of treatment.
Side effects of the GNRH agonists are similar. Most patients initially experience hot flashes. A systemic literature review27 reported:
- weight gain
- perspiration
- gynecomastia
- urinary incontinence
- hot flashes
- decreased growth of facial and body hair
- asthenia
- erectile failure
- muscle tenderness
- frequent bone demineralization.
Rare cases of pituitary apoplexy (a clinical syndrome secondary to infarction of the pituitary gland) have been reported, possibly related to an underlying pituitary adenoma.28
In a literature review that totalled 118 patients,27 GNRH agonists significantly decreased erections, ejaculations, paraphiliac fantasies, and paraphiliac behavior. Patients also reported feeling more relaxed, and recidivism rates were low. Some patients who failed to respond to CPA and MPA responded to GNRH agonists. Subsequent studies found similar results.29,30
Cyproterone acetate (CPA), which is available in Canada and Europe, is a synthetic steroid with structure similar to that of progesterone. CPA blocks androgen receptors, which makes it antiandrogenic, progestational, and antigonadotrophic.a
Dosages for paraphilias range from 100 to 500 mg/d orally or 100 to 600 mg intramuscularly every 1 or 2 weeks. b
Once stabilized, some individuals can be maintained on very low doses, such as 12.5 to 50 mg/d. a Lower doses may be appropriate for individuals who are self-motivated for treatment and who reliably report their sexual interests.
CPA reduces testosterone by approximately 50%. Side effects include decreases in:
- erections
- ejaculate volume
- spermatogenesis.
Some patients experience hypersomnia, neurasthenia, weight gain, hot flashes, decreased body and facial hair, and increased scalp hair. About 20% of patients may experience gynecomastia, particularly at higher doses.
Evidence shows CPA reduces sexual arousal, activity, fantasy, and masturbation.a In a systematic review of 7 studies that included 127 patients, the re-offense rate averaged 6%.c This is significantly lower than the expected recidivism of approximately 13.4%.d
References
a. Rösler A, Witztum E. Pharmacotherapy of paraphilias in the next millennium. Behav Sci Law. 2000;18:43-56.
b. Finn DA, Beadles-Bohling AS, Beckley EH, et al. A new look at the 5-alpha-reductase inhibitor finasteride. CNS Drug Rev. 2006;12(1):53-76.
c. Mantzoros CS, Georgiadis EI, Trichopoulos D. Contribution of dihydrotestosterone to male sexual behaviour. BMJ. 1995;310(6990):1289-1291.
d. Hanson R, Bussiere MT. Predicting relapse: A meta-analysis of sexual offender recidivism studies. J Consult Clin Psychology. 1998;66:348-362.
Table 2
Common side effects of antiandrogen therapy
| Depression |
| Erectile dysfunction |
| Fatigue |
| Gynecomastia |
| Hot flashes |
| Hypertension |
| Low libido |
| Myalgia |
| Osteopenia |
| Osteoporosis |
| Sweating |
| Thromboembolism |
| Weight gain |
Monitoring
Laboratory investigations are recommended to monitor for side effects of antiandrogen medications (Table 3).19,27,31 Medical contraindications to rule out before initiating antiandrogen medications include:
- thromboembolic diseases
- liver disease
- bone demineralization disorders
- hypersensitivity to the drug.
Measure prolactin to rule out pituitary adenomas. Monitor serum testosterone because some patients will not experience testosterone suppression from GNRH agonists or other antiandrogens. Noncompliant patients could potentially reverse the effects of MPA and GNRH agonists by taking exogenous testosterone.
Table 3
Monitoring patients receiving antiandrogen medications
| Pre-therapy workup | Periodic monitoring |
|---|---|
| Endocrinology or internist consultation Bone scan Weight Blood pressure Electrocardiogram CBC, renal function, liver function, fasting glucose, and lipids LH, FSH, testosterone, prolactin | Monthly: testosterone for the first 6 months Every 6 months: testosterone, LH, FSH, prolactin, CBC, renal function, liver function, fasting glucose and lipids, weight, blood pressure Yearly: bone scan |
| CBC: complete blood count; FSH: follicle-stimulating hormone; LH: luteinizing hormone | |
| Source: References 19,27,31 | |
Medication selection
The goals of pharmacologic treatment of sex offenders are to:
- reduce sexual offending and victimization
- suppress sexual drive to a controllable level
- possibly preferentially eliminate deviant arousal/thoughts
- allow normal sexual relationships.
Gauging risk. In determining which pharmacologic treatment to offer a patient, first evaluate the individual’s risk for recidivism. Actuarial scales32,33 suggest that recidivism risk can be categorized, based on clinical factors (Table 4).4,25,34
In addition to statistical risk factors, several other factors affect medication selection. Self-referred individuals may be more reliable in taking oral medications than those referred by the courts. A developmentally delayed individual may be a poor candidate for oral medication, unless he resides in a group home setting where compliance can be assured. Efficacy also guides medication choice. Finally, some patients will be legally required to provide proof of compliance, which only IM medications provide.
Treatment. Based on clinical experience and available literature, Bradford5 created an algorithm to help clinicians select appropriate pharmacologic interventions. Although it has not been validated, this algorithm provides a reasonable starting place.
In general, start treatment with an SSRI for low-risk individuals (Table 1).3-5 If this strategy is insufficient, consider augmentation with methylphenidate, naltrexone, or finasteride.
The next step would be to add oral MPA or CPA, 50 mg/d, which would partially inhibit testosterone and may allow some normal sexual functioning.4,23 Higher-dose oral MPA or CPA would be tried next. For higher-risk individuals or treatment failures, IM MPA or CPA would be offered next, followed by a GNRH agonist. For individuals at highest risk for re-offending, combinations of agents may be indicated.
This simple strategy is appealing, but in reality, treatment should be individualized. Choose medications based on the patient’s risk, wishes, and the previously mentioned clinical factors.
Table 4: Will my patient commit another sexual offense? Evaluating risk
Source: References 4,25,34Related resources
- Krueger RB, Kaplan MS. The paraphilic and hypersexual disorders: an overview. J Psychiatr Pract. 2001;7:391-403.
- Krueger RB, Kaplan MS. Behavioral and psychopharmacological treatment of the paraphilic and hypersexual disorders. J Psychiatr Pract. 2002;8:21-32.
- Association for the Treatment of Sexual Abusers. www.atsa.com.
Drug brand names
- Cyproterone acetate • Androcur
- Finasteride • Propecia, Proscar
- Fluoxetine • Prozac
- Flutamide • Eulexin
- Fluvoxamine • Luvox
- Goserelin • Zoladex
- Leuprolide • Eligard, Lupron
- Medroxyprogesterone acetate • Depo-Provera, Provera
- Methylphenidate • Ritalin
- Naltrexone • ReVia
- Paroxetine • Paxil
- Sertraline • Zoloft
- Triptorelin • Trelstar Depot
Disclosure
Dr. Booth reports no financial relationship with any company whose products are mentioned in the article or with manufacturers of competing products.
Sex offenders traditionally are managed by the criminal justice system, but psychiatrists are frequently called on to assess and treat these individuals. Part of the reason is the overlap of paraphilias (disorders of sexual preference) and sexual offending. Many sexual offenders do not meet DSM criteria for paraphilias,1 however, and individuals with paraphilias do not necessarily commit offenses or come into contact with the legal system.
As clinicians, we may need to assess and treat a wide range of sexual issues, from persons with paraphilias who are self-referred and have no legal involvement, to recurrent sexual offenders who are at a high risk of repeat offending. Successfully managing sex offenders includes psychological and pharmacologic interventions and possibly incarceration and post-incarceration surveillance. This article focuses on pharmacologic interventions for male sexual offenders.
Reducing sexual drive
Sex offending likely is the result of a complex interplay of environment and psychological and biologic factors. The biology of sexual function provides numerous targets for pharmacologic intervention, including:2
- endocrine factors, such as testosterone
- neurotransmitters, such as serotonin.
The use of pharmacologic treatments for sex offenders is off-label, and evidence is limited. In general, pharmacologic treatments are geared toward reducing sexual drive through nonhormonal or hormonal means (Table 1).3-5
Table 1: Pharmacologic treatment of male sex offenders: A risk-based approach
*Not available in the United States
†Some authors suggest administering this dosage once every 2 weeks
CPA: cyproterone acetate; GNRH: gonadotropin-releasing hormone; IM: intramuscular; MPA: medroxyprogesterone acetate; SSRI: selective serotonin reuptake inhibitor
Source: References 3-5
Nonhormonal treatments
SSRIs. Selective serotonin reuptake inhibitors act by blocking serotonin reuptake in the synaptic cleft. Soon after the first SSRIs were approved in 1988, reports appeared of SSRIs interfering with sexual functioning.6 This side effect quickly was exploited to assist the treatment of sexual offenders.7
The mechanism of action may include:8
- direct effects, such as general inhibition of sexual activity, reduced impulsiveness, and an effect on the hypothesized “obsessive-compulsive” nature of paraphilias9
- indirect reduction of testosterone.
A growing body of literature supports SSRIs’ effectiveness in treating paraphilias and sexual offenders. Greenberg7 reviewed case studies and open drug trials of nearly 200 patients receiving fluoxetine, fluvoxamine, or sertraline. Most studies showed response rates of 50% to 90%.10 Positive effects included decreases in:
- paraphiliac fantasies, urges, and sexual acts
- masturbation
- hypersexual activity.
Some studies reported a preferential decrease in paraphiliac interests with an increase in conventional sexual interests, although this may be related to placebo or halo effects—patients may have reported an increase in conventional interests because they noticed a decrease in paraphiliac interests. Negative side effects included decreased sexual desires, delayed ejaculation, decreased libido, and anorgasmia.
Adi et al11 completed a more rigorous literature evaluation that included 9 studies with a total of 225 patients receiving fluoxetine, fluvoxamine, sertraline, or paroxetine. Eight studies showed benefits; however, Adi noted that this preliminary evidence was “far from conclusive.”
SSRIs generally are well tolerated and may be more appealing to patients than the “chemical castration” of hormonal treatments. Dosing is similar to that used in depression or obsessive-compulsive disorder. Although most patients notice beneficial effects in 2 to 4 weeks, some notice the effect nearly immediately.
Naltrexone. An opioid antagonist thought to affect the CNS processes of pleasure and pain, naltrexone has been used to treat alcohol dependence and pathologic gambling. A few case studies12-14 and 1 study of 21 adolescent sex offenders15 have shown benefits in treating sexual offenders or paraphiliacs. Benefits were seen at 50 mg/d, with suggested dosing of 100 to 200 mg/d. Because data are very limited, consider naltrexone only on an individual basis or as a possible adjunctive treatment.
Psychostimulants. Methylphenidate was added to augment SSRI treatment in a study of 26 men with paraphilias or paraphilia-related disorders.16 Results included further significant decreases in total sexual outlets (orgasms per week) and average time spent per day in paraphilia and paraphilia-related behavior. These gains appeared to be independent of the presence of attention-deficit/hyperactivity disorder.
Again, because data are very limited, consider this strategy only on an individual basis or as a possible adjunctive treatment. Because sexual offenders have high rates of substance abuse,17 consider the potential for stimulant abuse.
Hormonal treatments
Because testosterone is required for healthy bone metabolism, the antiandrogen medications used in hormonal treatment can cause osteoporosis.18,19 Therefore, long-term antiandrogen treatment should include bone scans to monitor for osteopenia and osteoporosis. Some authors have suggested that monthly doses of 25 to 50 mg of testosterone could minimize this risk.20 Bisphosphonates, vitamin D, and calcium supplements at osteoporosis treatment levels might be helpful.18 Other common side effects of antiandrogen medications are listed in Table 2.
Finasteride is approved for treating benign prostatic hyperplasia and androgenetic alopecia. It works by preventing conversion of testosterone to dihydrotestosterone (DHT) by the type II isoenzyme.21 Serum DHT contributes to male sexual behavior and predicts frequency of orgasms in healthy volunteers.22 Although there have been no studies of finasteride in sex offenders, it may be more acceptable to patients than other hormonal treatments and have a theoretical benefit in reducing sexual drive. Clinically, some patients describe increased control over urges without substantial side effects. Because there is no evidence supporting finasteride use in sex offenders, consider this medication only on an individual basis or as a possible adjunctive treatment.
Cyproterone acetate (CPA) is a synthetic steroid that blocks androgen receptors.3,23 Some evidence supports its use in treating sex offenders,24 although this agent is not available in the United States. For more information about CPA, see Box.
Medroxyprogesterone acetate (MPA), a derivative of progesterone, lowers serum testosterone by inhibiting its production through reducing pituitary luteinizing hormone (LH).24 The typical dose range for use in sex offenders is 100 to 600 mg/d orally or 100 to 700 mg IM every week,3 although some authors suggest giving similar doses every 2 weeks.4
Side effects of MPA include hypersomnia; neurasthenia; weight gain; hot flashes; gynecomastia; increased scalp hair; and decreased erections, ejaculate volume, spermatogenesis, and body and facial hair. The drug decreases testosterone levels by about 50%. Positive effects include reduced interest in and energy spent on pursuing sexual goals, but preservation of nondeviant sexual arousal.4
MPA has been shown to effectively decrease deviant sexual arousal and recidivism. In a study of 100 patients receiving MPA (average 250 mg IM every 2 weeks) for an average of 3 years, only 1 re-offended while taking MPA.4
In a 5-year follow-up study25 of 275 men, subjects were classified into high risk/treatment with MPA, 200 to 400 mg IM every 2 weeks, and low risk/nontreatment groups. A portion of the high risk/treatment group did not receive MPA. No sexual re-offenses occurred among high-risk subjects who received MPA, whereas the recidivism rate was 18% among high-risk subjects who did not receive MPA. Subjects in the low risk/nontreatment group had a recidivism rate of 15%, which suggests the need for more liberal use of antiandrogens. One major confounding factor was that subjects in the high risk/treatment group had to report every 2 weeks for injections; this may have resulted in closer follow-up, monitoring, and support, which may have contributed to lower recidivism.
Gonadotropin-releasing hormone (GNRH) agonists. The terms gonadotropin-releasing hormone and luteinizing-releasing hormone are used interchangeably. Most body testosterone is produced and released by Leydig cells in the testes in response to stimulation by LH released by the pituitary gland. LH release is controlled by the pulsatile release of GNRH from the hypothalamus. GNRH agonists are high-potency analogs of GNRH that work by causing an initial surge of LH followed by down-regulation of gonadotroph cells, a drop in LH, and a drop in testosterone to castration levels.
The GNRH analogs leuprolide, goserelin, and triptorelin are used to treat paraphiliacs and sexual offenders.20 Leuprolide typically is dosed at 7.5 mg IM every month, 22.5 mg IM every 3 months, or 30 mg IM every 4 months. Goserelin is provided as a subcutaneous implant/depot injected as 3.6 mg every month or 10.8 mg every 3 months.
Triptorelin is FDA-approved as treatment for advanced prostate cancer. Triptorelin is given in depot formulation as 3.75 mg IM every month or in a long-acting form as 11.25 mg IM every 3 months.
When starting these medications, an initial surge of LH and testosterone can last up to 3 weeks.26 Theoretically, this could worsen paraphiliac interests. Many practitioners will use a testosterone blocker such as flutamide, 250 mg tid, for the initial weeks of treatment.
Side effects of the GNRH agonists are similar. Most patients initially experience hot flashes. A systemic literature review27 reported:
- weight gain
- perspiration
- gynecomastia
- urinary incontinence
- hot flashes
- decreased growth of facial and body hair
- asthenia
- erectile failure
- muscle tenderness
- frequent bone demineralization.
Rare cases of pituitary apoplexy (a clinical syndrome secondary to infarction of the pituitary gland) have been reported, possibly related to an underlying pituitary adenoma.28
In a literature review that totalled 118 patients,27 GNRH agonists significantly decreased erections, ejaculations, paraphiliac fantasies, and paraphiliac behavior. Patients also reported feeling more relaxed, and recidivism rates were low. Some patients who failed to respond to CPA and MPA responded to GNRH agonists. Subsequent studies found similar results.29,30
Cyproterone acetate (CPA), which is available in Canada and Europe, is a synthetic steroid with structure similar to that of progesterone. CPA blocks androgen receptors, which makes it antiandrogenic, progestational, and antigonadotrophic.a
Dosages for paraphilias range from 100 to 500 mg/d orally or 100 to 600 mg intramuscularly every 1 or 2 weeks. b
Once stabilized, some individuals can be maintained on very low doses, such as 12.5 to 50 mg/d. a Lower doses may be appropriate for individuals who are self-motivated for treatment and who reliably report their sexual interests.
CPA reduces testosterone by approximately 50%. Side effects include decreases in:
- erections
- ejaculate volume
- spermatogenesis.
Some patients experience hypersomnia, neurasthenia, weight gain, hot flashes, decreased body and facial hair, and increased scalp hair. About 20% of patients may experience gynecomastia, particularly at higher doses.
Evidence shows CPA reduces sexual arousal, activity, fantasy, and masturbation.a In a systematic review of 7 studies that included 127 patients, the re-offense rate averaged 6%.c This is significantly lower than the expected recidivism of approximately 13.4%.d
References
a. Rösler A, Witztum E. Pharmacotherapy of paraphilias in the next millennium. Behav Sci Law. 2000;18:43-56.
b. Finn DA, Beadles-Bohling AS, Beckley EH, et al. A new look at the 5-alpha-reductase inhibitor finasteride. CNS Drug Rev. 2006;12(1):53-76.
c. Mantzoros CS, Georgiadis EI, Trichopoulos D. Contribution of dihydrotestosterone to male sexual behaviour. BMJ. 1995;310(6990):1289-1291.
d. Hanson R, Bussiere MT. Predicting relapse: A meta-analysis of sexual offender recidivism studies. J Consult Clin Psychology. 1998;66:348-362.
Table 2
Common side effects of antiandrogen therapy
| Depression |
| Erectile dysfunction |
| Fatigue |
| Gynecomastia |
| Hot flashes |
| Hypertension |
| Low libido |
| Myalgia |
| Osteopenia |
| Osteoporosis |
| Sweating |
| Thromboembolism |
| Weight gain |
Monitoring
Laboratory investigations are recommended to monitor for side effects of antiandrogen medications (Table 3).19,27,31 Medical contraindications to rule out before initiating antiandrogen medications include:
- thromboembolic diseases
- liver disease
- bone demineralization disorders
- hypersensitivity to the drug.
Measure prolactin to rule out pituitary adenomas. Monitor serum testosterone because some patients will not experience testosterone suppression from GNRH agonists or other antiandrogens. Noncompliant patients could potentially reverse the effects of MPA and GNRH agonists by taking exogenous testosterone.
Table 3
Monitoring patients receiving antiandrogen medications
| Pre-therapy workup | Periodic monitoring |
|---|---|
| Endocrinology or internist consultation Bone scan Weight Blood pressure Electrocardiogram CBC, renal function, liver function, fasting glucose, and lipids LH, FSH, testosterone, prolactin | Monthly: testosterone for the first 6 months Every 6 months: testosterone, LH, FSH, prolactin, CBC, renal function, liver function, fasting glucose and lipids, weight, blood pressure Yearly: bone scan |
| CBC: complete blood count; FSH: follicle-stimulating hormone; LH: luteinizing hormone | |
| Source: References 19,27,31 | |
Medication selection
The goals of pharmacologic treatment of sex offenders are to:
- reduce sexual offending and victimization
- suppress sexual drive to a controllable level
- possibly preferentially eliminate deviant arousal/thoughts
- allow normal sexual relationships.
Gauging risk. In determining which pharmacologic treatment to offer a patient, first evaluate the individual’s risk for recidivism. Actuarial scales32,33 suggest that recidivism risk can be categorized, based on clinical factors (Table 4).4,25,34
In addition to statistical risk factors, several other factors affect medication selection. Self-referred individuals may be more reliable in taking oral medications than those referred by the courts. A developmentally delayed individual may be a poor candidate for oral medication, unless he resides in a group home setting where compliance can be assured. Efficacy also guides medication choice. Finally, some patients will be legally required to provide proof of compliance, which only IM medications provide.
Treatment. Based on clinical experience and available literature, Bradford5 created an algorithm to help clinicians select appropriate pharmacologic interventions. Although it has not been validated, this algorithm provides a reasonable starting place.
In general, start treatment with an SSRI for low-risk individuals (Table 1).3-5 If this strategy is insufficient, consider augmentation with methylphenidate, naltrexone, or finasteride.
The next step would be to add oral MPA or CPA, 50 mg/d, which would partially inhibit testosterone and may allow some normal sexual functioning.4,23 Higher-dose oral MPA or CPA would be tried next. For higher-risk individuals or treatment failures, IM MPA or CPA would be offered next, followed by a GNRH agonist. For individuals at highest risk for re-offending, combinations of agents may be indicated.
This simple strategy is appealing, but in reality, treatment should be individualized. Choose medications based on the patient’s risk, wishes, and the previously mentioned clinical factors.
Table 4: Will my patient commit another sexual offense? Evaluating risk
Source: References 4,25,34Related resources
- Krueger RB, Kaplan MS. The paraphilic and hypersexual disorders: an overview. J Psychiatr Pract. 2001;7:391-403.
- Krueger RB, Kaplan MS. Behavioral and psychopharmacological treatment of the paraphilic and hypersexual disorders. J Psychiatr Pract. 2002;8:21-32.
- Association for the Treatment of Sexual Abusers. www.atsa.com.
Drug brand names
- Cyproterone acetate • Androcur
- Finasteride • Propecia, Proscar
- Fluoxetine • Prozac
- Flutamide • Eulexin
- Fluvoxamine • Luvox
- Goserelin • Zoladex
- Leuprolide • Eligard, Lupron
- Medroxyprogesterone acetate • Depo-Provera, Provera
- Methylphenidate • Ritalin
- Naltrexone • ReVia
- Paroxetine • Paxil
- Sertraline • Zoloft
- Triptorelin • Trelstar Depot
Disclosure
Dr. Booth reports no financial relationship with any company whose products are mentioned in the article or with manufacturers of competing products.
1. First MB, Halon RL. Use of DSM paraphilia diagnoses in sexually violent predator commitment cases. J Am Acad Psychiatry Law. 2008;36(4):443-454.
2. Meston CM, Frohlich PF. The neurobiology of sexual function. Arch Gen Psychiatry. 2000;57:1012-1030.
3. Bezchlibnyk-Butler KZ, Jeffries JJ, eds. Clinical handbook of psychotropic drugs. 16th ed. Seattle, WA: Hogrefe & Huber Publishers; 2006:222–225.
4. Maletzky B. The use of medroxyprogesterone acetate to assist in the treatment of sexual offenders. Annals of Sex Research. 1991;4:117-129.
5. Bradford JM. The neurobiology, neuropharmacology, and pharmacological treatment of the paraphilias and compulsive sexual behaviour. Can J Psychiatry. 2001;46(1):26-34.
6. Baldwin DS. Sexual dysfunction associated with antidepressant drugs. Expert Opin Drug Saf. 2004;3(5):457-470.
7. Greenberg DM, Bradford JMW. Treatment of the paraphilic disorders: a review of the role of the selective serotonin reuptake inhibitors. Sex Abuse. 1997;9(4):349-360.
8. Hill A, Briken P, Kraus C, et al. Differential pharmacological treatment of paraphilias and sex offenders. Int J Offender Ther Comp Criminol. 2003;47(4):407-421.
9. Bradford JMW. The paraphilias, obsessive compulsive spectrum disorder, and the treatment of sexually deviant behaviour. Psychiatr Q. 1999;70(3):209-219.
10. Krueger RB, Kaplan MS. Behavioral and psychopharmacological treatment of the paraphilic and hypersexual disorders. J Psychiatr Prac. 2002;8(1):21-32.
11. Adi Y, Ashcroft D, Browne K, et al. Clinical effectiveness and cost-consequences of selective serotonin reuptake inhibitors in the treatment of sex offenders. Health Technol Assess. 2002;6(28):1-66.
12. Raymond NC, Grant JE, Kim SW, et al. Treatment of compulsive sexual behaviour with naltrexone and serotonin reuptake inhibitors: two case studies. Int Clin Psychopharm. 2002;17(4):201-205.
13. Grant JE, Kim SW. A case of kleptomania and compulsive sexual behavior treated with naltrexone. Ann Clin Psychiatry. 2001;13(4):229-231.
14. Sandyk R. Naltrexone suppresses abnormal sexual behavior in Tourette’s syndrome. Int J Neurosci. 1988;43(1-2):107-110.
15. Ryback RS. Naltrexone in the treatment of adolescent sexual offenders. J Clin Psychiatry. 2004;65(7):982-986.
16. Kafka M, Hennen J. Psychostimulant augmentation during treatment with selective serotonin reuptake inhibitors in men with paraphilias and paraphilia-related disorders: a case series. J Clin Psychiatry. 2000;61(9):664-670.
17. Langstrom N, Sjostedt G, Grann M. Psychiatric disorders and recidivism in sexual offenders. Sex Abuse. 2004;16(2):139-150.
18. Smith MR. Osteoporosis during androgen deprivation therapy for prostate cancer. Urology. 2002;60(3 suppl 1):79-85.
19. Grasswick LJ, Bradford JM. Osteoporosis associated with the treatment of paraphilias: a clinical review of seven case reports. J Forensic Sci. 2003;48:849-855.
20. Rösler A, Witztum E. Pharmacotherapy of paraphilias in the next millennium. Behav Sci Law. 2000;18:43-56.
21. Finn DA, Beadles-Bohling AS, Beckley EH, et al. A new look at the 5-alpha-reductase inhibitor finasteride. CNS Drug Rev. 2006;12(1):53-76.
22. Mantzoros CS, Georgiadis EI, Trichopoulos D. Contribution of dihydrotestosterone to male sexual behaviour. BMJ. 1995;310(6990):1289-1291.
23. Bradford JMW, Pawlak A. Double-blind placebo crossover study of cyproterone acetate in the treatment of the paraphilias. Arch Sex Behav. 1993;22(5):383-402.
24. Meyer WJ, Cole CM. Physical and chemical castration of sex offenders: a review. J Offender Rehab. 1997;25(3/4):1-18.
25. Maletzky BM, Tolan A, McFarland B. The Oregon depoProvera program: a five-year follow-up. Sex Abuse. 2006;18:303-316.
26. van Poppel H, Nilsson S. Testosterone surge: rationale for gonadotropin-releasing hormone blockers? Urology. 2008;71:1001-1006.
27. Briken P, Hill A, Berner W. Pharmacotherapy of paraphilias with long-acting agonists of luteinizing hormone–releasing hormone: a systematic review. J Clin Psychiatry. 2003;64:890-897.
28. Blaut K, Winiewski P, Syrenicz A, et al. Apoplexy of clinically silent pituitary adenoma during prostate cancer treatment with LHRH analog. Neuro Endocrinol Lett. 2006;27(5):569-572.
29. Schober JM, Kuhn PJ, Kovacs P, et al. Leuprolide acetate suppresses pedophilic urges and arousability. Arch Sex Behav. 2005;34(6):691-705.
30. Ösler A, Witztum E. Treatment of men with paraphilia with a long-acting analogue of gonadotropin-releasing hormone. N Engl J Med. 1998;338:416-422.
31. Reilly DR, Delva NJ, Hudson RW. Protocols for the use of cyproterone, medroxyprogesterone, and leuprolide in the treatment of paraphilia. Can J Psychiatry. 2000;45:559-563.
32. Quinsey VL, Harris GT, Rice ME, et al. Violent offenders: appraising and managing risk. Washington, DC: American Psychological Association; 1998.
33. Epperson DL, Kaul JD, Huot SJ, et al. Minnesota Sex Offender Screening Tool–revised (MnSOST-R). St. Paul, MN: Minnesota Department of Corrections; 1998.
34. Bradford JMW. The treatment of sexual deviation using a pharmacological approach. J Sex Res. 2000;37(3):248-257.
1. First MB, Halon RL. Use of DSM paraphilia diagnoses in sexually violent predator commitment cases. J Am Acad Psychiatry Law. 2008;36(4):443-454.
2. Meston CM, Frohlich PF. The neurobiology of sexual function. Arch Gen Psychiatry. 2000;57:1012-1030.
3. Bezchlibnyk-Butler KZ, Jeffries JJ, eds. Clinical handbook of psychotropic drugs. 16th ed. Seattle, WA: Hogrefe & Huber Publishers; 2006:222–225.
4. Maletzky B. The use of medroxyprogesterone acetate to assist in the treatment of sexual offenders. Annals of Sex Research. 1991;4:117-129.
5. Bradford JM. The neurobiology, neuropharmacology, and pharmacological treatment of the paraphilias and compulsive sexual behaviour. Can J Psychiatry. 2001;46(1):26-34.
6. Baldwin DS. Sexual dysfunction associated with antidepressant drugs. Expert Opin Drug Saf. 2004;3(5):457-470.
7. Greenberg DM, Bradford JMW. Treatment of the paraphilic disorders: a review of the role of the selective serotonin reuptake inhibitors. Sex Abuse. 1997;9(4):349-360.
8. Hill A, Briken P, Kraus C, et al. Differential pharmacological treatment of paraphilias and sex offenders. Int J Offender Ther Comp Criminol. 2003;47(4):407-421.
9. Bradford JMW. The paraphilias, obsessive compulsive spectrum disorder, and the treatment of sexually deviant behaviour. Psychiatr Q. 1999;70(3):209-219.
10. Krueger RB, Kaplan MS. Behavioral and psychopharmacological treatment of the paraphilic and hypersexual disorders. J Psychiatr Prac. 2002;8(1):21-32.
11. Adi Y, Ashcroft D, Browne K, et al. Clinical effectiveness and cost-consequences of selective serotonin reuptake inhibitors in the treatment of sex offenders. Health Technol Assess. 2002;6(28):1-66.
12. Raymond NC, Grant JE, Kim SW, et al. Treatment of compulsive sexual behaviour with naltrexone and serotonin reuptake inhibitors: two case studies. Int Clin Psychopharm. 2002;17(4):201-205.
13. Grant JE, Kim SW. A case of kleptomania and compulsive sexual behavior treated with naltrexone. Ann Clin Psychiatry. 2001;13(4):229-231.
14. Sandyk R. Naltrexone suppresses abnormal sexual behavior in Tourette’s syndrome. Int J Neurosci. 1988;43(1-2):107-110.
15. Ryback RS. Naltrexone in the treatment of adolescent sexual offenders. J Clin Psychiatry. 2004;65(7):982-986.
16. Kafka M, Hennen J. Psychostimulant augmentation during treatment with selective serotonin reuptake inhibitors in men with paraphilias and paraphilia-related disorders: a case series. J Clin Psychiatry. 2000;61(9):664-670.
17. Langstrom N, Sjostedt G, Grann M. Psychiatric disorders and recidivism in sexual offenders. Sex Abuse. 2004;16(2):139-150.
18. Smith MR. Osteoporosis during androgen deprivation therapy for prostate cancer. Urology. 2002;60(3 suppl 1):79-85.
19. Grasswick LJ, Bradford JM. Osteoporosis associated with the treatment of paraphilias: a clinical review of seven case reports. J Forensic Sci. 2003;48:849-855.
20. Rösler A, Witztum E. Pharmacotherapy of paraphilias in the next millennium. Behav Sci Law. 2000;18:43-56.
21. Finn DA, Beadles-Bohling AS, Beckley EH, et al. A new look at the 5-alpha-reductase inhibitor finasteride. CNS Drug Rev. 2006;12(1):53-76.
22. Mantzoros CS, Georgiadis EI, Trichopoulos D. Contribution of dihydrotestosterone to male sexual behaviour. BMJ. 1995;310(6990):1289-1291.
23. Bradford JMW, Pawlak A. Double-blind placebo crossover study of cyproterone acetate in the treatment of the paraphilias. Arch Sex Behav. 1993;22(5):383-402.
24. Meyer WJ, Cole CM. Physical and chemical castration of sex offenders: a review. J Offender Rehab. 1997;25(3/4):1-18.
25. Maletzky BM, Tolan A, McFarland B. The Oregon depoProvera program: a five-year follow-up. Sex Abuse. 2006;18:303-316.
26. van Poppel H, Nilsson S. Testosterone surge: rationale for gonadotropin-releasing hormone blockers? Urology. 2008;71:1001-1006.
27. Briken P, Hill A, Berner W. Pharmacotherapy of paraphilias with long-acting agonists of luteinizing hormone–releasing hormone: a systematic review. J Clin Psychiatry. 2003;64:890-897.
28. Blaut K, Winiewski P, Syrenicz A, et al. Apoplexy of clinically silent pituitary adenoma during prostate cancer treatment with LHRH analog. Neuro Endocrinol Lett. 2006;27(5):569-572.
29. Schober JM, Kuhn PJ, Kovacs P, et al. Leuprolide acetate suppresses pedophilic urges and arousability. Arch Sex Behav. 2005;34(6):691-705.
30. Ösler A, Witztum E. Treatment of men with paraphilia with a long-acting analogue of gonadotropin-releasing hormone. N Engl J Med. 1998;338:416-422.
31. Reilly DR, Delva NJ, Hudson RW. Protocols for the use of cyproterone, medroxyprogesterone, and leuprolide in the treatment of paraphilia. Can J Psychiatry. 2000;45:559-563.
32. Quinsey VL, Harris GT, Rice ME, et al. Violent offenders: appraising and managing risk. Washington, DC: American Psychological Association; 1998.
33. Epperson DL, Kaul JD, Huot SJ, et al. Minnesota Sex Offender Screening Tool–revised (MnSOST-R). St. Paul, MN: Minnesota Department of Corrections; 1998.
34. Bradford JMW. The treatment of sexual deviation using a pharmacological approach. J Sex Res. 2000;37(3):248-257.
Borderline personality disorder: STEPPS is practical, evidence-based, easier to use
Treatment of borderline personality disorder (BPD) often is viewed as challenging and the results so discouraging that some clinicians avoid referrals of BPD patients.1-3 Psychotherapy has been the treatment mainstay for decades, and supportive approaches are probably the most widely employed.4 Psychodynamic therapy often has been recommended.
This article introduces a new evidence-based group treatment program that we developed for BPD patients. Systems Training for Emotional Predictability and Problem Solving (STEPPS) is founded on the successes of better known psychoeducational models but is easier for practicing psychiatrists to implement.
A different approach to BPD
Linehan5 introduced dialectical behavior therapy (DBT)—a manualized, time-limited, cognitive-behavioral approach in which patients learn to regulate their emotions and behaviors rather than change their personality structure. Other evidence-based BPD treatments include transference-focused psychotherapy,6 schema-focused psychotherapy,7 and Bateman and Fonagy’s mentalization program.8 For a description of the unique challenges presented by BPD patients, see Box.
In the mid-1990s, we set out to create a treatment program for our BPD patients in response to managed care directives to lower the cost of care, decrease length of inpatient treatment, and reduce rehospitalization rates. Despite DBT’s many appealing features, we felt this model was too lengthy and labor-intensive for our treatment setting. We concluded that modifying a program developed by Bartels and Crotty9 would serve our needs. This 12-week psychoeducational program:
- employs established cognitive-behavioral techniques in group treatment intended to supplement but not replace patients’ ongoing treatment
- incorporates a “systems” component that recognizes the importance of the patient’s family and friends.
We eventually renamed the program Systems Training for Emotional Predictability and Problem Solving (STEPPS)10 and created a new manual (see Related Resources) to simplify group leader training and ensure fidelity to the model. Data from 5 studies, including 2 randomized controlled trials (Table 1), show that STEPPS has a robust antidepressant effect and leads to broad-based improvements in the affective, cognitive, impulsive, and disturbed relationship domains of BPD.11-15
Borderline personality disorder (BPD) is 1 of the most challenging mental health conditions. BPD is surprisingly common, with prevalence rates of 0.5% to 1% in the community, 10% in outpatient mental health settings, and up to 20% in inpatient psychiatric settings.a-c Patients with BPD experience substantial functional impairment in several areas (eg, difficulty maintaining employment, disturbed interpersonal relationships, and disrupted family relationships).a,d,e
Many borderline patients have childhood histories of abuse and continue to be victims of domestic and other violence through adulthood.f High utilization of medical and psychiatric health care services is common and costly.g BPD also is associated with substantial psychiatric comorbidity, particularly mood and anxiety disorders, substance use disorders, eating disorders, and other Axis II disorders.h,i
Persons with BPD experience intense dysphoria and intrapsychic pain. Characteristic features include affective intensity, reactivity, and lability; a pervasive pattern of unstable interpersonal relationships; marked behavioral impulsivity; unstable self-identity; intense anger; and extreme fear of abandonment.j
The symptom that probably makes the greatest demand on mental health resources is recurrent suicidal threats/attempts or episodes of self-mutilation, many prompted by disappointment in a relationship.k Two-thirds to three-quarters of BPD patients will attempt suicide, with up to 10% eventually completing suicide, often following multiple failed treatments.l
References
a. Gunderson J. Borderline personality disorder: A clinical guide. 2nd ed. Washington, DC: American Psychiatric Publishing; 2008.
b. Widiger TA, Frances AJ. Epidemiology, diagnosis, and co-morbidity of borderline personality disorder. In: Tasman A, Hales RE, Frances AJ (eds). American Psychiatric Press Review of Psychiatry, vol. 8. Washington, DC: American Psychiatric Press; 1989:8-24.
c. Swartz MS, Blazer D, George L, et al. Estimating the prevalence of borderline personality disorder in the community. J Personal Disord. 1990;4:257-272.
d. Nakao K, Gunderson JG, Phillips KA, et al. Functional impairment in personality disorders. J Personal Disord. 1992;6:24-31.
e. Skodol AE, Gunderson JG, McGlashan TH, et al. Functional impairment in patients with schizotypal, borderline, avoidant, or obsessive-compulsive personality disorder. Am J Psychiatry. 2002;159:276-283.
f. Zanarini MC. Childhood experiences associated with the development of borderline personality disorder. Psychiatr Clin North Am. 2000;23:89-101.
g. Bender DS, Dolan RT, Skodol AE, et al. Treatment utilization by patients with personality disorders. Am J Psychiatry. 2001;158:295-302.
h. Zanarini MC, Frankenburg FR, Dubo ED, et al. Axis I co-morbidity of borderline personality disorder. Am J Psychiatry. 1998;155:1733-1739.
i. Zimmerman M, Coryell W. DSM-III personality disorder diagnoses in a non-patient sample: demographic correlates and co-morbidity. Arch Gen Psychiatry. 1989;46:682-689.
j. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 1994.
k. Paris J. Social factors in personality disorders— a biopsychosocial approach to etiology and treatment. New York, NY: Cambridge University Press; 1996.
l. Soloff PH, Lynch KG, Kelly TM. Characteristics of suicide attempts of patients with major depressive episode and borderline personality disorder: a comparative study. Am J Psychiatry. 2000;157:601-608.
Table 1
STEPPS: Trials show improvement across BPD domains
| Study | Patients | Results |
|---|---|---|
| Uncontrolled trials | ||
| Blum et al, 200211 | 52 outpatients; 94% female; mean age 33 | Significant improvement in BEST score; significant drop in BDI score and the PANAS negative affect scale |
| Black et al, 200812 | 12 incarcerated women; mean age 35 | Significant improvement in BEST score; significant drop in BDI score and the PANAS negative affect scale |
| Freije et al, 200213 | 85 patients; 91% female; mean age 32 | Significant improvement in score on a Dutch version of BEST; significant improvement on SCL-90 subscales, especially those rating anxiety, depression, and interpersonal sensitivity |
| Randomized controlled trials | ||
| Blum et al, 200814 | 165 adults with BPD assigned to STEPPS plus treatment as usual or only treatment as usual | Patients receiving STEPPS plus treatment as usual experienced greater improvements in ZAN-BPD total score, impulsivity, negative affect, mood, and global functioning |
| van Wel, 200715 | 79 adults with BPD assigned to STEPPS plus treatment as usual or only treatment as usuals | Patients receiving STEPPS plus treatment as usual had greater improvements in global psychiatric symptoms using the SCL-90, BPD symptoms, and quality of life measures at the end of treatment and at 6-month follow-up |
| BDI: Beck Depression Inventory; BEST: Borderline Evaluation of Severity Over Time; BPD: borderline personality disorder; PANAS: Positive and Negative Affect Scale; SCL-90: Symptoms Checklist-90; STEPPS: Systems Training for Emotional Predictability and Problem Solving; ZAN-BPD: Zanarini Rating Scale for Borderline Personality Disorder | ||
STEPPS’ theoretical foundation
- does not disrupt the patient’s present regimen, and
- potentially enhances relationship skills by encouraging the patient to remain in longer relationships with professional and non-professional support.
STEPPS employs cognitive-behavioral methods, including identifying and challenging distorted thoughts and specific behavioral change, combined with psycho-education and skills training.11,12 The addition of a systems component that enlists the help of the patient’s family and friends is unique to STEPPS (Box 1).
Emotional intensity disorder. Many clinicians assume that the core deficit in BPD is inability to manage emotional intensity. In STEPPS, therapists reframe BPD as emotional intensity disorder (EID), a term patients find easier to understand and accept. Patients tend to “see themselves as driven by the disorder to seek relief from a painful illness through desperate behaviors that are reinforced by negative and distorted thinking.”16 Starting with the first session, STEPPS therapists validate the patients’ experience of BPD and provide hope by teaching that patients can acquire skills to manage the disorder.
In the first Systems Training for Emotional Predictability and Problem Solving (STEPPS) session, patients identify and utilize a “reinforcement team” that consists of any person or persons—family members, professionals, friends, coworkers, etc.—who agree to assist the patient in reinforcing STEPPS skills. The systems perspective emphasizes patients’ responsibility for responding to their system more effectively by using their skills and helps patients develop more realistic expectations of—and more helpful interactions with—their support system. Patients are:
- expected to become STEPPS experts and to teach their reinforcement team how to respond with the STEPPS “language”
- encouraged to share what they are learning in group sessions, including relevant handouts
- given “reinforcement team” cards that explain how team members should respond when the patient contacts them.
The cards also list the skills taught in STEPPS and provide questions for team members to ask when contacted by the participant (ie, “Where are you on the Emotional Intensity Continuum?” “Have you used your notebook?” “What skill can you use in this situation?” “How will you use it?”). The cards provide a common language and consistent interaction between patients and their support systems. Patients are instructed to give the cards to their reinforcement team members when they request their assistance.
After the first 4 to 6 STEPPS sessions, a 2-hour meeting is arranged for reinforcement team members, during which the facilitators describe diagnostic criteria and clinical symptoms of borderline personality disorder and discuss the STEPPS language and format. Team members are taught that their role is to reinforce and support the use of skills taught in STEPPS. They are shown how to use the reinforcement team cards.
Group format
STEPPS consists of 20 consecutive weekly, 2-hour sessions led by 2 therapists (we prefer the term “facilitators”). Sessions take place in a classroom setting and are highly structured, with specific facilitator guidelines for each session.
When they arrive at each session, patients fill out the Borderline Evaluation of Severity Over Time (BEST) scale (Box 2)11,17 and record the results on a graph to measure their progress. Each session has a specific handout, including an agenda, followed by the homework assignment for the next week. Participants read the handout material aloud during the group session and start the homework assignment to be sure they understand it.
Handouts also include poems, essays, drawings, and examples created by previous STEPPS participants; these provide a sense of ownership among participants past, present, and future. Participants are encouraged to share their own writings and drawings, as well as other resources they have found helpful to illustrate the skills being taught.
Skills training. Facilitators introduce a new skill at each session, and each skill builds on previously taught skills. A recurring theme in STEPPS is that “most of the work is done between sessions”—during the week, patients are expected to practice the skill taught at the previous session. Using the STEPPS skills is framed as “change from the outside in.” As patients challenge maladaptive filters and distorted cognitions, they find that negative feelings and dysfunctional behaviors change.
Patients identify their use of specific skills by completing a 5-point Emotional Intensity Continuum (EIC) scale. This abstract concept is made concrete with drawings of pots on a burner. At level 1 (baseline), there is no heat under the burner; at level 5, the pot is boiling over.
We developed the Borderline Evaluation of Severity Over Time (BEST) to rate severity and change in patients with borderline personality disorder (BPD).11,17 The self-rated scale has 15 items for which patients rate themselves on a 5-point scale; scores can range from 12 to 72.
The BEST shows evidence for good internal consistency and for both face and content validity because the items were constructed to assess behaviors relevant to BPD. We recently assessed the BEST in subjects who had participated in our randomized controlled trials and concluded that the scale is reliable, valid, and sensitive to clinical change as early as week 4.17 To obtain a copy of this scale, contact the authors.
3 components of STEPPS
Awareness of illness. The first step is for patients to replace misconceptions about BPD with awareness of the behaviors and feelings that define the disorder. They are provided with a printed handout listing DSM-IV criteria for BPD and given time to acknowledge examples in their own behavior. This is called “owning” the illness.
The second step is to introduce the concept of schemas, referred to as cognitive filters. With the author’s permission, we extracted 64 items from Young’s Schema Questionnaire,18 which helps patients identify their early maladaptive schemas. We encourage patients to understand the relationship among these filters, DSM-IV criteria, and their subsequent pattern of feelings, thoughts, and behaviors.
Emotion management skills taught in STEPPS are distancing, communicating, challenging, distracting, and managing problems (Table 2). Using these skills, participants learn to:
- predict the course of emotional states
- anticipate stressful situations
- develop functional coping strategies.
Table 2
Patient skills taught in STEPPS
| Awareness of illness |
| Understand what BPD is |
| Reframe BPD as ‘emotional intensity disorder’ |
| ‘Own’ the illness |
| Identify and challenge dysfunctional schemas |
| Emotion management skills |
| Distancing: Provide distance from emotional intensity |
| Communicating: Describe and define feelings, physical sensations, thoughts, filters, action urges, and behaviors |
| Challenging: Identify distorted thinking and develop alternate ways of thinking |
| Distracting: Identify and engage in behaviors that lower emotional intensity or assist in getting through an episode without resorting to damaging behaviors |
| Managing problems: Identify and define problems, then plan and carry out action steps |
| Behavior management skills |
| Setting goals: Identify specific goals and develop strategies to manage specific problematic behaviors |
| Eating: Balanced diet |
| Sleeping: Good sleep hygiene |
| Exercising: Regular and balanced exercise |
| Leisure: Regular leisure activities |
| Physical health: Manage medical problems |
| Abuse avoidance: Develop strategies to replace abusive behaviors (self-harm, substance abuse, gambling, etc.) |
| Relationship management: Identify and determine strategies to develop healthy relationships. Understand and implement healthy boundaries |
| BPD: borderline personality disorder; STEPPS: Systems Training for Emotional Predictability and Problem Solving |
Patient characteristics
Although some patients learn of STEPPS from previous participants, at our facility we usually request a formal professional referral. We then send potential participants a letter inviting them to attend the group, along with a brochure describing STEPPS and a dated syllabus. We generally begin with 12 to 15 patients but typically have 7 to 10 by the fifth session.
Patients with strong narcissistic or antisocial traits may have difficulty in group settings, probably because they prefer to be the center of attention. That said, we have successfully implemented STEPPS in Iowa prisons and have not experienced difficulties.12 Patients who are abusing substances or have active eating disorders (primarily anorexia nervosa) may not be cognitively able to benefit from STEPPS until these behaviors are better controlled. We recommend that patients seek treatment for these behaviors before—or concurrent with—STEPPS participation.
Persons who deal with conflict by physical threats or intimidation are potential threats to group integrity and are removed immediately. We avoid forming groups with a lone male participant because:
- he may come to represent all men to the rest of the group
- he may have difficulty identifying with problems unique to women with BPD.
In a recent study we found that patients who were rated as more symptomatic at baseline experienced the greatest improvement. Apart from this finding, there were few response predictors, but it was reassuring that both men and women improved.19 Members are cautiously encouraged to use each other as reinforcement team members between sessions, once they feel safe in the group. They are instructed to follow the reinforcement team guidelines.
The facilitators’ role
STEPPS groups are led by 2 facilitators with graduate level training in social sciences and psychotherapy experience. Therapists may be trained in STEPPS during a 1- to 2-day on-site workshop or by attending a 20-week group. These trainees are identified as professionals and do not participate in the sessions.
Using 1 male and 1 female facilitator for a STEPPS group allows modeling of relationship behaviors between genders, projects a healthy male role, and provides support for male participants, who in most groups are in the minority. Initially the facilitators’ stance is active and directive, although this tends to decrease as patients gradually are given increasing leadership responsibilities (such as leading brief reviews of homework assignments).
The therapists’ main tasks include:
- maintaining the psychoeducational format
- adhering to the guidelines
- avoiding involvement in individual issues and past traumas (providing individual psychotherapy in a group setting)
- maintaining focus on skills acquisition
- encouraging group cohesion through identification
- facilitating participants’ change of perspective from victims of EID to experts on managing EID.
Follow-up: STAIRWAYS
STAIRWAYS is a 1-year follow-up group that meets twice a month after the 20-week STEPPS program and consists of stand-alone modules addressing:
- Setting goals
- Trying new things (oriented toward long-term goals, such as obtaining a degree, employment, etc.)
- Anger management
- Impulsivity control
- Relationship management (emphasis on conflict management)
- Writing a script (identifying and preparing for future stressors)
- Assertiveness training
- Your choices (making healthy choices)
- Staying on track (relapse prevention).
- The STEPPS Model for Borderline Personality Disorder Manual. www.steppsforbpd.com.
- van Wel B, Kockmann I, Blum N, et al. STEPPS group treatment for borderline personality disorder in The Netherlands. Ann Clin Psychiatry. 2006;18(1):63-67.
Disclosures
Dr. Black receives research/grant support from AstraZeneca and Forest Laboratories and is a consultant to Jazz Pharmaceuticals.
Ms. Blum and Mr. St. John receive royalties from Blums’ Books LLC, publisher of the Systems Training for Emotional Predictability and Problem Solving (STEPPS) manual.
1. American Psychiatric Association. Practice guidelines for the treatment of patients with borderline personality disorder. Am J Psychiatry. 2001;158(suppl 1):1-52.
2. Soloff PH. Psychopharmacology of borderline personality disorder. Psychiatr Clin North Am. 2000;23:169-191.
3. Zanarini MC. Update on pharmacotherapy of borderline personality disorder. Curr Psychiatry Rep. 2004;6:66-70.
4. Paris J. Borderline personality disorder—a multidimensional approach. Washington, DC: American Psychiatric Press; 1994.
5. Linehan MM. Cognitive-behavioral treatment for borderline personality disorder. New York, NY: Guilford Press; 1993.
6. Clarkin JF, Levy KN, Lenzenweger MF, et al. Evaluating three treatments for borderline personality disorder: a multiwave study. Am J Psychiatry. 2007;164:922-928.
7. Giesen-Bloo J, van Dyck R, Spinhoven P, et al. Outpatient psychotherapy for borderline personality disorder: randomized trial of schema-focused therapy vs transference-focused therapy. Arch Gen Psychiatry. 2006;63:649-658.
8. Bateman A, Fonagy P. Effectiveness of partial hospitalization in the treatment of borderline personality disorder: a randomized controlled trial. Am J Psychiatry. 1999;156:1563-1569.
9. Bartels N, Crotty T. A systems approach to treatment: the borderline personality disorder skill training manual. Winfield, IL: EID Treatment Systems, Inc; 1992.
10. Blum N, Bartels N, St. John D, et al. STEPPS: Systems Training for Emotional Predictability and Problem Solving—group treatment for borderline personality disorder. Coralville, IA: Blum’s Books; 2002.
11. Blum N, Pfohl B, St. John D, et al. STEPPS: a cognitive behavioral systems based group treatment for outpatients with borderline personality disorder—a preliminary report. Compr Psychiatry. 2002;43:301-310.
12. Black DW, Blum N, Eichinger L, et al. Systems Training for Emotional Predictability and Problem Solving (STEPPS) in women offenders with borderline personality disorder in prison: a pilot study. CNS Spectr. 2008;13(10):881-886.
13. Freije H, Dietz B, Appelo M. Behandling van de borderline persoonlijk heidsstoornis met de VERS: de Vaardigheidstraining emotionele regulatiestoornis. Directive Therapies. 2002;4:367-378.
14. Blum N, Pfohl B, St. John D, et al. Systems Training for Emotional Predictability and Problem Solving (STEPPS) for outpatients with borderline personality disorder: a randomized controlled trial and 1-year follow-up. Am J Psychiatry. 2008;165:468-478.
15. van Wel B. VERS: RCT on Dutch STEPPS. Presented at: Annual Meeting of the International Society for the Study of Personality Disorders; September 21, 2007; Den Haag, The Netherlands.
16. Black DW, Blum N, Pfohl B, et al. The STEPPS group treatment program for outpatients with borderline personality disorder. Journal of Contemporary Psychotherapy. 2004;34:193-210.
17. Pfohl B, Blum N, St. John D, et al. Reliability and validity of the Borderline Evaluation of Severity over Time (BEST): a self-rated scale to measure severity and change in persons with borderline personality disorder. J Pers Disord. 2009;23(3):281-293.
18. Young J. Cognitive therapy for personality disorders: a schema-focused approach. Sarasota, FL: Professional Resource Press; 1994.
19. Black DW, Blum N, Pfohl B, et al. Predictors of response to Systems Training to Emotional Predictability and Problem Solving (STEPPS) for borderline personality disorder: an exploratory study. Acta Psychiatr Scand. 2009;120:53-61.
Treatment of borderline personality disorder (BPD) often is viewed as challenging and the results so discouraging that some clinicians avoid referrals of BPD patients.1-3 Psychotherapy has been the treatment mainstay for decades, and supportive approaches are probably the most widely employed.4 Psychodynamic therapy often has been recommended.
This article introduces a new evidence-based group treatment program that we developed for BPD patients. Systems Training for Emotional Predictability and Problem Solving (STEPPS) is founded on the successes of better known psychoeducational models but is easier for practicing psychiatrists to implement.
A different approach to BPD
Linehan5 introduced dialectical behavior therapy (DBT)—a manualized, time-limited, cognitive-behavioral approach in which patients learn to regulate their emotions and behaviors rather than change their personality structure. Other evidence-based BPD treatments include transference-focused psychotherapy,6 schema-focused psychotherapy,7 and Bateman and Fonagy’s mentalization program.8 For a description of the unique challenges presented by BPD patients, see Box.
In the mid-1990s, we set out to create a treatment program for our BPD patients in response to managed care directives to lower the cost of care, decrease length of inpatient treatment, and reduce rehospitalization rates. Despite DBT’s many appealing features, we felt this model was too lengthy and labor-intensive for our treatment setting. We concluded that modifying a program developed by Bartels and Crotty9 would serve our needs. This 12-week psychoeducational program:
- employs established cognitive-behavioral techniques in group treatment intended to supplement but not replace patients’ ongoing treatment
- incorporates a “systems” component that recognizes the importance of the patient’s family and friends.
We eventually renamed the program Systems Training for Emotional Predictability and Problem Solving (STEPPS)10 and created a new manual (see Related Resources) to simplify group leader training and ensure fidelity to the model. Data from 5 studies, including 2 randomized controlled trials (Table 1), show that STEPPS has a robust antidepressant effect and leads to broad-based improvements in the affective, cognitive, impulsive, and disturbed relationship domains of BPD.11-15
Borderline personality disorder (BPD) is 1 of the most challenging mental health conditions. BPD is surprisingly common, with prevalence rates of 0.5% to 1% in the community, 10% in outpatient mental health settings, and up to 20% in inpatient psychiatric settings.a-c Patients with BPD experience substantial functional impairment in several areas (eg, difficulty maintaining employment, disturbed interpersonal relationships, and disrupted family relationships).a,d,e
Many borderline patients have childhood histories of abuse and continue to be victims of domestic and other violence through adulthood.f High utilization of medical and psychiatric health care services is common and costly.g BPD also is associated with substantial psychiatric comorbidity, particularly mood and anxiety disorders, substance use disorders, eating disorders, and other Axis II disorders.h,i
Persons with BPD experience intense dysphoria and intrapsychic pain. Characteristic features include affective intensity, reactivity, and lability; a pervasive pattern of unstable interpersonal relationships; marked behavioral impulsivity; unstable self-identity; intense anger; and extreme fear of abandonment.j
The symptom that probably makes the greatest demand on mental health resources is recurrent suicidal threats/attempts or episodes of self-mutilation, many prompted by disappointment in a relationship.k Two-thirds to three-quarters of BPD patients will attempt suicide, with up to 10% eventually completing suicide, often following multiple failed treatments.l
References
a. Gunderson J. Borderline personality disorder: A clinical guide. 2nd ed. Washington, DC: American Psychiatric Publishing; 2008.
b. Widiger TA, Frances AJ. Epidemiology, diagnosis, and co-morbidity of borderline personality disorder. In: Tasman A, Hales RE, Frances AJ (eds). American Psychiatric Press Review of Psychiatry, vol. 8. Washington, DC: American Psychiatric Press; 1989:8-24.
c. Swartz MS, Blazer D, George L, et al. Estimating the prevalence of borderline personality disorder in the community. J Personal Disord. 1990;4:257-272.
d. Nakao K, Gunderson JG, Phillips KA, et al. Functional impairment in personality disorders. J Personal Disord. 1992;6:24-31.
e. Skodol AE, Gunderson JG, McGlashan TH, et al. Functional impairment in patients with schizotypal, borderline, avoidant, or obsessive-compulsive personality disorder. Am J Psychiatry. 2002;159:276-283.
f. Zanarini MC. Childhood experiences associated with the development of borderline personality disorder. Psychiatr Clin North Am. 2000;23:89-101.
g. Bender DS, Dolan RT, Skodol AE, et al. Treatment utilization by patients with personality disorders. Am J Psychiatry. 2001;158:295-302.
h. Zanarini MC, Frankenburg FR, Dubo ED, et al. Axis I co-morbidity of borderline personality disorder. Am J Psychiatry. 1998;155:1733-1739.
i. Zimmerman M, Coryell W. DSM-III personality disorder diagnoses in a non-patient sample: demographic correlates and co-morbidity. Arch Gen Psychiatry. 1989;46:682-689.
j. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 1994.
k. Paris J. Social factors in personality disorders— a biopsychosocial approach to etiology and treatment. New York, NY: Cambridge University Press; 1996.
l. Soloff PH, Lynch KG, Kelly TM. Characteristics of suicide attempts of patients with major depressive episode and borderline personality disorder: a comparative study. Am J Psychiatry. 2000;157:601-608.
Table 1
STEPPS: Trials show improvement across BPD domains
| Study | Patients | Results |
|---|---|---|
| Uncontrolled trials | ||
| Blum et al, 200211 | 52 outpatients; 94% female; mean age 33 | Significant improvement in BEST score; significant drop in BDI score and the PANAS negative affect scale |
| Black et al, 200812 | 12 incarcerated women; mean age 35 | Significant improvement in BEST score; significant drop in BDI score and the PANAS negative affect scale |
| Freije et al, 200213 | 85 patients; 91% female; mean age 32 | Significant improvement in score on a Dutch version of BEST; significant improvement on SCL-90 subscales, especially those rating anxiety, depression, and interpersonal sensitivity |
| Randomized controlled trials | ||
| Blum et al, 200814 | 165 adults with BPD assigned to STEPPS plus treatment as usual or only treatment as usual | Patients receiving STEPPS plus treatment as usual experienced greater improvements in ZAN-BPD total score, impulsivity, negative affect, mood, and global functioning |
| van Wel, 200715 | 79 adults with BPD assigned to STEPPS plus treatment as usual or only treatment as usuals | Patients receiving STEPPS plus treatment as usual had greater improvements in global psychiatric symptoms using the SCL-90, BPD symptoms, and quality of life measures at the end of treatment and at 6-month follow-up |
| BDI: Beck Depression Inventory; BEST: Borderline Evaluation of Severity Over Time; BPD: borderline personality disorder; PANAS: Positive and Negative Affect Scale; SCL-90: Symptoms Checklist-90; STEPPS: Systems Training for Emotional Predictability and Problem Solving; ZAN-BPD: Zanarini Rating Scale for Borderline Personality Disorder | ||
STEPPS’ theoretical foundation
- does not disrupt the patient’s present regimen, and
- potentially enhances relationship skills by encouraging the patient to remain in longer relationships with professional and non-professional support.
STEPPS employs cognitive-behavioral methods, including identifying and challenging distorted thoughts and specific behavioral change, combined with psycho-education and skills training.11,12 The addition of a systems component that enlists the help of the patient’s family and friends is unique to STEPPS (Box 1).
Emotional intensity disorder. Many clinicians assume that the core deficit in BPD is inability to manage emotional intensity. In STEPPS, therapists reframe BPD as emotional intensity disorder (EID), a term patients find easier to understand and accept. Patients tend to “see themselves as driven by the disorder to seek relief from a painful illness through desperate behaviors that are reinforced by negative and distorted thinking.”16 Starting with the first session, STEPPS therapists validate the patients’ experience of BPD and provide hope by teaching that patients can acquire skills to manage the disorder.
In the first Systems Training for Emotional Predictability and Problem Solving (STEPPS) session, patients identify and utilize a “reinforcement team” that consists of any person or persons—family members, professionals, friends, coworkers, etc.—who agree to assist the patient in reinforcing STEPPS skills. The systems perspective emphasizes patients’ responsibility for responding to their system more effectively by using their skills and helps patients develop more realistic expectations of—and more helpful interactions with—their support system. Patients are:
- expected to become STEPPS experts and to teach their reinforcement team how to respond with the STEPPS “language”
- encouraged to share what they are learning in group sessions, including relevant handouts
- given “reinforcement team” cards that explain how team members should respond when the patient contacts them.
The cards also list the skills taught in STEPPS and provide questions for team members to ask when contacted by the participant (ie, “Where are you on the Emotional Intensity Continuum?” “Have you used your notebook?” “What skill can you use in this situation?” “How will you use it?”). The cards provide a common language and consistent interaction between patients and their support systems. Patients are instructed to give the cards to their reinforcement team members when they request their assistance.
After the first 4 to 6 STEPPS sessions, a 2-hour meeting is arranged for reinforcement team members, during which the facilitators describe diagnostic criteria and clinical symptoms of borderline personality disorder and discuss the STEPPS language and format. Team members are taught that their role is to reinforce and support the use of skills taught in STEPPS. They are shown how to use the reinforcement team cards.
Group format
STEPPS consists of 20 consecutive weekly, 2-hour sessions led by 2 therapists (we prefer the term “facilitators”). Sessions take place in a classroom setting and are highly structured, with specific facilitator guidelines for each session.
When they arrive at each session, patients fill out the Borderline Evaluation of Severity Over Time (BEST) scale (Box 2)11,17 and record the results on a graph to measure their progress. Each session has a specific handout, including an agenda, followed by the homework assignment for the next week. Participants read the handout material aloud during the group session and start the homework assignment to be sure they understand it.
Handouts also include poems, essays, drawings, and examples created by previous STEPPS participants; these provide a sense of ownership among participants past, present, and future. Participants are encouraged to share their own writings and drawings, as well as other resources they have found helpful to illustrate the skills being taught.
Skills training. Facilitators introduce a new skill at each session, and each skill builds on previously taught skills. A recurring theme in STEPPS is that “most of the work is done between sessions”—during the week, patients are expected to practice the skill taught at the previous session. Using the STEPPS skills is framed as “change from the outside in.” As patients challenge maladaptive filters and distorted cognitions, they find that negative feelings and dysfunctional behaviors change.
Patients identify their use of specific skills by completing a 5-point Emotional Intensity Continuum (EIC) scale. This abstract concept is made concrete with drawings of pots on a burner. At level 1 (baseline), there is no heat under the burner; at level 5, the pot is boiling over.
We developed the Borderline Evaluation of Severity Over Time (BEST) to rate severity and change in patients with borderline personality disorder (BPD).11,17 The self-rated scale has 15 items for which patients rate themselves on a 5-point scale; scores can range from 12 to 72.
The BEST shows evidence for good internal consistency and for both face and content validity because the items were constructed to assess behaviors relevant to BPD. We recently assessed the BEST in subjects who had participated in our randomized controlled trials and concluded that the scale is reliable, valid, and sensitive to clinical change as early as week 4.17 To obtain a copy of this scale, contact the authors.
3 components of STEPPS
Awareness of illness. The first step is for patients to replace misconceptions about BPD with awareness of the behaviors and feelings that define the disorder. They are provided with a printed handout listing DSM-IV criteria for BPD and given time to acknowledge examples in their own behavior. This is called “owning” the illness.
The second step is to introduce the concept of schemas, referred to as cognitive filters. With the author’s permission, we extracted 64 items from Young’s Schema Questionnaire,18 which helps patients identify their early maladaptive schemas. We encourage patients to understand the relationship among these filters, DSM-IV criteria, and their subsequent pattern of feelings, thoughts, and behaviors.
Emotion management skills taught in STEPPS are distancing, communicating, challenging, distracting, and managing problems (Table 2). Using these skills, participants learn to:
- predict the course of emotional states
- anticipate stressful situations
- develop functional coping strategies.
Table 2
Patient skills taught in STEPPS
| Awareness of illness |
| Understand what BPD is |
| Reframe BPD as ‘emotional intensity disorder’ |
| ‘Own’ the illness |
| Identify and challenge dysfunctional schemas |
| Emotion management skills |
| Distancing: Provide distance from emotional intensity |
| Communicating: Describe and define feelings, physical sensations, thoughts, filters, action urges, and behaviors |
| Challenging: Identify distorted thinking and develop alternate ways of thinking |
| Distracting: Identify and engage in behaviors that lower emotional intensity or assist in getting through an episode without resorting to damaging behaviors |
| Managing problems: Identify and define problems, then plan and carry out action steps |
| Behavior management skills |
| Setting goals: Identify specific goals and develop strategies to manage specific problematic behaviors |
| Eating: Balanced diet |
| Sleeping: Good sleep hygiene |
| Exercising: Regular and balanced exercise |
| Leisure: Regular leisure activities |
| Physical health: Manage medical problems |
| Abuse avoidance: Develop strategies to replace abusive behaviors (self-harm, substance abuse, gambling, etc.) |
| Relationship management: Identify and determine strategies to develop healthy relationships. Understand and implement healthy boundaries |
| BPD: borderline personality disorder; STEPPS: Systems Training for Emotional Predictability and Problem Solving |
Patient characteristics
Although some patients learn of STEPPS from previous participants, at our facility we usually request a formal professional referral. We then send potential participants a letter inviting them to attend the group, along with a brochure describing STEPPS and a dated syllabus. We generally begin with 12 to 15 patients but typically have 7 to 10 by the fifth session.
Patients with strong narcissistic or antisocial traits may have difficulty in group settings, probably because they prefer to be the center of attention. That said, we have successfully implemented STEPPS in Iowa prisons and have not experienced difficulties.12 Patients who are abusing substances or have active eating disorders (primarily anorexia nervosa) may not be cognitively able to benefit from STEPPS until these behaviors are better controlled. We recommend that patients seek treatment for these behaviors before—or concurrent with—STEPPS participation.
Persons who deal with conflict by physical threats or intimidation are potential threats to group integrity and are removed immediately. We avoid forming groups with a lone male participant because:
- he may come to represent all men to the rest of the group
- he may have difficulty identifying with problems unique to women with BPD.
In a recent study we found that patients who were rated as more symptomatic at baseline experienced the greatest improvement. Apart from this finding, there were few response predictors, but it was reassuring that both men and women improved.19 Members are cautiously encouraged to use each other as reinforcement team members between sessions, once they feel safe in the group. They are instructed to follow the reinforcement team guidelines.
The facilitators’ role
STEPPS groups are led by 2 facilitators with graduate level training in social sciences and psychotherapy experience. Therapists may be trained in STEPPS during a 1- to 2-day on-site workshop or by attending a 20-week group. These trainees are identified as professionals and do not participate in the sessions.
Using 1 male and 1 female facilitator for a STEPPS group allows modeling of relationship behaviors between genders, projects a healthy male role, and provides support for male participants, who in most groups are in the minority. Initially the facilitators’ stance is active and directive, although this tends to decrease as patients gradually are given increasing leadership responsibilities (such as leading brief reviews of homework assignments).
The therapists’ main tasks include:
- maintaining the psychoeducational format
- adhering to the guidelines
- avoiding involvement in individual issues and past traumas (providing individual psychotherapy in a group setting)
- maintaining focus on skills acquisition
- encouraging group cohesion through identification
- facilitating participants’ change of perspective from victims of EID to experts on managing EID.
Follow-up: STAIRWAYS
STAIRWAYS is a 1-year follow-up group that meets twice a month after the 20-week STEPPS program and consists of stand-alone modules addressing:
- Setting goals
- Trying new things (oriented toward long-term goals, such as obtaining a degree, employment, etc.)
- Anger management
- Impulsivity control
- Relationship management (emphasis on conflict management)
- Writing a script (identifying and preparing for future stressors)
- Assertiveness training
- Your choices (making healthy choices)
- Staying on track (relapse prevention).
- The STEPPS Model for Borderline Personality Disorder Manual. www.steppsforbpd.com.
- van Wel B, Kockmann I, Blum N, et al. STEPPS group treatment for borderline personality disorder in The Netherlands. Ann Clin Psychiatry. 2006;18(1):63-67.
Disclosures
Dr. Black receives research/grant support from AstraZeneca and Forest Laboratories and is a consultant to Jazz Pharmaceuticals.
Ms. Blum and Mr. St. John receive royalties from Blums’ Books LLC, publisher of the Systems Training for Emotional Predictability and Problem Solving (STEPPS) manual.
Treatment of borderline personality disorder (BPD) often is viewed as challenging and the results so discouraging that some clinicians avoid referrals of BPD patients.1-3 Psychotherapy has been the treatment mainstay for decades, and supportive approaches are probably the most widely employed.4 Psychodynamic therapy often has been recommended.
This article introduces a new evidence-based group treatment program that we developed for BPD patients. Systems Training for Emotional Predictability and Problem Solving (STEPPS) is founded on the successes of better known psychoeducational models but is easier for practicing psychiatrists to implement.
A different approach to BPD
Linehan5 introduced dialectical behavior therapy (DBT)—a manualized, time-limited, cognitive-behavioral approach in which patients learn to regulate their emotions and behaviors rather than change their personality structure. Other evidence-based BPD treatments include transference-focused psychotherapy,6 schema-focused psychotherapy,7 and Bateman and Fonagy’s mentalization program.8 For a description of the unique challenges presented by BPD patients, see Box.
In the mid-1990s, we set out to create a treatment program for our BPD patients in response to managed care directives to lower the cost of care, decrease length of inpatient treatment, and reduce rehospitalization rates. Despite DBT’s many appealing features, we felt this model was too lengthy and labor-intensive for our treatment setting. We concluded that modifying a program developed by Bartels and Crotty9 would serve our needs. This 12-week psychoeducational program:
- employs established cognitive-behavioral techniques in group treatment intended to supplement but not replace patients’ ongoing treatment
- incorporates a “systems” component that recognizes the importance of the patient’s family and friends.
We eventually renamed the program Systems Training for Emotional Predictability and Problem Solving (STEPPS)10 and created a new manual (see Related Resources) to simplify group leader training and ensure fidelity to the model. Data from 5 studies, including 2 randomized controlled trials (Table 1), show that STEPPS has a robust antidepressant effect and leads to broad-based improvements in the affective, cognitive, impulsive, and disturbed relationship domains of BPD.11-15
Borderline personality disorder (BPD) is 1 of the most challenging mental health conditions. BPD is surprisingly common, with prevalence rates of 0.5% to 1% in the community, 10% in outpatient mental health settings, and up to 20% in inpatient psychiatric settings.a-c Patients with BPD experience substantial functional impairment in several areas (eg, difficulty maintaining employment, disturbed interpersonal relationships, and disrupted family relationships).a,d,e
Many borderline patients have childhood histories of abuse and continue to be victims of domestic and other violence through adulthood.f High utilization of medical and psychiatric health care services is common and costly.g BPD also is associated with substantial psychiatric comorbidity, particularly mood and anxiety disorders, substance use disorders, eating disorders, and other Axis II disorders.h,i
Persons with BPD experience intense dysphoria and intrapsychic pain. Characteristic features include affective intensity, reactivity, and lability; a pervasive pattern of unstable interpersonal relationships; marked behavioral impulsivity; unstable self-identity; intense anger; and extreme fear of abandonment.j
The symptom that probably makes the greatest demand on mental health resources is recurrent suicidal threats/attempts or episodes of self-mutilation, many prompted by disappointment in a relationship.k Two-thirds to three-quarters of BPD patients will attempt suicide, with up to 10% eventually completing suicide, often following multiple failed treatments.l
References
a. Gunderson J. Borderline personality disorder: A clinical guide. 2nd ed. Washington, DC: American Psychiatric Publishing; 2008.
b. Widiger TA, Frances AJ. Epidemiology, diagnosis, and co-morbidity of borderline personality disorder. In: Tasman A, Hales RE, Frances AJ (eds). American Psychiatric Press Review of Psychiatry, vol. 8. Washington, DC: American Psychiatric Press; 1989:8-24.
c. Swartz MS, Blazer D, George L, et al. Estimating the prevalence of borderline personality disorder in the community. J Personal Disord. 1990;4:257-272.
d. Nakao K, Gunderson JG, Phillips KA, et al. Functional impairment in personality disorders. J Personal Disord. 1992;6:24-31.
e. Skodol AE, Gunderson JG, McGlashan TH, et al. Functional impairment in patients with schizotypal, borderline, avoidant, or obsessive-compulsive personality disorder. Am J Psychiatry. 2002;159:276-283.
f. Zanarini MC. Childhood experiences associated with the development of borderline personality disorder. Psychiatr Clin North Am. 2000;23:89-101.
g. Bender DS, Dolan RT, Skodol AE, et al. Treatment utilization by patients with personality disorders. Am J Psychiatry. 2001;158:295-302.
h. Zanarini MC, Frankenburg FR, Dubo ED, et al. Axis I co-morbidity of borderline personality disorder. Am J Psychiatry. 1998;155:1733-1739.
i. Zimmerman M, Coryell W. DSM-III personality disorder diagnoses in a non-patient sample: demographic correlates and co-morbidity. Arch Gen Psychiatry. 1989;46:682-689.
j. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 1994.
k. Paris J. Social factors in personality disorders— a biopsychosocial approach to etiology and treatment. New York, NY: Cambridge University Press; 1996.
l. Soloff PH, Lynch KG, Kelly TM. Characteristics of suicide attempts of patients with major depressive episode and borderline personality disorder: a comparative study. Am J Psychiatry. 2000;157:601-608.
Table 1
STEPPS: Trials show improvement across BPD domains
| Study | Patients | Results |
|---|---|---|
| Uncontrolled trials | ||
| Blum et al, 200211 | 52 outpatients; 94% female; mean age 33 | Significant improvement in BEST score; significant drop in BDI score and the PANAS negative affect scale |
| Black et al, 200812 | 12 incarcerated women; mean age 35 | Significant improvement in BEST score; significant drop in BDI score and the PANAS negative affect scale |
| Freije et al, 200213 | 85 patients; 91% female; mean age 32 | Significant improvement in score on a Dutch version of BEST; significant improvement on SCL-90 subscales, especially those rating anxiety, depression, and interpersonal sensitivity |
| Randomized controlled trials | ||
| Blum et al, 200814 | 165 adults with BPD assigned to STEPPS plus treatment as usual or only treatment as usual | Patients receiving STEPPS plus treatment as usual experienced greater improvements in ZAN-BPD total score, impulsivity, negative affect, mood, and global functioning |
| van Wel, 200715 | 79 adults with BPD assigned to STEPPS plus treatment as usual or only treatment as usuals | Patients receiving STEPPS plus treatment as usual had greater improvements in global psychiatric symptoms using the SCL-90, BPD symptoms, and quality of life measures at the end of treatment and at 6-month follow-up |
| BDI: Beck Depression Inventory; BEST: Borderline Evaluation of Severity Over Time; BPD: borderline personality disorder; PANAS: Positive and Negative Affect Scale; SCL-90: Symptoms Checklist-90; STEPPS: Systems Training for Emotional Predictability and Problem Solving; ZAN-BPD: Zanarini Rating Scale for Borderline Personality Disorder | ||
STEPPS’ theoretical foundation
- does not disrupt the patient’s present regimen, and
- potentially enhances relationship skills by encouraging the patient to remain in longer relationships with professional and non-professional support.
STEPPS employs cognitive-behavioral methods, including identifying and challenging distorted thoughts and specific behavioral change, combined with psycho-education and skills training.11,12 The addition of a systems component that enlists the help of the patient’s family and friends is unique to STEPPS (Box 1).
Emotional intensity disorder. Many clinicians assume that the core deficit in BPD is inability to manage emotional intensity. In STEPPS, therapists reframe BPD as emotional intensity disorder (EID), a term patients find easier to understand and accept. Patients tend to “see themselves as driven by the disorder to seek relief from a painful illness through desperate behaviors that are reinforced by negative and distorted thinking.”16 Starting with the first session, STEPPS therapists validate the patients’ experience of BPD and provide hope by teaching that patients can acquire skills to manage the disorder.
In the first Systems Training for Emotional Predictability and Problem Solving (STEPPS) session, patients identify and utilize a “reinforcement team” that consists of any person or persons—family members, professionals, friends, coworkers, etc.—who agree to assist the patient in reinforcing STEPPS skills. The systems perspective emphasizes patients’ responsibility for responding to their system more effectively by using their skills and helps patients develop more realistic expectations of—and more helpful interactions with—their support system. Patients are:
- expected to become STEPPS experts and to teach their reinforcement team how to respond with the STEPPS “language”
- encouraged to share what they are learning in group sessions, including relevant handouts
- given “reinforcement team” cards that explain how team members should respond when the patient contacts them.
The cards also list the skills taught in STEPPS and provide questions for team members to ask when contacted by the participant (ie, “Where are you on the Emotional Intensity Continuum?” “Have you used your notebook?” “What skill can you use in this situation?” “How will you use it?”). The cards provide a common language and consistent interaction between patients and their support systems. Patients are instructed to give the cards to their reinforcement team members when they request their assistance.
After the first 4 to 6 STEPPS sessions, a 2-hour meeting is arranged for reinforcement team members, during which the facilitators describe diagnostic criteria and clinical symptoms of borderline personality disorder and discuss the STEPPS language and format. Team members are taught that their role is to reinforce and support the use of skills taught in STEPPS. They are shown how to use the reinforcement team cards.
Group format
STEPPS consists of 20 consecutive weekly, 2-hour sessions led by 2 therapists (we prefer the term “facilitators”). Sessions take place in a classroom setting and are highly structured, with specific facilitator guidelines for each session.
When they arrive at each session, patients fill out the Borderline Evaluation of Severity Over Time (BEST) scale (Box 2)11,17 and record the results on a graph to measure their progress. Each session has a specific handout, including an agenda, followed by the homework assignment for the next week. Participants read the handout material aloud during the group session and start the homework assignment to be sure they understand it.
Handouts also include poems, essays, drawings, and examples created by previous STEPPS participants; these provide a sense of ownership among participants past, present, and future. Participants are encouraged to share their own writings and drawings, as well as other resources they have found helpful to illustrate the skills being taught.
Skills training. Facilitators introduce a new skill at each session, and each skill builds on previously taught skills. A recurring theme in STEPPS is that “most of the work is done between sessions”—during the week, patients are expected to practice the skill taught at the previous session. Using the STEPPS skills is framed as “change from the outside in.” As patients challenge maladaptive filters and distorted cognitions, they find that negative feelings and dysfunctional behaviors change.
Patients identify their use of specific skills by completing a 5-point Emotional Intensity Continuum (EIC) scale. This abstract concept is made concrete with drawings of pots on a burner. At level 1 (baseline), there is no heat under the burner; at level 5, the pot is boiling over.
We developed the Borderline Evaluation of Severity Over Time (BEST) to rate severity and change in patients with borderline personality disorder (BPD).11,17 The self-rated scale has 15 items for which patients rate themselves on a 5-point scale; scores can range from 12 to 72.
The BEST shows evidence for good internal consistency and for both face and content validity because the items were constructed to assess behaviors relevant to BPD. We recently assessed the BEST in subjects who had participated in our randomized controlled trials and concluded that the scale is reliable, valid, and sensitive to clinical change as early as week 4.17 To obtain a copy of this scale, contact the authors.
3 components of STEPPS
Awareness of illness. The first step is for patients to replace misconceptions about BPD with awareness of the behaviors and feelings that define the disorder. They are provided with a printed handout listing DSM-IV criteria for BPD and given time to acknowledge examples in their own behavior. This is called “owning” the illness.
The second step is to introduce the concept of schemas, referred to as cognitive filters. With the author’s permission, we extracted 64 items from Young’s Schema Questionnaire,18 which helps patients identify their early maladaptive schemas. We encourage patients to understand the relationship among these filters, DSM-IV criteria, and their subsequent pattern of feelings, thoughts, and behaviors.
Emotion management skills taught in STEPPS are distancing, communicating, challenging, distracting, and managing problems (Table 2). Using these skills, participants learn to:
- predict the course of emotional states
- anticipate stressful situations
- develop functional coping strategies.
Table 2
Patient skills taught in STEPPS
| Awareness of illness |
| Understand what BPD is |
| Reframe BPD as ‘emotional intensity disorder’ |
| ‘Own’ the illness |
| Identify and challenge dysfunctional schemas |
| Emotion management skills |
| Distancing: Provide distance from emotional intensity |
| Communicating: Describe and define feelings, physical sensations, thoughts, filters, action urges, and behaviors |
| Challenging: Identify distorted thinking and develop alternate ways of thinking |
| Distracting: Identify and engage in behaviors that lower emotional intensity or assist in getting through an episode without resorting to damaging behaviors |
| Managing problems: Identify and define problems, then plan and carry out action steps |
| Behavior management skills |
| Setting goals: Identify specific goals and develop strategies to manage specific problematic behaviors |
| Eating: Balanced diet |
| Sleeping: Good sleep hygiene |
| Exercising: Regular and balanced exercise |
| Leisure: Regular leisure activities |
| Physical health: Manage medical problems |
| Abuse avoidance: Develop strategies to replace abusive behaviors (self-harm, substance abuse, gambling, etc.) |
| Relationship management: Identify and determine strategies to develop healthy relationships. Understand and implement healthy boundaries |
| BPD: borderline personality disorder; STEPPS: Systems Training for Emotional Predictability and Problem Solving |
Patient characteristics
Although some patients learn of STEPPS from previous participants, at our facility we usually request a formal professional referral. We then send potential participants a letter inviting them to attend the group, along with a brochure describing STEPPS and a dated syllabus. We generally begin with 12 to 15 patients but typically have 7 to 10 by the fifth session.
Patients with strong narcissistic or antisocial traits may have difficulty in group settings, probably because they prefer to be the center of attention. That said, we have successfully implemented STEPPS in Iowa prisons and have not experienced difficulties.12 Patients who are abusing substances or have active eating disorders (primarily anorexia nervosa) may not be cognitively able to benefit from STEPPS until these behaviors are better controlled. We recommend that patients seek treatment for these behaviors before—or concurrent with—STEPPS participation.
Persons who deal with conflict by physical threats or intimidation are potential threats to group integrity and are removed immediately. We avoid forming groups with a lone male participant because:
- he may come to represent all men to the rest of the group
- he may have difficulty identifying with problems unique to women with BPD.
In a recent study we found that patients who were rated as more symptomatic at baseline experienced the greatest improvement. Apart from this finding, there were few response predictors, but it was reassuring that both men and women improved.19 Members are cautiously encouraged to use each other as reinforcement team members between sessions, once they feel safe in the group. They are instructed to follow the reinforcement team guidelines.
The facilitators’ role
STEPPS groups are led by 2 facilitators with graduate level training in social sciences and psychotherapy experience. Therapists may be trained in STEPPS during a 1- to 2-day on-site workshop or by attending a 20-week group. These trainees are identified as professionals and do not participate in the sessions.
Using 1 male and 1 female facilitator for a STEPPS group allows modeling of relationship behaviors between genders, projects a healthy male role, and provides support for male participants, who in most groups are in the minority. Initially the facilitators’ stance is active and directive, although this tends to decrease as patients gradually are given increasing leadership responsibilities (such as leading brief reviews of homework assignments).
The therapists’ main tasks include:
- maintaining the psychoeducational format
- adhering to the guidelines
- avoiding involvement in individual issues and past traumas (providing individual psychotherapy in a group setting)
- maintaining focus on skills acquisition
- encouraging group cohesion through identification
- facilitating participants’ change of perspective from victims of EID to experts on managing EID.
Follow-up: STAIRWAYS
STAIRWAYS is a 1-year follow-up group that meets twice a month after the 20-week STEPPS program and consists of stand-alone modules addressing:
- Setting goals
- Trying new things (oriented toward long-term goals, such as obtaining a degree, employment, etc.)
- Anger management
- Impulsivity control
- Relationship management (emphasis on conflict management)
- Writing a script (identifying and preparing for future stressors)
- Assertiveness training
- Your choices (making healthy choices)
- Staying on track (relapse prevention).
- The STEPPS Model for Borderline Personality Disorder Manual. www.steppsforbpd.com.
- van Wel B, Kockmann I, Blum N, et al. STEPPS group treatment for borderline personality disorder in The Netherlands. Ann Clin Psychiatry. 2006;18(1):63-67.
Disclosures
Dr. Black receives research/grant support from AstraZeneca and Forest Laboratories and is a consultant to Jazz Pharmaceuticals.
Ms. Blum and Mr. St. John receive royalties from Blums’ Books LLC, publisher of the Systems Training for Emotional Predictability and Problem Solving (STEPPS) manual.
1. American Psychiatric Association. Practice guidelines for the treatment of patients with borderline personality disorder. Am J Psychiatry. 2001;158(suppl 1):1-52.
2. Soloff PH. Psychopharmacology of borderline personality disorder. Psychiatr Clin North Am. 2000;23:169-191.
3. Zanarini MC. Update on pharmacotherapy of borderline personality disorder. Curr Psychiatry Rep. 2004;6:66-70.
4. Paris J. Borderline personality disorder—a multidimensional approach. Washington, DC: American Psychiatric Press; 1994.
5. Linehan MM. Cognitive-behavioral treatment for borderline personality disorder. New York, NY: Guilford Press; 1993.
6. Clarkin JF, Levy KN, Lenzenweger MF, et al. Evaluating three treatments for borderline personality disorder: a multiwave study. Am J Psychiatry. 2007;164:922-928.
7. Giesen-Bloo J, van Dyck R, Spinhoven P, et al. Outpatient psychotherapy for borderline personality disorder: randomized trial of schema-focused therapy vs transference-focused therapy. Arch Gen Psychiatry. 2006;63:649-658.
8. Bateman A, Fonagy P. Effectiveness of partial hospitalization in the treatment of borderline personality disorder: a randomized controlled trial. Am J Psychiatry. 1999;156:1563-1569.
9. Bartels N, Crotty T. A systems approach to treatment: the borderline personality disorder skill training manual. Winfield, IL: EID Treatment Systems, Inc; 1992.
10. Blum N, Bartels N, St. John D, et al. STEPPS: Systems Training for Emotional Predictability and Problem Solving—group treatment for borderline personality disorder. Coralville, IA: Blum’s Books; 2002.
11. Blum N, Pfohl B, St. John D, et al. STEPPS: a cognitive behavioral systems based group treatment for outpatients with borderline personality disorder—a preliminary report. Compr Psychiatry. 2002;43:301-310.
12. Black DW, Blum N, Eichinger L, et al. Systems Training for Emotional Predictability and Problem Solving (STEPPS) in women offenders with borderline personality disorder in prison: a pilot study. CNS Spectr. 2008;13(10):881-886.
13. Freije H, Dietz B, Appelo M. Behandling van de borderline persoonlijk heidsstoornis met de VERS: de Vaardigheidstraining emotionele regulatiestoornis. Directive Therapies. 2002;4:367-378.
14. Blum N, Pfohl B, St. John D, et al. Systems Training for Emotional Predictability and Problem Solving (STEPPS) for outpatients with borderline personality disorder: a randomized controlled trial and 1-year follow-up. Am J Psychiatry. 2008;165:468-478.
15. van Wel B. VERS: RCT on Dutch STEPPS. Presented at: Annual Meeting of the International Society for the Study of Personality Disorders; September 21, 2007; Den Haag, The Netherlands.
16. Black DW, Blum N, Pfohl B, et al. The STEPPS group treatment program for outpatients with borderline personality disorder. Journal of Contemporary Psychotherapy. 2004;34:193-210.
17. Pfohl B, Blum N, St. John D, et al. Reliability and validity of the Borderline Evaluation of Severity over Time (BEST): a self-rated scale to measure severity and change in persons with borderline personality disorder. J Pers Disord. 2009;23(3):281-293.
18. Young J. Cognitive therapy for personality disorders: a schema-focused approach. Sarasota, FL: Professional Resource Press; 1994.
19. Black DW, Blum N, Pfohl B, et al. Predictors of response to Systems Training to Emotional Predictability and Problem Solving (STEPPS) for borderline personality disorder: an exploratory study. Acta Psychiatr Scand. 2009;120:53-61.
1. American Psychiatric Association. Practice guidelines for the treatment of patients with borderline personality disorder. Am J Psychiatry. 2001;158(suppl 1):1-52.
2. Soloff PH. Psychopharmacology of borderline personality disorder. Psychiatr Clin North Am. 2000;23:169-191.
3. Zanarini MC. Update on pharmacotherapy of borderline personality disorder. Curr Psychiatry Rep. 2004;6:66-70.
4. Paris J. Borderline personality disorder—a multidimensional approach. Washington, DC: American Psychiatric Press; 1994.
5. Linehan MM. Cognitive-behavioral treatment for borderline personality disorder. New York, NY: Guilford Press; 1993.
6. Clarkin JF, Levy KN, Lenzenweger MF, et al. Evaluating three treatments for borderline personality disorder: a multiwave study. Am J Psychiatry. 2007;164:922-928.
7. Giesen-Bloo J, van Dyck R, Spinhoven P, et al. Outpatient psychotherapy for borderline personality disorder: randomized trial of schema-focused therapy vs transference-focused therapy. Arch Gen Psychiatry. 2006;63:649-658.
8. Bateman A, Fonagy P. Effectiveness of partial hospitalization in the treatment of borderline personality disorder: a randomized controlled trial. Am J Psychiatry. 1999;156:1563-1569.
9. Bartels N, Crotty T. A systems approach to treatment: the borderline personality disorder skill training manual. Winfield, IL: EID Treatment Systems, Inc; 1992.
10. Blum N, Bartels N, St. John D, et al. STEPPS: Systems Training for Emotional Predictability and Problem Solving—group treatment for borderline personality disorder. Coralville, IA: Blum’s Books; 2002.
11. Blum N, Pfohl B, St. John D, et al. STEPPS: a cognitive behavioral systems based group treatment for outpatients with borderline personality disorder—a preliminary report. Compr Psychiatry. 2002;43:301-310.
12. Black DW, Blum N, Eichinger L, et al. Systems Training for Emotional Predictability and Problem Solving (STEPPS) in women offenders with borderline personality disorder in prison: a pilot study. CNS Spectr. 2008;13(10):881-886.
13. Freije H, Dietz B, Appelo M. Behandling van de borderline persoonlijk heidsstoornis met de VERS: de Vaardigheidstraining emotionele regulatiestoornis. Directive Therapies. 2002;4:367-378.
14. Blum N, Pfohl B, St. John D, et al. Systems Training for Emotional Predictability and Problem Solving (STEPPS) for outpatients with borderline personality disorder: a randomized controlled trial and 1-year follow-up. Am J Psychiatry. 2008;165:468-478.
15. van Wel B. VERS: RCT on Dutch STEPPS. Presented at: Annual Meeting of the International Society for the Study of Personality Disorders; September 21, 2007; Den Haag, The Netherlands.
16. Black DW, Blum N, Pfohl B, et al. The STEPPS group treatment program for outpatients with borderline personality disorder. Journal of Contemporary Psychotherapy. 2004;34:193-210.
17. Pfohl B, Blum N, St. John D, et al. Reliability and validity of the Borderline Evaluation of Severity over Time (BEST): a self-rated scale to measure severity and change in persons with borderline personality disorder. J Pers Disord. 2009;23(3):281-293.
18. Young J. Cognitive therapy for personality disorders: a schema-focused approach. Sarasota, FL: Professional Resource Press; 1994.
19. Black DW, Blum N, Pfohl B, et al. Predictors of response to Systems Training to Emotional Predictability and Problem Solving (STEPPS) for borderline personality disorder: an exploratory study. Acta Psychiatr Scand. 2009;120:53-61.
Help your bipolar disorder patients remain employed
Mrs. S, age 34, worked as an office manager with responsibilities for more than 40 employees for 5 years. Starting in her mid 20s she had repeated periods of depression, binge drinking, and risk-taking that were treated ineffectively with antidepressants. Ultimately, she was fired from her job.
Eventually Mrs. S was diagnosed as bipolar and over time responded well to a mood-stabilizing regimen. She now desires to return to work, both for financial reasons and for the sense of accomplishment that comes from working. Initially, personnel managers review her résumé and tell her she would be bored by the routine nature of entry-level positions, or they offer her jobs with major responsibilities. She accepts a high-level position but soon leaves, feeling overwhelmed by the stress.
Bipolar disorder’s long-term course presents a therapeutic challenge when patients desire to remain employed, seek temporary or permanent disability status, or—most commonly—attempt to return to employment after a period of inability to work. As the experience of Mrs. S illustrates, previous capabilities that appear higher than the person’s present or recent work experience are a key issue to address in interpersonal therapy.
Evidence-based research is informative, but ultimately you must apply judgment and flexibility in setting and revising goals with the bipolar individual. Attention to the disorder’s core characteristics can help you equip patients for work that contributes to their pursuit of health.
Obstacles to employment
Role function. Bipolar disorder impairs family and social function in approximately one-half of persons with this diagnosis, a higher impairment rate than in persons with major depression.1
Cognitive function. Bipolar disorder patients have subtle sustained impairments in cognitive function, particularly working memory.2,3 These deficits—although generally much less severe than in persons with schizophrenia—contribute to workplace and educational difficulties.
Unstable mood. Some symptoms associated with elevated mood contribute to functional impairment. These are not limited to mania or hypomania but also can be prominent in mixed states and depression.
A study from the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) found that two-thirds of 1,380 depressed bipolar I and II patients had ?1 concomitant symptoms principally associated with manic states. The most prominent were distractibility, pressured speech and thoughts, risky behavior, and agitation.4 Each of these—or, more often, all of these—can interfere with work responsibilities.
Circadian rhythm pattern. Sleep disturbances in bipolar disorder differ from those associated with other medical conditions. Bipolar patients’ tendency to increase their activity and interests in the evening may keep them awake into the early morning hours. Insufficient sleep and impaired daytime cognition and alertness related to idio syncratic circadian rhythms can interfere with job requirements.5 The structure of employment can help many bipolar patients maintain effective sleep patterns as well as waking activities (Box 1).
Some individuals recognize their disturbed activity pattern, but many view it simply as the way they approach a day. For the latter group, a sustained treatment effort is needed to help them recognize the adverse consequences of the pattern and develop a more effective daily routine.
Adverse treatment effects. Although important, this core medical issue is not central to the interpersonal focus of this article. The simple tolerability objective in prescribing medications—and less frequently therapies such as electroconvulsive treatment—is to avoid dosages that impair concentration, alertness, or motor speed and accuracy. Similarly, avoid medications that can cause physical changes noticeable to others—such as tremor, sleepiness, or significant weight gain—or adjust dosages to eliminate these side effects.
Work, defined as what we do to make a living, is useful for most individuals. For persons with bipolar disorder, work has additional benefits. Having a job aids in structuring their daily activities, which tend to be skewed by circadian rhythm-linked problems of inadequate sleep or sleep that starts too late and extends into the day. The routine expectations of a work schedule also can counteract the distractibility and unproductive multitasking common in some bipolar disorder patients.
These benefits are not guaranteed and vary considerably across occupational settings, but patients and family members readily understand this aspect of work. Its benefits can serve as an important impetus for patients to persist in efforts to attain employment, even in the face of obstacles.
Bipolar symptom domains
Anxiety is recognized as a separate and major domain in bipolar psychopathology,6 contributing strongly to poor outcomes. Although anxiety is somewhat more predominant in depression and mixed states, it is common in manic and recovered bipolar states as well.
Social anxiety and panic states appear to be most specifically associated with bipolar disorder.7 Because these types of anxiety entail excessive fearful responses, psychotherapeutic techniques including extinction approaches can be helpful.
Depression in bipolar disorder tends to manifest as slowed motor and cognitive function, which is likely to be evident in work situations. Additionally, loss of social interests—one of the most common and severe aspects of depression in bipolar disorders—is likely to be evident to coworkers and to negatively impact work effectiveness.
Irritability occurs most frequently in mixed bipolar states but also is characteristic of—though generally less intense in—depressed and manic clinical states. Even when strictly internal and subjective, irritability can reduce an individual’s confidence and work effectiveness. Expressed irritability, from minor annoyances to explosive outbursts, can have serious employment consequences, including termination.
Manic symptoms. The impulsivity that is common in bipolar mania can interfere with work. Acting without considering consequences, taking undue risks, or reaching conclusions on inadequate information can cause problems, including physical harm to self or coworkers. Excessive talking—usually associated with internally recognized racing thoughts—can be a nuisance when mild or problematic if it interferes with customer or coworker interactions.
Hyperactivity and increased energy may be perceived as behaviors that facilitate productivity at work (Box 2).8-10 The adaptive characteristics of many hypomanic states are infrequent or absent in depressive, manic, and mixed manic clinical states, however.
Psychosis is principally associated with manic episodes, but it can be a component of any symptomatic clinical state. Delusional ideas or persecutory thoughts are rarely compatible with a work environment, in part because of potential risks to others.
For some purposes, bipolar disease confers social and employment advantages. Common, frequently adaptive behavioral characteristics of hypomania include:
- perseverance
- high energy
- heightened perceptual sensitivity
- exuberance and playfulness
- optimism.
Increased energy and mild degrees of hyperactivity—as well as thinking along creative, multisystem lines—can benefit work productivity, customer interactions, and work group relations. Heightened confidence and social interests can be valuable in some sales and marketing activities.
Although these attitudes and behaviors can have constructive effects, patients need to understand their limits and destructive potential. This is not a straightforward issue, as patients may not have self-awareness of some adverse consequences of characteristics such as irritability, risk taking, or inappropriate sexual advances. A phenomenon little described in clinical literature but relatively common in biographical accounts of persons with bipolar disorder is that friends or coworkers may encourage, rationalize, and take advantage of an individual’s hypomanic energy, thwarting effective interventions.
Componential treatment
Bipolar disorder’s multiple symptom domains suggest a componential approach to treatment. It may be useful to convey this concept metaphorically to the patient. When working on a jigsaw puzzle, a section that has been put together can be largely left intact and attention turned to other sections of the puzzle. Similarly, once a particular bipolar component is well managed—whether via medication, lifestyle, attitudes, or combinations of these—that symptom is likely to remain stable, barring a new insult/stressor (such as a medical condition requiring drugs that interfere with the bipolar regimen).
If mood stabilizers control risky behavior, impulsivity, and affective lability, the regimen generally will remain effective. If residual or new problems develop in another area (such as anxiety, sleep cycle, or irritability), choose drug regimens and psychoeducation approaches that are compatible with the mood-stabilizing plan. This attitude toward treatment:
- is reassuring to most patients, who come to see a new or recurring problem in one domain as not inherently a harbinger of complete relapse
- can reduce patient- or clinician-initiated deletions and additions of medications in a regimen that has been established as effective.
Autobiographical accounts of persons with bipolar disorders can be useful in educating patients about the considerations presented here. Actress Patty Duke made these observations in describing the gradual development of an effective treatment for her severe bipolar disorder:
I work at not flying off the handle…and I’m much better at it. My general medical bills dropped by $50,000 a year since my bipolar diagnosis and treatment. Until then, I was always in the hospital for some phantom illness. I was there with real symptoms born of depression. I haven’t been in the hospital since I was diagnosed.
My recovery from manic-depression has been an evolution, not a sudden miracle. For someone who spent 50% of her life screaming and yelling about something, I am now down to, say, 5%.11
Psychosocial factors to consider
Stigma in the workplace. Although most coworkers are tolerant of and fair-minded about the functional difficulties common in symptomatic bipolar disorder, some will have biased, inaccurate views about psychiatric conditions. Advise bipolar individuals to make case-by-case decisions about whether to provide personal information to other employees and, if so, how much.
As with most medical conditions, the default choice will be to not discuss personal information in the workplace. Some coworkers, however, might appreciate learning of the bipolar condition (for example, a supervisor who seems empathic to an employee’s seeming stressed state).
Realistic expectations. Most clinicians recognize that relief from a syndromal bipolar state is achieved more quickly than a sustained recovered status in which symptoms are minimal. Attaining functional capacity in a normal range also lags, both in time and in the proportion of persons who ever achieve sustained good function.12 Patients, their families, and often employers may have unrealistic expectations about early resumption of work after a depressive or manic episode resolves.
Ethnic considerations. Some literature suggests ethnic differences in the initial presentation of bipolar disorder, with more severe manifestations in some populations particularly if psychosis is a component symptom.13 Additionally, some cultural views about stigma from illness can add to patients’ or family members’ reluctance to re-enter the workplace.
Socioeconomic status. Sometimes bipolar illness puts out of reach the occupational activities that an individual has previously undertaken or that are characteristic of the family’s experience and expectations. Resistance to a change in self-concept can add to the difficulty in successfully moving a patient to consider employment that is more routinized and less intellectual or decisional in nature (Box 3).14
Divergence in education vs work status. Persons with bipolar disorders often have substantial divergence between high educational attainment and lower work performance. When this is the case, all or most of the factors reviewed in this article probably have contributed. Mrs. S’s experience illustrates this aspect of our care for persons with bipolar disorders.
An employment barrier for some bipolar patients is that a brief, often long-past period of high intellectual or vocational performance serves as the benchmark for their capabilities. Patients with this characteristic resist revising their self-concept. Some treat the loss of this idealized image as an unfair consequence of their illness or society’s reaction to bipolar disorder. Their stubbornness tends to prevent realistic engagement socially or vocationally at levels that are presently feasible for them.
Resistance to change associated with this characteristic often is difficult to manage effectively with short, relatively infrequent medication-focused visits. Specific psychosocial interventions may be more effective.14
CASE CONTINUED: Finding a new balance
After leaving the stressful high-level job, Mrs. S next resolved to limit her search to half-time positions and took a job with limited responsibilities in a bookstore. Her work productivity was outstanding, but she became easily flustered when asked to assume additional responsibilities. Some of these required quick learning of new skills in inventory re-supply or interacting with dissatisfied customers.
As she became more confident and less fearful of being fired, Mrs. S talked with 2 supervisors about her illness management. This halted their well-intentioned efforts to promote her, based on their perception of her as talented and engaging.
Attention to these workplace issues took up approximately half of the time in her regular psychiatric appointments for more than 1 year. Through this process, Mrs. S developed increasingly effective insight into the complex mix of her accomplishments and resilience on one hand and her fluctuating social and vocational impairment on the other. She also recognized that subsyndromal symptoms continued at times, despite her overall good functional state. These insights and her greater self-confidence helped Mrs. S resolve and manage the divergences in her own and others’ perceptions of her capabilities and potential.
Related Resource
- Coping with depression or bipolar disorder at your job (patient information). Depression and Bipolar Support Alliance. www.dbsalliance.org/site/PageServer?pagename=Employment_Information.
Disclosure
Dr. Bowden reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Ware JE, Jr, Kosinski M, Bayliss MS, et al. Comparison of methods for the scoring and statistical analysis of SF-36 health profile and summary measures: summary of results from the Medical Outcomes Study. Med Care. 1995;33(suppl 4):AS264-AS279.
2. Glahn D, Bearden CE, Barguil M, et al. The neurocognitive signature of psychotic bipolar disorder. Biol Psychiatry. 2007;62:910-916.
3. Goodwin G, Martinez-Aran A, Glahn DC, et al. Cognitive impairment in bipolar disorder: neurodevelopment or neurodegeneration? An ECNP expert meeting report. Eur Neuropsychopharm. 2008;18:787-793.
4. Goldberg JF, Perlis RH, Bowden CL, et al. Manic symptoms during depressive episodes in 1,380 patients with bipolar disorder: findings from the STEP-BD. Am J Psychiatry. 2009;166(2):173-181.
5. Mansour HA, Wood J, Chowdari KV, et al. Circadian phase variation in bipolar I disorder. Chronobiol Int. 2005;22(3):571-584.
6. Feske U, Frank E, Mallinger AG, et al. Anxiety as a correlate of response to the acute treatment of bipolar I disorder. Am J Psychiatry. 2000;57:956-962.
7. Mantere O, Melartin TK, Suominen K, et al. Difference in axis I and II comorbidities between bipolar I and II disorder and major depressive disorder. J Clin Psychiatry. 2006;67:584-593.
8. Bowden CL. Bipolar disorder and creativity. In: Shaw MP, Runco MA, eds. Creativity and affect. Norwood, NJ: Ablex Publishing Corp; 1994:73-86.
9. Andreasen N, Powers S. Overinclusive thinking in mania and schizophrenia. Br J Psychiatry. 1974;125:452-456.
10. Solovay MR, Shenton ME, Holzman PS. Comparative studies of thought disorders. I. Mania and schizophrenia. Arch Gen Psychiatry. 1987;44:13-20.
11. Duke P, Hochman G. A brilliant madness: living with manic-depressive illness. New York, NY: Bantam Books; 1997.
12. Coryell W, Scheftner W, Keller M, et al. The enduring psychosocial consequences of mania and depression. Am J Psychiatry. 1993;150:720-727.
13. Kennedy N, Boydell J, van Os J, et al. Ethnic differences in first clinical presentation of bipolar disorder: results from an epidemiological study. J Affect Dis. 2004;83:161-168.
14. Mikowitz DJ, Goldstein MJ. Bipolar disorder: a family-focused approach. New York, NY: Guilford Press; 2006.
Mrs. S, age 34, worked as an office manager with responsibilities for more than 40 employees for 5 years. Starting in her mid 20s she had repeated periods of depression, binge drinking, and risk-taking that were treated ineffectively with antidepressants. Ultimately, she was fired from her job.
Eventually Mrs. S was diagnosed as bipolar and over time responded well to a mood-stabilizing regimen. She now desires to return to work, both for financial reasons and for the sense of accomplishment that comes from working. Initially, personnel managers review her résumé and tell her she would be bored by the routine nature of entry-level positions, or they offer her jobs with major responsibilities. She accepts a high-level position but soon leaves, feeling overwhelmed by the stress.
Bipolar disorder’s long-term course presents a therapeutic challenge when patients desire to remain employed, seek temporary or permanent disability status, or—most commonly—attempt to return to employment after a period of inability to work. As the experience of Mrs. S illustrates, previous capabilities that appear higher than the person’s present or recent work experience are a key issue to address in interpersonal therapy.
Evidence-based research is informative, but ultimately you must apply judgment and flexibility in setting and revising goals with the bipolar individual. Attention to the disorder’s core characteristics can help you equip patients for work that contributes to their pursuit of health.
Obstacles to employment
Role function. Bipolar disorder impairs family and social function in approximately one-half of persons with this diagnosis, a higher impairment rate than in persons with major depression.1
Cognitive function. Bipolar disorder patients have subtle sustained impairments in cognitive function, particularly working memory.2,3 These deficits—although generally much less severe than in persons with schizophrenia—contribute to workplace and educational difficulties.
Unstable mood. Some symptoms associated with elevated mood contribute to functional impairment. These are not limited to mania or hypomania but also can be prominent in mixed states and depression.
A study from the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) found that two-thirds of 1,380 depressed bipolar I and II patients had ?1 concomitant symptoms principally associated with manic states. The most prominent were distractibility, pressured speech and thoughts, risky behavior, and agitation.4 Each of these—or, more often, all of these—can interfere with work responsibilities.
Circadian rhythm pattern. Sleep disturbances in bipolar disorder differ from those associated with other medical conditions. Bipolar patients’ tendency to increase their activity and interests in the evening may keep them awake into the early morning hours. Insufficient sleep and impaired daytime cognition and alertness related to idio syncratic circadian rhythms can interfere with job requirements.5 The structure of employment can help many bipolar patients maintain effective sleep patterns as well as waking activities (Box 1).
Some individuals recognize their disturbed activity pattern, but many view it simply as the way they approach a day. For the latter group, a sustained treatment effort is needed to help them recognize the adverse consequences of the pattern and develop a more effective daily routine.
Adverse treatment effects. Although important, this core medical issue is not central to the interpersonal focus of this article. The simple tolerability objective in prescribing medications—and less frequently therapies such as electroconvulsive treatment—is to avoid dosages that impair concentration, alertness, or motor speed and accuracy. Similarly, avoid medications that can cause physical changes noticeable to others—such as tremor, sleepiness, or significant weight gain—or adjust dosages to eliminate these side effects.
Work, defined as what we do to make a living, is useful for most individuals. For persons with bipolar disorder, work has additional benefits. Having a job aids in structuring their daily activities, which tend to be skewed by circadian rhythm-linked problems of inadequate sleep or sleep that starts too late and extends into the day. The routine expectations of a work schedule also can counteract the distractibility and unproductive multitasking common in some bipolar disorder patients.
These benefits are not guaranteed and vary considerably across occupational settings, but patients and family members readily understand this aspect of work. Its benefits can serve as an important impetus for patients to persist in efforts to attain employment, even in the face of obstacles.
Bipolar symptom domains
Anxiety is recognized as a separate and major domain in bipolar psychopathology,6 contributing strongly to poor outcomes. Although anxiety is somewhat more predominant in depression and mixed states, it is common in manic and recovered bipolar states as well.
Social anxiety and panic states appear to be most specifically associated with bipolar disorder.7 Because these types of anxiety entail excessive fearful responses, psychotherapeutic techniques including extinction approaches can be helpful.
Depression in bipolar disorder tends to manifest as slowed motor and cognitive function, which is likely to be evident in work situations. Additionally, loss of social interests—one of the most common and severe aspects of depression in bipolar disorders—is likely to be evident to coworkers and to negatively impact work effectiveness.
Irritability occurs most frequently in mixed bipolar states but also is characteristic of—though generally less intense in—depressed and manic clinical states. Even when strictly internal and subjective, irritability can reduce an individual’s confidence and work effectiveness. Expressed irritability, from minor annoyances to explosive outbursts, can have serious employment consequences, including termination.
Manic symptoms. The impulsivity that is common in bipolar mania can interfere with work. Acting without considering consequences, taking undue risks, or reaching conclusions on inadequate information can cause problems, including physical harm to self or coworkers. Excessive talking—usually associated with internally recognized racing thoughts—can be a nuisance when mild or problematic if it interferes with customer or coworker interactions.
Hyperactivity and increased energy may be perceived as behaviors that facilitate productivity at work (Box 2).8-10 The adaptive characteristics of many hypomanic states are infrequent or absent in depressive, manic, and mixed manic clinical states, however.
Psychosis is principally associated with manic episodes, but it can be a component of any symptomatic clinical state. Delusional ideas or persecutory thoughts are rarely compatible with a work environment, in part because of potential risks to others.
For some purposes, bipolar disease confers social and employment advantages. Common, frequently adaptive behavioral characteristics of hypomania include:
- perseverance
- high energy
- heightened perceptual sensitivity
- exuberance and playfulness
- optimism.
Increased energy and mild degrees of hyperactivity—as well as thinking along creative, multisystem lines—can benefit work productivity, customer interactions, and work group relations. Heightened confidence and social interests can be valuable in some sales and marketing activities.
Although these attitudes and behaviors can have constructive effects, patients need to understand their limits and destructive potential. This is not a straightforward issue, as patients may not have self-awareness of some adverse consequences of characteristics such as irritability, risk taking, or inappropriate sexual advances. A phenomenon little described in clinical literature but relatively common in biographical accounts of persons with bipolar disorder is that friends or coworkers may encourage, rationalize, and take advantage of an individual’s hypomanic energy, thwarting effective interventions.
Componential treatment
Bipolar disorder’s multiple symptom domains suggest a componential approach to treatment. It may be useful to convey this concept metaphorically to the patient. When working on a jigsaw puzzle, a section that has been put together can be largely left intact and attention turned to other sections of the puzzle. Similarly, once a particular bipolar component is well managed—whether via medication, lifestyle, attitudes, or combinations of these—that symptom is likely to remain stable, barring a new insult/stressor (such as a medical condition requiring drugs that interfere with the bipolar regimen).
If mood stabilizers control risky behavior, impulsivity, and affective lability, the regimen generally will remain effective. If residual or new problems develop in another area (such as anxiety, sleep cycle, or irritability), choose drug regimens and psychoeducation approaches that are compatible with the mood-stabilizing plan. This attitude toward treatment:
- is reassuring to most patients, who come to see a new or recurring problem in one domain as not inherently a harbinger of complete relapse
- can reduce patient- or clinician-initiated deletions and additions of medications in a regimen that has been established as effective.
Autobiographical accounts of persons with bipolar disorders can be useful in educating patients about the considerations presented here. Actress Patty Duke made these observations in describing the gradual development of an effective treatment for her severe bipolar disorder:
I work at not flying off the handle…and I’m much better at it. My general medical bills dropped by $50,000 a year since my bipolar diagnosis and treatment. Until then, I was always in the hospital for some phantom illness. I was there with real symptoms born of depression. I haven’t been in the hospital since I was diagnosed.
My recovery from manic-depression has been an evolution, not a sudden miracle. For someone who spent 50% of her life screaming and yelling about something, I am now down to, say, 5%.11
Psychosocial factors to consider
Stigma in the workplace. Although most coworkers are tolerant of and fair-minded about the functional difficulties common in symptomatic bipolar disorder, some will have biased, inaccurate views about psychiatric conditions. Advise bipolar individuals to make case-by-case decisions about whether to provide personal information to other employees and, if so, how much.
As with most medical conditions, the default choice will be to not discuss personal information in the workplace. Some coworkers, however, might appreciate learning of the bipolar condition (for example, a supervisor who seems empathic to an employee’s seeming stressed state).
Realistic expectations. Most clinicians recognize that relief from a syndromal bipolar state is achieved more quickly than a sustained recovered status in which symptoms are minimal. Attaining functional capacity in a normal range also lags, both in time and in the proportion of persons who ever achieve sustained good function.12 Patients, their families, and often employers may have unrealistic expectations about early resumption of work after a depressive or manic episode resolves.
Ethnic considerations. Some literature suggests ethnic differences in the initial presentation of bipolar disorder, with more severe manifestations in some populations particularly if psychosis is a component symptom.13 Additionally, some cultural views about stigma from illness can add to patients’ or family members’ reluctance to re-enter the workplace.
Socioeconomic status. Sometimes bipolar illness puts out of reach the occupational activities that an individual has previously undertaken or that are characteristic of the family’s experience and expectations. Resistance to a change in self-concept can add to the difficulty in successfully moving a patient to consider employment that is more routinized and less intellectual or decisional in nature (Box 3).14
Divergence in education vs work status. Persons with bipolar disorders often have substantial divergence between high educational attainment and lower work performance. When this is the case, all or most of the factors reviewed in this article probably have contributed. Mrs. S’s experience illustrates this aspect of our care for persons with bipolar disorders.
An employment barrier for some bipolar patients is that a brief, often long-past period of high intellectual or vocational performance serves as the benchmark for their capabilities. Patients with this characteristic resist revising their self-concept. Some treat the loss of this idealized image as an unfair consequence of their illness or society’s reaction to bipolar disorder. Their stubbornness tends to prevent realistic engagement socially or vocationally at levels that are presently feasible for them.
Resistance to change associated with this characteristic often is difficult to manage effectively with short, relatively infrequent medication-focused visits. Specific psychosocial interventions may be more effective.14
CASE CONTINUED: Finding a new balance
After leaving the stressful high-level job, Mrs. S next resolved to limit her search to half-time positions and took a job with limited responsibilities in a bookstore. Her work productivity was outstanding, but she became easily flustered when asked to assume additional responsibilities. Some of these required quick learning of new skills in inventory re-supply or interacting with dissatisfied customers.
As she became more confident and less fearful of being fired, Mrs. S talked with 2 supervisors about her illness management. This halted their well-intentioned efforts to promote her, based on their perception of her as talented and engaging.
Attention to these workplace issues took up approximately half of the time in her regular psychiatric appointments for more than 1 year. Through this process, Mrs. S developed increasingly effective insight into the complex mix of her accomplishments and resilience on one hand and her fluctuating social and vocational impairment on the other. She also recognized that subsyndromal symptoms continued at times, despite her overall good functional state. These insights and her greater self-confidence helped Mrs. S resolve and manage the divergences in her own and others’ perceptions of her capabilities and potential.
Related Resource
- Coping with depression or bipolar disorder at your job (patient information). Depression and Bipolar Support Alliance. www.dbsalliance.org/site/PageServer?pagename=Employment_Information.
Disclosure
Dr. Bowden reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Mrs. S, age 34, worked as an office manager with responsibilities for more than 40 employees for 5 years. Starting in her mid 20s she had repeated periods of depression, binge drinking, and risk-taking that were treated ineffectively with antidepressants. Ultimately, she was fired from her job.
Eventually Mrs. S was diagnosed as bipolar and over time responded well to a mood-stabilizing regimen. She now desires to return to work, both for financial reasons and for the sense of accomplishment that comes from working. Initially, personnel managers review her résumé and tell her she would be bored by the routine nature of entry-level positions, or they offer her jobs with major responsibilities. She accepts a high-level position but soon leaves, feeling overwhelmed by the stress.
Bipolar disorder’s long-term course presents a therapeutic challenge when patients desire to remain employed, seek temporary or permanent disability status, or—most commonly—attempt to return to employment after a period of inability to work. As the experience of Mrs. S illustrates, previous capabilities that appear higher than the person’s present or recent work experience are a key issue to address in interpersonal therapy.
Evidence-based research is informative, but ultimately you must apply judgment and flexibility in setting and revising goals with the bipolar individual. Attention to the disorder’s core characteristics can help you equip patients for work that contributes to their pursuit of health.
Obstacles to employment
Role function. Bipolar disorder impairs family and social function in approximately one-half of persons with this diagnosis, a higher impairment rate than in persons with major depression.1
Cognitive function. Bipolar disorder patients have subtle sustained impairments in cognitive function, particularly working memory.2,3 These deficits—although generally much less severe than in persons with schizophrenia—contribute to workplace and educational difficulties.
Unstable mood. Some symptoms associated with elevated mood contribute to functional impairment. These are not limited to mania or hypomania but also can be prominent in mixed states and depression.
A study from the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) found that two-thirds of 1,380 depressed bipolar I and II patients had ?1 concomitant symptoms principally associated with manic states. The most prominent were distractibility, pressured speech and thoughts, risky behavior, and agitation.4 Each of these—or, more often, all of these—can interfere with work responsibilities.
Circadian rhythm pattern. Sleep disturbances in bipolar disorder differ from those associated with other medical conditions. Bipolar patients’ tendency to increase their activity and interests in the evening may keep them awake into the early morning hours. Insufficient sleep and impaired daytime cognition and alertness related to idio syncratic circadian rhythms can interfere with job requirements.5 The structure of employment can help many bipolar patients maintain effective sleep patterns as well as waking activities (Box 1).
Some individuals recognize their disturbed activity pattern, but many view it simply as the way they approach a day. For the latter group, a sustained treatment effort is needed to help them recognize the adverse consequences of the pattern and develop a more effective daily routine.
Adverse treatment effects. Although important, this core medical issue is not central to the interpersonal focus of this article. The simple tolerability objective in prescribing medications—and less frequently therapies such as electroconvulsive treatment—is to avoid dosages that impair concentration, alertness, or motor speed and accuracy. Similarly, avoid medications that can cause physical changes noticeable to others—such as tremor, sleepiness, or significant weight gain—or adjust dosages to eliminate these side effects.
Work, defined as what we do to make a living, is useful for most individuals. For persons with bipolar disorder, work has additional benefits. Having a job aids in structuring their daily activities, which tend to be skewed by circadian rhythm-linked problems of inadequate sleep or sleep that starts too late and extends into the day. The routine expectations of a work schedule also can counteract the distractibility and unproductive multitasking common in some bipolar disorder patients.
These benefits are not guaranteed and vary considerably across occupational settings, but patients and family members readily understand this aspect of work. Its benefits can serve as an important impetus for patients to persist in efforts to attain employment, even in the face of obstacles.
Bipolar symptom domains
Anxiety is recognized as a separate and major domain in bipolar psychopathology,6 contributing strongly to poor outcomes. Although anxiety is somewhat more predominant in depression and mixed states, it is common in manic and recovered bipolar states as well.
Social anxiety and panic states appear to be most specifically associated with bipolar disorder.7 Because these types of anxiety entail excessive fearful responses, psychotherapeutic techniques including extinction approaches can be helpful.
Depression in bipolar disorder tends to manifest as slowed motor and cognitive function, which is likely to be evident in work situations. Additionally, loss of social interests—one of the most common and severe aspects of depression in bipolar disorders—is likely to be evident to coworkers and to negatively impact work effectiveness.
Irritability occurs most frequently in mixed bipolar states but also is characteristic of—though generally less intense in—depressed and manic clinical states. Even when strictly internal and subjective, irritability can reduce an individual’s confidence and work effectiveness. Expressed irritability, from minor annoyances to explosive outbursts, can have serious employment consequences, including termination.
Manic symptoms. The impulsivity that is common in bipolar mania can interfere with work. Acting without considering consequences, taking undue risks, or reaching conclusions on inadequate information can cause problems, including physical harm to self or coworkers. Excessive talking—usually associated with internally recognized racing thoughts—can be a nuisance when mild or problematic if it interferes with customer or coworker interactions.
Hyperactivity and increased energy may be perceived as behaviors that facilitate productivity at work (Box 2).8-10 The adaptive characteristics of many hypomanic states are infrequent or absent in depressive, manic, and mixed manic clinical states, however.
Psychosis is principally associated with manic episodes, but it can be a component of any symptomatic clinical state. Delusional ideas or persecutory thoughts are rarely compatible with a work environment, in part because of potential risks to others.
For some purposes, bipolar disease confers social and employment advantages. Common, frequently adaptive behavioral characteristics of hypomania include:
- perseverance
- high energy
- heightened perceptual sensitivity
- exuberance and playfulness
- optimism.
Increased energy and mild degrees of hyperactivity—as well as thinking along creative, multisystem lines—can benefit work productivity, customer interactions, and work group relations. Heightened confidence and social interests can be valuable in some sales and marketing activities.
Although these attitudes and behaviors can have constructive effects, patients need to understand their limits and destructive potential. This is not a straightforward issue, as patients may not have self-awareness of some adverse consequences of characteristics such as irritability, risk taking, or inappropriate sexual advances. A phenomenon little described in clinical literature but relatively common in biographical accounts of persons with bipolar disorder is that friends or coworkers may encourage, rationalize, and take advantage of an individual’s hypomanic energy, thwarting effective interventions.
Componential treatment
Bipolar disorder’s multiple symptom domains suggest a componential approach to treatment. It may be useful to convey this concept metaphorically to the patient. When working on a jigsaw puzzle, a section that has been put together can be largely left intact and attention turned to other sections of the puzzle. Similarly, once a particular bipolar component is well managed—whether via medication, lifestyle, attitudes, or combinations of these—that symptom is likely to remain stable, barring a new insult/stressor (such as a medical condition requiring drugs that interfere with the bipolar regimen).
If mood stabilizers control risky behavior, impulsivity, and affective lability, the regimen generally will remain effective. If residual or new problems develop in another area (such as anxiety, sleep cycle, or irritability), choose drug regimens and psychoeducation approaches that are compatible with the mood-stabilizing plan. This attitude toward treatment:
- is reassuring to most patients, who come to see a new or recurring problem in one domain as not inherently a harbinger of complete relapse
- can reduce patient- or clinician-initiated deletions and additions of medications in a regimen that has been established as effective.
Autobiographical accounts of persons with bipolar disorders can be useful in educating patients about the considerations presented here. Actress Patty Duke made these observations in describing the gradual development of an effective treatment for her severe bipolar disorder:
I work at not flying off the handle…and I’m much better at it. My general medical bills dropped by $50,000 a year since my bipolar diagnosis and treatment. Until then, I was always in the hospital for some phantom illness. I was there with real symptoms born of depression. I haven’t been in the hospital since I was diagnosed.
My recovery from manic-depression has been an evolution, not a sudden miracle. For someone who spent 50% of her life screaming and yelling about something, I am now down to, say, 5%.11
Psychosocial factors to consider
Stigma in the workplace. Although most coworkers are tolerant of and fair-minded about the functional difficulties common in symptomatic bipolar disorder, some will have biased, inaccurate views about psychiatric conditions. Advise bipolar individuals to make case-by-case decisions about whether to provide personal information to other employees and, if so, how much.
As with most medical conditions, the default choice will be to not discuss personal information in the workplace. Some coworkers, however, might appreciate learning of the bipolar condition (for example, a supervisor who seems empathic to an employee’s seeming stressed state).
Realistic expectations. Most clinicians recognize that relief from a syndromal bipolar state is achieved more quickly than a sustained recovered status in which symptoms are minimal. Attaining functional capacity in a normal range also lags, both in time and in the proportion of persons who ever achieve sustained good function.12 Patients, their families, and often employers may have unrealistic expectations about early resumption of work after a depressive or manic episode resolves.
Ethnic considerations. Some literature suggests ethnic differences in the initial presentation of bipolar disorder, with more severe manifestations in some populations particularly if psychosis is a component symptom.13 Additionally, some cultural views about stigma from illness can add to patients’ or family members’ reluctance to re-enter the workplace.
Socioeconomic status. Sometimes bipolar illness puts out of reach the occupational activities that an individual has previously undertaken or that are characteristic of the family’s experience and expectations. Resistance to a change in self-concept can add to the difficulty in successfully moving a patient to consider employment that is more routinized and less intellectual or decisional in nature (Box 3).14
Divergence in education vs work status. Persons with bipolar disorders often have substantial divergence between high educational attainment and lower work performance. When this is the case, all or most of the factors reviewed in this article probably have contributed. Mrs. S’s experience illustrates this aspect of our care for persons with bipolar disorders.
An employment barrier for some bipolar patients is that a brief, often long-past period of high intellectual or vocational performance serves as the benchmark for their capabilities. Patients with this characteristic resist revising their self-concept. Some treat the loss of this idealized image as an unfair consequence of their illness or society’s reaction to bipolar disorder. Their stubbornness tends to prevent realistic engagement socially or vocationally at levels that are presently feasible for them.
Resistance to change associated with this characteristic often is difficult to manage effectively with short, relatively infrequent medication-focused visits. Specific psychosocial interventions may be more effective.14
CASE CONTINUED: Finding a new balance
After leaving the stressful high-level job, Mrs. S next resolved to limit her search to half-time positions and took a job with limited responsibilities in a bookstore. Her work productivity was outstanding, but she became easily flustered when asked to assume additional responsibilities. Some of these required quick learning of new skills in inventory re-supply or interacting with dissatisfied customers.
As she became more confident and less fearful of being fired, Mrs. S talked with 2 supervisors about her illness management. This halted their well-intentioned efforts to promote her, based on their perception of her as talented and engaging.
Attention to these workplace issues took up approximately half of the time in her regular psychiatric appointments for more than 1 year. Through this process, Mrs. S developed increasingly effective insight into the complex mix of her accomplishments and resilience on one hand and her fluctuating social and vocational impairment on the other. She also recognized that subsyndromal symptoms continued at times, despite her overall good functional state. These insights and her greater self-confidence helped Mrs. S resolve and manage the divergences in her own and others’ perceptions of her capabilities and potential.
Related Resource
- Coping with depression or bipolar disorder at your job (patient information). Depression and Bipolar Support Alliance. www.dbsalliance.org/site/PageServer?pagename=Employment_Information.
Disclosure
Dr. Bowden reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Ware JE, Jr, Kosinski M, Bayliss MS, et al. Comparison of methods for the scoring and statistical analysis of SF-36 health profile and summary measures: summary of results from the Medical Outcomes Study. Med Care. 1995;33(suppl 4):AS264-AS279.
2. Glahn D, Bearden CE, Barguil M, et al. The neurocognitive signature of psychotic bipolar disorder. Biol Psychiatry. 2007;62:910-916.
3. Goodwin G, Martinez-Aran A, Glahn DC, et al. Cognitive impairment in bipolar disorder: neurodevelopment or neurodegeneration? An ECNP expert meeting report. Eur Neuropsychopharm. 2008;18:787-793.
4. Goldberg JF, Perlis RH, Bowden CL, et al. Manic symptoms during depressive episodes in 1,380 patients with bipolar disorder: findings from the STEP-BD. Am J Psychiatry. 2009;166(2):173-181.
5. Mansour HA, Wood J, Chowdari KV, et al. Circadian phase variation in bipolar I disorder. Chronobiol Int. 2005;22(3):571-584.
6. Feske U, Frank E, Mallinger AG, et al. Anxiety as a correlate of response to the acute treatment of bipolar I disorder. Am J Psychiatry. 2000;57:956-962.
7. Mantere O, Melartin TK, Suominen K, et al. Difference in axis I and II comorbidities between bipolar I and II disorder and major depressive disorder. J Clin Psychiatry. 2006;67:584-593.
8. Bowden CL. Bipolar disorder and creativity. In: Shaw MP, Runco MA, eds. Creativity and affect. Norwood, NJ: Ablex Publishing Corp; 1994:73-86.
9. Andreasen N, Powers S. Overinclusive thinking in mania and schizophrenia. Br J Psychiatry. 1974;125:452-456.
10. Solovay MR, Shenton ME, Holzman PS. Comparative studies of thought disorders. I. Mania and schizophrenia. Arch Gen Psychiatry. 1987;44:13-20.
11. Duke P, Hochman G. A brilliant madness: living with manic-depressive illness. New York, NY: Bantam Books; 1997.
12. Coryell W, Scheftner W, Keller M, et al. The enduring psychosocial consequences of mania and depression. Am J Psychiatry. 1993;150:720-727.
13. Kennedy N, Boydell J, van Os J, et al. Ethnic differences in first clinical presentation of bipolar disorder: results from an epidemiological study. J Affect Dis. 2004;83:161-168.
14. Mikowitz DJ, Goldstein MJ. Bipolar disorder: a family-focused approach. New York, NY: Guilford Press; 2006.
1. Ware JE, Jr, Kosinski M, Bayliss MS, et al. Comparison of methods for the scoring and statistical analysis of SF-36 health profile and summary measures: summary of results from the Medical Outcomes Study. Med Care. 1995;33(suppl 4):AS264-AS279.
2. Glahn D, Bearden CE, Barguil M, et al. The neurocognitive signature of psychotic bipolar disorder. Biol Psychiatry. 2007;62:910-916.
3. Goodwin G, Martinez-Aran A, Glahn DC, et al. Cognitive impairment in bipolar disorder: neurodevelopment or neurodegeneration? An ECNP expert meeting report. Eur Neuropsychopharm. 2008;18:787-793.
4. Goldberg JF, Perlis RH, Bowden CL, et al. Manic symptoms during depressive episodes in 1,380 patients with bipolar disorder: findings from the STEP-BD. Am J Psychiatry. 2009;166(2):173-181.
5. Mansour HA, Wood J, Chowdari KV, et al. Circadian phase variation in bipolar I disorder. Chronobiol Int. 2005;22(3):571-584.
6. Feske U, Frank E, Mallinger AG, et al. Anxiety as a correlate of response to the acute treatment of bipolar I disorder. Am J Psychiatry. 2000;57:956-962.
7. Mantere O, Melartin TK, Suominen K, et al. Difference in axis I and II comorbidities between bipolar I and II disorder and major depressive disorder. J Clin Psychiatry. 2006;67:584-593.
8. Bowden CL. Bipolar disorder and creativity. In: Shaw MP, Runco MA, eds. Creativity and affect. Norwood, NJ: Ablex Publishing Corp; 1994:73-86.
9. Andreasen N, Powers S. Overinclusive thinking in mania and schizophrenia. Br J Psychiatry. 1974;125:452-456.
10. Solovay MR, Shenton ME, Holzman PS. Comparative studies of thought disorders. I. Mania and schizophrenia. Arch Gen Psychiatry. 1987;44:13-20.
11. Duke P, Hochman G. A brilliant madness: living with manic-depressive illness. New York, NY: Bantam Books; 1997.
12. Coryell W, Scheftner W, Keller M, et al. The enduring psychosocial consequences of mania and depression. Am J Psychiatry. 1993;150:720-727.
13. Kennedy N, Boydell J, van Os J, et al. Ethnic differences in first clinical presentation of bipolar disorder: results from an epidemiological study. J Affect Dis. 2004;83:161-168.
14. Mikowitz DJ, Goldstein MJ. Bipolar disorder: a family-focused approach. New York, NY: Guilford Press; 2006.
Transcend dread: 8 ways to transform your care of ‘difficult’ patients
In a psychiatric clinic, Dr. B treats Ms. D, a single 28-year-old, for depression. She has multiple pain and gastrointestinal complaints that have responded poorly to treatment, morbid obesity, chronic tiredness, irritability, and Cluster B personality traits. Ms. D is lonely, unemployed, and seems to be in perpetual crisis. She states “unless someone does something to make this better, I just might kill myself.” She blames Dr. B for failing to adequately treat her depression; he has tried many medications to no avail. In psychotherapy sessions, Ms. D complains instead of examining methods for improvement, and she does not complete psychotherapy homework. She is extremely passive in her approach to getting better.
Ms. D asks Dr. B fill out the necessary paperwork so she can qualify for disability. Dr. B informs her that he will not do so because he believes she is capable of employment and that receiving disability would make her less likely to improve. Ms. D and her parents file letters of complaint about Dr. B to the supervisor of the psychiatric clinic for lack of treatment efficacy and for not supporting her disability claim. Dr. B dreads seeing Ms. D on his appointment list, and realizes she repulses him.
Although “the difficult patient” is not a diagnosis or specific clinical entity, clinicians universally struggle with such patients and have an immediate sense of shared experience when describing the phenomenon. In primary care, O’Dowd1 aptly described this type of patient as the “heartsink” patient, meaning the practitioner often feels exasperation, defeat, or dislike when he or she sees the patient’s name on the schedule.
This article discusses the literature on this topic and provides strategies for dealing with difficult patients in psychiatric practice.
Patient characteristics
Most published reports of difficult patients involve descriptive case series or physician accounts, most often describing patients presenting in nonpsychiatric specialties, including family practice, emergency medicine, rheumatology, gastroenterology, plastic surgery, and dentistry, among others.2-7
In a survey of physicians in 4 primary care clinics, subjects rated 96 (15%) of 627 adult patients as “difficult.”8 Difficult patients were significantly more likely than others to have a mental disorder ( Table 1 ).8 They also had more functional impairment, higher health care utilization, and lower satisfaction with care.
A separate primary care clinic study found uncannily similar results—physicians rated 74 (15%) of 500 new walk-in patients as “difficult.”9 Compared with other patients, the difficult patients had:
- higher rates of psychiatric illness, somatization (>5 somatic complaints), and more severe symptoms
- poorer functional status, more unmet expectations, less satisfaction with care, and higher use of health services.
Fewer articles on difficult patients have been published in psychiatric literature, although some commonalities have emerged ( Box ).10-12 Often suffering from chronic conditions without well-defined treatment endpoints, difficult patients do worse clinically, have higher use of health services, and are less happy with their care than other patients.
Difficult patients challenge our competence as physicians and evoke personal distress. Physicians with less job satisfaction, less clinical experience, less training in counseling, and a poor attitude toward psychosocial problems are more likely to perceive a patient as difficult.13,14
Table 1
Common psychiatric disorders in difficult patients
| Multisomatoform disorder |
| Panic disorder |
| Dysthymia |
| Generalized anxiety disorder |
| Major depressive disorder |
| Alcohol abuse or dependence* |
| *Researchers categorized patients as having “probable” alcohol abuse or dependence but did not determine if they met DSM-IV-TR criteria for these disorders |
| Source: Reference 8 |
An Ovid Medline search of psychiatric literature for “difficult patients” found only 9 articles published from 1996 to 2008, and most were editorials or essays.10
Groves11 grouped difficult patients into 4 categories:
- dependent clingers
- entitled demanders
- manipulative help-rejecters
- self-destructive deniers.
For a description of the behaviors and personality traits associated with each of these 4 categories and strategies to address them, see “The nurse who worked the system,” Current Psychiatry, July 2009. Groves emphasized that a physician’s negative reactions evoked by such patients—once understood through introspection—may facilitate better understanding and psychological management in their care.
Hinshelwood12 wrote about the cognitive dissonance psychiatrists encounter when trying to balance the different responses evoked by patients with schizophrenia and severe personality disorders.
When confronted with a psychotic patient’s severely damaged reality testing, psychiatrists often depersonalize the patient in an effort to be “scientific.” Conversely, patients with severe personality disorders threaten the psychiatrist with their emotional instability. The psychiatrist loses the role of objective observer and instead becomes a “moral evaluator,” seeing the patient as “good” or “bad” instead of as a person in need of help.
Hinshelwood cautioned that patients such as this are difficult not because their treatment is complicated but because they challenge our identity as scientists and put us in personal difficulty.
Survival strategies for clinicians
Eight strategies can help improve your care of difficult patients ( Table 2 ).
Table 2
8 strategies for managing difficult patients
| 1. Acknowledge that the patient is difficult |
| 2. Develop empathy |
| 3. Seek out supervision/consultation |
| 4. Utilize a team approach |
| 5. Lower treatment goals |
| 6. Decompress the treatment timeline |
| 7. Use ‘plussing’ (positive comments and acknowledgements) |
| 8. Use imagery (visualize the patient as a character in an unfinished novel) |
2. Develop empathy. Empathy is identification with and understanding of why a person feels, thinks, and acts as he or she does. The best way to develop empathy for a difficult patient is to learn about him or her firsthand—directly from the patient, not from reading chart notes or from information passed among colleagues.
Learning about the patient firsthand means shifting from sign-and-symptom gathering to performing a genuine inquiry about how the person thinks or feels, including interests, loves, or background. Challenging clinical circumstances—such as seeing a patient in a busy emergency department or during a 15-minute medication check—can make this difficult. In some cases, however, the time needed to establish empathy can be surprisingly brief.
The more patients feel that the psychiatrist is “on their level,” the less likely they are to project internalized anguish or impulsively act on conflicted feelings.
3. Seek out supervision or consultation. You can gain new perspectives by taking a “step back” and looking at the case with a colleague. Seeking out consultation also allows you to decompress by “getting it off your chest.” Supervision often allows clinicians to develop:
- empathy toward a difficult patient
- increased energy and creativity in subsequent sessions.
If you cannot utilize a team to carry out treatment, this approach still may help you develop a treatment plan.
5. Lower treatment goals. The nature of difficult patients makes complete “cures” a rarity. A psychiatrist whose goal is to substantially help a patient may become chronically frustrated and feel inadequate in the face of a patient’s perpetual suffering. The clinician sometimes reacts by developing therapeutic nihilism and withdrawing energy from the case. The patient, of course, senses this and increases his or her general distress level, which intensifies the negative interaction.
By lowering goals—for example, aiming for stabilization rather than improvement—you can feel less like a failure and be more relaxed. A relaxed clinician is more tolerant and in a better position to help the patient. Other lowered goals might be to reduce harm from impulsive or dangerous behaviors instead of eliminating them or better coping with symptoms rather than symptom remission.
7. Use ‘plussing.’ Because we experience dread with difficult patients, clinicians often avoid, refrain from, or simply don’t see opportunities to use positive comments and acknowledgements (“plussing”) when they arise. Most patients (as well as clinicians) want to be liked, and small compliments—when genuinely and appropriately placed—sometimes can make a huge difference in patients’ willingness to cope or try new things.
This technique might allow you to see the humorous side of yourself as the hardworking, well-intentioned yet ineffectual psychiatrist. You don’t know how the story will unfold, but you can accept this as you would in any other unfinished novel.
CASE CONTINUED: A more effective approach
Dr. B realizes Ms. D is a difficult patient for him and takes the case into supervision. He is stunned when he is unable to answer several of his supervisor’s questions about Ms. D, including “What was her upbringing like?” and “What are her strengths or interests?” He realizes he knows little about Ms. D and becomes aware that he has focused most of their sessions on either fixing her immediate and never-ending crises or defending himself.
The supervisor points out that Dr. B’s lack of empathy for Ms. D keeps him from helping her—being anxious and defensive makes him less likely to be supportive or creative. Dr. B feels better after the supervision session. He experiences some catharsis and develops a plan to improve the situation.
Dr. B structures the next session to get to know Ms. D better. He mentally decompresses the treatment timeline and refocuses on the need to develop empathy instead of attempting to ameliorate symptoms. Dr. B begins by letting Ms. D know he wants to help her but doesn’t know much about her. She initially rejects his attempts at empathic communication, but with gentle persistence he learns about her upbringing and interests. Dr. B is able to genuinely compliment her on coping with previous traumas and begins to better understand her strengths. Over the next several weeks, Ms. D seems more able to accept supportive interventions and eventually begins a part-time job.
Related resources
- Colson DB. Difficult patients in extended psychiatric hospitalization: a research perspective on the patient, staff, and team. Psychiatry. 1990;53(4):369-382.
- Koekkoek B, van Meijel B, Hutschemaekers G. “Difficult patients” in mental health care: a review. Psychiatr Serv. 2006;57:795-802.
Dr. Battaglia reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. O’Dowd TC. Five years of heartsink patients in general practice. BMJ. 1988;297:528-530.
2. Smith HW. The difficult patient and doctor: origins and suggestions. Facial Plast Surg Clin North Am. 2008;16(2):177-178, vi.
3. Woods CD. The difficult patient: a psychodynamic perspective. J Calif Dent Assoc. 2007;35(3):186-191.
4. Müller-Lissner S. The difficult patient with constipation. Best Pract Res Clin Gastroenterol. 2007;21(3):473-484.
5. King K, Moss AH. The frequency and significance of the “difficult” patient: the nephrology community’s perceptions. Adv Chronic Kidney Dis. 2004;11(2):234-239.
6. Potter M, Gordon S, Hamer P. The difficult patient in private practice physiotherapy: a qualitative study. Aust J Physiother. 2003;49(1):53-61.
7. Fee C. Death of a difficult patient. Ann Emerg Med. 2001;37(3):354-355.
8. Hahn SR, Kroenke K, Spitzer RL, et al. The difficult patient: prevalence, psychopathology, and functional impairment. J Gen Intern Med. 1996;11(1):1-8.
9. Jackson JL, Kroenke K. Difficult patient encounters in the ambulatory clinic: clinical predictors and outcomes. Arch Intern Med. 1999;159(10):1069-1075.
10. Ovid Medline [database online]. New York, NY: Ovid Technologies, Inc; 2009.
11. Groves JE. Taking care of the hateful patient. N Engl J Med. 1978;298(16):883-887.
12. Hinshelwood RD. The difficult patient. The role of ‘scientific psychiatry’ in understanding patients with chronic schizophrenia or severe personality disorders. Br J Psychiatry. 1999;174:187-190.
13. Mathers N, Jones N, Hannay D. Heartsink patients: a study of their general practitioners. Br J Gen Pract. 1995;45(395):293-296.
14. Steinmetz D, Tabenkin H. The ‘difficult’ patient as perceived by family physicians. Fam Pract. 2001;18(5):495-500.
15. Maltsberger JT, Buie DH. Countertransference hate in the treatment of suicidal patients. Arch Gen Psychiatry. 1974;30(5):625-633.
In a psychiatric clinic, Dr. B treats Ms. D, a single 28-year-old, for depression. She has multiple pain and gastrointestinal complaints that have responded poorly to treatment, morbid obesity, chronic tiredness, irritability, and Cluster B personality traits. Ms. D is lonely, unemployed, and seems to be in perpetual crisis. She states “unless someone does something to make this better, I just might kill myself.” She blames Dr. B for failing to adequately treat her depression; he has tried many medications to no avail. In psychotherapy sessions, Ms. D complains instead of examining methods for improvement, and she does not complete psychotherapy homework. She is extremely passive in her approach to getting better.
Ms. D asks Dr. B fill out the necessary paperwork so she can qualify for disability. Dr. B informs her that he will not do so because he believes she is capable of employment and that receiving disability would make her less likely to improve. Ms. D and her parents file letters of complaint about Dr. B to the supervisor of the psychiatric clinic for lack of treatment efficacy and for not supporting her disability claim. Dr. B dreads seeing Ms. D on his appointment list, and realizes she repulses him.
Although “the difficult patient” is not a diagnosis or specific clinical entity, clinicians universally struggle with such patients and have an immediate sense of shared experience when describing the phenomenon. In primary care, O’Dowd1 aptly described this type of patient as the “heartsink” patient, meaning the practitioner often feels exasperation, defeat, or dislike when he or she sees the patient’s name on the schedule.
This article discusses the literature on this topic and provides strategies for dealing with difficult patients in psychiatric practice.
Patient characteristics
Most published reports of difficult patients involve descriptive case series or physician accounts, most often describing patients presenting in nonpsychiatric specialties, including family practice, emergency medicine, rheumatology, gastroenterology, plastic surgery, and dentistry, among others.2-7
In a survey of physicians in 4 primary care clinics, subjects rated 96 (15%) of 627 adult patients as “difficult.”8 Difficult patients were significantly more likely than others to have a mental disorder ( Table 1 ).8 They also had more functional impairment, higher health care utilization, and lower satisfaction with care.
A separate primary care clinic study found uncannily similar results—physicians rated 74 (15%) of 500 new walk-in patients as “difficult.”9 Compared with other patients, the difficult patients had:
- higher rates of psychiatric illness, somatization (>5 somatic complaints), and more severe symptoms
- poorer functional status, more unmet expectations, less satisfaction with care, and higher use of health services.
Fewer articles on difficult patients have been published in psychiatric literature, although some commonalities have emerged ( Box ).10-12 Often suffering from chronic conditions without well-defined treatment endpoints, difficult patients do worse clinically, have higher use of health services, and are less happy with their care than other patients.
Difficult patients challenge our competence as physicians and evoke personal distress. Physicians with less job satisfaction, less clinical experience, less training in counseling, and a poor attitude toward psychosocial problems are more likely to perceive a patient as difficult.13,14
Table 1
Common psychiatric disorders in difficult patients
| Multisomatoform disorder |
| Panic disorder |
| Dysthymia |
| Generalized anxiety disorder |
| Major depressive disorder |
| Alcohol abuse or dependence* |
| *Researchers categorized patients as having “probable” alcohol abuse or dependence but did not determine if they met DSM-IV-TR criteria for these disorders |
| Source: Reference 8 |
An Ovid Medline search of psychiatric literature for “difficult patients” found only 9 articles published from 1996 to 2008, and most were editorials or essays.10
Groves11 grouped difficult patients into 4 categories:
- dependent clingers
- entitled demanders
- manipulative help-rejecters
- self-destructive deniers.
For a description of the behaviors and personality traits associated with each of these 4 categories and strategies to address them, see “The nurse who worked the system,” Current Psychiatry, July 2009. Groves emphasized that a physician’s negative reactions evoked by such patients—once understood through introspection—may facilitate better understanding and psychological management in their care.
Hinshelwood12 wrote about the cognitive dissonance psychiatrists encounter when trying to balance the different responses evoked by patients with schizophrenia and severe personality disorders.
When confronted with a psychotic patient’s severely damaged reality testing, psychiatrists often depersonalize the patient in an effort to be “scientific.” Conversely, patients with severe personality disorders threaten the psychiatrist with their emotional instability. The psychiatrist loses the role of objective observer and instead becomes a “moral evaluator,” seeing the patient as “good” or “bad” instead of as a person in need of help.
Hinshelwood cautioned that patients such as this are difficult not because their treatment is complicated but because they challenge our identity as scientists and put us in personal difficulty.
Survival strategies for clinicians
Eight strategies can help improve your care of difficult patients ( Table 2 ).
Table 2
8 strategies for managing difficult patients
| 1. Acknowledge that the patient is difficult |
| 2. Develop empathy |
| 3. Seek out supervision/consultation |
| 4. Utilize a team approach |
| 5. Lower treatment goals |
| 6. Decompress the treatment timeline |
| 7. Use ‘plussing’ (positive comments and acknowledgements) |
| 8. Use imagery (visualize the patient as a character in an unfinished novel) |
2. Develop empathy. Empathy is identification with and understanding of why a person feels, thinks, and acts as he or she does. The best way to develop empathy for a difficult patient is to learn about him or her firsthand—directly from the patient, not from reading chart notes or from information passed among colleagues.
Learning about the patient firsthand means shifting from sign-and-symptom gathering to performing a genuine inquiry about how the person thinks or feels, including interests, loves, or background. Challenging clinical circumstances—such as seeing a patient in a busy emergency department or during a 15-minute medication check—can make this difficult. In some cases, however, the time needed to establish empathy can be surprisingly brief.
The more patients feel that the psychiatrist is “on their level,” the less likely they are to project internalized anguish or impulsively act on conflicted feelings.
3. Seek out supervision or consultation. You can gain new perspectives by taking a “step back” and looking at the case with a colleague. Seeking out consultation also allows you to decompress by “getting it off your chest.” Supervision often allows clinicians to develop:
- empathy toward a difficult patient
- increased energy and creativity in subsequent sessions.
If you cannot utilize a team to carry out treatment, this approach still may help you develop a treatment plan.
5. Lower treatment goals. The nature of difficult patients makes complete “cures” a rarity. A psychiatrist whose goal is to substantially help a patient may become chronically frustrated and feel inadequate in the face of a patient’s perpetual suffering. The clinician sometimes reacts by developing therapeutic nihilism and withdrawing energy from the case. The patient, of course, senses this and increases his or her general distress level, which intensifies the negative interaction.
By lowering goals—for example, aiming for stabilization rather than improvement—you can feel less like a failure and be more relaxed. A relaxed clinician is more tolerant and in a better position to help the patient. Other lowered goals might be to reduce harm from impulsive or dangerous behaviors instead of eliminating them or better coping with symptoms rather than symptom remission.
7. Use ‘plussing.’ Because we experience dread with difficult patients, clinicians often avoid, refrain from, or simply don’t see opportunities to use positive comments and acknowledgements (“plussing”) when they arise. Most patients (as well as clinicians) want to be liked, and small compliments—when genuinely and appropriately placed—sometimes can make a huge difference in patients’ willingness to cope or try new things.
This technique might allow you to see the humorous side of yourself as the hardworking, well-intentioned yet ineffectual psychiatrist. You don’t know how the story will unfold, but you can accept this as you would in any other unfinished novel.
CASE CONTINUED: A more effective approach
Dr. B realizes Ms. D is a difficult patient for him and takes the case into supervision. He is stunned when he is unable to answer several of his supervisor’s questions about Ms. D, including “What was her upbringing like?” and “What are her strengths or interests?” He realizes he knows little about Ms. D and becomes aware that he has focused most of their sessions on either fixing her immediate and never-ending crises or defending himself.
The supervisor points out that Dr. B’s lack of empathy for Ms. D keeps him from helping her—being anxious and defensive makes him less likely to be supportive or creative. Dr. B feels better after the supervision session. He experiences some catharsis and develops a plan to improve the situation.
Dr. B structures the next session to get to know Ms. D better. He mentally decompresses the treatment timeline and refocuses on the need to develop empathy instead of attempting to ameliorate symptoms. Dr. B begins by letting Ms. D know he wants to help her but doesn’t know much about her. She initially rejects his attempts at empathic communication, but with gentle persistence he learns about her upbringing and interests. Dr. B is able to genuinely compliment her on coping with previous traumas and begins to better understand her strengths. Over the next several weeks, Ms. D seems more able to accept supportive interventions and eventually begins a part-time job.
Related resources
- Colson DB. Difficult patients in extended psychiatric hospitalization: a research perspective on the patient, staff, and team. Psychiatry. 1990;53(4):369-382.
- Koekkoek B, van Meijel B, Hutschemaekers G. “Difficult patients” in mental health care: a review. Psychiatr Serv. 2006;57:795-802.
Dr. Battaglia reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
In a psychiatric clinic, Dr. B treats Ms. D, a single 28-year-old, for depression. She has multiple pain and gastrointestinal complaints that have responded poorly to treatment, morbid obesity, chronic tiredness, irritability, and Cluster B personality traits. Ms. D is lonely, unemployed, and seems to be in perpetual crisis. She states “unless someone does something to make this better, I just might kill myself.” She blames Dr. B for failing to adequately treat her depression; he has tried many medications to no avail. In psychotherapy sessions, Ms. D complains instead of examining methods for improvement, and she does not complete psychotherapy homework. She is extremely passive in her approach to getting better.
Ms. D asks Dr. B fill out the necessary paperwork so she can qualify for disability. Dr. B informs her that he will not do so because he believes she is capable of employment and that receiving disability would make her less likely to improve. Ms. D and her parents file letters of complaint about Dr. B to the supervisor of the psychiatric clinic for lack of treatment efficacy and for not supporting her disability claim. Dr. B dreads seeing Ms. D on his appointment list, and realizes she repulses him.
Although “the difficult patient” is not a diagnosis or specific clinical entity, clinicians universally struggle with such patients and have an immediate sense of shared experience when describing the phenomenon. In primary care, O’Dowd1 aptly described this type of patient as the “heartsink” patient, meaning the practitioner often feels exasperation, defeat, or dislike when he or she sees the patient’s name on the schedule.
This article discusses the literature on this topic and provides strategies for dealing with difficult patients in psychiatric practice.
Patient characteristics
Most published reports of difficult patients involve descriptive case series or physician accounts, most often describing patients presenting in nonpsychiatric specialties, including family practice, emergency medicine, rheumatology, gastroenterology, plastic surgery, and dentistry, among others.2-7
In a survey of physicians in 4 primary care clinics, subjects rated 96 (15%) of 627 adult patients as “difficult.”8 Difficult patients were significantly more likely than others to have a mental disorder ( Table 1 ).8 They also had more functional impairment, higher health care utilization, and lower satisfaction with care.
A separate primary care clinic study found uncannily similar results—physicians rated 74 (15%) of 500 new walk-in patients as “difficult.”9 Compared with other patients, the difficult patients had:
- higher rates of psychiatric illness, somatization (>5 somatic complaints), and more severe symptoms
- poorer functional status, more unmet expectations, less satisfaction with care, and higher use of health services.
Fewer articles on difficult patients have been published in psychiatric literature, although some commonalities have emerged ( Box ).10-12 Often suffering from chronic conditions without well-defined treatment endpoints, difficult patients do worse clinically, have higher use of health services, and are less happy with their care than other patients.
Difficult patients challenge our competence as physicians and evoke personal distress. Physicians with less job satisfaction, less clinical experience, less training in counseling, and a poor attitude toward psychosocial problems are more likely to perceive a patient as difficult.13,14
Table 1
Common psychiatric disorders in difficult patients
| Multisomatoform disorder |
| Panic disorder |
| Dysthymia |
| Generalized anxiety disorder |
| Major depressive disorder |
| Alcohol abuse or dependence* |
| *Researchers categorized patients as having “probable” alcohol abuse or dependence but did not determine if they met DSM-IV-TR criteria for these disorders |
| Source: Reference 8 |
An Ovid Medline search of psychiatric literature for “difficult patients” found only 9 articles published from 1996 to 2008, and most were editorials or essays.10
Groves11 grouped difficult patients into 4 categories:
- dependent clingers
- entitled demanders
- manipulative help-rejecters
- self-destructive deniers.
For a description of the behaviors and personality traits associated with each of these 4 categories and strategies to address them, see “The nurse who worked the system,” Current Psychiatry, July 2009. Groves emphasized that a physician’s negative reactions evoked by such patients—once understood through introspection—may facilitate better understanding and psychological management in their care.
Hinshelwood12 wrote about the cognitive dissonance psychiatrists encounter when trying to balance the different responses evoked by patients with schizophrenia and severe personality disorders.
When confronted with a psychotic patient’s severely damaged reality testing, psychiatrists often depersonalize the patient in an effort to be “scientific.” Conversely, patients with severe personality disorders threaten the psychiatrist with their emotional instability. The psychiatrist loses the role of objective observer and instead becomes a “moral evaluator,” seeing the patient as “good” or “bad” instead of as a person in need of help.
Hinshelwood cautioned that patients such as this are difficult not because their treatment is complicated but because they challenge our identity as scientists and put us in personal difficulty.
Survival strategies for clinicians
Eight strategies can help improve your care of difficult patients ( Table 2 ).
Table 2
8 strategies for managing difficult patients
| 1. Acknowledge that the patient is difficult |
| 2. Develop empathy |
| 3. Seek out supervision/consultation |
| 4. Utilize a team approach |
| 5. Lower treatment goals |
| 6. Decompress the treatment timeline |
| 7. Use ‘plussing’ (positive comments and acknowledgements) |
| 8. Use imagery (visualize the patient as a character in an unfinished novel) |
2. Develop empathy. Empathy is identification with and understanding of why a person feels, thinks, and acts as he or she does. The best way to develop empathy for a difficult patient is to learn about him or her firsthand—directly from the patient, not from reading chart notes or from information passed among colleagues.
Learning about the patient firsthand means shifting from sign-and-symptom gathering to performing a genuine inquiry about how the person thinks or feels, including interests, loves, or background. Challenging clinical circumstances—such as seeing a patient in a busy emergency department or during a 15-minute medication check—can make this difficult. In some cases, however, the time needed to establish empathy can be surprisingly brief.
The more patients feel that the psychiatrist is “on their level,” the less likely they are to project internalized anguish or impulsively act on conflicted feelings.
3. Seek out supervision or consultation. You can gain new perspectives by taking a “step back” and looking at the case with a colleague. Seeking out consultation also allows you to decompress by “getting it off your chest.” Supervision often allows clinicians to develop:
- empathy toward a difficult patient
- increased energy and creativity in subsequent sessions.
If you cannot utilize a team to carry out treatment, this approach still may help you develop a treatment plan.
5. Lower treatment goals. The nature of difficult patients makes complete “cures” a rarity. A psychiatrist whose goal is to substantially help a patient may become chronically frustrated and feel inadequate in the face of a patient’s perpetual suffering. The clinician sometimes reacts by developing therapeutic nihilism and withdrawing energy from the case. The patient, of course, senses this and increases his or her general distress level, which intensifies the negative interaction.
By lowering goals—for example, aiming for stabilization rather than improvement—you can feel less like a failure and be more relaxed. A relaxed clinician is more tolerant and in a better position to help the patient. Other lowered goals might be to reduce harm from impulsive or dangerous behaviors instead of eliminating them or better coping with symptoms rather than symptom remission.
7. Use ‘plussing.’ Because we experience dread with difficult patients, clinicians often avoid, refrain from, or simply don’t see opportunities to use positive comments and acknowledgements (“plussing”) when they arise. Most patients (as well as clinicians) want to be liked, and small compliments—when genuinely and appropriately placed—sometimes can make a huge difference in patients’ willingness to cope or try new things.
This technique might allow you to see the humorous side of yourself as the hardworking, well-intentioned yet ineffectual psychiatrist. You don’t know how the story will unfold, but you can accept this as you would in any other unfinished novel.
CASE CONTINUED: A more effective approach
Dr. B realizes Ms. D is a difficult patient for him and takes the case into supervision. He is stunned when he is unable to answer several of his supervisor’s questions about Ms. D, including “What was her upbringing like?” and “What are her strengths or interests?” He realizes he knows little about Ms. D and becomes aware that he has focused most of their sessions on either fixing her immediate and never-ending crises or defending himself.
The supervisor points out that Dr. B’s lack of empathy for Ms. D keeps him from helping her—being anxious and defensive makes him less likely to be supportive or creative. Dr. B feels better after the supervision session. He experiences some catharsis and develops a plan to improve the situation.
Dr. B structures the next session to get to know Ms. D better. He mentally decompresses the treatment timeline and refocuses on the need to develop empathy instead of attempting to ameliorate symptoms. Dr. B begins by letting Ms. D know he wants to help her but doesn’t know much about her. She initially rejects his attempts at empathic communication, but with gentle persistence he learns about her upbringing and interests. Dr. B is able to genuinely compliment her on coping with previous traumas and begins to better understand her strengths. Over the next several weeks, Ms. D seems more able to accept supportive interventions and eventually begins a part-time job.
Related resources
- Colson DB. Difficult patients in extended psychiatric hospitalization: a research perspective on the patient, staff, and team. Psychiatry. 1990;53(4):369-382.
- Koekkoek B, van Meijel B, Hutschemaekers G. “Difficult patients” in mental health care: a review. Psychiatr Serv. 2006;57:795-802.
Dr. Battaglia reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. O’Dowd TC. Five years of heartsink patients in general practice. BMJ. 1988;297:528-530.
2. Smith HW. The difficult patient and doctor: origins and suggestions. Facial Plast Surg Clin North Am. 2008;16(2):177-178, vi.
3. Woods CD. The difficult patient: a psychodynamic perspective. J Calif Dent Assoc. 2007;35(3):186-191.
4. Müller-Lissner S. The difficult patient with constipation. Best Pract Res Clin Gastroenterol. 2007;21(3):473-484.
5. King K, Moss AH. The frequency and significance of the “difficult” patient: the nephrology community’s perceptions. Adv Chronic Kidney Dis. 2004;11(2):234-239.
6. Potter M, Gordon S, Hamer P. The difficult patient in private practice physiotherapy: a qualitative study. Aust J Physiother. 2003;49(1):53-61.
7. Fee C. Death of a difficult patient. Ann Emerg Med. 2001;37(3):354-355.
8. Hahn SR, Kroenke K, Spitzer RL, et al. The difficult patient: prevalence, psychopathology, and functional impairment. J Gen Intern Med. 1996;11(1):1-8.
9. Jackson JL, Kroenke K. Difficult patient encounters in the ambulatory clinic: clinical predictors and outcomes. Arch Intern Med. 1999;159(10):1069-1075.
10. Ovid Medline [database online]. New York, NY: Ovid Technologies, Inc; 2009.
11. Groves JE. Taking care of the hateful patient. N Engl J Med. 1978;298(16):883-887.
12. Hinshelwood RD. The difficult patient. The role of ‘scientific psychiatry’ in understanding patients with chronic schizophrenia or severe personality disorders. Br J Psychiatry. 1999;174:187-190.
13. Mathers N, Jones N, Hannay D. Heartsink patients: a study of their general practitioners. Br J Gen Pract. 1995;45(395):293-296.
14. Steinmetz D, Tabenkin H. The ‘difficult’ patient as perceived by family physicians. Fam Pract. 2001;18(5):495-500.
15. Maltsberger JT, Buie DH. Countertransference hate in the treatment of suicidal patients. Arch Gen Psychiatry. 1974;30(5):625-633.
1. O’Dowd TC. Five years of heartsink patients in general practice. BMJ. 1988;297:528-530.
2. Smith HW. The difficult patient and doctor: origins and suggestions. Facial Plast Surg Clin North Am. 2008;16(2):177-178, vi.
3. Woods CD. The difficult patient: a psychodynamic perspective. J Calif Dent Assoc. 2007;35(3):186-191.
4. Müller-Lissner S. The difficult patient with constipation. Best Pract Res Clin Gastroenterol. 2007;21(3):473-484.
5. King K, Moss AH. The frequency and significance of the “difficult” patient: the nephrology community’s perceptions. Adv Chronic Kidney Dis. 2004;11(2):234-239.
6. Potter M, Gordon S, Hamer P. The difficult patient in private practice physiotherapy: a qualitative study. Aust J Physiother. 2003;49(1):53-61.
7. Fee C. Death of a difficult patient. Ann Emerg Med. 2001;37(3):354-355.
8. Hahn SR, Kroenke K, Spitzer RL, et al. The difficult patient: prevalence, psychopathology, and functional impairment. J Gen Intern Med. 1996;11(1):1-8.
9. Jackson JL, Kroenke K. Difficult patient encounters in the ambulatory clinic: clinical predictors and outcomes. Arch Intern Med. 1999;159(10):1069-1075.
10. Ovid Medline [database online]. New York, NY: Ovid Technologies, Inc; 2009.
11. Groves JE. Taking care of the hateful patient. N Engl J Med. 1978;298(16):883-887.
12. Hinshelwood RD. The difficult patient. The role of ‘scientific psychiatry’ in understanding patients with chronic schizophrenia or severe personality disorders. Br J Psychiatry. 1999;174:187-190.
13. Mathers N, Jones N, Hannay D. Heartsink patients: a study of their general practitioners. Br J Gen Pract. 1995;45(395):293-296.
14. Steinmetz D, Tabenkin H. The ‘difficult’ patient as perceived by family physicians. Fam Pract. 2001;18(5):495-500.
15. Maltsberger JT, Buie DH. Countertransference hate in the treatment of suicidal patients. Arch Gen Psychiatry. 1974;30(5):625-633.
Life after near death: What interventions work for a suicide survivor?
Completed suicide provokes a multitude of questions: What motivated it? What interventions could have diverted it? Could anyone or anything have prevented it? The question of who dies by suicide often overshadows the question of what lessons suicide attempt (SA) survivors can teach us. Their story does not end with the attempt episode. For these patients, we have ongoing opportunities for interventions to make a difference.
A history of SA strongly predicts eventual completion, so we must try to identify which survivors will reattempt and complete suicide. This article addresses what is known about the psychiatry of suicide survivors—suicide motives and methods, clinical management, and short- and long-term outcomes—from the perspective that suicidality in this population may be a trait, with SA or deliberate self-harm (DSH) as its state-driven manifestations. When viewed in this manner, it is not just a question of who survives a suicide attempt, but who survives suicidality.
CASE REPORT: End of the game
Ms. T, age 39, was admitted to the intensive care unit after an aspirin overdose. She had been living with a man in a southern state for 8 years since the demise of her first marriage, but kept deferring remarriage. She returned to Minnesota with her teenage daughter to visit her family and stayed 6 months. Her partner phoned Ms. T every day, telling her he wanted her to come back. One day he tired of the game and said, “Fine, don’t come back.” She immediately overdosed, then called him to tell him what she’d done. He called her daughter, telling her to go check on her mother and to call 911. When later asked why she did it, Ms. T said, “So he would know how much he loved me.”
Motive for self-harm
Ms. T’s suicide attempt was nonlethal, and she reported it immediately—characteristics of parasuicidal gesturing as a motive. A useful categorization of suicidal behavior divides it into discrete categories or narratives. Gardner and Cowdry describe 4: true suicidal acts, parasuicidal gesturing, self-mutilation, and retributive rage.1 We modify this schema with 4 additional categories: altruism, acute shame, command hallucinations, and panic ( Table 1 ).1-3 Categories are differentiated by affective state, motivation, and goal of behavior, but all involve situations in which the individual feels a lack of other options and resorts to maladaptive strategies.
Although this classification scheme helps clinicians understand a patient’s mindset, the specific motive underpinning DSH or SA is not consistently linked to its lethality. True suicidal acts frequently are marked by careful planning and high-lethality methods that increase the risk of completed suicide, but any motive can lead to a lethal act, whether or not death was intended.2,3
Factors that increase the risk of SA and completed suicide include male gender, age (adolescent or age >60), low socioeconomic status, and alcohol or drug abuse.4 An underlying mood disorder accounts for 73% of attributable risk of suicide or medically serious SA in older adults.5 This connection between mood and suicidality highlights the concept that emotional pain can cause so much suffering that patients seek release from distress by ending their lives.
A useful model by Shneidman6 casts psychological pain as 1 dimension in a 3-dimensional system that includes press and perturbation. In this model:
- Pain refers to psychological pain (from little or no pain to intolerable agony).
- Press means actual or imagined events in the inner or outer world that cause a person to react. It ranges from positive press (good fortune, happy events, protective factors) to negative press (stressors, failures, losses, persecution), which in turn decrease or increase the likelihood of suicide.
- Perturbation refers to the state of being disturbed or upset.
Certain risk factors make SA simultaneously more likely to occur but less likely to be lethal. For example, parental discord, nonheterosexual orientation, and female gender have been found to increase non-fatal attempts among adolescents.7 Borderline personality disorder increases the reattempt rate out of proportion to completion among adults.8 One might interpret a pattern of repeated nonlethal attempts to mean the patient has no real intent to die, but this is not always the case.
8
Table 1
8 categories or narratives of suicidal behavior
| Motive | Characteristics |
|---|---|
| True suicidal act | Release from intense baseline despair/hopelessness; self-nihilism as a permanent end to internal pain (entails highest intent to die and highest risk of completed suicide) |
| Self-mutilation | Relieving dysphoria or dissociation/depersonalization; acts of DSH designed to self-regulate or distract from emotional pain or other overwhelming affects |
| Retributive rage | Revenge; impulsiveness, vengefulness, and reduced capacity to conceive of other immediate options |
| Parasuicidal gesturing | Communication designed to extract a response from a significant other; often repetitive acts of DSH, strong dependency needs |
| Acute shame | Penance designed to escape from or to atone for a shameful act; often occurs within a short time after act is committed |
| Altruism | Relief of real or imagined burden on others; often occurs in setting of medical illness or substantial financial concerns |
| Command hallucinations | Acting in compliance with a command hallucination; often in setting of schizophrenia or depression with psychotic features |
| Panic | Driven by agitation, psychic anxiety, and/or panic attack; action intended as escape from real or imagined factor provoking agitation |
| DSH: deliberate self-harm | |
| Source: References 1-3 | |
CASE REPORT: Caught in the act
Mrs. L, age 35, works at a nail salon and took $12 from the cash register to buy gas so she could visit her husband in the next town. She’d never done anything like that before. She planned to return the money the next day, but her act was captured by a security camera and reported before she had a chance. Her boss said she had to go to the police.
Mrs. L was so ashamed that she decided she wanted to die. She drove her car to a remote hunting area where she tried to shoot herself in the head. The gun bucked, however, and shot her in the shoulder instead. She climbed into the front seat and drove herself to the hospital.
Method of self-harm
Survival of a suicide attempt depends in part on the lethality of the suicide method. Although she survived, Mrs. L’s attempt was intended to be quite lethal and illustrates shame as a motive.
The method’s lethality does not always correlate with the intent to die.9 Attempters with the highest suicidal intent do not reliably choose the most lethal method, either because they overestimate the lethality of methods such as cutting or overdose or because less lethal methods were most accessible.
Firearms, which are both accessible and lethal, remain the most common and deadly method in the United States, with more suicides from gunshot than all other methods combined.13 Cultural factors also are involved, such as in India where poisoning (especially with readily available organophosphates) is more common than gunshot.14 Suicidality screening in psychiatric practice and in the emergency department should always include questioning about convenient access to lethal means, especially those commonly used among the local population.
Clinical management
Treatment goals for patients who have demonstrated suicidal behavior may include decreasing the occurrence of suicidal thoughts, plans, gestures, or attempts. At a population level, accepted management strategies include:
- psychotherapy (cognitive-behavioral therapy [CBT], dialectical behavioral therapy)
- contracts for safety (widely employed but lacking evidence of efficacy)
- medications that target underlying disorders (antidepressants, mood stabilizers, antipsychotics).
Ineffective interventions? A study examining suicide trends since 1990 in the United States18 found disheartening evidence that although treatment dramatically increased, the incidence of suicidal thoughts, plans, gestures, or attempts did not significantly decrease ( Box 1 ).18–26 Based on a systematic review of 15 randomized controlled trials, Arensman et al19 offered 2 explanations for why studies of various psychosocial and pharmacologic interventions showed no significant effect on suicidality compared with usual care:
- the intervention had a negligible effect on patient outcomes
- the sample size was too small to detect clinically important differences in reattempt rates.
Suicide research deploys a single intervention for a diverse group of subjects rather than tailoring the approach to each particular case. A certain intervention may be highly effective for 1 patient because it is well matched to the specific blend of issues driving that patient’s suicidality, yet ineffective for another because it fails to address that individual’s underlying issues. Thus, a single treatment program standardized for research can be simultaneously a success and a failure, depending on which patient is assessed. The overall outcome is statistical insignificance because success is lost in the noise of failure.
Treating the individual. To individualize your treatment approach, it may be useful to recast the case and treatment strategy into Shneidman’s cubic model.6 Identifying the uniquely personal drivers behind a patient’s thoughts and actions helps point toward the most effective management approach. Tailored pharmacologic treatments and psychotherapy can be used to help guide the patient away from maximum suicide risk.
Table 2
Symptom-targeted pharmacologic treatment of suicidal patients
| Drug class | Impulsive-behavioral dyscontrol | Affective dysregulation | Psychotic features |
|---|---|---|---|
| SSRIs | Self-damaging behavior, impulsivity | Mood lability/mood crashes; anger; temper outbursts | |
| Antipsychotics | Anger, temper outbursts | Cognitive symptoms; perceptual symptoms | |
| Mood stabilizers (lithium, carbamazepine, valproic acid) | Self-damaging behavior, impulsivity | Mood lability/mood crashes, anger, temper outbursts | |
| SSRI: selective serotonin reuptake inhibitor | |||
| Source: References 16,17 | |||
Treatment of suicide attempt survivors has dramatically increased in the United States since 1990, but the incidence of suicidal thoughts, plans, gestures, or attempts has not significantly decreased.18 A systematic review of 15 randomized controlled trials using the search methods published by Arensman et al19 reveals very little difference in the suicide reattempt rate, despite extra treatment beyond the “usual standard of care.”
Intervention strategies shown to significantly decrease the rate of self-harm include home visits, behavioral therapy, and a “green card” strategy (patients were issued a card at the time of discharge explaining that a doctor was always available for them and how that doctor could be contacted).20-23
No significant difference in reattempt rate was found with other strategies, although benefits such as lower rates of depression and suicidal ideation or higher outpatient visit attendance were observed in some trials.24-26 Click here for a summary of the studies’ methodologies and results.
Outcomes of self-harm
When considering outcomes of SA, it is important to separate the short-term outcome of a single SA from the long-term outcome of suicidality. Short-term outcome depends on the characteristics and management of the acute episode, whereas long-term encompasses ongoing management of suicidality as a trait.
In the short term, surviving a SA depends heavily on the lethality of method and access to acute treatment. It also depends on medical fitness to withstand injury, which may help account for the higher death rate among elderly suicide attempters. A frail or medically ill person is less likely to survive the bodily insult of a SA.
Long-term outcomes are harder to predict. Some patients’ index attempts result from a transient state—an isolated incident that never will be repeated. In others, suicidality is a trait—a chronic maladaptive pattern that is potentially lethal. After an index attempt, the most reliable predictors for eventual death by suicide are:
- diagnosed mental illness
- high-lethality method on the index SA
- number of reattempts.4
Mood disorders impact long-term outcome, yet only a limited number of studies have found a reduction in suicide rates in response to mood disorder treatment. In a 44-year follow-up study, long-term treatment of depression and bipolar disorder with lithium significantly reduced the suicide rate.30 A meta-analysis of recurrent major affective disorder studies found that subjects on lithium maintenance treatment were 15 times less likely to commit suicidal acts, compared with those not on lithium.31
An important confounding factor in these findings is that effective lithium treatment requires long-term adherence, which implies a long-term doctor-patient relationship. As Cipriani et al32 noted, patients who can maintain an ongoing therapeutic relationship may be “less disturbed” than those who cannot, making them less likely to kill themselves regardless of pharmacologic treatment. Furthermore, patient interviews reveal that the therapeutic alliance created by a continuous relationship can be a protective support against further SA.33
Clinical implications
Suicide survivors often continue to struggle with suicidality well beyond the index attempt. This suicidality is a maladaptive problem-solving method that functions as a chronic morbid illness. As such, it is not enough to analyze the phenomenon of surviving an SA; one must examine the ongoing process of surviving suicidality.
Consider 3 factors. Consider all 3 factors— motive, method, and management—when addressing suicide survivorship.
Method lethality significantly influences survival likelihood. In clinical practice, we have observed that the index attempt is a learning experience for some patients that will inform their choice of method on the next attempt. When interacting with a suicide survivor, carefully assess the reasoning behind their initial choice of method and whether it has evolved toward higher lethality since the index attempt.
Management recommendations after SA continue to evolve. Risk factor management—such as treating underlying mood disorders, home visits to reduce social isolation, and prioritized “green card” contact with psychiatrists—has been shown to decrease reattempt rates, but many other interventions have not shown the expected benefit. Increased intervention rates have not yielded proportional decreases in suicidal ideation, attempts, or completion.
Suicide survivors often continue to struggle with suicidality well beyond the index attempt
Consider the SA motive and method when planning how to manage the survivor
Method lethality significantly influences survival likelihood (firearms are the most common and deadly method in the United States)
In many clinical trials, the incidence of suicidal thoughts, plans, gestures, or attempts has not significantly decreased when SA survivors received extra treatment
Management recommendations after SA continue to evolve; effective techniques appear to be keeping lines of communication open and providing individualized treatment
Individualize pharmacologic treatments and psychotherapy to help guide the patient away from maximum suicide risk
SA: suicide attempt
- American Association of Suicidology. www.suicidology.org.
- American Foundation for Suicide Prevention. www.afsp.org.
- Mayo Clinic Patient/Family Education. Suicide: What to do when someone is suicidal. www.mayoclinic.com/health/suicide/MH00058.
- Carbamazepine • Carbatrol
- Lithium • Eskalith, Lithobid
- Valproic acid • Depakene, Depakote
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Gardner DL, Cowdry RW. Suicidal and parasuicidal behavior in borderline personality disorder. Psychiatr Clin North Am. 1985;8(2):389-403.
2. Bostwick J, Levenson J. Suicidality. In: Levenson J, ed. The American Psychiatric Publishing textbook of psychosomatic medicine. Arlington, VA: American Psychiatric Publishing, Inc; 2004:219-234.
3. Bostwick JM, Cohen LM. Differentiating suicide from life-ending acts and end-of-life decisions: a model based on chronic kidney disease and dialysis. Psychosomatics. 2009;50(1):1-7.
4. Jeglic EL. Will my patient attempt suicide again? Current Psychiatry. 2008;7(11):19-28.
5. Beautrais A. A case control study of suicide and attempted suicide. Suicide Life Threat Behav. 2002;32(1):1-9.
6. Shneidman E. Overview: a multidimensional approach to suicide. In: Jacobs D, Brown H, eds. Suicide: understanding and responding. Madison, WI: International Universities Press; 1989:1-20.
7. Beautrais AL. Risk factors for suicide and attempted suicide among young people. Aust N Z J Psychiatry. 2000;34:420-436.
8. Black DW, Blum N, Pfohl B, et al. Suicidal behavior in borderline personality disorder: prevalence, risk factors, prediction, and prevention. J Pers Disord. 2004;18(3):226-239.
9. Plutchik R, van Praag HM, Picard S, et al. Is there a relation between the seriousness of suicidal intent and the lethality of suicide attempt? Psychiatric Res. 1988;27:71-79.
10. Schernhammer ES, Colditz GA. Suicide rates among physicians: a quantitative and gender assessment (meta-analysis). Am J Psychiatry. 2004;161(12):2295-2302.
11. Recupero PR, Harms SE, Noble JM. Googling suicide: surfing for suicide information on the internet. J Clin Psychiatry. 2008;69(6):878-888.
12. Gibb SJ, Beautrais AL, Fergusson DM. Mortality and further suicidal behaviour after an index suicide attempt: a 10-year study. Aust N Z J Psychiatry. 2005;39:95-100.
13. Miller M, Hemenway D. Guns and suicide in the United States. N Engl J Med. 2008;359(10):989-991.
14. Latha KS, Bhat SM, D’Souza P. Suicide attempters in general hospital unit in India: their socio-demographic and clinical profile—emphasis on cross-cultural aspects. Acta Psychiatra Scand. 1996;94(1):26-30.
15. Yildiz A, Sachs GS, Turgay A. Pharmacological management of agitation in emergency settings. Emerg Med J. 2003;20(4):339-346.
16. American Psychiatric Association practice guidelines. Treatment of patients with borderline personality disorder. 2001. Available at: http://www.psychiatryonline.com/pracGuide/pracGuideTopic_13.aspx. Accessed July 8, 2009.
17. Zanarini MC. Update on pharmacotherapy of borderline personality disorder. Curr Psychiatry Rep. 2004;6(1):66-70.
18. Kessler RC, Berglund P, Borges G, et al. Trends in suicide ideation, plans, gestures, and attempts in the United States, 1990-1992 to 2001-2003. JAMA. 2005;293(20):2487-2495.
19. Arensman E, Townsend E, Hawton K, et al. Psychosocial and pharmacological treatment of patients following deliberate self-harm: the methodological issues involved in evaluating effectiveness. Suicide Life Threat Behav. 2001;31(2):169-180.
20. Welu TC. A follow-up program for suicide attempters: evaluation of effectiveness. Suicide Life Threat Behav. 1977;7(1):17-20.
21. Linehan MM, Armstrong HE, Suarez A, et al. Cognitive-behavioral treatment of chronically parasuicidal borderline patients. Arch Gen Psychiatry. 1991;48(12):1060-1064.
22. McLeavey B, Daly R, Ludgate J, et al. Interpersonal problem-solving skills training in the treatment of self-poisoning patients. Suicide Life Threat Behav. 1994;24(4):382-394.
23. Morgan HG, Jones EM, Owen JH. Secondary prevention of non-fatal deliberate self-harm. The green card study. Br J Psychiatry. 1993;163:111-112.
24. Salkovskis PM, Atha C, Storer D. Cognitive-behavioural problem solving in the treatment of patients who repeatedly attempt suicide. A controlled trial. Br J Psychiatry. 1990;157:871-876.
25. Hirsch SR, Walsh C, Draper R. Parasuicide. A review of treatment interventions. J Affect Disord. 1982;4(4):299-311.
26. Hawton K, Bancroft J, Catalan J, et al. Domiciliary and outpatient treatment of self-poisoning patients by medical and non-medical staff. Psychol Med. 1981;11(1):169-177.
27. Brown GK, Ten Have T, Henriques GR, et al. Cognitive therapy for the prevention of suicide attempts: a randomized controlled trial. JAMA. 2005;294(5):563-570.
28. Bateman A, Fonagy P. 8-year follow-up of patients treated for borderline personality disorder: mentalization-based treatment versus treatment as usual. Am J Psychiatry. 2008;165(5):631-638.
29. Hawton K, Zahl D, Weatherall R. Suicide following deliberate self-harm: long-term follow-up of patients who presented to a general hospital. Br J Psychiatry. 2003;182:537-542.
30. Angst J, Angst F, Gerber-Werder R, et al. Suicide in 406 mood-disorder patients with and without long-term medication: a 40 to 44 years’ follow-up. Arch Suicide Res. 2005;9(3):279-300.
31. Baldessarini RJ, Tondo L, Hennen J. Lithium treatment and suicide risk in major affective disorders: update and new findings. J Clin Psychiatry. 2003;64(suppl 5):44-52.
32. Cipriani A, Pretty H, Hawton K, et al. Lithium in the prevention of suicidal behavior and all-cause mortality in patients with mood disorders: a systematic review of randomized trials. Am J Psychiatry. 2005;162(10):1805-1819.
33. Sinclair J, Green J. Understanding resolution of deliberate self harm: qualitative interview study of patients’ experiences. BMJ. 2005;330(7500):1112.-
Completed suicide provokes a multitude of questions: What motivated it? What interventions could have diverted it? Could anyone or anything have prevented it? The question of who dies by suicide often overshadows the question of what lessons suicide attempt (SA) survivors can teach us. Their story does not end with the attempt episode. For these patients, we have ongoing opportunities for interventions to make a difference.
A history of SA strongly predicts eventual completion, so we must try to identify which survivors will reattempt and complete suicide. This article addresses what is known about the psychiatry of suicide survivors—suicide motives and methods, clinical management, and short- and long-term outcomes—from the perspective that suicidality in this population may be a trait, with SA or deliberate self-harm (DSH) as its state-driven manifestations. When viewed in this manner, it is not just a question of who survives a suicide attempt, but who survives suicidality.
CASE REPORT: End of the game
Ms. T, age 39, was admitted to the intensive care unit after an aspirin overdose. She had been living with a man in a southern state for 8 years since the demise of her first marriage, but kept deferring remarriage. She returned to Minnesota with her teenage daughter to visit her family and stayed 6 months. Her partner phoned Ms. T every day, telling her he wanted her to come back. One day he tired of the game and said, “Fine, don’t come back.” She immediately overdosed, then called him to tell him what she’d done. He called her daughter, telling her to go check on her mother and to call 911. When later asked why she did it, Ms. T said, “So he would know how much he loved me.”
Motive for self-harm
Ms. T’s suicide attempt was nonlethal, and she reported it immediately—characteristics of parasuicidal gesturing as a motive. A useful categorization of suicidal behavior divides it into discrete categories or narratives. Gardner and Cowdry describe 4: true suicidal acts, parasuicidal gesturing, self-mutilation, and retributive rage.1 We modify this schema with 4 additional categories: altruism, acute shame, command hallucinations, and panic ( Table 1 ).1-3 Categories are differentiated by affective state, motivation, and goal of behavior, but all involve situations in which the individual feels a lack of other options and resorts to maladaptive strategies.
Although this classification scheme helps clinicians understand a patient’s mindset, the specific motive underpinning DSH or SA is not consistently linked to its lethality. True suicidal acts frequently are marked by careful planning and high-lethality methods that increase the risk of completed suicide, but any motive can lead to a lethal act, whether or not death was intended.2,3
Factors that increase the risk of SA and completed suicide include male gender, age (adolescent or age >60), low socioeconomic status, and alcohol or drug abuse.4 An underlying mood disorder accounts for 73% of attributable risk of suicide or medically serious SA in older adults.5 This connection between mood and suicidality highlights the concept that emotional pain can cause so much suffering that patients seek release from distress by ending their lives.
A useful model by Shneidman6 casts psychological pain as 1 dimension in a 3-dimensional system that includes press and perturbation. In this model:
- Pain refers to psychological pain (from little or no pain to intolerable agony).
- Press means actual or imagined events in the inner or outer world that cause a person to react. It ranges from positive press (good fortune, happy events, protective factors) to negative press (stressors, failures, losses, persecution), which in turn decrease or increase the likelihood of suicide.
- Perturbation refers to the state of being disturbed or upset.
Certain risk factors make SA simultaneously more likely to occur but less likely to be lethal. For example, parental discord, nonheterosexual orientation, and female gender have been found to increase non-fatal attempts among adolescents.7 Borderline personality disorder increases the reattempt rate out of proportion to completion among adults.8 One might interpret a pattern of repeated nonlethal attempts to mean the patient has no real intent to die, but this is not always the case.
8
Table 1
8 categories or narratives of suicidal behavior
| Motive | Characteristics |
|---|---|
| True suicidal act | Release from intense baseline despair/hopelessness; self-nihilism as a permanent end to internal pain (entails highest intent to die and highest risk of completed suicide) |
| Self-mutilation | Relieving dysphoria or dissociation/depersonalization; acts of DSH designed to self-regulate or distract from emotional pain or other overwhelming affects |
| Retributive rage | Revenge; impulsiveness, vengefulness, and reduced capacity to conceive of other immediate options |
| Parasuicidal gesturing | Communication designed to extract a response from a significant other; often repetitive acts of DSH, strong dependency needs |
| Acute shame | Penance designed to escape from or to atone for a shameful act; often occurs within a short time after act is committed |
| Altruism | Relief of real or imagined burden on others; often occurs in setting of medical illness or substantial financial concerns |
| Command hallucinations | Acting in compliance with a command hallucination; often in setting of schizophrenia or depression with psychotic features |
| Panic | Driven by agitation, psychic anxiety, and/or panic attack; action intended as escape from real or imagined factor provoking agitation |
| DSH: deliberate self-harm | |
| Source: References 1-3 | |
CASE REPORT: Caught in the act
Mrs. L, age 35, works at a nail salon and took $12 from the cash register to buy gas so she could visit her husband in the next town. She’d never done anything like that before. She planned to return the money the next day, but her act was captured by a security camera and reported before she had a chance. Her boss said she had to go to the police.
Mrs. L was so ashamed that she decided she wanted to die. She drove her car to a remote hunting area where she tried to shoot herself in the head. The gun bucked, however, and shot her in the shoulder instead. She climbed into the front seat and drove herself to the hospital.
Method of self-harm
Survival of a suicide attempt depends in part on the lethality of the suicide method. Although she survived, Mrs. L’s attempt was intended to be quite lethal and illustrates shame as a motive.
The method’s lethality does not always correlate with the intent to die.9 Attempters with the highest suicidal intent do not reliably choose the most lethal method, either because they overestimate the lethality of methods such as cutting or overdose or because less lethal methods were most accessible.
Firearms, which are both accessible and lethal, remain the most common and deadly method in the United States, with more suicides from gunshot than all other methods combined.13 Cultural factors also are involved, such as in India where poisoning (especially with readily available organophosphates) is more common than gunshot.14 Suicidality screening in psychiatric practice and in the emergency department should always include questioning about convenient access to lethal means, especially those commonly used among the local population.
Clinical management
Treatment goals for patients who have demonstrated suicidal behavior may include decreasing the occurrence of suicidal thoughts, plans, gestures, or attempts. At a population level, accepted management strategies include:
- psychotherapy (cognitive-behavioral therapy [CBT], dialectical behavioral therapy)
- contracts for safety (widely employed but lacking evidence of efficacy)
- medications that target underlying disorders (antidepressants, mood stabilizers, antipsychotics).
Ineffective interventions? A study examining suicide trends since 1990 in the United States18 found disheartening evidence that although treatment dramatically increased, the incidence of suicidal thoughts, plans, gestures, or attempts did not significantly decrease ( Box 1 ).18–26 Based on a systematic review of 15 randomized controlled trials, Arensman et al19 offered 2 explanations for why studies of various psychosocial and pharmacologic interventions showed no significant effect on suicidality compared with usual care:
- the intervention had a negligible effect on patient outcomes
- the sample size was too small to detect clinically important differences in reattempt rates.
Suicide research deploys a single intervention for a diverse group of subjects rather than tailoring the approach to each particular case. A certain intervention may be highly effective for 1 patient because it is well matched to the specific blend of issues driving that patient’s suicidality, yet ineffective for another because it fails to address that individual’s underlying issues. Thus, a single treatment program standardized for research can be simultaneously a success and a failure, depending on which patient is assessed. The overall outcome is statistical insignificance because success is lost in the noise of failure.
Treating the individual. To individualize your treatment approach, it may be useful to recast the case and treatment strategy into Shneidman’s cubic model.6 Identifying the uniquely personal drivers behind a patient’s thoughts and actions helps point toward the most effective management approach. Tailored pharmacologic treatments and psychotherapy can be used to help guide the patient away from maximum suicide risk.
Table 2
Symptom-targeted pharmacologic treatment of suicidal patients
| Drug class | Impulsive-behavioral dyscontrol | Affective dysregulation | Psychotic features |
|---|---|---|---|
| SSRIs | Self-damaging behavior, impulsivity | Mood lability/mood crashes; anger; temper outbursts | |
| Antipsychotics | Anger, temper outbursts | Cognitive symptoms; perceptual symptoms | |
| Mood stabilizers (lithium, carbamazepine, valproic acid) | Self-damaging behavior, impulsivity | Mood lability/mood crashes, anger, temper outbursts | |
| SSRI: selective serotonin reuptake inhibitor | |||
| Source: References 16,17 | |||
Treatment of suicide attempt survivors has dramatically increased in the United States since 1990, but the incidence of suicidal thoughts, plans, gestures, or attempts has not significantly decreased.18 A systematic review of 15 randomized controlled trials using the search methods published by Arensman et al19 reveals very little difference in the suicide reattempt rate, despite extra treatment beyond the “usual standard of care.”
Intervention strategies shown to significantly decrease the rate of self-harm include home visits, behavioral therapy, and a “green card” strategy (patients were issued a card at the time of discharge explaining that a doctor was always available for them and how that doctor could be contacted).20-23
No significant difference in reattempt rate was found with other strategies, although benefits such as lower rates of depression and suicidal ideation or higher outpatient visit attendance were observed in some trials.24-26 Click here for a summary of the studies’ methodologies and results.
Outcomes of self-harm
When considering outcomes of SA, it is important to separate the short-term outcome of a single SA from the long-term outcome of suicidality. Short-term outcome depends on the characteristics and management of the acute episode, whereas long-term encompasses ongoing management of suicidality as a trait.
In the short term, surviving a SA depends heavily on the lethality of method and access to acute treatment. It also depends on medical fitness to withstand injury, which may help account for the higher death rate among elderly suicide attempters. A frail or medically ill person is less likely to survive the bodily insult of a SA.
Long-term outcomes are harder to predict. Some patients’ index attempts result from a transient state—an isolated incident that never will be repeated. In others, suicidality is a trait—a chronic maladaptive pattern that is potentially lethal. After an index attempt, the most reliable predictors for eventual death by suicide are:
- diagnosed mental illness
- high-lethality method on the index SA
- number of reattempts.4
Mood disorders impact long-term outcome, yet only a limited number of studies have found a reduction in suicide rates in response to mood disorder treatment. In a 44-year follow-up study, long-term treatment of depression and bipolar disorder with lithium significantly reduced the suicide rate.30 A meta-analysis of recurrent major affective disorder studies found that subjects on lithium maintenance treatment were 15 times less likely to commit suicidal acts, compared with those not on lithium.31
An important confounding factor in these findings is that effective lithium treatment requires long-term adherence, which implies a long-term doctor-patient relationship. As Cipriani et al32 noted, patients who can maintain an ongoing therapeutic relationship may be “less disturbed” than those who cannot, making them less likely to kill themselves regardless of pharmacologic treatment. Furthermore, patient interviews reveal that the therapeutic alliance created by a continuous relationship can be a protective support against further SA.33
Clinical implications
Suicide survivors often continue to struggle with suicidality well beyond the index attempt. This suicidality is a maladaptive problem-solving method that functions as a chronic morbid illness. As such, it is not enough to analyze the phenomenon of surviving an SA; one must examine the ongoing process of surviving suicidality.
Consider 3 factors. Consider all 3 factors— motive, method, and management—when addressing suicide survivorship.
Method lethality significantly influences survival likelihood. In clinical practice, we have observed that the index attempt is a learning experience for some patients that will inform their choice of method on the next attempt. When interacting with a suicide survivor, carefully assess the reasoning behind their initial choice of method and whether it has evolved toward higher lethality since the index attempt.
Management recommendations after SA continue to evolve. Risk factor management—such as treating underlying mood disorders, home visits to reduce social isolation, and prioritized “green card” contact with psychiatrists—has been shown to decrease reattempt rates, but many other interventions have not shown the expected benefit. Increased intervention rates have not yielded proportional decreases in suicidal ideation, attempts, or completion.
Suicide survivors often continue to struggle with suicidality well beyond the index attempt
Consider the SA motive and method when planning how to manage the survivor
Method lethality significantly influences survival likelihood (firearms are the most common and deadly method in the United States)
In many clinical trials, the incidence of suicidal thoughts, plans, gestures, or attempts has not significantly decreased when SA survivors received extra treatment
Management recommendations after SA continue to evolve; effective techniques appear to be keeping lines of communication open and providing individualized treatment
Individualize pharmacologic treatments and psychotherapy to help guide the patient away from maximum suicide risk
SA: suicide attempt
- American Association of Suicidology. www.suicidology.org.
- American Foundation for Suicide Prevention. www.afsp.org.
- Mayo Clinic Patient/Family Education. Suicide: What to do when someone is suicidal. www.mayoclinic.com/health/suicide/MH00058.
- Carbamazepine • Carbatrol
- Lithium • Eskalith, Lithobid
- Valproic acid • Depakene, Depakote
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Completed suicide provokes a multitude of questions: What motivated it? What interventions could have diverted it? Could anyone or anything have prevented it? The question of who dies by suicide often overshadows the question of what lessons suicide attempt (SA) survivors can teach us. Their story does not end with the attempt episode. For these patients, we have ongoing opportunities for interventions to make a difference.
A history of SA strongly predicts eventual completion, so we must try to identify which survivors will reattempt and complete suicide. This article addresses what is known about the psychiatry of suicide survivors—suicide motives and methods, clinical management, and short- and long-term outcomes—from the perspective that suicidality in this population may be a trait, with SA or deliberate self-harm (DSH) as its state-driven manifestations. When viewed in this manner, it is not just a question of who survives a suicide attempt, but who survives suicidality.
CASE REPORT: End of the game
Ms. T, age 39, was admitted to the intensive care unit after an aspirin overdose. She had been living with a man in a southern state for 8 years since the demise of her first marriage, but kept deferring remarriage. She returned to Minnesota with her teenage daughter to visit her family and stayed 6 months. Her partner phoned Ms. T every day, telling her he wanted her to come back. One day he tired of the game and said, “Fine, don’t come back.” She immediately overdosed, then called him to tell him what she’d done. He called her daughter, telling her to go check on her mother and to call 911. When later asked why she did it, Ms. T said, “So he would know how much he loved me.”
Motive for self-harm
Ms. T’s suicide attempt was nonlethal, and she reported it immediately—characteristics of parasuicidal gesturing as a motive. A useful categorization of suicidal behavior divides it into discrete categories or narratives. Gardner and Cowdry describe 4: true suicidal acts, parasuicidal gesturing, self-mutilation, and retributive rage.1 We modify this schema with 4 additional categories: altruism, acute shame, command hallucinations, and panic ( Table 1 ).1-3 Categories are differentiated by affective state, motivation, and goal of behavior, but all involve situations in which the individual feels a lack of other options and resorts to maladaptive strategies.
Although this classification scheme helps clinicians understand a patient’s mindset, the specific motive underpinning DSH or SA is not consistently linked to its lethality. True suicidal acts frequently are marked by careful planning and high-lethality methods that increase the risk of completed suicide, but any motive can lead to a lethal act, whether or not death was intended.2,3
Factors that increase the risk of SA and completed suicide include male gender, age (adolescent or age >60), low socioeconomic status, and alcohol or drug abuse.4 An underlying mood disorder accounts for 73% of attributable risk of suicide or medically serious SA in older adults.5 This connection between mood and suicidality highlights the concept that emotional pain can cause so much suffering that patients seek release from distress by ending their lives.
A useful model by Shneidman6 casts psychological pain as 1 dimension in a 3-dimensional system that includes press and perturbation. In this model:
- Pain refers to psychological pain (from little or no pain to intolerable agony).
- Press means actual or imagined events in the inner or outer world that cause a person to react. It ranges from positive press (good fortune, happy events, protective factors) to negative press (stressors, failures, losses, persecution), which in turn decrease or increase the likelihood of suicide.
- Perturbation refers to the state of being disturbed or upset.
Certain risk factors make SA simultaneously more likely to occur but less likely to be lethal. For example, parental discord, nonheterosexual orientation, and female gender have been found to increase non-fatal attempts among adolescents.7 Borderline personality disorder increases the reattempt rate out of proportion to completion among adults.8 One might interpret a pattern of repeated nonlethal attempts to mean the patient has no real intent to die, but this is not always the case.
8
Table 1
8 categories or narratives of suicidal behavior
| Motive | Characteristics |
|---|---|
| True suicidal act | Release from intense baseline despair/hopelessness; self-nihilism as a permanent end to internal pain (entails highest intent to die and highest risk of completed suicide) |
| Self-mutilation | Relieving dysphoria or dissociation/depersonalization; acts of DSH designed to self-regulate or distract from emotional pain or other overwhelming affects |
| Retributive rage | Revenge; impulsiveness, vengefulness, and reduced capacity to conceive of other immediate options |
| Parasuicidal gesturing | Communication designed to extract a response from a significant other; often repetitive acts of DSH, strong dependency needs |
| Acute shame | Penance designed to escape from or to atone for a shameful act; often occurs within a short time after act is committed |
| Altruism | Relief of real or imagined burden on others; often occurs in setting of medical illness or substantial financial concerns |
| Command hallucinations | Acting in compliance with a command hallucination; often in setting of schizophrenia or depression with psychotic features |
| Panic | Driven by agitation, psychic anxiety, and/or panic attack; action intended as escape from real or imagined factor provoking agitation |
| DSH: deliberate self-harm | |
| Source: References 1-3 | |
CASE REPORT: Caught in the act
Mrs. L, age 35, works at a nail salon and took $12 from the cash register to buy gas so she could visit her husband in the next town. She’d never done anything like that before. She planned to return the money the next day, but her act was captured by a security camera and reported before she had a chance. Her boss said she had to go to the police.
Mrs. L was so ashamed that she decided she wanted to die. She drove her car to a remote hunting area where she tried to shoot herself in the head. The gun bucked, however, and shot her in the shoulder instead. She climbed into the front seat and drove herself to the hospital.
Method of self-harm
Survival of a suicide attempt depends in part on the lethality of the suicide method. Although she survived, Mrs. L’s attempt was intended to be quite lethal and illustrates shame as a motive.
The method’s lethality does not always correlate with the intent to die.9 Attempters with the highest suicidal intent do not reliably choose the most lethal method, either because they overestimate the lethality of methods such as cutting or overdose or because less lethal methods were most accessible.
Firearms, which are both accessible and lethal, remain the most common and deadly method in the United States, with more suicides from gunshot than all other methods combined.13 Cultural factors also are involved, such as in India where poisoning (especially with readily available organophosphates) is more common than gunshot.14 Suicidality screening in psychiatric practice and in the emergency department should always include questioning about convenient access to lethal means, especially those commonly used among the local population.
Clinical management
Treatment goals for patients who have demonstrated suicidal behavior may include decreasing the occurrence of suicidal thoughts, plans, gestures, or attempts. At a population level, accepted management strategies include:
- psychotherapy (cognitive-behavioral therapy [CBT], dialectical behavioral therapy)
- contracts for safety (widely employed but lacking evidence of efficacy)
- medications that target underlying disorders (antidepressants, mood stabilizers, antipsychotics).
Ineffective interventions? A study examining suicide trends since 1990 in the United States18 found disheartening evidence that although treatment dramatically increased, the incidence of suicidal thoughts, plans, gestures, or attempts did not significantly decrease ( Box 1 ).18–26 Based on a systematic review of 15 randomized controlled trials, Arensman et al19 offered 2 explanations for why studies of various psychosocial and pharmacologic interventions showed no significant effect on suicidality compared with usual care:
- the intervention had a negligible effect on patient outcomes
- the sample size was too small to detect clinically important differences in reattempt rates.
Suicide research deploys a single intervention for a diverse group of subjects rather than tailoring the approach to each particular case. A certain intervention may be highly effective for 1 patient because it is well matched to the specific blend of issues driving that patient’s suicidality, yet ineffective for another because it fails to address that individual’s underlying issues. Thus, a single treatment program standardized for research can be simultaneously a success and a failure, depending on which patient is assessed. The overall outcome is statistical insignificance because success is lost in the noise of failure.
Treating the individual. To individualize your treatment approach, it may be useful to recast the case and treatment strategy into Shneidman’s cubic model.6 Identifying the uniquely personal drivers behind a patient’s thoughts and actions helps point toward the most effective management approach. Tailored pharmacologic treatments and psychotherapy can be used to help guide the patient away from maximum suicide risk.
Table 2
Symptom-targeted pharmacologic treatment of suicidal patients
| Drug class | Impulsive-behavioral dyscontrol | Affective dysregulation | Psychotic features |
|---|---|---|---|
| SSRIs | Self-damaging behavior, impulsivity | Mood lability/mood crashes; anger; temper outbursts | |
| Antipsychotics | Anger, temper outbursts | Cognitive symptoms; perceptual symptoms | |
| Mood stabilizers (lithium, carbamazepine, valproic acid) | Self-damaging behavior, impulsivity | Mood lability/mood crashes, anger, temper outbursts | |
| SSRI: selective serotonin reuptake inhibitor | |||
| Source: References 16,17 | |||
Treatment of suicide attempt survivors has dramatically increased in the United States since 1990, but the incidence of suicidal thoughts, plans, gestures, or attempts has not significantly decreased.18 A systematic review of 15 randomized controlled trials using the search methods published by Arensman et al19 reveals very little difference in the suicide reattempt rate, despite extra treatment beyond the “usual standard of care.”
Intervention strategies shown to significantly decrease the rate of self-harm include home visits, behavioral therapy, and a “green card” strategy (patients were issued a card at the time of discharge explaining that a doctor was always available for them and how that doctor could be contacted).20-23
No significant difference in reattempt rate was found with other strategies, although benefits such as lower rates of depression and suicidal ideation or higher outpatient visit attendance were observed in some trials.24-26 Click here for a summary of the studies’ methodologies and results.
Outcomes of self-harm
When considering outcomes of SA, it is important to separate the short-term outcome of a single SA from the long-term outcome of suicidality. Short-term outcome depends on the characteristics and management of the acute episode, whereas long-term encompasses ongoing management of suicidality as a trait.
In the short term, surviving a SA depends heavily on the lethality of method and access to acute treatment. It also depends on medical fitness to withstand injury, which may help account for the higher death rate among elderly suicide attempters. A frail or medically ill person is less likely to survive the bodily insult of a SA.
Long-term outcomes are harder to predict. Some patients’ index attempts result from a transient state—an isolated incident that never will be repeated. In others, suicidality is a trait—a chronic maladaptive pattern that is potentially lethal. After an index attempt, the most reliable predictors for eventual death by suicide are:
- diagnosed mental illness
- high-lethality method on the index SA
- number of reattempts.4
Mood disorders impact long-term outcome, yet only a limited number of studies have found a reduction in suicide rates in response to mood disorder treatment. In a 44-year follow-up study, long-term treatment of depression and bipolar disorder with lithium significantly reduced the suicide rate.30 A meta-analysis of recurrent major affective disorder studies found that subjects on lithium maintenance treatment were 15 times less likely to commit suicidal acts, compared with those not on lithium.31
An important confounding factor in these findings is that effective lithium treatment requires long-term adherence, which implies a long-term doctor-patient relationship. As Cipriani et al32 noted, patients who can maintain an ongoing therapeutic relationship may be “less disturbed” than those who cannot, making them less likely to kill themselves regardless of pharmacologic treatment. Furthermore, patient interviews reveal that the therapeutic alliance created by a continuous relationship can be a protective support against further SA.33
Clinical implications
Suicide survivors often continue to struggle with suicidality well beyond the index attempt. This suicidality is a maladaptive problem-solving method that functions as a chronic morbid illness. As such, it is not enough to analyze the phenomenon of surviving an SA; one must examine the ongoing process of surviving suicidality.
Consider 3 factors. Consider all 3 factors— motive, method, and management—when addressing suicide survivorship.
Method lethality significantly influences survival likelihood. In clinical practice, we have observed that the index attempt is a learning experience for some patients that will inform their choice of method on the next attempt. When interacting with a suicide survivor, carefully assess the reasoning behind their initial choice of method and whether it has evolved toward higher lethality since the index attempt.
Management recommendations after SA continue to evolve. Risk factor management—such as treating underlying mood disorders, home visits to reduce social isolation, and prioritized “green card” contact with psychiatrists—has been shown to decrease reattempt rates, but many other interventions have not shown the expected benefit. Increased intervention rates have not yielded proportional decreases in suicidal ideation, attempts, or completion.
Suicide survivors often continue to struggle with suicidality well beyond the index attempt
Consider the SA motive and method when planning how to manage the survivor
Method lethality significantly influences survival likelihood (firearms are the most common and deadly method in the United States)
In many clinical trials, the incidence of suicidal thoughts, plans, gestures, or attempts has not significantly decreased when SA survivors received extra treatment
Management recommendations after SA continue to evolve; effective techniques appear to be keeping lines of communication open and providing individualized treatment
Individualize pharmacologic treatments and psychotherapy to help guide the patient away from maximum suicide risk
SA: suicide attempt
- American Association of Suicidology. www.suicidology.org.
- American Foundation for Suicide Prevention. www.afsp.org.
- Mayo Clinic Patient/Family Education. Suicide: What to do when someone is suicidal. www.mayoclinic.com/health/suicide/MH00058.
- Carbamazepine • Carbatrol
- Lithium • Eskalith, Lithobid
- Valproic acid • Depakene, Depakote
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Gardner DL, Cowdry RW. Suicidal and parasuicidal behavior in borderline personality disorder. Psychiatr Clin North Am. 1985;8(2):389-403.
2. Bostwick J, Levenson J. Suicidality. In: Levenson J, ed. The American Psychiatric Publishing textbook of psychosomatic medicine. Arlington, VA: American Psychiatric Publishing, Inc; 2004:219-234.
3. Bostwick JM, Cohen LM. Differentiating suicide from life-ending acts and end-of-life decisions: a model based on chronic kidney disease and dialysis. Psychosomatics. 2009;50(1):1-7.
4. Jeglic EL. Will my patient attempt suicide again? Current Psychiatry. 2008;7(11):19-28.
5. Beautrais A. A case control study of suicide and attempted suicide. Suicide Life Threat Behav. 2002;32(1):1-9.
6. Shneidman E. Overview: a multidimensional approach to suicide. In: Jacobs D, Brown H, eds. Suicide: understanding and responding. Madison, WI: International Universities Press; 1989:1-20.
7. Beautrais AL. Risk factors for suicide and attempted suicide among young people. Aust N Z J Psychiatry. 2000;34:420-436.
8. Black DW, Blum N, Pfohl B, et al. Suicidal behavior in borderline personality disorder: prevalence, risk factors, prediction, and prevention. J Pers Disord. 2004;18(3):226-239.
9. Plutchik R, van Praag HM, Picard S, et al. Is there a relation between the seriousness of suicidal intent and the lethality of suicide attempt? Psychiatric Res. 1988;27:71-79.
10. Schernhammer ES, Colditz GA. Suicide rates among physicians: a quantitative and gender assessment (meta-analysis). Am J Psychiatry. 2004;161(12):2295-2302.
11. Recupero PR, Harms SE, Noble JM. Googling suicide: surfing for suicide information on the internet. J Clin Psychiatry. 2008;69(6):878-888.
12. Gibb SJ, Beautrais AL, Fergusson DM. Mortality and further suicidal behaviour after an index suicide attempt: a 10-year study. Aust N Z J Psychiatry. 2005;39:95-100.
13. Miller M, Hemenway D. Guns and suicide in the United States. N Engl J Med. 2008;359(10):989-991.
14. Latha KS, Bhat SM, D’Souza P. Suicide attempters in general hospital unit in India: their socio-demographic and clinical profile—emphasis on cross-cultural aspects. Acta Psychiatra Scand. 1996;94(1):26-30.
15. Yildiz A, Sachs GS, Turgay A. Pharmacological management of agitation in emergency settings. Emerg Med J. 2003;20(4):339-346.
16. American Psychiatric Association practice guidelines. Treatment of patients with borderline personality disorder. 2001. Available at: http://www.psychiatryonline.com/pracGuide/pracGuideTopic_13.aspx. Accessed July 8, 2009.
17. Zanarini MC. Update on pharmacotherapy of borderline personality disorder. Curr Psychiatry Rep. 2004;6(1):66-70.
18. Kessler RC, Berglund P, Borges G, et al. Trends in suicide ideation, plans, gestures, and attempts in the United States, 1990-1992 to 2001-2003. JAMA. 2005;293(20):2487-2495.
19. Arensman E, Townsend E, Hawton K, et al. Psychosocial and pharmacological treatment of patients following deliberate self-harm: the methodological issues involved in evaluating effectiveness. Suicide Life Threat Behav. 2001;31(2):169-180.
20. Welu TC. A follow-up program for suicide attempters: evaluation of effectiveness. Suicide Life Threat Behav. 1977;7(1):17-20.
21. Linehan MM, Armstrong HE, Suarez A, et al. Cognitive-behavioral treatment of chronically parasuicidal borderline patients. Arch Gen Psychiatry. 1991;48(12):1060-1064.
22. McLeavey B, Daly R, Ludgate J, et al. Interpersonal problem-solving skills training in the treatment of self-poisoning patients. Suicide Life Threat Behav. 1994;24(4):382-394.
23. Morgan HG, Jones EM, Owen JH. Secondary prevention of non-fatal deliberate self-harm. The green card study. Br J Psychiatry. 1993;163:111-112.
24. Salkovskis PM, Atha C, Storer D. Cognitive-behavioural problem solving in the treatment of patients who repeatedly attempt suicide. A controlled trial. Br J Psychiatry. 1990;157:871-876.
25. Hirsch SR, Walsh C, Draper R. Parasuicide. A review of treatment interventions. J Affect Disord. 1982;4(4):299-311.
26. Hawton K, Bancroft J, Catalan J, et al. Domiciliary and outpatient treatment of self-poisoning patients by medical and non-medical staff. Psychol Med. 1981;11(1):169-177.
27. Brown GK, Ten Have T, Henriques GR, et al. Cognitive therapy for the prevention of suicide attempts: a randomized controlled trial. JAMA. 2005;294(5):563-570.
28. Bateman A, Fonagy P. 8-year follow-up of patients treated for borderline personality disorder: mentalization-based treatment versus treatment as usual. Am J Psychiatry. 2008;165(5):631-638.
29. Hawton K, Zahl D, Weatherall R. Suicide following deliberate self-harm: long-term follow-up of patients who presented to a general hospital. Br J Psychiatry. 2003;182:537-542.
30. Angst J, Angst F, Gerber-Werder R, et al. Suicide in 406 mood-disorder patients with and without long-term medication: a 40 to 44 years’ follow-up. Arch Suicide Res. 2005;9(3):279-300.
31. Baldessarini RJ, Tondo L, Hennen J. Lithium treatment and suicide risk in major affective disorders: update and new findings. J Clin Psychiatry. 2003;64(suppl 5):44-52.
32. Cipriani A, Pretty H, Hawton K, et al. Lithium in the prevention of suicidal behavior and all-cause mortality in patients with mood disorders: a systematic review of randomized trials. Am J Psychiatry. 2005;162(10):1805-1819.
33. Sinclair J, Green J. Understanding resolution of deliberate self harm: qualitative interview study of patients’ experiences. BMJ. 2005;330(7500):1112.-
1. Gardner DL, Cowdry RW. Suicidal and parasuicidal behavior in borderline personality disorder. Psychiatr Clin North Am. 1985;8(2):389-403.
2. Bostwick J, Levenson J. Suicidality. In: Levenson J, ed. The American Psychiatric Publishing textbook of psychosomatic medicine. Arlington, VA: American Psychiatric Publishing, Inc; 2004:219-234.
3. Bostwick JM, Cohen LM. Differentiating suicide from life-ending acts and end-of-life decisions: a model based on chronic kidney disease and dialysis. Psychosomatics. 2009;50(1):1-7.
4. Jeglic EL. Will my patient attempt suicide again? Current Psychiatry. 2008;7(11):19-28.
5. Beautrais A. A case control study of suicide and attempted suicide. Suicide Life Threat Behav. 2002;32(1):1-9.
6. Shneidman E. Overview: a multidimensional approach to suicide. In: Jacobs D, Brown H, eds. Suicide: understanding and responding. Madison, WI: International Universities Press; 1989:1-20.
7. Beautrais AL. Risk factors for suicide and attempted suicide among young people. Aust N Z J Psychiatry. 2000;34:420-436.
8. Black DW, Blum N, Pfohl B, et al. Suicidal behavior in borderline personality disorder: prevalence, risk factors, prediction, and prevention. J Pers Disord. 2004;18(3):226-239.
9. Plutchik R, van Praag HM, Picard S, et al. Is there a relation between the seriousness of suicidal intent and the lethality of suicide attempt? Psychiatric Res. 1988;27:71-79.
10. Schernhammer ES, Colditz GA. Suicide rates among physicians: a quantitative and gender assessment (meta-analysis). Am J Psychiatry. 2004;161(12):2295-2302.
11. Recupero PR, Harms SE, Noble JM. Googling suicide: surfing for suicide information on the internet. J Clin Psychiatry. 2008;69(6):878-888.
12. Gibb SJ, Beautrais AL, Fergusson DM. Mortality and further suicidal behaviour after an index suicide attempt: a 10-year study. Aust N Z J Psychiatry. 2005;39:95-100.
13. Miller M, Hemenway D. Guns and suicide in the United States. N Engl J Med. 2008;359(10):989-991.
14. Latha KS, Bhat SM, D’Souza P. Suicide attempters in general hospital unit in India: their socio-demographic and clinical profile—emphasis on cross-cultural aspects. Acta Psychiatra Scand. 1996;94(1):26-30.
15. Yildiz A, Sachs GS, Turgay A. Pharmacological management of agitation in emergency settings. Emerg Med J. 2003;20(4):339-346.
16. American Psychiatric Association practice guidelines. Treatment of patients with borderline personality disorder. 2001. Available at: http://www.psychiatryonline.com/pracGuide/pracGuideTopic_13.aspx. Accessed July 8, 2009.
17. Zanarini MC. Update on pharmacotherapy of borderline personality disorder. Curr Psychiatry Rep. 2004;6(1):66-70.
18. Kessler RC, Berglund P, Borges G, et al. Trends in suicide ideation, plans, gestures, and attempts in the United States, 1990-1992 to 2001-2003. JAMA. 2005;293(20):2487-2495.
19. Arensman E, Townsend E, Hawton K, et al. Psychosocial and pharmacological treatment of patients following deliberate self-harm: the methodological issues involved in evaluating effectiveness. Suicide Life Threat Behav. 2001;31(2):169-180.
20. Welu TC. A follow-up program for suicide attempters: evaluation of effectiveness. Suicide Life Threat Behav. 1977;7(1):17-20.
21. Linehan MM, Armstrong HE, Suarez A, et al. Cognitive-behavioral treatment of chronically parasuicidal borderline patients. Arch Gen Psychiatry. 1991;48(12):1060-1064.
22. McLeavey B, Daly R, Ludgate J, et al. Interpersonal problem-solving skills training in the treatment of self-poisoning patients. Suicide Life Threat Behav. 1994;24(4):382-394.
23. Morgan HG, Jones EM, Owen JH. Secondary prevention of non-fatal deliberate self-harm. The green card study. Br J Psychiatry. 1993;163:111-112.
24. Salkovskis PM, Atha C, Storer D. Cognitive-behavioural problem solving in the treatment of patients who repeatedly attempt suicide. A controlled trial. Br J Psychiatry. 1990;157:871-876.
25. Hirsch SR, Walsh C, Draper R. Parasuicide. A review of treatment interventions. J Affect Disord. 1982;4(4):299-311.
26. Hawton K, Bancroft J, Catalan J, et al. Domiciliary and outpatient treatment of self-poisoning patients by medical and non-medical staff. Psychol Med. 1981;11(1):169-177.
27. Brown GK, Ten Have T, Henriques GR, et al. Cognitive therapy for the prevention of suicide attempts: a randomized controlled trial. JAMA. 2005;294(5):563-570.
28. Bateman A, Fonagy P. 8-year follow-up of patients treated for borderline personality disorder: mentalization-based treatment versus treatment as usual. Am J Psychiatry. 2008;165(5):631-638.
29. Hawton K, Zahl D, Weatherall R. Suicide following deliberate self-harm: long-term follow-up of patients who presented to a general hospital. Br J Psychiatry. 2003;182:537-542.
30. Angst J, Angst F, Gerber-Werder R, et al. Suicide in 406 mood-disorder patients with and without long-term medication: a 40 to 44 years’ follow-up. Arch Suicide Res. 2005;9(3):279-300.
31. Baldessarini RJ, Tondo L, Hennen J. Lithium treatment and suicide risk in major affective disorders: update and new findings. J Clin Psychiatry. 2003;64(suppl 5):44-52.
32. Cipriani A, Pretty H, Hawton K, et al. Lithium in the prevention of suicidal behavior and all-cause mortality in patients with mood disorders: a systematic review of randomized trials. Am J Psychiatry. 2005;162(10):1805-1819.
33. Sinclair J, Green J. Understanding resolution of deliberate self harm: qualitative interview study of patients’ experiences. BMJ. 2005;330(7500):1112.-
How to reduce distress and repetitive behaviors in patients with OCD
Exposure and response (or ritual) prevention has been shown to be effective in improving the therapeutic outlook for patients with obsessive-compulsive disorder (OCD). Yet barriers—including patient unwillingness to enter into the intensive therapy—prevent more persons with OCD from achieving an improved quality of life.
This article focuses on the clinical picture of OCD and the multifaceted cognitive-behavioral therapy (CBT) that has received the most empirical support. We also describe initiatives to make CBT more accessible to OCD patients, such as providing twice-weekly instead of daily treatment sessions.
OCD definition: Anxiety/distress
OCD is a relatively common, debilitating condition that often develops early in life (Box 1).1,2 The obsessions of this disorder are not simply excessive worries about real-life problems. The compulsions are excessive or unreasonable and serve to reduce the discomfort associated with the obsessions. The Yale-Brown Obsessive Compulsive Scale (Y-BOCS) is the gold standard tool for quantifying OCD (Box 2).3,4
Obsessions vs compulsions. When diagnosing and treating OCD, it is important to ascertain the functional relationship between a patient’s obsessions and compulsions and anxiety/distress:
- Obsessions give rise to anxiety/distress.
- Compulsions aim to reduce this anxiety/distress.
The lifetime prevalence of obsessive-compulsive disorder (OCD) is 2% to 3%—approximately 2 to 3 times higher than that of schizophrenia. Onset of OCD often is in childhood or adolescence. OCD presents earlier in boys than girls, but by young adulthood the incidence is equally distributed in men and women.1 Its course typically is chronic and is associated with substantial suffering and functional impairment.
According to DSM-IV-TR criteria, OCD is characterized by:
- obsessions—persistent thoughts, impulses, or images that are experienced as intrusive, inappropriate, and distressing that an individual attempts to ignore, suppress, or neutralize with other thoughts or actions
- compulsions—repetitive behaviors or mental acts that are aimed at reducing distress or preventing a dreaded consequence.2
The 10-item, clinician-rated Yale-Brown Obsessive Compulsive Scale (Y-BOCS)3,4 has excellent psychometric properties. It is widely used in outcome studies and clinical practice to assess and monitor change and progress.
Y-BOCS consists of 5 questions about obsessions and 5 about compulsions; each symptom is rated on a scale of 0 (least severe) to 4 (most severe). Results are combined for a total score of 0 to 40, which is interpreted as:
- 0 to 7=subclinical
- 8 to 15=mild
- 16 to 23=moderate
- 24 to 31=severe
- 32 to 40=extreme.
OCD’s clinical picture
Classic vs nonclassic obsessions. Frequently reported obsessions in OCD include fears related to:
- contamination (dirt, germs, bodily waste, chemicals)
- making mistakes (locks, appliances, paperwork, decisions)
- having unwanted impulses (violent, sexual, religious, embarrassing)
- orderliness (neatness, symmetry, numbers).
- intrusive, irrational, or excessive worries about loss of identity, essence, or intelligence, mostly seen in teenagers or young adults
- contamination by “evil” or fear of becoming a “bad person”
- fear of harm to a newborn child by new parents
- fear of unintentionally performing socially inappropriate behaviors, such as shoplifting, molesting, or insulting someone.
A common theme among OCD patients is overwhelming distress associated with uncertainty. Patients with OCD often appraise low-probability events as extremely high-probability events, and as a result require reassurance and guarantees that dreaded outcomes will not occur. That reassurance can come in many forms:
- searching the Internet for answers
- asking family members, friends, or experts for confirmation or disconfirmation
- mentally checking and reevaluating whether they had opportunity or propensity to perform any of those acts.
Because guarantees often are impossible to secure, persons with OCD begin avoiding places and people where they may have an opportunity to encounter triggering stimuli. Phrases such as “just in case,” “yes, but what if…?” and “how do I know for sure?” are telltale signs of an OCD obsession.
A recent model of OCD may further advance our understanding of how obsessions and compulsions frequently appear together because of their functional link. This model clusters OCD symptoms into “symptom dimensions” that include:
Multifaceted CBT
OCD is conceptualized by both behavioral and cognitive theory (Box 3). Cognitive-behavioral treatment for OCD includes:
- exposure in vivo—repeated, prolonged confrontation with anxiety-evoking stimuli
- repeated, prolonged imaginal confrontation with feared disasters
- ritual prevention—blocking or preventing compulsions
- cognitive interventions—correcting erroneous cognitions about potential consequences if confrontation with feared situations is not followed by “ritualizing” (engaging in compulsive behavior).
The behavioral theory of obsessive-compulsive disorder (OCD) suggests that obsessions produce anxiety—and/or other forms of distress, such as disgust—and compulsions reduce obsessional anxiety. Compulsions are maintained because they are reinforced by briefly reducing obsessional anxiety; however, in the long term, they prevent the habituation of obsessional anxiety.
The cognitive theory of OCD maintains that the disorder is characterized by erroneous cognitions, including:
- unrealistic estimates of threat, and exaggerated sense of personal responsibility for harm
- the notion that absence of complete evidence of safety denotes danger
- the notion that obsessional anxiety can be reduced only by compulsions or avoidance of the triggering stimuli.
Imaginal exposure involves repeated confrontation (in imagination) with the disastrous consequences the patient anticipates if the rituals are not performed (eg, a parent’s children will contract a disease many years from now because of failure to protect them from harmful toxins).
Cognitive interventions involve discussing the changes that take place during in vivo and imaginal exposure, such as:
- the patient’s anxiety decreases with repeated exposure even without ritualistic behavior
- the feared consequences often do not materialize
- in some cases tolerance of uncertainty is what is being practiced.
Evidence supports EX/RP
Several randomized controlled trials (RCTs) have demonstrated the efficacy of EX/RP for reducing OCD symptoms.8–10 To address the potential generalizability of these results to typical clinical practice, Franklin et al11 compared findings from 4 RCTs of EX/RP with treatment outcome data from 110 outpatients receiving EX/RP. The outpatients had varying OCD severity, treatment histories, concomitant pharmacotherapy regimens, psychiatric comorbidity profiles, and ages. Following EX/RP, they achieved substantial and clinically meaningful reductions in their OCD and depressive symptoms that were comparable with those reported in the RCTs, which suggests the benefits of EX/RP are not limited to select patient samples.
Foa et al12 compared the relative and combined efficacy of clomipramine (maximum dosage 250 mg/d) and EX/RP for treating OCD in adults. At week 12, all active treatments were more effective than placebo. EX/RP and EX/RP plus clomipramine were comparable, and both were more effective than clomipramine alone. The study also suggested that with regular supervision, treatment modalities could be successfully implemented in clinics with differing expertise.
Most OCD patients who receive an adequate selective serotonin reuptake inhibitor (SSRI) trial (Table) continue to have clinically significant OCD symptoms. Simpson et al13 studied 108 outpatients with OCD and found that augmenting SSRIs with EX/RP further reduces OCD symptoms and is more effective than stress management training. However, the now-standard 17 sessions of EX/RP were not sufficient to help most patients achieve minimal symptoms, defined as a Y-BOCS score ≤12. Ongoing augmentation studies are examining ways to increase OCD remission rates and achieve greater palatability, accessibility, and duration of effects.
Table
SSRIs for OCD: Recommended dosages
| Medication | Recommended dosage |
|---|---|
| Citalopram* | 20 to 80 mg/d |
| Fluoxetine | 20 to 80 mg/d |
| Fluvoxamine | 100 to 300 mg/d |
| Paroxetine | 40 to 60 mg/d |
| Sertraline | 50 to 200 mg/d |
| *Not FDA-approved for OCD | |
| SSRI: selective serotonin reuptake inhibitor; OCD: obsessive-compulsive disorder | |
Using EX/RP in practice
When EX/RP was developed and studied in the 1960s and ‘70s, it was conducted daily. However, intensive OCD treatments are not always practical or readily available. This consideration prompted us to examine—in a nonrandomized study—the efficacy of a twice-weekly EX/RP program that is otherwise identical to the intensive treatment program at 3-month follow-up. Results indicated that this less intensive program was as effective as the intensive treatment.14
Less-intensive, once- or twice-weekly programs may be suitable for most OCD patients. Consider intensive EX/RP for:
- patients who wish to complete their treatment in a short period at expert centers
- patients for whom less intensive treatment fails to produce the desired outcome.
In addition to lack of adherence, other predictors of poorer outcome include:
- poor insight by the patient that the feared consequences were unrealistic16
- comorbid severe depression that interferes with utilization of EX/RP17
- family members’ expressed emotion (mainly hostility).18
To help patients and clinicians overcome barriers to effective OCD treatment, researchers are evaluating the cost-effectiveness and feasibility of a stepped-care model,20 in which effective treatment components are offered in phases, depending on need and availability. Phases include self-directed EX/RP, therapist-assisted EX/RP, intensifying frequency of sessions, and augmenting EX/RP with SSRIs.
Despite major improvements in OCD identification, treatment, and dissemination of knowledge to clinicians, the situation is far from ideal. Future research will help uncover additional factors for improving treatment outcome and portability.
Related resources
- Foa EB, Wilson R. Stop obsessing! New York, NY: Bantam Books; 2001.
- Abramowitz JS, Houts AC, eds. Concepts and controversies in obsessive-compulsive disorder. New York, NY: Springer; 2005.
- The Obsessive-Compulsive Foundation. www.ocfoundation.org
- Citalopram • Celexa
- Clomipramine • Anafranil
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Paroxetine • Paxil
- Sertraline • Zoloft
Dr. Yadin reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Foa receives research support from the American Psychiatric Association, BASF (formerly Ciba Geigy), Bristol-Myers Squibb, Cephalon, Eli Lilly and Company, Forest Pharmaceuticals, GlaxoSmithKline, Kali-Duphar, Pfizer Inc., and Solvay. She has been a speaker for the American Psychiatric Association, Forest Pharmaceuticals, GlaxoSmithKline, Jazz Pharmaceuticals, and Pfizer Inc. and a consultant to Actelion Pharmaceuticals.
1. Rasmussen SA, Eisen JL. Epidemiology of obsessive compulsive disorder. J Clin Psychiatry. 1990;51(suppl):10-14.
2. Diagnostic and statistical manual of mental disorders. 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000.
3. Goodman WK, Price LH, Rasmussen SA, et al. The Yale-Brown Obsessive Compulsive Scale. I. Development, use, and reliability. Arch Gen Psychiatry. 1989;46(11):1006-1011.
4. Goodman WK, Price LH, Rasmussen SA, et al. The Yale-Brown Obsessive Compulsive Scale. II. Validity. Arch Gen Psychiatry. 1989;46(11):1012-1016.
5. Mataix-Cols D, Rosario-Campos MC, Leckman JF. A multidimensional model of obsessive-compulsive disorder. Am J Psychiatry. 2005;162:228-238.
6. Hollander E, Kim S, Zohar J. OCSDs in the forthcoming DSM-V. CNS Spectr. 2007;12(5):320-323.
7. Storch EA, Abramowitz JS, Goodman WK. Where does obsessive-compulsive disorder belong in the DSM-V? Depress Anxiety. 2008;25:336-347.
8. Foa EB, Steketee G, Grayson JB, et al. Deliberate exposure and blocking of obsessive-compulsive rituals: immediate and long-term effects. Behav Ther. 1984;15:450-472.
9. Marks I. Behavior therapy for obsessive-compulsive disorder: a decade of progress. Can J Psychiatry. 1997;42:1021-1027.
10. Pediatric OCD Treatment Study (POTS) Team. Cognitive-behavior therapy, sertraline, and their combination for children and adolescents with obsessive-compulsive disorder. JAMA. 2004;292:1969-1976.
11. Franklin ME, Abramowitz JS, Kozak MJ, et al. Effectiveness of exposure and ritual prevention for obsessive-compulsive disorder: randomized compared with nonrandomized samples. J Consult Clin Psychol. 2000;68(4):594-602.
12. Foa EB, Liebowitz MR, Kozak MJ, et al. Randomized, placebo-controlled trial of exposure and ritual prevention, clomipramine, and their combination in the treatment of obsessive-compulsive disorder. Am J Psychiatry. 2005;162(1):151-161.
13. Simpson HB, Foa EB, Liebowitz MR, et al. A randomized, controlled trial of cognitive-behavioral therapy for augmenting pharmacotherapy in obsessive-compulsive disorder. Am J Psychiatry. 2008;165(5):621-630.
14. Abramowitz JS, Foa EB, Franklin ME. Exposure and ritual prevention for obsessive-compulsive disorder: effectiveness of intensive versus twice-weekly treatment sessions. J Consult Clin Psychol. 2003;71:394-398.
15. Simpson HB, Zuckoff A, Page JR, et al. Adding motivational interviewing to exposure and ritual prevention for obsessive-compulsive disorder: an open pilot trial. Cog Behav Ther. 2008;37(1):38-49.
16. Foa EB, Abramowitz JS, Franklin ME, et al. Feared consequences, fixity of belief, and treatment outcome in patients with obsessive-compulsive disorder. Behav Ther. 1999;30(4):717-724.
17. Abramowitz JS. The psychological treatment of obsessive-compulsive disorder. Can J Psychiatry. 2006;51(7):407-416.
18. Chambless DL, Steketee G. Expressed emotion and behavior therapy outcome: a prospective study with obsessive-compulsive and agoraphobic outpatients. J Consult Clin Psychol. 1999;67(5):658-665.
19. The Expert Consensus Panel for obsessive-compulsive disorder. Treatment of obsessive-compulsive disorder. J Clin Psychiatry. 1997;58(suppl 4):2-72.
20. Tolin DF, Diefenbach GJ, Maltby N, et al. Stepped care for obsessive-compulsive disorder: a pilot study. Cogn Behav Pract. 2005;12:403-414.
Exposure and response (or ritual) prevention has been shown to be effective in improving the therapeutic outlook for patients with obsessive-compulsive disorder (OCD). Yet barriers—including patient unwillingness to enter into the intensive therapy—prevent more persons with OCD from achieving an improved quality of life.
This article focuses on the clinical picture of OCD and the multifaceted cognitive-behavioral therapy (CBT) that has received the most empirical support. We also describe initiatives to make CBT more accessible to OCD patients, such as providing twice-weekly instead of daily treatment sessions.
OCD definition: Anxiety/distress
OCD is a relatively common, debilitating condition that often develops early in life (Box 1).1,2 The obsessions of this disorder are not simply excessive worries about real-life problems. The compulsions are excessive or unreasonable and serve to reduce the discomfort associated with the obsessions. The Yale-Brown Obsessive Compulsive Scale (Y-BOCS) is the gold standard tool for quantifying OCD (Box 2).3,4
Obsessions vs compulsions. When diagnosing and treating OCD, it is important to ascertain the functional relationship between a patient’s obsessions and compulsions and anxiety/distress:
- Obsessions give rise to anxiety/distress.
- Compulsions aim to reduce this anxiety/distress.
The lifetime prevalence of obsessive-compulsive disorder (OCD) is 2% to 3%—approximately 2 to 3 times higher than that of schizophrenia. Onset of OCD often is in childhood or adolescence. OCD presents earlier in boys than girls, but by young adulthood the incidence is equally distributed in men and women.1 Its course typically is chronic and is associated with substantial suffering and functional impairment.
According to DSM-IV-TR criteria, OCD is characterized by:
- obsessions—persistent thoughts, impulses, or images that are experienced as intrusive, inappropriate, and distressing that an individual attempts to ignore, suppress, or neutralize with other thoughts or actions
- compulsions—repetitive behaviors or mental acts that are aimed at reducing distress or preventing a dreaded consequence.2
The 10-item, clinician-rated Yale-Brown Obsessive Compulsive Scale (Y-BOCS)3,4 has excellent psychometric properties. It is widely used in outcome studies and clinical practice to assess and monitor change and progress.
Y-BOCS consists of 5 questions about obsessions and 5 about compulsions; each symptom is rated on a scale of 0 (least severe) to 4 (most severe). Results are combined for a total score of 0 to 40, which is interpreted as:
- 0 to 7=subclinical
- 8 to 15=mild
- 16 to 23=moderate
- 24 to 31=severe
- 32 to 40=extreme.
OCD’s clinical picture
Classic vs nonclassic obsessions. Frequently reported obsessions in OCD include fears related to:
- contamination (dirt, germs, bodily waste, chemicals)
- making mistakes (locks, appliances, paperwork, decisions)
- having unwanted impulses (violent, sexual, religious, embarrassing)
- orderliness (neatness, symmetry, numbers).
- intrusive, irrational, or excessive worries about loss of identity, essence, or intelligence, mostly seen in teenagers or young adults
- contamination by “evil” or fear of becoming a “bad person”
- fear of harm to a newborn child by new parents
- fear of unintentionally performing socially inappropriate behaviors, such as shoplifting, molesting, or insulting someone.
A common theme among OCD patients is overwhelming distress associated with uncertainty. Patients with OCD often appraise low-probability events as extremely high-probability events, and as a result require reassurance and guarantees that dreaded outcomes will not occur. That reassurance can come in many forms:
- searching the Internet for answers
- asking family members, friends, or experts for confirmation or disconfirmation
- mentally checking and reevaluating whether they had opportunity or propensity to perform any of those acts.
Because guarantees often are impossible to secure, persons with OCD begin avoiding places and people where they may have an opportunity to encounter triggering stimuli. Phrases such as “just in case,” “yes, but what if…?” and “how do I know for sure?” are telltale signs of an OCD obsession.
A recent model of OCD may further advance our understanding of how obsessions and compulsions frequently appear together because of their functional link. This model clusters OCD symptoms into “symptom dimensions” that include:
Multifaceted CBT
OCD is conceptualized by both behavioral and cognitive theory (Box 3). Cognitive-behavioral treatment for OCD includes:
- exposure in vivo—repeated, prolonged confrontation with anxiety-evoking stimuli
- repeated, prolonged imaginal confrontation with feared disasters
- ritual prevention—blocking or preventing compulsions
- cognitive interventions—correcting erroneous cognitions about potential consequences if confrontation with feared situations is not followed by “ritualizing” (engaging in compulsive behavior).
The behavioral theory of obsessive-compulsive disorder (OCD) suggests that obsessions produce anxiety—and/or other forms of distress, such as disgust—and compulsions reduce obsessional anxiety. Compulsions are maintained because they are reinforced by briefly reducing obsessional anxiety; however, in the long term, they prevent the habituation of obsessional anxiety.
The cognitive theory of OCD maintains that the disorder is characterized by erroneous cognitions, including:
- unrealistic estimates of threat, and exaggerated sense of personal responsibility for harm
- the notion that absence of complete evidence of safety denotes danger
- the notion that obsessional anxiety can be reduced only by compulsions or avoidance of the triggering stimuli.
Imaginal exposure involves repeated confrontation (in imagination) with the disastrous consequences the patient anticipates if the rituals are not performed (eg, a parent’s children will contract a disease many years from now because of failure to protect them from harmful toxins).
Cognitive interventions involve discussing the changes that take place during in vivo and imaginal exposure, such as:
- the patient’s anxiety decreases with repeated exposure even without ritualistic behavior
- the feared consequences often do not materialize
- in some cases tolerance of uncertainty is what is being practiced.
Evidence supports EX/RP
Several randomized controlled trials (RCTs) have demonstrated the efficacy of EX/RP for reducing OCD symptoms.8–10 To address the potential generalizability of these results to typical clinical practice, Franklin et al11 compared findings from 4 RCTs of EX/RP with treatment outcome data from 110 outpatients receiving EX/RP. The outpatients had varying OCD severity, treatment histories, concomitant pharmacotherapy regimens, psychiatric comorbidity profiles, and ages. Following EX/RP, they achieved substantial and clinically meaningful reductions in their OCD and depressive symptoms that were comparable with those reported in the RCTs, which suggests the benefits of EX/RP are not limited to select patient samples.
Foa et al12 compared the relative and combined efficacy of clomipramine (maximum dosage 250 mg/d) and EX/RP for treating OCD in adults. At week 12, all active treatments were more effective than placebo. EX/RP and EX/RP plus clomipramine were comparable, and both were more effective than clomipramine alone. The study also suggested that with regular supervision, treatment modalities could be successfully implemented in clinics with differing expertise.
Most OCD patients who receive an adequate selective serotonin reuptake inhibitor (SSRI) trial (Table) continue to have clinically significant OCD symptoms. Simpson et al13 studied 108 outpatients with OCD and found that augmenting SSRIs with EX/RP further reduces OCD symptoms and is more effective than stress management training. However, the now-standard 17 sessions of EX/RP were not sufficient to help most patients achieve minimal symptoms, defined as a Y-BOCS score ≤12. Ongoing augmentation studies are examining ways to increase OCD remission rates and achieve greater palatability, accessibility, and duration of effects.
Table
SSRIs for OCD: Recommended dosages
| Medication | Recommended dosage |
|---|---|
| Citalopram* | 20 to 80 mg/d |
| Fluoxetine | 20 to 80 mg/d |
| Fluvoxamine | 100 to 300 mg/d |
| Paroxetine | 40 to 60 mg/d |
| Sertraline | 50 to 200 mg/d |
| *Not FDA-approved for OCD | |
| SSRI: selective serotonin reuptake inhibitor; OCD: obsessive-compulsive disorder | |
Using EX/RP in practice
When EX/RP was developed and studied in the 1960s and ‘70s, it was conducted daily. However, intensive OCD treatments are not always practical or readily available. This consideration prompted us to examine—in a nonrandomized study—the efficacy of a twice-weekly EX/RP program that is otherwise identical to the intensive treatment program at 3-month follow-up. Results indicated that this less intensive program was as effective as the intensive treatment.14
Less-intensive, once- or twice-weekly programs may be suitable for most OCD patients. Consider intensive EX/RP for:
- patients who wish to complete their treatment in a short period at expert centers
- patients for whom less intensive treatment fails to produce the desired outcome.
In addition to lack of adherence, other predictors of poorer outcome include:
- poor insight by the patient that the feared consequences were unrealistic16
- comorbid severe depression that interferes with utilization of EX/RP17
- family members’ expressed emotion (mainly hostility).18
To help patients and clinicians overcome barriers to effective OCD treatment, researchers are evaluating the cost-effectiveness and feasibility of a stepped-care model,20 in which effective treatment components are offered in phases, depending on need and availability. Phases include self-directed EX/RP, therapist-assisted EX/RP, intensifying frequency of sessions, and augmenting EX/RP with SSRIs.
Despite major improvements in OCD identification, treatment, and dissemination of knowledge to clinicians, the situation is far from ideal. Future research will help uncover additional factors for improving treatment outcome and portability.
Related resources
- Foa EB, Wilson R. Stop obsessing! New York, NY: Bantam Books; 2001.
- Abramowitz JS, Houts AC, eds. Concepts and controversies in obsessive-compulsive disorder. New York, NY: Springer; 2005.
- The Obsessive-Compulsive Foundation. www.ocfoundation.org
- Citalopram • Celexa
- Clomipramine • Anafranil
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Paroxetine • Paxil
- Sertraline • Zoloft
Dr. Yadin reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Foa receives research support from the American Psychiatric Association, BASF (formerly Ciba Geigy), Bristol-Myers Squibb, Cephalon, Eli Lilly and Company, Forest Pharmaceuticals, GlaxoSmithKline, Kali-Duphar, Pfizer Inc., and Solvay. She has been a speaker for the American Psychiatric Association, Forest Pharmaceuticals, GlaxoSmithKline, Jazz Pharmaceuticals, and Pfizer Inc. and a consultant to Actelion Pharmaceuticals.
Exposure and response (or ritual) prevention has been shown to be effective in improving the therapeutic outlook for patients with obsessive-compulsive disorder (OCD). Yet barriers—including patient unwillingness to enter into the intensive therapy—prevent more persons with OCD from achieving an improved quality of life.
This article focuses on the clinical picture of OCD and the multifaceted cognitive-behavioral therapy (CBT) that has received the most empirical support. We also describe initiatives to make CBT more accessible to OCD patients, such as providing twice-weekly instead of daily treatment sessions.
OCD definition: Anxiety/distress
OCD is a relatively common, debilitating condition that often develops early in life (Box 1).1,2 The obsessions of this disorder are not simply excessive worries about real-life problems. The compulsions are excessive or unreasonable and serve to reduce the discomfort associated with the obsessions. The Yale-Brown Obsessive Compulsive Scale (Y-BOCS) is the gold standard tool for quantifying OCD (Box 2).3,4
Obsessions vs compulsions. When diagnosing and treating OCD, it is important to ascertain the functional relationship between a patient’s obsessions and compulsions and anxiety/distress:
- Obsessions give rise to anxiety/distress.
- Compulsions aim to reduce this anxiety/distress.
The lifetime prevalence of obsessive-compulsive disorder (OCD) is 2% to 3%—approximately 2 to 3 times higher than that of schizophrenia. Onset of OCD often is in childhood or adolescence. OCD presents earlier in boys than girls, but by young adulthood the incidence is equally distributed in men and women.1 Its course typically is chronic and is associated with substantial suffering and functional impairment.
According to DSM-IV-TR criteria, OCD is characterized by:
- obsessions—persistent thoughts, impulses, or images that are experienced as intrusive, inappropriate, and distressing that an individual attempts to ignore, suppress, or neutralize with other thoughts or actions
- compulsions—repetitive behaviors or mental acts that are aimed at reducing distress or preventing a dreaded consequence.2
The 10-item, clinician-rated Yale-Brown Obsessive Compulsive Scale (Y-BOCS)3,4 has excellent psychometric properties. It is widely used in outcome studies and clinical practice to assess and monitor change and progress.
Y-BOCS consists of 5 questions about obsessions and 5 about compulsions; each symptom is rated on a scale of 0 (least severe) to 4 (most severe). Results are combined for a total score of 0 to 40, which is interpreted as:
- 0 to 7=subclinical
- 8 to 15=mild
- 16 to 23=moderate
- 24 to 31=severe
- 32 to 40=extreme.
OCD’s clinical picture
Classic vs nonclassic obsessions. Frequently reported obsessions in OCD include fears related to:
- contamination (dirt, germs, bodily waste, chemicals)
- making mistakes (locks, appliances, paperwork, decisions)
- having unwanted impulses (violent, sexual, religious, embarrassing)
- orderliness (neatness, symmetry, numbers).
- intrusive, irrational, or excessive worries about loss of identity, essence, or intelligence, mostly seen in teenagers or young adults
- contamination by “evil” or fear of becoming a “bad person”
- fear of harm to a newborn child by new parents
- fear of unintentionally performing socially inappropriate behaviors, such as shoplifting, molesting, or insulting someone.
A common theme among OCD patients is overwhelming distress associated with uncertainty. Patients with OCD often appraise low-probability events as extremely high-probability events, and as a result require reassurance and guarantees that dreaded outcomes will not occur. That reassurance can come in many forms:
- searching the Internet for answers
- asking family members, friends, or experts for confirmation or disconfirmation
- mentally checking and reevaluating whether they had opportunity or propensity to perform any of those acts.
Because guarantees often are impossible to secure, persons with OCD begin avoiding places and people where they may have an opportunity to encounter triggering stimuli. Phrases such as “just in case,” “yes, but what if…?” and “how do I know for sure?” are telltale signs of an OCD obsession.
A recent model of OCD may further advance our understanding of how obsessions and compulsions frequently appear together because of their functional link. This model clusters OCD symptoms into “symptom dimensions” that include:
Multifaceted CBT
OCD is conceptualized by both behavioral and cognitive theory (Box 3). Cognitive-behavioral treatment for OCD includes:
- exposure in vivo—repeated, prolonged confrontation with anxiety-evoking stimuli
- repeated, prolonged imaginal confrontation with feared disasters
- ritual prevention—blocking or preventing compulsions
- cognitive interventions—correcting erroneous cognitions about potential consequences if confrontation with feared situations is not followed by “ritualizing” (engaging in compulsive behavior).
The behavioral theory of obsessive-compulsive disorder (OCD) suggests that obsessions produce anxiety—and/or other forms of distress, such as disgust—and compulsions reduce obsessional anxiety. Compulsions are maintained because they are reinforced by briefly reducing obsessional anxiety; however, in the long term, they prevent the habituation of obsessional anxiety.
The cognitive theory of OCD maintains that the disorder is characterized by erroneous cognitions, including:
- unrealistic estimates of threat, and exaggerated sense of personal responsibility for harm
- the notion that absence of complete evidence of safety denotes danger
- the notion that obsessional anxiety can be reduced only by compulsions or avoidance of the triggering stimuli.
Imaginal exposure involves repeated confrontation (in imagination) with the disastrous consequences the patient anticipates if the rituals are not performed (eg, a parent’s children will contract a disease many years from now because of failure to protect them from harmful toxins).
Cognitive interventions involve discussing the changes that take place during in vivo and imaginal exposure, such as:
- the patient’s anxiety decreases with repeated exposure even without ritualistic behavior
- the feared consequences often do not materialize
- in some cases tolerance of uncertainty is what is being practiced.
Evidence supports EX/RP
Several randomized controlled trials (RCTs) have demonstrated the efficacy of EX/RP for reducing OCD symptoms.8–10 To address the potential generalizability of these results to typical clinical practice, Franklin et al11 compared findings from 4 RCTs of EX/RP with treatment outcome data from 110 outpatients receiving EX/RP. The outpatients had varying OCD severity, treatment histories, concomitant pharmacotherapy regimens, psychiatric comorbidity profiles, and ages. Following EX/RP, they achieved substantial and clinically meaningful reductions in their OCD and depressive symptoms that were comparable with those reported in the RCTs, which suggests the benefits of EX/RP are not limited to select patient samples.
Foa et al12 compared the relative and combined efficacy of clomipramine (maximum dosage 250 mg/d) and EX/RP for treating OCD in adults. At week 12, all active treatments were more effective than placebo. EX/RP and EX/RP plus clomipramine were comparable, and both were more effective than clomipramine alone. The study also suggested that with regular supervision, treatment modalities could be successfully implemented in clinics with differing expertise.
Most OCD patients who receive an adequate selective serotonin reuptake inhibitor (SSRI) trial (Table) continue to have clinically significant OCD symptoms. Simpson et al13 studied 108 outpatients with OCD and found that augmenting SSRIs with EX/RP further reduces OCD symptoms and is more effective than stress management training. However, the now-standard 17 sessions of EX/RP were not sufficient to help most patients achieve minimal symptoms, defined as a Y-BOCS score ≤12. Ongoing augmentation studies are examining ways to increase OCD remission rates and achieve greater palatability, accessibility, and duration of effects.
Table
SSRIs for OCD: Recommended dosages
| Medication | Recommended dosage |
|---|---|
| Citalopram* | 20 to 80 mg/d |
| Fluoxetine | 20 to 80 mg/d |
| Fluvoxamine | 100 to 300 mg/d |
| Paroxetine | 40 to 60 mg/d |
| Sertraline | 50 to 200 mg/d |
| *Not FDA-approved for OCD | |
| SSRI: selective serotonin reuptake inhibitor; OCD: obsessive-compulsive disorder | |
Using EX/RP in practice
When EX/RP was developed and studied in the 1960s and ‘70s, it was conducted daily. However, intensive OCD treatments are not always practical or readily available. This consideration prompted us to examine—in a nonrandomized study—the efficacy of a twice-weekly EX/RP program that is otherwise identical to the intensive treatment program at 3-month follow-up. Results indicated that this less intensive program was as effective as the intensive treatment.14
Less-intensive, once- or twice-weekly programs may be suitable for most OCD patients. Consider intensive EX/RP for:
- patients who wish to complete their treatment in a short period at expert centers
- patients for whom less intensive treatment fails to produce the desired outcome.
In addition to lack of adherence, other predictors of poorer outcome include:
- poor insight by the patient that the feared consequences were unrealistic16
- comorbid severe depression that interferes with utilization of EX/RP17
- family members’ expressed emotion (mainly hostility).18
To help patients and clinicians overcome barriers to effective OCD treatment, researchers are evaluating the cost-effectiveness and feasibility of a stepped-care model,20 in which effective treatment components are offered in phases, depending on need and availability. Phases include self-directed EX/RP, therapist-assisted EX/RP, intensifying frequency of sessions, and augmenting EX/RP with SSRIs.
Despite major improvements in OCD identification, treatment, and dissemination of knowledge to clinicians, the situation is far from ideal. Future research will help uncover additional factors for improving treatment outcome and portability.
Related resources
- Foa EB, Wilson R. Stop obsessing! New York, NY: Bantam Books; 2001.
- Abramowitz JS, Houts AC, eds. Concepts and controversies in obsessive-compulsive disorder. New York, NY: Springer; 2005.
- The Obsessive-Compulsive Foundation. www.ocfoundation.org
- Citalopram • Celexa
- Clomipramine • Anafranil
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Paroxetine • Paxil
- Sertraline • Zoloft
Dr. Yadin reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Foa receives research support from the American Psychiatric Association, BASF (formerly Ciba Geigy), Bristol-Myers Squibb, Cephalon, Eli Lilly and Company, Forest Pharmaceuticals, GlaxoSmithKline, Kali-Duphar, Pfizer Inc., and Solvay. She has been a speaker for the American Psychiatric Association, Forest Pharmaceuticals, GlaxoSmithKline, Jazz Pharmaceuticals, and Pfizer Inc. and a consultant to Actelion Pharmaceuticals.
1. Rasmussen SA, Eisen JL. Epidemiology of obsessive compulsive disorder. J Clin Psychiatry. 1990;51(suppl):10-14.
2. Diagnostic and statistical manual of mental disorders. 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000.
3. Goodman WK, Price LH, Rasmussen SA, et al. The Yale-Brown Obsessive Compulsive Scale. I. Development, use, and reliability. Arch Gen Psychiatry. 1989;46(11):1006-1011.
4. Goodman WK, Price LH, Rasmussen SA, et al. The Yale-Brown Obsessive Compulsive Scale. II. Validity. Arch Gen Psychiatry. 1989;46(11):1012-1016.
5. Mataix-Cols D, Rosario-Campos MC, Leckman JF. A multidimensional model of obsessive-compulsive disorder. Am J Psychiatry. 2005;162:228-238.
6. Hollander E, Kim S, Zohar J. OCSDs in the forthcoming DSM-V. CNS Spectr. 2007;12(5):320-323.
7. Storch EA, Abramowitz JS, Goodman WK. Where does obsessive-compulsive disorder belong in the DSM-V? Depress Anxiety. 2008;25:336-347.
8. Foa EB, Steketee G, Grayson JB, et al. Deliberate exposure and blocking of obsessive-compulsive rituals: immediate and long-term effects. Behav Ther. 1984;15:450-472.
9. Marks I. Behavior therapy for obsessive-compulsive disorder: a decade of progress. Can J Psychiatry. 1997;42:1021-1027.
10. Pediatric OCD Treatment Study (POTS) Team. Cognitive-behavior therapy, sertraline, and their combination for children and adolescents with obsessive-compulsive disorder. JAMA. 2004;292:1969-1976.
11. Franklin ME, Abramowitz JS, Kozak MJ, et al. Effectiveness of exposure and ritual prevention for obsessive-compulsive disorder: randomized compared with nonrandomized samples. J Consult Clin Psychol. 2000;68(4):594-602.
12. Foa EB, Liebowitz MR, Kozak MJ, et al. Randomized, placebo-controlled trial of exposure and ritual prevention, clomipramine, and their combination in the treatment of obsessive-compulsive disorder. Am J Psychiatry. 2005;162(1):151-161.
13. Simpson HB, Foa EB, Liebowitz MR, et al. A randomized, controlled trial of cognitive-behavioral therapy for augmenting pharmacotherapy in obsessive-compulsive disorder. Am J Psychiatry. 2008;165(5):621-630.
14. Abramowitz JS, Foa EB, Franklin ME. Exposure and ritual prevention for obsessive-compulsive disorder: effectiveness of intensive versus twice-weekly treatment sessions. J Consult Clin Psychol. 2003;71:394-398.
15. Simpson HB, Zuckoff A, Page JR, et al. Adding motivational interviewing to exposure and ritual prevention for obsessive-compulsive disorder: an open pilot trial. Cog Behav Ther. 2008;37(1):38-49.
16. Foa EB, Abramowitz JS, Franklin ME, et al. Feared consequences, fixity of belief, and treatment outcome in patients with obsessive-compulsive disorder. Behav Ther. 1999;30(4):717-724.
17. Abramowitz JS. The psychological treatment of obsessive-compulsive disorder. Can J Psychiatry. 2006;51(7):407-416.
18. Chambless DL, Steketee G. Expressed emotion and behavior therapy outcome: a prospective study with obsessive-compulsive and agoraphobic outpatients. J Consult Clin Psychol. 1999;67(5):658-665.
19. The Expert Consensus Panel for obsessive-compulsive disorder. Treatment of obsessive-compulsive disorder. J Clin Psychiatry. 1997;58(suppl 4):2-72.
20. Tolin DF, Diefenbach GJ, Maltby N, et al. Stepped care for obsessive-compulsive disorder: a pilot study. Cogn Behav Pract. 2005;12:403-414.
1. Rasmussen SA, Eisen JL. Epidemiology of obsessive compulsive disorder. J Clin Psychiatry. 1990;51(suppl):10-14.
2. Diagnostic and statistical manual of mental disorders. 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000.
3. Goodman WK, Price LH, Rasmussen SA, et al. The Yale-Brown Obsessive Compulsive Scale. I. Development, use, and reliability. Arch Gen Psychiatry. 1989;46(11):1006-1011.
4. Goodman WK, Price LH, Rasmussen SA, et al. The Yale-Brown Obsessive Compulsive Scale. II. Validity. Arch Gen Psychiatry. 1989;46(11):1012-1016.
5. Mataix-Cols D, Rosario-Campos MC, Leckman JF. A multidimensional model of obsessive-compulsive disorder. Am J Psychiatry. 2005;162:228-238.
6. Hollander E, Kim S, Zohar J. OCSDs in the forthcoming DSM-V. CNS Spectr. 2007;12(5):320-323.
7. Storch EA, Abramowitz JS, Goodman WK. Where does obsessive-compulsive disorder belong in the DSM-V? Depress Anxiety. 2008;25:336-347.
8. Foa EB, Steketee G, Grayson JB, et al. Deliberate exposure and blocking of obsessive-compulsive rituals: immediate and long-term effects. Behav Ther. 1984;15:450-472.
9. Marks I. Behavior therapy for obsessive-compulsive disorder: a decade of progress. Can J Psychiatry. 1997;42:1021-1027.
10. Pediatric OCD Treatment Study (POTS) Team. Cognitive-behavior therapy, sertraline, and their combination for children and adolescents with obsessive-compulsive disorder. JAMA. 2004;292:1969-1976.
11. Franklin ME, Abramowitz JS, Kozak MJ, et al. Effectiveness of exposure and ritual prevention for obsessive-compulsive disorder: randomized compared with nonrandomized samples. J Consult Clin Psychol. 2000;68(4):594-602.
12. Foa EB, Liebowitz MR, Kozak MJ, et al. Randomized, placebo-controlled trial of exposure and ritual prevention, clomipramine, and their combination in the treatment of obsessive-compulsive disorder. Am J Psychiatry. 2005;162(1):151-161.
13. Simpson HB, Foa EB, Liebowitz MR, et al. A randomized, controlled trial of cognitive-behavioral therapy for augmenting pharmacotherapy in obsessive-compulsive disorder. Am J Psychiatry. 2008;165(5):621-630.
14. Abramowitz JS, Foa EB, Franklin ME. Exposure and ritual prevention for obsessive-compulsive disorder: effectiveness of intensive versus twice-weekly treatment sessions. J Consult Clin Psychol. 2003;71:394-398.
15. Simpson HB, Zuckoff A, Page JR, et al. Adding motivational interviewing to exposure and ritual prevention for obsessive-compulsive disorder: an open pilot trial. Cog Behav Ther. 2008;37(1):38-49.
16. Foa EB, Abramowitz JS, Franklin ME, et al. Feared consequences, fixity of belief, and treatment outcome in patients with obsessive-compulsive disorder. Behav Ther. 1999;30(4):717-724.
17. Abramowitz JS. The psychological treatment of obsessive-compulsive disorder. Can J Psychiatry. 2006;51(7):407-416.
18. Chambless DL, Steketee G. Expressed emotion and behavior therapy outcome: a prospective study with obsessive-compulsive and agoraphobic outpatients. J Consult Clin Psychol. 1999;67(5):658-665.
19. The Expert Consensus Panel for obsessive-compulsive disorder. Treatment of obsessive-compulsive disorder. J Clin Psychiatry. 1997;58(suppl 4):2-72.
20. Tolin DF, Diefenbach GJ, Maltby N, et al. Stepped care for obsessive-compulsive disorder: a pilot study. Cogn Behav Pract. 2005;12:403-414.
Choosing antipsychotics for children with schizophrenia: Evidence plus experience
“A patient I’ve seen for a number of years had been diagnosed in the pervasive developmental disorder spectrum, but she was quite atypical. Her perseverative thinking focused on a fantasy world, and she was so preoccupied that it was very difficult to pull her out of it. Now at age 12, she has a full-blown psychotic disorder, and the fantasy world is enveloping her. She hears people talking to her all day long.”
Jean A. Frazier, MD, who treats this patient and other children with psychotic disorders, was 1 of 4 principal investigators in the Treatment of Early-Onset Schizophrenia Spectrum Disorders (TEOSS) study, a randomized, double-blind, multisite trial funded by the National Institute of Mental Health. The study, published in November 2008,1 compared the efficacy and tolerability of 3 antipsychotics—olanzapine, risperidone, and molindone—in pediatric patients with schizophrenia or schizoaffective disorder (Box 1).
Dr. Frazier discusses the unexpected findings of the TEOSS trial with Current Psychiatry Section Editor Robert A. Kowatch, MD, PhD. Based on the trial findings and her experience, she tells how she makes decisions when prescribing antipsychotics for children and adolescents with schizophrenia and related disorders.
DR. KOWATCH: The TEOSS trial found no significant differences in efficacy between molindone and the atypical antipsychotics (olanzapine and risperidone) included in the study. You’ve prescribed both typical and atypical antipsychotics in research and in your clinical practice. Do you believe there’s any difference between the 2 classes?
DR. FRAZIER: There are some differences. For example, treatment-refractory patients, especially young children, sometimes need more D2 blockade than some atypical antipsychotics provide. I’ve seen more extra pyramidal side effects with the typical antipsychotics than the atypicals, although it’s not uncommon to see some akathisia with aripiprazole or some dystonia and dyskinesia with risperidone.
DR. KOWATCH: What are the benefits and risks of using antipsychotics in young children?
DR. FRAZIER: The benefit is that antipsychotics can decrease children’s suffering and get them more centered in reality so they can enjoy their friends and progress in school. And when that happens, it’s wonderful. What are the risks? With the atypicals my greatest concern is weight gain, and with the typical agents it’s tardive dyskinesia.
DR. KOWATCH: Have you changed the way you prescribe antipsychotics as a result of the TEOSS study?
DR. FRAZIER: Actually, I have. Clinicians have to be very careful about selecting psychotropic agents that can worsen pediatric-onset obesity. Olanzapine is an effective agent for targeting psychosis and mood symptoms, but the weight gain associated with it is a concern. I do not prescribe olanzapine as much as I have in the past, although I keep it in my armamentarium and tend to reserve it for third- or fourth-line therapy.
I have found molindone to be quite effective in children with schizophrenia or schizoaffective disorder, especially in those who have gained a lot of weight on atypical antipsychotics. They usually lose weight on molindone.
DR. KOWATCH: Do you think the TEOSS study had adequate power to demonstrate differences among molindone, olanzapine, and risperidone?
DR. FRAZIER: We enrolled 119 patients—which is large for a study such as this—but we did not reach our target of 168 patients, which might have increased our power to detect differences. Among the children we did enroll, the 3 antipsychotics showed no difference in efficacy, but the meaningful finding of this study to me was the side effect profile of these agents.
DR. KOWATCH: You mean weight gain with olanzapine and extrapyramidal symptoms with molindone?
DR. FRAZIER: Yes.
Managing side effects
DR. KOWATCH: How do you manage antipsychotic side effects?
DR. FRAZIER: For any of the antipsychotics’ side effects, you have to decide whether to continue the agent or switch to another anti psychotic. For example, I’ve had a number of children—many with significant weight problems—whose psychotic symptoms have responded only to risperidone. So we put them back on risperidone, and the decision then becomes what can we do to help with the weight gain while continuing that agent.
For weight gain, I think the best intervention is diet, exercise, and drinking a lot of water, but that can be effective only if you engage the patient’s entire family in the intervention as well. Short of that, a number of pharmacologic interventions have been studied, although not specifically in children.
In an open-label trial our group conducted with 11 children age 10 to 18 years who had gained weight while taking atypical antipsychotics, metformin decreased lipid levels and body mass index but not significantly. I’ve followed these children in my practice, however, and all those who continued taking metformin over a period of months lost weight.
TEOSS study adds to debate about efficacy and tolerability
The 5-year National Institute of Mental Health-funded Treatment of Early-Onset Schizophrenia Spectrum Disorders (TEOSS) trial began with an ambitious goal: to compare the efficacy and safety of 1 typical and 2 atypical antipsychotics in children age 8 to 19 with schizophrenia. The primary hypothesis was that atypical agents would show greater efficacy and tolerability when given for 8 weeks. Instead, the atypical agents showed no greater efficacy, and adverse effects occurred with all 3 antipsychotics. Because the trial was designed for 168 subjects but enrolled 119, it may not have been adequately powered to detect differences among the 3 agents.
Medications: Most of the 116 children who received medications were severely ill with psychotic symptoms when randomly assigned to 1 of the 3 antipsychotics for 8 weeks of double-blind treatment. Administration began at the lowest dose in a set range and usually was increased to midrange within 10 to 14 days. Dosing remained flexible within these ranges:
- molindone, 10 to 140 mg/d (mean endpoint dose 59.9 mg/d)
- olanzapine, 2.5 to 20 mg/d (mean endpoint dose 11.4 mg/d)
- risperidone, 0.5 to 6 mg/d (mean endpoint dose 2.8 mg/d).
Benztropine, ≥1 mg/d, was given to all patients treated with molindone, 14% of those treated with olanzapine, and 34% of those treated with risperidone to prevent or manage akathisia.
Efficacy: Two criteria defined treatment response: a Clinical Global Impression improvement score of 1 or 2 and a ≥20% reduction in baseline Positive and Negative Syndrome Scale (PANSS) score. Tolerability outcomes included neurologic side effects, weight changes, laboratory analyses, vital signs, ECG, serious adverse events, and treatment discontinuation. Extrapyramidal symptoms were monitored with involuntary movement and akathisia scales.
Observed PANSS total score by week of treatment
Mean Positive and Negative Symptom Scale (PANSS) total scores of observed cases during each week of the TEOSS trial. Minimum possible PANSS score is 30; scores >60 typically are viewed as problematic.
Among the 70 patients who completed treatment (25 of 40 with molindone, 17 of 35 olanzapine, and 28 of 41 with risperidone), more than one-half failed to achieve an adequate response. Response rates were 50% with molindone, 34% with olanzapine, and 46% with risperidone. The atypical antipsychotics did not show greater efficacy than molindone, and mean reductions in psychotic symptoms were modest (20% to 34% on the PANSS). Mean medication doses were midrange and considered moderate.
Tolerability: Sedation, irritability, and anxiety were frequent adverse events. Patients receiving molindone reported significantly higher rates of akathisia (P < .0008). Those receiving olanzapine reported significantly higher rates of weight gain (P < .0001) and were the only group with increased lipid and insulin serum levels and liver function tests. Patients in the risperidone group reported significantly higher rates of constipation (P < .021) and were the only group that experienced elevated serum prolactin.
Source: Sikich L, Frazier JA, McClellan J, et al. Double-blind comparison of first- and second-generation antipsychotics in early-onset schizophrenia and schizoaffective disorder: findings from the Treatment of Early-Onset Schizophrenia Spectrum disorders (TEOSS) study. Am J Psychiatry. 2008;165:1420-1431
Choosing antipsychotics
DR. KOWATCH: Let’s say you’re seeing psychosis in a 12-year-old whom you think is schizophrenic, and he or she has not yet received an antipsychotic. What are your top 3 treatment choices?
DR. FRAZIER: The first agent I usually select is risperidone. We have the most data on the use of this atypical antipsychotic in children and adolescents, and most psychotic children I see do better with a bit more D2 blockade than some of the other atypicals provide. That said, I remain concerned about risperidone’s side effects—such as weight gain and increased serum prolactin—so my usual second-line agent is aripiprazole.
I became interested in the complicated overlap between pervasive developmental disorders spectrum and psychotic disorders early in my training. More is known about prodromal symptoms in adolescents and adults than in children.
A group in the Netherlands5 compared 32 adolescents with severe early deficits in affect regulation, anxiety, disturbed social relationships, and thought disorder (characterized as “multiple complex developmental disorder” [MCDD]) with 80 adolescents with prodromal psychotic symptoms who met criteria for “at-risk mental state” (ARMS). Three-quarters of the children with MCDD (78%) were found to meet criteria for ARMS, and the 2 groups showed similar schizotypal traits, disorganization, and prodromal symptoms.
Signs of progression to psychosis and schizophrenia in children typically include:
- change in personality
- decrease in functioning or decline in ability to perform at school
- unusual thoughts or behaviors
- crippling anxiety
- supersensitivity to stress.
With experience, the clinician can more clearly differentiate the prodromal signs of psychosis from normal childhood behaviors. Children who are psychotic often don’t make good eye contact. When you try to engage them in discussion about hearing voices, they’re inattentive and internally preoccupied.
Normal vs psychotic children. You want a child in the latency age to have a rich fantasy life. If they do not, that raises concerns. Both normal and psychotic children sometimes say an imaginary friend told them something. Normal children eventually will admit this friend is imaginary. When children are psychotic, especially at an early age, you can’t pull them out of thinking about the imaginary friend, and they can’t distinguish fantasy from reality. Psychotic children also hear imaginary friends talking to them much more often.
Normal children usually are not afraid of their imaginary friends, whereas psychotic children—particularly adolescents—often are afraid of the voices they hear. However, if a psychotic child has heard voices from a young age, the voices aren’t always ego-dystonic. The girl I mentioned at the beginning of this article likes having the voices around. In fact, she gets uncomfortable when the voices are quiet.—Jean A. Frazier, MD
DR. KOWATCH: Why do you like aripiprazole for this patient population?
DR. FRAZIER: Aripiprazole doesn’t tend to be associated with as much weight gain as olanzapine or risperidone, although I’ve had children—especially in the autism spectrum—who have gained quite a bit of weight on aripiprazole. Clinically, I’ve noticed that aripiprazole seems to brighten up children’s affect. It also seems to help many children in my practice with attentional symptoms, although that’s anecdotal.
Although we don’t have a lot of data to inform this discussion about aripiprazole, a placebo-controlled study of 302 adolescents diagnosed with schizophrenia showed that aripiprazole, 10 mg/d, targeted negative symptoms fairly well, based on changes from baseline in PANSS (Positive and Negative Syndrome Scale) total scores. This was a 6-week multicenter, double-blind, randomized, trial.2
Ultimately, cognition in patients with schizophrenia is the strongest predictor of success in the workplace and in school. We need data on what happens to neurocognitive functioning with aripiprazole—and all the other atypical agents.
DR. KOWATCH: What would be your third-line agent?
DR. FRAZIER: Well, that varies for me. I’m trying to match the medication I use with the individual patient, and at this point I prescribe based on the side-effect profile more than anything else. I also consider if the child has a family member who has suffered from a similar condition and what agents the family member responded to.
Let’s say I have a child who has tried 1 or 2 atypical antipsychotics and has not had a good response. Many times I decide to try yet another atypical, and often I will try quetiapine. But after a patient has not responded to 2 atypicals, I might start thinking about a typical agent or clozapine. I use clozapine quite a bit. I find it is the most efficacious agent available, and the data speak to this as well.3,4 It has been truly remarkable for some children in my practice.
Less than 50% chance of efficacy?
DR. KOWATCH: The TEOSS study found 50% or lower response rates across 8 weeks of antipsychotic treatment. Clinically, what kind of response rates do you see with antipsychotics in children and adolescents?
DR. FRAZIER: I probably see about a 50% response rate in my practice as well. It’s variable, and the earlier the onset of the illness, the harder it is to treat.
DR. KOWATCH: Do you ever combine a typical antipsychotic with an atypical?
DR. FRAZIER: I try not to, but a number of children in the schizophrenia spectrum have enduring positive symptoms after 2 or 3 trials of atypical antipsychotics. Sometimes adding a touch of a typical agent can improve the situation. The typicals I usually try are perphenazine (around 8 to 16 mg/d) or molin done (around 20 to 60 mg/d). Sometimes I use a very low dose of haloperidol (such as 0.5 to 2 mg/d) with an atypical agent, and it can be quite effective.
DR. KOWATCH: That has been our experience as well; sometimes combining typical and atypical agents improves response. Besides medications, what do you consider an optimal treatment plan for a child or adolescent with psychosis?
DR. FRAZIER: These children need a multi-modal approach. Pharmacotherapy is the cornerstone because you want to decrease positive symptoms of psychosis, but often these children require therapeutic school placements or residential programs. If they’re old enough, cognitive-behavioral therapy can help by teaching them skills to manage ongoing psychotic symptoms. Older teens often have comorbid substance abuse and may require substance abuse intervention.
Are antipsychotics overused?
DR. KOWATCH: Do you think antipsychotics are overused in pediatric patients with psychosis?
DR. FRAZIER: In pediatric patients with psychosis? No.
DR. KOWATCH: What about in pediatric patients with behavioral disorders?
DR. FRAZIER: We need more studies to inform our practice and to be mindful of the evidence. Most children with schizophrenia have substantial developmental challenges (Box 2).5 In the autism spectrum, often an atypical antipsychotic is the only agent that can help a patient who is aggressive, self-injurious, or agitated.
In terms of bipolar disorder in children and adolescents, it would be ideal if we had more head-to-head comparator studies to inform our prescriptive practice. For example, we need more studies comparing traditional mood stabilizers such as lithium with the atypical agents.
Of course it would be ideal if we could use monotherapy in children who suffer from bipolar disorder and schizophrenia. But early-onset bipolar disorder—like early-onset schizophrenia—can be very difficult to treat and often requires more than 1 agent.
In a recent study of a pharmacotherapy algorithm for treating pediatric bipolar disorder,3 the children who did the best were on a combination of a mood stabilizer and an atypical antipsychotic. That has been my experience, too. I do my best to manage children on a mood stabilizer alone, but I rarely have been able to do that.
In terms of attention-deficit/hyperactivity disorder (ADHD), it depends on what’s going on with the child. Certain children with an ADHD diagnosis have complicated behavioral issues. First I would wonder if they have a different diagnosis, particularly if it gets to the point that an atypical agent is being considered. But sometimes it becomes a question of treating pronounced aggression. We need more studies to inform what we do. Some studies indicate that stimulants can be quite helpful for the aggressive child with ADHD.7
DR. KOWATCH: I don’t see any child and adolescent psychiatrist in the United States using antipsychotics to treat uncomplicated ADHD. The kids we see [at specialty clinics] have comorbid problems such as conduct disorder, oppositional-defiant disorder, mood instability—whatever you want to call it. And we’re seeing these patients because they haven’t done well on other medications, such as stimulants. Usually the parents are desperate because these children are moody and aggressive. I don’t think anybody wants to treat children with antipsychotics or mood stabilizers, but it’s what keeps these children well.
DR. FRAZIER: Yes, I agree.
Related resources
- Longitudinal assessment and monitoring of clinical status and brain function in adolescents and adults. Boston Center for Intervention Development and Applied Research (CIDAR) study. www.bostoncidar.org.
- Frazier JA, Hodge S, Breeze JL, et al. Diagnostic and sex effects on limbic volumes in early-onset bipolar disorder and schizophrenia. Schizophr Bull. 2008;34(1):37-46.
- Frazier JA, McClellan J, Findling RL, et al. Treatment of Early-Onset Schizophrenia Spectrum disorders (TEOSS): demographic and clinical characteristics. J Am Acad Child Adolesc Psychiatry. 2007;46:979-988.
Drug brand names
- Aripiprazole • Abilify
- Benztropine • Cogentin
- Clozapine • Clozaril
- Haloperidol • Haldol
- Metformin • Glucophage
- Molindone • Moban
- Olanzapine • Zyprexa
- Perphenazine • Trilafon
- Quetiapine • Seroquel
- Risperidone • Risperdal
Disclosures
Dr. Kowatch receives grant/research support from the Stanley Foundation, National Institute of Mental Health, National Institute of Child Health and Human Development, and the National Alliance for Research on Schizophrenia and Depression. He is a consultant to AstraZeneca and Forest Pharmaceuticals and a speaker for AstraZeneca.
Dr. Frazier receives grant/research support from Bristol-Myers Squibb, Eli Lilly and Company, GlaxoSmithKline, Johnson & Johnson, Neuropharm, Otsuka America Pharmaceuticals, and Pfizer Inc.
1. Sikich L, Frazier JA, McClellan J, et al. Double-blind comparison of first- and second-generation antipsychotics in early-onset schizophrenia and schizoaffective disorder: findings from the Treatment of Early-Onset Schizophrenia Spectrum Disorders (TEOSS) study. Am J Psychiatry. 2008;165:1420-1431.
2. Findling RL, Robb A, Nyilas M, et al. A multiple-center, randomized, double-blind, placebo-controlled study of oral aripiprazole for treatment of adolescents with schizophrenia. Am J Psychiatry. 2008;165(11):1432-1441.
3. Findling RL, Frazier JA, Gerbino-Rosen G, et al. Is there a role for clozapine in the treatment of children and adolescents? J Am Acad Child Adolesc Psychiatry. 2007;46(3):423-428.
4. Kim Y, Kim BN, Cho SC, et al. Long-term sustained benefits of clozapine treatment in refractory early onset schizophrenia: a retrospective study in Korean children and adolescents. Hum Psychopharmacol. 2008;23(8):715-722.
5. Sprong M, Becker HE, Schothorst PF, et al. Pathways to psychosis: a comparison of the pervasive developmental disorder subtype multiple complex developmental disorder and the “at risk mental state.” Schizophr Res. 2008;99:38-47.
6. Pavuluri MN, Henry DB, Devineni B, et al. A pharmacotherapy algorithm for stabilization and maintenance of pediatric bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2004;43(7):859-867.
7. Sinzig J, Döpfner M, Lehmkuhl G, et al. Long-acting methylphenidate has an effect on aggressive behavior in children with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2007;17(4):421-432.
“A patient I’ve seen for a number of years had been diagnosed in the pervasive developmental disorder spectrum, but she was quite atypical. Her perseverative thinking focused on a fantasy world, and she was so preoccupied that it was very difficult to pull her out of it. Now at age 12, she has a full-blown psychotic disorder, and the fantasy world is enveloping her. She hears people talking to her all day long.”
Jean A. Frazier, MD, who treats this patient and other children with psychotic disorders, was 1 of 4 principal investigators in the Treatment of Early-Onset Schizophrenia Spectrum Disorders (TEOSS) study, a randomized, double-blind, multisite trial funded by the National Institute of Mental Health. The study, published in November 2008,1 compared the efficacy and tolerability of 3 antipsychotics—olanzapine, risperidone, and molindone—in pediatric patients with schizophrenia or schizoaffective disorder (Box 1).
Dr. Frazier discusses the unexpected findings of the TEOSS trial with Current Psychiatry Section Editor Robert A. Kowatch, MD, PhD. Based on the trial findings and her experience, she tells how she makes decisions when prescribing antipsychotics for children and adolescents with schizophrenia and related disorders.
DR. KOWATCH: The TEOSS trial found no significant differences in efficacy between molindone and the atypical antipsychotics (olanzapine and risperidone) included in the study. You’ve prescribed both typical and atypical antipsychotics in research and in your clinical practice. Do you believe there’s any difference between the 2 classes?
DR. FRAZIER: There are some differences. For example, treatment-refractory patients, especially young children, sometimes need more D2 blockade than some atypical antipsychotics provide. I’ve seen more extra pyramidal side effects with the typical antipsychotics than the atypicals, although it’s not uncommon to see some akathisia with aripiprazole or some dystonia and dyskinesia with risperidone.
DR. KOWATCH: What are the benefits and risks of using antipsychotics in young children?
DR. FRAZIER: The benefit is that antipsychotics can decrease children’s suffering and get them more centered in reality so they can enjoy their friends and progress in school. And when that happens, it’s wonderful. What are the risks? With the atypicals my greatest concern is weight gain, and with the typical agents it’s tardive dyskinesia.
DR. KOWATCH: Have you changed the way you prescribe antipsychotics as a result of the TEOSS study?
DR. FRAZIER: Actually, I have. Clinicians have to be very careful about selecting psychotropic agents that can worsen pediatric-onset obesity. Olanzapine is an effective agent for targeting psychosis and mood symptoms, but the weight gain associated with it is a concern. I do not prescribe olanzapine as much as I have in the past, although I keep it in my armamentarium and tend to reserve it for third- or fourth-line therapy.
I have found molindone to be quite effective in children with schizophrenia or schizoaffective disorder, especially in those who have gained a lot of weight on atypical antipsychotics. They usually lose weight on molindone.
DR. KOWATCH: Do you think the TEOSS study had adequate power to demonstrate differences among molindone, olanzapine, and risperidone?
DR. FRAZIER: We enrolled 119 patients—which is large for a study such as this—but we did not reach our target of 168 patients, which might have increased our power to detect differences. Among the children we did enroll, the 3 antipsychotics showed no difference in efficacy, but the meaningful finding of this study to me was the side effect profile of these agents.
DR. KOWATCH: You mean weight gain with olanzapine and extrapyramidal symptoms with molindone?
DR. FRAZIER: Yes.
Managing side effects
DR. KOWATCH: How do you manage antipsychotic side effects?
DR. FRAZIER: For any of the antipsychotics’ side effects, you have to decide whether to continue the agent or switch to another anti psychotic. For example, I’ve had a number of children—many with significant weight problems—whose psychotic symptoms have responded only to risperidone. So we put them back on risperidone, and the decision then becomes what can we do to help with the weight gain while continuing that agent.
For weight gain, I think the best intervention is diet, exercise, and drinking a lot of water, but that can be effective only if you engage the patient’s entire family in the intervention as well. Short of that, a number of pharmacologic interventions have been studied, although not specifically in children.
In an open-label trial our group conducted with 11 children age 10 to 18 years who had gained weight while taking atypical antipsychotics, metformin decreased lipid levels and body mass index but not significantly. I’ve followed these children in my practice, however, and all those who continued taking metformin over a period of months lost weight.
TEOSS study adds to debate about efficacy and tolerability
The 5-year National Institute of Mental Health-funded Treatment of Early-Onset Schizophrenia Spectrum Disorders (TEOSS) trial began with an ambitious goal: to compare the efficacy and safety of 1 typical and 2 atypical antipsychotics in children age 8 to 19 with schizophrenia. The primary hypothesis was that atypical agents would show greater efficacy and tolerability when given for 8 weeks. Instead, the atypical agents showed no greater efficacy, and adverse effects occurred with all 3 antipsychotics. Because the trial was designed for 168 subjects but enrolled 119, it may not have been adequately powered to detect differences among the 3 agents.
Medications: Most of the 116 children who received medications were severely ill with psychotic symptoms when randomly assigned to 1 of the 3 antipsychotics for 8 weeks of double-blind treatment. Administration began at the lowest dose in a set range and usually was increased to midrange within 10 to 14 days. Dosing remained flexible within these ranges:
- molindone, 10 to 140 mg/d (mean endpoint dose 59.9 mg/d)
- olanzapine, 2.5 to 20 mg/d (mean endpoint dose 11.4 mg/d)
- risperidone, 0.5 to 6 mg/d (mean endpoint dose 2.8 mg/d).
Benztropine, ≥1 mg/d, was given to all patients treated with molindone, 14% of those treated with olanzapine, and 34% of those treated with risperidone to prevent or manage akathisia.
Efficacy: Two criteria defined treatment response: a Clinical Global Impression improvement score of 1 or 2 and a ≥20% reduction in baseline Positive and Negative Syndrome Scale (PANSS) score. Tolerability outcomes included neurologic side effects, weight changes, laboratory analyses, vital signs, ECG, serious adverse events, and treatment discontinuation. Extrapyramidal symptoms were monitored with involuntary movement and akathisia scales.
Observed PANSS total score by week of treatment
Mean Positive and Negative Symptom Scale (PANSS) total scores of observed cases during each week of the TEOSS trial. Minimum possible PANSS score is 30; scores >60 typically are viewed as problematic.
Among the 70 patients who completed treatment (25 of 40 with molindone, 17 of 35 olanzapine, and 28 of 41 with risperidone), more than one-half failed to achieve an adequate response. Response rates were 50% with molindone, 34% with olanzapine, and 46% with risperidone. The atypical antipsychotics did not show greater efficacy than molindone, and mean reductions in psychotic symptoms were modest (20% to 34% on the PANSS). Mean medication doses were midrange and considered moderate.
Tolerability: Sedation, irritability, and anxiety were frequent adverse events. Patients receiving molindone reported significantly higher rates of akathisia (P < .0008). Those receiving olanzapine reported significantly higher rates of weight gain (P < .0001) and were the only group with increased lipid and insulin serum levels and liver function tests. Patients in the risperidone group reported significantly higher rates of constipation (P < .021) and were the only group that experienced elevated serum prolactin.
Source: Sikich L, Frazier JA, McClellan J, et al. Double-blind comparison of first- and second-generation antipsychotics in early-onset schizophrenia and schizoaffective disorder: findings from the Treatment of Early-Onset Schizophrenia Spectrum disorders (TEOSS) study. Am J Psychiatry. 2008;165:1420-1431
Choosing antipsychotics
DR. KOWATCH: Let’s say you’re seeing psychosis in a 12-year-old whom you think is schizophrenic, and he or she has not yet received an antipsychotic. What are your top 3 treatment choices?
DR. FRAZIER: The first agent I usually select is risperidone. We have the most data on the use of this atypical antipsychotic in children and adolescents, and most psychotic children I see do better with a bit more D2 blockade than some of the other atypicals provide. That said, I remain concerned about risperidone’s side effects—such as weight gain and increased serum prolactin—so my usual second-line agent is aripiprazole.
I became interested in the complicated overlap between pervasive developmental disorders spectrum and psychotic disorders early in my training. More is known about prodromal symptoms in adolescents and adults than in children.
A group in the Netherlands5 compared 32 adolescents with severe early deficits in affect regulation, anxiety, disturbed social relationships, and thought disorder (characterized as “multiple complex developmental disorder” [MCDD]) with 80 adolescents with prodromal psychotic symptoms who met criteria for “at-risk mental state” (ARMS). Three-quarters of the children with MCDD (78%) were found to meet criteria for ARMS, and the 2 groups showed similar schizotypal traits, disorganization, and prodromal symptoms.
Signs of progression to psychosis and schizophrenia in children typically include:
- change in personality
- decrease in functioning or decline in ability to perform at school
- unusual thoughts or behaviors
- crippling anxiety
- supersensitivity to stress.
With experience, the clinician can more clearly differentiate the prodromal signs of psychosis from normal childhood behaviors. Children who are psychotic often don’t make good eye contact. When you try to engage them in discussion about hearing voices, they’re inattentive and internally preoccupied.
Normal vs psychotic children. You want a child in the latency age to have a rich fantasy life. If they do not, that raises concerns. Both normal and psychotic children sometimes say an imaginary friend told them something. Normal children eventually will admit this friend is imaginary. When children are psychotic, especially at an early age, you can’t pull them out of thinking about the imaginary friend, and they can’t distinguish fantasy from reality. Psychotic children also hear imaginary friends talking to them much more often.
Normal children usually are not afraid of their imaginary friends, whereas psychotic children—particularly adolescents—often are afraid of the voices they hear. However, if a psychotic child has heard voices from a young age, the voices aren’t always ego-dystonic. The girl I mentioned at the beginning of this article likes having the voices around. In fact, she gets uncomfortable when the voices are quiet.—Jean A. Frazier, MD
DR. KOWATCH: Why do you like aripiprazole for this patient population?
DR. FRAZIER: Aripiprazole doesn’t tend to be associated with as much weight gain as olanzapine or risperidone, although I’ve had children—especially in the autism spectrum—who have gained quite a bit of weight on aripiprazole. Clinically, I’ve noticed that aripiprazole seems to brighten up children’s affect. It also seems to help many children in my practice with attentional symptoms, although that’s anecdotal.
Although we don’t have a lot of data to inform this discussion about aripiprazole, a placebo-controlled study of 302 adolescents diagnosed with schizophrenia showed that aripiprazole, 10 mg/d, targeted negative symptoms fairly well, based on changes from baseline in PANSS (Positive and Negative Syndrome Scale) total scores. This was a 6-week multicenter, double-blind, randomized, trial.2
Ultimately, cognition in patients with schizophrenia is the strongest predictor of success in the workplace and in school. We need data on what happens to neurocognitive functioning with aripiprazole—and all the other atypical agents.
DR. KOWATCH: What would be your third-line agent?
DR. FRAZIER: Well, that varies for me. I’m trying to match the medication I use with the individual patient, and at this point I prescribe based on the side-effect profile more than anything else. I also consider if the child has a family member who has suffered from a similar condition and what agents the family member responded to.
Let’s say I have a child who has tried 1 or 2 atypical antipsychotics and has not had a good response. Many times I decide to try yet another atypical, and often I will try quetiapine. But after a patient has not responded to 2 atypicals, I might start thinking about a typical agent or clozapine. I use clozapine quite a bit. I find it is the most efficacious agent available, and the data speak to this as well.3,4 It has been truly remarkable for some children in my practice.
Less than 50% chance of efficacy?
DR. KOWATCH: The TEOSS study found 50% or lower response rates across 8 weeks of antipsychotic treatment. Clinically, what kind of response rates do you see with antipsychotics in children and adolescents?
DR. FRAZIER: I probably see about a 50% response rate in my practice as well. It’s variable, and the earlier the onset of the illness, the harder it is to treat.
DR. KOWATCH: Do you ever combine a typical antipsychotic with an atypical?
DR. FRAZIER: I try not to, but a number of children in the schizophrenia spectrum have enduring positive symptoms after 2 or 3 trials of atypical antipsychotics. Sometimes adding a touch of a typical agent can improve the situation. The typicals I usually try are perphenazine (around 8 to 16 mg/d) or molin done (around 20 to 60 mg/d). Sometimes I use a very low dose of haloperidol (such as 0.5 to 2 mg/d) with an atypical agent, and it can be quite effective.
DR. KOWATCH: That has been our experience as well; sometimes combining typical and atypical agents improves response. Besides medications, what do you consider an optimal treatment plan for a child or adolescent with psychosis?
DR. FRAZIER: These children need a multi-modal approach. Pharmacotherapy is the cornerstone because you want to decrease positive symptoms of psychosis, but often these children require therapeutic school placements or residential programs. If they’re old enough, cognitive-behavioral therapy can help by teaching them skills to manage ongoing psychotic symptoms. Older teens often have comorbid substance abuse and may require substance abuse intervention.
Are antipsychotics overused?
DR. KOWATCH: Do you think antipsychotics are overused in pediatric patients with psychosis?
DR. FRAZIER: In pediatric patients with psychosis? No.
DR. KOWATCH: What about in pediatric patients with behavioral disorders?
DR. FRAZIER: We need more studies to inform our practice and to be mindful of the evidence. Most children with schizophrenia have substantial developmental challenges (Box 2).5 In the autism spectrum, often an atypical antipsychotic is the only agent that can help a patient who is aggressive, self-injurious, or agitated.
In terms of bipolar disorder in children and adolescents, it would be ideal if we had more head-to-head comparator studies to inform our prescriptive practice. For example, we need more studies comparing traditional mood stabilizers such as lithium with the atypical agents.
Of course it would be ideal if we could use monotherapy in children who suffer from bipolar disorder and schizophrenia. But early-onset bipolar disorder—like early-onset schizophrenia—can be very difficult to treat and often requires more than 1 agent.
In a recent study of a pharmacotherapy algorithm for treating pediatric bipolar disorder,3 the children who did the best were on a combination of a mood stabilizer and an atypical antipsychotic. That has been my experience, too. I do my best to manage children on a mood stabilizer alone, but I rarely have been able to do that.
In terms of attention-deficit/hyperactivity disorder (ADHD), it depends on what’s going on with the child. Certain children with an ADHD diagnosis have complicated behavioral issues. First I would wonder if they have a different diagnosis, particularly if it gets to the point that an atypical agent is being considered. But sometimes it becomes a question of treating pronounced aggression. We need more studies to inform what we do. Some studies indicate that stimulants can be quite helpful for the aggressive child with ADHD.7
DR. KOWATCH: I don’t see any child and adolescent psychiatrist in the United States using antipsychotics to treat uncomplicated ADHD. The kids we see [at specialty clinics] have comorbid problems such as conduct disorder, oppositional-defiant disorder, mood instability—whatever you want to call it. And we’re seeing these patients because they haven’t done well on other medications, such as stimulants. Usually the parents are desperate because these children are moody and aggressive. I don’t think anybody wants to treat children with antipsychotics or mood stabilizers, but it’s what keeps these children well.
DR. FRAZIER: Yes, I agree.
Related resources
- Longitudinal assessment and monitoring of clinical status and brain function in adolescents and adults. Boston Center for Intervention Development and Applied Research (CIDAR) study. www.bostoncidar.org.
- Frazier JA, Hodge S, Breeze JL, et al. Diagnostic and sex effects on limbic volumes in early-onset bipolar disorder and schizophrenia. Schizophr Bull. 2008;34(1):37-46.
- Frazier JA, McClellan J, Findling RL, et al. Treatment of Early-Onset Schizophrenia Spectrum disorders (TEOSS): demographic and clinical characteristics. J Am Acad Child Adolesc Psychiatry. 2007;46:979-988.
Drug brand names
- Aripiprazole • Abilify
- Benztropine • Cogentin
- Clozapine • Clozaril
- Haloperidol • Haldol
- Metformin • Glucophage
- Molindone • Moban
- Olanzapine • Zyprexa
- Perphenazine • Trilafon
- Quetiapine • Seroquel
- Risperidone • Risperdal
Disclosures
Dr. Kowatch receives grant/research support from the Stanley Foundation, National Institute of Mental Health, National Institute of Child Health and Human Development, and the National Alliance for Research on Schizophrenia and Depression. He is a consultant to AstraZeneca and Forest Pharmaceuticals and a speaker for AstraZeneca.
Dr. Frazier receives grant/research support from Bristol-Myers Squibb, Eli Lilly and Company, GlaxoSmithKline, Johnson & Johnson, Neuropharm, Otsuka America Pharmaceuticals, and Pfizer Inc.
“A patient I’ve seen for a number of years had been diagnosed in the pervasive developmental disorder spectrum, but she was quite atypical. Her perseverative thinking focused on a fantasy world, and she was so preoccupied that it was very difficult to pull her out of it. Now at age 12, she has a full-blown psychotic disorder, and the fantasy world is enveloping her. She hears people talking to her all day long.”
Jean A. Frazier, MD, who treats this patient and other children with psychotic disorders, was 1 of 4 principal investigators in the Treatment of Early-Onset Schizophrenia Spectrum Disorders (TEOSS) study, a randomized, double-blind, multisite trial funded by the National Institute of Mental Health. The study, published in November 2008,1 compared the efficacy and tolerability of 3 antipsychotics—olanzapine, risperidone, and molindone—in pediatric patients with schizophrenia or schizoaffective disorder (Box 1).
Dr. Frazier discusses the unexpected findings of the TEOSS trial with Current Psychiatry Section Editor Robert A. Kowatch, MD, PhD. Based on the trial findings and her experience, she tells how she makes decisions when prescribing antipsychotics for children and adolescents with schizophrenia and related disorders.
DR. KOWATCH: The TEOSS trial found no significant differences in efficacy between molindone and the atypical antipsychotics (olanzapine and risperidone) included in the study. You’ve prescribed both typical and atypical antipsychotics in research and in your clinical practice. Do you believe there’s any difference between the 2 classes?
DR. FRAZIER: There are some differences. For example, treatment-refractory patients, especially young children, sometimes need more D2 blockade than some atypical antipsychotics provide. I’ve seen more extra pyramidal side effects with the typical antipsychotics than the atypicals, although it’s not uncommon to see some akathisia with aripiprazole or some dystonia and dyskinesia with risperidone.
DR. KOWATCH: What are the benefits and risks of using antipsychotics in young children?
DR. FRAZIER: The benefit is that antipsychotics can decrease children’s suffering and get them more centered in reality so they can enjoy their friends and progress in school. And when that happens, it’s wonderful. What are the risks? With the atypicals my greatest concern is weight gain, and with the typical agents it’s tardive dyskinesia.
DR. KOWATCH: Have you changed the way you prescribe antipsychotics as a result of the TEOSS study?
DR. FRAZIER: Actually, I have. Clinicians have to be very careful about selecting psychotropic agents that can worsen pediatric-onset obesity. Olanzapine is an effective agent for targeting psychosis and mood symptoms, but the weight gain associated with it is a concern. I do not prescribe olanzapine as much as I have in the past, although I keep it in my armamentarium and tend to reserve it for third- or fourth-line therapy.
I have found molindone to be quite effective in children with schizophrenia or schizoaffective disorder, especially in those who have gained a lot of weight on atypical antipsychotics. They usually lose weight on molindone.
DR. KOWATCH: Do you think the TEOSS study had adequate power to demonstrate differences among molindone, olanzapine, and risperidone?
DR. FRAZIER: We enrolled 119 patients—which is large for a study such as this—but we did not reach our target of 168 patients, which might have increased our power to detect differences. Among the children we did enroll, the 3 antipsychotics showed no difference in efficacy, but the meaningful finding of this study to me was the side effect profile of these agents.
DR. KOWATCH: You mean weight gain with olanzapine and extrapyramidal symptoms with molindone?
DR. FRAZIER: Yes.
Managing side effects
DR. KOWATCH: How do you manage antipsychotic side effects?
DR. FRAZIER: For any of the antipsychotics’ side effects, you have to decide whether to continue the agent or switch to another anti psychotic. For example, I’ve had a number of children—many with significant weight problems—whose psychotic symptoms have responded only to risperidone. So we put them back on risperidone, and the decision then becomes what can we do to help with the weight gain while continuing that agent.
For weight gain, I think the best intervention is diet, exercise, and drinking a lot of water, but that can be effective only if you engage the patient’s entire family in the intervention as well. Short of that, a number of pharmacologic interventions have been studied, although not specifically in children.
In an open-label trial our group conducted with 11 children age 10 to 18 years who had gained weight while taking atypical antipsychotics, metformin decreased lipid levels and body mass index but not significantly. I’ve followed these children in my practice, however, and all those who continued taking metformin over a period of months lost weight.
TEOSS study adds to debate about efficacy and tolerability
The 5-year National Institute of Mental Health-funded Treatment of Early-Onset Schizophrenia Spectrum Disorders (TEOSS) trial began with an ambitious goal: to compare the efficacy and safety of 1 typical and 2 atypical antipsychotics in children age 8 to 19 with schizophrenia. The primary hypothesis was that atypical agents would show greater efficacy and tolerability when given for 8 weeks. Instead, the atypical agents showed no greater efficacy, and adverse effects occurred with all 3 antipsychotics. Because the trial was designed for 168 subjects but enrolled 119, it may not have been adequately powered to detect differences among the 3 agents.
Medications: Most of the 116 children who received medications were severely ill with psychotic symptoms when randomly assigned to 1 of the 3 antipsychotics for 8 weeks of double-blind treatment. Administration began at the lowest dose in a set range and usually was increased to midrange within 10 to 14 days. Dosing remained flexible within these ranges:
- molindone, 10 to 140 mg/d (mean endpoint dose 59.9 mg/d)
- olanzapine, 2.5 to 20 mg/d (mean endpoint dose 11.4 mg/d)
- risperidone, 0.5 to 6 mg/d (mean endpoint dose 2.8 mg/d).
Benztropine, ≥1 mg/d, was given to all patients treated with molindone, 14% of those treated with olanzapine, and 34% of those treated with risperidone to prevent or manage akathisia.
Efficacy: Two criteria defined treatment response: a Clinical Global Impression improvement score of 1 or 2 and a ≥20% reduction in baseline Positive and Negative Syndrome Scale (PANSS) score. Tolerability outcomes included neurologic side effects, weight changes, laboratory analyses, vital signs, ECG, serious adverse events, and treatment discontinuation. Extrapyramidal symptoms were monitored with involuntary movement and akathisia scales.
Observed PANSS total score by week of treatment
Mean Positive and Negative Symptom Scale (PANSS) total scores of observed cases during each week of the TEOSS trial. Minimum possible PANSS score is 30; scores >60 typically are viewed as problematic.
Among the 70 patients who completed treatment (25 of 40 with molindone, 17 of 35 olanzapine, and 28 of 41 with risperidone), more than one-half failed to achieve an adequate response. Response rates were 50% with molindone, 34% with olanzapine, and 46% with risperidone. The atypical antipsychotics did not show greater efficacy than molindone, and mean reductions in psychotic symptoms were modest (20% to 34% on the PANSS). Mean medication doses were midrange and considered moderate.
Tolerability: Sedation, irritability, and anxiety were frequent adverse events. Patients receiving molindone reported significantly higher rates of akathisia (P < .0008). Those receiving olanzapine reported significantly higher rates of weight gain (P < .0001) and were the only group with increased lipid and insulin serum levels and liver function tests. Patients in the risperidone group reported significantly higher rates of constipation (P < .021) and were the only group that experienced elevated serum prolactin.
Source: Sikich L, Frazier JA, McClellan J, et al. Double-blind comparison of first- and second-generation antipsychotics in early-onset schizophrenia and schizoaffective disorder: findings from the Treatment of Early-Onset Schizophrenia Spectrum disorders (TEOSS) study. Am J Psychiatry. 2008;165:1420-1431
Choosing antipsychotics
DR. KOWATCH: Let’s say you’re seeing psychosis in a 12-year-old whom you think is schizophrenic, and he or she has not yet received an antipsychotic. What are your top 3 treatment choices?
DR. FRAZIER: The first agent I usually select is risperidone. We have the most data on the use of this atypical antipsychotic in children and adolescents, and most psychotic children I see do better with a bit more D2 blockade than some of the other atypicals provide. That said, I remain concerned about risperidone’s side effects—such as weight gain and increased serum prolactin—so my usual second-line agent is aripiprazole.
I became interested in the complicated overlap between pervasive developmental disorders spectrum and psychotic disorders early in my training. More is known about prodromal symptoms in adolescents and adults than in children.
A group in the Netherlands5 compared 32 adolescents with severe early deficits in affect regulation, anxiety, disturbed social relationships, and thought disorder (characterized as “multiple complex developmental disorder” [MCDD]) with 80 adolescents with prodromal psychotic symptoms who met criteria for “at-risk mental state” (ARMS). Three-quarters of the children with MCDD (78%) were found to meet criteria for ARMS, and the 2 groups showed similar schizotypal traits, disorganization, and prodromal symptoms.
Signs of progression to psychosis and schizophrenia in children typically include:
- change in personality
- decrease in functioning or decline in ability to perform at school
- unusual thoughts or behaviors
- crippling anxiety
- supersensitivity to stress.
With experience, the clinician can more clearly differentiate the prodromal signs of psychosis from normal childhood behaviors. Children who are psychotic often don’t make good eye contact. When you try to engage them in discussion about hearing voices, they’re inattentive and internally preoccupied.
Normal vs psychotic children. You want a child in the latency age to have a rich fantasy life. If they do not, that raises concerns. Both normal and psychotic children sometimes say an imaginary friend told them something. Normal children eventually will admit this friend is imaginary. When children are psychotic, especially at an early age, you can’t pull them out of thinking about the imaginary friend, and they can’t distinguish fantasy from reality. Psychotic children also hear imaginary friends talking to them much more often.
Normal children usually are not afraid of their imaginary friends, whereas psychotic children—particularly adolescents—often are afraid of the voices they hear. However, if a psychotic child has heard voices from a young age, the voices aren’t always ego-dystonic. The girl I mentioned at the beginning of this article likes having the voices around. In fact, she gets uncomfortable when the voices are quiet.—Jean A. Frazier, MD
DR. KOWATCH: Why do you like aripiprazole for this patient population?
DR. FRAZIER: Aripiprazole doesn’t tend to be associated with as much weight gain as olanzapine or risperidone, although I’ve had children—especially in the autism spectrum—who have gained quite a bit of weight on aripiprazole. Clinically, I’ve noticed that aripiprazole seems to brighten up children’s affect. It also seems to help many children in my practice with attentional symptoms, although that’s anecdotal.
Although we don’t have a lot of data to inform this discussion about aripiprazole, a placebo-controlled study of 302 adolescents diagnosed with schizophrenia showed that aripiprazole, 10 mg/d, targeted negative symptoms fairly well, based on changes from baseline in PANSS (Positive and Negative Syndrome Scale) total scores. This was a 6-week multicenter, double-blind, randomized, trial.2
Ultimately, cognition in patients with schizophrenia is the strongest predictor of success in the workplace and in school. We need data on what happens to neurocognitive functioning with aripiprazole—and all the other atypical agents.
DR. KOWATCH: What would be your third-line agent?
DR. FRAZIER: Well, that varies for me. I’m trying to match the medication I use with the individual patient, and at this point I prescribe based on the side-effect profile more than anything else. I also consider if the child has a family member who has suffered from a similar condition and what agents the family member responded to.
Let’s say I have a child who has tried 1 or 2 atypical antipsychotics and has not had a good response. Many times I decide to try yet another atypical, and often I will try quetiapine. But after a patient has not responded to 2 atypicals, I might start thinking about a typical agent or clozapine. I use clozapine quite a bit. I find it is the most efficacious agent available, and the data speak to this as well.3,4 It has been truly remarkable for some children in my practice.
Less than 50% chance of efficacy?
DR. KOWATCH: The TEOSS study found 50% or lower response rates across 8 weeks of antipsychotic treatment. Clinically, what kind of response rates do you see with antipsychotics in children and adolescents?
DR. FRAZIER: I probably see about a 50% response rate in my practice as well. It’s variable, and the earlier the onset of the illness, the harder it is to treat.
DR. KOWATCH: Do you ever combine a typical antipsychotic with an atypical?
DR. FRAZIER: I try not to, but a number of children in the schizophrenia spectrum have enduring positive symptoms after 2 or 3 trials of atypical antipsychotics. Sometimes adding a touch of a typical agent can improve the situation. The typicals I usually try are perphenazine (around 8 to 16 mg/d) or molin done (around 20 to 60 mg/d). Sometimes I use a very low dose of haloperidol (such as 0.5 to 2 mg/d) with an atypical agent, and it can be quite effective.
DR. KOWATCH: That has been our experience as well; sometimes combining typical and atypical agents improves response. Besides medications, what do you consider an optimal treatment plan for a child or adolescent with psychosis?
DR. FRAZIER: These children need a multi-modal approach. Pharmacotherapy is the cornerstone because you want to decrease positive symptoms of psychosis, but often these children require therapeutic school placements or residential programs. If they’re old enough, cognitive-behavioral therapy can help by teaching them skills to manage ongoing psychotic symptoms. Older teens often have comorbid substance abuse and may require substance abuse intervention.
Are antipsychotics overused?
DR. KOWATCH: Do you think antipsychotics are overused in pediatric patients with psychosis?
DR. FRAZIER: In pediatric patients with psychosis? No.
DR. KOWATCH: What about in pediatric patients with behavioral disorders?
DR. FRAZIER: We need more studies to inform our practice and to be mindful of the evidence. Most children with schizophrenia have substantial developmental challenges (Box 2).5 In the autism spectrum, often an atypical antipsychotic is the only agent that can help a patient who is aggressive, self-injurious, or agitated.
In terms of bipolar disorder in children and adolescents, it would be ideal if we had more head-to-head comparator studies to inform our prescriptive practice. For example, we need more studies comparing traditional mood stabilizers such as lithium with the atypical agents.
Of course it would be ideal if we could use monotherapy in children who suffer from bipolar disorder and schizophrenia. But early-onset bipolar disorder—like early-onset schizophrenia—can be very difficult to treat and often requires more than 1 agent.
In a recent study of a pharmacotherapy algorithm for treating pediatric bipolar disorder,3 the children who did the best were on a combination of a mood stabilizer and an atypical antipsychotic. That has been my experience, too. I do my best to manage children on a mood stabilizer alone, but I rarely have been able to do that.
In terms of attention-deficit/hyperactivity disorder (ADHD), it depends on what’s going on with the child. Certain children with an ADHD diagnosis have complicated behavioral issues. First I would wonder if they have a different diagnosis, particularly if it gets to the point that an atypical agent is being considered. But sometimes it becomes a question of treating pronounced aggression. We need more studies to inform what we do. Some studies indicate that stimulants can be quite helpful for the aggressive child with ADHD.7
DR. KOWATCH: I don’t see any child and adolescent psychiatrist in the United States using antipsychotics to treat uncomplicated ADHD. The kids we see [at specialty clinics] have comorbid problems such as conduct disorder, oppositional-defiant disorder, mood instability—whatever you want to call it. And we’re seeing these patients because they haven’t done well on other medications, such as stimulants. Usually the parents are desperate because these children are moody and aggressive. I don’t think anybody wants to treat children with antipsychotics or mood stabilizers, but it’s what keeps these children well.
DR. FRAZIER: Yes, I agree.
Related resources
- Longitudinal assessment and monitoring of clinical status and brain function in adolescents and adults. Boston Center for Intervention Development and Applied Research (CIDAR) study. www.bostoncidar.org.
- Frazier JA, Hodge S, Breeze JL, et al. Diagnostic and sex effects on limbic volumes in early-onset bipolar disorder and schizophrenia. Schizophr Bull. 2008;34(1):37-46.
- Frazier JA, McClellan J, Findling RL, et al. Treatment of Early-Onset Schizophrenia Spectrum disorders (TEOSS): demographic and clinical characteristics. J Am Acad Child Adolesc Psychiatry. 2007;46:979-988.
Drug brand names
- Aripiprazole • Abilify
- Benztropine • Cogentin
- Clozapine • Clozaril
- Haloperidol • Haldol
- Metformin • Glucophage
- Molindone • Moban
- Olanzapine • Zyprexa
- Perphenazine • Trilafon
- Quetiapine • Seroquel
- Risperidone • Risperdal
Disclosures
Dr. Kowatch receives grant/research support from the Stanley Foundation, National Institute of Mental Health, National Institute of Child Health and Human Development, and the National Alliance for Research on Schizophrenia and Depression. He is a consultant to AstraZeneca and Forest Pharmaceuticals and a speaker for AstraZeneca.
Dr. Frazier receives grant/research support from Bristol-Myers Squibb, Eli Lilly and Company, GlaxoSmithKline, Johnson & Johnson, Neuropharm, Otsuka America Pharmaceuticals, and Pfizer Inc.
1. Sikich L, Frazier JA, McClellan J, et al. Double-blind comparison of first- and second-generation antipsychotics in early-onset schizophrenia and schizoaffective disorder: findings from the Treatment of Early-Onset Schizophrenia Spectrum Disorders (TEOSS) study. Am J Psychiatry. 2008;165:1420-1431.
2. Findling RL, Robb A, Nyilas M, et al. A multiple-center, randomized, double-blind, placebo-controlled study of oral aripiprazole for treatment of adolescents with schizophrenia. Am J Psychiatry. 2008;165(11):1432-1441.
3. Findling RL, Frazier JA, Gerbino-Rosen G, et al. Is there a role for clozapine in the treatment of children and adolescents? J Am Acad Child Adolesc Psychiatry. 2007;46(3):423-428.
4. Kim Y, Kim BN, Cho SC, et al. Long-term sustained benefits of clozapine treatment in refractory early onset schizophrenia: a retrospective study in Korean children and adolescents. Hum Psychopharmacol. 2008;23(8):715-722.
5. Sprong M, Becker HE, Schothorst PF, et al. Pathways to psychosis: a comparison of the pervasive developmental disorder subtype multiple complex developmental disorder and the “at risk mental state.” Schizophr Res. 2008;99:38-47.
6. Pavuluri MN, Henry DB, Devineni B, et al. A pharmacotherapy algorithm for stabilization and maintenance of pediatric bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2004;43(7):859-867.
7. Sinzig J, Döpfner M, Lehmkuhl G, et al. Long-acting methylphenidate has an effect on aggressive behavior in children with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2007;17(4):421-432.
1. Sikich L, Frazier JA, McClellan J, et al. Double-blind comparison of first- and second-generation antipsychotics in early-onset schizophrenia and schizoaffective disorder: findings from the Treatment of Early-Onset Schizophrenia Spectrum Disorders (TEOSS) study. Am J Psychiatry. 2008;165:1420-1431.
2. Findling RL, Robb A, Nyilas M, et al. A multiple-center, randomized, double-blind, placebo-controlled study of oral aripiprazole for treatment of adolescents with schizophrenia. Am J Psychiatry. 2008;165(11):1432-1441.
3. Findling RL, Frazier JA, Gerbino-Rosen G, et al. Is there a role for clozapine in the treatment of children and adolescents? J Am Acad Child Adolesc Psychiatry. 2007;46(3):423-428.
4. Kim Y, Kim BN, Cho SC, et al. Long-term sustained benefits of clozapine treatment in refractory early onset schizophrenia: a retrospective study in Korean children and adolescents. Hum Psychopharmacol. 2008;23(8):715-722.
5. Sprong M, Becker HE, Schothorst PF, et al. Pathways to psychosis: a comparison of the pervasive developmental disorder subtype multiple complex developmental disorder and the “at risk mental state.” Schizophr Res. 2008;99:38-47.
6. Pavuluri MN, Henry DB, Devineni B, et al. A pharmacotherapy algorithm for stabilization and maintenance of pediatric bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2004;43(7):859-867.
7. Sinzig J, Döpfner M, Lehmkuhl G, et al. Long-acting methylphenidate has an effect on aggressive behavior in children with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2007;17(4):421-432.