User login
‘Morning sickness’ in pregnancy loses psychogenic stigma
Nausea and vomiting in pregnancy (NVP) is a misunderstood disorder associated with stress, anxiety, and depression. Prejudice toward women is thought to have guided the historical psychoanalytic concept of NVP as psychogenic, but this view is being replaced by newer biologic theories.
This article examines the evidence for psychological and organic causes of NVP to inform psychiatrists treating pregnant patients. We review guidelines for pharmacologic treatment of NVP and discuss potentially useful psychotherapies and alternative approaches.
Definitive cause unknown
“Morning sickness” affects 50% to 80% of pregnant women, occurring so commonly that NVP is often considered normal.1,2 Approximately 0.5% to 2% of women experience the most severe NVP—hyperemesis gravidarum (HG)3—characterized by intractable vomiting, weight loss, and electrolyte imbalance that can lead to hospitalization.
Without modern supportive care, HG can be lethal; although Charlotte Brontë’s death certificate states she died of “phthisis” (tuberculosis), the author of Jane Eyre is popularly believed to have succumbed to HG.4,5
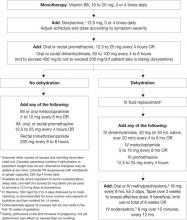
The search for effective NVP treatments has been disappointing, partly because no cause has been identified. After other conditions that may lead to nausea and vomiting are ruled out (Table 1), NVP medical management is supportive. Correcting dehydration and encouraging dietary and lifestyle changes (Table 2)6 are important adjuncts to step-wise pharmacologic treatment recommended by the American College of Obstetrics and Gynecology (Algorithm).7
Algorithm Pharmacologic treatment of nausea and vomiting in pregnancy*
Source: Adapted and reprinted with permission from Canadian Family Physician. Levichek Z, Atanackovic G, Oepkes D, et al. Nausea and vomiting of pregnancy. Evidence-based treatment algorithm. Can Fam Physician 2002;48:267-77Table 1
Medical causes of nausea and vomiting in pregnancy
| Possible cause | How to rule it out |
|---|---|
| Appendicitis | History; do physical, order imaging |
| Hepatitis | Jaundice; order liver function tests, antibody studies, imaging |
| Pancreatitis | History of alcohol use, abdominal pain; check amylase and lipase level |
| Gastrointestinal obstruction | History of surgeries; order imaging |
| Peptic ulcer disease | History; order upper GI series/endoscopy |
| Thyroid disease | Thyroid function tests |
| Urinary tract infection | Urinalysis, culture-sensitivity |
| Trophoblastic disease | Check hCG,* order ultrasound |
| * Elevated human chorionic gonadotropin (hCG) has shown evidence of an association with NVP20 | |
Table 2
Advice for patients: Strategies to manage NVP
| Correct dehydration |
| Drink small amount of fluids frequently |
| Dietary changes |
| Eat frequent, small meals |
| Avoid high-fat foods |
| Snack before getting out of bed and before going to sleep |
| Don’t force yourself to eat |
| Use candy and salty snacks to combat nausea |
| Avoid strong odors and scents; try cold foods, which may have less odor than hot foods |
| Take advantage of good days or good hours of the day for eating |
| Lifestyle changes |
| Get out of bed slowly |
| Lie down when nauseated |
| Avoid stressful situations |
| NVP: nausea and vomiting in pregnancy Source: Reference 6 |
Prejudice vs evidence
Psychological factors. Historically, psychological factors have been blamed for NVP, but support comes from a few poorly designed studies or case reports.3,4
Psychoanalytically, pregnancy and childbirth are significant events in a woman’s life and a rich environment for conflict that could lead to physical expression of symptoms. Freud believed pregnancy and childbirth involve the unconscious substitution of the penis with the child.8 Later writers viewed motherhood as woman’s most powerful wish and the primary organizer of her sexual drive and personality.8
NVP has been considered a conversion or somatization disorder in which symptoms are a “hysterical” expression of unconscious conflict. A psychoanalytic view contends that women who experience NVP are ambivalent about the pregnancy and seek to reject it.1,9 Vomiting, in this view, represents an oral abortion attempt.10 Others claim NVP is a rejection of femininity3 or that symptoms in women with overly attached maternal relationships mask unconscious aggressive feelings toward their mothers.11
Robertson11 proposed an association between NVP and a woman’s view of sexual experiences and her ability to achieve orgasm. He interviewed 100 women and found that 40 of 57 with NVP had “disturbed sexual functioning” or were “frigid” (defined as experiencing coitus as undesirable and unaccompanied by orgasm).
Higgins12 in 1887 proposed that the cause of NVP “is sexual intercourse, the husband too eager for it and the wife too adverse.” Additionally, NVP and HG have been associated with infantile, childish, immature, and hysterical personalities.12-14
Psychiatric comorbidities. Attempts have been made to associate NVP with other psychiatric disorders such as depression, bipolar disorder, schizophrenia, and anxiety disorders, including posttraumatic stress disorder (PTSD). No definitive association has been found between NVP and depressive illness, bipolar disorder, or schizophrenia15 or the use of antidepressants before or during early pregnancy.16 Studies reporting an association with depression have not established a cause-effect relationship.17
Seng18 reported increased NVP risk in women with PTSD. High levels of stress, anxiety, and depression found in women with NVP are thought to result from—rather than cause—NVP’s physical symptoms, however.3,19,20
Psychosocial stressors have been implicated, with higher NVP rates reported in unmarried women, those with unwanted or unplanned pregnancies, immigrants, and those living in crowded situations.21 NVP also is more frequent among women who experience emotionally disturbing events or interpersonal, economic, or occupational difficulties during pregnancy.22 Physical symptoms may provide secondary gain in attention and sympathy and a time-out from stressful home events.14
These psychosocial theories are poorly supported by data, but some clinicians may still believe NVP has a psychogenic cause. Lennane and Lennane23 proposed in 1973 that this perception may result from gender bias because:
- most conditions believed to have psychogenic causes affect women more than men
- the belief that NVP is psychogenic has been perpetuated primarily by male authors.
They argued that sexual prejudice may prevent women from receiving necessary symptomatic treatment and impede research into the cause of NVP.23
Gender bias continues to be found in the diagnosis of women with physical complaints. In 2006, Chiaramonte and Friend24 found strong, consistent gender bias among medical students and residents when evaluating women who reported coronary disease symptoms during stressful life events.
Organic theories
Organic theories view NVP as multifactorial, with contributions from evolution and multiple organ systems. Endocrine, vestibular, gastrointestinal, and CNS contributions have been described, but none have solved NVP’s etiologic mystery.
Evolutionary. NVP may provide an evolutionary advantage by protecting the embryo and mother. This theory states that potential toxins are present in many foods, especially if eaten in large quantities. NVP prevents the pregnant woman from eating very much and harming the embryo. Below-average miscarriage rates are seen in women with NVP.1,2,25
And because a woman’s immune system is depressed during pregnancy, NVP may be advantageous for the mother by limiting her ingestion of potential toxins.25
Endocrine. Human chorionic gonadotropin (hCG), estrogens, progesterone, and leptin, as well as adrenal cortex insufficiencies have been investigated for a role in NVP. Only hCG has shown clear evidence of an association, and some researchers believe it is the most likely cause of NVP.20
NVP rates are higher in pregnancies with elevated hCG. Molar and multiple-gestation pregnancies—each associated with elevated hCG—are complicated more frequently with the severest form of NVP.20,26 Conversely, NVP is less common in women who smoke, which is associated with lower hCG.26
During pregnancy, actions of hCG stimulate the thyroid. Hyperstimulation, leading to transient hyperthyroidism, has been implicated in NVP development.20,26 Symptom severity and the degree of thyroid stimulating hormone (TSH) suppression are closely correlated.26
Elevated hCG levels, hypersensitive TSH receptors, and the presence of a hyperactive hCG isoform have been proposed.20
Gastrointestinal disorders are believed to be involved in the pathogenesis of persistent NVP. Women with NVP usually lack structural or mucosal abnormalities and have normal endoscopic upper GI evaluations. They may, however, have disorders of the stomach’s neuromuscular function. Severe cases of gastric dysrhythmias and abnormalities of gastric tone may lead to gastroparesis.27
Stomach motility in pregnancy is influenced by neurohormonal changes, specifically in estrogen and thyroid hormones. Gastric motility abnormalities—evaluated by electrogastrography (EGG)—have been associated with NVP symptoms and normal EGGs with the absence of symptoms. Some women who had NVP and abnormal EGGs were retested after delivery when symptom-free and found to have normal myoelectric EGG patterns.27
Helicobacter pylori also may be involved in NVP, and at least 1 study found active H pylori infection and HG to be highly correlated. Pregnancy is not believed to predispose to H pylori infection, but active infection compounded by pregnancy’s hormonal changes may exacerbate NVP.28
NVP and motion sickness share many features, suggesting that NVP treatment could be targeted if a vestibular disorder could be discovered.29 Abnormal electroencephalography—particularly generalized slowing—that is not present in asymptomatic pregnant women has been reported in women with NVP.30
CNS contributions. Persistent NVP may be a learned behavior,24 a view based on findings of anticipatory nausea and vomiting in chemotherapy patients. Through conditioning, a pregnant woman may associate her physical symptoms with elements in her life that maintain the cycle of nausea and vomiting.31
Treating psychological symptoms
Brief psychotherapy to identify and correct sources of anxiety in pregnancy may alleviate a patient’s nausea and vomiting.32
- Progressive muscle relaxation training, often combined with guided imagery, can decrease nausea and vomiting associated with chemotherapy and may prevent anticipatory symptoms by decreasing anxiety.
- Systematic desensitization is successful in most chemotherapy patients who try it. In this technique, relaxation is counter-conditioned as a response to stimuli known to elicit symptoms.31
Hypnosis allows patients to achieve a physiologic state incompatible with nausea and vomiting31 and can terminate vomiting after 1 to 3 sessions.3
Medication. Similar to ondansetron, the antidepressant mirtazapine exhibits an antiemetic effect by blocking the 5-HT3 receptor. In treatment-resistant cases, mirtazapine, 30 mg/d, has been reported to ameliorate NVP symptoms, usually within 24 hours. Patients were able to return to normal diets and discontinue treatment after 6 to 10 days. Mirtazapine appears to be safe during pregnancy, based on animal studies using 17 and 20 times the maximum recommended human dose.33
For patients with anxiety symptoms, consider other medications—including selective serotonin reuptake inhibitors and benzodiazepines—only after counseling the patient about potential risks and benefits to her and the fetus.34
Alternative treatments. In traditional Indian medicine, a mixture of powdered ginger and honey is given to women with NVP. At least 2 studies demonstrate ginger’s efficacy.35
In traditional Chinese medicine, stimulating the Neiguan point (P6) on the wrist is believed to relieve nausea and vomiting. Although results are inconclusive, studies suggest that P6 stimulation can help control NVP.36 The FDA has approved wristbands that stimulate the P6 site, either electrically or by acupressure (Figure).36
Figure Wristband to manage nausea and vomiting in pregnancy
The FDA-cleared BioBand acupressure wristband may help control nausea and vomiting in pregnancy by stimulating the Neiguan point (P6) on the wrist.
Source: Reference 36Consider thiamine supplementation for women with severe symptoms, as Wernicke’s encephalopathy is a rare complication of prolonged NVP.19,37
Related resources
- Motherisk Program at The Hospital for Sick Children, Toronto, Ontario, Canada. Website with information on vomiting during pregnancy: www.motherisk.org/women/morningSickness.jsp. Nausea and vomiting of pregnancy (NVP) forum. www.motherisk.org/women/forum.jsp.
- BioBand acupuncture wristband. www.BioBands.com.
- Koren G, Bishai R, eds. Nausea and vomiting of pregnancy: state of the art 2000. Toronto, Ontario, Canada: The Motherisk Program; 2000.
Drug brand names
- Dimenhydrinate • Dramamine
- Doxylamine • Unisom
- Methylprednisolone • Medrol
- Metoclopramide • Reglan
- Mirtazapine • Remeron
- Ondansetron • Zofran
- Promethazine • Phenergan
- Trimethobenzamide • Tigan
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. el-Mallakh RS, Liebowitz NR, Hale MS. Hyperemesis gravidarum as conversion disorder. J Nerv Ment Dis 1990;178:655-9.
2. Davis M. Nausea and vomiting of pregnancy: an evidence-based review. J Perinat Neonatal Nurs 2004;18:312-28.
3. Buckwalter JG, Simpson SW. Psychological factors in the etiology and treatment of severe nausea and vomiting in pregnancy. Am J Obstet Gynecol 2002;186(5 suppl):S210-4.
4. Bogen JT. Neurosis: a Ms-diagnosis. Perspect Biol Med 1994;37(2):263-74.
5. Weiss G. The death of Charlotte Brontë. Obstet Gynecol 1991;78(4):705-8.
6. Lester EP, Notman MT. Pregnancy, developmental crisis and object relations: psychoanalytic considerations. Int J Psychoanal 1986;67(pt 3):357-66.
7. Sheehan P. Hyperemesis gravidarum—assessment and management. Aust Fam Physician 2007;36:698-701.
8. American College of Obstetrics and Gynecology. ACOG practice bulletin: nausea and vomiting of pregnancy. Obstet Gynecol 2004;103:803-14.
9. Iancu I, Kotler M, Spivak B, et al. Psychiatric aspects of hyperemesis gravidarum. Psychother Psychosom 1994;61:143-9.
10. Munch S. Chicken or the egg? The biological-psychological controversy surrounding hyperemesis gravidarum. Soc Sci Med 2002;55:1267-78.
11. Robertson GG. Nausea and vomiting of pregnancy: a study in psychosomatic and social medicine. Lancet 1946;336-45.
12. Fairweather DV. Nausea and vomiting in pregnancy. Am J Obstet Gynecol 1968;102:135-75.
13. Katon WJ, Ries RK, Bokan JA, Kleinman A. Hyperemesis gravidarum: a biopsychosocial perspective. Int J Psychiatry Med 1980;10:151-62.
14. Simpson SW, Goodwin TM, Robins SB, et al. Psychological factors and hyperemesis gravidarum. J Womens Health Gend Based Med 2001;10:471-7.
15. Majerus PW, Guze SB, Delong WB, Robins E. Psychologic factors and psychiatric disease in hyperemesis gravidarum: a follow-up study of 69 vomiters and 66 controls. Am J Psychiatry 1960;117:421-8.
16. Bozzo P, Koren G, Nava-Ocampo AA, Einarson A. The incidence of nausea and vomiting of pregnancy (NVP): a comparison between depressed women treated with antidepressants and non-depressed women. Clin Invest Med 2006;29(6):347-50.
17. Markl GE, Strunz-Lehner C, Egen-Lappe V, et al. The association of psychosocial factors with nausea and vomiting during pregnancy. J Psychosom Obstet Gynaecol 2007;1-6.
18. Seng JS, Oakley DJ, Sampselle CM, et al. Posttraumatic stress disorder and pregnancy complications. Obstet Gynecol 2001;97(1):17-22.
19. Ismail SK, Kenny L. Review on hyperemesis gravidarum. Best Pract Res Clin Gastroenterol 2007;21:755-69.
20. Verberg MF, Gillott DJ, Al-Fardan N, Grudzinskas JG. Hyperemesis gravidarum, a literature review. Hum Reprod Update 2005;11:527-39.
21. Deuchar N. Nausea and vomiting in pregnancy: a review of the problem with particular regard to psychological and social aspects. Br J Obstet Gynaecol 1995;102(1):6-8.
22. Iatrakis GM, Sakellaropoulos GG, Kourkoubas AH, Kabounia SE. Vomiting and nausea in the first 12 weeks of pregnancy. Psychother Psychosom 1988;49(1):22-4.
23. Lennane KJ, Lennane RJ. Alleged psychogenic disorders in women—a possible manifestation of sexual prejudice. N Engl J Med 1973;288(6):288-92.
24. Chiaramonte GR, Friend R. Medical students’ and residents’ gender bias in the diagnosis, treatment, and interpretation of coronary heart disease symptoms. Health Psychol 2006;25:255-66.
25. Sherman PW, Flaxman SM. Nausea and vomiting of pregnancy in an evolutionary perspective. Am J Obstet Gynecol 2002;186(5 suppl):S190-7.
26. Goodwin TM. Nausea and vomiting of pregnancy: an obstetric syndrome. Am J Obstet Gynecol 2002;186(5 suppl):S184-9.
27. Koch KL. Gastrointestinal factors in nausea and vomiting of pregnancy. Am J Obstet Gynecol 2002;186(5 suppl):S198-203.
28. Golberg D, Szilagyi A, Graves L. Hyperemesis gravidarum and Helicobacter pylori infection: a systematic review. Obstet Gynecol 2007;110:695-703.
29. Black FO. Maternal susceptibility to nausea and vomiting of pregnancy: is the vestibular system involved? Am J Obstet Gynecol 2002;186(5 suppl):S204-9.
30. Vaknin Z, Halperin R, Schneider D, et al. Hyperemesis gravidarum and nonspecific abnormal EEG findings: a preliminary report. J Reprod Med 2006;51:623-7.
31. Matteson S, Roscoe J, Hickok J, Morrow GR. The role of behavioral conditioning in the development of nausea. Am J Obstet Gynecol 2002;186(5 suppl):S239-43.
32. Zechnich R, Hammer T. Brief psychotherapy for hyperemesis gravidarum. Am Fam Physician 1982;26:179-81.
33. Guclu S, Gol M, Dogan E, Saygili U. Mirtazapine use in resistant hyperemesis gravidarum: report of three cases and review of the literature. Arch Gynecol Obstet 2005;272:298-300.
34. Raphael DB, Ross J, Brizendine L. Treating anxiety during pregnancy: Just how safe are SSRIs? Current Psychiatry 2008;7(2):39-52.
35. Niebyl JR, Goodwin TM. Overview of nausea and vomiting of pregnancy with an emphasis on vitamins and ginger. Am J Obstet Gynecol 2002;186(5 suppl):S253-5.
36. Roscoe JA, Matteson SE. Acupressure and acustimulation bands for control of nausea: a brief review. Am J Obstet Gynecol 2002;186(5 suppl):S244-7.
37. Chiossi G, Neri I, Cavazzuti M, et al. Hyperemesis gravidarum complicated by Wernicke encephalopathy: background, case report, and review of the literature. Obstet Gynecol Surv 2006;61:255-68.
Nausea and vomiting in pregnancy (NVP) is a misunderstood disorder associated with stress, anxiety, and depression. Prejudice toward women is thought to have guided the historical psychoanalytic concept of NVP as psychogenic, but this view is being replaced by newer biologic theories.
This article examines the evidence for psychological and organic causes of NVP to inform psychiatrists treating pregnant patients. We review guidelines for pharmacologic treatment of NVP and discuss potentially useful psychotherapies and alternative approaches.
Definitive cause unknown
“Morning sickness” affects 50% to 80% of pregnant women, occurring so commonly that NVP is often considered normal.1,2 Approximately 0.5% to 2% of women experience the most severe NVP—hyperemesis gravidarum (HG)3—characterized by intractable vomiting, weight loss, and electrolyte imbalance that can lead to hospitalization.
Without modern supportive care, HG can be lethal; although Charlotte Brontë’s death certificate states she died of “phthisis” (tuberculosis), the author of Jane Eyre is popularly believed to have succumbed to HG.4,5
The search for effective NVP treatments has been disappointing, partly because no cause has been identified. After other conditions that may lead to nausea and vomiting are ruled out (Table 1), NVP medical management is supportive. Correcting dehydration and encouraging dietary and lifestyle changes (Table 2)6 are important adjuncts to step-wise pharmacologic treatment recommended by the American College of Obstetrics and Gynecology (Algorithm).7
Algorithm Pharmacologic treatment of nausea and vomiting in pregnancy*
Source: Adapted and reprinted with permission from Canadian Family Physician. Levichek Z, Atanackovic G, Oepkes D, et al. Nausea and vomiting of pregnancy. Evidence-based treatment algorithm. Can Fam Physician 2002;48:267-77Table 1
Medical causes of nausea and vomiting in pregnancy
| Possible cause | How to rule it out |
|---|---|
| Appendicitis | History; do physical, order imaging |
| Hepatitis | Jaundice; order liver function tests, antibody studies, imaging |
| Pancreatitis | History of alcohol use, abdominal pain; check amylase and lipase level |
| Gastrointestinal obstruction | History of surgeries; order imaging |
| Peptic ulcer disease | History; order upper GI series/endoscopy |
| Thyroid disease | Thyroid function tests |
| Urinary tract infection | Urinalysis, culture-sensitivity |
| Trophoblastic disease | Check hCG,* order ultrasound |
| * Elevated human chorionic gonadotropin (hCG) has shown evidence of an association with NVP20 | |
Table 2
Advice for patients: Strategies to manage NVP
| Correct dehydration |
| Drink small amount of fluids frequently |
| Dietary changes |
| Eat frequent, small meals |
| Avoid high-fat foods |
| Snack before getting out of bed and before going to sleep |
| Don’t force yourself to eat |
| Use candy and salty snacks to combat nausea |
| Avoid strong odors and scents; try cold foods, which may have less odor than hot foods |
| Take advantage of good days or good hours of the day for eating |
| Lifestyle changes |
| Get out of bed slowly |
| Lie down when nauseated |
| Avoid stressful situations |
| NVP: nausea and vomiting in pregnancy Source: Reference 6 |
Prejudice vs evidence
Psychological factors. Historically, psychological factors have been blamed for NVP, but support comes from a few poorly designed studies or case reports.3,4
Psychoanalytically, pregnancy and childbirth are significant events in a woman’s life and a rich environment for conflict that could lead to physical expression of symptoms. Freud believed pregnancy and childbirth involve the unconscious substitution of the penis with the child.8 Later writers viewed motherhood as woman’s most powerful wish and the primary organizer of her sexual drive and personality.8
NVP has been considered a conversion or somatization disorder in which symptoms are a “hysterical” expression of unconscious conflict. A psychoanalytic view contends that women who experience NVP are ambivalent about the pregnancy and seek to reject it.1,9 Vomiting, in this view, represents an oral abortion attempt.10 Others claim NVP is a rejection of femininity3 or that symptoms in women with overly attached maternal relationships mask unconscious aggressive feelings toward their mothers.11
Robertson11 proposed an association between NVP and a woman’s view of sexual experiences and her ability to achieve orgasm. He interviewed 100 women and found that 40 of 57 with NVP had “disturbed sexual functioning” or were “frigid” (defined as experiencing coitus as undesirable and unaccompanied by orgasm).
Higgins12 in 1887 proposed that the cause of NVP “is sexual intercourse, the husband too eager for it and the wife too adverse.” Additionally, NVP and HG have been associated with infantile, childish, immature, and hysterical personalities.12-14
Psychiatric comorbidities. Attempts have been made to associate NVP with other psychiatric disorders such as depression, bipolar disorder, schizophrenia, and anxiety disorders, including posttraumatic stress disorder (PTSD). No definitive association has been found between NVP and depressive illness, bipolar disorder, or schizophrenia15 or the use of antidepressants before or during early pregnancy.16 Studies reporting an association with depression have not established a cause-effect relationship.17
Seng18 reported increased NVP risk in women with PTSD. High levels of stress, anxiety, and depression found in women with NVP are thought to result from—rather than cause—NVP’s physical symptoms, however.3,19,20
Psychosocial stressors have been implicated, with higher NVP rates reported in unmarried women, those with unwanted or unplanned pregnancies, immigrants, and those living in crowded situations.21 NVP also is more frequent among women who experience emotionally disturbing events or interpersonal, economic, or occupational difficulties during pregnancy.22 Physical symptoms may provide secondary gain in attention and sympathy and a time-out from stressful home events.14
These psychosocial theories are poorly supported by data, but some clinicians may still believe NVP has a psychogenic cause. Lennane and Lennane23 proposed in 1973 that this perception may result from gender bias because:
- most conditions believed to have psychogenic causes affect women more than men
- the belief that NVP is psychogenic has been perpetuated primarily by male authors.
They argued that sexual prejudice may prevent women from receiving necessary symptomatic treatment and impede research into the cause of NVP.23
Gender bias continues to be found in the diagnosis of women with physical complaints. In 2006, Chiaramonte and Friend24 found strong, consistent gender bias among medical students and residents when evaluating women who reported coronary disease symptoms during stressful life events.
Organic theories
Organic theories view NVP as multifactorial, with contributions from evolution and multiple organ systems. Endocrine, vestibular, gastrointestinal, and CNS contributions have been described, but none have solved NVP’s etiologic mystery.
Evolutionary. NVP may provide an evolutionary advantage by protecting the embryo and mother. This theory states that potential toxins are present in many foods, especially if eaten in large quantities. NVP prevents the pregnant woman from eating very much and harming the embryo. Below-average miscarriage rates are seen in women with NVP.1,2,25
And because a woman’s immune system is depressed during pregnancy, NVP may be advantageous for the mother by limiting her ingestion of potential toxins.25
Endocrine. Human chorionic gonadotropin (hCG), estrogens, progesterone, and leptin, as well as adrenal cortex insufficiencies have been investigated for a role in NVP. Only hCG has shown clear evidence of an association, and some researchers believe it is the most likely cause of NVP.20
NVP rates are higher in pregnancies with elevated hCG. Molar and multiple-gestation pregnancies—each associated with elevated hCG—are complicated more frequently with the severest form of NVP.20,26 Conversely, NVP is less common in women who smoke, which is associated with lower hCG.26
During pregnancy, actions of hCG stimulate the thyroid. Hyperstimulation, leading to transient hyperthyroidism, has been implicated in NVP development.20,26 Symptom severity and the degree of thyroid stimulating hormone (TSH) suppression are closely correlated.26
Elevated hCG levels, hypersensitive TSH receptors, and the presence of a hyperactive hCG isoform have been proposed.20
Gastrointestinal disorders are believed to be involved in the pathogenesis of persistent NVP. Women with NVP usually lack structural or mucosal abnormalities and have normal endoscopic upper GI evaluations. They may, however, have disorders of the stomach’s neuromuscular function. Severe cases of gastric dysrhythmias and abnormalities of gastric tone may lead to gastroparesis.27
Stomach motility in pregnancy is influenced by neurohormonal changes, specifically in estrogen and thyroid hormones. Gastric motility abnormalities—evaluated by electrogastrography (EGG)—have been associated with NVP symptoms and normal EGGs with the absence of symptoms. Some women who had NVP and abnormal EGGs were retested after delivery when symptom-free and found to have normal myoelectric EGG patterns.27
Helicobacter pylori also may be involved in NVP, and at least 1 study found active H pylori infection and HG to be highly correlated. Pregnancy is not believed to predispose to H pylori infection, but active infection compounded by pregnancy’s hormonal changes may exacerbate NVP.28
NVP and motion sickness share many features, suggesting that NVP treatment could be targeted if a vestibular disorder could be discovered.29 Abnormal electroencephalography—particularly generalized slowing—that is not present in asymptomatic pregnant women has been reported in women with NVP.30
CNS contributions. Persistent NVP may be a learned behavior,24 a view based on findings of anticipatory nausea and vomiting in chemotherapy patients. Through conditioning, a pregnant woman may associate her physical symptoms with elements in her life that maintain the cycle of nausea and vomiting.31
Treating psychological symptoms
Brief psychotherapy to identify and correct sources of anxiety in pregnancy may alleviate a patient’s nausea and vomiting.32
- Progressive muscle relaxation training, often combined with guided imagery, can decrease nausea and vomiting associated with chemotherapy and may prevent anticipatory symptoms by decreasing anxiety.
- Systematic desensitization is successful in most chemotherapy patients who try it. In this technique, relaxation is counter-conditioned as a response to stimuli known to elicit symptoms.31
Hypnosis allows patients to achieve a physiologic state incompatible with nausea and vomiting31 and can terminate vomiting after 1 to 3 sessions.3
Medication. Similar to ondansetron, the antidepressant mirtazapine exhibits an antiemetic effect by blocking the 5-HT3 receptor. In treatment-resistant cases, mirtazapine, 30 mg/d, has been reported to ameliorate NVP symptoms, usually within 24 hours. Patients were able to return to normal diets and discontinue treatment after 6 to 10 days. Mirtazapine appears to be safe during pregnancy, based on animal studies using 17 and 20 times the maximum recommended human dose.33
For patients with anxiety symptoms, consider other medications—including selective serotonin reuptake inhibitors and benzodiazepines—only after counseling the patient about potential risks and benefits to her and the fetus.34
Alternative treatments. In traditional Indian medicine, a mixture of powdered ginger and honey is given to women with NVP. At least 2 studies demonstrate ginger’s efficacy.35
In traditional Chinese medicine, stimulating the Neiguan point (P6) on the wrist is believed to relieve nausea and vomiting. Although results are inconclusive, studies suggest that P6 stimulation can help control NVP.36 The FDA has approved wristbands that stimulate the P6 site, either electrically or by acupressure (Figure).36
Figure Wristband to manage nausea and vomiting in pregnancy
The FDA-cleared BioBand acupressure wristband may help control nausea and vomiting in pregnancy by stimulating the Neiguan point (P6) on the wrist.
Source: Reference 36Consider thiamine supplementation for women with severe symptoms, as Wernicke’s encephalopathy is a rare complication of prolonged NVP.19,37
Related resources
- Motherisk Program at The Hospital for Sick Children, Toronto, Ontario, Canada. Website with information on vomiting during pregnancy: www.motherisk.org/women/morningSickness.jsp. Nausea and vomiting of pregnancy (NVP) forum. www.motherisk.org/women/forum.jsp.
- BioBand acupuncture wristband. www.BioBands.com.
- Koren G, Bishai R, eds. Nausea and vomiting of pregnancy: state of the art 2000. Toronto, Ontario, Canada: The Motherisk Program; 2000.
Drug brand names
- Dimenhydrinate • Dramamine
- Doxylamine • Unisom
- Methylprednisolone • Medrol
- Metoclopramide • Reglan
- Mirtazapine • Remeron
- Ondansetron • Zofran
- Promethazine • Phenergan
- Trimethobenzamide • Tigan
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Nausea and vomiting in pregnancy (NVP) is a misunderstood disorder associated with stress, anxiety, and depression. Prejudice toward women is thought to have guided the historical psychoanalytic concept of NVP as psychogenic, but this view is being replaced by newer biologic theories.
This article examines the evidence for psychological and organic causes of NVP to inform psychiatrists treating pregnant patients. We review guidelines for pharmacologic treatment of NVP and discuss potentially useful psychotherapies and alternative approaches.
Definitive cause unknown
“Morning sickness” affects 50% to 80% of pregnant women, occurring so commonly that NVP is often considered normal.1,2 Approximately 0.5% to 2% of women experience the most severe NVP—hyperemesis gravidarum (HG)3—characterized by intractable vomiting, weight loss, and electrolyte imbalance that can lead to hospitalization.
Without modern supportive care, HG can be lethal; although Charlotte Brontë’s death certificate states she died of “phthisis” (tuberculosis), the author of Jane Eyre is popularly believed to have succumbed to HG.4,5
The search for effective NVP treatments has been disappointing, partly because no cause has been identified. After other conditions that may lead to nausea and vomiting are ruled out (Table 1), NVP medical management is supportive. Correcting dehydration and encouraging dietary and lifestyle changes (Table 2)6 are important adjuncts to step-wise pharmacologic treatment recommended by the American College of Obstetrics and Gynecology (Algorithm).7
Algorithm Pharmacologic treatment of nausea and vomiting in pregnancy*
Source: Adapted and reprinted with permission from Canadian Family Physician. Levichek Z, Atanackovic G, Oepkes D, et al. Nausea and vomiting of pregnancy. Evidence-based treatment algorithm. Can Fam Physician 2002;48:267-77Table 1
Medical causes of nausea and vomiting in pregnancy
| Possible cause | How to rule it out |
|---|---|
| Appendicitis | History; do physical, order imaging |
| Hepatitis | Jaundice; order liver function tests, antibody studies, imaging |
| Pancreatitis | History of alcohol use, abdominal pain; check amylase and lipase level |
| Gastrointestinal obstruction | History of surgeries; order imaging |
| Peptic ulcer disease | History; order upper GI series/endoscopy |
| Thyroid disease | Thyroid function tests |
| Urinary tract infection | Urinalysis, culture-sensitivity |
| Trophoblastic disease | Check hCG,* order ultrasound |
| * Elevated human chorionic gonadotropin (hCG) has shown evidence of an association with NVP20 | |
Table 2
Advice for patients: Strategies to manage NVP
| Correct dehydration |
| Drink small amount of fluids frequently |
| Dietary changes |
| Eat frequent, small meals |
| Avoid high-fat foods |
| Snack before getting out of bed and before going to sleep |
| Don’t force yourself to eat |
| Use candy and salty snacks to combat nausea |
| Avoid strong odors and scents; try cold foods, which may have less odor than hot foods |
| Take advantage of good days or good hours of the day for eating |
| Lifestyle changes |
| Get out of bed slowly |
| Lie down when nauseated |
| Avoid stressful situations |
| NVP: nausea and vomiting in pregnancy Source: Reference 6 |
Prejudice vs evidence
Psychological factors. Historically, psychological factors have been blamed for NVP, but support comes from a few poorly designed studies or case reports.3,4
Psychoanalytically, pregnancy and childbirth are significant events in a woman’s life and a rich environment for conflict that could lead to physical expression of symptoms. Freud believed pregnancy and childbirth involve the unconscious substitution of the penis with the child.8 Later writers viewed motherhood as woman’s most powerful wish and the primary organizer of her sexual drive and personality.8
NVP has been considered a conversion or somatization disorder in which symptoms are a “hysterical” expression of unconscious conflict. A psychoanalytic view contends that women who experience NVP are ambivalent about the pregnancy and seek to reject it.1,9 Vomiting, in this view, represents an oral abortion attempt.10 Others claim NVP is a rejection of femininity3 or that symptoms in women with overly attached maternal relationships mask unconscious aggressive feelings toward their mothers.11
Robertson11 proposed an association between NVP and a woman’s view of sexual experiences and her ability to achieve orgasm. He interviewed 100 women and found that 40 of 57 with NVP had “disturbed sexual functioning” or were “frigid” (defined as experiencing coitus as undesirable and unaccompanied by orgasm).
Higgins12 in 1887 proposed that the cause of NVP “is sexual intercourse, the husband too eager for it and the wife too adverse.” Additionally, NVP and HG have been associated with infantile, childish, immature, and hysterical personalities.12-14
Psychiatric comorbidities. Attempts have been made to associate NVP with other psychiatric disorders such as depression, bipolar disorder, schizophrenia, and anxiety disorders, including posttraumatic stress disorder (PTSD). No definitive association has been found between NVP and depressive illness, bipolar disorder, or schizophrenia15 or the use of antidepressants before or during early pregnancy.16 Studies reporting an association with depression have not established a cause-effect relationship.17
Seng18 reported increased NVP risk in women with PTSD. High levels of stress, anxiety, and depression found in women with NVP are thought to result from—rather than cause—NVP’s physical symptoms, however.3,19,20
Psychosocial stressors have been implicated, with higher NVP rates reported in unmarried women, those with unwanted or unplanned pregnancies, immigrants, and those living in crowded situations.21 NVP also is more frequent among women who experience emotionally disturbing events or interpersonal, economic, or occupational difficulties during pregnancy.22 Physical symptoms may provide secondary gain in attention and sympathy and a time-out from stressful home events.14
These psychosocial theories are poorly supported by data, but some clinicians may still believe NVP has a psychogenic cause. Lennane and Lennane23 proposed in 1973 that this perception may result from gender bias because:
- most conditions believed to have psychogenic causes affect women more than men
- the belief that NVP is psychogenic has been perpetuated primarily by male authors.
They argued that sexual prejudice may prevent women from receiving necessary symptomatic treatment and impede research into the cause of NVP.23
Gender bias continues to be found in the diagnosis of women with physical complaints. In 2006, Chiaramonte and Friend24 found strong, consistent gender bias among medical students and residents when evaluating women who reported coronary disease symptoms during stressful life events.
Organic theories
Organic theories view NVP as multifactorial, with contributions from evolution and multiple organ systems. Endocrine, vestibular, gastrointestinal, and CNS contributions have been described, but none have solved NVP’s etiologic mystery.
Evolutionary. NVP may provide an evolutionary advantage by protecting the embryo and mother. This theory states that potential toxins are present in many foods, especially if eaten in large quantities. NVP prevents the pregnant woman from eating very much and harming the embryo. Below-average miscarriage rates are seen in women with NVP.1,2,25
And because a woman’s immune system is depressed during pregnancy, NVP may be advantageous for the mother by limiting her ingestion of potential toxins.25
Endocrine. Human chorionic gonadotropin (hCG), estrogens, progesterone, and leptin, as well as adrenal cortex insufficiencies have been investigated for a role in NVP. Only hCG has shown clear evidence of an association, and some researchers believe it is the most likely cause of NVP.20
NVP rates are higher in pregnancies with elevated hCG. Molar and multiple-gestation pregnancies—each associated with elevated hCG—are complicated more frequently with the severest form of NVP.20,26 Conversely, NVP is less common in women who smoke, which is associated with lower hCG.26
During pregnancy, actions of hCG stimulate the thyroid. Hyperstimulation, leading to transient hyperthyroidism, has been implicated in NVP development.20,26 Symptom severity and the degree of thyroid stimulating hormone (TSH) suppression are closely correlated.26
Elevated hCG levels, hypersensitive TSH receptors, and the presence of a hyperactive hCG isoform have been proposed.20
Gastrointestinal disorders are believed to be involved in the pathogenesis of persistent NVP. Women with NVP usually lack structural or mucosal abnormalities and have normal endoscopic upper GI evaluations. They may, however, have disorders of the stomach’s neuromuscular function. Severe cases of gastric dysrhythmias and abnormalities of gastric tone may lead to gastroparesis.27
Stomach motility in pregnancy is influenced by neurohormonal changes, specifically in estrogen and thyroid hormones. Gastric motility abnormalities—evaluated by electrogastrography (EGG)—have been associated with NVP symptoms and normal EGGs with the absence of symptoms. Some women who had NVP and abnormal EGGs were retested after delivery when symptom-free and found to have normal myoelectric EGG patterns.27
Helicobacter pylori also may be involved in NVP, and at least 1 study found active H pylori infection and HG to be highly correlated. Pregnancy is not believed to predispose to H pylori infection, but active infection compounded by pregnancy’s hormonal changes may exacerbate NVP.28
NVP and motion sickness share many features, suggesting that NVP treatment could be targeted if a vestibular disorder could be discovered.29 Abnormal electroencephalography—particularly generalized slowing—that is not present in asymptomatic pregnant women has been reported in women with NVP.30
CNS contributions. Persistent NVP may be a learned behavior,24 a view based on findings of anticipatory nausea and vomiting in chemotherapy patients. Through conditioning, a pregnant woman may associate her physical symptoms with elements in her life that maintain the cycle of nausea and vomiting.31
Treating psychological symptoms
Brief psychotherapy to identify and correct sources of anxiety in pregnancy may alleviate a patient’s nausea and vomiting.32
- Progressive muscle relaxation training, often combined with guided imagery, can decrease nausea and vomiting associated with chemotherapy and may prevent anticipatory symptoms by decreasing anxiety.
- Systematic desensitization is successful in most chemotherapy patients who try it. In this technique, relaxation is counter-conditioned as a response to stimuli known to elicit symptoms.31
Hypnosis allows patients to achieve a physiologic state incompatible with nausea and vomiting31 and can terminate vomiting after 1 to 3 sessions.3
Medication. Similar to ondansetron, the antidepressant mirtazapine exhibits an antiemetic effect by blocking the 5-HT3 receptor. In treatment-resistant cases, mirtazapine, 30 mg/d, has been reported to ameliorate NVP symptoms, usually within 24 hours. Patients were able to return to normal diets and discontinue treatment after 6 to 10 days. Mirtazapine appears to be safe during pregnancy, based on animal studies using 17 and 20 times the maximum recommended human dose.33
For patients with anxiety symptoms, consider other medications—including selective serotonin reuptake inhibitors and benzodiazepines—only after counseling the patient about potential risks and benefits to her and the fetus.34
Alternative treatments. In traditional Indian medicine, a mixture of powdered ginger and honey is given to women with NVP. At least 2 studies demonstrate ginger’s efficacy.35
In traditional Chinese medicine, stimulating the Neiguan point (P6) on the wrist is believed to relieve nausea and vomiting. Although results are inconclusive, studies suggest that P6 stimulation can help control NVP.36 The FDA has approved wristbands that stimulate the P6 site, either electrically or by acupressure (Figure).36
Figure Wristband to manage nausea and vomiting in pregnancy
The FDA-cleared BioBand acupressure wristband may help control nausea and vomiting in pregnancy by stimulating the Neiguan point (P6) on the wrist.
Source: Reference 36Consider thiamine supplementation for women with severe symptoms, as Wernicke’s encephalopathy is a rare complication of prolonged NVP.19,37
Related resources
- Motherisk Program at The Hospital for Sick Children, Toronto, Ontario, Canada. Website with information on vomiting during pregnancy: www.motherisk.org/women/morningSickness.jsp. Nausea and vomiting of pregnancy (NVP) forum. www.motherisk.org/women/forum.jsp.
- BioBand acupuncture wristband. www.BioBands.com.
- Koren G, Bishai R, eds. Nausea and vomiting of pregnancy: state of the art 2000. Toronto, Ontario, Canada: The Motherisk Program; 2000.
Drug brand names
- Dimenhydrinate • Dramamine
- Doxylamine • Unisom
- Methylprednisolone • Medrol
- Metoclopramide • Reglan
- Mirtazapine • Remeron
- Ondansetron • Zofran
- Promethazine • Phenergan
- Trimethobenzamide • Tigan
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. el-Mallakh RS, Liebowitz NR, Hale MS. Hyperemesis gravidarum as conversion disorder. J Nerv Ment Dis 1990;178:655-9.
2. Davis M. Nausea and vomiting of pregnancy: an evidence-based review. J Perinat Neonatal Nurs 2004;18:312-28.
3. Buckwalter JG, Simpson SW. Psychological factors in the etiology and treatment of severe nausea and vomiting in pregnancy. Am J Obstet Gynecol 2002;186(5 suppl):S210-4.
4. Bogen JT. Neurosis: a Ms-diagnosis. Perspect Biol Med 1994;37(2):263-74.
5. Weiss G. The death of Charlotte Brontë. Obstet Gynecol 1991;78(4):705-8.
6. Lester EP, Notman MT. Pregnancy, developmental crisis and object relations: psychoanalytic considerations. Int J Psychoanal 1986;67(pt 3):357-66.
7. Sheehan P. Hyperemesis gravidarum—assessment and management. Aust Fam Physician 2007;36:698-701.
8. American College of Obstetrics and Gynecology. ACOG practice bulletin: nausea and vomiting of pregnancy. Obstet Gynecol 2004;103:803-14.
9. Iancu I, Kotler M, Spivak B, et al. Psychiatric aspects of hyperemesis gravidarum. Psychother Psychosom 1994;61:143-9.
10. Munch S. Chicken or the egg? The biological-psychological controversy surrounding hyperemesis gravidarum. Soc Sci Med 2002;55:1267-78.
11. Robertson GG. Nausea and vomiting of pregnancy: a study in psychosomatic and social medicine. Lancet 1946;336-45.
12. Fairweather DV. Nausea and vomiting in pregnancy. Am J Obstet Gynecol 1968;102:135-75.
13. Katon WJ, Ries RK, Bokan JA, Kleinman A. Hyperemesis gravidarum: a biopsychosocial perspective. Int J Psychiatry Med 1980;10:151-62.
14. Simpson SW, Goodwin TM, Robins SB, et al. Psychological factors and hyperemesis gravidarum. J Womens Health Gend Based Med 2001;10:471-7.
15. Majerus PW, Guze SB, Delong WB, Robins E. Psychologic factors and psychiatric disease in hyperemesis gravidarum: a follow-up study of 69 vomiters and 66 controls. Am J Psychiatry 1960;117:421-8.
16. Bozzo P, Koren G, Nava-Ocampo AA, Einarson A. The incidence of nausea and vomiting of pregnancy (NVP): a comparison between depressed women treated with antidepressants and non-depressed women. Clin Invest Med 2006;29(6):347-50.
17. Markl GE, Strunz-Lehner C, Egen-Lappe V, et al. The association of psychosocial factors with nausea and vomiting during pregnancy. J Psychosom Obstet Gynaecol 2007;1-6.
18. Seng JS, Oakley DJ, Sampselle CM, et al. Posttraumatic stress disorder and pregnancy complications. Obstet Gynecol 2001;97(1):17-22.
19. Ismail SK, Kenny L. Review on hyperemesis gravidarum. Best Pract Res Clin Gastroenterol 2007;21:755-69.
20. Verberg MF, Gillott DJ, Al-Fardan N, Grudzinskas JG. Hyperemesis gravidarum, a literature review. Hum Reprod Update 2005;11:527-39.
21. Deuchar N. Nausea and vomiting in pregnancy: a review of the problem with particular regard to psychological and social aspects. Br J Obstet Gynaecol 1995;102(1):6-8.
22. Iatrakis GM, Sakellaropoulos GG, Kourkoubas AH, Kabounia SE. Vomiting and nausea in the first 12 weeks of pregnancy. Psychother Psychosom 1988;49(1):22-4.
23. Lennane KJ, Lennane RJ. Alleged psychogenic disorders in women—a possible manifestation of sexual prejudice. N Engl J Med 1973;288(6):288-92.
24. Chiaramonte GR, Friend R. Medical students’ and residents’ gender bias in the diagnosis, treatment, and interpretation of coronary heart disease symptoms. Health Psychol 2006;25:255-66.
25. Sherman PW, Flaxman SM. Nausea and vomiting of pregnancy in an evolutionary perspective. Am J Obstet Gynecol 2002;186(5 suppl):S190-7.
26. Goodwin TM. Nausea and vomiting of pregnancy: an obstetric syndrome. Am J Obstet Gynecol 2002;186(5 suppl):S184-9.
27. Koch KL. Gastrointestinal factors in nausea and vomiting of pregnancy. Am J Obstet Gynecol 2002;186(5 suppl):S198-203.
28. Golberg D, Szilagyi A, Graves L. Hyperemesis gravidarum and Helicobacter pylori infection: a systematic review. Obstet Gynecol 2007;110:695-703.
29. Black FO. Maternal susceptibility to nausea and vomiting of pregnancy: is the vestibular system involved? Am J Obstet Gynecol 2002;186(5 suppl):S204-9.
30. Vaknin Z, Halperin R, Schneider D, et al. Hyperemesis gravidarum and nonspecific abnormal EEG findings: a preliminary report. J Reprod Med 2006;51:623-7.
31. Matteson S, Roscoe J, Hickok J, Morrow GR. The role of behavioral conditioning in the development of nausea. Am J Obstet Gynecol 2002;186(5 suppl):S239-43.
32. Zechnich R, Hammer T. Brief psychotherapy for hyperemesis gravidarum. Am Fam Physician 1982;26:179-81.
33. Guclu S, Gol M, Dogan E, Saygili U. Mirtazapine use in resistant hyperemesis gravidarum: report of three cases and review of the literature. Arch Gynecol Obstet 2005;272:298-300.
34. Raphael DB, Ross J, Brizendine L. Treating anxiety during pregnancy: Just how safe are SSRIs? Current Psychiatry 2008;7(2):39-52.
35. Niebyl JR, Goodwin TM. Overview of nausea and vomiting of pregnancy with an emphasis on vitamins and ginger. Am J Obstet Gynecol 2002;186(5 suppl):S253-5.
36. Roscoe JA, Matteson SE. Acupressure and acustimulation bands for control of nausea: a brief review. Am J Obstet Gynecol 2002;186(5 suppl):S244-7.
37. Chiossi G, Neri I, Cavazzuti M, et al. Hyperemesis gravidarum complicated by Wernicke encephalopathy: background, case report, and review of the literature. Obstet Gynecol Surv 2006;61:255-68.
1. el-Mallakh RS, Liebowitz NR, Hale MS. Hyperemesis gravidarum as conversion disorder. J Nerv Ment Dis 1990;178:655-9.
2. Davis M. Nausea and vomiting of pregnancy: an evidence-based review. J Perinat Neonatal Nurs 2004;18:312-28.
3. Buckwalter JG, Simpson SW. Psychological factors in the etiology and treatment of severe nausea and vomiting in pregnancy. Am J Obstet Gynecol 2002;186(5 suppl):S210-4.
4. Bogen JT. Neurosis: a Ms-diagnosis. Perspect Biol Med 1994;37(2):263-74.
5. Weiss G. The death of Charlotte Brontë. Obstet Gynecol 1991;78(4):705-8.
6. Lester EP, Notman MT. Pregnancy, developmental crisis and object relations: psychoanalytic considerations. Int J Psychoanal 1986;67(pt 3):357-66.
7. Sheehan P. Hyperemesis gravidarum—assessment and management. Aust Fam Physician 2007;36:698-701.
8. American College of Obstetrics and Gynecology. ACOG practice bulletin: nausea and vomiting of pregnancy. Obstet Gynecol 2004;103:803-14.
9. Iancu I, Kotler M, Spivak B, et al. Psychiatric aspects of hyperemesis gravidarum. Psychother Psychosom 1994;61:143-9.
10. Munch S. Chicken or the egg? The biological-psychological controversy surrounding hyperemesis gravidarum. Soc Sci Med 2002;55:1267-78.
11. Robertson GG. Nausea and vomiting of pregnancy: a study in psychosomatic and social medicine. Lancet 1946;336-45.
12. Fairweather DV. Nausea and vomiting in pregnancy. Am J Obstet Gynecol 1968;102:135-75.
13. Katon WJ, Ries RK, Bokan JA, Kleinman A. Hyperemesis gravidarum: a biopsychosocial perspective. Int J Psychiatry Med 1980;10:151-62.
14. Simpson SW, Goodwin TM, Robins SB, et al. Psychological factors and hyperemesis gravidarum. J Womens Health Gend Based Med 2001;10:471-7.
15. Majerus PW, Guze SB, Delong WB, Robins E. Psychologic factors and psychiatric disease in hyperemesis gravidarum: a follow-up study of 69 vomiters and 66 controls. Am J Psychiatry 1960;117:421-8.
16. Bozzo P, Koren G, Nava-Ocampo AA, Einarson A. The incidence of nausea and vomiting of pregnancy (NVP): a comparison between depressed women treated with antidepressants and non-depressed women. Clin Invest Med 2006;29(6):347-50.
17. Markl GE, Strunz-Lehner C, Egen-Lappe V, et al. The association of psychosocial factors with nausea and vomiting during pregnancy. J Psychosom Obstet Gynaecol 2007;1-6.
18. Seng JS, Oakley DJ, Sampselle CM, et al. Posttraumatic stress disorder and pregnancy complications. Obstet Gynecol 2001;97(1):17-22.
19. Ismail SK, Kenny L. Review on hyperemesis gravidarum. Best Pract Res Clin Gastroenterol 2007;21:755-69.
20. Verberg MF, Gillott DJ, Al-Fardan N, Grudzinskas JG. Hyperemesis gravidarum, a literature review. Hum Reprod Update 2005;11:527-39.
21. Deuchar N. Nausea and vomiting in pregnancy: a review of the problem with particular regard to psychological and social aspects. Br J Obstet Gynaecol 1995;102(1):6-8.
22. Iatrakis GM, Sakellaropoulos GG, Kourkoubas AH, Kabounia SE. Vomiting and nausea in the first 12 weeks of pregnancy. Psychother Psychosom 1988;49(1):22-4.
23. Lennane KJ, Lennane RJ. Alleged psychogenic disorders in women—a possible manifestation of sexual prejudice. N Engl J Med 1973;288(6):288-92.
24. Chiaramonte GR, Friend R. Medical students’ and residents’ gender bias in the diagnosis, treatment, and interpretation of coronary heart disease symptoms. Health Psychol 2006;25:255-66.
25. Sherman PW, Flaxman SM. Nausea and vomiting of pregnancy in an evolutionary perspective. Am J Obstet Gynecol 2002;186(5 suppl):S190-7.
26. Goodwin TM. Nausea and vomiting of pregnancy: an obstetric syndrome. Am J Obstet Gynecol 2002;186(5 suppl):S184-9.
27. Koch KL. Gastrointestinal factors in nausea and vomiting of pregnancy. Am J Obstet Gynecol 2002;186(5 suppl):S198-203.
28. Golberg D, Szilagyi A, Graves L. Hyperemesis gravidarum and Helicobacter pylori infection: a systematic review. Obstet Gynecol 2007;110:695-703.
29. Black FO. Maternal susceptibility to nausea and vomiting of pregnancy: is the vestibular system involved? Am J Obstet Gynecol 2002;186(5 suppl):S204-9.
30. Vaknin Z, Halperin R, Schneider D, et al. Hyperemesis gravidarum and nonspecific abnormal EEG findings: a preliminary report. J Reprod Med 2006;51:623-7.
31. Matteson S, Roscoe J, Hickok J, Morrow GR. The role of behavioral conditioning in the development of nausea. Am J Obstet Gynecol 2002;186(5 suppl):S239-43.
32. Zechnich R, Hammer T. Brief psychotherapy for hyperemesis gravidarum. Am Fam Physician 1982;26:179-81.
33. Guclu S, Gol M, Dogan E, Saygili U. Mirtazapine use in resistant hyperemesis gravidarum: report of three cases and review of the literature. Arch Gynecol Obstet 2005;272:298-300.
34. Raphael DB, Ross J, Brizendine L. Treating anxiety during pregnancy: Just how safe are SSRIs? Current Psychiatry 2008;7(2):39-52.
35. Niebyl JR, Goodwin TM. Overview of nausea and vomiting of pregnancy with an emphasis on vitamins and ginger. Am J Obstet Gynecol 2002;186(5 suppl):S253-5.
36. Roscoe JA, Matteson SE. Acupressure and acustimulation bands for control of nausea: a brief review. Am J Obstet Gynecol 2002;186(5 suppl):S244-7.
37. Chiossi G, Neri I, Cavazzuti M, et al. Hyperemesis gravidarum complicated by Wernicke encephalopathy: background, case report, and review of the literature. Obstet Gynecol Surv 2006;61:255-68.
Double jeopardy: How to treat kids with comorbid anxiety and ADHD
Aaron, age 10, has been diagnosed with an anxiety disorder and attention-deficit/hyperactivity disorder (ADHD) but is not being treated with medication because his parents do not believe in psychopharmacology. They bring him to a specialized child anxiety clinic and ask for “urgent CBT” because his behavior at school is out of control.
Aaron rearranges the therapist’s office furniture during much of the assessment interview. He also acknowledges many anxiety symptoms. The therapist doubts that cognitive-behavioral therapy (CBT) would help without other interventions.
Children with anxiety disorders and ADHD—a common comorbid presentation—tend to be more impaired than those with either condition alone.1 Effective treatment usually requires 4 components (Table 1), including medication plus behavioral or cognitive-behavioral therapy. This article discusses clinical issues related to each component and describes how to successfully combine them into a treatment plan.
Table 1
Comorbid ADHD and anxiety: 4 treatment components
| Successful treatment usually involves combining 4 components: | |
| |
| Make individual adjustments as needed, depending on the child’s symptom profile, social context, and developmental level | |
| ADHD: attention-deficit/hyperactivity disorder | |
Medication options
Stimulants, atomoxetine, and selective serotonin reuptake inhibitors (SSRIs) have been advocated for children with anxiety and ADHD. Given the high risk of behavioral disinhibition with SSRIs in children,2 stimulants or atomoxetine are suggested as first-line medications.3,4
Stimulants target ADHD symptoms primarily, but anxiety decreases in some children (24% in a recent trial) as ADHD symptoms are controlled.4 Because it is a selective norepinephrine reuptake inhibitor (SNRI), atomoxetine may target both ADHD and anxiety symptoms. When initiating these medications, “start low and go slow.” Recommended dosing is no different for children with ADHD and anxiety than for those with ADHD alone (Table 2).5
Stimulant response rates for children with ADHD and anxiety vary among studies. Some report lower response rates than for children with ADHD alone and possibly more treatment-emergent side effects.6 The National Institute of Mental Health’s Multimodal Treatment Study of Children with ADHD (MTA) found that comorbid anxiety did not adversely affect behavioral response to stimulants but did moderate outcomes (Box 1).7,8 Adding intensive psychosocial intervention to stimulant treatment appeared to yield greater improvements in anxious children with ADHD, compared with stimulants alone.8
Cognitive impairments related to inattention do not consistently improve with stimulant treatment.9 This is clinically important because children with ADHD and comorbid anxiety disorders can be very cognitively impaired.10
Add an SSRI? Monotherapy is simpler and usually more acceptable to families, but a placebo-controlled study examined adding an SSRI (fluvoxamine) to methylphenidate treatment.4 Children with anxiety and ADHD who received adjunctive fluvoxamine did no better than those who received methylphenidate plus placebo.
Atomoxetine. A large, randomized, controlled trial of atomoxetine in this population found good tolerability and statistically significant reductions in ADHD and anxiety symptoms compared with placebo. Effect size was greater for ADHD symptoms than for anxiety symptoms,11 however, which supports smaller trials that show more consistent evidence of atomoxetine reducing ADHD symptoms than anxiety symptoms.
Similar to antidepressants with the SNRI chemical structure, atomoxetine’s effectiveness for a given child takes several weeks to determine. This can be a problem in children who are highly distressed or impaired and require rapid symptomatic improvement.
Recommendation. Consider a stimulant or atomoxetine initially for children with anxiety disorders and ADHD, and seek concurrent behavioral or cognitive-behavioral therapy. Caution families that:
- >1 medication trial might be needed, as response may not be as consistent as in children with ADHD alone
- medication-related improvements in ADHD symptoms will not necessarily be associated with reduced anxiety symptoms or improved academic ability
- improvements with atomoxetine might not be evident for several weeks.
Table 2
Medication dosing for children with ADHD*
| Medication | Recommended starting dosage | Recommended maximum dosage | 5 most common side effects in descending prevalence |
|---|---|---|---|
| Stimulants | |||
| Methylphenidate hydrochloride (Ritalin) | 5 mg tid | Total 60 mg/d | Insomnia, nervousness, decreased appetite, dizziness, nausea |
| Methylphenidate hydrochloride (Concerta) | 18 mg every morning | 54 mg every morning | Headache, abdominal pain, decreased appetite, vomiting, insomnia |
| Dextroamphetamine sulfate (Dexedrine) | 5 mg every morning | Total 40 mg/d | Palpitations, restlessness, dizziness, dry mouth, decreased appetite |
| Mixed amphetamine salts (Adderall) | 10 mg every morning | 30 mg every morning | Decreased appetite, insomnia, abdominal pain, emotional lability, vomiting |
| Nonstimulant | |||
| Atomoxetine (Strattera) | 0.5 mg/kg/d | 1.2 mg/kg/d | Decreased appetite, dizziness, stomach upset, fatigue, irritability |
| ADHD: attention-deficit/hyperactivity disorder | |||
| * Recommended dosing is no different for children with ADHD and anxiety than for children with only ADHD | |||
| Source: Reference 5 | |||
Psychological intervention
CBT has been shown effective for child-hood anxiety disorders in randomized controlled trials,12 but even those that included children with comorbid ADHD required that an anxiety disorder be the primary, most impairing diagnosis.13 Thus, little is known about CBT’s effectiveness for children with anxiety plus ADHD. Given the evidence for cognitive deficits in comorbid anxiety and ADHD10 and the challenge of working with highly distractible children, one would expect CBT to be more difficult in this population.
The potential for distraction to adversely affect learning of coping strategies is higher in group than in individual therapy, and children with anxiety and ADHD can be disruptive to other children in CBT groups. Consider individual CBT, and seek a therapist who has experience with this population. Having the child on medication for ADHD symptoms usually helps reduce these symptoms’ impact on sessions.
For children younger than about age 8 or too cognitively impaired to benefit from CBT, behavioral intervention alone may be helpful. The largely behavioral psychosocial intervention in the MTA study of ADHD children age 7 to 9 (Box 2)8,14 helped many of those with comorbid anxiety.
Although programs as intense as that used in the MTA study rarely are provided in community practice, consider behavior modification. For example:
- To reduce anxiety, have the child follow regular, predictable routines, and reward the child for gradually facing previously avoided situations.
- To reduce distractibility in class, have the child sit near the teacher, break work into small chunks, and reward completion of each chunk.
Even small improvements in the child’s home or school behavior may reduce negative interactions with others and the attendant effects on self-esteem.
CASE CONTINUED: Weighing the options
The therapist seeing Aaron’s family listens to their concerns about medication and reassures them that their son will not be denied psychotherapy. She tells them, however, that psychotherapy will not address his urgent school problems and is unlikely to work in the absence of medication, given Aaron’s behavior in the office. The therapist provides accurate information about the risks and benefits of medication and CBT, and the parents agree to think about all treatment options.
By the next office visit, the school has threatened to suspend Aaron. He and his parents agree to combined treatment with a stimulant medication and CBT and to having the therapist provide a behavioral consultation at the school.
shows best outcomes for ADHD with anxiety
The National Institute of Mental Health’s Multimodal Treatment Study of Children with ADHD—the largest study to date—found that comorbid anxiety did not adversely affect behavioral response to stimulants but did moderate outcomes.
In the parallel group design study, 579 ADHD children age 7 to 9 were enrolled at 6 treatment sites, thoroughly assessed, then randomly assigned to 4 groups: medication treatment alone, intensive psychosocial treatment alone, a combination of both treatments, or usual community care. The first 3 interventions were designed to reflect best practices for each approach, and these children were closely monitored and studied for 14 months. All 4 groups were reassessed periodically for 24 months, evaluating multiple outcomes.
For the total sample, combined and medication treatment were more effective than psychosocial treatment and community care. For ADHD children with comorbid anxiety disorders:
- combined treatment was more effective than either medication treatment alone or psychosocial treatment alone
- both monotherapies were superior to community care.
ADHD: attention-deficit/hyperactivity disorder
Family psychoeducation
With families of children with behavioral challenges, adopt a patient, educational approach rather than acquiescing to their wishes or arguing with them. Either can result in treatment failure. Discuss potential benefits and risks of all treatment options and the impact of comorbidity on treatment.
Parents’ rigid insistence on a particular course of action—such as refusing psychopharmacology—may be caused by anxiety or misinformation. Elicit the source of any anxiety, and provide realistic information and reassurance if possible.
Anxiety in family members may be constitutional—as anxiety is highly heritable15—or relate to aspects of treatment. Families may feel overwhelmed by:
- their child having 2 disorders rather than 1
- your suggestion to start medical and nonmedical intervention together
- hearing about the possibility of multiple medication trials.
Negotiating medication. Discuss with the family the difficulties of a child learning CBT strategies when ADHD is not well-controlled and the cognitive difficulties in many of these children that may necessitate individualized CBT. If the family remains reluctant to consider combining medication with CBT, try contracting for a limited number of CBT sessions (perhaps 3 or 4) before re-evaluating the need for medication.
The child’s perceptions (and potential anxieties) about his or her difficulties also must be understood, validated, and addressed. Children are more likely to engage in a treatment if they participate in the decision to adopt it.
Anxiety can heighten vigilance in the child or the parents to treatment-emergent side effects, which you may exacerbate by providing exhaustive lists of potential ad-verse events. Limit discussion to serious side effects—with emphasis on their rarity—and those that are common.
ADHD traits in families can affect treatment success. Because of their own distractibility and organizational difficulties, parents with ADHD traits may have difficulty ensuring the child’s medication adherence and treatment participation.16
Behavior modification can require a high degree of consistency in parents’ behavior toward the child. This may be difficult to achieve in families where:
- 1 or both parents are inattentive because of ADHD
- a high degree of conflict exists between parents.
To help these families, provide reminder calls about appointments and schedule sessions at a consistent time. To improve consistency of medication use:
- combine medication administration with an essential daily activity
- check adherence with pill counts or other means.
If the child participates in CBT, provide separate notebooks for in-session and homework exercises—anticipating some loss of homework notebooks.
Individualizing care
Individualized care is important to return each child to his or her best possible level of functioning. The child’s symptom profile, environment, and developmental level can affect treatment.
For example, in a child whose ADHD-related impairment is substantial but whose anxiety-related impairment is mild, pharmacotherapy for ADHD and some pa-rental guidance may be adequate to manage remaining anxiety symptoms.17 As mentioned, some children show decreased anxiety as their ADHD is better controlled.4 Conversely, if ADHD-related impairment is mild but the child is highly anxious, consider CBT alone—preferably on an individual basis—provided the child can manage the cognitive aspects of therapy.
School personnel can monitor change in relation to various interventions, as many of these children’s symptoms manifest in the classroom. Behavioral interventions are more likely to succeed if they are administered consistently across home and school environments8 and teachers participate in behavior modification.
To elicit cooperation from school personnel, listen to their concerns and observations and help them understand the child’s difficulties and the rationale for various treatments. This approach often reduces negative feedback toward the child, a benefit that may further improve outcomes.
Attention to peer relationships and social stressors is often needed. Because of their multiple difficulties, these children may lack social skills and be shunned by their peers.1 You may need to help them develop social skills and reconnect with their peers after symptoms are well-controlled.
Poverty or lack of social support can affect treatment. Children with ADHD and anxiety usually need multiple interventions, and it is difficult for families to at-tend to these consistently when struggling with social stressors.
The 14-month intensive behavioral intervention used in the National Institute of Mental Health’s Multimodal Treatment Study (MTA) of 579 children age 7 to 9 with ADHD included:
- weekly parent training initially, decreasing to monthly by the end
- biweekly teacher consultations in behavior management
- 8-week full-day therapeutic summer program for children, focusing on behavioral and cognitive behavioral intervention
- 12-week half-time behaviorally trained paraprofessional aide in the classroom to generalize gains from summer program
- parent coaching on collaborating with teacher long-term so therapeutic consultation could be faded.
ADHD: attention-deficit/hyperactivity disorder
Adolescent adjustments. ADHD and anxiety often are diagnosed in the early school years, so anticipate developmental effects on treatment as the child enters adolescence. Adolescents value autonomy and may need to be more involved in treatment decisions than younger children.
Ask about and address family disagreements about treatment options, which may reduce adherence. You may need to talk about peer pressure to “not take drugs” by clearly differentiating the reasons some people take street drugs and the reasons for taking prescribed medication. Also discuss in a frank, nonjudgmental manner the risks of experimenting with street drugs (especially with prescribed medication) or of “sharing” one’s medications with friends.
Increased cognitive sophistication in adolescence may increase the potential benefit of CBT, so explore this option with the teen, especially if it was not attempted in the past.
Related resources
- American Academy of Child and Adolescent Psychiatry. “ADHD—a guide for families,” under the Resources for Families tab. www.aacap.org.
- Watkins C. Stimulant medication and ADHD. www.ncpamd.com/Stimulants.htm.
- Manassis K. Keys to parenting your anxious child. 2nd ed. Hauppauge, NY: Barron’s Educational Series, Inc.; 2008.
Drug brand names
- Atomoxetine • Strattera
- Dextroamphetamine • Dexedrine
- Fluvoxamine • Luvox
- Methylphenidate • Ritalin, Concerta
- Mixed amphetamine salts • Adderall
Disclosures
Dr. Manassis reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Bowen R, Chavira DA, Bailey K, et al. Nature of anxiety comorbid with attention deficit hyperactivity disorder in children from a pediatric primary care setting. Psychiatry Res 2008;157:201-9.
2. Walkup JT, Labellarte MJ, Riddle MA, et al. Searching for moderators and mediators of pharmacological treatment in children and adolescents with anxiety disorders. J Am Acad Child Adolesc Psychiatry 2003;42:13-21.
3. Wiesegger G, Kienbacher C, Pellegrini E, et al. Pharmacotherapy of attention-deficit/hyperactivity disorder (ADHD) and comorbid disorders. Neuropsychiatr 2007;21:187-206.
4. Abikoff H, McGough J, Vitiello B, et al. Sequential pharmacotherapy for children with comorbid attention-deficit/hyperactivity and anxiety disorders. J Am Acad Child Adolesc Psychiatry 2005;44:418-27.
5. Compendium of pharmaceuticals and specialties. Ottawa, Canada: Canadian Pharmacists Association; 2008.
6. Goez H, Back-Bennet O, Zelnik N. Differential stimulant response on attention in children with comorbid anxiety and oppositional defiant disorder. J Child Neurol 2007;22:538-42.
7. Wells KC, Pelham WE, Kotkin RA, et al. Psychosocial treatment strategies in the MTA study: rationale, methods, and critical issues in design and implementation. J Abnorm Child Psychol 2000;28:483-505.
8. March JS, Swanson JM, Arnold EL, et al. Anxiety as a predictor and outcome variable in the Multimodal Treatment Study of Children with ADHD (MTA). J Abnorm Child Psychol 2000;28:527-41.
9. Tannock R, Ickowicz A, Schachar R. Differential effects of methylphenidate on working memory in ADHD children with and without anxiety. J Am Acad Child Adolesc Psychiatry 1995;34:886-96.
10. Manassis K, Tannock R, Young A, Francis-John S. Cognition in anxious children with attention deficit hyperactivity disorder: a comparison with clinical and normal children. Behav Brain Funct 2007;3-4.
11. Geller D, Donnelly C, Lopez F, et al. Atomoxetine treatment for pediatric patients with attention-deficit/hyperactivity disorder with comorbid anxiety disorder. J Am Acad Child Adolesc Psychiatry 2007;46:1119-27.
12. Compton SN, March JS, Brent D, et al. Cognitive behavioural psychotherapy for anxiety and depressive disorders in children and adolescents: an evidence-based medicine review. J Am Acad Child Adolesc Psychiatry 2004;43:930-59.
13. Manassis K, Mendlowitz SL, Scapillato D, et al. Group and individual cognitive-behavioral therapy for childhood anxiety disorders: a randomized trial. J Am Acad Child Adolesc Psychiatry 2002;41:1423-30.
14. Arnold LE, Abikoff HB, Cantwell DP, et al. National Institute of Mental Health Collaborative Multimodal Treatment Study of Children with ADHD (the MTA). Design challenges and choices. Arch Gen Psychiatry 1997;54:865-70.
15. Kagan J, Reznick JS, Snidman N. Biological basis of childhood shyness. Science 1990;240:167-71.
16. Van Cleave J, Leslie LK. Approaching ADHD as a chronic condition: implications for long-term adherence. Pediatr Ann 2008;37:19-26.
17. Manassis K, Monga S. therapeutic approach to children and adolescents with anxiety disorders and associated comorbid conditions. J Am Acad Child Adolesc Psychiatry 2001;40:115-7.
Aaron, age 10, has been diagnosed with an anxiety disorder and attention-deficit/hyperactivity disorder (ADHD) but is not being treated with medication because his parents do not believe in psychopharmacology. They bring him to a specialized child anxiety clinic and ask for “urgent CBT” because his behavior at school is out of control.
Aaron rearranges the therapist’s office furniture during much of the assessment interview. He also acknowledges many anxiety symptoms. The therapist doubts that cognitive-behavioral therapy (CBT) would help without other interventions.
Children with anxiety disorders and ADHD—a common comorbid presentation—tend to be more impaired than those with either condition alone.1 Effective treatment usually requires 4 components (Table 1), including medication plus behavioral or cognitive-behavioral therapy. This article discusses clinical issues related to each component and describes how to successfully combine them into a treatment plan.
Table 1
Comorbid ADHD and anxiety: 4 treatment components
| Successful treatment usually involves combining 4 components: | |
| |
| Make individual adjustments as needed, depending on the child’s symptom profile, social context, and developmental level | |
| ADHD: attention-deficit/hyperactivity disorder | |
Medication options
Stimulants, atomoxetine, and selective serotonin reuptake inhibitors (SSRIs) have been advocated for children with anxiety and ADHD. Given the high risk of behavioral disinhibition with SSRIs in children,2 stimulants or atomoxetine are suggested as first-line medications.3,4
Stimulants target ADHD symptoms primarily, but anxiety decreases in some children (24% in a recent trial) as ADHD symptoms are controlled.4 Because it is a selective norepinephrine reuptake inhibitor (SNRI), atomoxetine may target both ADHD and anxiety symptoms. When initiating these medications, “start low and go slow.” Recommended dosing is no different for children with ADHD and anxiety than for those with ADHD alone (Table 2).5
Stimulant response rates for children with ADHD and anxiety vary among studies. Some report lower response rates than for children with ADHD alone and possibly more treatment-emergent side effects.6 The National Institute of Mental Health’s Multimodal Treatment Study of Children with ADHD (MTA) found that comorbid anxiety did not adversely affect behavioral response to stimulants but did moderate outcomes (Box 1).7,8 Adding intensive psychosocial intervention to stimulant treatment appeared to yield greater improvements in anxious children with ADHD, compared with stimulants alone.8
Cognitive impairments related to inattention do not consistently improve with stimulant treatment.9 This is clinically important because children with ADHD and comorbid anxiety disorders can be very cognitively impaired.10
Add an SSRI? Monotherapy is simpler and usually more acceptable to families, but a placebo-controlled study examined adding an SSRI (fluvoxamine) to methylphenidate treatment.4 Children with anxiety and ADHD who received adjunctive fluvoxamine did no better than those who received methylphenidate plus placebo.
Atomoxetine. A large, randomized, controlled trial of atomoxetine in this population found good tolerability and statistically significant reductions in ADHD and anxiety symptoms compared with placebo. Effect size was greater for ADHD symptoms than for anxiety symptoms,11 however, which supports smaller trials that show more consistent evidence of atomoxetine reducing ADHD symptoms than anxiety symptoms.
Similar to antidepressants with the SNRI chemical structure, atomoxetine’s effectiveness for a given child takes several weeks to determine. This can be a problem in children who are highly distressed or impaired and require rapid symptomatic improvement.
Recommendation. Consider a stimulant or atomoxetine initially for children with anxiety disorders and ADHD, and seek concurrent behavioral or cognitive-behavioral therapy. Caution families that:
- >1 medication trial might be needed, as response may not be as consistent as in children with ADHD alone
- medication-related improvements in ADHD symptoms will not necessarily be associated with reduced anxiety symptoms or improved academic ability
- improvements with atomoxetine might not be evident for several weeks.
Table 2
Medication dosing for children with ADHD*
| Medication | Recommended starting dosage | Recommended maximum dosage | 5 most common side effects in descending prevalence |
|---|---|---|---|
| Stimulants | |||
| Methylphenidate hydrochloride (Ritalin) | 5 mg tid | Total 60 mg/d | Insomnia, nervousness, decreased appetite, dizziness, nausea |
| Methylphenidate hydrochloride (Concerta) | 18 mg every morning | 54 mg every morning | Headache, abdominal pain, decreased appetite, vomiting, insomnia |
| Dextroamphetamine sulfate (Dexedrine) | 5 mg every morning | Total 40 mg/d | Palpitations, restlessness, dizziness, dry mouth, decreased appetite |
| Mixed amphetamine salts (Adderall) | 10 mg every morning | 30 mg every morning | Decreased appetite, insomnia, abdominal pain, emotional lability, vomiting |
| Nonstimulant | |||
| Atomoxetine (Strattera) | 0.5 mg/kg/d | 1.2 mg/kg/d | Decreased appetite, dizziness, stomach upset, fatigue, irritability |
| ADHD: attention-deficit/hyperactivity disorder | |||
| * Recommended dosing is no different for children with ADHD and anxiety than for children with only ADHD | |||
| Source: Reference 5 | |||
Psychological intervention
CBT has been shown effective for child-hood anxiety disorders in randomized controlled trials,12 but even those that included children with comorbid ADHD required that an anxiety disorder be the primary, most impairing diagnosis.13 Thus, little is known about CBT’s effectiveness for children with anxiety plus ADHD. Given the evidence for cognitive deficits in comorbid anxiety and ADHD10 and the challenge of working with highly distractible children, one would expect CBT to be more difficult in this population.
The potential for distraction to adversely affect learning of coping strategies is higher in group than in individual therapy, and children with anxiety and ADHD can be disruptive to other children in CBT groups. Consider individual CBT, and seek a therapist who has experience with this population. Having the child on medication for ADHD symptoms usually helps reduce these symptoms’ impact on sessions.
For children younger than about age 8 or too cognitively impaired to benefit from CBT, behavioral intervention alone may be helpful. The largely behavioral psychosocial intervention in the MTA study of ADHD children age 7 to 9 (Box 2)8,14 helped many of those with comorbid anxiety.
Although programs as intense as that used in the MTA study rarely are provided in community practice, consider behavior modification. For example:
- To reduce anxiety, have the child follow regular, predictable routines, and reward the child for gradually facing previously avoided situations.
- To reduce distractibility in class, have the child sit near the teacher, break work into small chunks, and reward completion of each chunk.
Even small improvements in the child’s home or school behavior may reduce negative interactions with others and the attendant effects on self-esteem.
CASE CONTINUED: Weighing the options
The therapist seeing Aaron’s family listens to their concerns about medication and reassures them that their son will not be denied psychotherapy. She tells them, however, that psychotherapy will not address his urgent school problems and is unlikely to work in the absence of medication, given Aaron’s behavior in the office. The therapist provides accurate information about the risks and benefits of medication and CBT, and the parents agree to think about all treatment options.
By the next office visit, the school has threatened to suspend Aaron. He and his parents agree to combined treatment with a stimulant medication and CBT and to having the therapist provide a behavioral consultation at the school.
shows best outcomes for ADHD with anxiety
The National Institute of Mental Health’s Multimodal Treatment Study of Children with ADHD—the largest study to date—found that comorbid anxiety did not adversely affect behavioral response to stimulants but did moderate outcomes.
In the parallel group design study, 579 ADHD children age 7 to 9 were enrolled at 6 treatment sites, thoroughly assessed, then randomly assigned to 4 groups: medication treatment alone, intensive psychosocial treatment alone, a combination of both treatments, or usual community care. The first 3 interventions were designed to reflect best practices for each approach, and these children were closely monitored and studied for 14 months. All 4 groups were reassessed periodically for 24 months, evaluating multiple outcomes.
For the total sample, combined and medication treatment were more effective than psychosocial treatment and community care. For ADHD children with comorbid anxiety disorders:
- combined treatment was more effective than either medication treatment alone or psychosocial treatment alone
- both monotherapies were superior to community care.
ADHD: attention-deficit/hyperactivity disorder
Family psychoeducation
With families of children with behavioral challenges, adopt a patient, educational approach rather than acquiescing to their wishes or arguing with them. Either can result in treatment failure. Discuss potential benefits and risks of all treatment options and the impact of comorbidity on treatment.
Parents’ rigid insistence on a particular course of action—such as refusing psychopharmacology—may be caused by anxiety or misinformation. Elicit the source of any anxiety, and provide realistic information and reassurance if possible.
Anxiety in family members may be constitutional—as anxiety is highly heritable15—or relate to aspects of treatment. Families may feel overwhelmed by:
- their child having 2 disorders rather than 1
- your suggestion to start medical and nonmedical intervention together
- hearing about the possibility of multiple medication trials.
Negotiating medication. Discuss with the family the difficulties of a child learning CBT strategies when ADHD is not well-controlled and the cognitive difficulties in many of these children that may necessitate individualized CBT. If the family remains reluctant to consider combining medication with CBT, try contracting for a limited number of CBT sessions (perhaps 3 or 4) before re-evaluating the need for medication.
The child’s perceptions (and potential anxieties) about his or her difficulties also must be understood, validated, and addressed. Children are more likely to engage in a treatment if they participate in the decision to adopt it.
Anxiety can heighten vigilance in the child or the parents to treatment-emergent side effects, which you may exacerbate by providing exhaustive lists of potential ad-verse events. Limit discussion to serious side effects—with emphasis on their rarity—and those that are common.
ADHD traits in families can affect treatment success. Because of their own distractibility and organizational difficulties, parents with ADHD traits may have difficulty ensuring the child’s medication adherence and treatment participation.16
Behavior modification can require a high degree of consistency in parents’ behavior toward the child. This may be difficult to achieve in families where:
- 1 or both parents are inattentive because of ADHD
- a high degree of conflict exists between parents.
To help these families, provide reminder calls about appointments and schedule sessions at a consistent time. To improve consistency of medication use:
- combine medication administration with an essential daily activity
- check adherence with pill counts or other means.
If the child participates in CBT, provide separate notebooks for in-session and homework exercises—anticipating some loss of homework notebooks.
Individualizing care
Individualized care is important to return each child to his or her best possible level of functioning. The child’s symptom profile, environment, and developmental level can affect treatment.
For example, in a child whose ADHD-related impairment is substantial but whose anxiety-related impairment is mild, pharmacotherapy for ADHD and some pa-rental guidance may be adequate to manage remaining anxiety symptoms.17 As mentioned, some children show decreased anxiety as their ADHD is better controlled.4 Conversely, if ADHD-related impairment is mild but the child is highly anxious, consider CBT alone—preferably on an individual basis—provided the child can manage the cognitive aspects of therapy.
School personnel can monitor change in relation to various interventions, as many of these children’s symptoms manifest in the classroom. Behavioral interventions are more likely to succeed if they are administered consistently across home and school environments8 and teachers participate in behavior modification.
To elicit cooperation from school personnel, listen to their concerns and observations and help them understand the child’s difficulties and the rationale for various treatments. This approach often reduces negative feedback toward the child, a benefit that may further improve outcomes.
Attention to peer relationships and social stressors is often needed. Because of their multiple difficulties, these children may lack social skills and be shunned by their peers.1 You may need to help them develop social skills and reconnect with their peers after symptoms are well-controlled.
Poverty or lack of social support can affect treatment. Children with ADHD and anxiety usually need multiple interventions, and it is difficult for families to at-tend to these consistently when struggling with social stressors.
The 14-month intensive behavioral intervention used in the National Institute of Mental Health’s Multimodal Treatment Study (MTA) of 579 children age 7 to 9 with ADHD included:
- weekly parent training initially, decreasing to monthly by the end
- biweekly teacher consultations in behavior management
- 8-week full-day therapeutic summer program for children, focusing on behavioral and cognitive behavioral intervention
- 12-week half-time behaviorally trained paraprofessional aide in the classroom to generalize gains from summer program
- parent coaching on collaborating with teacher long-term so therapeutic consultation could be faded.
ADHD: attention-deficit/hyperactivity disorder
Adolescent adjustments. ADHD and anxiety often are diagnosed in the early school years, so anticipate developmental effects on treatment as the child enters adolescence. Adolescents value autonomy and may need to be more involved in treatment decisions than younger children.
Ask about and address family disagreements about treatment options, which may reduce adherence. You may need to talk about peer pressure to “not take drugs” by clearly differentiating the reasons some people take street drugs and the reasons for taking prescribed medication. Also discuss in a frank, nonjudgmental manner the risks of experimenting with street drugs (especially with prescribed medication) or of “sharing” one’s medications with friends.
Increased cognitive sophistication in adolescence may increase the potential benefit of CBT, so explore this option with the teen, especially if it was not attempted in the past.
Related resources
- American Academy of Child and Adolescent Psychiatry. “ADHD—a guide for families,” under the Resources for Families tab. www.aacap.org.
- Watkins C. Stimulant medication and ADHD. www.ncpamd.com/Stimulants.htm.
- Manassis K. Keys to parenting your anxious child. 2nd ed. Hauppauge, NY: Barron’s Educational Series, Inc.; 2008.
Drug brand names
- Atomoxetine • Strattera
- Dextroamphetamine • Dexedrine
- Fluvoxamine • Luvox
- Methylphenidate • Ritalin, Concerta
- Mixed amphetamine salts • Adderall
Disclosures
Dr. Manassis reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Aaron, age 10, has been diagnosed with an anxiety disorder and attention-deficit/hyperactivity disorder (ADHD) but is not being treated with medication because his parents do not believe in psychopharmacology. They bring him to a specialized child anxiety clinic and ask for “urgent CBT” because his behavior at school is out of control.
Aaron rearranges the therapist’s office furniture during much of the assessment interview. He also acknowledges many anxiety symptoms. The therapist doubts that cognitive-behavioral therapy (CBT) would help without other interventions.
Children with anxiety disorders and ADHD—a common comorbid presentation—tend to be more impaired than those with either condition alone.1 Effective treatment usually requires 4 components (Table 1), including medication plus behavioral or cognitive-behavioral therapy. This article discusses clinical issues related to each component and describes how to successfully combine them into a treatment plan.
Table 1
Comorbid ADHD and anxiety: 4 treatment components
| Successful treatment usually involves combining 4 components: | |
| |
| Make individual adjustments as needed, depending on the child’s symptom profile, social context, and developmental level | |
| ADHD: attention-deficit/hyperactivity disorder | |
Medication options
Stimulants, atomoxetine, and selective serotonin reuptake inhibitors (SSRIs) have been advocated for children with anxiety and ADHD. Given the high risk of behavioral disinhibition with SSRIs in children,2 stimulants or atomoxetine are suggested as first-line medications.3,4
Stimulants target ADHD symptoms primarily, but anxiety decreases in some children (24% in a recent trial) as ADHD symptoms are controlled.4 Because it is a selective norepinephrine reuptake inhibitor (SNRI), atomoxetine may target both ADHD and anxiety symptoms. When initiating these medications, “start low and go slow.” Recommended dosing is no different for children with ADHD and anxiety than for those with ADHD alone (Table 2).5
Stimulant response rates for children with ADHD and anxiety vary among studies. Some report lower response rates than for children with ADHD alone and possibly more treatment-emergent side effects.6 The National Institute of Mental Health’s Multimodal Treatment Study of Children with ADHD (MTA) found that comorbid anxiety did not adversely affect behavioral response to stimulants but did moderate outcomes (Box 1).7,8 Adding intensive psychosocial intervention to stimulant treatment appeared to yield greater improvements in anxious children with ADHD, compared with stimulants alone.8
Cognitive impairments related to inattention do not consistently improve with stimulant treatment.9 This is clinically important because children with ADHD and comorbid anxiety disorders can be very cognitively impaired.10
Add an SSRI? Monotherapy is simpler and usually more acceptable to families, but a placebo-controlled study examined adding an SSRI (fluvoxamine) to methylphenidate treatment.4 Children with anxiety and ADHD who received adjunctive fluvoxamine did no better than those who received methylphenidate plus placebo.
Atomoxetine. A large, randomized, controlled trial of atomoxetine in this population found good tolerability and statistically significant reductions in ADHD and anxiety symptoms compared with placebo. Effect size was greater for ADHD symptoms than for anxiety symptoms,11 however, which supports smaller trials that show more consistent evidence of atomoxetine reducing ADHD symptoms than anxiety symptoms.
Similar to antidepressants with the SNRI chemical structure, atomoxetine’s effectiveness for a given child takes several weeks to determine. This can be a problem in children who are highly distressed or impaired and require rapid symptomatic improvement.
Recommendation. Consider a stimulant or atomoxetine initially for children with anxiety disorders and ADHD, and seek concurrent behavioral or cognitive-behavioral therapy. Caution families that:
- >1 medication trial might be needed, as response may not be as consistent as in children with ADHD alone
- medication-related improvements in ADHD symptoms will not necessarily be associated with reduced anxiety symptoms or improved academic ability
- improvements with atomoxetine might not be evident for several weeks.
Table 2
Medication dosing for children with ADHD*
| Medication | Recommended starting dosage | Recommended maximum dosage | 5 most common side effects in descending prevalence |
|---|---|---|---|
| Stimulants | |||
| Methylphenidate hydrochloride (Ritalin) | 5 mg tid | Total 60 mg/d | Insomnia, nervousness, decreased appetite, dizziness, nausea |
| Methylphenidate hydrochloride (Concerta) | 18 mg every morning | 54 mg every morning | Headache, abdominal pain, decreased appetite, vomiting, insomnia |
| Dextroamphetamine sulfate (Dexedrine) | 5 mg every morning | Total 40 mg/d | Palpitations, restlessness, dizziness, dry mouth, decreased appetite |
| Mixed amphetamine salts (Adderall) | 10 mg every morning | 30 mg every morning | Decreased appetite, insomnia, abdominal pain, emotional lability, vomiting |
| Nonstimulant | |||
| Atomoxetine (Strattera) | 0.5 mg/kg/d | 1.2 mg/kg/d | Decreased appetite, dizziness, stomach upset, fatigue, irritability |
| ADHD: attention-deficit/hyperactivity disorder | |||
| * Recommended dosing is no different for children with ADHD and anxiety than for children with only ADHD | |||
| Source: Reference 5 | |||
Psychological intervention
CBT has been shown effective for child-hood anxiety disorders in randomized controlled trials,12 but even those that included children with comorbid ADHD required that an anxiety disorder be the primary, most impairing diagnosis.13 Thus, little is known about CBT’s effectiveness for children with anxiety plus ADHD. Given the evidence for cognitive deficits in comorbid anxiety and ADHD10 and the challenge of working with highly distractible children, one would expect CBT to be more difficult in this population.
The potential for distraction to adversely affect learning of coping strategies is higher in group than in individual therapy, and children with anxiety and ADHD can be disruptive to other children in CBT groups. Consider individual CBT, and seek a therapist who has experience with this population. Having the child on medication for ADHD symptoms usually helps reduce these symptoms’ impact on sessions.
For children younger than about age 8 or too cognitively impaired to benefit from CBT, behavioral intervention alone may be helpful. The largely behavioral psychosocial intervention in the MTA study of ADHD children age 7 to 9 (Box 2)8,14 helped many of those with comorbid anxiety.
Although programs as intense as that used in the MTA study rarely are provided in community practice, consider behavior modification. For example:
- To reduce anxiety, have the child follow regular, predictable routines, and reward the child for gradually facing previously avoided situations.
- To reduce distractibility in class, have the child sit near the teacher, break work into small chunks, and reward completion of each chunk.
Even small improvements in the child’s home or school behavior may reduce negative interactions with others and the attendant effects on self-esteem.
CASE CONTINUED: Weighing the options
The therapist seeing Aaron’s family listens to their concerns about medication and reassures them that their son will not be denied psychotherapy. She tells them, however, that psychotherapy will not address his urgent school problems and is unlikely to work in the absence of medication, given Aaron’s behavior in the office. The therapist provides accurate information about the risks and benefits of medication and CBT, and the parents agree to think about all treatment options.
By the next office visit, the school has threatened to suspend Aaron. He and his parents agree to combined treatment with a stimulant medication and CBT and to having the therapist provide a behavioral consultation at the school.
shows best outcomes for ADHD with anxiety
The National Institute of Mental Health’s Multimodal Treatment Study of Children with ADHD—the largest study to date—found that comorbid anxiety did not adversely affect behavioral response to stimulants but did moderate outcomes.
In the parallel group design study, 579 ADHD children age 7 to 9 were enrolled at 6 treatment sites, thoroughly assessed, then randomly assigned to 4 groups: medication treatment alone, intensive psychosocial treatment alone, a combination of both treatments, or usual community care. The first 3 interventions were designed to reflect best practices for each approach, and these children were closely monitored and studied for 14 months. All 4 groups were reassessed periodically for 24 months, evaluating multiple outcomes.
For the total sample, combined and medication treatment were more effective than psychosocial treatment and community care. For ADHD children with comorbid anxiety disorders:
- combined treatment was more effective than either medication treatment alone or psychosocial treatment alone
- both monotherapies were superior to community care.
ADHD: attention-deficit/hyperactivity disorder
Family psychoeducation
With families of children with behavioral challenges, adopt a patient, educational approach rather than acquiescing to their wishes or arguing with them. Either can result in treatment failure. Discuss potential benefits and risks of all treatment options and the impact of comorbidity on treatment.
Parents’ rigid insistence on a particular course of action—such as refusing psychopharmacology—may be caused by anxiety or misinformation. Elicit the source of any anxiety, and provide realistic information and reassurance if possible.
Anxiety in family members may be constitutional—as anxiety is highly heritable15—or relate to aspects of treatment. Families may feel overwhelmed by:
- their child having 2 disorders rather than 1
- your suggestion to start medical and nonmedical intervention together
- hearing about the possibility of multiple medication trials.
Negotiating medication. Discuss with the family the difficulties of a child learning CBT strategies when ADHD is not well-controlled and the cognitive difficulties in many of these children that may necessitate individualized CBT. If the family remains reluctant to consider combining medication with CBT, try contracting for a limited number of CBT sessions (perhaps 3 or 4) before re-evaluating the need for medication.
The child’s perceptions (and potential anxieties) about his or her difficulties also must be understood, validated, and addressed. Children are more likely to engage in a treatment if they participate in the decision to adopt it.
Anxiety can heighten vigilance in the child or the parents to treatment-emergent side effects, which you may exacerbate by providing exhaustive lists of potential ad-verse events. Limit discussion to serious side effects—with emphasis on their rarity—and those that are common.
ADHD traits in families can affect treatment success. Because of their own distractibility and organizational difficulties, parents with ADHD traits may have difficulty ensuring the child’s medication adherence and treatment participation.16
Behavior modification can require a high degree of consistency in parents’ behavior toward the child. This may be difficult to achieve in families where:
- 1 or both parents are inattentive because of ADHD
- a high degree of conflict exists between parents.
To help these families, provide reminder calls about appointments and schedule sessions at a consistent time. To improve consistency of medication use:
- combine medication administration with an essential daily activity
- check adherence with pill counts or other means.
If the child participates in CBT, provide separate notebooks for in-session and homework exercises—anticipating some loss of homework notebooks.
Individualizing care
Individualized care is important to return each child to his or her best possible level of functioning. The child’s symptom profile, environment, and developmental level can affect treatment.
For example, in a child whose ADHD-related impairment is substantial but whose anxiety-related impairment is mild, pharmacotherapy for ADHD and some pa-rental guidance may be adequate to manage remaining anxiety symptoms.17 As mentioned, some children show decreased anxiety as their ADHD is better controlled.4 Conversely, if ADHD-related impairment is mild but the child is highly anxious, consider CBT alone—preferably on an individual basis—provided the child can manage the cognitive aspects of therapy.
School personnel can monitor change in relation to various interventions, as many of these children’s symptoms manifest in the classroom. Behavioral interventions are more likely to succeed if they are administered consistently across home and school environments8 and teachers participate in behavior modification.
To elicit cooperation from school personnel, listen to their concerns and observations and help them understand the child’s difficulties and the rationale for various treatments. This approach often reduces negative feedback toward the child, a benefit that may further improve outcomes.
Attention to peer relationships and social stressors is often needed. Because of their multiple difficulties, these children may lack social skills and be shunned by their peers.1 You may need to help them develop social skills and reconnect with their peers after symptoms are well-controlled.
Poverty or lack of social support can affect treatment. Children with ADHD and anxiety usually need multiple interventions, and it is difficult for families to at-tend to these consistently when struggling with social stressors.
The 14-month intensive behavioral intervention used in the National Institute of Mental Health’s Multimodal Treatment Study (MTA) of 579 children age 7 to 9 with ADHD included:
- weekly parent training initially, decreasing to monthly by the end
- biweekly teacher consultations in behavior management
- 8-week full-day therapeutic summer program for children, focusing on behavioral and cognitive behavioral intervention
- 12-week half-time behaviorally trained paraprofessional aide in the classroom to generalize gains from summer program
- parent coaching on collaborating with teacher long-term so therapeutic consultation could be faded.
ADHD: attention-deficit/hyperactivity disorder
Adolescent adjustments. ADHD and anxiety often are diagnosed in the early school years, so anticipate developmental effects on treatment as the child enters adolescence. Adolescents value autonomy and may need to be more involved in treatment decisions than younger children.
Ask about and address family disagreements about treatment options, which may reduce adherence. You may need to talk about peer pressure to “not take drugs” by clearly differentiating the reasons some people take street drugs and the reasons for taking prescribed medication. Also discuss in a frank, nonjudgmental manner the risks of experimenting with street drugs (especially with prescribed medication) or of “sharing” one’s medications with friends.
Increased cognitive sophistication in adolescence may increase the potential benefit of CBT, so explore this option with the teen, especially if it was not attempted in the past.
Related resources
- American Academy of Child and Adolescent Psychiatry. “ADHD—a guide for families,” under the Resources for Families tab. www.aacap.org.
- Watkins C. Stimulant medication and ADHD. www.ncpamd.com/Stimulants.htm.
- Manassis K. Keys to parenting your anxious child. 2nd ed. Hauppauge, NY: Barron’s Educational Series, Inc.; 2008.
Drug brand names
- Atomoxetine • Strattera
- Dextroamphetamine • Dexedrine
- Fluvoxamine • Luvox
- Methylphenidate • Ritalin, Concerta
- Mixed amphetamine salts • Adderall
Disclosures
Dr. Manassis reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Bowen R, Chavira DA, Bailey K, et al. Nature of anxiety comorbid with attention deficit hyperactivity disorder in children from a pediatric primary care setting. Psychiatry Res 2008;157:201-9.
2. Walkup JT, Labellarte MJ, Riddle MA, et al. Searching for moderators and mediators of pharmacological treatment in children and adolescents with anxiety disorders. J Am Acad Child Adolesc Psychiatry 2003;42:13-21.
3. Wiesegger G, Kienbacher C, Pellegrini E, et al. Pharmacotherapy of attention-deficit/hyperactivity disorder (ADHD) and comorbid disorders. Neuropsychiatr 2007;21:187-206.
4. Abikoff H, McGough J, Vitiello B, et al. Sequential pharmacotherapy for children with comorbid attention-deficit/hyperactivity and anxiety disorders. J Am Acad Child Adolesc Psychiatry 2005;44:418-27.
5. Compendium of pharmaceuticals and specialties. Ottawa, Canada: Canadian Pharmacists Association; 2008.
6. Goez H, Back-Bennet O, Zelnik N. Differential stimulant response on attention in children with comorbid anxiety and oppositional defiant disorder. J Child Neurol 2007;22:538-42.
7. Wells KC, Pelham WE, Kotkin RA, et al. Psychosocial treatment strategies in the MTA study: rationale, methods, and critical issues in design and implementation. J Abnorm Child Psychol 2000;28:483-505.
8. March JS, Swanson JM, Arnold EL, et al. Anxiety as a predictor and outcome variable in the Multimodal Treatment Study of Children with ADHD (MTA). J Abnorm Child Psychol 2000;28:527-41.
9. Tannock R, Ickowicz A, Schachar R. Differential effects of methylphenidate on working memory in ADHD children with and without anxiety. J Am Acad Child Adolesc Psychiatry 1995;34:886-96.
10. Manassis K, Tannock R, Young A, Francis-John S. Cognition in anxious children with attention deficit hyperactivity disorder: a comparison with clinical and normal children. Behav Brain Funct 2007;3-4.
11. Geller D, Donnelly C, Lopez F, et al. Atomoxetine treatment for pediatric patients with attention-deficit/hyperactivity disorder with comorbid anxiety disorder. J Am Acad Child Adolesc Psychiatry 2007;46:1119-27.
12. Compton SN, March JS, Brent D, et al. Cognitive behavioural psychotherapy for anxiety and depressive disorders in children and adolescents: an evidence-based medicine review. J Am Acad Child Adolesc Psychiatry 2004;43:930-59.
13. Manassis K, Mendlowitz SL, Scapillato D, et al. Group and individual cognitive-behavioral therapy for childhood anxiety disorders: a randomized trial. J Am Acad Child Adolesc Psychiatry 2002;41:1423-30.
14. Arnold LE, Abikoff HB, Cantwell DP, et al. National Institute of Mental Health Collaborative Multimodal Treatment Study of Children with ADHD (the MTA). Design challenges and choices. Arch Gen Psychiatry 1997;54:865-70.
15. Kagan J, Reznick JS, Snidman N. Biological basis of childhood shyness. Science 1990;240:167-71.
16. Van Cleave J, Leslie LK. Approaching ADHD as a chronic condition: implications for long-term adherence. Pediatr Ann 2008;37:19-26.
17. Manassis K, Monga S. therapeutic approach to children and adolescents with anxiety disorders and associated comorbid conditions. J Am Acad Child Adolesc Psychiatry 2001;40:115-7.
1. Bowen R, Chavira DA, Bailey K, et al. Nature of anxiety comorbid with attention deficit hyperactivity disorder in children from a pediatric primary care setting. Psychiatry Res 2008;157:201-9.
2. Walkup JT, Labellarte MJ, Riddle MA, et al. Searching for moderators and mediators of pharmacological treatment in children and adolescents with anxiety disorders. J Am Acad Child Adolesc Psychiatry 2003;42:13-21.
3. Wiesegger G, Kienbacher C, Pellegrini E, et al. Pharmacotherapy of attention-deficit/hyperactivity disorder (ADHD) and comorbid disorders. Neuropsychiatr 2007;21:187-206.
4. Abikoff H, McGough J, Vitiello B, et al. Sequential pharmacotherapy for children with comorbid attention-deficit/hyperactivity and anxiety disorders. J Am Acad Child Adolesc Psychiatry 2005;44:418-27.
5. Compendium of pharmaceuticals and specialties. Ottawa, Canada: Canadian Pharmacists Association; 2008.
6. Goez H, Back-Bennet O, Zelnik N. Differential stimulant response on attention in children with comorbid anxiety and oppositional defiant disorder. J Child Neurol 2007;22:538-42.
7. Wells KC, Pelham WE, Kotkin RA, et al. Psychosocial treatment strategies in the MTA study: rationale, methods, and critical issues in design and implementation. J Abnorm Child Psychol 2000;28:483-505.
8. March JS, Swanson JM, Arnold EL, et al. Anxiety as a predictor and outcome variable in the Multimodal Treatment Study of Children with ADHD (MTA). J Abnorm Child Psychol 2000;28:527-41.
9. Tannock R, Ickowicz A, Schachar R. Differential effects of methylphenidate on working memory in ADHD children with and without anxiety. J Am Acad Child Adolesc Psychiatry 1995;34:886-96.
10. Manassis K, Tannock R, Young A, Francis-John S. Cognition in anxious children with attention deficit hyperactivity disorder: a comparison with clinical and normal children. Behav Brain Funct 2007;3-4.
11. Geller D, Donnelly C, Lopez F, et al. Atomoxetine treatment for pediatric patients with attention-deficit/hyperactivity disorder with comorbid anxiety disorder. J Am Acad Child Adolesc Psychiatry 2007;46:1119-27.
12. Compton SN, March JS, Brent D, et al. Cognitive behavioural psychotherapy for anxiety and depressive disorders in children and adolescents: an evidence-based medicine review. J Am Acad Child Adolesc Psychiatry 2004;43:930-59.
13. Manassis K, Mendlowitz SL, Scapillato D, et al. Group and individual cognitive-behavioral therapy for childhood anxiety disorders: a randomized trial. J Am Acad Child Adolesc Psychiatry 2002;41:1423-30.
14. Arnold LE, Abikoff HB, Cantwell DP, et al. National Institute of Mental Health Collaborative Multimodal Treatment Study of Children with ADHD (the MTA). Design challenges and choices. Arch Gen Psychiatry 1997;54:865-70.
15. Kagan J, Reznick JS, Snidman N. Biological basis of childhood shyness. Science 1990;240:167-71.
16. Van Cleave J, Leslie LK. Approaching ADHD as a chronic condition: implications for long-term adherence. Pediatr Ann 2008;37:19-26.
17. Manassis K, Monga S. therapeutic approach to children and adolescents with anxiety disorders and associated comorbid conditions. J Am Acad Child Adolesc Psychiatry 2001;40:115-7.
Antipsychotics in dementia: Beyond ‘black-box’ warnings
Diagnosed 7 years ago with Alzheimer’s disease (AD), Mrs. B, age 82, resides in an assisted living facility whose staff is trained to care for older persons with dementia. Over the past 2 months she has shown an escalating pattern of psychosis and aggression, despite one-to-one attention and verbal reassurance.
At first Mrs. B’s psychosis was restricted to occasional rape accusations during assisted bathing and aggression manifested by banging her hand repetitively on furniture, causing skin tears. In the last week, she has been accusing staff and patients of stealing her belongings and has assaulted a staff member and another resident. When supervisors at the facility advise Mrs. B’s husband that she can no longer stay there, he takes her to a local emergency room, from which she is admitted involuntarily to a geriatric psychiatry inpatient unit.
For many patients and families, the most problematic aspects of dementia are neuropsychiatric symptoms—depression, sleep disturbance, psychosis, and aggression. Psychosis affects approximately 40% of persons with AD, whereas ≥80% of persons with dementia experience agitation at some point in the illness.1 These symptoms can lead to:
- caregiver morbidity
- poor patient quality of life
- early patient institutionalization.2
Although no drug has been FDA-approved for treating dementia’s neuropsychiatric symptoms, psychiatrists often use off-label psychotropics—especially antipsychotics—to ameliorate them. This practice is controversial because of public perception that antipsychotics are used in dementia patients to create “zombies” to lighten healthcare workers’ burden. Nonetheless, because dementia patients with psychosis and severe agitation/aggression can pose risks to themselves and those around them, efforts to treat these symptoms are warranted.
The FDA warned prescribers in 2003 of increased risk of “cerebrovascular adverse events including stroke” in dementia patients treated with risperidone vs placebo. Similar cerebrovascular warnings have been issued for olanzapine and aripiprazole. Although the absolute risk difference was generally 1% to 2% between antipsychotic- and placebo-treated patients, the relative risk was approximately 2 times higher with antipsychotics because the prevalence of these events is low in both groups.3
Perhaps more daunting, after a meta-analysis of 17 trials using atypical antipsychotics in elderly patients with dementia-related psychosis, the FDA in 2005 issued a black-box warning of increased mortality risk with atypical antipsychotics (relative risk 1.6 to 1.7) vs placebo. The mortality rate in antipsychotic-treated patients was about 4.5%, compared with about 2.6% in the placebo group. Although causes of death varied, most were cardiovascular (heart failure, sudden death) or infectious (pneumonia). This warning was applied to atypical antipsychotics as a class. As with cerebrovascular risks, the absolute mortality risk difference was 1% to 2%.4
The FDA’s “black-box” warnings about using atypical agents in patients with dementia add another layer of complexity to your treatment decisions (Box).3,4 The public is well served by evidence identifying risks associated with prescription medications, but the FDA data do little to help millions of families answer the question, “And so, what now?”
Recognizing that solid empiric evidence is lacking, we attempt to address this lingering question for clinicians, patients, and caregivers who must deal with these symptoms while science tries to provide a more definitive answer.
5-step evaluation
A 5-step initial evaluation of persons with dementia who present with psychosis and/or agitation/aggression includes establishing the frequency, severity, and cause of these symptoms as well as the effectiveness of past treatments and strategies (Algorithm).5
Because adverse drug effects are a potentially reversible cause of psychosis and agitation, review the patient’s drug list—including “as needed” medications—from records at a facility or from family report. Mrs. B’s record from the assisted living facility reveals she was receiving:
- atenolol, 25 mg/d
- aspirin, 81 mg/d
- extended-release oxybutynin, 10 mg at bedtime
- psyllium, one packet daily
- hydrocodone/acetaminophen, 5/500 mg every 4 hours as needed for pain
- lorazepam, 1 mg every 6 hours as needed for agitation
- diphenhydramine, 25 mg at bedtime
- paroxetine, 20 mg/d
- haloperidol, 5 mg at bedtime
- memantine, 10 mg twice a day.
Mrs. B’s medication list is revealing for reasons that, unfortunately, are not rare. She is receiving 3 anticholinergic medications—oxybutynin, diphenhydramine, and paroxetine—that may be worsening her mental status and behavior directly through CNS effects, possibly in combination with frequent benzodiazepine use.
Anticholinergics also can lead to behavior changes via peripheral side effects. Constipation and urinary retention may cause discomfort that an aphasic patient “acts out.” A patient may be experiencing pain related to these side effects and receiving opioid analgesics, which can worsen constipation and urinary retention. Uncontrolled pain related to musculoskeletal disease or neuropathy may merit treatment that will reduce behavioral disturbances.
Mrs. B also was being catheterized every 8 hours as needed for urinary retention. The invasive and unpleasant nature of urinary catheterization is likely to worsen behavior and increases the risk of one of the most common “asymptomatic” etiologies of behavioral symptoms in dementia—urinary tract infection (UTI).
Algorithm
5-step evaluation of dementia patients
with psychosis and/or agitation/aggression*
1. How dangerous is the situation?
|
| ↓ |
2. Establish a clear diagnosis/etiology for the symptoms
|
| ↓ |
3. Establish symptom severity and frequency, including:
|
| ↓ |
| 4. Explore past treatments/caregiver strategies used to address the symptoms and their success and/or problematic outcomes |
| ↓ |
| 5. Discuss with the patient/decision-maker what is and is not known about possible risks and benefits of pharmacologic and nonpharmacologic treatments for psychosis and agitation/aggression in dementia |
| Source: Reference 5 |
| * Agitation is defined as “inappropriate verbal, vocal, or motor activity that is not judged by an outside observer to be an obvious outcome of the needs or confusion of the individual”24 |
CASE CONTINUED: Persistent agitation
After evaluating Mrs. B, the psychiatrist limits her medications to atenolol, aspirin, psyllium, and memantine, and begins to taper lorazepam and paroxetine. Laboratory, radiologic, and physical examinations reveal UTI, fecal impaction, bladder distension, and mild hyponatremia. She is given a phosphosoda enema and ciprofloxacin, 250 mg/d for 5 days.
Despite one-to-one nursing care, frequent reorientation, and attempts to interest her in art therapy, Mrs. B remains agitated and postures to strike staff members and other patients. She denies pain or discomfort. Fearing that someone might be injured, the nurse pages the on-duty psychiatrist.
The nurse then calls Mr. B, who has durable power of attorney for his wife’s healthcare. When the nurse advises Mr. B that the psychiatrist has ordered risperidone, 0.5 mg, he immediately interjects that the psychiatrist at the assisted living facility told him haloperidol should be used for his wife’s symptoms because other antipsychotics can cause strokes and death.
Typical vs atypical antipsychotics
Mrs. B’s nurse may have to delay administering risperidone while she puts Mr. B in contact with the psychiatrist. In an emergent situation when well-trained staff have assessed for common reversible causes of agitation and tried reasonable nonpharmacologic means to calm the patient, few people would argue against using medication to preserve the safety of the patient and others. To avoid questions such as this during a crisis, obtain informed consent at admission from the patient or (more likely) the proxy decision-maker for medications you anticipate the patient might receive during hospitalization.
The larger question is whether typical antipsychotics are preferred for dementia-related psychosis and agitation/aggression because the FDA has not issued the same global black-box warning for this class. Astute clinicians realize that a lack of evidence of harm is not evidence of a lack of harm. In fact, since the black-box warnings for atypical antipsychotics in dementia emerged, several studies have examined whether the same risks exist for typical agents.
Evidence regarding risk of stroke and death with the use of typical and atypical antipsychotics in patients with dementia is summarized in Table 1.6-13 Most evidence, including numerous studies in the past year, comes from retrospective database analyses. Prospective head-to-head comparisons of atypical and typical antipsychotics in dementia are scarce, and future prospective comparisons would be unethical.
No evidence suggests that typical antipsychotics mitigate the risks of stroke or death in dementia compared with atypical agents. Moreover, typical agents are more likely than atypicals to cause movement-related side effects—especially tardive dyskinesia and parkinsonism—in older adults with dementia.14
Table 1
Typical antipsychotics: Safer than atypicals for older patients?
| Study | Population | Summarized results |
|---|---|---|
| Mortality | ||
| Nasrallah et al6 | VA patients age ≥65 taking haloperidol or an atypical antipsychotic (n=1,553) | Approximately 4 times higher rate of death in those receiving haloperidol compared with those receiving atypicals |
| Wang et al8 | Pennsylvania adults age ≥65 with prescription coverage taking antipsychotics (n=22,890) | Typicals had higher relative risk (RR) of death at all time points over 180 days (RR 1.27 to 1.56), both in persons with and without dementia; higher risk associated with increased typical doses |
| Gill et al10 | Canadians age >65 with dementia (n=27,259 matched pairs) | Mortality rate was higher for users of typical vs atypical antipsychotics (RR 1.26 to 1.55) |
| Kales et al11 | VA patients age >65 prescribed psychotropics after a dementia diagnosis (n=10,615) | Risk of death similar for atypical and typical antipsychotics |
| Schneeweiss et al7 | Cancer-free Canadians age ≥65 taking antipsychotics (n=37,241) | Higher mortality rates for those taking typical antipsychotics than those taking atypicals (RR 1.47); higher mortality associated with higher typical doses |
| Trifirò et al9 | Adults age >65 with dementia receiving antipsychotics in Italy (n=2,385) | Equivalent rates of mortality in those taking typical and atypical antipsychotics |
| Stroke | ||
| Gill et al12 | Canadians age ≥65 with dementia receiving antipsychotics (n=32,710) | Equivalent rates of ischemic stroke in those taking atypical and typical agents compared with those receiving atypicals |
| Liperoti et al13 | Nursing home residents with dementia hospitalized for stroke or TIA and matched controls (n=4,788) | Rates of cerebrovascular adverse events equivalent between users of atypical and typical antipsychotics |
| VA: Veterans Affairs; TIA: transient ischemic attack | ||
CASE CONTINUED: Moderate relief from risperidone
After the psychiatrist explains the data on atypical vs typical antipsychotics in dementia—and the lack of FDA-approved treatments—Mr. B consents to the use of risperidone. He believes his wife would have wanted to try a medication with a moderate chance of relieving her internal distress and preventing her from harming anyone.
Risperidone provides moderate relief of Mrs. B’s aggression and paranoia. The next day Mr. B visits the unit and asks to speak with the psychiatrist. Although he appreciates the staff’s caring attitude, he says, “There must be safer or better ways to deal with these symptoms than medications like risperidone. I just don’t want the guilt of causing my wife to have a stroke or pass away.” He also asks, “How long will she have to take this medication?”
Evidence for efficacy
In addition to discussing antipsychotics’ risk in dementia, we also need to highlight their efficacy and effectiveness. A recent meta-analysis of 15 randomized controlled trials of atypical antipsychotics for agitation and/or psychosis in dementia included studies with risperidone, olanzapine, aripiprazole, and quetiapine.3 Most study participants were institutionalized, female, and had AD.
Psychosis scores improved in pooled studies of risperidone, whereas global neuropsychiatric disturbance improved with risperidone and aripiprazole. Effects were more notable in:
- persons without psychosis
- those living in nursing homes
- patients with severe cognitive impairment.
Subsequent placebo-controlled trials of risperidone, quetiapine, and aripiprazole—most focusing on patients with AD—reveal that atypical and typical antipsychotics have modest efficacy in reducing aggression and psychosis.15-19 However, to some extent the National Institute of Mental Health Clinical Antipsychotic Trial of Intervention Effectiveness Study for Alzheimer’s Disease (CATIE-AD)—the largest nonindustry-funded study conducted to address this question—called this conclusion into question.20 Risperidone and olanzapine (but not quetiapine) were efficacious in that fewer patients taking them vs placebo dropped out because of lack of efficacy. Antipsychotics were not effective overall, however, because the primary outcome—all-cause discontinuation rate—was similar for all 3 drugs and placebo. This indicates that on average these medications’ side effect burden may offset their efficacy, though individual patients’ responses may vary.
Alternatives to antipsychotics
Mr. B also raised the issue of treatment alternatives, such as no treatment, other psychotropics (Table 2),5,21 and nonpharmacologic methods (Table 3).22
“No treatment” does not imply a lack of assessment or intervention. Always examine patients for iatrogenic, medical, psychosocial, or other precipitants of behavioral symptoms. No treatment may be viable in mild to moderate cases but is impractical for patients with severe psychosis or agitation. Untreated, these symptoms could compromise safety or leave the patient without housing options.
Although possibly underused because of time constraints, reimbursement issues, or lack of training, nonpharmacologic strategies to treat aggression and psychosis in dementia are appealing alternatives to antipsychotics. Little empiric evidence supports nonpharmacologic strategies, however.22
Treatment decisions need to consider patients’ and caregivers’ value systems. Proxy decision-makers should examine treatment decisions in terms of how they believe the patient would view the alternatives. Without a specific advance directive, however, even well-intentioned decision-makers are likely to “contaminate” decisions with their own values and interests.
After discussing with the decision-maker various treatments’ risks and benefits, it might be useful to ask, for example, “If Mrs. B could have foreseen her behaviors 10 years ago, what do you think she would have wanted us to do? Some people might have been mortified by the thought of attacking other people, whereas other people would not mind this as much as the fear of being ‘overmedicated.’ Which end of the spectrum do you think she would have leaned toward?”
When medical research does not offer clear answers for the “right” next clinical step, clinicians can:
- acknowledge our own limits and those of human knowledge
- engage the caregiver (or, when appropriate, the patient) in shared decision-making, recognizing that some people will appreciate the opportunity for “equal partnership” whereas others will want us to decide based on our best clinical judgment.
Table 2
Pharmacologic alternatives to antipsychotics: What the evidence says
| Treatment | Evidence/results |
|---|---|
| Selective serotonin reuptake inhibitors | 2 positive studies with citalopram (more effective than placebo for agitation in 1 trial and equivalent to risperidone for psychosis and agitation with greater tolerability in the other); 2 negative trials with sertraline |
| Other antidepressants | 1 study showed trazodone was equivalent to haloperidol for agitation, with greater tolerability; another found trazodone was no different from placebo; other agents have only case reports or open-label trials |
| Anticonvulsants | 3 trials showed divalproex was equivalent to placebo; 2 positive trials for carbamazepine, but tolerability problems in both; other agents tried only in case reports or open-label trials |
| Benzodiazepines/anxiolytics | 3 trials showed oxazepam, alprazolam, diphenhydramine, and buspirone were equivalent to haloperidol in effects on agitation, but none used a placebo control; trials had problematic methodologies and indicated cognitive worsening with some agents (especially diphenhydramine) |
| Cognitive enhancers | Some evidence of modest benefit in mostly post-hoc data analyses in trials designed to assess cognitive variables and often among participants with overall mild psychiatric symptoms; prospective studies of rivastigmine and donepezil specifically designed to assess neuropsychiatric symptoms have found no difference compared with placebo |
| Miscellaneous drugs | Failed trial of transdermal estrogen in men; small study showed propranolol (average dose 106 mg/d) more effective than placebo |
| Source: References 5,21 | |
Table 3
How well do psychosocial/behavioral therapies manage
psychosis/agitation in dementia?*
| Treatment | Evidence/results |
|---|---|
| Caregiver psychoeducation/support | Several positive RCTs (evidence grade A) |
| Music therapy | 6 RCTs, generally positive in the short term (evidence grade B) |
| Cognitive stimulation therapy | Three-quarters of RCTs showed some benefit (evidence grade B) |
| Snoezelen therapy (controlled multisensory stimulation) | 3 RCTs with positive short-term benefits (evidence grade B) |
| Behavioral management therapies (by professionals) | Largest RCTs with some benefits (grade B) |
| Staff training/education | Several positive studies of fair-to-good methodologic quality (evidence grade B) |
| Reality orientation therapy | Best RCT showed no benefit (evidence grade D) |
| Teaching caregivers behavioral management techniques | Overall inconsistent results (evidence grade D) |
| Simulated presence therapy | Only 1 RCT which was negative (evidence grade D) |
| Validation therapy | 1-year RCT with mixed results (evidence grade D) |
| Reminiscence therapy | A few small studies with mixed methodologies (evidence grade D) |
| Therapeutic activity programs (such as exercise, puzzle play) | Varied methods and inconsistent results (evidence grade D) |
| Physical environmental stimulation (such as altered visual stimuli, mirrors, signs) | Generally poor methodology and inconsistent results; best results with obscuring exits to decrease exit-seeking (evidence grade D) |
| * Evidence grades from A (strongest) to D (weakest) were assigned in a review by Livingston G, Johnston K, Katona C, et al. Systematic review of psychological approaches to the management of neuropsychiatric symptoms of dementia. Am J Psychiatry 2005;162:1996-2021 | |
| RCT: randomized controlled trial | |
| Source: Reference 22 | |
Duration of treatment
Limited evidence leaves psychiatrists largely on our own in regards to how long to continue pharmacotherapy with antipsychotics. Neuropsychiatric symptoms such as psychosis and agitation exhibit variable patterns. Symptoms may wax and wane for unclear reasons.
Given the tenuous nature of the risk-benefit profile for atypical antipsychotics in dementia, consider a gradual taper for persons with dementia who remain asymptomatic after 3 to 6 months of atypical antipsychotic treatment. Monitor them closely for symptom recurrence.5
Carefully consider the necessary duration of antipsychotic therapy in patients (such as Mrs. B) in whom you can identify possibly reversible precipitants of psychosis and aggression. Patients may have a delayed beneficial response to the correction of precipitating factors such as medical illness, physical discomfort, or medication side effects.
Mrs. B received risperidone, but evidence for efficacy and safety in dementia-related psychosis or agitation does not yet significantly distinguish among the atypical agents (except that data are limited for ziprasidone and clozapine). Usual starting and target doses are provided in Table 4.23
Table 4
Atypicals in dementia: Starting and target doses
| Drug | Starting dose | Target dose |
|---|---|---|
| Aripiprazole | 2.5 to 5 mg/d | 7.5 to 12.5 mg/d |
| Olanzapine | 2.5 to 5 mg/d | 5 to 10 mg/d |
| Quetiapine | 12.5 to 25 mg/d | 50 to 200 mg/d |
| Risperidone | 0.25 to 0.5 mg/d | 0.5 to 1.5 mg/d |
| Source: Reference 23 | ||
Related resources
- Jeste DV, Blazer D, Casey D, et al. ACNP White Paper: update on use of antipsychotic drugs in elderly persons with dementia. Neuropsychopharmacology 2008;33(5):957-70.
- American Association for Geriatric Psychiatry position statement: principles of care for persons with dementia resulting from Alzheimer disease. www.aagponline.org/prof/position_caredmnalz.asp.
Drug brand names
- Alprazolam • Xanax
- Aripiprazole • Abilify
- Atenolol • Tenormin
- Buspirone • Buspar
- Carbamazepine • Tegretol
- Ciprofloxacin • Ciloxan
- Citalopram • Celexa
- Clozapine • Clozaril
- Diphenhydramine • Benadryl
- Divalproex • Depakote
- Donepezil • Aricept
- Haloperidol • Haldol
- Hydrocodone/acetaminophen • Lortab, Vicodin
- Lorazepam • Ativan
- Memantine • Namenda
- Olanzapine • Zyprexa
- Oxazepam • Serax
- Oxybutynin • Ditropan
- Paroxetine • Paxil
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Rivastigmine • Exelon
- Sertraline • Zoloft
- Trazodone • Desyrel
- Ziprasidone • Geodon
Disclosure
Dr. Meeks receives research/grant support from the John A. Hartford Foundation, the Mental Health Research Foundation, NARSAD, and the U.S. Department of Health and Human Services’ Health Resources and Services Administration.
Dr. Jeste receives research/grant support from the John A. Hartford Foundation, the National Institute of Aging, and the National Institute of Mental Health. AstraZeneca, Bristol-Myers Squibb, Eli Lilly and Company, and Janssen, L.P. provide free medications for an NIMH-funded study for which Dr. Jeste is the principal investigator.
1. Jeste DV, Meeks TW, Kim DS, Zubenko GS. Research agenda for DSM-V: diagnostic categories and criteria for neuropsychiatry syndromes in dementia. J Geriatr Psychiatry Neurol 2006;19:160-71.
2. Yaffe K, Fox P, Newcomer R, et al. Patient and caregiver characteristics and nursing home placement in patients with dementia. JAMA 2002;287:2090-7.
3. Schneider LS, Dagerman K, Insel PS. Efficacy and adverse effects of atypical antipsychotics for dementia: meta-analysis of randomized, placebo-controlled trials. Am J Geriatr Psychiatry 2006;14:191-210.
4. Schneider LS, Dagerman KS, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo-controlled trials. JAMA 2005;294:1934-43.
5. Jeste DV, Blazer D, Casey D, et al. ACNP White Paper: update on the use of antipsychotic drugs in elderly persons with dementia. Neuropsychopharmacology 2008;33(5):957-70.
6. Nasrallah HA, White T, Nasrallah AT. Lower mortality in geriatric patients receiving risperidone and olanzapine versus haloperidol: preliminary analysis of retrospective data. Am J Geriatr Psychiatry 2004;12:437-9.
7. Schneeweiss S, Setoguchi S, Brookhart A, et al. Risk of death associated with the use of conventional versus atypical antipsychotic drugs among elderly patients. CMAJ 2007;176:627-32.
8. Wang PS, Schneeweiss S, Avorn J, et al. Risk of death in elderly users of conventional vs. atypical antipsychotic medications. N Engl J Med 2005;353:2335-41.
9. Trifirò G, Verhamme KM, Ziere G, et al. All-cause mortality associated with atypical and typical antipsychotics in demented outpatients. Pharmacoepidemiol Drug Saf 2007;16:538-44.
10. Gill SS, Bronskill SE, Normand SL, et al. Antipsychotic drug use and mortality in older adults with dementia. Ann Intern Med 2007;146:775-86.
11. Kales HC, Valenstein M, Kim HM, et al. Mortality risk in patients with dementia treated with antipsychotics versus other psychiatric medications. Am J Psychiatry 2007;164:1568-76.
12. Gill SS, Rochon PA, Herrmann N, et al. Atypical antipsychotic drugs and risk of ischaemic stroke: population based retrospective cohort study. BMJ 2005;330:445.-
13. Liperoti R, Gambassi G, Lapane KL, et al. Cerebrovascular events among elderly nursing home patients treated with conventional or atypical antipsychotics. J Clin Psychiatry 2005;66:1090-6.
14. Jeste DV, Lacro JP, Nguyen HA, et al. Lower incidence of tardive dyskinesia with risperidone versus haloperidol. J Am Geriatr Soc 1999;47:716-9.
15. Rainer M, Haushofer M, Pfolz H, et al. Quetiapine versus risperidone in elderly patients with behavioural and psychological symptoms of dementia: efficacy, safety and cognitive function. Eur Psychiatry 2007;22:395-403.
16. Holmes C, Wilkinson D, Dean C, et al. Risperidone and rivastigmine and agitated behaviour in severe Alzheimer’s disease: a randomised double blind placebo controlled study. Int J Geriatr Psychiatry 2007;22:380-1.
17. Pollock BG, Mulsant BH, Rosen J, et al. A double-blind comparison of citalopram and risperidone for the treatment of behavioral and psychotic symptoms associated with dementia. Am J Geriatr Psychiatry 2007;15:942-52.
18. Zhong KX, Tariot PN, Mintzer J, et al. Quetiapine to treat agitation in dementia: a randomized, double-blind, placebo-controlled study. Curr Alzheimer Res 2007;4:81-93.
19. Mintzer JE, Tune LE, Breder CD, et al. Aripiprazole for the treatment of psychoses in institutionalized patients with Alzheimer dementia: a multicenter, randomized, double-blind, placebo-controlled assessment of three fixed doses. Am J Geriatr Psychiatry 2007;15:918-31.
20. Schneider LS, Tariot PN, Dagerman KS, et al. Effectiveness of atypical antipsychotic drugs in patients with Alzheimer’s disease. N Engl J Med 2006;355:1525-38.
21. Pollock BG, Mulsant BH, Rosen J, et al. A double-blind comparison of citalopram and risperidone for the treatment of behavioral and psychotic symptoms associated with dementia. Am J Geriatr Psychiatry 2007;15:942-52.
22. Livingston G, Johnston K, Katona C, et al. Systematic review of psychological approaches to the management of neuropsychiatric symptoms of dementia. Am J Psychiatry 2005;162:1996-2021.
23. Jeste D, Meeks T. To prescribe or not to prescribe? Atypical antipsychotic drugs in patients with dementia. South Med J 2007;100:961-3.
24. Cohen-Mansfield J. Nonpharmacologic interventions for inappropriate behaviors in dementia: a review and critique. Am J Geriatr Psychiatry 2001;9:361-81.
Diagnosed 7 years ago with Alzheimer’s disease (AD), Mrs. B, age 82, resides in an assisted living facility whose staff is trained to care for older persons with dementia. Over the past 2 months she has shown an escalating pattern of psychosis and aggression, despite one-to-one attention and verbal reassurance.
At first Mrs. B’s psychosis was restricted to occasional rape accusations during assisted bathing and aggression manifested by banging her hand repetitively on furniture, causing skin tears. In the last week, she has been accusing staff and patients of stealing her belongings and has assaulted a staff member and another resident. When supervisors at the facility advise Mrs. B’s husband that she can no longer stay there, he takes her to a local emergency room, from which she is admitted involuntarily to a geriatric psychiatry inpatient unit.
For many patients and families, the most problematic aspects of dementia are neuropsychiatric symptoms—depression, sleep disturbance, psychosis, and aggression. Psychosis affects approximately 40% of persons with AD, whereas ≥80% of persons with dementia experience agitation at some point in the illness.1 These symptoms can lead to:
- caregiver morbidity
- poor patient quality of life
- early patient institutionalization.2
Although no drug has been FDA-approved for treating dementia’s neuropsychiatric symptoms, psychiatrists often use off-label psychotropics—especially antipsychotics—to ameliorate them. This practice is controversial because of public perception that antipsychotics are used in dementia patients to create “zombies” to lighten healthcare workers’ burden. Nonetheless, because dementia patients with psychosis and severe agitation/aggression can pose risks to themselves and those around them, efforts to treat these symptoms are warranted.
The FDA warned prescribers in 2003 of increased risk of “cerebrovascular adverse events including stroke” in dementia patients treated with risperidone vs placebo. Similar cerebrovascular warnings have been issued for olanzapine and aripiprazole. Although the absolute risk difference was generally 1% to 2% between antipsychotic- and placebo-treated patients, the relative risk was approximately 2 times higher with antipsychotics because the prevalence of these events is low in both groups.3
Perhaps more daunting, after a meta-analysis of 17 trials using atypical antipsychotics in elderly patients with dementia-related psychosis, the FDA in 2005 issued a black-box warning of increased mortality risk with atypical antipsychotics (relative risk 1.6 to 1.7) vs placebo. The mortality rate in antipsychotic-treated patients was about 4.5%, compared with about 2.6% in the placebo group. Although causes of death varied, most were cardiovascular (heart failure, sudden death) or infectious (pneumonia). This warning was applied to atypical antipsychotics as a class. As with cerebrovascular risks, the absolute mortality risk difference was 1% to 2%.4
The FDA’s “black-box” warnings about using atypical agents in patients with dementia add another layer of complexity to your treatment decisions (Box).3,4 The public is well served by evidence identifying risks associated with prescription medications, but the FDA data do little to help millions of families answer the question, “And so, what now?”
Recognizing that solid empiric evidence is lacking, we attempt to address this lingering question for clinicians, patients, and caregivers who must deal with these symptoms while science tries to provide a more definitive answer.
5-step evaluation
A 5-step initial evaluation of persons with dementia who present with psychosis and/or agitation/aggression includes establishing the frequency, severity, and cause of these symptoms as well as the effectiveness of past treatments and strategies (Algorithm).5
Because adverse drug effects are a potentially reversible cause of psychosis and agitation, review the patient’s drug list—including “as needed” medications—from records at a facility or from family report. Mrs. B’s record from the assisted living facility reveals she was receiving:
- atenolol, 25 mg/d
- aspirin, 81 mg/d
- extended-release oxybutynin, 10 mg at bedtime
- psyllium, one packet daily
- hydrocodone/acetaminophen, 5/500 mg every 4 hours as needed for pain
- lorazepam, 1 mg every 6 hours as needed for agitation
- diphenhydramine, 25 mg at bedtime
- paroxetine, 20 mg/d
- haloperidol, 5 mg at bedtime
- memantine, 10 mg twice a day.
Mrs. B’s medication list is revealing for reasons that, unfortunately, are not rare. She is receiving 3 anticholinergic medications—oxybutynin, diphenhydramine, and paroxetine—that may be worsening her mental status and behavior directly through CNS effects, possibly in combination with frequent benzodiazepine use.
Anticholinergics also can lead to behavior changes via peripheral side effects. Constipation and urinary retention may cause discomfort that an aphasic patient “acts out.” A patient may be experiencing pain related to these side effects and receiving opioid analgesics, which can worsen constipation and urinary retention. Uncontrolled pain related to musculoskeletal disease or neuropathy may merit treatment that will reduce behavioral disturbances.
Mrs. B also was being catheterized every 8 hours as needed for urinary retention. The invasive and unpleasant nature of urinary catheterization is likely to worsen behavior and increases the risk of one of the most common “asymptomatic” etiologies of behavioral symptoms in dementia—urinary tract infection (UTI).
Algorithm
5-step evaluation of dementia patients
with psychosis and/or agitation/aggression*
1. How dangerous is the situation?
|
| ↓ |
2. Establish a clear diagnosis/etiology for the symptoms
|
| ↓ |
3. Establish symptom severity and frequency, including:
|
| ↓ |
| 4. Explore past treatments/caregiver strategies used to address the symptoms and their success and/or problematic outcomes |
| ↓ |
| 5. Discuss with the patient/decision-maker what is and is not known about possible risks and benefits of pharmacologic and nonpharmacologic treatments for psychosis and agitation/aggression in dementia |
| Source: Reference 5 |
| * Agitation is defined as “inappropriate verbal, vocal, or motor activity that is not judged by an outside observer to be an obvious outcome of the needs or confusion of the individual”24 |
CASE CONTINUED: Persistent agitation
After evaluating Mrs. B, the psychiatrist limits her medications to atenolol, aspirin, psyllium, and memantine, and begins to taper lorazepam and paroxetine. Laboratory, radiologic, and physical examinations reveal UTI, fecal impaction, bladder distension, and mild hyponatremia. She is given a phosphosoda enema and ciprofloxacin, 250 mg/d for 5 days.
Despite one-to-one nursing care, frequent reorientation, and attempts to interest her in art therapy, Mrs. B remains agitated and postures to strike staff members and other patients. She denies pain or discomfort. Fearing that someone might be injured, the nurse pages the on-duty psychiatrist.
The nurse then calls Mr. B, who has durable power of attorney for his wife’s healthcare. When the nurse advises Mr. B that the psychiatrist has ordered risperidone, 0.5 mg, he immediately interjects that the psychiatrist at the assisted living facility told him haloperidol should be used for his wife’s symptoms because other antipsychotics can cause strokes and death.
Typical vs atypical antipsychotics
Mrs. B’s nurse may have to delay administering risperidone while she puts Mr. B in contact with the psychiatrist. In an emergent situation when well-trained staff have assessed for common reversible causes of agitation and tried reasonable nonpharmacologic means to calm the patient, few people would argue against using medication to preserve the safety of the patient and others. To avoid questions such as this during a crisis, obtain informed consent at admission from the patient or (more likely) the proxy decision-maker for medications you anticipate the patient might receive during hospitalization.
The larger question is whether typical antipsychotics are preferred for dementia-related psychosis and agitation/aggression because the FDA has not issued the same global black-box warning for this class. Astute clinicians realize that a lack of evidence of harm is not evidence of a lack of harm. In fact, since the black-box warnings for atypical antipsychotics in dementia emerged, several studies have examined whether the same risks exist for typical agents.
Evidence regarding risk of stroke and death with the use of typical and atypical antipsychotics in patients with dementia is summarized in Table 1.6-13 Most evidence, including numerous studies in the past year, comes from retrospective database analyses. Prospective head-to-head comparisons of atypical and typical antipsychotics in dementia are scarce, and future prospective comparisons would be unethical.
No evidence suggests that typical antipsychotics mitigate the risks of stroke or death in dementia compared with atypical agents. Moreover, typical agents are more likely than atypicals to cause movement-related side effects—especially tardive dyskinesia and parkinsonism—in older adults with dementia.14
Table 1
Typical antipsychotics: Safer than atypicals for older patients?
| Study | Population | Summarized results |
|---|---|---|
| Mortality | ||
| Nasrallah et al6 | VA patients age ≥65 taking haloperidol or an atypical antipsychotic (n=1,553) | Approximately 4 times higher rate of death in those receiving haloperidol compared with those receiving atypicals |
| Wang et al8 | Pennsylvania adults age ≥65 with prescription coverage taking antipsychotics (n=22,890) | Typicals had higher relative risk (RR) of death at all time points over 180 days (RR 1.27 to 1.56), both in persons with and without dementia; higher risk associated with increased typical doses |
| Gill et al10 | Canadians age >65 with dementia (n=27,259 matched pairs) | Mortality rate was higher for users of typical vs atypical antipsychotics (RR 1.26 to 1.55) |
| Kales et al11 | VA patients age >65 prescribed psychotropics after a dementia diagnosis (n=10,615) | Risk of death similar for atypical and typical antipsychotics |
| Schneeweiss et al7 | Cancer-free Canadians age ≥65 taking antipsychotics (n=37,241) | Higher mortality rates for those taking typical antipsychotics than those taking atypicals (RR 1.47); higher mortality associated with higher typical doses |
| Trifirò et al9 | Adults age >65 with dementia receiving antipsychotics in Italy (n=2,385) | Equivalent rates of mortality in those taking typical and atypical antipsychotics |
| Stroke | ||
| Gill et al12 | Canadians age ≥65 with dementia receiving antipsychotics (n=32,710) | Equivalent rates of ischemic stroke in those taking atypical and typical agents compared with those receiving atypicals |
| Liperoti et al13 | Nursing home residents with dementia hospitalized for stroke or TIA and matched controls (n=4,788) | Rates of cerebrovascular adverse events equivalent between users of atypical and typical antipsychotics |
| VA: Veterans Affairs; TIA: transient ischemic attack | ||
CASE CONTINUED: Moderate relief from risperidone
After the psychiatrist explains the data on atypical vs typical antipsychotics in dementia—and the lack of FDA-approved treatments—Mr. B consents to the use of risperidone. He believes his wife would have wanted to try a medication with a moderate chance of relieving her internal distress and preventing her from harming anyone.
Risperidone provides moderate relief of Mrs. B’s aggression and paranoia. The next day Mr. B visits the unit and asks to speak with the psychiatrist. Although he appreciates the staff’s caring attitude, he says, “There must be safer or better ways to deal with these symptoms than medications like risperidone. I just don’t want the guilt of causing my wife to have a stroke or pass away.” He also asks, “How long will she have to take this medication?”
Evidence for efficacy
In addition to discussing antipsychotics’ risk in dementia, we also need to highlight their efficacy and effectiveness. A recent meta-analysis of 15 randomized controlled trials of atypical antipsychotics for agitation and/or psychosis in dementia included studies with risperidone, olanzapine, aripiprazole, and quetiapine.3 Most study participants were institutionalized, female, and had AD.
Psychosis scores improved in pooled studies of risperidone, whereas global neuropsychiatric disturbance improved with risperidone and aripiprazole. Effects were more notable in:
- persons without psychosis
- those living in nursing homes
- patients with severe cognitive impairment.
Subsequent placebo-controlled trials of risperidone, quetiapine, and aripiprazole—most focusing on patients with AD—reveal that atypical and typical antipsychotics have modest efficacy in reducing aggression and psychosis.15-19 However, to some extent the National Institute of Mental Health Clinical Antipsychotic Trial of Intervention Effectiveness Study for Alzheimer’s Disease (CATIE-AD)—the largest nonindustry-funded study conducted to address this question—called this conclusion into question.20 Risperidone and olanzapine (but not quetiapine) were efficacious in that fewer patients taking them vs placebo dropped out because of lack of efficacy. Antipsychotics were not effective overall, however, because the primary outcome—all-cause discontinuation rate—was similar for all 3 drugs and placebo. This indicates that on average these medications’ side effect burden may offset their efficacy, though individual patients’ responses may vary.
Alternatives to antipsychotics
Mr. B also raised the issue of treatment alternatives, such as no treatment, other psychotropics (Table 2),5,21 and nonpharmacologic methods (Table 3).22
“No treatment” does not imply a lack of assessment or intervention. Always examine patients for iatrogenic, medical, psychosocial, or other precipitants of behavioral symptoms. No treatment may be viable in mild to moderate cases but is impractical for patients with severe psychosis or agitation. Untreated, these symptoms could compromise safety or leave the patient without housing options.
Although possibly underused because of time constraints, reimbursement issues, or lack of training, nonpharmacologic strategies to treat aggression and psychosis in dementia are appealing alternatives to antipsychotics. Little empiric evidence supports nonpharmacologic strategies, however.22
Treatment decisions need to consider patients’ and caregivers’ value systems. Proxy decision-makers should examine treatment decisions in terms of how they believe the patient would view the alternatives. Without a specific advance directive, however, even well-intentioned decision-makers are likely to “contaminate” decisions with their own values and interests.
After discussing with the decision-maker various treatments’ risks and benefits, it might be useful to ask, for example, “If Mrs. B could have foreseen her behaviors 10 years ago, what do you think she would have wanted us to do? Some people might have been mortified by the thought of attacking other people, whereas other people would not mind this as much as the fear of being ‘overmedicated.’ Which end of the spectrum do you think she would have leaned toward?”
When medical research does not offer clear answers for the “right” next clinical step, clinicians can:
- acknowledge our own limits and those of human knowledge
- engage the caregiver (or, when appropriate, the patient) in shared decision-making, recognizing that some people will appreciate the opportunity for “equal partnership” whereas others will want us to decide based on our best clinical judgment.
Table 2
Pharmacologic alternatives to antipsychotics: What the evidence says
| Treatment | Evidence/results |
|---|---|
| Selective serotonin reuptake inhibitors | 2 positive studies with citalopram (more effective than placebo for agitation in 1 trial and equivalent to risperidone for psychosis and agitation with greater tolerability in the other); 2 negative trials with sertraline |
| Other antidepressants | 1 study showed trazodone was equivalent to haloperidol for agitation, with greater tolerability; another found trazodone was no different from placebo; other agents have only case reports or open-label trials |
| Anticonvulsants | 3 trials showed divalproex was equivalent to placebo; 2 positive trials for carbamazepine, but tolerability problems in both; other agents tried only in case reports or open-label trials |
| Benzodiazepines/anxiolytics | 3 trials showed oxazepam, alprazolam, diphenhydramine, and buspirone were equivalent to haloperidol in effects on agitation, but none used a placebo control; trials had problematic methodologies and indicated cognitive worsening with some agents (especially diphenhydramine) |
| Cognitive enhancers | Some evidence of modest benefit in mostly post-hoc data analyses in trials designed to assess cognitive variables and often among participants with overall mild psychiatric symptoms; prospective studies of rivastigmine and donepezil specifically designed to assess neuropsychiatric symptoms have found no difference compared with placebo |
| Miscellaneous drugs | Failed trial of transdermal estrogen in men; small study showed propranolol (average dose 106 mg/d) more effective than placebo |
| Source: References 5,21 | |
Table 3
How well do psychosocial/behavioral therapies manage
psychosis/agitation in dementia?*
| Treatment | Evidence/results |
|---|---|
| Caregiver psychoeducation/support | Several positive RCTs (evidence grade A) |
| Music therapy | 6 RCTs, generally positive in the short term (evidence grade B) |
| Cognitive stimulation therapy | Three-quarters of RCTs showed some benefit (evidence grade B) |
| Snoezelen therapy (controlled multisensory stimulation) | 3 RCTs with positive short-term benefits (evidence grade B) |
| Behavioral management therapies (by professionals) | Largest RCTs with some benefits (grade B) |
| Staff training/education | Several positive studies of fair-to-good methodologic quality (evidence grade B) |
| Reality orientation therapy | Best RCT showed no benefit (evidence grade D) |
| Teaching caregivers behavioral management techniques | Overall inconsistent results (evidence grade D) |
| Simulated presence therapy | Only 1 RCT which was negative (evidence grade D) |
| Validation therapy | 1-year RCT with mixed results (evidence grade D) |
| Reminiscence therapy | A few small studies with mixed methodologies (evidence grade D) |
| Therapeutic activity programs (such as exercise, puzzle play) | Varied methods and inconsistent results (evidence grade D) |
| Physical environmental stimulation (such as altered visual stimuli, mirrors, signs) | Generally poor methodology and inconsistent results; best results with obscuring exits to decrease exit-seeking (evidence grade D) |
| * Evidence grades from A (strongest) to D (weakest) were assigned in a review by Livingston G, Johnston K, Katona C, et al. Systematic review of psychological approaches to the management of neuropsychiatric symptoms of dementia. Am J Psychiatry 2005;162:1996-2021 | |
| RCT: randomized controlled trial | |
| Source: Reference 22 | |
Duration of treatment
Limited evidence leaves psychiatrists largely on our own in regards to how long to continue pharmacotherapy with antipsychotics. Neuropsychiatric symptoms such as psychosis and agitation exhibit variable patterns. Symptoms may wax and wane for unclear reasons.
Given the tenuous nature of the risk-benefit profile for atypical antipsychotics in dementia, consider a gradual taper for persons with dementia who remain asymptomatic after 3 to 6 months of atypical antipsychotic treatment. Monitor them closely for symptom recurrence.5
Carefully consider the necessary duration of antipsychotic therapy in patients (such as Mrs. B) in whom you can identify possibly reversible precipitants of psychosis and aggression. Patients may have a delayed beneficial response to the correction of precipitating factors such as medical illness, physical discomfort, or medication side effects.
Mrs. B received risperidone, but evidence for efficacy and safety in dementia-related psychosis or agitation does not yet significantly distinguish among the atypical agents (except that data are limited for ziprasidone and clozapine). Usual starting and target doses are provided in Table 4.23
Table 4
Atypicals in dementia: Starting and target doses
| Drug | Starting dose | Target dose |
|---|---|---|
| Aripiprazole | 2.5 to 5 mg/d | 7.5 to 12.5 mg/d |
| Olanzapine | 2.5 to 5 mg/d | 5 to 10 mg/d |
| Quetiapine | 12.5 to 25 mg/d | 50 to 200 mg/d |
| Risperidone | 0.25 to 0.5 mg/d | 0.5 to 1.5 mg/d |
| Source: Reference 23 | ||
Related resources
- Jeste DV, Blazer D, Casey D, et al. ACNP White Paper: update on use of antipsychotic drugs in elderly persons with dementia. Neuropsychopharmacology 2008;33(5):957-70.
- American Association for Geriatric Psychiatry position statement: principles of care for persons with dementia resulting from Alzheimer disease. www.aagponline.org/prof/position_caredmnalz.asp.
Drug brand names
- Alprazolam • Xanax
- Aripiprazole • Abilify
- Atenolol • Tenormin
- Buspirone • Buspar
- Carbamazepine • Tegretol
- Ciprofloxacin • Ciloxan
- Citalopram • Celexa
- Clozapine • Clozaril
- Diphenhydramine • Benadryl
- Divalproex • Depakote
- Donepezil • Aricept
- Haloperidol • Haldol
- Hydrocodone/acetaminophen • Lortab, Vicodin
- Lorazepam • Ativan
- Memantine • Namenda
- Olanzapine • Zyprexa
- Oxazepam • Serax
- Oxybutynin • Ditropan
- Paroxetine • Paxil
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Rivastigmine • Exelon
- Sertraline • Zoloft
- Trazodone • Desyrel
- Ziprasidone • Geodon
Disclosure
Dr. Meeks receives research/grant support from the John A. Hartford Foundation, the Mental Health Research Foundation, NARSAD, and the U.S. Department of Health and Human Services’ Health Resources and Services Administration.
Dr. Jeste receives research/grant support from the John A. Hartford Foundation, the National Institute of Aging, and the National Institute of Mental Health. AstraZeneca, Bristol-Myers Squibb, Eli Lilly and Company, and Janssen, L.P. provide free medications for an NIMH-funded study for which Dr. Jeste is the principal investigator.
Diagnosed 7 years ago with Alzheimer’s disease (AD), Mrs. B, age 82, resides in an assisted living facility whose staff is trained to care for older persons with dementia. Over the past 2 months she has shown an escalating pattern of psychosis and aggression, despite one-to-one attention and verbal reassurance.
At first Mrs. B’s psychosis was restricted to occasional rape accusations during assisted bathing and aggression manifested by banging her hand repetitively on furniture, causing skin tears. In the last week, she has been accusing staff and patients of stealing her belongings and has assaulted a staff member and another resident. When supervisors at the facility advise Mrs. B’s husband that she can no longer stay there, he takes her to a local emergency room, from which she is admitted involuntarily to a geriatric psychiatry inpatient unit.
For many patients and families, the most problematic aspects of dementia are neuropsychiatric symptoms—depression, sleep disturbance, psychosis, and aggression. Psychosis affects approximately 40% of persons with AD, whereas ≥80% of persons with dementia experience agitation at some point in the illness.1 These symptoms can lead to:
- caregiver morbidity
- poor patient quality of life
- early patient institutionalization.2
Although no drug has been FDA-approved for treating dementia’s neuropsychiatric symptoms, psychiatrists often use off-label psychotropics—especially antipsychotics—to ameliorate them. This practice is controversial because of public perception that antipsychotics are used in dementia patients to create “zombies” to lighten healthcare workers’ burden. Nonetheless, because dementia patients with psychosis and severe agitation/aggression can pose risks to themselves and those around them, efforts to treat these symptoms are warranted.
The FDA warned prescribers in 2003 of increased risk of “cerebrovascular adverse events including stroke” in dementia patients treated with risperidone vs placebo. Similar cerebrovascular warnings have been issued for olanzapine and aripiprazole. Although the absolute risk difference was generally 1% to 2% between antipsychotic- and placebo-treated patients, the relative risk was approximately 2 times higher with antipsychotics because the prevalence of these events is low in both groups.3
Perhaps more daunting, after a meta-analysis of 17 trials using atypical antipsychotics in elderly patients with dementia-related psychosis, the FDA in 2005 issued a black-box warning of increased mortality risk with atypical antipsychotics (relative risk 1.6 to 1.7) vs placebo. The mortality rate in antipsychotic-treated patients was about 4.5%, compared with about 2.6% in the placebo group. Although causes of death varied, most were cardiovascular (heart failure, sudden death) or infectious (pneumonia). This warning was applied to atypical antipsychotics as a class. As with cerebrovascular risks, the absolute mortality risk difference was 1% to 2%.4
The FDA’s “black-box” warnings about using atypical agents in patients with dementia add another layer of complexity to your treatment decisions (Box).3,4 The public is well served by evidence identifying risks associated with prescription medications, but the FDA data do little to help millions of families answer the question, “And so, what now?”
Recognizing that solid empiric evidence is lacking, we attempt to address this lingering question for clinicians, patients, and caregivers who must deal with these symptoms while science tries to provide a more definitive answer.
5-step evaluation
A 5-step initial evaluation of persons with dementia who present with psychosis and/or agitation/aggression includes establishing the frequency, severity, and cause of these symptoms as well as the effectiveness of past treatments and strategies (Algorithm).5
Because adverse drug effects are a potentially reversible cause of psychosis and agitation, review the patient’s drug list—including “as needed” medications—from records at a facility or from family report. Mrs. B’s record from the assisted living facility reveals she was receiving:
- atenolol, 25 mg/d
- aspirin, 81 mg/d
- extended-release oxybutynin, 10 mg at bedtime
- psyllium, one packet daily
- hydrocodone/acetaminophen, 5/500 mg every 4 hours as needed for pain
- lorazepam, 1 mg every 6 hours as needed for agitation
- diphenhydramine, 25 mg at bedtime
- paroxetine, 20 mg/d
- haloperidol, 5 mg at bedtime
- memantine, 10 mg twice a day.
Mrs. B’s medication list is revealing for reasons that, unfortunately, are not rare. She is receiving 3 anticholinergic medications—oxybutynin, diphenhydramine, and paroxetine—that may be worsening her mental status and behavior directly through CNS effects, possibly in combination with frequent benzodiazepine use.
Anticholinergics also can lead to behavior changes via peripheral side effects. Constipation and urinary retention may cause discomfort that an aphasic patient “acts out.” A patient may be experiencing pain related to these side effects and receiving opioid analgesics, which can worsen constipation and urinary retention. Uncontrolled pain related to musculoskeletal disease or neuropathy may merit treatment that will reduce behavioral disturbances.
Mrs. B also was being catheterized every 8 hours as needed for urinary retention. The invasive and unpleasant nature of urinary catheterization is likely to worsen behavior and increases the risk of one of the most common “asymptomatic” etiologies of behavioral symptoms in dementia—urinary tract infection (UTI).
Algorithm
5-step evaluation of dementia patients
with psychosis and/or agitation/aggression*
1. How dangerous is the situation?
|
| ↓ |
2. Establish a clear diagnosis/etiology for the symptoms
|
| ↓ |
3. Establish symptom severity and frequency, including:
|
| ↓ |
| 4. Explore past treatments/caregiver strategies used to address the symptoms and their success and/or problematic outcomes |
| ↓ |
| 5. Discuss with the patient/decision-maker what is and is not known about possible risks and benefits of pharmacologic and nonpharmacologic treatments for psychosis and agitation/aggression in dementia |
| Source: Reference 5 |
| * Agitation is defined as “inappropriate verbal, vocal, or motor activity that is not judged by an outside observer to be an obvious outcome of the needs or confusion of the individual”24 |
CASE CONTINUED: Persistent agitation
After evaluating Mrs. B, the psychiatrist limits her medications to atenolol, aspirin, psyllium, and memantine, and begins to taper lorazepam and paroxetine. Laboratory, radiologic, and physical examinations reveal UTI, fecal impaction, bladder distension, and mild hyponatremia. She is given a phosphosoda enema and ciprofloxacin, 250 mg/d for 5 days.
Despite one-to-one nursing care, frequent reorientation, and attempts to interest her in art therapy, Mrs. B remains agitated and postures to strike staff members and other patients. She denies pain or discomfort. Fearing that someone might be injured, the nurse pages the on-duty psychiatrist.
The nurse then calls Mr. B, who has durable power of attorney for his wife’s healthcare. When the nurse advises Mr. B that the psychiatrist has ordered risperidone, 0.5 mg, he immediately interjects that the psychiatrist at the assisted living facility told him haloperidol should be used for his wife’s symptoms because other antipsychotics can cause strokes and death.
Typical vs atypical antipsychotics
Mrs. B’s nurse may have to delay administering risperidone while she puts Mr. B in contact with the psychiatrist. In an emergent situation when well-trained staff have assessed for common reversible causes of agitation and tried reasonable nonpharmacologic means to calm the patient, few people would argue against using medication to preserve the safety of the patient and others. To avoid questions such as this during a crisis, obtain informed consent at admission from the patient or (more likely) the proxy decision-maker for medications you anticipate the patient might receive during hospitalization.
The larger question is whether typical antipsychotics are preferred for dementia-related psychosis and agitation/aggression because the FDA has not issued the same global black-box warning for this class. Astute clinicians realize that a lack of evidence of harm is not evidence of a lack of harm. In fact, since the black-box warnings for atypical antipsychotics in dementia emerged, several studies have examined whether the same risks exist for typical agents.
Evidence regarding risk of stroke and death with the use of typical and atypical antipsychotics in patients with dementia is summarized in Table 1.6-13 Most evidence, including numerous studies in the past year, comes from retrospective database analyses. Prospective head-to-head comparisons of atypical and typical antipsychotics in dementia are scarce, and future prospective comparisons would be unethical.
No evidence suggests that typical antipsychotics mitigate the risks of stroke or death in dementia compared with atypical agents. Moreover, typical agents are more likely than atypicals to cause movement-related side effects—especially tardive dyskinesia and parkinsonism—in older adults with dementia.14
Table 1
Typical antipsychotics: Safer than atypicals for older patients?
| Study | Population | Summarized results |
|---|---|---|
| Mortality | ||
| Nasrallah et al6 | VA patients age ≥65 taking haloperidol or an atypical antipsychotic (n=1,553) | Approximately 4 times higher rate of death in those receiving haloperidol compared with those receiving atypicals |
| Wang et al8 | Pennsylvania adults age ≥65 with prescription coverage taking antipsychotics (n=22,890) | Typicals had higher relative risk (RR) of death at all time points over 180 days (RR 1.27 to 1.56), both in persons with and without dementia; higher risk associated with increased typical doses |
| Gill et al10 | Canadians age >65 with dementia (n=27,259 matched pairs) | Mortality rate was higher for users of typical vs atypical antipsychotics (RR 1.26 to 1.55) |
| Kales et al11 | VA patients age >65 prescribed psychotropics after a dementia diagnosis (n=10,615) | Risk of death similar for atypical and typical antipsychotics |
| Schneeweiss et al7 | Cancer-free Canadians age ≥65 taking antipsychotics (n=37,241) | Higher mortality rates for those taking typical antipsychotics than those taking atypicals (RR 1.47); higher mortality associated with higher typical doses |
| Trifirò et al9 | Adults age >65 with dementia receiving antipsychotics in Italy (n=2,385) | Equivalent rates of mortality in those taking typical and atypical antipsychotics |
| Stroke | ||
| Gill et al12 | Canadians age ≥65 with dementia receiving antipsychotics (n=32,710) | Equivalent rates of ischemic stroke in those taking atypical and typical agents compared with those receiving atypicals |
| Liperoti et al13 | Nursing home residents with dementia hospitalized for stroke or TIA and matched controls (n=4,788) | Rates of cerebrovascular adverse events equivalent between users of atypical and typical antipsychotics |
| VA: Veterans Affairs; TIA: transient ischemic attack | ||
CASE CONTINUED: Moderate relief from risperidone
After the psychiatrist explains the data on atypical vs typical antipsychotics in dementia—and the lack of FDA-approved treatments—Mr. B consents to the use of risperidone. He believes his wife would have wanted to try a medication with a moderate chance of relieving her internal distress and preventing her from harming anyone.
Risperidone provides moderate relief of Mrs. B’s aggression and paranoia. The next day Mr. B visits the unit and asks to speak with the psychiatrist. Although he appreciates the staff’s caring attitude, he says, “There must be safer or better ways to deal with these symptoms than medications like risperidone. I just don’t want the guilt of causing my wife to have a stroke or pass away.” He also asks, “How long will she have to take this medication?”
Evidence for efficacy
In addition to discussing antipsychotics’ risk in dementia, we also need to highlight their efficacy and effectiveness. A recent meta-analysis of 15 randomized controlled trials of atypical antipsychotics for agitation and/or psychosis in dementia included studies with risperidone, olanzapine, aripiprazole, and quetiapine.3 Most study participants were institutionalized, female, and had AD.
Psychosis scores improved in pooled studies of risperidone, whereas global neuropsychiatric disturbance improved with risperidone and aripiprazole. Effects were more notable in:
- persons without psychosis
- those living in nursing homes
- patients with severe cognitive impairment.
Subsequent placebo-controlled trials of risperidone, quetiapine, and aripiprazole—most focusing on patients with AD—reveal that atypical and typical antipsychotics have modest efficacy in reducing aggression and psychosis.15-19 However, to some extent the National Institute of Mental Health Clinical Antipsychotic Trial of Intervention Effectiveness Study for Alzheimer’s Disease (CATIE-AD)—the largest nonindustry-funded study conducted to address this question—called this conclusion into question.20 Risperidone and olanzapine (but not quetiapine) were efficacious in that fewer patients taking them vs placebo dropped out because of lack of efficacy. Antipsychotics were not effective overall, however, because the primary outcome—all-cause discontinuation rate—was similar for all 3 drugs and placebo. This indicates that on average these medications’ side effect burden may offset their efficacy, though individual patients’ responses may vary.
Alternatives to antipsychotics
Mr. B also raised the issue of treatment alternatives, such as no treatment, other psychotropics (Table 2),5,21 and nonpharmacologic methods (Table 3).22
“No treatment” does not imply a lack of assessment or intervention. Always examine patients for iatrogenic, medical, psychosocial, or other precipitants of behavioral symptoms. No treatment may be viable in mild to moderate cases but is impractical for patients with severe psychosis or agitation. Untreated, these symptoms could compromise safety or leave the patient without housing options.
Although possibly underused because of time constraints, reimbursement issues, or lack of training, nonpharmacologic strategies to treat aggression and psychosis in dementia are appealing alternatives to antipsychotics. Little empiric evidence supports nonpharmacologic strategies, however.22
Treatment decisions need to consider patients’ and caregivers’ value systems. Proxy decision-makers should examine treatment decisions in terms of how they believe the patient would view the alternatives. Without a specific advance directive, however, even well-intentioned decision-makers are likely to “contaminate” decisions with their own values and interests.
After discussing with the decision-maker various treatments’ risks and benefits, it might be useful to ask, for example, “If Mrs. B could have foreseen her behaviors 10 years ago, what do you think she would have wanted us to do? Some people might have been mortified by the thought of attacking other people, whereas other people would not mind this as much as the fear of being ‘overmedicated.’ Which end of the spectrum do you think she would have leaned toward?”
When medical research does not offer clear answers for the “right” next clinical step, clinicians can:
- acknowledge our own limits and those of human knowledge
- engage the caregiver (or, when appropriate, the patient) in shared decision-making, recognizing that some people will appreciate the opportunity for “equal partnership” whereas others will want us to decide based on our best clinical judgment.
Table 2
Pharmacologic alternatives to antipsychotics: What the evidence says
| Treatment | Evidence/results |
|---|---|
| Selective serotonin reuptake inhibitors | 2 positive studies with citalopram (more effective than placebo for agitation in 1 trial and equivalent to risperidone for psychosis and agitation with greater tolerability in the other); 2 negative trials with sertraline |
| Other antidepressants | 1 study showed trazodone was equivalent to haloperidol for agitation, with greater tolerability; another found trazodone was no different from placebo; other agents have only case reports or open-label trials |
| Anticonvulsants | 3 trials showed divalproex was equivalent to placebo; 2 positive trials for carbamazepine, but tolerability problems in both; other agents tried only in case reports or open-label trials |
| Benzodiazepines/anxiolytics | 3 trials showed oxazepam, alprazolam, diphenhydramine, and buspirone were equivalent to haloperidol in effects on agitation, but none used a placebo control; trials had problematic methodologies and indicated cognitive worsening with some agents (especially diphenhydramine) |
| Cognitive enhancers | Some evidence of modest benefit in mostly post-hoc data analyses in trials designed to assess cognitive variables and often among participants with overall mild psychiatric symptoms; prospective studies of rivastigmine and donepezil specifically designed to assess neuropsychiatric symptoms have found no difference compared with placebo |
| Miscellaneous drugs | Failed trial of transdermal estrogen in men; small study showed propranolol (average dose 106 mg/d) more effective than placebo |
| Source: References 5,21 | |
Table 3
How well do psychosocial/behavioral therapies manage
psychosis/agitation in dementia?*
| Treatment | Evidence/results |
|---|---|
| Caregiver psychoeducation/support | Several positive RCTs (evidence grade A) |
| Music therapy | 6 RCTs, generally positive in the short term (evidence grade B) |
| Cognitive stimulation therapy | Three-quarters of RCTs showed some benefit (evidence grade B) |
| Snoezelen therapy (controlled multisensory stimulation) | 3 RCTs with positive short-term benefits (evidence grade B) |
| Behavioral management therapies (by professionals) | Largest RCTs with some benefits (grade B) |
| Staff training/education | Several positive studies of fair-to-good methodologic quality (evidence grade B) |
| Reality orientation therapy | Best RCT showed no benefit (evidence grade D) |
| Teaching caregivers behavioral management techniques | Overall inconsistent results (evidence grade D) |
| Simulated presence therapy | Only 1 RCT which was negative (evidence grade D) |
| Validation therapy | 1-year RCT with mixed results (evidence grade D) |
| Reminiscence therapy | A few small studies with mixed methodologies (evidence grade D) |
| Therapeutic activity programs (such as exercise, puzzle play) | Varied methods and inconsistent results (evidence grade D) |
| Physical environmental stimulation (such as altered visual stimuli, mirrors, signs) | Generally poor methodology and inconsistent results; best results with obscuring exits to decrease exit-seeking (evidence grade D) |
| * Evidence grades from A (strongest) to D (weakest) were assigned in a review by Livingston G, Johnston K, Katona C, et al. Systematic review of psychological approaches to the management of neuropsychiatric symptoms of dementia. Am J Psychiatry 2005;162:1996-2021 | |
| RCT: randomized controlled trial | |
| Source: Reference 22 | |
Duration of treatment
Limited evidence leaves psychiatrists largely on our own in regards to how long to continue pharmacotherapy with antipsychotics. Neuropsychiatric symptoms such as psychosis and agitation exhibit variable patterns. Symptoms may wax and wane for unclear reasons.
Given the tenuous nature of the risk-benefit profile for atypical antipsychotics in dementia, consider a gradual taper for persons with dementia who remain asymptomatic after 3 to 6 months of atypical antipsychotic treatment. Monitor them closely for symptom recurrence.5
Carefully consider the necessary duration of antipsychotic therapy in patients (such as Mrs. B) in whom you can identify possibly reversible precipitants of psychosis and aggression. Patients may have a delayed beneficial response to the correction of precipitating factors such as medical illness, physical discomfort, or medication side effects.
Mrs. B received risperidone, but evidence for efficacy and safety in dementia-related psychosis or agitation does not yet significantly distinguish among the atypical agents (except that data are limited for ziprasidone and clozapine). Usual starting and target doses are provided in Table 4.23
Table 4
Atypicals in dementia: Starting and target doses
| Drug | Starting dose | Target dose |
|---|---|---|
| Aripiprazole | 2.5 to 5 mg/d | 7.5 to 12.5 mg/d |
| Olanzapine | 2.5 to 5 mg/d | 5 to 10 mg/d |
| Quetiapine | 12.5 to 25 mg/d | 50 to 200 mg/d |
| Risperidone | 0.25 to 0.5 mg/d | 0.5 to 1.5 mg/d |
| Source: Reference 23 | ||
Related resources
- Jeste DV, Blazer D, Casey D, et al. ACNP White Paper: update on use of antipsychotic drugs in elderly persons with dementia. Neuropsychopharmacology 2008;33(5):957-70.
- American Association for Geriatric Psychiatry position statement: principles of care for persons with dementia resulting from Alzheimer disease. www.aagponline.org/prof/position_caredmnalz.asp.
Drug brand names
- Alprazolam • Xanax
- Aripiprazole • Abilify
- Atenolol • Tenormin
- Buspirone • Buspar
- Carbamazepine • Tegretol
- Ciprofloxacin • Ciloxan
- Citalopram • Celexa
- Clozapine • Clozaril
- Diphenhydramine • Benadryl
- Divalproex • Depakote
- Donepezil • Aricept
- Haloperidol • Haldol
- Hydrocodone/acetaminophen • Lortab, Vicodin
- Lorazepam • Ativan
- Memantine • Namenda
- Olanzapine • Zyprexa
- Oxazepam • Serax
- Oxybutynin • Ditropan
- Paroxetine • Paxil
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Rivastigmine • Exelon
- Sertraline • Zoloft
- Trazodone • Desyrel
- Ziprasidone • Geodon
Disclosure
Dr. Meeks receives research/grant support from the John A. Hartford Foundation, the Mental Health Research Foundation, NARSAD, and the U.S. Department of Health and Human Services’ Health Resources and Services Administration.
Dr. Jeste receives research/grant support from the John A. Hartford Foundation, the National Institute of Aging, and the National Institute of Mental Health. AstraZeneca, Bristol-Myers Squibb, Eli Lilly and Company, and Janssen, L.P. provide free medications for an NIMH-funded study for which Dr. Jeste is the principal investigator.
1. Jeste DV, Meeks TW, Kim DS, Zubenko GS. Research agenda for DSM-V: diagnostic categories and criteria for neuropsychiatry syndromes in dementia. J Geriatr Psychiatry Neurol 2006;19:160-71.
2. Yaffe K, Fox P, Newcomer R, et al. Patient and caregiver characteristics and nursing home placement in patients with dementia. JAMA 2002;287:2090-7.
3. Schneider LS, Dagerman K, Insel PS. Efficacy and adverse effects of atypical antipsychotics for dementia: meta-analysis of randomized, placebo-controlled trials. Am J Geriatr Psychiatry 2006;14:191-210.
4. Schneider LS, Dagerman KS, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo-controlled trials. JAMA 2005;294:1934-43.
5. Jeste DV, Blazer D, Casey D, et al. ACNP White Paper: update on the use of antipsychotic drugs in elderly persons with dementia. Neuropsychopharmacology 2008;33(5):957-70.
6. Nasrallah HA, White T, Nasrallah AT. Lower mortality in geriatric patients receiving risperidone and olanzapine versus haloperidol: preliminary analysis of retrospective data. Am J Geriatr Psychiatry 2004;12:437-9.
7. Schneeweiss S, Setoguchi S, Brookhart A, et al. Risk of death associated with the use of conventional versus atypical antipsychotic drugs among elderly patients. CMAJ 2007;176:627-32.
8. Wang PS, Schneeweiss S, Avorn J, et al. Risk of death in elderly users of conventional vs. atypical antipsychotic medications. N Engl J Med 2005;353:2335-41.
9. Trifirò G, Verhamme KM, Ziere G, et al. All-cause mortality associated with atypical and typical antipsychotics in demented outpatients. Pharmacoepidemiol Drug Saf 2007;16:538-44.
10. Gill SS, Bronskill SE, Normand SL, et al. Antipsychotic drug use and mortality in older adults with dementia. Ann Intern Med 2007;146:775-86.
11. Kales HC, Valenstein M, Kim HM, et al. Mortality risk in patients with dementia treated with antipsychotics versus other psychiatric medications. Am J Psychiatry 2007;164:1568-76.
12. Gill SS, Rochon PA, Herrmann N, et al. Atypical antipsychotic drugs and risk of ischaemic stroke: population based retrospective cohort study. BMJ 2005;330:445.-
13. Liperoti R, Gambassi G, Lapane KL, et al. Cerebrovascular events among elderly nursing home patients treated with conventional or atypical antipsychotics. J Clin Psychiatry 2005;66:1090-6.
14. Jeste DV, Lacro JP, Nguyen HA, et al. Lower incidence of tardive dyskinesia with risperidone versus haloperidol. J Am Geriatr Soc 1999;47:716-9.
15. Rainer M, Haushofer M, Pfolz H, et al. Quetiapine versus risperidone in elderly patients with behavioural and psychological symptoms of dementia: efficacy, safety and cognitive function. Eur Psychiatry 2007;22:395-403.
16. Holmes C, Wilkinson D, Dean C, et al. Risperidone and rivastigmine and agitated behaviour in severe Alzheimer’s disease: a randomised double blind placebo controlled study. Int J Geriatr Psychiatry 2007;22:380-1.
17. Pollock BG, Mulsant BH, Rosen J, et al. A double-blind comparison of citalopram and risperidone for the treatment of behavioral and psychotic symptoms associated with dementia. Am J Geriatr Psychiatry 2007;15:942-52.
18. Zhong KX, Tariot PN, Mintzer J, et al. Quetiapine to treat agitation in dementia: a randomized, double-blind, placebo-controlled study. Curr Alzheimer Res 2007;4:81-93.
19. Mintzer JE, Tune LE, Breder CD, et al. Aripiprazole for the treatment of psychoses in institutionalized patients with Alzheimer dementia: a multicenter, randomized, double-blind, placebo-controlled assessment of three fixed doses. Am J Geriatr Psychiatry 2007;15:918-31.
20. Schneider LS, Tariot PN, Dagerman KS, et al. Effectiveness of atypical antipsychotic drugs in patients with Alzheimer’s disease. N Engl J Med 2006;355:1525-38.
21. Pollock BG, Mulsant BH, Rosen J, et al. A double-blind comparison of citalopram and risperidone for the treatment of behavioral and psychotic symptoms associated with dementia. Am J Geriatr Psychiatry 2007;15:942-52.
22. Livingston G, Johnston K, Katona C, et al. Systematic review of psychological approaches to the management of neuropsychiatric symptoms of dementia. Am J Psychiatry 2005;162:1996-2021.
23. Jeste D, Meeks T. To prescribe or not to prescribe? Atypical antipsychotic drugs in patients with dementia. South Med J 2007;100:961-3.
24. Cohen-Mansfield J. Nonpharmacologic interventions for inappropriate behaviors in dementia: a review and critique. Am J Geriatr Psychiatry 2001;9:361-81.
1. Jeste DV, Meeks TW, Kim DS, Zubenko GS. Research agenda for DSM-V: diagnostic categories and criteria for neuropsychiatry syndromes in dementia. J Geriatr Psychiatry Neurol 2006;19:160-71.
2. Yaffe K, Fox P, Newcomer R, et al. Patient and caregiver characteristics and nursing home placement in patients with dementia. JAMA 2002;287:2090-7.
3. Schneider LS, Dagerman K, Insel PS. Efficacy and adverse effects of atypical antipsychotics for dementia: meta-analysis of randomized, placebo-controlled trials. Am J Geriatr Psychiatry 2006;14:191-210.
4. Schneider LS, Dagerman KS, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo-controlled trials. JAMA 2005;294:1934-43.
5. Jeste DV, Blazer D, Casey D, et al. ACNP White Paper: update on the use of antipsychotic drugs in elderly persons with dementia. Neuropsychopharmacology 2008;33(5):957-70.
6. Nasrallah HA, White T, Nasrallah AT. Lower mortality in geriatric patients receiving risperidone and olanzapine versus haloperidol: preliminary analysis of retrospective data. Am J Geriatr Psychiatry 2004;12:437-9.
7. Schneeweiss S, Setoguchi S, Brookhart A, et al. Risk of death associated with the use of conventional versus atypical antipsychotic drugs among elderly patients. CMAJ 2007;176:627-32.
8. Wang PS, Schneeweiss S, Avorn J, et al. Risk of death in elderly users of conventional vs. atypical antipsychotic medications. N Engl J Med 2005;353:2335-41.
9. Trifirò G, Verhamme KM, Ziere G, et al. All-cause mortality associated with atypical and typical antipsychotics in demented outpatients. Pharmacoepidemiol Drug Saf 2007;16:538-44.
10. Gill SS, Bronskill SE, Normand SL, et al. Antipsychotic drug use and mortality in older adults with dementia. Ann Intern Med 2007;146:775-86.
11. Kales HC, Valenstein M, Kim HM, et al. Mortality risk in patients with dementia treated with antipsychotics versus other psychiatric medications. Am J Psychiatry 2007;164:1568-76.
12. Gill SS, Rochon PA, Herrmann N, et al. Atypical antipsychotic drugs and risk of ischaemic stroke: population based retrospective cohort study. BMJ 2005;330:445.-
13. Liperoti R, Gambassi G, Lapane KL, et al. Cerebrovascular events among elderly nursing home patients treated with conventional or atypical antipsychotics. J Clin Psychiatry 2005;66:1090-6.
14. Jeste DV, Lacro JP, Nguyen HA, et al. Lower incidence of tardive dyskinesia with risperidone versus haloperidol. J Am Geriatr Soc 1999;47:716-9.
15. Rainer M, Haushofer M, Pfolz H, et al. Quetiapine versus risperidone in elderly patients with behavioural and psychological symptoms of dementia: efficacy, safety and cognitive function. Eur Psychiatry 2007;22:395-403.
16. Holmes C, Wilkinson D, Dean C, et al. Risperidone and rivastigmine and agitated behaviour in severe Alzheimer’s disease: a randomised double blind placebo controlled study. Int J Geriatr Psychiatry 2007;22:380-1.
17. Pollock BG, Mulsant BH, Rosen J, et al. A double-blind comparison of citalopram and risperidone for the treatment of behavioral and psychotic symptoms associated with dementia. Am J Geriatr Psychiatry 2007;15:942-52.
18. Zhong KX, Tariot PN, Mintzer J, et al. Quetiapine to treat agitation in dementia: a randomized, double-blind, placebo-controlled study. Curr Alzheimer Res 2007;4:81-93.
19. Mintzer JE, Tune LE, Breder CD, et al. Aripiprazole for the treatment of psychoses in institutionalized patients with Alzheimer dementia: a multicenter, randomized, double-blind, placebo-controlled assessment of three fixed doses. Am J Geriatr Psychiatry 2007;15:918-31.
20. Schneider LS, Tariot PN, Dagerman KS, et al. Effectiveness of atypical antipsychotic drugs in patients with Alzheimer’s disease. N Engl J Med 2006;355:1525-38.
21. Pollock BG, Mulsant BH, Rosen J, et al. A double-blind comparison of citalopram and risperidone for the treatment of behavioral and psychotic symptoms associated with dementia. Am J Geriatr Psychiatry 2007;15:942-52.
22. Livingston G, Johnston K, Katona C, et al. Systematic review of psychological approaches to the management of neuropsychiatric symptoms of dementia. Am J Psychiatry 2005;162:1996-2021.
23. Jeste D, Meeks T. To prescribe or not to prescribe? Atypical antipsychotic drugs in patients with dementia. South Med J 2007;100:961-3.
24. Cohen-Mansfield J. Nonpharmacologic interventions for inappropriate behaviors in dementia: a review and critique. Am J Geriatr Psychiatry 2001;9:361-81.
Every patient, every visit: Routine tests yield clinically useful data
General psychiatry practitioners such as myself traditionally have relied on writing case reports to describe our clinical experience. One obstacle to getting cases published is that many research journals require submitted articles to include large samples and rating scales as measures of change in the conditions of patients being studied.
I have published articles about my clinical experiences using patient data collected with the Clinical Global Impressions (CGI) scale and other standardized tests. Research instruments such as the CGI can gather empiric data and are easy to use in clinical practice.1
This article describes how routine standardized testing provides useful data for research and improves diagnostic accuracy—and patient outcomes—even before I meet my patients for the first time.
Why use standardized tests?
Benefits. All my new patients undergo screening before their first face-to-face meeting with a psychiatrist. This registration visit takes about 2 hours, after which they are scheduled for an appointment based on clinical urgency. We charge no fee for the screening visit; the benefits of gathering a comprehensive database before the clinical evaluation outweigh the cost of the tests, software, and staff time.
Along with completing insurance and biographical paperwork, patients perform self-administered psychosocial and medical histories and a battery of standardized tests. This information allows me to focus on interpersonal issues—rather than fact-finding—during the first interview. It also ensures a comprehensive patient history.
Bipolar disorder is difficult to diagnose in patients presenting with depressive symptoms. In a 5-year chart review,2 we used data from Structured Clinical Interview for DSM-IV (Mini-SCID) screening tests to assess this tool’s usefulness in diagnosing depressed patients. Data also included each patient’s demographic information, initial clinical diagnosis, current clinical diagnosis, and Symptom Checklist-90 (SCL-90) results.
Among 796 patients who took the Mini-SCID at their initial visit, 256 had a current clinical diagnosis of bipolar disorder and 540 had nonbipolar diagnoses. The Mini-SCID had a sensitivity of 0.58 and specificity of 0.63 in predicting a current diagnosis of bipolarity. This compared with a sensitivity of 0.35 and specificity of 0.98 for the clinician’s initial diagnosis. Among patients with bipolar II disorder, the MiniSCID’s sensitivity was 0.55, compared with 0.20 for the clinician’s initial diagnosis.
Patients who endorsed mania/hypomania on the Mini-SCID yet had a diagnosis of nonbipolar illness had SCL-90 profiles more like those of bipolar than unipolar patients. Therefore, using the Mini-SCID with the SCL-90 might improve in-office recognition of bipolar illness.
Limitations. One limitation to using rating scales to publish experiences in clinical practice is that clinical need, rather than a research protocol, determines the frequency of visits. Another is that we ask patients to rate symptoms they experience in the week before office visits. Thus, the data do not capture changes that occurred in other weeks.
Standardized tests we use
Except for the Quick Inventory of Depressive Symptomatology (QIDS), I selected the tests I use in the late 1980s because of:
- their ease of use and affordability
- my familiarity with them from my academic work
- their suitability for a mood disorder clinical practice such as mine.
Psychosocial history. Patients use an office computer to complete a questionnaire about family and developmental history, financial and employment history, education, health, alcohol and drug history, current stressors, and the presenting problem. Software from Multi-Health Systems (See Related Resources) allows me to add or remove questions as needed.
To ensure privacy when the next patient uses the computer, each patient’s report is deleted after it is printed. I receive the printed report, which details all responses and flags those that may require clarification.
Medical history. A standardized form asks patients about whether they have had most common medical conditions, their present symptoms, and family members’ health. An additional form inquires into psychiatric treatment, family history of psychiatric illnesses, and present medications.
Mini-SCID. The Mini-SCID has several advantages over the Structured Clinical Interview for DSM (SCID):
- Patients self-administer the test on a computer at the office.
- For research purposes, Mini-SCID results are protected from clinician biases because patients are interviewed using uniform questions and circumstances.
Being able to identify the bipolar nature of a depressive episode leads to better treatment and outcomes. In our private psychiatric clinic, we used the 39-item Temperament Evaluation of Memphis, Pisa, Paris and San Diego (TEMPS) to screen for temperaments of 783 consecutive mood disorder outpatients. We also examined their demographic information, clinical diagnoses by the treating psychiatrist, and Clinical Global Impressions (CGI) scores to measure response to treatment.6
- Patients with bipolar disorder scored significantly higher on cyclothymia, depression, and irritability scales, compared with patients diagnosed with unipolar depression.
- Bipolar II patients scored significantly higher on the same 3 scales than did patients with bipolar I disorder or unipolar depression.
Patients with higher cyclothymia scores tended also to have higher CGI-C scores, indicating greater treatment resistance.
Symptom Checklist-90 (SCL-90). This tool adds another layer of support for bipolar illness diagnosis (Box 1). It also is useful in conjunction with rating scales specific to other diagnostic categories, such as depression and anxiety.
The SCL-903 consists of 90 statements that measure the severity of 9 dimensions of psychopathology: somatization, obsession-compulsion, interpersonal sensitivity, depression, anxiety, hostility, phobic anxiety, paranoid ideation, and psychoticism. Using a scale of 0 (not at all) to 4 (a great deal), patients rate how much they are bothered by the feelings expressed in each statement.
In its standard scoring, the SCL-90 returns a score for 9 scales. Hunter et al4 developed an alternate set of 8 scales that uses SCL-90 questions to screen for depression, mania, schizophrenia, antisocial personality disorder, somatization disorder, obsessive-compulsive disorder, panic disorder, and agoraphobia. These SCL-90 diagnostic scales showed good reliability as an aid to the Mini-SCID in identifying diagnoses among 1,457 adult psychiatric outpatients.
Clinical Global Impressions scale. The CGI uses a 7-point Likert scale to describe the clinician’s impression of change in a patient’s condition. This scale:
- transcends symptom checklists by incorporating knowledge of the patient’s history, symptoms, and behaviors
- lends itself easily to repeated measures of change and severity of the condition being rated.1
Every office visit
At the screening visit and before every office visit, my patients complete 2 depression rating tests to document changes between visits and over time: a visual analog scale (VAS) and the QIDS.
The VAS’ 10-cm line with the left side marked “worst ever” and the right side marked “best ever” is a simple tool. It captures patients’ subjective impressions of their mood states in answer to the question, “How do you feel today?” I used the VAS as an outcome measure in a study of modafinil augmentation of antidepressant therapy.7
The QIDS is a 16-item screen that measures 9 depressive symptoms.8 It has been validated against the Hamilton Depression Rating Scale (HAM-D)9 and was used as the outcome measure in the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trial.10 The QIDS-16 is available online for free use in many languages (see Related Resources).11
Until recently, our office performed routine depression screening with the 52-item Carroll Depression Rating Scale (CDRS),13 a self-administered inventory designed to mirror results from the HAM-D. I published articles using the CDRS as the primary outcome measure in a chart review of long-term effectiveness of antidepressant monotherapy (Box 3)13,14 and in a study of modafinil’s effectiveness as adjunctive therapy in patients with unipolar depression.7
The Carroll Depression Rating Scale (CDRS) is lengthy (52 items), but its self-rating yes/no format makes it easy to administer and score.13 We used the CDRS as the primary outcome measure in a chart review of long-term effectiveness of antidepressant monotherapy in 346 patients with unipolar depression.14
Using baseline and follow-up CDRS scores over 5 years, we examined:
- changes in scores
- which medications most rapidly brought about remission (defined as CDRS score ≤7)
- which medication was most effective in preventing relapse.
We found that sertraline and to a lesser extent paroxetine were more effective than several other antidepressants in achieving remission and preventing relapse.
Logistical concerns
Patient feedback. Although some patients complain about having to complete depression rating scales at every visit, most accept this as equivalent to having routine blood pressure measurements. Many become interested in tracking their improvement by test scores in addition to subjective feelings.
- Multi-Health Systems. Publishers of mental health assessment tools. www.mhs.com.
- Inventory of Depressive Symptomatology (IDS) and Quick Inventory of Depressive Symptomatology (QIDS). www.ids-qids.org.
- Modafinil • Provigil
- Paroxetine • Paxil
- Sertraline • Zoloft
Dr. Nasr is a speaker for Takeda Pharmaceutical Company, Pfizer Inc, Eli Lilly and Company, Bristol-Myers Squibb, Forest Pharmaceuticals, and GlaxoSmithKline.
Acknowledgment
Dr. Nasr acknowledges the contribution of research assistant Burdette J. Wendt, who collects and analyzes the data referenced above and helped prepare this manuscript.
HIPAA. The Health Insurance Portability and Accountability Act (HIPAA) allows publication of large-scale studies that do not identify patients individually. We also obtained permission from the local Institutional Review Board to disseminate non-identifying cumulative data.
1. Busner J, Targum SD. The Clinical Global Impressions Scale: applying a research tool in clinical practice. Psychiatry 2007 2007;4(7):28-37.
2. Nasr S, Popli A, Wendt B. Can the MiniSCID improve the detection of bipolarity in private practice? J Affect Disord 2005;86:289-93.
3. Derogatis LR, Lipman RS, Covi L. SCL-90: an outpatient psychiatric rating scale—preliminary report. Psychopharmacol Bull 1973;9(1):13-28.
4. Hunter E, Penick E, Powell B, et al. Development of scales to screen for eight common psychiatric disorders. J Nerv Ment Dis 2005;193:131-5.
5. Akiskal HS, Akiskal KK, Haykal RF, et al. TEMPS-A: progress towards validation of a self-rated clinical version of the Temperament Evaluation of the Memphis, Pisa, Paris, and San Diego Autoquestionnaire. J Affect Disord 2005;85:3-16.
6. Nasr S, Wendt B. The TEMPS in outpatient practice. Poster presented at: Annual Meeting of the American Psychiatric Association; May 23, 2007; San Diego, CA.
7. Nasr S. Modafinil as adjunctive therapy in depressed outpatients. Ann Clin Psychiatry 2004;16:133-8.
8. Rush AJ, Trivedi MH, Ibrahim HM, et al. The 16-item Quick Inventory of Depressive Symptomatology (QIDS) clinician rating (QIDS-C) and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry 2003;54:573-83.
9. Rush AJ, Gullion CM, Basco MR, et al. The Inventory of Depressive Symptomatology (IDS): psychometric properties. Psychol Med 1996;26:477-86.
10. Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry 2006;163(11):1905-17.
11. IDS/QIDS. Instruments in English and multiple translations. University of Pittsburgh Epidemiology Data Center. Available at: http://www.ids-qids.org. Accessed March 7, 2008.
12. Nasr S, Wendt B. Sleeplessness despite remission in depressed outpatients. Poster presented at: Annual Meeting of the American Psychiatric Association; May 23, 2007; San Diego, CA.
13. Carroll BJM, Feinberg M, Smouse PE, et al. The Carroll rating scale for depression. I. Development, reliability and validation. Br J Psychiatry 1981;138:194-200.
14. Nasr S, Wendt B. Five-year comparison of antidepressant monotherapy. Int J Psychiatry Clin Pract 2006;10:297-9.
General psychiatry practitioners such as myself traditionally have relied on writing case reports to describe our clinical experience. One obstacle to getting cases published is that many research journals require submitted articles to include large samples and rating scales as measures of change in the conditions of patients being studied.
I have published articles about my clinical experiences using patient data collected with the Clinical Global Impressions (CGI) scale and other standardized tests. Research instruments such as the CGI can gather empiric data and are easy to use in clinical practice.1
This article describes how routine standardized testing provides useful data for research and improves diagnostic accuracy—and patient outcomes—even before I meet my patients for the first time.
Why use standardized tests?
Benefits. All my new patients undergo screening before their first face-to-face meeting with a psychiatrist. This registration visit takes about 2 hours, after which they are scheduled for an appointment based on clinical urgency. We charge no fee for the screening visit; the benefits of gathering a comprehensive database before the clinical evaluation outweigh the cost of the tests, software, and staff time.
Along with completing insurance and biographical paperwork, patients perform self-administered psychosocial and medical histories and a battery of standardized tests. This information allows me to focus on interpersonal issues—rather than fact-finding—during the first interview. It also ensures a comprehensive patient history.
Bipolar disorder is difficult to diagnose in patients presenting with depressive symptoms. In a 5-year chart review,2 we used data from Structured Clinical Interview for DSM-IV (Mini-SCID) screening tests to assess this tool’s usefulness in diagnosing depressed patients. Data also included each patient’s demographic information, initial clinical diagnosis, current clinical diagnosis, and Symptom Checklist-90 (SCL-90) results.
Among 796 patients who took the Mini-SCID at their initial visit, 256 had a current clinical diagnosis of bipolar disorder and 540 had nonbipolar diagnoses. The Mini-SCID had a sensitivity of 0.58 and specificity of 0.63 in predicting a current diagnosis of bipolarity. This compared with a sensitivity of 0.35 and specificity of 0.98 for the clinician’s initial diagnosis. Among patients with bipolar II disorder, the MiniSCID’s sensitivity was 0.55, compared with 0.20 for the clinician’s initial diagnosis.
Patients who endorsed mania/hypomania on the Mini-SCID yet had a diagnosis of nonbipolar illness had SCL-90 profiles more like those of bipolar than unipolar patients. Therefore, using the Mini-SCID with the SCL-90 might improve in-office recognition of bipolar illness.
Limitations. One limitation to using rating scales to publish experiences in clinical practice is that clinical need, rather than a research protocol, determines the frequency of visits. Another is that we ask patients to rate symptoms they experience in the week before office visits. Thus, the data do not capture changes that occurred in other weeks.
Standardized tests we use
Except for the Quick Inventory of Depressive Symptomatology (QIDS), I selected the tests I use in the late 1980s because of:
- their ease of use and affordability
- my familiarity with them from my academic work
- their suitability for a mood disorder clinical practice such as mine.
Psychosocial history. Patients use an office computer to complete a questionnaire about family and developmental history, financial and employment history, education, health, alcohol and drug history, current stressors, and the presenting problem. Software from Multi-Health Systems (See Related Resources) allows me to add or remove questions as needed.
To ensure privacy when the next patient uses the computer, each patient’s report is deleted after it is printed. I receive the printed report, which details all responses and flags those that may require clarification.
Medical history. A standardized form asks patients about whether they have had most common medical conditions, their present symptoms, and family members’ health. An additional form inquires into psychiatric treatment, family history of psychiatric illnesses, and present medications.
Mini-SCID. The Mini-SCID has several advantages over the Structured Clinical Interview for DSM (SCID):
- Patients self-administer the test on a computer at the office.
- For research purposes, Mini-SCID results are protected from clinician biases because patients are interviewed using uniform questions and circumstances.
Being able to identify the bipolar nature of a depressive episode leads to better treatment and outcomes. In our private psychiatric clinic, we used the 39-item Temperament Evaluation of Memphis, Pisa, Paris and San Diego (TEMPS) to screen for temperaments of 783 consecutive mood disorder outpatients. We also examined their demographic information, clinical diagnoses by the treating psychiatrist, and Clinical Global Impressions (CGI) scores to measure response to treatment.6
- Patients with bipolar disorder scored significantly higher on cyclothymia, depression, and irritability scales, compared with patients diagnosed with unipolar depression.
- Bipolar II patients scored significantly higher on the same 3 scales than did patients with bipolar I disorder or unipolar depression.
Patients with higher cyclothymia scores tended also to have higher CGI-C scores, indicating greater treatment resistance.
Symptom Checklist-90 (SCL-90). This tool adds another layer of support for bipolar illness diagnosis (Box 1). It also is useful in conjunction with rating scales specific to other diagnostic categories, such as depression and anxiety.
The SCL-903 consists of 90 statements that measure the severity of 9 dimensions of psychopathology: somatization, obsession-compulsion, interpersonal sensitivity, depression, anxiety, hostility, phobic anxiety, paranoid ideation, and psychoticism. Using a scale of 0 (not at all) to 4 (a great deal), patients rate how much they are bothered by the feelings expressed in each statement.
In its standard scoring, the SCL-90 returns a score for 9 scales. Hunter et al4 developed an alternate set of 8 scales that uses SCL-90 questions to screen for depression, mania, schizophrenia, antisocial personality disorder, somatization disorder, obsessive-compulsive disorder, panic disorder, and agoraphobia. These SCL-90 diagnostic scales showed good reliability as an aid to the Mini-SCID in identifying diagnoses among 1,457 adult psychiatric outpatients.
Clinical Global Impressions scale. The CGI uses a 7-point Likert scale to describe the clinician’s impression of change in a patient’s condition. This scale:
- transcends symptom checklists by incorporating knowledge of the patient’s history, symptoms, and behaviors
- lends itself easily to repeated measures of change and severity of the condition being rated.1
Every office visit
At the screening visit and before every office visit, my patients complete 2 depression rating tests to document changes between visits and over time: a visual analog scale (VAS) and the QIDS.
The VAS’ 10-cm line with the left side marked “worst ever” and the right side marked “best ever” is a simple tool. It captures patients’ subjective impressions of their mood states in answer to the question, “How do you feel today?” I used the VAS as an outcome measure in a study of modafinil augmentation of antidepressant therapy.7
The QIDS is a 16-item screen that measures 9 depressive symptoms.8 It has been validated against the Hamilton Depression Rating Scale (HAM-D)9 and was used as the outcome measure in the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trial.10 The QIDS-16 is available online for free use in many languages (see Related Resources).11
Until recently, our office performed routine depression screening with the 52-item Carroll Depression Rating Scale (CDRS),13 a self-administered inventory designed to mirror results from the HAM-D. I published articles using the CDRS as the primary outcome measure in a chart review of long-term effectiveness of antidepressant monotherapy (Box 3)13,14 and in a study of modafinil’s effectiveness as adjunctive therapy in patients with unipolar depression.7
The Carroll Depression Rating Scale (CDRS) is lengthy (52 items), but its self-rating yes/no format makes it easy to administer and score.13 We used the CDRS as the primary outcome measure in a chart review of long-term effectiveness of antidepressant monotherapy in 346 patients with unipolar depression.14
Using baseline and follow-up CDRS scores over 5 years, we examined:
- changes in scores
- which medications most rapidly brought about remission (defined as CDRS score ≤7)
- which medication was most effective in preventing relapse.
We found that sertraline and to a lesser extent paroxetine were more effective than several other antidepressants in achieving remission and preventing relapse.
Logistical concerns
Patient feedback. Although some patients complain about having to complete depression rating scales at every visit, most accept this as equivalent to having routine blood pressure measurements. Many become interested in tracking their improvement by test scores in addition to subjective feelings.
- Multi-Health Systems. Publishers of mental health assessment tools. www.mhs.com.
- Inventory of Depressive Symptomatology (IDS) and Quick Inventory of Depressive Symptomatology (QIDS). www.ids-qids.org.
- Modafinil • Provigil
- Paroxetine • Paxil
- Sertraline • Zoloft
Dr. Nasr is a speaker for Takeda Pharmaceutical Company, Pfizer Inc, Eli Lilly and Company, Bristol-Myers Squibb, Forest Pharmaceuticals, and GlaxoSmithKline.
Acknowledgment
Dr. Nasr acknowledges the contribution of research assistant Burdette J. Wendt, who collects and analyzes the data referenced above and helped prepare this manuscript.
HIPAA. The Health Insurance Portability and Accountability Act (HIPAA) allows publication of large-scale studies that do not identify patients individually. We also obtained permission from the local Institutional Review Board to disseminate non-identifying cumulative data.
General psychiatry practitioners such as myself traditionally have relied on writing case reports to describe our clinical experience. One obstacle to getting cases published is that many research journals require submitted articles to include large samples and rating scales as measures of change in the conditions of patients being studied.
I have published articles about my clinical experiences using patient data collected with the Clinical Global Impressions (CGI) scale and other standardized tests. Research instruments such as the CGI can gather empiric data and are easy to use in clinical practice.1
This article describes how routine standardized testing provides useful data for research and improves diagnostic accuracy—and patient outcomes—even before I meet my patients for the first time.
Why use standardized tests?
Benefits. All my new patients undergo screening before their first face-to-face meeting with a psychiatrist. This registration visit takes about 2 hours, after which they are scheduled for an appointment based on clinical urgency. We charge no fee for the screening visit; the benefits of gathering a comprehensive database before the clinical evaluation outweigh the cost of the tests, software, and staff time.
Along with completing insurance and biographical paperwork, patients perform self-administered psychosocial and medical histories and a battery of standardized tests. This information allows me to focus on interpersonal issues—rather than fact-finding—during the first interview. It also ensures a comprehensive patient history.
Bipolar disorder is difficult to diagnose in patients presenting with depressive symptoms. In a 5-year chart review,2 we used data from Structured Clinical Interview for DSM-IV (Mini-SCID) screening tests to assess this tool’s usefulness in diagnosing depressed patients. Data also included each patient’s demographic information, initial clinical diagnosis, current clinical diagnosis, and Symptom Checklist-90 (SCL-90) results.
Among 796 patients who took the Mini-SCID at their initial visit, 256 had a current clinical diagnosis of bipolar disorder and 540 had nonbipolar diagnoses. The Mini-SCID had a sensitivity of 0.58 and specificity of 0.63 in predicting a current diagnosis of bipolarity. This compared with a sensitivity of 0.35 and specificity of 0.98 for the clinician’s initial diagnosis. Among patients with bipolar II disorder, the MiniSCID’s sensitivity was 0.55, compared with 0.20 for the clinician’s initial diagnosis.
Patients who endorsed mania/hypomania on the Mini-SCID yet had a diagnosis of nonbipolar illness had SCL-90 profiles more like those of bipolar than unipolar patients. Therefore, using the Mini-SCID with the SCL-90 might improve in-office recognition of bipolar illness.
Limitations. One limitation to using rating scales to publish experiences in clinical practice is that clinical need, rather than a research protocol, determines the frequency of visits. Another is that we ask patients to rate symptoms they experience in the week before office visits. Thus, the data do not capture changes that occurred in other weeks.
Standardized tests we use
Except for the Quick Inventory of Depressive Symptomatology (QIDS), I selected the tests I use in the late 1980s because of:
- their ease of use and affordability
- my familiarity with them from my academic work
- their suitability for a mood disorder clinical practice such as mine.
Psychosocial history. Patients use an office computer to complete a questionnaire about family and developmental history, financial and employment history, education, health, alcohol and drug history, current stressors, and the presenting problem. Software from Multi-Health Systems (See Related Resources) allows me to add or remove questions as needed.
To ensure privacy when the next patient uses the computer, each patient’s report is deleted after it is printed. I receive the printed report, which details all responses and flags those that may require clarification.
Medical history. A standardized form asks patients about whether they have had most common medical conditions, their present symptoms, and family members’ health. An additional form inquires into psychiatric treatment, family history of psychiatric illnesses, and present medications.
Mini-SCID. The Mini-SCID has several advantages over the Structured Clinical Interview for DSM (SCID):
- Patients self-administer the test on a computer at the office.
- For research purposes, Mini-SCID results are protected from clinician biases because patients are interviewed using uniform questions and circumstances.
Being able to identify the bipolar nature of a depressive episode leads to better treatment and outcomes. In our private psychiatric clinic, we used the 39-item Temperament Evaluation of Memphis, Pisa, Paris and San Diego (TEMPS) to screen for temperaments of 783 consecutive mood disorder outpatients. We also examined their demographic information, clinical diagnoses by the treating psychiatrist, and Clinical Global Impressions (CGI) scores to measure response to treatment.6
- Patients with bipolar disorder scored significantly higher on cyclothymia, depression, and irritability scales, compared with patients diagnosed with unipolar depression.
- Bipolar II patients scored significantly higher on the same 3 scales than did patients with bipolar I disorder or unipolar depression.
Patients with higher cyclothymia scores tended also to have higher CGI-C scores, indicating greater treatment resistance.
Symptom Checklist-90 (SCL-90). This tool adds another layer of support for bipolar illness diagnosis (Box 1). It also is useful in conjunction with rating scales specific to other diagnostic categories, such as depression and anxiety.
The SCL-903 consists of 90 statements that measure the severity of 9 dimensions of psychopathology: somatization, obsession-compulsion, interpersonal sensitivity, depression, anxiety, hostility, phobic anxiety, paranoid ideation, and psychoticism. Using a scale of 0 (not at all) to 4 (a great deal), patients rate how much they are bothered by the feelings expressed in each statement.
In its standard scoring, the SCL-90 returns a score for 9 scales. Hunter et al4 developed an alternate set of 8 scales that uses SCL-90 questions to screen for depression, mania, schizophrenia, antisocial personality disorder, somatization disorder, obsessive-compulsive disorder, panic disorder, and agoraphobia. These SCL-90 diagnostic scales showed good reliability as an aid to the Mini-SCID in identifying diagnoses among 1,457 adult psychiatric outpatients.
Clinical Global Impressions scale. The CGI uses a 7-point Likert scale to describe the clinician’s impression of change in a patient’s condition. This scale:
- transcends symptom checklists by incorporating knowledge of the patient’s history, symptoms, and behaviors
- lends itself easily to repeated measures of change and severity of the condition being rated.1
Every office visit
At the screening visit and before every office visit, my patients complete 2 depression rating tests to document changes between visits and over time: a visual analog scale (VAS) and the QIDS.
The VAS’ 10-cm line with the left side marked “worst ever” and the right side marked “best ever” is a simple tool. It captures patients’ subjective impressions of their mood states in answer to the question, “How do you feel today?” I used the VAS as an outcome measure in a study of modafinil augmentation of antidepressant therapy.7
The QIDS is a 16-item screen that measures 9 depressive symptoms.8 It has been validated against the Hamilton Depression Rating Scale (HAM-D)9 and was used as the outcome measure in the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trial.10 The QIDS-16 is available online for free use in many languages (see Related Resources).11
Until recently, our office performed routine depression screening with the 52-item Carroll Depression Rating Scale (CDRS),13 a self-administered inventory designed to mirror results from the HAM-D. I published articles using the CDRS as the primary outcome measure in a chart review of long-term effectiveness of antidepressant monotherapy (Box 3)13,14 and in a study of modafinil’s effectiveness as adjunctive therapy in patients with unipolar depression.7
The Carroll Depression Rating Scale (CDRS) is lengthy (52 items), but its self-rating yes/no format makes it easy to administer and score.13 We used the CDRS as the primary outcome measure in a chart review of long-term effectiveness of antidepressant monotherapy in 346 patients with unipolar depression.14
Using baseline and follow-up CDRS scores over 5 years, we examined:
- changes in scores
- which medications most rapidly brought about remission (defined as CDRS score ≤7)
- which medication was most effective in preventing relapse.
We found that sertraline and to a lesser extent paroxetine were more effective than several other antidepressants in achieving remission and preventing relapse.
Logistical concerns
Patient feedback. Although some patients complain about having to complete depression rating scales at every visit, most accept this as equivalent to having routine blood pressure measurements. Many become interested in tracking their improvement by test scores in addition to subjective feelings.
- Multi-Health Systems. Publishers of mental health assessment tools. www.mhs.com.
- Inventory of Depressive Symptomatology (IDS) and Quick Inventory of Depressive Symptomatology (QIDS). www.ids-qids.org.
- Modafinil • Provigil
- Paroxetine • Paxil
- Sertraline • Zoloft
Dr. Nasr is a speaker for Takeda Pharmaceutical Company, Pfizer Inc, Eli Lilly and Company, Bristol-Myers Squibb, Forest Pharmaceuticals, and GlaxoSmithKline.
Acknowledgment
Dr. Nasr acknowledges the contribution of research assistant Burdette J. Wendt, who collects and analyzes the data referenced above and helped prepare this manuscript.
HIPAA. The Health Insurance Portability and Accountability Act (HIPAA) allows publication of large-scale studies that do not identify patients individually. We also obtained permission from the local Institutional Review Board to disseminate non-identifying cumulative data.
1. Busner J, Targum SD. The Clinical Global Impressions Scale: applying a research tool in clinical practice. Psychiatry 2007 2007;4(7):28-37.
2. Nasr S, Popli A, Wendt B. Can the MiniSCID improve the detection of bipolarity in private practice? J Affect Disord 2005;86:289-93.
3. Derogatis LR, Lipman RS, Covi L. SCL-90: an outpatient psychiatric rating scale—preliminary report. Psychopharmacol Bull 1973;9(1):13-28.
4. Hunter E, Penick E, Powell B, et al. Development of scales to screen for eight common psychiatric disorders. J Nerv Ment Dis 2005;193:131-5.
5. Akiskal HS, Akiskal KK, Haykal RF, et al. TEMPS-A: progress towards validation of a self-rated clinical version of the Temperament Evaluation of the Memphis, Pisa, Paris, and San Diego Autoquestionnaire. J Affect Disord 2005;85:3-16.
6. Nasr S, Wendt B. The TEMPS in outpatient practice. Poster presented at: Annual Meeting of the American Psychiatric Association; May 23, 2007; San Diego, CA.
7. Nasr S. Modafinil as adjunctive therapy in depressed outpatients. Ann Clin Psychiatry 2004;16:133-8.
8. Rush AJ, Trivedi MH, Ibrahim HM, et al. The 16-item Quick Inventory of Depressive Symptomatology (QIDS) clinician rating (QIDS-C) and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry 2003;54:573-83.
9. Rush AJ, Gullion CM, Basco MR, et al. The Inventory of Depressive Symptomatology (IDS): psychometric properties. Psychol Med 1996;26:477-86.
10. Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry 2006;163(11):1905-17.
11. IDS/QIDS. Instruments in English and multiple translations. University of Pittsburgh Epidemiology Data Center. Available at: http://www.ids-qids.org. Accessed March 7, 2008.
12. Nasr S, Wendt B. Sleeplessness despite remission in depressed outpatients. Poster presented at: Annual Meeting of the American Psychiatric Association; May 23, 2007; San Diego, CA.
13. Carroll BJM, Feinberg M, Smouse PE, et al. The Carroll rating scale for depression. I. Development, reliability and validation. Br J Psychiatry 1981;138:194-200.
14. Nasr S, Wendt B. Five-year comparison of antidepressant monotherapy. Int J Psychiatry Clin Pract 2006;10:297-9.
1. Busner J, Targum SD. The Clinical Global Impressions Scale: applying a research tool in clinical practice. Psychiatry 2007 2007;4(7):28-37.
2. Nasr S, Popli A, Wendt B. Can the MiniSCID improve the detection of bipolarity in private practice? J Affect Disord 2005;86:289-93.
3. Derogatis LR, Lipman RS, Covi L. SCL-90: an outpatient psychiatric rating scale—preliminary report. Psychopharmacol Bull 1973;9(1):13-28.
4. Hunter E, Penick E, Powell B, et al. Development of scales to screen for eight common psychiatric disorders. J Nerv Ment Dis 2005;193:131-5.
5. Akiskal HS, Akiskal KK, Haykal RF, et al. TEMPS-A: progress towards validation of a self-rated clinical version of the Temperament Evaluation of the Memphis, Pisa, Paris, and San Diego Autoquestionnaire. J Affect Disord 2005;85:3-16.
6. Nasr S, Wendt B. The TEMPS in outpatient practice. Poster presented at: Annual Meeting of the American Psychiatric Association; May 23, 2007; San Diego, CA.
7. Nasr S. Modafinil as adjunctive therapy in depressed outpatients. Ann Clin Psychiatry 2004;16:133-8.
8. Rush AJ, Trivedi MH, Ibrahim HM, et al. The 16-item Quick Inventory of Depressive Symptomatology (QIDS) clinician rating (QIDS-C) and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry 2003;54:573-83.
9. Rush AJ, Gullion CM, Basco MR, et al. The Inventory of Depressive Symptomatology (IDS): psychometric properties. Psychol Med 1996;26:477-86.
10. Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry 2006;163(11):1905-17.
11. IDS/QIDS. Instruments in English and multiple translations. University of Pittsburgh Epidemiology Data Center. Available at: http://www.ids-qids.org. Accessed March 7, 2008.
12. Nasr S, Wendt B. Sleeplessness despite remission in depressed outpatients. Poster presented at: Annual Meeting of the American Psychiatric Association; May 23, 2007; San Diego, CA.
13. Carroll BJM, Feinberg M, Smouse PE, et al. The Carroll rating scale for depression. I. Development, reliability and validation. Br J Psychiatry 1981;138:194-200.
14. Nasr S, Wendt B. Five-year comparison of antidepressant monotherapy. Int J Psychiatry Clin Pract 2006;10:297-9.
Restoring sexual function: Which medications show benefit?
Sexual disorders such as premature ejaculation, erectile dysfunction (ED), and low libido reduce quality of life in patients with depression, anxiety, and other psychiatric illnesses. In addition, sexual dysfunction is a side effect of many drugs used to treat psychiatric disorders.1
Psychiatry—with its biopsychosocial model—can easily assume the evaluation and treatment of sexual disorders. To inform your practice, this article provides an update on pharmacotherapy for the 3 most common sexual disorders. Its emphasis on biologic treatment is not intended to minimize the importance of psychological interventions.
Premature ejaculation
Premature ejaculation is one of the most common male sexual complaints. In some surveys, approximately 30% of men express concern about ejaculating too rapidly.2 Behavioral therapy often is effective (Box 1), but in my experience most male patients prefer a pharmacologic approach to sexual problems.
Anesthetic creams. Locally applied anesthetic creams—such as prilocaine, lidocaine mixtures, and creams consisting of natural herbs—can increase ejaculatory latency by approximately 7 to 10 minutes. The major side effect of these preparations is penile hypoanesthesia. The man also must use a condom or wash off the cream before vaginal penetration to minimize vaginal absorption.3
- With male lying on back, partner strokes penis until male signals that ejaculation will occur with continued stimulation†
- Stroking stops, and erection is allowed to subside, then stroking resumes
- Repeat steps 1 and 2 four times, 2 times/week
- Ejaculatory latency will increase
- Partner assumes female-superior position and moves up and down until male indicates ejaculation is imminent
* Behavioral therapy for heterosexual couples. Oral-genital stimulation can be utilized between steps 4 and 5
† Frenulum squeeze technique is similar except that partner squeezes frenulum of penis at sign of male excitement
Among the SSRIs, paroxetine appears to have the greatest effect on ejaculatory latency (Table 1).5 Most trials have found that the dose needed to delay ejaculation is similar to the dose necessary to treat depressive disorders.
Some case reports suggest that phosphodiesterase type 5 inhibitors (PDE-5 inhibitors) may help to delay ejaculation, but this effect has not been borne out in double-blind studies.6
Table 1
Drug treatment options for premature ejaculation
| Drug | Dosage | Common side effects |
|---|---|---|
| Paroxetine | 20 to 40 mg/d | Nausea, headache |
| Clomipramine | 25 to 50 mg 4 to 6 hours before sexual activity | Nausea, fatigue |
| Lorazepam | 0.5 to 1 mg 30 minutes before sexual activity | Sedation |
| Source: Reference 5 | ||
Erectile dysfunction
Men with major depressive disorder, anxiety disorders, and psychotic disorders have higher rates of ED, compared with the general male population. ED also can be a side effect of—and adversely affect adherence to—antidepressant and antipsychotic therapy.7 Restored erectile function can positively affect patients’ self-esteem and sense of personal efficacy and may facilitate recovery from depression.8
PDE-5 inhibitors. Nitric oxide release triggers the production of cyclic guanosine monophosphate, which leads to decreased intracellular calcium, smooth muscle relaxation, and penile erection. All available PDE-5 inhibitors work by inhibiting the degradation of cyclic guanosine monophosphate. They are highly specific, vary somewhat in selectivity for other phosphodiesterase enzyme types, and differ in duration of action (Table 2).
Common side effects include dyspepsia, stuffy nose, and headache. The use of PDE-5 inhibitors with nitrates is contraindicated because of the risk of severe hypotension. Use PDE-5 inhibitors cautiously:
- with alpha blockers because of the risk of hypotension
- in men with aortic stenosis, recent myocardial infarction, unstable angina, heart failure, arrhythmias, degenerative retinal disease, or poorly controlled hypertension.9
Table 2
Duration of action of PDE-5 inhibitors
| Drug | Duration |
|---|---|
| Sildenafil | 4 hours |
| Vardenafil | 4 hours |
| Tadalafil | 24 to 36 hours |
| PDE-5: phosphodiesterase type 5 | |
Other options. Other accepted options for treating ED include intracavernosal injection of vasoactive substances such as phentolamine or prostaglandin E1, or intraurethral insertion of prostaglandins.12 Since the advent of PDE-5 inhibitors, these approaches are rarely used.
Cabergoline. Off-label use of dopaminergic agents such as cabergoline may be moderately effective in treating ED in men who do not respond adequately to PDE-5 inhibitors. Cabergoline is a dopamine D2 receptor agonist used to treat hyperprolactinemia and Parkinson’s disease.
In a randomized, double-blind, placebo- controlled study, 402 men who did not respond to sildenafil received cabergoline, 0.5 to 1 mg weekly for 6 months. Among the 370 men (92%) who completed the trial, mean weekly intercourse episodes increased from 1.4 to 2.2, compared with 1.2 to 1.4 in men who received placebo.13
Cabergoline also improved erectile function and sexual satisfaction in a randomized, double-blind, placebo-controlled trial of 50 men with psychogenic erectile dysfunction.14
Treatment outcomes. Most studies report positive psychological responses to ED reversal in men using PDE-5 inhibitors. Successful therapy has been associated with increased self-esteem, satisfaction, and sexual satisfaction.8 Some studies have found increased sexual satisfaction in the partner as well.15
Approximately 50% of PDE-5 inhibitor prescriptions are not refilled, however, and some case reports suggest that restored erectile function can result in:
- divorce and marital discord
- no change in partner-related activity.16
Recommendation. Because erectile problems are common in psychiatric patients, most psychiatrists should feel comfortable treating ED with PDE-5 inhibitors. In general, these agents are relatively safe. Reversing drug-induced sexual dysfunction may improve patients’ adherence to psychotropics, increase self-esteem, and improve relationships with sexual partners.
Hypoactive sexual desire
PDE-5 inhibitors. The success of PDE-5 inhibitors in treating ED led to investigations into whether these agents also could treat female sexual disorders. Large multisite trials using sildenafil failed to find evidence of efficacy in treating female hypoactive sexual desire or arousal disorders, however.18 Similarly, trials of topical prostaglandin E1 and alpha blockers were unsuccessful in women.
Some small studies suggested that PDE-5 inhibitors might be useful in:
- women with normal libido but decreased arousal19
- young women with normal libido and inability to reach orgasm.20
Testosterone therapy. In the 1940s, case reports suggested increased libido as a side effect when women were treated with androgens for metastatic cancer. In a prospective study, Sherwin and Gelfand21 found increased sexual desire, sexual arousal, and rates of coitus and orgasm in women injected with testosterone/estrogen preparations after surgical menopausal.
More recently, Shifren et al22 showed in a randomized, placebo-controlled trial that transdermal testosterone, 150 or 300 micrograms/day for 12 weeks, improved sexual function and psychological wellbeing in women age 31 to 56 after surgical menopause. Since then, numerous large, multisite, double-blind studies have demonstrated the efficacy of transdermal testosterone in treating low sexual desire in surgically menopausal women.23 Transdermal testosterone has been approved for this indication in the European Union but not in the United States.
Testosterone also has been reported to increase sexual desire in women who experienced natural menopause and in normal premenopausal women.24 The study of the relationship of testosterone to libido in women is complicated by numerous factors, however (Box 2).23,24
Other agents. Recent research has focused on centrally active compounds’ effect on libido. One agent in development is flibanserin, a serotonergic 5HT2 antagonist and a 5HT1a agonist. Data from large, multisite studies indicates that this compound increases libido in women with low sexual desire.
Transdermal testosterone can increase sexual desire in surgically menopausal women—according to numerous large, multisite, double-blind studies23—and has been approved for this indication in the European Union. Testosterone also has been reported to increase sexual desire in women who experienced natural menopause and in normal premenopausal women.24
In the United States, testosterone is not FDA-approved for treating low sexual desire. Considerable off-label use occurs, although long-term safety is unknown.
Various factors complicate the study of testosterone’s relationship to female libido:
- 3 large population studies have found minimal evidence of a relationship between endogenous androgenic activity and measures of female sexual responsiveness; these results may reflect an absence of such a relationship or methodologic weaknesses.
- The sensitivity and specificity of available assays to detect testosterone in women are seriously limited.
- Much of the active testosterone in the female body is made by intracellular conversion or testosterone precursors and thus may not be detected by assays of serum androgen levels.24
Some evidence suggests that bupropion—which has noradrenergic and dopaminergic agonist properties—increases orgasm completion and other measures of sexual responsiveness in women with hypoactive sexual desire disorders.25
The investigational compound bremelanotide—a synthetic version of melanocytes stimulating hormone—is administered intranasally and appears to acutely influence libido in women.26 Trials have been delayed because of this agent’s effects on blood pressure.
Herbal compounds. Some herbal compounds are being sold for low-desire complaints. Web sites for 2 herbal compounds, Ziti and Alibi, cite unpublished double-blind studies attesting to their efficacy. Because these studies are unpublished, one cannot evaluate their methodologies.
One double-blind study of the herbal compound ArginMax—which contains ginseng, ginkgo, damiana, L-arginine, and multivitamins—suggests efficacy in a small group of women with poorly specified sexual problems.27 Zestra, a topical herbal compound, has been evaluated in large multisite studies and found to be effective in increasing female sexual responsiveness.28
Other approaches. Some clinicians advocate using the testosterone precursor dehydroepiandrosterone (DHEA) for low sexual desire, although evidence does not support its efficacy.29
A battery-operated device is FDA -approved for treating sexual dysfunction in women. The clitoral vacuum increases vaginal engorgement and various indices of sexual responsiveness. This device’s target population is not clearly defined.30
When medication side effects are causing hypoactive sexual desire, consider substituting another drug or using antidotes such as buspirone or bupropion. Unfortunately, however, most sexual desire problems are idiopathic.
Testosterone therapy has been shown to improve libido, although it is not FDA-approved for this indication. Considerable off-label use occurs, but long-term safety is unknown.
Related resource
- Medline Plus. Sexual problems overview. www.nlm.nih.gov/medlineplus/ency/article/001951.htm.
- Bupropion • Wellbutrin
- Buspirone • BuSpar
- Cabergoline • Dostinex
- Clomipramine • Anafranil
- Fluoxetine • Prozac
- Lorazepam • Ativan
- Paroxetine • Paxil
- Phentolamine • Regitine
- Prostaglandin E1 • Liprostin
- Sertraline • Zoloft
- Sildenafil • Viagr
- Tadalafil • Cialis
- Vardenafil • Levitra
Dr. Segraves receives grant/research support from Pfizer, Boehringer Ingelheim, AstraZeneca, Bristol-Myers Squibb, and Novartis and is a consultant to Eli Lilly and Company, GlaxoSmithKline, Boehringer Ingelheim, and Bristol-Myers Squibb.
1. Segraves RT. Female sexual disorders: psychiatric aspects. Can J Psychiatry 2002;47:419-25.
2. Segraves RT. Rapid ejaculation: a review of nosology, prevalence and treatment. Int J Impot Res 2006;18(suppl 1):S24-S32.
3. Riley A, Segraves RT. Treatment of premature ejaculation. Int J Clin Pract 2006;60(6):694-7.
4. Duterte E, Segraves R, Althof S. Psychotherapy and pharmacotherapy of sexual dysfunctions. In: Nathan P, Gorman J, eds. A guide to treatments that work. New York, NY: Oxford University Press; 2007:531-60.
5. Waldinger M. Male ejaculation and orgasmic disorders. In: Balon R, Segraves RT, eds. Handbook of sexual dysfunction. Boca Raton, FL: Taylor & Francis; 2005:215-48.
6. McMahon C, Stuckey B, Andersen M, et al. Efficacy of sildenafil citrate (Viagra) in men with premature ejaculation. J Sex Med 2005;2:368-75.
7. Segraves R. Treatment of erectile dysfunction: a psychiatric perspective. Primary Psychiatry 2004;11(12):35-45.
8. Hartmann U. Depression and sexual dysfunction. J Men’s Health Gender 2007;4:18-25.
9. Shabsigh R, Seftel A, Rosen R, et al. Review of time of onset and duration of clinical efficacy of phosphodiesterase inhibitors in treatment of erectile dysfunction. Urology 2006;68:689-96.
10. Mukherjee B, Shivakumar T. A case of sensorineural deafness following ingestion of sildenafil. J Laryngol Otol 2007;121(4):395-7.
11. Pomeranz HD, Bhavsar AR. Nonarteritic ischemic optic neuropathy developing soon after use of sildenafil (Viagra): a report of seven new cases. J Neuroophthalmol 2005;25(1):9-13.
12. Padma-Nathan H, Christ G, Adaikan G, et al. Pharmacotherapy for erectile dysfunction. In: Lue T, Basson R, Rosen R, et al, eds. Sexual medicine: sexual dysfunctions in men and women. Paris, France: Health Publications; 2004:505-68.
13. Safarinejad M. Salvage of sildenafil failures with cabergoline: a randomized, double-blind, placebo-controlled study. Int J Impot Res 2006;18:550-8.
14. Nickel M, Moleda D, Loew T, et al. Cabergoline treatment in men with psychogenic erectile dysfunction: a randomized placebo-controlled study. Int J Impot Res 2007;19:164-7.
15. Montorsi F, Althof S. Partner responses to sildenafil citrate (Viagra) treatment of erectile dysfunction. Urology 2004;63:762-7.
16. Wise T. Psychosocial effects of sildenafil therapy for erectile dysfunction. J Sex Marital Ther 1999;25(2):145-50.
17. Pallas J, Levine SB, Althof SE, Risen CB. A study using Viagra in a mental health practice. J Sex Marital Ther 2000;26:41-50.
18. Basson R, McInnes R, Smith MD, et al. Efficacy and safety of sildenafil citrate in women with sexual dysfunction associated with female sexual arousal disorder. J Womens Health Gend Based Med 2002;11:367-77.
19. Berman JR, Berman LA, Toler SM, et al. Safety and efficacy of sildenafil citrate for the treatment of female sexual arousal disorder: a double-blind, placebo controlled study. J Urol 2003;170(6 Pt 1):2333-8.
20. Caruso S, Intelisano G, Lupo L, Agnello C. Premenopausal women affected by sexual arousal disorder treated with sildenafil: a double-blind, cross-over, placebo-controlled study. BJOG 2001;108:623-8.
21. Sherwin BB, Gelfand MM. The role of androgen in the maintenance of sexual functioning in oophorectomized women. Psychosom Med 1987;49:397-409.
22. Shifren JL, Braunstein GD, Simon JA, et al. Transdermal testosterone treatment in women with impaired sexual function after oophorectomy. N Engl J Med 2000;343:682-8.
23. Segraves RT, Woodard T. Female hypoactive sexual desire disorder: history and current status. J Sex Med 2006;3:408-18.
24. Basaria S, Dobs AS. Clinical review: controversies regarding transdermal androgen therapy in postmenopausal women. J Clin Endocrin Metab 2006;91:4743-52.
25. Segraves RT, Clayton A, Croft H, et al. Bupropion for the treatment of hypoactive sexual desire disorder in premenopausal women. J Clin Psychopharamacol 2004;24:339-42.
26. Pfaus J, Giuliano F, Gelez H. Bremelanotide: an overview of preclinical CNS effects on female sexual function. J Sex Med 2007;4(suppl 4):269-79.
27. Ito T, Polan M, Whipple B, Trant A. The enhancement of female sexual function with ArginMax, a nutritional supplement, among women suffering in menopausal status. J Sex Marit Ther 2006;32:359-78.
28. Ferguson DM, Steidle CP, Singh GS, et al. Randomized, placebo-controlled, double blind, crossover design trial of the efficacy and safety of Zestra for Women in women with and without female sexual arousal disorder. J Sex Marital Ther 2003;29(suppl 1):33-44.
29. Panjari M, Davis S. DHEA therapy for women: effect on sexual functioning and wellbeing. Hum Reprod Update 2007;13:239-48.
30. Feldman J, Striepe M. Women’s sexual health. Clinics in Family Practice 2004;6:839-61.
Sexual disorders such as premature ejaculation, erectile dysfunction (ED), and low libido reduce quality of life in patients with depression, anxiety, and other psychiatric illnesses. In addition, sexual dysfunction is a side effect of many drugs used to treat psychiatric disorders.1
Psychiatry—with its biopsychosocial model—can easily assume the evaluation and treatment of sexual disorders. To inform your practice, this article provides an update on pharmacotherapy for the 3 most common sexual disorders. Its emphasis on biologic treatment is not intended to minimize the importance of psychological interventions.
Premature ejaculation
Premature ejaculation is one of the most common male sexual complaints. In some surveys, approximately 30% of men express concern about ejaculating too rapidly.2 Behavioral therapy often is effective (Box 1), but in my experience most male patients prefer a pharmacologic approach to sexual problems.
Anesthetic creams. Locally applied anesthetic creams—such as prilocaine, lidocaine mixtures, and creams consisting of natural herbs—can increase ejaculatory latency by approximately 7 to 10 minutes. The major side effect of these preparations is penile hypoanesthesia. The man also must use a condom or wash off the cream before vaginal penetration to minimize vaginal absorption.3
- With male lying on back, partner strokes penis until male signals that ejaculation will occur with continued stimulation†
- Stroking stops, and erection is allowed to subside, then stroking resumes
- Repeat steps 1 and 2 four times, 2 times/week
- Ejaculatory latency will increase
- Partner assumes female-superior position and moves up and down until male indicates ejaculation is imminent
* Behavioral therapy for heterosexual couples. Oral-genital stimulation can be utilized between steps 4 and 5
† Frenulum squeeze technique is similar except that partner squeezes frenulum of penis at sign of male excitement
Among the SSRIs, paroxetine appears to have the greatest effect on ejaculatory latency (Table 1).5 Most trials have found that the dose needed to delay ejaculation is similar to the dose necessary to treat depressive disorders.
Some case reports suggest that phosphodiesterase type 5 inhibitors (PDE-5 inhibitors) may help to delay ejaculation, but this effect has not been borne out in double-blind studies.6
Table 1
Drug treatment options for premature ejaculation
| Drug | Dosage | Common side effects |
|---|---|---|
| Paroxetine | 20 to 40 mg/d | Nausea, headache |
| Clomipramine | 25 to 50 mg 4 to 6 hours before sexual activity | Nausea, fatigue |
| Lorazepam | 0.5 to 1 mg 30 minutes before sexual activity | Sedation |
| Source: Reference 5 | ||
Erectile dysfunction
Men with major depressive disorder, anxiety disorders, and psychotic disorders have higher rates of ED, compared with the general male population. ED also can be a side effect of—and adversely affect adherence to—antidepressant and antipsychotic therapy.7 Restored erectile function can positively affect patients’ self-esteem and sense of personal efficacy and may facilitate recovery from depression.8
PDE-5 inhibitors. Nitric oxide release triggers the production of cyclic guanosine monophosphate, which leads to decreased intracellular calcium, smooth muscle relaxation, and penile erection. All available PDE-5 inhibitors work by inhibiting the degradation of cyclic guanosine monophosphate. They are highly specific, vary somewhat in selectivity for other phosphodiesterase enzyme types, and differ in duration of action (Table 2).
Common side effects include dyspepsia, stuffy nose, and headache. The use of PDE-5 inhibitors with nitrates is contraindicated because of the risk of severe hypotension. Use PDE-5 inhibitors cautiously:
- with alpha blockers because of the risk of hypotension
- in men with aortic stenosis, recent myocardial infarction, unstable angina, heart failure, arrhythmias, degenerative retinal disease, or poorly controlled hypertension.9
Table 2
Duration of action of PDE-5 inhibitors
| Drug | Duration |
|---|---|
| Sildenafil | 4 hours |
| Vardenafil | 4 hours |
| Tadalafil | 24 to 36 hours |
| PDE-5: phosphodiesterase type 5 | |
Other options. Other accepted options for treating ED include intracavernosal injection of vasoactive substances such as phentolamine or prostaglandin E1, or intraurethral insertion of prostaglandins.12 Since the advent of PDE-5 inhibitors, these approaches are rarely used.
Cabergoline. Off-label use of dopaminergic agents such as cabergoline may be moderately effective in treating ED in men who do not respond adequately to PDE-5 inhibitors. Cabergoline is a dopamine D2 receptor agonist used to treat hyperprolactinemia and Parkinson’s disease.
In a randomized, double-blind, placebo- controlled study, 402 men who did not respond to sildenafil received cabergoline, 0.5 to 1 mg weekly for 6 months. Among the 370 men (92%) who completed the trial, mean weekly intercourse episodes increased from 1.4 to 2.2, compared with 1.2 to 1.4 in men who received placebo.13
Cabergoline also improved erectile function and sexual satisfaction in a randomized, double-blind, placebo-controlled trial of 50 men with psychogenic erectile dysfunction.14
Treatment outcomes. Most studies report positive psychological responses to ED reversal in men using PDE-5 inhibitors. Successful therapy has been associated with increased self-esteem, satisfaction, and sexual satisfaction.8 Some studies have found increased sexual satisfaction in the partner as well.15
Approximately 50% of PDE-5 inhibitor prescriptions are not refilled, however, and some case reports suggest that restored erectile function can result in:
- divorce and marital discord
- no change in partner-related activity.16
Recommendation. Because erectile problems are common in psychiatric patients, most psychiatrists should feel comfortable treating ED with PDE-5 inhibitors. In general, these agents are relatively safe. Reversing drug-induced sexual dysfunction may improve patients’ adherence to psychotropics, increase self-esteem, and improve relationships with sexual partners.
Hypoactive sexual desire
PDE-5 inhibitors. The success of PDE-5 inhibitors in treating ED led to investigations into whether these agents also could treat female sexual disorders. Large multisite trials using sildenafil failed to find evidence of efficacy in treating female hypoactive sexual desire or arousal disorders, however.18 Similarly, trials of topical prostaglandin E1 and alpha blockers were unsuccessful in women.
Some small studies suggested that PDE-5 inhibitors might be useful in:
- women with normal libido but decreased arousal19
- young women with normal libido and inability to reach orgasm.20
Testosterone therapy. In the 1940s, case reports suggested increased libido as a side effect when women were treated with androgens for metastatic cancer. In a prospective study, Sherwin and Gelfand21 found increased sexual desire, sexual arousal, and rates of coitus and orgasm in women injected with testosterone/estrogen preparations after surgical menopausal.
More recently, Shifren et al22 showed in a randomized, placebo-controlled trial that transdermal testosterone, 150 or 300 micrograms/day for 12 weeks, improved sexual function and psychological wellbeing in women age 31 to 56 after surgical menopause. Since then, numerous large, multisite, double-blind studies have demonstrated the efficacy of transdermal testosterone in treating low sexual desire in surgically menopausal women.23 Transdermal testosterone has been approved for this indication in the European Union but not in the United States.
Testosterone also has been reported to increase sexual desire in women who experienced natural menopause and in normal premenopausal women.24 The study of the relationship of testosterone to libido in women is complicated by numerous factors, however (Box 2).23,24
Other agents. Recent research has focused on centrally active compounds’ effect on libido. One agent in development is flibanserin, a serotonergic 5HT2 antagonist and a 5HT1a agonist. Data from large, multisite studies indicates that this compound increases libido in women with low sexual desire.
Transdermal testosterone can increase sexual desire in surgically menopausal women—according to numerous large, multisite, double-blind studies23—and has been approved for this indication in the European Union. Testosterone also has been reported to increase sexual desire in women who experienced natural menopause and in normal premenopausal women.24
In the United States, testosterone is not FDA-approved for treating low sexual desire. Considerable off-label use occurs, although long-term safety is unknown.
Various factors complicate the study of testosterone’s relationship to female libido:
- 3 large population studies have found minimal evidence of a relationship between endogenous androgenic activity and measures of female sexual responsiveness; these results may reflect an absence of such a relationship or methodologic weaknesses.
- The sensitivity and specificity of available assays to detect testosterone in women are seriously limited.
- Much of the active testosterone in the female body is made by intracellular conversion or testosterone precursors and thus may not be detected by assays of serum androgen levels.24
Some evidence suggests that bupropion—which has noradrenergic and dopaminergic agonist properties—increases orgasm completion and other measures of sexual responsiveness in women with hypoactive sexual desire disorders.25
The investigational compound bremelanotide—a synthetic version of melanocytes stimulating hormone—is administered intranasally and appears to acutely influence libido in women.26 Trials have been delayed because of this agent’s effects on blood pressure.
Herbal compounds. Some herbal compounds are being sold for low-desire complaints. Web sites for 2 herbal compounds, Ziti and Alibi, cite unpublished double-blind studies attesting to their efficacy. Because these studies are unpublished, one cannot evaluate their methodologies.
One double-blind study of the herbal compound ArginMax—which contains ginseng, ginkgo, damiana, L-arginine, and multivitamins—suggests efficacy in a small group of women with poorly specified sexual problems.27 Zestra, a topical herbal compound, has been evaluated in large multisite studies and found to be effective in increasing female sexual responsiveness.28
Other approaches. Some clinicians advocate using the testosterone precursor dehydroepiandrosterone (DHEA) for low sexual desire, although evidence does not support its efficacy.29
A battery-operated device is FDA -approved for treating sexual dysfunction in women. The clitoral vacuum increases vaginal engorgement and various indices of sexual responsiveness. This device’s target population is not clearly defined.30
When medication side effects are causing hypoactive sexual desire, consider substituting another drug or using antidotes such as buspirone or bupropion. Unfortunately, however, most sexual desire problems are idiopathic.
Testosterone therapy has been shown to improve libido, although it is not FDA-approved for this indication. Considerable off-label use occurs, but long-term safety is unknown.
Related resource
- Medline Plus. Sexual problems overview. www.nlm.nih.gov/medlineplus/ency/article/001951.htm.
- Bupropion • Wellbutrin
- Buspirone • BuSpar
- Cabergoline • Dostinex
- Clomipramine • Anafranil
- Fluoxetine • Prozac
- Lorazepam • Ativan
- Paroxetine • Paxil
- Phentolamine • Regitine
- Prostaglandin E1 • Liprostin
- Sertraline • Zoloft
- Sildenafil • Viagr
- Tadalafil • Cialis
- Vardenafil • Levitra
Dr. Segraves receives grant/research support from Pfizer, Boehringer Ingelheim, AstraZeneca, Bristol-Myers Squibb, and Novartis and is a consultant to Eli Lilly and Company, GlaxoSmithKline, Boehringer Ingelheim, and Bristol-Myers Squibb.
Sexual disorders such as premature ejaculation, erectile dysfunction (ED), and low libido reduce quality of life in patients with depression, anxiety, and other psychiatric illnesses. In addition, sexual dysfunction is a side effect of many drugs used to treat psychiatric disorders.1
Psychiatry—with its biopsychosocial model—can easily assume the evaluation and treatment of sexual disorders. To inform your practice, this article provides an update on pharmacotherapy for the 3 most common sexual disorders. Its emphasis on biologic treatment is not intended to minimize the importance of psychological interventions.
Premature ejaculation
Premature ejaculation is one of the most common male sexual complaints. In some surveys, approximately 30% of men express concern about ejaculating too rapidly.2 Behavioral therapy often is effective (Box 1), but in my experience most male patients prefer a pharmacologic approach to sexual problems.
Anesthetic creams. Locally applied anesthetic creams—such as prilocaine, lidocaine mixtures, and creams consisting of natural herbs—can increase ejaculatory latency by approximately 7 to 10 minutes. The major side effect of these preparations is penile hypoanesthesia. The man also must use a condom or wash off the cream before vaginal penetration to minimize vaginal absorption.3
- With male lying on back, partner strokes penis until male signals that ejaculation will occur with continued stimulation†
- Stroking stops, and erection is allowed to subside, then stroking resumes
- Repeat steps 1 and 2 four times, 2 times/week
- Ejaculatory latency will increase
- Partner assumes female-superior position and moves up and down until male indicates ejaculation is imminent
* Behavioral therapy for heterosexual couples. Oral-genital stimulation can be utilized between steps 4 and 5
† Frenulum squeeze technique is similar except that partner squeezes frenulum of penis at sign of male excitement
Among the SSRIs, paroxetine appears to have the greatest effect on ejaculatory latency (Table 1).5 Most trials have found that the dose needed to delay ejaculation is similar to the dose necessary to treat depressive disorders.
Some case reports suggest that phosphodiesterase type 5 inhibitors (PDE-5 inhibitors) may help to delay ejaculation, but this effect has not been borne out in double-blind studies.6
Table 1
Drug treatment options for premature ejaculation
| Drug | Dosage | Common side effects |
|---|---|---|
| Paroxetine | 20 to 40 mg/d | Nausea, headache |
| Clomipramine | 25 to 50 mg 4 to 6 hours before sexual activity | Nausea, fatigue |
| Lorazepam | 0.5 to 1 mg 30 minutes before sexual activity | Sedation |
| Source: Reference 5 | ||
Erectile dysfunction
Men with major depressive disorder, anxiety disorders, and psychotic disorders have higher rates of ED, compared with the general male population. ED also can be a side effect of—and adversely affect adherence to—antidepressant and antipsychotic therapy.7 Restored erectile function can positively affect patients’ self-esteem and sense of personal efficacy and may facilitate recovery from depression.8
PDE-5 inhibitors. Nitric oxide release triggers the production of cyclic guanosine monophosphate, which leads to decreased intracellular calcium, smooth muscle relaxation, and penile erection. All available PDE-5 inhibitors work by inhibiting the degradation of cyclic guanosine monophosphate. They are highly specific, vary somewhat in selectivity for other phosphodiesterase enzyme types, and differ in duration of action (Table 2).
Common side effects include dyspepsia, stuffy nose, and headache. The use of PDE-5 inhibitors with nitrates is contraindicated because of the risk of severe hypotension. Use PDE-5 inhibitors cautiously:
- with alpha blockers because of the risk of hypotension
- in men with aortic stenosis, recent myocardial infarction, unstable angina, heart failure, arrhythmias, degenerative retinal disease, or poorly controlled hypertension.9
Table 2
Duration of action of PDE-5 inhibitors
| Drug | Duration |
|---|---|
| Sildenafil | 4 hours |
| Vardenafil | 4 hours |
| Tadalafil | 24 to 36 hours |
| PDE-5: phosphodiesterase type 5 | |
Other options. Other accepted options for treating ED include intracavernosal injection of vasoactive substances such as phentolamine or prostaglandin E1, or intraurethral insertion of prostaglandins.12 Since the advent of PDE-5 inhibitors, these approaches are rarely used.
Cabergoline. Off-label use of dopaminergic agents such as cabergoline may be moderately effective in treating ED in men who do not respond adequately to PDE-5 inhibitors. Cabergoline is a dopamine D2 receptor agonist used to treat hyperprolactinemia and Parkinson’s disease.
In a randomized, double-blind, placebo- controlled study, 402 men who did not respond to sildenafil received cabergoline, 0.5 to 1 mg weekly for 6 months. Among the 370 men (92%) who completed the trial, mean weekly intercourse episodes increased from 1.4 to 2.2, compared with 1.2 to 1.4 in men who received placebo.13
Cabergoline also improved erectile function and sexual satisfaction in a randomized, double-blind, placebo-controlled trial of 50 men with psychogenic erectile dysfunction.14
Treatment outcomes. Most studies report positive psychological responses to ED reversal in men using PDE-5 inhibitors. Successful therapy has been associated with increased self-esteem, satisfaction, and sexual satisfaction.8 Some studies have found increased sexual satisfaction in the partner as well.15
Approximately 50% of PDE-5 inhibitor prescriptions are not refilled, however, and some case reports suggest that restored erectile function can result in:
- divorce and marital discord
- no change in partner-related activity.16
Recommendation. Because erectile problems are common in psychiatric patients, most psychiatrists should feel comfortable treating ED with PDE-5 inhibitors. In general, these agents are relatively safe. Reversing drug-induced sexual dysfunction may improve patients’ adherence to psychotropics, increase self-esteem, and improve relationships with sexual partners.
Hypoactive sexual desire
PDE-5 inhibitors. The success of PDE-5 inhibitors in treating ED led to investigations into whether these agents also could treat female sexual disorders. Large multisite trials using sildenafil failed to find evidence of efficacy in treating female hypoactive sexual desire or arousal disorders, however.18 Similarly, trials of topical prostaglandin E1 and alpha blockers were unsuccessful in women.
Some small studies suggested that PDE-5 inhibitors might be useful in:
- women with normal libido but decreased arousal19
- young women with normal libido and inability to reach orgasm.20
Testosterone therapy. In the 1940s, case reports suggested increased libido as a side effect when women were treated with androgens for metastatic cancer. In a prospective study, Sherwin and Gelfand21 found increased sexual desire, sexual arousal, and rates of coitus and orgasm in women injected with testosterone/estrogen preparations after surgical menopausal.
More recently, Shifren et al22 showed in a randomized, placebo-controlled trial that transdermal testosterone, 150 or 300 micrograms/day for 12 weeks, improved sexual function and psychological wellbeing in women age 31 to 56 after surgical menopause. Since then, numerous large, multisite, double-blind studies have demonstrated the efficacy of transdermal testosterone in treating low sexual desire in surgically menopausal women.23 Transdermal testosterone has been approved for this indication in the European Union but not in the United States.
Testosterone also has been reported to increase sexual desire in women who experienced natural menopause and in normal premenopausal women.24 The study of the relationship of testosterone to libido in women is complicated by numerous factors, however (Box 2).23,24
Other agents. Recent research has focused on centrally active compounds’ effect on libido. One agent in development is flibanserin, a serotonergic 5HT2 antagonist and a 5HT1a agonist. Data from large, multisite studies indicates that this compound increases libido in women with low sexual desire.
Transdermal testosterone can increase sexual desire in surgically menopausal women—according to numerous large, multisite, double-blind studies23—and has been approved for this indication in the European Union. Testosterone also has been reported to increase sexual desire in women who experienced natural menopause and in normal premenopausal women.24
In the United States, testosterone is not FDA-approved for treating low sexual desire. Considerable off-label use occurs, although long-term safety is unknown.
Various factors complicate the study of testosterone’s relationship to female libido:
- 3 large population studies have found minimal evidence of a relationship between endogenous androgenic activity and measures of female sexual responsiveness; these results may reflect an absence of such a relationship or methodologic weaknesses.
- The sensitivity and specificity of available assays to detect testosterone in women are seriously limited.
- Much of the active testosterone in the female body is made by intracellular conversion or testosterone precursors and thus may not be detected by assays of serum androgen levels.24
Some evidence suggests that bupropion—which has noradrenergic and dopaminergic agonist properties—increases orgasm completion and other measures of sexual responsiveness in women with hypoactive sexual desire disorders.25
The investigational compound bremelanotide—a synthetic version of melanocytes stimulating hormone—is administered intranasally and appears to acutely influence libido in women.26 Trials have been delayed because of this agent’s effects on blood pressure.
Herbal compounds. Some herbal compounds are being sold for low-desire complaints. Web sites for 2 herbal compounds, Ziti and Alibi, cite unpublished double-blind studies attesting to their efficacy. Because these studies are unpublished, one cannot evaluate their methodologies.
One double-blind study of the herbal compound ArginMax—which contains ginseng, ginkgo, damiana, L-arginine, and multivitamins—suggests efficacy in a small group of women with poorly specified sexual problems.27 Zestra, a topical herbal compound, has been evaluated in large multisite studies and found to be effective in increasing female sexual responsiveness.28
Other approaches. Some clinicians advocate using the testosterone precursor dehydroepiandrosterone (DHEA) for low sexual desire, although evidence does not support its efficacy.29
A battery-operated device is FDA -approved for treating sexual dysfunction in women. The clitoral vacuum increases vaginal engorgement and various indices of sexual responsiveness. This device’s target population is not clearly defined.30
When medication side effects are causing hypoactive sexual desire, consider substituting another drug or using antidotes such as buspirone or bupropion. Unfortunately, however, most sexual desire problems are idiopathic.
Testosterone therapy has been shown to improve libido, although it is not FDA-approved for this indication. Considerable off-label use occurs, but long-term safety is unknown.
Related resource
- Medline Plus. Sexual problems overview. www.nlm.nih.gov/medlineplus/ency/article/001951.htm.
- Bupropion • Wellbutrin
- Buspirone • BuSpar
- Cabergoline • Dostinex
- Clomipramine • Anafranil
- Fluoxetine • Prozac
- Lorazepam • Ativan
- Paroxetine • Paxil
- Phentolamine • Regitine
- Prostaglandin E1 • Liprostin
- Sertraline • Zoloft
- Sildenafil • Viagr
- Tadalafil • Cialis
- Vardenafil • Levitra
Dr. Segraves receives grant/research support from Pfizer, Boehringer Ingelheim, AstraZeneca, Bristol-Myers Squibb, and Novartis and is a consultant to Eli Lilly and Company, GlaxoSmithKline, Boehringer Ingelheim, and Bristol-Myers Squibb.
1. Segraves RT. Female sexual disorders: psychiatric aspects. Can J Psychiatry 2002;47:419-25.
2. Segraves RT. Rapid ejaculation: a review of nosology, prevalence and treatment. Int J Impot Res 2006;18(suppl 1):S24-S32.
3. Riley A, Segraves RT. Treatment of premature ejaculation. Int J Clin Pract 2006;60(6):694-7.
4. Duterte E, Segraves R, Althof S. Psychotherapy and pharmacotherapy of sexual dysfunctions. In: Nathan P, Gorman J, eds. A guide to treatments that work. New York, NY: Oxford University Press; 2007:531-60.
5. Waldinger M. Male ejaculation and orgasmic disorders. In: Balon R, Segraves RT, eds. Handbook of sexual dysfunction. Boca Raton, FL: Taylor & Francis; 2005:215-48.
6. McMahon C, Stuckey B, Andersen M, et al. Efficacy of sildenafil citrate (Viagra) in men with premature ejaculation. J Sex Med 2005;2:368-75.
7. Segraves R. Treatment of erectile dysfunction: a psychiatric perspective. Primary Psychiatry 2004;11(12):35-45.
8. Hartmann U. Depression and sexual dysfunction. J Men’s Health Gender 2007;4:18-25.
9. Shabsigh R, Seftel A, Rosen R, et al. Review of time of onset and duration of clinical efficacy of phosphodiesterase inhibitors in treatment of erectile dysfunction. Urology 2006;68:689-96.
10. Mukherjee B, Shivakumar T. A case of sensorineural deafness following ingestion of sildenafil. J Laryngol Otol 2007;121(4):395-7.
11. Pomeranz HD, Bhavsar AR. Nonarteritic ischemic optic neuropathy developing soon after use of sildenafil (Viagra): a report of seven new cases. J Neuroophthalmol 2005;25(1):9-13.
12. Padma-Nathan H, Christ G, Adaikan G, et al. Pharmacotherapy for erectile dysfunction. In: Lue T, Basson R, Rosen R, et al, eds. Sexual medicine: sexual dysfunctions in men and women. Paris, France: Health Publications; 2004:505-68.
13. Safarinejad M. Salvage of sildenafil failures with cabergoline: a randomized, double-blind, placebo-controlled study. Int J Impot Res 2006;18:550-8.
14. Nickel M, Moleda D, Loew T, et al. Cabergoline treatment in men with psychogenic erectile dysfunction: a randomized placebo-controlled study. Int J Impot Res 2007;19:164-7.
15. Montorsi F, Althof S. Partner responses to sildenafil citrate (Viagra) treatment of erectile dysfunction. Urology 2004;63:762-7.
16. Wise T. Psychosocial effects of sildenafil therapy for erectile dysfunction. J Sex Marital Ther 1999;25(2):145-50.
17. Pallas J, Levine SB, Althof SE, Risen CB. A study using Viagra in a mental health practice. J Sex Marital Ther 2000;26:41-50.
18. Basson R, McInnes R, Smith MD, et al. Efficacy and safety of sildenafil citrate in women with sexual dysfunction associated with female sexual arousal disorder. J Womens Health Gend Based Med 2002;11:367-77.
19. Berman JR, Berman LA, Toler SM, et al. Safety and efficacy of sildenafil citrate for the treatment of female sexual arousal disorder: a double-blind, placebo controlled study. J Urol 2003;170(6 Pt 1):2333-8.
20. Caruso S, Intelisano G, Lupo L, Agnello C. Premenopausal women affected by sexual arousal disorder treated with sildenafil: a double-blind, cross-over, placebo-controlled study. BJOG 2001;108:623-8.
21. Sherwin BB, Gelfand MM. The role of androgen in the maintenance of sexual functioning in oophorectomized women. Psychosom Med 1987;49:397-409.
22. Shifren JL, Braunstein GD, Simon JA, et al. Transdermal testosterone treatment in women with impaired sexual function after oophorectomy. N Engl J Med 2000;343:682-8.
23. Segraves RT, Woodard T. Female hypoactive sexual desire disorder: history and current status. J Sex Med 2006;3:408-18.
24. Basaria S, Dobs AS. Clinical review: controversies regarding transdermal androgen therapy in postmenopausal women. J Clin Endocrin Metab 2006;91:4743-52.
25. Segraves RT, Clayton A, Croft H, et al. Bupropion for the treatment of hypoactive sexual desire disorder in premenopausal women. J Clin Psychopharamacol 2004;24:339-42.
26. Pfaus J, Giuliano F, Gelez H. Bremelanotide: an overview of preclinical CNS effects on female sexual function. J Sex Med 2007;4(suppl 4):269-79.
27. Ito T, Polan M, Whipple B, Trant A. The enhancement of female sexual function with ArginMax, a nutritional supplement, among women suffering in menopausal status. J Sex Marit Ther 2006;32:359-78.
28. Ferguson DM, Steidle CP, Singh GS, et al. Randomized, placebo-controlled, double blind, crossover design trial of the efficacy and safety of Zestra for Women in women with and without female sexual arousal disorder. J Sex Marital Ther 2003;29(suppl 1):33-44.
29. Panjari M, Davis S. DHEA therapy for women: effect on sexual functioning and wellbeing. Hum Reprod Update 2007;13:239-48.
30. Feldman J, Striepe M. Women’s sexual health. Clinics in Family Practice 2004;6:839-61.
1. Segraves RT. Female sexual disorders: psychiatric aspects. Can J Psychiatry 2002;47:419-25.
2. Segraves RT. Rapid ejaculation: a review of nosology, prevalence and treatment. Int J Impot Res 2006;18(suppl 1):S24-S32.
3. Riley A, Segraves RT. Treatment of premature ejaculation. Int J Clin Pract 2006;60(6):694-7.
4. Duterte E, Segraves R, Althof S. Psychotherapy and pharmacotherapy of sexual dysfunctions. In: Nathan P, Gorman J, eds. A guide to treatments that work. New York, NY: Oxford University Press; 2007:531-60.
5. Waldinger M. Male ejaculation and orgasmic disorders. In: Balon R, Segraves RT, eds. Handbook of sexual dysfunction. Boca Raton, FL: Taylor & Francis; 2005:215-48.
6. McMahon C, Stuckey B, Andersen M, et al. Efficacy of sildenafil citrate (Viagra) in men with premature ejaculation. J Sex Med 2005;2:368-75.
7. Segraves R. Treatment of erectile dysfunction: a psychiatric perspective. Primary Psychiatry 2004;11(12):35-45.
8. Hartmann U. Depression and sexual dysfunction. J Men’s Health Gender 2007;4:18-25.
9. Shabsigh R, Seftel A, Rosen R, et al. Review of time of onset and duration of clinical efficacy of phosphodiesterase inhibitors in treatment of erectile dysfunction. Urology 2006;68:689-96.
10. Mukherjee B, Shivakumar T. A case of sensorineural deafness following ingestion of sildenafil. J Laryngol Otol 2007;121(4):395-7.
11. Pomeranz HD, Bhavsar AR. Nonarteritic ischemic optic neuropathy developing soon after use of sildenafil (Viagra): a report of seven new cases. J Neuroophthalmol 2005;25(1):9-13.
12. Padma-Nathan H, Christ G, Adaikan G, et al. Pharmacotherapy for erectile dysfunction. In: Lue T, Basson R, Rosen R, et al, eds. Sexual medicine: sexual dysfunctions in men and women. Paris, France: Health Publications; 2004:505-68.
13. Safarinejad M. Salvage of sildenafil failures with cabergoline: a randomized, double-blind, placebo-controlled study. Int J Impot Res 2006;18:550-8.
14. Nickel M, Moleda D, Loew T, et al. Cabergoline treatment in men with psychogenic erectile dysfunction: a randomized placebo-controlled study. Int J Impot Res 2007;19:164-7.
15. Montorsi F, Althof S. Partner responses to sildenafil citrate (Viagra) treatment of erectile dysfunction. Urology 2004;63:762-7.
16. Wise T. Psychosocial effects of sildenafil therapy for erectile dysfunction. J Sex Marital Ther 1999;25(2):145-50.
17. Pallas J, Levine SB, Althof SE, Risen CB. A study using Viagra in a mental health practice. J Sex Marital Ther 2000;26:41-50.
18. Basson R, McInnes R, Smith MD, et al. Efficacy and safety of sildenafil citrate in women with sexual dysfunction associated with female sexual arousal disorder. J Womens Health Gend Based Med 2002;11:367-77.
19. Berman JR, Berman LA, Toler SM, et al. Safety and efficacy of sildenafil citrate for the treatment of female sexual arousal disorder: a double-blind, placebo controlled study. J Urol 2003;170(6 Pt 1):2333-8.
20. Caruso S, Intelisano G, Lupo L, Agnello C. Premenopausal women affected by sexual arousal disorder treated with sildenafil: a double-blind, cross-over, placebo-controlled study. BJOG 2001;108:623-8.
21. Sherwin BB, Gelfand MM. The role of androgen in the maintenance of sexual functioning in oophorectomized women. Psychosom Med 1987;49:397-409.
22. Shifren JL, Braunstein GD, Simon JA, et al. Transdermal testosterone treatment in women with impaired sexual function after oophorectomy. N Engl J Med 2000;343:682-8.
23. Segraves RT, Woodard T. Female hypoactive sexual desire disorder: history and current status. J Sex Med 2006;3:408-18.
24. Basaria S, Dobs AS. Clinical review: controversies regarding transdermal androgen therapy in postmenopausal women. J Clin Endocrin Metab 2006;91:4743-52.
25. Segraves RT, Clayton A, Croft H, et al. Bupropion for the treatment of hypoactive sexual desire disorder in premenopausal women. J Clin Psychopharamacol 2004;24:339-42.
26. Pfaus J, Giuliano F, Gelez H. Bremelanotide: an overview of preclinical CNS effects on female sexual function. J Sex Med 2007;4(suppl 4):269-79.
27. Ito T, Polan M, Whipple B, Trant A. The enhancement of female sexual function with ArginMax, a nutritional supplement, among women suffering in menopausal status. J Sex Marit Ther 2006;32:359-78.
28. Ferguson DM, Steidle CP, Singh GS, et al. Randomized, placebo-controlled, double blind, crossover design trial of the efficacy and safety of Zestra for Women in women with and without female sexual arousal disorder. J Sex Marital Ther 2003;29(suppl 1):33-44.
29. Panjari M, Davis S. DHEA therapy for women: effect on sexual functioning and wellbeing. Hum Reprod Update 2007;13:239-48.
30. Feldman J, Striepe M. Women’s sexual health. Clinics in Family Practice 2004;6:839-61.
Insomnia in patients with addictions: A safer way to break the cycle
From alcohol to opioids, most addictive substances can induce sleep disturbances that persist despite abstinence and may increase the risk for relapse. Nearly all FDA-approved hypnotics are Schedule IV controlled substances that—although safe and effective for most populations—are prone to abuse by patients with substance use disorders.
You’re not alone if you hesitate to prescribe hypnotics to these patients; a study of 311 addiction medicine physicians found that they prescribed sleep-promoting medication to only 30% of their alcohol-dependent patients with insomnia.1
This article presents evidence on how alcohol and other substances disturb sleep in patients with addictions. We discuss the usefulness of hypnotics, off-label sedatives, and cognitive-behavioral therapy (CBT). Our goal is to help you reduce your patients’ risk of relapse by addressing their sleep complaints.
Workup: 3 principles
Insomnia is multifactorial. Don’t assume that substance abuse is the only cause of prominent insomnia complaints. Insomnia in patients with substance use disorders may be a manifestation of protracted withdrawal or a primary sleep disorder. Evaluate your patient’s:
- other illnesses (psychiatric, medical, and other sleep disorders)
- sleep-impairing medications (such as activating antidepressants and theophylline)
- inadequate sleep hygiene
- dysfunctional beliefs about sleep.
Insomnia is a clinical diagnosis that does not require an overnight sleep laboratory study (polysomnography [PSG]). Diagnose insomnia when a patient meets DSM-IV-TR criteria (has difficulty falling asleep or staying asleep or feels that sleep is not refreshing for at least 1 month; and the sleep problem impairs daytime functioning and/or causes clinically significant distress). In addition, consider:
- PSG if you suspect other sleep disorders, particularly obstructive sleep apnea (OSA) and periodic limb movement disorder (PLMD)
- an overnight sleep study for treatment-resistant insomnia, when you have adequately treated other causes.
Sleep logs are useful. Ask patients to keep a sleep log for 2 weeks during early recovery, after acute withdrawal subsides. These diaries help assess sleep patterns over time, document improvement with abstinence, and engage the patient in treatment. The National Sleep Foundation can provide examples (see Related Resources).
Alcohol and sleep disturbances
Insomnia is extremely common in active drinkers and in those who are in treatment after having stopped drinking. Across 7 studies of 1,577 alcohol-dependent patients undergoing treatment, more than one-half reported insomnia symptoms (mean 58%, range 36% to 91%),2,3 substantially higher than the rate in the general population (33%). Nicotine, marijuana, cocaine and other stimulants, and opioids also can disrupt sleep (Table 1).
Which came first? Sleep problems may be a pathway by which problematic substance use develops. In 1 study, sleep problems reported by mothers in boys ages 3 to 5 predicted onset of alcohol and drug use by ages 12 to 14.4 This relationship was not mediated by attention problems, anxiety/depression, or aggression. Thus, insomnia may increase the risk for early substance use.
In an epidemiologic study of >10,000 adults, the incidence of new alcohol use disorders after 1 year in those without psychiatric disorders at baseline was twice as high in persons with persistent insomnia as in those without insomnia.5
Patients with sleep disturbances may use alcohol to self-medicate,6 and tolerance to alcohol’s sedating effects develops quickly. As patients consume larger quantities with greater frequency to produce sleep, the risk for dependence may increase.
Comorbid sleep disorders. Alcohol-dependent patients with difficulty falling asleep may have abnormal circadian rhythms, as suggested by delayed onset of nocturnal melatonin secretion.7 They also may have low homeostatic sleep drive, another factor required to promote sleep.8
Habitual alcohol consumption before bedtime (1 to 3 standard drinks) is associated with mild sleep-disordered breathing (SDB) in men but not in women.9 SDB also may be more prevalent in alcohol-dependent men age >60.10
Consuming >2 drinks/day has been associated with restless legs and increased periodic limb movements during sleep. Twice as many women reporting high alcohol use were diagnosed with PLMD, compared with women reporting normal alcohol consumption.10-11 Recovering alcohol-dependent patients have significantly more periodic limb movements associated with arousals (PLMA) from sleep than controls. Moreover, PLMA can predict 80% of abstainers and 44% of relapsers after 6 months of abstinence.12
Table 1
Sleep disruptions caused by substances of abuse
| Substance | Effect on sleep |
|---|---|
| Nicotine | Difficulty falling asleep, sleep fragmentation, less restful sleep compared with nonsmokers, increased risk for OSA and SDBa-e |
| Marijuana | Short-term difficulty falling asleep and decreased slow-wave sleep percentage during withdrawalf-j |
| Cocaine | Prolonged sleep latency, decreased sleep efficiency, and decreased REM sleep with intranasal self-administration; hypersomnia during withdrawalk-m |
| Other stimulants (amphetamine, methamphetamine, methylphenidate) | Sleep complaints similar to those reported with cocaine use disordersn |
| Opioids | Decreased slow-wave sleep, increased stage-2 sleep, but minimal impact on sleep continuity; dreams and nightmares; central sleep apneao-t |
| OSA: obstructive sleep apnea; SDB: sleep-disordered breathing; REM: rapid eye movement | |
| Reference Citations: click here | |
Multifaceted treatment
A thorough history is essential to evaluate sleep and guide treatment decisions. Refer patients to an accredited sleep disorders center if their history shows:
- loud snoring
- cessation of breathing
- frequent kicking during sleep
- excessive daytime sleepiness.
Chronic insomnia. Patients who report chronic insomnia and behaviors incompatible with sleep may be good candidates for cognitive-behavioral therapy for insomnia (CBT-I). Patient education can change maladaptive behaviors, such as staying in bed for long periods of time to compensate for sleep loss, using the bed for activities other than sleep, or worrying excessively about sleep (Box 1).13
Pharmacotherapy may be preferred:
- for patients with unstable physical or mental illness
- when CBT-I could exacerbate a comorbid condition (such as restricting sleep in a patient with bipolar disorder)
- for patients with low motivation for behavior change
- when trained CBT-I providers or resources to pay for CBT-I are limited.
| Step 1. Get into bed to go to sleep only when you are sleepy |
| ↓ |
| Step 2. Avoid using the bed for activities other than sleep; for example, do not read, watch TV, eat, or worry in bed. Sexual activity is the only exception; on these occasions, follow the next steps when you intend to go to sleep |
| ↓ |
| Step 3. If you are unable to fall asleep within 15 to 20 minutes, get out of bed and go into another room. Remember, the goal is to associate your bed with falling asleep quickly. Return to bed intending to go to sleep only when you are very sleepy |
| ↓ |
| Step 4. While out of bed during the night, engage in activities that are quiet but of interest to you. Do not exercise, eat, smoke, or take warm showers or baths. Do not lie down or fall asleep when not in bed |
| ↓ |
| Step 5. If you return to bed and still cannot fall asleep within 15 to 20 minutes, repeat Step 3. Do this as often as necessary throughout the night |
| ↓ |
| Step 6. Set your alarm and get up at the same time every morning, regardless of how much sleep you got during the night. This will help your body acquire a sleep-wake rhythm |
| ↓ |
| Step 7. Do not nap during the day |
| Source. Adapted from Bootzin R, Nicassio P. Behavioral treatments for insomnia. In: Hersen M, Eissler R, Miller P, eds. Progress in behavior modification, vol. 6. New York: Academic Press; 1978:30 |
In older adults with insomnia but no history of addiction, CBT-I was more effective than placebo and as effective as a hypnotic alone (temazepam, 7.5 and 30 mg qhs) and a hypnotic/CBT-I combination in reducing nighttime wakefulness, increasing total sleep time, and increasing sleep efficiency. After 2 years, patients treated with CBT-I alone were most likely to maintain these initial treatment gains.15
Limited data exist on CBT-I’s effectiveness in patients with addiction. In 2 studies, alcohol-dependent patients reported improved sleep.16,17 CBT-I also improved measures of anxiety and depression, fatigue, and some quality-of-life items.16
Stimulus control (SC). Patients with chronic insomnia may watch television, talk on the telephone, or worry about not sleeping while lying in bed. The goal of SC is to alter this association by reestablishing the bed and bedroom with the pleasant experience of falling asleep and staying asleep.13 Instructions for SC (Box 1) are commonly provided with sleep restriction.
Sleep restriction (SR) addresses the excessive time that patients with insomnia spend in bed not sleeping. SR temporarily restricts time spent in bed and prohibits sleep at other times. The resulting mild sleep deprivation may promote consolidated sleep, leading to improved patient-reported sleep quality.14
Sleep hygiene (SH) addresses behaviors that may help or hinder sleep. Patients with addiction may benefit from learning how drug use and withdrawal affects sleep or how substance use for sleep may exacerbate sleep problems. Other SH recommendations include avoiding caffeine, nicotine, and exercise in close proximity to bedtime.
Cognitive therapy. Goals are to:
- identify and explore dysfunctional beliefs that cause patients anxiety about sleep problems
- replace these beliefs with more appropriate self-statements that promote sleep-healthy behaviors.
Common themes address patients’ unrealistic sleep expectations, inability to control or predict sleep, and faulty beliefs about sleep-promoting practices.
Precautions about hypnotics. The newer alpha-1-selective benzodiazepine receptor agonists (zolpidem, zaleplon, and eszopiclone) and the older nonselective benzodiazepines (such as flurazepam, temazepam, and triazolam) share an equivalent range of abuse liability.18 Consequently, all benzodiazepine receptor agonists are classified as Schedule IV controlled substances and should be used with caution, if at all, in substance-abusing or substance-dependent patients (Table 2).
In general, most physicians who specialize in treating addictions would not recommend these drug classes as first choice in postwithdrawal, substance-dependent patients complaining of chronic insomnia. Nevertheless, you are likely to encounter patients with a history of substance abuse/dependence who are taking legally prescribed benzodiazepine receptor agonists for insomnia, and they may be very reluctant to discontinue these medications.
Weigh and discuss with the patient the risks and benefits of taking vs discontinuing the hypnotic, as well as alternatives. Because chronic hypnotic use may interfere with addiction recovery, it is important to discuss the patient’s recovery plan.
If you decide to prescribe a hypnotic with abuse liability, the newer alpha-1-selective benzodiazepine receptor agonists are preferable—as they would be for non-addicted patients—because they are less likely to disrupt sleep architecture. They are also less likely than the long-acting benzodiazepines (such as flurazepam) to accumulate over time and result in daytime impairment.
Patient contracts. A written agreement can be useful whenever you prescribe a controlled substance for a patient with an addiction history. Include these issues:
- frequency of clinic visits for monitoring response and refills, requests for early refills, and telephone refills
- obtaining prescriptions from only one prescriber and one pharmacy
- abstinence from other abused substances
- urine drug screens and pill counts
- authorization for you to share information with other care providers or significant others
- an addiction recovery plan for other abused substances
- consequences of nonadherence.
Table 2
FDA-approved benzodiazepine receptor agonists for insomnia*
| Agent | Dose range (mg) | TMAX (hr) | T½ (hr) |
|---|---|---|---|
| Benzodiazepine receptor agonists (benzodiazepine structures) | |||
| Estazolam | 1 to 2 | 0.5 to 1.6 | 10 to 24 |
| Flurazepam | 15 to 30 | 3 to 6 | 50 to 100† |
| Quazepam | 7.5 to 15 | 2 | 25 to 100† |
| Temazepam | 15 to 30 | 2 to 3 | 10 to 17 |
| Triazolam | 0.125 to 0.5 | 1 to 2 | 1.5 to 5.5 |
| Selective benzodiazepine receptor agonists (nonbenzodiazepine structures)‡ | |||
| Eszopiclone | 1 to 3 | 1 | ~6 |
| Zaleplon | 5 to 20 | 1 | ~1 |
| Zolpidem | 5 to 10 | 1.6 | 2.5 (1.5 to 3.8) |
| Zolpidem CR | 6.25 to 12.5 | 1.5 | 2.8 (1.6 to 4) |
| TMAX: time to reach maximal plasma concentrations; T½: elimination half-life (all values are approximate for any given individual) | |||
| * All benzodiazepine receptor agonists are Schedule IV controlled substances. Use with caution, if at all, in alcohol-dependent patients | |||
| † Including active metabolites | |||
| ‡ Selective GABAA receptor agonists bind the alpha-1 protein subunit of GABAA receptors. Alpha-1 containing GABAA receptors are thought to mediate sedative and amnesic effects but not antianxiety or muscle relaxant effects of the GABA system | |||
Off-label sedatives for insomnia
Like ramelteon, sedating agents that do not have abuse liability are first-choice medications for patients with addiction and co-occurring insomnia (Table 3):
- The most studied are gabapentin and trazodone.
- Quetiapine and mirtazapine may be considered as second-choice options.
In 2 open-label pilot studies of alcohol-dependent patients with insomnia:
- gabapentin (mean dose 953 mg) significantly improved sleep quality over 4 to 6 weeks20
- both gabapentin (mean 888 mg qhs) and trazodone (mean 105 mg qhs) significantly improved Sleep Problems Questionnaire scores, but patients receiving gabapentin were less likely than those taking trazodone to feel tired upon awakening.21
Although gabapentin and the anticonvulsant pregabalin increase slow-wave sleep in healthy control subjects, evidence of a similar effect is lacking in alcohol-dependent patients.
Trazodone is the most commonly prescribed antidepressant for insomnia because of its sedating effect and low abuse potential. Trazodone was associated with greater sleep improvements vs placebo as measured via PSG in a randomized, double-blind trial of alcohol-dependent patients with insomnia.23 In a second study, sleep outcomes were better with trazodone vs placebo over 12 weeks in alcohol-dependent patients, although patients in the trazodone group drank more heavily.24
Other sedating antidepressants such as mirtazapine and doxepin have not been studied in patients with substance use disorders.
Quetiapine is a second-generation antipsychotic with sedating properties. When quetiapine, 25 to 200 mg/d, was given to alcohol-dependent veterans with sleep complaints, they remained abstinent more days and had fewer hospitalizations than veterans not receiving quetiapine.25 Both groups had high rates of psychiatric comorbidity, and 90% had posttraumatic stress disorder. Improved abstinence was thought to result from improved sleep, but no sleep measures were included to test this hypothesis.
A recently published, randomized controlled pilot study reported significantly reduced drinking and craving in severely alcohol-dependent patients receiving quetiapine vs placebo, although sleep data were not included.26
Other options. Tricyclic antidepressants carry risks of cardiotoxicity and other side effects but can be useful when other options have not worked or patients have comorbidities such as neuropathic pain or migraine headaches. Combinations of agents also may be considered for treatment-resistant insomnia.
Nonprescription remedies such as antihistamines, valerian root extract (from the herb Valeriana officinalis), and melatonin are commonly used for sleep, although data are limited in substance-abusing patients.
Table 3
Noncontrolled sedating agents for treating insomnia
in patients with a history of substance abuse
| Agent | Dose range (mg) | TMAX (hr) | T½ (hr) |
|---|---|---|---|
| Melatonin receptor agonist | |||
| Ramelteon | 8 | 0.5 to 1.5 | 1 to 2.6 |
| Sedating anticonvulsant | |||
| Gabapentin | 300 to 1,500 | 2 to 3 | 6 to 7 |
| Sedating antidepressants | |||
| Amitriptyline† | 25 to 150 | 2 to 8 | 5 to 45 |
| Doxepin† | 25 to 150 | 2 to 8 | 10 to 30 |
| Mirtazapine | 7.5 to 45‡ | 1 to 3 | 20 to 40 |
| Nefazodone | 50 to 150 | 1 | 6 to 18* |
| Nortriptyline† | 10 to 75§ | 2 to 8 | 20 to 55 |
| Trazodone | 25 to 300 | 1 to 2 | 3 to 9¶ |
| Sedating second-generation antipsychotic | |||
| Quetiapine | 25 to 100 | 1.5 | 6 |
| TMAX: time to reach maximal plasma concentrations; T½: elimination half-life (all values are approximate for any given individual) | |||
| * Including active metabolites | |||
| † Tricyclic antidepressants | |||
| ‡ Antihistaminergic effects predominate at low doses (7.5 to 15 mg) | |||
| § Can be titrated to morning serum level (50 to 150 mcg/mL) 12 hr after bedtime dose if no effect at lower doses | |||
| ¶ Major metabolite, mCPP, has 14-hour half-life | |||
- American Academy of Sleep Medicine. www.sleepeducation.com.
- National Sleep Foundation. Sleep logs for downloading. www.sleepfoundation.org.
- Restless Legs Syndrome Foundation. www.rls.org.
- Brower KJ. Insomnia, alcoholism and relapse. Sleep Med Rev 2003;7:523-39.
- Amitriptyline • Elavil, Endep
- Doxepin • Sinequan
- Estazolam • ProSom
- Eszopiclone • Lunesta
- Flurazepam • Dalmane
- Gabapentin • Neurontin
- Methamphetamine • Desoxyn
- Methylphenidate • Concerta, Ritalin, others
- Mirtazapine • Remeron
- Nefazodone • Serzone
- Nortriptyline • Pamelor
- Pregabalin • Lyrica
- Quazepam • Doral
- Quetiapine • Seroquel
- Ramelteon • Rozerem
- Temazepam • Restoril
- Theophylline • Theo-24, others
- Trazodone • Desyrel
- Triazolam • Halcion
- Zaleplon • Sonata
- Zolpidem • Ambien, Ambien CR
Dr. Conroy and Dr. Arnedt report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products. Dr. Brower is a consultant to Pfizer.
Acknowledgment
This work was supported by an NIH grant to Dr. Brower (2K24 AA00304).
1. Friedmann PD, Herman DS, Freedman S, et al. Treatment of sleep disturbance in alcohol recovery: a national survey of addiction medicine physicians. J Addict Dis 2003;22:91-103.
2. Brower KJ. Alcohol’s effects on sleep in alcoholics. Alcohol Res Health 2001;25:110-25.
3. Cohn TJ, Foster JH, Peters TJ. Sequential studies of sleep disturbance and quality of life in abstaining alcoholics. Addict Biol 2003;8(4):455-62.
4. Wong MM, Brower KJ, Fitzgerald HE, Zucker RA. Sleep problems in early childhood and early onset of alcohol and other drug use in adolescence. Alcohol Clin Exp Res 2004;28(4):578-87.
5. Weissman MM, Greenwald S, Nino-Murcia G, Dement WC. The morbidity of insomnia uncomplicated by psychiatric disorders. Gen Hosp Psychiatry 1997;19:245-50.
6. Brower KJ, Aldrich MS, Robinson EAR, et al. Insomnia, self-medication, and relapse to alcoholism. Am J Psychiatry 2001;158:399-404.
7. Kuhlwein E, Hauger RL, Irwin MR. Abnormal nocturnal melatonin secretion and disordered sleep in abstinent alcoholics. Biol Psychiatry 2003;54(12):1437-43.
8. Irwin M, Gillin JC, Dang J, et al. Sleep deprivation as a probe of homeostatic sleep regulation in primary alcoholics. Biol Psychiatry 2002;51(8):632-41.
9. Peppard PE, Austin D, Brown RL. Association of alcohol consumption and sleep disordered breathing in men and women. J Clin Sleep Med 2007;3(3):265-70.
10. Aldrich MS, Shipley JE, Tandon R, et al. Sleep-disordered breathing in alcoholics: association with age. Alcohol Clin Exp Res 1993;17:1179-83.
11. Aldrich MS, Shipley JE. Alcohol use and periodic limb movements of sleep. Alcohol Clin Exp Res 1993;17:192-6.
12. Gann H, Feige B, Fasihi S, et al. Periodic limb movements during sleep in alcohol dependent patients. Eur Arch Psychiatry Clin Neurosci 2002;252(3):124-9.
13. Bootzin R, Nicassio P. Behavioral treatments for insomnia. In: Hersen M, Eissler R, Miller P, eds. Progress in behavior modification. Vol. 6. New York, NY: Academic Press; 1978:1-45.
14. Spielman AJ, Saskin P, Thorpy MJ. Treatment of chronic insomnia by restriction of time in bed. Sleep 1987;10:45-55.
15. Morin CM, Colecchi C, Stone J, et al. Behavioral and pharmacological therapies for late-life insomnia: a randomized controlled trial. JAMA 1999;281(11):991-9.
16. Arnedt JT, Conroy D, Rutt J, et al. An open trial of cognitivebehavioral treatment for insomnia comorbid with alcohol dependence. Sleep Med 2007;8:176-80.
17. Currie SR, Clark S, Hodgins DC, el-Guebaly N. Randomized controlled trial of brief cognitive-behavioural interventions for insomnia in recovering alcoholics. Addiction 2004;99:1121-32.
18. Griffiths RR, Johnson MW. Relative abuse liability of hypnotic drugs: a conceptual framework and algorithm for differentiating among compounds. J Clin Psychiatry 2005;66(suppl 9):31-41.
19. Johnson MW, Suess PE, Griffiths RR. Ramelteon: a novel hypnotic lacking abuse liability and sedative adverse side effects. Arch Gen Psychiatry 2006;63:1149-57.
20. Karam-Hage M, Brower KJ. Gabapentin treatment for insomnia associated with alcohol dependence [letter]. Am J Psychiatry 2000;157:151.-
21. Karam-Hage M, Brower KJ. Open pilot study of gabapentin versus trazodone to treat insomnia in alcoholic outpatients. Psychiatry Clini Neurosci 2003;57:542-4.
22. Brower KJ, Kim HM, Karam-Hage M, et al. A double-blind randomized clinical trial of gabapentin vs. placebo for treating alcohol dependence. Biol Psychiatry 2003;53(8S):84S-85S.
23. Le Bon O, Murphy JR, Staner L, et al. Double-blind, placebo-controlled study of the efficacy of trazodone in alcohol post-withdrawal syndrome: polysomnographic and clinical evaluations. J Clin Psychopharmacol 2003;23(4):377-83.
24. Friedmann PD, Rose JS, Swift RM, et al. Trazodone for sleep disturbance after detoxification from alcohol dependence: a double-blind, placebo-controlled trial. Paper presented at: Annual Meeting of the American Academy of Addiction Psychiatry, December 1-2, 2007; Coronado, CA.
25. Monnelly EP, Ciraulo DA, Knapp C, et al. Quetiapine for treatment of alcohol dependence. J Clin Psychopharmacol 2004;24(5):532-5.
26. Kampman KM, Pettinati HM, Lynch KG, et al. A double-blind, placebo-controlled pilot trial of quetiapine for the treatment of Type A and Type B alcoholism. J Clin Psychopharmacol 2007;27:344-51.
From alcohol to opioids, most addictive substances can induce sleep disturbances that persist despite abstinence and may increase the risk for relapse. Nearly all FDA-approved hypnotics are Schedule IV controlled substances that—although safe and effective for most populations—are prone to abuse by patients with substance use disorders.
You’re not alone if you hesitate to prescribe hypnotics to these patients; a study of 311 addiction medicine physicians found that they prescribed sleep-promoting medication to only 30% of their alcohol-dependent patients with insomnia.1
This article presents evidence on how alcohol and other substances disturb sleep in patients with addictions. We discuss the usefulness of hypnotics, off-label sedatives, and cognitive-behavioral therapy (CBT). Our goal is to help you reduce your patients’ risk of relapse by addressing their sleep complaints.
Workup: 3 principles
Insomnia is multifactorial. Don’t assume that substance abuse is the only cause of prominent insomnia complaints. Insomnia in patients with substance use disorders may be a manifestation of protracted withdrawal or a primary sleep disorder. Evaluate your patient’s:
- other illnesses (psychiatric, medical, and other sleep disorders)
- sleep-impairing medications (such as activating antidepressants and theophylline)
- inadequate sleep hygiene
- dysfunctional beliefs about sleep.
Insomnia is a clinical diagnosis that does not require an overnight sleep laboratory study (polysomnography [PSG]). Diagnose insomnia when a patient meets DSM-IV-TR criteria (has difficulty falling asleep or staying asleep or feels that sleep is not refreshing for at least 1 month; and the sleep problem impairs daytime functioning and/or causes clinically significant distress). In addition, consider:
- PSG if you suspect other sleep disorders, particularly obstructive sleep apnea (OSA) and periodic limb movement disorder (PLMD)
- an overnight sleep study for treatment-resistant insomnia, when you have adequately treated other causes.
Sleep logs are useful. Ask patients to keep a sleep log for 2 weeks during early recovery, after acute withdrawal subsides. These diaries help assess sleep patterns over time, document improvement with abstinence, and engage the patient in treatment. The National Sleep Foundation can provide examples (see Related Resources).
Alcohol and sleep disturbances
Insomnia is extremely common in active drinkers and in those who are in treatment after having stopped drinking. Across 7 studies of 1,577 alcohol-dependent patients undergoing treatment, more than one-half reported insomnia symptoms (mean 58%, range 36% to 91%),2,3 substantially higher than the rate in the general population (33%). Nicotine, marijuana, cocaine and other stimulants, and opioids also can disrupt sleep (Table 1).
Which came first? Sleep problems may be a pathway by which problematic substance use develops. In 1 study, sleep problems reported by mothers in boys ages 3 to 5 predicted onset of alcohol and drug use by ages 12 to 14.4 This relationship was not mediated by attention problems, anxiety/depression, or aggression. Thus, insomnia may increase the risk for early substance use.
In an epidemiologic study of >10,000 adults, the incidence of new alcohol use disorders after 1 year in those without psychiatric disorders at baseline was twice as high in persons with persistent insomnia as in those without insomnia.5
Patients with sleep disturbances may use alcohol to self-medicate,6 and tolerance to alcohol’s sedating effects develops quickly. As patients consume larger quantities with greater frequency to produce sleep, the risk for dependence may increase.
Comorbid sleep disorders. Alcohol-dependent patients with difficulty falling asleep may have abnormal circadian rhythms, as suggested by delayed onset of nocturnal melatonin secretion.7 They also may have low homeostatic sleep drive, another factor required to promote sleep.8
Habitual alcohol consumption before bedtime (1 to 3 standard drinks) is associated with mild sleep-disordered breathing (SDB) in men but not in women.9 SDB also may be more prevalent in alcohol-dependent men age >60.10
Consuming >2 drinks/day has been associated with restless legs and increased periodic limb movements during sleep. Twice as many women reporting high alcohol use were diagnosed with PLMD, compared with women reporting normal alcohol consumption.10-11 Recovering alcohol-dependent patients have significantly more periodic limb movements associated with arousals (PLMA) from sleep than controls. Moreover, PLMA can predict 80% of abstainers and 44% of relapsers after 6 months of abstinence.12
Table 1
Sleep disruptions caused by substances of abuse
| Substance | Effect on sleep |
|---|---|
| Nicotine | Difficulty falling asleep, sleep fragmentation, less restful sleep compared with nonsmokers, increased risk for OSA and SDBa-e |
| Marijuana | Short-term difficulty falling asleep and decreased slow-wave sleep percentage during withdrawalf-j |
| Cocaine | Prolonged sleep latency, decreased sleep efficiency, and decreased REM sleep with intranasal self-administration; hypersomnia during withdrawalk-m |
| Other stimulants (amphetamine, methamphetamine, methylphenidate) | Sleep complaints similar to those reported with cocaine use disordersn |
| Opioids | Decreased slow-wave sleep, increased stage-2 sleep, but minimal impact on sleep continuity; dreams and nightmares; central sleep apneao-t |
| OSA: obstructive sleep apnea; SDB: sleep-disordered breathing; REM: rapid eye movement | |
| Reference Citations: click here | |
Multifaceted treatment
A thorough history is essential to evaluate sleep and guide treatment decisions. Refer patients to an accredited sleep disorders center if their history shows:
- loud snoring
- cessation of breathing
- frequent kicking during sleep
- excessive daytime sleepiness.
Chronic insomnia. Patients who report chronic insomnia and behaviors incompatible with sleep may be good candidates for cognitive-behavioral therapy for insomnia (CBT-I). Patient education can change maladaptive behaviors, such as staying in bed for long periods of time to compensate for sleep loss, using the bed for activities other than sleep, or worrying excessively about sleep (Box 1).13
Pharmacotherapy may be preferred:
- for patients with unstable physical or mental illness
- when CBT-I could exacerbate a comorbid condition (such as restricting sleep in a patient with bipolar disorder)
- for patients with low motivation for behavior change
- when trained CBT-I providers or resources to pay for CBT-I are limited.
| Step 1. Get into bed to go to sleep only when you are sleepy |
| ↓ |
| Step 2. Avoid using the bed for activities other than sleep; for example, do not read, watch TV, eat, or worry in bed. Sexual activity is the only exception; on these occasions, follow the next steps when you intend to go to sleep |
| ↓ |
| Step 3. If you are unable to fall asleep within 15 to 20 minutes, get out of bed and go into another room. Remember, the goal is to associate your bed with falling asleep quickly. Return to bed intending to go to sleep only when you are very sleepy |
| ↓ |
| Step 4. While out of bed during the night, engage in activities that are quiet but of interest to you. Do not exercise, eat, smoke, or take warm showers or baths. Do not lie down or fall asleep when not in bed |
| ↓ |
| Step 5. If you return to bed and still cannot fall asleep within 15 to 20 minutes, repeat Step 3. Do this as often as necessary throughout the night |
| ↓ |
| Step 6. Set your alarm and get up at the same time every morning, regardless of how much sleep you got during the night. This will help your body acquire a sleep-wake rhythm |
| ↓ |
| Step 7. Do not nap during the day |
| Source. Adapted from Bootzin R, Nicassio P. Behavioral treatments for insomnia. In: Hersen M, Eissler R, Miller P, eds. Progress in behavior modification, vol. 6. New York: Academic Press; 1978:30 |
In older adults with insomnia but no history of addiction, CBT-I was more effective than placebo and as effective as a hypnotic alone (temazepam, 7.5 and 30 mg qhs) and a hypnotic/CBT-I combination in reducing nighttime wakefulness, increasing total sleep time, and increasing sleep efficiency. After 2 years, patients treated with CBT-I alone were most likely to maintain these initial treatment gains.15
Limited data exist on CBT-I’s effectiveness in patients with addiction. In 2 studies, alcohol-dependent patients reported improved sleep.16,17 CBT-I also improved measures of anxiety and depression, fatigue, and some quality-of-life items.16
Stimulus control (SC). Patients with chronic insomnia may watch television, talk on the telephone, or worry about not sleeping while lying in bed. The goal of SC is to alter this association by reestablishing the bed and bedroom with the pleasant experience of falling asleep and staying asleep.13 Instructions for SC (Box 1) are commonly provided with sleep restriction.
Sleep restriction (SR) addresses the excessive time that patients with insomnia spend in bed not sleeping. SR temporarily restricts time spent in bed and prohibits sleep at other times. The resulting mild sleep deprivation may promote consolidated sleep, leading to improved patient-reported sleep quality.14
Sleep hygiene (SH) addresses behaviors that may help or hinder sleep. Patients with addiction may benefit from learning how drug use and withdrawal affects sleep or how substance use for sleep may exacerbate sleep problems. Other SH recommendations include avoiding caffeine, nicotine, and exercise in close proximity to bedtime.
Cognitive therapy. Goals are to:
- identify and explore dysfunctional beliefs that cause patients anxiety about sleep problems
- replace these beliefs with more appropriate self-statements that promote sleep-healthy behaviors.
Common themes address patients’ unrealistic sleep expectations, inability to control or predict sleep, and faulty beliefs about sleep-promoting practices.
Precautions about hypnotics. The newer alpha-1-selective benzodiazepine receptor agonists (zolpidem, zaleplon, and eszopiclone) and the older nonselective benzodiazepines (such as flurazepam, temazepam, and triazolam) share an equivalent range of abuse liability.18 Consequently, all benzodiazepine receptor agonists are classified as Schedule IV controlled substances and should be used with caution, if at all, in substance-abusing or substance-dependent patients (Table 2).
In general, most physicians who specialize in treating addictions would not recommend these drug classes as first choice in postwithdrawal, substance-dependent patients complaining of chronic insomnia. Nevertheless, you are likely to encounter patients with a history of substance abuse/dependence who are taking legally prescribed benzodiazepine receptor agonists for insomnia, and they may be very reluctant to discontinue these medications.
Weigh and discuss with the patient the risks and benefits of taking vs discontinuing the hypnotic, as well as alternatives. Because chronic hypnotic use may interfere with addiction recovery, it is important to discuss the patient’s recovery plan.
If you decide to prescribe a hypnotic with abuse liability, the newer alpha-1-selective benzodiazepine receptor agonists are preferable—as they would be for non-addicted patients—because they are less likely to disrupt sleep architecture. They are also less likely than the long-acting benzodiazepines (such as flurazepam) to accumulate over time and result in daytime impairment.
Patient contracts. A written agreement can be useful whenever you prescribe a controlled substance for a patient with an addiction history. Include these issues:
- frequency of clinic visits for monitoring response and refills, requests for early refills, and telephone refills
- obtaining prescriptions from only one prescriber and one pharmacy
- abstinence from other abused substances
- urine drug screens and pill counts
- authorization for you to share information with other care providers or significant others
- an addiction recovery plan for other abused substances
- consequences of nonadherence.
Table 2
FDA-approved benzodiazepine receptor agonists for insomnia*
| Agent | Dose range (mg) | TMAX (hr) | T½ (hr) |
|---|---|---|---|
| Benzodiazepine receptor agonists (benzodiazepine structures) | |||
| Estazolam | 1 to 2 | 0.5 to 1.6 | 10 to 24 |
| Flurazepam | 15 to 30 | 3 to 6 | 50 to 100† |
| Quazepam | 7.5 to 15 | 2 | 25 to 100† |
| Temazepam | 15 to 30 | 2 to 3 | 10 to 17 |
| Triazolam | 0.125 to 0.5 | 1 to 2 | 1.5 to 5.5 |
| Selective benzodiazepine receptor agonists (nonbenzodiazepine structures)‡ | |||
| Eszopiclone | 1 to 3 | 1 | ~6 |
| Zaleplon | 5 to 20 | 1 | ~1 |
| Zolpidem | 5 to 10 | 1.6 | 2.5 (1.5 to 3.8) |
| Zolpidem CR | 6.25 to 12.5 | 1.5 | 2.8 (1.6 to 4) |
| TMAX: time to reach maximal plasma concentrations; T½: elimination half-life (all values are approximate for any given individual) | |||
| * All benzodiazepine receptor agonists are Schedule IV controlled substances. Use with caution, if at all, in alcohol-dependent patients | |||
| † Including active metabolites | |||
| ‡ Selective GABAA receptor agonists bind the alpha-1 protein subunit of GABAA receptors. Alpha-1 containing GABAA receptors are thought to mediate sedative and amnesic effects but not antianxiety or muscle relaxant effects of the GABA system | |||
Off-label sedatives for insomnia
Like ramelteon, sedating agents that do not have abuse liability are first-choice medications for patients with addiction and co-occurring insomnia (Table 3):
- The most studied are gabapentin and trazodone.
- Quetiapine and mirtazapine may be considered as second-choice options.
In 2 open-label pilot studies of alcohol-dependent patients with insomnia:
- gabapentin (mean dose 953 mg) significantly improved sleep quality over 4 to 6 weeks20
- both gabapentin (mean 888 mg qhs) and trazodone (mean 105 mg qhs) significantly improved Sleep Problems Questionnaire scores, but patients receiving gabapentin were less likely than those taking trazodone to feel tired upon awakening.21
Although gabapentin and the anticonvulsant pregabalin increase slow-wave sleep in healthy control subjects, evidence of a similar effect is lacking in alcohol-dependent patients.
Trazodone is the most commonly prescribed antidepressant for insomnia because of its sedating effect and low abuse potential. Trazodone was associated with greater sleep improvements vs placebo as measured via PSG in a randomized, double-blind trial of alcohol-dependent patients with insomnia.23 In a second study, sleep outcomes were better with trazodone vs placebo over 12 weeks in alcohol-dependent patients, although patients in the trazodone group drank more heavily.24
Other sedating antidepressants such as mirtazapine and doxepin have not been studied in patients with substance use disorders.
Quetiapine is a second-generation antipsychotic with sedating properties. When quetiapine, 25 to 200 mg/d, was given to alcohol-dependent veterans with sleep complaints, they remained abstinent more days and had fewer hospitalizations than veterans not receiving quetiapine.25 Both groups had high rates of psychiatric comorbidity, and 90% had posttraumatic stress disorder. Improved abstinence was thought to result from improved sleep, but no sleep measures were included to test this hypothesis.
A recently published, randomized controlled pilot study reported significantly reduced drinking and craving in severely alcohol-dependent patients receiving quetiapine vs placebo, although sleep data were not included.26
Other options. Tricyclic antidepressants carry risks of cardiotoxicity and other side effects but can be useful when other options have not worked or patients have comorbidities such as neuropathic pain or migraine headaches. Combinations of agents also may be considered for treatment-resistant insomnia.
Nonprescription remedies such as antihistamines, valerian root extract (from the herb Valeriana officinalis), and melatonin are commonly used for sleep, although data are limited in substance-abusing patients.
Table 3
Noncontrolled sedating agents for treating insomnia
in patients with a history of substance abuse
| Agent | Dose range (mg) | TMAX (hr) | T½ (hr) |
|---|---|---|---|
| Melatonin receptor agonist | |||
| Ramelteon | 8 | 0.5 to 1.5 | 1 to 2.6 |
| Sedating anticonvulsant | |||
| Gabapentin | 300 to 1,500 | 2 to 3 | 6 to 7 |
| Sedating antidepressants | |||
| Amitriptyline† | 25 to 150 | 2 to 8 | 5 to 45 |
| Doxepin† | 25 to 150 | 2 to 8 | 10 to 30 |
| Mirtazapine | 7.5 to 45‡ | 1 to 3 | 20 to 40 |
| Nefazodone | 50 to 150 | 1 | 6 to 18* |
| Nortriptyline† | 10 to 75§ | 2 to 8 | 20 to 55 |
| Trazodone | 25 to 300 | 1 to 2 | 3 to 9¶ |
| Sedating second-generation antipsychotic | |||
| Quetiapine | 25 to 100 | 1.5 | 6 |
| TMAX: time to reach maximal plasma concentrations; T½: elimination half-life (all values are approximate for any given individual) | |||
| * Including active metabolites | |||
| † Tricyclic antidepressants | |||
| ‡ Antihistaminergic effects predominate at low doses (7.5 to 15 mg) | |||
| § Can be titrated to morning serum level (50 to 150 mcg/mL) 12 hr after bedtime dose if no effect at lower doses | |||
| ¶ Major metabolite, mCPP, has 14-hour half-life | |||
- American Academy of Sleep Medicine. www.sleepeducation.com.
- National Sleep Foundation. Sleep logs for downloading. www.sleepfoundation.org.
- Restless Legs Syndrome Foundation. www.rls.org.
- Brower KJ. Insomnia, alcoholism and relapse. Sleep Med Rev 2003;7:523-39.
- Amitriptyline • Elavil, Endep
- Doxepin • Sinequan
- Estazolam • ProSom
- Eszopiclone • Lunesta
- Flurazepam • Dalmane
- Gabapentin • Neurontin
- Methamphetamine • Desoxyn
- Methylphenidate • Concerta, Ritalin, others
- Mirtazapine • Remeron
- Nefazodone • Serzone
- Nortriptyline • Pamelor
- Pregabalin • Lyrica
- Quazepam • Doral
- Quetiapine • Seroquel
- Ramelteon • Rozerem
- Temazepam • Restoril
- Theophylline • Theo-24, others
- Trazodone • Desyrel
- Triazolam • Halcion
- Zaleplon • Sonata
- Zolpidem • Ambien, Ambien CR
Dr. Conroy and Dr. Arnedt report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products. Dr. Brower is a consultant to Pfizer.
Acknowledgment
This work was supported by an NIH grant to Dr. Brower (2K24 AA00304).
From alcohol to opioids, most addictive substances can induce sleep disturbances that persist despite abstinence and may increase the risk for relapse. Nearly all FDA-approved hypnotics are Schedule IV controlled substances that—although safe and effective for most populations—are prone to abuse by patients with substance use disorders.
You’re not alone if you hesitate to prescribe hypnotics to these patients; a study of 311 addiction medicine physicians found that they prescribed sleep-promoting medication to only 30% of their alcohol-dependent patients with insomnia.1
This article presents evidence on how alcohol and other substances disturb sleep in patients with addictions. We discuss the usefulness of hypnotics, off-label sedatives, and cognitive-behavioral therapy (CBT). Our goal is to help you reduce your patients’ risk of relapse by addressing their sleep complaints.
Workup: 3 principles
Insomnia is multifactorial. Don’t assume that substance abuse is the only cause of prominent insomnia complaints. Insomnia in patients with substance use disorders may be a manifestation of protracted withdrawal or a primary sleep disorder. Evaluate your patient’s:
- other illnesses (psychiatric, medical, and other sleep disorders)
- sleep-impairing medications (such as activating antidepressants and theophylline)
- inadequate sleep hygiene
- dysfunctional beliefs about sleep.
Insomnia is a clinical diagnosis that does not require an overnight sleep laboratory study (polysomnography [PSG]). Diagnose insomnia when a patient meets DSM-IV-TR criteria (has difficulty falling asleep or staying asleep or feels that sleep is not refreshing for at least 1 month; and the sleep problem impairs daytime functioning and/or causes clinically significant distress). In addition, consider:
- PSG if you suspect other sleep disorders, particularly obstructive sleep apnea (OSA) and periodic limb movement disorder (PLMD)
- an overnight sleep study for treatment-resistant insomnia, when you have adequately treated other causes.
Sleep logs are useful. Ask patients to keep a sleep log for 2 weeks during early recovery, after acute withdrawal subsides. These diaries help assess sleep patterns over time, document improvement with abstinence, and engage the patient in treatment. The National Sleep Foundation can provide examples (see Related Resources).
Alcohol and sleep disturbances
Insomnia is extremely common in active drinkers and in those who are in treatment after having stopped drinking. Across 7 studies of 1,577 alcohol-dependent patients undergoing treatment, more than one-half reported insomnia symptoms (mean 58%, range 36% to 91%),2,3 substantially higher than the rate in the general population (33%). Nicotine, marijuana, cocaine and other stimulants, and opioids also can disrupt sleep (Table 1).
Which came first? Sleep problems may be a pathway by which problematic substance use develops. In 1 study, sleep problems reported by mothers in boys ages 3 to 5 predicted onset of alcohol and drug use by ages 12 to 14.4 This relationship was not mediated by attention problems, anxiety/depression, or aggression. Thus, insomnia may increase the risk for early substance use.
In an epidemiologic study of >10,000 adults, the incidence of new alcohol use disorders after 1 year in those without psychiatric disorders at baseline was twice as high in persons with persistent insomnia as in those without insomnia.5
Patients with sleep disturbances may use alcohol to self-medicate,6 and tolerance to alcohol’s sedating effects develops quickly. As patients consume larger quantities with greater frequency to produce sleep, the risk for dependence may increase.
Comorbid sleep disorders. Alcohol-dependent patients with difficulty falling asleep may have abnormal circadian rhythms, as suggested by delayed onset of nocturnal melatonin secretion.7 They also may have low homeostatic sleep drive, another factor required to promote sleep.8
Habitual alcohol consumption before bedtime (1 to 3 standard drinks) is associated with mild sleep-disordered breathing (SDB) in men but not in women.9 SDB also may be more prevalent in alcohol-dependent men age >60.10
Consuming >2 drinks/day has been associated with restless legs and increased periodic limb movements during sleep. Twice as many women reporting high alcohol use were diagnosed with PLMD, compared with women reporting normal alcohol consumption.10-11 Recovering alcohol-dependent patients have significantly more periodic limb movements associated with arousals (PLMA) from sleep than controls. Moreover, PLMA can predict 80% of abstainers and 44% of relapsers after 6 months of abstinence.12
Table 1
Sleep disruptions caused by substances of abuse
| Substance | Effect on sleep |
|---|---|
| Nicotine | Difficulty falling asleep, sleep fragmentation, less restful sleep compared with nonsmokers, increased risk for OSA and SDBa-e |
| Marijuana | Short-term difficulty falling asleep and decreased slow-wave sleep percentage during withdrawalf-j |
| Cocaine | Prolonged sleep latency, decreased sleep efficiency, and decreased REM sleep with intranasal self-administration; hypersomnia during withdrawalk-m |
| Other stimulants (amphetamine, methamphetamine, methylphenidate) | Sleep complaints similar to those reported with cocaine use disordersn |
| Opioids | Decreased slow-wave sleep, increased stage-2 sleep, but minimal impact on sleep continuity; dreams and nightmares; central sleep apneao-t |
| OSA: obstructive sleep apnea; SDB: sleep-disordered breathing; REM: rapid eye movement | |
| Reference Citations: click here | |
Multifaceted treatment
A thorough history is essential to evaluate sleep and guide treatment decisions. Refer patients to an accredited sleep disorders center if their history shows:
- loud snoring
- cessation of breathing
- frequent kicking during sleep
- excessive daytime sleepiness.
Chronic insomnia. Patients who report chronic insomnia and behaviors incompatible with sleep may be good candidates for cognitive-behavioral therapy for insomnia (CBT-I). Patient education can change maladaptive behaviors, such as staying in bed for long periods of time to compensate for sleep loss, using the bed for activities other than sleep, or worrying excessively about sleep (Box 1).13
Pharmacotherapy may be preferred:
- for patients with unstable physical or mental illness
- when CBT-I could exacerbate a comorbid condition (such as restricting sleep in a patient with bipolar disorder)
- for patients with low motivation for behavior change
- when trained CBT-I providers or resources to pay for CBT-I are limited.
| Step 1. Get into bed to go to sleep only when you are sleepy |
| ↓ |
| Step 2. Avoid using the bed for activities other than sleep; for example, do not read, watch TV, eat, or worry in bed. Sexual activity is the only exception; on these occasions, follow the next steps when you intend to go to sleep |
| ↓ |
| Step 3. If you are unable to fall asleep within 15 to 20 minutes, get out of bed and go into another room. Remember, the goal is to associate your bed with falling asleep quickly. Return to bed intending to go to sleep only when you are very sleepy |
| ↓ |
| Step 4. While out of bed during the night, engage in activities that are quiet but of interest to you. Do not exercise, eat, smoke, or take warm showers or baths. Do not lie down or fall asleep when not in bed |
| ↓ |
| Step 5. If you return to bed and still cannot fall asleep within 15 to 20 minutes, repeat Step 3. Do this as often as necessary throughout the night |
| ↓ |
| Step 6. Set your alarm and get up at the same time every morning, regardless of how much sleep you got during the night. This will help your body acquire a sleep-wake rhythm |
| ↓ |
| Step 7. Do not nap during the day |
| Source. Adapted from Bootzin R, Nicassio P. Behavioral treatments for insomnia. In: Hersen M, Eissler R, Miller P, eds. Progress in behavior modification, vol. 6. New York: Academic Press; 1978:30 |
In older adults with insomnia but no history of addiction, CBT-I was more effective than placebo and as effective as a hypnotic alone (temazepam, 7.5 and 30 mg qhs) and a hypnotic/CBT-I combination in reducing nighttime wakefulness, increasing total sleep time, and increasing sleep efficiency. After 2 years, patients treated with CBT-I alone were most likely to maintain these initial treatment gains.15
Limited data exist on CBT-I’s effectiveness in patients with addiction. In 2 studies, alcohol-dependent patients reported improved sleep.16,17 CBT-I also improved measures of anxiety and depression, fatigue, and some quality-of-life items.16
Stimulus control (SC). Patients with chronic insomnia may watch television, talk on the telephone, or worry about not sleeping while lying in bed. The goal of SC is to alter this association by reestablishing the bed and bedroom with the pleasant experience of falling asleep and staying asleep.13 Instructions for SC (Box 1) are commonly provided with sleep restriction.
Sleep restriction (SR) addresses the excessive time that patients with insomnia spend in bed not sleeping. SR temporarily restricts time spent in bed and prohibits sleep at other times. The resulting mild sleep deprivation may promote consolidated sleep, leading to improved patient-reported sleep quality.14
Sleep hygiene (SH) addresses behaviors that may help or hinder sleep. Patients with addiction may benefit from learning how drug use and withdrawal affects sleep or how substance use for sleep may exacerbate sleep problems. Other SH recommendations include avoiding caffeine, nicotine, and exercise in close proximity to bedtime.
Cognitive therapy. Goals are to:
- identify and explore dysfunctional beliefs that cause patients anxiety about sleep problems
- replace these beliefs with more appropriate self-statements that promote sleep-healthy behaviors.
Common themes address patients’ unrealistic sleep expectations, inability to control or predict sleep, and faulty beliefs about sleep-promoting practices.
Precautions about hypnotics. The newer alpha-1-selective benzodiazepine receptor agonists (zolpidem, zaleplon, and eszopiclone) and the older nonselective benzodiazepines (such as flurazepam, temazepam, and triazolam) share an equivalent range of abuse liability.18 Consequently, all benzodiazepine receptor agonists are classified as Schedule IV controlled substances and should be used with caution, if at all, in substance-abusing or substance-dependent patients (Table 2).
In general, most physicians who specialize in treating addictions would not recommend these drug classes as first choice in postwithdrawal, substance-dependent patients complaining of chronic insomnia. Nevertheless, you are likely to encounter patients with a history of substance abuse/dependence who are taking legally prescribed benzodiazepine receptor agonists for insomnia, and they may be very reluctant to discontinue these medications.
Weigh and discuss with the patient the risks and benefits of taking vs discontinuing the hypnotic, as well as alternatives. Because chronic hypnotic use may interfere with addiction recovery, it is important to discuss the patient’s recovery plan.
If you decide to prescribe a hypnotic with abuse liability, the newer alpha-1-selective benzodiazepine receptor agonists are preferable—as they would be for non-addicted patients—because they are less likely to disrupt sleep architecture. They are also less likely than the long-acting benzodiazepines (such as flurazepam) to accumulate over time and result in daytime impairment.
Patient contracts. A written agreement can be useful whenever you prescribe a controlled substance for a patient with an addiction history. Include these issues:
- frequency of clinic visits for monitoring response and refills, requests for early refills, and telephone refills
- obtaining prescriptions from only one prescriber and one pharmacy
- abstinence from other abused substances
- urine drug screens and pill counts
- authorization for you to share information with other care providers or significant others
- an addiction recovery plan for other abused substances
- consequences of nonadherence.
Table 2
FDA-approved benzodiazepine receptor agonists for insomnia*
| Agent | Dose range (mg) | TMAX (hr) | T½ (hr) |
|---|---|---|---|
| Benzodiazepine receptor agonists (benzodiazepine structures) | |||
| Estazolam | 1 to 2 | 0.5 to 1.6 | 10 to 24 |
| Flurazepam | 15 to 30 | 3 to 6 | 50 to 100† |
| Quazepam | 7.5 to 15 | 2 | 25 to 100† |
| Temazepam | 15 to 30 | 2 to 3 | 10 to 17 |
| Triazolam | 0.125 to 0.5 | 1 to 2 | 1.5 to 5.5 |
| Selective benzodiazepine receptor agonists (nonbenzodiazepine structures)‡ | |||
| Eszopiclone | 1 to 3 | 1 | ~6 |
| Zaleplon | 5 to 20 | 1 | ~1 |
| Zolpidem | 5 to 10 | 1.6 | 2.5 (1.5 to 3.8) |
| Zolpidem CR | 6.25 to 12.5 | 1.5 | 2.8 (1.6 to 4) |
| TMAX: time to reach maximal plasma concentrations; T½: elimination half-life (all values are approximate for any given individual) | |||
| * All benzodiazepine receptor agonists are Schedule IV controlled substances. Use with caution, if at all, in alcohol-dependent patients | |||
| † Including active metabolites | |||
| ‡ Selective GABAA receptor agonists bind the alpha-1 protein subunit of GABAA receptors. Alpha-1 containing GABAA receptors are thought to mediate sedative and amnesic effects but not antianxiety or muscle relaxant effects of the GABA system | |||
Off-label sedatives for insomnia
Like ramelteon, sedating agents that do not have abuse liability are first-choice medications for patients with addiction and co-occurring insomnia (Table 3):
- The most studied are gabapentin and trazodone.
- Quetiapine and mirtazapine may be considered as second-choice options.
In 2 open-label pilot studies of alcohol-dependent patients with insomnia:
- gabapentin (mean dose 953 mg) significantly improved sleep quality over 4 to 6 weeks20
- both gabapentin (mean 888 mg qhs) and trazodone (mean 105 mg qhs) significantly improved Sleep Problems Questionnaire scores, but patients receiving gabapentin were less likely than those taking trazodone to feel tired upon awakening.21
Although gabapentin and the anticonvulsant pregabalin increase slow-wave sleep in healthy control subjects, evidence of a similar effect is lacking in alcohol-dependent patients.
Trazodone is the most commonly prescribed antidepressant for insomnia because of its sedating effect and low abuse potential. Trazodone was associated with greater sleep improvements vs placebo as measured via PSG in a randomized, double-blind trial of alcohol-dependent patients with insomnia.23 In a second study, sleep outcomes were better with trazodone vs placebo over 12 weeks in alcohol-dependent patients, although patients in the trazodone group drank more heavily.24
Other sedating antidepressants such as mirtazapine and doxepin have not been studied in patients with substance use disorders.
Quetiapine is a second-generation antipsychotic with sedating properties. When quetiapine, 25 to 200 mg/d, was given to alcohol-dependent veterans with sleep complaints, they remained abstinent more days and had fewer hospitalizations than veterans not receiving quetiapine.25 Both groups had high rates of psychiatric comorbidity, and 90% had posttraumatic stress disorder. Improved abstinence was thought to result from improved sleep, but no sleep measures were included to test this hypothesis.
A recently published, randomized controlled pilot study reported significantly reduced drinking and craving in severely alcohol-dependent patients receiving quetiapine vs placebo, although sleep data were not included.26
Other options. Tricyclic antidepressants carry risks of cardiotoxicity and other side effects but can be useful when other options have not worked or patients have comorbidities such as neuropathic pain or migraine headaches. Combinations of agents also may be considered for treatment-resistant insomnia.
Nonprescription remedies such as antihistamines, valerian root extract (from the herb Valeriana officinalis), and melatonin are commonly used for sleep, although data are limited in substance-abusing patients.
Table 3
Noncontrolled sedating agents for treating insomnia
in patients with a history of substance abuse
| Agent | Dose range (mg) | TMAX (hr) | T½ (hr) |
|---|---|---|---|
| Melatonin receptor agonist | |||
| Ramelteon | 8 | 0.5 to 1.5 | 1 to 2.6 |
| Sedating anticonvulsant | |||
| Gabapentin | 300 to 1,500 | 2 to 3 | 6 to 7 |
| Sedating antidepressants | |||
| Amitriptyline† | 25 to 150 | 2 to 8 | 5 to 45 |
| Doxepin† | 25 to 150 | 2 to 8 | 10 to 30 |
| Mirtazapine | 7.5 to 45‡ | 1 to 3 | 20 to 40 |
| Nefazodone | 50 to 150 | 1 | 6 to 18* |
| Nortriptyline† | 10 to 75§ | 2 to 8 | 20 to 55 |
| Trazodone | 25 to 300 | 1 to 2 | 3 to 9¶ |
| Sedating second-generation antipsychotic | |||
| Quetiapine | 25 to 100 | 1.5 | 6 |
| TMAX: time to reach maximal plasma concentrations; T½: elimination half-life (all values are approximate for any given individual) | |||
| * Including active metabolites | |||
| † Tricyclic antidepressants | |||
| ‡ Antihistaminergic effects predominate at low doses (7.5 to 15 mg) | |||
| § Can be titrated to morning serum level (50 to 150 mcg/mL) 12 hr after bedtime dose if no effect at lower doses | |||
| ¶ Major metabolite, mCPP, has 14-hour half-life | |||
- American Academy of Sleep Medicine. www.sleepeducation.com.
- National Sleep Foundation. Sleep logs for downloading. www.sleepfoundation.org.
- Restless Legs Syndrome Foundation. www.rls.org.
- Brower KJ. Insomnia, alcoholism and relapse. Sleep Med Rev 2003;7:523-39.
- Amitriptyline • Elavil, Endep
- Doxepin • Sinequan
- Estazolam • ProSom
- Eszopiclone • Lunesta
- Flurazepam • Dalmane
- Gabapentin • Neurontin
- Methamphetamine • Desoxyn
- Methylphenidate • Concerta, Ritalin, others
- Mirtazapine • Remeron
- Nefazodone • Serzone
- Nortriptyline • Pamelor
- Pregabalin • Lyrica
- Quazepam • Doral
- Quetiapine • Seroquel
- Ramelteon • Rozerem
- Temazepam • Restoril
- Theophylline • Theo-24, others
- Trazodone • Desyrel
- Triazolam • Halcion
- Zaleplon • Sonata
- Zolpidem • Ambien, Ambien CR
Dr. Conroy and Dr. Arnedt report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products. Dr. Brower is a consultant to Pfizer.
Acknowledgment
This work was supported by an NIH grant to Dr. Brower (2K24 AA00304).
1. Friedmann PD, Herman DS, Freedman S, et al. Treatment of sleep disturbance in alcohol recovery: a national survey of addiction medicine physicians. J Addict Dis 2003;22:91-103.
2. Brower KJ. Alcohol’s effects on sleep in alcoholics. Alcohol Res Health 2001;25:110-25.
3. Cohn TJ, Foster JH, Peters TJ. Sequential studies of sleep disturbance and quality of life in abstaining alcoholics. Addict Biol 2003;8(4):455-62.
4. Wong MM, Brower KJ, Fitzgerald HE, Zucker RA. Sleep problems in early childhood and early onset of alcohol and other drug use in adolescence. Alcohol Clin Exp Res 2004;28(4):578-87.
5. Weissman MM, Greenwald S, Nino-Murcia G, Dement WC. The morbidity of insomnia uncomplicated by psychiatric disorders. Gen Hosp Psychiatry 1997;19:245-50.
6. Brower KJ, Aldrich MS, Robinson EAR, et al. Insomnia, self-medication, and relapse to alcoholism. Am J Psychiatry 2001;158:399-404.
7. Kuhlwein E, Hauger RL, Irwin MR. Abnormal nocturnal melatonin secretion and disordered sleep in abstinent alcoholics. Biol Psychiatry 2003;54(12):1437-43.
8. Irwin M, Gillin JC, Dang J, et al. Sleep deprivation as a probe of homeostatic sleep regulation in primary alcoholics. Biol Psychiatry 2002;51(8):632-41.
9. Peppard PE, Austin D, Brown RL. Association of alcohol consumption and sleep disordered breathing in men and women. J Clin Sleep Med 2007;3(3):265-70.
10. Aldrich MS, Shipley JE, Tandon R, et al. Sleep-disordered breathing in alcoholics: association with age. Alcohol Clin Exp Res 1993;17:1179-83.
11. Aldrich MS, Shipley JE. Alcohol use and periodic limb movements of sleep. Alcohol Clin Exp Res 1993;17:192-6.
12. Gann H, Feige B, Fasihi S, et al. Periodic limb movements during sleep in alcohol dependent patients. Eur Arch Psychiatry Clin Neurosci 2002;252(3):124-9.
13. Bootzin R, Nicassio P. Behavioral treatments for insomnia. In: Hersen M, Eissler R, Miller P, eds. Progress in behavior modification. Vol. 6. New York, NY: Academic Press; 1978:1-45.
14. Spielman AJ, Saskin P, Thorpy MJ. Treatment of chronic insomnia by restriction of time in bed. Sleep 1987;10:45-55.
15. Morin CM, Colecchi C, Stone J, et al. Behavioral and pharmacological therapies for late-life insomnia: a randomized controlled trial. JAMA 1999;281(11):991-9.
16. Arnedt JT, Conroy D, Rutt J, et al. An open trial of cognitivebehavioral treatment for insomnia comorbid with alcohol dependence. Sleep Med 2007;8:176-80.
17. Currie SR, Clark S, Hodgins DC, el-Guebaly N. Randomized controlled trial of brief cognitive-behavioural interventions for insomnia in recovering alcoholics. Addiction 2004;99:1121-32.
18. Griffiths RR, Johnson MW. Relative abuse liability of hypnotic drugs: a conceptual framework and algorithm for differentiating among compounds. J Clin Psychiatry 2005;66(suppl 9):31-41.
19. Johnson MW, Suess PE, Griffiths RR. Ramelteon: a novel hypnotic lacking abuse liability and sedative adverse side effects. Arch Gen Psychiatry 2006;63:1149-57.
20. Karam-Hage M, Brower KJ. Gabapentin treatment for insomnia associated with alcohol dependence [letter]. Am J Psychiatry 2000;157:151.-
21. Karam-Hage M, Brower KJ. Open pilot study of gabapentin versus trazodone to treat insomnia in alcoholic outpatients. Psychiatry Clini Neurosci 2003;57:542-4.
22. Brower KJ, Kim HM, Karam-Hage M, et al. A double-blind randomized clinical trial of gabapentin vs. placebo for treating alcohol dependence. Biol Psychiatry 2003;53(8S):84S-85S.
23. Le Bon O, Murphy JR, Staner L, et al. Double-blind, placebo-controlled study of the efficacy of trazodone in alcohol post-withdrawal syndrome: polysomnographic and clinical evaluations. J Clin Psychopharmacol 2003;23(4):377-83.
24. Friedmann PD, Rose JS, Swift RM, et al. Trazodone for sleep disturbance after detoxification from alcohol dependence: a double-blind, placebo-controlled trial. Paper presented at: Annual Meeting of the American Academy of Addiction Psychiatry, December 1-2, 2007; Coronado, CA.
25. Monnelly EP, Ciraulo DA, Knapp C, et al. Quetiapine for treatment of alcohol dependence. J Clin Psychopharmacol 2004;24(5):532-5.
26. Kampman KM, Pettinati HM, Lynch KG, et al. A double-blind, placebo-controlled pilot trial of quetiapine for the treatment of Type A and Type B alcoholism. J Clin Psychopharmacol 2007;27:344-51.
1. Friedmann PD, Herman DS, Freedman S, et al. Treatment of sleep disturbance in alcohol recovery: a national survey of addiction medicine physicians. J Addict Dis 2003;22:91-103.
2. Brower KJ. Alcohol’s effects on sleep in alcoholics. Alcohol Res Health 2001;25:110-25.
3. Cohn TJ, Foster JH, Peters TJ. Sequential studies of sleep disturbance and quality of life in abstaining alcoholics. Addict Biol 2003;8(4):455-62.
4. Wong MM, Brower KJ, Fitzgerald HE, Zucker RA. Sleep problems in early childhood and early onset of alcohol and other drug use in adolescence. Alcohol Clin Exp Res 2004;28(4):578-87.
5. Weissman MM, Greenwald S, Nino-Murcia G, Dement WC. The morbidity of insomnia uncomplicated by psychiatric disorders. Gen Hosp Psychiatry 1997;19:245-50.
6. Brower KJ, Aldrich MS, Robinson EAR, et al. Insomnia, self-medication, and relapse to alcoholism. Am J Psychiatry 2001;158:399-404.
7. Kuhlwein E, Hauger RL, Irwin MR. Abnormal nocturnal melatonin secretion and disordered sleep in abstinent alcoholics. Biol Psychiatry 2003;54(12):1437-43.
8. Irwin M, Gillin JC, Dang J, et al. Sleep deprivation as a probe of homeostatic sleep regulation in primary alcoholics. Biol Psychiatry 2002;51(8):632-41.
9. Peppard PE, Austin D, Brown RL. Association of alcohol consumption and sleep disordered breathing in men and women. J Clin Sleep Med 2007;3(3):265-70.
10. Aldrich MS, Shipley JE, Tandon R, et al. Sleep-disordered breathing in alcoholics: association with age. Alcohol Clin Exp Res 1993;17:1179-83.
11. Aldrich MS, Shipley JE. Alcohol use and periodic limb movements of sleep. Alcohol Clin Exp Res 1993;17:192-6.
12. Gann H, Feige B, Fasihi S, et al. Periodic limb movements during sleep in alcohol dependent patients. Eur Arch Psychiatry Clin Neurosci 2002;252(3):124-9.
13. Bootzin R, Nicassio P. Behavioral treatments for insomnia. In: Hersen M, Eissler R, Miller P, eds. Progress in behavior modification. Vol. 6. New York, NY: Academic Press; 1978:1-45.
14. Spielman AJ, Saskin P, Thorpy MJ. Treatment of chronic insomnia by restriction of time in bed. Sleep 1987;10:45-55.
15. Morin CM, Colecchi C, Stone J, et al. Behavioral and pharmacological therapies for late-life insomnia: a randomized controlled trial. JAMA 1999;281(11):991-9.
16. Arnedt JT, Conroy D, Rutt J, et al. An open trial of cognitivebehavioral treatment for insomnia comorbid with alcohol dependence. Sleep Med 2007;8:176-80.
17. Currie SR, Clark S, Hodgins DC, el-Guebaly N. Randomized controlled trial of brief cognitive-behavioural interventions for insomnia in recovering alcoholics. Addiction 2004;99:1121-32.
18. Griffiths RR, Johnson MW. Relative abuse liability of hypnotic drugs: a conceptual framework and algorithm for differentiating among compounds. J Clin Psychiatry 2005;66(suppl 9):31-41.
19. Johnson MW, Suess PE, Griffiths RR. Ramelteon: a novel hypnotic lacking abuse liability and sedative adverse side effects. Arch Gen Psychiatry 2006;63:1149-57.
20. Karam-Hage M, Brower KJ. Gabapentin treatment for insomnia associated with alcohol dependence [letter]. Am J Psychiatry 2000;157:151.-
21. Karam-Hage M, Brower KJ. Open pilot study of gabapentin versus trazodone to treat insomnia in alcoholic outpatients. Psychiatry Clini Neurosci 2003;57:542-4.
22. Brower KJ, Kim HM, Karam-Hage M, et al. A double-blind randomized clinical trial of gabapentin vs. placebo for treating alcohol dependence. Biol Psychiatry 2003;53(8S):84S-85S.
23. Le Bon O, Murphy JR, Staner L, et al. Double-blind, placebo-controlled study of the efficacy of trazodone in alcohol post-withdrawal syndrome: polysomnographic and clinical evaluations. J Clin Psychopharmacol 2003;23(4):377-83.
24. Friedmann PD, Rose JS, Swift RM, et al. Trazodone for sleep disturbance after detoxification from alcohol dependence: a double-blind, placebo-controlled trial. Paper presented at: Annual Meeting of the American Academy of Addiction Psychiatry, December 1-2, 2007; Coronado, CA.
25. Monnelly EP, Ciraulo DA, Knapp C, et al. Quetiapine for treatment of alcohol dependence. J Clin Psychopharmacol 2004;24(5):532-5.
26. Kampman KM, Pettinati HM, Lynch KG, et al. A double-blind, placebo-controlled pilot trial of quetiapine for the treatment of Type A and Type B alcoholism. J Clin Psychopharmacol 2007;27:344-51.
Postpartum depression: What to tell patients who breast-feed
Whether you encounter postpartum depression (PPD) in a patient you have been treating or in one referred by her obstetrician, early, aggressive treatment is essential. Although PPD shares some symptoms with major depressive disorder (MDD)—and may be a subtype of that disorder—it also has distinguishing characteristics, such as timing of symptom onset (Box 1).1,2 Two screening tools facilitate diagnosis (Box 2).2-4
Women with PPD usually respond to pharmacotherapy, but antidepressants’ potential effects on a nursing mother’s newborn are important to consider.
HPA axis dysregulation
Although the precise cause of PPD remains unclear, a better understanding is emerging of the complicated interplay of estrogen and progesterone with the hypothalamic-pituitary-adrenal (HPA) axis and other neuroregulatory systems associated with depressive illness. Two lines of evidence implicate hormonal dysregulation:
- Despite normal reproductive hormone levels, women with PPD may have an abnormal response to changes in these levels.5
- Abnormalities in HPA axis activity appear to be associated with reproductive endocrine-related mood disorders in vulnerable women, particularly during the transition from childbirth to the immediate postpartum period.
Most women will have mild mood and anxiety symptoms in the first few days to weeks postpartum—often referred to as the ‘baby blues’—but these symptoms usually resolve spontaneously. More severe and persistent depressed mood and anxiety should arouse suspicion of postpartum depression (PPD).
Although not categorized as a distinct disorder in the DSM-IV-TR, PPD is diagnosed using DSM-IV-TR criteria for a major depressive episode, including feelings of being overwhelmed, guilt or worthlessness, tearfulness, appetite change, difficulty sleeping (even when the baby is sleeping), difficulty concentrating, and loss of interest or pleasure in activities.2
PPD symptoms differ, however, in some important ways from those of nonpuerperal depression. Distinguishing characteristics of PPD are:
- severe worry, anxiety, and/or agitation
- fears of hurting the baby or oneself
- not having any interest in the baby.2
PPD usually begins within the first month postpartum but may occur later; the first 3 months appear to be the most vulnerable period.1
- high estrogen and progesterone levels
- a hyperactive HPA axis (normal during pregnancy)
- high plasma cortisol level, stimulated in part by high levels of estrogen and progesterone.7,8
Differences in HPA reactivity. In a normal HPA axis, the delivery of CRH from the paraventricular nucleus of the hypothalamus triggers the stimulation of adrenocorticotropic hormone (ACTH) from the anterior pituitary and, consequently, cortisol from the adrenal cortex. This hormonal system is regulated by negative feedback mediated by cortisol receptors on the anterior pituitary, hypothalamus, and hippocampus, as well as ACTH receptors in the anterior pituitary and CRH autoreceptors in the hypothalamus.10
A hallmark feature of the HPA axis in depression is altered response to stress and inability to maintain regulation:
- In MDD, HPA axis hyperactivity is one of the most robust biological findings.11 In general, women with MDD exhibit high baseline cortisol and an exaggerated response to the dexamethasone/corticotropin releasing hormone test.
- In contrast, women with PPD experience a more blunted ACTH response to CRH, which may reflect a hyporeactive HPA axis.9
It has been hypothesized that both increased cortisol and decreased cortisol (observed under conditions of sustained elevated gonadal steroid levels or withdrawal of gonadal steroids) may result in insufficient glucocorticoid signaling.13 Impaired glucocorticoid signaling may be the “final common pathway” leading to psychiatric disturbance in MDD and PPD.
Understanding the characteristics of HPA axis reactivity in women with PPD could improve early identification and, theoretically, prevention or immediate treatment for at-risk women. In addition to HPA axis dysregulation, disturbances in other endocrine systems may play a role in PPD. Women with antenatal total and free thyroxine concentrations in the lower euthyroid range may be at increased risk of developing postpartum depressive symptoms.14
Two well-validated, simple-to-administer postpartum depression (PPD) screening instruments are useful during the postnatal period:
- the Edinburgh Postnatal Depression Scale (EPDS),3 a 10-item self-report questionnaire that asks about mood, anxiety, guilt, and suicide ideation
- the Postpartum Depression Screening Scale (PDSS),4 a 35-item self-report questionnaire that asks about sleeping/eating disturbances, anxiety/insecurity, emotional lability, mental confusion, loss of self, guilt/shame, and suicide ideation.
If screening indicates a patient has PPD, her psychiatric history will influence your treatment selection. Pay particular attention to:
- past episodes of depression, hypomania, or mania
- severity and timing of those episodes
- treatment history, including documentation of response to antidepressants.2
Risks with or without treatment
PPD has potentially serious adverse consequences and needs to be aggressively treated. Ethical and practical challenges have hindered PPD research, however, and evidence to guide treatment is limited.15
Approximately 70% of mothers in the United States breast-feed their infants at least for the first 3 months.16 With any patient with PPD who is breast-feeding, carefully discuss the risk of antidepressant side effects for the mother and child.17
Also discuss potential risks and benefits of treatment vs no treatment.17 Potential risks of untreated depression include:
- impaired mother/child bonding because of ongoing maternal depressive illness
- impaired cognitive, emotional, and social development in the child.18
- educate the patient about potential antidepressant side effects for mother and baby
- avoid communicating “mixed messages” to the patient about the risk and benefits of treatment
- ensure the health of mother and baby.17
Antidepressants. In general, women with PPD respond well to antidepressant therapy. They may be hesitant to take any medication while breast-feeding because of possible harmful effects to their babies, but most studies examining antidepressant use by lactating women found low rates of adverse events in infants exposed to antidepressants (Table).19-24 Potential adverse effects include:
- sedation
- changes in sleep or feeding
- irritability.17
SSRIs. Few adverse events have been reported with sertraline, paroxetine, and fluvoxamine during lactation.20 However, paroxetine may be associated with increased risk of cardiac abnormalities in infants exposed during the first trimester of pregnancy.26 Two agents in this class may be less desirable:
- fluoxetine, because it has a long half-life
- citalopram, because of potentially high breast milk concentration.20
Tricyclics might be indicated for patients who responded to them previously or who have not responded to SSRIs. No adverse effects have been reported in breast-feeding infants receiving amitriptyline, clomipramine, desipramine, imipramine, or nortriptyline.25 Avoid doxepin, however, because it has the longest half-life among tricyclics, and adverse effects in infants—including respiratory distress, drowsiness, and vomiting—have been reported.
Other antidepressants. Venlafaxine and duloxetine are not recommended because of limited data about use of these agents during lactation. Bupropion poses a small increased risk of seizures in newborns but is not absolutely contraindicated.24 Trazodone also has limited data, but in clinical practice it has been used safely at low doses for many years.20
Psychotherapeutic techniques—including individual or group therapy—also can effectively reduce depressive symptoms in women with PPD.27
Table
Antidepressants for postpartum depression
| Medication | Starting dosage | Maximum dosage during lactation | Potential adverse event(s) |
|---|---|---|---|
| Selective serotonin reuptake inhibitors | |||
| Citalopram | 10 mg | 60 mg | High milk/plasma concentration at higher doses20 |
| Escitalopram | 10 mg | 20 mg | Very limited data to date show lower milk/plasma concentrations compared with citalopram21 |
| Fluoxetine | 10 mg | 60 mg | Long half-life can increase the potential for accumulation20 |
| Sertraline | 25 mg | 150 to 200 mg | Minimal detection of drug in infants’ serum19,20 |
| Paroxetine | 10 mg | 50 mg | Minimal detection of drug in infants’ serum19,20 |
| Tricyclics | |||
| Desipramine | 25 mg | 200 mg | Minimal detection of drug in infants’ serum19,22 |
| Imipramine | 25 mg | 200 mg | Minimal detection of drug in infants’ serum19,22 |
| Nortriptyline | 25 mg | 125 to 150 mg | Minimal detection of drug in infants’ serum19,22 |
| Others | |||
| Bupropion | 75 to 150 mg | 300 mg | Limited data available. Small increased risk of infant seizure (case report)24 |
| Mirtazapine | 7.5 mg | 45 mg | Limited data available. Well tolerated in a small study.23 Always monitor for changes in sleep (sedation and activation) and eating behaviors |
| Note: Clinical monitoring of the infant for adverse effects—including sedation, changes in sleep or feeding, and irritability—should be part of routine care | |||
Related resources
Clinician resource
- Cuijpers P, Brännmark JG, van Straten A. Psychological treatment of postpartum depression: a meta-analysis. J Clin Psychol 2008;64(1):103-18.
- American Psychiatric Association. Postpartum depression. www.healthyminds.org/postpartumdepression.cfm.
- Amitriptyline • Elavil, Endep
- Bupropion • Wellbutrin
- Citalopram • Celexa
- Clomipramine • Anafranil
- Desipramine • Norpramin
- Doxepin • Sinequan
- Duloxetine • Cymbalta
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Imipramine • Tofranil
- Mirtazapine • Remeron
- Nortriptyline • Aventyl
- Paroxetine • Paxil
- Sertraline • Zoloft
- Sertraline • Zoloft
- Venlafaxine • Effexor
Dr. Meltzer-Brody receives research/grant support from AstraZeneca, GlaxoSmithKline, and The Foundation of Hope.
Dr. Payne receives research/grant support from AstraZeneca, Novartis, Stanley Medical Research Institute, and Wyeth Pharmaceuticals.
Dr. Rubin reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Munk-Olsen T, Laursen TM, Pedersen CB, et al. New parents and mental disorders: a population-based register study. JAMA 2006;296:2592-9.
2. Gaynes BN, Gavin N, Meltzer-Brody S, et al. Perinatal depression: prevalence, screening accuracy, and screening outcomes. Evid Rep Technol Assess (Summ) 2005;(119):1-8.
3. Cox JL, Holden JM, Sagovsk R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry 1987;150:782-6.
4. Beck CT, Gable RK. Comparative analysis of the performance of the Postpartum Depression Screening Scale with two other depression instruments. Nurs Res 2001;50:242-50.
5. Bloch M, Schmidt PJ, Danaceau M, et al. Effects of gonadal steroids in women with a history of postpartum depression. Am J Psychiatry 2000;157(6):924-30.
6. Mastorakos G, Ilias I. Maternal and fetal hypothalamic-pituitary-adrenal axes during pregnancy and postpartum. Ann N Y Acad Sci 2003;997:136-49.
7. Noltern WE, Lindheimer MD, Rueckert PA, et al. Diurnal patterns and regulation of cortisol secretion in pregnancy. J Clin Endocrinology Metab 1980;51:466-72.
8. Bloch M, Daly RC, Rubinow DR. Endocrine factors in the etiology of postpartum depression. Compr Psychiatry 2003;44(3):234-46.
9. Magiakou MA, Mastorakos G, Rabin D. Hypothalamic-cortico-releasing hormone suppression during the postpartum period: implications for the increase in psychiatric manifestations at this time. J Clin Endocrinol Metab 1996;81:1912-7.
10. Jolley SN, Elmore S, Barnard KE, Carr D. Dysregulation of the hypothalamic-pituitary-adrenal axis in postpartum depression. Biol Res Nurs 2007;8:210-22.
11. Gold PW, Gabry KE, Yasuda MR, Chrousos GP. Divergent endocrine abnormalities in melancholic and atypical depression: clinical and pathophysiologic implications. Endocrinol Metab Clin North Am 2002;31:37-62.
12. Bloch M, Rubinow DR, Schmidt PJ. Cortisol response to ovine corticotropin-releasing hormone in a model of pregnancy and parturition in euthymic women with and without a history of postpartum depression. J Clin Endocrinol Metab 2005;90(2):695-9.
13. Raison CL, Miller AH. When not enough is too much: the role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. Am J Psychiatry 2003;160:1554-65.
14. Pedersen CA, Johnson JL, Silva S, et al. Antenatal thyroid correlates of postpartum depression. Psychoneuroendocrinology 2007;32(3):235-45.
15. Yonkers KA. The treatment of women suffering from depression who are either pregnant or breastfeeding. Am J Psychiatry 2007;164(10):1457-9.
16. Ryan AS, Wenjun Z, Acosta A. Breastfeeding continues to increase into the new millennium. Pediatrics 2002;110:1103-9.
17. Payne J. Antidepressant use in the postpartum period: practical considerations. Am J Psychiatry 2007;164:1329-32.
18. Murray L, Sinclair D, Cooper PJ, et al. The socioemotional development of 5-year old children of postnatally depressed mothers. J Child Psychol Psychiatry 1999;40:1259-71.
19. Weissman AM, Levt BT, Hartz AJ, et al. Pooled analysis of antidepressant levels in lactating mothers, breast milk, and nursing infants. Am J Psychiatry 2004;161(6):1066-78.
20. Eberhard-Gran M, Eskild A, Opjordsmoen S. Use of psychotropic medications in treating mood disorders during lactation: practical recommendations. CNS Drugs 2006;20:187-98.
21. Rampono J, Hackett LP, Kristensen JH, et al. Transfer of escitalopram and its metabolite demethylescitalopram into breastmilk. Br J Clin Pharmacol 2006;62(3):316-22.
22. Wisner KL, Perel JM, Findling RL. Antidepressant treatment during breast-feeding. Am J Psychiatry 1996;153(9):1132-7.
23. Kristensen JH, Ilett KF, Rampono J, et al. Transfer of the antidepressant mirtazapine into breast milk. Br J Clin Pharmacol 2007;63(3):322-7.
24. Chaudron LH, Schoenecker CJ. Bupropion and breastfeeding: a case of a possible infant seizure. J Clin Psychiatry 2004;65(6):881-2.
25. Misri S, Kostaras X. Postpartum depression: is there an Andrea Yates in your practice? Current Psychiatry 2002;1(5):22-9.
26. Louik C, Lin AE, Werler MM, et al. First-trimester use of selective serotonin-reuptake inhibitors and the risk of birth defects. N Engl J Med 2007;356(26):2675-83.
27. Dennis CL, Hodnett E. Psychosocial and psychological interventions for treating postpartum depression. Cochrane Database Syst Rev 2007;(4):CD006116.-
Whether you encounter postpartum depression (PPD) in a patient you have been treating or in one referred by her obstetrician, early, aggressive treatment is essential. Although PPD shares some symptoms with major depressive disorder (MDD)—and may be a subtype of that disorder—it also has distinguishing characteristics, such as timing of symptom onset (Box 1).1,2 Two screening tools facilitate diagnosis (Box 2).2-4
Women with PPD usually respond to pharmacotherapy, but antidepressants’ potential effects on a nursing mother’s newborn are important to consider.
HPA axis dysregulation
Although the precise cause of PPD remains unclear, a better understanding is emerging of the complicated interplay of estrogen and progesterone with the hypothalamic-pituitary-adrenal (HPA) axis and other neuroregulatory systems associated with depressive illness. Two lines of evidence implicate hormonal dysregulation:
- Despite normal reproductive hormone levels, women with PPD may have an abnormal response to changes in these levels.5
- Abnormalities in HPA axis activity appear to be associated with reproductive endocrine-related mood disorders in vulnerable women, particularly during the transition from childbirth to the immediate postpartum period.
Most women will have mild mood and anxiety symptoms in the first few days to weeks postpartum—often referred to as the ‘baby blues’—but these symptoms usually resolve spontaneously. More severe and persistent depressed mood and anxiety should arouse suspicion of postpartum depression (PPD).
Although not categorized as a distinct disorder in the DSM-IV-TR, PPD is diagnosed using DSM-IV-TR criteria for a major depressive episode, including feelings of being overwhelmed, guilt or worthlessness, tearfulness, appetite change, difficulty sleeping (even when the baby is sleeping), difficulty concentrating, and loss of interest or pleasure in activities.2
PPD symptoms differ, however, in some important ways from those of nonpuerperal depression. Distinguishing characteristics of PPD are:
- severe worry, anxiety, and/or agitation
- fears of hurting the baby or oneself
- not having any interest in the baby.2
PPD usually begins within the first month postpartum but may occur later; the first 3 months appear to be the most vulnerable period.1
- high estrogen and progesterone levels
- a hyperactive HPA axis (normal during pregnancy)
- high plasma cortisol level, stimulated in part by high levels of estrogen and progesterone.7,8
Differences in HPA reactivity. In a normal HPA axis, the delivery of CRH from the paraventricular nucleus of the hypothalamus triggers the stimulation of adrenocorticotropic hormone (ACTH) from the anterior pituitary and, consequently, cortisol from the adrenal cortex. This hormonal system is regulated by negative feedback mediated by cortisol receptors on the anterior pituitary, hypothalamus, and hippocampus, as well as ACTH receptors in the anterior pituitary and CRH autoreceptors in the hypothalamus.10
A hallmark feature of the HPA axis in depression is altered response to stress and inability to maintain regulation:
- In MDD, HPA axis hyperactivity is one of the most robust biological findings.11 In general, women with MDD exhibit high baseline cortisol and an exaggerated response to the dexamethasone/corticotropin releasing hormone test.
- In contrast, women with PPD experience a more blunted ACTH response to CRH, which may reflect a hyporeactive HPA axis.9
It has been hypothesized that both increased cortisol and decreased cortisol (observed under conditions of sustained elevated gonadal steroid levels or withdrawal of gonadal steroids) may result in insufficient glucocorticoid signaling.13 Impaired glucocorticoid signaling may be the “final common pathway” leading to psychiatric disturbance in MDD and PPD.
Understanding the characteristics of HPA axis reactivity in women with PPD could improve early identification and, theoretically, prevention or immediate treatment for at-risk women. In addition to HPA axis dysregulation, disturbances in other endocrine systems may play a role in PPD. Women with antenatal total and free thyroxine concentrations in the lower euthyroid range may be at increased risk of developing postpartum depressive symptoms.14
Two well-validated, simple-to-administer postpartum depression (PPD) screening instruments are useful during the postnatal period:
- the Edinburgh Postnatal Depression Scale (EPDS),3 a 10-item self-report questionnaire that asks about mood, anxiety, guilt, and suicide ideation
- the Postpartum Depression Screening Scale (PDSS),4 a 35-item self-report questionnaire that asks about sleeping/eating disturbances, anxiety/insecurity, emotional lability, mental confusion, loss of self, guilt/shame, and suicide ideation.
If screening indicates a patient has PPD, her psychiatric history will influence your treatment selection. Pay particular attention to:
- past episodes of depression, hypomania, or mania
- severity and timing of those episodes
- treatment history, including documentation of response to antidepressants.2
Risks with or without treatment
PPD has potentially serious adverse consequences and needs to be aggressively treated. Ethical and practical challenges have hindered PPD research, however, and evidence to guide treatment is limited.15
Approximately 70% of mothers in the United States breast-feed their infants at least for the first 3 months.16 With any patient with PPD who is breast-feeding, carefully discuss the risk of antidepressant side effects for the mother and child.17
Also discuss potential risks and benefits of treatment vs no treatment.17 Potential risks of untreated depression include:
- impaired mother/child bonding because of ongoing maternal depressive illness
- impaired cognitive, emotional, and social development in the child.18
- educate the patient about potential antidepressant side effects for mother and baby
- avoid communicating “mixed messages” to the patient about the risk and benefits of treatment
- ensure the health of mother and baby.17
Antidepressants. In general, women with PPD respond well to antidepressant therapy. They may be hesitant to take any medication while breast-feeding because of possible harmful effects to their babies, but most studies examining antidepressant use by lactating women found low rates of adverse events in infants exposed to antidepressants (Table).19-24 Potential adverse effects include:
- sedation
- changes in sleep or feeding
- irritability.17
SSRIs. Few adverse events have been reported with sertraline, paroxetine, and fluvoxamine during lactation.20 However, paroxetine may be associated with increased risk of cardiac abnormalities in infants exposed during the first trimester of pregnancy.26 Two agents in this class may be less desirable:
- fluoxetine, because it has a long half-life
- citalopram, because of potentially high breast milk concentration.20
Tricyclics might be indicated for patients who responded to them previously or who have not responded to SSRIs. No adverse effects have been reported in breast-feeding infants receiving amitriptyline, clomipramine, desipramine, imipramine, or nortriptyline.25 Avoid doxepin, however, because it has the longest half-life among tricyclics, and adverse effects in infants—including respiratory distress, drowsiness, and vomiting—have been reported.
Other antidepressants. Venlafaxine and duloxetine are not recommended because of limited data about use of these agents during lactation. Bupropion poses a small increased risk of seizures in newborns but is not absolutely contraindicated.24 Trazodone also has limited data, but in clinical practice it has been used safely at low doses for many years.20
Psychotherapeutic techniques—including individual or group therapy—also can effectively reduce depressive symptoms in women with PPD.27
Table
Antidepressants for postpartum depression
| Medication | Starting dosage | Maximum dosage during lactation | Potential adverse event(s) |
|---|---|---|---|
| Selective serotonin reuptake inhibitors | |||
| Citalopram | 10 mg | 60 mg | High milk/plasma concentration at higher doses20 |
| Escitalopram | 10 mg | 20 mg | Very limited data to date show lower milk/plasma concentrations compared with citalopram21 |
| Fluoxetine | 10 mg | 60 mg | Long half-life can increase the potential for accumulation20 |
| Sertraline | 25 mg | 150 to 200 mg | Minimal detection of drug in infants’ serum19,20 |
| Paroxetine | 10 mg | 50 mg | Minimal detection of drug in infants’ serum19,20 |
| Tricyclics | |||
| Desipramine | 25 mg | 200 mg | Minimal detection of drug in infants’ serum19,22 |
| Imipramine | 25 mg | 200 mg | Minimal detection of drug in infants’ serum19,22 |
| Nortriptyline | 25 mg | 125 to 150 mg | Minimal detection of drug in infants’ serum19,22 |
| Others | |||
| Bupropion | 75 to 150 mg | 300 mg | Limited data available. Small increased risk of infant seizure (case report)24 |
| Mirtazapine | 7.5 mg | 45 mg | Limited data available. Well tolerated in a small study.23 Always monitor for changes in sleep (sedation and activation) and eating behaviors |
| Note: Clinical monitoring of the infant for adverse effects—including sedation, changes in sleep or feeding, and irritability—should be part of routine care | |||
Related resources
Clinician resource
- Cuijpers P, Brännmark JG, van Straten A. Psychological treatment of postpartum depression: a meta-analysis. J Clin Psychol 2008;64(1):103-18.
- American Psychiatric Association. Postpartum depression. www.healthyminds.org/postpartumdepression.cfm.
- Amitriptyline • Elavil, Endep
- Bupropion • Wellbutrin
- Citalopram • Celexa
- Clomipramine • Anafranil
- Desipramine • Norpramin
- Doxepin • Sinequan
- Duloxetine • Cymbalta
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Imipramine • Tofranil
- Mirtazapine • Remeron
- Nortriptyline • Aventyl
- Paroxetine • Paxil
- Sertraline • Zoloft
- Sertraline • Zoloft
- Venlafaxine • Effexor
Dr. Meltzer-Brody receives research/grant support from AstraZeneca, GlaxoSmithKline, and The Foundation of Hope.
Dr. Payne receives research/grant support from AstraZeneca, Novartis, Stanley Medical Research Institute, and Wyeth Pharmaceuticals.
Dr. Rubin reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Whether you encounter postpartum depression (PPD) in a patient you have been treating or in one referred by her obstetrician, early, aggressive treatment is essential. Although PPD shares some symptoms with major depressive disorder (MDD)—and may be a subtype of that disorder—it also has distinguishing characteristics, such as timing of symptom onset (Box 1).1,2 Two screening tools facilitate diagnosis (Box 2).2-4
Women with PPD usually respond to pharmacotherapy, but antidepressants’ potential effects on a nursing mother’s newborn are important to consider.
HPA axis dysregulation
Although the precise cause of PPD remains unclear, a better understanding is emerging of the complicated interplay of estrogen and progesterone with the hypothalamic-pituitary-adrenal (HPA) axis and other neuroregulatory systems associated with depressive illness. Two lines of evidence implicate hormonal dysregulation:
- Despite normal reproductive hormone levels, women with PPD may have an abnormal response to changes in these levels.5
- Abnormalities in HPA axis activity appear to be associated with reproductive endocrine-related mood disorders in vulnerable women, particularly during the transition from childbirth to the immediate postpartum period.
Most women will have mild mood and anxiety symptoms in the first few days to weeks postpartum—often referred to as the ‘baby blues’—but these symptoms usually resolve spontaneously. More severe and persistent depressed mood and anxiety should arouse suspicion of postpartum depression (PPD).
Although not categorized as a distinct disorder in the DSM-IV-TR, PPD is diagnosed using DSM-IV-TR criteria for a major depressive episode, including feelings of being overwhelmed, guilt or worthlessness, tearfulness, appetite change, difficulty sleeping (even when the baby is sleeping), difficulty concentrating, and loss of interest or pleasure in activities.2
PPD symptoms differ, however, in some important ways from those of nonpuerperal depression. Distinguishing characteristics of PPD are:
- severe worry, anxiety, and/or agitation
- fears of hurting the baby or oneself
- not having any interest in the baby.2
PPD usually begins within the first month postpartum but may occur later; the first 3 months appear to be the most vulnerable period.1
- high estrogen and progesterone levels
- a hyperactive HPA axis (normal during pregnancy)
- high plasma cortisol level, stimulated in part by high levels of estrogen and progesterone.7,8
Differences in HPA reactivity. In a normal HPA axis, the delivery of CRH from the paraventricular nucleus of the hypothalamus triggers the stimulation of adrenocorticotropic hormone (ACTH) from the anterior pituitary and, consequently, cortisol from the adrenal cortex. This hormonal system is regulated by negative feedback mediated by cortisol receptors on the anterior pituitary, hypothalamus, and hippocampus, as well as ACTH receptors in the anterior pituitary and CRH autoreceptors in the hypothalamus.10
A hallmark feature of the HPA axis in depression is altered response to stress and inability to maintain regulation:
- In MDD, HPA axis hyperactivity is one of the most robust biological findings.11 In general, women with MDD exhibit high baseline cortisol and an exaggerated response to the dexamethasone/corticotropin releasing hormone test.
- In contrast, women with PPD experience a more blunted ACTH response to CRH, which may reflect a hyporeactive HPA axis.9
It has been hypothesized that both increased cortisol and decreased cortisol (observed under conditions of sustained elevated gonadal steroid levels or withdrawal of gonadal steroids) may result in insufficient glucocorticoid signaling.13 Impaired glucocorticoid signaling may be the “final common pathway” leading to psychiatric disturbance in MDD and PPD.
Understanding the characteristics of HPA axis reactivity in women with PPD could improve early identification and, theoretically, prevention or immediate treatment for at-risk women. In addition to HPA axis dysregulation, disturbances in other endocrine systems may play a role in PPD. Women with antenatal total and free thyroxine concentrations in the lower euthyroid range may be at increased risk of developing postpartum depressive symptoms.14
Two well-validated, simple-to-administer postpartum depression (PPD) screening instruments are useful during the postnatal period:
- the Edinburgh Postnatal Depression Scale (EPDS),3 a 10-item self-report questionnaire that asks about mood, anxiety, guilt, and suicide ideation
- the Postpartum Depression Screening Scale (PDSS),4 a 35-item self-report questionnaire that asks about sleeping/eating disturbances, anxiety/insecurity, emotional lability, mental confusion, loss of self, guilt/shame, and suicide ideation.
If screening indicates a patient has PPD, her psychiatric history will influence your treatment selection. Pay particular attention to:
- past episodes of depression, hypomania, or mania
- severity and timing of those episodes
- treatment history, including documentation of response to antidepressants.2
Risks with or without treatment
PPD has potentially serious adverse consequences and needs to be aggressively treated. Ethical and practical challenges have hindered PPD research, however, and evidence to guide treatment is limited.15
Approximately 70% of mothers in the United States breast-feed their infants at least for the first 3 months.16 With any patient with PPD who is breast-feeding, carefully discuss the risk of antidepressant side effects for the mother and child.17
Also discuss potential risks and benefits of treatment vs no treatment.17 Potential risks of untreated depression include:
- impaired mother/child bonding because of ongoing maternal depressive illness
- impaired cognitive, emotional, and social development in the child.18
- educate the patient about potential antidepressant side effects for mother and baby
- avoid communicating “mixed messages” to the patient about the risk and benefits of treatment
- ensure the health of mother and baby.17
Antidepressants. In general, women with PPD respond well to antidepressant therapy. They may be hesitant to take any medication while breast-feeding because of possible harmful effects to their babies, but most studies examining antidepressant use by lactating women found low rates of adverse events in infants exposed to antidepressants (Table).19-24 Potential adverse effects include:
- sedation
- changes in sleep or feeding
- irritability.17
SSRIs. Few adverse events have been reported with sertraline, paroxetine, and fluvoxamine during lactation.20 However, paroxetine may be associated with increased risk of cardiac abnormalities in infants exposed during the first trimester of pregnancy.26 Two agents in this class may be less desirable:
- fluoxetine, because it has a long half-life
- citalopram, because of potentially high breast milk concentration.20
Tricyclics might be indicated for patients who responded to them previously or who have not responded to SSRIs. No adverse effects have been reported in breast-feeding infants receiving amitriptyline, clomipramine, desipramine, imipramine, or nortriptyline.25 Avoid doxepin, however, because it has the longest half-life among tricyclics, and adverse effects in infants—including respiratory distress, drowsiness, and vomiting—have been reported.
Other antidepressants. Venlafaxine and duloxetine are not recommended because of limited data about use of these agents during lactation. Bupropion poses a small increased risk of seizures in newborns but is not absolutely contraindicated.24 Trazodone also has limited data, but in clinical practice it has been used safely at low doses for many years.20
Psychotherapeutic techniques—including individual or group therapy—also can effectively reduce depressive symptoms in women with PPD.27
Table
Antidepressants for postpartum depression
| Medication | Starting dosage | Maximum dosage during lactation | Potential adverse event(s) |
|---|---|---|---|
| Selective serotonin reuptake inhibitors | |||
| Citalopram | 10 mg | 60 mg | High milk/plasma concentration at higher doses20 |
| Escitalopram | 10 mg | 20 mg | Very limited data to date show lower milk/plasma concentrations compared with citalopram21 |
| Fluoxetine | 10 mg | 60 mg | Long half-life can increase the potential for accumulation20 |
| Sertraline | 25 mg | 150 to 200 mg | Minimal detection of drug in infants’ serum19,20 |
| Paroxetine | 10 mg | 50 mg | Minimal detection of drug in infants’ serum19,20 |
| Tricyclics | |||
| Desipramine | 25 mg | 200 mg | Minimal detection of drug in infants’ serum19,22 |
| Imipramine | 25 mg | 200 mg | Minimal detection of drug in infants’ serum19,22 |
| Nortriptyline | 25 mg | 125 to 150 mg | Minimal detection of drug in infants’ serum19,22 |
| Others | |||
| Bupropion | 75 to 150 mg | 300 mg | Limited data available. Small increased risk of infant seizure (case report)24 |
| Mirtazapine | 7.5 mg | 45 mg | Limited data available. Well tolerated in a small study.23 Always monitor for changes in sleep (sedation and activation) and eating behaviors |
| Note: Clinical monitoring of the infant for adverse effects—including sedation, changes in sleep or feeding, and irritability—should be part of routine care | |||
Related resources
Clinician resource
- Cuijpers P, Brännmark JG, van Straten A. Psychological treatment of postpartum depression: a meta-analysis. J Clin Psychol 2008;64(1):103-18.
- American Psychiatric Association. Postpartum depression. www.healthyminds.org/postpartumdepression.cfm.
- Amitriptyline • Elavil, Endep
- Bupropion • Wellbutrin
- Citalopram • Celexa
- Clomipramine • Anafranil
- Desipramine • Norpramin
- Doxepin • Sinequan
- Duloxetine • Cymbalta
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Imipramine • Tofranil
- Mirtazapine • Remeron
- Nortriptyline • Aventyl
- Paroxetine • Paxil
- Sertraline • Zoloft
- Sertraline • Zoloft
- Venlafaxine • Effexor
Dr. Meltzer-Brody receives research/grant support from AstraZeneca, GlaxoSmithKline, and The Foundation of Hope.
Dr. Payne receives research/grant support from AstraZeneca, Novartis, Stanley Medical Research Institute, and Wyeth Pharmaceuticals.
Dr. Rubin reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Munk-Olsen T, Laursen TM, Pedersen CB, et al. New parents and mental disorders: a population-based register study. JAMA 2006;296:2592-9.
2. Gaynes BN, Gavin N, Meltzer-Brody S, et al. Perinatal depression: prevalence, screening accuracy, and screening outcomes. Evid Rep Technol Assess (Summ) 2005;(119):1-8.
3. Cox JL, Holden JM, Sagovsk R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry 1987;150:782-6.
4. Beck CT, Gable RK. Comparative analysis of the performance of the Postpartum Depression Screening Scale with two other depression instruments. Nurs Res 2001;50:242-50.
5. Bloch M, Schmidt PJ, Danaceau M, et al. Effects of gonadal steroids in women with a history of postpartum depression. Am J Psychiatry 2000;157(6):924-30.
6. Mastorakos G, Ilias I. Maternal and fetal hypothalamic-pituitary-adrenal axes during pregnancy and postpartum. Ann N Y Acad Sci 2003;997:136-49.
7. Noltern WE, Lindheimer MD, Rueckert PA, et al. Diurnal patterns and regulation of cortisol secretion in pregnancy. J Clin Endocrinology Metab 1980;51:466-72.
8. Bloch M, Daly RC, Rubinow DR. Endocrine factors in the etiology of postpartum depression. Compr Psychiatry 2003;44(3):234-46.
9. Magiakou MA, Mastorakos G, Rabin D. Hypothalamic-cortico-releasing hormone suppression during the postpartum period: implications for the increase in psychiatric manifestations at this time. J Clin Endocrinol Metab 1996;81:1912-7.
10. Jolley SN, Elmore S, Barnard KE, Carr D. Dysregulation of the hypothalamic-pituitary-adrenal axis in postpartum depression. Biol Res Nurs 2007;8:210-22.
11. Gold PW, Gabry KE, Yasuda MR, Chrousos GP. Divergent endocrine abnormalities in melancholic and atypical depression: clinical and pathophysiologic implications. Endocrinol Metab Clin North Am 2002;31:37-62.
12. Bloch M, Rubinow DR, Schmidt PJ. Cortisol response to ovine corticotropin-releasing hormone in a model of pregnancy and parturition in euthymic women with and without a history of postpartum depression. J Clin Endocrinol Metab 2005;90(2):695-9.
13. Raison CL, Miller AH. When not enough is too much: the role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. Am J Psychiatry 2003;160:1554-65.
14. Pedersen CA, Johnson JL, Silva S, et al. Antenatal thyroid correlates of postpartum depression. Psychoneuroendocrinology 2007;32(3):235-45.
15. Yonkers KA. The treatment of women suffering from depression who are either pregnant or breastfeeding. Am J Psychiatry 2007;164(10):1457-9.
16. Ryan AS, Wenjun Z, Acosta A. Breastfeeding continues to increase into the new millennium. Pediatrics 2002;110:1103-9.
17. Payne J. Antidepressant use in the postpartum period: practical considerations. Am J Psychiatry 2007;164:1329-32.
18. Murray L, Sinclair D, Cooper PJ, et al. The socioemotional development of 5-year old children of postnatally depressed mothers. J Child Psychol Psychiatry 1999;40:1259-71.
19. Weissman AM, Levt BT, Hartz AJ, et al. Pooled analysis of antidepressant levels in lactating mothers, breast milk, and nursing infants. Am J Psychiatry 2004;161(6):1066-78.
20. Eberhard-Gran M, Eskild A, Opjordsmoen S. Use of psychotropic medications in treating mood disorders during lactation: practical recommendations. CNS Drugs 2006;20:187-98.
21. Rampono J, Hackett LP, Kristensen JH, et al. Transfer of escitalopram and its metabolite demethylescitalopram into breastmilk. Br J Clin Pharmacol 2006;62(3):316-22.
22. Wisner KL, Perel JM, Findling RL. Antidepressant treatment during breast-feeding. Am J Psychiatry 1996;153(9):1132-7.
23. Kristensen JH, Ilett KF, Rampono J, et al. Transfer of the antidepressant mirtazapine into breast milk. Br J Clin Pharmacol 2007;63(3):322-7.
24. Chaudron LH, Schoenecker CJ. Bupropion and breastfeeding: a case of a possible infant seizure. J Clin Psychiatry 2004;65(6):881-2.
25. Misri S, Kostaras X. Postpartum depression: is there an Andrea Yates in your practice? Current Psychiatry 2002;1(5):22-9.
26. Louik C, Lin AE, Werler MM, et al. First-trimester use of selective serotonin-reuptake inhibitors and the risk of birth defects. N Engl J Med 2007;356(26):2675-83.
27. Dennis CL, Hodnett E. Psychosocial and psychological interventions for treating postpartum depression. Cochrane Database Syst Rev 2007;(4):CD006116.-
1. Munk-Olsen T, Laursen TM, Pedersen CB, et al. New parents and mental disorders: a population-based register study. JAMA 2006;296:2592-9.
2. Gaynes BN, Gavin N, Meltzer-Brody S, et al. Perinatal depression: prevalence, screening accuracy, and screening outcomes. Evid Rep Technol Assess (Summ) 2005;(119):1-8.
3. Cox JL, Holden JM, Sagovsk R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry 1987;150:782-6.
4. Beck CT, Gable RK. Comparative analysis of the performance of the Postpartum Depression Screening Scale with two other depression instruments. Nurs Res 2001;50:242-50.
5. Bloch M, Schmidt PJ, Danaceau M, et al. Effects of gonadal steroids in women with a history of postpartum depression. Am J Psychiatry 2000;157(6):924-30.
6. Mastorakos G, Ilias I. Maternal and fetal hypothalamic-pituitary-adrenal axes during pregnancy and postpartum. Ann N Y Acad Sci 2003;997:136-49.
7. Noltern WE, Lindheimer MD, Rueckert PA, et al. Diurnal patterns and regulation of cortisol secretion in pregnancy. J Clin Endocrinology Metab 1980;51:466-72.
8. Bloch M, Daly RC, Rubinow DR. Endocrine factors in the etiology of postpartum depression. Compr Psychiatry 2003;44(3):234-46.
9. Magiakou MA, Mastorakos G, Rabin D. Hypothalamic-cortico-releasing hormone suppression during the postpartum period: implications for the increase in psychiatric manifestations at this time. J Clin Endocrinol Metab 1996;81:1912-7.
10. Jolley SN, Elmore S, Barnard KE, Carr D. Dysregulation of the hypothalamic-pituitary-adrenal axis in postpartum depression. Biol Res Nurs 2007;8:210-22.
11. Gold PW, Gabry KE, Yasuda MR, Chrousos GP. Divergent endocrine abnormalities in melancholic and atypical depression: clinical and pathophysiologic implications. Endocrinol Metab Clin North Am 2002;31:37-62.
12. Bloch M, Rubinow DR, Schmidt PJ. Cortisol response to ovine corticotropin-releasing hormone in a model of pregnancy and parturition in euthymic women with and without a history of postpartum depression. J Clin Endocrinol Metab 2005;90(2):695-9.
13. Raison CL, Miller AH. When not enough is too much: the role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. Am J Psychiatry 2003;160:1554-65.
14. Pedersen CA, Johnson JL, Silva S, et al. Antenatal thyroid correlates of postpartum depression. Psychoneuroendocrinology 2007;32(3):235-45.
15. Yonkers KA. The treatment of women suffering from depression who are either pregnant or breastfeeding. Am J Psychiatry 2007;164(10):1457-9.
16. Ryan AS, Wenjun Z, Acosta A. Breastfeeding continues to increase into the new millennium. Pediatrics 2002;110:1103-9.
17. Payne J. Antidepressant use in the postpartum period: practical considerations. Am J Psychiatry 2007;164:1329-32.
18. Murray L, Sinclair D, Cooper PJ, et al. The socioemotional development of 5-year old children of postnatally depressed mothers. J Child Psychol Psychiatry 1999;40:1259-71.
19. Weissman AM, Levt BT, Hartz AJ, et al. Pooled analysis of antidepressant levels in lactating mothers, breast milk, and nursing infants. Am J Psychiatry 2004;161(6):1066-78.
20. Eberhard-Gran M, Eskild A, Opjordsmoen S. Use of psychotropic medications in treating mood disorders during lactation: practical recommendations. CNS Drugs 2006;20:187-98.
21. Rampono J, Hackett LP, Kristensen JH, et al. Transfer of escitalopram and its metabolite demethylescitalopram into breastmilk. Br J Clin Pharmacol 2006;62(3):316-22.
22. Wisner KL, Perel JM, Findling RL. Antidepressant treatment during breast-feeding. Am J Psychiatry 1996;153(9):1132-7.
23. Kristensen JH, Ilett KF, Rampono J, et al. Transfer of the antidepressant mirtazapine into breast milk. Br J Clin Pharmacol 2007;63(3):322-7.
24. Chaudron LH, Schoenecker CJ. Bupropion and breastfeeding: a case of a possible infant seizure. J Clin Psychiatry 2004;65(6):881-2.
25. Misri S, Kostaras X. Postpartum depression: is there an Andrea Yates in your practice? Current Psychiatry 2002;1(5):22-9.
26. Louik C, Lin AE, Werler MM, et al. First-trimester use of selective serotonin-reuptake inhibitors and the risk of birth defects. N Engl J Med 2007;356(26):2675-83.
27. Dennis CL, Hodnett E. Psychosocial and psychological interventions for treating postpartum depression. Cochrane Database Syst Rev 2007;(4):CD006116.-
Dissociative disorders unclear? Think ‘rainbows from pain blows’
Mr. D, age 45, presents to his primary care physician with panic attacks, nausea, shortness of breath, nightmares, and dizziness 6 months after being assaulted and robbed at an ATM. Following a routine medical workup, the physician diagnoses posttraumatic stress disorder (PTSD) and refers Mr. D for exposure and response prevention therapy.
During graded exposure sessions, Mr. D’s eyes sometimes glaze over and he seems to “float away” from the discussion. When the therapist asks about these symptoms, Mr. D reports having had them as long as he can remember. In school, he says, teachers thought he was a slow learner, a daydreamer, or had attention-deficit/hyperactivity disorder. From what he can recall of his childhood, he describes a history of trauma and neglect with a violent, drug-abusing father and absent mother.
Patients with a history of early abuse or neglect are at risk for dissociative phenomena and other trauma-related psychiatric disorders.1 The heterogeneous dissociative disorders are often hidden and unrecognized2 —as in Mr. D’s case—or present with unfamiliar or atypical symptoms. Understanding and identifying dissociative symptoms is important because:
- Dissociative symptoms worsen prognosis, whether patients have conversion disorders1 or psychogenic seizures3 or are in psychotherapy.4
- Dissociative states may impair memory encoding5 and decrease patients’ ability to remember therapeutic information.
- Symptoms (such as hearing voices in multiple personality disorder) can be confused with those of disorders with different treatment strategies (such as psychotic disorders).6
- Peritraumatic dissociation may be a risk factor for PTSD.7
This article presents a practical model for understanding dissociation, reviews clinical characteristics of this family of symptoms, and offers suggestions for assessing and treating patients with dissociative disorders.
Coming together, falling apart
Since Pierre Janet’s first reports on dissociative disorders, a number of theories and models of dissociation have been proposed,8 including empirically based, taxonomic models that address DSM-IV-TR categories (Table 1). The model I propose—which attaches a visual metaphor to dissociative phenomena—answers the question, “What is ‘dissociated’ in dissociation disorders?”
Table 1
DSM-IV-TR classification of dissociative disorders
| Disorder | Symptoms |
|---|---|
| Dissociative amnesia | A reversible loss of memory, typically preceded by a stressor |
| Dissociative fugue | Loss of memory and identity, along with travel away from home |
| Dissociative identity disorder (formerly multiple personality disorder) | Presence of different identity states, often with lack of connection between them; current models highlight the presence of recurrent dissociative intrusions into many aspects of executive function and self |
| Depersonalization disorder | Detachment from oneself as a present, feeling person (depersonalization) and the world (derealization) |
| Dissociative identity disorder NOS | Functionally disturbing dissociative symptoms that do not fit into any of the above |
| NOS: not otherwise specified | |
| Source: Diagnostic and statistical manual of mental disorders. 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000 | |
One paired component is a detached “observer” and a more embodied, feeling “experiencer.” The observer is a perspective that begets metacognition (thinking about one’s inner world) and self-observation; it resides in the same body as soma-based “feelings” that unconsciously contribute to the sense of “being present” with oneself and the world in the moment.9
A second component is voluntary access to one’s autobiographical memories (memories about the self in time), which are constantly “updated” and integrated with current experiences. This component allows one to distinguish between remembered (past) experiences and “firsthand” (present) experience.
Three other components of normal consciousness are:
- a sense of agency and voluntary control over one’s mental contents, mental activity, and bodily movements
- an ongoing connection with one’s body and mind and an understanding of where sensations and images come from
- a sense of sequential experience, with relatively smooth transitions (from self at work to self at home, self a week ago to self today, etc) that have a singular referent (an identity).
- separation of the “observer” and “experiencer” occurs in depersonalization disorder
- reversible loss of ability to access memories characterizes dissociative amnesia
- disconnection between sequential experiences is a part of dissociative identity disorder.
10 Functional neuroimaging of dissociation supports an understanding of these symptoms as “disconnection syndromes” (Box).
From a neurophysiologic perspective, mental states may be viewed as arising from synchronized integration of the activity of functionally specialized brain regions. Functional neuroimaging of dissociation supports an understanding of these symptoms as ‘disconnection syndromes.’
Functional neuroimaging. Different ‘identities’—sometimes called a traumatic personality state and neutral personality state—demonstrate different patterns of cerebral blood flow, subjective reports, and peripheral physiologic parameters (blood pressure, heart rate).a
Functional imaging of traumatic dissociation shows active suppression of limbic regions (amygdala) and increased activity in dorsolateral prefrontal areas.b Similarly, neuroimaging of depersonalization disorder show increased neural activity in prefrontal regions associated with affect regulation and decreased activity in emotion-related areas.c,d
Speed. Dissociative responses occur extremely rapidly. Using EEG, which allows finer temporal resolution than functional imaging studies, Kirino et ale showed reversible attenuation of a specific EEG signal within 300 msec during dissociative episodes. This ultra-rapid neural reflex was correlated with allocation of attentional and working memory resources, perhaps with the goal of minimizing memory activation and resurgence of affect-laden memories.e
Hormonal. Stress-related disorders cause perturbations in neurohormonal function. Simeon et alf found a distinct pattern of stress-induced HPA axis dysregulation in dissociative patients compared with PTSD patients and healthy controls. Similar results were seen in patients with borderline personality disorder and dissociative symptoms.g
Structural imaging. Stress-related neurohormonal perturbations are known to affect critical neural structures, including the hippocampus. Using MRI, Vermetten et alh found significantly decreased amygdala and hippocampal volumes in patients with dissociative identity disorder.
EEG: electroencephalography; HPA: hypothalamic-pituitary-adrenal; PTSD: posttraumatic stress disorder
Reference Citations: click here
Causes of dissociative disorders
As with many psychiatric disorders, the etiology of dissociative phenomena is thought to include the individual patient’s temperamental or constitutional predispositions11 as well as a strong contribution of environmental trauma (early abuse, neglect).12
Constitutional predisposition for developing a dissociative disorder may include personality traits such as being easily hypnotized, mental absorption, suggestibility, and a tendency to fantasize.13 These characteristics fueled concerns in the 1990s that therapists may contribute to dissociative identity disorder by “digging” for repressed memories in susceptible patients and creating “pseudomemories” of events that did not happen.14
The issue of repressed traumatic memory and its role in therapy is extremely controversial and contributes to the complexity of psychotherapeutic treatment of dissociation.15
- shame and secrecy of early sexual or physical abuse and potential for victims to repress traumatic memories
- lability of memory, potential for suggestibility, and difficulty with verification.14
- early relationships are one of the primary ways that humans learn to regulate distress
- early trauma frequently includes pathology in caregiving relationships, including overt role reversal, abuse, and neglect.
Finally, remember that transient dissociative symptoms can be considered normal in high-stress situations. Intensive military training has been found to be associated with a very high incidence (96%) of dissociative symptoms in army recruits.17
Identifying ‘hidden’ phenomena
Dissociative disorders have been called “diseases of hiddenness”18 because:
- Many of their clinical characteristics— sense of identity, memory, connectedness, somatosensory phenomena—are alterations in subjective phenomena that lack clearly observable symptoms.
- Patients are often reluctant to seek help or divulge their symptoms to clinicians.
- When dissociative symptoms are obvious—such as multiple personalities or sudden loss of memories—they may be dismissed or evoke skepticism because of their dramatic presentation.
For more targeted screening, the self-report Dissociative Experiences Scale (DES)21 is useful for clinical assessment in conjunction with the clinician-administered diagnostic Structured Clinical Interview for DSM-IV Dissociative Disorder (SCID-D).22
Table 2
With these findings, consider screening for dissociation
| Posttraumatic stress disorder |
| Certain personality disorders (especially borderline personality disorder) |
| Somatoform disorders (conversion disorders and nonepileptic seizures) |
| Eating disorders |
| Substance use disorders |
| Extensive history of trauma or neglect |
| Self-harm behavior |
As in Mr. D’s case, dissociative phenomena may attenuate the benefit of post-trauma therapeutic interventions, especially those involving exposure. Therefore:
- assess post-trauma patients for dissociation before you start treatment
- make specific alterations in psychotherapy for such patients, as described below.
Table 3
Differential diagnosis: Dissociation ‘look-alikes’
| Dissociation symptom | Can be confused with: |
|---|---|
| Visual or auditory hallucinations, other ‘first-rank’ psychotic symptoms in dissociative identity disorder | Psychotic disorder |
| ‘Blanking out’ (cognitive disruption) | ADHD, seizures |
| Somatoform (conversion) symptoms | A variety of nonpsychiatric medical problems, including pelvic or abdominal pathology and headaches |
| Dissociative memory lapses | Learning disability, not paying attention |
| ‘Switching’ between states | Bipolar disorder, rapid cycling |
| Lack of emotional reaction to traumatic stimuli(numbing response) | Healthy coping |
| ADHD: attention-deficit/hyperactivity disorder | |
Recreational drugs such as ketamine, methylenedioxymethamphetamine (“Ecstasy”), hallucinogens, marijuana, and dextromethorphan also can induce dissociative states. Consider evaluating for use of these substances, some of which may not be detected on a routine drug screen.24
CASE CONTINUED: A tactical shift
Internal distress—such as when remembering painful events—clearly is linked with the appearance of Mr. D’s symptoms. The therapist—recognizing unacknowledged dissociative phenomena—changes Mr. D’s therapeutic strategy from exposure therapy to affect and anxiety regulation, with an explicit focus on attachment security (safety).
The therapist explains to Mr. D that dissociation symptoms are a response to distress, and he can learn more adaptive distress regulation in therapy. The in-session focus shifts to include more direct attention to components of the therapy relationship, including overt disclosure of the therapist’s positive regard and commitment to help the patient and frequent pauses to “check in” that the patient feels present, safe, and understood. With this new focus, Mr. D’s dissociative symptoms resolve and he feels more ready to face and overcome his fear and avoided memories.
Psychotherapy: Putting pieces together
Psychotherapy is the primary treatment, based on understanding dissociative disorders as manifestations of distress-related, traumatic fragmentation of the sense of self, interpersonal relatedness, and capacity for adaptive affect regulation (Table 4).
Table 4
Tips for conceptualizing dissociative disorders
| Ground your understanding of this class of disorders as distress-related breakdowns in functional connection and integration among components of normal consciousness |
| Consider the overlap among dissociation, certain somatoform disorders (conversion symptoms, pseudoseizures), and PTSD |
| Maintain a high index of suspicion for dissociative symptoms in patients with early trauma or neglect (consider screening for this); do further evaluation with dissociative-specific tools |
| Avoid the tendency to assume that reversible, unfamiliar, or peculiar symptoms imply volition or lack of an organic basis |
| PTSD: posttraumatic stress disorder |
Safety, stabilization, and symptom reduction. Providing a safe therapeutic relationship is a primary and necessary part of DID treatment. On that platform, a first step in reintegrating distressing material into the self involves building the patient’s capacity for conscious, flexible affect regulation. This keeps anxiety and distress within a therapeutic “window.”
Integration of identity and person. Treatment ends when formerly unintegrated or dissociated experiences or parts of the self are integrated into a coherent whole, and the patient can deal adaptively with inter-personal relationships and distress without fragmentation.
Adjunctive medications
Few studies have addressed using psychopharmacologic interventions in the heterogeneous dissociative disorders. GABAA antagonism and 5-HT2a/2c agonism have induced psychotic and dissociative-like symptoms in healthy men,29 and alterations in enzymes such as catechol-O-methyltransferase (COMT) may explain individual vulnerability to trauma.30 Reports of dissociation related to ketamine31 and marijuana32 implicate other neurotransmitter systems in their etiology.
DID. Similar to guidelines for borderline personality disorder,33 guidelines for DID suggest using medications to treat the most prominent symptom clusters such as insomnia, affective instability, and posttraumatic intrusions.
Depersonalization disorder. Trials of fluoxetine and lamotrigine showed no benefit in depersonalization disorder.34,35 In an open trial of 14 patients, naloxone (mean 120 mg/d) reduced depersonalization symptoms by 30%, as measured by 3 validated scales.36
Related Resources
- International Society for the Study of Trauma and Dissociation. Site for professionals. www.isst-d.org
- National Alliance on Mental Illness (NAMI). Patient education on dissociative disorders. www.nami.org/Content/ContentGroups/Helpline1/Dissociative_Disorders.htm
- Fluoxetine • Prozac
- Lamotrigine • Lamictal
- Naloxone • Narcan
- Paroxetine • Paxil
Dr. MacDonald is a speaker for Eli Lilly and Company, Janssen, L.P., and Pfizer Inc.
1. Sar V, Akyuz G, Kundakci T, et al. Childhood trauma, dissociation, and psychiatric comorbidity in patients with conversion disorder. Am J Psychiatry 2004;161:2271-6.
2. Foote B, Smolin Y, Kaplan M, et al. Prevalence of dissociative disorders in psychiatric outpatients. Am J Psychiatry 2006;163:623-9.
3. Reuber M, Pukrop R, Bauer J, et al. Outcome in psychogenic nonepileptic seizures: 1 to 10-year follow-up in 164 patients. Ann Neurol 2003;53:305-11.
4. Spitzer C, Barnow S, Freyberger HJ, Grabe HJ. Dissociation predicts symptom-related treatment outcome in short-term inpatient psychotherapy. Aust N Z J Psychiatry 2007;41:682-7.
5. Allen JG, Console DA, Lewis L. Dissociative detachment and memory impairment: reversible amnesia or encoding failure? Compr Psychiatry 1999;40:160-71.
6. Dell PF. A new model of dissociative identity disorder. Psychiatr Clin North Am 2006;29:1-26.
7. Shalev AY, Freedman S. PTSD following terrorist attacks: a prospective evaluation. Am J Psychiatry 2005;162:1188-91.
8. Steinberg M, Rounsaville B, Cicchetti DV. The Structured Clinical Interview for DSM-III-R Dissociative Disorders: preliminary report on a new diagnostic instrument. Am J Psychiatry 1990;147:76-82.
9. Damasio A. The feeling of what happens: body and emotion in the making of consciousness. New York, NY: Harcourt, Inc; 1999.
10. Alkire MT, Miller J. General anesthesia and the neural correlates of consciousness. Prog Brain Res 2005;150:229-44.
11. Simeon D, Guralnik O, Knutelska M, Schmeidler J. Personality factors associated with dissociation: temperament, defenses, and cognitive schemata. Am J Psychiatry 2002;159(3):489-91.
12. Kihlstrom JF. Dissociative disorders. Annu Rev Clin Psychol 2005;1:227-53.
13. Isaac M, Chand PK. Dissociative and conversion disorders: defining boundaries. Curr Opin Psychiatry 2006;19(1):61-6.
14. Laney C, Loftus EF. Traumatic memories are not necessarily accurate memories. Can J Psychiatry 2005;50(13):823-8.
15. Loftus EF, Davis D. Recovered memories. Annu Rev Clin Psychol 2006;2:469-98.
16. Lyons-Ruth K, Dutra L, Schuder MR, Bianchi I. From infant attachment disorganization to adult dissociation: relational adaptations or traumatic experiences? Psychiatr Clin North Am 2006;29(1):63-86.
17. Morgan CA, 3rd, Hazlett G, Wang S, et al. Symptoms of dissociation in humans experiencing acute, uncontrollable stress: a prospective investigation. Am J Psychiatry 2001;158(8):1239-47.
18. Spiegel D. Recognizing traumatic dissociation. Am J Psychiatry 2006;163(4):566-8.
19. Scher CD, Stein MB, Asmundson GJ, et al. The childhood trauma questionnaire in a community sample: psychometric properties and normative data. J Trauma Stress 2001;14:843-57.
20. Teicher MH, Andersen SL, Polcari A, et al. The neurobiological consequences of early stress and childhood maltreatment. Neurosci Biobehav Rev 2003;27:33-44.
21. Bernstein EM, Putnam FW. Development, reliability, and validity of a dissociation scale. J Nerv Ment Dis 1986;174(12):727-35.
22. Steinberg M, Rounsaville B, Cicchetti D. Detection of dissociative disorders in psychiatric patients by a screening instrument and a structured diagnostic interview. Am J Psychiatry 1991;148(8):1050-4.
23. Devinsky O, Putnam F, Grafman J, et al. Dissociative states and epilepsy. Neurology 1989;39:835-40.
24. Schonenberg M, Reichwald U, Domes G, et al. Effects of peritraumatic ketamine medication on early and sustained posttraumatic stress symptoms in moderately injured accident victims. Psychopharmacology (Berl) 2005;182(3):420-5.
25. Hunter EC, Phillips ML, Chalder T, et al. Depersonalisation disorder: a cognitive-behavioural conceptualisation. Behav Res Ther 2003;41:1451-67.
26. Guidelines for treating dissociative identity disorder in adults (2005). J Trauma Dissociation 2005;6(4):69-149.
27. van der Hart O, Nijenhuis E. Generalized dissociative amnesia: episodic, semantic and procedural memories lost and found. Aust N Z J Psychiatry 2001;35:589-600.
28. Holmes EA, Brown RJ, Mansell W, et al. Are there two qualitatively distinct forms of dissociation? A review and some clinical implications. Clin Psychol Rev 2005;25(1):1-23.
29. D’Souza DC, Gil RB, Zuzarte E, et al. gamma-Aminobutyric acid-serotonin interactions in healthy men: implications for network models of psychosis and dissociation. Biol Psychiatry 2006;59(2):128-37.
30. Savitz JB, van der Merwe L, Newman TK, et al. The relationship between childhood abuse and dissociation. Is it influenced by catechol-O-methyltransferase (COMT) activity? Int J Neuropsychopharmacol 2008;11:149-61.
31. Curran HV, Morgan C. Cognitive, dissociative and psychotogenic effects of ketamine in recreational users on the night of drug use and 3 days later. Addiction 2000;95:575-90.
32. Mathew RJ, Wilson WH, Humphreys D, et al. Depersonalization after marijuana smoking. Biol Psychiatry 1993;33:431-41.
33. American Psychiatric Association. Practice guideline for the treatment of patients with borderline personality disorder. Am J Psychiatry 2001;158(10 suppl):1-52.
34. Sierra M, Phillips ML, Ivin G, et al. A placebo-controlled, cross-over trial of lamotrigine in depersonalization disorder. J Psychopharmacol 2003;17:103-5.
35. Simeon D, Guralnik O, Schmeidler J, Knutelska M. Fluoxetine therapy in depersonalisation disorder: randomised controlled trial. Br J Psychiatry 2004;185:31-6.
36. Simeon D, Knutelska M. An open trial of naltrexone in the treatment of depersonalization disorder. J Clin Psychopharmacol 2005;25:267-70.
37. Stein DJ, Ipser JC, Seedat S. Pharmacotherapy for post traumatic stress disorder (PTSD). Cochrane Database Syst Rev 2006(1):CD002795.-
38. Marshall RD, Lewis-Fernandez R, Blanco C, et al. A controlled trial of paroxetine for chronic PTSD, dissociation, and interpersonal problems in mostly minority adults. Depress Anxiety 2007;24:77-84.
Mr. D, age 45, presents to his primary care physician with panic attacks, nausea, shortness of breath, nightmares, and dizziness 6 months after being assaulted and robbed at an ATM. Following a routine medical workup, the physician diagnoses posttraumatic stress disorder (PTSD) and refers Mr. D for exposure and response prevention therapy.
During graded exposure sessions, Mr. D’s eyes sometimes glaze over and he seems to “float away” from the discussion. When the therapist asks about these symptoms, Mr. D reports having had them as long as he can remember. In school, he says, teachers thought he was a slow learner, a daydreamer, or had attention-deficit/hyperactivity disorder. From what he can recall of his childhood, he describes a history of trauma and neglect with a violent, drug-abusing father and absent mother.
Patients with a history of early abuse or neglect are at risk for dissociative phenomena and other trauma-related psychiatric disorders.1 The heterogeneous dissociative disorders are often hidden and unrecognized2 —as in Mr. D’s case—or present with unfamiliar or atypical symptoms. Understanding and identifying dissociative symptoms is important because:
- Dissociative symptoms worsen prognosis, whether patients have conversion disorders1 or psychogenic seizures3 or are in psychotherapy.4
- Dissociative states may impair memory encoding5 and decrease patients’ ability to remember therapeutic information.
- Symptoms (such as hearing voices in multiple personality disorder) can be confused with those of disorders with different treatment strategies (such as psychotic disorders).6
- Peritraumatic dissociation may be a risk factor for PTSD.7
This article presents a practical model for understanding dissociation, reviews clinical characteristics of this family of symptoms, and offers suggestions for assessing and treating patients with dissociative disorders.
Coming together, falling apart
Since Pierre Janet’s first reports on dissociative disorders, a number of theories and models of dissociation have been proposed,8 including empirically based, taxonomic models that address DSM-IV-TR categories (Table 1). The model I propose—which attaches a visual metaphor to dissociative phenomena—answers the question, “What is ‘dissociated’ in dissociation disorders?”
Table 1
DSM-IV-TR classification of dissociative disorders
| Disorder | Symptoms |
|---|---|
| Dissociative amnesia | A reversible loss of memory, typically preceded by a stressor |
| Dissociative fugue | Loss of memory and identity, along with travel away from home |
| Dissociative identity disorder (formerly multiple personality disorder) | Presence of different identity states, often with lack of connection between them; current models highlight the presence of recurrent dissociative intrusions into many aspects of executive function and self |
| Depersonalization disorder | Detachment from oneself as a present, feeling person (depersonalization) and the world (derealization) |
| Dissociative identity disorder NOS | Functionally disturbing dissociative symptoms that do not fit into any of the above |
| NOS: not otherwise specified | |
| Source: Diagnostic and statistical manual of mental disorders. 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000 | |
One paired component is a detached “observer” and a more embodied, feeling “experiencer.” The observer is a perspective that begets metacognition (thinking about one’s inner world) and self-observation; it resides in the same body as soma-based “feelings” that unconsciously contribute to the sense of “being present” with oneself and the world in the moment.9
A second component is voluntary access to one’s autobiographical memories (memories about the self in time), which are constantly “updated” and integrated with current experiences. This component allows one to distinguish between remembered (past) experiences and “firsthand” (present) experience.
Three other components of normal consciousness are:
- a sense of agency and voluntary control over one’s mental contents, mental activity, and bodily movements
- an ongoing connection with one’s body and mind and an understanding of where sensations and images come from
- a sense of sequential experience, with relatively smooth transitions (from self at work to self at home, self a week ago to self today, etc) that have a singular referent (an identity).
- separation of the “observer” and “experiencer” occurs in depersonalization disorder
- reversible loss of ability to access memories characterizes dissociative amnesia
- disconnection between sequential experiences is a part of dissociative identity disorder.
10 Functional neuroimaging of dissociation supports an understanding of these symptoms as “disconnection syndromes” (Box).
From a neurophysiologic perspective, mental states may be viewed as arising from synchronized integration of the activity of functionally specialized brain regions. Functional neuroimaging of dissociation supports an understanding of these symptoms as ‘disconnection syndromes.’
Functional neuroimaging. Different ‘identities’—sometimes called a traumatic personality state and neutral personality state—demonstrate different patterns of cerebral blood flow, subjective reports, and peripheral physiologic parameters (blood pressure, heart rate).a
Functional imaging of traumatic dissociation shows active suppression of limbic regions (amygdala) and increased activity in dorsolateral prefrontal areas.b Similarly, neuroimaging of depersonalization disorder show increased neural activity in prefrontal regions associated with affect regulation and decreased activity in emotion-related areas.c,d
Speed. Dissociative responses occur extremely rapidly. Using EEG, which allows finer temporal resolution than functional imaging studies, Kirino et ale showed reversible attenuation of a specific EEG signal within 300 msec during dissociative episodes. This ultra-rapid neural reflex was correlated with allocation of attentional and working memory resources, perhaps with the goal of minimizing memory activation and resurgence of affect-laden memories.e
Hormonal. Stress-related disorders cause perturbations in neurohormonal function. Simeon et alf found a distinct pattern of stress-induced HPA axis dysregulation in dissociative patients compared with PTSD patients and healthy controls. Similar results were seen in patients with borderline personality disorder and dissociative symptoms.g
Structural imaging. Stress-related neurohormonal perturbations are known to affect critical neural structures, including the hippocampus. Using MRI, Vermetten et alh found significantly decreased amygdala and hippocampal volumes in patients with dissociative identity disorder.
EEG: electroencephalography; HPA: hypothalamic-pituitary-adrenal; PTSD: posttraumatic stress disorder
Reference Citations: click here
Causes of dissociative disorders
As with many psychiatric disorders, the etiology of dissociative phenomena is thought to include the individual patient’s temperamental or constitutional predispositions11 as well as a strong contribution of environmental trauma (early abuse, neglect).12
Constitutional predisposition for developing a dissociative disorder may include personality traits such as being easily hypnotized, mental absorption, suggestibility, and a tendency to fantasize.13 These characteristics fueled concerns in the 1990s that therapists may contribute to dissociative identity disorder by “digging” for repressed memories in susceptible patients and creating “pseudomemories” of events that did not happen.14
The issue of repressed traumatic memory and its role in therapy is extremely controversial and contributes to the complexity of psychotherapeutic treatment of dissociation.15
- shame and secrecy of early sexual or physical abuse and potential for victims to repress traumatic memories
- lability of memory, potential for suggestibility, and difficulty with verification.14
- early relationships are one of the primary ways that humans learn to regulate distress
- early trauma frequently includes pathology in caregiving relationships, including overt role reversal, abuse, and neglect.
Finally, remember that transient dissociative symptoms can be considered normal in high-stress situations. Intensive military training has been found to be associated with a very high incidence (96%) of dissociative symptoms in army recruits.17
Identifying ‘hidden’ phenomena
Dissociative disorders have been called “diseases of hiddenness”18 because:
- Many of their clinical characteristics— sense of identity, memory, connectedness, somatosensory phenomena—are alterations in subjective phenomena that lack clearly observable symptoms.
- Patients are often reluctant to seek help or divulge their symptoms to clinicians.
- When dissociative symptoms are obvious—such as multiple personalities or sudden loss of memories—they may be dismissed or evoke skepticism because of their dramatic presentation.
For more targeted screening, the self-report Dissociative Experiences Scale (DES)21 is useful for clinical assessment in conjunction with the clinician-administered diagnostic Structured Clinical Interview for DSM-IV Dissociative Disorder (SCID-D).22
Table 2
With these findings, consider screening for dissociation
| Posttraumatic stress disorder |
| Certain personality disorders (especially borderline personality disorder) |
| Somatoform disorders (conversion disorders and nonepileptic seizures) |
| Eating disorders |
| Substance use disorders |
| Extensive history of trauma or neglect |
| Self-harm behavior |
As in Mr. D’s case, dissociative phenomena may attenuate the benefit of post-trauma therapeutic interventions, especially those involving exposure. Therefore:
- assess post-trauma patients for dissociation before you start treatment
- make specific alterations in psychotherapy for such patients, as described below.
Table 3
Differential diagnosis: Dissociation ‘look-alikes’
| Dissociation symptom | Can be confused with: |
|---|---|
| Visual or auditory hallucinations, other ‘first-rank’ psychotic symptoms in dissociative identity disorder | Psychotic disorder |
| ‘Blanking out’ (cognitive disruption) | ADHD, seizures |
| Somatoform (conversion) symptoms | A variety of nonpsychiatric medical problems, including pelvic or abdominal pathology and headaches |
| Dissociative memory lapses | Learning disability, not paying attention |
| ‘Switching’ between states | Bipolar disorder, rapid cycling |
| Lack of emotional reaction to traumatic stimuli(numbing response) | Healthy coping |
| ADHD: attention-deficit/hyperactivity disorder | |
Recreational drugs such as ketamine, methylenedioxymethamphetamine (“Ecstasy”), hallucinogens, marijuana, and dextromethorphan also can induce dissociative states. Consider evaluating for use of these substances, some of which may not be detected on a routine drug screen.24
CASE CONTINUED: A tactical shift
Internal distress—such as when remembering painful events—clearly is linked with the appearance of Mr. D’s symptoms. The therapist—recognizing unacknowledged dissociative phenomena—changes Mr. D’s therapeutic strategy from exposure therapy to affect and anxiety regulation, with an explicit focus on attachment security (safety).
The therapist explains to Mr. D that dissociation symptoms are a response to distress, and he can learn more adaptive distress regulation in therapy. The in-session focus shifts to include more direct attention to components of the therapy relationship, including overt disclosure of the therapist’s positive regard and commitment to help the patient and frequent pauses to “check in” that the patient feels present, safe, and understood. With this new focus, Mr. D’s dissociative symptoms resolve and he feels more ready to face and overcome his fear and avoided memories.
Psychotherapy: Putting pieces together
Psychotherapy is the primary treatment, based on understanding dissociative disorders as manifestations of distress-related, traumatic fragmentation of the sense of self, interpersonal relatedness, and capacity for adaptive affect regulation (Table 4).
Table 4
Tips for conceptualizing dissociative disorders
| Ground your understanding of this class of disorders as distress-related breakdowns in functional connection and integration among components of normal consciousness |
| Consider the overlap among dissociation, certain somatoform disorders (conversion symptoms, pseudoseizures), and PTSD |
| Maintain a high index of suspicion for dissociative symptoms in patients with early trauma or neglect (consider screening for this); do further evaluation with dissociative-specific tools |
| Avoid the tendency to assume that reversible, unfamiliar, or peculiar symptoms imply volition or lack of an organic basis |
| PTSD: posttraumatic stress disorder |
Safety, stabilization, and symptom reduction. Providing a safe therapeutic relationship is a primary and necessary part of DID treatment. On that platform, a first step in reintegrating distressing material into the self involves building the patient’s capacity for conscious, flexible affect regulation. This keeps anxiety and distress within a therapeutic “window.”
Integration of identity and person. Treatment ends when formerly unintegrated or dissociated experiences or parts of the self are integrated into a coherent whole, and the patient can deal adaptively with inter-personal relationships and distress without fragmentation.
Adjunctive medications
Few studies have addressed using psychopharmacologic interventions in the heterogeneous dissociative disorders. GABAA antagonism and 5-HT2a/2c agonism have induced psychotic and dissociative-like symptoms in healthy men,29 and alterations in enzymes such as catechol-O-methyltransferase (COMT) may explain individual vulnerability to trauma.30 Reports of dissociation related to ketamine31 and marijuana32 implicate other neurotransmitter systems in their etiology.
DID. Similar to guidelines for borderline personality disorder,33 guidelines for DID suggest using medications to treat the most prominent symptom clusters such as insomnia, affective instability, and posttraumatic intrusions.
Depersonalization disorder. Trials of fluoxetine and lamotrigine showed no benefit in depersonalization disorder.34,35 In an open trial of 14 patients, naloxone (mean 120 mg/d) reduced depersonalization symptoms by 30%, as measured by 3 validated scales.36
Related Resources
- International Society for the Study of Trauma and Dissociation. Site for professionals. www.isst-d.org
- National Alliance on Mental Illness (NAMI). Patient education on dissociative disorders. www.nami.org/Content/ContentGroups/Helpline1/Dissociative_Disorders.htm
- Fluoxetine • Prozac
- Lamotrigine • Lamictal
- Naloxone • Narcan
- Paroxetine • Paxil
Dr. MacDonald is a speaker for Eli Lilly and Company, Janssen, L.P., and Pfizer Inc.
Mr. D, age 45, presents to his primary care physician with panic attacks, nausea, shortness of breath, nightmares, and dizziness 6 months after being assaulted and robbed at an ATM. Following a routine medical workup, the physician diagnoses posttraumatic stress disorder (PTSD) and refers Mr. D for exposure and response prevention therapy.
During graded exposure sessions, Mr. D’s eyes sometimes glaze over and he seems to “float away” from the discussion. When the therapist asks about these symptoms, Mr. D reports having had them as long as he can remember. In school, he says, teachers thought he was a slow learner, a daydreamer, or had attention-deficit/hyperactivity disorder. From what he can recall of his childhood, he describes a history of trauma and neglect with a violent, drug-abusing father and absent mother.
Patients with a history of early abuse or neglect are at risk for dissociative phenomena and other trauma-related psychiatric disorders.1 The heterogeneous dissociative disorders are often hidden and unrecognized2 —as in Mr. D’s case—or present with unfamiliar or atypical symptoms. Understanding and identifying dissociative symptoms is important because:
- Dissociative symptoms worsen prognosis, whether patients have conversion disorders1 or psychogenic seizures3 or are in psychotherapy.4
- Dissociative states may impair memory encoding5 and decrease patients’ ability to remember therapeutic information.
- Symptoms (such as hearing voices in multiple personality disorder) can be confused with those of disorders with different treatment strategies (such as psychotic disorders).6
- Peritraumatic dissociation may be a risk factor for PTSD.7
This article presents a practical model for understanding dissociation, reviews clinical characteristics of this family of symptoms, and offers suggestions for assessing and treating patients with dissociative disorders.
Coming together, falling apart
Since Pierre Janet’s first reports on dissociative disorders, a number of theories and models of dissociation have been proposed,8 including empirically based, taxonomic models that address DSM-IV-TR categories (Table 1). The model I propose—which attaches a visual metaphor to dissociative phenomena—answers the question, “What is ‘dissociated’ in dissociation disorders?”
Table 1
DSM-IV-TR classification of dissociative disorders
| Disorder | Symptoms |
|---|---|
| Dissociative amnesia | A reversible loss of memory, typically preceded by a stressor |
| Dissociative fugue | Loss of memory and identity, along with travel away from home |
| Dissociative identity disorder (formerly multiple personality disorder) | Presence of different identity states, often with lack of connection between them; current models highlight the presence of recurrent dissociative intrusions into many aspects of executive function and self |
| Depersonalization disorder | Detachment from oneself as a present, feeling person (depersonalization) and the world (derealization) |
| Dissociative identity disorder NOS | Functionally disturbing dissociative symptoms that do not fit into any of the above |
| NOS: not otherwise specified | |
| Source: Diagnostic and statistical manual of mental disorders. 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000 | |
One paired component is a detached “observer” and a more embodied, feeling “experiencer.” The observer is a perspective that begets metacognition (thinking about one’s inner world) and self-observation; it resides in the same body as soma-based “feelings” that unconsciously contribute to the sense of “being present” with oneself and the world in the moment.9
A second component is voluntary access to one’s autobiographical memories (memories about the self in time), which are constantly “updated” and integrated with current experiences. This component allows one to distinguish between remembered (past) experiences and “firsthand” (present) experience.
Three other components of normal consciousness are:
- a sense of agency and voluntary control over one’s mental contents, mental activity, and bodily movements
- an ongoing connection with one’s body and mind and an understanding of where sensations and images come from
- a sense of sequential experience, with relatively smooth transitions (from self at work to self at home, self a week ago to self today, etc) that have a singular referent (an identity).
- separation of the “observer” and “experiencer” occurs in depersonalization disorder
- reversible loss of ability to access memories characterizes dissociative amnesia
- disconnection between sequential experiences is a part of dissociative identity disorder.
10 Functional neuroimaging of dissociation supports an understanding of these symptoms as “disconnection syndromes” (Box).
From a neurophysiologic perspective, mental states may be viewed as arising from synchronized integration of the activity of functionally specialized brain regions. Functional neuroimaging of dissociation supports an understanding of these symptoms as ‘disconnection syndromes.’
Functional neuroimaging. Different ‘identities’—sometimes called a traumatic personality state and neutral personality state—demonstrate different patterns of cerebral blood flow, subjective reports, and peripheral physiologic parameters (blood pressure, heart rate).a
Functional imaging of traumatic dissociation shows active suppression of limbic regions (amygdala) and increased activity in dorsolateral prefrontal areas.b Similarly, neuroimaging of depersonalization disorder show increased neural activity in prefrontal regions associated with affect regulation and decreased activity in emotion-related areas.c,d
Speed. Dissociative responses occur extremely rapidly. Using EEG, which allows finer temporal resolution than functional imaging studies, Kirino et ale showed reversible attenuation of a specific EEG signal within 300 msec during dissociative episodes. This ultra-rapid neural reflex was correlated with allocation of attentional and working memory resources, perhaps with the goal of minimizing memory activation and resurgence of affect-laden memories.e
Hormonal. Stress-related disorders cause perturbations in neurohormonal function. Simeon et alf found a distinct pattern of stress-induced HPA axis dysregulation in dissociative patients compared with PTSD patients and healthy controls. Similar results were seen in patients with borderline personality disorder and dissociative symptoms.g
Structural imaging. Stress-related neurohormonal perturbations are known to affect critical neural structures, including the hippocampus. Using MRI, Vermetten et alh found significantly decreased amygdala and hippocampal volumes in patients with dissociative identity disorder.
EEG: electroencephalography; HPA: hypothalamic-pituitary-adrenal; PTSD: posttraumatic stress disorder
Reference Citations: click here
Causes of dissociative disorders
As with many psychiatric disorders, the etiology of dissociative phenomena is thought to include the individual patient’s temperamental or constitutional predispositions11 as well as a strong contribution of environmental trauma (early abuse, neglect).12
Constitutional predisposition for developing a dissociative disorder may include personality traits such as being easily hypnotized, mental absorption, suggestibility, and a tendency to fantasize.13 These characteristics fueled concerns in the 1990s that therapists may contribute to dissociative identity disorder by “digging” for repressed memories in susceptible patients and creating “pseudomemories” of events that did not happen.14
The issue of repressed traumatic memory and its role in therapy is extremely controversial and contributes to the complexity of psychotherapeutic treatment of dissociation.15
- shame and secrecy of early sexual or physical abuse and potential for victims to repress traumatic memories
- lability of memory, potential for suggestibility, and difficulty with verification.14
- early relationships are one of the primary ways that humans learn to regulate distress
- early trauma frequently includes pathology in caregiving relationships, including overt role reversal, abuse, and neglect.
Finally, remember that transient dissociative symptoms can be considered normal in high-stress situations. Intensive military training has been found to be associated with a very high incidence (96%) of dissociative symptoms in army recruits.17
Identifying ‘hidden’ phenomena
Dissociative disorders have been called “diseases of hiddenness”18 because:
- Many of their clinical characteristics— sense of identity, memory, connectedness, somatosensory phenomena—are alterations in subjective phenomena that lack clearly observable symptoms.
- Patients are often reluctant to seek help or divulge their symptoms to clinicians.
- When dissociative symptoms are obvious—such as multiple personalities or sudden loss of memories—they may be dismissed or evoke skepticism because of their dramatic presentation.
For more targeted screening, the self-report Dissociative Experiences Scale (DES)21 is useful for clinical assessment in conjunction with the clinician-administered diagnostic Structured Clinical Interview for DSM-IV Dissociative Disorder (SCID-D).22
Table 2
With these findings, consider screening for dissociation
| Posttraumatic stress disorder |
| Certain personality disorders (especially borderline personality disorder) |
| Somatoform disorders (conversion disorders and nonepileptic seizures) |
| Eating disorders |
| Substance use disorders |
| Extensive history of trauma or neglect |
| Self-harm behavior |
As in Mr. D’s case, dissociative phenomena may attenuate the benefit of post-trauma therapeutic interventions, especially those involving exposure. Therefore:
- assess post-trauma patients for dissociation before you start treatment
- make specific alterations in psychotherapy for such patients, as described below.
Table 3
Differential diagnosis: Dissociation ‘look-alikes’
| Dissociation symptom | Can be confused with: |
|---|---|
| Visual or auditory hallucinations, other ‘first-rank’ psychotic symptoms in dissociative identity disorder | Psychotic disorder |
| ‘Blanking out’ (cognitive disruption) | ADHD, seizures |
| Somatoform (conversion) symptoms | A variety of nonpsychiatric medical problems, including pelvic or abdominal pathology and headaches |
| Dissociative memory lapses | Learning disability, not paying attention |
| ‘Switching’ between states | Bipolar disorder, rapid cycling |
| Lack of emotional reaction to traumatic stimuli(numbing response) | Healthy coping |
| ADHD: attention-deficit/hyperactivity disorder | |
Recreational drugs such as ketamine, methylenedioxymethamphetamine (“Ecstasy”), hallucinogens, marijuana, and dextromethorphan also can induce dissociative states. Consider evaluating for use of these substances, some of which may not be detected on a routine drug screen.24
CASE CONTINUED: A tactical shift
Internal distress—such as when remembering painful events—clearly is linked with the appearance of Mr. D’s symptoms. The therapist—recognizing unacknowledged dissociative phenomena—changes Mr. D’s therapeutic strategy from exposure therapy to affect and anxiety regulation, with an explicit focus on attachment security (safety).
The therapist explains to Mr. D that dissociation symptoms are a response to distress, and he can learn more adaptive distress regulation in therapy. The in-session focus shifts to include more direct attention to components of the therapy relationship, including overt disclosure of the therapist’s positive regard and commitment to help the patient and frequent pauses to “check in” that the patient feels present, safe, and understood. With this new focus, Mr. D’s dissociative symptoms resolve and he feels more ready to face and overcome his fear and avoided memories.
Psychotherapy: Putting pieces together
Psychotherapy is the primary treatment, based on understanding dissociative disorders as manifestations of distress-related, traumatic fragmentation of the sense of self, interpersonal relatedness, and capacity for adaptive affect regulation (Table 4).
Table 4
Tips for conceptualizing dissociative disorders
| Ground your understanding of this class of disorders as distress-related breakdowns in functional connection and integration among components of normal consciousness |
| Consider the overlap among dissociation, certain somatoform disorders (conversion symptoms, pseudoseizures), and PTSD |
| Maintain a high index of suspicion for dissociative symptoms in patients with early trauma or neglect (consider screening for this); do further evaluation with dissociative-specific tools |
| Avoid the tendency to assume that reversible, unfamiliar, or peculiar symptoms imply volition or lack of an organic basis |
| PTSD: posttraumatic stress disorder |
Safety, stabilization, and symptom reduction. Providing a safe therapeutic relationship is a primary and necessary part of DID treatment. On that platform, a first step in reintegrating distressing material into the self involves building the patient’s capacity for conscious, flexible affect regulation. This keeps anxiety and distress within a therapeutic “window.”
Integration of identity and person. Treatment ends when formerly unintegrated or dissociated experiences or parts of the self are integrated into a coherent whole, and the patient can deal adaptively with inter-personal relationships and distress without fragmentation.
Adjunctive medications
Few studies have addressed using psychopharmacologic interventions in the heterogeneous dissociative disorders. GABAA antagonism and 5-HT2a/2c agonism have induced psychotic and dissociative-like symptoms in healthy men,29 and alterations in enzymes such as catechol-O-methyltransferase (COMT) may explain individual vulnerability to trauma.30 Reports of dissociation related to ketamine31 and marijuana32 implicate other neurotransmitter systems in their etiology.
DID. Similar to guidelines for borderline personality disorder,33 guidelines for DID suggest using medications to treat the most prominent symptom clusters such as insomnia, affective instability, and posttraumatic intrusions.
Depersonalization disorder. Trials of fluoxetine and lamotrigine showed no benefit in depersonalization disorder.34,35 In an open trial of 14 patients, naloxone (mean 120 mg/d) reduced depersonalization symptoms by 30%, as measured by 3 validated scales.36
Related Resources
- International Society for the Study of Trauma and Dissociation. Site for professionals. www.isst-d.org
- National Alliance on Mental Illness (NAMI). Patient education on dissociative disorders. www.nami.org/Content/ContentGroups/Helpline1/Dissociative_Disorders.htm
- Fluoxetine • Prozac
- Lamotrigine • Lamictal
- Naloxone • Narcan
- Paroxetine • Paxil
Dr. MacDonald is a speaker for Eli Lilly and Company, Janssen, L.P., and Pfizer Inc.
1. Sar V, Akyuz G, Kundakci T, et al. Childhood trauma, dissociation, and psychiatric comorbidity in patients with conversion disorder. Am J Psychiatry 2004;161:2271-6.
2. Foote B, Smolin Y, Kaplan M, et al. Prevalence of dissociative disorders in psychiatric outpatients. Am J Psychiatry 2006;163:623-9.
3. Reuber M, Pukrop R, Bauer J, et al. Outcome in psychogenic nonepileptic seizures: 1 to 10-year follow-up in 164 patients. Ann Neurol 2003;53:305-11.
4. Spitzer C, Barnow S, Freyberger HJ, Grabe HJ. Dissociation predicts symptom-related treatment outcome in short-term inpatient psychotherapy. Aust N Z J Psychiatry 2007;41:682-7.
5. Allen JG, Console DA, Lewis L. Dissociative detachment and memory impairment: reversible amnesia or encoding failure? Compr Psychiatry 1999;40:160-71.
6. Dell PF. A new model of dissociative identity disorder. Psychiatr Clin North Am 2006;29:1-26.
7. Shalev AY, Freedman S. PTSD following terrorist attacks: a prospective evaluation. Am J Psychiatry 2005;162:1188-91.
8. Steinberg M, Rounsaville B, Cicchetti DV. The Structured Clinical Interview for DSM-III-R Dissociative Disorders: preliminary report on a new diagnostic instrument. Am J Psychiatry 1990;147:76-82.
9. Damasio A. The feeling of what happens: body and emotion in the making of consciousness. New York, NY: Harcourt, Inc; 1999.
10. Alkire MT, Miller J. General anesthesia and the neural correlates of consciousness. Prog Brain Res 2005;150:229-44.
11. Simeon D, Guralnik O, Knutelska M, Schmeidler J. Personality factors associated with dissociation: temperament, defenses, and cognitive schemata. Am J Psychiatry 2002;159(3):489-91.
12. Kihlstrom JF. Dissociative disorders. Annu Rev Clin Psychol 2005;1:227-53.
13. Isaac M, Chand PK. Dissociative and conversion disorders: defining boundaries. Curr Opin Psychiatry 2006;19(1):61-6.
14. Laney C, Loftus EF. Traumatic memories are not necessarily accurate memories. Can J Psychiatry 2005;50(13):823-8.
15. Loftus EF, Davis D. Recovered memories. Annu Rev Clin Psychol 2006;2:469-98.
16. Lyons-Ruth K, Dutra L, Schuder MR, Bianchi I. From infant attachment disorganization to adult dissociation: relational adaptations or traumatic experiences? Psychiatr Clin North Am 2006;29(1):63-86.
17. Morgan CA, 3rd, Hazlett G, Wang S, et al. Symptoms of dissociation in humans experiencing acute, uncontrollable stress: a prospective investigation. Am J Psychiatry 2001;158(8):1239-47.
18. Spiegel D. Recognizing traumatic dissociation. Am J Psychiatry 2006;163(4):566-8.
19. Scher CD, Stein MB, Asmundson GJ, et al. The childhood trauma questionnaire in a community sample: psychometric properties and normative data. J Trauma Stress 2001;14:843-57.
20. Teicher MH, Andersen SL, Polcari A, et al. The neurobiological consequences of early stress and childhood maltreatment. Neurosci Biobehav Rev 2003;27:33-44.
21. Bernstein EM, Putnam FW. Development, reliability, and validity of a dissociation scale. J Nerv Ment Dis 1986;174(12):727-35.
22. Steinberg M, Rounsaville B, Cicchetti D. Detection of dissociative disorders in psychiatric patients by a screening instrument and a structured diagnostic interview. Am J Psychiatry 1991;148(8):1050-4.
23. Devinsky O, Putnam F, Grafman J, et al. Dissociative states and epilepsy. Neurology 1989;39:835-40.
24. Schonenberg M, Reichwald U, Domes G, et al. Effects of peritraumatic ketamine medication on early and sustained posttraumatic stress symptoms in moderately injured accident victims. Psychopharmacology (Berl) 2005;182(3):420-5.
25. Hunter EC, Phillips ML, Chalder T, et al. Depersonalisation disorder: a cognitive-behavioural conceptualisation. Behav Res Ther 2003;41:1451-67.
26. Guidelines for treating dissociative identity disorder in adults (2005). J Trauma Dissociation 2005;6(4):69-149.
27. van der Hart O, Nijenhuis E. Generalized dissociative amnesia: episodic, semantic and procedural memories lost and found. Aust N Z J Psychiatry 2001;35:589-600.
28. Holmes EA, Brown RJ, Mansell W, et al. Are there two qualitatively distinct forms of dissociation? A review and some clinical implications. Clin Psychol Rev 2005;25(1):1-23.
29. D’Souza DC, Gil RB, Zuzarte E, et al. gamma-Aminobutyric acid-serotonin interactions in healthy men: implications for network models of psychosis and dissociation. Biol Psychiatry 2006;59(2):128-37.
30. Savitz JB, van der Merwe L, Newman TK, et al. The relationship between childhood abuse and dissociation. Is it influenced by catechol-O-methyltransferase (COMT) activity? Int J Neuropsychopharmacol 2008;11:149-61.
31. Curran HV, Morgan C. Cognitive, dissociative and psychotogenic effects of ketamine in recreational users on the night of drug use and 3 days later. Addiction 2000;95:575-90.
32. Mathew RJ, Wilson WH, Humphreys D, et al. Depersonalization after marijuana smoking. Biol Psychiatry 1993;33:431-41.
33. American Psychiatric Association. Practice guideline for the treatment of patients with borderline personality disorder. Am J Psychiatry 2001;158(10 suppl):1-52.
34. Sierra M, Phillips ML, Ivin G, et al. A placebo-controlled, cross-over trial of lamotrigine in depersonalization disorder. J Psychopharmacol 2003;17:103-5.
35. Simeon D, Guralnik O, Schmeidler J, Knutelska M. Fluoxetine therapy in depersonalisation disorder: randomised controlled trial. Br J Psychiatry 2004;185:31-6.
36. Simeon D, Knutelska M. An open trial of naltrexone in the treatment of depersonalization disorder. J Clin Psychopharmacol 2005;25:267-70.
37. Stein DJ, Ipser JC, Seedat S. Pharmacotherapy for post traumatic stress disorder (PTSD). Cochrane Database Syst Rev 2006(1):CD002795.-
38. Marshall RD, Lewis-Fernandez R, Blanco C, et al. A controlled trial of paroxetine for chronic PTSD, dissociation, and interpersonal problems in mostly minority adults. Depress Anxiety 2007;24:77-84.
1. Sar V, Akyuz G, Kundakci T, et al. Childhood trauma, dissociation, and psychiatric comorbidity in patients with conversion disorder. Am J Psychiatry 2004;161:2271-6.
2. Foote B, Smolin Y, Kaplan M, et al. Prevalence of dissociative disorders in psychiatric outpatients. Am J Psychiatry 2006;163:623-9.
3. Reuber M, Pukrop R, Bauer J, et al. Outcome in psychogenic nonepileptic seizures: 1 to 10-year follow-up in 164 patients. Ann Neurol 2003;53:305-11.
4. Spitzer C, Barnow S, Freyberger HJ, Grabe HJ. Dissociation predicts symptom-related treatment outcome in short-term inpatient psychotherapy. Aust N Z J Psychiatry 2007;41:682-7.
5. Allen JG, Console DA, Lewis L. Dissociative detachment and memory impairment: reversible amnesia or encoding failure? Compr Psychiatry 1999;40:160-71.
6. Dell PF. A new model of dissociative identity disorder. Psychiatr Clin North Am 2006;29:1-26.
7. Shalev AY, Freedman S. PTSD following terrorist attacks: a prospective evaluation. Am J Psychiatry 2005;162:1188-91.
8. Steinberg M, Rounsaville B, Cicchetti DV. The Structured Clinical Interview for DSM-III-R Dissociative Disorders: preliminary report on a new diagnostic instrument. Am J Psychiatry 1990;147:76-82.
9. Damasio A. The feeling of what happens: body and emotion in the making of consciousness. New York, NY: Harcourt, Inc; 1999.
10. Alkire MT, Miller J. General anesthesia and the neural correlates of consciousness. Prog Brain Res 2005;150:229-44.
11. Simeon D, Guralnik O, Knutelska M, Schmeidler J. Personality factors associated with dissociation: temperament, defenses, and cognitive schemata. Am J Psychiatry 2002;159(3):489-91.
12. Kihlstrom JF. Dissociative disorders. Annu Rev Clin Psychol 2005;1:227-53.
13. Isaac M, Chand PK. Dissociative and conversion disorders: defining boundaries. Curr Opin Psychiatry 2006;19(1):61-6.
14. Laney C, Loftus EF. Traumatic memories are not necessarily accurate memories. Can J Psychiatry 2005;50(13):823-8.
15. Loftus EF, Davis D. Recovered memories. Annu Rev Clin Psychol 2006;2:469-98.
16. Lyons-Ruth K, Dutra L, Schuder MR, Bianchi I. From infant attachment disorganization to adult dissociation: relational adaptations or traumatic experiences? Psychiatr Clin North Am 2006;29(1):63-86.
17. Morgan CA, 3rd, Hazlett G, Wang S, et al. Symptoms of dissociation in humans experiencing acute, uncontrollable stress: a prospective investigation. Am J Psychiatry 2001;158(8):1239-47.
18. Spiegel D. Recognizing traumatic dissociation. Am J Psychiatry 2006;163(4):566-8.
19. Scher CD, Stein MB, Asmundson GJ, et al. The childhood trauma questionnaire in a community sample: psychometric properties and normative data. J Trauma Stress 2001;14:843-57.
20. Teicher MH, Andersen SL, Polcari A, et al. The neurobiological consequences of early stress and childhood maltreatment. Neurosci Biobehav Rev 2003;27:33-44.
21. Bernstein EM, Putnam FW. Development, reliability, and validity of a dissociation scale. J Nerv Ment Dis 1986;174(12):727-35.
22. Steinberg M, Rounsaville B, Cicchetti D. Detection of dissociative disorders in psychiatric patients by a screening instrument and a structured diagnostic interview. Am J Psychiatry 1991;148(8):1050-4.
23. Devinsky O, Putnam F, Grafman J, et al. Dissociative states and epilepsy. Neurology 1989;39:835-40.
24. Schonenberg M, Reichwald U, Domes G, et al. Effects of peritraumatic ketamine medication on early and sustained posttraumatic stress symptoms in moderately injured accident victims. Psychopharmacology (Berl) 2005;182(3):420-5.
25. Hunter EC, Phillips ML, Chalder T, et al. Depersonalisation disorder: a cognitive-behavioural conceptualisation. Behav Res Ther 2003;41:1451-67.
26. Guidelines for treating dissociative identity disorder in adults (2005). J Trauma Dissociation 2005;6(4):69-149.
27. van der Hart O, Nijenhuis E. Generalized dissociative amnesia: episodic, semantic and procedural memories lost and found. Aust N Z J Psychiatry 2001;35:589-600.
28. Holmes EA, Brown RJ, Mansell W, et al. Are there two qualitatively distinct forms of dissociation? A review and some clinical implications. Clin Psychol Rev 2005;25(1):1-23.
29. D’Souza DC, Gil RB, Zuzarte E, et al. gamma-Aminobutyric acid-serotonin interactions in healthy men: implications for network models of psychosis and dissociation. Biol Psychiatry 2006;59(2):128-37.
30. Savitz JB, van der Merwe L, Newman TK, et al. The relationship between childhood abuse and dissociation. Is it influenced by catechol-O-methyltransferase (COMT) activity? Int J Neuropsychopharmacol 2008;11:149-61.
31. Curran HV, Morgan C. Cognitive, dissociative and psychotogenic effects of ketamine in recreational users on the night of drug use and 3 days later. Addiction 2000;95:575-90.
32. Mathew RJ, Wilson WH, Humphreys D, et al. Depersonalization after marijuana smoking. Biol Psychiatry 1993;33:431-41.
33. American Psychiatric Association. Practice guideline for the treatment of patients with borderline personality disorder. Am J Psychiatry 2001;158(10 suppl):1-52.
34. Sierra M, Phillips ML, Ivin G, et al. A placebo-controlled, cross-over trial of lamotrigine in depersonalization disorder. J Psychopharmacol 2003;17:103-5.
35. Simeon D, Guralnik O, Schmeidler J, Knutelska M. Fluoxetine therapy in depersonalisation disorder: randomised controlled trial. Br J Psychiatry 2004;185:31-6.
36. Simeon D, Knutelska M. An open trial of naltrexone in the treatment of depersonalization disorder. J Clin Psychopharmacol 2005;25:267-70.
37. Stein DJ, Ipser JC, Seedat S. Pharmacotherapy for post traumatic stress disorder (PTSD). Cochrane Database Syst Rev 2006(1):CD002795.-
38. Marshall RD, Lewis-Fernandez R, Blanco C, et al. A controlled trial of paroxetine for chronic PTSD, dissociation, and interpersonal problems in mostly minority adults. Depress Anxiety 2007;24:77-84.
5-step plan to treat constipation in psychiatric patients
Mr. W, age 50, presents to the psychiatry clinic with obsessive-compulsive disorder (OCD) symptoms. At his first interview, he says he spends every waking hour obsessing over whether or not he does things “right.” These thoughts force him to compulsively check and recheck everything he does, from simple body movements to complex computer tasks.
He has a history of OCD since age 8, with intermittent episodes of major depression. He reports that several years ago, he had a “miraculous” response to clomipramine for several weeks but has not responded to any other medication. Nevertheless, he continues taking clomipramine, 50 mg/d, hoping that it “might eventually do some good.” He adds that when he tried to increase the dose, he suffered from “terrible constipation” despite regular use of a methylcellulose fiber supplement.
The psychiatrist discontinues clomipramine and starts Mr. W on duloxetine, 90 mg/d. At the next visit, Mr. W complains that his constipation is much worse, so the psychiatrist decreases duloxetine to 60 mg/d, which eventually provides some relief. Because Mr. W has minimal response to duloxetine after 6 months, the psychiatrist adds olanzapine. Although this agent is anticholinergic, the patient had responded to a previous trial of this antipsychotic. Soon after, Mr. W experiences severe constipation.
Psychiatric patients face a host of potential causes of constipation, including:
- use of psychotropics and other medications
- decreased eating or physical activity as a result of depression or another psychiatric disorder
- medical comorbidities that decrease gastrointestinal (GI) motility.
Constipation carries a tremendous cost in terms of resources and quality of life.1-7 This condition also can make patients stop taking medications. You can help patients avoid the discomfort and quality-of-life consequences by promptly diagnosing constipation and following a 5-step treatment algorithm that has shown value in our clinical practice.
- 2 or more of the following
- Loose stools are rarely present unless the patient takes a laxative
- Patient does not meet criteria for irritable bowel syndrome
* Must be present during ≥25% of defecations
Source: Reference 8
What to look for
When evaluating a patient who complains of constipation, first determine what he or she means by “constipation.” Do not rely on frequency of bowel movements as the only criterion for diagnosis. Under Rome Committee for Functional Gastrointestinal Disorders guidelines for diagnosis of chronic (or functional) constipation, patients who move their bowels daily may meet criteria for chronic constipation if they experience straining, incomplete evacuation, or other symptoms (Box 1).8
Many patients who complain of constipation have daily, regular bowel movements that produce hard, difficult-to-pass stool or require straining or manual maneuvers. Take a careful history including:
- stool frequency and quality
- straining
- manual maneuvers (disimpaction or manual pelvic floor support)
- sensation of blockage or incomplete evacuation.
‘Alarm’ symptoms. For psychiatrists, the most important part of the Rome guidelines are the “alarm” symptoms:
- age ≥50 years
- family history of colon cancer or polyps
- family history of inflammatory bowel disease (ulcerative colitis or Crohn’s disease)
- rectal bleeding, anemia
- weight loss >10 pounds
- new onset of chronic constipation without apparent cause in an elderly patient
- severe, persistent constipation refractory to conservative management.9
Table 1
Colorectal cancer screening recommendations*
| Test | Frequency |
|---|---|
| Fecal occult blood testing (FOBT) | Annually |
| Sigmoidoscopy | Every 5 years |
| FOBT and sigmoidoscopy | Every 5 years |
| Double contrast barium enema | Every 5 years |
| Colonoscopy | Every 10 years |
| * For patients age=50. For higher-risk patients, it is reasonable to begin screening at a younger age | |
| Source: Reference 10 | |
Determining the cause
Common causes of constipation include altered visceral sensitivity, decreased GI motility, alterations in pelvic and anorectal musculature, and alterations in the enteric nervous system. Systemic causes are less common and include electrolyte abnormalities (hypercalcemia and hypokalemia) and endocrine disorders (hypothyroidism and diabetes mellitus).
Some patients’ constipation is caused by involuntarily contracting the pelvic floor muscles or suppressing the urge to defecate (Box 2).1,11,12 Suspect this in patients who strain repeatedly to pass soft or liquid stool.
Medication side effects are probably the most common constipation cause psychiatrists will encounter. Many psychotropics have anticholinergic effects that decrease GI motility and cause constipation. The most commonly implicated drugs are:
- older tricyclic antidepressants (such as amitriptyline)
- antipsychotics.
Outlet obstruction, caused by inappropriately contracting posterior pelvic floor muscles during defecatory effort, is the cause of 5% to 10% of constipation cases.1 Patients are not aware of this pelvic floor incoordination. Often, they will give a history of straining even for soft or liquid stool.
Consider outlet obstruction in women with history of multiple vaginal childbirths or pelvic or gynecologic surgery, particularly if they fail to respond to usual measures to treat constipation. For adequate relief, these patients often require anorectal biofeedback, which teaches them to relax the posterior pelvic floor.11,12
Habitually suppressing the gastrocolic reflex—the urge to defecate after eating—causes some patients difficulty moving their bowels. Counsel these patients to sit on the toilet for several minutes after the morning meal to relearn this behavior. Some may need several weeks of daily enema or glycerine suppository use to retrain themselves to have bowel movements after the morning meal.
Other psychiatric-related causes. Patients with depression may experience decreased stool output because of a lack of food intake or physical activity. These causes may be effectively addressed by treating the depression.
Give special consideration to patients with eating disorders and those who routinely use laxatives. A patient who is not eating will not produce the same amount of stool as one who eats regularly.
Constipated patients may require escalating doses of laxatives to obtain symptom relief; this does not constitute laxative abuse but rather tachyphylaxis. Chronic laxative use has not been shown to permanently decrease colonic motility,14 but patients who use laxatives chronically may have altered expectations of what is normal.
CASE CONTINUED: Recurring symptoms
After discontinuing Mr. W’s olanzapine and duloxetine, the psychiatrist prescribes polyethylene glycol solution (MiraLax) and instructs Mr. W to increase his daily fluid and fiber intake. Although the solution works well, Mr. W complains of the cost. He then resumes methyl cellulose and starts taking magnesium hydroxide chewable tablets (Milk of Magnesia) every 2 to 4 days as needed for constipation.
The psychiatrist prescribes mirtazapine for OCD symptoms, but soon stops this regimen because Mr. W complains of worsening constipation. Next Mr. W is started on fluvoxamine, which he had tried briefly many years before. The dosage is gradually titrated to 150 mg/d. Although Mr. W’s OCD improves somewhat, he complains of agitation and once again of worsening constipation.
Treatment algorithm
To minimize trial and error, we use a stepwise approach to treating constipation (Algorithm).8,11,15 Although many standard recommendations have not been evaluated in large randomized controlled trials, they are supported by decades of observed actions among clinicians and thus remain valuable.
Multiple nonprescription agents are available to treat constipation, including:
- bulking agents (fiber supplements)
- lubricating agents
- stool softening agents
- stimulant and osmotic laxatives (Table 2).8
Steps 1 & 2. When initial attempts at increasing physical activity, fluid, and dietary fiber fail to yield a response, fiber supplements are commonly used as a second step in managing constipation. We advocate beginning with a supplement that contains psyllium—such as Fiber-all or Metamucil—because psyllium has been shown to increase stool frequency. Supplements that contain methylcellulose (Citrucel), polycarbophil (such as Equalactin and Mitrolan), or bran have either not shown efficacy or have not been studied rigorously enough to merit recommendation.10 Some patients respond to other fiber products, but start a fibernaïve patient with a psyllium-containing supplement.
Fiber supplements may cause increased gas and bloating, so start at a low dose and gradually increase over several weeks to mitigate these side effects.
In our experience, patients usually have tried bisacodyl before seeking treatment for constipation. Although bisacodyl may be effective for some patients, others may need something stronger. Many gastroenterologists prefer prescribing osmotic or prescription laxatives.
Step 4. Osmotic laxatives generally are liquids, including magnesium hydroxide, polyethylene glycol solution, and the prescription agent lactulose. Magnesium hydroxide is inexpensive and can be taken chronically.
Algorithm
A stepwise approach to managing constipation
| Step 1 | |
| Recommendation | Comments |
| Increase activity or daily walking | Not rigorously studied in constipated patients; exercise is associated with decreased orocecal transit time15 |
| Increase fluid intake | Not rigorously studied in constipated patients8 |
| Increase dietary fiber intake | Not rigorously studied in constipated patients8 |
| ↓ | |
| Step 2 | |
| Recommendation | Comments |
| Fiber supplements | Psyllium compounds may be superior to methylcellulose, polycarbophil, and bran11 |
| ↓ | |
| Step 3 | |
| Recommendation | Comments |
| Over-the-counter laxative pills | Senna compounds are derived from plants |
| ↓ | |
| Step 4 | |
| Recommendation | Comments |
| Over-the-counter laxative solutions | Milk of Magnesia is very inexpensive |
| ↓ | |
| Step 5 | |
| Recommendation | Comments |
| Prescription laxatives | Lubiprostone causes fetal loss in animals; tegaserod is available only under a treatment investigational new drug protocol |
Table 2
Commonly used laxatives: Mechanisms of action
| Category | Agents |
|---|---|
| Bulk-forming | Methylcellulose (Citrucel), polycarbophil (Equalactin, Mitrolan, others), psyllium (Fiberall, Metamucil, others) |
| Lubricating | Glycerin (Sani-Supp), magnesium hydroxide and mineral oil (Magnolax), mineral oil (Fleet Mineral Oil, Zymenol, others) |
| Stool softener | Docusate sodium (Colace) |
| Osmotic | Magnesium hydroxide (Milk of Magnesia), polyethylene glycol (MiraLax), lactulose* (Cholac Syrup, Constulose, others), lubiprostone* (Amitiza) |
| Stimulant | Bisacodyl (Correctol, Dulcolax, others), castor oil (Alphamul, Emulsoil, others), senna/sennosides (Ex-Lax, Senokot, others), sodium bicarbonate and potassium bitartrate (Ceo-Two evacuant) |
| * Available by prescription only | |
| Source: Reference 8 | |
Prescription medications
Tegaserod is a partial 5-HT4 agonist and stimulator of GI motility and secretion. It also decreases visceral sensitivity.16 Tegaserod’s manufacturer voluntarily withdrew the drug from the market because it may increase risk of cardiovascular ischemic events, including angina, heart attack, and stroke. Tegaserod is available only under a treatment investigational new drug (IND) protocol that includes obtaining approval from a local institutional review board. We recommend that psychiatrists should not prescribe tegaserod but refer patients to experienced gastroenterologists or other GI specialists.
Lubiprostone is a selective chloride channel activator that works only in the gut and results in net fluid excretion and increased stool frequency. The molecule is a prostaglandin derivative and is poorly absorbed.17
Because lubiprostone has been shown to cause fetal loss in animals (at the equivalent of 2 and 6 times the recommended human dose), women of reproductive age should use contraception while taking lubiprostone and carefully consider the risks and benefits of lubiprostone use during pregnancy.
CASE CONTINUED: Finding an effective strategy
The psychiatrist prescribes lubiprostone, 24 mcg bid, but Mr. W once again complains of the expense and says the drug does not work well. He quickly returns to his intermittent use of magnesium hydroxide tablets and occasionally takes bisacodyl tablets.
To address Mr. W’s OCD, the psychiatrist adds risperidone, 0.5 mg bid, to Mr. W’s regimen. He has a modest response in OCD symptoms—30% of his day is now symptom- free— without worsening his constipation.
Probiotics and prebiotics
Emerging therapies for constipation include probiotics and prebiotics, which attempt to alter the gut flora and milieu. The primary bacterial agents are Lactobacillus species and Bifidobacterium species. At least one probiotic Bifidobacterium product—Activia—is being marketed in the United States as a fortified yogurt.
Investigational medications. Renzapride is a 5HT4 receptor agonist and 5HT3 receptor antagonist that has shown promise in a pilot study18 and is in phase III trials. Linaclotide is a peptide that activates chloride and bicarbonate secretion in the gut and may reduce visceral hypersensitivity. It too has shown promise in a pilot study.19
Related resources
- Rome Foundation. Functional gastrointestinal disorders. www.romecriteria.org.
- Bleser S, Brunton S, Carmichael B, et al. Management of chronic constipation: recommendations from a consensus panel. J Fam Pract 2005;54(8):691-8.
- Amitriptyline • Elavil, Endep
- Chlorpromazine • Thorazine
- Clomipramine • Anafranil
- Clozapine • Clozaril
- Duloxetine • Cymbalta
- Fluvoxamine • Luvox
- Lactulose • Cholac Syrup, Constulose, others
- Lubiprostone • Amitiza
- Mirtazapine • Remeron
- Olanzapine • Zyprexa
- Risperidone • Risperdal
- Thioridazine • Mellaril
- Tegaserod • Zelnorm
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgment
This project was partially supported by grant number 5 T32 HS013852 from the Agency for Healthcare Research and Quality.
1. Stewart WF, Liberman JN, Sandler RS, et al. Epidemiology of constipation (EPOC) study in the United States: relation of clinical subtypes to sociodemographic features. Am J Gastroenterol 1999;94(12):3530-40.
2. Choung RS, Locke GR, 3rd, Schleck CD, et al. Cumulative incidence of chronic constipation: a population-based study 1988-2003. Aliment Pharmacol Ther 2007;26(11-12):1521-8.
3. Agency for Healthcare Research and Quality. Healthcare Cost and Utilization Project (HCUPnet). Available at: http://hcupnet.ahrq.gov. Accessed March 19, 2008.
4. Sonnenberg A, Koch TR. Physician visits in the United States for constipation: 1958 to 1986. Dig Dis Sci 1989;34(4):606-11.
5. Sonnenberg A, Koch TR. Epidemiology of constipation in the United States. Dis Colon Rectum 1989;32(1):1-8.
6. Dennison C, Prasad M, Lloyd A, et al. The health-related quality of life and economic burden of constipation. Pharmacoeconomics 2005;23(5):461-76.
7. Donald IP, Smith RG, Cruikshank JG, et al. A study of constipation in the elderly living at home. Gerontology 1985;31(2):112-8.
8. Longstreth GF, Thompson WG, Chey WD, et al. Functional bowel disorders. Gastroenterology 2006;130(5):1480-91.
9. American College of Gastroenterology Chronic Constipation Task Force. An evidence-based approach to the management of chronic constipation in North America. Am J Gastroenterol 2005;(100 suppl 1):S1-4.
10. U.S. Preventive Services Task Force. Colorectal cancer screening. Available at: http://www.ahrq.gov/clinic/3rduspstf/colorectal. Accessed March 19, 2008.
11. Chiotakakou-Faliakou E, Kamm MA, Roy AJ, et al. Biofeedback provides long-term benefit for patients with intractable, slow and normal transit constipation. Gut 1998;42(4):517-21.
12. Kawimbe BM, Papachrysostomou M, Binnie NR, et al. Outlet obstruction constipation (anismus) managed by biofeedback. Gut 1991;32(10):1175-9.
13. Richelson E. Receptor pharmacology of neuroleptics: relation to clinical effects. J Clin Psychiatry 1999;(60 suppl 10):5-14.
14. Muller-Lissner SA, Kamm MA, Scarpignato C, Wald A. Myths and misconceptions about chronic constipation. Am J Gastroenterol 2005;100(1):232-42.
15. Keeling WF, Harris A, Martin BJ. Orocecal transit during mild exercise in women. J Appl Physiol 1990;68(4):1350-3.
16. Tegaserod [package insert]. East Hanover, NJ: Novartis Pharmaceuticals; 2006.
17. Amitiza [package insert]. Bethesda, MD: Sucampo Pharmaceuticals; 2007.
18. Tack J, Middleton SJ, Horne MC, et al. Pilot study of the efficacy of renzapride on gastrointestinal motility and symptoms in patients with constipation-predominant irritable bowel syndrome. Aliment Pharmacol Ther 2006;23(11):1655-65.
19. Andresen V, Camilleri M, Busciglio IA, et al. Effect of 5 days linaclotide on transit and bowel function in females with constipation-predominant irritable bowel syndrome. Gastroenterology 2007;133(3):761-8.
Mr. W, age 50, presents to the psychiatry clinic with obsessive-compulsive disorder (OCD) symptoms. At his first interview, he says he spends every waking hour obsessing over whether or not he does things “right.” These thoughts force him to compulsively check and recheck everything he does, from simple body movements to complex computer tasks.
He has a history of OCD since age 8, with intermittent episodes of major depression. He reports that several years ago, he had a “miraculous” response to clomipramine for several weeks but has not responded to any other medication. Nevertheless, he continues taking clomipramine, 50 mg/d, hoping that it “might eventually do some good.” He adds that when he tried to increase the dose, he suffered from “terrible constipation” despite regular use of a methylcellulose fiber supplement.
The psychiatrist discontinues clomipramine and starts Mr. W on duloxetine, 90 mg/d. At the next visit, Mr. W complains that his constipation is much worse, so the psychiatrist decreases duloxetine to 60 mg/d, which eventually provides some relief. Because Mr. W has minimal response to duloxetine after 6 months, the psychiatrist adds olanzapine. Although this agent is anticholinergic, the patient had responded to a previous trial of this antipsychotic. Soon after, Mr. W experiences severe constipation.
Psychiatric patients face a host of potential causes of constipation, including:
- use of psychotropics and other medications
- decreased eating or physical activity as a result of depression or another psychiatric disorder
- medical comorbidities that decrease gastrointestinal (GI) motility.
Constipation carries a tremendous cost in terms of resources and quality of life.1-7 This condition also can make patients stop taking medications. You can help patients avoid the discomfort and quality-of-life consequences by promptly diagnosing constipation and following a 5-step treatment algorithm that has shown value in our clinical practice.
- 2 or more of the following
- Loose stools are rarely present unless the patient takes a laxative
- Patient does not meet criteria for irritable bowel syndrome
* Must be present during ≥25% of defecations
Source: Reference 8
What to look for
When evaluating a patient who complains of constipation, first determine what he or she means by “constipation.” Do not rely on frequency of bowel movements as the only criterion for diagnosis. Under Rome Committee for Functional Gastrointestinal Disorders guidelines for diagnosis of chronic (or functional) constipation, patients who move their bowels daily may meet criteria for chronic constipation if they experience straining, incomplete evacuation, or other symptoms (Box 1).8
Many patients who complain of constipation have daily, regular bowel movements that produce hard, difficult-to-pass stool or require straining or manual maneuvers. Take a careful history including:
- stool frequency and quality
- straining
- manual maneuvers (disimpaction or manual pelvic floor support)
- sensation of blockage or incomplete evacuation.
‘Alarm’ symptoms. For psychiatrists, the most important part of the Rome guidelines are the “alarm” symptoms:
- age ≥50 years
- family history of colon cancer or polyps
- family history of inflammatory bowel disease (ulcerative colitis or Crohn’s disease)
- rectal bleeding, anemia
- weight loss >10 pounds
- new onset of chronic constipation without apparent cause in an elderly patient
- severe, persistent constipation refractory to conservative management.9
Table 1
Colorectal cancer screening recommendations*
| Test | Frequency |
|---|---|
| Fecal occult blood testing (FOBT) | Annually |
| Sigmoidoscopy | Every 5 years |
| FOBT and sigmoidoscopy | Every 5 years |
| Double contrast barium enema | Every 5 years |
| Colonoscopy | Every 10 years |
| * For patients age=50. For higher-risk patients, it is reasonable to begin screening at a younger age | |
| Source: Reference 10 | |
Determining the cause
Common causes of constipation include altered visceral sensitivity, decreased GI motility, alterations in pelvic and anorectal musculature, and alterations in the enteric nervous system. Systemic causes are less common and include electrolyte abnormalities (hypercalcemia and hypokalemia) and endocrine disorders (hypothyroidism and diabetes mellitus).
Some patients’ constipation is caused by involuntarily contracting the pelvic floor muscles or suppressing the urge to defecate (Box 2).1,11,12 Suspect this in patients who strain repeatedly to pass soft or liquid stool.
Medication side effects are probably the most common constipation cause psychiatrists will encounter. Many psychotropics have anticholinergic effects that decrease GI motility and cause constipation. The most commonly implicated drugs are:
- older tricyclic antidepressants (such as amitriptyline)
- antipsychotics.
Outlet obstruction, caused by inappropriately contracting posterior pelvic floor muscles during defecatory effort, is the cause of 5% to 10% of constipation cases.1 Patients are not aware of this pelvic floor incoordination. Often, they will give a history of straining even for soft or liquid stool.
Consider outlet obstruction in women with history of multiple vaginal childbirths or pelvic or gynecologic surgery, particularly if they fail to respond to usual measures to treat constipation. For adequate relief, these patients often require anorectal biofeedback, which teaches them to relax the posterior pelvic floor.11,12
Habitually suppressing the gastrocolic reflex—the urge to defecate after eating—causes some patients difficulty moving their bowels. Counsel these patients to sit on the toilet for several minutes after the morning meal to relearn this behavior. Some may need several weeks of daily enema or glycerine suppository use to retrain themselves to have bowel movements after the morning meal.
Other psychiatric-related causes. Patients with depression may experience decreased stool output because of a lack of food intake or physical activity. These causes may be effectively addressed by treating the depression.
Give special consideration to patients with eating disorders and those who routinely use laxatives. A patient who is not eating will not produce the same amount of stool as one who eats regularly.
Constipated patients may require escalating doses of laxatives to obtain symptom relief; this does not constitute laxative abuse but rather tachyphylaxis. Chronic laxative use has not been shown to permanently decrease colonic motility,14 but patients who use laxatives chronically may have altered expectations of what is normal.
CASE CONTINUED: Recurring symptoms
After discontinuing Mr. W’s olanzapine and duloxetine, the psychiatrist prescribes polyethylene glycol solution (MiraLax) and instructs Mr. W to increase his daily fluid and fiber intake. Although the solution works well, Mr. W complains of the cost. He then resumes methyl cellulose and starts taking magnesium hydroxide chewable tablets (Milk of Magnesia) every 2 to 4 days as needed for constipation.
The psychiatrist prescribes mirtazapine for OCD symptoms, but soon stops this regimen because Mr. W complains of worsening constipation. Next Mr. W is started on fluvoxamine, which he had tried briefly many years before. The dosage is gradually titrated to 150 mg/d. Although Mr. W’s OCD improves somewhat, he complains of agitation and once again of worsening constipation.
Treatment algorithm
To minimize trial and error, we use a stepwise approach to treating constipation (Algorithm).8,11,15 Although many standard recommendations have not been evaluated in large randomized controlled trials, they are supported by decades of observed actions among clinicians and thus remain valuable.
Multiple nonprescription agents are available to treat constipation, including:
- bulking agents (fiber supplements)
- lubricating agents
- stool softening agents
- stimulant and osmotic laxatives (Table 2).8
Steps 1 & 2. When initial attempts at increasing physical activity, fluid, and dietary fiber fail to yield a response, fiber supplements are commonly used as a second step in managing constipation. We advocate beginning with a supplement that contains psyllium—such as Fiber-all or Metamucil—because psyllium has been shown to increase stool frequency. Supplements that contain methylcellulose (Citrucel), polycarbophil (such as Equalactin and Mitrolan), or bran have either not shown efficacy or have not been studied rigorously enough to merit recommendation.10 Some patients respond to other fiber products, but start a fibernaïve patient with a psyllium-containing supplement.
Fiber supplements may cause increased gas and bloating, so start at a low dose and gradually increase over several weeks to mitigate these side effects.
In our experience, patients usually have tried bisacodyl before seeking treatment for constipation. Although bisacodyl may be effective for some patients, others may need something stronger. Many gastroenterologists prefer prescribing osmotic or prescription laxatives.
Step 4. Osmotic laxatives generally are liquids, including magnesium hydroxide, polyethylene glycol solution, and the prescription agent lactulose. Magnesium hydroxide is inexpensive and can be taken chronically.
Algorithm
A stepwise approach to managing constipation
| Step 1 | |
| Recommendation | Comments |
| Increase activity or daily walking | Not rigorously studied in constipated patients; exercise is associated with decreased orocecal transit time15 |
| Increase fluid intake | Not rigorously studied in constipated patients8 |
| Increase dietary fiber intake | Not rigorously studied in constipated patients8 |
| ↓ | |
| Step 2 | |
| Recommendation | Comments |
| Fiber supplements | Psyllium compounds may be superior to methylcellulose, polycarbophil, and bran11 |
| ↓ | |
| Step 3 | |
| Recommendation | Comments |
| Over-the-counter laxative pills | Senna compounds are derived from plants |
| ↓ | |
| Step 4 | |
| Recommendation | Comments |
| Over-the-counter laxative solutions | Milk of Magnesia is very inexpensive |
| ↓ | |
| Step 5 | |
| Recommendation | Comments |
| Prescription laxatives | Lubiprostone causes fetal loss in animals; tegaserod is available only under a treatment investigational new drug protocol |
Table 2
Commonly used laxatives: Mechanisms of action
| Category | Agents |
|---|---|
| Bulk-forming | Methylcellulose (Citrucel), polycarbophil (Equalactin, Mitrolan, others), psyllium (Fiberall, Metamucil, others) |
| Lubricating | Glycerin (Sani-Supp), magnesium hydroxide and mineral oil (Magnolax), mineral oil (Fleet Mineral Oil, Zymenol, others) |
| Stool softener | Docusate sodium (Colace) |
| Osmotic | Magnesium hydroxide (Milk of Magnesia), polyethylene glycol (MiraLax), lactulose* (Cholac Syrup, Constulose, others), lubiprostone* (Amitiza) |
| Stimulant | Bisacodyl (Correctol, Dulcolax, others), castor oil (Alphamul, Emulsoil, others), senna/sennosides (Ex-Lax, Senokot, others), sodium bicarbonate and potassium bitartrate (Ceo-Two evacuant) |
| * Available by prescription only | |
| Source: Reference 8 | |
Prescription medications
Tegaserod is a partial 5-HT4 agonist and stimulator of GI motility and secretion. It also decreases visceral sensitivity.16 Tegaserod’s manufacturer voluntarily withdrew the drug from the market because it may increase risk of cardiovascular ischemic events, including angina, heart attack, and stroke. Tegaserod is available only under a treatment investigational new drug (IND) protocol that includes obtaining approval from a local institutional review board. We recommend that psychiatrists should not prescribe tegaserod but refer patients to experienced gastroenterologists or other GI specialists.
Lubiprostone is a selective chloride channel activator that works only in the gut and results in net fluid excretion and increased stool frequency. The molecule is a prostaglandin derivative and is poorly absorbed.17
Because lubiprostone has been shown to cause fetal loss in animals (at the equivalent of 2 and 6 times the recommended human dose), women of reproductive age should use contraception while taking lubiprostone and carefully consider the risks and benefits of lubiprostone use during pregnancy.
CASE CONTINUED: Finding an effective strategy
The psychiatrist prescribes lubiprostone, 24 mcg bid, but Mr. W once again complains of the expense and says the drug does not work well. He quickly returns to his intermittent use of magnesium hydroxide tablets and occasionally takes bisacodyl tablets.
To address Mr. W’s OCD, the psychiatrist adds risperidone, 0.5 mg bid, to Mr. W’s regimen. He has a modest response in OCD symptoms—30% of his day is now symptom- free— without worsening his constipation.
Probiotics and prebiotics
Emerging therapies for constipation include probiotics and prebiotics, which attempt to alter the gut flora and milieu. The primary bacterial agents are Lactobacillus species and Bifidobacterium species. At least one probiotic Bifidobacterium product—Activia—is being marketed in the United States as a fortified yogurt.
Investigational medications. Renzapride is a 5HT4 receptor agonist and 5HT3 receptor antagonist that has shown promise in a pilot study18 and is in phase III trials. Linaclotide is a peptide that activates chloride and bicarbonate secretion in the gut and may reduce visceral hypersensitivity. It too has shown promise in a pilot study.19
Related resources
- Rome Foundation. Functional gastrointestinal disorders. www.romecriteria.org.
- Bleser S, Brunton S, Carmichael B, et al. Management of chronic constipation: recommendations from a consensus panel. J Fam Pract 2005;54(8):691-8.
- Amitriptyline • Elavil, Endep
- Chlorpromazine • Thorazine
- Clomipramine • Anafranil
- Clozapine • Clozaril
- Duloxetine • Cymbalta
- Fluvoxamine • Luvox
- Lactulose • Cholac Syrup, Constulose, others
- Lubiprostone • Amitiza
- Mirtazapine • Remeron
- Olanzapine • Zyprexa
- Risperidone • Risperdal
- Thioridazine • Mellaril
- Tegaserod • Zelnorm
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgment
This project was partially supported by grant number 5 T32 HS013852 from the Agency for Healthcare Research and Quality.
Mr. W, age 50, presents to the psychiatry clinic with obsessive-compulsive disorder (OCD) symptoms. At his first interview, he says he spends every waking hour obsessing over whether or not he does things “right.” These thoughts force him to compulsively check and recheck everything he does, from simple body movements to complex computer tasks.
He has a history of OCD since age 8, with intermittent episodes of major depression. He reports that several years ago, he had a “miraculous” response to clomipramine for several weeks but has not responded to any other medication. Nevertheless, he continues taking clomipramine, 50 mg/d, hoping that it “might eventually do some good.” He adds that when he tried to increase the dose, he suffered from “terrible constipation” despite regular use of a methylcellulose fiber supplement.
The psychiatrist discontinues clomipramine and starts Mr. W on duloxetine, 90 mg/d. At the next visit, Mr. W complains that his constipation is much worse, so the psychiatrist decreases duloxetine to 60 mg/d, which eventually provides some relief. Because Mr. W has minimal response to duloxetine after 6 months, the psychiatrist adds olanzapine. Although this agent is anticholinergic, the patient had responded to a previous trial of this antipsychotic. Soon after, Mr. W experiences severe constipation.
Psychiatric patients face a host of potential causes of constipation, including:
- use of psychotropics and other medications
- decreased eating or physical activity as a result of depression or another psychiatric disorder
- medical comorbidities that decrease gastrointestinal (GI) motility.
Constipation carries a tremendous cost in terms of resources and quality of life.1-7 This condition also can make patients stop taking medications. You can help patients avoid the discomfort and quality-of-life consequences by promptly diagnosing constipation and following a 5-step treatment algorithm that has shown value in our clinical practice.
- 2 or more of the following
- Loose stools are rarely present unless the patient takes a laxative
- Patient does not meet criteria for irritable bowel syndrome
* Must be present during ≥25% of defecations
Source: Reference 8
What to look for
When evaluating a patient who complains of constipation, first determine what he or she means by “constipation.” Do not rely on frequency of bowel movements as the only criterion for diagnosis. Under Rome Committee for Functional Gastrointestinal Disorders guidelines for diagnosis of chronic (or functional) constipation, patients who move their bowels daily may meet criteria for chronic constipation if they experience straining, incomplete evacuation, or other symptoms (Box 1).8
Many patients who complain of constipation have daily, regular bowel movements that produce hard, difficult-to-pass stool or require straining or manual maneuvers. Take a careful history including:
- stool frequency and quality
- straining
- manual maneuvers (disimpaction or manual pelvic floor support)
- sensation of blockage or incomplete evacuation.
‘Alarm’ symptoms. For psychiatrists, the most important part of the Rome guidelines are the “alarm” symptoms:
- age ≥50 years
- family history of colon cancer or polyps
- family history of inflammatory bowel disease (ulcerative colitis or Crohn’s disease)
- rectal bleeding, anemia
- weight loss >10 pounds
- new onset of chronic constipation without apparent cause in an elderly patient
- severe, persistent constipation refractory to conservative management.9
Table 1
Colorectal cancer screening recommendations*
| Test | Frequency |
|---|---|
| Fecal occult blood testing (FOBT) | Annually |
| Sigmoidoscopy | Every 5 years |
| FOBT and sigmoidoscopy | Every 5 years |
| Double contrast barium enema | Every 5 years |
| Colonoscopy | Every 10 years |
| * For patients age=50. For higher-risk patients, it is reasonable to begin screening at a younger age | |
| Source: Reference 10 | |
Determining the cause
Common causes of constipation include altered visceral sensitivity, decreased GI motility, alterations in pelvic and anorectal musculature, and alterations in the enteric nervous system. Systemic causes are less common and include electrolyte abnormalities (hypercalcemia and hypokalemia) and endocrine disorders (hypothyroidism and diabetes mellitus).
Some patients’ constipation is caused by involuntarily contracting the pelvic floor muscles or suppressing the urge to defecate (Box 2).1,11,12 Suspect this in patients who strain repeatedly to pass soft or liquid stool.
Medication side effects are probably the most common constipation cause psychiatrists will encounter. Many psychotropics have anticholinergic effects that decrease GI motility and cause constipation. The most commonly implicated drugs are:
- older tricyclic antidepressants (such as amitriptyline)
- antipsychotics.
Outlet obstruction, caused by inappropriately contracting posterior pelvic floor muscles during defecatory effort, is the cause of 5% to 10% of constipation cases.1 Patients are not aware of this pelvic floor incoordination. Often, they will give a history of straining even for soft or liquid stool.
Consider outlet obstruction in women with history of multiple vaginal childbirths or pelvic or gynecologic surgery, particularly if they fail to respond to usual measures to treat constipation. For adequate relief, these patients often require anorectal biofeedback, which teaches them to relax the posterior pelvic floor.11,12
Habitually suppressing the gastrocolic reflex—the urge to defecate after eating—causes some patients difficulty moving their bowels. Counsel these patients to sit on the toilet for several minutes after the morning meal to relearn this behavior. Some may need several weeks of daily enema or glycerine suppository use to retrain themselves to have bowel movements after the morning meal.
Other psychiatric-related causes. Patients with depression may experience decreased stool output because of a lack of food intake or physical activity. These causes may be effectively addressed by treating the depression.
Give special consideration to patients with eating disorders and those who routinely use laxatives. A patient who is not eating will not produce the same amount of stool as one who eats regularly.
Constipated patients may require escalating doses of laxatives to obtain symptom relief; this does not constitute laxative abuse but rather tachyphylaxis. Chronic laxative use has not been shown to permanently decrease colonic motility,14 but patients who use laxatives chronically may have altered expectations of what is normal.
CASE CONTINUED: Recurring symptoms
After discontinuing Mr. W’s olanzapine and duloxetine, the psychiatrist prescribes polyethylene glycol solution (MiraLax) and instructs Mr. W to increase his daily fluid and fiber intake. Although the solution works well, Mr. W complains of the cost. He then resumes methyl cellulose and starts taking magnesium hydroxide chewable tablets (Milk of Magnesia) every 2 to 4 days as needed for constipation.
The psychiatrist prescribes mirtazapine for OCD symptoms, but soon stops this regimen because Mr. W complains of worsening constipation. Next Mr. W is started on fluvoxamine, which he had tried briefly many years before. The dosage is gradually titrated to 150 mg/d. Although Mr. W’s OCD improves somewhat, he complains of agitation and once again of worsening constipation.
Treatment algorithm
To minimize trial and error, we use a stepwise approach to treating constipation (Algorithm).8,11,15 Although many standard recommendations have not been evaluated in large randomized controlled trials, they are supported by decades of observed actions among clinicians and thus remain valuable.
Multiple nonprescription agents are available to treat constipation, including:
- bulking agents (fiber supplements)
- lubricating agents
- stool softening agents
- stimulant and osmotic laxatives (Table 2).8
Steps 1 & 2. When initial attempts at increasing physical activity, fluid, and dietary fiber fail to yield a response, fiber supplements are commonly used as a second step in managing constipation. We advocate beginning with a supplement that contains psyllium—such as Fiber-all or Metamucil—because psyllium has been shown to increase stool frequency. Supplements that contain methylcellulose (Citrucel), polycarbophil (such as Equalactin and Mitrolan), or bran have either not shown efficacy or have not been studied rigorously enough to merit recommendation.10 Some patients respond to other fiber products, but start a fibernaïve patient with a psyllium-containing supplement.
Fiber supplements may cause increased gas and bloating, so start at a low dose and gradually increase over several weeks to mitigate these side effects.
In our experience, patients usually have tried bisacodyl before seeking treatment for constipation. Although bisacodyl may be effective for some patients, others may need something stronger. Many gastroenterologists prefer prescribing osmotic or prescription laxatives.
Step 4. Osmotic laxatives generally are liquids, including magnesium hydroxide, polyethylene glycol solution, and the prescription agent lactulose. Magnesium hydroxide is inexpensive and can be taken chronically.
Algorithm
A stepwise approach to managing constipation
| Step 1 | |
| Recommendation | Comments |
| Increase activity or daily walking | Not rigorously studied in constipated patients; exercise is associated with decreased orocecal transit time15 |
| Increase fluid intake | Not rigorously studied in constipated patients8 |
| Increase dietary fiber intake | Not rigorously studied in constipated patients8 |
| ↓ | |
| Step 2 | |
| Recommendation | Comments |
| Fiber supplements | Psyllium compounds may be superior to methylcellulose, polycarbophil, and bran11 |
| ↓ | |
| Step 3 | |
| Recommendation | Comments |
| Over-the-counter laxative pills | Senna compounds are derived from plants |
| ↓ | |
| Step 4 | |
| Recommendation | Comments |
| Over-the-counter laxative solutions | Milk of Magnesia is very inexpensive |
| ↓ | |
| Step 5 | |
| Recommendation | Comments |
| Prescription laxatives | Lubiprostone causes fetal loss in animals; tegaserod is available only under a treatment investigational new drug protocol |
Table 2
Commonly used laxatives: Mechanisms of action
| Category | Agents |
|---|---|
| Bulk-forming | Methylcellulose (Citrucel), polycarbophil (Equalactin, Mitrolan, others), psyllium (Fiberall, Metamucil, others) |
| Lubricating | Glycerin (Sani-Supp), magnesium hydroxide and mineral oil (Magnolax), mineral oil (Fleet Mineral Oil, Zymenol, others) |
| Stool softener | Docusate sodium (Colace) |
| Osmotic | Magnesium hydroxide (Milk of Magnesia), polyethylene glycol (MiraLax), lactulose* (Cholac Syrup, Constulose, others), lubiprostone* (Amitiza) |
| Stimulant | Bisacodyl (Correctol, Dulcolax, others), castor oil (Alphamul, Emulsoil, others), senna/sennosides (Ex-Lax, Senokot, others), sodium bicarbonate and potassium bitartrate (Ceo-Two evacuant) |
| * Available by prescription only | |
| Source: Reference 8 | |
Prescription medications
Tegaserod is a partial 5-HT4 agonist and stimulator of GI motility and secretion. It also decreases visceral sensitivity.16 Tegaserod’s manufacturer voluntarily withdrew the drug from the market because it may increase risk of cardiovascular ischemic events, including angina, heart attack, and stroke. Tegaserod is available only under a treatment investigational new drug (IND) protocol that includes obtaining approval from a local institutional review board. We recommend that psychiatrists should not prescribe tegaserod but refer patients to experienced gastroenterologists or other GI specialists.
Lubiprostone is a selective chloride channel activator that works only in the gut and results in net fluid excretion and increased stool frequency. The molecule is a prostaglandin derivative and is poorly absorbed.17
Because lubiprostone has been shown to cause fetal loss in animals (at the equivalent of 2 and 6 times the recommended human dose), women of reproductive age should use contraception while taking lubiprostone and carefully consider the risks and benefits of lubiprostone use during pregnancy.
CASE CONTINUED: Finding an effective strategy
The psychiatrist prescribes lubiprostone, 24 mcg bid, but Mr. W once again complains of the expense and says the drug does not work well. He quickly returns to his intermittent use of magnesium hydroxide tablets and occasionally takes bisacodyl tablets.
To address Mr. W’s OCD, the psychiatrist adds risperidone, 0.5 mg bid, to Mr. W’s regimen. He has a modest response in OCD symptoms—30% of his day is now symptom- free— without worsening his constipation.
Probiotics and prebiotics
Emerging therapies for constipation include probiotics and prebiotics, which attempt to alter the gut flora and milieu. The primary bacterial agents are Lactobacillus species and Bifidobacterium species. At least one probiotic Bifidobacterium product—Activia—is being marketed in the United States as a fortified yogurt.
Investigational medications. Renzapride is a 5HT4 receptor agonist and 5HT3 receptor antagonist that has shown promise in a pilot study18 and is in phase III trials. Linaclotide is a peptide that activates chloride and bicarbonate secretion in the gut and may reduce visceral hypersensitivity. It too has shown promise in a pilot study.19
Related resources
- Rome Foundation. Functional gastrointestinal disorders. www.romecriteria.org.
- Bleser S, Brunton S, Carmichael B, et al. Management of chronic constipation: recommendations from a consensus panel. J Fam Pract 2005;54(8):691-8.
- Amitriptyline • Elavil, Endep
- Chlorpromazine • Thorazine
- Clomipramine • Anafranil
- Clozapine • Clozaril
- Duloxetine • Cymbalta
- Fluvoxamine • Luvox
- Lactulose • Cholac Syrup, Constulose, others
- Lubiprostone • Amitiza
- Mirtazapine • Remeron
- Olanzapine • Zyprexa
- Risperidone • Risperdal
- Thioridazine • Mellaril
- Tegaserod • Zelnorm
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgment
This project was partially supported by grant number 5 T32 HS013852 from the Agency for Healthcare Research and Quality.
1. Stewart WF, Liberman JN, Sandler RS, et al. Epidemiology of constipation (EPOC) study in the United States: relation of clinical subtypes to sociodemographic features. Am J Gastroenterol 1999;94(12):3530-40.
2. Choung RS, Locke GR, 3rd, Schleck CD, et al. Cumulative incidence of chronic constipation: a population-based study 1988-2003. Aliment Pharmacol Ther 2007;26(11-12):1521-8.
3. Agency for Healthcare Research and Quality. Healthcare Cost and Utilization Project (HCUPnet). Available at: http://hcupnet.ahrq.gov. Accessed March 19, 2008.
4. Sonnenberg A, Koch TR. Physician visits in the United States for constipation: 1958 to 1986. Dig Dis Sci 1989;34(4):606-11.
5. Sonnenberg A, Koch TR. Epidemiology of constipation in the United States. Dis Colon Rectum 1989;32(1):1-8.
6. Dennison C, Prasad M, Lloyd A, et al. The health-related quality of life and economic burden of constipation. Pharmacoeconomics 2005;23(5):461-76.
7. Donald IP, Smith RG, Cruikshank JG, et al. A study of constipation in the elderly living at home. Gerontology 1985;31(2):112-8.
8. Longstreth GF, Thompson WG, Chey WD, et al. Functional bowel disorders. Gastroenterology 2006;130(5):1480-91.
9. American College of Gastroenterology Chronic Constipation Task Force. An evidence-based approach to the management of chronic constipation in North America. Am J Gastroenterol 2005;(100 suppl 1):S1-4.
10. U.S. Preventive Services Task Force. Colorectal cancer screening. Available at: http://www.ahrq.gov/clinic/3rduspstf/colorectal. Accessed March 19, 2008.
11. Chiotakakou-Faliakou E, Kamm MA, Roy AJ, et al. Biofeedback provides long-term benefit for patients with intractable, slow and normal transit constipation. Gut 1998;42(4):517-21.
12. Kawimbe BM, Papachrysostomou M, Binnie NR, et al. Outlet obstruction constipation (anismus) managed by biofeedback. Gut 1991;32(10):1175-9.
13. Richelson E. Receptor pharmacology of neuroleptics: relation to clinical effects. J Clin Psychiatry 1999;(60 suppl 10):5-14.
14. Muller-Lissner SA, Kamm MA, Scarpignato C, Wald A. Myths and misconceptions about chronic constipation. Am J Gastroenterol 2005;100(1):232-42.
15. Keeling WF, Harris A, Martin BJ. Orocecal transit during mild exercise in women. J Appl Physiol 1990;68(4):1350-3.
16. Tegaserod [package insert]. East Hanover, NJ: Novartis Pharmaceuticals; 2006.
17. Amitiza [package insert]. Bethesda, MD: Sucampo Pharmaceuticals; 2007.
18. Tack J, Middleton SJ, Horne MC, et al. Pilot study of the efficacy of renzapride on gastrointestinal motility and symptoms in patients with constipation-predominant irritable bowel syndrome. Aliment Pharmacol Ther 2006;23(11):1655-65.
19. Andresen V, Camilleri M, Busciglio IA, et al. Effect of 5 days linaclotide on transit and bowel function in females with constipation-predominant irritable bowel syndrome. Gastroenterology 2007;133(3):761-8.
1. Stewart WF, Liberman JN, Sandler RS, et al. Epidemiology of constipation (EPOC) study in the United States: relation of clinical subtypes to sociodemographic features. Am J Gastroenterol 1999;94(12):3530-40.
2. Choung RS, Locke GR, 3rd, Schleck CD, et al. Cumulative incidence of chronic constipation: a population-based study 1988-2003. Aliment Pharmacol Ther 2007;26(11-12):1521-8.
3. Agency for Healthcare Research and Quality. Healthcare Cost and Utilization Project (HCUPnet). Available at: http://hcupnet.ahrq.gov. Accessed March 19, 2008.
4. Sonnenberg A, Koch TR. Physician visits in the United States for constipation: 1958 to 1986. Dig Dis Sci 1989;34(4):606-11.
5. Sonnenberg A, Koch TR. Epidemiology of constipation in the United States. Dis Colon Rectum 1989;32(1):1-8.
6. Dennison C, Prasad M, Lloyd A, et al. The health-related quality of life and economic burden of constipation. Pharmacoeconomics 2005;23(5):461-76.
7. Donald IP, Smith RG, Cruikshank JG, et al. A study of constipation in the elderly living at home. Gerontology 1985;31(2):112-8.
8. Longstreth GF, Thompson WG, Chey WD, et al. Functional bowel disorders. Gastroenterology 2006;130(5):1480-91.
9. American College of Gastroenterology Chronic Constipation Task Force. An evidence-based approach to the management of chronic constipation in North America. Am J Gastroenterol 2005;(100 suppl 1):S1-4.
10. U.S. Preventive Services Task Force. Colorectal cancer screening. Available at: http://www.ahrq.gov/clinic/3rduspstf/colorectal. Accessed March 19, 2008.
11. Chiotakakou-Faliakou E, Kamm MA, Roy AJ, et al. Biofeedback provides long-term benefit for patients with intractable, slow and normal transit constipation. Gut 1998;42(4):517-21.
12. Kawimbe BM, Papachrysostomou M, Binnie NR, et al. Outlet obstruction constipation (anismus) managed by biofeedback. Gut 1991;32(10):1175-9.
13. Richelson E. Receptor pharmacology of neuroleptics: relation to clinical effects. J Clin Psychiatry 1999;(60 suppl 10):5-14.
14. Muller-Lissner SA, Kamm MA, Scarpignato C, Wald A. Myths and misconceptions about chronic constipation. Am J Gastroenterol 2005;100(1):232-42.
15. Keeling WF, Harris A, Martin BJ. Orocecal transit during mild exercise in women. J Appl Physiol 1990;68(4):1350-3.
16. Tegaserod [package insert]. East Hanover, NJ: Novartis Pharmaceuticals; 2006.
17. Amitiza [package insert]. Bethesda, MD: Sucampo Pharmaceuticals; 2007.
18. Tack J, Middleton SJ, Horne MC, et al. Pilot study of the efficacy of renzapride on gastrointestinal motility and symptoms in patients with constipation-predominant irritable bowel syndrome. Aliment Pharmacol Ther 2006;23(11):1655-65.
19. Andresen V, Camilleri M, Busciglio IA, et al. Effect of 5 days linaclotide on transit and bowel function in females with constipation-predominant irritable bowel syndrome. Gastroenterology 2007;133(3):761-8.
Drug eruptions: 6 dangerous rashes
The best intervention for a potentially life-threatening drug rash can happen before you choose a psychotropic. Carefully evaluating your patient’s risk for an adverse cutaneous drug reaction (ACDR) will guide safer prescribing. If your patient develops a rash, differentiating serious from benign reactions can help prevent morbidity, which can range from work loss or hospitalization to disfigurement or death.
In the first installment of this 2-part article on drug eruptions, we discussed how to recognize and manage benign rashes.1 Here we explain how to reduce ACDR risk and identify 6 serious rashes.
Risk reduction strategies
Although it is impossible to eliminate drug rashes, you may be able to reduce ACDR risk by using sound prescribing methods. Ultimately your choice of a psychotropic comes down to whether the drug’s benefits outweigh the risks to your patient. Factors affecting ACDR risk fall into 3 categories:
- historical
- pharmacokinetic
- environmental/other.
A patient who has had an ACDR also may be hypersensitive to other drugs in the same class. One example is anticonvulsant hypersensitivity syndrome. Phenytoin, carbamazepine, and phenobarbital may be cross-reactive.3 A patient who is hypersensitive to carbamazepine may have a ≥30% risk of reacting to oxcarbazepine.4 A major predictor of rash associated with lamotrigine is history of a rash from another antiepileptic.5 Cross-reactivity also may occur among antidepressants, particularly selective serotonin reuptake inhibitors.6
Knowles et al3 suggests warning close relatives of a patient with anticonvulsant hypersensitivity syndrome about the risk of using potentially cross-reactive anticonvulsants.
If your patient reports that a relative had an ACDR—particularly a severe reaction—to a drug you are considering prescribing, reduce this patient’s risk by choosing an alternate drug or proceeding cautiously by slowly titrating the dosage and monitoring carefully.
Pharmacokinetic factors. In general, ACDRs and dosage are not correlated,2 but anticonvulsants may be an exception. For example:
- lowering the starting dosage of lamotrigine reduces ACDR risk9
- rapid increase in dosages and high serum concentrations of phenytoin and carbamazepine appear to increase the risk of rash.10
Be vigilant for potential interactions between drugs. For instance, valproic acid inhibits lamotrigine metabolism, so when prescribing these 2 medications together, take steps to avoid a serious, life-threatening rash such as Stevens-Johnson syndrome (SJS). For bipolar patients age >12 taking valproic acid, titrate lamotrigine in a special regimen (initially 25 mg every other day, then gradually increased to ≤100 mg/d).11 Remain in close contact with the patient’s other prescribers to ensure that all are aware of potential adverse reactions if the patient’s medications are changed.
Environmental /other factors. Psychotropic medications—particularly antipsychotics—are associated with ACDRs related to sun exposure.12-14 Advise patients to use sunscreen and wear protective clothing, and consider recommending antioxidant supplements to help prevent photosensitive reactions.15
Populations at increased risk of developing a drug rash include African-Americans and persons age >70.7 Women have higher incidence of rash from lamotrigine use compared with men.9 Underlying diseases, such as human immunodeficiency virus, may increase ACDR risk.7 Strategies for reducing ACDR risk are summarized in Table 1.
Table 1
Steps to reduce ACDR risk
| Identify patients at risk |
| Use lowest effective dosages |
| Titrate medications according to latest recommendations |
| Consider the effects of polypharmacy, particularly on drug metabolism |
| Remain in contact with patients’ other providers to stay informed of medication changes |
| Advise patients that limiting sun exposure may reduce ACDR risk of certain drugs |
| Educate patients about ACDRs, including how to identify ‘red flags’ that indicate a serious reaction and when to seek medical attention |
| ACDR: adverse cutaneous drug reaction |
Serious drug eruptions
Most drug rashes are benign, but some can be life-threatening and require immediate drug discontinuation. Six serious ACDRs associated with psychotropics are listed in Table 2.
Table 2
Serious rashes associated with psychotropics*
| Rash | Suspect drugs/classes |
|---|---|
| Erythema multiforme | Bupropion, carbamazepine, clozapine, duloxetine, eszopiclone, fluoxetine, lamotrigine, methylphenidate, oxcarbazepine, paroxetine, quetiapine, risperidone, sertraline, topiramate, trazodone, valproic acid, venlafaxine |
| Stevens-Johnson syndrome/toxic epidermal necrolysis | Alprazolam, bupropion, carbamazepine, chlorpromazine, clozapine, duloxetine, fluoxetine, fluvoxamine, lamotrigine, mixed amphetamine salts, oxcarbazepine, paroxetine, quetiapine, sertraline, topiramate, valproic acid, venlafaxine |
| Hypersensitivity syndrome | Amitriptyline, carbamazepine, clomipramine, desipramine, fluoxetine, lamotrigine, methylphenidate, olanzapine, oxcarbazepine, valproic acid |
| Vasculitis | Carbamazepine, clozapine, diazepam, fluoxetine, fluvoxamine, haloperidol, lamotrigine, maprotiline, paroxetine, sertraline, thioridazine, trazodone |
| Erythroderma | Aripiprazole, bupropion, carbamazepine, duloxetine, fluoxetine, lamotrigine, lithium, methylphenidate, mirtazapine, paroxetine, phenothiazines, quetiapine, risperidone, TCAs (most), venlafaxine, ziprasidone |
| Erythema nodosum | Carbamazepine, fluoxetine, paroxetine, venlafaxine |
| * Suspect any drug with any reaction | |
| TCAs: tricyclic antidepressants | |
| Source: For reference citations, see this article on CurrentPsychiatry.com | |
As described in part 1 of this article, general strategies for identifying and treating potential ACDRs include identifying the lesion by taking a history and performing a physical examination (Box). Look for “red flags” that indicate a potentially serious reaction:
- constitutional symptoms (fever, sore throat, malaise, arthralgia, lymphadenopathy, cough)
- facial involvement
- mucous membrane involvement
- skin tenderness or blistering, particularly if there is full-thickness epidermal detachment
- purpura.16,17
Table 3
Managing a serious rash
| Discontinue the offending drug immediately |
| Consult with a dermatologist and other specialists |
| Hospitalize the patient if indicated for supportive care |
| Report the case to the FDA and the drug manufacturer if the eruption is atypical or uncommon |
© 2001-2008, DermAtlas
Erythema multiforme: Erythematous target or iris-like papules and vesicobullous eruptions that present on extremities and palmoplantar surfaces. The patient might present with detachment of the epidermis from the dermis. If this consider SJS spectrum disease (see below).2,13,18,19
Because EM may be a harbinger of a more severe skin reaction, consult a dermatologist and—if the rash involves the eyes—an ophthalmologist.12 Antihistamines and topical corticosteroids may be used to treat EM.18 Depending on the severity of the reaction, hospitalization might be indicated.
Stevens-Johnson syndrome/toxic epidermal necrolysis (TEN) are considered a spectrum of reactive skin disorders; TEN is the more severe variant. Patients may present with a prodrome of fever, cough, and malaise. Oral lesions—such as mucosal blistering (Photo 2)—may precede skin lesions. Look for widespread distribution of flat, atypical target lesions characterized by blisters on purpuric macules.2 Compared with EM, SJS/TEN lesions are more far-reaching, and the more extensive mucous membrane involvement can affect the mouth, esophagus, and genitalia. Ocular involvement might lead to blindness.20-23
© 2001-2008, DermAtlas
Stevens-Johnson syndrome/toxic epidermal necrosis: Mucosal blistering, widespread flat skin lesions, and epidermal detachment. Epidermal detachment also may be widespread. SJS and TEN are differentiated by the extent of skin detachment:
- 10% to 30% detachment is SJS/TEN
- >30% is TEN.2
Advise patients who have had TEN to alert relatives that they also may be at increased risk of an ACDR to the offending drug.22 Because SJS/TEN can cause blindness, an ophthalmologist typically will be involved in the patient’s care.20
Hypersensitivity syndrome—known as drug rash with eosinophilia and systemic symptoms (DRESS)—is a potentially life-threatening syndrome that presents as a triad of fever, rash, and internal organ involvement.26 These symptoms typically present 2 to 6 weeks after the patient starts the offending drug.
Early symptoms may include fever, malaise, pharyngitis, and lymphadenopathy.2 Cutaneous manifestations range from relatively benign exanthematous eruptions to more serious eruptions such as erythroderma or TEN.
Laboratory findings might show abnormalities of the liver, kidneys, lungs, or thyroid. Atypical lymphocytes and eosinophilia may be present.
Because hypersensitivity syndrome may present like a benign condition, consider the diagnosis when assessing any drug rash, particularly if the patient is receiving an anticonvulsant.20,22,27 Appropriate, timely care may be best delivered in an inpatient setting, so hospitalization might be indicated. Laboratory tests to assess organ function may include complete blood count (CBC), urine analysis (UA), creatinine, liver function tests, and thyroid stimulating hormone (TSH).
Treatment is supportive. Note that unlike those with SJS/TEN, patients with hypersensitivity syndrome may be treated with systemic corticosteroids.27 As with TEN, patients should alert relatives to a possible increased risk of a severe reaction to the offending drug.22
Vasculitis may present with palpable purpura, fever, and rash generally in dependent areas (Photo 3). Patients often develop morbilliform or urticarial eruptions, and the condition might affect internal organs. Differential diagnosis includes:
- Henoch-Schönlein (allergic) purpura
- Wegener’s granulomatosis
- infections
- collagen vascular diseases.2
© 2001-2008, DermAtlas
Vasculitis: Palpable purpura, fever, and rash generally in dependent areas.
Pharmacotherapy depends on the severity of presentation and ranges from topical agents to immunosuppressants.2 Other treatments are rest, elevation, support stockings, and antihistamines.28
Erythroderma, also known as exfoliative dermatitis, can present as sudden, pruritic erythema that can generalize (Photo 4). Scaling will appear, followed by desquamation. Patients typically complain of irritation, feeling cold, and a sensation of tightness. Dilated dermal vessels can result in high-output cardiac failure. This potentially life-threatening condition can develop within 1 week of starting a drug.2,29
© 2001-2008, DermAtlas
Erythroderma: Sudden, pruritic erythema that can generalize. Scaling precedes desquamation.
Erythema nodosum may present as painful erythematous nodules—usually in the lower extremities (Photo 5)—that are the result of fat necrosis.13,30 Treatment typically involves best rest, nonsteroidal anti-inflammatory drugs, and potassium iodide.30 Systemic corticosteroids also may be used.31
© 2001-2008, DermAtlas
Erythema nodosum: Painful erythematous nodules, usually in the lower extremities.
Resuming psychiatric treatment
Although medically necessary for patients with a serious rash, abruptly discontinuing a psychotropic might place them at risk for rapid psychiatric decompensation. Whenever possible, wait 2 weeks before restarting psychopharmacotherapy in a patient who has been treated for an ACDR. If that is not feasible because (for example) the patient is psychotic and agitated, you can cross-taper with a different medication from another class.
If your patient has experienced a serious ACDR, follow the 3 “A’s” to protect against recurrence (Table 4).
Desquamation: skin falling off in scales or layers; exfoliation
Erythema: redness of the skin
Macule: a discolored lesion on the skin that is not elevated above the surface
Morbilliform: resembling measles
Nodule: a small lump, swelling, or collection of tissue
Papule: a small circumscribed, superficial, solid elevation of the skin
Purpura: red or purple discolorations on the skin caused by bleeding underneath the skin
Urticaria: a vascular reaction in the upper dermis characterized by pruritic hives
Vesicobullous: denoting an eruption of fluid-containing lesions of various sizes
Source: Dorland’s illustrated medical dictionary. 30th ed. Philadelphia, PA: Saunders; 2003.
3 ‘As’ to protect patients after a life-threatening ACDR
| Allergy. Add the offending drug to the patient’s allergy list to ensure it is not given again |
| Alert. Tell the patient he or she should wear a medical alert bracelet to prevent being given the drug |
| Advise. Inform the patients’ close relatives that they may be at risk for a similar reaction to the same drug or drugs from the same class |
| ACDR: adverse cutaneous drug reactions |
- Knowles SR, Shear NH. Recognition and management of severe cutaneous drug reactions. Dermatol Clin 2007;25(2):245-53.
- Dermatology Image Atlas. www.dermatlas.org.
- American Academy of Dermatology. www.aad.org.
- Alprazolam • Xanax
- Amitriptyline • Elavil
- Aripiprazole • Abilify
- Bupropion • Wellbutrin
- Carbamazepine • Tegretol
- Chlorpromazine • Thorazine
- Clomipramine • Anafranil
- Clozapine • Clozaril
- Cyclosporine • Neoral, Sandimmune
- Desipramine • Norpramin
- Diazepam • Valium
- Duloxetine • Cymbalta
- Eszopiclone • Lunesta
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Haloperidol • Haldol
- Lamotrigine • Lamictal
- Methylphenidate • Ritalin
- Mirtazapine • Remeron
- Maprotiline • Ludiomil
- Olanzapine • Zyprexa
- Oxcarbazepine • Trileptal
- Paroxetine • Paxil
- Phenytoin • Dilantin
- Phenobarbital • Luminal
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Sertraline • Zoloft
- Thioridazine • Mellaril
- Topiramate • Topamax
- Lithium • Lithobid, Eskalith
- Trazodone • Desyrel
- Valproic acid • Depakote
- Venlafaxine • Effexor
- Ziprasidone • Geodon
Dr. Skonicki reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Warnock receives research/grant support from Boehringer Ingelheim, Forest Pharmaceuticals, and Wyeth Pharmaceuticals.
1. Warnock JK, Skonicki J. Drug eruptions: Is your patient’s rash serious or benign? Current Psychiatry 2008;7(3):42-56.
2. Kimyai-Asadi A, Harris JC, Nousari HC. Critical overview: adverse cutaneous reactions to psychotropic medications. J Clin Psychiatry 1999;60(10):714-25.
3. Knowles SR, Shapiro LE, Shear NH. Anticonvulsant hypersensitivity syndrome: incidence, prevention, and management. Drug Saf 1999;21(6):489-501.
4. Trileptal [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2007.
5. Hirsch LJ, Weintraub DB, Buchsbaum R, et al. Predictors of lamotrigine-associated rash. Epilepsia 2006;47(2):318-22.
6. Warnock CA, Azadian AG. Cross-sensitivity between paroxetine and sertraline. Ann Pharmacother 2002;36(4):631-3.
7. Babu KS, Belgi G. Management of cutaneous drug reactions. Curr Allergy Asthma Rep 2002;2(1):26-33.
8. Olfson M, Wilner MT. A family case history of fluoxetine-induced skin reactions. J Nerv Ment Dis 1991;179(8):504-5.
9. Wong IC, Mawer GE, Sander JW. Factors influencing the incidence of lamotrigine-related skin rash. Ann Pharmacother 1999;33(10):1037-42.
10. Chadwick D, Shaw MDM, Foy P, et al. Serum anticonvulsant concentrations and the risk of drug induced skin eruptions. J Neurol Neurosurg Psychiatry 1984;47(6):642-4.
11. Lamictal [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2007.
12. Warnock JK, Morris DW. Adverse cutaneous reactions to mood stabilizers. Am J Clin Dermatol 2003;4(1):21-30.
13. Warnock JK, Morris DW. Adverse cutaneous reactions to antidepressants. Am J Clin Dermatol 2002;3(5):329-39.
14. Warnock JK, Morris DW. Adverse cutaneous reactions to antipsychotics. Am J Clin Dermatol 2002;3(9):629-36.
15. Moore DE. Drug-induced cutaneous photosensitivity: incidence, mechanism, prevention, and management. Drug Saf 2002;25(5):345-72.
16. Shear NH, Knowles SR, Sullivan JR, Shapiro L. Cutaneous reactions to drugs. In: Freedburg IM, Eisen AZ, Wolff K, et al, eds. Fitzpatrick’s dermatology in general medicine. 6th ed. New York, NY: McGraw-Hill; 2003:1330-7.
17. Chosidow OM, Stern RS, Wintroub BU. Cutaneous drug reactions. In: Kasper DL, Braunwald E, Fauci AS, et al, eds. Harrisons’s principles of internal medicine. 16th ed. New York, NY: McGraw-Hill; 2005:318-24.
18. Brushan M, Craven N. Erythema multiforme. In: Lebwohl MG, Heymann WR, Berth-Jones J, Coulson I, eds.Treatment of skin disease: comprehensive therapeutic strategies. London, UK: Mosby; 2002:196-9.
19. Al-Joani KA, Fedele S, Porter SR. Erythema multiforme and related disorders. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2007;103(5):642-54.
20. Wolf R, Orion E, Marcos B, Matz H. Life-threatening acute adverse cutaneous drug reactions. Clin Dermatol 2005;23(2):171-81.
21. Pereira FA, Mudgil AV, Rosmarin DM. Toxic epidermal necrolysis. J Am Acad Dermatol 2007;56(2):181-200.
22. Rojeau JC, Stern RS. Medical progress: severe adverse cutaneous reactions to drugs. N Engl J Med 1994;331(19):1272-85.
23. Bastuji-Garin S, Rzany B, Stern R, et al. Clinical classification of cases of toxic epidermal necrolysis, Stevens-Johnson syndrome, and erythema multiforme. Arch Dermatol 1993;129(1):92-6.
24. Craven N. Toxic epidermal necrolysis and Stevens-Johnson syndrome. In: Lebwohl MG, Heymann WR, Berth-Jones J, Coulson I, eds. Treatment of skin disease: comprehensive therapeutic strategies. London, UK: Mosby; 2002:633-6.
25. Chave TA, Mortimer NJ, Sladden MJ, et al. Toxic epidermal necrolysis: current evidence, practical management and future directions. Br J Dermatol 2005;153(2):241-53.
26. Bachot N, Roujeau JC. Differential diagnosis of severe cutaneous drug eruptions. Am J Clin Dermatol 2003;4(8):561-72.
27. Knowles SR, Shear NH. Recognition and management of severe cutaneous drug reactions. Dermatol Clin 2007;25(2):245-53.
28. Callen JP. Leukocytoclastic vasculitis. In: Lebwohl MG, Heymann WR, Berth-Jones J, Coulson I, eds. Treatment of skin disease: comprehensive therapeutic strategies. London, UK: Mosby; 2002:340-3.
29. Berth-Jones J. Erythroderma. In: Lebwohl MG, Heymann WR, Berth-Jones J, Coulson I, eds. Treatment of skin disease: comprehensive therapeutic strategies. London, UK: Mosby; 2002:205-8.
30. Woodall TG, Spielvogel RL. Erythema nodosum. In: Lebwohl MG, Heymann WR, Berth-Jones J, Coulson I, eds. Treatment of skin disease: comprehensive therapeutic strategies. London, UK: Mosby; 2002:200-2.
31. Schwartz RA, Nervi SJ. Erythema nodosum: a sign of systemic disease. Am Fam Physician 2007;75(5):695-700.
The best intervention for a potentially life-threatening drug rash can happen before you choose a psychotropic. Carefully evaluating your patient’s risk for an adverse cutaneous drug reaction (ACDR) will guide safer prescribing. If your patient develops a rash, differentiating serious from benign reactions can help prevent morbidity, which can range from work loss or hospitalization to disfigurement or death.
In the first installment of this 2-part article on drug eruptions, we discussed how to recognize and manage benign rashes.1 Here we explain how to reduce ACDR risk and identify 6 serious rashes.
Risk reduction strategies
Although it is impossible to eliminate drug rashes, you may be able to reduce ACDR risk by using sound prescribing methods. Ultimately your choice of a psychotropic comes down to whether the drug’s benefits outweigh the risks to your patient. Factors affecting ACDR risk fall into 3 categories:
- historical
- pharmacokinetic
- environmental/other.
A patient who has had an ACDR also may be hypersensitive to other drugs in the same class. One example is anticonvulsant hypersensitivity syndrome. Phenytoin, carbamazepine, and phenobarbital may be cross-reactive.3 A patient who is hypersensitive to carbamazepine may have a ≥30% risk of reacting to oxcarbazepine.4 A major predictor of rash associated with lamotrigine is history of a rash from another antiepileptic.5 Cross-reactivity also may occur among antidepressants, particularly selective serotonin reuptake inhibitors.6
Knowles et al3 suggests warning close relatives of a patient with anticonvulsant hypersensitivity syndrome about the risk of using potentially cross-reactive anticonvulsants.
If your patient reports that a relative had an ACDR—particularly a severe reaction—to a drug you are considering prescribing, reduce this patient’s risk by choosing an alternate drug or proceeding cautiously by slowly titrating the dosage and monitoring carefully.
Pharmacokinetic factors. In general, ACDRs and dosage are not correlated,2 but anticonvulsants may be an exception. For example:
- lowering the starting dosage of lamotrigine reduces ACDR risk9
- rapid increase in dosages and high serum concentrations of phenytoin and carbamazepine appear to increase the risk of rash.10
Be vigilant for potential interactions between drugs. For instance, valproic acid inhibits lamotrigine metabolism, so when prescribing these 2 medications together, take steps to avoid a serious, life-threatening rash such as Stevens-Johnson syndrome (SJS). For bipolar patients age >12 taking valproic acid, titrate lamotrigine in a special regimen (initially 25 mg every other day, then gradually increased to ≤100 mg/d).11 Remain in close contact with the patient’s other prescribers to ensure that all are aware of potential adverse reactions if the patient’s medications are changed.
Environmental /other factors. Psychotropic medications—particularly antipsychotics—are associated with ACDRs related to sun exposure.12-14 Advise patients to use sunscreen and wear protective clothing, and consider recommending antioxidant supplements to help prevent photosensitive reactions.15
Populations at increased risk of developing a drug rash include African-Americans and persons age >70.7 Women have higher incidence of rash from lamotrigine use compared with men.9 Underlying diseases, such as human immunodeficiency virus, may increase ACDR risk.7 Strategies for reducing ACDR risk are summarized in Table 1.
Table 1
Steps to reduce ACDR risk
| Identify patients at risk |
| Use lowest effective dosages |
| Titrate medications according to latest recommendations |
| Consider the effects of polypharmacy, particularly on drug metabolism |
| Remain in contact with patients’ other providers to stay informed of medication changes |
| Advise patients that limiting sun exposure may reduce ACDR risk of certain drugs |
| Educate patients about ACDRs, including how to identify ‘red flags’ that indicate a serious reaction and when to seek medical attention |
| ACDR: adverse cutaneous drug reaction |
Serious drug eruptions
Most drug rashes are benign, but some can be life-threatening and require immediate drug discontinuation. Six serious ACDRs associated with psychotropics are listed in Table 2.
Table 2
Serious rashes associated with psychotropics*
| Rash | Suspect drugs/classes |
|---|---|
| Erythema multiforme | Bupropion, carbamazepine, clozapine, duloxetine, eszopiclone, fluoxetine, lamotrigine, methylphenidate, oxcarbazepine, paroxetine, quetiapine, risperidone, sertraline, topiramate, trazodone, valproic acid, venlafaxine |
| Stevens-Johnson syndrome/toxic epidermal necrolysis | Alprazolam, bupropion, carbamazepine, chlorpromazine, clozapine, duloxetine, fluoxetine, fluvoxamine, lamotrigine, mixed amphetamine salts, oxcarbazepine, paroxetine, quetiapine, sertraline, topiramate, valproic acid, venlafaxine |
| Hypersensitivity syndrome | Amitriptyline, carbamazepine, clomipramine, desipramine, fluoxetine, lamotrigine, methylphenidate, olanzapine, oxcarbazepine, valproic acid |
| Vasculitis | Carbamazepine, clozapine, diazepam, fluoxetine, fluvoxamine, haloperidol, lamotrigine, maprotiline, paroxetine, sertraline, thioridazine, trazodone |
| Erythroderma | Aripiprazole, bupropion, carbamazepine, duloxetine, fluoxetine, lamotrigine, lithium, methylphenidate, mirtazapine, paroxetine, phenothiazines, quetiapine, risperidone, TCAs (most), venlafaxine, ziprasidone |
| Erythema nodosum | Carbamazepine, fluoxetine, paroxetine, venlafaxine |
| * Suspect any drug with any reaction | |
| TCAs: tricyclic antidepressants | |
| Source: For reference citations, see this article on CurrentPsychiatry.com | |
As described in part 1 of this article, general strategies for identifying and treating potential ACDRs include identifying the lesion by taking a history and performing a physical examination (Box). Look for “red flags” that indicate a potentially serious reaction:
- constitutional symptoms (fever, sore throat, malaise, arthralgia, lymphadenopathy, cough)
- facial involvement
- mucous membrane involvement
- skin tenderness or blistering, particularly if there is full-thickness epidermal detachment
- purpura.16,17
Table 3
Managing a serious rash
| Discontinue the offending drug immediately |
| Consult with a dermatologist and other specialists |
| Hospitalize the patient if indicated for supportive care |
| Report the case to the FDA and the drug manufacturer if the eruption is atypical or uncommon |
© 2001-2008, DermAtlas
Erythema multiforme: Erythematous target or iris-like papules and vesicobullous eruptions that present on extremities and palmoplantar surfaces. The patient might present with detachment of the epidermis from the dermis. If this consider SJS spectrum disease (see below).2,13,18,19
Because EM may be a harbinger of a more severe skin reaction, consult a dermatologist and—if the rash involves the eyes—an ophthalmologist.12 Antihistamines and topical corticosteroids may be used to treat EM.18 Depending on the severity of the reaction, hospitalization might be indicated.
Stevens-Johnson syndrome/toxic epidermal necrolysis (TEN) are considered a spectrum of reactive skin disorders; TEN is the more severe variant. Patients may present with a prodrome of fever, cough, and malaise. Oral lesions—such as mucosal blistering (Photo 2)—may precede skin lesions. Look for widespread distribution of flat, atypical target lesions characterized by blisters on purpuric macules.2 Compared with EM, SJS/TEN lesions are more far-reaching, and the more extensive mucous membrane involvement can affect the mouth, esophagus, and genitalia. Ocular involvement might lead to blindness.20-23
© 2001-2008, DermAtlas
Stevens-Johnson syndrome/toxic epidermal necrosis: Mucosal blistering, widespread flat skin lesions, and epidermal detachment. Epidermal detachment also may be widespread. SJS and TEN are differentiated by the extent of skin detachment:
- 10% to 30% detachment is SJS/TEN
- >30% is TEN.2
Advise patients who have had TEN to alert relatives that they also may be at increased risk of an ACDR to the offending drug.22 Because SJS/TEN can cause blindness, an ophthalmologist typically will be involved in the patient’s care.20
Hypersensitivity syndrome—known as drug rash with eosinophilia and systemic symptoms (DRESS)—is a potentially life-threatening syndrome that presents as a triad of fever, rash, and internal organ involvement.26 These symptoms typically present 2 to 6 weeks after the patient starts the offending drug.
Early symptoms may include fever, malaise, pharyngitis, and lymphadenopathy.2 Cutaneous manifestations range from relatively benign exanthematous eruptions to more serious eruptions such as erythroderma or TEN.
Laboratory findings might show abnormalities of the liver, kidneys, lungs, or thyroid. Atypical lymphocytes and eosinophilia may be present.
Because hypersensitivity syndrome may present like a benign condition, consider the diagnosis when assessing any drug rash, particularly if the patient is receiving an anticonvulsant.20,22,27 Appropriate, timely care may be best delivered in an inpatient setting, so hospitalization might be indicated. Laboratory tests to assess organ function may include complete blood count (CBC), urine analysis (UA), creatinine, liver function tests, and thyroid stimulating hormone (TSH).
Treatment is supportive. Note that unlike those with SJS/TEN, patients with hypersensitivity syndrome may be treated with systemic corticosteroids.27 As with TEN, patients should alert relatives to a possible increased risk of a severe reaction to the offending drug.22
Vasculitis may present with palpable purpura, fever, and rash generally in dependent areas (Photo 3). Patients often develop morbilliform or urticarial eruptions, and the condition might affect internal organs. Differential diagnosis includes:
- Henoch-Schönlein (allergic) purpura
- Wegener’s granulomatosis
- infections
- collagen vascular diseases.2
© 2001-2008, DermAtlas
Vasculitis: Palpable purpura, fever, and rash generally in dependent areas.
Pharmacotherapy depends on the severity of presentation and ranges from topical agents to immunosuppressants.2 Other treatments are rest, elevation, support stockings, and antihistamines.28
Erythroderma, also known as exfoliative dermatitis, can present as sudden, pruritic erythema that can generalize (Photo 4). Scaling will appear, followed by desquamation. Patients typically complain of irritation, feeling cold, and a sensation of tightness. Dilated dermal vessels can result in high-output cardiac failure. This potentially life-threatening condition can develop within 1 week of starting a drug.2,29
© 2001-2008, DermAtlas
Erythroderma: Sudden, pruritic erythema that can generalize. Scaling precedes desquamation.
Erythema nodosum may present as painful erythematous nodules—usually in the lower extremities (Photo 5)—that are the result of fat necrosis.13,30 Treatment typically involves best rest, nonsteroidal anti-inflammatory drugs, and potassium iodide.30 Systemic corticosteroids also may be used.31
© 2001-2008, DermAtlas
Erythema nodosum: Painful erythematous nodules, usually in the lower extremities.
Resuming psychiatric treatment
Although medically necessary for patients with a serious rash, abruptly discontinuing a psychotropic might place them at risk for rapid psychiatric decompensation. Whenever possible, wait 2 weeks before restarting psychopharmacotherapy in a patient who has been treated for an ACDR. If that is not feasible because (for example) the patient is psychotic and agitated, you can cross-taper with a different medication from another class.
If your patient has experienced a serious ACDR, follow the 3 “A’s” to protect against recurrence (Table 4).
Desquamation: skin falling off in scales or layers; exfoliation
Erythema: redness of the skin
Macule: a discolored lesion on the skin that is not elevated above the surface
Morbilliform: resembling measles
Nodule: a small lump, swelling, or collection of tissue
Papule: a small circumscribed, superficial, solid elevation of the skin
Purpura: red or purple discolorations on the skin caused by bleeding underneath the skin
Urticaria: a vascular reaction in the upper dermis characterized by pruritic hives
Vesicobullous: denoting an eruption of fluid-containing lesions of various sizes
Source: Dorland’s illustrated medical dictionary. 30th ed. Philadelphia, PA: Saunders; 2003.
3 ‘As’ to protect patients after a life-threatening ACDR
| Allergy. Add the offending drug to the patient’s allergy list to ensure it is not given again |
| Alert. Tell the patient he or she should wear a medical alert bracelet to prevent being given the drug |
| Advise. Inform the patients’ close relatives that they may be at risk for a similar reaction to the same drug or drugs from the same class |
| ACDR: adverse cutaneous drug reactions |
- Knowles SR, Shear NH. Recognition and management of severe cutaneous drug reactions. Dermatol Clin 2007;25(2):245-53.
- Dermatology Image Atlas. www.dermatlas.org.
- American Academy of Dermatology. www.aad.org.
- Alprazolam • Xanax
- Amitriptyline • Elavil
- Aripiprazole • Abilify
- Bupropion • Wellbutrin
- Carbamazepine • Tegretol
- Chlorpromazine • Thorazine
- Clomipramine • Anafranil
- Clozapine • Clozaril
- Cyclosporine • Neoral, Sandimmune
- Desipramine • Norpramin
- Diazepam • Valium
- Duloxetine • Cymbalta
- Eszopiclone • Lunesta
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Haloperidol • Haldol
- Lamotrigine • Lamictal
- Methylphenidate • Ritalin
- Mirtazapine • Remeron
- Maprotiline • Ludiomil
- Olanzapine • Zyprexa
- Oxcarbazepine • Trileptal
- Paroxetine • Paxil
- Phenytoin • Dilantin
- Phenobarbital • Luminal
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Sertraline • Zoloft
- Thioridazine • Mellaril
- Topiramate • Topamax
- Lithium • Lithobid, Eskalith
- Trazodone • Desyrel
- Valproic acid • Depakote
- Venlafaxine • Effexor
- Ziprasidone • Geodon
Dr. Skonicki reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Warnock receives research/grant support from Boehringer Ingelheim, Forest Pharmaceuticals, and Wyeth Pharmaceuticals.
The best intervention for a potentially life-threatening drug rash can happen before you choose a psychotropic. Carefully evaluating your patient’s risk for an adverse cutaneous drug reaction (ACDR) will guide safer prescribing. If your patient develops a rash, differentiating serious from benign reactions can help prevent morbidity, which can range from work loss or hospitalization to disfigurement or death.
In the first installment of this 2-part article on drug eruptions, we discussed how to recognize and manage benign rashes.1 Here we explain how to reduce ACDR risk and identify 6 serious rashes.
Risk reduction strategies
Although it is impossible to eliminate drug rashes, you may be able to reduce ACDR risk by using sound prescribing methods. Ultimately your choice of a psychotropic comes down to whether the drug’s benefits outweigh the risks to your patient. Factors affecting ACDR risk fall into 3 categories:
- historical
- pharmacokinetic
- environmental/other.
A patient who has had an ACDR also may be hypersensitive to other drugs in the same class. One example is anticonvulsant hypersensitivity syndrome. Phenytoin, carbamazepine, and phenobarbital may be cross-reactive.3 A patient who is hypersensitive to carbamazepine may have a ≥30% risk of reacting to oxcarbazepine.4 A major predictor of rash associated with lamotrigine is history of a rash from another antiepileptic.5 Cross-reactivity also may occur among antidepressants, particularly selective serotonin reuptake inhibitors.6
Knowles et al3 suggests warning close relatives of a patient with anticonvulsant hypersensitivity syndrome about the risk of using potentially cross-reactive anticonvulsants.
If your patient reports that a relative had an ACDR—particularly a severe reaction—to a drug you are considering prescribing, reduce this patient’s risk by choosing an alternate drug or proceeding cautiously by slowly titrating the dosage and monitoring carefully.
Pharmacokinetic factors. In general, ACDRs and dosage are not correlated,2 but anticonvulsants may be an exception. For example:
- lowering the starting dosage of lamotrigine reduces ACDR risk9
- rapid increase in dosages and high serum concentrations of phenytoin and carbamazepine appear to increase the risk of rash.10
Be vigilant for potential interactions between drugs. For instance, valproic acid inhibits lamotrigine metabolism, so when prescribing these 2 medications together, take steps to avoid a serious, life-threatening rash such as Stevens-Johnson syndrome (SJS). For bipolar patients age >12 taking valproic acid, titrate lamotrigine in a special regimen (initially 25 mg every other day, then gradually increased to ≤100 mg/d).11 Remain in close contact with the patient’s other prescribers to ensure that all are aware of potential adverse reactions if the patient’s medications are changed.
Environmental /other factors. Psychotropic medications—particularly antipsychotics—are associated with ACDRs related to sun exposure.12-14 Advise patients to use sunscreen and wear protective clothing, and consider recommending antioxidant supplements to help prevent photosensitive reactions.15
Populations at increased risk of developing a drug rash include African-Americans and persons age >70.7 Women have higher incidence of rash from lamotrigine use compared with men.9 Underlying diseases, such as human immunodeficiency virus, may increase ACDR risk.7 Strategies for reducing ACDR risk are summarized in Table 1.
Table 1
Steps to reduce ACDR risk
| Identify patients at risk |
| Use lowest effective dosages |
| Titrate medications according to latest recommendations |
| Consider the effects of polypharmacy, particularly on drug metabolism |
| Remain in contact with patients’ other providers to stay informed of medication changes |
| Advise patients that limiting sun exposure may reduce ACDR risk of certain drugs |
| Educate patients about ACDRs, including how to identify ‘red flags’ that indicate a serious reaction and when to seek medical attention |
| ACDR: adverse cutaneous drug reaction |
Serious drug eruptions
Most drug rashes are benign, but some can be life-threatening and require immediate drug discontinuation. Six serious ACDRs associated with psychotropics are listed in Table 2.
Table 2
Serious rashes associated with psychotropics*
| Rash | Suspect drugs/classes |
|---|---|
| Erythema multiforme | Bupropion, carbamazepine, clozapine, duloxetine, eszopiclone, fluoxetine, lamotrigine, methylphenidate, oxcarbazepine, paroxetine, quetiapine, risperidone, sertraline, topiramate, trazodone, valproic acid, venlafaxine |
| Stevens-Johnson syndrome/toxic epidermal necrolysis | Alprazolam, bupropion, carbamazepine, chlorpromazine, clozapine, duloxetine, fluoxetine, fluvoxamine, lamotrigine, mixed amphetamine salts, oxcarbazepine, paroxetine, quetiapine, sertraline, topiramate, valproic acid, venlafaxine |
| Hypersensitivity syndrome | Amitriptyline, carbamazepine, clomipramine, desipramine, fluoxetine, lamotrigine, methylphenidate, olanzapine, oxcarbazepine, valproic acid |
| Vasculitis | Carbamazepine, clozapine, diazepam, fluoxetine, fluvoxamine, haloperidol, lamotrigine, maprotiline, paroxetine, sertraline, thioridazine, trazodone |
| Erythroderma | Aripiprazole, bupropion, carbamazepine, duloxetine, fluoxetine, lamotrigine, lithium, methylphenidate, mirtazapine, paroxetine, phenothiazines, quetiapine, risperidone, TCAs (most), venlafaxine, ziprasidone |
| Erythema nodosum | Carbamazepine, fluoxetine, paroxetine, venlafaxine |
| * Suspect any drug with any reaction | |
| TCAs: tricyclic antidepressants | |
| Source: For reference citations, see this article on CurrentPsychiatry.com | |
As described in part 1 of this article, general strategies for identifying and treating potential ACDRs include identifying the lesion by taking a history and performing a physical examination (Box). Look for “red flags” that indicate a potentially serious reaction:
- constitutional symptoms (fever, sore throat, malaise, arthralgia, lymphadenopathy, cough)
- facial involvement
- mucous membrane involvement
- skin tenderness or blistering, particularly if there is full-thickness epidermal detachment
- purpura.16,17
Table 3
Managing a serious rash
| Discontinue the offending drug immediately |
| Consult with a dermatologist and other specialists |
| Hospitalize the patient if indicated for supportive care |
| Report the case to the FDA and the drug manufacturer if the eruption is atypical or uncommon |
© 2001-2008, DermAtlas
Erythema multiforme: Erythematous target or iris-like papules and vesicobullous eruptions that present on extremities and palmoplantar surfaces. The patient might present with detachment of the epidermis from the dermis. If this consider SJS spectrum disease (see below).2,13,18,19
Because EM may be a harbinger of a more severe skin reaction, consult a dermatologist and—if the rash involves the eyes—an ophthalmologist.12 Antihistamines and topical corticosteroids may be used to treat EM.18 Depending on the severity of the reaction, hospitalization might be indicated.
Stevens-Johnson syndrome/toxic epidermal necrolysis (TEN) are considered a spectrum of reactive skin disorders; TEN is the more severe variant. Patients may present with a prodrome of fever, cough, and malaise. Oral lesions—such as mucosal blistering (Photo 2)—may precede skin lesions. Look for widespread distribution of flat, atypical target lesions characterized by blisters on purpuric macules.2 Compared with EM, SJS/TEN lesions are more far-reaching, and the more extensive mucous membrane involvement can affect the mouth, esophagus, and genitalia. Ocular involvement might lead to blindness.20-23
© 2001-2008, DermAtlas
Stevens-Johnson syndrome/toxic epidermal necrosis: Mucosal blistering, widespread flat skin lesions, and epidermal detachment. Epidermal detachment also may be widespread. SJS and TEN are differentiated by the extent of skin detachment:
- 10% to 30% detachment is SJS/TEN
- >30% is TEN.2
Advise patients who have had TEN to alert relatives that they also may be at increased risk of an ACDR to the offending drug.22 Because SJS/TEN can cause blindness, an ophthalmologist typically will be involved in the patient’s care.20
Hypersensitivity syndrome—known as drug rash with eosinophilia and systemic symptoms (DRESS)—is a potentially life-threatening syndrome that presents as a triad of fever, rash, and internal organ involvement.26 These symptoms typically present 2 to 6 weeks after the patient starts the offending drug.
Early symptoms may include fever, malaise, pharyngitis, and lymphadenopathy.2 Cutaneous manifestations range from relatively benign exanthematous eruptions to more serious eruptions such as erythroderma or TEN.
Laboratory findings might show abnormalities of the liver, kidneys, lungs, or thyroid. Atypical lymphocytes and eosinophilia may be present.
Because hypersensitivity syndrome may present like a benign condition, consider the diagnosis when assessing any drug rash, particularly if the patient is receiving an anticonvulsant.20,22,27 Appropriate, timely care may be best delivered in an inpatient setting, so hospitalization might be indicated. Laboratory tests to assess organ function may include complete blood count (CBC), urine analysis (UA), creatinine, liver function tests, and thyroid stimulating hormone (TSH).
Treatment is supportive. Note that unlike those with SJS/TEN, patients with hypersensitivity syndrome may be treated with systemic corticosteroids.27 As with TEN, patients should alert relatives to a possible increased risk of a severe reaction to the offending drug.22
Vasculitis may present with palpable purpura, fever, and rash generally in dependent areas (Photo 3). Patients often develop morbilliform or urticarial eruptions, and the condition might affect internal organs. Differential diagnosis includes:
- Henoch-Schönlein (allergic) purpura
- Wegener’s granulomatosis
- infections
- collagen vascular diseases.2
© 2001-2008, DermAtlas
Vasculitis: Palpable purpura, fever, and rash generally in dependent areas.
Pharmacotherapy depends on the severity of presentation and ranges from topical agents to immunosuppressants.2 Other treatments are rest, elevation, support stockings, and antihistamines.28
Erythroderma, also known as exfoliative dermatitis, can present as sudden, pruritic erythema that can generalize (Photo 4). Scaling will appear, followed by desquamation. Patients typically complain of irritation, feeling cold, and a sensation of tightness. Dilated dermal vessels can result in high-output cardiac failure. This potentially life-threatening condition can develop within 1 week of starting a drug.2,29
© 2001-2008, DermAtlas
Erythroderma: Sudden, pruritic erythema that can generalize. Scaling precedes desquamation.
Erythema nodosum may present as painful erythematous nodules—usually in the lower extremities (Photo 5)—that are the result of fat necrosis.13,30 Treatment typically involves best rest, nonsteroidal anti-inflammatory drugs, and potassium iodide.30 Systemic corticosteroids also may be used.31
© 2001-2008, DermAtlas
Erythema nodosum: Painful erythematous nodules, usually in the lower extremities.
Resuming psychiatric treatment
Although medically necessary for patients with a serious rash, abruptly discontinuing a psychotropic might place them at risk for rapid psychiatric decompensation. Whenever possible, wait 2 weeks before restarting psychopharmacotherapy in a patient who has been treated for an ACDR. If that is not feasible because (for example) the patient is psychotic and agitated, you can cross-taper with a different medication from another class.
If your patient has experienced a serious ACDR, follow the 3 “A’s” to protect against recurrence (Table 4).
Desquamation: skin falling off in scales or layers; exfoliation
Erythema: redness of the skin
Macule: a discolored lesion on the skin that is not elevated above the surface
Morbilliform: resembling measles
Nodule: a small lump, swelling, or collection of tissue
Papule: a small circumscribed, superficial, solid elevation of the skin
Purpura: red or purple discolorations on the skin caused by bleeding underneath the skin
Urticaria: a vascular reaction in the upper dermis characterized by pruritic hives
Vesicobullous: denoting an eruption of fluid-containing lesions of various sizes
Source: Dorland’s illustrated medical dictionary. 30th ed. Philadelphia, PA: Saunders; 2003.
3 ‘As’ to protect patients after a life-threatening ACDR
| Allergy. Add the offending drug to the patient’s allergy list to ensure it is not given again |
| Alert. Tell the patient he or she should wear a medical alert bracelet to prevent being given the drug |
| Advise. Inform the patients’ close relatives that they may be at risk for a similar reaction to the same drug or drugs from the same class |
| ACDR: adverse cutaneous drug reactions |
- Knowles SR, Shear NH. Recognition and management of severe cutaneous drug reactions. Dermatol Clin 2007;25(2):245-53.
- Dermatology Image Atlas. www.dermatlas.org.
- American Academy of Dermatology. www.aad.org.
- Alprazolam • Xanax
- Amitriptyline • Elavil
- Aripiprazole • Abilify
- Bupropion • Wellbutrin
- Carbamazepine • Tegretol
- Chlorpromazine • Thorazine
- Clomipramine • Anafranil
- Clozapine • Clozaril
- Cyclosporine • Neoral, Sandimmune
- Desipramine • Norpramin
- Diazepam • Valium
- Duloxetine • Cymbalta
- Eszopiclone • Lunesta
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Haloperidol • Haldol
- Lamotrigine • Lamictal
- Methylphenidate • Ritalin
- Mirtazapine • Remeron
- Maprotiline • Ludiomil
- Olanzapine • Zyprexa
- Oxcarbazepine • Trileptal
- Paroxetine • Paxil
- Phenytoin • Dilantin
- Phenobarbital • Luminal
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Sertraline • Zoloft
- Thioridazine • Mellaril
- Topiramate • Topamax
- Lithium • Lithobid, Eskalith
- Trazodone • Desyrel
- Valproic acid • Depakote
- Venlafaxine • Effexor
- Ziprasidone • Geodon
Dr. Skonicki reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Warnock receives research/grant support from Boehringer Ingelheim, Forest Pharmaceuticals, and Wyeth Pharmaceuticals.
1. Warnock JK, Skonicki J. Drug eruptions: Is your patient’s rash serious or benign? Current Psychiatry 2008;7(3):42-56.
2. Kimyai-Asadi A, Harris JC, Nousari HC. Critical overview: adverse cutaneous reactions to psychotropic medications. J Clin Psychiatry 1999;60(10):714-25.
3. Knowles SR, Shapiro LE, Shear NH. Anticonvulsant hypersensitivity syndrome: incidence, prevention, and management. Drug Saf 1999;21(6):489-501.
4. Trileptal [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2007.
5. Hirsch LJ, Weintraub DB, Buchsbaum R, et al. Predictors of lamotrigine-associated rash. Epilepsia 2006;47(2):318-22.
6. Warnock CA, Azadian AG. Cross-sensitivity between paroxetine and sertraline. Ann Pharmacother 2002;36(4):631-3.
7. Babu KS, Belgi G. Management of cutaneous drug reactions. Curr Allergy Asthma Rep 2002;2(1):26-33.
8. Olfson M, Wilner MT. A family case history of fluoxetine-induced skin reactions. J Nerv Ment Dis 1991;179(8):504-5.
9. Wong IC, Mawer GE, Sander JW. Factors influencing the incidence of lamotrigine-related skin rash. Ann Pharmacother 1999;33(10):1037-42.
10. Chadwick D, Shaw MDM, Foy P, et al. Serum anticonvulsant concentrations and the risk of drug induced skin eruptions. J Neurol Neurosurg Psychiatry 1984;47(6):642-4.
11. Lamictal [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2007.
12. Warnock JK, Morris DW. Adverse cutaneous reactions to mood stabilizers. Am J Clin Dermatol 2003;4(1):21-30.
13. Warnock JK, Morris DW. Adverse cutaneous reactions to antidepressants. Am J Clin Dermatol 2002;3(5):329-39.
14. Warnock JK, Morris DW. Adverse cutaneous reactions to antipsychotics. Am J Clin Dermatol 2002;3(9):629-36.
15. Moore DE. Drug-induced cutaneous photosensitivity: incidence, mechanism, prevention, and management. Drug Saf 2002;25(5):345-72.
16. Shear NH, Knowles SR, Sullivan JR, Shapiro L. Cutaneous reactions to drugs. In: Freedburg IM, Eisen AZ, Wolff K, et al, eds. Fitzpatrick’s dermatology in general medicine. 6th ed. New York, NY: McGraw-Hill; 2003:1330-7.
17. Chosidow OM, Stern RS, Wintroub BU. Cutaneous drug reactions. In: Kasper DL, Braunwald E, Fauci AS, et al, eds. Harrisons’s principles of internal medicine. 16th ed. New York, NY: McGraw-Hill; 2005:318-24.
18. Brushan M, Craven N. Erythema multiforme. In: Lebwohl MG, Heymann WR, Berth-Jones J, Coulson I, eds.Treatment of skin disease: comprehensive therapeutic strategies. London, UK: Mosby; 2002:196-9.
19. Al-Joani KA, Fedele S, Porter SR. Erythema multiforme and related disorders. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2007;103(5):642-54.
20. Wolf R, Orion E, Marcos B, Matz H. Life-threatening acute adverse cutaneous drug reactions. Clin Dermatol 2005;23(2):171-81.
21. Pereira FA, Mudgil AV, Rosmarin DM. Toxic epidermal necrolysis. J Am Acad Dermatol 2007;56(2):181-200.
22. Rojeau JC, Stern RS. Medical progress: severe adverse cutaneous reactions to drugs. N Engl J Med 1994;331(19):1272-85.
23. Bastuji-Garin S, Rzany B, Stern R, et al. Clinical classification of cases of toxic epidermal necrolysis, Stevens-Johnson syndrome, and erythema multiforme. Arch Dermatol 1993;129(1):92-6.
24. Craven N. Toxic epidermal necrolysis and Stevens-Johnson syndrome. In: Lebwohl MG, Heymann WR, Berth-Jones J, Coulson I, eds. Treatment of skin disease: comprehensive therapeutic strategies. London, UK: Mosby; 2002:633-6.
25. Chave TA, Mortimer NJ, Sladden MJ, et al. Toxic epidermal necrolysis: current evidence, practical management and future directions. Br J Dermatol 2005;153(2):241-53.
26. Bachot N, Roujeau JC. Differential diagnosis of severe cutaneous drug eruptions. Am J Clin Dermatol 2003;4(8):561-72.
27. Knowles SR, Shear NH. Recognition and management of severe cutaneous drug reactions. Dermatol Clin 2007;25(2):245-53.
28. Callen JP. Leukocytoclastic vasculitis. In: Lebwohl MG, Heymann WR, Berth-Jones J, Coulson I, eds. Treatment of skin disease: comprehensive therapeutic strategies. London, UK: Mosby; 2002:340-3.
29. Berth-Jones J. Erythroderma. In: Lebwohl MG, Heymann WR, Berth-Jones J, Coulson I, eds. Treatment of skin disease: comprehensive therapeutic strategies. London, UK: Mosby; 2002:205-8.
30. Woodall TG, Spielvogel RL. Erythema nodosum. In: Lebwohl MG, Heymann WR, Berth-Jones J, Coulson I, eds. Treatment of skin disease: comprehensive therapeutic strategies. London, UK: Mosby; 2002:200-2.
31. Schwartz RA, Nervi SJ. Erythema nodosum: a sign of systemic disease. Am Fam Physician 2007;75(5):695-700.
1. Warnock JK, Skonicki J. Drug eruptions: Is your patient’s rash serious or benign? Current Psychiatry 2008;7(3):42-56.
2. Kimyai-Asadi A, Harris JC, Nousari HC. Critical overview: adverse cutaneous reactions to psychotropic medications. J Clin Psychiatry 1999;60(10):714-25.
3. Knowles SR, Shapiro LE, Shear NH. Anticonvulsant hypersensitivity syndrome: incidence, prevention, and management. Drug Saf 1999;21(6):489-501.
4. Trileptal [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2007.
5. Hirsch LJ, Weintraub DB, Buchsbaum R, et al. Predictors of lamotrigine-associated rash. Epilepsia 2006;47(2):318-22.
6. Warnock CA, Azadian AG. Cross-sensitivity between paroxetine and sertraline. Ann Pharmacother 2002;36(4):631-3.
7. Babu KS, Belgi G. Management of cutaneous drug reactions. Curr Allergy Asthma Rep 2002;2(1):26-33.
8. Olfson M, Wilner MT. A family case history of fluoxetine-induced skin reactions. J Nerv Ment Dis 1991;179(8):504-5.
9. Wong IC, Mawer GE, Sander JW. Factors influencing the incidence of lamotrigine-related skin rash. Ann Pharmacother 1999;33(10):1037-42.
10. Chadwick D, Shaw MDM, Foy P, et al. Serum anticonvulsant concentrations and the risk of drug induced skin eruptions. J Neurol Neurosurg Psychiatry 1984;47(6):642-4.
11. Lamictal [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2007.
12. Warnock JK, Morris DW. Adverse cutaneous reactions to mood stabilizers. Am J Clin Dermatol 2003;4(1):21-30.
13. Warnock JK, Morris DW. Adverse cutaneous reactions to antidepressants. Am J Clin Dermatol 2002;3(5):329-39.
14. Warnock JK, Morris DW. Adverse cutaneous reactions to antipsychotics. Am J Clin Dermatol 2002;3(9):629-36.
15. Moore DE. Drug-induced cutaneous photosensitivity: incidence, mechanism, prevention, and management. Drug Saf 2002;25(5):345-72.
16. Shear NH, Knowles SR, Sullivan JR, Shapiro L. Cutaneous reactions to drugs. In: Freedburg IM, Eisen AZ, Wolff K, et al, eds. Fitzpatrick’s dermatology in general medicine. 6th ed. New York, NY: McGraw-Hill; 2003:1330-7.
17. Chosidow OM, Stern RS, Wintroub BU. Cutaneous drug reactions. In: Kasper DL, Braunwald E, Fauci AS, et al, eds. Harrisons’s principles of internal medicine. 16th ed. New York, NY: McGraw-Hill; 2005:318-24.
18. Brushan M, Craven N. Erythema multiforme. In: Lebwohl MG, Heymann WR, Berth-Jones J, Coulson I, eds.Treatment of skin disease: comprehensive therapeutic strategies. London, UK: Mosby; 2002:196-9.
19. Al-Joani KA, Fedele S, Porter SR. Erythema multiforme and related disorders. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2007;103(5):642-54.
20. Wolf R, Orion E, Marcos B, Matz H. Life-threatening acute adverse cutaneous drug reactions. Clin Dermatol 2005;23(2):171-81.
21. Pereira FA, Mudgil AV, Rosmarin DM. Toxic epidermal necrolysis. J Am Acad Dermatol 2007;56(2):181-200.
22. Rojeau JC, Stern RS. Medical progress: severe adverse cutaneous reactions to drugs. N Engl J Med 1994;331(19):1272-85.
23. Bastuji-Garin S, Rzany B, Stern R, et al. Clinical classification of cases of toxic epidermal necrolysis, Stevens-Johnson syndrome, and erythema multiforme. Arch Dermatol 1993;129(1):92-6.
24. Craven N. Toxic epidermal necrolysis and Stevens-Johnson syndrome. In: Lebwohl MG, Heymann WR, Berth-Jones J, Coulson I, eds. Treatment of skin disease: comprehensive therapeutic strategies. London, UK: Mosby; 2002:633-6.
25. Chave TA, Mortimer NJ, Sladden MJ, et al. Toxic epidermal necrolysis: current evidence, practical management and future directions. Br J Dermatol 2005;153(2):241-53.
26. Bachot N, Roujeau JC. Differential diagnosis of severe cutaneous drug eruptions. Am J Clin Dermatol 2003;4(8):561-72.
27. Knowles SR, Shear NH. Recognition and management of severe cutaneous drug reactions. Dermatol Clin 2007;25(2):245-53.
28. Callen JP. Leukocytoclastic vasculitis. In: Lebwohl MG, Heymann WR, Berth-Jones J, Coulson I, eds. Treatment of skin disease: comprehensive therapeutic strategies. London, UK: Mosby; 2002:340-3.
29. Berth-Jones J. Erythroderma. In: Lebwohl MG, Heymann WR, Berth-Jones J, Coulson I, eds. Treatment of skin disease: comprehensive therapeutic strategies. London, UK: Mosby; 2002:205-8.
30. Woodall TG, Spielvogel RL. Erythema nodosum. In: Lebwohl MG, Heymann WR, Berth-Jones J, Coulson I, eds. Treatment of skin disease: comprehensive therapeutic strategies. London, UK: Mosby; 2002:200-2.
31. Schwartz RA, Nervi SJ. Erythema nodosum: a sign of systemic disease. Am Fam Physician 2007;75(5):695-700.