User login
Are sweeping efforts to reduce primary CD rates associated with an increase in maternal or neonatal AEs?
EXPERT COMMENTARY
Main EK, Chang SC, Cape V, et al. Safety assessment of a large-scale improvement collaborative to reduce nulliparous cesarean delivery rates. Obstet Gynecol. 2019;133:613-623.
Cesarean delivery can be lifesaving for both mother and infant. When compared with successful vaginal delivery, however, CD is associated with higher maternal complication rates (including excessive blood loss requiring blood product transfusion, infectious morbidity, and venous thromboembolic events), longer hospital length of stay, and higher cost. While the optimal CD rate is not well defined, it is generally accepted that the CD rate in the United States is excessively high. As such, efforts to reduce the CD rate should be encouraged, but not at the expense of patient safety.
Details about the study
In keeping with the dictum that the most important CD to prevent is the first one, the California Maternal Quality Care Collaborative (CMQCC) in 2016 introduced a large-scale quality improvement project designed to reduce nulliparous, term, singleton, vertex (NTSV) CDs across the state. This bundle included education around joint guidelines issued by the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine on reducing primary CDs,1 introduction of a CMQCC toolkit, increased nursing labor support, and monthly meetings to share best practices across all collaborating sites. The NTSV CD rate in these hospitals did decrease from 29.3% in 2015 to 25.0% in 2017 (adjusted odds ratio, 0.76; 95% confidence interval, 0.73–0.78).
Whether or not implementation of the bundle resulted in an inappropriate delay in indicated CDs and, as such, in an increase in maternal or neonatal morbidity is not known. To address this issue, Main and colleagues collected cross-sectional data from more than 50 hospitals with more than 119,000 deliveries throughout California and measured rates of chorioamnionitis, blood transfusions, third- or fourth-degree perineal lacerations, operative vaginal delivery, severe unexpected newborn complications, and 5-minute Apgar scores of less than 5. None of the 6 safety measures showed any difference when comparing 2017 (after implementation of the CMQCC bundle) to 2015 (before implementation), suggesting that patient safety was not compromised significantly.
Study strengths and weaknesses
Strengths of this study include its large sample size and multicenter design with inclusion of a variety of collaborating hospitals. Earlier studies examining the effect of standardized protocols to reduce CD rates have been largely underpowered and conducted at single institutions.2-6 Moreover, results have been mixed, with some studies reporting an increase in maternal/neonatal adverse events,2-4 while others suggesting an improvement in select newborn quality outcome metrics.5 The current study provides reassurance to providers and institutions employing strategies to reduce NTSV CD rates that such efforts are safe.
Continue to: This study has several limitations...
This study has several limitations. Data collection relied on birth certificate and discharge diagnoses without a robust quality audit. As such, ascertainment bias, random error, and undercounting cannot be excluded. Although the population was heterogeneous, most women had more than a high school education and private insurance, and only 1 in 5 were obese. Whether these findings are generalizable to other areas within the United States is not known.
All reasonable efforts to decrease the CD rate in the United States should be encouraged, with particular attention paid to avoiding the first CD. However, this should not be done at the expense of patient safety. Large-scale quality improvement initiatives, similar to CMQCC efforts in California in 2016, appear to be one such strategy. Other successful strategies may include, for example, routine induction of labor for all low-risk nulliparous women at 39 weeks' gestation.7 The current report suggests that implementing a large-scale quality improvement initiative to reduce the primary CD rate can likely be done safely, without a significant increase in maternal or neonatal morbidity.
- American College of Obstetricians and Gynecologists; Society for Maternal-Fetal Medicine. ACOG Obstetric care consensus no. 1: safe prevention of the primary cesarean delivery. Obstet Gynecol. 2014;123:693-711.
- Rosenbloom JI, Stout MJ, Tuuli MG, et al. New labor management guidelines and changes in cesarean delivery patterns. Am J Obstet Gynecol. 2017;217:689.e1-689.e8.
- Vadnais MA, Hacker MR, Shah NT, et al. Quality improvement initiatives lead to reduction in nulliparous term singleton vertex cesarean delivery rate. Jt Comm J Qual Patient Saf. 2017;43:53-61.
- Zipori Y, Grunwald O, Ginsberg Y, et al. The impact of extending the second stage of labor to prevent primary cesarean delivery on maternal and neonatal outcomes. Am J Obstet Gynecol. 2019; 220:191.e1-191.e7.
- Thuillier C, Roy S, Peyronnet V, et al. Impact of recommended changes in labor management for prevention of the primary cesarean delivery. Am J Obstet Gynecol. 2018;218:341.e1-341.e9.
- Gimovsky AC, Berghella V. Randomized controlled trial of prolonged second stage: extending the time limit vs usual guidelines. Am J Obstet Gynecol. 2016;214:361.e1-361.e6.
- Grobman WA, Rice MM, Reddy UM, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Labor induction versus expectant management in low-risk nulliparous women. N Engl J Med. 2018;379:513-523.
EXPERT COMMENTARY
Main EK, Chang SC, Cape V, et al. Safety assessment of a large-scale improvement collaborative to reduce nulliparous cesarean delivery rates. Obstet Gynecol. 2019;133:613-623.
Cesarean delivery can be lifesaving for both mother and infant. When compared with successful vaginal delivery, however, CD is associated with higher maternal complication rates (including excessive blood loss requiring blood product transfusion, infectious morbidity, and venous thromboembolic events), longer hospital length of stay, and higher cost. While the optimal CD rate is not well defined, it is generally accepted that the CD rate in the United States is excessively high. As such, efforts to reduce the CD rate should be encouraged, but not at the expense of patient safety.
Details about the study
In keeping with the dictum that the most important CD to prevent is the first one, the California Maternal Quality Care Collaborative (CMQCC) in 2016 introduced a large-scale quality improvement project designed to reduce nulliparous, term, singleton, vertex (NTSV) CDs across the state. This bundle included education around joint guidelines issued by the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine on reducing primary CDs,1 introduction of a CMQCC toolkit, increased nursing labor support, and monthly meetings to share best practices across all collaborating sites. The NTSV CD rate in these hospitals did decrease from 29.3% in 2015 to 25.0% in 2017 (adjusted odds ratio, 0.76; 95% confidence interval, 0.73–0.78).
Whether or not implementation of the bundle resulted in an inappropriate delay in indicated CDs and, as such, in an increase in maternal or neonatal morbidity is not known. To address this issue, Main and colleagues collected cross-sectional data from more than 50 hospitals with more than 119,000 deliveries throughout California and measured rates of chorioamnionitis, blood transfusions, third- or fourth-degree perineal lacerations, operative vaginal delivery, severe unexpected newborn complications, and 5-minute Apgar scores of less than 5. None of the 6 safety measures showed any difference when comparing 2017 (after implementation of the CMQCC bundle) to 2015 (before implementation), suggesting that patient safety was not compromised significantly.
Study strengths and weaknesses
Strengths of this study include its large sample size and multicenter design with inclusion of a variety of collaborating hospitals. Earlier studies examining the effect of standardized protocols to reduce CD rates have been largely underpowered and conducted at single institutions.2-6 Moreover, results have been mixed, with some studies reporting an increase in maternal/neonatal adverse events,2-4 while others suggesting an improvement in select newborn quality outcome metrics.5 The current study provides reassurance to providers and institutions employing strategies to reduce NTSV CD rates that such efforts are safe.
Continue to: This study has several limitations...
This study has several limitations. Data collection relied on birth certificate and discharge diagnoses without a robust quality audit. As such, ascertainment bias, random error, and undercounting cannot be excluded. Although the population was heterogeneous, most women had more than a high school education and private insurance, and only 1 in 5 were obese. Whether these findings are generalizable to other areas within the United States is not known.
All reasonable efforts to decrease the CD rate in the United States should be encouraged, with particular attention paid to avoiding the first CD. However, this should not be done at the expense of patient safety. Large-scale quality improvement initiatives, similar to CMQCC efforts in California in 2016, appear to be one such strategy. Other successful strategies may include, for example, routine induction of labor for all low-risk nulliparous women at 39 weeks' gestation.7 The current report suggests that implementing a large-scale quality improvement initiative to reduce the primary CD rate can likely be done safely, without a significant increase in maternal or neonatal morbidity.
EXPERT COMMENTARY
Main EK, Chang SC, Cape V, et al. Safety assessment of a large-scale improvement collaborative to reduce nulliparous cesarean delivery rates. Obstet Gynecol. 2019;133:613-623.
Cesarean delivery can be lifesaving for both mother and infant. When compared with successful vaginal delivery, however, CD is associated with higher maternal complication rates (including excessive blood loss requiring blood product transfusion, infectious morbidity, and venous thromboembolic events), longer hospital length of stay, and higher cost. While the optimal CD rate is not well defined, it is generally accepted that the CD rate in the United States is excessively high. As such, efforts to reduce the CD rate should be encouraged, but not at the expense of patient safety.
Details about the study
In keeping with the dictum that the most important CD to prevent is the first one, the California Maternal Quality Care Collaborative (CMQCC) in 2016 introduced a large-scale quality improvement project designed to reduce nulliparous, term, singleton, vertex (NTSV) CDs across the state. This bundle included education around joint guidelines issued by the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine on reducing primary CDs,1 introduction of a CMQCC toolkit, increased nursing labor support, and monthly meetings to share best practices across all collaborating sites. The NTSV CD rate in these hospitals did decrease from 29.3% in 2015 to 25.0% in 2017 (adjusted odds ratio, 0.76; 95% confidence interval, 0.73–0.78).
Whether or not implementation of the bundle resulted in an inappropriate delay in indicated CDs and, as such, in an increase in maternal or neonatal morbidity is not known. To address this issue, Main and colleagues collected cross-sectional data from more than 50 hospitals with more than 119,000 deliveries throughout California and measured rates of chorioamnionitis, blood transfusions, third- or fourth-degree perineal lacerations, operative vaginal delivery, severe unexpected newborn complications, and 5-minute Apgar scores of less than 5. None of the 6 safety measures showed any difference when comparing 2017 (after implementation of the CMQCC bundle) to 2015 (before implementation), suggesting that patient safety was not compromised significantly.
Study strengths and weaknesses
Strengths of this study include its large sample size and multicenter design with inclusion of a variety of collaborating hospitals. Earlier studies examining the effect of standardized protocols to reduce CD rates have been largely underpowered and conducted at single institutions.2-6 Moreover, results have been mixed, with some studies reporting an increase in maternal/neonatal adverse events,2-4 while others suggesting an improvement in select newborn quality outcome metrics.5 The current study provides reassurance to providers and institutions employing strategies to reduce NTSV CD rates that such efforts are safe.
Continue to: This study has several limitations...
This study has several limitations. Data collection relied on birth certificate and discharge diagnoses without a robust quality audit. As such, ascertainment bias, random error, and undercounting cannot be excluded. Although the population was heterogeneous, most women had more than a high school education and private insurance, and only 1 in 5 were obese. Whether these findings are generalizable to other areas within the United States is not known.
All reasonable efforts to decrease the CD rate in the United States should be encouraged, with particular attention paid to avoiding the first CD. However, this should not be done at the expense of patient safety. Large-scale quality improvement initiatives, similar to CMQCC efforts in California in 2016, appear to be one such strategy. Other successful strategies may include, for example, routine induction of labor for all low-risk nulliparous women at 39 weeks' gestation.7 The current report suggests that implementing a large-scale quality improvement initiative to reduce the primary CD rate can likely be done safely, without a significant increase in maternal or neonatal morbidity.
- American College of Obstetricians and Gynecologists; Society for Maternal-Fetal Medicine. ACOG Obstetric care consensus no. 1: safe prevention of the primary cesarean delivery. Obstet Gynecol. 2014;123:693-711.
- Rosenbloom JI, Stout MJ, Tuuli MG, et al. New labor management guidelines and changes in cesarean delivery patterns. Am J Obstet Gynecol. 2017;217:689.e1-689.e8.
- Vadnais MA, Hacker MR, Shah NT, et al. Quality improvement initiatives lead to reduction in nulliparous term singleton vertex cesarean delivery rate. Jt Comm J Qual Patient Saf. 2017;43:53-61.
- Zipori Y, Grunwald O, Ginsberg Y, et al. The impact of extending the second stage of labor to prevent primary cesarean delivery on maternal and neonatal outcomes. Am J Obstet Gynecol. 2019; 220:191.e1-191.e7.
- Thuillier C, Roy S, Peyronnet V, et al. Impact of recommended changes in labor management for prevention of the primary cesarean delivery. Am J Obstet Gynecol. 2018;218:341.e1-341.e9.
- Gimovsky AC, Berghella V. Randomized controlled trial of prolonged second stage: extending the time limit vs usual guidelines. Am J Obstet Gynecol. 2016;214:361.e1-361.e6.
- Grobman WA, Rice MM, Reddy UM, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Labor induction versus expectant management in low-risk nulliparous women. N Engl J Med. 2018;379:513-523.
- American College of Obstetricians and Gynecologists; Society for Maternal-Fetal Medicine. ACOG Obstetric care consensus no. 1: safe prevention of the primary cesarean delivery. Obstet Gynecol. 2014;123:693-711.
- Rosenbloom JI, Stout MJ, Tuuli MG, et al. New labor management guidelines and changes in cesarean delivery patterns. Am J Obstet Gynecol. 2017;217:689.e1-689.e8.
- Vadnais MA, Hacker MR, Shah NT, et al. Quality improvement initiatives lead to reduction in nulliparous term singleton vertex cesarean delivery rate. Jt Comm J Qual Patient Saf. 2017;43:53-61.
- Zipori Y, Grunwald O, Ginsberg Y, et al. The impact of extending the second stage of labor to prevent primary cesarean delivery on maternal and neonatal outcomes. Am J Obstet Gynecol. 2019; 220:191.e1-191.e7.
- Thuillier C, Roy S, Peyronnet V, et al. Impact of recommended changes in labor management for prevention of the primary cesarean delivery. Am J Obstet Gynecol. 2018;218:341.e1-341.e9.
- Gimovsky AC, Berghella V. Randomized controlled trial of prolonged second stage: extending the time limit vs usual guidelines. Am J Obstet Gynecol. 2016;214:361.e1-361.e6.
- Grobman WA, Rice MM, Reddy UM, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Labor induction versus expectant management in low-risk nulliparous women. N Engl J Med. 2018;379:513-523.
The ARRIVE trial: Women’s desideratum versus logistical concerns
Of the 1.5 million nulliparous women who deliver annually in the United States, more than 50% are low-risk pregnancies. Among clinicians, there is a hesitancy to offer elective induction of labor to low-risk nulliparous women, mainly due to early observational studies that noted an association between elective induction of labor and higher rates of cesarean delivery (CD) and other adverse maternal and perinatal outcomes. 1-3 This reluctance over time has permeated throughout the ObGyn specialty and is culturally embedded in contemporary practice. The early observational studies lacked proper comparison groups because outcomes of women undergoing induction (elective and medically indicated) were compared to those in spontaneous labor. Since women who are being induced do not have the option to be in spontaneous labor, the appropriate comparator group for women undergoing elective induction is women who are being managed expectantly.
ARRIVE addresses appropriate comparator groups
Challenging this pervaded practice, in August 2018, Grobman and colleagues published the findings of the ARRIVE trial (A Randomized Trial of Induction Versus Expectant Management).4 This trial, conducted by Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal–Fetal Medicine Units Network, recruited participants from 41 geographically dispersed centers in the United States. Nulliparous women with low-risk pregnancies between 34 0/7 and 38 6/7 weeks were randomly assigned to either induction of labor at 39 0/7 to 39 4/7 weeks or to expectant management, which was defined as delaying induction until 40 5/7 to 42 2/7 weeks. The objective of the ARRIVE trial was to determine if, among low-risk nulliparous women, elective induction of labor at 39 weeks, compared with expectant management, would reduce the rate of adverse outcomes.
The primary outcome was a composite: perinatal death or severe neonatal complications (need for respiratory support within 72 hours of birth, Apgar score of ≤ 3 at 5 minutes, hypoxic-ischemic encephalopathy, seizures, infection [confirmed sepsis or pneumonia], meconium aspiration syndrome, birth trauma [bone fracture, neurologic injury, or retinal damage], intracranial or subgaleal hemorrhage, or hypotension requiring vasopressor support). The secondary outcomes included CD, hypertensive disorders of pregnancy, number of hours in the labor and delivery (L&D) unit, length of postpartum hospital stay, and assessment of satisfaction with labor process.
Mothers induced at 39 weeks fared better, while neonatal outcomes were similar. Of 22,533 eligible women, 6,106 (27%) were randomized: 3,062 were assigned to the induction group, and, 3,044 to the expectant management group. The primary composite outcome—perinatal death or severe neonatal complications—was similar in both groups (4.3% in the induction group vs 5.4% in the expectant management group).
However, women who were induced had significantly lower rates of:
- CD (18.6% with induction vs 22.2% for expectant management; relative risk [RR], 0.84; 95% confidence interval [CI], 0.76–0.93)
- hypertensive disorders of pregnancy (9.1% vs 14.1%; RR, 0.64; 95% CI, 0.56–0.74)
- neonatal respiratory support (3.0% vs. 4.2%; RR, 0.71; 95% CI, 0.55–0.93).
In addition, although women in the induction group had a longer stay in the L&D unit (an expected outcome), the overall postpartum length of stay was shorter. Finally, women in the induction group had higher patient satisfaction scores, with less pain and more control reported during labor.
Continue to: What about uncommon adverse outcomes compared at 39 vs 41 weeks?
What about uncommon adverse outcomes compared at 39 vs 41 weeks?
Due to the study’s sample size, ARRIVE investigators could not ascertain if uncommon adverse outcomes (maternal admission to intensive care unit or neonatal seizure) are significantly more common at 40 and 41 weeks, than at 39 weeks.
To address the issue of uncommon adverse outcomes, Chen and colleagues analyzed the US Vital Statistics datasets to compare composite maternal and neonatal morbidity among low-risk nulliparous women with nonanomalous singleton gestations who labored at 39 to 41 weeks.5 The primary outcome was composite neonatal morbidity that included Apgar score < 5 at 5 minutes, assisted ventilation longer than 6 hours, seizure, or neonatal mortality. The secondary outcome was composite maternal morbidity that included intensive care unit admission, blood transfusion, uterine rupture, or unplanned hysterectomy.
The investigators found that from 2011–2015, among 19.8 million live births in the United States, there were 3.3 million live births among low-risk nulliparous women. Among these women, 43% delivered at 39 weeks’ gestation, 41% at 40 weeks, and 15% at 41 weeks. The overall rate of composite neonatal morbidity was 8.8 per 1,000 live births; compared with those who delivered at 39 weeks, composite neonatal morbidity was significantly higher for those delivered at 40 (adjusted RR [aRR], 1.22; 95% CI, 1.19–1.25) and 41 weeks (aRR, 1.53; 95% CI, 1.49–1.58).
The secondary outcome, the overall rate of composite maternal morbidity, was 2.8 per 1,000 live births. As with composite neonatal morbidity, the risk of composite maternal morbidity was also significantly higher for those delivered at 40 (aRR, 1.19; 95% CI, 1.14–1.25) and 41 weeks’ gestation (aRR, 1.56; 95% CI, 1.47–1.65) than at 39 weeks.
Thus, among low-risk nulliparous pregnancies, there is an incremental increase in the rates of composite neonatal and maternal morbidity from 39 to 41 weeks.
Is induction of labor at 39 weeks feasible?
As the evidence demonstrating multiple benefits of 39-week inductions increases, concerns regarding the feasibility and cost of implementation in the current US health care system mount. A planned secondary analysis of the ARRIVE trial evaluated medical resource utilization among low-risk nulliparous women randomly assigned to elective induction at 39 weeks or expectant management.6 Resource utilization was compared between the 2 groups during the antepartum period, delivery admission, and from discharge to 8 weeks postpartum.
For the antepartum period, women in the induction group were significantly less likely than women undergoing expectant management to have at least 1: office visit for routine prenatal care (32.4% vs 68.4%), unanticipated office visit (0.5% vs 2.6%), urgent care/emergency department/triage visit (16.2% vs 44.3%), or hospital admission (0.8% vs 2.2%). When admitted for delivery, as expected, women in the induction group spent significantly more time on the L&D unit (14 hours vs 20 hours) and were more likely to receive interventions for induction (cervical ripening, oxytocin, intrauterine pressure catheter placement). However, they required magnesium sulfate and antibiotics significantly less frequently. For the postpartum group comparison, women in the induction group and their neonates had a significantly shorter duration of hospital stay.
In summary, the investigators found that, compared to women undergoing expectant management, women undergoing elective induction spent longer duration in L&D units and utilized more resources, but they required significantly fewer antepartum clinic and hospital visits, treatments for hypertensive disorders or chorioamnionitis, and had shorter duration of postpartum length of stay.
Continue to: Is induction of labor at 39 weeks cost-effective?
Is induction of labor at 39 weeks cost-effective?
Hersh and colleagues performed a cost-effectiveness analysis for induction of labor at 39 weeks versus expectant management for low-risk nulliparous women.7 Based on 2016 National Vital Statistics Data, there were 3.5 million term births in the United States. Following the exclusion of high-risk pregnancies and term parous low-risk pregnancies, a theoretical cohort of 1.6 million low-risk nulliparous women was included in the analysis. A decision-tree analytic model was created, in which the initial node stratified low-risk nulliparous women into 2 categories: elective induction at 39 weeks and expectant management. Probabilities of maternal and neonatal outcomes were derived from the literature.
Maternal outcomes included hypertensive disorders of pregnancy and delivery mode. Neonatal outcomes included macrosomia, shoulder dystocia, brachial plexus injury, stillbirth, and neonatal death. Costs of clinic and triage visits, induction of labor, modes of delivery, and maternal and neonatal outcomes were derived from previous studies and adjusted for inflation to 2018 dollars. Finally, quality-adjusted life years (QALYs) were calculated for mothers and neonates and were then used to estimate the incremental cost-effectiveness ratio (ICER) of elective induction of labor at 39 weeks. Following accepted standards, the threshold for cost-effectiveness was set at $100,000/QALYs or less.
Induction at 39 weeks comes in lower cost-wise than the standard threshold for QALY. In their analysis, the investigators found that if all 1.6 million women in their theoretical cohort underwent an elective induction of labor at 39 weeks (rather than expectant management), there would be 54,498 fewer CDs, 79,152 fewer cases of hypertensive disorders, 795 fewer cases of stillbirth, and 11 fewer neonatal deaths. Due to the decreased CD rates, the investigators did project an estimated 86 additional cases of neonatal brachial plexus injury. Using these estimates, costs, and utilities, the authors demonstrated that, compared with expectant management, elective induction of labor at 39 weeks was marginally cost-effective with an ICER of $87,692 per QALY, which was lower than the cost-effectiveness threshold of $100,000 per QALY.
Based on additional sensitivity analyses, the authors concluded that cost-effectiveness of elective induction of labor varied based on variations in model inputs. Specifically, the authors demonstrated that cost-effectiveness of induction of labor varied based on labor induction techniques, modes of delivery, and fluctuations in the rates of CD in induction versus expectant management groups.
Despite these theoretically imputed findings, the authors acknowledged the limitations of their study. Their cost-effectiveness model did not account for costs associated with long-term health impact of CD and hypertensive disease of pregnancy. Additionally, their model did not account for an increase in cost and resource utilization associated with increased time on L&D units to accommodate women undergoing induction. Furthermore, the analysis did not take into account the bundled payments for vaginal versus CDs, which are increasing in prevalence. Lastly, the analysis did not consider the incremental increase in severe neonatal and maternal morbidity from 39 to 41 weeks that Chen et al found in their study.5
Will ARRIVE finally arrive?
Cognizant of the medical and economic benefits of 39-week inductions, the Society for Maternal-Fetal Medicine and the American College of Obstetrics and Gynecology published a joint practice advisory recommending “shared decision-making” when counseling low-risk women about induction.8 While more research is needed to validate the aforementioned findings, particularly in regard to resource utilization, the ARRIVE trial and its associated analyses suggest that a reconsideration to deliver term low-risk nulliparous women at 39 weeks is warranted.
In summary, the overwhelming evidence suggests that, among low-risk nulliparous women there are maternal and neonatal benefits with delivery at 39 weeks, as compared with expectant management. Logistical concerns should not interfere with women’s desideratum for optimal outcomes.
- Vardo JH, Thornburg LL, Glantz JC. Maternal and neonatal morbidity among nulliparous women undergoing elective induction of labor. J Reprod Med. 2011;56:25-30.
- Dunne C, Da Silva O, Schmidt G, Natale R. Outcomes of elective labour induction and elective caesarean section in low-risk pregnancies between 37 and 41 weeks’ gestation. J Obstet Gynaecol Can . 2009;31:1124-1130.
- Guerra GV, Cecatti JG, Souza JP, et al; WHO Global Survey on Maternal Perinatal Health in Latin America Study Group. Elective induction versus spontaneous labour in Latin America. Bull World Health Organ. 2011;89:657-665.
- Grobman WA, Rice MM, Reddy UM, et al. Labor induction versus expectant management in low-risk nulliparous women. N Engl J Med. 2018;379:513-523.
- Chen HY, Grobman WA, Blackwell SC, et al. Women at 39-41 weeks of gestation among low-risk nulliparous women, several adverse outcomes—including neonatal mortality—are significantly more frequent with delivery at 40 or 41 weeks of gestation than at 39 weeks. Obstet Gynecol. 2019;133:729-737.
- Grobman WA, et al. Resource utilization among low-risk nulliparas randomized to elective induction at 39 weeks or expectant management. Oral presentation at: Society for Maternal-Fetal Medicine 39th Annual Pregnancy Meeting; February 11-16, 2019; Las Vegas, NV.
- Hersh AR, Skeith AE, Sargent JA, et al. Induction of labor at 39 weeks of gestation vs. expectant management for low-risk nulliparous women: a cost-effectiveness analysis. Am J Obstet Gynecol . February 12, 2019. doi: https://doi.org/10.1016/ j.ajog.2019.02.017.
- Society for Maternal-Fetal Medicine (SMFM) Publications Committee. SMFM statement on elective induction of labor in low-risk nulliparous women at term: the ARRIVE trial. Am J Obstet Gynecol. August 9, 2018. doi: 10.1016/j.ajog.2018.08.009.
Of the 1.5 million nulliparous women who deliver annually in the United States, more than 50% are low-risk pregnancies. Among clinicians, there is a hesitancy to offer elective induction of labor to low-risk nulliparous women, mainly due to early observational studies that noted an association between elective induction of labor and higher rates of cesarean delivery (CD) and other adverse maternal and perinatal outcomes. 1-3 This reluctance over time has permeated throughout the ObGyn specialty and is culturally embedded in contemporary practice. The early observational studies lacked proper comparison groups because outcomes of women undergoing induction (elective and medically indicated) were compared to those in spontaneous labor. Since women who are being induced do not have the option to be in spontaneous labor, the appropriate comparator group for women undergoing elective induction is women who are being managed expectantly.
ARRIVE addresses appropriate comparator groups
Challenging this pervaded practice, in August 2018, Grobman and colleagues published the findings of the ARRIVE trial (A Randomized Trial of Induction Versus Expectant Management).4 This trial, conducted by Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal–Fetal Medicine Units Network, recruited participants from 41 geographically dispersed centers in the United States. Nulliparous women with low-risk pregnancies between 34 0/7 and 38 6/7 weeks were randomly assigned to either induction of labor at 39 0/7 to 39 4/7 weeks or to expectant management, which was defined as delaying induction until 40 5/7 to 42 2/7 weeks. The objective of the ARRIVE trial was to determine if, among low-risk nulliparous women, elective induction of labor at 39 weeks, compared with expectant management, would reduce the rate of adverse outcomes.
The primary outcome was a composite: perinatal death or severe neonatal complications (need for respiratory support within 72 hours of birth, Apgar score of ≤ 3 at 5 minutes, hypoxic-ischemic encephalopathy, seizures, infection [confirmed sepsis or pneumonia], meconium aspiration syndrome, birth trauma [bone fracture, neurologic injury, or retinal damage], intracranial or subgaleal hemorrhage, or hypotension requiring vasopressor support). The secondary outcomes included CD, hypertensive disorders of pregnancy, number of hours in the labor and delivery (L&D) unit, length of postpartum hospital stay, and assessment of satisfaction with labor process.
Mothers induced at 39 weeks fared better, while neonatal outcomes were similar. Of 22,533 eligible women, 6,106 (27%) were randomized: 3,062 were assigned to the induction group, and, 3,044 to the expectant management group. The primary composite outcome—perinatal death or severe neonatal complications—was similar in both groups (4.3% in the induction group vs 5.4% in the expectant management group).
However, women who were induced had significantly lower rates of:
- CD (18.6% with induction vs 22.2% for expectant management; relative risk [RR], 0.84; 95% confidence interval [CI], 0.76–0.93)
- hypertensive disorders of pregnancy (9.1% vs 14.1%; RR, 0.64; 95% CI, 0.56–0.74)
- neonatal respiratory support (3.0% vs. 4.2%; RR, 0.71; 95% CI, 0.55–0.93).
In addition, although women in the induction group had a longer stay in the L&D unit (an expected outcome), the overall postpartum length of stay was shorter. Finally, women in the induction group had higher patient satisfaction scores, with less pain and more control reported during labor.
Continue to: What about uncommon adverse outcomes compared at 39 vs 41 weeks?
What about uncommon adverse outcomes compared at 39 vs 41 weeks?
Due to the study’s sample size, ARRIVE investigators could not ascertain if uncommon adverse outcomes (maternal admission to intensive care unit or neonatal seizure) are significantly more common at 40 and 41 weeks, than at 39 weeks.
To address the issue of uncommon adverse outcomes, Chen and colleagues analyzed the US Vital Statistics datasets to compare composite maternal and neonatal morbidity among low-risk nulliparous women with nonanomalous singleton gestations who labored at 39 to 41 weeks.5 The primary outcome was composite neonatal morbidity that included Apgar score < 5 at 5 minutes, assisted ventilation longer than 6 hours, seizure, or neonatal mortality. The secondary outcome was composite maternal morbidity that included intensive care unit admission, blood transfusion, uterine rupture, or unplanned hysterectomy.
The investigators found that from 2011–2015, among 19.8 million live births in the United States, there were 3.3 million live births among low-risk nulliparous women. Among these women, 43% delivered at 39 weeks’ gestation, 41% at 40 weeks, and 15% at 41 weeks. The overall rate of composite neonatal morbidity was 8.8 per 1,000 live births; compared with those who delivered at 39 weeks, composite neonatal morbidity was significantly higher for those delivered at 40 (adjusted RR [aRR], 1.22; 95% CI, 1.19–1.25) and 41 weeks (aRR, 1.53; 95% CI, 1.49–1.58).
The secondary outcome, the overall rate of composite maternal morbidity, was 2.8 per 1,000 live births. As with composite neonatal morbidity, the risk of composite maternal morbidity was also significantly higher for those delivered at 40 (aRR, 1.19; 95% CI, 1.14–1.25) and 41 weeks’ gestation (aRR, 1.56; 95% CI, 1.47–1.65) than at 39 weeks.
Thus, among low-risk nulliparous pregnancies, there is an incremental increase in the rates of composite neonatal and maternal morbidity from 39 to 41 weeks.
Is induction of labor at 39 weeks feasible?
As the evidence demonstrating multiple benefits of 39-week inductions increases, concerns regarding the feasibility and cost of implementation in the current US health care system mount. A planned secondary analysis of the ARRIVE trial evaluated medical resource utilization among low-risk nulliparous women randomly assigned to elective induction at 39 weeks or expectant management.6 Resource utilization was compared between the 2 groups during the antepartum period, delivery admission, and from discharge to 8 weeks postpartum.
For the antepartum period, women in the induction group were significantly less likely than women undergoing expectant management to have at least 1: office visit for routine prenatal care (32.4% vs 68.4%), unanticipated office visit (0.5% vs 2.6%), urgent care/emergency department/triage visit (16.2% vs 44.3%), or hospital admission (0.8% vs 2.2%). When admitted for delivery, as expected, women in the induction group spent significantly more time on the L&D unit (14 hours vs 20 hours) and were more likely to receive interventions for induction (cervical ripening, oxytocin, intrauterine pressure catheter placement). However, they required magnesium sulfate and antibiotics significantly less frequently. For the postpartum group comparison, women in the induction group and their neonates had a significantly shorter duration of hospital stay.
In summary, the investigators found that, compared to women undergoing expectant management, women undergoing elective induction spent longer duration in L&D units and utilized more resources, but they required significantly fewer antepartum clinic and hospital visits, treatments for hypertensive disorders or chorioamnionitis, and had shorter duration of postpartum length of stay.
Continue to: Is induction of labor at 39 weeks cost-effective?
Is induction of labor at 39 weeks cost-effective?
Hersh and colleagues performed a cost-effectiveness analysis for induction of labor at 39 weeks versus expectant management for low-risk nulliparous women.7 Based on 2016 National Vital Statistics Data, there were 3.5 million term births in the United States. Following the exclusion of high-risk pregnancies and term parous low-risk pregnancies, a theoretical cohort of 1.6 million low-risk nulliparous women was included in the analysis. A decision-tree analytic model was created, in which the initial node stratified low-risk nulliparous women into 2 categories: elective induction at 39 weeks and expectant management. Probabilities of maternal and neonatal outcomes were derived from the literature.
Maternal outcomes included hypertensive disorders of pregnancy and delivery mode. Neonatal outcomes included macrosomia, shoulder dystocia, brachial plexus injury, stillbirth, and neonatal death. Costs of clinic and triage visits, induction of labor, modes of delivery, and maternal and neonatal outcomes were derived from previous studies and adjusted for inflation to 2018 dollars. Finally, quality-adjusted life years (QALYs) were calculated for mothers and neonates and were then used to estimate the incremental cost-effectiveness ratio (ICER) of elective induction of labor at 39 weeks. Following accepted standards, the threshold for cost-effectiveness was set at $100,000/QALYs or less.
Induction at 39 weeks comes in lower cost-wise than the standard threshold for QALY. In their analysis, the investigators found that if all 1.6 million women in their theoretical cohort underwent an elective induction of labor at 39 weeks (rather than expectant management), there would be 54,498 fewer CDs, 79,152 fewer cases of hypertensive disorders, 795 fewer cases of stillbirth, and 11 fewer neonatal deaths. Due to the decreased CD rates, the investigators did project an estimated 86 additional cases of neonatal brachial plexus injury. Using these estimates, costs, and utilities, the authors demonstrated that, compared with expectant management, elective induction of labor at 39 weeks was marginally cost-effective with an ICER of $87,692 per QALY, which was lower than the cost-effectiveness threshold of $100,000 per QALY.
Based on additional sensitivity analyses, the authors concluded that cost-effectiveness of elective induction of labor varied based on variations in model inputs. Specifically, the authors demonstrated that cost-effectiveness of induction of labor varied based on labor induction techniques, modes of delivery, and fluctuations in the rates of CD in induction versus expectant management groups.
Despite these theoretically imputed findings, the authors acknowledged the limitations of their study. Their cost-effectiveness model did not account for costs associated with long-term health impact of CD and hypertensive disease of pregnancy. Additionally, their model did not account for an increase in cost and resource utilization associated with increased time on L&D units to accommodate women undergoing induction. Furthermore, the analysis did not take into account the bundled payments for vaginal versus CDs, which are increasing in prevalence. Lastly, the analysis did not consider the incremental increase in severe neonatal and maternal morbidity from 39 to 41 weeks that Chen et al found in their study.5
Will ARRIVE finally arrive?
Cognizant of the medical and economic benefits of 39-week inductions, the Society for Maternal-Fetal Medicine and the American College of Obstetrics and Gynecology published a joint practice advisory recommending “shared decision-making” when counseling low-risk women about induction.8 While more research is needed to validate the aforementioned findings, particularly in regard to resource utilization, the ARRIVE trial and its associated analyses suggest that a reconsideration to deliver term low-risk nulliparous women at 39 weeks is warranted.
In summary, the overwhelming evidence suggests that, among low-risk nulliparous women there are maternal and neonatal benefits with delivery at 39 weeks, as compared with expectant management. Logistical concerns should not interfere with women’s desideratum for optimal outcomes.
Of the 1.5 million nulliparous women who deliver annually in the United States, more than 50% are low-risk pregnancies. Among clinicians, there is a hesitancy to offer elective induction of labor to low-risk nulliparous women, mainly due to early observational studies that noted an association between elective induction of labor and higher rates of cesarean delivery (CD) and other adverse maternal and perinatal outcomes. 1-3 This reluctance over time has permeated throughout the ObGyn specialty and is culturally embedded in contemporary practice. The early observational studies lacked proper comparison groups because outcomes of women undergoing induction (elective and medically indicated) were compared to those in spontaneous labor. Since women who are being induced do not have the option to be in spontaneous labor, the appropriate comparator group for women undergoing elective induction is women who are being managed expectantly.
ARRIVE addresses appropriate comparator groups
Challenging this pervaded practice, in August 2018, Grobman and colleagues published the findings of the ARRIVE trial (A Randomized Trial of Induction Versus Expectant Management).4 This trial, conducted by Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal–Fetal Medicine Units Network, recruited participants from 41 geographically dispersed centers in the United States. Nulliparous women with low-risk pregnancies between 34 0/7 and 38 6/7 weeks were randomly assigned to either induction of labor at 39 0/7 to 39 4/7 weeks or to expectant management, which was defined as delaying induction until 40 5/7 to 42 2/7 weeks. The objective of the ARRIVE trial was to determine if, among low-risk nulliparous women, elective induction of labor at 39 weeks, compared with expectant management, would reduce the rate of adverse outcomes.
The primary outcome was a composite: perinatal death or severe neonatal complications (need for respiratory support within 72 hours of birth, Apgar score of ≤ 3 at 5 minutes, hypoxic-ischemic encephalopathy, seizures, infection [confirmed sepsis or pneumonia], meconium aspiration syndrome, birth trauma [bone fracture, neurologic injury, or retinal damage], intracranial or subgaleal hemorrhage, or hypotension requiring vasopressor support). The secondary outcomes included CD, hypertensive disorders of pregnancy, number of hours in the labor and delivery (L&D) unit, length of postpartum hospital stay, and assessment of satisfaction with labor process.
Mothers induced at 39 weeks fared better, while neonatal outcomes were similar. Of 22,533 eligible women, 6,106 (27%) were randomized: 3,062 were assigned to the induction group, and, 3,044 to the expectant management group. The primary composite outcome—perinatal death or severe neonatal complications—was similar in both groups (4.3% in the induction group vs 5.4% in the expectant management group).
However, women who were induced had significantly lower rates of:
- CD (18.6% with induction vs 22.2% for expectant management; relative risk [RR], 0.84; 95% confidence interval [CI], 0.76–0.93)
- hypertensive disorders of pregnancy (9.1% vs 14.1%; RR, 0.64; 95% CI, 0.56–0.74)
- neonatal respiratory support (3.0% vs. 4.2%; RR, 0.71; 95% CI, 0.55–0.93).
In addition, although women in the induction group had a longer stay in the L&D unit (an expected outcome), the overall postpartum length of stay was shorter. Finally, women in the induction group had higher patient satisfaction scores, with less pain and more control reported during labor.
Continue to: What about uncommon adverse outcomes compared at 39 vs 41 weeks?
What about uncommon adverse outcomes compared at 39 vs 41 weeks?
Due to the study’s sample size, ARRIVE investigators could not ascertain if uncommon adverse outcomes (maternal admission to intensive care unit or neonatal seizure) are significantly more common at 40 and 41 weeks, than at 39 weeks.
To address the issue of uncommon adverse outcomes, Chen and colleagues analyzed the US Vital Statistics datasets to compare composite maternal and neonatal morbidity among low-risk nulliparous women with nonanomalous singleton gestations who labored at 39 to 41 weeks.5 The primary outcome was composite neonatal morbidity that included Apgar score < 5 at 5 minutes, assisted ventilation longer than 6 hours, seizure, or neonatal mortality. The secondary outcome was composite maternal morbidity that included intensive care unit admission, blood transfusion, uterine rupture, or unplanned hysterectomy.
The investigators found that from 2011–2015, among 19.8 million live births in the United States, there were 3.3 million live births among low-risk nulliparous women. Among these women, 43% delivered at 39 weeks’ gestation, 41% at 40 weeks, and 15% at 41 weeks. The overall rate of composite neonatal morbidity was 8.8 per 1,000 live births; compared with those who delivered at 39 weeks, composite neonatal morbidity was significantly higher for those delivered at 40 (adjusted RR [aRR], 1.22; 95% CI, 1.19–1.25) and 41 weeks (aRR, 1.53; 95% CI, 1.49–1.58).
The secondary outcome, the overall rate of composite maternal morbidity, was 2.8 per 1,000 live births. As with composite neonatal morbidity, the risk of composite maternal morbidity was also significantly higher for those delivered at 40 (aRR, 1.19; 95% CI, 1.14–1.25) and 41 weeks’ gestation (aRR, 1.56; 95% CI, 1.47–1.65) than at 39 weeks.
Thus, among low-risk nulliparous pregnancies, there is an incremental increase in the rates of composite neonatal and maternal morbidity from 39 to 41 weeks.
Is induction of labor at 39 weeks feasible?
As the evidence demonstrating multiple benefits of 39-week inductions increases, concerns regarding the feasibility and cost of implementation in the current US health care system mount. A planned secondary analysis of the ARRIVE trial evaluated medical resource utilization among low-risk nulliparous women randomly assigned to elective induction at 39 weeks or expectant management.6 Resource utilization was compared between the 2 groups during the antepartum period, delivery admission, and from discharge to 8 weeks postpartum.
For the antepartum period, women in the induction group were significantly less likely than women undergoing expectant management to have at least 1: office visit for routine prenatal care (32.4% vs 68.4%), unanticipated office visit (0.5% vs 2.6%), urgent care/emergency department/triage visit (16.2% vs 44.3%), or hospital admission (0.8% vs 2.2%). When admitted for delivery, as expected, women in the induction group spent significantly more time on the L&D unit (14 hours vs 20 hours) and were more likely to receive interventions for induction (cervical ripening, oxytocin, intrauterine pressure catheter placement). However, they required magnesium sulfate and antibiotics significantly less frequently. For the postpartum group comparison, women in the induction group and their neonates had a significantly shorter duration of hospital stay.
In summary, the investigators found that, compared to women undergoing expectant management, women undergoing elective induction spent longer duration in L&D units and utilized more resources, but they required significantly fewer antepartum clinic and hospital visits, treatments for hypertensive disorders or chorioamnionitis, and had shorter duration of postpartum length of stay.
Continue to: Is induction of labor at 39 weeks cost-effective?
Is induction of labor at 39 weeks cost-effective?
Hersh and colleagues performed a cost-effectiveness analysis for induction of labor at 39 weeks versus expectant management for low-risk nulliparous women.7 Based on 2016 National Vital Statistics Data, there were 3.5 million term births in the United States. Following the exclusion of high-risk pregnancies and term parous low-risk pregnancies, a theoretical cohort of 1.6 million low-risk nulliparous women was included in the analysis. A decision-tree analytic model was created, in which the initial node stratified low-risk nulliparous women into 2 categories: elective induction at 39 weeks and expectant management. Probabilities of maternal and neonatal outcomes were derived from the literature.
Maternal outcomes included hypertensive disorders of pregnancy and delivery mode. Neonatal outcomes included macrosomia, shoulder dystocia, brachial plexus injury, stillbirth, and neonatal death. Costs of clinic and triage visits, induction of labor, modes of delivery, and maternal and neonatal outcomes were derived from previous studies and adjusted for inflation to 2018 dollars. Finally, quality-adjusted life years (QALYs) were calculated for mothers and neonates and were then used to estimate the incremental cost-effectiveness ratio (ICER) of elective induction of labor at 39 weeks. Following accepted standards, the threshold for cost-effectiveness was set at $100,000/QALYs or less.
Induction at 39 weeks comes in lower cost-wise than the standard threshold for QALY. In their analysis, the investigators found that if all 1.6 million women in their theoretical cohort underwent an elective induction of labor at 39 weeks (rather than expectant management), there would be 54,498 fewer CDs, 79,152 fewer cases of hypertensive disorders, 795 fewer cases of stillbirth, and 11 fewer neonatal deaths. Due to the decreased CD rates, the investigators did project an estimated 86 additional cases of neonatal brachial plexus injury. Using these estimates, costs, and utilities, the authors demonstrated that, compared with expectant management, elective induction of labor at 39 weeks was marginally cost-effective with an ICER of $87,692 per QALY, which was lower than the cost-effectiveness threshold of $100,000 per QALY.
Based on additional sensitivity analyses, the authors concluded that cost-effectiveness of elective induction of labor varied based on variations in model inputs. Specifically, the authors demonstrated that cost-effectiveness of induction of labor varied based on labor induction techniques, modes of delivery, and fluctuations in the rates of CD in induction versus expectant management groups.
Despite these theoretically imputed findings, the authors acknowledged the limitations of their study. Their cost-effectiveness model did not account for costs associated with long-term health impact of CD and hypertensive disease of pregnancy. Additionally, their model did not account for an increase in cost and resource utilization associated with increased time on L&D units to accommodate women undergoing induction. Furthermore, the analysis did not take into account the bundled payments for vaginal versus CDs, which are increasing in prevalence. Lastly, the analysis did not consider the incremental increase in severe neonatal and maternal morbidity from 39 to 41 weeks that Chen et al found in their study.5
Will ARRIVE finally arrive?
Cognizant of the medical and economic benefits of 39-week inductions, the Society for Maternal-Fetal Medicine and the American College of Obstetrics and Gynecology published a joint practice advisory recommending “shared decision-making” when counseling low-risk women about induction.8 While more research is needed to validate the aforementioned findings, particularly in regard to resource utilization, the ARRIVE trial and its associated analyses suggest that a reconsideration to deliver term low-risk nulliparous women at 39 weeks is warranted.
In summary, the overwhelming evidence suggests that, among low-risk nulliparous women there are maternal and neonatal benefits with delivery at 39 weeks, as compared with expectant management. Logistical concerns should not interfere with women’s desideratum for optimal outcomes.
- Vardo JH, Thornburg LL, Glantz JC. Maternal and neonatal morbidity among nulliparous women undergoing elective induction of labor. J Reprod Med. 2011;56:25-30.
- Dunne C, Da Silva O, Schmidt G, Natale R. Outcomes of elective labour induction and elective caesarean section in low-risk pregnancies between 37 and 41 weeks’ gestation. J Obstet Gynaecol Can . 2009;31:1124-1130.
- Guerra GV, Cecatti JG, Souza JP, et al; WHO Global Survey on Maternal Perinatal Health in Latin America Study Group. Elective induction versus spontaneous labour in Latin America. Bull World Health Organ. 2011;89:657-665.
- Grobman WA, Rice MM, Reddy UM, et al. Labor induction versus expectant management in low-risk nulliparous women. N Engl J Med. 2018;379:513-523.
- Chen HY, Grobman WA, Blackwell SC, et al. Women at 39-41 weeks of gestation among low-risk nulliparous women, several adverse outcomes—including neonatal mortality—are significantly more frequent with delivery at 40 or 41 weeks of gestation than at 39 weeks. Obstet Gynecol. 2019;133:729-737.
- Grobman WA, et al. Resource utilization among low-risk nulliparas randomized to elective induction at 39 weeks or expectant management. Oral presentation at: Society for Maternal-Fetal Medicine 39th Annual Pregnancy Meeting; February 11-16, 2019; Las Vegas, NV.
- Hersh AR, Skeith AE, Sargent JA, et al. Induction of labor at 39 weeks of gestation vs. expectant management for low-risk nulliparous women: a cost-effectiveness analysis. Am J Obstet Gynecol . February 12, 2019. doi: https://doi.org/10.1016/ j.ajog.2019.02.017.
- Society for Maternal-Fetal Medicine (SMFM) Publications Committee. SMFM statement on elective induction of labor in low-risk nulliparous women at term: the ARRIVE trial. Am J Obstet Gynecol. August 9, 2018. doi: 10.1016/j.ajog.2018.08.009.
- Vardo JH, Thornburg LL, Glantz JC. Maternal and neonatal morbidity among nulliparous women undergoing elective induction of labor. J Reprod Med. 2011;56:25-30.
- Dunne C, Da Silva O, Schmidt G, Natale R. Outcomes of elective labour induction and elective caesarean section in low-risk pregnancies between 37 and 41 weeks’ gestation. J Obstet Gynaecol Can . 2009;31:1124-1130.
- Guerra GV, Cecatti JG, Souza JP, et al; WHO Global Survey on Maternal Perinatal Health in Latin America Study Group. Elective induction versus spontaneous labour in Latin America. Bull World Health Organ. 2011;89:657-665.
- Grobman WA, Rice MM, Reddy UM, et al. Labor induction versus expectant management in low-risk nulliparous women. N Engl J Med. 2018;379:513-523.
- Chen HY, Grobman WA, Blackwell SC, et al. Women at 39-41 weeks of gestation among low-risk nulliparous women, several adverse outcomes—including neonatal mortality—are significantly more frequent with delivery at 40 or 41 weeks of gestation than at 39 weeks. Obstet Gynecol. 2019;133:729-737.
- Grobman WA, et al. Resource utilization among low-risk nulliparas randomized to elective induction at 39 weeks or expectant management. Oral presentation at: Society for Maternal-Fetal Medicine 39th Annual Pregnancy Meeting; February 11-16, 2019; Las Vegas, NV.
- Hersh AR, Skeith AE, Sargent JA, et al. Induction of labor at 39 weeks of gestation vs. expectant management for low-risk nulliparous women: a cost-effectiveness analysis. Am J Obstet Gynecol . February 12, 2019. doi: https://doi.org/10.1016/ j.ajog.2019.02.017.
- Society for Maternal-Fetal Medicine (SMFM) Publications Committee. SMFM statement on elective induction of labor in low-risk nulliparous women at term: the ARRIVE trial. Am J Obstet Gynecol. August 9, 2018. doi: 10.1016/j.ajog.2018.08.009.
Treating the pregnant patient with opioid addiction
OBG Management : How has the opioid crisis affected women in general?
Mishka Terplan, MD: Everyone is aware that we are experiencing a massive opioid crisis in the United States, and from a historical perspective, this is at least the third or fourth significant opioid epidemic in our nation’s history.1 It is similar in some ways to the very first one, which also featured a large proportion of women and also was driven initially by physician prescribing practices. However, the magnitude of this crisis is unparalleled compared with prior opioid epidemics.
There are lots of reasons why women are overrepresented in this crisis. There are gender-based differences in pain—chronic pain syndromes are more common in women. In addition, we have a gender bias in prescribing opioids and prescribe more opioids to women (especially older women) than to men. Cultural differences also contribute. As providers, we tend not to think of women as people who use drugs or people who develop addictions the same way as we think of these risks and behaviors for men. Therefore, compared with men, we are less likely to screen, assess, or refer women for substance use, misuse, and addiction. All of this adds up to creating a crisis in which women are increasingly the face of the epidemic.
OBG Management : What are the concerns about opioid addiction and pregnant women specifically?
Dr. Terplan: Addiction is a chronic condition, just like diabetes or depression, and the same principles that we think of in terms of optimizing maternal and newborn health apply to addiction. Ideally, we want, for women with chronic diseases to have stable disease at the time of conception and through pregnancy. We know this maximizes birth outcomes.
Unfortunately, there is a massive treatment gap in the United States. Most people with addiction receive no treatment. Only 11% of people with a substance use disorder report receipt of treatment. By contrast, more than 70% of people with depression, hypertension, or diabetes receive care. This treatment gap is also present in pregnancy. Among use disorders, treatment receipt is highest for opioid use disorder; however, nationally, at best, 25% of pregnant women with opioid addiction receive any care.
In other words, when we encounter addiction clinically, it is often untreated addiction. Therefore, many times providers will have women presenting to care who are both pregnant and have untreated addiction. From both a public health and a clinical practice perspective, the salient distinction is not between people with addiction and those without but between people with treated disease and people with untreated disease.
Untreated addiction is a serious medical condition. It is associated with preterm delivery and low birth weight infants. It is associated with acquisition and transmission of HIV and hepatitis C. It is associated with overdose and overdose death. By contrast, treated addiction is associated with term delivery and normal weight infants. Pharmacotherapies for opioid use disorder stabilize the intrauterine environment and allow for normal fetal growth. Pharmacotherapies for opioid use disorder help to structure and stabilize the mom’s social circumstance, providing a platform to deliver prenatal care and essential social services. And pharmacotherapies for opioid use disorder protect women and their fetuses from overdose and from overdose deaths. The goal of management of addiction in pregnancy is treatment of the underlying condition, treating the addiction.
Continue to: OBG Management...
OBG Management : What should the ObGyn do when faced with a patient who might have an addiction?
Dr. Terplan: The good news is that there are lots of recently published guidance documents from the World Health Organization,2 the American College of Obstetricians and Gynecologists (ACOG),3 and the Substance Abuse and Mental Health Services Administration (SAMHSA),4 and there have been a whole series of trainings throughout the United States organized by both ACOG and SAMHSA.
There is also a collaboration between ACOG and the American Society of Addiction Medicine (ASAM) to provide buprenorphine waiver trainings specifically designed for ObGyns. Check both the ACOG and ASAM pages for details. I encourage every provider to get a waiver to prescribe buprenorphine. There are about 30 ObGyns who are also board certified in addiction medicine in the United States, and all of us are more than happy to help our colleagues in the clinical care of this population, a population that all of us really enjoy taking care of.
Although care in pregnancy is important, we must not forget about the postpartum period. Generally speaking, women do quite well during pregnancy in terms of treatment. Postpartum, however, is a vulnerable period, where relapse happens, where gaps in care happen, where child welfare involvement and sometimes child removal happens, which can be very stressful for anyone much less somebody with a substance use disorder. Recent data demonstrate that one of the leading causes of maternal mortality in the US in from overdose, and most of these deaths occur in the postpartum period.5 Regardless of what happens during pregnancy, it is essential that we be able to link and continue care for women with opioid use disorder throughout the postpartum period.
OBG Management : How do you treat opioid use disorder in pregnancy?
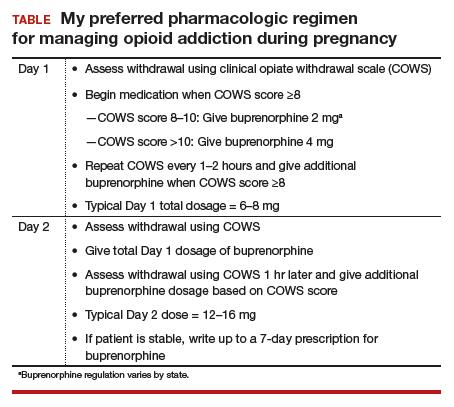
Dr. Terplan: The standard of care for treatment of opioid use disorder in pregnancy is pharmacotherapy with either methadone or buprenorphine (TABLE) plus behavioral counseling—ideally, co-located with prenatal care. The evidence base for pharmacotherapy for opioid use disorder in pregnancy is supported by every single professional society that has ever issued guidance on this, from the World Health Organization to ACOG, to ASAM, to the Royal College in the UK as well as Canadian and Australian obstetrics and gynecology societies; literally every single professional society supports medication.
The core principle of maternal fetal medicine rests upon the fact that chronic conditions need to be treated and that treated illness improves birth outcomes. For both maternal and fetal health, treated addiction is way better than untreated addiction. One concern people have regarding methadone and buprenorphine is the development of dependence. Dependence is a physiologic effect of medication and occurs with opioids, as well as with many other medications, such as antidepressants and most hypertensive agents. For the fetus, dependence means that at the time of delivery, the infant may go into withdrawal, which is also called neonatal abstinence syndrome. Neonatal abstinence syndrome is an expected outcome of in-utero opioid exposure. It is a time-limited and treatable condition. Prospective data do not demonstrate any long-term harms among infants whose mothers received pharmacotherapy for opioid use disorder during pregnancy.6
The treatment for neonatal abstinence syndrome is costly, especially when in a neonatal intensive care unit. It can be quite concerning to a new mother to have an infant that has to spend extra time in the hospital and sometimes be medicated for management of withdrawal.
There has been a renewed interest amongst ObGyns in investigating medically-supervised withdrawal during pregnancy. Although there are remaining questions, overall, the literature does not support withdrawal during pregnancy—mostly because withdrawal is associated with relapse, and relapse is associated with cessation of care (both prenatal care and addiction treatment), acquisition and transmission of HIV and Hepatitis C, and overdose and overdose death. The pertinent clinical and public health goal is the treatment of the chronic condition of addiction during pregnancy. The standard of care remains pharmacotherapy plus behavioral counseling for the treatment of opioid use disorder in pregnancy.
Clinical care, however, is both evidence-based and person-centered. All of us who have worked in this field, long before there was attention to the opioid crisis, all of us have provided medically-supervised withdrawal of a pregnant person, and that is because we understand the principles of care. When evidence-based care conflicts with person-centered care, the ethical course is the provision of person-centered care. Patients have the right of refusal. If someone wants to discontinue medication, I have tapered the medication during pregnancy, but continued to provide (and often increase) behavioral counseling and prenatal care.
Treated addiction is better for the fetus than untreated addiction. Untreated opioid addiction is associated with preterm birth and low birth weight. These obstetric risks are not because of the opioid per se, but because of the repeated cycles of withdrawal that an individual with untreated addiction experiences. People with untreated addiction are not getting “high” when they use, they are just becoming a little bit less sick. It is this repeated cycle of withdrawal that stresses the fetus, which leads to preterm delivery and low birth weight.
Medications for opioid use disorder are long-acting and dosed daily. In contrast to the repeated cycles of fetal withdrawal in untreated addiction, pharmacotherapy stabilizes the intrauterine environment. There is no cyclic, repeated, stressful withdrawal, and consequentially, the fetus grows normally and delivers at term. Obstetric risk is from repeated cyclic withdrawal more than from opioid exposure itself.
Continue to: OBG Management...
OBG Management : Research reports that women are not using all of the opioids that are prescribed to them after a cesarean delivery. What are the risks for addiction in this setting?
Dr. Terplan: I mark a distinction between use (ie, using something as prescribed) and misuse, which means using a prescribed medication not in the manner in which it was prescribed, or using somebody else’s medications, or using an illicit substance. And I differentiate use and misuse from addiction, which is a behavioral condition, a disease. There has been a lot of attention paid to opioid prescribing in general and in particular postdelivery and post–cesarean delivery, which is one of the most common operative procedures in the United States.
It seems clear from the literature that we have overprescribed opioids postdelivery, and a small number of women, about 1 in 300 will continue an opioid script.7 This means that 1 in 300 women who received an opioid prescription following delivery present for care and get another opioid prescription filled. Now, that is a small number at the level of the individual, but because we do so many cesarean deliveries, this is a large number of women at the level of the population. This does not mean, however, that 1 in 300 women who received opioids after cesarean delivery are going to become addicted to them. It just means that 1 in 300 will continue the prescription. Prescription continuation is a risk factor for opioid misuse, and opioid misuse is on the pathway toward addiction.
Most people who use substances do not develop an addiction to that substance. We know from the opioid literature that at most only 10% of people who receive chronic opioid therapy will meet criteria for opioid use disorder.8 Now 10% is not 100%, nor is it 0%, but because we prescribed so many opioids to so many people for so long, the absolute number of people with opioid use disorder from physician opioid prescribing is large, even though the risk at the level of the individual is not as large as people think.
OBG Management : From your experience in treating addiction during pregnancy, are there clinical pearls you would like to share with ObGyns?
Dr. Terplan: There are a couple of takeaways. One is that all women are motivated to maximize their health and that of their baby to be, and every pregnant woman engages in behavioral change; in fact most women quit or cutback substance use during pregnancy. But some can’t. Those that can’t likely have a substance use disorder. We think of addiction as a chronic condition, centered in the brain, but the primary symptoms of addiction are behaviors. The salient feature of addiction is continued use despite adverse consequences; using something that you know is harming yourself and others but you can’t stop using it. In other words, continuing substance use during pregnancy. When we see clinically a pregnant woman who is using a substance, 99% of the time we are seeing a pregnant woman who has the condition of addiction, and what she needs is treatment. She does not need to be told that injecting heroin is unsafe for her and her fetus, she knows that. What she needs is treatment.
The second point is that pregnant women who use drugs and pregnant women with addiction experience a real specific and strong form of discrimination by providers, by other people with addiction, by the legal system, and by their friends and families. Caring for people who have substance use disorder is grounded in human rights, which means treating people with dignity and respect. It is important for providers to have empathy, especially for pregnant people who use drugs, to counter the discrimination they experience from society and from other health care providers.
Continue to: OBG Management...
OBG Management : Are there specific ways in which ObGyns can show empathy when speaking with a pregnant woman who likely has addiction?
Dr. Terplan: In general when we talk to people about drug use, it is important to ask their permission to talk about it. For example, “Is it okay if I ask you some questions about smoking, drinking, and other drugs?” If someone says, “No, I don’t want you to ask those questions,” we have to respect that. Assessment of substance use should be a universal part of all medical care, as substance use, misuse, and addiction are essential domains of wellness, but I think we should ask permission before screening.
One of the really good things about prenatal care is that people come back; we have multiple visits across the gestational period. The behavioral work of addiction treatment rests upon a strong therapeutic alliance. If you do not respect your patient, then there is no way you can achieve a therapeutic alliance. Asking permission, and then respecting somebody’s answers, I think goes a really long way to establishing a strong therapeutic alliance, which is the basis of any medical care.
- Terplan M. Women and the opioid crisis: historical context and public health solutions. Fertil Steril. 2017;108:195-199.
- Management of substance abuse. World Health Organization website. https://www.who.int/substance_abuse/activities/treatment_opioid_dependence/en/. Accessed March 20, 2019.
- Committee on Obstetric Practice. Committee Opinion No. 711: Opioid use and opioid use disorder in pregnancy. Obstet Gynecol. 2017;130(2):e81-e94.
- Substance Abuse and Mental Health Services Administration. Clinical guidance for treating pregnant and parenting women with opioid use disorder and their infants. HHS Publication No. (SMA) 18-5054. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2018.
- Metz TD, Royner P, Hoffman MC, et al. Maternal deaths from suicide and overdose in Colorado, 2004-2012. Obstet Gynecol. 2016;128:1233-1240.
- Kaltenbach K, O’Grady E, Heil SH, et al. Prenatal exposure to methadone or buprenorphine: early childhood developmental outcomes. Drug Alcohol Depend. 2018;185:40-49.
- Bateman BT, Franklin JM, Bykov K, et al. Persistent opioid use following cesarean delivery: patterns and predictors among opioid-naive women. Am J Obstet Gynecol. 2016;215:353.e1-353.e18.
OBG Management : How has the opioid crisis affected women in general?
Mishka Terplan, MD: Everyone is aware that we are experiencing a massive opioid crisis in the United States, and from a historical perspective, this is at least the third or fourth significant opioid epidemic in our nation’s history.1 It is similar in some ways to the very first one, which also featured a large proportion of women and also was driven initially by physician prescribing practices. However, the magnitude of this crisis is unparalleled compared with prior opioid epidemics.
There are lots of reasons why women are overrepresented in this crisis. There are gender-based differences in pain—chronic pain syndromes are more common in women. In addition, we have a gender bias in prescribing opioids and prescribe more opioids to women (especially older women) than to men. Cultural differences also contribute. As providers, we tend not to think of women as people who use drugs or people who develop addictions the same way as we think of these risks and behaviors for men. Therefore, compared with men, we are less likely to screen, assess, or refer women for substance use, misuse, and addiction. All of this adds up to creating a crisis in which women are increasingly the face of the epidemic.
OBG Management : What are the concerns about opioid addiction and pregnant women specifically?
Dr. Terplan: Addiction is a chronic condition, just like diabetes or depression, and the same principles that we think of in terms of optimizing maternal and newborn health apply to addiction. Ideally, we want, for women with chronic diseases to have stable disease at the time of conception and through pregnancy. We know this maximizes birth outcomes.
Unfortunately, there is a massive treatment gap in the United States. Most people with addiction receive no treatment. Only 11% of people with a substance use disorder report receipt of treatment. By contrast, more than 70% of people with depression, hypertension, or diabetes receive care. This treatment gap is also present in pregnancy. Among use disorders, treatment receipt is highest for opioid use disorder; however, nationally, at best, 25% of pregnant women with opioid addiction receive any care.
In other words, when we encounter addiction clinically, it is often untreated addiction. Therefore, many times providers will have women presenting to care who are both pregnant and have untreated addiction. From both a public health and a clinical practice perspective, the salient distinction is not between people with addiction and those without but between people with treated disease and people with untreated disease.
Untreated addiction is a serious medical condition. It is associated with preterm delivery and low birth weight infants. It is associated with acquisition and transmission of HIV and hepatitis C. It is associated with overdose and overdose death. By contrast, treated addiction is associated with term delivery and normal weight infants. Pharmacotherapies for opioid use disorder stabilize the intrauterine environment and allow for normal fetal growth. Pharmacotherapies for opioid use disorder help to structure and stabilize the mom’s social circumstance, providing a platform to deliver prenatal care and essential social services. And pharmacotherapies for opioid use disorder protect women and their fetuses from overdose and from overdose deaths. The goal of management of addiction in pregnancy is treatment of the underlying condition, treating the addiction.
Continue to: OBG Management...
OBG Management : What should the ObGyn do when faced with a patient who might have an addiction?
Dr. Terplan: The good news is that there are lots of recently published guidance documents from the World Health Organization,2 the American College of Obstetricians and Gynecologists (ACOG),3 and the Substance Abuse and Mental Health Services Administration (SAMHSA),4 and there have been a whole series of trainings throughout the United States organized by both ACOG and SAMHSA.
There is also a collaboration between ACOG and the American Society of Addiction Medicine (ASAM) to provide buprenorphine waiver trainings specifically designed for ObGyns. Check both the ACOG and ASAM pages for details. I encourage every provider to get a waiver to prescribe buprenorphine. There are about 30 ObGyns who are also board certified in addiction medicine in the United States, and all of us are more than happy to help our colleagues in the clinical care of this population, a population that all of us really enjoy taking care of.
Although care in pregnancy is important, we must not forget about the postpartum period. Generally speaking, women do quite well during pregnancy in terms of treatment. Postpartum, however, is a vulnerable period, where relapse happens, where gaps in care happen, where child welfare involvement and sometimes child removal happens, which can be very stressful for anyone much less somebody with a substance use disorder. Recent data demonstrate that one of the leading causes of maternal mortality in the US in from overdose, and most of these deaths occur in the postpartum period.5 Regardless of what happens during pregnancy, it is essential that we be able to link and continue care for women with opioid use disorder throughout the postpartum period.
OBG Management : How do you treat opioid use disorder in pregnancy?
Dr. Terplan: The standard of care for treatment of opioid use disorder in pregnancy is pharmacotherapy with either methadone or buprenorphine (TABLE) plus behavioral counseling—ideally, co-located with prenatal care. The evidence base for pharmacotherapy for opioid use disorder in pregnancy is supported by every single professional society that has ever issued guidance on this, from the World Health Organization to ACOG, to ASAM, to the Royal College in the UK as well as Canadian and Australian obstetrics and gynecology societies; literally every single professional society supports medication.
The core principle of maternal fetal medicine rests upon the fact that chronic conditions need to be treated and that treated illness improves birth outcomes. For both maternal and fetal health, treated addiction is way better than untreated addiction. One concern people have regarding methadone and buprenorphine is the development of dependence. Dependence is a physiologic effect of medication and occurs with opioids, as well as with many other medications, such as antidepressants and most hypertensive agents. For the fetus, dependence means that at the time of delivery, the infant may go into withdrawal, which is also called neonatal abstinence syndrome. Neonatal abstinence syndrome is an expected outcome of in-utero opioid exposure. It is a time-limited and treatable condition. Prospective data do not demonstrate any long-term harms among infants whose mothers received pharmacotherapy for opioid use disorder during pregnancy.6
The treatment for neonatal abstinence syndrome is costly, especially when in a neonatal intensive care unit. It can be quite concerning to a new mother to have an infant that has to spend extra time in the hospital and sometimes be medicated for management of withdrawal.
There has been a renewed interest amongst ObGyns in investigating medically-supervised withdrawal during pregnancy. Although there are remaining questions, overall, the literature does not support withdrawal during pregnancy—mostly because withdrawal is associated with relapse, and relapse is associated with cessation of care (both prenatal care and addiction treatment), acquisition and transmission of HIV and Hepatitis C, and overdose and overdose death. The pertinent clinical and public health goal is the treatment of the chronic condition of addiction during pregnancy. The standard of care remains pharmacotherapy plus behavioral counseling for the treatment of opioid use disorder in pregnancy.
Clinical care, however, is both evidence-based and person-centered. All of us who have worked in this field, long before there was attention to the opioid crisis, all of us have provided medically-supervised withdrawal of a pregnant person, and that is because we understand the principles of care. When evidence-based care conflicts with person-centered care, the ethical course is the provision of person-centered care. Patients have the right of refusal. If someone wants to discontinue medication, I have tapered the medication during pregnancy, but continued to provide (and often increase) behavioral counseling and prenatal care.
Treated addiction is better for the fetus than untreated addiction. Untreated opioid addiction is associated with preterm birth and low birth weight. These obstetric risks are not because of the opioid per se, but because of the repeated cycles of withdrawal that an individual with untreated addiction experiences. People with untreated addiction are not getting “high” when they use, they are just becoming a little bit less sick. It is this repeated cycle of withdrawal that stresses the fetus, which leads to preterm delivery and low birth weight.
Medications for opioid use disorder are long-acting and dosed daily. In contrast to the repeated cycles of fetal withdrawal in untreated addiction, pharmacotherapy stabilizes the intrauterine environment. There is no cyclic, repeated, stressful withdrawal, and consequentially, the fetus grows normally and delivers at term. Obstetric risk is from repeated cyclic withdrawal more than from opioid exposure itself.
Continue to: OBG Management...
OBG Management : Research reports that women are not using all of the opioids that are prescribed to them after a cesarean delivery. What are the risks for addiction in this setting?
Dr. Terplan: I mark a distinction between use (ie, using something as prescribed) and misuse, which means using a prescribed medication not in the manner in which it was prescribed, or using somebody else’s medications, or using an illicit substance. And I differentiate use and misuse from addiction, which is a behavioral condition, a disease. There has been a lot of attention paid to opioid prescribing in general and in particular postdelivery and post–cesarean delivery, which is one of the most common operative procedures in the United States.
It seems clear from the literature that we have overprescribed opioids postdelivery, and a small number of women, about 1 in 300 will continue an opioid script.7 This means that 1 in 300 women who received an opioid prescription following delivery present for care and get another opioid prescription filled. Now, that is a small number at the level of the individual, but because we do so many cesarean deliveries, this is a large number of women at the level of the population. This does not mean, however, that 1 in 300 women who received opioids after cesarean delivery are going to become addicted to them. It just means that 1 in 300 will continue the prescription. Prescription continuation is a risk factor for opioid misuse, and opioid misuse is on the pathway toward addiction.
Most people who use substances do not develop an addiction to that substance. We know from the opioid literature that at most only 10% of people who receive chronic opioid therapy will meet criteria for opioid use disorder.8 Now 10% is not 100%, nor is it 0%, but because we prescribed so many opioids to so many people for so long, the absolute number of people with opioid use disorder from physician opioid prescribing is large, even though the risk at the level of the individual is not as large as people think.
OBG Management : From your experience in treating addiction during pregnancy, are there clinical pearls you would like to share with ObGyns?
Dr. Terplan: There are a couple of takeaways. One is that all women are motivated to maximize their health and that of their baby to be, and every pregnant woman engages in behavioral change; in fact most women quit or cutback substance use during pregnancy. But some can’t. Those that can’t likely have a substance use disorder. We think of addiction as a chronic condition, centered in the brain, but the primary symptoms of addiction are behaviors. The salient feature of addiction is continued use despite adverse consequences; using something that you know is harming yourself and others but you can’t stop using it. In other words, continuing substance use during pregnancy. When we see clinically a pregnant woman who is using a substance, 99% of the time we are seeing a pregnant woman who has the condition of addiction, and what she needs is treatment. She does not need to be told that injecting heroin is unsafe for her and her fetus, she knows that. What she needs is treatment.
The second point is that pregnant women who use drugs and pregnant women with addiction experience a real specific and strong form of discrimination by providers, by other people with addiction, by the legal system, and by their friends and families. Caring for people who have substance use disorder is grounded in human rights, which means treating people with dignity and respect. It is important for providers to have empathy, especially for pregnant people who use drugs, to counter the discrimination they experience from society and from other health care providers.
Continue to: OBG Management...
OBG Management : Are there specific ways in which ObGyns can show empathy when speaking with a pregnant woman who likely has addiction?
Dr. Terplan: In general when we talk to people about drug use, it is important to ask their permission to talk about it. For example, “Is it okay if I ask you some questions about smoking, drinking, and other drugs?” If someone says, “No, I don’t want you to ask those questions,” we have to respect that. Assessment of substance use should be a universal part of all medical care, as substance use, misuse, and addiction are essential domains of wellness, but I think we should ask permission before screening.
One of the really good things about prenatal care is that people come back; we have multiple visits across the gestational period. The behavioral work of addiction treatment rests upon a strong therapeutic alliance. If you do not respect your patient, then there is no way you can achieve a therapeutic alliance. Asking permission, and then respecting somebody’s answers, I think goes a really long way to establishing a strong therapeutic alliance, which is the basis of any medical care.
OBG Management : How has the opioid crisis affected women in general?
Mishka Terplan, MD: Everyone is aware that we are experiencing a massive opioid crisis in the United States, and from a historical perspective, this is at least the third or fourth significant opioid epidemic in our nation’s history.1 It is similar in some ways to the very first one, which also featured a large proportion of women and also was driven initially by physician prescribing practices. However, the magnitude of this crisis is unparalleled compared with prior opioid epidemics.
There are lots of reasons why women are overrepresented in this crisis. There are gender-based differences in pain—chronic pain syndromes are more common in women. In addition, we have a gender bias in prescribing opioids and prescribe more opioids to women (especially older women) than to men. Cultural differences also contribute. As providers, we tend not to think of women as people who use drugs or people who develop addictions the same way as we think of these risks and behaviors for men. Therefore, compared with men, we are less likely to screen, assess, or refer women for substance use, misuse, and addiction. All of this adds up to creating a crisis in which women are increasingly the face of the epidemic.
OBG Management : What are the concerns about opioid addiction and pregnant women specifically?
Dr. Terplan: Addiction is a chronic condition, just like diabetes or depression, and the same principles that we think of in terms of optimizing maternal and newborn health apply to addiction. Ideally, we want, for women with chronic diseases to have stable disease at the time of conception and through pregnancy. We know this maximizes birth outcomes.
Unfortunately, there is a massive treatment gap in the United States. Most people with addiction receive no treatment. Only 11% of people with a substance use disorder report receipt of treatment. By contrast, more than 70% of people with depression, hypertension, or diabetes receive care. This treatment gap is also present in pregnancy. Among use disorders, treatment receipt is highest for opioid use disorder; however, nationally, at best, 25% of pregnant women with opioid addiction receive any care.
In other words, when we encounter addiction clinically, it is often untreated addiction. Therefore, many times providers will have women presenting to care who are both pregnant and have untreated addiction. From both a public health and a clinical practice perspective, the salient distinction is not between people with addiction and those without but between people with treated disease and people with untreated disease.
Untreated addiction is a serious medical condition. It is associated with preterm delivery and low birth weight infants. It is associated with acquisition and transmission of HIV and hepatitis C. It is associated with overdose and overdose death. By contrast, treated addiction is associated with term delivery and normal weight infants. Pharmacotherapies for opioid use disorder stabilize the intrauterine environment and allow for normal fetal growth. Pharmacotherapies for opioid use disorder help to structure and stabilize the mom’s social circumstance, providing a platform to deliver prenatal care and essential social services. And pharmacotherapies for opioid use disorder protect women and their fetuses from overdose and from overdose deaths. The goal of management of addiction in pregnancy is treatment of the underlying condition, treating the addiction.
Continue to: OBG Management...
OBG Management : What should the ObGyn do when faced with a patient who might have an addiction?
Dr. Terplan: The good news is that there are lots of recently published guidance documents from the World Health Organization,2 the American College of Obstetricians and Gynecologists (ACOG),3 and the Substance Abuse and Mental Health Services Administration (SAMHSA),4 and there have been a whole series of trainings throughout the United States organized by both ACOG and SAMHSA.
There is also a collaboration between ACOG and the American Society of Addiction Medicine (ASAM) to provide buprenorphine waiver trainings specifically designed for ObGyns. Check both the ACOG and ASAM pages for details. I encourage every provider to get a waiver to prescribe buprenorphine. There are about 30 ObGyns who are also board certified in addiction medicine in the United States, and all of us are more than happy to help our colleagues in the clinical care of this population, a population that all of us really enjoy taking care of.
Although care in pregnancy is important, we must not forget about the postpartum period. Generally speaking, women do quite well during pregnancy in terms of treatment. Postpartum, however, is a vulnerable period, where relapse happens, where gaps in care happen, where child welfare involvement and sometimes child removal happens, which can be very stressful for anyone much less somebody with a substance use disorder. Recent data demonstrate that one of the leading causes of maternal mortality in the US in from overdose, and most of these deaths occur in the postpartum period.5 Regardless of what happens during pregnancy, it is essential that we be able to link and continue care for women with opioid use disorder throughout the postpartum period.
OBG Management : How do you treat opioid use disorder in pregnancy?
Dr. Terplan: The standard of care for treatment of opioid use disorder in pregnancy is pharmacotherapy with either methadone or buprenorphine (TABLE) plus behavioral counseling—ideally, co-located with prenatal care. The evidence base for pharmacotherapy for opioid use disorder in pregnancy is supported by every single professional society that has ever issued guidance on this, from the World Health Organization to ACOG, to ASAM, to the Royal College in the UK as well as Canadian and Australian obstetrics and gynecology societies; literally every single professional society supports medication.
The core principle of maternal fetal medicine rests upon the fact that chronic conditions need to be treated and that treated illness improves birth outcomes. For both maternal and fetal health, treated addiction is way better than untreated addiction. One concern people have regarding methadone and buprenorphine is the development of dependence. Dependence is a physiologic effect of medication and occurs with opioids, as well as with many other medications, such as antidepressants and most hypertensive agents. For the fetus, dependence means that at the time of delivery, the infant may go into withdrawal, which is also called neonatal abstinence syndrome. Neonatal abstinence syndrome is an expected outcome of in-utero opioid exposure. It is a time-limited and treatable condition. Prospective data do not demonstrate any long-term harms among infants whose mothers received pharmacotherapy for opioid use disorder during pregnancy.6
The treatment for neonatal abstinence syndrome is costly, especially when in a neonatal intensive care unit. It can be quite concerning to a new mother to have an infant that has to spend extra time in the hospital and sometimes be medicated for management of withdrawal.
There has been a renewed interest amongst ObGyns in investigating medically-supervised withdrawal during pregnancy. Although there are remaining questions, overall, the literature does not support withdrawal during pregnancy—mostly because withdrawal is associated with relapse, and relapse is associated with cessation of care (both prenatal care and addiction treatment), acquisition and transmission of HIV and Hepatitis C, and overdose and overdose death. The pertinent clinical and public health goal is the treatment of the chronic condition of addiction during pregnancy. The standard of care remains pharmacotherapy plus behavioral counseling for the treatment of opioid use disorder in pregnancy.
Clinical care, however, is both evidence-based and person-centered. All of us who have worked in this field, long before there was attention to the opioid crisis, all of us have provided medically-supervised withdrawal of a pregnant person, and that is because we understand the principles of care. When evidence-based care conflicts with person-centered care, the ethical course is the provision of person-centered care. Patients have the right of refusal. If someone wants to discontinue medication, I have tapered the medication during pregnancy, but continued to provide (and often increase) behavioral counseling and prenatal care.
Treated addiction is better for the fetus than untreated addiction. Untreated opioid addiction is associated with preterm birth and low birth weight. These obstetric risks are not because of the opioid per se, but because of the repeated cycles of withdrawal that an individual with untreated addiction experiences. People with untreated addiction are not getting “high” when they use, they are just becoming a little bit less sick. It is this repeated cycle of withdrawal that stresses the fetus, which leads to preterm delivery and low birth weight.
Medications for opioid use disorder are long-acting and dosed daily. In contrast to the repeated cycles of fetal withdrawal in untreated addiction, pharmacotherapy stabilizes the intrauterine environment. There is no cyclic, repeated, stressful withdrawal, and consequentially, the fetus grows normally and delivers at term. Obstetric risk is from repeated cyclic withdrawal more than from opioid exposure itself.
Continue to: OBG Management...
OBG Management : Research reports that women are not using all of the opioids that are prescribed to them after a cesarean delivery. What are the risks for addiction in this setting?
Dr. Terplan: I mark a distinction between use (ie, using something as prescribed) and misuse, which means using a prescribed medication not in the manner in which it was prescribed, or using somebody else’s medications, or using an illicit substance. And I differentiate use and misuse from addiction, which is a behavioral condition, a disease. There has been a lot of attention paid to opioid prescribing in general and in particular postdelivery and post–cesarean delivery, which is one of the most common operative procedures in the United States.
It seems clear from the literature that we have overprescribed opioids postdelivery, and a small number of women, about 1 in 300 will continue an opioid script.7 This means that 1 in 300 women who received an opioid prescription following delivery present for care and get another opioid prescription filled. Now, that is a small number at the level of the individual, but because we do so many cesarean deliveries, this is a large number of women at the level of the population. This does not mean, however, that 1 in 300 women who received opioids after cesarean delivery are going to become addicted to them. It just means that 1 in 300 will continue the prescription. Prescription continuation is a risk factor for opioid misuse, and opioid misuse is on the pathway toward addiction.
Most people who use substances do not develop an addiction to that substance. We know from the opioid literature that at most only 10% of people who receive chronic opioid therapy will meet criteria for opioid use disorder.8 Now 10% is not 100%, nor is it 0%, but because we prescribed so many opioids to so many people for so long, the absolute number of people with opioid use disorder from physician opioid prescribing is large, even though the risk at the level of the individual is not as large as people think.
OBG Management : From your experience in treating addiction during pregnancy, are there clinical pearls you would like to share with ObGyns?
Dr. Terplan: There are a couple of takeaways. One is that all women are motivated to maximize their health and that of their baby to be, and every pregnant woman engages in behavioral change; in fact most women quit or cutback substance use during pregnancy. But some can’t. Those that can’t likely have a substance use disorder. We think of addiction as a chronic condition, centered in the brain, but the primary symptoms of addiction are behaviors. The salient feature of addiction is continued use despite adverse consequences; using something that you know is harming yourself and others but you can’t stop using it. In other words, continuing substance use during pregnancy. When we see clinically a pregnant woman who is using a substance, 99% of the time we are seeing a pregnant woman who has the condition of addiction, and what she needs is treatment. She does not need to be told that injecting heroin is unsafe for her and her fetus, she knows that. What she needs is treatment.
The second point is that pregnant women who use drugs and pregnant women with addiction experience a real specific and strong form of discrimination by providers, by other people with addiction, by the legal system, and by their friends and families. Caring for people who have substance use disorder is grounded in human rights, which means treating people with dignity and respect. It is important for providers to have empathy, especially for pregnant people who use drugs, to counter the discrimination they experience from society and from other health care providers.
Continue to: OBG Management...
OBG Management : Are there specific ways in which ObGyns can show empathy when speaking with a pregnant woman who likely has addiction?
Dr. Terplan: In general when we talk to people about drug use, it is important to ask their permission to talk about it. For example, “Is it okay if I ask you some questions about smoking, drinking, and other drugs?” If someone says, “No, I don’t want you to ask those questions,” we have to respect that. Assessment of substance use should be a universal part of all medical care, as substance use, misuse, and addiction are essential domains of wellness, but I think we should ask permission before screening.
One of the really good things about prenatal care is that people come back; we have multiple visits across the gestational period. The behavioral work of addiction treatment rests upon a strong therapeutic alliance. If you do not respect your patient, then there is no way you can achieve a therapeutic alliance. Asking permission, and then respecting somebody’s answers, I think goes a really long way to establishing a strong therapeutic alliance, which is the basis of any medical care.
- Terplan M. Women and the opioid crisis: historical context and public health solutions. Fertil Steril. 2017;108:195-199.
- Management of substance abuse. World Health Organization website. https://www.who.int/substance_abuse/activities/treatment_opioid_dependence/en/. Accessed March 20, 2019.
- Committee on Obstetric Practice. Committee Opinion No. 711: Opioid use and opioid use disorder in pregnancy. Obstet Gynecol. 2017;130(2):e81-e94.
- Substance Abuse and Mental Health Services Administration. Clinical guidance for treating pregnant and parenting women with opioid use disorder and their infants. HHS Publication No. (SMA) 18-5054. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2018.
- Metz TD, Royner P, Hoffman MC, et al. Maternal deaths from suicide and overdose in Colorado, 2004-2012. Obstet Gynecol. 2016;128:1233-1240.
- Kaltenbach K, O’Grady E, Heil SH, et al. Prenatal exposure to methadone or buprenorphine: early childhood developmental outcomes. Drug Alcohol Depend. 2018;185:40-49.
- Bateman BT, Franklin JM, Bykov K, et al. Persistent opioid use following cesarean delivery: patterns and predictors among opioid-naive women. Am J Obstet Gynecol. 2016;215:353.e1-353.e18.
- Terplan M. Women and the opioid crisis: historical context and public health solutions. Fertil Steril. 2017;108:195-199.
- Management of substance abuse. World Health Organization website. https://www.who.int/substance_abuse/activities/treatment_opioid_dependence/en/. Accessed March 20, 2019.
- Committee on Obstetric Practice. Committee Opinion No. 711: Opioid use and opioid use disorder in pregnancy. Obstet Gynecol. 2017;130(2):e81-e94.
- Substance Abuse and Mental Health Services Administration. Clinical guidance for treating pregnant and parenting women with opioid use disorder and their infants. HHS Publication No. (SMA) 18-5054. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2018.
- Metz TD, Royner P, Hoffman MC, et al. Maternal deaths from suicide and overdose in Colorado, 2004-2012. Obstet Gynecol. 2016;128:1233-1240.
- Kaltenbach K, O’Grady E, Heil SH, et al. Prenatal exposure to methadone or buprenorphine: early childhood developmental outcomes. Drug Alcohol Depend. 2018;185:40-49.
- Bateman BT, Franklin JM, Bykov K, et al. Persistent opioid use following cesarean delivery: patterns and predictors among opioid-naive women. Am J Obstet Gynecol. 2016;215:353.e1-353.e18.
Rising to the challenges in gynecologic surgical care
As the face of health care changes and physicians are presented with new challenges, we need to keep focused on our priorities: maintain outstanding patient care, continue to grow ourselves as physicians, and train the next generation of women’s health care providers. The theme of the SGS 2019 annual scientific meeting in Tucson, Arizona, “Looking Forward:
The excellent postgraduate workshops included courses on simulation of laparoscopic suturing, surgical strategies for fibroid management, and a quality improvement boot camp. In addition, Rebecca Rogers, MD, Cassandra Carberry, MD, and Danielle Antosh, MD, along with physical therapist Uchenna Ossai, PT, DPT, WCS, ran a course on pelvic surgery and its impact on sexual function, tackling an important, often difficult topic for gynecologic surgeons. In part 2 of this special section, these authors highlight current knowledge on sexual function related to surgery and offer an initial evaluation and treatment approach for women with sexual dysfunction after surgery.
Peter Jeppson, MD, Audra Jolyn Hill, MD, and Sunil Balgobin, MD, have been integral leaders of the SGS Pelvic Anatomy Group, which has a mission to educate physicians about pelvic anatomy. Early discussions made it clear that standardized terms needed to be established and used for pelvic structures. In this special section, these authors illustrate the importance of standard terminology to optimize patient care, and they review pertinent vaginal compartment structures for the gynecologist.
Along with outstanding plenary talks focusing on surgical education research by Gary Dunnington, MD, and health disparities in gynecologic surgery by Marcela del Carmen, MD, MPH, 2 special focus speakers were featured. Sean Dowdy, MD, highlighted advances in the perioperative care of gynecologic surgery patients. In this special section, he reviews best practices for enhanced recovery after surgery (ERAS) and describes his experience with implementing a successful ERAS program.
Cheryl Iglesia, MD, covered energy-based therapies in female genital cosmetic surgery. In part 2 of this special section, she highlights, with Sarah Ward, MD, the salient points from her presentation, including the mechanism of action of laser therapy on tissue remodeling as well as some therapeutic uses for and outcomes of laser therapy in gynecologic care.
I hope you enjoy the content of this special section (part 2 will follow in the May issue) and find that it helps you achieve excellence in gynecologic surgery for yourself, your learners, and your patients!
As the face of health care changes and physicians are presented with new challenges, we need to keep focused on our priorities: maintain outstanding patient care, continue to grow ourselves as physicians, and train the next generation of women’s health care providers. The theme of the SGS 2019 annual scientific meeting in Tucson, Arizona, “Looking Forward:
The excellent postgraduate workshops included courses on simulation of laparoscopic suturing, surgical strategies for fibroid management, and a quality improvement boot camp. In addition, Rebecca Rogers, MD, Cassandra Carberry, MD, and Danielle Antosh, MD, along with physical therapist Uchenna Ossai, PT, DPT, WCS, ran a course on pelvic surgery and its impact on sexual function, tackling an important, often difficult topic for gynecologic surgeons. In part 2 of this special section, these authors highlight current knowledge on sexual function related to surgery and offer an initial evaluation and treatment approach for women with sexual dysfunction after surgery.
Peter Jeppson, MD, Audra Jolyn Hill, MD, and Sunil Balgobin, MD, have been integral leaders of the SGS Pelvic Anatomy Group, which has a mission to educate physicians about pelvic anatomy. Early discussions made it clear that standardized terms needed to be established and used for pelvic structures. In this special section, these authors illustrate the importance of standard terminology to optimize patient care, and they review pertinent vaginal compartment structures for the gynecologist.
Along with outstanding plenary talks focusing on surgical education research by Gary Dunnington, MD, and health disparities in gynecologic surgery by Marcela del Carmen, MD, MPH, 2 special focus speakers were featured. Sean Dowdy, MD, highlighted advances in the perioperative care of gynecologic surgery patients. In this special section, he reviews best practices for enhanced recovery after surgery (ERAS) and describes his experience with implementing a successful ERAS program.
Cheryl Iglesia, MD, covered energy-based therapies in female genital cosmetic surgery. In part 2 of this special section, she highlights, with Sarah Ward, MD, the salient points from her presentation, including the mechanism of action of laser therapy on tissue remodeling as well as some therapeutic uses for and outcomes of laser therapy in gynecologic care.
I hope you enjoy the content of this special section (part 2 will follow in the May issue) and find that it helps you achieve excellence in gynecologic surgery for yourself, your learners, and your patients!
As the face of health care changes and physicians are presented with new challenges, we need to keep focused on our priorities: maintain outstanding patient care, continue to grow ourselves as physicians, and train the next generation of women’s health care providers. The theme of the SGS 2019 annual scientific meeting in Tucson, Arizona, “Looking Forward:
The excellent postgraduate workshops included courses on simulation of laparoscopic suturing, surgical strategies for fibroid management, and a quality improvement boot camp. In addition, Rebecca Rogers, MD, Cassandra Carberry, MD, and Danielle Antosh, MD, along with physical therapist Uchenna Ossai, PT, DPT, WCS, ran a course on pelvic surgery and its impact on sexual function, tackling an important, often difficult topic for gynecologic surgeons. In part 2 of this special section, these authors highlight current knowledge on sexual function related to surgery and offer an initial evaluation and treatment approach for women with sexual dysfunction after surgery.
Peter Jeppson, MD, Audra Jolyn Hill, MD, and Sunil Balgobin, MD, have been integral leaders of the SGS Pelvic Anatomy Group, which has a mission to educate physicians about pelvic anatomy. Early discussions made it clear that standardized terms needed to be established and used for pelvic structures. In this special section, these authors illustrate the importance of standard terminology to optimize patient care, and they review pertinent vaginal compartment structures for the gynecologist.
Along with outstanding plenary talks focusing on surgical education research by Gary Dunnington, MD, and health disparities in gynecologic surgery by Marcela del Carmen, MD, MPH, 2 special focus speakers were featured. Sean Dowdy, MD, highlighted advances in the perioperative care of gynecologic surgery patients. In this special section, he reviews best practices for enhanced recovery after surgery (ERAS) and describes his experience with implementing a successful ERAS program.
Cheryl Iglesia, MD, covered energy-based therapies in female genital cosmetic surgery. In part 2 of this special section, she highlights, with Sarah Ward, MD, the salient points from her presentation, including the mechanism of action of laser therapy on tissue remodeling as well as some therapeutic uses for and outcomes of laser therapy in gynecologic care.
I hope you enjoy the content of this special section (part 2 will follow in the May issue) and find that it helps you achieve excellence in gynecologic surgery for yourself, your learners, and your patients!
What is the association of menopausal HT use and risk of Alzheimer disease?
EXPERT COMMENTARY
Savolainen-Peltonen H, Rahkola-Soisalo P, Hoti F, et al. Use of postmenopausal hormone therapy and risk of Alzheimer’s disease in Finland: nationwide case-control study. BMJ. 2019;364:1665.
Alzheimer disease represents the most common cause of dementia. Although sex hormones may play a role in the etiology of AD in women, studies addressing the impact of menopausal HT on risk of AD have conflicting findings.
Finnish researchers Savolainen-Peltonen and colleagues aimed to compare postmenopausal HT use in women with and without AD. They used national drug and population registries to identify patients with AD, control women without a diagnosis of AD, and data on postmenopausal HT use.
Details of the study
In Finland, reimbursement for treatment related to AD requires cognitive testing, brain imaging, and a statement from a specialist physician. Using national records, the study investigators identified 84,739 women with a diagnosis of AD during the years 1999–2013 and the same number of control women (without AD) during the same period. A national drug reimbursement registry was used to identify HT use from the year 1994.
Findings. Women diagnosed with AD were more likely to have been current or former users of systemic HT than controls (18.6% vs 17.0%, P<.001). The odds ratios (ORs) for AD were 1.09 for the estradiol-only group and 1.17 for the estrogen-progestin group (P<.05 for both comparisons).
Initiation of HT prior to age 60 was less common among AD cases than controls (P = .006). As a continuous variable, age was not a determinant for disease risk in estradiol-only users (OR, 1.0), estrogen-progestin users (OR, 1.0), or any HT use (OR, 1.0).
The exclusive use of vaginal estrogen therapy was not associated with an elevated risk of AD (OR, 0.99).
Study strengths and limitations
This study on the association between HT and AD included a very large number of participants from a national population registry, and the use of HT was objectively determined from a controlled registry (not self-reported). In addition, AD was accurately diagnosed and differentiated from other forms of dementia.
Limitations of the study include the lack of baseline demographic data for AD risk factors for both HT users and controls. Further, an increased risk of AD may have been a cause for HT use and not a consequence, given that initial cognitive impairments may occur 7 to 8 years prior to AD diagnosis and the possibility exists that such women may have sought help for cognitive symptoms from HT. In addition, the lack of brain imaging or neurologic examination to exclude AD might also account for undiagnosed disease in controls. The authors noted that they were unable to compare the use of oral and transdermal HT preparations or the use of cyclic and continuous estrogen-progestin therapy.
Alzheimer disease is more prevalent in women, and women are more likely to be caregivers for individuals with AD than men, making AD an issue of particular concern to midlife and older women. Current guidance from The North American Menopause Society and other organizations does not recommend use of systemic HT to prevent AD.1 As Savolainen-Peltonen and colleagues note in their observational study, the small risk increases for AD with use of HT are subject to bias. Editorialists agree with this concern and point out that a conclusive large randomized trial assessing HT's impact on AD is unlikely to be performed.2 I agree with the editorialists that the findings of this Finnish study should not change current practice. For recently menopausal women who have bothersome vasomotor symptoms and no contraindications, I will continue to counsel that initiating systemic HT is appropriate.
ANDREW M. KAUNITZ, MD
- The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24:728-753.
- Maki PM, Girard LM, Manson JE. Menopausal hormone therapy and cognition. BMJ. 2019;364:1877.
EXPERT COMMENTARY
Savolainen-Peltonen H, Rahkola-Soisalo P, Hoti F, et al. Use of postmenopausal hormone therapy and risk of Alzheimer’s disease in Finland: nationwide case-control study. BMJ. 2019;364:1665.
Alzheimer disease represents the most common cause of dementia. Although sex hormones may play a role in the etiology of AD in women, studies addressing the impact of menopausal HT on risk of AD have conflicting findings.
Finnish researchers Savolainen-Peltonen and colleagues aimed to compare postmenopausal HT use in women with and without AD. They used national drug and population registries to identify patients with AD, control women without a diagnosis of AD, and data on postmenopausal HT use.
Details of the study
In Finland, reimbursement for treatment related to AD requires cognitive testing, brain imaging, and a statement from a specialist physician. Using national records, the study investigators identified 84,739 women with a diagnosis of AD during the years 1999–2013 and the same number of control women (without AD) during the same period. A national drug reimbursement registry was used to identify HT use from the year 1994.
Findings. Women diagnosed with AD were more likely to have been current or former users of systemic HT than controls (18.6% vs 17.0%, P<.001). The odds ratios (ORs) for AD were 1.09 for the estradiol-only group and 1.17 for the estrogen-progestin group (P<.05 for both comparisons).
Initiation of HT prior to age 60 was less common among AD cases than controls (P = .006). As a continuous variable, age was not a determinant for disease risk in estradiol-only users (OR, 1.0), estrogen-progestin users (OR, 1.0), or any HT use (OR, 1.0).
The exclusive use of vaginal estrogen therapy was not associated with an elevated risk of AD (OR, 0.99).
Study strengths and limitations
This study on the association between HT and AD included a very large number of participants from a national population registry, and the use of HT was objectively determined from a controlled registry (not self-reported). In addition, AD was accurately diagnosed and differentiated from other forms of dementia.
Limitations of the study include the lack of baseline demographic data for AD risk factors for both HT users and controls. Further, an increased risk of AD may have been a cause for HT use and not a consequence, given that initial cognitive impairments may occur 7 to 8 years prior to AD diagnosis and the possibility exists that such women may have sought help for cognitive symptoms from HT. In addition, the lack of brain imaging or neurologic examination to exclude AD might also account for undiagnosed disease in controls. The authors noted that they were unable to compare the use of oral and transdermal HT preparations or the use of cyclic and continuous estrogen-progestin therapy.
Alzheimer disease is more prevalent in women, and women are more likely to be caregivers for individuals with AD than men, making AD an issue of particular concern to midlife and older women. Current guidance from The North American Menopause Society and other organizations does not recommend use of systemic HT to prevent AD.1 As Savolainen-Peltonen and colleagues note in their observational study, the small risk increases for AD with use of HT are subject to bias. Editorialists agree with this concern and point out that a conclusive large randomized trial assessing HT's impact on AD is unlikely to be performed.2 I agree with the editorialists that the findings of this Finnish study should not change current practice. For recently menopausal women who have bothersome vasomotor symptoms and no contraindications, I will continue to counsel that initiating systemic HT is appropriate.
ANDREW M. KAUNITZ, MD
EXPERT COMMENTARY
Savolainen-Peltonen H, Rahkola-Soisalo P, Hoti F, et al. Use of postmenopausal hormone therapy and risk of Alzheimer’s disease in Finland: nationwide case-control study. BMJ. 2019;364:1665.
Alzheimer disease represents the most common cause of dementia. Although sex hormones may play a role in the etiology of AD in women, studies addressing the impact of menopausal HT on risk of AD have conflicting findings.
Finnish researchers Savolainen-Peltonen and colleagues aimed to compare postmenopausal HT use in women with and without AD. They used national drug and population registries to identify patients with AD, control women without a diagnosis of AD, and data on postmenopausal HT use.
Details of the study
In Finland, reimbursement for treatment related to AD requires cognitive testing, brain imaging, and a statement from a specialist physician. Using national records, the study investigators identified 84,739 women with a diagnosis of AD during the years 1999–2013 and the same number of control women (without AD) during the same period. A national drug reimbursement registry was used to identify HT use from the year 1994.
Findings. Women diagnosed with AD were more likely to have been current or former users of systemic HT than controls (18.6% vs 17.0%, P<.001). The odds ratios (ORs) for AD were 1.09 for the estradiol-only group and 1.17 for the estrogen-progestin group (P<.05 for both comparisons).
Initiation of HT prior to age 60 was less common among AD cases than controls (P = .006). As a continuous variable, age was not a determinant for disease risk in estradiol-only users (OR, 1.0), estrogen-progestin users (OR, 1.0), or any HT use (OR, 1.0).
The exclusive use of vaginal estrogen therapy was not associated with an elevated risk of AD (OR, 0.99).
Study strengths and limitations
This study on the association between HT and AD included a very large number of participants from a national population registry, and the use of HT was objectively determined from a controlled registry (not self-reported). In addition, AD was accurately diagnosed and differentiated from other forms of dementia.
Limitations of the study include the lack of baseline demographic data for AD risk factors for both HT users and controls. Further, an increased risk of AD may have been a cause for HT use and not a consequence, given that initial cognitive impairments may occur 7 to 8 years prior to AD diagnosis and the possibility exists that such women may have sought help for cognitive symptoms from HT. In addition, the lack of brain imaging or neurologic examination to exclude AD might also account for undiagnosed disease in controls. The authors noted that they were unable to compare the use of oral and transdermal HT preparations or the use of cyclic and continuous estrogen-progestin therapy.
Alzheimer disease is more prevalent in women, and women are more likely to be caregivers for individuals with AD than men, making AD an issue of particular concern to midlife and older women. Current guidance from The North American Menopause Society and other organizations does not recommend use of systemic HT to prevent AD.1 As Savolainen-Peltonen and colleagues note in their observational study, the small risk increases for AD with use of HT are subject to bias. Editorialists agree with this concern and point out that a conclusive large randomized trial assessing HT's impact on AD is unlikely to be performed.2 I agree with the editorialists that the findings of this Finnish study should not change current practice. For recently menopausal women who have bothersome vasomotor symptoms and no contraindications, I will continue to counsel that initiating systemic HT is appropriate.
ANDREW M. KAUNITZ, MD
- The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24:728-753.
- Maki PM, Girard LM, Manson JE. Menopausal hormone therapy and cognition. BMJ. 2019;364:1877.
- The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24:728-753.
- Maki PM, Girard LM, Manson JE. Menopausal hormone therapy and cognition. BMJ. 2019;364:1877.
Pre-exposure prophylaxis for the prevention of HIV infection: Ready for prime time
The first cases of HIV infection in the United States were reported in 1981. Since that time, more than 700,000 individuals in our country have died of AIDS. Slightly more than 1 million persons in the United States are currently living with HIV infection; approximately 15% of them are unaware of their infection. Men who have sex with men (MSM) and African American and Hispanic/Latino men and women are disproportionately affected by HIV infection.1 Among men, MSM is the most common method of infection transmission, accounting for 83% of infections. Heterosexual contact accounts for 9.4% of new infections and injection drug use for 4.0%. Among women in the United States, heterosexual contact is the most common mechanism of transmission, accounting for about 87% of cases; injection drug use accounts for about 12%.1 Perinatal transmission rates are extremely low—less than 1%—when women receive effective treatment during pregnancy and their infants are treated in the neonatal period.1,2
The prognosis for HIV-infected patients has improved dramatically in recent years with the availability of many new and exceptionally effective highly-active antiretroviral treatment regimens. Nevertheless, the disease is not yet completely curable. Therefore, preventive measures are of great importance in reducing the enormous toll imposed by this condition.2
Evaluating effectiveness of PrEP
At the request of the US Preventive Services Task Force, Chou and colleagues recently conducted a systematic review to determine the effectiveness of pre-exposure prophylaxis (PrEP) in preventing the horizontal transmission of HIV infection.1 The authors’ secondary objectives included assessing the relationship between degree of adherence to the prophylactic regimen and degree of effectiveness and evaluating the accuracy of various screening systems for identifying patients at high risk for acquiring HIV infection.
The authors reviewed prospective, randomized controlled trials (treatment versus no treatment or treatment versus placebo) published through 2018. Pregnant women were excluded from the studies, as were women who became pregnant after enrollment.
Two different prophylactic regimens were used in the reviewed studies: 1) the combination of tenofovir disoproxil fumarate 300 mg or 245 mg plus emtricitabine 200 mg and 2) tenofovir 300 mg alone. Most trials used the combination regimen. With the exception of one trial, the medications were given daily to uninfected patients at high risk of acquiring HIV infection. In one investigation, the administration of prophylaxis was event driven (administered after a specific high-risk exposure).
Key study findings
PrEP decreased HIV transmission in high-risk patients. Chou and colleagues found that high-risk patients included primarily MSM who did not use condoms consistently or who had a high number of sex partners, individuals in an HIV-serodiscordant relationship, and intravenous drug users who shared injection equipment.
In these high-risk patients, PrEP was associated with a significantly decreased risk of HIV transmission. Observations from 11 trials demonstrated a relative risk (RR) of 0.46 (95% confidence interval [CI], 0.33–0.66). The absolute risk reduction was -2.0% (95% CI, -2.8% to -1.2%). The duration of follow up ranged from 4 months to 4 years.
Continue to: Better medication adherence = greater prophylaxis effectiveness...
Better medication adherence = greater prophylaxis effectiveness. When adherence was ≥70%, the RR was 0.27 (95% CI, 0.19–0.39). When adherence was 40% to 70%, the RR was 0.51 (95% CI, 0.38–0.70). When adherence was ≤40%, the relative risk was 0.93 (95% CI, 0.72–1.20). Adherence was better with daily administration, as opposed to event-driven administration.
Although the combination prophylactic regimen (tenofovir plus emtricitabine) was most frequently used in the clinical trials, tenofovir alone was comparable in effectiveness.
PrEP resulted in more mild adverse effects. Patients who received PrEP were more likely to develop gastrointestinal adverse effects and renal function abnormalities when compared with patients in the control arms of the studies. These adverse effects were virtually always mild and did not necessitate discontinuation of treatment.
No increase in promiscuous sexual behavior with PrEP. Specifically, investigators did not document an increased incidence of new sexually transmitted infections (STIs) in treated patients.
PrEP did not increase adverse pregnancy outcomes. In women who became pregnant while on PrEP, and who then discontinued treatment, there was no increase in the frequency of spontaneous abortion, congenital anomalies, or other adverse pregnancy outcomes.
In addition, PrEP posed a low risk for causing drug resistance in patients who became infected despite prophylaxis. Finally, the authors found that screening instruments for identifying patients at highest risk for acquiring HIV infection had low to modest sensitivity.
My recommendations for practice
Based on the study by Chou and colleagues, and on a recent commentary by Marcus et al, I believe that the following actions are justified1–3:
- For prophylaxis to be effective, we must identify all infected patients. Therefore, screening of asymptomatic individuals during routine health encounters is essential.
- All patients should have access to easy-to-understand information related to risk factors for HIV infection.
- Every effort should be made to promote safe sex practices, such as use of latex condoms, avoidance of sex during menses and in the presence of ulcerative genital lesions, and avoidance of use of contaminated drug-injection needles.
- All high-risk patients, as defined above, should be offered PrEP.
- To the greatest extent possible, financial barriers to PrEP should be eliminated.
- Patients receiving PrEP should be monitored for evidence of renal dysfunction. Should they become infected despite prophylaxis, they should be evaluated carefully to detect drug-resistant viral strains.
- Although PrEP is definitely effective in reducing the risk of transmission of HIV infection, it does not prevent the transmission of other STIs, such as syphilis, gonorrhea, and chlamydia.
In my practice, I administer prophyaxis on a daily basis rather than just before, or after, a high-risk exposure. This approach enhances patient adherence and, hopefully, will lead to maximum effectiveness over time. I also use the combination of tenofovir disoproxil fumarate plus emtricitabine rather than tenofovir alone because there is more published information regarding the effectiveness of the combination regimen.
- Chou R, Evans C, Hoverman A, et al. Pre-exposure Prophylaxis for the Prevention of HIV Infection: A Systematic Review for the U.S. Preventive Services Task Force. AHRQ Publication No. 18-05247-EF-1; November 2018.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TR, Green MF, Copel JA, Silver RM (eds). Creasy & Resnik's Maternal-Fetal Medicine. Principles and Practice (8th ed). Philadelphia, PA: Elsevier; 2019.
- Marcus JL, Katz KA, Krakower DS, et al. Risk compensation and clinical decision making--the case of HIV preexposure prophylaxis. N Engl J Med. 2019;380:510-512.
The first cases of HIV infection in the United States were reported in 1981. Since that time, more than 700,000 individuals in our country have died of AIDS. Slightly more than 1 million persons in the United States are currently living with HIV infection; approximately 15% of them are unaware of their infection. Men who have sex with men (MSM) and African American and Hispanic/Latino men and women are disproportionately affected by HIV infection.1 Among men, MSM is the most common method of infection transmission, accounting for 83% of infections. Heterosexual contact accounts for 9.4% of new infections and injection drug use for 4.0%. Among women in the United States, heterosexual contact is the most common mechanism of transmission, accounting for about 87% of cases; injection drug use accounts for about 12%.1 Perinatal transmission rates are extremely low—less than 1%—when women receive effective treatment during pregnancy and their infants are treated in the neonatal period.1,2
The prognosis for HIV-infected patients has improved dramatically in recent years with the availability of many new and exceptionally effective highly-active antiretroviral treatment regimens. Nevertheless, the disease is not yet completely curable. Therefore, preventive measures are of great importance in reducing the enormous toll imposed by this condition.2
Evaluating effectiveness of PrEP
At the request of the US Preventive Services Task Force, Chou and colleagues recently conducted a systematic review to determine the effectiveness of pre-exposure prophylaxis (PrEP) in preventing the horizontal transmission of HIV infection.1 The authors’ secondary objectives included assessing the relationship between degree of adherence to the prophylactic regimen and degree of effectiveness and evaluating the accuracy of various screening systems for identifying patients at high risk for acquiring HIV infection.
The authors reviewed prospective, randomized controlled trials (treatment versus no treatment or treatment versus placebo) published through 2018. Pregnant women were excluded from the studies, as were women who became pregnant after enrollment.
Two different prophylactic regimens were used in the reviewed studies: 1) the combination of tenofovir disoproxil fumarate 300 mg or 245 mg plus emtricitabine 200 mg and 2) tenofovir 300 mg alone. Most trials used the combination regimen. With the exception of one trial, the medications were given daily to uninfected patients at high risk of acquiring HIV infection. In one investigation, the administration of prophylaxis was event driven (administered after a specific high-risk exposure).
Key study findings
PrEP decreased HIV transmission in high-risk patients. Chou and colleagues found that high-risk patients included primarily MSM who did not use condoms consistently or who had a high number of sex partners, individuals in an HIV-serodiscordant relationship, and intravenous drug users who shared injection equipment.
In these high-risk patients, PrEP was associated with a significantly decreased risk of HIV transmission. Observations from 11 trials demonstrated a relative risk (RR) of 0.46 (95% confidence interval [CI], 0.33–0.66). The absolute risk reduction was -2.0% (95% CI, -2.8% to -1.2%). The duration of follow up ranged from 4 months to 4 years.
Continue to: Better medication adherence = greater prophylaxis effectiveness...
Better medication adherence = greater prophylaxis effectiveness. When adherence was ≥70%, the RR was 0.27 (95% CI, 0.19–0.39). When adherence was 40% to 70%, the RR was 0.51 (95% CI, 0.38–0.70). When adherence was ≤40%, the relative risk was 0.93 (95% CI, 0.72–1.20). Adherence was better with daily administration, as opposed to event-driven administration.
Although the combination prophylactic regimen (tenofovir plus emtricitabine) was most frequently used in the clinical trials, tenofovir alone was comparable in effectiveness.
PrEP resulted in more mild adverse effects. Patients who received PrEP were more likely to develop gastrointestinal adverse effects and renal function abnormalities when compared with patients in the control arms of the studies. These adverse effects were virtually always mild and did not necessitate discontinuation of treatment.
No increase in promiscuous sexual behavior with PrEP. Specifically, investigators did not document an increased incidence of new sexually transmitted infections (STIs) in treated patients.
PrEP did not increase adverse pregnancy outcomes. In women who became pregnant while on PrEP, and who then discontinued treatment, there was no increase in the frequency of spontaneous abortion, congenital anomalies, or other adverse pregnancy outcomes.
In addition, PrEP posed a low risk for causing drug resistance in patients who became infected despite prophylaxis. Finally, the authors found that screening instruments for identifying patients at highest risk for acquiring HIV infection had low to modest sensitivity.
My recommendations for practice
Based on the study by Chou and colleagues, and on a recent commentary by Marcus et al, I believe that the following actions are justified1–3:
- For prophylaxis to be effective, we must identify all infected patients. Therefore, screening of asymptomatic individuals during routine health encounters is essential.
- All patients should have access to easy-to-understand information related to risk factors for HIV infection.
- Every effort should be made to promote safe sex practices, such as use of latex condoms, avoidance of sex during menses and in the presence of ulcerative genital lesions, and avoidance of use of contaminated drug-injection needles.
- All high-risk patients, as defined above, should be offered PrEP.
- To the greatest extent possible, financial barriers to PrEP should be eliminated.
- Patients receiving PrEP should be monitored for evidence of renal dysfunction. Should they become infected despite prophylaxis, they should be evaluated carefully to detect drug-resistant viral strains.
- Although PrEP is definitely effective in reducing the risk of transmission of HIV infection, it does not prevent the transmission of other STIs, such as syphilis, gonorrhea, and chlamydia.
In my practice, I administer prophyaxis on a daily basis rather than just before, or after, a high-risk exposure. This approach enhances patient adherence and, hopefully, will lead to maximum effectiveness over time. I also use the combination of tenofovir disoproxil fumarate plus emtricitabine rather than tenofovir alone because there is more published information regarding the effectiveness of the combination regimen.
The first cases of HIV infection in the United States were reported in 1981. Since that time, more than 700,000 individuals in our country have died of AIDS. Slightly more than 1 million persons in the United States are currently living with HIV infection; approximately 15% of them are unaware of their infection. Men who have sex with men (MSM) and African American and Hispanic/Latino men and women are disproportionately affected by HIV infection.1 Among men, MSM is the most common method of infection transmission, accounting for 83% of infections. Heterosexual contact accounts for 9.4% of new infections and injection drug use for 4.0%. Among women in the United States, heterosexual contact is the most common mechanism of transmission, accounting for about 87% of cases; injection drug use accounts for about 12%.1 Perinatal transmission rates are extremely low—less than 1%—when women receive effective treatment during pregnancy and their infants are treated in the neonatal period.1,2
The prognosis for HIV-infected patients has improved dramatically in recent years with the availability of many new and exceptionally effective highly-active antiretroviral treatment regimens. Nevertheless, the disease is not yet completely curable. Therefore, preventive measures are of great importance in reducing the enormous toll imposed by this condition.2
Evaluating effectiveness of PrEP
At the request of the US Preventive Services Task Force, Chou and colleagues recently conducted a systematic review to determine the effectiveness of pre-exposure prophylaxis (PrEP) in preventing the horizontal transmission of HIV infection.1 The authors’ secondary objectives included assessing the relationship between degree of adherence to the prophylactic regimen and degree of effectiveness and evaluating the accuracy of various screening systems for identifying patients at high risk for acquiring HIV infection.
The authors reviewed prospective, randomized controlled trials (treatment versus no treatment or treatment versus placebo) published through 2018. Pregnant women were excluded from the studies, as were women who became pregnant after enrollment.
Two different prophylactic regimens were used in the reviewed studies: 1) the combination of tenofovir disoproxil fumarate 300 mg or 245 mg plus emtricitabine 200 mg and 2) tenofovir 300 mg alone. Most trials used the combination regimen. With the exception of one trial, the medications were given daily to uninfected patients at high risk of acquiring HIV infection. In one investigation, the administration of prophylaxis was event driven (administered after a specific high-risk exposure).
Key study findings
PrEP decreased HIV transmission in high-risk patients. Chou and colleagues found that high-risk patients included primarily MSM who did not use condoms consistently or who had a high number of sex partners, individuals in an HIV-serodiscordant relationship, and intravenous drug users who shared injection equipment.
In these high-risk patients, PrEP was associated with a significantly decreased risk of HIV transmission. Observations from 11 trials demonstrated a relative risk (RR) of 0.46 (95% confidence interval [CI], 0.33–0.66). The absolute risk reduction was -2.0% (95% CI, -2.8% to -1.2%). The duration of follow up ranged from 4 months to 4 years.
Continue to: Better medication adherence = greater prophylaxis effectiveness...
Better medication adherence = greater prophylaxis effectiveness. When adherence was ≥70%, the RR was 0.27 (95% CI, 0.19–0.39). When adherence was 40% to 70%, the RR was 0.51 (95% CI, 0.38–0.70). When adherence was ≤40%, the relative risk was 0.93 (95% CI, 0.72–1.20). Adherence was better with daily administration, as opposed to event-driven administration.
Although the combination prophylactic regimen (tenofovir plus emtricitabine) was most frequently used in the clinical trials, tenofovir alone was comparable in effectiveness.
PrEP resulted in more mild adverse effects. Patients who received PrEP were more likely to develop gastrointestinal adverse effects and renal function abnormalities when compared with patients in the control arms of the studies. These adverse effects were virtually always mild and did not necessitate discontinuation of treatment.
No increase in promiscuous sexual behavior with PrEP. Specifically, investigators did not document an increased incidence of new sexually transmitted infections (STIs) in treated patients.
PrEP did not increase adverse pregnancy outcomes. In women who became pregnant while on PrEP, and who then discontinued treatment, there was no increase in the frequency of spontaneous abortion, congenital anomalies, or other adverse pregnancy outcomes.
In addition, PrEP posed a low risk for causing drug resistance in patients who became infected despite prophylaxis. Finally, the authors found that screening instruments for identifying patients at highest risk for acquiring HIV infection had low to modest sensitivity.
My recommendations for practice
Based on the study by Chou and colleagues, and on a recent commentary by Marcus et al, I believe that the following actions are justified1–3:
- For prophylaxis to be effective, we must identify all infected patients. Therefore, screening of asymptomatic individuals during routine health encounters is essential.
- All patients should have access to easy-to-understand information related to risk factors for HIV infection.
- Every effort should be made to promote safe sex practices, such as use of latex condoms, avoidance of sex during menses and in the presence of ulcerative genital lesions, and avoidance of use of contaminated drug-injection needles.
- All high-risk patients, as defined above, should be offered PrEP.
- To the greatest extent possible, financial barriers to PrEP should be eliminated.
- Patients receiving PrEP should be monitored for evidence of renal dysfunction. Should they become infected despite prophylaxis, they should be evaluated carefully to detect drug-resistant viral strains.
- Although PrEP is definitely effective in reducing the risk of transmission of HIV infection, it does not prevent the transmission of other STIs, such as syphilis, gonorrhea, and chlamydia.
In my practice, I administer prophyaxis on a daily basis rather than just before, or after, a high-risk exposure. This approach enhances patient adherence and, hopefully, will lead to maximum effectiveness over time. I also use the combination of tenofovir disoproxil fumarate plus emtricitabine rather than tenofovir alone because there is more published information regarding the effectiveness of the combination regimen.
- Chou R, Evans C, Hoverman A, et al. Pre-exposure Prophylaxis for the Prevention of HIV Infection: A Systematic Review for the U.S. Preventive Services Task Force. AHRQ Publication No. 18-05247-EF-1; November 2018.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TR, Green MF, Copel JA, Silver RM (eds). Creasy & Resnik's Maternal-Fetal Medicine. Principles and Practice (8th ed). Philadelphia, PA: Elsevier; 2019.
- Marcus JL, Katz KA, Krakower DS, et al. Risk compensation and clinical decision making--the case of HIV preexposure prophylaxis. N Engl J Med. 2019;380:510-512.
- Chou R, Evans C, Hoverman A, et al. Pre-exposure Prophylaxis for the Prevention of HIV Infection: A Systematic Review for the U.S. Preventive Services Task Force. AHRQ Publication No. 18-05247-EF-1; November 2018.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TR, Green MF, Copel JA, Silver RM (eds). Creasy & Resnik's Maternal-Fetal Medicine. Principles and Practice (8th ed). Philadelphia, PA: Elsevier; 2019.
- Marcus JL, Katz KA, Krakower DS, et al. Risk compensation and clinical decision making--the case of HIV preexposure prophylaxis. N Engl J Med. 2019;380:510-512.
Society of Gynecologic Surgeons 2019 meeting: Daily reporting from Fellow Scholar
WEDNESDAY, 4/3/2019. DAY 4 OF SGS.
Sadly, the annual Society of Gynecologic Surgeons meeting is wrapping up, and we will soon be leaving sunny Tucson! The last morning of conference proceedings was jam-packed with more outstanding oral and video presentations. We heard about topics such as the burden of postoperative catheterization, dietary patterns associated with postoperative defecatory symptoms, and more surgical tips and tricks to take back to our own institutions. At the end of the morning, the Distinguished Surgeon award was presented to the talented and deserving J. Marion Sims Endowed Chair of Obstetrics and Gynecology at UAB Medicine in Birmingham Dr. Holly E. Richter. The SGS Presidential Gavel was then passed from current SGS President Dr. Rajiv Gala to the incoming 46th President Dr. Peter Rosenblatt, Director of the Urogynecology and Pelvic Reconstructive Surgery Division at Mount Auburn Hospital in Cambridge, Massachusetts.
#SGS2019 was an amazingly successful conference! Beautiful surroundings, emerging science and education, and respectful inquiry was plentiful. I enjoyed all of the networking, reconnecting, and relaxing, and could not ask for a better community of GYN surgeons to have shared this with. I can’t wait to return to Pittsburgh to implement all the new things that I have learned. Thanks to the Society of Gynecologic Surgeons, OBG MANAGEMENT, and all the sponsors of the Fellows Scholar Program for supporting each of the scholars and this blog!
If you were at all intrigued by the happenings reported here, please consider attending the SGS meeting in 2020! The conference will be located in Jacksonville, Florida! See you there!
Thanks for following along! #SGS2019 out.
Continue to: TUESDAY, 4/2/19. DAY 3...
TUESDAY, 4/2/19. DAY 3.
The third day of the annual meeting of the Society of Gynecologic Surgeons started off with several academic roundtables hosted by experts in the field. The general session got underway with more fantastic oral and video presentations and, as usual, plenty of lively discussion and education ensued! The 45th SGS President Dr. Rajiv Gala (@rgala_nola) gave his presidential address, where he spoke so genuinely about how SGS is looking forward in our field. After all, the best way to predict your future is to create it! Be on the lookout on Twitter for Dr. Gala’s selfie with his “SGS Family” that he took during his address!
This year’s Telinde Lecture was given by Dr. Marcela G. del Carmen, titled “Health Care Disparities in Gynecologic Oncology Surgery.” She gave an informative and eye-opening lecture on the disparities that still exist in our field, specifically in patients with cancer. The morning session was rounded out with a mentoring panel, featuring Drs. B. Star Hampton, Bobby Shull, Peggy Norton, Tom Nolan, and Deborah Myers. Plenty of sage advice was offered. Thanks to Dr. Shull for reminding us to “be gracious; kindness never goes out of style,” and to be “a citizen of the world.”
Conference goers took the afternoon to enjoy leisure activities in the beautiful Arizona surroundings, including mountain biking, yoga, golf, and poolside lounging. The evening was filled with the excitement of the annual “SGS Got Talent” show! Fabulous performances and delicious food and drinks were just half of the fun, though. The life-size play on hungry, hungry hippos—“Hungry, Hungry Surgeons”—competition was the hit of the night!
Tomorrow is the last day of #SGS2019. Be sure to follow along for the final day of coverage!
Continue to: MONDAY, 4/1/19. DAY 2...
MONDAY, 4/1/19. DAY 2.
The first day of the general sessions started off with a cleverly titled breakfast symposium, “Postmenopausal sexuality: A bit dry but a must-have conversation,” by the brilliant and entertaining duo of Cheryl Iglesia (@cheryliglesia) and Sheryl Kingsberg (@SherylKingsburg) #CherylandSheryl.
Cheryl Iglesia, MD
The new members of the Society of Gynecologic Surgeons were recognized, and there were several outstanding oral and video presentations throughout the morning. A range of topics were discussed, including vaginal surgery education, patient perspectives on adverse events, and postoperative pain management. In addition, Dr. Gary Dunnington (@GLDunnington), Chair of Surgery at Indiana University School of Medicine in Indianapolis, gave the keynote lecture on “Measuring and improving performance in surgical training,” reminding us to continually strive for change.
After a brief lunch and stroll around the exhibit hall, the afternoon session kicked off with a special guest lecture on vaginal rejuvenation and energy-based therapies for female genital cosmetic surgery by Cheryl Iglesia (@cheryliglesia). Next, a distinguished panel of experts from all gynecologic subspecialties gave their opinions on “Working together to shape the future of gynecologic surgery.” What a treat to see such important topics discussed by all the giants of our field sitting in one room: Society of Gynecologic Surgeons President Rajiv Gala, MD; ACOG President Elect Ted Anderson, MD; American Urogynecologic Society President Geoffrey W. Cundiff, MD; Society of Gynecologic Oncology President Elect Warner Huh, MD; Society of Reproductive Surgeons Immediate Past President Samantha Pfeifer, MD; and AAGL President Marie Fidela R. Paraiso, MD.
Supplemented by popcorn, the Videofest featured a series of informative and impressive videos—from management of removal of the Essure hysteroscopic contraceptive device to tips and tricks to navigate a pelvic kidney. The Fellows’ Pelvic Research Network (FPRN), a network of fellows from both minimally invasive gynecologic surgery and urogynecology programs that facilitates multicenter research, met and discussed ongoing and upcoming studies. Exciting work is coming your way thanks to the collaboration of the FPRN!
We concluded an excellent first day of general sessions with an awards ceremony and President’s reception. It was an evening filled with networking, catching up with old colleagues, and meeting new friends. I look forward to another day of scholarship and education tomorrow! Follow @lauraknewcomb, @GynSurgery, and @MDedgeObGyn on Twitter for updates.
Continue to: SUNDAY, 3/31/19. DAY 1 AT SGS...
SUNDAY, 3/31/19. DAY 1 AT SGS.
Hello from Tucson! I woke up to a beautiful Arizona sunrise, with cacti as far as the eye can see; a great start to what is surely going to be an educational scientific conference of the Society of Gynecologic Surgeons! Be sure to follow me on Twitter to stay in the loop real-time: @lauraknewcomb. And don’t forget to check out our conference hashtag #SGS2019.
Postgrad courses kick off
Quality improvement bootcamp
Dr. Bob Flora (@RFFlora) gave a great “Teach the Teacher” session, reviewing different methods for performing quality improvement projects in your own workspace, including the Institute for Healthcare Improvement (IHI) Model for Improvement (www.IHI.org). We also had the opportunity to learn and play with QI Macros (KnowWare International Inc) and Lucid Chart (Lucid Software Inc) programs—which are excellent tools to assist in quality improvement data analysis and presentation. Try them out if you have never used them before!
Sex and surgery
The sex and surgery postgraduate course was a lively discussion centering on:
- the links between gynecologic surgery and sexual function
- how to measure sexual function and incorporate discussion into our pre- and post-operative counseling
- how to approach the patient with postoperative sexual concerns.
As surgeons, we admitted that an anatomic approach with surgery will not always be successful in treating sexual complaints, as sexuality encompasses psychological, social/cultural, interpersonal, and biological aspects. We agreed that further studies are needed to examine the issue, using sexual function as a primary endpoint, because the concern is of critical importance to our patients.
Social media workshop
The talented SGS Social Media Committee, including influencers Dr. Mireille Truong (@MIS_MDT) and Dr. Elisa Jorgensen (@ejiorgensenmd) gave us the run-down on how to host a successful Twitter journal club and how to be a responsible and influential influencer on various social media avenues. They encouraged us to take advantage of the virtual space that connects so many more people than we could interact with without it!
Hands-on laparoscopic suturing simulation
This course was an excellent comprehensive laparoscopic suturing course. It began with a detailed outline of basic principles and slowly built on these concepts until we were performing laparoscopic myomectomies on a high-fidelity model. We can’t wait to implement these principles in the operating room next week! Thanks to the talented faculty who taught all the tips and tricks of the experts!
Conservative and definitive surgical strategies for fibroid management
Drs. Megan Wasson (@WassonMegan), Arnold Advincula (@arnieadvincula), and others taught all the nuances of managing fibroids and difficult surgical cases. Participants learned several tips, tricks, and techniques to use to manage fibroids—for example the “bow and arrow” and “push and tuck” techniques when performing a hysteroscopic myomectomy with a resectoscope.
Women’s leadership forum
During the evening women’s leadership forum, Drs. Catherine Matthews and Kimberly Kenton (@KimKenton1) highlighted the differences between mentorship and sponsorship. While most female physicians identify meaningful mentorship relationships, women lack sponsorship to advance their careers. Furthermore, more women-to-women sponsorship relationships are needed to improve and achieve gender equality.
Lastly, we all enjoyed the Arizona sunset with a welcome reception on the lawn. It was a great first day and we are all looking forward to an exciting general session on Monday! Stay tuned for more!

#SGS2019 attendees enjoying the welcome reception
WEDNESDAY, 4/3/2019. DAY 4 OF SGS.
Sadly, the annual Society of Gynecologic Surgeons meeting is wrapping up, and we will soon be leaving sunny Tucson! The last morning of conference proceedings was jam-packed with more outstanding oral and video presentations. We heard about topics such as the burden of postoperative catheterization, dietary patterns associated with postoperative defecatory symptoms, and more surgical tips and tricks to take back to our own institutions. At the end of the morning, the Distinguished Surgeon award was presented to the talented and deserving J. Marion Sims Endowed Chair of Obstetrics and Gynecology at UAB Medicine in Birmingham Dr. Holly E. Richter. The SGS Presidential Gavel was then passed from current SGS President Dr. Rajiv Gala to the incoming 46th President Dr. Peter Rosenblatt, Director of the Urogynecology and Pelvic Reconstructive Surgery Division at Mount Auburn Hospital in Cambridge, Massachusetts.
#SGS2019 was an amazingly successful conference! Beautiful surroundings, emerging science and education, and respectful inquiry was plentiful. I enjoyed all of the networking, reconnecting, and relaxing, and could not ask for a better community of GYN surgeons to have shared this with. I can’t wait to return to Pittsburgh to implement all the new things that I have learned. Thanks to the Society of Gynecologic Surgeons, OBG MANAGEMENT, and all the sponsors of the Fellows Scholar Program for supporting each of the scholars and this blog!
If you were at all intrigued by the happenings reported here, please consider attending the SGS meeting in 2020! The conference will be located in Jacksonville, Florida! See you there!
Thanks for following along! #SGS2019 out.
Continue to: TUESDAY, 4/2/19. DAY 3...
TUESDAY, 4/2/19. DAY 3.
The third day of the annual meeting of the Society of Gynecologic Surgeons started off with several academic roundtables hosted by experts in the field. The general session got underway with more fantastic oral and video presentations and, as usual, plenty of lively discussion and education ensued! The 45th SGS President Dr. Rajiv Gala (@rgala_nola) gave his presidential address, where he spoke so genuinely about how SGS is looking forward in our field. After all, the best way to predict your future is to create it! Be on the lookout on Twitter for Dr. Gala’s selfie with his “SGS Family” that he took during his address!
This year’s Telinde Lecture was given by Dr. Marcela G. del Carmen, titled “Health Care Disparities in Gynecologic Oncology Surgery.” She gave an informative and eye-opening lecture on the disparities that still exist in our field, specifically in patients with cancer. The morning session was rounded out with a mentoring panel, featuring Drs. B. Star Hampton, Bobby Shull, Peggy Norton, Tom Nolan, and Deborah Myers. Plenty of sage advice was offered. Thanks to Dr. Shull for reminding us to “be gracious; kindness never goes out of style,” and to be “a citizen of the world.”
Conference goers took the afternoon to enjoy leisure activities in the beautiful Arizona surroundings, including mountain biking, yoga, golf, and poolside lounging. The evening was filled with the excitement of the annual “SGS Got Talent” show! Fabulous performances and delicious food and drinks were just half of the fun, though. The life-size play on hungry, hungry hippos—“Hungry, Hungry Surgeons”—competition was the hit of the night!
Tomorrow is the last day of #SGS2019. Be sure to follow along for the final day of coverage!
Continue to: MONDAY, 4/1/19. DAY 2...
MONDAY, 4/1/19. DAY 2.
The first day of the general sessions started off with a cleverly titled breakfast symposium, “Postmenopausal sexuality: A bit dry but a must-have conversation,” by the brilliant and entertaining duo of Cheryl Iglesia (@cheryliglesia) and Sheryl Kingsberg (@SherylKingsburg) #CherylandSheryl.
Cheryl Iglesia, MD
The new members of the Society of Gynecologic Surgeons were recognized, and there were several outstanding oral and video presentations throughout the morning. A range of topics were discussed, including vaginal surgery education, patient perspectives on adverse events, and postoperative pain management. In addition, Dr. Gary Dunnington (@GLDunnington), Chair of Surgery at Indiana University School of Medicine in Indianapolis, gave the keynote lecture on “Measuring and improving performance in surgical training,” reminding us to continually strive for change.
After a brief lunch and stroll around the exhibit hall, the afternoon session kicked off with a special guest lecture on vaginal rejuvenation and energy-based therapies for female genital cosmetic surgery by Cheryl Iglesia (@cheryliglesia). Next, a distinguished panel of experts from all gynecologic subspecialties gave their opinions on “Working together to shape the future of gynecologic surgery.” What a treat to see such important topics discussed by all the giants of our field sitting in one room: Society of Gynecologic Surgeons President Rajiv Gala, MD; ACOG President Elect Ted Anderson, MD; American Urogynecologic Society President Geoffrey W. Cundiff, MD; Society of Gynecologic Oncology President Elect Warner Huh, MD; Society of Reproductive Surgeons Immediate Past President Samantha Pfeifer, MD; and AAGL President Marie Fidela R. Paraiso, MD.
Supplemented by popcorn, the Videofest featured a series of informative and impressive videos—from management of removal of the Essure hysteroscopic contraceptive device to tips and tricks to navigate a pelvic kidney. The Fellows’ Pelvic Research Network (FPRN), a network of fellows from both minimally invasive gynecologic surgery and urogynecology programs that facilitates multicenter research, met and discussed ongoing and upcoming studies. Exciting work is coming your way thanks to the collaboration of the FPRN!
We concluded an excellent first day of general sessions with an awards ceremony and President’s reception. It was an evening filled with networking, catching up with old colleagues, and meeting new friends. I look forward to another day of scholarship and education tomorrow! Follow @lauraknewcomb, @GynSurgery, and @MDedgeObGyn on Twitter for updates.
Continue to: SUNDAY, 3/31/19. DAY 1 AT SGS...
SUNDAY, 3/31/19. DAY 1 AT SGS.
Hello from Tucson! I woke up to a beautiful Arizona sunrise, with cacti as far as the eye can see; a great start to what is surely going to be an educational scientific conference of the Society of Gynecologic Surgeons! Be sure to follow me on Twitter to stay in the loop real-time: @lauraknewcomb. And don’t forget to check out our conference hashtag #SGS2019.
Postgrad courses kick off
Quality improvement bootcamp
Dr. Bob Flora (@RFFlora) gave a great “Teach the Teacher” session, reviewing different methods for performing quality improvement projects in your own workspace, including the Institute for Healthcare Improvement (IHI) Model for Improvement (www.IHI.org). We also had the opportunity to learn and play with QI Macros (KnowWare International Inc) and Lucid Chart (Lucid Software Inc) programs—which are excellent tools to assist in quality improvement data analysis and presentation. Try them out if you have never used them before!
Sex and surgery
The sex and surgery postgraduate course was a lively discussion centering on:
- the links between gynecologic surgery and sexual function
- how to measure sexual function and incorporate discussion into our pre- and post-operative counseling
- how to approach the patient with postoperative sexual concerns.
As surgeons, we admitted that an anatomic approach with surgery will not always be successful in treating sexual complaints, as sexuality encompasses psychological, social/cultural, interpersonal, and biological aspects. We agreed that further studies are needed to examine the issue, using sexual function as a primary endpoint, because the concern is of critical importance to our patients.
Social media workshop
The talented SGS Social Media Committee, including influencers Dr. Mireille Truong (@MIS_MDT) and Dr. Elisa Jorgensen (@ejiorgensenmd) gave us the run-down on how to host a successful Twitter journal club and how to be a responsible and influential influencer on various social media avenues. They encouraged us to take advantage of the virtual space that connects so many more people than we could interact with without it!
Hands-on laparoscopic suturing simulation
This course was an excellent comprehensive laparoscopic suturing course. It began with a detailed outline of basic principles and slowly built on these concepts until we were performing laparoscopic myomectomies on a high-fidelity model. We can’t wait to implement these principles in the operating room next week! Thanks to the talented faculty who taught all the tips and tricks of the experts!
Conservative and definitive surgical strategies for fibroid management
Drs. Megan Wasson (@WassonMegan), Arnold Advincula (@arnieadvincula), and others taught all the nuances of managing fibroids and difficult surgical cases. Participants learned several tips, tricks, and techniques to use to manage fibroids—for example the “bow and arrow” and “push and tuck” techniques when performing a hysteroscopic myomectomy with a resectoscope.
Women’s leadership forum
During the evening women’s leadership forum, Drs. Catherine Matthews and Kimberly Kenton (@KimKenton1) highlighted the differences between mentorship and sponsorship. While most female physicians identify meaningful mentorship relationships, women lack sponsorship to advance their careers. Furthermore, more women-to-women sponsorship relationships are needed to improve and achieve gender equality.
Lastly, we all enjoyed the Arizona sunset with a welcome reception on the lawn. It was a great first day and we are all looking forward to an exciting general session on Monday! Stay tuned for more!

#SGS2019 attendees enjoying the welcome reception
WEDNESDAY, 4/3/2019. DAY 4 OF SGS.
Sadly, the annual Society of Gynecologic Surgeons meeting is wrapping up, and we will soon be leaving sunny Tucson! The last morning of conference proceedings was jam-packed with more outstanding oral and video presentations. We heard about topics such as the burden of postoperative catheterization, dietary patterns associated with postoperative defecatory symptoms, and more surgical tips and tricks to take back to our own institutions. At the end of the morning, the Distinguished Surgeon award was presented to the talented and deserving J. Marion Sims Endowed Chair of Obstetrics and Gynecology at UAB Medicine in Birmingham Dr. Holly E. Richter. The SGS Presidential Gavel was then passed from current SGS President Dr. Rajiv Gala to the incoming 46th President Dr. Peter Rosenblatt, Director of the Urogynecology and Pelvic Reconstructive Surgery Division at Mount Auburn Hospital in Cambridge, Massachusetts.
#SGS2019 was an amazingly successful conference! Beautiful surroundings, emerging science and education, and respectful inquiry was plentiful. I enjoyed all of the networking, reconnecting, and relaxing, and could not ask for a better community of GYN surgeons to have shared this with. I can’t wait to return to Pittsburgh to implement all the new things that I have learned. Thanks to the Society of Gynecologic Surgeons, OBG MANAGEMENT, and all the sponsors of the Fellows Scholar Program for supporting each of the scholars and this blog!
If you were at all intrigued by the happenings reported here, please consider attending the SGS meeting in 2020! The conference will be located in Jacksonville, Florida! See you there!
Thanks for following along! #SGS2019 out.
Continue to: TUESDAY, 4/2/19. DAY 3...
TUESDAY, 4/2/19. DAY 3.
The third day of the annual meeting of the Society of Gynecologic Surgeons started off with several academic roundtables hosted by experts in the field. The general session got underway with more fantastic oral and video presentations and, as usual, plenty of lively discussion and education ensued! The 45th SGS President Dr. Rajiv Gala (@rgala_nola) gave his presidential address, where he spoke so genuinely about how SGS is looking forward in our field. After all, the best way to predict your future is to create it! Be on the lookout on Twitter for Dr. Gala’s selfie with his “SGS Family” that he took during his address!
This year’s Telinde Lecture was given by Dr. Marcela G. del Carmen, titled “Health Care Disparities in Gynecologic Oncology Surgery.” She gave an informative and eye-opening lecture on the disparities that still exist in our field, specifically in patients with cancer. The morning session was rounded out with a mentoring panel, featuring Drs. B. Star Hampton, Bobby Shull, Peggy Norton, Tom Nolan, and Deborah Myers. Plenty of sage advice was offered. Thanks to Dr. Shull for reminding us to “be gracious; kindness never goes out of style,” and to be “a citizen of the world.”
Conference goers took the afternoon to enjoy leisure activities in the beautiful Arizona surroundings, including mountain biking, yoga, golf, and poolside lounging. The evening was filled with the excitement of the annual “SGS Got Talent” show! Fabulous performances and delicious food and drinks were just half of the fun, though. The life-size play on hungry, hungry hippos—“Hungry, Hungry Surgeons”—competition was the hit of the night!
Tomorrow is the last day of #SGS2019. Be sure to follow along for the final day of coverage!
Continue to: MONDAY, 4/1/19. DAY 2...
MONDAY, 4/1/19. DAY 2.
The first day of the general sessions started off with a cleverly titled breakfast symposium, “Postmenopausal sexuality: A bit dry but a must-have conversation,” by the brilliant and entertaining duo of Cheryl Iglesia (@cheryliglesia) and Sheryl Kingsberg (@SherylKingsburg) #CherylandSheryl.
Cheryl Iglesia, MD
The new members of the Society of Gynecologic Surgeons were recognized, and there were several outstanding oral and video presentations throughout the morning. A range of topics were discussed, including vaginal surgery education, patient perspectives on adverse events, and postoperative pain management. In addition, Dr. Gary Dunnington (@GLDunnington), Chair of Surgery at Indiana University School of Medicine in Indianapolis, gave the keynote lecture on “Measuring and improving performance in surgical training,” reminding us to continually strive for change.
After a brief lunch and stroll around the exhibit hall, the afternoon session kicked off with a special guest lecture on vaginal rejuvenation and energy-based therapies for female genital cosmetic surgery by Cheryl Iglesia (@cheryliglesia). Next, a distinguished panel of experts from all gynecologic subspecialties gave their opinions on “Working together to shape the future of gynecologic surgery.” What a treat to see such important topics discussed by all the giants of our field sitting in one room: Society of Gynecologic Surgeons President Rajiv Gala, MD; ACOG President Elect Ted Anderson, MD; American Urogynecologic Society President Geoffrey W. Cundiff, MD; Society of Gynecologic Oncology President Elect Warner Huh, MD; Society of Reproductive Surgeons Immediate Past President Samantha Pfeifer, MD; and AAGL President Marie Fidela R. Paraiso, MD.
Supplemented by popcorn, the Videofest featured a series of informative and impressive videos—from management of removal of the Essure hysteroscopic contraceptive device to tips and tricks to navigate a pelvic kidney. The Fellows’ Pelvic Research Network (FPRN), a network of fellows from both minimally invasive gynecologic surgery and urogynecology programs that facilitates multicenter research, met and discussed ongoing and upcoming studies. Exciting work is coming your way thanks to the collaboration of the FPRN!
We concluded an excellent first day of general sessions with an awards ceremony and President’s reception. It was an evening filled with networking, catching up with old colleagues, and meeting new friends. I look forward to another day of scholarship and education tomorrow! Follow @lauraknewcomb, @GynSurgery, and @MDedgeObGyn on Twitter for updates.
Continue to: SUNDAY, 3/31/19. DAY 1 AT SGS...
SUNDAY, 3/31/19. DAY 1 AT SGS.
Hello from Tucson! I woke up to a beautiful Arizona sunrise, with cacti as far as the eye can see; a great start to what is surely going to be an educational scientific conference of the Society of Gynecologic Surgeons! Be sure to follow me on Twitter to stay in the loop real-time: @lauraknewcomb. And don’t forget to check out our conference hashtag #SGS2019.
Postgrad courses kick off
Quality improvement bootcamp
Dr. Bob Flora (@RFFlora) gave a great “Teach the Teacher” session, reviewing different methods for performing quality improvement projects in your own workspace, including the Institute for Healthcare Improvement (IHI) Model for Improvement (www.IHI.org). We also had the opportunity to learn and play with QI Macros (KnowWare International Inc) and Lucid Chart (Lucid Software Inc) programs—which are excellent tools to assist in quality improvement data analysis and presentation. Try them out if you have never used them before!
Sex and surgery
The sex and surgery postgraduate course was a lively discussion centering on:
- the links between gynecologic surgery and sexual function
- how to measure sexual function and incorporate discussion into our pre- and post-operative counseling
- how to approach the patient with postoperative sexual concerns.
As surgeons, we admitted that an anatomic approach with surgery will not always be successful in treating sexual complaints, as sexuality encompasses psychological, social/cultural, interpersonal, and biological aspects. We agreed that further studies are needed to examine the issue, using sexual function as a primary endpoint, because the concern is of critical importance to our patients.
Social media workshop
The talented SGS Social Media Committee, including influencers Dr. Mireille Truong (@MIS_MDT) and Dr. Elisa Jorgensen (@ejiorgensenmd) gave us the run-down on how to host a successful Twitter journal club and how to be a responsible and influential influencer on various social media avenues. They encouraged us to take advantage of the virtual space that connects so many more people than we could interact with without it!
Hands-on laparoscopic suturing simulation
This course was an excellent comprehensive laparoscopic suturing course. It began with a detailed outline of basic principles and slowly built on these concepts until we were performing laparoscopic myomectomies on a high-fidelity model. We can’t wait to implement these principles in the operating room next week! Thanks to the talented faculty who taught all the tips and tricks of the experts!
Conservative and definitive surgical strategies for fibroid management
Drs. Megan Wasson (@WassonMegan), Arnold Advincula (@arnieadvincula), and others taught all the nuances of managing fibroids and difficult surgical cases. Participants learned several tips, tricks, and techniques to use to manage fibroids—for example the “bow and arrow” and “push and tuck” techniques when performing a hysteroscopic myomectomy with a resectoscope.
Women’s leadership forum
During the evening women’s leadership forum, Drs. Catherine Matthews and Kimberly Kenton (@KimKenton1) highlighted the differences between mentorship and sponsorship. While most female physicians identify meaningful mentorship relationships, women lack sponsorship to advance their careers. Furthermore, more women-to-women sponsorship relationships are needed to improve and achieve gender equality.
Lastly, we all enjoyed the Arizona sunset with a welcome reception on the lawn. It was a great first day and we are all looking forward to an exciting general session on Monday! Stay tuned for more!

#SGS2019 attendees enjoying the welcome reception