User login
Is oral or IV iron therapy more beneficial for postpartum anemia?
EXPERT COMMENTARY
Sultan P, Bampoe S, Shah R, et al. Oral versus intravenous iron therapy for postpartum anemia: a systematic review and meta-analysis. Am J Obstet Gynecol. Published online December 19, 2018. DOI:10.1016/j.ajog.2018.12.016.
Iron deficiency anemia in pregnancy is associated with increased risk for adverse birth outcomes, including preterm delivery, cesarean delivery, and need for blood transfusion.1,2 Although the outcomes with postpartum iron deficiency anemia are more difficult to study, this condition is associated with increased risk of maternal fatigue and depression, and it is often overlooked as a significant issue during the postpartum period.
In a recent systematic review, Sultan and colleagues sought to provide an updated assessment of IV versus oral iron treatment for postpartum anemia. The 6-week postpartum hemoglobin concentration was the primary outcome.
Details of the study
The authors screened 2,744 articles for randomized controlled trials (RCTs) comparing oral and IV iron in the treatment of postpartum anemia. Fifteen RCTs were included in the review, with 1,001 women receiving oral iron therapy and 1,181 women receiving IV iron. The baseline postpartum hemoglobin concentration in the 15 studies ranged from less than 8 g/dL to 10.5 g/dL.
In all but 1 study, the women in the IV treatment arm experienced a significant increase in postpartum hemoglobin concentration, with the mean difference being 1.0 g/dL at postpartum week 1 (95% confidence interval [CI], 0.5–1.5; P<.0001) and 0.9 g/dL at postpartum week 6 (95% CI, 0.4–1.3; P = .0003).
Only 4 studies were included in the meta-analysis; specifically, 6-week postpartum hemoglobin levels were measured in 251 women who received IV iron and in 134 who received oral iron. Significant differences were seen in the IV iron group compared with the oral iron group for 3 of the secondary outcomes evaluated: flushing (odds ratio [OR], 6.95), decreased constipation (OR, 0.08), and decreased dyspepsia (OR, 0.07).
None of the other secondary outcomes associated with IV iron (muscle cramps, headache, urticaria, rash, or anaphylaxis) occurred at statistically significant rates. Notably, adherence was not assessed in the majority of the studies. Although constipation was increased in the oral iron therapy group, it was reported at only 12%.
Study strengths and weaknesses
Results of this study support previous findings that IV iron is better tolerated, with fewer gastrointestinal adverse effects, than oral iron, and they re-emphasize that IV iron therapy is both safe (the authors identified only 2 cases of anaphylaxis) and effective in improving hematologic indices.
Continue to: The systematic review included...
The systematic review included studies, however, that excluded women treated for antepartum anemia, a group that may benefit from aggressive correction of iron deficiency. Another study weakness is that all the oral iron regimens used were dosed either daily or multiple times per day, which may lead to difficulty with adherence and can decrease overall iron absorption compared with an every-other-day regimen.3
Future studies are needed to determine 1) which women with what level of anemia will benefit the most from postpartum IV iron and 2) the hemoglobin level at which IV iron is a cost-effective therapy.
Given the efficacy and reduced adverse effects associated with IV iron therapy demonstrated in the systematic review by Sultan and colleagues, I recommend treatment with IV iron for women with moderate to severe postpartum anemia (defined in pregnancy as a hemoglobin level less than 10 g/dL and ferritin less than 40 µg/L) who have not received blood products or for women who are unable to tolerate or absorb oral iron (such as those with a history of bariatric surgery, gastritis, or inflammatory bowel disease). In our institution, we frequently give IV iron sucrose 300 mg prior to discharge due to ease of administration. For women with mild iron deficiency anemia (hemoglobin greater than 10 g/dL), I prescribe every-other-day oral iron in the form of ferrous sulfate 325 mg, which effectively raises the hemoglobin level and limits the gastrointestinal side effects associated with more frequent dosing.
Julianna Schantz-Dunn, MD, MPH
- Drukker L, Hants Y, Farkash R, et al. Iron deficiency anemia at admission for labor and delivery is associated with an increased risk for Cesarean section and adverse maternal and neonatal outcomes. Transfusion. 2015;55:2799-2806.
- Rahman MM, Abe SK, Rahman MS, et al. Maternal anemia and risk of adverse birth and health outcomes in low- and middle-income countries: systematic review and meta-analysis. Am J Clin Nutr. 2016;103:495-504.
- Stoffel NU, Cercamondi CI, Brittenham G, et al. Iron absorption from oral iron supplements given on consecutive versus alternate days and as single morning doses versus twice-daily split dosing in iron-depleted women: two open-label, randomised controlled trials. Lancet Haematol. 2017;4:e524-e533.
EXPERT COMMENTARY
Sultan P, Bampoe S, Shah R, et al. Oral versus intravenous iron therapy for postpartum anemia: a systematic review and meta-analysis. Am J Obstet Gynecol. Published online December 19, 2018. DOI:10.1016/j.ajog.2018.12.016.
Iron deficiency anemia in pregnancy is associated with increased risk for adverse birth outcomes, including preterm delivery, cesarean delivery, and need for blood transfusion.1,2 Although the outcomes with postpartum iron deficiency anemia are more difficult to study, this condition is associated with increased risk of maternal fatigue and depression, and it is often overlooked as a significant issue during the postpartum period.
In a recent systematic review, Sultan and colleagues sought to provide an updated assessment of IV versus oral iron treatment for postpartum anemia. The 6-week postpartum hemoglobin concentration was the primary outcome.
Details of the study
The authors screened 2,744 articles for randomized controlled trials (RCTs) comparing oral and IV iron in the treatment of postpartum anemia. Fifteen RCTs were included in the review, with 1,001 women receiving oral iron therapy and 1,181 women receiving IV iron. The baseline postpartum hemoglobin concentration in the 15 studies ranged from less than 8 g/dL to 10.5 g/dL.
In all but 1 study, the women in the IV treatment arm experienced a significant increase in postpartum hemoglobin concentration, with the mean difference being 1.0 g/dL at postpartum week 1 (95% confidence interval [CI], 0.5–1.5; P<.0001) and 0.9 g/dL at postpartum week 6 (95% CI, 0.4–1.3; P = .0003).
Only 4 studies were included in the meta-analysis; specifically, 6-week postpartum hemoglobin levels were measured in 251 women who received IV iron and in 134 who received oral iron. Significant differences were seen in the IV iron group compared with the oral iron group for 3 of the secondary outcomes evaluated: flushing (odds ratio [OR], 6.95), decreased constipation (OR, 0.08), and decreased dyspepsia (OR, 0.07).
None of the other secondary outcomes associated with IV iron (muscle cramps, headache, urticaria, rash, or anaphylaxis) occurred at statistically significant rates. Notably, adherence was not assessed in the majority of the studies. Although constipation was increased in the oral iron therapy group, it was reported at only 12%.
Study strengths and weaknesses
Results of this study support previous findings that IV iron is better tolerated, with fewer gastrointestinal adverse effects, than oral iron, and they re-emphasize that IV iron therapy is both safe (the authors identified only 2 cases of anaphylaxis) and effective in improving hematologic indices.
Continue to: The systematic review included...
The systematic review included studies, however, that excluded women treated for antepartum anemia, a group that may benefit from aggressive correction of iron deficiency. Another study weakness is that all the oral iron regimens used were dosed either daily or multiple times per day, which may lead to difficulty with adherence and can decrease overall iron absorption compared with an every-other-day regimen.3
Future studies are needed to determine 1) which women with what level of anemia will benefit the most from postpartum IV iron and 2) the hemoglobin level at which IV iron is a cost-effective therapy.
Given the efficacy and reduced adverse effects associated with IV iron therapy demonstrated in the systematic review by Sultan and colleagues, I recommend treatment with IV iron for women with moderate to severe postpartum anemia (defined in pregnancy as a hemoglobin level less than 10 g/dL and ferritin less than 40 µg/L) who have not received blood products or for women who are unable to tolerate or absorb oral iron (such as those with a history of bariatric surgery, gastritis, or inflammatory bowel disease). In our institution, we frequently give IV iron sucrose 300 mg prior to discharge due to ease of administration. For women with mild iron deficiency anemia (hemoglobin greater than 10 g/dL), I prescribe every-other-day oral iron in the form of ferrous sulfate 325 mg, which effectively raises the hemoglobin level and limits the gastrointestinal side effects associated with more frequent dosing.
Julianna Schantz-Dunn, MD, MPH
EXPERT COMMENTARY
Sultan P, Bampoe S, Shah R, et al. Oral versus intravenous iron therapy for postpartum anemia: a systematic review and meta-analysis. Am J Obstet Gynecol. Published online December 19, 2018. DOI:10.1016/j.ajog.2018.12.016.
Iron deficiency anemia in pregnancy is associated with increased risk for adverse birth outcomes, including preterm delivery, cesarean delivery, and need for blood transfusion.1,2 Although the outcomes with postpartum iron deficiency anemia are more difficult to study, this condition is associated with increased risk of maternal fatigue and depression, and it is often overlooked as a significant issue during the postpartum period.
In a recent systematic review, Sultan and colleagues sought to provide an updated assessment of IV versus oral iron treatment for postpartum anemia. The 6-week postpartum hemoglobin concentration was the primary outcome.
Details of the study
The authors screened 2,744 articles for randomized controlled trials (RCTs) comparing oral and IV iron in the treatment of postpartum anemia. Fifteen RCTs were included in the review, with 1,001 women receiving oral iron therapy and 1,181 women receiving IV iron. The baseline postpartum hemoglobin concentration in the 15 studies ranged from less than 8 g/dL to 10.5 g/dL.
In all but 1 study, the women in the IV treatment arm experienced a significant increase in postpartum hemoglobin concentration, with the mean difference being 1.0 g/dL at postpartum week 1 (95% confidence interval [CI], 0.5–1.5; P<.0001) and 0.9 g/dL at postpartum week 6 (95% CI, 0.4–1.3; P = .0003).
Only 4 studies were included in the meta-analysis; specifically, 6-week postpartum hemoglobin levels were measured in 251 women who received IV iron and in 134 who received oral iron. Significant differences were seen in the IV iron group compared with the oral iron group for 3 of the secondary outcomes evaluated: flushing (odds ratio [OR], 6.95), decreased constipation (OR, 0.08), and decreased dyspepsia (OR, 0.07).
None of the other secondary outcomes associated with IV iron (muscle cramps, headache, urticaria, rash, or anaphylaxis) occurred at statistically significant rates. Notably, adherence was not assessed in the majority of the studies. Although constipation was increased in the oral iron therapy group, it was reported at only 12%.
Study strengths and weaknesses
Results of this study support previous findings that IV iron is better tolerated, with fewer gastrointestinal adverse effects, than oral iron, and they re-emphasize that IV iron therapy is both safe (the authors identified only 2 cases of anaphylaxis) and effective in improving hematologic indices.
Continue to: The systematic review included...
The systematic review included studies, however, that excluded women treated for antepartum anemia, a group that may benefit from aggressive correction of iron deficiency. Another study weakness is that all the oral iron regimens used were dosed either daily or multiple times per day, which may lead to difficulty with adherence and can decrease overall iron absorption compared with an every-other-day regimen.3
Future studies are needed to determine 1) which women with what level of anemia will benefit the most from postpartum IV iron and 2) the hemoglobin level at which IV iron is a cost-effective therapy.
Given the efficacy and reduced adverse effects associated with IV iron therapy demonstrated in the systematic review by Sultan and colleagues, I recommend treatment with IV iron for women with moderate to severe postpartum anemia (defined in pregnancy as a hemoglobin level less than 10 g/dL and ferritin less than 40 µg/L) who have not received blood products or for women who are unable to tolerate or absorb oral iron (such as those with a history of bariatric surgery, gastritis, or inflammatory bowel disease). In our institution, we frequently give IV iron sucrose 300 mg prior to discharge due to ease of administration. For women with mild iron deficiency anemia (hemoglobin greater than 10 g/dL), I prescribe every-other-day oral iron in the form of ferrous sulfate 325 mg, which effectively raises the hemoglobin level and limits the gastrointestinal side effects associated with more frequent dosing.
Julianna Schantz-Dunn, MD, MPH
- Drukker L, Hants Y, Farkash R, et al. Iron deficiency anemia at admission for labor and delivery is associated with an increased risk for Cesarean section and adverse maternal and neonatal outcomes. Transfusion. 2015;55:2799-2806.
- Rahman MM, Abe SK, Rahman MS, et al. Maternal anemia and risk of adverse birth and health outcomes in low- and middle-income countries: systematic review and meta-analysis. Am J Clin Nutr. 2016;103:495-504.
- Stoffel NU, Cercamondi CI, Brittenham G, et al. Iron absorption from oral iron supplements given on consecutive versus alternate days and as single morning doses versus twice-daily split dosing in iron-depleted women: two open-label, randomised controlled trials. Lancet Haematol. 2017;4:e524-e533.
- Drukker L, Hants Y, Farkash R, et al. Iron deficiency anemia at admission for labor and delivery is associated with an increased risk for Cesarean section and adverse maternal and neonatal outcomes. Transfusion. 2015;55:2799-2806.
- Rahman MM, Abe SK, Rahman MS, et al. Maternal anemia and risk of adverse birth and health outcomes in low- and middle-income countries: systematic review and meta-analysis. Am J Clin Nutr. 2016;103:495-504.
- Stoffel NU, Cercamondi CI, Brittenham G, et al. Iron absorption from oral iron supplements given on consecutive versus alternate days and as single morning doses versus twice-daily split dosing in iron-depleted women: two open-label, randomised controlled trials. Lancet Haematol. 2017;4:e524-e533.
Is vaginal estrogen used for GSM associated with a higher risk of CVD or cancer?
Expert Commentary
Bhupathiraju SN, Grodstein F, Stampfer MJ, et al. Vaginal estrogen use and chronic disease risk in the Nurses’ Health Study. Menopause. December 17, 2018. doi: 10.1097/GME.0000000000001284.
GSM, a chronic and often progressive condition, occurs in almost 50% of postmenopausal women and has been shown to impair sexual function and quality of life.1 Symptoms include vaginal dryness, vulvar or vaginal itching, dyspareunia, urinary urgency or frequency, and increased urinary tract infections. Although lubricants or vaginal moisturizers may be sufficient to treat GSM, targeted hormonal therapy may be needed to improve the symptoms and resolve the underlying cause, due to vaginal hormone loss.
Despite lack of any observational or clinical trial evidence for chronic health disease risks related to low-dose vaginal estrogen use, there remains an US Food and Drug Administration boxed warning on the package label for low-dose vaginal estrogen related to risks of heart disease, stroke, venous thromboembolism, pdementia, and breast cancer. The objective of the investigation by Bhupathiraju and colleagues was to evaluate associations between vaginal estrogen use and health outcomes, including CVD (myocardial infarction, stroke, and pulmonary embolism/deep vein thrombosis), cancer (total invasive, breast, endometrial, ovarian, and colorectal), and hip fracture.
Details of the study
The prospective analysis included 896 postmenopausal current users of vaginal estrogen in the Nurses’ Health Study (NHS; 1982–2012), compared with 52,901 nonusers. Eighteen years of follow-up was evaluated. Users of systemic hormone therapy were excluded from the analysis. For the NHS, self-reported data were collected every 2 years on questionnaires for vaginal estrogen use and health outcomes. Investigators used medical records to confirm health outcomes.
After adjusting for covariates, no significant differences in risks were found for CVD, cancer, and hip fracture between users and nonusers of vaginal estrogen, regardless of hysterectomy status.
Key findings
After adjusting for multiple variables (including age, race, physical activity, age at menopause, hysterectomy, aspirin use, parental history of cancer, etc), health outcomes for CVDs, all cancers, and hip fracture were:
- myocardial infarction: hazard ratio (HR), 0.73 (95% confidence interval [CI], 0.47–1.13)
- stroke: HR, 0.85 (95% CI, 0.56–1.29)
- pulmonary embolism/deep vein thrombosis: HR, 1.06 (95% CI, 0.58–1.93)
- hip fracture: HR, 0.91 (95% CI, 0.60–1.38)
- all cancers: HR, 1.05 (95% CI, 0.89–1.25).
Continue to: Health outcomes for specific invasive cancers
Health outcomes for specific invasive cancers (risk for endometrial cancer included only women with an intact uterus) were:
- invasive breast cancer: HR, 1.07 (95% CI, 0.78–1.47)
- ovarian cancer: HR, 1.17 (95% CI, 0.52–2.65)
- endometrial cancer: HR, 1.62 (95% CI, 0.88–2.97)
- colorectal cancer: HR, 0.77 (95% CI, 0.45–1.34).
Study strengths and weaknesses
A causal relationship cannot be proven as the study was observational. However, a strength included the 18 years of follow-up. Women used vaginal estrogen for an average of 3 years, which provided longer-term safety data than available 12-month clinical trial data. Data were collected through self-report on questionnaires every 2 years, which is a drawback; however, participants were registered nurses, who have been shown to provide reliable health-related information. Comparisons between therapies were not possible as data were not collected about type or dosage of vaginal estrogen. Available therapies during the NHS included vaginal estrogen tablets, creams, and an estradiol ring, with higher doses available during earlier parts of the study than the lower doses commonly prescribed in current day.
Overall
The findings from this long-term follow-up of the NHS provide support for the safety of vaginal estrogen for treatment of GSM. No statistically significant increased health risks were found for users of vaginal estrogen, similar to earlier reported findings from the large Women’s Health Initiative.2 Low-dose vaginal estrogen is recommended for treatment of GSM by The North American Menopause Society, the American College of Obstetricians and Gynecologists, and the Endocrine Society.
Absorption of low-dose vaginal estrogen preparations appears minimal, and they are effective and generally safe for the treatment of GSM for women at any age. Progesterone is not recommended with low-dose vaginal estrogen therapies, based primarily on randomized clinical trial safety data of 12 months.3 Postmenopausal bleeding, however, needs to be thoroughly evaluated. For women with breast cancer, include the oncologist in decision making about the use of low-dose vaginal estrogen.
Despite the boxed warning on vaginal estrogen, the findings from this study support the safety of vaginal estrogen use for effective relief of GSM in women with and without a uterus.
JOANN V. PINKERTON, MD, NCMP
- Gandhi J, Chen A, Dagur G, et al. Genitourinary syndrome of menopause: an overview of clinical manifestations, pathophysiology, etiology, evaluation, and management. Am J Obstet Gynecol. 2016;251:704-711.
- Crandall CJ, Hovey KM, Andrews CA, et al. Breast cancer, endometrial cancer, and cardiovascular events in participants who used vaginal estrogen in the Women's Health Initiative Observational Study. Menopause. 2018;25:11-20.
- The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24:728-753.
Expert Commentary
Bhupathiraju SN, Grodstein F, Stampfer MJ, et al. Vaginal estrogen use and chronic disease risk in the Nurses’ Health Study. Menopause. December 17, 2018. doi: 10.1097/GME.0000000000001284.
GSM, a chronic and often progressive condition, occurs in almost 50% of postmenopausal women and has been shown to impair sexual function and quality of life.1 Symptoms include vaginal dryness, vulvar or vaginal itching, dyspareunia, urinary urgency or frequency, and increased urinary tract infections. Although lubricants or vaginal moisturizers may be sufficient to treat GSM, targeted hormonal therapy may be needed to improve the symptoms and resolve the underlying cause, due to vaginal hormone loss.
Despite lack of any observational or clinical trial evidence for chronic health disease risks related to low-dose vaginal estrogen use, there remains an US Food and Drug Administration boxed warning on the package label for low-dose vaginal estrogen related to risks of heart disease, stroke, venous thromboembolism, pdementia, and breast cancer. The objective of the investigation by Bhupathiraju and colleagues was to evaluate associations between vaginal estrogen use and health outcomes, including CVD (myocardial infarction, stroke, and pulmonary embolism/deep vein thrombosis), cancer (total invasive, breast, endometrial, ovarian, and colorectal), and hip fracture.
Details of the study
The prospective analysis included 896 postmenopausal current users of vaginal estrogen in the Nurses’ Health Study (NHS; 1982–2012), compared with 52,901 nonusers. Eighteen years of follow-up was evaluated. Users of systemic hormone therapy were excluded from the analysis. For the NHS, self-reported data were collected every 2 years on questionnaires for vaginal estrogen use and health outcomes. Investigators used medical records to confirm health outcomes.
After adjusting for covariates, no significant differences in risks were found for CVD, cancer, and hip fracture between users and nonusers of vaginal estrogen, regardless of hysterectomy status.
Key findings
After adjusting for multiple variables (including age, race, physical activity, age at menopause, hysterectomy, aspirin use, parental history of cancer, etc), health outcomes for CVDs, all cancers, and hip fracture were:
- myocardial infarction: hazard ratio (HR), 0.73 (95% confidence interval [CI], 0.47–1.13)
- stroke: HR, 0.85 (95% CI, 0.56–1.29)
- pulmonary embolism/deep vein thrombosis: HR, 1.06 (95% CI, 0.58–1.93)
- hip fracture: HR, 0.91 (95% CI, 0.60–1.38)
- all cancers: HR, 1.05 (95% CI, 0.89–1.25).
Continue to: Health outcomes for specific invasive cancers
Health outcomes for specific invasive cancers (risk for endometrial cancer included only women with an intact uterus) were:
- invasive breast cancer: HR, 1.07 (95% CI, 0.78–1.47)
- ovarian cancer: HR, 1.17 (95% CI, 0.52–2.65)
- endometrial cancer: HR, 1.62 (95% CI, 0.88–2.97)
- colorectal cancer: HR, 0.77 (95% CI, 0.45–1.34).
Study strengths and weaknesses
A causal relationship cannot be proven as the study was observational. However, a strength included the 18 years of follow-up. Women used vaginal estrogen for an average of 3 years, which provided longer-term safety data than available 12-month clinical trial data. Data were collected through self-report on questionnaires every 2 years, which is a drawback; however, participants were registered nurses, who have been shown to provide reliable health-related information. Comparisons between therapies were not possible as data were not collected about type or dosage of vaginal estrogen. Available therapies during the NHS included vaginal estrogen tablets, creams, and an estradiol ring, with higher doses available during earlier parts of the study than the lower doses commonly prescribed in current day.
Overall
The findings from this long-term follow-up of the NHS provide support for the safety of vaginal estrogen for treatment of GSM. No statistically significant increased health risks were found for users of vaginal estrogen, similar to earlier reported findings from the large Women’s Health Initiative.2 Low-dose vaginal estrogen is recommended for treatment of GSM by The North American Menopause Society, the American College of Obstetricians and Gynecologists, and the Endocrine Society.
Absorption of low-dose vaginal estrogen preparations appears minimal, and they are effective and generally safe for the treatment of GSM for women at any age. Progesterone is not recommended with low-dose vaginal estrogen therapies, based primarily on randomized clinical trial safety data of 12 months.3 Postmenopausal bleeding, however, needs to be thoroughly evaluated. For women with breast cancer, include the oncologist in decision making about the use of low-dose vaginal estrogen.
Despite the boxed warning on vaginal estrogen, the findings from this study support the safety of vaginal estrogen use for effective relief of GSM in women with and without a uterus.
JOANN V. PINKERTON, MD, NCMP
Expert Commentary
Bhupathiraju SN, Grodstein F, Stampfer MJ, et al. Vaginal estrogen use and chronic disease risk in the Nurses’ Health Study. Menopause. December 17, 2018. doi: 10.1097/GME.0000000000001284.
GSM, a chronic and often progressive condition, occurs in almost 50% of postmenopausal women and has been shown to impair sexual function and quality of life.1 Symptoms include vaginal dryness, vulvar or vaginal itching, dyspareunia, urinary urgency or frequency, and increased urinary tract infections. Although lubricants or vaginal moisturizers may be sufficient to treat GSM, targeted hormonal therapy may be needed to improve the symptoms and resolve the underlying cause, due to vaginal hormone loss.
Despite lack of any observational or clinical trial evidence for chronic health disease risks related to low-dose vaginal estrogen use, there remains an US Food and Drug Administration boxed warning on the package label for low-dose vaginal estrogen related to risks of heart disease, stroke, venous thromboembolism, pdementia, and breast cancer. The objective of the investigation by Bhupathiraju and colleagues was to evaluate associations between vaginal estrogen use and health outcomes, including CVD (myocardial infarction, stroke, and pulmonary embolism/deep vein thrombosis), cancer (total invasive, breast, endometrial, ovarian, and colorectal), and hip fracture.
Details of the study
The prospective analysis included 896 postmenopausal current users of vaginal estrogen in the Nurses’ Health Study (NHS; 1982–2012), compared with 52,901 nonusers. Eighteen years of follow-up was evaluated. Users of systemic hormone therapy were excluded from the analysis. For the NHS, self-reported data were collected every 2 years on questionnaires for vaginal estrogen use and health outcomes. Investigators used medical records to confirm health outcomes.
After adjusting for covariates, no significant differences in risks were found for CVD, cancer, and hip fracture between users and nonusers of vaginal estrogen, regardless of hysterectomy status.
Key findings
After adjusting for multiple variables (including age, race, physical activity, age at menopause, hysterectomy, aspirin use, parental history of cancer, etc), health outcomes for CVDs, all cancers, and hip fracture were:
- myocardial infarction: hazard ratio (HR), 0.73 (95% confidence interval [CI], 0.47–1.13)
- stroke: HR, 0.85 (95% CI, 0.56–1.29)
- pulmonary embolism/deep vein thrombosis: HR, 1.06 (95% CI, 0.58–1.93)
- hip fracture: HR, 0.91 (95% CI, 0.60–1.38)
- all cancers: HR, 1.05 (95% CI, 0.89–1.25).
Continue to: Health outcomes for specific invasive cancers
Health outcomes for specific invasive cancers (risk for endometrial cancer included only women with an intact uterus) were:
- invasive breast cancer: HR, 1.07 (95% CI, 0.78–1.47)
- ovarian cancer: HR, 1.17 (95% CI, 0.52–2.65)
- endometrial cancer: HR, 1.62 (95% CI, 0.88–2.97)
- colorectal cancer: HR, 0.77 (95% CI, 0.45–1.34).
Study strengths and weaknesses
A causal relationship cannot be proven as the study was observational. However, a strength included the 18 years of follow-up. Women used vaginal estrogen for an average of 3 years, which provided longer-term safety data than available 12-month clinical trial data. Data were collected through self-report on questionnaires every 2 years, which is a drawback; however, participants were registered nurses, who have been shown to provide reliable health-related information. Comparisons between therapies were not possible as data were not collected about type or dosage of vaginal estrogen. Available therapies during the NHS included vaginal estrogen tablets, creams, and an estradiol ring, with higher doses available during earlier parts of the study than the lower doses commonly prescribed in current day.
Overall
The findings from this long-term follow-up of the NHS provide support for the safety of vaginal estrogen for treatment of GSM. No statistically significant increased health risks were found for users of vaginal estrogen, similar to earlier reported findings from the large Women’s Health Initiative.2 Low-dose vaginal estrogen is recommended for treatment of GSM by The North American Menopause Society, the American College of Obstetricians and Gynecologists, and the Endocrine Society.
Absorption of low-dose vaginal estrogen preparations appears minimal, and they are effective and generally safe for the treatment of GSM for women at any age. Progesterone is not recommended with low-dose vaginal estrogen therapies, based primarily on randomized clinical trial safety data of 12 months.3 Postmenopausal bleeding, however, needs to be thoroughly evaluated. For women with breast cancer, include the oncologist in decision making about the use of low-dose vaginal estrogen.
Despite the boxed warning on vaginal estrogen, the findings from this study support the safety of vaginal estrogen use for effective relief of GSM in women with and without a uterus.
JOANN V. PINKERTON, MD, NCMP
- Gandhi J, Chen A, Dagur G, et al. Genitourinary syndrome of menopause: an overview of clinical manifestations, pathophysiology, etiology, evaluation, and management. Am J Obstet Gynecol. 2016;251:704-711.
- Crandall CJ, Hovey KM, Andrews CA, et al. Breast cancer, endometrial cancer, and cardiovascular events in participants who used vaginal estrogen in the Women's Health Initiative Observational Study. Menopause. 2018;25:11-20.
- The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24:728-753.
- Gandhi J, Chen A, Dagur G, et al. Genitourinary syndrome of menopause: an overview of clinical manifestations, pathophysiology, etiology, evaluation, and management. Am J Obstet Gynecol. 2016;251:704-711.
- Crandall CJ, Hovey KM, Andrews CA, et al. Breast cancer, endometrial cancer, and cardiovascular events in participants who used vaginal estrogen in the Women's Health Initiative Observational Study. Menopause. 2018;25:11-20.
- The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24:728-753.
Does the type of menopausal HT used increase the risk of venous thromboembolism?
EXPERT COMMENTARY
Vinogradova Y, Coupland C, Hippisley-Cox J. Use of hormone replacement therapy and risk of venous thromboembolism: nested case-control studies using the QResearch and CPRD databases. BMJ. 2019;364:k4810.
The Women’s Health Initiative trials, in which menopausal women were randomly assigned to treatment with oral CEE or placebo, found that statistically the largest risk associated with menopausal hormone therapy (HT) was increased VTE.1 Recently, investigators in the United Kingdom (UK) published results of their research aimed at determining the association between the risk of VTE and the use of different types of HT.2
Details of the study
Vinogradova and colleagues used 2 UK primary care research databases, QResearch and Clinical Practice Research Datalink, to identify cases of incident VTE in general practice records, hospital admissions, and mortality records. They identified 80,396 women (aged 40 to 79 years) diagnosed with VTE between 1998 and 2017 and 391,494 control women matched by age and general practice. The mean age of the case and control women was approximately 64 years; the great majority of women were white. Analyses were adjusted for smoking, body mass index (BMI), family history of VTE, and comorbidities associated with VTE.
Types of HT used. The investigators found that 5,795 (7.2%) women with VTE and 21,670 (5.5%) controls were exposed to HT in the 90 days before the index date (the first date of VTE diagnosis for cases became the index date for matched controls). In those exposed to HT:
- 4,915 (85%) cases and 16,938 (78%) controls used oral preparations (including 102 [1.8%] cases and 312 [1.4%] controls who also had transdermal preparations)
- 880 (14%) cases and 4,731 (19%) controls used transdermal HT only.
Association of VTE with HT. Risk of VTE was increased with all oral HT formulations, including combined (estrogen plus progestogen) and estrogen-only preparations. Use of oral CEE (odds ratio [OR], 1.49) and estradiol (OR, 1.27) were both associated with an elevated risk of VTE (P<.05 for both comparisons). In contrast, use of transdermal estradiol (the great majority of which was administered by patch) was not associated with an elevated risk of VTE (OR, 0.96).
Direct comparison of oral estradiol and CEE found that the lower VTE risk with oral estradiol achieved statistical significance (P = .005). Direct comparison of oral and transdermal estrogen revealed an OR of 1.7 for the oral route of administration (P<.001)
Continue to: Study strengths and weaknesses
Study strengths and weaknesses
This study used data from the 2 largest primary care databases in the United Kingdom. Analyses were adjusted for numerous confounding factors, including acute and chronic conditions, lifestyle factors, and social deprivation. Additional sensitivity analyses were conducted and yielded results similar to those of the main analysis.
Several limitations could have resulted in some residual confounding bias. For example, drug exposure information was based on HT prescriptions and not actual use; data on some factors were not available, such as indications for HT, age at menopause, and education level; and for a small proportion of women, some data (smoking status, alcohol consumption, BMI) were missing and had to be imputed for analysis.
Although randomized trials have not compared VTE risk with oral versus transdermal estrogen, prior observational studies have consistently suggested that transdermal estrogen does not elevate VTE risk; this is consistent with the results from this large UK study. In my practice, congruent with the authors’ suggestions, I recommend transdermal rather than oral estrogen for patients (notably, those who are obese) who at baseline have risk factors for VTE. For menopausal women for whom use of oral estrogen is indicated, I recommend estradiol rather than CEE, since estradiol is less expensive and, based on this study’s results, may be safer than CEE.
ANDREW M. KAUNITZ, MD
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women's Health Initiative randomized trials. JAMA. 2013;310:1353-1368.
- Vinogradova Y, Coupland C, Hippisley-Cox J. Use of hormone replacement therapy and risk of venous thromboembolism: nested case-control studies using the QResearch and CPRD databases. BMJ. 2019;364:k4810.
EXPERT COMMENTARY
Vinogradova Y, Coupland C, Hippisley-Cox J. Use of hormone replacement therapy and risk of venous thromboembolism: nested case-control studies using the QResearch and CPRD databases. BMJ. 2019;364:k4810.
The Women’s Health Initiative trials, in which menopausal women were randomly assigned to treatment with oral CEE or placebo, found that statistically the largest risk associated with menopausal hormone therapy (HT) was increased VTE.1 Recently, investigators in the United Kingdom (UK) published results of their research aimed at determining the association between the risk of VTE and the use of different types of HT.2
Details of the study
Vinogradova and colleagues used 2 UK primary care research databases, QResearch and Clinical Practice Research Datalink, to identify cases of incident VTE in general practice records, hospital admissions, and mortality records. They identified 80,396 women (aged 40 to 79 years) diagnosed with VTE between 1998 and 2017 and 391,494 control women matched by age and general practice. The mean age of the case and control women was approximately 64 years; the great majority of women were white. Analyses were adjusted for smoking, body mass index (BMI), family history of VTE, and comorbidities associated with VTE.
Types of HT used. The investigators found that 5,795 (7.2%) women with VTE and 21,670 (5.5%) controls were exposed to HT in the 90 days before the index date (the first date of VTE diagnosis for cases became the index date for matched controls). In those exposed to HT:
- 4,915 (85%) cases and 16,938 (78%) controls used oral preparations (including 102 [1.8%] cases and 312 [1.4%] controls who also had transdermal preparations)
- 880 (14%) cases and 4,731 (19%) controls used transdermal HT only.
Association of VTE with HT. Risk of VTE was increased with all oral HT formulations, including combined (estrogen plus progestogen) and estrogen-only preparations. Use of oral CEE (odds ratio [OR], 1.49) and estradiol (OR, 1.27) were both associated with an elevated risk of VTE (P<.05 for both comparisons). In contrast, use of transdermal estradiol (the great majority of which was administered by patch) was not associated with an elevated risk of VTE (OR, 0.96).
Direct comparison of oral estradiol and CEE found that the lower VTE risk with oral estradiol achieved statistical significance (P = .005). Direct comparison of oral and transdermal estrogen revealed an OR of 1.7 for the oral route of administration (P<.001)
Continue to: Study strengths and weaknesses
Study strengths and weaknesses
This study used data from the 2 largest primary care databases in the United Kingdom. Analyses were adjusted for numerous confounding factors, including acute and chronic conditions, lifestyle factors, and social deprivation. Additional sensitivity analyses were conducted and yielded results similar to those of the main analysis.
Several limitations could have resulted in some residual confounding bias. For example, drug exposure information was based on HT prescriptions and not actual use; data on some factors were not available, such as indications for HT, age at menopause, and education level; and for a small proportion of women, some data (smoking status, alcohol consumption, BMI) were missing and had to be imputed for analysis.
Although randomized trials have not compared VTE risk with oral versus transdermal estrogen, prior observational studies have consistently suggested that transdermal estrogen does not elevate VTE risk; this is consistent with the results from this large UK study. In my practice, congruent with the authors’ suggestions, I recommend transdermal rather than oral estrogen for patients (notably, those who are obese) who at baseline have risk factors for VTE. For menopausal women for whom use of oral estrogen is indicated, I recommend estradiol rather than CEE, since estradiol is less expensive and, based on this study’s results, may be safer than CEE.
ANDREW M. KAUNITZ, MD
EXPERT COMMENTARY
Vinogradova Y, Coupland C, Hippisley-Cox J. Use of hormone replacement therapy and risk of venous thromboembolism: nested case-control studies using the QResearch and CPRD databases. BMJ. 2019;364:k4810.
The Women’s Health Initiative trials, in which menopausal women were randomly assigned to treatment with oral CEE or placebo, found that statistically the largest risk associated with menopausal hormone therapy (HT) was increased VTE.1 Recently, investigators in the United Kingdom (UK) published results of their research aimed at determining the association between the risk of VTE and the use of different types of HT.2
Details of the study
Vinogradova and colleagues used 2 UK primary care research databases, QResearch and Clinical Practice Research Datalink, to identify cases of incident VTE in general practice records, hospital admissions, and mortality records. They identified 80,396 women (aged 40 to 79 years) diagnosed with VTE between 1998 and 2017 and 391,494 control women matched by age and general practice. The mean age of the case and control women was approximately 64 years; the great majority of women were white. Analyses were adjusted for smoking, body mass index (BMI), family history of VTE, and comorbidities associated with VTE.
Types of HT used. The investigators found that 5,795 (7.2%) women with VTE and 21,670 (5.5%) controls were exposed to HT in the 90 days before the index date (the first date of VTE diagnosis for cases became the index date for matched controls). In those exposed to HT:
- 4,915 (85%) cases and 16,938 (78%) controls used oral preparations (including 102 [1.8%] cases and 312 [1.4%] controls who also had transdermal preparations)
- 880 (14%) cases and 4,731 (19%) controls used transdermal HT only.
Association of VTE with HT. Risk of VTE was increased with all oral HT formulations, including combined (estrogen plus progestogen) and estrogen-only preparations. Use of oral CEE (odds ratio [OR], 1.49) and estradiol (OR, 1.27) were both associated with an elevated risk of VTE (P<.05 for both comparisons). In contrast, use of transdermal estradiol (the great majority of which was administered by patch) was not associated with an elevated risk of VTE (OR, 0.96).
Direct comparison of oral estradiol and CEE found that the lower VTE risk with oral estradiol achieved statistical significance (P = .005). Direct comparison of oral and transdermal estrogen revealed an OR of 1.7 for the oral route of administration (P<.001)
Continue to: Study strengths and weaknesses
Study strengths and weaknesses
This study used data from the 2 largest primary care databases in the United Kingdom. Analyses were adjusted for numerous confounding factors, including acute and chronic conditions, lifestyle factors, and social deprivation. Additional sensitivity analyses were conducted and yielded results similar to those of the main analysis.
Several limitations could have resulted in some residual confounding bias. For example, drug exposure information was based on HT prescriptions and not actual use; data on some factors were not available, such as indications for HT, age at menopause, and education level; and for a small proportion of women, some data (smoking status, alcohol consumption, BMI) were missing and had to be imputed for analysis.
Although randomized trials have not compared VTE risk with oral versus transdermal estrogen, prior observational studies have consistently suggested that transdermal estrogen does not elevate VTE risk; this is consistent with the results from this large UK study. In my practice, congruent with the authors’ suggestions, I recommend transdermal rather than oral estrogen for patients (notably, those who are obese) who at baseline have risk factors for VTE. For menopausal women for whom use of oral estrogen is indicated, I recommend estradiol rather than CEE, since estradiol is less expensive and, based on this study’s results, may be safer than CEE.
ANDREW M. KAUNITZ, MD
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women's Health Initiative randomized trials. JAMA. 2013;310:1353-1368.
- Vinogradova Y, Coupland C, Hippisley-Cox J. Use of hormone replacement therapy and risk of venous thromboembolism: nested case-control studies using the QResearch and CPRD databases. BMJ. 2019;364:k4810.
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women's Health Initiative randomized trials. JAMA. 2013;310:1353-1368.
- Vinogradova Y, Coupland C, Hippisley-Cox J. Use of hormone replacement therapy and risk of venous thromboembolism: nested case-control studies using the QResearch and CPRD databases. BMJ. 2019;364:k4810.
Intimate partner violence, guns, and the ObGyn
On the afternoon of November 19, 2018, Dr. Tamara O’Neal was shot and killed by her ex-fiancé outside Mercy Hospital and Medical Center in Chicago, Illinois. After killing Dr. O’Neal, the gunman ran into the hospital where he exchanged gunfire with police, killing a pharmacy resident and a police officer, before he was killed by police.1
This horrific encounter between a woman and her former partner begs for a conversation about intimate partner violence (IPV). A data brief of The National Intimate Partner and Sexual Violence Survey was published in November 2018. According to this report, 30.6% of women experienced physical violence by an intimate partner in 2015, with 21.4% of women experiencing severe physical violence. In addition, 31.0% of men experienced physical violence by an intimate partner in 2015; 14.9% of men experienced severe physical violence.2

Intimate partner violence is “our lane”
The shooting at Mercy Hospital occurred amongst a backdrop of controversy between the National Rifle Association (NRA) and the medical community. On November 7, 2018, the NRA tweeted that doctors should “stay in their lane” with regard to gun control after a position paper from the American College of Physicians on reducing firearm deaths and injuries was published in the Annals of Internal Medicine.3 Doctors from every field and from all over the country responded through social media by stating that treating bullet wounds and caring for those affected by gun violence was “their lane.”4
It is time for us as a community to recognize that gun violence affects us all. The majority of mass shooters have a history of IPV and often target their current or prior partner during the shooting.5 At this intersection of IPV and gun control, the physician has a unique role. We not only treat those affected by gun violence and advocate for better gun control but we also have a duty to screen our patients for IPV. Part of the sacred patient-physician relationship is being present for our patients when they need us most. The American College of Obstetricians and Gynecologists (ACOG) recommends that ObGyns screen patients for IPV at regular intervals and recognizes that it may take several conversations before a patient discloses her history of IPV.6 Additionally, given the increased risk of gun injuries and death, it behooves us to also screen for gun safety in the home.
Ask patients about IPV, and ask again
The shooting at Mercy Hospital was a stark reminder that IPV can affect any of us. With nearly one-third of women and more than one-quarter of men experiencing IPV in their lifetime, action must be taken. The first step is to routinely screen patients for IPV, offering support and community resources. (see “Screening for intimate partner violence). The second step is to work to decrease the access perpetrators of IPV have to weapons with which to enact violence—through legislation, community engagement, and using our physician voices.
States that have passed legislation that prohibits persons with active restraining orders or a history of IPV or domestic violence from possessing firearms has seen a decrease in IPV firearm homicide rates.7 These policies can make a profound impact on the safety of our patients. Women who are in violent relationships are 5 times more likely to die if their partner has access to a firearm.5
Continue to: #BreakTheCycle...
#BreakTheCycle
The 116th Congress convened in January. We have an opportunity to make real gun legislation reform and work to keep our communities and our patients at risk for IPV safer. Tweet your representatives with #BreakTheCycle, and be on the lookout for important legislation to enact real change.
To sign the open letter from American Healthcare Professionals to the NRA regarding their recent comments and our medical experiences with gun violence, click here. Currently, there are more than 41,000 signatures.
There are numerous verified screening tools available to assess for intimate partner violence (IPV) for both pregnant and nonpregnant patients. Many recommended tools are accessible on the Centers for Disease Control and Prevention (CDC) website: https://www.cdc.gov/violenceprevention/pdf/ipv/ipvandsvscreening.pdf. In our office, the tool most commonly used is a 3-part question assessing domestic violence and IPV. It is important to recognize IPV can affect everyone—all races and religions regardless of socioeconomic background, sexual orientation, and pregnancy status. All patients deserve screening for IPV, and it should never be assumed a patient is not at risk. During an annual gynecology visit for return and new patients or a new obstetric intake visit, we use the following script obtained from ACOG’s Committee Opinion 518 on IPV1 :
Because violence is so common in many women’s lives and because there is help available for women being abused, I now ask every patient about domestic violence:
1. Within the past year (or since you have become pregnant) have you been hit, slapped, kicked, or otherwise physically hurt by someone?
2. Are you in a relationship with a person who threatens or physically hurts you?
3. Has anyone forced you to have sexual activities that made you feel uncomfortable?
If a patient screens positive, we assess their immediate safety. If a social worker is readily available, we arrange an urgent meeting with the patient. If offices do not have immediate access to this service, online information can be provided to patients, including the National Resource Center on Domestic Violence (https://nnedv.org/) and a toll-free number to the National Domestic Violence Hotline: 1-800-799-7233. Additionally, we ask patients about any history of verbal, physical, or sexual violence with prior partners, family members, acquaintances, coworkers, etc. Although the patient might not be at immediate risk, prior experiences with abuse can cause fear and anxiety around gynecologic and obstetric exams. Acknowledging this history can help the clinician adjust his or her physical exam and support the patient during, what may be, a triggering experience.
As an additional resource, Dr. Katherine Hicks-Courant, a resident at Tufts Medical Center, in Boston, Massachusetts, created a tool kit for providers working with pregnant patients with a history of sexual assault. It can be accessed without login online under the Junior Fellow Initiative Toolkit section at http://www.acog.org.
References
1. American College of Obstetricians and Gynecologists. Committee Opinion No. 518: intimate partner violence. Obstet Gynecol. 2012;119:412-417.
If you, or someone you know, needs help, please call The National Domestic Violence Hotline at 1-800-799-7233.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. Buckley M, Gorner J, Greene M. “Chicago hospital shooting: Young cop, doctor, pharmacy resident and gunman die in Mercy Hospital attack. Chicago Tribune. Nov. 20, 2018.
2. Smith SG, Zhang X, Basile KC, et al. The National Intimate Partner
and Sexual Violence Survey (NISVS): 2015 data brief – updated release. Atlanta, GA: National Center for Injury Prevention and Control, Centers for Disease Control and Prevention; November 2018.
3. Butkus R, Doherty R, Bornstein SS; for the Health and Public Policy Committee of the American College of Physicians. Reducing firearm injuries and deaths in the United States: a position paper from the American College of Physicians. Ann Intern Med. 2018;169:704-707.
4. Papenfuss M. NRA Tweets Warning to Anti-Gun Doctors: ‘Stay In Your Lane’. The Huffington Post. November 8, 2018.
5. Everytown for Gun Safety website. Mass Shootings in the United States: 2009–2016. Available at https://everytownresearch.org/reports/mass-shootings-analysis/. Accessed January 17, 2019.
6. The American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 518: intimate partner violence. Obstet Gynecol. 2012;119(2 pt 1):412-417. https://www.acog.org/Clinical-Guidance-and-Publications/Committee-Opinions/Committee-on-Health-Care-for-Underserved-Women/Intimate-Partner-Violence.
7. Zeoli AM, McCourt A, Buggs S, et al. Analysis of the strength of legal firearms restrictions for perpetrators of domestic violence and their associations with intimate partner homicide. Am J Epidemiol. 2018;187:2365-2371.
On the afternoon of November 19, 2018, Dr. Tamara O’Neal was shot and killed by her ex-fiancé outside Mercy Hospital and Medical Center in Chicago, Illinois. After killing Dr. O’Neal, the gunman ran into the hospital where he exchanged gunfire with police, killing a pharmacy resident and a police officer, before he was killed by police.1
This horrific encounter between a woman and her former partner begs for a conversation about intimate partner violence (IPV). A data brief of The National Intimate Partner and Sexual Violence Survey was published in November 2018. According to this report, 30.6% of women experienced physical violence by an intimate partner in 2015, with 21.4% of women experiencing severe physical violence. In addition, 31.0% of men experienced physical violence by an intimate partner in 2015; 14.9% of men experienced severe physical violence.2

Intimate partner violence is “our lane”
The shooting at Mercy Hospital occurred amongst a backdrop of controversy between the National Rifle Association (NRA) and the medical community. On November 7, 2018, the NRA tweeted that doctors should “stay in their lane” with regard to gun control after a position paper from the American College of Physicians on reducing firearm deaths and injuries was published in the Annals of Internal Medicine.3 Doctors from every field and from all over the country responded through social media by stating that treating bullet wounds and caring for those affected by gun violence was “their lane.”4
It is time for us as a community to recognize that gun violence affects us all. The majority of mass shooters have a history of IPV and often target their current or prior partner during the shooting.5 At this intersection of IPV and gun control, the physician has a unique role. We not only treat those affected by gun violence and advocate for better gun control but we also have a duty to screen our patients for IPV. Part of the sacred patient-physician relationship is being present for our patients when they need us most. The American College of Obstetricians and Gynecologists (ACOG) recommends that ObGyns screen patients for IPV at regular intervals and recognizes that it may take several conversations before a patient discloses her history of IPV.6 Additionally, given the increased risk of gun injuries and death, it behooves us to also screen for gun safety in the home.
Ask patients about IPV, and ask again
The shooting at Mercy Hospital was a stark reminder that IPV can affect any of us. With nearly one-third of women and more than one-quarter of men experiencing IPV in their lifetime, action must be taken. The first step is to routinely screen patients for IPV, offering support and community resources. (see “Screening for intimate partner violence). The second step is to work to decrease the access perpetrators of IPV have to weapons with which to enact violence—through legislation, community engagement, and using our physician voices.
States that have passed legislation that prohibits persons with active restraining orders or a history of IPV or domestic violence from possessing firearms has seen a decrease in IPV firearm homicide rates.7 These policies can make a profound impact on the safety of our patients. Women who are in violent relationships are 5 times more likely to die if their partner has access to a firearm.5
Continue to: #BreakTheCycle...
#BreakTheCycle
The 116th Congress convened in January. We have an opportunity to make real gun legislation reform and work to keep our communities and our patients at risk for IPV safer. Tweet your representatives with #BreakTheCycle, and be on the lookout for important legislation to enact real change.
To sign the open letter from American Healthcare Professionals to the NRA regarding their recent comments and our medical experiences with gun violence, click here. Currently, there are more than 41,000 signatures.
There are numerous verified screening tools available to assess for intimate partner violence (IPV) for both pregnant and nonpregnant patients. Many recommended tools are accessible on the Centers for Disease Control and Prevention (CDC) website: https://www.cdc.gov/violenceprevention/pdf/ipv/ipvandsvscreening.pdf. In our office, the tool most commonly used is a 3-part question assessing domestic violence and IPV. It is important to recognize IPV can affect everyone—all races and religions regardless of socioeconomic background, sexual orientation, and pregnancy status. All patients deserve screening for IPV, and it should never be assumed a patient is not at risk. During an annual gynecology visit for return and new patients or a new obstetric intake visit, we use the following script obtained from ACOG’s Committee Opinion 518 on IPV1 :
Because violence is so common in many women’s lives and because there is help available for women being abused, I now ask every patient about domestic violence:
1. Within the past year (or since you have become pregnant) have you been hit, slapped, kicked, or otherwise physically hurt by someone?
2. Are you in a relationship with a person who threatens or physically hurts you?
3. Has anyone forced you to have sexual activities that made you feel uncomfortable?
If a patient screens positive, we assess their immediate safety. If a social worker is readily available, we arrange an urgent meeting with the patient. If offices do not have immediate access to this service, online information can be provided to patients, including the National Resource Center on Domestic Violence (https://nnedv.org/) and a toll-free number to the National Domestic Violence Hotline: 1-800-799-7233. Additionally, we ask patients about any history of verbal, physical, or sexual violence with prior partners, family members, acquaintances, coworkers, etc. Although the patient might not be at immediate risk, prior experiences with abuse can cause fear and anxiety around gynecologic and obstetric exams. Acknowledging this history can help the clinician adjust his or her physical exam and support the patient during, what may be, a triggering experience.
As an additional resource, Dr. Katherine Hicks-Courant, a resident at Tufts Medical Center, in Boston, Massachusetts, created a tool kit for providers working with pregnant patients with a history of sexual assault. It can be accessed without login online under the Junior Fellow Initiative Toolkit section at http://www.acog.org.
References
1. American College of Obstetricians and Gynecologists. Committee Opinion No. 518: intimate partner violence. Obstet Gynecol. 2012;119:412-417.
If you, or someone you know, needs help, please call The National Domestic Violence Hotline at 1-800-799-7233.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
On the afternoon of November 19, 2018, Dr. Tamara O’Neal was shot and killed by her ex-fiancé outside Mercy Hospital and Medical Center in Chicago, Illinois. After killing Dr. O’Neal, the gunman ran into the hospital where he exchanged gunfire with police, killing a pharmacy resident and a police officer, before he was killed by police.1
This horrific encounter between a woman and her former partner begs for a conversation about intimate partner violence (IPV). A data brief of The National Intimate Partner and Sexual Violence Survey was published in November 2018. According to this report, 30.6% of women experienced physical violence by an intimate partner in 2015, with 21.4% of women experiencing severe physical violence. In addition, 31.0% of men experienced physical violence by an intimate partner in 2015; 14.9% of men experienced severe physical violence.2

Intimate partner violence is “our lane”
The shooting at Mercy Hospital occurred amongst a backdrop of controversy between the National Rifle Association (NRA) and the medical community. On November 7, 2018, the NRA tweeted that doctors should “stay in their lane” with regard to gun control after a position paper from the American College of Physicians on reducing firearm deaths and injuries was published in the Annals of Internal Medicine.3 Doctors from every field and from all over the country responded through social media by stating that treating bullet wounds and caring for those affected by gun violence was “their lane.”4
It is time for us as a community to recognize that gun violence affects us all. The majority of mass shooters have a history of IPV and often target their current or prior partner during the shooting.5 At this intersection of IPV and gun control, the physician has a unique role. We not only treat those affected by gun violence and advocate for better gun control but we also have a duty to screen our patients for IPV. Part of the sacred patient-physician relationship is being present for our patients when they need us most. The American College of Obstetricians and Gynecologists (ACOG) recommends that ObGyns screen patients for IPV at regular intervals and recognizes that it may take several conversations before a patient discloses her history of IPV.6 Additionally, given the increased risk of gun injuries and death, it behooves us to also screen for gun safety in the home.
Ask patients about IPV, and ask again
The shooting at Mercy Hospital was a stark reminder that IPV can affect any of us. With nearly one-third of women and more than one-quarter of men experiencing IPV in their lifetime, action must be taken. The first step is to routinely screen patients for IPV, offering support and community resources. (see “Screening for intimate partner violence). The second step is to work to decrease the access perpetrators of IPV have to weapons with which to enact violence—through legislation, community engagement, and using our physician voices.
States that have passed legislation that prohibits persons with active restraining orders or a history of IPV or domestic violence from possessing firearms has seen a decrease in IPV firearm homicide rates.7 These policies can make a profound impact on the safety of our patients. Women who are in violent relationships are 5 times more likely to die if their partner has access to a firearm.5
Continue to: #BreakTheCycle...
#BreakTheCycle
The 116th Congress convened in January. We have an opportunity to make real gun legislation reform and work to keep our communities and our patients at risk for IPV safer. Tweet your representatives with #BreakTheCycle, and be on the lookout for important legislation to enact real change.
To sign the open letter from American Healthcare Professionals to the NRA regarding their recent comments and our medical experiences with gun violence, click here. Currently, there are more than 41,000 signatures.
There are numerous verified screening tools available to assess for intimate partner violence (IPV) for both pregnant and nonpregnant patients. Many recommended tools are accessible on the Centers for Disease Control and Prevention (CDC) website: https://www.cdc.gov/violenceprevention/pdf/ipv/ipvandsvscreening.pdf. In our office, the tool most commonly used is a 3-part question assessing domestic violence and IPV. It is important to recognize IPV can affect everyone—all races and religions regardless of socioeconomic background, sexual orientation, and pregnancy status. All patients deserve screening for IPV, and it should never be assumed a patient is not at risk. During an annual gynecology visit for return and new patients or a new obstetric intake visit, we use the following script obtained from ACOG’s Committee Opinion 518 on IPV1 :
Because violence is so common in many women’s lives and because there is help available for women being abused, I now ask every patient about domestic violence:
1. Within the past year (or since you have become pregnant) have you been hit, slapped, kicked, or otherwise physically hurt by someone?
2. Are you in a relationship with a person who threatens or physically hurts you?
3. Has anyone forced you to have sexual activities that made you feel uncomfortable?
If a patient screens positive, we assess their immediate safety. If a social worker is readily available, we arrange an urgent meeting with the patient. If offices do not have immediate access to this service, online information can be provided to patients, including the National Resource Center on Domestic Violence (https://nnedv.org/) and a toll-free number to the National Domestic Violence Hotline: 1-800-799-7233. Additionally, we ask patients about any history of verbal, physical, or sexual violence with prior partners, family members, acquaintances, coworkers, etc. Although the patient might not be at immediate risk, prior experiences with abuse can cause fear and anxiety around gynecologic and obstetric exams. Acknowledging this history can help the clinician adjust his or her physical exam and support the patient during, what may be, a triggering experience.
As an additional resource, Dr. Katherine Hicks-Courant, a resident at Tufts Medical Center, in Boston, Massachusetts, created a tool kit for providers working with pregnant patients with a history of sexual assault. It can be accessed without login online under the Junior Fellow Initiative Toolkit section at http://www.acog.org.
References
1. American College of Obstetricians and Gynecologists. Committee Opinion No. 518: intimate partner violence. Obstet Gynecol. 2012;119:412-417.
If you, or someone you know, needs help, please call The National Domestic Violence Hotline at 1-800-799-7233.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. Buckley M, Gorner J, Greene M. “Chicago hospital shooting: Young cop, doctor, pharmacy resident and gunman die in Mercy Hospital attack. Chicago Tribune. Nov. 20, 2018.
2. Smith SG, Zhang X, Basile KC, et al. The National Intimate Partner
and Sexual Violence Survey (NISVS): 2015 data brief – updated release. Atlanta, GA: National Center for Injury Prevention and Control, Centers for Disease Control and Prevention; November 2018.
3. Butkus R, Doherty R, Bornstein SS; for the Health and Public Policy Committee of the American College of Physicians. Reducing firearm injuries and deaths in the United States: a position paper from the American College of Physicians. Ann Intern Med. 2018;169:704-707.
4. Papenfuss M. NRA Tweets Warning to Anti-Gun Doctors: ‘Stay In Your Lane’. The Huffington Post. November 8, 2018.
5. Everytown for Gun Safety website. Mass Shootings in the United States: 2009–2016. Available at https://everytownresearch.org/reports/mass-shootings-analysis/. Accessed January 17, 2019.
6. The American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 518: intimate partner violence. Obstet Gynecol. 2012;119(2 pt 1):412-417. https://www.acog.org/Clinical-Guidance-and-Publications/Committee-Opinions/Committee-on-Health-Care-for-Underserved-Women/Intimate-Partner-Violence.
7. Zeoli AM, McCourt A, Buggs S, et al. Analysis of the strength of legal firearms restrictions for perpetrators of domestic violence and their associations with intimate partner homicide. Am J Epidemiol. 2018;187:2365-2371.
1. Buckley M, Gorner J, Greene M. “Chicago hospital shooting: Young cop, doctor, pharmacy resident and gunman die in Mercy Hospital attack. Chicago Tribune. Nov. 20, 2018.
2. Smith SG, Zhang X, Basile KC, et al. The National Intimate Partner
and Sexual Violence Survey (NISVS): 2015 data brief – updated release. Atlanta, GA: National Center for Injury Prevention and Control, Centers for Disease Control and Prevention; November 2018.
3. Butkus R, Doherty R, Bornstein SS; for the Health and Public Policy Committee of the American College of Physicians. Reducing firearm injuries and deaths in the United States: a position paper from the American College of Physicians. Ann Intern Med. 2018;169:704-707.
4. Papenfuss M. NRA Tweets Warning to Anti-Gun Doctors: ‘Stay In Your Lane’. The Huffington Post. November 8, 2018.
5. Everytown for Gun Safety website. Mass Shootings in the United States: 2009–2016. Available at https://everytownresearch.org/reports/mass-shootings-analysis/. Accessed January 17, 2019.
6. The American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 518: intimate partner violence. Obstet Gynecol. 2012;119(2 pt 1):412-417. https://www.acog.org/Clinical-Guidance-and-Publications/Committee-Opinions/Committee-on-Health-Care-for-Underserved-Women/Intimate-Partner-Violence.
7. Zeoli AM, McCourt A, Buggs S, et al. Analysis of the strength of legal firearms restrictions for perpetrators of domestic violence and their associations with intimate partner homicide. Am J Epidemiol. 2018;187:2365-2371.
How does HT in recent and 10+ years past menopause affect atherosclerosis progression?
Expert Commentary
Sriprasert I, Hodis HN, Karim R, et al. Differential effect of plasma estradiol on subclinical atherosclerosis progression in early versus late postmenopause. J Clin Endocrinol Metab. 2019;104:293-300. doi:10.1210/jc.2018-01600.
In 2016, the primary findings of the Early versus Late Intervention Trial with Estradiol (ELITE) demonstrated that oral E2 administered to women who were less than 6 years postmenopause slowed progression of subclinical atherosclerosis as assessed by carotid artery intima-media thickness (CIMT), while it had no effect in women who were at least 10 years postmenopause.1
That trial included 643 healthy women without cardiovascular disease who at enrollment had a median age of 55.4 years in the early postmenopause group (median 3.5 years since menopause) and 63.6 years in the late postmenopause group (median 14.3 years since menopause). The study medications were oral estradiol 1 mg daily plus progesterone vaginal gel for women with a uterus or placebo and placebo gel for a median of 5 years.
The investigators found also that, in contrast with CIMT, cardiac computed tomography (CT) measures of atherosclerosis did not differ significantly between the estradiol and placebo groups, regardless of age.1
Posttrial data analysis revealed a new finding
In a secondary analysis of data from the ELITE trial, Sriprasert and colleagues dug deeper to assess the impact of plasma E2 levels on progression of subclinical atherosclerosis.2
Among 596 women (69.6% white non-Hispanic, 8.7% black, 13.3% Hispanic, and 8.4% Asian/Pacific Islander), E2 levels were available in 248 women in early postmenopause (mean age, 54.7 years) and 348 women in late postmenopause (median age, 63.6 years).
For women in the estradiol-treated group, mean E2 levels during the trial as well as change of E2 levels from baseline were significantly higher in the early postmenopause group than in the late postmenopause group, even though both groups had similar adherence based on pill count. For those in the placebo group, mean E2 levels and change of E2 levels from baseline were equivalent in early and late menopause.
In the E2-treated group and the placebo group combined, the mixed effects analysis of the CIMT progression rate (based on the mean E2 level during the trial) demonstrated that a higher level of E2 was inversely associated with the CIMT progression rate in early postmenopausal women (beta coefficient = -0.04 [95% confidence interval (CI), -0.09 to -0.001] μm CIMT per year per 1 pg/mL estradiol; P = .04). However, a higher level of E2 was positively associated (beta coefficient = 0.063 [95% CI, 0.018 to 0.107] μm CIMT per year per 1 pg/mL estradiol; P = .006) with CIMT progression rate in the late postmenopausal women.
Continue to: Bottom line...
Bottom line. E2 levels resulting from administration of oral estradiol were inversely associated with atherosclerosis progression in women in early menopause, but they were positively associated with progression in late postmenopause participants.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
These new findings from a posttrial analysis of ELITE data provide yet further support for the hormone therapy (HT) “timing hypothesis,” which postulates that HT slows atherosclerosis progression in recently menopausal women but has neutral or adverse effects in women who are at least a decade past menopause onset. As the authors suggest, the favorable vascular effects of E2 appear limited to those women (most often in early menopause) who have not yet developed atherosclerosis. Whether or not HT should be considered for cardioprotection remains unresolved (and controversial). By contrast, these data, along with findings from the Women’s Health Initiative,3 provide reassurance regarding the cardiovascular safety of HT when prescribed for recently menopausal women with bothersome vasomotor symptoms.
ANDREW M. KAUNITZ, MD
References
1. Hodis HN, Mack WJ, Henderson VW, et al; for the ELITE Research Group. Vascular effects of early versus late postmenopausal treatment with estradiol. N Engl J Med. 2016;374;1221-1231.
2. Sriprasert I, Hodis HN, Karim R, et al. Differential effect of plasma estradiol on subclinical atherosclerosis progression in early versus late postmenopause. J Clin Endocrinol Metab. 2019;104:293-300. doi:10.1210/jc.2018-01600.
3. Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310:1353-1368.
Expert Commentary
Sriprasert I, Hodis HN, Karim R, et al. Differential effect of plasma estradiol on subclinical atherosclerosis progression in early versus late postmenopause. J Clin Endocrinol Metab. 2019;104:293-300. doi:10.1210/jc.2018-01600.
In 2016, the primary findings of the Early versus Late Intervention Trial with Estradiol (ELITE) demonstrated that oral E2 administered to women who were less than 6 years postmenopause slowed progression of subclinical atherosclerosis as assessed by carotid artery intima-media thickness (CIMT), while it had no effect in women who were at least 10 years postmenopause.1
That trial included 643 healthy women without cardiovascular disease who at enrollment had a median age of 55.4 years in the early postmenopause group (median 3.5 years since menopause) and 63.6 years in the late postmenopause group (median 14.3 years since menopause). The study medications were oral estradiol 1 mg daily plus progesterone vaginal gel for women with a uterus or placebo and placebo gel for a median of 5 years.
The investigators found also that, in contrast with CIMT, cardiac computed tomography (CT) measures of atherosclerosis did not differ significantly between the estradiol and placebo groups, regardless of age.1
Posttrial data analysis revealed a new finding
In a secondary analysis of data from the ELITE trial, Sriprasert and colleagues dug deeper to assess the impact of plasma E2 levels on progression of subclinical atherosclerosis.2
Among 596 women (69.6% white non-Hispanic, 8.7% black, 13.3% Hispanic, and 8.4% Asian/Pacific Islander), E2 levels were available in 248 women in early postmenopause (mean age, 54.7 years) and 348 women in late postmenopause (median age, 63.6 years).
For women in the estradiol-treated group, mean E2 levels during the trial as well as change of E2 levels from baseline were significantly higher in the early postmenopause group than in the late postmenopause group, even though both groups had similar adherence based on pill count. For those in the placebo group, mean E2 levels and change of E2 levels from baseline were equivalent in early and late menopause.
In the E2-treated group and the placebo group combined, the mixed effects analysis of the CIMT progression rate (based on the mean E2 level during the trial) demonstrated that a higher level of E2 was inversely associated with the CIMT progression rate in early postmenopausal women (beta coefficient = -0.04 [95% confidence interval (CI), -0.09 to -0.001] μm CIMT per year per 1 pg/mL estradiol; P = .04). However, a higher level of E2 was positively associated (beta coefficient = 0.063 [95% CI, 0.018 to 0.107] μm CIMT per year per 1 pg/mL estradiol; P = .006) with CIMT progression rate in the late postmenopausal women.
Continue to: Bottom line...
Bottom line. E2 levels resulting from administration of oral estradiol were inversely associated with atherosclerosis progression in women in early menopause, but they were positively associated with progression in late postmenopause participants.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
These new findings from a posttrial analysis of ELITE data provide yet further support for the hormone therapy (HT) “timing hypothesis,” which postulates that HT slows atherosclerosis progression in recently menopausal women but has neutral or adverse effects in women who are at least a decade past menopause onset. As the authors suggest, the favorable vascular effects of E2 appear limited to those women (most often in early menopause) who have not yet developed atherosclerosis. Whether or not HT should be considered for cardioprotection remains unresolved (and controversial). By contrast, these data, along with findings from the Women’s Health Initiative,3 provide reassurance regarding the cardiovascular safety of HT when prescribed for recently menopausal women with bothersome vasomotor symptoms.
ANDREW M. KAUNITZ, MD
References
1. Hodis HN, Mack WJ, Henderson VW, et al; for the ELITE Research Group. Vascular effects of early versus late postmenopausal treatment with estradiol. N Engl J Med. 2016;374;1221-1231.
2. Sriprasert I, Hodis HN, Karim R, et al. Differential effect of plasma estradiol on subclinical atherosclerosis progression in early versus late postmenopause. J Clin Endocrinol Metab. 2019;104:293-300. doi:10.1210/jc.2018-01600.
3. Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310:1353-1368.
Expert Commentary
Sriprasert I, Hodis HN, Karim R, et al. Differential effect of plasma estradiol on subclinical atherosclerosis progression in early versus late postmenopause. J Clin Endocrinol Metab. 2019;104:293-300. doi:10.1210/jc.2018-01600.
In 2016, the primary findings of the Early versus Late Intervention Trial with Estradiol (ELITE) demonstrated that oral E2 administered to women who were less than 6 years postmenopause slowed progression of subclinical atherosclerosis as assessed by carotid artery intima-media thickness (CIMT), while it had no effect in women who were at least 10 years postmenopause.1
That trial included 643 healthy women without cardiovascular disease who at enrollment had a median age of 55.4 years in the early postmenopause group (median 3.5 years since menopause) and 63.6 years in the late postmenopause group (median 14.3 years since menopause). The study medications were oral estradiol 1 mg daily plus progesterone vaginal gel for women with a uterus or placebo and placebo gel for a median of 5 years.
The investigators found also that, in contrast with CIMT, cardiac computed tomography (CT) measures of atherosclerosis did not differ significantly between the estradiol and placebo groups, regardless of age.1
Posttrial data analysis revealed a new finding
In a secondary analysis of data from the ELITE trial, Sriprasert and colleagues dug deeper to assess the impact of plasma E2 levels on progression of subclinical atherosclerosis.2
Among 596 women (69.6% white non-Hispanic, 8.7% black, 13.3% Hispanic, and 8.4% Asian/Pacific Islander), E2 levels were available in 248 women in early postmenopause (mean age, 54.7 years) and 348 women in late postmenopause (median age, 63.6 years).
For women in the estradiol-treated group, mean E2 levels during the trial as well as change of E2 levels from baseline were significantly higher in the early postmenopause group than in the late postmenopause group, even though both groups had similar adherence based on pill count. For those in the placebo group, mean E2 levels and change of E2 levels from baseline were equivalent in early and late menopause.
In the E2-treated group and the placebo group combined, the mixed effects analysis of the CIMT progression rate (based on the mean E2 level during the trial) demonstrated that a higher level of E2 was inversely associated with the CIMT progression rate in early postmenopausal women (beta coefficient = -0.04 [95% confidence interval (CI), -0.09 to -0.001] μm CIMT per year per 1 pg/mL estradiol; P = .04). However, a higher level of E2 was positively associated (beta coefficient = 0.063 [95% CI, 0.018 to 0.107] μm CIMT per year per 1 pg/mL estradiol; P = .006) with CIMT progression rate in the late postmenopausal women.
Continue to: Bottom line...
Bottom line. E2 levels resulting from administration of oral estradiol were inversely associated with atherosclerosis progression in women in early menopause, but they were positively associated with progression in late postmenopause participants.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
These new findings from a posttrial analysis of ELITE data provide yet further support for the hormone therapy (HT) “timing hypothesis,” which postulates that HT slows atherosclerosis progression in recently menopausal women but has neutral or adverse effects in women who are at least a decade past menopause onset. As the authors suggest, the favorable vascular effects of E2 appear limited to those women (most often in early menopause) who have not yet developed atherosclerosis. Whether or not HT should be considered for cardioprotection remains unresolved (and controversial). By contrast, these data, along with findings from the Women’s Health Initiative,3 provide reassurance regarding the cardiovascular safety of HT when prescribed for recently menopausal women with bothersome vasomotor symptoms.
ANDREW M. KAUNITZ, MD
References
1. Hodis HN, Mack WJ, Henderson VW, et al; for the ELITE Research Group. Vascular effects of early versus late postmenopausal treatment with estradiol. N Engl J Med. 2016;374;1221-1231.
2. Sriprasert I, Hodis HN, Karim R, et al. Differential effect of plasma estradiol on subclinical atherosclerosis progression in early versus late postmenopause. J Clin Endocrinol Metab. 2019;104:293-300. doi:10.1210/jc.2018-01600.
3. Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310:1353-1368.
Should we abandon minimally invasive surgery for cervical cancer?
A minimally invasive approach for gynecologic surgery increasingly has become the surgical modality of choice (vs open surgery) due to decreased perioperative and postoperative morbidity for many gynecologic cancers.1-3 This has included radical hysterectomy for cervical cancers. Until recently, retrospective evidence supported its use, suggesting decreased perioperative and postoperative complications with similar survival outcomes between patients undergoing minimally invasive and open radical hysterectomy.4,5 In November 2018, two new studies were published in the New England Journal of Medicine, and another study was presented at the American Society of Clinical Oncology (ASCO) annual meeting challenging this practice paradigm. These studies reveal a higher risk of disease recurrence and decreased overall survival with minimally invasive surgery (MIS) compared with open surgery for Stages IA–IB1 cervical cancer. These findings have resulted in a change in practice nationwide.
RCT findings astonish specialty
The first study, the Laparoscopic Approach to Cervical Cancer (LACC) trial, authored by Ramirez and colleagues was a noninferiority randomized controlled trial evaluating MIS versus open radical hysterectomy for patients with cervical cancer (Stage 1A–1B1) conducted from 2008–2017.6 The primary outcome was disease-free survival at 4.5 years. Secondary outcomes included recurrence and overall survival rates. Power analysis suggested a sample size of 740 patients to provide greater than 80% power with a noninferiority margin of -7.2% between disease-free rates of the two groups. However, the study was closed prematurely at enrollment of 631 patients (85% recruitment) by the Data Safety Monitoring Committee due to the astounding differences in survival between the two groups.
The rate of disease-free survival at 4.5 years was 86.0% with MIS and 96% with open surgery. There were 27 recurrences (8.5%) in the MIS group and only 7 (2.2%) in the open-surgery group, accounting for a hazard ratio (HR) for disease recurrence or death from cervical cancer of 3.74 (95% confidence interval [CI], 1.63–8.58). This difference remained after adjusting for confounding variables. There were 22 deaths—19 (5.9%) in the MIS group and 3 (0.1%) in the open-surgery group (HR, 6.56). Although patient characteristics between groups appeared to be similar, more than one-third of patients in each group had missing data regarding histology at the time of surgery, grade, tumor size, lymphovascular space invasion, and depth of invasion. Interestingly, intraoperative, perioperative, and postoperative complications between the two groups were similar (with rates of 11%, about 40%, and about 25%, respectively).
Surprising findings continue in NEJM
The second study, by Melamed and colleagues, was a retrospective cohort study using data from the National Cancer Database (NCDB) and the Surveillance, Epidemiology, and End Results (SEER) database evaluating women with stage IA2 or IB1 cervical cancer who underwent either minimally invasive or open radical hysterectomy between 2010 and 2013.7 The primary outcome was time to death.
Participant characteristics. A total of 2,461 women were included: 49.8% underwent MIS and 50.2% underwent open surgery. According to the raw data, patients undergoing MIS were more likely to be white, privately insured, reside in an area associated with higher income, undergo surgery at a nonacademic institution, have adenocarcinoma, and have smaller, lower-grade tumors. After propensity-score weighting, demographic and clinical characteristics were similar between groups. Median follow-up was 45 months.
Results. A total of 164 deaths occurred: 94 in the MIS and 70 in the open-surgery group. The risk of death during study follow-up was 9.1% in the MIS group versus 5.3% in the open-surgery group, and women who underwent MIS had shorter overall survival (P = .002; HR, 1.65; 95% CI, 1.22–2.22). Mortality rates remained higher in the MIS group after adjusting for adjuvant therapy (HR, 1.62; 95% CI, 1.2–2.19). However, the HR for death with MIS was not statistically significant in a subgroup analysis evaluating tumors 2 cm in size or less (HR, 1.46; 95% CI, 0.70–3.02). The authors demonstrated that the adoption of MIS for radical hysterectomy corresponded to a drop in the 4-year survival rate of 0.8% per year (P = .01).
Continue to: ASCO meeting data emphasize lower...
ASCO meeting data emphasize lower mortality and survival rates for MIS
A third important, but less publicized study, is a retrospective cohort study by Marguland and colleagues that was presented at the ASCO annual meeting and is pending publication. This study evaluated the 5-year survival of women with stage IB1 cervical cancer after MIS or open radical hysterectomy from 2010 to 2013.8 The findings demonstrated similar results to the above studies with decreased 5-year survival rates in patients with a tumor size of 2 cm or greater in the MIS group (81.3% vs 90.8; HR, 2.14; 95% CI, 1.36–3.38; P<.001). These results hold true when controlling for confounding clinical variables. Interestingly, in a subset analysis evaluating patients with tumors less than 2 cm, survival rates were similar between groups. This study confirms decreased morbidity and cost associated with MIS radical hysterectomy.
A consistent message emerges from 3 independent studies
We must take the study findings seriously and evaluate the quality of the evidence. There are many strengths to the above studies. First and most importantly, the LACC study is the only prospective randomized controlled trial (RCT) to evaluate this very important clinical question. RCTs are the gold standard for understanding the effectiveness and safety of an intervention compared with an established treatment. The study was well designed in that the study population was clearly defined with detailed inclusion and exclusion criteria. The intention to treat analysis was similar to the per-protocol analysis, and the study followed Consolidated Standards of Reporting Trials (CONSORT) guidelines. While the study was stopped early, there was still 84% power for the primary outcome. Therefore, when it comes to MIS for cervical cancer, this study provides the soundest data we have available. It is also extremely noteworthy that two additional large retrospective studies evaluating this question separately found similar results.
Criticisms remain, but older research has drawbacks
A main concern with these studies is that the findings challenge previously published research, which overall suggest similar survival outcomes between MIS and open surgical approaches. However, in evaluating the previously published retrospective data it is clear that the studies have considerable limitations.
Long-term survival not always evaluated in research. First, the majority of studies comparing MIS and open treatment modalities specifically evaluated perioperative complications and did not consider long-term survival.4,9,10 Of those studies that did consider survival outcomes, the groups often were not balanced and were skewed toward the open surgery patients having larger tumors and higher-stage disease.5
Difficult to compare “apples to apples.” These findings are complicated by the fact that open radical hysterectomies were essentially replaced by MIS radical hysterectomies, and therefore, the comparisons are not equivalent since they are comparing different treatment times. For instance, throughout the time period many of these studies were conducted, the treatment paradigm for early-stage cervical cancer changed regarding who received adjuvant therapy and imaging techniques. Therefore, these studies are not comparing apples to apples.11,12
Are we going to increase morbidity? Another common concern when considering abandoning MIS for cervical cancer is the increase in morbidity that our patients may incur immediately postoperatively due to open surgery. Multiple studies have associated minimally invasive radical hysterectomies with decreased blood loss, shorter hospital stay, lower transfusion rates, and decreased time until return of bowel function.4,10,13
Continue to: While we recognize that...
While we recognize that open surgery is associated with increased morbidity, we do argue that, with the almost-universal implementation of Enhanced Recovery Pathways (ERP) in gynecologic oncology, the disparities between the two groups will be minimized and likely are much smaller than that reported in historical literature.14 Notably, there were no differences in peri-, intra-, or postoperative complications between the two groups in the LACC study, indicating that MIS may not be saving our patients as much morbidity as we think.
Surgical ability differences. Despite the vast strengths associated with the studies we have discussed they certainly embody limitations as well. First, surgical aptitude is difficult to evaluate and tease out. This is extremely pertinent given perioperative, and postoperative, outcomes in cervical cancer, as well as survival outcomes, in multiple surgically managed cancers, which are directly associated with the volume and proficiency of the surgeon.15-19 Additionally, the mode of minimally invasive surgery that was most commonly utilized was different from practice in the United States. Eighty four percent of the patients in the MIS group of the LACC study underwent laparoscopic and 13.6% underwent robot-assisted radical hysterectomy. This is starkly different from US practice, where 75% of gynecologic oncologists report performing radical hysterectomies only robotically.20
Take-home points
Consider this latest evidence in your surgical planning. Most importantly, the evidence is the evidence. In other words, we can attempt to explain away the findings, but despite arguments against these studies, these data are the most reliable evidence we have to date regarding outcomes for cervical cancer with MIS versus open approaches. These data demonstrate that MIS may be harming our patients and so we must take this into careful consideration during surgical planning.
For small cancers, MIS may be the best option. MIS radical hysterectomy may still be the best approach for patients with tumors less than 2 cm in size. The LACC study is not powered to evaluate oncologic outcomes in this subset of patients and the two retrospective studies suggest no difference in survival in this cohort.
We must work to understand the driving force between the disparate outcomes. Are the increased rates due to the open surgical approach, the uterine manipulator, circulating CO2 gas, or tumor exposure to the intraperitoneal cavity as the authors suggest? Or is it due to surgical expertise, tumor biology, tumor size, or mode of MIS? At this point the impelling cause is unknown.
New NCCN guidelines are to come. Up to this point the National Comprehensive Cancer Network (NCCN) guidelines stated that “radical hysterectomy procedure may be performed either via laparotomy or laparoscopy.” Given these recent studies, however, new NCCN guidelines will be released cautioning the use of the MIS approach. In short, these data have transformed the standard of care.
At our institution, the majority of radical hysterectomies will be performed open. Continued discussion remains regarding small lesions, but even in these cases most surgeons will proceed with open surgery in an attempt to maximize survival.
As providers, it is our duty to honestly reflect on published data and comprehensively counsel patients about the risks and benefits associated with each approach, including the fact that recurrence may be higher with a minimally invasive approach. Patients and providers must then collectively decide what is best for each individual case.
- Walker JL, Piedmonte MR, Spirtos NM, et al. Laparoscopy compared with laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group Study LAP2. J Clin Oncol. 2009;27:5331-5336.
- Zanagnolo V, Minig L, Rollo D, et al. Clinical and oncologic outcomes of robotic versus abdominal radical hysterectomy for women with cervical cancer: experience at a referral cancer center. Int J Gynecol Cancer. 2016;26:568-574.
- Wallin E, Floter Radestad A, et al. Introduction of robot-assisted radical hysterectomy for early stage cervical cancer: impact on complications, costs and oncologic outcome. Acta Obstet Gynecol Scand. 2017;96:536-542.
- Sert BM, Boggess JF, Ahmad S, et al. Robot-assisted versus open radical hysterectomy: a multi-institutional experience for early-stage cervical cancer. Euro J Surg Oncol. 2016;42:513-522.
- Shah CA, Beck T, Liao JB, et al. Surgical and oncologic outcomes after robotic radical hysterectomy as compared to open radical hysterectomy in the treatment of early cervical cancer. J Gynecol Oncol. 2017;28:e82.
- Ramirez PT, Frumovitz M, Pareja R, et al. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N Engl J Med. 2018;379:1895-1904.
- Melamed A, Margul DJ, Chen L, et al. Survival after minimally invasive radical hysterectomy for early stage cervical cancer. N Engl J Med. 2018;379:1905-1914.
- Margul DJ, Yang J, Seagle BL, et al. Outcomes and costs of open, robotic, and laparoscopic radical hysterectomy for stage IB1 cervical cancer. J Clin Oncol. 2018;36(15 suppl):5502.
- Geetha P, Nair MK. Laparoscopic, robotic and open method of radical hysterectomy for cervical cancer: a systematic review. J Minim Access Surg. 2012;8:67-73.
- Jin YM, Liu SS, Chen J, et al. Robotic radical hysterectomy is superior to laparoscopic radical hysterectomy and open radical hysterectomy in the treatment of cervical cancer. PloS One. 2018;13:e0193033.
- Rotman M, Sedlis A, Piedmonte MR, et al. A phase III randomized trial of postoperative pelvic irradiation in Stage IB cervical carcinoma with poor prognostic features: follow-up of a gynecologic oncology group study. Int J Radiation Oncol, Biol, Phys. 2006;65:169-176.
- Peters WA 3rd, Liu PY, Barrett RJ 2nd, et al. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J Clin Oncol. 2000;18:1606-1613.
- Uppal S, Liu RJ, Reynolds KR, et al. Trends and comparative effectiveness of inpatient radical hysterectomy for cervical cancer in the United States (2012-2015). Gynecol Oncol. 2018. pii: S0090-8258(18)31246-0.
- Barber EL, Van Le L. Enhanced Recovery Pathways in Gynecology and Gynecologic Oncology. Obstetr Gynecol Surv. 2015;70:780-792.
- Morche J, Mathes T, Pieper D. Relationship between surgeon volume and outcomes: a systematic review of systematic reviews. Syst Rev. 2016;5:204.
- Persson J, Reynisson P, Borgfeldt C, et al. Robot assisted laparoscopic radical hysterectomy and pelvic lymphadenectomy with short and long term morbidity data. Gynecol Oncol. 2009;113:185-190.
- Woelk JL, Casiano ER, Weaver AL, et al. The learning curve of robotic hysterectomy. Obstetr Gynecol. 2013;121:87-95.
- Yim GW, Kim SW, Nam EJ, et al. Learning curve analysis of robot-assisted radical hysterectomy for cervical cancer: initial experience at a single institution. J Gynecol Oncol. 2013;24:303-312.
- Vickers AJ, Bianco FJ, Serio AM, et al. The surgical learning curve for prostate cancer control after radical prostatectomy. J Natl Can Inst. 2007;99:1171-1177.
- Conrad LB, Ramirez PT, Burke W, et al. Role of minimally invasive surgery in gynecologic oncology: an updated survey of members of the Society of Gynecologic Oncology. Int J Gynecol Cancer. 2015;25:1121-1127.
A minimally invasive approach for gynecologic surgery increasingly has become the surgical modality of choice (vs open surgery) due to decreased perioperative and postoperative morbidity for many gynecologic cancers.1-3 This has included radical hysterectomy for cervical cancers. Until recently, retrospective evidence supported its use, suggesting decreased perioperative and postoperative complications with similar survival outcomes between patients undergoing minimally invasive and open radical hysterectomy.4,5 In November 2018, two new studies were published in the New England Journal of Medicine, and another study was presented at the American Society of Clinical Oncology (ASCO) annual meeting challenging this practice paradigm. These studies reveal a higher risk of disease recurrence and decreased overall survival with minimally invasive surgery (MIS) compared with open surgery for Stages IA–IB1 cervical cancer. These findings have resulted in a change in practice nationwide.
RCT findings astonish specialty
The first study, the Laparoscopic Approach to Cervical Cancer (LACC) trial, authored by Ramirez and colleagues was a noninferiority randomized controlled trial evaluating MIS versus open radical hysterectomy for patients with cervical cancer (Stage 1A–1B1) conducted from 2008–2017.6 The primary outcome was disease-free survival at 4.5 years. Secondary outcomes included recurrence and overall survival rates. Power analysis suggested a sample size of 740 patients to provide greater than 80% power with a noninferiority margin of -7.2% between disease-free rates of the two groups. However, the study was closed prematurely at enrollment of 631 patients (85% recruitment) by the Data Safety Monitoring Committee due to the astounding differences in survival between the two groups.
The rate of disease-free survival at 4.5 years was 86.0% with MIS and 96% with open surgery. There were 27 recurrences (8.5%) in the MIS group and only 7 (2.2%) in the open-surgery group, accounting for a hazard ratio (HR) for disease recurrence or death from cervical cancer of 3.74 (95% confidence interval [CI], 1.63–8.58). This difference remained after adjusting for confounding variables. There were 22 deaths—19 (5.9%) in the MIS group and 3 (0.1%) in the open-surgery group (HR, 6.56). Although patient characteristics between groups appeared to be similar, more than one-third of patients in each group had missing data regarding histology at the time of surgery, grade, tumor size, lymphovascular space invasion, and depth of invasion. Interestingly, intraoperative, perioperative, and postoperative complications between the two groups were similar (with rates of 11%, about 40%, and about 25%, respectively).
Surprising findings continue in NEJM
The second study, by Melamed and colleagues, was a retrospective cohort study using data from the National Cancer Database (NCDB) and the Surveillance, Epidemiology, and End Results (SEER) database evaluating women with stage IA2 or IB1 cervical cancer who underwent either minimally invasive or open radical hysterectomy between 2010 and 2013.7 The primary outcome was time to death.
Participant characteristics. A total of 2,461 women were included: 49.8% underwent MIS and 50.2% underwent open surgery. According to the raw data, patients undergoing MIS were more likely to be white, privately insured, reside in an area associated with higher income, undergo surgery at a nonacademic institution, have adenocarcinoma, and have smaller, lower-grade tumors. After propensity-score weighting, demographic and clinical characteristics were similar between groups. Median follow-up was 45 months.
Results. A total of 164 deaths occurred: 94 in the MIS and 70 in the open-surgery group. The risk of death during study follow-up was 9.1% in the MIS group versus 5.3% in the open-surgery group, and women who underwent MIS had shorter overall survival (P = .002; HR, 1.65; 95% CI, 1.22–2.22). Mortality rates remained higher in the MIS group after adjusting for adjuvant therapy (HR, 1.62; 95% CI, 1.2–2.19). However, the HR for death with MIS was not statistically significant in a subgroup analysis evaluating tumors 2 cm in size or less (HR, 1.46; 95% CI, 0.70–3.02). The authors demonstrated that the adoption of MIS for radical hysterectomy corresponded to a drop in the 4-year survival rate of 0.8% per year (P = .01).
Continue to: ASCO meeting data emphasize lower...
ASCO meeting data emphasize lower mortality and survival rates for MIS
A third important, but less publicized study, is a retrospective cohort study by Marguland and colleagues that was presented at the ASCO annual meeting and is pending publication. This study evaluated the 5-year survival of women with stage IB1 cervical cancer after MIS or open radical hysterectomy from 2010 to 2013.8 The findings demonstrated similar results to the above studies with decreased 5-year survival rates in patients with a tumor size of 2 cm or greater in the MIS group (81.3% vs 90.8; HR, 2.14; 95% CI, 1.36–3.38; P<.001). These results hold true when controlling for confounding clinical variables. Interestingly, in a subset analysis evaluating patients with tumors less than 2 cm, survival rates were similar between groups. This study confirms decreased morbidity and cost associated with MIS radical hysterectomy.
A consistent message emerges from 3 independent studies
We must take the study findings seriously and evaluate the quality of the evidence. There are many strengths to the above studies. First and most importantly, the LACC study is the only prospective randomized controlled trial (RCT) to evaluate this very important clinical question. RCTs are the gold standard for understanding the effectiveness and safety of an intervention compared with an established treatment. The study was well designed in that the study population was clearly defined with detailed inclusion and exclusion criteria. The intention to treat analysis was similar to the per-protocol analysis, and the study followed Consolidated Standards of Reporting Trials (CONSORT) guidelines. While the study was stopped early, there was still 84% power for the primary outcome. Therefore, when it comes to MIS for cervical cancer, this study provides the soundest data we have available. It is also extremely noteworthy that two additional large retrospective studies evaluating this question separately found similar results.
Criticisms remain, but older research has drawbacks
A main concern with these studies is that the findings challenge previously published research, which overall suggest similar survival outcomes between MIS and open surgical approaches. However, in evaluating the previously published retrospective data it is clear that the studies have considerable limitations.
Long-term survival not always evaluated in research. First, the majority of studies comparing MIS and open treatment modalities specifically evaluated perioperative complications and did not consider long-term survival.4,9,10 Of those studies that did consider survival outcomes, the groups often were not balanced and were skewed toward the open surgery patients having larger tumors and higher-stage disease.5
Difficult to compare “apples to apples.” These findings are complicated by the fact that open radical hysterectomies were essentially replaced by MIS radical hysterectomies, and therefore, the comparisons are not equivalent since they are comparing different treatment times. For instance, throughout the time period many of these studies were conducted, the treatment paradigm for early-stage cervical cancer changed regarding who received adjuvant therapy and imaging techniques. Therefore, these studies are not comparing apples to apples.11,12
Are we going to increase morbidity? Another common concern when considering abandoning MIS for cervical cancer is the increase in morbidity that our patients may incur immediately postoperatively due to open surgery. Multiple studies have associated minimally invasive radical hysterectomies with decreased blood loss, shorter hospital stay, lower transfusion rates, and decreased time until return of bowel function.4,10,13
Continue to: While we recognize that...
While we recognize that open surgery is associated with increased morbidity, we do argue that, with the almost-universal implementation of Enhanced Recovery Pathways (ERP) in gynecologic oncology, the disparities between the two groups will be minimized and likely are much smaller than that reported in historical literature.14 Notably, there were no differences in peri-, intra-, or postoperative complications between the two groups in the LACC study, indicating that MIS may not be saving our patients as much morbidity as we think.
Surgical ability differences. Despite the vast strengths associated with the studies we have discussed they certainly embody limitations as well. First, surgical aptitude is difficult to evaluate and tease out. This is extremely pertinent given perioperative, and postoperative, outcomes in cervical cancer, as well as survival outcomes, in multiple surgically managed cancers, which are directly associated with the volume and proficiency of the surgeon.15-19 Additionally, the mode of minimally invasive surgery that was most commonly utilized was different from practice in the United States. Eighty four percent of the patients in the MIS group of the LACC study underwent laparoscopic and 13.6% underwent robot-assisted radical hysterectomy. This is starkly different from US practice, where 75% of gynecologic oncologists report performing radical hysterectomies only robotically.20
Take-home points
Consider this latest evidence in your surgical planning. Most importantly, the evidence is the evidence. In other words, we can attempt to explain away the findings, but despite arguments against these studies, these data are the most reliable evidence we have to date regarding outcomes for cervical cancer with MIS versus open approaches. These data demonstrate that MIS may be harming our patients and so we must take this into careful consideration during surgical planning.
For small cancers, MIS may be the best option. MIS radical hysterectomy may still be the best approach for patients with tumors less than 2 cm in size. The LACC study is not powered to evaluate oncologic outcomes in this subset of patients and the two retrospective studies suggest no difference in survival in this cohort.
We must work to understand the driving force between the disparate outcomes. Are the increased rates due to the open surgical approach, the uterine manipulator, circulating CO2 gas, or tumor exposure to the intraperitoneal cavity as the authors suggest? Or is it due to surgical expertise, tumor biology, tumor size, or mode of MIS? At this point the impelling cause is unknown.
New NCCN guidelines are to come. Up to this point the National Comprehensive Cancer Network (NCCN) guidelines stated that “radical hysterectomy procedure may be performed either via laparotomy or laparoscopy.” Given these recent studies, however, new NCCN guidelines will be released cautioning the use of the MIS approach. In short, these data have transformed the standard of care.
At our institution, the majority of radical hysterectomies will be performed open. Continued discussion remains regarding small lesions, but even in these cases most surgeons will proceed with open surgery in an attempt to maximize survival.
As providers, it is our duty to honestly reflect on published data and comprehensively counsel patients about the risks and benefits associated with each approach, including the fact that recurrence may be higher with a minimally invasive approach. Patients and providers must then collectively decide what is best for each individual case.
A minimally invasive approach for gynecologic surgery increasingly has become the surgical modality of choice (vs open surgery) due to decreased perioperative and postoperative morbidity for many gynecologic cancers.1-3 This has included radical hysterectomy for cervical cancers. Until recently, retrospective evidence supported its use, suggesting decreased perioperative and postoperative complications with similar survival outcomes between patients undergoing minimally invasive and open radical hysterectomy.4,5 In November 2018, two new studies were published in the New England Journal of Medicine, and another study was presented at the American Society of Clinical Oncology (ASCO) annual meeting challenging this practice paradigm. These studies reveal a higher risk of disease recurrence and decreased overall survival with minimally invasive surgery (MIS) compared with open surgery for Stages IA–IB1 cervical cancer. These findings have resulted in a change in practice nationwide.
RCT findings astonish specialty
The first study, the Laparoscopic Approach to Cervical Cancer (LACC) trial, authored by Ramirez and colleagues was a noninferiority randomized controlled trial evaluating MIS versus open radical hysterectomy for patients with cervical cancer (Stage 1A–1B1) conducted from 2008–2017.6 The primary outcome was disease-free survival at 4.5 years. Secondary outcomes included recurrence and overall survival rates. Power analysis suggested a sample size of 740 patients to provide greater than 80% power with a noninferiority margin of -7.2% between disease-free rates of the two groups. However, the study was closed prematurely at enrollment of 631 patients (85% recruitment) by the Data Safety Monitoring Committee due to the astounding differences in survival between the two groups.
The rate of disease-free survival at 4.5 years was 86.0% with MIS and 96% with open surgery. There were 27 recurrences (8.5%) in the MIS group and only 7 (2.2%) in the open-surgery group, accounting for a hazard ratio (HR) for disease recurrence or death from cervical cancer of 3.74 (95% confidence interval [CI], 1.63–8.58). This difference remained after adjusting for confounding variables. There were 22 deaths—19 (5.9%) in the MIS group and 3 (0.1%) in the open-surgery group (HR, 6.56). Although patient characteristics between groups appeared to be similar, more than one-third of patients in each group had missing data regarding histology at the time of surgery, grade, tumor size, lymphovascular space invasion, and depth of invasion. Interestingly, intraoperative, perioperative, and postoperative complications between the two groups were similar (with rates of 11%, about 40%, and about 25%, respectively).
Surprising findings continue in NEJM
The second study, by Melamed and colleagues, was a retrospective cohort study using data from the National Cancer Database (NCDB) and the Surveillance, Epidemiology, and End Results (SEER) database evaluating women with stage IA2 or IB1 cervical cancer who underwent either minimally invasive or open radical hysterectomy between 2010 and 2013.7 The primary outcome was time to death.
Participant characteristics. A total of 2,461 women were included: 49.8% underwent MIS and 50.2% underwent open surgery. According to the raw data, patients undergoing MIS were more likely to be white, privately insured, reside in an area associated with higher income, undergo surgery at a nonacademic institution, have adenocarcinoma, and have smaller, lower-grade tumors. After propensity-score weighting, demographic and clinical characteristics were similar between groups. Median follow-up was 45 months.
Results. A total of 164 deaths occurred: 94 in the MIS and 70 in the open-surgery group. The risk of death during study follow-up was 9.1% in the MIS group versus 5.3% in the open-surgery group, and women who underwent MIS had shorter overall survival (P = .002; HR, 1.65; 95% CI, 1.22–2.22). Mortality rates remained higher in the MIS group after adjusting for adjuvant therapy (HR, 1.62; 95% CI, 1.2–2.19). However, the HR for death with MIS was not statistically significant in a subgroup analysis evaluating tumors 2 cm in size or less (HR, 1.46; 95% CI, 0.70–3.02). The authors demonstrated that the adoption of MIS for radical hysterectomy corresponded to a drop in the 4-year survival rate of 0.8% per year (P = .01).
Continue to: ASCO meeting data emphasize lower...
ASCO meeting data emphasize lower mortality and survival rates for MIS
A third important, but less publicized study, is a retrospective cohort study by Marguland and colleagues that was presented at the ASCO annual meeting and is pending publication. This study evaluated the 5-year survival of women with stage IB1 cervical cancer after MIS or open radical hysterectomy from 2010 to 2013.8 The findings demonstrated similar results to the above studies with decreased 5-year survival rates in patients with a tumor size of 2 cm or greater in the MIS group (81.3% vs 90.8; HR, 2.14; 95% CI, 1.36–3.38; P<.001). These results hold true when controlling for confounding clinical variables. Interestingly, in a subset analysis evaluating patients with tumors less than 2 cm, survival rates were similar between groups. This study confirms decreased morbidity and cost associated with MIS radical hysterectomy.
A consistent message emerges from 3 independent studies
We must take the study findings seriously and evaluate the quality of the evidence. There are many strengths to the above studies. First and most importantly, the LACC study is the only prospective randomized controlled trial (RCT) to evaluate this very important clinical question. RCTs are the gold standard for understanding the effectiveness and safety of an intervention compared with an established treatment. The study was well designed in that the study population was clearly defined with detailed inclusion and exclusion criteria. The intention to treat analysis was similar to the per-protocol analysis, and the study followed Consolidated Standards of Reporting Trials (CONSORT) guidelines. While the study was stopped early, there was still 84% power for the primary outcome. Therefore, when it comes to MIS for cervical cancer, this study provides the soundest data we have available. It is also extremely noteworthy that two additional large retrospective studies evaluating this question separately found similar results.
Criticisms remain, but older research has drawbacks
A main concern with these studies is that the findings challenge previously published research, which overall suggest similar survival outcomes between MIS and open surgical approaches. However, in evaluating the previously published retrospective data it is clear that the studies have considerable limitations.
Long-term survival not always evaluated in research. First, the majority of studies comparing MIS and open treatment modalities specifically evaluated perioperative complications and did not consider long-term survival.4,9,10 Of those studies that did consider survival outcomes, the groups often were not balanced and were skewed toward the open surgery patients having larger tumors and higher-stage disease.5
Difficult to compare “apples to apples.” These findings are complicated by the fact that open radical hysterectomies were essentially replaced by MIS radical hysterectomies, and therefore, the comparisons are not equivalent since they are comparing different treatment times. For instance, throughout the time period many of these studies were conducted, the treatment paradigm for early-stage cervical cancer changed regarding who received adjuvant therapy and imaging techniques. Therefore, these studies are not comparing apples to apples.11,12
Are we going to increase morbidity? Another common concern when considering abandoning MIS for cervical cancer is the increase in morbidity that our patients may incur immediately postoperatively due to open surgery. Multiple studies have associated minimally invasive radical hysterectomies with decreased blood loss, shorter hospital stay, lower transfusion rates, and decreased time until return of bowel function.4,10,13
Continue to: While we recognize that...
While we recognize that open surgery is associated with increased morbidity, we do argue that, with the almost-universal implementation of Enhanced Recovery Pathways (ERP) in gynecologic oncology, the disparities between the two groups will be minimized and likely are much smaller than that reported in historical literature.14 Notably, there were no differences in peri-, intra-, or postoperative complications between the two groups in the LACC study, indicating that MIS may not be saving our patients as much morbidity as we think.
Surgical ability differences. Despite the vast strengths associated with the studies we have discussed they certainly embody limitations as well. First, surgical aptitude is difficult to evaluate and tease out. This is extremely pertinent given perioperative, and postoperative, outcomes in cervical cancer, as well as survival outcomes, in multiple surgically managed cancers, which are directly associated with the volume and proficiency of the surgeon.15-19 Additionally, the mode of minimally invasive surgery that was most commonly utilized was different from practice in the United States. Eighty four percent of the patients in the MIS group of the LACC study underwent laparoscopic and 13.6% underwent robot-assisted radical hysterectomy. This is starkly different from US practice, where 75% of gynecologic oncologists report performing radical hysterectomies only robotically.20
Take-home points
Consider this latest evidence in your surgical planning. Most importantly, the evidence is the evidence. In other words, we can attempt to explain away the findings, but despite arguments against these studies, these data are the most reliable evidence we have to date regarding outcomes for cervical cancer with MIS versus open approaches. These data demonstrate that MIS may be harming our patients and so we must take this into careful consideration during surgical planning.
For small cancers, MIS may be the best option. MIS radical hysterectomy may still be the best approach for patients with tumors less than 2 cm in size. The LACC study is not powered to evaluate oncologic outcomes in this subset of patients and the two retrospective studies suggest no difference in survival in this cohort.
We must work to understand the driving force between the disparate outcomes. Are the increased rates due to the open surgical approach, the uterine manipulator, circulating CO2 gas, or tumor exposure to the intraperitoneal cavity as the authors suggest? Or is it due to surgical expertise, tumor biology, tumor size, or mode of MIS? At this point the impelling cause is unknown.
New NCCN guidelines are to come. Up to this point the National Comprehensive Cancer Network (NCCN) guidelines stated that “radical hysterectomy procedure may be performed either via laparotomy or laparoscopy.” Given these recent studies, however, new NCCN guidelines will be released cautioning the use of the MIS approach. In short, these data have transformed the standard of care.
At our institution, the majority of radical hysterectomies will be performed open. Continued discussion remains regarding small lesions, but even in these cases most surgeons will proceed with open surgery in an attempt to maximize survival.
As providers, it is our duty to honestly reflect on published data and comprehensively counsel patients about the risks and benefits associated with each approach, including the fact that recurrence may be higher with a minimally invasive approach. Patients and providers must then collectively decide what is best for each individual case.
- Walker JL, Piedmonte MR, Spirtos NM, et al. Laparoscopy compared with laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group Study LAP2. J Clin Oncol. 2009;27:5331-5336.
- Zanagnolo V, Minig L, Rollo D, et al. Clinical and oncologic outcomes of robotic versus abdominal radical hysterectomy for women with cervical cancer: experience at a referral cancer center. Int J Gynecol Cancer. 2016;26:568-574.
- Wallin E, Floter Radestad A, et al. Introduction of robot-assisted radical hysterectomy for early stage cervical cancer: impact on complications, costs and oncologic outcome. Acta Obstet Gynecol Scand. 2017;96:536-542.
- Sert BM, Boggess JF, Ahmad S, et al. Robot-assisted versus open radical hysterectomy: a multi-institutional experience for early-stage cervical cancer. Euro J Surg Oncol. 2016;42:513-522.
- Shah CA, Beck T, Liao JB, et al. Surgical and oncologic outcomes after robotic radical hysterectomy as compared to open radical hysterectomy in the treatment of early cervical cancer. J Gynecol Oncol. 2017;28:e82.
- Ramirez PT, Frumovitz M, Pareja R, et al. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N Engl J Med. 2018;379:1895-1904.
- Melamed A, Margul DJ, Chen L, et al. Survival after minimally invasive radical hysterectomy for early stage cervical cancer. N Engl J Med. 2018;379:1905-1914.
- Margul DJ, Yang J, Seagle BL, et al. Outcomes and costs of open, robotic, and laparoscopic radical hysterectomy for stage IB1 cervical cancer. J Clin Oncol. 2018;36(15 suppl):5502.
- Geetha P, Nair MK. Laparoscopic, robotic and open method of radical hysterectomy for cervical cancer: a systematic review. J Minim Access Surg. 2012;8:67-73.
- Jin YM, Liu SS, Chen J, et al. Robotic radical hysterectomy is superior to laparoscopic radical hysterectomy and open radical hysterectomy in the treatment of cervical cancer. PloS One. 2018;13:e0193033.
- Rotman M, Sedlis A, Piedmonte MR, et al. A phase III randomized trial of postoperative pelvic irradiation in Stage IB cervical carcinoma with poor prognostic features: follow-up of a gynecologic oncology group study. Int J Radiation Oncol, Biol, Phys. 2006;65:169-176.
- Peters WA 3rd, Liu PY, Barrett RJ 2nd, et al. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J Clin Oncol. 2000;18:1606-1613.
- Uppal S, Liu RJ, Reynolds KR, et al. Trends and comparative effectiveness of inpatient radical hysterectomy for cervical cancer in the United States (2012-2015). Gynecol Oncol. 2018. pii: S0090-8258(18)31246-0.
- Barber EL, Van Le L. Enhanced Recovery Pathways in Gynecology and Gynecologic Oncology. Obstetr Gynecol Surv. 2015;70:780-792.
- Morche J, Mathes T, Pieper D. Relationship between surgeon volume and outcomes: a systematic review of systematic reviews. Syst Rev. 2016;5:204.
- Persson J, Reynisson P, Borgfeldt C, et al. Robot assisted laparoscopic radical hysterectomy and pelvic lymphadenectomy with short and long term morbidity data. Gynecol Oncol. 2009;113:185-190.
- Woelk JL, Casiano ER, Weaver AL, et al. The learning curve of robotic hysterectomy. Obstetr Gynecol. 2013;121:87-95.
- Yim GW, Kim SW, Nam EJ, et al. Learning curve analysis of robot-assisted radical hysterectomy for cervical cancer: initial experience at a single institution. J Gynecol Oncol. 2013;24:303-312.
- Vickers AJ, Bianco FJ, Serio AM, et al. The surgical learning curve for prostate cancer control after radical prostatectomy. J Natl Can Inst. 2007;99:1171-1177.
- Conrad LB, Ramirez PT, Burke W, et al. Role of minimally invasive surgery in gynecologic oncology: an updated survey of members of the Society of Gynecologic Oncology. Int J Gynecol Cancer. 2015;25:1121-1127.
- Walker JL, Piedmonte MR, Spirtos NM, et al. Laparoscopy compared with laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group Study LAP2. J Clin Oncol. 2009;27:5331-5336.
- Zanagnolo V, Minig L, Rollo D, et al. Clinical and oncologic outcomes of robotic versus abdominal radical hysterectomy for women with cervical cancer: experience at a referral cancer center. Int J Gynecol Cancer. 2016;26:568-574.
- Wallin E, Floter Radestad A, et al. Introduction of robot-assisted radical hysterectomy for early stage cervical cancer: impact on complications, costs and oncologic outcome. Acta Obstet Gynecol Scand. 2017;96:536-542.
- Sert BM, Boggess JF, Ahmad S, et al. Robot-assisted versus open radical hysterectomy: a multi-institutional experience for early-stage cervical cancer. Euro J Surg Oncol. 2016;42:513-522.
- Shah CA, Beck T, Liao JB, et al. Surgical and oncologic outcomes after robotic radical hysterectomy as compared to open radical hysterectomy in the treatment of early cervical cancer. J Gynecol Oncol. 2017;28:e82.
- Ramirez PT, Frumovitz M, Pareja R, et al. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N Engl J Med. 2018;379:1895-1904.
- Melamed A, Margul DJ, Chen L, et al. Survival after minimally invasive radical hysterectomy for early stage cervical cancer. N Engl J Med. 2018;379:1905-1914.
- Margul DJ, Yang J, Seagle BL, et al. Outcomes and costs of open, robotic, and laparoscopic radical hysterectomy for stage IB1 cervical cancer. J Clin Oncol. 2018;36(15 suppl):5502.
- Geetha P, Nair MK. Laparoscopic, robotic and open method of radical hysterectomy for cervical cancer: a systematic review. J Minim Access Surg. 2012;8:67-73.
- Jin YM, Liu SS, Chen J, et al. Robotic radical hysterectomy is superior to laparoscopic radical hysterectomy and open radical hysterectomy in the treatment of cervical cancer. PloS One. 2018;13:e0193033.
- Rotman M, Sedlis A, Piedmonte MR, et al. A phase III randomized trial of postoperative pelvic irradiation in Stage IB cervical carcinoma with poor prognostic features: follow-up of a gynecologic oncology group study. Int J Radiation Oncol, Biol, Phys. 2006;65:169-176.
- Peters WA 3rd, Liu PY, Barrett RJ 2nd, et al. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J Clin Oncol. 2000;18:1606-1613.
- Uppal S, Liu RJ, Reynolds KR, et al. Trends and comparative effectiveness of inpatient radical hysterectomy for cervical cancer in the United States (2012-2015). Gynecol Oncol. 2018. pii: S0090-8258(18)31246-0.
- Barber EL, Van Le L. Enhanced Recovery Pathways in Gynecology and Gynecologic Oncology. Obstetr Gynecol Surv. 2015;70:780-792.
- Morche J, Mathes T, Pieper D. Relationship between surgeon volume and outcomes: a systematic review of systematic reviews. Syst Rev. 2016;5:204.
- Persson J, Reynisson P, Borgfeldt C, et al. Robot assisted laparoscopic radical hysterectomy and pelvic lymphadenectomy with short and long term morbidity data. Gynecol Oncol. 2009;113:185-190.
- Woelk JL, Casiano ER, Weaver AL, et al. The learning curve of robotic hysterectomy. Obstetr Gynecol. 2013;121:87-95.
- Yim GW, Kim SW, Nam EJ, et al. Learning curve analysis of robot-assisted radical hysterectomy for cervical cancer: initial experience at a single institution. J Gynecol Oncol. 2013;24:303-312.
- Vickers AJ, Bianco FJ, Serio AM, et al. The surgical learning curve for prostate cancer control after radical prostatectomy. J Natl Can Inst. 2007;99:1171-1177.
- Conrad LB, Ramirez PT, Burke W, et al. Role of minimally invasive surgery in gynecologic oncology: an updated survey of members of the Society of Gynecologic Oncology. Int J Gynecol Cancer. 2015;25:1121-1127.
The HPV vaccine is now recommended for adults aged 27–45: Counseling implications
The US Food and Drug Administration (FDA) recently extended the approval for Gardasil 9 (to prevent HPV-associated cancers, cancer precursors, and genital lesions) to men and women aged 27 to 45.1 In this editorial, we discuss the evolution of the HPV vaccine since its initial approval more than 10 years ago, the benefits of primary prevention with the HPV vaccine, and the case for the FDA’s recent extension of coverage to older men and women.
The evolution of the HPV vaccine
Since recognition in the 1980s and 90s that high-risk strains of HPV, notably HPV types 16 and 18, were linked to cervical cancer, there have been exciting advances in detection and prevention of high-risk HPV infection. About 70% of cervical cancers are attributable to these 2 oncogenic types.2 The first vaccine licensed, Gardasil (Merck), was approved in 2006 for girls and women aged 9 through 26 to prevent HPV-related diseases caused by types 6, 11, 16, and 18.3 The vaccine was effective for prevention of cervical cancer; genital warts; and grades 2 and 3 of cervical, vulvar, and vaginal intraepithelial neoplasia. In 2008, prevention of vulvar and vaginal cancers was added to the indication. By 2009, prevention of genital warts was added, and use in males aged 9 to 15 was approved. By 2010 sufficient data were accumulated to document prevention of anal cancer and anal intraepithelial neoplasia in men and women, and this indication was added.
In 2014 Gardasil 9 was approved to extend coverage to an additional 5 oncogenic HPV types (31, 33, 45, 52, and 58), now covering an additional 20% of cervical cancers, and in 2015 Gardasil 9 indications were expanded to include boys and men 9 to 26 years of age. Immunogenicity studies were performed to infer effectiveness of a 2-dose regimen in boys and girls aged 9 to 14 years, which was recommended by the Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC) in late 2016.4
Until October 2018, Gardasil 9 was indicated for prevention of genital warts, cervical, vaginal, vulvar and anal cancers and cancer precursors for males and females aged 9 to 26 years. In October the FDA extended approval of the 3-dose vaccine regimen to men and women up to age 45.
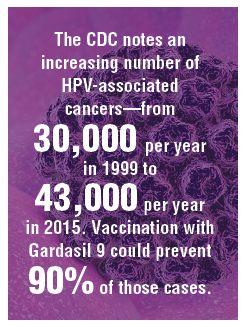
HPV vaccine uptake
HPV vaccination has been underutilized in the United States. In 2017, a disappointing 49% of adolescents were up to date on vaccination, and 66% had received at least one dose.5 In rural areas the vaccination rates are 11 points lower than in urban regions.6 The CDC notes an increasing number of HPV-associated cancers—from 30,000 per year in 1999 to 43,000 per year in 2015—due mostly to increases in oral and anal carcinomas. Vaccination with Gardasil 9 could prevent 90% of those cases.7
Non-US successes. HPV vaccine uptake in Australia provides an excellent opportunity to study the impact of universally available, school-based vaccinations. In 2007 Australia implemented a program of free HPV vaccination distributed through schools. Boys and girls aged 12 and 13 were targeted that year, with catch-up vaccinations for those aged 13 to 18 in 2007-2009 in schools and for those aged 18 to 26 reached in the community.8
Continue to: Ali and colleagues studied the...
Ali and colleagues studied the preprogram and postprogram incidence of genital warts.9 About 83% received at least 1 dose of vaccine, and 73% of the eligible population completed the 3-dose regimen. There was a significant reduction in warts in both men and women younger than age 21 from 2007 to 2011 (12.1% to 2.2% in men and 11.5% to 0.85% in women). In the 21 to 30 age group there were similar reductions. This study demonstrates that with universal access and public implementation, the rates of HPV-associated disease can be reduced dramatically.
Data informing expanded vaccination ages
Will vaccination of an older population, with presumably many of whom sexually active and at risk for prior exposure to multiple HPV types, have a reasonable impact on lowering HPV-associated cancers? Are HPV-detected lesions in 27- to 45-year-old women the result of reactivation of latent HPV infection, or are they related to new-onset exposure? The FDA reviewed data from 3 studies of HPV vaccination in women aged 27 to 45. The first enrolled women who were naïve to oncogenic HPV types and provided all 3 doses of quadrivalent vaccine were followed for 4 years, along with a comparison group of nonvaccinated women. The second study allowed the nonvaccinated group to receive vaccine in year 4. Both groups were followed up to 10 years with the relevant outcome defined as cumulative incidence of HPV 6/11/16/18-related CIN and condyloma. The third study looked at the same outcomes in a set of all women—whether HPV high-risk naïve or not—after receiving vaccine and followed more than 10 years.7 This last study is most relevant to ObGyns, as it is closest to how we would consider vaccinating our patients.
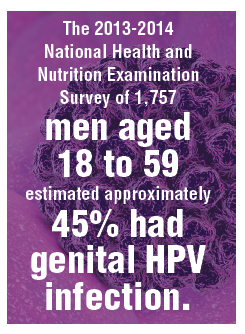
The study findings are reassuring: A large proportion of HPV infections in women between 27 and 45 are the result of new exposure/infection. A study of 420 online daters aged 25 to 65 showed an annual incidence of high-risk HPV types in vaginal swabs of 25.4%, of which 64% were likely new acquisitions.10 The 2013-2014 National Health and Nutrition Examination Survey of 1,757 men aged 18 to 59 estimated approximately 45% had genital HPV infection. There was a bimodal distribution of disease with peaks at 28 to 32 and a larger second peak at 58 to 59 years of age.11 Bottom line: Men and women older than age 26 who are sexually active likely acquire new HPV infections with oncogenic types. Exposure to high-risk HPV types prior to vaccination—as we would expect in the real-world setting—did not eliminate the substantial benefit of immunization.
Based on these study results, and extrapolation to the 9-valent vaccine, the FDA extended the approval of Gardasil 9 to men and women from age 9 to 45. The indications and usage will remain the same: for prevention of cervical, vulvar, vaginal, and anal cancer and genital warts as well as precancerous or dysplastic lesions of the cervix, vulva, vagina, and anus related to HPV types 6, 11, 16, 18, 31, 33, 45, 52, and 58.
Continue to: Impact of the new...
Impact of the new indication on HPV-related disease
As described above, widespread vaccination of young girls and boys is going to have major impact on HPV-related disease, including precancer and cancer. Because there is evidence that older women and men are at risk for new HPV infection,10 there likely will be some benefit from vaccination of adults. It is difficult, however, to extrapolate the degree to which adult vaccination will impact HPV-related disease. This is because we do not fully understand the rates at which new HPV infection in the cervices of older women will progress to high-grade dysplasia or cancer. Further, the pathophysiology of HPV-related cancers at other anogenital sites and new oral-pharyngeal infection is poorly understood in comparison with our knowledge of the natural history of high-risk HPV infection in younger women. That said, because of the outstanding efficacy of HPV vaccination and the low-risk profile, even if the actual impact on prevention of cancer or morbidity from dysplasia is relatively low, adult vaccination benefits outweigh the limited risks.
It may be that increased vaccination and awareness of vaccination for adults may enhance the adherence and acceptance of widespread vaccination of boys and girls. Adult vaccination could create a cultural shift toward HPV vaccination acceptance when adult parents and loved ones of vaccine-age boys and girls have been vaccinated themselves.
Current and future insurance coverage
The Affordable Care Act, otherwise known as Obamacare, mandates coverage for all immunizations recommended by the ACIP. HPV vaccination up to age 26 is fully covered, without copay or deductible. The ACIP did consider extension of the indications for HPV vaccination to men and women up to age 45 at their October 2018 meeting. They are tasked with considering not only safety and efficacy but also the cost effectiveness of implementing vaccination. They continue to study the costs and potential benefits of extending HPV vaccination to age 45. Their recommendations may be determined at the February 2019 meeting—or even later in 2019. The American College of Obstetricians and Gynecologists (ACOG) relies upon ACIP for practice guidance. Once the ACIP has made a determination, and if new guidelines are published in the Morbidity and Mortality Weekly Report, insurance coverage and ACOG guidance will be updated.
How should we react and change practice based on this new indication?
Given the information reviewed by the FDA, ObGyns will want to discuss the availability of Gardasil 9 with our patients between ages 27 and 45 who have not been previously immunized.

Especially for our patients with exposure to multiple or new sexual partners, immunization against oncogenic HPV viral types is effective in providing protection from cancer precursors and cancers of the cervix, vulva, vagina, and anus—and of course from genital warts. They should understand that, until formal recommendations are published by the ACIP, they are likely to be responsible for the cost of the vaccination series. These conversations will also remind our patients to immunize their teens against HPV. The more conversation we have regarding the benefits of vaccination against high-risk HPV types, the more likely we are to be able to achieve the impressive results seen in Australia.
- US Food and Drug Administration website. FDA approves expanded use of Gardasil 9 to include individuals 27 through 45 years old. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm622715.htm. Updated October 9, 2018. Accessed December 27, 2018.
- World Health Organization website. Human papillomavirus (HPV) and cervical cancer. https://www.who.int/news-room/fact-sheets/detail/human-papillomavirus-(hpv)-and-cervical-cancer. February 15, 2018. Accessed December 27, 2018.
- Centers for Disease Control and Prevention website. Human papillomavirus (HPV) vaccine safety. https://www.cdc.gov/vaccinesafety/vaccines/hpv-vaccine.html. Last reviewed October 27, 2015. Accessed December 27, 2018.
- Meites E, Kempe A, Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination—updated recommendations of the Advisory Committee on Immunization Practices. MMWR. 2016;65(49):1405–1408.
- AAP News website. Jenko M. CDC: 49% of teens up to date on HPV vaccine. http://www.aappublications.org/news/2018/08/23/vac cinationrates082318. August 23, 2018. Accessed December 27, 2018.
- Walker TY, Elam-Evans LD, Yankey D, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years—United States, 2017. MMWR Morb Mortal Wkly Rep. 2018;67:909-917.
- Montague L. Summary basis for regulatory action. October 5, 2018. https://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM622941.pdf. Accessed December 27, 2018.
- Tabrizi SN, Brotherton JM, Kaldor JM, et al. Fall in human papillomavirus prevalence following a national vaccination program. J Infect Dis. 2012;206:1645-1651.
- Ali H, Donovan B, Wand H, et al. Genital warts in young Australians five years into national human papillomavirus vaccination programme: national surveillance data [published correction appears in BMJ. 2013;346:F2942]. BMJ. 2013;346:F2032.
- Winer RL, Hughes JP, Feng Q, et al. Incident detection of high-risk human papillomavirus infections in a cohort of high-risk women aged 25-65 years. J Infect Dis. 2016;214:665-675.
- Han JJ, Beltran TH, Song JW, et al. Prevalence of genital human papillomavirus infection and human papillomavirus vaccination rates among US adult men: National Health and Nutrition Examination Survey (NHANES) 2013-2014. JAMA Oncol. 2017;3:810-816.
The US Food and Drug Administration (FDA) recently extended the approval for Gardasil 9 (to prevent HPV-associated cancers, cancer precursors, and genital lesions) to men and women aged 27 to 45.1 In this editorial, we discuss the evolution of the HPV vaccine since its initial approval more than 10 years ago, the benefits of primary prevention with the HPV vaccine, and the case for the FDA’s recent extension of coverage to older men and women.
The evolution of the HPV vaccine
Since recognition in the 1980s and 90s that high-risk strains of HPV, notably HPV types 16 and 18, were linked to cervical cancer, there have been exciting advances in detection and prevention of high-risk HPV infection. About 70% of cervical cancers are attributable to these 2 oncogenic types.2 The first vaccine licensed, Gardasil (Merck), was approved in 2006 for girls and women aged 9 through 26 to prevent HPV-related diseases caused by types 6, 11, 16, and 18.3 The vaccine was effective for prevention of cervical cancer; genital warts; and grades 2 and 3 of cervical, vulvar, and vaginal intraepithelial neoplasia. In 2008, prevention of vulvar and vaginal cancers was added to the indication. By 2009, prevention of genital warts was added, and use in males aged 9 to 15 was approved. By 2010 sufficient data were accumulated to document prevention of anal cancer and anal intraepithelial neoplasia in men and women, and this indication was added.
In 2014 Gardasil 9 was approved to extend coverage to an additional 5 oncogenic HPV types (31, 33, 45, 52, and 58), now covering an additional 20% of cervical cancers, and in 2015 Gardasil 9 indications were expanded to include boys and men 9 to 26 years of age. Immunogenicity studies were performed to infer effectiveness of a 2-dose regimen in boys and girls aged 9 to 14 years, which was recommended by the Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC) in late 2016.4
Until October 2018, Gardasil 9 was indicated for prevention of genital warts, cervical, vaginal, vulvar and anal cancers and cancer precursors for males and females aged 9 to 26 years. In October the FDA extended approval of the 3-dose vaccine regimen to men and women up to age 45.

HPV vaccine uptake
HPV vaccination has been underutilized in the United States. In 2017, a disappointing 49% of adolescents were up to date on vaccination, and 66% had received at least one dose.5 In rural areas the vaccination rates are 11 points lower than in urban regions.6 The CDC notes an increasing number of HPV-associated cancers—from 30,000 per year in 1999 to 43,000 per year in 2015—due mostly to increases in oral and anal carcinomas. Vaccination with Gardasil 9 could prevent 90% of those cases.7
Non-US successes. HPV vaccine uptake in Australia provides an excellent opportunity to study the impact of universally available, school-based vaccinations. In 2007 Australia implemented a program of free HPV vaccination distributed through schools. Boys and girls aged 12 and 13 were targeted that year, with catch-up vaccinations for those aged 13 to 18 in 2007-2009 in schools and for those aged 18 to 26 reached in the community.8
Continue to: Ali and colleagues studied the...
Ali and colleagues studied the preprogram and postprogram incidence of genital warts.9 About 83% received at least 1 dose of vaccine, and 73% of the eligible population completed the 3-dose regimen. There was a significant reduction in warts in both men and women younger than age 21 from 2007 to 2011 (12.1% to 2.2% in men and 11.5% to 0.85% in women). In the 21 to 30 age group there were similar reductions. This study demonstrates that with universal access and public implementation, the rates of HPV-associated disease can be reduced dramatically.
Data informing expanded vaccination ages
Will vaccination of an older population, with presumably many of whom sexually active and at risk for prior exposure to multiple HPV types, have a reasonable impact on lowering HPV-associated cancers? Are HPV-detected lesions in 27- to 45-year-old women the result of reactivation of latent HPV infection, or are they related to new-onset exposure? The FDA reviewed data from 3 studies of HPV vaccination in women aged 27 to 45. The first enrolled women who were naïve to oncogenic HPV types and provided all 3 doses of quadrivalent vaccine were followed for 4 years, along with a comparison group of nonvaccinated women. The second study allowed the nonvaccinated group to receive vaccine in year 4. Both groups were followed up to 10 years with the relevant outcome defined as cumulative incidence of HPV 6/11/16/18-related CIN and condyloma. The third study looked at the same outcomes in a set of all women—whether HPV high-risk naïve or not—after receiving vaccine and followed more than 10 years.7 This last study is most relevant to ObGyns, as it is closest to how we would consider vaccinating our patients.

The study findings are reassuring: A large proportion of HPV infections in women between 27 and 45 are the result of new exposure/infection. A study of 420 online daters aged 25 to 65 showed an annual incidence of high-risk HPV types in vaginal swabs of 25.4%, of which 64% were likely new acquisitions.10 The 2013-2014 National Health and Nutrition Examination Survey of 1,757 men aged 18 to 59 estimated approximately 45% had genital HPV infection. There was a bimodal distribution of disease with peaks at 28 to 32 and a larger second peak at 58 to 59 years of age.11 Bottom line: Men and women older than age 26 who are sexually active likely acquire new HPV infections with oncogenic types. Exposure to high-risk HPV types prior to vaccination—as we would expect in the real-world setting—did not eliminate the substantial benefit of immunization.
Based on these study results, and extrapolation to the 9-valent vaccine, the FDA extended the approval of Gardasil 9 to men and women from age 9 to 45. The indications and usage will remain the same: for prevention of cervical, vulvar, vaginal, and anal cancer and genital warts as well as precancerous or dysplastic lesions of the cervix, vulva, vagina, and anus related to HPV types 6, 11, 16, 18, 31, 33, 45, 52, and 58.
Continue to: Impact of the new...
Impact of the new indication on HPV-related disease
As described above, widespread vaccination of young girls and boys is going to have major impact on HPV-related disease, including precancer and cancer. Because there is evidence that older women and men are at risk for new HPV infection,10 there likely will be some benefit from vaccination of adults. It is difficult, however, to extrapolate the degree to which adult vaccination will impact HPV-related disease. This is because we do not fully understand the rates at which new HPV infection in the cervices of older women will progress to high-grade dysplasia or cancer. Further, the pathophysiology of HPV-related cancers at other anogenital sites and new oral-pharyngeal infection is poorly understood in comparison with our knowledge of the natural history of high-risk HPV infection in younger women. That said, because of the outstanding efficacy of HPV vaccination and the low-risk profile, even if the actual impact on prevention of cancer or morbidity from dysplasia is relatively low, adult vaccination benefits outweigh the limited risks.
It may be that increased vaccination and awareness of vaccination for adults may enhance the adherence and acceptance of widespread vaccination of boys and girls. Adult vaccination could create a cultural shift toward HPV vaccination acceptance when adult parents and loved ones of vaccine-age boys and girls have been vaccinated themselves.
Current and future insurance coverage
The Affordable Care Act, otherwise known as Obamacare, mandates coverage for all immunizations recommended by the ACIP. HPV vaccination up to age 26 is fully covered, without copay or deductible. The ACIP did consider extension of the indications for HPV vaccination to men and women up to age 45 at their October 2018 meeting. They are tasked with considering not only safety and efficacy but also the cost effectiveness of implementing vaccination. They continue to study the costs and potential benefits of extending HPV vaccination to age 45. Their recommendations may be determined at the February 2019 meeting—or even later in 2019. The American College of Obstetricians and Gynecologists (ACOG) relies upon ACIP for practice guidance. Once the ACIP has made a determination, and if new guidelines are published in the Morbidity and Mortality Weekly Report, insurance coverage and ACOG guidance will be updated.
How should we react and change practice based on this new indication?
Given the information reviewed by the FDA, ObGyns will want to discuss the availability of Gardasil 9 with our patients between ages 27 and 45 who have not been previously immunized.

Especially for our patients with exposure to multiple or new sexual partners, immunization against oncogenic HPV viral types is effective in providing protection from cancer precursors and cancers of the cervix, vulva, vagina, and anus—and of course from genital warts. They should understand that, until formal recommendations are published by the ACIP, they are likely to be responsible for the cost of the vaccination series. These conversations will also remind our patients to immunize their teens against HPV. The more conversation we have regarding the benefits of vaccination against high-risk HPV types, the more likely we are to be able to achieve the impressive results seen in Australia.
The US Food and Drug Administration (FDA) recently extended the approval for Gardasil 9 (to prevent HPV-associated cancers, cancer precursors, and genital lesions) to men and women aged 27 to 45.1 In this editorial, we discuss the evolution of the HPV vaccine since its initial approval more than 10 years ago, the benefits of primary prevention with the HPV vaccine, and the case for the FDA’s recent extension of coverage to older men and women.
The evolution of the HPV vaccine
Since recognition in the 1980s and 90s that high-risk strains of HPV, notably HPV types 16 and 18, were linked to cervical cancer, there have been exciting advances in detection and prevention of high-risk HPV infection. About 70% of cervical cancers are attributable to these 2 oncogenic types.2 The first vaccine licensed, Gardasil (Merck), was approved in 2006 for girls and women aged 9 through 26 to prevent HPV-related diseases caused by types 6, 11, 16, and 18.3 The vaccine was effective for prevention of cervical cancer; genital warts; and grades 2 and 3 of cervical, vulvar, and vaginal intraepithelial neoplasia. In 2008, prevention of vulvar and vaginal cancers was added to the indication. By 2009, prevention of genital warts was added, and use in males aged 9 to 15 was approved. By 2010 sufficient data were accumulated to document prevention of anal cancer and anal intraepithelial neoplasia in men and women, and this indication was added.
In 2014 Gardasil 9 was approved to extend coverage to an additional 5 oncogenic HPV types (31, 33, 45, 52, and 58), now covering an additional 20% of cervical cancers, and in 2015 Gardasil 9 indications were expanded to include boys and men 9 to 26 years of age. Immunogenicity studies were performed to infer effectiveness of a 2-dose regimen in boys and girls aged 9 to 14 years, which was recommended by the Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC) in late 2016.4
Until October 2018, Gardasil 9 was indicated for prevention of genital warts, cervical, vaginal, vulvar and anal cancers and cancer precursors for males and females aged 9 to 26 years. In October the FDA extended approval of the 3-dose vaccine regimen to men and women up to age 45.

HPV vaccine uptake
HPV vaccination has been underutilized in the United States. In 2017, a disappointing 49% of adolescents were up to date on vaccination, and 66% had received at least one dose.5 In rural areas the vaccination rates are 11 points lower than in urban regions.6 The CDC notes an increasing number of HPV-associated cancers—from 30,000 per year in 1999 to 43,000 per year in 2015—due mostly to increases in oral and anal carcinomas. Vaccination with Gardasil 9 could prevent 90% of those cases.7
Non-US successes. HPV vaccine uptake in Australia provides an excellent opportunity to study the impact of universally available, school-based vaccinations. In 2007 Australia implemented a program of free HPV vaccination distributed through schools. Boys and girls aged 12 and 13 were targeted that year, with catch-up vaccinations for those aged 13 to 18 in 2007-2009 in schools and for those aged 18 to 26 reached in the community.8
Continue to: Ali and colleagues studied the...
Ali and colleagues studied the preprogram and postprogram incidence of genital warts.9 About 83% received at least 1 dose of vaccine, and 73% of the eligible population completed the 3-dose regimen. There was a significant reduction in warts in both men and women younger than age 21 from 2007 to 2011 (12.1% to 2.2% in men and 11.5% to 0.85% in women). In the 21 to 30 age group there were similar reductions. This study demonstrates that with universal access and public implementation, the rates of HPV-associated disease can be reduced dramatically.
Data informing expanded vaccination ages
Will vaccination of an older population, with presumably many of whom sexually active and at risk for prior exposure to multiple HPV types, have a reasonable impact on lowering HPV-associated cancers? Are HPV-detected lesions in 27- to 45-year-old women the result of reactivation of latent HPV infection, or are they related to new-onset exposure? The FDA reviewed data from 3 studies of HPV vaccination in women aged 27 to 45. The first enrolled women who were naïve to oncogenic HPV types and provided all 3 doses of quadrivalent vaccine were followed for 4 years, along with a comparison group of nonvaccinated women. The second study allowed the nonvaccinated group to receive vaccine in year 4. Both groups were followed up to 10 years with the relevant outcome defined as cumulative incidence of HPV 6/11/16/18-related CIN and condyloma. The third study looked at the same outcomes in a set of all women—whether HPV high-risk naïve or not—after receiving vaccine and followed more than 10 years.7 This last study is most relevant to ObGyns, as it is closest to how we would consider vaccinating our patients.

The study findings are reassuring: A large proportion of HPV infections in women between 27 and 45 are the result of new exposure/infection. A study of 420 online daters aged 25 to 65 showed an annual incidence of high-risk HPV types in vaginal swabs of 25.4%, of which 64% were likely new acquisitions.10 The 2013-2014 National Health and Nutrition Examination Survey of 1,757 men aged 18 to 59 estimated approximately 45% had genital HPV infection. There was a bimodal distribution of disease with peaks at 28 to 32 and a larger second peak at 58 to 59 years of age.11 Bottom line: Men and women older than age 26 who are sexually active likely acquire new HPV infections with oncogenic types. Exposure to high-risk HPV types prior to vaccination—as we would expect in the real-world setting—did not eliminate the substantial benefit of immunization.
Based on these study results, and extrapolation to the 9-valent vaccine, the FDA extended the approval of Gardasil 9 to men and women from age 9 to 45. The indications and usage will remain the same: for prevention of cervical, vulvar, vaginal, and anal cancer and genital warts as well as precancerous or dysplastic lesions of the cervix, vulva, vagina, and anus related to HPV types 6, 11, 16, 18, 31, 33, 45, 52, and 58.
Continue to: Impact of the new...
Impact of the new indication on HPV-related disease
As described above, widespread vaccination of young girls and boys is going to have major impact on HPV-related disease, including precancer and cancer. Because there is evidence that older women and men are at risk for new HPV infection,10 there likely will be some benefit from vaccination of adults. It is difficult, however, to extrapolate the degree to which adult vaccination will impact HPV-related disease. This is because we do not fully understand the rates at which new HPV infection in the cervices of older women will progress to high-grade dysplasia or cancer. Further, the pathophysiology of HPV-related cancers at other anogenital sites and new oral-pharyngeal infection is poorly understood in comparison with our knowledge of the natural history of high-risk HPV infection in younger women. That said, because of the outstanding efficacy of HPV vaccination and the low-risk profile, even if the actual impact on prevention of cancer or morbidity from dysplasia is relatively low, adult vaccination benefits outweigh the limited risks.
It may be that increased vaccination and awareness of vaccination for adults may enhance the adherence and acceptance of widespread vaccination of boys and girls. Adult vaccination could create a cultural shift toward HPV vaccination acceptance when adult parents and loved ones of vaccine-age boys and girls have been vaccinated themselves.
Current and future insurance coverage
The Affordable Care Act, otherwise known as Obamacare, mandates coverage for all immunizations recommended by the ACIP. HPV vaccination up to age 26 is fully covered, without copay or deductible. The ACIP did consider extension of the indications for HPV vaccination to men and women up to age 45 at their October 2018 meeting. They are tasked with considering not only safety and efficacy but also the cost effectiveness of implementing vaccination. They continue to study the costs and potential benefits of extending HPV vaccination to age 45. Their recommendations may be determined at the February 2019 meeting—or even later in 2019. The American College of Obstetricians and Gynecologists (ACOG) relies upon ACIP for practice guidance. Once the ACIP has made a determination, and if new guidelines are published in the Morbidity and Mortality Weekly Report, insurance coverage and ACOG guidance will be updated.
How should we react and change practice based on this new indication?
Given the information reviewed by the FDA, ObGyns will want to discuss the availability of Gardasil 9 with our patients between ages 27 and 45 who have not been previously immunized.

Especially for our patients with exposure to multiple or new sexual partners, immunization against oncogenic HPV viral types is effective in providing protection from cancer precursors and cancers of the cervix, vulva, vagina, and anus—and of course from genital warts. They should understand that, until formal recommendations are published by the ACIP, they are likely to be responsible for the cost of the vaccination series. These conversations will also remind our patients to immunize their teens against HPV. The more conversation we have regarding the benefits of vaccination against high-risk HPV types, the more likely we are to be able to achieve the impressive results seen in Australia.
- US Food and Drug Administration website. FDA approves expanded use of Gardasil 9 to include individuals 27 through 45 years old. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm622715.htm. Updated October 9, 2018. Accessed December 27, 2018.
- World Health Organization website. Human papillomavirus (HPV) and cervical cancer. https://www.who.int/news-room/fact-sheets/detail/human-papillomavirus-(hpv)-and-cervical-cancer. February 15, 2018. Accessed December 27, 2018.
- Centers for Disease Control and Prevention website. Human papillomavirus (HPV) vaccine safety. https://www.cdc.gov/vaccinesafety/vaccines/hpv-vaccine.html. Last reviewed October 27, 2015. Accessed December 27, 2018.
- Meites E, Kempe A, Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination—updated recommendations of the Advisory Committee on Immunization Practices. MMWR. 2016;65(49):1405–1408.
- AAP News website. Jenko M. CDC: 49% of teens up to date on HPV vaccine. http://www.aappublications.org/news/2018/08/23/vac cinationrates082318. August 23, 2018. Accessed December 27, 2018.
- Walker TY, Elam-Evans LD, Yankey D, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years—United States, 2017. MMWR Morb Mortal Wkly Rep. 2018;67:909-917.
- Montague L. Summary basis for regulatory action. October 5, 2018. https://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM622941.pdf. Accessed December 27, 2018.
- Tabrizi SN, Brotherton JM, Kaldor JM, et al. Fall in human papillomavirus prevalence following a national vaccination program. J Infect Dis. 2012;206:1645-1651.
- Ali H, Donovan B, Wand H, et al. Genital warts in young Australians five years into national human papillomavirus vaccination programme: national surveillance data [published correction appears in BMJ. 2013;346:F2942]. BMJ. 2013;346:F2032.
- Winer RL, Hughes JP, Feng Q, et al. Incident detection of high-risk human papillomavirus infections in a cohort of high-risk women aged 25-65 years. J Infect Dis. 2016;214:665-675.
- Han JJ, Beltran TH, Song JW, et al. Prevalence of genital human papillomavirus infection and human papillomavirus vaccination rates among US adult men: National Health and Nutrition Examination Survey (NHANES) 2013-2014. JAMA Oncol. 2017;3:810-816.
- US Food and Drug Administration website. FDA approves expanded use of Gardasil 9 to include individuals 27 through 45 years old. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm622715.htm. Updated October 9, 2018. Accessed December 27, 2018.
- World Health Organization website. Human papillomavirus (HPV) and cervical cancer. https://www.who.int/news-room/fact-sheets/detail/human-papillomavirus-(hpv)-and-cervical-cancer. February 15, 2018. Accessed December 27, 2018.
- Centers for Disease Control and Prevention website. Human papillomavirus (HPV) vaccine safety. https://www.cdc.gov/vaccinesafety/vaccines/hpv-vaccine.html. Last reviewed October 27, 2015. Accessed December 27, 2018.
- Meites E, Kempe A, Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination—updated recommendations of the Advisory Committee on Immunization Practices. MMWR. 2016;65(49):1405–1408.
- AAP News website. Jenko M. CDC: 49% of teens up to date on HPV vaccine. http://www.aappublications.org/news/2018/08/23/vac cinationrates082318. August 23, 2018. Accessed December 27, 2018.
- Walker TY, Elam-Evans LD, Yankey D, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years—United States, 2017. MMWR Morb Mortal Wkly Rep. 2018;67:909-917.
- Montague L. Summary basis for regulatory action. October 5, 2018. https://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM622941.pdf. Accessed December 27, 2018.
- Tabrizi SN, Brotherton JM, Kaldor JM, et al. Fall in human papillomavirus prevalence following a national vaccination program. J Infect Dis. 2012;206:1645-1651.
- Ali H, Donovan B, Wand H, et al. Genital warts in young Australians five years into national human papillomavirus vaccination programme: national surveillance data [published correction appears in BMJ. 2013;346:F2942]. BMJ. 2013;346:F2032.
- Winer RL, Hughes JP, Feng Q, et al. Incident detection of high-risk human papillomavirus infections in a cohort of high-risk women aged 25-65 years. J Infect Dis. 2016;214:665-675.
- Han JJ, Beltran TH, Song JW, et al. Prevalence of genital human papillomavirus infection and human papillomavirus vaccination rates among US adult men: National Health and Nutrition Examination Survey (NHANES) 2013-2014. JAMA Oncol. 2017;3:810-816.
Is an IUD a good contraceptive choice for a never sexually active teen?
Expert Commentary
Data demonstrate efficacy and safety of the IUD in adolescents. In addition, IUDs (particularly the levonorgestrel-containing IUD) have many noncontraceptive benefits. There is still reluctance, however, among clinicians to use IUDs in adolescents. In a sample of fellows of the American College of Obstetricians and Gynecologists, only 43% considered adolescents appropriate candidates for use of an IUD.1
Study details
In this retrospective chart review, Kebodeaux and Schwartz sought to compare successful IUD insertion rates on first attempt in 120 sexually active (SA) and 82 never sexually active (NSA) adolescents. The IUD type used for all women was the 52-mg levonorgestrel IUD (Mirena), except for 3 copper IUDs (Paragard) used in the SA group. The primary indications for IUD use were contraception (85.2%) in the SA group and abnormal uterine bleeding (43.9%) and menstrual suppression (24.4%) in the NSA group.
In the NSA group, 82.9% of adolescents had had some type of prior treatment affecting the menstrual cycle, compared with 60.9% in the SA group (P = .001).
Non–office insertion. Either a sedation unit or operating room was utilized in 5.5% of the IUD insertions in the SA group and 47.6% of the NSA group. Among the 39 adolescents in the NSA group undergoing non–office insertion, 19 (48.7%) had special needs (learning or intellectual disabilities, autism/autism spectrum, or physical disabilities, such as cerebral palsy). Only 1 adolescent with special needs in the NSA group had an office insertion compared with 5 out of 6 in the SA group.
The performance of another procedure other than the IUD insertion (including diagnostic laparoscopy and hymenectomy) was common among adolescents undergoing procedures in the sedation unit or operating room who did not have special needs. It is also important to note that adolescents with special needs were routinely offered insertion under anesthesia while SA adolescents were offered insertion under anesthesia only if they were undergoing another procedure as well.
Study strengths and weaknesses
The study’s strengths include IUD insertions performed at a children’s hospital by providers with experience working with adolescent populations. This likely accounts for the high rates of “tolerance of the procedure well” (93.8% in the SA group vs 81.7% in the NSA group; P = .006). The study also included a patient population—adolescents with special needs—that has not been studied relative to IUD use previously.
A significant weakness of the study, however, is that there are no long-term follow-up data, particularly related to continuation rates.
These study findings provides further support to combat the myth that adolescents, particularly if nulliparous or not sexually active, are not suitable candidates for IUD use. However, if they have never been sexually active or have special needs, IUD insertion under sedation or in an operating room may be necessary. It is also likely that selection of the IUD as an option by an adolescent and overall tolerance of the insertion procedure requires providers with experience in caring for adolescents as well as providers possessing good counseling skills.
—Ronald T. Burkman, MD
1. Luchowski AT, Anderson BL, Power ML, Reglan GB, Espey E, Shulkin J. Obstetrician-gynecologists and contraception: practice and opinions about the use of IUDs in nulliparous women, adolescents and other patient populations. Contraception. 2014;89:572-577.
Expert Commentary
Data demonstrate efficacy and safety of the IUD in adolescents. In addition, IUDs (particularly the levonorgestrel-containing IUD) have many noncontraceptive benefits. There is still reluctance, however, among clinicians to use IUDs in adolescents. In a sample of fellows of the American College of Obstetricians and Gynecologists, only 43% considered adolescents appropriate candidates for use of an IUD.1
Study details
In this retrospective chart review, Kebodeaux and Schwartz sought to compare successful IUD insertion rates on first attempt in 120 sexually active (SA) and 82 never sexually active (NSA) adolescents. The IUD type used for all women was the 52-mg levonorgestrel IUD (Mirena), except for 3 copper IUDs (Paragard) used in the SA group. The primary indications for IUD use were contraception (85.2%) in the SA group and abnormal uterine bleeding (43.9%) and menstrual suppression (24.4%) in the NSA group.
In the NSA group, 82.9% of adolescents had had some type of prior treatment affecting the menstrual cycle, compared with 60.9% in the SA group (P = .001).
Non–office insertion. Either a sedation unit or operating room was utilized in 5.5% of the IUD insertions in the SA group and 47.6% of the NSA group. Among the 39 adolescents in the NSA group undergoing non–office insertion, 19 (48.7%) had special needs (learning or intellectual disabilities, autism/autism spectrum, or physical disabilities, such as cerebral palsy). Only 1 adolescent with special needs in the NSA group had an office insertion compared with 5 out of 6 in the SA group.
The performance of another procedure other than the IUD insertion (including diagnostic laparoscopy and hymenectomy) was common among adolescents undergoing procedures in the sedation unit or operating room who did not have special needs. It is also important to note that adolescents with special needs were routinely offered insertion under anesthesia while SA adolescents were offered insertion under anesthesia only if they were undergoing another procedure as well.
Study strengths and weaknesses
The study’s strengths include IUD insertions performed at a children’s hospital by providers with experience working with adolescent populations. This likely accounts for the high rates of “tolerance of the procedure well” (93.8% in the SA group vs 81.7% in the NSA group; P = .006). The study also included a patient population—adolescents with special needs—that has not been studied relative to IUD use previously.
A significant weakness of the study, however, is that there are no long-term follow-up data, particularly related to continuation rates.
These study findings provides further support to combat the myth that adolescents, particularly if nulliparous or not sexually active, are not suitable candidates for IUD use. However, if they have never been sexually active or have special needs, IUD insertion under sedation or in an operating room may be necessary. It is also likely that selection of the IUD as an option by an adolescent and overall tolerance of the insertion procedure requires providers with experience in caring for adolescents as well as providers possessing good counseling skills.
—Ronald T. Burkman, MD
Expert Commentary
Data demonstrate efficacy and safety of the IUD in adolescents. In addition, IUDs (particularly the levonorgestrel-containing IUD) have many noncontraceptive benefits. There is still reluctance, however, among clinicians to use IUDs in adolescents. In a sample of fellows of the American College of Obstetricians and Gynecologists, only 43% considered adolescents appropriate candidates for use of an IUD.1
Study details
In this retrospective chart review, Kebodeaux and Schwartz sought to compare successful IUD insertion rates on first attempt in 120 sexually active (SA) and 82 never sexually active (NSA) adolescents. The IUD type used for all women was the 52-mg levonorgestrel IUD (Mirena), except for 3 copper IUDs (Paragard) used in the SA group. The primary indications for IUD use were contraception (85.2%) in the SA group and abnormal uterine bleeding (43.9%) and menstrual suppression (24.4%) in the NSA group.
In the NSA group, 82.9% of adolescents had had some type of prior treatment affecting the menstrual cycle, compared with 60.9% in the SA group (P = .001).
Non–office insertion. Either a sedation unit or operating room was utilized in 5.5% of the IUD insertions in the SA group and 47.6% of the NSA group. Among the 39 adolescents in the NSA group undergoing non–office insertion, 19 (48.7%) had special needs (learning or intellectual disabilities, autism/autism spectrum, or physical disabilities, such as cerebral palsy). Only 1 adolescent with special needs in the NSA group had an office insertion compared with 5 out of 6 in the SA group.
The performance of another procedure other than the IUD insertion (including diagnostic laparoscopy and hymenectomy) was common among adolescents undergoing procedures in the sedation unit or operating room who did not have special needs. It is also important to note that adolescents with special needs were routinely offered insertion under anesthesia while SA adolescents were offered insertion under anesthesia only if they were undergoing another procedure as well.
Study strengths and weaknesses
The study’s strengths include IUD insertions performed at a children’s hospital by providers with experience working with adolescent populations. This likely accounts for the high rates of “tolerance of the procedure well” (93.8% in the SA group vs 81.7% in the NSA group; P = .006). The study also included a patient population—adolescents with special needs—that has not been studied relative to IUD use previously.
A significant weakness of the study, however, is that there are no long-term follow-up data, particularly related to continuation rates.
These study findings provides further support to combat the myth that adolescents, particularly if nulliparous or not sexually active, are not suitable candidates for IUD use. However, if they have never been sexually active or have special needs, IUD insertion under sedation or in an operating room may be necessary. It is also likely that selection of the IUD as an option by an adolescent and overall tolerance of the insertion procedure requires providers with experience in caring for adolescents as well as providers possessing good counseling skills.
—Ronald T. Burkman, MD
1. Luchowski AT, Anderson BL, Power ML, Reglan GB, Espey E, Shulkin J. Obstetrician-gynecologists and contraception: practice and opinions about the use of IUDs in nulliparous women, adolescents and other patient populations. Contraception. 2014;89:572-577.
1. Luchowski AT, Anderson BL, Power ML, Reglan GB, Espey E, Shulkin J. Obstetrician-gynecologists and contraception: practice and opinions about the use of IUDs in nulliparous women, adolescents and other patient populations. Contraception. 2014;89:572-577.