User login
Is vaginal laser therapy more efficacious in improving vaginal menopausal symptoms compared with sham therapy?
Li FG, Maheux-Lacroix S, Deans R, et al. Effect of fractional carbon dioxide laser vs sham treatment on symptom severity in women with postmenopausal vaginal symptoms: a randomized clinical trial. JAMA. 2021;326:1381-1389. doi: 10.1001/jama.2021.14892.
EXPERT COMMENTARY
Symptomatic vaginal atrophy, also referred to as genitourinary syndrome of menopause (GSM), is common and tends to progress without treatment. When use of over-the-counter lubricants and/or moisturizers are not sufficient to address symptoms, vaginal estrogen has represented the mainstay of treatment for this condition and effectively addresses GSM symptoms.1 In recent years, some physicians have been offering vaginal carbon dioxide (CO2) laser therapy as an alternative to vaginal estrogen in the treatment of GSM; however, the efficacy of laser therapy in this setting has been uncertain.
Li and colleagues conducted a double-blind randomized trial in postmenopausal women with bothersome vaginal symptoms to compare the efficacy of the fractional CO2 vaginal laser with that of sham treatment.
Details of the study
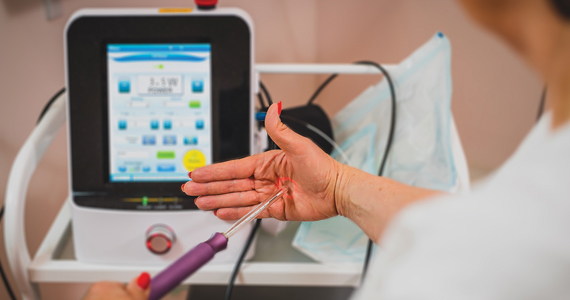
Investigators (who received no funding from any relevant commercial entity) at a teaching hospital in Sydney, Australia, randomly assigned 85 women with menopausal symptoms suggestive of GSM to laser (n = 43) or sham (n = 42) treatment. Participants underwent 3 treatments at monthly intervals. Laser treatments were performed with standard settings (40-watt power), while sham treatments were conducted with low settings that have no tissue effect. Local anesthesia cream was employed for all procedures, and a plume evacuator was used to remove visual and olfactory effects from laser smoke.
To maintain blinding, different clinicians performed assessments and treatments. Symptom severity assessments were based on a visual analog scale (VAS) and the Vulvovaginal Symptom Questionnaire (VSQ), with a minimal clinically important difference specified as a 50% decrease in severity scores of both assessment tools. Change in severity of symptoms, including dyspareunia, dysuria, vaginal dryness, and burning and itching, was assessed at 12 months. Quality of life, the Vaginal Health Index (VHI) score, and vaginal histology were among the secondary outcomes. In addition, vaginal biopsies were performed at baseline and 6 months after study treatment.
Among the 78 women (91.7%) who completed the 12-month evaluations, the mean age was approximately 57, more than 95% were White, and approximately half were sexually active.
Results. For the laser and sham treatment groups, at 12 months no significant differences were noted for change in overall symptoms or in the most severe symptom. Many participants who received laser or sham treatment reported an improvement in vaginal symptoms 12 months following treatment.
The VAS score for a change in symptom severity in the laser-treated group compared with the sham-treated group was -17.2 versus -26.6, a difference of 9.4 (95% confidence interval [CI], -28.6 to 47.5), while the VAS score for the most severe symptom was -24.5 versus -20.4, a difference of -4.1 (95% CI, -32.5 to 24.3). The VSQ score was, respectively, -3.1 versus -1.6 (difference, -1.5 [95% CI, -5.9 to 3.0]). The mean quality of life score showed no significant differences between the laser and the sham group (6.3 vs 1.4, a difference of 4.8 [95% CI, -3.9 to 13.5]). The VHI score was 0.9 in the laser group versus 1.3 in the sham group, for a difference of -0.4 (95% CI, -4.3 to 3.6). Likewise, the proportion of participants who noted a reduction of more than 50% in bother from their most severe symptoms was similar in the 2 groups. Similarly, changes in vaginal histology were similar in the laser and sham groups.
The proportion of participants who reported adverse events, including transient vaginal discomfort, discharge, or urinary tract symptoms, was similar in the 2 groups.
Study strengths and limitations
Although other randomized studies of fractionated laser therapy for GSM have been reported, this Australian trial is the largest and longest to date and also is the first to have used sham-treated controls.
Breast cancer survivors represent a group of patients for whom treatment of GSM can be a major conundrum—induced menopause that often results when combination chemotherapy is employed in premenopausal survivors can result in severe GSM; use of aromatase inhibitors likewise can cause bothersome GSM symptoms. Since the US Food and Drug Administration lists a personal history of breast cancer as a contraindication to use of any estrogen formulation, breast cancer survivors represent a population targeted by physicians offering vaginal laser treatment. Accordingly, that approximately 50% of trial participants were breast cancer survivors means the investigators were assessing the impact of laser therapy in a population of particular clinical relevance. Of note, as with participants overall, laser therapy when employed in breast cancer survivors did not result in outcomes distinct from sham treatments.2 ●
We agree with editorialists that outside of clinical trials, we should not recommend laser for treatment of menopausal vaginal symptoms.3 Currently, a US multisite randomized trial of fractionated laser versus sham for dyspareunia in menopausal women is planned.
ANDREW M. KAUNITZ, MD, NCMP,
AND CHERYL B. IGLESIA, MD
- The 2020 genitourinary syndrome of menopause position statement of The North American Menopause Society. Menopause. 2020;27:976- 992. doi: 10.1097/GME.0000000000001609.
- Li FG, Maheux-Lacroix S, Deans R, et al. Effect of fractional carbon dioxide laser vs sham treatment on symptom severity in women with postmenopausal vaginal symptoms: a randomized clinical trial. JAMA. 2021;326:1381-1389. doi: 10.1001/jama.2021.14892.
- Adelman M, Nygaard IE. Time for a “pause” on the use of vaginal laser. JAMA. 2021;326:1378-1380. doi: 10.1001/jama.2021.14809.
Li FG, Maheux-Lacroix S, Deans R, et al. Effect of fractional carbon dioxide laser vs sham treatment on symptom severity in women with postmenopausal vaginal symptoms: a randomized clinical trial. JAMA. 2021;326:1381-1389. doi: 10.1001/jama.2021.14892.
EXPERT COMMENTARY
Symptomatic vaginal atrophy, also referred to as genitourinary syndrome of menopause (GSM), is common and tends to progress without treatment. When use of over-the-counter lubricants and/or moisturizers are not sufficient to address symptoms, vaginal estrogen has represented the mainstay of treatment for this condition and effectively addresses GSM symptoms.1 In recent years, some physicians have been offering vaginal carbon dioxide (CO2) laser therapy as an alternative to vaginal estrogen in the treatment of GSM; however, the efficacy of laser therapy in this setting has been uncertain.
Li and colleagues conducted a double-blind randomized trial in postmenopausal women with bothersome vaginal symptoms to compare the efficacy of the fractional CO2 vaginal laser with that of sham treatment.
Details of the study
Investigators (who received no funding from any relevant commercial entity) at a teaching hospital in Sydney, Australia, randomly assigned 85 women with menopausal symptoms suggestive of GSM to laser (n = 43) or sham (n = 42) treatment. Participants underwent 3 treatments at monthly intervals. Laser treatments were performed with standard settings (40-watt power), while sham treatments were conducted with low settings that have no tissue effect. Local anesthesia cream was employed for all procedures, and a plume evacuator was used to remove visual and olfactory effects from laser smoke.
To maintain blinding, different clinicians performed assessments and treatments. Symptom severity assessments were based on a visual analog scale (VAS) and the Vulvovaginal Symptom Questionnaire (VSQ), with a minimal clinically important difference specified as a 50% decrease in severity scores of both assessment tools. Change in severity of symptoms, including dyspareunia, dysuria, vaginal dryness, and burning and itching, was assessed at 12 months. Quality of life, the Vaginal Health Index (VHI) score, and vaginal histology were among the secondary outcomes. In addition, vaginal biopsies were performed at baseline and 6 months after study treatment.
Among the 78 women (91.7%) who completed the 12-month evaluations, the mean age was approximately 57, more than 95% were White, and approximately half were sexually active.
Results. For the laser and sham treatment groups, at 12 months no significant differences were noted for change in overall symptoms or in the most severe symptom. Many participants who received laser or sham treatment reported an improvement in vaginal symptoms 12 months following treatment.
The VAS score for a change in symptom severity in the laser-treated group compared with the sham-treated group was -17.2 versus -26.6, a difference of 9.4 (95% confidence interval [CI], -28.6 to 47.5), while the VAS score for the most severe symptom was -24.5 versus -20.4, a difference of -4.1 (95% CI, -32.5 to 24.3). The VSQ score was, respectively, -3.1 versus -1.6 (difference, -1.5 [95% CI, -5.9 to 3.0]). The mean quality of life score showed no significant differences between the laser and the sham group (6.3 vs 1.4, a difference of 4.8 [95% CI, -3.9 to 13.5]). The VHI score was 0.9 in the laser group versus 1.3 in the sham group, for a difference of -0.4 (95% CI, -4.3 to 3.6). Likewise, the proportion of participants who noted a reduction of more than 50% in bother from their most severe symptoms was similar in the 2 groups. Similarly, changes in vaginal histology were similar in the laser and sham groups.
The proportion of participants who reported adverse events, including transient vaginal discomfort, discharge, or urinary tract symptoms, was similar in the 2 groups.
Study strengths and limitations
Although other randomized studies of fractionated laser therapy for GSM have been reported, this Australian trial is the largest and longest to date and also is the first to have used sham-treated controls.
Breast cancer survivors represent a group of patients for whom treatment of GSM can be a major conundrum—induced menopause that often results when combination chemotherapy is employed in premenopausal survivors can result in severe GSM; use of aromatase inhibitors likewise can cause bothersome GSM symptoms. Since the US Food and Drug Administration lists a personal history of breast cancer as a contraindication to use of any estrogen formulation, breast cancer survivors represent a population targeted by physicians offering vaginal laser treatment. Accordingly, that approximately 50% of trial participants were breast cancer survivors means the investigators were assessing the impact of laser therapy in a population of particular clinical relevance. Of note, as with participants overall, laser therapy when employed in breast cancer survivors did not result in outcomes distinct from sham treatments.2 ●
We agree with editorialists that outside of clinical trials, we should not recommend laser for treatment of menopausal vaginal symptoms.3 Currently, a US multisite randomized trial of fractionated laser versus sham for dyspareunia in menopausal women is planned.
ANDREW M. KAUNITZ, MD, NCMP,
AND CHERYL B. IGLESIA, MD
Li FG, Maheux-Lacroix S, Deans R, et al. Effect of fractional carbon dioxide laser vs sham treatment on symptom severity in women with postmenopausal vaginal symptoms: a randomized clinical trial. JAMA. 2021;326:1381-1389. doi: 10.1001/jama.2021.14892.
EXPERT COMMENTARY
Symptomatic vaginal atrophy, also referred to as genitourinary syndrome of menopause (GSM), is common and tends to progress without treatment. When use of over-the-counter lubricants and/or moisturizers are not sufficient to address symptoms, vaginal estrogen has represented the mainstay of treatment for this condition and effectively addresses GSM symptoms.1 In recent years, some physicians have been offering vaginal carbon dioxide (CO2) laser therapy as an alternative to vaginal estrogen in the treatment of GSM; however, the efficacy of laser therapy in this setting has been uncertain.
Li and colleagues conducted a double-blind randomized trial in postmenopausal women with bothersome vaginal symptoms to compare the efficacy of the fractional CO2 vaginal laser with that of sham treatment.
Details of the study
Investigators (who received no funding from any relevant commercial entity) at a teaching hospital in Sydney, Australia, randomly assigned 85 women with menopausal symptoms suggestive of GSM to laser (n = 43) or sham (n = 42) treatment. Participants underwent 3 treatments at monthly intervals. Laser treatments were performed with standard settings (40-watt power), while sham treatments were conducted with low settings that have no tissue effect. Local anesthesia cream was employed for all procedures, and a plume evacuator was used to remove visual and olfactory effects from laser smoke.
To maintain blinding, different clinicians performed assessments and treatments. Symptom severity assessments were based on a visual analog scale (VAS) and the Vulvovaginal Symptom Questionnaire (VSQ), with a minimal clinically important difference specified as a 50% decrease in severity scores of both assessment tools. Change in severity of symptoms, including dyspareunia, dysuria, vaginal dryness, and burning and itching, was assessed at 12 months. Quality of life, the Vaginal Health Index (VHI) score, and vaginal histology were among the secondary outcomes. In addition, vaginal biopsies were performed at baseline and 6 months after study treatment.
Among the 78 women (91.7%) who completed the 12-month evaluations, the mean age was approximately 57, more than 95% were White, and approximately half were sexually active.
Results. For the laser and sham treatment groups, at 12 months no significant differences were noted for change in overall symptoms or in the most severe symptom. Many participants who received laser or sham treatment reported an improvement in vaginal symptoms 12 months following treatment.
The VAS score for a change in symptom severity in the laser-treated group compared with the sham-treated group was -17.2 versus -26.6, a difference of 9.4 (95% confidence interval [CI], -28.6 to 47.5), while the VAS score for the most severe symptom was -24.5 versus -20.4, a difference of -4.1 (95% CI, -32.5 to 24.3). The VSQ score was, respectively, -3.1 versus -1.6 (difference, -1.5 [95% CI, -5.9 to 3.0]). The mean quality of life score showed no significant differences between the laser and the sham group (6.3 vs 1.4, a difference of 4.8 [95% CI, -3.9 to 13.5]). The VHI score was 0.9 in the laser group versus 1.3 in the sham group, for a difference of -0.4 (95% CI, -4.3 to 3.6). Likewise, the proportion of participants who noted a reduction of more than 50% in bother from their most severe symptoms was similar in the 2 groups. Similarly, changes in vaginal histology were similar in the laser and sham groups.
The proportion of participants who reported adverse events, including transient vaginal discomfort, discharge, or urinary tract symptoms, was similar in the 2 groups.
Study strengths and limitations
Although other randomized studies of fractionated laser therapy for GSM have been reported, this Australian trial is the largest and longest to date and also is the first to have used sham-treated controls.
Breast cancer survivors represent a group of patients for whom treatment of GSM can be a major conundrum—induced menopause that often results when combination chemotherapy is employed in premenopausal survivors can result in severe GSM; use of aromatase inhibitors likewise can cause bothersome GSM symptoms. Since the US Food and Drug Administration lists a personal history of breast cancer as a contraindication to use of any estrogen formulation, breast cancer survivors represent a population targeted by physicians offering vaginal laser treatment. Accordingly, that approximately 50% of trial participants were breast cancer survivors means the investigators were assessing the impact of laser therapy in a population of particular clinical relevance. Of note, as with participants overall, laser therapy when employed in breast cancer survivors did not result in outcomes distinct from sham treatments.2 ●
We agree with editorialists that outside of clinical trials, we should not recommend laser for treatment of menopausal vaginal symptoms.3 Currently, a US multisite randomized trial of fractionated laser versus sham for dyspareunia in menopausal women is planned.
ANDREW M. KAUNITZ, MD, NCMP,
AND CHERYL B. IGLESIA, MD
- The 2020 genitourinary syndrome of menopause position statement of The North American Menopause Society. Menopause. 2020;27:976- 992. doi: 10.1097/GME.0000000000001609.
- Li FG, Maheux-Lacroix S, Deans R, et al. Effect of fractional carbon dioxide laser vs sham treatment on symptom severity in women with postmenopausal vaginal symptoms: a randomized clinical trial. JAMA. 2021;326:1381-1389. doi: 10.1001/jama.2021.14892.
- Adelman M, Nygaard IE. Time for a “pause” on the use of vaginal laser. JAMA. 2021;326:1378-1380. doi: 10.1001/jama.2021.14809.
- The 2020 genitourinary syndrome of menopause position statement of The North American Menopause Society. Menopause. 2020;27:976- 992. doi: 10.1097/GME.0000000000001609.
- Li FG, Maheux-Lacroix S, Deans R, et al. Effect of fractional carbon dioxide laser vs sham treatment on symptom severity in women with postmenopausal vaginal symptoms: a randomized clinical trial. JAMA. 2021;326:1381-1389. doi: 10.1001/jama.2021.14892.
- Adelman M, Nygaard IE. Time for a “pause” on the use of vaginal laser. JAMA. 2021;326:1378-1380. doi: 10.1001/jama.2021.14809.
Remote and in-home prenatal care: Safe, inclusive, and here to stay

For much of the general public, in-home care from a physician is akin to the rotary telephone: a feature of a bygone age, long since replaced by vastly different systems. While approximately 40% of physician-patient interactions in 1930 were house calls, by the early 1980s this had dwindled to less than 1%,1 with almost all physician-patient encounters taking place in a clinical setting, whether in a hospital or in a free-standing clinic. In the last 2 decades, a smattering of primary care and medical subspecialty clinicians started to incorporate some in-home care into their practices in the form of telemedicine, using video and telephone technology to facilitate care outside of the clinical setting, and by 2016, approximately 15% of physicians reported using some form of telemedicine in their interactions with patients.2
Despite these advances, prior to the COVID-19 pandemic, obstetricians lagged significantly behind in their use of at-home or remote care. Although there were some efforts to promote a hybrid care model that incorporated prenatal telemedicine,3 pre-pandemic ObGyn was one of the least likely fields to offer telemedicine to their patients, with only 9% of practices offering such services.2 In this article, we discuss how the COVID-19 pandemic resulted in a shift from traditional, in-person care to a hybrid remote model and how this may benefit obstetrics patients as well as clinicians.
Pre-pandemic patient management
The traditional model of prenatal care presents a particularly intense time period for patients in terms of its demands. Women who are pregnant and start care in their first trimester typically have 12 to 14 visits during the subsequent 6 to 7 months, with additional visits for those with high-risk pregnancies. Although some of these visits coincide with the need for in-person laboratory work or imaging, many are chiefly oriented around assessment of vital signs or counseling. These frequent prenatal visits represent a significant commitment from patients in terms of transportation, time off work, and childcare resources—all of which may be exacerbated for patients who need to receive their care from overbooked, high-risk specialists.
After delivery, attending an in-person postpartum visit with a newborn can be even more daunting. Despite the increased recognition from professional groups of the importance of postpartum care to support breastfeeding, physical recovery, and mental health, as many as 40% of recently delivered patients do not attend their scheduled postpartum visit(s).4 Still, before 2020, few obstetricians had revised their workflows to “meet patients where they are,” with many continuing to only offer in-person care and assessments.
COVID-19: An impetus for change
As with so many things, the COVID-19 pandemic has challenged our ideas of what is normal. In a sense, the pandemic has catalyzed a revolution in the prenatal care model. The very real risks of exposure and contagion during the pandemic—for clinicians and patients alike—has forced ObGyns to reexamine the actual risks and benefits of in-person and in-clinic prenatal care. As a result, many ObGyns have rapidly adopted telemedicine into practices that were strictly in-person. For example, a national survey of 172 clinicians who offered contraception counseling during the pandemic found that 91% of them were now offering telemedicine services, with 78% of those clinicians new to telemedicine.5 Similarly, although a minority of surveyed obstetricians in New York City reported using telemedicine pre-pandemic, 89% planned to continue using such technology in the future.6
Continue to: Incorporating mobile technology...
Incorporating mobile technology
Obstetricians, forced to consolidate and maximize their in-person care to protect their patients’ safety, have started to realize that many of the conversations and counseling offered to patients can be managed equally effectively with telemedicine. Furthermore, basic home monitoring devices, such as blood pressure machines, can be safely and accurately used by patients without requiring them to come to the office.
More recent research into mobile medical devices suggests that patients can safely and appropriately manage more complex tools. One such example is a mobile, self-operated, ultrasound transducer that is controlled through a smartphone (Instinct, Pulsenmore Ltd). This device was evaluated in an observational, noninterventional trial of 100 women carrying a singleton fetus at 14/0 weeks’ to 39/6 weeks’ gestation. Patients performed 1,360 self-scans, which were reviewed by a clinician in real time online or subsequently off-line. Results showed successful detection rates of 95.3% for fetal heart activity, 88.3% for body movements, 69.4% for tone, 23.8% for breathing movements, and 92.2% for normal amniotic fluid volume.7 The authors concluded that this represents a feasible solution for remote sonographic fetal assessment.
Coordinating care with health care extenders
Remote monitoring options allow patients to be safely monitored during their pregnancies while remaining at home more often, especially when used in conjunction with trained health care extenders such as registered nurses, primary care associates, or “maternity navigators” who can facilitate off-site care. In fact, many aspects of prenatal care are particularly amenable to remote medicine or non–physician-based home care. Different variations of this model of “hybrid” prenatal care may be appropriate depending upon the needs of the patient population served by a given obstetrics practice. Ideally, a prenatal care model personalizes care based on the known risk factors that are identified at the beginning of prenatal care, the anticipated barriers to care, and the patient’s own preferences. As a result, alternatives to the traditional model may be to alternate in-person and telemedicine visits,3,8 to incorporate in-person or remote group prenatal visits,9,10 or to incorporate staff with basic health care skills to serve as health care extenders in the community and provide home visits for basic monitoring, laboratory work, and patient education.11
Benefits of hybrid prenatal models
As we look ahead to the end of the pandemic, how should obstetricians view these hybrid prenatal care models? Are these models safe for patients? Were they only worthwhile to minimize infection risk, or do they have potential benefits for patients going forward?
In fact, data on the use of telemedicine in prenatal care indicate that these models may be equally as safe as the traditional model in terms of clinical outcomes and may have important additional benefits with regard to patient convenience and access to and satisfaction with care. Even audio-only prenatal televisits have been found to be equivalent to in-person visits in terms of serious perinatal outcomes.12 Common pregnancy diagnoses are also well-served by telemedicine. For example, several recent investigations of patients with gestational diabetes have found that telemedicine was as effective as standard care for glucose control.13,14 Management of hypertension during pregnancy, another antenatal condition that is commonly managed with frequent in-person check-ups, also was found to be adequately feasible with telemedicine using home monitors and symptom checklists, with high rates of patient satisfaction.15
With good evidence for safety, the added potential for patients to benefit in such hybrid models is multifactorial. For one, despite our collective hopes, the COVID-19 pandemic may have a long tail. Vaccine hesitancy and COVID-19 variants may mean that clinicians will have to consider the real threat of infection risk in the clinic setting for years to come. In-home prenatal care also provides a wide variety of social, economic, and psychological benefits for pregnant women across various patient populations. The pandemic has introduced many patients to the full potential of working and meeting remotely; pregnant patients are becoming more familiar with these technology platforms and appreciate its incorporation into their busy lives.5 Furthermore, hybrid models actually can provide otherwise “nonadherent” patients with better access to care. From the patient perspective, an in-person 15-minute health care provider visit actually represents a significant commitment of time and resources (ie, hours spent on public transportation, lost wages for those with inflexible work schedules, and childcare costs for patients discouraged from bringing their children to prenatal visits). Especially for patients with fewer socioeconomic resources, these barriers to in-person clinic visits may be daunting, if not insurmountable; the option of remote visits or house calls reduces these barriers and facilitates care.16
Such hybrid models benefit prenatal clinicians as well. In addition to a decreased risk of infection, clinicians may be able to attract a wider potential prenatal patient population with telemedicine by appealing to younger and potentially more technology-savvy patients.17 Importantly, telemedicine is increasingly recognized as on par with in-person visits in many billing algorithms. Changes during the pandemic led Medicare to cover telemedicine visits as well as in-person visits18,19; among other groundbreaking changes, new patients can have an initial billable visit via telemedicine. Although the billing landscape will likely continue to evolve, such changes allow clinicians to focus on patient safety and convenience without financial risk to their practices.
The future of prenatal appointment scheduling
The future of prenatal care certainly doesn’t look like a dozen 15-minute visits in a private physician’s office. While these emerging hybrid models of prenatal care certainly can benefit patients with low-risk uncomplicated pregnancies, they are already being adopted by clinicians who care for patients with antenatal complications that require specialist consultation; for those with conditions that require frequent, low-complexity check-ins (gestational diabetes, chronic hypertension, history of pre-term birth, etc.); and for patients who struggle with financial or logistical barriers to in-person care. Although obstetrics may have lagged behind other subspecialties in revising its traditional health care models, the pandemic has opened up a new world of possibilities of remote and in-home care for this field. ●
- Kao H, Conant R, Soriano T, et al. The past, present, and future of house calls. Clin Geriatr Med. 2009;25:19-34. doi:10.1016/j.cger.2008.10.005.
- Kane CK, Gillis K. The use of telemedicine by physicians: still the exception rather than the rule. Health Aff (Millwood). 2018;37:1923-1930. doi:10.1377/hlthaff.2018.05077.
- Weigel G, Frederiksen B, Ranji U. Telemedicine and pregnancy care. Kaiser Family Foundation website. https://www.kff.org/womens-health-policy/issue-brief/telemedicine-and-pregnancy-care. Accessed August 23, 2021.
- ACOG Committee Opinion No. 736: optimizing postpartum care. Obstet Gynecol. 2018;131:e140-e150. doi:10.1097/AOG.0000000000002633.
- Stifani BM, Avila K, Levi EE. Telemedicine for contraceptive counseling: an exploratory survey of US family planning providers following rapid adoption of services during the COVID-19 pandemic. Contraception. 2021;103:157-162. doi:10.1016/j.contraception.2020.11.006.
- Madden N, Emeruwa UN, Friedman AM, et al. Telehealth uptake into prenatal care and provider attitudes during the COVID-19 pandemic in New York City: a quantitative and qualitative analysis. Am J Perinatol. 2020;37:1005-1014. doi:10.1055/s-0040-1712939.
- Hadar E, Wolff L, Tenenbaum-Gavish K, et al. Mobile self-operated home ultrasound system for remote fetal assessment during pregnancy. Telemed J E Health. 2021. doi:10.1089/tmj.2020.0541.
- Thomas Jefferson University Division of Maternal Fetal Medicine. Jefferson Maternal Fetal Medicine COVID19 Preparedness. Version 2.1. March 19, 2020. https://communities.smfm.org/HigherLogic/System/DownloadDocumentFile.ashx?DocumentFileKey=a109df77-74fe-462b-87fb-895d6ee7d0e6. Accessed August 23, 2021.
- Ickovics JR, Kershaw TS, Westdahl C, et al. Group prenatal care and perinatal outcomes. Obstet Gynecol. 2007;110(2 pt 1):330-339. doi:10.1097/01.AOG.0000275284.24298.23.
- Wicklund M. Oakland launches telehealth program for Black prenatal, postpartum care. Telehealth News. https://mhealthintelligence.com/news/oakland-launches-telehealth-program-for-black-prenatal-postpartum-care. Accessed August 23, 2021.
- Home-based pregnancy care. CayabaCare website. https://www.cayabacare.com. Accessed August 23, 2021.
- Duryea EL, Adhikari EH, Ambia A, et al. Comparison between in-person and audio-only virtual prenatal visits and perinatal outcomes. JAMA Netw Open. 2021;4:e215854. doi:10.1001/jamanetworkopen.2021.5854.
- Ming WK, Mackillop LH, Farmer AJ, et al. Telemedicine technologies for diabetes in pregnancy: a systematic review and meta-analysis. J Med Internet Res. 2016;18:e290. doi:10.2196/jmir.6556.
- Tian Y, Zhang S, Huang F, et al. Comparing the efficacies of telemedicine and standard prenatal care on blood glucose control in women with gestational diabetes mellitus: randomized controlled trial. JMIR Mhealth Uhealth. 2021;9:e22881. doi:10.2196/22881.
- van den Heuvel JFM, Kariman SS, van Solinge WW, et al. SAFE@HOME – feasibility study of a telemonitoring platform combining blood pressure and preeclampsia symptoms in pregnancy care. Eur J Obstet Gynecol Reprod Biol. 2019;240:226-231. doi:10.1016/j.ejogrb.2019.07.012.
- Dixon-Shambley K, Gabbe PT. Using telehealth approaches to address social determinants of health and improve pregnancy and postpartum outcomes. Clin Obstet Gynecol. 2021;64:333-344. doi:10.1097/GRF.0000000000000611.
- Eruchalu CN, Pichardo MS, Bharadwaj M, et al. The expanding digital divide: digital health access inequities during the COVID-19 pandemic in New York City. J Urban Health. 2021;98:183-186. doi:10.1007/s11524-020-00508-9.
- COVID-19 FAQs for obstetrician-gynecologists, telehealth. The American College of Obstetricians and Gynecologists website. https://www.acog.org/clinical-information/physician-faqs/covid-19-faqs-for-ob-gyns-telehealth. Accessed August 23, 2021.
- Managing patients remotely: billing for digital and telehealth services. The American College of Obstetricians and Gynecologists website. Updated October 19, 2020. https://www.acog.org/practice-management/coding/coding-library/managing-patients-remotely-billing-for-digital-and-telehealth-services. Accessed August 23, 2021.

For much of the general public, in-home care from a physician is akin to the rotary telephone: a feature of a bygone age, long since replaced by vastly different systems. While approximately 40% of physician-patient interactions in 1930 were house calls, by the early 1980s this had dwindled to less than 1%,1 with almost all physician-patient encounters taking place in a clinical setting, whether in a hospital or in a free-standing clinic. In the last 2 decades, a smattering of primary care and medical subspecialty clinicians started to incorporate some in-home care into their practices in the form of telemedicine, using video and telephone technology to facilitate care outside of the clinical setting, and by 2016, approximately 15% of physicians reported using some form of telemedicine in their interactions with patients.2
Despite these advances, prior to the COVID-19 pandemic, obstetricians lagged significantly behind in their use of at-home or remote care. Although there were some efforts to promote a hybrid care model that incorporated prenatal telemedicine,3 pre-pandemic ObGyn was one of the least likely fields to offer telemedicine to their patients, with only 9% of practices offering such services.2 In this article, we discuss how the COVID-19 pandemic resulted in a shift from traditional, in-person care to a hybrid remote model and how this may benefit obstetrics patients as well as clinicians.
Pre-pandemic patient management
The traditional model of prenatal care presents a particularly intense time period for patients in terms of its demands. Women who are pregnant and start care in their first trimester typically have 12 to 14 visits during the subsequent 6 to 7 months, with additional visits for those with high-risk pregnancies. Although some of these visits coincide with the need for in-person laboratory work or imaging, many are chiefly oriented around assessment of vital signs or counseling. These frequent prenatal visits represent a significant commitment from patients in terms of transportation, time off work, and childcare resources—all of which may be exacerbated for patients who need to receive their care from overbooked, high-risk specialists.
After delivery, attending an in-person postpartum visit with a newborn can be even more daunting. Despite the increased recognition from professional groups of the importance of postpartum care to support breastfeeding, physical recovery, and mental health, as many as 40% of recently delivered patients do not attend their scheduled postpartum visit(s).4 Still, before 2020, few obstetricians had revised their workflows to “meet patients where they are,” with many continuing to only offer in-person care and assessments.
COVID-19: An impetus for change
As with so many things, the COVID-19 pandemic has challenged our ideas of what is normal. In a sense, the pandemic has catalyzed a revolution in the prenatal care model. The very real risks of exposure and contagion during the pandemic—for clinicians and patients alike—has forced ObGyns to reexamine the actual risks and benefits of in-person and in-clinic prenatal care. As a result, many ObGyns have rapidly adopted telemedicine into practices that were strictly in-person. For example, a national survey of 172 clinicians who offered contraception counseling during the pandemic found that 91% of them were now offering telemedicine services, with 78% of those clinicians new to telemedicine.5 Similarly, although a minority of surveyed obstetricians in New York City reported using telemedicine pre-pandemic, 89% planned to continue using such technology in the future.6
Continue to: Incorporating mobile technology...
Incorporating mobile technology
Obstetricians, forced to consolidate and maximize their in-person care to protect their patients’ safety, have started to realize that many of the conversations and counseling offered to patients can be managed equally effectively with telemedicine. Furthermore, basic home monitoring devices, such as blood pressure machines, can be safely and accurately used by patients without requiring them to come to the office.
More recent research into mobile medical devices suggests that patients can safely and appropriately manage more complex tools. One such example is a mobile, self-operated, ultrasound transducer that is controlled through a smartphone (Instinct, Pulsenmore Ltd). This device was evaluated in an observational, noninterventional trial of 100 women carrying a singleton fetus at 14/0 weeks’ to 39/6 weeks’ gestation. Patients performed 1,360 self-scans, which were reviewed by a clinician in real time online or subsequently off-line. Results showed successful detection rates of 95.3% for fetal heart activity, 88.3% for body movements, 69.4% for tone, 23.8% for breathing movements, and 92.2% for normal amniotic fluid volume.7 The authors concluded that this represents a feasible solution for remote sonographic fetal assessment.
Coordinating care with health care extenders
Remote monitoring options allow patients to be safely monitored during their pregnancies while remaining at home more often, especially when used in conjunction with trained health care extenders such as registered nurses, primary care associates, or “maternity navigators” who can facilitate off-site care. In fact, many aspects of prenatal care are particularly amenable to remote medicine or non–physician-based home care. Different variations of this model of “hybrid” prenatal care may be appropriate depending upon the needs of the patient population served by a given obstetrics practice. Ideally, a prenatal care model personalizes care based on the known risk factors that are identified at the beginning of prenatal care, the anticipated barriers to care, and the patient’s own preferences. As a result, alternatives to the traditional model may be to alternate in-person and telemedicine visits,3,8 to incorporate in-person or remote group prenatal visits,9,10 or to incorporate staff with basic health care skills to serve as health care extenders in the community and provide home visits for basic monitoring, laboratory work, and patient education.11
Benefits of hybrid prenatal models
As we look ahead to the end of the pandemic, how should obstetricians view these hybrid prenatal care models? Are these models safe for patients? Were they only worthwhile to minimize infection risk, or do they have potential benefits for patients going forward?
In fact, data on the use of telemedicine in prenatal care indicate that these models may be equally as safe as the traditional model in terms of clinical outcomes and may have important additional benefits with regard to patient convenience and access to and satisfaction with care. Even audio-only prenatal televisits have been found to be equivalent to in-person visits in terms of serious perinatal outcomes.12 Common pregnancy diagnoses are also well-served by telemedicine. For example, several recent investigations of patients with gestational diabetes have found that telemedicine was as effective as standard care for glucose control.13,14 Management of hypertension during pregnancy, another antenatal condition that is commonly managed with frequent in-person check-ups, also was found to be adequately feasible with telemedicine using home monitors and symptom checklists, with high rates of patient satisfaction.15
With good evidence for safety, the added potential for patients to benefit in such hybrid models is multifactorial. For one, despite our collective hopes, the COVID-19 pandemic may have a long tail. Vaccine hesitancy and COVID-19 variants may mean that clinicians will have to consider the real threat of infection risk in the clinic setting for years to come. In-home prenatal care also provides a wide variety of social, economic, and psychological benefits for pregnant women across various patient populations. The pandemic has introduced many patients to the full potential of working and meeting remotely; pregnant patients are becoming more familiar with these technology platforms and appreciate its incorporation into their busy lives.5 Furthermore, hybrid models actually can provide otherwise “nonadherent” patients with better access to care. From the patient perspective, an in-person 15-minute health care provider visit actually represents a significant commitment of time and resources (ie, hours spent on public transportation, lost wages for those with inflexible work schedules, and childcare costs for patients discouraged from bringing their children to prenatal visits). Especially for patients with fewer socioeconomic resources, these barriers to in-person clinic visits may be daunting, if not insurmountable; the option of remote visits or house calls reduces these barriers and facilitates care.16
Such hybrid models benefit prenatal clinicians as well. In addition to a decreased risk of infection, clinicians may be able to attract a wider potential prenatal patient population with telemedicine by appealing to younger and potentially more technology-savvy patients.17 Importantly, telemedicine is increasingly recognized as on par with in-person visits in many billing algorithms. Changes during the pandemic led Medicare to cover telemedicine visits as well as in-person visits18,19; among other groundbreaking changes, new patients can have an initial billable visit via telemedicine. Although the billing landscape will likely continue to evolve, such changes allow clinicians to focus on patient safety and convenience without financial risk to their practices.
The future of prenatal appointment scheduling
The future of prenatal care certainly doesn’t look like a dozen 15-minute visits in a private physician’s office. While these emerging hybrid models of prenatal care certainly can benefit patients with low-risk uncomplicated pregnancies, they are already being adopted by clinicians who care for patients with antenatal complications that require specialist consultation; for those with conditions that require frequent, low-complexity check-ins (gestational diabetes, chronic hypertension, history of pre-term birth, etc.); and for patients who struggle with financial or logistical barriers to in-person care. Although obstetrics may have lagged behind other subspecialties in revising its traditional health care models, the pandemic has opened up a new world of possibilities of remote and in-home care for this field. ●

For much of the general public, in-home care from a physician is akin to the rotary telephone: a feature of a bygone age, long since replaced by vastly different systems. While approximately 40% of physician-patient interactions in 1930 were house calls, by the early 1980s this had dwindled to less than 1%,1 with almost all physician-patient encounters taking place in a clinical setting, whether in a hospital or in a free-standing clinic. In the last 2 decades, a smattering of primary care and medical subspecialty clinicians started to incorporate some in-home care into their practices in the form of telemedicine, using video and telephone technology to facilitate care outside of the clinical setting, and by 2016, approximately 15% of physicians reported using some form of telemedicine in their interactions with patients.2
Despite these advances, prior to the COVID-19 pandemic, obstetricians lagged significantly behind in their use of at-home or remote care. Although there were some efforts to promote a hybrid care model that incorporated prenatal telemedicine,3 pre-pandemic ObGyn was one of the least likely fields to offer telemedicine to their patients, with only 9% of practices offering such services.2 In this article, we discuss how the COVID-19 pandemic resulted in a shift from traditional, in-person care to a hybrid remote model and how this may benefit obstetrics patients as well as clinicians.
Pre-pandemic patient management
The traditional model of prenatal care presents a particularly intense time period for patients in terms of its demands. Women who are pregnant and start care in their first trimester typically have 12 to 14 visits during the subsequent 6 to 7 months, with additional visits for those with high-risk pregnancies. Although some of these visits coincide with the need for in-person laboratory work or imaging, many are chiefly oriented around assessment of vital signs or counseling. These frequent prenatal visits represent a significant commitment from patients in terms of transportation, time off work, and childcare resources—all of which may be exacerbated for patients who need to receive their care from overbooked, high-risk specialists.
After delivery, attending an in-person postpartum visit with a newborn can be even more daunting. Despite the increased recognition from professional groups of the importance of postpartum care to support breastfeeding, physical recovery, and mental health, as many as 40% of recently delivered patients do not attend their scheduled postpartum visit(s).4 Still, before 2020, few obstetricians had revised their workflows to “meet patients where they are,” with many continuing to only offer in-person care and assessments.
COVID-19: An impetus for change
As with so many things, the COVID-19 pandemic has challenged our ideas of what is normal. In a sense, the pandemic has catalyzed a revolution in the prenatal care model. The very real risks of exposure and contagion during the pandemic—for clinicians and patients alike—has forced ObGyns to reexamine the actual risks and benefits of in-person and in-clinic prenatal care. As a result, many ObGyns have rapidly adopted telemedicine into practices that were strictly in-person. For example, a national survey of 172 clinicians who offered contraception counseling during the pandemic found that 91% of them were now offering telemedicine services, with 78% of those clinicians new to telemedicine.5 Similarly, although a minority of surveyed obstetricians in New York City reported using telemedicine pre-pandemic, 89% planned to continue using such technology in the future.6
Continue to: Incorporating mobile technology...
Incorporating mobile technology
Obstetricians, forced to consolidate and maximize their in-person care to protect their patients’ safety, have started to realize that many of the conversations and counseling offered to patients can be managed equally effectively with telemedicine. Furthermore, basic home monitoring devices, such as blood pressure machines, can be safely and accurately used by patients without requiring them to come to the office.
More recent research into mobile medical devices suggests that patients can safely and appropriately manage more complex tools. One such example is a mobile, self-operated, ultrasound transducer that is controlled through a smartphone (Instinct, Pulsenmore Ltd). This device was evaluated in an observational, noninterventional trial of 100 women carrying a singleton fetus at 14/0 weeks’ to 39/6 weeks’ gestation. Patients performed 1,360 self-scans, which were reviewed by a clinician in real time online or subsequently off-line. Results showed successful detection rates of 95.3% for fetal heart activity, 88.3% for body movements, 69.4% for tone, 23.8% for breathing movements, and 92.2% for normal amniotic fluid volume.7 The authors concluded that this represents a feasible solution for remote sonographic fetal assessment.
Coordinating care with health care extenders
Remote monitoring options allow patients to be safely monitored during their pregnancies while remaining at home more often, especially when used in conjunction with trained health care extenders such as registered nurses, primary care associates, or “maternity navigators” who can facilitate off-site care. In fact, many aspects of prenatal care are particularly amenable to remote medicine or non–physician-based home care. Different variations of this model of “hybrid” prenatal care may be appropriate depending upon the needs of the patient population served by a given obstetrics practice. Ideally, a prenatal care model personalizes care based on the known risk factors that are identified at the beginning of prenatal care, the anticipated barriers to care, and the patient’s own preferences. As a result, alternatives to the traditional model may be to alternate in-person and telemedicine visits,3,8 to incorporate in-person or remote group prenatal visits,9,10 or to incorporate staff with basic health care skills to serve as health care extenders in the community and provide home visits for basic monitoring, laboratory work, and patient education.11
Benefits of hybrid prenatal models
As we look ahead to the end of the pandemic, how should obstetricians view these hybrid prenatal care models? Are these models safe for patients? Were they only worthwhile to minimize infection risk, or do they have potential benefits for patients going forward?
In fact, data on the use of telemedicine in prenatal care indicate that these models may be equally as safe as the traditional model in terms of clinical outcomes and may have important additional benefits with regard to patient convenience and access to and satisfaction with care. Even audio-only prenatal televisits have been found to be equivalent to in-person visits in terms of serious perinatal outcomes.12 Common pregnancy diagnoses are also well-served by telemedicine. For example, several recent investigations of patients with gestational diabetes have found that telemedicine was as effective as standard care for glucose control.13,14 Management of hypertension during pregnancy, another antenatal condition that is commonly managed with frequent in-person check-ups, also was found to be adequately feasible with telemedicine using home monitors and symptom checklists, with high rates of patient satisfaction.15
With good evidence for safety, the added potential for patients to benefit in such hybrid models is multifactorial. For one, despite our collective hopes, the COVID-19 pandemic may have a long tail. Vaccine hesitancy and COVID-19 variants may mean that clinicians will have to consider the real threat of infection risk in the clinic setting for years to come. In-home prenatal care also provides a wide variety of social, economic, and psychological benefits for pregnant women across various patient populations. The pandemic has introduced many patients to the full potential of working and meeting remotely; pregnant patients are becoming more familiar with these technology platforms and appreciate its incorporation into their busy lives.5 Furthermore, hybrid models actually can provide otherwise “nonadherent” patients with better access to care. From the patient perspective, an in-person 15-minute health care provider visit actually represents a significant commitment of time and resources (ie, hours spent on public transportation, lost wages for those with inflexible work schedules, and childcare costs for patients discouraged from bringing their children to prenatal visits). Especially for patients with fewer socioeconomic resources, these barriers to in-person clinic visits may be daunting, if not insurmountable; the option of remote visits or house calls reduces these barriers and facilitates care.16
Such hybrid models benefit prenatal clinicians as well. In addition to a decreased risk of infection, clinicians may be able to attract a wider potential prenatal patient population with telemedicine by appealing to younger and potentially more technology-savvy patients.17 Importantly, telemedicine is increasingly recognized as on par with in-person visits in many billing algorithms. Changes during the pandemic led Medicare to cover telemedicine visits as well as in-person visits18,19; among other groundbreaking changes, new patients can have an initial billable visit via telemedicine. Although the billing landscape will likely continue to evolve, such changes allow clinicians to focus on patient safety and convenience without financial risk to their practices.
The future of prenatal appointment scheduling
The future of prenatal care certainly doesn’t look like a dozen 15-minute visits in a private physician’s office. While these emerging hybrid models of prenatal care certainly can benefit patients with low-risk uncomplicated pregnancies, they are already being adopted by clinicians who care for patients with antenatal complications that require specialist consultation; for those with conditions that require frequent, low-complexity check-ins (gestational diabetes, chronic hypertension, history of pre-term birth, etc.); and for patients who struggle with financial or logistical barriers to in-person care. Although obstetrics may have lagged behind other subspecialties in revising its traditional health care models, the pandemic has opened up a new world of possibilities of remote and in-home care for this field. ●
- Kao H, Conant R, Soriano T, et al. The past, present, and future of house calls. Clin Geriatr Med. 2009;25:19-34. doi:10.1016/j.cger.2008.10.005.
- Kane CK, Gillis K. The use of telemedicine by physicians: still the exception rather than the rule. Health Aff (Millwood). 2018;37:1923-1930. doi:10.1377/hlthaff.2018.05077.
- Weigel G, Frederiksen B, Ranji U. Telemedicine and pregnancy care. Kaiser Family Foundation website. https://www.kff.org/womens-health-policy/issue-brief/telemedicine-and-pregnancy-care. Accessed August 23, 2021.
- ACOG Committee Opinion No. 736: optimizing postpartum care. Obstet Gynecol. 2018;131:e140-e150. doi:10.1097/AOG.0000000000002633.
- Stifani BM, Avila K, Levi EE. Telemedicine for contraceptive counseling: an exploratory survey of US family planning providers following rapid adoption of services during the COVID-19 pandemic. Contraception. 2021;103:157-162. doi:10.1016/j.contraception.2020.11.006.
- Madden N, Emeruwa UN, Friedman AM, et al. Telehealth uptake into prenatal care and provider attitudes during the COVID-19 pandemic in New York City: a quantitative and qualitative analysis. Am J Perinatol. 2020;37:1005-1014. doi:10.1055/s-0040-1712939.
- Hadar E, Wolff L, Tenenbaum-Gavish K, et al. Mobile self-operated home ultrasound system for remote fetal assessment during pregnancy. Telemed J E Health. 2021. doi:10.1089/tmj.2020.0541.
- Thomas Jefferson University Division of Maternal Fetal Medicine. Jefferson Maternal Fetal Medicine COVID19 Preparedness. Version 2.1. March 19, 2020. https://communities.smfm.org/HigherLogic/System/DownloadDocumentFile.ashx?DocumentFileKey=a109df77-74fe-462b-87fb-895d6ee7d0e6. Accessed August 23, 2021.
- Ickovics JR, Kershaw TS, Westdahl C, et al. Group prenatal care and perinatal outcomes. Obstet Gynecol. 2007;110(2 pt 1):330-339. doi:10.1097/01.AOG.0000275284.24298.23.
- Wicklund M. Oakland launches telehealth program for Black prenatal, postpartum care. Telehealth News. https://mhealthintelligence.com/news/oakland-launches-telehealth-program-for-black-prenatal-postpartum-care. Accessed August 23, 2021.
- Home-based pregnancy care. CayabaCare website. https://www.cayabacare.com. Accessed August 23, 2021.
- Duryea EL, Adhikari EH, Ambia A, et al. Comparison between in-person and audio-only virtual prenatal visits and perinatal outcomes. JAMA Netw Open. 2021;4:e215854. doi:10.1001/jamanetworkopen.2021.5854.
- Ming WK, Mackillop LH, Farmer AJ, et al. Telemedicine technologies for diabetes in pregnancy: a systematic review and meta-analysis. J Med Internet Res. 2016;18:e290. doi:10.2196/jmir.6556.
- Tian Y, Zhang S, Huang F, et al. Comparing the efficacies of telemedicine and standard prenatal care on blood glucose control in women with gestational diabetes mellitus: randomized controlled trial. JMIR Mhealth Uhealth. 2021;9:e22881. doi:10.2196/22881.
- van den Heuvel JFM, Kariman SS, van Solinge WW, et al. SAFE@HOME – feasibility study of a telemonitoring platform combining blood pressure and preeclampsia symptoms in pregnancy care. Eur J Obstet Gynecol Reprod Biol. 2019;240:226-231. doi:10.1016/j.ejogrb.2019.07.012.
- Dixon-Shambley K, Gabbe PT. Using telehealth approaches to address social determinants of health and improve pregnancy and postpartum outcomes. Clin Obstet Gynecol. 2021;64:333-344. doi:10.1097/GRF.0000000000000611.
- Eruchalu CN, Pichardo MS, Bharadwaj M, et al. The expanding digital divide: digital health access inequities during the COVID-19 pandemic in New York City. J Urban Health. 2021;98:183-186. doi:10.1007/s11524-020-00508-9.
- COVID-19 FAQs for obstetrician-gynecologists, telehealth. The American College of Obstetricians and Gynecologists website. https://www.acog.org/clinical-information/physician-faqs/covid-19-faqs-for-ob-gyns-telehealth. Accessed August 23, 2021.
- Managing patients remotely: billing for digital and telehealth services. The American College of Obstetricians and Gynecologists website. Updated October 19, 2020. https://www.acog.org/practice-management/coding/coding-library/managing-patients-remotely-billing-for-digital-and-telehealth-services. Accessed August 23, 2021.
- Kao H, Conant R, Soriano T, et al. The past, present, and future of house calls. Clin Geriatr Med. 2009;25:19-34. doi:10.1016/j.cger.2008.10.005.
- Kane CK, Gillis K. The use of telemedicine by physicians: still the exception rather than the rule. Health Aff (Millwood). 2018;37:1923-1930. doi:10.1377/hlthaff.2018.05077.
- Weigel G, Frederiksen B, Ranji U. Telemedicine and pregnancy care. Kaiser Family Foundation website. https://www.kff.org/womens-health-policy/issue-brief/telemedicine-and-pregnancy-care. Accessed August 23, 2021.
- ACOG Committee Opinion No. 736: optimizing postpartum care. Obstet Gynecol. 2018;131:e140-e150. doi:10.1097/AOG.0000000000002633.
- Stifani BM, Avila K, Levi EE. Telemedicine for contraceptive counseling: an exploratory survey of US family planning providers following rapid adoption of services during the COVID-19 pandemic. Contraception. 2021;103:157-162. doi:10.1016/j.contraception.2020.11.006.
- Madden N, Emeruwa UN, Friedman AM, et al. Telehealth uptake into prenatal care and provider attitudes during the COVID-19 pandemic in New York City: a quantitative and qualitative analysis. Am J Perinatol. 2020;37:1005-1014. doi:10.1055/s-0040-1712939.
- Hadar E, Wolff L, Tenenbaum-Gavish K, et al. Mobile self-operated home ultrasound system for remote fetal assessment during pregnancy. Telemed J E Health. 2021. doi:10.1089/tmj.2020.0541.
- Thomas Jefferson University Division of Maternal Fetal Medicine. Jefferson Maternal Fetal Medicine COVID19 Preparedness. Version 2.1. March 19, 2020. https://communities.smfm.org/HigherLogic/System/DownloadDocumentFile.ashx?DocumentFileKey=a109df77-74fe-462b-87fb-895d6ee7d0e6. Accessed August 23, 2021.
- Ickovics JR, Kershaw TS, Westdahl C, et al. Group prenatal care and perinatal outcomes. Obstet Gynecol. 2007;110(2 pt 1):330-339. doi:10.1097/01.AOG.0000275284.24298.23.
- Wicklund M. Oakland launches telehealth program for Black prenatal, postpartum care. Telehealth News. https://mhealthintelligence.com/news/oakland-launches-telehealth-program-for-black-prenatal-postpartum-care. Accessed August 23, 2021.
- Home-based pregnancy care. CayabaCare website. https://www.cayabacare.com. Accessed August 23, 2021.
- Duryea EL, Adhikari EH, Ambia A, et al. Comparison between in-person and audio-only virtual prenatal visits and perinatal outcomes. JAMA Netw Open. 2021;4:e215854. doi:10.1001/jamanetworkopen.2021.5854.
- Ming WK, Mackillop LH, Farmer AJ, et al. Telemedicine technologies for diabetes in pregnancy: a systematic review and meta-analysis. J Med Internet Res. 2016;18:e290. doi:10.2196/jmir.6556.
- Tian Y, Zhang S, Huang F, et al. Comparing the efficacies of telemedicine and standard prenatal care on blood glucose control in women with gestational diabetes mellitus: randomized controlled trial. JMIR Mhealth Uhealth. 2021;9:e22881. doi:10.2196/22881.
- van den Heuvel JFM, Kariman SS, van Solinge WW, et al. SAFE@HOME – feasibility study of a telemonitoring platform combining blood pressure and preeclampsia symptoms in pregnancy care. Eur J Obstet Gynecol Reprod Biol. 2019;240:226-231. doi:10.1016/j.ejogrb.2019.07.012.
- Dixon-Shambley K, Gabbe PT. Using telehealth approaches to address social determinants of health and improve pregnancy and postpartum outcomes. Clin Obstet Gynecol. 2021;64:333-344. doi:10.1097/GRF.0000000000000611.
- Eruchalu CN, Pichardo MS, Bharadwaj M, et al. The expanding digital divide: digital health access inequities during the COVID-19 pandemic in New York City. J Urban Health. 2021;98:183-186. doi:10.1007/s11524-020-00508-9.
- COVID-19 FAQs for obstetrician-gynecologists, telehealth. The American College of Obstetricians and Gynecologists website. https://www.acog.org/clinical-information/physician-faqs/covid-19-faqs-for-ob-gyns-telehealth. Accessed August 23, 2021.
- Managing patients remotely: billing for digital and telehealth services. The American College of Obstetricians and Gynecologists website. Updated October 19, 2020. https://www.acog.org/practice-management/coding/coding-library/managing-patients-remotely-billing-for-digital-and-telehealth-services. Accessed August 23, 2021.
Time to retire race- and ethnicity-based carrier screening
The social reckoning of 2020 has led to many discussions and conversations around equity and disparities. With the COVID-19 pandemic, there has been a particular spotlight on health care disparities and race-based medicine. Racism in medicine is pervasive; little has been done over the years to dismantle and unlearn practices that continue to contribute to existing gaps and disparities. Race and ethnicity are both social constructs that have long been used within medical practice and in dictating the type of care an individual receives. Without a universal definition, race, ethnicity, and ancestry have long been used interchangeably within medicine and society. Appreciating that race and ethnicity-based constructs can have other social implications in health care, with their impact on structural racism beyond health care settings, these constructs may still be part of assessments and key modifiers to understanding health differences. It is imperative that medical providers examine the use of race and ethnicity within the care that they provide.
While racial determinants of health cannot be removed from historical access, utilization, and barriers related to reproductive care, guidelines structured around historical ethnicity and race further restrict universal access to carrier screening and informed reproductive testing decisions.
Carrier screening
The goal of preconception and prenatal carrier screening is to provide individuals and reproductive partners with information to optimize pregnancy outcomes based on personal values and preferences.1 The practice of carrier screening began almost half a century ago with screening for individual conditions seen more frequently in certain populations, such as Tay-Sachs disease in those of Ashkenazi Jewish descent and sickle cell disease in those of African descent. Cystic fibrosis carrier screening was first recommended for individuals of Northern European descent in 2001 before being recommended for pan ethnic screening a decade later. Other individual conditions are also recommended for screening based on race/ethnicity (eg, Canavan disease in the Ashkenazi Jewish population, Tay-Sachs disease in individuals of Cajun or French-Canadian descent).2-4 Practice guidelines from professional societies recommend offering carrier screening for individual conditions based on condition severity, race or ethnicity, prevalence, carrier frequency, detection rates, and residual risk.1 However, this process can be problematic, as the data frequently used in updating guidelines and recommendations come primarily from studies and databases where much of the cohort is White.5,6 Failing to identify genetic associations in diverse populations limits the ability to illuminate new discoveries that inform risk management and treatment, especially for populations that are disproportionately underserved in medicine.7
Need for expanded carrier screening
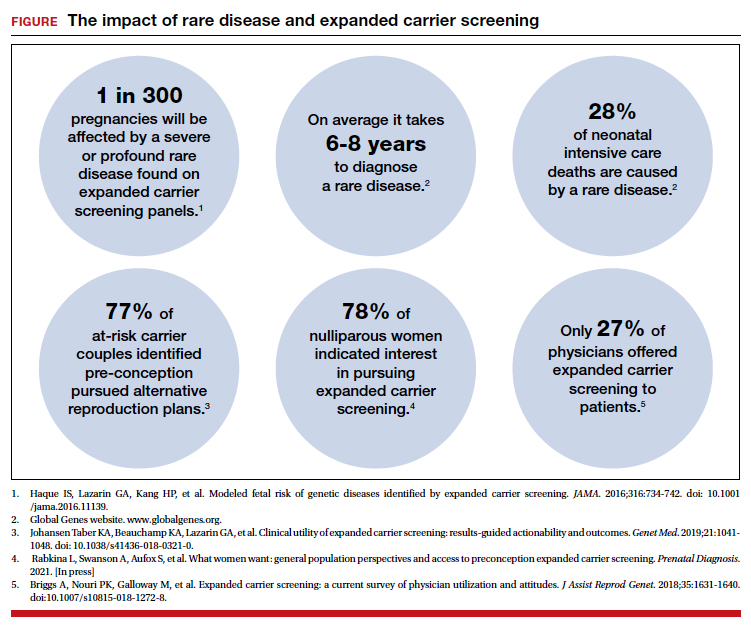
The evolution of genomics and technology within the realm of carrier screening has enabled the simultaneous screening for many serious Mendelian diseases, known as expanded carrier screening (ECS). A 2016 study illustrated that, in most racial/ethnic categories, the cumulative risk of severe and profound conditions found on ECS panels outside the guideline recommendations are greater than the risk identified by guideline-based panels.8 Additionally, a 2020 study showed that self-reported ethnicity was an imperfect indicator of genetic ancestry, with 9% of those in the cohort having a >50% genetic ancestry from a lineage inconsistent with their self-reported ethnicity.9 Data over the past decade have established the clinical utility,10 clinical validity,11 analytical validity,12 and cost-effectiveness13 of pan-ethnic ECS. In 2021, American College of Medical Genetics and Genomics (ACMG) recommended a panel of pan-ethnic conditions that should be offered to all patients due to smaller ethnicity-based panels failing to provide equitable evaluation of all racial and ethnic groups.14 The guidelines from the American College of Obstetricians and Gynecologists (ACOG) fall short of recommending that ECS be offered to all individuals in lieu of screening based on self-reported ethnicity.3,4
Phasing out ethnicity-based carrier screening
This begs the question: Do race, ethnicity, or ancestry have a role in carrier screening? While each may have had a role at the inception of offering carrier screening due to high costs of technology, recent studies have shown the limitations of using self-reported ethnicity in screening. Guideline-based carrier screenings miss a significant percentage of pregnancies (13% to 94%) affected by serious conditions on expanded carrier screening panels.8 Additionally, 40% of Americans cannot identify the ethnicity of all 4 grandparents.15
Founder mutations due to ancestry patterns are still present; however, stratification of care should only be pursued when the presence or absence of these markers would alter clinical management. While the reproductive risk an individual may receive varies based on their self-reported ethnicity, the clinically indicated follow-up testing is the same: offering carrier screening for the reproductive partner or gamete donor. With increased detection rates via sequencing for most autosomal recessive conditions, if the reproductive partner or gamete donor is not identified as a carrier, no further testing is generally indicated regardless of ancestry. Genotyping platforms should not be used for partner carrier screening as they primarily target common pathogenic variants based on dominant ancestry groups and do not provide the same risk reduction.
Continue to: Variant reporting...
Variant reporting
We have long known that databases and registries in the United States have an increased representation of individuals from European ancestries.5,6 However, there have been limited conversations about how the lack of representation within our databases and registries leads to inequities in guidelines and the care that we provide to patients. As a result, studies have shown higher rates of variants of uncertain significance (VUS) identified during genetic testing in non-White individuals than in Whites.16 When it comes to reporting of variants, carrier screening laboratories follow guidelines set forth by the ACMG, and most laboratories only report likely pathogenic or pathogenic variants.17 It is unknown how the higher rate of VUSs in the non-White population, and lack of data and representation in databases and software used to calculate predicted phenotype, impacts identification of at-risk carrier couples in these underrepresented populations. It is imperative that we increase knowledge and representation of variants across ethnicities to improve sensitivity and specificity across the population and not just for those of European descent.
Moving forward
Being aware of social- and race-based biases in carrier screening is important, but modifying structural systems to increase representation, access, and utility of carrier screening is a critical next step. Organizations like ACOG and ACMG have committed not only to understanding but also to addressing factors that have led to disparities and inequities in health care delivery and access.18,19 Actionable steps include offering a universal carrier screening program to all preconception and prenatal patients that addresses conditions with increased carrier frequency, in any population, defined as severe and moderate phenotype with established natural history.3,4 Educational materials should be provided to detail risks, benefits, and limitations of carrier screening, as well as shared decision making between patient and provider to align the patient’s wishes for the information provided by carrier screening.
A broader number of conditions offered through carrier screening will increase the likelihood of positive carrier results. The increase in carriers identified should be viewed as more accurate reproductive risk assessment in the context of equitable care, rather than justification for panels to be limited to specific ancestries. Simultaneous or tandem reproductive partner or donor testing can be considered to reduce clinical workload and time for results return.
In addition, increased representation of individuals who are from diverse ancestries in promotional and educational resources can reinforce that risk for Mendelian conditions is not specific to single ancestries or for targeted conditions. Future research should be conducted to examine the role of racial disparities related to carrier screening and greater inclusion and recruitment of diverse populations in data sets and research studies.
Learned biases toward race, religion, gender identity, sexual orientation, and economic status in the context of carrier screening should be examined and challenged to increase access for all patients who may benefit from this testing. For example, the use of gendered language within carrier screening guidelines and policies and how such screening is offered to patients should be examined. Guidelines do not specify what to do when someone is adopted, for instance, or does not know their ethnicity. It is important that, as genomic testing becomes more available, individuals and groups are not left behind and existing gaps are not further widened. Assessing for genetic variation that modifies for disease or treatment will be more powerful than stratifying based on race. Carrier screening panels should be comprehensive regardless of ancestry to ensure coverage for global genetic variation and to increase access for all patients to risk assessments that promote informed reproductive decision making.
Health equity requires unlearning certain behaviors
As clinicians we all have a commitment to educate and empower one another to offer care that helps promote health equity. Equitable care requires us to look at the current gaps and figure out what programs and initiatives need to be designed to address those gaps. Carrier screening is one such area in which we can work together to improve the overall care that our patients receive, but it is imperative that we examine our practices and unlearn behaviors that contribute to existing disparities. ●
- Edwards JG, Feldman G, Goldberg J, et al. Expanded carrier screening in reproductive medicine—points to consider: a joint statement of the American College of Medical Genetics and Genomics, American College of Obstetricians and Gynecologists, National Society of Genetic Counselors, Perinatal Quality Foundation, and Society for Maternal-Fetal Medicine. Obstet Gynecol. 2015;125:653-662. doi: 10.1097 /AOG.0000000000000666.
- Grody WW, Thompson BH, Gregg AR, et al. ACMG position statement on prenatal/preconception expanded carrier screening. Genet Med. 2013;15:482-483. doi: 10.1038/gim.2013.47.
- Committee Opinion No. 690. Summary: carrier screening in the age of genomic medicine. Obstet Gynecol. 2017;129: 595-596. doi: 10.1097/AOG.0000000000001947.
- Committee Opinion No. 691. Carrier screening for genetic conditions. Obstet Gynecol. 2017;129:e41-e55. doi: 10.1097 /AOG.0000000000001952.
- Need AC, Goldstein DB. Next generation disparities in human genomics: concerns and remedies. Trends Genet. 2009;25:489-494. doi: 10.1016/j.tig.2009.09.012.
- Popejoy A, Fullerton S. Genomics is failing on diversity. Nature. 2016;538;161-164. doi: 10.1038/538161a.
- Ewing A. Reimagining health equity in genetic testing. Medpage Today. June 17, 2021. https://www.medpagetoday.com /opinion/second-opinions/93173. Accessed October 27, 2021.
- Haque IS, Lazarin GA, Kang HP, et al. Modeled fetal risk of genetic diseases identified by expanded carrier screening. JAMA. 2016;316:734-742. doi: 10.1001/jama.2016.11139.
- Kaseniit KE, Haque IS, Goldberg JD, et al. Genetic ancestry analysis on >93,000 individuals undergoing expanded carrier screening reveals limitations of ethnicity-based medical guidelines. Genet Med. 2020;22:1694-1702. doi: 10 .1038/s41436-020-0869-3.
- Johansen Taber KA, Beauchamp KA, Lazarin GA, et al. Clinical utility of expanded carrier screening: results-guided actionability and outcomes. Genet Med. 2019;21:1041-1048. doi: 10.1038/s41436-018-0321-0.
- Balzotti M, Meng L, Muzzey D, et al. Clinical validity of expanded carrier screening: Evaluating the gene-disease relationship in more than 200 conditions. Hum Mutat. 2020;41:1365-1371. doi: 10.1002/humu.24033.
- Hogan GJ, Vysotskaia VS, Beauchamp KA, et al. Validation of an expanded carrier screen that optimizes sensitivity via full-exon sequencing and panel-wide copy number variant identification. Clin Chem. 2018;64:1063-1073. doi: 10.1373 /clinchem.2018.286823.
- Beauchamp KA, Johansen Taber KA, Muzzey D. Clinical impact and cost-effectiveness of a 176-condition expanded carrier screen. Genet Med. 2019;21:1948-1957. doi: 10.1038/s41436-019-0455-8.
- Gregg AR, Aarabi M, Klugman S, et al. Screening for autosomal recessive and X-linked conditions during pregnancy and preconception: a practice resource of the American College of Medical Genetics and Genomics (ACMG). Genet Med. 2021;23:1793-1806. doi: 10.1038/s41436-021-01203-z.
- Condit C, Templeton A, Bates BR, et al. Attitudinal barriers to delivery of race-targeted pharmacogenomics among informed lay persons. Genet Med. 2003;5:385-392. doi: 10 .1097/01.gim.0000087990.30961.72.
- Caswell-Jin J, Gupta T, Hall E, et al. Racial/ethnic differences in multiple-gene sequencing results for hereditary cancer risk. Genet Med. 2018;20:234-239.
- Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405-424. doi:10.1038/gim.2015.30.
- Gregg AR. Message from ACMG President: overcoming disparities. Genet Med. 2020;22:1758.
The social reckoning of 2020 has led to many discussions and conversations around equity and disparities. With the COVID-19 pandemic, there has been a particular spotlight on health care disparities and race-based medicine. Racism in medicine is pervasive; little has been done over the years to dismantle and unlearn practices that continue to contribute to existing gaps and disparities. Race and ethnicity are both social constructs that have long been used within medical practice and in dictating the type of care an individual receives. Without a universal definition, race, ethnicity, and ancestry have long been used interchangeably within medicine and society. Appreciating that race and ethnicity-based constructs can have other social implications in health care, with their impact on structural racism beyond health care settings, these constructs may still be part of assessments and key modifiers to understanding health differences. It is imperative that medical providers examine the use of race and ethnicity within the care that they provide.
While racial determinants of health cannot be removed from historical access, utilization, and barriers related to reproductive care, guidelines structured around historical ethnicity and race further restrict universal access to carrier screening and informed reproductive testing decisions.
Carrier screening
The goal of preconception and prenatal carrier screening is to provide individuals and reproductive partners with information to optimize pregnancy outcomes based on personal values and preferences.1 The practice of carrier screening began almost half a century ago with screening for individual conditions seen more frequently in certain populations, such as Tay-Sachs disease in those of Ashkenazi Jewish descent and sickle cell disease in those of African descent. Cystic fibrosis carrier screening was first recommended for individuals of Northern European descent in 2001 before being recommended for pan ethnic screening a decade later. Other individual conditions are also recommended for screening based on race/ethnicity (eg, Canavan disease in the Ashkenazi Jewish population, Tay-Sachs disease in individuals of Cajun or French-Canadian descent).2-4 Practice guidelines from professional societies recommend offering carrier screening for individual conditions based on condition severity, race or ethnicity, prevalence, carrier frequency, detection rates, and residual risk.1 However, this process can be problematic, as the data frequently used in updating guidelines and recommendations come primarily from studies and databases where much of the cohort is White.5,6 Failing to identify genetic associations in diverse populations limits the ability to illuminate new discoveries that inform risk management and treatment, especially for populations that are disproportionately underserved in medicine.7
Need for expanded carrier screening
The evolution of genomics and technology within the realm of carrier screening has enabled the simultaneous screening for many serious Mendelian diseases, known as expanded carrier screening (ECS). A 2016 study illustrated that, in most racial/ethnic categories, the cumulative risk of severe and profound conditions found on ECS panels outside the guideline recommendations are greater than the risk identified by guideline-based panels.8 Additionally, a 2020 study showed that self-reported ethnicity was an imperfect indicator of genetic ancestry, with 9% of those in the cohort having a >50% genetic ancestry from a lineage inconsistent with their self-reported ethnicity.9 Data over the past decade have established the clinical utility,10 clinical validity,11 analytical validity,12 and cost-effectiveness13 of pan-ethnic ECS. In 2021, American College of Medical Genetics and Genomics (ACMG) recommended a panel of pan-ethnic conditions that should be offered to all patients due to smaller ethnicity-based panels failing to provide equitable evaluation of all racial and ethnic groups.14 The guidelines from the American College of Obstetricians and Gynecologists (ACOG) fall short of recommending that ECS be offered to all individuals in lieu of screening based on self-reported ethnicity.3,4
Phasing out ethnicity-based carrier screening
This begs the question: Do race, ethnicity, or ancestry have a role in carrier screening? While each may have had a role at the inception of offering carrier screening due to high costs of technology, recent studies have shown the limitations of using self-reported ethnicity in screening. Guideline-based carrier screenings miss a significant percentage of pregnancies (13% to 94%) affected by serious conditions on expanded carrier screening panels.8 Additionally, 40% of Americans cannot identify the ethnicity of all 4 grandparents.15
Founder mutations due to ancestry patterns are still present; however, stratification of care should only be pursued when the presence or absence of these markers would alter clinical management. While the reproductive risk an individual may receive varies based on their self-reported ethnicity, the clinically indicated follow-up testing is the same: offering carrier screening for the reproductive partner or gamete donor. With increased detection rates via sequencing for most autosomal recessive conditions, if the reproductive partner or gamete donor is not identified as a carrier, no further testing is generally indicated regardless of ancestry. Genotyping platforms should not be used for partner carrier screening as they primarily target common pathogenic variants based on dominant ancestry groups and do not provide the same risk reduction.

Continue to: Variant reporting...
Variant reporting
We have long known that databases and registries in the United States have an increased representation of individuals from European ancestries.5,6 However, there have been limited conversations about how the lack of representation within our databases and registries leads to inequities in guidelines and the care that we provide to patients. As a result, studies have shown higher rates of variants of uncertain significance (VUS) identified during genetic testing in non-White individuals than in Whites.16 When it comes to reporting of variants, carrier screening laboratories follow guidelines set forth by the ACMG, and most laboratories only report likely pathogenic or pathogenic variants.17 It is unknown how the higher rate of VUSs in the non-White population, and lack of data and representation in databases and software used to calculate predicted phenotype, impacts identification of at-risk carrier couples in these underrepresented populations. It is imperative that we increase knowledge and representation of variants across ethnicities to improve sensitivity and specificity across the population and not just for those of European descent.
Moving forward
Being aware of social- and race-based biases in carrier screening is important, but modifying structural systems to increase representation, access, and utility of carrier screening is a critical next step. Organizations like ACOG and ACMG have committed not only to understanding but also to addressing factors that have led to disparities and inequities in health care delivery and access.18,19 Actionable steps include offering a universal carrier screening program to all preconception and prenatal patients that addresses conditions with increased carrier frequency, in any population, defined as severe and moderate phenotype with established natural history.3,4 Educational materials should be provided to detail risks, benefits, and limitations of carrier screening, as well as shared decision making between patient and provider to align the patient’s wishes for the information provided by carrier screening.
A broader number of conditions offered through carrier screening will increase the likelihood of positive carrier results. The increase in carriers identified should be viewed as more accurate reproductive risk assessment in the context of equitable care, rather than justification for panels to be limited to specific ancestries. Simultaneous or tandem reproductive partner or donor testing can be considered to reduce clinical workload and time for results return.
In addition, increased representation of individuals who are from diverse ancestries in promotional and educational resources can reinforce that risk for Mendelian conditions is not specific to single ancestries or for targeted conditions. Future research should be conducted to examine the role of racial disparities related to carrier screening and greater inclusion and recruitment of diverse populations in data sets and research studies.
Learned biases toward race, religion, gender identity, sexual orientation, and economic status in the context of carrier screening should be examined and challenged to increase access for all patients who may benefit from this testing. For example, the use of gendered language within carrier screening guidelines and policies and how such screening is offered to patients should be examined. Guidelines do not specify what to do when someone is adopted, for instance, or does not know their ethnicity. It is important that, as genomic testing becomes more available, individuals and groups are not left behind and existing gaps are not further widened. Assessing for genetic variation that modifies for disease or treatment will be more powerful than stratifying based on race. Carrier screening panels should be comprehensive regardless of ancestry to ensure coverage for global genetic variation and to increase access for all patients to risk assessments that promote informed reproductive decision making.
Health equity requires unlearning certain behaviors
As clinicians we all have a commitment to educate and empower one another to offer care that helps promote health equity. Equitable care requires us to look at the current gaps and figure out what programs and initiatives need to be designed to address those gaps. Carrier screening is one such area in which we can work together to improve the overall care that our patients receive, but it is imperative that we examine our practices and unlearn behaviors that contribute to existing disparities. ●
The social reckoning of 2020 has led to many discussions and conversations around equity and disparities. With the COVID-19 pandemic, there has been a particular spotlight on health care disparities and race-based medicine. Racism in medicine is pervasive; little has been done over the years to dismantle and unlearn practices that continue to contribute to existing gaps and disparities. Race and ethnicity are both social constructs that have long been used within medical practice and in dictating the type of care an individual receives. Without a universal definition, race, ethnicity, and ancestry have long been used interchangeably within medicine and society. Appreciating that race and ethnicity-based constructs can have other social implications in health care, with their impact on structural racism beyond health care settings, these constructs may still be part of assessments and key modifiers to understanding health differences. It is imperative that medical providers examine the use of race and ethnicity within the care that they provide.
While racial determinants of health cannot be removed from historical access, utilization, and barriers related to reproductive care, guidelines structured around historical ethnicity and race further restrict universal access to carrier screening and informed reproductive testing decisions.
Carrier screening
The goal of preconception and prenatal carrier screening is to provide individuals and reproductive partners with information to optimize pregnancy outcomes based on personal values and preferences.1 The practice of carrier screening began almost half a century ago with screening for individual conditions seen more frequently in certain populations, such as Tay-Sachs disease in those of Ashkenazi Jewish descent and sickle cell disease in those of African descent. Cystic fibrosis carrier screening was first recommended for individuals of Northern European descent in 2001 before being recommended for pan ethnic screening a decade later. Other individual conditions are also recommended for screening based on race/ethnicity (eg, Canavan disease in the Ashkenazi Jewish population, Tay-Sachs disease in individuals of Cajun or French-Canadian descent).2-4 Practice guidelines from professional societies recommend offering carrier screening for individual conditions based on condition severity, race or ethnicity, prevalence, carrier frequency, detection rates, and residual risk.1 However, this process can be problematic, as the data frequently used in updating guidelines and recommendations come primarily from studies and databases where much of the cohort is White.5,6 Failing to identify genetic associations in diverse populations limits the ability to illuminate new discoveries that inform risk management and treatment, especially for populations that are disproportionately underserved in medicine.7
Need for expanded carrier screening
The evolution of genomics and technology within the realm of carrier screening has enabled the simultaneous screening for many serious Mendelian diseases, known as expanded carrier screening (ECS). A 2016 study illustrated that, in most racial/ethnic categories, the cumulative risk of severe and profound conditions found on ECS panels outside the guideline recommendations are greater than the risk identified by guideline-based panels.8 Additionally, a 2020 study showed that self-reported ethnicity was an imperfect indicator of genetic ancestry, with 9% of those in the cohort having a >50% genetic ancestry from a lineage inconsistent with their self-reported ethnicity.9 Data over the past decade have established the clinical utility,10 clinical validity,11 analytical validity,12 and cost-effectiveness13 of pan-ethnic ECS. In 2021, American College of Medical Genetics and Genomics (ACMG) recommended a panel of pan-ethnic conditions that should be offered to all patients due to smaller ethnicity-based panels failing to provide equitable evaluation of all racial and ethnic groups.14 The guidelines from the American College of Obstetricians and Gynecologists (ACOG) fall short of recommending that ECS be offered to all individuals in lieu of screening based on self-reported ethnicity.3,4
Phasing out ethnicity-based carrier screening
This begs the question: Do race, ethnicity, or ancestry have a role in carrier screening? While each may have had a role at the inception of offering carrier screening due to high costs of technology, recent studies have shown the limitations of using self-reported ethnicity in screening. Guideline-based carrier screenings miss a significant percentage of pregnancies (13% to 94%) affected by serious conditions on expanded carrier screening panels.8 Additionally, 40% of Americans cannot identify the ethnicity of all 4 grandparents.15
Founder mutations due to ancestry patterns are still present; however, stratification of care should only be pursued when the presence or absence of these markers would alter clinical management. While the reproductive risk an individual may receive varies based on their self-reported ethnicity, the clinically indicated follow-up testing is the same: offering carrier screening for the reproductive partner or gamete donor. With increased detection rates via sequencing for most autosomal recessive conditions, if the reproductive partner or gamete donor is not identified as a carrier, no further testing is generally indicated regardless of ancestry. Genotyping platforms should not be used for partner carrier screening as they primarily target common pathogenic variants based on dominant ancestry groups and do not provide the same risk reduction.

Continue to: Variant reporting...
Variant reporting
We have long known that databases and registries in the United States have an increased representation of individuals from European ancestries.5,6 However, there have been limited conversations about how the lack of representation within our databases and registries leads to inequities in guidelines and the care that we provide to patients. As a result, studies have shown higher rates of variants of uncertain significance (VUS) identified during genetic testing in non-White individuals than in Whites.16 When it comes to reporting of variants, carrier screening laboratories follow guidelines set forth by the ACMG, and most laboratories only report likely pathogenic or pathogenic variants.17 It is unknown how the higher rate of VUSs in the non-White population, and lack of data and representation in databases and software used to calculate predicted phenotype, impacts identification of at-risk carrier couples in these underrepresented populations. It is imperative that we increase knowledge and representation of variants across ethnicities to improve sensitivity and specificity across the population and not just for those of European descent.
Moving forward
Being aware of social- and race-based biases in carrier screening is important, but modifying structural systems to increase representation, access, and utility of carrier screening is a critical next step. Organizations like ACOG and ACMG have committed not only to understanding but also to addressing factors that have led to disparities and inequities in health care delivery and access.18,19 Actionable steps include offering a universal carrier screening program to all preconception and prenatal patients that addresses conditions with increased carrier frequency, in any population, defined as severe and moderate phenotype with established natural history.3,4 Educational materials should be provided to detail risks, benefits, and limitations of carrier screening, as well as shared decision making between patient and provider to align the patient’s wishes for the information provided by carrier screening.
A broader number of conditions offered through carrier screening will increase the likelihood of positive carrier results. The increase in carriers identified should be viewed as more accurate reproductive risk assessment in the context of equitable care, rather than justification for panels to be limited to specific ancestries. Simultaneous or tandem reproductive partner or donor testing can be considered to reduce clinical workload and time for results return.
In addition, increased representation of individuals who are from diverse ancestries in promotional and educational resources can reinforce that risk for Mendelian conditions is not specific to single ancestries or for targeted conditions. Future research should be conducted to examine the role of racial disparities related to carrier screening and greater inclusion and recruitment of diverse populations in data sets and research studies.
Learned biases toward race, religion, gender identity, sexual orientation, and economic status in the context of carrier screening should be examined and challenged to increase access for all patients who may benefit from this testing. For example, the use of gendered language within carrier screening guidelines and policies and how such screening is offered to patients should be examined. Guidelines do not specify what to do when someone is adopted, for instance, or does not know their ethnicity. It is important that, as genomic testing becomes more available, individuals and groups are not left behind and existing gaps are not further widened. Assessing for genetic variation that modifies for disease or treatment will be more powerful than stratifying based on race. Carrier screening panels should be comprehensive regardless of ancestry to ensure coverage for global genetic variation and to increase access for all patients to risk assessments that promote informed reproductive decision making.
Health equity requires unlearning certain behaviors
As clinicians we all have a commitment to educate and empower one another to offer care that helps promote health equity. Equitable care requires us to look at the current gaps and figure out what programs and initiatives need to be designed to address those gaps. Carrier screening is one such area in which we can work together to improve the overall care that our patients receive, but it is imperative that we examine our practices and unlearn behaviors that contribute to existing disparities. ●
- Edwards JG, Feldman G, Goldberg J, et al. Expanded carrier screening in reproductive medicine—points to consider: a joint statement of the American College of Medical Genetics and Genomics, American College of Obstetricians and Gynecologists, National Society of Genetic Counselors, Perinatal Quality Foundation, and Society for Maternal-Fetal Medicine. Obstet Gynecol. 2015;125:653-662. doi: 10.1097 /AOG.0000000000000666.
- Grody WW, Thompson BH, Gregg AR, et al. ACMG position statement on prenatal/preconception expanded carrier screening. Genet Med. 2013;15:482-483. doi: 10.1038/gim.2013.47.
- Committee Opinion No. 690. Summary: carrier screening in the age of genomic medicine. Obstet Gynecol. 2017;129: 595-596. doi: 10.1097/AOG.0000000000001947.
- Committee Opinion No. 691. Carrier screening for genetic conditions. Obstet Gynecol. 2017;129:e41-e55. doi: 10.1097 /AOG.0000000000001952.
- Need AC, Goldstein DB. Next generation disparities in human genomics: concerns and remedies. Trends Genet. 2009;25:489-494. doi: 10.1016/j.tig.2009.09.012.
- Popejoy A, Fullerton S. Genomics is failing on diversity. Nature. 2016;538;161-164. doi: 10.1038/538161a.
- Ewing A. Reimagining health equity in genetic testing. Medpage Today. June 17, 2021. https://www.medpagetoday.com /opinion/second-opinions/93173. Accessed October 27, 2021.
- Haque IS, Lazarin GA, Kang HP, et al. Modeled fetal risk of genetic diseases identified by expanded carrier screening. JAMA. 2016;316:734-742. doi: 10.1001/jama.2016.11139.
- Kaseniit KE, Haque IS, Goldberg JD, et al. Genetic ancestry analysis on >93,000 individuals undergoing expanded carrier screening reveals limitations of ethnicity-based medical guidelines. Genet Med. 2020;22:1694-1702. doi: 10 .1038/s41436-020-0869-3.
- Johansen Taber KA, Beauchamp KA, Lazarin GA, et al. Clinical utility of expanded carrier screening: results-guided actionability and outcomes. Genet Med. 2019;21:1041-1048. doi: 10.1038/s41436-018-0321-0.
- Balzotti M, Meng L, Muzzey D, et al. Clinical validity of expanded carrier screening: Evaluating the gene-disease relationship in more than 200 conditions. Hum Mutat. 2020;41:1365-1371. doi: 10.1002/humu.24033.
- Hogan GJ, Vysotskaia VS, Beauchamp KA, et al. Validation of an expanded carrier screen that optimizes sensitivity via full-exon sequencing and panel-wide copy number variant identification. Clin Chem. 2018;64:1063-1073. doi: 10.1373 /clinchem.2018.286823.
- Beauchamp KA, Johansen Taber KA, Muzzey D. Clinical impact and cost-effectiveness of a 176-condition expanded carrier screen. Genet Med. 2019;21:1948-1957. doi: 10.1038/s41436-019-0455-8.
- Gregg AR, Aarabi M, Klugman S, et al. Screening for autosomal recessive and X-linked conditions during pregnancy and preconception: a practice resource of the American College of Medical Genetics and Genomics (ACMG). Genet Med. 2021;23:1793-1806. doi: 10.1038/s41436-021-01203-z.
- Condit C, Templeton A, Bates BR, et al. Attitudinal barriers to delivery of race-targeted pharmacogenomics among informed lay persons. Genet Med. 2003;5:385-392. doi: 10 .1097/01.gim.0000087990.30961.72.
- Caswell-Jin J, Gupta T, Hall E, et al. Racial/ethnic differences in multiple-gene sequencing results for hereditary cancer risk. Genet Med. 2018;20:234-239.
- Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405-424. doi:10.1038/gim.2015.30.
- Gregg AR. Message from ACMG President: overcoming disparities. Genet Med. 2020;22:1758.
- Edwards JG, Feldman G, Goldberg J, et al. Expanded carrier screening in reproductive medicine—points to consider: a joint statement of the American College of Medical Genetics and Genomics, American College of Obstetricians and Gynecologists, National Society of Genetic Counselors, Perinatal Quality Foundation, and Society for Maternal-Fetal Medicine. Obstet Gynecol. 2015;125:653-662. doi: 10.1097 /AOG.0000000000000666.
- Grody WW, Thompson BH, Gregg AR, et al. ACMG position statement on prenatal/preconception expanded carrier screening. Genet Med. 2013;15:482-483. doi: 10.1038/gim.2013.47.
- Committee Opinion No. 690. Summary: carrier screening in the age of genomic medicine. Obstet Gynecol. 2017;129: 595-596. doi: 10.1097/AOG.0000000000001947.
- Committee Opinion No. 691. Carrier screening for genetic conditions. Obstet Gynecol. 2017;129:e41-e55. doi: 10.1097 /AOG.0000000000001952.
- Need AC, Goldstein DB. Next generation disparities in human genomics: concerns and remedies. Trends Genet. 2009;25:489-494. doi: 10.1016/j.tig.2009.09.012.
- Popejoy A, Fullerton S. Genomics is failing on diversity. Nature. 2016;538;161-164. doi: 10.1038/538161a.
- Ewing A. Reimagining health equity in genetic testing. Medpage Today. June 17, 2021. https://www.medpagetoday.com /opinion/second-opinions/93173. Accessed October 27, 2021.
- Haque IS, Lazarin GA, Kang HP, et al. Modeled fetal risk of genetic diseases identified by expanded carrier screening. JAMA. 2016;316:734-742. doi: 10.1001/jama.2016.11139.
- Kaseniit KE, Haque IS, Goldberg JD, et al. Genetic ancestry analysis on >93,000 individuals undergoing expanded carrier screening reveals limitations of ethnicity-based medical guidelines. Genet Med. 2020;22:1694-1702. doi: 10 .1038/s41436-020-0869-3.
- Johansen Taber KA, Beauchamp KA, Lazarin GA, et al. Clinical utility of expanded carrier screening: results-guided actionability and outcomes. Genet Med. 2019;21:1041-1048. doi: 10.1038/s41436-018-0321-0.
- Balzotti M, Meng L, Muzzey D, et al. Clinical validity of expanded carrier screening: Evaluating the gene-disease relationship in more than 200 conditions. Hum Mutat. 2020;41:1365-1371. doi: 10.1002/humu.24033.
- Hogan GJ, Vysotskaia VS, Beauchamp KA, et al. Validation of an expanded carrier screen that optimizes sensitivity via full-exon sequencing and panel-wide copy number variant identification. Clin Chem. 2018;64:1063-1073. doi: 10.1373 /clinchem.2018.286823.
- Beauchamp KA, Johansen Taber KA, Muzzey D. Clinical impact and cost-effectiveness of a 176-condition expanded carrier screen. Genet Med. 2019;21:1948-1957. doi: 10.1038/s41436-019-0455-8.
- Gregg AR, Aarabi M, Klugman S, et al. Screening for autosomal recessive and X-linked conditions during pregnancy and preconception: a practice resource of the American College of Medical Genetics and Genomics (ACMG). Genet Med. 2021;23:1793-1806. doi: 10.1038/s41436-021-01203-z.
- Condit C, Templeton A, Bates BR, et al. Attitudinal barriers to delivery of race-targeted pharmacogenomics among informed lay persons. Genet Med. 2003;5:385-392. doi: 10 .1097/01.gim.0000087990.30961.72.
- Caswell-Jin J, Gupta T, Hall E, et al. Racial/ethnic differences in multiple-gene sequencing results for hereditary cancer risk. Genet Med. 2018;20:234-239.
- Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405-424. doi:10.1038/gim.2015.30.
- Gregg AR. Message from ACMG President: overcoming disparities. Genet Med. 2020;22:1758.
Is active (vs expectant) management of a persistent PUL more effective?
Barnhart K, Hansen KR, Stephenson MD, et al; Reproductive Medicine Network. Effect of an active vs expectant management strategy on successful resolution of pregnancy among patients with a persisting pregnancy of unknown location: the ACT or NOT randomized clinical trial. JAMA. 2021;326:390-400.
EXPERT COMMENTARY
Among patients with persistent PUL, it can be difficult to distinguish between ectopic pregnancy and an early nonviable intrauterine pregnancy.1 If untreated, ectopic pregnancy can lead to serious morbidity and mortality.2 Management options for persistent PUL include expectant management, empirical methotrexate, or diagnostic uterine evacuation with methotrexate as needed. Data on the potential for these options to achieve pregnancy resolution is valuable for patients and clinicians choosing a treatment plan.
Details of the study
Barnhart and colleagues conducted a multicenter, randomized controlled trial that enrolled 225 women with persistent PUL (defined by transvaginal ultrasound imaging without a definitive intrauterine or extrauterine gestation and at least 2 consecutive human chorionic gonadotropin [hCG] values with less than a 15% rise per day). Participants were randomly assigned to 1 of 3 treatment groups: expectant management, empirical methotrexate, or uterine evacuation followed by methotrexate if needed.
The primary outcome was pregnancy resolution without a change in management strategy. A secondary outcome was noninferiority of empirical methotrexate compared with uterine evacuation with methotrexate as needed in achieving pregnancy resolution.
Results. The active management groups were significantly more likely to achieve pregnancy resolution without changing strategies than the expectant management group (51.5% vs 36.0%; difference, 15.4%). However, 39% of enrolled participants declined their randomized allocation and crossed over into a different management strategy.
Empirical methotrexate was found to be noninferior to uterine evacuation followed by methotrexate as needed in achieving pregnancy resolution (54.9% vs 48.3%; difference, 6.6%).
Study strengths and limitations
Prior studies of hemodynamically stable patients with persistent PUL or stable tubal ectopic pregnancy and low initial hCG values (<2,000 IU/L) failed to demonstrate that active management with methotrexate or uterine evacuation leads to more successful or faster pregnancy resolution.3-5 Barnhart and colleagues’ study results, however, found that active management with 2-dose empirical methotrexate or uterine evacuation was more likely to lead to pregnancy resolution without requiring a change in management plan than was expectant management. The authors performed both an intention-to-treat and an as-treated analysis to confirm results.
The 39% crossover rate between the treatment groups likely reflected both patient preference and clinical presentation, potentially biasing the results. The low overall rate of adverse events confirms the safety and acceptability of a patient-centered approach to persistent PUL management. ●
Patients with a persistent PUL who undergo active management with either empirical methotrexate or uterine evacuation followed by methotrexate are more likely to experience pregnancy resolution without a change in management strategy than those who undergo expectant management. Given the safety of all 3 options and demonstrated patient preferences, shared decision making should be used when determining a management plan.
SARAH GUTMAN, MD, MSPH, AND
COURTNEY A. SCHRIEBER, MD, MPH
- van Mello NM, Mol F, Opmeer BC, et al. Diagnostic value of serum hCG on the outcome of pregnancy of unknown location: a systematic review and meta-analysis. Hum Reprod Update. 2012:18:603-617.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Gynecology. ACOG practice bulletin no. 193: tubal ectopic pregnancy. Obstet Gynecol. 2018;131:e91-e103.
- van Mello NM, Mol F, Verhoeve HR, et al. Methotrexate or expectant management in women with an ectopic pregnancy or pregnancy of unknown location and low serum hCG concentrations? A randomized comparison. Hum Reprod. 2013;28:60-67.
- Jurkovic D, Memtsa M, Sawyer E, et al. Single-dose systemic methotrexate vs expectant management for treatment of tubal ectopic pregnancy: a placebo-controlled randomized trial. Ultrasound Obstet Gynecol. 2017;49:171-176.
- Silva PM, Araujo Junior E, Ceccino GN, et al. Effectiveness of expectant management versus methotrexate in tubal ectopic pregnancy: a double-blind randomized trial. Arch Gynecol Obstet. 2015;291:939-943.
Barnhart K, Hansen KR, Stephenson MD, et al; Reproductive Medicine Network. Effect of an active vs expectant management strategy on successful resolution of pregnancy among patients with a persisting pregnancy of unknown location: the ACT or NOT randomized clinical trial. JAMA. 2021;326:390-400.
EXPERT COMMENTARY
Among patients with persistent PUL, it can be difficult to distinguish between ectopic pregnancy and an early nonviable intrauterine pregnancy.1 If untreated, ectopic pregnancy can lead to serious morbidity and mortality.2 Management options for persistent PUL include expectant management, empirical methotrexate, or diagnostic uterine evacuation with methotrexate as needed. Data on the potential for these options to achieve pregnancy resolution is valuable for patients and clinicians choosing a treatment plan.
Details of the study
Barnhart and colleagues conducted a multicenter, randomized controlled trial that enrolled 225 women with persistent PUL (defined by transvaginal ultrasound imaging without a definitive intrauterine or extrauterine gestation and at least 2 consecutive human chorionic gonadotropin [hCG] values with less than a 15% rise per day). Participants were randomly assigned to 1 of 3 treatment groups: expectant management, empirical methotrexate, or uterine evacuation followed by methotrexate if needed.
The primary outcome was pregnancy resolution without a change in management strategy. A secondary outcome was noninferiority of empirical methotrexate compared with uterine evacuation with methotrexate as needed in achieving pregnancy resolution.
Results. The active management groups were significantly more likely to achieve pregnancy resolution without changing strategies than the expectant management group (51.5% vs 36.0%; difference, 15.4%). However, 39% of enrolled participants declined their randomized allocation and crossed over into a different management strategy.
Empirical methotrexate was found to be noninferior to uterine evacuation followed by methotrexate as needed in achieving pregnancy resolution (54.9% vs 48.3%; difference, 6.6%).
Study strengths and limitations
Prior studies of hemodynamically stable patients with persistent PUL or stable tubal ectopic pregnancy and low initial hCG values (<2,000 IU/L) failed to demonstrate that active management with methotrexate or uterine evacuation leads to more successful or faster pregnancy resolution.3-5 Barnhart and colleagues’ study results, however, found that active management with 2-dose empirical methotrexate or uterine evacuation was more likely to lead to pregnancy resolution without requiring a change in management plan than was expectant management. The authors performed both an intention-to-treat and an as-treated analysis to confirm results.
The 39% crossover rate between the treatment groups likely reflected both patient preference and clinical presentation, potentially biasing the results. The low overall rate of adverse events confirms the safety and acceptability of a patient-centered approach to persistent PUL management. ●
Patients with a persistent PUL who undergo active management with either empirical methotrexate or uterine evacuation followed by methotrexate are more likely to experience pregnancy resolution without a change in management strategy than those who undergo expectant management. Given the safety of all 3 options and demonstrated patient preferences, shared decision making should be used when determining a management plan.
SARAH GUTMAN, MD, MSPH, AND
COURTNEY A. SCHRIEBER, MD, MPH
Barnhart K, Hansen KR, Stephenson MD, et al; Reproductive Medicine Network. Effect of an active vs expectant management strategy on successful resolution of pregnancy among patients with a persisting pregnancy of unknown location: the ACT or NOT randomized clinical trial. JAMA. 2021;326:390-400.
EXPERT COMMENTARY
Among patients with persistent PUL, it can be difficult to distinguish between ectopic pregnancy and an early nonviable intrauterine pregnancy.1 If untreated, ectopic pregnancy can lead to serious morbidity and mortality.2 Management options for persistent PUL include expectant management, empirical methotrexate, or diagnostic uterine evacuation with methotrexate as needed. Data on the potential for these options to achieve pregnancy resolution is valuable for patients and clinicians choosing a treatment plan.
Details of the study
Barnhart and colleagues conducted a multicenter, randomized controlled trial that enrolled 225 women with persistent PUL (defined by transvaginal ultrasound imaging without a definitive intrauterine or extrauterine gestation and at least 2 consecutive human chorionic gonadotropin [hCG] values with less than a 15% rise per day). Participants were randomly assigned to 1 of 3 treatment groups: expectant management, empirical methotrexate, or uterine evacuation followed by methotrexate if needed.
The primary outcome was pregnancy resolution without a change in management strategy. A secondary outcome was noninferiority of empirical methotrexate compared with uterine evacuation with methotrexate as needed in achieving pregnancy resolution.
Results. The active management groups were significantly more likely to achieve pregnancy resolution without changing strategies than the expectant management group (51.5% vs 36.0%; difference, 15.4%). However, 39% of enrolled participants declined their randomized allocation and crossed over into a different management strategy.
Empirical methotrexate was found to be noninferior to uterine evacuation followed by methotrexate as needed in achieving pregnancy resolution (54.9% vs 48.3%; difference, 6.6%).
Study strengths and limitations
Prior studies of hemodynamically stable patients with persistent PUL or stable tubal ectopic pregnancy and low initial hCG values (<2,000 IU/L) failed to demonstrate that active management with methotrexate or uterine evacuation leads to more successful or faster pregnancy resolution.3-5 Barnhart and colleagues’ study results, however, found that active management with 2-dose empirical methotrexate or uterine evacuation was more likely to lead to pregnancy resolution without requiring a change in management plan than was expectant management. The authors performed both an intention-to-treat and an as-treated analysis to confirm results.
The 39% crossover rate between the treatment groups likely reflected both patient preference and clinical presentation, potentially biasing the results. The low overall rate of adverse events confirms the safety and acceptability of a patient-centered approach to persistent PUL management. ●
Patients with a persistent PUL who undergo active management with either empirical methotrexate or uterine evacuation followed by methotrexate are more likely to experience pregnancy resolution without a change in management strategy than those who undergo expectant management. Given the safety of all 3 options and demonstrated patient preferences, shared decision making should be used when determining a management plan.
SARAH GUTMAN, MD, MSPH, AND
COURTNEY A. SCHRIEBER, MD, MPH
- van Mello NM, Mol F, Opmeer BC, et al. Diagnostic value of serum hCG on the outcome of pregnancy of unknown location: a systematic review and meta-analysis. Hum Reprod Update. 2012:18:603-617.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Gynecology. ACOG practice bulletin no. 193: tubal ectopic pregnancy. Obstet Gynecol. 2018;131:e91-e103.
- van Mello NM, Mol F, Verhoeve HR, et al. Methotrexate or expectant management in women with an ectopic pregnancy or pregnancy of unknown location and low serum hCG concentrations? A randomized comparison. Hum Reprod. 2013;28:60-67.
- Jurkovic D, Memtsa M, Sawyer E, et al. Single-dose systemic methotrexate vs expectant management for treatment of tubal ectopic pregnancy: a placebo-controlled randomized trial. Ultrasound Obstet Gynecol. 2017;49:171-176.
- Silva PM, Araujo Junior E, Ceccino GN, et al. Effectiveness of expectant management versus methotrexate in tubal ectopic pregnancy: a double-blind randomized trial. Arch Gynecol Obstet. 2015;291:939-943.
- van Mello NM, Mol F, Opmeer BC, et al. Diagnostic value of serum hCG on the outcome of pregnancy of unknown location: a systematic review and meta-analysis. Hum Reprod Update. 2012:18:603-617.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Gynecology. ACOG practice bulletin no. 193: tubal ectopic pregnancy. Obstet Gynecol. 2018;131:e91-e103.
- van Mello NM, Mol F, Verhoeve HR, et al. Methotrexate or expectant management in women with an ectopic pregnancy or pregnancy of unknown location and low serum hCG concentrations? A randomized comparison. Hum Reprod. 2013;28:60-67.
- Jurkovic D, Memtsa M, Sawyer E, et al. Single-dose systemic methotrexate vs expectant management for treatment of tubal ectopic pregnancy: a placebo-controlled randomized trial. Ultrasound Obstet Gynecol. 2017;49:171-176.
- Silva PM, Araujo Junior E, Ceccino GN, et al. Effectiveness of expectant management versus methotrexate in tubal ectopic pregnancy: a double-blind randomized trial. Arch Gynecol Obstet. 2015;291:939-943.
Can we return to the ABCs of crafting a medical record note?
Prior to 1980, medical record notes were generally hand-written, short, and to the point. Senior physicians often wrote their 3-line notes using a fountain pen in an elegant cursive. With the transition to electronic medical records, notes have become bloated with irrelevant information and frequently lack a focus on the critical clinical insights that optimize patient care. The use of smart phrases to pull vast amounts of raw data into the note is a major contributor to note bloat. The unrestrained use of the copy and paste functionality generates a sequence of cloned notes that grow in length as new information is added and little information from prior notes removed. With each subsequent clone the note often becomes less accurate, lengthier, and more difficult for a reader to understand. In one survey of 253 physicians who wrote electronic notes, 90% reported that they used the copy and paste function, with 71% reporting that use of this function caused inconsistencies within and among notes and increased the repetitive presentation of outdated information in the note.1 Although the surveyed clinicians recognized that the copy and paste function caused problems, 80% reported that they planned to continue to use the copy and paste function.1
The SOAP note
The problem-oriented SOAP note is written in the classic structure of subjective and objective information, followed by an assessment and plan.2 The structure of the SOAP note emphasizes the logical and sequential collection of data followed by data analysis, resulting in a focused assessment and plan. When notes were hand-written and short, the entire SOAP note could be viewed on one page. Like a dashboard, the eye could quickly scan each key component of the note, facilitating the simultaneous integration of all 4 components of the note, facilitating understanding of the patient’s clinical situation. When the SOAP note structure is used to create a multipage electronic note, the result is a note that often confuses rather than enlightens the reader. A 5- to 10-page SOAP note is often useless for patient care but demonstrates the ability of computer-savvy clinicians to quickly generate a note thousands of words in length.
The APSO note, a response to note bloat
When a medical record note becomes a multipage document, clinicians should consider switching from the SOAP note structure to the APSO note, where the assessment and plan are at the top of the note, and the subjective and objective information is below the assessment and plan. The APSO format permits the reader to more quickly grasp the critical thinking of the author and facilitates a focus on key points relevant to the patient’s condition. The note can be written in the SOAP format, but then the assessment and plan are brought to the top of the note. In my clinical experience fewer than 10% of clinicians are using an APSO note structure. I believe that, with a multipage note, the APSO structure improves the experience of the reader and should be more widely utilized, especially by clinicians who are prone to crafting a bloated note. In a survey of more than 3,000 clinicians, approximately two-thirds of the respondents reported that, compared with SOAP notes, APSO notes were easier and faster to read, and APSO notes made it easier to follow the clinical reasoning of the author.3
Continue to: New evaluation and management billing guidelines—An opportunity to reduce note bloat...
New evaluation and management billing guidelines—An opportunity to reduce note bloat
Previous evaluation and management federal billing guidelines emphasized documentation of a myriad of clinically irrelevant details contributing to note bloat. The new federal evaluation and management billing guidelines pivot the focus of the note to the quality and complexity of medical decision making as demonstrated in the assessment and plan.4 Prioritizing the assessment and plan as the key feature of the medical record note should help reduce the length of notes. The American College of Physicians recently recommended deleting the complete review of systems and prior histories from most notes unless relevant to medical decision making and the assessment and plan.5
The open note
The open note mandate was contained in federal regulations developed to implement the 21st Century Cures Act, which required patients to have access to the information in their medical record. In order to comply with the regulation, health systems are sending most notes and test results to the patient through the health system’s patient gateway. The open note process entered my practice through a stealthy progression, from an initial step of permitting a clinician to easily share their note with a patient to a top-down edict that all notes, except some notes that have a high risk of causing patient harm, must be sent immediately to the patient. Obviously, an open note supports “transparency,” but I am unaware of high quality evidence that open notes improve the health of a population or reduce morbidity or mortality from health problems.
The federal mandate that clinicians share their notes or risk fiscal penalties is coercive and undermines the independence of health professionals. Open notes may have many benefits, including:
- improving a patient’s comprehension and sense of control over their health issues
- increasing patient trust in their health system
- increasing the number of questions patients ask their clinician.6
Open notes may also cause unintended adverse emotional trauma to patients, especially when the note communicates “bad news.” In one study of 100 oncology patients, approximately 25% of respondents reported that reading clinical notes was emotionally difficult, and they sometimes regretted having read the note.6 One patient reported, “I think MyChart is great but in this whole cancer thing MyChart has not been a good thing.” Another patient reported, “Reading serious stuff like that is just too taxing for me to be honest with you.”6 An additional finding of the study was that patients reported their notes were written with too much medical jargon and repetition of information.
Open laboratory, pathology, and imaging data—Helpful or harmful?
A component of the open note mandate is that laboratory, pathology, and imaging data must be shared timely with patients. Some health systems incorporate a 3-day pause prior to sharing such data, in order to provide the clinical team with time to communicate with the patient before the test results are shared. Some health systems, including my health system, have engineered the open note data-sharing system to immediately share the results of most completed laboratory, pathology, and imaging studies with the patient. Immediate sharing of data may result in the patient first learning that they have a serious, life-threatening health problem, such as cancer, from their patient portal rather than from a clinician. As an example, a patient may first learn that they have metastatic cancer from a CT scan that was ordered for a benign indication.
Another example is that a patient may first learn that they have an HIV infection from their patient portal. This can be a shocking and emotionally damaging experience for the patient. For many test results, it would be best if a clinician were able to communicate the result to the patient, providing support and context to the meaning of the result, rather than sending sensitive, life-altering information directly from the laboratory or imaging department to the patient. Leaders in medical education have spent decades teaching clinicians how to communicate “bad news” in a sensitive, supportive, and effective manner. The open sharing of laboratory, pathology, and imaging data short-circuits the superior process of relying on a highly capable clinician to communicate bad news.
Continue to: Crafting the open medical record note...
Crafting the open medical record note
Building on the advice that “when life gives you lemons, make lemonade,” I have begun to pivot the purpose of my medical notes from a product useful to myself and other clinicians to a product whose primary purpose is to be helpful for the patient. The open note can facilitate building a trusting relationship with the patient. My notes are becoming a series of written conversations with the patient, emphasizing compassion and empathy. I am increasing significantly the amount of educational information in the note to help the patient understand their situation. In addition, I am replacing traditional medical terms with verbiage more appropriate in the context of a conversation with the patient, reducing the use of medical jargon. For example, I have stopped using “chief complaint” and replaced it with “health issues.” I am diligently avoiding the use of medical terms that have negative connotations, including “obese,” “psychosomatic,” “alcoholic,” and “drug addiction.” I include encouragement and positive comments in many of my notes. For example, “Ms. X is successfully managing her health issues and experiencing improved health. It is a pleasure collaborating with her on achieving optimal health.”
Can we bring sanity back to medical note writing?
The primary role of a clinician is to spend as much time as possible listening to patients, understanding their needs, and helping them achieve optimal health. There are many benefits to an electronic medical record, including legibility, accessibility, interoperability, and efficiency. However, in current practice “note bloat” undermines the potential of the electronic medical record and makes many notes ineffective to the process of advancing the patient’s health. We are competent and highly trained clinicians. We can craft notes that are simple, specific, story-driven, compassionate, and empathetic. If we return to the ABCs of note writing, focusing on accuracy, brevity, and clarity, we will make note writing and reading more rewarding and improve patient care. ●
- O’Donnell HC, Kaushal R, Barron Y, et al. Physicians’ attitudes towards copy and pasting in the electronic note writing. J Gen Intern Med. 2009;24:63-68.
- Weed LL. Medical records, patient care and medical education. Ir J Med Sci. 1964;462:271-282.
- Sieja A, Pell J, Markley K, et al. Successful implementation of APSO notes across a major health system. Am J Account Care. 2017;5:29-34.
- Barbieri RL, Levy B. Major changes in Medicare billing are planned for January 2021: some specialists fare better that others. OBG Manag. 2020;32:9, 10, 12, 14.
- State of the note summit, 2021. Medical specialty dos and don’ts. https://www.acponline.org/system/files/documents/practice-resources/business-resources/coding/state-of-the-note-summit-2021/sotn21-specialtycare.pdf. Accessed September 21, 2021.
- Kayashtha N, Pollak KI, LeBLanc TW. Open oncology notes: a qualitative study of oncology patients’ experiences reading their cancer care notes. Am Soc Clin Oncol. 2018;14:e251-e257.
Prior to 1980, medical record notes were generally hand-written, short, and to the point. Senior physicians often wrote their 3-line notes using a fountain pen in an elegant cursive. With the transition to electronic medical records, notes have become bloated with irrelevant information and frequently lack a focus on the critical clinical insights that optimize patient care. The use of smart phrases to pull vast amounts of raw data into the note is a major contributor to note bloat. The unrestrained use of the copy and paste functionality generates a sequence of cloned notes that grow in length as new information is added and little information from prior notes removed. With each subsequent clone the note often becomes less accurate, lengthier, and more difficult for a reader to understand. In one survey of 253 physicians who wrote electronic notes, 90% reported that they used the copy and paste function, with 71% reporting that use of this function caused inconsistencies within and among notes and increased the repetitive presentation of outdated information in the note.1 Although the surveyed clinicians recognized that the copy and paste function caused problems, 80% reported that they planned to continue to use the copy and paste function.1
The SOAP note
The problem-oriented SOAP note is written in the classic structure of subjective and objective information, followed by an assessment and plan.2 The structure of the SOAP note emphasizes the logical and sequential collection of data followed by data analysis, resulting in a focused assessment and plan. When notes were hand-written and short, the entire SOAP note could be viewed on one page. Like a dashboard, the eye could quickly scan each key component of the note, facilitating the simultaneous integration of all 4 components of the note, facilitating understanding of the patient’s clinical situation. When the SOAP note structure is used to create a multipage electronic note, the result is a note that often confuses rather than enlightens the reader. A 5- to 10-page SOAP note is often useless for patient care but demonstrates the ability of computer-savvy clinicians to quickly generate a note thousands of words in length.
The APSO note, a response to note bloat
When a medical record note becomes a multipage document, clinicians should consider switching from the SOAP note structure to the APSO note, where the assessment and plan are at the top of the note, and the subjective and objective information is below the assessment and plan. The APSO format permits the reader to more quickly grasp the critical thinking of the author and facilitates a focus on key points relevant to the patient’s condition. The note can be written in the SOAP format, but then the assessment and plan are brought to the top of the note. In my clinical experience fewer than 10% of clinicians are using an APSO note structure. I believe that, with a multipage note, the APSO structure improves the experience of the reader and should be more widely utilized, especially by clinicians who are prone to crafting a bloated note. In a survey of more than 3,000 clinicians, approximately two-thirds of the respondents reported that, compared with SOAP notes, APSO notes were easier and faster to read, and APSO notes made it easier to follow the clinical reasoning of the author.3
Continue to: New evaluation and management billing guidelines—An opportunity to reduce note bloat...
New evaluation and management billing guidelines—An opportunity to reduce note bloat
Previous evaluation and management federal billing guidelines emphasized documentation of a myriad of clinically irrelevant details contributing to note bloat. The new federal evaluation and management billing guidelines pivot the focus of the note to the quality and complexity of medical decision making as demonstrated in the assessment and plan.4 Prioritizing the assessment and plan as the key feature of the medical record note should help reduce the length of notes. The American College of Physicians recently recommended deleting the complete review of systems and prior histories from most notes unless relevant to medical decision making and the assessment and plan.5
The open note
The open note mandate was contained in federal regulations developed to implement the 21st Century Cures Act, which required patients to have access to the information in their medical record. In order to comply with the regulation, health systems are sending most notes and test results to the patient through the health system’s patient gateway. The open note process entered my practice through a stealthy progression, from an initial step of permitting a clinician to easily share their note with a patient to a top-down edict that all notes, except some notes that have a high risk of causing patient harm, must be sent immediately to the patient. Obviously, an open note supports “transparency,” but I am unaware of high quality evidence that open notes improve the health of a population or reduce morbidity or mortality from health problems.
The federal mandate that clinicians share their notes or risk fiscal penalties is coercive and undermines the independence of health professionals. Open notes may have many benefits, including:
- improving a patient’s comprehension and sense of control over their health issues
- increasing patient trust in their health system
- increasing the number of questions patients ask their clinician.6
Open notes may also cause unintended adverse emotional trauma to patients, especially when the note communicates “bad news.” In one study of 100 oncology patients, approximately 25% of respondents reported that reading clinical notes was emotionally difficult, and they sometimes regretted having read the note.6 One patient reported, “I think MyChart is great but in this whole cancer thing MyChart has not been a good thing.” Another patient reported, “Reading serious stuff like that is just too taxing for me to be honest with you.”6 An additional finding of the study was that patients reported their notes were written with too much medical jargon and repetition of information.
Open laboratory, pathology, and imaging data—Helpful or harmful?
A component of the open note mandate is that laboratory, pathology, and imaging data must be shared timely with patients. Some health systems incorporate a 3-day pause prior to sharing such data, in order to provide the clinical team with time to communicate with the patient before the test results are shared. Some health systems, including my health system, have engineered the open note data-sharing system to immediately share the results of most completed laboratory, pathology, and imaging studies with the patient. Immediate sharing of data may result in the patient first learning that they have a serious, life-threatening health problem, such as cancer, from their patient portal rather than from a clinician. As an example, a patient may first learn that they have metastatic cancer from a CT scan that was ordered for a benign indication.
Another example is that a patient may first learn that they have an HIV infection from their patient portal. This can be a shocking and emotionally damaging experience for the patient. For many test results, it would be best if a clinician were able to communicate the result to the patient, providing support and context to the meaning of the result, rather than sending sensitive, life-altering information directly from the laboratory or imaging department to the patient. Leaders in medical education have spent decades teaching clinicians how to communicate “bad news” in a sensitive, supportive, and effective manner. The open sharing of laboratory, pathology, and imaging data short-circuits the superior process of relying on a highly capable clinician to communicate bad news.
Continue to: Crafting the open medical record note...
Crafting the open medical record note
Building on the advice that “when life gives you lemons, make lemonade,” I have begun to pivot the purpose of my medical notes from a product useful to myself and other clinicians to a product whose primary purpose is to be helpful for the patient. The open note can facilitate building a trusting relationship with the patient. My notes are becoming a series of written conversations with the patient, emphasizing compassion and empathy. I am increasing significantly the amount of educational information in the note to help the patient understand their situation. In addition, I am replacing traditional medical terms with verbiage more appropriate in the context of a conversation with the patient, reducing the use of medical jargon. For example, I have stopped using “chief complaint” and replaced it with “health issues.” I am diligently avoiding the use of medical terms that have negative connotations, including “obese,” “psychosomatic,” “alcoholic,” and “drug addiction.” I include encouragement and positive comments in many of my notes. For example, “Ms. X is successfully managing her health issues and experiencing improved health. It is a pleasure collaborating with her on achieving optimal health.”
Can we bring sanity back to medical note writing?
The primary role of a clinician is to spend as much time as possible listening to patients, understanding their needs, and helping them achieve optimal health. There are many benefits to an electronic medical record, including legibility, accessibility, interoperability, and efficiency. However, in current practice “note bloat” undermines the potential of the electronic medical record and makes many notes ineffective to the process of advancing the patient’s health. We are competent and highly trained clinicians. We can craft notes that are simple, specific, story-driven, compassionate, and empathetic. If we return to the ABCs of note writing, focusing on accuracy, brevity, and clarity, we will make note writing and reading more rewarding and improve patient care. ●
Prior to 1980, medical record notes were generally hand-written, short, and to the point. Senior physicians often wrote their 3-line notes using a fountain pen in an elegant cursive. With the transition to electronic medical records, notes have become bloated with irrelevant information and frequently lack a focus on the critical clinical insights that optimize patient care. The use of smart phrases to pull vast amounts of raw data into the note is a major contributor to note bloat. The unrestrained use of the copy and paste functionality generates a sequence of cloned notes that grow in length as new information is added and little information from prior notes removed. With each subsequent clone the note often becomes less accurate, lengthier, and more difficult for a reader to understand. In one survey of 253 physicians who wrote electronic notes, 90% reported that they used the copy and paste function, with 71% reporting that use of this function caused inconsistencies within and among notes and increased the repetitive presentation of outdated information in the note.1 Although the surveyed clinicians recognized that the copy and paste function caused problems, 80% reported that they planned to continue to use the copy and paste function.1
The SOAP note
The problem-oriented SOAP note is written in the classic structure of subjective and objective information, followed by an assessment and plan.2 The structure of the SOAP note emphasizes the logical and sequential collection of data followed by data analysis, resulting in a focused assessment and plan. When notes were hand-written and short, the entire SOAP note could be viewed on one page. Like a dashboard, the eye could quickly scan each key component of the note, facilitating the simultaneous integration of all 4 components of the note, facilitating understanding of the patient’s clinical situation. When the SOAP note structure is used to create a multipage electronic note, the result is a note that often confuses rather than enlightens the reader. A 5- to 10-page SOAP note is often useless for patient care but demonstrates the ability of computer-savvy clinicians to quickly generate a note thousands of words in length.
The APSO note, a response to note bloat
When a medical record note becomes a multipage document, clinicians should consider switching from the SOAP note structure to the APSO note, where the assessment and plan are at the top of the note, and the subjective and objective information is below the assessment and plan. The APSO format permits the reader to more quickly grasp the critical thinking of the author and facilitates a focus on key points relevant to the patient’s condition. The note can be written in the SOAP format, but then the assessment and plan are brought to the top of the note. In my clinical experience fewer than 10% of clinicians are using an APSO note structure. I believe that, with a multipage note, the APSO structure improves the experience of the reader and should be more widely utilized, especially by clinicians who are prone to crafting a bloated note. In a survey of more than 3,000 clinicians, approximately two-thirds of the respondents reported that, compared with SOAP notes, APSO notes were easier and faster to read, and APSO notes made it easier to follow the clinical reasoning of the author.3
Continue to: New evaluation and management billing guidelines—An opportunity to reduce note bloat...
New evaluation and management billing guidelines—An opportunity to reduce note bloat
Previous evaluation and management federal billing guidelines emphasized documentation of a myriad of clinically irrelevant details contributing to note bloat. The new federal evaluation and management billing guidelines pivot the focus of the note to the quality and complexity of medical decision making as demonstrated in the assessment and plan.4 Prioritizing the assessment and plan as the key feature of the medical record note should help reduce the length of notes. The American College of Physicians recently recommended deleting the complete review of systems and prior histories from most notes unless relevant to medical decision making and the assessment and plan.5
The open note
The open note mandate was contained in federal regulations developed to implement the 21st Century Cures Act, which required patients to have access to the information in their medical record. In order to comply with the regulation, health systems are sending most notes and test results to the patient through the health system’s patient gateway. The open note process entered my practice through a stealthy progression, from an initial step of permitting a clinician to easily share their note with a patient to a top-down edict that all notes, except some notes that have a high risk of causing patient harm, must be sent immediately to the patient. Obviously, an open note supports “transparency,” but I am unaware of high quality evidence that open notes improve the health of a population or reduce morbidity or mortality from health problems.
The federal mandate that clinicians share their notes or risk fiscal penalties is coercive and undermines the independence of health professionals. Open notes may have many benefits, including:
- improving a patient’s comprehension and sense of control over their health issues
- increasing patient trust in their health system
- increasing the number of questions patients ask their clinician.6
Open notes may also cause unintended adverse emotional trauma to patients, especially when the note communicates “bad news.” In one study of 100 oncology patients, approximately 25% of respondents reported that reading clinical notes was emotionally difficult, and they sometimes regretted having read the note.6 One patient reported, “I think MyChart is great but in this whole cancer thing MyChart has not been a good thing.” Another patient reported, “Reading serious stuff like that is just too taxing for me to be honest with you.”6 An additional finding of the study was that patients reported their notes were written with too much medical jargon and repetition of information.
Open laboratory, pathology, and imaging data—Helpful or harmful?
A component of the open note mandate is that laboratory, pathology, and imaging data must be shared timely with patients. Some health systems incorporate a 3-day pause prior to sharing such data, in order to provide the clinical team with time to communicate with the patient before the test results are shared. Some health systems, including my health system, have engineered the open note data-sharing system to immediately share the results of most completed laboratory, pathology, and imaging studies with the patient. Immediate sharing of data may result in the patient first learning that they have a serious, life-threatening health problem, such as cancer, from their patient portal rather than from a clinician. As an example, a patient may first learn that they have metastatic cancer from a CT scan that was ordered for a benign indication.
Another example is that a patient may first learn that they have an HIV infection from their patient portal. This can be a shocking and emotionally damaging experience for the patient. For many test results, it would be best if a clinician were able to communicate the result to the patient, providing support and context to the meaning of the result, rather than sending sensitive, life-altering information directly from the laboratory or imaging department to the patient. Leaders in medical education have spent decades teaching clinicians how to communicate “bad news” in a sensitive, supportive, and effective manner. The open sharing of laboratory, pathology, and imaging data short-circuits the superior process of relying on a highly capable clinician to communicate bad news.
Continue to: Crafting the open medical record note...
Crafting the open medical record note
Building on the advice that “when life gives you lemons, make lemonade,” I have begun to pivot the purpose of my medical notes from a product useful to myself and other clinicians to a product whose primary purpose is to be helpful for the patient. The open note can facilitate building a trusting relationship with the patient. My notes are becoming a series of written conversations with the patient, emphasizing compassion and empathy. I am increasing significantly the amount of educational information in the note to help the patient understand their situation. In addition, I am replacing traditional medical terms with verbiage more appropriate in the context of a conversation with the patient, reducing the use of medical jargon. For example, I have stopped using “chief complaint” and replaced it with “health issues.” I am diligently avoiding the use of medical terms that have negative connotations, including “obese,” “psychosomatic,” “alcoholic,” and “drug addiction.” I include encouragement and positive comments in many of my notes. For example, “Ms. X is successfully managing her health issues and experiencing improved health. It is a pleasure collaborating with her on achieving optimal health.”
Can we bring sanity back to medical note writing?
The primary role of a clinician is to spend as much time as possible listening to patients, understanding their needs, and helping them achieve optimal health. There are many benefits to an electronic medical record, including legibility, accessibility, interoperability, and efficiency. However, in current practice “note bloat” undermines the potential of the electronic medical record and makes many notes ineffective to the process of advancing the patient’s health. We are competent and highly trained clinicians. We can craft notes that are simple, specific, story-driven, compassionate, and empathetic. If we return to the ABCs of note writing, focusing on accuracy, brevity, and clarity, we will make note writing and reading more rewarding and improve patient care. ●
- O’Donnell HC, Kaushal R, Barron Y, et al. Physicians’ attitudes towards copy and pasting in the electronic note writing. J Gen Intern Med. 2009;24:63-68.
- Weed LL. Medical records, patient care and medical education. Ir J Med Sci. 1964;462:271-282.
- Sieja A, Pell J, Markley K, et al. Successful implementation of APSO notes across a major health system. Am J Account Care. 2017;5:29-34.
- Barbieri RL, Levy B. Major changes in Medicare billing are planned for January 2021: some specialists fare better that others. OBG Manag. 2020;32:9, 10, 12, 14.
- State of the note summit, 2021. Medical specialty dos and don’ts. https://www.acponline.org/system/files/documents/practice-resources/business-resources/coding/state-of-the-note-summit-2021/sotn21-specialtycare.pdf. Accessed September 21, 2021.
- Kayashtha N, Pollak KI, LeBLanc TW. Open oncology notes: a qualitative study of oncology patients’ experiences reading their cancer care notes. Am Soc Clin Oncol. 2018;14:e251-e257.
- O’Donnell HC, Kaushal R, Barron Y, et al. Physicians’ attitudes towards copy and pasting in the electronic note writing. J Gen Intern Med. 2009;24:63-68.
- Weed LL. Medical records, patient care and medical education. Ir J Med Sci. 1964;462:271-282.
- Sieja A, Pell J, Markley K, et al. Successful implementation of APSO notes across a major health system. Am J Account Care. 2017;5:29-34.
- Barbieri RL, Levy B. Major changes in Medicare billing are planned for January 2021: some specialists fare better that others. OBG Manag. 2020;32:9, 10, 12, 14.
- State of the note summit, 2021. Medical specialty dos and don’ts. https://www.acponline.org/system/files/documents/practice-resources/business-resources/coding/state-of-the-note-summit-2021/sotn21-specialtycare.pdf. Accessed September 21, 2021.
- Kayashtha N, Pollak KI, LeBLanc TW. Open oncology notes: a qualitative study of oncology patients’ experiences reading their cancer care notes. Am Soc Clin Oncol. 2018;14:e251-e257.
Gender equity and gynecologic surgery: Ensuring a culture of diversity and inclusion
A workplace environment conducive to success includes equal access to resources and opportunities, work-life integration, freedom from gender discrimination and sexual harassment, and supportive leadership. With focused leadership that is accountable for actionable interventions through measurable outcomes, it is possible to create an equitable, safe, and dignified workplace for all ObGyns.
Recently, obstetrics and gynecology has become the only surgical specialty in which a majority of practitioners are women. Since the 1990s, women in ObGyn have composed the majority of trainees, and 2012 marked the first year that more than half of the American College of Obstetricians and Gynecologists (ACOG) Fellows in practice were women.1
Despite the large proportion of women within the specialty, ongoing gender-based inequities continue. Many of these inequities are rooted in our pervasive societal views of behavioral norms based on biologic or perceived sex, otherwise known as “gender,” roles.2 The cultural gender role for men embodies characteristics that are bold, competitive, decisive, analytical; qualities for women include modesty, nurturing, and accommodating in interactions with others. Such male-typed traits and behaviors are termed “agentic” because they involve human agency, whereas female-typed traits and behaviors are termed “communal.”3
Gender biases remain widespread, even among health care providers.4 When gender roles are applied to medical specialties, there is an assumption that women tend toward “communal” specialties, such as pediatrics or family practice, whereas men are better suited for technical or procedural specialties.5 ObGyn is an outlier in this schema because its procedural and surgical aspects would characterize the specialty as “agentic,” yet the majority of ObGyn trainees and physicians are women.
Biases related to gender impact many aspects of practice for the ObGyn, including:
- surgical education and training
- the gender wage gap
- interpersonal interactions and sexual harassment
- advancement and promotion.
Surgical education and training
The message that desirable characteristics for leadership and autonomy are aligned with masculinity is enforced early in medical culture, and it supports the ubiquity of deep-seated stereotypes about gender roles in medicine. For example, the language used for letters of recommendation for women applying to residency and fellowship highlight communal language (nurturing, warm), whereas those for men more typically use agentic terms (decisive, strong, future leader).6 During ObGyn surgical training, women residents receive more negative evaluations than men from nurses throughout training, and they report spending more effort to nurture these relationships, including changing communication in order to engage assistance from nurses.7
Similarly, women trainees receive harsher and more contradictory feedback from attending physicians.8 For example, a woman resident may be criticized for failing to develop independence and execute complete plans for patient care; later, she might be labeled as “rogue” and told that she should engage with and seek input from supervising faculty when independently executing a treatment plan.
Even when attempting to apply feedback in the operating room, women trainees are afforded less surgical autonomy than men trainees.9 These factors contribute to lower surgical confidence in women trainees despite their having the same technical skills as men, as measured by the Fundamentals of Laparoscopic Surgery skills exam.10
Continue to: The gender wage gap...
The gender wage gap
The mean salary for women ObGyns remains lower than that for men at every academic rank, with the differences ranging from $54,700 at the assistant professor rank to $183,200 for the department chair position.11 Notably, the pay discrepancy persists after adjustments are made for common salary-influencing metrics, such as experience, practice construct, and academic productivity.12 The gender salary gap is further identified for women subspecialists, as women reproductive endocrinology and infertility specialists and gynecologic oncologists earn $67,000 and $120,000 less, respectively, than men colleagues.13,14
While the gender wage gap often is attributed to women’s desire to work part time, similar rates of graduating women and men medical students in 2018 ranked schedule flexibility as important, suggesting that work-life balance is related to an individual’s generation rather than gender.11
Parenting status specifically adversely affects women physicians, with an ascribed “motherhood penalty” and “fatherhood bonus” phenomenon: women physicians who became parents lost an additional 6% salary, whereas men physicians saw a salary increase of 4% with parenthood.15
Most worrisome for the specialty is evidence of declining wages for ObGyns relative to other fields. “Occupational segregation”16 refers to the pronounced negative effect on earnings as more women enter a given field, which has been described in other professions.17 Overall, ObGyn salaries are the lowest among surgical specialties18 and show evidence of decline corresponding to the increasing numbers of women in the field.16
Interpersonal interactions and sexual harassment
In the workplace, women in ObGyn face more interpersonal relationship friction than men. Practicing women ObGyns report differing treatment by nurses as compared to men,19 noting that additional time and effort are required to nurture professional relationships. Additionally, nurses and trainees20 evaluate practicing women ObGyns more harshly than they evaluate men. Further, women gynecologic surgeons experience gender bias from patients, as patients endorse a preference to have a woman gynecologist but prefer a man gynecologic surgeon.21
In addition to gender bias, the experience of gender harassment, including sexual harassment, is common, as two-thirds of women gynecologists report workplace harassment, 90% of which is attributed to gender.22 This rate is 3 times higher than that for men, with a senior colleague in a position of power within the same organization reported to be the harasser to women in 91% of occurrences.
Advancement and promotion
Within academia, women faculty face specific career-limiting barriers related to gender. Rates of academic promotion and leadership opportunities remain lower for women than for men faculty. Although there has been more women representation in ObGyn over the past 20 years, the number of women serving as department chairs, cancer center directors, editors-in-chief, or on a board of directors remains lower than what would be expected by representation ratios.23 (Representation ratios were calculated as the proportion of ObGyn department-based leadership roles held by women in 2019 divided by the proportion of women ObGyn residents in 1990; representation ratios <1.0 indicate underrepresentation of women). This lag in attainment of leadership roles is compounded by the difficulties women faculty experience in finding mentorship and sponsorship,24 which are known benefits to career advancement.
Having fewer women hold leadership roles also negatively influences those in training. For example, a survey of emergency medicine and ObGyn residents identified an implicit gender bias that men and women residents favored men for leadership roles.25 This difference, however, was not significant when division chiefs and department chairs were women, which suggests that visibility of women leaders positively influences the stereotype perception of men and women trainees.4
Continue to: Blueprint for change...
Blueprint for change
While the issues surrounding gender bias are widespread, solutions exist to create gender equity within ObGyn. Efforts to change individual behavior and organizational culture should start with an understanding of the current environment.
Multiple studies have promoted the concept of “culture change,”26,27 which parallels a standard change process. A critical aspect of change is that individuals and organizations maintain the status quo until something prompts a desire to achieve a different way of being. As data regarding the breadth and impact of gender bias emerge and awareness is raised, there is recognition that the status quo is not achieving the goals of the department or institution. This may occur through the result of loss of physician talent, reduced access for vulnerable patient populations, or lower financial productivity.
Once change is considered, it must deliberately be pursued through a specific process. The first actionable step is to assess the existing state and then identify prior barriers to and current opportunities for success. A validated instrument that has been applied for this purpose is the Diversity Engagement Survey, a 22-item questionnaire that assesses 8 domains of organizational inclusion on a 5-point Likert scale (see TABLE).28 This tool not only provides a measure of institutional culture but also obtains characteristics of the respondents so that it additionally assesses how engaged specific groups are within the organization. Once baseline data are obtained, an action plan can be formulated and enacted. This cycle of assessment, system influences, plan, and act should be continued until the desired changes are achieved.
It is critically important to identify objective, measurable outcomes to assure that the interventions are moving the culture toward enhanced gender equity. As the ideal state is achieved, development of practices and enforceable policies help to ensure the longevity of cultural changes. Furthermore, periodic re-evaluation of the existing organizational culture will confirm the maintenance of gender equity objectives.
Solutions toward gender equity
Gender inequity may arise from societal gender roles, but it is incumbent on health care organizations to create an environment free from gender bias and gender harassment. An imperative first step is to identify the occurrence of gender discrimination.
The HITS (Hurt, Insulted, Threatened with harm, or Screamed at) screening tool has been used effectively with surgical residents to identify the prevalence of and most common types of abuse.29 This instrument could be adapted and administered to ObGyns in practice or in training. These data should inform the need for system-level antisexist training as well as enforcement of zero-tolerance policies.
Organizations have the ability to create a salary-only compensation model for physicians within the same specialty regardless of academic rank or academic productivity, which has been demonstrated to eliminate gender pay disparity.30 Additional measures to achieve gender equity involve antisexist hiring processes31 and transparency in metrics for job performance, salary, and promotion.32
While health care organizations are obliged to construct a gender-equitable culture, efforts can be made on the individual level. Implicit bias is ascribed to the unconscious attitudes and stereotypes people conclude about groups. The Implicit Association Test (IAT) is a validated instrument that provides the respondent with information about one’s own implicit biases. By uncovering gender bias “blind spots,” an individual can work to consciously overcome these stereotypes. Extending from the mental reframing required for overturning implicit biases, individuals can learn to identify and intervene in real-world situations. This concept of “being an upstander” denotes stepping in and standing up when an inappropriate situation arises33 (see “Case example: Being an upstander”). The targeted individual may not have the ability or safety to navigate through a confrontation, but an upstander might be able to assist the target with empowerment, verbalization of needs, and support.
Lastly, mentorship and sponsorship are critical factors for professional development and career advancement. Bidirectional mentorship identifies benefit for the mentee and the mentor whereby the junior faculty obtain career development and support and the senior faculty may learn new teaching or communication skills.34

A final word
As recognized advocates for women’s health, we must intentionally move toward a workplace that is equitable, safe, and dignified for all ObGyns. Ensuring gender equity within obstetrics and gynecology is everyone’s responsibility. ●
Dr. Bethany Wain is attending a departmental conference and is talking with another member of her division when Dr. Joselle, her division director, approaches. He is accompanied by the Visiting Professor, an internationally reputable and dynamic man, a content expert in the field of work in which Dr. Wain is interested and has published. Dr. Joselle introduces the Visiting Professor formally, using his title of “doctor.” He then introduces Dr. Wain by her abridged first name, Beth.
As an upstander, the Visiting Professor quickly addresses Dr. Wain by her title and uses the situation as a platform to highlight the need to maintain professional address in the professional environment. He then adds that women, who are usually junior in academic rank, confer more benefit to being addressed formally and receiving visibility and respect for their work in a public forum. In this way, the Visiting Professor amplifies Dr. Wain’s work and status and demonstrates the standard of using professional address for women and men.
- Rayburn WF. The Obstetrician-Gynecologist Workforce in the United States: Facts, Figures, and Implications, 2017. Washington, DC: American Congress of Obstetricians and Gynecologists; 2017.
- Carnes M. Commentary: deconstructing gender differences. Acad Med. 2010;85:575-577.
- Eagly AH. The his and hers of prosocial behavior: an examination of the social psychology of gender. Am Psychol. 2009;64:644-658.
- Salles A, Awad M, Goldin L, et al. Estimating implicit and explicit gender bias among health care professionals and surgeons. JAMA Netw Open. 2019;2:e196545.
- Carnes M, Bartels CM, Kaatz A, et al. Why is John more likely to become department chair than Jennifer? Trans Am Clin Climatol Assoc. 2015;126:197-214.
- Hoffman A, Grant, W, McCormick, et al. Gendered differences in letters of recommendation for transplant surgery fellowship applicants. J Surg Edu. 2019;76:427-432.
- Galvin SL, Parlier AB, Martino E, et al. Gender bias in nurse evaluations of residents in obstetrics and gynecology. Obstet Gynecol. 2015;126(suppl 4):7S-12S.
- Gerull KM, Loe M, Seiler K, et al. Assessing gender bias in qualitative evaluations of surgical residents. Am J Surg. 2019;217:306-313.
- Meyerson SL, Sternbach JM, Zwischenberger JB, et al. The effect of gender on resident autonomy in the operating room. J Surg Educ. 2017;74:e111-e118.
- Flyckt RL, White EE, Goodman LR, et al. The use of laparoscopy simulation to explore gender differences in resident surgical confidence. Obstet Gynecol Int. 2017;2017:1945801.
- Heisler CA, Mark K, Ton J, et al. Has a critical mass of women resulted in gender equity in gynecologic surgery? Am J Obstet Gynecol. 2020;223:665-673.
- Warner AS, Lehmann LS. Gender wage disparities in medicine: time to close the gap. J Gen Intern Med. 2019;34:1334-1336.
- Gilbert SB, Allshouse A, Skaznik-Wikiel ME. Gender inequality in salaries among reproductive endocrinology and infertility specialists in the United States. Fertil Steril. 2019;111:1194-1200.
- Croft KM, Rauh LA, Orr JW, et al. Compensation differences by gender in gynecologic oncology. Society of Gynecologic Oncology Annual Meeting on Women’s Cancer. 2020. https://sgo.confex.com /sgo/2020/meetingapp.cgi/Paper/15762. 2020. Accessed April 1, 2020.
- Wang SS, Ackerman S. The motherhood penalty: is it alive and well in 2020? J Am Coll Radiol. 2020;17:688-689.
- Pelley E, Carnes M. When a specialty becomes “women’s work”: trends in and implications of specialty gender segregation in medicine. Acad Med. 2020;95:1499-1506.
- Hegewisch A, Hartmann H. Occupational segregation and the gender wage gap: a job half done. Institute for Women’s Policy Research. 2014. https://iwpr.org/iwpr-issues/employment-and-earnings /occupational-segregation-and-the-gender-wage-gap-a-job-half -done/. Accessed August 26, 2021.
- Greenberg CC. Association for Academic Surgery presidential address: sticky floors and glass ceilings. J Surg Res. 2017;219:ix-xviii.
- Dossett LA, Vitous CA, Lindquist K, et al. Women surgeons’ experiences of interprofessional workplace conflict. JAMA Netw Open. 2020;3:e2019843.
- Morgan HK, Purkis JA, Porter AC, et al. Student evaluation of faculty physicians: gender differences in teaching evaluations. J Womens Health (Larchmt). 2016;25:453-456.
- Childs AJ, Friedman WH, Schwartz MP, et al. Female patients’ sex preferences in selection of gynecologists and surgeons. South Med J. 2005;98:405-408.
- Brown J, Drury L, Raub K, et al. Workplace harassment and discrimination in gynecology: results of the AAGL Member Survey. J Minim Invasive Gynecol. 2019;26:838-846.
- Temkin AM, Rubinsak L, Benoit MF, et al. Take me to your leader: reporting structures and equity in academic gynecologic oncology. Gynecol Oncol. 2020;157:759-764.
- Shakil S, Redberg RF. Gender disparities in sponsorship—how they perpetuate the glass ceiling. JAMA Intern Med. 2017;177:582.
- Hansen M, Schoonover A, Skarica B, et al. Implicit gender bias among US resident physicians. BMC Med Ed. 2019;19:396.
- Estrada M, Burnett M, Campbell AG, et al. Improving underrepresented minority student persistence in STEM. CBE Life Sci Educ. 2016;15:es5.
- Carnes M, Handelsman J, Sheridan J. Diversity in academic medicine: the stages of change model. J Womens Health (Larchmt). 2005;14:471-475.
- Person SD, Jordan CG, Allison JJ, et al. Measuring diversity and inclusion in academic medicine. The Diversity Engagement Survey. Acad Med. 2015;90:1675-1683.
- Fitzgerald CA, Smith RN, Luo-Owen X, et al. Screening for harassment, abuse, and discrimination among surgery residents: an EAST multicenter trial. Am Surg. 2019;85:456-461.
- Hayes SN, Noseworthy JH, Farrugia G. A structured compensation plan results in equitable physician compensation: a single-center analysis. Mayo Clin Proc. 2020;95:35-43.
- Devine PG, Forscher PS, Cox WT, et al. A gender bias habit-breaking intervention led to increased hiring of female faculty in STEMM departments. J Exp Soc Psychol. 2017;73:211-215.
- Morgan AU, Chaiyachati KH, Weissman GE, et al. Eliminating genderbased bias in academic medicine: more than naming the “elephant in the room.” J Gen Intern Med. 2018;33:966-968.
- Mello MM, Jagsi R. Standing up against gender bias and harassment— a matter of professional ethics. N Engl J Med. 2020;382:1385-1387.
- Burgess A, van Diggele C, Mellis C. Mentorship in the health profession: a review. Clin Teach. 2018;15:197-202.
A workplace environment conducive to success includes equal access to resources and opportunities, work-life integration, freedom from gender discrimination and sexual harassment, and supportive leadership. With focused leadership that is accountable for actionable interventions through measurable outcomes, it is possible to create an equitable, safe, and dignified workplace for all ObGyns.
Recently, obstetrics and gynecology has become the only surgical specialty in which a majority of practitioners are women. Since the 1990s, women in ObGyn have composed the majority of trainees, and 2012 marked the first year that more than half of the American College of Obstetricians and Gynecologists (ACOG) Fellows in practice were women.1
Despite the large proportion of women within the specialty, ongoing gender-based inequities continue. Many of these inequities are rooted in our pervasive societal views of behavioral norms based on biologic or perceived sex, otherwise known as “gender,” roles.2 The cultural gender role for men embodies characteristics that are bold, competitive, decisive, analytical; qualities for women include modesty, nurturing, and accommodating in interactions with others. Such male-typed traits and behaviors are termed “agentic” because they involve human agency, whereas female-typed traits and behaviors are termed “communal.”3
Gender biases remain widespread, even among health care providers.4 When gender roles are applied to medical specialties, there is an assumption that women tend toward “communal” specialties, such as pediatrics or family practice, whereas men are better suited for technical or procedural specialties.5 ObGyn is an outlier in this schema because its procedural and surgical aspects would characterize the specialty as “agentic,” yet the majority of ObGyn trainees and physicians are women.
Biases related to gender impact many aspects of practice for the ObGyn, including:
- surgical education and training
- the gender wage gap
- interpersonal interactions and sexual harassment
- advancement and promotion.
Surgical education and training
The message that desirable characteristics for leadership and autonomy are aligned with masculinity is enforced early in medical culture, and it supports the ubiquity of deep-seated stereotypes about gender roles in medicine. For example, the language used for letters of recommendation for women applying to residency and fellowship highlight communal language (nurturing, warm), whereas those for men more typically use agentic terms (decisive, strong, future leader).6 During ObGyn surgical training, women residents receive more negative evaluations than men from nurses throughout training, and they report spending more effort to nurture these relationships, including changing communication in order to engage assistance from nurses.7
Similarly, women trainees receive harsher and more contradictory feedback from attending physicians.8 For example, a woman resident may be criticized for failing to develop independence and execute complete plans for patient care; later, she might be labeled as “rogue” and told that she should engage with and seek input from supervising faculty when independently executing a treatment plan.
Even when attempting to apply feedback in the operating room, women trainees are afforded less surgical autonomy than men trainees.9 These factors contribute to lower surgical confidence in women trainees despite their having the same technical skills as men, as measured by the Fundamentals of Laparoscopic Surgery skills exam.10
Continue to: The gender wage gap...
The gender wage gap
The mean salary for women ObGyns remains lower than that for men at every academic rank, with the differences ranging from $54,700 at the assistant professor rank to $183,200 for the department chair position.11 Notably, the pay discrepancy persists after adjustments are made for common salary-influencing metrics, such as experience, practice construct, and academic productivity.12 The gender salary gap is further identified for women subspecialists, as women reproductive endocrinology and infertility specialists and gynecologic oncologists earn $67,000 and $120,000 less, respectively, than men colleagues.13,14
While the gender wage gap often is attributed to women’s desire to work part time, similar rates of graduating women and men medical students in 2018 ranked schedule flexibility as important, suggesting that work-life balance is related to an individual’s generation rather than gender.11
Parenting status specifically adversely affects women physicians, with an ascribed “motherhood penalty” and “fatherhood bonus” phenomenon: women physicians who became parents lost an additional 6% salary, whereas men physicians saw a salary increase of 4% with parenthood.15
Most worrisome for the specialty is evidence of declining wages for ObGyns relative to other fields. “Occupational segregation”16 refers to the pronounced negative effect on earnings as more women enter a given field, which has been described in other professions.17 Overall, ObGyn salaries are the lowest among surgical specialties18 and show evidence of decline corresponding to the increasing numbers of women in the field.16
Interpersonal interactions and sexual harassment
In the workplace, women in ObGyn face more interpersonal relationship friction than men. Practicing women ObGyns report differing treatment by nurses as compared to men,19 noting that additional time and effort are required to nurture professional relationships. Additionally, nurses and trainees20 evaluate practicing women ObGyns more harshly than they evaluate men. Further, women gynecologic surgeons experience gender bias from patients, as patients endorse a preference to have a woman gynecologist but prefer a man gynecologic surgeon.21
In addition to gender bias, the experience of gender harassment, including sexual harassment, is common, as two-thirds of women gynecologists report workplace harassment, 90% of which is attributed to gender.22 This rate is 3 times higher than that for men, with a senior colleague in a position of power within the same organization reported to be the harasser to women in 91% of occurrences.
Advancement and promotion
Within academia, women faculty face specific career-limiting barriers related to gender. Rates of academic promotion and leadership opportunities remain lower for women than for men faculty. Although there has been more women representation in ObGyn over the past 20 years, the number of women serving as department chairs, cancer center directors, editors-in-chief, or on a board of directors remains lower than what would be expected by representation ratios.23 (Representation ratios were calculated as the proportion of ObGyn department-based leadership roles held by women in 2019 divided by the proportion of women ObGyn residents in 1990; representation ratios <1.0 indicate underrepresentation of women). This lag in attainment of leadership roles is compounded by the difficulties women faculty experience in finding mentorship and sponsorship,24 which are known benefits to career advancement.
Having fewer women hold leadership roles also negatively influences those in training. For example, a survey of emergency medicine and ObGyn residents identified an implicit gender bias that men and women residents favored men for leadership roles.25 This difference, however, was not significant when division chiefs and department chairs were women, which suggests that visibility of women leaders positively influences the stereotype perception of men and women trainees.4
Continue to: Blueprint for change...
Blueprint for change
While the issues surrounding gender bias are widespread, solutions exist to create gender equity within ObGyn. Efforts to change individual behavior and organizational culture should start with an understanding of the current environment.
Multiple studies have promoted the concept of “culture change,”26,27 which parallels a standard change process. A critical aspect of change is that individuals and organizations maintain the status quo until something prompts a desire to achieve a different way of being. As data regarding the breadth and impact of gender bias emerge and awareness is raised, there is recognition that the status quo is not achieving the goals of the department or institution. This may occur through the result of loss of physician talent, reduced access for vulnerable patient populations, or lower financial productivity.
Once change is considered, it must deliberately be pursued through a specific process. The first actionable step is to assess the existing state and then identify prior barriers to and current opportunities for success. A validated instrument that has been applied for this purpose is the Diversity Engagement Survey, a 22-item questionnaire that assesses 8 domains of organizational inclusion on a 5-point Likert scale (see TABLE).28 This tool not only provides a measure of institutional culture but also obtains characteristics of the respondents so that it additionally assesses how engaged specific groups are within the organization. Once baseline data are obtained, an action plan can be formulated and enacted. This cycle of assessment, system influences, plan, and act should be continued until the desired changes are achieved.
It is critically important to identify objective, measurable outcomes to assure that the interventions are moving the culture toward enhanced gender equity. As the ideal state is achieved, development of practices and enforceable policies help to ensure the longevity of cultural changes. Furthermore, periodic re-evaluation of the existing organizational culture will confirm the maintenance of gender equity objectives.

Solutions toward gender equity
Gender inequity may arise from societal gender roles, but it is incumbent on health care organizations to create an environment free from gender bias and gender harassment. An imperative first step is to identify the occurrence of gender discrimination.
The HITS (Hurt, Insulted, Threatened with harm, or Screamed at) screening tool has been used effectively with surgical residents to identify the prevalence of and most common types of abuse.29 This instrument could be adapted and administered to ObGyns in practice or in training. These data should inform the need for system-level antisexist training as well as enforcement of zero-tolerance policies.
Organizations have the ability to create a salary-only compensation model for physicians within the same specialty regardless of academic rank or academic productivity, which has been demonstrated to eliminate gender pay disparity.30 Additional measures to achieve gender equity involve antisexist hiring processes31 and transparency in metrics for job performance, salary, and promotion.32
While health care organizations are obliged to construct a gender-equitable culture, efforts can be made on the individual level. Implicit bias is ascribed to the unconscious attitudes and stereotypes people conclude about groups. The Implicit Association Test (IAT) is a validated instrument that provides the respondent with information about one’s own implicit biases. By uncovering gender bias “blind spots,” an individual can work to consciously overcome these stereotypes. Extending from the mental reframing required for overturning implicit biases, individuals can learn to identify and intervene in real-world situations. This concept of “being an upstander” denotes stepping in and standing up when an inappropriate situation arises33 (see “Case example: Being an upstander”). The targeted individual may not have the ability or safety to navigate through a confrontation, but an upstander might be able to assist the target with empowerment, verbalization of needs, and support.
Lastly, mentorship and sponsorship are critical factors for professional development and career advancement. Bidirectional mentorship identifies benefit for the mentee and the mentor whereby the junior faculty obtain career development and support and the senior faculty may learn new teaching or communication skills.34

A final word
As recognized advocates for women’s health, we must intentionally move toward a workplace that is equitable, safe, and dignified for all ObGyns. Ensuring gender equity within obstetrics and gynecology is everyone’s responsibility. ●
Dr. Bethany Wain is attending a departmental conference and is talking with another member of her division when Dr. Joselle, her division director, approaches. He is accompanied by the Visiting Professor, an internationally reputable and dynamic man, a content expert in the field of work in which Dr. Wain is interested and has published. Dr. Joselle introduces the Visiting Professor formally, using his title of “doctor.” He then introduces Dr. Wain by her abridged first name, Beth.
As an upstander, the Visiting Professor quickly addresses Dr. Wain by her title and uses the situation as a platform to highlight the need to maintain professional address in the professional environment. He then adds that women, who are usually junior in academic rank, confer more benefit to being addressed formally and receiving visibility and respect for their work in a public forum. In this way, the Visiting Professor amplifies Dr. Wain’s work and status and demonstrates the standard of using professional address for women and men.
A workplace environment conducive to success includes equal access to resources and opportunities, work-life integration, freedom from gender discrimination and sexual harassment, and supportive leadership. With focused leadership that is accountable for actionable interventions through measurable outcomes, it is possible to create an equitable, safe, and dignified workplace for all ObGyns.
Recently, obstetrics and gynecology has become the only surgical specialty in which a majority of practitioners are women. Since the 1990s, women in ObGyn have composed the majority of trainees, and 2012 marked the first year that more than half of the American College of Obstetricians and Gynecologists (ACOG) Fellows in practice were women.1
Despite the large proportion of women within the specialty, ongoing gender-based inequities continue. Many of these inequities are rooted in our pervasive societal views of behavioral norms based on biologic or perceived sex, otherwise known as “gender,” roles.2 The cultural gender role for men embodies characteristics that are bold, competitive, decisive, analytical; qualities for women include modesty, nurturing, and accommodating in interactions with others. Such male-typed traits and behaviors are termed “agentic” because they involve human agency, whereas female-typed traits and behaviors are termed “communal.”3
Gender biases remain widespread, even among health care providers.4 When gender roles are applied to medical specialties, there is an assumption that women tend toward “communal” specialties, such as pediatrics or family practice, whereas men are better suited for technical or procedural specialties.5 ObGyn is an outlier in this schema because its procedural and surgical aspects would characterize the specialty as “agentic,” yet the majority of ObGyn trainees and physicians are women.
Biases related to gender impact many aspects of practice for the ObGyn, including:
- surgical education and training
- the gender wage gap
- interpersonal interactions and sexual harassment
- advancement and promotion.
Surgical education and training
The message that desirable characteristics for leadership and autonomy are aligned with masculinity is enforced early in medical culture, and it supports the ubiquity of deep-seated stereotypes about gender roles in medicine. For example, the language used for letters of recommendation for women applying to residency and fellowship highlight communal language (nurturing, warm), whereas those for men more typically use agentic terms (decisive, strong, future leader).6 During ObGyn surgical training, women residents receive more negative evaluations than men from nurses throughout training, and they report spending more effort to nurture these relationships, including changing communication in order to engage assistance from nurses.7
Similarly, women trainees receive harsher and more contradictory feedback from attending physicians.8 For example, a woman resident may be criticized for failing to develop independence and execute complete plans for patient care; later, she might be labeled as “rogue” and told that she should engage with and seek input from supervising faculty when independently executing a treatment plan.
Even when attempting to apply feedback in the operating room, women trainees are afforded less surgical autonomy than men trainees.9 These factors contribute to lower surgical confidence in women trainees despite their having the same technical skills as men, as measured by the Fundamentals of Laparoscopic Surgery skills exam.10
Continue to: The gender wage gap...
The gender wage gap
The mean salary for women ObGyns remains lower than that for men at every academic rank, with the differences ranging from $54,700 at the assistant professor rank to $183,200 for the department chair position.11 Notably, the pay discrepancy persists after adjustments are made for common salary-influencing metrics, such as experience, practice construct, and academic productivity.12 The gender salary gap is further identified for women subspecialists, as women reproductive endocrinology and infertility specialists and gynecologic oncologists earn $67,000 and $120,000 less, respectively, than men colleagues.13,14
While the gender wage gap often is attributed to women’s desire to work part time, similar rates of graduating women and men medical students in 2018 ranked schedule flexibility as important, suggesting that work-life balance is related to an individual’s generation rather than gender.11
Parenting status specifically adversely affects women physicians, with an ascribed “motherhood penalty” and “fatherhood bonus” phenomenon: women physicians who became parents lost an additional 6% salary, whereas men physicians saw a salary increase of 4% with parenthood.15
Most worrisome for the specialty is evidence of declining wages for ObGyns relative to other fields. “Occupational segregation”16 refers to the pronounced negative effect on earnings as more women enter a given field, which has been described in other professions.17 Overall, ObGyn salaries are the lowest among surgical specialties18 and show evidence of decline corresponding to the increasing numbers of women in the field.16
Interpersonal interactions and sexual harassment
In the workplace, women in ObGyn face more interpersonal relationship friction than men. Practicing women ObGyns report differing treatment by nurses as compared to men,19 noting that additional time and effort are required to nurture professional relationships. Additionally, nurses and trainees20 evaluate practicing women ObGyns more harshly than they evaluate men. Further, women gynecologic surgeons experience gender bias from patients, as patients endorse a preference to have a woman gynecologist but prefer a man gynecologic surgeon.21
In addition to gender bias, the experience of gender harassment, including sexual harassment, is common, as two-thirds of women gynecologists report workplace harassment, 90% of which is attributed to gender.22 This rate is 3 times higher than that for men, with a senior colleague in a position of power within the same organization reported to be the harasser to women in 91% of occurrences.
Advancement and promotion
Within academia, women faculty face specific career-limiting barriers related to gender. Rates of academic promotion and leadership opportunities remain lower for women than for men faculty. Although there has been more women representation in ObGyn over the past 20 years, the number of women serving as department chairs, cancer center directors, editors-in-chief, or on a board of directors remains lower than what would be expected by representation ratios.23 (Representation ratios were calculated as the proportion of ObGyn department-based leadership roles held by women in 2019 divided by the proportion of women ObGyn residents in 1990; representation ratios <1.0 indicate underrepresentation of women). This lag in attainment of leadership roles is compounded by the difficulties women faculty experience in finding mentorship and sponsorship,24 which are known benefits to career advancement.
Having fewer women hold leadership roles also negatively influences those in training. For example, a survey of emergency medicine and ObGyn residents identified an implicit gender bias that men and women residents favored men for leadership roles.25 This difference, however, was not significant when division chiefs and department chairs were women, which suggests that visibility of women leaders positively influences the stereotype perception of men and women trainees.4
Continue to: Blueprint for change...
Blueprint for change
While the issues surrounding gender bias are widespread, solutions exist to create gender equity within ObGyn. Efforts to change individual behavior and organizational culture should start with an understanding of the current environment.
Multiple studies have promoted the concept of “culture change,”26,27 which parallels a standard change process. A critical aspect of change is that individuals and organizations maintain the status quo until something prompts a desire to achieve a different way of being. As data regarding the breadth and impact of gender bias emerge and awareness is raised, there is recognition that the status quo is not achieving the goals of the department or institution. This may occur through the result of loss of physician talent, reduced access for vulnerable patient populations, or lower financial productivity.
Once change is considered, it must deliberately be pursued through a specific process. The first actionable step is to assess the existing state and then identify prior barriers to and current opportunities for success. A validated instrument that has been applied for this purpose is the Diversity Engagement Survey, a 22-item questionnaire that assesses 8 domains of organizational inclusion on a 5-point Likert scale (see TABLE).28 This tool not only provides a measure of institutional culture but also obtains characteristics of the respondents so that it additionally assesses how engaged specific groups are within the organization. Once baseline data are obtained, an action plan can be formulated and enacted. This cycle of assessment, system influences, plan, and act should be continued until the desired changes are achieved.
It is critically important to identify objective, measurable outcomes to assure that the interventions are moving the culture toward enhanced gender equity. As the ideal state is achieved, development of practices and enforceable policies help to ensure the longevity of cultural changes. Furthermore, periodic re-evaluation of the existing organizational culture will confirm the maintenance of gender equity objectives.

Solutions toward gender equity
Gender inequity may arise from societal gender roles, but it is incumbent on health care organizations to create an environment free from gender bias and gender harassment. An imperative first step is to identify the occurrence of gender discrimination.
The HITS (Hurt, Insulted, Threatened with harm, or Screamed at) screening tool has been used effectively with surgical residents to identify the prevalence of and most common types of abuse.29 This instrument could be adapted and administered to ObGyns in practice or in training. These data should inform the need for system-level antisexist training as well as enforcement of zero-tolerance policies.
Organizations have the ability to create a salary-only compensation model for physicians within the same specialty regardless of academic rank or academic productivity, which has been demonstrated to eliminate gender pay disparity.30 Additional measures to achieve gender equity involve antisexist hiring processes31 and transparency in metrics for job performance, salary, and promotion.32
While health care organizations are obliged to construct a gender-equitable culture, efforts can be made on the individual level. Implicit bias is ascribed to the unconscious attitudes and stereotypes people conclude about groups. The Implicit Association Test (IAT) is a validated instrument that provides the respondent with information about one’s own implicit biases. By uncovering gender bias “blind spots,” an individual can work to consciously overcome these stereotypes. Extending from the mental reframing required for overturning implicit biases, individuals can learn to identify and intervene in real-world situations. This concept of “being an upstander” denotes stepping in and standing up when an inappropriate situation arises33 (see “Case example: Being an upstander”). The targeted individual may not have the ability or safety to navigate through a confrontation, but an upstander might be able to assist the target with empowerment, verbalization of needs, and support.
Lastly, mentorship and sponsorship are critical factors for professional development and career advancement. Bidirectional mentorship identifies benefit for the mentee and the mentor whereby the junior faculty obtain career development and support and the senior faculty may learn new teaching or communication skills.34

A final word
As recognized advocates for women’s health, we must intentionally move toward a workplace that is equitable, safe, and dignified for all ObGyns. Ensuring gender equity within obstetrics and gynecology is everyone’s responsibility. ●
Dr. Bethany Wain is attending a departmental conference and is talking with another member of her division when Dr. Joselle, her division director, approaches. He is accompanied by the Visiting Professor, an internationally reputable and dynamic man, a content expert in the field of work in which Dr. Wain is interested and has published. Dr. Joselle introduces the Visiting Professor formally, using his title of “doctor.” He then introduces Dr. Wain by her abridged first name, Beth.
As an upstander, the Visiting Professor quickly addresses Dr. Wain by her title and uses the situation as a platform to highlight the need to maintain professional address in the professional environment. He then adds that women, who are usually junior in academic rank, confer more benefit to being addressed formally and receiving visibility and respect for their work in a public forum. In this way, the Visiting Professor amplifies Dr. Wain’s work and status and demonstrates the standard of using professional address for women and men.
- Rayburn WF. The Obstetrician-Gynecologist Workforce in the United States: Facts, Figures, and Implications, 2017. Washington, DC: American Congress of Obstetricians and Gynecologists; 2017.
- Carnes M. Commentary: deconstructing gender differences. Acad Med. 2010;85:575-577.
- Eagly AH. The his and hers of prosocial behavior: an examination of the social psychology of gender. Am Psychol. 2009;64:644-658.
- Salles A, Awad M, Goldin L, et al. Estimating implicit and explicit gender bias among health care professionals and surgeons. JAMA Netw Open. 2019;2:e196545.
- Carnes M, Bartels CM, Kaatz A, et al. Why is John more likely to become department chair than Jennifer? Trans Am Clin Climatol Assoc. 2015;126:197-214.
- Hoffman A, Grant, W, McCormick, et al. Gendered differences in letters of recommendation for transplant surgery fellowship applicants. J Surg Edu. 2019;76:427-432.
- Galvin SL, Parlier AB, Martino E, et al. Gender bias in nurse evaluations of residents in obstetrics and gynecology. Obstet Gynecol. 2015;126(suppl 4):7S-12S.
- Gerull KM, Loe M, Seiler K, et al. Assessing gender bias in qualitative evaluations of surgical residents. Am J Surg. 2019;217:306-313.
- Meyerson SL, Sternbach JM, Zwischenberger JB, et al. The effect of gender on resident autonomy in the operating room. J Surg Educ. 2017;74:e111-e118.
- Flyckt RL, White EE, Goodman LR, et al. The use of laparoscopy simulation to explore gender differences in resident surgical confidence. Obstet Gynecol Int. 2017;2017:1945801.
- Heisler CA, Mark K, Ton J, et al. Has a critical mass of women resulted in gender equity in gynecologic surgery? Am J Obstet Gynecol. 2020;223:665-673.
- Warner AS, Lehmann LS. Gender wage disparities in medicine: time to close the gap. J Gen Intern Med. 2019;34:1334-1336.
- Gilbert SB, Allshouse A, Skaznik-Wikiel ME. Gender inequality in salaries among reproductive endocrinology and infertility specialists in the United States. Fertil Steril. 2019;111:1194-1200.
- Croft KM, Rauh LA, Orr JW, et al. Compensation differences by gender in gynecologic oncology. Society of Gynecologic Oncology Annual Meeting on Women’s Cancer. 2020. https://sgo.confex.com /sgo/2020/meetingapp.cgi/Paper/15762. 2020. Accessed April 1, 2020.
- Wang SS, Ackerman S. The motherhood penalty: is it alive and well in 2020? J Am Coll Radiol. 2020;17:688-689.
- Pelley E, Carnes M. When a specialty becomes “women’s work”: trends in and implications of specialty gender segregation in medicine. Acad Med. 2020;95:1499-1506.
- Hegewisch A, Hartmann H. Occupational segregation and the gender wage gap: a job half done. Institute for Women’s Policy Research. 2014. https://iwpr.org/iwpr-issues/employment-and-earnings /occupational-segregation-and-the-gender-wage-gap-a-job-half -done/. Accessed August 26, 2021.
- Greenberg CC. Association for Academic Surgery presidential address: sticky floors and glass ceilings. J Surg Res. 2017;219:ix-xviii.
- Dossett LA, Vitous CA, Lindquist K, et al. Women surgeons’ experiences of interprofessional workplace conflict. JAMA Netw Open. 2020;3:e2019843.
- Morgan HK, Purkis JA, Porter AC, et al. Student evaluation of faculty physicians: gender differences in teaching evaluations. J Womens Health (Larchmt). 2016;25:453-456.
- Childs AJ, Friedman WH, Schwartz MP, et al. Female patients’ sex preferences in selection of gynecologists and surgeons. South Med J. 2005;98:405-408.
- Brown J, Drury L, Raub K, et al. Workplace harassment and discrimination in gynecology: results of the AAGL Member Survey. J Minim Invasive Gynecol. 2019;26:838-846.
- Temkin AM, Rubinsak L, Benoit MF, et al. Take me to your leader: reporting structures and equity in academic gynecologic oncology. Gynecol Oncol. 2020;157:759-764.
- Shakil S, Redberg RF. Gender disparities in sponsorship—how they perpetuate the glass ceiling. JAMA Intern Med. 2017;177:582.
- Hansen M, Schoonover A, Skarica B, et al. Implicit gender bias among US resident physicians. BMC Med Ed. 2019;19:396.
- Estrada M, Burnett M, Campbell AG, et al. Improving underrepresented minority student persistence in STEM. CBE Life Sci Educ. 2016;15:es5.
- Carnes M, Handelsman J, Sheridan J. Diversity in academic medicine: the stages of change model. J Womens Health (Larchmt). 2005;14:471-475.
- Person SD, Jordan CG, Allison JJ, et al. Measuring diversity and inclusion in academic medicine. The Diversity Engagement Survey. Acad Med. 2015;90:1675-1683.
- Fitzgerald CA, Smith RN, Luo-Owen X, et al. Screening for harassment, abuse, and discrimination among surgery residents: an EAST multicenter trial. Am Surg. 2019;85:456-461.
- Hayes SN, Noseworthy JH, Farrugia G. A structured compensation plan results in equitable physician compensation: a single-center analysis. Mayo Clin Proc. 2020;95:35-43.
- Devine PG, Forscher PS, Cox WT, et al. A gender bias habit-breaking intervention led to increased hiring of female faculty in STEMM departments. J Exp Soc Psychol. 2017;73:211-215.
- Morgan AU, Chaiyachati KH, Weissman GE, et al. Eliminating genderbased bias in academic medicine: more than naming the “elephant in the room.” J Gen Intern Med. 2018;33:966-968.
- Mello MM, Jagsi R. Standing up against gender bias and harassment— a matter of professional ethics. N Engl J Med. 2020;382:1385-1387.
- Burgess A, van Diggele C, Mellis C. Mentorship in the health profession: a review. Clin Teach. 2018;15:197-202.
- Rayburn WF. The Obstetrician-Gynecologist Workforce in the United States: Facts, Figures, and Implications, 2017. Washington, DC: American Congress of Obstetricians and Gynecologists; 2017.
- Carnes M. Commentary: deconstructing gender differences. Acad Med. 2010;85:575-577.
- Eagly AH. The his and hers of prosocial behavior: an examination of the social psychology of gender. Am Psychol. 2009;64:644-658.
- Salles A, Awad M, Goldin L, et al. Estimating implicit and explicit gender bias among health care professionals and surgeons. JAMA Netw Open. 2019;2:e196545.
- Carnes M, Bartels CM, Kaatz A, et al. Why is John more likely to become department chair than Jennifer? Trans Am Clin Climatol Assoc. 2015;126:197-214.
- Hoffman A, Grant, W, McCormick, et al. Gendered differences in letters of recommendation for transplant surgery fellowship applicants. J Surg Edu. 2019;76:427-432.
- Galvin SL, Parlier AB, Martino E, et al. Gender bias in nurse evaluations of residents in obstetrics and gynecology. Obstet Gynecol. 2015;126(suppl 4):7S-12S.
- Gerull KM, Loe M, Seiler K, et al. Assessing gender bias in qualitative evaluations of surgical residents. Am J Surg. 2019;217:306-313.
- Meyerson SL, Sternbach JM, Zwischenberger JB, et al. The effect of gender on resident autonomy in the operating room. J Surg Educ. 2017;74:e111-e118.
- Flyckt RL, White EE, Goodman LR, et al. The use of laparoscopy simulation to explore gender differences in resident surgical confidence. Obstet Gynecol Int. 2017;2017:1945801.
- Heisler CA, Mark K, Ton J, et al. Has a critical mass of women resulted in gender equity in gynecologic surgery? Am J Obstet Gynecol. 2020;223:665-673.
- Warner AS, Lehmann LS. Gender wage disparities in medicine: time to close the gap. J Gen Intern Med. 2019;34:1334-1336.
- Gilbert SB, Allshouse A, Skaznik-Wikiel ME. Gender inequality in salaries among reproductive endocrinology and infertility specialists in the United States. Fertil Steril. 2019;111:1194-1200.
- Croft KM, Rauh LA, Orr JW, et al. Compensation differences by gender in gynecologic oncology. Society of Gynecologic Oncology Annual Meeting on Women’s Cancer. 2020. https://sgo.confex.com /sgo/2020/meetingapp.cgi/Paper/15762. 2020. Accessed April 1, 2020.
- Wang SS, Ackerman S. The motherhood penalty: is it alive and well in 2020? J Am Coll Radiol. 2020;17:688-689.
- Pelley E, Carnes M. When a specialty becomes “women’s work”: trends in and implications of specialty gender segregation in medicine. Acad Med. 2020;95:1499-1506.
- Hegewisch A, Hartmann H. Occupational segregation and the gender wage gap: a job half done. Institute for Women’s Policy Research. 2014. https://iwpr.org/iwpr-issues/employment-and-earnings /occupational-segregation-and-the-gender-wage-gap-a-job-half -done/. Accessed August 26, 2021.
- Greenberg CC. Association for Academic Surgery presidential address: sticky floors and glass ceilings. J Surg Res. 2017;219:ix-xviii.
- Dossett LA, Vitous CA, Lindquist K, et al. Women surgeons’ experiences of interprofessional workplace conflict. JAMA Netw Open. 2020;3:e2019843.
- Morgan HK, Purkis JA, Porter AC, et al. Student evaluation of faculty physicians: gender differences in teaching evaluations. J Womens Health (Larchmt). 2016;25:453-456.
- Childs AJ, Friedman WH, Schwartz MP, et al. Female patients’ sex preferences in selection of gynecologists and surgeons. South Med J. 2005;98:405-408.
- Brown J, Drury L, Raub K, et al. Workplace harassment and discrimination in gynecology: results of the AAGL Member Survey. J Minim Invasive Gynecol. 2019;26:838-846.
- Temkin AM, Rubinsak L, Benoit MF, et al. Take me to your leader: reporting structures and equity in academic gynecologic oncology. Gynecol Oncol. 2020;157:759-764.
- Shakil S, Redberg RF. Gender disparities in sponsorship—how they perpetuate the glass ceiling. JAMA Intern Med. 2017;177:582.
- Hansen M, Schoonover A, Skarica B, et al. Implicit gender bias among US resident physicians. BMC Med Ed. 2019;19:396.
- Estrada M, Burnett M, Campbell AG, et al. Improving underrepresented minority student persistence in STEM. CBE Life Sci Educ. 2016;15:es5.
- Carnes M, Handelsman J, Sheridan J. Diversity in academic medicine: the stages of change model. J Womens Health (Larchmt). 2005;14:471-475.
- Person SD, Jordan CG, Allison JJ, et al. Measuring diversity and inclusion in academic medicine. The Diversity Engagement Survey. Acad Med. 2015;90:1675-1683.
- Fitzgerald CA, Smith RN, Luo-Owen X, et al. Screening for harassment, abuse, and discrimination among surgery residents: an EAST multicenter trial. Am Surg. 2019;85:456-461.
- Hayes SN, Noseworthy JH, Farrugia G. A structured compensation plan results in equitable physician compensation: a single-center analysis. Mayo Clin Proc. 2020;95:35-43.
- Devine PG, Forscher PS, Cox WT, et al. A gender bias habit-breaking intervention led to increased hiring of female faculty in STEMM departments. J Exp Soc Psychol. 2017;73:211-215.
- Morgan AU, Chaiyachati KH, Weissman GE, et al. Eliminating genderbased bias in academic medicine: more than naming the “elephant in the room.” J Gen Intern Med. 2018;33:966-968.
- Mello MM, Jagsi R. Standing up against gender bias and harassment— a matter of professional ethics. N Engl J Med. 2020;382:1385-1387.
- Burgess A, van Diggele C, Mellis C. Mentorship in the health profession: a review. Clin Teach. 2018;15:197-202.
Is the 52-mg LNG-IUD effective as emergency contraception?
Turok DK, Gero A, Simmons RG, et al. Levonorgestrel vs copper intrauterine devices for emergency contraception. N Engl J Med. 2021;384:335-344.
EXPERT COMMENTARY
Emergency contraception refers to therapies used to prevent pregnancy after inadequately protected intercourse.1 Evidence-based forms of EC available in the United States include oral LNG, oral ulipristal acetate, and the copper IUD. The copper IUD provides not only EC but also highly effective contraception after placement.2 The LNG-IUD has a favorable side effect profile compared with the copper IUD and is theorized to act as EC through direct interference with sperm and oviduct transport.3 Recently, Turok and colleagues conducted a noninferiority trial designed to investigate the EC effectiveness of the LNG-IUD compared with the copper IUD.3
Details of the study
Turok and colleagues recruited participants aged 18 to 35 who requested EC from 6 family planning clinics in Utah from 2016 to 2019. Participants who reported unprotected intercourse within the past 120 hours and who desired an IUD to prevent pregnancy for at least 1 year were randomly assigned to receive either the LNG-IUD or the copper IUD. Individuals were excluded from the trial if they had contraindications to IUD placement, were breastfeeding, had abnormal uterine bleeding, had irregular menses, were currently using highly effective contraception, or had recent EC use. Researchers determined pregnancy status at 1 month through a pregnancy test or clinical records review.
Results. Of 711 participants randomly assigned, 317 who received the LNG-IUD and 321 who received the copper IUD provided 1-month outcome data. Pregnancy 1 month after IUD placement occurred in 1 participant (0.3%) in the LNG-IUD group and in no participants in the copper IUD group (0%). The between-group difference of 0.3 percentage points was within the margin of noninferiority and was not significant.
Study strengths and limitations
This large, multicenter randomized controlled trial contributes novel information about the effectiveness and noninferiority of the LNG-IUD as EC. Unlike prior studies of oral EC, which commonly limited participants to 1 episode of unprotected intercourse, this trial enrolled women at potentially higher risk of pregnancy with multiple episodes of intercourse and found fewer pregnancies than expected. Randomization ensured equivalence between groups, with the exception of the reason for needing EC.
Study limitations include a higher than expected rate of loss to follow-up, requiring clinical records and survey data to confirm pregnancy status. After randomization, clinicians were unable to place IUDs in more than 5% of participants in both groups; noninferiority was demonstrated nonetheless. This study did not include participants receiving oral EC, so direct comparison of effectiveness is not possible. Pregnancy rates among IUD users in this study were favorable to rates reported in previous studies of oral EC.4
When choosing an IUD for contraception, more women select the LNG-IUD for its favorable side effect profile and reduction in menstrual bleeding. In this randomized IUD study, only 7% of eligible participants enrolled, potentially introducing selection bias. The majority who declined enrollment did not want an IUD. Previous studies that allowed participants to choose their IUD had higher enrollment rates. ●
The study by Turok and colleagues is the largest randomized controlled trial to date of IUDs as EC. It demonstrated that LNG-IUDs are noninferior to copper IUDs in preventing pregnancy when placed within 5 days of unprotected intercourse. IUDs offer advantages over oral EC methods: only IUDs provide ongoing contraception after EC, and IUD efficacy does not vary by body mass index. It is reasonable for clinicians and patients to consider LNG-IUDs among EC options after shared decision making.
This study suggests that quick-start placement of the LNG-IUD at any time in the menstrual cycle is reasonable given its effectiveness as EC. Additionally, there were no pregnancies among 138 study participants who resumed intercourse within 7 days of LNG-IUD placement, most of whom did not use backup contraception.5 While current guidelines still recommend backup contraception after LNG-IUD placement, clinicians may reassure patients with unprotected intercourse following any type of IUD placement about the low risk of pregnancy.
LISA HOFLER, MD, MPH, MBA,
AND SMITA CARROLL, MD, MBA
- ACOG Committee on Practice Bulletins–Gynecology. Practice bulletin no. 152: emergency contraception. Obstet Gynecol. 2015;126:e1-e11.
- Cleland K, Zhu H, Goldstuck N, et al. The efficacy of intrauterine devices for emergency contraception: a systematic review of 35 years of experience. Hum Reprod. 2012;27:1994-2000.
- Turok DK, Gero A, Simmons RG, et al. Levonorgestrel vs copper intrauterine devices for emergency contraception. N Engl J Med. 2021;384:335-344.
- Glasier AF, Cameron ST, Fine PM, et al. Ulipristal acetate versus levonorgestrel for emergency contraception: a randomized non-inferiority trial and meta-analysis. Lancet. 2010;375:555-562.
- Fay KE, Clement AC, Gero A, et al. Rates of pregnancy among levonorgestrel and copper intrauterine emergency contraception initiators: implications for backup contraception recommendations. Contraception. 2021;S0010-7824(21)00210-9.
Turok DK, Gero A, Simmons RG, et al. Levonorgestrel vs copper intrauterine devices for emergency contraception. N Engl J Med. 2021;384:335-344.
EXPERT COMMENTARY
Emergency contraception refers to therapies used to prevent pregnancy after inadequately protected intercourse.1 Evidence-based forms of EC available in the United States include oral LNG, oral ulipristal acetate, and the copper IUD. The copper IUD provides not only EC but also highly effective contraception after placement.2 The LNG-IUD has a favorable side effect profile compared with the copper IUD and is theorized to act as EC through direct interference with sperm and oviduct transport.3 Recently, Turok and colleagues conducted a noninferiority trial designed to investigate the EC effectiveness of the LNG-IUD compared with the copper IUD.3
Details of the study
Turok and colleagues recruited participants aged 18 to 35 who requested EC from 6 family planning clinics in Utah from 2016 to 2019. Participants who reported unprotected intercourse within the past 120 hours and who desired an IUD to prevent pregnancy for at least 1 year were randomly assigned to receive either the LNG-IUD or the copper IUD. Individuals were excluded from the trial if they had contraindications to IUD placement, were breastfeeding, had abnormal uterine bleeding, had irregular menses, were currently using highly effective contraception, or had recent EC use. Researchers determined pregnancy status at 1 month through a pregnancy test or clinical records review.
Results. Of 711 participants randomly assigned, 317 who received the LNG-IUD and 321 who received the copper IUD provided 1-month outcome data. Pregnancy 1 month after IUD placement occurred in 1 participant (0.3%) in the LNG-IUD group and in no participants in the copper IUD group (0%). The between-group difference of 0.3 percentage points was within the margin of noninferiority and was not significant.
Study strengths and limitations
This large, multicenter randomized controlled trial contributes novel information about the effectiveness and noninferiority of the LNG-IUD as EC. Unlike prior studies of oral EC, which commonly limited participants to 1 episode of unprotected intercourse, this trial enrolled women at potentially higher risk of pregnancy with multiple episodes of intercourse and found fewer pregnancies than expected. Randomization ensured equivalence between groups, with the exception of the reason for needing EC.
Study limitations include a higher than expected rate of loss to follow-up, requiring clinical records and survey data to confirm pregnancy status. After randomization, clinicians were unable to place IUDs in more than 5% of participants in both groups; noninferiority was demonstrated nonetheless. This study did not include participants receiving oral EC, so direct comparison of effectiveness is not possible. Pregnancy rates among IUD users in this study were favorable to rates reported in previous studies of oral EC.4
When choosing an IUD for contraception, more women select the LNG-IUD for its favorable side effect profile and reduction in menstrual bleeding. In this randomized IUD study, only 7% of eligible participants enrolled, potentially introducing selection bias. The majority who declined enrollment did not want an IUD. Previous studies that allowed participants to choose their IUD had higher enrollment rates. ●
The study by Turok and colleagues is the largest randomized controlled trial to date of IUDs as EC. It demonstrated that LNG-IUDs are noninferior to copper IUDs in preventing pregnancy when placed within 5 days of unprotected intercourse. IUDs offer advantages over oral EC methods: only IUDs provide ongoing contraception after EC, and IUD efficacy does not vary by body mass index. It is reasonable for clinicians and patients to consider LNG-IUDs among EC options after shared decision making.
This study suggests that quick-start placement of the LNG-IUD at any time in the menstrual cycle is reasonable given its effectiveness as EC. Additionally, there were no pregnancies among 138 study participants who resumed intercourse within 7 days of LNG-IUD placement, most of whom did not use backup contraception.5 While current guidelines still recommend backup contraception after LNG-IUD placement, clinicians may reassure patients with unprotected intercourse following any type of IUD placement about the low risk of pregnancy.
LISA HOFLER, MD, MPH, MBA,
AND SMITA CARROLL, MD, MBA
Turok DK, Gero A, Simmons RG, et al. Levonorgestrel vs copper intrauterine devices for emergency contraception. N Engl J Med. 2021;384:335-344.
EXPERT COMMENTARY
Emergency contraception refers to therapies used to prevent pregnancy after inadequately protected intercourse.1 Evidence-based forms of EC available in the United States include oral LNG, oral ulipristal acetate, and the copper IUD. The copper IUD provides not only EC but also highly effective contraception after placement.2 The LNG-IUD has a favorable side effect profile compared with the copper IUD and is theorized to act as EC through direct interference with sperm and oviduct transport.3 Recently, Turok and colleagues conducted a noninferiority trial designed to investigate the EC effectiveness of the LNG-IUD compared with the copper IUD.3
Details of the study
Turok and colleagues recruited participants aged 18 to 35 who requested EC from 6 family planning clinics in Utah from 2016 to 2019. Participants who reported unprotected intercourse within the past 120 hours and who desired an IUD to prevent pregnancy for at least 1 year were randomly assigned to receive either the LNG-IUD or the copper IUD. Individuals were excluded from the trial if they had contraindications to IUD placement, were breastfeeding, had abnormal uterine bleeding, had irregular menses, were currently using highly effective contraception, or had recent EC use. Researchers determined pregnancy status at 1 month through a pregnancy test or clinical records review.
Results. Of 711 participants randomly assigned, 317 who received the LNG-IUD and 321 who received the copper IUD provided 1-month outcome data. Pregnancy 1 month after IUD placement occurred in 1 participant (0.3%) in the LNG-IUD group and in no participants in the copper IUD group (0%). The between-group difference of 0.3 percentage points was within the margin of noninferiority and was not significant.
Study strengths and limitations
This large, multicenter randomized controlled trial contributes novel information about the effectiveness and noninferiority of the LNG-IUD as EC. Unlike prior studies of oral EC, which commonly limited participants to 1 episode of unprotected intercourse, this trial enrolled women at potentially higher risk of pregnancy with multiple episodes of intercourse and found fewer pregnancies than expected. Randomization ensured equivalence between groups, with the exception of the reason for needing EC.
Study limitations include a higher than expected rate of loss to follow-up, requiring clinical records and survey data to confirm pregnancy status. After randomization, clinicians were unable to place IUDs in more than 5% of participants in both groups; noninferiority was demonstrated nonetheless. This study did not include participants receiving oral EC, so direct comparison of effectiveness is not possible. Pregnancy rates among IUD users in this study were favorable to rates reported in previous studies of oral EC.4
When choosing an IUD for contraception, more women select the LNG-IUD for its favorable side effect profile and reduction in menstrual bleeding. In this randomized IUD study, only 7% of eligible participants enrolled, potentially introducing selection bias. The majority who declined enrollment did not want an IUD. Previous studies that allowed participants to choose their IUD had higher enrollment rates. ●
The study by Turok and colleagues is the largest randomized controlled trial to date of IUDs as EC. It demonstrated that LNG-IUDs are noninferior to copper IUDs in preventing pregnancy when placed within 5 days of unprotected intercourse. IUDs offer advantages over oral EC methods: only IUDs provide ongoing contraception after EC, and IUD efficacy does not vary by body mass index. It is reasonable for clinicians and patients to consider LNG-IUDs among EC options after shared decision making.
This study suggests that quick-start placement of the LNG-IUD at any time in the menstrual cycle is reasonable given its effectiveness as EC. Additionally, there were no pregnancies among 138 study participants who resumed intercourse within 7 days of LNG-IUD placement, most of whom did not use backup contraception.5 While current guidelines still recommend backup contraception after LNG-IUD placement, clinicians may reassure patients with unprotected intercourse following any type of IUD placement about the low risk of pregnancy.
LISA HOFLER, MD, MPH, MBA,
AND SMITA CARROLL, MD, MBA
- ACOG Committee on Practice Bulletins–Gynecology. Practice bulletin no. 152: emergency contraception. Obstet Gynecol. 2015;126:e1-e11.
- Cleland K, Zhu H, Goldstuck N, et al. The efficacy of intrauterine devices for emergency contraception: a systematic review of 35 years of experience. Hum Reprod. 2012;27:1994-2000.
- Turok DK, Gero A, Simmons RG, et al. Levonorgestrel vs copper intrauterine devices for emergency contraception. N Engl J Med. 2021;384:335-344.
- Glasier AF, Cameron ST, Fine PM, et al. Ulipristal acetate versus levonorgestrel for emergency contraception: a randomized non-inferiority trial and meta-analysis. Lancet. 2010;375:555-562.
- Fay KE, Clement AC, Gero A, et al. Rates of pregnancy among levonorgestrel and copper intrauterine emergency contraception initiators: implications for backup contraception recommendations. Contraception. 2021;S0010-7824(21)00210-9.
- ACOG Committee on Practice Bulletins–Gynecology. Practice bulletin no. 152: emergency contraception. Obstet Gynecol. 2015;126:e1-e11.
- Cleland K, Zhu H, Goldstuck N, et al. The efficacy of intrauterine devices for emergency contraception: a systematic review of 35 years of experience. Hum Reprod. 2012;27:1994-2000.
- Turok DK, Gero A, Simmons RG, et al. Levonorgestrel vs copper intrauterine devices for emergency contraception. N Engl J Med. 2021;384:335-344.
- Glasier AF, Cameron ST, Fine PM, et al. Ulipristal acetate versus levonorgestrel for emergency contraception: a randomized non-inferiority trial and meta-analysis. Lancet. 2010;375:555-562.
- Fay KE, Clement AC, Gero A, et al. Rates of pregnancy among levonorgestrel and copper intrauterine emergency contraception initiators: implications for backup contraception recommendations. Contraception. 2021;S0010-7824(21)00210-9.
Relugolix combination therapy: A novel hormonal treatment for AUB associated with uterine fibroids
When gonadotropin-releasing hormone (GnRH) agonist and antagonist peptide medications were first approved for use in the 1980s and 1990s, the available agents could only be administered by injection or nasal spray. The innovative development of orally active, nonpeptide GnRH antagonists, including relugolix and elagolix (FIGURE 1), is a major breakthrough in women’s health. Orally active GnRH antagonists provide gynecologists with a unique way to regulate hypothalamic-pituitary-ovarian-uterus function. GnRH antagonists bind to the pituitary GnRH receptor, reducing pituitary secretion of luteinizing hormone (LH) and follicle-stimulating hormone (FSH). In turn, reduction in LH and FSH suppresses ovarian follicle development, reducing ovarian secretion of estradiol and progesterone. The uterine endometrium becomes less active in response to low levels of estradiol and progesterone, resulting in oligomenorrhea or amenorrhea. The hypoestrogenic adverse effects of GnRH antagonist treatment, including bone loss and vasomotor symptoms can be minimized by adding back a low dose of estrogen and progestin, such as oral estradiol 1 mg and norethindrone acetate 0.5 mg.
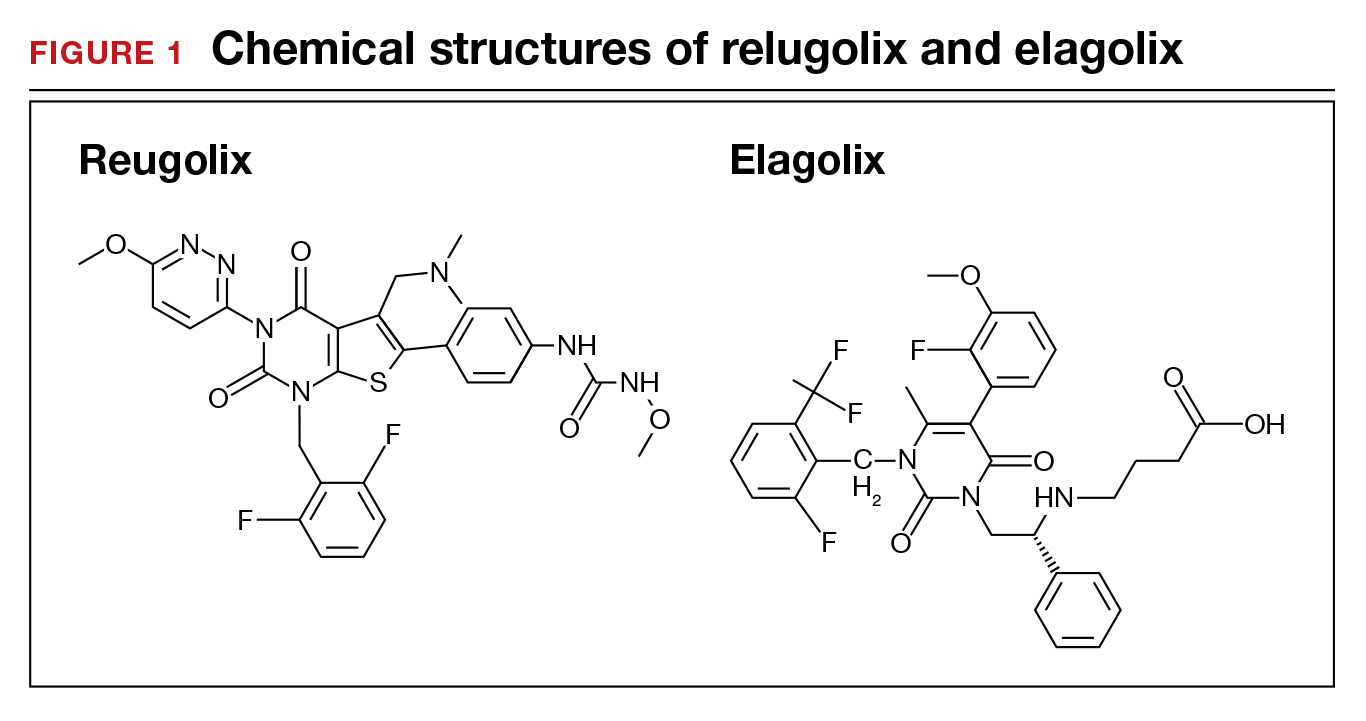
Recently, the US Food and Drug Administration (FDA) approved oral relugolix combination therapy (Myfembree, Myovant Sciences and Pfizer Inc; relugolix 40 mg, estradiol 1 mg, and norethindrone acetate 0.5 mg) once daily for the treatment of abnormal uterine bleeding (AUB) associated with uterine leiomyomata (fibroids) in premenopausal women for up to 24 months.1 This editorial will focus on key clinical issues when using relugolix combination therapy.
Relugolix combination treatment is superior to placebo for AUB from fibroids
In 2 clinical trials, 770 women with symptomatic uterine fibroids were randomly assigned to 1 of 3 groups2:
- placebo for 24 weeks
- relugolix combination therapy (consisting of relugolix 40 mg, estradiol 1 mg, and norethindrone acetate 0.5 mg) daily for 24 weeks
- relugolix monotherapy (40 mg daily for 12 weeks) followed by relugolix combination therapy for 12 additional weeks (delayed combination therapy group).
The women’s mean age was approximately 42 years, and they had a mean menstrual blood loss at baseline of approximately 230 mL and mean uterine volume by ultrasound measurement of 408 cm3.2 Prior to entry into the study all the women had an endometrial biopsy and a transvaginal ultrasound study of the pelvis. Women with a baseline bone mineral density Z-score of less than -2.0 at the spine, total hip, or femoral neck were excluded from the study because of low bone mass.2
At 24 weeks of treatment, approximately 72% of the women in the relugolix combination therapy groups had less than 80 mL of menstrual blood volume loss and ≥50% reduction in menstrual blood loss from baseline compared with 17% of women in the placebo group.2 At 8 weeks of treatment mean percent changes in menstrual blood loss from baseline were approximately 80% and 20% for the women receiving relugolix combination and placebo, respectively. Those differences persisted from 8 weeks through 24 weeks of treatment.1 In the last 35 days of treatment, amenorrhea was reported by approximately 51% and 4.5% of women receiving relugolix combination or placebo treatment, respectively.2 Compared with the placebo group, the relugolix combination groups reported significant improvement in bleeding and pelvic discomfort and had a higher hemoglobin concentration. Compared with placebo, relugolix combination treatment resulted in a greater percentage decrease in uterine volume (-12.9% vs +2.2%, respectively; P< .001).2
Continue to: Relugolix combination treatment is associated with fewer side effects than relugolix monotherapy...
Relugolix combination treatment is associated with fewer side effects than relugolix monotherapy
Compared with relugolix combination therapy, women treated with relugolix monotherapy for 12 weeks followed by 12 weeks of relugolix combination therapy lost more bone density as measured by dual-energy X-ray absorptiometry and reported more vasomotor symptoms. This is an expected finding because GnRH antagonist monotherapy is known to significantly reduce ovarian estradiol and progesterone levels, causing bone loss and vasomotor symptoms. Relugolix combination treatment minimizes bone density loss and vasomotor symptoms because the combination of estradiol and norethindrone helps to preserve bone density and reduce hot flashes. Based on these and other findings, the FDA approved relugolix combination therapy for up to 24 months of treatment.1
Contraindications
Contraindications to relugolix combination therapy include: 1) pregnancy, 2) undiagnosed abnormal uterine bleeding, 3) current or personal history of breast cancer or other hormone-sensitive cancer, 4) known osteoporosis, 5) liver disease, 6) high risk of thrombosis, and 7) hypersensitivity to components of the medication.1
Adverse reactions
Serious adverse reactions were reported by 3.1% and 2.3% of women treated with the relugolix combination and placebo, respectively. Women taking relugolix combination reported the following adverse effects: 10.6% hot flashes, 6.3% AUB, 3.5% alopecia, and 3.1% decreased libido. Women taking placebo reported the following adverse effects: 6.6% hot flashes, 1.2% AUB, 0.8% alopecia, and 0.4% decreased libido. Among women taking relugolix combination, the following events occurred, each reported once by different women: myoma expulsion with menorrhagia, myoma prolapse without menorrhagia, cholecystitis, and pelvic pain.1
Bone loss
In women taking relugolix combination or placebo for 6 months, lumbar spine bone density change from baseline, as measured by DEXA, were -0.23% and +0.18%, respectively.1 After 12 months of relugolix combination treatment, lumbar spine bone density had decreased by -0.8% from baseline. These changes in lumbar bone density are minimal, and in my opinion of no clinical importance.
Reported mental health effects
Compared with placebo, more women taking relugolix combination reported depression, depressed mood, or mood swings (2.4% vs 0.8%), irritability (2.4% vs 0%), and anxiety (1.2% vs 0.8%).1
Continue to: Options for the treatment of AUB caused by fibroids...
Options for the treatment of AUB caused by fibroids
There are many options for the treatment of AUB caused by fibroids, including surgical, hormonal, and nonhormonal therapies. Women with bothersome fibroids strongly prefer to be involved in the decision-making process and select the treatment plan that is best for their situation.3 The patient’s preferences can be explored by discussing the main benefits and common complications and side effects of each treatment option.
Surgical options for the treatment of AUB caused by fibroids include, but are not limited to, hysterectomy (laparoscopic, vaginal, or laparotomy), myomectomy (hysteroscopic, laparoscopic, or laparotomy), uterine artery embolization, focused ultrasound surgery, radiofrequency ablation, cryomyolysis, endometrial ablation, and occlusion of the uterine arteries.4 The FIGO classification system provides a consensus nomenclature for describing fibroid location (see FIGURE 2).5 The selection of a treatment option is greatly influenced by the location of the fibroids in the uterus.6 Most experts recommend hysteroscopic myomectomy to treat Type 0 and Type 1 fibroids causing AUB.6 For Type 2 fibroids, hysteroscopic myomectomy, if technically feasible, is associated with a high rate of resolution of AUB with minimal complications. Hormonal treatment of Type 0 and Type 1 fibroids may result in red degeneration of the fibroid with significant menorrhagia.7,8 In my practice, I generally advise patients that hysteroscopic myomectomy is the first-line treatment option for Types 0, 1, and 2 fibroids causing AUB.
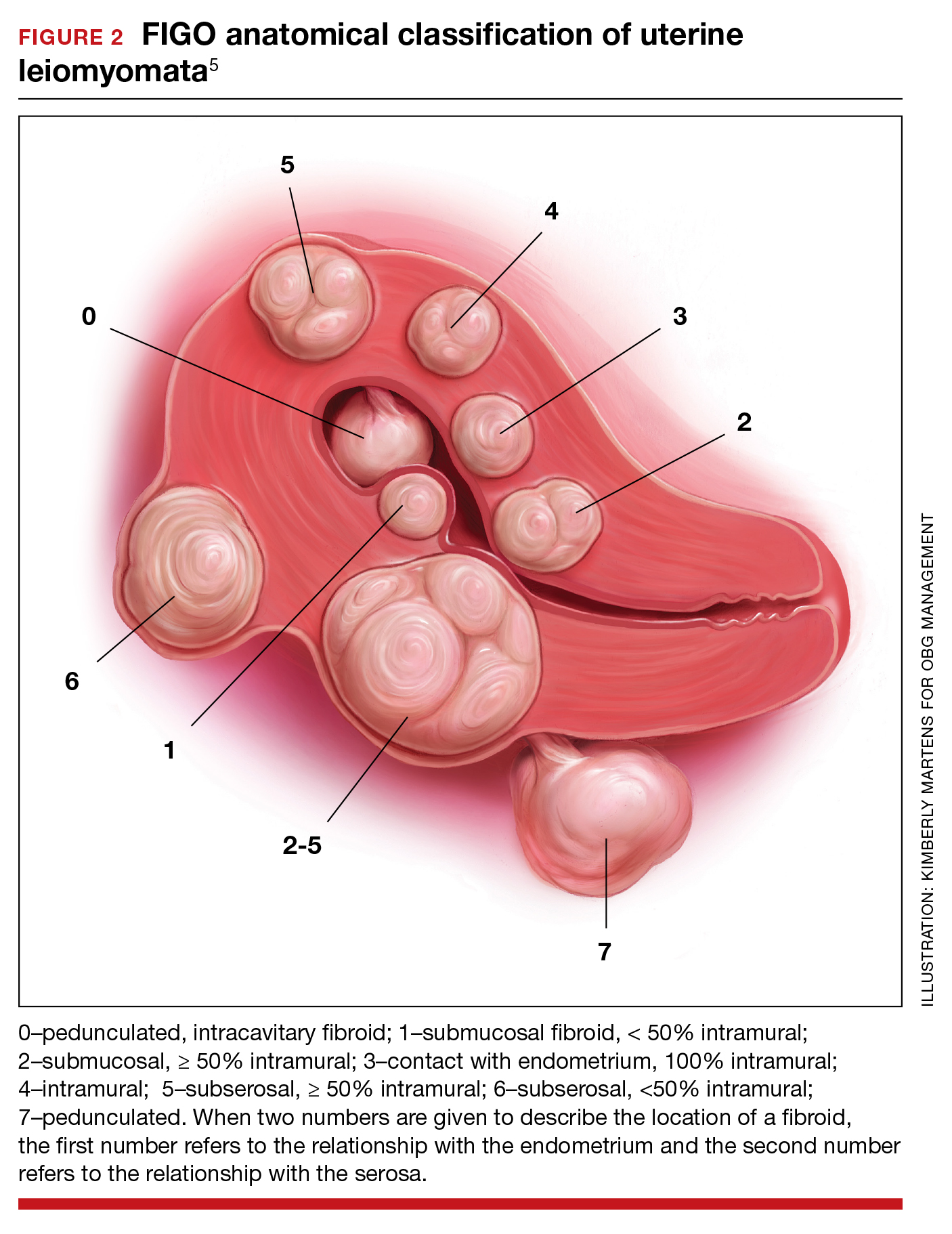
The FDA has approved the hormonal options of relugolix combination therapy (Myfembree)2 and elagolix combination therapy (Oriahnn)9,10 for the treatment of AUB associated with fibroids. Of note, elagolix combination therapy contains the same daily dose of estradiol (1 mg) and norethindrone acetate (0.5 mg) as relugolix combination therapy. Relugolix and elagolix combination therapy for fibroids are good options for women who have FIGO Type 2 to 5 fibroids and who prefer a nonsurgical option. If GnRH antagonist combination therapy results in a meaningful reduction in AUB, treatment can be continued for up to 2 years. If the patient reports an insufficient decrease in AUB, an alternative surgical, hormonal, or nonhormonal option can be offered.
Other hormonal treatments that may reduce AUB due to fibroids include combination estrogen-progestin contraceptives,11 the levonorgestrel-releasing intrauterine device (LNG-IUD),12 progestins, and leuprolide.13 Leuprolide plus iron therapy is approved by the FDA for improving red blood cell concentration prior to surgery in women with fibroids, AUB, and anemia.14 The Mirena LNG-IUD is FDA approved for the treatment of heavy menstrual bleeding among women who choose to use an IUD for contraception.15 However, a recent systematic review and meta-analysis concluded that because of very low-quality evidence it was difficult to assess the efficacy of the LNG-IUD and progestins for the treatment of fibroids.16 Tranexamic acid is a nonhormonal option, FDA approved for the treatment of cyclic heavy management of AUB caused by fibroids, and may be an option for women who are near menopause.
New hormonal treatment adds options for women
Fibroids are the most common pelvic tumor of women. Women with fibroids often present for clinical care due to AUB, pelvic pain, and/or lower abdominal discomfort. For women with symptomatic fibroids it may be difficult to effectively complete employment-related tasks and home responsibilities. In one study, women with symptomatic fibroids reported that their symptoms negatively impacted approximately 20 hours per month of employment-related work and 12 hours per month of home responsibilities, reducing productivity in both settings.19 Relugolix combination therapy adds another important option for the hormonal treatment of the problems caused by these prevalent and bothersome tumors, improving health and the quality of contributions at work and home. ●
- Orgovyx [package insert]. Brisbane, CA: Myovant Sciences, Inc; December 2020.
- Al-Hendy A, Lukes AS, Poindexter AN, et al. Treatment of uterine fibroid symptoms with relugolix combination therapy. N Engl J Med. 2021;384:630-642. doi: 10.1056/NEJMoa2008283.
- Solberg LI, Asche SE, Anderson LH, et al. Evaluating preference-sensitive care for uterine fibroids: it’s not so simple. J Women’s Health. 2009;18:1071-1079. doi: 10.1089/jwh.2008.0948.
- Stewart EA. Uterine Fibroids. N Engl J Med. 2015;372:1646-1655. doi: 10.1056/NEJMcp1411029.
- Munro MG, Critchley HO, Broder MS, et al. FIGO Working Group on Menstrual Disorders. FIGO classification system (PALM-COEIN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. Int J Gynaecol Obstet. 2011;113:3-13. doi: 10.1016/j.ijgo.2010.11.011.
- Donnez J, Dolmans MM. Uterine fibroid management: from the present to the future. Hum Reprod Update. 2016;22:665-686. doi: 10.1093/humupd/dmw023.
- Furui T, Imai A, Takagi A, et al. Differential efficacy of gonadotropin-releasing hormone (GnRH) agonist treatment on pedunculated and degenerated myomas: a retrospective study of 630 women. J Obstet Gynaecol. 2000;20:504-506. doi: 10.1080/014436100434703.
- Takeuchi M, Matsuzaki K, Bando Y, et al. Evaluation of red degeneration of uterine leiomyoma with susceptibility-weighted MR imaging. Magn Reson Med Sci. 2019;18:158-162. doi: 10.2463/mrms.mp.2018-0074.
- Schlaff WD, Ackerman RT, Al-Hendy A, et al. Elagolix for heavy menstrual bleeding in women with uterine fibroids. N Engl J Med. 2020;382:328-340. doi: 10.1056/NEJMoa1904351.
- Simon JA, Al-Hendy A, Archer DE, et al. Elagolix treatment for up to 12 months in women with heavy menstrual bleeding and uterine leiomyomas. Obstet Gynecol. 2020;135:1313-1326. doi: 10.1097/AOG.0000000000003869.
- Yao X, Stewart EA, Laughlin-Tommaso SK, et al. Medical therapies for heavy menstrual bleeding in women with uterine fibroids: a retrospective analysis of a large commercially insured population in the USA. BJOG. 2017;124:322-330. doi: 10.1111/1471-0528.14383.
- Zapata LB, Whiteman MK, Tepper NK, et al. Intrauterine device use among women with uterine fibroids: a systematic review. Contraception. 2010;82:41-55. doi: 10.1016/j.contraception.2010.02.011.
- Donnez J, Tomaszewski J, Vazquez F, et al. Ulipristal acetate versus leuprolide acetate for uterine fibroids. N Engl J Med. 2012;366:421-432. doi: 10.1056/NEJMoa1103180.
- Lupron Depot [package insert]. Osaka, Japan: Takeda; Revised March 2012.
- Mirena [package insert]. Whippany, NJ: Bayer Healthcare Pharmaceuticals, Inc; Revised August 2020.
- Sangkormkamhang US, Lumbiganon P, Pattanittum P. Progestogens or progestogen-releasing intrauterine systems for uterine fibroids (other than preoperative medical therapy). Cochrane Database Syst Rev. 2020;CD008994. doi: 10.1002/14651858.CD008994.pub3.
- Lysteda [package insert]. Parsippany, NJ: Ferring Pharmaceuticals Inc; Revised October 2013.
- Eder S, Baker J, Gersten J, et al. Efficacy and safety of oral tranexamic acid in women with heavy menstrual bleeding and fibroids. Women’s Health. 2013;9:397-403. doi: 10.2217/whe.13.28.
Solimon AM, Anand SB, Coyne KS, et al. Examining the relationship between symptomatic burden and self-reported productivity losses among patients with uterine fibroids in the United States. J Occup Environ Med. 2017;59:974-981. doi: 10.1097/JOM.0000000000001105.
When gonadotropin-releasing hormone (GnRH) agonist and antagonist peptide medications were first approved for use in the 1980s and 1990s, the available agents could only be administered by injection or nasal spray. The innovative development of orally active, nonpeptide GnRH antagonists, including relugolix and elagolix (FIGURE 1), is a major breakthrough in women’s health. Orally active GnRH antagonists provide gynecologists with a unique way to regulate hypothalamic-pituitary-ovarian-uterus function. GnRH antagonists bind to the pituitary GnRH receptor, reducing pituitary secretion of luteinizing hormone (LH) and follicle-stimulating hormone (FSH). In turn, reduction in LH and FSH suppresses ovarian follicle development, reducing ovarian secretion of estradiol and progesterone. The uterine endometrium becomes less active in response to low levels of estradiol and progesterone, resulting in oligomenorrhea or amenorrhea. The hypoestrogenic adverse effects of GnRH antagonist treatment, including bone loss and vasomotor symptoms can be minimized by adding back a low dose of estrogen and progestin, such as oral estradiol 1 mg and norethindrone acetate 0.5 mg.

Recently, the US Food and Drug Administration (FDA) approved oral relugolix combination therapy (Myfembree, Myovant Sciences and Pfizer Inc; relugolix 40 mg, estradiol 1 mg, and norethindrone acetate 0.5 mg) once daily for the treatment of abnormal uterine bleeding (AUB) associated with uterine leiomyomata (fibroids) in premenopausal women for up to 24 months.1 This editorial will focus on key clinical issues when using relugolix combination therapy.
Relugolix combination treatment is superior to placebo for AUB from fibroids
In 2 clinical trials, 770 women with symptomatic uterine fibroids were randomly assigned to 1 of 3 groups2:
- placebo for 24 weeks
- relugolix combination therapy (consisting of relugolix 40 mg, estradiol 1 mg, and norethindrone acetate 0.5 mg) daily for 24 weeks
- relugolix monotherapy (40 mg daily for 12 weeks) followed by relugolix combination therapy for 12 additional weeks (delayed combination therapy group).
The women’s mean age was approximately 42 years, and they had a mean menstrual blood loss at baseline of approximately 230 mL and mean uterine volume by ultrasound measurement of 408 cm3.2 Prior to entry into the study all the women had an endometrial biopsy and a transvaginal ultrasound study of the pelvis. Women with a baseline bone mineral density Z-score of less than -2.0 at the spine, total hip, or femoral neck were excluded from the study because of low bone mass.2
At 24 weeks of treatment, approximately 72% of the women in the relugolix combination therapy groups had less than 80 mL of menstrual blood volume loss and ≥50% reduction in menstrual blood loss from baseline compared with 17% of women in the placebo group.2 At 8 weeks of treatment mean percent changes in menstrual blood loss from baseline were approximately 80% and 20% for the women receiving relugolix combination and placebo, respectively. Those differences persisted from 8 weeks through 24 weeks of treatment.1 In the last 35 days of treatment, amenorrhea was reported by approximately 51% and 4.5% of women receiving relugolix combination or placebo treatment, respectively.2 Compared with the placebo group, the relugolix combination groups reported significant improvement in bleeding and pelvic discomfort and had a higher hemoglobin concentration. Compared with placebo, relugolix combination treatment resulted in a greater percentage decrease in uterine volume (-12.9% vs +2.2%, respectively; P< .001).2
Continue to: Relugolix combination treatment is associated with fewer side effects than relugolix monotherapy...
Relugolix combination treatment is associated with fewer side effects than relugolix monotherapy
Compared with relugolix combination therapy, women treated with relugolix monotherapy for 12 weeks followed by 12 weeks of relugolix combination therapy lost more bone density as measured by dual-energy X-ray absorptiometry and reported more vasomotor symptoms. This is an expected finding because GnRH antagonist monotherapy is known to significantly reduce ovarian estradiol and progesterone levels, causing bone loss and vasomotor symptoms. Relugolix combination treatment minimizes bone density loss and vasomotor symptoms because the combination of estradiol and norethindrone helps to preserve bone density and reduce hot flashes. Based on these and other findings, the FDA approved relugolix combination therapy for up to 24 months of treatment.1
Contraindications
Contraindications to relugolix combination therapy include: 1) pregnancy, 2) undiagnosed abnormal uterine bleeding, 3) current or personal history of breast cancer or other hormone-sensitive cancer, 4) known osteoporosis, 5) liver disease, 6) high risk of thrombosis, and 7) hypersensitivity to components of the medication.1
Adverse reactions
Serious adverse reactions were reported by 3.1% and 2.3% of women treated with the relugolix combination and placebo, respectively. Women taking relugolix combination reported the following adverse effects: 10.6% hot flashes, 6.3% AUB, 3.5% alopecia, and 3.1% decreased libido. Women taking placebo reported the following adverse effects: 6.6% hot flashes, 1.2% AUB, 0.8% alopecia, and 0.4% decreased libido. Among women taking relugolix combination, the following events occurred, each reported once by different women: myoma expulsion with menorrhagia, myoma prolapse without menorrhagia, cholecystitis, and pelvic pain.1
Bone loss
In women taking relugolix combination or placebo for 6 months, lumbar spine bone density change from baseline, as measured by DEXA, were -0.23% and +0.18%, respectively.1 After 12 months of relugolix combination treatment, lumbar spine bone density had decreased by -0.8% from baseline. These changes in lumbar bone density are minimal, and in my opinion of no clinical importance.
Reported mental health effects
Compared with placebo, more women taking relugolix combination reported depression, depressed mood, or mood swings (2.4% vs 0.8%), irritability (2.4% vs 0%), and anxiety (1.2% vs 0.8%).1
Continue to: Options for the treatment of AUB caused by fibroids...
Options for the treatment of AUB caused by fibroids
There are many options for the treatment of AUB caused by fibroids, including surgical, hormonal, and nonhormonal therapies. Women with bothersome fibroids strongly prefer to be involved in the decision-making process and select the treatment plan that is best for their situation.3 The patient’s preferences can be explored by discussing the main benefits and common complications and side effects of each treatment option.
Surgical options for the treatment of AUB caused by fibroids include, but are not limited to, hysterectomy (laparoscopic, vaginal, or laparotomy), myomectomy (hysteroscopic, laparoscopic, or laparotomy), uterine artery embolization, focused ultrasound surgery, radiofrequency ablation, cryomyolysis, endometrial ablation, and occlusion of the uterine arteries.4 The FIGO classification system provides a consensus nomenclature for describing fibroid location (see FIGURE 2).5 The selection of a treatment option is greatly influenced by the location of the fibroids in the uterus.6 Most experts recommend hysteroscopic myomectomy to treat Type 0 and Type 1 fibroids causing AUB.6 For Type 2 fibroids, hysteroscopic myomectomy, if technically feasible, is associated with a high rate of resolution of AUB with minimal complications. Hormonal treatment of Type 0 and Type 1 fibroids may result in red degeneration of the fibroid with significant menorrhagia.7,8 In my practice, I generally advise patients that hysteroscopic myomectomy is the first-line treatment option for Types 0, 1, and 2 fibroids causing AUB.

The FDA has approved the hormonal options of relugolix combination therapy (Myfembree)2 and elagolix combination therapy (Oriahnn)9,10 for the treatment of AUB associated with fibroids. Of note, elagolix combination therapy contains the same daily dose of estradiol (1 mg) and norethindrone acetate (0.5 mg) as relugolix combination therapy. Relugolix and elagolix combination therapy for fibroids are good options for women who have FIGO Type 2 to 5 fibroids and who prefer a nonsurgical option. If GnRH antagonist combination therapy results in a meaningful reduction in AUB, treatment can be continued for up to 2 years. If the patient reports an insufficient decrease in AUB, an alternative surgical, hormonal, or nonhormonal option can be offered.
Other hormonal treatments that may reduce AUB due to fibroids include combination estrogen-progestin contraceptives,11 the levonorgestrel-releasing intrauterine device (LNG-IUD),12 progestins, and leuprolide.13 Leuprolide plus iron therapy is approved by the FDA for improving red blood cell concentration prior to surgery in women with fibroids, AUB, and anemia.14 The Mirena LNG-IUD is FDA approved for the treatment of heavy menstrual bleeding among women who choose to use an IUD for contraception.15 However, a recent systematic review and meta-analysis concluded that because of very low-quality evidence it was difficult to assess the efficacy of the LNG-IUD and progestins for the treatment of fibroids.16 Tranexamic acid is a nonhormonal option, FDA approved for the treatment of cyclic heavy management of AUB caused by fibroids, and may be an option for women who are near menopause.
New hormonal treatment adds options for women
Fibroids are the most common pelvic tumor of women. Women with fibroids often present for clinical care due to AUB, pelvic pain, and/or lower abdominal discomfort. For women with symptomatic fibroids it may be difficult to effectively complete employment-related tasks and home responsibilities. In one study, women with symptomatic fibroids reported that their symptoms negatively impacted approximately 20 hours per month of employment-related work and 12 hours per month of home responsibilities, reducing productivity in both settings.19 Relugolix combination therapy adds another important option for the hormonal treatment of the problems caused by these prevalent and bothersome tumors, improving health and the quality of contributions at work and home. ●
When gonadotropin-releasing hormone (GnRH) agonist and antagonist peptide medications were first approved for use in the 1980s and 1990s, the available agents could only be administered by injection or nasal spray. The innovative development of orally active, nonpeptide GnRH antagonists, including relugolix and elagolix (FIGURE 1), is a major breakthrough in women’s health. Orally active GnRH antagonists provide gynecologists with a unique way to regulate hypothalamic-pituitary-ovarian-uterus function. GnRH antagonists bind to the pituitary GnRH receptor, reducing pituitary secretion of luteinizing hormone (LH) and follicle-stimulating hormone (FSH). In turn, reduction in LH and FSH suppresses ovarian follicle development, reducing ovarian secretion of estradiol and progesterone. The uterine endometrium becomes less active in response to low levels of estradiol and progesterone, resulting in oligomenorrhea or amenorrhea. The hypoestrogenic adverse effects of GnRH antagonist treatment, including bone loss and vasomotor symptoms can be minimized by adding back a low dose of estrogen and progestin, such as oral estradiol 1 mg and norethindrone acetate 0.5 mg.

Recently, the US Food and Drug Administration (FDA) approved oral relugolix combination therapy (Myfembree, Myovant Sciences and Pfizer Inc; relugolix 40 mg, estradiol 1 mg, and norethindrone acetate 0.5 mg) once daily for the treatment of abnormal uterine bleeding (AUB) associated with uterine leiomyomata (fibroids) in premenopausal women for up to 24 months.1 This editorial will focus on key clinical issues when using relugolix combination therapy.
Relugolix combination treatment is superior to placebo for AUB from fibroids
In 2 clinical trials, 770 women with symptomatic uterine fibroids were randomly assigned to 1 of 3 groups2:
- placebo for 24 weeks
- relugolix combination therapy (consisting of relugolix 40 mg, estradiol 1 mg, and norethindrone acetate 0.5 mg) daily for 24 weeks
- relugolix monotherapy (40 mg daily for 12 weeks) followed by relugolix combination therapy for 12 additional weeks (delayed combination therapy group).
The women’s mean age was approximately 42 years, and they had a mean menstrual blood loss at baseline of approximately 230 mL and mean uterine volume by ultrasound measurement of 408 cm3.2 Prior to entry into the study all the women had an endometrial biopsy and a transvaginal ultrasound study of the pelvis. Women with a baseline bone mineral density Z-score of less than -2.0 at the spine, total hip, or femoral neck were excluded from the study because of low bone mass.2
At 24 weeks of treatment, approximately 72% of the women in the relugolix combination therapy groups had less than 80 mL of menstrual blood volume loss and ≥50% reduction in menstrual blood loss from baseline compared with 17% of women in the placebo group.2 At 8 weeks of treatment mean percent changes in menstrual blood loss from baseline were approximately 80% and 20% for the women receiving relugolix combination and placebo, respectively. Those differences persisted from 8 weeks through 24 weeks of treatment.1 In the last 35 days of treatment, amenorrhea was reported by approximately 51% and 4.5% of women receiving relugolix combination or placebo treatment, respectively.2 Compared with the placebo group, the relugolix combination groups reported significant improvement in bleeding and pelvic discomfort and had a higher hemoglobin concentration. Compared with placebo, relugolix combination treatment resulted in a greater percentage decrease in uterine volume (-12.9% vs +2.2%, respectively; P< .001).2
Continue to: Relugolix combination treatment is associated with fewer side effects than relugolix monotherapy...
Relugolix combination treatment is associated with fewer side effects than relugolix monotherapy
Compared with relugolix combination therapy, women treated with relugolix monotherapy for 12 weeks followed by 12 weeks of relugolix combination therapy lost more bone density as measured by dual-energy X-ray absorptiometry and reported more vasomotor symptoms. This is an expected finding because GnRH antagonist monotherapy is known to significantly reduce ovarian estradiol and progesterone levels, causing bone loss and vasomotor symptoms. Relugolix combination treatment minimizes bone density loss and vasomotor symptoms because the combination of estradiol and norethindrone helps to preserve bone density and reduce hot flashes. Based on these and other findings, the FDA approved relugolix combination therapy for up to 24 months of treatment.1
Contraindications
Contraindications to relugolix combination therapy include: 1) pregnancy, 2) undiagnosed abnormal uterine bleeding, 3) current or personal history of breast cancer or other hormone-sensitive cancer, 4) known osteoporosis, 5) liver disease, 6) high risk of thrombosis, and 7) hypersensitivity to components of the medication.1
Adverse reactions
Serious adverse reactions were reported by 3.1% and 2.3% of women treated with the relugolix combination and placebo, respectively. Women taking relugolix combination reported the following adverse effects: 10.6% hot flashes, 6.3% AUB, 3.5% alopecia, and 3.1% decreased libido. Women taking placebo reported the following adverse effects: 6.6% hot flashes, 1.2% AUB, 0.8% alopecia, and 0.4% decreased libido. Among women taking relugolix combination, the following events occurred, each reported once by different women: myoma expulsion with menorrhagia, myoma prolapse without menorrhagia, cholecystitis, and pelvic pain.1
Bone loss
In women taking relugolix combination or placebo for 6 months, lumbar spine bone density change from baseline, as measured by DEXA, were -0.23% and +0.18%, respectively.1 After 12 months of relugolix combination treatment, lumbar spine bone density had decreased by -0.8% from baseline. These changes in lumbar bone density are minimal, and in my opinion of no clinical importance.
Reported mental health effects
Compared with placebo, more women taking relugolix combination reported depression, depressed mood, or mood swings (2.4% vs 0.8%), irritability (2.4% vs 0%), and anxiety (1.2% vs 0.8%).1
Continue to: Options for the treatment of AUB caused by fibroids...
Options for the treatment of AUB caused by fibroids
There are many options for the treatment of AUB caused by fibroids, including surgical, hormonal, and nonhormonal therapies. Women with bothersome fibroids strongly prefer to be involved in the decision-making process and select the treatment plan that is best for their situation.3 The patient’s preferences can be explored by discussing the main benefits and common complications and side effects of each treatment option.
Surgical options for the treatment of AUB caused by fibroids include, but are not limited to, hysterectomy (laparoscopic, vaginal, or laparotomy), myomectomy (hysteroscopic, laparoscopic, or laparotomy), uterine artery embolization, focused ultrasound surgery, radiofrequency ablation, cryomyolysis, endometrial ablation, and occlusion of the uterine arteries.4 The FIGO classification system provides a consensus nomenclature for describing fibroid location (see FIGURE 2).5 The selection of a treatment option is greatly influenced by the location of the fibroids in the uterus.6 Most experts recommend hysteroscopic myomectomy to treat Type 0 and Type 1 fibroids causing AUB.6 For Type 2 fibroids, hysteroscopic myomectomy, if technically feasible, is associated with a high rate of resolution of AUB with minimal complications. Hormonal treatment of Type 0 and Type 1 fibroids may result in red degeneration of the fibroid with significant menorrhagia.7,8 In my practice, I generally advise patients that hysteroscopic myomectomy is the first-line treatment option for Types 0, 1, and 2 fibroids causing AUB.

The FDA has approved the hormonal options of relugolix combination therapy (Myfembree)2 and elagolix combination therapy (Oriahnn)9,10 for the treatment of AUB associated with fibroids. Of note, elagolix combination therapy contains the same daily dose of estradiol (1 mg) and norethindrone acetate (0.5 mg) as relugolix combination therapy. Relugolix and elagolix combination therapy for fibroids are good options for women who have FIGO Type 2 to 5 fibroids and who prefer a nonsurgical option. If GnRH antagonist combination therapy results in a meaningful reduction in AUB, treatment can be continued for up to 2 years. If the patient reports an insufficient decrease in AUB, an alternative surgical, hormonal, or nonhormonal option can be offered.
Other hormonal treatments that may reduce AUB due to fibroids include combination estrogen-progestin contraceptives,11 the levonorgestrel-releasing intrauterine device (LNG-IUD),12 progestins, and leuprolide.13 Leuprolide plus iron therapy is approved by the FDA for improving red blood cell concentration prior to surgery in women with fibroids, AUB, and anemia.14 The Mirena LNG-IUD is FDA approved for the treatment of heavy menstrual bleeding among women who choose to use an IUD for contraception.15 However, a recent systematic review and meta-analysis concluded that because of very low-quality evidence it was difficult to assess the efficacy of the LNG-IUD and progestins for the treatment of fibroids.16 Tranexamic acid is a nonhormonal option, FDA approved for the treatment of cyclic heavy management of AUB caused by fibroids, and may be an option for women who are near menopause.
New hormonal treatment adds options for women
Fibroids are the most common pelvic tumor of women. Women with fibroids often present for clinical care due to AUB, pelvic pain, and/or lower abdominal discomfort. For women with symptomatic fibroids it may be difficult to effectively complete employment-related tasks and home responsibilities. In one study, women with symptomatic fibroids reported that their symptoms negatively impacted approximately 20 hours per month of employment-related work and 12 hours per month of home responsibilities, reducing productivity in both settings.19 Relugolix combination therapy adds another important option for the hormonal treatment of the problems caused by these prevalent and bothersome tumors, improving health and the quality of contributions at work and home. ●
- Orgovyx [package insert]. Brisbane, CA: Myovant Sciences, Inc; December 2020.
- Al-Hendy A, Lukes AS, Poindexter AN, et al. Treatment of uterine fibroid symptoms with relugolix combination therapy. N Engl J Med. 2021;384:630-642. doi: 10.1056/NEJMoa2008283.
- Solberg LI, Asche SE, Anderson LH, et al. Evaluating preference-sensitive care for uterine fibroids: it’s not so simple. J Women’s Health. 2009;18:1071-1079. doi: 10.1089/jwh.2008.0948.
- Stewart EA. Uterine Fibroids. N Engl J Med. 2015;372:1646-1655. doi: 10.1056/NEJMcp1411029.
- Munro MG, Critchley HO, Broder MS, et al. FIGO Working Group on Menstrual Disorders. FIGO classification system (PALM-COEIN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. Int J Gynaecol Obstet. 2011;113:3-13. doi: 10.1016/j.ijgo.2010.11.011.
- Donnez J, Dolmans MM. Uterine fibroid management: from the present to the future. Hum Reprod Update. 2016;22:665-686. doi: 10.1093/humupd/dmw023.
- Furui T, Imai A, Takagi A, et al. Differential efficacy of gonadotropin-releasing hormone (GnRH) agonist treatment on pedunculated and degenerated myomas: a retrospective study of 630 women. J Obstet Gynaecol. 2000;20:504-506. doi: 10.1080/014436100434703.
- Takeuchi M, Matsuzaki K, Bando Y, et al. Evaluation of red degeneration of uterine leiomyoma with susceptibility-weighted MR imaging. Magn Reson Med Sci. 2019;18:158-162. doi: 10.2463/mrms.mp.2018-0074.
- Schlaff WD, Ackerman RT, Al-Hendy A, et al. Elagolix for heavy menstrual bleeding in women with uterine fibroids. N Engl J Med. 2020;382:328-340. doi: 10.1056/NEJMoa1904351.
- Simon JA, Al-Hendy A, Archer DE, et al. Elagolix treatment for up to 12 months in women with heavy menstrual bleeding and uterine leiomyomas. Obstet Gynecol. 2020;135:1313-1326. doi: 10.1097/AOG.0000000000003869.
- Yao X, Stewart EA, Laughlin-Tommaso SK, et al. Medical therapies for heavy menstrual bleeding in women with uterine fibroids: a retrospective analysis of a large commercially insured population in the USA. BJOG. 2017;124:322-330. doi: 10.1111/1471-0528.14383.
- Zapata LB, Whiteman MK, Tepper NK, et al. Intrauterine device use among women with uterine fibroids: a systematic review. Contraception. 2010;82:41-55. doi: 10.1016/j.contraception.2010.02.011.
- Donnez J, Tomaszewski J, Vazquez F, et al. Ulipristal acetate versus leuprolide acetate for uterine fibroids. N Engl J Med. 2012;366:421-432. doi: 10.1056/NEJMoa1103180.
- Lupron Depot [package insert]. Osaka, Japan: Takeda; Revised March 2012.
- Mirena [package insert]. Whippany, NJ: Bayer Healthcare Pharmaceuticals, Inc; Revised August 2020.
- Sangkormkamhang US, Lumbiganon P, Pattanittum P. Progestogens or progestogen-releasing intrauterine systems for uterine fibroids (other than preoperative medical therapy). Cochrane Database Syst Rev. 2020;CD008994. doi: 10.1002/14651858.CD008994.pub3.
- Lysteda [package insert]. Parsippany, NJ: Ferring Pharmaceuticals Inc; Revised October 2013.
- Eder S, Baker J, Gersten J, et al. Efficacy and safety of oral tranexamic acid in women with heavy menstrual bleeding and fibroids. Women’s Health. 2013;9:397-403. doi: 10.2217/whe.13.28.
Solimon AM, Anand SB, Coyne KS, et al. Examining the relationship between symptomatic burden and self-reported productivity losses among patients with uterine fibroids in the United States. J Occup Environ Med. 2017;59:974-981. doi: 10.1097/JOM.0000000000001105.
- Orgovyx [package insert]. Brisbane, CA: Myovant Sciences, Inc; December 2020.
- Al-Hendy A, Lukes AS, Poindexter AN, et al. Treatment of uterine fibroid symptoms with relugolix combination therapy. N Engl J Med. 2021;384:630-642. doi: 10.1056/NEJMoa2008283.
- Solberg LI, Asche SE, Anderson LH, et al. Evaluating preference-sensitive care for uterine fibroids: it’s not so simple. J Women’s Health. 2009;18:1071-1079. doi: 10.1089/jwh.2008.0948.
- Stewart EA. Uterine Fibroids. N Engl J Med. 2015;372:1646-1655. doi: 10.1056/NEJMcp1411029.
- Munro MG, Critchley HO, Broder MS, et al. FIGO Working Group on Menstrual Disorders. FIGO classification system (PALM-COEIN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. Int J Gynaecol Obstet. 2011;113:3-13. doi: 10.1016/j.ijgo.2010.11.011.
- Donnez J, Dolmans MM. Uterine fibroid management: from the present to the future. Hum Reprod Update. 2016;22:665-686. doi: 10.1093/humupd/dmw023.
- Furui T, Imai A, Takagi A, et al. Differential efficacy of gonadotropin-releasing hormone (GnRH) agonist treatment on pedunculated and degenerated myomas: a retrospective study of 630 women. J Obstet Gynaecol. 2000;20:504-506. doi: 10.1080/014436100434703.
- Takeuchi M, Matsuzaki K, Bando Y, et al. Evaluation of red degeneration of uterine leiomyoma with susceptibility-weighted MR imaging. Magn Reson Med Sci. 2019;18:158-162. doi: 10.2463/mrms.mp.2018-0074.
- Schlaff WD, Ackerman RT, Al-Hendy A, et al. Elagolix for heavy menstrual bleeding in women with uterine fibroids. N Engl J Med. 2020;382:328-340. doi: 10.1056/NEJMoa1904351.
- Simon JA, Al-Hendy A, Archer DE, et al. Elagolix treatment for up to 12 months in women with heavy menstrual bleeding and uterine leiomyomas. Obstet Gynecol. 2020;135:1313-1326. doi: 10.1097/AOG.0000000000003869.
- Yao X, Stewart EA, Laughlin-Tommaso SK, et al. Medical therapies for heavy menstrual bleeding in women with uterine fibroids: a retrospective analysis of a large commercially insured population in the USA. BJOG. 2017;124:322-330. doi: 10.1111/1471-0528.14383.
- Zapata LB, Whiteman MK, Tepper NK, et al. Intrauterine device use among women with uterine fibroids: a systematic review. Contraception. 2010;82:41-55. doi: 10.1016/j.contraception.2010.02.011.
- Donnez J, Tomaszewski J, Vazquez F, et al. Ulipristal acetate versus leuprolide acetate for uterine fibroids. N Engl J Med. 2012;366:421-432. doi: 10.1056/NEJMoa1103180.
- Lupron Depot [package insert]. Osaka, Japan: Takeda; Revised March 2012.
- Mirena [package insert]. Whippany, NJ: Bayer Healthcare Pharmaceuticals, Inc; Revised August 2020.
- Sangkormkamhang US, Lumbiganon P, Pattanittum P. Progestogens or progestogen-releasing intrauterine systems for uterine fibroids (other than preoperative medical therapy). Cochrane Database Syst Rev. 2020;CD008994. doi: 10.1002/14651858.CD008994.pub3.
- Lysteda [package insert]. Parsippany, NJ: Ferring Pharmaceuticals Inc; Revised October 2013.
- Eder S, Baker J, Gersten J, et al. Efficacy and safety of oral tranexamic acid in women with heavy menstrual bleeding and fibroids. Women’s Health. 2013;9:397-403. doi: 10.2217/whe.13.28.
Solimon AM, Anand SB, Coyne KS, et al. Examining the relationship between symptomatic burden and self-reported productivity losses among patients with uterine fibroids in the United States. J Occup Environ Med. 2017;59:974-981. doi: 10.1097/JOM.0000000000001105.