User login
Outpatient antibiotics ABRS vs. AOM
Acute bacterial rhinosinusitis (ABRS) has been suggested as a parallel pyogenic infection to acute otitis media (AOM). Like AOM, ABRS is due to obstruction of the normal drainage system into the nasopharynx from a normally aerated pouch(es) within the bone of the skull. Potential pathogens from the nasopharynx, having refluxed into the aerated spaces, begin to replicate and induce inflammation, at least in part due to the obstruction and the inflammation-induced deficiency of the normal cleansing system. For the middle ear, this system is the eustachian tube complex. For the sinuses, it is the osteomeatal complex. The similarities have led some to designate ABRS as "AOM in the middle of the face."
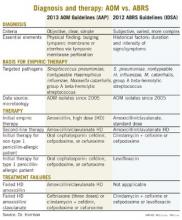
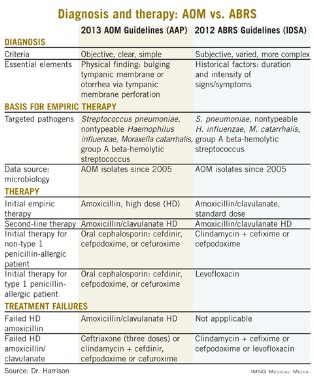
Other parallels are striking, including the microbiology, although 21st century data are less available for the microbiology of ABRS compared with AOM. The table lists some comparisons between the 2013 American Academy of Pediatrics (AAP) guidelines on managing AOM (Pediatrics 2013;131;e964-e999) and the 2012 Infectious Diseases Society of America (IDSA) ABRS guidelines (Clin. Infect. Dis. 2012;54:e72-e112).
So the question arises: Why was high-dose amoxicillin reaffirmed as the drug of choice for uncomplicated AOM in normal hosts in the 2013 AAP AOM guidelines, whereas the most recent guidelines for ABRS (2012 from IDSA) recommend standard-dose amoxicillin plus clavulanate? Amoxicillin is an inexpensive and reasonably palatable drug with a low adverse effect (AE) profile. Amoxicillin-clavulanate is a broader-spectrum, more expensive, somewhat bitter-tasting drug with a moderate AE profile. When the extra spectrum is needed, the added expense and AEs are acceptable. But they seem excessive for a first-line drug.
Do differences in diagnostic criteria lessen the impact on antimicrobial resistance from use of a broader-spectrum first-line drug for ABRS compared to AOM?
Compared with the 2013 AAP otitis media guidelines, which provide objective, clear, and simple criteria, the 2012 IDSA ABRS Guidelines have less objective and less precise criteria. For an AOM diagnosis, the tympanic membrane (TM) must be bulging or be perforated with purulent drainage. Both result from an expanding inflammatory process that stretches the TM. Using this single criterion in the presence of an effusion, clinicians have a clear understanding of what constitutes AOM. No more need to rely on history of acute onset, or a particular color or opacity, or lack of mobility on pneumatic otoscopy. One need only see a bulging TM and note that there is an inflammatory effusion. Bingo – this is AOM.
So, diagnosis of AOM is easier and can be more precise, eliminating "uncertain AOM" from the options. With these firm diagnostic criteria, the question then is whether the AOM episode requires antibiotics. That question is also addressed in the 2013 guidelines and will not be discussed here. The end result is that the 2013 AOM guidelines should decrease the number of AOM diagnoses and thereby antibiotic overuse.
Based on the 2012 IDSA Guideline for ABRS, in contrast, there are three sets of circumstances whereby an ABRS diagnosis can be made. For the most part these involve historical data about duration and intensity of symptoms reported by patients or parents. Thus these are varied, mostly subjective, and more complex with multiple nuances. There is more art and no real reliance on objective physical findings in diagnosing ABRS. This is due to there being no reliable physical findings to diagnose uncomplicated ABRS. There also is no reliable, inexpensive, and safe laboratory or radiological modality for ABRS diagnosis. This results in considerable wiggle room and subjective clinical judgment about the diagnosis.
And the 2012 IDSA ABRS guidelines state that antibiotic treatment should begin whenever an ABRS diagnosis is made. There is some verbiage that one could consider observation without antibiotics if the symptoms are mild, but there are no specifics about what constitutes "mild." This seems like the perfect storm for potential overdiagnosis and overuse of antibiotics, so a broader-spectrum drug would be less desirable from an antibiotic stewardship perspective.
Are pathogens in routine uncomplicated ABRS more resistant to amoxicillin than in AOM so that addition of clavulanate to neutralize beta-lactamase is warranted?
The 2012 ABRS guidelines indicate that the basis for recommending amoxicillin-clavulanate was the microbiology of AOM. There has been little pediatric ABRS microbiology in the past 25 years because sinus punctures are needed to have the best data. Such punctures have not been used in controlled trials in decades. So it is logical to use AOM data, given that pneumococcal conjugate vaccines (PCVs) have produced shifts in pneumococcal serotypes, and there continues to be an evolving distribution of serotypes and their accompanying antibiotic resistance patterns since the 2010 shift to PCV13.
The current expectation is that serotype 19A, the most frequently multidrug-resistant serotype that emerged after PCV7 was introduced in 2000, will decline by the end of 2013. Other classic pneumococcal otopathogen serotypes expressing resistance to amoxicillin have declined since 2004, as has the overall prevalence of AOM due to pneumococcus. Since 2004, more than 50% of recently antibiotic-treated or recurrent AOM appear to be due to nontypeable Haemophilus influenzae (ntHi), and more than half of these produce beta-lactamase. (Pediatr. Infect. Dis. J. 2004;23:829-33; Pediatr. Infect Dis. J. 2010;29:304-9). So more than 25% of recently antibiotic-treated AOM patients would be expected to have amoxicillin-resistant pathogens by virtue of beta-lactamase.
Is this a reasonable rationale for the first-line therapy for both AOM and ABRS to be standard (some would call low) dose, but beta-lactamase stable, amoxicillin-clavulanate at 45 mg/kg per day divided twice daily? This is the argument utilized in the 2012 IDSA ABRS guidelines. However, based on the same data, the AAP 2013 AOM guidelines conclude that high-dose amoxicillin without clavulanate should be used for first-line empiric therapy of AOM.
A powerful argument for the AAP AOM guidelines is the expectation that half of all ntHi, including those that produce beta-lactamase, will spontaneously clear without antibiotics. This is more frequent than for pneumococcus, which has only a 20% spontaneous remission. Data from our laboratory in Kansas City showed that up to 50% of the ntHi in persistent or recurrent AOM produce beta-lactamase; however, less than 15% do so in AOM when not recently treated with antibiotics (Harrison, C.J. The Changing Microbiology of Acute Otitis Media, in "Acute Otitis Media: Translating Science into Clinical Practice," International Congress and Symposium Series. 265:22-35. Royal Society of Medicine Press, London, 2007). How powerful then is the argument to add clavulanate and to use low-dose amoxicillin?
ntHi considered
First consider the contribution to amoxicillin failures by ntHi. Choosing a worst-case scenario of all ABRS having the microbiology of recently treated AOM, we will assume that 60% of persistent/recurrent AOM (and by extrapolation ABRS) is due to ntHi, and 50% of these produce beta-lactamase. Now factor in that 50% of all ntHi clear without antibiotics. The overall expected clinical failure rate for amoxicillin due to beta-lactamase producing ntHi in recurrent/persistent AOM (and by extrapolation ABRS) is 15% (0.6 × 0.5 × 0.5 = 0.15).
In contrast, let us assume that recently untreated ABRS has the same microbiology as recently untreated AOM. Then 45% would be due to ntHi, and 15% of those produce beta-lactamase. Again 50% of all the ntHi spontaneously clear without antibiotics. The expected clinical failure rate for amoxicillin would be 3%-4% due to beta-lactamase–producing ntHi (0.45 × 0.15 × 0.50 = 0.034). This relatively low rate of expected amoxicillin failure for a noninvasive AOM or ABRS pathogen does not seem to mandate addition of clavulanate.
Further, the higher resistance based on beta-lactamase production in ntHi that was quoted in the ABRS 2012 IDSA guidelines were from isolates of children who had tympanocentesis mostly for persistent or recurrent AOM. So, my deduction is that it is logical to use the beta-lactamase–stable drug combination as second-line therapy, that is, in persistent or recurrent AOM and by extrapolation, also in persistent or recurrent ABRS, but not as first-line therapy.
I also am concerned about using a lower dose of amoxicillin because this regimen would be expected to cover less than half of pneumococci with intermediate resistance to penicillin and none with high levels of penicillin resistance. Because pneumococcus is the potentially invasive and yet still common oto- and sinus pathogen, it seems logical to optimize coverage for pneumococcus rather than ntHi in as many young children as possible, particularly those not yet fully PCV13 immunized. This means high-dose amoxicillin, not standard-dose amoxicillin.
This high-dose amoxicillin is what is recommended in the 2013 AAP AOM guidelines. So I feel comfortable, based on the available AOM data, using high-dose amoxicillin (90 mg/kg per day divided in two daily doses) as empiric first-line therapy for non–penicillin-allergic ABRS patients. I would, however, use high-dose amoxicillin-clavulanate as second-line therapy for recurrent or persistent ABRS.
Summary
Most of us wish to follow rules and recommendations from groups of experts who laboriously review the literature and work many hours crafting them. However, sometimes we must remember that such rules are, as was stated in "Pirates of the Caribbean" in regard to "parlay," still only guidelines. When guidelines conflict and practicing clinicians are caught in the middle, we must consider the data and reasons underpinning the conflicting recommendations. Given the AAP AOM 2013 guidelines and examination of the available data, I am comfortable and feel that I am doing my part for antibiotic stewardship by using the same first- and second-line drugs for ABRS as recommended for AOM in the 2013 AOM guidelines.
Dr. Harrison is a professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. Dr. Harrison said he has no relevant financial disclosures.
Acute bacterial rhinosinusitis (ABRS) has been suggested as a parallel pyogenic infection to acute otitis media (AOM). Like AOM, ABRS is due to obstruction of the normal drainage system into the nasopharynx from a normally aerated pouch(es) within the bone of the skull. Potential pathogens from the nasopharynx, having refluxed into the aerated spaces, begin to replicate and induce inflammation, at least in part due to the obstruction and the inflammation-induced deficiency of the normal cleansing system. For the middle ear, this system is the eustachian tube complex. For the sinuses, it is the osteomeatal complex. The similarities have led some to designate ABRS as "AOM in the middle of the face."
Other parallels are striking, including the microbiology, although 21st century data are less available for the microbiology of ABRS compared with AOM. The table lists some comparisons between the 2013 American Academy of Pediatrics (AAP) guidelines on managing AOM (Pediatrics 2013;131;e964-e999) and the 2012 Infectious Diseases Society of America (IDSA) ABRS guidelines (Clin. Infect. Dis. 2012;54:e72-e112).
So the question arises: Why was high-dose amoxicillin reaffirmed as the drug of choice for uncomplicated AOM in normal hosts in the 2013 AAP AOM guidelines, whereas the most recent guidelines for ABRS (2012 from IDSA) recommend standard-dose amoxicillin plus clavulanate? Amoxicillin is an inexpensive and reasonably palatable drug with a low adverse effect (AE) profile. Amoxicillin-clavulanate is a broader-spectrum, more expensive, somewhat bitter-tasting drug with a moderate AE profile. When the extra spectrum is needed, the added expense and AEs are acceptable. But they seem excessive for a first-line drug.
Do differences in diagnostic criteria lessen the impact on antimicrobial resistance from use of a broader-spectrum first-line drug for ABRS compared to AOM?
Compared with the 2013 AAP otitis media guidelines, which provide objective, clear, and simple criteria, the 2012 IDSA ABRS Guidelines have less objective and less precise criteria. For an AOM diagnosis, the tympanic membrane (TM) must be bulging or be perforated with purulent drainage. Both result from an expanding inflammatory process that stretches the TM. Using this single criterion in the presence of an effusion, clinicians have a clear understanding of what constitutes AOM. No more need to rely on history of acute onset, or a particular color or opacity, or lack of mobility on pneumatic otoscopy. One need only see a bulging TM and note that there is an inflammatory effusion. Bingo – this is AOM.
So, diagnosis of AOM is easier and can be more precise, eliminating "uncertain AOM" from the options. With these firm diagnostic criteria, the question then is whether the AOM episode requires antibiotics. That question is also addressed in the 2013 guidelines and will not be discussed here. The end result is that the 2013 AOM guidelines should decrease the number of AOM diagnoses and thereby antibiotic overuse.
Based on the 2012 IDSA Guideline for ABRS, in contrast, there are three sets of circumstances whereby an ABRS diagnosis can be made. For the most part these involve historical data about duration and intensity of symptoms reported by patients or parents. Thus these are varied, mostly subjective, and more complex with multiple nuances. There is more art and no real reliance on objective physical findings in diagnosing ABRS. This is due to there being no reliable physical findings to diagnose uncomplicated ABRS. There also is no reliable, inexpensive, and safe laboratory or radiological modality for ABRS diagnosis. This results in considerable wiggle room and subjective clinical judgment about the diagnosis.
And the 2012 IDSA ABRS guidelines state that antibiotic treatment should begin whenever an ABRS diagnosis is made. There is some verbiage that one could consider observation without antibiotics if the symptoms are mild, but there are no specifics about what constitutes "mild." This seems like the perfect storm for potential overdiagnosis and overuse of antibiotics, so a broader-spectrum drug would be less desirable from an antibiotic stewardship perspective.
Are pathogens in routine uncomplicated ABRS more resistant to amoxicillin than in AOM so that addition of clavulanate to neutralize beta-lactamase is warranted?
The 2012 ABRS guidelines indicate that the basis for recommending amoxicillin-clavulanate was the microbiology of AOM. There has been little pediatric ABRS microbiology in the past 25 years because sinus punctures are needed to have the best data. Such punctures have not been used in controlled trials in decades. So it is logical to use AOM data, given that pneumococcal conjugate vaccines (PCVs) have produced shifts in pneumococcal serotypes, and there continues to be an evolving distribution of serotypes and their accompanying antibiotic resistance patterns since the 2010 shift to PCV13.
The current expectation is that serotype 19A, the most frequently multidrug-resistant serotype that emerged after PCV7 was introduced in 2000, will decline by the end of 2013. Other classic pneumococcal otopathogen serotypes expressing resistance to amoxicillin have declined since 2004, as has the overall prevalence of AOM due to pneumococcus. Since 2004, more than 50% of recently antibiotic-treated or recurrent AOM appear to be due to nontypeable Haemophilus influenzae (ntHi), and more than half of these produce beta-lactamase. (Pediatr. Infect. Dis. J. 2004;23:829-33; Pediatr. Infect Dis. J. 2010;29:304-9). So more than 25% of recently antibiotic-treated AOM patients would be expected to have amoxicillin-resistant pathogens by virtue of beta-lactamase.
Is this a reasonable rationale for the first-line therapy for both AOM and ABRS to be standard (some would call low) dose, but beta-lactamase stable, amoxicillin-clavulanate at 45 mg/kg per day divided twice daily? This is the argument utilized in the 2012 IDSA ABRS guidelines. However, based on the same data, the AAP 2013 AOM guidelines conclude that high-dose amoxicillin without clavulanate should be used for first-line empiric therapy of AOM.
A powerful argument for the AAP AOM guidelines is the expectation that half of all ntHi, including those that produce beta-lactamase, will spontaneously clear without antibiotics. This is more frequent than for pneumococcus, which has only a 20% spontaneous remission. Data from our laboratory in Kansas City showed that up to 50% of the ntHi in persistent or recurrent AOM produce beta-lactamase; however, less than 15% do so in AOM when not recently treated with antibiotics (Harrison, C.J. The Changing Microbiology of Acute Otitis Media, in "Acute Otitis Media: Translating Science into Clinical Practice," International Congress and Symposium Series. 265:22-35. Royal Society of Medicine Press, London, 2007). How powerful then is the argument to add clavulanate and to use low-dose amoxicillin?
ntHi considered
First consider the contribution to amoxicillin failures by ntHi. Choosing a worst-case scenario of all ABRS having the microbiology of recently treated AOM, we will assume that 60% of persistent/recurrent AOM (and by extrapolation ABRS) is due to ntHi, and 50% of these produce beta-lactamase. Now factor in that 50% of all ntHi clear without antibiotics. The overall expected clinical failure rate for amoxicillin due to beta-lactamase producing ntHi in recurrent/persistent AOM (and by extrapolation ABRS) is 15% (0.6 × 0.5 × 0.5 = 0.15).
In contrast, let us assume that recently untreated ABRS has the same microbiology as recently untreated AOM. Then 45% would be due to ntHi, and 15% of those produce beta-lactamase. Again 50% of all the ntHi spontaneously clear without antibiotics. The expected clinical failure rate for amoxicillin would be 3%-4% due to beta-lactamase–producing ntHi (0.45 × 0.15 × 0.50 = 0.034). This relatively low rate of expected amoxicillin failure for a noninvasive AOM or ABRS pathogen does not seem to mandate addition of clavulanate.
Further, the higher resistance based on beta-lactamase production in ntHi that was quoted in the ABRS 2012 IDSA guidelines were from isolates of children who had tympanocentesis mostly for persistent or recurrent AOM. So, my deduction is that it is logical to use the beta-lactamase–stable drug combination as second-line therapy, that is, in persistent or recurrent AOM and by extrapolation, also in persistent or recurrent ABRS, but not as first-line therapy.
I also am concerned about using a lower dose of amoxicillin because this regimen would be expected to cover less than half of pneumococci with intermediate resistance to penicillin and none with high levels of penicillin resistance. Because pneumococcus is the potentially invasive and yet still common oto- and sinus pathogen, it seems logical to optimize coverage for pneumococcus rather than ntHi in as many young children as possible, particularly those not yet fully PCV13 immunized. This means high-dose amoxicillin, not standard-dose amoxicillin.
This high-dose amoxicillin is what is recommended in the 2013 AAP AOM guidelines. So I feel comfortable, based on the available AOM data, using high-dose amoxicillin (90 mg/kg per day divided in two daily doses) as empiric first-line therapy for non–penicillin-allergic ABRS patients. I would, however, use high-dose amoxicillin-clavulanate as second-line therapy for recurrent or persistent ABRS.
Summary
Most of us wish to follow rules and recommendations from groups of experts who laboriously review the literature and work many hours crafting them. However, sometimes we must remember that such rules are, as was stated in "Pirates of the Caribbean" in regard to "parlay," still only guidelines. When guidelines conflict and practicing clinicians are caught in the middle, we must consider the data and reasons underpinning the conflicting recommendations. Given the AAP AOM 2013 guidelines and examination of the available data, I am comfortable and feel that I am doing my part for antibiotic stewardship by using the same first- and second-line drugs for ABRS as recommended for AOM in the 2013 AOM guidelines.
Dr. Harrison is a professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. Dr. Harrison said he has no relevant financial disclosures.
Acute bacterial rhinosinusitis (ABRS) has been suggested as a parallel pyogenic infection to acute otitis media (AOM). Like AOM, ABRS is due to obstruction of the normal drainage system into the nasopharynx from a normally aerated pouch(es) within the bone of the skull. Potential pathogens from the nasopharynx, having refluxed into the aerated spaces, begin to replicate and induce inflammation, at least in part due to the obstruction and the inflammation-induced deficiency of the normal cleansing system. For the middle ear, this system is the eustachian tube complex. For the sinuses, it is the osteomeatal complex. The similarities have led some to designate ABRS as "AOM in the middle of the face."
Other parallels are striking, including the microbiology, although 21st century data are less available for the microbiology of ABRS compared with AOM. The table lists some comparisons between the 2013 American Academy of Pediatrics (AAP) guidelines on managing AOM (Pediatrics 2013;131;e964-e999) and the 2012 Infectious Diseases Society of America (IDSA) ABRS guidelines (Clin. Infect. Dis. 2012;54:e72-e112).
So the question arises: Why was high-dose amoxicillin reaffirmed as the drug of choice for uncomplicated AOM in normal hosts in the 2013 AAP AOM guidelines, whereas the most recent guidelines for ABRS (2012 from IDSA) recommend standard-dose amoxicillin plus clavulanate? Amoxicillin is an inexpensive and reasonably palatable drug with a low adverse effect (AE) profile. Amoxicillin-clavulanate is a broader-spectrum, more expensive, somewhat bitter-tasting drug with a moderate AE profile. When the extra spectrum is needed, the added expense and AEs are acceptable. But they seem excessive for a first-line drug.
Do differences in diagnostic criteria lessen the impact on antimicrobial resistance from use of a broader-spectrum first-line drug for ABRS compared to AOM?
Compared with the 2013 AAP otitis media guidelines, which provide objective, clear, and simple criteria, the 2012 IDSA ABRS Guidelines have less objective and less precise criteria. For an AOM diagnosis, the tympanic membrane (TM) must be bulging or be perforated with purulent drainage. Both result from an expanding inflammatory process that stretches the TM. Using this single criterion in the presence of an effusion, clinicians have a clear understanding of what constitutes AOM. No more need to rely on history of acute onset, or a particular color or opacity, or lack of mobility on pneumatic otoscopy. One need only see a bulging TM and note that there is an inflammatory effusion. Bingo – this is AOM.
So, diagnosis of AOM is easier and can be more precise, eliminating "uncertain AOM" from the options. With these firm diagnostic criteria, the question then is whether the AOM episode requires antibiotics. That question is also addressed in the 2013 guidelines and will not be discussed here. The end result is that the 2013 AOM guidelines should decrease the number of AOM diagnoses and thereby antibiotic overuse.
Based on the 2012 IDSA Guideline for ABRS, in contrast, there are three sets of circumstances whereby an ABRS diagnosis can be made. For the most part these involve historical data about duration and intensity of symptoms reported by patients or parents. Thus these are varied, mostly subjective, and more complex with multiple nuances. There is more art and no real reliance on objective physical findings in diagnosing ABRS. This is due to there being no reliable physical findings to diagnose uncomplicated ABRS. There also is no reliable, inexpensive, and safe laboratory or radiological modality for ABRS diagnosis. This results in considerable wiggle room and subjective clinical judgment about the diagnosis.
And the 2012 IDSA ABRS guidelines state that antibiotic treatment should begin whenever an ABRS diagnosis is made. There is some verbiage that one could consider observation without antibiotics if the symptoms are mild, but there are no specifics about what constitutes "mild." This seems like the perfect storm for potential overdiagnosis and overuse of antibiotics, so a broader-spectrum drug would be less desirable from an antibiotic stewardship perspective.
Are pathogens in routine uncomplicated ABRS more resistant to amoxicillin than in AOM so that addition of clavulanate to neutralize beta-lactamase is warranted?
The 2012 ABRS guidelines indicate that the basis for recommending amoxicillin-clavulanate was the microbiology of AOM. There has been little pediatric ABRS microbiology in the past 25 years because sinus punctures are needed to have the best data. Such punctures have not been used in controlled trials in decades. So it is logical to use AOM data, given that pneumococcal conjugate vaccines (PCVs) have produced shifts in pneumococcal serotypes, and there continues to be an evolving distribution of serotypes and their accompanying antibiotic resistance patterns since the 2010 shift to PCV13.
The current expectation is that serotype 19A, the most frequently multidrug-resistant serotype that emerged after PCV7 was introduced in 2000, will decline by the end of 2013. Other classic pneumococcal otopathogen serotypes expressing resistance to amoxicillin have declined since 2004, as has the overall prevalence of AOM due to pneumococcus. Since 2004, more than 50% of recently antibiotic-treated or recurrent AOM appear to be due to nontypeable Haemophilus influenzae (ntHi), and more than half of these produce beta-lactamase. (Pediatr. Infect. Dis. J. 2004;23:829-33; Pediatr. Infect Dis. J. 2010;29:304-9). So more than 25% of recently antibiotic-treated AOM patients would be expected to have amoxicillin-resistant pathogens by virtue of beta-lactamase.
Is this a reasonable rationale for the first-line therapy for both AOM and ABRS to be standard (some would call low) dose, but beta-lactamase stable, amoxicillin-clavulanate at 45 mg/kg per day divided twice daily? This is the argument utilized in the 2012 IDSA ABRS guidelines. However, based on the same data, the AAP 2013 AOM guidelines conclude that high-dose amoxicillin without clavulanate should be used for first-line empiric therapy of AOM.
A powerful argument for the AAP AOM guidelines is the expectation that half of all ntHi, including those that produce beta-lactamase, will spontaneously clear without antibiotics. This is more frequent than for pneumococcus, which has only a 20% spontaneous remission. Data from our laboratory in Kansas City showed that up to 50% of the ntHi in persistent or recurrent AOM produce beta-lactamase; however, less than 15% do so in AOM when not recently treated with antibiotics (Harrison, C.J. The Changing Microbiology of Acute Otitis Media, in "Acute Otitis Media: Translating Science into Clinical Practice," International Congress and Symposium Series. 265:22-35. Royal Society of Medicine Press, London, 2007). How powerful then is the argument to add clavulanate and to use low-dose amoxicillin?
ntHi considered
First consider the contribution to amoxicillin failures by ntHi. Choosing a worst-case scenario of all ABRS having the microbiology of recently treated AOM, we will assume that 60% of persistent/recurrent AOM (and by extrapolation ABRS) is due to ntHi, and 50% of these produce beta-lactamase. Now factor in that 50% of all ntHi clear without antibiotics. The overall expected clinical failure rate for amoxicillin due to beta-lactamase producing ntHi in recurrent/persistent AOM (and by extrapolation ABRS) is 15% (0.6 × 0.5 × 0.5 = 0.15).
In contrast, let us assume that recently untreated ABRS has the same microbiology as recently untreated AOM. Then 45% would be due to ntHi, and 15% of those produce beta-lactamase. Again 50% of all the ntHi spontaneously clear without antibiotics. The expected clinical failure rate for amoxicillin would be 3%-4% due to beta-lactamase–producing ntHi (0.45 × 0.15 × 0.50 = 0.034). This relatively low rate of expected amoxicillin failure for a noninvasive AOM or ABRS pathogen does not seem to mandate addition of clavulanate.
Further, the higher resistance based on beta-lactamase production in ntHi that was quoted in the ABRS 2012 IDSA guidelines were from isolates of children who had tympanocentesis mostly for persistent or recurrent AOM. So, my deduction is that it is logical to use the beta-lactamase–stable drug combination as second-line therapy, that is, in persistent or recurrent AOM and by extrapolation, also in persistent or recurrent ABRS, but not as first-line therapy.
I also am concerned about using a lower dose of amoxicillin because this regimen would be expected to cover less than half of pneumococci with intermediate resistance to penicillin and none with high levels of penicillin resistance. Because pneumococcus is the potentially invasive and yet still common oto- and sinus pathogen, it seems logical to optimize coverage for pneumococcus rather than ntHi in as many young children as possible, particularly those not yet fully PCV13 immunized. This means high-dose amoxicillin, not standard-dose amoxicillin.
This high-dose amoxicillin is what is recommended in the 2013 AAP AOM guidelines. So I feel comfortable, based on the available AOM data, using high-dose amoxicillin (90 mg/kg per day divided in two daily doses) as empiric first-line therapy for non–penicillin-allergic ABRS patients. I would, however, use high-dose amoxicillin-clavulanate as second-line therapy for recurrent or persistent ABRS.
Summary
Most of us wish to follow rules and recommendations from groups of experts who laboriously review the literature and work many hours crafting them. However, sometimes we must remember that such rules are, as was stated in "Pirates of the Caribbean" in regard to "parlay," still only guidelines. When guidelines conflict and practicing clinicians are caught in the middle, we must consider the data and reasons underpinning the conflicting recommendations. Given the AAP AOM 2013 guidelines and examination of the available data, I am comfortable and feel that I am doing my part for antibiotic stewardship by using the same first- and second-line drugs for ABRS as recommended for AOM in the 2013 AOM guidelines.
Dr. Harrison is a professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. Dr. Harrison said he has no relevant financial disclosures.
Chronic suppurative otitis media
Chronic suppurative otitis media remains a global burden for children despite the declining incidence in industrialized countries and advances in diagnosis and management in developing countries. The World Health Organization cites chronic suppurative otitis media (CSOM) as a major cause of acquired hearing loss, primarily in developing countries and indigenous peoples.
CSOM is characterized by a persistent discharge from the middle ear lasting for a minimum of 2 weeks. In industrialized countries, the major risk factor is tympanostomy tube placement; in developing nations, the major risk factor is early bacterial colonization with Streptococcus pneumoniae and nontypable Haemophilus influenzae and early onset of acute bacterial otitis media with perforation. In both situations, biofilms are thought to underlie the pathogenesis with S. pneumoniae and nontypable H. influenzae found on mucosal biopsies using specific fluorescent in situ hybridization assays on specimens from children with chronic suppurative otitis media or recurrent acute otitis media (ROM). Dr. Ruth B. Thornton and her colleagues reported that 11 of 17 (65%) middle-ear mucosal biopsies from children with CSOM or ROM showed evidence of bacterial biofilm, and 12 (71%) demonstrated intracellular bacteria (Pediatrics 2011;11:94).
Microbiologic studies in children with otorrhea, through either a perforation or tympanostomy tube, demonstrate primarily Staphylococcus aureus, both methicillin sensitive and resistant isolates, and Pseudomonas aeruginosa. However, it is recognized that the early pathogens are S. pneumoniae and nontypable H. influenzae in these children recovered both from cultures of ear drainage and from molecular studies of middle-ear mucosal biopsies. Amanda J. Leach, Ph.D., and Peter S. Morris, Ph.D., reported that cultures from ear discharge in Aborigine children with acute perforations identified nontypable H. influenzae in 57%, S. pneumoniae in 34%, and both in 21% (Pediatr. Inf. Dis. J. 2007;26:S4-7).
The high rate of mixed infection has also been reported in Bedouin children with recurrent and persistent otitis. In children with otorrhea from a tympanostomy tube, a dichotomy in microbiology etiology was found. In young children, nasopharyngeal pathogens (S. pneumoniae and nontypable H. influenzae) dominated and in older children, external ear commensals (Staph. aureus and P. aeruginosa) predominated (Int. J. Pediatr. Otorhinolaryngol. 2003;67:1317-23).
In industrialized countries, successful treatment of young children with otorrhea through a tympanostomy tube has been reported with both oral amoxicillin/clavulanate and topical fluoroquinolones, reflecting the frequent role of S. pneumoniae and nontypable H. influenzae in young children. However, in older children, in those with foul-smelling discharge, and in those who fail amoxicillin/clavulanate, topical fluoroquinolone is the treatment of choice. Guidelines for the treatment of otorrhea through a tympanostomy tube have been published with a recommendation that topical therapy be used as the first choice when systemic signs of illness are not present (J. Otolaryngol. 2005;34[suppl. 2]:S60-3). Treatment failures are most often due to methicillin-resistant Staph. aureus (MRSA) and often require a combination of oral therapy with an agent active against MRSA such as trimethoprim/sulfamethoxazole and topical therapy with a fluoroquinolone; removal of the tympanostomy tube also may be necessary to achieve a cure.
The prevention of chronic suppurative otitis media has proven elusive. Studies of 7-valent pneumococcal conjugate (PCV7) vaccine in Dutch children with established ROM demonstrated no reduction in episodes. In fact, more episodes of AOM or otorrhea were observed in the vaccine group, despite good immunogenicity and a reduction in colonization with vaccine-type S. pneumoniae (Int. J. Pediatr. Otorhinolaryngol. 2006;70:275-85).
In studies of PCV7 administered at 2, 4, and 6 months of age to Aborigine infants, only a marginal benefit was observed when they were compared with a historical birth cohort. By 12 months of age, 89% of those vaccinated had experienced AOM; 34%, AOM with perforation; and 14%, CSOM. Although not statistically significant, this represented a 40% decrease in CSOM at 1 year of age (BMC Pediatr. 2009;9:14).
CSOM persists as an important cause of morbidity in indigenous children and in children in developing countries. It is a major cause of acquired hearing loss and impacts dramatically on the quality of life of affected children. We have made important advances in identifying the bacterial antecedents and understanding the pathogenesis of disease, yet morbidity remains substantial. Further research in the treatment and prevention of middle-ear biofilms is likely to be critical to reducing the burden of ear disease in children.
Dr. Pelton is chief of pediatric infectious disease and also is the coordinator of the maternal-child HIV program at Boston Medical Center. He disclosed that he has received honoraria and investigator-initiated research funding from Pfizer and Merck, and honoraria from GlaxoSmithKline related to pneumococcal vaccines. E-mail him at [email protected].
Chronic suppurative otitis media remains a global burden for children despite the declining incidence in industrialized countries and advances in diagnosis and management in developing countries. The World Health Organization cites chronic suppurative otitis media (CSOM) as a major cause of acquired hearing loss, primarily in developing countries and indigenous peoples.
CSOM is characterized by a persistent discharge from the middle ear lasting for a minimum of 2 weeks. In industrialized countries, the major risk factor is tympanostomy tube placement; in developing nations, the major risk factor is early bacterial colonization with Streptococcus pneumoniae and nontypable Haemophilus influenzae and early onset of acute bacterial otitis media with perforation. In both situations, biofilms are thought to underlie the pathogenesis with S. pneumoniae and nontypable H. influenzae found on mucosal biopsies using specific fluorescent in situ hybridization assays on specimens from children with chronic suppurative otitis media or recurrent acute otitis media (ROM). Dr. Ruth B. Thornton and her colleagues reported that 11 of 17 (65%) middle-ear mucosal biopsies from children with CSOM or ROM showed evidence of bacterial biofilm, and 12 (71%) demonstrated intracellular bacteria (Pediatrics 2011;11:94).
Microbiologic studies in children with otorrhea, through either a perforation or tympanostomy tube, demonstrate primarily Staphylococcus aureus, both methicillin sensitive and resistant isolates, and Pseudomonas aeruginosa. However, it is recognized that the early pathogens are S. pneumoniae and nontypable H. influenzae in these children recovered both from cultures of ear drainage and from molecular studies of middle-ear mucosal biopsies. Amanda J. Leach, Ph.D., and Peter S. Morris, Ph.D., reported that cultures from ear discharge in Aborigine children with acute perforations identified nontypable H. influenzae in 57%, S. pneumoniae in 34%, and both in 21% (Pediatr. Inf. Dis. J. 2007;26:S4-7).
The high rate of mixed infection has also been reported in Bedouin children with recurrent and persistent otitis. In children with otorrhea from a tympanostomy tube, a dichotomy in microbiology etiology was found. In young children, nasopharyngeal pathogens (S. pneumoniae and nontypable H. influenzae) dominated and in older children, external ear commensals (Staph. aureus and P. aeruginosa) predominated (Int. J. Pediatr. Otorhinolaryngol. 2003;67:1317-23).
In industrialized countries, successful treatment of young children with otorrhea through a tympanostomy tube has been reported with both oral amoxicillin/clavulanate and topical fluoroquinolones, reflecting the frequent role of S. pneumoniae and nontypable H. influenzae in young children. However, in older children, in those with foul-smelling discharge, and in those who fail amoxicillin/clavulanate, topical fluoroquinolone is the treatment of choice. Guidelines for the treatment of otorrhea through a tympanostomy tube have been published with a recommendation that topical therapy be used as the first choice when systemic signs of illness are not present (J. Otolaryngol. 2005;34[suppl. 2]:S60-3). Treatment failures are most often due to methicillin-resistant Staph. aureus (MRSA) and often require a combination of oral therapy with an agent active against MRSA such as trimethoprim/sulfamethoxazole and topical therapy with a fluoroquinolone; removal of the tympanostomy tube also may be necessary to achieve a cure.
The prevention of chronic suppurative otitis media has proven elusive. Studies of 7-valent pneumococcal conjugate (PCV7) vaccine in Dutch children with established ROM demonstrated no reduction in episodes. In fact, more episodes of AOM or otorrhea were observed in the vaccine group, despite good immunogenicity and a reduction in colonization with vaccine-type S. pneumoniae (Int. J. Pediatr. Otorhinolaryngol. 2006;70:275-85).
In studies of PCV7 administered at 2, 4, and 6 months of age to Aborigine infants, only a marginal benefit was observed when they were compared with a historical birth cohort. By 12 months of age, 89% of those vaccinated had experienced AOM; 34%, AOM with perforation; and 14%, CSOM. Although not statistically significant, this represented a 40% decrease in CSOM at 1 year of age (BMC Pediatr. 2009;9:14).
CSOM persists as an important cause of morbidity in indigenous children and in children in developing countries. It is a major cause of acquired hearing loss and impacts dramatically on the quality of life of affected children. We have made important advances in identifying the bacterial antecedents and understanding the pathogenesis of disease, yet morbidity remains substantial. Further research in the treatment and prevention of middle-ear biofilms is likely to be critical to reducing the burden of ear disease in children.
Dr. Pelton is chief of pediatric infectious disease and also is the coordinator of the maternal-child HIV program at Boston Medical Center. He disclosed that he has received honoraria and investigator-initiated research funding from Pfizer and Merck, and honoraria from GlaxoSmithKline related to pneumococcal vaccines. E-mail him at [email protected].
Chronic suppurative otitis media remains a global burden for children despite the declining incidence in industrialized countries and advances in diagnosis and management in developing countries. The World Health Organization cites chronic suppurative otitis media (CSOM) as a major cause of acquired hearing loss, primarily in developing countries and indigenous peoples.
CSOM is characterized by a persistent discharge from the middle ear lasting for a minimum of 2 weeks. In industrialized countries, the major risk factor is tympanostomy tube placement; in developing nations, the major risk factor is early bacterial colonization with Streptococcus pneumoniae and nontypable Haemophilus influenzae and early onset of acute bacterial otitis media with perforation. In both situations, biofilms are thought to underlie the pathogenesis with S. pneumoniae and nontypable H. influenzae found on mucosal biopsies using specific fluorescent in situ hybridization assays on specimens from children with chronic suppurative otitis media or recurrent acute otitis media (ROM). Dr. Ruth B. Thornton and her colleagues reported that 11 of 17 (65%) middle-ear mucosal biopsies from children with CSOM or ROM showed evidence of bacterial biofilm, and 12 (71%) demonstrated intracellular bacteria (Pediatrics 2011;11:94).
Microbiologic studies in children with otorrhea, through either a perforation or tympanostomy tube, demonstrate primarily Staphylococcus aureus, both methicillin sensitive and resistant isolates, and Pseudomonas aeruginosa. However, it is recognized that the early pathogens are S. pneumoniae and nontypable H. influenzae in these children recovered both from cultures of ear drainage and from molecular studies of middle-ear mucosal biopsies. Amanda J. Leach, Ph.D., and Peter S. Morris, Ph.D., reported that cultures from ear discharge in Aborigine children with acute perforations identified nontypable H. influenzae in 57%, S. pneumoniae in 34%, and both in 21% (Pediatr. Inf. Dis. J. 2007;26:S4-7).
The high rate of mixed infection has also been reported in Bedouin children with recurrent and persistent otitis. In children with otorrhea from a tympanostomy tube, a dichotomy in microbiology etiology was found. In young children, nasopharyngeal pathogens (S. pneumoniae and nontypable H. influenzae) dominated and in older children, external ear commensals (Staph. aureus and P. aeruginosa) predominated (Int. J. Pediatr. Otorhinolaryngol. 2003;67:1317-23).
In industrialized countries, successful treatment of young children with otorrhea through a tympanostomy tube has been reported with both oral amoxicillin/clavulanate and topical fluoroquinolones, reflecting the frequent role of S. pneumoniae and nontypable H. influenzae in young children. However, in older children, in those with foul-smelling discharge, and in those who fail amoxicillin/clavulanate, topical fluoroquinolone is the treatment of choice. Guidelines for the treatment of otorrhea through a tympanostomy tube have been published with a recommendation that topical therapy be used as the first choice when systemic signs of illness are not present (J. Otolaryngol. 2005;34[suppl. 2]:S60-3). Treatment failures are most often due to methicillin-resistant Staph. aureus (MRSA) and often require a combination of oral therapy with an agent active against MRSA such as trimethoprim/sulfamethoxazole and topical therapy with a fluoroquinolone; removal of the tympanostomy tube also may be necessary to achieve a cure.
The prevention of chronic suppurative otitis media has proven elusive. Studies of 7-valent pneumococcal conjugate (PCV7) vaccine in Dutch children with established ROM demonstrated no reduction in episodes. In fact, more episodes of AOM or otorrhea were observed in the vaccine group, despite good immunogenicity and a reduction in colonization with vaccine-type S. pneumoniae (Int. J. Pediatr. Otorhinolaryngol. 2006;70:275-85).
In studies of PCV7 administered at 2, 4, and 6 months of age to Aborigine infants, only a marginal benefit was observed when they were compared with a historical birth cohort. By 12 months of age, 89% of those vaccinated had experienced AOM; 34%, AOM with perforation; and 14%, CSOM. Although not statistically significant, this represented a 40% decrease in CSOM at 1 year of age (BMC Pediatr. 2009;9:14).
CSOM persists as an important cause of morbidity in indigenous children and in children in developing countries. It is a major cause of acquired hearing loss and impacts dramatically on the quality of life of affected children. We have made important advances in identifying the bacterial antecedents and understanding the pathogenesis of disease, yet morbidity remains substantial. Further research in the treatment and prevention of middle-ear biofilms is likely to be critical to reducing the burden of ear disease in children.
Dr. Pelton is chief of pediatric infectious disease and also is the coordinator of the maternal-child HIV program at Boston Medical Center. He disclosed that he has received honoraria and investigator-initiated research funding from Pfizer and Merck, and honoraria from GlaxoSmithKline related to pneumococcal vaccines. E-mail him at [email protected].
ID Predictions for 2013
It is that time of year when 2013 predictions come your way, with insights into upcoming changes and/or developments in the specialty of pediatric infectious diseases. The theme this year: drugs, bugs, and the new immunization schedule.
Antimicrobial resistance for Gram negative organisms will reach new heights in 2013, new antibiotics will not likely appear on the market, and you will see an increase in emphasis on judicious antibiotic use in other venues such as the animal industry.
Particularly worrisome is the increased rate of hospital acquired carbapenem-resistant Klebsiella pneumoniae infections as few good therapeutic options currently exist for these pathogens. Judicious use of antibiotics in all instances is key, and pediatricians should particularly focus on their practice patterns for common infections (streptococcal pharyngitis, otitis media, and sinusitis), and avoiding antibiotics for upper respiratory infections and bronchitis.
The United States is the fifth greatest user of antibiotics in the world (France, Greece, Italy, and Belgium exceed us), and Kentucky, West Virginia, Tennessee, Mississippi, and Louisiana are the states with the highest use. Check out the map of this data to see antibiotic use for your state.
The winter scourge of rotavirus infection has virtually disappeared following the introduction of rotavirus vaccine but two diarrheal pathogens you’ll likely hear more about in 2013 are norovirus and cryptosporidia.
Norovirus (think cruise ship diarrhea) moves front and center as the most important cause of diarrheal outbreaks in the United States. While foodborne disease occurs, most outbreaks relate to person-to-person transmission, and you are most likely to see disease this time of year (November through April). This might be explained by the fact that infected individuals shed billions of norovirus particles, and it only takes 18 particles to infect another, plus folks are more likely to be closely quartered in winter months.
In terms of cryptosporidiosis, famous outbreaks have followed contamination of drinking water, and sporadic cases are often seen in summer following recreational water exposure. While self-limited in the healthy child, cryptosporidiosis is hard to treat and causes significant morbidity in immunocompromised individuals, such as organ transplant patients. Pediatricians should alert parents to the risk related to recreational water exposure for high-risk patients who should avoid ingesting such water, and particularly avoid pools where diapered children may contaminate the water.
Speaking of diarrhea, as rates for Clostridium difficile associated disease (CDAD) in children have been increasing over the last decade, I suspect clinicians will need to gain a better understanding of the specifics regarding newer C. difficile tests. Many institutions have gone to molecular assays. Polymerase chain reaction (PCR) testing, for instance, has been introduced, which is very sensitive, and doubled the rate of positivity (compared with enzyme immunoassay) in some studies. We know that asymptomatic carriage of C. difficile is common in infants younger than 12 months of age, but several studies suggest that 25%-33% of 0- to 36-month control patients had stools that were positive for C. difficile toxin. Take a highly sensitive test, high rates of asymptomatic colonization, and the overall low prevalence CDAD, and you are likely to see diagnosis and treatment instituted inappropriately in some cases. The key to diagnosis of CDAD is to perform testing only on liquid stools and to make sure that other etiologies of diarrhea have been excluded in those less than 3 years of age. Don’t test young infants younger than 1 year (unless they have Hirschsprung’s disease), and do not perform tests to check for cure. See the new guideline published in the January issue of Pediatrics (2013; 131:196-200).
We may still be months away from knowing the full extent of the 2012 national fungal meningitis outbreak; however, based on what we know now, there is a clear need for legislation to ensure safe practices in compounding pharmacies, and I predict this will come in 2013. The first case of fungal meningitis cases was reported Sept. 18, 2012, in a man in Tennessee, and within a week, seven other cases were diagnosed; all had epidural steroid injections at the same center (N. Engl. J. Med. 2012 Dec. 19 [doi: 10.1056/NEJMoa1213978]).
Since then, a Centers for Disease Control and Prevention investigation has found that more than 600 infected patients and 39 patients have died. Three lots of methylprednisolone products from a compounding pharmacy in New England were found to be the source, and the CDC investigation found that more than 14,000 individuals in 70-plus clinics in 22 states were exposed to the products, mostly adult patients with chronic back pain. The organism in all but one case is an unusual environmental fungus (Exserohilum rostratum) that likely was introduced into the products during drug preparation. The Food and Drug Administration has since inspected the company’s processing room and noted a number of different issues that may have resulted in contamination. Products have been recalled from the implicated pharmacy (New England Compounding Center), and a sister pharmacy (Ameridose) has voluntarily recalled its products. This is not the first time that an outbreak has been tracked to contamination at a compounding pharmacies, but the extent of this outbreak emphasizes the need for definitive action to prevent this from ever happening again.
The 2013 Immunization Schedule will be out soon, and I predict practitioners may be happy to see a comprehensive footnote table, a harmonized schedule for those 0-18 years, and separate tables for the high-risk patient and for those requiring catch-up schedules.
In terms of vaccines, an important goal for practitioners may be to increase vaccine coverage in teens. Human papillomavirus (HPV) coverage rates are still dismal; 35% of girls and 1% of boys completed three vaccines in 2011, according to the National Immunization Survey–Teen. Parents who refused HPV vaccines in their daughters more likely cited safety concerns, but those who refused for their sons were more likely not to be aware of the recommendation for vaccination, according to data from the NIS-Teen. Geographic disparities also have been noted, with the southeastern U.S. states having lowest rates for immunization and some of the highest rates for cervical cancer. Recommend HPV vaccine every time another teen platform vaccine is recommended, and use a standing order in your practice so every encounter is an opportunity to immunize.
I wish you blessings in the coming year and hope that at least some of my predictions have utility for those of you in practice.
Dr. Jackson is the chief of infectious diseases at Children’s Mercy Hospitals and Clinics in Kansas City, Mo., and professor of pediatrics at the University of Missouri–Kansas City. She said she has no relevant financial disclosures. E-mail her at [email protected].
It is that time of year when 2013 predictions come your way, with insights into upcoming changes and/or developments in the specialty of pediatric infectious diseases. The theme this year: drugs, bugs, and the new immunization schedule.
Antimicrobial resistance for Gram negative organisms will reach new heights in 2013, new antibiotics will not likely appear on the market, and you will see an increase in emphasis on judicious antibiotic use in other venues such as the animal industry.
Particularly worrisome is the increased rate of hospital acquired carbapenem-resistant Klebsiella pneumoniae infections as few good therapeutic options currently exist for these pathogens. Judicious use of antibiotics in all instances is key, and pediatricians should particularly focus on their practice patterns for common infections (streptococcal pharyngitis, otitis media, and sinusitis), and avoiding antibiotics for upper respiratory infections and bronchitis.
The United States is the fifth greatest user of antibiotics in the world (France, Greece, Italy, and Belgium exceed us), and Kentucky, West Virginia, Tennessee, Mississippi, and Louisiana are the states with the highest use. Check out the map of this data to see antibiotic use for your state.
The winter scourge of rotavirus infection has virtually disappeared following the introduction of rotavirus vaccine but two diarrheal pathogens you’ll likely hear more about in 2013 are norovirus and cryptosporidia.
Norovirus (think cruise ship diarrhea) moves front and center as the most important cause of diarrheal outbreaks in the United States. While foodborne disease occurs, most outbreaks relate to person-to-person transmission, and you are most likely to see disease this time of year (November through April). This might be explained by the fact that infected individuals shed billions of norovirus particles, and it only takes 18 particles to infect another, plus folks are more likely to be closely quartered in winter months.
In terms of cryptosporidiosis, famous outbreaks have followed contamination of drinking water, and sporadic cases are often seen in summer following recreational water exposure. While self-limited in the healthy child, cryptosporidiosis is hard to treat and causes significant morbidity in immunocompromised individuals, such as organ transplant patients. Pediatricians should alert parents to the risk related to recreational water exposure for high-risk patients who should avoid ingesting such water, and particularly avoid pools where diapered children may contaminate the water.
Speaking of diarrhea, as rates for Clostridium difficile associated disease (CDAD) in children have been increasing over the last decade, I suspect clinicians will need to gain a better understanding of the specifics regarding newer C. difficile tests. Many institutions have gone to molecular assays. Polymerase chain reaction (PCR) testing, for instance, has been introduced, which is very sensitive, and doubled the rate of positivity (compared with enzyme immunoassay) in some studies. We know that asymptomatic carriage of C. difficile is common in infants younger than 12 months of age, but several studies suggest that 25%-33% of 0- to 36-month control patients had stools that were positive for C. difficile toxin. Take a highly sensitive test, high rates of asymptomatic colonization, and the overall low prevalence CDAD, and you are likely to see diagnosis and treatment instituted inappropriately in some cases. The key to diagnosis of CDAD is to perform testing only on liquid stools and to make sure that other etiologies of diarrhea have been excluded in those less than 3 years of age. Don’t test young infants younger than 1 year (unless they have Hirschsprung’s disease), and do not perform tests to check for cure. See the new guideline published in the January issue of Pediatrics (2013; 131:196-200).
We may still be months away from knowing the full extent of the 2012 national fungal meningitis outbreak; however, based on what we know now, there is a clear need for legislation to ensure safe practices in compounding pharmacies, and I predict this will come in 2013. The first case of fungal meningitis cases was reported Sept. 18, 2012, in a man in Tennessee, and within a week, seven other cases were diagnosed; all had epidural steroid injections at the same center (N. Engl. J. Med. 2012 Dec. 19 [doi: 10.1056/NEJMoa1213978]).
Since then, a Centers for Disease Control and Prevention investigation has found that more than 600 infected patients and 39 patients have died. Three lots of methylprednisolone products from a compounding pharmacy in New England were found to be the source, and the CDC investigation found that more than 14,000 individuals in 70-plus clinics in 22 states were exposed to the products, mostly adult patients with chronic back pain. The organism in all but one case is an unusual environmental fungus (Exserohilum rostratum) that likely was introduced into the products during drug preparation. The Food and Drug Administration has since inspected the company’s processing room and noted a number of different issues that may have resulted in contamination. Products have been recalled from the implicated pharmacy (New England Compounding Center), and a sister pharmacy (Ameridose) has voluntarily recalled its products. This is not the first time that an outbreak has been tracked to contamination at a compounding pharmacies, but the extent of this outbreak emphasizes the need for definitive action to prevent this from ever happening again.
The 2013 Immunization Schedule will be out soon, and I predict practitioners may be happy to see a comprehensive footnote table, a harmonized schedule for those 0-18 years, and separate tables for the high-risk patient and for those requiring catch-up schedules.
In terms of vaccines, an important goal for practitioners may be to increase vaccine coverage in teens. Human papillomavirus (HPV) coverage rates are still dismal; 35% of girls and 1% of boys completed three vaccines in 2011, according to the National Immunization Survey–Teen. Parents who refused HPV vaccines in their daughters more likely cited safety concerns, but those who refused for their sons were more likely not to be aware of the recommendation for vaccination, according to data from the NIS-Teen. Geographic disparities also have been noted, with the southeastern U.S. states having lowest rates for immunization and some of the highest rates for cervical cancer. Recommend HPV vaccine every time another teen platform vaccine is recommended, and use a standing order in your practice so every encounter is an opportunity to immunize.
I wish you blessings in the coming year and hope that at least some of my predictions have utility for those of you in practice.
Dr. Jackson is the chief of infectious diseases at Children’s Mercy Hospitals and Clinics in Kansas City, Mo., and professor of pediatrics at the University of Missouri–Kansas City. She said she has no relevant financial disclosures. E-mail her at [email protected].
It is that time of year when 2013 predictions come your way, with insights into upcoming changes and/or developments in the specialty of pediatric infectious diseases. The theme this year: drugs, bugs, and the new immunization schedule.
Antimicrobial resistance for Gram negative organisms will reach new heights in 2013, new antibiotics will not likely appear on the market, and you will see an increase in emphasis on judicious antibiotic use in other venues such as the animal industry.
Particularly worrisome is the increased rate of hospital acquired carbapenem-resistant Klebsiella pneumoniae infections as few good therapeutic options currently exist for these pathogens. Judicious use of antibiotics in all instances is key, and pediatricians should particularly focus on their practice patterns for common infections (streptococcal pharyngitis, otitis media, and sinusitis), and avoiding antibiotics for upper respiratory infections and bronchitis.
The United States is the fifth greatest user of antibiotics in the world (France, Greece, Italy, and Belgium exceed us), and Kentucky, West Virginia, Tennessee, Mississippi, and Louisiana are the states with the highest use. Check out the map of this data to see antibiotic use for your state.
The winter scourge of rotavirus infection has virtually disappeared following the introduction of rotavirus vaccine but two diarrheal pathogens you’ll likely hear more about in 2013 are norovirus and cryptosporidia.
Norovirus (think cruise ship diarrhea) moves front and center as the most important cause of diarrheal outbreaks in the United States. While foodborne disease occurs, most outbreaks relate to person-to-person transmission, and you are most likely to see disease this time of year (November through April). This might be explained by the fact that infected individuals shed billions of norovirus particles, and it only takes 18 particles to infect another, plus folks are more likely to be closely quartered in winter months.
In terms of cryptosporidiosis, famous outbreaks have followed contamination of drinking water, and sporadic cases are often seen in summer following recreational water exposure. While self-limited in the healthy child, cryptosporidiosis is hard to treat and causes significant morbidity in immunocompromised individuals, such as organ transplant patients. Pediatricians should alert parents to the risk related to recreational water exposure for high-risk patients who should avoid ingesting such water, and particularly avoid pools where diapered children may contaminate the water.
Speaking of diarrhea, as rates for Clostridium difficile associated disease (CDAD) in children have been increasing over the last decade, I suspect clinicians will need to gain a better understanding of the specifics regarding newer C. difficile tests. Many institutions have gone to molecular assays. Polymerase chain reaction (PCR) testing, for instance, has been introduced, which is very sensitive, and doubled the rate of positivity (compared with enzyme immunoassay) in some studies. We know that asymptomatic carriage of C. difficile is common in infants younger than 12 months of age, but several studies suggest that 25%-33% of 0- to 36-month control patients had stools that were positive for C. difficile toxin. Take a highly sensitive test, high rates of asymptomatic colonization, and the overall low prevalence CDAD, and you are likely to see diagnosis and treatment instituted inappropriately in some cases. The key to diagnosis of CDAD is to perform testing only on liquid stools and to make sure that other etiologies of diarrhea have been excluded in those less than 3 years of age. Don’t test young infants younger than 1 year (unless they have Hirschsprung’s disease), and do not perform tests to check for cure. See the new guideline published in the January issue of Pediatrics (2013; 131:196-200).
We may still be months away from knowing the full extent of the 2012 national fungal meningitis outbreak; however, based on what we know now, there is a clear need for legislation to ensure safe practices in compounding pharmacies, and I predict this will come in 2013. The first case of fungal meningitis cases was reported Sept. 18, 2012, in a man in Tennessee, and within a week, seven other cases were diagnosed; all had epidural steroid injections at the same center (N. Engl. J. Med. 2012 Dec. 19 [doi: 10.1056/NEJMoa1213978]).
Since then, a Centers for Disease Control and Prevention investigation has found that more than 600 infected patients and 39 patients have died. Three lots of methylprednisolone products from a compounding pharmacy in New England were found to be the source, and the CDC investigation found that more than 14,000 individuals in 70-plus clinics in 22 states were exposed to the products, mostly adult patients with chronic back pain. The organism in all but one case is an unusual environmental fungus (Exserohilum rostratum) that likely was introduced into the products during drug preparation. The Food and Drug Administration has since inspected the company’s processing room and noted a number of different issues that may have resulted in contamination. Products have been recalled from the implicated pharmacy (New England Compounding Center), and a sister pharmacy (Ameridose) has voluntarily recalled its products. This is not the first time that an outbreak has been tracked to contamination at a compounding pharmacies, but the extent of this outbreak emphasizes the need for definitive action to prevent this from ever happening again.
The 2013 Immunization Schedule will be out soon, and I predict practitioners may be happy to see a comprehensive footnote table, a harmonized schedule for those 0-18 years, and separate tables for the high-risk patient and for those requiring catch-up schedules.
In terms of vaccines, an important goal for practitioners may be to increase vaccine coverage in teens. Human papillomavirus (HPV) coverage rates are still dismal; 35% of girls and 1% of boys completed three vaccines in 2011, according to the National Immunization Survey–Teen. Parents who refused HPV vaccines in their daughters more likely cited safety concerns, but those who refused for their sons were more likely not to be aware of the recommendation for vaccination, according to data from the NIS-Teen. Geographic disparities also have been noted, with the southeastern U.S. states having lowest rates for immunization and some of the highest rates for cervical cancer. Recommend HPV vaccine every time another teen platform vaccine is recommended, and use a standing order in your practice so every encounter is an opportunity to immunize.
I wish you blessings in the coming year and hope that at least some of my predictions have utility for those of you in practice.
Dr. Jackson is the chief of infectious diseases at Children’s Mercy Hospitals and Clinics in Kansas City, Mo., and professor of pediatrics at the University of Missouri–Kansas City. She said she has no relevant financial disclosures. E-mail her at [email protected].
Cold weather and diarrhea: Don't forget yersiniosis
The genus Yersinia includes 11 species. Three species are generally associated with human disease; Y. enterocolitica, Y. pestis, and Y. pseudotuberculosis. Yersinia pestis is the causative agent of plague. Yersinia pseudotuberculosis can manifest with fever, abdominal pain, and scarlatiniform rash. Additional symptoms include diarrhea, sterile joint effusions, erythema nodosum, and septicemia; these symptoms can be indistinguishable from Kawasaki Disease. By report, almost 10 % of cases of Kawasaki Disease in Japan have serologic or bacteriologic evidence of Y. pseudotuberculosis infection [Redbook: 2012 Report of the Committee on Infectious Diseases, 795-7]. Y. enterocolitica is most often associated with yersiniosis.
Although Y. enterocolitica is not the most common cause of diarrheal illness in the United States, it is one of the nine pathogens that have been monitored by the Foodborne Diseases Active Surveillance Network (FoodNet) since 1996. In the United States, it is estimated that Y. enterocolitica causes slightly over 115,000 infections annually (Emerg. Infect. Dis. 2011;17:7-15). The disease is more common in cooler months. It is transmitted by consumption of contaminated food, especially raw or undercooked pork products.
Only a few outbreaks have been reported in the United States, and these were usually associated with consumption of pork, specifically chitterlings (pig intestines), a winter holiday dish prepared most frequently in black households in the South (MMWR 1990;39:819-20). Transmission to infants and young children is thought to occur from caretakers preparing chitterlings who have not adequately cleaned their hands prior to touching objects subsequently handled by the child.
The incubation period is usually 4-6 days (range, 1-14 days). The duration of diarrhea is variable and can persist up to 3 weeks. Organisms can be excreted an average of 6 weeks. Clinical manifestations vary by age. Younger children usually present with fever and diarrhea. Stools frequently contain blood and leucocytes. Vomiting is also reported in most series. In contrast, older children and adults often present with a pseudoappendicitis syndrome with right-sided abdominal pain and fever. Leukocytosis is often present. At surgery, mesenteric adenitis is observed, and the appendix generally is normal.
Bacteremia can occur and is usually associated with infection in children less than 1 year of age and in those with iron-overloaded states, including persons with sickle cell disease, beta-thalassemia, and those receiving deferoxamine therapy. While uncommon, focal manifestations including pharyngitis, osteomyelitis, pyomyositis, pneumonia, empyema, and meningitis may occur.
Diagnosis is confirmed by isolation of the organism from stool, blood, peritoneal fluid, lymph nodes, and throat cultures. Most laboratories do not routinely test for Yersinia in stool cultures. If Y. enterocolitica is suspected, you should notify the laboratory so the stool can be plated on appropriate media (CIN agar). Serologic tests to detect a rise in serum antibody titers to confirm infection are available in reference and research laboratories, but are not generally used for diagnosis. Cross reactivity with Brucella, Salmonella, Vibrio, and Rickettsia may lead to false positive titer results. Y. enterocolitica antibodies also have antigenic similarity with thyroid tissue. You may see persistent elevation of titers in patients with thyroid disease.
Benefit of antimicrobial therapy for isolated Y. enterocolitica gastrointestinal disease and Y. pseudotuberculosis has not been established. Therapy may decrease the duration of fecal shedding. Treatment is indicated for immunocompromised hosts and persons with septicemia and focal infections. Y. enterocolitica and Y. pseudotuberculosis are usually sensitive to trimethoprim-sulfamethoxazole, aminoglycosides, cefotaxime, fluoroquinolones (persons greater than 18 years of age or older), and tetracycline or doxycycline (for children at least 8 years of age and older).
So what is the actual incidence and when should the practitioner be concerned? Initial population based surveillance data for Y. enterocolitica infections in FoodNet sites between 1996 and 1999 reported an overall incidence of 0.9 cases per 100,000 population. The highest incidence was among black and Asian individuals and was 3.2 cases and 1.5 cases per 100,000 population, respectively. The incidence in Hispanics and whites was 0.6 and 0.4 cases per 100,000 respectively. Incidence increased with decreasing age in all racial/ethnic groups. Blacks infants had the highest incidence, 141.9 cases/100,000 population, and the highest incidence in infants was reported from Georgia (207 cases/100,000). Seasonal variation in incidence was noted only in black individuals with peak activity occurring in December (Clin. Infect. Dis. 2004;38[Suppl 3]:S181-9).
The most recent data from FoodNet (1996-2009) reveals an overall incidence of 0.5/100,000. There was a decline in incidence in all racial and ethnic groups. The highest incidence is still observed in black and Asians (0.9 and 0.7 per 100,000). The most dramatic decline occurred in black individuals (3.2 vs. 0.9 per 100,000). In 1998, an educational campaign was initiated in Georgia that targeted high-risk individuals and provided information on the safe handling and preparation of chitterlings. The state of Georgia reported the greatest decline to 0.4/100,000, which has almost eliminated the racial disparity reported in 2009. It is unclear if this campaign was the only reason for the decline in Georgia. The incidence in whites is 0.2/100,000. Since 2007, the incidence in Asian children less than 5 years of ages has been the highest amongst all racial and ethnic groups. Pork consumption is still assumed to be the major source. Seasonal variability persists amongst Black children less 5 years of age, implying that chitterlings may still be the source of infection for individuals in this group (Clin. Infect. Dis. 2012:54 [Suppl 5]:S385-S90).
In general, yersiniosis should be included in the differential of a febrile diarrheal illness, particularly during the cooler months and holiday season. It is prudent to determine if consumption and/or preparation of chitterlings or other pork products by the patient or caretakers has occurred. This will enable you to alert the laboratory so stool specimens can be cultured on the appropriate medium (CIN agar). Consumption of chitterlings is not limited to any specific racial or ethnic group. Individuals from rural and farming areas may also consume this product.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures. Write to Dr. Word at [email protected].
The genus Yersinia includes 11 species. Three species are generally associated with human disease; Y. enterocolitica, Y. pestis, and Y. pseudotuberculosis. Yersinia pestis is the causative agent of plague. Yersinia pseudotuberculosis can manifest with fever, abdominal pain, and scarlatiniform rash. Additional symptoms include diarrhea, sterile joint effusions, erythema nodosum, and septicemia; these symptoms can be indistinguishable from Kawasaki Disease. By report, almost 10 % of cases of Kawasaki Disease in Japan have serologic or bacteriologic evidence of Y. pseudotuberculosis infection [Redbook: 2012 Report of the Committee on Infectious Diseases, 795-7]. Y. enterocolitica is most often associated with yersiniosis.
Although Y. enterocolitica is not the most common cause of diarrheal illness in the United States, it is one of the nine pathogens that have been monitored by the Foodborne Diseases Active Surveillance Network (FoodNet) since 1996. In the United States, it is estimated that Y. enterocolitica causes slightly over 115,000 infections annually (Emerg. Infect. Dis. 2011;17:7-15). The disease is more common in cooler months. It is transmitted by consumption of contaminated food, especially raw or undercooked pork products.
Only a few outbreaks have been reported in the United States, and these were usually associated with consumption of pork, specifically chitterlings (pig intestines), a winter holiday dish prepared most frequently in black households in the South (MMWR 1990;39:819-20). Transmission to infants and young children is thought to occur from caretakers preparing chitterlings who have not adequately cleaned their hands prior to touching objects subsequently handled by the child.
The incubation period is usually 4-6 days (range, 1-14 days). The duration of diarrhea is variable and can persist up to 3 weeks. Organisms can be excreted an average of 6 weeks. Clinical manifestations vary by age. Younger children usually present with fever and diarrhea. Stools frequently contain blood and leucocytes. Vomiting is also reported in most series. In contrast, older children and adults often present with a pseudoappendicitis syndrome with right-sided abdominal pain and fever. Leukocytosis is often present. At surgery, mesenteric adenitis is observed, and the appendix generally is normal.
Bacteremia can occur and is usually associated with infection in children less than 1 year of age and in those with iron-overloaded states, including persons with sickle cell disease, beta-thalassemia, and those receiving deferoxamine therapy. While uncommon, focal manifestations including pharyngitis, osteomyelitis, pyomyositis, pneumonia, empyema, and meningitis may occur.
Diagnosis is confirmed by isolation of the organism from stool, blood, peritoneal fluid, lymph nodes, and throat cultures. Most laboratories do not routinely test for Yersinia in stool cultures. If Y. enterocolitica is suspected, you should notify the laboratory so the stool can be plated on appropriate media (CIN agar). Serologic tests to detect a rise in serum antibody titers to confirm infection are available in reference and research laboratories, but are not generally used for diagnosis. Cross reactivity with Brucella, Salmonella, Vibrio, and Rickettsia may lead to false positive titer results. Y. enterocolitica antibodies also have antigenic similarity with thyroid tissue. You may see persistent elevation of titers in patients with thyroid disease.
Benefit of antimicrobial therapy for isolated Y. enterocolitica gastrointestinal disease and Y. pseudotuberculosis has not been established. Therapy may decrease the duration of fecal shedding. Treatment is indicated for immunocompromised hosts and persons with septicemia and focal infections. Y. enterocolitica and Y. pseudotuberculosis are usually sensitive to trimethoprim-sulfamethoxazole, aminoglycosides, cefotaxime, fluoroquinolones (persons greater than 18 years of age or older), and tetracycline or doxycycline (for children at least 8 years of age and older).
So what is the actual incidence and when should the practitioner be concerned? Initial population based surveillance data for Y. enterocolitica infections in FoodNet sites between 1996 and 1999 reported an overall incidence of 0.9 cases per 100,000 population. The highest incidence was among black and Asian individuals and was 3.2 cases and 1.5 cases per 100,000 population, respectively. The incidence in Hispanics and whites was 0.6 and 0.4 cases per 100,000 respectively. Incidence increased with decreasing age in all racial/ethnic groups. Blacks infants had the highest incidence, 141.9 cases/100,000 population, and the highest incidence in infants was reported from Georgia (207 cases/100,000). Seasonal variation in incidence was noted only in black individuals with peak activity occurring in December (Clin. Infect. Dis. 2004;38[Suppl 3]:S181-9).
The most recent data from FoodNet (1996-2009) reveals an overall incidence of 0.5/100,000. There was a decline in incidence in all racial and ethnic groups. The highest incidence is still observed in black and Asians (0.9 and 0.7 per 100,000). The most dramatic decline occurred in black individuals (3.2 vs. 0.9 per 100,000). In 1998, an educational campaign was initiated in Georgia that targeted high-risk individuals and provided information on the safe handling and preparation of chitterlings. The state of Georgia reported the greatest decline to 0.4/100,000, which has almost eliminated the racial disparity reported in 2009. It is unclear if this campaign was the only reason for the decline in Georgia. The incidence in whites is 0.2/100,000. Since 2007, the incidence in Asian children less than 5 years of ages has been the highest amongst all racial and ethnic groups. Pork consumption is still assumed to be the major source. Seasonal variability persists amongst Black children less 5 years of age, implying that chitterlings may still be the source of infection for individuals in this group (Clin. Infect. Dis. 2012:54 [Suppl 5]:S385-S90).
In general, yersiniosis should be included in the differential of a febrile diarrheal illness, particularly during the cooler months and holiday season. It is prudent to determine if consumption and/or preparation of chitterlings or other pork products by the patient or caretakers has occurred. This will enable you to alert the laboratory so stool specimens can be cultured on the appropriate medium (CIN agar). Consumption of chitterlings is not limited to any specific racial or ethnic group. Individuals from rural and farming areas may also consume this product.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures. Write to Dr. Word at [email protected].
The genus Yersinia includes 11 species. Three species are generally associated with human disease; Y. enterocolitica, Y. pestis, and Y. pseudotuberculosis. Yersinia pestis is the causative agent of plague. Yersinia pseudotuberculosis can manifest with fever, abdominal pain, and scarlatiniform rash. Additional symptoms include diarrhea, sterile joint effusions, erythema nodosum, and septicemia; these symptoms can be indistinguishable from Kawasaki Disease. By report, almost 10 % of cases of Kawasaki Disease in Japan have serologic or bacteriologic evidence of Y. pseudotuberculosis infection [Redbook: 2012 Report of the Committee on Infectious Diseases, 795-7]. Y. enterocolitica is most often associated with yersiniosis.
Although Y. enterocolitica is not the most common cause of diarrheal illness in the United States, it is one of the nine pathogens that have been monitored by the Foodborne Diseases Active Surveillance Network (FoodNet) since 1996. In the United States, it is estimated that Y. enterocolitica causes slightly over 115,000 infections annually (Emerg. Infect. Dis. 2011;17:7-15). The disease is more common in cooler months. It is transmitted by consumption of contaminated food, especially raw or undercooked pork products.
Only a few outbreaks have been reported in the United States, and these were usually associated with consumption of pork, specifically chitterlings (pig intestines), a winter holiday dish prepared most frequently in black households in the South (MMWR 1990;39:819-20). Transmission to infants and young children is thought to occur from caretakers preparing chitterlings who have not adequately cleaned their hands prior to touching objects subsequently handled by the child.
The incubation period is usually 4-6 days (range, 1-14 days). The duration of diarrhea is variable and can persist up to 3 weeks. Organisms can be excreted an average of 6 weeks. Clinical manifestations vary by age. Younger children usually present with fever and diarrhea. Stools frequently contain blood and leucocytes. Vomiting is also reported in most series. In contrast, older children and adults often present with a pseudoappendicitis syndrome with right-sided abdominal pain and fever. Leukocytosis is often present. At surgery, mesenteric adenitis is observed, and the appendix generally is normal.
Bacteremia can occur and is usually associated with infection in children less than 1 year of age and in those with iron-overloaded states, including persons with sickle cell disease, beta-thalassemia, and those receiving deferoxamine therapy. While uncommon, focal manifestations including pharyngitis, osteomyelitis, pyomyositis, pneumonia, empyema, and meningitis may occur.
Diagnosis is confirmed by isolation of the organism from stool, blood, peritoneal fluid, lymph nodes, and throat cultures. Most laboratories do not routinely test for Yersinia in stool cultures. If Y. enterocolitica is suspected, you should notify the laboratory so the stool can be plated on appropriate media (CIN agar). Serologic tests to detect a rise in serum antibody titers to confirm infection are available in reference and research laboratories, but are not generally used for diagnosis. Cross reactivity with Brucella, Salmonella, Vibrio, and Rickettsia may lead to false positive titer results. Y. enterocolitica antibodies also have antigenic similarity with thyroid tissue. You may see persistent elevation of titers in patients with thyroid disease.
Benefit of antimicrobial therapy for isolated Y. enterocolitica gastrointestinal disease and Y. pseudotuberculosis has not been established. Therapy may decrease the duration of fecal shedding. Treatment is indicated for immunocompromised hosts and persons with septicemia and focal infections. Y. enterocolitica and Y. pseudotuberculosis are usually sensitive to trimethoprim-sulfamethoxazole, aminoglycosides, cefotaxime, fluoroquinolones (persons greater than 18 years of age or older), and tetracycline or doxycycline (for children at least 8 years of age and older).
So what is the actual incidence and when should the practitioner be concerned? Initial population based surveillance data for Y. enterocolitica infections in FoodNet sites between 1996 and 1999 reported an overall incidence of 0.9 cases per 100,000 population. The highest incidence was among black and Asian individuals and was 3.2 cases and 1.5 cases per 100,000 population, respectively. The incidence in Hispanics and whites was 0.6 and 0.4 cases per 100,000 respectively. Incidence increased with decreasing age in all racial/ethnic groups. Blacks infants had the highest incidence, 141.9 cases/100,000 population, and the highest incidence in infants was reported from Georgia (207 cases/100,000). Seasonal variation in incidence was noted only in black individuals with peak activity occurring in December (Clin. Infect. Dis. 2004;38[Suppl 3]:S181-9).
The most recent data from FoodNet (1996-2009) reveals an overall incidence of 0.5/100,000. There was a decline in incidence in all racial and ethnic groups. The highest incidence is still observed in black and Asians (0.9 and 0.7 per 100,000). The most dramatic decline occurred in black individuals (3.2 vs. 0.9 per 100,000). In 1998, an educational campaign was initiated in Georgia that targeted high-risk individuals and provided information on the safe handling and preparation of chitterlings. The state of Georgia reported the greatest decline to 0.4/100,000, which has almost eliminated the racial disparity reported in 2009. It is unclear if this campaign was the only reason for the decline in Georgia. The incidence in whites is 0.2/100,000. Since 2007, the incidence in Asian children less than 5 years of ages has been the highest amongst all racial and ethnic groups. Pork consumption is still assumed to be the major source. Seasonal variability persists amongst Black children less 5 years of age, implying that chitterlings may still be the source of infection for individuals in this group (Clin. Infect. Dis. 2012:54 [Suppl 5]:S385-S90).
In general, yersiniosis should be included in the differential of a febrile diarrheal illness, particularly during the cooler months and holiday season. It is prudent to determine if consumption and/or preparation of chitterlings or other pork products by the patient or caretakers has occurred. This will enable you to alert the laboratory so stool specimens can be cultured on the appropriate medium (CIN agar). Consumption of chitterlings is not limited to any specific racial or ethnic group. Individuals from rural and farming areas may also consume this product.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures. Write to Dr. Word at [email protected].
Infant Meningococcal Vaccine: Why Not?
Haemophilus influenzae type B-Neisseria meningitidis serogroups C (MenC) and Y (MenY)-tetanus toxoid (Hib-MenCY-TT, MenHibrix) vaccine has been approved by the Food and Drug Administration for an infant indication to be administered according to the standard 2-, 4-, 6-, and 12 months vaccine schedule in the United States as endorsed by the American Academy of Pediatrics (AAP), the American Academy of Family Physicians (AAFP), and the Centers for Disease Control and Prevention (CDC).
According to the presentation given at the CDC meeting Oct. 14, 2011, by Dr. Ismael Ortega-Sanchez, giving the vaccine could prevent 130 of the projected 377 (34.5%) cases of meningococcal infection in the 4-million-child birth cohort of the United States cumulatively to age 10 years. Also infant vaccination would prevent one death per 642,000 infants (seven deaths/year). The vaccine could be given along with a DTaP/inactivated polio vaccine/hepatitis b vaccine (DTaP/IPV/HepB, Pediarix) and the 13-valent pneumococcal conjugate vaccine (PCV13, Prevnar13) without increasing the total number of shots in a visit. The vaccine has been proven safe and effective.
Yet at the October meeting of the CDC Advisory Committee on Immunization Practices (ACIP), Hib-MenCY-TT (MenHibrix) vaccine was not recommended for universal use. Instead, it was recommended for high risk children as previously defined as complement deficient and asplenic. Why the restricted recommendation?*
• It isn’t the right time. When the ACIP/AAP/AAFP endorsed meningococcal vaccination for 11- to 12-year-olds 7 years ago, the annual incidence of meningococcal disease was fivefold higher than it is now. The drop in incidence cannot be fully attributed to the initiation of our national vaccination campaign. It was known before the meningococcal conjugate vaccine was recommended that meningococcal disease had a cyclical pattern with high and low years of incidence. But, had the incidence been as low as it is now, then one might speculate that the vote to recommend universal vaccination might have been different. The passionate pleas of concerned parents and the desire by all of us in health care to protect every single adolescent against the devastation of meningococcal infections carried the day, even though the cost for prevention of those cases and deaths was the highest ever seen up to that time. So, if the incidence of meningococcal infections is now at an all time low, the calculations of cost to prevent cases and deaths would be a multiple of what it was 7 years ago.
• The vaccine doesn’t include all the serotypes. Hib-MenCY-TT has meningococcal serotypes C and Y. The vaccine does not include serotype A or serotype W-135 (because these serotypes are virtually absent and uncommon, respectively, in the United States and other developed countries. So, a concern could be that serotype replacement might occur over time as we have seen with Prevnar7, now replaced in the United States with Prevnar13 because of serotype replacement. But more importantly is the absence of serotype B in the vaccine. Serotype B meningococci cause 60%-65% of meningococcal disease in the United States in infants. That is why the number of cases projected to be prevented with Hib-MenCY-TT is about one-third of all cases among infants.
• The total number of shots goes up per visit unless GlaxoSmithKline vaccines are preferentially used. Hib-MenCY-TT was developed and is licensed by GlaxoSmithKline, a world leader in pediatric vaccines, and they are building a portfolio of vaccines that can fit together well. There is nothing wrong with that – it is good marketing. Sanofi Pasteur Vaccines is doing the same thing and, as more products are forthcoming from Pfizer Vaccines, Novartis Vaccines, and others, we can expect the same strategy. However, the CDC, AAP, and AAFP do not want to endorse products that limit choices and/or provide any single company with a competitive advantage. So, to endorse Hib-MenCY-TT that clearly fits best with only GlaxoSmithKline vaccine products may be an unspoken concern.
• The National Immunization Program cannot afford it. Going back to the presentation to ACIP at the CDC in October 2011, a key aspect was the cost of vaccination calculated against cases prevented and lives saved. The calculation for Quality-Adjusted Life Year (QALY) saved for a two-dose schedule among adolescents came out to $157,000/case. For the infant vaccination, the numbers were pretty staggering at $3.6 million/case, based on the current incidence of meningococcal infections in the United States (see sidebar). Even if the incidence of meningococcal infections were currently as high as they were back in 1997-1999, the cost would be $0.5 million/case. For those thinking about the option of toddler vaccination with the quadrivalent meningococcal conjugate vaccine (Menactra), the calculations for QALYs concluded that such a strategy prevented half as many cases at half the cost.
So where do we go from here? The lack of an endorsement by ACIP/AAP/AAFP for universal use* normally means that the vaccine will not be available within the Vaccines for Children free program, and it will not be covered by commercial health insurance plans except for the specific indications endorsed by the recommending bodies.* So these are huge barriers to use. Nevertheless, it is a licensed vaccine and it is safe and effective, just not perfect and not cost-effective for widespread public use at government expense. How much is a child’s life worth? If it is your child, then the life is priceless. But in public health there are limits to what can be afforded. We will see more of these types of issues in the future. Another unspoken concern of the ACIP/AAP/AAFP is that a two-tiered vaccine access situation develops. In other words, for those who can afford to pay, Hib-MenCY-TT is available and, if they can pay for it out-of-pocket, then they can buy it to protect their child.
The following are cost-effectiveness analysis conclusions that were presented to the CDC:
• Vaccinating infants or toddlers with meningococcal vaccine has a high cost per case prevented – even at a low vaccine price.
• Cost estimates are much higher than prior analyses because of declining incidence and shorter duration of protection.
• Infant vaccination prevents twice as many cases as toddler vaccination but at twice the cost – cost per QALY saved is similar for both strategies.
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Rochester (N.Y.) General Hospital Research Institute. He is also a pediatrician at Legacy Pediatrics in Rochester. Dr. Pichichero disclosed that in the past 3 years, he has served as a consultant to Sanofi Pasteur, Pfizer, Novartis, and Crucell for their vaccines currently licensed and in development. Dr. Pichichero also disclosed that in the past 3 years, his academic institution has received research grants to support vaccine work from GlaxoSmithKline, Sanofi Pasteur, Pfizer, Novartis, and Crucell, including studies involving Hib-MenCY-TT produced by GlaxoSmithKline and Quadrivalent meningococcal vaccine by Sanofi Pasteur and Novartis.
* This article was updated on 10/26/12.
Haemophilus influenzae type B-Neisseria meningitidis serogroups C (MenC) and Y (MenY)-tetanus toxoid (Hib-MenCY-TT, MenHibrix) vaccine has been approved by the Food and Drug Administration for an infant indication to be administered according to the standard 2-, 4-, 6-, and 12 months vaccine schedule in the United States as endorsed by the American Academy of Pediatrics (AAP), the American Academy of Family Physicians (AAFP), and the Centers for Disease Control and Prevention (CDC).
According to the presentation given at the CDC meeting Oct. 14, 2011, by Dr. Ismael Ortega-Sanchez, giving the vaccine could prevent 130 of the projected 377 (34.5%) cases of meningococcal infection in the 4-million-child birth cohort of the United States cumulatively to age 10 years. Also infant vaccination would prevent one death per 642,000 infants (seven deaths/year). The vaccine could be given along with a DTaP/inactivated polio vaccine/hepatitis b vaccine (DTaP/IPV/HepB, Pediarix) and the 13-valent pneumococcal conjugate vaccine (PCV13, Prevnar13) without increasing the total number of shots in a visit. The vaccine has been proven safe and effective.
Yet at the October meeting of the CDC Advisory Committee on Immunization Practices (ACIP), Hib-MenCY-TT (MenHibrix) vaccine was not recommended for universal use. Instead, it was recommended for high risk children as previously defined as complement deficient and asplenic. Why the restricted recommendation?*
• It isn’t the right time. When the ACIP/AAP/AAFP endorsed meningococcal vaccination for 11- to 12-year-olds 7 years ago, the annual incidence of meningococcal disease was fivefold higher than it is now. The drop in incidence cannot be fully attributed to the initiation of our national vaccination campaign. It was known before the meningococcal conjugate vaccine was recommended that meningococcal disease had a cyclical pattern with high and low years of incidence. But, had the incidence been as low as it is now, then one might speculate that the vote to recommend universal vaccination might have been different. The passionate pleas of concerned parents and the desire by all of us in health care to protect every single adolescent against the devastation of meningococcal infections carried the day, even though the cost for prevention of those cases and deaths was the highest ever seen up to that time. So, if the incidence of meningococcal infections is now at an all time low, the calculations of cost to prevent cases and deaths would be a multiple of what it was 7 years ago.
• The vaccine doesn’t include all the serotypes. Hib-MenCY-TT has meningococcal serotypes C and Y. The vaccine does not include serotype A or serotype W-135 (because these serotypes are virtually absent and uncommon, respectively, in the United States and other developed countries. So, a concern could be that serotype replacement might occur over time as we have seen with Prevnar7, now replaced in the United States with Prevnar13 because of serotype replacement. But more importantly is the absence of serotype B in the vaccine. Serotype B meningococci cause 60%-65% of meningococcal disease in the United States in infants. That is why the number of cases projected to be prevented with Hib-MenCY-TT is about one-third of all cases among infants.
• The total number of shots goes up per visit unless GlaxoSmithKline vaccines are preferentially used. Hib-MenCY-TT was developed and is licensed by GlaxoSmithKline, a world leader in pediatric vaccines, and they are building a portfolio of vaccines that can fit together well. There is nothing wrong with that – it is good marketing. Sanofi Pasteur Vaccines is doing the same thing and, as more products are forthcoming from Pfizer Vaccines, Novartis Vaccines, and others, we can expect the same strategy. However, the CDC, AAP, and AAFP do not want to endorse products that limit choices and/or provide any single company with a competitive advantage. So, to endorse Hib-MenCY-TT that clearly fits best with only GlaxoSmithKline vaccine products may be an unspoken concern.
• The National Immunization Program cannot afford it. Going back to the presentation to ACIP at the CDC in October 2011, a key aspect was the cost of vaccination calculated against cases prevented and lives saved. The calculation for Quality-Adjusted Life Year (QALY) saved for a two-dose schedule among adolescents came out to $157,000/case. For the infant vaccination, the numbers were pretty staggering at $3.6 million/case, based on the current incidence of meningococcal infections in the United States (see sidebar). Even if the incidence of meningococcal infections were currently as high as they were back in 1997-1999, the cost would be $0.5 million/case. For those thinking about the option of toddler vaccination with the quadrivalent meningococcal conjugate vaccine (Menactra), the calculations for QALYs concluded that such a strategy prevented half as many cases at half the cost.
So where do we go from here? The lack of an endorsement by ACIP/AAP/AAFP for universal use* normally means that the vaccine will not be available within the Vaccines for Children free program, and it will not be covered by commercial health insurance plans except for the specific indications endorsed by the recommending bodies.* So these are huge barriers to use. Nevertheless, it is a licensed vaccine and it is safe and effective, just not perfect and not cost-effective for widespread public use at government expense. How much is a child’s life worth? If it is your child, then the life is priceless. But in public health there are limits to what can be afforded. We will see more of these types of issues in the future. Another unspoken concern of the ACIP/AAP/AAFP is that a two-tiered vaccine access situation develops. In other words, for those who can afford to pay, Hib-MenCY-TT is available and, if they can pay for it out-of-pocket, then they can buy it to protect their child.
The following are cost-effectiveness analysis conclusions that were presented to the CDC:
• Vaccinating infants or toddlers with meningococcal vaccine has a high cost per case prevented – even at a low vaccine price.
• Cost estimates are much higher than prior analyses because of declining incidence and shorter duration of protection.
• Infant vaccination prevents twice as many cases as toddler vaccination but at twice the cost – cost per QALY saved is similar for both strategies.
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Rochester (N.Y.) General Hospital Research Institute. He is also a pediatrician at Legacy Pediatrics in Rochester. Dr. Pichichero disclosed that in the past 3 years, he has served as a consultant to Sanofi Pasteur, Pfizer, Novartis, and Crucell for their vaccines currently licensed and in development. Dr. Pichichero also disclosed that in the past 3 years, his academic institution has received research grants to support vaccine work from GlaxoSmithKline, Sanofi Pasteur, Pfizer, Novartis, and Crucell, including studies involving Hib-MenCY-TT produced by GlaxoSmithKline and Quadrivalent meningococcal vaccine by Sanofi Pasteur and Novartis.
* This article was updated on 10/26/12.
Haemophilus influenzae type B-Neisseria meningitidis serogroups C (MenC) and Y (MenY)-tetanus toxoid (Hib-MenCY-TT, MenHibrix) vaccine has been approved by the Food and Drug Administration for an infant indication to be administered according to the standard 2-, 4-, 6-, and 12 months vaccine schedule in the United States as endorsed by the American Academy of Pediatrics (AAP), the American Academy of Family Physicians (AAFP), and the Centers for Disease Control and Prevention (CDC).
According to the presentation given at the CDC meeting Oct. 14, 2011, by Dr. Ismael Ortega-Sanchez, giving the vaccine could prevent 130 of the projected 377 (34.5%) cases of meningococcal infection in the 4-million-child birth cohort of the United States cumulatively to age 10 years. Also infant vaccination would prevent one death per 642,000 infants (seven deaths/year). The vaccine could be given along with a DTaP/inactivated polio vaccine/hepatitis b vaccine (DTaP/IPV/HepB, Pediarix) and the 13-valent pneumococcal conjugate vaccine (PCV13, Prevnar13) without increasing the total number of shots in a visit. The vaccine has been proven safe and effective.
Yet at the October meeting of the CDC Advisory Committee on Immunization Practices (ACIP), Hib-MenCY-TT (MenHibrix) vaccine was not recommended for universal use. Instead, it was recommended for high risk children as previously defined as complement deficient and asplenic. Why the restricted recommendation?*
• It isn’t the right time. When the ACIP/AAP/AAFP endorsed meningococcal vaccination for 11- to 12-year-olds 7 years ago, the annual incidence of meningococcal disease was fivefold higher than it is now. The drop in incidence cannot be fully attributed to the initiation of our national vaccination campaign. It was known before the meningococcal conjugate vaccine was recommended that meningococcal disease had a cyclical pattern with high and low years of incidence. But, had the incidence been as low as it is now, then one might speculate that the vote to recommend universal vaccination might have been different. The passionate pleas of concerned parents and the desire by all of us in health care to protect every single adolescent against the devastation of meningococcal infections carried the day, even though the cost for prevention of those cases and deaths was the highest ever seen up to that time. So, if the incidence of meningococcal infections is now at an all time low, the calculations of cost to prevent cases and deaths would be a multiple of what it was 7 years ago.
• The vaccine doesn’t include all the serotypes. Hib-MenCY-TT has meningococcal serotypes C and Y. The vaccine does not include serotype A or serotype W-135 (because these serotypes are virtually absent and uncommon, respectively, in the United States and other developed countries. So, a concern could be that serotype replacement might occur over time as we have seen with Prevnar7, now replaced in the United States with Prevnar13 because of serotype replacement. But more importantly is the absence of serotype B in the vaccine. Serotype B meningococci cause 60%-65% of meningococcal disease in the United States in infants. That is why the number of cases projected to be prevented with Hib-MenCY-TT is about one-third of all cases among infants.
• The total number of shots goes up per visit unless GlaxoSmithKline vaccines are preferentially used. Hib-MenCY-TT was developed and is licensed by GlaxoSmithKline, a world leader in pediatric vaccines, and they are building a portfolio of vaccines that can fit together well. There is nothing wrong with that – it is good marketing. Sanofi Pasteur Vaccines is doing the same thing and, as more products are forthcoming from Pfizer Vaccines, Novartis Vaccines, and others, we can expect the same strategy. However, the CDC, AAP, and AAFP do not want to endorse products that limit choices and/or provide any single company with a competitive advantage. So, to endorse Hib-MenCY-TT that clearly fits best with only GlaxoSmithKline vaccine products may be an unspoken concern.
• The National Immunization Program cannot afford it. Going back to the presentation to ACIP at the CDC in October 2011, a key aspect was the cost of vaccination calculated against cases prevented and lives saved. The calculation for Quality-Adjusted Life Year (QALY) saved for a two-dose schedule among adolescents came out to $157,000/case. For the infant vaccination, the numbers were pretty staggering at $3.6 million/case, based on the current incidence of meningococcal infections in the United States (see sidebar). Even if the incidence of meningococcal infections were currently as high as they were back in 1997-1999, the cost would be $0.5 million/case. For those thinking about the option of toddler vaccination with the quadrivalent meningococcal conjugate vaccine (Menactra), the calculations for QALYs concluded that such a strategy prevented half as many cases at half the cost.
So where do we go from here? The lack of an endorsement by ACIP/AAP/AAFP for universal use* normally means that the vaccine will not be available within the Vaccines for Children free program, and it will not be covered by commercial health insurance plans except for the specific indications endorsed by the recommending bodies.* So these are huge barriers to use. Nevertheless, it is a licensed vaccine and it is safe and effective, just not perfect and not cost-effective for widespread public use at government expense. How much is a child’s life worth? If it is your child, then the life is priceless. But in public health there are limits to what can be afforded. We will see more of these types of issues in the future. Another unspoken concern of the ACIP/AAP/AAFP is that a two-tiered vaccine access situation develops. In other words, for those who can afford to pay, Hib-MenCY-TT is available and, if they can pay for it out-of-pocket, then they can buy it to protect their child.
The following are cost-effectiveness analysis conclusions that were presented to the CDC:
• Vaccinating infants or toddlers with meningococcal vaccine has a high cost per case prevented – even at a low vaccine price.
• Cost estimates are much higher than prior analyses because of declining incidence and shorter duration of protection.
• Infant vaccination prevents twice as many cases as toddler vaccination but at twice the cost – cost per QALY saved is similar for both strategies.
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Rochester (N.Y.) General Hospital Research Institute. He is also a pediatrician at Legacy Pediatrics in Rochester. Dr. Pichichero disclosed that in the past 3 years, he has served as a consultant to Sanofi Pasteur, Pfizer, Novartis, and Crucell for their vaccines currently licensed and in development. Dr. Pichichero also disclosed that in the past 3 years, his academic institution has received research grants to support vaccine work from GlaxoSmithKline, Sanofi Pasteur, Pfizer, Novartis, and Crucell, including studies involving Hib-MenCY-TT produced by GlaxoSmithKline and Quadrivalent meningococcal vaccine by Sanofi Pasteur and Novartis.
* This article was updated on 10/26/12.
Answers to Your Questions About Flu and Flu Vaccine
Although the 2011-2012 influenza season was milder than usual and associated with fewer outpatient visits, lower hospitalization rates, and fewer pediatric deaths, practitioners should be aware that this virus is still the leading cause of vaccine-preventable deaths in children. Hopefully, practitioners are already providing influenza immunization utilizing the 2012-2013 vaccine, which contains the same influenza A (H1N1) antigen as the 2011-2012 seasonal vaccine but new influenza A (H3N2) and B antigens: These are A/California/7/2009 (H1N1)–like antigen, A/Victoria/361/2011(H3N2)–like antigen, and B/Wisconsin/1/2010–like antigen.
Here are the answers to some of the most common questions related to this year’s influenza season:
• How many doses are recommended this year for children between 6 months through 8 years of age?
For a child in this age group who had two or more doses of seasonal vaccine since July 1, 2010, or in whom you can document one dose of a pandemic H1N1–containing vaccine and at least two seasonal vaccines from any season, only one dose is needed. All others in this age group should receive two doses. As always, those 9 years of age and older receive one dose of vaccine.
• Given recent data that suggested a slight increased risk of a febrile seizure following trivalent inactivated vaccine (TIV) in children less than 4 years, have vaccine recommendations changed?
A suggestion of an increased risk for febrile seizures in young children after TIV was noted in the United States in 2010-2011. This followed enhanced monitoring after the observation in Australia in 2010 of an association with an increased risk of febrile seizures (greater than or equal to nine per 1,000 doses) that were related to a particular influenza vaccine. (Afluria vaccine is approved for use in those greater than age 5 years, but current recommendations from the American Academy of Pediatrics and the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices (ACIP) state this vaccine should not be used in those less than 9 years of age. It could be considered for a high-risk patient between 5 through 8 years if no alternative TIV is available and after discussion of the seizure risk with parents.)
To further study this potential association, the CDC tracked more than 200,000 children in the United States who received TIV or PCV13 (Prevnar) vaccine at different visits or both together at the same visit. Rarely, a febrile seizure was noted in children less than 5 years who got TIV or PCV13 vaccine given at separate visits. Those 12- to 23-month-olds who received both at the same visit had a slightly increased risk for an uncomplicated febrile seizure in the 24 hours following vaccine receipt. This is the age group where febrile seizures peak in general and is equivalent to one excess seizure for every 2,000-3,000 vaccine doses. Based on this low risk and the uncomplicated course in such patients, coupled with the benefits of immunization, both the AAP and ACIP recommended no change in either the TIV or PCV13 vaccine policies. And remember that neither a prior febrile seizure history nor a preexisting seizure disorder is considered a contraindication for influenza vaccine.
• Who can get the intradermal formulation of influenza vaccine?
Fluzone Intradermal, which made its debut in the 2011-2012 season, is licensed for those 18-64 years of age, and is a preservative-free, trivalent inactivated influenza vaccine. It comes in a prefilled microinjection syringe, which for those who require TIV and are needle averse, may be preferred. Local reactions seen with intramuscular TIV (with the exception of pain), including redness, swelling, and itching at the site seem to be a bit more common with the intradermal product, but such reactions abate in 3-7 days. This vaccine could be utilized in an older adolescent who might opt for a vaccine that has a needle that is 90% shorter than the needle used for intramuscular injection of TIV.
• Is oseltamivir still the drug of choice to treat influenza?
Last year only 1.4% of strains were resistant to oseltamivir, and this year is expected to be the same. Treatment and prophylaxis dosing is the same as last year. The AAP and ACIP continue to emphasize early treatment for all children in high-risk groups who develop influenza, regardless of influenza immunization status. Treatment is also recommended for all who are ill enough to require hospitalization. For patients with influenzalike illness, the decision to treat should not be based on rapid antigen testing results. A negative test does not "rule out" influenza as commercially available tests are not sufficiently sensitive. You might want to check with your hospital to find out what other influenza testing is available in your locale.
• Which of my "egg-allergic" patients can receive influenza vaccine?
Decision making related to TIV depends on the type of prior reaction the patient had. Those with mild reactions, defined as hives alone, may receive TIV followed by a 30-minute observation period. Use the same vaccine for those who require a second dose, if at all possible. Consult an allergist for those with severe reactions including cardiovascular changes, respiratory and/or gastrointestinal tract symptoms, or reactions that required the use of epinephrine. An algorithm is available from the CDC that can be used to guide decision making in such cases.
Dr. Jackson is the chief of infectious diseases at Children’s Mercy Hospitals and Clinics in Kansas City, Mo., and professor of pediatrics at the University of Missouri–Kansas City. She said she has no relevant financial disclosures. E-mail her at [email protected].
Although the 2011-2012 influenza season was milder than usual and associated with fewer outpatient visits, lower hospitalization rates, and fewer pediatric deaths, practitioners should be aware that this virus is still the leading cause of vaccine-preventable deaths in children. Hopefully, practitioners are already providing influenza immunization utilizing the 2012-2013 vaccine, which contains the same influenza A (H1N1) antigen as the 2011-2012 seasonal vaccine but new influenza A (H3N2) and B antigens: These are A/California/7/2009 (H1N1)–like antigen, A/Victoria/361/2011(H3N2)–like antigen, and B/Wisconsin/1/2010–like antigen.
Here are the answers to some of the most common questions related to this year’s influenza season:
• How many doses are recommended this year for children between 6 months through 8 years of age?
For a child in this age group who had two or more doses of seasonal vaccine since July 1, 2010, or in whom you can document one dose of a pandemic H1N1–containing vaccine and at least two seasonal vaccines from any season, only one dose is needed. All others in this age group should receive two doses. As always, those 9 years of age and older receive one dose of vaccine.
• Given recent data that suggested a slight increased risk of a febrile seizure following trivalent inactivated vaccine (TIV) in children less than 4 years, have vaccine recommendations changed?
A suggestion of an increased risk for febrile seizures in young children after TIV was noted in the United States in 2010-2011. This followed enhanced monitoring after the observation in Australia in 2010 of an association with an increased risk of febrile seizures (greater than or equal to nine per 1,000 doses) that were related to a particular influenza vaccine. (Afluria vaccine is approved for use in those greater than age 5 years, but current recommendations from the American Academy of Pediatrics and the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices (ACIP) state this vaccine should not be used in those less than 9 years of age. It could be considered for a high-risk patient between 5 through 8 years if no alternative TIV is available and after discussion of the seizure risk with parents.)
To further study this potential association, the CDC tracked more than 200,000 children in the United States who received TIV or PCV13 (Prevnar) vaccine at different visits or both together at the same visit. Rarely, a febrile seizure was noted in children less than 5 years who got TIV or PCV13 vaccine given at separate visits. Those 12- to 23-month-olds who received both at the same visit had a slightly increased risk for an uncomplicated febrile seizure in the 24 hours following vaccine receipt. This is the age group where febrile seizures peak in general and is equivalent to one excess seizure for every 2,000-3,000 vaccine doses. Based on this low risk and the uncomplicated course in such patients, coupled with the benefits of immunization, both the AAP and ACIP recommended no change in either the TIV or PCV13 vaccine policies. And remember that neither a prior febrile seizure history nor a preexisting seizure disorder is considered a contraindication for influenza vaccine.
• Who can get the intradermal formulation of influenza vaccine?
Fluzone Intradermal, which made its debut in the 2011-2012 season, is licensed for those 18-64 years of age, and is a preservative-free, trivalent inactivated influenza vaccine. It comes in a prefilled microinjection syringe, which for those who require TIV and are needle averse, may be preferred. Local reactions seen with intramuscular TIV (with the exception of pain), including redness, swelling, and itching at the site seem to be a bit more common with the intradermal product, but such reactions abate in 3-7 days. This vaccine could be utilized in an older adolescent who might opt for a vaccine that has a needle that is 90% shorter than the needle used for intramuscular injection of TIV.
• Is oseltamivir still the drug of choice to treat influenza?
Last year only 1.4% of strains were resistant to oseltamivir, and this year is expected to be the same. Treatment and prophylaxis dosing is the same as last year. The AAP and ACIP continue to emphasize early treatment for all children in high-risk groups who develop influenza, regardless of influenza immunization status. Treatment is also recommended for all who are ill enough to require hospitalization. For patients with influenzalike illness, the decision to treat should not be based on rapid antigen testing results. A negative test does not "rule out" influenza as commercially available tests are not sufficiently sensitive. You might want to check with your hospital to find out what other influenza testing is available in your locale.
• Which of my "egg-allergic" patients can receive influenza vaccine?
Decision making related to TIV depends on the type of prior reaction the patient had. Those with mild reactions, defined as hives alone, may receive TIV followed by a 30-minute observation period. Use the same vaccine for those who require a second dose, if at all possible. Consult an allergist for those with severe reactions including cardiovascular changes, respiratory and/or gastrointestinal tract symptoms, or reactions that required the use of epinephrine. An algorithm is available from the CDC that can be used to guide decision making in such cases.
Dr. Jackson is the chief of infectious diseases at Children’s Mercy Hospitals and Clinics in Kansas City, Mo., and professor of pediatrics at the University of Missouri–Kansas City. She said she has no relevant financial disclosures. E-mail her at [email protected].
Although the 2011-2012 influenza season was milder than usual and associated with fewer outpatient visits, lower hospitalization rates, and fewer pediatric deaths, practitioners should be aware that this virus is still the leading cause of vaccine-preventable deaths in children. Hopefully, practitioners are already providing influenza immunization utilizing the 2012-2013 vaccine, which contains the same influenza A (H1N1) antigen as the 2011-2012 seasonal vaccine but new influenza A (H3N2) and B antigens: These are A/California/7/2009 (H1N1)–like antigen, A/Victoria/361/2011(H3N2)–like antigen, and B/Wisconsin/1/2010–like antigen.
Here are the answers to some of the most common questions related to this year’s influenza season:
• How many doses are recommended this year for children between 6 months through 8 years of age?
For a child in this age group who had two or more doses of seasonal vaccine since July 1, 2010, or in whom you can document one dose of a pandemic H1N1–containing vaccine and at least two seasonal vaccines from any season, only one dose is needed. All others in this age group should receive two doses. As always, those 9 years of age and older receive one dose of vaccine.
• Given recent data that suggested a slight increased risk of a febrile seizure following trivalent inactivated vaccine (TIV) in children less than 4 years, have vaccine recommendations changed?
A suggestion of an increased risk for febrile seizures in young children after TIV was noted in the United States in 2010-2011. This followed enhanced monitoring after the observation in Australia in 2010 of an association with an increased risk of febrile seizures (greater than or equal to nine per 1,000 doses) that were related to a particular influenza vaccine. (Afluria vaccine is approved for use in those greater than age 5 years, but current recommendations from the American Academy of Pediatrics and the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices (ACIP) state this vaccine should not be used in those less than 9 years of age. It could be considered for a high-risk patient between 5 through 8 years if no alternative TIV is available and after discussion of the seizure risk with parents.)
To further study this potential association, the CDC tracked more than 200,000 children in the United States who received TIV or PCV13 (Prevnar) vaccine at different visits or both together at the same visit. Rarely, a febrile seizure was noted in children less than 5 years who got TIV or PCV13 vaccine given at separate visits. Those 12- to 23-month-olds who received both at the same visit had a slightly increased risk for an uncomplicated febrile seizure in the 24 hours following vaccine receipt. This is the age group where febrile seizures peak in general and is equivalent to one excess seizure for every 2,000-3,000 vaccine doses. Based on this low risk and the uncomplicated course in such patients, coupled with the benefits of immunization, both the AAP and ACIP recommended no change in either the TIV or PCV13 vaccine policies. And remember that neither a prior febrile seizure history nor a preexisting seizure disorder is considered a contraindication for influenza vaccine.
• Who can get the intradermal formulation of influenza vaccine?
Fluzone Intradermal, which made its debut in the 2011-2012 season, is licensed for those 18-64 years of age, and is a preservative-free, trivalent inactivated influenza vaccine. It comes in a prefilled microinjection syringe, which for those who require TIV and are needle averse, may be preferred. Local reactions seen with intramuscular TIV (with the exception of pain), including redness, swelling, and itching at the site seem to be a bit more common with the intradermal product, but such reactions abate in 3-7 days. This vaccine could be utilized in an older adolescent who might opt for a vaccine that has a needle that is 90% shorter than the needle used for intramuscular injection of TIV.
• Is oseltamivir still the drug of choice to treat influenza?
Last year only 1.4% of strains were resistant to oseltamivir, and this year is expected to be the same. Treatment and prophylaxis dosing is the same as last year. The AAP and ACIP continue to emphasize early treatment for all children in high-risk groups who develop influenza, regardless of influenza immunization status. Treatment is also recommended for all who are ill enough to require hospitalization. For patients with influenzalike illness, the decision to treat should not be based on rapid antigen testing results. A negative test does not "rule out" influenza as commercially available tests are not sufficiently sensitive. You might want to check with your hospital to find out what other influenza testing is available in your locale.
• Which of my "egg-allergic" patients can receive influenza vaccine?
Decision making related to TIV depends on the type of prior reaction the patient had. Those with mild reactions, defined as hives alone, may receive TIV followed by a 30-minute observation period. Use the same vaccine for those who require a second dose, if at all possible. Consult an allergist for those with severe reactions including cardiovascular changes, respiratory and/or gastrointestinal tract symptoms, or reactions that required the use of epinephrine. An algorithm is available from the CDC that can be used to guide decision making in such cases.
Dr. Jackson is the chief of infectious diseases at Children’s Mercy Hospitals and Clinics in Kansas City, Mo., and professor of pediatrics at the University of Missouri–Kansas City. She said she has no relevant financial disclosures. E-mail her at [email protected].
To Combat West Nile Virus, Emphasize Prevention
The best way to deal with the recent resurgence in West Nile virus is to emphasize prevention.
West Nile virus (WNV) is back with a vengeance this year. As of Sept. 4, 2012, there were 1,993 reported cases of WNV disease in people, including 1,069 with neuroinvasive disease and 87 deaths. My state, Texas, is leading the pack with a total of 888 cases, 443 neuroinvasive disease cases, and 35 deaths. Texas’ West Nile problem is clearly the worst in the country. The next-highest number of total cases is only 119, in South Dakota.
According to the Centers for Disease Control and Prevention, the total of 1,993 cases is the highest number of WNV disease cases reported to the CDC through the first week in September since the virus was first detected in the United States in 1999. It’s not clear why this resurgence is happening now, or why Texas has been so hard hit. Some say that rising temperatures have resulted in an increased mosquito population, but here in Texas there is also a drought and mosquitos need moisture, so I’m not sure about that.
An arbovirus, WNV is usually transmitted to humans after a bite from an infected Culex mosquito. The transmission cycle is maintained between mosquito and vertebrate hosts, usually birds. Humans are actually an incidental and dead-end host. Though rare, person-to-person transmission has been documented through both blood transfusion and solid organ transplantation (N. Engl. J. Med. 2003;348:2196-203).
When speaking with your patients, it’s worth reemphasizing the methods for prevention. Parents should be instructed to apply one of the Environmental Protection Agency–registered insect repellents to their children before they go outside, using just enough to protect exposed skin. Products containing up to 30% DEET – but not higher – can be used in children older than 2 months of age. Products containing both DEET and a sunscreen should be avoided, since sunscreen needs to be applied more frequently.
Children should be covered up with clothing as much as possible when outside, and netting should be used over infant carriers. Outdoor exposure should be limited at dusk and dawn, when mosquitos are most active. Holes in screen doors should be repaired. Standing water, which attracts mosquitos, is a major hazard and should be avoided. Birdba ths and blow-up pools need to be emptied out often, and children should steer clear of puddles.
Here in Texas, the state health department has issued a statement preparing people for aerial spraying of chemicals to control the mosquitos. Each state most likely will develop its own recommendations, which should be available on the state health department website.
Routine screening of the U.S. blood supply was initiated in 2003, and no cases have been identified in donated blood since then. A single case of congenital infection also has been reported (MMWR 2002;51:1135-6). There are specific management guidelines for mother, fetus, and newborn if women are diagnosed with WNV during pregnancy (MMWR 2004;53:154-7).
Most people who become infected with WNV will be asymptomatic. About 1 in 5 who are infected will develop symptoms such as fever, headache, body aches, joint pains, vomiting, diarrhea, or rash after a 2-14 day incubation period. Less than 1% will develop neuroinvasive disease, but of those who do, about 10% are fatal.
It’s important to include WNV in your differential diagnosis for children who present with a febrile illness, meningitis, encephalitis, or acute flaccid paralysis, particularly if they’ve had exposure to mosquitos during the summer and early fall in endemic areas. The clinical presentation of neuroinvasive WNV is indistinguishable from those of other causes of viral meningitis and/or encephalitis. While the epidemiological characteristics of WNV disease in children are similar to those in adults, neuroinvasive disease due to WNV is more likely to manifest as meningitis in children than in older adults, who are more likely to develop encephalitis (Pediatrics 2009;123:e1084-9).
Because there is no specific treatment for WNV, and the majority of patients have a self-limited course, the diagnosis need not be made in every febrile patient. A definitive diagnosis should be sought in individuals with fever and acute neurologic symptoms who have recently been exposed to mosquitos, are solid organ transplant recipients, or are pregnant.
The presence of WNV-reactive IgM antibody in serum or cerebrospinal fluid supports a recent infection. However, anti-WNV IgM can persist up to a year in some people, so its presence may represent a prior infection. Moreover, if serum is tested within the first 10 days of illness, IgM antibody may not always be detected. Convalescent titers should be obtained 2-3 weeks following the onset of illness.
Treatment is supportive care. Standard precautions are recommended for hospitalized patients in the American Academy of Pediatrics Red Book on pages 792-5 (Red Book: 2012 Report of the Committee on Infectious Diseases. L.K. Pickering, Ed. 29th ed. Elk Grove Village, Ill.: American Academy of Pediatrics). Most people will recover from even the neuroinvasive manifestations of WNV, although symptoms may last for several weeks and those with severe cases may require hospitalization for supportive treatment. Serious sequelae can occur among those with underlying immune deficiencies.
For the most up-to-date information on WNV statistics from the CDC, click here.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures. Write to Dr. Word at [email protected].
The best way to deal with the recent resurgence in West Nile virus is to emphasize prevention.
West Nile virus (WNV) is back with a vengeance this year. As of Sept. 4, 2012, there were 1,993 reported cases of WNV disease in people, including 1,069 with neuroinvasive disease and 87 deaths. My state, Texas, is leading the pack with a total of 888 cases, 443 neuroinvasive disease cases, and 35 deaths. Texas’ West Nile problem is clearly the worst in the country. The next-highest number of total cases is only 119, in South Dakota.
According to the Centers for Disease Control and Prevention, the total of 1,993 cases is the highest number of WNV disease cases reported to the CDC through the first week in September since the virus was first detected in the United States in 1999. It’s not clear why this resurgence is happening now, or why Texas has been so hard hit. Some say that rising temperatures have resulted in an increased mosquito population, but here in Texas there is also a drought and mosquitos need moisture, so I’m not sure about that.
An arbovirus, WNV is usually transmitted to humans after a bite from an infected Culex mosquito. The transmission cycle is maintained between mosquito and vertebrate hosts, usually birds. Humans are actually an incidental and dead-end host. Though rare, person-to-person transmission has been documented through both blood transfusion and solid organ transplantation (N. Engl. J. Med. 2003;348:2196-203).
When speaking with your patients, it’s worth reemphasizing the methods for prevention. Parents should be instructed to apply one of the Environmental Protection Agency–registered insect repellents to their children before they go outside, using just enough to protect exposed skin. Products containing up to 30% DEET – but not higher – can be used in children older than 2 months of age. Products containing both DEET and a sunscreen should be avoided, since sunscreen needs to be applied more frequently.
Children should be covered up with clothing as much as possible when outside, and netting should be used over infant carriers. Outdoor exposure should be limited at dusk and dawn, when mosquitos are most active. Holes in screen doors should be repaired. Standing water, which attracts mosquitos, is a major hazard and should be avoided. Birdba ths and blow-up pools need to be emptied out often, and children should steer clear of puddles.
Here in Texas, the state health department has issued a statement preparing people for aerial spraying of chemicals to control the mosquitos. Each state most likely will develop its own recommendations, which should be available on the state health department website.
Routine screening of the U.S. blood supply was initiated in 2003, and no cases have been identified in donated blood since then. A single case of congenital infection also has been reported (MMWR 2002;51:1135-6). There are specific management guidelines for mother, fetus, and newborn if women are diagnosed with WNV during pregnancy (MMWR 2004;53:154-7).
Most people who become infected with WNV will be asymptomatic. About 1 in 5 who are infected will develop symptoms such as fever, headache, body aches, joint pains, vomiting, diarrhea, or rash after a 2-14 day incubation period. Less than 1% will develop neuroinvasive disease, but of those who do, about 10% are fatal.
It’s important to include WNV in your differential diagnosis for children who present with a febrile illness, meningitis, encephalitis, or acute flaccid paralysis, particularly if they’ve had exposure to mosquitos during the summer and early fall in endemic areas. The clinical presentation of neuroinvasive WNV is indistinguishable from those of other causes of viral meningitis and/or encephalitis. While the epidemiological characteristics of WNV disease in children are similar to those in adults, neuroinvasive disease due to WNV is more likely to manifest as meningitis in children than in older adults, who are more likely to develop encephalitis (Pediatrics 2009;123:e1084-9).
Because there is no specific treatment for WNV, and the majority of patients have a self-limited course, the diagnosis need not be made in every febrile patient. A definitive diagnosis should be sought in individuals with fever and acute neurologic symptoms who have recently been exposed to mosquitos, are solid organ transplant recipients, or are pregnant.
The presence of WNV-reactive IgM antibody in serum or cerebrospinal fluid supports a recent infection. However, anti-WNV IgM can persist up to a year in some people, so its presence may represent a prior infection. Moreover, if serum is tested within the first 10 days of illness, IgM antibody may not always be detected. Convalescent titers should be obtained 2-3 weeks following the onset of illness.
Treatment is supportive care. Standard precautions are recommended for hospitalized patients in the American Academy of Pediatrics Red Book on pages 792-5 (Red Book: 2012 Report of the Committee on Infectious Diseases. L.K. Pickering, Ed. 29th ed. Elk Grove Village, Ill.: American Academy of Pediatrics). Most people will recover from even the neuroinvasive manifestations of WNV, although symptoms may last for several weeks and those with severe cases may require hospitalization for supportive treatment. Serious sequelae can occur among those with underlying immune deficiencies.
For the most up-to-date information on WNV statistics from the CDC, click here.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures. Write to Dr. Word at [email protected].
The best way to deal with the recent resurgence in West Nile virus is to emphasize prevention.
West Nile virus (WNV) is back with a vengeance this year. As of Sept. 4, 2012, there were 1,993 reported cases of WNV disease in people, including 1,069 with neuroinvasive disease and 87 deaths. My state, Texas, is leading the pack with a total of 888 cases, 443 neuroinvasive disease cases, and 35 deaths. Texas’ West Nile problem is clearly the worst in the country. The next-highest number of total cases is only 119, in South Dakota.
According to the Centers for Disease Control and Prevention, the total of 1,993 cases is the highest number of WNV disease cases reported to the CDC through the first week in September since the virus was first detected in the United States in 1999. It’s not clear why this resurgence is happening now, or why Texas has been so hard hit. Some say that rising temperatures have resulted in an increased mosquito population, but here in Texas there is also a drought and mosquitos need moisture, so I’m not sure about that.
An arbovirus, WNV is usually transmitted to humans after a bite from an infected Culex mosquito. The transmission cycle is maintained between mosquito and vertebrate hosts, usually birds. Humans are actually an incidental and dead-end host. Though rare, person-to-person transmission has been documented through both blood transfusion and solid organ transplantation (N. Engl. J. Med. 2003;348:2196-203).
When speaking with your patients, it’s worth reemphasizing the methods for prevention. Parents should be instructed to apply one of the Environmental Protection Agency–registered insect repellents to their children before they go outside, using just enough to protect exposed skin. Products containing up to 30% DEET – but not higher – can be used in children older than 2 months of age. Products containing both DEET and a sunscreen should be avoided, since sunscreen needs to be applied more frequently.
Children should be covered up with clothing as much as possible when outside, and netting should be used over infant carriers. Outdoor exposure should be limited at dusk and dawn, when mosquitos are most active. Holes in screen doors should be repaired. Standing water, which attracts mosquitos, is a major hazard and should be avoided. Birdba ths and blow-up pools need to be emptied out often, and children should steer clear of puddles.
Here in Texas, the state health department has issued a statement preparing people for aerial spraying of chemicals to control the mosquitos. Each state most likely will develop its own recommendations, which should be available on the state health department website.
Routine screening of the U.S. blood supply was initiated in 2003, and no cases have been identified in donated blood since then. A single case of congenital infection also has been reported (MMWR 2002;51:1135-6). There are specific management guidelines for mother, fetus, and newborn if women are diagnosed with WNV during pregnancy (MMWR 2004;53:154-7).
Most people who become infected with WNV will be asymptomatic. About 1 in 5 who are infected will develop symptoms such as fever, headache, body aches, joint pains, vomiting, diarrhea, or rash after a 2-14 day incubation period. Less than 1% will develop neuroinvasive disease, but of those who do, about 10% are fatal.
It’s important to include WNV in your differential diagnosis for children who present with a febrile illness, meningitis, encephalitis, or acute flaccid paralysis, particularly if they’ve had exposure to mosquitos during the summer and early fall in endemic areas. The clinical presentation of neuroinvasive WNV is indistinguishable from those of other causes of viral meningitis and/or encephalitis. While the epidemiological characteristics of WNV disease in children are similar to those in adults, neuroinvasive disease due to WNV is more likely to manifest as meningitis in children than in older adults, who are more likely to develop encephalitis (Pediatrics 2009;123:e1084-9).
Because there is no specific treatment for WNV, and the majority of patients have a self-limited course, the diagnosis need not be made in every febrile patient. A definitive diagnosis should be sought in individuals with fever and acute neurologic symptoms who have recently been exposed to mosquitos, are solid organ transplant recipients, or are pregnant.
The presence of WNV-reactive IgM antibody in serum or cerebrospinal fluid supports a recent infection. However, anti-WNV IgM can persist up to a year in some people, so its presence may represent a prior infection. Moreover, if serum is tested within the first 10 days of illness, IgM antibody may not always be detected. Convalescent titers should be obtained 2-3 weeks following the onset of illness.
Treatment is supportive care. Standard precautions are recommended for hospitalized patients in the American Academy of Pediatrics Red Book on pages 792-5 (Red Book: 2012 Report of the Committee on Infectious Diseases. L.K. Pickering, Ed. 29th ed. Elk Grove Village, Ill.: American Academy of Pediatrics). Most people will recover from even the neuroinvasive manifestations of WNV, although symptoms may last for several weeks and those with severe cases may require hospitalization for supportive treatment. Serious sequelae can occur among those with underlying immune deficiencies.
For the most up-to-date information on WNV statistics from the CDC, click here.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures. Write to Dr. Word at [email protected].
New Paradigms in HIV Prevention
The recent International AIDS Conference held in Washington highlighted new paradigms in HIV prevention. The biennial meeting brought more than 20,000 attendees together to focus on the political and social – as well as the clinical – aspects of the HIV/AIDS epidemic.
A major discussion topic was the new approach to HIV prevention. Back in the early 1980s, "safe sex" via condoms and abstinence was the focus of prevention strategies. In 2006, the Centers for Disease Control and Prevention recommended routine testing for everyone, in order to broaden the potential for both treatment and prevention (MMWR 2006;55(RR-14);1-17). But in the past 3-4 years, we’ve expanded the concept of prevention in the following three ways:
• Verbal consent. In July, Massachusetts became the 49th state to stop requiring written consent for HIV testing. Other states have done the same over the last 18-24 months. Now the test is explained to the patient, and the patient can simply agree verbally to be tested for HIV, without having to sign a form. We believe that’s a significant step forward, because the requirement for a signature made HIV testing different from any other medical test, which often scared patients and resulted in their reluctance to be tested.
• Treatment as prevention. In August 2011, the landmark HIV Prevention Trial 052 (HPTN 052) showed that early antiviral therapy in HIV-infected individuals who were in serodiscordant sexual relationships reduced transmission to their uninfected partners by 96% (N. Engl. J. Med. 2011;365:493-505). This means that now, part of the clinical decision about starting treatment involves consideration of the patient’s sexual contacts and their protection as well.
There is still concern about the side effects (such as insomnia, gastrointestinal problems, and bone demineralization) of antiretrovirals, as well as uncertainty about long-term adherence when treatment is started early. However, the new data on transmission have shifted the risk/benefit equation in favor of treating more people. In HPTN 052, treatment was started at CD4 counts of 350-550 cells per mcL. Now, many experts advocate starting HIV-infected individuals at T cell counts of 500 or greater, for both improved outcome in the individual and reduction in the risk of HIV transmission.
• Prophylaxis as prevention. With the approval of the combination emtricitabine and tenofovir disoproxil fumarate (Truvada) for pre-exposure prophylaxis, we now have the option of not only treating the HIV-infected partner in serodiscordant couples, but also prophylaxing the uninfected partner in order to prevent transmission.
To do this, the individual must be tested and prove to be HIV negative prior to starting on pre-exposure prophylaxis. Regular testing must also be done every 3-6 months thereafter for as long as the person is taking Truvada. Such testing is necessary because Truvada (which comprises two reverse transcriptase inhibitors) does not sufficiently suppress viral replication by itself, and must be used in combination with antiretrovirals of other classes in the treatment of HIV-infected individuals. If a person were to become HIV infected while taking only Truvada without other classes of antiretrovirals, there is a high risk for the development of resistance as a result of mutations in the replicating virus.
Of course, it could be argued that it is more ethical to treat the person who actually has the disease than to subject an uninfected person to potential antiretroviral toxicity. However, consider the following case I saw recently: A pregnant woman said that her HIV-infected partner was taking his medication, but the partner’s physician said he hadn\'t seen the patient in many months and didn’t know if he was still taking the antiretrovirals. The woman was in labor, and said she’d had sex with the man recently. If there had been time, we would have started her on nevirapine and AZT (zidovudine) prior to delivery. But in this case, she delivered too quickly. So we put the infant on antiretrovirals and tested the mother a month later. She was negative, so we were able to take the infant off the drugs.
There are many examples like this, in which the real world doesn’t quite line up with what we try to achieve through research and guidelines.
Of course, cost is a consideration as well. Insurance will typically cover some or all of the cost ($800-$1,000 per month) for antiretroviral treatment, but it’s too early to know whether that coverage will extend to prophylaxis. I think the cost will limit its use in that capacity, at least until reasonable clinical guidelines are developed to determine which patients would be best targeted with this approach. And just to note: Although Truvada is approved for treating those as young as age 12 years, thus far its use as prophylaxis is restricted to adults aged 18 years and older.
Dr. Pelton is chief of pediatric infectious disease and coordinator of the maternal-child HIV program at Boston Medical Center. He said he had no relevant financial disclosures.
The recent International AIDS Conference held in Washington highlighted new paradigms in HIV prevention. The biennial meeting brought more than 20,000 attendees together to focus on the political and social – as well as the clinical – aspects of the HIV/AIDS epidemic.
A major discussion topic was the new approach to HIV prevention. Back in the early 1980s, "safe sex" via condoms and abstinence was the focus of prevention strategies. In 2006, the Centers for Disease Control and Prevention recommended routine testing for everyone, in order to broaden the potential for both treatment and prevention (MMWR 2006;55(RR-14);1-17). But in the past 3-4 years, we’ve expanded the concept of prevention in the following three ways:
• Verbal consent. In July, Massachusetts became the 49th state to stop requiring written consent for HIV testing. Other states have done the same over the last 18-24 months. Now the test is explained to the patient, and the patient can simply agree verbally to be tested for HIV, without having to sign a form. We believe that’s a significant step forward, because the requirement for a signature made HIV testing different from any other medical test, which often scared patients and resulted in their reluctance to be tested.
• Treatment as prevention. In August 2011, the landmark HIV Prevention Trial 052 (HPTN 052) showed that early antiviral therapy in HIV-infected individuals who were in serodiscordant sexual relationships reduced transmission to their uninfected partners by 96% (N. Engl. J. Med. 2011;365:493-505). This means that now, part of the clinical decision about starting treatment involves consideration of the patient’s sexual contacts and their protection as well.
There is still concern about the side effects (such as insomnia, gastrointestinal problems, and bone demineralization) of antiretrovirals, as well as uncertainty about long-term adherence when treatment is started early. However, the new data on transmission have shifted the risk/benefit equation in favor of treating more people. In HPTN 052, treatment was started at CD4 counts of 350-550 cells per mcL. Now, many experts advocate starting HIV-infected individuals at T cell counts of 500 or greater, for both improved outcome in the individual and reduction in the risk of HIV transmission.
• Prophylaxis as prevention. With the approval of the combination emtricitabine and tenofovir disoproxil fumarate (Truvada) for pre-exposure prophylaxis, we now have the option of not only treating the HIV-infected partner in serodiscordant couples, but also prophylaxing the uninfected partner in order to prevent transmission.
To do this, the individual must be tested and prove to be HIV negative prior to starting on pre-exposure prophylaxis. Regular testing must also be done every 3-6 months thereafter for as long as the person is taking Truvada. Such testing is necessary because Truvada (which comprises two reverse transcriptase inhibitors) does not sufficiently suppress viral replication by itself, and must be used in combination with antiretrovirals of other classes in the treatment of HIV-infected individuals. If a person were to become HIV infected while taking only Truvada without other classes of antiretrovirals, there is a high risk for the development of resistance as a result of mutations in the replicating virus.
Of course, it could be argued that it is more ethical to treat the person who actually has the disease than to subject an uninfected person to potential antiretroviral toxicity. However, consider the following case I saw recently: A pregnant woman said that her HIV-infected partner was taking his medication, but the partner’s physician said he hadn\'t seen the patient in many months and didn’t know if he was still taking the antiretrovirals. The woman was in labor, and said she’d had sex with the man recently. If there had been time, we would have started her on nevirapine and AZT (zidovudine) prior to delivery. But in this case, she delivered too quickly. So we put the infant on antiretrovirals and tested the mother a month later. She was negative, so we were able to take the infant off the drugs.
There are many examples like this, in which the real world doesn’t quite line up with what we try to achieve through research and guidelines.
Of course, cost is a consideration as well. Insurance will typically cover some or all of the cost ($800-$1,000 per month) for antiretroviral treatment, but it’s too early to know whether that coverage will extend to prophylaxis. I think the cost will limit its use in that capacity, at least until reasonable clinical guidelines are developed to determine which patients would be best targeted with this approach. And just to note: Although Truvada is approved for treating those as young as age 12 years, thus far its use as prophylaxis is restricted to adults aged 18 years and older.
Dr. Pelton is chief of pediatric infectious disease and coordinator of the maternal-child HIV program at Boston Medical Center. He said he had no relevant financial disclosures.
The recent International AIDS Conference held in Washington highlighted new paradigms in HIV prevention. The biennial meeting brought more than 20,000 attendees together to focus on the political and social – as well as the clinical – aspects of the HIV/AIDS epidemic.
A major discussion topic was the new approach to HIV prevention. Back in the early 1980s, "safe sex" via condoms and abstinence was the focus of prevention strategies. In 2006, the Centers for Disease Control and Prevention recommended routine testing for everyone, in order to broaden the potential for both treatment and prevention (MMWR 2006;55(RR-14);1-17). But in the past 3-4 years, we’ve expanded the concept of prevention in the following three ways:
• Verbal consent. In July, Massachusetts became the 49th state to stop requiring written consent for HIV testing. Other states have done the same over the last 18-24 months. Now the test is explained to the patient, and the patient can simply agree verbally to be tested for HIV, without having to sign a form. We believe that’s a significant step forward, because the requirement for a signature made HIV testing different from any other medical test, which often scared patients and resulted in their reluctance to be tested.
• Treatment as prevention. In August 2011, the landmark HIV Prevention Trial 052 (HPTN 052) showed that early antiviral therapy in HIV-infected individuals who were in serodiscordant sexual relationships reduced transmission to their uninfected partners by 96% (N. Engl. J. Med. 2011;365:493-505). This means that now, part of the clinical decision about starting treatment involves consideration of the patient’s sexual contacts and their protection as well.
There is still concern about the side effects (such as insomnia, gastrointestinal problems, and bone demineralization) of antiretrovirals, as well as uncertainty about long-term adherence when treatment is started early. However, the new data on transmission have shifted the risk/benefit equation in favor of treating more people. In HPTN 052, treatment was started at CD4 counts of 350-550 cells per mcL. Now, many experts advocate starting HIV-infected individuals at T cell counts of 500 or greater, for both improved outcome in the individual and reduction in the risk of HIV transmission.
• Prophylaxis as prevention. With the approval of the combination emtricitabine and tenofovir disoproxil fumarate (Truvada) for pre-exposure prophylaxis, we now have the option of not only treating the HIV-infected partner in serodiscordant couples, but also prophylaxing the uninfected partner in order to prevent transmission.
To do this, the individual must be tested and prove to be HIV negative prior to starting on pre-exposure prophylaxis. Regular testing must also be done every 3-6 months thereafter for as long as the person is taking Truvada. Such testing is necessary because Truvada (which comprises two reverse transcriptase inhibitors) does not sufficiently suppress viral replication by itself, and must be used in combination with antiretrovirals of other classes in the treatment of HIV-infected individuals. If a person were to become HIV infected while taking only Truvada without other classes of antiretrovirals, there is a high risk for the development of resistance as a result of mutations in the replicating virus.
Of course, it could be argued that it is more ethical to treat the person who actually has the disease than to subject an uninfected person to potential antiretroviral toxicity. However, consider the following case I saw recently: A pregnant woman said that her HIV-infected partner was taking his medication, but the partner’s physician said he hadn\'t seen the patient in many months and didn’t know if he was still taking the antiretrovirals. The woman was in labor, and said she’d had sex with the man recently. If there had been time, we would have started her on nevirapine and AZT (zidovudine) prior to delivery. But in this case, she delivered too quickly. So we put the infant on antiretrovirals and tested the mother a month later. She was negative, so we were able to take the infant off the drugs.
There are many examples like this, in which the real world doesn’t quite line up with what we try to achieve through research and guidelines.
Of course, cost is a consideration as well. Insurance will typically cover some or all of the cost ($800-$1,000 per month) for antiretroviral treatment, but it’s too early to know whether that coverage will extend to prophylaxis. I think the cost will limit its use in that capacity, at least until reasonable clinical guidelines are developed to determine which patients would be best targeted with this approach. And just to note: Although Truvada is approved for treating those as young as age 12 years, thus far its use as prophylaxis is restricted to adults aged 18 years and older.
Dr. Pelton is chief of pediatric infectious disease and coordinator of the maternal-child HIV program at Boston Medical Center. He said he had no relevant financial disclosures.
WBC Count Prevents Unnecessary Antibiotics for Enterovirus
It’s summertime, which means that pediatric and family medicine offices are filled with children who have enteroviral infections, and too many of them will receive inappropriate antibiotic prescriptions.
From June through September, children who present with fever and other flulike symptoms nearly always have a nonpolio enterovirus (NPEV) infection, with coxsackievirus groups A and B, echoviruses, and the newer numbered enteroviruses being the most common in the United States. Epidemiologic surveillance suggests that 10 million to 15 million illnesses attributable to NPEV occur in the United States each year. I tell parents that their child has a "summer flu," which helps to communicate that it is usually self-limited and that antibiotics will not work to treat it.
A sudden high fever is usually what brings the child in, and the classic blistering hand-foot-mouth presentation of enterovirus helps us nail down the diagnosis. But sometimes, especially in younger children, symptoms such as myalgia, malaise, irritability, upper respiratory symptoms, or gastrointestinal symptoms may be nonspecific. In addition, the fever can precede hand-foot-mouth blisters by several days. A 9-month-old with a sudden fever of 103° F and a mildly red-looking throat can cause panic for parents, and a physician will often be led to prescribe an antibiotic out of an abundance of caution. In fact, more than 80% of inappropriate antibiotic use in the summertime is for febrile illness related to early enteroviral infection combined with worried parents and physicians.
Obtaining a complete blood count and differential can solve this problem, but it’s often not done because typically it involves sending the patient out for the blood work and waiting 4-6 hours for the results. Depending on when the test is done, results may not be available until the following day. It’s much simpler to "cover" with an antibiotic than to order the diagnostic test.
But I believe we should be using the white blood cell (WBC) count rather than prescribing antibiotics. The WBC count will almost always be low, and if a differential is added it will show a predominance of lymphocytes, indicating a viral infection. With that, parents and physicians can be reassured it’s enterovirus, and that no antibiotic is required.
In recent years, large pediatric practices and urgent care centers have begun purchasing a point-of-care WBC test made by Hemacue. Currently, it is licensed for use only in level 3 Clinical Laboratory Improvement Amendments (CLIA) facilities. A couple of years ago, I testified before a Food and Drug Administration device panel urging broader availability for the machine in clinical practice, but the panel had concerns that physicians would overrely on the WBC – the machine doesn’t give the differential – and possibly miss a more significant illness, such as leukemia.
Of course, the current widespread practice of empirically prescribing an antibiotic with no lab testing won’t pick up leukemia, either. In my view, the point-of-care test is an aid. It’s a piece of information you add to your clinical history and physical exam that assists in your diagnosis. I believe the benefits of avoiding unnecessary antibiotic use in millions of children every summer far outweigh the theoretical possibility that pediatricians would not rely on the broader context, including the time of year, disease rates in the community, and their clinical judgment. I really believe that WBC testing constitutes better care.
My colleagues and I have published two papers regarding the use of the WBC test to aid in judicious antibiotic use. In one, a prospective, 3-year study of 1,956 patients aged 3 months to 21 years with acute upper respiratory illness and fever, 737 did not have a diagnosis established by history and physical. Of those patients, we had WBC counts done for 351 children who appeared ill, had a temperature greater than 101° F, and parents who were demanding an antibiotic or physicians who were inclined to give an antibiotic. Of those, just 14 had a WBC count of 15,000/mcL or greater, and an antibiotic was prescribed for 13 of them. With the selective use of WBC testing, no child had significant bacterial illness that was missed (Clin. Pediatr. [Phila] 2003;42:113-9).
In another study of 120 acutely ill children and potential antibiotic recipients, we found that the point-of-care Hemacue WBC device produced comparable results to the Cell-Dyn countertop machine for total WBC counts (Clin. Pediatr. [Phila] 2009;48:291-4).
I’d just like to add a few more points about enterovirus to keep in mind when you advise patients and families. There is a long-held notion that summer viruses enteroviruses in particular – are of shorter duration than are winter viruses such as influenza, parainfluenza, and respiratory syncytial virus. That’s actually not true. Several years ago, my colleagues and I prospectively studied 380 children aged 4-18 years with systemic NPEV syndromes who presented to private suburban pediatric practices. Overall, NPEV infections were virologically confirmed in 122 of 372 patients (33%). The median duration of illness was 6 days for those with rash, 7 days for those with hand-foot-mouth and viral meningitis, 8 days for those with pleurodynia, and 9 days for those with myalgia/malaise (Pediatrics 1998;102:1126-34). Many children were ill for 10 days to 2 weeks.
Interestingly, in that study we also found that in more than half of the families, there was more than one individual with NPEV illness that was often similar but sometimes had a different clinical presentation. For example, one child might have classic hand-foot-mouth while another just has upper respiratory symptoms or gastrointestinal manifestations. This creates a confusing picture for the family because it is not intuitive to attribute different clinical presentations to the same viral etiology and, as a consequence, medical care may be sought more often.
We also found that 20% of the mothers caught the illness from the child, compared with fewer than 10% of the fathers. Luckily, the mother’s illness was usually less severe.
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Rochester (N.Y.) General Research Institute. He is also a pediatrician at Legacy Pediatrics in Rochester. Dr. Pichichero’s institution received a research grant in 2008 from Hemacue, and he has served as a paid consultant to testify before the FDA in 2009 for Hemacue. He said he has no other conflicts to declare.
It’s summertime, which means that pediatric and family medicine offices are filled with children who have enteroviral infections, and too many of them will receive inappropriate antibiotic prescriptions.
From June through September, children who present with fever and other flulike symptoms nearly always have a nonpolio enterovirus (NPEV) infection, with coxsackievirus groups A and B, echoviruses, and the newer numbered enteroviruses being the most common in the United States. Epidemiologic surveillance suggests that 10 million to 15 million illnesses attributable to NPEV occur in the United States each year. I tell parents that their child has a "summer flu," which helps to communicate that it is usually self-limited and that antibiotics will not work to treat it.
A sudden high fever is usually what brings the child in, and the classic blistering hand-foot-mouth presentation of enterovirus helps us nail down the diagnosis. But sometimes, especially in younger children, symptoms such as myalgia, malaise, irritability, upper respiratory symptoms, or gastrointestinal symptoms may be nonspecific. In addition, the fever can precede hand-foot-mouth blisters by several days. A 9-month-old with a sudden fever of 103° F and a mildly red-looking throat can cause panic for parents, and a physician will often be led to prescribe an antibiotic out of an abundance of caution. In fact, more than 80% of inappropriate antibiotic use in the summertime is for febrile illness related to early enteroviral infection combined with worried parents and physicians.
Obtaining a complete blood count and differential can solve this problem, but it’s often not done because typically it involves sending the patient out for the blood work and waiting 4-6 hours for the results. Depending on when the test is done, results may not be available until the following day. It’s much simpler to "cover" with an antibiotic than to order the diagnostic test.
But I believe we should be using the white blood cell (WBC) count rather than prescribing antibiotics. The WBC count will almost always be low, and if a differential is added it will show a predominance of lymphocytes, indicating a viral infection. With that, parents and physicians can be reassured it’s enterovirus, and that no antibiotic is required.
In recent years, large pediatric practices and urgent care centers have begun purchasing a point-of-care WBC test made by Hemacue. Currently, it is licensed for use only in level 3 Clinical Laboratory Improvement Amendments (CLIA) facilities. A couple of years ago, I testified before a Food and Drug Administration device panel urging broader availability for the machine in clinical practice, but the panel had concerns that physicians would overrely on the WBC – the machine doesn’t give the differential – and possibly miss a more significant illness, such as leukemia.
Of course, the current widespread practice of empirically prescribing an antibiotic with no lab testing won’t pick up leukemia, either. In my view, the point-of-care test is an aid. It’s a piece of information you add to your clinical history and physical exam that assists in your diagnosis. I believe the benefits of avoiding unnecessary antibiotic use in millions of children every summer far outweigh the theoretical possibility that pediatricians would not rely on the broader context, including the time of year, disease rates in the community, and their clinical judgment. I really believe that WBC testing constitutes better care.
My colleagues and I have published two papers regarding the use of the WBC test to aid in judicious antibiotic use. In one, a prospective, 3-year study of 1,956 patients aged 3 months to 21 years with acute upper respiratory illness and fever, 737 did not have a diagnosis established by history and physical. Of those patients, we had WBC counts done for 351 children who appeared ill, had a temperature greater than 101° F, and parents who were demanding an antibiotic or physicians who were inclined to give an antibiotic. Of those, just 14 had a WBC count of 15,000/mcL or greater, and an antibiotic was prescribed for 13 of them. With the selective use of WBC testing, no child had significant bacterial illness that was missed (Clin. Pediatr. [Phila] 2003;42:113-9).
In another study of 120 acutely ill children and potential antibiotic recipients, we found that the point-of-care Hemacue WBC device produced comparable results to the Cell-Dyn countertop machine for total WBC counts (Clin. Pediatr. [Phila] 2009;48:291-4).
I’d just like to add a few more points about enterovirus to keep in mind when you advise patients and families. There is a long-held notion that summer viruses enteroviruses in particular – are of shorter duration than are winter viruses such as influenza, parainfluenza, and respiratory syncytial virus. That’s actually not true. Several years ago, my colleagues and I prospectively studied 380 children aged 4-18 years with systemic NPEV syndromes who presented to private suburban pediatric practices. Overall, NPEV infections were virologically confirmed in 122 of 372 patients (33%). The median duration of illness was 6 days for those with rash, 7 days for those with hand-foot-mouth and viral meningitis, 8 days for those with pleurodynia, and 9 days for those with myalgia/malaise (Pediatrics 1998;102:1126-34). Many children were ill for 10 days to 2 weeks.
Interestingly, in that study we also found that in more than half of the families, there was more than one individual with NPEV illness that was often similar but sometimes had a different clinical presentation. For example, one child might have classic hand-foot-mouth while another just has upper respiratory symptoms or gastrointestinal manifestations. This creates a confusing picture for the family because it is not intuitive to attribute different clinical presentations to the same viral etiology and, as a consequence, medical care may be sought more often.
We also found that 20% of the mothers caught the illness from the child, compared with fewer than 10% of the fathers. Luckily, the mother’s illness was usually less severe.
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Rochester (N.Y.) General Research Institute. He is also a pediatrician at Legacy Pediatrics in Rochester. Dr. Pichichero’s institution received a research grant in 2008 from Hemacue, and he has served as a paid consultant to testify before the FDA in 2009 for Hemacue. He said he has no other conflicts to declare.
It’s summertime, which means that pediatric and family medicine offices are filled with children who have enteroviral infections, and too many of them will receive inappropriate antibiotic prescriptions.
From June through September, children who present with fever and other flulike symptoms nearly always have a nonpolio enterovirus (NPEV) infection, with coxsackievirus groups A and B, echoviruses, and the newer numbered enteroviruses being the most common in the United States. Epidemiologic surveillance suggests that 10 million to 15 million illnesses attributable to NPEV occur in the United States each year. I tell parents that their child has a "summer flu," which helps to communicate that it is usually self-limited and that antibiotics will not work to treat it.
A sudden high fever is usually what brings the child in, and the classic blistering hand-foot-mouth presentation of enterovirus helps us nail down the diagnosis. But sometimes, especially in younger children, symptoms such as myalgia, malaise, irritability, upper respiratory symptoms, or gastrointestinal symptoms may be nonspecific. In addition, the fever can precede hand-foot-mouth blisters by several days. A 9-month-old with a sudden fever of 103° F and a mildly red-looking throat can cause panic for parents, and a physician will often be led to prescribe an antibiotic out of an abundance of caution. In fact, more than 80% of inappropriate antibiotic use in the summertime is for febrile illness related to early enteroviral infection combined with worried parents and physicians.
Obtaining a complete blood count and differential can solve this problem, but it’s often not done because typically it involves sending the patient out for the blood work and waiting 4-6 hours for the results. Depending on when the test is done, results may not be available until the following day. It’s much simpler to "cover" with an antibiotic than to order the diagnostic test.
But I believe we should be using the white blood cell (WBC) count rather than prescribing antibiotics. The WBC count will almost always be low, and if a differential is added it will show a predominance of lymphocytes, indicating a viral infection. With that, parents and physicians can be reassured it’s enterovirus, and that no antibiotic is required.
In recent years, large pediatric practices and urgent care centers have begun purchasing a point-of-care WBC test made by Hemacue. Currently, it is licensed for use only in level 3 Clinical Laboratory Improvement Amendments (CLIA) facilities. A couple of years ago, I testified before a Food and Drug Administration device panel urging broader availability for the machine in clinical practice, but the panel had concerns that physicians would overrely on the WBC – the machine doesn’t give the differential – and possibly miss a more significant illness, such as leukemia.
Of course, the current widespread practice of empirically prescribing an antibiotic with no lab testing won’t pick up leukemia, either. In my view, the point-of-care test is an aid. It’s a piece of information you add to your clinical history and physical exam that assists in your diagnosis. I believe the benefits of avoiding unnecessary antibiotic use in millions of children every summer far outweigh the theoretical possibility that pediatricians would not rely on the broader context, including the time of year, disease rates in the community, and their clinical judgment. I really believe that WBC testing constitutes better care.
My colleagues and I have published two papers regarding the use of the WBC test to aid in judicious antibiotic use. In one, a prospective, 3-year study of 1,956 patients aged 3 months to 21 years with acute upper respiratory illness and fever, 737 did not have a diagnosis established by history and physical. Of those patients, we had WBC counts done for 351 children who appeared ill, had a temperature greater than 101° F, and parents who were demanding an antibiotic or physicians who were inclined to give an antibiotic. Of those, just 14 had a WBC count of 15,000/mcL or greater, and an antibiotic was prescribed for 13 of them. With the selective use of WBC testing, no child had significant bacterial illness that was missed (Clin. Pediatr. [Phila] 2003;42:113-9).
In another study of 120 acutely ill children and potential antibiotic recipients, we found that the point-of-care Hemacue WBC device produced comparable results to the Cell-Dyn countertop machine for total WBC counts (Clin. Pediatr. [Phila] 2009;48:291-4).
I’d just like to add a few more points about enterovirus to keep in mind when you advise patients and families. There is a long-held notion that summer viruses enteroviruses in particular – are of shorter duration than are winter viruses such as influenza, parainfluenza, and respiratory syncytial virus. That’s actually not true. Several years ago, my colleagues and I prospectively studied 380 children aged 4-18 years with systemic NPEV syndromes who presented to private suburban pediatric practices. Overall, NPEV infections were virologically confirmed in 122 of 372 patients (33%). The median duration of illness was 6 days for those with rash, 7 days for those with hand-foot-mouth and viral meningitis, 8 days for those with pleurodynia, and 9 days for those with myalgia/malaise (Pediatrics 1998;102:1126-34). Many children were ill for 10 days to 2 weeks.
Interestingly, in that study we also found that in more than half of the families, there was more than one individual with NPEV illness that was often similar but sometimes had a different clinical presentation. For example, one child might have classic hand-foot-mouth while another just has upper respiratory symptoms or gastrointestinal manifestations. This creates a confusing picture for the family because it is not intuitive to attribute different clinical presentations to the same viral etiology and, as a consequence, medical care may be sought more often.
We also found that 20% of the mothers caught the illness from the child, compared with fewer than 10% of the fathers. Luckily, the mother’s illness was usually less severe.
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Rochester (N.Y.) General Research Institute. He is also a pediatrician at Legacy Pediatrics in Rochester. Dr. Pichichero’s institution received a research grant in 2008 from Hemacue, and he has served as a paid consultant to testify before the FDA in 2009 for Hemacue. He said he has no other conflicts to declare.
Measles Is Now a Worldwide Threat
Measles is a threat that we need to take seriously, both domestically and when traveling anywhere abroad.
During 2011, the United States had more cases of measles than we have had in 15 years. There were 222 cases and 17 outbreaks of measles reported to the Centers for Disease Control and Prevention from 31 different states. This is a concern and a warning. It’s the highest number since 1996, when there were 508 cases.
Of those 222 cases, 200 were associated with importations from other countries. This includes both people who themselves traveled and became ill and/or imported the virus and those who were exposed to imported cases and themselves became ill. Most of the cases were not vaccinated against measles (65%) or didn’t know if they were vaccinated (21%). Of the 166 U.S. residents who were unvaccinated or had unknown vaccination status, 85% were eligible to get the measles-mumps-rubella (MMR) vaccine based on age and other factors. Of those 141 people, 66 (47%) were between the ages of 16 months and 19 years of age, and another 18 (11%) were under 1 year and, therefore, too young for vaccination. Of the 66 between 16 months and 19 years of age, 50 (76%) had not been vaccinated because of a philosophic, religious, or personal objection (MMWR 2012;61:253-7).
People who choose not to vaccinate themselves or their children are putting themselves and others at risk, particularly infants who are too young to receive the vaccine, and those who are immunocompromised and can’t receive the live attenuated MMR vaccine. Globally, 164,000 people die each year from measles. Nobody has died in the U.S. outbreak so far, but 70 of the 222 (32%) in 2011 were hospitalized.
Nearly half of the 72 known actual importations from other countries were from Europe. We don’t typically think of Europe as a high-risk region for infectious disease, but measles has taken hold there in a big way. Of the 30,567 measles cases reported in the continent in 2011, half – 15,206 – were in France and another 5,181 in Italy, both popular destinations for U.S. travelers.
There is growing concern now about the risk of measles transmission at the Olympics, to be held in July in London. The United Kingdom reported 1,083 cases last year. This year, hundreds of thousands of visitors are
expected for the Olympics. The infectious disease community is advising all travelers to receive measles vaccination at least 2 weeks prior to traveling.
The concern is not limited to travelers to Europe, of course, but extends throughout the world, as outbreaks have been reported in New Zealand, Ecuador, much of Africa, and even Canada. Montreal is currently experiencing an outbreak, with 776 cases reported in the province of Quebec between Jan. 1 and March 31 of this year.
The CDC’s Advisory Committee on Immunization Practices, the American Academy of Pediatrics, and the American Academy of Family Physicians recommend that people of all ages receive MMR. It is given routinely to children at 1 year of age and again at kindergarten entry (4-6 years). The travelers’ recommendation is that any child aged 6-12 months who is eligible receives one MMR dose prior to travel and everyone aged 12 months or older who is eligible receives two MMR doses prior to travel, separated by 28 days.
The MMR vaccine recommendation for travelers has been the same for more than 20 years, but people never really thought about it in terms of travel to Europe or Canada. Also, many adults assume that they were vaccinated as a child. Adults born prior to 1957 are presumed to have been exposed to natural measles and are therefore generally assumed to be immune. Routine measles immunization with one dose began in the mid-1960s, and the two-dose schedule was first recommended in 1989. Since approximately 5% of people don’t respond to the first vaccine dose and many people are unsure of their vaccination history, a substantial proportion of adults are currently susceptible.
Physicians should be prepared to respond to questions regarding MMR vaccine eligibility and should look for documentation of two MMR vaccines.
Summer is travel time, and measles vaccine is now a priority for our patients who are traveling.
Dr. Jackson is the chief of infectious diseases at Children’s Mercy Hospitals and Clinics in Kansas City, Mo., and professor of pediatrics at the University of Missouri–Kansas City. She said she has no relevant financial disclosures.
Measles is a threat that we need to take seriously, both domestically and when traveling anywhere abroad.
During 2011, the United States had more cases of measles than we have had in 15 years. There were 222 cases and 17 outbreaks of measles reported to the Centers for Disease Control and Prevention from 31 different states. This is a concern and a warning. It’s the highest number since 1996, when there were 508 cases.
Of those 222 cases, 200 were associated with importations from other countries. This includes both people who themselves traveled and became ill and/or imported the virus and those who were exposed to imported cases and themselves became ill. Most of the cases were not vaccinated against measles (65%) or didn’t know if they were vaccinated (21%). Of the 166 U.S. residents who were unvaccinated or had unknown vaccination status, 85% were eligible to get the measles-mumps-rubella (MMR) vaccine based on age and other factors. Of those 141 people, 66 (47%) were between the ages of 16 months and 19 years of age, and another 18 (11%) were under 1 year and, therefore, too young for vaccination. Of the 66 between 16 months and 19 years of age, 50 (76%) had not been vaccinated because of a philosophic, religious, or personal objection (MMWR 2012;61:253-7).
People who choose not to vaccinate themselves or their children are putting themselves and others at risk, particularly infants who are too young to receive the vaccine, and those who are immunocompromised and can’t receive the live attenuated MMR vaccine. Globally, 164,000 people die each year from measles. Nobody has died in the U.S. outbreak so far, but 70 of the 222 (32%) in 2011 were hospitalized.
Nearly half of the 72 known actual importations from other countries were from Europe. We don’t typically think of Europe as a high-risk region for infectious disease, but measles has taken hold there in a big way. Of the 30,567 measles cases reported in the continent in 2011, half – 15,206 – were in France and another 5,181 in Italy, both popular destinations for U.S. travelers.
There is growing concern now about the risk of measles transmission at the Olympics, to be held in July in London. The United Kingdom reported 1,083 cases last year. This year, hundreds of thousands of visitors are
expected for the Olympics. The infectious disease community is advising all travelers to receive measles vaccination at least 2 weeks prior to traveling.
The concern is not limited to travelers to Europe, of course, but extends throughout the world, as outbreaks have been reported in New Zealand, Ecuador, much of Africa, and even Canada. Montreal is currently experiencing an outbreak, with 776 cases reported in the province of Quebec between Jan. 1 and March 31 of this year.
The CDC’s Advisory Committee on Immunization Practices, the American Academy of Pediatrics, and the American Academy of Family Physicians recommend that people of all ages receive MMR. It is given routinely to children at 1 year of age and again at kindergarten entry (4-6 years). The travelers’ recommendation is that any child aged 6-12 months who is eligible receives one MMR dose prior to travel and everyone aged 12 months or older who is eligible receives two MMR doses prior to travel, separated by 28 days.
The MMR vaccine recommendation for travelers has been the same for more than 20 years, but people never really thought about it in terms of travel to Europe or Canada. Also, many adults assume that they were vaccinated as a child. Adults born prior to 1957 are presumed to have been exposed to natural measles and are therefore generally assumed to be immune. Routine measles immunization with one dose began in the mid-1960s, and the two-dose schedule was first recommended in 1989. Since approximately 5% of people don’t respond to the first vaccine dose and many people are unsure of their vaccination history, a substantial proportion of adults are currently susceptible.
Physicians should be prepared to respond to questions regarding MMR vaccine eligibility and should look for documentation of two MMR vaccines.
Summer is travel time, and measles vaccine is now a priority for our patients who are traveling.
Dr. Jackson is the chief of infectious diseases at Children’s Mercy Hospitals and Clinics in Kansas City, Mo., and professor of pediatrics at the University of Missouri–Kansas City. She said she has no relevant financial disclosures.
Measles is a threat that we need to take seriously, both domestically and when traveling anywhere abroad.
During 2011, the United States had more cases of measles than we have had in 15 years. There were 222 cases and 17 outbreaks of measles reported to the Centers for Disease Control and Prevention from 31 different states. This is a concern and a warning. It’s the highest number since 1996, when there were 508 cases.
Of those 222 cases, 200 were associated with importations from other countries. This includes both people who themselves traveled and became ill and/or imported the virus and those who were exposed to imported cases and themselves became ill. Most of the cases were not vaccinated against measles (65%) or didn’t know if they were vaccinated (21%). Of the 166 U.S. residents who were unvaccinated or had unknown vaccination status, 85% were eligible to get the measles-mumps-rubella (MMR) vaccine based on age and other factors. Of those 141 people, 66 (47%) were between the ages of 16 months and 19 years of age, and another 18 (11%) were under 1 year and, therefore, too young for vaccination. Of the 66 between 16 months and 19 years of age, 50 (76%) had not been vaccinated because of a philosophic, religious, or personal objection (MMWR 2012;61:253-7).
People who choose not to vaccinate themselves or their children are putting themselves and others at risk, particularly infants who are too young to receive the vaccine, and those who are immunocompromised and can’t receive the live attenuated MMR vaccine. Globally, 164,000 people die each year from measles. Nobody has died in the U.S. outbreak so far, but 70 of the 222 (32%) in 2011 were hospitalized.
Nearly half of the 72 known actual importations from other countries were from Europe. We don’t typically think of Europe as a high-risk region for infectious disease, but measles has taken hold there in a big way. Of the 30,567 measles cases reported in the continent in 2011, half – 15,206 – were in France and another 5,181 in Italy, both popular destinations for U.S. travelers.
There is growing concern now about the risk of measles transmission at the Olympics, to be held in July in London. The United Kingdom reported 1,083 cases last year. This year, hundreds of thousands of visitors are
expected for the Olympics. The infectious disease community is advising all travelers to receive measles vaccination at least 2 weeks prior to traveling.
The concern is not limited to travelers to Europe, of course, but extends throughout the world, as outbreaks have been reported in New Zealand, Ecuador, much of Africa, and even Canada. Montreal is currently experiencing an outbreak, with 776 cases reported in the province of Quebec between Jan. 1 and March 31 of this year.
The CDC’s Advisory Committee on Immunization Practices, the American Academy of Pediatrics, and the American Academy of Family Physicians recommend that people of all ages receive MMR. It is given routinely to children at 1 year of age and again at kindergarten entry (4-6 years). The travelers’ recommendation is that any child aged 6-12 months who is eligible receives one MMR dose prior to travel and everyone aged 12 months or older who is eligible receives two MMR doses prior to travel, separated by 28 days.
The MMR vaccine recommendation for travelers has been the same for more than 20 years, but people never really thought about it in terms of travel to Europe or Canada. Also, many adults assume that they were vaccinated as a child. Adults born prior to 1957 are presumed to have been exposed to natural measles and are therefore generally assumed to be immune. Routine measles immunization with one dose began in the mid-1960s, and the two-dose schedule was first recommended in 1989. Since approximately 5% of people don’t respond to the first vaccine dose and many people are unsure of their vaccination history, a substantial proportion of adults are currently susceptible.
Physicians should be prepared to respond to questions regarding MMR vaccine eligibility and should look for documentation of two MMR vaccines.
Summer is travel time, and measles vaccine is now a priority for our patients who are traveling.
Dr. Jackson is the chief of infectious diseases at Children’s Mercy Hospitals and Clinics in Kansas City, Mo., and professor of pediatrics at the University of Missouri–Kansas City. She said she has no relevant financial disclosures.