User login
AGA News
Coronavirus 101 for gastroenterologists
Now in AGA University: Gain a clear understanding of the lifespan and gastrointestinal manifestations of SARS-CoV-2 and best practices for protecting yourself while working with at-risk patients. http://agau.gastro.org/diweb/catalog/item/eid/COVID-19
Get the latest information and resources on coronavirus by visiting www.gastro.org/COVID.
A message from our president to the GI community
“Our commitment at AGA is to support you. We’ll get through this together,” says AGA President Hashem El-Serag, MD, MPH, AGAF.
Dear colleagues,
The coronavirus pandemic has affected every facet of society, bringing almost unprecedented challenges to our world, and especially to our world of health care.
But our profession has been ignited in the way only a crisis can spark. Many of you are working on the front lines of patient care, at personal risk, lacking sufficient information and adequate resources. This is heroic work.
AGA’s priority during this time of disruption is to get practical guidance into your hands to help you treat patients, and protect yourselves and your coworkers. We’re also advocating on your behalf to get the resources you need and economic relief necessitated by the measures taken to fight the pandemic.
We are continually updating our COVID-19 website, www.gastro.org/covid. Check it for the latest clinical guidance, practice management information, and advocacy initiatives.
Our journals have started a collection of submissions related to COVID-19. Your AGA colleagues on the Clinical Guidelines Committee and Clinical Practice Updates Committee have been hard at work developing guidance for questions that you have asked us on Twitter, @AmerGastroAssn, and the AGA Community. So join us there where resources and insights are being shared in real time.
Your commitment to our patients is a testament to your professionalism. Our commitment at AGA is to support you.
We’ll get through this together.
Hashem B. El-Serag, MD, MPH, AGAF
President, AGA Institute
AGA selects two new social media editors
Congratulations to our new social media editors, Mindy Engevik, PhD, and Sultan Mahmood, MD.
Both editors will have the opportunity to positively impact AGA journal engagement by increasing the dissemination of each of the AGA publications’ content across their social media platforms. Dr. Engevik will focus on basic and translational science research and Dr. Mahmood will focus on clinical research. They will be sharing noteworthy research and news across AGA’s diverse publication portfolio, which includes Gastroenterology, Clinical Gastroenterology and Hepatology, Cellular and Molecular Gastroenterology and Hepatology, Techniques and Innovations in Gastrointestinal Endoscopy, GI & Hepatology News, and the New Gastroenterologist.
Melinda Engevik, PhD
@MicroMindy
Dr. Engevik is an instructor at Baylor College of Medicine, Houston, in the department of pathology and immunology. She has a PhD in systems biology and physiology from the University of Cincinnati and completed her postdoctoral training at Baylor College of Medicine. Her research focuses on microbe-epithelial interactions in the gastrointestinal tract, with a particular focus on infection and inflammatory bowel disease. Dr. Engevik currently serves as an AGA Young Delegate and enjoys her involvement in the GI community.
Sultan Mahmood, MD
@SultanMahmoodMD
Dr. Mahmood finished his medical school in King Edward Medical University in Lahore, Pakistan. He did his internal medicine residency as well as GI fellowship training in University of Oklahoma Health Sciences Center, Oklahoma City, where he also served as the chief fellow from 2017 to 2018. He is the cofounder of @GIjournal, which is a weekly GI journal club on Twitter. He is currently an assistant professor in the department of medicine, division of gastroenterology, at the University at Buffalo (N.Y.). He also serves the role of coprogram director of the GI fellowship program in University at Buffalo. His research interests include medical education, work-life balance, quality improvement in the endoscopy suite, and cold snare.
The journals’ board of editors and editorial staff congratulate the new social media editors and are excited to work with them over the next 3 years.
Diversify GI: Fola May
We’re celebrating diversity in our field with a new series spotlighting members of the AGA Diversity Committee and AGA FORWARD Program.
The University of California, Los Angeles, Women’s Basketball Program recognized AGA FORWARD Scholar Fola May, MD, PhD, MPhil, for exemplifying their values of being “uncommon” and going above and beyond.
You’ll find proof she meets these criteria through extracurriculars like her participation in the AGA FORWARD program – a National Institute of Health–funded initiative that supports underrepresented minority physician scientists – and as a GI patient advocate on Capitol Hill.
Dr. May’s unconventional career path is also testament to her ability to color outside the lines while creating a masterpiece.
“Realizing late in my training that I wanted a career in research, I joined the STAR program at UCLA which allowed me to complete a PhD in health policy and management [a health services research degree] during my GI fellowship. With this training, I have been able to pursue a career in research and clinical care far beyond what I ever imagined.”
But she noticed a void along her career path that she couldn’t fill on her own: limited access to diverse research leaders in the field who can serve as her mentors, supporters, and advocators.
“Though I have a wonderful mentorship team that has been instrumental to my success thus far, there are currently no senior health services researchers in gastroenterology or gastroenterologists of color at my institution.”
At Dr. May’s institution, there are about 60 faculty members – only 1 Hispanic female. At the academic health center where Dr. May works, she is the only African American gastroenterologist. Other divisions and departments do not look much different, she explained.
“We serve a massive, diverse urban center. I don’t understand it, and I feel strongly that we can do better.”
She stressed that the key to breaking unjust cultural norms is for white colleagues to acknowledge the issues minorities face and to make intentional efforts to increase diversity in the workforce.
“We can’t expect black and brown faculty to do it on their own. The ‘minority tax’ that we face is a heavy toll and has the potential to paralyze our careers. We need members of the majority populations to also embrace diversity issues.”
Let’s get personal
- What do you know now that you wish someone told you when you started your career? “I wish that someone told me earlier that there will come a time when you will transition from working hard to check off all the check boxes to working hard in the things that make you happy. So much of medical school and residency is about doing what you are told you have to do to succeed. Finally, I feel that I am encouraged to find the research topics and patient populations that I am most passionate about. In dedicating ourselves to the things we care most about we have the best opportunity for real impact.”
- Who is your professional hero and why? “Wow. Honestly, I do not have one. Maybe Michelle Obama. I know she is not in medicine, but I pick her because she is an African American women who I know has been put through a lot and has to put up with a lot. But she keeps her head up high and stays strong. Reading her book transformed me. You can’t tell by just looking at her, all that she’s had to deal with. I would like to be seen as someone who is strong despite all of the background noise.”
- Something you may not know about me is: “I am pretty obsessed with CrossFit and fitness. I really enjoy staying active.”
- If I weren’t in gastroenterology, I would be: “A television/movie writer or movie producer.”
- In my free time I like to: “Spend time with my husband and kids, travel, stay active.”
Coronavirus 101 for gastroenterologists
Now in AGA University: Gain a clear understanding of the lifespan and gastrointestinal manifestations of SARS-CoV-2 and best practices for protecting yourself while working with at-risk patients. http://agau.gastro.org/diweb/catalog/item/eid/COVID-19
Get the latest information and resources on coronavirus by visiting www.gastro.org/COVID.
A message from our president to the GI community
“Our commitment at AGA is to support you. We’ll get through this together,” says AGA President Hashem El-Serag, MD, MPH, AGAF.
Dear colleagues,
The coronavirus pandemic has affected every facet of society, bringing almost unprecedented challenges to our world, and especially to our world of health care.
But our profession has been ignited in the way only a crisis can spark. Many of you are working on the front lines of patient care, at personal risk, lacking sufficient information and adequate resources. This is heroic work.
AGA’s priority during this time of disruption is to get practical guidance into your hands to help you treat patients, and protect yourselves and your coworkers. We’re also advocating on your behalf to get the resources you need and economic relief necessitated by the measures taken to fight the pandemic.
We are continually updating our COVID-19 website, www.gastro.org/covid. Check it for the latest clinical guidance, practice management information, and advocacy initiatives.
Our journals have started a collection of submissions related to COVID-19. Your AGA colleagues on the Clinical Guidelines Committee and Clinical Practice Updates Committee have been hard at work developing guidance for questions that you have asked us on Twitter, @AmerGastroAssn, and the AGA Community. So join us there where resources and insights are being shared in real time.
Your commitment to our patients is a testament to your professionalism. Our commitment at AGA is to support you.
We’ll get through this together.
Hashem B. El-Serag, MD, MPH, AGAF
President, AGA Institute
AGA selects two new social media editors
Congratulations to our new social media editors, Mindy Engevik, PhD, and Sultan Mahmood, MD.
Both editors will have the opportunity to positively impact AGA journal engagement by increasing the dissemination of each of the AGA publications’ content across their social media platforms. Dr. Engevik will focus on basic and translational science research and Dr. Mahmood will focus on clinical research. They will be sharing noteworthy research and news across AGA’s diverse publication portfolio, which includes Gastroenterology, Clinical Gastroenterology and Hepatology, Cellular and Molecular Gastroenterology and Hepatology, Techniques and Innovations in Gastrointestinal Endoscopy, GI & Hepatology News, and the New Gastroenterologist.
Melinda Engevik, PhD
@MicroMindy
Dr. Engevik is an instructor at Baylor College of Medicine, Houston, in the department of pathology and immunology. She has a PhD in systems biology and physiology from the University of Cincinnati and completed her postdoctoral training at Baylor College of Medicine. Her research focuses on microbe-epithelial interactions in the gastrointestinal tract, with a particular focus on infection and inflammatory bowel disease. Dr. Engevik currently serves as an AGA Young Delegate and enjoys her involvement in the GI community.
Sultan Mahmood, MD
@SultanMahmoodMD
Dr. Mahmood finished his medical school in King Edward Medical University in Lahore, Pakistan. He did his internal medicine residency as well as GI fellowship training in University of Oklahoma Health Sciences Center, Oklahoma City, where he also served as the chief fellow from 2017 to 2018. He is the cofounder of @GIjournal, which is a weekly GI journal club on Twitter. He is currently an assistant professor in the department of medicine, division of gastroenterology, at the University at Buffalo (N.Y.). He also serves the role of coprogram director of the GI fellowship program in University at Buffalo. His research interests include medical education, work-life balance, quality improvement in the endoscopy suite, and cold snare.
The journals’ board of editors and editorial staff congratulate the new social media editors and are excited to work with them over the next 3 years.
Diversify GI: Fola May
We’re celebrating diversity in our field with a new series spotlighting members of the AGA Diversity Committee and AGA FORWARD Program.
The University of California, Los Angeles, Women’s Basketball Program recognized AGA FORWARD Scholar Fola May, MD, PhD, MPhil, for exemplifying their values of being “uncommon” and going above and beyond.
You’ll find proof she meets these criteria through extracurriculars like her participation in the AGA FORWARD program – a National Institute of Health–funded initiative that supports underrepresented minority physician scientists – and as a GI patient advocate on Capitol Hill.
Dr. May’s unconventional career path is also testament to her ability to color outside the lines while creating a masterpiece.
“Realizing late in my training that I wanted a career in research, I joined the STAR program at UCLA which allowed me to complete a PhD in health policy and management [a health services research degree] during my GI fellowship. With this training, I have been able to pursue a career in research and clinical care far beyond what I ever imagined.”
But she noticed a void along her career path that she couldn’t fill on her own: limited access to diverse research leaders in the field who can serve as her mentors, supporters, and advocators.
“Though I have a wonderful mentorship team that has been instrumental to my success thus far, there are currently no senior health services researchers in gastroenterology or gastroenterologists of color at my institution.”
At Dr. May’s institution, there are about 60 faculty members – only 1 Hispanic female. At the academic health center where Dr. May works, she is the only African American gastroenterologist. Other divisions and departments do not look much different, she explained.
“We serve a massive, diverse urban center. I don’t understand it, and I feel strongly that we can do better.”
She stressed that the key to breaking unjust cultural norms is for white colleagues to acknowledge the issues minorities face and to make intentional efforts to increase diversity in the workforce.
“We can’t expect black and brown faculty to do it on their own. The ‘minority tax’ that we face is a heavy toll and has the potential to paralyze our careers. We need members of the majority populations to also embrace diversity issues.”
Let’s get personal
- What do you know now that you wish someone told you when you started your career? “I wish that someone told me earlier that there will come a time when you will transition from working hard to check off all the check boxes to working hard in the things that make you happy. So much of medical school and residency is about doing what you are told you have to do to succeed. Finally, I feel that I am encouraged to find the research topics and patient populations that I am most passionate about. In dedicating ourselves to the things we care most about we have the best opportunity for real impact.”
- Who is your professional hero and why? “Wow. Honestly, I do not have one. Maybe Michelle Obama. I know she is not in medicine, but I pick her because she is an African American women who I know has been put through a lot and has to put up with a lot. But she keeps her head up high and stays strong. Reading her book transformed me. You can’t tell by just looking at her, all that she’s had to deal with. I would like to be seen as someone who is strong despite all of the background noise.”
- Something you may not know about me is: “I am pretty obsessed with CrossFit and fitness. I really enjoy staying active.”
- If I weren’t in gastroenterology, I would be: “A television/movie writer or movie producer.”
- In my free time I like to: “Spend time with my husband and kids, travel, stay active.”
Coronavirus 101 for gastroenterologists
Now in AGA University: Gain a clear understanding of the lifespan and gastrointestinal manifestations of SARS-CoV-2 and best practices for protecting yourself while working with at-risk patients. http://agau.gastro.org/diweb/catalog/item/eid/COVID-19
Get the latest information and resources on coronavirus by visiting www.gastro.org/COVID.
A message from our president to the GI community
“Our commitment at AGA is to support you. We’ll get through this together,” says AGA President Hashem El-Serag, MD, MPH, AGAF.
Dear colleagues,
The coronavirus pandemic has affected every facet of society, bringing almost unprecedented challenges to our world, and especially to our world of health care.
But our profession has been ignited in the way only a crisis can spark. Many of you are working on the front lines of patient care, at personal risk, lacking sufficient information and adequate resources. This is heroic work.
AGA’s priority during this time of disruption is to get practical guidance into your hands to help you treat patients, and protect yourselves and your coworkers. We’re also advocating on your behalf to get the resources you need and economic relief necessitated by the measures taken to fight the pandemic.
We are continually updating our COVID-19 website, www.gastro.org/covid. Check it for the latest clinical guidance, practice management information, and advocacy initiatives.
Our journals have started a collection of submissions related to COVID-19. Your AGA colleagues on the Clinical Guidelines Committee and Clinical Practice Updates Committee have been hard at work developing guidance for questions that you have asked us on Twitter, @AmerGastroAssn, and the AGA Community. So join us there where resources and insights are being shared in real time.
Your commitment to our patients is a testament to your professionalism. Our commitment at AGA is to support you.
We’ll get through this together.
Hashem B. El-Serag, MD, MPH, AGAF
President, AGA Institute
AGA selects two new social media editors
Congratulations to our new social media editors, Mindy Engevik, PhD, and Sultan Mahmood, MD.
Both editors will have the opportunity to positively impact AGA journal engagement by increasing the dissemination of each of the AGA publications’ content across their social media platforms. Dr. Engevik will focus on basic and translational science research and Dr. Mahmood will focus on clinical research. They will be sharing noteworthy research and news across AGA’s diverse publication portfolio, which includes Gastroenterology, Clinical Gastroenterology and Hepatology, Cellular and Molecular Gastroenterology and Hepatology, Techniques and Innovations in Gastrointestinal Endoscopy, GI & Hepatology News, and the New Gastroenterologist.
Melinda Engevik, PhD
@MicroMindy
Dr. Engevik is an instructor at Baylor College of Medicine, Houston, in the department of pathology and immunology. She has a PhD in systems biology and physiology from the University of Cincinnati and completed her postdoctoral training at Baylor College of Medicine. Her research focuses on microbe-epithelial interactions in the gastrointestinal tract, with a particular focus on infection and inflammatory bowel disease. Dr. Engevik currently serves as an AGA Young Delegate and enjoys her involvement in the GI community.
Sultan Mahmood, MD
@SultanMahmoodMD
Dr. Mahmood finished his medical school in King Edward Medical University in Lahore, Pakistan. He did his internal medicine residency as well as GI fellowship training in University of Oklahoma Health Sciences Center, Oklahoma City, where he also served as the chief fellow from 2017 to 2018. He is the cofounder of @GIjournal, which is a weekly GI journal club on Twitter. He is currently an assistant professor in the department of medicine, division of gastroenterology, at the University at Buffalo (N.Y.). He also serves the role of coprogram director of the GI fellowship program in University at Buffalo. His research interests include medical education, work-life balance, quality improvement in the endoscopy suite, and cold snare.
The journals’ board of editors and editorial staff congratulate the new social media editors and are excited to work with them over the next 3 years.
Diversify GI: Fola May
We’re celebrating diversity in our field with a new series spotlighting members of the AGA Diversity Committee and AGA FORWARD Program.
The University of California, Los Angeles, Women’s Basketball Program recognized AGA FORWARD Scholar Fola May, MD, PhD, MPhil, for exemplifying their values of being “uncommon” and going above and beyond.
You’ll find proof she meets these criteria through extracurriculars like her participation in the AGA FORWARD program – a National Institute of Health–funded initiative that supports underrepresented minority physician scientists – and as a GI patient advocate on Capitol Hill.
Dr. May’s unconventional career path is also testament to her ability to color outside the lines while creating a masterpiece.
“Realizing late in my training that I wanted a career in research, I joined the STAR program at UCLA which allowed me to complete a PhD in health policy and management [a health services research degree] during my GI fellowship. With this training, I have been able to pursue a career in research and clinical care far beyond what I ever imagined.”
But she noticed a void along her career path that she couldn’t fill on her own: limited access to diverse research leaders in the field who can serve as her mentors, supporters, and advocators.
“Though I have a wonderful mentorship team that has been instrumental to my success thus far, there are currently no senior health services researchers in gastroenterology or gastroenterologists of color at my institution.”
At Dr. May’s institution, there are about 60 faculty members – only 1 Hispanic female. At the academic health center where Dr. May works, she is the only African American gastroenterologist. Other divisions and departments do not look much different, she explained.
“We serve a massive, diverse urban center. I don’t understand it, and I feel strongly that we can do better.”
She stressed that the key to breaking unjust cultural norms is for white colleagues to acknowledge the issues minorities face and to make intentional efforts to increase diversity in the workforce.
“We can’t expect black and brown faculty to do it on their own. The ‘minority tax’ that we face is a heavy toll and has the potential to paralyze our careers. We need members of the majority populations to also embrace diversity issues.”
Let’s get personal
- What do you know now that you wish someone told you when you started your career? “I wish that someone told me earlier that there will come a time when you will transition from working hard to check off all the check boxes to working hard in the things that make you happy. So much of medical school and residency is about doing what you are told you have to do to succeed. Finally, I feel that I am encouraged to find the research topics and patient populations that I am most passionate about. In dedicating ourselves to the things we care most about we have the best opportunity for real impact.”
- Who is your professional hero and why? “Wow. Honestly, I do not have one. Maybe Michelle Obama. I know she is not in medicine, but I pick her because she is an African American women who I know has been put through a lot and has to put up with a lot. But she keeps her head up high and stays strong. Reading her book transformed me. You can’t tell by just looking at her, all that she’s had to deal with. I would like to be seen as someone who is strong despite all of the background noise.”
- Something you may not know about me is: “I am pretty obsessed with CrossFit and fitness. I really enjoy staying active.”
- If I weren’t in gastroenterology, I would be: “A television/movie writer or movie producer.”
- In my free time I like to: “Spend time with my husband and kids, travel, stay active.”
May 2020 – ICYMI
Gastroenterology
February 2020
Gastric electrical stimulation reduces refractory vomiting in a randomized crossover trial. Philippe Ducrotte et al. 2020 Feb;158(3):506-14.e2. doi: 10.1053/j.gastro.2019.10.018
Efficacy and safety of vedolizumab subcutaneous formulation in a randomized trial of patients with ulcerative colitis. William J. Sandborn et al. 2020 Feb;158(3)562-72.e12. doi: 10.1053/j.gastro.2019.08.027
AGA Clinical Practice Guidelines on management of gastric intestinal metaplasia. Samir Gupta et al. 2020 Feb;158(3):693-702. doi: 10.1053/j.gastro.2019.12.003
March 2020
Approaches and challenges to management of pediatric and adult patients with eosinophilic esophagitis. Ikuo Hirano, Glenn T. Furuta. 2020 Mar;158(4):840-51. doi: 10.1053/j.gastro.2019.09.052
Uptake of colorectal cancer screening by physicians is associated with greater uptake by their patients. Owen Litwin et al. 2020 Mar;158(4):905-14. doi: 10.1053/j.gastro.2019.10.027
Recommendations for follow-up after colonoscopy and polypectomy: A consensus update by the US Multi-Society Task Force on Colorectal Cancer. Samir Gupta et al. 2020 Mar;158(4):1131-53.e5. doi: 10.1053/j.gastro.2019.10.026
Differences in fecal microbiomes and metabolomes of people with vs without irritable bowel syndrome and bile acid malabsorption. Ian B. Jeffery et al. 2020 Mar;158(4):1016-28.e8. doi: 10.1053/j.gastro.2019.11.301
April 2020
How to set up a successful motility lab. Rena Yadlapati et al. 2020 April;158(5):1202-10. doi: 10.1053/j.gastro.2020.01.030
Mechanisms, evaluation, and management of chronic constipation. Adil E. Bharucha, Brian E. Lacy. 2020 April;158(5):1232-49.e3. doi: 10.1053/j.gastro.2019.12.034.
Incidence of venous thromboembolism in patients with newly diagnosed pancreatic cancer and factors associated with outcomes. Corinne Frere et al. 2020 April;158(5):1346-58.e4. doi: 10.1053/j.gastro.2019.12.009
Clinical Gastroenterology and Hepatology
February 2020
Increased incidence and mortality of gastric cancer in immigrant populations from high to low regions of incidence: A systematic review and meta-analysis. Baldeep S. Pabla et al. 2020 Feb;18(2):347-59.e5. doi: 10.1016/j.cgh.2019.05.032
Risk of gastrointestinal bleeding increases with combinations of antithrombotic agents and patient age. Neena S. Abraham et al. 2020 Feb;18(2):337-46.e19. doi: 10.1016/j.cgh.2019.05.017
Alcohol rehabilitation within 30 days of hospital discharge is associated with reduced readmission, relapse, and death in patients with alcoholic hepatitis. Thoetchai (Bee) Peeraphatdit et al. 2020 Feb;18(2):477-85.e5. doi: 10.1016/j.cgh.2019.04.048
March 2020
Telemedicine in gastroenterology: A value-added service for patients. Theresa Lee, Lawrence Kim. 2020 Mar;18(3):530-3. doi: 10.1016/j.cgh.2019.12.005
Best practices in teaching endoscopy based on a Delphi survey of gastroenterology program directors and experts in endoscopy education. Navin L. Kumar et al. 2020 Mar;18(3):574-9.e1. doi: 10.1016/j.cgh.2019.05.023
Consumption of fish and long-chain n-3 polyunsaturated fatty acids is associated with reduced risk of colorectal cancer in a large European cohort. Elom K. Aglago et al. 2020 Mar;18(3):654-66.e6. doi: 10.1016/j.cgh.2019.06.031
April 2020
Low incidence of aerodigestive cancers in patients with negative results from colonoscopies, regardless of findings from multitarget stool DNA tests. Barry M. Berger et al. 2020 April;18(4):864-71. doi: 10.1016/j.cgh.2019.07.057
Lifetime economic burden of Crohn’s disease and ulcerative colitis by age at diagnosis. Gary R. Lichtenstein et al. 2020 April;18(4):889-97.e10. doi: 10.1016/j.cgh.2019.07.022
Clinical and Molecular Gastroenterology and Hepatology
Etiopathogenetic mechanisms in diverticular disease of the colon. Michael Camilleri et al. 2020;9(1):15-32. doi: 10.1016/j.jcmgh.2019.07.007
Gastroenterology
February 2020
Gastric electrical stimulation reduces refractory vomiting in a randomized crossover trial. Philippe Ducrotte et al. 2020 Feb;158(3):506-14.e2. doi: 10.1053/j.gastro.2019.10.018
Efficacy and safety of vedolizumab subcutaneous formulation in a randomized trial of patients with ulcerative colitis. William J. Sandborn et al. 2020 Feb;158(3)562-72.e12. doi: 10.1053/j.gastro.2019.08.027
AGA Clinical Practice Guidelines on management of gastric intestinal metaplasia. Samir Gupta et al. 2020 Feb;158(3):693-702. doi: 10.1053/j.gastro.2019.12.003
March 2020
Approaches and challenges to management of pediatric and adult patients with eosinophilic esophagitis. Ikuo Hirano, Glenn T. Furuta. 2020 Mar;158(4):840-51. doi: 10.1053/j.gastro.2019.09.052
Uptake of colorectal cancer screening by physicians is associated with greater uptake by their patients. Owen Litwin et al. 2020 Mar;158(4):905-14. doi: 10.1053/j.gastro.2019.10.027
Recommendations for follow-up after colonoscopy and polypectomy: A consensus update by the US Multi-Society Task Force on Colorectal Cancer. Samir Gupta et al. 2020 Mar;158(4):1131-53.e5. doi: 10.1053/j.gastro.2019.10.026
Differences in fecal microbiomes and metabolomes of people with vs without irritable bowel syndrome and bile acid malabsorption. Ian B. Jeffery et al. 2020 Mar;158(4):1016-28.e8. doi: 10.1053/j.gastro.2019.11.301
April 2020
How to set up a successful motility lab. Rena Yadlapati et al. 2020 April;158(5):1202-10. doi: 10.1053/j.gastro.2020.01.030
Mechanisms, evaluation, and management of chronic constipation. Adil E. Bharucha, Brian E. Lacy. 2020 April;158(5):1232-49.e3. doi: 10.1053/j.gastro.2019.12.034.
Incidence of venous thromboembolism in patients with newly diagnosed pancreatic cancer and factors associated with outcomes. Corinne Frere et al. 2020 April;158(5):1346-58.e4. doi: 10.1053/j.gastro.2019.12.009
Clinical Gastroenterology and Hepatology
February 2020
Increased incidence and mortality of gastric cancer in immigrant populations from high to low regions of incidence: A systematic review and meta-analysis. Baldeep S. Pabla et al. 2020 Feb;18(2):347-59.e5. doi: 10.1016/j.cgh.2019.05.032
Risk of gastrointestinal bleeding increases with combinations of antithrombotic agents and patient age. Neena S. Abraham et al. 2020 Feb;18(2):337-46.e19. doi: 10.1016/j.cgh.2019.05.017
Alcohol rehabilitation within 30 days of hospital discharge is associated with reduced readmission, relapse, and death in patients with alcoholic hepatitis. Thoetchai (Bee) Peeraphatdit et al. 2020 Feb;18(2):477-85.e5. doi: 10.1016/j.cgh.2019.04.048
March 2020
Telemedicine in gastroenterology: A value-added service for patients. Theresa Lee, Lawrence Kim. 2020 Mar;18(3):530-3. doi: 10.1016/j.cgh.2019.12.005
Best practices in teaching endoscopy based on a Delphi survey of gastroenterology program directors and experts in endoscopy education. Navin L. Kumar et al. 2020 Mar;18(3):574-9.e1. doi: 10.1016/j.cgh.2019.05.023
Consumption of fish and long-chain n-3 polyunsaturated fatty acids is associated with reduced risk of colorectal cancer in a large European cohort. Elom K. Aglago et al. 2020 Mar;18(3):654-66.e6. doi: 10.1016/j.cgh.2019.06.031
April 2020
Low incidence of aerodigestive cancers in patients with negative results from colonoscopies, regardless of findings from multitarget stool DNA tests. Barry M. Berger et al. 2020 April;18(4):864-71. doi: 10.1016/j.cgh.2019.07.057
Lifetime economic burden of Crohn’s disease and ulcerative colitis by age at diagnosis. Gary R. Lichtenstein et al. 2020 April;18(4):889-97.e10. doi: 10.1016/j.cgh.2019.07.022
Clinical and Molecular Gastroenterology and Hepatology
Etiopathogenetic mechanisms in diverticular disease of the colon. Michael Camilleri et al. 2020;9(1):15-32. doi: 10.1016/j.jcmgh.2019.07.007
Gastroenterology
February 2020
Gastric electrical stimulation reduces refractory vomiting in a randomized crossover trial. Philippe Ducrotte et al. 2020 Feb;158(3):506-14.e2. doi: 10.1053/j.gastro.2019.10.018
Efficacy and safety of vedolizumab subcutaneous formulation in a randomized trial of patients with ulcerative colitis. William J. Sandborn et al. 2020 Feb;158(3)562-72.e12. doi: 10.1053/j.gastro.2019.08.027
AGA Clinical Practice Guidelines on management of gastric intestinal metaplasia. Samir Gupta et al. 2020 Feb;158(3):693-702. doi: 10.1053/j.gastro.2019.12.003
March 2020
Approaches and challenges to management of pediatric and adult patients with eosinophilic esophagitis. Ikuo Hirano, Glenn T. Furuta. 2020 Mar;158(4):840-51. doi: 10.1053/j.gastro.2019.09.052
Uptake of colorectal cancer screening by physicians is associated with greater uptake by their patients. Owen Litwin et al. 2020 Mar;158(4):905-14. doi: 10.1053/j.gastro.2019.10.027
Recommendations for follow-up after colonoscopy and polypectomy: A consensus update by the US Multi-Society Task Force on Colorectal Cancer. Samir Gupta et al. 2020 Mar;158(4):1131-53.e5. doi: 10.1053/j.gastro.2019.10.026
Differences in fecal microbiomes and metabolomes of people with vs without irritable bowel syndrome and bile acid malabsorption. Ian B. Jeffery et al. 2020 Mar;158(4):1016-28.e8. doi: 10.1053/j.gastro.2019.11.301
April 2020
How to set up a successful motility lab. Rena Yadlapati et al. 2020 April;158(5):1202-10. doi: 10.1053/j.gastro.2020.01.030
Mechanisms, evaluation, and management of chronic constipation. Adil E. Bharucha, Brian E. Lacy. 2020 April;158(5):1232-49.e3. doi: 10.1053/j.gastro.2019.12.034.
Incidence of venous thromboembolism in patients with newly diagnosed pancreatic cancer and factors associated with outcomes. Corinne Frere et al. 2020 April;158(5):1346-58.e4. doi: 10.1053/j.gastro.2019.12.009
Clinical Gastroenterology and Hepatology
February 2020
Increased incidence and mortality of gastric cancer in immigrant populations from high to low regions of incidence: A systematic review and meta-analysis. Baldeep S. Pabla et al. 2020 Feb;18(2):347-59.e5. doi: 10.1016/j.cgh.2019.05.032
Risk of gastrointestinal bleeding increases with combinations of antithrombotic agents and patient age. Neena S. Abraham et al. 2020 Feb;18(2):337-46.e19. doi: 10.1016/j.cgh.2019.05.017
Alcohol rehabilitation within 30 days of hospital discharge is associated with reduced readmission, relapse, and death in patients with alcoholic hepatitis. Thoetchai (Bee) Peeraphatdit et al. 2020 Feb;18(2):477-85.e5. doi: 10.1016/j.cgh.2019.04.048
March 2020
Telemedicine in gastroenterology: A value-added service for patients. Theresa Lee, Lawrence Kim. 2020 Mar;18(3):530-3. doi: 10.1016/j.cgh.2019.12.005
Best practices in teaching endoscopy based on a Delphi survey of gastroenterology program directors and experts in endoscopy education. Navin L. Kumar et al. 2020 Mar;18(3):574-9.e1. doi: 10.1016/j.cgh.2019.05.023
Consumption of fish and long-chain n-3 polyunsaturated fatty acids is associated with reduced risk of colorectal cancer in a large European cohort. Elom K. Aglago et al. 2020 Mar;18(3):654-66.e6. doi: 10.1016/j.cgh.2019.06.031
April 2020
Low incidence of aerodigestive cancers in patients with negative results from colonoscopies, regardless of findings from multitarget stool DNA tests. Barry M. Berger et al. 2020 April;18(4):864-71. doi: 10.1016/j.cgh.2019.07.057
Lifetime economic burden of Crohn’s disease and ulcerative colitis by age at diagnosis. Gary R. Lichtenstein et al. 2020 April;18(4):889-97.e10. doi: 10.1016/j.cgh.2019.07.022
Clinical and Molecular Gastroenterology and Hepatology
Etiopathogenetic mechanisms in diverticular disease of the colon. Michael Camilleri et al. 2020;9(1):15-32. doi: 10.1016/j.jcmgh.2019.07.007
Fellowship Burnout: What can we do to identify those at risk and minimize the impact?
Jeff is a high-performing first-year gastroenterology fellow who started with eagerness and enthusiasm. He seemed to enjoy talking to patients, wrote thorough notes, and often participated during case discussions at morning report. He initiated a quality improvement project and joined a hospital committee. Over the past few months, he has interacted less with his peers in the fellow’s office and stayed late to complete his patient encounters. He now frequently arrives late to work, is unprepared for rounds, and forgets to place important orders. One day, you notice him shuffling through several papers when the attending asks him a question about his patient. Later that day, he snapped at a nurse who paged to ask a question about a patient who just had a colonoscopy. When you ask him how he is doing, he becomes tearful and reports that he is under a lot of stress between work and home and does not feel the work he is doing is meaningful.
Introduction
The above scenario is all too familiar. Gastroenterology training can be a stressful period in an individual’s life. Long hours, steep learning curves for new cognitive and mechanical skill sets, as well as managing personal relationships and responsibilities at home all contribute to the stress of training and finding appropriate work-life balance. These stressors can result in burnout. The last decade has brought about a renewed emphasis on mitigating the impact of occupational burnout and improving trainee lifestyle through interventions such as work-hour restrictions, resiliency training, instruction on the importance of sleep, and team-building activities.
The problem
The World Health Organization (WHO) defines occupational burnout as chronic work-related stress, which may be characterized by feelings of energy depletion, mental distance from one’s job or feelings of negativity toward it, and reduced professional efficacy. Occupational burnout has been identified as an increasing problem both in practicing providers and trainees. Surveys in gastroenterologists show rates of burnout ranging between 37% and 50%,1 with trainees and early-career physicians disproportionately affected.1,2Physicians along the entire training spectrum are more likely to report high emotional exhaustion, high depersonalization, and burnout than a population control sample.2
Several individual factors identified for those at increased risk for burnout include younger age, not being married, and being male.2 Individuals spending less than 20% of their time working on activities they find meaningful and productive were more likely to show evidence of burnout.1

Symptoms of burnout can have a profound impact on trainees’ work performance, personal interactions, and the learning environment as a whole. The Accreditation Council for Graduate Medical Education (ACGME) annual survey of trainees asks them how strongly they agree or disagree on various components of burnout such as how meaningful they find their work, if they have enough time to think and reflect, if they feel emotionally drained at work, and if they feel worn out and weary after work. The intent of these questions is to provide anonymous feedback to training programs to help identify year to year trends and intervene early to prevent occupational burnout from becoming an increasing issue.
The solution
Considerations for any intervention should take several factors into account: the impact it may have on training and the development of a competent physician in their individualized specialty, the sustainability of the intervention, and whether it is something that will be accepted by the invested parties.

One method proposed for preventing burnout during fellowship has been designated as the three R’s: relaxation, reflection, and regrouping.3
- Relax. In order to relax, trainees need ways to decompress. Activities such as exercise and social events can be helpful. Within our own program the fellows have started their own group exercise program, playing wallyball weekly before clinical duties. We also encourage use of vacation days and build comradery by organizing potluck dinners for major holidays, graduation parties at the program director’s house, and an end-of-the-year golf outing in which trainees play against staff followed by a discussion regarding the state of the program. More recently we have added one half-day per a quarter for morale and team building. During this first year, the activities in which trainees have collectively decided to participate include an escape room, a rock-climbing facility, and laser tag. The addition of more team-building days has been well received by our program’s trainees and the simple addition of these team-building days has resulted in the trainees interacting more together outside of work, particularly in the form of group dinners.

Walter Reed National Military Medical Center fellows gathering for wallyball. - Reflect. They describe reflection as a necessary checkpoint which typically occurs every 6 months.3 These “checkpoints” provide an opportunity to provide feedback to the fellow as well as check in on their well-being and receive feedback about the program. We give frequent feedback to fellows in the form of spot, rotational, and mid-/end-of-year feedback. Additionally, we have developed a unique feedback system in which the trainees meet at the end of the year to discuss collective feedback for the staff and the program. This feedback is collated by the chief fellow and given to the program director as anonymous feedback, which is then passed to the individual staff.
- Regroup. Finally, regrouping to form new strategies.3 This regrouping provides an opportunity to improve on areas in which the trainee may have a deficiency and build on their strengths. To facilitate regrouping, we identify a mentor within the department and occasionally in other departments to meet regularly with the trainee. A successful mentor ensures effective regrouping and can help the trainee avoid pitfalls that they may have experienced in similar situations.
Moving forward
Occupational burnout is a systemic problem within the medical field, with trainees disproportionately affected. It is imperative that training programs continue to work toward creating a culture that prevents development of burnout. Along with the ideas presented here, the ACGME has launched AWARE, which is a suite of resources directed specifically at the GME community, with a goal of mitigating stress and preventing burnout. No one approach will be universally applicable but continued awareness and efforts to address this on an individual and programmatic level should be encouraged.
Dr. Ordway is a chief fellow, Dr. Tritsch and Dr. Singla are associate program directors, and Dr. Torres the program director, division of gastroenterology and hepatology, Walter Reed National Military Medical Center, Bethesda, Md.
References
1. Barnes EL et al. Dig Dis Sci. 2019;64(2):302-6.
2. Dyrbye LN et al. Acad Med. 2014;89(3):443-51.
3. Waldo OA. J Am Coll Cardiol. 2015;66(11):1303-6.
Jeff is a high-performing first-year gastroenterology fellow who started with eagerness and enthusiasm. He seemed to enjoy talking to patients, wrote thorough notes, and often participated during case discussions at morning report. He initiated a quality improvement project and joined a hospital committee. Over the past few months, he has interacted less with his peers in the fellow’s office and stayed late to complete his patient encounters. He now frequently arrives late to work, is unprepared for rounds, and forgets to place important orders. One day, you notice him shuffling through several papers when the attending asks him a question about his patient. Later that day, he snapped at a nurse who paged to ask a question about a patient who just had a colonoscopy. When you ask him how he is doing, he becomes tearful and reports that he is under a lot of stress between work and home and does not feel the work he is doing is meaningful.
Introduction
The above scenario is all too familiar. Gastroenterology training can be a stressful period in an individual’s life. Long hours, steep learning curves for new cognitive and mechanical skill sets, as well as managing personal relationships and responsibilities at home all contribute to the stress of training and finding appropriate work-life balance. These stressors can result in burnout. The last decade has brought about a renewed emphasis on mitigating the impact of occupational burnout and improving trainee lifestyle through interventions such as work-hour restrictions, resiliency training, instruction on the importance of sleep, and team-building activities.
The problem
The World Health Organization (WHO) defines occupational burnout as chronic work-related stress, which may be characterized by feelings of energy depletion, mental distance from one’s job or feelings of negativity toward it, and reduced professional efficacy. Occupational burnout has been identified as an increasing problem both in practicing providers and trainees. Surveys in gastroenterologists show rates of burnout ranging between 37% and 50%,1 with trainees and early-career physicians disproportionately affected.1,2Physicians along the entire training spectrum are more likely to report high emotional exhaustion, high depersonalization, and burnout than a population control sample.2
Several individual factors identified for those at increased risk for burnout include younger age, not being married, and being male.2 Individuals spending less than 20% of their time working on activities they find meaningful and productive were more likely to show evidence of burnout.1

Symptoms of burnout can have a profound impact on trainees’ work performance, personal interactions, and the learning environment as a whole. The Accreditation Council for Graduate Medical Education (ACGME) annual survey of trainees asks them how strongly they agree or disagree on various components of burnout such as how meaningful they find their work, if they have enough time to think and reflect, if they feel emotionally drained at work, and if they feel worn out and weary after work. The intent of these questions is to provide anonymous feedback to training programs to help identify year to year trends and intervene early to prevent occupational burnout from becoming an increasing issue.
The solution
Considerations for any intervention should take several factors into account: the impact it may have on training and the development of a competent physician in their individualized specialty, the sustainability of the intervention, and whether it is something that will be accepted by the invested parties.

One method proposed for preventing burnout during fellowship has been designated as the three R’s: relaxation, reflection, and regrouping.3
- Relax. In order to relax, trainees need ways to decompress. Activities such as exercise and social events can be helpful. Within our own program the fellows have started their own group exercise program, playing wallyball weekly before clinical duties. We also encourage use of vacation days and build comradery by organizing potluck dinners for major holidays, graduation parties at the program director’s house, and an end-of-the-year golf outing in which trainees play against staff followed by a discussion regarding the state of the program. More recently we have added one half-day per a quarter for morale and team building. During this first year, the activities in which trainees have collectively decided to participate include an escape room, a rock-climbing facility, and laser tag. The addition of more team-building days has been well received by our program’s trainees and the simple addition of these team-building days has resulted in the trainees interacting more together outside of work, particularly in the form of group dinners.

Walter Reed National Military Medical Center fellows gathering for wallyball. - Reflect. They describe reflection as a necessary checkpoint which typically occurs every 6 months.3 These “checkpoints” provide an opportunity to provide feedback to the fellow as well as check in on their well-being and receive feedback about the program. We give frequent feedback to fellows in the form of spot, rotational, and mid-/end-of-year feedback. Additionally, we have developed a unique feedback system in which the trainees meet at the end of the year to discuss collective feedback for the staff and the program. This feedback is collated by the chief fellow and given to the program director as anonymous feedback, which is then passed to the individual staff.
- Regroup. Finally, regrouping to form new strategies.3 This regrouping provides an opportunity to improve on areas in which the trainee may have a deficiency and build on their strengths. To facilitate regrouping, we identify a mentor within the department and occasionally in other departments to meet regularly with the trainee. A successful mentor ensures effective regrouping and can help the trainee avoid pitfalls that they may have experienced in similar situations.
Moving forward
Occupational burnout is a systemic problem within the medical field, with trainees disproportionately affected. It is imperative that training programs continue to work toward creating a culture that prevents development of burnout. Along with the ideas presented here, the ACGME has launched AWARE, which is a suite of resources directed specifically at the GME community, with a goal of mitigating stress and preventing burnout. No one approach will be universally applicable but continued awareness and efforts to address this on an individual and programmatic level should be encouraged.
Dr. Ordway is a chief fellow, Dr. Tritsch and Dr. Singla are associate program directors, and Dr. Torres the program director, division of gastroenterology and hepatology, Walter Reed National Military Medical Center, Bethesda, Md.
References
1. Barnes EL et al. Dig Dis Sci. 2019;64(2):302-6.
2. Dyrbye LN et al. Acad Med. 2014;89(3):443-51.
3. Waldo OA. J Am Coll Cardiol. 2015;66(11):1303-6.
Jeff is a high-performing first-year gastroenterology fellow who started with eagerness and enthusiasm. He seemed to enjoy talking to patients, wrote thorough notes, and often participated during case discussions at morning report. He initiated a quality improvement project and joined a hospital committee. Over the past few months, he has interacted less with his peers in the fellow’s office and stayed late to complete his patient encounters. He now frequently arrives late to work, is unprepared for rounds, and forgets to place important orders. One day, you notice him shuffling through several papers when the attending asks him a question about his patient. Later that day, he snapped at a nurse who paged to ask a question about a patient who just had a colonoscopy. When you ask him how he is doing, he becomes tearful and reports that he is under a lot of stress between work and home and does not feel the work he is doing is meaningful.
Introduction
The above scenario is all too familiar. Gastroenterology training can be a stressful period in an individual’s life. Long hours, steep learning curves for new cognitive and mechanical skill sets, as well as managing personal relationships and responsibilities at home all contribute to the stress of training and finding appropriate work-life balance. These stressors can result in burnout. The last decade has brought about a renewed emphasis on mitigating the impact of occupational burnout and improving trainee lifestyle through interventions such as work-hour restrictions, resiliency training, instruction on the importance of sleep, and team-building activities.
The problem
The World Health Organization (WHO) defines occupational burnout as chronic work-related stress, which may be characterized by feelings of energy depletion, mental distance from one’s job or feelings of negativity toward it, and reduced professional efficacy. Occupational burnout has been identified as an increasing problem both in practicing providers and trainees. Surveys in gastroenterologists show rates of burnout ranging between 37% and 50%,1 with trainees and early-career physicians disproportionately affected.1,2Physicians along the entire training spectrum are more likely to report high emotional exhaustion, high depersonalization, and burnout than a population control sample.2
Several individual factors identified for those at increased risk for burnout include younger age, not being married, and being male.2 Individuals spending less than 20% of their time working on activities they find meaningful and productive were more likely to show evidence of burnout.1

Symptoms of burnout can have a profound impact on trainees’ work performance, personal interactions, and the learning environment as a whole. The Accreditation Council for Graduate Medical Education (ACGME) annual survey of trainees asks them how strongly they agree or disagree on various components of burnout such as how meaningful they find their work, if they have enough time to think and reflect, if they feel emotionally drained at work, and if they feel worn out and weary after work. The intent of these questions is to provide anonymous feedback to training programs to help identify year to year trends and intervene early to prevent occupational burnout from becoming an increasing issue.
The solution
Considerations for any intervention should take several factors into account: the impact it may have on training and the development of a competent physician in their individualized specialty, the sustainability of the intervention, and whether it is something that will be accepted by the invested parties.

One method proposed for preventing burnout during fellowship has been designated as the three R’s: relaxation, reflection, and regrouping.3
- Relax. In order to relax, trainees need ways to decompress. Activities such as exercise and social events can be helpful. Within our own program the fellows have started their own group exercise program, playing wallyball weekly before clinical duties. We also encourage use of vacation days and build comradery by organizing potluck dinners for major holidays, graduation parties at the program director’s house, and an end-of-the-year golf outing in which trainees play against staff followed by a discussion regarding the state of the program. More recently we have added one half-day per a quarter for morale and team building. During this first year, the activities in which trainees have collectively decided to participate include an escape room, a rock-climbing facility, and laser tag. The addition of more team-building days has been well received by our program’s trainees and the simple addition of these team-building days has resulted in the trainees interacting more together outside of work, particularly in the form of group dinners.

Walter Reed National Military Medical Center fellows gathering for wallyball. - Reflect. They describe reflection as a necessary checkpoint which typically occurs every 6 months.3 These “checkpoints” provide an opportunity to provide feedback to the fellow as well as check in on their well-being and receive feedback about the program. We give frequent feedback to fellows in the form of spot, rotational, and mid-/end-of-year feedback. Additionally, we have developed a unique feedback system in which the trainees meet at the end of the year to discuss collective feedback for the staff and the program. This feedback is collated by the chief fellow and given to the program director as anonymous feedback, which is then passed to the individual staff.
- Regroup. Finally, regrouping to form new strategies.3 This regrouping provides an opportunity to improve on areas in which the trainee may have a deficiency and build on their strengths. To facilitate regrouping, we identify a mentor within the department and occasionally in other departments to meet regularly with the trainee. A successful mentor ensures effective regrouping and can help the trainee avoid pitfalls that they may have experienced in similar situations.
Moving forward
Occupational burnout is a systemic problem within the medical field, with trainees disproportionately affected. It is imperative that training programs continue to work toward creating a culture that prevents development of burnout. Along with the ideas presented here, the ACGME has launched AWARE, which is a suite of resources directed specifically at the GME community, with a goal of mitigating stress and preventing burnout. No one approach will be universally applicable but continued awareness and efforts to address this on an individual and programmatic level should be encouraged.
Dr. Ordway is a chief fellow, Dr. Tritsch and Dr. Singla are associate program directors, and Dr. Torres the program director, division of gastroenterology and hepatology, Walter Reed National Military Medical Center, Bethesda, Md.
References
1. Barnes EL et al. Dig Dis Sci. 2019;64(2):302-6.
2. Dyrbye LN et al. Acad Med. 2014;89(3):443-51.
3. Waldo OA. J Am Coll Cardiol. 2015;66(11):1303-6.
Early liver transplantation for alcoholic hepatitis
Case
A 45-year-old male was admitted to the hospital with severe alcoholic hepatitis. After several days of supportive care and medical therapy, the patient continued to show clinical decline. The patient is now admitted to the intensive-care unit with a Maddrey’s Discriminant Function score of 45 and a Model for End-Stage Liver Disease score of 38. He has no other significant medical comorbidities. On rounds, the patient’s wife, who is at the bedside, asks the team whether her husband would be a candidate for liver transplantation.
Should this patient be offered liver transplantation? What medical and psychosocial factors should we consider? What ethical principles should we consider?
Medical considerations
With the advent of direct-acting antivirals (DAAs), there has been a decline in the number of liver transplants performed for hepatitis C virus–related cirrhosis.1 Instead, alcohol-related liver disease (ALD) has become the most common indication for liver transplant in the United States.2 The 6-month abstinence requirement was a widespread practice within the transplant community that would exclude any patients who were actively drinking from being considered for liver transplant. However, data are inconclusive whether the 6-month rule serves as a predictor of future drinking or poor outcomes after liver transplant.3,4 Unfortunately, many patients with severe alcoholic hepatitis will not survive long enough to fulfill the 6-month requirement.5
In 2011, Mathurin and colleagues led the pivotal European trial demonstrating the effectiveness of liver transplant as a rescue option for highly selected patients with severe alcoholic hepatitis.5 The selection criteria included patients with severe alcoholic hepatitis unresponsive to medical therapy, first liver-decompensating event, presence of close supportive family members, absence of severe psychiatric disorders, and agreement by patients to adhere to lifelong total alcohol abstinence. The study showed that the 6-month survival rate of patients who received early liver transplant was 77%, compared with 25% among those who did not. The positive outcomes were subsequently replicated at several centers in the United States, and this led to a wider adoption of early liver transplant for severe alcoholic hepatitis.6-8
Psychosocial considerations
At present, we do not have well-validated consensus selection criteria to identify patients with alcoholic hepatitis most suitable for liver transplant. Each transplant center employs its own set of selection criteria with slight variations from the original European trial which prompted a national expert consensus meeting in Dallas in 2019.9 The consortium published a set of guidelines for centers planning to or already performing alcoholic hepatitis transplants. The proposed criteria to determine liver transplant candidacy are the following: 1) patients presenting for the first time with decompensated liver disease who are nonresponders to medical therapy; 2) assessment by a multidisciplinary psychosocial team including a social worker and an additional specialist; 3) no repeated unsuccessful attempts at addiction rehabilitation; 4) lack of other substance use/dependence; 5) insight with a commitment to sobriety; 6) presence of close supportive family members. The goal was to select candidates with the least likelihood of relapse in the hope of preventing poor outcomes after liver transplant. A study by a Johns Hopkins group comparing patients with severe alcoholic hepatitis who underwent careful psychosocial evaluation versus alcoholic cirrhosis with at least 6 months abstinence found that the survival and alcohol relapse rates were similar between the two groups.7
Ethical considerations
Expanding liver transplant indications to include some patients with severe alcoholic hepatitis will uphold the principle of beneficence given clear evidence of a survival benefit. In addition, graft survival rates were comparable with those of patients who underwent liver transplant for other causes.10 However, in an era of persistent organ shortage, it is important to balance justice or fairness to the patient with utilitarian policies that optimize outcomes for all who are in need of liver transplantation.
Justice
Justice means fair and equal distribution of scarce health resources to patients without bias on account of sex, race, wealth, and the nature of a patient’s disease. Based on the principle of justice, a patient with alcoholic hepatitis should be afforded opportunities for liver transplant equal to patients with other etiologies of liver disease.
Opponents of adoption of liver transplant for alcoholic hepatitis often base their reluctance on the following: patients’ failure to gain control of their alcohol use disorder, fears of alcohol relapse, and ultimately perceptions that these patients may be less deserving, compared with patients with other etiologies of liver disease. But, is this fair to the patient?
Alcohol use disorder, in general, is stigmatized and is considered by some to be a self-inflicted condition. As a medical community, we do not withhold life-saving treatment from patients who had inflicted their own injuries. Nevertheless, the stigma against alcoholism is so entrenched in our society that some fear transplanting a patient who is actively drinking would negatively affect the public’s perception of the transplant community and thus diminish the organ donation rate and harm the common good. Interestingly, a public opinion survey actually showed that the majority of respondents were at least neutral about the idea of transplanting patients with alcoholic hepatitis.11
Utility
Utility means achieving the greatest good for the greatest number of patients. The absolute scarcity of available organs imposes a need for a strict allocation decision. A liver that is used for a patient with severe alcoholic hepatitis is an organ not used for another patient suffering chronic liver disease. It is worth noting that about 20% of patients with severe alcoholic hepatitis might recover without a transplant.5 That means about one out of five liver transplants performed for alcoholic hepatitis may have been done in a patient who would have recovered without a transplant. Does this policy optimize the greatest good for everyone who is on the wait list?
Moss and Siegler argued that it was not the alcoholism that made patients with alcoholic liver disease less deserving of liver transplant, but rather their failure to seek treatment for alcoholism that made their claim for liver transplant weaker, compared with those who developed cirrhosis through no fault of their own.12 This argument is problematic. For example, patients with nonalcoholic steatohepatitis (NASH) are often compared with patients with alcoholic liver disease when it comes to modifiable behaviors that affect their health, whether it is through weight loss or abstinence, respectively. Yet, there is very little argument for lower priority for NASH patients who failed to lose weight. Secondly, alcohol use disorder is a psychosocially complex disease that requires a multidisciplinary treatment approach. Substance abuse rehabilitation is not readily available to most patients and could single out vulnerable patients from lower socioeconomic classes who are at higher risk for developing alcohol use disorder. Imposing a strict abstinence period regardless of a patient’s medical need is punitive and does not treat the underlying disease. Instead of focusing on disease causality, we ought to advocate for medical treatment of the underlying disease.
Conclusions
Liver transplant effectively functions as a zero-sum game. Efforts to save individual patients with severe alcoholic hepatitis can result in trade-offs to other patients on the wait list. Balancing the ethical principles of utility and justice is challenging. A strict 6-month rule, while convenient, does not strike the balance. The decision to transplant a patient with alcoholic hepatitis should be made on a case-by-case basis. As stewards of donor organs, transplant centers have a duty to carefully evaluate a potential candidate based on medical needs and recipient outcome without the influence of bias. We feel that, when considering liver transplant in patients with severe alcoholic hepatitis, the principle of justice or fairness to the patient is the overriding ethical principle. Provided the patient meets medical and psychosocial criteria that available evidence suggests would result in long-term survival post transplantation, we would support listing for liver transplantation.
References
1. Goldberg D et al. Gastroenterology, 2017;152(5):1090-9.e1.
2. Cholankeril G, Ahmed A. Clin Gastroenterol Hepatol. 2018;16(8):1356-8.
3. Neuberger J et al. J Hepatol. 2002;36(1):130-7.
4. DiMartini A, et al. Clin Liver Dis. 2011;15(4):727-51.
5. Mathurin P et al. N Engl J Med, 2011;365(19):1790-800.
6. Im GY et al. Am J Transplant. 2016;16(3):841-9.
7. Lee BP et al. Ann Surg. 2017;265(1):20-9.
8. Lee BP et al. Gastroenterology. 2018. 155(2):422-30.e1.
9. Asrani SK et al. Liver Transpl. 2020;26(1):127-40.
10. Singal AK et al. Transplantation. 2013;95(5):755-60.
11. Stroh G et al. Am J Transplant. 2015;15(6):1598-604.
12. Moss AH, Siegler M. JAMA. 1991;265(10):1295-8.
Dr. Wang is a gastroenterology fellow in the division of gastroenterology, hepatology, and nutrition, department of internal medicine, University of Chicago Medicine; Dr. Aronsohn is associate professor in the division of gastroenterology, hepatology, and nutrition, department of internal medicine, University of Chicago Medicine.
Case
A 45-year-old male was admitted to the hospital with severe alcoholic hepatitis. After several days of supportive care and medical therapy, the patient continued to show clinical decline. The patient is now admitted to the intensive-care unit with a Maddrey’s Discriminant Function score of 45 and a Model for End-Stage Liver Disease score of 38. He has no other significant medical comorbidities. On rounds, the patient’s wife, who is at the bedside, asks the team whether her husband would be a candidate for liver transplantation.
Should this patient be offered liver transplantation? What medical and psychosocial factors should we consider? What ethical principles should we consider?
Medical considerations
With the advent of direct-acting antivirals (DAAs), there has been a decline in the number of liver transplants performed for hepatitis C virus–related cirrhosis.1 Instead, alcohol-related liver disease (ALD) has become the most common indication for liver transplant in the United States.2 The 6-month abstinence requirement was a widespread practice within the transplant community that would exclude any patients who were actively drinking from being considered for liver transplant. However, data are inconclusive whether the 6-month rule serves as a predictor of future drinking or poor outcomes after liver transplant.3,4 Unfortunately, many patients with severe alcoholic hepatitis will not survive long enough to fulfill the 6-month requirement.5
In 2011, Mathurin and colleagues led the pivotal European trial demonstrating the effectiveness of liver transplant as a rescue option for highly selected patients with severe alcoholic hepatitis.5 The selection criteria included patients with severe alcoholic hepatitis unresponsive to medical therapy, first liver-decompensating event, presence of close supportive family members, absence of severe psychiatric disorders, and agreement by patients to adhere to lifelong total alcohol abstinence. The study showed that the 6-month survival rate of patients who received early liver transplant was 77%, compared with 25% among those who did not. The positive outcomes were subsequently replicated at several centers in the United States, and this led to a wider adoption of early liver transplant for severe alcoholic hepatitis.6-8
Psychosocial considerations
At present, we do not have well-validated consensus selection criteria to identify patients with alcoholic hepatitis most suitable for liver transplant. Each transplant center employs its own set of selection criteria with slight variations from the original European trial which prompted a national expert consensus meeting in Dallas in 2019.9 The consortium published a set of guidelines for centers planning to or already performing alcoholic hepatitis transplants. The proposed criteria to determine liver transplant candidacy are the following: 1) patients presenting for the first time with decompensated liver disease who are nonresponders to medical therapy; 2) assessment by a multidisciplinary psychosocial team including a social worker and an additional specialist; 3) no repeated unsuccessful attempts at addiction rehabilitation; 4) lack of other substance use/dependence; 5) insight with a commitment to sobriety; 6) presence of close supportive family members. The goal was to select candidates with the least likelihood of relapse in the hope of preventing poor outcomes after liver transplant. A study by a Johns Hopkins group comparing patients with severe alcoholic hepatitis who underwent careful psychosocial evaluation versus alcoholic cirrhosis with at least 6 months abstinence found that the survival and alcohol relapse rates were similar between the two groups.7
Ethical considerations
Expanding liver transplant indications to include some patients with severe alcoholic hepatitis will uphold the principle of beneficence given clear evidence of a survival benefit. In addition, graft survival rates were comparable with those of patients who underwent liver transplant for other causes.10 However, in an era of persistent organ shortage, it is important to balance justice or fairness to the patient with utilitarian policies that optimize outcomes for all who are in need of liver transplantation.
Justice
Justice means fair and equal distribution of scarce health resources to patients without bias on account of sex, race, wealth, and the nature of a patient’s disease. Based on the principle of justice, a patient with alcoholic hepatitis should be afforded opportunities for liver transplant equal to patients with other etiologies of liver disease.
Opponents of adoption of liver transplant for alcoholic hepatitis often base their reluctance on the following: patients’ failure to gain control of their alcohol use disorder, fears of alcohol relapse, and ultimately perceptions that these patients may be less deserving, compared with patients with other etiologies of liver disease. But, is this fair to the patient?
Alcohol use disorder, in general, is stigmatized and is considered by some to be a self-inflicted condition. As a medical community, we do not withhold life-saving treatment from patients who had inflicted their own injuries. Nevertheless, the stigma against alcoholism is so entrenched in our society that some fear transplanting a patient who is actively drinking would negatively affect the public’s perception of the transplant community and thus diminish the organ donation rate and harm the common good. Interestingly, a public opinion survey actually showed that the majority of respondents were at least neutral about the idea of transplanting patients with alcoholic hepatitis.11
Utility
Utility means achieving the greatest good for the greatest number of patients. The absolute scarcity of available organs imposes a need for a strict allocation decision. A liver that is used for a patient with severe alcoholic hepatitis is an organ not used for another patient suffering chronic liver disease. It is worth noting that about 20% of patients with severe alcoholic hepatitis might recover without a transplant.5 That means about one out of five liver transplants performed for alcoholic hepatitis may have been done in a patient who would have recovered without a transplant. Does this policy optimize the greatest good for everyone who is on the wait list?
Moss and Siegler argued that it was not the alcoholism that made patients with alcoholic liver disease less deserving of liver transplant, but rather their failure to seek treatment for alcoholism that made their claim for liver transplant weaker, compared with those who developed cirrhosis through no fault of their own.12 This argument is problematic. For example, patients with nonalcoholic steatohepatitis (NASH) are often compared with patients with alcoholic liver disease when it comes to modifiable behaviors that affect their health, whether it is through weight loss or abstinence, respectively. Yet, there is very little argument for lower priority for NASH patients who failed to lose weight. Secondly, alcohol use disorder is a psychosocially complex disease that requires a multidisciplinary treatment approach. Substance abuse rehabilitation is not readily available to most patients and could single out vulnerable patients from lower socioeconomic classes who are at higher risk for developing alcohol use disorder. Imposing a strict abstinence period regardless of a patient’s medical need is punitive and does not treat the underlying disease. Instead of focusing on disease causality, we ought to advocate for medical treatment of the underlying disease.
Conclusions
Liver transplant effectively functions as a zero-sum game. Efforts to save individual patients with severe alcoholic hepatitis can result in trade-offs to other patients on the wait list. Balancing the ethical principles of utility and justice is challenging. A strict 6-month rule, while convenient, does not strike the balance. The decision to transplant a patient with alcoholic hepatitis should be made on a case-by-case basis. As stewards of donor organs, transplant centers have a duty to carefully evaluate a potential candidate based on medical needs and recipient outcome without the influence of bias. We feel that, when considering liver transplant in patients with severe alcoholic hepatitis, the principle of justice or fairness to the patient is the overriding ethical principle. Provided the patient meets medical and psychosocial criteria that available evidence suggests would result in long-term survival post transplantation, we would support listing for liver transplantation.
References
1. Goldberg D et al. Gastroenterology, 2017;152(5):1090-9.e1.
2. Cholankeril G, Ahmed A. Clin Gastroenterol Hepatol. 2018;16(8):1356-8.
3. Neuberger J et al. J Hepatol. 2002;36(1):130-7.
4. DiMartini A, et al. Clin Liver Dis. 2011;15(4):727-51.
5. Mathurin P et al. N Engl J Med, 2011;365(19):1790-800.
6. Im GY et al. Am J Transplant. 2016;16(3):841-9.
7. Lee BP et al. Ann Surg. 2017;265(1):20-9.
8. Lee BP et al. Gastroenterology. 2018. 155(2):422-30.e1.
9. Asrani SK et al. Liver Transpl. 2020;26(1):127-40.
10. Singal AK et al. Transplantation. 2013;95(5):755-60.
11. Stroh G et al. Am J Transplant. 2015;15(6):1598-604.
12. Moss AH, Siegler M. JAMA. 1991;265(10):1295-8.
Dr. Wang is a gastroenterology fellow in the division of gastroenterology, hepatology, and nutrition, department of internal medicine, University of Chicago Medicine; Dr. Aronsohn is associate professor in the division of gastroenterology, hepatology, and nutrition, department of internal medicine, University of Chicago Medicine.
Case
A 45-year-old male was admitted to the hospital with severe alcoholic hepatitis. After several days of supportive care and medical therapy, the patient continued to show clinical decline. The patient is now admitted to the intensive-care unit with a Maddrey’s Discriminant Function score of 45 and a Model for End-Stage Liver Disease score of 38. He has no other significant medical comorbidities. On rounds, the patient’s wife, who is at the bedside, asks the team whether her husband would be a candidate for liver transplantation.
Should this patient be offered liver transplantation? What medical and psychosocial factors should we consider? What ethical principles should we consider?
Medical considerations
With the advent of direct-acting antivirals (DAAs), there has been a decline in the number of liver transplants performed for hepatitis C virus–related cirrhosis.1 Instead, alcohol-related liver disease (ALD) has become the most common indication for liver transplant in the United States.2 The 6-month abstinence requirement was a widespread practice within the transplant community that would exclude any patients who were actively drinking from being considered for liver transplant. However, data are inconclusive whether the 6-month rule serves as a predictor of future drinking or poor outcomes after liver transplant.3,4 Unfortunately, many patients with severe alcoholic hepatitis will not survive long enough to fulfill the 6-month requirement.5
In 2011, Mathurin and colleagues led the pivotal European trial demonstrating the effectiveness of liver transplant as a rescue option for highly selected patients with severe alcoholic hepatitis.5 The selection criteria included patients with severe alcoholic hepatitis unresponsive to medical therapy, first liver-decompensating event, presence of close supportive family members, absence of severe psychiatric disorders, and agreement by patients to adhere to lifelong total alcohol abstinence. The study showed that the 6-month survival rate of patients who received early liver transplant was 77%, compared with 25% among those who did not. The positive outcomes were subsequently replicated at several centers in the United States, and this led to a wider adoption of early liver transplant for severe alcoholic hepatitis.6-8
Psychosocial considerations
At present, we do not have well-validated consensus selection criteria to identify patients with alcoholic hepatitis most suitable for liver transplant. Each transplant center employs its own set of selection criteria with slight variations from the original European trial which prompted a national expert consensus meeting in Dallas in 2019.9 The consortium published a set of guidelines for centers planning to or already performing alcoholic hepatitis transplants. The proposed criteria to determine liver transplant candidacy are the following: 1) patients presenting for the first time with decompensated liver disease who are nonresponders to medical therapy; 2) assessment by a multidisciplinary psychosocial team including a social worker and an additional specialist; 3) no repeated unsuccessful attempts at addiction rehabilitation; 4) lack of other substance use/dependence; 5) insight with a commitment to sobriety; 6) presence of close supportive family members. The goal was to select candidates with the least likelihood of relapse in the hope of preventing poor outcomes after liver transplant. A study by a Johns Hopkins group comparing patients with severe alcoholic hepatitis who underwent careful psychosocial evaluation versus alcoholic cirrhosis with at least 6 months abstinence found that the survival and alcohol relapse rates were similar between the two groups.7
Ethical considerations
Expanding liver transplant indications to include some patients with severe alcoholic hepatitis will uphold the principle of beneficence given clear evidence of a survival benefit. In addition, graft survival rates were comparable with those of patients who underwent liver transplant for other causes.10 However, in an era of persistent organ shortage, it is important to balance justice or fairness to the patient with utilitarian policies that optimize outcomes for all who are in need of liver transplantation.
Justice
Justice means fair and equal distribution of scarce health resources to patients without bias on account of sex, race, wealth, and the nature of a patient’s disease. Based on the principle of justice, a patient with alcoholic hepatitis should be afforded opportunities for liver transplant equal to patients with other etiologies of liver disease.
Opponents of adoption of liver transplant for alcoholic hepatitis often base their reluctance on the following: patients’ failure to gain control of their alcohol use disorder, fears of alcohol relapse, and ultimately perceptions that these patients may be less deserving, compared with patients with other etiologies of liver disease. But, is this fair to the patient?
Alcohol use disorder, in general, is stigmatized and is considered by some to be a self-inflicted condition. As a medical community, we do not withhold life-saving treatment from patients who had inflicted their own injuries. Nevertheless, the stigma against alcoholism is so entrenched in our society that some fear transplanting a patient who is actively drinking would negatively affect the public’s perception of the transplant community and thus diminish the organ donation rate and harm the common good. Interestingly, a public opinion survey actually showed that the majority of respondents were at least neutral about the idea of transplanting patients with alcoholic hepatitis.11
Utility
Utility means achieving the greatest good for the greatest number of patients. The absolute scarcity of available organs imposes a need for a strict allocation decision. A liver that is used for a patient with severe alcoholic hepatitis is an organ not used for another patient suffering chronic liver disease. It is worth noting that about 20% of patients with severe alcoholic hepatitis might recover without a transplant.5 That means about one out of five liver transplants performed for alcoholic hepatitis may have been done in a patient who would have recovered without a transplant. Does this policy optimize the greatest good for everyone who is on the wait list?
Moss and Siegler argued that it was not the alcoholism that made patients with alcoholic liver disease less deserving of liver transplant, but rather their failure to seek treatment for alcoholism that made their claim for liver transplant weaker, compared with those who developed cirrhosis through no fault of their own.12 This argument is problematic. For example, patients with nonalcoholic steatohepatitis (NASH) are often compared with patients with alcoholic liver disease when it comes to modifiable behaviors that affect their health, whether it is through weight loss or abstinence, respectively. Yet, there is very little argument for lower priority for NASH patients who failed to lose weight. Secondly, alcohol use disorder is a psychosocially complex disease that requires a multidisciplinary treatment approach. Substance abuse rehabilitation is not readily available to most patients and could single out vulnerable patients from lower socioeconomic classes who are at higher risk for developing alcohol use disorder. Imposing a strict abstinence period regardless of a patient’s medical need is punitive and does not treat the underlying disease. Instead of focusing on disease causality, we ought to advocate for medical treatment of the underlying disease.
Conclusions
Liver transplant effectively functions as a zero-sum game. Efforts to save individual patients with severe alcoholic hepatitis can result in trade-offs to other patients on the wait list. Balancing the ethical principles of utility and justice is challenging. A strict 6-month rule, while convenient, does not strike the balance. The decision to transplant a patient with alcoholic hepatitis should be made on a case-by-case basis. As stewards of donor organs, transplant centers have a duty to carefully evaluate a potential candidate based on medical needs and recipient outcome without the influence of bias. We feel that, when considering liver transplant in patients with severe alcoholic hepatitis, the principle of justice or fairness to the patient is the overriding ethical principle. Provided the patient meets medical and psychosocial criteria that available evidence suggests would result in long-term survival post transplantation, we would support listing for liver transplantation.
References
1. Goldberg D et al. Gastroenterology, 2017;152(5):1090-9.e1.
2. Cholankeril G, Ahmed A. Clin Gastroenterol Hepatol. 2018;16(8):1356-8.
3. Neuberger J et al. J Hepatol. 2002;36(1):130-7.
4. DiMartini A, et al. Clin Liver Dis. 2011;15(4):727-51.
5. Mathurin P et al. N Engl J Med, 2011;365(19):1790-800.
6. Im GY et al. Am J Transplant. 2016;16(3):841-9.
7. Lee BP et al. Ann Surg. 2017;265(1):20-9.
8. Lee BP et al. Gastroenterology. 2018. 155(2):422-30.e1.
9. Asrani SK et al. Liver Transpl. 2020;26(1):127-40.
10. Singal AK et al. Transplantation. 2013;95(5):755-60.
11. Stroh G et al. Am J Transplant. 2015;15(6):1598-604.
12. Moss AH, Siegler M. JAMA. 1991;265(10):1295-8.
Dr. Wang is a gastroenterology fellow in the division of gastroenterology, hepatology, and nutrition, department of internal medicine, University of Chicago Medicine; Dr. Aronsohn is associate professor in the division of gastroenterology, hepatology, and nutrition, department of internal medicine, University of Chicago Medicine.
Ergonomics 101 for trainees
To the early trainee, often the goal of performing a colonoscopy is to reach the cecum using whatever technique necessary. Although the recommended amount of colonoscopies for safe independent practice is 140 (with some sources stating more than 500), this only relates to the safety of the patient.1 We receive scant education on how to form good procedural habits to preserve our own safety and efficiency over the course of our career. Here are some tips on how to prevent injury:
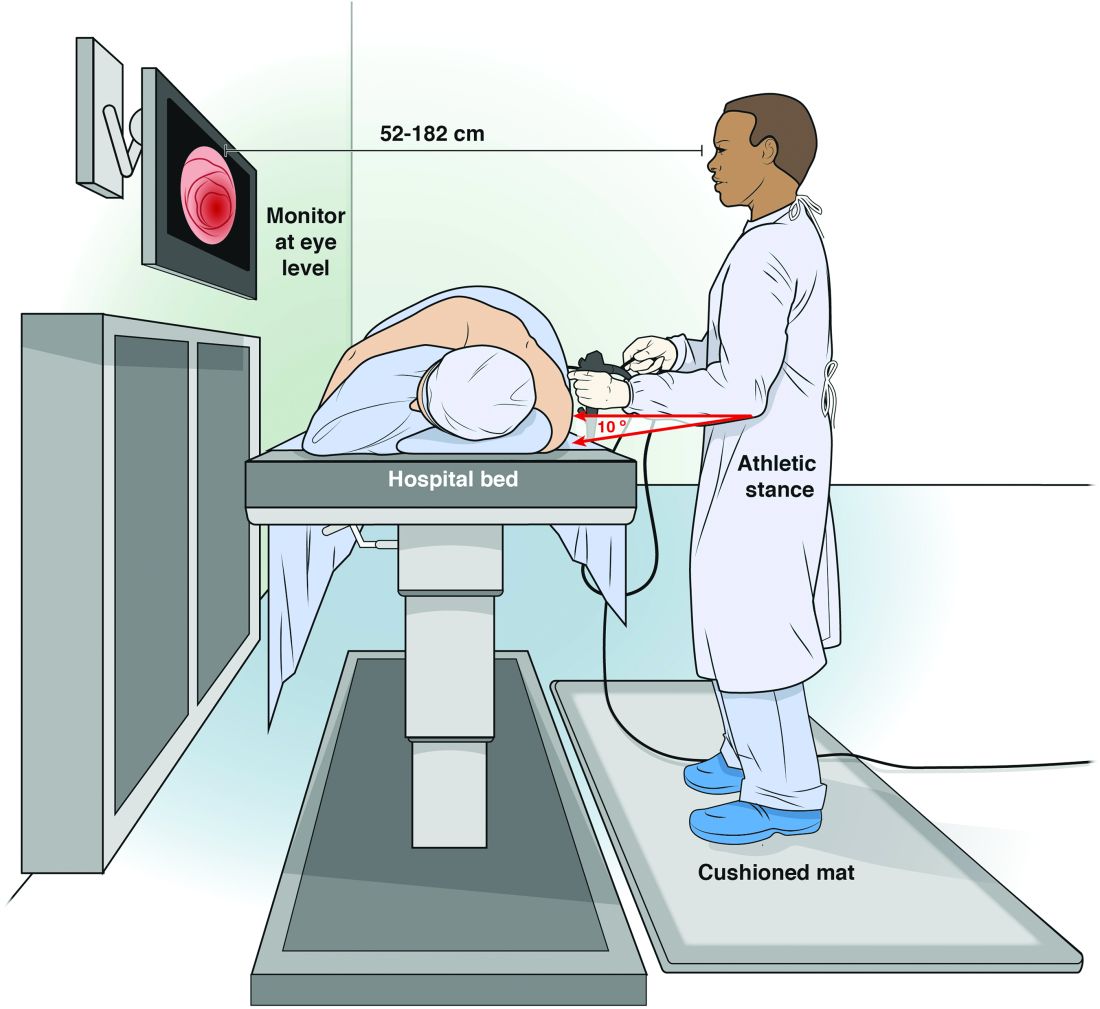
Maintain an appropriate stance. The optimal stance during endoscopy is an athletic stance: chest out, shoulders back to facilitate ease of neck movements, and a slight bend in the knees to facilitate good blood return and distribute weight. Feet should be hip width apart with toes pointed at the endoscopy screen to allow for easy pivoting of the hips and torque of upper body if needed. Ideally, this stance is complemented by the use of proper footwear and a cushioned mat to facilitate weight distribution while standing. An athletic stance facilitates a fluidity for movements from head to toe and an ability to use larger muscles groups to accomplish fine movements.
Handle the endoscope properly. Preserve energy by understanding your equipment and how to manipulate it. Orienting the endoscope directly in front of the endoscopist for upper endoscopy, and at a 45-degree angle for colonoscopy, places the instrument at optimal location to complete the procedure.5 Reviewing how to perform common techniques such as retroflexion, scope reduction, and instrumentation can also facilitate improved ergonomics and adjustment of incorrect techniques at an early stage of endoscopic training. An area of particular concern for most early trainees is the amount of rotational force placed on the right wrist with administration of torque to the endoscope. This is a foreign movement for most endoscopists and requires use of smaller muscle groups of the forearms. We suggest attempting torque with internal and external rotation of the left shoulder to utilize larger muscle groups. We can also combat fatigue during the procedure with the use of microrests intermittently to reduce prolonged muscle contraction. A common way to utilize microrests is by pinning the scope to the patient’s bed with the endoscopist’s hip to provide stability of endoscope and allow removal and relaxation of the right hand. This can be done periodically throughout the procedure to provide the ability to regroup mentally and physically.
Seek feedback. Because it is difficult to focus on ergonomics while performing a diagnostic procedure, utilize your team of observers to facilitate proper form during procedure. This includes your attending gastroenterologists, nurses, and technicians who can observe posture and technique to help detect incorrect positioning early and make corrections. A common practice is to discuss areas of desired improvement before procedures to facilitate a more vigilant observation of areas for improvement.
Assess and adjust often. As early trainees, these endoscopists perform all endoscopies under the direct supervision and often with significant assistance from a supervising gastroenterologist. This can lead to a sharp differential in psychological size; it can be hard to adjust a room to your needs when you have an intimidating and demanding attending physician who has different needs. Despite this disparity, we strongly encourage all trainees to be vigilant about adjusting the room (monitors and beds) to their own needs rather than their attendings’. A great way to head off potential conflict is to discuss the ergonomic positioning of the room before you start endoscopy with your attending, nurse, and technicians so that everyone is in agreement.
Conclusion
We offer this article as a guide for the novice endoscopist to make small changes early to prevent injuries later. Reaching competency with our skills is difficult, and we hope it can be achieved safely with our health in mind.
Dr. Magee, first-year fellow, NCC Gastroenterology; Dr. Singla, associate program director, NCC Gastroenterology, and gastroenterology service, department of internal medicine, Walter Reed National Military Medical Center, Bethesda, Md.
References
1. Spier B et al. Colonoscopy training in gastroenterology fellowships: determining competence. Gastrointest Endosc. 2010 Feb;71(2):319-24G.
2. Malmström EM et al. A slouched body posture decreases arm mobility and changes muscle recruitment in the neck and shoulder region. Eur J Appl Physiol. 2015;115(12):2491-503.
3. Singla M et al. Training the endo-athlete: an update in ergonomics in endoscopy. Clin Gastroenterol Hepatol. 2018 Jul;16(7):1003-6.
4. Bexander CS, et al. Effect of gaze direction on neck muscle activity during cervical rotation. Exp Brain Res. 2005 Dec;167(3):422-32.
5. Soetikno R et al. Holding and manipulating the endoscope: A user’s guide. Techn Gastrointest Endosc. 2019;21:124-32.
To the early trainee, often the goal of performing a colonoscopy is to reach the cecum using whatever technique necessary. Although the recommended amount of colonoscopies for safe independent practice is 140 (with some sources stating more than 500), this only relates to the safety of the patient.1 We receive scant education on how to form good procedural habits to preserve our own safety and efficiency over the course of our career. Here are some tips on how to prevent injury:

Maintain an appropriate stance. The optimal stance during endoscopy is an athletic stance: chest out, shoulders back to facilitate ease of neck movements, and a slight bend in the knees to facilitate good blood return and distribute weight. Feet should be hip width apart with toes pointed at the endoscopy screen to allow for easy pivoting of the hips and torque of upper body if needed. Ideally, this stance is complemented by the use of proper footwear and a cushioned mat to facilitate weight distribution while standing. An athletic stance facilitates a fluidity for movements from head to toe and an ability to use larger muscles groups to accomplish fine movements.
Handle the endoscope properly. Preserve energy by understanding your equipment and how to manipulate it. Orienting the endoscope directly in front of the endoscopist for upper endoscopy, and at a 45-degree angle for colonoscopy, places the instrument at optimal location to complete the procedure.5 Reviewing how to perform common techniques such as retroflexion, scope reduction, and instrumentation can also facilitate improved ergonomics and adjustment of incorrect techniques at an early stage of endoscopic training. An area of particular concern for most early trainees is the amount of rotational force placed on the right wrist with administration of torque to the endoscope. This is a foreign movement for most endoscopists and requires use of smaller muscle groups of the forearms. We suggest attempting torque with internal and external rotation of the left shoulder to utilize larger muscle groups. We can also combat fatigue during the procedure with the use of microrests intermittently to reduce prolonged muscle contraction. A common way to utilize microrests is by pinning the scope to the patient’s bed with the endoscopist’s hip to provide stability of endoscope and allow removal and relaxation of the right hand. This can be done periodically throughout the procedure to provide the ability to regroup mentally and physically.
Seek feedback. Because it is difficult to focus on ergonomics while performing a diagnostic procedure, utilize your team of observers to facilitate proper form during procedure. This includes your attending gastroenterologists, nurses, and technicians who can observe posture and technique to help detect incorrect positioning early and make corrections. A common practice is to discuss areas of desired improvement before procedures to facilitate a more vigilant observation of areas for improvement.
Assess and adjust often. As early trainees, these endoscopists perform all endoscopies under the direct supervision and often with significant assistance from a supervising gastroenterologist. This can lead to a sharp differential in psychological size; it can be hard to adjust a room to your needs when you have an intimidating and demanding attending physician who has different needs. Despite this disparity, we strongly encourage all trainees to be vigilant about adjusting the room (monitors and beds) to their own needs rather than their attendings’. A great way to head off potential conflict is to discuss the ergonomic positioning of the room before you start endoscopy with your attending, nurse, and technicians so that everyone is in agreement.
Conclusion
We offer this article as a guide for the novice endoscopist to make small changes early to prevent injuries later. Reaching competency with our skills is difficult, and we hope it can be achieved safely with our health in mind.
Dr. Magee, first-year fellow, NCC Gastroenterology; Dr. Singla, associate program director, NCC Gastroenterology, and gastroenterology service, department of internal medicine, Walter Reed National Military Medical Center, Bethesda, Md.
References
1. Spier B et al. Colonoscopy training in gastroenterology fellowships: determining competence. Gastrointest Endosc. 2010 Feb;71(2):319-24G.
2. Malmström EM et al. A slouched body posture decreases arm mobility and changes muscle recruitment in the neck and shoulder region. Eur J Appl Physiol. 2015;115(12):2491-503.
3. Singla M et al. Training the endo-athlete: an update in ergonomics in endoscopy. Clin Gastroenterol Hepatol. 2018 Jul;16(7):1003-6.
4. Bexander CS, et al. Effect of gaze direction on neck muscle activity during cervical rotation. Exp Brain Res. 2005 Dec;167(3):422-32.
5. Soetikno R et al. Holding and manipulating the endoscope: A user’s guide. Techn Gastrointest Endosc. 2019;21:124-32.
To the early trainee, often the goal of performing a colonoscopy is to reach the cecum using whatever technique necessary. Although the recommended amount of colonoscopies for safe independent practice is 140 (with some sources stating more than 500), this only relates to the safety of the patient.1 We receive scant education on how to form good procedural habits to preserve our own safety and efficiency over the course of our career. Here are some tips on how to prevent injury:

Maintain an appropriate stance. The optimal stance during endoscopy is an athletic stance: chest out, shoulders back to facilitate ease of neck movements, and a slight bend in the knees to facilitate good blood return and distribute weight. Feet should be hip width apart with toes pointed at the endoscopy screen to allow for easy pivoting of the hips and torque of upper body if needed. Ideally, this stance is complemented by the use of proper footwear and a cushioned mat to facilitate weight distribution while standing. An athletic stance facilitates a fluidity for movements from head to toe and an ability to use larger muscles groups to accomplish fine movements.
Handle the endoscope properly. Preserve energy by understanding your equipment and how to manipulate it. Orienting the endoscope directly in front of the endoscopist for upper endoscopy, and at a 45-degree angle for colonoscopy, places the instrument at optimal location to complete the procedure.5 Reviewing how to perform common techniques such as retroflexion, scope reduction, and instrumentation can also facilitate improved ergonomics and adjustment of incorrect techniques at an early stage of endoscopic training. An area of particular concern for most early trainees is the amount of rotational force placed on the right wrist with administration of torque to the endoscope. This is a foreign movement for most endoscopists and requires use of smaller muscle groups of the forearms. We suggest attempting torque with internal and external rotation of the left shoulder to utilize larger muscle groups. We can also combat fatigue during the procedure with the use of microrests intermittently to reduce prolonged muscle contraction. A common way to utilize microrests is by pinning the scope to the patient’s bed with the endoscopist’s hip to provide stability of endoscope and allow removal and relaxation of the right hand. This can be done periodically throughout the procedure to provide the ability to regroup mentally and physically.
Seek feedback. Because it is difficult to focus on ergonomics while performing a diagnostic procedure, utilize your team of observers to facilitate proper form during procedure. This includes your attending gastroenterologists, nurses, and technicians who can observe posture and technique to help detect incorrect positioning early and make corrections. A common practice is to discuss areas of desired improvement before procedures to facilitate a more vigilant observation of areas for improvement.
Assess and adjust often. As early trainees, these endoscopists perform all endoscopies under the direct supervision and often with significant assistance from a supervising gastroenterologist. This can lead to a sharp differential in psychological size; it can be hard to adjust a room to your needs when you have an intimidating and demanding attending physician who has different needs. Despite this disparity, we strongly encourage all trainees to be vigilant about adjusting the room (monitors and beds) to their own needs rather than their attendings’. A great way to head off potential conflict is to discuss the ergonomic positioning of the room before you start endoscopy with your attending, nurse, and technicians so that everyone is in agreement.
Conclusion
We offer this article as a guide for the novice endoscopist to make small changes early to prevent injuries later. Reaching competency with our skills is difficult, and we hope it can be achieved safely with our health in mind.
Dr. Magee, first-year fellow, NCC Gastroenterology; Dr. Singla, associate program director, NCC Gastroenterology, and gastroenterology service, department of internal medicine, Walter Reed National Military Medical Center, Bethesda, Md.
References
1. Spier B et al. Colonoscopy training in gastroenterology fellowships: determining competence. Gastrointest Endosc. 2010 Feb;71(2):319-24G.
2. Malmström EM et al. A slouched body posture decreases arm mobility and changes muscle recruitment in the neck and shoulder region. Eur J Appl Physiol. 2015;115(12):2491-503.
3. Singla M et al. Training the endo-athlete: an update in ergonomics in endoscopy. Clin Gastroenterol Hepatol. 2018 Jul;16(7):1003-6.
4. Bexander CS, et al. Effect of gaze direction on neck muscle activity during cervical rotation. Exp Brain Res. 2005 Dec;167(3):422-32.
5. Soetikno R et al. Holding and manipulating the endoscope: A user’s guide. Techn Gastrointest Endosc. 2019;21:124-32.
Student loan management: An introduction for the young gastroenterologist
The young gastroenterologist has no shortage of personal finance topics to juggle, ranging from investments, to life and disability coverage, and planning for retirement. But the elephant in the room is student loan management. Average medical student debt today is approximately $240,000, and debt burdens greater than $300,000 are becoming common.1,2 With this staggering amount of debt, it is understandable why student loans are a major source of anxiety. Here, I will provide a brief introduction to student loan management for gastroenterologists.
Student loans: Basic strategy
It is important to distinguish between two major types of loans: private student loans and direct federal loans. With private student loans the best strategy in most cases is to refinance to a lower interest rate. For direct federal loans, however, the decision making is more complex. There are two major approaches to these federal loans – either 1) refinance, or 2) go for public service loan forgiveness (PSLF). See Figure 1 for a flowchart summarizing my general approach to student loan management.
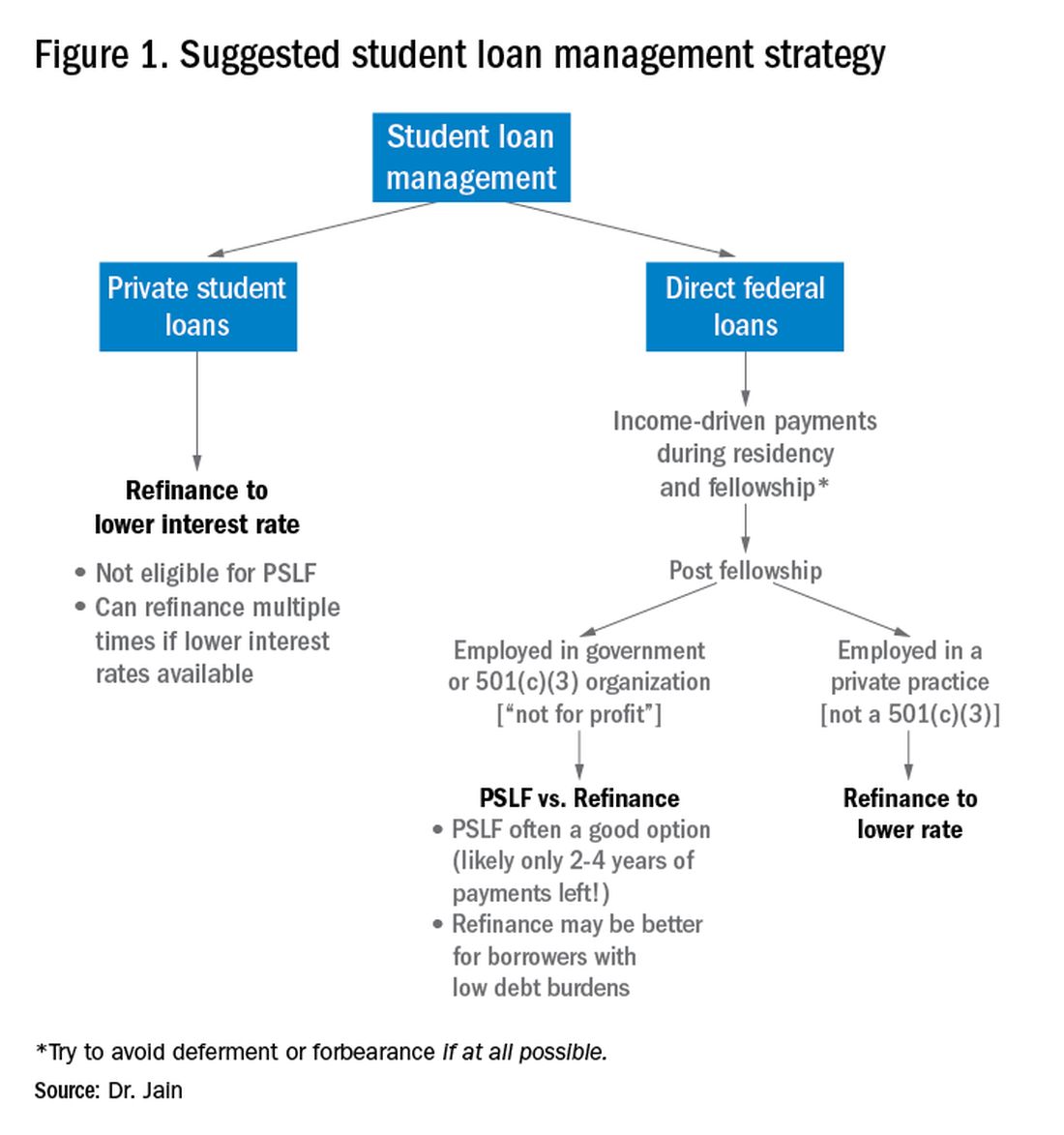
Refinance basics
One potential approach is to refinance your federal loans. Most federal loans today are at a relatively high interest rate of 6%-8%.3 Private refinancing can yield rates in the 3%-5% range, depending on the type of loan and other factors. For a loan balance of $200,000, the savings by refinancing could be approximately $2,000-$10,000 per year in interest alone. However, refinancing your loans with a private company eliminates the possibility of PSLF. Hence, you should only refinance federal loans once you are sure that you will not be pursuing PSLF. You may refinance your private loans anytime since they do not qualify for PSLF. There are multiple companies that provide student loan refinancing. The process can be done online, sometimes in as little as 30 minutes. There is generally little or no cost to refinancing, and many companies even provide a small cash-back incentive to refinance.
PSLF basics
The PSLF program allows borrowers to have the remainder of their direct loans forgiven after 10 years (120 monthly payments) under a qualifying income-driven repayment (IDR) plan.4 Figure 2 shows an overview of the various IDR plans. During the 120 payments, the borrower must work full time for a qualifying employer, which includes a government employer or a not-for-profit 501(c)(3) organization. Loan forgiveness with PSLF is completely tax free. Importantly, the PSLF program only applies to direct federal loans. You can see your federal loan types and balances by visiting https://studentaid.gov/.
To PSLF or not to PSLF?
With direct federal loans, the decision to refinance or go for PSLF is a major fork in the road. PSLF can be a good option for borrowers with long training programs and with high student loan burdens (e.g., loan-to-income ratios of 1:1, 2:1 or higher). By contrast, borrowers with short training programs or relatively small loan burdens may be better off refinancing to a low interest rate and paying off loans quickly. Virtually all institutions that train residents and fellows are qualified government or 501(c)(3) organizations. Hence, a gastroenterology graduate generally will have completed at least 6 out of 10 years of payments by the end of training. Trainees who did a chief resident year or gastroenterology research track may have completed 7 or 8 years of qualifying payments already.
For trainees who are already planning an academic career, PSLF is often a good option. While PSLF can be a nice benefit, I would not advise making a career decision purely based on PSLF. Private practice jobs generally come with substantially higher salaries than academic and government jobs. This salary differential typically more than compensates for the loss of access to PSLF. Hence, I advise trainees to choose the practice setting that is best for their personal and career satisfaction, and then build a student loan management plan around that. The exception may be the trainee who has a very large student loan burden (e.g., loan-to-income ratio of 2:1 or 3:1).
Caveats with PSLF
There have been well publicized concerns about the future of PSLF, including proposals to eliminate or cap the program.5,6 However, most proposed legislation has only recommended changes to PSLF for new borrowers. If you currently have existing federal loans, you would very likely be grandfathered into the existing PSLF terms. All federal master promissory notes since 2007 have cited PSLF as a loan repayment option.7 Hence, eliminating PSLF for existing borrowers seems unlikely since it would be changing the terms of an executed contract.8
There have also been widespread reports of high numbers of borrowers being denied applications for PSLF.9,10 However, the majority of these applicants did not have correct types of loans, had not worked full time for qualifying employers or had not made the full 120 payments.11 Yet some denials have apparently resulted from errors in tracking qualifying payments by FedLoan servicing.12 Therefore it would be prudent to keep your own careful records of all qualifying payments towards PSLF.
The nuclear option: 20- to 25-year IDR-based forgiveness
An additional option allows borrowers to make IDRs for 20-25 years (details in Figure 2) and then having their remaining loan balance forgiven.13 This option is completely independent of PSLF. Borrowers can work full time or part time and can work for any employer, including private employers.
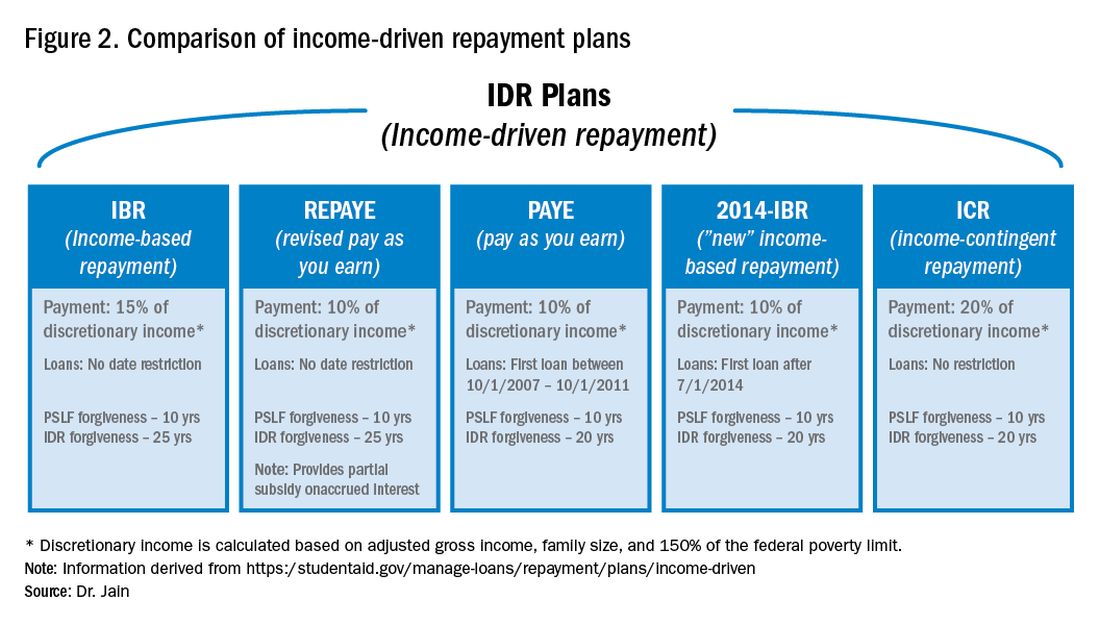
One additional option: NIH loan repayment programs
One additional solution to consider are the NIH Loan Repayment Programs (LRPs). These programs can provide substantial loan repayment (up to $50,000 annually) for trainees and attendings engaged in research that aligns with NIH priorities, including clinical research or health disparities research.14 Notably, the applicant’s research does not have to be NIH sponsored research.
Getting more information
The approach above is a general overview of student loan concepts for gastroenterologists. However, there are countless nuances and tactics that are beyond the scope of this introductory article. I encourage everyone to get additional information and advice when making your own loan management plan. There are many helpful online resources, podcasts, and books discussing the topic. Several companies provide detailed consultation on managing student loans. Such services may cost a few hundred dollars but could potentially save tens of thousands of dollars on student loan costs.
Dr. Jain is assistant professor of medicine, division of gastroenterology & hepatology, department of medicine, University of North Carolina School of Medicine, Chapel Hill. Dr. Jain has no conflicts of interest and no funding source.
References
1. https://nces.ed.gov/programs/digest/d18/tables/dt18_332.45.asp
2. https://www.credible.com/blog/statistics/average-medical-school-debt/
3. https://studentaid.gov/understand-aid/types/loans/interest-rates
4. https://studentaid.gov/manage-loans/forgiveness-cancellation/public-service
5. https://www.forbes.com/sites/robertfarrington/2019/09/24/how-to-get-your-public-service-loan-forgiveness-qualifying-payments-recounted/#18567f061f5d
6. https://www.cbo.gov/budget-options/2018/54721
7. https://static.studentloans.gov/images/ApplicationAndPromissoryNote.pdf
8. https://www.biglawinvestor.com/pslf-promissory-note/
9. https://bostonstudentloanlawyer.com/scary-stats-for-public-service-loan-forgiveness/
10. https://www.marketwatch.com/story/this-government-loan-forgiveness-program-has-rejected-99-of-borrowers-so-far-2018-09-20
11. https://studentaid.gov/data-center/student/loan-forgiveness/pslf-data
12. https://www.nytimes.com/2019/04/12/your-money/public-service-loan-forgiveness.html
13. https://studentaid.gov/manage-loans/repayment/plans/income-driven
14. https://www.lrp.nih.gov/eligibility-programs
The young gastroenterologist has no shortage of personal finance topics to juggle, ranging from investments, to life and disability coverage, and planning for retirement. But the elephant in the room is student loan management. Average medical student debt today is approximately $240,000, and debt burdens greater than $300,000 are becoming common.1,2 With this staggering amount of debt, it is understandable why student loans are a major source of anxiety. Here, I will provide a brief introduction to student loan management for gastroenterologists.
Student loans: Basic strategy
It is important to distinguish between two major types of loans: private student loans and direct federal loans. With private student loans the best strategy in most cases is to refinance to a lower interest rate. For direct federal loans, however, the decision making is more complex. There are two major approaches to these federal loans – either 1) refinance, or 2) go for public service loan forgiveness (PSLF). See Figure 1 for a flowchart summarizing my general approach to student loan management.

Refinance basics
One potential approach is to refinance your federal loans. Most federal loans today are at a relatively high interest rate of 6%-8%.3 Private refinancing can yield rates in the 3%-5% range, depending on the type of loan and other factors. For a loan balance of $200,000, the savings by refinancing could be approximately $2,000-$10,000 per year in interest alone. However, refinancing your loans with a private company eliminates the possibility of PSLF. Hence, you should only refinance federal loans once you are sure that you will not be pursuing PSLF. You may refinance your private loans anytime since they do not qualify for PSLF. There are multiple companies that provide student loan refinancing. The process can be done online, sometimes in as little as 30 minutes. There is generally little or no cost to refinancing, and many companies even provide a small cash-back incentive to refinance.
PSLF basics
The PSLF program allows borrowers to have the remainder of their direct loans forgiven after 10 years (120 monthly payments) under a qualifying income-driven repayment (IDR) plan.4 Figure 2 shows an overview of the various IDR plans. During the 120 payments, the borrower must work full time for a qualifying employer, which includes a government employer or a not-for-profit 501(c)(3) organization. Loan forgiveness with PSLF is completely tax free. Importantly, the PSLF program only applies to direct federal loans. You can see your federal loan types and balances by visiting https://studentaid.gov/.
To PSLF or not to PSLF?
With direct federal loans, the decision to refinance or go for PSLF is a major fork in the road. PSLF can be a good option for borrowers with long training programs and with high student loan burdens (e.g., loan-to-income ratios of 1:1, 2:1 or higher). By contrast, borrowers with short training programs or relatively small loan burdens may be better off refinancing to a low interest rate and paying off loans quickly. Virtually all institutions that train residents and fellows are qualified government or 501(c)(3) organizations. Hence, a gastroenterology graduate generally will have completed at least 6 out of 10 years of payments by the end of training. Trainees who did a chief resident year or gastroenterology research track may have completed 7 or 8 years of qualifying payments already.
For trainees who are already planning an academic career, PSLF is often a good option. While PSLF can be a nice benefit, I would not advise making a career decision purely based on PSLF. Private practice jobs generally come with substantially higher salaries than academic and government jobs. This salary differential typically more than compensates for the loss of access to PSLF. Hence, I advise trainees to choose the practice setting that is best for their personal and career satisfaction, and then build a student loan management plan around that. The exception may be the trainee who has a very large student loan burden (e.g., loan-to-income ratio of 2:1 or 3:1).
Caveats with PSLF
There have been well publicized concerns about the future of PSLF, including proposals to eliminate or cap the program.5,6 However, most proposed legislation has only recommended changes to PSLF for new borrowers. If you currently have existing federal loans, you would very likely be grandfathered into the existing PSLF terms. All federal master promissory notes since 2007 have cited PSLF as a loan repayment option.7 Hence, eliminating PSLF for existing borrowers seems unlikely since it would be changing the terms of an executed contract.8
There have also been widespread reports of high numbers of borrowers being denied applications for PSLF.9,10 However, the majority of these applicants did not have correct types of loans, had not worked full time for qualifying employers or had not made the full 120 payments.11 Yet some denials have apparently resulted from errors in tracking qualifying payments by FedLoan servicing.12 Therefore it would be prudent to keep your own careful records of all qualifying payments towards PSLF.
The nuclear option: 20- to 25-year IDR-based forgiveness
An additional option allows borrowers to make IDRs for 20-25 years (details in Figure 2) and then having their remaining loan balance forgiven.13 This option is completely independent of PSLF. Borrowers can work full time or part time and can work for any employer, including private employers.

One additional option: NIH loan repayment programs
One additional solution to consider are the NIH Loan Repayment Programs (LRPs). These programs can provide substantial loan repayment (up to $50,000 annually) for trainees and attendings engaged in research that aligns with NIH priorities, including clinical research or health disparities research.14 Notably, the applicant’s research does not have to be NIH sponsored research.
Getting more information
The approach above is a general overview of student loan concepts for gastroenterologists. However, there are countless nuances and tactics that are beyond the scope of this introductory article. I encourage everyone to get additional information and advice when making your own loan management plan. There are many helpful online resources, podcasts, and books discussing the topic. Several companies provide detailed consultation on managing student loans. Such services may cost a few hundred dollars but could potentially save tens of thousands of dollars on student loan costs.
Dr. Jain is assistant professor of medicine, division of gastroenterology & hepatology, department of medicine, University of North Carolina School of Medicine, Chapel Hill. Dr. Jain has no conflicts of interest and no funding source.
References
1. https://nces.ed.gov/programs/digest/d18/tables/dt18_332.45.asp
2. https://www.credible.com/blog/statistics/average-medical-school-debt/
3. https://studentaid.gov/understand-aid/types/loans/interest-rates
4. https://studentaid.gov/manage-loans/forgiveness-cancellation/public-service
5. https://www.forbes.com/sites/robertfarrington/2019/09/24/how-to-get-your-public-service-loan-forgiveness-qualifying-payments-recounted/#18567f061f5d
6. https://www.cbo.gov/budget-options/2018/54721
7. https://static.studentloans.gov/images/ApplicationAndPromissoryNote.pdf
8. https://www.biglawinvestor.com/pslf-promissory-note/
9. https://bostonstudentloanlawyer.com/scary-stats-for-public-service-loan-forgiveness/
10. https://www.marketwatch.com/story/this-government-loan-forgiveness-program-has-rejected-99-of-borrowers-so-far-2018-09-20
11. https://studentaid.gov/data-center/student/loan-forgiveness/pslf-data
12. https://www.nytimes.com/2019/04/12/your-money/public-service-loan-forgiveness.html
13. https://studentaid.gov/manage-loans/repayment/plans/income-driven
14. https://www.lrp.nih.gov/eligibility-programs
The young gastroenterologist has no shortage of personal finance topics to juggle, ranging from investments, to life and disability coverage, and planning for retirement. But the elephant in the room is student loan management. Average medical student debt today is approximately $240,000, and debt burdens greater than $300,000 are becoming common.1,2 With this staggering amount of debt, it is understandable why student loans are a major source of anxiety. Here, I will provide a brief introduction to student loan management for gastroenterologists.
Student loans: Basic strategy
It is important to distinguish between two major types of loans: private student loans and direct federal loans. With private student loans the best strategy in most cases is to refinance to a lower interest rate. For direct federal loans, however, the decision making is more complex. There are two major approaches to these federal loans – either 1) refinance, or 2) go for public service loan forgiveness (PSLF). See Figure 1 for a flowchart summarizing my general approach to student loan management.

Refinance basics
One potential approach is to refinance your federal loans. Most federal loans today are at a relatively high interest rate of 6%-8%.3 Private refinancing can yield rates in the 3%-5% range, depending on the type of loan and other factors. For a loan balance of $200,000, the savings by refinancing could be approximately $2,000-$10,000 per year in interest alone. However, refinancing your loans with a private company eliminates the possibility of PSLF. Hence, you should only refinance federal loans once you are sure that you will not be pursuing PSLF. You may refinance your private loans anytime since they do not qualify for PSLF. There are multiple companies that provide student loan refinancing. The process can be done online, sometimes in as little as 30 minutes. There is generally little or no cost to refinancing, and many companies even provide a small cash-back incentive to refinance.
PSLF basics
The PSLF program allows borrowers to have the remainder of their direct loans forgiven after 10 years (120 monthly payments) under a qualifying income-driven repayment (IDR) plan.4 Figure 2 shows an overview of the various IDR plans. During the 120 payments, the borrower must work full time for a qualifying employer, which includes a government employer or a not-for-profit 501(c)(3) organization. Loan forgiveness with PSLF is completely tax free. Importantly, the PSLF program only applies to direct federal loans. You can see your federal loan types and balances by visiting https://studentaid.gov/.
To PSLF or not to PSLF?
With direct federal loans, the decision to refinance or go for PSLF is a major fork in the road. PSLF can be a good option for borrowers with long training programs and with high student loan burdens (e.g., loan-to-income ratios of 1:1, 2:1 or higher). By contrast, borrowers with short training programs or relatively small loan burdens may be better off refinancing to a low interest rate and paying off loans quickly. Virtually all institutions that train residents and fellows are qualified government or 501(c)(3) organizations. Hence, a gastroenterology graduate generally will have completed at least 6 out of 10 years of payments by the end of training. Trainees who did a chief resident year or gastroenterology research track may have completed 7 or 8 years of qualifying payments already.
For trainees who are already planning an academic career, PSLF is often a good option. While PSLF can be a nice benefit, I would not advise making a career decision purely based on PSLF. Private practice jobs generally come with substantially higher salaries than academic and government jobs. This salary differential typically more than compensates for the loss of access to PSLF. Hence, I advise trainees to choose the practice setting that is best for their personal and career satisfaction, and then build a student loan management plan around that. The exception may be the trainee who has a very large student loan burden (e.g., loan-to-income ratio of 2:1 or 3:1).
Caveats with PSLF
There have been well publicized concerns about the future of PSLF, including proposals to eliminate or cap the program.5,6 However, most proposed legislation has only recommended changes to PSLF for new borrowers. If you currently have existing federal loans, you would very likely be grandfathered into the existing PSLF terms. All federal master promissory notes since 2007 have cited PSLF as a loan repayment option.7 Hence, eliminating PSLF for existing borrowers seems unlikely since it would be changing the terms of an executed contract.8
There have also been widespread reports of high numbers of borrowers being denied applications for PSLF.9,10 However, the majority of these applicants did not have correct types of loans, had not worked full time for qualifying employers or had not made the full 120 payments.11 Yet some denials have apparently resulted from errors in tracking qualifying payments by FedLoan servicing.12 Therefore it would be prudent to keep your own careful records of all qualifying payments towards PSLF.
The nuclear option: 20- to 25-year IDR-based forgiveness
An additional option allows borrowers to make IDRs for 20-25 years (details in Figure 2) and then having their remaining loan balance forgiven.13 This option is completely independent of PSLF. Borrowers can work full time or part time and can work for any employer, including private employers.

One additional option: NIH loan repayment programs
One additional solution to consider are the NIH Loan Repayment Programs (LRPs). These programs can provide substantial loan repayment (up to $50,000 annually) for trainees and attendings engaged in research that aligns with NIH priorities, including clinical research or health disparities research.14 Notably, the applicant’s research does not have to be NIH sponsored research.
Getting more information
The approach above is a general overview of student loan concepts for gastroenterologists. However, there are countless nuances and tactics that are beyond the scope of this introductory article. I encourage everyone to get additional information and advice when making your own loan management plan. There are many helpful online resources, podcasts, and books discussing the topic. Several companies provide detailed consultation on managing student loans. Such services may cost a few hundred dollars but could potentially save tens of thousands of dollars on student loan costs.
Dr. Jain is assistant professor of medicine, division of gastroenterology & hepatology, department of medicine, University of North Carolina School of Medicine, Chapel Hill. Dr. Jain has no conflicts of interest and no funding source.
References
1. https://nces.ed.gov/programs/digest/d18/tables/dt18_332.45.asp
2. https://www.credible.com/blog/statistics/average-medical-school-debt/
3. https://studentaid.gov/understand-aid/types/loans/interest-rates
4. https://studentaid.gov/manage-loans/forgiveness-cancellation/public-service
5. https://www.forbes.com/sites/robertfarrington/2019/09/24/how-to-get-your-public-service-loan-forgiveness-qualifying-payments-recounted/#18567f061f5d
6. https://www.cbo.gov/budget-options/2018/54721
7. https://static.studentloans.gov/images/ApplicationAndPromissoryNote.pdf
8. https://www.biglawinvestor.com/pslf-promissory-note/
9. https://bostonstudentloanlawyer.com/scary-stats-for-public-service-loan-forgiveness/
10. https://www.marketwatch.com/story/this-government-loan-forgiveness-program-has-rejected-99-of-borrowers-so-far-2018-09-20
11. https://studentaid.gov/data-center/student/loan-forgiveness/pslf-data
12. https://www.nytimes.com/2019/04/12/your-money/public-service-loan-forgiveness.html
13. https://studentaid.gov/manage-loans/repayment/plans/income-driven
14. https://www.lrp.nih.gov/eligibility-programs
Promoting diversity through the AGA
As gastroenterologists and gastrointestinal researchers, we work with an increasingly diverse patient population amid known disparities in health care delivery and health outcomes. The American Gastroenterological Association values diversity and inclusion, and part of its strategic plan is to increase and diversify its membership and leaders. The AGA Diversity Committee supports this strategic goal by fostering and promoting involvement, advancement, and recognition of underrepresented diverse constituencies. This is accomplished through policy recommendations and programs, providing resources to AGA members for addressing barriers to access and utilization of health care services among diverse patient populations with attention to linguistic, racial, cultural, religious, sexual orientation, gender identity, disability, age, and economic diversity.
There are eight clinician and investigator members, including the chair as well as one trainee member on the AGA Diversity Committee. Four members are appointed at-large and three members are appointed from the AGA Institute Committees and the AGA Institute Council. The committee has set out to achieve mission-driven goals with several initiatives that align with its intention to cultivate a diverse, inclusive, and engaged membership, armed with the necessary tools to provide the highest quality care and perform the most effective research that will benefit our patient population.
The communications task force highlights programs and topics that support the committee’s missions. Members of the committee coauthored a paper on colorectal disparities published in GI & Hepatology News in May 2018.1 In addition to presenting the disparities in colorectal screening, it provided ways to close this gap. A more recent publication in AGA Perspectives focused on unconscious bias as a prelude to the committee’s workshop at Digestive Disease Week® (DDW) 2019.2 An important initiative has been promoting Black History Month in February and Pride Month in June by posting cases or displaying prominent trailblazers on the AGA Community. In the upcoming months, profiles of several committee members will be featured in eDigest, GI & Hepatology News, and on AGA’s social media platforms.
DDW programming sponsored by the Diversity Committee is an important way to engage our members. At DDW 2019, the committee sponsored a clinical symposium title “Beyond Starbucks: Mitigating Bias Through Awareness.” This session was inspired by the 2018 incidence hallmarked by the inappropriate arrest of two African American men at a Philadelphia Starbucks. This event led to a nationwide educational breakout for all employees aimed at providing unconscious bias training. The Diversity Committee drew inspiration from these events, holding a symposium with set goals of defining unconscious bias and identifying areas within health care where unconscious bias can influence patient care. The committee invited Allyson Dylan Robinson, a portfolio lead from the Association of American Medical Colleges–endorsed Cook Ross Firm, who is nationally recognized as a leader in unconscious bias training. The assembly began with an introductory lecture followed by breakout sessions where small groups reviewed selected patient cases to determine the influence of unconscious bias in clinical scenarios. It was a well-attended symposium and was complementary to the wide array of didactic lectures offered at DDW. Bringing to light significant issues and barriers to health care is one key aspect of the mission set forth by the Diversity Committee.
The AGA Diversity Committee e-poster tour at DDW 2019 promoted the research led by scientist and physician members of underrepresented groups in medicine and/or research focused on gastrointestinal diseases in underrepresented populations. Several high-quality abstracts were reviewed and four were selected for the e-poster tour. Each scientist presented their research in front of an enthusiastic audience of DDW attendees, followed by a question and answer session. Gonzalo Parodi, BS (Cedars-Sinai Medical Center, Los Angeles), presented an elegant study showing that the antibiotic changes to intestinal microbiome were sex specific in mice. Alexis Rivera, MD (University of Puerto Rico, San Juan), found no association between inflammatory bowel disease (IBD) serologic markers and risk of surgery in patients with IBD in Puerto Rico. Maria Gonzalez-Pons, PhD (University of Puerto Rico Comprehensive Cancer Center, San Juan), presented the first study looking at the mutational landscape of early-onset colorectal cancer tumors in Puerto Rican Hispanics, as a first step toward personalizing early screening in this population. Finally, Sushrut Jangi, MD (Brigham and Women’s Hospital, Boston), presented the first longitudinal study describing the unique demographic and phenotypic characteristics of IBD in South Asians living in the United States, showing that smoking was less relevant as a risk factor, and that Crohn’s presented with a more aggressive penetrating phenotype in this population. At the conclusion of the e-poster tour, attendees and presenters had the opportunity to exchange future research ideas or follow-up and network.
The upcoming DDW 2020 AGA Diversity Committee–sponsored symposium entitled “GI Health Disparities and Creating Affirming Environments for the LGBT+ Community: The Gastroenterologist, Patient, Researcher and Educator Perspectives” will provide attendees with the opportunity to learn not only about challenges faced by the LGBTQ+ community as patients, learners, and scientists, but how we as educators, researchers, clinicians, and leaders can strategically address these challenges and intentionally create inclusive spaces in an effort to reduce health care disparities and inequities in both clinical and academic environments.
A current initiative is the creation of an archive of notable underrepresented gastroenterologists and GI scientists. The database will serve as a resource for conference organizers and committee members to identify junior speakers and mentors from diverse minority, ethnic, and racial backgrounds. This will be a platform for divisional chairs, program directors, and mentors to recommend and promote upcoming stars in their designated fields. Once the website and cloud database have been built, the diversity committee will reach out to divisional chairs, program directors, committee members, and leaders in the field to recommend physicians and scientists to include in this database. We will then reach out to nominees with an invitation and link to complete their profile in the database. We believe that this will be an opportunity for young physicians and scientists and a resource to promote diversity in medicine and science.
While we share many common experiences as ethnic minorities, the gastroenterologists comprising the AGA Diversity Committee come from various cultural backgrounds, ethnicities, and clinical practice settings. Yet rather than creating contention, our differences are the strength of this committee. Our diverse backgrounds lead to a plethora of innovative ideas and perspectives in group discussions, resulting in very robust and productive meetings. In recognizing that a diverse group can render novel solutions to many topics and issues, one of our goals is to increase membership of underrepresented groups in the AGA, as well as participation in AGA committees. This entails direct outreach to gastroenterologists in these groups and acquainting them with the ways active participation in the numerous AGA committees will support the issues affecting their profession and patients.
The process of serving on an AGA committee is simple. Interested members can nominate themselves or be nominated by another AGA member and fill out a short application. The list of AGA committees, responsibilities, open positions, and application can be found at https://www.gastro.org/aga-leadership/committees. We believe committee participation is personally and professionally rewarding, and serving on the Diversity Committee is particularly gratifying, as we can address pertinent issues that may otherwise be neglected.
Dr. Badurdeen is assistant professor at Johns Hopkins Medicine, Columbia, Md; Dr. Charabaty Pishvaian is associate professor, clinical director of the GI division, and director of the IBD Center at Sibley Memorial Hospital, Washington; Dr. Malespin is assistant professor at the University of South Florida, Tampa, and transplant hepatologist, Tampa General Hospital; Dr. Oduyebo is a gastroenterologist for Mid-Atlantic Permanente Medical Group, Shady Grove, Md; Dr. Quezada is associate professor and assistant dean for academic and multicultural affairs, University of Maryland, Baltimore County; and Dr. Stephen is a gastroenterologist at Annadel Medical Group, Santa Rosa, Calif.
References
1. Oduyebo I et al. Underserved populations and colorectal cancer screening: Patient perceptions of barriers to care and effective interventions. GI & Hepatology News. May 2018.
2. Munroe CA et al. The AGA Diversity Committee: Opening up a conversation about unconscious bias in GI practice. AGA Perspectives. July 2019.
As gastroenterologists and gastrointestinal researchers, we work with an increasingly diverse patient population amid known disparities in health care delivery and health outcomes. The American Gastroenterological Association values diversity and inclusion, and part of its strategic plan is to increase and diversify its membership and leaders. The AGA Diversity Committee supports this strategic goal by fostering and promoting involvement, advancement, and recognition of underrepresented diverse constituencies. This is accomplished through policy recommendations and programs, providing resources to AGA members for addressing barriers to access and utilization of health care services among diverse patient populations with attention to linguistic, racial, cultural, religious, sexual orientation, gender identity, disability, age, and economic diversity.
There are eight clinician and investigator members, including the chair as well as one trainee member on the AGA Diversity Committee. Four members are appointed at-large and three members are appointed from the AGA Institute Committees and the AGA Institute Council. The committee has set out to achieve mission-driven goals with several initiatives that align with its intention to cultivate a diverse, inclusive, and engaged membership, armed with the necessary tools to provide the highest quality care and perform the most effective research that will benefit our patient population.
The communications task force highlights programs and topics that support the committee’s missions. Members of the committee coauthored a paper on colorectal disparities published in GI & Hepatology News in May 2018.1 In addition to presenting the disparities in colorectal screening, it provided ways to close this gap. A more recent publication in AGA Perspectives focused on unconscious bias as a prelude to the committee’s workshop at Digestive Disease Week® (DDW) 2019.2 An important initiative has been promoting Black History Month in February and Pride Month in June by posting cases or displaying prominent trailblazers on the AGA Community. In the upcoming months, profiles of several committee members will be featured in eDigest, GI & Hepatology News, and on AGA’s social media platforms.
DDW programming sponsored by the Diversity Committee is an important way to engage our members. At DDW 2019, the committee sponsored a clinical symposium title “Beyond Starbucks: Mitigating Bias Through Awareness.” This session was inspired by the 2018 incidence hallmarked by the inappropriate arrest of two African American men at a Philadelphia Starbucks. This event led to a nationwide educational breakout for all employees aimed at providing unconscious bias training. The Diversity Committee drew inspiration from these events, holding a symposium with set goals of defining unconscious bias and identifying areas within health care where unconscious bias can influence patient care. The committee invited Allyson Dylan Robinson, a portfolio lead from the Association of American Medical Colleges–endorsed Cook Ross Firm, who is nationally recognized as a leader in unconscious bias training. The assembly began with an introductory lecture followed by breakout sessions where small groups reviewed selected patient cases to determine the influence of unconscious bias in clinical scenarios. It was a well-attended symposium and was complementary to the wide array of didactic lectures offered at DDW. Bringing to light significant issues and barriers to health care is one key aspect of the mission set forth by the Diversity Committee.
The AGA Diversity Committee e-poster tour at DDW 2019 promoted the research led by scientist and physician members of underrepresented groups in medicine and/or research focused on gastrointestinal diseases in underrepresented populations. Several high-quality abstracts were reviewed and four were selected for the e-poster tour. Each scientist presented their research in front of an enthusiastic audience of DDW attendees, followed by a question and answer session. Gonzalo Parodi, BS (Cedars-Sinai Medical Center, Los Angeles), presented an elegant study showing that the antibiotic changes to intestinal microbiome were sex specific in mice. Alexis Rivera, MD (University of Puerto Rico, San Juan), found no association between inflammatory bowel disease (IBD) serologic markers and risk of surgery in patients with IBD in Puerto Rico. Maria Gonzalez-Pons, PhD (University of Puerto Rico Comprehensive Cancer Center, San Juan), presented the first study looking at the mutational landscape of early-onset colorectal cancer tumors in Puerto Rican Hispanics, as a first step toward personalizing early screening in this population. Finally, Sushrut Jangi, MD (Brigham and Women’s Hospital, Boston), presented the first longitudinal study describing the unique demographic and phenotypic characteristics of IBD in South Asians living in the United States, showing that smoking was less relevant as a risk factor, and that Crohn’s presented with a more aggressive penetrating phenotype in this population. At the conclusion of the e-poster tour, attendees and presenters had the opportunity to exchange future research ideas or follow-up and network.
The upcoming DDW 2020 AGA Diversity Committee–sponsored symposium entitled “GI Health Disparities and Creating Affirming Environments for the LGBT+ Community: The Gastroenterologist, Patient, Researcher and Educator Perspectives” will provide attendees with the opportunity to learn not only about challenges faced by the LGBTQ+ community as patients, learners, and scientists, but how we as educators, researchers, clinicians, and leaders can strategically address these challenges and intentionally create inclusive spaces in an effort to reduce health care disparities and inequities in both clinical and academic environments.
A current initiative is the creation of an archive of notable underrepresented gastroenterologists and GI scientists. The database will serve as a resource for conference organizers and committee members to identify junior speakers and mentors from diverse minority, ethnic, and racial backgrounds. This will be a platform for divisional chairs, program directors, and mentors to recommend and promote upcoming stars in their designated fields. Once the website and cloud database have been built, the diversity committee will reach out to divisional chairs, program directors, committee members, and leaders in the field to recommend physicians and scientists to include in this database. We will then reach out to nominees with an invitation and link to complete their profile in the database. We believe that this will be an opportunity for young physicians and scientists and a resource to promote diversity in medicine and science.
While we share many common experiences as ethnic minorities, the gastroenterologists comprising the AGA Diversity Committee come from various cultural backgrounds, ethnicities, and clinical practice settings. Yet rather than creating contention, our differences are the strength of this committee. Our diverse backgrounds lead to a plethora of innovative ideas and perspectives in group discussions, resulting in very robust and productive meetings. In recognizing that a diverse group can render novel solutions to many topics and issues, one of our goals is to increase membership of underrepresented groups in the AGA, as well as participation in AGA committees. This entails direct outreach to gastroenterologists in these groups and acquainting them with the ways active participation in the numerous AGA committees will support the issues affecting their profession and patients.
The process of serving on an AGA committee is simple. Interested members can nominate themselves or be nominated by another AGA member and fill out a short application. The list of AGA committees, responsibilities, open positions, and application can be found at https://www.gastro.org/aga-leadership/committees. We believe committee participation is personally and professionally rewarding, and serving on the Diversity Committee is particularly gratifying, as we can address pertinent issues that may otherwise be neglected.
Dr. Badurdeen is assistant professor at Johns Hopkins Medicine, Columbia, Md; Dr. Charabaty Pishvaian is associate professor, clinical director of the GI division, and director of the IBD Center at Sibley Memorial Hospital, Washington; Dr. Malespin is assistant professor at the University of South Florida, Tampa, and transplant hepatologist, Tampa General Hospital; Dr. Oduyebo is a gastroenterologist for Mid-Atlantic Permanente Medical Group, Shady Grove, Md; Dr. Quezada is associate professor and assistant dean for academic and multicultural affairs, University of Maryland, Baltimore County; and Dr. Stephen is a gastroenterologist at Annadel Medical Group, Santa Rosa, Calif.
References
1. Oduyebo I et al. Underserved populations and colorectal cancer screening: Patient perceptions of barriers to care and effective interventions. GI & Hepatology News. May 2018.
2. Munroe CA et al. The AGA Diversity Committee: Opening up a conversation about unconscious bias in GI practice. AGA Perspectives. July 2019.
As gastroenterologists and gastrointestinal researchers, we work with an increasingly diverse patient population amid known disparities in health care delivery and health outcomes. The American Gastroenterological Association values diversity and inclusion, and part of its strategic plan is to increase and diversify its membership and leaders. The AGA Diversity Committee supports this strategic goal by fostering and promoting involvement, advancement, and recognition of underrepresented diverse constituencies. This is accomplished through policy recommendations and programs, providing resources to AGA members for addressing barriers to access and utilization of health care services among diverse patient populations with attention to linguistic, racial, cultural, religious, sexual orientation, gender identity, disability, age, and economic diversity.
There are eight clinician and investigator members, including the chair as well as one trainee member on the AGA Diversity Committee. Four members are appointed at-large and three members are appointed from the AGA Institute Committees and the AGA Institute Council. The committee has set out to achieve mission-driven goals with several initiatives that align with its intention to cultivate a diverse, inclusive, and engaged membership, armed with the necessary tools to provide the highest quality care and perform the most effective research that will benefit our patient population.
The communications task force highlights programs and topics that support the committee’s missions. Members of the committee coauthored a paper on colorectal disparities published in GI & Hepatology News in May 2018.1 In addition to presenting the disparities in colorectal screening, it provided ways to close this gap. A more recent publication in AGA Perspectives focused on unconscious bias as a prelude to the committee’s workshop at Digestive Disease Week® (DDW) 2019.2 An important initiative has been promoting Black History Month in February and Pride Month in June by posting cases or displaying prominent trailblazers on the AGA Community. In the upcoming months, profiles of several committee members will be featured in eDigest, GI & Hepatology News, and on AGA’s social media platforms.
DDW programming sponsored by the Diversity Committee is an important way to engage our members. At DDW 2019, the committee sponsored a clinical symposium title “Beyond Starbucks: Mitigating Bias Through Awareness.” This session was inspired by the 2018 incidence hallmarked by the inappropriate arrest of two African American men at a Philadelphia Starbucks. This event led to a nationwide educational breakout for all employees aimed at providing unconscious bias training. The Diversity Committee drew inspiration from these events, holding a symposium with set goals of defining unconscious bias and identifying areas within health care where unconscious bias can influence patient care. The committee invited Allyson Dylan Robinson, a portfolio lead from the Association of American Medical Colleges–endorsed Cook Ross Firm, who is nationally recognized as a leader in unconscious bias training. The assembly began with an introductory lecture followed by breakout sessions where small groups reviewed selected patient cases to determine the influence of unconscious bias in clinical scenarios. It was a well-attended symposium and was complementary to the wide array of didactic lectures offered at DDW. Bringing to light significant issues and barriers to health care is one key aspect of the mission set forth by the Diversity Committee.
The AGA Diversity Committee e-poster tour at DDW 2019 promoted the research led by scientist and physician members of underrepresented groups in medicine and/or research focused on gastrointestinal diseases in underrepresented populations. Several high-quality abstracts were reviewed and four were selected for the e-poster tour. Each scientist presented their research in front of an enthusiastic audience of DDW attendees, followed by a question and answer session. Gonzalo Parodi, BS (Cedars-Sinai Medical Center, Los Angeles), presented an elegant study showing that the antibiotic changes to intestinal microbiome were sex specific in mice. Alexis Rivera, MD (University of Puerto Rico, San Juan), found no association between inflammatory bowel disease (IBD) serologic markers and risk of surgery in patients with IBD in Puerto Rico. Maria Gonzalez-Pons, PhD (University of Puerto Rico Comprehensive Cancer Center, San Juan), presented the first study looking at the mutational landscape of early-onset colorectal cancer tumors in Puerto Rican Hispanics, as a first step toward personalizing early screening in this population. Finally, Sushrut Jangi, MD (Brigham and Women’s Hospital, Boston), presented the first longitudinal study describing the unique demographic and phenotypic characteristics of IBD in South Asians living in the United States, showing that smoking was less relevant as a risk factor, and that Crohn’s presented with a more aggressive penetrating phenotype in this population. At the conclusion of the e-poster tour, attendees and presenters had the opportunity to exchange future research ideas or follow-up and network.
The upcoming DDW 2020 AGA Diversity Committee–sponsored symposium entitled “GI Health Disparities and Creating Affirming Environments for the LGBT+ Community: The Gastroenterologist, Patient, Researcher and Educator Perspectives” will provide attendees with the opportunity to learn not only about challenges faced by the LGBTQ+ community as patients, learners, and scientists, but how we as educators, researchers, clinicians, and leaders can strategically address these challenges and intentionally create inclusive spaces in an effort to reduce health care disparities and inequities in both clinical and academic environments.
A current initiative is the creation of an archive of notable underrepresented gastroenterologists and GI scientists. The database will serve as a resource for conference organizers and committee members to identify junior speakers and mentors from diverse minority, ethnic, and racial backgrounds. This will be a platform for divisional chairs, program directors, and mentors to recommend and promote upcoming stars in their designated fields. Once the website and cloud database have been built, the diversity committee will reach out to divisional chairs, program directors, committee members, and leaders in the field to recommend physicians and scientists to include in this database. We will then reach out to nominees with an invitation and link to complete their profile in the database. We believe that this will be an opportunity for young physicians and scientists and a resource to promote diversity in medicine and science.
While we share many common experiences as ethnic minorities, the gastroenterologists comprising the AGA Diversity Committee come from various cultural backgrounds, ethnicities, and clinical practice settings. Yet rather than creating contention, our differences are the strength of this committee. Our diverse backgrounds lead to a plethora of innovative ideas and perspectives in group discussions, resulting in very robust and productive meetings. In recognizing that a diverse group can render novel solutions to many topics and issues, one of our goals is to increase membership of underrepresented groups in the AGA, as well as participation in AGA committees. This entails direct outreach to gastroenterologists in these groups and acquainting them with the ways active participation in the numerous AGA committees will support the issues affecting their profession and patients.
The process of serving on an AGA committee is simple. Interested members can nominate themselves or be nominated by another AGA member and fill out a short application. The list of AGA committees, responsibilities, open positions, and application can be found at https://www.gastro.org/aga-leadership/committees. We believe committee participation is personally and professionally rewarding, and serving on the Diversity Committee is particularly gratifying, as we can address pertinent issues that may otherwise be neglected.
Dr. Badurdeen is assistant professor at Johns Hopkins Medicine, Columbia, Md; Dr. Charabaty Pishvaian is associate professor, clinical director of the GI division, and director of the IBD Center at Sibley Memorial Hospital, Washington; Dr. Malespin is assistant professor at the University of South Florida, Tampa, and transplant hepatologist, Tampa General Hospital; Dr. Oduyebo is a gastroenterologist for Mid-Atlantic Permanente Medical Group, Shady Grove, Md; Dr. Quezada is associate professor and assistant dean for academic and multicultural affairs, University of Maryland, Baltimore County; and Dr. Stephen is a gastroenterologist at Annadel Medical Group, Santa Rosa, Calif.
References
1. Oduyebo I et al. Underserved populations and colorectal cancer screening: Patient perceptions of barriers to care and effective interventions. GI & Hepatology News. May 2018.
2. Munroe CA et al. The AGA Diversity Committee: Opening up a conversation about unconscious bias in GI practice. AGA Perspectives. July 2019.
The pros and cons of pathology lab ownership: What early career GI doctors need to know
From colonoscopies to endoscopic ultrasound, gastroenterology is fundamentally a procedure-based specialty. Given that reality, making a decision to have as much control as possible over the entire process just makes sense for many GI practices.
Back in 2008, I was in charge of the process to develop a pathology lab at Arizona Digestive Health, a physician group with 26 locations throughout the state, as part of our decision to form a supergroup with eight ambulatory surgery centers. For us, having ambulatory surgery centers had been a game changer. We learned we could double our efficiency with procedures when we controlled the process from start to finish. We began to consider other processes – in this case pathology – that we could improve.
Prior to running our own pathology lab, doctors who read our slides were general pathologists who did not always understand the language of gastroenterology. We had results that came back by fax that were often cumbersome to read and did not always give us the information we needed in the way we needed it. Consistency was a problem. We knew we needed a change.
I cannot lie – setting up and running your own pathology lab is not always easy. But with the right factors in place, here are some benefits to consider when you are making a decision about joining a practice.
Quality, efficiency can lead to opportunity
Our lab has three GI fellowship–trained pathologists reading our slides. That means they are highly specialized and know exactly what we are looking for in a pathology report. We have a 24-hour turnaround for results. A courier service delivers biopsy specimens from our endoscopy centers to our path lab every day, and each morning our gastropathologists have a stack of pathology slides waiting for them. It’s added predictability and stability to the process, and we get the level of quality, specificity, and uniformity we need in a report.
The efficiencies are beneficial and it has given us more leverage in our negotiations with payers. We know what our costs are and have great quality metrics as well as read rates that we can provide. This signals to health plans that quality is a top priority for us.
We have also gained a reputational benefit with patients. Although much of the work is happening behind the scenes for our patients, they get results faster and a consistency with costs. It also allows us to easily access the slides of patients we have been seeing for years, giving us a richer data set and more confidence in our diagnosis.
Now that we have our own lab, we can look at our pathology data and conduct studies that will benefit all patients. For example, a few of our GI fellows were able to work with our pathologists to conduct a study on adenoma detection rates, exhausting a tissue block when no adenoma was found on initial review. We found a significant increase in adenoma detection using this method; we plan to publish results soon. The ability to conduct this kind of research is worth considering when early career gastroenterologists are selecting a practice to join.
And last but not least, having our own pathology lab acts as a unifying force for our group, which is spread out across 26 offices. When diagnoses are available and we get a call from our pathologist, we know to pick up the phone immediately.
What to consider before jumping in
Setting up our own pathology lab from the ground up was a learning process. We had enough patient volume for the move to make sense, so it is possible that smaller practices might not be able to make the investment if they have lower patient volume or cannot control their specimen flow. One option is to set up a technical lab and contract out for the slide reading. We felt it was important for our pathologists to also be our practice partners, and time has proven this to be a good decision for us.
We designed a lab with our work flow in mind, and it helped to have a pathologist on board from the beginning who knows gastroenterology. We even created our own lab information system with the help of a software engineer. It took a little bit over a year from conception to a functioning comprehensive lab.
Of course, there are regulatory factors to consider – the federal physician self-referral (Stark) law and the federal Anti-Markup Rule – come to mind. But we made sure to get a legal opinion that allows us to comply with the law. That’s something anyone who wants to make a move in this direction should do.
Looking back over the experience, I would not do anything differently. Yes, there are startup costs and a learning curve. But the quality we get from having our own pathology lab dedicated to GI and the efficiencies we have gained are well worth it.
Dr. Berggreen is the president of Arizona Digestive Health and chief strategy officer of the GI Alliance. He is also a board member of the Digestive Health Physicians Association. He received his doctorate at Louisiana State University, New Orleans. He is the former site director of the Good Samaritan GI Fellowship Program and named one of Phoenix Magazine’s “Top Docs.”
From colonoscopies to endoscopic ultrasound, gastroenterology is fundamentally a procedure-based specialty. Given that reality, making a decision to have as much control as possible over the entire process just makes sense for many GI practices.
Back in 2008, I was in charge of the process to develop a pathology lab at Arizona Digestive Health, a physician group with 26 locations throughout the state, as part of our decision to form a supergroup with eight ambulatory surgery centers. For us, having ambulatory surgery centers had been a game changer. We learned we could double our efficiency with procedures when we controlled the process from start to finish. We began to consider other processes – in this case pathology – that we could improve.
Prior to running our own pathology lab, doctors who read our slides were general pathologists who did not always understand the language of gastroenterology. We had results that came back by fax that were often cumbersome to read and did not always give us the information we needed in the way we needed it. Consistency was a problem. We knew we needed a change.
I cannot lie – setting up and running your own pathology lab is not always easy. But with the right factors in place, here are some benefits to consider when you are making a decision about joining a practice.
Quality, efficiency can lead to opportunity
Our lab has three GI fellowship–trained pathologists reading our slides. That means they are highly specialized and know exactly what we are looking for in a pathology report. We have a 24-hour turnaround for results. A courier service delivers biopsy specimens from our endoscopy centers to our path lab every day, and each morning our gastropathologists have a stack of pathology slides waiting for them. It’s added predictability and stability to the process, and we get the level of quality, specificity, and uniformity we need in a report.
The efficiencies are beneficial and it has given us more leverage in our negotiations with payers. We know what our costs are and have great quality metrics as well as read rates that we can provide. This signals to health plans that quality is a top priority for us.
We have also gained a reputational benefit with patients. Although much of the work is happening behind the scenes for our patients, they get results faster and a consistency with costs. It also allows us to easily access the slides of patients we have been seeing for years, giving us a richer data set and more confidence in our diagnosis.
Now that we have our own lab, we can look at our pathology data and conduct studies that will benefit all patients. For example, a few of our GI fellows were able to work with our pathologists to conduct a study on adenoma detection rates, exhausting a tissue block when no adenoma was found on initial review. We found a significant increase in adenoma detection using this method; we plan to publish results soon. The ability to conduct this kind of research is worth considering when early career gastroenterologists are selecting a practice to join.
And last but not least, having our own pathology lab acts as a unifying force for our group, which is spread out across 26 offices. When diagnoses are available and we get a call from our pathologist, we know to pick up the phone immediately.
What to consider before jumping in
Setting up our own pathology lab from the ground up was a learning process. We had enough patient volume for the move to make sense, so it is possible that smaller practices might not be able to make the investment if they have lower patient volume or cannot control their specimen flow. One option is to set up a technical lab and contract out for the slide reading. We felt it was important for our pathologists to also be our practice partners, and time has proven this to be a good decision for us.
We designed a lab with our work flow in mind, and it helped to have a pathologist on board from the beginning who knows gastroenterology. We even created our own lab information system with the help of a software engineer. It took a little bit over a year from conception to a functioning comprehensive lab.
Of course, there are regulatory factors to consider – the federal physician self-referral (Stark) law and the federal Anti-Markup Rule – come to mind. But we made sure to get a legal opinion that allows us to comply with the law. That’s something anyone who wants to make a move in this direction should do.
Looking back over the experience, I would not do anything differently. Yes, there are startup costs and a learning curve. But the quality we get from having our own pathology lab dedicated to GI and the efficiencies we have gained are well worth it.
Dr. Berggreen is the president of Arizona Digestive Health and chief strategy officer of the GI Alliance. He is also a board member of the Digestive Health Physicians Association. He received his doctorate at Louisiana State University, New Orleans. He is the former site director of the Good Samaritan GI Fellowship Program and named one of Phoenix Magazine’s “Top Docs.”
From colonoscopies to endoscopic ultrasound, gastroenterology is fundamentally a procedure-based specialty. Given that reality, making a decision to have as much control as possible over the entire process just makes sense for many GI practices.
Back in 2008, I was in charge of the process to develop a pathology lab at Arizona Digestive Health, a physician group with 26 locations throughout the state, as part of our decision to form a supergroup with eight ambulatory surgery centers. For us, having ambulatory surgery centers had been a game changer. We learned we could double our efficiency with procedures when we controlled the process from start to finish. We began to consider other processes – in this case pathology – that we could improve.
Prior to running our own pathology lab, doctors who read our slides were general pathologists who did not always understand the language of gastroenterology. We had results that came back by fax that were often cumbersome to read and did not always give us the information we needed in the way we needed it. Consistency was a problem. We knew we needed a change.
I cannot lie – setting up and running your own pathology lab is not always easy. But with the right factors in place, here are some benefits to consider when you are making a decision about joining a practice.
Quality, efficiency can lead to opportunity
Our lab has three GI fellowship–trained pathologists reading our slides. That means they are highly specialized and know exactly what we are looking for in a pathology report. We have a 24-hour turnaround for results. A courier service delivers biopsy specimens from our endoscopy centers to our path lab every day, and each morning our gastropathologists have a stack of pathology slides waiting for them. It’s added predictability and stability to the process, and we get the level of quality, specificity, and uniformity we need in a report.
The efficiencies are beneficial and it has given us more leverage in our negotiations with payers. We know what our costs are and have great quality metrics as well as read rates that we can provide. This signals to health plans that quality is a top priority for us.
We have also gained a reputational benefit with patients. Although much of the work is happening behind the scenes for our patients, they get results faster and a consistency with costs. It also allows us to easily access the slides of patients we have been seeing for years, giving us a richer data set and more confidence in our diagnosis.
Now that we have our own lab, we can look at our pathology data and conduct studies that will benefit all patients. For example, a few of our GI fellows were able to work with our pathologists to conduct a study on adenoma detection rates, exhausting a tissue block when no adenoma was found on initial review. We found a significant increase in adenoma detection using this method; we plan to publish results soon. The ability to conduct this kind of research is worth considering when early career gastroenterologists are selecting a practice to join.
And last but not least, having our own pathology lab acts as a unifying force for our group, which is spread out across 26 offices. When diagnoses are available and we get a call from our pathologist, we know to pick up the phone immediately.
What to consider before jumping in
Setting up our own pathology lab from the ground up was a learning process. We had enough patient volume for the move to make sense, so it is possible that smaller practices might not be able to make the investment if they have lower patient volume or cannot control their specimen flow. One option is to set up a technical lab and contract out for the slide reading. We felt it was important for our pathologists to also be our practice partners, and time has proven this to be a good decision for us.
We designed a lab with our work flow in mind, and it helped to have a pathologist on board from the beginning who knows gastroenterology. We even created our own lab information system with the help of a software engineer. It took a little bit over a year from conception to a functioning comprehensive lab.
Of course, there are regulatory factors to consider – the federal physician self-referral (Stark) law and the federal Anti-Markup Rule – come to mind. But we made sure to get a legal opinion that allows us to comply with the law. That’s something anyone who wants to make a move in this direction should do.
Looking back over the experience, I would not do anything differently. Yes, there are startup costs and a learning curve. But the quality we get from having our own pathology lab dedicated to GI and the efficiencies we have gained are well worth it.
Dr. Berggreen is the president of Arizona Digestive Health and chief strategy officer of the GI Alliance. He is also a board member of the Digestive Health Physicians Association. He received his doctorate at Louisiana State University, New Orleans. He is the former site director of the Good Samaritan GI Fellowship Program and named one of Phoenix Magazine’s “Top Docs.”
New medical ethics series debuts
Dear colleagues,
The first issue of The New Gastroenterologist in 2020 consists of a particularly interesting array of articles and the introduction of a new medical ethics series!
This month’s “In Focus” article, brought to you by Jennifer Maratt (Indiana University) and Elena Stoffel (University of Michigan), provides a high yield overview of hereditary colorectal cancer and polyposis syndromes, with guidance on when a referral to a high risk cancer specialist and geneticist is warranted.
Daniel Mills (Cunningham, Meyer & Vedrine P.C.) gives us a valuable legal perspective of the role of electronic patient portals in the dissemination of information and medical advice to patients – such an important topic for everyone to be aware of as the nature of patient communication now strongly relies on electronic messaging.
R. Thomas Finn III (Palo Alto Medical Foundation) and David Leiman (Duke) nicely broach the issue of patient satisfaction. This is a timely topic as many institutions are not only publishing patient reviews online so that they are readily available to the public, but are also making financial incentives contingent on high patient ratings. The article discusses the evolution of the emphasis placed on patient satisfaction throughout the years with tips on how to navigate some of the distinct challenges within gastroenterology.
As part of our DHPA Private Practice Perspectives series, David Stokesberry (Digestive Disease Specialists Inc, Oklahoma City) discusses the nuts and bolts of ambulatory endoscopy centers and some of the challenges and benefits that accompany ownership of such centers.
An often overlooked aspect of gastroenterology training is nutrition. In our postfellowship pathways section, Dejan Micic (University of Chicago) outlines his decision to pursue a career in nutrition support, small bowel disorders, and the practice of deep enteroscopy.
Finally, this quarter’s newsletter features the start of a new section, which I am very excited to introduce – a case based series which will address issues in clinical medical ethics specific to gastroenterology. Lauren Feld (University of Washington) writes the inaugural piece for the section, providing a systematic approach to the patient with an existing do-not-resuscitate (DNR) order that is about to undergo endoscopy.
If you have interest in contributing or have ideas for future TNG topics, please contact me ([email protected]), or Ryan Farrell ([email protected]), managing editor of TNG.
Sincerely,
Vijaya L. Rao, MD
Editor in Chief
Dear colleagues,
The first issue of The New Gastroenterologist in 2020 consists of a particularly interesting array of articles and the introduction of a new medical ethics series!
This month’s “In Focus” article, brought to you by Jennifer Maratt (Indiana University) and Elena Stoffel (University of Michigan), provides a high yield overview of hereditary colorectal cancer and polyposis syndromes, with guidance on when a referral to a high risk cancer specialist and geneticist is warranted.
Daniel Mills (Cunningham, Meyer & Vedrine P.C.) gives us a valuable legal perspective of the role of electronic patient portals in the dissemination of information and medical advice to patients – such an important topic for everyone to be aware of as the nature of patient communication now strongly relies on electronic messaging.
R. Thomas Finn III (Palo Alto Medical Foundation) and David Leiman (Duke) nicely broach the issue of patient satisfaction. This is a timely topic as many institutions are not only publishing patient reviews online so that they are readily available to the public, but are also making financial incentives contingent on high patient ratings. The article discusses the evolution of the emphasis placed on patient satisfaction throughout the years with tips on how to navigate some of the distinct challenges within gastroenterology.
As part of our DHPA Private Practice Perspectives series, David Stokesberry (Digestive Disease Specialists Inc, Oklahoma City) discusses the nuts and bolts of ambulatory endoscopy centers and some of the challenges and benefits that accompany ownership of such centers.
An often overlooked aspect of gastroenterology training is nutrition. In our postfellowship pathways section, Dejan Micic (University of Chicago) outlines his decision to pursue a career in nutrition support, small bowel disorders, and the practice of deep enteroscopy.
Finally, this quarter’s newsletter features the start of a new section, which I am very excited to introduce – a case based series which will address issues in clinical medical ethics specific to gastroenterology. Lauren Feld (University of Washington) writes the inaugural piece for the section, providing a systematic approach to the patient with an existing do-not-resuscitate (DNR) order that is about to undergo endoscopy.
If you have interest in contributing or have ideas for future TNG topics, please contact me ([email protected]), or Ryan Farrell ([email protected]), managing editor of TNG.
Sincerely,
Vijaya L. Rao, MD
Editor in Chief
Dear colleagues,
The first issue of The New Gastroenterologist in 2020 consists of a particularly interesting array of articles and the introduction of a new medical ethics series!
This month’s “In Focus” article, brought to you by Jennifer Maratt (Indiana University) and Elena Stoffel (University of Michigan), provides a high yield overview of hereditary colorectal cancer and polyposis syndromes, with guidance on when a referral to a high risk cancer specialist and geneticist is warranted.
Daniel Mills (Cunningham, Meyer & Vedrine P.C.) gives us a valuable legal perspective of the role of electronic patient portals in the dissemination of information and medical advice to patients – such an important topic for everyone to be aware of as the nature of patient communication now strongly relies on electronic messaging.
R. Thomas Finn III (Palo Alto Medical Foundation) and David Leiman (Duke) nicely broach the issue of patient satisfaction. This is a timely topic as many institutions are not only publishing patient reviews online so that they are readily available to the public, but are also making financial incentives contingent on high patient ratings. The article discusses the evolution of the emphasis placed on patient satisfaction throughout the years with tips on how to navigate some of the distinct challenges within gastroenterology.
As part of our DHPA Private Practice Perspectives series, David Stokesberry (Digestive Disease Specialists Inc, Oklahoma City) discusses the nuts and bolts of ambulatory endoscopy centers and some of the challenges and benefits that accompany ownership of such centers.
An often overlooked aspect of gastroenterology training is nutrition. In our postfellowship pathways section, Dejan Micic (University of Chicago) outlines his decision to pursue a career in nutrition support, small bowel disorders, and the practice of deep enteroscopy.
Finally, this quarter’s newsletter features the start of a new section, which I am very excited to introduce – a case based series which will address issues in clinical medical ethics specific to gastroenterology. Lauren Feld (University of Washington) writes the inaugural piece for the section, providing a systematic approach to the patient with an existing do-not-resuscitate (DNR) order that is about to undergo endoscopy.
If you have interest in contributing or have ideas for future TNG topics, please contact me ([email protected]), or Ryan Farrell ([email protected]), managing editor of TNG.
Sincerely,
Vijaya L. Rao, MD
Editor in Chief
Colorectal polyps and cancer – when to refer to genetics
Introduction
Genetic predisposition to colorectal polyps and colorectal cancer (CRC) is more common than previously recognized. Approximately 5%-10% of all individuals diagnosed with CRC have a known genetic association. However, among those with early-onset CRC (diagnosed at age less than 50 years), recent studies show that up to 20% have an associated genetic mutation.1,2 In addition, the risk of CRC in patients with certain hereditary syndromes, such as familial adenomatous polyposis (FAP), approaches 80%-90% without timely management.3 This overall high risk of CRC and extracolonic malignancies in patients with a hereditary syndrome, along with the rising rates of early-onset CRC, underscores the importance of early diagnosis and management of a hereditary condition.
Despite increasing awareness of hereditary polyposis and nonpolyposis syndromes, referral rates for genetic counseling and testing remain low.4 As gastroenterologists we have several unique opportunities, in clinic and in endoscopy, to identify patients at risk for hereditary syndromes.
Risk stratification
Personal and family history
Reviewing personal medical history and family history in detail should be a routine part of our practice. This is often when initial signs of a potential hereditary syndrome can be detected. For example, if a patient reports a personal or family history of colorectal polyps or CRC, additional information that becomes important includes age at time of diagnosis, polyp burden (number and histologic subtype), presence of inflammatory bowel disease, and history of any extracolonic malignancies. Patients with multiple colorectal polyps (e.g. more than 10-20 adenomas or more than 2 hamartomas) and those with CRC diagnosed at a young age (younger than 50 years) should be considered candidates for genetic evaluation.5
Lynch syndrome (LS), an autosomal dominant condition caused by loss of DNA mismatch repair (MMR) genes, is the most common hereditary CRC syndrome, accounting for 2%-4% of all CRCs.3,6 Extracolonic LS-associated cancers to keep in mind while reviewing personal and family histories include those involving the gastrointestinal (GI) tract such as gastric, pancreatic, biliary tract, and small intestine cancers, and also non-GI tract cancers including endometrial, ovarian, urinary tract, and renal cancers along with brain tumors, and skin lesions including sebaceous adenomas, sebaceous carcinomas, and keratoacanthomas. Notably, after CRC, endometrial cancer is the second most common cancer among women with LS. Prior diagnosis of endometrial cancer should also prompt additional history-taking and evaluation for LS.
As the National Comprehensive Cancer Network (NCCN) highlights in its recent guidelines, several key findings in family history that should prompt referral to genetics for evaluation and testing for LS include: one or more first-degree relatives (FDR) with CRC or endometrial cancer diagnosed at less than 50 years of age, one or more FDR with CRC or endometrial cancer and another synchronous or metachronous LS-related cancer, two or more FDR or second-degree relatives (SDR) with LS-related cancer (including at least one diagnosed at age less than 50 years), and three or more FDR or SDR with LS-related cancers (regardless of age).5
Comprehensive assessment of family history should include all cancer diagnoses in first- and second-degree relatives, including age at diagnosis and cancer type, as well as ethnicity, as these inform the likelihood that the patient harbors a germline pathogenic variant associated with cancer predisposition.5 Given the difficulty of eliciting this level of detail, the family histories elicited in clinical settings are often limited or incomplete. Unknown family history should not be mistaken for unremarkable family history. Alternatively, if family history is unimpressive, this is not necessarily reassuring, as there can be variability in disease penetrance, including autosomal recessive syndromes that may skip generations, and de novo mutations do occur. In fact, among individuals with early-onset CRC diagnosed at age less than 50, only half of mutation carriers reported a family history of CRC in an FDR.2 Thus, individuals with concerning personal histories should undergo a genetic evaluation even if family history is not concerning.
Polyp phenotype
In addition to personal and family history, colon polyp history (including number, size, and histology) can provide important clues to identifying individuals with genetic predisposition to CRC. Table 1 highlights hereditary syndromes and polyp phenotypes associated with increased CRC risk. Based on consensus guidelines, individuals with a history of greater than 10-20 adenomas, 2 or more hamartomas, or 5 or more sessile serrated polyps should be referred for genetic testing.5,7 Serrated polyposis syndrome (SPS) is diagnosed based on at least one of the following criteria: 1) 5 or more serrated polyps, all at least 5 mm in size, proximal to the rectum including at least 2 that are 10 mm or larger in size, or 2) more than 20 serrated polyps distributed throughout the colon with at least 5 proximal to the rectum.8 Pathogenic germline variants in RNF43, a tumor suppressor gene, have been associated with SPS in rare families; however, in most cases genetic testing is uninformative and further genetic and environmental discovery studies are needed to determine the underlying cause.8,9

Risk prediction models
Models have been developed that integrate family history and phenotype data to help identify patients who may be at risk for LS. The Amsterdam criteria (more than 3 relatives with LS-associated cancers, more than 2 generations involving LS-associated cancers, and more than 1 cancer diagnosed before the age of 50; “3:2:1” criteria) were initially developed for research purposes to identify individuals who were likely to be carriers of mutations of LS based on CRC and later revised to include extracolonic malignancies (Amsterdam II).11 However, they have limited sensitivity for identifying high-risk patients. Similarly, the Bethesda guidelines have also been modified and revised to identify patients at risk for LS whose tumors should be tested with microsatellite instability (MSI), but also with limited sensitivity.12
Several risk prediction models have been developed that perform better than the Amsterdam criteria or Bethesda guidelines for determining which patients should be referred for genetic testing for LS. These include MMRPredict, MMRpro, and PREMM5.13-16 These models use clinical data (personal and family history of cancer and tumor phenotypes) to calculate the probability of a germline mutation in one of the mismatch repair (MMR) genes associated with LS. The current threshold at which to refer a patient for genetic counseling and testing is a predicted probability of 5% or greater using any one of these models, though some have proposed lowering the threshold to 2.5%.16,17
Universal tumor testing
Because of the limitations of relying on clinical family history, such as with the Amsterdam criteria and the Bethesda guidelines,18,19 as of 2014 the NCCN recommended universal tumor screening for DNA MMR deficiency associated with LS. This approach, also known as “universal testing,” has been shown to be cost effective and more sensitive in identifying at-risk patients than clinical criteria alone.20,21 Specifically, the NCCN recommends that tumor specimens of all patients diagnosed with CRC undergo testing for microsatellite instability (MSI) or loss of MMR proteins (MLH1, MSH2, MSH6, PMS2) expression by immunohistochemistry (IHC).5 Loss of MMR proteins or MSI-high findings should prompt a referral to genetics for counseling and consideration of testing for germline mutations. Universal testing of CRC and endometrial cancers is considered the most reliable way to screen patients for LS.

Universal testing by MSI or IHC may be performed on premalignant or malignant lesions. However, it is important to recognize that DNA MMR deficiency testing may not be as reliable when applied to colorectal polyps. Using data from three cancer registries (Dana-Farber Cancer Institute, University of Michigan, MD Anderson Cancer Center), Yurgelun and colleagues investigated the yield of MSI and IHC in colorectal polyps removed from patients with known LS.22 Overall, high-level MSI was found in only 41% of Lynch-associated adenomas and loss of MMR protein expression was evident in only 50%. While adenomas 8 mm in size or greater were more likely to have MSI-high or loss of MMR protein expression compared with those less than 8 mm in size, MMR-deficiency phenotype was less reliable in smaller adenomas. Consequently, results of MSI and/or IHC should therefore be interpreted with caution and in the context of the specimen upon which they are performed.
Considerations for clinical genetic testing
Genetic testing for cancer susceptibility should include informed consent and counseling for patients regarding potential risks and benefits. Clinicians ordering genetic testing should have the expertise necessary to interpret test results, which may be positive (pathogenic or likely pathogenic germline variant identified), or negative (no variants identified), or may yield one or more variants of uncertain clinical significance. Individuals found to carry a pathogenic or likely pathogenic germline variant associated with cancer susceptibility should be referred for additional genetic counseling and may require additional expert consultation for management of extracolonic cancer risks. It is important that individuals diagnosed with a hereditary cancer syndrome be informed that this diagnosis has implications for family members, who may also be at risk for the condition and may benefit from genetic testing.
Practical considerations
Given the difficulty in obtaining a detailed family history while in clinic or in endoscopy, several studies have investigated strategies that may be integrated into practice to identify high-risk patients without substantial burden on providers or patients. Kastrinos and colleagues identified the following three high-yield questions as part of a CRC Risk Assessment Tool that can be used while performing a precolonoscopy assessment: 1) Do you have a first-degree relative with CRC or LS-related cancer diagnosed before age 50?; 2) Have you had CRC or polyps diagnosed prior to age 50?; and 3) Do you have three or more relatives with CRC? The authors found that these three questions alone identified 77% of high-risk individuals.23 In addition, implementation of family history screening instruments using standardized surveys or self-administered risk prediction models at the time of colonoscopy have been shown to improve ascertainment of high-risk patients.24,25 Such strategies may become increasingly easier to implement with integration into patients’ electronic medical records.
Conclusions
Hereditary CRC syndromes are becoming increasingly important to identify, especially in an era where we are seeing rising rates of early-onset CRC. Early identification of high-risk features (Table 2) can lead to timely diagnosis with the goal to implement preventive strategies for screening and/or surveillance, ideally prior to development of cancers.
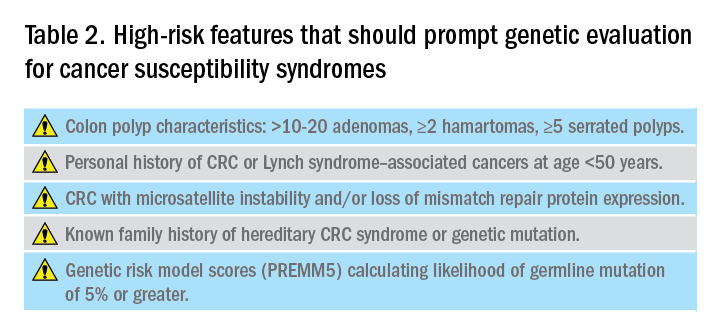
As gastroenterologists, we have several unique opportunities to identify these individuals and must maintain a high level of suspicion with careful attention when obtaining personal and family history details in clinic and in endoscopy.
Dr. Maratt is assistant professor, Indiana University, Richard L. Roudebush VA Medical Center, Indianapolis. Dr. Stoffel is assistant professor, University of Michigan; director of Cancer Genetic Clinic, Rogel Cancer Center, Ann Arbor. They have no conflicts of interest.
References
1. Pearlman R et al. JAMA Oncol. 2017;3(4):464-71.
2. Stoffel EM et al. Gastroenterology. 2018;154(4):897-905.
3. Kanth P et al. Am J Gastroenterol. 2017;112:1509-25.
4. Brennan B et al. Ther Adv Gastroenterol. 2017;10:361-71.
5. National Comprehensive Cancer Network. Available at: nccn.org.
6. Lynch HT et al. Nat Rev Cancer. 2015;15:181-94.
7. Syngal S et al. Am J Gastroenterol. 2015;110:223-62.
8. Mankaney G et al. Clin Gastroenterol Hepatol. 2020:(in press)
9. Yan HHN et al. Gut 2017;66:1645-56.
10. Ma H et al. Pathology. 2018;50:49-59.
11. Vasen H et al. Gastroenterology 1999;116:1453-6.
12. Umar A et al. J Natl Cancer Inst. 2004;96:261-8.
13. Kastrinos F et al. J Natl Cancer Inst. 2015;108(2):1-9.
14. Chen S et al. JAMA. 2006;296(12):1479-87.
15. Barnetson RA et al. N Engl J Med. 2006;354(26):2751-63.
16. Kastrinos F et al. J Clin Oncol. 2017;35:2165-72.
17. Kastrinos F et al. Fam Cancer. 2018;17:567-67.
18. Cohen SA et al. Annu Rev Genomics Hum Genet. 2019;20:293-307.
19. Matloff J et al. J Natl Compr Canc Netw. 2013;11:1380-5.
20. Ladabaum U et al. Ann Intern Med. 2011;155(2):69-79.
21. Hampel H et al. N Engl J Med. 2005;352(18):1851-60.
22. Yurgelun MB et al. Cancer Prev Res. 2012;5:574-82.
23. Kastrinos F et al. Am J Gastroenterol. 2009;104:1508-18.
24. Luba DG et al. Clin Gastroenterol Hepatol. 2018;16:49-58.
25. Guivatchian T et al. Gastrointest Endosc. 2017;86:684-91.
Introduction
Genetic predisposition to colorectal polyps and colorectal cancer (CRC) is more common than previously recognized. Approximately 5%-10% of all individuals diagnosed with CRC have a known genetic association. However, among those with early-onset CRC (diagnosed at age less than 50 years), recent studies show that up to 20% have an associated genetic mutation.1,2 In addition, the risk of CRC in patients with certain hereditary syndromes, such as familial adenomatous polyposis (FAP), approaches 80%-90% without timely management.3 This overall high risk of CRC and extracolonic malignancies in patients with a hereditary syndrome, along with the rising rates of early-onset CRC, underscores the importance of early diagnosis and management of a hereditary condition.
Despite increasing awareness of hereditary polyposis and nonpolyposis syndromes, referral rates for genetic counseling and testing remain low.4 As gastroenterologists we have several unique opportunities, in clinic and in endoscopy, to identify patients at risk for hereditary syndromes.
Risk stratification
Personal and family history
Reviewing personal medical history and family history in detail should be a routine part of our practice. This is often when initial signs of a potential hereditary syndrome can be detected. For example, if a patient reports a personal or family history of colorectal polyps or CRC, additional information that becomes important includes age at time of diagnosis, polyp burden (number and histologic subtype), presence of inflammatory bowel disease, and history of any extracolonic malignancies. Patients with multiple colorectal polyps (e.g. more than 10-20 adenomas or more than 2 hamartomas) and those with CRC diagnosed at a young age (younger than 50 years) should be considered candidates for genetic evaluation.5
Lynch syndrome (LS), an autosomal dominant condition caused by loss of DNA mismatch repair (MMR) genes, is the most common hereditary CRC syndrome, accounting for 2%-4% of all CRCs.3,6 Extracolonic LS-associated cancers to keep in mind while reviewing personal and family histories include those involving the gastrointestinal (GI) tract such as gastric, pancreatic, biliary tract, and small intestine cancers, and also non-GI tract cancers including endometrial, ovarian, urinary tract, and renal cancers along with brain tumors, and skin lesions including sebaceous adenomas, sebaceous carcinomas, and keratoacanthomas. Notably, after CRC, endometrial cancer is the second most common cancer among women with LS. Prior diagnosis of endometrial cancer should also prompt additional history-taking and evaluation for LS.
As the National Comprehensive Cancer Network (NCCN) highlights in its recent guidelines, several key findings in family history that should prompt referral to genetics for evaluation and testing for LS include: one or more first-degree relatives (FDR) with CRC or endometrial cancer diagnosed at less than 50 years of age, one or more FDR with CRC or endometrial cancer and another synchronous or metachronous LS-related cancer, two or more FDR or second-degree relatives (SDR) with LS-related cancer (including at least one diagnosed at age less than 50 years), and three or more FDR or SDR with LS-related cancers (regardless of age).5
Comprehensive assessment of family history should include all cancer diagnoses in first- and second-degree relatives, including age at diagnosis and cancer type, as well as ethnicity, as these inform the likelihood that the patient harbors a germline pathogenic variant associated with cancer predisposition.5 Given the difficulty of eliciting this level of detail, the family histories elicited in clinical settings are often limited or incomplete. Unknown family history should not be mistaken for unremarkable family history. Alternatively, if family history is unimpressive, this is not necessarily reassuring, as there can be variability in disease penetrance, including autosomal recessive syndromes that may skip generations, and de novo mutations do occur. In fact, among individuals with early-onset CRC diagnosed at age less than 50, only half of mutation carriers reported a family history of CRC in an FDR.2 Thus, individuals with concerning personal histories should undergo a genetic evaluation even if family history is not concerning.
Polyp phenotype
In addition to personal and family history, colon polyp history (including number, size, and histology) can provide important clues to identifying individuals with genetic predisposition to CRC. Table 1 highlights hereditary syndromes and polyp phenotypes associated with increased CRC risk. Based on consensus guidelines, individuals with a history of greater than 10-20 adenomas, 2 or more hamartomas, or 5 or more sessile serrated polyps should be referred for genetic testing.5,7 Serrated polyposis syndrome (SPS) is diagnosed based on at least one of the following criteria: 1) 5 or more serrated polyps, all at least 5 mm in size, proximal to the rectum including at least 2 that are 10 mm or larger in size, or 2) more than 20 serrated polyps distributed throughout the colon with at least 5 proximal to the rectum.8 Pathogenic germline variants in RNF43, a tumor suppressor gene, have been associated with SPS in rare families; however, in most cases genetic testing is uninformative and further genetic and environmental discovery studies are needed to determine the underlying cause.8,9

Risk prediction models
Models have been developed that integrate family history and phenotype data to help identify patients who may be at risk for LS. The Amsterdam criteria (more than 3 relatives with LS-associated cancers, more than 2 generations involving LS-associated cancers, and more than 1 cancer diagnosed before the age of 50; “3:2:1” criteria) were initially developed for research purposes to identify individuals who were likely to be carriers of mutations of LS based on CRC and later revised to include extracolonic malignancies (Amsterdam II).11 However, they have limited sensitivity for identifying high-risk patients. Similarly, the Bethesda guidelines have also been modified and revised to identify patients at risk for LS whose tumors should be tested with microsatellite instability (MSI), but also with limited sensitivity.12
Several risk prediction models have been developed that perform better than the Amsterdam criteria or Bethesda guidelines for determining which patients should be referred for genetic testing for LS. These include MMRPredict, MMRpro, and PREMM5.13-16 These models use clinical data (personal and family history of cancer and tumor phenotypes) to calculate the probability of a germline mutation in one of the mismatch repair (MMR) genes associated with LS. The current threshold at which to refer a patient for genetic counseling and testing is a predicted probability of 5% or greater using any one of these models, though some have proposed lowering the threshold to 2.5%.16,17
Universal tumor testing
Because of the limitations of relying on clinical family history, such as with the Amsterdam criteria and the Bethesda guidelines,18,19 as of 2014 the NCCN recommended universal tumor screening for DNA MMR deficiency associated with LS. This approach, also known as “universal testing,” has been shown to be cost effective and more sensitive in identifying at-risk patients than clinical criteria alone.20,21 Specifically, the NCCN recommends that tumor specimens of all patients diagnosed with CRC undergo testing for microsatellite instability (MSI) or loss of MMR proteins (MLH1, MSH2, MSH6, PMS2) expression by immunohistochemistry (IHC).5 Loss of MMR proteins or MSI-high findings should prompt a referral to genetics for counseling and consideration of testing for germline mutations. Universal testing of CRC and endometrial cancers is considered the most reliable way to screen patients for LS.

Universal testing by MSI or IHC may be performed on premalignant or malignant lesions. However, it is important to recognize that DNA MMR deficiency testing may not be as reliable when applied to colorectal polyps. Using data from three cancer registries (Dana-Farber Cancer Institute, University of Michigan, MD Anderson Cancer Center), Yurgelun and colleagues investigated the yield of MSI and IHC in colorectal polyps removed from patients with known LS.22 Overall, high-level MSI was found in only 41% of Lynch-associated adenomas and loss of MMR protein expression was evident in only 50%. While adenomas 8 mm in size or greater were more likely to have MSI-high or loss of MMR protein expression compared with those less than 8 mm in size, MMR-deficiency phenotype was less reliable in smaller adenomas. Consequently, results of MSI and/or IHC should therefore be interpreted with caution and in the context of the specimen upon which they are performed.
Considerations for clinical genetic testing
Genetic testing for cancer susceptibility should include informed consent and counseling for patients regarding potential risks and benefits. Clinicians ordering genetic testing should have the expertise necessary to interpret test results, which may be positive (pathogenic or likely pathogenic germline variant identified), or negative (no variants identified), or may yield one or more variants of uncertain clinical significance. Individuals found to carry a pathogenic or likely pathogenic germline variant associated with cancer susceptibility should be referred for additional genetic counseling and may require additional expert consultation for management of extracolonic cancer risks. It is important that individuals diagnosed with a hereditary cancer syndrome be informed that this diagnosis has implications for family members, who may also be at risk for the condition and may benefit from genetic testing.
Practical considerations
Given the difficulty in obtaining a detailed family history while in clinic or in endoscopy, several studies have investigated strategies that may be integrated into practice to identify high-risk patients without substantial burden on providers or patients. Kastrinos and colleagues identified the following three high-yield questions as part of a CRC Risk Assessment Tool that can be used while performing a precolonoscopy assessment: 1) Do you have a first-degree relative with CRC or LS-related cancer diagnosed before age 50?; 2) Have you had CRC or polyps diagnosed prior to age 50?; and 3) Do you have three or more relatives with CRC? The authors found that these three questions alone identified 77% of high-risk individuals.23 In addition, implementation of family history screening instruments using standardized surveys or self-administered risk prediction models at the time of colonoscopy have been shown to improve ascertainment of high-risk patients.24,25 Such strategies may become increasingly easier to implement with integration into patients’ electronic medical records.
Conclusions
Hereditary CRC syndromes are becoming increasingly important to identify, especially in an era where we are seeing rising rates of early-onset CRC. Early identification of high-risk features (Table 2) can lead to timely diagnosis with the goal to implement preventive strategies for screening and/or surveillance, ideally prior to development of cancers.

As gastroenterologists, we have several unique opportunities to identify these individuals and must maintain a high level of suspicion with careful attention when obtaining personal and family history details in clinic and in endoscopy.
Dr. Maratt is assistant professor, Indiana University, Richard L. Roudebush VA Medical Center, Indianapolis. Dr. Stoffel is assistant professor, University of Michigan; director of Cancer Genetic Clinic, Rogel Cancer Center, Ann Arbor. They have no conflicts of interest.
References
1. Pearlman R et al. JAMA Oncol. 2017;3(4):464-71.
2. Stoffel EM et al. Gastroenterology. 2018;154(4):897-905.
3. Kanth P et al. Am J Gastroenterol. 2017;112:1509-25.
4. Brennan B et al. Ther Adv Gastroenterol. 2017;10:361-71.
5. National Comprehensive Cancer Network. Available at: nccn.org.
6. Lynch HT et al. Nat Rev Cancer. 2015;15:181-94.
7. Syngal S et al. Am J Gastroenterol. 2015;110:223-62.
8. Mankaney G et al. Clin Gastroenterol Hepatol. 2020:(in press)
9. Yan HHN et al. Gut 2017;66:1645-56.
10. Ma H et al. Pathology. 2018;50:49-59.
11. Vasen H et al. Gastroenterology 1999;116:1453-6.
12. Umar A et al. J Natl Cancer Inst. 2004;96:261-8.
13. Kastrinos F et al. J Natl Cancer Inst. 2015;108(2):1-9.
14. Chen S et al. JAMA. 2006;296(12):1479-87.
15. Barnetson RA et al. N Engl J Med. 2006;354(26):2751-63.
16. Kastrinos F et al. J Clin Oncol. 2017;35:2165-72.
17. Kastrinos F et al. Fam Cancer. 2018;17:567-67.
18. Cohen SA et al. Annu Rev Genomics Hum Genet. 2019;20:293-307.
19. Matloff J et al. J Natl Compr Canc Netw. 2013;11:1380-5.
20. Ladabaum U et al. Ann Intern Med. 2011;155(2):69-79.
21. Hampel H et al. N Engl J Med. 2005;352(18):1851-60.
22. Yurgelun MB et al. Cancer Prev Res. 2012;5:574-82.
23. Kastrinos F et al. Am J Gastroenterol. 2009;104:1508-18.
24. Luba DG et al. Clin Gastroenterol Hepatol. 2018;16:49-58.
25. Guivatchian T et al. Gastrointest Endosc. 2017;86:684-91.
Introduction
Genetic predisposition to colorectal polyps and colorectal cancer (CRC) is more common than previously recognized. Approximately 5%-10% of all individuals diagnosed with CRC have a known genetic association. However, among those with early-onset CRC (diagnosed at age less than 50 years), recent studies show that up to 20% have an associated genetic mutation.1,2 In addition, the risk of CRC in patients with certain hereditary syndromes, such as familial adenomatous polyposis (FAP), approaches 80%-90% without timely management.3 This overall high risk of CRC and extracolonic malignancies in patients with a hereditary syndrome, along with the rising rates of early-onset CRC, underscores the importance of early diagnosis and management of a hereditary condition.
Despite increasing awareness of hereditary polyposis and nonpolyposis syndromes, referral rates for genetic counseling and testing remain low.4 As gastroenterologists we have several unique opportunities, in clinic and in endoscopy, to identify patients at risk for hereditary syndromes.
Risk stratification
Personal and family history
Reviewing personal medical history and family history in detail should be a routine part of our practice. This is often when initial signs of a potential hereditary syndrome can be detected. For example, if a patient reports a personal or family history of colorectal polyps or CRC, additional information that becomes important includes age at time of diagnosis, polyp burden (number and histologic subtype), presence of inflammatory bowel disease, and history of any extracolonic malignancies. Patients with multiple colorectal polyps (e.g. more than 10-20 adenomas or more than 2 hamartomas) and those with CRC diagnosed at a young age (younger than 50 years) should be considered candidates for genetic evaluation.5
Lynch syndrome (LS), an autosomal dominant condition caused by loss of DNA mismatch repair (MMR) genes, is the most common hereditary CRC syndrome, accounting for 2%-4% of all CRCs.3,6 Extracolonic LS-associated cancers to keep in mind while reviewing personal and family histories include those involving the gastrointestinal (GI) tract such as gastric, pancreatic, biliary tract, and small intestine cancers, and also non-GI tract cancers including endometrial, ovarian, urinary tract, and renal cancers along with brain tumors, and skin lesions including sebaceous adenomas, sebaceous carcinomas, and keratoacanthomas. Notably, after CRC, endometrial cancer is the second most common cancer among women with LS. Prior diagnosis of endometrial cancer should also prompt additional history-taking and evaluation for LS.
As the National Comprehensive Cancer Network (NCCN) highlights in its recent guidelines, several key findings in family history that should prompt referral to genetics for evaluation and testing for LS include: one or more first-degree relatives (FDR) with CRC or endometrial cancer diagnosed at less than 50 years of age, one or more FDR with CRC or endometrial cancer and another synchronous or metachronous LS-related cancer, two or more FDR or second-degree relatives (SDR) with LS-related cancer (including at least one diagnosed at age less than 50 years), and three or more FDR or SDR with LS-related cancers (regardless of age).5
Comprehensive assessment of family history should include all cancer diagnoses in first- and second-degree relatives, including age at diagnosis and cancer type, as well as ethnicity, as these inform the likelihood that the patient harbors a germline pathogenic variant associated with cancer predisposition.5 Given the difficulty of eliciting this level of detail, the family histories elicited in clinical settings are often limited or incomplete. Unknown family history should not be mistaken for unremarkable family history. Alternatively, if family history is unimpressive, this is not necessarily reassuring, as there can be variability in disease penetrance, including autosomal recessive syndromes that may skip generations, and de novo mutations do occur. In fact, among individuals with early-onset CRC diagnosed at age less than 50, only half of mutation carriers reported a family history of CRC in an FDR.2 Thus, individuals with concerning personal histories should undergo a genetic evaluation even if family history is not concerning.
Polyp phenotype
In addition to personal and family history, colon polyp history (including number, size, and histology) can provide important clues to identifying individuals with genetic predisposition to CRC. Table 1 highlights hereditary syndromes and polyp phenotypes associated with increased CRC risk. Based on consensus guidelines, individuals with a history of greater than 10-20 adenomas, 2 or more hamartomas, or 5 or more sessile serrated polyps should be referred for genetic testing.5,7 Serrated polyposis syndrome (SPS) is diagnosed based on at least one of the following criteria: 1) 5 or more serrated polyps, all at least 5 mm in size, proximal to the rectum including at least 2 that are 10 mm or larger in size, or 2) more than 20 serrated polyps distributed throughout the colon with at least 5 proximal to the rectum.8 Pathogenic germline variants in RNF43, a tumor suppressor gene, have been associated with SPS in rare families; however, in most cases genetic testing is uninformative and further genetic and environmental discovery studies are needed to determine the underlying cause.8,9

Risk prediction models
Models have been developed that integrate family history and phenotype data to help identify patients who may be at risk for LS. The Amsterdam criteria (more than 3 relatives with LS-associated cancers, more than 2 generations involving LS-associated cancers, and more than 1 cancer diagnosed before the age of 50; “3:2:1” criteria) were initially developed for research purposes to identify individuals who were likely to be carriers of mutations of LS based on CRC and later revised to include extracolonic malignancies (Amsterdam II).11 However, they have limited sensitivity for identifying high-risk patients. Similarly, the Bethesda guidelines have also been modified and revised to identify patients at risk for LS whose tumors should be tested with microsatellite instability (MSI), but also with limited sensitivity.12
Several risk prediction models have been developed that perform better than the Amsterdam criteria or Bethesda guidelines for determining which patients should be referred for genetic testing for LS. These include MMRPredict, MMRpro, and PREMM5.13-16 These models use clinical data (personal and family history of cancer and tumor phenotypes) to calculate the probability of a germline mutation in one of the mismatch repair (MMR) genes associated with LS. The current threshold at which to refer a patient for genetic counseling and testing is a predicted probability of 5% or greater using any one of these models, though some have proposed lowering the threshold to 2.5%.16,17
Universal tumor testing
Because of the limitations of relying on clinical family history, such as with the Amsterdam criteria and the Bethesda guidelines,18,19 as of 2014 the NCCN recommended universal tumor screening for DNA MMR deficiency associated with LS. This approach, also known as “universal testing,” has been shown to be cost effective and more sensitive in identifying at-risk patients than clinical criteria alone.20,21 Specifically, the NCCN recommends that tumor specimens of all patients diagnosed with CRC undergo testing for microsatellite instability (MSI) or loss of MMR proteins (MLH1, MSH2, MSH6, PMS2) expression by immunohistochemistry (IHC).5 Loss of MMR proteins or MSI-high findings should prompt a referral to genetics for counseling and consideration of testing for germline mutations. Universal testing of CRC and endometrial cancers is considered the most reliable way to screen patients for LS.

Universal testing by MSI or IHC may be performed on premalignant or malignant lesions. However, it is important to recognize that DNA MMR deficiency testing may not be as reliable when applied to colorectal polyps. Using data from three cancer registries (Dana-Farber Cancer Institute, University of Michigan, MD Anderson Cancer Center), Yurgelun and colleagues investigated the yield of MSI and IHC in colorectal polyps removed from patients with known LS.22 Overall, high-level MSI was found in only 41% of Lynch-associated adenomas and loss of MMR protein expression was evident in only 50%. While adenomas 8 mm in size or greater were more likely to have MSI-high or loss of MMR protein expression compared with those less than 8 mm in size, MMR-deficiency phenotype was less reliable in smaller adenomas. Consequently, results of MSI and/or IHC should therefore be interpreted with caution and in the context of the specimen upon which they are performed.
Considerations for clinical genetic testing
Genetic testing for cancer susceptibility should include informed consent and counseling for patients regarding potential risks and benefits. Clinicians ordering genetic testing should have the expertise necessary to interpret test results, which may be positive (pathogenic or likely pathogenic germline variant identified), or negative (no variants identified), or may yield one or more variants of uncertain clinical significance. Individuals found to carry a pathogenic or likely pathogenic germline variant associated with cancer susceptibility should be referred for additional genetic counseling and may require additional expert consultation for management of extracolonic cancer risks. It is important that individuals diagnosed with a hereditary cancer syndrome be informed that this diagnosis has implications for family members, who may also be at risk for the condition and may benefit from genetic testing.
Practical considerations
Given the difficulty in obtaining a detailed family history while in clinic or in endoscopy, several studies have investigated strategies that may be integrated into practice to identify high-risk patients without substantial burden on providers or patients. Kastrinos and colleagues identified the following three high-yield questions as part of a CRC Risk Assessment Tool that can be used while performing a precolonoscopy assessment: 1) Do you have a first-degree relative with CRC or LS-related cancer diagnosed before age 50?; 2) Have you had CRC or polyps diagnosed prior to age 50?; and 3) Do you have three or more relatives with CRC? The authors found that these three questions alone identified 77% of high-risk individuals.23 In addition, implementation of family history screening instruments using standardized surveys or self-administered risk prediction models at the time of colonoscopy have been shown to improve ascertainment of high-risk patients.24,25 Such strategies may become increasingly easier to implement with integration into patients’ electronic medical records.
Conclusions
Hereditary CRC syndromes are becoming increasingly important to identify, especially in an era where we are seeing rising rates of early-onset CRC. Early identification of high-risk features (Table 2) can lead to timely diagnosis with the goal to implement preventive strategies for screening and/or surveillance, ideally prior to development of cancers.

As gastroenterologists, we have several unique opportunities to identify these individuals and must maintain a high level of suspicion with careful attention when obtaining personal and family history details in clinic and in endoscopy.
Dr. Maratt is assistant professor, Indiana University, Richard L. Roudebush VA Medical Center, Indianapolis. Dr. Stoffel is assistant professor, University of Michigan; director of Cancer Genetic Clinic, Rogel Cancer Center, Ann Arbor. They have no conflicts of interest.
References
1. Pearlman R et al. JAMA Oncol. 2017;3(4):464-71.
2. Stoffel EM et al. Gastroenterology. 2018;154(4):897-905.
3. Kanth P et al. Am J Gastroenterol. 2017;112:1509-25.
4. Brennan B et al. Ther Adv Gastroenterol. 2017;10:361-71.
5. National Comprehensive Cancer Network. Available at: nccn.org.
6. Lynch HT et al. Nat Rev Cancer. 2015;15:181-94.
7. Syngal S et al. Am J Gastroenterol. 2015;110:223-62.
8. Mankaney G et al. Clin Gastroenterol Hepatol. 2020:(in press)
9. Yan HHN et al. Gut 2017;66:1645-56.
10. Ma H et al. Pathology. 2018;50:49-59.
11. Vasen H et al. Gastroenterology 1999;116:1453-6.
12. Umar A et al. J Natl Cancer Inst. 2004;96:261-8.
13. Kastrinos F et al. J Natl Cancer Inst. 2015;108(2):1-9.
14. Chen S et al. JAMA. 2006;296(12):1479-87.
15. Barnetson RA et al. N Engl J Med. 2006;354(26):2751-63.
16. Kastrinos F et al. J Clin Oncol. 2017;35:2165-72.
17. Kastrinos F et al. Fam Cancer. 2018;17:567-67.
18. Cohen SA et al. Annu Rev Genomics Hum Genet. 2019;20:293-307.
19. Matloff J et al. J Natl Compr Canc Netw. 2013;11:1380-5.
20. Ladabaum U et al. Ann Intern Med. 2011;155(2):69-79.
21. Hampel H et al. N Engl J Med. 2005;352(18):1851-60.
22. Yurgelun MB et al. Cancer Prev Res. 2012;5:574-82.
23. Kastrinos F et al. Am J Gastroenterol. 2009;104:1508-18.
24. Luba DG et al. Clin Gastroenterol Hepatol. 2018;16:49-58.
25. Guivatchian T et al. Gastrointest Endosc. 2017;86:684-91.
























