User login
Lessons learned as a gastroenterologist on social media
I have always been a strong believer in meeting patients where they obtain their health information. Early in my clinical training, I realized that patients are exposed to health information through traditional media formats and, increasingly, social media, rather than brief clinical encounters. Unlike traditional media, social media allows individuals the opportunity to post information without a third-party filter. However, this opens the door for untrained individuals to spread misinformation and disinformation. In health care, this could potentially disrupt public health efforts. Even innocent mistakes like overlooking the appropriate clinical context can cause issues. Traditional media outlets also have agendas that may leave certain conditions, therapies, and other facets of health care underrepresented. My belief is that experts should therefore be trained and incentivized to be spokespeople for their own areas of expertise. Furthermore, social media provides a novel opportunity to improve health literacy while humanizing and restoring fading trust in health care.
There are several items to consider before initiating on one’s social media journey: whether you are committed to exploring the space, what one’s purpose is on social media, who the intended target audience is, which platform is most appropriate to serve that purpose and audience, and what potential pitfalls there may be.
The first question to ask oneself is whether you are prepared to devote time to cultivating a social media presence and speak or be heard publicly. Regardless of the platform, a social media presence requires consistency and audience interaction. The decision to partake can be personal; I view social media as an extension of in-person interaction, but not everyone is willing to commit to increased accessibility and visibility. Social media can still be valuable to those who choose to observe and learn rather than post.
Next is what one’s purpose is with being on social media. This can vary from peer education, boosting health literacy for patients, or using social media as a news source, networking tool, or a creative outlet. While my social media activity supports all these, my primary purpose is the distribution of accurate health information as a trained expert. When I started, I was one of few academic gastroenterologists uniquely positioned to bridge the elusive gap between the young, Gen Z crowd and academic medicine. Of similar importance is defining one’s target audience: patients, trainees, colleagues, or the general public.
Because there are numerous social media platforms, and only more to come in the future, it is critical to focus only on platforms that will serve one’s purpose and audience. Additionally, some may find more joy or agility in using one platform over the other. While I am one of the few clinicians who are adept at building communities across multiple rapidly evolving social media platforms, I will be the first to admit that it takes time to fully understand each platform with its ever-growing array of features. I find myself better at some platforms over others and, depending on my goals, I often will shift my focus from one to another.
Each platform has its pros and cons. Twitter is perhaps the most appropriate platform for starters. Easy to use with the least preparation necessary for every post, it also serves as the primary platform for academic discussion among all the popular social media platforms. Over the past few years, hundreds of gastroenterologists have become active on Twitter, which allows for ample networking opportunities and potential collaborations. The space has evolved to house various structured chats and learning opportunities as described by accounts like @MondayNightIBD, @ScopingSundays, #TracingTuesday, and @GIJournal. All major GI journals and societies are also present on Twitter and disseminating the latest information. Now a vestige of the past when text within tweets was not searchable, hashtags were used to curate discussion because searching by hashtag could reveal the latest discussion surrounding a topic and help identify others with a similar interest. Hashtags now remain relevant when crafting tweets, as the strategic inclusion of hashtags can help your content reach those who share an interest. A hashtag ontology was previously published to standardize academic conversation online in gastroenterology. Twitter also boasts features like polls that also help audiences engage.
Twitter has its disadvantages, however. Conversation is often siloed and difficult to reach audiences who don’t already follow you or others associated with you. Tweets disappear quickly in one’s feed and are often not seen by your followers. It lacks the visual appeal of other image- and video-based platforms that tend to attract more members of the general public. (Twitter lags behind these other platforms in monthly users) Other platforms like Facebook, Instagram, YouTube, LinkedIn, and TikTok have other benefits. Facebook may help foster community discussions in groups and business pages are also helpful for practice promotion. Instagram has gained popularity for educational purposes over the past 2 years, given its pairing with imagery and room for a lengthier caption. It has a variety of additional features like the temporary Instagram Stories that last 24 hours (which also allows for polling), question and answer, and livestream options. Other platforms like YouTube and TikTok have greater potential to reach audiences who otherwise would not see your content, with the former having the benefit of being highly searchable and the latter being the social media app with fastest growing popularity.
Having grown up with the Internet-based instant messaging and social media platforms, I have always enjoyed the medium as a way to connect with others. However, productive engagement on these platforms came much later. During a brief stint as part of the ABC News medical unit, I learned how Twitter was used to facilitate weekly chats around a specific topic online. I began exploring my own social media voice, which quickly gave way to live-tweeting medical conferences, hosting and participating Twitter chats myself, and guiding colleagues and professional societies to greater adoption of social media. In an attempt to introduce a divisional social media account during my fellowship, I learned of institutional barriers including antiquated policies that actively dissuaded social media use. I became increasingly involved on committees in our main GI societies after engaging in multiple research projects using social media data looking at how GI journals promote their content online, the associations between social media presence and institutional ranking, social media behavior at medical conferences, and the evolving perspectives of training program leadership regarding social media.
The pitfalls of social media remain a major concern for physicians and employers alike. First and foremost, it is important to review one’s institutional social media policy prior to starting, as individuals are ultimately held to their local policies. Not only can social media activity be a major liability for a health care employer, but also in the general public’s trust in health professionals. Protecting patient privacy and safety are of utmost concern, and physicians must be mindful not to inadvertently reveal patient identity. HIPAA violations are not limited to only naming patients by name or photo; descriptions of procedural cases and posting patient-related images such as radiographs or endoscopic images may reveal patient identity if there are unique details on these images (e.g., a radio-opaque necklace on x-ray or a particular swallowed foreign body).
Another disadvantage of social media is being approached with personal medical questions. I universally decline to answer these inquiries, citing the need to perform a comprehensive review of one’s medical chart and perform an in-person physical exam to fully assess a patient. The distinction between education and advice is subtle, yet important to recognize. Similarly, the need to uphold professionalism online is important. Short messages on social media can be misinterpreted by colleagues and the public. Not only can these interactions be potentially detrimental to one’s career, but it can further erode trust in health care if patients perceive this as fragmentation of the health care system. On platforms that encourage humor and creativity like TikTok, there have also been medical professionals and students publicly criticized and penalized for posting unprofessional content mocking patients.
With the introduction of social media influencers in recent years, some professionals have amassed followings, introducing yet another set of concerns. One is being approached with sponsorship and endorsement offers, as any agreements must be in accordance with institutional policy. As one’s following grows, there may be other concerns of safety both online and in real life. Online concerns include issues with impersonation and use of photos or written content without permission. On the surface this may not seem like a significant concern, but there have been situations where family photos are distributed to intended audiences or one’s likeness is used to endorse a product.
In addition to physical safety, another unintended consequence of social media use is its impact on one’s mental health. As social media tends to be a highlight reel, it is easy to be consumed by comparison with colleagues and their lives on social media, whether it truly reflects one’s actual life or not.
My ability to understand multiple social media platforms and anticipate a growing set of risks and concerns with using social media is what led to my involvement with multiple GI societies and appointment by my institution’s CEO to serve as the first chief medical social media officer. My desire to help other professionals with the journey also led to the formation of the Association for Healthcare Social Media, the first 501(c)(3) nonprofit professional organization devoted to health professionals on social media. There is tremendous opportunity to impact public health through social media, especially with regards to raising awareness about underrepresented conditions and presenting information that is accurate. Many barriers remain to the widespread adoption of social media by health professionals, such as the lack of financial or academic incentives. For now, there is every indication that social media is here to stay, and it will likely continue to play an important role in how we communicate with our patients.
AGA can be found online at @AmerGastroAssn (Facebook, Instagram, and Twitter) and @AGA_Gastro, @AGA_CGH, and @AGA_CMGH (Facebook and Twitter).
Dr. Chiang is assistant professor of medicine, division of gastroenterology & hepatology, director, endoscopic bariatric program, chief medical social media officer, Jefferson Health, Philadelphia, and president, Association for Healthcare Social Media, @austinchiangmd
I have always been a strong believer in meeting patients where they obtain their health information. Early in my clinical training, I realized that patients are exposed to health information through traditional media formats and, increasingly, social media, rather than brief clinical encounters. Unlike traditional media, social media allows individuals the opportunity to post information without a third-party filter. However, this opens the door for untrained individuals to spread misinformation and disinformation. In health care, this could potentially disrupt public health efforts. Even innocent mistakes like overlooking the appropriate clinical context can cause issues. Traditional media outlets also have agendas that may leave certain conditions, therapies, and other facets of health care underrepresented. My belief is that experts should therefore be trained and incentivized to be spokespeople for their own areas of expertise. Furthermore, social media provides a novel opportunity to improve health literacy while humanizing and restoring fading trust in health care.
There are several items to consider before initiating on one’s social media journey: whether you are committed to exploring the space, what one’s purpose is on social media, who the intended target audience is, which platform is most appropriate to serve that purpose and audience, and what potential pitfalls there may be.
The first question to ask oneself is whether you are prepared to devote time to cultivating a social media presence and speak or be heard publicly. Regardless of the platform, a social media presence requires consistency and audience interaction. The decision to partake can be personal; I view social media as an extension of in-person interaction, but not everyone is willing to commit to increased accessibility and visibility. Social media can still be valuable to those who choose to observe and learn rather than post.
Next is what one’s purpose is with being on social media. This can vary from peer education, boosting health literacy for patients, or using social media as a news source, networking tool, or a creative outlet. While my social media activity supports all these, my primary purpose is the distribution of accurate health information as a trained expert. When I started, I was one of few academic gastroenterologists uniquely positioned to bridge the elusive gap between the young, Gen Z crowd and academic medicine. Of similar importance is defining one’s target audience: patients, trainees, colleagues, or the general public.
Because there are numerous social media platforms, and only more to come in the future, it is critical to focus only on platforms that will serve one’s purpose and audience. Additionally, some may find more joy or agility in using one platform over the other. While I am one of the few clinicians who are adept at building communities across multiple rapidly evolving social media platforms, I will be the first to admit that it takes time to fully understand each platform with its ever-growing array of features. I find myself better at some platforms over others and, depending on my goals, I often will shift my focus from one to another.
Each platform has its pros and cons. Twitter is perhaps the most appropriate platform for starters. Easy to use with the least preparation necessary for every post, it also serves as the primary platform for academic discussion among all the popular social media platforms. Over the past few years, hundreds of gastroenterologists have become active on Twitter, which allows for ample networking opportunities and potential collaborations. The space has evolved to house various structured chats and learning opportunities as described by accounts like @MondayNightIBD, @ScopingSundays, #TracingTuesday, and @GIJournal. All major GI journals and societies are also present on Twitter and disseminating the latest information. Now a vestige of the past when text within tweets was not searchable, hashtags were used to curate discussion because searching by hashtag could reveal the latest discussion surrounding a topic and help identify others with a similar interest. Hashtags now remain relevant when crafting tweets, as the strategic inclusion of hashtags can help your content reach those who share an interest. A hashtag ontology was previously published to standardize academic conversation online in gastroenterology. Twitter also boasts features like polls that also help audiences engage.
Twitter has its disadvantages, however. Conversation is often siloed and difficult to reach audiences who don’t already follow you or others associated with you. Tweets disappear quickly in one’s feed and are often not seen by your followers. It lacks the visual appeal of other image- and video-based platforms that tend to attract more members of the general public. (Twitter lags behind these other platforms in monthly users) Other platforms like Facebook, Instagram, YouTube, LinkedIn, and TikTok have other benefits. Facebook may help foster community discussions in groups and business pages are also helpful for practice promotion. Instagram has gained popularity for educational purposes over the past 2 years, given its pairing with imagery and room for a lengthier caption. It has a variety of additional features like the temporary Instagram Stories that last 24 hours (which also allows for polling), question and answer, and livestream options. Other platforms like YouTube and TikTok have greater potential to reach audiences who otherwise would not see your content, with the former having the benefit of being highly searchable and the latter being the social media app with fastest growing popularity.
Having grown up with the Internet-based instant messaging and social media platforms, I have always enjoyed the medium as a way to connect with others. However, productive engagement on these platforms came much later. During a brief stint as part of the ABC News medical unit, I learned how Twitter was used to facilitate weekly chats around a specific topic online. I began exploring my own social media voice, which quickly gave way to live-tweeting medical conferences, hosting and participating Twitter chats myself, and guiding colleagues and professional societies to greater adoption of social media. In an attempt to introduce a divisional social media account during my fellowship, I learned of institutional barriers including antiquated policies that actively dissuaded social media use. I became increasingly involved on committees in our main GI societies after engaging in multiple research projects using social media data looking at how GI journals promote their content online, the associations between social media presence and institutional ranking, social media behavior at medical conferences, and the evolving perspectives of training program leadership regarding social media.
The pitfalls of social media remain a major concern for physicians and employers alike. First and foremost, it is important to review one’s institutional social media policy prior to starting, as individuals are ultimately held to their local policies. Not only can social media activity be a major liability for a health care employer, but also in the general public’s trust in health professionals. Protecting patient privacy and safety are of utmost concern, and physicians must be mindful not to inadvertently reveal patient identity. HIPAA violations are not limited to only naming patients by name or photo; descriptions of procedural cases and posting patient-related images such as radiographs or endoscopic images may reveal patient identity if there are unique details on these images (e.g., a radio-opaque necklace on x-ray or a particular swallowed foreign body).
Another disadvantage of social media is being approached with personal medical questions. I universally decline to answer these inquiries, citing the need to perform a comprehensive review of one’s medical chart and perform an in-person physical exam to fully assess a patient. The distinction between education and advice is subtle, yet important to recognize. Similarly, the need to uphold professionalism online is important. Short messages on social media can be misinterpreted by colleagues and the public. Not only can these interactions be potentially detrimental to one’s career, but it can further erode trust in health care if patients perceive this as fragmentation of the health care system. On platforms that encourage humor and creativity like TikTok, there have also been medical professionals and students publicly criticized and penalized for posting unprofessional content mocking patients.
With the introduction of social media influencers in recent years, some professionals have amassed followings, introducing yet another set of concerns. One is being approached with sponsorship and endorsement offers, as any agreements must be in accordance with institutional policy. As one’s following grows, there may be other concerns of safety both online and in real life. Online concerns include issues with impersonation and use of photos or written content without permission. On the surface this may not seem like a significant concern, but there have been situations where family photos are distributed to intended audiences or one’s likeness is used to endorse a product.
In addition to physical safety, another unintended consequence of social media use is its impact on one’s mental health. As social media tends to be a highlight reel, it is easy to be consumed by comparison with colleagues and their lives on social media, whether it truly reflects one’s actual life or not.
My ability to understand multiple social media platforms and anticipate a growing set of risks and concerns with using social media is what led to my involvement with multiple GI societies and appointment by my institution’s CEO to serve as the first chief medical social media officer. My desire to help other professionals with the journey also led to the formation of the Association for Healthcare Social Media, the first 501(c)(3) nonprofit professional organization devoted to health professionals on social media. There is tremendous opportunity to impact public health through social media, especially with regards to raising awareness about underrepresented conditions and presenting information that is accurate. Many barriers remain to the widespread adoption of social media by health professionals, such as the lack of financial or academic incentives. For now, there is every indication that social media is here to stay, and it will likely continue to play an important role in how we communicate with our patients.
AGA can be found online at @AmerGastroAssn (Facebook, Instagram, and Twitter) and @AGA_Gastro, @AGA_CGH, and @AGA_CMGH (Facebook and Twitter).
Dr. Chiang is assistant professor of medicine, division of gastroenterology & hepatology, director, endoscopic bariatric program, chief medical social media officer, Jefferson Health, Philadelphia, and president, Association for Healthcare Social Media, @austinchiangmd
I have always been a strong believer in meeting patients where they obtain their health information. Early in my clinical training, I realized that patients are exposed to health information through traditional media formats and, increasingly, social media, rather than brief clinical encounters. Unlike traditional media, social media allows individuals the opportunity to post information without a third-party filter. However, this opens the door for untrained individuals to spread misinformation and disinformation. In health care, this could potentially disrupt public health efforts. Even innocent mistakes like overlooking the appropriate clinical context can cause issues. Traditional media outlets also have agendas that may leave certain conditions, therapies, and other facets of health care underrepresented. My belief is that experts should therefore be trained and incentivized to be spokespeople for their own areas of expertise. Furthermore, social media provides a novel opportunity to improve health literacy while humanizing and restoring fading trust in health care.
There are several items to consider before initiating on one’s social media journey: whether you are committed to exploring the space, what one’s purpose is on social media, who the intended target audience is, which platform is most appropriate to serve that purpose and audience, and what potential pitfalls there may be.
The first question to ask oneself is whether you are prepared to devote time to cultivating a social media presence and speak or be heard publicly. Regardless of the platform, a social media presence requires consistency and audience interaction. The decision to partake can be personal; I view social media as an extension of in-person interaction, but not everyone is willing to commit to increased accessibility and visibility. Social media can still be valuable to those who choose to observe and learn rather than post.
Next is what one’s purpose is with being on social media. This can vary from peer education, boosting health literacy for patients, or using social media as a news source, networking tool, or a creative outlet. While my social media activity supports all these, my primary purpose is the distribution of accurate health information as a trained expert. When I started, I was one of few academic gastroenterologists uniquely positioned to bridge the elusive gap between the young, Gen Z crowd and academic medicine. Of similar importance is defining one’s target audience: patients, trainees, colleagues, or the general public.
Because there are numerous social media platforms, and only more to come in the future, it is critical to focus only on platforms that will serve one’s purpose and audience. Additionally, some may find more joy or agility in using one platform over the other. While I am one of the few clinicians who are adept at building communities across multiple rapidly evolving social media platforms, I will be the first to admit that it takes time to fully understand each platform with its ever-growing array of features. I find myself better at some platforms over others and, depending on my goals, I often will shift my focus from one to another.
Each platform has its pros and cons. Twitter is perhaps the most appropriate platform for starters. Easy to use with the least preparation necessary for every post, it also serves as the primary platform for academic discussion among all the popular social media platforms. Over the past few years, hundreds of gastroenterologists have become active on Twitter, which allows for ample networking opportunities and potential collaborations. The space has evolved to house various structured chats and learning opportunities as described by accounts like @MondayNightIBD, @ScopingSundays, #TracingTuesday, and @GIJournal. All major GI journals and societies are also present on Twitter and disseminating the latest information. Now a vestige of the past when text within tweets was not searchable, hashtags were used to curate discussion because searching by hashtag could reveal the latest discussion surrounding a topic and help identify others with a similar interest. Hashtags now remain relevant when crafting tweets, as the strategic inclusion of hashtags can help your content reach those who share an interest. A hashtag ontology was previously published to standardize academic conversation online in gastroenterology. Twitter also boasts features like polls that also help audiences engage.
Twitter has its disadvantages, however. Conversation is often siloed and difficult to reach audiences who don’t already follow you or others associated with you. Tweets disappear quickly in one’s feed and are often not seen by your followers. It lacks the visual appeal of other image- and video-based platforms that tend to attract more members of the general public. (Twitter lags behind these other platforms in monthly users) Other platforms like Facebook, Instagram, YouTube, LinkedIn, and TikTok have other benefits. Facebook may help foster community discussions in groups and business pages are also helpful for practice promotion. Instagram has gained popularity for educational purposes over the past 2 years, given its pairing with imagery and room for a lengthier caption. It has a variety of additional features like the temporary Instagram Stories that last 24 hours (which also allows for polling), question and answer, and livestream options. Other platforms like YouTube and TikTok have greater potential to reach audiences who otherwise would not see your content, with the former having the benefit of being highly searchable and the latter being the social media app with fastest growing popularity.
Having grown up with the Internet-based instant messaging and social media platforms, I have always enjoyed the medium as a way to connect with others. However, productive engagement on these platforms came much later. During a brief stint as part of the ABC News medical unit, I learned how Twitter was used to facilitate weekly chats around a specific topic online. I began exploring my own social media voice, which quickly gave way to live-tweeting medical conferences, hosting and participating Twitter chats myself, and guiding colleagues and professional societies to greater adoption of social media. In an attempt to introduce a divisional social media account during my fellowship, I learned of institutional barriers including antiquated policies that actively dissuaded social media use. I became increasingly involved on committees in our main GI societies after engaging in multiple research projects using social media data looking at how GI journals promote their content online, the associations between social media presence and institutional ranking, social media behavior at medical conferences, and the evolving perspectives of training program leadership regarding social media.
The pitfalls of social media remain a major concern for physicians and employers alike. First and foremost, it is important to review one’s institutional social media policy prior to starting, as individuals are ultimately held to their local policies. Not only can social media activity be a major liability for a health care employer, but also in the general public’s trust in health professionals. Protecting patient privacy and safety are of utmost concern, and physicians must be mindful not to inadvertently reveal patient identity. HIPAA violations are not limited to only naming patients by name or photo; descriptions of procedural cases and posting patient-related images such as radiographs or endoscopic images may reveal patient identity if there are unique details on these images (e.g., a radio-opaque necklace on x-ray or a particular swallowed foreign body).
Another disadvantage of social media is being approached with personal medical questions. I universally decline to answer these inquiries, citing the need to perform a comprehensive review of one’s medical chart and perform an in-person physical exam to fully assess a patient. The distinction between education and advice is subtle, yet important to recognize. Similarly, the need to uphold professionalism online is important. Short messages on social media can be misinterpreted by colleagues and the public. Not only can these interactions be potentially detrimental to one’s career, but it can further erode trust in health care if patients perceive this as fragmentation of the health care system. On platforms that encourage humor and creativity like TikTok, there have also been medical professionals and students publicly criticized and penalized for posting unprofessional content mocking patients.
With the introduction of social media influencers in recent years, some professionals have amassed followings, introducing yet another set of concerns. One is being approached with sponsorship and endorsement offers, as any agreements must be in accordance with institutional policy. As one’s following grows, there may be other concerns of safety both online and in real life. Online concerns include issues with impersonation and use of photos or written content without permission. On the surface this may not seem like a significant concern, but there have been situations where family photos are distributed to intended audiences or one’s likeness is used to endorse a product.
In addition to physical safety, another unintended consequence of social media use is its impact on one’s mental health. As social media tends to be a highlight reel, it is easy to be consumed by comparison with colleagues and their lives on social media, whether it truly reflects one’s actual life or not.
My ability to understand multiple social media platforms and anticipate a growing set of risks and concerns with using social media is what led to my involvement with multiple GI societies and appointment by my institution’s CEO to serve as the first chief medical social media officer. My desire to help other professionals with the journey also led to the formation of the Association for Healthcare Social Media, the first 501(c)(3) nonprofit professional organization devoted to health professionals on social media. There is tremendous opportunity to impact public health through social media, especially with regards to raising awareness about underrepresented conditions and presenting information that is accurate. Many barriers remain to the widespread adoption of social media by health professionals, such as the lack of financial or academic incentives. For now, there is every indication that social media is here to stay, and it will likely continue to play an important role in how we communicate with our patients.
AGA can be found online at @AmerGastroAssn (Facebook, Instagram, and Twitter) and @AGA_Gastro, @AGA_CGH, and @AGA_CMGH (Facebook and Twitter).
Dr. Chiang is assistant professor of medicine, division of gastroenterology & hepatology, director, endoscopic bariatric program, chief medical social media officer, Jefferson Health, Philadelphia, and president, Association for Healthcare Social Media, @austinchiangmd
Navigating a pandemic: The importance of preparedness in independent GI practices
It was early March, and our second day of advocacy on Capitol Hill with the Digestive Health Physicians Association (DHPA) was cut short when congressional offices were shuttered because of the COVID-19 pandemic. Sitting with several of my GI physician colleagues from across the country, we knew that our practices, our patients, and our communities would be impacted by the coronavirus. None of us could have known the extent.
We also didn’t know in that moment that our advocacy work through DHPA would be one of the most important factors in ensuring that our practices were prepared to weather the pandemic. Our membership, legal counsel, and legislative lobbyists helped us remain informed about new legislation and regulations and ensured that we had much-needed access to government resources.
Just a few months into what is now the COVID-19 pandemic, independent GI practice leaders have learned a lot about how to strengthen our practices to respond to future crises – and what early-career GIs should look for in the practices they are considering.
First and foremost, practice leadership is key. One thing most successful GI practices have in common is that they hire really smart executives and administrative teams who excel at taking care of the business side of things so that physicians like me can do what we do best: treat patients.
Stay informed about state and federal policies
As a member of DHPA, Capital Digestive Care was well positioned to keep up to date on the government response to the coronavirus and the support it provided to small businesses and to health care providers.
Over the past 5 years, DHPA physician leaders have established strong relationships with our elected federal leaders. During our Capitol Hill visits in early March, we discussed the coronavirus in addition to our policy priorities.
The relationships we’ve built with policymakers have helped us educate them about how private practices were being affected and make the case that it was crucial to include private practices in health care stimulus packages.
Without this federal financial support, many medical groups may have had to close their doors – leaving a large gap in care once the pandemic subsides.
In addition to the federal government’s financial support, our policy advocacy efforts kept us informed about federal health agencies’ decisions on telehealth coverage. We were able to educate our physicians and staff about state and federal adjustments to telehealth rules for the pandemic, on the guidelines for elective procedures, on employee furlough and leave rules, as well as other congressional and state actions that would impact our practice.
You can’t be an independent physician without being open to learning about the business of health care and understanding how health policies affect your ability to practice medicine and care for people in your community. Every early-career physician who is looking to join a practice should ask how its leadership remains informed about health policy at the state and federal levels.
Make plans, be flexible
Implementing telehealth was critical in responding to the coronavirus pandemic. We were able to get up and running quickly on telemedicine because we had already invested in telehealth and had conducted a pilot of the platform with a smaller group of providers well before the pandemic hit.
In March, we were able to expand the telehealth platform to accommodate virtual visits by all of our providers. We also had to figure out how to shift our employees to telework, develop remote desktop and VPN solutions, and make sure that our scheduling and revenue cycle team members were fully operational.
The overriding goal was the safety of patients, staff, and our providers while continuing to provide medical care. Our inflammatory bowel disease patients needing visits to receive medication infusions took over an entire office so that there could be appropriate spacing and limited contacts with staff and other patients.
Our administrators knew early on that we needed a back-up plan and worked with physicians and providers doing telehealth visits to provide the flexibility to switch to Centers for Medicare & Medicaid–approved platforms (including Facetime) for those instances in which patients were uncomfortable using our main platform or when it was strained by bandwidth issues – a common challenge with any platform. Virtual check-in and check-out procedures were developed utilizing our usual office staff from remote locations.
For patients who had indications for gastroenterology procedures, we established a prioritization system, based on state guidelines, for those that were needed urgently or routinely as our endoscopy centers began to reopen. Safety measures were put into place including screening questionnaires, preprocedure COVID rt-PCT testing, personal protective equipment, and workflow changes to achieve social distancing.
As an early-career GI physician who is considering private practice, you’ll likely have several conversations with administrative leaders when deciding what practice to join. Ask about how the practice responded to COVID-19, and what processes it has in place to prepare for future emergencies.
During the early weeks of the pandemic, the CDC Board of Managers met two to three times per week. Task forces to discuss office operations and planning for ambulatory surgery center opening were established with participation by nearly every provider and manager. Communication between all providers and managers was important to decrease the obvious anxiety everyone was experiencing.
Old financial models may no longer work
Most practices develop budgets based on historical data. We quickly figured out that budgets from historical forecasts no longer worked and that we needed to understand the impact to budgets almost in real time.
We immediately looked to conserve cash and reduce expenses, requesting that our large vendors extend payment terms or provide a period of forbearance. We looked at everything from our EMR costs to lab supplies and everything in between.
Changing how we modeled our budgets and reducing costs made some of our hard decisions less difficult. While we had to furlough staff, our models for reducing physician compensation and lowering our costs allowed us to create a model for the return to work that included the use of paid time off and paid health care for our furloughed employees.
Our operations team also set up systems to gather information that was needed to apply for and report on federal loans and grants. They also set up ways to track revenue per visit and appeals for denied telehealth and other services in an effort to create new models and budgets as COVID-19 progressed. The revenue cycle team focused on unpaid older accounts receivable.
Focused on the future
It’s an understatement to say that COVID-19 has forever changed the practice of medicine. The health care industry will need to transform.
For some time now, GI practices have discussed the consequence of disruptive innovation affecting utilization of endoscopic procedures. We were looking at technology that might eventually replace office personnel. No one was thinking about a pandemic that would cause nearly overnight closure of endoscopy suites and curtail the entire in-office administrative workforce. The coronavirus pandemic is likely to be the catalyst that brings many innovations into the mainstream.
We’ll most likely see a transition to the virtual medical office for those visits that don’t require a patient to see a physician in person. This will make online scheduling and registration, on-demand messaging, and remote patient monitoring and chronic care management necessities.
We may also see more rapid adoption of technologies that allow information from health trackers and wearables to be integrated into EMRs that easily follow the patient from physician to physician. Administrative support and patient assistance from remote locations will become the norm.
Inquiring about how practices plan for emergencies and how their leadership thinks about the future of gastroenterology is a great way to show that you’re thinking holistically about health care delivery and how medicine is practiced now and in the future.
So much has changed in the decades I’ve been practicing medicine and so much is yet to change. As early-career GI physicians who are familiar with new technologies, you are in a great position to lead the practices you join into the future of gastroenterology.
Dr. Weinstein is president and CEO of Capital Digestive Care and the immediate past president of the Digestive Health Physicians Association.
It was early March, and our second day of advocacy on Capitol Hill with the Digestive Health Physicians Association (DHPA) was cut short when congressional offices were shuttered because of the COVID-19 pandemic. Sitting with several of my GI physician colleagues from across the country, we knew that our practices, our patients, and our communities would be impacted by the coronavirus. None of us could have known the extent.
We also didn’t know in that moment that our advocacy work through DHPA would be one of the most important factors in ensuring that our practices were prepared to weather the pandemic. Our membership, legal counsel, and legislative lobbyists helped us remain informed about new legislation and regulations and ensured that we had much-needed access to government resources.
Just a few months into what is now the COVID-19 pandemic, independent GI practice leaders have learned a lot about how to strengthen our practices to respond to future crises – and what early-career GIs should look for in the practices they are considering.
First and foremost, practice leadership is key. One thing most successful GI practices have in common is that they hire really smart executives and administrative teams who excel at taking care of the business side of things so that physicians like me can do what we do best: treat patients.
Stay informed about state and federal policies
As a member of DHPA, Capital Digestive Care was well positioned to keep up to date on the government response to the coronavirus and the support it provided to small businesses and to health care providers.
Over the past 5 years, DHPA physician leaders have established strong relationships with our elected federal leaders. During our Capitol Hill visits in early March, we discussed the coronavirus in addition to our policy priorities.
The relationships we’ve built with policymakers have helped us educate them about how private practices were being affected and make the case that it was crucial to include private practices in health care stimulus packages.
Without this federal financial support, many medical groups may have had to close their doors – leaving a large gap in care once the pandemic subsides.
In addition to the federal government’s financial support, our policy advocacy efforts kept us informed about federal health agencies’ decisions on telehealth coverage. We were able to educate our physicians and staff about state and federal adjustments to telehealth rules for the pandemic, on the guidelines for elective procedures, on employee furlough and leave rules, as well as other congressional and state actions that would impact our practice.
You can’t be an independent physician without being open to learning about the business of health care and understanding how health policies affect your ability to practice medicine and care for people in your community. Every early-career physician who is looking to join a practice should ask how its leadership remains informed about health policy at the state and federal levels.
Make plans, be flexible
Implementing telehealth was critical in responding to the coronavirus pandemic. We were able to get up and running quickly on telemedicine because we had already invested in telehealth and had conducted a pilot of the platform with a smaller group of providers well before the pandemic hit.
In March, we were able to expand the telehealth platform to accommodate virtual visits by all of our providers. We also had to figure out how to shift our employees to telework, develop remote desktop and VPN solutions, and make sure that our scheduling and revenue cycle team members were fully operational.
The overriding goal was the safety of patients, staff, and our providers while continuing to provide medical care. Our inflammatory bowel disease patients needing visits to receive medication infusions took over an entire office so that there could be appropriate spacing and limited contacts with staff and other patients.
Our administrators knew early on that we needed a back-up plan and worked with physicians and providers doing telehealth visits to provide the flexibility to switch to Centers for Medicare & Medicaid–approved platforms (including Facetime) for those instances in which patients were uncomfortable using our main platform or when it was strained by bandwidth issues – a common challenge with any platform. Virtual check-in and check-out procedures were developed utilizing our usual office staff from remote locations.
For patients who had indications for gastroenterology procedures, we established a prioritization system, based on state guidelines, for those that were needed urgently or routinely as our endoscopy centers began to reopen. Safety measures were put into place including screening questionnaires, preprocedure COVID rt-PCT testing, personal protective equipment, and workflow changes to achieve social distancing.
As an early-career GI physician who is considering private practice, you’ll likely have several conversations with administrative leaders when deciding what practice to join. Ask about how the practice responded to COVID-19, and what processes it has in place to prepare for future emergencies.
During the early weeks of the pandemic, the CDC Board of Managers met two to three times per week. Task forces to discuss office operations and planning for ambulatory surgery center opening were established with participation by nearly every provider and manager. Communication between all providers and managers was important to decrease the obvious anxiety everyone was experiencing.
Old financial models may no longer work
Most practices develop budgets based on historical data. We quickly figured out that budgets from historical forecasts no longer worked and that we needed to understand the impact to budgets almost in real time.
We immediately looked to conserve cash and reduce expenses, requesting that our large vendors extend payment terms or provide a period of forbearance. We looked at everything from our EMR costs to lab supplies and everything in between.
Changing how we modeled our budgets and reducing costs made some of our hard decisions less difficult. While we had to furlough staff, our models for reducing physician compensation and lowering our costs allowed us to create a model for the return to work that included the use of paid time off and paid health care for our furloughed employees.
Our operations team also set up systems to gather information that was needed to apply for and report on federal loans and grants. They also set up ways to track revenue per visit and appeals for denied telehealth and other services in an effort to create new models and budgets as COVID-19 progressed. The revenue cycle team focused on unpaid older accounts receivable.
Focused on the future
It’s an understatement to say that COVID-19 has forever changed the practice of medicine. The health care industry will need to transform.
For some time now, GI practices have discussed the consequence of disruptive innovation affecting utilization of endoscopic procedures. We were looking at technology that might eventually replace office personnel. No one was thinking about a pandemic that would cause nearly overnight closure of endoscopy suites and curtail the entire in-office administrative workforce. The coronavirus pandemic is likely to be the catalyst that brings many innovations into the mainstream.
We’ll most likely see a transition to the virtual medical office for those visits that don’t require a patient to see a physician in person. This will make online scheduling and registration, on-demand messaging, and remote patient monitoring and chronic care management necessities.
We may also see more rapid adoption of technologies that allow information from health trackers and wearables to be integrated into EMRs that easily follow the patient from physician to physician. Administrative support and patient assistance from remote locations will become the norm.
Inquiring about how practices plan for emergencies and how their leadership thinks about the future of gastroenterology is a great way to show that you’re thinking holistically about health care delivery and how medicine is practiced now and in the future.
So much has changed in the decades I’ve been practicing medicine and so much is yet to change. As early-career GI physicians who are familiar with new technologies, you are in a great position to lead the practices you join into the future of gastroenterology.
Dr. Weinstein is president and CEO of Capital Digestive Care and the immediate past president of the Digestive Health Physicians Association.
It was early March, and our second day of advocacy on Capitol Hill with the Digestive Health Physicians Association (DHPA) was cut short when congressional offices were shuttered because of the COVID-19 pandemic. Sitting with several of my GI physician colleagues from across the country, we knew that our practices, our patients, and our communities would be impacted by the coronavirus. None of us could have known the extent.
We also didn’t know in that moment that our advocacy work through DHPA would be one of the most important factors in ensuring that our practices were prepared to weather the pandemic. Our membership, legal counsel, and legislative lobbyists helped us remain informed about new legislation and regulations and ensured that we had much-needed access to government resources.
Just a few months into what is now the COVID-19 pandemic, independent GI practice leaders have learned a lot about how to strengthen our practices to respond to future crises – and what early-career GIs should look for in the practices they are considering.
First and foremost, practice leadership is key. One thing most successful GI practices have in common is that they hire really smart executives and administrative teams who excel at taking care of the business side of things so that physicians like me can do what we do best: treat patients.
Stay informed about state and federal policies
As a member of DHPA, Capital Digestive Care was well positioned to keep up to date on the government response to the coronavirus and the support it provided to small businesses and to health care providers.
Over the past 5 years, DHPA physician leaders have established strong relationships with our elected federal leaders. During our Capitol Hill visits in early March, we discussed the coronavirus in addition to our policy priorities.
The relationships we’ve built with policymakers have helped us educate them about how private practices were being affected and make the case that it was crucial to include private practices in health care stimulus packages.
Without this federal financial support, many medical groups may have had to close their doors – leaving a large gap in care once the pandemic subsides.
In addition to the federal government’s financial support, our policy advocacy efforts kept us informed about federal health agencies’ decisions on telehealth coverage. We were able to educate our physicians and staff about state and federal adjustments to telehealth rules for the pandemic, on the guidelines for elective procedures, on employee furlough and leave rules, as well as other congressional and state actions that would impact our practice.
You can’t be an independent physician without being open to learning about the business of health care and understanding how health policies affect your ability to practice medicine and care for people in your community. Every early-career physician who is looking to join a practice should ask how its leadership remains informed about health policy at the state and federal levels.
Make plans, be flexible
Implementing telehealth was critical in responding to the coronavirus pandemic. We were able to get up and running quickly on telemedicine because we had already invested in telehealth and had conducted a pilot of the platform with a smaller group of providers well before the pandemic hit.
In March, we were able to expand the telehealth platform to accommodate virtual visits by all of our providers. We also had to figure out how to shift our employees to telework, develop remote desktop and VPN solutions, and make sure that our scheduling and revenue cycle team members were fully operational.
The overriding goal was the safety of patients, staff, and our providers while continuing to provide medical care. Our inflammatory bowel disease patients needing visits to receive medication infusions took over an entire office so that there could be appropriate spacing and limited contacts with staff and other patients.
Our administrators knew early on that we needed a back-up plan and worked with physicians and providers doing telehealth visits to provide the flexibility to switch to Centers for Medicare & Medicaid–approved platforms (including Facetime) for those instances in which patients were uncomfortable using our main platform or when it was strained by bandwidth issues – a common challenge with any platform. Virtual check-in and check-out procedures were developed utilizing our usual office staff from remote locations.
For patients who had indications for gastroenterology procedures, we established a prioritization system, based on state guidelines, for those that were needed urgently or routinely as our endoscopy centers began to reopen. Safety measures were put into place including screening questionnaires, preprocedure COVID rt-PCT testing, personal protective equipment, and workflow changes to achieve social distancing.
As an early-career GI physician who is considering private practice, you’ll likely have several conversations with administrative leaders when deciding what practice to join. Ask about how the practice responded to COVID-19, and what processes it has in place to prepare for future emergencies.
During the early weeks of the pandemic, the CDC Board of Managers met two to three times per week. Task forces to discuss office operations and planning for ambulatory surgery center opening were established with participation by nearly every provider and manager. Communication between all providers and managers was important to decrease the obvious anxiety everyone was experiencing.
Old financial models may no longer work
Most practices develop budgets based on historical data. We quickly figured out that budgets from historical forecasts no longer worked and that we needed to understand the impact to budgets almost in real time.
We immediately looked to conserve cash and reduce expenses, requesting that our large vendors extend payment terms or provide a period of forbearance. We looked at everything from our EMR costs to lab supplies and everything in between.
Changing how we modeled our budgets and reducing costs made some of our hard decisions less difficult. While we had to furlough staff, our models for reducing physician compensation and lowering our costs allowed us to create a model for the return to work that included the use of paid time off and paid health care for our furloughed employees.
Our operations team also set up systems to gather information that was needed to apply for and report on federal loans and grants. They also set up ways to track revenue per visit and appeals for denied telehealth and other services in an effort to create new models and budgets as COVID-19 progressed. The revenue cycle team focused on unpaid older accounts receivable.
Focused on the future
It’s an understatement to say that COVID-19 has forever changed the practice of medicine. The health care industry will need to transform.
For some time now, GI practices have discussed the consequence of disruptive innovation affecting utilization of endoscopic procedures. We were looking at technology that might eventually replace office personnel. No one was thinking about a pandemic that would cause nearly overnight closure of endoscopy suites and curtail the entire in-office administrative workforce. The coronavirus pandemic is likely to be the catalyst that brings many innovations into the mainstream.
We’ll most likely see a transition to the virtual medical office for those visits that don’t require a patient to see a physician in person. This will make online scheduling and registration, on-demand messaging, and remote patient monitoring and chronic care management necessities.
We may also see more rapid adoption of technologies that allow information from health trackers and wearables to be integrated into EMRs that easily follow the patient from physician to physician. Administrative support and patient assistance from remote locations will become the norm.
Inquiring about how practices plan for emergencies and how their leadership thinks about the future of gastroenterology is a great way to show that you’re thinking holistically about health care delivery and how medicine is practiced now and in the future.
So much has changed in the decades I’ve been practicing medicine and so much is yet to change. As early-career GI physicians who are familiar with new technologies, you are in a great position to lead the practices you join into the future of gastroenterology.
Dr. Weinstein is president and CEO of Capital Digestive Care and the immediate past president of the Digestive Health Physicians Association.
Coronavirus impact on medical education: Thoughts from two GI fellows’ perspectives
Introduction
We are living in an unprecedented time. During March 2020, in response to the COVID-19 (coronavirus disease 2019) outbreak, our institution removed all medical students from rotations with direct patient contact to prioritize their safety and well-being, following recommendations made by the Association of American Medical Colleges (AAMC).1 Similarly, we as gastroenterology fellows experienced an upheaval in our usual schedules and routines. Some of us were redeployed to other areas of the hospital, such as inpatient wards and emergency departments, to meet the needs of our patients and our health system. These changes were difficult, not only because we were practicing in different roles, but also because unknown situations commonly incite fear and anxiety.
Among the repercussions of the COVID-19 pandemic were the changes thrust upon medical students who suddenly found themselves without clinical exposure (both on core clerkships and electives) for the duration of the academic year.2 We too lost many of our educational and teaching opportunities as we adapted to our changing circumstances and new reality. Therefore, . We used the lessons we learned because of the changes in our own medical education to anticipate the best ways to provide learning opportunities for our students.
GI fellows’ experiences
The changes to our schedules and lack of in-person educational conferences seemingly happened overnight – the shock of being pulled from clinics, consults, and endoscopy left us feeling scared and lonely. We were quickly transitioned from knowing our roles and responsibilities as GI providers to taking over care for hospitalist patients as the “primary team,” working in the COVID emergency department (ED), and losing our clinic space. Redeployment to other clinical environments was anxiety-provoking. Self-doubt and fear were the most cited concerns as we asked ourselves: Do I remember enough general medicine to be an effective hospitalist? How do I place admission orders or perform a medication reconciliation on discharge? What can I expect in the COVID ED? Will I have to intubate someone? What about possible PPE shortages? Are my family members safe at home? Should I stay in a hotel? Do we have estimates on how long this will last?
Clinical schedules were reconfigured to consolidate the use of inpatient fellows and allow for reserves of fellows to be redeployed if needed. Schedules for the following 7 days were made just 48 hours prior to the start of each workweek. The anticipation and fear of the unknown were perhaps the hardest parts of the changes in our clinical learning environment. Little time was provided to make child care arrangements, coordinate with the schedules of significant others, or review topics and skills we might need in the next week that had gone unused for some time.
Our conference schedule was pared down considerably as fellows and attendings adjusted to their new responsibilities and a virtual platform for fellows’ education. While the transition to online lectures was seamless, the spirit of conference certainly changed. Impromptu questions and conversations that oftentimes arise organically during case conferences no longer occurred as virtual meetings do not offer the same space to foster these discussions as we awkwardly muted and unmuted ourselves. Participation in lectures seemed disjointed, which translated in some ways to less effective learning opportunities. Our involvement in endoscopy was also removed as only urgent cases were being performed and PPE conservation was of the utmost priority. This was especially concerning for third-year fellows on the cusp of graduation who would soon be independent practitioners without recent procedural practice. In general, the fellowship felt isolated and uncertain, which our program director addressed with weekly virtual COVID-19 “happy hour” updates.
GI fellows’ contribution
As our program encouraged us to come together during this time to support each other, we realized that while our clinical duties may look different during the COVID-19 crisis, our responsibility to learners was more important than ever. At many academic institutions, GI fellows are referred to as “the face of the division” owed in large part to our consistent presence on consult services and roles as teachers for medical students and residents who rotate with us. In an effort to assist the medical school’s charge to rapidly generate at-home curriculum for our students, we created an online curriculum for medical students to complete during the time they were previously scheduled to rotate with us on consults either as third- or fourth-year students.
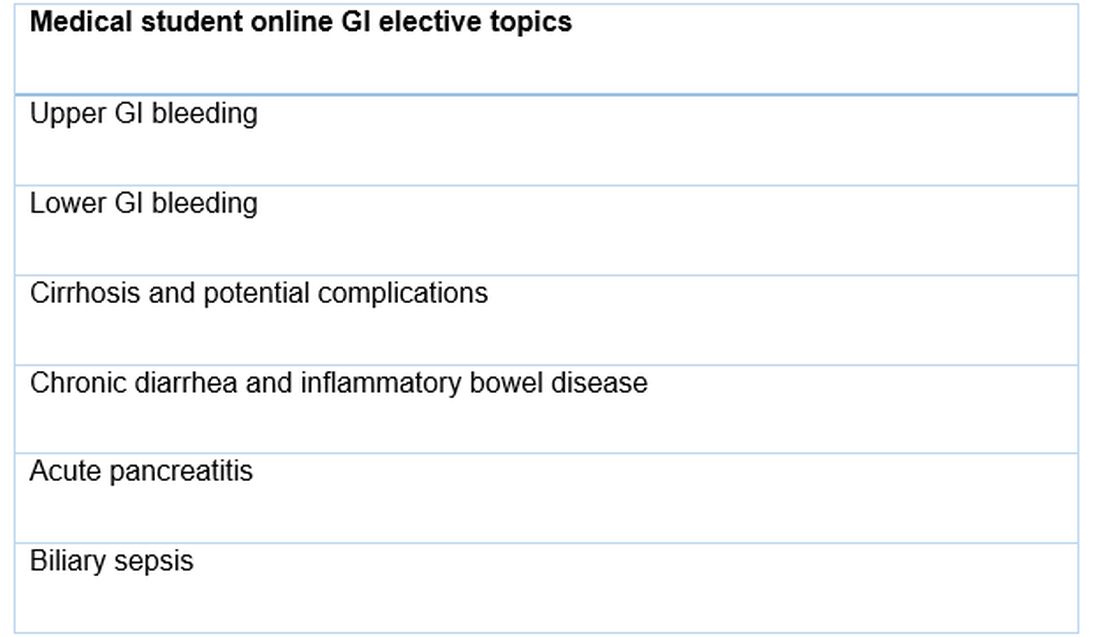
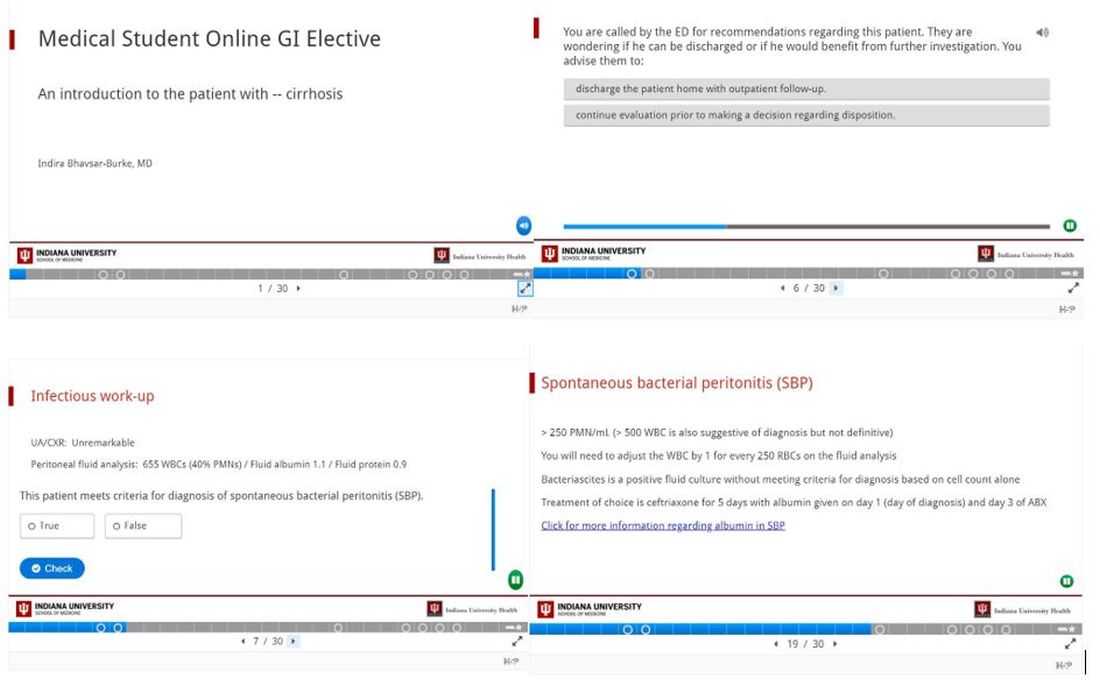
We designed a series of interactive podcasts covering six topics that are commonly encountered issues on the GI consult service: upper GI bleeding, lower GI bleeding, biliary sepsis, acute pancreatitis, chronic diarrhea with a new diagnosis of inflammatory bowel disease, as well as cirrhosis and its associated complications.
Conclusion
The COVID-19 pandemic brought about significant change in the daily activities of GI fellows including new responsibilities and a great need for adaptation. We hope that the lessons the COVID-19 pandemic has taught us – to think of others and make our talents available to those who need them, to look for ways to adapt to challenges, to live in the present but focus on the future, and to spread creativity when able – will continue long after the curve has flattened.
References
1. Murphy B. American Medical Association website. https://www.ama-assn.org/residents-students/medical-school-life/online-learning-during-covid-19-tips-help-med-students. Apr 3, 2020.
2. Murphy B. American Medical Association website. https://www.ama-assn.org/delivering-care/public-health/covid-19-how-virus-impacting-medical-schools. Mar 20, 2020.
3. “H5P: Create, share and reuse interactive HTML5 content in your browser.” H5P website. https://h5p.org.
Dr. Bhavsar-Burke and Dr. Jansson-Knodell are GI fellows in the division of gastroenterology and hepatology, department of medicine, Indiana University, Indianapolis. The authors have no conflicts of interest.
Introduction
We are living in an unprecedented time. During March 2020, in response to the COVID-19 (coronavirus disease 2019) outbreak, our institution removed all medical students from rotations with direct patient contact to prioritize their safety and well-being, following recommendations made by the Association of American Medical Colleges (AAMC).1 Similarly, we as gastroenterology fellows experienced an upheaval in our usual schedules and routines. Some of us were redeployed to other areas of the hospital, such as inpatient wards and emergency departments, to meet the needs of our patients and our health system. These changes were difficult, not only because we were practicing in different roles, but also because unknown situations commonly incite fear and anxiety.
Among the repercussions of the COVID-19 pandemic were the changes thrust upon medical students who suddenly found themselves without clinical exposure (both on core clerkships and electives) for the duration of the academic year.2 We too lost many of our educational and teaching opportunities as we adapted to our changing circumstances and new reality. Therefore, . We used the lessons we learned because of the changes in our own medical education to anticipate the best ways to provide learning opportunities for our students.
GI fellows’ experiences
The changes to our schedules and lack of in-person educational conferences seemingly happened overnight – the shock of being pulled from clinics, consults, and endoscopy left us feeling scared and lonely. We were quickly transitioned from knowing our roles and responsibilities as GI providers to taking over care for hospitalist patients as the “primary team,” working in the COVID emergency department (ED), and losing our clinic space. Redeployment to other clinical environments was anxiety-provoking. Self-doubt and fear were the most cited concerns as we asked ourselves: Do I remember enough general medicine to be an effective hospitalist? How do I place admission orders or perform a medication reconciliation on discharge? What can I expect in the COVID ED? Will I have to intubate someone? What about possible PPE shortages? Are my family members safe at home? Should I stay in a hotel? Do we have estimates on how long this will last?
Clinical schedules were reconfigured to consolidate the use of inpatient fellows and allow for reserves of fellows to be redeployed if needed. Schedules for the following 7 days were made just 48 hours prior to the start of each workweek. The anticipation and fear of the unknown were perhaps the hardest parts of the changes in our clinical learning environment. Little time was provided to make child care arrangements, coordinate with the schedules of significant others, or review topics and skills we might need in the next week that had gone unused for some time.
Our conference schedule was pared down considerably as fellows and attendings adjusted to their new responsibilities and a virtual platform for fellows’ education. While the transition to online lectures was seamless, the spirit of conference certainly changed. Impromptu questions and conversations that oftentimes arise organically during case conferences no longer occurred as virtual meetings do not offer the same space to foster these discussions as we awkwardly muted and unmuted ourselves. Participation in lectures seemed disjointed, which translated in some ways to less effective learning opportunities. Our involvement in endoscopy was also removed as only urgent cases were being performed and PPE conservation was of the utmost priority. This was especially concerning for third-year fellows on the cusp of graduation who would soon be independent practitioners without recent procedural practice. In general, the fellowship felt isolated and uncertain, which our program director addressed with weekly virtual COVID-19 “happy hour” updates.
GI fellows’ contribution
As our program encouraged us to come together during this time to support each other, we realized that while our clinical duties may look different during the COVID-19 crisis, our responsibility to learners was more important than ever. At many academic institutions, GI fellows are referred to as “the face of the division” owed in large part to our consistent presence on consult services and roles as teachers for medical students and residents who rotate with us. In an effort to assist the medical school’s charge to rapidly generate at-home curriculum for our students, we created an online curriculum for medical students to complete during the time they were previously scheduled to rotate with us on consults either as third- or fourth-year students.
We designed a series of interactive podcasts covering six topics that are commonly encountered issues on the GI consult service: upper GI bleeding, lower GI bleeding, biliary sepsis, acute pancreatitis, chronic diarrhea with a new diagnosis of inflammatory bowel disease, as well as cirrhosis and its associated complications.


Conclusion
The COVID-19 pandemic brought about significant change in the daily activities of GI fellows including new responsibilities and a great need for adaptation. We hope that the lessons the COVID-19 pandemic has taught us – to think of others and make our talents available to those who need them, to look for ways to adapt to challenges, to live in the present but focus on the future, and to spread creativity when able – will continue long after the curve has flattened.
References
1. Murphy B. American Medical Association website. https://www.ama-assn.org/residents-students/medical-school-life/online-learning-during-covid-19-tips-help-med-students. Apr 3, 2020.
2. Murphy B. American Medical Association website. https://www.ama-assn.org/delivering-care/public-health/covid-19-how-virus-impacting-medical-schools. Mar 20, 2020.
3. “H5P: Create, share and reuse interactive HTML5 content in your browser.” H5P website. https://h5p.org.
Dr. Bhavsar-Burke and Dr. Jansson-Knodell are GI fellows in the division of gastroenterology and hepatology, department of medicine, Indiana University, Indianapolis. The authors have no conflicts of interest.
Introduction
We are living in an unprecedented time. During March 2020, in response to the COVID-19 (coronavirus disease 2019) outbreak, our institution removed all medical students from rotations with direct patient contact to prioritize their safety and well-being, following recommendations made by the Association of American Medical Colleges (AAMC).1 Similarly, we as gastroenterology fellows experienced an upheaval in our usual schedules and routines. Some of us were redeployed to other areas of the hospital, such as inpatient wards and emergency departments, to meet the needs of our patients and our health system. These changes were difficult, not only because we were practicing in different roles, but also because unknown situations commonly incite fear and anxiety.
Among the repercussions of the COVID-19 pandemic were the changes thrust upon medical students who suddenly found themselves without clinical exposure (both on core clerkships and electives) for the duration of the academic year.2 We too lost many of our educational and teaching opportunities as we adapted to our changing circumstances and new reality. Therefore, . We used the lessons we learned because of the changes in our own medical education to anticipate the best ways to provide learning opportunities for our students.
GI fellows’ experiences
The changes to our schedules and lack of in-person educational conferences seemingly happened overnight – the shock of being pulled from clinics, consults, and endoscopy left us feeling scared and lonely. We were quickly transitioned from knowing our roles and responsibilities as GI providers to taking over care for hospitalist patients as the “primary team,” working in the COVID emergency department (ED), and losing our clinic space. Redeployment to other clinical environments was anxiety-provoking. Self-doubt and fear were the most cited concerns as we asked ourselves: Do I remember enough general medicine to be an effective hospitalist? How do I place admission orders or perform a medication reconciliation on discharge? What can I expect in the COVID ED? Will I have to intubate someone? What about possible PPE shortages? Are my family members safe at home? Should I stay in a hotel? Do we have estimates on how long this will last?
Clinical schedules were reconfigured to consolidate the use of inpatient fellows and allow for reserves of fellows to be redeployed if needed. Schedules for the following 7 days were made just 48 hours prior to the start of each workweek. The anticipation and fear of the unknown were perhaps the hardest parts of the changes in our clinical learning environment. Little time was provided to make child care arrangements, coordinate with the schedules of significant others, or review topics and skills we might need in the next week that had gone unused for some time.
Our conference schedule was pared down considerably as fellows and attendings adjusted to their new responsibilities and a virtual platform for fellows’ education. While the transition to online lectures was seamless, the spirit of conference certainly changed. Impromptu questions and conversations that oftentimes arise organically during case conferences no longer occurred as virtual meetings do not offer the same space to foster these discussions as we awkwardly muted and unmuted ourselves. Participation in lectures seemed disjointed, which translated in some ways to less effective learning opportunities. Our involvement in endoscopy was also removed as only urgent cases were being performed and PPE conservation was of the utmost priority. This was especially concerning for third-year fellows on the cusp of graduation who would soon be independent practitioners without recent procedural practice. In general, the fellowship felt isolated and uncertain, which our program director addressed with weekly virtual COVID-19 “happy hour” updates.
GI fellows’ contribution
As our program encouraged us to come together during this time to support each other, we realized that while our clinical duties may look different during the COVID-19 crisis, our responsibility to learners was more important than ever. At many academic institutions, GI fellows are referred to as “the face of the division” owed in large part to our consistent presence on consult services and roles as teachers for medical students and residents who rotate with us. In an effort to assist the medical school’s charge to rapidly generate at-home curriculum for our students, we created an online curriculum for medical students to complete during the time they were previously scheduled to rotate with us on consults either as third- or fourth-year students.
We designed a series of interactive podcasts covering six topics that are commonly encountered issues on the GI consult service: upper GI bleeding, lower GI bleeding, biliary sepsis, acute pancreatitis, chronic diarrhea with a new diagnosis of inflammatory bowel disease, as well as cirrhosis and its associated complications.


Conclusion
The COVID-19 pandemic brought about significant change in the daily activities of GI fellows including new responsibilities and a great need for adaptation. We hope that the lessons the COVID-19 pandemic has taught us – to think of others and make our talents available to those who need them, to look for ways to adapt to challenges, to live in the present but focus on the future, and to spread creativity when able – will continue long after the curve has flattened.
References
1. Murphy B. American Medical Association website. https://www.ama-assn.org/residents-students/medical-school-life/online-learning-during-covid-19-tips-help-med-students. Apr 3, 2020.
2. Murphy B. American Medical Association website. https://www.ama-assn.org/delivering-care/public-health/covid-19-how-virus-impacting-medical-schools. Mar 20, 2020.
3. “H5P: Create, share and reuse interactive HTML5 content in your browser.” H5P website. https://h5p.org.
Dr. Bhavsar-Burke and Dr. Jansson-Knodell are GI fellows in the division of gastroenterology and hepatology, department of medicine, Indiana University, Indianapolis. The authors have no conflicts of interest.
Choosing a career in health equity and health care policy
Dr. Anyane-Yeboa is a Commonwealth Fund Fellow in Minority Health Policy at Harvard University and a recent graduate of the Harvard T.H. Chan School of Public Health. She previously completed her gastroenterology fellowship at the University of Chicago. She will be an academic gastroenterologist at Massachusetts General Hospital starting in the fall of 2020.
How did your career pathway lead you to a career in health equity and policy?
I have been passionate about issues related to health equity, workforce diversity, and care of vulnerable populations since the early years of my career. For instance, as undergraduates my friends and I received a grant to start a program to provide mentorship for endangered youth in Boston. During my residency and chief residency, I advocated for increased resident diversity and created programs for underrepresented minority medical students to increase minority representation in medicine. During my gastroenterology fellowship, I remained passionate about the care of minority and underserved populations. During my second year of fellowship, I looked for advanced training opportunities where I could learn the skills to tackle health disparities in minority communities, and almost serendipitously came across the Commonwealth Fund Fellowship in Minority Health Policy. When I decided to apply for the fellowship, I knew that this would be a nontraditional path for most gastroenterology fellows, but the right path for me.
About the Commonwealth Fund Fellowship
The purpose of the Commonwealth Fund Fellowship in Minority Health Policy at Harvard University is to train the next generation of leaders in health care. The program is based at Harvard Medical School and supported by the Commonwealth Fund whose mission is to “provide affordable quality health care for all.” To date, the fellowship has trained more than 130 physicians who are advancing health care across the nation as leaders in public health, academic medicine, and health policy.
The fellowship is a year-long, full-time, degree-granting program. Fellows are eligible for a master’s in public health with a concentration in health management or health policy from the Harvard T.H. Chan School of Public Health or a master’s in public administration from the Harvard Kennedy School.
The fellowship program and experiences have been transformative for me. The structure of the program consists of visits to the Massachusetts Department of Public Health, the Boston Public Health Commission, and the Commonwealth Fund, as well as lectures, seminars, and journal club sessions with national leaders in public health, health policy, and health care delivery reform. Additional opportunities include one-on-one shadowing experiences with leaders in hospital administration at academic institutions in Boston and private meetings with leaders and staff at several government agencies in Washington, including the Centers for Medicaid & Medicare Services, the Office of Minority Health, the Food and Drug Administration, the Health Resources & Services Administration, and the National Institutes of Health.
The program has given me an opportunity to meet and learn from physicians who have chosen a variety of different career paths. Through the program I have had exposure to physicians in academic medicine, health care administration, health policy, and public service as well as those who have chosen a combination of clinical practice with any of the above. This experience has opened my eyes to the different possibilities for physician careers and has encouraged me to be open if new opportunities should arise.
As part of the fellowship, we also have regular meetings with Joan Reede, MD, MPH, who is the director of the fellowship and has been with the program since its inception; she is also the Dean of Diversity and Inclusion at Harvard Medical School. Dr. Reede is an incredibly wise, insightful, and caring mentor, but also a powerhouse in issues surrounding workforce diversity, mentorship, policy, care of underserved communities, and being an advocate for change. To have access to such a powerful individual who has dedicated her career to the mentorship of individuals like myself, who cares deeply about the impact of our careers, and who genuinely values each fellow almost as her own child is a unique gift that is hard to describe in words.
The Commonwealth Fund Fellowship also provides a large network of mentors and advisers. My direct mentor for the program is Monica Bharel, MD, MPH, who is a former Commonwealth Fund fellow and the current Commissioner of the Massachusetts Department of Public Health. However, I also have a wealth of other mentors and advisers in the alumni fellows, including Darrell Gray II, MD, MPH, a former fellow and gastroenterologist at the Ohio State University College of Medicine, as well as the other faculty associated with the program. I never imagined that I would have access to leaders in so many different sectors of health care and policy who are genuinely and passionately rooting for my success. In addition, my cofellows and I have created a uniquely special bond, and they will likely continue as my close network of peer advisers as I move forward throughout my career.
After the fellowship
I have no doubt that the Commonwealth Fund Fellowship will alter the trajectory of my career. It has already affected my career path in ways that I could not have anticipated years ago. The knowledge that I have gained in health care policy, innovation, and equity, as well as the networks that I have access to as a fellow, will be invaluable as I move forward. In terms of next steps, I will be working as an academic gastroenterologist; I will continue to lead initiatives, perform research, and participate in projects to elevate the voices of underserved communities and work toward health equity in gastroenterology. I am particularly passionate about ending disparities in colorectal cancer in minority communities and increasing awareness around minorities with inflammatory bowel disease.
I plan to work with health centers, city- and state-level organizations, and community partners to raise awareness around issues of equity in gastroenterology and develop interventions to create change. I will also work with local legislators and community-based organizations to advocate for policies that remove barriers to screening both locally and nationally. Further down the line, I am open to exploring careers in the public sector or health care administration if that is where my career takes me. The exposure that I had to these fields as part of the fellowship has shown me that it is possible to be a practicing gastroenterologist and simultaneously work in the public sector, health policy, or health care administration. If you are interested in applying to the Commonwealth Fund Fellowship in Minority Health Policy at Harvard University, please feel free to contact me at [email protected]. More information about the program and how to apply can be found at https://cff.hms.harvard.edu/.
Dr. Anyane-Yeboa is a Commonwealth Fund Fellow in Minority Health Policy at Harvard University and a recent graduate of the Harvard T.H. Chan School of Public Health. She previously completed her gastroenterology fellowship at the University of Chicago. She will be an academic gastroenterologist at Massachusetts General Hospital starting in the fall of 2020.
How did your career pathway lead you to a career in health equity and policy?
I have been passionate about issues related to health equity, workforce diversity, and care of vulnerable populations since the early years of my career. For instance, as undergraduates my friends and I received a grant to start a program to provide mentorship for endangered youth in Boston. During my residency and chief residency, I advocated for increased resident diversity and created programs for underrepresented minority medical students to increase minority representation in medicine. During my gastroenterology fellowship, I remained passionate about the care of minority and underserved populations. During my second year of fellowship, I looked for advanced training opportunities where I could learn the skills to tackle health disparities in minority communities, and almost serendipitously came across the Commonwealth Fund Fellowship in Minority Health Policy. When I decided to apply for the fellowship, I knew that this would be a nontraditional path for most gastroenterology fellows, but the right path for me.
About the Commonwealth Fund Fellowship
The purpose of the Commonwealth Fund Fellowship in Minority Health Policy at Harvard University is to train the next generation of leaders in health care. The program is based at Harvard Medical School and supported by the Commonwealth Fund whose mission is to “provide affordable quality health care for all.” To date, the fellowship has trained more than 130 physicians who are advancing health care across the nation as leaders in public health, academic medicine, and health policy.
The fellowship is a year-long, full-time, degree-granting program. Fellows are eligible for a master’s in public health with a concentration in health management or health policy from the Harvard T.H. Chan School of Public Health or a master’s in public administration from the Harvard Kennedy School.
The fellowship program and experiences have been transformative for me. The structure of the program consists of visits to the Massachusetts Department of Public Health, the Boston Public Health Commission, and the Commonwealth Fund, as well as lectures, seminars, and journal club sessions with national leaders in public health, health policy, and health care delivery reform. Additional opportunities include one-on-one shadowing experiences with leaders in hospital administration at academic institutions in Boston and private meetings with leaders and staff at several government agencies in Washington, including the Centers for Medicaid & Medicare Services, the Office of Minority Health, the Food and Drug Administration, the Health Resources & Services Administration, and the National Institutes of Health.
The program has given me an opportunity to meet and learn from physicians who have chosen a variety of different career paths. Through the program I have had exposure to physicians in academic medicine, health care administration, health policy, and public service as well as those who have chosen a combination of clinical practice with any of the above. This experience has opened my eyes to the different possibilities for physician careers and has encouraged me to be open if new opportunities should arise.
As part of the fellowship, we also have regular meetings with Joan Reede, MD, MPH, who is the director of the fellowship and has been with the program since its inception; she is also the Dean of Diversity and Inclusion at Harvard Medical School. Dr. Reede is an incredibly wise, insightful, and caring mentor, but also a powerhouse in issues surrounding workforce diversity, mentorship, policy, care of underserved communities, and being an advocate for change. To have access to such a powerful individual who has dedicated her career to the mentorship of individuals like myself, who cares deeply about the impact of our careers, and who genuinely values each fellow almost as her own child is a unique gift that is hard to describe in words.
The Commonwealth Fund Fellowship also provides a large network of mentors and advisers. My direct mentor for the program is Monica Bharel, MD, MPH, who is a former Commonwealth Fund fellow and the current Commissioner of the Massachusetts Department of Public Health. However, I also have a wealth of other mentors and advisers in the alumni fellows, including Darrell Gray II, MD, MPH, a former fellow and gastroenterologist at the Ohio State University College of Medicine, as well as the other faculty associated with the program. I never imagined that I would have access to leaders in so many different sectors of health care and policy who are genuinely and passionately rooting for my success. In addition, my cofellows and I have created a uniquely special bond, and they will likely continue as my close network of peer advisers as I move forward throughout my career.
After the fellowship
I have no doubt that the Commonwealth Fund Fellowship will alter the trajectory of my career. It has already affected my career path in ways that I could not have anticipated years ago. The knowledge that I have gained in health care policy, innovation, and equity, as well as the networks that I have access to as a fellow, will be invaluable as I move forward. In terms of next steps, I will be working as an academic gastroenterologist; I will continue to lead initiatives, perform research, and participate in projects to elevate the voices of underserved communities and work toward health equity in gastroenterology. I am particularly passionate about ending disparities in colorectal cancer in minority communities and increasing awareness around minorities with inflammatory bowel disease.
I plan to work with health centers, city- and state-level organizations, and community partners to raise awareness around issues of equity in gastroenterology and develop interventions to create change. I will also work with local legislators and community-based organizations to advocate for policies that remove barriers to screening both locally and nationally. Further down the line, I am open to exploring careers in the public sector or health care administration if that is where my career takes me. The exposure that I had to these fields as part of the fellowship has shown me that it is possible to be a practicing gastroenterologist and simultaneously work in the public sector, health policy, or health care administration. If you are interested in applying to the Commonwealth Fund Fellowship in Minority Health Policy at Harvard University, please feel free to contact me at [email protected]. More information about the program and how to apply can be found at https://cff.hms.harvard.edu/.
Dr. Anyane-Yeboa is a Commonwealth Fund Fellow in Minority Health Policy at Harvard University and a recent graduate of the Harvard T.H. Chan School of Public Health. She previously completed her gastroenterology fellowship at the University of Chicago. She will be an academic gastroenterologist at Massachusetts General Hospital starting in the fall of 2020.
How did your career pathway lead you to a career in health equity and policy?
I have been passionate about issues related to health equity, workforce diversity, and care of vulnerable populations since the early years of my career. For instance, as undergraduates my friends and I received a grant to start a program to provide mentorship for endangered youth in Boston. During my residency and chief residency, I advocated for increased resident diversity and created programs for underrepresented minority medical students to increase minority representation in medicine. During my gastroenterology fellowship, I remained passionate about the care of minority and underserved populations. During my second year of fellowship, I looked for advanced training opportunities where I could learn the skills to tackle health disparities in minority communities, and almost serendipitously came across the Commonwealth Fund Fellowship in Minority Health Policy. When I decided to apply for the fellowship, I knew that this would be a nontraditional path for most gastroenterology fellows, but the right path for me.
About the Commonwealth Fund Fellowship
The purpose of the Commonwealth Fund Fellowship in Minority Health Policy at Harvard University is to train the next generation of leaders in health care. The program is based at Harvard Medical School and supported by the Commonwealth Fund whose mission is to “provide affordable quality health care for all.” To date, the fellowship has trained more than 130 physicians who are advancing health care across the nation as leaders in public health, academic medicine, and health policy.
The fellowship is a year-long, full-time, degree-granting program. Fellows are eligible for a master’s in public health with a concentration in health management or health policy from the Harvard T.H. Chan School of Public Health or a master’s in public administration from the Harvard Kennedy School.
The fellowship program and experiences have been transformative for me. The structure of the program consists of visits to the Massachusetts Department of Public Health, the Boston Public Health Commission, and the Commonwealth Fund, as well as lectures, seminars, and journal club sessions with national leaders in public health, health policy, and health care delivery reform. Additional opportunities include one-on-one shadowing experiences with leaders in hospital administration at academic institutions in Boston and private meetings with leaders and staff at several government agencies in Washington, including the Centers for Medicaid & Medicare Services, the Office of Minority Health, the Food and Drug Administration, the Health Resources & Services Administration, and the National Institutes of Health.
The program has given me an opportunity to meet and learn from physicians who have chosen a variety of different career paths. Through the program I have had exposure to physicians in academic medicine, health care administration, health policy, and public service as well as those who have chosen a combination of clinical practice with any of the above. This experience has opened my eyes to the different possibilities for physician careers and has encouraged me to be open if new opportunities should arise.
As part of the fellowship, we also have regular meetings with Joan Reede, MD, MPH, who is the director of the fellowship and has been with the program since its inception; she is also the Dean of Diversity and Inclusion at Harvard Medical School. Dr. Reede is an incredibly wise, insightful, and caring mentor, but also a powerhouse in issues surrounding workforce diversity, mentorship, policy, care of underserved communities, and being an advocate for change. To have access to such a powerful individual who has dedicated her career to the mentorship of individuals like myself, who cares deeply about the impact of our careers, and who genuinely values each fellow almost as her own child is a unique gift that is hard to describe in words.
The Commonwealth Fund Fellowship also provides a large network of mentors and advisers. My direct mentor for the program is Monica Bharel, MD, MPH, who is a former Commonwealth Fund fellow and the current Commissioner of the Massachusetts Department of Public Health. However, I also have a wealth of other mentors and advisers in the alumni fellows, including Darrell Gray II, MD, MPH, a former fellow and gastroenterologist at the Ohio State University College of Medicine, as well as the other faculty associated with the program. I never imagined that I would have access to leaders in so many different sectors of health care and policy who are genuinely and passionately rooting for my success. In addition, my cofellows and I have created a uniquely special bond, and they will likely continue as my close network of peer advisers as I move forward throughout my career.
After the fellowship
I have no doubt that the Commonwealth Fund Fellowship will alter the trajectory of my career. It has already affected my career path in ways that I could not have anticipated years ago. The knowledge that I have gained in health care policy, innovation, and equity, as well as the networks that I have access to as a fellow, will be invaluable as I move forward. In terms of next steps, I will be working as an academic gastroenterologist; I will continue to lead initiatives, perform research, and participate in projects to elevate the voices of underserved communities and work toward health equity in gastroenterology. I am particularly passionate about ending disparities in colorectal cancer in minority communities and increasing awareness around minorities with inflammatory bowel disease.
I plan to work with health centers, city- and state-level organizations, and community partners to raise awareness around issues of equity in gastroenterology and develop interventions to create change. I will also work with local legislators and community-based organizations to advocate for policies that remove barriers to screening both locally and nationally. Further down the line, I am open to exploring careers in the public sector or health care administration if that is where my career takes me. The exposure that I had to these fields as part of the fellowship has shown me that it is possible to be a practicing gastroenterologist and simultaneously work in the public sector, health policy, or health care administration. If you are interested in applying to the Commonwealth Fund Fellowship in Minority Health Policy at Harvard University, please feel free to contact me at [email protected]. More information about the program and how to apply can be found at https://cff.hms.harvard.edu/.
Ethical considerations in nutrition support because of provider bias
Case:
A 37-year-old woman presents with severe emaciation (body mass index, 9.4 kg/m2) because of chronic severe avoidant/restrictive food intake disorder. She had asked for parenteral nutrition (PN) for several years, whenever her providers pushed her to accept nutrition support, as she had experienced extreme distress because of presumed gastroparesis with enteral feeds or any time she tried to eat. All of her many physicians refused the request for PN on the basis that her intestine was believed to be functioning and her symptoms were functional, so they insisted on tube feeding. The medical team was angered by the request for PN, and very concerned that providing it would support her belief that she could not eat, which they likened to a delusion. They opined that refusal of appropriate therapy (enteral nutrition) did not constitute an indication for inappropriate therapy (PN). They also deemed her to have capacity, so her refusal of tube feeding was honored. She continued to deteriorate, and because of her inability to travel, along with financial and insurance-related issues, was unable to seek alternative care providers. The family provided access to highly credible external consultants, and begged that her providers initiate PN as a life-saving measure. Both were declined. She was taken by her family to the emergency department when she began to have difficulty ambulating and increasing confusion. In recognition of the severity of her starvation, she was to be admitted to the critical care unit. With minimal monitoring while awaiting transfer from the emergency department overnight, she developed severe hypoglycemia and sustained cardiac arrest. Although spontaneous circulation was resumed, she sustained anoxic brain injury, and died after removal of life-sustaining treatment.
Ethical considerations
This case illustrates how the practice of caring for certain patients may come with deep unconscious determinants and conflicts of expectation – the duty to treat can be unclear in cases of refractory eating disorders. Multiple clinical teams were angry at the patient and her family for requesting PN and refused external input.
Although other eating disorders have received more attention, there is little research specific to avoidant/restrictive food intake disorder. There is some consensus that someone at a very low weight because of anorexia nervosa cannot, by definition, have decisional capacity with regard to feeding. Certainly, reviews cite cognitive dysfunction as a common finding, far worse during starvation, in patients with anorexia nervosa,1,2 and nourishment over objection has been advised.3 Further, it is known that gastric dysfunction occurs with some frequency in the presence of starvation in patients with eating disorders.4 Moreover, the potential risks of PN should be contextualized and compared with the certainty of death in someone this starved. Finally, if the patient’s refusal to eat or be tube fed were a delusion, which is by definition “fixed,” refusing to provide PN, and allowing further starvation, would not be expected to have benefit in resolution of the delusion.
Issues related to nourishment can be highly emotive – from “starving to death” on the one hand and “force feeding” on the other. Delivery of adequate nutrition and hydration is considered a basic human right, and must be offered as part of basic care. At the same time, we have observed that the request for nutrition support creates severe moral distress and anger among clinicians treating patients with eating disorders or with fatal illness. Does a delusion preclude feeding, even if by less than ideal means? How should a physician react to feeding treatments they deem excessive or unnecessary? Does a treating team have a duty to consider input from specialists with expertise specific to the patient when such conflict occurs between the patient/family and the treating team? Speculation exists that onset of anorexia nervosa may be linked to a postinfectious condition – a post–viral disease brain reprogramming.5,6 Would an organic explanation change our attitude toward patients with eating disorders?
Medicine’s emotive harms
Clinicians hold more negative attitudes toward certain patients – our implicit bias. It has been suggested that nice patients may be preferred by clinicians and therefore receive more humanistic care.7 Clinicians hold more negative attitudes toward patients with eating disorders than toward other patients. Cases of starvation caused by eating disorders are often seen by clinicians as a form of deviance, which provokes a visceral reaction of anger and frustration. These reactions have been associated with patients’ lack of improvement and personality pathology and with clinicians’ stigmatizing beliefs and inexperience.8 One could argue that this type of unconscious partiality may be worse than intentional harm.
Families and patients often request a treatment as a way to exert their agency. We clinicians may experience ethical dissonance as a result, whether because of ego or because the desired treatment is less favorable (for example, parenteral vs. enteral nutrition). Should maintaining clinical obstinance overrule patient and family autonomy, particularly in the face of the availability of life-saving intervention, even if less desirable than other standard treatments?
Should the physicians have better considered the relative risk of PN? What is the true potential harm? Would it benefit the patient or family? While PN’s benefit is usually life prolongation, it is not without risk of infection, potential mucosal atrophy of the unused gut, hepatic dysfunction, high cost, and an increased complexity of care. However, the incidence of blood stream infections in hospitalized patients receiving PN is only 1 episode for every 100 patient-days of treatment.9 On the other hand, weight regain is a significant determinant of success for treating eating disorders.10 Does the small risk of line-related sepsis, unlikely to be fatal, outweigh the certainty of death from starvation? What is the source of providers’ anger toward such patients? Even when providers feel any hope of improved outcome to be unreasonable, does refusal to provide nourishment, even if less than ideally, improve the likelihood the family will “come to grips” with the situation? Is there an obligation to consider our contribution to the emotional harm to the family because of our refusal, especially if coupled with anger?
Duty of life-saving care
Treating a competent patient without consent is unlawful. Autonomy is the dominant ethical principle, and a mentally competent person has the right to refuse consent to medical treatment for any reason, even when that decision may lead to death. Authors urge that patient lives should not be intentionally shortened, including the withholding of life-prolonging medical treatments or interventions.11,12 Although starvation can compromise capacity, whether patients with severe starvation have truly lost their mental competence and right to self-determination is debated.13 Do physicians have a duty to provide nutrition support by whatever route a patient will accept as a life-saving measure or at least until nutritional stability and improved mental status can be attained?
Next steps
Despite potential concerns clinicians may have over the risks and disadvantages of PN, reeducation of clinician emotional responses toward providing it is needed. As illustrated by this case study, there are likely situations, not fitting the norm, when PN is warranted as a life-saving measure. An awareness of implicit bias we may experience is paramount in all situations. Case-by-case multidisciplinary evaluations are warranted based on guidelines from professional organizations,14 alongside core ethical principles, when considering nutrition support.
References
1. Guillaume S et al. Psychol Med. 2015 Dec;45(16):3377-91.
2. Katzman DK et al. Semin Clin Neuropsychiatry. 2001 Apr;6(2):146-52.
3. Elzakkers IF et al. Int J Eat Disord. 2014 Dec;47(8):845-52.
4. Robinson PH et al. Gut. 1988 Apr;29(4):458-64.
5. Breithaupt L et al. JAMA Psychiatry. 2019 Apr 24;76(8):800-9.
6. Sokol MS. J Child Adolesc Psychopharmacol. 2000;10(2):133-45.
7. Detsky AS, Baerlocher MO. JAMA. 2011 Jul;306(1):94-5.
8. Thompson-Brenner H et al. Psychiatr Serv. 2012 Jan;63(1):73-8.
9. Fonseca G et al. JPEN J Parenter Enteral Nutr. 2018 Jan;42(1):171-5.
10. National Collaborating Centre for Mental Health. In: Eating disorders: Core interventions in the treatment and management of anorexia nervosa, bulimia nervosa and related eating disorders. Leicester, United Kingdom: British Psychological Society, 2004.
11. Keown J. Leg Stud. 2000 Mar;20(1):66-84.
12. Sayers GM et al. Postgrad Med J. 2006 Feb;82(964):79-83.
13. Miller I. BioSocieties. 2017;12:89-108.
14. A.S.P.E.N. Ethics Position Paper Task Force; Barrocas A et al. Nutr Clin Pract. 2010 Dec;25(6):672-9.
Dr. Anderson (@dochitect) is a clinical fellow in geriatric medicine at the University of California, San Francisco; Dr. Seres (@davidseres1) is an associate professor of medicine in the Institute of Human Nutrition, director of medical nutrition, and associate clinical ethicist at Columbia University Irving Medical Center, New York. They have no funding sources to declare and no conflicts of interest.
Case:
A 37-year-old woman presents with severe emaciation (body mass index, 9.4 kg/m2) because of chronic severe avoidant/restrictive food intake disorder. She had asked for parenteral nutrition (PN) for several years, whenever her providers pushed her to accept nutrition support, as she had experienced extreme distress because of presumed gastroparesis with enteral feeds or any time she tried to eat. All of her many physicians refused the request for PN on the basis that her intestine was believed to be functioning and her symptoms were functional, so they insisted on tube feeding. The medical team was angered by the request for PN, and very concerned that providing it would support her belief that she could not eat, which they likened to a delusion. They opined that refusal of appropriate therapy (enteral nutrition) did not constitute an indication for inappropriate therapy (PN). They also deemed her to have capacity, so her refusal of tube feeding was honored. She continued to deteriorate, and because of her inability to travel, along with financial and insurance-related issues, was unable to seek alternative care providers. The family provided access to highly credible external consultants, and begged that her providers initiate PN as a life-saving measure. Both were declined. She was taken by her family to the emergency department when she began to have difficulty ambulating and increasing confusion. In recognition of the severity of her starvation, she was to be admitted to the critical care unit. With minimal monitoring while awaiting transfer from the emergency department overnight, she developed severe hypoglycemia and sustained cardiac arrest. Although spontaneous circulation was resumed, she sustained anoxic brain injury, and died after removal of life-sustaining treatment.
Ethical considerations
This case illustrates how the practice of caring for certain patients may come with deep unconscious determinants and conflicts of expectation – the duty to treat can be unclear in cases of refractory eating disorders. Multiple clinical teams were angry at the patient and her family for requesting PN and refused external input.
Although other eating disorders have received more attention, there is little research specific to avoidant/restrictive food intake disorder. There is some consensus that someone at a very low weight because of anorexia nervosa cannot, by definition, have decisional capacity with regard to feeding. Certainly, reviews cite cognitive dysfunction as a common finding, far worse during starvation, in patients with anorexia nervosa,1,2 and nourishment over objection has been advised.3 Further, it is known that gastric dysfunction occurs with some frequency in the presence of starvation in patients with eating disorders.4 Moreover, the potential risks of PN should be contextualized and compared with the certainty of death in someone this starved. Finally, if the patient’s refusal to eat or be tube fed were a delusion, which is by definition “fixed,” refusing to provide PN, and allowing further starvation, would not be expected to have benefit in resolution of the delusion.
Issues related to nourishment can be highly emotive – from “starving to death” on the one hand and “force feeding” on the other. Delivery of adequate nutrition and hydration is considered a basic human right, and must be offered as part of basic care. At the same time, we have observed that the request for nutrition support creates severe moral distress and anger among clinicians treating patients with eating disorders or with fatal illness. Does a delusion preclude feeding, even if by less than ideal means? How should a physician react to feeding treatments they deem excessive or unnecessary? Does a treating team have a duty to consider input from specialists with expertise specific to the patient when such conflict occurs between the patient/family and the treating team? Speculation exists that onset of anorexia nervosa may be linked to a postinfectious condition – a post–viral disease brain reprogramming.5,6 Would an organic explanation change our attitude toward patients with eating disorders?
Medicine’s emotive harms
Clinicians hold more negative attitudes toward certain patients – our implicit bias. It has been suggested that nice patients may be preferred by clinicians and therefore receive more humanistic care.7 Clinicians hold more negative attitudes toward patients with eating disorders than toward other patients. Cases of starvation caused by eating disorders are often seen by clinicians as a form of deviance, which provokes a visceral reaction of anger and frustration. These reactions have been associated with patients’ lack of improvement and personality pathology and with clinicians’ stigmatizing beliefs and inexperience.8 One could argue that this type of unconscious partiality may be worse than intentional harm.
Families and patients often request a treatment as a way to exert their agency. We clinicians may experience ethical dissonance as a result, whether because of ego or because the desired treatment is less favorable (for example, parenteral vs. enteral nutrition). Should maintaining clinical obstinance overrule patient and family autonomy, particularly in the face of the availability of life-saving intervention, even if less desirable than other standard treatments?
Should the physicians have better considered the relative risk of PN? What is the true potential harm? Would it benefit the patient or family? While PN’s benefit is usually life prolongation, it is not without risk of infection, potential mucosal atrophy of the unused gut, hepatic dysfunction, high cost, and an increased complexity of care. However, the incidence of blood stream infections in hospitalized patients receiving PN is only 1 episode for every 100 patient-days of treatment.9 On the other hand, weight regain is a significant determinant of success for treating eating disorders.10 Does the small risk of line-related sepsis, unlikely to be fatal, outweigh the certainty of death from starvation? What is the source of providers’ anger toward such patients? Even when providers feel any hope of improved outcome to be unreasonable, does refusal to provide nourishment, even if less than ideally, improve the likelihood the family will “come to grips” with the situation? Is there an obligation to consider our contribution to the emotional harm to the family because of our refusal, especially if coupled with anger?
Duty of life-saving care
Treating a competent patient without consent is unlawful. Autonomy is the dominant ethical principle, and a mentally competent person has the right to refuse consent to medical treatment for any reason, even when that decision may lead to death. Authors urge that patient lives should not be intentionally shortened, including the withholding of life-prolonging medical treatments or interventions.11,12 Although starvation can compromise capacity, whether patients with severe starvation have truly lost their mental competence and right to self-determination is debated.13 Do physicians have a duty to provide nutrition support by whatever route a patient will accept as a life-saving measure or at least until nutritional stability and improved mental status can be attained?
Next steps
Despite potential concerns clinicians may have over the risks and disadvantages of PN, reeducation of clinician emotional responses toward providing it is needed. As illustrated by this case study, there are likely situations, not fitting the norm, when PN is warranted as a life-saving measure. An awareness of implicit bias we may experience is paramount in all situations. Case-by-case multidisciplinary evaluations are warranted based on guidelines from professional organizations,14 alongside core ethical principles, when considering nutrition support.
References
1. Guillaume S et al. Psychol Med. 2015 Dec;45(16):3377-91.
2. Katzman DK et al. Semin Clin Neuropsychiatry. 2001 Apr;6(2):146-52.
3. Elzakkers IF et al. Int J Eat Disord. 2014 Dec;47(8):845-52.
4. Robinson PH et al. Gut. 1988 Apr;29(4):458-64.
5. Breithaupt L et al. JAMA Psychiatry. 2019 Apr 24;76(8):800-9.
6. Sokol MS. J Child Adolesc Psychopharmacol. 2000;10(2):133-45.
7. Detsky AS, Baerlocher MO. JAMA. 2011 Jul;306(1):94-5.
8. Thompson-Brenner H et al. Psychiatr Serv. 2012 Jan;63(1):73-8.
9. Fonseca G et al. JPEN J Parenter Enteral Nutr. 2018 Jan;42(1):171-5.
10. National Collaborating Centre for Mental Health. In: Eating disorders: Core interventions in the treatment and management of anorexia nervosa, bulimia nervosa and related eating disorders. Leicester, United Kingdom: British Psychological Society, 2004.
11. Keown J. Leg Stud. 2000 Mar;20(1):66-84.
12. Sayers GM et al. Postgrad Med J. 2006 Feb;82(964):79-83.
13. Miller I. BioSocieties. 2017;12:89-108.
14. A.S.P.E.N. Ethics Position Paper Task Force; Barrocas A et al. Nutr Clin Pract. 2010 Dec;25(6):672-9.
Dr. Anderson (@dochitect) is a clinical fellow in geriatric medicine at the University of California, San Francisco; Dr. Seres (@davidseres1) is an associate professor of medicine in the Institute of Human Nutrition, director of medical nutrition, and associate clinical ethicist at Columbia University Irving Medical Center, New York. They have no funding sources to declare and no conflicts of interest.
Case:
A 37-year-old woman presents with severe emaciation (body mass index, 9.4 kg/m2) because of chronic severe avoidant/restrictive food intake disorder. She had asked for parenteral nutrition (PN) for several years, whenever her providers pushed her to accept nutrition support, as she had experienced extreme distress because of presumed gastroparesis with enteral feeds or any time she tried to eat. All of her many physicians refused the request for PN on the basis that her intestine was believed to be functioning and her symptoms were functional, so they insisted on tube feeding. The medical team was angered by the request for PN, and very concerned that providing it would support her belief that she could not eat, which they likened to a delusion. They opined that refusal of appropriate therapy (enteral nutrition) did not constitute an indication for inappropriate therapy (PN). They also deemed her to have capacity, so her refusal of tube feeding was honored. She continued to deteriorate, and because of her inability to travel, along with financial and insurance-related issues, was unable to seek alternative care providers. The family provided access to highly credible external consultants, and begged that her providers initiate PN as a life-saving measure. Both were declined. She was taken by her family to the emergency department when she began to have difficulty ambulating and increasing confusion. In recognition of the severity of her starvation, she was to be admitted to the critical care unit. With minimal monitoring while awaiting transfer from the emergency department overnight, she developed severe hypoglycemia and sustained cardiac arrest. Although spontaneous circulation was resumed, she sustained anoxic brain injury, and died after removal of life-sustaining treatment.
Ethical considerations
This case illustrates how the practice of caring for certain patients may come with deep unconscious determinants and conflicts of expectation – the duty to treat can be unclear in cases of refractory eating disorders. Multiple clinical teams were angry at the patient and her family for requesting PN and refused external input.
Although other eating disorders have received more attention, there is little research specific to avoidant/restrictive food intake disorder. There is some consensus that someone at a very low weight because of anorexia nervosa cannot, by definition, have decisional capacity with regard to feeding. Certainly, reviews cite cognitive dysfunction as a common finding, far worse during starvation, in patients with anorexia nervosa,1,2 and nourishment over objection has been advised.3 Further, it is known that gastric dysfunction occurs with some frequency in the presence of starvation in patients with eating disorders.4 Moreover, the potential risks of PN should be contextualized and compared with the certainty of death in someone this starved. Finally, if the patient’s refusal to eat or be tube fed were a delusion, which is by definition “fixed,” refusing to provide PN, and allowing further starvation, would not be expected to have benefit in resolution of the delusion.
Issues related to nourishment can be highly emotive – from “starving to death” on the one hand and “force feeding” on the other. Delivery of adequate nutrition and hydration is considered a basic human right, and must be offered as part of basic care. At the same time, we have observed that the request for nutrition support creates severe moral distress and anger among clinicians treating patients with eating disorders or with fatal illness. Does a delusion preclude feeding, even if by less than ideal means? How should a physician react to feeding treatments they deem excessive or unnecessary? Does a treating team have a duty to consider input from specialists with expertise specific to the patient when such conflict occurs between the patient/family and the treating team? Speculation exists that onset of anorexia nervosa may be linked to a postinfectious condition – a post–viral disease brain reprogramming.5,6 Would an organic explanation change our attitude toward patients with eating disorders?
Medicine’s emotive harms
Clinicians hold more negative attitudes toward certain patients – our implicit bias. It has been suggested that nice patients may be preferred by clinicians and therefore receive more humanistic care.7 Clinicians hold more negative attitudes toward patients with eating disorders than toward other patients. Cases of starvation caused by eating disorders are often seen by clinicians as a form of deviance, which provokes a visceral reaction of anger and frustration. These reactions have been associated with patients’ lack of improvement and personality pathology and with clinicians’ stigmatizing beliefs and inexperience.8 One could argue that this type of unconscious partiality may be worse than intentional harm.
Families and patients often request a treatment as a way to exert their agency. We clinicians may experience ethical dissonance as a result, whether because of ego or because the desired treatment is less favorable (for example, parenteral vs. enteral nutrition). Should maintaining clinical obstinance overrule patient and family autonomy, particularly in the face of the availability of life-saving intervention, even if less desirable than other standard treatments?
Should the physicians have better considered the relative risk of PN? What is the true potential harm? Would it benefit the patient or family? While PN’s benefit is usually life prolongation, it is not without risk of infection, potential mucosal atrophy of the unused gut, hepatic dysfunction, high cost, and an increased complexity of care. However, the incidence of blood stream infections in hospitalized patients receiving PN is only 1 episode for every 100 patient-days of treatment.9 On the other hand, weight regain is a significant determinant of success for treating eating disorders.10 Does the small risk of line-related sepsis, unlikely to be fatal, outweigh the certainty of death from starvation? What is the source of providers’ anger toward such patients? Even when providers feel any hope of improved outcome to be unreasonable, does refusal to provide nourishment, even if less than ideally, improve the likelihood the family will “come to grips” with the situation? Is there an obligation to consider our contribution to the emotional harm to the family because of our refusal, especially if coupled with anger?
Duty of life-saving care
Treating a competent patient without consent is unlawful. Autonomy is the dominant ethical principle, and a mentally competent person has the right to refuse consent to medical treatment for any reason, even when that decision may lead to death. Authors urge that patient lives should not be intentionally shortened, including the withholding of life-prolonging medical treatments or interventions.11,12 Although starvation can compromise capacity, whether patients with severe starvation have truly lost their mental competence and right to self-determination is debated.13 Do physicians have a duty to provide nutrition support by whatever route a patient will accept as a life-saving measure or at least until nutritional stability and improved mental status can be attained?
Next steps
Despite potential concerns clinicians may have over the risks and disadvantages of PN, reeducation of clinician emotional responses toward providing it is needed. As illustrated by this case study, there are likely situations, not fitting the norm, when PN is warranted as a life-saving measure. An awareness of implicit bias we may experience is paramount in all situations. Case-by-case multidisciplinary evaluations are warranted based on guidelines from professional organizations,14 alongside core ethical principles, when considering nutrition support.
References
1. Guillaume S et al. Psychol Med. 2015 Dec;45(16):3377-91.
2. Katzman DK et al. Semin Clin Neuropsychiatry. 2001 Apr;6(2):146-52.
3. Elzakkers IF et al. Int J Eat Disord. 2014 Dec;47(8):845-52.
4. Robinson PH et al. Gut. 1988 Apr;29(4):458-64.
5. Breithaupt L et al. JAMA Psychiatry. 2019 Apr 24;76(8):800-9.
6. Sokol MS. J Child Adolesc Psychopharmacol. 2000;10(2):133-45.
7. Detsky AS, Baerlocher MO. JAMA. 2011 Jul;306(1):94-5.
8. Thompson-Brenner H et al. Psychiatr Serv. 2012 Jan;63(1):73-8.
9. Fonseca G et al. JPEN J Parenter Enteral Nutr. 2018 Jan;42(1):171-5.
10. National Collaborating Centre for Mental Health. In: Eating disorders: Core interventions in the treatment and management of anorexia nervosa, bulimia nervosa and related eating disorders. Leicester, United Kingdom: British Psychological Society, 2004.
11. Keown J. Leg Stud. 2000 Mar;20(1):66-84.
12. Sayers GM et al. Postgrad Med J. 2006 Feb;82(964):79-83.
13. Miller I. BioSocieties. 2017;12:89-108.
14. A.S.P.E.N. Ethics Position Paper Task Force; Barrocas A et al. Nutr Clin Pract. 2010 Dec;25(6):672-9.
Dr. Anderson (@dochitect) is a clinical fellow in geriatric medicine at the University of California, San Francisco; Dr. Seres (@davidseres1) is an associate professor of medicine in the Institute of Human Nutrition, director of medical nutrition, and associate clinical ethicist at Columbia University Irving Medical Center, New York. They have no funding sources to declare and no conflicts of interest.
So you want to be an expert witness?
Acting as an expert witness in a legal matter can be a nice way to compliment your practice. However, it is important to understand the role of experts, as well as their duties and obligations. Expert witnesses are called to testify on the basis of their specialized knowledge, not necessarily their direct knowledge of events and issues in the case.
Medical experts often play an important role in the evaluation, development, and preparation of a case long before it ever goes to trial. In some states, to even file a medical malpractice complaint a plaintiff is required to have the case evaluated by an expert and obtain a written report outlining why the plaintiff has a reasonable and meritorious cause for filing such an action.
There are different types of expert witness testimony. Experts can give opinion testimony as a physician who provided treatment to the plaintiff and whose conduct is not at issue. The second type of expert witness is a retained or controlled expert witness. This is a person giving opinion testimony after being retained by a lawyer on behalf of one of the parties to the lawsuit.
Before you give deposition or trial testimony, your opinions must be disclosed in writing and provided to the other parties in the case. In federal court, this is governed by Federal Rule of Civil Procedure 26. If the case is pending in state court, your written opinions are governed by local court rules. In both cases, the written opinions should be thorough and complete because you will not be allowed to testify to new opinions at the time of trial but will generally be allowed to expand upon those disclosed in writing at your deposition trial.
In order for a jury to hear your opinions at trial, your opinions must be reliable. In federal court, expert testimony is governed by Federal Rule of Evidence 702, which states:
A witness who is qualified as an expert by knowledge, skill, experience, training, or education may testify in the form of an opinion or otherwise if:
a) the expert’s scientific, technical, or other specialized knowledge will help the trier of fact to understand the evidence or to determine a fact in issue;
b) the testimony is based on sufficient facts or data;
c) the testimony is the product of reliable principles and methods; and
d) the expert has reliably applied the principles and methods to the facts of the case.
This means, that if a fact or evidence at issue involves scientific, technical, or specialized knowledge that is outside the scope of an ordinary layman’s experience, or involves complex issues challenging a layman’s comprehension, expert testimony is required. The scientific evidence must not just be relevant but also reliable. Expert opinions will be scrutinized to see if they are based on scientific testing or review of scientific data rather than just assumptions or speculation. Additionally, the experts must be qualified by their knowledge, skill, experience, training, or education. Given these parameters, it should come as no surprise that expert trial testimony is required for all medical malpractice cases.
Some states follow the “new or novel rule” which dictates that expert testimony is only admissible if the methodology or scientific principal on which the opinion is based is sufficiently established to have gained general acceptance in the particular field in which it belongs. This means that the evidence must be generally accepted as reliable in the relevant scientific community. New or novel techniques will be placed under the scrutiny of this standard. Courts will look at papers, books, journals, and case law to make a determination as to the reliability and general acceptance. Failure to meet the requisite standards may render a physician ineligible to testify.
If you are considering acting as an expert witness there are a few basic dos and don’ts to keep in mind:
Do be mindful of your criticism. If testifying in a medical malpractice case, you will be giving sworn testimony as to whether another physician deviated from the standard of care. Be aware that your testimony can later be used against you if your conduct is ever at issue, or if you contradict yourself in another case. Attorneys often look for prior testimony to use when questioning you at deposition and trial.
Do be aware of any applicable professional society guidelines. Many professional societies publish ethical guidelines as it relates to expert medical testimony. Be aware of those and know that you may be asked about them, especially if you are a member of that society.
Do be prepared for basic areas of cross-examination. There are a few tried and true areas that will always be the subject of cross-examination. Any perceived bias you may have, your fees, and whether you do more work for plaintiffs versus defendants are a just few examples. You should also be prepared to be cross-examined on the differences between personal practice (what you do) and an actual deviation from the standard of care.
Do keep written communication to a minimum. All communication between the expert physician and the attorney is potentially discoverable by the other side. The rules differ for state and federal courts. Emails, draft reports, and written questions all cause the creation of unnecessary side issues and areas of cross-examination. The best practice is for all substantive communication to be done by phone.
Do be clear in what you are charging. It is not unusual for an expert to charge one hourly rate for record review, and a different rate for testimony. Your fee schedule should also note that any travel expenses you incur will also be invoiced. Your hourly rate should be appropriate for your area of practice. In our experience, gastroenterologists typically charge $400.00-$600.00 an hour for record review, and $550.00-$700.00 an hour for testimony.
Do not submit an invoice until after your deposition. Submitting invoices before your deposition creates unnecessary cross-examination issues. At the time of retention, speak to the attorney and ask if you will be able to submit invoices as you work. Most attorneys prefer invoices be submitted after your deposition. Because the wheels of justice often turn slowly, you could be waiting an equally long time to submit an invoice and get paid. One way to avoid this dilemma is to require a retainer at the time of retention.
Do not sign up with an expert finder service. Resist the urge to sign up with an expert finder service. The best medical experts come from referrals from other attorneys or physicians. Expert retention via an expert finder service creates the impression that you are a “hired gun” in the business of being a professional expert and can diminish your credibility. The finder services also charge a commission or fee.
As a gastroenterologist, you have the specialized knowledge to provide expert testimony regarding the cause of an injury and extent of damages in cases where you have treated a patient. You also have the type of education and training necessary to serve as an independent expert. Doing so is a serious task that can be time consuming and stressful. However, it can also be rewarding and allow you to make sure a fair and just outcome occurs.
This article is for general informational purposes only. Please consult your own attorney if you have questions. This information is not intended to create an attorney-client relationship.
Mr. Mills is an equity partner at Cunningham, Meyer & Vedrine PC in Chicago. Ms. Lindbert is a partner at Cunningham, Meyer & Vedrine PC. Both focus their practices on defending doctors and hospitals in medical malpractice actions.
Acting as an expert witness in a legal matter can be a nice way to compliment your practice. However, it is important to understand the role of experts, as well as their duties and obligations. Expert witnesses are called to testify on the basis of their specialized knowledge, not necessarily their direct knowledge of events and issues in the case.
Medical experts often play an important role in the evaluation, development, and preparation of a case long before it ever goes to trial. In some states, to even file a medical malpractice complaint a plaintiff is required to have the case evaluated by an expert and obtain a written report outlining why the plaintiff has a reasonable and meritorious cause for filing such an action.
There are different types of expert witness testimony. Experts can give opinion testimony as a physician who provided treatment to the plaintiff and whose conduct is not at issue. The second type of expert witness is a retained or controlled expert witness. This is a person giving opinion testimony after being retained by a lawyer on behalf of one of the parties to the lawsuit.
Before you give deposition or trial testimony, your opinions must be disclosed in writing and provided to the other parties in the case. In federal court, this is governed by Federal Rule of Civil Procedure 26. If the case is pending in state court, your written opinions are governed by local court rules. In both cases, the written opinions should be thorough and complete because you will not be allowed to testify to new opinions at the time of trial but will generally be allowed to expand upon those disclosed in writing at your deposition trial.
In order for a jury to hear your opinions at trial, your opinions must be reliable. In federal court, expert testimony is governed by Federal Rule of Evidence 702, which states:
A witness who is qualified as an expert by knowledge, skill, experience, training, or education may testify in the form of an opinion or otherwise if:
a) the expert’s scientific, technical, or other specialized knowledge will help the trier of fact to understand the evidence or to determine a fact in issue;
b) the testimony is based on sufficient facts or data;
c) the testimony is the product of reliable principles and methods; and
d) the expert has reliably applied the principles and methods to the facts of the case.
This means, that if a fact or evidence at issue involves scientific, technical, or specialized knowledge that is outside the scope of an ordinary layman’s experience, or involves complex issues challenging a layman’s comprehension, expert testimony is required. The scientific evidence must not just be relevant but also reliable. Expert opinions will be scrutinized to see if they are based on scientific testing or review of scientific data rather than just assumptions or speculation. Additionally, the experts must be qualified by their knowledge, skill, experience, training, or education. Given these parameters, it should come as no surprise that expert trial testimony is required for all medical malpractice cases.
Some states follow the “new or novel rule” which dictates that expert testimony is only admissible if the methodology or scientific principal on which the opinion is based is sufficiently established to have gained general acceptance in the particular field in which it belongs. This means that the evidence must be generally accepted as reliable in the relevant scientific community. New or novel techniques will be placed under the scrutiny of this standard. Courts will look at papers, books, journals, and case law to make a determination as to the reliability and general acceptance. Failure to meet the requisite standards may render a physician ineligible to testify.
If you are considering acting as an expert witness there are a few basic dos and don’ts to keep in mind:
Do be mindful of your criticism. If testifying in a medical malpractice case, you will be giving sworn testimony as to whether another physician deviated from the standard of care. Be aware that your testimony can later be used against you if your conduct is ever at issue, or if you contradict yourself in another case. Attorneys often look for prior testimony to use when questioning you at deposition and trial.
Do be aware of any applicable professional society guidelines. Many professional societies publish ethical guidelines as it relates to expert medical testimony. Be aware of those and know that you may be asked about them, especially if you are a member of that society.
Do be prepared for basic areas of cross-examination. There are a few tried and true areas that will always be the subject of cross-examination. Any perceived bias you may have, your fees, and whether you do more work for plaintiffs versus defendants are a just few examples. You should also be prepared to be cross-examined on the differences between personal practice (what you do) and an actual deviation from the standard of care.
Do keep written communication to a minimum. All communication between the expert physician and the attorney is potentially discoverable by the other side. The rules differ for state and federal courts. Emails, draft reports, and written questions all cause the creation of unnecessary side issues and areas of cross-examination. The best practice is for all substantive communication to be done by phone.
Do be clear in what you are charging. It is not unusual for an expert to charge one hourly rate for record review, and a different rate for testimony. Your fee schedule should also note that any travel expenses you incur will also be invoiced. Your hourly rate should be appropriate for your area of practice. In our experience, gastroenterologists typically charge $400.00-$600.00 an hour for record review, and $550.00-$700.00 an hour for testimony.
Do not submit an invoice until after your deposition. Submitting invoices before your deposition creates unnecessary cross-examination issues. At the time of retention, speak to the attorney and ask if you will be able to submit invoices as you work. Most attorneys prefer invoices be submitted after your deposition. Because the wheels of justice often turn slowly, you could be waiting an equally long time to submit an invoice and get paid. One way to avoid this dilemma is to require a retainer at the time of retention.
Do not sign up with an expert finder service. Resist the urge to sign up with an expert finder service. The best medical experts come from referrals from other attorneys or physicians. Expert retention via an expert finder service creates the impression that you are a “hired gun” in the business of being a professional expert and can diminish your credibility. The finder services also charge a commission or fee.
As a gastroenterologist, you have the specialized knowledge to provide expert testimony regarding the cause of an injury and extent of damages in cases where you have treated a patient. You also have the type of education and training necessary to serve as an independent expert. Doing so is a serious task that can be time consuming and stressful. However, it can also be rewarding and allow you to make sure a fair and just outcome occurs.
This article is for general informational purposes only. Please consult your own attorney if you have questions. This information is not intended to create an attorney-client relationship.
Mr. Mills is an equity partner at Cunningham, Meyer & Vedrine PC in Chicago. Ms. Lindbert is a partner at Cunningham, Meyer & Vedrine PC. Both focus their practices on defending doctors and hospitals in medical malpractice actions.
Acting as an expert witness in a legal matter can be a nice way to compliment your practice. However, it is important to understand the role of experts, as well as their duties and obligations. Expert witnesses are called to testify on the basis of their specialized knowledge, not necessarily their direct knowledge of events and issues in the case.
Medical experts often play an important role in the evaluation, development, and preparation of a case long before it ever goes to trial. In some states, to even file a medical malpractice complaint a plaintiff is required to have the case evaluated by an expert and obtain a written report outlining why the plaintiff has a reasonable and meritorious cause for filing such an action.
There are different types of expert witness testimony. Experts can give opinion testimony as a physician who provided treatment to the plaintiff and whose conduct is not at issue. The second type of expert witness is a retained or controlled expert witness. This is a person giving opinion testimony after being retained by a lawyer on behalf of one of the parties to the lawsuit.
Before you give deposition or trial testimony, your opinions must be disclosed in writing and provided to the other parties in the case. In federal court, this is governed by Federal Rule of Civil Procedure 26. If the case is pending in state court, your written opinions are governed by local court rules. In both cases, the written opinions should be thorough and complete because you will not be allowed to testify to new opinions at the time of trial but will generally be allowed to expand upon those disclosed in writing at your deposition trial.
In order for a jury to hear your opinions at trial, your opinions must be reliable. In federal court, expert testimony is governed by Federal Rule of Evidence 702, which states:
A witness who is qualified as an expert by knowledge, skill, experience, training, or education may testify in the form of an opinion or otherwise if:
a) the expert’s scientific, technical, or other specialized knowledge will help the trier of fact to understand the evidence or to determine a fact in issue;
b) the testimony is based on sufficient facts or data;
c) the testimony is the product of reliable principles and methods; and
d) the expert has reliably applied the principles and methods to the facts of the case.
This means, that if a fact or evidence at issue involves scientific, technical, or specialized knowledge that is outside the scope of an ordinary layman’s experience, or involves complex issues challenging a layman’s comprehension, expert testimony is required. The scientific evidence must not just be relevant but also reliable. Expert opinions will be scrutinized to see if they are based on scientific testing or review of scientific data rather than just assumptions or speculation. Additionally, the experts must be qualified by their knowledge, skill, experience, training, or education. Given these parameters, it should come as no surprise that expert trial testimony is required for all medical malpractice cases.
Some states follow the “new or novel rule” which dictates that expert testimony is only admissible if the methodology or scientific principal on which the opinion is based is sufficiently established to have gained general acceptance in the particular field in which it belongs. This means that the evidence must be generally accepted as reliable in the relevant scientific community. New or novel techniques will be placed under the scrutiny of this standard. Courts will look at papers, books, journals, and case law to make a determination as to the reliability and general acceptance. Failure to meet the requisite standards may render a physician ineligible to testify.
If you are considering acting as an expert witness there are a few basic dos and don’ts to keep in mind:
Do be mindful of your criticism. If testifying in a medical malpractice case, you will be giving sworn testimony as to whether another physician deviated from the standard of care. Be aware that your testimony can later be used against you if your conduct is ever at issue, or if you contradict yourself in another case. Attorneys often look for prior testimony to use when questioning you at deposition and trial.
Do be aware of any applicable professional society guidelines. Many professional societies publish ethical guidelines as it relates to expert medical testimony. Be aware of those and know that you may be asked about them, especially if you are a member of that society.
Do be prepared for basic areas of cross-examination. There are a few tried and true areas that will always be the subject of cross-examination. Any perceived bias you may have, your fees, and whether you do more work for plaintiffs versus defendants are a just few examples. You should also be prepared to be cross-examined on the differences between personal practice (what you do) and an actual deviation from the standard of care.
Do keep written communication to a minimum. All communication between the expert physician and the attorney is potentially discoverable by the other side. The rules differ for state and federal courts. Emails, draft reports, and written questions all cause the creation of unnecessary side issues and areas of cross-examination. The best practice is for all substantive communication to be done by phone.
Do be clear in what you are charging. It is not unusual for an expert to charge one hourly rate for record review, and a different rate for testimony. Your fee schedule should also note that any travel expenses you incur will also be invoiced. Your hourly rate should be appropriate for your area of practice. In our experience, gastroenterologists typically charge $400.00-$600.00 an hour for record review, and $550.00-$700.00 an hour for testimony.
Do not submit an invoice until after your deposition. Submitting invoices before your deposition creates unnecessary cross-examination issues. At the time of retention, speak to the attorney and ask if you will be able to submit invoices as you work. Most attorneys prefer invoices be submitted after your deposition. Because the wheels of justice often turn slowly, you could be waiting an equally long time to submit an invoice and get paid. One way to avoid this dilemma is to require a retainer at the time of retention.
Do not sign up with an expert finder service. Resist the urge to sign up with an expert finder service. The best medical experts come from referrals from other attorneys or physicians. Expert retention via an expert finder service creates the impression that you are a “hired gun” in the business of being a professional expert and can diminish your credibility. The finder services also charge a commission or fee.
As a gastroenterologist, you have the specialized knowledge to provide expert testimony regarding the cause of an injury and extent of damages in cases where you have treated a patient. You also have the type of education and training necessary to serve as an independent expert. Doing so is a serious task that can be time consuming and stressful. However, it can also be rewarding and allow you to make sure a fair and just outcome occurs.
This article is for general informational purposes only. Please consult your own attorney if you have questions. This information is not intended to create an attorney-client relationship.
Mr. Mills is an equity partner at Cunningham, Meyer & Vedrine PC in Chicago. Ms. Lindbert is a partner at Cunningham, Meyer & Vedrine PC. Both focus their practices on defending doctors and hospitals in medical malpractice actions.
Remaining connected
Dear colleagues,
We bring you the spring edition of The New Gastroenterologist amid a backdrop of uncertainty in the setting of the novel coronavirus disease 2019 (COVID-19) pandemic. As physicians, we are poised to view this unprecedented situation in modern medicine through a unique lens. At the time of this writing, we are experiencing significant interruptions to our work as gastroenterologists coupled with the possibility of reassignments in order to care for COVID-19 patients to meet the demand of the precipitous rise in cases. Weighing these responsibilities, along with the heightened concern about the threat of exposure to ourselves and our families, is a formidable challenge, but one that we can navigate together.
My sincere hope is that this quarter’s newsletter can provide, at the very least, a brief reprieve from some of these constant stressors. It is during times like this that remaining connected to our colleagues through digital platforms and publications such as The New Gastroenterologist remains of utmost importance.
That being said, I felt it was prudent to first address some common concerns regarding the COVID-19 pandemic, specifically, its implications within gastroenterology. In conjunction with Krishna Rao (University of Michigan), a specialist in infectious diseases, we attempt to shed some light on what is a rapidly evolving situation. For more resources from the American Gastroenterological Association (AGA) on up-to-date clinical guidance and research, you can also visit https://www.gastro.org/practice-guidance/practice-updates/covid-19.
Moving on to our “In Focus” feature, Thangam Ventakesan and Harrison Mooers (Medical College of Wisconsin) provide a comprehensive overview of cyclic vomiting syndrome. This is a valuable read as cyclic vomiting syndrome has been gaining increased recognition among adults, and Dr. Ventakesan and Dr. Mooers elucidate a thorough approach to the diagnosis and treatment of this disorder.
A facet of endoscopy that is extremely important, but frequently overlooked, is ergonomics. Manish Singla and Jared Magee (Walter Reed National Military Medical Center) compile a high-yield list of recommendations on the best practices to preserve our own safety and health as endoscopists.
We continue our medical ethics series with Jennifer Wang and Andrew Aronsohn (University of Chicago) who offer a thought-provoking discussion on the role of early liver transplantation for alcoholic hepatitis, including an analysis of the medical, psychosocial, and ethical considerations.
Also in this issue, Animesh Jain (University of North Carolina) gives us some excellent financial advice on student loan management, outlining a basic strategy of repayment with clear explanations of the available options including refinancing, public service loan forgiveness, and income-driven repayment.
Dilhana Badurdeen (Johns Hopkins), Aline Charabaty Pishvaian (Sibley Memorial Hospital), Miguel Malespin (University of South Florida), Ibironke Oduyebo (Midatlantic Permanente Medical Group), and Sandra Quezada (University of Maryland) give us an in-depth summary of the efforts of the AGA’s Diversity Committee, including publications, events, and future initiatives.
This quarter’s DHPA Private Practice Perspectives series features Paul Berggreen (Arizona Digestive Health), who reviews the advantages and disadvantages of pathology lab ownership as a gastroenterologist. Lastly, Sarah Ordway, Dawn Torres, Manish Singla, and Adam Tritsch (Walter Reed National Military Medical Center) broach the issue of fellowship burnout by providing guidance on how to identify signs and those at risk in addition to providing tangible solutions that any fellowship can incorporate.
Although the cancellation of the upcoming DDW meetings in Chicago is a disappointment, I hope that we can all take this time to prioritize the well-being of ourselves and our communities until we meet again.
As always, if you have interest in contributing or have ideas for future TNG topics, please contact me ([email protected]), or Ryan Farrell ([email protected]), managing editor of TNG.
Best wishes to stay safe and healthy.
Vijaya L. Rao, MD
Editor in Chief
Dr. Rao is assistant professor of medicine, University of Chicago, section of gastroenterology, hepatology & nutrition.
Dear colleagues,
We bring you the spring edition of The New Gastroenterologist amid a backdrop of uncertainty in the setting of the novel coronavirus disease 2019 (COVID-19) pandemic. As physicians, we are poised to view this unprecedented situation in modern medicine through a unique lens. At the time of this writing, we are experiencing significant interruptions to our work as gastroenterologists coupled with the possibility of reassignments in order to care for COVID-19 patients to meet the demand of the precipitous rise in cases. Weighing these responsibilities, along with the heightened concern about the threat of exposure to ourselves and our families, is a formidable challenge, but one that we can navigate together.
My sincere hope is that this quarter’s newsletter can provide, at the very least, a brief reprieve from some of these constant stressors. It is during times like this that remaining connected to our colleagues through digital platforms and publications such as The New Gastroenterologist remains of utmost importance.
That being said, I felt it was prudent to first address some common concerns regarding the COVID-19 pandemic, specifically, its implications within gastroenterology. In conjunction with Krishna Rao (University of Michigan), a specialist in infectious diseases, we attempt to shed some light on what is a rapidly evolving situation. For more resources from the American Gastroenterological Association (AGA) on up-to-date clinical guidance and research, you can also visit https://www.gastro.org/practice-guidance/practice-updates/covid-19.
Moving on to our “In Focus” feature, Thangam Ventakesan and Harrison Mooers (Medical College of Wisconsin) provide a comprehensive overview of cyclic vomiting syndrome. This is a valuable read as cyclic vomiting syndrome has been gaining increased recognition among adults, and Dr. Ventakesan and Dr. Mooers elucidate a thorough approach to the diagnosis and treatment of this disorder.
A facet of endoscopy that is extremely important, but frequently overlooked, is ergonomics. Manish Singla and Jared Magee (Walter Reed National Military Medical Center) compile a high-yield list of recommendations on the best practices to preserve our own safety and health as endoscopists.
We continue our medical ethics series with Jennifer Wang and Andrew Aronsohn (University of Chicago) who offer a thought-provoking discussion on the role of early liver transplantation for alcoholic hepatitis, including an analysis of the medical, psychosocial, and ethical considerations.
Also in this issue, Animesh Jain (University of North Carolina) gives us some excellent financial advice on student loan management, outlining a basic strategy of repayment with clear explanations of the available options including refinancing, public service loan forgiveness, and income-driven repayment.
Dilhana Badurdeen (Johns Hopkins), Aline Charabaty Pishvaian (Sibley Memorial Hospital), Miguel Malespin (University of South Florida), Ibironke Oduyebo (Midatlantic Permanente Medical Group), and Sandra Quezada (University of Maryland) give us an in-depth summary of the efforts of the AGA’s Diversity Committee, including publications, events, and future initiatives.
This quarter’s DHPA Private Practice Perspectives series features Paul Berggreen (Arizona Digestive Health), who reviews the advantages and disadvantages of pathology lab ownership as a gastroenterologist. Lastly, Sarah Ordway, Dawn Torres, Manish Singla, and Adam Tritsch (Walter Reed National Military Medical Center) broach the issue of fellowship burnout by providing guidance on how to identify signs and those at risk in addition to providing tangible solutions that any fellowship can incorporate.
Although the cancellation of the upcoming DDW meetings in Chicago is a disappointment, I hope that we can all take this time to prioritize the well-being of ourselves and our communities until we meet again.
As always, if you have interest in contributing or have ideas for future TNG topics, please contact me ([email protected]), or Ryan Farrell ([email protected]), managing editor of TNG.
Best wishes to stay safe and healthy.
Vijaya L. Rao, MD
Editor in Chief
Dr. Rao is assistant professor of medicine, University of Chicago, section of gastroenterology, hepatology & nutrition.
Dear colleagues,
We bring you the spring edition of The New Gastroenterologist amid a backdrop of uncertainty in the setting of the novel coronavirus disease 2019 (COVID-19) pandemic. As physicians, we are poised to view this unprecedented situation in modern medicine through a unique lens. At the time of this writing, we are experiencing significant interruptions to our work as gastroenterologists coupled with the possibility of reassignments in order to care for COVID-19 patients to meet the demand of the precipitous rise in cases. Weighing these responsibilities, along with the heightened concern about the threat of exposure to ourselves and our families, is a formidable challenge, but one that we can navigate together.
My sincere hope is that this quarter’s newsletter can provide, at the very least, a brief reprieve from some of these constant stressors. It is during times like this that remaining connected to our colleagues through digital platforms and publications such as The New Gastroenterologist remains of utmost importance.
That being said, I felt it was prudent to first address some common concerns regarding the COVID-19 pandemic, specifically, its implications within gastroenterology. In conjunction with Krishna Rao (University of Michigan), a specialist in infectious diseases, we attempt to shed some light on what is a rapidly evolving situation. For more resources from the American Gastroenterological Association (AGA) on up-to-date clinical guidance and research, you can also visit https://www.gastro.org/practice-guidance/practice-updates/covid-19.
Moving on to our “In Focus” feature, Thangam Ventakesan and Harrison Mooers (Medical College of Wisconsin) provide a comprehensive overview of cyclic vomiting syndrome. This is a valuable read as cyclic vomiting syndrome has been gaining increased recognition among adults, and Dr. Ventakesan and Dr. Mooers elucidate a thorough approach to the diagnosis and treatment of this disorder.
A facet of endoscopy that is extremely important, but frequently overlooked, is ergonomics. Manish Singla and Jared Magee (Walter Reed National Military Medical Center) compile a high-yield list of recommendations on the best practices to preserve our own safety and health as endoscopists.
We continue our medical ethics series with Jennifer Wang and Andrew Aronsohn (University of Chicago) who offer a thought-provoking discussion on the role of early liver transplantation for alcoholic hepatitis, including an analysis of the medical, psychosocial, and ethical considerations.
Also in this issue, Animesh Jain (University of North Carolina) gives us some excellent financial advice on student loan management, outlining a basic strategy of repayment with clear explanations of the available options including refinancing, public service loan forgiveness, and income-driven repayment.
Dilhana Badurdeen (Johns Hopkins), Aline Charabaty Pishvaian (Sibley Memorial Hospital), Miguel Malespin (University of South Florida), Ibironke Oduyebo (Midatlantic Permanente Medical Group), and Sandra Quezada (University of Maryland) give us an in-depth summary of the efforts of the AGA’s Diversity Committee, including publications, events, and future initiatives.
This quarter’s DHPA Private Practice Perspectives series features Paul Berggreen (Arizona Digestive Health), who reviews the advantages and disadvantages of pathology lab ownership as a gastroenterologist. Lastly, Sarah Ordway, Dawn Torres, Manish Singla, and Adam Tritsch (Walter Reed National Military Medical Center) broach the issue of fellowship burnout by providing guidance on how to identify signs and those at risk in addition to providing tangible solutions that any fellowship can incorporate.
Although the cancellation of the upcoming DDW meetings in Chicago is a disappointment, I hope that we can all take this time to prioritize the well-being of ourselves and our communities until we meet again.
As always, if you have interest in contributing or have ideas for future TNG topics, please contact me ([email protected]), or Ryan Farrell ([email protected]), managing editor of TNG.
Best wishes to stay safe and healthy.
Vijaya L. Rao, MD
Editor in Chief
Dr. Rao is assistant professor of medicine, University of Chicago, section of gastroenterology, hepatology & nutrition.
Cyclic vomiting syndrome: A GI primer
Introduction
Cyclic vomiting syndrome (CVS) is a chronic disorder of gut-brain interaction (DGBI) and is characterized by recurrent episodes of severe nausea, vomiting, and often, abdominal pain. Patients are usually asymptomatic in between episodes.1 CVS was considered a pediatric disease but is now known to be as common in adults. The prevalence of CVS in adults was 2% in a recent population-based study.2 Patients are predominantly white. Both males and females are affected with some studies showing a female preponderance. The mean age of onset is 5 years in children and 35 years in adults.3
The etiology of CVS is not known, but various hypotheses have been proposed. Zaki et al. showed that two mitochondrial DNA polymorphisms 16519T and 3010A were associated with a 17-fold increased odds of having CVS in children.4 These polymorphisms were not associated with CVS in adults.5 Alterations in the brain-gut axis also have been shown in CVS. Functional neuroimaging studies demonstrate that patients with CVS displayed increased connectivity between insula and salience networks with concomitant decrease in connectivity to somatosensory networks.6 Recent data also indicate that the endocannabinoid system (ECS) and the hypothalamic-pituitary-adrenal axis are implicated in CVS with an increase in serum endocannabinoid concentration during an episode.7 The same study also showed a significant increase in salivary cortisol in CVS patients who used cannabis. Further, single nucleotide polymorphisms (SNPs) in the gene that encodes for the cannabinoid receptor type 1 (CB1R) are implicated in CVS.8 The CB1R is part of the ECS and is densely expressed in brain areas involved in emesis, such as the dorsal vagal complex consisting of the area postrema (AP), nucleus of the solitary tract (NTS), and also the dorsal motor nucleus of the vagus.9 Wasilewski et al. showed an increased risk of CVS among individuals with AG and GG genotypes of CNR1 rs806380 (P less than .01), whereas the CC genotype of CNR1 rs806368 was associated with a decreased risk of CVS (P less than .05).8 CB1R agonists – endocannabinoids and tetrahydrocannabinol (THC) – have acute antiemetic and anxiolytic effects.9-11 The apparent paradoxical effects of cannabis in this patient population are yet to be explained and need further study.
Diagnosis and clinical features of CVS
Figure 1: Phases of Cyclic Vomiting Syndrome12
Adapted from Fleisher DR, Gornowicz B, Adams K, Burch R, Feldman EJ. Cyclic Vomiting Syndrome in 41 adults: The illness, the patients, and problems of management. BMC Med 2005;3:20. This work is licensed under the Creative Commons Attribution 4.0 International License https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use, distribution, modification, and reproduction in any medium.
CVS consists of four phases which include the a) prodromal phase, b) the episodic phase, c) recovery phase, and d) the interepisodic phase; and was first described by David Fleisher.12 The phases of CVS are important for clinicians and patients alike as they have therapeutic implications. The administration of abortive medications during a prodrome can terminate an episode. The phases of CVS are shown above.
Most patients (~ 93%) have a prodromal phase. Symptoms during this phase can include nausea, abdominal pain, diaphoresis, fatigue, weakness, hot flashes, chills, shivering, increased thirst, loss of appetite, burping, lightheadedness, and paresthesia.13 Some patients report a sense of impending doom and many have symptoms consistent with panic. If untreated, this progresses to the emetic phase and patients have unrelenting nausea, retching, vomiting, and other symptoms. During an episode, patients may vomit up to 20 times per hour and the episode may last several hours to days. During this phase, patients are sometimes described as being in a “conscious coma” and exhibit lethargy, listlessness, withdrawal, and sometimes disorientation.14,15 The emetic phase is followed by the recovery phase, during which symptoms subside and patients are able to resume oral intake. Patients are usually asymptomatic between episodes but ~ 30% can have interepisodic nausea and dyspepsia. In some patients, episodes become progressively longer and the interepisodic phase is considerably shortened and patients have a “coalescence of symptoms.”12 It is important to elicit a thorough history in all patients with vomiting in order to make an accurate diagnosis of CVS since coalescence of symptoms only occurs over a period of time. Episodes often are triggered by psychological stress, both positive and negative. Common triggers can include positive events such as birthdays, holidays, and negative ones like examinations, the death of a loved one, etc. Sleep deprivation and physical exhaustion also can trigger an episode.12
CVS remains a clinical diagnosis since there are no biomarkers. While there is a lack of data on the optimal work-up in these patients, experts recommend an upper endoscopy or upper GI series in order to rule out alternative gastric and intestinal pathology (e.g., malrotation with volvulus).16 Of note, a gastric-emptying study is not recommended as part of the routine work-up as per recent guidelines because of the poor specificity of this test in establishing a diagnosis of CVS.16 Biochemical testing including a complete blood count, serum electrolytes, serum glucose, liver panel, and urinalysis is also warranted. Any additional testing is indicated when clinical features suggest an alternative diagnosis. For instance, neurologic symptoms might warrant a cranial MRI to exclude an intracerebral tumor or other lesions of the brain.

The severity and unpredictable nature of symptoms makes it difficult for some patients to attend school or work; one study found that 32% of patients with CVS were completely disabled.12 Despite increasing awareness of this disorder, patients often are misdiagnosed. The prevalence of CVS in an outpatient gastroenterology clinic in the United Kingdom was 11% and was markedly underdiagnosed in the community.17 Only 5% of patients who were subsequently diagnosed with CVS were initially diagnosed accurately by their referring physician despite meeting criteria for the disorder.17 A subset of patients with CVS even undergo futile surgeries.13 Fleisher et al. noted that 30% of a 41-patient cohort underwent cholecystectomy for CVS symptoms without any improvement in disease.12 Prompt diagnosis and appropriate therapy is essential to improve patient outcomes and improve quality of life.
CVS is associated with various comorbidities such as migraine, anxiety, depression and dysautonomia, which can further impair quality of life.18,19 Approximately 70% of CVS patients report a personal or family history of migraine. Anxiety and depression affects nearly half of patients with CVS.13 Cannabis use is significantly more prevalent among patients with CVS than patients without CVS.20
Role of cannabis in CVS
The role of cannabis in the pathogenesis of symptoms in CVS is controversial. While cannabis has antiemetic properties, there is a strong link between its use and CVS. The use of cannabis has increased over the past decade with increasing legalization.21 Several studies have shown that 40%-80% of patients with CVS use cannabis.22,23 Following this, cannabinoid hyperemesis syndrome (CHS) was coined as a separate entity based on this statistical association, though there are no data to support the notion that cannabis causes vomiting.24,25 CHS has clinical features that are indistinguishable from CVS except for the chronic heavy cannabis use. A peculiar bathing behavior called “compulsive hot-water bathing” has been described and was thought to be pathognomonic of cannabis use.26 During an episode, patients will take multiple hot showers/baths, which temporarily alleviate their symptoms. Many patients even report running out of hot water and sometimes check into a hotel for a continuous supply of hot water. A small number of patients may sustain burns from the hot-water bathing. However, studies show that this hot-water bathing behavior also is seen in about 50% of patents with CVS who do not use cannabis.22
CHS is now defined by Rome IV criteria, which include episodes of nausea and vomiting similar to CVS preceded by chronic, heavy cannabis use. Patients must have complete resolution of symptoms following cessation.1 A recent systematic review of 376 cases of purported CHS showed that only 59 (15.7%) met Rome IV criteria for this disorder.27 This is because of considerable heterogeneity in how the diagnosis of CHS was made and the lack of standard diagnostic criteria at the time. Some cases of CHS were diagnosed merely based on an association of vomiting, hot-water bathing, and cannabis use.28 Only a minority of patients (71,19%) had a duration of follow-up more than 4 weeks, which would make it impossible to establish a diagnosis of CHS. A period of at least a year or a duration of time that spans at least three episodes is generally recommended to determine if abstinence from cannabis causes a true resolution of symptoms.27 Whether CHS is a separate entity or a subtype of CVS remains to be determined. The paradoxical effects of cannabis may happen because of the use of highly potent cannabis products that are currently in use. A complete discussion of the role of cannabis in CVS is beyond the scope of this article, and the reader is referred to a recent systematic review and discussion.27
Treatment
CVS should be treated based on a biopsychosocial model with a multidisciplinary team that includes a gastroenterologist with knowledge of CVS, primary care physician, psychologist, psychiatrist, and sleep specialist if needed.16 Initiating prophylactic treatment is based on the severity of disease. An algorithm for the treatment of CVS based on severity of symptoms is shown below.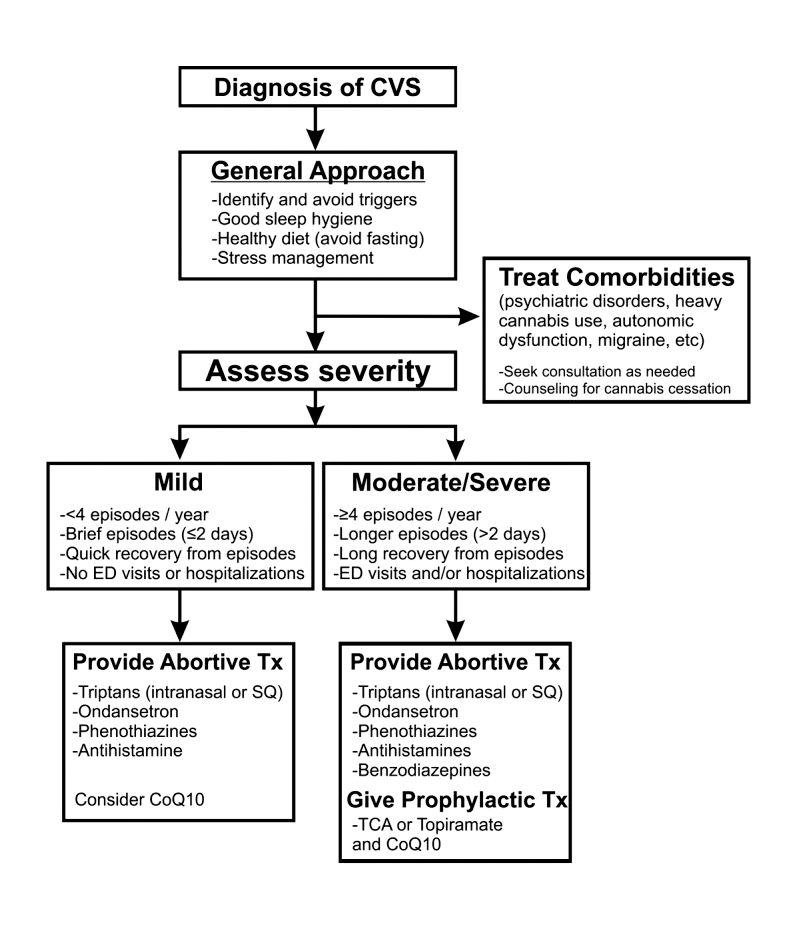
Figure 2. Adapted and reprinted by permission from the Licensor: Springer Nature, Current Treatment Options in Gastroenterology, Bhandari S, Venkatesan T. Novel Treatments for Cyclic Vomiting Syndrome: Beyond Ondansetron and Amitriptyline, 14:495-506, Copyright 2016.
Patients who have mild disease (defined as fewer than four episodes/year, episodes lasting up to 2 days, quick recovery from episodes, or episodes not requiring ED care or hospitalization) are usually prescribed abortive medications.16 These medications are best administered during the prodromal phase and can prevent progression to the emetic phase. Medications used for aborting episodes include sumatriptan (20 mg intranasal or 6 mg subcutaneous), ondansetron (8 mg sublingual), and diphenhydramine (25-50 mg).30,31 This combination can help abort symptoms and potentially avoid ED visits or hospitalizations. Patients with moderate-to-severe CVS are offered prophylactic therapy in addition to abortive therapy.16
Recent guidelines recommend tricyclic antidepressants (TCAs) as the first-line agent in the prophylaxis of CVS episodes. Data from 14 studies determined that 70% (413/600) of patients responded partially or completely to TCAs.16 An open-label study of 46 patients by Hejazi et al. noted a decline in the number of CVS episodes from 17 to 3, in the duration of a CVS episode from 6 to 2 days, and in the number of ED visits/ hospitalizations from 15 to 3.3.32Amitriptyline should be started at 25 mg at night and titrated up by 10-25 mg each week to minimize emergence of side effects. The mean effective dose is 75-100 mg or 1.0-1.5 mg/kg. An EKG should be checked at baseline and during titration to monitor the QT interval. Unfortunately, side effects from TCAs are quite common and include cognitive impairment, drowsiness, dryness of mouth, weight gain, constipation, and mood changes, which may warrant dose reduction or discontinuation. Antiepileptics such as topiramate, mitochondrial supplements such as Coenzyme Q10 and riboflavin are alternative prophylactic agents in CVS.33 Aprepitant, a newer NK1 receptor antagonist has been found to be effective in refractory CVS.34 In addition to pharmacotherapy, addressing comorbid conditions such as anxiety and depression and counseling patients to abstain from heavy cannabis use is also important to achieve good health care outcomes.
In summary, CVS is a common, chronic functional GI disorder with episodic nausea, vomiting, and often, abdominal pain. Symptoms can be disabling, and prompt diagnosis and therapy is important. CVS is associated with multiple comorbid conditions such as migraine, anxiety and depression, and a biopsychosocial model of care is essential. Medications such as amitriptyline are effective in the prophylaxis of CVS, but side effects hamper their use. Recent recommendations for management of CVS have been published.16 Cannabis is frequently used by patients for symptom relief but use of high potency products may cause worsening of symptoms or unmask symptoms in genetically predisposed individuals.23 Studies to elucidate the pathophysiology of CVS should help in the development of better therapies.
Dr. Mooers is PGY-2, an internal medicine resident in the department of medicine, Medical College of Wisconsin, Milwaukee; Dr. Venkatesan is professor of medicine, division of gastroenterology and hepatology, department of medicine, Medical College of Wisconsin, Milwaukee. The authors have no conflicts to disclose.
References
1. Stanghellini V et al. Gastroenterology. 2016;150:1380-92.
2. Aziz I et al. Clin Gastroenterol Hepatol. 2019 Apr;17(5):878-86.
3. Kovacic K et al. Curr Gastroenterol Rep. 2018;20(10):46.
4. Zaki EA et al. Cephalalgia. 2009;29:719-28.
5. Venkatesan T et al. BMC Gastroenterol. 2014;14:181.
6. Ellingsen DM et al. Neurogastroenterol Motil. 2017;29 (6)e13004 9.
7. Venkatesan T et al. Neurogastroenterol Motil. 2016;28:1409-18.
8. Wasilewski A et al. Am J Gastroenterol. 2017;112:933-9.
9. van Sickle MD et al. Am J Physiol Gastrointest Liver Physiol 2003;285:G566-76.
10. Parker LA et al. Br J Pharmacol. 2011;163:1411-22.
11. van Sickle MD et al. Gastroenterology. 2001;121:767-74.
12. Fleisher DR et al. BMC Med. 2005;3:20.
13. Kumar N et al. BMC Gastroenterol. 2012;12:52.
14. Li BU et al. J Pediatr Gastroenterol Nutr. 2008;47:379-93.
15. Bhandari S et al. Clin Auton Res. 2018 Apr;28(2):203-9.
16. Venkatesan T et al. Neurogastroenterol Motil. 2019;31 Suppl 2:e13604. doi: 10.1111/nmo.13604.
17. Sagar RC et al. Neurogastroenterol Motil. 2018;30. doi: 10.1111/nmo.13174.
18. Taranukha T et al. Neurogastroenterol Motil. 2018 Apr;30(4):e13245. doi: 10.1111/nmo.13245.
19. Bhandari S and Venkatesan T. Dig Dis Sci. 2017;62:2035-44.
20. Choung RS et al. Neurogastroenterol Motil. 2012;24:20-6, e21. doi: 10.1111/j.1365-2982.2011.01791.x.
21. Bhandari S et al. Intern Med J. 2019 May;49(5):649-55.
22. Venkatesan T et al. Exp Brain Res. 2014; 232:2563-70.
23. Venkatesan T et al. Clin Gastroenterol Hepatol. 2019 Jul 25. doi: 10.1016/j.cgh.2019.07.039.
24. Simonetto DA et al. Mayo Clin Proc. 2012;87:114-9.
25. Wallace EA et al. South Med J. 2011;104:659-64.
26. Allen JH et al. Gut. 2004;53:1566-70.
27. Venkatesan T et al. Neurogastroenterol Motil. 2019;31 Suppl 2:e13606. doi: 10.1111/nmo.13606.
28. Habboushe J et al. Basic Clin Pharmacol Toxicol. 2018;122:660-2.
29. Bhandari S and Venkatesan T. Curr Treat Options Gastroenterol. 2016;14:495-506.
30. Hikita T et al. Cephalalgia. 2011;31:504-7.
31. Fuseau E et al. Clin Pharmacokinet 2002;41:801-11.
32. Hejazi RA et al. J Clin Gastroenterol. 2010;44:18-21.
33. Sezer OB and Sezer T. J Neurogastroenterol Motil. 2016;22:656-60.
34. Cristofori F et al. Aliment Pharmacol Ther. 2014;40:309-17.
Introduction
Cyclic vomiting syndrome (CVS) is a chronic disorder of gut-brain interaction (DGBI) and is characterized by recurrent episodes of severe nausea, vomiting, and often, abdominal pain. Patients are usually asymptomatic in between episodes.1 CVS was considered a pediatric disease but is now known to be as common in adults. The prevalence of CVS in adults was 2% in a recent population-based study.2 Patients are predominantly white. Both males and females are affected with some studies showing a female preponderance. The mean age of onset is 5 years in children and 35 years in adults.3
The etiology of CVS is not known, but various hypotheses have been proposed. Zaki et al. showed that two mitochondrial DNA polymorphisms 16519T and 3010A were associated with a 17-fold increased odds of having CVS in children.4 These polymorphisms were not associated with CVS in adults.5 Alterations in the brain-gut axis also have been shown in CVS. Functional neuroimaging studies demonstrate that patients with CVS displayed increased connectivity between insula and salience networks with concomitant decrease in connectivity to somatosensory networks.6 Recent data also indicate that the endocannabinoid system (ECS) and the hypothalamic-pituitary-adrenal axis are implicated in CVS with an increase in serum endocannabinoid concentration during an episode.7 The same study also showed a significant increase in salivary cortisol in CVS patients who used cannabis. Further, single nucleotide polymorphisms (SNPs) in the gene that encodes for the cannabinoid receptor type 1 (CB1R) are implicated in CVS.8 The CB1R is part of the ECS and is densely expressed in brain areas involved in emesis, such as the dorsal vagal complex consisting of the area postrema (AP), nucleus of the solitary tract (NTS), and also the dorsal motor nucleus of the vagus.9 Wasilewski et al. showed an increased risk of CVS among individuals with AG and GG genotypes of CNR1 rs806380 (P less than .01), whereas the CC genotype of CNR1 rs806368 was associated with a decreased risk of CVS (P less than .05).8 CB1R agonists – endocannabinoids and tetrahydrocannabinol (THC) – have acute antiemetic and anxiolytic effects.9-11 The apparent paradoxical effects of cannabis in this patient population are yet to be explained and need further study.
Diagnosis and clinical features of CVS

Figure 1: Phases of Cyclic Vomiting Syndrome12
Adapted from Fleisher DR, Gornowicz B, Adams K, Burch R, Feldman EJ. Cyclic Vomiting Syndrome in 41 adults: The illness, the patients, and problems of management. BMC Med 2005;3:20. This work is licensed under the Creative Commons Attribution 4.0 International License https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use, distribution, modification, and reproduction in any medium.
CVS consists of four phases which include the a) prodromal phase, b) the episodic phase, c) recovery phase, and d) the interepisodic phase; and was first described by David Fleisher.12 The phases of CVS are important for clinicians and patients alike as they have therapeutic implications. The administration of abortive medications during a prodrome can terminate an episode. The phases of CVS are shown above.
Most patients (~ 93%) have a prodromal phase. Symptoms during this phase can include nausea, abdominal pain, diaphoresis, fatigue, weakness, hot flashes, chills, shivering, increased thirst, loss of appetite, burping, lightheadedness, and paresthesia.13 Some patients report a sense of impending doom and many have symptoms consistent with panic. If untreated, this progresses to the emetic phase and patients have unrelenting nausea, retching, vomiting, and other symptoms. During an episode, patients may vomit up to 20 times per hour and the episode may last several hours to days. During this phase, patients are sometimes described as being in a “conscious coma” and exhibit lethargy, listlessness, withdrawal, and sometimes disorientation.14,15 The emetic phase is followed by the recovery phase, during which symptoms subside and patients are able to resume oral intake. Patients are usually asymptomatic between episodes but ~ 30% can have interepisodic nausea and dyspepsia. In some patients, episodes become progressively longer and the interepisodic phase is considerably shortened and patients have a “coalescence of symptoms.”12 It is important to elicit a thorough history in all patients with vomiting in order to make an accurate diagnosis of CVS since coalescence of symptoms only occurs over a period of time. Episodes often are triggered by psychological stress, both positive and negative. Common triggers can include positive events such as birthdays, holidays, and negative ones like examinations, the death of a loved one, etc. Sleep deprivation and physical exhaustion also can trigger an episode.12
CVS remains a clinical diagnosis since there are no biomarkers. While there is a lack of data on the optimal work-up in these patients, experts recommend an upper endoscopy or upper GI series in order to rule out alternative gastric and intestinal pathology (e.g., malrotation with volvulus).16 Of note, a gastric-emptying study is not recommended as part of the routine work-up as per recent guidelines because of the poor specificity of this test in establishing a diagnosis of CVS.16 Biochemical testing including a complete blood count, serum electrolytes, serum glucose, liver panel, and urinalysis is also warranted. Any additional testing is indicated when clinical features suggest an alternative diagnosis. For instance, neurologic symptoms might warrant a cranial MRI to exclude an intracerebral tumor or other lesions of the brain.

The severity and unpredictable nature of symptoms makes it difficult for some patients to attend school or work; one study found that 32% of patients with CVS were completely disabled.12 Despite increasing awareness of this disorder, patients often are misdiagnosed. The prevalence of CVS in an outpatient gastroenterology clinic in the United Kingdom was 11% and was markedly underdiagnosed in the community.17 Only 5% of patients who were subsequently diagnosed with CVS were initially diagnosed accurately by their referring physician despite meeting criteria for the disorder.17 A subset of patients with CVS even undergo futile surgeries.13 Fleisher et al. noted that 30% of a 41-patient cohort underwent cholecystectomy for CVS symptoms without any improvement in disease.12 Prompt diagnosis and appropriate therapy is essential to improve patient outcomes and improve quality of life.
CVS is associated with various comorbidities such as migraine, anxiety, depression and dysautonomia, which can further impair quality of life.18,19 Approximately 70% of CVS patients report a personal or family history of migraine. Anxiety and depression affects nearly half of patients with CVS.13 Cannabis use is significantly more prevalent among patients with CVS than patients without CVS.20
Role of cannabis in CVS
The role of cannabis in the pathogenesis of symptoms in CVS is controversial. While cannabis has antiemetic properties, there is a strong link between its use and CVS. The use of cannabis has increased over the past decade with increasing legalization.21 Several studies have shown that 40%-80% of patients with CVS use cannabis.22,23 Following this, cannabinoid hyperemesis syndrome (CHS) was coined as a separate entity based on this statistical association, though there are no data to support the notion that cannabis causes vomiting.24,25 CHS has clinical features that are indistinguishable from CVS except for the chronic heavy cannabis use. A peculiar bathing behavior called “compulsive hot-water bathing” has been described and was thought to be pathognomonic of cannabis use.26 During an episode, patients will take multiple hot showers/baths, which temporarily alleviate their symptoms. Many patients even report running out of hot water and sometimes check into a hotel for a continuous supply of hot water. A small number of patients may sustain burns from the hot-water bathing. However, studies show that this hot-water bathing behavior also is seen in about 50% of patents with CVS who do not use cannabis.22

CHS is now defined by Rome IV criteria, which include episodes of nausea and vomiting similar to CVS preceded by chronic, heavy cannabis use. Patients must have complete resolution of symptoms following cessation.1 A recent systematic review of 376 cases of purported CHS showed that only 59 (15.7%) met Rome IV criteria for this disorder.27 This is because of considerable heterogeneity in how the diagnosis of CHS was made and the lack of standard diagnostic criteria at the time. Some cases of CHS were diagnosed merely based on an association of vomiting, hot-water bathing, and cannabis use.28 Only a minority of patients (71,19%) had a duration of follow-up more than 4 weeks, which would make it impossible to establish a diagnosis of CHS. A period of at least a year or a duration of time that spans at least three episodes is generally recommended to determine if abstinence from cannabis causes a true resolution of symptoms.27 Whether CHS is a separate entity or a subtype of CVS remains to be determined. The paradoxical effects of cannabis may happen because of the use of highly potent cannabis products that are currently in use. A complete discussion of the role of cannabis in CVS is beyond the scope of this article, and the reader is referred to a recent systematic review and discussion.27
Treatment
CVS should be treated based on a biopsychosocial model with a multidisciplinary team that includes a gastroenterologist with knowledge of CVS, primary care physician, psychologist, psychiatrist, and sleep specialist if needed.16 Initiating prophylactic treatment is based on the severity of disease. An algorithm for the treatment of CVS based on severity of symptoms is shown below.
Figure 2. Adapted and reprinted by permission from the Licensor: Springer Nature, Current Treatment Options in Gastroenterology, Bhandari S, Venkatesan T. Novel Treatments for Cyclic Vomiting Syndrome: Beyond Ondansetron and Amitriptyline, 14:495-506, Copyright 2016.
Patients who have mild disease (defined as fewer than four episodes/year, episodes lasting up to 2 days, quick recovery from episodes, or episodes not requiring ED care or hospitalization) are usually prescribed abortive medications.16 These medications are best administered during the prodromal phase and can prevent progression to the emetic phase. Medications used for aborting episodes include sumatriptan (20 mg intranasal or 6 mg subcutaneous), ondansetron (8 mg sublingual), and diphenhydramine (25-50 mg).30,31 This combination can help abort symptoms and potentially avoid ED visits or hospitalizations. Patients with moderate-to-severe CVS are offered prophylactic therapy in addition to abortive therapy.16
Recent guidelines recommend tricyclic antidepressants (TCAs) as the first-line agent in the prophylaxis of CVS episodes. Data from 14 studies determined that 70% (413/600) of patients responded partially or completely to TCAs.16 An open-label study of 46 patients by Hejazi et al. noted a decline in the number of CVS episodes from 17 to 3, in the duration of a CVS episode from 6 to 2 days, and in the number of ED visits/ hospitalizations from 15 to 3.3.32Amitriptyline should be started at 25 mg at night and titrated up by 10-25 mg each week to minimize emergence of side effects. The mean effective dose is 75-100 mg or 1.0-1.5 mg/kg. An EKG should be checked at baseline and during titration to monitor the QT interval. Unfortunately, side effects from TCAs are quite common and include cognitive impairment, drowsiness, dryness of mouth, weight gain, constipation, and mood changes, which may warrant dose reduction or discontinuation. Antiepileptics such as topiramate, mitochondrial supplements such as Coenzyme Q10 and riboflavin are alternative prophylactic agents in CVS.33 Aprepitant, a newer NK1 receptor antagonist has been found to be effective in refractory CVS.34 In addition to pharmacotherapy, addressing comorbid conditions such as anxiety and depression and counseling patients to abstain from heavy cannabis use is also important to achieve good health care outcomes.
In summary, CVS is a common, chronic functional GI disorder with episodic nausea, vomiting, and often, abdominal pain. Symptoms can be disabling, and prompt diagnosis and therapy is important. CVS is associated with multiple comorbid conditions such as migraine, anxiety and depression, and a biopsychosocial model of care is essential. Medications such as amitriptyline are effective in the prophylaxis of CVS, but side effects hamper their use. Recent recommendations for management of CVS have been published.16 Cannabis is frequently used by patients for symptom relief but use of high potency products may cause worsening of symptoms or unmask symptoms in genetically predisposed individuals.23 Studies to elucidate the pathophysiology of CVS should help in the development of better therapies.
Dr. Mooers is PGY-2, an internal medicine resident in the department of medicine, Medical College of Wisconsin, Milwaukee; Dr. Venkatesan is professor of medicine, division of gastroenterology and hepatology, department of medicine, Medical College of Wisconsin, Milwaukee. The authors have no conflicts to disclose.
References
1. Stanghellini V et al. Gastroenterology. 2016;150:1380-92.
2. Aziz I et al. Clin Gastroenterol Hepatol. 2019 Apr;17(5):878-86.
3. Kovacic K et al. Curr Gastroenterol Rep. 2018;20(10):46.
4. Zaki EA et al. Cephalalgia. 2009;29:719-28.
5. Venkatesan T et al. BMC Gastroenterol. 2014;14:181.
6. Ellingsen DM et al. Neurogastroenterol Motil. 2017;29 (6)e13004 9.
7. Venkatesan T et al. Neurogastroenterol Motil. 2016;28:1409-18.
8. Wasilewski A et al. Am J Gastroenterol. 2017;112:933-9.
9. van Sickle MD et al. Am J Physiol Gastrointest Liver Physiol 2003;285:G566-76.
10. Parker LA et al. Br J Pharmacol. 2011;163:1411-22.
11. van Sickle MD et al. Gastroenterology. 2001;121:767-74.
12. Fleisher DR et al. BMC Med. 2005;3:20.
13. Kumar N et al. BMC Gastroenterol. 2012;12:52.
14. Li BU et al. J Pediatr Gastroenterol Nutr. 2008;47:379-93.
15. Bhandari S et al. Clin Auton Res. 2018 Apr;28(2):203-9.
16. Venkatesan T et al. Neurogastroenterol Motil. 2019;31 Suppl 2:e13604. doi: 10.1111/nmo.13604.
17. Sagar RC et al. Neurogastroenterol Motil. 2018;30. doi: 10.1111/nmo.13174.
18. Taranukha T et al. Neurogastroenterol Motil. 2018 Apr;30(4):e13245. doi: 10.1111/nmo.13245.
19. Bhandari S and Venkatesan T. Dig Dis Sci. 2017;62:2035-44.
20. Choung RS et al. Neurogastroenterol Motil. 2012;24:20-6, e21. doi: 10.1111/j.1365-2982.2011.01791.x.
21. Bhandari S et al. Intern Med J. 2019 May;49(5):649-55.
22. Venkatesan T et al. Exp Brain Res. 2014; 232:2563-70.
23. Venkatesan T et al. Clin Gastroenterol Hepatol. 2019 Jul 25. doi: 10.1016/j.cgh.2019.07.039.
24. Simonetto DA et al. Mayo Clin Proc. 2012;87:114-9.
25. Wallace EA et al. South Med J. 2011;104:659-64.
26. Allen JH et al. Gut. 2004;53:1566-70.
27. Venkatesan T et al. Neurogastroenterol Motil. 2019;31 Suppl 2:e13606. doi: 10.1111/nmo.13606.
28. Habboushe J et al. Basic Clin Pharmacol Toxicol. 2018;122:660-2.
29. Bhandari S and Venkatesan T. Curr Treat Options Gastroenterol. 2016;14:495-506.
30. Hikita T et al. Cephalalgia. 2011;31:504-7.
31. Fuseau E et al. Clin Pharmacokinet 2002;41:801-11.
32. Hejazi RA et al. J Clin Gastroenterol. 2010;44:18-21.
33. Sezer OB and Sezer T. J Neurogastroenterol Motil. 2016;22:656-60.
34. Cristofori F et al. Aliment Pharmacol Ther. 2014;40:309-17.
Introduction
Cyclic vomiting syndrome (CVS) is a chronic disorder of gut-brain interaction (DGBI) and is characterized by recurrent episodes of severe nausea, vomiting, and often, abdominal pain. Patients are usually asymptomatic in between episodes.1 CVS was considered a pediatric disease but is now known to be as common in adults. The prevalence of CVS in adults was 2% in a recent population-based study.2 Patients are predominantly white. Both males and females are affected with some studies showing a female preponderance. The mean age of onset is 5 years in children and 35 years in adults.3
The etiology of CVS is not known, but various hypotheses have been proposed. Zaki et al. showed that two mitochondrial DNA polymorphisms 16519T and 3010A were associated with a 17-fold increased odds of having CVS in children.4 These polymorphisms were not associated with CVS in adults.5 Alterations in the brain-gut axis also have been shown in CVS. Functional neuroimaging studies demonstrate that patients with CVS displayed increased connectivity between insula and salience networks with concomitant decrease in connectivity to somatosensory networks.6 Recent data also indicate that the endocannabinoid system (ECS) and the hypothalamic-pituitary-adrenal axis are implicated in CVS with an increase in serum endocannabinoid concentration during an episode.7 The same study also showed a significant increase in salivary cortisol in CVS patients who used cannabis. Further, single nucleotide polymorphisms (SNPs) in the gene that encodes for the cannabinoid receptor type 1 (CB1R) are implicated in CVS.8 The CB1R is part of the ECS and is densely expressed in brain areas involved in emesis, such as the dorsal vagal complex consisting of the area postrema (AP), nucleus of the solitary tract (NTS), and also the dorsal motor nucleus of the vagus.9 Wasilewski et al. showed an increased risk of CVS among individuals with AG and GG genotypes of CNR1 rs806380 (P less than .01), whereas the CC genotype of CNR1 rs806368 was associated with a decreased risk of CVS (P less than .05).8 CB1R agonists – endocannabinoids and tetrahydrocannabinol (THC) – have acute antiemetic and anxiolytic effects.9-11 The apparent paradoxical effects of cannabis in this patient population are yet to be explained and need further study.
Diagnosis and clinical features of CVS

Figure 1: Phases of Cyclic Vomiting Syndrome12
Adapted from Fleisher DR, Gornowicz B, Adams K, Burch R, Feldman EJ. Cyclic Vomiting Syndrome in 41 adults: The illness, the patients, and problems of management. BMC Med 2005;3:20. This work is licensed under the Creative Commons Attribution 4.0 International License https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use, distribution, modification, and reproduction in any medium.
CVS consists of four phases which include the a) prodromal phase, b) the episodic phase, c) recovery phase, and d) the interepisodic phase; and was first described by David Fleisher.12 The phases of CVS are important for clinicians and patients alike as they have therapeutic implications. The administration of abortive medications during a prodrome can terminate an episode. The phases of CVS are shown above.
Most patients (~ 93%) have a prodromal phase. Symptoms during this phase can include nausea, abdominal pain, diaphoresis, fatigue, weakness, hot flashes, chills, shivering, increased thirst, loss of appetite, burping, lightheadedness, and paresthesia.13 Some patients report a sense of impending doom and many have symptoms consistent with panic. If untreated, this progresses to the emetic phase and patients have unrelenting nausea, retching, vomiting, and other symptoms. During an episode, patients may vomit up to 20 times per hour and the episode may last several hours to days. During this phase, patients are sometimes described as being in a “conscious coma” and exhibit lethargy, listlessness, withdrawal, and sometimes disorientation.14,15 The emetic phase is followed by the recovery phase, during which symptoms subside and patients are able to resume oral intake. Patients are usually asymptomatic between episodes but ~ 30% can have interepisodic nausea and dyspepsia. In some patients, episodes become progressively longer and the interepisodic phase is considerably shortened and patients have a “coalescence of symptoms.”12 It is important to elicit a thorough history in all patients with vomiting in order to make an accurate diagnosis of CVS since coalescence of symptoms only occurs over a period of time. Episodes often are triggered by psychological stress, both positive and negative. Common triggers can include positive events such as birthdays, holidays, and negative ones like examinations, the death of a loved one, etc. Sleep deprivation and physical exhaustion also can trigger an episode.12
CVS remains a clinical diagnosis since there are no biomarkers. While there is a lack of data on the optimal work-up in these patients, experts recommend an upper endoscopy or upper GI series in order to rule out alternative gastric and intestinal pathology (e.g., malrotation with volvulus).16 Of note, a gastric-emptying study is not recommended as part of the routine work-up as per recent guidelines because of the poor specificity of this test in establishing a diagnosis of CVS.16 Biochemical testing including a complete blood count, serum electrolytes, serum glucose, liver panel, and urinalysis is also warranted. Any additional testing is indicated when clinical features suggest an alternative diagnosis. For instance, neurologic symptoms might warrant a cranial MRI to exclude an intracerebral tumor or other lesions of the brain.

The severity and unpredictable nature of symptoms makes it difficult for some patients to attend school or work; one study found that 32% of patients with CVS were completely disabled.12 Despite increasing awareness of this disorder, patients often are misdiagnosed. The prevalence of CVS in an outpatient gastroenterology clinic in the United Kingdom was 11% and was markedly underdiagnosed in the community.17 Only 5% of patients who were subsequently diagnosed with CVS were initially diagnosed accurately by their referring physician despite meeting criteria for the disorder.17 A subset of patients with CVS even undergo futile surgeries.13 Fleisher et al. noted that 30% of a 41-patient cohort underwent cholecystectomy for CVS symptoms without any improvement in disease.12 Prompt diagnosis and appropriate therapy is essential to improve patient outcomes and improve quality of life.
CVS is associated with various comorbidities such as migraine, anxiety, depression and dysautonomia, which can further impair quality of life.18,19 Approximately 70% of CVS patients report a personal or family history of migraine. Anxiety and depression affects nearly half of patients with CVS.13 Cannabis use is significantly more prevalent among patients with CVS than patients without CVS.20
Role of cannabis in CVS
The role of cannabis in the pathogenesis of symptoms in CVS is controversial. While cannabis has antiemetic properties, there is a strong link between its use and CVS. The use of cannabis has increased over the past decade with increasing legalization.21 Several studies have shown that 40%-80% of patients with CVS use cannabis.22,23 Following this, cannabinoid hyperemesis syndrome (CHS) was coined as a separate entity based on this statistical association, though there are no data to support the notion that cannabis causes vomiting.24,25 CHS has clinical features that are indistinguishable from CVS except for the chronic heavy cannabis use. A peculiar bathing behavior called “compulsive hot-water bathing” has been described and was thought to be pathognomonic of cannabis use.26 During an episode, patients will take multiple hot showers/baths, which temporarily alleviate their symptoms. Many patients even report running out of hot water and sometimes check into a hotel for a continuous supply of hot water. A small number of patients may sustain burns from the hot-water bathing. However, studies show that this hot-water bathing behavior also is seen in about 50% of patents with CVS who do not use cannabis.22

CHS is now defined by Rome IV criteria, which include episodes of nausea and vomiting similar to CVS preceded by chronic, heavy cannabis use. Patients must have complete resolution of symptoms following cessation.1 A recent systematic review of 376 cases of purported CHS showed that only 59 (15.7%) met Rome IV criteria for this disorder.27 This is because of considerable heterogeneity in how the diagnosis of CHS was made and the lack of standard diagnostic criteria at the time. Some cases of CHS were diagnosed merely based on an association of vomiting, hot-water bathing, and cannabis use.28 Only a minority of patients (71,19%) had a duration of follow-up more than 4 weeks, which would make it impossible to establish a diagnosis of CHS. A period of at least a year or a duration of time that spans at least three episodes is generally recommended to determine if abstinence from cannabis causes a true resolution of symptoms.27 Whether CHS is a separate entity or a subtype of CVS remains to be determined. The paradoxical effects of cannabis may happen because of the use of highly potent cannabis products that are currently in use. A complete discussion of the role of cannabis in CVS is beyond the scope of this article, and the reader is referred to a recent systematic review and discussion.27
Treatment
CVS should be treated based on a biopsychosocial model with a multidisciplinary team that includes a gastroenterologist with knowledge of CVS, primary care physician, psychologist, psychiatrist, and sleep specialist if needed.16 Initiating prophylactic treatment is based on the severity of disease. An algorithm for the treatment of CVS based on severity of symptoms is shown below.
Figure 2. Adapted and reprinted by permission from the Licensor: Springer Nature, Current Treatment Options in Gastroenterology, Bhandari S, Venkatesan T. Novel Treatments for Cyclic Vomiting Syndrome: Beyond Ondansetron and Amitriptyline, 14:495-506, Copyright 2016.
Patients who have mild disease (defined as fewer than four episodes/year, episodes lasting up to 2 days, quick recovery from episodes, or episodes not requiring ED care or hospitalization) are usually prescribed abortive medications.16 These medications are best administered during the prodromal phase and can prevent progression to the emetic phase. Medications used for aborting episodes include sumatriptan (20 mg intranasal or 6 mg subcutaneous), ondansetron (8 mg sublingual), and diphenhydramine (25-50 mg).30,31 This combination can help abort symptoms and potentially avoid ED visits or hospitalizations. Patients with moderate-to-severe CVS are offered prophylactic therapy in addition to abortive therapy.16
Recent guidelines recommend tricyclic antidepressants (TCAs) as the first-line agent in the prophylaxis of CVS episodes. Data from 14 studies determined that 70% (413/600) of patients responded partially or completely to TCAs.16 An open-label study of 46 patients by Hejazi et al. noted a decline in the number of CVS episodes from 17 to 3, in the duration of a CVS episode from 6 to 2 days, and in the number of ED visits/ hospitalizations from 15 to 3.3.32Amitriptyline should be started at 25 mg at night and titrated up by 10-25 mg each week to minimize emergence of side effects. The mean effective dose is 75-100 mg or 1.0-1.5 mg/kg. An EKG should be checked at baseline and during titration to monitor the QT interval. Unfortunately, side effects from TCAs are quite common and include cognitive impairment, drowsiness, dryness of mouth, weight gain, constipation, and mood changes, which may warrant dose reduction or discontinuation. Antiepileptics such as topiramate, mitochondrial supplements such as Coenzyme Q10 and riboflavin are alternative prophylactic agents in CVS.33 Aprepitant, a newer NK1 receptor antagonist has been found to be effective in refractory CVS.34 In addition to pharmacotherapy, addressing comorbid conditions such as anxiety and depression and counseling patients to abstain from heavy cannabis use is also important to achieve good health care outcomes.
In summary, CVS is a common, chronic functional GI disorder with episodic nausea, vomiting, and often, abdominal pain. Symptoms can be disabling, and prompt diagnosis and therapy is important. CVS is associated with multiple comorbid conditions such as migraine, anxiety and depression, and a biopsychosocial model of care is essential. Medications such as amitriptyline are effective in the prophylaxis of CVS, but side effects hamper their use. Recent recommendations for management of CVS have been published.16 Cannabis is frequently used by patients for symptom relief but use of high potency products may cause worsening of symptoms or unmask symptoms in genetically predisposed individuals.23 Studies to elucidate the pathophysiology of CVS should help in the development of better therapies.
Dr. Mooers is PGY-2, an internal medicine resident in the department of medicine, Medical College of Wisconsin, Milwaukee; Dr. Venkatesan is professor of medicine, division of gastroenterology and hepatology, department of medicine, Medical College of Wisconsin, Milwaukee. The authors have no conflicts to disclose.
References
1. Stanghellini V et al. Gastroenterology. 2016;150:1380-92.
2. Aziz I et al. Clin Gastroenterol Hepatol. 2019 Apr;17(5):878-86.
3. Kovacic K et al. Curr Gastroenterol Rep. 2018;20(10):46.
4. Zaki EA et al. Cephalalgia. 2009;29:719-28.
5. Venkatesan T et al. BMC Gastroenterol. 2014;14:181.
6. Ellingsen DM et al. Neurogastroenterol Motil. 2017;29 (6)e13004 9.
7. Venkatesan T et al. Neurogastroenterol Motil. 2016;28:1409-18.
8. Wasilewski A et al. Am J Gastroenterol. 2017;112:933-9.
9. van Sickle MD et al. Am J Physiol Gastrointest Liver Physiol 2003;285:G566-76.
10. Parker LA et al. Br J Pharmacol. 2011;163:1411-22.
11. van Sickle MD et al. Gastroenterology. 2001;121:767-74.
12. Fleisher DR et al. BMC Med. 2005;3:20.
13. Kumar N et al. BMC Gastroenterol. 2012;12:52.
14. Li BU et al. J Pediatr Gastroenterol Nutr. 2008;47:379-93.
15. Bhandari S et al. Clin Auton Res. 2018 Apr;28(2):203-9.
16. Venkatesan T et al. Neurogastroenterol Motil. 2019;31 Suppl 2:e13604. doi: 10.1111/nmo.13604.
17. Sagar RC et al. Neurogastroenterol Motil. 2018;30. doi: 10.1111/nmo.13174.
18. Taranukha T et al. Neurogastroenterol Motil. 2018 Apr;30(4):e13245. doi: 10.1111/nmo.13245.
19. Bhandari S and Venkatesan T. Dig Dis Sci. 2017;62:2035-44.
20. Choung RS et al. Neurogastroenterol Motil. 2012;24:20-6, e21. doi: 10.1111/j.1365-2982.2011.01791.x.
21. Bhandari S et al. Intern Med J. 2019 May;49(5):649-55.
22. Venkatesan T et al. Exp Brain Res. 2014; 232:2563-70.
23. Venkatesan T et al. Clin Gastroenterol Hepatol. 2019 Jul 25. doi: 10.1016/j.cgh.2019.07.039.
24. Simonetto DA et al. Mayo Clin Proc. 2012;87:114-9.
25. Wallace EA et al. South Med J. 2011;104:659-64.
26. Allen JH et al. Gut. 2004;53:1566-70.
27. Venkatesan T et al. Neurogastroenterol Motil. 2019;31 Suppl 2:e13606. doi: 10.1111/nmo.13606.
28. Habboushe J et al. Basic Clin Pharmacol Toxicol. 2018;122:660-2.
29. Bhandari S and Venkatesan T. Curr Treat Options Gastroenterol. 2016;14:495-506.
30. Hikita T et al. Cephalalgia. 2011;31:504-7.
31. Fuseau E et al. Clin Pharmacokinet 2002;41:801-11.
32. Hejazi RA et al. J Clin Gastroenterol. 2010;44:18-21.
33. Sezer OB and Sezer T. J Neurogastroenterol Motil. 2016;22:656-60.
34. Cristofori F et al. Aliment Pharmacol Ther. 2014;40:309-17.
COVID-19: Implications in gastroenterology
What is coronavirus disease 2019 (COVID-19)?
COVID-19 is a viral respiratory illness that can be potentially life-threatening and is caused by a novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-COV-2). The constellation of symptoms varies in severity but most often includes fever, fatigue, myalgias, cough, and dyspnea. Digestive symptoms such as anorexia, nausea, and diarrhea have also been reported.1 The incubation period of the virus appears to range from 1 to 14 days, most commonly between 3 and 7 days.2 The virus is characterized by its efficient person-to-person transmission, with each case leading to 1.4-3.9 additional infected individuals on average, which has led to a global pandemic and one of the most significant public health crises in modern history.
What are the most vulnerable patient populations within a typical gastroenterology practice?
While the virus can affect anyone, and there are increasing reports of young individuals requiring intensive care, older patients are thought to be at the highest risk for severe disease – particularly those older than age 60 years. Those who developed disease requiring admission to an ICU in Wuhan, China, had a median age of 66 years with comorbid conditions including hypertension, diabetes, and cardiovascular and cerebrovascular disease.3 In addition to these, the Centers for Disease Control and Prevention identifies those who live in a nursing home or long-term care facility to be at high risk, and patients with chronic lung disease, severe obesity, renal failure, or liver disease also may be at increased risk.4 There is often a question if patients on immunosuppression, such as those with inflammatory bowel disease, are at increased risk for the development of infection. At the time of writing, there are not available data that demonstrate this association. Regarding pregnant and lactating women, limited studies done on pregnant patients with COVID-19 revealed that the virus was not transmitted to the fetus in later stages of pregnancy or into breast milk.5 As there is much that has yet to be clearly elucidated, it is prudent to recommend that all patients adhere to social distancing guidelines (including working from home when possible) as well as frequent and thorough hand washing, avoidance of touching one’s face, and avoidance of sick contacts.
Can COVID-19 present with gastrointestinal symptoms?
While initial reports did not describe this as a common presentation, a subsequent multicenter study out of the Hubei province in China reported that nearly half of all patients in the study with COVID-19 had one or more digestive symptoms as their chief complaint. Of note, the study cited the most common digestive complaint as anorexia, which is not necessarily specific to the gastrointestinal tract. Twenty percent of the patients in their cohort did report either abdominal pain, vomiting, or diarrhea.1,6 The majority had concomitant respiratory symptoms, though a small minority (7%) had digestive symptoms only. In patients reporting diarrhea, it was not described as high volume or clinically severe, but the digestive symptoms worsened with severity of the overall disease. Interestingly, the first patient with COVID-19 in the United States presented with nausea, vomiting, and diarrhea; ultimately, stool and respiratory specimens tested positive for the virus. This has led to the question of fecal-oral transmission in addition to, or in lieu of, aerosolization, which has been thought to be the primary mode of transmission.7 There have also been increasing reports of ageusia and anosmia, sometimes as the presenting complaint.8 More data are certainly needed; however, the possibility of gastrointestinal symptoms as a manifestation of COVID-19 and of fecal-oral transmission should be kept in mind when evaluating patients and performing procedures.
What kind of personal protective equipment (PPE) should I wear while performing endoscopy?
An early publication from Italy suggested a risk-stratification system in order to dictate the type of PPE to wear for endoscopy; however, official recommendations from the American Gastroenterological Association (AGA) have since emerged.9,10 For both upper and lower endoscopic procedures, regardless of COVID-19 status, it is recommended to wear a respirator mask, which is specifically designed to block aerosols (N95, N99, or powered air purifying respirator). Given that upper endoscopic procedures are aerosol-generating procedures and there is a theoretic risk to aerosolization during colonoscopy (especially during insertion of instruments through the biopsy channel), respirator masks will provide the most protection to the endoscopist. In addition, the presence of SARS-CoV-2 RNA in fecal samples, although of unclear clinical significance at this time, led to the recommended use of respirators for lower endoscopic procedures as well.
Furthermore, endoscopists should double-glove for all endoscopic procedures in order to reduce viral transmission from contaminated PPE to hands or clothing. Also, in known or presumptive COVID-19 positive patients, negative pressure rooms for endoscopy should be utilized when available.10
If I have been exposed or if I develop symptoms suspicious for COVID-19, what should I do?
First and foremost, a health care provider should reach out to their physician as well as department leadership if in either situation. The CDC recommends immediate self-quarantine if there is any suspicion you may have COVID-19 to minimize further person-to-person transmission.11 This means staying home from work, avoiding public places, and if possible, separating yourself from others in your home. The decision for testing may be individualized based on regional availability of tests, nature of exposure, or severity of symptoms. Many institutions have a sick health care worker triage number in place to advise further. Be cognizant of your symptoms, particularly your respiratory status, and if your condition appears to be worsening seek prompt medical attention and, if possible, call ahead to facilitate being triaged appropriately upon arrival.
As a trainee, how can I minimize my risk while continuing medical education?
Most institutions are implementing ways to minimize exposure of trainees to patients. Ways of doing so include limiting the number of individuals on bedside rounds, providing consultative care and recommendations remotely, conducting team discussions of patients remotely, avoiding workrooms or common areas, and practicing social distancing at the hospital. Some institutions are also consolidating inpatient fellows/services in order to limit fellow time in the hospital, recommending against fellow participation in endoscopy and in-person ambulatory care in order to protect fellows as well as preserve PPE. The reduction in in-person clinical care should be tempered by continuing to prioritize medical education during this time. Fellows can still be involved in an outpatient clinic setting by conducting virtual visits and engaging in telehealth, as many specialties are instituting. Furthermore, clinical conferences, board reviews, and journal club can still be conducted through digital platforms and remain interactive. Trainees can also wisely utilize this unexpected period away from the hospital to complete research projects, case reports, and review articles, thereby strengthening resumes for upcoming job searches or advanced fellowship applications.
To engage in more discussion on how to navigate educational activities in fellowship at this time, visit the AGA community.
To learn more about COVID-19 and its implications for gastroenterologists, visit the AGA university site which features helpful educational modules.
Lastly, the Joint GI Society message on COVID-19 can be found here.
References
1. Pan L et al. Clinical characteristics of COVID-19 patients with digestive symptoms in Hubei, China: a descriptive, cross-sectional, multicenter study. Am J Gastro. 2020. doi: 10.14309/ajg.0000000000000620.
2. Huang C et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506.
3. Wang D et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020 Feb 7;323(11):1061-9.
4. Centers for Disease Control and Prevention. Information for Healthcare Professionals: COVID-19 and Underlying Conditions. Accessed March 22, 2020.
5. Schwartz DA. An analysis of 38 pregnant women with COVID-19, their newborn infants, and maternal-fetal transmission of SARS-CoV-2: Maternal coronavirus infections and pregnancy outcomes. Arch Pathol Lab Med. 2020. doi: 10.5858/arpa.2020-0901-SA.
6. Guan W et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020 Feb 28. doi: 10.1056/NEJMoa2002032.
7. Gu J et al. COVID-19: Gastrointestinal manifestations and potential fecal-oral transmission. Gastroenterology. 2020 Mar 3. doi: 10.1053/j.gastro.2020.02.054.
8. The New York Times. Roni Caryn Rabin, “Lost Sense of Smell May Be a Clue to Coronavirus Infection.” Accessed March 24, 2020.
9. Repici A et al. Coronavirus (COVID-19) outbreak: What the department of endoscopy should know. Gastrointest Endosc. 2020 Mar 14. doi: 10.1016/j.gie.2020.03.019.
10. Sultan S et al. AGA Institute rapid recommendations for gastrointestinal procedures during the COVID-19 pandemic. Gastroenterology. 2020 Mar 31. doi: 10.1053/j.gastro.2020.03.072.
11. Centers for Disease Control and Prevention. COVID-19: What to do if you are sick. Accessed March 22, 2020.
Dr. V.L. Rao is assistant professor of medicine, section of gastroenterology, hepatology, nutrition, department of internal medicine, University of Chicago Medicine; Dr. K. Rao is assistant professor, division of infectious diseases, department of internal medicine, University of Michigan Medical School, Ann Arbor.
What is coronavirus disease 2019 (COVID-19)?
COVID-19 is a viral respiratory illness that can be potentially life-threatening and is caused by a novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-COV-2). The constellation of symptoms varies in severity but most often includes fever, fatigue, myalgias, cough, and dyspnea. Digestive symptoms such as anorexia, nausea, and diarrhea have also been reported.1 The incubation period of the virus appears to range from 1 to 14 days, most commonly between 3 and 7 days.2 The virus is characterized by its efficient person-to-person transmission, with each case leading to 1.4-3.9 additional infected individuals on average, which has led to a global pandemic and one of the most significant public health crises in modern history.
What are the most vulnerable patient populations within a typical gastroenterology practice?
While the virus can affect anyone, and there are increasing reports of young individuals requiring intensive care, older patients are thought to be at the highest risk for severe disease – particularly those older than age 60 years. Those who developed disease requiring admission to an ICU in Wuhan, China, had a median age of 66 years with comorbid conditions including hypertension, diabetes, and cardiovascular and cerebrovascular disease.3 In addition to these, the Centers for Disease Control and Prevention identifies those who live in a nursing home or long-term care facility to be at high risk, and patients with chronic lung disease, severe obesity, renal failure, or liver disease also may be at increased risk.4 There is often a question if patients on immunosuppression, such as those with inflammatory bowel disease, are at increased risk for the development of infection. At the time of writing, there are not available data that demonstrate this association. Regarding pregnant and lactating women, limited studies done on pregnant patients with COVID-19 revealed that the virus was not transmitted to the fetus in later stages of pregnancy or into breast milk.5 As there is much that has yet to be clearly elucidated, it is prudent to recommend that all patients adhere to social distancing guidelines (including working from home when possible) as well as frequent and thorough hand washing, avoidance of touching one’s face, and avoidance of sick contacts.
Can COVID-19 present with gastrointestinal symptoms?
While initial reports did not describe this as a common presentation, a subsequent multicenter study out of the Hubei province in China reported that nearly half of all patients in the study with COVID-19 had one or more digestive symptoms as their chief complaint. Of note, the study cited the most common digestive complaint as anorexia, which is not necessarily specific to the gastrointestinal tract. Twenty percent of the patients in their cohort did report either abdominal pain, vomiting, or diarrhea.1,6 The majority had concomitant respiratory symptoms, though a small minority (7%) had digestive symptoms only. In patients reporting diarrhea, it was not described as high volume or clinically severe, but the digestive symptoms worsened with severity of the overall disease. Interestingly, the first patient with COVID-19 in the United States presented with nausea, vomiting, and diarrhea; ultimately, stool and respiratory specimens tested positive for the virus. This has led to the question of fecal-oral transmission in addition to, or in lieu of, aerosolization, which has been thought to be the primary mode of transmission.7 There have also been increasing reports of ageusia and anosmia, sometimes as the presenting complaint.8 More data are certainly needed; however, the possibility of gastrointestinal symptoms as a manifestation of COVID-19 and of fecal-oral transmission should be kept in mind when evaluating patients and performing procedures.
What kind of personal protective equipment (PPE) should I wear while performing endoscopy?
An early publication from Italy suggested a risk-stratification system in order to dictate the type of PPE to wear for endoscopy; however, official recommendations from the American Gastroenterological Association (AGA) have since emerged.9,10 For both upper and lower endoscopic procedures, regardless of COVID-19 status, it is recommended to wear a respirator mask, which is specifically designed to block aerosols (N95, N99, or powered air purifying respirator). Given that upper endoscopic procedures are aerosol-generating procedures and there is a theoretic risk to aerosolization during colonoscopy (especially during insertion of instruments through the biopsy channel), respirator masks will provide the most protection to the endoscopist. In addition, the presence of SARS-CoV-2 RNA in fecal samples, although of unclear clinical significance at this time, led to the recommended use of respirators for lower endoscopic procedures as well.
Furthermore, endoscopists should double-glove for all endoscopic procedures in order to reduce viral transmission from contaminated PPE to hands or clothing. Also, in known or presumptive COVID-19 positive patients, negative pressure rooms for endoscopy should be utilized when available.10
If I have been exposed or if I develop symptoms suspicious for COVID-19, what should I do?
First and foremost, a health care provider should reach out to their physician as well as department leadership if in either situation. The CDC recommends immediate self-quarantine if there is any suspicion you may have COVID-19 to minimize further person-to-person transmission.11 This means staying home from work, avoiding public places, and if possible, separating yourself from others in your home. The decision for testing may be individualized based on regional availability of tests, nature of exposure, or severity of symptoms. Many institutions have a sick health care worker triage number in place to advise further. Be cognizant of your symptoms, particularly your respiratory status, and if your condition appears to be worsening seek prompt medical attention and, if possible, call ahead to facilitate being triaged appropriately upon arrival.
As a trainee, how can I minimize my risk while continuing medical education?
Most institutions are implementing ways to minimize exposure of trainees to patients. Ways of doing so include limiting the number of individuals on bedside rounds, providing consultative care and recommendations remotely, conducting team discussions of patients remotely, avoiding workrooms or common areas, and practicing social distancing at the hospital. Some institutions are also consolidating inpatient fellows/services in order to limit fellow time in the hospital, recommending against fellow participation in endoscopy and in-person ambulatory care in order to protect fellows as well as preserve PPE. The reduction in in-person clinical care should be tempered by continuing to prioritize medical education during this time. Fellows can still be involved in an outpatient clinic setting by conducting virtual visits and engaging in telehealth, as many specialties are instituting. Furthermore, clinical conferences, board reviews, and journal club can still be conducted through digital platforms and remain interactive. Trainees can also wisely utilize this unexpected period away from the hospital to complete research projects, case reports, and review articles, thereby strengthening resumes for upcoming job searches or advanced fellowship applications.
To engage in more discussion on how to navigate educational activities in fellowship at this time, visit the AGA community.
To learn more about COVID-19 and its implications for gastroenterologists, visit the AGA university site which features helpful educational modules.
Lastly, the Joint GI Society message on COVID-19 can be found here.
References
1. Pan L et al. Clinical characteristics of COVID-19 patients with digestive symptoms in Hubei, China: a descriptive, cross-sectional, multicenter study. Am J Gastro. 2020. doi: 10.14309/ajg.0000000000000620.
2. Huang C et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506.
3. Wang D et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020 Feb 7;323(11):1061-9.
4. Centers for Disease Control and Prevention. Information for Healthcare Professionals: COVID-19 and Underlying Conditions. Accessed March 22, 2020.
5. Schwartz DA. An analysis of 38 pregnant women with COVID-19, their newborn infants, and maternal-fetal transmission of SARS-CoV-2: Maternal coronavirus infections and pregnancy outcomes. Arch Pathol Lab Med. 2020. doi: 10.5858/arpa.2020-0901-SA.
6. Guan W et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020 Feb 28. doi: 10.1056/NEJMoa2002032.
7. Gu J et al. COVID-19: Gastrointestinal manifestations and potential fecal-oral transmission. Gastroenterology. 2020 Mar 3. doi: 10.1053/j.gastro.2020.02.054.
8. The New York Times. Roni Caryn Rabin, “Lost Sense of Smell May Be a Clue to Coronavirus Infection.” Accessed March 24, 2020.
9. Repici A et al. Coronavirus (COVID-19) outbreak: What the department of endoscopy should know. Gastrointest Endosc. 2020 Mar 14. doi: 10.1016/j.gie.2020.03.019.
10. Sultan S et al. AGA Institute rapid recommendations for gastrointestinal procedures during the COVID-19 pandemic. Gastroenterology. 2020 Mar 31. doi: 10.1053/j.gastro.2020.03.072.
11. Centers for Disease Control and Prevention. COVID-19: What to do if you are sick. Accessed March 22, 2020.
Dr. V.L. Rao is assistant professor of medicine, section of gastroenterology, hepatology, nutrition, department of internal medicine, University of Chicago Medicine; Dr. K. Rao is assistant professor, division of infectious diseases, department of internal medicine, University of Michigan Medical School, Ann Arbor.
What is coronavirus disease 2019 (COVID-19)?
COVID-19 is a viral respiratory illness that can be potentially life-threatening and is caused by a novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-COV-2). The constellation of symptoms varies in severity but most often includes fever, fatigue, myalgias, cough, and dyspnea. Digestive symptoms such as anorexia, nausea, and diarrhea have also been reported.1 The incubation period of the virus appears to range from 1 to 14 days, most commonly between 3 and 7 days.2 The virus is characterized by its efficient person-to-person transmission, with each case leading to 1.4-3.9 additional infected individuals on average, which has led to a global pandemic and one of the most significant public health crises in modern history.
What are the most vulnerable patient populations within a typical gastroenterology practice?
While the virus can affect anyone, and there are increasing reports of young individuals requiring intensive care, older patients are thought to be at the highest risk for severe disease – particularly those older than age 60 years. Those who developed disease requiring admission to an ICU in Wuhan, China, had a median age of 66 years with comorbid conditions including hypertension, diabetes, and cardiovascular and cerebrovascular disease.3 In addition to these, the Centers for Disease Control and Prevention identifies those who live in a nursing home or long-term care facility to be at high risk, and patients with chronic lung disease, severe obesity, renal failure, or liver disease also may be at increased risk.4 There is often a question if patients on immunosuppression, such as those with inflammatory bowel disease, are at increased risk for the development of infection. At the time of writing, there are not available data that demonstrate this association. Regarding pregnant and lactating women, limited studies done on pregnant patients with COVID-19 revealed that the virus was not transmitted to the fetus in later stages of pregnancy or into breast milk.5 As there is much that has yet to be clearly elucidated, it is prudent to recommend that all patients adhere to social distancing guidelines (including working from home when possible) as well as frequent and thorough hand washing, avoidance of touching one’s face, and avoidance of sick contacts.
Can COVID-19 present with gastrointestinal symptoms?
While initial reports did not describe this as a common presentation, a subsequent multicenter study out of the Hubei province in China reported that nearly half of all patients in the study with COVID-19 had one or more digestive symptoms as their chief complaint. Of note, the study cited the most common digestive complaint as anorexia, which is not necessarily specific to the gastrointestinal tract. Twenty percent of the patients in their cohort did report either abdominal pain, vomiting, or diarrhea.1,6 The majority had concomitant respiratory symptoms, though a small minority (7%) had digestive symptoms only. In patients reporting diarrhea, it was not described as high volume or clinically severe, but the digestive symptoms worsened with severity of the overall disease. Interestingly, the first patient with COVID-19 in the United States presented with nausea, vomiting, and diarrhea; ultimately, stool and respiratory specimens tested positive for the virus. This has led to the question of fecal-oral transmission in addition to, or in lieu of, aerosolization, which has been thought to be the primary mode of transmission.7 There have also been increasing reports of ageusia and anosmia, sometimes as the presenting complaint.8 More data are certainly needed; however, the possibility of gastrointestinal symptoms as a manifestation of COVID-19 and of fecal-oral transmission should be kept in mind when evaluating patients and performing procedures.
What kind of personal protective equipment (PPE) should I wear while performing endoscopy?
An early publication from Italy suggested a risk-stratification system in order to dictate the type of PPE to wear for endoscopy; however, official recommendations from the American Gastroenterological Association (AGA) have since emerged.9,10 For both upper and lower endoscopic procedures, regardless of COVID-19 status, it is recommended to wear a respirator mask, which is specifically designed to block aerosols (N95, N99, or powered air purifying respirator). Given that upper endoscopic procedures are aerosol-generating procedures and there is a theoretic risk to aerosolization during colonoscopy (especially during insertion of instruments through the biopsy channel), respirator masks will provide the most protection to the endoscopist. In addition, the presence of SARS-CoV-2 RNA in fecal samples, although of unclear clinical significance at this time, led to the recommended use of respirators for lower endoscopic procedures as well.
Furthermore, endoscopists should double-glove for all endoscopic procedures in order to reduce viral transmission from contaminated PPE to hands or clothing. Also, in known or presumptive COVID-19 positive patients, negative pressure rooms for endoscopy should be utilized when available.10
If I have been exposed or if I develop symptoms suspicious for COVID-19, what should I do?
First and foremost, a health care provider should reach out to their physician as well as department leadership if in either situation. The CDC recommends immediate self-quarantine if there is any suspicion you may have COVID-19 to minimize further person-to-person transmission.11 This means staying home from work, avoiding public places, and if possible, separating yourself from others in your home. The decision for testing may be individualized based on regional availability of tests, nature of exposure, or severity of symptoms. Many institutions have a sick health care worker triage number in place to advise further. Be cognizant of your symptoms, particularly your respiratory status, and if your condition appears to be worsening seek prompt medical attention and, if possible, call ahead to facilitate being triaged appropriately upon arrival.
As a trainee, how can I minimize my risk while continuing medical education?
Most institutions are implementing ways to minimize exposure of trainees to patients. Ways of doing so include limiting the number of individuals on bedside rounds, providing consultative care and recommendations remotely, conducting team discussions of patients remotely, avoiding workrooms or common areas, and practicing social distancing at the hospital. Some institutions are also consolidating inpatient fellows/services in order to limit fellow time in the hospital, recommending against fellow participation in endoscopy and in-person ambulatory care in order to protect fellows as well as preserve PPE. The reduction in in-person clinical care should be tempered by continuing to prioritize medical education during this time. Fellows can still be involved in an outpatient clinic setting by conducting virtual visits and engaging in telehealth, as many specialties are instituting. Furthermore, clinical conferences, board reviews, and journal club can still be conducted through digital platforms and remain interactive. Trainees can also wisely utilize this unexpected period away from the hospital to complete research projects, case reports, and review articles, thereby strengthening resumes for upcoming job searches or advanced fellowship applications.
To engage in more discussion on how to navigate educational activities in fellowship at this time, visit the AGA community.
To learn more about COVID-19 and its implications for gastroenterologists, visit the AGA university site which features helpful educational modules.
Lastly, the Joint GI Society message on COVID-19 can be found here.
References
1. Pan L et al. Clinical characteristics of COVID-19 patients with digestive symptoms in Hubei, China: a descriptive, cross-sectional, multicenter study. Am J Gastro. 2020. doi: 10.14309/ajg.0000000000000620.
2. Huang C et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506.
3. Wang D et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020 Feb 7;323(11):1061-9.
4. Centers for Disease Control and Prevention. Information for Healthcare Professionals: COVID-19 and Underlying Conditions. Accessed March 22, 2020.
5. Schwartz DA. An analysis of 38 pregnant women with COVID-19, their newborn infants, and maternal-fetal transmission of SARS-CoV-2: Maternal coronavirus infections and pregnancy outcomes. Arch Pathol Lab Med. 2020. doi: 10.5858/arpa.2020-0901-SA.
6. Guan W et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020 Feb 28. doi: 10.1056/NEJMoa2002032.
7. Gu J et al. COVID-19: Gastrointestinal manifestations and potential fecal-oral transmission. Gastroenterology. 2020 Mar 3. doi: 10.1053/j.gastro.2020.02.054.
8. The New York Times. Roni Caryn Rabin, “Lost Sense of Smell May Be a Clue to Coronavirus Infection.” Accessed March 24, 2020.
9. Repici A et al. Coronavirus (COVID-19) outbreak: What the department of endoscopy should know. Gastrointest Endosc. 2020 Mar 14. doi: 10.1016/j.gie.2020.03.019.
10. Sultan S et al. AGA Institute rapid recommendations for gastrointestinal procedures during the COVID-19 pandemic. Gastroenterology. 2020 Mar 31. doi: 10.1053/j.gastro.2020.03.072.
11. Centers for Disease Control and Prevention. COVID-19: What to do if you are sick. Accessed March 22, 2020.
Dr. V.L. Rao is assistant professor of medicine, section of gastroenterology, hepatology, nutrition, department of internal medicine, University of Chicago Medicine; Dr. K. Rao is assistant professor, division of infectious diseases, department of internal medicine, University of Michigan Medical School, Ann Arbor.
Calendar
For more information about upcoming events and award deadlines, please visit http://agau.gastro.org and http://www.gastro.org/research-funding.
UPCOMING EVENTS
May 2-5, 2020
Digestive Disease Week® (DDW)
DDW® 2020 and all associated events have been canceled. While we are disappointed to miss the science, education and networking that are hallmarks of DDW®, we must focus on the health and safety of our community. Certainly, this cancellation raises many questions. We have attempted to answer them in this FAQ and remain committed to keeping you informed of new details as they form.
Aug. 14-15, 2020
James W. Freston Single Topic Conference: Gastrointestinal Organoids and Engineered Organ Systems
AGA is actively evaluating developments concerning coronavirus. We expect the 2020 James W. Freston Conference will take place as scheduled and continue to monitor the situation.
Chicago, IL
Aug. 14-16, 2020
2020 Principles of GI for the NP and PA
AGA is actively evaluating developments concerning coronavirus. We expect the 2020 Principles of GI for the NP and PA will take place as scheduled and continue to monitor the situation.
Denver, CO
For more information about upcoming events and award deadlines, please visit http://agau.gastro.org and http://www.gastro.org/research-funding.
UPCOMING EVENTS
May 2-5, 2020
Digestive Disease Week® (DDW)
DDW® 2020 and all associated events have been canceled. While we are disappointed to miss the science, education and networking that are hallmarks of DDW®, we must focus on the health and safety of our community. Certainly, this cancellation raises many questions. We have attempted to answer them in this FAQ and remain committed to keeping you informed of new details as they form.
Aug. 14-15, 2020
James W. Freston Single Topic Conference: Gastrointestinal Organoids and Engineered Organ Systems
AGA is actively evaluating developments concerning coronavirus. We expect the 2020 James W. Freston Conference will take place as scheduled and continue to monitor the situation.
Chicago, IL
Aug. 14-16, 2020
2020 Principles of GI for the NP and PA
AGA is actively evaluating developments concerning coronavirus. We expect the 2020 Principles of GI for the NP and PA will take place as scheduled and continue to monitor the situation.
Denver, CO
For more information about upcoming events and award deadlines, please visit http://agau.gastro.org and http://www.gastro.org/research-funding.
UPCOMING EVENTS
May 2-5, 2020
Digestive Disease Week® (DDW)
DDW® 2020 and all associated events have been canceled. While we are disappointed to miss the science, education and networking that are hallmarks of DDW®, we must focus on the health and safety of our community. Certainly, this cancellation raises many questions. We have attempted to answer them in this FAQ and remain committed to keeping you informed of new details as they form.
Aug. 14-15, 2020
James W. Freston Single Topic Conference: Gastrointestinal Organoids and Engineered Organ Systems
AGA is actively evaluating developments concerning coronavirus. We expect the 2020 James W. Freston Conference will take place as scheduled and continue to monitor the situation.
Chicago, IL
Aug. 14-16, 2020
2020 Principles of GI for the NP and PA
AGA is actively evaluating developments concerning coronavirus. We expect the 2020 Principles of GI for the NP and PA will take place as scheduled and continue to monitor the situation.
Denver, CO























