User login
2019 Update in perioperative cardiovascular medicine
Perioperative medicine is an evolving field with a rapidly growing body of literature, particularly in cardiology.
In this update, we review 6 articles to answer questions related to preoperative cardiac risk assessment, perioperative medication management, and postoperative cardiac complications. We surveyed perioperative literature from February 2018 through January 2019 and chose the final articles by consensus, based on relevance to clinicians who provide preoperative evaluations and postoperative care to surgical patients.
These summaries are derived from “Updates in Perioperative Medicine” presented at the 14th Annual Perioperative Medicine Summit (Orlando, FL, February 13–16, 2019) and the 2019 Society of Hospital Medicine Annual Meeting (National Harbor, MD, March 24–27, 2019).
PREOPERATIVE CARDIAC EVALUATION
How well do measures of functional capacity predict perioperative complications and mortality in noncardiac surgical patients?
Functional capacity is commonly assessed in preoperative evaluations to estimate patients’ risks of perioperative complications and death. The American College of Cardiology/American Heart Association1 and the European Society of Cardiology2 guidelines both include estimation of cardiopulmonary fitness as a step in preoperative assessment before major noncardiac surgery.
“Subjective assessment” is one way to estimate functional capacity. Simply put, clinicians try to form a rough idea about the fitness of patients by asking questions about routine activities such as walking or climbing stairs. Although commonly used, subjective assessment of functional capacity lacks strong evidence that it predicts adverse perioperative events.
Cardiopulmonary exercise testing is a third option. It measures peak oxygen consumption and anaerobic threshold during exercise. It is probably the best objective measurement of functional capacity, but not necessarily for predicting postoperative cardiac complications, and it is performed relatively infrequently.
[Wijeysundera DN, Pearse RM, Sulman MA, et al. Assessment of functional capacity before major non-cardiac surgery: an international, prospective cohort study. Lancet 2018; 391(10140):2631–2640. doi:10.1016/S0140-6736(18)31131-0]
In a multicenter, prospective cohort study, Wijeysundera et al4 compared subjective functional capacity assessment, the Duke Activity Status Index, cardiopulmonary exercise testing, and the preoperative N-terminal pro-B-type natriuretic peptide (NT-proBNP) level in their ability to predict complications and death in 1,401 noncardiac surgery patients older than 40 with at least 1 cardiovascular risk factor. After surgery, patients had daily electrocardiograms and troponin measurements until postoperative day 3 or discharge.
The primary outcome was the 30-day incidence of death or myocardial infarction (MI). Additional outcomes included the 30-day incidence of death or myocardial injury after noncardiac surgery (MINS), the 1-year mortality rate, and moderate to severe in-hospital perioperative complications.
Findings. Two percent of patients died or had an MI within 30 days of surgery.4
Subjective assessment had only a 19.2% sensitivity (95% confidence interval [CI] 14.2–25) but a 94.7% specificity (95% CI 93.2–95.9) for predicting inability to attain 4 metabolic equivalents during exercise.4
A lower Duke Activity Status Index predicted the primary outcome of death or MI within 30 days (adjusted odds ratio [OR] 0.96, 95% CI 0.83–0.99, P = .03), and it was the only measure that did so. Additionally, the Duke index and NT-proBNP level predicted the risk of death or MINS within 30 days.4
Only elevated NT-proBNP was associated with death at 1 year.4
On exercise testing, low peak oxygen consumption was significantly associated with perioperative complications.
Limitations. The number of primary outcome events (death and MI) was low, potentially affecting the statistical power of the study.
Conclusions. Subjective assessment of functional capacity misclassifies too many patients as being at low risk of perioperative complications and should not be used for preoperative risk stratification. Other tools, such as the Duke Activity Status Index and NT-proBNP levels, are better predictors of adverse perioperative cardiovascular outcomes and should be considered for use in preoperative cardiac risk assessment.
Although the Duke Activity Status Index is a better predictor of adverse outcomes than subjective functional capacity assessment, a specific perioperative threshold for risk classification has not been established. Its correlate for metabolic equivalents should be considered for use in clinical practice at this point.
PERIOPERATIVE MEDICATION MANAGEMENT
Is perioperative aspirin beneficial in patients undergoing vascular surgery?
The Perioperative Ischemic Evaluation 2 (POISE-2) trial,5 a 2-by-2 factorial randomized controlled trial in which patients received perioperative aspirin, clonidine, both, or neither, demonstrated that perioperative aspirin did not reduce cardiovascular events and increased major bleeding. Patients with recently placed coronary stents and those undergoing carotid endarterectomy were excluded because aspirin is known to have a beneficial effect in these patients.
A subsequent substudy6 found perioperative aspirin to be beneficial in patients with coronary stents placed more than a year before noncardiac surgery. Whether perioperative aspirin is beneficial in other subgroups was unknown.
[Biccard BM, Sigamani A, Chan MTV, et al. Effect of aspirin in vascular surgery in patients from a randomized clinical trial (POISE-2). Br J Surg 2018; 105(12):1591–1597. doi:10.1002/bjs.10925]
Biccard et al7 investigated the effect of perioperative aspirin in the subgroup of patients from the POISE-2 trial who underwent vascular surgery. The primary outcome was death or MI within 30 days. Secondary outcomes in this substudy included vascular occlusive complications (amputation and peripheral arterial thrombosis) and major or life-threatening bleeding.
Limitations. There were few adverse events, and this substudy was underpowered for the primary and secondary outcomes.
Conclusion. Starting or continuing aspirin did not improve outcomes, and withdrawing it did not increase cardiovascular or occlusive complications.
Do ACE inhibitors affect risk in noncardiac nonvascular surgery?
Angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs) are some of the most commonly used medications for treating hypertension. But whether patients should continue receiving them on the day of surgery or whether they should be held remains unclear.
Although current recommendations are inconsistent, the most recent American College of Cardiology/American Heart Association1 perioperative practice guidelines say that continuing ACE inhibitors or ARBs is reasonable perioperatively. This recommendation, however, acknowledges that published evidence is limited. There is general agreement that preoperative exposure to ACE inhibitors and ARBs is associated with intraoperative hypotension, but whether this increases the risk of adverse clinical outcomes remains unclear. Needed was a study to determine the effect on perioperative morbidity and mortality of continuing vs withholding ACE inhibitors and ARBs before surgery.
[Shiffermiller JF, Monson BJ, Vokoun CW, et al. Prospective randomized evaluation of preoperative angiotensin-converting enzyme inhibition (PREOP-ACEI). J Hosp Med 2018; 13(10):661–667. doi:10.12788/jhm.3036]
Shiffermiller et al8 performed a randomized controlled trial comparing the effect of 2 preoperative ACE inhibitor management protocols in patients undergoing noncardiac nonvascular surgery. Patients were randomized to either receive or not receive their final preoperative ACE inhibitor dose, whether scheduled on the morning of surgery or the night before.
Exclusion criteria included hypotension or hypertension at their preoperative clinic appointment (defined as systolic blood pressure < 90 or ≥ 160 mm Hg, and diastolic blood pressure < 60 or ≥ 95 mm Hg), moderate to severe heart failure, and end-stage renal disease requiring dialysis. Excluded surgery types were cardiac, vascular, organ transplant, oncologic, and all outpatient procedures. Patients taking ARBs were also excluded.
The primary outcome was intraoperative hypotension defined as any systolic blood pressure less than 80 mm Hg from the time of anesthesia induction until transfer to the postanesthesia care unit. Secondary outcomes were measured until hospital discharge and included postoperative acute kidney injury, postoperative hypotension (systolic pressure < 90 mm Hg) and hypertension (systolic pressure > 180 mm Hg), major cardiac events (composite of acute coronary syndrome, acute heart failure, or new-onset arrhythmia), and death.
Findings. A total of 453 patients were screened for eligibility, and of these, 291 were included for randomization. Their average age was 64, 48% were men, and 87% were white. About 50% underwent general anesthesia, 25% spinal, and 25% regional. Over half of the surgeries were orthopedic, and 20% were spine surgeries.
The primary outcome of intraoperative hypotension occurred significantly less often in patients randomized to ACE inhibitor omission than in the continuation group (55% vs 69%, relative risk [RR] 0.81, 95% CI 0.67–0.97, P = .03). This translates to 1 case of intraoperative hypotension for every 7.5 patients continuing an ACE inhibitor perioperatively (number needed to harm 7.5). Intraoperative hypotension associated with vasopressor administration also occurred significantly less frequently in the ACE inhibitor omission group.
Patients in the ACE inhibitor omission group were also less likely to experience postoperative hypotension, but on the other hand, they were more likely to experience severe postoperative hypertension (defined as any systolic blood pressure > 180 mm Hg). The two groups fared the same in terms of rates of acute kidney injury and major adverse cardiac events (MACE) and hospital length of stay, and no patients died in either group.
Limitations. Several factors limit the generalizability of this single-center study, including the many exclusion criteria, the predominance of orthopedic and spine surgeries, and the low-risk patient population (the average Revised Cardiac Risk Index score was 0, range 0–3). Other limitations include not controlling for the specific ACE inhibitor used and not including the precise timing of the final dose in relation to surgery. Lastly, this study lacked power to measure postoperative outcomes.
Conclusions. Continuing ACE inhibitor treatment before noncardiac nonvascular surgery is associated with a greater frequency and duration of intraoperative hypotension, but it did not increase the incidences of acute kidney injury, MACE, or death nor the hospital length of stay.
[Hollmann C, Fernandes NL, Biccard BM. A systematic review of outcomes associated with withholding or continuing angiotensin-converting enzyme inhibitors and angiotensin receptor blockers before noncardiac surgery. Anesth Analg 2018; 127(3):678–687. doi:10.1213/ANE.0000000000002837]
Hollmann et al9 performed a meta-analysis to determine whether it is better to continue or withhold ACE inhibitors and ARBs before surgery. The patients were adults undergoing noncardiac surgery and receiving an ACE inhibitor or ARB, which was either withheld or continued on the morning of surgery.
Primary outcomes were all-cause mortality and MACE, while secondary outcomes included the incidence of acute kidney injury, heart failure, stroke, intraoperative and postoperative hypotension, and length of hospital stay. Randomized controlled trials and observational studies were included, while case reports and case-control studies were excluded.
Findings. This meta-analysis included 5 randomized controlled trials and 4 cohort studies, with a total of 6,022 patients; 1,816 had their ACE inhibitor or ARB withheld before surgery, while 4,206 continued therapy. It found no difference between the 2 groups in the incidence of death or MACE, and there were not enough data to determine a difference in heart failure, stroke, acute kidney injury, or hospital length of stay.
Seven studies, with 5,414 patients, examined intraoperative hypotension. The overall incidence was 30%, but was significantly lower if the ACE inhibitor or ARB was withheld (OR 0.63, 95% CI 0.47–0.85, P = .002). Findings were similar in an analysis of only the randomized controlled trials. No difference was observed in postoperative hypotension.
Limitations. There was no standard definition of the morbidity outcomes, including hypotension and MACE. The assessment of MACE included data only for MI and not MINS. The specific duration of hypotension was not reported, and this meta-analysis did not take into account different anesthetic techniques. The duration of follow-up varied widely among studies, ranging from the day of hospital discharge to 30 days after surgery. And the randomized controlled trial performed by Shiffermiller et al8 was not included.
Conclusions. While continuing ACE inhibitors or ARBs before noncardiac surgery was associated with intraoperative hypotension, it did not seem to affect other outcomes, including death and MACE. The authors propose that a large randomized controlled trial is needed to determine whether continuing or withholding ACE inhibitor or ARB therapy before surgery is safer.
POSTOPERATIVE CARDIAC COMPLICATIONS
How should we treat MINS?
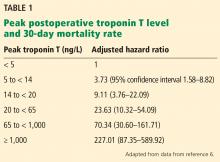
MINS is associated with an increased risk of cardiovascular events and death in both the short term and long term. MINS is defined as an elevated postoperative troponin level related to an ischemic etiology. However, whether to routinely measure troponin after surgery is unclear, as most patients do not present with ischemic symptoms, and there is no standard of care for treatment of this entity. Limited observational data suggest that starting or intensifying cardiac medications, particularly aspirin and statins, may be beneficial in terms of reducing 30-day mortality rates in patients with MI or cardiac events at 1 year in vascular surgery patients with MINS.
The Management of Myocardial Injury After Noncardiac Surgery (MANAGE) trial was designed to evaluate the potential of the anticoagulant dabigatran to prevent major vascular complications in patients with MINS.
[Devereaux PJ, Duceppe E, Guyatt G, et al. Dabigatran in patients with myocardial injury after non-cardiac surgery (MANAGE): an international, randomised, placebo-controlled trial. Lancet 2018; 391(10137):2325–2334. doi:10.1016/S0140-6736(18)30832-8]
Devereaux et al10 randomized patients who were at least 45 years old and had developed MINS within the previous 35 days to receive dabigatran 110 mg orally twice daily or placebo for up to 2 years. Patients not already taking a proton pump inhibitor were also randomized to take either omeprazole 20 mg once daily or placebo.
The primary efficacy outcome initially was major vascular complications, which included vascular mortality, nonfatal MI, nonhemorrhagic stroke, and peripheral arterial thrombosis. However, amputation and symptomatic venous thromboembolism were subsequently added during the study.
The primary safety outcome was a composite of life-threatening, major, and critical organ bleeding. Major bleeding required a decrease in hemoglobin of at least 4 g/dL, transfusion of at least 3 units of red blood cells within a 24-hour period, or a procedure to stop the bleeding.
Findings. The original goal was to recruit 3,200 patients, but due to slow enrollment and loss of funding, the sample was reduced to 1,754 patients (877 in each group). Approximately 45% of each group stopped taking the study drug prematurely.
The primary efficacy outcome occurred in significantly fewer patients receiving dabigatran (97, 11%) than placebo (133, 15%, HR 0.72, 95% CI 0.55–0.93, P = .0115). The incidence of the primary safety outcome was similar in both groups: 3% with dabigatran and 4% with placebo (HR 0.92, 95% CI 0.55–1.53, P = .76). The only individual efficacy outcome meeting statistical significance was a lower rate of nonhemorrhagic stroke in the dabigatran group. Subgroup analyses showed a trend benefiting patients randomized within 5 days of MINS or with a diagnosis of MI, although it was not statistically significant.
Limitations. The efficacy outcomes were expanded to include venous thromboembolism and others not directly related to MINS, raising questions about the conclusions. Further, as defined by the protocol, bleeding had to be fairly severe to be deemed major. The high number of patients who discontinued the study drug is another limitation of this study.
Conclusion. Dabigatran lowered the risk of major vascular complications with no significant increase in major bleeding in patients with MINS.
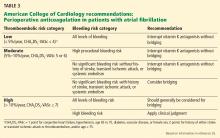
What is the risk of thromboembolism in postoperative atrial fibrillation, and what are the benefits of anticoagulation?
Although nonvalvular atrial fibrillation is associated with increased risks of ischemic stroke and systemic embolic events in nonsurgical patients, the association of new-onset postoperative atrial fibrillation with long-term thromboembolic events in the noncardiac surgical population is not well established.
[Butt JH, Olesen JB, Havers-Borgersen E, et al. Risk of thromboembolism associated with atrial fibrillation following noncardiac surgery. J Am Coll Cardiol 2018; 72(17):2027–2036. doi:10.1016/j.jacc.2018.07.088]
In this retrospective cohort study using a nationwide registry in Denmark, Butt et al11 assessed the long-term risk of thromboembolic events in noncardiac surgical patients with new postoperative atrial fibrillation. Patients were identified who had no previous history of atrial fibrillation and developed it after noncardiac, nonobstetric surgeries, and were matched in a 1:4 ratio with patients who developed nonvalvular atrial fibrillation during nonsurgical hospitalizations. Matching was based on age, sex, heart failure, hypertension, diabetes, known history of thromboembolic events, ischemic heart disease, and the year patients presented with new atrial fibrillation.
Patients were excluded if they received antiarrhythmic drugs or oral anticoagulants before hospitalization or surgery, had cancer in the year prior, or died in the hospital.
The primary outcome of the study was thromboembolic events—a composite of ischemic stroke, transient cerebral ischemia, and peripheral arterial thrombosis or embolism. Secondary outcomes included rehospitalization for atrial fibrillation and all-cause mortality.
Findings. Overall, 0.4% of patients developed new postoperative atrial fibrillation, of whom 3,380 were matched with 15,320 patients with nonvalvular atrial fibrillation. Over a median follow-up of 3.2 years, the risk of thromboembolic events was similar in both groups (31.7 and 29.9 per 1,000 person-years, HR 0.95, 95% CI 0.85–1.07). The groups did not differ in their CHA2DS2-VASc risk scores, HAS-BLED risk scores, or year in which patients were diagnosed.
Anticoagulation lowered the risk of thromboembolic events to a similar extent in both groups compared with no anticoagulation:
- In postoperative atrial fibrillation—HR 0.57, 95% CI 0.40–0.67
- In nonvalvular atrial fibrillation—HR 0.56, 95% CI 0.51–0.62.
Despite the similar reduction in thromboembolic events, only 24.4% of the postoperative atrial fibrillation patients were started on anticoagulation therapy within 30 days of discharge, compared with 41.5% of those with nonvalvular atrial fibrillation.
Limitations. Although this was a large study with excellent follow-up data, it was observational. It may have underestimated the number of patients who developed postoperative atrial fibrillation because episodes that were judged not to be clinically significant may not have been charted. Many patients are not monitored with continuous telemetry postoperatively, which also may have led to underestimation of the number of atrial fibrillation events.
The study also did not examine the number of atrial fibrillation episodes per patient, the heart rhythm at discharge or long-term, or indication for and duration of anticoagulation. There were no data regarding international normalized ratio levels.
Conclusions. Postoperative atrial fibrillation is associated with outcomes similar to those of nonsurgical nonvalvular atrial fibrillation. Anticoagulation decreases the risks of stroke and death. However, substantially fewer patients with postoperative atrial fibrillation receive anticoagulation. Anticoagulation should be considered in these patients, while noting bleeding risk.
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol 2014; 64(22):e77–137. doi:10.1016/j.jacc.2014.07.944
- Kristensen SD, Knuuti J, Saraste A, et al. 2014 ESC/ESA Guidelines on non-cardiac surgery: cardiovascular assessment and management: the Joint Task Force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur Heart J 2014; 35(35):2383–2431. doi:10.1093/eurheartj/ehu282
- Hlatky MA, Boineau RE, Higginbotham MB, et al. A brief self-administered questionnaire to determine functional capacity (The Duke Activity Status Index). Am J Cardiol 1989; 64(10):651–654. doi:10.1016/0002-9149(89)90496-7
- Wijeysundera DN, Pearse RM, Sulman MA, et al. Assessment of functional capacity before major non-cardiac surgery: an international, prospective cohort study. Lancet 2018; 391(10140):2631–2640. doi:10.1016/S0140-6736(18)31131-0
- Devereaux PJ, Mrkobrada M, Sessler DI, et al; POISE-2 Investigators. Aspirin in patients undergoing noncardiac surgery. N Engl J Med 2014; 370(16):1494–1503. doi:10.1056/NEJMoa1401105
- Graham MM, Sessler DI, Parlow JL, et al. Aspirin in patients with previous percutaneous coronary intervention undergoing noncardiac surgery. Ann Intern Med 2018;168(4):237–244. pmid:29132159
- Biccard BM, Sigamani A, Chan MTV, et al. Effect of aspirin in vascular surgery in patients from a randomized clinical trial (POISE-2). Br J Surg 2018; 105(12):1591–1597. doi:10.1002/bjs.10925
- Shiffermiller JF, Monson BJ, Vokoun CW, et al. Prospective randomized evaluation of preoperative angiotensin-converting enzyme inhibition (PREOP-ACEI). J Hosp Med 2018; 13(10):661–667. doi:10.12788/jhm.3036
- Hollmann C, Fernandes NL, Biccard BM. A systematic review of outcomes associated with withholding or continuing angiotensin-converting enzyme inhibitors and angiotensin receptor blockers before noncardiac surgery. Anesth Analg 2018; 127(3):678–687. doi:10.1213/ANE.0000000000002837
- Devereaux PJ, Duceppe E, Guyatt G, et al. Dabigatran in patients with myocardial injury after non-cardiac surgery (MANAGE): an international, randomised, placebo-controlled trial. Lancet 2018; 391(10137):2325–2334. doi:10.1016/S0140-6736(18)30832-8
- Butt JH, Olesen JB, Havers-Borgersen E, et al. Risk of thromboembolism associated with atrial fibrillation following noncardiac surgery. J Am Coll Cardiol 2018; 72(17):2027–2036. doi:10.1016/j.jacc.2018.07.088
Perioperative medicine is an evolving field with a rapidly growing body of literature, particularly in cardiology.
In this update, we review 6 articles to answer questions related to preoperative cardiac risk assessment, perioperative medication management, and postoperative cardiac complications. We surveyed perioperative literature from February 2018 through January 2019 and chose the final articles by consensus, based on relevance to clinicians who provide preoperative evaluations and postoperative care to surgical patients.
These summaries are derived from “Updates in Perioperative Medicine” presented at the 14th Annual Perioperative Medicine Summit (Orlando, FL, February 13–16, 2019) and the 2019 Society of Hospital Medicine Annual Meeting (National Harbor, MD, March 24–27, 2019).
PREOPERATIVE CARDIAC EVALUATION
How well do measures of functional capacity predict perioperative complications and mortality in noncardiac surgical patients?
Functional capacity is commonly assessed in preoperative evaluations to estimate patients’ risks of perioperative complications and death. The American College of Cardiology/American Heart Association1 and the European Society of Cardiology2 guidelines both include estimation of cardiopulmonary fitness as a step in preoperative assessment before major noncardiac surgery.
“Subjective assessment” is one way to estimate functional capacity. Simply put, clinicians try to form a rough idea about the fitness of patients by asking questions about routine activities such as walking or climbing stairs. Although commonly used, subjective assessment of functional capacity lacks strong evidence that it predicts adverse perioperative events.
Cardiopulmonary exercise testing is a third option. It measures peak oxygen consumption and anaerobic threshold during exercise. It is probably the best objective measurement of functional capacity, but not necessarily for predicting postoperative cardiac complications, and it is performed relatively infrequently.
[Wijeysundera DN, Pearse RM, Sulman MA, et al. Assessment of functional capacity before major non-cardiac surgery: an international, prospective cohort study. Lancet 2018; 391(10140):2631–2640. doi:10.1016/S0140-6736(18)31131-0]
In a multicenter, prospective cohort study, Wijeysundera et al4 compared subjective functional capacity assessment, the Duke Activity Status Index, cardiopulmonary exercise testing, and the preoperative N-terminal pro-B-type natriuretic peptide (NT-proBNP) level in their ability to predict complications and death in 1,401 noncardiac surgery patients older than 40 with at least 1 cardiovascular risk factor. After surgery, patients had daily electrocardiograms and troponin measurements until postoperative day 3 or discharge.
The primary outcome was the 30-day incidence of death or myocardial infarction (MI). Additional outcomes included the 30-day incidence of death or myocardial injury after noncardiac surgery (MINS), the 1-year mortality rate, and moderate to severe in-hospital perioperative complications.
Findings. Two percent of patients died or had an MI within 30 days of surgery.4
Subjective assessment had only a 19.2% sensitivity (95% confidence interval [CI] 14.2–25) but a 94.7% specificity (95% CI 93.2–95.9) for predicting inability to attain 4 metabolic equivalents during exercise.4
A lower Duke Activity Status Index predicted the primary outcome of death or MI within 30 days (adjusted odds ratio [OR] 0.96, 95% CI 0.83–0.99, P = .03), and it was the only measure that did so. Additionally, the Duke index and NT-proBNP level predicted the risk of death or MINS within 30 days.4
Only elevated NT-proBNP was associated with death at 1 year.4
On exercise testing, low peak oxygen consumption was significantly associated with perioperative complications.
Limitations. The number of primary outcome events (death and MI) was low, potentially affecting the statistical power of the study.
Conclusions. Subjective assessment of functional capacity misclassifies too many patients as being at low risk of perioperative complications and should not be used for preoperative risk stratification. Other tools, such as the Duke Activity Status Index and NT-proBNP levels, are better predictors of adverse perioperative cardiovascular outcomes and should be considered for use in preoperative cardiac risk assessment.
Although the Duke Activity Status Index is a better predictor of adverse outcomes than subjective functional capacity assessment, a specific perioperative threshold for risk classification has not been established. Its correlate for metabolic equivalents should be considered for use in clinical practice at this point.
PERIOPERATIVE MEDICATION MANAGEMENT
Is perioperative aspirin beneficial in patients undergoing vascular surgery?
The Perioperative Ischemic Evaluation 2 (POISE-2) trial,5 a 2-by-2 factorial randomized controlled trial in which patients received perioperative aspirin, clonidine, both, or neither, demonstrated that perioperative aspirin did not reduce cardiovascular events and increased major bleeding. Patients with recently placed coronary stents and those undergoing carotid endarterectomy were excluded because aspirin is known to have a beneficial effect in these patients.
A subsequent substudy6 found perioperative aspirin to be beneficial in patients with coronary stents placed more than a year before noncardiac surgery. Whether perioperative aspirin is beneficial in other subgroups was unknown.
[Biccard BM, Sigamani A, Chan MTV, et al. Effect of aspirin in vascular surgery in patients from a randomized clinical trial (POISE-2). Br J Surg 2018; 105(12):1591–1597. doi:10.1002/bjs.10925]
Biccard et al7 investigated the effect of perioperative aspirin in the subgroup of patients from the POISE-2 trial who underwent vascular surgery. The primary outcome was death or MI within 30 days. Secondary outcomes in this substudy included vascular occlusive complications (amputation and peripheral arterial thrombosis) and major or life-threatening bleeding.
Limitations. There were few adverse events, and this substudy was underpowered for the primary and secondary outcomes.
Conclusion. Starting or continuing aspirin did not improve outcomes, and withdrawing it did not increase cardiovascular or occlusive complications.
Do ACE inhibitors affect risk in noncardiac nonvascular surgery?
Angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs) are some of the most commonly used medications for treating hypertension. But whether patients should continue receiving them on the day of surgery or whether they should be held remains unclear.
Although current recommendations are inconsistent, the most recent American College of Cardiology/American Heart Association1 perioperative practice guidelines say that continuing ACE inhibitors or ARBs is reasonable perioperatively. This recommendation, however, acknowledges that published evidence is limited. There is general agreement that preoperative exposure to ACE inhibitors and ARBs is associated with intraoperative hypotension, but whether this increases the risk of adverse clinical outcomes remains unclear. Needed was a study to determine the effect on perioperative morbidity and mortality of continuing vs withholding ACE inhibitors and ARBs before surgery.
[Shiffermiller JF, Monson BJ, Vokoun CW, et al. Prospective randomized evaluation of preoperative angiotensin-converting enzyme inhibition (PREOP-ACEI). J Hosp Med 2018; 13(10):661–667. doi:10.12788/jhm.3036]
Shiffermiller et al8 performed a randomized controlled trial comparing the effect of 2 preoperative ACE inhibitor management protocols in patients undergoing noncardiac nonvascular surgery. Patients were randomized to either receive or not receive their final preoperative ACE inhibitor dose, whether scheduled on the morning of surgery or the night before.
Exclusion criteria included hypotension or hypertension at their preoperative clinic appointment (defined as systolic blood pressure < 90 or ≥ 160 mm Hg, and diastolic blood pressure < 60 or ≥ 95 mm Hg), moderate to severe heart failure, and end-stage renal disease requiring dialysis. Excluded surgery types were cardiac, vascular, organ transplant, oncologic, and all outpatient procedures. Patients taking ARBs were also excluded.
The primary outcome was intraoperative hypotension defined as any systolic blood pressure less than 80 mm Hg from the time of anesthesia induction until transfer to the postanesthesia care unit. Secondary outcomes were measured until hospital discharge and included postoperative acute kidney injury, postoperative hypotension (systolic pressure < 90 mm Hg) and hypertension (systolic pressure > 180 mm Hg), major cardiac events (composite of acute coronary syndrome, acute heart failure, or new-onset arrhythmia), and death.
Findings. A total of 453 patients were screened for eligibility, and of these, 291 were included for randomization. Their average age was 64, 48% were men, and 87% were white. About 50% underwent general anesthesia, 25% spinal, and 25% regional. Over half of the surgeries were orthopedic, and 20% were spine surgeries.
The primary outcome of intraoperative hypotension occurred significantly less often in patients randomized to ACE inhibitor omission than in the continuation group (55% vs 69%, relative risk [RR] 0.81, 95% CI 0.67–0.97, P = .03). This translates to 1 case of intraoperative hypotension for every 7.5 patients continuing an ACE inhibitor perioperatively (number needed to harm 7.5). Intraoperative hypotension associated with vasopressor administration also occurred significantly less frequently in the ACE inhibitor omission group.
Patients in the ACE inhibitor omission group were also less likely to experience postoperative hypotension, but on the other hand, they were more likely to experience severe postoperative hypertension (defined as any systolic blood pressure > 180 mm Hg). The two groups fared the same in terms of rates of acute kidney injury and major adverse cardiac events (MACE) and hospital length of stay, and no patients died in either group.
Limitations. Several factors limit the generalizability of this single-center study, including the many exclusion criteria, the predominance of orthopedic and spine surgeries, and the low-risk patient population (the average Revised Cardiac Risk Index score was 0, range 0–3). Other limitations include not controlling for the specific ACE inhibitor used and not including the precise timing of the final dose in relation to surgery. Lastly, this study lacked power to measure postoperative outcomes.
Conclusions. Continuing ACE inhibitor treatment before noncardiac nonvascular surgery is associated with a greater frequency and duration of intraoperative hypotension, but it did not increase the incidences of acute kidney injury, MACE, or death nor the hospital length of stay.
[Hollmann C, Fernandes NL, Biccard BM. A systematic review of outcomes associated with withholding or continuing angiotensin-converting enzyme inhibitors and angiotensin receptor blockers before noncardiac surgery. Anesth Analg 2018; 127(3):678–687. doi:10.1213/ANE.0000000000002837]
Hollmann et al9 performed a meta-analysis to determine whether it is better to continue or withhold ACE inhibitors and ARBs before surgery. The patients were adults undergoing noncardiac surgery and receiving an ACE inhibitor or ARB, which was either withheld or continued on the morning of surgery.
Primary outcomes were all-cause mortality and MACE, while secondary outcomes included the incidence of acute kidney injury, heart failure, stroke, intraoperative and postoperative hypotension, and length of hospital stay. Randomized controlled trials and observational studies were included, while case reports and case-control studies were excluded.
Findings. This meta-analysis included 5 randomized controlled trials and 4 cohort studies, with a total of 6,022 patients; 1,816 had their ACE inhibitor or ARB withheld before surgery, while 4,206 continued therapy. It found no difference between the 2 groups in the incidence of death or MACE, and there were not enough data to determine a difference in heart failure, stroke, acute kidney injury, or hospital length of stay.
Seven studies, with 5,414 patients, examined intraoperative hypotension. The overall incidence was 30%, but was significantly lower if the ACE inhibitor or ARB was withheld (OR 0.63, 95% CI 0.47–0.85, P = .002). Findings were similar in an analysis of only the randomized controlled trials. No difference was observed in postoperative hypotension.
Limitations. There was no standard definition of the morbidity outcomes, including hypotension and MACE. The assessment of MACE included data only for MI and not MINS. The specific duration of hypotension was not reported, and this meta-analysis did not take into account different anesthetic techniques. The duration of follow-up varied widely among studies, ranging from the day of hospital discharge to 30 days after surgery. And the randomized controlled trial performed by Shiffermiller et al8 was not included.
Conclusions. While continuing ACE inhibitors or ARBs before noncardiac surgery was associated with intraoperative hypotension, it did not seem to affect other outcomes, including death and MACE. The authors propose that a large randomized controlled trial is needed to determine whether continuing or withholding ACE inhibitor or ARB therapy before surgery is safer.
POSTOPERATIVE CARDIAC COMPLICATIONS
How should we treat MINS?
MINS is associated with an increased risk of cardiovascular events and death in both the short term and long term. MINS is defined as an elevated postoperative troponin level related to an ischemic etiology. However, whether to routinely measure troponin after surgery is unclear, as most patients do not present with ischemic symptoms, and there is no standard of care for treatment of this entity. Limited observational data suggest that starting or intensifying cardiac medications, particularly aspirin and statins, may be beneficial in terms of reducing 30-day mortality rates in patients with MI or cardiac events at 1 year in vascular surgery patients with MINS.
The Management of Myocardial Injury After Noncardiac Surgery (MANAGE) trial was designed to evaluate the potential of the anticoagulant dabigatran to prevent major vascular complications in patients with MINS.
[Devereaux PJ, Duceppe E, Guyatt G, et al. Dabigatran in patients with myocardial injury after non-cardiac surgery (MANAGE): an international, randomised, placebo-controlled trial. Lancet 2018; 391(10137):2325–2334. doi:10.1016/S0140-6736(18)30832-8]
Devereaux et al10 randomized patients who were at least 45 years old and had developed MINS within the previous 35 days to receive dabigatran 110 mg orally twice daily or placebo for up to 2 years. Patients not already taking a proton pump inhibitor were also randomized to take either omeprazole 20 mg once daily or placebo.
The primary efficacy outcome initially was major vascular complications, which included vascular mortality, nonfatal MI, nonhemorrhagic stroke, and peripheral arterial thrombosis. However, amputation and symptomatic venous thromboembolism were subsequently added during the study.
The primary safety outcome was a composite of life-threatening, major, and critical organ bleeding. Major bleeding required a decrease in hemoglobin of at least 4 g/dL, transfusion of at least 3 units of red blood cells within a 24-hour period, or a procedure to stop the bleeding.
Findings. The original goal was to recruit 3,200 patients, but due to slow enrollment and loss of funding, the sample was reduced to 1,754 patients (877 in each group). Approximately 45% of each group stopped taking the study drug prematurely.
The primary efficacy outcome occurred in significantly fewer patients receiving dabigatran (97, 11%) than placebo (133, 15%, HR 0.72, 95% CI 0.55–0.93, P = .0115). The incidence of the primary safety outcome was similar in both groups: 3% with dabigatran and 4% with placebo (HR 0.92, 95% CI 0.55–1.53, P = .76). The only individual efficacy outcome meeting statistical significance was a lower rate of nonhemorrhagic stroke in the dabigatran group. Subgroup analyses showed a trend benefiting patients randomized within 5 days of MINS or with a diagnosis of MI, although it was not statistically significant.
Limitations. The efficacy outcomes were expanded to include venous thromboembolism and others not directly related to MINS, raising questions about the conclusions. Further, as defined by the protocol, bleeding had to be fairly severe to be deemed major. The high number of patients who discontinued the study drug is another limitation of this study.
Conclusion. Dabigatran lowered the risk of major vascular complications with no significant increase in major bleeding in patients with MINS.
What is the risk of thromboembolism in postoperative atrial fibrillation, and what are the benefits of anticoagulation?
Although nonvalvular atrial fibrillation is associated with increased risks of ischemic stroke and systemic embolic events in nonsurgical patients, the association of new-onset postoperative atrial fibrillation with long-term thromboembolic events in the noncardiac surgical population is not well established.
[Butt JH, Olesen JB, Havers-Borgersen E, et al. Risk of thromboembolism associated with atrial fibrillation following noncardiac surgery. J Am Coll Cardiol 2018; 72(17):2027–2036. doi:10.1016/j.jacc.2018.07.088]
In this retrospective cohort study using a nationwide registry in Denmark, Butt et al11 assessed the long-term risk of thromboembolic events in noncardiac surgical patients with new postoperative atrial fibrillation. Patients were identified who had no previous history of atrial fibrillation and developed it after noncardiac, nonobstetric surgeries, and were matched in a 1:4 ratio with patients who developed nonvalvular atrial fibrillation during nonsurgical hospitalizations. Matching was based on age, sex, heart failure, hypertension, diabetes, known history of thromboembolic events, ischemic heart disease, and the year patients presented with new atrial fibrillation.
Patients were excluded if they received antiarrhythmic drugs or oral anticoagulants before hospitalization or surgery, had cancer in the year prior, or died in the hospital.
The primary outcome of the study was thromboembolic events—a composite of ischemic stroke, transient cerebral ischemia, and peripheral arterial thrombosis or embolism. Secondary outcomes included rehospitalization for atrial fibrillation and all-cause mortality.
Findings. Overall, 0.4% of patients developed new postoperative atrial fibrillation, of whom 3,380 were matched with 15,320 patients with nonvalvular atrial fibrillation. Over a median follow-up of 3.2 years, the risk of thromboembolic events was similar in both groups (31.7 and 29.9 per 1,000 person-years, HR 0.95, 95% CI 0.85–1.07). The groups did not differ in their CHA2DS2-VASc risk scores, HAS-BLED risk scores, or year in which patients were diagnosed.
Anticoagulation lowered the risk of thromboembolic events to a similar extent in both groups compared with no anticoagulation:
- In postoperative atrial fibrillation—HR 0.57, 95% CI 0.40–0.67
- In nonvalvular atrial fibrillation—HR 0.56, 95% CI 0.51–0.62.
Despite the similar reduction in thromboembolic events, only 24.4% of the postoperative atrial fibrillation patients were started on anticoagulation therapy within 30 days of discharge, compared with 41.5% of those with nonvalvular atrial fibrillation.
Limitations. Although this was a large study with excellent follow-up data, it was observational. It may have underestimated the number of patients who developed postoperative atrial fibrillation because episodes that were judged not to be clinically significant may not have been charted. Many patients are not monitored with continuous telemetry postoperatively, which also may have led to underestimation of the number of atrial fibrillation events.
The study also did not examine the number of atrial fibrillation episodes per patient, the heart rhythm at discharge or long-term, or indication for and duration of anticoagulation. There were no data regarding international normalized ratio levels.
Conclusions. Postoperative atrial fibrillation is associated with outcomes similar to those of nonsurgical nonvalvular atrial fibrillation. Anticoagulation decreases the risks of stroke and death. However, substantially fewer patients with postoperative atrial fibrillation receive anticoagulation. Anticoagulation should be considered in these patients, while noting bleeding risk.
Perioperative medicine is an evolving field with a rapidly growing body of literature, particularly in cardiology.
In this update, we review 6 articles to answer questions related to preoperative cardiac risk assessment, perioperative medication management, and postoperative cardiac complications. We surveyed perioperative literature from February 2018 through January 2019 and chose the final articles by consensus, based on relevance to clinicians who provide preoperative evaluations and postoperative care to surgical patients.
These summaries are derived from “Updates in Perioperative Medicine” presented at the 14th Annual Perioperative Medicine Summit (Orlando, FL, February 13–16, 2019) and the 2019 Society of Hospital Medicine Annual Meeting (National Harbor, MD, March 24–27, 2019).
PREOPERATIVE CARDIAC EVALUATION
How well do measures of functional capacity predict perioperative complications and mortality in noncardiac surgical patients?
Functional capacity is commonly assessed in preoperative evaluations to estimate patients’ risks of perioperative complications and death. The American College of Cardiology/American Heart Association1 and the European Society of Cardiology2 guidelines both include estimation of cardiopulmonary fitness as a step in preoperative assessment before major noncardiac surgery.
“Subjective assessment” is one way to estimate functional capacity. Simply put, clinicians try to form a rough idea about the fitness of patients by asking questions about routine activities such as walking or climbing stairs. Although commonly used, subjective assessment of functional capacity lacks strong evidence that it predicts adverse perioperative events.
Cardiopulmonary exercise testing is a third option. It measures peak oxygen consumption and anaerobic threshold during exercise. It is probably the best objective measurement of functional capacity, but not necessarily for predicting postoperative cardiac complications, and it is performed relatively infrequently.
[Wijeysundera DN, Pearse RM, Sulman MA, et al. Assessment of functional capacity before major non-cardiac surgery: an international, prospective cohort study. Lancet 2018; 391(10140):2631–2640. doi:10.1016/S0140-6736(18)31131-0]
In a multicenter, prospective cohort study, Wijeysundera et al4 compared subjective functional capacity assessment, the Duke Activity Status Index, cardiopulmonary exercise testing, and the preoperative N-terminal pro-B-type natriuretic peptide (NT-proBNP) level in their ability to predict complications and death in 1,401 noncardiac surgery patients older than 40 with at least 1 cardiovascular risk factor. After surgery, patients had daily electrocardiograms and troponin measurements until postoperative day 3 or discharge.
The primary outcome was the 30-day incidence of death or myocardial infarction (MI). Additional outcomes included the 30-day incidence of death or myocardial injury after noncardiac surgery (MINS), the 1-year mortality rate, and moderate to severe in-hospital perioperative complications.
Findings. Two percent of patients died or had an MI within 30 days of surgery.4
Subjective assessment had only a 19.2% sensitivity (95% confidence interval [CI] 14.2–25) but a 94.7% specificity (95% CI 93.2–95.9) for predicting inability to attain 4 metabolic equivalents during exercise.4
A lower Duke Activity Status Index predicted the primary outcome of death or MI within 30 days (adjusted odds ratio [OR] 0.96, 95% CI 0.83–0.99, P = .03), and it was the only measure that did so. Additionally, the Duke index and NT-proBNP level predicted the risk of death or MINS within 30 days.4
Only elevated NT-proBNP was associated with death at 1 year.4
On exercise testing, low peak oxygen consumption was significantly associated with perioperative complications.
Limitations. The number of primary outcome events (death and MI) was low, potentially affecting the statistical power of the study.
Conclusions. Subjective assessment of functional capacity misclassifies too many patients as being at low risk of perioperative complications and should not be used for preoperative risk stratification. Other tools, such as the Duke Activity Status Index and NT-proBNP levels, are better predictors of adverse perioperative cardiovascular outcomes and should be considered for use in preoperative cardiac risk assessment.
Although the Duke Activity Status Index is a better predictor of adverse outcomes than subjective functional capacity assessment, a specific perioperative threshold for risk classification has not been established. Its correlate for metabolic equivalents should be considered for use in clinical practice at this point.
PERIOPERATIVE MEDICATION MANAGEMENT
Is perioperative aspirin beneficial in patients undergoing vascular surgery?
The Perioperative Ischemic Evaluation 2 (POISE-2) trial,5 a 2-by-2 factorial randomized controlled trial in which patients received perioperative aspirin, clonidine, both, or neither, demonstrated that perioperative aspirin did not reduce cardiovascular events and increased major bleeding. Patients with recently placed coronary stents and those undergoing carotid endarterectomy were excluded because aspirin is known to have a beneficial effect in these patients.
A subsequent substudy6 found perioperative aspirin to be beneficial in patients with coronary stents placed more than a year before noncardiac surgery. Whether perioperative aspirin is beneficial in other subgroups was unknown.
[Biccard BM, Sigamani A, Chan MTV, et al. Effect of aspirin in vascular surgery in patients from a randomized clinical trial (POISE-2). Br J Surg 2018; 105(12):1591–1597. doi:10.1002/bjs.10925]
Biccard et al7 investigated the effect of perioperative aspirin in the subgroup of patients from the POISE-2 trial who underwent vascular surgery. The primary outcome was death or MI within 30 days. Secondary outcomes in this substudy included vascular occlusive complications (amputation and peripheral arterial thrombosis) and major or life-threatening bleeding.
Limitations. There were few adverse events, and this substudy was underpowered for the primary and secondary outcomes.
Conclusion. Starting or continuing aspirin did not improve outcomes, and withdrawing it did not increase cardiovascular or occlusive complications.
Do ACE inhibitors affect risk in noncardiac nonvascular surgery?
Angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs) are some of the most commonly used medications for treating hypertension. But whether patients should continue receiving them on the day of surgery or whether they should be held remains unclear.
Although current recommendations are inconsistent, the most recent American College of Cardiology/American Heart Association1 perioperative practice guidelines say that continuing ACE inhibitors or ARBs is reasonable perioperatively. This recommendation, however, acknowledges that published evidence is limited. There is general agreement that preoperative exposure to ACE inhibitors and ARBs is associated with intraoperative hypotension, but whether this increases the risk of adverse clinical outcomes remains unclear. Needed was a study to determine the effect on perioperative morbidity and mortality of continuing vs withholding ACE inhibitors and ARBs before surgery.
[Shiffermiller JF, Monson BJ, Vokoun CW, et al. Prospective randomized evaluation of preoperative angiotensin-converting enzyme inhibition (PREOP-ACEI). J Hosp Med 2018; 13(10):661–667. doi:10.12788/jhm.3036]
Shiffermiller et al8 performed a randomized controlled trial comparing the effect of 2 preoperative ACE inhibitor management protocols in patients undergoing noncardiac nonvascular surgery. Patients were randomized to either receive or not receive their final preoperative ACE inhibitor dose, whether scheduled on the morning of surgery or the night before.
Exclusion criteria included hypotension or hypertension at their preoperative clinic appointment (defined as systolic blood pressure < 90 or ≥ 160 mm Hg, and diastolic blood pressure < 60 or ≥ 95 mm Hg), moderate to severe heart failure, and end-stage renal disease requiring dialysis. Excluded surgery types were cardiac, vascular, organ transplant, oncologic, and all outpatient procedures. Patients taking ARBs were also excluded.
The primary outcome was intraoperative hypotension defined as any systolic blood pressure less than 80 mm Hg from the time of anesthesia induction until transfer to the postanesthesia care unit. Secondary outcomes were measured until hospital discharge and included postoperative acute kidney injury, postoperative hypotension (systolic pressure < 90 mm Hg) and hypertension (systolic pressure > 180 mm Hg), major cardiac events (composite of acute coronary syndrome, acute heart failure, or new-onset arrhythmia), and death.
Findings. A total of 453 patients were screened for eligibility, and of these, 291 were included for randomization. Their average age was 64, 48% were men, and 87% were white. About 50% underwent general anesthesia, 25% spinal, and 25% regional. Over half of the surgeries were orthopedic, and 20% were spine surgeries.
The primary outcome of intraoperative hypotension occurred significantly less often in patients randomized to ACE inhibitor omission than in the continuation group (55% vs 69%, relative risk [RR] 0.81, 95% CI 0.67–0.97, P = .03). This translates to 1 case of intraoperative hypotension for every 7.5 patients continuing an ACE inhibitor perioperatively (number needed to harm 7.5). Intraoperative hypotension associated with vasopressor administration also occurred significantly less frequently in the ACE inhibitor omission group.
Patients in the ACE inhibitor omission group were also less likely to experience postoperative hypotension, but on the other hand, they were more likely to experience severe postoperative hypertension (defined as any systolic blood pressure > 180 mm Hg). The two groups fared the same in terms of rates of acute kidney injury and major adverse cardiac events (MACE) and hospital length of stay, and no patients died in either group.
Limitations. Several factors limit the generalizability of this single-center study, including the many exclusion criteria, the predominance of orthopedic and spine surgeries, and the low-risk patient population (the average Revised Cardiac Risk Index score was 0, range 0–3). Other limitations include not controlling for the specific ACE inhibitor used and not including the precise timing of the final dose in relation to surgery. Lastly, this study lacked power to measure postoperative outcomes.
Conclusions. Continuing ACE inhibitor treatment before noncardiac nonvascular surgery is associated with a greater frequency and duration of intraoperative hypotension, but it did not increase the incidences of acute kidney injury, MACE, or death nor the hospital length of stay.
[Hollmann C, Fernandes NL, Biccard BM. A systematic review of outcomes associated with withholding or continuing angiotensin-converting enzyme inhibitors and angiotensin receptor blockers before noncardiac surgery. Anesth Analg 2018; 127(3):678–687. doi:10.1213/ANE.0000000000002837]
Hollmann et al9 performed a meta-analysis to determine whether it is better to continue or withhold ACE inhibitors and ARBs before surgery. The patients were adults undergoing noncardiac surgery and receiving an ACE inhibitor or ARB, which was either withheld or continued on the morning of surgery.
Primary outcomes were all-cause mortality and MACE, while secondary outcomes included the incidence of acute kidney injury, heart failure, stroke, intraoperative and postoperative hypotension, and length of hospital stay. Randomized controlled trials and observational studies were included, while case reports and case-control studies were excluded.
Findings. This meta-analysis included 5 randomized controlled trials and 4 cohort studies, with a total of 6,022 patients; 1,816 had their ACE inhibitor or ARB withheld before surgery, while 4,206 continued therapy. It found no difference between the 2 groups in the incidence of death or MACE, and there were not enough data to determine a difference in heart failure, stroke, acute kidney injury, or hospital length of stay.
Seven studies, with 5,414 patients, examined intraoperative hypotension. The overall incidence was 30%, but was significantly lower if the ACE inhibitor or ARB was withheld (OR 0.63, 95% CI 0.47–0.85, P = .002). Findings were similar in an analysis of only the randomized controlled trials. No difference was observed in postoperative hypotension.
Limitations. There was no standard definition of the morbidity outcomes, including hypotension and MACE. The assessment of MACE included data only for MI and not MINS. The specific duration of hypotension was not reported, and this meta-analysis did not take into account different anesthetic techniques. The duration of follow-up varied widely among studies, ranging from the day of hospital discharge to 30 days after surgery. And the randomized controlled trial performed by Shiffermiller et al8 was not included.
Conclusions. While continuing ACE inhibitors or ARBs before noncardiac surgery was associated with intraoperative hypotension, it did not seem to affect other outcomes, including death and MACE. The authors propose that a large randomized controlled trial is needed to determine whether continuing or withholding ACE inhibitor or ARB therapy before surgery is safer.
POSTOPERATIVE CARDIAC COMPLICATIONS
How should we treat MINS?
MINS is associated with an increased risk of cardiovascular events and death in both the short term and long term. MINS is defined as an elevated postoperative troponin level related to an ischemic etiology. However, whether to routinely measure troponin after surgery is unclear, as most patients do not present with ischemic symptoms, and there is no standard of care for treatment of this entity. Limited observational data suggest that starting or intensifying cardiac medications, particularly aspirin and statins, may be beneficial in terms of reducing 30-day mortality rates in patients with MI or cardiac events at 1 year in vascular surgery patients with MINS.
The Management of Myocardial Injury After Noncardiac Surgery (MANAGE) trial was designed to evaluate the potential of the anticoagulant dabigatran to prevent major vascular complications in patients with MINS.
[Devereaux PJ, Duceppe E, Guyatt G, et al. Dabigatran in patients with myocardial injury after non-cardiac surgery (MANAGE): an international, randomised, placebo-controlled trial. Lancet 2018; 391(10137):2325–2334. doi:10.1016/S0140-6736(18)30832-8]
Devereaux et al10 randomized patients who were at least 45 years old and had developed MINS within the previous 35 days to receive dabigatran 110 mg orally twice daily or placebo for up to 2 years. Patients not already taking a proton pump inhibitor were also randomized to take either omeprazole 20 mg once daily or placebo.
The primary efficacy outcome initially was major vascular complications, which included vascular mortality, nonfatal MI, nonhemorrhagic stroke, and peripheral arterial thrombosis. However, amputation and symptomatic venous thromboembolism were subsequently added during the study.
The primary safety outcome was a composite of life-threatening, major, and critical organ bleeding. Major bleeding required a decrease in hemoglobin of at least 4 g/dL, transfusion of at least 3 units of red blood cells within a 24-hour period, or a procedure to stop the bleeding.
Findings. The original goal was to recruit 3,200 patients, but due to slow enrollment and loss of funding, the sample was reduced to 1,754 patients (877 in each group). Approximately 45% of each group stopped taking the study drug prematurely.
The primary efficacy outcome occurred in significantly fewer patients receiving dabigatran (97, 11%) than placebo (133, 15%, HR 0.72, 95% CI 0.55–0.93, P = .0115). The incidence of the primary safety outcome was similar in both groups: 3% with dabigatran and 4% with placebo (HR 0.92, 95% CI 0.55–1.53, P = .76). The only individual efficacy outcome meeting statistical significance was a lower rate of nonhemorrhagic stroke in the dabigatran group. Subgroup analyses showed a trend benefiting patients randomized within 5 days of MINS or with a diagnosis of MI, although it was not statistically significant.
Limitations. The efficacy outcomes were expanded to include venous thromboembolism and others not directly related to MINS, raising questions about the conclusions. Further, as defined by the protocol, bleeding had to be fairly severe to be deemed major. The high number of patients who discontinued the study drug is another limitation of this study.
Conclusion. Dabigatran lowered the risk of major vascular complications with no significant increase in major bleeding in patients with MINS.
What is the risk of thromboembolism in postoperative atrial fibrillation, and what are the benefits of anticoagulation?
Although nonvalvular atrial fibrillation is associated with increased risks of ischemic stroke and systemic embolic events in nonsurgical patients, the association of new-onset postoperative atrial fibrillation with long-term thromboembolic events in the noncardiac surgical population is not well established.
[Butt JH, Olesen JB, Havers-Borgersen E, et al. Risk of thromboembolism associated with atrial fibrillation following noncardiac surgery. J Am Coll Cardiol 2018; 72(17):2027–2036. doi:10.1016/j.jacc.2018.07.088]
In this retrospective cohort study using a nationwide registry in Denmark, Butt et al11 assessed the long-term risk of thromboembolic events in noncardiac surgical patients with new postoperative atrial fibrillation. Patients were identified who had no previous history of atrial fibrillation and developed it after noncardiac, nonobstetric surgeries, and were matched in a 1:4 ratio with patients who developed nonvalvular atrial fibrillation during nonsurgical hospitalizations. Matching was based on age, sex, heart failure, hypertension, diabetes, known history of thromboembolic events, ischemic heart disease, and the year patients presented with new atrial fibrillation.
Patients were excluded if they received antiarrhythmic drugs or oral anticoagulants before hospitalization or surgery, had cancer in the year prior, or died in the hospital.
The primary outcome of the study was thromboembolic events—a composite of ischemic stroke, transient cerebral ischemia, and peripheral arterial thrombosis or embolism. Secondary outcomes included rehospitalization for atrial fibrillation and all-cause mortality.
Findings. Overall, 0.4% of patients developed new postoperative atrial fibrillation, of whom 3,380 were matched with 15,320 patients with nonvalvular atrial fibrillation. Over a median follow-up of 3.2 years, the risk of thromboembolic events was similar in both groups (31.7 and 29.9 per 1,000 person-years, HR 0.95, 95% CI 0.85–1.07). The groups did not differ in their CHA2DS2-VASc risk scores, HAS-BLED risk scores, or year in which patients were diagnosed.
Anticoagulation lowered the risk of thromboembolic events to a similar extent in both groups compared with no anticoagulation:
- In postoperative atrial fibrillation—HR 0.57, 95% CI 0.40–0.67
- In nonvalvular atrial fibrillation—HR 0.56, 95% CI 0.51–0.62.
Despite the similar reduction in thromboembolic events, only 24.4% of the postoperative atrial fibrillation patients were started on anticoagulation therapy within 30 days of discharge, compared with 41.5% of those with nonvalvular atrial fibrillation.
Limitations. Although this was a large study with excellent follow-up data, it was observational. It may have underestimated the number of patients who developed postoperative atrial fibrillation because episodes that were judged not to be clinically significant may not have been charted. Many patients are not monitored with continuous telemetry postoperatively, which also may have led to underestimation of the number of atrial fibrillation events.
The study also did not examine the number of atrial fibrillation episodes per patient, the heart rhythm at discharge or long-term, or indication for and duration of anticoagulation. There were no data regarding international normalized ratio levels.
Conclusions. Postoperative atrial fibrillation is associated with outcomes similar to those of nonsurgical nonvalvular atrial fibrillation. Anticoagulation decreases the risks of stroke and death. However, substantially fewer patients with postoperative atrial fibrillation receive anticoagulation. Anticoagulation should be considered in these patients, while noting bleeding risk.
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol 2014; 64(22):e77–137. doi:10.1016/j.jacc.2014.07.944
- Kristensen SD, Knuuti J, Saraste A, et al. 2014 ESC/ESA Guidelines on non-cardiac surgery: cardiovascular assessment and management: the Joint Task Force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur Heart J 2014; 35(35):2383–2431. doi:10.1093/eurheartj/ehu282
- Hlatky MA, Boineau RE, Higginbotham MB, et al. A brief self-administered questionnaire to determine functional capacity (The Duke Activity Status Index). Am J Cardiol 1989; 64(10):651–654. doi:10.1016/0002-9149(89)90496-7
- Wijeysundera DN, Pearse RM, Sulman MA, et al. Assessment of functional capacity before major non-cardiac surgery: an international, prospective cohort study. Lancet 2018; 391(10140):2631–2640. doi:10.1016/S0140-6736(18)31131-0
- Devereaux PJ, Mrkobrada M, Sessler DI, et al; POISE-2 Investigators. Aspirin in patients undergoing noncardiac surgery. N Engl J Med 2014; 370(16):1494–1503. doi:10.1056/NEJMoa1401105
- Graham MM, Sessler DI, Parlow JL, et al. Aspirin in patients with previous percutaneous coronary intervention undergoing noncardiac surgery. Ann Intern Med 2018;168(4):237–244. pmid:29132159
- Biccard BM, Sigamani A, Chan MTV, et al. Effect of aspirin in vascular surgery in patients from a randomized clinical trial (POISE-2). Br J Surg 2018; 105(12):1591–1597. doi:10.1002/bjs.10925
- Shiffermiller JF, Monson BJ, Vokoun CW, et al. Prospective randomized evaluation of preoperative angiotensin-converting enzyme inhibition (PREOP-ACEI). J Hosp Med 2018; 13(10):661–667. doi:10.12788/jhm.3036
- Hollmann C, Fernandes NL, Biccard BM. A systematic review of outcomes associated with withholding or continuing angiotensin-converting enzyme inhibitors and angiotensin receptor blockers before noncardiac surgery. Anesth Analg 2018; 127(3):678–687. doi:10.1213/ANE.0000000000002837
- Devereaux PJ, Duceppe E, Guyatt G, et al. Dabigatran in patients with myocardial injury after non-cardiac surgery (MANAGE): an international, randomised, placebo-controlled trial. Lancet 2018; 391(10137):2325–2334. doi:10.1016/S0140-6736(18)30832-8
- Butt JH, Olesen JB, Havers-Borgersen E, et al. Risk of thromboembolism associated with atrial fibrillation following noncardiac surgery. J Am Coll Cardiol 2018; 72(17):2027–2036. doi:10.1016/j.jacc.2018.07.088
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol 2014; 64(22):e77–137. doi:10.1016/j.jacc.2014.07.944
- Kristensen SD, Knuuti J, Saraste A, et al. 2014 ESC/ESA Guidelines on non-cardiac surgery: cardiovascular assessment and management: the Joint Task Force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur Heart J 2014; 35(35):2383–2431. doi:10.1093/eurheartj/ehu282
- Hlatky MA, Boineau RE, Higginbotham MB, et al. A brief self-administered questionnaire to determine functional capacity (The Duke Activity Status Index). Am J Cardiol 1989; 64(10):651–654. doi:10.1016/0002-9149(89)90496-7
- Wijeysundera DN, Pearse RM, Sulman MA, et al. Assessment of functional capacity before major non-cardiac surgery: an international, prospective cohort study. Lancet 2018; 391(10140):2631–2640. doi:10.1016/S0140-6736(18)31131-0
- Devereaux PJ, Mrkobrada M, Sessler DI, et al; POISE-2 Investigators. Aspirin in patients undergoing noncardiac surgery. N Engl J Med 2014; 370(16):1494–1503. doi:10.1056/NEJMoa1401105
- Graham MM, Sessler DI, Parlow JL, et al. Aspirin in patients with previous percutaneous coronary intervention undergoing noncardiac surgery. Ann Intern Med 2018;168(4):237–244. pmid:29132159
- Biccard BM, Sigamani A, Chan MTV, et al. Effect of aspirin in vascular surgery in patients from a randomized clinical trial (POISE-2). Br J Surg 2018; 105(12):1591–1597. doi:10.1002/bjs.10925
- Shiffermiller JF, Monson BJ, Vokoun CW, et al. Prospective randomized evaluation of preoperative angiotensin-converting enzyme inhibition (PREOP-ACEI). J Hosp Med 2018; 13(10):661–667. doi:10.12788/jhm.3036
- Hollmann C, Fernandes NL, Biccard BM. A systematic review of outcomes associated with withholding or continuing angiotensin-converting enzyme inhibitors and angiotensin receptor blockers before noncardiac surgery. Anesth Analg 2018; 127(3):678–687. doi:10.1213/ANE.0000000000002837
- Devereaux PJ, Duceppe E, Guyatt G, et al. Dabigatran in patients with myocardial injury after non-cardiac surgery (MANAGE): an international, randomised, placebo-controlled trial. Lancet 2018; 391(10137):2325–2334. doi:10.1016/S0140-6736(18)30832-8
- Butt JH, Olesen JB, Havers-Borgersen E, et al. Risk of thromboembolism associated with atrial fibrillation following noncardiac surgery. J Am Coll Cardiol 2018; 72(17):2027–2036. doi:10.1016/j.jacc.2018.07.088
KEY POINTS
- The Duke Activity Status Index is a better tool for assessing cardiopulmonary fitness than subjective assessment, and it should be considered for use in guideline algorithms.
- Aspirin should not be given perioperatively in patients undergoing vascular surgery other than carotid endarterectomy.
- Angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs) are associated with intraoperative hypotension if given before surgery. Further study is needed to determined how best to manage ACE inhibitors and ARBs perioperatively.
- In a study, dabigatran given to patients with myocardial injury after noncardiac surgery lowered the risk of major vascular complications, with no significant increase in major bleeding. But the study had major limitations.
- Postoperative atrial fibrillation is associated with outcomes similar to those of nonsurgical nonvalvular atrial fibrillation. Anticoagulation decreases its stroke and mortality risk.
Update in Hospital Medicine: Practical Lessons from Current Literature
Hospital medicine continues to expand with respect to the number of practitioners as well as the scope of the practice of those practitioners. In addition, the commitment to, and rigor of, scientific inquiry in the field continues to grow. The authors of this article conducted a review of the medical literature, including articles published between March 2017 and March 2018. The key articles reported studies with high methodological quality, clear findings, and a high potential for impact on clinical practice. The literature was independently reviewed by each author, and candidate works were chosen on the basis of relevance to hospital medicine and expected clinical impact. The articles were organized by subject matter, ranked by applicability to the audience, and selected to meet the time constraints of each talk. Twenty-nine articles were presented at the Update in Hospital Medicine at the 2018 Society of Hospital Medicine and Society of General Internal Medicine annual meetings (B Sharpe, A Burger at SGIM and B Slawski, C Cooper at SHM). Nine articles were included in this review through an iterative voting process. Each author ranked their top five articles from one to five. Points were tallied for each article, and the five articles with the highest points were included. A second round of voting identified the remaining four articles for inclusion. Ties were adjudicated by group discussion. Each article is summarized below, and their key points are highlighted in the table.
KEY PUBLICATIONS
Aspirin in Patients with Previous Percutaneous Coronary Intervention Undergoing Noncardiac Surgery. Graham MM et al. Ann Intern Med. 2018;168(4):237-244.1
Background
The Perioperative Ischemic Evaluation 2 (POISE-2) trial found that perioperative aspirin use had no significant effect on the risk of perioperative death and nonfatal myocardial infarction (MI) in patients who are at risk for vascular complications; however, the risk of major bleeding increased with aspirin use in these patients.2 Nevertheless, the POISE-2 trial did not specifically address the role of aspirin in patients who had undergone previous percutaneous coronary intervention (PCI).
Methods
A post hoc subgroup analysis of POISE-2 evaluated 470 PCI patients (234 aspirin-treated and 236 placebo-treated patients) aged >45 years, 90% of whom had stents. The administration of the study drug was initiated within four hours preoperatively and continued postoperatively. Patients who had bare metal stents placed within the six weeks prior to the study or drug-eluting stents placed within the preceding 12 months were excluded.
Findings
The composite endpoint of risk of death and nonfatal MI was 11.5% in the placebo group and 6% in aspirin-treated patients (HR 0.50; CI, 0.26-0.95). Most of the difference in primary outcome was attributed to an increase in nonfatal MI in the placebo group. Major and life-threatening bleeding were not substantially increased in PCI patients but increased in the overall POISE-2 trial (absolute risk increase 0.8% for major bleeding [95% CI, 0.1%-1.6%]; HR 1.22 [95% CI, 1.01-1.48]). Stent type had no effect on death and nonfatal MI.
Cautions
This was a non-prespecified subgroup analysis with a small sample size.
Implications
Perioperative aspirin use in patients with previous PCI appears to provide more benefit than harm, unless a substantial bleeding risk exists.
Association Between Wait Time and 30-Day Mortality in Adults Undergoing Hip Fracture Surgery. Pincus D et al. JAMA. 2017;318(20):1994-2003.3
Background
Wait times to hip fracture surgery have been associated with mortality in previous studies; however, the wait time associated with complications remains controversial.4,5
Methods
This retrospective cohort study of 42,230 adults modeled the probability of complications in accordance with wait time from hospital arrival to hip fracture surgery. It aimed to identify the optimal time window in which to conduct surgery before complications increased. This window to increased complications was used to define early and delayed surgery. The matched cohorts of early and delayed patients were then used to compare outcomes.
Findings
Overall 30-day mortality was 7%. Complication rates increased when wait times reached 24 hours. Comparing the propensity-matched early (<24 hours) and late (>24 hours) surgery patients revealed that late surgery patients had significantly higher 30-day mortality (6.5% vs 5.8%; % absolute RD 0.79; 95% CI, 0.23-1.35) than early surgery patients and the composite outcome of mortality or other medical complications (MI, DVT, PE, and pneumonia; 12.2% vs 10.1%; % absolute RD 2.16; 95% CI, 1.43-2.89).
Cautions
Only 34% of patients in this study had surgery within 24 hours. The observational cohort study design may result in unmeasured confounders, eg, less sick patients go to surgery more quickly than sicker patients.
Implications
A preoperative wait time of 24 hours appears to represent a threshold of increased risk for 30-day perioperative complications and mortality in hip fracture surgery.
When are Oral Antibiotics a Safe and Effective Choice for Bacterial Bloodstream Infections? An Evidence-Based Narrative Review. Hale AJ et al. J Hosp Med. 2018;13(5):328-335.6
Background
Bloodstream infections (BSIs) are significant causes of morbidity and mortality in the United States. Traditionally, clinicians have relied on intravenous antibiotics for treatment. A recent “Choosing Wisely®” initiative recommends that clinicians should use “oral formulations of highly bioavailable antimicrobials wherever possible.”7 Thus, the authors searched for evidence for scenarios wherein BSIs could be safely treated with oral antibiotics.
Methods
A narrative review was conducted given that robust clinical data for an extensive systematic review were insufficient.
Findings
Key decision points on the use of an oral antibiotic for a diagnosed BSI are as follows: (1) Source control must be attained prior to the consideration of oral antibiotics. (2) A highly bioavailable oral option to which the pathogen is sensitive must be available. (3) Patients must be able to comply with the therapy for the full course and not be on interfering medications. Good evidence for use of oral antibiotics against sensitive gram-negative bacilli other than Pseudomonas exists. Evidence for treating Streptococcus pneumoniae with early transition (within three days) to oral antibiotics is robust when treating bacteremia and pneumonia but not for other primary sites of infection. Evidence for the use of oral antibiotics for B-hemolytic streptococcus, including necrotizing fasciitis and Enterococcus, is insufficient. The evidence supports at least two weeks of IV antibiotics for the treatment of Staphylococcus aureus.
Cautions
This is a narrative review due to limited evidence.
Implications
The early use of oral antibiotics in the setting of bacteremia may be appropriate in select clinical situations.
Prevalence of Pulmonary Embolism in Patients with Syncope. Costantino et al. JAMA Intern Med. 2018;178(3):356-362.8
Background
Data on the prevalence of pulmonary embolism in patients presenting with syncope are conflicting.
Methods
This was a retrospective observational study involving five databases in four countries of >1.6 million adults identified through syncope ICD codes. The rates of pulmonary embolism at first evaluation and pulmonary embolism or venous thromboembolism within 90 days were calculated for emergency room patients and a hospitalized subgroup.
Findings
Pulmonary embolism was rare in patients with syncope, eg, less than 3% for hospitalized patients in this database study.
Cautions
The results of this study are based on the use of administrative databases to confirm the diagnosis of syncope. Additionally, the results include hospitalized and nonhospitalized patients. The design of this study differs significantly from those of the PESIT study, which showed a prevalence of 17% in hospitalized patients.9 The PESIT study specifically sought the diagnosis of pulmonary embolism even when other etiologies for syncope existed.
Implications
Ultimately, the clinical impetus to search for pulmonary embolism in hospitalized patients admitted with syncope will depend on individual presentations. The authors argued that pulmonary embolism is rare in syncope and much lower than 17% but should be considered in appropriate patients.
Balanced Crystalloids versus Saline in Noncritically Ill Patients. Self WH et al. N Engl J Med. 2018;378(9):819-828.10
Background
Data on the optimal composition of intravenous fluids (IVF) are limited. Limited experimental evidence suggests that IVF-induced hyperchloremia results in renal vasoconstriction and acute kidney injury.
Methods
This was a single-center, open-label, multiple crossover trial of >13,000 non-ICU hospitalized patients admitted from the Emergency Department. Patients were randomized to receive either only normal saline or a “balanced crystalloid,” eg, either Lactated Ringer’s or Plasmalyte. The primary outcome was hospital-free days. Secondary outcomes were major adverse kidney events (MAKE) at 30 days.
Findings
The study found no difference in the primary outcome of hospital-free days. However, balanced IVF resulted in a lower incidence of hyperchloremia and a slightly reduced incidence of MAKE 30 (4.7% vs 5.6%; adjusted OR 0.82).
Cautions
The incidence of acute kidney injury was low in this single-center ED population. This study, however, did not include hospitalized patients. The long-term effects on renal function could not be ascertained.
Implications
Hospital-free days after inpatient randomization to either normal saline or “balanced IVF” were not significantly different. “Balanced IVF” may be beneficial in select high renal-risk populations.
Speaker Introductions at Internal Medicine Grand Rounds: Forms of Address Reveal Speaker Bias. Files et al. J Womens Health. 2017;26(5):413-419.11
Background
Gender bias is known to contribute to leadership disparities between men and women in several academic medical centers.
Methods
This was a retrospective observational study reviewing video-archived introductions at Internal Medicine Grand Rounds at two connected institutions. All speakers had doctoral degrees. The outcome measured was the use of a speaker’s professional title during his/her introduction as a function of the introducer’s gender.
Findings
Women were more likely than men to introduce speakers of any gender by their professional title in the 321 forms of address analyzed (96% vs 66%, P < .001). When the introducer and speaker were of different genders, women were more likely to introduce male speakers with formal titles than men introducing female speakers (95% vs 49%, P < .001).
Cautions
This study was done at two associated academic institutions and may not reflect the practice or customs of physicians in other departments or institutions.
Implications
Despite the study’s limitations, it supports a theme of prevalent gender bias within academic medical institutions that may affect the outcomes of leadership, promotion, and scholarship.
Edoxaban for the Treatment of Cancer-Associated Venous Thromboembolism. Raskob GE et al. N Engl J Med. 2018;378(7):615-624.12
Background
Low-molecular-weight heparin (LMWH) is the standard of care for the treatment of venous thromboembolism (VTE) in patients with cancer. Direct oral anticoagulants have not been studied for this indication.
Methods
This open-label, noninferiority trial randomized patients with cancer and acute VTE to either LMWH for a minimum of five days followed by oral edoxaban vs subcutaneous dalteparin.
Findings
A total of 1,046 patients were included in the modified intention-to-treat analysis. Patients received treatment for six to twelve months total. A composite outcome of recurrent VTE or major bleed within 12 months occurred in 67 of 522 (12.8%) of patients in the edoxaban group vs 71 of 524 (13.5%) of patients in the dalteparin group (HR 0.91, 95% CI 0.70-1.36, P = .006 for noninferiority). Recurrent VTE occurred more commonly with dalteparin than with edoxaban (11.3% vs 7.9%), whereas major bleeding was less common with dalteparin than with edoxaban (4% vs 6.9%). The increased bleeding rate with edoxaban was predominantly in patients with an upper gastrointestinal (GI) malignancy.
Cautions
This was an open-label study. Patients in the edoxaban still received five days of LMWH prior to oral edoxaban. More patients in the edoxaban group continued treatment for the entire 12-month period, which contributes to the observed decreased bleeding and increased VTE rates in the dalteparin group.
Implications
Oral edoxaban is noninferior to subcutaneous dalteparin for the primary composite endpoint of VTE and bleeding. Notably, the patients in the edoxaban group experienced a lower rate of recurrent VTE and a higher rate of major bleeding than the patients in the dalteparin group. Additional caution about bleeding risk in those with a GI malignancy is recommended.
Can High-flow Nasal Cannula Reduce the Rate of Endotracheal Intubation in Adult Patients with Acute Respiratory Failure Compared with Conventional Oxygen Therapy and Noninvasive Positive Pressure Ventilation? Ni Y-N et al. Chest. 2017;151(4):764-775.13
Background
High-flow nasal cannula (HFNC) can deliver heated and humidified oxygen at rates of up to 60 L/min. Evidence on the benefits of HFNC over usual oxygen therapy or noninvasive positive pressure ventilation (NIPPV) is conflicting.
Methods
This systematic review and meta-analysis included 18 studies (12 RCTs, four retrospective, and two prospective cohort studies) with 3,881 patients with respiratory failure (medical and surgical causes). The included studies compared HFNC with usual oxygen therapy or NIPPV.
Findings
HFNC was associated with lower rates of endotracheal intubation (OR 0.47, 95% CI 0.27-0.84, P = .01) relative to oxygen therapy. Intubation rates did not differ between HFNC and NIPPV (OR 0.73, 95% CI 0.47-1.13, P = .16). No differences in ICU mortality or ICU length of stay (LOS) were found when HFNC was compared with either usual oxygen therapy or NIPPV.
Cautions
The significant heterogeneity in study design across studies is mainly attributable to varying causes of respiratory failure and differences in flow rate, oxygen concentration, and treatment duration across studies.
Implications
In patients with respiratory failure, HFNC may reduce intubation when compared with usual oxygen therapy and has similar ICU mortality when compared with usual oxygen and NIPPV.
Errors in the Diagnosis of Spinal Epidural Abscesses in the Era of Electronic Health Records. Bhise V et al. Am J Med. 2017;130(8):975-981.14
Background
Diagnostic errors are common in patients with spinal epidural abscess, but the main contributing factors are unclear.15
Methods
All patients who were newly diagnosed with spinal epidural abscess in 2013 were identified from the Veterans Affairs (VA) national database. Charts were reviewed for diagnostic delay and contributing factors, including the presence of “red flag” symptoms (eg, fever and neurological deficits).
Findings
Of the 119 patients with a new diagnosis of spinal epidural abscess, 66 (56%) had a diagnostic error. The median time to diagnosis in those with a diagnostic error was 12 days vs four days in those without error (P < .01). Common missed red flags in error cases included fever (n = 57, 86.4%), focal neurologic deficit (n = 54, 81.8%), and active infection (n = 54, 81.8%). Most errors occurred during the provider–patient encounter (eg, information not gathered during the history or physical). The magnitude of harm was serious for most patients (n = 40, 60.6%) and contributed to death in eight patients (12.1%).
Cautions
The study may not be generalizable because it was limited to the VA health system.
Implications
Diagnostic errors are common in patients with spinal epidural abscesses and can lead to serious harm. Health systems should build mechanisms to support providers in the evaluation of patients with back pain.
1. Graham MM, Sessler DI, Parlow JL, et al. Aspirin in patients with previous percutaneous coronary intervention undergoing noncardiac surgery. Ann Intern Med. 2018;168(4):237-244. doi: 10.7326/M17-2341.
2. Devereaux PJ, Mrkobrada M, Sessler DI, et al. Aspirin in patients undergoing noncardiac surgery. N Engl J Med. 2014;370(16):1494-1503. doi: 10.1056/NEJMoa1401105
3. Pincus D, Ravi B, Wasserstein D, et al. Association between wait time and 30-day mortality in adults undergoing hip fracture surgery. JAMA. 2017;318(20):1994-2003. doi: 10.1001/jama.2017.17606.
4. Simunovic N, Devereaux PJ, Sprague S, et al. Effect of early surgery after hip fracture on mortality and complications: systematic review and meta-analysis. CMAJ. 2010;182(15):1609-1616. doi: 10.1503/cmaj.092220.
5. Shiga T, Wajima Z, Ohe Y. Is operative delay associated with increased mortality of hip fracture patients? ystematic review, meta-analysis, and meta-regression. Can J Anaesth. 2008;55(3):146-154. doi: 10.1007/BF03016088.
6. Hale AJ, Snyder GM, Ahern JW, Eliopoulos G, Ricotta D, Alston WK. When are oral antibiotics a safe and effective choice for bacterial bloodstream infections? An evidence-based narrative review. J Hosp Med. 2018;13(5):328-335. doi: 10.12788/jhm.2949.
7. Lehmann C, Berner R, Bogner JR, et al. The “Choosing Wisely” initiative in infectious diseases. Infection. 2017;45(3):263-268. doi: 10.1007/s15010-017-0997-0.
8. Costantino G, Ruwald MH, Quinn J, et al. Prevalence of pulmonary embolism in patients with syncope. JAMA Intern Med. 2018;178(3):356-362. doi: 10.1001/jamainternmed.2017.8175.
9. Prandoni P, Lensing AW, Prins MH, et al. Prevalence of pulmonary embolism among patients hospitalized for syncope. N Engl J Med. 2016;375(16):1524-1531. doi: 10.1056/NEJMoa1602172
10. Self WH, Semler MW, Wanderer JP, et al. Balanced crystalloids versus saline in noncritically ill adults. N Engl J Med. 2018;378(9):819-828. doi: 10.1056/NEJMoa1711586.
11. Files JA, Mayer AP, Ko MG, et al. Speaker introductions at internal medicine grand rounds: forms of address reveal gender bias. J Womens Health (Larchmt). 2017;26(5):413-419. doi: 10.1089/jwh.2016.6044.
12. Raskob GE, van Es N, Verhamme P, et al. Edoxaban for the treatment of cancer-associated venous thromboembolism. N Engl J Med. 2018;378(7):615-624. doi: 10.1056/NEJMoa1711948.
13. Ni YN, Luo J, Yu H, et al. Can high-flow nasal cannula reduce the rate of endotracheal intubation in adult patients with acute respiratory failure compared with conventional oxygen therapy and noninvasive positive pressure ventilation?: A systematic review and meta-analysis. Chest. 2017;151(4):764-775. doi: 10.1016/j.chest.2017.01.004.
14. Bhise V, Meyer AND, Singh H, et al. Errors in diagnosis of spinal epidural abscesses in the era of electronic health records. Am J Med. 2017;130(8):975-981. doi: 10.1016/j.amjmed.2017.03.009
15. Davis DP, Wold RM, Patel RJ, et al. The clinical presentation and impact of diagnostic delays on emergency department patients with spinal epidural abscess. J Emerg Med. 2004;26(3):285-291. doi: 10.1016/j.jemermed.2003.11.013.
Hospital medicine continues to expand with respect to the number of practitioners as well as the scope of the practice of those practitioners. In addition, the commitment to, and rigor of, scientific inquiry in the field continues to grow. The authors of this article conducted a review of the medical literature, including articles published between March 2017 and March 2018. The key articles reported studies with high methodological quality, clear findings, and a high potential for impact on clinical practice. The literature was independently reviewed by each author, and candidate works were chosen on the basis of relevance to hospital medicine and expected clinical impact. The articles were organized by subject matter, ranked by applicability to the audience, and selected to meet the time constraints of each talk. Twenty-nine articles were presented at the Update in Hospital Medicine at the 2018 Society of Hospital Medicine and Society of General Internal Medicine annual meetings (B Sharpe, A Burger at SGIM and B Slawski, C Cooper at SHM). Nine articles were included in this review through an iterative voting process. Each author ranked their top five articles from one to five. Points were tallied for each article, and the five articles with the highest points were included. A second round of voting identified the remaining four articles for inclusion. Ties were adjudicated by group discussion. Each article is summarized below, and their key points are highlighted in the table.
KEY PUBLICATIONS
Aspirin in Patients with Previous Percutaneous Coronary Intervention Undergoing Noncardiac Surgery. Graham MM et al. Ann Intern Med. 2018;168(4):237-244.1
Background
The Perioperative Ischemic Evaluation 2 (POISE-2) trial found that perioperative aspirin use had no significant effect on the risk of perioperative death and nonfatal myocardial infarction (MI) in patients who are at risk for vascular complications; however, the risk of major bleeding increased with aspirin use in these patients.2 Nevertheless, the POISE-2 trial did not specifically address the role of aspirin in patients who had undergone previous percutaneous coronary intervention (PCI).
Methods
A post hoc subgroup analysis of POISE-2 evaluated 470 PCI patients (234 aspirin-treated and 236 placebo-treated patients) aged >45 years, 90% of whom had stents. The administration of the study drug was initiated within four hours preoperatively and continued postoperatively. Patients who had bare metal stents placed within the six weeks prior to the study or drug-eluting stents placed within the preceding 12 months were excluded.
Findings
The composite endpoint of risk of death and nonfatal MI was 11.5% in the placebo group and 6% in aspirin-treated patients (HR 0.50; CI, 0.26-0.95). Most of the difference in primary outcome was attributed to an increase in nonfatal MI in the placebo group. Major and life-threatening bleeding were not substantially increased in PCI patients but increased in the overall POISE-2 trial (absolute risk increase 0.8% for major bleeding [95% CI, 0.1%-1.6%]; HR 1.22 [95% CI, 1.01-1.48]). Stent type had no effect on death and nonfatal MI.
Cautions
This was a non-prespecified subgroup analysis with a small sample size.
Implications
Perioperative aspirin use in patients with previous PCI appears to provide more benefit than harm, unless a substantial bleeding risk exists.
Association Between Wait Time and 30-Day Mortality in Adults Undergoing Hip Fracture Surgery. Pincus D et al. JAMA. 2017;318(20):1994-2003.3
Background
Wait times to hip fracture surgery have been associated with mortality in previous studies; however, the wait time associated with complications remains controversial.4,5
Methods
This retrospective cohort study of 42,230 adults modeled the probability of complications in accordance with wait time from hospital arrival to hip fracture surgery. It aimed to identify the optimal time window in which to conduct surgery before complications increased. This window to increased complications was used to define early and delayed surgery. The matched cohorts of early and delayed patients were then used to compare outcomes.
Findings
Overall 30-day mortality was 7%. Complication rates increased when wait times reached 24 hours. Comparing the propensity-matched early (<24 hours) and late (>24 hours) surgery patients revealed that late surgery patients had significantly higher 30-day mortality (6.5% vs 5.8%; % absolute RD 0.79; 95% CI, 0.23-1.35) than early surgery patients and the composite outcome of mortality or other medical complications (MI, DVT, PE, and pneumonia; 12.2% vs 10.1%; % absolute RD 2.16; 95% CI, 1.43-2.89).
Cautions
Only 34% of patients in this study had surgery within 24 hours. The observational cohort study design may result in unmeasured confounders, eg, less sick patients go to surgery more quickly than sicker patients.
Implications
A preoperative wait time of 24 hours appears to represent a threshold of increased risk for 30-day perioperative complications and mortality in hip fracture surgery.
When are Oral Antibiotics a Safe and Effective Choice for Bacterial Bloodstream Infections? An Evidence-Based Narrative Review. Hale AJ et al. J Hosp Med. 2018;13(5):328-335.6
Background
Bloodstream infections (BSIs) are significant causes of morbidity and mortality in the United States. Traditionally, clinicians have relied on intravenous antibiotics for treatment. A recent “Choosing Wisely®” initiative recommends that clinicians should use “oral formulations of highly bioavailable antimicrobials wherever possible.”7 Thus, the authors searched for evidence for scenarios wherein BSIs could be safely treated with oral antibiotics.
Methods
A narrative review was conducted given that robust clinical data for an extensive systematic review were insufficient.
Findings
Key decision points on the use of an oral antibiotic for a diagnosed BSI are as follows: (1) Source control must be attained prior to the consideration of oral antibiotics. (2) A highly bioavailable oral option to which the pathogen is sensitive must be available. (3) Patients must be able to comply with the therapy for the full course and not be on interfering medications. Good evidence for use of oral antibiotics against sensitive gram-negative bacilli other than Pseudomonas exists. Evidence for treating Streptococcus pneumoniae with early transition (within three days) to oral antibiotics is robust when treating bacteremia and pneumonia but not for other primary sites of infection. Evidence for the use of oral antibiotics for B-hemolytic streptococcus, including necrotizing fasciitis and Enterococcus, is insufficient. The evidence supports at least two weeks of IV antibiotics for the treatment of Staphylococcus aureus.
Cautions
This is a narrative review due to limited evidence.
Implications
The early use of oral antibiotics in the setting of bacteremia may be appropriate in select clinical situations.
Prevalence of Pulmonary Embolism in Patients with Syncope. Costantino et al. JAMA Intern Med. 2018;178(3):356-362.8
Background
Data on the prevalence of pulmonary embolism in patients presenting with syncope are conflicting.
Methods
This was a retrospective observational study involving five databases in four countries of >1.6 million adults identified through syncope ICD codes. The rates of pulmonary embolism at first evaluation and pulmonary embolism or venous thromboembolism within 90 days were calculated for emergency room patients and a hospitalized subgroup.
Findings
Pulmonary embolism was rare in patients with syncope, eg, less than 3% for hospitalized patients in this database study.
Cautions
The results of this study are based on the use of administrative databases to confirm the diagnosis of syncope. Additionally, the results include hospitalized and nonhospitalized patients. The design of this study differs significantly from those of the PESIT study, which showed a prevalence of 17% in hospitalized patients.9 The PESIT study specifically sought the diagnosis of pulmonary embolism even when other etiologies for syncope existed.
Implications
Ultimately, the clinical impetus to search for pulmonary embolism in hospitalized patients admitted with syncope will depend on individual presentations. The authors argued that pulmonary embolism is rare in syncope and much lower than 17% but should be considered in appropriate patients.
Balanced Crystalloids versus Saline in Noncritically Ill Patients. Self WH et al. N Engl J Med. 2018;378(9):819-828.10
Background
Data on the optimal composition of intravenous fluids (IVF) are limited. Limited experimental evidence suggests that IVF-induced hyperchloremia results in renal vasoconstriction and acute kidney injury.
Methods
This was a single-center, open-label, multiple crossover trial of >13,000 non-ICU hospitalized patients admitted from the Emergency Department. Patients were randomized to receive either only normal saline or a “balanced crystalloid,” eg, either Lactated Ringer’s or Plasmalyte. The primary outcome was hospital-free days. Secondary outcomes were major adverse kidney events (MAKE) at 30 days.
Findings
The study found no difference in the primary outcome of hospital-free days. However, balanced IVF resulted in a lower incidence of hyperchloremia and a slightly reduced incidence of MAKE 30 (4.7% vs 5.6%; adjusted OR 0.82).
Cautions
The incidence of acute kidney injury was low in this single-center ED population. This study, however, did not include hospitalized patients. The long-term effects on renal function could not be ascertained.
Implications
Hospital-free days after inpatient randomization to either normal saline or “balanced IVF” were not significantly different. “Balanced IVF” may be beneficial in select high renal-risk populations.
Speaker Introductions at Internal Medicine Grand Rounds: Forms of Address Reveal Speaker Bias. Files et al. J Womens Health. 2017;26(5):413-419.11
Background
Gender bias is known to contribute to leadership disparities between men and women in several academic medical centers.
Methods
This was a retrospective observational study reviewing video-archived introductions at Internal Medicine Grand Rounds at two connected institutions. All speakers had doctoral degrees. The outcome measured was the use of a speaker’s professional title during his/her introduction as a function of the introducer’s gender.
Findings
Women were more likely than men to introduce speakers of any gender by their professional title in the 321 forms of address analyzed (96% vs 66%, P < .001). When the introducer and speaker were of different genders, women were more likely to introduce male speakers with formal titles than men introducing female speakers (95% vs 49%, P < .001).
Cautions
This study was done at two associated academic institutions and may not reflect the practice or customs of physicians in other departments or institutions.
Implications
Despite the study’s limitations, it supports a theme of prevalent gender bias within academic medical institutions that may affect the outcomes of leadership, promotion, and scholarship.
Edoxaban for the Treatment of Cancer-Associated Venous Thromboembolism. Raskob GE et al. N Engl J Med. 2018;378(7):615-624.12
Background
Low-molecular-weight heparin (LMWH) is the standard of care for the treatment of venous thromboembolism (VTE) in patients with cancer. Direct oral anticoagulants have not been studied for this indication.
Methods
This open-label, noninferiority trial randomized patients with cancer and acute VTE to either LMWH for a minimum of five days followed by oral edoxaban vs subcutaneous dalteparin.
Findings
A total of 1,046 patients were included in the modified intention-to-treat analysis. Patients received treatment for six to twelve months total. A composite outcome of recurrent VTE or major bleed within 12 months occurred in 67 of 522 (12.8%) of patients in the edoxaban group vs 71 of 524 (13.5%) of patients in the dalteparin group (HR 0.91, 95% CI 0.70-1.36, P = .006 for noninferiority). Recurrent VTE occurred more commonly with dalteparin than with edoxaban (11.3% vs 7.9%), whereas major bleeding was less common with dalteparin than with edoxaban (4% vs 6.9%). The increased bleeding rate with edoxaban was predominantly in patients with an upper gastrointestinal (GI) malignancy.
Cautions
This was an open-label study. Patients in the edoxaban still received five days of LMWH prior to oral edoxaban. More patients in the edoxaban group continued treatment for the entire 12-month period, which contributes to the observed decreased bleeding and increased VTE rates in the dalteparin group.
Implications
Oral edoxaban is noninferior to subcutaneous dalteparin for the primary composite endpoint of VTE and bleeding. Notably, the patients in the edoxaban group experienced a lower rate of recurrent VTE and a higher rate of major bleeding than the patients in the dalteparin group. Additional caution about bleeding risk in those with a GI malignancy is recommended.
Can High-flow Nasal Cannula Reduce the Rate of Endotracheal Intubation in Adult Patients with Acute Respiratory Failure Compared with Conventional Oxygen Therapy and Noninvasive Positive Pressure Ventilation? Ni Y-N et al. Chest. 2017;151(4):764-775.13
Background
High-flow nasal cannula (HFNC) can deliver heated and humidified oxygen at rates of up to 60 L/min. Evidence on the benefits of HFNC over usual oxygen therapy or noninvasive positive pressure ventilation (NIPPV) is conflicting.
Methods
This systematic review and meta-analysis included 18 studies (12 RCTs, four retrospective, and two prospective cohort studies) with 3,881 patients with respiratory failure (medical and surgical causes). The included studies compared HFNC with usual oxygen therapy or NIPPV.
Findings
HFNC was associated with lower rates of endotracheal intubation (OR 0.47, 95% CI 0.27-0.84, P = .01) relative to oxygen therapy. Intubation rates did not differ between HFNC and NIPPV (OR 0.73, 95% CI 0.47-1.13, P = .16). No differences in ICU mortality or ICU length of stay (LOS) were found when HFNC was compared with either usual oxygen therapy or NIPPV.
Cautions
The significant heterogeneity in study design across studies is mainly attributable to varying causes of respiratory failure and differences in flow rate, oxygen concentration, and treatment duration across studies.
Implications
In patients with respiratory failure, HFNC may reduce intubation when compared with usual oxygen therapy and has similar ICU mortality when compared with usual oxygen and NIPPV.
Errors in the Diagnosis of Spinal Epidural Abscesses in the Era of Electronic Health Records. Bhise V et al. Am J Med. 2017;130(8):975-981.14
Background
Diagnostic errors are common in patients with spinal epidural abscess, but the main contributing factors are unclear.15
Methods
All patients who were newly diagnosed with spinal epidural abscess in 2013 were identified from the Veterans Affairs (VA) national database. Charts were reviewed for diagnostic delay and contributing factors, including the presence of “red flag” symptoms (eg, fever and neurological deficits).
Findings
Of the 119 patients with a new diagnosis of spinal epidural abscess, 66 (56%) had a diagnostic error. The median time to diagnosis in those with a diagnostic error was 12 days vs four days in those without error (P < .01). Common missed red flags in error cases included fever (n = 57, 86.4%), focal neurologic deficit (n = 54, 81.8%), and active infection (n = 54, 81.8%). Most errors occurred during the provider–patient encounter (eg, information not gathered during the history or physical). The magnitude of harm was serious for most patients (n = 40, 60.6%) and contributed to death in eight patients (12.1%).
Cautions
The study may not be generalizable because it was limited to the VA health system.
Implications
Diagnostic errors are common in patients with spinal epidural abscesses and can lead to serious harm. Health systems should build mechanisms to support providers in the evaluation of patients with back pain.
Hospital medicine continues to expand with respect to the number of practitioners as well as the scope of the practice of those practitioners. In addition, the commitment to, and rigor of, scientific inquiry in the field continues to grow. The authors of this article conducted a review of the medical literature, including articles published between March 2017 and March 2018. The key articles reported studies with high methodological quality, clear findings, and a high potential for impact on clinical practice. The literature was independently reviewed by each author, and candidate works were chosen on the basis of relevance to hospital medicine and expected clinical impact. The articles were organized by subject matter, ranked by applicability to the audience, and selected to meet the time constraints of each talk. Twenty-nine articles were presented at the Update in Hospital Medicine at the 2018 Society of Hospital Medicine and Society of General Internal Medicine annual meetings (B Sharpe, A Burger at SGIM and B Slawski, C Cooper at SHM). Nine articles were included in this review through an iterative voting process. Each author ranked their top five articles from one to five. Points were tallied for each article, and the five articles with the highest points were included. A second round of voting identified the remaining four articles for inclusion. Ties were adjudicated by group discussion. Each article is summarized below, and their key points are highlighted in the table.
KEY PUBLICATIONS
Aspirin in Patients with Previous Percutaneous Coronary Intervention Undergoing Noncardiac Surgery. Graham MM et al. Ann Intern Med. 2018;168(4):237-244.1
Background
The Perioperative Ischemic Evaluation 2 (POISE-2) trial found that perioperative aspirin use had no significant effect on the risk of perioperative death and nonfatal myocardial infarction (MI) in patients who are at risk for vascular complications; however, the risk of major bleeding increased with aspirin use in these patients.2 Nevertheless, the POISE-2 trial did not specifically address the role of aspirin in patients who had undergone previous percutaneous coronary intervention (PCI).
Methods
A post hoc subgroup analysis of POISE-2 evaluated 470 PCI patients (234 aspirin-treated and 236 placebo-treated patients) aged >45 years, 90% of whom had stents. The administration of the study drug was initiated within four hours preoperatively and continued postoperatively. Patients who had bare metal stents placed within the six weeks prior to the study or drug-eluting stents placed within the preceding 12 months were excluded.
Findings
The composite endpoint of risk of death and nonfatal MI was 11.5% in the placebo group and 6% in aspirin-treated patients (HR 0.50; CI, 0.26-0.95). Most of the difference in primary outcome was attributed to an increase in nonfatal MI in the placebo group. Major and life-threatening bleeding were not substantially increased in PCI patients but increased in the overall POISE-2 trial (absolute risk increase 0.8% for major bleeding [95% CI, 0.1%-1.6%]; HR 1.22 [95% CI, 1.01-1.48]). Stent type had no effect on death and nonfatal MI.
Cautions
This was a non-prespecified subgroup analysis with a small sample size.
Implications
Perioperative aspirin use in patients with previous PCI appears to provide more benefit than harm, unless a substantial bleeding risk exists.
Association Between Wait Time and 30-Day Mortality in Adults Undergoing Hip Fracture Surgery. Pincus D et al. JAMA. 2017;318(20):1994-2003.3
Background
Wait times to hip fracture surgery have been associated with mortality in previous studies; however, the wait time associated with complications remains controversial.4,5
Methods
This retrospective cohort study of 42,230 adults modeled the probability of complications in accordance with wait time from hospital arrival to hip fracture surgery. It aimed to identify the optimal time window in which to conduct surgery before complications increased. This window to increased complications was used to define early and delayed surgery. The matched cohorts of early and delayed patients were then used to compare outcomes.
Findings
Overall 30-day mortality was 7%. Complication rates increased when wait times reached 24 hours. Comparing the propensity-matched early (<24 hours) and late (>24 hours) surgery patients revealed that late surgery patients had significantly higher 30-day mortality (6.5% vs 5.8%; % absolute RD 0.79; 95% CI, 0.23-1.35) than early surgery patients and the composite outcome of mortality or other medical complications (MI, DVT, PE, and pneumonia; 12.2% vs 10.1%; % absolute RD 2.16; 95% CI, 1.43-2.89).
Cautions
Only 34% of patients in this study had surgery within 24 hours. The observational cohort study design may result in unmeasured confounders, eg, less sick patients go to surgery more quickly than sicker patients.
Implications
A preoperative wait time of 24 hours appears to represent a threshold of increased risk for 30-day perioperative complications and mortality in hip fracture surgery.
When are Oral Antibiotics a Safe and Effective Choice for Bacterial Bloodstream Infections? An Evidence-Based Narrative Review. Hale AJ et al. J Hosp Med. 2018;13(5):328-335.6
Background
Bloodstream infections (BSIs) are significant causes of morbidity and mortality in the United States. Traditionally, clinicians have relied on intravenous antibiotics for treatment. A recent “Choosing Wisely®” initiative recommends that clinicians should use “oral formulations of highly bioavailable antimicrobials wherever possible.”7 Thus, the authors searched for evidence for scenarios wherein BSIs could be safely treated with oral antibiotics.
Methods
A narrative review was conducted given that robust clinical data for an extensive systematic review were insufficient.
Findings
Key decision points on the use of an oral antibiotic for a diagnosed BSI are as follows: (1) Source control must be attained prior to the consideration of oral antibiotics. (2) A highly bioavailable oral option to which the pathogen is sensitive must be available. (3) Patients must be able to comply with the therapy for the full course and not be on interfering medications. Good evidence for use of oral antibiotics against sensitive gram-negative bacilli other than Pseudomonas exists. Evidence for treating Streptococcus pneumoniae with early transition (within three days) to oral antibiotics is robust when treating bacteremia and pneumonia but not for other primary sites of infection. Evidence for the use of oral antibiotics for B-hemolytic streptococcus, including necrotizing fasciitis and Enterococcus, is insufficient. The evidence supports at least two weeks of IV antibiotics for the treatment of Staphylococcus aureus.
Cautions
This is a narrative review due to limited evidence.
Implications
The early use of oral antibiotics in the setting of bacteremia may be appropriate in select clinical situations.
Prevalence of Pulmonary Embolism in Patients with Syncope. Costantino et al. JAMA Intern Med. 2018;178(3):356-362.8
Background
Data on the prevalence of pulmonary embolism in patients presenting with syncope are conflicting.
Methods
This was a retrospective observational study involving five databases in four countries of >1.6 million adults identified through syncope ICD codes. The rates of pulmonary embolism at first evaluation and pulmonary embolism or venous thromboembolism within 90 days were calculated for emergency room patients and a hospitalized subgroup.
Findings
Pulmonary embolism was rare in patients with syncope, eg, less than 3% for hospitalized patients in this database study.
Cautions
The results of this study are based on the use of administrative databases to confirm the diagnosis of syncope. Additionally, the results include hospitalized and nonhospitalized patients. The design of this study differs significantly from those of the PESIT study, which showed a prevalence of 17% in hospitalized patients.9 The PESIT study specifically sought the diagnosis of pulmonary embolism even when other etiologies for syncope existed.
Implications
Ultimately, the clinical impetus to search for pulmonary embolism in hospitalized patients admitted with syncope will depend on individual presentations. The authors argued that pulmonary embolism is rare in syncope and much lower than 17% but should be considered in appropriate patients.
Balanced Crystalloids versus Saline in Noncritically Ill Patients. Self WH et al. N Engl J Med. 2018;378(9):819-828.10
Background
Data on the optimal composition of intravenous fluids (IVF) are limited. Limited experimental evidence suggests that IVF-induced hyperchloremia results in renal vasoconstriction and acute kidney injury.
Methods
This was a single-center, open-label, multiple crossover trial of >13,000 non-ICU hospitalized patients admitted from the Emergency Department. Patients were randomized to receive either only normal saline or a “balanced crystalloid,” eg, either Lactated Ringer’s or Plasmalyte. The primary outcome was hospital-free days. Secondary outcomes were major adverse kidney events (MAKE) at 30 days.
Findings
The study found no difference in the primary outcome of hospital-free days. However, balanced IVF resulted in a lower incidence of hyperchloremia and a slightly reduced incidence of MAKE 30 (4.7% vs 5.6%; adjusted OR 0.82).
Cautions
The incidence of acute kidney injury was low in this single-center ED population. This study, however, did not include hospitalized patients. The long-term effects on renal function could not be ascertained.
Implications
Hospital-free days after inpatient randomization to either normal saline or “balanced IVF” were not significantly different. “Balanced IVF” may be beneficial in select high renal-risk populations.
Speaker Introductions at Internal Medicine Grand Rounds: Forms of Address Reveal Speaker Bias. Files et al. J Womens Health. 2017;26(5):413-419.11
Background
Gender bias is known to contribute to leadership disparities between men and women in several academic medical centers.
Methods
This was a retrospective observational study reviewing video-archived introductions at Internal Medicine Grand Rounds at two connected institutions. All speakers had doctoral degrees. The outcome measured was the use of a speaker’s professional title during his/her introduction as a function of the introducer’s gender.
Findings
Women were more likely than men to introduce speakers of any gender by their professional title in the 321 forms of address analyzed (96% vs 66%, P < .001). When the introducer and speaker were of different genders, women were more likely to introduce male speakers with formal titles than men introducing female speakers (95% vs 49%, P < .001).
Cautions
This study was done at two associated academic institutions and may not reflect the practice or customs of physicians in other departments or institutions.
Implications
Despite the study’s limitations, it supports a theme of prevalent gender bias within academic medical institutions that may affect the outcomes of leadership, promotion, and scholarship.
Edoxaban for the Treatment of Cancer-Associated Venous Thromboembolism. Raskob GE et al. N Engl J Med. 2018;378(7):615-624.12
Background
Low-molecular-weight heparin (LMWH) is the standard of care for the treatment of venous thromboembolism (VTE) in patients with cancer. Direct oral anticoagulants have not been studied for this indication.
Methods
This open-label, noninferiority trial randomized patients with cancer and acute VTE to either LMWH for a minimum of five days followed by oral edoxaban vs subcutaneous dalteparin.
Findings
A total of 1,046 patients were included in the modified intention-to-treat analysis. Patients received treatment for six to twelve months total. A composite outcome of recurrent VTE or major bleed within 12 months occurred in 67 of 522 (12.8%) of patients in the edoxaban group vs 71 of 524 (13.5%) of patients in the dalteparin group (HR 0.91, 95% CI 0.70-1.36, P = .006 for noninferiority). Recurrent VTE occurred more commonly with dalteparin than with edoxaban (11.3% vs 7.9%), whereas major bleeding was less common with dalteparin than with edoxaban (4% vs 6.9%). The increased bleeding rate with edoxaban was predominantly in patients with an upper gastrointestinal (GI) malignancy.
Cautions
This was an open-label study. Patients in the edoxaban still received five days of LMWH prior to oral edoxaban. More patients in the edoxaban group continued treatment for the entire 12-month period, which contributes to the observed decreased bleeding and increased VTE rates in the dalteparin group.
Implications
Oral edoxaban is noninferior to subcutaneous dalteparin for the primary composite endpoint of VTE and bleeding. Notably, the patients in the edoxaban group experienced a lower rate of recurrent VTE and a higher rate of major bleeding than the patients in the dalteparin group. Additional caution about bleeding risk in those with a GI malignancy is recommended.
Can High-flow Nasal Cannula Reduce the Rate of Endotracheal Intubation in Adult Patients with Acute Respiratory Failure Compared with Conventional Oxygen Therapy and Noninvasive Positive Pressure Ventilation? Ni Y-N et al. Chest. 2017;151(4):764-775.13
Background
High-flow nasal cannula (HFNC) can deliver heated and humidified oxygen at rates of up to 60 L/min. Evidence on the benefits of HFNC over usual oxygen therapy or noninvasive positive pressure ventilation (NIPPV) is conflicting.
Methods
This systematic review and meta-analysis included 18 studies (12 RCTs, four retrospective, and two prospective cohort studies) with 3,881 patients with respiratory failure (medical and surgical causes). The included studies compared HFNC with usual oxygen therapy or NIPPV.
Findings
HFNC was associated with lower rates of endotracheal intubation (OR 0.47, 95% CI 0.27-0.84, P = .01) relative to oxygen therapy. Intubation rates did not differ between HFNC and NIPPV (OR 0.73, 95% CI 0.47-1.13, P = .16). No differences in ICU mortality or ICU length of stay (LOS) were found when HFNC was compared with either usual oxygen therapy or NIPPV.
Cautions
The significant heterogeneity in study design across studies is mainly attributable to varying causes of respiratory failure and differences in flow rate, oxygen concentration, and treatment duration across studies.
Implications
In patients with respiratory failure, HFNC may reduce intubation when compared with usual oxygen therapy and has similar ICU mortality when compared with usual oxygen and NIPPV.
Errors in the Diagnosis of Spinal Epidural Abscesses in the Era of Electronic Health Records. Bhise V et al. Am J Med. 2017;130(8):975-981.14
Background
Diagnostic errors are common in patients with spinal epidural abscess, but the main contributing factors are unclear.15
Methods
All patients who were newly diagnosed with spinal epidural abscess in 2013 were identified from the Veterans Affairs (VA) national database. Charts were reviewed for diagnostic delay and contributing factors, including the presence of “red flag” symptoms (eg, fever and neurological deficits).
Findings
Of the 119 patients with a new diagnosis of spinal epidural abscess, 66 (56%) had a diagnostic error. The median time to diagnosis in those with a diagnostic error was 12 days vs four days in those without error (P < .01). Common missed red flags in error cases included fever (n = 57, 86.4%), focal neurologic deficit (n = 54, 81.8%), and active infection (n = 54, 81.8%). Most errors occurred during the provider–patient encounter (eg, information not gathered during the history or physical). The magnitude of harm was serious for most patients (n = 40, 60.6%) and contributed to death in eight patients (12.1%).
Cautions
The study may not be generalizable because it was limited to the VA health system.
Implications
Diagnostic errors are common in patients with spinal epidural abscesses and can lead to serious harm. Health systems should build mechanisms to support providers in the evaluation of patients with back pain.
1. Graham MM, Sessler DI, Parlow JL, et al. Aspirin in patients with previous percutaneous coronary intervention undergoing noncardiac surgery. Ann Intern Med. 2018;168(4):237-244. doi: 10.7326/M17-2341.
2. Devereaux PJ, Mrkobrada M, Sessler DI, et al. Aspirin in patients undergoing noncardiac surgery. N Engl J Med. 2014;370(16):1494-1503. doi: 10.1056/NEJMoa1401105
3. Pincus D, Ravi B, Wasserstein D, et al. Association between wait time and 30-day mortality in adults undergoing hip fracture surgery. JAMA. 2017;318(20):1994-2003. doi: 10.1001/jama.2017.17606.
4. Simunovic N, Devereaux PJ, Sprague S, et al. Effect of early surgery after hip fracture on mortality and complications: systematic review and meta-analysis. CMAJ. 2010;182(15):1609-1616. doi: 10.1503/cmaj.092220.
5. Shiga T, Wajima Z, Ohe Y. Is operative delay associated with increased mortality of hip fracture patients? ystematic review, meta-analysis, and meta-regression. Can J Anaesth. 2008;55(3):146-154. doi: 10.1007/BF03016088.
6. Hale AJ, Snyder GM, Ahern JW, Eliopoulos G, Ricotta D, Alston WK. When are oral antibiotics a safe and effective choice for bacterial bloodstream infections? An evidence-based narrative review. J Hosp Med. 2018;13(5):328-335. doi: 10.12788/jhm.2949.
7. Lehmann C, Berner R, Bogner JR, et al. The “Choosing Wisely” initiative in infectious diseases. Infection. 2017;45(3):263-268. doi: 10.1007/s15010-017-0997-0.
8. Costantino G, Ruwald MH, Quinn J, et al. Prevalence of pulmonary embolism in patients with syncope. JAMA Intern Med. 2018;178(3):356-362. doi: 10.1001/jamainternmed.2017.8175.
9. Prandoni P, Lensing AW, Prins MH, et al. Prevalence of pulmonary embolism among patients hospitalized for syncope. N Engl J Med. 2016;375(16):1524-1531. doi: 10.1056/NEJMoa1602172
10. Self WH, Semler MW, Wanderer JP, et al. Balanced crystalloids versus saline in noncritically ill adults. N Engl J Med. 2018;378(9):819-828. doi: 10.1056/NEJMoa1711586.
11. Files JA, Mayer AP, Ko MG, et al. Speaker introductions at internal medicine grand rounds: forms of address reveal gender bias. J Womens Health (Larchmt). 2017;26(5):413-419. doi: 10.1089/jwh.2016.6044.
12. Raskob GE, van Es N, Verhamme P, et al. Edoxaban for the treatment of cancer-associated venous thromboembolism. N Engl J Med. 2018;378(7):615-624. doi: 10.1056/NEJMoa1711948.
13. Ni YN, Luo J, Yu H, et al. Can high-flow nasal cannula reduce the rate of endotracheal intubation in adult patients with acute respiratory failure compared with conventional oxygen therapy and noninvasive positive pressure ventilation?: A systematic review and meta-analysis. Chest. 2017;151(4):764-775. doi: 10.1016/j.chest.2017.01.004.
14. Bhise V, Meyer AND, Singh H, et al. Errors in diagnosis of spinal epidural abscesses in the era of electronic health records. Am J Med. 2017;130(8):975-981. doi: 10.1016/j.amjmed.2017.03.009
15. Davis DP, Wold RM, Patel RJ, et al. The clinical presentation and impact of diagnostic delays on emergency department patients with spinal epidural abscess. J Emerg Med. 2004;26(3):285-291. doi: 10.1016/j.jemermed.2003.11.013.
1. Graham MM, Sessler DI, Parlow JL, et al. Aspirin in patients with previous percutaneous coronary intervention undergoing noncardiac surgery. Ann Intern Med. 2018;168(4):237-244. doi: 10.7326/M17-2341.
2. Devereaux PJ, Mrkobrada M, Sessler DI, et al. Aspirin in patients undergoing noncardiac surgery. N Engl J Med. 2014;370(16):1494-1503. doi: 10.1056/NEJMoa1401105
3. Pincus D, Ravi B, Wasserstein D, et al. Association between wait time and 30-day mortality in adults undergoing hip fracture surgery. JAMA. 2017;318(20):1994-2003. doi: 10.1001/jama.2017.17606.
4. Simunovic N, Devereaux PJ, Sprague S, et al. Effect of early surgery after hip fracture on mortality and complications: systematic review and meta-analysis. CMAJ. 2010;182(15):1609-1616. doi: 10.1503/cmaj.092220.
5. Shiga T, Wajima Z, Ohe Y. Is operative delay associated with increased mortality of hip fracture patients? ystematic review, meta-analysis, and meta-regression. Can J Anaesth. 2008;55(3):146-154. doi: 10.1007/BF03016088.
6. Hale AJ, Snyder GM, Ahern JW, Eliopoulos G, Ricotta D, Alston WK. When are oral antibiotics a safe and effective choice for bacterial bloodstream infections? An evidence-based narrative review. J Hosp Med. 2018;13(5):328-335. doi: 10.12788/jhm.2949.
7. Lehmann C, Berner R, Bogner JR, et al. The “Choosing Wisely” initiative in infectious diseases. Infection. 2017;45(3):263-268. doi: 10.1007/s15010-017-0997-0.
8. Costantino G, Ruwald MH, Quinn J, et al. Prevalence of pulmonary embolism in patients with syncope. JAMA Intern Med. 2018;178(3):356-362. doi: 10.1001/jamainternmed.2017.8175.
9. Prandoni P, Lensing AW, Prins MH, et al. Prevalence of pulmonary embolism among patients hospitalized for syncope. N Engl J Med. 2016;375(16):1524-1531. doi: 10.1056/NEJMoa1602172
10. Self WH, Semler MW, Wanderer JP, et al. Balanced crystalloids versus saline in noncritically ill adults. N Engl J Med. 2018;378(9):819-828. doi: 10.1056/NEJMoa1711586.
11. Files JA, Mayer AP, Ko MG, et al. Speaker introductions at internal medicine grand rounds: forms of address reveal gender bias. J Womens Health (Larchmt). 2017;26(5):413-419. doi: 10.1089/jwh.2016.6044.
12. Raskob GE, van Es N, Verhamme P, et al. Edoxaban for the treatment of cancer-associated venous thromboembolism. N Engl J Med. 2018;378(7):615-624. doi: 10.1056/NEJMoa1711948.
13. Ni YN, Luo J, Yu H, et al. Can high-flow nasal cannula reduce the rate of endotracheal intubation in adult patients with acute respiratory failure compared with conventional oxygen therapy and noninvasive positive pressure ventilation?: A systematic review and meta-analysis. Chest. 2017;151(4):764-775. doi: 10.1016/j.chest.2017.01.004.
14. Bhise V, Meyer AND, Singh H, et al. Errors in diagnosis of spinal epidural abscesses in the era of electronic health records. Am J Med. 2017;130(8):975-981. doi: 10.1016/j.amjmed.2017.03.009
15. Davis DP, Wold RM, Patel RJ, et al. The clinical presentation and impact of diagnostic delays on emergency department patients with spinal epidural abscess. J Emerg Med. 2004;26(3):285-291. doi: 10.1016/j.jemermed.2003.11.013.
© 2019 Society of Hospital Medicine
Perioperative cardiovascular medicine: 5 questions for 2018
A plethora of studies are under way in the field of perioperative medicine. As a result, evidence-based care of surgical patients is evolving at an exponential rate.
We performed a literature search and, using consensus, identified recent articles we believe will have a great impact on perioperative cardiovascular medicine. These articles report studies that were presented at national meetings in 2018, including the Perioperative Medicine Summit, Society of General Internal Medicine, and Society of Hospital Medicine. These articles are grouped under 5 questions that will help guide clinical practice in perioperative cardiovascular medicine.
SHOULD ASPIRIN BE CONTINUED PERIOPERATIVELY IN PATIENTS WITH A CORONARY STENT?
The Perioperative Ischemic Evaluation 2 (POISE-2) trial1 found that giving aspirin before surgery and throughout the early postoperative period had no significant effect on the rate of a composite of death or nonfatal myocardial infarction; moreover, aspirin increased the risk of major bleeding. However, many experts felt uncomfortable stopping aspirin preoperatively in patients taking it for secondary prophylaxis, particularly patients with a coronary stent.
[Graham MM, Sessler DI, Parlow JL, et al. Aspirin in patients with previous percutaneous coronary intervention undergoing noncardiac surgery. Ann Intern Med 2018; 168(4):237–244.]
This post hoc subgroup analysis2 of POISE-2 evaluated the benefit and harm of perioperative aspirin in patients who had previously undergone percutaneous coronary intervention, more than 90% of whom had received a stent. Patients were age 45 or older with atherosclerotic heart disease or risk factors for it who had previously undergone percutaneous coronary intervention and were now undergoing noncardiac surgery.
Patients who had received a bare-metal stent within the previous 6 weeks or a drug-eluting stent within 12 months before surgery were excluded because guidelines at that time said to continue dual antiplatelet therapy for that long. Recommendations have since changed; the optimal duration for dual antiplatelet therapy with drug-eluting stents is now 6 months. Second-generation drug-eluting stents pose a lower risk of stent thrombosis and require a shorter duration of dual antiplatelet therapy than first-generation drug-eluting stents. Approximately 25% of the percutaneous coronary intervention subgroup had a drug-eluting stent, but the authors did not specify the type of drug-eluting stent.
The post hoc analysis2 included a subgroup of 234 of 4,998 patients receiving aspirin and 236 of 5,012 patients receiving placebo initiated within 4 hours before surgery and continued postoperatively. The primary outcome measured was the rate of death or nonfatal myocardial infarction within 30 days after surgery, and bleeding was a secondary outcome.
Findings. Although the overall POISE-2 study found no benefit from aspirin, in the subgroup who had previously undergone percutaneous coronary intervention, aspirin significantly reduced the risk of the primary outcome, which occurred in 6% vs 11.5% of the patients:
- Absolute risk reduction 5.5% (95% confidence interval 0.4%–10.5%)
- Hazard ratio 0.50 (0.26–0.95).
The reduction was primarily due to fewer myocardial infarctions:
- Absolute risk reduction 5.9% (1.0%–10.8%)
- Hazard ratio 0.44 (0.22–0.87).
The type of stent had no effect on the primary outcome, although this subgroup analysis had limited power. In the nonpercutaneous coronary intervention subgroup, there was no significant difference in outcomes between the aspirin and placebo groups. This subgroup analysis was underpowered to evaluate the effect of aspirin on the composite of major and life-threatening bleeding in patients with prior percutaneous coronary intervention, which was reported as “uncertain” due to wide confidence intervals (absolute risk increase 1.3%, 95% confidence interval –2.6% to 5.2%), but the increased risk of major or life-threatening bleeding with aspirin demonstrated in the overall POISE-2 study population likely applies:
- Absolute risk increase 0.8% (0.1%–1.6%)
- Hazard ratio 1.22 (1.01–1.48).
Limitations. This was a nonspecified subgroup analysis that was underpowered and had a relatively small sample size with few events.
Conclusion. In the absence of a very high bleeding risk, continuing aspirin perioperatively in patients with prior percutaneous coronary intervention undergoing noncardiac surgery is more likely to result in benefit than harm. This finding is in agreement with current recommendations from the American College Cardiology and American Heart Association (class I; level of evidence C).3
WHAT IS THE INCIDENCE OF MINS? IS MEASURING TROPONIN USEFUL?
Despite advances in anesthesia and surgical techniques, about 1% of patients over age 45 die within 30 days of noncardiac surgery.4 Studies have demonstrated a high mortality rate in patients who experience myocardial injury after noncardiac surgery (MINS), defined as elevations of troponin T with or without ischemic symptoms or electrocardiographic changes.5 Most of these studies used earlier, “non-high-sensitivity” troponin T assays. Fifth-generation, highly sensitive troponin T assays are now available that can detect troponin T at lower concentrations, but their utility in predicting postoperative outcomes remains uncertain. Two recent studies provide further insight into these issues.
[Writing Committee for the VISION Study Investigators, Devereaux PJ, Biccard BM, Sigamani A, et al. Association of postoperative high-sensitivity troponin levels with myocardial injury and 30-day mortality among patients undergoing noncardiac surgery. JAMA 2017; 317(16):1642–1651.]
The Vascular Events in Noncardiac Surgery Patients Cohort Evaluation (VISION) study5 was an international, prospective cohort study that initially evaluated the association between MINS and the 30-day mortality rate using a non-high-sensitivity troponin T assay (Roche fourth-generation Elecsys TnT assay) in patients age 45 or older undergoing noncardiac surgery and requiring hospital admission for at least 1 night. After the first 15,000 patients, the study switched to the Roche fifth-generation assay, with measurements at 6 to 12 hours after surgery and on postoperative days 1, 2, and 3.
A 2017 analysis by Devereaux et al6 included only these later-enrolled patients and correlated their high-sensitivity troponin T levels with 30-day mortality rates. Patients with a level 14 ng/L or higher, the upper limit of normal in this study, were also assessed for ischemic symptoms and electrocardiographic changes. Although not required by the study, more than 7,800 patients had their troponin T levels measured before surgery, and the absolute change was also analyzed for an association with the 30-day mortality rate.
Findings. Of the 21,842 patients, about two-thirds underwent some form of major surgery; some of them had more than 1 type. A total of 1.2% of the patients died within 30 days of surgery.
Based on their analysis, the authors proposed that MINS be defined as:
- A postoperative troponin T level of 65 ng/L or higher, or
- A level in the range of 20 ng/L to less than 65 ng/L with an absolute increase from the preoperative level at least 5 ng/L, not attributable to a nonischemic cause.
Seventeen percent of the study patients met these criteria, and of these, 21.7% met the universal definition of myocardial infarction, although only 6.9% had symptoms of it.
Limitations. Only 40.4% of the patients had a preoperative high-sensitivity troponin T measurement for comparison, and in 13.8% of patients who had an elevated perioperative measurement, their preoperative value was the same or higher than their postoperative one. Thus, the incidence of MINS may have been overestimated if patients were otherwise not known to have troponin T elevations before surgery.
[Puelacher C, Lurati Buse G, Seeberger D, et al. Perioperative myocardial injury after noncardiac surgery: incidence, mortality, and characterization. Circulation 2018; 137(12):1221–1232.]
Puelacher et al7 investigated the prevalence of MINS in 2,018 patients at increased cardiovascular risk (age ≥ 65, or age ≥ 45 with a history of coronary artery disease, peripheral vascular disease, or stroke) who underwent major noncardiac surgery (planned overnight stay ≥ 24 hours) at a university hospital in Switzerland. Patients had their troponin T measured with a high-sensitivity assay within 30 days before surgery and on postoperative days 1 and 2.
Instead of MINS, the investigators used the term “perioperative myocardial injury” (PMI), defined as an absolute increase in troponin T of at least 14 ng/L from before surgery to the peak postoperative reading. Similar to MINS, PMI did not require ischemic features, but in this study, noncardiac triggers (sepsis, stroke, or pulmonary embolus) were not excluded.
Findings. PMI occurred in 16% of surgeries, and of the patients with PMI, 6% had typical chest pain and 18% had any ischemic symptoms. Unlike in the POISE-2 study discussed above, PMI triggered an automatic referral to a cardiologist.
The unadjusted 30-day mortality rate was 8.9% among patients with PMI and 1.5% in those without. Multivariable logistic regression analysis showed an adjusted hazard ratio for 30-day mortality of 2.7 (95% CI 1.5–4.8) for those with PMI vs without, and this difference persisted for at least 1 year.
In patients with PMI, the authors compared the 30-day mortality rate of those with no ischemic signs or symptoms (71% of the patients) with those who met the criteria for myocardial infarction and found no difference. Patients with PMI triggered by a noncardiac event had a worse prognosis than those with a presumed cardiac etiology.
Limitations. Despite the multivariate analysis that included adjustment for age, nonelective surgery, and Revised Cardiac Risk Index (RCRI), the increased risk associated with PMI could simply reflect higher risk at baseline. Although PMI resulted in automatic referral to a cardiologist, only 10% of patients eventually underwent coronary angiography; a similar percentage were discharged with additional medical therapy such as aspirin, a statin, or a beta-blocker. The effect of these interventions is not known.
Conclusions. MINS is common and has a strong association with mortality risk proportional to the degree of troponin T elevation using high-sensitivity assays, consistent with data from previous studies of earlier assays. Because the mechanism of MINS may differ from that of myocardial infarction, its prevention and treatment may differ, and it remains unclear how serial measurement in postoperative patients should change clinical practice.
The recently published Dabigatran in Patients With Myocardial Injury After Non-cardiac Surgery (MANAGE) trial8 suggests that dabigatran may reduce arterial and venous complications in patients with MINS, but the study had a number of limitations that may restrict the clinical applicability of this finding.
While awaiting further clinical outcomes data, pre- and postoperative troponin T measurement may be beneficial in higher-risk patients (such as those with cardiovascular disease or multiple RCRI risk factors) if the information will change perioperative management.
WHAT IS THE ROLE OF HYPOTENSION OR BLOOD PRESSURE CONTROL?
Intraoperative hypotension is associated with organ ischemia, which may cause postoperative myocardial infarction, myocardial injury, and acute kidney injury.9 Traditional anesthesia practice is to maintain intraoperative blood pressure within 20% of the preoperative baseline, based on the notion that hypertensive patients require higher perfusion pressures.
[Futier E, Lefrant J-Y, Guinot P-G, et al. Effect of individualized vs standard blood pressure management strategies on postoperative organ dysfunction among high-risk patients undergoing major surgery: a randomized clinical trial. JAMA 2017; 318(14):1346–1357.]
Futier et al10 sought to address uncertainty in intraoperative and immediate postoperative management of systolic blood pressure. In this multicenter, randomized, parallel-group trial, 298 patients at increased risk of postoperative renal complications were randomized to blood pressure management that was either “individualized” (within 10% of resting systolic pressure) or “standard” (≥ 80 mm Hg or ≥ 40% of resting systolic pressure) from induction to 4 hours postoperatively.
Blood pressure was monitored using radial arterial lines and maintained using a combination of intravenous fluids, norepinephrine (the first-line agent for the individualized group), and ephedrine (in the standard treatment group only). The primary outcome was a composite of systemic inflammatory response syndrome (SIRS) and organ dysfunction affecting at least 1 organ system (cardiovascular, respiratory, renal, hematologic, or neurologic).
Findings. Data on the primary outcome were available for 292 of 298 patients enrolled. The mean age was 70 years, 15% were women, and 82% had previously diagnosed hypertension. Despite the requirement for an elevated risk of acute kidney injury, only 13% of the patients had a baseline estimated glomerular filtration rate of less than 60 mL/min/1.73 m2, and the median was 88 mL/min/1.73 m2. Ninety-five percent of patients underwent abdominal surgery, and 50% of the surgeries were elective.
The mean systolic blood pressure was 123 mm Hg in the individualized treatment group compared with 116 mm Hg in the standard treatment group. Despite this small difference, 96% of individualized treatment patients received norepinephrine, compared with 26% in the standard treatment group.
The primary outcome of SIRS with organ dysfunction occurred in 38.1% of patients in the individualized treatment group and 51.7% of those in the standard treatment group. After adjusting for center, surgical urgency, surgical site, and acute kidney injury risk index, the relative risk of developing SIRS in those receiving individualized management was 0.73 (P = .02). Renal dysfunction (based on Acute Dialysis Quality Initiative criteria11) occurred in 32.7% of individualized treatment patients and 49% of standardized treatment patients.
Limitations of this study included differences in pharmacologic approach to maintain blood pressure in the 2 protocols (ephedrine and fluids vs norepinephrine) and a modest sample size.
Conclusions. Despite this, the difference in organ dysfunction was striking, with a number needed to treat of only 7 patients. This intervention extended 4 hours postoperatively, a time when many of these patients have left the postanesthesia care unit and have returned to hospitalist care on inpatient wards.
While optimal management of intraoperative and immediate postoperative blood pressure may not be settled, this study suggests that even mild relative hypotension may justify immediate action. Further studies may be useful to delineate high- and low-risk populations, the timing of greatest risk, and indications for intraarterial blood pressure monitoring.
[Salmasi V, Maheswari K, Yang D, et al. Relationship between intraoperative hypotension, defined by either reduction from baseline or absolute thresholds, and acute kidney and myocardial injury after noncardiac surgery: a retrospective cohort analysis. Anesthesiology 2017; 126(1):47–65.]
This retrospective cohort study12 assessed the association between myocardial or kidney injury and absolute or relative thresholds of intraoperative mean arterial pressure. It included 57,315 adults who underwent inpatient noncardiac surgery, had a preoperative and at least 1 postoperative serum creatinine measurement within 7 days, and had blood pressure recorded in preoperative appointments within 6 months. Patients with chronic kidney disease (glomerular filtration rate < 60 mL/min/1.73 m2) and those on dialysis were excluded. The outcomes were MINS5 and acute kidney injury as defined by the Acute Kidney Injury Network.9
Findings. A mean arterial pressure below an absolute threshold of 65 mm Hg or a relative threshold of 20% lower than baseline value was associated with myocardial and kidney injury. At each threshold, prolonged periods of hypotension were associated with progressively increased risk.
An important conclusion of the study was that relative thresholds of mean arterial pressure were not any more predictive than absolute thresholds. Absolute thresholds are easier to use intraoperatively, especially when baseline values are not available. The authors did not find a clinically significant interaction between baseline blood pressure and the association of hypotension and myocardial and kidney injury.
Limitations included use of cardiac enzymes postoperatively to define MINS. Since these were not routinely collected, clinically silent myocardial injury may have been missed. Baseline blood pressure may have important implications in other forms of organ injury (ie, cerebral ischemia) that were not studied.
Summary. The lowest absolute mean arterial pressure is as predictive of postoperative myocardial and kidney injury as the relative pressure reduction, at least in patients with normal renal function. Limiting exposure to intraoperative hypotension is important. Baseline blood pressure values may have limited utility for intraoperative management.
In combination, these studies confirm that intraoperative hypotension is a predictor of postoperative organ dysfunction, but the definition and management remain unclear. While aggressive intraoperative management is likely beneficial, how to manage the antihypertensive therapy the patient has been taking as an outpatient when he or she comes into the hospital for surgery remains uncertain.
DOES PATENT FORAMEN OVALE INCREASE THE RISK OF STROKE?
Perioperative stroke is an uncommon, severe complication of noncardiac surgery. The pathophysiology has been better defined in cardiac than in noncardiac surgeries. In nonsurgical patients, patent foramen ovale (PFO) is associated with stroke, even in patients considered to be at low risk.13 Perioperative patients have additional risk for venous thromboembolism and may have periprocedural antithrombotic medications altered, increasing their risk of paradoxical embolism through the PFO.
[Ng PY, Ng AK, Subramaniam B, et al. Association of preoperatively diagnosed patent foramen ovale with perioperative ischemic stroke. JAMA 2018; 319(5):452–462.]
This retrospective cohort study of noncardiac surgery patients at 3 hospitals14 sought to determine the association of preoperatively diagnosed PFO with the risk of perioperative ischemic stroke identified by International Classification of Diseases diagnoses.
Of 150,198 patients, 1.0% had a preoperative diagnosis of PFO, and at baseline, those with PFO had significantly more comorbidities than those without PFO. Stroke occurred in 3.2% of patients with PFO vs 0.5% of those without. Patients known to have a PFO were much more likely to have cardiovascular and thromboembolic risk factors for stroke. In the adjusted analysis, the absolute risk difference between groups was 0.4% (95% CI 0.2–0.6%), with an estimated perioperative stroke risk of 5.9 per 1,000 in patients with known patent foramen ovale and 2.2 per 1,000 in those without. A diagnosis of PFO was also associated with increased risk of large-vessel-territory stroke and more severe neurologic deficit.
Further attempts to adjust for baseline risk factors and other potential bias, including a propensity score-matched cohort analysis and an analysis limited to patients who had echocardiography performed in the same healthcare system, still showed a higher risk of perioperative stroke among patients with preoperatively detected patent foramen ovale.
Limitations. The study was retrospective and observational, used administrative data, and had a low rate of PFO diagnosis (1%), compared with about 25% in population-based studies.15 Indications for preoperative echocardiography are unknown. In addition, the study specifically examined preoperatively diagnosed PFO, rather than including those diagnosed in the postoperative period.
Discussion. How does this study affect clinical practice? The absolute stroke risk was increased by 0.4% in patients with PFO compared with those without. Although this is a relatively small increase, millions of patients undergo noncardiac surgery annually. The risks of therapeutic anticoagulation or PFO closure are likely too high in this context; however, clinicians may approach the perioperative management of antiplatelet agents and venous thromboembolism prophylaxis in patients with known PFO with additional caution.
HOW DOES TIMING OF EMERGENCY SURGERY AFTER PRIOR STROKE AFFECT OUTCOMES?
A history of stroke or transient ischemic attack is a known risk factor for perioperative vascular complications. A recent large cohort study demonstrated that a history of stroke within 9 months of elective surgery was associated with increased adverse outcomes.16 Little is known, however, of the perioperative risk in patients with a history of stroke who undergo emergency surgery.
[Christiansen MN, Andersson C, Gislason GH, et al. Risks of cardiovascular adverse events and death in patients with previous stroke undergoing emergency noncardiac, nonintracranial surgery: the importance of operative timing. Anesthesiology 2017; 127(1):9–19.]
In this study,17 all emergency noncardiac and nonintracranial surgeries from 2005 to 2011 were analyzed using multiple national patient registries in Denmark according to time elapsed between previous stroke and surgery. Primary outcomes were 30-day all-cause mortality and 30-day major adverse cardiac events (MACE), defined as nonfatal ischemic stroke, nonfatal myocardial infarction, and cardiovascular death. Statistical analysis to assess the risk of adverse outcomes included logistic regression models, spline analyses, and propensity-score matching.
Findings. The authors identified 146,694 emergency surgeries, with 7,861 patients (5.4%) having had a previous stroke (transient ischemic attacks and hemorrhagic strokes were not included). Rates of postoperative stroke were as follows:
- 9.9% in patents with a history of ischemic stroke within 3 months of surgery
- 2.8% in patients with a history of stroke 3 to 9 months before surgery
- 0.3% in patients with no previous stroke.
The risk plateaued when the time between stroke and surgery exceeded 4 to 5 months.15
Interestingly, in patients who underwent emergency surgery within 14 days of stroke, the risk of MACE was significantly lower immediately after surgery (1–3 days after stroke) compared with surgery that took place 4 to 14 days after stroke. The authors hypothesized that because cerebral autoregulation does not become compromised until approximately 5 days after a stroke, the risk was lower 1 to 3 days after surgery and increased thereafter.
Limitations of this study included the possibility of residual confounding, given its retrospective design using administrative data, not accounting for preoperative antithrombotic and anticoagulation therapy, and lack of information regarding the etiology of recurrent stroke (eg, thromboembolic, atherothrombotic, hypoperfusion).
Conclusions. Although it would be impractical to postpone emergency surgery in a patient who recently had a stroke, this study shows that the incidence rates of postoperative recurrent stroke and MACE are high. Therefore, it is important that the patient and perioperative team be aware of the risk. Further research is needed to confirm these estimates of postoperative adverse events in more diverse patient populations.
- Devereaux PJ, Mrkobrada M, Sessler DI, et al. Aspirin in patients undergoing noncardiac surgery. N Engl J Med 2014; 370(16):1494–1503. doi:10.1056/NEJMoa1401105
- Graham MM, Sessler DI, Parlow JL, et al. Aspirin in patients with previous percutaneous coronary intervention undergoing noncardiac surgery. Ann Intern Med 2018; 168(4):237–244. doi:10.7326/M17-2341
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 130(24):2215–2245. doi:10.1161/CIR.0000000000000105
- Smilowitz NR, Gupta N, Ramakrishna H, Guo Y, Berger JS, Bangalore S. Perioperative major adverse cardiovascular and cerebrovascular events associated with noncardiac surgery. JAMA Cardiol 2017; 2(2):181–187. doi:10.1001/jamacardio.2016.4792
- Botto F, Alonso-Coello P, Chan MT, et al. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology 2014; 120(3):564–578. doi:10.1097/ALN.0000000000000113
- Writing Committee for the VISION Study Investigators, Devereaux PJ, Biccard BM, Sigamani A, et al. Association of postoperative high-sensitivity troponin levels with myocardial injury and 30-day mortality among patients undergoing noncardiac surgery. JAMA 2017; 317(16):1642–1651. doi:10.1001/jama.2017.4360
- Puelacher C, Lurati Buse G, Seeberger D, et al. Perioperative myocardial injury after noncardiac surgery: incidence, mortality, and characterization. Circulation 2018; 137(12):1221–1232. doi:10.1161/CIRCULATIONAHA.117.030114
- Devereaux PJ, Duceppe E, Guyatt G, et al. Dabigatran in patients with myocardial injury after non-cardiac surgery (MANAGE): an international, randomised, placebo-controlled trial. Lancet 2018; 391(10137):2325–2334. doi:10.1016/S0140-6736(18)30832-8
- Walsh M, Devereaux PJ, Garg AX, et al. Relationship between intraoperative mean arterial pressure and clinical outcomes after noncardiac surgery: toward an empirical definition of hypotension. Anesthesiology 2013; 119(3):507–515. doi:10.1097/ALN.0b013e3182a10e26
- Futier E, Lefrant JY, Guinot PG, et al. Effect of individualized vs standard blood pressure management strategies on postoperative organ dysfunction among high-risk patients undergoing major surgery: a randomized clinical trial. JAMA 2017; 318(14):1346–1357. doi:10.1001/jama.2017.14172
- Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P; Acute Dialysis Quality Initiative workgroup. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) group. Crit Care 2004; 8:R204. doi:10.1186/cc2872
- Salmasi V, Maheswari K, Yang D, et al. Relationship between intraoperative hypotension, defined by either reduction from baseline or absolute thresholds, and acute kidney and myocardial injury after noncardiac surgery: a retrospective cohort analysis. Anesthesiology 2017; 126(1):47–65. doi:10.1097/ALN.0000000000001432
- Lechat P, Mas JL, Lascault G, et al. Prevalence of patent foramen ovale in patients with stroke. N Engl J Med 1988; 318(18):1148–1152. doi:10.1056/NEJM198805053181802
- Ng PY, Ng AK, Subramaniam B, et al. Association of preoperatively diagnosed patent foramen ovale with perioperative ischemic stroke. JAMA 2018; 319(5):452–462. doi:10.1001/jama.2017.21899
- Meissner I, Whisnant JP, Khandheria BK, et al. Prevalence of potential risk factors for stroke assessed by transesophageal echocardiography and carotid ultrasonography: the SPARC study. Stroke Prevention: Assessment of Risk in a Community. Mayo Clin Proc 1999; 74(9):862–869. pmid:10488786
- Jørgensen ME, Torp-Pedersen C, Gislason GH, et al. Time elapsed after ischemic stroke and risk of adverse cardiovascular events and mortality following elective noncardiac surgery. JAMA 2014; 312:269–277. doi:10.1001/jama.2014.8165
- Christiansen MN, Andersson C, Gislason GH, et al. Risks of cardiovascular adverse events and death in patients with previous stroke undergoing emergency noncardiac, nonintracranial surgery: the importance of operative timing. Anesthesiology 2017; 127(1):9–19. doi:10.1097/ALN.0000000000001685
A plethora of studies are under way in the field of perioperative medicine. As a result, evidence-based care of surgical patients is evolving at an exponential rate.
We performed a literature search and, using consensus, identified recent articles we believe will have a great impact on perioperative cardiovascular medicine. These articles report studies that were presented at national meetings in 2018, including the Perioperative Medicine Summit, Society of General Internal Medicine, and Society of Hospital Medicine. These articles are grouped under 5 questions that will help guide clinical practice in perioperative cardiovascular medicine.
SHOULD ASPIRIN BE CONTINUED PERIOPERATIVELY IN PATIENTS WITH A CORONARY STENT?
The Perioperative Ischemic Evaluation 2 (POISE-2) trial1 found that giving aspirin before surgery and throughout the early postoperative period had no significant effect on the rate of a composite of death or nonfatal myocardial infarction; moreover, aspirin increased the risk of major bleeding. However, many experts felt uncomfortable stopping aspirin preoperatively in patients taking it for secondary prophylaxis, particularly patients with a coronary stent.
[Graham MM, Sessler DI, Parlow JL, et al. Aspirin in patients with previous percutaneous coronary intervention undergoing noncardiac surgery. Ann Intern Med 2018; 168(4):237–244.]
This post hoc subgroup analysis2 of POISE-2 evaluated the benefit and harm of perioperative aspirin in patients who had previously undergone percutaneous coronary intervention, more than 90% of whom had received a stent. Patients were age 45 or older with atherosclerotic heart disease or risk factors for it who had previously undergone percutaneous coronary intervention and were now undergoing noncardiac surgery.
Patients who had received a bare-metal stent within the previous 6 weeks or a drug-eluting stent within 12 months before surgery were excluded because guidelines at that time said to continue dual antiplatelet therapy for that long. Recommendations have since changed; the optimal duration for dual antiplatelet therapy with drug-eluting stents is now 6 months. Second-generation drug-eluting stents pose a lower risk of stent thrombosis and require a shorter duration of dual antiplatelet therapy than first-generation drug-eluting stents. Approximately 25% of the percutaneous coronary intervention subgroup had a drug-eluting stent, but the authors did not specify the type of drug-eluting stent.
The post hoc analysis2 included a subgroup of 234 of 4,998 patients receiving aspirin and 236 of 5,012 patients receiving placebo initiated within 4 hours before surgery and continued postoperatively. The primary outcome measured was the rate of death or nonfatal myocardial infarction within 30 days after surgery, and bleeding was a secondary outcome.
Findings. Although the overall POISE-2 study found no benefit from aspirin, in the subgroup who had previously undergone percutaneous coronary intervention, aspirin significantly reduced the risk of the primary outcome, which occurred in 6% vs 11.5% of the patients:
- Absolute risk reduction 5.5% (95% confidence interval 0.4%–10.5%)
- Hazard ratio 0.50 (0.26–0.95).
The reduction was primarily due to fewer myocardial infarctions:
- Absolute risk reduction 5.9% (1.0%–10.8%)
- Hazard ratio 0.44 (0.22–0.87).
The type of stent had no effect on the primary outcome, although this subgroup analysis had limited power. In the nonpercutaneous coronary intervention subgroup, there was no significant difference in outcomes between the aspirin and placebo groups. This subgroup analysis was underpowered to evaluate the effect of aspirin on the composite of major and life-threatening bleeding in patients with prior percutaneous coronary intervention, which was reported as “uncertain” due to wide confidence intervals (absolute risk increase 1.3%, 95% confidence interval –2.6% to 5.2%), but the increased risk of major or life-threatening bleeding with aspirin demonstrated in the overall POISE-2 study population likely applies:
- Absolute risk increase 0.8% (0.1%–1.6%)
- Hazard ratio 1.22 (1.01–1.48).
Limitations. This was a nonspecified subgroup analysis that was underpowered and had a relatively small sample size with few events.
Conclusion. In the absence of a very high bleeding risk, continuing aspirin perioperatively in patients with prior percutaneous coronary intervention undergoing noncardiac surgery is more likely to result in benefit than harm. This finding is in agreement with current recommendations from the American College Cardiology and American Heart Association (class I; level of evidence C).3
WHAT IS THE INCIDENCE OF MINS? IS MEASURING TROPONIN USEFUL?
Despite advances in anesthesia and surgical techniques, about 1% of patients over age 45 die within 30 days of noncardiac surgery.4 Studies have demonstrated a high mortality rate in patients who experience myocardial injury after noncardiac surgery (MINS), defined as elevations of troponin T with or without ischemic symptoms or electrocardiographic changes.5 Most of these studies used earlier, “non-high-sensitivity” troponin T assays. Fifth-generation, highly sensitive troponin T assays are now available that can detect troponin T at lower concentrations, but their utility in predicting postoperative outcomes remains uncertain. Two recent studies provide further insight into these issues.
[Writing Committee for the VISION Study Investigators, Devereaux PJ, Biccard BM, Sigamani A, et al. Association of postoperative high-sensitivity troponin levels with myocardial injury and 30-day mortality among patients undergoing noncardiac surgery. JAMA 2017; 317(16):1642–1651.]
The Vascular Events in Noncardiac Surgery Patients Cohort Evaluation (VISION) study5 was an international, prospective cohort study that initially evaluated the association between MINS and the 30-day mortality rate using a non-high-sensitivity troponin T assay (Roche fourth-generation Elecsys TnT assay) in patients age 45 or older undergoing noncardiac surgery and requiring hospital admission for at least 1 night. After the first 15,000 patients, the study switched to the Roche fifth-generation assay, with measurements at 6 to 12 hours after surgery and on postoperative days 1, 2, and 3.
A 2017 analysis by Devereaux et al6 included only these later-enrolled patients and correlated their high-sensitivity troponin T levels with 30-day mortality rates. Patients with a level 14 ng/L or higher, the upper limit of normal in this study, were also assessed for ischemic symptoms and electrocardiographic changes. Although not required by the study, more than 7,800 patients had their troponin T levels measured before surgery, and the absolute change was also analyzed for an association with the 30-day mortality rate.
Findings. Of the 21,842 patients, about two-thirds underwent some form of major surgery; some of them had more than 1 type. A total of 1.2% of the patients died within 30 days of surgery.
Based on their analysis, the authors proposed that MINS be defined as:
- A postoperative troponin T level of 65 ng/L or higher, or
- A level in the range of 20 ng/L to less than 65 ng/L with an absolute increase from the preoperative level at least 5 ng/L, not attributable to a nonischemic cause.
Seventeen percent of the study patients met these criteria, and of these, 21.7% met the universal definition of myocardial infarction, although only 6.9% had symptoms of it.
Limitations. Only 40.4% of the patients had a preoperative high-sensitivity troponin T measurement for comparison, and in 13.8% of patients who had an elevated perioperative measurement, their preoperative value was the same or higher than their postoperative one. Thus, the incidence of MINS may have been overestimated if patients were otherwise not known to have troponin T elevations before surgery.
[Puelacher C, Lurati Buse G, Seeberger D, et al. Perioperative myocardial injury after noncardiac surgery: incidence, mortality, and characterization. Circulation 2018; 137(12):1221–1232.]
Puelacher et al7 investigated the prevalence of MINS in 2,018 patients at increased cardiovascular risk (age ≥ 65, or age ≥ 45 with a history of coronary artery disease, peripheral vascular disease, or stroke) who underwent major noncardiac surgery (planned overnight stay ≥ 24 hours) at a university hospital in Switzerland. Patients had their troponin T measured with a high-sensitivity assay within 30 days before surgery and on postoperative days 1 and 2.
Instead of MINS, the investigators used the term “perioperative myocardial injury” (PMI), defined as an absolute increase in troponin T of at least 14 ng/L from before surgery to the peak postoperative reading. Similar to MINS, PMI did not require ischemic features, but in this study, noncardiac triggers (sepsis, stroke, or pulmonary embolus) were not excluded.
Findings. PMI occurred in 16% of surgeries, and of the patients with PMI, 6% had typical chest pain and 18% had any ischemic symptoms. Unlike in the POISE-2 study discussed above, PMI triggered an automatic referral to a cardiologist.
The unadjusted 30-day mortality rate was 8.9% among patients with PMI and 1.5% in those without. Multivariable logistic regression analysis showed an adjusted hazard ratio for 30-day mortality of 2.7 (95% CI 1.5–4.8) for those with PMI vs without, and this difference persisted for at least 1 year.
In patients with PMI, the authors compared the 30-day mortality rate of those with no ischemic signs or symptoms (71% of the patients) with those who met the criteria for myocardial infarction and found no difference. Patients with PMI triggered by a noncardiac event had a worse prognosis than those with a presumed cardiac etiology.
Limitations. Despite the multivariate analysis that included adjustment for age, nonelective surgery, and Revised Cardiac Risk Index (RCRI), the increased risk associated with PMI could simply reflect higher risk at baseline. Although PMI resulted in automatic referral to a cardiologist, only 10% of patients eventually underwent coronary angiography; a similar percentage were discharged with additional medical therapy such as aspirin, a statin, or a beta-blocker. The effect of these interventions is not known.
Conclusions. MINS is common and has a strong association with mortality risk proportional to the degree of troponin T elevation using high-sensitivity assays, consistent with data from previous studies of earlier assays. Because the mechanism of MINS may differ from that of myocardial infarction, its prevention and treatment may differ, and it remains unclear how serial measurement in postoperative patients should change clinical practice.
The recently published Dabigatran in Patients With Myocardial Injury After Non-cardiac Surgery (MANAGE) trial8 suggests that dabigatran may reduce arterial and venous complications in patients with MINS, but the study had a number of limitations that may restrict the clinical applicability of this finding.
While awaiting further clinical outcomes data, pre- and postoperative troponin T measurement may be beneficial in higher-risk patients (such as those with cardiovascular disease or multiple RCRI risk factors) if the information will change perioperative management.
WHAT IS THE ROLE OF HYPOTENSION OR BLOOD PRESSURE CONTROL?
Intraoperative hypotension is associated with organ ischemia, which may cause postoperative myocardial infarction, myocardial injury, and acute kidney injury.9 Traditional anesthesia practice is to maintain intraoperative blood pressure within 20% of the preoperative baseline, based on the notion that hypertensive patients require higher perfusion pressures.
[Futier E, Lefrant J-Y, Guinot P-G, et al. Effect of individualized vs standard blood pressure management strategies on postoperative organ dysfunction among high-risk patients undergoing major surgery: a randomized clinical trial. JAMA 2017; 318(14):1346–1357.]
Futier et al10 sought to address uncertainty in intraoperative and immediate postoperative management of systolic blood pressure. In this multicenter, randomized, parallel-group trial, 298 patients at increased risk of postoperative renal complications were randomized to blood pressure management that was either “individualized” (within 10% of resting systolic pressure) or “standard” (≥ 80 mm Hg or ≥ 40% of resting systolic pressure) from induction to 4 hours postoperatively.
Blood pressure was monitored using radial arterial lines and maintained using a combination of intravenous fluids, norepinephrine (the first-line agent for the individualized group), and ephedrine (in the standard treatment group only). The primary outcome was a composite of systemic inflammatory response syndrome (SIRS) and organ dysfunction affecting at least 1 organ system (cardiovascular, respiratory, renal, hematologic, or neurologic).
Findings. Data on the primary outcome were available for 292 of 298 patients enrolled. The mean age was 70 years, 15% were women, and 82% had previously diagnosed hypertension. Despite the requirement for an elevated risk of acute kidney injury, only 13% of the patients had a baseline estimated glomerular filtration rate of less than 60 mL/min/1.73 m2, and the median was 88 mL/min/1.73 m2. Ninety-five percent of patients underwent abdominal surgery, and 50% of the surgeries were elective.
The mean systolic blood pressure was 123 mm Hg in the individualized treatment group compared with 116 mm Hg in the standard treatment group. Despite this small difference, 96% of individualized treatment patients received norepinephrine, compared with 26% in the standard treatment group.
The primary outcome of SIRS with organ dysfunction occurred in 38.1% of patients in the individualized treatment group and 51.7% of those in the standard treatment group. After adjusting for center, surgical urgency, surgical site, and acute kidney injury risk index, the relative risk of developing SIRS in those receiving individualized management was 0.73 (P = .02). Renal dysfunction (based on Acute Dialysis Quality Initiative criteria11) occurred in 32.7% of individualized treatment patients and 49% of standardized treatment patients.
Limitations of this study included differences in pharmacologic approach to maintain blood pressure in the 2 protocols (ephedrine and fluids vs norepinephrine) and a modest sample size.
Conclusions. Despite this, the difference in organ dysfunction was striking, with a number needed to treat of only 7 patients. This intervention extended 4 hours postoperatively, a time when many of these patients have left the postanesthesia care unit and have returned to hospitalist care on inpatient wards.
While optimal management of intraoperative and immediate postoperative blood pressure may not be settled, this study suggests that even mild relative hypotension may justify immediate action. Further studies may be useful to delineate high- and low-risk populations, the timing of greatest risk, and indications for intraarterial blood pressure monitoring.
[Salmasi V, Maheswari K, Yang D, et al. Relationship between intraoperative hypotension, defined by either reduction from baseline or absolute thresholds, and acute kidney and myocardial injury after noncardiac surgery: a retrospective cohort analysis. Anesthesiology 2017; 126(1):47–65.]
This retrospective cohort study12 assessed the association between myocardial or kidney injury and absolute or relative thresholds of intraoperative mean arterial pressure. It included 57,315 adults who underwent inpatient noncardiac surgery, had a preoperative and at least 1 postoperative serum creatinine measurement within 7 days, and had blood pressure recorded in preoperative appointments within 6 months. Patients with chronic kidney disease (glomerular filtration rate < 60 mL/min/1.73 m2) and those on dialysis were excluded. The outcomes were MINS5 and acute kidney injury as defined by the Acute Kidney Injury Network.9
Findings. A mean arterial pressure below an absolute threshold of 65 mm Hg or a relative threshold of 20% lower than baseline value was associated with myocardial and kidney injury. At each threshold, prolonged periods of hypotension were associated with progressively increased risk.
An important conclusion of the study was that relative thresholds of mean arterial pressure were not any more predictive than absolute thresholds. Absolute thresholds are easier to use intraoperatively, especially when baseline values are not available. The authors did not find a clinically significant interaction between baseline blood pressure and the association of hypotension and myocardial and kidney injury.
Limitations included use of cardiac enzymes postoperatively to define MINS. Since these were not routinely collected, clinically silent myocardial injury may have been missed. Baseline blood pressure may have important implications in other forms of organ injury (ie, cerebral ischemia) that were not studied.
Summary. The lowest absolute mean arterial pressure is as predictive of postoperative myocardial and kidney injury as the relative pressure reduction, at least in patients with normal renal function. Limiting exposure to intraoperative hypotension is important. Baseline blood pressure values may have limited utility for intraoperative management.
In combination, these studies confirm that intraoperative hypotension is a predictor of postoperative organ dysfunction, but the definition and management remain unclear. While aggressive intraoperative management is likely beneficial, how to manage the antihypertensive therapy the patient has been taking as an outpatient when he or she comes into the hospital for surgery remains uncertain.
DOES PATENT FORAMEN OVALE INCREASE THE RISK OF STROKE?
Perioperative stroke is an uncommon, severe complication of noncardiac surgery. The pathophysiology has been better defined in cardiac than in noncardiac surgeries. In nonsurgical patients, patent foramen ovale (PFO) is associated with stroke, even in patients considered to be at low risk.13 Perioperative patients have additional risk for venous thromboembolism and may have periprocedural antithrombotic medications altered, increasing their risk of paradoxical embolism through the PFO.
[Ng PY, Ng AK, Subramaniam B, et al. Association of preoperatively diagnosed patent foramen ovale with perioperative ischemic stroke. JAMA 2018; 319(5):452–462.]
This retrospective cohort study of noncardiac surgery patients at 3 hospitals14 sought to determine the association of preoperatively diagnosed PFO with the risk of perioperative ischemic stroke identified by International Classification of Diseases diagnoses.
Of 150,198 patients, 1.0% had a preoperative diagnosis of PFO, and at baseline, those with PFO had significantly more comorbidities than those without PFO. Stroke occurred in 3.2% of patients with PFO vs 0.5% of those without. Patients known to have a PFO were much more likely to have cardiovascular and thromboembolic risk factors for stroke. In the adjusted analysis, the absolute risk difference between groups was 0.4% (95% CI 0.2–0.6%), with an estimated perioperative stroke risk of 5.9 per 1,000 in patients with known patent foramen ovale and 2.2 per 1,000 in those without. A diagnosis of PFO was also associated with increased risk of large-vessel-territory stroke and more severe neurologic deficit.
Further attempts to adjust for baseline risk factors and other potential bias, including a propensity score-matched cohort analysis and an analysis limited to patients who had echocardiography performed in the same healthcare system, still showed a higher risk of perioperative stroke among patients with preoperatively detected patent foramen ovale.
Limitations. The study was retrospective and observational, used administrative data, and had a low rate of PFO diagnosis (1%), compared with about 25% in population-based studies.15 Indications for preoperative echocardiography are unknown. In addition, the study specifically examined preoperatively diagnosed PFO, rather than including those diagnosed in the postoperative period.
Discussion. How does this study affect clinical practice? The absolute stroke risk was increased by 0.4% in patients with PFO compared with those without. Although this is a relatively small increase, millions of patients undergo noncardiac surgery annually. The risks of therapeutic anticoagulation or PFO closure are likely too high in this context; however, clinicians may approach the perioperative management of antiplatelet agents and venous thromboembolism prophylaxis in patients with known PFO with additional caution.
HOW DOES TIMING OF EMERGENCY SURGERY AFTER PRIOR STROKE AFFECT OUTCOMES?
A history of stroke or transient ischemic attack is a known risk factor for perioperative vascular complications. A recent large cohort study demonstrated that a history of stroke within 9 months of elective surgery was associated with increased adverse outcomes.16 Little is known, however, of the perioperative risk in patients with a history of stroke who undergo emergency surgery.
[Christiansen MN, Andersson C, Gislason GH, et al. Risks of cardiovascular adverse events and death in patients with previous stroke undergoing emergency noncardiac, nonintracranial surgery: the importance of operative timing. Anesthesiology 2017; 127(1):9–19.]
In this study,17 all emergency noncardiac and nonintracranial surgeries from 2005 to 2011 were analyzed using multiple national patient registries in Denmark according to time elapsed between previous stroke and surgery. Primary outcomes were 30-day all-cause mortality and 30-day major adverse cardiac events (MACE), defined as nonfatal ischemic stroke, nonfatal myocardial infarction, and cardiovascular death. Statistical analysis to assess the risk of adverse outcomes included logistic regression models, spline analyses, and propensity-score matching.
Findings. The authors identified 146,694 emergency surgeries, with 7,861 patients (5.4%) having had a previous stroke (transient ischemic attacks and hemorrhagic strokes were not included). Rates of postoperative stroke were as follows:
- 9.9% in patents with a history of ischemic stroke within 3 months of surgery
- 2.8% in patients with a history of stroke 3 to 9 months before surgery
- 0.3% in patients with no previous stroke.
The risk plateaued when the time between stroke and surgery exceeded 4 to 5 months.15
Interestingly, in patients who underwent emergency surgery within 14 days of stroke, the risk of MACE was significantly lower immediately after surgery (1–3 days after stroke) compared with surgery that took place 4 to 14 days after stroke. The authors hypothesized that because cerebral autoregulation does not become compromised until approximately 5 days after a stroke, the risk was lower 1 to 3 days after surgery and increased thereafter.
Limitations of this study included the possibility of residual confounding, given its retrospective design using administrative data, not accounting for preoperative antithrombotic and anticoagulation therapy, and lack of information regarding the etiology of recurrent stroke (eg, thromboembolic, atherothrombotic, hypoperfusion).
Conclusions. Although it would be impractical to postpone emergency surgery in a patient who recently had a stroke, this study shows that the incidence rates of postoperative recurrent stroke and MACE are high. Therefore, it is important that the patient and perioperative team be aware of the risk. Further research is needed to confirm these estimates of postoperative adverse events in more diverse patient populations.
A plethora of studies are under way in the field of perioperative medicine. As a result, evidence-based care of surgical patients is evolving at an exponential rate.
We performed a literature search and, using consensus, identified recent articles we believe will have a great impact on perioperative cardiovascular medicine. These articles report studies that were presented at national meetings in 2018, including the Perioperative Medicine Summit, Society of General Internal Medicine, and Society of Hospital Medicine. These articles are grouped under 5 questions that will help guide clinical practice in perioperative cardiovascular medicine.
SHOULD ASPIRIN BE CONTINUED PERIOPERATIVELY IN PATIENTS WITH A CORONARY STENT?
The Perioperative Ischemic Evaluation 2 (POISE-2) trial1 found that giving aspirin before surgery and throughout the early postoperative period had no significant effect on the rate of a composite of death or nonfatal myocardial infarction; moreover, aspirin increased the risk of major bleeding. However, many experts felt uncomfortable stopping aspirin preoperatively in patients taking it for secondary prophylaxis, particularly patients with a coronary stent.
[Graham MM, Sessler DI, Parlow JL, et al. Aspirin in patients with previous percutaneous coronary intervention undergoing noncardiac surgery. Ann Intern Med 2018; 168(4):237–244.]
This post hoc subgroup analysis2 of POISE-2 evaluated the benefit and harm of perioperative aspirin in patients who had previously undergone percutaneous coronary intervention, more than 90% of whom had received a stent. Patients were age 45 or older with atherosclerotic heart disease or risk factors for it who had previously undergone percutaneous coronary intervention and were now undergoing noncardiac surgery.
Patients who had received a bare-metal stent within the previous 6 weeks or a drug-eluting stent within 12 months before surgery were excluded because guidelines at that time said to continue dual antiplatelet therapy for that long. Recommendations have since changed; the optimal duration for dual antiplatelet therapy with drug-eluting stents is now 6 months. Second-generation drug-eluting stents pose a lower risk of stent thrombosis and require a shorter duration of dual antiplatelet therapy than first-generation drug-eluting stents. Approximately 25% of the percutaneous coronary intervention subgroup had a drug-eluting stent, but the authors did not specify the type of drug-eluting stent.
The post hoc analysis2 included a subgroup of 234 of 4,998 patients receiving aspirin and 236 of 5,012 patients receiving placebo initiated within 4 hours before surgery and continued postoperatively. The primary outcome measured was the rate of death or nonfatal myocardial infarction within 30 days after surgery, and bleeding was a secondary outcome.
Findings. Although the overall POISE-2 study found no benefit from aspirin, in the subgroup who had previously undergone percutaneous coronary intervention, aspirin significantly reduced the risk of the primary outcome, which occurred in 6% vs 11.5% of the patients:
- Absolute risk reduction 5.5% (95% confidence interval 0.4%–10.5%)
- Hazard ratio 0.50 (0.26–0.95).
The reduction was primarily due to fewer myocardial infarctions:
- Absolute risk reduction 5.9% (1.0%–10.8%)
- Hazard ratio 0.44 (0.22–0.87).
The type of stent had no effect on the primary outcome, although this subgroup analysis had limited power. In the nonpercutaneous coronary intervention subgroup, there was no significant difference in outcomes between the aspirin and placebo groups. This subgroup analysis was underpowered to evaluate the effect of aspirin on the composite of major and life-threatening bleeding in patients with prior percutaneous coronary intervention, which was reported as “uncertain” due to wide confidence intervals (absolute risk increase 1.3%, 95% confidence interval –2.6% to 5.2%), but the increased risk of major or life-threatening bleeding with aspirin demonstrated in the overall POISE-2 study population likely applies:
- Absolute risk increase 0.8% (0.1%–1.6%)
- Hazard ratio 1.22 (1.01–1.48).
Limitations. This was a nonspecified subgroup analysis that was underpowered and had a relatively small sample size with few events.
Conclusion. In the absence of a very high bleeding risk, continuing aspirin perioperatively in patients with prior percutaneous coronary intervention undergoing noncardiac surgery is more likely to result in benefit than harm. This finding is in agreement with current recommendations from the American College Cardiology and American Heart Association (class I; level of evidence C).3
WHAT IS THE INCIDENCE OF MINS? IS MEASURING TROPONIN USEFUL?
Despite advances in anesthesia and surgical techniques, about 1% of patients over age 45 die within 30 days of noncardiac surgery.4 Studies have demonstrated a high mortality rate in patients who experience myocardial injury after noncardiac surgery (MINS), defined as elevations of troponin T with or without ischemic symptoms or electrocardiographic changes.5 Most of these studies used earlier, “non-high-sensitivity” troponin T assays. Fifth-generation, highly sensitive troponin T assays are now available that can detect troponin T at lower concentrations, but their utility in predicting postoperative outcomes remains uncertain. Two recent studies provide further insight into these issues.
[Writing Committee for the VISION Study Investigators, Devereaux PJ, Biccard BM, Sigamani A, et al. Association of postoperative high-sensitivity troponin levels with myocardial injury and 30-day mortality among patients undergoing noncardiac surgery. JAMA 2017; 317(16):1642–1651.]
The Vascular Events in Noncardiac Surgery Patients Cohort Evaluation (VISION) study5 was an international, prospective cohort study that initially evaluated the association between MINS and the 30-day mortality rate using a non-high-sensitivity troponin T assay (Roche fourth-generation Elecsys TnT assay) in patients age 45 or older undergoing noncardiac surgery and requiring hospital admission for at least 1 night. After the first 15,000 patients, the study switched to the Roche fifth-generation assay, with measurements at 6 to 12 hours after surgery and on postoperative days 1, 2, and 3.
A 2017 analysis by Devereaux et al6 included only these later-enrolled patients and correlated their high-sensitivity troponin T levels with 30-day mortality rates. Patients with a level 14 ng/L or higher, the upper limit of normal in this study, were also assessed for ischemic symptoms and electrocardiographic changes. Although not required by the study, more than 7,800 patients had their troponin T levels measured before surgery, and the absolute change was also analyzed for an association with the 30-day mortality rate.
Findings. Of the 21,842 patients, about two-thirds underwent some form of major surgery; some of them had more than 1 type. A total of 1.2% of the patients died within 30 days of surgery.
Based on their analysis, the authors proposed that MINS be defined as:
- A postoperative troponin T level of 65 ng/L or higher, or
- A level in the range of 20 ng/L to less than 65 ng/L with an absolute increase from the preoperative level at least 5 ng/L, not attributable to a nonischemic cause.
Seventeen percent of the study patients met these criteria, and of these, 21.7% met the universal definition of myocardial infarction, although only 6.9% had symptoms of it.
Limitations. Only 40.4% of the patients had a preoperative high-sensitivity troponin T measurement for comparison, and in 13.8% of patients who had an elevated perioperative measurement, their preoperative value was the same or higher than their postoperative one. Thus, the incidence of MINS may have been overestimated if patients were otherwise not known to have troponin T elevations before surgery.
[Puelacher C, Lurati Buse G, Seeberger D, et al. Perioperative myocardial injury after noncardiac surgery: incidence, mortality, and characterization. Circulation 2018; 137(12):1221–1232.]
Puelacher et al7 investigated the prevalence of MINS in 2,018 patients at increased cardiovascular risk (age ≥ 65, or age ≥ 45 with a history of coronary artery disease, peripheral vascular disease, or stroke) who underwent major noncardiac surgery (planned overnight stay ≥ 24 hours) at a university hospital in Switzerland. Patients had their troponin T measured with a high-sensitivity assay within 30 days before surgery and on postoperative days 1 and 2.
Instead of MINS, the investigators used the term “perioperative myocardial injury” (PMI), defined as an absolute increase in troponin T of at least 14 ng/L from before surgery to the peak postoperative reading. Similar to MINS, PMI did not require ischemic features, but in this study, noncardiac triggers (sepsis, stroke, or pulmonary embolus) were not excluded.
Findings. PMI occurred in 16% of surgeries, and of the patients with PMI, 6% had typical chest pain and 18% had any ischemic symptoms. Unlike in the POISE-2 study discussed above, PMI triggered an automatic referral to a cardiologist.
The unadjusted 30-day mortality rate was 8.9% among patients with PMI and 1.5% in those without. Multivariable logistic regression analysis showed an adjusted hazard ratio for 30-day mortality of 2.7 (95% CI 1.5–4.8) for those with PMI vs without, and this difference persisted for at least 1 year.
In patients with PMI, the authors compared the 30-day mortality rate of those with no ischemic signs or symptoms (71% of the patients) with those who met the criteria for myocardial infarction and found no difference. Patients with PMI triggered by a noncardiac event had a worse prognosis than those with a presumed cardiac etiology.
Limitations. Despite the multivariate analysis that included adjustment for age, nonelective surgery, and Revised Cardiac Risk Index (RCRI), the increased risk associated with PMI could simply reflect higher risk at baseline. Although PMI resulted in automatic referral to a cardiologist, only 10% of patients eventually underwent coronary angiography; a similar percentage were discharged with additional medical therapy such as aspirin, a statin, or a beta-blocker. The effect of these interventions is not known.
Conclusions. MINS is common and has a strong association with mortality risk proportional to the degree of troponin T elevation using high-sensitivity assays, consistent with data from previous studies of earlier assays. Because the mechanism of MINS may differ from that of myocardial infarction, its prevention and treatment may differ, and it remains unclear how serial measurement in postoperative patients should change clinical practice.
The recently published Dabigatran in Patients With Myocardial Injury After Non-cardiac Surgery (MANAGE) trial8 suggests that dabigatran may reduce arterial and venous complications in patients with MINS, but the study had a number of limitations that may restrict the clinical applicability of this finding.
While awaiting further clinical outcomes data, pre- and postoperative troponin T measurement may be beneficial in higher-risk patients (such as those with cardiovascular disease or multiple RCRI risk factors) if the information will change perioperative management.
WHAT IS THE ROLE OF HYPOTENSION OR BLOOD PRESSURE CONTROL?
Intraoperative hypotension is associated with organ ischemia, which may cause postoperative myocardial infarction, myocardial injury, and acute kidney injury.9 Traditional anesthesia practice is to maintain intraoperative blood pressure within 20% of the preoperative baseline, based on the notion that hypertensive patients require higher perfusion pressures.
[Futier E, Lefrant J-Y, Guinot P-G, et al. Effect of individualized vs standard blood pressure management strategies on postoperative organ dysfunction among high-risk patients undergoing major surgery: a randomized clinical trial. JAMA 2017; 318(14):1346–1357.]
Futier et al10 sought to address uncertainty in intraoperative and immediate postoperative management of systolic blood pressure. In this multicenter, randomized, parallel-group trial, 298 patients at increased risk of postoperative renal complications were randomized to blood pressure management that was either “individualized” (within 10% of resting systolic pressure) or “standard” (≥ 80 mm Hg or ≥ 40% of resting systolic pressure) from induction to 4 hours postoperatively.
Blood pressure was monitored using radial arterial lines and maintained using a combination of intravenous fluids, norepinephrine (the first-line agent for the individualized group), and ephedrine (in the standard treatment group only). The primary outcome was a composite of systemic inflammatory response syndrome (SIRS) and organ dysfunction affecting at least 1 organ system (cardiovascular, respiratory, renal, hematologic, or neurologic).
Findings. Data on the primary outcome were available for 292 of 298 patients enrolled. The mean age was 70 years, 15% were women, and 82% had previously diagnosed hypertension. Despite the requirement for an elevated risk of acute kidney injury, only 13% of the patients had a baseline estimated glomerular filtration rate of less than 60 mL/min/1.73 m2, and the median was 88 mL/min/1.73 m2. Ninety-five percent of patients underwent abdominal surgery, and 50% of the surgeries were elective.
The mean systolic blood pressure was 123 mm Hg in the individualized treatment group compared with 116 mm Hg in the standard treatment group. Despite this small difference, 96% of individualized treatment patients received norepinephrine, compared with 26% in the standard treatment group.
The primary outcome of SIRS with organ dysfunction occurred in 38.1% of patients in the individualized treatment group and 51.7% of those in the standard treatment group. After adjusting for center, surgical urgency, surgical site, and acute kidney injury risk index, the relative risk of developing SIRS in those receiving individualized management was 0.73 (P = .02). Renal dysfunction (based on Acute Dialysis Quality Initiative criteria11) occurred in 32.7% of individualized treatment patients and 49% of standardized treatment patients.
Limitations of this study included differences in pharmacologic approach to maintain blood pressure in the 2 protocols (ephedrine and fluids vs norepinephrine) and a modest sample size.
Conclusions. Despite this, the difference in organ dysfunction was striking, with a number needed to treat of only 7 patients. This intervention extended 4 hours postoperatively, a time when many of these patients have left the postanesthesia care unit and have returned to hospitalist care on inpatient wards.
While optimal management of intraoperative and immediate postoperative blood pressure may not be settled, this study suggests that even mild relative hypotension may justify immediate action. Further studies may be useful to delineate high- and low-risk populations, the timing of greatest risk, and indications for intraarterial blood pressure monitoring.
[Salmasi V, Maheswari K, Yang D, et al. Relationship between intraoperative hypotension, defined by either reduction from baseline or absolute thresholds, and acute kidney and myocardial injury after noncardiac surgery: a retrospective cohort analysis. Anesthesiology 2017; 126(1):47–65.]
This retrospective cohort study12 assessed the association between myocardial or kidney injury and absolute or relative thresholds of intraoperative mean arterial pressure. It included 57,315 adults who underwent inpatient noncardiac surgery, had a preoperative and at least 1 postoperative serum creatinine measurement within 7 days, and had blood pressure recorded in preoperative appointments within 6 months. Patients with chronic kidney disease (glomerular filtration rate < 60 mL/min/1.73 m2) and those on dialysis were excluded. The outcomes were MINS5 and acute kidney injury as defined by the Acute Kidney Injury Network.9
Findings. A mean arterial pressure below an absolute threshold of 65 mm Hg or a relative threshold of 20% lower than baseline value was associated with myocardial and kidney injury. At each threshold, prolonged periods of hypotension were associated with progressively increased risk.
An important conclusion of the study was that relative thresholds of mean arterial pressure were not any more predictive than absolute thresholds. Absolute thresholds are easier to use intraoperatively, especially when baseline values are not available. The authors did not find a clinically significant interaction between baseline blood pressure and the association of hypotension and myocardial and kidney injury.
Limitations included use of cardiac enzymes postoperatively to define MINS. Since these were not routinely collected, clinically silent myocardial injury may have been missed. Baseline blood pressure may have important implications in other forms of organ injury (ie, cerebral ischemia) that were not studied.
Summary. The lowest absolute mean arterial pressure is as predictive of postoperative myocardial and kidney injury as the relative pressure reduction, at least in patients with normal renal function. Limiting exposure to intraoperative hypotension is important. Baseline blood pressure values may have limited utility for intraoperative management.
In combination, these studies confirm that intraoperative hypotension is a predictor of postoperative organ dysfunction, but the definition and management remain unclear. While aggressive intraoperative management is likely beneficial, how to manage the antihypertensive therapy the patient has been taking as an outpatient when he or she comes into the hospital for surgery remains uncertain.
DOES PATENT FORAMEN OVALE INCREASE THE RISK OF STROKE?
Perioperative stroke is an uncommon, severe complication of noncardiac surgery. The pathophysiology has been better defined in cardiac than in noncardiac surgeries. In nonsurgical patients, patent foramen ovale (PFO) is associated with stroke, even in patients considered to be at low risk.13 Perioperative patients have additional risk for venous thromboembolism and may have periprocedural antithrombotic medications altered, increasing their risk of paradoxical embolism through the PFO.
[Ng PY, Ng AK, Subramaniam B, et al. Association of preoperatively diagnosed patent foramen ovale with perioperative ischemic stroke. JAMA 2018; 319(5):452–462.]
This retrospective cohort study of noncardiac surgery patients at 3 hospitals14 sought to determine the association of preoperatively diagnosed PFO with the risk of perioperative ischemic stroke identified by International Classification of Diseases diagnoses.
Of 150,198 patients, 1.0% had a preoperative diagnosis of PFO, and at baseline, those with PFO had significantly more comorbidities than those without PFO. Stroke occurred in 3.2% of patients with PFO vs 0.5% of those without. Patients known to have a PFO were much more likely to have cardiovascular and thromboembolic risk factors for stroke. In the adjusted analysis, the absolute risk difference between groups was 0.4% (95% CI 0.2–0.6%), with an estimated perioperative stroke risk of 5.9 per 1,000 in patients with known patent foramen ovale and 2.2 per 1,000 in those without. A diagnosis of PFO was also associated with increased risk of large-vessel-territory stroke and more severe neurologic deficit.
Further attempts to adjust for baseline risk factors and other potential bias, including a propensity score-matched cohort analysis and an analysis limited to patients who had echocardiography performed in the same healthcare system, still showed a higher risk of perioperative stroke among patients with preoperatively detected patent foramen ovale.
Limitations. The study was retrospective and observational, used administrative data, and had a low rate of PFO diagnosis (1%), compared with about 25% in population-based studies.15 Indications for preoperative echocardiography are unknown. In addition, the study specifically examined preoperatively diagnosed PFO, rather than including those diagnosed in the postoperative period.
Discussion. How does this study affect clinical practice? The absolute stroke risk was increased by 0.4% in patients with PFO compared with those without. Although this is a relatively small increase, millions of patients undergo noncardiac surgery annually. The risks of therapeutic anticoagulation or PFO closure are likely too high in this context; however, clinicians may approach the perioperative management of antiplatelet agents and venous thromboembolism prophylaxis in patients with known PFO with additional caution.
HOW DOES TIMING OF EMERGENCY SURGERY AFTER PRIOR STROKE AFFECT OUTCOMES?
A history of stroke or transient ischemic attack is a known risk factor for perioperative vascular complications. A recent large cohort study demonstrated that a history of stroke within 9 months of elective surgery was associated with increased adverse outcomes.16 Little is known, however, of the perioperative risk in patients with a history of stroke who undergo emergency surgery.
[Christiansen MN, Andersson C, Gislason GH, et al. Risks of cardiovascular adverse events and death in patients with previous stroke undergoing emergency noncardiac, nonintracranial surgery: the importance of operative timing. Anesthesiology 2017; 127(1):9–19.]
In this study,17 all emergency noncardiac and nonintracranial surgeries from 2005 to 2011 were analyzed using multiple national patient registries in Denmark according to time elapsed between previous stroke and surgery. Primary outcomes were 30-day all-cause mortality and 30-day major adverse cardiac events (MACE), defined as nonfatal ischemic stroke, nonfatal myocardial infarction, and cardiovascular death. Statistical analysis to assess the risk of adverse outcomes included logistic regression models, spline analyses, and propensity-score matching.
Findings. The authors identified 146,694 emergency surgeries, with 7,861 patients (5.4%) having had a previous stroke (transient ischemic attacks and hemorrhagic strokes were not included). Rates of postoperative stroke were as follows:
- 9.9% in patents with a history of ischemic stroke within 3 months of surgery
- 2.8% in patients with a history of stroke 3 to 9 months before surgery
- 0.3% in patients with no previous stroke.
The risk plateaued when the time between stroke and surgery exceeded 4 to 5 months.15
Interestingly, in patients who underwent emergency surgery within 14 days of stroke, the risk of MACE was significantly lower immediately after surgery (1–3 days after stroke) compared with surgery that took place 4 to 14 days after stroke. The authors hypothesized that because cerebral autoregulation does not become compromised until approximately 5 days after a stroke, the risk was lower 1 to 3 days after surgery and increased thereafter.
Limitations of this study included the possibility of residual confounding, given its retrospective design using administrative data, not accounting for preoperative antithrombotic and anticoagulation therapy, and lack of information regarding the etiology of recurrent stroke (eg, thromboembolic, atherothrombotic, hypoperfusion).
Conclusions. Although it would be impractical to postpone emergency surgery in a patient who recently had a stroke, this study shows that the incidence rates of postoperative recurrent stroke and MACE are high. Therefore, it is important that the patient and perioperative team be aware of the risk. Further research is needed to confirm these estimates of postoperative adverse events in more diverse patient populations.
- Devereaux PJ, Mrkobrada M, Sessler DI, et al. Aspirin in patients undergoing noncardiac surgery. N Engl J Med 2014; 370(16):1494–1503. doi:10.1056/NEJMoa1401105
- Graham MM, Sessler DI, Parlow JL, et al. Aspirin in patients with previous percutaneous coronary intervention undergoing noncardiac surgery. Ann Intern Med 2018; 168(4):237–244. doi:10.7326/M17-2341
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 130(24):2215–2245. doi:10.1161/CIR.0000000000000105
- Smilowitz NR, Gupta N, Ramakrishna H, Guo Y, Berger JS, Bangalore S. Perioperative major adverse cardiovascular and cerebrovascular events associated with noncardiac surgery. JAMA Cardiol 2017; 2(2):181–187. doi:10.1001/jamacardio.2016.4792
- Botto F, Alonso-Coello P, Chan MT, et al. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology 2014; 120(3):564–578. doi:10.1097/ALN.0000000000000113
- Writing Committee for the VISION Study Investigators, Devereaux PJ, Biccard BM, Sigamani A, et al. Association of postoperative high-sensitivity troponin levels with myocardial injury and 30-day mortality among patients undergoing noncardiac surgery. JAMA 2017; 317(16):1642–1651. doi:10.1001/jama.2017.4360
- Puelacher C, Lurati Buse G, Seeberger D, et al. Perioperative myocardial injury after noncardiac surgery: incidence, mortality, and characterization. Circulation 2018; 137(12):1221–1232. doi:10.1161/CIRCULATIONAHA.117.030114
- Devereaux PJ, Duceppe E, Guyatt G, et al. Dabigatran in patients with myocardial injury after non-cardiac surgery (MANAGE): an international, randomised, placebo-controlled trial. Lancet 2018; 391(10137):2325–2334. doi:10.1016/S0140-6736(18)30832-8
- Walsh M, Devereaux PJ, Garg AX, et al. Relationship between intraoperative mean arterial pressure and clinical outcomes after noncardiac surgery: toward an empirical definition of hypotension. Anesthesiology 2013; 119(3):507–515. doi:10.1097/ALN.0b013e3182a10e26
- Futier E, Lefrant JY, Guinot PG, et al. Effect of individualized vs standard blood pressure management strategies on postoperative organ dysfunction among high-risk patients undergoing major surgery: a randomized clinical trial. JAMA 2017; 318(14):1346–1357. doi:10.1001/jama.2017.14172
- Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P; Acute Dialysis Quality Initiative workgroup. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) group. Crit Care 2004; 8:R204. doi:10.1186/cc2872
- Salmasi V, Maheswari K, Yang D, et al. Relationship between intraoperative hypotension, defined by either reduction from baseline or absolute thresholds, and acute kidney and myocardial injury after noncardiac surgery: a retrospective cohort analysis. Anesthesiology 2017; 126(1):47–65. doi:10.1097/ALN.0000000000001432
- Lechat P, Mas JL, Lascault G, et al. Prevalence of patent foramen ovale in patients with stroke. N Engl J Med 1988; 318(18):1148–1152. doi:10.1056/NEJM198805053181802
- Ng PY, Ng AK, Subramaniam B, et al. Association of preoperatively diagnosed patent foramen ovale with perioperative ischemic stroke. JAMA 2018; 319(5):452–462. doi:10.1001/jama.2017.21899
- Meissner I, Whisnant JP, Khandheria BK, et al. Prevalence of potential risk factors for stroke assessed by transesophageal echocardiography and carotid ultrasonography: the SPARC study. Stroke Prevention: Assessment of Risk in a Community. Mayo Clin Proc 1999; 74(9):862–869. pmid:10488786
- Jørgensen ME, Torp-Pedersen C, Gislason GH, et al. Time elapsed after ischemic stroke and risk of adverse cardiovascular events and mortality following elective noncardiac surgery. JAMA 2014; 312:269–277. doi:10.1001/jama.2014.8165
- Christiansen MN, Andersson C, Gislason GH, et al. Risks of cardiovascular adverse events and death in patients with previous stroke undergoing emergency noncardiac, nonintracranial surgery: the importance of operative timing. Anesthesiology 2017; 127(1):9–19. doi:10.1097/ALN.0000000000001685
- Devereaux PJ, Mrkobrada M, Sessler DI, et al. Aspirin in patients undergoing noncardiac surgery. N Engl J Med 2014; 370(16):1494–1503. doi:10.1056/NEJMoa1401105
- Graham MM, Sessler DI, Parlow JL, et al. Aspirin in patients with previous percutaneous coronary intervention undergoing noncardiac surgery. Ann Intern Med 2018; 168(4):237–244. doi:10.7326/M17-2341
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 130(24):2215–2245. doi:10.1161/CIR.0000000000000105
- Smilowitz NR, Gupta N, Ramakrishna H, Guo Y, Berger JS, Bangalore S. Perioperative major adverse cardiovascular and cerebrovascular events associated with noncardiac surgery. JAMA Cardiol 2017; 2(2):181–187. doi:10.1001/jamacardio.2016.4792
- Botto F, Alonso-Coello P, Chan MT, et al. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology 2014; 120(3):564–578. doi:10.1097/ALN.0000000000000113
- Writing Committee for the VISION Study Investigators, Devereaux PJ, Biccard BM, Sigamani A, et al. Association of postoperative high-sensitivity troponin levels with myocardial injury and 30-day mortality among patients undergoing noncardiac surgery. JAMA 2017; 317(16):1642–1651. doi:10.1001/jama.2017.4360
- Puelacher C, Lurati Buse G, Seeberger D, et al. Perioperative myocardial injury after noncardiac surgery: incidence, mortality, and characterization. Circulation 2018; 137(12):1221–1232. doi:10.1161/CIRCULATIONAHA.117.030114
- Devereaux PJ, Duceppe E, Guyatt G, et al. Dabigatran in patients with myocardial injury after non-cardiac surgery (MANAGE): an international, randomised, placebo-controlled trial. Lancet 2018; 391(10137):2325–2334. doi:10.1016/S0140-6736(18)30832-8
- Walsh M, Devereaux PJ, Garg AX, et al. Relationship between intraoperative mean arterial pressure and clinical outcomes after noncardiac surgery: toward an empirical definition of hypotension. Anesthesiology 2013; 119(3):507–515. doi:10.1097/ALN.0b013e3182a10e26
- Futier E, Lefrant JY, Guinot PG, et al. Effect of individualized vs standard blood pressure management strategies on postoperative organ dysfunction among high-risk patients undergoing major surgery: a randomized clinical trial. JAMA 2017; 318(14):1346–1357. doi:10.1001/jama.2017.14172
- Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P; Acute Dialysis Quality Initiative workgroup. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) group. Crit Care 2004; 8:R204. doi:10.1186/cc2872
- Salmasi V, Maheswari K, Yang D, et al. Relationship between intraoperative hypotension, defined by either reduction from baseline or absolute thresholds, and acute kidney and myocardial injury after noncardiac surgery: a retrospective cohort analysis. Anesthesiology 2017; 126(1):47–65. doi:10.1097/ALN.0000000000001432
- Lechat P, Mas JL, Lascault G, et al. Prevalence of patent foramen ovale in patients with stroke. N Engl J Med 1988; 318(18):1148–1152. doi:10.1056/NEJM198805053181802
- Ng PY, Ng AK, Subramaniam B, et al. Association of preoperatively diagnosed patent foramen ovale with perioperative ischemic stroke. JAMA 2018; 319(5):452–462. doi:10.1001/jama.2017.21899
- Meissner I, Whisnant JP, Khandheria BK, et al. Prevalence of potential risk factors for stroke assessed by transesophageal echocardiography and carotid ultrasonography: the SPARC study. Stroke Prevention: Assessment of Risk in a Community. Mayo Clin Proc 1999; 74(9):862–869. pmid:10488786
- Jørgensen ME, Torp-Pedersen C, Gislason GH, et al. Time elapsed after ischemic stroke and risk of adverse cardiovascular events and mortality following elective noncardiac surgery. JAMA 2014; 312:269–277. doi:10.1001/jama.2014.8165
- Christiansen MN, Andersson C, Gislason GH, et al. Risks of cardiovascular adverse events and death in patients with previous stroke undergoing emergency noncardiac, nonintracranial surgery: the importance of operative timing. Anesthesiology 2017; 127(1):9–19. doi:10.1097/ALN.0000000000001685
KEY POINTS
- Patients undergoing noncardiac surgery who have a history of percutaneous coronary intervention will benefit from continuing aspirin perioperatively if they are not at very high risk of bleeding.
- Myocardial injury after noncardiac surgery is strongly associated with a risk of death, and the higher the troponin level, the higher the risk. Measuring troponin T before and after surgery may be beneficial in patients at high risk if the information leads to a change in management.
- Perioperative hypotension can lead to end-organ dysfunction postoperatively. There is conflicting evidence whether the absolute or relative reduction in blood pressure is more predictive.
- Perioperative risk of stroke is higher in patients with patent foramen ovale than in those without.
- Many patients who recently had a stroke suffer recurrent stroke and major adverse cardiac events if they undergo emergency surgery.
Renal disease and the surgical patient: Minimizing the impact
Chronic kidney disease (CKD) is estimated to affect 14% of Americans, but it is likely underdiagnosed because it is often asymptomatic.1,2 Its prevalence is even higher in patients who undergo surgery—up to 30% in cardiac surgery.3 Its impact on surgical outcomes is substantial.4 Importantly, patients with CKD are at higher risk of postoperative acute kidney injury (AKI), which is also associated with adverse outcomes. Thus, it is important to recognize, assess, and manage abnormal renal function in surgical patients.
WHAT IS THE IMPACT ON POSTOPERATIVE OUTCOMES?
Cardiac surgery outcomes
Moreover, in patients undergoing coronary artery bypass grafting (CABG), the worse the renal dysfunction, the higher the long-term mortality rate. Patients with moderate (stage 3) CKD had a 3.5 times higher odds of in-hospital mortality compared with patients with normal renal function, rising to 8.8 with severe (stage 4) and to 9.6 with dialysis-dependent (stage 5) CKD.11
The mechanisms linking CKD with negative cardiac outcomes are unclear, but many possibilities exist. CKD is an independent risk factor for coronary artery disease and shares underlying risk factors such as hypertension and diabetes. Cardiac surgery patients with CKD are also more likely to have diabetes, left ventricular dysfunction, and peripheral vascular disease.
Noncardiac surgery outcomes
CKD is also associated with adverse outcomes in noncardiac surgery patients, especially at higher levels of renal dysfunction.12–14 For example, in patients who underwent major noncardiac surgery, compared with patients in stage 1 (estimated GFR > 90 mL/min/1.73 m2), the odds ratios for all-cause mortality were as follows:
- 0.8 for patients with stage 2 CKD
- 2.2 in stage 3a
- 2.8 in stage 3b
- 11.3 in stage 4
- 5.8 in stage 5.14
The association between estimated GFR and all-cause mortality was not statistically significant (P = .071), but statistically significant associations were observed between estimated GFR and major adverse cardiovascular events (P < .001) and hospital length of stay (P < .001).
The association of CKD with major adverse outcomes and death in both cardiac and noncardiac surgical patients demonstrates the importance of understanding this risk, identifying patients with CKD preoperatively, and taking steps to lower the risk.
WHAT IS THE IMPACT OF ACUTE KIDNEY INJURY?
AKI is a common and serious complication of surgery, especially cardiac surgery. It has been associated with higher rates of morbidity, mortality, and cardiovascular events, longer hospital length of stay, and higher cost.
Several groups have proposed criteria for defining AKI and its severity; the KDIGO criteria are the most widely accepted.15 These define AKI as an increase in serum creatinine concentration of 0.3 mg/dL or more within 48 hours or at least 1.5 times the baseline value within 7 days, or urine volume less than 0.5 mL/kg/hour for more than 6 hours. There are 3 stages of severity:
- Stage 1—an increase in serum creatinine of 1.5 to 1.9 times baseline, an absolute increase of at least 0.3 mg/dL, or urine output less than 0.5 mL/kg/hour for 6 to 12 hours
- Stage 2—an increase in serum creatinine of 2.0 to 2.9 times baseline or urine output less than 0.5 mmL/kg/hour for 12 or more hours
- Stage 3—an increase in serum creatinine of 3 times baseline, an absolute increase of at least 4 mg/dL, initiation of renal replacement therapy, urine output less than 0.3 mL/kg/hour for 24 or more hours, or anuria for 12 or more hours.15
Multiple factors associated with surgery may contribute to AKI, including hemodynamic instability, volume shifts, blood loss, use of heart-lung bypass, new medications, activation of the inflammatory cascade, oxidative stress, and anemia.
AKI in cardiac surgery
The incidence of AKI is high in cardiac surgery. In a meta-analysis of 46 studies (N = 242,000), its incidence in cardiopulmonary bypass surgery was about 18%, with 2.1% of patients needing renal replacement therapy.16 However, the incidence varied considerably from study to study, ranging from 1% to 53%, and was influenced by the definition of AKI, the type of cardiac surgery, and the patient population.16
Cardiac surgery-associated AKI adversely affects outcomes. Several studies have shown that cardiac surgery patients who develop AKI have higher rates of death and stroke.16–21 More severe AKI confers higher mortality rates, with the highest mortality rate in patients who need renal replacement therapy, approximately 37%.17 Patients with cardiac surgery-associated AKI also have a longer hospital length of stay and significantly higher costs of care.17,18
Long-term outcomes are also negatively affected by AKI. In cardiac surgery patients with AKI who had completely recovered renal function by the time they left the hospital, the 2-year incidence rate of CKD was 6.8%, significantly higher than the 0.2% rate in patients who did not develop AKI.19 The 2-year survival rates also were significantly worse for patients who developed postoperative AKI (82.3% vs 93.7%). Similarly, in patients undergoing CABG who had normal renal function before surgery, those who developed AKI postoperatively had significantly shorter long-term survival rates.20 The effect does not require a large change in renal function. An increase in creatinine as small as 0.3 mg/dL has been associated with a higher rate of death and a long-term risk of end-stage renal disease that is 3 times higher.21
WHAT ARE THE RISK FACTORS FOR ACUTE KIDNEY INJURY?
Cardiac surgery
CKD is a risk factor not only after cardiac surgery but also after percutaneous procedures. In a meta-analysis of 4,992 patients with CKD who underwent transcatheter aortic valve replacement, both moderate and severe CKD increased the odds of AKI, early stroke, the need for dialysis, and all-cause and cardiovascular mortality at 1 year.22,23 Increased rates of AKI also have been found in patients with CKD undergoing CABG surgery.24 These results point to a synergistic effect between AKI and CKD, with outcomes much worse in combination than alone.
In cardiac surgery, the most important patient risk factors associated with a higher incidence of postoperative AKI are age older than 75, CKD, preoperative heart failure, and prior myocardial infarction.19,25 Diabetes is an additional independent risk factor, with type 1 conferring higher risk than type 2.26 Preoperative use of angiotensin-converting enzyme (ACE) inhibitors may or may not be a risk factor for cardiac surgery-associated AKI, with some studies finding increased risk and others finding reduced rates.27,28
Anemia, which may be related to either patient or surgical risk factors (eg, intraoperative blood loss), also increases the risk of AKI in cardiac surgery.29,30 A retrospective study of CABG surgery patients found that intraoperative hemoglobin levels below 8 g/dL were associated with a 25% to 30% incidence of AKI, compared with 15% to 20% with hemoglobin levels above 9 g/dL.29 Additionally, having severe hypotension (mean arterial pressure < 50 mm Hg) significantly increased the AKI rates in the low-hemoglobin group.29 Similar results were reported in a later study.30
Among surgical factors, several randomized controlled trials have shown that off-pump CABG is associated with a significantly lower risk of postoperative AKI than on-pump CABG; however, this difference did not translate into any long-term difference in mortality rates.31,32 Longer cardiopulmonary bypass time is strongly associated with a higher incidence of AKI and postoperative death.33
Noncardiac surgery
AKI is less common after noncardiac surgery; however, outcomes are severe in patients in whom it occurs. In a study of 15,102 noncardiac surgery patients, only 0.8% developed AKI and 0.1% required renal replacement therapy.34
Risk factors after noncardiac surgery are similar to those after cardiac surgery (Table 3).34–36 Factors with the greatest impact are older age, peripheral vascular occlusive disease, chronic obstructive pulmonary disease necessitating chronic bronchodilator therapy, high-risk surgery, hepatic disease, emergent or urgent surgery, and high body mass index.
Surgical risk factors include total vasopressor dose administered, use of a vasopressor infusion, and diuretic administration.34 In addition, intraoperative hypotension is associated with a higher risk of AKI, major adverse cardiac events, and 30-day mortality.37
Noncardiac surgery patients with postoperative AKI have significantly higher rates of 30-day readmissions, 1-year progression to end-stage renal disease, and mortality than patients who do not develop AKI.35 Additionally, patients with AKI have significantly higher rates of cardiovascular complications (33.3% vs 11.3%) and death (6.1% vs 0.9%), as well as a significantly longer length of hospital stay.34,36
CAN WE DECREASE THE IMPACT OF RENAL DISEASE IN SURGERY?
Before surgery, practitioners need to identify patients at risk of AKI, implement possible risk-reduction measures, and, afterward, treat it early in its course if it occurs.
The preoperative visit is the ideal time to assess a patient’s risk of postoperative renal dysfunction. Laboratory tests can identify risks based on surgery type, age, hypertension, the presence of CKD, and medications that affect renal function. However, the basic chemistry panel is abnormal in only 8.2% of patients and affects management in just 2.6%, requiring the clinician to target testing to patients at high risk.38
Patients with a significant degree of renal dysfunction, particularly those previously undiagnosed, may benefit from additional preoperative testing and medication management. Perioperative management of medications that could adversely affect renal function should be carefully considered during the preoperative visit. In addition, the postoperative inpatient team needs to be informed about potentially nephrotoxic medications and medications that are renally cleared. Attention needs to be given to the renal impact of common perioperative medications such as nonsteroidal anti-inflammatory drugs, antibiotics, intravenous contrast, low-molecular-weight heparins, diuretics, ACE inhibitors, and angiotensin II receptor blockers. With the emphasis on opioid-sparing analgesics, it is particularly important to assess the risk of AKI if nonsteroidal anti-inflammatory drugs are part of the pain control plan.
Nephrology referral may help, especially for patients with a GFR less than 45 mL/min. This information enables more informed decision-making regarding the risks of adverse outcomes related to kidney disease.
WHAT TOOLS DO WE HAVE TO DIAGNOSE RENAL INJURY?
Several risk-prediction models have been developed to assess the postoperative risk of AKI in both cardiac and major noncardiac surgery patients. Although these models can identify risk factors, their clinical accuracy and utility have been questioned.
Biomarkers
Early diagnosis is the first step in managing AKI, allowing time to implement measures to minimize its impact.
Serum creatinine testing is widely used to measure renal function and diagnose AKI; however, it does not detect small reductions in renal function, and there is a time lag between renal insult and a rise in creatinine. The result is a delay to diagnosis of AKI.
Biomarkers other than creatinine have been studied for early detection of intraoperative and postoperative renal insult. These novel renal injury markers include the following:
Neutrophil gelatinase-associated lipocalin (NGAL). Two studies looked at plasma NGAL as an early marker of AKI in patients with CKD who were undergoing cardiac surgery.39,40 One study found that by using NGAL instead of creatinine, postoperative AKI could be diagnosed an average of 20 hours earlier.39 In addition, NGAL helped detect renal recovery earlier than creatinine.40 The diagnostic cut-off values of NGAL were different for patients with CKD than for those without CKD.39,40
Other novel markers include:
- Kidney injury marker 1
- N-acetyl-beta-D-glucosaminidase
- Cysteine C.
Although these biomarkers show some ability to detect renal injury, they provide only modest discrimination and are not widely available for clinical use.41 Current evidence does not support routine use of these markers in clinical settings.
CAN WE PROTECT RENAL FUNCTION?
Interventions to prevent or ameliorate the impact of CKD and AKI on surgical outcomes have been studied most extensively in cardiac surgery patients.
Aspirin. A retrospective study of 3,585 cardiac surgery patients with CKD found that preoperative aspirin use significantly lowered the incidence of postoperative AKI and 30-day mortality compared with patients not using aspirin.42 Aspirin use reduced 30-day mortality in CKD stages 1, 2, and 3 by 23.3%, 58%, and 70%, respectively. On the other hand, in the Perioperative Ischemic Evaluation (POISE) trial, in noncardiac surgery patients, neither aspirin nor clonidine started 2 to 4 hours preoperatively and continued up to 30 days after surgery altered the risk of AKI significantly more than placebo.43
Statins have been ineffective in reducing the incidence of AKI in cardiac surgery patients. In fact, a meta-analysis of 8 interventional trials found an increased incidence of AKI in patients in whom statins were started perioperatively.44 Erythropoietin was also found to be ineffective in the prevention of perioperative AKI in cardiac surgery patients in a separate study.45
The evidence regarding other therapies has also varied.
N-acetylcysteine in high doses reduced the incidence of AKI in patients with CKD stage 3 and 4 undergoing CABG.46 Another meta-analysis of 10 studies in cardiac surgery patients published recently did not show any benefit of N-acetylcysteine in reducing AKI.47
Human atrial natriuretic peptide, given preoperatively to patients with CKD, reduced the acute and long-term creatinine rise as well as the number of cardiac events after CABG; however, it did not reduce mortality rates.48
Renin-angiotensin system inhibitors, given preoperatively to patients with heart failure was associated with a decrease in the incidence of AKI in 1 study.49
Dexmedetomidine is a highly selective alpha 2 adrenoreceptor agonist. A recent meta-analysis of 10 clinical trials found it beneficial in reducing the risk of perioperative AKI in cardiac surgery patients.50 An earlier meta-analysis had similar results.51
Levosimendan is an inotropic vasodilator that improves cardiac output and renal perfusion in patients with systolic heart failure, and it has been hypothesized to decrease the risk of AKI after cardiac surgery. Previous data demonstrated that this drug reduced AKI and mortality; however, analysis was limited by small sample size and varying definitions of AKI.52 A recent meta-analysis showed that levosimendan was associated with a lower incidence of AKI but was also associated with an increased incidence of atrial fibrillation and no reduction in 30-day mortality.53
Remote ischemic preconditioning is a procedure that subjects the kidneys to brief episodes of ischemia before surgery, protecting them when they are later subjected to prolonged ischemia or reperfusion injury. It has shown initial promising results in preventing AKI. In a randomized controlled trial in 240 patients at high risk of AKI, those who received remote ischemic preconditioning had an AKI incidence of 37.5% compared with 52.5% for controls (P = .02); however, the mortality rate was the same.54 Similarly, remote ischemic preconditioning significantly lowered the incidence of AKI in nondiabetic patients undergoing CABG surgery compared with controls.55
Fluid management. Renal perfusion is intimately related to the development of AKI, and there is evidence that both hypovolemia and excessive fluid resuscitation can increase the risk of AKI in noncardiac surgery patients.56 Because of this, fluid management has also received attention in perioperative AKI. Goal-directed fluid management has been evaluated in noncardiac surgery patients, and it did not show any benefit in preventing AKI.57 However, in a more recent retrospective study, postoperative positive fluid balance was associated with increased incidence of AKI compared with zero fluid balance. Negative fluid balance did not appear to have a detrimental effect.58
RECOMMENDATIONS
No prophylactic therapy has yet been shown to definitively decrease the risk of postoperative AKI in all patients. Nevertheless, it is important to identify patients at risk during the preoperative visit, especially those with CKD. Many patients undergoing surgery have CKD, placing them at high risk of developing AKI in the perioperative period. The risk is particularly high with cardiac surgery.
Serum creatinine and urine output should be closely monitored perioperatively in at-risk patients. If AKI is diagnosed, practitioners need to identify and ameliorate the cause as early as possible.
Recommendations from KDIGO for perioperative prevention and management of AKI are listed in Table 4.15 These include avoiding additional nephrotoxic medications and adjusting the doses of renally cleared medications. Also, some patients may benefit from preoperative counseling and specialist referral.
- Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA 2007; 298(17):2038–2047. doi:10.1001/jama.298.17.2038
- National Institute of Diabetes and Digestive and Kidney Diseases. Kidney Disease Statistics for the United States. www.niddk.nih.gov/health-information/health-statistics/kidney-disease. Accessed June 11, 2018.
- Rosner MH, Okusa MD. Acute kidney injury associated with cardiac surgery. Clin J Am Soc Nephrol 2006; 1(1):19–32. doi:10.2215/CJN.00240605
- Meersch M, Schmidt C, Zarbock A. Patient with chronic renal failure undergoing surgery. Curr Opin Anaesthesiol 2016; 29(3):413–420. doi:10.1097/ACO.0000000000000329
- Stevens PE, Levin A; Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members. Evaluation and management of chronic kidney disease: synopsis of the Kidney Disease: Improving Global Outcomes 2012 clinical practice guideline. Ann Intern Med 2013; 158(11):825–830. doi:10.7326/0003-4819-158-11-201306040-00007
- Levey AS, Eckardt KU, Tsukamoto Y, et al. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 2005; 67(6):2089–2100. doi:10.1111/j.1523-1755.2005.00365.x
- Saitoh M, Takahashi T, Sakurada K, et al. Factors determining achievement of early postoperative cardiac rehabilitation goal in patients with or without preoperative kidney dysfunction undergoing isolated cardiac surgery. J Cardiol 2013; 61(4):299–303. doi:10.1016/j.jjcc.2012.12.014
- Minakata K, Bando K, Tanaka S, et al. Preoperative chronic kidney disease as a strong predictor of postoperative infection and mortality after coronary artery bypass grafting. Circ J 2014; 78(9):2225–2231. doi:10.1253/circj.CJ-14-0328
- Domoto S, Tagusari O, Nakamura Y, et al. Preoperative estimated glomerular filtration rate as a significant predictor of long-term outcomes after coronary artery bypass grafting in Japanese patients. Gen Thorac Cardiovasc Surg 2014; 62(2):95–102. doi:10.1007/s11748-013-0306-5
- Hedley AJ, Roberts MA, Hayward PA, et al. Impact of chronic kidney disease on patient outcome following cardiac surgery. Heart Lung Circ 2010; 19(8):453–459. doi:10.1016/j.hlc.2010.03.005
- Boulton BJ, Kilgo P, Guyton RA, et al. Impact of preoperative renal dysfunction in patients undergoing off-pump versus on-pump coronary artery bypass. Ann Thorac Surg 2011; 92(2):595–601. doi:10.1016/j.athoracsur.2011.04.023
- Prowle JR, Kam EP, Ahmad T, Smith NC, Protopapa K, Pearse RM. Preoperative renal dysfunction and mortality after non-cardiac surgery. Br J Surg 2016; 103(10):1316–1325. doi:10.1002/bjs.10186
- Gaber AO, Moore LW, Aloia TA, et al. Cross-sectional and case-control analyses of the association of kidney function staging with adverse postoperative outcomes in general and vascular surgery. Ann Surg 2013; 258(1):169–177. doi:10.1097/SLA.0b013e318288e18e
- Mases A, Sabaté S, Guilera N, et al. Preoperative estimated glomerular filtration rate and the risk of major adverse cardiovascular and cerebrovascular events in non-cardiac surgery. Br J Anaesth 2014; 113(4):644–651. doi:10.1093/bja/aeu134
- Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clinical Practice 2012; 120(4):c179–c184. doi:10.1159/000339789
- Pickering JW, James MT, Palmer SC. Acute kidney injury and prognosis after cardiopulmonary bypass: a meta-analysis of cohort studies. Am J Kidney Dis 2015; 65(2):283–293. doi:10.1053/j.ajkd.2014.09.008
- Dasta JF, Kane-Gill SL, Durtschi AJ, Pathak DS, Kellum JA. Costs and outcomes of acute kidney injury (AKI) following cardiac surgery. Nephrol Dial Transplant 2008; 23(6):1970-1974. doi:10.1093/ndt/gfm908
- Karkouti K, Wijeysundera DN, Yau TM, et al. Acute kidney injury after cardiac surgery focus on modifiable risk factors. Circulation 2009; 119(4):495–502. doi:10.1161/CIRCULATIONAHA.108.786913
- Xu JR, Zhu JM, Jiang J, et al. Risk factors for long-term mortality and progressive chronic kidney disease associated with acute kidney injury after cardiac surgery. Medicine (Baltimore) 2015; 94(45):e2025. doi:10.1097/MD.0000000000002025
- Chalmers J, Mediratta N, McShane J, Shaw M, Pullan M, Poullis M. The long-term effects of developing renal failure post-coronary artery bypass surgery, in patients with normal preoperative renal function. Eur J Cardiothorac Surg 2013; 43(3):555–559. doi:10.1093/ejcts/ezs329
- Ryden L, Sartipy U, Evans M, Holzmann MJ. Acute kidney injury after coronary artery bypass grafting and long-term risk of end-stage renal disease. Circulation 2014; 130(23):2005–2011. doi:10.1161/CIRCULATIONAHA.114.010622
- Gargiulo G, Capodanno D, Sannino A, et al. Impact of moderate preoperative chronic kidney disease on mortality after transcatheter aortic valve implantation. Int J Cardiol 2015; 189:77–78. doi:10.1016/j.ijcard.2015.04.077
- Gargiulo G, Capodanno D, Sannino A, et al. Moderate and severe preoperative chronic kidney disease worsen clinical outcomes after transcatheter aortic valve implantation meta-analysis of 4,992 patients. Circ Cardiovasc Interv 2015; 8(2):e002220. doi:10.1161/CIRCINTERVENTIONS.114.002220
- Han SS, Shin N, Baek SH, et al. Effects of acute kidney injury and chronic kidney disease on long-term mortality after coronary artery bypass grafting. Am Heart J 2015; 169(3):419–425. doi:10.1016/j.ahj.2014.12.019
- Aronson S, Fontes ML, Miao Y, Mangano DT; Investigators of the Multicenter Study of Perioperative Ischemia Research Group; Ischemia Research and Education Foundation. Risk index for perioperative renal dysfunction/failure: critical dependence on pulse pressure hypertension. Circulation 2007; 115(6):733–742. doi:10.1161/CIRCULATIONAHA.106.623538
- Hertzberg D, Sartipy U, Holzmann MJ. Type 1 and type 2 diabetes mellitus and risk of acute kidney injury after coronary artery bypass grafting. Am Heart J 2015; 170(5):895–902. doi:10.1016/j.ahj.2015.08.013
- Benedetto U, Sciarretta S, Roscitano A, et al. Preoperative angiotensin-converting enzyme inhibitors and acute kidney injury after coronary artery bypass grafting. Ann Thorac Surg 2008; 86(4):1160–1165. doi:10.1016/j.athoracsur.2008.06.018
- Arora P, Rajagopalam S, Ranjan R, et al. Preoperative use of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers is associated with increased risk for acute kidney injury after cardiovascular surgery. Clin J Am Soc Nephrol 2008; 3(5):1266–1273. doi:10.2215/CJN.05271107
- Haase M, Bellomo R, Story D, et al. Effect of mean arterial pressure, haemoglobin and blood transfusion during cardiopulmonary bypass on post-operative acute kidney injury. Nephrol Dial Transplant 2012; 27(1):153–160. doi:10.1093/ndt/gfr275
- Ono M, Arnaoutakis GJ, Fine DM, et al. Blood pressure excursions below the cerebral autoregulation threshold during cardiac surgery are associated with acute kidney injury. Crit Care Med 2013; 41(2):464-471. doi:10.1097/CCM.0b013e31826ab3a1
- Seabra VF, Alobaidi S, Balk EM, Poon AH, Jaber BL. Off-pump coronary artery bypass surgery and acute kidney injury: a meta-analysis of randomized controlled trials. Clin J Am Soc Nephrol 2010; 5(10):1734–1744. doi:10.2215/CJN.02800310
- Garg AX, Devereaux PJ, Yusuf S, et al; CORONARY Investigators. Kidney function after off-pump or on-pump coronary artery bypass graft surgery: a randomized clinical trial. JAMA 2014; 311(21):2191–2198. doi:10.1001/jama.2014.4952
- Kumar AB, Suneja M, Bayman EO, Weide GD, Tarasi M. Association between postoperative acute kidney injury and duration of cardiopulmonary bypass: a meta-analysis. J Cardiothorac Vasc Anesth 2012; 26(1):64–69. doi:10.1053/j.jvca.2011.07.007
- Kheterpal S, Tremper KK, Englesbe MJ, et al. Predictors of postoperative acute renal failure after noncardiac surgery in patients with previously normal renal function. Anesthesiology 2007; 107(6):892–902. doi:10.1097/01.anes.0000290588.29668.38
- Grams ME, Sang Y, Coresh J, et al. Acute kidney injury after major surgery: a retrospective analysis of Veterans Health Administration data. Am J Kidney Dis 2016; 67(6):872–880. doi:10.1053/j.ajkd.2015.07.022
- Biteker M, Dayan A, Tekkesin AI, et al. Incidence, risk factors, and outcomes of perioperative acute kidney injury in noncardiac and nonvascular surgery. Am J Surg 2014: 207(1):53–59. doi:10.1016/j.amjsurg.2013.04.006
- Gu W-J, Hou B-L, Kwong JS, et al. Association between intraoperative hypotension and 30-day mortality, major adverse cardiac events, and acute kidney injury after non-cardiac surgery: a meta-analysis of cohort studies. Int J Cardiol 2018; 258:68–73. doi:10.1016/j.ijcard.2018.01.137
- Smetana GW, Macpherson DS. The case against routine preoperative laboratory testing. Med Clin North Am 2003; 87(1):7–40. pmid:12575882
- Perrotti A, Miltgen G, Chevet-Noel A, et al. Neutrophil gelatinase-associated lipocalin as early predictor of acute kidney injury after cardiac surgery in adults with chronic kidney failure. Ann Thorac Surg 2015; 99(3):864–869. doi:10.1016/j.athoracsur.2014.10.011
- Doi K, Urata M, Katagiri D, et al. Plasma neutrophil gelatinase-associated lipocalin in acute kidney injury superimposed on chronic kidney disease after cardiac surgery: a multicenter prospective study. Crit Care 2013; 17(6):R270. doi:10.1186/cc13104
- Ho J, Tangri N, Komenda P, et al. Urinary, plasma, and serum biomarkers’ utility for predicting acute kidney injury associated with cardiac surgery in adults: a meta-analysis. Am J Kidney Dis 2015; 66(6):993–1005. doi:10.1053/j.ajkd.2015.06.018
- Yao L, Young N, Liu H, et al. Evidence for preoperative aspirin improving major outcomes in patients with chronic kidney disease undergoing cardiac surgery: a cohort study. Ann Surg 2015; 261(1):207–212. doi:10.1097/SLA.0000000000000641
- Garg AX, Kurz A, Sessler DI, et al; POISE-2 Investigators. Aspirin and clonidine in non-cardiac surgery: acute kidney injury substudy protocol of the perioperative ischaemic evaluation (POISE) 2 randomised controlled trial. BMJ open 2014; 4(2):e004886. doi:10.1136/bmjopen-2014-004886
- He SJ, Liu Q, Li HQ, Tian F, Chen SY, Weng JX. Role of statins in preventing cardiac surgery-associated acute kidney injury: an updated meta-analysis of randomized controlled trials. Ther Clin Risk Manag 2018; 14:475–482. doi:10.2147/TCRM.S160298
- Tie HT, Luo MZ, Lin D, Zhang M, Wan JY, Wu QC. Erythropoietin administration for prevention of cardiac surgery-associated acute kidney injury: a meta-analysis of randomized controlled trials. Eur J Cardiothorac Surg 2015; 48(1):32–39. doi:10.1093/ejcts/ezu378
- Santana-Santos E, Gowdak LH, Gaiotto FA, et al. High dose of N-acetylcystein prevents acute kidney injury in chronic kidney disease patients undergoing myocardial revascularization. Ann Thorac Surg 2014; 97(5):1617–1623. doi:10.1016/j.athoracsur.2014.01.056
- Mei M, Zhao HW, Pan QG, Pu YM, Tang MZ, Shen BB. Efficacy of N-acetylcysteine in preventing acute kidney injury after cardiac surgery: a meta-analysis study. J Invest Surg 2018; 31(1):14–23. doi:10.1080/08941939.2016.1269853
- Sezai A, Hata M, Niino T, et al. Results of low-dose human atrial natriuretic peptide infusion in nondialysis patients with chronic kidney disease undergoing coronary artery bypass grafting: the NU-HIT (Nihon University working group study of low-dose HANP infusion therapy during cardiac surgery) trial for CKD. J Am Coll Cardiol 2011; 58(9):897–903. doi:10.1016/j.jacc.2011.03.056
- Xu N, Long Q, He T, et al. Association between preoperative renin-angiotensin system inhibitor use and postoperative acute kidney injury risk in patients with hypertension. Clin Nephrol 2018; 89(6):403–414. doi:10.5414/CN109319
- Liu Y, Sheng B, Wang S, Lu F, Zhen J, Chen W. Dexmedetomidine prevents acute kidney injury after adult cardiac surgery: a meta-analysis of randomized controlled trials. BMC Anesthesiol 2018; 18(1):7. doi:10.1186/s12871-018-0472-1
- Shi R, Tie H-T. Dexmedetomidine as a promising prevention strategy for cardiac surgery-associated acute kidney injury: a meta-analysis. Critical Care 2017; 21(1):198. doi:10.1186/s13054-017-1776-0
- Zhou C, Gong J, Chen D, Wang W, Liu M, Liu B. Levosimendan for prevention of acute kidney injury after cardiac surgery: a meta-analysis of randomized controlled trials. Am J Kidney Dis 2016; 67(3):408–416. doi:10.1053/j.ajkd.2015.09.015
- Elbadawi A, Elgendy IY, Saad M, et al. Meta-analysis of trials on prophylactic use of levosimendan in patients undergoing cardiac surgery. Ann Thorac Surg 2018; 105(5):1403–1410. doi:10.1016/j.athoracsur.2017.11.027
- Zarbock A, Schmidt C, Van Aken H, et al; RenalRIPC Investigators. Effect of remote ischemic preconditioning on kidney injury among high-risk patients undergoing cardiac surgery: a randomized clinical trial. JAMA 2015; 313(21):2133–2141. doi:10.1001/jama.2015.4189
- Venugopal V, Laing CM, Ludman A, Yellon DM, Hausenloy D. Effect of remote ischemic preconditioning on acute kidney injury in nondiabetic patients undergoing coronary artery bypass graft surgery: a secondary analysis of 2 small randomized trials. Am J Kidney Dis 2010; 56(6):1043–1049. doi:10.1053/j.ajkd.2010.07.014
- Futier E, Constantin JM, Petit A, et al. Conservative vs restrictive individualized goal-directed fluid replacement strategy in major abdominal surgery: a prospective randomized trial. Arch Surg 2010; 145(12):1193–1200. doi:10.1001/archsurg.2010.275
- Patel A, Prowle JR, Ackland GL. Postoperative goal-directed therapy and development of acute kidney injury following major elective noncardiac surgery: post-hoc analysis of POM-O randomized controlled trial. Clin Kidney J 2017; 10(3):348–356. doi:10.1093/ckj/sfw118
- Shen Y, Zhang W, Cheng X, Ying M. Association between postoperative fluid balance and acute kidney injury in patients after cardiac surgery: a retrospective cohort study. J Crit Care 2018; 44:273–277. doi:10.1016/j.jcrc.2017.11.041
Chronic kidney disease (CKD) is estimated to affect 14% of Americans, but it is likely underdiagnosed because it is often asymptomatic.1,2 Its prevalence is even higher in patients who undergo surgery—up to 30% in cardiac surgery.3 Its impact on surgical outcomes is substantial.4 Importantly, patients with CKD are at higher risk of postoperative acute kidney injury (AKI), which is also associated with adverse outcomes. Thus, it is important to recognize, assess, and manage abnormal renal function in surgical patients.
WHAT IS THE IMPACT ON POSTOPERATIVE OUTCOMES?
Cardiac surgery outcomes
Moreover, in patients undergoing coronary artery bypass grafting (CABG), the worse the renal dysfunction, the higher the long-term mortality rate. Patients with moderate (stage 3) CKD had a 3.5 times higher odds of in-hospital mortality compared with patients with normal renal function, rising to 8.8 with severe (stage 4) and to 9.6 with dialysis-dependent (stage 5) CKD.11
The mechanisms linking CKD with negative cardiac outcomes are unclear, but many possibilities exist. CKD is an independent risk factor for coronary artery disease and shares underlying risk factors such as hypertension and diabetes. Cardiac surgery patients with CKD are also more likely to have diabetes, left ventricular dysfunction, and peripheral vascular disease.
Noncardiac surgery outcomes
CKD is also associated with adverse outcomes in noncardiac surgery patients, especially at higher levels of renal dysfunction.12–14 For example, in patients who underwent major noncardiac surgery, compared with patients in stage 1 (estimated GFR > 90 mL/min/1.73 m2), the odds ratios for all-cause mortality were as follows:
- 0.8 for patients with stage 2 CKD
- 2.2 in stage 3a
- 2.8 in stage 3b
- 11.3 in stage 4
- 5.8 in stage 5.14
The association between estimated GFR and all-cause mortality was not statistically significant (P = .071), but statistically significant associations were observed between estimated GFR and major adverse cardiovascular events (P < .001) and hospital length of stay (P < .001).
The association of CKD with major adverse outcomes and death in both cardiac and noncardiac surgical patients demonstrates the importance of understanding this risk, identifying patients with CKD preoperatively, and taking steps to lower the risk.
WHAT IS THE IMPACT OF ACUTE KIDNEY INJURY?
AKI is a common and serious complication of surgery, especially cardiac surgery. It has been associated with higher rates of morbidity, mortality, and cardiovascular events, longer hospital length of stay, and higher cost.
Several groups have proposed criteria for defining AKI and its severity; the KDIGO criteria are the most widely accepted.15 These define AKI as an increase in serum creatinine concentration of 0.3 mg/dL or more within 48 hours or at least 1.5 times the baseline value within 7 days, or urine volume less than 0.5 mL/kg/hour for more than 6 hours. There are 3 stages of severity:
- Stage 1—an increase in serum creatinine of 1.5 to 1.9 times baseline, an absolute increase of at least 0.3 mg/dL, or urine output less than 0.5 mL/kg/hour for 6 to 12 hours
- Stage 2—an increase in serum creatinine of 2.0 to 2.9 times baseline or urine output less than 0.5 mmL/kg/hour for 12 or more hours
- Stage 3—an increase in serum creatinine of 3 times baseline, an absolute increase of at least 4 mg/dL, initiation of renal replacement therapy, urine output less than 0.3 mL/kg/hour for 24 or more hours, or anuria for 12 or more hours.15
Multiple factors associated with surgery may contribute to AKI, including hemodynamic instability, volume shifts, blood loss, use of heart-lung bypass, new medications, activation of the inflammatory cascade, oxidative stress, and anemia.
AKI in cardiac surgery
The incidence of AKI is high in cardiac surgery. In a meta-analysis of 46 studies (N = 242,000), its incidence in cardiopulmonary bypass surgery was about 18%, with 2.1% of patients needing renal replacement therapy.16 However, the incidence varied considerably from study to study, ranging from 1% to 53%, and was influenced by the definition of AKI, the type of cardiac surgery, and the patient population.16
Cardiac surgery-associated AKI adversely affects outcomes. Several studies have shown that cardiac surgery patients who develop AKI have higher rates of death and stroke.16–21 More severe AKI confers higher mortality rates, with the highest mortality rate in patients who need renal replacement therapy, approximately 37%.17 Patients with cardiac surgery-associated AKI also have a longer hospital length of stay and significantly higher costs of care.17,18
Long-term outcomes are also negatively affected by AKI. In cardiac surgery patients with AKI who had completely recovered renal function by the time they left the hospital, the 2-year incidence rate of CKD was 6.8%, significantly higher than the 0.2% rate in patients who did not develop AKI.19 The 2-year survival rates also were significantly worse for patients who developed postoperative AKI (82.3% vs 93.7%). Similarly, in patients undergoing CABG who had normal renal function before surgery, those who developed AKI postoperatively had significantly shorter long-term survival rates.20 The effect does not require a large change in renal function. An increase in creatinine as small as 0.3 mg/dL has been associated with a higher rate of death and a long-term risk of end-stage renal disease that is 3 times higher.21
WHAT ARE THE RISK FACTORS FOR ACUTE KIDNEY INJURY?
Cardiac surgery
CKD is a risk factor not only after cardiac surgery but also after percutaneous procedures. In a meta-analysis of 4,992 patients with CKD who underwent transcatheter aortic valve replacement, both moderate and severe CKD increased the odds of AKI, early stroke, the need for dialysis, and all-cause and cardiovascular mortality at 1 year.22,23 Increased rates of AKI also have been found in patients with CKD undergoing CABG surgery.24 These results point to a synergistic effect between AKI and CKD, with outcomes much worse in combination than alone.
In cardiac surgery, the most important patient risk factors associated with a higher incidence of postoperative AKI are age older than 75, CKD, preoperative heart failure, and prior myocardial infarction.19,25 Diabetes is an additional independent risk factor, with type 1 conferring higher risk than type 2.26 Preoperative use of angiotensin-converting enzyme (ACE) inhibitors may or may not be a risk factor for cardiac surgery-associated AKI, with some studies finding increased risk and others finding reduced rates.27,28
Anemia, which may be related to either patient or surgical risk factors (eg, intraoperative blood loss), also increases the risk of AKI in cardiac surgery.29,30 A retrospective study of CABG surgery patients found that intraoperative hemoglobin levels below 8 g/dL were associated with a 25% to 30% incidence of AKI, compared with 15% to 20% with hemoglobin levels above 9 g/dL.29 Additionally, having severe hypotension (mean arterial pressure < 50 mm Hg) significantly increased the AKI rates in the low-hemoglobin group.29 Similar results were reported in a later study.30
Among surgical factors, several randomized controlled trials have shown that off-pump CABG is associated with a significantly lower risk of postoperative AKI than on-pump CABG; however, this difference did not translate into any long-term difference in mortality rates.31,32 Longer cardiopulmonary bypass time is strongly associated with a higher incidence of AKI and postoperative death.33
Noncardiac surgery
AKI is less common after noncardiac surgery; however, outcomes are severe in patients in whom it occurs. In a study of 15,102 noncardiac surgery patients, only 0.8% developed AKI and 0.1% required renal replacement therapy.34
Risk factors after noncardiac surgery are similar to those after cardiac surgery (Table 3).34–36 Factors with the greatest impact are older age, peripheral vascular occlusive disease, chronic obstructive pulmonary disease necessitating chronic bronchodilator therapy, high-risk surgery, hepatic disease, emergent or urgent surgery, and high body mass index.
Surgical risk factors include total vasopressor dose administered, use of a vasopressor infusion, and diuretic administration.34 In addition, intraoperative hypotension is associated with a higher risk of AKI, major adverse cardiac events, and 30-day mortality.37
Noncardiac surgery patients with postoperative AKI have significantly higher rates of 30-day readmissions, 1-year progression to end-stage renal disease, and mortality than patients who do not develop AKI.35 Additionally, patients with AKI have significantly higher rates of cardiovascular complications (33.3% vs 11.3%) and death (6.1% vs 0.9%), as well as a significantly longer length of hospital stay.34,36
CAN WE DECREASE THE IMPACT OF RENAL DISEASE IN SURGERY?
Before surgery, practitioners need to identify patients at risk of AKI, implement possible risk-reduction measures, and, afterward, treat it early in its course if it occurs.
The preoperative visit is the ideal time to assess a patient’s risk of postoperative renal dysfunction. Laboratory tests can identify risks based on surgery type, age, hypertension, the presence of CKD, and medications that affect renal function. However, the basic chemistry panel is abnormal in only 8.2% of patients and affects management in just 2.6%, requiring the clinician to target testing to patients at high risk.38
Patients with a significant degree of renal dysfunction, particularly those previously undiagnosed, may benefit from additional preoperative testing and medication management. Perioperative management of medications that could adversely affect renal function should be carefully considered during the preoperative visit. In addition, the postoperative inpatient team needs to be informed about potentially nephrotoxic medications and medications that are renally cleared. Attention needs to be given to the renal impact of common perioperative medications such as nonsteroidal anti-inflammatory drugs, antibiotics, intravenous contrast, low-molecular-weight heparins, diuretics, ACE inhibitors, and angiotensin II receptor blockers. With the emphasis on opioid-sparing analgesics, it is particularly important to assess the risk of AKI if nonsteroidal anti-inflammatory drugs are part of the pain control plan.
Nephrology referral may help, especially for patients with a GFR less than 45 mL/min. This information enables more informed decision-making regarding the risks of adverse outcomes related to kidney disease.
WHAT TOOLS DO WE HAVE TO DIAGNOSE RENAL INJURY?
Several risk-prediction models have been developed to assess the postoperative risk of AKI in both cardiac and major noncardiac surgery patients. Although these models can identify risk factors, their clinical accuracy and utility have been questioned.
Biomarkers
Early diagnosis is the first step in managing AKI, allowing time to implement measures to minimize its impact.
Serum creatinine testing is widely used to measure renal function and diagnose AKI; however, it does not detect small reductions in renal function, and there is a time lag between renal insult and a rise in creatinine. The result is a delay to diagnosis of AKI.
Biomarkers other than creatinine have been studied for early detection of intraoperative and postoperative renal insult. These novel renal injury markers include the following:
Neutrophil gelatinase-associated lipocalin (NGAL). Two studies looked at plasma NGAL as an early marker of AKI in patients with CKD who were undergoing cardiac surgery.39,40 One study found that by using NGAL instead of creatinine, postoperative AKI could be diagnosed an average of 20 hours earlier.39 In addition, NGAL helped detect renal recovery earlier than creatinine.40 The diagnostic cut-off values of NGAL were different for patients with CKD than for those without CKD.39,40
Other novel markers include:
- Kidney injury marker 1
- N-acetyl-beta-D-glucosaminidase
- Cysteine C.
Although these biomarkers show some ability to detect renal injury, they provide only modest discrimination and are not widely available for clinical use.41 Current evidence does not support routine use of these markers in clinical settings.
CAN WE PROTECT RENAL FUNCTION?
Interventions to prevent or ameliorate the impact of CKD and AKI on surgical outcomes have been studied most extensively in cardiac surgery patients.
Aspirin. A retrospective study of 3,585 cardiac surgery patients with CKD found that preoperative aspirin use significantly lowered the incidence of postoperative AKI and 30-day mortality compared with patients not using aspirin.42 Aspirin use reduced 30-day mortality in CKD stages 1, 2, and 3 by 23.3%, 58%, and 70%, respectively. On the other hand, in the Perioperative Ischemic Evaluation (POISE) trial, in noncardiac surgery patients, neither aspirin nor clonidine started 2 to 4 hours preoperatively and continued up to 30 days after surgery altered the risk of AKI significantly more than placebo.43
Statins have been ineffective in reducing the incidence of AKI in cardiac surgery patients. In fact, a meta-analysis of 8 interventional trials found an increased incidence of AKI in patients in whom statins were started perioperatively.44 Erythropoietin was also found to be ineffective in the prevention of perioperative AKI in cardiac surgery patients in a separate study.45
The evidence regarding other therapies has also varied.
N-acetylcysteine in high doses reduced the incidence of AKI in patients with CKD stage 3 and 4 undergoing CABG.46 Another meta-analysis of 10 studies in cardiac surgery patients published recently did not show any benefit of N-acetylcysteine in reducing AKI.47
Human atrial natriuretic peptide, given preoperatively to patients with CKD, reduced the acute and long-term creatinine rise as well as the number of cardiac events after CABG; however, it did not reduce mortality rates.48
Renin-angiotensin system inhibitors, given preoperatively to patients with heart failure was associated with a decrease in the incidence of AKI in 1 study.49
Dexmedetomidine is a highly selective alpha 2 adrenoreceptor agonist. A recent meta-analysis of 10 clinical trials found it beneficial in reducing the risk of perioperative AKI in cardiac surgery patients.50 An earlier meta-analysis had similar results.51
Levosimendan is an inotropic vasodilator that improves cardiac output and renal perfusion in patients with systolic heart failure, and it has been hypothesized to decrease the risk of AKI after cardiac surgery. Previous data demonstrated that this drug reduced AKI and mortality; however, analysis was limited by small sample size and varying definitions of AKI.52 A recent meta-analysis showed that levosimendan was associated with a lower incidence of AKI but was also associated with an increased incidence of atrial fibrillation and no reduction in 30-day mortality.53
Remote ischemic preconditioning is a procedure that subjects the kidneys to brief episodes of ischemia before surgery, protecting them when they are later subjected to prolonged ischemia or reperfusion injury. It has shown initial promising results in preventing AKI. In a randomized controlled trial in 240 patients at high risk of AKI, those who received remote ischemic preconditioning had an AKI incidence of 37.5% compared with 52.5% for controls (P = .02); however, the mortality rate was the same.54 Similarly, remote ischemic preconditioning significantly lowered the incidence of AKI in nondiabetic patients undergoing CABG surgery compared with controls.55
Fluid management. Renal perfusion is intimately related to the development of AKI, and there is evidence that both hypovolemia and excessive fluid resuscitation can increase the risk of AKI in noncardiac surgery patients.56 Because of this, fluid management has also received attention in perioperative AKI. Goal-directed fluid management has been evaluated in noncardiac surgery patients, and it did not show any benefit in preventing AKI.57 However, in a more recent retrospective study, postoperative positive fluid balance was associated with increased incidence of AKI compared with zero fluid balance. Negative fluid balance did not appear to have a detrimental effect.58
RECOMMENDATIONS
No prophylactic therapy has yet been shown to definitively decrease the risk of postoperative AKI in all patients. Nevertheless, it is important to identify patients at risk during the preoperative visit, especially those with CKD. Many patients undergoing surgery have CKD, placing them at high risk of developing AKI in the perioperative period. The risk is particularly high with cardiac surgery.
Serum creatinine and urine output should be closely monitored perioperatively in at-risk patients. If AKI is diagnosed, practitioners need to identify and ameliorate the cause as early as possible.
Recommendations from KDIGO for perioperative prevention and management of AKI are listed in Table 4.15 These include avoiding additional nephrotoxic medications and adjusting the doses of renally cleared medications. Also, some patients may benefit from preoperative counseling and specialist referral.
Chronic kidney disease (CKD) is estimated to affect 14% of Americans, but it is likely underdiagnosed because it is often asymptomatic.1,2 Its prevalence is even higher in patients who undergo surgery—up to 30% in cardiac surgery.3 Its impact on surgical outcomes is substantial.4 Importantly, patients with CKD are at higher risk of postoperative acute kidney injury (AKI), which is also associated with adverse outcomes. Thus, it is important to recognize, assess, and manage abnormal renal function in surgical patients.
WHAT IS THE IMPACT ON POSTOPERATIVE OUTCOMES?
Cardiac surgery outcomes
Moreover, in patients undergoing coronary artery bypass grafting (CABG), the worse the renal dysfunction, the higher the long-term mortality rate. Patients with moderate (stage 3) CKD had a 3.5 times higher odds of in-hospital mortality compared with patients with normal renal function, rising to 8.8 with severe (stage 4) and to 9.6 with dialysis-dependent (stage 5) CKD.11
The mechanisms linking CKD with negative cardiac outcomes are unclear, but many possibilities exist. CKD is an independent risk factor for coronary artery disease and shares underlying risk factors such as hypertension and diabetes. Cardiac surgery patients with CKD are also more likely to have diabetes, left ventricular dysfunction, and peripheral vascular disease.
Noncardiac surgery outcomes
CKD is also associated with adverse outcomes in noncardiac surgery patients, especially at higher levels of renal dysfunction.12–14 For example, in patients who underwent major noncardiac surgery, compared with patients in stage 1 (estimated GFR > 90 mL/min/1.73 m2), the odds ratios for all-cause mortality were as follows:
- 0.8 for patients with stage 2 CKD
- 2.2 in stage 3a
- 2.8 in stage 3b
- 11.3 in stage 4
- 5.8 in stage 5.14
The association between estimated GFR and all-cause mortality was not statistically significant (P = .071), but statistically significant associations were observed between estimated GFR and major adverse cardiovascular events (P < .001) and hospital length of stay (P < .001).
The association of CKD with major adverse outcomes and death in both cardiac and noncardiac surgical patients demonstrates the importance of understanding this risk, identifying patients with CKD preoperatively, and taking steps to lower the risk.
WHAT IS THE IMPACT OF ACUTE KIDNEY INJURY?
AKI is a common and serious complication of surgery, especially cardiac surgery. It has been associated with higher rates of morbidity, mortality, and cardiovascular events, longer hospital length of stay, and higher cost.
Several groups have proposed criteria for defining AKI and its severity; the KDIGO criteria are the most widely accepted.15 These define AKI as an increase in serum creatinine concentration of 0.3 mg/dL or more within 48 hours or at least 1.5 times the baseline value within 7 days, or urine volume less than 0.5 mL/kg/hour for more than 6 hours. There are 3 stages of severity:
- Stage 1—an increase in serum creatinine of 1.5 to 1.9 times baseline, an absolute increase of at least 0.3 mg/dL, or urine output less than 0.5 mL/kg/hour for 6 to 12 hours
- Stage 2—an increase in serum creatinine of 2.0 to 2.9 times baseline or urine output less than 0.5 mmL/kg/hour for 12 or more hours
- Stage 3—an increase in serum creatinine of 3 times baseline, an absolute increase of at least 4 mg/dL, initiation of renal replacement therapy, urine output less than 0.3 mL/kg/hour for 24 or more hours, or anuria for 12 or more hours.15
Multiple factors associated with surgery may contribute to AKI, including hemodynamic instability, volume shifts, blood loss, use of heart-lung bypass, new medications, activation of the inflammatory cascade, oxidative stress, and anemia.
AKI in cardiac surgery
The incidence of AKI is high in cardiac surgery. In a meta-analysis of 46 studies (N = 242,000), its incidence in cardiopulmonary bypass surgery was about 18%, with 2.1% of patients needing renal replacement therapy.16 However, the incidence varied considerably from study to study, ranging from 1% to 53%, and was influenced by the definition of AKI, the type of cardiac surgery, and the patient population.16
Cardiac surgery-associated AKI adversely affects outcomes. Several studies have shown that cardiac surgery patients who develop AKI have higher rates of death and stroke.16–21 More severe AKI confers higher mortality rates, with the highest mortality rate in patients who need renal replacement therapy, approximately 37%.17 Patients with cardiac surgery-associated AKI also have a longer hospital length of stay and significantly higher costs of care.17,18
Long-term outcomes are also negatively affected by AKI. In cardiac surgery patients with AKI who had completely recovered renal function by the time they left the hospital, the 2-year incidence rate of CKD was 6.8%, significantly higher than the 0.2% rate in patients who did not develop AKI.19 The 2-year survival rates also were significantly worse for patients who developed postoperative AKI (82.3% vs 93.7%). Similarly, in patients undergoing CABG who had normal renal function before surgery, those who developed AKI postoperatively had significantly shorter long-term survival rates.20 The effect does not require a large change in renal function. An increase in creatinine as small as 0.3 mg/dL has been associated with a higher rate of death and a long-term risk of end-stage renal disease that is 3 times higher.21
WHAT ARE THE RISK FACTORS FOR ACUTE KIDNEY INJURY?
Cardiac surgery
CKD is a risk factor not only after cardiac surgery but also after percutaneous procedures. In a meta-analysis of 4,992 patients with CKD who underwent transcatheter aortic valve replacement, both moderate and severe CKD increased the odds of AKI, early stroke, the need for dialysis, and all-cause and cardiovascular mortality at 1 year.22,23 Increased rates of AKI also have been found in patients with CKD undergoing CABG surgery.24 These results point to a synergistic effect between AKI and CKD, with outcomes much worse in combination than alone.
In cardiac surgery, the most important patient risk factors associated with a higher incidence of postoperative AKI are age older than 75, CKD, preoperative heart failure, and prior myocardial infarction.19,25 Diabetes is an additional independent risk factor, with type 1 conferring higher risk than type 2.26 Preoperative use of angiotensin-converting enzyme (ACE) inhibitors may or may not be a risk factor for cardiac surgery-associated AKI, with some studies finding increased risk and others finding reduced rates.27,28
Anemia, which may be related to either patient or surgical risk factors (eg, intraoperative blood loss), also increases the risk of AKI in cardiac surgery.29,30 A retrospective study of CABG surgery patients found that intraoperative hemoglobin levels below 8 g/dL were associated with a 25% to 30% incidence of AKI, compared with 15% to 20% with hemoglobin levels above 9 g/dL.29 Additionally, having severe hypotension (mean arterial pressure < 50 mm Hg) significantly increased the AKI rates in the low-hemoglobin group.29 Similar results were reported in a later study.30
Among surgical factors, several randomized controlled trials have shown that off-pump CABG is associated with a significantly lower risk of postoperative AKI than on-pump CABG; however, this difference did not translate into any long-term difference in mortality rates.31,32 Longer cardiopulmonary bypass time is strongly associated with a higher incidence of AKI and postoperative death.33
Noncardiac surgery
AKI is less common after noncardiac surgery; however, outcomes are severe in patients in whom it occurs. In a study of 15,102 noncardiac surgery patients, only 0.8% developed AKI and 0.1% required renal replacement therapy.34
Risk factors after noncardiac surgery are similar to those after cardiac surgery (Table 3).34–36 Factors with the greatest impact are older age, peripheral vascular occlusive disease, chronic obstructive pulmonary disease necessitating chronic bronchodilator therapy, high-risk surgery, hepatic disease, emergent or urgent surgery, and high body mass index.
Surgical risk factors include total vasopressor dose administered, use of a vasopressor infusion, and diuretic administration.34 In addition, intraoperative hypotension is associated with a higher risk of AKI, major adverse cardiac events, and 30-day mortality.37
Noncardiac surgery patients with postoperative AKI have significantly higher rates of 30-day readmissions, 1-year progression to end-stage renal disease, and mortality than patients who do not develop AKI.35 Additionally, patients with AKI have significantly higher rates of cardiovascular complications (33.3% vs 11.3%) and death (6.1% vs 0.9%), as well as a significantly longer length of hospital stay.34,36
CAN WE DECREASE THE IMPACT OF RENAL DISEASE IN SURGERY?
Before surgery, practitioners need to identify patients at risk of AKI, implement possible risk-reduction measures, and, afterward, treat it early in its course if it occurs.
The preoperative visit is the ideal time to assess a patient’s risk of postoperative renal dysfunction. Laboratory tests can identify risks based on surgery type, age, hypertension, the presence of CKD, and medications that affect renal function. However, the basic chemistry panel is abnormal in only 8.2% of patients and affects management in just 2.6%, requiring the clinician to target testing to patients at high risk.38
Patients with a significant degree of renal dysfunction, particularly those previously undiagnosed, may benefit from additional preoperative testing and medication management. Perioperative management of medications that could adversely affect renal function should be carefully considered during the preoperative visit. In addition, the postoperative inpatient team needs to be informed about potentially nephrotoxic medications and medications that are renally cleared. Attention needs to be given to the renal impact of common perioperative medications such as nonsteroidal anti-inflammatory drugs, antibiotics, intravenous contrast, low-molecular-weight heparins, diuretics, ACE inhibitors, and angiotensin II receptor blockers. With the emphasis on opioid-sparing analgesics, it is particularly important to assess the risk of AKI if nonsteroidal anti-inflammatory drugs are part of the pain control plan.
Nephrology referral may help, especially for patients with a GFR less than 45 mL/min. This information enables more informed decision-making regarding the risks of adverse outcomes related to kidney disease.
WHAT TOOLS DO WE HAVE TO DIAGNOSE RENAL INJURY?
Several risk-prediction models have been developed to assess the postoperative risk of AKI in both cardiac and major noncardiac surgery patients. Although these models can identify risk factors, their clinical accuracy and utility have been questioned.
Biomarkers
Early diagnosis is the first step in managing AKI, allowing time to implement measures to minimize its impact.
Serum creatinine testing is widely used to measure renal function and diagnose AKI; however, it does not detect small reductions in renal function, and there is a time lag between renal insult and a rise in creatinine. The result is a delay to diagnosis of AKI.
Biomarkers other than creatinine have been studied for early detection of intraoperative and postoperative renal insult. These novel renal injury markers include the following:
Neutrophil gelatinase-associated lipocalin (NGAL). Two studies looked at plasma NGAL as an early marker of AKI in patients with CKD who were undergoing cardiac surgery.39,40 One study found that by using NGAL instead of creatinine, postoperative AKI could be diagnosed an average of 20 hours earlier.39 In addition, NGAL helped detect renal recovery earlier than creatinine.40 The diagnostic cut-off values of NGAL were different for patients with CKD than for those without CKD.39,40
Other novel markers include:
- Kidney injury marker 1
- N-acetyl-beta-D-glucosaminidase
- Cysteine C.
Although these biomarkers show some ability to detect renal injury, they provide only modest discrimination and are not widely available for clinical use.41 Current evidence does not support routine use of these markers in clinical settings.
CAN WE PROTECT RENAL FUNCTION?
Interventions to prevent or ameliorate the impact of CKD and AKI on surgical outcomes have been studied most extensively in cardiac surgery patients.
Aspirin. A retrospective study of 3,585 cardiac surgery patients with CKD found that preoperative aspirin use significantly lowered the incidence of postoperative AKI and 30-day mortality compared with patients not using aspirin.42 Aspirin use reduced 30-day mortality in CKD stages 1, 2, and 3 by 23.3%, 58%, and 70%, respectively. On the other hand, in the Perioperative Ischemic Evaluation (POISE) trial, in noncardiac surgery patients, neither aspirin nor clonidine started 2 to 4 hours preoperatively and continued up to 30 days after surgery altered the risk of AKI significantly more than placebo.43
Statins have been ineffective in reducing the incidence of AKI in cardiac surgery patients. In fact, a meta-analysis of 8 interventional trials found an increased incidence of AKI in patients in whom statins were started perioperatively.44 Erythropoietin was also found to be ineffective in the prevention of perioperative AKI in cardiac surgery patients in a separate study.45
The evidence regarding other therapies has also varied.
N-acetylcysteine in high doses reduced the incidence of AKI in patients with CKD stage 3 and 4 undergoing CABG.46 Another meta-analysis of 10 studies in cardiac surgery patients published recently did not show any benefit of N-acetylcysteine in reducing AKI.47
Human atrial natriuretic peptide, given preoperatively to patients with CKD, reduced the acute and long-term creatinine rise as well as the number of cardiac events after CABG; however, it did not reduce mortality rates.48
Renin-angiotensin system inhibitors, given preoperatively to patients with heart failure was associated with a decrease in the incidence of AKI in 1 study.49
Dexmedetomidine is a highly selective alpha 2 adrenoreceptor agonist. A recent meta-analysis of 10 clinical trials found it beneficial in reducing the risk of perioperative AKI in cardiac surgery patients.50 An earlier meta-analysis had similar results.51
Levosimendan is an inotropic vasodilator that improves cardiac output and renal perfusion in patients with systolic heart failure, and it has been hypothesized to decrease the risk of AKI after cardiac surgery. Previous data demonstrated that this drug reduced AKI and mortality; however, analysis was limited by small sample size and varying definitions of AKI.52 A recent meta-analysis showed that levosimendan was associated with a lower incidence of AKI but was also associated with an increased incidence of atrial fibrillation and no reduction in 30-day mortality.53
Remote ischemic preconditioning is a procedure that subjects the kidneys to brief episodes of ischemia before surgery, protecting them when they are later subjected to prolonged ischemia or reperfusion injury. It has shown initial promising results in preventing AKI. In a randomized controlled trial in 240 patients at high risk of AKI, those who received remote ischemic preconditioning had an AKI incidence of 37.5% compared with 52.5% for controls (P = .02); however, the mortality rate was the same.54 Similarly, remote ischemic preconditioning significantly lowered the incidence of AKI in nondiabetic patients undergoing CABG surgery compared with controls.55
Fluid management. Renal perfusion is intimately related to the development of AKI, and there is evidence that both hypovolemia and excessive fluid resuscitation can increase the risk of AKI in noncardiac surgery patients.56 Because of this, fluid management has also received attention in perioperative AKI. Goal-directed fluid management has been evaluated in noncardiac surgery patients, and it did not show any benefit in preventing AKI.57 However, in a more recent retrospective study, postoperative positive fluid balance was associated with increased incidence of AKI compared with zero fluid balance. Negative fluid balance did not appear to have a detrimental effect.58
RECOMMENDATIONS
No prophylactic therapy has yet been shown to definitively decrease the risk of postoperative AKI in all patients. Nevertheless, it is important to identify patients at risk during the preoperative visit, especially those with CKD. Many patients undergoing surgery have CKD, placing them at high risk of developing AKI in the perioperative period. The risk is particularly high with cardiac surgery.
Serum creatinine and urine output should be closely monitored perioperatively in at-risk patients. If AKI is diagnosed, practitioners need to identify and ameliorate the cause as early as possible.
Recommendations from KDIGO for perioperative prevention and management of AKI are listed in Table 4.15 These include avoiding additional nephrotoxic medications and adjusting the doses of renally cleared medications. Also, some patients may benefit from preoperative counseling and specialist referral.
- Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA 2007; 298(17):2038–2047. doi:10.1001/jama.298.17.2038
- National Institute of Diabetes and Digestive and Kidney Diseases. Kidney Disease Statistics for the United States. www.niddk.nih.gov/health-information/health-statistics/kidney-disease. Accessed June 11, 2018.
- Rosner MH, Okusa MD. Acute kidney injury associated with cardiac surgery. Clin J Am Soc Nephrol 2006; 1(1):19–32. doi:10.2215/CJN.00240605
- Meersch M, Schmidt C, Zarbock A. Patient with chronic renal failure undergoing surgery. Curr Opin Anaesthesiol 2016; 29(3):413–420. doi:10.1097/ACO.0000000000000329
- Stevens PE, Levin A; Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members. Evaluation and management of chronic kidney disease: synopsis of the Kidney Disease: Improving Global Outcomes 2012 clinical practice guideline. Ann Intern Med 2013; 158(11):825–830. doi:10.7326/0003-4819-158-11-201306040-00007
- Levey AS, Eckardt KU, Tsukamoto Y, et al. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 2005; 67(6):2089–2100. doi:10.1111/j.1523-1755.2005.00365.x
- Saitoh M, Takahashi T, Sakurada K, et al. Factors determining achievement of early postoperative cardiac rehabilitation goal in patients with or without preoperative kidney dysfunction undergoing isolated cardiac surgery. J Cardiol 2013; 61(4):299–303. doi:10.1016/j.jjcc.2012.12.014
- Minakata K, Bando K, Tanaka S, et al. Preoperative chronic kidney disease as a strong predictor of postoperative infection and mortality after coronary artery bypass grafting. Circ J 2014; 78(9):2225–2231. doi:10.1253/circj.CJ-14-0328
- Domoto S, Tagusari O, Nakamura Y, et al. Preoperative estimated glomerular filtration rate as a significant predictor of long-term outcomes after coronary artery bypass grafting in Japanese patients. Gen Thorac Cardiovasc Surg 2014; 62(2):95–102. doi:10.1007/s11748-013-0306-5
- Hedley AJ, Roberts MA, Hayward PA, et al. Impact of chronic kidney disease on patient outcome following cardiac surgery. Heart Lung Circ 2010; 19(8):453–459. doi:10.1016/j.hlc.2010.03.005
- Boulton BJ, Kilgo P, Guyton RA, et al. Impact of preoperative renal dysfunction in patients undergoing off-pump versus on-pump coronary artery bypass. Ann Thorac Surg 2011; 92(2):595–601. doi:10.1016/j.athoracsur.2011.04.023
- Prowle JR, Kam EP, Ahmad T, Smith NC, Protopapa K, Pearse RM. Preoperative renal dysfunction and mortality after non-cardiac surgery. Br J Surg 2016; 103(10):1316–1325. doi:10.1002/bjs.10186
- Gaber AO, Moore LW, Aloia TA, et al. Cross-sectional and case-control analyses of the association of kidney function staging with adverse postoperative outcomes in general and vascular surgery. Ann Surg 2013; 258(1):169–177. doi:10.1097/SLA.0b013e318288e18e
- Mases A, Sabaté S, Guilera N, et al. Preoperative estimated glomerular filtration rate and the risk of major adverse cardiovascular and cerebrovascular events in non-cardiac surgery. Br J Anaesth 2014; 113(4):644–651. doi:10.1093/bja/aeu134
- Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clinical Practice 2012; 120(4):c179–c184. doi:10.1159/000339789
- Pickering JW, James MT, Palmer SC. Acute kidney injury and prognosis after cardiopulmonary bypass: a meta-analysis of cohort studies. Am J Kidney Dis 2015; 65(2):283–293. doi:10.1053/j.ajkd.2014.09.008
- Dasta JF, Kane-Gill SL, Durtschi AJ, Pathak DS, Kellum JA. Costs and outcomes of acute kidney injury (AKI) following cardiac surgery. Nephrol Dial Transplant 2008; 23(6):1970-1974. doi:10.1093/ndt/gfm908
- Karkouti K, Wijeysundera DN, Yau TM, et al. Acute kidney injury after cardiac surgery focus on modifiable risk factors. Circulation 2009; 119(4):495–502. doi:10.1161/CIRCULATIONAHA.108.786913
- Xu JR, Zhu JM, Jiang J, et al. Risk factors for long-term mortality and progressive chronic kidney disease associated with acute kidney injury after cardiac surgery. Medicine (Baltimore) 2015; 94(45):e2025. doi:10.1097/MD.0000000000002025
- Chalmers J, Mediratta N, McShane J, Shaw M, Pullan M, Poullis M. The long-term effects of developing renal failure post-coronary artery bypass surgery, in patients with normal preoperative renal function. Eur J Cardiothorac Surg 2013; 43(3):555–559. doi:10.1093/ejcts/ezs329
- Ryden L, Sartipy U, Evans M, Holzmann MJ. Acute kidney injury after coronary artery bypass grafting and long-term risk of end-stage renal disease. Circulation 2014; 130(23):2005–2011. doi:10.1161/CIRCULATIONAHA.114.010622
- Gargiulo G, Capodanno D, Sannino A, et al. Impact of moderate preoperative chronic kidney disease on mortality after transcatheter aortic valve implantation. Int J Cardiol 2015; 189:77–78. doi:10.1016/j.ijcard.2015.04.077
- Gargiulo G, Capodanno D, Sannino A, et al. Moderate and severe preoperative chronic kidney disease worsen clinical outcomes after transcatheter aortic valve implantation meta-analysis of 4,992 patients. Circ Cardiovasc Interv 2015; 8(2):e002220. doi:10.1161/CIRCINTERVENTIONS.114.002220
- Han SS, Shin N, Baek SH, et al. Effects of acute kidney injury and chronic kidney disease on long-term mortality after coronary artery bypass grafting. Am Heart J 2015; 169(3):419–425. doi:10.1016/j.ahj.2014.12.019
- Aronson S, Fontes ML, Miao Y, Mangano DT; Investigators of the Multicenter Study of Perioperative Ischemia Research Group; Ischemia Research and Education Foundation. Risk index for perioperative renal dysfunction/failure: critical dependence on pulse pressure hypertension. Circulation 2007; 115(6):733–742. doi:10.1161/CIRCULATIONAHA.106.623538
- Hertzberg D, Sartipy U, Holzmann MJ. Type 1 and type 2 diabetes mellitus and risk of acute kidney injury after coronary artery bypass grafting. Am Heart J 2015; 170(5):895–902. doi:10.1016/j.ahj.2015.08.013
- Benedetto U, Sciarretta S, Roscitano A, et al. Preoperative angiotensin-converting enzyme inhibitors and acute kidney injury after coronary artery bypass grafting. Ann Thorac Surg 2008; 86(4):1160–1165. doi:10.1016/j.athoracsur.2008.06.018
- Arora P, Rajagopalam S, Ranjan R, et al. Preoperative use of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers is associated with increased risk for acute kidney injury after cardiovascular surgery. Clin J Am Soc Nephrol 2008; 3(5):1266–1273. doi:10.2215/CJN.05271107
- Haase M, Bellomo R, Story D, et al. Effect of mean arterial pressure, haemoglobin and blood transfusion during cardiopulmonary bypass on post-operative acute kidney injury. Nephrol Dial Transplant 2012; 27(1):153–160. doi:10.1093/ndt/gfr275
- Ono M, Arnaoutakis GJ, Fine DM, et al. Blood pressure excursions below the cerebral autoregulation threshold during cardiac surgery are associated with acute kidney injury. Crit Care Med 2013; 41(2):464-471. doi:10.1097/CCM.0b013e31826ab3a1
- Seabra VF, Alobaidi S, Balk EM, Poon AH, Jaber BL. Off-pump coronary artery bypass surgery and acute kidney injury: a meta-analysis of randomized controlled trials. Clin J Am Soc Nephrol 2010; 5(10):1734–1744. doi:10.2215/CJN.02800310
- Garg AX, Devereaux PJ, Yusuf S, et al; CORONARY Investigators. Kidney function after off-pump or on-pump coronary artery bypass graft surgery: a randomized clinical trial. JAMA 2014; 311(21):2191–2198. doi:10.1001/jama.2014.4952
- Kumar AB, Suneja M, Bayman EO, Weide GD, Tarasi M. Association between postoperative acute kidney injury and duration of cardiopulmonary bypass: a meta-analysis. J Cardiothorac Vasc Anesth 2012; 26(1):64–69. doi:10.1053/j.jvca.2011.07.007
- Kheterpal S, Tremper KK, Englesbe MJ, et al. Predictors of postoperative acute renal failure after noncardiac surgery in patients with previously normal renal function. Anesthesiology 2007; 107(6):892–902. doi:10.1097/01.anes.0000290588.29668.38
- Grams ME, Sang Y, Coresh J, et al. Acute kidney injury after major surgery: a retrospective analysis of Veterans Health Administration data. Am J Kidney Dis 2016; 67(6):872–880. doi:10.1053/j.ajkd.2015.07.022
- Biteker M, Dayan A, Tekkesin AI, et al. Incidence, risk factors, and outcomes of perioperative acute kidney injury in noncardiac and nonvascular surgery. Am J Surg 2014: 207(1):53–59. doi:10.1016/j.amjsurg.2013.04.006
- Gu W-J, Hou B-L, Kwong JS, et al. Association between intraoperative hypotension and 30-day mortality, major adverse cardiac events, and acute kidney injury after non-cardiac surgery: a meta-analysis of cohort studies. Int J Cardiol 2018; 258:68–73. doi:10.1016/j.ijcard.2018.01.137
- Smetana GW, Macpherson DS. The case against routine preoperative laboratory testing. Med Clin North Am 2003; 87(1):7–40. pmid:12575882
- Perrotti A, Miltgen G, Chevet-Noel A, et al. Neutrophil gelatinase-associated lipocalin as early predictor of acute kidney injury after cardiac surgery in adults with chronic kidney failure. Ann Thorac Surg 2015; 99(3):864–869. doi:10.1016/j.athoracsur.2014.10.011
- Doi K, Urata M, Katagiri D, et al. Plasma neutrophil gelatinase-associated lipocalin in acute kidney injury superimposed on chronic kidney disease after cardiac surgery: a multicenter prospective study. Crit Care 2013; 17(6):R270. doi:10.1186/cc13104
- Ho J, Tangri N, Komenda P, et al. Urinary, plasma, and serum biomarkers’ utility for predicting acute kidney injury associated with cardiac surgery in adults: a meta-analysis. Am J Kidney Dis 2015; 66(6):993–1005. doi:10.1053/j.ajkd.2015.06.018
- Yao L, Young N, Liu H, et al. Evidence for preoperative aspirin improving major outcomes in patients with chronic kidney disease undergoing cardiac surgery: a cohort study. Ann Surg 2015; 261(1):207–212. doi:10.1097/SLA.0000000000000641
- Garg AX, Kurz A, Sessler DI, et al; POISE-2 Investigators. Aspirin and clonidine in non-cardiac surgery: acute kidney injury substudy protocol of the perioperative ischaemic evaluation (POISE) 2 randomised controlled trial. BMJ open 2014; 4(2):e004886. doi:10.1136/bmjopen-2014-004886
- He SJ, Liu Q, Li HQ, Tian F, Chen SY, Weng JX. Role of statins in preventing cardiac surgery-associated acute kidney injury: an updated meta-analysis of randomized controlled trials. Ther Clin Risk Manag 2018; 14:475–482. doi:10.2147/TCRM.S160298
- Tie HT, Luo MZ, Lin D, Zhang M, Wan JY, Wu QC. Erythropoietin administration for prevention of cardiac surgery-associated acute kidney injury: a meta-analysis of randomized controlled trials. Eur J Cardiothorac Surg 2015; 48(1):32–39. doi:10.1093/ejcts/ezu378
- Santana-Santos E, Gowdak LH, Gaiotto FA, et al. High dose of N-acetylcystein prevents acute kidney injury in chronic kidney disease patients undergoing myocardial revascularization. Ann Thorac Surg 2014; 97(5):1617–1623. doi:10.1016/j.athoracsur.2014.01.056
- Mei M, Zhao HW, Pan QG, Pu YM, Tang MZ, Shen BB. Efficacy of N-acetylcysteine in preventing acute kidney injury after cardiac surgery: a meta-analysis study. J Invest Surg 2018; 31(1):14–23. doi:10.1080/08941939.2016.1269853
- Sezai A, Hata M, Niino T, et al. Results of low-dose human atrial natriuretic peptide infusion in nondialysis patients with chronic kidney disease undergoing coronary artery bypass grafting: the NU-HIT (Nihon University working group study of low-dose HANP infusion therapy during cardiac surgery) trial for CKD. J Am Coll Cardiol 2011; 58(9):897–903. doi:10.1016/j.jacc.2011.03.056
- Xu N, Long Q, He T, et al. Association between preoperative renin-angiotensin system inhibitor use and postoperative acute kidney injury risk in patients with hypertension. Clin Nephrol 2018; 89(6):403–414. doi:10.5414/CN109319
- Liu Y, Sheng B, Wang S, Lu F, Zhen J, Chen W. Dexmedetomidine prevents acute kidney injury after adult cardiac surgery: a meta-analysis of randomized controlled trials. BMC Anesthesiol 2018; 18(1):7. doi:10.1186/s12871-018-0472-1
- Shi R, Tie H-T. Dexmedetomidine as a promising prevention strategy for cardiac surgery-associated acute kidney injury: a meta-analysis. Critical Care 2017; 21(1):198. doi:10.1186/s13054-017-1776-0
- Zhou C, Gong J, Chen D, Wang W, Liu M, Liu B. Levosimendan for prevention of acute kidney injury after cardiac surgery: a meta-analysis of randomized controlled trials. Am J Kidney Dis 2016; 67(3):408–416. doi:10.1053/j.ajkd.2015.09.015
- Elbadawi A, Elgendy IY, Saad M, et al. Meta-analysis of trials on prophylactic use of levosimendan in patients undergoing cardiac surgery. Ann Thorac Surg 2018; 105(5):1403–1410. doi:10.1016/j.athoracsur.2017.11.027
- Zarbock A, Schmidt C, Van Aken H, et al; RenalRIPC Investigators. Effect of remote ischemic preconditioning on kidney injury among high-risk patients undergoing cardiac surgery: a randomized clinical trial. JAMA 2015; 313(21):2133–2141. doi:10.1001/jama.2015.4189
- Venugopal V, Laing CM, Ludman A, Yellon DM, Hausenloy D. Effect of remote ischemic preconditioning on acute kidney injury in nondiabetic patients undergoing coronary artery bypass graft surgery: a secondary analysis of 2 small randomized trials. Am J Kidney Dis 2010; 56(6):1043–1049. doi:10.1053/j.ajkd.2010.07.014
- Futier E, Constantin JM, Petit A, et al. Conservative vs restrictive individualized goal-directed fluid replacement strategy in major abdominal surgery: a prospective randomized trial. Arch Surg 2010; 145(12):1193–1200. doi:10.1001/archsurg.2010.275
- Patel A, Prowle JR, Ackland GL. Postoperative goal-directed therapy and development of acute kidney injury following major elective noncardiac surgery: post-hoc analysis of POM-O randomized controlled trial. Clin Kidney J 2017; 10(3):348–356. doi:10.1093/ckj/sfw118
- Shen Y, Zhang W, Cheng X, Ying M. Association between postoperative fluid balance and acute kidney injury in patients after cardiac surgery: a retrospective cohort study. J Crit Care 2018; 44:273–277. doi:10.1016/j.jcrc.2017.11.041
- Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA 2007; 298(17):2038–2047. doi:10.1001/jama.298.17.2038
- National Institute of Diabetes and Digestive and Kidney Diseases. Kidney Disease Statistics for the United States. www.niddk.nih.gov/health-information/health-statistics/kidney-disease. Accessed June 11, 2018.
- Rosner MH, Okusa MD. Acute kidney injury associated with cardiac surgery. Clin J Am Soc Nephrol 2006; 1(1):19–32. doi:10.2215/CJN.00240605
- Meersch M, Schmidt C, Zarbock A. Patient with chronic renal failure undergoing surgery. Curr Opin Anaesthesiol 2016; 29(3):413–420. doi:10.1097/ACO.0000000000000329
- Stevens PE, Levin A; Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members. Evaluation and management of chronic kidney disease: synopsis of the Kidney Disease: Improving Global Outcomes 2012 clinical practice guideline. Ann Intern Med 2013; 158(11):825–830. doi:10.7326/0003-4819-158-11-201306040-00007
- Levey AS, Eckardt KU, Tsukamoto Y, et al. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 2005; 67(6):2089–2100. doi:10.1111/j.1523-1755.2005.00365.x
- Saitoh M, Takahashi T, Sakurada K, et al. Factors determining achievement of early postoperative cardiac rehabilitation goal in patients with or without preoperative kidney dysfunction undergoing isolated cardiac surgery. J Cardiol 2013; 61(4):299–303. doi:10.1016/j.jjcc.2012.12.014
- Minakata K, Bando K, Tanaka S, et al. Preoperative chronic kidney disease as a strong predictor of postoperative infection and mortality after coronary artery bypass grafting. Circ J 2014; 78(9):2225–2231. doi:10.1253/circj.CJ-14-0328
- Domoto S, Tagusari O, Nakamura Y, et al. Preoperative estimated glomerular filtration rate as a significant predictor of long-term outcomes after coronary artery bypass grafting in Japanese patients. Gen Thorac Cardiovasc Surg 2014; 62(2):95–102. doi:10.1007/s11748-013-0306-5
- Hedley AJ, Roberts MA, Hayward PA, et al. Impact of chronic kidney disease on patient outcome following cardiac surgery. Heart Lung Circ 2010; 19(8):453–459. doi:10.1016/j.hlc.2010.03.005
- Boulton BJ, Kilgo P, Guyton RA, et al. Impact of preoperative renal dysfunction in patients undergoing off-pump versus on-pump coronary artery bypass. Ann Thorac Surg 2011; 92(2):595–601. doi:10.1016/j.athoracsur.2011.04.023
- Prowle JR, Kam EP, Ahmad T, Smith NC, Protopapa K, Pearse RM. Preoperative renal dysfunction and mortality after non-cardiac surgery. Br J Surg 2016; 103(10):1316–1325. doi:10.1002/bjs.10186
- Gaber AO, Moore LW, Aloia TA, et al. Cross-sectional and case-control analyses of the association of kidney function staging with adverse postoperative outcomes in general and vascular surgery. Ann Surg 2013; 258(1):169–177. doi:10.1097/SLA.0b013e318288e18e
- Mases A, Sabaté S, Guilera N, et al. Preoperative estimated glomerular filtration rate and the risk of major adverse cardiovascular and cerebrovascular events in non-cardiac surgery. Br J Anaesth 2014; 113(4):644–651. doi:10.1093/bja/aeu134
- Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clinical Practice 2012; 120(4):c179–c184. doi:10.1159/000339789
- Pickering JW, James MT, Palmer SC. Acute kidney injury and prognosis after cardiopulmonary bypass: a meta-analysis of cohort studies. Am J Kidney Dis 2015; 65(2):283–293. doi:10.1053/j.ajkd.2014.09.008
- Dasta JF, Kane-Gill SL, Durtschi AJ, Pathak DS, Kellum JA. Costs and outcomes of acute kidney injury (AKI) following cardiac surgery. Nephrol Dial Transplant 2008; 23(6):1970-1974. doi:10.1093/ndt/gfm908
- Karkouti K, Wijeysundera DN, Yau TM, et al. Acute kidney injury after cardiac surgery focus on modifiable risk factors. Circulation 2009; 119(4):495–502. doi:10.1161/CIRCULATIONAHA.108.786913
- Xu JR, Zhu JM, Jiang J, et al. Risk factors for long-term mortality and progressive chronic kidney disease associated with acute kidney injury after cardiac surgery. Medicine (Baltimore) 2015; 94(45):e2025. doi:10.1097/MD.0000000000002025
- Chalmers J, Mediratta N, McShane J, Shaw M, Pullan M, Poullis M. The long-term effects of developing renal failure post-coronary artery bypass surgery, in patients with normal preoperative renal function. Eur J Cardiothorac Surg 2013; 43(3):555–559. doi:10.1093/ejcts/ezs329
- Ryden L, Sartipy U, Evans M, Holzmann MJ. Acute kidney injury after coronary artery bypass grafting and long-term risk of end-stage renal disease. Circulation 2014; 130(23):2005–2011. doi:10.1161/CIRCULATIONAHA.114.010622
- Gargiulo G, Capodanno D, Sannino A, et al. Impact of moderate preoperative chronic kidney disease on mortality after transcatheter aortic valve implantation. Int J Cardiol 2015; 189:77–78. doi:10.1016/j.ijcard.2015.04.077
- Gargiulo G, Capodanno D, Sannino A, et al. Moderate and severe preoperative chronic kidney disease worsen clinical outcomes after transcatheter aortic valve implantation meta-analysis of 4,992 patients. Circ Cardiovasc Interv 2015; 8(2):e002220. doi:10.1161/CIRCINTERVENTIONS.114.002220
- Han SS, Shin N, Baek SH, et al. Effects of acute kidney injury and chronic kidney disease on long-term mortality after coronary artery bypass grafting. Am Heart J 2015; 169(3):419–425. doi:10.1016/j.ahj.2014.12.019
- Aronson S, Fontes ML, Miao Y, Mangano DT; Investigators of the Multicenter Study of Perioperative Ischemia Research Group; Ischemia Research and Education Foundation. Risk index for perioperative renal dysfunction/failure: critical dependence on pulse pressure hypertension. Circulation 2007; 115(6):733–742. doi:10.1161/CIRCULATIONAHA.106.623538
- Hertzberg D, Sartipy U, Holzmann MJ. Type 1 and type 2 diabetes mellitus and risk of acute kidney injury after coronary artery bypass grafting. Am Heart J 2015; 170(5):895–902. doi:10.1016/j.ahj.2015.08.013
- Benedetto U, Sciarretta S, Roscitano A, et al. Preoperative angiotensin-converting enzyme inhibitors and acute kidney injury after coronary artery bypass grafting. Ann Thorac Surg 2008; 86(4):1160–1165. doi:10.1016/j.athoracsur.2008.06.018
- Arora P, Rajagopalam S, Ranjan R, et al. Preoperative use of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers is associated with increased risk for acute kidney injury after cardiovascular surgery. Clin J Am Soc Nephrol 2008; 3(5):1266–1273. doi:10.2215/CJN.05271107
- Haase M, Bellomo R, Story D, et al. Effect of mean arterial pressure, haemoglobin and blood transfusion during cardiopulmonary bypass on post-operative acute kidney injury. Nephrol Dial Transplant 2012; 27(1):153–160. doi:10.1093/ndt/gfr275
- Ono M, Arnaoutakis GJ, Fine DM, et al. Blood pressure excursions below the cerebral autoregulation threshold during cardiac surgery are associated with acute kidney injury. Crit Care Med 2013; 41(2):464-471. doi:10.1097/CCM.0b013e31826ab3a1
- Seabra VF, Alobaidi S, Balk EM, Poon AH, Jaber BL. Off-pump coronary artery bypass surgery and acute kidney injury: a meta-analysis of randomized controlled trials. Clin J Am Soc Nephrol 2010; 5(10):1734–1744. doi:10.2215/CJN.02800310
- Garg AX, Devereaux PJ, Yusuf S, et al; CORONARY Investigators. Kidney function after off-pump or on-pump coronary artery bypass graft surgery: a randomized clinical trial. JAMA 2014; 311(21):2191–2198. doi:10.1001/jama.2014.4952
- Kumar AB, Suneja M, Bayman EO, Weide GD, Tarasi M. Association between postoperative acute kidney injury and duration of cardiopulmonary bypass: a meta-analysis. J Cardiothorac Vasc Anesth 2012; 26(1):64–69. doi:10.1053/j.jvca.2011.07.007
- Kheterpal S, Tremper KK, Englesbe MJ, et al. Predictors of postoperative acute renal failure after noncardiac surgery in patients with previously normal renal function. Anesthesiology 2007; 107(6):892–902. doi:10.1097/01.anes.0000290588.29668.38
- Grams ME, Sang Y, Coresh J, et al. Acute kidney injury after major surgery: a retrospective analysis of Veterans Health Administration data. Am J Kidney Dis 2016; 67(6):872–880. doi:10.1053/j.ajkd.2015.07.022
- Biteker M, Dayan A, Tekkesin AI, et al. Incidence, risk factors, and outcomes of perioperative acute kidney injury in noncardiac and nonvascular surgery. Am J Surg 2014: 207(1):53–59. doi:10.1016/j.amjsurg.2013.04.006
- Gu W-J, Hou B-L, Kwong JS, et al. Association between intraoperative hypotension and 30-day mortality, major adverse cardiac events, and acute kidney injury after non-cardiac surgery: a meta-analysis of cohort studies. Int J Cardiol 2018; 258:68–73. doi:10.1016/j.ijcard.2018.01.137
- Smetana GW, Macpherson DS. The case against routine preoperative laboratory testing. Med Clin North Am 2003; 87(1):7–40. pmid:12575882
- Perrotti A, Miltgen G, Chevet-Noel A, et al. Neutrophil gelatinase-associated lipocalin as early predictor of acute kidney injury after cardiac surgery in adults with chronic kidney failure. Ann Thorac Surg 2015; 99(3):864–869. doi:10.1016/j.athoracsur.2014.10.011
- Doi K, Urata M, Katagiri D, et al. Plasma neutrophil gelatinase-associated lipocalin in acute kidney injury superimposed on chronic kidney disease after cardiac surgery: a multicenter prospective study. Crit Care 2013; 17(6):R270. doi:10.1186/cc13104
- Ho J, Tangri N, Komenda P, et al. Urinary, plasma, and serum biomarkers’ utility for predicting acute kidney injury associated with cardiac surgery in adults: a meta-analysis. Am J Kidney Dis 2015; 66(6):993–1005. doi:10.1053/j.ajkd.2015.06.018
- Yao L, Young N, Liu H, et al. Evidence for preoperative aspirin improving major outcomes in patients with chronic kidney disease undergoing cardiac surgery: a cohort study. Ann Surg 2015; 261(1):207–212. doi:10.1097/SLA.0000000000000641
- Garg AX, Kurz A, Sessler DI, et al; POISE-2 Investigators. Aspirin and clonidine in non-cardiac surgery: acute kidney injury substudy protocol of the perioperative ischaemic evaluation (POISE) 2 randomised controlled trial. BMJ open 2014; 4(2):e004886. doi:10.1136/bmjopen-2014-004886
- He SJ, Liu Q, Li HQ, Tian F, Chen SY, Weng JX. Role of statins in preventing cardiac surgery-associated acute kidney injury: an updated meta-analysis of randomized controlled trials. Ther Clin Risk Manag 2018; 14:475–482. doi:10.2147/TCRM.S160298
- Tie HT, Luo MZ, Lin D, Zhang M, Wan JY, Wu QC. Erythropoietin administration for prevention of cardiac surgery-associated acute kidney injury: a meta-analysis of randomized controlled trials. Eur J Cardiothorac Surg 2015; 48(1):32–39. doi:10.1093/ejcts/ezu378
- Santana-Santos E, Gowdak LH, Gaiotto FA, et al. High dose of N-acetylcystein prevents acute kidney injury in chronic kidney disease patients undergoing myocardial revascularization. Ann Thorac Surg 2014; 97(5):1617–1623. doi:10.1016/j.athoracsur.2014.01.056
- Mei M, Zhao HW, Pan QG, Pu YM, Tang MZ, Shen BB. Efficacy of N-acetylcysteine in preventing acute kidney injury after cardiac surgery: a meta-analysis study. J Invest Surg 2018; 31(1):14–23. doi:10.1080/08941939.2016.1269853
- Sezai A, Hata M, Niino T, et al. Results of low-dose human atrial natriuretic peptide infusion in nondialysis patients with chronic kidney disease undergoing coronary artery bypass grafting: the NU-HIT (Nihon University working group study of low-dose HANP infusion therapy during cardiac surgery) trial for CKD. J Am Coll Cardiol 2011; 58(9):897–903. doi:10.1016/j.jacc.2011.03.056
- Xu N, Long Q, He T, et al. Association between preoperative renin-angiotensin system inhibitor use and postoperative acute kidney injury risk in patients with hypertension. Clin Nephrol 2018; 89(6):403–414. doi:10.5414/CN109319
- Liu Y, Sheng B, Wang S, Lu F, Zhen J, Chen W. Dexmedetomidine prevents acute kidney injury after adult cardiac surgery: a meta-analysis of randomized controlled trials. BMC Anesthesiol 2018; 18(1):7. doi:10.1186/s12871-018-0472-1
- Shi R, Tie H-T. Dexmedetomidine as a promising prevention strategy for cardiac surgery-associated acute kidney injury: a meta-analysis. Critical Care 2017; 21(1):198. doi:10.1186/s13054-017-1776-0
- Zhou C, Gong J, Chen D, Wang W, Liu M, Liu B. Levosimendan for prevention of acute kidney injury after cardiac surgery: a meta-analysis of randomized controlled trials. Am J Kidney Dis 2016; 67(3):408–416. doi:10.1053/j.ajkd.2015.09.015
- Elbadawi A, Elgendy IY, Saad M, et al. Meta-analysis of trials on prophylactic use of levosimendan in patients undergoing cardiac surgery. Ann Thorac Surg 2018; 105(5):1403–1410. doi:10.1016/j.athoracsur.2017.11.027
- Zarbock A, Schmidt C, Van Aken H, et al; RenalRIPC Investigators. Effect of remote ischemic preconditioning on kidney injury among high-risk patients undergoing cardiac surgery: a randomized clinical trial. JAMA 2015; 313(21):2133–2141. doi:10.1001/jama.2015.4189
- Venugopal V, Laing CM, Ludman A, Yellon DM, Hausenloy D. Effect of remote ischemic preconditioning on acute kidney injury in nondiabetic patients undergoing coronary artery bypass graft surgery: a secondary analysis of 2 small randomized trials. Am J Kidney Dis 2010; 56(6):1043–1049. doi:10.1053/j.ajkd.2010.07.014
- Futier E, Constantin JM, Petit A, et al. Conservative vs restrictive individualized goal-directed fluid replacement strategy in major abdominal surgery: a prospective randomized trial. Arch Surg 2010; 145(12):1193–1200. doi:10.1001/archsurg.2010.275
- Patel A, Prowle JR, Ackland GL. Postoperative goal-directed therapy and development of acute kidney injury following major elective noncardiac surgery: post-hoc analysis of POM-O randomized controlled trial. Clin Kidney J 2017; 10(3):348–356. doi:10.1093/ckj/sfw118
- Shen Y, Zhang W, Cheng X, Ying M. Association between postoperative fluid balance and acute kidney injury in patients after cardiac surgery: a retrospective cohort study. J Crit Care 2018; 44:273–277. doi:10.1016/j.jcrc.2017.11.041
KEY POINTS
- Many patients undergoing surgery have CKD—up to 30% in some cardiac surgery populations.
- CKD is a risk factor for perioperative complications including acute kidney injury and death.
- Although challenging, early detection of renal injury is crucial to improving outcomes in this patient population. New biomarkers are being investigated.
- Preoperative assessment and perioperative management of renal dysfunction may reduce the risk of adverse postoperative outcomes.
In reply: Perioperative interruption of dual antiplatelet therapy
In Reply: We reported on publications from 2016–2017 and, unfortunately, at the time we were writing our paper, the European Society of Cardiology (ESC) update on dual antiplatelet therapy1 had not yet been published. We presented the recommendations from the American College of Cardiology (ACC) and American Heart Association (AHA),2 which differ from the recently published ESC guidelines. The ESC suggests that the minimum waiting period after drug-eluting stent placement before noncardiac surgery should be 1 month rather than 3 months but acknowledges that in the setting of complex stenting or recent acute coronary syndrome, 6 months is preferred. The recommendation in this latter scenario is a class IIb C recommendation—essentially expert consensus opinion.
Further, in the study by Egholm et al,3 the event rates in patients undergoing noncardiac surgery in the 1- to 2-month period were numerically higher than in the control group, and no adjusted odds ratios were given. The numbers of events were very low, and a change of only 1 or 2 events in the other direction in the groups would likely make it statistically significant.
All of these recommendations are based on observational studies and registry data, as there are no randomized controlled trials to address this issue. There are many complexities to be accounted for including the type of stent, timing, circumstances surrounding stenting, anatomy, number of stents, patient comorbidities (particularly age, diabetes mellitus, cardiac disease), type of surgery and anesthesia, and perioperative management of antiplatelet therapy. While we acknowledge the ESC recommendation, we would urge caution in the recommendation to wait only 1 month, and in the United States most would prefer to wait 3 months if possible.
- Valgimigli M, Bueno H, Byrne RA, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2018; 39(3):213–260. doi:10.1093/eurheartj/ehx419
- Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease. Circulation 2016; 134(10):e123–e155. doi:10.1161/CIR.0000000000000404
- Egholm G, Kristensen SD, Thim T, et al. Risk associated with surgery within 12 months after coronary drug-eluting stent implantation. J Am Coll Cardiol 2016; 68(24):2622–2632. doi:10.1016/j.jacc.2016.09.967
In Reply: We reported on publications from 2016–2017 and, unfortunately, at the time we were writing our paper, the European Society of Cardiology (ESC) update on dual antiplatelet therapy1 had not yet been published. We presented the recommendations from the American College of Cardiology (ACC) and American Heart Association (AHA),2 which differ from the recently published ESC guidelines. The ESC suggests that the minimum waiting period after drug-eluting stent placement before noncardiac surgery should be 1 month rather than 3 months but acknowledges that in the setting of complex stenting or recent acute coronary syndrome, 6 months is preferred. The recommendation in this latter scenario is a class IIb C recommendation—essentially expert consensus opinion.
Further, in the study by Egholm et al,3 the event rates in patients undergoing noncardiac surgery in the 1- to 2-month period were numerically higher than in the control group, and no adjusted odds ratios were given. The numbers of events were very low, and a change of only 1 or 2 events in the other direction in the groups would likely make it statistically significant.
All of these recommendations are based on observational studies and registry data, as there are no randomized controlled trials to address this issue. There are many complexities to be accounted for including the type of stent, timing, circumstances surrounding stenting, anatomy, number of stents, patient comorbidities (particularly age, diabetes mellitus, cardiac disease), type of surgery and anesthesia, and perioperative management of antiplatelet therapy. While we acknowledge the ESC recommendation, we would urge caution in the recommendation to wait only 1 month, and in the United States most would prefer to wait 3 months if possible.
In Reply: We reported on publications from 2016–2017 and, unfortunately, at the time we were writing our paper, the European Society of Cardiology (ESC) update on dual antiplatelet therapy1 had not yet been published. We presented the recommendations from the American College of Cardiology (ACC) and American Heart Association (AHA),2 which differ from the recently published ESC guidelines. The ESC suggests that the minimum waiting period after drug-eluting stent placement before noncardiac surgery should be 1 month rather than 3 months but acknowledges that in the setting of complex stenting or recent acute coronary syndrome, 6 months is preferred. The recommendation in this latter scenario is a class IIb C recommendation—essentially expert consensus opinion.
Further, in the study by Egholm et al,3 the event rates in patients undergoing noncardiac surgery in the 1- to 2-month period were numerically higher than in the control group, and no adjusted odds ratios were given. The numbers of events were very low, and a change of only 1 or 2 events in the other direction in the groups would likely make it statistically significant.
All of these recommendations are based on observational studies and registry data, as there are no randomized controlled trials to address this issue. There are many complexities to be accounted for including the type of stent, timing, circumstances surrounding stenting, anatomy, number of stents, patient comorbidities (particularly age, diabetes mellitus, cardiac disease), type of surgery and anesthesia, and perioperative management of antiplatelet therapy. While we acknowledge the ESC recommendation, we would urge caution in the recommendation to wait only 1 month, and in the United States most would prefer to wait 3 months if possible.
- Valgimigli M, Bueno H, Byrne RA, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2018; 39(3):213–260. doi:10.1093/eurheartj/ehx419
- Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease. Circulation 2016; 134(10):e123–e155. doi:10.1161/CIR.0000000000000404
- Egholm G, Kristensen SD, Thim T, et al. Risk associated with surgery within 12 months after coronary drug-eluting stent implantation. J Am Coll Cardiol 2016; 68(24):2622–2632. doi:10.1016/j.jacc.2016.09.967
- Valgimigli M, Bueno H, Byrne RA, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2018; 39(3):213–260. doi:10.1093/eurheartj/ehx419
- Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease. Circulation 2016; 134(10):e123–e155. doi:10.1161/CIR.0000000000000404
- Egholm G, Kristensen SD, Thim T, et al. Risk associated with surgery within 12 months after coronary drug-eluting stent implantation. J Am Coll Cardiol 2016; 68(24):2622–2632. doi:10.1016/j.jacc.2016.09.967
2017 Update in perioperative medicine: 6 questions answered
Perioperative care is increasingly complex, and the rapid evolution of literature in this field makes it a challenge for clinicians to stay up-to-date. To help meet this challenge, we used a systematic approach to identify appropriate articles in the medical literature and then, by consensus, to develop a list of 6 clinical questions based on their novelty and potential to change perioperative medical practice:
- How should we screen for cardiac risk in patients undergoing noncardiac surgery?
- What is the appropriate timing for surgery after coronary intervention?
- Can we use statin therapy to reduce perioperative cardiac risk?
- How should we manage sleep apnea risk perioperatively?
- Which patients with atrial fibrillation should receive perioperative bridging anticoagulation?
- Is frailty screening beneficial for elderly patients before noncardiac surgery?
The summaries in this article are a composite of perioperative medicine updates presented at the Perioperative Medicine Summit and the annual meetings of the Society for General Internal Medicine and the Society of Hospital Medicine. “Perioperative care is complex and changing”1–10 (page 864) offers a brief overview.
HOW TO SCREEN FOR CARDIAC RISK BEFORE NONCARDIAC SURGERY
Perioperative cardiac risk can be estimated by clinical risk indexes (based on history, physical examination, common blood tests, and electrocardiography), cardiac biomarkers (natriuretic peptide or troponin levels), and noninvasive cardiac tests.

American and European guidelines
In 2014, the American College of Cardiology/American Heart Association2 and the European Society of Cardiology11 published guidelines on perioperative cardiovascular evaluation and management. They recommended several tools to calculate the risk of postoperative cardiac complications but did not specify a preference. These tools include:
- The Revised Cardiac Risk Index (RCRI)12 (www.mdcalc.com/revised-cardiac-risk-index-pre-operative-risk), which has been externally validated in multiple studies and is the most widely used
- The American College of Surgeons surgical risk calculator13 (www.riskcalculator.facs.org), derived from the National Surgery Quality Improvement Program (NSQIP) database
- The myocardial infarction or cardiac arrest (MICA) calculator14 (www.surgicalriskcalculator.com/miorcardiacarrest), also derived from the NSQIP database.
2017 Canadian guidelines differ
In 2017, the Canadian Cardiovascular Society published its own guidelines on perioperative risk assessment and management.1 These differ from the American and European guidelines on several points.
RCRI recommended. The Canadian guidelines suggested using the RCRI over the other risk predictors, which despite superior discrimination lacked external validation (conditional recommendation; low-quality evidence). Additionally, the Canadians believed that the NSQIP risk indexes underestimated cardiac risk because patients did not undergo routine biomarker screening.
Biomarker measurement. The Canadian guidelines went a step further in their algorithm (Figure 1) and recommended measuring N-terminal-pro B-type natriuretic peptide (NT-proBNP) or BNP preoperatively to improve risk prediction in 3 groups (strong recommendation; moderate-quality evidence):
- Patients ages 65 and older
- Patients ages 45 to 64 with significant cardiovascular disease
- Patients with an RCRI score of 1 or more.
This differs from the American guidelines, which did not recommend measuring preoperative biomarkers but did acknowledge that they may provide incremental value. The American College of Cardiology/American Heart Association authors felt that there were no data to suggest that targeting these biomarkers for treatment and intervention would reduce postoperative risk. The European guidelines did not recommend routinely using biomarkers, but stated that they may be considered in high-risk patients (who have a functional capacity ≤ 4 metabolic equivalents or an RCRI score > 1 undergoing vascular surgery, or > 2 undergoing nonvascular surgery).
Stress testing deemphasized. The Canadian guidelines recommended biomarker testing rather than noninvasive tests to enhance risk assessment based on cost, potential delays in surgery, and absence of evidence of an overall absolute net improvement in risk reclassification. This contrasts with the American and European guidelines and algorithms, which recommended pharmacologic stress testing in patients at elevated risk with poor functional capacity undergoing intermediate- to high-risk surgery if the results would change how they are managed.
Postoperative monitoring. The Canadian guidelines recommended that if patients have an NT-proBNP level higher than 300 mg/L or a BNP level higher than 92 mg/L, they should receive postoperative monitoring with electrocardiography in the postanesthesia care unit and daily troponin measurements for 48 to 72 hours. The American guidelines recommended postoperative electrocardiography and troponin measurement only for patients suspected of having myocardial ischemia, and the European guidelines said postoperative biomarkers may be considered in patients at high risk.
Physician judgment needed
While guidelines and risk calculators are potentially helpful in risk assessment, the lack of consensus and the conflicting recommendations force the physician to weigh the evidence and make individual decisions based on his or her interpretation of the data.
Until there are studies directly comparing the various risk calculators, physicians will most likely use the RCRI, which is simple and has been externally validated, in conjunction with the American guidelines.
At this time, it is unclear how biomarkers should be used—preoperatively, postoperatively, or both—because there are no studies demonstrating that management strategies based on the results lead to better outcomes. We do not believe that biomarker testing will be accepted in lieu of stress testing by our surgery, anesthesiology, or cardiology colleagues, but going forward, it will probably be used more frequently postoperatively, particularly in patients at moderate to high risk.
WHAT IS THE APPROPRIATE TIMING FOR SURGERY AFTER PCI?
A 2014 American College of Cardiology/American Heart Association guideline recommended delaying noncardiac surgery for 1 month after percutaneous coronary intervention (PCI) with bare-metal stents and 1 year after PCI with drug-eluting stents.15 The guideline suggested that surgery may be performed 6 months after drug-eluting stent placement if the risks of delaying surgery outweigh the risk of thrombosis.15
The primary rationale behind these timeframes was to provide dual antiplatelet therapy for a minimally acceptable duration before temporary interruption for a procedure. These recommendations were influenced largely by observational studies of first-generation devices, which are no longer used. Studies of newer-generation stents have suggested that the risk of stent thrombosis reaches a plateau considerably earlier than 6 to 12 months after PCI.
2016 Revised guideline on dual antiplatelet therapy
Although not separately delineated in the recommendations, risk factors for stent thrombosis that should influence the decision include smoking, multivessel coronary artery disease, and suboptimally controlled diabetes mellitus or hyperlipidemia.17 The presence of such stent thrombosis risk factors should be factored into the decision about proceeding with surgery within 3 to 6 months after drug-eluting stent placement.
Holcomb et al: Higher postoperative risk after PCI for myocardial infarction
Another important consideration is the indication for which PCI was performed. In a recent study, Holcomb et al16 found an association between postoperative major adverse cardiac events and PCI for myocardial infarction (MI) that was independent of stent type.
Compared with patients who underwent PCI not associated with acute coronary syndrome, the odds ratios and 95% confidence intervals (CIs) for major adverse cardiac events in those who underwent PCI for MI were:
- 5.25 (4.08–6.75) in the first 3 months
- 2.45 (1.80–3.35) in months 3 to 6
- 2.50 (1.90–3.28) in months 6 to 12.
In absolute terms, patients with stenting performed for an MI had an incidence of major adverse cardiac events of:
- 22.2% in the first 3 months
- 9.4% in months 3 to 6
- 5.8% in months 6 to 12
- 4.4% in months 12 to 24.
The perioperative risks were reduced after 12 months but still remained greater in patients whose PCI was performed for MI rather than another indication.16
The authors of this study suggested delaying noncardiac surgery for up to 6 months after PCI for MI, regardless of stent type.16
A careful, individualized approach
Optimal timing of noncardiac surgery PCI requires a careful, individualized approach and should always be coordinated with the patient’s cardiologist, surgeon, and anesthesiologist.3,15 For most patients, surgery should be delayed for 30 days after bare-metal stent placement and 6 months after drug-eluting stent placement.3 However, for those with greater surgical need and less thrombotic risk, noncardiac surgery can be considered 3 to 6 months after drug-eluting stent placement.3
Additional discussion of the prolonged increased risk of postoperative major adverse cardiac events is warranted in patients whose PCI was performed for MI, in whom delaying noncardiac surgery for up to 6 months (irrespective of stent type) should be considered.16
CAN WE USE STATINS TO REDUCE PERIOPERATIVE RISK?
Current recommendations from the American College of Cardiology/American Heart Association support continuing statins in the perioperative period, but the evidence supporting starting statins in this period has yet to be fully determined. In 2013, a Cochrane review18 found insufficient evidence to conclude that statins reduced perioperative adverse cardiac events, though several large studies were excluded due to controversial methods and data.
In contrast, the Vascular Events in Noncardiac Surgery Patients Cohort Evaluation (VISION) study,4 a multicenter, prospective, cohort-matched study of approximately 7,200 patients, found a lower risk of a composite primary outcome of all-cause mortality, myocardial injury after noncardiac surgery, or stroke at 30 days for patients exposed to statin therapy (relative risk [RR] 0.83, 95% CI 0.73–0.95, P = .007).4
London et al retrospective study: 30-day mortality rate is lower with statins
In 2017, London et al5 published the results of a very large retrospective, observational cohort study of approximately 96,000 elective or emergency surgery patients in Department of Veterans Affairs hospitals. The patients were propensity-matched and evaluated for exposure to statins on the day of or the day after surgery, for a total of approximately 48,000 pairs.
The primary outcome was death at 30 days, and statin exposure was associated with a significant reduction (RR 0.82; 95% CI 0.75–0.89; P < .001). Significant risk reductions were demonstrated in nearly all secondary end points as well, except for stroke or coma and thrombosis (pulmonary embolism, deep vein thrombosis, or graft failure). Overall, the number needed to treat to prevent any complication was 67. Statin therapy did not show significant harm, though on subgroup analysis, those who received high-intensity statin therapy had a slightly higher risk of renal injury (odds ratio 1.18, 95% CI 1.02–1.37, P = .03). Also on subgroup analysis, after propensity matching, patients on long-term moderate- or high-intensity statin therapy for 6 to 12 months before surgery had a small risk reduction for many of the outcomes, including death.
The authors also noted that only 62% of the patients who were prescribed statins as outpatients received them in the hospital, which suggests that improvement is necessary in educating perioperative physicians about the benefits and widespread support for continuing statins perioperatively.5
LOAD trial: No benefit from starting statins
Both London et al5 and the VISION investigators4 called for a large randomized controlled trial of perioperative statin initiation. The Lowering the Risk of Operative Complications Using Atorvastatin Loading Dose (LOAD) trial attempted to answer this call.6
This trial randomized 648 statin-naïve Brazilian patients at high risk of perioperative cardiac events to receive either atorvastatin or placebo before surgery and then continuously for another 7 days. The primary outcomes were the rates of death, nonfatal myocardial injury after noncardiac surgery, and cerebrovascular accident at 30 days.6
The investigators found no significant difference in outcomes between the two groups and estimated that the sample size would need to be approximately 7,000 patients to demonstrate a significant benefit. Nonetheless, this trial established that a prospective perioperative statin trial is feasible.
When to continue or start statins
Although we cannot recommend starting statins for all perioperative patients, perioperative statins clearly can carry significant benefit and should be continued in all patients who have been taking them. It is also likely beneficial to initiate statins in those patients who would otherwise warrant therapy based on the American College of Cardiology/American Heart Association Pooled Cohort Equations Risk calculator.19
HOW SHOULD WE MANAGE SLEEP APNEA RISK PERIOPERATIVELY?
From 20% to 30% of US men and 10% to 15% of US women have obstructive sleep apnea, and many are undiagnosed. Obstructive sleep apnea increases the risk of perioperative respiratory failure, unplanned reintubation, unplanned transfer to the intensive care unit, and death.20 Sentinel events (unexpected respiratory arrest after surgery on general surgical wards) have prompted the development of guidelines that aim to identify patients with previously undiagnosed obstructive sleep apnea before surgery and to develop approaches to reduce perioperative morbidity and mortality.
Kaw et al: Beware obesity hypoventilation syndrome
A 2016 study suggested that patients with obstructive sleep apnea and obesity hypoventilation syndrome may be at particularly high risk of perioperative complications.21
Kaw et al21 queried a database of patients with obstructive sleep apnea undergoing elective noncardiac surgery at Cleveland Clinic. All patients (N = 519) had obstructive sleep apnea confirmed by polysomnography, and a body mass index greater than 30 kg/m2. The authors considered a patient to have obesity hypoventilation syndrome (n = 194) if he or she also had hypercapnia (Paco2 ≥ 45 mm Hg) on at least 2 occasions before or after surgery.
In an adjusted analysis, the odds ratios and 95% CIs for adverse outcomes in patients with obesity hypoventilation syndrome were:
- 10.9 (3.7–32.3) for respiratory failure
- 5.4 (1.9–15.7) for heart failure
- 10.9 (3.7–32.3) for intensive care unit transfer.
The absolute increases in risk in the presence of obesity hypoventilation syndrome were:
- 19% (21% vs 2%) for respiratory failure
- 8% (8% vs 0) for heart failure
- 15% (21% vs 6%) for intensive care unit transfer.
There was no difference in rates of perioperative mortality.21
The authors proposed an algorithm to identify patients with possible obesity hypoventilation syndrome before surgery that included prior sleep study results, STOP-BANG score (Table 2),22 and serum bicarbonate level.
Important limitations of the study were that most patients with obesity hypoventilation syndrome were undiagnosed at the time of surgery. Still, the study does offer a tool to potentially identify patients at high risk for perioperative morbidity due to obesity hypoventilation syndrome. Clinicians could then choose to cancel nonessential surgery, propose a lower-risk alternative procedure, or maximize the use of strategies known to reduce perioperative risk for patients with obstructive sleep apnea in general.
Two guidelines on obstructive sleep apnea
Two professional societies have issued guidelines aiming to improve detection of previously undiagnosed obstructive sleep apnea and perioperative outcomes in patients known to have it or suspected of having it:
- The American Society of Anesthesiologists in 201423
- The Society of Anesthesia and Sleep Medicine in 2016.7
Both guidelines recommend that each institution develop a local protocol to screen patients for possible obstructive sleep apnea before elective surgery. The American Society of Anesthesiologists does not recommend any particular tool, but does recommend taking a history and performing a focused examination that includes evaluation of the airway, nasopharyngeal characteristics, neck circumference, and tonsil and tongue size. The Society of Anesthesia and Sleep Medicine recommends using a validated tool such as the STOP-BANG score to estimate the risk of obstructive sleep apnea.
If this screening suggests that a patient has obstructive sleep apnea, should surgery be delayed until a formal sleep study can be done? Or should the patient be treated empirically as if he or she has obstructive sleep apnea? Both professional societies recommend shared decision-making with the patient in this situation, with the Society of Anesthesia and Sleep Medicine recommending additional cardiopulmonary evaluation for patients with hypoventilation, severe pulmonary hypertension, or resting hypoxemia.
Both recommend using continuous positive airway pressure (CPAP) after surgery in patients with known obstructive sleep apnea, although there is not enough evidence to determine if empiric CPAP for screening-positive patients (without polysomnography-diagnosed obstructive sleep apnea) is beneficial. The Society of Anesthesia and Sleep Medicine advises that it is safe to proceed to surgery if obstructive sleep apnea is suspected as long as monitoring and risk-reduction strategies are implemented after surgery to reduce complication rates.
During surgery, the American Society of Anesthesiologists advises peripheral nerve blocks when appropriate, general anesthesia with a secure airway rather than deep sedation, capnography when using moderate sedation, awake extubation, and full reversal of neuromuscular blockade before extubation. After surgery, they recommend reducing opioid use, minimizing postoperative sedatives, supplemental oxygen, and continuous pulse oximetry. The Society of Anesthesia and Sleep Medicine guideline addresses preoperative assessment and therefore makes no recommendations regarding postoperative care.
In conclusion, use of pertinent findings from the history and physical examination and a validated obstructive sleep apnea screening tool such as STOP-BANG before surgery are recommended, with joint decision-making as to proceeding with surgery with empiric CPAP vs a formal sleep study for patients who screen as high risk. The Society of Anesthesia and Sleep Medicine recommends further cardiopulmonary evaluation if there is evidence of hypoventilation, hypoxemia, or pulmonary hypertension in addition to likely obstructive sleep apnea.
WHICH ATRIAL FIBRILLATION PATIENTS NEED BRIDGING ANTICOAGULATION?
When patients receiving anticoagulation need surgery, we need to carefully assess the risks of thromboembolism without anticoagulation vs bleeding with anticoagulation.
Historically, we tended to worry more about thromboembolism24; however, recent studies have revealed a significant risk of bleeding when long-term anticoagulant therapy is bridged (ie, interrupted and replaced with a shorter-acting agent in the perioperative period), with minimal to no decrease in thromboembolic events.25–27
American College of Cardiology guideline
In 2017, the American College of Cardiology8 published a guideline on periprocedural management of anticoagulation in patients with nonvalvular atrial fibrillation. The guideline includes a series of decision algorithms on whether and when to interrupt anticoagulation, whether and how to provide bridging anticoagulation, and how to restart postprocedural anticoagulation.
When deciding whether to interrupt anticoagulation, we need to consider the risk of bleeding posed both by patient-specific factors and by the type of surgery. Bridging anticoagulation is not indicated when direct oral anticoagulants (eg, dabigatran, apixaban, edoxaban, rivaroxaban) are interrupted for procedures.
Unlike an earlier guideline statement by the American College of Chest Physicians,24 this consensus statement emphasizes using the CHA2DS2-VASc score as a predictor of thromboembolic events rather than the CHADS2 core.
Table 3 summarizes the key points in the guidance statement about which patients should receive periprocedural bridging anticoagulation.
As evidence continues to evolve in this complicated area of perioperative medicine, it will remain important to continue to create patient management plans that take individual patient and procedural risks into account.
IS FRAILTY SCREENING BENEFICIAL BEFORE NONCARDIAC SURGERY?
Frailty, defined as a composite score of a patient’s age and comorbidities, has great potential to become an obligatory factor in perioperative risk assessment. However, it remains difficult to incorporate frailty scoring into clinical practice due to variations among scoring systems,28 uncertain outcome data, and the imprecise role of socioeconomic factors. In particular, the effect of frailty on perioperative mortality over longer periods of time is uncertain.
McIsaac et al: Higher risk in frail patients
McIsaac and colleagues at the University of Ottawa used a frailty scoring system developed at Johns Hopkins University to evaluate the effect of frailty on all-cause postoperative mortality in approximately 202,000 patients over a 10-year period.9 Although this scoring system is proprietary, it is based on factors such as malnutrition, dementia, impaired vision, decubitus ulcers, urinary incontinence, weight loss, poverty, barriers to access of care, difficulty in walking, and falls.
After adjusting for the procedure risk, patient age, sex, and neighborhood income quintile, the 1-year mortality risk was significantly higher in the frail group (absolute risk 13.6% vs 4.8%; adjusted hazard ratio 2.23; 95% CI 2.08–2.40). The risk of death in the first 3 days was much higher in frail than in nonfrail patients (hazard ratio 35.58; 95% CI 29.78–40.1), but the hazard ratio decreased to approximately 2.4 by day 90.
The authors emphasize that the elevated risk for frail patients warrants particular perioperative planning, though it is not yet clear what frailty-specific interventions should be performed. Further study is needed into the benefit of “prehabilitation” (ie, exercise training to “build up” a patient before surgery) for perioperative risk reduction.
Hall et al: Better care for frail patients
Hall et al10 instituted a quality improvement initiative for perioperative care of patients at the Omaha Veterans Affairs Hospital. Frail patients were identified using the Risk Analysis Index, a 14-question screening tool previously developed and validated over several years using Veterans Administration databases.29 Questions in the Risk Analysis Index cover living situation, any diagnosis of cancer, ability to perform activities of daily living, and others.
To maximize compliance, a Risk Analysis Index score was required to schedule a surgery. Patients with high scores underwent further review by a designated team of physicians who initiated informal and formal consultations with anesthesiologists, critical care physicians, surgeons, and palliative care providers, with the goals of minimizing risk, clarifying patient goals or resuscitation wishes, and developing comprehensive perioperative planning.10
Approximately 9,100 patients were included in the cohort. The authors demonstrated a significant improvement in mortality for frail patients at 30, 180, and 365 days, but noted an improvement in postoperative mortality for the nonfrail patients as well, perhaps due to increased focus on geriatric patient care. In particular, the mortality rate at 365 days dropped from 34.5% to 11.7% for frail patients who underwent this intervention.
While this quality improvement initiative was unable to examine how surgical rates changed in frail patients, it is highly likely that very high-risk patients opted out of surgery or had their surgical plan change, though the authors point out that the overall surgical volume at the institution did not change significantly. As well, it remains unclear which particular interventions may have had the most effect in improving survival, as the perioperative plans were individualized and continually adjusted throughout the study period.
Nonetheless, this article highlights how higher vigilance, individualized planning and appreciation of the high risks of frail patients is associated with improved patient survival postoperatively. Although frailty screening is still in its early stages and further work is needed, it is likely that performing frailty screening in elderly patients and utilizing interdisciplinary collaboration for comprehensive management of frail patients can improve their postoperative course.
- Duceppe E, Parlow J, MacDonald P, et al. Canadian Cardiovascular Society guidelines on perioperative cardiac risk assessment and management for patients who undergo noncardiac surgery. Can J Cardiol 2017; 33:17–32.
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol 2014; 64:2373–2405.
- Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease. Circulation 2016; 134:e123–e155.
- Berwanger O, Le Manach Y, Suzumura EA, et al. Association between pre-operative statin use and major cardiovascular complications among patients undergoing non-cardiac surgery: the VISION study. Eur Heart J 2016; 37:177–185.
- London MJ, Schwartz GG, Hur K, Henderson WG. Association of perioperative statin use with mortality and morbidity after major noncardiac surgery. JAMA Intern Med 2017; 177:231–242.
- Berwanger O, de Barros E Silva PG, Barbosa RR, et al. Atorvastatin for high-risk statin-naïve patients undergoing noncardiac surgery: the Lowering the Risk of Operative Complications Using Atorvastatin Loading Dose (LOAD) randomized trial. Am Heart J 2017; 184:88–96.
- Chung F, Memtsoudis SG, Ramachandran SK, et al. Society of Anesthesia and Sleep Medicine guidelines on preoperative screening and assessment of adult patients with obstructive sleep apnea. Anesth Analg 2016; 123:452–473.
- Doherty JU, Gluckman TJ, Hucker W, et al. 2017 ACC expert consensus decision pathway for periprocedural management of anticoagulation in patients with nonvalvular atrial fibrillation: a report of the American College of Cardiology Clinical Expert Consensus Document Task Force. J Am Coll Cardiol 2017; 69:871–898.
- McIsaac DI, Bryson GL, van Walraven C. Association of frailty and 1-year postoperative mortality following major elective noncardiac surgery: a population-based cohort study. JAMA Surg 2016; 151:538–545.
- Hall DE, Arya S, Schmid KK, et al. Association of a frailty screening initiative with postoperative survival at 30, 180, and 365 days. JAMA Surg 2017; 152:233–240.
- Kristensen SD, Knuuti J, Saraste A, et al. 2014 ESC/ESA Guidelines on non-cardiac surgery: cardiovascular assessment and management: The Joint Task Force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur Heart J 2014; 35:2383–2431.
- Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999; 100:1043–1049.
- Bilimoria KY, Liu Y, Paruch JL, Zhou L, Kmiecik TE, Ko CY, Cohen ME. Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aid and informed consent tool for patients and surgeons. J Am Coll Surg 2013; 217:833–842.
- Gupta PK, Gupta H, Sundaram A, et al. Development and validation of a risk calculator for prediction of cardiac risk after surgery. Circulation 2011; 124:381–387.
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; 64:e77–e137.
- Holcomb CN, Hollis RH, Graham LA, et al. Association of coronary stent indication with postoperative outcomes following noncardiac surgery. JAMA Surg 2016; 151:462–469.
- Lemesle G, Tricot O, Meurice T, et al. Incident myocardial infarction and very late stent thrombosis in outpatients with stable coronary artery disease. J Am Coll Cardiol 2017; 69:2149–2156.
- Sanders RD, Nicholson A, Lewis SR, Smith AF, Alderson P. Perioperative statin therapy for improving outcomes during and after noncardiac vascular surgery. Cochrane Database Syst Rev 2013; 7:CD009971.
- Goff DC, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; 63:2935–2959.
- Kaw R, Pasupuleti V, Walker E, et al. Postoperative complications in patients with obstructive sleep apnea. Chest 2012; 141:436–441.
- Kaw R, Bhateja P, Mar HP, et al. Postoperative complications in patients with unrecognized obesity hypoventilation syndrome undergoing elective noncardiac surgery. Chest 2016; 149:84–91.
- Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology 2008; 108:812–821.
- Gross JB, Apfelbaum JL, Caplan RA, et al. Practice guidelines for the perioperative management of patients with obstructive sleep apnea: an updated report by the American Society of Anesthesiologists Task Force on Perioperative Management of Patients with Obstructive Sleep Apnea. Anesthesiology 2014; 120:268–286.
- Douketis JD, Spyropoulos AC, Spencer FA, et al. Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(2 suppl):e326S–e350S.
- Siegal D, Yudin J, Kaatz S, Douketis JD, Lim W, Spyropoulos AC. Periprocedural heparin bridging in patients receiving vitamin K antagonists: systematic review and meta-analysis of bleeding and thromboembolic rates. Circulation 2012; 126:1630–1639.
- Clark NP, Witt DM, Davies LE, et al. Bleeding, recurrent venous thromboembolism, and mortality risks during warfarin interruption for invasive procedures. JAMA Intern Med 2015; 175:1163–1168.
- Douketis JD, Spyropoulos AC, Kaatz S, et al. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med 2015; 373:823–833.
- Theou O, Brothers TD, Mitnitski A, Rockwood K. Operationalization of frailty using eight commonly used scales and comparison of their ability to predict all-cause mortality. J Am Geriatr Soc 2013; 61:1537–1551.
- Hall DE, Arya S, Schmid KK, et al. Development and initial validation of the risk analysis index for measuring frailty in surgical populations. JAMA Surg 2017; 152:175–182.
Perioperative care is increasingly complex, and the rapid evolution of literature in this field makes it a challenge for clinicians to stay up-to-date. To help meet this challenge, we used a systematic approach to identify appropriate articles in the medical literature and then, by consensus, to develop a list of 6 clinical questions based on their novelty and potential to change perioperative medical practice:
- How should we screen for cardiac risk in patients undergoing noncardiac surgery?
- What is the appropriate timing for surgery after coronary intervention?
- Can we use statin therapy to reduce perioperative cardiac risk?
- How should we manage sleep apnea risk perioperatively?
- Which patients with atrial fibrillation should receive perioperative bridging anticoagulation?
- Is frailty screening beneficial for elderly patients before noncardiac surgery?
The summaries in this article are a composite of perioperative medicine updates presented at the Perioperative Medicine Summit and the annual meetings of the Society for General Internal Medicine and the Society of Hospital Medicine. “Perioperative care is complex and changing”1–10 (page 864) offers a brief overview.
HOW TO SCREEN FOR CARDIAC RISK BEFORE NONCARDIAC SURGERY
Perioperative cardiac risk can be estimated by clinical risk indexes (based on history, physical examination, common blood tests, and electrocardiography), cardiac biomarkers (natriuretic peptide or troponin levels), and noninvasive cardiac tests.

American and European guidelines
In 2014, the American College of Cardiology/American Heart Association2 and the European Society of Cardiology11 published guidelines on perioperative cardiovascular evaluation and management. They recommended several tools to calculate the risk of postoperative cardiac complications but did not specify a preference. These tools include:
- The Revised Cardiac Risk Index (RCRI)12 (www.mdcalc.com/revised-cardiac-risk-index-pre-operative-risk), which has been externally validated in multiple studies and is the most widely used
- The American College of Surgeons surgical risk calculator13 (www.riskcalculator.facs.org), derived from the National Surgery Quality Improvement Program (NSQIP) database
- The myocardial infarction or cardiac arrest (MICA) calculator14 (www.surgicalriskcalculator.com/miorcardiacarrest), also derived from the NSQIP database.
2017 Canadian guidelines differ
In 2017, the Canadian Cardiovascular Society published its own guidelines on perioperative risk assessment and management.1 These differ from the American and European guidelines on several points.
RCRI recommended. The Canadian guidelines suggested using the RCRI over the other risk predictors, which despite superior discrimination lacked external validation (conditional recommendation; low-quality evidence). Additionally, the Canadians believed that the NSQIP risk indexes underestimated cardiac risk because patients did not undergo routine biomarker screening.
Biomarker measurement. The Canadian guidelines went a step further in their algorithm (Figure 1) and recommended measuring N-terminal-pro B-type natriuretic peptide (NT-proBNP) or BNP preoperatively to improve risk prediction in 3 groups (strong recommendation; moderate-quality evidence):
- Patients ages 65 and older
- Patients ages 45 to 64 with significant cardiovascular disease
- Patients with an RCRI score of 1 or more.
This differs from the American guidelines, which did not recommend measuring preoperative biomarkers but did acknowledge that they may provide incremental value. The American College of Cardiology/American Heart Association authors felt that there were no data to suggest that targeting these biomarkers for treatment and intervention would reduce postoperative risk. The European guidelines did not recommend routinely using biomarkers, but stated that they may be considered in high-risk patients (who have a functional capacity ≤ 4 metabolic equivalents or an RCRI score > 1 undergoing vascular surgery, or > 2 undergoing nonvascular surgery).
Stress testing deemphasized. The Canadian guidelines recommended biomarker testing rather than noninvasive tests to enhance risk assessment based on cost, potential delays in surgery, and absence of evidence of an overall absolute net improvement in risk reclassification. This contrasts with the American and European guidelines and algorithms, which recommended pharmacologic stress testing in patients at elevated risk with poor functional capacity undergoing intermediate- to high-risk surgery if the results would change how they are managed.
Postoperative monitoring. The Canadian guidelines recommended that if patients have an NT-proBNP level higher than 300 mg/L or a BNP level higher than 92 mg/L, they should receive postoperative monitoring with electrocardiography in the postanesthesia care unit and daily troponin measurements for 48 to 72 hours. The American guidelines recommended postoperative electrocardiography and troponin measurement only for patients suspected of having myocardial ischemia, and the European guidelines said postoperative biomarkers may be considered in patients at high risk.
Physician judgment needed
While guidelines and risk calculators are potentially helpful in risk assessment, the lack of consensus and the conflicting recommendations force the physician to weigh the evidence and make individual decisions based on his or her interpretation of the data.
Until there are studies directly comparing the various risk calculators, physicians will most likely use the RCRI, which is simple and has been externally validated, in conjunction with the American guidelines.
At this time, it is unclear how biomarkers should be used—preoperatively, postoperatively, or both—because there are no studies demonstrating that management strategies based on the results lead to better outcomes. We do not believe that biomarker testing will be accepted in lieu of stress testing by our surgery, anesthesiology, or cardiology colleagues, but going forward, it will probably be used more frequently postoperatively, particularly in patients at moderate to high risk.
WHAT IS THE APPROPRIATE TIMING FOR SURGERY AFTER PCI?
A 2014 American College of Cardiology/American Heart Association guideline recommended delaying noncardiac surgery for 1 month after percutaneous coronary intervention (PCI) with bare-metal stents and 1 year after PCI with drug-eluting stents.15 The guideline suggested that surgery may be performed 6 months after drug-eluting stent placement if the risks of delaying surgery outweigh the risk of thrombosis.15
The primary rationale behind these timeframes was to provide dual antiplatelet therapy for a minimally acceptable duration before temporary interruption for a procedure. These recommendations were influenced largely by observational studies of first-generation devices, which are no longer used. Studies of newer-generation stents have suggested that the risk of stent thrombosis reaches a plateau considerably earlier than 6 to 12 months after PCI.
2016 Revised guideline on dual antiplatelet therapy
Although not separately delineated in the recommendations, risk factors for stent thrombosis that should influence the decision include smoking, multivessel coronary artery disease, and suboptimally controlled diabetes mellitus or hyperlipidemia.17 The presence of such stent thrombosis risk factors should be factored into the decision about proceeding with surgery within 3 to 6 months after drug-eluting stent placement.
Holcomb et al: Higher postoperative risk after PCI for myocardial infarction
Another important consideration is the indication for which PCI was performed. In a recent study, Holcomb et al16 found an association between postoperative major adverse cardiac events and PCI for myocardial infarction (MI) that was independent of stent type.
Compared with patients who underwent PCI not associated with acute coronary syndrome, the odds ratios and 95% confidence intervals (CIs) for major adverse cardiac events in those who underwent PCI for MI were:
- 5.25 (4.08–6.75) in the first 3 months
- 2.45 (1.80–3.35) in months 3 to 6
- 2.50 (1.90–3.28) in months 6 to 12.
In absolute terms, patients with stenting performed for an MI had an incidence of major adverse cardiac events of:
- 22.2% in the first 3 months
- 9.4% in months 3 to 6
- 5.8% in months 6 to 12
- 4.4% in months 12 to 24.
The perioperative risks were reduced after 12 months but still remained greater in patients whose PCI was performed for MI rather than another indication.16
The authors of this study suggested delaying noncardiac surgery for up to 6 months after PCI for MI, regardless of stent type.16
A careful, individualized approach
Optimal timing of noncardiac surgery PCI requires a careful, individualized approach and should always be coordinated with the patient’s cardiologist, surgeon, and anesthesiologist.3,15 For most patients, surgery should be delayed for 30 days after bare-metal stent placement and 6 months after drug-eluting stent placement.3 However, for those with greater surgical need and less thrombotic risk, noncardiac surgery can be considered 3 to 6 months after drug-eluting stent placement.3
Additional discussion of the prolonged increased risk of postoperative major adverse cardiac events is warranted in patients whose PCI was performed for MI, in whom delaying noncardiac surgery for up to 6 months (irrespective of stent type) should be considered.16
CAN WE USE STATINS TO REDUCE PERIOPERATIVE RISK?
Current recommendations from the American College of Cardiology/American Heart Association support continuing statins in the perioperative period, but the evidence supporting starting statins in this period has yet to be fully determined. In 2013, a Cochrane review18 found insufficient evidence to conclude that statins reduced perioperative adverse cardiac events, though several large studies were excluded due to controversial methods and data.
In contrast, the Vascular Events in Noncardiac Surgery Patients Cohort Evaluation (VISION) study,4 a multicenter, prospective, cohort-matched study of approximately 7,200 patients, found a lower risk of a composite primary outcome of all-cause mortality, myocardial injury after noncardiac surgery, or stroke at 30 days for patients exposed to statin therapy (relative risk [RR] 0.83, 95% CI 0.73–0.95, P = .007).4
London et al retrospective study: 30-day mortality rate is lower with statins
In 2017, London et al5 published the results of a very large retrospective, observational cohort study of approximately 96,000 elective or emergency surgery patients in Department of Veterans Affairs hospitals. The patients were propensity-matched and evaluated for exposure to statins on the day of or the day after surgery, for a total of approximately 48,000 pairs.
The primary outcome was death at 30 days, and statin exposure was associated with a significant reduction (RR 0.82; 95% CI 0.75–0.89; P < .001). Significant risk reductions were demonstrated in nearly all secondary end points as well, except for stroke or coma and thrombosis (pulmonary embolism, deep vein thrombosis, or graft failure). Overall, the number needed to treat to prevent any complication was 67. Statin therapy did not show significant harm, though on subgroup analysis, those who received high-intensity statin therapy had a slightly higher risk of renal injury (odds ratio 1.18, 95% CI 1.02–1.37, P = .03). Also on subgroup analysis, after propensity matching, patients on long-term moderate- or high-intensity statin therapy for 6 to 12 months before surgery had a small risk reduction for many of the outcomes, including death.
The authors also noted that only 62% of the patients who were prescribed statins as outpatients received them in the hospital, which suggests that improvement is necessary in educating perioperative physicians about the benefits and widespread support for continuing statins perioperatively.5
LOAD trial: No benefit from starting statins
Both London et al5 and the VISION investigators4 called for a large randomized controlled trial of perioperative statin initiation. The Lowering the Risk of Operative Complications Using Atorvastatin Loading Dose (LOAD) trial attempted to answer this call.6
This trial randomized 648 statin-naïve Brazilian patients at high risk of perioperative cardiac events to receive either atorvastatin or placebo before surgery and then continuously for another 7 days. The primary outcomes were the rates of death, nonfatal myocardial injury after noncardiac surgery, and cerebrovascular accident at 30 days.6
The investigators found no significant difference in outcomes between the two groups and estimated that the sample size would need to be approximately 7,000 patients to demonstrate a significant benefit. Nonetheless, this trial established that a prospective perioperative statin trial is feasible.
When to continue or start statins
Although we cannot recommend starting statins for all perioperative patients, perioperative statins clearly can carry significant benefit and should be continued in all patients who have been taking them. It is also likely beneficial to initiate statins in those patients who would otherwise warrant therapy based on the American College of Cardiology/American Heart Association Pooled Cohort Equations Risk calculator.19
HOW SHOULD WE MANAGE SLEEP APNEA RISK PERIOPERATIVELY?
From 20% to 30% of US men and 10% to 15% of US women have obstructive sleep apnea, and many are undiagnosed. Obstructive sleep apnea increases the risk of perioperative respiratory failure, unplanned reintubation, unplanned transfer to the intensive care unit, and death.20 Sentinel events (unexpected respiratory arrest after surgery on general surgical wards) have prompted the development of guidelines that aim to identify patients with previously undiagnosed obstructive sleep apnea before surgery and to develop approaches to reduce perioperative morbidity and mortality.
Kaw et al: Beware obesity hypoventilation syndrome
A 2016 study suggested that patients with obstructive sleep apnea and obesity hypoventilation syndrome may be at particularly high risk of perioperative complications.21
Kaw et al21 queried a database of patients with obstructive sleep apnea undergoing elective noncardiac surgery at Cleveland Clinic. All patients (N = 519) had obstructive sleep apnea confirmed by polysomnography, and a body mass index greater than 30 kg/m2. The authors considered a patient to have obesity hypoventilation syndrome (n = 194) if he or she also had hypercapnia (Paco2 ≥ 45 mm Hg) on at least 2 occasions before or after surgery.
In an adjusted analysis, the odds ratios and 95% CIs for adverse outcomes in patients with obesity hypoventilation syndrome were:
- 10.9 (3.7–32.3) for respiratory failure
- 5.4 (1.9–15.7) for heart failure
- 10.9 (3.7–32.3) for intensive care unit transfer.
The absolute increases in risk in the presence of obesity hypoventilation syndrome were:
- 19% (21% vs 2%) for respiratory failure
- 8% (8% vs 0) for heart failure
- 15% (21% vs 6%) for intensive care unit transfer.
There was no difference in rates of perioperative mortality.21
The authors proposed an algorithm to identify patients with possible obesity hypoventilation syndrome before surgery that included prior sleep study results, STOP-BANG score (Table 2),22 and serum bicarbonate level.
Important limitations of the study were that most patients with obesity hypoventilation syndrome were undiagnosed at the time of surgery. Still, the study does offer a tool to potentially identify patients at high risk for perioperative morbidity due to obesity hypoventilation syndrome. Clinicians could then choose to cancel nonessential surgery, propose a lower-risk alternative procedure, or maximize the use of strategies known to reduce perioperative risk for patients with obstructive sleep apnea in general.
Two guidelines on obstructive sleep apnea
Two professional societies have issued guidelines aiming to improve detection of previously undiagnosed obstructive sleep apnea and perioperative outcomes in patients known to have it or suspected of having it:
- The American Society of Anesthesiologists in 201423
- The Society of Anesthesia and Sleep Medicine in 2016.7
Both guidelines recommend that each institution develop a local protocol to screen patients for possible obstructive sleep apnea before elective surgery. The American Society of Anesthesiologists does not recommend any particular tool, but does recommend taking a history and performing a focused examination that includes evaluation of the airway, nasopharyngeal characteristics, neck circumference, and tonsil and tongue size. The Society of Anesthesia and Sleep Medicine recommends using a validated tool such as the STOP-BANG score to estimate the risk of obstructive sleep apnea.
If this screening suggests that a patient has obstructive sleep apnea, should surgery be delayed until a formal sleep study can be done? Or should the patient be treated empirically as if he or she has obstructive sleep apnea? Both professional societies recommend shared decision-making with the patient in this situation, with the Society of Anesthesia and Sleep Medicine recommending additional cardiopulmonary evaluation for patients with hypoventilation, severe pulmonary hypertension, or resting hypoxemia.
Both recommend using continuous positive airway pressure (CPAP) after surgery in patients with known obstructive sleep apnea, although there is not enough evidence to determine if empiric CPAP for screening-positive patients (without polysomnography-diagnosed obstructive sleep apnea) is beneficial. The Society of Anesthesia and Sleep Medicine advises that it is safe to proceed to surgery if obstructive sleep apnea is suspected as long as monitoring and risk-reduction strategies are implemented after surgery to reduce complication rates.
During surgery, the American Society of Anesthesiologists advises peripheral nerve blocks when appropriate, general anesthesia with a secure airway rather than deep sedation, capnography when using moderate sedation, awake extubation, and full reversal of neuromuscular blockade before extubation. After surgery, they recommend reducing opioid use, minimizing postoperative sedatives, supplemental oxygen, and continuous pulse oximetry. The Society of Anesthesia and Sleep Medicine guideline addresses preoperative assessment and therefore makes no recommendations regarding postoperative care.
In conclusion, use of pertinent findings from the history and physical examination and a validated obstructive sleep apnea screening tool such as STOP-BANG before surgery are recommended, with joint decision-making as to proceeding with surgery with empiric CPAP vs a formal sleep study for patients who screen as high risk. The Society of Anesthesia and Sleep Medicine recommends further cardiopulmonary evaluation if there is evidence of hypoventilation, hypoxemia, or pulmonary hypertension in addition to likely obstructive sleep apnea.
WHICH ATRIAL FIBRILLATION PATIENTS NEED BRIDGING ANTICOAGULATION?
When patients receiving anticoagulation need surgery, we need to carefully assess the risks of thromboembolism without anticoagulation vs bleeding with anticoagulation.
Historically, we tended to worry more about thromboembolism24; however, recent studies have revealed a significant risk of bleeding when long-term anticoagulant therapy is bridged (ie, interrupted and replaced with a shorter-acting agent in the perioperative period), with minimal to no decrease in thromboembolic events.25–27
American College of Cardiology guideline
In 2017, the American College of Cardiology8 published a guideline on periprocedural management of anticoagulation in patients with nonvalvular atrial fibrillation. The guideline includes a series of decision algorithms on whether and when to interrupt anticoagulation, whether and how to provide bridging anticoagulation, and how to restart postprocedural anticoagulation.
When deciding whether to interrupt anticoagulation, we need to consider the risk of bleeding posed both by patient-specific factors and by the type of surgery. Bridging anticoagulation is not indicated when direct oral anticoagulants (eg, dabigatran, apixaban, edoxaban, rivaroxaban) are interrupted for procedures.
Unlike an earlier guideline statement by the American College of Chest Physicians,24 this consensus statement emphasizes using the CHA2DS2-VASc score as a predictor of thromboembolic events rather than the CHADS2 core.
Table 3 summarizes the key points in the guidance statement about which patients should receive periprocedural bridging anticoagulation.
As evidence continues to evolve in this complicated area of perioperative medicine, it will remain important to continue to create patient management plans that take individual patient and procedural risks into account.
IS FRAILTY SCREENING BENEFICIAL BEFORE NONCARDIAC SURGERY?
Frailty, defined as a composite score of a patient’s age and comorbidities, has great potential to become an obligatory factor in perioperative risk assessment. However, it remains difficult to incorporate frailty scoring into clinical practice due to variations among scoring systems,28 uncertain outcome data, and the imprecise role of socioeconomic factors. In particular, the effect of frailty on perioperative mortality over longer periods of time is uncertain.
McIsaac et al: Higher risk in frail patients
McIsaac and colleagues at the University of Ottawa used a frailty scoring system developed at Johns Hopkins University to evaluate the effect of frailty on all-cause postoperative mortality in approximately 202,000 patients over a 10-year period.9 Although this scoring system is proprietary, it is based on factors such as malnutrition, dementia, impaired vision, decubitus ulcers, urinary incontinence, weight loss, poverty, barriers to access of care, difficulty in walking, and falls.
After adjusting for the procedure risk, patient age, sex, and neighborhood income quintile, the 1-year mortality risk was significantly higher in the frail group (absolute risk 13.6% vs 4.8%; adjusted hazard ratio 2.23; 95% CI 2.08–2.40). The risk of death in the first 3 days was much higher in frail than in nonfrail patients (hazard ratio 35.58; 95% CI 29.78–40.1), but the hazard ratio decreased to approximately 2.4 by day 90.
The authors emphasize that the elevated risk for frail patients warrants particular perioperative planning, though it is not yet clear what frailty-specific interventions should be performed. Further study is needed into the benefit of “prehabilitation” (ie, exercise training to “build up” a patient before surgery) for perioperative risk reduction.
Hall et al: Better care for frail patients
Hall et al10 instituted a quality improvement initiative for perioperative care of patients at the Omaha Veterans Affairs Hospital. Frail patients were identified using the Risk Analysis Index, a 14-question screening tool previously developed and validated over several years using Veterans Administration databases.29 Questions in the Risk Analysis Index cover living situation, any diagnosis of cancer, ability to perform activities of daily living, and others.
To maximize compliance, a Risk Analysis Index score was required to schedule a surgery. Patients with high scores underwent further review by a designated team of physicians who initiated informal and formal consultations with anesthesiologists, critical care physicians, surgeons, and palliative care providers, with the goals of minimizing risk, clarifying patient goals or resuscitation wishes, and developing comprehensive perioperative planning.10
Approximately 9,100 patients were included in the cohort. The authors demonstrated a significant improvement in mortality for frail patients at 30, 180, and 365 days, but noted an improvement in postoperative mortality for the nonfrail patients as well, perhaps due to increased focus on geriatric patient care. In particular, the mortality rate at 365 days dropped from 34.5% to 11.7% for frail patients who underwent this intervention.
While this quality improvement initiative was unable to examine how surgical rates changed in frail patients, it is highly likely that very high-risk patients opted out of surgery or had their surgical plan change, though the authors point out that the overall surgical volume at the institution did not change significantly. As well, it remains unclear which particular interventions may have had the most effect in improving survival, as the perioperative plans were individualized and continually adjusted throughout the study period.
Nonetheless, this article highlights how higher vigilance, individualized planning and appreciation of the high risks of frail patients is associated with improved patient survival postoperatively. Although frailty screening is still in its early stages and further work is needed, it is likely that performing frailty screening in elderly patients and utilizing interdisciplinary collaboration for comprehensive management of frail patients can improve their postoperative course.
Perioperative care is increasingly complex, and the rapid evolution of literature in this field makes it a challenge for clinicians to stay up-to-date. To help meet this challenge, we used a systematic approach to identify appropriate articles in the medical literature and then, by consensus, to develop a list of 6 clinical questions based on their novelty and potential to change perioperative medical practice:
- How should we screen for cardiac risk in patients undergoing noncardiac surgery?
- What is the appropriate timing for surgery after coronary intervention?
- Can we use statin therapy to reduce perioperative cardiac risk?
- How should we manage sleep apnea risk perioperatively?
- Which patients with atrial fibrillation should receive perioperative bridging anticoagulation?
- Is frailty screening beneficial for elderly patients before noncardiac surgery?
The summaries in this article are a composite of perioperative medicine updates presented at the Perioperative Medicine Summit and the annual meetings of the Society for General Internal Medicine and the Society of Hospital Medicine. “Perioperative care is complex and changing”1–10 (page 864) offers a brief overview.
HOW TO SCREEN FOR CARDIAC RISK BEFORE NONCARDIAC SURGERY
Perioperative cardiac risk can be estimated by clinical risk indexes (based on history, physical examination, common blood tests, and electrocardiography), cardiac biomarkers (natriuretic peptide or troponin levels), and noninvasive cardiac tests.

American and European guidelines
In 2014, the American College of Cardiology/American Heart Association2 and the European Society of Cardiology11 published guidelines on perioperative cardiovascular evaluation and management. They recommended several tools to calculate the risk of postoperative cardiac complications but did not specify a preference. These tools include:
- The Revised Cardiac Risk Index (RCRI)12 (www.mdcalc.com/revised-cardiac-risk-index-pre-operative-risk), which has been externally validated in multiple studies and is the most widely used
- The American College of Surgeons surgical risk calculator13 (www.riskcalculator.facs.org), derived from the National Surgery Quality Improvement Program (NSQIP) database
- The myocardial infarction or cardiac arrest (MICA) calculator14 (www.surgicalriskcalculator.com/miorcardiacarrest), also derived from the NSQIP database.
2017 Canadian guidelines differ
In 2017, the Canadian Cardiovascular Society published its own guidelines on perioperative risk assessment and management.1 These differ from the American and European guidelines on several points.
RCRI recommended. The Canadian guidelines suggested using the RCRI over the other risk predictors, which despite superior discrimination lacked external validation (conditional recommendation; low-quality evidence). Additionally, the Canadians believed that the NSQIP risk indexes underestimated cardiac risk because patients did not undergo routine biomarker screening.
Biomarker measurement. The Canadian guidelines went a step further in their algorithm (Figure 1) and recommended measuring N-terminal-pro B-type natriuretic peptide (NT-proBNP) or BNP preoperatively to improve risk prediction in 3 groups (strong recommendation; moderate-quality evidence):
- Patients ages 65 and older
- Patients ages 45 to 64 with significant cardiovascular disease
- Patients with an RCRI score of 1 or more.
This differs from the American guidelines, which did not recommend measuring preoperative biomarkers but did acknowledge that they may provide incremental value. The American College of Cardiology/American Heart Association authors felt that there were no data to suggest that targeting these biomarkers for treatment and intervention would reduce postoperative risk. The European guidelines did not recommend routinely using biomarkers, but stated that they may be considered in high-risk patients (who have a functional capacity ≤ 4 metabolic equivalents or an RCRI score > 1 undergoing vascular surgery, or > 2 undergoing nonvascular surgery).
Stress testing deemphasized. The Canadian guidelines recommended biomarker testing rather than noninvasive tests to enhance risk assessment based on cost, potential delays in surgery, and absence of evidence of an overall absolute net improvement in risk reclassification. This contrasts with the American and European guidelines and algorithms, which recommended pharmacologic stress testing in patients at elevated risk with poor functional capacity undergoing intermediate- to high-risk surgery if the results would change how they are managed.
Postoperative monitoring. The Canadian guidelines recommended that if patients have an NT-proBNP level higher than 300 mg/L or a BNP level higher than 92 mg/L, they should receive postoperative monitoring with electrocardiography in the postanesthesia care unit and daily troponin measurements for 48 to 72 hours. The American guidelines recommended postoperative electrocardiography and troponin measurement only for patients suspected of having myocardial ischemia, and the European guidelines said postoperative biomarkers may be considered in patients at high risk.
Physician judgment needed
While guidelines and risk calculators are potentially helpful in risk assessment, the lack of consensus and the conflicting recommendations force the physician to weigh the evidence and make individual decisions based on his or her interpretation of the data.
Until there are studies directly comparing the various risk calculators, physicians will most likely use the RCRI, which is simple and has been externally validated, in conjunction with the American guidelines.
At this time, it is unclear how biomarkers should be used—preoperatively, postoperatively, or both—because there are no studies demonstrating that management strategies based on the results lead to better outcomes. We do not believe that biomarker testing will be accepted in lieu of stress testing by our surgery, anesthesiology, or cardiology colleagues, but going forward, it will probably be used more frequently postoperatively, particularly in patients at moderate to high risk.
WHAT IS THE APPROPRIATE TIMING FOR SURGERY AFTER PCI?
A 2014 American College of Cardiology/American Heart Association guideline recommended delaying noncardiac surgery for 1 month after percutaneous coronary intervention (PCI) with bare-metal stents and 1 year after PCI with drug-eluting stents.15 The guideline suggested that surgery may be performed 6 months after drug-eluting stent placement if the risks of delaying surgery outweigh the risk of thrombosis.15
The primary rationale behind these timeframes was to provide dual antiplatelet therapy for a minimally acceptable duration before temporary interruption for a procedure. These recommendations were influenced largely by observational studies of first-generation devices, which are no longer used. Studies of newer-generation stents have suggested that the risk of stent thrombosis reaches a plateau considerably earlier than 6 to 12 months after PCI.
2016 Revised guideline on dual antiplatelet therapy
Although not separately delineated in the recommendations, risk factors for stent thrombosis that should influence the decision include smoking, multivessel coronary artery disease, and suboptimally controlled diabetes mellitus or hyperlipidemia.17 The presence of such stent thrombosis risk factors should be factored into the decision about proceeding with surgery within 3 to 6 months after drug-eluting stent placement.
Holcomb et al: Higher postoperative risk after PCI for myocardial infarction
Another important consideration is the indication for which PCI was performed. In a recent study, Holcomb et al16 found an association between postoperative major adverse cardiac events and PCI for myocardial infarction (MI) that was independent of stent type.
Compared with patients who underwent PCI not associated with acute coronary syndrome, the odds ratios and 95% confidence intervals (CIs) for major adverse cardiac events in those who underwent PCI for MI were:
- 5.25 (4.08–6.75) in the first 3 months
- 2.45 (1.80–3.35) in months 3 to 6
- 2.50 (1.90–3.28) in months 6 to 12.
In absolute terms, patients with stenting performed for an MI had an incidence of major adverse cardiac events of:
- 22.2% in the first 3 months
- 9.4% in months 3 to 6
- 5.8% in months 6 to 12
- 4.4% in months 12 to 24.
The perioperative risks were reduced after 12 months but still remained greater in patients whose PCI was performed for MI rather than another indication.16
The authors of this study suggested delaying noncardiac surgery for up to 6 months after PCI for MI, regardless of stent type.16
A careful, individualized approach
Optimal timing of noncardiac surgery PCI requires a careful, individualized approach and should always be coordinated with the patient’s cardiologist, surgeon, and anesthesiologist.3,15 For most patients, surgery should be delayed for 30 days after bare-metal stent placement and 6 months after drug-eluting stent placement.3 However, for those with greater surgical need and less thrombotic risk, noncardiac surgery can be considered 3 to 6 months after drug-eluting stent placement.3
Additional discussion of the prolonged increased risk of postoperative major adverse cardiac events is warranted in patients whose PCI was performed for MI, in whom delaying noncardiac surgery for up to 6 months (irrespective of stent type) should be considered.16
CAN WE USE STATINS TO REDUCE PERIOPERATIVE RISK?
Current recommendations from the American College of Cardiology/American Heart Association support continuing statins in the perioperative period, but the evidence supporting starting statins in this period has yet to be fully determined. In 2013, a Cochrane review18 found insufficient evidence to conclude that statins reduced perioperative adverse cardiac events, though several large studies were excluded due to controversial methods and data.
In contrast, the Vascular Events in Noncardiac Surgery Patients Cohort Evaluation (VISION) study,4 a multicenter, prospective, cohort-matched study of approximately 7,200 patients, found a lower risk of a composite primary outcome of all-cause mortality, myocardial injury after noncardiac surgery, or stroke at 30 days for patients exposed to statin therapy (relative risk [RR] 0.83, 95% CI 0.73–0.95, P = .007).4
London et al retrospective study: 30-day mortality rate is lower with statins
In 2017, London et al5 published the results of a very large retrospective, observational cohort study of approximately 96,000 elective or emergency surgery patients in Department of Veterans Affairs hospitals. The patients were propensity-matched and evaluated for exposure to statins on the day of or the day after surgery, for a total of approximately 48,000 pairs.
The primary outcome was death at 30 days, and statin exposure was associated with a significant reduction (RR 0.82; 95% CI 0.75–0.89; P < .001). Significant risk reductions were demonstrated in nearly all secondary end points as well, except for stroke or coma and thrombosis (pulmonary embolism, deep vein thrombosis, or graft failure). Overall, the number needed to treat to prevent any complication was 67. Statin therapy did not show significant harm, though on subgroup analysis, those who received high-intensity statin therapy had a slightly higher risk of renal injury (odds ratio 1.18, 95% CI 1.02–1.37, P = .03). Also on subgroup analysis, after propensity matching, patients on long-term moderate- or high-intensity statin therapy for 6 to 12 months before surgery had a small risk reduction for many of the outcomes, including death.
The authors also noted that only 62% of the patients who were prescribed statins as outpatients received them in the hospital, which suggests that improvement is necessary in educating perioperative physicians about the benefits and widespread support for continuing statins perioperatively.5
LOAD trial: No benefit from starting statins
Both London et al5 and the VISION investigators4 called for a large randomized controlled trial of perioperative statin initiation. The Lowering the Risk of Operative Complications Using Atorvastatin Loading Dose (LOAD) trial attempted to answer this call.6
This trial randomized 648 statin-naïve Brazilian patients at high risk of perioperative cardiac events to receive either atorvastatin or placebo before surgery and then continuously for another 7 days. The primary outcomes were the rates of death, nonfatal myocardial injury after noncardiac surgery, and cerebrovascular accident at 30 days.6
The investigators found no significant difference in outcomes between the two groups and estimated that the sample size would need to be approximately 7,000 patients to demonstrate a significant benefit. Nonetheless, this trial established that a prospective perioperative statin trial is feasible.
When to continue or start statins
Although we cannot recommend starting statins for all perioperative patients, perioperative statins clearly can carry significant benefit and should be continued in all patients who have been taking them. It is also likely beneficial to initiate statins in those patients who would otherwise warrant therapy based on the American College of Cardiology/American Heart Association Pooled Cohort Equations Risk calculator.19
HOW SHOULD WE MANAGE SLEEP APNEA RISK PERIOPERATIVELY?
From 20% to 30% of US men and 10% to 15% of US women have obstructive sleep apnea, and many are undiagnosed. Obstructive sleep apnea increases the risk of perioperative respiratory failure, unplanned reintubation, unplanned transfer to the intensive care unit, and death.20 Sentinel events (unexpected respiratory arrest after surgery on general surgical wards) have prompted the development of guidelines that aim to identify patients with previously undiagnosed obstructive sleep apnea before surgery and to develop approaches to reduce perioperative morbidity and mortality.
Kaw et al: Beware obesity hypoventilation syndrome
A 2016 study suggested that patients with obstructive sleep apnea and obesity hypoventilation syndrome may be at particularly high risk of perioperative complications.21
Kaw et al21 queried a database of patients with obstructive sleep apnea undergoing elective noncardiac surgery at Cleveland Clinic. All patients (N = 519) had obstructive sleep apnea confirmed by polysomnography, and a body mass index greater than 30 kg/m2. The authors considered a patient to have obesity hypoventilation syndrome (n = 194) if he or she also had hypercapnia (Paco2 ≥ 45 mm Hg) on at least 2 occasions before or after surgery.
In an adjusted analysis, the odds ratios and 95% CIs for adverse outcomes in patients with obesity hypoventilation syndrome were:
- 10.9 (3.7–32.3) for respiratory failure
- 5.4 (1.9–15.7) for heart failure
- 10.9 (3.7–32.3) for intensive care unit transfer.
The absolute increases in risk in the presence of obesity hypoventilation syndrome were:
- 19% (21% vs 2%) for respiratory failure
- 8% (8% vs 0) for heart failure
- 15% (21% vs 6%) for intensive care unit transfer.
There was no difference in rates of perioperative mortality.21
The authors proposed an algorithm to identify patients with possible obesity hypoventilation syndrome before surgery that included prior sleep study results, STOP-BANG score (Table 2),22 and serum bicarbonate level.
Important limitations of the study were that most patients with obesity hypoventilation syndrome were undiagnosed at the time of surgery. Still, the study does offer a tool to potentially identify patients at high risk for perioperative morbidity due to obesity hypoventilation syndrome. Clinicians could then choose to cancel nonessential surgery, propose a lower-risk alternative procedure, or maximize the use of strategies known to reduce perioperative risk for patients with obstructive sleep apnea in general.
Two guidelines on obstructive sleep apnea
Two professional societies have issued guidelines aiming to improve detection of previously undiagnosed obstructive sleep apnea and perioperative outcomes in patients known to have it or suspected of having it:
- The American Society of Anesthesiologists in 201423
- The Society of Anesthesia and Sleep Medicine in 2016.7
Both guidelines recommend that each institution develop a local protocol to screen patients for possible obstructive sleep apnea before elective surgery. The American Society of Anesthesiologists does not recommend any particular tool, but does recommend taking a history and performing a focused examination that includes evaluation of the airway, nasopharyngeal characteristics, neck circumference, and tonsil and tongue size. The Society of Anesthesia and Sleep Medicine recommends using a validated tool such as the STOP-BANG score to estimate the risk of obstructive sleep apnea.
If this screening suggests that a patient has obstructive sleep apnea, should surgery be delayed until a formal sleep study can be done? Or should the patient be treated empirically as if he or she has obstructive sleep apnea? Both professional societies recommend shared decision-making with the patient in this situation, with the Society of Anesthesia and Sleep Medicine recommending additional cardiopulmonary evaluation for patients with hypoventilation, severe pulmonary hypertension, or resting hypoxemia.
Both recommend using continuous positive airway pressure (CPAP) after surgery in patients with known obstructive sleep apnea, although there is not enough evidence to determine if empiric CPAP for screening-positive patients (without polysomnography-diagnosed obstructive sleep apnea) is beneficial. The Society of Anesthesia and Sleep Medicine advises that it is safe to proceed to surgery if obstructive sleep apnea is suspected as long as monitoring and risk-reduction strategies are implemented after surgery to reduce complication rates.
During surgery, the American Society of Anesthesiologists advises peripheral nerve blocks when appropriate, general anesthesia with a secure airway rather than deep sedation, capnography when using moderate sedation, awake extubation, and full reversal of neuromuscular blockade before extubation. After surgery, they recommend reducing opioid use, minimizing postoperative sedatives, supplemental oxygen, and continuous pulse oximetry. The Society of Anesthesia and Sleep Medicine guideline addresses preoperative assessment and therefore makes no recommendations regarding postoperative care.
In conclusion, use of pertinent findings from the history and physical examination and a validated obstructive sleep apnea screening tool such as STOP-BANG before surgery are recommended, with joint decision-making as to proceeding with surgery with empiric CPAP vs a formal sleep study for patients who screen as high risk. The Society of Anesthesia and Sleep Medicine recommends further cardiopulmonary evaluation if there is evidence of hypoventilation, hypoxemia, or pulmonary hypertension in addition to likely obstructive sleep apnea.
WHICH ATRIAL FIBRILLATION PATIENTS NEED BRIDGING ANTICOAGULATION?
When patients receiving anticoagulation need surgery, we need to carefully assess the risks of thromboembolism without anticoagulation vs bleeding with anticoagulation.
Historically, we tended to worry more about thromboembolism24; however, recent studies have revealed a significant risk of bleeding when long-term anticoagulant therapy is bridged (ie, interrupted and replaced with a shorter-acting agent in the perioperative period), with minimal to no decrease in thromboembolic events.25–27
American College of Cardiology guideline
In 2017, the American College of Cardiology8 published a guideline on periprocedural management of anticoagulation in patients with nonvalvular atrial fibrillation. The guideline includes a series of decision algorithms on whether and when to interrupt anticoagulation, whether and how to provide bridging anticoagulation, and how to restart postprocedural anticoagulation.
When deciding whether to interrupt anticoagulation, we need to consider the risk of bleeding posed both by patient-specific factors and by the type of surgery. Bridging anticoagulation is not indicated when direct oral anticoagulants (eg, dabigatran, apixaban, edoxaban, rivaroxaban) are interrupted for procedures.
Unlike an earlier guideline statement by the American College of Chest Physicians,24 this consensus statement emphasizes using the CHA2DS2-VASc score as a predictor of thromboembolic events rather than the CHADS2 core.
Table 3 summarizes the key points in the guidance statement about which patients should receive periprocedural bridging anticoagulation.
As evidence continues to evolve in this complicated area of perioperative medicine, it will remain important to continue to create patient management plans that take individual patient and procedural risks into account.
IS FRAILTY SCREENING BENEFICIAL BEFORE NONCARDIAC SURGERY?
Frailty, defined as a composite score of a patient’s age and comorbidities, has great potential to become an obligatory factor in perioperative risk assessment. However, it remains difficult to incorporate frailty scoring into clinical practice due to variations among scoring systems,28 uncertain outcome data, and the imprecise role of socioeconomic factors. In particular, the effect of frailty on perioperative mortality over longer periods of time is uncertain.
McIsaac et al: Higher risk in frail patients
McIsaac and colleagues at the University of Ottawa used a frailty scoring system developed at Johns Hopkins University to evaluate the effect of frailty on all-cause postoperative mortality in approximately 202,000 patients over a 10-year period.9 Although this scoring system is proprietary, it is based on factors such as malnutrition, dementia, impaired vision, decubitus ulcers, urinary incontinence, weight loss, poverty, barriers to access of care, difficulty in walking, and falls.
After adjusting for the procedure risk, patient age, sex, and neighborhood income quintile, the 1-year mortality risk was significantly higher in the frail group (absolute risk 13.6% vs 4.8%; adjusted hazard ratio 2.23; 95% CI 2.08–2.40). The risk of death in the first 3 days was much higher in frail than in nonfrail patients (hazard ratio 35.58; 95% CI 29.78–40.1), but the hazard ratio decreased to approximately 2.4 by day 90.
The authors emphasize that the elevated risk for frail patients warrants particular perioperative planning, though it is not yet clear what frailty-specific interventions should be performed. Further study is needed into the benefit of “prehabilitation” (ie, exercise training to “build up” a patient before surgery) for perioperative risk reduction.
Hall et al: Better care for frail patients
Hall et al10 instituted a quality improvement initiative for perioperative care of patients at the Omaha Veterans Affairs Hospital. Frail patients were identified using the Risk Analysis Index, a 14-question screening tool previously developed and validated over several years using Veterans Administration databases.29 Questions in the Risk Analysis Index cover living situation, any diagnosis of cancer, ability to perform activities of daily living, and others.
To maximize compliance, a Risk Analysis Index score was required to schedule a surgery. Patients with high scores underwent further review by a designated team of physicians who initiated informal and formal consultations with anesthesiologists, critical care physicians, surgeons, and palliative care providers, with the goals of minimizing risk, clarifying patient goals or resuscitation wishes, and developing comprehensive perioperative planning.10
Approximately 9,100 patients were included in the cohort. The authors demonstrated a significant improvement in mortality for frail patients at 30, 180, and 365 days, but noted an improvement in postoperative mortality for the nonfrail patients as well, perhaps due to increased focus on geriatric patient care. In particular, the mortality rate at 365 days dropped from 34.5% to 11.7% for frail patients who underwent this intervention.
While this quality improvement initiative was unable to examine how surgical rates changed in frail patients, it is highly likely that very high-risk patients opted out of surgery or had their surgical plan change, though the authors point out that the overall surgical volume at the institution did not change significantly. As well, it remains unclear which particular interventions may have had the most effect in improving survival, as the perioperative plans were individualized and continually adjusted throughout the study period.
Nonetheless, this article highlights how higher vigilance, individualized planning and appreciation of the high risks of frail patients is associated with improved patient survival postoperatively. Although frailty screening is still in its early stages and further work is needed, it is likely that performing frailty screening in elderly patients and utilizing interdisciplinary collaboration for comprehensive management of frail patients can improve their postoperative course.
- Duceppe E, Parlow J, MacDonald P, et al. Canadian Cardiovascular Society guidelines on perioperative cardiac risk assessment and management for patients who undergo noncardiac surgery. Can J Cardiol 2017; 33:17–32.
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol 2014; 64:2373–2405.
- Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease. Circulation 2016; 134:e123–e155.
- Berwanger O, Le Manach Y, Suzumura EA, et al. Association between pre-operative statin use and major cardiovascular complications among patients undergoing non-cardiac surgery: the VISION study. Eur Heart J 2016; 37:177–185.
- London MJ, Schwartz GG, Hur K, Henderson WG. Association of perioperative statin use with mortality and morbidity after major noncardiac surgery. JAMA Intern Med 2017; 177:231–242.
- Berwanger O, de Barros E Silva PG, Barbosa RR, et al. Atorvastatin for high-risk statin-naïve patients undergoing noncardiac surgery: the Lowering the Risk of Operative Complications Using Atorvastatin Loading Dose (LOAD) randomized trial. Am Heart J 2017; 184:88–96.
- Chung F, Memtsoudis SG, Ramachandran SK, et al. Society of Anesthesia and Sleep Medicine guidelines on preoperative screening and assessment of adult patients with obstructive sleep apnea. Anesth Analg 2016; 123:452–473.
- Doherty JU, Gluckman TJ, Hucker W, et al. 2017 ACC expert consensus decision pathway for periprocedural management of anticoagulation in patients with nonvalvular atrial fibrillation: a report of the American College of Cardiology Clinical Expert Consensus Document Task Force. J Am Coll Cardiol 2017; 69:871–898.
- McIsaac DI, Bryson GL, van Walraven C. Association of frailty and 1-year postoperative mortality following major elective noncardiac surgery: a population-based cohort study. JAMA Surg 2016; 151:538–545.
- Hall DE, Arya S, Schmid KK, et al. Association of a frailty screening initiative with postoperative survival at 30, 180, and 365 days. JAMA Surg 2017; 152:233–240.
- Kristensen SD, Knuuti J, Saraste A, et al. 2014 ESC/ESA Guidelines on non-cardiac surgery: cardiovascular assessment and management: The Joint Task Force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur Heart J 2014; 35:2383–2431.
- Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999; 100:1043–1049.
- Bilimoria KY, Liu Y, Paruch JL, Zhou L, Kmiecik TE, Ko CY, Cohen ME. Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aid and informed consent tool for patients and surgeons. J Am Coll Surg 2013; 217:833–842.
- Gupta PK, Gupta H, Sundaram A, et al. Development and validation of a risk calculator for prediction of cardiac risk after surgery. Circulation 2011; 124:381–387.
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; 64:e77–e137.
- Holcomb CN, Hollis RH, Graham LA, et al. Association of coronary stent indication with postoperative outcomes following noncardiac surgery. JAMA Surg 2016; 151:462–469.
- Lemesle G, Tricot O, Meurice T, et al. Incident myocardial infarction and very late stent thrombosis in outpatients with stable coronary artery disease. J Am Coll Cardiol 2017; 69:2149–2156.
- Sanders RD, Nicholson A, Lewis SR, Smith AF, Alderson P. Perioperative statin therapy for improving outcomes during and after noncardiac vascular surgery. Cochrane Database Syst Rev 2013; 7:CD009971.
- Goff DC, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; 63:2935–2959.
- Kaw R, Pasupuleti V, Walker E, et al. Postoperative complications in patients with obstructive sleep apnea. Chest 2012; 141:436–441.
- Kaw R, Bhateja P, Mar HP, et al. Postoperative complications in patients with unrecognized obesity hypoventilation syndrome undergoing elective noncardiac surgery. Chest 2016; 149:84–91.
- Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology 2008; 108:812–821.
- Gross JB, Apfelbaum JL, Caplan RA, et al. Practice guidelines for the perioperative management of patients with obstructive sleep apnea: an updated report by the American Society of Anesthesiologists Task Force on Perioperative Management of Patients with Obstructive Sleep Apnea. Anesthesiology 2014; 120:268–286.
- Douketis JD, Spyropoulos AC, Spencer FA, et al. Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(2 suppl):e326S–e350S.
- Siegal D, Yudin J, Kaatz S, Douketis JD, Lim W, Spyropoulos AC. Periprocedural heparin bridging in patients receiving vitamin K antagonists: systematic review and meta-analysis of bleeding and thromboembolic rates. Circulation 2012; 126:1630–1639.
- Clark NP, Witt DM, Davies LE, et al. Bleeding, recurrent venous thromboembolism, and mortality risks during warfarin interruption for invasive procedures. JAMA Intern Med 2015; 175:1163–1168.
- Douketis JD, Spyropoulos AC, Kaatz S, et al. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med 2015; 373:823–833.
- Theou O, Brothers TD, Mitnitski A, Rockwood K. Operationalization of frailty using eight commonly used scales and comparison of their ability to predict all-cause mortality. J Am Geriatr Soc 2013; 61:1537–1551.
- Hall DE, Arya S, Schmid KK, et al. Development and initial validation of the risk analysis index for measuring frailty in surgical populations. JAMA Surg 2017; 152:175–182.
- Duceppe E, Parlow J, MacDonald P, et al. Canadian Cardiovascular Society guidelines on perioperative cardiac risk assessment and management for patients who undergo noncardiac surgery. Can J Cardiol 2017; 33:17–32.
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol 2014; 64:2373–2405.
- Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease. Circulation 2016; 134:e123–e155.
- Berwanger O, Le Manach Y, Suzumura EA, et al. Association between pre-operative statin use and major cardiovascular complications among patients undergoing non-cardiac surgery: the VISION study. Eur Heart J 2016; 37:177–185.
- London MJ, Schwartz GG, Hur K, Henderson WG. Association of perioperative statin use with mortality and morbidity after major noncardiac surgery. JAMA Intern Med 2017; 177:231–242.
- Berwanger O, de Barros E Silva PG, Barbosa RR, et al. Atorvastatin for high-risk statin-naïve patients undergoing noncardiac surgery: the Lowering the Risk of Operative Complications Using Atorvastatin Loading Dose (LOAD) randomized trial. Am Heart J 2017; 184:88–96.
- Chung F, Memtsoudis SG, Ramachandran SK, et al. Society of Anesthesia and Sleep Medicine guidelines on preoperative screening and assessment of adult patients with obstructive sleep apnea. Anesth Analg 2016; 123:452–473.
- Doherty JU, Gluckman TJ, Hucker W, et al. 2017 ACC expert consensus decision pathway for periprocedural management of anticoagulation in patients with nonvalvular atrial fibrillation: a report of the American College of Cardiology Clinical Expert Consensus Document Task Force. J Am Coll Cardiol 2017; 69:871–898.
- McIsaac DI, Bryson GL, van Walraven C. Association of frailty and 1-year postoperative mortality following major elective noncardiac surgery: a population-based cohort study. JAMA Surg 2016; 151:538–545.
- Hall DE, Arya S, Schmid KK, et al. Association of a frailty screening initiative with postoperative survival at 30, 180, and 365 days. JAMA Surg 2017; 152:233–240.
- Kristensen SD, Knuuti J, Saraste A, et al. 2014 ESC/ESA Guidelines on non-cardiac surgery: cardiovascular assessment and management: The Joint Task Force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur Heart J 2014; 35:2383–2431.
- Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999; 100:1043–1049.
- Bilimoria KY, Liu Y, Paruch JL, Zhou L, Kmiecik TE, Ko CY, Cohen ME. Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aid and informed consent tool for patients and surgeons. J Am Coll Surg 2013; 217:833–842.
- Gupta PK, Gupta H, Sundaram A, et al. Development and validation of a risk calculator for prediction of cardiac risk after surgery. Circulation 2011; 124:381–387.
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; 64:e77–e137.
- Holcomb CN, Hollis RH, Graham LA, et al. Association of coronary stent indication with postoperative outcomes following noncardiac surgery. JAMA Surg 2016; 151:462–469.
- Lemesle G, Tricot O, Meurice T, et al. Incident myocardial infarction and very late stent thrombosis in outpatients with stable coronary artery disease. J Am Coll Cardiol 2017; 69:2149–2156.
- Sanders RD, Nicholson A, Lewis SR, Smith AF, Alderson P. Perioperative statin therapy for improving outcomes during and after noncardiac vascular surgery. Cochrane Database Syst Rev 2013; 7:CD009971.
- Goff DC, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; 63:2935–2959.
- Kaw R, Pasupuleti V, Walker E, et al. Postoperative complications in patients with obstructive sleep apnea. Chest 2012; 141:436–441.
- Kaw R, Bhateja P, Mar HP, et al. Postoperative complications in patients with unrecognized obesity hypoventilation syndrome undergoing elective noncardiac surgery. Chest 2016; 149:84–91.
- Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology 2008; 108:812–821.
- Gross JB, Apfelbaum JL, Caplan RA, et al. Practice guidelines for the perioperative management of patients with obstructive sleep apnea: an updated report by the American Society of Anesthesiologists Task Force on Perioperative Management of Patients with Obstructive Sleep Apnea. Anesthesiology 2014; 120:268–286.
- Douketis JD, Spyropoulos AC, Spencer FA, et al. Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(2 suppl):e326S–e350S.
- Siegal D, Yudin J, Kaatz S, Douketis JD, Lim W, Spyropoulos AC. Periprocedural heparin bridging in patients receiving vitamin K antagonists: systematic review and meta-analysis of bleeding and thromboembolic rates. Circulation 2012; 126:1630–1639.
- Clark NP, Witt DM, Davies LE, et al. Bleeding, recurrent venous thromboembolism, and mortality risks during warfarin interruption for invasive procedures. JAMA Intern Med 2015; 175:1163–1168.
- Douketis JD, Spyropoulos AC, Kaatz S, et al. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med 2015; 373:823–833.
- Theou O, Brothers TD, Mitnitski A, Rockwood K. Operationalization of frailty using eight commonly used scales and comparison of their ability to predict all-cause mortality. J Am Geriatr Soc 2013; 61:1537–1551.
- Hall DE, Arya S, Schmid KK, et al. Development and initial validation of the risk analysis index for measuring frailty in surgical populations. JAMA Surg 2017; 152:175–182.
KEY POINTS
- Noncardiac surgery after drug-eluting stent placement can be considered after 3 to 6 months for those with greater surgical need and lower risk of stent thrombosis.
- Perioperative statin use continues to show benefits with minimal risk in large cohort studies, but significant randomized controlled trial data are lacking.
- Patients should be screened for obstructive sleep apnea before surgery, and further cardiopulmonary testing should be performed if the patient has evidence of significant sequelae from obstructive sleep apnea.
- For patients with atrial fibrillation on vitamin K antagonists, bridging can be considered for those with a CHA2DS2-VASc score of 5 or 6 and a history of stroke, transient ischemic attack, or systemic thromboembolism. Direct oral anticoagulation should not be bridged.
- Frailty carries significant perioperative mortality risk; systems-based changes to minimize these patients’ risks can be beneficial and warrant further study.