User login
Case Management Improves Quality of Life for Cancer Survivors
After cancer treatment, a new challenge emerges as patients negotiate reentry into their everyday lives. These patients often need multifaceted, simultaneous, and ongoing help with physical, emotional, and psychological issues. And they’re often dealing with a multiplicity of health care providers.
Researchers from University of Zurich and Centres for Addiction Medicine in Zurich, Switzerland, saw similarities between these needs and those of patients with chronic medical conditions. They hypothesized that case management—like that for chronic diseases—could work for cancer survivors perhaps even better than usual care. Among other duties, case managers can assess individual needs, identify barriers, ensure coordination among care providers, and perhaps most important, promote empowering self-management skills and self-efficacy. All of which could help cancer survivors cope with the long-term consequences of cancer and improve health-related quality of life (QOL).
Related: Putting the Focus on Quality of Life in Cancer Care
The researchers designed an intervention study in which 5 oncology nurse case managers met with 95 patients at least once a month for 3 months, then conducted telephone follow-ups for 9 months. Questionnaires measured health-related QOL at 12 months via the Functional Assessment of Cancer Therapy-General (FACT-G), self-efficacy, and concordance of received care with the Patient Assessment of Chronic Illness Care (PACIC).
Although the researchers’ study did not show a significant absolute difference between the groups in FACT-G after 12 months, all scores improved in the case management group compared with the usual care group. Overall, case management clearly boosted QOL and self-efficacy and aligned health care with the chronic care model.
Related: Quality of Supportive Care for Patients With Advanced Lung Cancer in the VHA
According to the researchers, their study is the first, to their knowledge, to examine the effect of case management on the QOL of early cancer survivors. Several factors help explain the intervention’s success: (1) The case managers provided important information on long-term symptoms and available services and therapies; (2) They offered a continuity of care when treatment appointments ceased and medical follow-up visits were less frequent; and (3) They offered support to cope with the psychological issues of the reentry phase.
All in all, the researchers say their findings show case management is a practical approach to bridging a “fragmented oncological health care system” and addressing the heterogenic needs of cancer survivors.
Source:
Scherz N, Bachmann-Mettler I, Chmiel C. BMC Cancer. 2017;17(1):223.
doi: 10.1186/s12885-017-3213-9.
After cancer treatment, a new challenge emerges as patients negotiate reentry into their everyday lives. These patients often need multifaceted, simultaneous, and ongoing help with physical, emotional, and psychological issues. And they’re often dealing with a multiplicity of health care providers.
Researchers from University of Zurich and Centres for Addiction Medicine in Zurich, Switzerland, saw similarities between these needs and those of patients with chronic medical conditions. They hypothesized that case management—like that for chronic diseases—could work for cancer survivors perhaps even better than usual care. Among other duties, case managers can assess individual needs, identify barriers, ensure coordination among care providers, and perhaps most important, promote empowering self-management skills and self-efficacy. All of which could help cancer survivors cope with the long-term consequences of cancer and improve health-related quality of life (QOL).
Related: Putting the Focus on Quality of Life in Cancer Care
The researchers designed an intervention study in which 5 oncology nurse case managers met with 95 patients at least once a month for 3 months, then conducted telephone follow-ups for 9 months. Questionnaires measured health-related QOL at 12 months via the Functional Assessment of Cancer Therapy-General (FACT-G), self-efficacy, and concordance of received care with the Patient Assessment of Chronic Illness Care (PACIC).
Although the researchers’ study did not show a significant absolute difference between the groups in FACT-G after 12 months, all scores improved in the case management group compared with the usual care group. Overall, case management clearly boosted QOL and self-efficacy and aligned health care with the chronic care model.
Related: Quality of Supportive Care for Patients With Advanced Lung Cancer in the VHA
According to the researchers, their study is the first, to their knowledge, to examine the effect of case management on the QOL of early cancer survivors. Several factors help explain the intervention’s success: (1) The case managers provided important information on long-term symptoms and available services and therapies; (2) They offered a continuity of care when treatment appointments ceased and medical follow-up visits were less frequent; and (3) They offered support to cope with the psychological issues of the reentry phase.
All in all, the researchers say their findings show case management is a practical approach to bridging a “fragmented oncological health care system” and addressing the heterogenic needs of cancer survivors.
Source:
Scherz N, Bachmann-Mettler I, Chmiel C. BMC Cancer. 2017;17(1):223.
doi: 10.1186/s12885-017-3213-9.
After cancer treatment, a new challenge emerges as patients negotiate reentry into their everyday lives. These patients often need multifaceted, simultaneous, and ongoing help with physical, emotional, and psychological issues. And they’re often dealing with a multiplicity of health care providers.
Researchers from University of Zurich and Centres for Addiction Medicine in Zurich, Switzerland, saw similarities between these needs and those of patients with chronic medical conditions. They hypothesized that case management—like that for chronic diseases—could work for cancer survivors perhaps even better than usual care. Among other duties, case managers can assess individual needs, identify barriers, ensure coordination among care providers, and perhaps most important, promote empowering self-management skills and self-efficacy. All of which could help cancer survivors cope with the long-term consequences of cancer and improve health-related quality of life (QOL).
Related: Putting the Focus on Quality of Life in Cancer Care
The researchers designed an intervention study in which 5 oncology nurse case managers met with 95 patients at least once a month for 3 months, then conducted telephone follow-ups for 9 months. Questionnaires measured health-related QOL at 12 months via the Functional Assessment of Cancer Therapy-General (FACT-G), self-efficacy, and concordance of received care with the Patient Assessment of Chronic Illness Care (PACIC).
Although the researchers’ study did not show a significant absolute difference between the groups in FACT-G after 12 months, all scores improved in the case management group compared with the usual care group. Overall, case management clearly boosted QOL and self-efficacy and aligned health care with the chronic care model.
Related: Quality of Supportive Care for Patients With Advanced Lung Cancer in the VHA
According to the researchers, their study is the first, to their knowledge, to examine the effect of case management on the QOL of early cancer survivors. Several factors help explain the intervention’s success: (1) The case managers provided important information on long-term symptoms and available services and therapies; (2) They offered a continuity of care when treatment appointments ceased and medical follow-up visits were less frequent; and (3) They offered support to cope with the psychological issues of the reentry phase.
All in all, the researchers say their findings show case management is a practical approach to bridging a “fragmented oncological health care system” and addressing the heterogenic needs of cancer survivors.
Source:
Scherz N, Bachmann-Mettler I, Chmiel C. BMC Cancer. 2017;17(1):223.
doi: 10.1186/s12885-017-3213-9.
Orthorexia Nervosa: An Obsession With Healthy Eating
First named by Steven Bratman in 1997, orthorexia nervosa (ON) from the Greek ortho, meaning correct, and orexi, meaning appetite, is classified as an unspecified feeding and eating disorder in the Diagnostic and Statistical Manual of Mental Disorders 5th edition (DSM-5).1,2
Hypothetical Case
Mr. P is a 30-year-old male who presented to the mental health clinic with his wife. The patient recounted that he had wanted to “be healthy” since childhood and has focused on exercise and proper diet, but anxiety about diet and food intake have steadily increased. Two years ago, he adopted a vegetarian diet by progressively eliminating several foods and food groups from his diet. He now feels “proud” to eat certain organically grown fruits, vegetables, nuts, beans, and drink only fruit or vegetable juice.
His wife stated that he spent between 3 and 5 hours daily preparing food or talking to friends and family about “correct foods to eat.” He also believed that errors in dietary habits caused physical or mental illnesses. He reported significant guilt and shame whenever he “slips up” on his dietary regimen and eats anything containing seafood, beef, or pork products, which he corrects by a day of fasting. His wife was frustrated because he refused to go to restaurants and started declining offers from friends to eat dinner at their homes unless he could bring his prepared food. He describes feeling “annoyed” when he sees other people eating fast food or meat.
Mr. P reported no significant medical or surgical history. His family history was significant for anxiety in his mother. He used to drink alcohol socially but ceased a few years ago due to its carbohydrate content. He never smoked or used illicit drugs.
A mental status exam revealed a thin male who appeared his stated age. He was cooperative, casually dressed, and made fair eye contact. He spoke clearly with an anxious tone and appropriate rate and volume. His affect was congruent with stated anxious mood. He was alert, awake, and oriented to person, place, and time. He reported no paranoia, auditory or visual hallucinations, and suicidal or homicidal ideation.
A physical exam revealed a thin male in no distress who measured 5 feet 10 inches tall and weighed 145 pounds, which yielded a body mass index of 20.8. His vitals included temperature of 98° F, blood pressure 115/76, pulse 74, and oxygen saturation 98% on room air. The remaining physical examination revealed no abnormalities. A complete blood count, thyroid function, urinalysis, and urine drug screens were within normal limits. Comprehensive metabolic profile revealed decreased sodium of 130 meq/L. Electrocardiogram revealed bradycardia.
An ON diagnosis is made primarily through a clinical interview. Collateral information from individuals familiar with the patient can be helpful. Experts have proposed and recently revised criteria for ON (Table). Although the ORTO-15 assessment tool may assist with diagnosis, the tool does not substitute for the clinical interview.
Discussion
There is no reliable measure of prevalence of ON, though Varga and colleagues initially estimated ON to occur in 6.9% of the general population, and ON may occur more frequently in health care professionals and performance artists.3 However, these may be overestimates, as the assessment tool used in the study does not adequately separate people with healthy eating habits from those with ON.4,5
Most prevalence studies were conducted in Europe and Turkey, and prevalence of ON may differ in the U.S. population. A recent assessment determined a prevalence of about 1%, similar to that of other eating disorders.5 No study has reported a correlation between ON and gender, but a survey of 448 college students in the U.S. (mean age 22 years) reported highest ON tendencies in Hispanic/Latino and overweight/obese students.6
Relationship to Other Illnesses
There is significant debate whether ON is a single syndrome, a variance of other syndromes, or a behavioral and culturally influenced attitude.7,8 Although ON may lead to or be comorbid with anorexia nervosa (AN) or obsessive-compulsive disorder (OCD), subtle differences exist between ON and these conditions.
To meet DSM-5 diagnostic criteria for AN, patients must weigh below minimally normal weight for their height and age, have an intense fear of gaining weight or becoming fat, and have a disturbed experience of their weight or body shape or cannot recognize the severity of the low weight.2 In contrast, an individual with ON may possess normal or low-normal weight. Patients with AN focus on food quantity, while patients with ON tend to focus on food quality. As summarized by Bratman, “People are ashamed of their anorexia, but they actively evangelize their orthorexia. People with anorexia skip meals; people with orthorexia do not (unless they are fasting). Those with anorexia focus only on avoiding foods, while those with orthorexia both avoid foods they think are bad and embrace foods they think are super-healthy.”9
Similarities between ON and OCD include anxiety, a need to exert control, and perfectionism. However, patients with OCD tend to report distress from compulsive behavior and a desire to change, thus exhibiting insight into their illness.8,10 Similarities between obsessive-compulsive personality disorder (OCPD) and ON include perfectionism, rigid thinking, excessive devotion, hypermorality, and a preoccupation with details and perceived rules.11
While no studies have yet described ON as a feature of somatoform disorders, some experts have hypothesized that preoccupation with illness in a patient with somatization disorder may engender a preoccupation with food and diet as a way to combat either real or perceived illness.11 Finally, there is a report of ON associated with the prodromal phase of schizophrenia, and the development of ON may increase risk for future psychotic disorders.11,12
Pathophysiology
The exact cause of ON is unknown, though it is likely multifactorial. Individuals with ON have neurocognitive deficits similar to those seen in patients with AN and OCD, including impairments in set-shifting (flexible problem solving), external attention, and working memory.11,13 Given these cognitive deficits as well as similar symptomatology, there may be analogous brain dysfunction in patients with ON and AN or OCD. Neuroimaging studies of patients with AN have revealed dysregulation of dopamine transmission in the reward circuitry of the ventral striatum and the food regulatory mechanism in the hypothalamus.14
Dysmorphology of and dysfunction in neural circuitry, particularly the cortico-striato-thalamo-cortical pathway, have been implicated in OCD.15 Neuroimaging studies have revealed increased volume and activation of the orbitofrontal cortex, which may be associated with obsessions and difficulty with extinction recall.14,15 In contrast, decreased volume and activity of the thalamus may impair its ability to inhibit the orbitofrontal cortex.15,16 Decreased volume and activity of the cingulate gyrus may be associated with difficulty in error monitoring and fear conditioning, while overactivation of the parietal lobe and cerebellum may be associated with compulsive behaviors.15,16
Risk Factors
Factors that contribute to the development of AN and possibly ON include development of food preferences, inherited differences in taste perception, food neophobia or pickiness, being premorbidly overweight or obese, parental feeding practices, and a history of parental eating disorders.14 One survey associated orthorexic tendencies with perfectionism, appearance orientation, overweight preoccupation, self-classified weight, and fearful and dismissing attachment styles.17 Significant predictors of ON included overweight preoccupation, appearance orientation, and a history of an eating disorder.17
Treatment
In contrast to patients with AN, patients with ON may be easily amenable to treatment, given their pursuit of and emphasis on wellness.18 Experts recommend a multidisciplinary team approach that includes physicians, psychotherapists, and dieticians.11 Treatment may be undertaken in an outpatient setting, but hospitalization for refeeding is recommended in cases with significant weight loss or malnourishment.11 Physical examination and laboratory studies are warranted, as excessive dietary restrictions can lead to weight loss and medical complications similar to those seen in AN, including osteopenia, anemia, hyponatremia, pancytopenia, bradycardia, and even pneumothorax and pneumomediastinum.19-21
There are no reported studies exploring the efficacy of psychotherapy or psychotropic medications for patients with ON. However, several treatments have been proposed given the symptom overlap with AN. Serotonin reuptake inhibitors may be beneficial for anxiety and obsessive-compulsive traits.18 However, patients with ON may refuse medications as unnatural substances.18
Cognitive behavioral therapy may be beneficial to address perfectionism and cognitive distortions, and exposure and response prevention may reduce obsessive-compulsive behaviors.11 Relaxation therapy may reduce mealtime anxiety. Psychoeducation may correct inaccurate beliefs about food groups, purity, and preparation, but it may induce emotional stress for the patient with ON.11
Conclusion
Orthorexia nervosa is perhaps best summarized as an obsession with healthy eating with associated restrictive behaviors. However, the attempt to attain optimum health through attention to diet may lead to malnourishment, loss of relationships, and poor quality of life.11 It is a little-understood disorder with uncertain etiology, imprecise assessment tools, and no formal diagnostic criteria or classification. Orthorexic characteristics vary from normal to pathologic in degree, and making a diagnosis remains a clinical judgment.22 Further research is needed to develop valid diagnostic tools and determining whether ON should be classified as a unique illness or a variation of other eating or anxiety disorders. Further research also may identify the etiology of ON, thus enabling targeted multidisciplinary treatment.
1. Bratman S. Health food junkie. Yoga J. 1997;136:42-50.
2. American Psychiatric Association. Feeding and eating disorders. In: Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013:329-354.
3. Varga M, Dukay-Szabó S, Túry F, van Furth EF. Evidence and gaps in the literature on orthorexia nervosa. Eat Weight Disord. 2013;18(2):103-111.
4. Donini LM, Marsili D, Graziani MP, Imbriale M, Cannella C. Orthorexia nervosa: validation of a diagnosis questionnaire. Eat Weight Disord. 2005;10(2):e28-e32.
5. Dunn TM, Gibbs J, Whitney N, Starosta A. Prevalence of orthorexia nervosa is less than 1 %: data from a US sample. Eat Weight Disord. 2016;22(1):185-192.
6. Bundros J, Clifford D, Silliman K, Neyman Morris M. Prevalence of orthorexia nervosa among college students based on Bratman’s test and associated tendencies. Appetite. 2016;101:86-94.
7. Vandereycken W. Media hype, diagnostic fad or genuine disorder? Professionals’ opinions about night eating syndrome, orthorexia, muscle dysmorphia, and emetophobia. Eat Disord. 2011;19(2):145-155.
8. Dell’Osso L, Abelli M, Carpita B, et al. Historical evolution of the concept of anorexia nervosa and relationships with orthorexia nervosa, autism, and obsessive-compulsive spectrum. Neuropsychiatr Dis Treat. 2016;12:1651-1660.
9. Bratman S. Orthorexia: an update. http://www.orthorexia.com/orthorexia-an-update. Updated October 5, 2015. Accessed April 18, 2017.
10. Dunn TM, Bratman S. On orthorexia nervosa: a review of the literature and proposed diagnostic criteria. Eat Behav. 2016;21:11-17.
11. Koven NS, Abry AW. The clinical basis of orthorexia nervosa: emerging perspectives. Neuropsychiatr Dis Treat. 2015;11:385-394.
12. Saddichha S, Babu GN, Chandra P. Orthorexia nervosa presenting as prodrome of schizophrenia. Schizophr Res. 2012;134(1):110.
13. Koven NS, Senbonmatsu R. A neuropsychological evaluation of orthorexia nervosa. Open J Psychiatry. 2013;3(2):214-222.
14. Gorwood P, Blanchet-Collet C, Chartrel N, et al. New insights in anorexia nervosa. Front Neurosci. 2016;10:256.
15. Milad MR, Rauch SL. Obsessive-compulsive disorder: beyond segregated cortico-striatal pathways. Trends Cogn Sci. 2012;16(1):43-51.
16. Tang W, Zhu Q, Gong X, Zhu C, Wang Y, Chen S. Cortico-striato-thalamo-cortical circuit abnormalities in obsessive-compulsive disorder: A voxel-based morphometric and fMRI study of the whole brain. Behav Brain Res. 2016;313:17-22.
17. Barnes MA, Caltabiano ML. The interrelationship between orthorexia nervosa, perfectionism, body image and attachment style. Eat Weight Disord. 2017;22(1):177-184.
18. Mathieu J. What is orthorexia? J Am Diet Assoc. 2005;105(10):1510-1512.
19. Catalina Zamora ML, Bote Bonaechea B, García Sánchez F, Ríos Rial B. Orthorexia nervosa. A new eating behavior disorder? [in Spanish]. Actas Esp Psiquiatr. 2005;33(1):66-68.
20. Moroze RM, Dunn TM, Craig Holland J, Yager J, Weintraub P. Microthinking about micronutrients: a case of transition from obsessions about healthy eating to near-fatal “orthorexia nervosa” and proposed diagnostic criteria. Psychosomatics. 2015;56(4):397-403.
21. Park SW, Kim JY, Go GJ, Jeon ES, Pyo HJ, Kwon YJ. Orthorexia nervosa with hyponatremia, subcutaneous emphysema, pneumomediastimum, pneumothorax, and pancytopenia. Electrolyte Blood Press. 2011;9(1):32-37.
22. Mogallapu RNG, Aynampudi AR, Scarff JR, Lippmann S. Orthorexia nervosa. The Kentucky Psychiatrist. 2012;22(3):3-6.
First named by Steven Bratman in 1997, orthorexia nervosa (ON) from the Greek ortho, meaning correct, and orexi, meaning appetite, is classified as an unspecified feeding and eating disorder in the Diagnostic and Statistical Manual of Mental Disorders 5th edition (DSM-5).1,2
Hypothetical Case
Mr. P is a 30-year-old male who presented to the mental health clinic with his wife. The patient recounted that he had wanted to “be healthy” since childhood and has focused on exercise and proper diet, but anxiety about diet and food intake have steadily increased. Two years ago, he adopted a vegetarian diet by progressively eliminating several foods and food groups from his diet. He now feels “proud” to eat certain organically grown fruits, vegetables, nuts, beans, and drink only fruit or vegetable juice.
His wife stated that he spent between 3 and 5 hours daily preparing food or talking to friends and family about “correct foods to eat.” He also believed that errors in dietary habits caused physical or mental illnesses. He reported significant guilt and shame whenever he “slips up” on his dietary regimen and eats anything containing seafood, beef, or pork products, which he corrects by a day of fasting. His wife was frustrated because he refused to go to restaurants and started declining offers from friends to eat dinner at their homes unless he could bring his prepared food. He describes feeling “annoyed” when he sees other people eating fast food or meat.
Mr. P reported no significant medical or surgical history. His family history was significant for anxiety in his mother. He used to drink alcohol socially but ceased a few years ago due to its carbohydrate content. He never smoked or used illicit drugs.
A mental status exam revealed a thin male who appeared his stated age. He was cooperative, casually dressed, and made fair eye contact. He spoke clearly with an anxious tone and appropriate rate and volume. His affect was congruent with stated anxious mood. He was alert, awake, and oriented to person, place, and time. He reported no paranoia, auditory or visual hallucinations, and suicidal or homicidal ideation.
A physical exam revealed a thin male in no distress who measured 5 feet 10 inches tall and weighed 145 pounds, which yielded a body mass index of 20.8. His vitals included temperature of 98° F, blood pressure 115/76, pulse 74, and oxygen saturation 98% on room air. The remaining physical examination revealed no abnormalities. A complete blood count, thyroid function, urinalysis, and urine drug screens were within normal limits. Comprehensive metabolic profile revealed decreased sodium of 130 meq/L. Electrocardiogram revealed bradycardia.
An ON diagnosis is made primarily through a clinical interview. Collateral information from individuals familiar with the patient can be helpful. Experts have proposed and recently revised criteria for ON (Table). Although the ORTO-15 assessment tool may assist with diagnosis, the tool does not substitute for the clinical interview.
Discussion
There is no reliable measure of prevalence of ON, though Varga and colleagues initially estimated ON to occur in 6.9% of the general population, and ON may occur more frequently in health care professionals and performance artists.3 However, these may be overestimates, as the assessment tool used in the study does not adequately separate people with healthy eating habits from those with ON.4,5
Most prevalence studies were conducted in Europe and Turkey, and prevalence of ON may differ in the U.S. population. A recent assessment determined a prevalence of about 1%, similar to that of other eating disorders.5 No study has reported a correlation between ON and gender, but a survey of 448 college students in the U.S. (mean age 22 years) reported highest ON tendencies in Hispanic/Latino and overweight/obese students.6
Relationship to Other Illnesses
There is significant debate whether ON is a single syndrome, a variance of other syndromes, or a behavioral and culturally influenced attitude.7,8 Although ON may lead to or be comorbid with anorexia nervosa (AN) or obsessive-compulsive disorder (OCD), subtle differences exist between ON and these conditions.
To meet DSM-5 diagnostic criteria for AN, patients must weigh below minimally normal weight for their height and age, have an intense fear of gaining weight or becoming fat, and have a disturbed experience of their weight or body shape or cannot recognize the severity of the low weight.2 In contrast, an individual with ON may possess normal or low-normal weight. Patients with AN focus on food quantity, while patients with ON tend to focus on food quality. As summarized by Bratman, “People are ashamed of their anorexia, but they actively evangelize their orthorexia. People with anorexia skip meals; people with orthorexia do not (unless they are fasting). Those with anorexia focus only on avoiding foods, while those with orthorexia both avoid foods they think are bad and embrace foods they think are super-healthy.”9
Similarities between ON and OCD include anxiety, a need to exert control, and perfectionism. However, patients with OCD tend to report distress from compulsive behavior and a desire to change, thus exhibiting insight into their illness.8,10 Similarities between obsessive-compulsive personality disorder (OCPD) and ON include perfectionism, rigid thinking, excessive devotion, hypermorality, and a preoccupation with details and perceived rules.11
While no studies have yet described ON as a feature of somatoform disorders, some experts have hypothesized that preoccupation with illness in a patient with somatization disorder may engender a preoccupation with food and diet as a way to combat either real or perceived illness.11 Finally, there is a report of ON associated with the prodromal phase of schizophrenia, and the development of ON may increase risk for future psychotic disorders.11,12
Pathophysiology
The exact cause of ON is unknown, though it is likely multifactorial. Individuals with ON have neurocognitive deficits similar to those seen in patients with AN and OCD, including impairments in set-shifting (flexible problem solving), external attention, and working memory.11,13 Given these cognitive deficits as well as similar symptomatology, there may be analogous brain dysfunction in patients with ON and AN or OCD. Neuroimaging studies of patients with AN have revealed dysregulation of dopamine transmission in the reward circuitry of the ventral striatum and the food regulatory mechanism in the hypothalamus.14
Dysmorphology of and dysfunction in neural circuitry, particularly the cortico-striato-thalamo-cortical pathway, have been implicated in OCD.15 Neuroimaging studies have revealed increased volume and activation of the orbitofrontal cortex, which may be associated with obsessions and difficulty with extinction recall.14,15 In contrast, decreased volume and activity of the thalamus may impair its ability to inhibit the orbitofrontal cortex.15,16 Decreased volume and activity of the cingulate gyrus may be associated with difficulty in error monitoring and fear conditioning, while overactivation of the parietal lobe and cerebellum may be associated with compulsive behaviors.15,16
Risk Factors
Factors that contribute to the development of AN and possibly ON include development of food preferences, inherited differences in taste perception, food neophobia or pickiness, being premorbidly overweight or obese, parental feeding practices, and a history of parental eating disorders.14 One survey associated orthorexic tendencies with perfectionism, appearance orientation, overweight preoccupation, self-classified weight, and fearful and dismissing attachment styles.17 Significant predictors of ON included overweight preoccupation, appearance orientation, and a history of an eating disorder.17
Treatment
In contrast to patients with AN, patients with ON may be easily amenable to treatment, given their pursuit of and emphasis on wellness.18 Experts recommend a multidisciplinary team approach that includes physicians, psychotherapists, and dieticians.11 Treatment may be undertaken in an outpatient setting, but hospitalization for refeeding is recommended in cases with significant weight loss or malnourishment.11 Physical examination and laboratory studies are warranted, as excessive dietary restrictions can lead to weight loss and medical complications similar to those seen in AN, including osteopenia, anemia, hyponatremia, pancytopenia, bradycardia, and even pneumothorax and pneumomediastinum.19-21
There are no reported studies exploring the efficacy of psychotherapy or psychotropic medications for patients with ON. However, several treatments have been proposed given the symptom overlap with AN. Serotonin reuptake inhibitors may be beneficial for anxiety and obsessive-compulsive traits.18 However, patients with ON may refuse medications as unnatural substances.18
Cognitive behavioral therapy may be beneficial to address perfectionism and cognitive distortions, and exposure and response prevention may reduce obsessive-compulsive behaviors.11 Relaxation therapy may reduce mealtime anxiety. Psychoeducation may correct inaccurate beliefs about food groups, purity, and preparation, but it may induce emotional stress for the patient with ON.11
Conclusion
Orthorexia nervosa is perhaps best summarized as an obsession with healthy eating with associated restrictive behaviors. However, the attempt to attain optimum health through attention to diet may lead to malnourishment, loss of relationships, and poor quality of life.11 It is a little-understood disorder with uncertain etiology, imprecise assessment tools, and no formal diagnostic criteria or classification. Orthorexic characteristics vary from normal to pathologic in degree, and making a diagnosis remains a clinical judgment.22 Further research is needed to develop valid diagnostic tools and determining whether ON should be classified as a unique illness or a variation of other eating or anxiety disorders. Further research also may identify the etiology of ON, thus enabling targeted multidisciplinary treatment.
First named by Steven Bratman in 1997, orthorexia nervosa (ON) from the Greek ortho, meaning correct, and orexi, meaning appetite, is classified as an unspecified feeding and eating disorder in the Diagnostic and Statistical Manual of Mental Disorders 5th edition (DSM-5).1,2
Hypothetical Case
Mr. P is a 30-year-old male who presented to the mental health clinic with his wife. The patient recounted that he had wanted to “be healthy” since childhood and has focused on exercise and proper diet, but anxiety about diet and food intake have steadily increased. Two years ago, he adopted a vegetarian diet by progressively eliminating several foods and food groups from his diet. He now feels “proud” to eat certain organically grown fruits, vegetables, nuts, beans, and drink only fruit or vegetable juice.
His wife stated that he spent between 3 and 5 hours daily preparing food or talking to friends and family about “correct foods to eat.” He also believed that errors in dietary habits caused physical or mental illnesses. He reported significant guilt and shame whenever he “slips up” on his dietary regimen and eats anything containing seafood, beef, or pork products, which he corrects by a day of fasting. His wife was frustrated because he refused to go to restaurants and started declining offers from friends to eat dinner at their homes unless he could bring his prepared food. He describes feeling “annoyed” when he sees other people eating fast food or meat.
Mr. P reported no significant medical or surgical history. His family history was significant for anxiety in his mother. He used to drink alcohol socially but ceased a few years ago due to its carbohydrate content. He never smoked or used illicit drugs.
A mental status exam revealed a thin male who appeared his stated age. He was cooperative, casually dressed, and made fair eye contact. He spoke clearly with an anxious tone and appropriate rate and volume. His affect was congruent with stated anxious mood. He was alert, awake, and oriented to person, place, and time. He reported no paranoia, auditory or visual hallucinations, and suicidal or homicidal ideation.
A physical exam revealed a thin male in no distress who measured 5 feet 10 inches tall and weighed 145 pounds, which yielded a body mass index of 20.8. His vitals included temperature of 98° F, blood pressure 115/76, pulse 74, and oxygen saturation 98% on room air. The remaining physical examination revealed no abnormalities. A complete blood count, thyroid function, urinalysis, and urine drug screens were within normal limits. Comprehensive metabolic profile revealed decreased sodium of 130 meq/L. Electrocardiogram revealed bradycardia.
An ON diagnosis is made primarily through a clinical interview. Collateral information from individuals familiar with the patient can be helpful. Experts have proposed and recently revised criteria for ON (Table). Although the ORTO-15 assessment tool may assist with diagnosis, the tool does not substitute for the clinical interview.
Discussion
There is no reliable measure of prevalence of ON, though Varga and colleagues initially estimated ON to occur in 6.9% of the general population, and ON may occur more frequently in health care professionals and performance artists.3 However, these may be overestimates, as the assessment tool used in the study does not adequately separate people with healthy eating habits from those with ON.4,5
Most prevalence studies were conducted in Europe and Turkey, and prevalence of ON may differ in the U.S. population. A recent assessment determined a prevalence of about 1%, similar to that of other eating disorders.5 No study has reported a correlation between ON and gender, but a survey of 448 college students in the U.S. (mean age 22 years) reported highest ON tendencies in Hispanic/Latino and overweight/obese students.6
Relationship to Other Illnesses
There is significant debate whether ON is a single syndrome, a variance of other syndromes, or a behavioral and culturally influenced attitude.7,8 Although ON may lead to or be comorbid with anorexia nervosa (AN) or obsessive-compulsive disorder (OCD), subtle differences exist between ON and these conditions.
To meet DSM-5 diagnostic criteria for AN, patients must weigh below minimally normal weight for their height and age, have an intense fear of gaining weight or becoming fat, and have a disturbed experience of their weight or body shape or cannot recognize the severity of the low weight.2 In contrast, an individual with ON may possess normal or low-normal weight. Patients with AN focus on food quantity, while patients with ON tend to focus on food quality. As summarized by Bratman, “People are ashamed of their anorexia, but they actively evangelize their orthorexia. People with anorexia skip meals; people with orthorexia do not (unless they are fasting). Those with anorexia focus only on avoiding foods, while those with orthorexia both avoid foods they think are bad and embrace foods they think are super-healthy.”9
Similarities between ON and OCD include anxiety, a need to exert control, and perfectionism. However, patients with OCD tend to report distress from compulsive behavior and a desire to change, thus exhibiting insight into their illness.8,10 Similarities between obsessive-compulsive personality disorder (OCPD) and ON include perfectionism, rigid thinking, excessive devotion, hypermorality, and a preoccupation with details and perceived rules.11
While no studies have yet described ON as a feature of somatoform disorders, some experts have hypothesized that preoccupation with illness in a patient with somatization disorder may engender a preoccupation with food and diet as a way to combat either real or perceived illness.11 Finally, there is a report of ON associated with the prodromal phase of schizophrenia, and the development of ON may increase risk for future psychotic disorders.11,12
Pathophysiology
The exact cause of ON is unknown, though it is likely multifactorial. Individuals with ON have neurocognitive deficits similar to those seen in patients with AN and OCD, including impairments in set-shifting (flexible problem solving), external attention, and working memory.11,13 Given these cognitive deficits as well as similar symptomatology, there may be analogous brain dysfunction in patients with ON and AN or OCD. Neuroimaging studies of patients with AN have revealed dysregulation of dopamine transmission in the reward circuitry of the ventral striatum and the food regulatory mechanism in the hypothalamus.14
Dysmorphology of and dysfunction in neural circuitry, particularly the cortico-striato-thalamo-cortical pathway, have been implicated in OCD.15 Neuroimaging studies have revealed increased volume and activation of the orbitofrontal cortex, which may be associated with obsessions and difficulty with extinction recall.14,15 In contrast, decreased volume and activity of the thalamus may impair its ability to inhibit the orbitofrontal cortex.15,16 Decreased volume and activity of the cingulate gyrus may be associated with difficulty in error monitoring and fear conditioning, while overactivation of the parietal lobe and cerebellum may be associated with compulsive behaviors.15,16
Risk Factors
Factors that contribute to the development of AN and possibly ON include development of food preferences, inherited differences in taste perception, food neophobia or pickiness, being premorbidly overweight or obese, parental feeding practices, and a history of parental eating disorders.14 One survey associated orthorexic tendencies with perfectionism, appearance orientation, overweight preoccupation, self-classified weight, and fearful and dismissing attachment styles.17 Significant predictors of ON included overweight preoccupation, appearance orientation, and a history of an eating disorder.17
Treatment
In contrast to patients with AN, patients with ON may be easily amenable to treatment, given their pursuit of and emphasis on wellness.18 Experts recommend a multidisciplinary team approach that includes physicians, psychotherapists, and dieticians.11 Treatment may be undertaken in an outpatient setting, but hospitalization for refeeding is recommended in cases with significant weight loss or malnourishment.11 Physical examination and laboratory studies are warranted, as excessive dietary restrictions can lead to weight loss and medical complications similar to those seen in AN, including osteopenia, anemia, hyponatremia, pancytopenia, bradycardia, and even pneumothorax and pneumomediastinum.19-21
There are no reported studies exploring the efficacy of psychotherapy or psychotropic medications for patients with ON. However, several treatments have been proposed given the symptom overlap with AN. Serotonin reuptake inhibitors may be beneficial for anxiety and obsessive-compulsive traits.18 However, patients with ON may refuse medications as unnatural substances.18
Cognitive behavioral therapy may be beneficial to address perfectionism and cognitive distortions, and exposure and response prevention may reduce obsessive-compulsive behaviors.11 Relaxation therapy may reduce mealtime anxiety. Psychoeducation may correct inaccurate beliefs about food groups, purity, and preparation, but it may induce emotional stress for the patient with ON.11
Conclusion
Orthorexia nervosa is perhaps best summarized as an obsession with healthy eating with associated restrictive behaviors. However, the attempt to attain optimum health through attention to diet may lead to malnourishment, loss of relationships, and poor quality of life.11 It is a little-understood disorder with uncertain etiology, imprecise assessment tools, and no formal diagnostic criteria or classification. Orthorexic characteristics vary from normal to pathologic in degree, and making a diagnosis remains a clinical judgment.22 Further research is needed to develop valid diagnostic tools and determining whether ON should be classified as a unique illness or a variation of other eating or anxiety disorders. Further research also may identify the etiology of ON, thus enabling targeted multidisciplinary treatment.
1. Bratman S. Health food junkie. Yoga J. 1997;136:42-50.
2. American Psychiatric Association. Feeding and eating disorders. In: Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013:329-354.
3. Varga M, Dukay-Szabó S, Túry F, van Furth EF. Evidence and gaps in the literature on orthorexia nervosa. Eat Weight Disord. 2013;18(2):103-111.
4. Donini LM, Marsili D, Graziani MP, Imbriale M, Cannella C. Orthorexia nervosa: validation of a diagnosis questionnaire. Eat Weight Disord. 2005;10(2):e28-e32.
5. Dunn TM, Gibbs J, Whitney N, Starosta A. Prevalence of orthorexia nervosa is less than 1 %: data from a US sample. Eat Weight Disord. 2016;22(1):185-192.
6. Bundros J, Clifford D, Silliman K, Neyman Morris M. Prevalence of orthorexia nervosa among college students based on Bratman’s test and associated tendencies. Appetite. 2016;101:86-94.
7. Vandereycken W. Media hype, diagnostic fad or genuine disorder? Professionals’ opinions about night eating syndrome, orthorexia, muscle dysmorphia, and emetophobia. Eat Disord. 2011;19(2):145-155.
8. Dell’Osso L, Abelli M, Carpita B, et al. Historical evolution of the concept of anorexia nervosa and relationships with orthorexia nervosa, autism, and obsessive-compulsive spectrum. Neuropsychiatr Dis Treat. 2016;12:1651-1660.
9. Bratman S. Orthorexia: an update. http://www.orthorexia.com/orthorexia-an-update. Updated October 5, 2015. Accessed April 18, 2017.
10. Dunn TM, Bratman S. On orthorexia nervosa: a review of the literature and proposed diagnostic criteria. Eat Behav. 2016;21:11-17.
11. Koven NS, Abry AW. The clinical basis of orthorexia nervosa: emerging perspectives. Neuropsychiatr Dis Treat. 2015;11:385-394.
12. Saddichha S, Babu GN, Chandra P. Orthorexia nervosa presenting as prodrome of schizophrenia. Schizophr Res. 2012;134(1):110.
13. Koven NS, Senbonmatsu R. A neuropsychological evaluation of orthorexia nervosa. Open J Psychiatry. 2013;3(2):214-222.
14. Gorwood P, Blanchet-Collet C, Chartrel N, et al. New insights in anorexia nervosa. Front Neurosci. 2016;10:256.
15. Milad MR, Rauch SL. Obsessive-compulsive disorder: beyond segregated cortico-striatal pathways. Trends Cogn Sci. 2012;16(1):43-51.
16. Tang W, Zhu Q, Gong X, Zhu C, Wang Y, Chen S. Cortico-striato-thalamo-cortical circuit abnormalities in obsessive-compulsive disorder: A voxel-based morphometric and fMRI study of the whole brain. Behav Brain Res. 2016;313:17-22.
17. Barnes MA, Caltabiano ML. The interrelationship between orthorexia nervosa, perfectionism, body image and attachment style. Eat Weight Disord. 2017;22(1):177-184.
18. Mathieu J. What is orthorexia? J Am Diet Assoc. 2005;105(10):1510-1512.
19. Catalina Zamora ML, Bote Bonaechea B, García Sánchez F, Ríos Rial B. Orthorexia nervosa. A new eating behavior disorder? [in Spanish]. Actas Esp Psiquiatr. 2005;33(1):66-68.
20. Moroze RM, Dunn TM, Craig Holland J, Yager J, Weintraub P. Microthinking about micronutrients: a case of transition from obsessions about healthy eating to near-fatal “orthorexia nervosa” and proposed diagnostic criteria. Psychosomatics. 2015;56(4):397-403.
21. Park SW, Kim JY, Go GJ, Jeon ES, Pyo HJ, Kwon YJ. Orthorexia nervosa with hyponatremia, subcutaneous emphysema, pneumomediastimum, pneumothorax, and pancytopenia. Electrolyte Blood Press. 2011;9(1):32-37.
22. Mogallapu RNG, Aynampudi AR, Scarff JR, Lippmann S. Orthorexia nervosa. The Kentucky Psychiatrist. 2012;22(3):3-6.
1. Bratman S. Health food junkie. Yoga J. 1997;136:42-50.
2. American Psychiatric Association. Feeding and eating disorders. In: Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013:329-354.
3. Varga M, Dukay-Szabó S, Túry F, van Furth EF. Evidence and gaps in the literature on orthorexia nervosa. Eat Weight Disord. 2013;18(2):103-111.
4. Donini LM, Marsili D, Graziani MP, Imbriale M, Cannella C. Orthorexia nervosa: validation of a diagnosis questionnaire. Eat Weight Disord. 2005;10(2):e28-e32.
5. Dunn TM, Gibbs J, Whitney N, Starosta A. Prevalence of orthorexia nervosa is less than 1 %: data from a US sample. Eat Weight Disord. 2016;22(1):185-192.
6. Bundros J, Clifford D, Silliman K, Neyman Morris M. Prevalence of orthorexia nervosa among college students based on Bratman’s test and associated tendencies. Appetite. 2016;101:86-94.
7. Vandereycken W. Media hype, diagnostic fad or genuine disorder? Professionals’ opinions about night eating syndrome, orthorexia, muscle dysmorphia, and emetophobia. Eat Disord. 2011;19(2):145-155.
8. Dell’Osso L, Abelli M, Carpita B, et al. Historical evolution of the concept of anorexia nervosa and relationships with orthorexia nervosa, autism, and obsessive-compulsive spectrum. Neuropsychiatr Dis Treat. 2016;12:1651-1660.
9. Bratman S. Orthorexia: an update. http://www.orthorexia.com/orthorexia-an-update. Updated October 5, 2015. Accessed April 18, 2017.
10. Dunn TM, Bratman S. On orthorexia nervosa: a review of the literature and proposed diagnostic criteria. Eat Behav. 2016;21:11-17.
11. Koven NS, Abry AW. The clinical basis of orthorexia nervosa: emerging perspectives. Neuropsychiatr Dis Treat. 2015;11:385-394.
12. Saddichha S, Babu GN, Chandra P. Orthorexia nervosa presenting as prodrome of schizophrenia. Schizophr Res. 2012;134(1):110.
13. Koven NS, Senbonmatsu R. A neuropsychological evaluation of orthorexia nervosa. Open J Psychiatry. 2013;3(2):214-222.
14. Gorwood P, Blanchet-Collet C, Chartrel N, et al. New insights in anorexia nervosa. Front Neurosci. 2016;10:256.
15. Milad MR, Rauch SL. Obsessive-compulsive disorder: beyond segregated cortico-striatal pathways. Trends Cogn Sci. 2012;16(1):43-51.
16. Tang W, Zhu Q, Gong X, Zhu C, Wang Y, Chen S. Cortico-striato-thalamo-cortical circuit abnormalities in obsessive-compulsive disorder: A voxel-based morphometric and fMRI study of the whole brain. Behav Brain Res. 2016;313:17-22.
17. Barnes MA, Caltabiano ML. The interrelationship between orthorexia nervosa, perfectionism, body image and attachment style. Eat Weight Disord. 2017;22(1):177-184.
18. Mathieu J. What is orthorexia? J Am Diet Assoc. 2005;105(10):1510-1512.
19. Catalina Zamora ML, Bote Bonaechea B, García Sánchez F, Ríos Rial B. Orthorexia nervosa. A new eating behavior disorder? [in Spanish]. Actas Esp Psiquiatr. 2005;33(1):66-68.
20. Moroze RM, Dunn TM, Craig Holland J, Yager J, Weintraub P. Microthinking about micronutrients: a case of transition from obsessions about healthy eating to near-fatal “orthorexia nervosa” and proposed diagnostic criteria. Psychosomatics. 2015;56(4):397-403.
21. Park SW, Kim JY, Go GJ, Jeon ES, Pyo HJ, Kwon YJ. Orthorexia nervosa with hyponatremia, subcutaneous emphysema, pneumomediastimum, pneumothorax, and pancytopenia. Electrolyte Blood Press. 2011;9(1):32-37.
22. Mogallapu RNG, Aynampudi AR, Scarff JR, Lippmann S. Orthorexia nervosa. The Kentucky Psychiatrist. 2012;22(3):3-6.
Interprofessional Education in Patient Aligned Care Team Primary Care-Mental Health Integration
Over the past 10 years, the VHA has been a national leader in primary care-mental health integration (PC-MHI) within patient aligned care teams (PACTs).1,2 Studies of the PC-MHI collaborative care model consistently have shown increased access to MH services, higher levels of MH treatment engagement, improved MH treatment outcomes, and high patient and provider satisfaction.3-7 Primary care-mental health integration relies heavily on interprofessional team-based practice with providers from diverse educational and clinical backgrounds who work together to deliver integrated mental and behavioral health services within PACTs. This model requires a unique blending of professional cultures and communication and practice styles.
To sustain PC-MHI in PACT, health care professionals (HCPs) must be well trained to work effectively in interprofessional teams. Across health care organizations, training in collaborative interprofessional team-based practice has been identified as an important and challenging task.8-11
Integrating educational experiences among different HCP learners is an approach to developing competency in interprofessional collaboration early in training. The World Health Organization defined interprofessional education (IPE) as occurring “when students from two or more professions learn about, from, and with each other to enable effective collaboration and improve health outcomes.”9 Fundamental to this definition is the belief that interaction among learners from different disciplines during their training develops competency in subsequent effective collaborative practice. Studies of IPE in MH professional training have found that prelicensure IPE contributes to increased knowledge of roles and responsibilities of different disciplines, improved interprofessional communication and attitudes, and increased willingness to work in teams.12-17
Interprofessional education is a valuable training model, but developing interprofessional learning experiences in a system of diverse and often siloed training programs is difficult. More information about design and implementation of IPE training experiences is needed, particularly in outpatient settings in which integration of traditionally separate discipline-specific care is central to the health care mission. The VA PACT PC-MHI is a strong team-based care model that represents a unique opportunity for training across disciplines in interprofessional collaborative care.
To find innovative approaches to meeting the need for IPE in PACT PC-MHI, the authors developed a new IPE program in PC-MHI at the William S. Middleton Memorial Veterans Hospital (WSMMVH) in Madison, Wisconsin. This article reviews the development, implementation, and first-year evaluation of the training program and discusses the challenges and the IPE areas in need of improvement in PACT PC-MHI.
Methods
In 2012, the VHA launched phase 1 of the Mental Health Education Expansion Initiative (MHEEI), a collaboration of the Office of Academic Affiliations (OAA), VHA Mental Health Services (VHA-MHS), and the Office of Mental Health Operations (OMHO).18 The MHEEI was intended to “increase expertise in critical areas of need, expand the recruitment pipeline of well-trained, highly qualified health care providers in behavioral and mental health disciplines, and promote the utilization of interprofessional team-based care.”18 In response, WSMMVH organized a planning committee and submitted a funding request through the section of MHEEI called PACT With Integrated Behavioral Health Providers. The planning committee included training program directors and staff from psychiatry, pharmacy, social work, psychology, and primary care. The authors received funding for trainees in psychiatry (postgraduate year 4 [PGY-4]), pharmacy/MH residency (PGY-2), pharmacy/ambulatory care (PGY-1), and social work (interns).
Curriculum Development
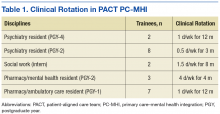
The planning committee met regularly for 6 months to develop the organization, learning objectives, educational strategies, and implementation plan for the IPE program. The program was organized as a 4- to 12-month clinical rotation with the PC-MHI team in PACT, combined with 12 months of protected weekly IPE time (Table 1).
Learning Objectives
To better understand the educational needs and foci for learning objectives, the interprofessional planning committee reviewed guidelines on training in integrated care and collaborative team-based practice.2,9,10,19-21 These guidelines were compared with existing training opportunities for each discipline to identify training gaps and needs.
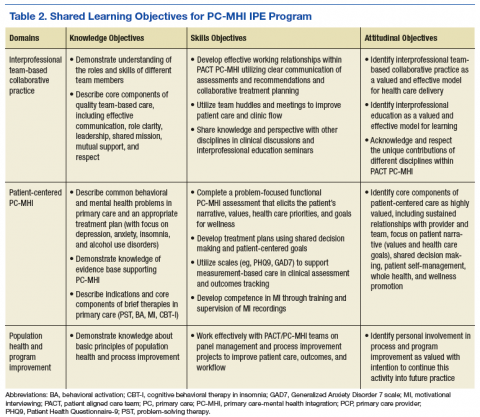
Learning objectives were organized into 3 domains: patient-centered PC-MHI, collaborative team-based practice, and population health and program improvement. Table 2 outlines the shared learning objectives linked to each domain that were common to the psychiatry, pharmacy, and social work disciplines. Although many of the learning objectives were shared among all disciplines, each trainee also had discipline-specific clinical activities and learning objectives. Psychiatry and pharmacy residents focused on primary care psychiatric medication consultation and care management for antidepressant medication starts. Social work interns focused on psychosocial and functional assessment and brief problem-focused psychotherapies. Learning objectives were met through direct veteran care in the primary care clinic as part of the PACT PC-MHI team and through interprofessional learning activities during protected weekly education time.
Implementation
Critical stakeholders in implementing the IPE program involved themselves early and throughout the planning process. Stakeholders included VAMC leadership, primary care and MH service line chiefs and clinic managers, training program directors, and PACT staff. Planning committee members gave presentations on the IPE program at MH service line and PACT meetings in the 2 months before program initiation in order to orient staff to learning objectives, program structure, and impact on PACT PC-MHI operations. Throughout the first year, the planning committee continued to meet every 2 weeks to review progress, solve implementation problems, and revise learning objectives and activities.
Trainee Clinical Activities
A wide range of educational strategies were planned to meet learning objectives across the 3 domains. There was strong emphasis on experiential learning through daily PACT and PC-MHI clinical work, team huddles and meetings, and trainee-led program improvement projects.
Psychiatry and PGY-2 pharmacy/MH residents focused on direct and indirect medication consultation and problem-focused assessments. Their clinical activities included PC-MHI medication evaluation and follow-up visits; chart reviews and e-consults for medication recommendations to PACT providers; reviews of care management data and consultations on veterans enrolled in depression and anxiety care management; “curbside consultations” for providers in PACT huddles and meetings and throughout the clinic day; and “warm handoffs,” same-day initial PC-MHI problem-focused assessments performed on PACT provider request. The residents were part of a pool of staff and trainees who performed these assessments.
PGY-1 pharmacy residents made care management phone calls for antidepressant trials for depression and anxiety. These residents were trained in motivational interviewing (MI). They applied their MI skills during care management calls focused on medication adherence and behavioral interventions for depression (eg, exercise, planning pleasurable activity) and during other clinical rotations, including tobacco cessation and medication management for diabetes and hypertension. Particularly challenging veteran cases from these clinics were cosupervised with medication management and PC-MHIstaff for added consultation on engagement, behavior change, and treatment plan adherence.
Social work interns completed initial PC-MHI psychosocial and functional assessments by phone and directly by same-day warm handoffs from PACT staff. The PC-MHI therapies they provided included problem-solving therapy, behavioral activation, stress management based on cognitive behavioral therapy, and brief alcohol interventions.
Group IPE Activities
All trainees had a weekly protected block of 3 hours during which they came together for group IPE that was designed to elicit active participation; facilitate interprofessional communication; and develop an understanding of and respect for the knowledge, culture, and practice style of the different disciplines.
Trainees participated in a Herrmann Brain Dominance Instrument (HBDI) workshop focused on developing a better understanding of individual differences in thinking and problem solving, with the goal of improving communication and learning within teams.22 In a seminar series on professionalism and boundaries in health care, trainees from each discipline gave a presentation on the traditional structure and content of their discipline’s training and discussed similarities and differences in their disciplines’ professional oaths, codes of ethics, and boundary guidelines.
Motivational interviewing training was conducted early in the year so trainees would be prepared to apply MI skills in their daily PACT PC-MHI clinical work. Motivational inteviewing is a patient-centered approach to engaging patients in health promoting behavior change. It is defined as a “directive, client-centered counseling style for eliciting behavior change by helping clients to explore and resolve ambivalence.”23
Trainees recorded MI sessions with at least 2 live-patient visits and at least 2 simulated-patient interviews (with staff serving as patient actors). The structure of MI training and supervision was deliberately designed to facilitate interprofessional communication and learning. In accord with a group supervision model for MI recorded reviews, the trainees presented their tapes to the entire learning group in the presence of a facilitating supervisor. Trainees had the opportunity to observe different interview styles and exchange feedback within a peer group of interprofessional learners.
Seminars were focused on core PC-MHI clinical content (eg, depression, anxiety, alcohol use disorders) and organized around case-based learning. Trainees divided into small teams in which representatives of each discipline offered their perspective on how to approach planning patient assessment and treatment. During the seminars, the authors engaged trainees as teachers and leaders whenever possible. All trainees presented on a topic in which they had some discipline expertise. For example, social work interns led a seminar on support and social services for victims of domestic violence, and PGY-1 pharmacy/ambulatory care residents led seminars and a panel management project focused on diabetes and depression.
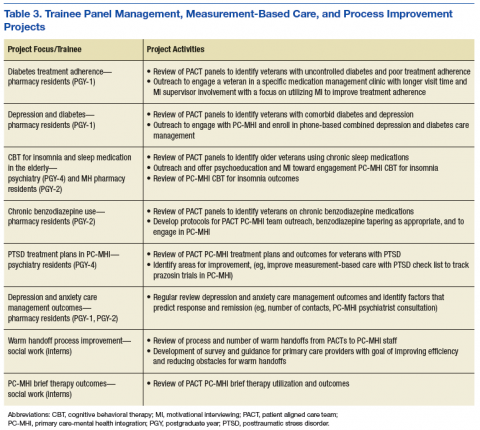
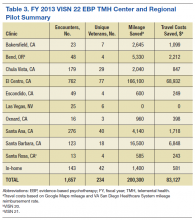
Trainees participated in several PACT PC-MHI projects focused on population- and measurement-based care, panel management, and program improvement (Table 3). Protected IPE time was used to teach trainees about population health principles and different tools for process improvement (eg, Vision-Analysis Team-Aim-Map-Measure-Change-Sustain) and provide a forum in which trainees could share their work with one another.
Evaluations
Several tools were used for trainee and program evaluations. Clinical skills were evaluated during daily supervision. Trainees began the year with PC-MHI staff directly observing all their clinical contacts with veterans. Staff evaluated and offered feedback on trainee clinical interviewing and on assessment and treatment planning skills. Once they were assessed to be ready to see veterans on their own, trainees were advanced by staff to “drop-in” direct supervision: Toward the end of a veteran’s visit, a staff preceptor entered the room to review relevant clinical findings, assessment and finalized treatment planning with the trainee and veteran. When appropriate for trainee competence level, clinical contacts were indirectly supervised: Trainees discussed their assessment and treatment plan with a staff supervisor at the end of the day.
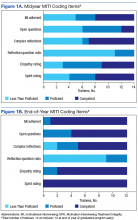
Motivational interviewing recordings were reviewed during group supervision. To objectively evaluate MI skills, supervisors who were VA-certified in MI used the Motivational Interviewing Treatment Integrity (MITI) coding tool to review and code both the live- and simulated-patient recordings.24 The MITI coding involves quantitative and qualitative analysis using standardized coding items.
Quantitative items included percentage of open-ended questions (Proficiency: 50%; competency: 70%); percentage of reflections considered complex reflections, or reflective statements adding substantial meaning or emphasis and conveying a deeper or more complex picture of what patients say (Proficiency: 40%; competency: 50%); reflection-to-question ratio (Proficiency: 1:1; competency: 2:1); and percentage of MI-adherent provider statements (Proficiency: 90%; competency: 100%).
Qualitative coding items were a global rating of therapist “empathy,” which evaluated the extent to which the trainee understood or made an effort to grasp the patient’s perspective, and “MI spirit.” This coding intended to capture the overall competence of the trainee in emphasizing collaboration, patient autonomy, and evocation of the patient experience (Proficiency score: 5; competency score: 6).
The PC-MHI teaching staff met midyear and end of year as a team to complete trainee evaluations focused on the 3 areas of learning objectives: patient-centered PC-MHI, collaborative team-based practice, and population health and program improvement. Patient-centered PC-MHI involves direct observation and supervision of trainee clinical contacts with veterans, including assessment and treatment planning, clinical documentation, and review of live- and simulated-patient MI recordings. Collaborative team-based practice involves review of trainee participation in day-to-day teamwork, huddles, team meetings, and IPE activities. Population health and program improvement involves review of trainee work on a panel measurement-based care management or program improvement project. In each learning objectives area, trainees were rated on a 3-point scale: needs improvement (1); satisfactory (2); achieved (3). Core knowledge about PC-MHI evidence base, structure, and clinical topics was assessed with case-based written examination at midyear and end of year.
Surveys and qualitative interviews were used to assess trainee perceptions about the IPE program. A midyear and end-of-year survey assessed trainee satisfaction and perceived efficacy of the IPE training program in meeting core learning objectives. A midyear survey designed by pharmacy residents as part of their program improvement project evaluated attitudes around interprofessional learning and team practice. Trainees met individually with the PC-MHI IPE director at midyear and end of year to gather qualitative feedback on the IPE program.
Outcomes
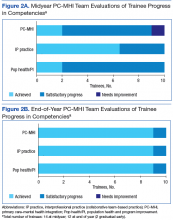
All trainees advanced to the expected level of supervision for clinical contacts (drop-in or indirect clinical supervision). Over the year, there was significant improvement in trainees’ MI skills as measured by MITI coding of at least 2 live-patient or 2 simulated-patient recordings (Figures 1A and 1B). By end of year, most trainees had reached proficiency or competency in several MITI coded items: percentage of open-ended questions (4/12 proficient, 8/12 competent), percentage of complex reflections (2/12 less than proficient, 3/12 proficient, 7/12 competent), MI adherence (1/12 proficient, 11/12 competent), global empathy rating (2/12 proficient, 10/12 competent), and global MI spirit rating (12/12 competent). Average reflection-to-question ratio for the trainee group increased from 0.63 to 0.96 from midyear to end of year, but only 3 of 12 trainees reached the proficiency level of a 1:1 ratio, and no trainee reached the competency level of a 2:1 ratio.
According to the PC-MHI team’s midyear evaluation, most trainees were already making satisfactory progress in the 3 domains of learning objectives for the training program. At end of year, 13 of 14 trainees were assessed as competent in all 3 domains (Figures 2A and B). All trainees passed the midyear and end-of-year written examinations with overall high scores (average score, 82%) demonstrating acquisition of core PC-MHI clinical knowledge.
Trainee evaluations of the IPE program were overall highly favorable at both midyear and at end of year. Trainees rated the program effective or extremely effective in developing their skills in patient-centered care, interprofessional communication, and collaborative team-based practice. They also rated the program highly effective in preparing them to use team-based practice skills in other settings. On a midyear survey, trainees moderately to strongly agreed with several positive beliefs and attitudes about team-based care.
In qualitative interviews at program completion, trainees across disciplines rated the MI training with group supervision as one of their most valuable interprofessional learning experiences. Other highly valued training experiences were PACT PC-MHI panel management projects, team-based clinical case reviews, and cross-disciplinary supervision.
Discussion
This article describes the successful development and implementation of a VA-based IPE program in PACT PC-MHI. Interprofessional clinical training and educational experiences were highly valued, and trainees identified positive attitudes and improved skills related to team-based care. These findings support previous findings that IPE is associated with high satisfaction and positive attitudes toward team-based collaborative practice.12-17 Program implementation presented several challenges: nonsynchronous academic calendars and rotation schedules, cross-disciplinary supervision regulations, variations in clinical and supervisory requirements for accreditation standards, the traditional health care hierarchy, and measurement of the impact of IPE experiences.11,25,26
Rotation schedules and academic calendars varied across the psychiatry, pharmacy, and social work home programs. Organizing different trainee rotation schedules was a significant challenge. Collaboration with training directors and support staff was crucial in planning rotations that offered a longitudinal training experience in PACT PC-MHI. Given the participants’ different starting dates, protected IPE time early in the calendar year was focused on developing clinical skills specific to the pharmacy and psychiatry trainees who would be starting in July, and IPE activities that required the presence of all trainee disciplines (eg, MI training, HBDI) were planned for after the September start of the social work interns.
Cross-disciplinary supervision was highly valued by trainees because it offered exposure to the disciplines’ different communication styles and approaches to clinical assessment and decision making. Throughout the year, however, trainees encountered several obstacles to cross-disciplinary supervision, with respect to coding/billing and home program supervisory policies. The authors worked closely with the VA administration and each training program to develop and revise supervising policies and procedures to meet the necessary administrative and program supervisory requirements for accreditation standards.
In some cases, this work resulted in dual supervision by a preceptor of the same discipline (to meet requirements for coding/billing and home program supervision) and clinical supervision by a preceptor of a different discipline (eg, in team meetings or in clinical case reviews during protected IPE time). Preceptors from each discipline met regularly to discuss challenges in cross-disciplinary supervision, review scope-of-practice issues, share information on discipline-specific training, and revise supervisory procedures.
Interprofessional education activities during weekly protected time were designed to improve collaboration and to challenge trainees to examine traditional hierarchical roles and patterns of communication in health care. An emphasis on case-based learning in small groups encouraged trainees to share perspectives on their discipline-specific approach to assessment and treatment planning. Motivational interviewing training, one of the IPE experiences most valued by trainees and staff, created the opportunity for a truly shared learning environment, as trainees largely started at about the same skill level despite having different educational backgrounds. Group supervision of MI recordings offered trainees the opportunity to learn from each other and develop comfort in offering and receiving interprofessional constructive feedback.
Limitations
There were considerable methodologic limitations in the authors’ efforts to evaluate the impact of this training program on trainee attitudes and skills in collaborative team-based practice. Although trainee surveys revealed highly positive attitudes and beliefs about team-based care as well as perceived competence in collaborative practice, these were not validated surveys, and changes could not be accurately measured over time (trainees were not assessed at baseline). Trainees also were involved in other clinical teams within the VA during the year, and it is difficult to assess the specific impact of their PC-MHI IPE experiences. The PC-MHI staff evaluations of trainee competence in collaborative team-based practice were largely observational and potentially vulnerable to biases, as the staff evaluating trainee competence also were part of the IPE program planning process and invested in its success.
To address these limitations in the future, the authors will use better assessment tools at baseline and end of year to more effectively evaluate the impact of the training program on teamwork skills as well changes in attitudes and beliefs about interprofessional learning and teamwork. The Attitudes Toward Interprofessional Health Care Teams Scale and the Perceptions of Effective Interpersonal Teams Scale should be considered as they have published reliability and validity.27,28 The authors could improve the reliability and depth of trainee evaluations with use of a “360-degree evaluation” model for trainee evaluations that includes other PACT members (beyond PC-MHI staff) as well as veterans.
Assessment of MI skills and competency was limited by use of both live- and simulated-patient recordings. Simulation is a valuable learning tool but often does not accurately represent actual clinical situations and challenges. To appropriately assess MI competence, future MI training should emphasize live-patient recordings over simulated-patient visits. Furthermore, whereas trainees reached competency in several MI coding items, none reached competency in the reflection-to-question ratio, and only about half reached competency in percentage of complex reflections. Future MI training will need to focus on further development of reflection skills.
Trainee intentions to remain involved in program improvement and collaborative team-based care in future professional work were core attitudinal learning objectives, but neither was adequately assessed in end-of-year surveys. Ideally, future evaluations will assess subjective trainee intentions and goals around team-based work and objectively measure future professional choices and activities. For example, it would be interesting to determine whether trainees who participated in this program will choose to practice in an interprofessional team-based model or participate in program improvement activities.
Last, the absence of psychology interns was considered a weakness in the learning environment, resulting in a relatively “prescriber-heavy” balance in discipline perspectives for IPE-focused case discussions and other training. Furthermore, the discipline representation in the IPE program did not exemplify the typical PC-MHI team in most VA clinic settings and community practices in which psychology has a strong presence. Adding psychology trainees was an important goal for the IPE program leadership. In 2015, WSMMVH was awarded additional funding for a PACT PC-MHI predoctoral psychology intern through the phase 4 MHEEI. The PC-MHI track psychology intern joined the IPE program in the 2016-2017 training year.
Conclusion
There is broad consensus that interprofessional team-based practice is crucial for providers in the VA and all health care systems. Primary care-mental health integration is an area of VA care in which interprofessional collaboration is uniquely important to implementation and sustainability of the model. Interprofessional education is an effective approach for preparing HCPs for team-based practice, but implementation is challenging. Several factors crucially contributed to the successful implementation of this program: collaboration of the interprofessional planning team with representation from key stakeholders in different departments and training programs; a well-established PACT PC-MHI team with experience in collaborative team-based care; a curriculum structure that emphasized experiential educational strategies designed to promote interprofessional learning and communication; VA leadership support at the national level (MHEEI funding) and local level; and PACT PC-MHI clinical staff committed to teaching and to the IPE mission.
1. Wray LO, Szymanski BR, Kearney LK, McCarthy JF. Implementation of primary care-mental health integration services in the Veterans Health Administration: program activity and associations with engagement in specialty mental health services. J Clin Psychol Med Settings. 2012;19(1):105-116.
2. Kearney LK, Post EP, Pomerantz AS, Zeiss AM. Applying the interprofessional patient aligned care team in the Department of Veterans Affairs: transforming primary care. Am Psychol. 2014;69(4):399-408.
3. Gilbody S, Bower P, Fletcher J, Richards D, Sutton AJ. Collaborative care for depression: a cumulative meta-analysis and review of loner-term outcomes. Arch Intern Med. 2006;166(21):2314-2321.
4. Unützer J, Katon W, Callahan CM, et al; IMPACT (Improving Mood-Promoting Access to Collaborative Treatment) Investigators. Collaborative care management in late-life depression in the primary care setting: a randomized control trial. JAMA. 2002;288(22):2836-2845.
5. Katon W, Unützer J. Consultation psychiatry in the medical home and accountable care organizations: achieving the triple aim. Gen Hosp Psychiatry. 2011;3(4):305-310.
6. Bruce ML, Tenhave TR, Reynolds CF III, et al. Reducing suicidal ideation and depressive symptoms in depressed older primary care patients: a randomized controlled trial. JAMA. 2004;291(9):1081-1091.
7. Alexopoulos GS, Reynolds CF III , Bruce ML, et al; PROSPECT Group. Reducing suicidal ideation and depression in older primary care patients: 24-month outcomes of the PROSPECT study. Am J Psychiatry. 2009;166(8):882-890.
8. Cox M, Cuff P, Brandt B, Reeves S, Zierler B. Measuring the impact of interprofessional education on collaborative practice and patient outcomes. J Interprof Care. 2016;30(1):1-3.
9. World Health Organization, Study Group on Interprofessional Education and Collaborative Practice. Framework for Action on Interprofessional Education and Collaborative Practice. Geneva, Switzerland: World Health Organization; 2010.
10. Interprofessional Education Collaborative Expert Panel. Core Competencies for Interprofessional Collaborative Practice: Report of an Expert Panel. Washington, DC: Interprofessional Education Collaborative; 2011.
11. Blue AV, Mitcham M, Smith T, Raymond J, Greenburg R. Changing the future of health professions: embedding interprofessional education within an academic health center. Acad Med. 2010;85(8):1290-1295.
12. Priest HM, Roberts P, Dent H, Blincoe C, Lawton D, Armstrong C. Interprofessional education and working in mental health: in search of the evidence base. J Nurs Manag. 2008;16(4):474-485.
13. Reeves S. A systematic review of the effects of interprofessional education on staff involved in the care of adults with mental health problems. J Psychiatr Ment Health Nurs. 2001;8(6):533-542.
14. Carpenter J, Barnes D, Dickinson C, Wooff D. Outcomes of interprofessional education for community mental health services in England: the longitudinal evaluation of a postgraduate programme. J Interprof Care. 2006;20(2):145-161.
15. Hammick M, Freeth D, Koppel I, Reeves S, Barr H. A best evidence systematic review of interprofessional education: BEME guide no. 9. Med Teach. 2007;29(8):735-751.
16. Pauzé E, Reeves S. Examining the effects of interprofessional education on mental health providers: findings from an updated systematic review. J Ment Health. 2010;19(3):258-271.
17. Curran V, Heath O, Adey T, et al. An approach to integrating interprofessional education in collaborative mental health care. Acad Psychiatry. 2012;36(2):91-95.
18. Veterans Health Administration. VA Interprofessional Mental Health Education Expansion Initiative, Phase I. Washington, DC; Department of Veterans Affairs; 2012.
19. Dundon M, Dollar K, Schohn M, Lantinga LJ. Primary care–mental health integration: co-located, collaborative care: an operations manual. https://www.mirecc.va.gov/cih-visn2/Documents/Clinical/MH-IPC_CCC_Operations_Manual_Version_2_1.pdf. Published February 2011. Accessed May 19, 2017.
20. McDaniel SH, Grus CL, Cubic BA, et al. Competencies for psychology practice in primary care. Am Psychol. 2014;69(4):409-429.
21. Cowley D, Dunaway K, Forstein M, et al. Teaching psychiatry residents to work at the interface of mental health and primary care. Acad Psychiatry. 2014;38(4):398-404.
22. Herrmann N. The creative brain. J Creat Behav. 1991;25:275-295.
23. Rollnick S, Miller WR. What is motivational interviewing? Behav Cogn Psychother. 1995;23(4):325-334.
24. Pierson HM, Hayes SC, Gifford EV, et al. An examination of the Motivational Interviewing Treatment Integrity code. J Subst Abuse Treat. 2007;32(1):11-17.
25. Shaw D, Blue A. Should psychiatry champion interprofessional education? Acad Psychiatry. 2012;36(3):163-166.
26. Gilbert JH. Interprofessional learning and higher education structural barriers. J Interprof Care. 2005;19(suppl 1):87-106.
27. Heinemann GD, Schmitt MH, Farrell PP, Brallier SA. Development of an Attitudes Toward Health Care Teams Scale. Eval Health Prof. 1999;22(1):123-142.
28. Hepburn K, Tsukuda RA, Fasser C. Team Skills Scale. In: Heinemann GD, Zeiss AM, eds. Team Performance in Healthcare: Assessment and Development. New York, NY: Kluwer Academic/Plenum; 2002:159-163.
Over the past 10 years, the VHA has been a national leader in primary care-mental health integration (PC-MHI) within patient aligned care teams (PACTs).1,2 Studies of the PC-MHI collaborative care model consistently have shown increased access to MH services, higher levels of MH treatment engagement, improved MH treatment outcomes, and high patient and provider satisfaction.3-7 Primary care-mental health integration relies heavily on interprofessional team-based practice with providers from diverse educational and clinical backgrounds who work together to deliver integrated mental and behavioral health services within PACTs. This model requires a unique blending of professional cultures and communication and practice styles.
To sustain PC-MHI in PACT, health care professionals (HCPs) must be well trained to work effectively in interprofessional teams. Across health care organizations, training in collaborative interprofessional team-based practice has been identified as an important and challenging task.8-11
Integrating educational experiences among different HCP learners is an approach to developing competency in interprofessional collaboration early in training. The World Health Organization defined interprofessional education (IPE) as occurring “when students from two or more professions learn about, from, and with each other to enable effective collaboration and improve health outcomes.”9 Fundamental to this definition is the belief that interaction among learners from different disciplines during their training develops competency in subsequent effective collaborative practice. Studies of IPE in MH professional training have found that prelicensure IPE contributes to increased knowledge of roles and responsibilities of different disciplines, improved interprofessional communication and attitudes, and increased willingness to work in teams.12-17
Interprofessional education is a valuable training model, but developing interprofessional learning experiences in a system of diverse and often siloed training programs is difficult. More information about design and implementation of IPE training experiences is needed, particularly in outpatient settings in which integration of traditionally separate discipline-specific care is central to the health care mission. The VA PACT PC-MHI is a strong team-based care model that represents a unique opportunity for training across disciplines in interprofessional collaborative care.
To find innovative approaches to meeting the need for IPE in PACT PC-MHI, the authors developed a new IPE program in PC-MHI at the William S. Middleton Memorial Veterans Hospital (WSMMVH) in Madison, Wisconsin. This article reviews the development, implementation, and first-year evaluation of the training program and discusses the challenges and the IPE areas in need of improvement in PACT PC-MHI.
Methods
In 2012, the VHA launched phase 1 of the Mental Health Education Expansion Initiative (MHEEI), a collaboration of the Office of Academic Affiliations (OAA), VHA Mental Health Services (VHA-MHS), and the Office of Mental Health Operations (OMHO).18 The MHEEI was intended to “increase expertise in critical areas of need, expand the recruitment pipeline of well-trained, highly qualified health care providers in behavioral and mental health disciplines, and promote the utilization of interprofessional team-based care.”18 In response, WSMMVH organized a planning committee and submitted a funding request through the section of MHEEI called PACT With Integrated Behavioral Health Providers. The planning committee included training program directors and staff from psychiatry, pharmacy, social work, psychology, and primary care. The authors received funding for trainees in psychiatry (postgraduate year 4 [PGY-4]), pharmacy/MH residency (PGY-2), pharmacy/ambulatory care (PGY-1), and social work (interns).
Curriculum Development
The planning committee met regularly for 6 months to develop the organization, learning objectives, educational strategies, and implementation plan for the IPE program. The program was organized as a 4- to 12-month clinical rotation with the PC-MHI team in PACT, combined with 12 months of protected weekly IPE time (Table 1).
Learning Objectives
To better understand the educational needs and foci for learning objectives, the interprofessional planning committee reviewed guidelines on training in integrated care and collaborative team-based practice.2,9,10,19-21 These guidelines were compared with existing training opportunities for each discipline to identify training gaps and needs.
Learning objectives were organized into 3 domains: patient-centered PC-MHI, collaborative team-based practice, and population health and program improvement. Table 2 outlines the shared learning objectives linked to each domain that were common to the psychiatry, pharmacy, and social work disciplines. Although many of the learning objectives were shared among all disciplines, each trainee also had discipline-specific clinical activities and learning objectives. Psychiatry and pharmacy residents focused on primary care psychiatric medication consultation and care management for antidepressant medication starts. Social work interns focused on psychosocial and functional assessment and brief problem-focused psychotherapies. Learning objectives were met through direct veteran care in the primary care clinic as part of the PACT PC-MHI team and through interprofessional learning activities during protected weekly education time.
Implementation
Critical stakeholders in implementing the IPE program involved themselves early and throughout the planning process. Stakeholders included VAMC leadership, primary care and MH service line chiefs and clinic managers, training program directors, and PACT staff. Planning committee members gave presentations on the IPE program at MH service line and PACT meetings in the 2 months before program initiation in order to orient staff to learning objectives, program structure, and impact on PACT PC-MHI operations. Throughout the first year, the planning committee continued to meet every 2 weeks to review progress, solve implementation problems, and revise learning objectives and activities.
Trainee Clinical Activities
A wide range of educational strategies were planned to meet learning objectives across the 3 domains. There was strong emphasis on experiential learning through daily PACT and PC-MHI clinical work, team huddles and meetings, and trainee-led program improvement projects.
Psychiatry and PGY-2 pharmacy/MH residents focused on direct and indirect medication consultation and problem-focused assessments. Their clinical activities included PC-MHI medication evaluation and follow-up visits; chart reviews and e-consults for medication recommendations to PACT providers; reviews of care management data and consultations on veterans enrolled in depression and anxiety care management; “curbside consultations” for providers in PACT huddles and meetings and throughout the clinic day; and “warm handoffs,” same-day initial PC-MHI problem-focused assessments performed on PACT provider request. The residents were part of a pool of staff and trainees who performed these assessments.
PGY-1 pharmacy residents made care management phone calls for antidepressant trials for depression and anxiety. These residents were trained in motivational interviewing (MI). They applied their MI skills during care management calls focused on medication adherence and behavioral interventions for depression (eg, exercise, planning pleasurable activity) and during other clinical rotations, including tobacco cessation and medication management for diabetes and hypertension. Particularly challenging veteran cases from these clinics were cosupervised with medication management and PC-MHIstaff for added consultation on engagement, behavior change, and treatment plan adherence.
Social work interns completed initial PC-MHI psychosocial and functional assessments by phone and directly by same-day warm handoffs from PACT staff. The PC-MHI therapies they provided included problem-solving therapy, behavioral activation, stress management based on cognitive behavioral therapy, and brief alcohol interventions.
Group IPE Activities
All trainees had a weekly protected block of 3 hours during which they came together for group IPE that was designed to elicit active participation; facilitate interprofessional communication; and develop an understanding of and respect for the knowledge, culture, and practice style of the different disciplines.
Trainees participated in a Herrmann Brain Dominance Instrument (HBDI) workshop focused on developing a better understanding of individual differences in thinking and problem solving, with the goal of improving communication and learning within teams.22 In a seminar series on professionalism and boundaries in health care, trainees from each discipline gave a presentation on the traditional structure and content of their discipline’s training and discussed similarities and differences in their disciplines’ professional oaths, codes of ethics, and boundary guidelines.
Motivational interviewing training was conducted early in the year so trainees would be prepared to apply MI skills in their daily PACT PC-MHI clinical work. Motivational inteviewing is a patient-centered approach to engaging patients in health promoting behavior change. It is defined as a “directive, client-centered counseling style for eliciting behavior change by helping clients to explore and resolve ambivalence.”23
Trainees recorded MI sessions with at least 2 live-patient visits and at least 2 simulated-patient interviews (with staff serving as patient actors). The structure of MI training and supervision was deliberately designed to facilitate interprofessional communication and learning. In accord with a group supervision model for MI recorded reviews, the trainees presented their tapes to the entire learning group in the presence of a facilitating supervisor. Trainees had the opportunity to observe different interview styles and exchange feedback within a peer group of interprofessional learners.
Seminars were focused on core PC-MHI clinical content (eg, depression, anxiety, alcohol use disorders) and organized around case-based learning. Trainees divided into small teams in which representatives of each discipline offered their perspective on how to approach planning patient assessment and treatment. During the seminars, the authors engaged trainees as teachers and leaders whenever possible. All trainees presented on a topic in which they had some discipline expertise. For example, social work interns led a seminar on support and social services for victims of domestic violence, and PGY-1 pharmacy/ambulatory care residents led seminars and a panel management project focused on diabetes and depression.
Trainees participated in several PACT PC-MHI projects focused on population- and measurement-based care, panel management, and program improvement (Table 3). Protected IPE time was used to teach trainees about population health principles and different tools for process improvement (eg, Vision-Analysis Team-Aim-Map-Measure-Change-Sustain) and provide a forum in which trainees could share their work with one another.
Evaluations
Several tools were used for trainee and program evaluations. Clinical skills were evaluated during daily supervision. Trainees began the year with PC-MHI staff directly observing all their clinical contacts with veterans. Staff evaluated and offered feedback on trainee clinical interviewing and on assessment and treatment planning skills. Once they were assessed to be ready to see veterans on their own, trainees were advanced by staff to “drop-in” direct supervision: Toward the end of a veteran’s visit, a staff preceptor entered the room to review relevant clinical findings, assessment and finalized treatment planning with the trainee and veteran. When appropriate for trainee competence level, clinical contacts were indirectly supervised: Trainees discussed their assessment and treatment plan with a staff supervisor at the end of the day.
Motivational interviewing recordings were reviewed during group supervision. To objectively evaluate MI skills, supervisors who were VA-certified in MI used the Motivational Interviewing Treatment Integrity (MITI) coding tool to review and code both the live- and simulated-patient recordings.24 The MITI coding involves quantitative and qualitative analysis using standardized coding items.
Quantitative items included percentage of open-ended questions (Proficiency: 50%; competency: 70%); percentage of reflections considered complex reflections, or reflective statements adding substantial meaning or emphasis and conveying a deeper or more complex picture of what patients say (Proficiency: 40%; competency: 50%); reflection-to-question ratio (Proficiency: 1:1; competency: 2:1); and percentage of MI-adherent provider statements (Proficiency: 90%; competency: 100%).
Qualitative coding items were a global rating of therapist “empathy,” which evaluated the extent to which the trainee understood or made an effort to grasp the patient’s perspective, and “MI spirit.” This coding intended to capture the overall competence of the trainee in emphasizing collaboration, patient autonomy, and evocation of the patient experience (Proficiency score: 5; competency score: 6).
The PC-MHI teaching staff met midyear and end of year as a team to complete trainee evaluations focused on the 3 areas of learning objectives: patient-centered PC-MHI, collaborative team-based practice, and population health and program improvement. Patient-centered PC-MHI involves direct observation and supervision of trainee clinical contacts with veterans, including assessment and treatment planning, clinical documentation, and review of live- and simulated-patient MI recordings. Collaborative team-based practice involves review of trainee participation in day-to-day teamwork, huddles, team meetings, and IPE activities. Population health and program improvement involves review of trainee work on a panel measurement-based care management or program improvement project. In each learning objectives area, trainees were rated on a 3-point scale: needs improvement (1); satisfactory (2); achieved (3). Core knowledge about PC-MHI evidence base, structure, and clinical topics was assessed with case-based written examination at midyear and end of year.
Surveys and qualitative interviews were used to assess trainee perceptions about the IPE program. A midyear and end-of-year survey assessed trainee satisfaction and perceived efficacy of the IPE training program in meeting core learning objectives. A midyear survey designed by pharmacy residents as part of their program improvement project evaluated attitudes around interprofessional learning and team practice. Trainees met individually with the PC-MHI IPE director at midyear and end of year to gather qualitative feedback on the IPE program.
Outcomes
All trainees advanced to the expected level of supervision for clinical contacts (drop-in or indirect clinical supervision). Over the year, there was significant improvement in trainees’ MI skills as measured by MITI coding of at least 2 live-patient or 2 simulated-patient recordings (Figures 1A and 1B). By end of year, most trainees had reached proficiency or competency in several MITI coded items: percentage of open-ended questions (4/12 proficient, 8/12 competent), percentage of complex reflections (2/12 less than proficient, 3/12 proficient, 7/12 competent), MI adherence (1/12 proficient, 11/12 competent), global empathy rating (2/12 proficient, 10/12 competent), and global MI spirit rating (12/12 competent). Average reflection-to-question ratio for the trainee group increased from 0.63 to 0.96 from midyear to end of year, but only 3 of 12 trainees reached the proficiency level of a 1:1 ratio, and no trainee reached the competency level of a 2:1 ratio.
According to the PC-MHI team’s midyear evaluation, most trainees were already making satisfactory progress in the 3 domains of learning objectives for the training program. At end of year, 13 of 14 trainees were assessed as competent in all 3 domains (Figures 2A and B). All trainees passed the midyear and end-of-year written examinations with overall high scores (average score, 82%) demonstrating acquisition of core PC-MHI clinical knowledge.
Trainee evaluations of the IPE program were overall highly favorable at both midyear and at end of year. Trainees rated the program effective or extremely effective in developing their skills in patient-centered care, interprofessional communication, and collaborative team-based practice. They also rated the program highly effective in preparing them to use team-based practice skills in other settings. On a midyear survey, trainees moderately to strongly agreed with several positive beliefs and attitudes about team-based care.
In qualitative interviews at program completion, trainees across disciplines rated the MI training with group supervision as one of their most valuable interprofessional learning experiences. Other highly valued training experiences were PACT PC-MHI panel management projects, team-based clinical case reviews, and cross-disciplinary supervision.
Discussion
This article describes the successful development and implementation of a VA-based IPE program in PACT PC-MHI. Interprofessional clinical training and educational experiences were highly valued, and trainees identified positive attitudes and improved skills related to team-based care. These findings support previous findings that IPE is associated with high satisfaction and positive attitudes toward team-based collaborative practice.12-17 Program implementation presented several challenges: nonsynchronous academic calendars and rotation schedules, cross-disciplinary supervision regulations, variations in clinical and supervisory requirements for accreditation standards, the traditional health care hierarchy, and measurement of the impact of IPE experiences.11,25,26
Rotation schedules and academic calendars varied across the psychiatry, pharmacy, and social work home programs. Organizing different trainee rotation schedules was a significant challenge. Collaboration with training directors and support staff was crucial in planning rotations that offered a longitudinal training experience in PACT PC-MHI. Given the participants’ different starting dates, protected IPE time early in the calendar year was focused on developing clinical skills specific to the pharmacy and psychiatry trainees who would be starting in July, and IPE activities that required the presence of all trainee disciplines (eg, MI training, HBDI) were planned for after the September start of the social work interns.
Cross-disciplinary supervision was highly valued by trainees because it offered exposure to the disciplines’ different communication styles and approaches to clinical assessment and decision making. Throughout the year, however, trainees encountered several obstacles to cross-disciplinary supervision, with respect to coding/billing and home program supervisory policies. The authors worked closely with the VA administration and each training program to develop and revise supervising policies and procedures to meet the necessary administrative and program supervisory requirements for accreditation standards.
In some cases, this work resulted in dual supervision by a preceptor of the same discipline (to meet requirements for coding/billing and home program supervision) and clinical supervision by a preceptor of a different discipline (eg, in team meetings or in clinical case reviews during protected IPE time). Preceptors from each discipline met regularly to discuss challenges in cross-disciplinary supervision, review scope-of-practice issues, share information on discipline-specific training, and revise supervisory procedures.
Interprofessional education activities during weekly protected time were designed to improve collaboration and to challenge trainees to examine traditional hierarchical roles and patterns of communication in health care. An emphasis on case-based learning in small groups encouraged trainees to share perspectives on their discipline-specific approach to assessment and treatment planning. Motivational interviewing training, one of the IPE experiences most valued by trainees and staff, created the opportunity for a truly shared learning environment, as trainees largely started at about the same skill level despite having different educational backgrounds. Group supervision of MI recordings offered trainees the opportunity to learn from each other and develop comfort in offering and receiving interprofessional constructive feedback.
Limitations
There were considerable methodologic limitations in the authors’ efforts to evaluate the impact of this training program on trainee attitudes and skills in collaborative team-based practice. Although trainee surveys revealed highly positive attitudes and beliefs about team-based care as well as perceived competence in collaborative practice, these were not validated surveys, and changes could not be accurately measured over time (trainees were not assessed at baseline). Trainees also were involved in other clinical teams within the VA during the year, and it is difficult to assess the specific impact of their PC-MHI IPE experiences. The PC-MHI staff evaluations of trainee competence in collaborative team-based practice were largely observational and potentially vulnerable to biases, as the staff evaluating trainee competence also were part of the IPE program planning process and invested in its success.
To address these limitations in the future, the authors will use better assessment tools at baseline and end of year to more effectively evaluate the impact of the training program on teamwork skills as well changes in attitudes and beliefs about interprofessional learning and teamwork. The Attitudes Toward Interprofessional Health Care Teams Scale and the Perceptions of Effective Interpersonal Teams Scale should be considered as they have published reliability and validity.27,28 The authors could improve the reliability and depth of trainee evaluations with use of a “360-degree evaluation” model for trainee evaluations that includes other PACT members (beyond PC-MHI staff) as well as veterans.
Assessment of MI skills and competency was limited by use of both live- and simulated-patient recordings. Simulation is a valuable learning tool but often does not accurately represent actual clinical situations and challenges. To appropriately assess MI competence, future MI training should emphasize live-patient recordings over simulated-patient visits. Furthermore, whereas trainees reached competency in several MI coding items, none reached competency in the reflection-to-question ratio, and only about half reached competency in percentage of complex reflections. Future MI training will need to focus on further development of reflection skills.
Trainee intentions to remain involved in program improvement and collaborative team-based care in future professional work were core attitudinal learning objectives, but neither was adequately assessed in end-of-year surveys. Ideally, future evaluations will assess subjective trainee intentions and goals around team-based work and objectively measure future professional choices and activities. For example, it would be interesting to determine whether trainees who participated in this program will choose to practice in an interprofessional team-based model or participate in program improvement activities.
Last, the absence of psychology interns was considered a weakness in the learning environment, resulting in a relatively “prescriber-heavy” balance in discipline perspectives for IPE-focused case discussions and other training. Furthermore, the discipline representation in the IPE program did not exemplify the typical PC-MHI team in most VA clinic settings and community practices in which psychology has a strong presence. Adding psychology trainees was an important goal for the IPE program leadership. In 2015, WSMMVH was awarded additional funding for a PACT PC-MHI predoctoral psychology intern through the phase 4 MHEEI. The PC-MHI track psychology intern joined the IPE program in the 2016-2017 training year.
Conclusion
There is broad consensus that interprofessional team-based practice is crucial for providers in the VA and all health care systems. Primary care-mental health integration is an area of VA care in which interprofessional collaboration is uniquely important to implementation and sustainability of the model. Interprofessional education is an effective approach for preparing HCPs for team-based practice, but implementation is challenging. Several factors crucially contributed to the successful implementation of this program: collaboration of the interprofessional planning team with representation from key stakeholders in different departments and training programs; a well-established PACT PC-MHI team with experience in collaborative team-based care; a curriculum structure that emphasized experiential educational strategies designed to promote interprofessional learning and communication; VA leadership support at the national level (MHEEI funding) and local level; and PACT PC-MHI clinical staff committed to teaching and to the IPE mission.
Over the past 10 years, the VHA has been a national leader in primary care-mental health integration (PC-MHI) within patient aligned care teams (PACTs).1,2 Studies of the PC-MHI collaborative care model consistently have shown increased access to MH services, higher levels of MH treatment engagement, improved MH treatment outcomes, and high patient and provider satisfaction.3-7 Primary care-mental health integration relies heavily on interprofessional team-based practice with providers from diverse educational and clinical backgrounds who work together to deliver integrated mental and behavioral health services within PACTs. This model requires a unique blending of professional cultures and communication and practice styles.
To sustain PC-MHI in PACT, health care professionals (HCPs) must be well trained to work effectively in interprofessional teams. Across health care organizations, training in collaborative interprofessional team-based practice has been identified as an important and challenging task.8-11
Integrating educational experiences among different HCP learners is an approach to developing competency in interprofessional collaboration early in training. The World Health Organization defined interprofessional education (IPE) as occurring “when students from two or more professions learn about, from, and with each other to enable effective collaboration and improve health outcomes.”9 Fundamental to this definition is the belief that interaction among learners from different disciplines during their training develops competency in subsequent effective collaborative practice. Studies of IPE in MH professional training have found that prelicensure IPE contributes to increased knowledge of roles and responsibilities of different disciplines, improved interprofessional communication and attitudes, and increased willingness to work in teams.12-17
Interprofessional education is a valuable training model, but developing interprofessional learning experiences in a system of diverse and often siloed training programs is difficult. More information about design and implementation of IPE training experiences is needed, particularly in outpatient settings in which integration of traditionally separate discipline-specific care is central to the health care mission. The VA PACT PC-MHI is a strong team-based care model that represents a unique opportunity for training across disciplines in interprofessional collaborative care.
To find innovative approaches to meeting the need for IPE in PACT PC-MHI, the authors developed a new IPE program in PC-MHI at the William S. Middleton Memorial Veterans Hospital (WSMMVH) in Madison, Wisconsin. This article reviews the development, implementation, and first-year evaluation of the training program and discusses the challenges and the IPE areas in need of improvement in PACT PC-MHI.
Methods
In 2012, the VHA launched phase 1 of the Mental Health Education Expansion Initiative (MHEEI), a collaboration of the Office of Academic Affiliations (OAA), VHA Mental Health Services (VHA-MHS), and the Office of Mental Health Operations (OMHO).18 The MHEEI was intended to “increase expertise in critical areas of need, expand the recruitment pipeline of well-trained, highly qualified health care providers in behavioral and mental health disciplines, and promote the utilization of interprofessional team-based care.”18 In response, WSMMVH organized a planning committee and submitted a funding request through the section of MHEEI called PACT With Integrated Behavioral Health Providers. The planning committee included training program directors and staff from psychiatry, pharmacy, social work, psychology, and primary care. The authors received funding for trainees in psychiatry (postgraduate year 4 [PGY-4]), pharmacy/MH residency (PGY-2), pharmacy/ambulatory care (PGY-1), and social work (interns).
Curriculum Development
The planning committee met regularly for 6 months to develop the organization, learning objectives, educational strategies, and implementation plan for the IPE program. The program was organized as a 4- to 12-month clinical rotation with the PC-MHI team in PACT, combined with 12 months of protected weekly IPE time (Table 1).
Learning Objectives
To better understand the educational needs and foci for learning objectives, the interprofessional planning committee reviewed guidelines on training in integrated care and collaborative team-based practice.2,9,10,19-21 These guidelines were compared with existing training opportunities for each discipline to identify training gaps and needs.
Learning objectives were organized into 3 domains: patient-centered PC-MHI, collaborative team-based practice, and population health and program improvement. Table 2 outlines the shared learning objectives linked to each domain that were common to the psychiatry, pharmacy, and social work disciplines. Although many of the learning objectives were shared among all disciplines, each trainee also had discipline-specific clinical activities and learning objectives. Psychiatry and pharmacy residents focused on primary care psychiatric medication consultation and care management for antidepressant medication starts. Social work interns focused on psychosocial and functional assessment and brief problem-focused psychotherapies. Learning objectives were met through direct veteran care in the primary care clinic as part of the PACT PC-MHI team and through interprofessional learning activities during protected weekly education time.
Implementation
Critical stakeholders in implementing the IPE program involved themselves early and throughout the planning process. Stakeholders included VAMC leadership, primary care and MH service line chiefs and clinic managers, training program directors, and PACT staff. Planning committee members gave presentations on the IPE program at MH service line and PACT meetings in the 2 months before program initiation in order to orient staff to learning objectives, program structure, and impact on PACT PC-MHI operations. Throughout the first year, the planning committee continued to meet every 2 weeks to review progress, solve implementation problems, and revise learning objectives and activities.
Trainee Clinical Activities
A wide range of educational strategies were planned to meet learning objectives across the 3 domains. There was strong emphasis on experiential learning through daily PACT and PC-MHI clinical work, team huddles and meetings, and trainee-led program improvement projects.
Psychiatry and PGY-2 pharmacy/MH residents focused on direct and indirect medication consultation and problem-focused assessments. Their clinical activities included PC-MHI medication evaluation and follow-up visits; chart reviews and e-consults for medication recommendations to PACT providers; reviews of care management data and consultations on veterans enrolled in depression and anxiety care management; “curbside consultations” for providers in PACT huddles and meetings and throughout the clinic day; and “warm handoffs,” same-day initial PC-MHI problem-focused assessments performed on PACT provider request. The residents were part of a pool of staff and trainees who performed these assessments.
PGY-1 pharmacy residents made care management phone calls for antidepressant trials for depression and anxiety. These residents were trained in motivational interviewing (MI). They applied their MI skills during care management calls focused on medication adherence and behavioral interventions for depression (eg, exercise, planning pleasurable activity) and during other clinical rotations, including tobacco cessation and medication management for diabetes and hypertension. Particularly challenging veteran cases from these clinics were cosupervised with medication management and PC-MHIstaff for added consultation on engagement, behavior change, and treatment plan adherence.
Social work interns completed initial PC-MHI psychosocial and functional assessments by phone and directly by same-day warm handoffs from PACT staff. The PC-MHI therapies they provided included problem-solving therapy, behavioral activation, stress management based on cognitive behavioral therapy, and brief alcohol interventions.
Group IPE Activities
All trainees had a weekly protected block of 3 hours during which they came together for group IPE that was designed to elicit active participation; facilitate interprofessional communication; and develop an understanding of and respect for the knowledge, culture, and practice style of the different disciplines.
Trainees participated in a Herrmann Brain Dominance Instrument (HBDI) workshop focused on developing a better understanding of individual differences in thinking and problem solving, with the goal of improving communication and learning within teams.22 In a seminar series on professionalism and boundaries in health care, trainees from each discipline gave a presentation on the traditional structure and content of their discipline’s training and discussed similarities and differences in their disciplines’ professional oaths, codes of ethics, and boundary guidelines.
Motivational interviewing training was conducted early in the year so trainees would be prepared to apply MI skills in their daily PACT PC-MHI clinical work. Motivational inteviewing is a patient-centered approach to engaging patients in health promoting behavior change. It is defined as a “directive, client-centered counseling style for eliciting behavior change by helping clients to explore and resolve ambivalence.”23
Trainees recorded MI sessions with at least 2 live-patient visits and at least 2 simulated-patient interviews (with staff serving as patient actors). The structure of MI training and supervision was deliberately designed to facilitate interprofessional communication and learning. In accord with a group supervision model for MI recorded reviews, the trainees presented their tapes to the entire learning group in the presence of a facilitating supervisor. Trainees had the opportunity to observe different interview styles and exchange feedback within a peer group of interprofessional learners.
Seminars were focused on core PC-MHI clinical content (eg, depression, anxiety, alcohol use disorders) and organized around case-based learning. Trainees divided into small teams in which representatives of each discipline offered their perspective on how to approach planning patient assessment and treatment. During the seminars, the authors engaged trainees as teachers and leaders whenever possible. All trainees presented on a topic in which they had some discipline expertise. For example, social work interns led a seminar on support and social services for victims of domestic violence, and PGY-1 pharmacy/ambulatory care residents led seminars and a panel management project focused on diabetes and depression.
Trainees participated in several PACT PC-MHI projects focused on population- and measurement-based care, panel management, and program improvement (Table 3). Protected IPE time was used to teach trainees about population health principles and different tools for process improvement (eg, Vision-Analysis Team-Aim-Map-Measure-Change-Sustain) and provide a forum in which trainees could share their work with one another.
Evaluations
Several tools were used for trainee and program evaluations. Clinical skills were evaluated during daily supervision. Trainees began the year with PC-MHI staff directly observing all their clinical contacts with veterans. Staff evaluated and offered feedback on trainee clinical interviewing and on assessment and treatment planning skills. Once they were assessed to be ready to see veterans on their own, trainees were advanced by staff to “drop-in” direct supervision: Toward the end of a veteran’s visit, a staff preceptor entered the room to review relevant clinical findings, assessment and finalized treatment planning with the trainee and veteran. When appropriate for trainee competence level, clinical contacts were indirectly supervised: Trainees discussed their assessment and treatment plan with a staff supervisor at the end of the day.
Motivational interviewing recordings were reviewed during group supervision. To objectively evaluate MI skills, supervisors who were VA-certified in MI used the Motivational Interviewing Treatment Integrity (MITI) coding tool to review and code both the live- and simulated-patient recordings.24 The MITI coding involves quantitative and qualitative analysis using standardized coding items.
Quantitative items included percentage of open-ended questions (Proficiency: 50%; competency: 70%); percentage of reflections considered complex reflections, or reflective statements adding substantial meaning or emphasis and conveying a deeper or more complex picture of what patients say (Proficiency: 40%; competency: 50%); reflection-to-question ratio (Proficiency: 1:1; competency: 2:1); and percentage of MI-adherent provider statements (Proficiency: 90%; competency: 100%).
Qualitative coding items were a global rating of therapist “empathy,” which evaluated the extent to which the trainee understood or made an effort to grasp the patient’s perspective, and “MI spirit.” This coding intended to capture the overall competence of the trainee in emphasizing collaboration, patient autonomy, and evocation of the patient experience (Proficiency score: 5; competency score: 6).
The PC-MHI teaching staff met midyear and end of year as a team to complete trainee evaluations focused on the 3 areas of learning objectives: patient-centered PC-MHI, collaborative team-based practice, and population health and program improvement. Patient-centered PC-MHI involves direct observation and supervision of trainee clinical contacts with veterans, including assessment and treatment planning, clinical documentation, and review of live- and simulated-patient MI recordings. Collaborative team-based practice involves review of trainee participation in day-to-day teamwork, huddles, team meetings, and IPE activities. Population health and program improvement involves review of trainee work on a panel measurement-based care management or program improvement project. In each learning objectives area, trainees were rated on a 3-point scale: needs improvement (1); satisfactory (2); achieved (3). Core knowledge about PC-MHI evidence base, structure, and clinical topics was assessed with case-based written examination at midyear and end of year.
Surveys and qualitative interviews were used to assess trainee perceptions about the IPE program. A midyear and end-of-year survey assessed trainee satisfaction and perceived efficacy of the IPE training program in meeting core learning objectives. A midyear survey designed by pharmacy residents as part of their program improvement project evaluated attitudes around interprofessional learning and team practice. Trainees met individually with the PC-MHI IPE director at midyear and end of year to gather qualitative feedback on the IPE program.
Outcomes
All trainees advanced to the expected level of supervision for clinical contacts (drop-in or indirect clinical supervision). Over the year, there was significant improvement in trainees’ MI skills as measured by MITI coding of at least 2 live-patient or 2 simulated-patient recordings (Figures 1A and 1B). By end of year, most trainees had reached proficiency or competency in several MITI coded items: percentage of open-ended questions (4/12 proficient, 8/12 competent), percentage of complex reflections (2/12 less than proficient, 3/12 proficient, 7/12 competent), MI adherence (1/12 proficient, 11/12 competent), global empathy rating (2/12 proficient, 10/12 competent), and global MI spirit rating (12/12 competent). Average reflection-to-question ratio for the trainee group increased from 0.63 to 0.96 from midyear to end of year, but only 3 of 12 trainees reached the proficiency level of a 1:1 ratio, and no trainee reached the competency level of a 2:1 ratio.
According to the PC-MHI team’s midyear evaluation, most trainees were already making satisfactory progress in the 3 domains of learning objectives for the training program. At end of year, 13 of 14 trainees were assessed as competent in all 3 domains (Figures 2A and B). All trainees passed the midyear and end-of-year written examinations with overall high scores (average score, 82%) demonstrating acquisition of core PC-MHI clinical knowledge.
Trainee evaluations of the IPE program were overall highly favorable at both midyear and at end of year. Trainees rated the program effective or extremely effective in developing their skills in patient-centered care, interprofessional communication, and collaborative team-based practice. They also rated the program highly effective in preparing them to use team-based practice skills in other settings. On a midyear survey, trainees moderately to strongly agreed with several positive beliefs and attitudes about team-based care.
In qualitative interviews at program completion, trainees across disciplines rated the MI training with group supervision as one of their most valuable interprofessional learning experiences. Other highly valued training experiences were PACT PC-MHI panel management projects, team-based clinical case reviews, and cross-disciplinary supervision.
Discussion
This article describes the successful development and implementation of a VA-based IPE program in PACT PC-MHI. Interprofessional clinical training and educational experiences were highly valued, and trainees identified positive attitudes and improved skills related to team-based care. These findings support previous findings that IPE is associated with high satisfaction and positive attitudes toward team-based collaborative practice.12-17 Program implementation presented several challenges: nonsynchronous academic calendars and rotation schedules, cross-disciplinary supervision regulations, variations in clinical and supervisory requirements for accreditation standards, the traditional health care hierarchy, and measurement of the impact of IPE experiences.11,25,26
Rotation schedules and academic calendars varied across the psychiatry, pharmacy, and social work home programs. Organizing different trainee rotation schedules was a significant challenge. Collaboration with training directors and support staff was crucial in planning rotations that offered a longitudinal training experience in PACT PC-MHI. Given the participants’ different starting dates, protected IPE time early in the calendar year was focused on developing clinical skills specific to the pharmacy and psychiatry trainees who would be starting in July, and IPE activities that required the presence of all trainee disciplines (eg, MI training, HBDI) were planned for after the September start of the social work interns.
Cross-disciplinary supervision was highly valued by trainees because it offered exposure to the disciplines’ different communication styles and approaches to clinical assessment and decision making. Throughout the year, however, trainees encountered several obstacles to cross-disciplinary supervision, with respect to coding/billing and home program supervisory policies. The authors worked closely with the VA administration and each training program to develop and revise supervising policies and procedures to meet the necessary administrative and program supervisory requirements for accreditation standards.
In some cases, this work resulted in dual supervision by a preceptor of the same discipline (to meet requirements for coding/billing and home program supervision) and clinical supervision by a preceptor of a different discipline (eg, in team meetings or in clinical case reviews during protected IPE time). Preceptors from each discipline met regularly to discuss challenges in cross-disciplinary supervision, review scope-of-practice issues, share information on discipline-specific training, and revise supervisory procedures.
Interprofessional education activities during weekly protected time were designed to improve collaboration and to challenge trainees to examine traditional hierarchical roles and patterns of communication in health care. An emphasis on case-based learning in small groups encouraged trainees to share perspectives on their discipline-specific approach to assessment and treatment planning. Motivational interviewing training, one of the IPE experiences most valued by trainees and staff, created the opportunity for a truly shared learning environment, as trainees largely started at about the same skill level despite having different educational backgrounds. Group supervision of MI recordings offered trainees the opportunity to learn from each other and develop comfort in offering and receiving interprofessional constructive feedback.
Limitations
There were considerable methodologic limitations in the authors’ efforts to evaluate the impact of this training program on trainee attitudes and skills in collaborative team-based practice. Although trainee surveys revealed highly positive attitudes and beliefs about team-based care as well as perceived competence in collaborative practice, these were not validated surveys, and changes could not be accurately measured over time (trainees were not assessed at baseline). Trainees also were involved in other clinical teams within the VA during the year, and it is difficult to assess the specific impact of their PC-MHI IPE experiences. The PC-MHI staff evaluations of trainee competence in collaborative team-based practice were largely observational and potentially vulnerable to biases, as the staff evaluating trainee competence also were part of the IPE program planning process and invested in its success.
To address these limitations in the future, the authors will use better assessment tools at baseline and end of year to more effectively evaluate the impact of the training program on teamwork skills as well changes in attitudes and beliefs about interprofessional learning and teamwork. The Attitudes Toward Interprofessional Health Care Teams Scale and the Perceptions of Effective Interpersonal Teams Scale should be considered as they have published reliability and validity.27,28 The authors could improve the reliability and depth of trainee evaluations with use of a “360-degree evaluation” model for trainee evaluations that includes other PACT members (beyond PC-MHI staff) as well as veterans.
Assessment of MI skills and competency was limited by use of both live- and simulated-patient recordings. Simulation is a valuable learning tool but often does not accurately represent actual clinical situations and challenges. To appropriately assess MI competence, future MI training should emphasize live-patient recordings over simulated-patient visits. Furthermore, whereas trainees reached competency in several MI coding items, none reached competency in the reflection-to-question ratio, and only about half reached competency in percentage of complex reflections. Future MI training will need to focus on further development of reflection skills.
Trainee intentions to remain involved in program improvement and collaborative team-based care in future professional work were core attitudinal learning objectives, but neither was adequately assessed in end-of-year surveys. Ideally, future evaluations will assess subjective trainee intentions and goals around team-based work and objectively measure future professional choices and activities. For example, it would be interesting to determine whether trainees who participated in this program will choose to practice in an interprofessional team-based model or participate in program improvement activities.
Last, the absence of psychology interns was considered a weakness in the learning environment, resulting in a relatively “prescriber-heavy” balance in discipline perspectives for IPE-focused case discussions and other training. Furthermore, the discipline representation in the IPE program did not exemplify the typical PC-MHI team in most VA clinic settings and community practices in which psychology has a strong presence. Adding psychology trainees was an important goal for the IPE program leadership. In 2015, WSMMVH was awarded additional funding for a PACT PC-MHI predoctoral psychology intern through the phase 4 MHEEI. The PC-MHI track psychology intern joined the IPE program in the 2016-2017 training year.
Conclusion
There is broad consensus that interprofessional team-based practice is crucial for providers in the VA and all health care systems. Primary care-mental health integration is an area of VA care in which interprofessional collaboration is uniquely important to implementation and sustainability of the model. Interprofessional education is an effective approach for preparing HCPs for team-based practice, but implementation is challenging. Several factors crucially contributed to the successful implementation of this program: collaboration of the interprofessional planning team with representation from key stakeholders in different departments and training programs; a well-established PACT PC-MHI team with experience in collaborative team-based care; a curriculum structure that emphasized experiential educational strategies designed to promote interprofessional learning and communication; VA leadership support at the national level (MHEEI funding) and local level; and PACT PC-MHI clinical staff committed to teaching and to the IPE mission.
1. Wray LO, Szymanski BR, Kearney LK, McCarthy JF. Implementation of primary care-mental health integration services in the Veterans Health Administration: program activity and associations with engagement in specialty mental health services. J Clin Psychol Med Settings. 2012;19(1):105-116.
2. Kearney LK, Post EP, Pomerantz AS, Zeiss AM. Applying the interprofessional patient aligned care team in the Department of Veterans Affairs: transforming primary care. Am Psychol. 2014;69(4):399-408.
3. Gilbody S, Bower P, Fletcher J, Richards D, Sutton AJ. Collaborative care for depression: a cumulative meta-analysis and review of loner-term outcomes. Arch Intern Med. 2006;166(21):2314-2321.
4. Unützer J, Katon W, Callahan CM, et al; IMPACT (Improving Mood-Promoting Access to Collaborative Treatment) Investigators. Collaborative care management in late-life depression in the primary care setting: a randomized control trial. JAMA. 2002;288(22):2836-2845.
5. Katon W, Unützer J. Consultation psychiatry in the medical home and accountable care organizations: achieving the triple aim. Gen Hosp Psychiatry. 2011;3(4):305-310.
6. Bruce ML, Tenhave TR, Reynolds CF III, et al. Reducing suicidal ideation and depressive symptoms in depressed older primary care patients: a randomized controlled trial. JAMA. 2004;291(9):1081-1091.
7. Alexopoulos GS, Reynolds CF III , Bruce ML, et al; PROSPECT Group. Reducing suicidal ideation and depression in older primary care patients: 24-month outcomes of the PROSPECT study. Am J Psychiatry. 2009;166(8):882-890.
8. Cox M, Cuff P, Brandt B, Reeves S, Zierler B. Measuring the impact of interprofessional education on collaborative practice and patient outcomes. J Interprof Care. 2016;30(1):1-3.
9. World Health Organization, Study Group on Interprofessional Education and Collaborative Practice. Framework for Action on Interprofessional Education and Collaborative Practice. Geneva, Switzerland: World Health Organization; 2010.
10. Interprofessional Education Collaborative Expert Panel. Core Competencies for Interprofessional Collaborative Practice: Report of an Expert Panel. Washington, DC: Interprofessional Education Collaborative; 2011.
11. Blue AV, Mitcham M, Smith T, Raymond J, Greenburg R. Changing the future of health professions: embedding interprofessional education within an academic health center. Acad Med. 2010;85(8):1290-1295.
12. Priest HM, Roberts P, Dent H, Blincoe C, Lawton D, Armstrong C. Interprofessional education and working in mental health: in search of the evidence base. J Nurs Manag. 2008;16(4):474-485.
13. Reeves S. A systematic review of the effects of interprofessional education on staff involved in the care of adults with mental health problems. J Psychiatr Ment Health Nurs. 2001;8(6):533-542.
14. Carpenter J, Barnes D, Dickinson C, Wooff D. Outcomes of interprofessional education for community mental health services in England: the longitudinal evaluation of a postgraduate programme. J Interprof Care. 2006;20(2):145-161.
15. Hammick M, Freeth D, Koppel I, Reeves S, Barr H. A best evidence systematic review of interprofessional education: BEME guide no. 9. Med Teach. 2007;29(8):735-751.
16. Pauzé E, Reeves S. Examining the effects of interprofessional education on mental health providers: findings from an updated systematic review. J Ment Health. 2010;19(3):258-271.
17. Curran V, Heath O, Adey T, et al. An approach to integrating interprofessional education in collaborative mental health care. Acad Psychiatry. 2012;36(2):91-95.
18. Veterans Health Administration. VA Interprofessional Mental Health Education Expansion Initiative, Phase I. Washington, DC; Department of Veterans Affairs; 2012.
19. Dundon M, Dollar K, Schohn M, Lantinga LJ. Primary care–mental health integration: co-located, collaborative care: an operations manual. https://www.mirecc.va.gov/cih-visn2/Documents/Clinical/MH-IPC_CCC_Operations_Manual_Version_2_1.pdf. Published February 2011. Accessed May 19, 2017.
20. McDaniel SH, Grus CL, Cubic BA, et al. Competencies for psychology practice in primary care. Am Psychol. 2014;69(4):409-429.
21. Cowley D, Dunaway K, Forstein M, et al. Teaching psychiatry residents to work at the interface of mental health and primary care. Acad Psychiatry. 2014;38(4):398-404.
22. Herrmann N. The creative brain. J Creat Behav. 1991;25:275-295.
23. Rollnick S, Miller WR. What is motivational interviewing? Behav Cogn Psychother. 1995;23(4):325-334.
24. Pierson HM, Hayes SC, Gifford EV, et al. An examination of the Motivational Interviewing Treatment Integrity code. J Subst Abuse Treat. 2007;32(1):11-17.
25. Shaw D, Blue A. Should psychiatry champion interprofessional education? Acad Psychiatry. 2012;36(3):163-166.
26. Gilbert JH. Interprofessional learning and higher education structural barriers. J Interprof Care. 2005;19(suppl 1):87-106.
27. Heinemann GD, Schmitt MH, Farrell PP, Brallier SA. Development of an Attitudes Toward Health Care Teams Scale. Eval Health Prof. 1999;22(1):123-142.
28. Hepburn K, Tsukuda RA, Fasser C. Team Skills Scale. In: Heinemann GD, Zeiss AM, eds. Team Performance in Healthcare: Assessment and Development. New York, NY: Kluwer Academic/Plenum; 2002:159-163.
1. Wray LO, Szymanski BR, Kearney LK, McCarthy JF. Implementation of primary care-mental health integration services in the Veterans Health Administration: program activity and associations with engagement in specialty mental health services. J Clin Psychol Med Settings. 2012;19(1):105-116.
2. Kearney LK, Post EP, Pomerantz AS, Zeiss AM. Applying the interprofessional patient aligned care team in the Department of Veterans Affairs: transforming primary care. Am Psychol. 2014;69(4):399-408.
3. Gilbody S, Bower P, Fletcher J, Richards D, Sutton AJ. Collaborative care for depression: a cumulative meta-analysis and review of loner-term outcomes. Arch Intern Med. 2006;166(21):2314-2321.
4. Unützer J, Katon W, Callahan CM, et al; IMPACT (Improving Mood-Promoting Access to Collaborative Treatment) Investigators. Collaborative care management in late-life depression in the primary care setting: a randomized control trial. JAMA. 2002;288(22):2836-2845.
5. Katon W, Unützer J. Consultation psychiatry in the medical home and accountable care organizations: achieving the triple aim. Gen Hosp Psychiatry. 2011;3(4):305-310.
6. Bruce ML, Tenhave TR, Reynolds CF III, et al. Reducing suicidal ideation and depressive symptoms in depressed older primary care patients: a randomized controlled trial. JAMA. 2004;291(9):1081-1091.
7. Alexopoulos GS, Reynolds CF III , Bruce ML, et al; PROSPECT Group. Reducing suicidal ideation and depression in older primary care patients: 24-month outcomes of the PROSPECT study. Am J Psychiatry. 2009;166(8):882-890.
8. Cox M, Cuff P, Brandt B, Reeves S, Zierler B. Measuring the impact of interprofessional education on collaborative practice and patient outcomes. J Interprof Care. 2016;30(1):1-3.
9. World Health Organization, Study Group on Interprofessional Education and Collaborative Practice. Framework for Action on Interprofessional Education and Collaborative Practice. Geneva, Switzerland: World Health Organization; 2010.
10. Interprofessional Education Collaborative Expert Panel. Core Competencies for Interprofessional Collaborative Practice: Report of an Expert Panel. Washington, DC: Interprofessional Education Collaborative; 2011.
11. Blue AV, Mitcham M, Smith T, Raymond J, Greenburg R. Changing the future of health professions: embedding interprofessional education within an academic health center. Acad Med. 2010;85(8):1290-1295.
12. Priest HM, Roberts P, Dent H, Blincoe C, Lawton D, Armstrong C. Interprofessional education and working in mental health: in search of the evidence base. J Nurs Manag. 2008;16(4):474-485.
13. Reeves S. A systematic review of the effects of interprofessional education on staff involved in the care of adults with mental health problems. J Psychiatr Ment Health Nurs. 2001;8(6):533-542.
14. Carpenter J, Barnes D, Dickinson C, Wooff D. Outcomes of interprofessional education for community mental health services in England: the longitudinal evaluation of a postgraduate programme. J Interprof Care. 2006;20(2):145-161.
15. Hammick M, Freeth D, Koppel I, Reeves S, Barr H. A best evidence systematic review of interprofessional education: BEME guide no. 9. Med Teach. 2007;29(8):735-751.
16. Pauzé E, Reeves S. Examining the effects of interprofessional education on mental health providers: findings from an updated systematic review. J Ment Health. 2010;19(3):258-271.
17. Curran V, Heath O, Adey T, et al. An approach to integrating interprofessional education in collaborative mental health care. Acad Psychiatry. 2012;36(2):91-95.
18. Veterans Health Administration. VA Interprofessional Mental Health Education Expansion Initiative, Phase I. Washington, DC; Department of Veterans Affairs; 2012.
19. Dundon M, Dollar K, Schohn M, Lantinga LJ. Primary care–mental health integration: co-located, collaborative care: an operations manual. https://www.mirecc.va.gov/cih-visn2/Documents/Clinical/MH-IPC_CCC_Operations_Manual_Version_2_1.pdf. Published February 2011. Accessed May 19, 2017.
20. McDaniel SH, Grus CL, Cubic BA, et al. Competencies for psychology practice in primary care. Am Psychol. 2014;69(4):409-429.
21. Cowley D, Dunaway K, Forstein M, et al. Teaching psychiatry residents to work at the interface of mental health and primary care. Acad Psychiatry. 2014;38(4):398-404.
22. Herrmann N. The creative brain. J Creat Behav. 1991;25:275-295.
23. Rollnick S, Miller WR. What is motivational interviewing? Behav Cogn Psychother. 1995;23(4):325-334.
24. Pierson HM, Hayes SC, Gifford EV, et al. An examination of the Motivational Interviewing Treatment Integrity code. J Subst Abuse Treat. 2007;32(1):11-17.
25. Shaw D, Blue A. Should psychiatry champion interprofessional education? Acad Psychiatry. 2012;36(3):163-166.
26. Gilbert JH. Interprofessional learning and higher education structural barriers. J Interprof Care. 2005;19(suppl 1):87-106.
27. Heinemann GD, Schmitt MH, Farrell PP, Brallier SA. Development of an Attitudes Toward Health Care Teams Scale. Eval Health Prof. 1999;22(1):123-142.
28. Hepburn K, Tsukuda RA, Fasser C. Team Skills Scale. In: Heinemann GD, Zeiss AM, eds. Team Performance in Healthcare: Assessment and Development. New York, NY: Kluwer Academic/Plenum; 2002:159-163.
Psychotherapy Telemental Health Center and Regional Pilot
Within VHA, telemental health (TMH) refers to behavioral health services that are provided remotely, using secure communication technologies, to veterans who are separated by distance from their mental health providers.1 Telemental health sometimes involves video teleconferencing (VTC) technology, where a veteran (or group of veterans) in one location and a provider in a different location are able to communicate in real time through a computer monitor or television screen.2 In the VHA, TMH visits are typically conducted from a central location (such as a medical center hospital) to a community-based outpatient clinic (CBOC), but pilot projects have also tested VTC in homes as well.1,3,4
In addition to providing timely access to behavioral health services in rural or underserved locations, TMH eliminates travel that may be disruptive or costly and allows mental health providers to consult with or provide supervision to one another. Telemental health can be used to make diagnoses, manage care, perform checkups, and provide long-term, follow-up care. Other uses for TMH include clinical assessment, individual and group psychotherapy, psycho-educational interventions, cognitive testing, and general psychiatric care.1,5,6 More recently, TMH has been used to provide evidence-based psychotherapies (EBPs) to individuals with posttraumatic stress disorder (PTSD) and other mental health diagnoses.6,7 Such care may be particularly advantageous for veterans with PTSD, because traveling can be a burden for them or a trigger for PTSD symptoms.
Although interactive video technology is becoming widely available, its use is limited in health care systems due to lack of knowledge, education, logistical guidance, and technical training. The authors have conducted EBPs using VTC across VISN 22 in both office-to-office and office-to-home modalities and are providing EBPs using VTC to CBOCs in other VISNs across the western U.S. This article addresses these issues, outlining the necessary steps required to establish a TMH clinic and to share the successes of the EBP TMH Center and Regional Pilot used at VISN 22.
Telemental Health
Telemental health is an effective alternative to in-person treatment and is well regarded by both mental health providers and veterans. Overall, mental health providers believe it can help reduce the stigma associated with traditional mental health care and ease transportation-related issues for veterans. Telemental health allows access to care for veterans living in rural or remote areas in addition to those who are incarcerated or are otherwise unable to attend visits at primary VA facilities.2,8-10 In an assessment of TMH services in 40 CBOCs across VISN 16, most CBOC mental health providers found it to be an acceptable alternative to face-to-face care, recognize the value of TMH, and endorse a willingness to use and expand TMH programs within their clinics.11
Veterans who participated in TMH via VTC have expressed satisfaction with the decreased travel time and expenses, fewer interactions with crowds, and fewer parking problems.12 Several studies suggested that veterans preferred TMH to in-person contact due to more rapid access to care and specialists who would otherwise be unavailable at remote locations.5,10 Similarly, veterans who avoid in-person mental health care were more open to remote therapy for many of the reasons listed earlier. Studies suggest that veterans from both rural and urban locations are generally receptive to receiving mental health services via TMH.5,10
Several studies have found that TMH services may have advantages over standard in-person care. These advantages include decreasing transportation costs, travel time, and time missed at work and increasing system coverage area.13 Overall, both veterans and providers reported similar satisfaction between VTC and in-person sessions and, in some cases, prefer VTC interactions due to a sense of “easing into” intense therapies or having a “therapeutic distance” as treatment begins.12
Utility
Previous studies have shown that TMH can be used successfully to provide psychopharmacologic treatment to veterans who have major depressive disorder or schizophrenia, among other psychiatric disorders.5,8,14 Recent studies have focused on the feasibility of providing EBPs via TMH, particularly for the treatment of PTSD.12,15 Studies have shown that TMH services via VTC can be used successfully to provide cognitive behavioral therapy (CBT), cognitive processing therapy (CPT), and prolonged exposure therapy (PE).16-21 In these studies, both PE and CPT delivered via TMH were found to be as efficacious as in-person formats. Furthermore, TMH services were successfully used in individual and group sessions.
Research has emphasized the benefits of TMH for veterans who are uncomfortable in crowds, waiting rooms, or hospital lobbies.7,12,18 For patients with PTSD who are initially limited by fears related to driving, TMH can facilitate access to care. Veterans with PTSD often avoid reminders of trauma (ie, uniforms, evidence of physical injury, artwork, photographs related to war), which can often be found at the larger VAMCs. These veterans may find mental health care services in their homes or at local CBOCs more appealing.7,12,18
Implementation
Prior to the implementation of telehealth services, many CBOC providers would refer veterans in need of specialty care to the nearest VAMC, which were sometimes many hours away.1 In response to travel and access concerns, the VA has implemented various telehealth modalities, including TMH.
In 2008, about 230,000 veterans received mental health services via real-time clinical VTC at 300 VA CBOCs, and about 40,000 veterans enrolled in the In-Home Telehealth program.22 By 2011, > 380,000 veterans used clinic-based telehealth services and about 100,000 veterans used the in-home program.1 Between 2006 and 2008, the 98,000 veterans who used TMH modalities had fewer hospital admissions compared with those who did not; overall, the need for hospital services decreased by about 25% for those using TMH services.23
Although research suggests that TMH is an effective treatment modality, it does have limitations. A recent study noted several visual and audio difficulties that can emerge, including pixilation, “tracer” images with movement, low resolution, “frozen” or “choppy” images, delays in sound, echoes, or “mechanical sounding” voices.12 In some cases, physical details, such as crying, sniffling, or fidgeting, could not be clearly observed.12 Overall, these unforeseen issues can impact the ability to give and receive care through TMH modalities. Proper procedures need to be developed and implemented for each site.
Getting Started
Using TMH to provide mental health care at other VHA facilities requires planning and preparation. Logistics, such as preparation of the room and equipment, should be considered. Similarly, veteran and provider convenience must be considered.2,11 Before starting TMH at any VA facility, professionals working with the audiovisual technology and providing TMH care must complete necessary VA Talent Management System courses and obtain copies of certificates to assure they have met the appropriate training criteria. Providers must be credentialed to provide TMH services, including the telehealth curriculum offered by VA Employee Educational Services.2,24 An appropriate memorandum of understanding (MOU) must be created, and credentialing and privileging must also be acquired.
In addition to provider training, an information technology representative who can administer technical support as needed must be selected for both the provider and remote locations. Technologic complications can make TMH implementation much more challenging.12 As such, it is important to assure that both the veteran and the provider have the necessary TMH equipment. The selected communication device must be compatible with the technology requirements at the provider and remote facilities.12
In addition to designated technical support, the VISN TMH coordinator needs to have point-of-contact information for those who can assist with each site’s telehealth services and address the demand for EBP for PTSD or other desired services. After this information has been obtained, relationships must be developed and maintained with local leadership at each site, associated telehealth coordinators, and evidence-based therapy coordinators.
After contact has been established with remote facilities and the demand for services has been determined, there are several agreements and procedures to put in place before starting TMH services. An initial step is to develop a MOU agreement between the VISN TMH center and remote
sites that allows providers’ credentials and privileges to be shared. Also, it is important to establish a service agreement that outlines the procedures for staff at the remote site. This agreement includes checking in veterans, setting up the TMH rooms, transferring homework to VISN TMH providers, and connecting with the VISN TMH provider. In addition to service agreements, emergency procedures must be in place to ensure the safety of the veterans and the staff.24
After these agreements have been completed, the VISN TMH providers will have to complete request forms to obtain access to the Computerized Patient Record System at the remote facilities, which then must be approved by the Information Security Officer at that site. This is separate from the request at the provider’s site.12 It is essential to have points of contact for questions regarding this process. In order to facilitate referrals for TMH, electronic interfacility consult requests must be developed. Local staff need to collaborate with VISN TMH staff to ensure that the consult addresses the referral facilities need to meet the appropriate requirements.
Before the initiation of TMH services, each TMH provider has to establish clinics for scheduling appointments and obtaining workload credit. Program support assistants at the provider and remote sites must work together to ensure clinics are established correctly. This collaboration is essential for coding of visits and clinic mapping. After the clinics are “built,” appointment times will be set up based on the availability of the provider, support staff, and rooms at the remote site for the TMH session.
Once a consult is initiated, the VISN TMH EBP coordinator will review the consult and the veteran’s chart to ensure initial inclusion/exclusion criteria are met before accepting or canceling the consult. If the consult is accepted, a VISN TMH provider is assigned to the case and contacts the veteran to discuss the referral and (if the veteran is appropriate and interested) initiate services at the closest CBOC or at home. The VISN TMH regional center staff enter the appointment time for the veteran at both facility sites. The VISN TMH provider also coordinates with the CBOC staff to ensure that the veteran is checked in to the appointment and is provided with any questionnaires and necessary homework.
During the first session, the provider obtains consent from the veteran to engage in TMH services, conducts an assessment, and establishes rapport. The provider works with the veteran to develop a treatment plan for PTSD or other mental health diagnosis that will include the type of EBP. At the end of the first session, the next appointment is scheduled, and treatment materials are either mailed to the veteran or given to him or her onsite. After completing EBP, the VISN TMH center works with the referring provider to find follow-up services for the veteran.
The various steps necessary to begin an interfacility TMH clinic are summarized in Table 1.
Provider Training
Despite strong evidence of success, many providers remained skeptical about the efficacy of TMH. One study indicated that several providers in VISN 16 rarely used the established TMH programs because they were not familiar with them and applied TMH only for medication checks and consults.11 This skepticism was present in providers preparing to offer TMH as well as in providers referring veterans for TMH services. However, once providers better understood the TMH programs and had more experience using them, they were significantly more likely to use TMH for initial evaluations and ongoing psychotherapy. For these reasons, proper training and educational opportunities for practicing providers are vital to TMH implementation.9,11
To be proficient, providers need to become familiar with various TMH applications.10 Health care networks implementing TMH must ensure that their providers are well trained and prepared to give and receive proper consultation and support. Providers must also acquire several skills and familiarize themselves with available tools.9 In educating providers on the process and use of TMH, the authors suggest the following steps for TMH application:
- Learn new ways to chart in multiple systems and know how to troubleshoot during connectivity issues.
- Have an established administrative support collaborator at outpatient clinics to fax and exchange veteran homework.12
- The TMH clinic culture must be embedded where the veteran is being served in order to allow for a more realistic therapeutic feel. This type of clinic setting will allow for referrals at the veteran site and the availability to coordinate emergency procedures in the remote clinic.
Clinical Issues
Ongoing clinical issues need to be addressed continuously. Initially, referrals may be plentiful but not always appropriate. It is important to have an understanding with referring providers and remote sites about what constitutes a “good referral” as well as alternate referral options. It is imperative to outline inclusion and exclusion criteria that are clear and concise for referring providers. It is often helpful to revisit these criteria with potential referral sources after initiating services.
With the ability to provide inhome services, it is important to identify specific inclusion/exclusion criteria. Recommendations are based on research and clinical applications for exclusions, which are available on the Office of Technology Services website. These include imminent suicidality or homicidality, serious personality disorder or problematic character traits, acute substance disorders, psychotic disorders, and bipolar disorder. It is important to use sound clinical judgment, because the usual safeguards present in a remote clinic are not available for inhome services. Emergency planning is one of the most important aspects of the in-home TMH health services that are provided. The information for the emergency plan is obtained prior to initiation of services.
Emergency Plans
Each remote clinic that provides services to veterans must have an emergency plan that details procedures, phone numbers, and resources in case of medical and psychological emergencies as well as natural disasters. The VISN TMH provider will need to have a copy of the emergency plan as well as a list of contacts in case of an emergency during a TMH session.
It is recommended that TMH providers have several ways to contact key staff who can assist during an emergency. Usually the clinical coordinator and telehealth technician are the first responders to be alerted by the TMH provider during an emergency. They will then institute the remote clinic’s emergency protocol. Discussing these procedures and reviewing them with staff regularly is advisable, as key contacts may change.
In a psychological emergency, the VISN TMH provider may assist in implementing emergency procedures until a clinical counterpart at the remote site can be alerted. In the authors’ experience, VISN TMH providers have successfully de-escalated and diffused potentially emergent situations by maintaining constant realtime communication with veterans and staff by using VTC as well as interoffice communication. By offering assistance to veterans and staff during challenging situations, the VISN TMH provider will not only decrease concerns of veterans, but oftentimes integrate themselves into the treatment team of the remote clinic. The role of a VISN TMH provider can be isolative, with minimal contact with remote clinic staff, so it is important to increase visibility among staff at a remote site by communication with them even when there is not an emergency.
Treatment protocols may be determined by either administrative or clinical factors. With certain TMH interventions, the rooms used for veterans may be available for only certain periods, which may or may not fit with treatment protocols. For example, if a room is available for only an hour but a treatment protocol session is for 90 minutes, then another time slot needs to be found or a different treatment considered and offered. Although it is not ideal to have treatment protocols determined by scheduling factors, the reality of shared space at remote sites requires flexibility.
Sharing Materials and Homework Another clinical issue that is often overlooked is how to implement specific treatment protocols that entail the exchange of materials between VISN TMH providers and veterans. If materials will need to be exchanged between provider and veteran, a plan will have to be in place to facilitate this. The service agreement addresses these details, but remote staff may not always be aware of the details.
If a TMH provider opts to use faxes to send materials between a veteran and a provider, a desktop faxing program is recommended so veteran privacy is not compromised. Often, providers will wait to begin sessions until after they have received materials, but this may result in a delayed
session. One solution TMH providers can implement is mailing the materials and questionnaires to veterans before the session with clear instructions to complete them beforehand. Once the veteran arrives for the TMH session, she or he will verbally respond to the questionnaire and treatment materials. This will add time to a session but minimizes potential delays. Many of the clinical VTC units have movable cameras, so veterans can tilt the camera to show providers the forms and questionnaires.
The various steps necessary to address TMH clinical issues are summarized in Table 2.
VISIN 22 Pilot Project
The VISN 22 EBP TMH Center and Regional Pilot, based at the VA San Diego Healthcare System, was tasked with developing and providing TMH EBP services for PTSD across VISN 22 and adjacent West Coast VISNs. In addition to creating standardized procedures, troubleshooting guides were established to assist other programs with implementation. The primary focus was to increase access to EBPs for veterans with PTSD in areas where there was either no available trained providers or delays for specific services. The program established 16 clinics as well as in-home
services in VISN 22, VISN 21, and VISN 20. In fiscal year (FY) 2013, the VISN 22 EBP TMH Center and Regional Pilot provided 1,657 EBP encounters via TMH to 234 unique veterans with PTSD (Table 3).
The pilot project collected data to evaluate program effectiveness. The data were de-identified before being sent to the VA Central Office (VACO) TMH program manager. The following items were collected for the pilot: (1) clinical information; (2) consent to engage in treatment and telehealth; (3) release of information to share de-identified data to VACO for program monitoring; (4) demographic form; (5) Beck Depression Inventory-II (every other week); (6) PTSD Checklist (every other week); (7) World Health Organization Quality of Life (sessions 1, 7, final); (8) Wechsler Adult Intelligence Scale-Revised (sessions 3, 7, final); (9) satisfaction survey (final); (10) mileage not driven by veterans who receive TMH services; (11) travel pay saved by VA; (12) no-show rates; and (13) veteran, TMH provider, and referral provider satisfaction.
The growth in number of encounters and number of unique veterans has increased steadily from the first quarter of FY14 through the second quarter of FY15 (Figure 1).
In January 2013, in-home TMH services were piloted. Although occasional technical difficulties occurred, 143 EBP encounters via TMH were provided to 42 unique veterans in 2013. The service has continued to expand, and in the first half of FY14, services were provided to 64 unique veterans for a total of 278 encounters, saving veterans 3,220 travel miles and saving the VA $1,336 in travel reimbursement. In-home TMH services will continue to expand as more providers in a variety of programs are being trained by the San Diego staff on how to provide these services to veterans in their homes. In addition to decreasing mileage and travel pay, the no-show rates are lower for TMH appointments in general (averaged 8%-10% vs facility no-show rate average of 13.5%) and with the use of inhome TMH, no-show rates were kept to 2%. The growth in the number of in-home encounters and the number of unique veterans has also increased steadily from the first quarter of FY14 thru the second quarter of FY15 (Figure 2).
In-Home TMH Services
The VISN 22 EBP TMH Center and Regional Pilot often requests to have an in-person meeting with a veteran before starting TMH services in order to complete a waiver to download the software used by the VA for real-time video in-home services, a Release of Information for a Primary Support Person form, and an emergency plan.
It is also recommended that information about the veteran’s Internet connection, type of computer, type of software, presence of a camera and speakers, e-mail address, and access to secure messaging are obtained. During the initial contact with a veteran, the provider will discuss the rules and requirements to ensure HIPAA compliance. The veteran will need to have a private area for the call (not a restaurant, car, or other place where Wi-Fi is offered). Even with these discussions, some veterans will initiate services from a public place or a room in their home where family members will enter and exit frequently.
Although not required, it is recommended to have the veteran identify a primary support person and complete a release form to allow the TMH provider to contact that person in an emergency. The support person may be a person in the home (adult family member or caregiver) or someone nearby (neighbor, friend, or family member) who can contact emergency services if needed. After the necessary information is gathered and the veteran agrees to the conditions of participation, a test call will be completed. The TMH provider is often the person to conduct this call, but if available, a telehealth technician or facility telehealth coordinator may assist. The TMH provider may help the veteran download the appropriate software that is sent from the VA Scheduler software. The veteran initiates the call with the provider. Once the connection is made, the session may begin. Sites that are currently conducting in-home services have provided guides to veterans and newer TMH providers to outline the necessary steps for initiating services.
It is recommended that any provider interested in providing in-home TMH services use the Office of Technological Services help desk to assist in troubleshooting difficulties with connectivity. Challenges have included the software used for in-home TMH, periodic Internet outages, and compatibility issues.
Veteran Satisfaction
Veteran satisfaction was measured through a self-report satisfaction survey. The survey included 12 questions assessing overall experience in using TMH services. Eleven of the 12 questions included a 5-point Likert scale (1 = strongly disagree, 5 = strongly agree); the last question was openended for additional comments.
A summary of the survey response of the initial 29 veterans who received TMH services suggested the following: (1) Veterans felt comfortable with using the TMH equipment and were able to see their clinician clearly; (2) Technical assistance was sufficient; (3) During the TMH session, they related to the provider as if it were a face-to-face meeting and that their needs were met; and (4) Veterans reported extremely high satisfaction with TMH and would refer TMH care to other veterans. Veterans found clinic locations very convenient and preferred the TMH modality of mental health services delivery to the alternative of travelling a long distance to see their provider (Table 4).
Written comments and recommendations from veterans supported the survey results. Most reported that they saved time and the convenience of the clinic allowed them to receive the treatment they need without interfering with their work schedule. However, some veterans still experienced trouble with travel to the remote clinic. Others felt their experience was different from the one they expected or they had a good experience via TMH but preferred face-to-face care.
Conclusion
The VISN 22 EBP TMH Center and Regional Pilot have established the infrastructure of interfacility clinics to use EBPs for the treatment of PTSD. Also, the center has provided consultation and guidance to facilities interested in developing their own TMH programs. The TMH Center now plans to expand mental health services and include medication management and EBP services for non-PTSD psychiatric diagnoses. The established infrastructure will allow providers from one facility to cover the mental health service needs of other facilities when there are absences or gaps due to leave or delays/challenges in hiring in rural locations. Finally, TMH offers the potential to offer after-hours services to veterans in other time zones during providers’ regular tours of duty.
Several other TMH programs are now expanding services into veterans’ homes. There are several sites within the VHA that have piloted this TMH modality and developed guidelines and recommendations for further expansion. Currently VACO is encouraging all VHA facilities to increase in-home telehealth services, and the Office of Telehealth Services provides details on implementation. Interested parties are encouraged to routinely visit the VACO website for updated information.
Developing and implementing a new TMH program can be an arduous task, but the program has great potential to provide veteran-centered care. As TMH sessions progress, the provider and veteran become less aware of the camera and software and more aware of the therapeutic process. Challenges and delays in implementation are to be expected—these can occur frequently during the development and implementation stages of a TMH program. Maintaining consistent communication with staff at remote sites is essential for the success of any program.
As the VHA focuses on veterancentered care, TMH services will improve access to providers with specific, needed expertise. The authors hope these experiences can facilitate the continued growth of TMH and assuage any concerns a facility or provider may have about this modality of care. Delivery of TMH care can be challenging, but the ability to provide these services to veterans at times and locations convenient to them makes these challenges worthwhile.
Acknowledgments
Dr. Hauser wishes to thank Cathy, Anika, Jirina, Katia, and Max Hauser, and Alba Pillwein for their continued support. In memory of Beverly Ostroski.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. U.S. Department of Veterans Affairs. What is telehealth? U.S. Department of Veterans Affairs Website. http://www.telehealth.va.gov. Update May 13, 2014. Accessed April 30, 2015.
2. Morland LA, Greene CJ, Rosen C, Mauldin PD, Frueh CB. Issues in the design of a randomized noninferiority clinical trial of telemental health psychotherapy for rural combat veterans with PTSD. Contemp Clin Trials. 2009;30(6):513-522.
3. Strachan M, Gros DF, Ruggiero KJ, Lejuez CW, Acierno R. An integrated approach to delivering exposure-based treatment for symptoms of PTSD and depression in OIF/OEF veterans: preliminary findings. Behav Ther. 2012;43(3):560-569.
4. Yuen EK, Gros DF, Price M, et al. Randomized controlled trial of home-based telehealth versus in-person prolonged exposure for combat-related PTSD in veterans: preliminary results. J Clin Psychol. 2015;71(6):500-512.
5. Ruskin PE, Reed S, Kumar R, et al. Reliability and acceptability of psychiatric diagnosis via telecommunication and audiovisual technology. Psychiatr Serv. 1998;49(8):1086-1088.
6. Gros DF, Morland LA, Greene CJ, et al. Delivery of evidence-based psychotherapy via video telehealth. J Psychopathol Behav Assess. 2013;35(4):506-521.
7. Backhaus A, Agha Z, Maglione ML, et al. Videoconferencing psychotherapy: a systematic review. Psychol Serv. 2012;9(2):111-131.
8. Egede LE, Frueh CB, Richardson LK, et al. Rationale and design: telepsychology service delivery for depressed elderly veterans. Trials. 2009;10:22.
9. Frueh BC, Deitsch SE, Santos AB, et al. Procedural and methodological issues in telepsychiatry research and program development. Psychiatr Serv. 2000;51(12):1522-1527.
10. Grubaugh AL, Cain GD, Elhai JD, Patrick SL, Frueh BC. Attitudes toward medical and mental health care delivered via telehealth applications among rural and urban primary care patients. J Nerv Ment Dis. 2008;196(2):166-170.
11. Jameson JP, Farmer MS, Head KJ, Fortney J, Teal CR. VA community mental health service providers’ utilization of and attitudes towards telemental health care: the gatekeeper’s perspective. J Rural Health. 2011;27(4):425-432.
12. Thorp SR, Fidler J, Moreno L, Floto E, Agha Z. Lessons learned from studies of psychotherapy for posttraumatic stress disorder via video teleconferencing. Psychol Serv. 2012;9(2):197-199.
13. Gros DF, Yoder M, Tuerk PW, Lozano BE, Acierno R. Exposure therapy for PTSD delivered to veterans via telehealth: predictors of treatment completion and outcome and comparison to treatment delivered in person. Behav Ther. 2011;42(2):276-283.
14. Zarate CA Jr, Weinstock L, Cukor P, et al. Applicability of telemedicine for assessing patients with schizophrenia: acceptance and reliability. J Clin Psychiatry. 1997;58(1):22-25.
15. Jones AM, Shealy KM, Reid-Quiñones K, et al. Guidelines for establishing a telemental health program to provide evidence-based therapy for trauma-exposed children and families. Psychol Serv. 2014;11(4):398-409.
16. Frueh BC, Monnier J, Grubaugh AL, Elhai JD, Yim E, Knapp R. Therapist adherence and competence with manualized cognitive-behavioral therapy for PTSD delivered via videoconferencing technology. Behav Modif. 2007;31(6):856-866.
17. Morland LA, Hynes AK, Mackintosh MA, Resick PA, Chard KM. Group cognitive processing therapy delivered to veterans via telehealth: a pilot cohort. J Trauma Stress. 2011;24(4):465-469.
18. Tuerk PW, Yoder M, Ruggiero KJ, Gros DF, Acierno R. A pilot study of prolonged exposure therapy for posttraumatic stress disorder delivered via telehealth technology. J Trauma Stress. 2010;23(1):116-123.
19. Fortney JC, Pyne JM, Kimbrell TA, et al. Telemedicine- based collaborative care for posttraumatic stress disorder: a randomized clinical trial. JAMA Psychiatry. 2015;72(1):58-67.
20. Germain V, Marchand A, Bouchard S, Drouin MS, Guay S. Effectiveness of cognitive behavioural therapy administered by videoconference for posttraumatic stress disorder. Cogn Behav Ther. 2009;38(1):42-53.
21. Morland LA, Mackintosh M, Greene CJ, et al. Cognitive processing therapy for posttraumatic stress disorder delivered to rural veterans via telemental health: a randomized noninferiority clinical trial. J Clin Psychiatry. 2014;75(5):470-476.
22. Tuerk PW, Fortney J, Bosworth HB, et al. Toward the development of national telehealth services: the role of Veterans Health Administration and future directions for research. Telemed J E Health. 2010;16(1):115-117.
23. Godleski L, Darkins A, Peters J. Outcomes of 98,609 U.S. Department of Veterans Affairs patients enrolled in telemental health services, 2006-2010. Psychiatr Serv. 2012;63(4):383-385.
24. Strachan M, Gros DF, Yuen E, Ruggiero KJ, Foa EB, Acierno R. Home-based telehealth to deliver evidence-based psychotherapy in veterans with PTSD. Contemp Clin Trials. 2012;33(2):402-409.
Within VHA, telemental health (TMH) refers to behavioral health services that are provided remotely, using secure communication technologies, to veterans who are separated by distance from their mental health providers.1 Telemental health sometimes involves video teleconferencing (VTC) technology, where a veteran (or group of veterans) in one location and a provider in a different location are able to communicate in real time through a computer monitor or television screen.2 In the VHA, TMH visits are typically conducted from a central location (such as a medical center hospital) to a community-based outpatient clinic (CBOC), but pilot projects have also tested VTC in homes as well.1,3,4
In addition to providing timely access to behavioral health services in rural or underserved locations, TMH eliminates travel that may be disruptive or costly and allows mental health providers to consult with or provide supervision to one another. Telemental health can be used to make diagnoses, manage care, perform checkups, and provide long-term, follow-up care. Other uses for TMH include clinical assessment, individual and group psychotherapy, psycho-educational interventions, cognitive testing, and general psychiatric care.1,5,6 More recently, TMH has been used to provide evidence-based psychotherapies (EBPs) to individuals with posttraumatic stress disorder (PTSD) and other mental health diagnoses.6,7 Such care may be particularly advantageous for veterans with PTSD, because traveling can be a burden for them or a trigger for PTSD symptoms.
Although interactive video technology is becoming widely available, its use is limited in health care systems due to lack of knowledge, education, logistical guidance, and technical training. The authors have conducted EBPs using VTC across VISN 22 in both office-to-office and office-to-home modalities and are providing EBPs using VTC to CBOCs in other VISNs across the western U.S. This article addresses these issues, outlining the necessary steps required to establish a TMH clinic and to share the successes of the EBP TMH Center and Regional Pilot used at VISN 22.
Telemental Health
Telemental health is an effective alternative to in-person treatment and is well regarded by both mental health providers and veterans. Overall, mental health providers believe it can help reduce the stigma associated with traditional mental health care and ease transportation-related issues for veterans. Telemental health allows access to care for veterans living in rural or remote areas in addition to those who are incarcerated or are otherwise unable to attend visits at primary VA facilities.2,8-10 In an assessment of TMH services in 40 CBOCs across VISN 16, most CBOC mental health providers found it to be an acceptable alternative to face-to-face care, recognize the value of TMH, and endorse a willingness to use and expand TMH programs within their clinics.11
Veterans who participated in TMH via VTC have expressed satisfaction with the decreased travel time and expenses, fewer interactions with crowds, and fewer parking problems.12 Several studies suggested that veterans preferred TMH to in-person contact due to more rapid access to care and specialists who would otherwise be unavailable at remote locations.5,10 Similarly, veterans who avoid in-person mental health care were more open to remote therapy for many of the reasons listed earlier. Studies suggest that veterans from both rural and urban locations are generally receptive to receiving mental health services via TMH.5,10
Several studies have found that TMH services may have advantages over standard in-person care. These advantages include decreasing transportation costs, travel time, and time missed at work and increasing system coverage area.13 Overall, both veterans and providers reported similar satisfaction between VTC and in-person sessions and, in some cases, prefer VTC interactions due to a sense of “easing into” intense therapies or having a “therapeutic distance” as treatment begins.12
Utility
Previous studies have shown that TMH can be used successfully to provide psychopharmacologic treatment to veterans who have major depressive disorder or schizophrenia, among other psychiatric disorders.5,8,14 Recent studies have focused on the feasibility of providing EBPs via TMH, particularly for the treatment of PTSD.12,15 Studies have shown that TMH services via VTC can be used successfully to provide cognitive behavioral therapy (CBT), cognitive processing therapy (CPT), and prolonged exposure therapy (PE).16-21 In these studies, both PE and CPT delivered via TMH were found to be as efficacious as in-person formats. Furthermore, TMH services were successfully used in individual and group sessions.
Research has emphasized the benefits of TMH for veterans who are uncomfortable in crowds, waiting rooms, or hospital lobbies.7,12,18 For patients with PTSD who are initially limited by fears related to driving, TMH can facilitate access to care. Veterans with PTSD often avoid reminders of trauma (ie, uniforms, evidence of physical injury, artwork, photographs related to war), which can often be found at the larger VAMCs. These veterans may find mental health care services in their homes or at local CBOCs more appealing.7,12,18
Implementation
Prior to the implementation of telehealth services, many CBOC providers would refer veterans in need of specialty care to the nearest VAMC, which were sometimes many hours away.1 In response to travel and access concerns, the VA has implemented various telehealth modalities, including TMH.
In 2008, about 230,000 veterans received mental health services via real-time clinical VTC at 300 VA CBOCs, and about 40,000 veterans enrolled in the In-Home Telehealth program.22 By 2011, > 380,000 veterans used clinic-based telehealth services and about 100,000 veterans used the in-home program.1 Between 2006 and 2008, the 98,000 veterans who used TMH modalities had fewer hospital admissions compared with those who did not; overall, the need for hospital services decreased by about 25% for those using TMH services.23
Although research suggests that TMH is an effective treatment modality, it does have limitations. A recent study noted several visual and audio difficulties that can emerge, including pixilation, “tracer” images with movement, low resolution, “frozen” or “choppy” images, delays in sound, echoes, or “mechanical sounding” voices.12 In some cases, physical details, such as crying, sniffling, or fidgeting, could not be clearly observed.12 Overall, these unforeseen issues can impact the ability to give and receive care through TMH modalities. Proper procedures need to be developed and implemented for each site.
Getting Started
Using TMH to provide mental health care at other VHA facilities requires planning and preparation. Logistics, such as preparation of the room and equipment, should be considered. Similarly, veteran and provider convenience must be considered.2,11 Before starting TMH at any VA facility, professionals working with the audiovisual technology and providing TMH care must complete necessary VA Talent Management System courses and obtain copies of certificates to assure they have met the appropriate training criteria. Providers must be credentialed to provide TMH services, including the telehealth curriculum offered by VA Employee Educational Services.2,24 An appropriate memorandum of understanding (MOU) must be created, and credentialing and privileging must also be acquired.
In addition to provider training, an information technology representative who can administer technical support as needed must be selected for both the provider and remote locations. Technologic complications can make TMH implementation much more challenging.12 As such, it is important to assure that both the veteran and the provider have the necessary TMH equipment. The selected communication device must be compatible with the technology requirements at the provider and remote facilities.12
In addition to designated technical support, the VISN TMH coordinator needs to have point-of-contact information for those who can assist with each site’s telehealth services and address the demand for EBP for PTSD or other desired services. After this information has been obtained, relationships must be developed and maintained with local leadership at each site, associated telehealth coordinators, and evidence-based therapy coordinators.
After contact has been established with remote facilities and the demand for services has been determined, there are several agreements and procedures to put in place before starting TMH services. An initial step is to develop a MOU agreement between the VISN TMH center and remote
sites that allows providers’ credentials and privileges to be shared. Also, it is important to establish a service agreement that outlines the procedures for staff at the remote site. This agreement includes checking in veterans, setting up the TMH rooms, transferring homework to VISN TMH providers, and connecting with the VISN TMH provider. In addition to service agreements, emergency procedures must be in place to ensure the safety of the veterans and the staff.24
After these agreements have been completed, the VISN TMH providers will have to complete request forms to obtain access to the Computerized Patient Record System at the remote facilities, which then must be approved by the Information Security Officer at that site. This is separate from the request at the provider’s site.12 It is essential to have points of contact for questions regarding this process. In order to facilitate referrals for TMH, electronic interfacility consult requests must be developed. Local staff need to collaborate with VISN TMH staff to ensure that the consult addresses the referral facilities need to meet the appropriate requirements.
Before the initiation of TMH services, each TMH provider has to establish clinics for scheduling appointments and obtaining workload credit. Program support assistants at the provider and remote sites must work together to ensure clinics are established correctly. This collaboration is essential for coding of visits and clinic mapping. After the clinics are “built,” appointment times will be set up based on the availability of the provider, support staff, and rooms at the remote site for the TMH session.
Once a consult is initiated, the VISN TMH EBP coordinator will review the consult and the veteran’s chart to ensure initial inclusion/exclusion criteria are met before accepting or canceling the consult. If the consult is accepted, a VISN TMH provider is assigned to the case and contacts the veteran to discuss the referral and (if the veteran is appropriate and interested) initiate services at the closest CBOC or at home. The VISN TMH regional center staff enter the appointment time for the veteran at both facility sites. The VISN TMH provider also coordinates with the CBOC staff to ensure that the veteran is checked in to the appointment and is provided with any questionnaires and necessary homework.
During the first session, the provider obtains consent from the veteran to engage in TMH services, conducts an assessment, and establishes rapport. The provider works with the veteran to develop a treatment plan for PTSD or other mental health diagnosis that will include the type of EBP. At the end of the first session, the next appointment is scheduled, and treatment materials are either mailed to the veteran or given to him or her onsite. After completing EBP, the VISN TMH center works with the referring provider to find follow-up services for the veteran.
The various steps necessary to begin an interfacility TMH clinic are summarized in Table 1.
Provider Training
Despite strong evidence of success, many providers remained skeptical about the efficacy of TMH. One study indicated that several providers in VISN 16 rarely used the established TMH programs because they were not familiar with them and applied TMH only for medication checks and consults.11 This skepticism was present in providers preparing to offer TMH as well as in providers referring veterans for TMH services. However, once providers better understood the TMH programs and had more experience using them, they were significantly more likely to use TMH for initial evaluations and ongoing psychotherapy. For these reasons, proper training and educational opportunities for practicing providers are vital to TMH implementation.9,11
To be proficient, providers need to become familiar with various TMH applications.10 Health care networks implementing TMH must ensure that their providers are well trained and prepared to give and receive proper consultation and support. Providers must also acquire several skills and familiarize themselves with available tools.9 In educating providers on the process and use of TMH, the authors suggest the following steps for TMH application:
- Learn new ways to chart in multiple systems and know how to troubleshoot during connectivity issues.
- Have an established administrative support collaborator at outpatient clinics to fax and exchange veteran homework.12
- The TMH clinic culture must be embedded where the veteran is being served in order to allow for a more realistic therapeutic feel. This type of clinic setting will allow for referrals at the veteran site and the availability to coordinate emergency procedures in the remote clinic.
Clinical Issues
Ongoing clinical issues need to be addressed continuously. Initially, referrals may be plentiful but not always appropriate. It is important to have an understanding with referring providers and remote sites about what constitutes a “good referral” as well as alternate referral options. It is imperative to outline inclusion and exclusion criteria that are clear and concise for referring providers. It is often helpful to revisit these criteria with potential referral sources after initiating services.
With the ability to provide inhome services, it is important to identify specific inclusion/exclusion criteria. Recommendations are based on research and clinical applications for exclusions, which are available on the Office of Technology Services website. These include imminent suicidality or homicidality, serious personality disorder or problematic character traits, acute substance disorders, psychotic disorders, and bipolar disorder. It is important to use sound clinical judgment, because the usual safeguards present in a remote clinic are not available for inhome services. Emergency planning is one of the most important aspects of the in-home TMH health services that are provided. The information for the emergency plan is obtained prior to initiation of services.
Emergency Plans
Each remote clinic that provides services to veterans must have an emergency plan that details procedures, phone numbers, and resources in case of medical and psychological emergencies as well as natural disasters. The VISN TMH provider will need to have a copy of the emergency plan as well as a list of contacts in case of an emergency during a TMH session.
It is recommended that TMH providers have several ways to contact key staff who can assist during an emergency. Usually the clinical coordinator and telehealth technician are the first responders to be alerted by the TMH provider during an emergency. They will then institute the remote clinic’s emergency protocol. Discussing these procedures and reviewing them with staff regularly is advisable, as key contacts may change.
In a psychological emergency, the VISN TMH provider may assist in implementing emergency procedures until a clinical counterpart at the remote site can be alerted. In the authors’ experience, VISN TMH providers have successfully de-escalated and diffused potentially emergent situations by maintaining constant realtime communication with veterans and staff by using VTC as well as interoffice communication. By offering assistance to veterans and staff during challenging situations, the VISN TMH provider will not only decrease concerns of veterans, but oftentimes integrate themselves into the treatment team of the remote clinic. The role of a VISN TMH provider can be isolative, with minimal contact with remote clinic staff, so it is important to increase visibility among staff at a remote site by communication with them even when there is not an emergency.
Treatment protocols may be determined by either administrative or clinical factors. With certain TMH interventions, the rooms used for veterans may be available for only certain periods, which may or may not fit with treatment protocols. For example, if a room is available for only an hour but a treatment protocol session is for 90 minutes, then another time slot needs to be found or a different treatment considered and offered. Although it is not ideal to have treatment protocols determined by scheduling factors, the reality of shared space at remote sites requires flexibility.
Sharing Materials and Homework Another clinical issue that is often overlooked is how to implement specific treatment protocols that entail the exchange of materials between VISN TMH providers and veterans. If materials will need to be exchanged between provider and veteran, a plan will have to be in place to facilitate this. The service agreement addresses these details, but remote staff may not always be aware of the details.
If a TMH provider opts to use faxes to send materials between a veteran and a provider, a desktop faxing program is recommended so veteran privacy is not compromised. Often, providers will wait to begin sessions until after they have received materials, but this may result in a delayed
session. One solution TMH providers can implement is mailing the materials and questionnaires to veterans before the session with clear instructions to complete them beforehand. Once the veteran arrives for the TMH session, she or he will verbally respond to the questionnaire and treatment materials. This will add time to a session but minimizes potential delays. Many of the clinical VTC units have movable cameras, so veterans can tilt the camera to show providers the forms and questionnaires.
The various steps necessary to address TMH clinical issues are summarized in Table 2.
VISIN 22 Pilot Project
The VISN 22 EBP TMH Center and Regional Pilot, based at the VA San Diego Healthcare System, was tasked with developing and providing TMH EBP services for PTSD across VISN 22 and adjacent West Coast VISNs. In addition to creating standardized procedures, troubleshooting guides were established to assist other programs with implementation. The primary focus was to increase access to EBPs for veterans with PTSD in areas where there was either no available trained providers or delays for specific services. The program established 16 clinics as well as in-home
services in VISN 22, VISN 21, and VISN 20. In fiscal year (FY) 2013, the VISN 22 EBP TMH Center and Regional Pilot provided 1,657 EBP encounters via TMH to 234 unique veterans with PTSD (Table 3).
The pilot project collected data to evaluate program effectiveness. The data were de-identified before being sent to the VA Central Office (VACO) TMH program manager. The following items were collected for the pilot: (1) clinical information; (2) consent to engage in treatment and telehealth; (3) release of information to share de-identified data to VACO for program monitoring; (4) demographic form; (5) Beck Depression Inventory-II (every other week); (6) PTSD Checklist (every other week); (7) World Health Organization Quality of Life (sessions 1, 7, final); (8) Wechsler Adult Intelligence Scale-Revised (sessions 3, 7, final); (9) satisfaction survey (final); (10) mileage not driven by veterans who receive TMH services; (11) travel pay saved by VA; (12) no-show rates; and (13) veteran, TMH provider, and referral provider satisfaction.
The growth in number of encounters and number of unique veterans has increased steadily from the first quarter of FY14 through the second quarter of FY15 (Figure 1).
In January 2013, in-home TMH services were piloted. Although occasional technical difficulties occurred, 143 EBP encounters via TMH were provided to 42 unique veterans in 2013. The service has continued to expand, and in the first half of FY14, services were provided to 64 unique veterans for a total of 278 encounters, saving veterans 3,220 travel miles and saving the VA $1,336 in travel reimbursement. In-home TMH services will continue to expand as more providers in a variety of programs are being trained by the San Diego staff on how to provide these services to veterans in their homes. In addition to decreasing mileage and travel pay, the no-show rates are lower for TMH appointments in general (averaged 8%-10% vs facility no-show rate average of 13.5%) and with the use of inhome TMH, no-show rates were kept to 2%. The growth in the number of in-home encounters and the number of unique veterans has also increased steadily from the first quarter of FY14 thru the second quarter of FY15 (Figure 2).
In-Home TMH Services
The VISN 22 EBP TMH Center and Regional Pilot often requests to have an in-person meeting with a veteran before starting TMH services in order to complete a waiver to download the software used by the VA for real-time video in-home services, a Release of Information for a Primary Support Person form, and an emergency plan.
It is also recommended that information about the veteran’s Internet connection, type of computer, type of software, presence of a camera and speakers, e-mail address, and access to secure messaging are obtained. During the initial contact with a veteran, the provider will discuss the rules and requirements to ensure HIPAA compliance. The veteran will need to have a private area for the call (not a restaurant, car, or other place where Wi-Fi is offered). Even with these discussions, some veterans will initiate services from a public place or a room in their home where family members will enter and exit frequently.
Although not required, it is recommended to have the veteran identify a primary support person and complete a release form to allow the TMH provider to contact that person in an emergency. The support person may be a person in the home (adult family member or caregiver) or someone nearby (neighbor, friend, or family member) who can contact emergency services if needed. After the necessary information is gathered and the veteran agrees to the conditions of participation, a test call will be completed. The TMH provider is often the person to conduct this call, but if available, a telehealth technician or facility telehealth coordinator may assist. The TMH provider may help the veteran download the appropriate software that is sent from the VA Scheduler software. The veteran initiates the call with the provider. Once the connection is made, the session may begin. Sites that are currently conducting in-home services have provided guides to veterans and newer TMH providers to outline the necessary steps for initiating services.
It is recommended that any provider interested in providing in-home TMH services use the Office of Technological Services help desk to assist in troubleshooting difficulties with connectivity. Challenges have included the software used for in-home TMH, periodic Internet outages, and compatibility issues.
Veteran Satisfaction
Veteran satisfaction was measured through a self-report satisfaction survey. The survey included 12 questions assessing overall experience in using TMH services. Eleven of the 12 questions included a 5-point Likert scale (1 = strongly disagree, 5 = strongly agree); the last question was openended for additional comments.
A summary of the survey response of the initial 29 veterans who received TMH services suggested the following: (1) Veterans felt comfortable with using the TMH equipment and were able to see their clinician clearly; (2) Technical assistance was sufficient; (3) During the TMH session, they related to the provider as if it were a face-to-face meeting and that their needs were met; and (4) Veterans reported extremely high satisfaction with TMH and would refer TMH care to other veterans. Veterans found clinic locations very convenient and preferred the TMH modality of mental health services delivery to the alternative of travelling a long distance to see their provider (Table 4).
Written comments and recommendations from veterans supported the survey results. Most reported that they saved time and the convenience of the clinic allowed them to receive the treatment they need without interfering with their work schedule. However, some veterans still experienced trouble with travel to the remote clinic. Others felt their experience was different from the one they expected or they had a good experience via TMH but preferred face-to-face care.
Conclusion
The VISN 22 EBP TMH Center and Regional Pilot have established the infrastructure of interfacility clinics to use EBPs for the treatment of PTSD. Also, the center has provided consultation and guidance to facilities interested in developing their own TMH programs. The TMH Center now plans to expand mental health services and include medication management and EBP services for non-PTSD psychiatric diagnoses. The established infrastructure will allow providers from one facility to cover the mental health service needs of other facilities when there are absences or gaps due to leave or delays/challenges in hiring in rural locations. Finally, TMH offers the potential to offer after-hours services to veterans in other time zones during providers’ regular tours of duty.
Several other TMH programs are now expanding services into veterans’ homes. There are several sites within the VHA that have piloted this TMH modality and developed guidelines and recommendations for further expansion. Currently VACO is encouraging all VHA facilities to increase in-home telehealth services, and the Office of Telehealth Services provides details on implementation. Interested parties are encouraged to routinely visit the VACO website for updated information.
Developing and implementing a new TMH program can be an arduous task, but the program has great potential to provide veteran-centered care. As TMH sessions progress, the provider and veteran become less aware of the camera and software and more aware of the therapeutic process. Challenges and delays in implementation are to be expected—these can occur frequently during the development and implementation stages of a TMH program. Maintaining consistent communication with staff at remote sites is essential for the success of any program.
As the VHA focuses on veterancentered care, TMH services will improve access to providers with specific, needed expertise. The authors hope these experiences can facilitate the continued growth of TMH and assuage any concerns a facility or provider may have about this modality of care. Delivery of TMH care can be challenging, but the ability to provide these services to veterans at times and locations convenient to them makes these challenges worthwhile.
Acknowledgments
Dr. Hauser wishes to thank Cathy, Anika, Jirina, Katia, and Max Hauser, and Alba Pillwein for their continued support. In memory of Beverly Ostroski.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Within VHA, telemental health (TMH) refers to behavioral health services that are provided remotely, using secure communication technologies, to veterans who are separated by distance from their mental health providers.1 Telemental health sometimes involves video teleconferencing (VTC) technology, where a veteran (or group of veterans) in one location and a provider in a different location are able to communicate in real time through a computer monitor or television screen.2 In the VHA, TMH visits are typically conducted from a central location (such as a medical center hospital) to a community-based outpatient clinic (CBOC), but pilot projects have also tested VTC in homes as well.1,3,4
In addition to providing timely access to behavioral health services in rural or underserved locations, TMH eliminates travel that may be disruptive or costly and allows mental health providers to consult with or provide supervision to one another. Telemental health can be used to make diagnoses, manage care, perform checkups, and provide long-term, follow-up care. Other uses for TMH include clinical assessment, individual and group psychotherapy, psycho-educational interventions, cognitive testing, and general psychiatric care.1,5,6 More recently, TMH has been used to provide evidence-based psychotherapies (EBPs) to individuals with posttraumatic stress disorder (PTSD) and other mental health diagnoses.6,7 Such care may be particularly advantageous for veterans with PTSD, because traveling can be a burden for them or a trigger for PTSD symptoms.
Although interactive video technology is becoming widely available, its use is limited in health care systems due to lack of knowledge, education, logistical guidance, and technical training. The authors have conducted EBPs using VTC across VISN 22 in both office-to-office and office-to-home modalities and are providing EBPs using VTC to CBOCs in other VISNs across the western U.S. This article addresses these issues, outlining the necessary steps required to establish a TMH clinic and to share the successes of the EBP TMH Center and Regional Pilot used at VISN 22.
Telemental Health
Telemental health is an effective alternative to in-person treatment and is well regarded by both mental health providers and veterans. Overall, mental health providers believe it can help reduce the stigma associated with traditional mental health care and ease transportation-related issues for veterans. Telemental health allows access to care for veterans living in rural or remote areas in addition to those who are incarcerated or are otherwise unable to attend visits at primary VA facilities.2,8-10 In an assessment of TMH services in 40 CBOCs across VISN 16, most CBOC mental health providers found it to be an acceptable alternative to face-to-face care, recognize the value of TMH, and endorse a willingness to use and expand TMH programs within their clinics.11
Veterans who participated in TMH via VTC have expressed satisfaction with the decreased travel time and expenses, fewer interactions with crowds, and fewer parking problems.12 Several studies suggested that veterans preferred TMH to in-person contact due to more rapid access to care and specialists who would otherwise be unavailable at remote locations.5,10 Similarly, veterans who avoid in-person mental health care were more open to remote therapy for many of the reasons listed earlier. Studies suggest that veterans from both rural and urban locations are generally receptive to receiving mental health services via TMH.5,10
Several studies have found that TMH services may have advantages over standard in-person care. These advantages include decreasing transportation costs, travel time, and time missed at work and increasing system coverage area.13 Overall, both veterans and providers reported similar satisfaction between VTC and in-person sessions and, in some cases, prefer VTC interactions due to a sense of “easing into” intense therapies or having a “therapeutic distance” as treatment begins.12
Utility
Previous studies have shown that TMH can be used successfully to provide psychopharmacologic treatment to veterans who have major depressive disorder or schizophrenia, among other psychiatric disorders.5,8,14 Recent studies have focused on the feasibility of providing EBPs via TMH, particularly for the treatment of PTSD.12,15 Studies have shown that TMH services via VTC can be used successfully to provide cognitive behavioral therapy (CBT), cognitive processing therapy (CPT), and prolonged exposure therapy (PE).16-21 In these studies, both PE and CPT delivered via TMH were found to be as efficacious as in-person formats. Furthermore, TMH services were successfully used in individual and group sessions.
Research has emphasized the benefits of TMH for veterans who are uncomfortable in crowds, waiting rooms, or hospital lobbies.7,12,18 For patients with PTSD who are initially limited by fears related to driving, TMH can facilitate access to care. Veterans with PTSD often avoid reminders of trauma (ie, uniforms, evidence of physical injury, artwork, photographs related to war), which can often be found at the larger VAMCs. These veterans may find mental health care services in their homes or at local CBOCs more appealing.7,12,18
Implementation
Prior to the implementation of telehealth services, many CBOC providers would refer veterans in need of specialty care to the nearest VAMC, which were sometimes many hours away.1 In response to travel and access concerns, the VA has implemented various telehealth modalities, including TMH.
In 2008, about 230,000 veterans received mental health services via real-time clinical VTC at 300 VA CBOCs, and about 40,000 veterans enrolled in the In-Home Telehealth program.22 By 2011, > 380,000 veterans used clinic-based telehealth services and about 100,000 veterans used the in-home program.1 Between 2006 and 2008, the 98,000 veterans who used TMH modalities had fewer hospital admissions compared with those who did not; overall, the need for hospital services decreased by about 25% for those using TMH services.23
Although research suggests that TMH is an effective treatment modality, it does have limitations. A recent study noted several visual and audio difficulties that can emerge, including pixilation, “tracer” images with movement, low resolution, “frozen” or “choppy” images, delays in sound, echoes, or “mechanical sounding” voices.12 In some cases, physical details, such as crying, sniffling, or fidgeting, could not be clearly observed.12 Overall, these unforeseen issues can impact the ability to give and receive care through TMH modalities. Proper procedures need to be developed and implemented for each site.
Getting Started
Using TMH to provide mental health care at other VHA facilities requires planning and preparation. Logistics, such as preparation of the room and equipment, should be considered. Similarly, veteran and provider convenience must be considered.2,11 Before starting TMH at any VA facility, professionals working with the audiovisual technology and providing TMH care must complete necessary VA Talent Management System courses and obtain copies of certificates to assure they have met the appropriate training criteria. Providers must be credentialed to provide TMH services, including the telehealth curriculum offered by VA Employee Educational Services.2,24 An appropriate memorandum of understanding (MOU) must be created, and credentialing and privileging must also be acquired.
In addition to provider training, an information technology representative who can administer technical support as needed must be selected for both the provider and remote locations. Technologic complications can make TMH implementation much more challenging.12 As such, it is important to assure that both the veteran and the provider have the necessary TMH equipment. The selected communication device must be compatible with the technology requirements at the provider and remote facilities.12
In addition to designated technical support, the VISN TMH coordinator needs to have point-of-contact information for those who can assist with each site’s telehealth services and address the demand for EBP for PTSD or other desired services. After this information has been obtained, relationships must be developed and maintained with local leadership at each site, associated telehealth coordinators, and evidence-based therapy coordinators.
After contact has been established with remote facilities and the demand for services has been determined, there are several agreements and procedures to put in place before starting TMH services. An initial step is to develop a MOU agreement between the VISN TMH center and remote
sites that allows providers’ credentials and privileges to be shared. Also, it is important to establish a service agreement that outlines the procedures for staff at the remote site. This agreement includes checking in veterans, setting up the TMH rooms, transferring homework to VISN TMH providers, and connecting with the VISN TMH provider. In addition to service agreements, emergency procedures must be in place to ensure the safety of the veterans and the staff.24
After these agreements have been completed, the VISN TMH providers will have to complete request forms to obtain access to the Computerized Patient Record System at the remote facilities, which then must be approved by the Information Security Officer at that site. This is separate from the request at the provider’s site.12 It is essential to have points of contact for questions regarding this process. In order to facilitate referrals for TMH, electronic interfacility consult requests must be developed. Local staff need to collaborate with VISN TMH staff to ensure that the consult addresses the referral facilities need to meet the appropriate requirements.
Before the initiation of TMH services, each TMH provider has to establish clinics for scheduling appointments and obtaining workload credit. Program support assistants at the provider and remote sites must work together to ensure clinics are established correctly. This collaboration is essential for coding of visits and clinic mapping. After the clinics are “built,” appointment times will be set up based on the availability of the provider, support staff, and rooms at the remote site for the TMH session.
Once a consult is initiated, the VISN TMH EBP coordinator will review the consult and the veteran’s chart to ensure initial inclusion/exclusion criteria are met before accepting or canceling the consult. If the consult is accepted, a VISN TMH provider is assigned to the case and contacts the veteran to discuss the referral and (if the veteran is appropriate and interested) initiate services at the closest CBOC or at home. The VISN TMH regional center staff enter the appointment time for the veteran at both facility sites. The VISN TMH provider also coordinates with the CBOC staff to ensure that the veteran is checked in to the appointment and is provided with any questionnaires and necessary homework.
During the first session, the provider obtains consent from the veteran to engage in TMH services, conducts an assessment, and establishes rapport. The provider works with the veteran to develop a treatment plan for PTSD or other mental health diagnosis that will include the type of EBP. At the end of the first session, the next appointment is scheduled, and treatment materials are either mailed to the veteran or given to him or her onsite. After completing EBP, the VISN TMH center works with the referring provider to find follow-up services for the veteran.
The various steps necessary to begin an interfacility TMH clinic are summarized in Table 1.
Provider Training
Despite strong evidence of success, many providers remained skeptical about the efficacy of TMH. One study indicated that several providers in VISN 16 rarely used the established TMH programs because they were not familiar with them and applied TMH only for medication checks and consults.11 This skepticism was present in providers preparing to offer TMH as well as in providers referring veterans for TMH services. However, once providers better understood the TMH programs and had more experience using them, they were significantly more likely to use TMH for initial evaluations and ongoing psychotherapy. For these reasons, proper training and educational opportunities for practicing providers are vital to TMH implementation.9,11
To be proficient, providers need to become familiar with various TMH applications.10 Health care networks implementing TMH must ensure that their providers are well trained and prepared to give and receive proper consultation and support. Providers must also acquire several skills and familiarize themselves with available tools.9 In educating providers on the process and use of TMH, the authors suggest the following steps for TMH application:
- Learn new ways to chart in multiple systems and know how to troubleshoot during connectivity issues.
- Have an established administrative support collaborator at outpatient clinics to fax and exchange veteran homework.12
- The TMH clinic culture must be embedded where the veteran is being served in order to allow for a more realistic therapeutic feel. This type of clinic setting will allow for referrals at the veteran site and the availability to coordinate emergency procedures in the remote clinic.
Clinical Issues
Ongoing clinical issues need to be addressed continuously. Initially, referrals may be plentiful but not always appropriate. It is important to have an understanding with referring providers and remote sites about what constitutes a “good referral” as well as alternate referral options. It is imperative to outline inclusion and exclusion criteria that are clear and concise for referring providers. It is often helpful to revisit these criteria with potential referral sources after initiating services.
With the ability to provide inhome services, it is important to identify specific inclusion/exclusion criteria. Recommendations are based on research and clinical applications for exclusions, which are available on the Office of Technology Services website. These include imminent suicidality or homicidality, serious personality disorder or problematic character traits, acute substance disorders, psychotic disorders, and bipolar disorder. It is important to use sound clinical judgment, because the usual safeguards present in a remote clinic are not available for inhome services. Emergency planning is one of the most important aspects of the in-home TMH health services that are provided. The information for the emergency plan is obtained prior to initiation of services.
Emergency Plans
Each remote clinic that provides services to veterans must have an emergency plan that details procedures, phone numbers, and resources in case of medical and psychological emergencies as well as natural disasters. The VISN TMH provider will need to have a copy of the emergency plan as well as a list of contacts in case of an emergency during a TMH session.
It is recommended that TMH providers have several ways to contact key staff who can assist during an emergency. Usually the clinical coordinator and telehealth technician are the first responders to be alerted by the TMH provider during an emergency. They will then institute the remote clinic’s emergency protocol. Discussing these procedures and reviewing them with staff regularly is advisable, as key contacts may change.
In a psychological emergency, the VISN TMH provider may assist in implementing emergency procedures until a clinical counterpart at the remote site can be alerted. In the authors’ experience, VISN TMH providers have successfully de-escalated and diffused potentially emergent situations by maintaining constant realtime communication with veterans and staff by using VTC as well as interoffice communication. By offering assistance to veterans and staff during challenging situations, the VISN TMH provider will not only decrease concerns of veterans, but oftentimes integrate themselves into the treatment team of the remote clinic. The role of a VISN TMH provider can be isolative, with minimal contact with remote clinic staff, so it is important to increase visibility among staff at a remote site by communication with them even when there is not an emergency.
Treatment protocols may be determined by either administrative or clinical factors. With certain TMH interventions, the rooms used for veterans may be available for only certain periods, which may or may not fit with treatment protocols. For example, if a room is available for only an hour but a treatment protocol session is for 90 minutes, then another time slot needs to be found or a different treatment considered and offered. Although it is not ideal to have treatment protocols determined by scheduling factors, the reality of shared space at remote sites requires flexibility.
Sharing Materials and Homework Another clinical issue that is often overlooked is how to implement specific treatment protocols that entail the exchange of materials between VISN TMH providers and veterans. If materials will need to be exchanged between provider and veteran, a plan will have to be in place to facilitate this. The service agreement addresses these details, but remote staff may not always be aware of the details.
If a TMH provider opts to use faxes to send materials between a veteran and a provider, a desktop faxing program is recommended so veteran privacy is not compromised. Often, providers will wait to begin sessions until after they have received materials, but this may result in a delayed
session. One solution TMH providers can implement is mailing the materials and questionnaires to veterans before the session with clear instructions to complete them beforehand. Once the veteran arrives for the TMH session, she or he will verbally respond to the questionnaire and treatment materials. This will add time to a session but minimizes potential delays. Many of the clinical VTC units have movable cameras, so veterans can tilt the camera to show providers the forms and questionnaires.
The various steps necessary to address TMH clinical issues are summarized in Table 2.
VISIN 22 Pilot Project
The VISN 22 EBP TMH Center and Regional Pilot, based at the VA San Diego Healthcare System, was tasked with developing and providing TMH EBP services for PTSD across VISN 22 and adjacent West Coast VISNs. In addition to creating standardized procedures, troubleshooting guides were established to assist other programs with implementation. The primary focus was to increase access to EBPs for veterans with PTSD in areas where there was either no available trained providers or delays for specific services. The program established 16 clinics as well as in-home
services in VISN 22, VISN 21, and VISN 20. In fiscal year (FY) 2013, the VISN 22 EBP TMH Center and Regional Pilot provided 1,657 EBP encounters via TMH to 234 unique veterans with PTSD (Table 3).
The pilot project collected data to evaluate program effectiveness. The data were de-identified before being sent to the VA Central Office (VACO) TMH program manager. The following items were collected for the pilot: (1) clinical information; (2) consent to engage in treatment and telehealth; (3) release of information to share de-identified data to VACO for program monitoring; (4) demographic form; (5) Beck Depression Inventory-II (every other week); (6) PTSD Checklist (every other week); (7) World Health Organization Quality of Life (sessions 1, 7, final); (8) Wechsler Adult Intelligence Scale-Revised (sessions 3, 7, final); (9) satisfaction survey (final); (10) mileage not driven by veterans who receive TMH services; (11) travel pay saved by VA; (12) no-show rates; and (13) veteran, TMH provider, and referral provider satisfaction.
The growth in number of encounters and number of unique veterans has increased steadily from the first quarter of FY14 through the second quarter of FY15 (Figure 1).
In January 2013, in-home TMH services were piloted. Although occasional technical difficulties occurred, 143 EBP encounters via TMH were provided to 42 unique veterans in 2013. The service has continued to expand, and in the first half of FY14, services were provided to 64 unique veterans for a total of 278 encounters, saving veterans 3,220 travel miles and saving the VA $1,336 in travel reimbursement. In-home TMH services will continue to expand as more providers in a variety of programs are being trained by the San Diego staff on how to provide these services to veterans in their homes. In addition to decreasing mileage and travel pay, the no-show rates are lower for TMH appointments in general (averaged 8%-10% vs facility no-show rate average of 13.5%) and with the use of inhome TMH, no-show rates were kept to 2%. The growth in the number of in-home encounters and the number of unique veterans has also increased steadily from the first quarter of FY14 thru the second quarter of FY15 (Figure 2).
In-Home TMH Services
The VISN 22 EBP TMH Center and Regional Pilot often requests to have an in-person meeting with a veteran before starting TMH services in order to complete a waiver to download the software used by the VA for real-time video in-home services, a Release of Information for a Primary Support Person form, and an emergency plan.
It is also recommended that information about the veteran’s Internet connection, type of computer, type of software, presence of a camera and speakers, e-mail address, and access to secure messaging are obtained. During the initial contact with a veteran, the provider will discuss the rules and requirements to ensure HIPAA compliance. The veteran will need to have a private area for the call (not a restaurant, car, or other place where Wi-Fi is offered). Even with these discussions, some veterans will initiate services from a public place or a room in their home where family members will enter and exit frequently.
Although not required, it is recommended to have the veteran identify a primary support person and complete a release form to allow the TMH provider to contact that person in an emergency. The support person may be a person in the home (adult family member or caregiver) or someone nearby (neighbor, friend, or family member) who can contact emergency services if needed. After the necessary information is gathered and the veteran agrees to the conditions of participation, a test call will be completed. The TMH provider is often the person to conduct this call, but if available, a telehealth technician or facility telehealth coordinator may assist. The TMH provider may help the veteran download the appropriate software that is sent from the VA Scheduler software. The veteran initiates the call with the provider. Once the connection is made, the session may begin. Sites that are currently conducting in-home services have provided guides to veterans and newer TMH providers to outline the necessary steps for initiating services.
It is recommended that any provider interested in providing in-home TMH services use the Office of Technological Services help desk to assist in troubleshooting difficulties with connectivity. Challenges have included the software used for in-home TMH, periodic Internet outages, and compatibility issues.
Veteran Satisfaction
Veteran satisfaction was measured through a self-report satisfaction survey. The survey included 12 questions assessing overall experience in using TMH services. Eleven of the 12 questions included a 5-point Likert scale (1 = strongly disagree, 5 = strongly agree); the last question was openended for additional comments.
A summary of the survey response of the initial 29 veterans who received TMH services suggested the following: (1) Veterans felt comfortable with using the TMH equipment and were able to see their clinician clearly; (2) Technical assistance was sufficient; (3) During the TMH session, they related to the provider as if it were a face-to-face meeting and that their needs were met; and (4) Veterans reported extremely high satisfaction with TMH and would refer TMH care to other veterans. Veterans found clinic locations very convenient and preferred the TMH modality of mental health services delivery to the alternative of travelling a long distance to see their provider (Table 4).
Written comments and recommendations from veterans supported the survey results. Most reported that they saved time and the convenience of the clinic allowed them to receive the treatment they need without interfering with their work schedule. However, some veterans still experienced trouble with travel to the remote clinic. Others felt their experience was different from the one they expected or they had a good experience via TMH but preferred face-to-face care.
Conclusion
The VISN 22 EBP TMH Center and Regional Pilot have established the infrastructure of interfacility clinics to use EBPs for the treatment of PTSD. Also, the center has provided consultation and guidance to facilities interested in developing their own TMH programs. The TMH Center now plans to expand mental health services and include medication management and EBP services for non-PTSD psychiatric diagnoses. The established infrastructure will allow providers from one facility to cover the mental health service needs of other facilities when there are absences or gaps due to leave or delays/challenges in hiring in rural locations. Finally, TMH offers the potential to offer after-hours services to veterans in other time zones during providers’ regular tours of duty.
Several other TMH programs are now expanding services into veterans’ homes. There are several sites within the VHA that have piloted this TMH modality and developed guidelines and recommendations for further expansion. Currently VACO is encouraging all VHA facilities to increase in-home telehealth services, and the Office of Telehealth Services provides details on implementation. Interested parties are encouraged to routinely visit the VACO website for updated information.
Developing and implementing a new TMH program can be an arduous task, but the program has great potential to provide veteran-centered care. As TMH sessions progress, the provider and veteran become less aware of the camera and software and more aware of the therapeutic process. Challenges and delays in implementation are to be expected—these can occur frequently during the development and implementation stages of a TMH program. Maintaining consistent communication with staff at remote sites is essential for the success of any program.
As the VHA focuses on veterancentered care, TMH services will improve access to providers with specific, needed expertise. The authors hope these experiences can facilitate the continued growth of TMH and assuage any concerns a facility or provider may have about this modality of care. Delivery of TMH care can be challenging, but the ability to provide these services to veterans at times and locations convenient to them makes these challenges worthwhile.
Acknowledgments
Dr. Hauser wishes to thank Cathy, Anika, Jirina, Katia, and Max Hauser, and Alba Pillwein for their continued support. In memory of Beverly Ostroski.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. U.S. Department of Veterans Affairs. What is telehealth? U.S. Department of Veterans Affairs Website. http://www.telehealth.va.gov. Update May 13, 2014. Accessed April 30, 2015.
2. Morland LA, Greene CJ, Rosen C, Mauldin PD, Frueh CB. Issues in the design of a randomized noninferiority clinical trial of telemental health psychotherapy for rural combat veterans with PTSD. Contemp Clin Trials. 2009;30(6):513-522.
3. Strachan M, Gros DF, Ruggiero KJ, Lejuez CW, Acierno R. An integrated approach to delivering exposure-based treatment for symptoms of PTSD and depression in OIF/OEF veterans: preliminary findings. Behav Ther. 2012;43(3):560-569.
4. Yuen EK, Gros DF, Price M, et al. Randomized controlled trial of home-based telehealth versus in-person prolonged exposure for combat-related PTSD in veterans: preliminary results. J Clin Psychol. 2015;71(6):500-512.
5. Ruskin PE, Reed S, Kumar R, et al. Reliability and acceptability of psychiatric diagnosis via telecommunication and audiovisual technology. Psychiatr Serv. 1998;49(8):1086-1088.
6. Gros DF, Morland LA, Greene CJ, et al. Delivery of evidence-based psychotherapy via video telehealth. J Psychopathol Behav Assess. 2013;35(4):506-521.
7. Backhaus A, Agha Z, Maglione ML, et al. Videoconferencing psychotherapy: a systematic review. Psychol Serv. 2012;9(2):111-131.
8. Egede LE, Frueh CB, Richardson LK, et al. Rationale and design: telepsychology service delivery for depressed elderly veterans. Trials. 2009;10:22.
9. Frueh BC, Deitsch SE, Santos AB, et al. Procedural and methodological issues in telepsychiatry research and program development. Psychiatr Serv. 2000;51(12):1522-1527.
10. Grubaugh AL, Cain GD, Elhai JD, Patrick SL, Frueh BC. Attitudes toward medical and mental health care delivered via telehealth applications among rural and urban primary care patients. J Nerv Ment Dis. 2008;196(2):166-170.
11. Jameson JP, Farmer MS, Head KJ, Fortney J, Teal CR. VA community mental health service providers’ utilization of and attitudes towards telemental health care: the gatekeeper’s perspective. J Rural Health. 2011;27(4):425-432.
12. Thorp SR, Fidler J, Moreno L, Floto E, Agha Z. Lessons learned from studies of psychotherapy for posttraumatic stress disorder via video teleconferencing. Psychol Serv. 2012;9(2):197-199.
13. Gros DF, Yoder M, Tuerk PW, Lozano BE, Acierno R. Exposure therapy for PTSD delivered to veterans via telehealth: predictors of treatment completion and outcome and comparison to treatment delivered in person. Behav Ther. 2011;42(2):276-283.
14. Zarate CA Jr, Weinstock L, Cukor P, et al. Applicability of telemedicine for assessing patients with schizophrenia: acceptance and reliability. J Clin Psychiatry. 1997;58(1):22-25.
15. Jones AM, Shealy KM, Reid-Quiñones K, et al. Guidelines for establishing a telemental health program to provide evidence-based therapy for trauma-exposed children and families. Psychol Serv. 2014;11(4):398-409.
16. Frueh BC, Monnier J, Grubaugh AL, Elhai JD, Yim E, Knapp R. Therapist adherence and competence with manualized cognitive-behavioral therapy for PTSD delivered via videoconferencing technology. Behav Modif. 2007;31(6):856-866.
17. Morland LA, Hynes AK, Mackintosh MA, Resick PA, Chard KM. Group cognitive processing therapy delivered to veterans via telehealth: a pilot cohort. J Trauma Stress. 2011;24(4):465-469.
18. Tuerk PW, Yoder M, Ruggiero KJ, Gros DF, Acierno R. A pilot study of prolonged exposure therapy for posttraumatic stress disorder delivered via telehealth technology. J Trauma Stress. 2010;23(1):116-123.
19. Fortney JC, Pyne JM, Kimbrell TA, et al. Telemedicine- based collaborative care for posttraumatic stress disorder: a randomized clinical trial. JAMA Psychiatry. 2015;72(1):58-67.
20. Germain V, Marchand A, Bouchard S, Drouin MS, Guay S. Effectiveness of cognitive behavioural therapy administered by videoconference for posttraumatic stress disorder. Cogn Behav Ther. 2009;38(1):42-53.
21. Morland LA, Mackintosh M, Greene CJ, et al. Cognitive processing therapy for posttraumatic stress disorder delivered to rural veterans via telemental health: a randomized noninferiority clinical trial. J Clin Psychiatry. 2014;75(5):470-476.
22. Tuerk PW, Fortney J, Bosworth HB, et al. Toward the development of national telehealth services: the role of Veterans Health Administration and future directions for research. Telemed J E Health. 2010;16(1):115-117.
23. Godleski L, Darkins A, Peters J. Outcomes of 98,609 U.S. Department of Veterans Affairs patients enrolled in telemental health services, 2006-2010. Psychiatr Serv. 2012;63(4):383-385.
24. Strachan M, Gros DF, Yuen E, Ruggiero KJ, Foa EB, Acierno R. Home-based telehealth to deliver evidence-based psychotherapy in veterans with PTSD. Contemp Clin Trials. 2012;33(2):402-409.
1. U.S. Department of Veterans Affairs. What is telehealth? U.S. Department of Veterans Affairs Website. http://www.telehealth.va.gov. Update May 13, 2014. Accessed April 30, 2015.
2. Morland LA, Greene CJ, Rosen C, Mauldin PD, Frueh CB. Issues in the design of a randomized noninferiority clinical trial of telemental health psychotherapy for rural combat veterans with PTSD. Contemp Clin Trials. 2009;30(6):513-522.
3. Strachan M, Gros DF, Ruggiero KJ, Lejuez CW, Acierno R. An integrated approach to delivering exposure-based treatment for symptoms of PTSD and depression in OIF/OEF veterans: preliminary findings. Behav Ther. 2012;43(3):560-569.
4. Yuen EK, Gros DF, Price M, et al. Randomized controlled trial of home-based telehealth versus in-person prolonged exposure for combat-related PTSD in veterans: preliminary results. J Clin Psychol. 2015;71(6):500-512.
5. Ruskin PE, Reed S, Kumar R, et al. Reliability and acceptability of psychiatric diagnosis via telecommunication and audiovisual technology. Psychiatr Serv. 1998;49(8):1086-1088.
6. Gros DF, Morland LA, Greene CJ, et al. Delivery of evidence-based psychotherapy via video telehealth. J Psychopathol Behav Assess. 2013;35(4):506-521.
7. Backhaus A, Agha Z, Maglione ML, et al. Videoconferencing psychotherapy: a systematic review. Psychol Serv. 2012;9(2):111-131.
8. Egede LE, Frueh CB, Richardson LK, et al. Rationale and design: telepsychology service delivery for depressed elderly veterans. Trials. 2009;10:22.
9. Frueh BC, Deitsch SE, Santos AB, et al. Procedural and methodological issues in telepsychiatry research and program development. Psychiatr Serv. 2000;51(12):1522-1527.
10. Grubaugh AL, Cain GD, Elhai JD, Patrick SL, Frueh BC. Attitudes toward medical and mental health care delivered via telehealth applications among rural and urban primary care patients. J Nerv Ment Dis. 2008;196(2):166-170.
11. Jameson JP, Farmer MS, Head KJ, Fortney J, Teal CR. VA community mental health service providers’ utilization of and attitudes towards telemental health care: the gatekeeper’s perspective. J Rural Health. 2011;27(4):425-432.
12. Thorp SR, Fidler J, Moreno L, Floto E, Agha Z. Lessons learned from studies of psychotherapy for posttraumatic stress disorder via video teleconferencing. Psychol Serv. 2012;9(2):197-199.
13. Gros DF, Yoder M, Tuerk PW, Lozano BE, Acierno R. Exposure therapy for PTSD delivered to veterans via telehealth: predictors of treatment completion and outcome and comparison to treatment delivered in person. Behav Ther. 2011;42(2):276-283.
14. Zarate CA Jr, Weinstock L, Cukor P, et al. Applicability of telemedicine for assessing patients with schizophrenia: acceptance and reliability. J Clin Psychiatry. 1997;58(1):22-25.
15. Jones AM, Shealy KM, Reid-Quiñones K, et al. Guidelines for establishing a telemental health program to provide evidence-based therapy for trauma-exposed children and families. Psychol Serv. 2014;11(4):398-409.
16. Frueh BC, Monnier J, Grubaugh AL, Elhai JD, Yim E, Knapp R. Therapist adherence and competence with manualized cognitive-behavioral therapy for PTSD delivered via videoconferencing technology. Behav Modif. 2007;31(6):856-866.
17. Morland LA, Hynes AK, Mackintosh MA, Resick PA, Chard KM. Group cognitive processing therapy delivered to veterans via telehealth: a pilot cohort. J Trauma Stress. 2011;24(4):465-469.
18. Tuerk PW, Yoder M, Ruggiero KJ, Gros DF, Acierno R. A pilot study of prolonged exposure therapy for posttraumatic stress disorder delivered via telehealth technology. J Trauma Stress. 2010;23(1):116-123.
19. Fortney JC, Pyne JM, Kimbrell TA, et al. Telemedicine- based collaborative care for posttraumatic stress disorder: a randomized clinical trial. JAMA Psychiatry. 2015;72(1):58-67.
20. Germain V, Marchand A, Bouchard S, Drouin MS, Guay S. Effectiveness of cognitive behavioural therapy administered by videoconference for posttraumatic stress disorder. Cogn Behav Ther. 2009;38(1):42-53.
21. Morland LA, Mackintosh M, Greene CJ, et al. Cognitive processing therapy for posttraumatic stress disorder delivered to rural veterans via telemental health: a randomized noninferiority clinical trial. J Clin Psychiatry. 2014;75(5):470-476.
22. Tuerk PW, Fortney J, Bosworth HB, et al. Toward the development of national telehealth services: the role of Veterans Health Administration and future directions for research. Telemed J E Health. 2010;16(1):115-117.
23. Godleski L, Darkins A, Peters J. Outcomes of 98,609 U.S. Department of Veterans Affairs patients enrolled in telemental health services, 2006-2010. Psychiatr Serv. 2012;63(4):383-385.
24. Strachan M, Gros DF, Yuen E, Ruggiero KJ, Foa EB, Acierno R. Home-based telehealth to deliver evidence-based psychotherapy in veterans with PTSD. Contemp Clin Trials. 2012;33(2):402-409.
Quality of Supportive Care for Patients With Advanced Lung Cancer in the VHA
Background Morbidity related to cancer and its treatment remains a significant source of human suffering and a challenge to the delivery of high-quality care.
Objective To develop and apply quality indicators to evaluate quality of supportive care for advanced lung cancer in the Veterans Health Administration (VHA) and examine facility-level predictors of quality.
Methods We evaluated supportive care quality using 12 quality indicators. Data were taken from VHA electronic health records for incident lung cancer cases occurring during 2007. Organizational characteristics of 111 VHA facilities were examined for association with receipt of care.
Results Rates of care-receipt were high, especially in the treatment toxicity (89%) and pain management (79%-98%) domains, but were lower in the palliative cancer treatment (60%-90%) and hospice (75%) domains, with substantial facility-level variation. Presence of a care tracking method that was monitored by a midlevel practitioner seemed to be associated with better quality for treatment toxicity (OR, 3.38; 95% CI, 1.87-6.10) and referral to hospice (OR, 1.60; 95% CI, 1.22-2.28); having a psychologist for cancer patients was associated with higher odds for pain management (OR, 1.76; 95% CI, 1.16-2.66).
Limitations Not all supportive care was evaluated. Care processes identified as present at facilities may not have been applied to cohort patients. Facility-level results may be influenced by errors in attributing a patient’s care to the correct facility.
Conclusions Quality indicators for supportive cancer care can be developed and applied in large evaluations using electronic health record review. This study confirmed high-quality supportive care, while identifying significant facility-level variation in VHA.
Funding Veterans Health Administration Office of Informatics and Analytics.
Morbidity related to cancer and its treatment remains a significant source of human suffering and a national challenge to delivery of high-quality cancer care. Quality care refers to the delivery of state-of-the-art treatments intended to achieve cure or prolong life as well as the supportive processes that address the disease- and treatment-related burdens of living with cancer. These processes span the cancer care continuum from diagnosis to end of life, and include pain-, symptom-, and side effect-management; psychosocial support; communication needs; and support for caregivers.1-5
A 2006 report from the Agency for Healthcare Research and Quality concluded that “a large number of measures are available for addressing palliative cancer care, but testing them in relevant populations is urgently needed.”6 Since then, evidence-based standards have been translated into “quality indicators” that may be used to identify outcome targets indicative of quality care, such as patient reports of pain reduction; or, they may specify facility-level care processes associated with these outcomes, such as pain screening, treatment, and follow-up assessment.1,7-9 Quality indicators can be used as the basis for tools to
measure processes that are critical to ensuring highquality supportive cancer care and identifying specific processes and practice sites that should be targeted for quality improvement efforts.10,11 Such tools can help characterize care quality for patient populations served by a health care system as a whole, while revealing important variations at individual facilities within the system.
Quality indicators for supportive and end-of-life care have been successfully applied to electronic health record (EHR) data in research studies assessing care quality in patients with different cancer types and showing supportive care for cancer to be generally in need of quality improvement.10-15 In this paper, we examine supportive care findings from the first largescale application of quality indicators in a system-wide evaluation of lung cancer care in the Veterans Health Administration (VHA). The evaluation was part of a series of operationally motivated projects to identify targets for cancer care improvement in VHA. Lung cancer, the most common malignancy in the United States, accounts for one-fifth of all tumors diagnosed each year within this large, integrated health care system and is responsible for a significant proportion of the system’s supportive care needs. Recently demonstrated benefits of early palliative care on the quality of life and end-of-life care in patients with advanced lung cancer point to the particular importance of assessing supportive care in this population.16 We report VHA evaluation findings and subsequent research analyses seeking to identify patient and facility characteristics associated with better quality supportive lung cancer care.
Methods
Quality Indicators
Quality indicators for supportive cancer care were identified from a systematic review of existing measures and expert guidelines. A panel of 9 national palliative and supportive cancer care experts rated the validity and feasibility of the indicators for use in the VHA using a modified Delphi panel method adapted from the RAND-UCLA appropriateness method17 and prioritized candidate indicators in a ranking exercise. Those with low validity or feasibility scores, or low priority rankings, were excluded, resulting in a final set of 12 quality indicators (8 supportive care, 4 end-of-life care) for use in the national evaluation.
Evaluation Population
All incident non-small-cell (NSCLC) and small-cell (SCLC) lung cancer cases diagnosed within the VHA during 2007 (N = 7,816) were identified through the Veterans Affairs Central Cancer Registry (VACCR). Cases were eligible for the study if the patient had advanced cancer (extensive small-cell or metastatic non-small-cell lung cancer) and had lived long enough to be eligible for the supportive care that was being evaluated. Patients were excluded if the health record did not confirm the lung cancer diagnosis (eg, no pathologic diagnosis or diagnosis outside the VHA, n = 1,297); they had a preexisting or concurrent diagnosis of metastatic neoplasm other than lung cancer (n = 540); had died or enrolled in hospice ≤ 30 days after diagnosis (n = 947); had documented
“comfort measures only” ≤ 30 days after diagnosis (n = 91); had documented life expectancy of ≤ 6 months at time of diagnosis (n = 39); or enrolled in a clinical trial (as care received as part of a trial may not be completely captured in the VHA record (n = 57). Of the patients meeting inclusion criteria, 2,969 were eligible for at least one of the supportive care quality indicators.
Data Collection
EHRs were reviewed remotely by abstractors from the West Virginia Medical Institute, the VHA’s contractor for its external peer-review program. Data were collected retrospectively from the year before to the 2007 diagnosis, with follow-up through 2009. Stage was determined through the VACCR or by abstraction if no VACCR stage was available. The VACCR also provided sociodemographic information including race, which is abstracted from patient charts by cancer registrars. Urban or rural residence was determined by using the rural-urban commuting area codes. Case-level quality indicator results were provided to each facility for review to identify any missed documentation, and abstractors updated data as appropriate. Facility characteristics for the Veterans Administration Medical Centers (VAMCs) at which patients received care were obtained from the 2009 Veterans Health Administration (VHA) Oncology Services
Survey.18 The study was approved by the Veterans Administration Greater Los Angeles Healthcare System Institutional Review Board Subcommittee on Human Studies.
Dependent Variables
For each quality indicator, cases eligible for the indicator were categorized as having received the specified care or not. Patient refusals of specified care or documentation of a contraindication were also considered to have met criteria for receipt of appropriate care. For bivariate and multivariate analyses, indicators were grouped into 4 domains: treatment toxicity (1 indicator), pain screening and management (6), palliative cancer treatment (4), and hospice (1). For these domain analyses, cases were considered to have met criteria for receipt of recommended supportive care if they received the care specified
for all of the indicators for which they were eligible in the domain.
Independent Variables
Patient characteristics included stage of disease, age, gender, race (white, black, other/unknown), marital status (married/living with partner, other), urban/rural residence, and performance status (poor/not poor), as shown in Table 1. Performance status was abstracted from the medical record as ECOG (Eastern Cooperative Oncology Group) or Karnofsky when present, but most of the time it was recorded in patients’ charts qualitatively (eg, “poor performance status”). Cases were attributed to facilities based on the VACCR attribution, which is usually the VHA facility where treatment is initiated. Facility characteristics included geographic region; number of unique patients; facility complexity level; chemotherapy availability on-site; radiation therapy availability on-site; and types of palliative care services, psychosocial support, and patient tracking and case management available on-site (Table 2).
Statistical Analyses
Bivariate analyses were performed to determine which patient and facility characteristics were associated with quality of care for treatment toxicity, pain, palliative treatment, and hospice. Multilevel logistic regression models were developed for each of these 4 domains to examine the relationship between indicator care receipt and patient and facility characteristics. Collinearity was assessed by examining Pearson correlations between independent variables, and highly collinear variables were not included in multivariate analyses.
Independent variables significantly associated with dependent variables in bivariate analyses or considered necessary for adjustment from conceptual standpoints were included in the multivariate models (cancer type [NSCLC or SCLC], age, sex, race, marital status, urban/rural classification, poor performance status, Veterans Integrated Service Network location, facility complexity level, facility region, as well as the facility having the following cancer-related services: tumor board, palliative care unit, palliative care services, chaplain, method for tracking patients through treatment and posttreatment care (to ensure receipt of timely and appropriate care), and presence of a case manager]. Testing yielded no significant interactions between any of the variables; thus interaction terms were not included in the final regressions. All analyses were performed using SAS statistical software, version 9.1 (Cary, NC).
Results
Patient Characteristics
Table 1 summarizes patient characteristics of this elderly (mean age, 67 years; SD, 9), mostly white, and mostly male veteran cohort. In all, 15% of the cohort was black, nearly one-third lived in a rural area, and 46% were married or living with a partner. Eighteen percent had poor performance status, 38% were referred for palliative care, and 81% died during the study period.
Organizational Characteristics
Patients in the cohort were cared for at one or more of 111 VAMC facilities (Table 2), with the largest proportion (45%) seen at facilities located in the South. Most patients (76%) were seen in low-complexity facilities (54% of facilities) and almost all (95%) used a facility reporting having at least 1 tumor board (76% of facilities) in the 2009 VHA Oncology Services Survey. Most facilities (90%) reported having chemotherapy on-site, with 99% of patients in our cohort receiving care at one of these facilities. Radiation therapy at most sites was provided by referral to a non-VA facility, although 31% of facilities had radiation therapy available on-site and these VAMCs provided care to 40% of the patients. A substantial proportion of facilities offered inpatient (91%) and outpatient (76%) palliative care consultation, serving the majority of patients, 95% and 78%, respectively. Various forms of psychosocial support were reported at facilities serving over half of the study patients. Forty-five percent of veterans were seen at facilities that provided care tracking for lung cancer patients (41% of facilities) and at 14% of facilities (12% of patients) the tracking method was monitored by a nurse practitioner or physician assistant. Sixty-eight percent of patients were cared for at facilities reporting the presence of a case manager (69%). (The fact that a site has chemotherapy or any of the other aforementioned services in-house does not imply that patients receiving care at this site were eligible for or received these particular services, but rather that they were cared for at a facility reporting presence of the service.)
About 8% of patients were diagnosed at a VAMC different from the VACCR attribution. Six percent received specialty service consultations or treatment at multiple VAMCs, and about 25% received at least one specialty service consultation or treatment at a non-VHA facility.
Quality Indicator Eligibility and Rates of Receiving Recommended Care
Patient eligibility for the quality indicators (Table 3) ranged from 1% (treatment of spinal cord compression) to 68% (prevention of chemotherapy-related nausea and vomiting). Few patients were eligible for palliative treatment indicators (palliative radiation therapy and treatment of spinal cord compression), with only 2% of patients (50 individuals) eligible for the imaging indicator. Eligibility for the two indicators related to use of shortacting opioids for break-through pain was also low (2%-4%).
Mean national rates for recommended support ive care were generally high (above 85% for 8 of the 12 indicators), ranging from 98% for outpatient pain screening to a low of 60% for spine MRI or myelography within 24 hours for suspected spinal cord compression (Table 3). There was pronounced variability across facilities, with facility rates in every domain ranging from 0%-100% (Figure 1). Many facilities scored 100% in at least 1 domain (eg, 44 of 109 facilities for pain screening; 51 of 94 for palliative cancer therapy). Eight (of 111) facilities did not provide recommended care in 1 domain and 1 did not provide recommended care in 2 domains (ie, 0% of recommended care).
In bivariate analyses (data not shown), referral to palliative care was associated with better care for treatment toxicity (P = .04) but not the other domains. Patients with poor performance status were more likely to be referred to hospice (81% vs 74% for those with good performance status, P = .005) as were patients who lived in an urban zip code area (77% vs 71% for rural zip code, P = .03). In multivariate analyses (Table 4), small-cell cancer type, though not significant in the bivariate analyses, was associated with a higher odds of receiving recommended care [OR, 2.71; 95% CI, 1.89-3.86)] in the
treatment toxicity domain and lower odds in the pain domain [OR, 0.50; 95% CI, 0.33-0.77)]; and poor performance remained associated with higher odds of referral to hospice [OR, 1.49; 95% CI, 1.12-1.99)] after adjusting for other patient and facility characteristics.
In multivariate analysis, moderate facility complexity was associated with lower quality in the toxicity domain than was low facility complexity [OR, 0.28; 95% CI, 0.18-0.44)]; however there was no such difference between the low- and high-complexity facilities. The presence of a patient tracking method appeared to be associated with better quality of care in the treatment toxicity domain [OR, 3.38; 95% CI, 1.87-6.10)] and a higher likelihood of referral to hospice [OR, 1.60; 95% CI, 1.22-2.28)] when the method was monitored by a midlevel practitioner (nurse practitioner or physician assistant), but no association was found for methods monitored by other types of staff. In the pain domain, having a psychologist specializing in cancer was associated with a higher odds of receiving recommended care [OR, 1.76; 95% CI, 1.16-2.66)]. No patient or facility characteristics were associated with receiving recommended palliative cancer therapy, although statistical power was limited by the small numbers of eligible events.
Discussion
Use of evidence-based measures to assess care quality proved feasible in this evaluation of supportive care for advanced lung cancer in a large national integrated health care system and confirmed that gaps found in smaller studies represent critical unmet needs for patients. The methods permitted individualized feedback to facilities, accompanied by support for quality improvement efforts. The effectiveness of providing facility feedback with quality improvement strategies will be evaluated in follow-up studies.
Although quality of supportive care as measured by this specific set of indicators was quite high compared to previous reports,10,13,15 substantial variation across facilities was observed even for measures with high overall performance. Comparatively high levels of supportive care may reflect greater emphasis on supportive measures for advanced lung cancer patients whose expectation of cure is low (other studies evaluated cohorts with mixed cancer types), or the fact that previous studies did not account for documentation of a reason for forgoing indicated care. Tough high quality of supportive care for lung cancer in VHA nationally is encouraging, variability across individual facilities is of concern. Such variation may well exist in any large health care system, with patients potentially experiencing different standards of care depending on where they live.
In this study, facilities located in the South, treating 45% of advanced lung cancer patients in the cohort, were associated with worse care in the treatment toxicity and hospice domains, and those in the Northeast appeared to generally have worse care for pain. Although reasons for these differences are unclear, geographic variation in Medicare hospice use has been related to a variety of determinants,20-23 some of which may also apply to VHA. Referral to palliative care or hospice was among the lowest scoring quality indicators in the evaluation, with a 75% national rate and 43% of facilities documenting referral for less than 75% of eligible cases. Although our measures determined only referral to palliative care or hospice (versus actual receipt of services), low rates may in part reflect provider anticipation of low local availability of hospice. Although the VHA Hospice and Palliative Care program has made progress in reducing variability in hospice access for Veterans, surveys conducted by Hospice-Veteran Partnerships showed lack of shared knowledge about the different systems of benefits and health care available for veterans, misunderstandings about referral processes among health care providers and payment for hospice services, and difficulties in caring for veterans across multiple care settings to be barriers to Veteran access to community services.24 Targeted inquiry at lowreferral facilities may elucidate which of these and other possible challenges may suggest points of intervention for improvement in hospice referral patterns and support of appropriate hospice use.
Attempting to identify facility-related explanations for overall variability, we explored the potential role of a number of structural variables, including presence of a tumor board, a palliative care unit, outpatient palliative care services, chaplain services, presence of a cancer care tracking system, and presence of a case manager. Tough facilities with a psychologist specializing in cancer provided better care for pain, and those with midlevel practitioners (nurse practitioner or physician assistant) monitoring patient tracking through care were more likely to appropriately refer to hospice and have better care for treatment toxicity, many facilities without such designated professionals also provided quality care in these domains. Because of limited methodological feasibility of demonstrating patient receipt of psychosocial/spiritual support and care tracking using medical record review, the final set of quality indicators did not include measures for these forms of care; however, psychosocial care is considered integral to supportive care in cancer25 and care tracking has been associated with improved cancer care in non-VA settings.26 The presence of appropriate professionals providing cancer-specific psychological support and tracking may be directly associated with improved care, not only in the domains noted above, but also in the important domain of existential and emotional well-being not evaluated here. These services may also be part of larger facility-level quality improvement mechanisms contributing to cancer care quality that are worth identifying in future research. Lack of consistent explanations for the overall facility-level variation in this study suggests the need to identify data sources that can be used to measure additional organizational characteristics in future research.
Our study has several limitations. The scope of the national evaluation did not include indicators for all aspects of supportive care, including care for dyspnea, depression, and psychosocial distress; support for care givers; and care planning assistance. Small numbers of eligible events per facility for some indicators limit interpretation, particularly in the palliative treatment domain, where the largest range was 1-15 eligible events per facility. Facility characteristics used for analyses were identified from a surveyand may be subject to respondent error. Potentially useful processes, such as a midlevel practitioner monitored tracking system were identified as “present” at the facility, but use for cohort patients was not possible to confirm, limiting direct linkage with better care. Some facility-level results may be influenced by errors in VACCR attribution to a facility that was not directly involved with the patient’s care, specialty service receipt at multiple VAMCs, or through contract care at non-VHA facilities. Quality-of-care coordination between different VHA and non-VHA facilities for services not provided at the diagnosing facility, or to reduce travel burden if the VAMC was far from the patient’s home may be associated with better or worse care quality and should be evaluated in future work.
In conclusion, use of quality indicators to evaluate quality of supportive lung cancer care in a large integrated health care system proved feasible, confirmed
provision of overall high-quality supportive care, but identified a high degree of variability across individual facilities in the system. Facility characteristics examined did not explain this variation; however, the data suggested that, while not accounting for the overall variation, having professionals providing cancer-specific psychological support and care-tracking may contribute to better quality of care in some domains. Difficulties in identifying predictors of quality suggest that future research should include qualitative comparisons of facilities with varying rates of providing recommended supportive care to identify potentially impactful organizational factors not examined in this study.
Disclaimer and Disclosures
Accepted for publication February 24, 2014. Correspondence: Sabine Oishi, PhD, MSPH; [email protected]. Disclosures: Dr Ryoo was supported in part by the Robert Wood Johnson Foundation Clinical Scholars Program and by the UCLA Jonsson Comprehensive Cancer Center R25 grant. The authors have no direct conflicts of interest to disclose. Dr Karl Lorenz is a consultant as a member of the Data Monitoring Committee for a Phase II trial of Sativex being conducted by Otsuka Pharmaceuticals. The current study has no direct financial implications related to his participation and was conducted entirely outside of the timeframe of his consultancy; however, it may be perceived as indirectly related. The views and opinions of authors expressed herein do not necessarily state or reflect that of the Department of Veterans Affairs, the United States Government, Kaiser Permanente, Stanford University, or the University of California.
Click here to read the digital edition.
1. Lorenz KA, Dy SM, Naeim A, et al. Quality measures for supportive cancer care: the Cancer Quality-ASSIST Project. J Pain Symptom Manage. 2009;37(6):943-964.
2. Dy SM, Asch SM, Naeim A, Sanati H, Walling A, Lorenz KA. Evidence-based standards for cancer pain management. J Clin Oncol. 2008;26(23):3879-3885.
3. Gordon, DB, Dahl, JL, Miakowski, C. American Pain Society recommendations for improving the quality of acute and cancer pain management. Arch Intern Med. 2005;165(14):1574-1580.
4. Lorenz, KA, Lynn, J, Dy, SM, et al. Evidence for improving palliative care at the end of life: a systematic review. Ann Intern Med. 2008;148(2):147-159.
5. Seow, H, Snyder, CF, Shugarman, LR, et al. Developing quality indicators for cancer end-of-life care: proceedings from a national symposium. Cancer. 2009;115(17):3820-3829.
6. Lorenz KA, Lynn J, Dy SM, et al. Agency for Healthcare Research and Quality (US). Cancer care quality measures: symptoms and end-of-life care. http://www.ncbi.nlm.nih.gov/books/NBK38028/. Published 2006. Accessed December 17, 2012.
7. Lorenz KA, Lynn J, Dy S, et al. Quality measures for symptoms and advance care planning in cancer: a systematic review. J Clin Oncol. 2006;24(30):4933-4938.
8. Lorenz KA, Rosenfeld K, Wenger N. Quality indicators for palliative and end-of-life care in vulnerable elders. JAGS. 2007;55(suppl 2):S318-S326.
9. Dy SM, Lorenz KA, O’Neill SM, et al. Cancer Quality-ASSIST supportive oncology quality indicator set. Cancer. 2010;116(13):3267-3275.
10. Jacobson JO, Neuss MN, McNiff KK, et al. Improvement in oncology practice performance through voluntary participation in the Quality Oncology Practice Initiative. J Clin Oncol. 2008;26(11):1893-1898.
11. Jacobsen PB, Shibata D, Siegel E, et al. Evaluating the quality of psychosocial care in outpatient medical oncology settings using performance indicators. Psycho-oncology. 2010; 20(11):1221-1227.
12. Walling AM, Asch SM, Lorenz KA, et al. The quality of care provided to hospitalized patients at the end of life. Arch Intern Med. 2010;170(12):1057-1063.
13. Dy SM, Asch SM, Lorenz KA, et al. Quality of end-of-life care for patients with advanced cancer in an academic medical center. J Palliat Med. 2011;14(4):451-457.
14. Gonsalves WI, Tsewang T, Krishnamurthy J, et al. Effect of palliative care services on the aggressiveness of end-of-life care in the Veteran’s Affairs cancer population. J Palliat Med. 2011;14(11):1231-1235.
15. Malin JL, O’Neill SM, Asch SM, et al. Quality of supportive care for patients with advanced cancer in a VA medical center. J Palliat Med. 2011;14(5):573-577.
16. Temel JS, Greer JA, Admane S, et al. Longitudinal perceptions of prognosis and goals of therapy in patients with metastatic non-small-cell lung cancer: results of a randomized study of early palliative care. J Clin Oncol. 2011;29(17):2319-2326.
17. Brook RH, Chassin MR, Fink A, Solomon DH, Kosecoff J, Park RE. A method for the detailed assessment of the appropriateness of medical technologies. Int J Technol Assess Health Care. 1986;2(1):53-63.
18. Department of Veterans Affairs, Veterans Health Administration, Office of the Assistant Deputy Under Secretary for Health for Policy and Planning. 2009 VHA Oncology Services Survey. April, 2010. Accessed November 1, 2010.
19. US Census Bureau. Census Bureau regions and divisions with state FIPS codes. Available from URL: https://www.census.gov/geo/ maps-data/maps/pdfs/reference/us_regdiv.pdf. Accessed December 17, 2012.
20. Virnig BA. Toward a better understanding of the role of geography in intensity of end-of-life care: must we first come to an understanding of end-of-life care? Medical Care. 2007;45(5):374-376.
21. Virnig BA, Kind S, McBean M, Fisher E. Geographic variation in hospice use prior to death. JAGS. 2000;48(9):117-11.
22. McCarthy EP, Burns RB, Davis RB, Phillips RS. Barriers to hospice care among older patients dying with lung and colorectal cancer. J Clin Oncol. 2003;21(4): 728-735.
23. Virnig BA, Ma H, Hartman LK, Moscovice I, Carlin B. Access to home-based hospice care for rural populations: identification of areas lacking service. J Palliat Med. 2006;9(6):1292-1299.
24. Jones D, Edes T, Shreve S. You won’t know if you’re improving unless you measure: recommendations for evaluating hospice-veteran partnerships. J Pain Symptom Manage. 2006;32(5):488-496.
25. Surbone A, Baider L, Weitzman TS, Brames MJ, Rittenberg CN, Johnson J, et al. Psychosocial care for patients and their families is integral to supportive care in cancer: MASCC position statement. Support Care Cancer. 2010;18(2):255-263.
26. Callahan M, Sanderson, J. A breast cancer tracking system. The Permanente Journal. 2000;4:36-39. http://www.thepermanentejournal.org/files/Fall2000/BreastCancer.pdf. Accessed June 1, 2012.
Background Morbidity related to cancer and its treatment remains a significant source of human suffering and a challenge to the delivery of high-quality care.
Objective To develop and apply quality indicators to evaluate quality of supportive care for advanced lung cancer in the Veterans Health Administration (VHA) and examine facility-level predictors of quality.
Methods We evaluated supportive care quality using 12 quality indicators. Data were taken from VHA electronic health records for incident lung cancer cases occurring during 2007. Organizational characteristics of 111 VHA facilities were examined for association with receipt of care.
Results Rates of care-receipt were high, especially in the treatment toxicity (89%) and pain management (79%-98%) domains, but were lower in the palliative cancer treatment (60%-90%) and hospice (75%) domains, with substantial facility-level variation. Presence of a care tracking method that was monitored by a midlevel practitioner seemed to be associated with better quality for treatment toxicity (OR, 3.38; 95% CI, 1.87-6.10) and referral to hospice (OR, 1.60; 95% CI, 1.22-2.28); having a psychologist for cancer patients was associated with higher odds for pain management (OR, 1.76; 95% CI, 1.16-2.66).
Limitations Not all supportive care was evaluated. Care processes identified as present at facilities may not have been applied to cohort patients. Facility-level results may be influenced by errors in attributing a patient’s care to the correct facility.
Conclusions Quality indicators for supportive cancer care can be developed and applied in large evaluations using electronic health record review. This study confirmed high-quality supportive care, while identifying significant facility-level variation in VHA.
Funding Veterans Health Administration Office of Informatics and Analytics.
Morbidity related to cancer and its treatment remains a significant source of human suffering and a national challenge to delivery of high-quality cancer care. Quality care refers to the delivery of state-of-the-art treatments intended to achieve cure or prolong life as well as the supportive processes that address the disease- and treatment-related burdens of living with cancer. These processes span the cancer care continuum from diagnosis to end of life, and include pain-, symptom-, and side effect-management; psychosocial support; communication needs; and support for caregivers.1-5
A 2006 report from the Agency for Healthcare Research and Quality concluded that “a large number of measures are available for addressing palliative cancer care, but testing them in relevant populations is urgently needed.”6 Since then, evidence-based standards have been translated into “quality indicators” that may be used to identify outcome targets indicative of quality care, such as patient reports of pain reduction; or, they may specify facility-level care processes associated with these outcomes, such as pain screening, treatment, and follow-up assessment.1,7-9 Quality indicators can be used as the basis for tools to
measure processes that are critical to ensuring highquality supportive cancer care and identifying specific processes and practice sites that should be targeted for quality improvement efforts.10,11 Such tools can help characterize care quality for patient populations served by a health care system as a whole, while revealing important variations at individual facilities within the system.
Quality indicators for supportive and end-of-life care have been successfully applied to electronic health record (EHR) data in research studies assessing care quality in patients with different cancer types and showing supportive care for cancer to be generally in need of quality improvement.10-15 In this paper, we examine supportive care findings from the first largescale application of quality indicators in a system-wide evaluation of lung cancer care in the Veterans Health Administration (VHA). The evaluation was part of a series of operationally motivated projects to identify targets for cancer care improvement in VHA. Lung cancer, the most common malignancy in the United States, accounts for one-fifth of all tumors diagnosed each year within this large, integrated health care system and is responsible for a significant proportion of the system’s supportive care needs. Recently demonstrated benefits of early palliative care on the quality of life and end-of-life care in patients with advanced lung cancer point to the particular importance of assessing supportive care in this population.16 We report VHA evaluation findings and subsequent research analyses seeking to identify patient and facility characteristics associated with better quality supportive lung cancer care.
Methods
Quality Indicators
Quality indicators for supportive cancer care were identified from a systematic review of existing measures and expert guidelines. A panel of 9 national palliative and supportive cancer care experts rated the validity and feasibility of the indicators for use in the VHA using a modified Delphi panel method adapted from the RAND-UCLA appropriateness method17 and prioritized candidate indicators in a ranking exercise. Those with low validity or feasibility scores, or low priority rankings, were excluded, resulting in a final set of 12 quality indicators (8 supportive care, 4 end-of-life care) for use in the national evaluation.
Evaluation Population
All incident non-small-cell (NSCLC) and small-cell (SCLC) lung cancer cases diagnosed within the VHA during 2007 (N = 7,816) were identified through the Veterans Affairs Central Cancer Registry (VACCR). Cases were eligible for the study if the patient had advanced cancer (extensive small-cell or metastatic non-small-cell lung cancer) and had lived long enough to be eligible for the supportive care that was being evaluated. Patients were excluded if the health record did not confirm the lung cancer diagnosis (eg, no pathologic diagnosis or diagnosis outside the VHA, n = 1,297); they had a preexisting or concurrent diagnosis of metastatic neoplasm other than lung cancer (n = 540); had died or enrolled in hospice ≤ 30 days after diagnosis (n = 947); had documented
“comfort measures only” ≤ 30 days after diagnosis (n = 91); had documented life expectancy of ≤ 6 months at time of diagnosis (n = 39); or enrolled in a clinical trial (as care received as part of a trial may not be completely captured in the VHA record (n = 57). Of the patients meeting inclusion criteria, 2,969 were eligible for at least one of the supportive care quality indicators.
Data Collection
EHRs were reviewed remotely by abstractors from the West Virginia Medical Institute, the VHA’s contractor for its external peer-review program. Data were collected retrospectively from the year before to the 2007 diagnosis, with follow-up through 2009. Stage was determined through the VACCR or by abstraction if no VACCR stage was available. The VACCR also provided sociodemographic information including race, which is abstracted from patient charts by cancer registrars. Urban or rural residence was determined by using the rural-urban commuting area codes. Case-level quality indicator results were provided to each facility for review to identify any missed documentation, and abstractors updated data as appropriate. Facility characteristics for the Veterans Administration Medical Centers (VAMCs) at which patients received care were obtained from the 2009 Veterans Health Administration (VHA) Oncology Services
Survey.18 The study was approved by the Veterans Administration Greater Los Angeles Healthcare System Institutional Review Board Subcommittee on Human Studies.
Dependent Variables
For each quality indicator, cases eligible for the indicator were categorized as having received the specified care or not. Patient refusals of specified care or documentation of a contraindication were also considered to have met criteria for receipt of appropriate care. For bivariate and multivariate analyses, indicators were grouped into 4 domains: treatment toxicity (1 indicator), pain screening and management (6), palliative cancer treatment (4), and hospice (1). For these domain analyses, cases were considered to have met criteria for receipt of recommended supportive care if they received the care specified
for all of the indicators for which they were eligible in the domain.
Independent Variables
Patient characteristics included stage of disease, age, gender, race (white, black, other/unknown), marital status (married/living with partner, other), urban/rural residence, and performance status (poor/not poor), as shown in Table 1. Performance status was abstracted from the medical record as ECOG (Eastern Cooperative Oncology Group) or Karnofsky when present, but most of the time it was recorded in patients’ charts qualitatively (eg, “poor performance status”). Cases were attributed to facilities based on the VACCR attribution, which is usually the VHA facility where treatment is initiated. Facility characteristics included geographic region; number of unique patients; facility complexity level; chemotherapy availability on-site; radiation therapy availability on-site; and types of palliative care services, psychosocial support, and patient tracking and case management available on-site (Table 2).
Statistical Analyses
Bivariate analyses were performed to determine which patient and facility characteristics were associated with quality of care for treatment toxicity, pain, palliative treatment, and hospice. Multilevel logistic regression models were developed for each of these 4 domains to examine the relationship between indicator care receipt and patient and facility characteristics. Collinearity was assessed by examining Pearson correlations between independent variables, and highly collinear variables were not included in multivariate analyses.
Independent variables significantly associated with dependent variables in bivariate analyses or considered necessary for adjustment from conceptual standpoints were included in the multivariate models (cancer type [NSCLC or SCLC], age, sex, race, marital status, urban/rural classification, poor performance status, Veterans Integrated Service Network location, facility complexity level, facility region, as well as the facility having the following cancer-related services: tumor board, palliative care unit, palliative care services, chaplain, method for tracking patients through treatment and posttreatment care (to ensure receipt of timely and appropriate care), and presence of a case manager]. Testing yielded no significant interactions between any of the variables; thus interaction terms were not included in the final regressions. All analyses were performed using SAS statistical software, version 9.1 (Cary, NC).
Results
Patient Characteristics
Table 1 summarizes patient characteristics of this elderly (mean age, 67 years; SD, 9), mostly white, and mostly male veteran cohort. In all, 15% of the cohort was black, nearly one-third lived in a rural area, and 46% were married or living with a partner. Eighteen percent had poor performance status, 38% were referred for palliative care, and 81% died during the study period.
Organizational Characteristics
Patients in the cohort were cared for at one or more of 111 VAMC facilities (Table 2), with the largest proportion (45%) seen at facilities located in the South. Most patients (76%) were seen in low-complexity facilities (54% of facilities) and almost all (95%) used a facility reporting having at least 1 tumor board (76% of facilities) in the 2009 VHA Oncology Services Survey. Most facilities (90%) reported having chemotherapy on-site, with 99% of patients in our cohort receiving care at one of these facilities. Radiation therapy at most sites was provided by referral to a non-VA facility, although 31% of facilities had radiation therapy available on-site and these VAMCs provided care to 40% of the patients. A substantial proportion of facilities offered inpatient (91%) and outpatient (76%) palliative care consultation, serving the majority of patients, 95% and 78%, respectively. Various forms of psychosocial support were reported at facilities serving over half of the study patients. Forty-five percent of veterans were seen at facilities that provided care tracking for lung cancer patients (41% of facilities) and at 14% of facilities (12% of patients) the tracking method was monitored by a nurse practitioner or physician assistant. Sixty-eight percent of patients were cared for at facilities reporting the presence of a case manager (69%). (The fact that a site has chemotherapy or any of the other aforementioned services in-house does not imply that patients receiving care at this site were eligible for or received these particular services, but rather that they were cared for at a facility reporting presence of the service.)
About 8% of patients were diagnosed at a VAMC different from the VACCR attribution. Six percent received specialty service consultations or treatment at multiple VAMCs, and about 25% received at least one specialty service consultation or treatment at a non-VHA facility.
Quality Indicator Eligibility and Rates of Receiving Recommended Care
Patient eligibility for the quality indicators (Table 3) ranged from 1% (treatment of spinal cord compression) to 68% (prevention of chemotherapy-related nausea and vomiting). Few patients were eligible for palliative treatment indicators (palliative radiation therapy and treatment of spinal cord compression), with only 2% of patients (50 individuals) eligible for the imaging indicator. Eligibility for the two indicators related to use of shortacting opioids for break-through pain was also low (2%-4%).
Mean national rates for recommended support ive care were generally high (above 85% for 8 of the 12 indicators), ranging from 98% for outpatient pain screening to a low of 60% for spine MRI or myelography within 24 hours for suspected spinal cord compression (Table 3). There was pronounced variability across facilities, with facility rates in every domain ranging from 0%-100% (Figure 1). Many facilities scored 100% in at least 1 domain (eg, 44 of 109 facilities for pain screening; 51 of 94 for palliative cancer therapy). Eight (of 111) facilities did not provide recommended care in 1 domain and 1 did not provide recommended care in 2 domains (ie, 0% of recommended care).
In bivariate analyses (data not shown), referral to palliative care was associated with better care for treatment toxicity (P = .04) but not the other domains. Patients with poor performance status were more likely to be referred to hospice (81% vs 74% for those with good performance status, P = .005) as were patients who lived in an urban zip code area (77% vs 71% for rural zip code, P = .03). In multivariate analyses (Table 4), small-cell cancer type, though not significant in the bivariate analyses, was associated with a higher odds of receiving recommended care [OR, 2.71; 95% CI, 1.89-3.86)] in the
treatment toxicity domain and lower odds in the pain domain [OR, 0.50; 95% CI, 0.33-0.77)]; and poor performance remained associated with higher odds of referral to hospice [OR, 1.49; 95% CI, 1.12-1.99)] after adjusting for other patient and facility characteristics.
In multivariate analysis, moderate facility complexity was associated with lower quality in the toxicity domain than was low facility complexity [OR, 0.28; 95% CI, 0.18-0.44)]; however there was no such difference between the low- and high-complexity facilities. The presence of a patient tracking method appeared to be associated with better quality of care in the treatment toxicity domain [OR, 3.38; 95% CI, 1.87-6.10)] and a higher likelihood of referral to hospice [OR, 1.60; 95% CI, 1.22-2.28)] when the method was monitored by a midlevel practitioner (nurse practitioner or physician assistant), but no association was found for methods monitored by other types of staff. In the pain domain, having a psychologist specializing in cancer was associated with a higher odds of receiving recommended care [OR, 1.76; 95% CI, 1.16-2.66)]. No patient or facility characteristics were associated with receiving recommended palliative cancer therapy, although statistical power was limited by the small numbers of eligible events.
Discussion
Use of evidence-based measures to assess care quality proved feasible in this evaluation of supportive care for advanced lung cancer in a large national integrated health care system and confirmed that gaps found in smaller studies represent critical unmet needs for patients. The methods permitted individualized feedback to facilities, accompanied by support for quality improvement efforts. The effectiveness of providing facility feedback with quality improvement strategies will be evaluated in follow-up studies.
Although quality of supportive care as measured by this specific set of indicators was quite high compared to previous reports,10,13,15 substantial variation across facilities was observed even for measures with high overall performance. Comparatively high levels of supportive care may reflect greater emphasis on supportive measures for advanced lung cancer patients whose expectation of cure is low (other studies evaluated cohorts with mixed cancer types), or the fact that previous studies did not account for documentation of a reason for forgoing indicated care. Tough high quality of supportive care for lung cancer in VHA nationally is encouraging, variability across individual facilities is of concern. Such variation may well exist in any large health care system, with patients potentially experiencing different standards of care depending on where they live.
In this study, facilities located in the South, treating 45% of advanced lung cancer patients in the cohort, were associated with worse care in the treatment toxicity and hospice domains, and those in the Northeast appeared to generally have worse care for pain. Although reasons for these differences are unclear, geographic variation in Medicare hospice use has been related to a variety of determinants,20-23 some of which may also apply to VHA. Referral to palliative care or hospice was among the lowest scoring quality indicators in the evaluation, with a 75% national rate and 43% of facilities documenting referral for less than 75% of eligible cases. Although our measures determined only referral to palliative care or hospice (versus actual receipt of services), low rates may in part reflect provider anticipation of low local availability of hospice. Although the VHA Hospice and Palliative Care program has made progress in reducing variability in hospice access for Veterans, surveys conducted by Hospice-Veteran Partnerships showed lack of shared knowledge about the different systems of benefits and health care available for veterans, misunderstandings about referral processes among health care providers and payment for hospice services, and difficulties in caring for veterans across multiple care settings to be barriers to Veteran access to community services.24 Targeted inquiry at lowreferral facilities may elucidate which of these and other possible challenges may suggest points of intervention for improvement in hospice referral patterns and support of appropriate hospice use.
Attempting to identify facility-related explanations for overall variability, we explored the potential role of a number of structural variables, including presence of a tumor board, a palliative care unit, outpatient palliative care services, chaplain services, presence of a cancer care tracking system, and presence of a case manager. Tough facilities with a psychologist specializing in cancer provided better care for pain, and those with midlevel practitioners (nurse practitioner or physician assistant) monitoring patient tracking through care were more likely to appropriately refer to hospice and have better care for treatment toxicity, many facilities without such designated professionals also provided quality care in these domains. Because of limited methodological feasibility of demonstrating patient receipt of psychosocial/spiritual support and care tracking using medical record review, the final set of quality indicators did not include measures for these forms of care; however, psychosocial care is considered integral to supportive care in cancer25 and care tracking has been associated with improved cancer care in non-VA settings.26 The presence of appropriate professionals providing cancer-specific psychological support and tracking may be directly associated with improved care, not only in the domains noted above, but also in the important domain of existential and emotional well-being not evaluated here. These services may also be part of larger facility-level quality improvement mechanisms contributing to cancer care quality that are worth identifying in future research. Lack of consistent explanations for the overall facility-level variation in this study suggests the need to identify data sources that can be used to measure additional organizational characteristics in future research.
Our study has several limitations. The scope of the national evaluation did not include indicators for all aspects of supportive care, including care for dyspnea, depression, and psychosocial distress; support for care givers; and care planning assistance. Small numbers of eligible events per facility for some indicators limit interpretation, particularly in the palliative treatment domain, where the largest range was 1-15 eligible events per facility. Facility characteristics used for analyses were identified from a surveyand may be subject to respondent error. Potentially useful processes, such as a midlevel practitioner monitored tracking system were identified as “present” at the facility, but use for cohort patients was not possible to confirm, limiting direct linkage with better care. Some facility-level results may be influenced by errors in VACCR attribution to a facility that was not directly involved with the patient’s care, specialty service receipt at multiple VAMCs, or through contract care at non-VHA facilities. Quality-of-care coordination between different VHA and non-VHA facilities for services not provided at the diagnosing facility, or to reduce travel burden if the VAMC was far from the patient’s home may be associated with better or worse care quality and should be evaluated in future work.
In conclusion, use of quality indicators to evaluate quality of supportive lung cancer care in a large integrated health care system proved feasible, confirmed
provision of overall high-quality supportive care, but identified a high degree of variability across individual facilities in the system. Facility characteristics examined did not explain this variation; however, the data suggested that, while not accounting for the overall variation, having professionals providing cancer-specific psychological support and care-tracking may contribute to better quality of care in some domains. Difficulties in identifying predictors of quality suggest that future research should include qualitative comparisons of facilities with varying rates of providing recommended supportive care to identify potentially impactful organizational factors not examined in this study.
Disclaimer and Disclosures
Accepted for publication February 24, 2014. Correspondence: Sabine Oishi, PhD, MSPH; [email protected]. Disclosures: Dr Ryoo was supported in part by the Robert Wood Johnson Foundation Clinical Scholars Program and by the UCLA Jonsson Comprehensive Cancer Center R25 grant. The authors have no direct conflicts of interest to disclose. Dr Karl Lorenz is a consultant as a member of the Data Monitoring Committee for a Phase II trial of Sativex being conducted by Otsuka Pharmaceuticals. The current study has no direct financial implications related to his participation and was conducted entirely outside of the timeframe of his consultancy; however, it may be perceived as indirectly related. The views and opinions of authors expressed herein do not necessarily state or reflect that of the Department of Veterans Affairs, the United States Government, Kaiser Permanente, Stanford University, or the University of California.
Click here to read the digital edition.
Background Morbidity related to cancer and its treatment remains a significant source of human suffering and a challenge to the delivery of high-quality care.
Objective To develop and apply quality indicators to evaluate quality of supportive care for advanced lung cancer in the Veterans Health Administration (VHA) and examine facility-level predictors of quality.
Methods We evaluated supportive care quality using 12 quality indicators. Data were taken from VHA electronic health records for incident lung cancer cases occurring during 2007. Organizational characteristics of 111 VHA facilities were examined for association with receipt of care.
Results Rates of care-receipt were high, especially in the treatment toxicity (89%) and pain management (79%-98%) domains, but were lower in the palliative cancer treatment (60%-90%) and hospice (75%) domains, with substantial facility-level variation. Presence of a care tracking method that was monitored by a midlevel practitioner seemed to be associated with better quality for treatment toxicity (OR, 3.38; 95% CI, 1.87-6.10) and referral to hospice (OR, 1.60; 95% CI, 1.22-2.28); having a psychologist for cancer patients was associated with higher odds for pain management (OR, 1.76; 95% CI, 1.16-2.66).
Limitations Not all supportive care was evaluated. Care processes identified as present at facilities may not have been applied to cohort patients. Facility-level results may be influenced by errors in attributing a patient’s care to the correct facility.
Conclusions Quality indicators for supportive cancer care can be developed and applied in large evaluations using electronic health record review. This study confirmed high-quality supportive care, while identifying significant facility-level variation in VHA.
Funding Veterans Health Administration Office of Informatics and Analytics.
Morbidity related to cancer and its treatment remains a significant source of human suffering and a national challenge to delivery of high-quality cancer care. Quality care refers to the delivery of state-of-the-art treatments intended to achieve cure or prolong life as well as the supportive processes that address the disease- and treatment-related burdens of living with cancer. These processes span the cancer care continuum from diagnosis to end of life, and include pain-, symptom-, and side effect-management; psychosocial support; communication needs; and support for caregivers.1-5
A 2006 report from the Agency for Healthcare Research and Quality concluded that “a large number of measures are available for addressing palliative cancer care, but testing them in relevant populations is urgently needed.”6 Since then, evidence-based standards have been translated into “quality indicators” that may be used to identify outcome targets indicative of quality care, such as patient reports of pain reduction; or, they may specify facility-level care processes associated with these outcomes, such as pain screening, treatment, and follow-up assessment.1,7-9 Quality indicators can be used as the basis for tools to
measure processes that are critical to ensuring highquality supportive cancer care and identifying specific processes and practice sites that should be targeted for quality improvement efforts.10,11 Such tools can help characterize care quality for patient populations served by a health care system as a whole, while revealing important variations at individual facilities within the system.
Quality indicators for supportive and end-of-life care have been successfully applied to electronic health record (EHR) data in research studies assessing care quality in patients with different cancer types and showing supportive care for cancer to be generally in need of quality improvement.10-15 In this paper, we examine supportive care findings from the first largescale application of quality indicators in a system-wide evaluation of lung cancer care in the Veterans Health Administration (VHA). The evaluation was part of a series of operationally motivated projects to identify targets for cancer care improvement in VHA. Lung cancer, the most common malignancy in the United States, accounts for one-fifth of all tumors diagnosed each year within this large, integrated health care system and is responsible for a significant proportion of the system’s supportive care needs. Recently demonstrated benefits of early palliative care on the quality of life and end-of-life care in patients with advanced lung cancer point to the particular importance of assessing supportive care in this population.16 We report VHA evaluation findings and subsequent research analyses seeking to identify patient and facility characteristics associated with better quality supportive lung cancer care.
Methods
Quality Indicators
Quality indicators for supportive cancer care were identified from a systematic review of existing measures and expert guidelines. A panel of 9 national palliative and supportive cancer care experts rated the validity and feasibility of the indicators for use in the VHA using a modified Delphi panel method adapted from the RAND-UCLA appropriateness method17 and prioritized candidate indicators in a ranking exercise. Those with low validity or feasibility scores, or low priority rankings, were excluded, resulting in a final set of 12 quality indicators (8 supportive care, 4 end-of-life care) for use in the national evaluation.
Evaluation Population
All incident non-small-cell (NSCLC) and small-cell (SCLC) lung cancer cases diagnosed within the VHA during 2007 (N = 7,816) were identified through the Veterans Affairs Central Cancer Registry (VACCR). Cases were eligible for the study if the patient had advanced cancer (extensive small-cell or metastatic non-small-cell lung cancer) and had lived long enough to be eligible for the supportive care that was being evaluated. Patients were excluded if the health record did not confirm the lung cancer diagnosis (eg, no pathologic diagnosis or diagnosis outside the VHA, n = 1,297); they had a preexisting or concurrent diagnosis of metastatic neoplasm other than lung cancer (n = 540); had died or enrolled in hospice ≤ 30 days after diagnosis (n = 947); had documented
“comfort measures only” ≤ 30 days after diagnosis (n = 91); had documented life expectancy of ≤ 6 months at time of diagnosis (n = 39); or enrolled in a clinical trial (as care received as part of a trial may not be completely captured in the VHA record (n = 57). Of the patients meeting inclusion criteria, 2,969 were eligible for at least one of the supportive care quality indicators.
Data Collection
EHRs were reviewed remotely by abstractors from the West Virginia Medical Institute, the VHA’s contractor for its external peer-review program. Data were collected retrospectively from the year before to the 2007 diagnosis, with follow-up through 2009. Stage was determined through the VACCR or by abstraction if no VACCR stage was available. The VACCR also provided sociodemographic information including race, which is abstracted from patient charts by cancer registrars. Urban or rural residence was determined by using the rural-urban commuting area codes. Case-level quality indicator results were provided to each facility for review to identify any missed documentation, and abstractors updated data as appropriate. Facility characteristics for the Veterans Administration Medical Centers (VAMCs) at which patients received care were obtained from the 2009 Veterans Health Administration (VHA) Oncology Services
Survey.18 The study was approved by the Veterans Administration Greater Los Angeles Healthcare System Institutional Review Board Subcommittee on Human Studies.
Dependent Variables
For each quality indicator, cases eligible for the indicator were categorized as having received the specified care or not. Patient refusals of specified care or documentation of a contraindication were also considered to have met criteria for receipt of appropriate care. For bivariate and multivariate analyses, indicators were grouped into 4 domains: treatment toxicity (1 indicator), pain screening and management (6), palliative cancer treatment (4), and hospice (1). For these domain analyses, cases were considered to have met criteria for receipt of recommended supportive care if they received the care specified
for all of the indicators for which they were eligible in the domain.
Independent Variables
Patient characteristics included stage of disease, age, gender, race (white, black, other/unknown), marital status (married/living with partner, other), urban/rural residence, and performance status (poor/not poor), as shown in Table 1. Performance status was abstracted from the medical record as ECOG (Eastern Cooperative Oncology Group) or Karnofsky when present, but most of the time it was recorded in patients’ charts qualitatively (eg, “poor performance status”). Cases were attributed to facilities based on the VACCR attribution, which is usually the VHA facility where treatment is initiated. Facility characteristics included geographic region; number of unique patients; facility complexity level; chemotherapy availability on-site; radiation therapy availability on-site; and types of palliative care services, psychosocial support, and patient tracking and case management available on-site (Table 2).
Statistical Analyses
Bivariate analyses were performed to determine which patient and facility characteristics were associated with quality of care for treatment toxicity, pain, palliative treatment, and hospice. Multilevel logistic regression models were developed for each of these 4 domains to examine the relationship between indicator care receipt and patient and facility characteristics. Collinearity was assessed by examining Pearson correlations between independent variables, and highly collinear variables were not included in multivariate analyses.
Independent variables significantly associated with dependent variables in bivariate analyses or considered necessary for adjustment from conceptual standpoints were included in the multivariate models (cancer type [NSCLC or SCLC], age, sex, race, marital status, urban/rural classification, poor performance status, Veterans Integrated Service Network location, facility complexity level, facility region, as well as the facility having the following cancer-related services: tumor board, palliative care unit, palliative care services, chaplain, method for tracking patients through treatment and posttreatment care (to ensure receipt of timely and appropriate care), and presence of a case manager]. Testing yielded no significant interactions between any of the variables; thus interaction terms were not included in the final regressions. All analyses were performed using SAS statistical software, version 9.1 (Cary, NC).
Results
Patient Characteristics
Table 1 summarizes patient characteristics of this elderly (mean age, 67 years; SD, 9), mostly white, and mostly male veteran cohort. In all, 15% of the cohort was black, nearly one-third lived in a rural area, and 46% were married or living with a partner. Eighteen percent had poor performance status, 38% were referred for palliative care, and 81% died during the study period.
Organizational Characteristics
Patients in the cohort were cared for at one or more of 111 VAMC facilities (Table 2), with the largest proportion (45%) seen at facilities located in the South. Most patients (76%) were seen in low-complexity facilities (54% of facilities) and almost all (95%) used a facility reporting having at least 1 tumor board (76% of facilities) in the 2009 VHA Oncology Services Survey. Most facilities (90%) reported having chemotherapy on-site, with 99% of patients in our cohort receiving care at one of these facilities. Radiation therapy at most sites was provided by referral to a non-VA facility, although 31% of facilities had radiation therapy available on-site and these VAMCs provided care to 40% of the patients. A substantial proportion of facilities offered inpatient (91%) and outpatient (76%) palliative care consultation, serving the majority of patients, 95% and 78%, respectively. Various forms of psychosocial support were reported at facilities serving over half of the study patients. Forty-five percent of veterans were seen at facilities that provided care tracking for lung cancer patients (41% of facilities) and at 14% of facilities (12% of patients) the tracking method was monitored by a nurse practitioner or physician assistant. Sixty-eight percent of patients were cared for at facilities reporting the presence of a case manager (69%). (The fact that a site has chemotherapy or any of the other aforementioned services in-house does not imply that patients receiving care at this site were eligible for or received these particular services, but rather that they were cared for at a facility reporting presence of the service.)
About 8% of patients were diagnosed at a VAMC different from the VACCR attribution. Six percent received specialty service consultations or treatment at multiple VAMCs, and about 25% received at least one specialty service consultation or treatment at a non-VHA facility.
Quality Indicator Eligibility and Rates of Receiving Recommended Care
Patient eligibility for the quality indicators (Table 3) ranged from 1% (treatment of spinal cord compression) to 68% (prevention of chemotherapy-related nausea and vomiting). Few patients were eligible for palliative treatment indicators (palliative radiation therapy and treatment of spinal cord compression), with only 2% of patients (50 individuals) eligible for the imaging indicator. Eligibility for the two indicators related to use of shortacting opioids for break-through pain was also low (2%-4%).
Mean national rates for recommended support ive care were generally high (above 85% for 8 of the 12 indicators), ranging from 98% for outpatient pain screening to a low of 60% for spine MRI or myelography within 24 hours for suspected spinal cord compression (Table 3). There was pronounced variability across facilities, with facility rates in every domain ranging from 0%-100% (Figure 1). Many facilities scored 100% in at least 1 domain (eg, 44 of 109 facilities for pain screening; 51 of 94 for palliative cancer therapy). Eight (of 111) facilities did not provide recommended care in 1 domain and 1 did not provide recommended care in 2 domains (ie, 0% of recommended care).
In bivariate analyses (data not shown), referral to palliative care was associated with better care for treatment toxicity (P = .04) but not the other domains. Patients with poor performance status were more likely to be referred to hospice (81% vs 74% for those with good performance status, P = .005) as were patients who lived in an urban zip code area (77% vs 71% for rural zip code, P = .03). In multivariate analyses (Table 4), small-cell cancer type, though not significant in the bivariate analyses, was associated with a higher odds of receiving recommended care [OR, 2.71; 95% CI, 1.89-3.86)] in the
treatment toxicity domain and lower odds in the pain domain [OR, 0.50; 95% CI, 0.33-0.77)]; and poor performance remained associated with higher odds of referral to hospice [OR, 1.49; 95% CI, 1.12-1.99)] after adjusting for other patient and facility characteristics.
In multivariate analysis, moderate facility complexity was associated with lower quality in the toxicity domain than was low facility complexity [OR, 0.28; 95% CI, 0.18-0.44)]; however there was no such difference between the low- and high-complexity facilities. The presence of a patient tracking method appeared to be associated with better quality of care in the treatment toxicity domain [OR, 3.38; 95% CI, 1.87-6.10)] and a higher likelihood of referral to hospice [OR, 1.60; 95% CI, 1.22-2.28)] when the method was monitored by a midlevel practitioner (nurse practitioner or physician assistant), but no association was found for methods monitored by other types of staff. In the pain domain, having a psychologist specializing in cancer was associated with a higher odds of receiving recommended care [OR, 1.76; 95% CI, 1.16-2.66)]. No patient or facility characteristics were associated with receiving recommended palliative cancer therapy, although statistical power was limited by the small numbers of eligible events.
Discussion
Use of evidence-based measures to assess care quality proved feasible in this evaluation of supportive care for advanced lung cancer in a large national integrated health care system and confirmed that gaps found in smaller studies represent critical unmet needs for patients. The methods permitted individualized feedback to facilities, accompanied by support for quality improvement efforts. The effectiveness of providing facility feedback with quality improvement strategies will be evaluated in follow-up studies.
Although quality of supportive care as measured by this specific set of indicators was quite high compared to previous reports,10,13,15 substantial variation across facilities was observed even for measures with high overall performance. Comparatively high levels of supportive care may reflect greater emphasis on supportive measures for advanced lung cancer patients whose expectation of cure is low (other studies evaluated cohorts with mixed cancer types), or the fact that previous studies did not account for documentation of a reason for forgoing indicated care. Tough high quality of supportive care for lung cancer in VHA nationally is encouraging, variability across individual facilities is of concern. Such variation may well exist in any large health care system, with patients potentially experiencing different standards of care depending on where they live.
In this study, facilities located in the South, treating 45% of advanced lung cancer patients in the cohort, were associated with worse care in the treatment toxicity and hospice domains, and those in the Northeast appeared to generally have worse care for pain. Although reasons for these differences are unclear, geographic variation in Medicare hospice use has been related to a variety of determinants,20-23 some of which may also apply to VHA. Referral to palliative care or hospice was among the lowest scoring quality indicators in the evaluation, with a 75% national rate and 43% of facilities documenting referral for less than 75% of eligible cases. Although our measures determined only referral to palliative care or hospice (versus actual receipt of services), low rates may in part reflect provider anticipation of low local availability of hospice. Although the VHA Hospice and Palliative Care program has made progress in reducing variability in hospice access for Veterans, surveys conducted by Hospice-Veteran Partnerships showed lack of shared knowledge about the different systems of benefits and health care available for veterans, misunderstandings about referral processes among health care providers and payment for hospice services, and difficulties in caring for veterans across multiple care settings to be barriers to Veteran access to community services.24 Targeted inquiry at lowreferral facilities may elucidate which of these and other possible challenges may suggest points of intervention for improvement in hospice referral patterns and support of appropriate hospice use.
Attempting to identify facility-related explanations for overall variability, we explored the potential role of a number of structural variables, including presence of a tumor board, a palliative care unit, outpatient palliative care services, chaplain services, presence of a cancer care tracking system, and presence of a case manager. Tough facilities with a psychologist specializing in cancer provided better care for pain, and those with midlevel practitioners (nurse practitioner or physician assistant) monitoring patient tracking through care were more likely to appropriately refer to hospice and have better care for treatment toxicity, many facilities without such designated professionals also provided quality care in these domains. Because of limited methodological feasibility of demonstrating patient receipt of psychosocial/spiritual support and care tracking using medical record review, the final set of quality indicators did not include measures for these forms of care; however, psychosocial care is considered integral to supportive care in cancer25 and care tracking has been associated with improved cancer care in non-VA settings.26 The presence of appropriate professionals providing cancer-specific psychological support and tracking may be directly associated with improved care, not only in the domains noted above, but also in the important domain of existential and emotional well-being not evaluated here. These services may also be part of larger facility-level quality improvement mechanisms contributing to cancer care quality that are worth identifying in future research. Lack of consistent explanations for the overall facility-level variation in this study suggests the need to identify data sources that can be used to measure additional organizational characteristics in future research.
Our study has several limitations. The scope of the national evaluation did not include indicators for all aspects of supportive care, including care for dyspnea, depression, and psychosocial distress; support for care givers; and care planning assistance. Small numbers of eligible events per facility for some indicators limit interpretation, particularly in the palliative treatment domain, where the largest range was 1-15 eligible events per facility. Facility characteristics used for analyses were identified from a surveyand may be subject to respondent error. Potentially useful processes, such as a midlevel practitioner monitored tracking system were identified as “present” at the facility, but use for cohort patients was not possible to confirm, limiting direct linkage with better care. Some facility-level results may be influenced by errors in VACCR attribution to a facility that was not directly involved with the patient’s care, specialty service receipt at multiple VAMCs, or through contract care at non-VHA facilities. Quality-of-care coordination between different VHA and non-VHA facilities for services not provided at the diagnosing facility, or to reduce travel burden if the VAMC was far from the patient’s home may be associated with better or worse care quality and should be evaluated in future work.
In conclusion, use of quality indicators to evaluate quality of supportive lung cancer care in a large integrated health care system proved feasible, confirmed
provision of overall high-quality supportive care, but identified a high degree of variability across individual facilities in the system. Facility characteristics examined did not explain this variation; however, the data suggested that, while not accounting for the overall variation, having professionals providing cancer-specific psychological support and care-tracking may contribute to better quality of care in some domains. Difficulties in identifying predictors of quality suggest that future research should include qualitative comparisons of facilities with varying rates of providing recommended supportive care to identify potentially impactful organizational factors not examined in this study.
Disclaimer and Disclosures
Accepted for publication February 24, 2014. Correspondence: Sabine Oishi, PhD, MSPH; [email protected]. Disclosures: Dr Ryoo was supported in part by the Robert Wood Johnson Foundation Clinical Scholars Program and by the UCLA Jonsson Comprehensive Cancer Center R25 grant. The authors have no direct conflicts of interest to disclose. Dr Karl Lorenz is a consultant as a member of the Data Monitoring Committee for a Phase II trial of Sativex being conducted by Otsuka Pharmaceuticals. The current study has no direct financial implications related to his participation and was conducted entirely outside of the timeframe of his consultancy; however, it may be perceived as indirectly related. The views and opinions of authors expressed herein do not necessarily state or reflect that of the Department of Veterans Affairs, the United States Government, Kaiser Permanente, Stanford University, or the University of California.
Click here to read the digital edition.
1. Lorenz KA, Dy SM, Naeim A, et al. Quality measures for supportive cancer care: the Cancer Quality-ASSIST Project. J Pain Symptom Manage. 2009;37(6):943-964.
2. Dy SM, Asch SM, Naeim A, Sanati H, Walling A, Lorenz KA. Evidence-based standards for cancer pain management. J Clin Oncol. 2008;26(23):3879-3885.
3. Gordon, DB, Dahl, JL, Miakowski, C. American Pain Society recommendations for improving the quality of acute and cancer pain management. Arch Intern Med. 2005;165(14):1574-1580.
4. Lorenz, KA, Lynn, J, Dy, SM, et al. Evidence for improving palliative care at the end of life: a systematic review. Ann Intern Med. 2008;148(2):147-159.
5. Seow, H, Snyder, CF, Shugarman, LR, et al. Developing quality indicators for cancer end-of-life care: proceedings from a national symposium. Cancer. 2009;115(17):3820-3829.
6. Lorenz KA, Lynn J, Dy SM, et al. Agency for Healthcare Research and Quality (US). Cancer care quality measures: symptoms and end-of-life care. http://www.ncbi.nlm.nih.gov/books/NBK38028/. Published 2006. Accessed December 17, 2012.
7. Lorenz KA, Lynn J, Dy S, et al. Quality measures for symptoms and advance care planning in cancer: a systematic review. J Clin Oncol. 2006;24(30):4933-4938.
8. Lorenz KA, Rosenfeld K, Wenger N. Quality indicators for palliative and end-of-life care in vulnerable elders. JAGS. 2007;55(suppl 2):S318-S326.
9. Dy SM, Lorenz KA, O’Neill SM, et al. Cancer Quality-ASSIST supportive oncology quality indicator set. Cancer. 2010;116(13):3267-3275.
10. Jacobson JO, Neuss MN, McNiff KK, et al. Improvement in oncology practice performance through voluntary participation in the Quality Oncology Practice Initiative. J Clin Oncol. 2008;26(11):1893-1898.
11. Jacobsen PB, Shibata D, Siegel E, et al. Evaluating the quality of psychosocial care in outpatient medical oncology settings using performance indicators. Psycho-oncology. 2010; 20(11):1221-1227.
12. Walling AM, Asch SM, Lorenz KA, et al. The quality of care provided to hospitalized patients at the end of life. Arch Intern Med. 2010;170(12):1057-1063.
13. Dy SM, Asch SM, Lorenz KA, et al. Quality of end-of-life care for patients with advanced cancer in an academic medical center. J Palliat Med. 2011;14(4):451-457.
14. Gonsalves WI, Tsewang T, Krishnamurthy J, et al. Effect of palliative care services on the aggressiveness of end-of-life care in the Veteran’s Affairs cancer population. J Palliat Med. 2011;14(11):1231-1235.
15. Malin JL, O’Neill SM, Asch SM, et al. Quality of supportive care for patients with advanced cancer in a VA medical center. J Palliat Med. 2011;14(5):573-577.
16. Temel JS, Greer JA, Admane S, et al. Longitudinal perceptions of prognosis and goals of therapy in patients with metastatic non-small-cell lung cancer: results of a randomized study of early palliative care. J Clin Oncol. 2011;29(17):2319-2326.
17. Brook RH, Chassin MR, Fink A, Solomon DH, Kosecoff J, Park RE. A method for the detailed assessment of the appropriateness of medical technologies. Int J Technol Assess Health Care. 1986;2(1):53-63.
18. Department of Veterans Affairs, Veterans Health Administration, Office of the Assistant Deputy Under Secretary for Health for Policy and Planning. 2009 VHA Oncology Services Survey. April, 2010. Accessed November 1, 2010.
19. US Census Bureau. Census Bureau regions and divisions with state FIPS codes. Available from URL: https://www.census.gov/geo/ maps-data/maps/pdfs/reference/us_regdiv.pdf. Accessed December 17, 2012.
20. Virnig BA. Toward a better understanding of the role of geography in intensity of end-of-life care: must we first come to an understanding of end-of-life care? Medical Care. 2007;45(5):374-376.
21. Virnig BA, Kind S, McBean M, Fisher E. Geographic variation in hospice use prior to death. JAGS. 2000;48(9):117-11.
22. McCarthy EP, Burns RB, Davis RB, Phillips RS. Barriers to hospice care among older patients dying with lung and colorectal cancer. J Clin Oncol. 2003;21(4): 728-735.
23. Virnig BA, Ma H, Hartman LK, Moscovice I, Carlin B. Access to home-based hospice care for rural populations: identification of areas lacking service. J Palliat Med. 2006;9(6):1292-1299.
24. Jones D, Edes T, Shreve S. You won’t know if you’re improving unless you measure: recommendations for evaluating hospice-veteran partnerships. J Pain Symptom Manage. 2006;32(5):488-496.
25. Surbone A, Baider L, Weitzman TS, Brames MJ, Rittenberg CN, Johnson J, et al. Psychosocial care for patients and their families is integral to supportive care in cancer: MASCC position statement. Support Care Cancer. 2010;18(2):255-263.
26. Callahan M, Sanderson, J. A breast cancer tracking system. The Permanente Journal. 2000;4:36-39. http://www.thepermanentejournal.org/files/Fall2000/BreastCancer.pdf. Accessed June 1, 2012.
1. Lorenz KA, Dy SM, Naeim A, et al. Quality measures for supportive cancer care: the Cancer Quality-ASSIST Project. J Pain Symptom Manage. 2009;37(6):943-964.
2. Dy SM, Asch SM, Naeim A, Sanati H, Walling A, Lorenz KA. Evidence-based standards for cancer pain management. J Clin Oncol. 2008;26(23):3879-3885.
3. Gordon, DB, Dahl, JL, Miakowski, C. American Pain Society recommendations for improving the quality of acute and cancer pain management. Arch Intern Med. 2005;165(14):1574-1580.
4. Lorenz, KA, Lynn, J, Dy, SM, et al. Evidence for improving palliative care at the end of life: a systematic review. Ann Intern Med. 2008;148(2):147-159.
5. Seow, H, Snyder, CF, Shugarman, LR, et al. Developing quality indicators for cancer end-of-life care: proceedings from a national symposium. Cancer. 2009;115(17):3820-3829.
6. Lorenz KA, Lynn J, Dy SM, et al. Agency for Healthcare Research and Quality (US). Cancer care quality measures: symptoms and end-of-life care. http://www.ncbi.nlm.nih.gov/books/NBK38028/. Published 2006. Accessed December 17, 2012.
7. Lorenz KA, Lynn J, Dy S, et al. Quality measures for symptoms and advance care planning in cancer: a systematic review. J Clin Oncol. 2006;24(30):4933-4938.
8. Lorenz KA, Rosenfeld K, Wenger N. Quality indicators for palliative and end-of-life care in vulnerable elders. JAGS. 2007;55(suppl 2):S318-S326.
9. Dy SM, Lorenz KA, O’Neill SM, et al. Cancer Quality-ASSIST supportive oncology quality indicator set. Cancer. 2010;116(13):3267-3275.
10. Jacobson JO, Neuss MN, McNiff KK, et al. Improvement in oncology practice performance through voluntary participation in the Quality Oncology Practice Initiative. J Clin Oncol. 2008;26(11):1893-1898.
11. Jacobsen PB, Shibata D, Siegel E, et al. Evaluating the quality of psychosocial care in outpatient medical oncology settings using performance indicators. Psycho-oncology. 2010; 20(11):1221-1227.
12. Walling AM, Asch SM, Lorenz KA, et al. The quality of care provided to hospitalized patients at the end of life. Arch Intern Med. 2010;170(12):1057-1063.
13. Dy SM, Asch SM, Lorenz KA, et al. Quality of end-of-life care for patients with advanced cancer in an academic medical center. J Palliat Med. 2011;14(4):451-457.
14. Gonsalves WI, Tsewang T, Krishnamurthy J, et al. Effect of palliative care services on the aggressiveness of end-of-life care in the Veteran’s Affairs cancer population. J Palliat Med. 2011;14(11):1231-1235.
15. Malin JL, O’Neill SM, Asch SM, et al. Quality of supportive care for patients with advanced cancer in a VA medical center. J Palliat Med. 2011;14(5):573-577.
16. Temel JS, Greer JA, Admane S, et al. Longitudinal perceptions of prognosis and goals of therapy in patients with metastatic non-small-cell lung cancer: results of a randomized study of early palliative care. J Clin Oncol. 2011;29(17):2319-2326.
17. Brook RH, Chassin MR, Fink A, Solomon DH, Kosecoff J, Park RE. A method for the detailed assessment of the appropriateness of medical technologies. Int J Technol Assess Health Care. 1986;2(1):53-63.
18. Department of Veterans Affairs, Veterans Health Administration, Office of the Assistant Deputy Under Secretary for Health for Policy and Planning. 2009 VHA Oncology Services Survey. April, 2010. Accessed November 1, 2010.
19. US Census Bureau. Census Bureau regions and divisions with state FIPS codes. Available from URL: https://www.census.gov/geo/ maps-data/maps/pdfs/reference/us_regdiv.pdf. Accessed December 17, 2012.
20. Virnig BA. Toward a better understanding of the role of geography in intensity of end-of-life care: must we first come to an understanding of end-of-life care? Medical Care. 2007;45(5):374-376.
21. Virnig BA, Kind S, McBean M, Fisher E. Geographic variation in hospice use prior to death. JAGS. 2000;48(9):117-11.
22. McCarthy EP, Burns RB, Davis RB, Phillips RS. Barriers to hospice care among older patients dying with lung and colorectal cancer. J Clin Oncol. 2003;21(4): 728-735.
23. Virnig BA, Ma H, Hartman LK, Moscovice I, Carlin B. Access to home-based hospice care for rural populations: identification of areas lacking service. J Palliat Med. 2006;9(6):1292-1299.
24. Jones D, Edes T, Shreve S. You won’t know if you’re improving unless you measure: recommendations for evaluating hospice-veteran partnerships. J Pain Symptom Manage. 2006;32(5):488-496.
25. Surbone A, Baider L, Weitzman TS, Brames MJ, Rittenberg CN, Johnson J, et al. Psychosocial care for patients and their families is integral to supportive care in cancer: MASCC position statement. Support Care Cancer. 2010;18(2):255-263.
26. Callahan M, Sanderson, J. A breast cancer tracking system. The Permanente Journal. 2000;4:36-39. http://www.thepermanentejournal.org/files/Fall2000/BreastCancer.pdf. Accessed June 1, 2012.
Palliative care ‘in my hands’
A randomized controlled multicenter study published by Carson et al. in JAMA concluded that, for patients with “chronic critical illness” (defined as requiring 7 days of mechanical ventilation), palliative care team-led informational and emotional support meetings did not reduce anxiety or depression for families and may have increased posttraumatic stress disorder symptoms (2016:316[1]:51-62. doi: 10.1001/jama.2016.8474).
This report may surprise surgeons, as well as practitioners in other specialties, as the disconnect between palliative care and critical care services has been previously perceived as an education and access issue, not an outcome problem.
Carson and colleagues point out that fidelity to some components of the meeting “templates” was low, suggesting that there was some flexibility baked into the study design. However, as Russ and Kaufman aptly described, patients and families vary greatly in their appetite for explicit information about prognosis (Cult Med Psychiatry 2005;29[1]:103-23). Conversely, the hypothesis that direct communication about prognosis will be welcomed by families is a core element of the Carson study. The manuscript supplement reports that discussion of the patient’s condition and prognosis took place in 100% of initial meetings. If the same variability in family receptiveness to this information exists in this population as was described by Russ and Kaufman, it is not hard to see why some families experienced negative consequences because of these discussions.
Furthermore, the authors of the Carson study point out that it was not intended to replicate the components of specialist palliative care (JAMA. 2016;316[15]:1598-9).
Essential elements of specialist palliative care include symptom management, a multidisciplinary approach, and fairly close contact in the acute care setting. These features were lacking in the study protocol. Experienced providers of palliative care will often use symptom assessment and symptom management optimization as a conduit for building rapport and to avoid focusing on prognosis until trust has been established. A period of delay before broaching challenging subjects also allows the palliative care team to develop an understanding of the patient’s or surrogates’ preferences regarding the amount and type of information communicated. Palliative care providers benefit from the deepening of relationships with patients and families over time, as much as or possibly more so than providers of other specialties.
The necessity of the multidisciplinary approach to successful palliative care outcomes cannot be overstated. In many programs, patients seen for specialist palliative care consultation are seen by a physician or advanced practitioner, a chaplain, and a social worker within 24-48 hours of initial referral, and these providers have key roles in addressing the sequelae of anxiety, depression, and stress that were the key outcomes in the JAMA study. In the study, the “support and information team” included a palliative care physician and an advanced practice nurse but not a chaplain or social worker, despite the significance of existential/spiritual and social consequences of ventilator withdrawal or progression to tracheostomy for long-term vent support.
Palliative care providers consider the family meeting to be the “procedure” of their field, a belief that may seem incongruous with a surgical understanding of the nature of procedures but is informative as a framework for understanding the results of the Carson study. Just as surgical procedures carry risk of complications or adverse outcomes, family meetings have risk for worsening instead of improving the coping of families and surrogates. And, as surgical technique can be connected to complications, the family meeting technique applied by Carson et al. may be related to its results. Although there was formalized communication between the ICU team and the palliative care team regarding the patient’s condition, prognosis, and treatment plan, there was not a representative from the critical care team present during the majority of the support and information team led family meetings. This represents a marked deviation from common practice at our institution and many others. Our usual practice is to have a member of the ICU team present for discussions focused on patient prognosis, in order to make sure that there is alignment between the messages of the ICU and palliative care teams and also to prevent the crippling of palliative support that occurs when it becomes the sole repository of unwelcome news.
Because the relief of suffering is a core value of surgery and palliative care, there are countless ways these disciplines can inform one another. The outcome of the Carson study is a cautionary tale about the fallibility of the integration of surgical and palliative care teams, both of which would acknowledge the importance of the multidisciplinary approach, relationships developed over time, and symptom management. As surgeons intuitively understand from their operative experience, the “procedure” (the family meeting) has the potential for both risk and benefit, the outcome of which may be determined “in my hands.”
Dr. Rivet is a colon and rectal surgeon with training and board certification in hospice and palliative medicine. She is an assistant professor, departments of surgery and internal medicine, Virginia Commonwealth University, Richmond. She has no disclosures.
A randomized controlled multicenter study published by Carson et al. in JAMA concluded that, for patients with “chronic critical illness” (defined as requiring 7 days of mechanical ventilation), palliative care team-led informational and emotional support meetings did not reduce anxiety or depression for families and may have increased posttraumatic stress disorder symptoms (2016:316[1]:51-62. doi: 10.1001/jama.2016.8474).
This report may surprise surgeons, as well as practitioners in other specialties, as the disconnect between palliative care and critical care services has been previously perceived as an education and access issue, not an outcome problem.
Carson and colleagues point out that fidelity to some components of the meeting “templates” was low, suggesting that there was some flexibility baked into the study design. However, as Russ and Kaufman aptly described, patients and families vary greatly in their appetite for explicit information about prognosis (Cult Med Psychiatry 2005;29[1]:103-23). Conversely, the hypothesis that direct communication about prognosis will be welcomed by families is a core element of the Carson study. The manuscript supplement reports that discussion of the patient’s condition and prognosis took place in 100% of initial meetings. If the same variability in family receptiveness to this information exists in this population as was described by Russ and Kaufman, it is not hard to see why some families experienced negative consequences because of these discussions.
Furthermore, the authors of the Carson study point out that it was not intended to replicate the components of specialist palliative care (JAMA. 2016;316[15]:1598-9).
Essential elements of specialist palliative care include symptom management, a multidisciplinary approach, and fairly close contact in the acute care setting. These features were lacking in the study protocol. Experienced providers of palliative care will often use symptom assessment and symptom management optimization as a conduit for building rapport and to avoid focusing on prognosis until trust has been established. A period of delay before broaching challenging subjects also allows the palliative care team to develop an understanding of the patient’s or surrogates’ preferences regarding the amount and type of information communicated. Palliative care providers benefit from the deepening of relationships with patients and families over time, as much as or possibly more so than providers of other specialties.
The necessity of the multidisciplinary approach to successful palliative care outcomes cannot be overstated. In many programs, patients seen for specialist palliative care consultation are seen by a physician or advanced practitioner, a chaplain, and a social worker within 24-48 hours of initial referral, and these providers have key roles in addressing the sequelae of anxiety, depression, and stress that were the key outcomes in the JAMA study. In the study, the “support and information team” included a palliative care physician and an advanced practice nurse but not a chaplain or social worker, despite the significance of existential/spiritual and social consequences of ventilator withdrawal or progression to tracheostomy for long-term vent support.
Palliative care providers consider the family meeting to be the “procedure” of their field, a belief that may seem incongruous with a surgical understanding of the nature of procedures but is informative as a framework for understanding the results of the Carson study. Just as surgical procedures carry risk of complications or adverse outcomes, family meetings have risk for worsening instead of improving the coping of families and surrogates. And, as surgical technique can be connected to complications, the family meeting technique applied by Carson et al. may be related to its results. Although there was formalized communication between the ICU team and the palliative care team regarding the patient’s condition, prognosis, and treatment plan, there was not a representative from the critical care team present during the majority of the support and information team led family meetings. This represents a marked deviation from common practice at our institution and many others. Our usual practice is to have a member of the ICU team present for discussions focused on patient prognosis, in order to make sure that there is alignment between the messages of the ICU and palliative care teams and also to prevent the crippling of palliative support that occurs when it becomes the sole repository of unwelcome news.
Because the relief of suffering is a core value of surgery and palliative care, there are countless ways these disciplines can inform one another. The outcome of the Carson study is a cautionary tale about the fallibility of the integration of surgical and palliative care teams, both of which would acknowledge the importance of the multidisciplinary approach, relationships developed over time, and symptom management. As surgeons intuitively understand from their operative experience, the “procedure” (the family meeting) has the potential for both risk and benefit, the outcome of which may be determined “in my hands.”
Dr. Rivet is a colon and rectal surgeon with training and board certification in hospice and palliative medicine. She is an assistant professor, departments of surgery and internal medicine, Virginia Commonwealth University, Richmond. She has no disclosures.
A randomized controlled multicenter study published by Carson et al. in JAMA concluded that, for patients with “chronic critical illness” (defined as requiring 7 days of mechanical ventilation), palliative care team-led informational and emotional support meetings did not reduce anxiety or depression for families and may have increased posttraumatic stress disorder symptoms (2016:316[1]:51-62. doi: 10.1001/jama.2016.8474).
This report may surprise surgeons, as well as practitioners in other specialties, as the disconnect between palliative care and critical care services has been previously perceived as an education and access issue, not an outcome problem.
Carson and colleagues point out that fidelity to some components of the meeting “templates” was low, suggesting that there was some flexibility baked into the study design. However, as Russ and Kaufman aptly described, patients and families vary greatly in their appetite for explicit information about prognosis (Cult Med Psychiatry 2005;29[1]:103-23). Conversely, the hypothesis that direct communication about prognosis will be welcomed by families is a core element of the Carson study. The manuscript supplement reports that discussion of the patient’s condition and prognosis took place in 100% of initial meetings. If the same variability in family receptiveness to this information exists in this population as was described by Russ and Kaufman, it is not hard to see why some families experienced negative consequences because of these discussions.
Furthermore, the authors of the Carson study point out that it was not intended to replicate the components of specialist palliative care (JAMA. 2016;316[15]:1598-9).
Essential elements of specialist palliative care include symptom management, a multidisciplinary approach, and fairly close contact in the acute care setting. These features were lacking in the study protocol. Experienced providers of palliative care will often use symptom assessment and symptom management optimization as a conduit for building rapport and to avoid focusing on prognosis until trust has been established. A period of delay before broaching challenging subjects also allows the palliative care team to develop an understanding of the patient’s or surrogates’ preferences regarding the amount and type of information communicated. Palliative care providers benefit from the deepening of relationships with patients and families over time, as much as or possibly more so than providers of other specialties.
The necessity of the multidisciplinary approach to successful palliative care outcomes cannot be overstated. In many programs, patients seen for specialist palliative care consultation are seen by a physician or advanced practitioner, a chaplain, and a social worker within 24-48 hours of initial referral, and these providers have key roles in addressing the sequelae of anxiety, depression, and stress that were the key outcomes in the JAMA study. In the study, the “support and information team” included a palliative care physician and an advanced practice nurse but not a chaplain or social worker, despite the significance of existential/spiritual and social consequences of ventilator withdrawal or progression to tracheostomy for long-term vent support.
Palliative care providers consider the family meeting to be the “procedure” of their field, a belief that may seem incongruous with a surgical understanding of the nature of procedures but is informative as a framework for understanding the results of the Carson study. Just as surgical procedures carry risk of complications or adverse outcomes, family meetings have risk for worsening instead of improving the coping of families and surrogates. And, as surgical technique can be connected to complications, the family meeting technique applied by Carson et al. may be related to its results. Although there was formalized communication between the ICU team and the palliative care team regarding the patient’s condition, prognosis, and treatment plan, there was not a representative from the critical care team present during the majority of the support and information team led family meetings. This represents a marked deviation from common practice at our institution and many others. Our usual practice is to have a member of the ICU team present for discussions focused on patient prognosis, in order to make sure that there is alignment between the messages of the ICU and palliative care teams and also to prevent the crippling of palliative support that occurs when it becomes the sole repository of unwelcome news.
Because the relief of suffering is a core value of surgery and palliative care, there are countless ways these disciplines can inform one another. The outcome of the Carson study is a cautionary tale about the fallibility of the integration of surgical and palliative care teams, both of which would acknowledge the importance of the multidisciplinary approach, relationships developed over time, and symptom management. As surgeons intuitively understand from their operative experience, the “procedure” (the family meeting) has the potential for both risk and benefit, the outcome of which may be determined “in my hands.”
Dr. Rivet is a colon and rectal surgeon with training and board certification in hospice and palliative medicine. She is an assistant professor, departments of surgery and internal medicine, Virginia Commonwealth University, Richmond. She has no disclosures.
COVER to COVER: Connecting Older Veterans (Especially Rural) to Community or Veteran-Eligible Resources
According to the VA, 23% of veterans in the U.S., nearly 5.2 million individuals, live in rural areas.1 The VHA serves more than 3 million rural veterans, and 56% of those enrolled in the VA system are aged ≥ 65 years.1 Thus, aging veterans in rural areas constitute a substantial group who need support and assistance from the VA. Fortunately, the VA offers numerous benefits for veterans that support aging in place and improve quality of life through the VHA, Veterans Benefits Administration (VBA), and National Cemetery Administration (NCA).
Despite the opportunities, many VA benefits go unclaimed. In some cases, veterans simply do not know these benefits exist.2 In a 2010 VA report, only 41% of veterans indicated that they understood their benefits “a lot” or “some.”2 However, their understanding of specific benefits tended to be lower. For example, many veterans stated that they had “heard about” burial options at VA cemeteries (41.5%), but few understood specific benefits, such as cash burial allowances (10.6%).2
Many veterans also hold misperceptions about eligibility, which prevents them from applying. For example, some veterans believe that a high income or lack of combat service disqualifies them from receiving VA benefits.3 Some veterans believe that others are more deserving of VA services, and they don’t want to “take a spot someone else needs.”3 Finally, some veterans hold negative attitudes about the VA, making them less likely to claim VA benefits, such as health care.4
For rural veterans, accessing benefits can be especially difficult, because most VA facilities that offer assistance are in urban centers.5 Though online access to benefit information is improving through programs like My HealtheVet and public facing websites, some older adults do not use computers, and Internet and mobile phone connectivity are often limited in rural communities.6 Nearly 43% of rural veterans do not have broadband Internet in their homes.1 Moreover, the complexity of navigating benefits information via the Internet can be a frustrating and confusing process for older veterans.6
Accessing services and benefits in the community is similarly difficult. For more than 30 years, community organizations have noted the frustration that clients experienced with navigating a complex network of community providers who provide long-term services and supports (LTSS).7,8 In 2003, the Administration on Aging and the Centers for Medicare and Medicaid Services developed the Aging and Disability Resource Center (ADRC) program to promote a “no wrong door” approach for LTSS. Aging and Disability Resource Centers are a single point of entry into a network of community, state, and federal LTSS for older adults and individuals with disabilities.8 Options counselors at ADRCs provide information, counseling, and assistance with connecting to a vast network of programs such as Social Security, Medicaid, local transportation, Meals on Wheels, and housing assistance through a single office.
Backround
In 2012, VHA Office of Rural Health Resource (ORH) and Utah ADRC conducted a national survey of ADRC sites about their experiences working with veterans and found that 95% of ADRCs always or usually asked clients about their veteran status. The survey found that veteran clients present to ADRCs with diverse needs, many of which could be addressed through a VA benefit. However, the majority (58%) of ADRC respondents reported that they had never attempted to help a veteran apply for VA benefits (unpublished data, 2012).
Respondents reported a limited understanding of VA benefits, infrequent contact with VA, and frustrations with the VA system. Although familiar with several sources for information about VA benefits (eg, toll-free number, websites, local VA facilities, etc), respondents generally found these sources unhelpful and insufficient for answering their questions. The only positive anecdotal comments that respondents made regarding VA were from those with personal relationships with employees at the VA who could help with veteran needs. Finally, all respondents reported a need for more information about VA benefits and to assist them with helping veteran clients.
Survey Response
In 2013, the ORH and the VA Salt Lake City Geriatric Research Education and Clinical Center (GRECC), under the sponsorship of the VA Office of Geriatrics and Extended Care (GEC), developed a collaborative demonstration with the Utah ADRC to address the needs identified in this survey. Connecting Older Veterans (Especially Rural) to Community or Veteran Eligible Resources (COVER to COVER) is a demonstration project designed to create a new access point for VA benefits for veterans living in rural areas. The pilot had 2 aims: (1) train ADRC options counselors as Veteran Benefits Specialists (VBSs); and (2) build relationships between the ADRCs and VA to facilitate information and referral.
Between 2013 and 2015, the demonstration was housed at the VA Salt Lake City Health Care System GRECC as part of the clinical demonstration portfolio. The GRECC staff provided administrative support and mentorship for the developing partnerships. Subsequently, the demonstration was selected as a Promising Practice for enterprise-wide implementation. Both ORH and GEC coordinated opportunities for broad dissemination.
Program Elements
In Utah, 5 pilot ADRC agencies cover 19 counties, 14 of which are entirely rural. The remaining counties contain populations that are 20% to 49% urbanized (1 county), 50% to 80% urbanized (1 county), and 80% to 100% urbanized (3 counties). More than 95,000 veterans (12,857 in the 14 rural counties) live in the participating counties. The average income for veterans in all participating counties is $36,699 for men and $30,915 for women.9 Furthermore, about 53% of veterans in all these counties are aged > 65 years.9
For this pilot, each ADRC site assigned an existing options counselor as a dedicated VBS. Each VBS completed 80 to 100 hours of training in VA benefits. To facilitate the amount of training required to become experts, the ORH funded a portion of the salary for each VBS.
An outreach specialist at the VA Salt Lake City Regional Benefit Office, a geriatric social worker at the VA Salt Lake City Health Care System, and an outreach specialist at the Utah Department of Veterans and Military Affairs (UDVMA) were primary trainers for this pilot. Trainers provided 15 training sessions between February 2013 and September 2015, totaling 74 hours. The 5 designated VBSs attended all trainings, but meetings were opened to all ADRC staff and other community organizations; 115 individuals from Utah, Idaho, Nevada, New Mexico, and Wyoming attended at least 1 training. In the first year and a half, trainings ranged from 1.5 to 4.5 hours and provided a general overview of benefits. As the value of these trainings increased among the ADRCs and other community providers, longer seminars were offered, the longest lasting 2 days, which provided in-depth training.
Training topics comprised the following 4 general categories:
- Core—VA structure, military culture
- VHA—health care, enrollment and eligibility, in-home services
- VBA—pension, aid and attendance, disability compensation, nursing home, dependency and indemnity compensation
- NCA burial benefits
In response to participant requests for training on other VA benefits, additional VA staff presented topics such as mental health, homelessness, telehealth, Vet Centers, and My HealtheVet. Information on the Veterans Choice Program was incorporated into later trainings.
In addition to the training provided by COVER to COVER, the 5 ADRC VBSs completed the 25-hour Training Responsibility Involvement and Preparation of Claims (TRIP) online course. This coursework qualified them to take the examination to become certified veterans service officers.
With the information received in training, ADRC VBSs assist veteran clients and their families to learn about and apply for VA benefits. Veterans or family members contact the ADRC with a variety of needs, such as difficulty paying utilities, functional limitations, etc. All ADRC staff screen callers for veteran status and refer willing veterans or family members to the VBS who provides information about LTSS options and screens for eligibility for VA benefits.
Through these training events, VBSs also formed relationships with the VA trainers, resulting in the ability to refer to and coordinate with the VA on cases when needed. The VBSs often work closely with the UDVMA by helping veterans organize needed documents and coordinate with UDVMA staff to complete VA benefit claims. Furthermore, VBSs can help veterans navigate the VA system and advocate for their needs in coordination with the VA trainers.
The VBAs have described numerous positive outcomes from the COVER to COVER program. They universally report improved knowledge and confidence in assisting veteran clients. In many cases, simply identifying clients’ veteran status in the normal ADRC intake protocol has placed them in touch with many veterans without any significant change in their workload. One specialist reported that COVER to COVER has improved the quality of services she can provide to veterans and that connecting veteran clients to VA frees public resources for other clients in need. Finally, they report that the trainings introduced them to key VA contacts and laid the groundwork for developing relationships with new partners. The following case is representative of the types of client experiences VBSs routinely describe.
Case Study
“Larry,” a 94 year-old World War II veteran who had never applied for VA benefits, presented to a rural ADRC for assistance with paying his utility bills. Larry had numerous health issues, including early stage dementia. He relied on his 96-year-old wife, “Sandy,” to assist him with activities of daily living (ADLs) and instrumental activities of living (IADLs). However, Sandy also had health problems that limited her ability to help. The couple wanted to stay in their home but worried they could not do it without help.
An ADRC staff member referred Larry and Sandy to the VBS, who helped the couple enroll in a community LTSS program called Aging Alternatives for in-home services. During this time, Sandy passed away, but the VBS continued to work with Larry and helped him apply for VA disability compensation, enroll in VHA health care, and connect to VA’s Veteran Directed Home and Community Based Services (VDHCBS) program for in-home services.
Larry received a 70% service-connected disability rating and started receiving monthly compensation from the VA. Although Larry wants to stay at home, the rating of 70% service connection allows VA to cover nursing home placement should it be needed. He established a VA primary care physician and uses VDHCBS to purchase in-home services. Since Larry receives in-home services from the VHA, he was discharged from the Aging Alternatives program. This allowed the ADRC to reallocate this resource to another person in need. Larry is still living at home.
Future Directions
This case study highlights the benefits for veterans of COVER to COVER program through its emphasis on productive relationships between VA and community partners. More extensive data collection related to veteran outcomes is ongoing and will be essential for sustaining the program locally and to support broader dissemination to other states. Ideally, expansion to other sites will include temporary pilot funding to offset the time needed to gain the knowledgeand skills to become a VBS and to provide consultation and training to other ADRC staff. Once the pilot funding ends, the ADRC staff would have the necessary knowledge, skills, and relationships to continue providing services to veterans.
Acknowledgments
This project was supported by the VHA Office of Rural Health. The authors thank all ADRC, UDVMA, and VHA staff who participated in the project.
1. VHA Office of Rural Health. Office of rural health annual report: thrive 2015. http://www.rural-health.va.gov/docs/ORH_Annual_Report_2015_FINAL.pdf. Published 2015. Accessed January 9, 2017.
2. National Center for Veterans Analysis and Statistics. 2010 national survey of veterans: understanding and knowledge of VA benefits and services. http://www.va.gov/VETDATA/docs/SpecialReports/2010NSV_Awareness_FINAL.pdf. Published November 2011. Accessed January 9, 2017.
3. Wittrock S, Ono S, Stewart K, Reisinger HS, Charlton M. Unclaimed health care benefits: a mixed-method analysis of rural veterans. J Rural Health. 2015;31(1):35-46.
4. Fox AB, Meyer EC, Vogt D. Attitudes about the VA health-care setting, mental illness, and mental health treatment and their relationship with VA mental health service use among female and male OEF/OIF veterans. Psychol Serv. 2015;12(1):49-58.
5. Helm MD. System worth saving report on rural healthcare 2012. http://archive.legion.org/handle/123456789/1951. Published 2012. Accessed January 9, 2017.
6. Lee B, Chen Y, Hewitt L. Age differences in constraints encountered by seniors in their use of computers and the Internet. Comput Human Behav. 2011;27(3):1231-1237.
7. Kane RL, Kane RA. A guide through the maze of long-term care. West J Med. 1981;135(6):503-510.
8. O’Shaughnessy CV. Aging and disability resource centers can help consumers navigate the maze of long-term services and supports. Generations. 2011;35(1):64-68.
9. United States Census Bureau. Veteran status: 2010-2014 American community survey 5-year estimates. http://factfinder.census.gov/faces/tableservices/jsf/pages/productview.xhtml?pid=ACS_14_5YR_S2101&prodType=table. Accessed January 9, 2017.
According to the VA, 23% of veterans in the U.S., nearly 5.2 million individuals, live in rural areas.1 The VHA serves more than 3 million rural veterans, and 56% of those enrolled in the VA system are aged ≥ 65 years.1 Thus, aging veterans in rural areas constitute a substantial group who need support and assistance from the VA. Fortunately, the VA offers numerous benefits for veterans that support aging in place and improve quality of life through the VHA, Veterans Benefits Administration (VBA), and National Cemetery Administration (NCA).
Despite the opportunities, many VA benefits go unclaimed. In some cases, veterans simply do not know these benefits exist.2 In a 2010 VA report, only 41% of veterans indicated that they understood their benefits “a lot” or “some.”2 However, their understanding of specific benefits tended to be lower. For example, many veterans stated that they had “heard about” burial options at VA cemeteries (41.5%), but few understood specific benefits, such as cash burial allowances (10.6%).2
Many veterans also hold misperceptions about eligibility, which prevents them from applying. For example, some veterans believe that a high income or lack of combat service disqualifies them from receiving VA benefits.3 Some veterans believe that others are more deserving of VA services, and they don’t want to “take a spot someone else needs.”3 Finally, some veterans hold negative attitudes about the VA, making them less likely to claim VA benefits, such as health care.4
For rural veterans, accessing benefits can be especially difficult, because most VA facilities that offer assistance are in urban centers.5 Though online access to benefit information is improving through programs like My HealtheVet and public facing websites, some older adults do not use computers, and Internet and mobile phone connectivity are often limited in rural communities.6 Nearly 43% of rural veterans do not have broadband Internet in their homes.1 Moreover, the complexity of navigating benefits information via the Internet can be a frustrating and confusing process for older veterans.6
Accessing services and benefits in the community is similarly difficult. For more than 30 years, community organizations have noted the frustration that clients experienced with navigating a complex network of community providers who provide long-term services and supports (LTSS).7,8 In 2003, the Administration on Aging and the Centers for Medicare and Medicaid Services developed the Aging and Disability Resource Center (ADRC) program to promote a “no wrong door” approach for LTSS. Aging and Disability Resource Centers are a single point of entry into a network of community, state, and federal LTSS for older adults and individuals with disabilities.8 Options counselors at ADRCs provide information, counseling, and assistance with connecting to a vast network of programs such as Social Security, Medicaid, local transportation, Meals on Wheels, and housing assistance through a single office.
Backround
In 2012, VHA Office of Rural Health Resource (ORH) and Utah ADRC conducted a national survey of ADRC sites about their experiences working with veterans and found that 95% of ADRCs always or usually asked clients about their veteran status. The survey found that veteran clients present to ADRCs with diverse needs, many of which could be addressed through a VA benefit. However, the majority (58%) of ADRC respondents reported that they had never attempted to help a veteran apply for VA benefits (unpublished data, 2012).
Respondents reported a limited understanding of VA benefits, infrequent contact with VA, and frustrations with the VA system. Although familiar with several sources for information about VA benefits (eg, toll-free number, websites, local VA facilities, etc), respondents generally found these sources unhelpful and insufficient for answering their questions. The only positive anecdotal comments that respondents made regarding VA were from those with personal relationships with employees at the VA who could help with veteran needs. Finally, all respondents reported a need for more information about VA benefits and to assist them with helping veteran clients.
Survey Response
In 2013, the ORH and the VA Salt Lake City Geriatric Research Education and Clinical Center (GRECC), under the sponsorship of the VA Office of Geriatrics and Extended Care (GEC), developed a collaborative demonstration with the Utah ADRC to address the needs identified in this survey. Connecting Older Veterans (Especially Rural) to Community or Veteran Eligible Resources (COVER to COVER) is a demonstration project designed to create a new access point for VA benefits for veterans living in rural areas. The pilot had 2 aims: (1) train ADRC options counselors as Veteran Benefits Specialists (VBSs); and (2) build relationships between the ADRCs and VA to facilitate information and referral.
Between 2013 and 2015, the demonstration was housed at the VA Salt Lake City Health Care System GRECC as part of the clinical demonstration portfolio. The GRECC staff provided administrative support and mentorship for the developing partnerships. Subsequently, the demonstration was selected as a Promising Practice for enterprise-wide implementation. Both ORH and GEC coordinated opportunities for broad dissemination.
Program Elements
In Utah, 5 pilot ADRC agencies cover 19 counties, 14 of which are entirely rural. The remaining counties contain populations that are 20% to 49% urbanized (1 county), 50% to 80% urbanized (1 county), and 80% to 100% urbanized (3 counties). More than 95,000 veterans (12,857 in the 14 rural counties) live in the participating counties. The average income for veterans in all participating counties is $36,699 for men and $30,915 for women.9 Furthermore, about 53% of veterans in all these counties are aged > 65 years.9
For this pilot, each ADRC site assigned an existing options counselor as a dedicated VBS. Each VBS completed 80 to 100 hours of training in VA benefits. To facilitate the amount of training required to become experts, the ORH funded a portion of the salary for each VBS.
An outreach specialist at the VA Salt Lake City Regional Benefit Office, a geriatric social worker at the VA Salt Lake City Health Care System, and an outreach specialist at the Utah Department of Veterans and Military Affairs (UDVMA) were primary trainers for this pilot. Trainers provided 15 training sessions between February 2013 and September 2015, totaling 74 hours. The 5 designated VBSs attended all trainings, but meetings were opened to all ADRC staff and other community organizations; 115 individuals from Utah, Idaho, Nevada, New Mexico, and Wyoming attended at least 1 training. In the first year and a half, trainings ranged from 1.5 to 4.5 hours and provided a general overview of benefits. As the value of these trainings increased among the ADRCs and other community providers, longer seminars were offered, the longest lasting 2 days, which provided in-depth training.
Training topics comprised the following 4 general categories:
- Core—VA structure, military culture
- VHA—health care, enrollment and eligibility, in-home services
- VBA—pension, aid and attendance, disability compensation, nursing home, dependency and indemnity compensation
- NCA burial benefits
In response to participant requests for training on other VA benefits, additional VA staff presented topics such as mental health, homelessness, telehealth, Vet Centers, and My HealtheVet. Information on the Veterans Choice Program was incorporated into later trainings.
In addition to the training provided by COVER to COVER, the 5 ADRC VBSs completed the 25-hour Training Responsibility Involvement and Preparation of Claims (TRIP) online course. This coursework qualified them to take the examination to become certified veterans service officers.
With the information received in training, ADRC VBSs assist veteran clients and their families to learn about and apply for VA benefits. Veterans or family members contact the ADRC with a variety of needs, such as difficulty paying utilities, functional limitations, etc. All ADRC staff screen callers for veteran status and refer willing veterans or family members to the VBS who provides information about LTSS options and screens for eligibility for VA benefits.
Through these training events, VBSs also formed relationships with the VA trainers, resulting in the ability to refer to and coordinate with the VA on cases when needed. The VBSs often work closely with the UDVMA by helping veterans organize needed documents and coordinate with UDVMA staff to complete VA benefit claims. Furthermore, VBSs can help veterans navigate the VA system and advocate for their needs in coordination with the VA trainers.
The VBAs have described numerous positive outcomes from the COVER to COVER program. They universally report improved knowledge and confidence in assisting veteran clients. In many cases, simply identifying clients’ veteran status in the normal ADRC intake protocol has placed them in touch with many veterans without any significant change in their workload. One specialist reported that COVER to COVER has improved the quality of services she can provide to veterans and that connecting veteran clients to VA frees public resources for other clients in need. Finally, they report that the trainings introduced them to key VA contacts and laid the groundwork for developing relationships with new partners. The following case is representative of the types of client experiences VBSs routinely describe.
Case Study
“Larry,” a 94 year-old World War II veteran who had never applied for VA benefits, presented to a rural ADRC for assistance with paying his utility bills. Larry had numerous health issues, including early stage dementia. He relied on his 96-year-old wife, “Sandy,” to assist him with activities of daily living (ADLs) and instrumental activities of living (IADLs). However, Sandy also had health problems that limited her ability to help. The couple wanted to stay in their home but worried they could not do it without help.
An ADRC staff member referred Larry and Sandy to the VBS, who helped the couple enroll in a community LTSS program called Aging Alternatives for in-home services. During this time, Sandy passed away, but the VBS continued to work with Larry and helped him apply for VA disability compensation, enroll in VHA health care, and connect to VA’s Veteran Directed Home and Community Based Services (VDHCBS) program for in-home services.
Larry received a 70% service-connected disability rating and started receiving monthly compensation from the VA. Although Larry wants to stay at home, the rating of 70% service connection allows VA to cover nursing home placement should it be needed. He established a VA primary care physician and uses VDHCBS to purchase in-home services. Since Larry receives in-home services from the VHA, he was discharged from the Aging Alternatives program. This allowed the ADRC to reallocate this resource to another person in need. Larry is still living at home.
Future Directions
This case study highlights the benefits for veterans of COVER to COVER program through its emphasis on productive relationships between VA and community partners. More extensive data collection related to veteran outcomes is ongoing and will be essential for sustaining the program locally and to support broader dissemination to other states. Ideally, expansion to other sites will include temporary pilot funding to offset the time needed to gain the knowledgeand skills to become a VBS and to provide consultation and training to other ADRC staff. Once the pilot funding ends, the ADRC staff would have the necessary knowledge, skills, and relationships to continue providing services to veterans.
Acknowledgments
This project was supported by the VHA Office of Rural Health. The authors thank all ADRC, UDVMA, and VHA staff who participated in the project.
According to the VA, 23% of veterans in the U.S., nearly 5.2 million individuals, live in rural areas.1 The VHA serves more than 3 million rural veterans, and 56% of those enrolled in the VA system are aged ≥ 65 years.1 Thus, aging veterans in rural areas constitute a substantial group who need support and assistance from the VA. Fortunately, the VA offers numerous benefits for veterans that support aging in place and improve quality of life through the VHA, Veterans Benefits Administration (VBA), and National Cemetery Administration (NCA).
Despite the opportunities, many VA benefits go unclaimed. In some cases, veterans simply do not know these benefits exist.2 In a 2010 VA report, only 41% of veterans indicated that they understood their benefits “a lot” or “some.”2 However, their understanding of specific benefits tended to be lower. For example, many veterans stated that they had “heard about” burial options at VA cemeteries (41.5%), but few understood specific benefits, such as cash burial allowances (10.6%).2
Many veterans also hold misperceptions about eligibility, which prevents them from applying. For example, some veterans believe that a high income or lack of combat service disqualifies them from receiving VA benefits.3 Some veterans believe that others are more deserving of VA services, and they don’t want to “take a spot someone else needs.”3 Finally, some veterans hold negative attitudes about the VA, making them less likely to claim VA benefits, such as health care.4
For rural veterans, accessing benefits can be especially difficult, because most VA facilities that offer assistance are in urban centers.5 Though online access to benefit information is improving through programs like My HealtheVet and public facing websites, some older adults do not use computers, and Internet and mobile phone connectivity are often limited in rural communities.6 Nearly 43% of rural veterans do not have broadband Internet in their homes.1 Moreover, the complexity of navigating benefits information via the Internet can be a frustrating and confusing process for older veterans.6
Accessing services and benefits in the community is similarly difficult. For more than 30 years, community organizations have noted the frustration that clients experienced with navigating a complex network of community providers who provide long-term services and supports (LTSS).7,8 In 2003, the Administration on Aging and the Centers for Medicare and Medicaid Services developed the Aging and Disability Resource Center (ADRC) program to promote a “no wrong door” approach for LTSS. Aging and Disability Resource Centers are a single point of entry into a network of community, state, and federal LTSS for older adults and individuals with disabilities.8 Options counselors at ADRCs provide information, counseling, and assistance with connecting to a vast network of programs such as Social Security, Medicaid, local transportation, Meals on Wheels, and housing assistance through a single office.
Backround
In 2012, VHA Office of Rural Health Resource (ORH) and Utah ADRC conducted a national survey of ADRC sites about their experiences working with veterans and found that 95% of ADRCs always or usually asked clients about their veteran status. The survey found that veteran clients present to ADRCs with diverse needs, many of which could be addressed through a VA benefit. However, the majority (58%) of ADRC respondents reported that they had never attempted to help a veteran apply for VA benefits (unpublished data, 2012).
Respondents reported a limited understanding of VA benefits, infrequent contact with VA, and frustrations with the VA system. Although familiar with several sources for information about VA benefits (eg, toll-free number, websites, local VA facilities, etc), respondents generally found these sources unhelpful and insufficient for answering their questions. The only positive anecdotal comments that respondents made regarding VA were from those with personal relationships with employees at the VA who could help with veteran needs. Finally, all respondents reported a need for more information about VA benefits and to assist them with helping veteran clients.
Survey Response
In 2013, the ORH and the VA Salt Lake City Geriatric Research Education and Clinical Center (GRECC), under the sponsorship of the VA Office of Geriatrics and Extended Care (GEC), developed a collaborative demonstration with the Utah ADRC to address the needs identified in this survey. Connecting Older Veterans (Especially Rural) to Community or Veteran Eligible Resources (COVER to COVER) is a demonstration project designed to create a new access point for VA benefits for veterans living in rural areas. The pilot had 2 aims: (1) train ADRC options counselors as Veteran Benefits Specialists (VBSs); and (2) build relationships between the ADRCs and VA to facilitate information and referral.
Between 2013 and 2015, the demonstration was housed at the VA Salt Lake City Health Care System GRECC as part of the clinical demonstration portfolio. The GRECC staff provided administrative support and mentorship for the developing partnerships. Subsequently, the demonstration was selected as a Promising Practice for enterprise-wide implementation. Both ORH and GEC coordinated opportunities for broad dissemination.
Program Elements
In Utah, 5 pilot ADRC agencies cover 19 counties, 14 of which are entirely rural. The remaining counties contain populations that are 20% to 49% urbanized (1 county), 50% to 80% urbanized (1 county), and 80% to 100% urbanized (3 counties). More than 95,000 veterans (12,857 in the 14 rural counties) live in the participating counties. The average income for veterans in all participating counties is $36,699 for men and $30,915 for women.9 Furthermore, about 53% of veterans in all these counties are aged > 65 years.9
For this pilot, each ADRC site assigned an existing options counselor as a dedicated VBS. Each VBS completed 80 to 100 hours of training in VA benefits. To facilitate the amount of training required to become experts, the ORH funded a portion of the salary for each VBS.
An outreach specialist at the VA Salt Lake City Regional Benefit Office, a geriatric social worker at the VA Salt Lake City Health Care System, and an outreach specialist at the Utah Department of Veterans and Military Affairs (UDVMA) were primary trainers for this pilot. Trainers provided 15 training sessions between February 2013 and September 2015, totaling 74 hours. The 5 designated VBSs attended all trainings, but meetings were opened to all ADRC staff and other community organizations; 115 individuals from Utah, Idaho, Nevada, New Mexico, and Wyoming attended at least 1 training. In the first year and a half, trainings ranged from 1.5 to 4.5 hours and provided a general overview of benefits. As the value of these trainings increased among the ADRCs and other community providers, longer seminars were offered, the longest lasting 2 days, which provided in-depth training.
Training topics comprised the following 4 general categories:
- Core—VA structure, military culture
- VHA—health care, enrollment and eligibility, in-home services
- VBA—pension, aid and attendance, disability compensation, nursing home, dependency and indemnity compensation
- NCA burial benefits
In response to participant requests for training on other VA benefits, additional VA staff presented topics such as mental health, homelessness, telehealth, Vet Centers, and My HealtheVet. Information on the Veterans Choice Program was incorporated into later trainings.
In addition to the training provided by COVER to COVER, the 5 ADRC VBSs completed the 25-hour Training Responsibility Involvement and Preparation of Claims (TRIP) online course. This coursework qualified them to take the examination to become certified veterans service officers.
With the information received in training, ADRC VBSs assist veteran clients and their families to learn about and apply for VA benefits. Veterans or family members contact the ADRC with a variety of needs, such as difficulty paying utilities, functional limitations, etc. All ADRC staff screen callers for veteran status and refer willing veterans or family members to the VBS who provides information about LTSS options and screens for eligibility for VA benefits.
Through these training events, VBSs also formed relationships with the VA trainers, resulting in the ability to refer to and coordinate with the VA on cases when needed. The VBSs often work closely with the UDVMA by helping veterans organize needed documents and coordinate with UDVMA staff to complete VA benefit claims. Furthermore, VBSs can help veterans navigate the VA system and advocate for their needs in coordination with the VA trainers.
The VBAs have described numerous positive outcomes from the COVER to COVER program. They universally report improved knowledge and confidence in assisting veteran clients. In many cases, simply identifying clients’ veteran status in the normal ADRC intake protocol has placed them in touch with many veterans without any significant change in their workload. One specialist reported that COVER to COVER has improved the quality of services she can provide to veterans and that connecting veteran clients to VA frees public resources for other clients in need. Finally, they report that the trainings introduced them to key VA contacts and laid the groundwork for developing relationships with new partners. The following case is representative of the types of client experiences VBSs routinely describe.
Case Study
“Larry,” a 94 year-old World War II veteran who had never applied for VA benefits, presented to a rural ADRC for assistance with paying his utility bills. Larry had numerous health issues, including early stage dementia. He relied on his 96-year-old wife, “Sandy,” to assist him with activities of daily living (ADLs) and instrumental activities of living (IADLs). However, Sandy also had health problems that limited her ability to help. The couple wanted to stay in their home but worried they could not do it without help.
An ADRC staff member referred Larry and Sandy to the VBS, who helped the couple enroll in a community LTSS program called Aging Alternatives for in-home services. During this time, Sandy passed away, but the VBS continued to work with Larry and helped him apply for VA disability compensation, enroll in VHA health care, and connect to VA’s Veteran Directed Home and Community Based Services (VDHCBS) program for in-home services.
Larry received a 70% service-connected disability rating and started receiving monthly compensation from the VA. Although Larry wants to stay at home, the rating of 70% service connection allows VA to cover nursing home placement should it be needed. He established a VA primary care physician and uses VDHCBS to purchase in-home services. Since Larry receives in-home services from the VHA, he was discharged from the Aging Alternatives program. This allowed the ADRC to reallocate this resource to another person in need. Larry is still living at home.
Future Directions
This case study highlights the benefits for veterans of COVER to COVER program through its emphasis on productive relationships between VA and community partners. More extensive data collection related to veteran outcomes is ongoing and will be essential for sustaining the program locally and to support broader dissemination to other states. Ideally, expansion to other sites will include temporary pilot funding to offset the time needed to gain the knowledgeand skills to become a VBS and to provide consultation and training to other ADRC staff. Once the pilot funding ends, the ADRC staff would have the necessary knowledge, skills, and relationships to continue providing services to veterans.
Acknowledgments
This project was supported by the VHA Office of Rural Health. The authors thank all ADRC, UDVMA, and VHA staff who participated in the project.
1. VHA Office of Rural Health. Office of rural health annual report: thrive 2015. http://www.rural-health.va.gov/docs/ORH_Annual_Report_2015_FINAL.pdf. Published 2015. Accessed January 9, 2017.
2. National Center for Veterans Analysis and Statistics. 2010 national survey of veterans: understanding and knowledge of VA benefits and services. http://www.va.gov/VETDATA/docs/SpecialReports/2010NSV_Awareness_FINAL.pdf. Published November 2011. Accessed January 9, 2017.
3. Wittrock S, Ono S, Stewart K, Reisinger HS, Charlton M. Unclaimed health care benefits: a mixed-method analysis of rural veterans. J Rural Health. 2015;31(1):35-46.
4. Fox AB, Meyer EC, Vogt D. Attitudes about the VA health-care setting, mental illness, and mental health treatment and their relationship with VA mental health service use among female and male OEF/OIF veterans. Psychol Serv. 2015;12(1):49-58.
5. Helm MD. System worth saving report on rural healthcare 2012. http://archive.legion.org/handle/123456789/1951. Published 2012. Accessed January 9, 2017.
6. Lee B, Chen Y, Hewitt L. Age differences in constraints encountered by seniors in their use of computers and the Internet. Comput Human Behav. 2011;27(3):1231-1237.
7. Kane RL, Kane RA. A guide through the maze of long-term care. West J Med. 1981;135(6):503-510.
8. O’Shaughnessy CV. Aging and disability resource centers can help consumers navigate the maze of long-term services and supports. Generations. 2011;35(1):64-68.
9. United States Census Bureau. Veteran status: 2010-2014 American community survey 5-year estimates. http://factfinder.census.gov/faces/tableservices/jsf/pages/productview.xhtml?pid=ACS_14_5YR_S2101&prodType=table. Accessed January 9, 2017.
1. VHA Office of Rural Health. Office of rural health annual report: thrive 2015. http://www.rural-health.va.gov/docs/ORH_Annual_Report_2015_FINAL.pdf. Published 2015. Accessed January 9, 2017.
2. National Center for Veterans Analysis and Statistics. 2010 national survey of veterans: understanding and knowledge of VA benefits and services. http://www.va.gov/VETDATA/docs/SpecialReports/2010NSV_Awareness_FINAL.pdf. Published November 2011. Accessed January 9, 2017.
3. Wittrock S, Ono S, Stewart K, Reisinger HS, Charlton M. Unclaimed health care benefits: a mixed-method analysis of rural veterans. J Rural Health. 2015;31(1):35-46.
4. Fox AB, Meyer EC, Vogt D. Attitudes about the VA health-care setting, mental illness, and mental health treatment and their relationship with VA mental health service use among female and male OEF/OIF veterans. Psychol Serv. 2015;12(1):49-58.
5. Helm MD. System worth saving report on rural healthcare 2012. http://archive.legion.org/handle/123456789/1951. Published 2012. Accessed January 9, 2017.
6. Lee B, Chen Y, Hewitt L. Age differences in constraints encountered by seniors in their use of computers and the Internet. Comput Human Behav. 2011;27(3):1231-1237.
7. Kane RL, Kane RA. A guide through the maze of long-term care. West J Med. 1981;135(6):503-510.
8. O’Shaughnessy CV. Aging and disability resource centers can help consumers navigate the maze of long-term services and supports. Generations. 2011;35(1):64-68.
9. United States Census Bureau. Veteran status: 2010-2014 American community survey 5-year estimates. http://factfinder.census.gov/faces/tableservices/jsf/pages/productview.xhtml?pid=ACS_14_5YR_S2101&prodType=table. Accessed January 9, 2017.
Memory Skills Classes to Address Cognitive Concerns in Older Veterans With a History of Posttraumatic Stress Disorder
The Geriatric Research Education and Clinical Center (GRECC) Memory Disorders Clinic at the VA Puget Sound Health Care System (VAPSHCS) in Seattle, Washington, receives referrals from primary and specialty care. About a decade ago, this clinic began to see an influx of Vietnam-era veterans who presented with a variety of symptoms: not remembering where they were going when driving, forgetting why they went into another room, not remembering what their spouse told them, and feeling “out of it.” These symptoms were not associated with the loss of independence, but they were cause for concern. Family members and care providers typically corroborated the symptom description and perception of decline. Yet during workups, these veterans showed no primary medical causes for cognitive impairments and on neuropsychological evaluation demonstrated essentially normal cognition.
Memory Disorders Clinic staff largely were at a loss to know how to care for these patients. The simple reassurance, “You do not have dementia now,” seemed unsatisfactory given the patients’ ongoing concerns and the established risk factors for neurodegenerative disease.1,2 One theme emerged when talking with these veterans and their families: They all had a diagnosis of or history of treatment for posttraumatic stress disorder (PTSD).
To help these veterans, the VAPSHCS GRECC sought to address their key areas of concern related to memory. With input from veterans and their families, a quality improvement project was developed with the following goals: (1) to educate veterans and their families about PTSD and cognitive changes; (2) to build and field test a psychoeducational class to teach memory skills in this population; and (3) to inform VA staff about PTSD and cognitive change. In this article, the authors focus on how the first 2 goals were addressed and present preliminary results related to quality improvement.
Memory Skills Classes
Several strategies might promote memory skills, including printed materials for self-directed learning, individual sessions, interactive technologies, or groups. Given the patients’ reports about concentration problems, asking them to work through structured materials independently seemed unproductive. Individual clinical evaluations and cognitive interventions likely would not meet the demand or be cost-effective. Groups have long been used to treat PTSD, and Norrie and colleagues reported that at-risk adults benefited from a group psychoeducation program targeting healthy brain aging.3 At the same time, the Memory Disorders Clinic sought to distinguish itself from PTSD groups, because these groups tend to focus on treating active PTSD.
A better fit for this offering was the description of the sessions as classes. Although the focus was on promoting memory skills among those capable of learning them, the authors were mindful that some veterans might truly have prodromal dementia or acute PTSD symptoms that would require clinical management. The classes were not intended to address all these issues, and there was a plan to refer participants either before or during the class if warranted.
There was no formal evaluation of memory prior to starting the class. These classes were not developed as a research intervention and were exempt from institutional review board (IRB) approval requirements, according to prescreening by the VAPSHCS IRB and a memo from the GRECC director.
Core Components of Memory Skills
It may not be evident at first glance that PTSD or a history of PTSD influences memory. The symptom criteria for PTSD (involving reexperiencing, hyperarousal, and avoidance) might be described as “too much remembering” rather than forgetting. Yet problems with attention and concentration often occur in the setting of intrusive memories and alterations in reactivity. Research has found that older adults with PTSD have deficits of memory, especially new learning.
To appreciate these effects, it was important for participants in the memory skills classes to have some understanding of how memory works. The authors developed the Memory Model (Figure) as a visual aid and reference point to discuss the stages of new learning and how different aspects of brain activity are required for new learning and for memory to occur. This straightforward model is based on cognitive science and presented in layman’s terms. An important part of this model is the “filter” stage, which controls the information and stimuli that are available to the brain. Posttraumatic stress disorder involves involuntary emotional responses and efforts to avoid them and selects and colors the information that is processed in some situations (eg, avoidance of situations associated with trauma or dissociation of extreme memories). At other times, such as when a powerful stimulus is presented (eg, a helicopter flying close overhead), the filter may try to block out all inputs in order to preserve safety. The Memory Model also served as a visual aid during class discussions of normal cognitive aging.
Class sessions incorporated specific, measurable, attainable, realistic, and timely (SMART) goals, regular exercises based on mindfulness-based stress reduction approaches, and principles of behavioral activation.5 The SMART goals structure the sessions and permit customization of learning for participants. Class leaders record a goal for each participant and use these throughout the sessions to build rapport, develop communication, and teach memory skills.
Mindfulness-based stress reduction is an evidence-based treatment used in PTSD.6 It provides a counterpoint to the more didactic memory skills and is a method that even those with objective memory impairments can practice and apply successfully. Being in the current moment and emotional regulation are important skills to teach veterans as they learn to exert
Organization
Class sessions occurred weekly for 1 hour for a total of 8 sessions. The weekly class topics included introduction to memory; mood disorders, cognition, and cognitive disorders; barriers to effective memory: assessing readiness for change; developing a routine and becoming organized; attention and concentration; memory improvement (strategies internal and external aids); and reassessing goals.
Over the 3 years of classes reported in this article, the class sizes varied from 4 to 12 participants based on veteran interest, retention, and room size. The classes were structured so that important content areas were covered but with enough elasticity so leaders and veterans would develop a rapport and explore in greater depth the topics that resonated most for the attendees. Group participation was strongly encouraged. Veterans were expressly informed that the class was not for treatment of PTSD and that evidence-based therapies were encouraged to address PTSD especially if their symptoms flared up when compared with previous levels. The attendees also understood that they did not receive formal cognitive or memory testing but were encouraged to pursue testing if they showed significant deficits.
Preliminary Findings
From spring 2012 until spring 2015, 69 veterans agreed to participate and attended at least 1 memory skills class. Eighty-seven percent of participants (n = 60) attended 4 or more classes. The mean age (SD) was 67.3 years (4.2). All the participants were men, and the race/ethnic distribution was similar to that of the aging veteran population and very close to racial demographics for Washington state: 80% white, 14% African American, 2% Asian/Pacific Islander, 2% Native American, and 2% unknown.
Attendees were asked, but not required, to complete questionnaires before the classes began and again at completion. These questionnaires included self-assessments of cognitive strategies and compensatory methods used; an assessment of concern regarding cognition, life satisfaction, and community integration; the PTSD CheckList-Civilian Version (PCL-C); and the Geriatric Depression Scale (GDS).7,8 The questionnaire also included open response questions to providefeedback on what attendees liked about the classes and recommendations for improvements. The majority of comments for improvement focused on attendees’ desire for longer sessions and repeat offerings. Five veterans did not complete the full set of questionnaires at the beginning of the classes, and 7 did not complete the questionnaires at completion (the 2 subsets did not perfectly overlap).
At the start of the class, on average, veteran participants were experiencing mild depression and moderate symptoms of PTSD as measured by the GDS (n = 54) and the PCL-C (n = 56), respectively. Preliminary comparisons of ratings pre- and post-classes, using simple paired t tests, indicated a reduction in symptoms of depression on the GDS, improved sense of mastery over their memory symptoms, as well as improved quality of life ratings (all P < .01, no corrections). There was no evidence for a significant reduction in PTSD symptoms or report of elimination of cognitive difficulties. With the small sample and modest effects, the clinical significance of these scores cannot be determined. The authors are planning more detailed analyses on a larger set of participants, including measures of health care utilization before and after the class.
Future Directions
1. Chopra MP, Zhang H, Pless Kaiser A, et al. PTSD is a chronic, fluctuating disorder affecting the mental quality of life in older adults. Am J Geriatr Psychiatry. 2014;22(1):86-97.
2. Donovan NJ, Amariglio RE, Zoller AS, et al. Subjective cognitive concerns and neuropsychiatric predictors of progression to the early clinical stages of Alzheimer disease. Am J Geriatr Psychiatry. 2014;22(12):1642-1651.
3. Norrie LM, Diamond K, Hickie IB, Rogers NL, Fearns S, Naismith SL. Can older “at risk” adults benefit from psychoeducation targeting healthy brain aging? Int Psychogeriatr. 2011;23(3):413-424.
4. Hopko DR, Robertson SMC, Lejuez CW. Behavioral activation for anxiety disorders. Behav Anal Today. 2006;7(2):212-232.
5. Schuitevoerder S, Rosen JW, Twamley EW, et al. A meta-analysis of cognitive functioning in older adults with PTSD. J Anxiety Disord. 2013;27(6):550-558.
6. Polusny MA, Erbes CR, Thuras P, et al. Mindfulness-based stress reduction for posttraumatic stress disorder among veterans: a randomized clinical trial. JAMA. 2015;314(5):456-465.
7. Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982-1983;17(1):37-49.
8. Mental Illness Research, Education and Clinical Center. PTSD CheckList-Civilian Version (PCL-C). http://www.mirecc.va.gov/docs/visn6/3_PTSD _CheckList_and_Scoring.pdf Published December 2013. Accessed November 3, 2016.
9. Scott JC, Matt GE, Wrocklage KM, et al. A quantitative meta-analysis of neurocognitive functioning in posttraumatic stress disorder. Psychol Bull. 2015;141(1):105-140.
10. Wrocklage KM, Schweinsburg BC, Krystal JH, et al. Neuropsychological functioning in veterans with posttraumatic stress disorder: associations with performance validity, comorbidities, and functional outcomes. J Int Neuropsychol Soc. 2016;22(4):399-411.
11. Cook JM, O’Donnell C. Assessment and psychological treatment of posttraumatic stress disorder in older adults. J Geriatr Psychiatry Neurol. 2005;18(2):61-71.
12. Mota N, Tsai J, Kirwin PD, et al. Late-life exacerbation of PTSD symptoms in US veterans: results from the National Health and Resilience in Veterans Study. J Clin Psychiatry. 2016;77(3):348-354.
13. Yaffe K, Vittinghoff E, Lindquist K, et al. Posttraumatic stress disorder and risk of dementia among US veterans. Arch Gen Psychiatry. 2010;67(6):608-613.
The Geriatric Research Education and Clinical Center (GRECC) Memory Disorders Clinic at the VA Puget Sound Health Care System (VAPSHCS) in Seattle, Washington, receives referrals from primary and specialty care. About a decade ago, this clinic began to see an influx of Vietnam-era veterans who presented with a variety of symptoms: not remembering where they were going when driving, forgetting why they went into another room, not remembering what their spouse told them, and feeling “out of it.” These symptoms were not associated with the loss of independence, but they were cause for concern. Family members and care providers typically corroborated the symptom description and perception of decline. Yet during workups, these veterans showed no primary medical causes for cognitive impairments and on neuropsychological evaluation demonstrated essentially normal cognition.
Memory Disorders Clinic staff largely were at a loss to know how to care for these patients. The simple reassurance, “You do not have dementia now,” seemed unsatisfactory given the patients’ ongoing concerns and the established risk factors for neurodegenerative disease.1,2 One theme emerged when talking with these veterans and their families: They all had a diagnosis of or history of treatment for posttraumatic stress disorder (PTSD).
To help these veterans, the VAPSHCS GRECC sought to address their key areas of concern related to memory. With input from veterans and their families, a quality improvement project was developed with the following goals: (1) to educate veterans and their families about PTSD and cognitive changes; (2) to build and field test a psychoeducational class to teach memory skills in this population; and (3) to inform VA staff about PTSD and cognitive change. In this article, the authors focus on how the first 2 goals were addressed and present preliminary results related to quality improvement.
Memory Skills Classes
Several strategies might promote memory skills, including printed materials for self-directed learning, individual sessions, interactive technologies, or groups. Given the patients’ reports about concentration problems, asking them to work through structured materials independently seemed unproductive. Individual clinical evaluations and cognitive interventions likely would not meet the demand or be cost-effective. Groups have long been used to treat PTSD, and Norrie and colleagues reported that at-risk adults benefited from a group psychoeducation program targeting healthy brain aging.3 At the same time, the Memory Disorders Clinic sought to distinguish itself from PTSD groups, because these groups tend to focus on treating active PTSD.
A better fit for this offering was the description of the sessions as classes. Although the focus was on promoting memory skills among those capable of learning them, the authors were mindful that some veterans might truly have prodromal dementia or acute PTSD symptoms that would require clinical management. The classes were not intended to address all these issues, and there was a plan to refer participants either before or during the class if warranted.
There was no formal evaluation of memory prior to starting the class. These classes were not developed as a research intervention and were exempt from institutional review board (IRB) approval requirements, according to prescreening by the VAPSHCS IRB and a memo from the GRECC director.
Core Components of Memory Skills
It may not be evident at first glance that PTSD or a history of PTSD influences memory. The symptom criteria for PTSD (involving reexperiencing, hyperarousal, and avoidance) might be described as “too much remembering” rather than forgetting. Yet problems with attention and concentration often occur in the setting of intrusive memories and alterations in reactivity. Research has found that older adults with PTSD have deficits of memory, especially new learning.
To appreciate these effects, it was important for participants in the memory skills classes to have some understanding of how memory works. The authors developed the Memory Model (Figure) as a visual aid and reference point to discuss the stages of new learning and how different aspects of brain activity are required for new learning and for memory to occur. This straightforward model is based on cognitive science and presented in layman’s terms. An important part of this model is the “filter” stage, which controls the information and stimuli that are available to the brain. Posttraumatic stress disorder involves involuntary emotional responses and efforts to avoid them and selects and colors the information that is processed in some situations (eg, avoidance of situations associated with trauma or dissociation of extreme memories). At other times, such as when a powerful stimulus is presented (eg, a helicopter flying close overhead), the filter may try to block out all inputs in order to preserve safety. The Memory Model also served as a visual aid during class discussions of normal cognitive aging.
Class sessions incorporated specific, measurable, attainable, realistic, and timely (SMART) goals, regular exercises based on mindfulness-based stress reduction approaches, and principles of behavioral activation.5 The SMART goals structure the sessions and permit customization of learning for participants. Class leaders record a goal for each participant and use these throughout the sessions to build rapport, develop communication, and teach memory skills.
Mindfulness-based stress reduction is an evidence-based treatment used in PTSD.6 It provides a counterpoint to the more didactic memory skills and is a method that even those with objective memory impairments can practice and apply successfully. Being in the current moment and emotional regulation are important skills to teach veterans as they learn to exert
Organization
Class sessions occurred weekly for 1 hour for a total of 8 sessions. The weekly class topics included introduction to memory; mood disorders, cognition, and cognitive disorders; barriers to effective memory: assessing readiness for change; developing a routine and becoming organized; attention and concentration; memory improvement (strategies internal and external aids); and reassessing goals.
Over the 3 years of classes reported in this article, the class sizes varied from 4 to 12 participants based on veteran interest, retention, and room size. The classes were structured so that important content areas were covered but with enough elasticity so leaders and veterans would develop a rapport and explore in greater depth the topics that resonated most for the attendees. Group participation was strongly encouraged. Veterans were expressly informed that the class was not for treatment of PTSD and that evidence-based therapies were encouraged to address PTSD especially if their symptoms flared up when compared with previous levels. The attendees also understood that they did not receive formal cognitive or memory testing but were encouraged to pursue testing if they showed significant deficits.
Preliminary Findings
From spring 2012 until spring 2015, 69 veterans agreed to participate and attended at least 1 memory skills class. Eighty-seven percent of participants (n = 60) attended 4 or more classes. The mean age (SD) was 67.3 years (4.2). All the participants were men, and the race/ethnic distribution was similar to that of the aging veteran population and very close to racial demographics for Washington state: 80% white, 14% African American, 2% Asian/Pacific Islander, 2% Native American, and 2% unknown.
Attendees were asked, but not required, to complete questionnaires before the classes began and again at completion. These questionnaires included self-assessments of cognitive strategies and compensatory methods used; an assessment of concern regarding cognition, life satisfaction, and community integration; the PTSD CheckList-Civilian Version (PCL-C); and the Geriatric Depression Scale (GDS).7,8 The questionnaire also included open response questions to providefeedback on what attendees liked about the classes and recommendations for improvements. The majority of comments for improvement focused on attendees’ desire for longer sessions and repeat offerings. Five veterans did not complete the full set of questionnaires at the beginning of the classes, and 7 did not complete the questionnaires at completion (the 2 subsets did not perfectly overlap).
At the start of the class, on average, veteran participants were experiencing mild depression and moderate symptoms of PTSD as measured by the GDS (n = 54) and the PCL-C (n = 56), respectively. Preliminary comparisons of ratings pre- and post-classes, using simple paired t tests, indicated a reduction in symptoms of depression on the GDS, improved sense of mastery over their memory symptoms, as well as improved quality of life ratings (all P < .01, no corrections). There was no evidence for a significant reduction in PTSD symptoms or report of elimination of cognitive difficulties. With the small sample and modest effects, the clinical significance of these scores cannot be determined. The authors are planning more detailed analyses on a larger set of participants, including measures of health care utilization before and after the class.
Future Directions
The Geriatric Research Education and Clinical Center (GRECC) Memory Disorders Clinic at the VA Puget Sound Health Care System (VAPSHCS) in Seattle, Washington, receives referrals from primary and specialty care. About a decade ago, this clinic began to see an influx of Vietnam-era veterans who presented with a variety of symptoms: not remembering where they were going when driving, forgetting why they went into another room, not remembering what their spouse told them, and feeling “out of it.” These symptoms were not associated with the loss of independence, but they were cause for concern. Family members and care providers typically corroborated the symptom description and perception of decline. Yet during workups, these veterans showed no primary medical causes for cognitive impairments and on neuropsychological evaluation demonstrated essentially normal cognition.
Memory Disorders Clinic staff largely were at a loss to know how to care for these patients. The simple reassurance, “You do not have dementia now,” seemed unsatisfactory given the patients’ ongoing concerns and the established risk factors for neurodegenerative disease.1,2 One theme emerged when talking with these veterans and their families: They all had a diagnosis of or history of treatment for posttraumatic stress disorder (PTSD).
To help these veterans, the VAPSHCS GRECC sought to address their key areas of concern related to memory. With input from veterans and their families, a quality improvement project was developed with the following goals: (1) to educate veterans and their families about PTSD and cognitive changes; (2) to build and field test a psychoeducational class to teach memory skills in this population; and (3) to inform VA staff about PTSD and cognitive change. In this article, the authors focus on how the first 2 goals were addressed and present preliminary results related to quality improvement.
Memory Skills Classes
Several strategies might promote memory skills, including printed materials for self-directed learning, individual sessions, interactive technologies, or groups. Given the patients’ reports about concentration problems, asking them to work through structured materials independently seemed unproductive. Individual clinical evaluations and cognitive interventions likely would not meet the demand or be cost-effective. Groups have long been used to treat PTSD, and Norrie and colleagues reported that at-risk adults benefited from a group psychoeducation program targeting healthy brain aging.3 At the same time, the Memory Disorders Clinic sought to distinguish itself from PTSD groups, because these groups tend to focus on treating active PTSD.
A better fit for this offering was the description of the sessions as classes. Although the focus was on promoting memory skills among those capable of learning them, the authors were mindful that some veterans might truly have prodromal dementia or acute PTSD symptoms that would require clinical management. The classes were not intended to address all these issues, and there was a plan to refer participants either before or during the class if warranted.
There was no formal evaluation of memory prior to starting the class. These classes were not developed as a research intervention and were exempt from institutional review board (IRB) approval requirements, according to prescreening by the VAPSHCS IRB and a memo from the GRECC director.
Core Components of Memory Skills
It may not be evident at first glance that PTSD or a history of PTSD influences memory. The symptom criteria for PTSD (involving reexperiencing, hyperarousal, and avoidance) might be described as “too much remembering” rather than forgetting. Yet problems with attention and concentration often occur in the setting of intrusive memories and alterations in reactivity. Research has found that older adults with PTSD have deficits of memory, especially new learning.
To appreciate these effects, it was important for participants in the memory skills classes to have some understanding of how memory works. The authors developed the Memory Model (Figure) as a visual aid and reference point to discuss the stages of new learning and how different aspects of brain activity are required for new learning and for memory to occur. This straightforward model is based on cognitive science and presented in layman’s terms. An important part of this model is the “filter” stage, which controls the information and stimuli that are available to the brain. Posttraumatic stress disorder involves involuntary emotional responses and efforts to avoid them and selects and colors the information that is processed in some situations (eg, avoidance of situations associated with trauma or dissociation of extreme memories). At other times, such as when a powerful stimulus is presented (eg, a helicopter flying close overhead), the filter may try to block out all inputs in order to preserve safety. The Memory Model also served as a visual aid during class discussions of normal cognitive aging.
Class sessions incorporated specific, measurable, attainable, realistic, and timely (SMART) goals, regular exercises based on mindfulness-based stress reduction approaches, and principles of behavioral activation.5 The SMART goals structure the sessions and permit customization of learning for participants. Class leaders record a goal for each participant and use these throughout the sessions to build rapport, develop communication, and teach memory skills.
Mindfulness-based stress reduction is an evidence-based treatment used in PTSD.6 It provides a counterpoint to the more didactic memory skills and is a method that even those with objective memory impairments can practice and apply successfully. Being in the current moment and emotional regulation are important skills to teach veterans as they learn to exert
Organization
Class sessions occurred weekly for 1 hour for a total of 8 sessions. The weekly class topics included introduction to memory; mood disorders, cognition, and cognitive disorders; barriers to effective memory: assessing readiness for change; developing a routine and becoming organized; attention and concentration; memory improvement (strategies internal and external aids); and reassessing goals.
Over the 3 years of classes reported in this article, the class sizes varied from 4 to 12 participants based on veteran interest, retention, and room size. The classes were structured so that important content areas were covered but with enough elasticity so leaders and veterans would develop a rapport and explore in greater depth the topics that resonated most for the attendees. Group participation was strongly encouraged. Veterans were expressly informed that the class was not for treatment of PTSD and that evidence-based therapies were encouraged to address PTSD especially if their symptoms flared up when compared with previous levels. The attendees also understood that they did not receive formal cognitive or memory testing but were encouraged to pursue testing if they showed significant deficits.
Preliminary Findings
From spring 2012 until spring 2015, 69 veterans agreed to participate and attended at least 1 memory skills class. Eighty-seven percent of participants (n = 60) attended 4 or more classes. The mean age (SD) was 67.3 years (4.2). All the participants were men, and the race/ethnic distribution was similar to that of the aging veteran population and very close to racial demographics for Washington state: 80% white, 14% African American, 2% Asian/Pacific Islander, 2% Native American, and 2% unknown.
Attendees were asked, but not required, to complete questionnaires before the classes began and again at completion. These questionnaires included self-assessments of cognitive strategies and compensatory methods used; an assessment of concern regarding cognition, life satisfaction, and community integration; the PTSD CheckList-Civilian Version (PCL-C); and the Geriatric Depression Scale (GDS).7,8 The questionnaire also included open response questions to providefeedback on what attendees liked about the classes and recommendations for improvements. The majority of comments for improvement focused on attendees’ desire for longer sessions and repeat offerings. Five veterans did not complete the full set of questionnaires at the beginning of the classes, and 7 did not complete the questionnaires at completion (the 2 subsets did not perfectly overlap).
At the start of the class, on average, veteran participants were experiencing mild depression and moderate symptoms of PTSD as measured by the GDS (n = 54) and the PCL-C (n = 56), respectively. Preliminary comparisons of ratings pre- and post-classes, using simple paired t tests, indicated a reduction in symptoms of depression on the GDS, improved sense of mastery over their memory symptoms, as well as improved quality of life ratings (all P < .01, no corrections). There was no evidence for a significant reduction in PTSD symptoms or report of elimination of cognitive difficulties. With the small sample and modest effects, the clinical significance of these scores cannot be determined. The authors are planning more detailed analyses on a larger set of participants, including measures of health care utilization before and after the class.
Future Directions
1. Chopra MP, Zhang H, Pless Kaiser A, et al. PTSD is a chronic, fluctuating disorder affecting the mental quality of life in older adults. Am J Geriatr Psychiatry. 2014;22(1):86-97.
2. Donovan NJ, Amariglio RE, Zoller AS, et al. Subjective cognitive concerns and neuropsychiatric predictors of progression to the early clinical stages of Alzheimer disease. Am J Geriatr Psychiatry. 2014;22(12):1642-1651.
3. Norrie LM, Diamond K, Hickie IB, Rogers NL, Fearns S, Naismith SL. Can older “at risk” adults benefit from psychoeducation targeting healthy brain aging? Int Psychogeriatr. 2011;23(3):413-424.
4. Hopko DR, Robertson SMC, Lejuez CW. Behavioral activation for anxiety disorders. Behav Anal Today. 2006;7(2):212-232.
5. Schuitevoerder S, Rosen JW, Twamley EW, et al. A meta-analysis of cognitive functioning in older adults with PTSD. J Anxiety Disord. 2013;27(6):550-558.
6. Polusny MA, Erbes CR, Thuras P, et al. Mindfulness-based stress reduction for posttraumatic stress disorder among veterans: a randomized clinical trial. JAMA. 2015;314(5):456-465.
7. Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982-1983;17(1):37-49.
8. Mental Illness Research, Education and Clinical Center. PTSD CheckList-Civilian Version (PCL-C). http://www.mirecc.va.gov/docs/visn6/3_PTSD _CheckList_and_Scoring.pdf Published December 2013. Accessed November 3, 2016.
9. Scott JC, Matt GE, Wrocklage KM, et al. A quantitative meta-analysis of neurocognitive functioning in posttraumatic stress disorder. Psychol Bull. 2015;141(1):105-140.
10. Wrocklage KM, Schweinsburg BC, Krystal JH, et al. Neuropsychological functioning in veterans with posttraumatic stress disorder: associations with performance validity, comorbidities, and functional outcomes. J Int Neuropsychol Soc. 2016;22(4):399-411.
11. Cook JM, O’Donnell C. Assessment and psychological treatment of posttraumatic stress disorder in older adults. J Geriatr Psychiatry Neurol. 2005;18(2):61-71.
12. Mota N, Tsai J, Kirwin PD, et al. Late-life exacerbation of PTSD symptoms in US veterans: results from the National Health and Resilience in Veterans Study. J Clin Psychiatry. 2016;77(3):348-354.
13. Yaffe K, Vittinghoff E, Lindquist K, et al. Posttraumatic stress disorder and risk of dementia among US veterans. Arch Gen Psychiatry. 2010;67(6):608-613.
1. Chopra MP, Zhang H, Pless Kaiser A, et al. PTSD is a chronic, fluctuating disorder affecting the mental quality of life in older adults. Am J Geriatr Psychiatry. 2014;22(1):86-97.
2. Donovan NJ, Amariglio RE, Zoller AS, et al. Subjective cognitive concerns and neuropsychiatric predictors of progression to the early clinical stages of Alzheimer disease. Am J Geriatr Psychiatry. 2014;22(12):1642-1651.
3. Norrie LM, Diamond K, Hickie IB, Rogers NL, Fearns S, Naismith SL. Can older “at risk” adults benefit from psychoeducation targeting healthy brain aging? Int Psychogeriatr. 2011;23(3):413-424.
4. Hopko DR, Robertson SMC, Lejuez CW. Behavioral activation for anxiety disorders. Behav Anal Today. 2006;7(2):212-232.
5. Schuitevoerder S, Rosen JW, Twamley EW, et al. A meta-analysis of cognitive functioning in older adults with PTSD. J Anxiety Disord. 2013;27(6):550-558.
6. Polusny MA, Erbes CR, Thuras P, et al. Mindfulness-based stress reduction for posttraumatic stress disorder among veterans: a randomized clinical trial. JAMA. 2015;314(5):456-465.
7. Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982-1983;17(1):37-49.
8. Mental Illness Research, Education and Clinical Center. PTSD CheckList-Civilian Version (PCL-C). http://www.mirecc.va.gov/docs/visn6/3_PTSD _CheckList_and_Scoring.pdf Published December 2013. Accessed November 3, 2016.
9. Scott JC, Matt GE, Wrocklage KM, et al. A quantitative meta-analysis of neurocognitive functioning in posttraumatic stress disorder. Psychol Bull. 2015;141(1):105-140.
10. Wrocklage KM, Schweinsburg BC, Krystal JH, et al. Neuropsychological functioning in veterans with posttraumatic stress disorder: associations with performance validity, comorbidities, and functional outcomes. J Int Neuropsychol Soc. 2016;22(4):399-411.
11. Cook JM, O’Donnell C. Assessment and psychological treatment of posttraumatic stress disorder in older adults. J Geriatr Psychiatry Neurol. 2005;18(2):61-71.
12. Mota N, Tsai J, Kirwin PD, et al. Late-life exacerbation of PTSD symptoms in US veterans: results from the National Health and Resilience in Veterans Study. J Clin Psychiatry. 2016;77(3):348-354.
13. Yaffe K, Vittinghoff E, Lindquist K, et al. Posttraumatic stress disorder and risk of dementia among US veterans. Arch Gen Psychiatry. 2010;67(6):608-613.
Integrating Palliative Care and Oncology Care for Veterans With Cancer
Purpose: This literature review was performed to better understand how to facilitate the integration of palliative care and oncology care at the onset of treatment for patients at a VAMC facility.
Background: Palliative care is defined by the World Health Organization (WHO) as an approach to care aimed at improving the quality of life of patients and their families who are facing obstacles associated with a serious or terminal illness (WHO). The intention of palliative care is not curative; it does however address the prevention, early identification and treatment of pain, and problems which may be physical, psychosocial and spiritual. Palliative care should be integrated early in the course of the disease and in conjunction with life-saving treatments (Greer et al, 2013).
Methods: A search of databases included Google Scholar, PubMed, Ovid, and CINAHL identified randomized clinical trials, systematic reviews and expert reviews regarding the integration of palliative care at the initial diagnosis and when cancer treatment begins.
Results: Several interventions for integration of palliative care with standard cancer were identified as well as increase patient outcomes. The National Comprehensive Care Network,(NCCN), the American Society of Clinical Oncology, (ASCO), and the Institute of Medicine (IOM), all advocate for the initiation of palliative care at the onset of treatment. Guidelines for screening, assessments, and interventions are available to begin the process of integration. There are several barriers, however, affecting the integration of palliative care with comprehensive oncology care. These barriers include inadequate staffing, inadequate training, standardized assessment and screening tools. The process also requires the cooperation and support of facility leadership and administration.
Implications: There is strong evidence for the integration of palliative and oncology care for Veterans receiving cancer care at VAMC facilities. Oncology would continue to focus on the treatment of disease with the primary assessment of pain, other symptom management, and goals of care. Palliative care would be able to assist the patient with the more complicated symptomatology, psychosocial support, advance care planning and easier transitions into hospice care. Each discipline has a role in the improved outcomes and improved quality of life for patients.
Purpose: This literature review was performed to better understand how to facilitate the integration of palliative care and oncology care at the onset of treatment for patients at a VAMC facility.
Background: Palliative care is defined by the World Health Organization (WHO) as an approach to care aimed at improving the quality of life of patients and their families who are facing obstacles associated with a serious or terminal illness (WHO). The intention of palliative care is not curative; it does however address the prevention, early identification and treatment of pain, and problems which may be physical, psychosocial and spiritual. Palliative care should be integrated early in the course of the disease and in conjunction with life-saving treatments (Greer et al, 2013).
Methods: A search of databases included Google Scholar, PubMed, Ovid, and CINAHL identified randomized clinical trials, systematic reviews and expert reviews regarding the integration of palliative care at the initial diagnosis and when cancer treatment begins.
Results: Several interventions for integration of palliative care with standard cancer were identified as well as increase patient outcomes. The National Comprehensive Care Network,(NCCN), the American Society of Clinical Oncology, (ASCO), and the Institute of Medicine (IOM), all advocate for the initiation of palliative care at the onset of treatment. Guidelines for screening, assessments, and interventions are available to begin the process of integration. There are several barriers, however, affecting the integration of palliative care with comprehensive oncology care. These barriers include inadequate staffing, inadequate training, standardized assessment and screening tools. The process also requires the cooperation and support of facility leadership and administration.
Implications: There is strong evidence for the integration of palliative and oncology care for Veterans receiving cancer care at VAMC facilities. Oncology would continue to focus on the treatment of disease with the primary assessment of pain, other symptom management, and goals of care. Palliative care would be able to assist the patient with the more complicated symptomatology, psychosocial support, advance care planning and easier transitions into hospice care. Each discipline has a role in the improved outcomes and improved quality of life for patients.
Purpose: This literature review was performed to better understand how to facilitate the integration of palliative care and oncology care at the onset of treatment for patients at a VAMC facility.
Background: Palliative care is defined by the World Health Organization (WHO) as an approach to care aimed at improving the quality of life of patients and their families who are facing obstacles associated with a serious or terminal illness (WHO). The intention of palliative care is not curative; it does however address the prevention, early identification and treatment of pain, and problems which may be physical, psychosocial and spiritual. Palliative care should be integrated early in the course of the disease and in conjunction with life-saving treatments (Greer et al, 2013).
Methods: A search of databases included Google Scholar, PubMed, Ovid, and CINAHL identified randomized clinical trials, systematic reviews and expert reviews regarding the integration of palliative care at the initial diagnosis and when cancer treatment begins.
Results: Several interventions for integration of palliative care with standard cancer were identified as well as increase patient outcomes. The National Comprehensive Care Network,(NCCN), the American Society of Clinical Oncology, (ASCO), and the Institute of Medicine (IOM), all advocate for the initiation of palliative care at the onset of treatment. Guidelines for screening, assessments, and interventions are available to begin the process of integration. There are several barriers, however, affecting the integration of palliative care with comprehensive oncology care. These barriers include inadequate staffing, inadequate training, standardized assessment and screening tools. The process also requires the cooperation and support of facility leadership and administration.
Implications: There is strong evidence for the integration of palliative and oncology care for Veterans receiving cancer care at VAMC facilities. Oncology would continue to focus on the treatment of disease with the primary assessment of pain, other symptom management, and goals of care. Palliative care would be able to assist the patient with the more complicated symptomatology, psychosocial support, advance care planning and easier transitions into hospice care. Each discipline has a role in the improved outcomes and improved quality of life for patients.
Advance care planning discussions: Talk is no longer cheap
Clinicians outside of the surgical specialties may consider surgeons primarily providers of technical services, but those of us who provide surgical care fully appreciate that communicating with patients and families is a large component of routine surgical practice. Typical communications in surgical practice include obtaining a history of present illness, which is a key element in the ultimate decision to offer a surgical intervention, or not; discussing the risks, benefits, and alternatives of any operation being considered; and the numerous discussions held following any surgical procedure. What many surgeons may not fully appreciate, however, is how these routine communication events can fall under the general category of advance care planning (ACP).
ACP is defined as a process in which physicians (and other health care providers) discuss a patient’s goals, values, and beliefs and determine how these inform a patient’s desire for current or future medical care. Hickman et al. (Hastings Center Report Special Report 35, no. 6 (2005):S26-S30) note that ACP should focus on defining “good” care for each patient. Furthermore, changes in a patient’s medical condition represent an opportune time to revisit a patient’s hopes and goals. Consideration of surgical intervention often represents a major change in a patient’s medical condition and therefore is an excellent opportunity to engage a patient in an ACP discussion.
Given that ACP discussions are likely occurring in surgical practices on a regular basis, surgeons need to be aware of a recent change in the Physician Fee Schedule that took effect Jan. 1, 2016. Effective this date, Current Procedural Terminology (CPT) codes 99497 and 99498 now allow for billing for ACP services. CPT code 99497 includes ACP “including the explanation and discussion of advance directives such as standard forms (with the completion of such forms, when performed), by the physician or other qualified health care professional; first 30 minutes, face-to-face with patient, family member(s) and/or surrogate.” CPT code 99498 is used for each additional 30 minutes spent in such face-to-face ACP counseling.
The nuts and bolts of how these ACP CPT codes work:
How many times can these code(s) be used? There are no limits on the number of times ACP can be reported for a given beneficiary in a given time period. For example, if an ACP discussion was held with a patient and/or family member and/or surrogate prior to a major elective procedure and again in the postoperative period, the above CPT codes could be used twice. In each instance, the ACP discussion must be documented, along with any relevant change in the patient’s clinical status that prompted another ACP discussion.
Can a patient or their family member/surrogate refuse ACP services? ACP services are voluntary; therefore, a patient or their family member/surrogate can refuse ACP services. These CPT codes only can be used if a patient or family member/surrogate consents for ACP services.
What must be documented in ACP services? Physicians should consult their Medicare Administrative Contractors for documentation requirements. Examples of elements to be included in the documentation are a brief description of the discussion with the patient or family/surrogate regarding the voluntary nature of ACP services, an explanation of advance directives and documentation if an advance directive is completed, who was present during the discussion, and time spent in the face-to-face encounter.
Does an advance directive have to be completed to bill the service? No. If an advance directive is completed, this should be documented (see above), but completion of the directive is not a requirement for billing the service.
Can ACP be reported in addition to an evaluation and management (E/M) service (such as an office visit)? Yes. CPT codes 99497 and 99498 may be billed on the same day or a different day as most other E/M services. They may be billed within the global surgical period.
Is a specific diagnosis required to use the ACP CPT codes? No, a specific diagnosis is not required for the ACP codes to be billed.
According to the 2016 Medicare Physician Fee Schedule, the reimbursement is $85.99 for CPT 99497 and $74.88 for CPT 99498. For comparison, the reimbursement for E/M CPT 99203 (30-minute initial evaluation) = $108.85, CPT 99204 (45-minute initial evaluation) = $166.13, and CPT 99205 (60-minute initial evaluation) = $208.38. Far more important than the financial remuneration for these discussions, however, is the critical need for surgeons to have and document their ACP discussions with their patients and/or their family member/surrogate. As surgeons, we are often called to see patients when they are facing a significant change in their health – whether that is a new diagnosis of cancer or after a traumatic injury. Understanding a patient’s values, hopes, and concerns is an essential component to ensuring that our patients receive the best care, as defined by them.
Dr. Fahy is associate professor of surgery and internal medicine at the University of New Mexico, Albuquerque. She is a surgical oncologist who is also board certified in hospice and palliative medicine. Dr. Fahy does not have any relevant conflicts of interest to disclose.
Clinicians outside of the surgical specialties may consider surgeons primarily providers of technical services, but those of us who provide surgical care fully appreciate that communicating with patients and families is a large component of routine surgical practice. Typical communications in surgical practice include obtaining a history of present illness, which is a key element in the ultimate decision to offer a surgical intervention, or not; discussing the risks, benefits, and alternatives of any operation being considered; and the numerous discussions held following any surgical procedure. What many surgeons may not fully appreciate, however, is how these routine communication events can fall under the general category of advance care planning (ACP).
ACP is defined as a process in which physicians (and other health care providers) discuss a patient’s goals, values, and beliefs and determine how these inform a patient’s desire for current or future medical care. Hickman et al. (Hastings Center Report Special Report 35, no. 6 (2005):S26-S30) note that ACP should focus on defining “good” care for each patient. Furthermore, changes in a patient’s medical condition represent an opportune time to revisit a patient’s hopes and goals. Consideration of surgical intervention often represents a major change in a patient’s medical condition and therefore is an excellent opportunity to engage a patient in an ACP discussion.
Given that ACP discussions are likely occurring in surgical practices on a regular basis, surgeons need to be aware of a recent change in the Physician Fee Schedule that took effect Jan. 1, 2016. Effective this date, Current Procedural Terminology (CPT) codes 99497 and 99498 now allow for billing for ACP services. CPT code 99497 includes ACP “including the explanation and discussion of advance directives such as standard forms (with the completion of such forms, when performed), by the physician or other qualified health care professional; first 30 minutes, face-to-face with patient, family member(s) and/or surrogate.” CPT code 99498 is used for each additional 30 minutes spent in such face-to-face ACP counseling.
The nuts and bolts of how these ACP CPT codes work:
How many times can these code(s) be used? There are no limits on the number of times ACP can be reported for a given beneficiary in a given time period. For example, if an ACP discussion was held with a patient and/or family member and/or surrogate prior to a major elective procedure and again in the postoperative period, the above CPT codes could be used twice. In each instance, the ACP discussion must be documented, along with any relevant change in the patient’s clinical status that prompted another ACP discussion.
Can a patient or their family member/surrogate refuse ACP services? ACP services are voluntary; therefore, a patient or their family member/surrogate can refuse ACP services. These CPT codes only can be used if a patient or family member/surrogate consents for ACP services.
What must be documented in ACP services? Physicians should consult their Medicare Administrative Contractors for documentation requirements. Examples of elements to be included in the documentation are a brief description of the discussion with the patient or family/surrogate regarding the voluntary nature of ACP services, an explanation of advance directives and documentation if an advance directive is completed, who was present during the discussion, and time spent in the face-to-face encounter.
Does an advance directive have to be completed to bill the service? No. If an advance directive is completed, this should be documented (see above), but completion of the directive is not a requirement for billing the service.
Can ACP be reported in addition to an evaluation and management (E/M) service (such as an office visit)? Yes. CPT codes 99497 and 99498 may be billed on the same day or a different day as most other E/M services. They may be billed within the global surgical period.
Is a specific diagnosis required to use the ACP CPT codes? No, a specific diagnosis is not required for the ACP codes to be billed.
According to the 2016 Medicare Physician Fee Schedule, the reimbursement is $85.99 for CPT 99497 and $74.88 for CPT 99498. For comparison, the reimbursement for E/M CPT 99203 (30-minute initial evaluation) = $108.85, CPT 99204 (45-minute initial evaluation) = $166.13, and CPT 99205 (60-minute initial evaluation) = $208.38. Far more important than the financial remuneration for these discussions, however, is the critical need for surgeons to have and document their ACP discussions with their patients and/or their family member/surrogate. As surgeons, we are often called to see patients when they are facing a significant change in their health – whether that is a new diagnosis of cancer or after a traumatic injury. Understanding a patient’s values, hopes, and concerns is an essential component to ensuring that our patients receive the best care, as defined by them.
Dr. Fahy is associate professor of surgery and internal medicine at the University of New Mexico, Albuquerque. She is a surgical oncologist who is also board certified in hospice and palliative medicine. Dr. Fahy does not have any relevant conflicts of interest to disclose.
Clinicians outside of the surgical specialties may consider surgeons primarily providers of technical services, but those of us who provide surgical care fully appreciate that communicating with patients and families is a large component of routine surgical practice. Typical communications in surgical practice include obtaining a history of present illness, which is a key element in the ultimate decision to offer a surgical intervention, or not; discussing the risks, benefits, and alternatives of any operation being considered; and the numerous discussions held following any surgical procedure. What many surgeons may not fully appreciate, however, is how these routine communication events can fall under the general category of advance care planning (ACP).
ACP is defined as a process in which physicians (and other health care providers) discuss a patient’s goals, values, and beliefs and determine how these inform a patient’s desire for current or future medical care. Hickman et al. (Hastings Center Report Special Report 35, no. 6 (2005):S26-S30) note that ACP should focus on defining “good” care for each patient. Furthermore, changes in a patient’s medical condition represent an opportune time to revisit a patient’s hopes and goals. Consideration of surgical intervention often represents a major change in a patient’s medical condition and therefore is an excellent opportunity to engage a patient in an ACP discussion.
Given that ACP discussions are likely occurring in surgical practices on a regular basis, surgeons need to be aware of a recent change in the Physician Fee Schedule that took effect Jan. 1, 2016. Effective this date, Current Procedural Terminology (CPT) codes 99497 and 99498 now allow for billing for ACP services. CPT code 99497 includes ACP “including the explanation and discussion of advance directives such as standard forms (with the completion of such forms, when performed), by the physician or other qualified health care professional; first 30 minutes, face-to-face with patient, family member(s) and/or surrogate.” CPT code 99498 is used for each additional 30 minutes spent in such face-to-face ACP counseling.
The nuts and bolts of how these ACP CPT codes work:
How many times can these code(s) be used? There are no limits on the number of times ACP can be reported for a given beneficiary in a given time period. For example, if an ACP discussion was held with a patient and/or family member and/or surrogate prior to a major elective procedure and again in the postoperative period, the above CPT codes could be used twice. In each instance, the ACP discussion must be documented, along with any relevant change in the patient’s clinical status that prompted another ACP discussion.
Can a patient or their family member/surrogate refuse ACP services? ACP services are voluntary; therefore, a patient or their family member/surrogate can refuse ACP services. These CPT codes only can be used if a patient or family member/surrogate consents for ACP services.
What must be documented in ACP services? Physicians should consult their Medicare Administrative Contractors for documentation requirements. Examples of elements to be included in the documentation are a brief description of the discussion with the patient or family/surrogate regarding the voluntary nature of ACP services, an explanation of advance directives and documentation if an advance directive is completed, who was present during the discussion, and time spent in the face-to-face encounter.
Does an advance directive have to be completed to bill the service? No. If an advance directive is completed, this should be documented (see above), but completion of the directive is not a requirement for billing the service.
Can ACP be reported in addition to an evaluation and management (E/M) service (such as an office visit)? Yes. CPT codes 99497 and 99498 may be billed on the same day or a different day as most other E/M services. They may be billed within the global surgical period.
Is a specific diagnosis required to use the ACP CPT codes? No, a specific diagnosis is not required for the ACP codes to be billed.
According to the 2016 Medicare Physician Fee Schedule, the reimbursement is $85.99 for CPT 99497 and $74.88 for CPT 99498. For comparison, the reimbursement for E/M CPT 99203 (30-minute initial evaluation) = $108.85, CPT 99204 (45-minute initial evaluation) = $166.13, and CPT 99205 (60-minute initial evaluation) = $208.38. Far more important than the financial remuneration for these discussions, however, is the critical need for surgeons to have and document their ACP discussions with their patients and/or their family member/surrogate. As surgeons, we are often called to see patients when they are facing a significant change in their health – whether that is a new diagnosis of cancer or after a traumatic injury. Understanding a patient’s values, hopes, and concerns is an essential component to ensuring that our patients receive the best care, as defined by them.
Dr. Fahy is associate professor of surgery and internal medicine at the University of New Mexico, Albuquerque. She is a surgical oncologist who is also board certified in hospice and palliative medicine. Dr. Fahy does not have any relevant conflicts of interest to disclose.