User login
Initiative to Minimize Pharmaceutical Risk in Older Veterans (IMPROVE) Polypharmacy Clinic
An interprofessional polypharmacy clinic for intensive management of medication regimens helps high-risk patients manage their medications.
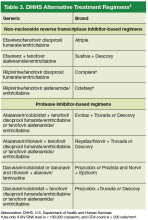
In 2011, 5 VA medical centers (VAMCs) were selected by the Office of Academic Affiliations (OAA) to establish CoEPCE. Part of the VA New Models of Care initiative, the 5 Centers of Excellence (CoE) in Boise, Idaho; Cleveland, Ohio; San Francisco, California; Seattle, Washington; and West Haven, Connecticut, are utilizing VA primary care settings to develop and test innovative approaches to prepare physician residents and students, advanced practice nurse residents and undergraduate nursing students, and other professions of health trainees (eg, pharmacy, social work, psychology, physician assistants [PAs], physical therapists) for primary care practice in the 21st century. The CoEs are developing, implementing, and evaluating curricula designed to prepare learners from relevant professions to practice in patient-centered, interprofessional team-based primary care settings. The curricula at all CoEs must address 4 core domains (Table).
Health care professional education programs do not have many opportunities for workplace learning where trainees from different professions can learn and work together to provide care to patients in real time.
The VA Connecticut Healthcare System CoEPCE developed and implemented an education and practice-based immersion learning model with physician residents, nurse practitioner (NP) residents and NP students, pharmacy residents, postdoctorate psychology learners, and PA and physical therapy learners and faculty. This interprofessional, collaborative team model breaks from the traditional independent model of siloed primary care providers (PCPs) caring for a panel of patients.
Methods
In 2015, OAA evaluators reviewed background documents and conducted open-ended interviews with 12 West Haven CoEPCE staff, participating trainees, VA faculty, VA facility leadership, and affiliate faculty. Informants described their involvement, challenges encountered, and benefits of the Initiative to Minimize Pharmaceutical Risk in Older Veterans (IMPROVE) program to trainees, veterans, and the VA.
Lack of Clinical Approaches to Interprofessional Education and Care
Polypharmacy is a common problem among older adults with multiple chronic conditions, which places patients at higher risk for multiple negative health outcomes.2,3 The typical primary care visit rarely allows for a thorough review of a patient’s medications, much less the identification of strategies to reduce polypharmacy and improve medication management. Rather, the complexity inherent to polypharmacy makes it an ideal challenge for a team-based approach.
Team Approach to Medication Needs
A key CoEPCE program aim is to expand workplace learning instruction strategies and to create more clinical opportunities for CoEPCE trainees to work together as a team to anticipate and address the health care needs of veterans. To address this training need, the West Haven CoEPCE developed IMPROVE to focus on high-need patients and provides a venue in which trainees and supervisors from different professions can collaborate on a specific patient case, using a patient-centered framework. IMPROVE can be easily applied to a range of medication-related aims, such as reducing medications, managing medications and adherence, and addressing adverse effects (AEs). These goals are 2-fold: (1) implement a trainee-led performance improvement project that reduces polypharmacy in elderly veterans; and (2) develop a hands-on, experiential geriatrics training program that enhances trainee skills and knowledge related to safe prescribing.
Planning and Implementation
IMPROVE has its origins in a scholarly project developed by a West Haven CoE physician resident trainee. Development of the IMPROVE program involved VA health psychology, internal medicine faculty, geriatric medicine faculty, NP faculty, and geriatric pharmacy residents and faculty. Planning started in 2013 with a series of pilot clinics and became an official project of the West Haven CoE in September 2014. The intervention required no change in West Haven VAMC policy. However, the initiative required buy-in from West Haven CoE leadership and the director of the West Haven primary care clinic.
Curriculum
IMPROVE is an educational, workplace learning, and clinical activity that combines a 1-hour trainee teaching session, a 45-minute group visit, and a 60-minute individual clinic visit to address the complex problem of polypharmacy. It emphasizes the sharing of trainee and faculty backgrounds by serving as a venue for interprofessional trainees and providers to discuss pharmacologic and nonpharmacologic treatment in the elderly and brainstorm strategies to optimize treatment regimens, minimize risk, and execute medication plans with patients.
All CoEPCE trainees in West Haven are required to participate in IMPROVE and on average, each trainee presents and sees one of their patients at least 3 times per year in the program. Up to 5 trainees participate in each IMPROVE session. Trainees are responsible for reviewing their panels to identify patients who might benefit from participation, followed by inviting the patient to participate. Patients are instructed to bring their pill bottles to the visit. To prepare for the polypharmacy clinic, the trainees, the geriatrician, and the geriatric pharmacist perform an extensive medication chart review, using the medication review worksheet developed by West Haven VAMC providers.4 They also work with a protocol for medication discontinuation, which was compiled by West Haven VAMC clinicians. The teams use a variety of tools that guide appropriate prescribing in older adult populations.5,6 During a preclinic conference, trainees present their patients to the interprofessional team for discussion and participate in a short discussion led by a pharmacist, geriatrician, or health psychologist on a topic related to prescribing safety in older adults or nonpharmacologic treatments.
IMPROVE emphasizes a patient-centered approach to develop, execute, and monitor medication plans. Patients and their family members are invited by their trainee clinician to participate in a group visit. Typically, trainees invite patients aged ≥ 65 years who have ≥ 10 medications and are considered appropriate for a group visit.
The group visit process is based on health psychology strategies, which often incorporate group-based engagement with patients. The health psychologist can give advice to facilitate the visit and optimize participant involvement. There is a discussion facilitator guide that lists the education points to be covered by a designated trainee facilitator and sample questions to guide the discussion.7 A health psychology resident and other rotating trainees cofacilitate the group visit with a goal to reach out to each group member, including family members, and have them discuss perceptions and share concerns and treatment goals. There is shared responsibility among the trainees to address the educational material as well as involve their respective patients during the sessions.
Immediately following the group visit, trainees conduct a 1-hour clinic session that includes medication reconciliation, a review of an IMPROVE questionnaire, orthostatic vital signs, and the St. Louis University Mental Status (SLUMS) exam to assess changes in cognition.7,8 Discussion involved the patient’s medication list as well as possible changes that could be made to the list. Using shared decision-making techniques, this conversation considers the patients’ treatment goals, feelings about the medications, which medications they would like to stop, and AEs they may be experiencing. After the individual visit is completed, the trainee participates in a 10-minute interprofessional precepting session, which may include a geriatrician, a pharmacist, and a health psychologist. In the session they may discuss adjustments to medications and a safe follow-up plan, including appropriate referrals. Trainees discuss the plan with the patient and send a letter describing the plan shortly after the visit.
IMPROVE combines didactic teaching with experiential education. It embodies the 4 core domains that shape the CoEPCE curriculum. First, trainees learn interprofessional collaboration concepts, including highlighting the roles of each profession and working with an interprofessional team to solve problems. Second, CoEPCE trainees learn performance improvement under the supervision of faculty. Third, IMPROVE allows trainees to develop sustained relationships with other team members while improving the quality of the clinic experience as well as with patients through increased continuity of care. Trainees see patients on their panel and are responsible for outreach before and after the visit. Finally, with a focus on personalized patient goals, trainees have the opportunity to further develop skills in shared decision making (SDM).
Related: Reducing Benzodiazepine Prescribing in Older Veterans: A Direct-to-Consumer Educational Brochure
The IMPROVE model continues to evolve. The original curriculum involved an hour-long preclinic preparation session before the group visit in which trainees and faculty discussed the medication review for each patient scheduled that day. This preparation session was later shortened to 40 minutes, and a 20-minute didactic component was added to create the current preclinic session. The didactic component focused on a specific topic in appropriate prescribing for older patients. For example, one didactic lesson is on a particular class of medications, its common AEs, and practical prescribing and “deprescribing” strategies for that class. Initially, the oldest patients or patients who could be grouped thematically, such as those taking both narcotics and benzodiazepines, were invited to participate, but that limited the number of appropriate patients within the CoEPCE. Currently, trainees identify patients from their panels who might benefit, based on age, number of medications, or potential medication-related concerns, such as falls, cognitive impairment, or other concerns for adverse drug effects. These trainees have the unique opportunity to apply learned strategies to their patients to continue to optimize the medication regimen even after the IMPROVE visit. Another significant change was the inclusion of veterans who are comanaged with PCPs outside the VA, because we found that patients with multiple providers could benefit from improved coordination of care.
Faculty Role
CoE faculty and non-CoE VA faculty participate in supervisory, consulting, teaching and precepting roles. Some faculty members such as the health psychologists are already located in or near the VA primary care clinic, so they can assist in curriculum development and execution during their regular clinic duties. The geriatrician reviews the patients’ health records before the patients come into the clinic, participates in the group visit, and coprecepts during the 1:1 patient visits. Collaboration is inherent in IMPROVE. For example, the geriatrician works with the geriatric pharmacist to identify and teach an educational topic. IMPROVE is characterized by a strong faculty/trainee partnership, with trainees playing roles as both teacher and facilitator in addition to learning how to take a team approach to polypharmacy.
Resources
IMPROVE requires administrative and academic support, especially faculty and trainee preparation of education sessions. The CoEPCE internal medicine resident and the internal medicine chief resident work with the health technicians for each patient aligned care team (PACT) to enter the information into the VA medical scheduling system. Trainee clinic time is blocked for their group visits in advance. Patients are scheduled 1 to 3 weeks in advance. Trainees and faculty are expected to review the medication review worksheet and resources prior to the visit. One CoEPCE faculty member reviews patients prior to the preclinic session (about an hour of preparation per session). Sufficient space also is required: a room large enough to accommodate up to 10 people for both didactic lessons and preclinic sessions, a facility patient education conference room for the group visit, and up to 5 clinic exam rooms. CoEPCE staff developed a templated note in the VA Computerized Patient Record System (CPRS), the VA electronic health record system to guide trainees step-by-step through the clinic visit and allow them to directly enter information into the system.7
Monitoring and Assessment
CoEPCE staff are evaluating IMPROVE by building a database for patient-level and trainee-level outcomes, including changes in trainee knowledge and attitudes over time. The CoEPCE also validated the polypharmacy knowledge assessment tool for medicine and NP trainees.
Partnerships
IMPROVE has greatly benefited from partnerships with facility department leadership, particularly involvement of pharmacy staff. In addition, we have partnered with both the health psychology and pharmacy faculty and trainees to participate in the program. Geriatrics faculty and trainees also have contributed extensively to IMPROVE. Future goals include offering the program to non-COEPCE patients throughout primary care.
The Yale Primary Care Internal Medicine Residency program and the Yale Categorical Internal Medicine Residency Program are integral partners to the CoEPCE. IMPROVE supports their mandate to encourage interprofessional teamwork in primary care, meet the Accreditation Council for Graduate Medical Education interprofessional milestones, and promote individual trainee scholarship and performance improvement in areas of broad applicability. IMPROVE also is an opportunity to share ideas across institutions and stimulate new collaborations and dissemination of the model to other primary care settings outside the VA.
Challenges and Solutions
The demand for increased direct patient care pressures programs like IMPROVE, which is a time-intensive process with high impact on a few complex patients. The assumption is that managing medications will save money in the long run, but in the short-term, a strong case has to be made for securing resources, particularly blocking provider time and securing an education room for group visits and clinic exam rooms for individual visits. First, decision makers need to be convinced that polypharmacy is important and should be a training priority. The CoEPCE has tried different configurations to increase the number of patients being seen, such as having ≥ 1 IMPROVE session in an afternoon, but trainees found this to be labor intensive and stressful.
Second, patients with medications prescribed by providers outside the VA require additional communication and coordination to reduce medications. The CoEPCE initially excluded these patients, but after realizing that some of these patients needed the most help, it developed a process for reaching out to non-VA providers and coordinating care. Additionally, there is significant diversity in patient polypharmacy needs. These can range from adherence problems to the challenge of complex psychosocial needs that are more easily (but less effectively) addressed with medications. The issue of polypharmacy is further complicated by evolving understanding of medications’ relative risks and benefits in older adults with multiple chronic conditions. IMPROVE is an effective vehicle for synthesizing current science in medications and their management, especially in complex older patients with multiple chronic conditions.
Other challenges include developing a templated CPRS electronic note that interfaces with the VA information technology system. The process of creating a template, obtaining approval from the forms committee, and working with information technology personnel to implement the template was more time intensive than anticipated and required multiple iterations of proofreading and editing.
Factors for Success
The commitment to support new models of trainee education by West Haven CoEPCE faculty and leadership, and West Haven VAMC and primary care clinic leadership facilitated the implementation of IMPROVE. Additionally, there is strong CoEPCE collaboration at all levels—codirectors, faculty, and trainees—for the program. High interprofessional trainee interest, organizational insight, and an academic orientation were critical for developing and launching IMPROVE.
Additionally, there is synergy with other team-based professions. Geriatrics has a tradition of working in multidisciplinary teams as well as working with SDM concepts as part of care discussions. High interest and collaboration by a geriatrician and an experienced geriatric pharmacist has been key. The 2 specialties complement each other and address the complex health needs of participating veterans. Health psychologists transition patients to nonpharmacologic treatments, such as sleep hygiene education and cognitive behavioral therapy, in addition to exploring barriers to behavior change.
Another factor for success has been the CoEPCE framework and expertise in interprofessional education. While refining the model, program planners tapped into existing expertise in polypharmacy within the VA from the geriatrics, pharmacy, and clinical health psychology departments. The success of the individual components—the preparation session, the group visit, and the 1:1 patient visit—is in large part the result of a collective effort by CoEPCE staff and the integration of CoEPCE staff through coordination, communications, logistics, quality improvement, and faculty involvement from multiple professions.
The IMPROVE model is flexible and can accommodate diverse patient interests and issues. Model components are based on sound practices that have demonstrated success in other arenas, such as diabetes mellitus group visits. The model can also accommodate diverse trainee levels. Senior trainees can be more independent in developing their care plans, teaching the didactic topic, or precepting during the 1:1 patient exam.
Accomplishments and Benefits
Trainees are using team skills to provide patient-centered care. They are strengthening their clinical skills through exposure to patients in a group visit and 1:1 clinic visit. There have been significant improvements in the trainees’ provision of individual patient care. Key IMPROVE outcomes are outlined below.
Interprofessional Education
Unlike a traditional didactic, IMPROVE is an opportunity for health care professionals to work together to provide care in a clinic setting. It also expands CoEPCE interprofessional education capacity through colocation of different trainee and faculty professions during the conference session. This combination trains participants to work as a team and reflect on patients together, which has strengthened communications among professions. The model provides sufficient time and expertise to discuss the medications in detail and as a team, something that would not normally happen during a regular primary care visit.
CoEPCE trainees learn about medication management, its importance in preventing complications and improving patient health outcomes. Trainees of all professions learn to translate the skills they learn in IMPROVE to other patients, such as how to perform a complete medication reconciliation or lead a discussion using SDM. IMPROVE also provides techniques useful in other contexts, such as group visits and consideration of different medication options for patients who have been cared for by other (VA and non-VA) providers.
Interprofessional Collaboration
Understanding and leveraging the expertise of trainees and faculty from different professions is a primary goal of IMPROVE. Education sessions, the group visit, and precepting model are intentionally designed to break down silos and foster a team approach to care, which supports the PACT team model. Trainees and faculty all have their unique strengths and look at the issue from a different perspective, which increases the likelihood that the patient will hear a cohesive solution or strategy. The result is that trainees are more well rounded and become better practitioners who seek advice from other professions and work well in teams.
Trainees are expected to learn about other professions and their skill sets. For example, trainees learn early about the roles and scopes of practice of pharmacists and health psychologists for more effective referrals. Discussions during the session before the group visit may bring conditions like depression or dementia to the trainees’ attention. This is significant because issues like patient motivation may be better handled from a behavioral perspective.
Expanded Clinical Performance
IMPROVE is an opportunity for CoEPCE trainees to expand their clinical expertise. It provides exposure to a variety of patients and patient care needs and is an opportunity to present a high-risk patient to colleagues of various professions. As of December 2015, about 30 internal medicine residents and 6 NP residents have seen patients in the polypharmacy clinic. Each year, 4 NP residents, 2 health psychology residents, 4 clinical pharmacy residents, and 1 geriatric pharmacy resident participate in the IMPROVE clinic during their yearlong training program. During their 3-year training program, 17 to 19 internal medicine residents participate in IMPROVE.
A structured forum for discussing patients and their care options supports professionals’ utilization of the full scope of their practice. Trainees learn and apply team skills, such as communication and the warm handoff, which can be used in other clinic settings. A warm handoff is often described as an intervention in which “a clinician directly introduces a patient to another clinician at the time of the patient’s visit and often a brief encounter between the patient and the health care professional occurs.”9 An interprofessional care plan supports trainee clinical performance, providing a more robust approach to patient care than individual providers might on their own.
Patient Outcomes
IMPROVE is an enriched care plan informed by multiple professions with the potential to improve medication use and provide better care. Veterans also are receiving better medication education as well as access to a health psychologist who can help them with goal setting and effective behavioral interventions. On average, 5 patients participate each month. As of December 2015, 68 patients have participated in IMPROVE.
The group visit and the 1:1 patient visits focus exclusively on medication issues and solutions, which would be less common in a typical primary care visit with a complex patient who brings a list of agenda items. In addition to taking a thorough look at their medications and related problems, it also educates patients on related issues such as sleep hygiene. Participating veterans also are encouraged to share their concerns, experiences, and solutions with the group, which may increase the saliency of the message beyond what is offered in counseling from a provider.
To date, preliminary data suggest that in some patients, cognition (as measured with SLUMS after 6 months) has modestly improved after decreasing their medications. Other outcomes being monitored in follow-up are utilization of care, reported history of falls, number of medications, and vital signs at initial and follow-up visits.
Patients experience increased continuity of care because the patient now has a team focusing on his or her care. Team members have a shared understanding of the patient’s situation and are better able to establish therapeutic rapport with patients during the group visit. Moreover, CoEPCE trainees and faculty try to ensure that everyone knows about and concurs with medication changes, including outside providers and family members.
Satisfaction Questionnaire
Patients that are presented at IMPROVE can be particularly challenging, and there may be a psychological benefit to working with a team to develop a new care plan. Providers are able to get input and look at the patient in a new light.
Results of postvisit patient satisfaction questionnaires are encouraging and result in a high level of patient satisfaction and perception of clinical benefit. Patients identify an improvement in the understanding of their medications, feel they are able to safely decrease their medications, and are interested in participating again.
CoEPCE Benefits
IMPROVE expands the prevention and treatment options for populations at risk of hospitalization and adverse outcomes from medication complications, such as AEs and drug-drug or drug-disease interactions. Embedding the polypharmacy clinic within the primary care setting rather than in a separate specialty clinic results in an increased likelihood of implementation of pharmacist and geriatrician recommendations for polypharmacy and allows for direct interprofessional education and collaboration.
IMPROVE also combines key components of interprofessional education—an enriched clinical training model and knowledge of medications in an elderly population—into a training activity that complements other CoEPCE activities. The model not only has strengthened CoEPCE partnerships with other VA departments and specialties, but also revealed opportunities for collaboration with academic affiliates as a means to break down traditional silos among medicine, nursing, pharmacy, geriatrics, and psychology.
IMPROVE combines key components of interprofessional education, including all 4 CoEPCE core domains, to provide hands-on experience with knowledge learned in other aspects of the CoEPCE training program (eg, shared decision-making strategies for eliciting patient goals, weighing risks and benefits in complex clinical situations). Physician and NP trainees work together with trainees in pharmacy and health psychology in the complex approach to polypharmacy. IMPROVE provides the framework for an interprofessional clinic that could be used in the treatment of other complex or high-risk chronic conditions.
The Future
An opportunity for improvement and expansion includes increased patient involvement (as patients continue to learn they have a team working on their behalf). Opportunities exist to connect with patients who have several clinicians prescribing medications outside the CoEPCE to provide comprehensive care and decrease medication complexity.
The CoEPCE has been proactive in increasing the visibility of IMPROVE through multiple presentations at local and national meetings, facilitating collaborations and greater adoption in primary care. Individual and collective IMPROVE components can be adapted to other contexts. For example, the 20-minute geriatrics education session and the forms completed prior and during the patient visit can be readily applied to other complex patients that trainees meet in clinic. Under stage 2 of the CoEPCE program, the CoEPCE is developing an implementation kit that describes the training process and includes the medication worksheet, assessment tools, and directions for conducting the group visit.
It is hoped that working collaboratively with the West Haven COEPCE polypharmacy faculty, a similar model of education and training will be implemented at other health professional training sites at Yale University in New Haven, Connecticut. Additionally, the West Haven CoEPCE is planning to partner with the other original CoEPCE program sites to implement similar interprofessional polypharmacy clinics.
1. US Department of Health and Human Services, Agency for Health Research and Quality. Transforming the organization and delivery of primary care. http://www.pcmh.ahrq .gov/. Accessed August 14, 2018.
2. Kantor ED, Rehm CD, Haas JS, Chan AT, Giovannucci EL. Trends in prescription drug use among adults in the United States from 1999-2012. JAMA. 2015;314(17):1818-1831.
3. Fried TR, O’Leary J, Towle V, Goldstein MK, Trentalange M, Martin DK. Health outcomes associated with polypharmacy in community-dwelling older adults: a systematic review. J Am Geriatr Soc. 2014;62(12):2261-2272.
4. Mecca M, Niehoff K, Grammas M. Medication review worksheet 2015. http://pogoe.org/productid/21872. Accessed August 14, 2018.
5. American Geriatrics Society 2015 Beers criteria update expert panel. American Geriatrics Society 2015 updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63(11):2227-2246.
6. O’Mahony D, O’Sullivan D, Byrne S, O’Connor MN, Ryan C, Gallagher P. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing. 2015;44(2):213-218.
7. Yale University. IMPROVE Polypharmacy Project. http://improvepolypharmacy.yale.edu. Accessed August 14, 2018.
8. Tariq SH, Tumosa N, Chibnall JT, Perry MH III, Morley JE. Comparison of the Saint Louis University mental status examination and the mini-mental state examination for detecting dementia and mild neurocognitive disorder—a pilot study. Am J Geriatr Psychiatry. 2006;14(11):900-910.
9. Cohen DJ, Balasubramanian BA, Davis M, et al. Understanding care integration from the ground up: Five organizing constructs that shape integrated practices. J Am Board Fam Med. 2015;28(suppl):S7-S20.
An interprofessional polypharmacy clinic for intensive management of medication regimens helps high-risk patients manage their medications.
An interprofessional polypharmacy clinic for intensive management of medication regimens helps high-risk patients manage their medications.
In 2011, 5 VA medical centers (VAMCs) were selected by the Office of Academic Affiliations (OAA) to establish CoEPCE. Part of the VA New Models of Care initiative, the 5 Centers of Excellence (CoE) in Boise, Idaho; Cleveland, Ohio; San Francisco, California; Seattle, Washington; and West Haven, Connecticut, are utilizing VA primary care settings to develop and test innovative approaches to prepare physician residents and students, advanced practice nurse residents and undergraduate nursing students, and other professions of health trainees (eg, pharmacy, social work, psychology, physician assistants [PAs], physical therapists) for primary care practice in the 21st century. The CoEs are developing, implementing, and evaluating curricula designed to prepare learners from relevant professions to practice in patient-centered, interprofessional team-based primary care settings. The curricula at all CoEs must address 4 core domains (Table).
Health care professional education programs do not have many opportunities for workplace learning where trainees from different professions can learn and work together to provide care to patients in real time.
The VA Connecticut Healthcare System CoEPCE developed and implemented an education and practice-based immersion learning model with physician residents, nurse practitioner (NP) residents and NP students, pharmacy residents, postdoctorate psychology learners, and PA and physical therapy learners and faculty. This interprofessional, collaborative team model breaks from the traditional independent model of siloed primary care providers (PCPs) caring for a panel of patients.
Methods
In 2015, OAA evaluators reviewed background documents and conducted open-ended interviews with 12 West Haven CoEPCE staff, participating trainees, VA faculty, VA facility leadership, and affiliate faculty. Informants described their involvement, challenges encountered, and benefits of the Initiative to Minimize Pharmaceutical Risk in Older Veterans (IMPROVE) program to trainees, veterans, and the VA.
Lack of Clinical Approaches to Interprofessional Education and Care
Polypharmacy is a common problem among older adults with multiple chronic conditions, which places patients at higher risk for multiple negative health outcomes.2,3 The typical primary care visit rarely allows for a thorough review of a patient’s medications, much less the identification of strategies to reduce polypharmacy and improve medication management. Rather, the complexity inherent to polypharmacy makes it an ideal challenge for a team-based approach.
Team Approach to Medication Needs
A key CoEPCE program aim is to expand workplace learning instruction strategies and to create more clinical opportunities for CoEPCE trainees to work together as a team to anticipate and address the health care needs of veterans. To address this training need, the West Haven CoEPCE developed IMPROVE to focus on high-need patients and provides a venue in which trainees and supervisors from different professions can collaborate on a specific patient case, using a patient-centered framework. IMPROVE can be easily applied to a range of medication-related aims, such as reducing medications, managing medications and adherence, and addressing adverse effects (AEs). These goals are 2-fold: (1) implement a trainee-led performance improvement project that reduces polypharmacy in elderly veterans; and (2) develop a hands-on, experiential geriatrics training program that enhances trainee skills and knowledge related to safe prescribing.
Planning and Implementation
IMPROVE has its origins in a scholarly project developed by a West Haven CoE physician resident trainee. Development of the IMPROVE program involved VA health psychology, internal medicine faculty, geriatric medicine faculty, NP faculty, and geriatric pharmacy residents and faculty. Planning started in 2013 with a series of pilot clinics and became an official project of the West Haven CoE in September 2014. The intervention required no change in West Haven VAMC policy. However, the initiative required buy-in from West Haven CoE leadership and the director of the West Haven primary care clinic.
Curriculum
IMPROVE is an educational, workplace learning, and clinical activity that combines a 1-hour trainee teaching session, a 45-minute group visit, and a 60-minute individual clinic visit to address the complex problem of polypharmacy. It emphasizes the sharing of trainee and faculty backgrounds by serving as a venue for interprofessional trainees and providers to discuss pharmacologic and nonpharmacologic treatment in the elderly and brainstorm strategies to optimize treatment regimens, minimize risk, and execute medication plans with patients.
All CoEPCE trainees in West Haven are required to participate in IMPROVE and on average, each trainee presents and sees one of their patients at least 3 times per year in the program. Up to 5 trainees participate in each IMPROVE session. Trainees are responsible for reviewing their panels to identify patients who might benefit from participation, followed by inviting the patient to participate. Patients are instructed to bring their pill bottles to the visit. To prepare for the polypharmacy clinic, the trainees, the geriatrician, and the geriatric pharmacist perform an extensive medication chart review, using the medication review worksheet developed by West Haven VAMC providers.4 They also work with a protocol for medication discontinuation, which was compiled by West Haven VAMC clinicians. The teams use a variety of tools that guide appropriate prescribing in older adult populations.5,6 During a preclinic conference, trainees present their patients to the interprofessional team for discussion and participate in a short discussion led by a pharmacist, geriatrician, or health psychologist on a topic related to prescribing safety in older adults or nonpharmacologic treatments.
IMPROVE emphasizes a patient-centered approach to develop, execute, and monitor medication plans. Patients and their family members are invited by their trainee clinician to participate in a group visit. Typically, trainees invite patients aged ≥ 65 years who have ≥ 10 medications and are considered appropriate for a group visit.
The group visit process is based on health psychology strategies, which often incorporate group-based engagement with patients. The health psychologist can give advice to facilitate the visit and optimize participant involvement. There is a discussion facilitator guide that lists the education points to be covered by a designated trainee facilitator and sample questions to guide the discussion.7 A health psychology resident and other rotating trainees cofacilitate the group visit with a goal to reach out to each group member, including family members, and have them discuss perceptions and share concerns and treatment goals. There is shared responsibility among the trainees to address the educational material as well as involve their respective patients during the sessions.
Immediately following the group visit, trainees conduct a 1-hour clinic session that includes medication reconciliation, a review of an IMPROVE questionnaire, orthostatic vital signs, and the St. Louis University Mental Status (SLUMS) exam to assess changes in cognition.7,8 Discussion involved the patient’s medication list as well as possible changes that could be made to the list. Using shared decision-making techniques, this conversation considers the patients’ treatment goals, feelings about the medications, which medications they would like to stop, and AEs they may be experiencing. After the individual visit is completed, the trainee participates in a 10-minute interprofessional precepting session, which may include a geriatrician, a pharmacist, and a health psychologist. In the session they may discuss adjustments to medications and a safe follow-up plan, including appropriate referrals. Trainees discuss the plan with the patient and send a letter describing the plan shortly after the visit.
IMPROVE combines didactic teaching with experiential education. It embodies the 4 core domains that shape the CoEPCE curriculum. First, trainees learn interprofessional collaboration concepts, including highlighting the roles of each profession and working with an interprofessional team to solve problems. Second, CoEPCE trainees learn performance improvement under the supervision of faculty. Third, IMPROVE allows trainees to develop sustained relationships with other team members while improving the quality of the clinic experience as well as with patients through increased continuity of care. Trainees see patients on their panel and are responsible for outreach before and after the visit. Finally, with a focus on personalized patient goals, trainees have the opportunity to further develop skills in shared decision making (SDM).
Related: Reducing Benzodiazepine Prescribing in Older Veterans: A Direct-to-Consumer Educational Brochure
The IMPROVE model continues to evolve. The original curriculum involved an hour-long preclinic preparation session before the group visit in which trainees and faculty discussed the medication review for each patient scheduled that day. This preparation session was later shortened to 40 minutes, and a 20-minute didactic component was added to create the current preclinic session. The didactic component focused on a specific topic in appropriate prescribing for older patients. For example, one didactic lesson is on a particular class of medications, its common AEs, and practical prescribing and “deprescribing” strategies for that class. Initially, the oldest patients or patients who could be grouped thematically, such as those taking both narcotics and benzodiazepines, were invited to participate, but that limited the number of appropriate patients within the CoEPCE. Currently, trainees identify patients from their panels who might benefit, based on age, number of medications, or potential medication-related concerns, such as falls, cognitive impairment, or other concerns for adverse drug effects. These trainees have the unique opportunity to apply learned strategies to their patients to continue to optimize the medication regimen even after the IMPROVE visit. Another significant change was the inclusion of veterans who are comanaged with PCPs outside the VA, because we found that patients with multiple providers could benefit from improved coordination of care.
Faculty Role
CoE faculty and non-CoE VA faculty participate in supervisory, consulting, teaching and precepting roles. Some faculty members such as the health psychologists are already located in or near the VA primary care clinic, so they can assist in curriculum development and execution during their regular clinic duties. The geriatrician reviews the patients’ health records before the patients come into the clinic, participates in the group visit, and coprecepts during the 1:1 patient visits. Collaboration is inherent in IMPROVE. For example, the geriatrician works with the geriatric pharmacist to identify and teach an educational topic. IMPROVE is characterized by a strong faculty/trainee partnership, with trainees playing roles as both teacher and facilitator in addition to learning how to take a team approach to polypharmacy.
Resources
IMPROVE requires administrative and academic support, especially faculty and trainee preparation of education sessions. The CoEPCE internal medicine resident and the internal medicine chief resident work with the health technicians for each patient aligned care team (PACT) to enter the information into the VA medical scheduling system. Trainee clinic time is blocked for their group visits in advance. Patients are scheduled 1 to 3 weeks in advance. Trainees and faculty are expected to review the medication review worksheet and resources prior to the visit. One CoEPCE faculty member reviews patients prior to the preclinic session (about an hour of preparation per session). Sufficient space also is required: a room large enough to accommodate up to 10 people for both didactic lessons and preclinic sessions, a facility patient education conference room for the group visit, and up to 5 clinic exam rooms. CoEPCE staff developed a templated note in the VA Computerized Patient Record System (CPRS), the VA electronic health record system to guide trainees step-by-step through the clinic visit and allow them to directly enter information into the system.7
Monitoring and Assessment
CoEPCE staff are evaluating IMPROVE by building a database for patient-level and trainee-level outcomes, including changes in trainee knowledge and attitudes over time. The CoEPCE also validated the polypharmacy knowledge assessment tool for medicine and NP trainees.
Partnerships
IMPROVE has greatly benefited from partnerships with facility department leadership, particularly involvement of pharmacy staff. In addition, we have partnered with both the health psychology and pharmacy faculty and trainees to participate in the program. Geriatrics faculty and trainees also have contributed extensively to IMPROVE. Future goals include offering the program to non-COEPCE patients throughout primary care.
The Yale Primary Care Internal Medicine Residency program and the Yale Categorical Internal Medicine Residency Program are integral partners to the CoEPCE. IMPROVE supports their mandate to encourage interprofessional teamwork in primary care, meet the Accreditation Council for Graduate Medical Education interprofessional milestones, and promote individual trainee scholarship and performance improvement in areas of broad applicability. IMPROVE also is an opportunity to share ideas across institutions and stimulate new collaborations and dissemination of the model to other primary care settings outside the VA.
Challenges and Solutions
The demand for increased direct patient care pressures programs like IMPROVE, which is a time-intensive process with high impact on a few complex patients. The assumption is that managing medications will save money in the long run, but in the short-term, a strong case has to be made for securing resources, particularly blocking provider time and securing an education room for group visits and clinic exam rooms for individual visits. First, decision makers need to be convinced that polypharmacy is important and should be a training priority. The CoEPCE has tried different configurations to increase the number of patients being seen, such as having ≥ 1 IMPROVE session in an afternoon, but trainees found this to be labor intensive and stressful.
Second, patients with medications prescribed by providers outside the VA require additional communication and coordination to reduce medications. The CoEPCE initially excluded these patients, but after realizing that some of these patients needed the most help, it developed a process for reaching out to non-VA providers and coordinating care. Additionally, there is significant diversity in patient polypharmacy needs. These can range from adherence problems to the challenge of complex psychosocial needs that are more easily (but less effectively) addressed with medications. The issue of polypharmacy is further complicated by evolving understanding of medications’ relative risks and benefits in older adults with multiple chronic conditions. IMPROVE is an effective vehicle for synthesizing current science in medications and their management, especially in complex older patients with multiple chronic conditions.
Other challenges include developing a templated CPRS electronic note that interfaces with the VA information technology system. The process of creating a template, obtaining approval from the forms committee, and working with information technology personnel to implement the template was more time intensive than anticipated and required multiple iterations of proofreading and editing.
Factors for Success
The commitment to support new models of trainee education by West Haven CoEPCE faculty and leadership, and West Haven VAMC and primary care clinic leadership facilitated the implementation of IMPROVE. Additionally, there is strong CoEPCE collaboration at all levels—codirectors, faculty, and trainees—for the program. High interprofessional trainee interest, organizational insight, and an academic orientation were critical for developing and launching IMPROVE.
Additionally, there is synergy with other team-based professions. Geriatrics has a tradition of working in multidisciplinary teams as well as working with SDM concepts as part of care discussions. High interest and collaboration by a geriatrician and an experienced geriatric pharmacist has been key. The 2 specialties complement each other and address the complex health needs of participating veterans. Health psychologists transition patients to nonpharmacologic treatments, such as sleep hygiene education and cognitive behavioral therapy, in addition to exploring barriers to behavior change.
Another factor for success has been the CoEPCE framework and expertise in interprofessional education. While refining the model, program planners tapped into existing expertise in polypharmacy within the VA from the geriatrics, pharmacy, and clinical health psychology departments. The success of the individual components—the preparation session, the group visit, and the 1:1 patient visit—is in large part the result of a collective effort by CoEPCE staff and the integration of CoEPCE staff through coordination, communications, logistics, quality improvement, and faculty involvement from multiple professions.
The IMPROVE model is flexible and can accommodate diverse patient interests and issues. Model components are based on sound practices that have demonstrated success in other arenas, such as diabetes mellitus group visits. The model can also accommodate diverse trainee levels. Senior trainees can be more independent in developing their care plans, teaching the didactic topic, or precepting during the 1:1 patient exam.
Accomplishments and Benefits
Trainees are using team skills to provide patient-centered care. They are strengthening their clinical skills through exposure to patients in a group visit and 1:1 clinic visit. There have been significant improvements in the trainees’ provision of individual patient care. Key IMPROVE outcomes are outlined below.
Interprofessional Education
Unlike a traditional didactic, IMPROVE is an opportunity for health care professionals to work together to provide care in a clinic setting. It also expands CoEPCE interprofessional education capacity through colocation of different trainee and faculty professions during the conference session. This combination trains participants to work as a team and reflect on patients together, which has strengthened communications among professions. The model provides sufficient time and expertise to discuss the medications in detail and as a team, something that would not normally happen during a regular primary care visit.
CoEPCE trainees learn about medication management, its importance in preventing complications and improving patient health outcomes. Trainees of all professions learn to translate the skills they learn in IMPROVE to other patients, such as how to perform a complete medication reconciliation or lead a discussion using SDM. IMPROVE also provides techniques useful in other contexts, such as group visits and consideration of different medication options for patients who have been cared for by other (VA and non-VA) providers.
Interprofessional Collaboration
Understanding and leveraging the expertise of trainees and faculty from different professions is a primary goal of IMPROVE. Education sessions, the group visit, and precepting model are intentionally designed to break down silos and foster a team approach to care, which supports the PACT team model. Trainees and faculty all have their unique strengths and look at the issue from a different perspective, which increases the likelihood that the patient will hear a cohesive solution or strategy. The result is that trainees are more well rounded and become better practitioners who seek advice from other professions and work well in teams.
Trainees are expected to learn about other professions and their skill sets. For example, trainees learn early about the roles and scopes of practice of pharmacists and health psychologists for more effective referrals. Discussions during the session before the group visit may bring conditions like depression or dementia to the trainees’ attention. This is significant because issues like patient motivation may be better handled from a behavioral perspective.
Expanded Clinical Performance
IMPROVE is an opportunity for CoEPCE trainees to expand their clinical expertise. It provides exposure to a variety of patients and patient care needs and is an opportunity to present a high-risk patient to colleagues of various professions. As of December 2015, about 30 internal medicine residents and 6 NP residents have seen patients in the polypharmacy clinic. Each year, 4 NP residents, 2 health psychology residents, 4 clinical pharmacy residents, and 1 geriatric pharmacy resident participate in the IMPROVE clinic during their yearlong training program. During their 3-year training program, 17 to 19 internal medicine residents participate in IMPROVE.
A structured forum for discussing patients and their care options supports professionals’ utilization of the full scope of their practice. Trainees learn and apply team skills, such as communication and the warm handoff, which can be used in other clinic settings. A warm handoff is often described as an intervention in which “a clinician directly introduces a patient to another clinician at the time of the patient’s visit and often a brief encounter between the patient and the health care professional occurs.”9 An interprofessional care plan supports trainee clinical performance, providing a more robust approach to patient care than individual providers might on their own.
Patient Outcomes
IMPROVE is an enriched care plan informed by multiple professions with the potential to improve medication use and provide better care. Veterans also are receiving better medication education as well as access to a health psychologist who can help them with goal setting and effective behavioral interventions. On average, 5 patients participate each month. As of December 2015, 68 patients have participated in IMPROVE.
The group visit and the 1:1 patient visits focus exclusively on medication issues and solutions, which would be less common in a typical primary care visit with a complex patient who brings a list of agenda items. In addition to taking a thorough look at their medications and related problems, it also educates patients on related issues such as sleep hygiene. Participating veterans also are encouraged to share their concerns, experiences, and solutions with the group, which may increase the saliency of the message beyond what is offered in counseling from a provider.
To date, preliminary data suggest that in some patients, cognition (as measured with SLUMS after 6 months) has modestly improved after decreasing their medications. Other outcomes being monitored in follow-up are utilization of care, reported history of falls, number of medications, and vital signs at initial and follow-up visits.
Patients experience increased continuity of care because the patient now has a team focusing on his or her care. Team members have a shared understanding of the patient’s situation and are better able to establish therapeutic rapport with patients during the group visit. Moreover, CoEPCE trainees and faculty try to ensure that everyone knows about and concurs with medication changes, including outside providers and family members.
Satisfaction Questionnaire
Patients that are presented at IMPROVE can be particularly challenging, and there may be a psychological benefit to working with a team to develop a new care plan. Providers are able to get input and look at the patient in a new light.
Results of postvisit patient satisfaction questionnaires are encouraging and result in a high level of patient satisfaction and perception of clinical benefit. Patients identify an improvement in the understanding of their medications, feel they are able to safely decrease their medications, and are interested in participating again.
CoEPCE Benefits
IMPROVE expands the prevention and treatment options for populations at risk of hospitalization and adverse outcomes from medication complications, such as AEs and drug-drug or drug-disease interactions. Embedding the polypharmacy clinic within the primary care setting rather than in a separate specialty clinic results in an increased likelihood of implementation of pharmacist and geriatrician recommendations for polypharmacy and allows for direct interprofessional education and collaboration.
IMPROVE also combines key components of interprofessional education—an enriched clinical training model and knowledge of medications in an elderly population—into a training activity that complements other CoEPCE activities. The model not only has strengthened CoEPCE partnerships with other VA departments and specialties, but also revealed opportunities for collaboration with academic affiliates as a means to break down traditional silos among medicine, nursing, pharmacy, geriatrics, and psychology.
IMPROVE combines key components of interprofessional education, including all 4 CoEPCE core domains, to provide hands-on experience with knowledge learned in other aspects of the CoEPCE training program (eg, shared decision-making strategies for eliciting patient goals, weighing risks and benefits in complex clinical situations). Physician and NP trainees work together with trainees in pharmacy and health psychology in the complex approach to polypharmacy. IMPROVE provides the framework for an interprofessional clinic that could be used in the treatment of other complex or high-risk chronic conditions.
The Future
An opportunity for improvement and expansion includes increased patient involvement (as patients continue to learn they have a team working on their behalf). Opportunities exist to connect with patients who have several clinicians prescribing medications outside the CoEPCE to provide comprehensive care and decrease medication complexity.
The CoEPCE has been proactive in increasing the visibility of IMPROVE through multiple presentations at local and national meetings, facilitating collaborations and greater adoption in primary care. Individual and collective IMPROVE components can be adapted to other contexts. For example, the 20-minute geriatrics education session and the forms completed prior and during the patient visit can be readily applied to other complex patients that trainees meet in clinic. Under stage 2 of the CoEPCE program, the CoEPCE is developing an implementation kit that describes the training process and includes the medication worksheet, assessment tools, and directions for conducting the group visit.
It is hoped that working collaboratively with the West Haven COEPCE polypharmacy faculty, a similar model of education and training will be implemented at other health professional training sites at Yale University in New Haven, Connecticut. Additionally, the West Haven CoEPCE is planning to partner with the other original CoEPCE program sites to implement similar interprofessional polypharmacy clinics.
In 2011, 5 VA medical centers (VAMCs) were selected by the Office of Academic Affiliations (OAA) to establish CoEPCE. Part of the VA New Models of Care initiative, the 5 Centers of Excellence (CoE) in Boise, Idaho; Cleveland, Ohio; San Francisco, California; Seattle, Washington; and West Haven, Connecticut, are utilizing VA primary care settings to develop and test innovative approaches to prepare physician residents and students, advanced practice nurse residents and undergraduate nursing students, and other professions of health trainees (eg, pharmacy, social work, psychology, physician assistants [PAs], physical therapists) for primary care practice in the 21st century. The CoEs are developing, implementing, and evaluating curricula designed to prepare learners from relevant professions to practice in patient-centered, interprofessional team-based primary care settings. The curricula at all CoEs must address 4 core domains (Table).
Health care professional education programs do not have many opportunities for workplace learning where trainees from different professions can learn and work together to provide care to patients in real time.
The VA Connecticut Healthcare System CoEPCE developed and implemented an education and practice-based immersion learning model with physician residents, nurse practitioner (NP) residents and NP students, pharmacy residents, postdoctorate psychology learners, and PA and physical therapy learners and faculty. This interprofessional, collaborative team model breaks from the traditional independent model of siloed primary care providers (PCPs) caring for a panel of patients.
Methods
In 2015, OAA evaluators reviewed background documents and conducted open-ended interviews with 12 West Haven CoEPCE staff, participating trainees, VA faculty, VA facility leadership, and affiliate faculty. Informants described their involvement, challenges encountered, and benefits of the Initiative to Minimize Pharmaceutical Risk in Older Veterans (IMPROVE) program to trainees, veterans, and the VA.
Lack of Clinical Approaches to Interprofessional Education and Care
Polypharmacy is a common problem among older adults with multiple chronic conditions, which places patients at higher risk for multiple negative health outcomes.2,3 The typical primary care visit rarely allows for a thorough review of a patient’s medications, much less the identification of strategies to reduce polypharmacy and improve medication management. Rather, the complexity inherent to polypharmacy makes it an ideal challenge for a team-based approach.
Team Approach to Medication Needs
A key CoEPCE program aim is to expand workplace learning instruction strategies and to create more clinical opportunities for CoEPCE trainees to work together as a team to anticipate and address the health care needs of veterans. To address this training need, the West Haven CoEPCE developed IMPROVE to focus on high-need patients and provides a venue in which trainees and supervisors from different professions can collaborate on a specific patient case, using a patient-centered framework. IMPROVE can be easily applied to a range of medication-related aims, such as reducing medications, managing medications and adherence, and addressing adverse effects (AEs). These goals are 2-fold: (1) implement a trainee-led performance improvement project that reduces polypharmacy in elderly veterans; and (2) develop a hands-on, experiential geriatrics training program that enhances trainee skills and knowledge related to safe prescribing.
Planning and Implementation
IMPROVE has its origins in a scholarly project developed by a West Haven CoE physician resident trainee. Development of the IMPROVE program involved VA health psychology, internal medicine faculty, geriatric medicine faculty, NP faculty, and geriatric pharmacy residents and faculty. Planning started in 2013 with a series of pilot clinics and became an official project of the West Haven CoE in September 2014. The intervention required no change in West Haven VAMC policy. However, the initiative required buy-in from West Haven CoE leadership and the director of the West Haven primary care clinic.
Curriculum
IMPROVE is an educational, workplace learning, and clinical activity that combines a 1-hour trainee teaching session, a 45-minute group visit, and a 60-minute individual clinic visit to address the complex problem of polypharmacy. It emphasizes the sharing of trainee and faculty backgrounds by serving as a venue for interprofessional trainees and providers to discuss pharmacologic and nonpharmacologic treatment in the elderly and brainstorm strategies to optimize treatment regimens, minimize risk, and execute medication plans with patients.
All CoEPCE trainees in West Haven are required to participate in IMPROVE and on average, each trainee presents and sees one of their patients at least 3 times per year in the program. Up to 5 trainees participate in each IMPROVE session. Trainees are responsible for reviewing their panels to identify patients who might benefit from participation, followed by inviting the patient to participate. Patients are instructed to bring their pill bottles to the visit. To prepare for the polypharmacy clinic, the trainees, the geriatrician, and the geriatric pharmacist perform an extensive medication chart review, using the medication review worksheet developed by West Haven VAMC providers.4 They also work with a protocol for medication discontinuation, which was compiled by West Haven VAMC clinicians. The teams use a variety of tools that guide appropriate prescribing in older adult populations.5,6 During a preclinic conference, trainees present their patients to the interprofessional team for discussion and participate in a short discussion led by a pharmacist, geriatrician, or health psychologist on a topic related to prescribing safety in older adults or nonpharmacologic treatments.
IMPROVE emphasizes a patient-centered approach to develop, execute, and monitor medication plans. Patients and their family members are invited by their trainee clinician to participate in a group visit. Typically, trainees invite patients aged ≥ 65 years who have ≥ 10 medications and are considered appropriate for a group visit.
The group visit process is based on health psychology strategies, which often incorporate group-based engagement with patients. The health psychologist can give advice to facilitate the visit and optimize participant involvement. There is a discussion facilitator guide that lists the education points to be covered by a designated trainee facilitator and sample questions to guide the discussion.7 A health psychology resident and other rotating trainees cofacilitate the group visit with a goal to reach out to each group member, including family members, and have them discuss perceptions and share concerns and treatment goals. There is shared responsibility among the trainees to address the educational material as well as involve their respective patients during the sessions.
Immediately following the group visit, trainees conduct a 1-hour clinic session that includes medication reconciliation, a review of an IMPROVE questionnaire, orthostatic vital signs, and the St. Louis University Mental Status (SLUMS) exam to assess changes in cognition.7,8 Discussion involved the patient’s medication list as well as possible changes that could be made to the list. Using shared decision-making techniques, this conversation considers the patients’ treatment goals, feelings about the medications, which medications they would like to stop, and AEs they may be experiencing. After the individual visit is completed, the trainee participates in a 10-minute interprofessional precepting session, which may include a geriatrician, a pharmacist, and a health psychologist. In the session they may discuss adjustments to medications and a safe follow-up plan, including appropriate referrals. Trainees discuss the plan with the patient and send a letter describing the plan shortly after the visit.
IMPROVE combines didactic teaching with experiential education. It embodies the 4 core domains that shape the CoEPCE curriculum. First, trainees learn interprofessional collaboration concepts, including highlighting the roles of each profession and working with an interprofessional team to solve problems. Second, CoEPCE trainees learn performance improvement under the supervision of faculty. Third, IMPROVE allows trainees to develop sustained relationships with other team members while improving the quality of the clinic experience as well as with patients through increased continuity of care. Trainees see patients on their panel and are responsible for outreach before and after the visit. Finally, with a focus on personalized patient goals, trainees have the opportunity to further develop skills in shared decision making (SDM).
Related: Reducing Benzodiazepine Prescribing in Older Veterans: A Direct-to-Consumer Educational Brochure
The IMPROVE model continues to evolve. The original curriculum involved an hour-long preclinic preparation session before the group visit in which trainees and faculty discussed the medication review for each patient scheduled that day. This preparation session was later shortened to 40 minutes, and a 20-minute didactic component was added to create the current preclinic session. The didactic component focused on a specific topic in appropriate prescribing for older patients. For example, one didactic lesson is on a particular class of medications, its common AEs, and practical prescribing and “deprescribing” strategies for that class. Initially, the oldest patients or patients who could be grouped thematically, such as those taking both narcotics and benzodiazepines, were invited to participate, but that limited the number of appropriate patients within the CoEPCE. Currently, trainees identify patients from their panels who might benefit, based on age, number of medications, or potential medication-related concerns, such as falls, cognitive impairment, or other concerns for adverse drug effects. These trainees have the unique opportunity to apply learned strategies to their patients to continue to optimize the medication regimen even after the IMPROVE visit. Another significant change was the inclusion of veterans who are comanaged with PCPs outside the VA, because we found that patients with multiple providers could benefit from improved coordination of care.
Faculty Role
CoE faculty and non-CoE VA faculty participate in supervisory, consulting, teaching and precepting roles. Some faculty members such as the health psychologists are already located in or near the VA primary care clinic, so they can assist in curriculum development and execution during their regular clinic duties. The geriatrician reviews the patients’ health records before the patients come into the clinic, participates in the group visit, and coprecepts during the 1:1 patient visits. Collaboration is inherent in IMPROVE. For example, the geriatrician works with the geriatric pharmacist to identify and teach an educational topic. IMPROVE is characterized by a strong faculty/trainee partnership, with trainees playing roles as both teacher and facilitator in addition to learning how to take a team approach to polypharmacy.
Resources
IMPROVE requires administrative and academic support, especially faculty and trainee preparation of education sessions. The CoEPCE internal medicine resident and the internal medicine chief resident work with the health technicians for each patient aligned care team (PACT) to enter the information into the VA medical scheduling system. Trainee clinic time is blocked for their group visits in advance. Patients are scheduled 1 to 3 weeks in advance. Trainees and faculty are expected to review the medication review worksheet and resources prior to the visit. One CoEPCE faculty member reviews patients prior to the preclinic session (about an hour of preparation per session). Sufficient space also is required: a room large enough to accommodate up to 10 people for both didactic lessons and preclinic sessions, a facility patient education conference room for the group visit, and up to 5 clinic exam rooms. CoEPCE staff developed a templated note in the VA Computerized Patient Record System (CPRS), the VA electronic health record system to guide trainees step-by-step through the clinic visit and allow them to directly enter information into the system.7
Monitoring and Assessment
CoEPCE staff are evaluating IMPROVE by building a database for patient-level and trainee-level outcomes, including changes in trainee knowledge and attitudes over time. The CoEPCE also validated the polypharmacy knowledge assessment tool for medicine and NP trainees.
Partnerships
IMPROVE has greatly benefited from partnerships with facility department leadership, particularly involvement of pharmacy staff. In addition, we have partnered with both the health psychology and pharmacy faculty and trainees to participate in the program. Geriatrics faculty and trainees also have contributed extensively to IMPROVE. Future goals include offering the program to non-COEPCE patients throughout primary care.
The Yale Primary Care Internal Medicine Residency program and the Yale Categorical Internal Medicine Residency Program are integral partners to the CoEPCE. IMPROVE supports their mandate to encourage interprofessional teamwork in primary care, meet the Accreditation Council for Graduate Medical Education interprofessional milestones, and promote individual trainee scholarship and performance improvement in areas of broad applicability. IMPROVE also is an opportunity to share ideas across institutions and stimulate new collaborations and dissemination of the model to other primary care settings outside the VA.
Challenges and Solutions
The demand for increased direct patient care pressures programs like IMPROVE, which is a time-intensive process with high impact on a few complex patients. The assumption is that managing medications will save money in the long run, but in the short-term, a strong case has to be made for securing resources, particularly blocking provider time and securing an education room for group visits and clinic exam rooms for individual visits. First, decision makers need to be convinced that polypharmacy is important and should be a training priority. The CoEPCE has tried different configurations to increase the number of patients being seen, such as having ≥ 1 IMPROVE session in an afternoon, but trainees found this to be labor intensive and stressful.
Second, patients with medications prescribed by providers outside the VA require additional communication and coordination to reduce medications. The CoEPCE initially excluded these patients, but after realizing that some of these patients needed the most help, it developed a process for reaching out to non-VA providers and coordinating care. Additionally, there is significant diversity in patient polypharmacy needs. These can range from adherence problems to the challenge of complex psychosocial needs that are more easily (but less effectively) addressed with medications. The issue of polypharmacy is further complicated by evolving understanding of medications’ relative risks and benefits in older adults with multiple chronic conditions. IMPROVE is an effective vehicle for synthesizing current science in medications and their management, especially in complex older patients with multiple chronic conditions.
Other challenges include developing a templated CPRS electronic note that interfaces with the VA information technology system. The process of creating a template, obtaining approval from the forms committee, and working with information technology personnel to implement the template was more time intensive than anticipated and required multiple iterations of proofreading and editing.
Factors for Success
The commitment to support new models of trainee education by West Haven CoEPCE faculty and leadership, and West Haven VAMC and primary care clinic leadership facilitated the implementation of IMPROVE. Additionally, there is strong CoEPCE collaboration at all levels—codirectors, faculty, and trainees—for the program. High interprofessional trainee interest, organizational insight, and an academic orientation were critical for developing and launching IMPROVE.
Additionally, there is synergy with other team-based professions. Geriatrics has a tradition of working in multidisciplinary teams as well as working with SDM concepts as part of care discussions. High interest and collaboration by a geriatrician and an experienced geriatric pharmacist has been key. The 2 specialties complement each other and address the complex health needs of participating veterans. Health psychologists transition patients to nonpharmacologic treatments, such as sleep hygiene education and cognitive behavioral therapy, in addition to exploring barriers to behavior change.
Another factor for success has been the CoEPCE framework and expertise in interprofessional education. While refining the model, program planners tapped into existing expertise in polypharmacy within the VA from the geriatrics, pharmacy, and clinical health psychology departments. The success of the individual components—the preparation session, the group visit, and the 1:1 patient visit—is in large part the result of a collective effort by CoEPCE staff and the integration of CoEPCE staff through coordination, communications, logistics, quality improvement, and faculty involvement from multiple professions.
The IMPROVE model is flexible and can accommodate diverse patient interests and issues. Model components are based on sound practices that have demonstrated success in other arenas, such as diabetes mellitus group visits. The model can also accommodate diverse trainee levels. Senior trainees can be more independent in developing their care plans, teaching the didactic topic, or precepting during the 1:1 patient exam.
Accomplishments and Benefits
Trainees are using team skills to provide patient-centered care. They are strengthening their clinical skills through exposure to patients in a group visit and 1:1 clinic visit. There have been significant improvements in the trainees’ provision of individual patient care. Key IMPROVE outcomes are outlined below.
Interprofessional Education
Unlike a traditional didactic, IMPROVE is an opportunity for health care professionals to work together to provide care in a clinic setting. It also expands CoEPCE interprofessional education capacity through colocation of different trainee and faculty professions during the conference session. This combination trains participants to work as a team and reflect on patients together, which has strengthened communications among professions. The model provides sufficient time and expertise to discuss the medications in detail and as a team, something that would not normally happen during a regular primary care visit.
CoEPCE trainees learn about medication management, its importance in preventing complications and improving patient health outcomes. Trainees of all professions learn to translate the skills they learn in IMPROVE to other patients, such as how to perform a complete medication reconciliation or lead a discussion using SDM. IMPROVE also provides techniques useful in other contexts, such as group visits and consideration of different medication options for patients who have been cared for by other (VA and non-VA) providers.
Interprofessional Collaboration
Understanding and leveraging the expertise of trainees and faculty from different professions is a primary goal of IMPROVE. Education sessions, the group visit, and precepting model are intentionally designed to break down silos and foster a team approach to care, which supports the PACT team model. Trainees and faculty all have their unique strengths and look at the issue from a different perspective, which increases the likelihood that the patient will hear a cohesive solution or strategy. The result is that trainees are more well rounded and become better practitioners who seek advice from other professions and work well in teams.
Trainees are expected to learn about other professions and their skill sets. For example, trainees learn early about the roles and scopes of practice of pharmacists and health psychologists for more effective referrals. Discussions during the session before the group visit may bring conditions like depression or dementia to the trainees’ attention. This is significant because issues like patient motivation may be better handled from a behavioral perspective.
Expanded Clinical Performance
IMPROVE is an opportunity for CoEPCE trainees to expand their clinical expertise. It provides exposure to a variety of patients and patient care needs and is an opportunity to present a high-risk patient to colleagues of various professions. As of December 2015, about 30 internal medicine residents and 6 NP residents have seen patients in the polypharmacy clinic. Each year, 4 NP residents, 2 health psychology residents, 4 clinical pharmacy residents, and 1 geriatric pharmacy resident participate in the IMPROVE clinic during their yearlong training program. During their 3-year training program, 17 to 19 internal medicine residents participate in IMPROVE.
A structured forum for discussing patients and their care options supports professionals’ utilization of the full scope of their practice. Trainees learn and apply team skills, such as communication and the warm handoff, which can be used in other clinic settings. A warm handoff is often described as an intervention in which “a clinician directly introduces a patient to another clinician at the time of the patient’s visit and often a brief encounter between the patient and the health care professional occurs.”9 An interprofessional care plan supports trainee clinical performance, providing a more robust approach to patient care than individual providers might on their own.
Patient Outcomes
IMPROVE is an enriched care plan informed by multiple professions with the potential to improve medication use and provide better care. Veterans also are receiving better medication education as well as access to a health psychologist who can help them with goal setting and effective behavioral interventions. On average, 5 patients participate each month. As of December 2015, 68 patients have participated in IMPROVE.
The group visit and the 1:1 patient visits focus exclusively on medication issues and solutions, which would be less common in a typical primary care visit with a complex patient who brings a list of agenda items. In addition to taking a thorough look at their medications and related problems, it also educates patients on related issues such as sleep hygiene. Participating veterans also are encouraged to share their concerns, experiences, and solutions with the group, which may increase the saliency of the message beyond what is offered in counseling from a provider.
To date, preliminary data suggest that in some patients, cognition (as measured with SLUMS after 6 months) has modestly improved after decreasing their medications. Other outcomes being monitored in follow-up are utilization of care, reported history of falls, number of medications, and vital signs at initial and follow-up visits.
Patients experience increased continuity of care because the patient now has a team focusing on his or her care. Team members have a shared understanding of the patient’s situation and are better able to establish therapeutic rapport with patients during the group visit. Moreover, CoEPCE trainees and faculty try to ensure that everyone knows about and concurs with medication changes, including outside providers and family members.
Satisfaction Questionnaire
Patients that are presented at IMPROVE can be particularly challenging, and there may be a psychological benefit to working with a team to develop a new care plan. Providers are able to get input and look at the patient in a new light.
Results of postvisit patient satisfaction questionnaires are encouraging and result in a high level of patient satisfaction and perception of clinical benefit. Patients identify an improvement in the understanding of their medications, feel they are able to safely decrease their medications, and are interested in participating again.
CoEPCE Benefits
IMPROVE expands the prevention and treatment options for populations at risk of hospitalization and adverse outcomes from medication complications, such as AEs and drug-drug or drug-disease interactions. Embedding the polypharmacy clinic within the primary care setting rather than in a separate specialty clinic results in an increased likelihood of implementation of pharmacist and geriatrician recommendations for polypharmacy and allows for direct interprofessional education and collaboration.
IMPROVE also combines key components of interprofessional education—an enriched clinical training model and knowledge of medications in an elderly population—into a training activity that complements other CoEPCE activities. The model not only has strengthened CoEPCE partnerships with other VA departments and specialties, but also revealed opportunities for collaboration with academic affiliates as a means to break down traditional silos among medicine, nursing, pharmacy, geriatrics, and psychology.
IMPROVE combines key components of interprofessional education, including all 4 CoEPCE core domains, to provide hands-on experience with knowledge learned in other aspects of the CoEPCE training program (eg, shared decision-making strategies for eliciting patient goals, weighing risks and benefits in complex clinical situations). Physician and NP trainees work together with trainees in pharmacy and health psychology in the complex approach to polypharmacy. IMPROVE provides the framework for an interprofessional clinic that could be used in the treatment of other complex or high-risk chronic conditions.
The Future
An opportunity for improvement and expansion includes increased patient involvement (as patients continue to learn they have a team working on their behalf). Opportunities exist to connect with patients who have several clinicians prescribing medications outside the CoEPCE to provide comprehensive care and decrease medication complexity.
The CoEPCE has been proactive in increasing the visibility of IMPROVE through multiple presentations at local and national meetings, facilitating collaborations and greater adoption in primary care. Individual and collective IMPROVE components can be adapted to other contexts. For example, the 20-minute geriatrics education session and the forms completed prior and during the patient visit can be readily applied to other complex patients that trainees meet in clinic. Under stage 2 of the CoEPCE program, the CoEPCE is developing an implementation kit that describes the training process and includes the medication worksheet, assessment tools, and directions for conducting the group visit.
It is hoped that working collaboratively with the West Haven COEPCE polypharmacy faculty, a similar model of education and training will be implemented at other health professional training sites at Yale University in New Haven, Connecticut. Additionally, the West Haven CoEPCE is planning to partner with the other original CoEPCE program sites to implement similar interprofessional polypharmacy clinics.
1. US Department of Health and Human Services, Agency for Health Research and Quality. Transforming the organization and delivery of primary care. http://www.pcmh.ahrq .gov/. Accessed August 14, 2018.
2. Kantor ED, Rehm CD, Haas JS, Chan AT, Giovannucci EL. Trends in prescription drug use among adults in the United States from 1999-2012. JAMA. 2015;314(17):1818-1831.
3. Fried TR, O’Leary J, Towle V, Goldstein MK, Trentalange M, Martin DK. Health outcomes associated with polypharmacy in community-dwelling older adults: a systematic review. J Am Geriatr Soc. 2014;62(12):2261-2272.
4. Mecca M, Niehoff K, Grammas M. Medication review worksheet 2015. http://pogoe.org/productid/21872. Accessed August 14, 2018.
5. American Geriatrics Society 2015 Beers criteria update expert panel. American Geriatrics Society 2015 updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63(11):2227-2246.
6. O’Mahony D, O’Sullivan D, Byrne S, O’Connor MN, Ryan C, Gallagher P. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing. 2015;44(2):213-218.
7. Yale University. IMPROVE Polypharmacy Project. http://improvepolypharmacy.yale.edu. Accessed August 14, 2018.
8. Tariq SH, Tumosa N, Chibnall JT, Perry MH III, Morley JE. Comparison of the Saint Louis University mental status examination and the mini-mental state examination for detecting dementia and mild neurocognitive disorder—a pilot study. Am J Geriatr Psychiatry. 2006;14(11):900-910.
9. Cohen DJ, Balasubramanian BA, Davis M, et al. Understanding care integration from the ground up: Five organizing constructs that shape integrated practices. J Am Board Fam Med. 2015;28(suppl):S7-S20.
1. US Department of Health and Human Services, Agency for Health Research and Quality. Transforming the organization and delivery of primary care. http://www.pcmh.ahrq .gov/. Accessed August 14, 2018.
2. Kantor ED, Rehm CD, Haas JS, Chan AT, Giovannucci EL. Trends in prescription drug use among adults in the United States from 1999-2012. JAMA. 2015;314(17):1818-1831.
3. Fried TR, O’Leary J, Towle V, Goldstein MK, Trentalange M, Martin DK. Health outcomes associated with polypharmacy in community-dwelling older adults: a systematic review. J Am Geriatr Soc. 2014;62(12):2261-2272.
4. Mecca M, Niehoff K, Grammas M. Medication review worksheet 2015. http://pogoe.org/productid/21872. Accessed August 14, 2018.
5. American Geriatrics Society 2015 Beers criteria update expert panel. American Geriatrics Society 2015 updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63(11):2227-2246.
6. O’Mahony D, O’Sullivan D, Byrne S, O’Connor MN, Ryan C, Gallagher P. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing. 2015;44(2):213-218.
7. Yale University. IMPROVE Polypharmacy Project. http://improvepolypharmacy.yale.edu. Accessed August 14, 2018.
8. Tariq SH, Tumosa N, Chibnall JT, Perry MH III, Morley JE. Comparison of the Saint Louis University mental status examination and the mini-mental state examination for detecting dementia and mild neurocognitive disorder—a pilot study. Am J Geriatr Psychiatry. 2006;14(11):900-910.
9. Cohen DJ, Balasubramanian BA, Davis M, et al. Understanding care integration from the ground up: Five organizing constructs that shape integrated practices. J Am Board Fam Med. 2015;28(suppl):S7-S20.
The Pharmacist’s Role in Medication Optimization for Patients With Chronic Heart Failure (FULL)
In the U.S., about 5.1 million people have clinically manifested heart failure (HF).1 The absolute mortality rate for HF is about 50% within 5 years of diagnosis, and 1 in 9 death certificates in the U.S. list HF as a cause of death.2 Heart failure is the primary diagnosis in more than 1 million hospitalizations annually.1 Patients with HF who are at risk for all-cause rehospitalization have a 1-month readmission rate of 25%, and their median survival time decreases with each hospitalization.3,4 Heart failure is the top reason for discharge of veterans treated within the VA health care system.5
Some medications decrease morbidity and mortality in patients with systolic dysfunction.6 These medications include angiotensin-converting enzyme inhibitors (ACEIs), angiotensin receptor blockers (ARBs), and β-blockers (BBs). Studies have demonstrated effectiveness of these medications in patients with reduced ejection fraction (EF) of less or equal to 40%. Other medications with proven success include aldosterone antagonists, hydralazine in combination with a nitrate, and digoxin. The American College of Cardiology Foundation/American Heart Association (ACCF/AHA) guidelines provide medication recommendations based on the ACCF/AHA stages of HF and the New York Heart Association (NYHA) functional classifications, designated as guideline-directed medical therapy (GDMT).6,7 Therapeutic interventions are aimed at reducing morbidity and mortality for ACCF/AHA stage C HF. The ACCF/AHA guidelines also recommend establishing multidisciplinary HF disease management programs for patients at high risk for hospital readmission to facilitate implementation of GDMT, address different barriers to behavior change, and reduce the risk of subsequent rehospitalization for HF.6
In October 2010, the Jesse Brown VAMC (JBVAMC) in Chicago, Illinois, opened its Heart Failure Disease Management Program (HFDMP) to prevent readmissions by targeting patients discharged after HF exacerbations and arranging follow-up in the HFDMP clinic. Enrollment in the clinic is initiated when an inpatient physician places a consultation. A cardiology nurse practitioner (NP) receives the consultation and schedules an in-clinic appointment for the patient within 1 week of discharge. The patient goes to the clinic on average every 2 weeks until he or she is on a stable, optimal medication regimen and is competent in self-management. After 3 months, the patient transitions to the general cardiology clinic. This process allows the HFDMP to see new patients in need of intense care and education for HF. The multidisciplinary HFDMP began with a NP and a cardiologist and 6 months later in April 2011 added a pharmacist.
After enrolling in HFDMP, the patient can be referred to the pharmacist for independent optimization of medication therapy in the Pharmacy Medication Titration Clinic (PMTC). The PMTC at JBVAMC is different from other HF clinics in that the pharmacist has prescribing authority and can interact face-to-face with patients to titrate medications. Once a patient is on an optimal medication regimen, he or she is referred to the NP and cardiologist. The PMTC is open 4 hours twice per month and offers 30-minute time slots. The authors conducted a study of the effectiveness of face-to-face PMTC appointments within the HFDMP.
Methods
This study, approved by the institutional review board at the University of Illinois at Chicago and the research and development service at JBVAMC, was a retrospective electronic chart review of patients enrolled in the HFDMP. Study patients were aged ≥ 18 years and were enrolled in the HFDMP between April 15, 2011 and April 15, 2013. Exclusion criteria included EF higher than 40%, 1 or no appointment attended, and enrollment before April 15, 2011. There were 2 study groups: HFDMP patients enrolled in PMTC (PMTC group) and HFDMP patients not enrolled in PMTC (no-PMTC group). For 1:1 comparison, the number of patients who met the criteria for the PMTC group was used to determine the number of patients to include in the no-PMTC group. Baseline date was the date of enrollment into either HFDMP or PMTC.
Data collected at baseline included demographics, NYHA class of HF, blood pressure (BP), heart rate, ejection fraction (EF), date of HF diagnosis, number of hospitalizations for HF within previous 6 months, serum creatinine level, height, weight, comorbidities, and HF medications. Data collected at the end date included NYHA class of HF; BP; heart rate; EF; HF medications; reason for not achieving target dose of medication or GDMT; ACEI, ARB, or BB adherence, defined as 80% of medication refills 6 months after date of discharge from group; readmission for HF within 30 days and 90 days; length of stay (LOS), including bed type if readmitted; emergency department (ED) visits for HF within 6 months of date of discharge from group; and death within 6 months of date of discharge from group. Clinical GDMT was defined as reaching the maximum tolerable or target dose of each HF medication for each patient depending on clinical presentation, as recommended by the ACCF/AHA guidelines for HF.6 It incorporated NYHA class of HF, contraindications, hypotension, bradycardia, dizziness, and hyperkalemia as well as the prescribing of aldosterone antagonists, hydralazine and isosorbide dinitrate, and digoxin.
Study Outcomes
There were 2 primary endpoints: difference in percentage of patients who achieved target ACEI or ARB doses and difference in percentage of patients who achieved target BB doses. Secondary endpoints were difference between PMTC and no-PMTC groups in percentage of patient achievement in clinical GDMT; percentage medication adherence; percentage of patients with change in NYHA class of HF; percentage of patients with change in EF, including mean change; percentage of patients with 30-day and 90-day readmissions for HF; mean LOS if readmitted within 30 days and 90 days; percentage of patients with ED visits for HF within 6 months after baseline; and percentage mortality within 6 months after baseline.
Statistical Analysis
A 2-tailed Fisher exact test was used for nominal data, and a Student t test for continuous data. Statistical significance was set at P < .05.
Results
Of the 228 HFDMP enrollees, 29 were seen in the PMTC during the study period, and 199 were not seen in the PMTC. Of the 29 patients seen in the PMTC, 24 met the criteria for the PMTC study group. Charts of 106 of the 199 patients not seen in the PMTC were randomly reviewed until 24 patients who met the study criteria were selected for the no-PMTC study group. Thus, the PMTC group and the no-PMTC group each had 24 patients for 1:1 comparison. Eighty-seven patients were excluded from the study: 22 with EF > 40%, 50 with 1 or no appointment attended, and 15 who were enrolled in HFDMP before April 15, 2011.
Mean age was 66 years. All patients were male, and most were African American. The baseline characteristics of the PMTC and no-PMTC groups were similar, except a higher percentage of patients in the PMTC group had NYHA class II or III of HF (Table 1).
The ACEI or ARB target dose was achieved by a higher percentage of patients in the PMTC group, 79.2% (n = 19) vs 50% (n = 12), but the difference was not significant (P = .07) (Figure 1). However, BB target dose was achieved by a significantly higher (P = .01) percentage of patients in the PMTC group, 87.5% (n = 21) vs 20.8% (n = 5)(Figure 2). Furthermore, a significantly higher (P = .02) percentage of patients in the PMTC group, 62.5% (n = 15) vs 25% (n = 6), achieved composite clinical GDMT (Table 2). Last, there was not a statistically significant difference in 80% adherence with ACEI or ARB dosing or with BB dosing between the PMTC group (70.8%; n = 17) and the no-PMTC group (75%; n = 18).
For a higher percentage of patients in the PMTC group, 50% (n = 12) vs 29.2% (n = 7), NYHA class of HF improved, but the difference was not significant (P = .24). In addition, EF improved in a higher percentage of patients in the PMTC group, 71.4% (n = 10) vs 41.7% (n = 5), and mean (SD) improvement in EF was higher in the PMTC group, 12.5% (12%) vs 5.4% (13%), but neither difference was significant (P = .23 and P = .16, respectively).
A higher percentage of patients in the no-PMTC group were readmitted for HF within 30 days, 8.3% (n = 2) vs 0%, but the difference was not significant. Likewise, a higher percentage of patients in the no-PMTC group were readmitted for HF within 90 days, 20.8% (n = 5) vs 4.2% (n = 1; P = .19). Mean LOS for these readmissions was longer in the PMTC group, 8 days (n = 1) vs 6.5 days (n = 8). There was a higher percentage of ED visits for HF in the no-PMTC group, 37.5% (n = 9) vs 20.8% (n = 5), but did not reach statistical significance (P = .34). Last, the no-PMTC group had a higher percentage of deaths within 6 months after baseline, 37.5% (n = 9) vs 20.8% (n = 9), which also was not significant.
Discussion
This study demonstrated that, within HFDMPs, there is a role for a pharmacist who has prescribing authority and interacts face-to-face with patients in the clinic. Significantly more patients in the PMTC group achieved target BB doses by the end of the study. Target doses of BBs have been found to decrease morbidity and mortality.8-10
The present study also found a positive trend toward achieving target ACEI or ARB doses. Reasons for not achieving target doses included contraindication to the medication, medication discontinuation during hospital admission, hypotension, hyperkalemia, and titration not complete by end of study period. Two of the many reasons for titration not being complete were clinic enrollment timing and nonadherence. Although achievement of target ACEI or ARB doses did not reach statistical significance, statistically significantly more patients achieved composite clinical GDMT.
As defined in the study, clinical GDMT captured the prescribing of hydralazine and isosorbide dinitrate in patients intolerant to ACEIs and ARBs. This study, the first known to evaluate achievement in GDMT, demonstrated a pharmacist’s ability to titrate more than just ACEI, ARB, and BB doses. This finding is clinically important in that appropriate pharmacologic therapy can reduce the number of hospitalizations for HF and improve survival, even though the study found that its PMTC group showed only trends toward fewer 30-day and 90-day readmissions for HF, fewer ED visits for HF, and less mortality.6 This finding may be attributable to the small number of readmissions for HF and deaths among the study groups.
One endpoint that did not show an expected difference with pharmacist intervention was medication adherence. However, medication nonadherence likely was a reason for patient referral to the PMTC. Baseline medication adherence was not determined, so improvement in adherence could not be assessed. Findings might have been different, too, if medication adherence had been evaluated with patient interviews and refill history, not just refill history.
In the PMTC group, LOS for readmissions for HF did not improve. However, the group had only 1 readmission, which may have skewed the result. No studies have linked outpatient pharmacist intervention to decreased LOS for readmission for HF. The endpoint was evaluated to assess whether medication stability leads to reduced LOS and to complete a limited cost analysis. Analysis of mean cost based on number of readmissions, bed type, and LOS revealed a cost savings of $167,556.82 for the PMTC group (Table 3). Other potential cost savings that are difficult to quantify and that were not accounted for include extended time between ED visits or readmissions for HF and increased quality of life and daily functioning.
This is the first study known to evaluate a pharmacist who had prescribing authority and interacted face-to-face with patients. Other studies have evaluated the role of the pharmacist in the multidisciplinary management of patients with HF. In 1999, the Pharmacist in Heart Failure Assessment Recommendation and Monitoring (PHARM) study reported the effect of direct HF-related patient care by a pharmacist who performed medication evaluations, provided patient education, and made medication recommendations to a physician.11
After a medication dosing change, the pharmacist provided telephone follow-up to assess for problems with the drug therapy and then, if any were identified, referred patients to the physician. Pharmacist intervention demonstrated a decrease in all-cause mortality and HF events and an increase in ACEI doses. Unlike the pharmacist in the present study, the pharmacist had to get recommendations approved and prescribed by a physician. The present PMTC allows for pharmacist intervention, including medication therapy changes and follow-up appointments without consultation with a physician. If needed, the HF cardiologist is available in the HFDMP clinic or by telephone.
Jain and colleagues evaluated a protocol-driven medication titration clinic staffed by nurse and pharmacist specialists.12 Although their study was limited by its descriptive nature, the authors concluded that the clinic increased the number of patients who achieved target ACEI/ARB or target BB doses. In the present study, the percentage of PMTC patients who achieved target doses increased during the study period, from 50% to 79.2% (ACEI/ARB) and from 20.8% to 87.5% (BB). Unlike other clinic pharmacists, however, the PMTC pharmacist titrates medications independently and does not follow a set clinic protocol.
Similar to the PHARM study, the Heart Failure Optimal Outcomes From Pharmacy Study (HOOPS) evaluated pharmacists who worked collaboratively with physicians to optimize HF therapy.13 As in the PMTC, patients and pharmacists had 30-minute appointments together. In HOOPS, however, physician agreement was needed before pharmacist recommendations were implemented. That study found that more patients with pharmacist intervention started an ACEI or ARB, had the medication titrated, and received recommended doses. More patients also either started a BB or increased its dose, but this did not increase the number of patients who received recommended BB doses. In addition, pharmacist intervention did not affect clinical outcomes. The authors acknowledged this finding might be attributable to the pharmacists’ lack of proper HF management training. Patients in the study were also more stable, whereas PMTC patients arrived after HF discharge and were followed until medication therapy was optimized. The HOOPS followed patients for only 3 or 4 visits, regardless of target dose achievement status.
In a study conducted within the VA health care system, Martinez and colleagues evaluated the use of pharmacists who had prescribing authority and were permitted to order laboratory tests under a scope of practice similar to that in the present study.14 However, their coordination agreement allowed them only to initiate and adjust doses of certain HF medications according to defined protocols. The pharmacist conducted monthly education classes and had medication titration appointments with individual patients by telephone over 2-week intervals. Face-to-face appointments were limited to medication reconciliations, whereas all appointments in the present study were face-to-face. In addition, Martinez and colleagues found that a higher percentage of patients who attended pharmacist appointments achieved target ACEI, ARB, and BB doses, whereas the present study found a higher percentage only of achieved target BB doses, not ACEI or ARB. However, the present study also found increased composite clinical GDMT achievement, which Martinez and colleagues did not evaluate. As mentioned, GDMT achievement may be a broader evaluation of optimal HF medical therapy, as it incorporates ACEI or ARB intolerance.
Martinez and colleagues acknowledged several study limitations different from those of the present study.14 Most members of their study population were white men, unlike this study’s population. Combining these 2 studies’ results may support use of pharmacist intervention for both white and African American men. In addition, the authors noted that patients often forgot their medications or were confused about doses, and concluded that forgetfulness and confusion may stem from having only telephone interviews and lacking written instructions for the interval between clinic appointments. By contrast, all PMTC patients were seen face-to-face, and handouts detailing any changes helped minimized confusion. Even with face-to-face appointments, however, patient nonadherence persisted, making it difficult to optimize HF therapy. Patients did not always follow instructions to bring HF medications (or medication lists) and daily weight measurements to clinic visits, which complicated medication reconciliations, interventions, and educational efforts regarding dose changes. Furthermore, patients sometimes missed face-to-face appointments, often because of transportation difficulties. In these situations, telephone appointments may be beneficial. The obstacle of transportation is removed, and, during the at-home telephone call, the patient has easy access to medications and measurements.
Limitations
This study had several limitations. It was retrospective, and its sample size was too small for conclusions regarding morbidity and mortality. As the population was predominantly African American males, results may not be applicable to other races and females. Furthermore, not evaluating HF causes at baseline could have confounded results, as disease progression, response to medications, and prognosis can vary, depending on etiology. Moreover, as patients are referred from the HFDMP to the PMTC, there may have been a bias in patient selection for the PMTC group. Patients in the PMTC group may have been more clinically stable yet had a larger knowledge deficit, an adherence issue, or a need for difficult, frequent titrations. Patients also may have been less likely to be seen during the first 30 days after discharge. In addition, it could have been beneficial to match patients on NYHA class of HF at baseline to ensure HF severity was balanced between groups. Last, the adherence analysis may not be accurate, as it relied on refill history, which may not reflect how medications were taken at home.
It would be beneficial to expand this initial study with a larger sample. Presumed HF causes and medication adherence should be captured at baseline. Additional endpoints, including quality of life and patient cognition, could enhance results. Furthermore, comparing the HFDMP with the general cardiology clinic may reveal other benefits of a focused HFDMP and its PMTC. Last, evaluating patients who are recently discharged from HF admission yet not enrolled in the HFDMP may provide more information regarding the utility of both the HFDMP and the PMTC.
Conclusion
For the PMTC group in this study, achievement of target BB doses and achievement in composite clinical GDMT were significant, but achievement of target ACEI/ARB doses were not.
Click here to read the digital edition.
1. Go AS, Mozaffarian D, Roger VL, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2013 update: a report from the American Heart Association. Circulation. 2013;127(1):e6-e245.
2. Roger VL, Weston SA, Redfield MM, et al. Trends in heart failure incidence and survival in a community-based population. JAMA. 2004;292(3):344-350.
3. Krumholz HM, Merrill AR, Schone EM, et al. Patterns of hospital performance in acute myocardial infarction and heart failure 30-day mortality and readmission. Circ Cardiovasc Qual Outcomes. 2009;2(5):407-413.
4. Setoguchi S, Stevenson LW, Schneeweiss S. Repeated hospitalizations predict mortality in the community population with heart failure. Am Heart J. 2007;154(2):260-266.
5. U.S. Department of Veterans Affairs, VA Office of Research and Development, Executive Committee, Chronic Heart Failure Quality Enhancement Research Initiative (CHF-QUERI), Health Services Research and Development Service. Chronic heart failure [QUERI fact sheet]. https://www.queri.research.va.gov/about/factsheets/chf_factsheet.pdf. Published July 2014. Accessed October 9, 2017.
6. Yancy CW, Jessup M, Bozkurt B, et al; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62(16):e147-e239.
7. New York Heart Association Criteria Committee. Nomenclature and Criteria for Diagnosis of Diseases of the Heart and Great Vessels. 9th ed. Boston, MA: Little Brown; 1994.
8. Parker M, Bristow MR, Cohn JN, et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. U.S. Carvedilol Heart Failure Study Group. N Engl J Med. 1996;334(21):1349-1355.
9. CIBIS-II Investigators and Committees. The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet. 1999;353(9146):9-13.
10. MERIT-HF Study Group. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet. 1999;353(9169):2001-2007.
11. Gattis WA, Hasselblad V, Whellan DJ, O’Connor CM. Reduction in heart failure events by the addition of a clinical pharmacist to the heart failure management team: results of the Pharmacist in Heart Failure Assessment Recommendation and Monitoring (PHARM) study. Arch Intern Med. 1999;159(16):1939-1945.
12. Jain A, Mills P, Nunn LM, et al. Success of a multidisciplinary heart failure clinic for initiation and up-titration of key therapeutic agents. Eur J Heart Fail. 2005;7(3):405-410.
13. Lowrie R, Mair FS, Greenlaw N, et al; Heart Failure Optimal Outcomes From Pharmacy Study (HOOPS) Investigators. Pharmacist intervention in primary care to improve outcomes in patients with left ventricular systolic dysfunction. Eur Heart J. 2012;33(3):314-324.
14. Martinez AS, Saef J, Paszczuk A, Bhatt-Chugani H. Implementation of a pharmacist-managed heart failure medication titration clinic. Am J Health Syst Pharm. 2013;70(12):1070-1076.
In the U.S., about 5.1 million people have clinically manifested heart failure (HF).1 The absolute mortality rate for HF is about 50% within 5 years of diagnosis, and 1 in 9 death certificates in the U.S. list HF as a cause of death.2 Heart failure is the primary diagnosis in more than 1 million hospitalizations annually.1 Patients with HF who are at risk for all-cause rehospitalization have a 1-month readmission rate of 25%, and their median survival time decreases with each hospitalization.3,4 Heart failure is the top reason for discharge of veterans treated within the VA health care system.5
Some medications decrease morbidity and mortality in patients with systolic dysfunction.6 These medications include angiotensin-converting enzyme inhibitors (ACEIs), angiotensin receptor blockers (ARBs), and β-blockers (BBs). Studies have demonstrated effectiveness of these medications in patients with reduced ejection fraction (EF) of less or equal to 40%. Other medications with proven success include aldosterone antagonists, hydralazine in combination with a nitrate, and digoxin. The American College of Cardiology Foundation/American Heart Association (ACCF/AHA) guidelines provide medication recommendations based on the ACCF/AHA stages of HF and the New York Heart Association (NYHA) functional classifications, designated as guideline-directed medical therapy (GDMT).6,7 Therapeutic interventions are aimed at reducing morbidity and mortality for ACCF/AHA stage C HF. The ACCF/AHA guidelines also recommend establishing multidisciplinary HF disease management programs for patients at high risk for hospital readmission to facilitate implementation of GDMT, address different barriers to behavior change, and reduce the risk of subsequent rehospitalization for HF.6
In October 2010, the Jesse Brown VAMC (JBVAMC) in Chicago, Illinois, opened its Heart Failure Disease Management Program (HFDMP) to prevent readmissions by targeting patients discharged after HF exacerbations and arranging follow-up in the HFDMP clinic. Enrollment in the clinic is initiated when an inpatient physician places a consultation. A cardiology nurse practitioner (NP) receives the consultation and schedules an in-clinic appointment for the patient within 1 week of discharge. The patient goes to the clinic on average every 2 weeks until he or she is on a stable, optimal medication regimen and is competent in self-management. After 3 months, the patient transitions to the general cardiology clinic. This process allows the HFDMP to see new patients in need of intense care and education for HF. The multidisciplinary HFDMP began with a NP and a cardiologist and 6 months later in April 2011 added a pharmacist.
After enrolling in HFDMP, the patient can be referred to the pharmacist for independent optimization of medication therapy in the Pharmacy Medication Titration Clinic (PMTC). The PMTC at JBVAMC is different from other HF clinics in that the pharmacist has prescribing authority and can interact face-to-face with patients to titrate medications. Once a patient is on an optimal medication regimen, he or she is referred to the NP and cardiologist. The PMTC is open 4 hours twice per month and offers 30-minute time slots. The authors conducted a study of the effectiveness of face-to-face PMTC appointments within the HFDMP.
Methods
This study, approved by the institutional review board at the University of Illinois at Chicago and the research and development service at JBVAMC, was a retrospective electronic chart review of patients enrolled in the HFDMP. Study patients were aged ≥ 18 years and were enrolled in the HFDMP between April 15, 2011 and April 15, 2013. Exclusion criteria included EF higher than 40%, 1 or no appointment attended, and enrollment before April 15, 2011. There were 2 study groups: HFDMP patients enrolled in PMTC (PMTC group) and HFDMP patients not enrolled in PMTC (no-PMTC group). For 1:1 comparison, the number of patients who met the criteria for the PMTC group was used to determine the number of patients to include in the no-PMTC group. Baseline date was the date of enrollment into either HFDMP or PMTC.
Data collected at baseline included demographics, NYHA class of HF, blood pressure (BP), heart rate, ejection fraction (EF), date of HF diagnosis, number of hospitalizations for HF within previous 6 months, serum creatinine level, height, weight, comorbidities, and HF medications. Data collected at the end date included NYHA class of HF; BP; heart rate; EF; HF medications; reason for not achieving target dose of medication or GDMT; ACEI, ARB, or BB adherence, defined as 80% of medication refills 6 months after date of discharge from group; readmission for HF within 30 days and 90 days; length of stay (LOS), including bed type if readmitted; emergency department (ED) visits for HF within 6 months of date of discharge from group; and death within 6 months of date of discharge from group. Clinical GDMT was defined as reaching the maximum tolerable or target dose of each HF medication for each patient depending on clinical presentation, as recommended by the ACCF/AHA guidelines for HF.6 It incorporated NYHA class of HF, contraindications, hypotension, bradycardia, dizziness, and hyperkalemia as well as the prescribing of aldosterone antagonists, hydralazine and isosorbide dinitrate, and digoxin.
Study Outcomes
There were 2 primary endpoints: difference in percentage of patients who achieved target ACEI or ARB doses and difference in percentage of patients who achieved target BB doses. Secondary endpoints were difference between PMTC and no-PMTC groups in percentage of patient achievement in clinical GDMT; percentage medication adherence; percentage of patients with change in NYHA class of HF; percentage of patients with change in EF, including mean change; percentage of patients with 30-day and 90-day readmissions for HF; mean LOS if readmitted within 30 days and 90 days; percentage of patients with ED visits for HF within 6 months after baseline; and percentage mortality within 6 months after baseline.
Statistical Analysis
A 2-tailed Fisher exact test was used for nominal data, and a Student t test for continuous data. Statistical significance was set at P < .05.
Results
Of the 228 HFDMP enrollees, 29 were seen in the PMTC during the study period, and 199 were not seen in the PMTC. Of the 29 patients seen in the PMTC, 24 met the criteria for the PMTC study group. Charts of 106 of the 199 patients not seen in the PMTC were randomly reviewed until 24 patients who met the study criteria were selected for the no-PMTC study group. Thus, the PMTC group and the no-PMTC group each had 24 patients for 1:1 comparison. Eighty-seven patients were excluded from the study: 22 with EF > 40%, 50 with 1 or no appointment attended, and 15 who were enrolled in HFDMP before April 15, 2011.
Mean age was 66 years. All patients were male, and most were African American. The baseline characteristics of the PMTC and no-PMTC groups were similar, except a higher percentage of patients in the PMTC group had NYHA class II or III of HF (Table 1).
The ACEI or ARB target dose was achieved by a higher percentage of patients in the PMTC group, 79.2% (n = 19) vs 50% (n = 12), but the difference was not significant (P = .07) (Figure 1). However, BB target dose was achieved by a significantly higher (P = .01) percentage of patients in the PMTC group, 87.5% (n = 21) vs 20.8% (n = 5)(Figure 2). Furthermore, a significantly higher (P = .02) percentage of patients in the PMTC group, 62.5% (n = 15) vs 25% (n = 6), achieved composite clinical GDMT (Table 2). Last, there was not a statistically significant difference in 80% adherence with ACEI or ARB dosing or with BB dosing between the PMTC group (70.8%; n = 17) and the no-PMTC group (75%; n = 18).
For a higher percentage of patients in the PMTC group, 50% (n = 12) vs 29.2% (n = 7), NYHA class of HF improved, but the difference was not significant (P = .24). In addition, EF improved in a higher percentage of patients in the PMTC group, 71.4% (n = 10) vs 41.7% (n = 5), and mean (SD) improvement in EF was higher in the PMTC group, 12.5% (12%) vs 5.4% (13%), but neither difference was significant (P = .23 and P = .16, respectively).
A higher percentage of patients in the no-PMTC group were readmitted for HF within 30 days, 8.3% (n = 2) vs 0%, but the difference was not significant. Likewise, a higher percentage of patients in the no-PMTC group were readmitted for HF within 90 days, 20.8% (n = 5) vs 4.2% (n = 1; P = .19). Mean LOS for these readmissions was longer in the PMTC group, 8 days (n = 1) vs 6.5 days (n = 8). There was a higher percentage of ED visits for HF in the no-PMTC group, 37.5% (n = 9) vs 20.8% (n = 5), but did not reach statistical significance (P = .34). Last, the no-PMTC group had a higher percentage of deaths within 6 months after baseline, 37.5% (n = 9) vs 20.8% (n = 9), which also was not significant.
Discussion
This study demonstrated that, within HFDMPs, there is a role for a pharmacist who has prescribing authority and interacts face-to-face with patients in the clinic. Significantly more patients in the PMTC group achieved target BB doses by the end of the study. Target doses of BBs have been found to decrease morbidity and mortality.8-10
The present study also found a positive trend toward achieving target ACEI or ARB doses. Reasons for not achieving target doses included contraindication to the medication, medication discontinuation during hospital admission, hypotension, hyperkalemia, and titration not complete by end of study period. Two of the many reasons for titration not being complete were clinic enrollment timing and nonadherence. Although achievement of target ACEI or ARB doses did not reach statistical significance, statistically significantly more patients achieved composite clinical GDMT.
As defined in the study, clinical GDMT captured the prescribing of hydralazine and isosorbide dinitrate in patients intolerant to ACEIs and ARBs. This study, the first known to evaluate achievement in GDMT, demonstrated a pharmacist’s ability to titrate more than just ACEI, ARB, and BB doses. This finding is clinically important in that appropriate pharmacologic therapy can reduce the number of hospitalizations for HF and improve survival, even though the study found that its PMTC group showed only trends toward fewer 30-day and 90-day readmissions for HF, fewer ED visits for HF, and less mortality.6 This finding may be attributable to the small number of readmissions for HF and deaths among the study groups.
One endpoint that did not show an expected difference with pharmacist intervention was medication adherence. However, medication nonadherence likely was a reason for patient referral to the PMTC. Baseline medication adherence was not determined, so improvement in adherence could not be assessed. Findings might have been different, too, if medication adherence had been evaluated with patient interviews and refill history, not just refill history.
In the PMTC group, LOS for readmissions for HF did not improve. However, the group had only 1 readmission, which may have skewed the result. No studies have linked outpatient pharmacist intervention to decreased LOS for readmission for HF. The endpoint was evaluated to assess whether medication stability leads to reduced LOS and to complete a limited cost analysis. Analysis of mean cost based on number of readmissions, bed type, and LOS revealed a cost savings of $167,556.82 for the PMTC group (Table 3). Other potential cost savings that are difficult to quantify and that were not accounted for include extended time between ED visits or readmissions for HF and increased quality of life and daily functioning.
This is the first study known to evaluate a pharmacist who had prescribing authority and interacted face-to-face with patients. Other studies have evaluated the role of the pharmacist in the multidisciplinary management of patients with HF. In 1999, the Pharmacist in Heart Failure Assessment Recommendation and Monitoring (PHARM) study reported the effect of direct HF-related patient care by a pharmacist who performed medication evaluations, provided patient education, and made medication recommendations to a physician.11
After a medication dosing change, the pharmacist provided telephone follow-up to assess for problems with the drug therapy and then, if any were identified, referred patients to the physician. Pharmacist intervention demonstrated a decrease in all-cause mortality and HF events and an increase in ACEI doses. Unlike the pharmacist in the present study, the pharmacist had to get recommendations approved and prescribed by a physician. The present PMTC allows for pharmacist intervention, including medication therapy changes and follow-up appointments without consultation with a physician. If needed, the HF cardiologist is available in the HFDMP clinic or by telephone.
Jain and colleagues evaluated a protocol-driven medication titration clinic staffed by nurse and pharmacist specialists.12 Although their study was limited by its descriptive nature, the authors concluded that the clinic increased the number of patients who achieved target ACEI/ARB or target BB doses. In the present study, the percentage of PMTC patients who achieved target doses increased during the study period, from 50% to 79.2% (ACEI/ARB) and from 20.8% to 87.5% (BB). Unlike other clinic pharmacists, however, the PMTC pharmacist titrates medications independently and does not follow a set clinic protocol.
Similar to the PHARM study, the Heart Failure Optimal Outcomes From Pharmacy Study (HOOPS) evaluated pharmacists who worked collaboratively with physicians to optimize HF therapy.13 As in the PMTC, patients and pharmacists had 30-minute appointments together. In HOOPS, however, physician agreement was needed before pharmacist recommendations were implemented. That study found that more patients with pharmacist intervention started an ACEI or ARB, had the medication titrated, and received recommended doses. More patients also either started a BB or increased its dose, but this did not increase the number of patients who received recommended BB doses. In addition, pharmacist intervention did not affect clinical outcomes. The authors acknowledged this finding might be attributable to the pharmacists’ lack of proper HF management training. Patients in the study were also more stable, whereas PMTC patients arrived after HF discharge and were followed until medication therapy was optimized. The HOOPS followed patients for only 3 or 4 visits, regardless of target dose achievement status.
In a study conducted within the VA health care system, Martinez and colleagues evaluated the use of pharmacists who had prescribing authority and were permitted to order laboratory tests under a scope of practice similar to that in the present study.14 However, their coordination agreement allowed them only to initiate and adjust doses of certain HF medications according to defined protocols. The pharmacist conducted monthly education classes and had medication titration appointments with individual patients by telephone over 2-week intervals. Face-to-face appointments were limited to medication reconciliations, whereas all appointments in the present study were face-to-face. In addition, Martinez and colleagues found that a higher percentage of patients who attended pharmacist appointments achieved target ACEI, ARB, and BB doses, whereas the present study found a higher percentage only of achieved target BB doses, not ACEI or ARB. However, the present study also found increased composite clinical GDMT achievement, which Martinez and colleagues did not evaluate. As mentioned, GDMT achievement may be a broader evaluation of optimal HF medical therapy, as it incorporates ACEI or ARB intolerance.
Martinez and colleagues acknowledged several study limitations different from those of the present study.14 Most members of their study population were white men, unlike this study’s population. Combining these 2 studies’ results may support use of pharmacist intervention for both white and African American men. In addition, the authors noted that patients often forgot their medications or were confused about doses, and concluded that forgetfulness and confusion may stem from having only telephone interviews and lacking written instructions for the interval between clinic appointments. By contrast, all PMTC patients were seen face-to-face, and handouts detailing any changes helped minimized confusion. Even with face-to-face appointments, however, patient nonadherence persisted, making it difficult to optimize HF therapy. Patients did not always follow instructions to bring HF medications (or medication lists) and daily weight measurements to clinic visits, which complicated medication reconciliations, interventions, and educational efforts regarding dose changes. Furthermore, patients sometimes missed face-to-face appointments, often because of transportation difficulties. In these situations, telephone appointments may be beneficial. The obstacle of transportation is removed, and, during the at-home telephone call, the patient has easy access to medications and measurements.
Limitations
This study had several limitations. It was retrospective, and its sample size was too small for conclusions regarding morbidity and mortality. As the population was predominantly African American males, results may not be applicable to other races and females. Furthermore, not evaluating HF causes at baseline could have confounded results, as disease progression, response to medications, and prognosis can vary, depending on etiology. Moreover, as patients are referred from the HFDMP to the PMTC, there may have been a bias in patient selection for the PMTC group. Patients in the PMTC group may have been more clinically stable yet had a larger knowledge deficit, an adherence issue, or a need for difficult, frequent titrations. Patients also may have been less likely to be seen during the first 30 days after discharge. In addition, it could have been beneficial to match patients on NYHA class of HF at baseline to ensure HF severity was balanced between groups. Last, the adherence analysis may not be accurate, as it relied on refill history, which may not reflect how medications were taken at home.
It would be beneficial to expand this initial study with a larger sample. Presumed HF causes and medication adherence should be captured at baseline. Additional endpoints, including quality of life and patient cognition, could enhance results. Furthermore, comparing the HFDMP with the general cardiology clinic may reveal other benefits of a focused HFDMP and its PMTC. Last, evaluating patients who are recently discharged from HF admission yet not enrolled in the HFDMP may provide more information regarding the utility of both the HFDMP and the PMTC.
Conclusion
For the PMTC group in this study, achievement of target BB doses and achievement in composite clinical GDMT were significant, but achievement of target ACEI/ARB doses were not.
Click here to read the digital edition.
In the U.S., about 5.1 million people have clinically manifested heart failure (HF).1 The absolute mortality rate for HF is about 50% within 5 years of diagnosis, and 1 in 9 death certificates in the U.S. list HF as a cause of death.2 Heart failure is the primary diagnosis in more than 1 million hospitalizations annually.1 Patients with HF who are at risk for all-cause rehospitalization have a 1-month readmission rate of 25%, and their median survival time decreases with each hospitalization.3,4 Heart failure is the top reason for discharge of veterans treated within the VA health care system.5
Some medications decrease morbidity and mortality in patients with systolic dysfunction.6 These medications include angiotensin-converting enzyme inhibitors (ACEIs), angiotensin receptor blockers (ARBs), and β-blockers (BBs). Studies have demonstrated effectiveness of these medications in patients with reduced ejection fraction (EF) of less or equal to 40%. Other medications with proven success include aldosterone antagonists, hydralazine in combination with a nitrate, and digoxin. The American College of Cardiology Foundation/American Heart Association (ACCF/AHA) guidelines provide medication recommendations based on the ACCF/AHA stages of HF and the New York Heart Association (NYHA) functional classifications, designated as guideline-directed medical therapy (GDMT).6,7 Therapeutic interventions are aimed at reducing morbidity and mortality for ACCF/AHA stage C HF. The ACCF/AHA guidelines also recommend establishing multidisciplinary HF disease management programs for patients at high risk for hospital readmission to facilitate implementation of GDMT, address different barriers to behavior change, and reduce the risk of subsequent rehospitalization for HF.6
In October 2010, the Jesse Brown VAMC (JBVAMC) in Chicago, Illinois, opened its Heart Failure Disease Management Program (HFDMP) to prevent readmissions by targeting patients discharged after HF exacerbations and arranging follow-up in the HFDMP clinic. Enrollment in the clinic is initiated when an inpatient physician places a consultation. A cardiology nurse practitioner (NP) receives the consultation and schedules an in-clinic appointment for the patient within 1 week of discharge. The patient goes to the clinic on average every 2 weeks until he or she is on a stable, optimal medication regimen and is competent in self-management. After 3 months, the patient transitions to the general cardiology clinic. This process allows the HFDMP to see new patients in need of intense care and education for HF. The multidisciplinary HFDMP began with a NP and a cardiologist and 6 months later in April 2011 added a pharmacist.
After enrolling in HFDMP, the patient can be referred to the pharmacist for independent optimization of medication therapy in the Pharmacy Medication Titration Clinic (PMTC). The PMTC at JBVAMC is different from other HF clinics in that the pharmacist has prescribing authority and can interact face-to-face with patients to titrate medications. Once a patient is on an optimal medication regimen, he or she is referred to the NP and cardiologist. The PMTC is open 4 hours twice per month and offers 30-minute time slots. The authors conducted a study of the effectiveness of face-to-face PMTC appointments within the HFDMP.
Methods
This study, approved by the institutional review board at the University of Illinois at Chicago and the research and development service at JBVAMC, was a retrospective electronic chart review of patients enrolled in the HFDMP. Study patients were aged ≥ 18 years and were enrolled in the HFDMP between April 15, 2011 and April 15, 2013. Exclusion criteria included EF higher than 40%, 1 or no appointment attended, and enrollment before April 15, 2011. There were 2 study groups: HFDMP patients enrolled in PMTC (PMTC group) and HFDMP patients not enrolled in PMTC (no-PMTC group). For 1:1 comparison, the number of patients who met the criteria for the PMTC group was used to determine the number of patients to include in the no-PMTC group. Baseline date was the date of enrollment into either HFDMP or PMTC.
Data collected at baseline included demographics, NYHA class of HF, blood pressure (BP), heart rate, ejection fraction (EF), date of HF diagnosis, number of hospitalizations for HF within previous 6 months, serum creatinine level, height, weight, comorbidities, and HF medications. Data collected at the end date included NYHA class of HF; BP; heart rate; EF; HF medications; reason for not achieving target dose of medication or GDMT; ACEI, ARB, or BB adherence, defined as 80% of medication refills 6 months after date of discharge from group; readmission for HF within 30 days and 90 days; length of stay (LOS), including bed type if readmitted; emergency department (ED) visits for HF within 6 months of date of discharge from group; and death within 6 months of date of discharge from group. Clinical GDMT was defined as reaching the maximum tolerable or target dose of each HF medication for each patient depending on clinical presentation, as recommended by the ACCF/AHA guidelines for HF.6 It incorporated NYHA class of HF, contraindications, hypotension, bradycardia, dizziness, and hyperkalemia as well as the prescribing of aldosterone antagonists, hydralazine and isosorbide dinitrate, and digoxin.
Study Outcomes
There were 2 primary endpoints: difference in percentage of patients who achieved target ACEI or ARB doses and difference in percentage of patients who achieved target BB doses. Secondary endpoints were difference between PMTC and no-PMTC groups in percentage of patient achievement in clinical GDMT; percentage medication adherence; percentage of patients with change in NYHA class of HF; percentage of patients with change in EF, including mean change; percentage of patients with 30-day and 90-day readmissions for HF; mean LOS if readmitted within 30 days and 90 days; percentage of patients with ED visits for HF within 6 months after baseline; and percentage mortality within 6 months after baseline.
Statistical Analysis
A 2-tailed Fisher exact test was used for nominal data, and a Student t test for continuous data. Statistical significance was set at P < .05.
Results
Of the 228 HFDMP enrollees, 29 were seen in the PMTC during the study period, and 199 were not seen in the PMTC. Of the 29 patients seen in the PMTC, 24 met the criteria for the PMTC study group. Charts of 106 of the 199 patients not seen in the PMTC were randomly reviewed until 24 patients who met the study criteria were selected for the no-PMTC study group. Thus, the PMTC group and the no-PMTC group each had 24 patients for 1:1 comparison. Eighty-seven patients were excluded from the study: 22 with EF > 40%, 50 with 1 or no appointment attended, and 15 who were enrolled in HFDMP before April 15, 2011.
Mean age was 66 years. All patients were male, and most were African American. The baseline characteristics of the PMTC and no-PMTC groups were similar, except a higher percentage of patients in the PMTC group had NYHA class II or III of HF (Table 1).
The ACEI or ARB target dose was achieved by a higher percentage of patients in the PMTC group, 79.2% (n = 19) vs 50% (n = 12), but the difference was not significant (P = .07) (Figure 1). However, BB target dose was achieved by a significantly higher (P = .01) percentage of patients in the PMTC group, 87.5% (n = 21) vs 20.8% (n = 5)(Figure 2). Furthermore, a significantly higher (P = .02) percentage of patients in the PMTC group, 62.5% (n = 15) vs 25% (n = 6), achieved composite clinical GDMT (Table 2). Last, there was not a statistically significant difference in 80% adherence with ACEI or ARB dosing or with BB dosing between the PMTC group (70.8%; n = 17) and the no-PMTC group (75%; n = 18).
For a higher percentage of patients in the PMTC group, 50% (n = 12) vs 29.2% (n = 7), NYHA class of HF improved, but the difference was not significant (P = .24). In addition, EF improved in a higher percentage of patients in the PMTC group, 71.4% (n = 10) vs 41.7% (n = 5), and mean (SD) improvement in EF was higher in the PMTC group, 12.5% (12%) vs 5.4% (13%), but neither difference was significant (P = .23 and P = .16, respectively).
A higher percentage of patients in the no-PMTC group were readmitted for HF within 30 days, 8.3% (n = 2) vs 0%, but the difference was not significant. Likewise, a higher percentage of patients in the no-PMTC group were readmitted for HF within 90 days, 20.8% (n = 5) vs 4.2% (n = 1; P = .19). Mean LOS for these readmissions was longer in the PMTC group, 8 days (n = 1) vs 6.5 days (n = 8). There was a higher percentage of ED visits for HF in the no-PMTC group, 37.5% (n = 9) vs 20.8% (n = 5), but did not reach statistical significance (P = .34). Last, the no-PMTC group had a higher percentage of deaths within 6 months after baseline, 37.5% (n = 9) vs 20.8% (n = 9), which also was not significant.
Discussion
This study demonstrated that, within HFDMPs, there is a role for a pharmacist who has prescribing authority and interacts face-to-face with patients in the clinic. Significantly more patients in the PMTC group achieved target BB doses by the end of the study. Target doses of BBs have been found to decrease morbidity and mortality.8-10
The present study also found a positive trend toward achieving target ACEI or ARB doses. Reasons for not achieving target doses included contraindication to the medication, medication discontinuation during hospital admission, hypotension, hyperkalemia, and titration not complete by end of study period. Two of the many reasons for titration not being complete were clinic enrollment timing and nonadherence. Although achievement of target ACEI or ARB doses did not reach statistical significance, statistically significantly more patients achieved composite clinical GDMT.
As defined in the study, clinical GDMT captured the prescribing of hydralazine and isosorbide dinitrate in patients intolerant to ACEIs and ARBs. This study, the first known to evaluate achievement in GDMT, demonstrated a pharmacist’s ability to titrate more than just ACEI, ARB, and BB doses. This finding is clinically important in that appropriate pharmacologic therapy can reduce the number of hospitalizations for HF and improve survival, even though the study found that its PMTC group showed only trends toward fewer 30-day and 90-day readmissions for HF, fewer ED visits for HF, and less mortality.6 This finding may be attributable to the small number of readmissions for HF and deaths among the study groups.
One endpoint that did not show an expected difference with pharmacist intervention was medication adherence. However, medication nonadherence likely was a reason for patient referral to the PMTC. Baseline medication adherence was not determined, so improvement in adherence could not be assessed. Findings might have been different, too, if medication adherence had been evaluated with patient interviews and refill history, not just refill history.
In the PMTC group, LOS for readmissions for HF did not improve. However, the group had only 1 readmission, which may have skewed the result. No studies have linked outpatient pharmacist intervention to decreased LOS for readmission for HF. The endpoint was evaluated to assess whether medication stability leads to reduced LOS and to complete a limited cost analysis. Analysis of mean cost based on number of readmissions, bed type, and LOS revealed a cost savings of $167,556.82 for the PMTC group (Table 3). Other potential cost savings that are difficult to quantify and that were not accounted for include extended time between ED visits or readmissions for HF and increased quality of life and daily functioning.
This is the first study known to evaluate a pharmacist who had prescribing authority and interacted face-to-face with patients. Other studies have evaluated the role of the pharmacist in the multidisciplinary management of patients with HF. In 1999, the Pharmacist in Heart Failure Assessment Recommendation and Monitoring (PHARM) study reported the effect of direct HF-related patient care by a pharmacist who performed medication evaluations, provided patient education, and made medication recommendations to a physician.11
After a medication dosing change, the pharmacist provided telephone follow-up to assess for problems with the drug therapy and then, if any were identified, referred patients to the physician. Pharmacist intervention demonstrated a decrease in all-cause mortality and HF events and an increase in ACEI doses. Unlike the pharmacist in the present study, the pharmacist had to get recommendations approved and prescribed by a physician. The present PMTC allows for pharmacist intervention, including medication therapy changes and follow-up appointments without consultation with a physician. If needed, the HF cardiologist is available in the HFDMP clinic or by telephone.
Jain and colleagues evaluated a protocol-driven medication titration clinic staffed by nurse and pharmacist specialists.12 Although their study was limited by its descriptive nature, the authors concluded that the clinic increased the number of patients who achieved target ACEI/ARB or target BB doses. In the present study, the percentage of PMTC patients who achieved target doses increased during the study period, from 50% to 79.2% (ACEI/ARB) and from 20.8% to 87.5% (BB). Unlike other clinic pharmacists, however, the PMTC pharmacist titrates medications independently and does not follow a set clinic protocol.
Similar to the PHARM study, the Heart Failure Optimal Outcomes From Pharmacy Study (HOOPS) evaluated pharmacists who worked collaboratively with physicians to optimize HF therapy.13 As in the PMTC, patients and pharmacists had 30-minute appointments together. In HOOPS, however, physician agreement was needed before pharmacist recommendations were implemented. That study found that more patients with pharmacist intervention started an ACEI or ARB, had the medication titrated, and received recommended doses. More patients also either started a BB or increased its dose, but this did not increase the number of patients who received recommended BB doses. In addition, pharmacist intervention did not affect clinical outcomes. The authors acknowledged this finding might be attributable to the pharmacists’ lack of proper HF management training. Patients in the study were also more stable, whereas PMTC patients arrived after HF discharge and were followed until medication therapy was optimized. The HOOPS followed patients for only 3 or 4 visits, regardless of target dose achievement status.
In a study conducted within the VA health care system, Martinez and colleagues evaluated the use of pharmacists who had prescribing authority and were permitted to order laboratory tests under a scope of practice similar to that in the present study.14 However, their coordination agreement allowed them only to initiate and adjust doses of certain HF medications according to defined protocols. The pharmacist conducted monthly education classes and had medication titration appointments with individual patients by telephone over 2-week intervals. Face-to-face appointments were limited to medication reconciliations, whereas all appointments in the present study were face-to-face. In addition, Martinez and colleagues found that a higher percentage of patients who attended pharmacist appointments achieved target ACEI, ARB, and BB doses, whereas the present study found a higher percentage only of achieved target BB doses, not ACEI or ARB. However, the present study also found increased composite clinical GDMT achievement, which Martinez and colleagues did not evaluate. As mentioned, GDMT achievement may be a broader evaluation of optimal HF medical therapy, as it incorporates ACEI or ARB intolerance.
Martinez and colleagues acknowledged several study limitations different from those of the present study.14 Most members of their study population were white men, unlike this study’s population. Combining these 2 studies’ results may support use of pharmacist intervention for both white and African American men. In addition, the authors noted that patients often forgot their medications or were confused about doses, and concluded that forgetfulness and confusion may stem from having only telephone interviews and lacking written instructions for the interval between clinic appointments. By contrast, all PMTC patients were seen face-to-face, and handouts detailing any changes helped minimized confusion. Even with face-to-face appointments, however, patient nonadherence persisted, making it difficult to optimize HF therapy. Patients did not always follow instructions to bring HF medications (or medication lists) and daily weight measurements to clinic visits, which complicated medication reconciliations, interventions, and educational efforts regarding dose changes. Furthermore, patients sometimes missed face-to-face appointments, often because of transportation difficulties. In these situations, telephone appointments may be beneficial. The obstacle of transportation is removed, and, during the at-home telephone call, the patient has easy access to medications and measurements.
Limitations
This study had several limitations. It was retrospective, and its sample size was too small for conclusions regarding morbidity and mortality. As the population was predominantly African American males, results may not be applicable to other races and females. Furthermore, not evaluating HF causes at baseline could have confounded results, as disease progression, response to medications, and prognosis can vary, depending on etiology. Moreover, as patients are referred from the HFDMP to the PMTC, there may have been a bias in patient selection for the PMTC group. Patients in the PMTC group may have been more clinically stable yet had a larger knowledge deficit, an adherence issue, or a need for difficult, frequent titrations. Patients also may have been less likely to be seen during the first 30 days after discharge. In addition, it could have been beneficial to match patients on NYHA class of HF at baseline to ensure HF severity was balanced between groups. Last, the adherence analysis may not be accurate, as it relied on refill history, which may not reflect how medications were taken at home.
It would be beneficial to expand this initial study with a larger sample. Presumed HF causes and medication adherence should be captured at baseline. Additional endpoints, including quality of life and patient cognition, could enhance results. Furthermore, comparing the HFDMP with the general cardiology clinic may reveal other benefits of a focused HFDMP and its PMTC. Last, evaluating patients who are recently discharged from HF admission yet not enrolled in the HFDMP may provide more information regarding the utility of both the HFDMP and the PMTC.
Conclusion
For the PMTC group in this study, achievement of target BB doses and achievement in composite clinical GDMT were significant, but achievement of target ACEI/ARB doses were not.
Click here to read the digital edition.
1. Go AS, Mozaffarian D, Roger VL, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2013 update: a report from the American Heart Association. Circulation. 2013;127(1):e6-e245.
2. Roger VL, Weston SA, Redfield MM, et al. Trends in heart failure incidence and survival in a community-based population. JAMA. 2004;292(3):344-350.
3. Krumholz HM, Merrill AR, Schone EM, et al. Patterns of hospital performance in acute myocardial infarction and heart failure 30-day mortality and readmission. Circ Cardiovasc Qual Outcomes. 2009;2(5):407-413.
4. Setoguchi S, Stevenson LW, Schneeweiss S. Repeated hospitalizations predict mortality in the community population with heart failure. Am Heart J. 2007;154(2):260-266.
5. U.S. Department of Veterans Affairs, VA Office of Research and Development, Executive Committee, Chronic Heart Failure Quality Enhancement Research Initiative (CHF-QUERI), Health Services Research and Development Service. Chronic heart failure [QUERI fact sheet]. https://www.queri.research.va.gov/about/factsheets/chf_factsheet.pdf. Published July 2014. Accessed October 9, 2017.
6. Yancy CW, Jessup M, Bozkurt B, et al; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62(16):e147-e239.
7. New York Heart Association Criteria Committee. Nomenclature and Criteria for Diagnosis of Diseases of the Heart and Great Vessels. 9th ed. Boston, MA: Little Brown; 1994.
8. Parker M, Bristow MR, Cohn JN, et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. U.S. Carvedilol Heart Failure Study Group. N Engl J Med. 1996;334(21):1349-1355.
9. CIBIS-II Investigators and Committees. The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet. 1999;353(9146):9-13.
10. MERIT-HF Study Group. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet. 1999;353(9169):2001-2007.
11. Gattis WA, Hasselblad V, Whellan DJ, O’Connor CM. Reduction in heart failure events by the addition of a clinical pharmacist to the heart failure management team: results of the Pharmacist in Heart Failure Assessment Recommendation and Monitoring (PHARM) study. Arch Intern Med. 1999;159(16):1939-1945.
12. Jain A, Mills P, Nunn LM, et al. Success of a multidisciplinary heart failure clinic for initiation and up-titration of key therapeutic agents. Eur J Heart Fail. 2005;7(3):405-410.
13. Lowrie R, Mair FS, Greenlaw N, et al; Heart Failure Optimal Outcomes From Pharmacy Study (HOOPS) Investigators. Pharmacist intervention in primary care to improve outcomes in patients with left ventricular systolic dysfunction. Eur Heart J. 2012;33(3):314-324.
14. Martinez AS, Saef J, Paszczuk A, Bhatt-Chugani H. Implementation of a pharmacist-managed heart failure medication titration clinic. Am J Health Syst Pharm. 2013;70(12):1070-1076.
1. Go AS, Mozaffarian D, Roger VL, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2013 update: a report from the American Heart Association. Circulation. 2013;127(1):e6-e245.
2. Roger VL, Weston SA, Redfield MM, et al. Trends in heart failure incidence and survival in a community-based population. JAMA. 2004;292(3):344-350.
3. Krumholz HM, Merrill AR, Schone EM, et al. Patterns of hospital performance in acute myocardial infarction and heart failure 30-day mortality and readmission. Circ Cardiovasc Qual Outcomes. 2009;2(5):407-413.
4. Setoguchi S, Stevenson LW, Schneeweiss S. Repeated hospitalizations predict mortality in the community population with heart failure. Am Heart J. 2007;154(2):260-266.
5. U.S. Department of Veterans Affairs, VA Office of Research and Development, Executive Committee, Chronic Heart Failure Quality Enhancement Research Initiative (CHF-QUERI), Health Services Research and Development Service. Chronic heart failure [QUERI fact sheet]. https://www.queri.research.va.gov/about/factsheets/chf_factsheet.pdf. Published July 2014. Accessed October 9, 2017.
6. Yancy CW, Jessup M, Bozkurt B, et al; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62(16):e147-e239.
7. New York Heart Association Criteria Committee. Nomenclature and Criteria for Diagnosis of Diseases of the Heart and Great Vessels. 9th ed. Boston, MA: Little Brown; 1994.
8. Parker M, Bristow MR, Cohn JN, et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. U.S. Carvedilol Heart Failure Study Group. N Engl J Med. 1996;334(21):1349-1355.
9. CIBIS-II Investigators and Committees. The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet. 1999;353(9146):9-13.
10. MERIT-HF Study Group. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet. 1999;353(9169):2001-2007.
11. Gattis WA, Hasselblad V, Whellan DJ, O’Connor CM. Reduction in heart failure events by the addition of a clinical pharmacist to the heart failure management team: results of the Pharmacist in Heart Failure Assessment Recommendation and Monitoring (PHARM) study. Arch Intern Med. 1999;159(16):1939-1945.
12. Jain A, Mills P, Nunn LM, et al. Success of a multidisciplinary heart failure clinic for initiation and up-titration of key therapeutic agents. Eur J Heart Fail. 2005;7(3):405-410.
13. Lowrie R, Mair FS, Greenlaw N, et al; Heart Failure Optimal Outcomes From Pharmacy Study (HOOPS) Investigators. Pharmacist intervention in primary care to improve outcomes in patients with left ventricular systolic dysfunction. Eur Heart J. 2012;33(3):314-324.
14. Martinez AS, Saef J, Paszczuk A, Bhatt-Chugani H. Implementation of a pharmacist-managed heart failure medication titration clinic. Am J Health Syst Pharm. 2013;70(12):1070-1076.
FDA Boxed Warnings Updates: October 2018
The FDA’s MedWatch program safety labeling changes for boxed warnings are compiled quarterly for drugs and therapeutic biologics where important changes have been made to the safety information. These and other label changes are searchable in the Drug Safety Labeling Changes (SLC) database, where data are available to the public in downloadable and searchable formats. Boxed warnings are ordinarily used to highlight either adverse reactions so serious in proportion to the potential benefi t from the drug that it is essential that it be considered in assessing the risks and benefi ts of using the drug; or serious adverse reactions that can be prevented/reduced in frequency or severity by appropriate use of the drug; or FDA approved the drug with restrictions to ensure safe use because FDA concluded that the drug can be safely used only if distribution or use is restricted. For complete FDA Drug Safety Labeling changes, please visit http://www.accessdata.fda.gov/scripts/cder/safetylabelingchanges.
DESOGEN (DESOGESTREL AND ETHINYL ESTRADIOL TABLETS
- Edited boxed warning, June 2018
WARNING: CIGARETTE SMOKING AND SERIOUS CARDIOVASCULAR EVENTS
Cigarette smoking increases the risk of serious cardiovascular events from combination oral contraceptive (COC) use. This risk increases with age, particularly in women over 35 years of age, and with the number of cigarettes smoked. For this reason, COCs are contraindicated in women who are over 35 years of age, and smoke.
TRIZIVIR (ABACAVIR, LAMIVUDINE, AND ZIDOVUDINE TABLETS)
- Edited boxed warning, April 2018
Lactic Acidosis and Severe Hepatomegaly with Steatosis: Lactic acidosis and severe hepatomegaly with steatosis, including fatal cases, have been reported with the use of nucleoside analogues, including abacavir, lamivudine, and zidovudine (components of TRIZIVIR). Discontinue TRIZIVIR if clinical or laboratory findings suggestive of lactic acidosis or pronounced hepatotoxicity occur [see Warnings and Precautions (5.4)].
ERBITUX (CETUXIMAB)
- Edited boxed warning, June 2018
WARNING: INFUSION REACTIONS and CARDIOPULMONARY ARREST
Infusion Reactions: ERBITUX can cause serious and fatal infusion reactions [see Warnings and Precautions (5.1), Adverse Reactions (6)]. Immediately interrupt and permanently discontinue ERBITUX for serious infusion reactions [see Dosage and Administration (2.4)].
Cardiopulmonary Arrest: Cardiopulmonary arrest or sudden death occurred in patients with squamous cell carcinoma of the head and neck receiving ERBITUX with radiation therapy or a cetuximab product with platinum-based therapy and fluorouracil. Monitor serum electrolytes, including serum magnesium, potassium, and calcium, during and after ERBITUX administration [see Warnings and Precautions (5.2, 5.6)].
AUSTEDO (DEUTETRABENAZINE)
- Edited boxed warning, June 2018
WARNING: DEPRESSION AND SUICIDALITY IN PATIENTS WITH HUNTINGTON’S DISEASE
AUSTEDO can increase the risk of depression …
EXJADE (DEFERASIROX)
- Edited boxed warning, May 2018
Renal Failure: EXJADE can cause acute renal failure and death, particularly in patients with comorbidities and those who are in the advanced stages of their hematologic disorders. Evaluate baseline renal function prior to starting or increasing Exjade dosing in all patients. Exjade is contraindicated in adult and pediatric patients with eGFR less than 40 mL/min/1.73 m2. Measure serum creatinine in duplicate prior to initiation of therapy. Monitor renal function at least monthly. For patients with baseline renal impairment or increased risk of acute renal failure, monitor renal function weekly for the first month, then at least monthly. Reduce the starting dose in patients with pre-existing renal disease. During therapy, increase the frequency of monitoring and modify the dose for patients with an increased risk of renal impairment, including use of concomitant nephrotoxic drugs, and pediatric patients with volume depletion or overchelation.
TUXARIN ER (CODEINE PHOSPHATE AND CHLORPHENIRAMINE MALEATE)
- Edited boxed warning, June 2018
WARNING: ADDICTION, ABUSE, AND MISUSE; LIFE-THREATENING RESPIRATORY DEPRESSION; ACCIDENTAL INGESTION; ULTRA-RAPID METABOLISM OF CODEINE AND OTHER RISK FACTORS FOR LIFE-THREATENING RESPIRATORY DEPRESSION IN CHILDREN; MEDICATION ERRORS; INTERACTIONS WITH DRUGS AFFECTING CYTOCHROME P450 ISOENZYMES; CONCOMITANT USE WITH BENZODIAZEPINES OR OTHER CNS DEPRESSANTS; NEONATAL OPIOID WITHDRAWAL SYNDROME.
Addiction, Abuse, and Misuse: TUXARIN ER exposes patients and other users to the risks of opioid addiction, abuse, and misuse,
which can lead to overdose and death. Reserve TUXARIN ER for use in adult patients for whom the benefits of cough suppression
are expected to outweigh the risks, and in whom an adequate assessment of the etiology of the cough has been made. Assess each
patient’s risk prior to prescribing TUXARIN ER, prescribe TUXARIN ER for the shortest duration that is consistent with individual patient
treatment goals, monitor all patients regularly for the development of addition or abuse, and refill only after reevaluation of the need for continued treatment. [see Warnings and Precautions (5.1)]
Life-Threatening Respiratory Depression: Serious, life-threatening, or fatal respiratory depression may occur with use of TUXARIN ER.
Monitor for respiratory depression, especially during initiation of TUXARIN ER therapy or when used in patients at higher risk [see Warnings and Precautions (5.2)].
Accidental Ingestion: Accidental ingestion of even one dose of TUXARIN ER, especially by children, can result in a fatal overdose of codeine [see Warnings and Precautions (5.2)].
Ultra-Rapid Metabolism of Codeine and Other Risk Factors for Life-Threatening Respiratory Depression in Children: Life threatening
respiratory depression and death have occurred in children who received codeine. Most of the reported cases occurred following tonsillectomy and/or adenoidectomy, and many of the children had evidence of being an ultra-rapid metabolizer of codeine due to a
CYP2D6 polymorphism. [see Warnings and Precautions (5.3)]. TUXARIN ER is contraindicated in children younger than 12 years of age and
in children younger than 18 years of age following tonsillectomy and/or adenoidectomy [see Contraindications (4)]. Avoid the use of TUXARIN ER in adolescents 12 to 18 years of age who have other risk factors that may increase their sensitivity to the respiratory depressant effects of codeine. [see Warnings and Precautions (5.1)].
Interactions with Drugs Affecting Cytochrome P450 Isoenzymes: The effects of concomitant use or discontinuation of cytochrome P450 3A4 inducers, 3A4 inhibitors, or 2D6 inhibitors with codeine are complex, requiring careful consideration of the effects on the parent drug, codeine, and the active metabolite, morphine. Avoid the use of TUXARIN ER in patients who are taking a CYP3A4 inhibitor, CYP3A4 inducer, or 2D6 inhibitor [see Warnings and Precautions (5.8), Drug Interactions (7.1,7.2, 7.4)]
Risks from Concomitant Use with Benzodiazepines, CNS Depressants: Concomitant use of opioids with benzodiazepines or other central nervous system (CNS) depressants, including alcohol, may result in profound sedation, respiratory depression, coma, and death. Avoid use of TUXARIN ER in patients taking benzodiazepines, other CNS depressants, or alcohol. [see Warning and Precautions (5.9) Drug Interactions (7.5)].
Neonatal Opioid Withdrawal Syndrome: TUXARIN ER is not recommended for use in pregnant women [see Use in Specific Populations
(8.1)]. Prolonged use of TUXARIN ER during pregnancy can result in neonatal opioid withdrawal syndrome, which may be life-threatening if not recognized and treated, and requires management according to protocols developed by neonatology experts. If TUXARIN ER is used for a prolonged period in a pregnant woman, advise the patient of the risk of neonatal opioid withdrawal syndrome and ensure that appropriate treatment will be available [see Warnings and Precautions (5.15)].
PROGRAF (TACROLIMUS)
- Edited boxed warning, May 2018
Increased risk for developing serious infections and malignancies with PROGRAF or other immunosuppressants that may lead to hospitalization or death [(see Warnings and Precautions 5.1,5.2)].
SAMSCA (TOLVAPTAN)
- Edited boxed warning, April 2018
Addition of the following:
WARNING: NOT FOR USE FOR AUTOSOMAL DOMINANT POLYCYSTIC KIDNEY DISEASE (ADPKD)
Because of the risk of hepatoxicity, tolvaptan should not be used for ADPKD outside of the FDA-approved REMS
GLUCOPHAGE/GLUCOPHAGE XR (METFORMIN HYDROCHLORIDE)
- Edited boxed warning, May 2018
PLR conversion; Lactic Acidosis warning is highlighted as boxed warning.
The FDA’s MedWatch program safety labeling changes for boxed warnings are compiled quarterly for drugs and therapeutic biologics where important changes have been made to the safety information. These and other label changes are searchable in the Drug Safety Labeling Changes (SLC) database, where data are available to the public in downloadable and searchable formats. Boxed warnings are ordinarily used to highlight either adverse reactions so serious in proportion to the potential benefi t from the drug that it is essential that it be considered in assessing the risks and benefi ts of using the drug; or serious adverse reactions that can be prevented/reduced in frequency or severity by appropriate use of the drug; or FDA approved the drug with restrictions to ensure safe use because FDA concluded that the drug can be safely used only if distribution or use is restricted. For complete FDA Drug Safety Labeling changes, please visit http://www.accessdata.fda.gov/scripts/cder/safetylabelingchanges.
DESOGEN (DESOGESTREL AND ETHINYL ESTRADIOL TABLETS
- Edited boxed warning, June 2018
WARNING: CIGARETTE SMOKING AND SERIOUS CARDIOVASCULAR EVENTS
Cigarette smoking increases the risk of serious cardiovascular events from combination oral contraceptive (COC) use. This risk increases with age, particularly in women over 35 years of age, and with the number of cigarettes smoked. For this reason, COCs are contraindicated in women who are over 35 years of age, and smoke.
TRIZIVIR (ABACAVIR, LAMIVUDINE, AND ZIDOVUDINE TABLETS)
- Edited boxed warning, April 2018
Lactic Acidosis and Severe Hepatomegaly with Steatosis: Lactic acidosis and severe hepatomegaly with steatosis, including fatal cases, have been reported with the use of nucleoside analogues, including abacavir, lamivudine, and zidovudine (components of TRIZIVIR). Discontinue TRIZIVIR if clinical or laboratory findings suggestive of lactic acidosis or pronounced hepatotoxicity occur [see Warnings and Precautions (5.4)].
ERBITUX (CETUXIMAB)
- Edited boxed warning, June 2018
WARNING: INFUSION REACTIONS and CARDIOPULMONARY ARREST
Infusion Reactions: ERBITUX can cause serious and fatal infusion reactions [see Warnings and Precautions (5.1), Adverse Reactions (6)]. Immediately interrupt and permanently discontinue ERBITUX for serious infusion reactions [see Dosage and Administration (2.4)].
Cardiopulmonary Arrest: Cardiopulmonary arrest or sudden death occurred in patients with squamous cell carcinoma of the head and neck receiving ERBITUX with radiation therapy or a cetuximab product with platinum-based therapy and fluorouracil. Monitor serum electrolytes, including serum magnesium, potassium, and calcium, during and after ERBITUX administration [see Warnings and Precautions (5.2, 5.6)].
AUSTEDO (DEUTETRABENAZINE)
- Edited boxed warning, June 2018
WARNING: DEPRESSION AND SUICIDALITY IN PATIENTS WITH HUNTINGTON’S DISEASE
AUSTEDO can increase the risk of depression …
EXJADE (DEFERASIROX)
- Edited boxed warning, May 2018
Renal Failure: EXJADE can cause acute renal failure and death, particularly in patients with comorbidities and those who are in the advanced stages of their hematologic disorders. Evaluate baseline renal function prior to starting or increasing Exjade dosing in all patients. Exjade is contraindicated in adult and pediatric patients with eGFR less than 40 mL/min/1.73 m2. Measure serum creatinine in duplicate prior to initiation of therapy. Monitor renal function at least monthly. For patients with baseline renal impairment or increased risk of acute renal failure, monitor renal function weekly for the first month, then at least monthly. Reduce the starting dose in patients with pre-existing renal disease. During therapy, increase the frequency of monitoring and modify the dose for patients with an increased risk of renal impairment, including use of concomitant nephrotoxic drugs, and pediatric patients with volume depletion or overchelation.
TUXARIN ER (CODEINE PHOSPHATE AND CHLORPHENIRAMINE MALEATE)
- Edited boxed warning, June 2018
WARNING: ADDICTION, ABUSE, AND MISUSE; LIFE-THREATENING RESPIRATORY DEPRESSION; ACCIDENTAL INGESTION; ULTRA-RAPID METABOLISM OF CODEINE AND OTHER RISK FACTORS FOR LIFE-THREATENING RESPIRATORY DEPRESSION IN CHILDREN; MEDICATION ERRORS; INTERACTIONS WITH DRUGS AFFECTING CYTOCHROME P450 ISOENZYMES; CONCOMITANT USE WITH BENZODIAZEPINES OR OTHER CNS DEPRESSANTS; NEONATAL OPIOID WITHDRAWAL SYNDROME.
Addiction, Abuse, and Misuse: TUXARIN ER exposes patients and other users to the risks of opioid addiction, abuse, and misuse,
which can lead to overdose and death. Reserve TUXARIN ER for use in adult patients for whom the benefits of cough suppression
are expected to outweigh the risks, and in whom an adequate assessment of the etiology of the cough has been made. Assess each
patient’s risk prior to prescribing TUXARIN ER, prescribe TUXARIN ER for the shortest duration that is consistent with individual patient
treatment goals, monitor all patients regularly for the development of addition or abuse, and refill only after reevaluation of the need for continued treatment. [see Warnings and Precautions (5.1)]
Life-Threatening Respiratory Depression: Serious, life-threatening, or fatal respiratory depression may occur with use of TUXARIN ER.
Monitor for respiratory depression, especially during initiation of TUXARIN ER therapy or when used in patients at higher risk [see Warnings and Precautions (5.2)].
Accidental Ingestion: Accidental ingestion of even one dose of TUXARIN ER, especially by children, can result in a fatal overdose of codeine [see Warnings and Precautions (5.2)].
Ultra-Rapid Metabolism of Codeine and Other Risk Factors for Life-Threatening Respiratory Depression in Children: Life threatening
respiratory depression and death have occurred in children who received codeine. Most of the reported cases occurred following tonsillectomy and/or adenoidectomy, and many of the children had evidence of being an ultra-rapid metabolizer of codeine due to a
CYP2D6 polymorphism. [see Warnings and Precautions (5.3)]. TUXARIN ER is contraindicated in children younger than 12 years of age and
in children younger than 18 years of age following tonsillectomy and/or adenoidectomy [see Contraindications (4)]. Avoid the use of TUXARIN ER in adolescents 12 to 18 years of age who have other risk factors that may increase their sensitivity to the respiratory depressant effects of codeine. [see Warnings and Precautions (5.1)].
Interactions with Drugs Affecting Cytochrome P450 Isoenzymes: The effects of concomitant use or discontinuation of cytochrome P450 3A4 inducers, 3A4 inhibitors, or 2D6 inhibitors with codeine are complex, requiring careful consideration of the effects on the parent drug, codeine, and the active metabolite, morphine. Avoid the use of TUXARIN ER in patients who are taking a CYP3A4 inhibitor, CYP3A4 inducer, or 2D6 inhibitor [see Warnings and Precautions (5.8), Drug Interactions (7.1,7.2, 7.4)]
Risks from Concomitant Use with Benzodiazepines, CNS Depressants: Concomitant use of opioids with benzodiazepines or other central nervous system (CNS) depressants, including alcohol, may result in profound sedation, respiratory depression, coma, and death. Avoid use of TUXARIN ER in patients taking benzodiazepines, other CNS depressants, or alcohol. [see Warning and Precautions (5.9) Drug Interactions (7.5)].
Neonatal Opioid Withdrawal Syndrome: TUXARIN ER is not recommended for use in pregnant women [see Use in Specific Populations
(8.1)]. Prolonged use of TUXARIN ER during pregnancy can result in neonatal opioid withdrawal syndrome, which may be life-threatening if not recognized and treated, and requires management according to protocols developed by neonatology experts. If TUXARIN ER is used for a prolonged period in a pregnant woman, advise the patient of the risk of neonatal opioid withdrawal syndrome and ensure that appropriate treatment will be available [see Warnings and Precautions (5.15)].
PROGRAF (TACROLIMUS)
- Edited boxed warning, May 2018
Increased risk for developing serious infections and malignancies with PROGRAF or other immunosuppressants that may lead to hospitalization or death [(see Warnings and Precautions 5.1,5.2)].
SAMSCA (TOLVAPTAN)
- Edited boxed warning, April 2018
Addition of the following:
WARNING: NOT FOR USE FOR AUTOSOMAL DOMINANT POLYCYSTIC KIDNEY DISEASE (ADPKD)
Because of the risk of hepatoxicity, tolvaptan should not be used for ADPKD outside of the FDA-approved REMS
GLUCOPHAGE/GLUCOPHAGE XR (METFORMIN HYDROCHLORIDE)
- Edited boxed warning, May 2018
PLR conversion; Lactic Acidosis warning is highlighted as boxed warning.
The FDA’s MedWatch program safety labeling changes for boxed warnings are compiled quarterly for drugs and therapeutic biologics where important changes have been made to the safety information. These and other label changes are searchable in the Drug Safety Labeling Changes (SLC) database, where data are available to the public in downloadable and searchable formats. Boxed warnings are ordinarily used to highlight either adverse reactions so serious in proportion to the potential benefi t from the drug that it is essential that it be considered in assessing the risks and benefi ts of using the drug; or serious adverse reactions that can be prevented/reduced in frequency or severity by appropriate use of the drug; or FDA approved the drug with restrictions to ensure safe use because FDA concluded that the drug can be safely used only if distribution or use is restricted. For complete FDA Drug Safety Labeling changes, please visit http://www.accessdata.fda.gov/scripts/cder/safetylabelingchanges.
DESOGEN (DESOGESTREL AND ETHINYL ESTRADIOL TABLETS
- Edited boxed warning, June 2018
WARNING: CIGARETTE SMOKING AND SERIOUS CARDIOVASCULAR EVENTS
Cigarette smoking increases the risk of serious cardiovascular events from combination oral contraceptive (COC) use. This risk increases with age, particularly in women over 35 years of age, and with the number of cigarettes smoked. For this reason, COCs are contraindicated in women who are over 35 years of age, and smoke.
TRIZIVIR (ABACAVIR, LAMIVUDINE, AND ZIDOVUDINE TABLETS)
- Edited boxed warning, April 2018
Lactic Acidosis and Severe Hepatomegaly with Steatosis: Lactic acidosis and severe hepatomegaly with steatosis, including fatal cases, have been reported with the use of nucleoside analogues, including abacavir, lamivudine, and zidovudine (components of TRIZIVIR). Discontinue TRIZIVIR if clinical or laboratory findings suggestive of lactic acidosis or pronounced hepatotoxicity occur [see Warnings and Precautions (5.4)].
ERBITUX (CETUXIMAB)
- Edited boxed warning, June 2018
WARNING: INFUSION REACTIONS and CARDIOPULMONARY ARREST
Infusion Reactions: ERBITUX can cause serious and fatal infusion reactions [see Warnings and Precautions (5.1), Adverse Reactions (6)]. Immediately interrupt and permanently discontinue ERBITUX for serious infusion reactions [see Dosage and Administration (2.4)].
Cardiopulmonary Arrest: Cardiopulmonary arrest or sudden death occurred in patients with squamous cell carcinoma of the head and neck receiving ERBITUX with radiation therapy or a cetuximab product with platinum-based therapy and fluorouracil. Monitor serum electrolytes, including serum magnesium, potassium, and calcium, during and after ERBITUX administration [see Warnings and Precautions (5.2, 5.6)].
AUSTEDO (DEUTETRABENAZINE)
- Edited boxed warning, June 2018
WARNING: DEPRESSION AND SUICIDALITY IN PATIENTS WITH HUNTINGTON’S DISEASE
AUSTEDO can increase the risk of depression …
EXJADE (DEFERASIROX)
- Edited boxed warning, May 2018
Renal Failure: EXJADE can cause acute renal failure and death, particularly in patients with comorbidities and those who are in the advanced stages of their hematologic disorders. Evaluate baseline renal function prior to starting or increasing Exjade dosing in all patients. Exjade is contraindicated in adult and pediatric patients with eGFR less than 40 mL/min/1.73 m2. Measure serum creatinine in duplicate prior to initiation of therapy. Monitor renal function at least monthly. For patients with baseline renal impairment or increased risk of acute renal failure, monitor renal function weekly for the first month, then at least monthly. Reduce the starting dose in patients with pre-existing renal disease. During therapy, increase the frequency of monitoring and modify the dose for patients with an increased risk of renal impairment, including use of concomitant nephrotoxic drugs, and pediatric patients with volume depletion or overchelation.
TUXARIN ER (CODEINE PHOSPHATE AND CHLORPHENIRAMINE MALEATE)
- Edited boxed warning, June 2018
WARNING: ADDICTION, ABUSE, AND MISUSE; LIFE-THREATENING RESPIRATORY DEPRESSION; ACCIDENTAL INGESTION; ULTRA-RAPID METABOLISM OF CODEINE AND OTHER RISK FACTORS FOR LIFE-THREATENING RESPIRATORY DEPRESSION IN CHILDREN; MEDICATION ERRORS; INTERACTIONS WITH DRUGS AFFECTING CYTOCHROME P450 ISOENZYMES; CONCOMITANT USE WITH BENZODIAZEPINES OR OTHER CNS DEPRESSANTS; NEONATAL OPIOID WITHDRAWAL SYNDROME.
Addiction, Abuse, and Misuse: TUXARIN ER exposes patients and other users to the risks of opioid addiction, abuse, and misuse,
which can lead to overdose and death. Reserve TUXARIN ER for use in adult patients for whom the benefits of cough suppression
are expected to outweigh the risks, and in whom an adequate assessment of the etiology of the cough has been made. Assess each
patient’s risk prior to prescribing TUXARIN ER, prescribe TUXARIN ER for the shortest duration that is consistent with individual patient
treatment goals, monitor all patients regularly for the development of addition or abuse, and refill only after reevaluation of the need for continued treatment. [see Warnings and Precautions (5.1)]
Life-Threatening Respiratory Depression: Serious, life-threatening, or fatal respiratory depression may occur with use of TUXARIN ER.
Monitor for respiratory depression, especially during initiation of TUXARIN ER therapy or when used in patients at higher risk [see Warnings and Precautions (5.2)].
Accidental Ingestion: Accidental ingestion of even one dose of TUXARIN ER, especially by children, can result in a fatal overdose of codeine [see Warnings and Precautions (5.2)].
Ultra-Rapid Metabolism of Codeine and Other Risk Factors for Life-Threatening Respiratory Depression in Children: Life threatening
respiratory depression and death have occurred in children who received codeine. Most of the reported cases occurred following tonsillectomy and/or adenoidectomy, and many of the children had evidence of being an ultra-rapid metabolizer of codeine due to a
CYP2D6 polymorphism. [see Warnings and Precautions (5.3)]. TUXARIN ER is contraindicated in children younger than 12 years of age and
in children younger than 18 years of age following tonsillectomy and/or adenoidectomy [see Contraindications (4)]. Avoid the use of TUXARIN ER in adolescents 12 to 18 years of age who have other risk factors that may increase their sensitivity to the respiratory depressant effects of codeine. [see Warnings and Precautions (5.1)].
Interactions with Drugs Affecting Cytochrome P450 Isoenzymes: The effects of concomitant use or discontinuation of cytochrome P450 3A4 inducers, 3A4 inhibitors, or 2D6 inhibitors with codeine are complex, requiring careful consideration of the effects on the parent drug, codeine, and the active metabolite, morphine. Avoid the use of TUXARIN ER in patients who are taking a CYP3A4 inhibitor, CYP3A4 inducer, or 2D6 inhibitor [see Warnings and Precautions (5.8), Drug Interactions (7.1,7.2, 7.4)]
Risks from Concomitant Use with Benzodiazepines, CNS Depressants: Concomitant use of opioids with benzodiazepines or other central nervous system (CNS) depressants, including alcohol, may result in profound sedation, respiratory depression, coma, and death. Avoid use of TUXARIN ER in patients taking benzodiazepines, other CNS depressants, or alcohol. [see Warning and Precautions (5.9) Drug Interactions (7.5)].
Neonatal Opioid Withdrawal Syndrome: TUXARIN ER is not recommended for use in pregnant women [see Use in Specific Populations
(8.1)]. Prolonged use of TUXARIN ER during pregnancy can result in neonatal opioid withdrawal syndrome, which may be life-threatening if not recognized and treated, and requires management according to protocols developed by neonatology experts. If TUXARIN ER is used for a prolonged period in a pregnant woman, advise the patient of the risk of neonatal opioid withdrawal syndrome and ensure that appropriate treatment will be available [see Warnings and Precautions (5.15)].
PROGRAF (TACROLIMUS)
- Edited boxed warning, May 2018
Increased risk for developing serious infections and malignancies with PROGRAF or other immunosuppressants that may lead to hospitalization or death [(see Warnings and Precautions 5.1,5.2)].
SAMSCA (TOLVAPTAN)
- Edited boxed warning, April 2018
Addition of the following:
WARNING: NOT FOR USE FOR AUTOSOMAL DOMINANT POLYCYSTIC KIDNEY DISEASE (ADPKD)
Because of the risk of hepatoxicity, tolvaptan should not be used for ADPKD outside of the FDA-approved REMS
GLUCOPHAGE/GLUCOPHAGE XR (METFORMIN HYDROCHLORIDE)
- Edited boxed warning, May 2018
PLR conversion; Lactic Acidosis warning is highlighted as boxed warning.
Tedizolid Use in Immunocompromised Patients
Immunocompromised patients are often susceptible to opportunistic infections, including those caused by multidrug-resistant organisms (MDROs). Transplant recipients are at high risk for developing infections due to lifelong immunosuppressive therapy.1,2 Additionally, patients receiving chemotherapy and those with HIV and AIDS are in an immunocompromised state.3-8
Regardless of the etiology for immunosuppression, decreased absolute neutrophil and platelet counts are seen in this condition. Although immunosuppressed individuals may be at increased risk of Gram-negative or Gram-positive infections, this review focuses on the treatment of Gram-positive bacterial infections. Of particular concern are opportunistic infections caused by Gram-positive MDROs, including methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant Enterococcus species (VRE), penicillin-resistant Streptococcus pneumoniae, and Nocardia species. Treatment of infections in the immunocompromised patient population warrants careful antimicrobial selection to ensure that a patient’s immune system is not further compromised due to adverse effects (AEs) secondary to therapy. As such, clinicians are exploring alternative antimicrobials, such as tedizolid, to treat various opportunistic infections.
Recently, requests at the William S. Middleton Memorial Veterans Hospital in Madison, Wisconsin, for off-label use of tedizolid have increased despite having other cheaper alternatives with comparable Gram-positive coverage. This review examines available literature regarding off-label use of tedizolid with a focus on use in immunocompromised patients.
Tedizolid phosphate (Sivextro) is an oxazolidinone antibiotic prodrug that joined linezolid as the second in its class in 2014. Oxazolidinones inhibit bacterial protein synthesis by binding to the 50S subunit of bacterial ribosomes in Gram-positive bacteria and are often used to treat MRSA and VRE infections.9 In vitro, oxazolidinones have shown bacteriostatic activity against Enterococcus and Staphylococcus species while exhibiting bactericidal activity against most Streptococcus species.10 Tedizolid has a US Food and Drug Administration (FDA) -approved, simplified dosing profile of 200 mg daily for 6 days compared with linezolid 400 to 600 mg twice daily for 10 to 14 days. Both medications are highly bioavailable with direct IV to oral conversion.11,12 Potential, expanded use of tedizolid against Gram-positive MDROs rests on a more favorable AE profile than does its linezolid predecessor. Tedizolid has been associated with less antibiotic-induced myelosuppression, which could prove valuable for immunocompromised patients.13
Tedizolid is approved for the sole indication of acute bacterial skin and skin structure infections (ABSSSI), whereas its predecessor has many approved indications and has been used extensively for off-label indications (Table).
Adverse reactions, as determined by 2 phase 2 and 2 phase 3 clinical trials evaluating 1,050 patients treated with tedizolid and 662 patients treated with linezolid, were similar between the oxazolidinones. Nausea was the most common AE and was reported in 8% and 12% of patients taking tedizolid and linezolid, respectively. Other common AEs (1%-6%) reported for both agents included vomiting, diarrhea, headache, and dizziness.11 Myelosuppression, peripheral neuropathy, and optic nerve disorders were the most common severe AEs reported with oxazolidinones. Tedizolid demonstrated a significantly decreased incidence of neutropenia (3%), defined by absolute neutrophil count 9/L compared with that of linezolid (7%) (P = .024).13 Evaluation of peripheral neuropathy and optic nerve disorders within the tedizolid and linezolid groups revealed similar incidences (peripheral neuropathy 1.2% vs 0.6%; optic nerve disorders 0.3% vs 0.2%, respectively).11
There is one preclinical trial that described the use of tedizolid in a murine model. A murine model study compared the antistaphylococcal killing effect of doses of tedizolid equivalent to human exposures ranging from 200 to 3,200 mg/d in both granulocytopenic and normal mice. The mice were evaluated at 24, 48, and 72 hours after therapy initiation. The presence of granulocytes had a dramatic effect on the antimicrobial effect of tedizolid. Dose response, demonstrated by the ratio of the area under the curve over the minimum inhibitory concentration, was on average > 25-fold for nonneutropenic vs neutropenic models. Near maximal effect of the nonneutropenic group, irrespective of duration of therapy, was achieved at the lowest dose tested (an exposure of about 200-mg tedizolid phosphate per day in humans).This study suggests that immunocompromised patients may warrant higher doses of tedizolid than the currently FDA-approved dose due to a decreased number of granulocytes available for modulating bacterial infections.15
Use of tedizolid doses higher than that which is FDA-approved may negate the favorable AE profile. A phase 1 clinical study was conducted to evaluate the safety, tolerability, and pharmacokinetics of tedizolid compared with those of linezolid in 40 healthy volunteers in a 21-day multiple ascending dose study.16 Subjects were stratified into 5 treatment cohorts: 200-, 300-, or 400-mg tedizolid orally once a day, 600-mg linezolid orally twice a day, and placebo. Tedizolid given at 200 mg had a hematologic safety profile similar to that of placebo. However, mean platelet counts decreased over time in a dose-dependent manner for tedizolid, with the 400-mg tedizolid and linezolid groups reporting similar reductions in platelet counts.16
Some evidence is available examining linezolid in neutropenic patients. Rafailidis and colleagues reviewed available literature regarding linezolid in neutropenic patients with Gram-positive infections. Evaluation of linezolid administration at usual doses to 438 neutropenic patients from 2 prospective comparative studies, a prospective cohort study, 2 retrospective studies, and 8 case reports was performed. Results of the evaluation revealed a clinical cure rate between 57% and 87% in the intention-to-treat population of the prospective studies.17 Given the similarities in bacterial spectrum of activity between linezolid and tedizolid, it may be reasonable to infer that tedizolid’s decreased myelosuppression profile would make it useful in the setting of neutropenia in immunocompromised patients.
There is little evidence regarding the use of tedizolid in immunocompromised patients, as only 2 case reports were found. The first described a 60-year-old male postrenal transplant complicated with VRE bacteremia, rhabdomyolysis, and thrombocytopenia. This patient was treated with prolonged tedizolid 200 mg daily due to multiple contraindications for treatment with other antibiotics. The patient was cured with a 14-day course of tedizolid without any noted AEs.18
The second identified case report described the use of tedizolid for the treatment of central nervous system (CNS) manifestations secondary to nocardiosis. Effective treatment of CNS nocardiosis requires high concentrations and prolonged duration of antimicrobial exposure. This case report described a 68-year-old, chronically immunocompromised female patient with multiple myeloma who was hospitalized for 3 months for the treatment of a CNS nocardiosis infection. After discharge, the patient was treated with an oral regimen of 200-mg tedizolid daily in combination with sulfamethoxazole/trimethoprim (800 mg/160 mg) 3 times daily. After 6 months of combination therapy, magnetic resonance imaging revealed complete resolution of nocardiosis-related central lesions. Although the patient’s malignancy advanced during combination antibiotic therapy, the patient’s absolute neutrophil count remained stable and showed an increase in absolute CD4+ cell counts with no other documented AEs.19
Tedizolid is the latest FDA-approved oxazolidinone antibiotic for susceptible Gram-positive acute bacterial skin and skin structure infections. It has a simplified and shorter duration of treatment and imparts similar AEs at improved rates compared with that of linezolid, most notably in relation to hematologic AEs. Due to the lack of established literature and an agreed-upon dosing strategy for the use of tedizolid in immunocompromised patients, tedizolid therapy for Gram-positive infections in immunocompromised patients should be reserved for salvage therapy when more established Gram-positive antibiotic agents lack efficacy or when patient contraindications to their use exist.
1. Fishman JA, Issa NC. Infection in organ transplantation: risk factors and evolving patterns of infection. Infect Dis Clin North Am. 2010;24(2):273-283.
2. Fishman JA. Infection in solid-organ transplant recipients. N Engl J Med. 2007;357(25):2601-2614.
3. Nightingale SD, Byrd LT, Southern PM, Jockusch JD, Cal SX, Wynne BA. Incidence of mycobacterium avium-intracellulare complex bacteremia in human immunodeficiency virus-positive patients. J Infect Dis. 1992;165(6):1082-1085.
4. O’Brien S, Kantarjian H, Beran M, et al. Results of fludarabine and prednisone therapy in 264 patients with chronic lymphocytic leukemia with multivariate analysis-derived prognostic model for response to treatment. Blood. 1993;82(6):1695-1700.
5. Anaissie E, Kontoyiannis DP, Kantarjian H, Elting L, Robertson LE, Keating M. Listeriosis in patients with chronic lymphocytic leukemia who were treated with fludarabine and prednisone. Ann Intern Med. 1992;117(6):466-469.
6. Morrison VA, Rai KR, Peterson BL, et al. Impact of therapy with chlorambucil, fludarabine, or fludarabine plus chlorambucil on infections in patients with chronic lymphocytic leukemia: Intergroup Study Cancer and Leukemia Group B 9011. J Clin Oncol. 2001;19(16):3611-3621.
7. Nucci M, Anaissie E. Infections in patients with multiple myeloma in the era of high-dose therapy and novel agents. Clin Infect Dis. 2009;49(8):1211-1225.
8. Naseer M, Dailey FE, Juboori AA, Samiullah S, Tahan V. Epidemiology, determinants, and managements of AIDS cholangiopathy: a review. World J Gastroenterol. 2018;24(7):767-774.
9. Radunz S, Juntermanns B, Kaiser GM, et al. Efficacy and safety of linezolid in liver transplant patients. Transpl Infect Dis. 2011;13(4):353-358.
10. Roger C, Roberts JA, Muller L. Clinical pharmacokinetics and pharmacodynamics of oxazolidinones. Clin Pharmacokinet. 2018;57(5):559-575.
11. Sivextro [package insert]. Whitehouse Station, NJ: Merck & Co Inc; 2016.
12. Zyvox [package insert]. New York, NY: Pfizer Inc; 2018.
13. Moran GJ, Fang E, Corey GR, Das AF, De Anda C, Prokocimer P. Tedizolid for 6 days versus linezolid for 10 days for acute bacterial skin and skin-structure infections (ESTABLISH-2): a randomized, double-blind, phase 3, non-inferiority trial. Lancet Infect Dis. 2014;14(8):696-705.
14. Liu C, Bayer A, Cosgrove S, et al; Infectious Diseases Society of America. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52(3):e18-e55.
15. Drusano GL, Liu W, Kulawy R, Louie A. Impact of granulocytes on the antimicrobial effect of tedizolid in a mouse thigh infection model. Antimicrob Agents Chemother. 2011;55(11):5300-5305.
16. Lodise TP, Bidell MR, Flanagan SD, Zasowski EJ, Minassian SL, Prokocimer P. Characterization of the haematological profile of 21 days of tedizolid in healthy subjects. J Antimicrob Chemother. 2016;71(9):2553-2558.
17. Rafailidis PI, Kouranos VD, Christodoulou C, Falagas ME. Linezolid for patients with neutropenia: are bacteriostatic agents appropriate? Expert Rev Anti Infect Ther. 2009;7(4):415-422.
18. Sudhindra P, Lee L, Wang G, Dhand A. Tedizolid for treatment of enterococcal bacteremia. Open Forum Infect Dis. 2016;3(suppl 1):1344.
19. Matin A, Sharma S, Mathur P, Apewokin SK. Myelosuppression-sparing treatment of central nervous system nocardiosis in a multiple myeloma patient utilizing a tedizolid-based regimen: a case report. Int J Antimicrob Agents. 2017;49(4):488-492.
20. Dryden MS. Alternative clinical indications for novel antibiotics licensed for skin and soft tissue infections? Curr Opin Infect Dis. 2015;28(2):117-124.
21. Milstein M, Brzezinski A, Varaine F, Mitnick CD. (Re)moving the needle: prospects for all-oral treatment for multidrug-resistant tuberculosis. Int J Tuberc Lung Dis. 2016;20(12):18-23.
22. Winthrop KL, Ku JH, Marras TK, et al. The tolerability of linezolid in the treatment of nontuberculous mycobacterial disease. Eur Respir J. 2015;45(4):1177-1179.
23. Yuste JR, Bertó J, Del Pozo JL, Leiva J. Prolonged use of tedizolid in a pulmonary non-tuberculosis mycobacterial infection after linezlid-induced toxicity. J Antimicrob Chemother. 2017;72(2):625-628.
Immunocompromised patients are often susceptible to opportunistic infections, including those caused by multidrug-resistant organisms (MDROs). Transplant recipients are at high risk for developing infections due to lifelong immunosuppressive therapy.1,2 Additionally, patients receiving chemotherapy and those with HIV and AIDS are in an immunocompromised state.3-8
Regardless of the etiology for immunosuppression, decreased absolute neutrophil and platelet counts are seen in this condition. Although immunosuppressed individuals may be at increased risk of Gram-negative or Gram-positive infections, this review focuses on the treatment of Gram-positive bacterial infections. Of particular concern are opportunistic infections caused by Gram-positive MDROs, including methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant Enterococcus species (VRE), penicillin-resistant Streptococcus pneumoniae, and Nocardia species. Treatment of infections in the immunocompromised patient population warrants careful antimicrobial selection to ensure that a patient’s immune system is not further compromised due to adverse effects (AEs) secondary to therapy. As such, clinicians are exploring alternative antimicrobials, such as tedizolid, to treat various opportunistic infections.
Recently, requests at the William S. Middleton Memorial Veterans Hospital in Madison, Wisconsin, for off-label use of tedizolid have increased despite having other cheaper alternatives with comparable Gram-positive coverage. This review examines available literature regarding off-label use of tedizolid with a focus on use in immunocompromised patients.
Tedizolid phosphate (Sivextro) is an oxazolidinone antibiotic prodrug that joined linezolid as the second in its class in 2014. Oxazolidinones inhibit bacterial protein synthesis by binding to the 50S subunit of bacterial ribosomes in Gram-positive bacteria and are often used to treat MRSA and VRE infections.9 In vitro, oxazolidinones have shown bacteriostatic activity against Enterococcus and Staphylococcus species while exhibiting bactericidal activity against most Streptococcus species.10 Tedizolid has a US Food and Drug Administration (FDA) -approved, simplified dosing profile of 200 mg daily for 6 days compared with linezolid 400 to 600 mg twice daily for 10 to 14 days. Both medications are highly bioavailable with direct IV to oral conversion.11,12 Potential, expanded use of tedizolid against Gram-positive MDROs rests on a more favorable AE profile than does its linezolid predecessor. Tedizolid has been associated with less antibiotic-induced myelosuppression, which could prove valuable for immunocompromised patients.13
Tedizolid is approved for the sole indication of acute bacterial skin and skin structure infections (ABSSSI), whereas its predecessor has many approved indications and has been used extensively for off-label indications (Table).
Adverse reactions, as determined by 2 phase 2 and 2 phase 3 clinical trials evaluating 1,050 patients treated with tedizolid and 662 patients treated with linezolid, were similar between the oxazolidinones. Nausea was the most common AE and was reported in 8% and 12% of patients taking tedizolid and linezolid, respectively. Other common AEs (1%-6%) reported for both agents included vomiting, diarrhea, headache, and dizziness.11 Myelosuppression, peripheral neuropathy, and optic nerve disorders were the most common severe AEs reported with oxazolidinones. Tedizolid demonstrated a significantly decreased incidence of neutropenia (3%), defined by absolute neutrophil count 9/L compared with that of linezolid (7%) (P = .024).13 Evaluation of peripheral neuropathy and optic nerve disorders within the tedizolid and linezolid groups revealed similar incidences (peripheral neuropathy 1.2% vs 0.6%; optic nerve disorders 0.3% vs 0.2%, respectively).11
There is one preclinical trial that described the use of tedizolid in a murine model. A murine model study compared the antistaphylococcal killing effect of doses of tedizolid equivalent to human exposures ranging from 200 to 3,200 mg/d in both granulocytopenic and normal mice. The mice were evaluated at 24, 48, and 72 hours after therapy initiation. The presence of granulocytes had a dramatic effect on the antimicrobial effect of tedizolid. Dose response, demonstrated by the ratio of the area under the curve over the minimum inhibitory concentration, was on average > 25-fold for nonneutropenic vs neutropenic models. Near maximal effect of the nonneutropenic group, irrespective of duration of therapy, was achieved at the lowest dose tested (an exposure of about 200-mg tedizolid phosphate per day in humans).This study suggests that immunocompromised patients may warrant higher doses of tedizolid than the currently FDA-approved dose due to a decreased number of granulocytes available for modulating bacterial infections.15
Use of tedizolid doses higher than that which is FDA-approved may negate the favorable AE profile. A phase 1 clinical study was conducted to evaluate the safety, tolerability, and pharmacokinetics of tedizolid compared with those of linezolid in 40 healthy volunteers in a 21-day multiple ascending dose study.16 Subjects were stratified into 5 treatment cohorts: 200-, 300-, or 400-mg tedizolid orally once a day, 600-mg linezolid orally twice a day, and placebo. Tedizolid given at 200 mg had a hematologic safety profile similar to that of placebo. However, mean platelet counts decreased over time in a dose-dependent manner for tedizolid, with the 400-mg tedizolid and linezolid groups reporting similar reductions in platelet counts.16
Some evidence is available examining linezolid in neutropenic patients. Rafailidis and colleagues reviewed available literature regarding linezolid in neutropenic patients with Gram-positive infections. Evaluation of linezolid administration at usual doses to 438 neutropenic patients from 2 prospective comparative studies, a prospective cohort study, 2 retrospective studies, and 8 case reports was performed. Results of the evaluation revealed a clinical cure rate between 57% and 87% in the intention-to-treat population of the prospective studies.17 Given the similarities in bacterial spectrum of activity between linezolid and tedizolid, it may be reasonable to infer that tedizolid’s decreased myelosuppression profile would make it useful in the setting of neutropenia in immunocompromised patients.
There is little evidence regarding the use of tedizolid in immunocompromised patients, as only 2 case reports were found. The first described a 60-year-old male postrenal transplant complicated with VRE bacteremia, rhabdomyolysis, and thrombocytopenia. This patient was treated with prolonged tedizolid 200 mg daily due to multiple contraindications for treatment with other antibiotics. The patient was cured with a 14-day course of tedizolid without any noted AEs.18
The second identified case report described the use of tedizolid for the treatment of central nervous system (CNS) manifestations secondary to nocardiosis. Effective treatment of CNS nocardiosis requires high concentrations and prolonged duration of antimicrobial exposure. This case report described a 68-year-old, chronically immunocompromised female patient with multiple myeloma who was hospitalized for 3 months for the treatment of a CNS nocardiosis infection. After discharge, the patient was treated with an oral regimen of 200-mg tedizolid daily in combination with sulfamethoxazole/trimethoprim (800 mg/160 mg) 3 times daily. After 6 months of combination therapy, magnetic resonance imaging revealed complete resolution of nocardiosis-related central lesions. Although the patient’s malignancy advanced during combination antibiotic therapy, the patient’s absolute neutrophil count remained stable and showed an increase in absolute CD4+ cell counts with no other documented AEs.19
Tedizolid is the latest FDA-approved oxazolidinone antibiotic for susceptible Gram-positive acute bacterial skin and skin structure infections. It has a simplified and shorter duration of treatment and imparts similar AEs at improved rates compared with that of linezolid, most notably in relation to hematologic AEs. Due to the lack of established literature and an agreed-upon dosing strategy for the use of tedizolid in immunocompromised patients, tedizolid therapy for Gram-positive infections in immunocompromised patients should be reserved for salvage therapy when more established Gram-positive antibiotic agents lack efficacy or when patient contraindications to their use exist.
Immunocompromised patients are often susceptible to opportunistic infections, including those caused by multidrug-resistant organisms (MDROs). Transplant recipients are at high risk for developing infections due to lifelong immunosuppressive therapy.1,2 Additionally, patients receiving chemotherapy and those with HIV and AIDS are in an immunocompromised state.3-8
Regardless of the etiology for immunosuppression, decreased absolute neutrophil and platelet counts are seen in this condition. Although immunosuppressed individuals may be at increased risk of Gram-negative or Gram-positive infections, this review focuses on the treatment of Gram-positive bacterial infections. Of particular concern are opportunistic infections caused by Gram-positive MDROs, including methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant Enterococcus species (VRE), penicillin-resistant Streptococcus pneumoniae, and Nocardia species. Treatment of infections in the immunocompromised patient population warrants careful antimicrobial selection to ensure that a patient’s immune system is not further compromised due to adverse effects (AEs) secondary to therapy. As such, clinicians are exploring alternative antimicrobials, such as tedizolid, to treat various opportunistic infections.
Recently, requests at the William S. Middleton Memorial Veterans Hospital in Madison, Wisconsin, for off-label use of tedizolid have increased despite having other cheaper alternatives with comparable Gram-positive coverage. This review examines available literature regarding off-label use of tedizolid with a focus on use in immunocompromised patients.
Tedizolid phosphate (Sivextro) is an oxazolidinone antibiotic prodrug that joined linezolid as the second in its class in 2014. Oxazolidinones inhibit bacterial protein synthesis by binding to the 50S subunit of bacterial ribosomes in Gram-positive bacteria and are often used to treat MRSA and VRE infections.9 In vitro, oxazolidinones have shown bacteriostatic activity against Enterococcus and Staphylococcus species while exhibiting bactericidal activity against most Streptococcus species.10 Tedizolid has a US Food and Drug Administration (FDA) -approved, simplified dosing profile of 200 mg daily for 6 days compared with linezolid 400 to 600 mg twice daily for 10 to 14 days. Both medications are highly bioavailable with direct IV to oral conversion.11,12 Potential, expanded use of tedizolid against Gram-positive MDROs rests on a more favorable AE profile than does its linezolid predecessor. Tedizolid has been associated with less antibiotic-induced myelosuppression, which could prove valuable for immunocompromised patients.13
Tedizolid is approved for the sole indication of acute bacterial skin and skin structure infections (ABSSSI), whereas its predecessor has many approved indications and has been used extensively for off-label indications (Table).
Adverse reactions, as determined by 2 phase 2 and 2 phase 3 clinical trials evaluating 1,050 patients treated with tedizolid and 662 patients treated with linezolid, were similar between the oxazolidinones. Nausea was the most common AE and was reported in 8% and 12% of patients taking tedizolid and linezolid, respectively. Other common AEs (1%-6%) reported for both agents included vomiting, diarrhea, headache, and dizziness.11 Myelosuppression, peripheral neuropathy, and optic nerve disorders were the most common severe AEs reported with oxazolidinones. Tedizolid demonstrated a significantly decreased incidence of neutropenia (3%), defined by absolute neutrophil count 9/L compared with that of linezolid (7%) (P = .024).13 Evaluation of peripheral neuropathy and optic nerve disorders within the tedizolid and linezolid groups revealed similar incidences (peripheral neuropathy 1.2% vs 0.6%; optic nerve disorders 0.3% vs 0.2%, respectively).11
There is one preclinical trial that described the use of tedizolid in a murine model. A murine model study compared the antistaphylococcal killing effect of doses of tedizolid equivalent to human exposures ranging from 200 to 3,200 mg/d in both granulocytopenic and normal mice. The mice were evaluated at 24, 48, and 72 hours after therapy initiation. The presence of granulocytes had a dramatic effect on the antimicrobial effect of tedizolid. Dose response, demonstrated by the ratio of the area under the curve over the minimum inhibitory concentration, was on average > 25-fold for nonneutropenic vs neutropenic models. Near maximal effect of the nonneutropenic group, irrespective of duration of therapy, was achieved at the lowest dose tested (an exposure of about 200-mg tedizolid phosphate per day in humans).This study suggests that immunocompromised patients may warrant higher doses of tedizolid than the currently FDA-approved dose due to a decreased number of granulocytes available for modulating bacterial infections.15
Use of tedizolid doses higher than that which is FDA-approved may negate the favorable AE profile. A phase 1 clinical study was conducted to evaluate the safety, tolerability, and pharmacokinetics of tedizolid compared with those of linezolid in 40 healthy volunteers in a 21-day multiple ascending dose study.16 Subjects were stratified into 5 treatment cohorts: 200-, 300-, or 400-mg tedizolid orally once a day, 600-mg linezolid orally twice a day, and placebo. Tedizolid given at 200 mg had a hematologic safety profile similar to that of placebo. However, mean platelet counts decreased over time in a dose-dependent manner for tedizolid, with the 400-mg tedizolid and linezolid groups reporting similar reductions in platelet counts.16
Some evidence is available examining linezolid in neutropenic patients. Rafailidis and colleagues reviewed available literature regarding linezolid in neutropenic patients with Gram-positive infections. Evaluation of linezolid administration at usual doses to 438 neutropenic patients from 2 prospective comparative studies, a prospective cohort study, 2 retrospective studies, and 8 case reports was performed. Results of the evaluation revealed a clinical cure rate between 57% and 87% in the intention-to-treat population of the prospective studies.17 Given the similarities in bacterial spectrum of activity between linezolid and tedizolid, it may be reasonable to infer that tedizolid’s decreased myelosuppression profile would make it useful in the setting of neutropenia in immunocompromised patients.
There is little evidence regarding the use of tedizolid in immunocompromised patients, as only 2 case reports were found. The first described a 60-year-old male postrenal transplant complicated with VRE bacteremia, rhabdomyolysis, and thrombocytopenia. This patient was treated with prolonged tedizolid 200 mg daily due to multiple contraindications for treatment with other antibiotics. The patient was cured with a 14-day course of tedizolid without any noted AEs.18
The second identified case report described the use of tedizolid for the treatment of central nervous system (CNS) manifestations secondary to nocardiosis. Effective treatment of CNS nocardiosis requires high concentrations and prolonged duration of antimicrobial exposure. This case report described a 68-year-old, chronically immunocompromised female patient with multiple myeloma who was hospitalized for 3 months for the treatment of a CNS nocardiosis infection. After discharge, the patient was treated with an oral regimen of 200-mg tedizolid daily in combination with sulfamethoxazole/trimethoprim (800 mg/160 mg) 3 times daily. After 6 months of combination therapy, magnetic resonance imaging revealed complete resolution of nocardiosis-related central lesions. Although the patient’s malignancy advanced during combination antibiotic therapy, the patient’s absolute neutrophil count remained stable and showed an increase in absolute CD4+ cell counts with no other documented AEs.19
Tedizolid is the latest FDA-approved oxazolidinone antibiotic for susceptible Gram-positive acute bacterial skin and skin structure infections. It has a simplified and shorter duration of treatment and imparts similar AEs at improved rates compared with that of linezolid, most notably in relation to hematologic AEs. Due to the lack of established literature and an agreed-upon dosing strategy for the use of tedizolid in immunocompromised patients, tedizolid therapy for Gram-positive infections in immunocompromised patients should be reserved for salvage therapy when more established Gram-positive antibiotic agents lack efficacy or when patient contraindications to their use exist.
1. Fishman JA, Issa NC. Infection in organ transplantation: risk factors and evolving patterns of infection. Infect Dis Clin North Am. 2010;24(2):273-283.
2. Fishman JA. Infection in solid-organ transplant recipients. N Engl J Med. 2007;357(25):2601-2614.
3. Nightingale SD, Byrd LT, Southern PM, Jockusch JD, Cal SX, Wynne BA. Incidence of mycobacterium avium-intracellulare complex bacteremia in human immunodeficiency virus-positive patients. J Infect Dis. 1992;165(6):1082-1085.
4. O’Brien S, Kantarjian H, Beran M, et al. Results of fludarabine and prednisone therapy in 264 patients with chronic lymphocytic leukemia with multivariate analysis-derived prognostic model for response to treatment. Blood. 1993;82(6):1695-1700.
5. Anaissie E, Kontoyiannis DP, Kantarjian H, Elting L, Robertson LE, Keating M. Listeriosis in patients with chronic lymphocytic leukemia who were treated with fludarabine and prednisone. Ann Intern Med. 1992;117(6):466-469.
6. Morrison VA, Rai KR, Peterson BL, et al. Impact of therapy with chlorambucil, fludarabine, or fludarabine plus chlorambucil on infections in patients with chronic lymphocytic leukemia: Intergroup Study Cancer and Leukemia Group B 9011. J Clin Oncol. 2001;19(16):3611-3621.
7. Nucci M, Anaissie E. Infections in patients with multiple myeloma in the era of high-dose therapy and novel agents. Clin Infect Dis. 2009;49(8):1211-1225.
8. Naseer M, Dailey FE, Juboori AA, Samiullah S, Tahan V. Epidemiology, determinants, and managements of AIDS cholangiopathy: a review. World J Gastroenterol. 2018;24(7):767-774.
9. Radunz S, Juntermanns B, Kaiser GM, et al. Efficacy and safety of linezolid in liver transplant patients. Transpl Infect Dis. 2011;13(4):353-358.
10. Roger C, Roberts JA, Muller L. Clinical pharmacokinetics and pharmacodynamics of oxazolidinones. Clin Pharmacokinet. 2018;57(5):559-575.
11. Sivextro [package insert]. Whitehouse Station, NJ: Merck & Co Inc; 2016.
12. Zyvox [package insert]. New York, NY: Pfizer Inc; 2018.
13. Moran GJ, Fang E, Corey GR, Das AF, De Anda C, Prokocimer P. Tedizolid for 6 days versus linezolid for 10 days for acute bacterial skin and skin-structure infections (ESTABLISH-2): a randomized, double-blind, phase 3, non-inferiority trial. Lancet Infect Dis. 2014;14(8):696-705.
14. Liu C, Bayer A, Cosgrove S, et al; Infectious Diseases Society of America. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52(3):e18-e55.
15. Drusano GL, Liu W, Kulawy R, Louie A. Impact of granulocytes on the antimicrobial effect of tedizolid in a mouse thigh infection model. Antimicrob Agents Chemother. 2011;55(11):5300-5305.
16. Lodise TP, Bidell MR, Flanagan SD, Zasowski EJ, Minassian SL, Prokocimer P. Characterization of the haematological profile of 21 days of tedizolid in healthy subjects. J Antimicrob Chemother. 2016;71(9):2553-2558.
17. Rafailidis PI, Kouranos VD, Christodoulou C, Falagas ME. Linezolid for patients with neutropenia: are bacteriostatic agents appropriate? Expert Rev Anti Infect Ther. 2009;7(4):415-422.
18. Sudhindra P, Lee L, Wang G, Dhand A. Tedizolid for treatment of enterococcal bacteremia. Open Forum Infect Dis. 2016;3(suppl 1):1344.
19. Matin A, Sharma S, Mathur P, Apewokin SK. Myelosuppression-sparing treatment of central nervous system nocardiosis in a multiple myeloma patient utilizing a tedizolid-based regimen: a case report. Int J Antimicrob Agents. 2017;49(4):488-492.
20. Dryden MS. Alternative clinical indications for novel antibiotics licensed for skin and soft tissue infections? Curr Opin Infect Dis. 2015;28(2):117-124.
21. Milstein M, Brzezinski A, Varaine F, Mitnick CD. (Re)moving the needle: prospects for all-oral treatment for multidrug-resistant tuberculosis. Int J Tuberc Lung Dis. 2016;20(12):18-23.
22. Winthrop KL, Ku JH, Marras TK, et al. The tolerability of linezolid in the treatment of nontuberculous mycobacterial disease. Eur Respir J. 2015;45(4):1177-1179.
23. Yuste JR, Bertó J, Del Pozo JL, Leiva J. Prolonged use of tedizolid in a pulmonary non-tuberculosis mycobacterial infection after linezlid-induced toxicity. J Antimicrob Chemother. 2017;72(2):625-628.
1. Fishman JA, Issa NC. Infection in organ transplantation: risk factors and evolving patterns of infection. Infect Dis Clin North Am. 2010;24(2):273-283.
2. Fishman JA. Infection in solid-organ transplant recipients. N Engl J Med. 2007;357(25):2601-2614.
3. Nightingale SD, Byrd LT, Southern PM, Jockusch JD, Cal SX, Wynne BA. Incidence of mycobacterium avium-intracellulare complex bacteremia in human immunodeficiency virus-positive patients. J Infect Dis. 1992;165(6):1082-1085.
4. O’Brien S, Kantarjian H, Beran M, et al. Results of fludarabine and prednisone therapy in 264 patients with chronic lymphocytic leukemia with multivariate analysis-derived prognostic model for response to treatment. Blood. 1993;82(6):1695-1700.
5. Anaissie E, Kontoyiannis DP, Kantarjian H, Elting L, Robertson LE, Keating M. Listeriosis in patients with chronic lymphocytic leukemia who were treated with fludarabine and prednisone. Ann Intern Med. 1992;117(6):466-469.
6. Morrison VA, Rai KR, Peterson BL, et al. Impact of therapy with chlorambucil, fludarabine, or fludarabine plus chlorambucil on infections in patients with chronic lymphocytic leukemia: Intergroup Study Cancer and Leukemia Group B 9011. J Clin Oncol. 2001;19(16):3611-3621.
7. Nucci M, Anaissie E. Infections in patients with multiple myeloma in the era of high-dose therapy and novel agents. Clin Infect Dis. 2009;49(8):1211-1225.
8. Naseer M, Dailey FE, Juboori AA, Samiullah S, Tahan V. Epidemiology, determinants, and managements of AIDS cholangiopathy: a review. World J Gastroenterol. 2018;24(7):767-774.
9. Radunz S, Juntermanns B, Kaiser GM, et al. Efficacy and safety of linezolid in liver transplant patients. Transpl Infect Dis. 2011;13(4):353-358.
10. Roger C, Roberts JA, Muller L. Clinical pharmacokinetics and pharmacodynamics of oxazolidinones. Clin Pharmacokinet. 2018;57(5):559-575.
11. Sivextro [package insert]. Whitehouse Station, NJ: Merck & Co Inc; 2016.
12. Zyvox [package insert]. New York, NY: Pfizer Inc; 2018.
13. Moran GJ, Fang E, Corey GR, Das AF, De Anda C, Prokocimer P. Tedizolid for 6 days versus linezolid for 10 days for acute bacterial skin and skin-structure infections (ESTABLISH-2): a randomized, double-blind, phase 3, non-inferiority trial. Lancet Infect Dis. 2014;14(8):696-705.
14. Liu C, Bayer A, Cosgrove S, et al; Infectious Diseases Society of America. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52(3):e18-e55.
15. Drusano GL, Liu W, Kulawy R, Louie A. Impact of granulocytes on the antimicrobial effect of tedizolid in a mouse thigh infection model. Antimicrob Agents Chemother. 2011;55(11):5300-5305.
16. Lodise TP, Bidell MR, Flanagan SD, Zasowski EJ, Minassian SL, Prokocimer P. Characterization of the haematological profile of 21 days of tedizolid in healthy subjects. J Antimicrob Chemother. 2016;71(9):2553-2558.
17. Rafailidis PI, Kouranos VD, Christodoulou C, Falagas ME. Linezolid for patients with neutropenia: are bacteriostatic agents appropriate? Expert Rev Anti Infect Ther. 2009;7(4):415-422.
18. Sudhindra P, Lee L, Wang G, Dhand A. Tedizolid for treatment of enterococcal bacteremia. Open Forum Infect Dis. 2016;3(suppl 1):1344.
19. Matin A, Sharma S, Mathur P, Apewokin SK. Myelosuppression-sparing treatment of central nervous system nocardiosis in a multiple myeloma patient utilizing a tedizolid-based regimen: a case report. Int J Antimicrob Agents. 2017;49(4):488-492.
20. Dryden MS. Alternative clinical indications for novel antibiotics licensed for skin and soft tissue infections? Curr Opin Infect Dis. 2015;28(2):117-124.
21. Milstein M, Brzezinski A, Varaine F, Mitnick CD. (Re)moving the needle: prospects for all-oral treatment for multidrug-resistant tuberculosis. Int J Tuberc Lung Dis. 2016;20(12):18-23.
22. Winthrop KL, Ku JH, Marras TK, et al. The tolerability of linezolid in the treatment of nontuberculous mycobacterial disease. Eur Respir J. 2015;45(4):1177-1179.
23. Yuste JR, Bertó J, Del Pozo JL, Leiva J. Prolonged use of tedizolid in a pulmonary non-tuberculosis mycobacterial infection after linezlid-induced toxicity. J Antimicrob Chemother. 2017;72(2):625-628.
FDA Boxed Warning Updates: June 2018
The FDA’s MedWatch program safety labeling changes for boxed warnings are compiled quarterly for drugs and therapeutic biologics where important changes have been made to the safety information. These and other label changes are searchable in the Drug Safety Labeling Changes (SLC) database, where data are available to the public in downloadable and searchable formats. Boxed warnings are ordinarily used to highlight either adverse reactions so serious in proportion to the potential benefi t from the drug that it is essential that it be considered in assessing the risks and benefi ts of using the drug; or serious adverse reactions that can be prevented/reduced in frequency or severity by appropriate use of the drug; or FDA approved the drug with restrictions to ensure safe use because FDA concluded that the drug can be safely used only if distribution or use is restricted. For complete FDA Drug Safety Labeling changes, please visit http://www.accessdata.fda.gov/scripts/cder/safetylabelingchanges.
VIDEX AND VIDEX EC (DIDANOSINE):
- Edited boxed warning, January 2018
WARNING: PANCREATITIS, LACTIC ACIDOSIS and HEPATOMEGALY with STEATOSIS
Coadministration of VIDEX or VIDEX EC and stavudine is contraindicated because of increased risk of serious and/or life-threatening events. Suspend treatment if clinical or laboratory findings suggestive of lactic acidosis or pronounced hepatotoxicity occurs.
ZYDELIG (IDELALISIB):
- Edited boxed warning, January 2018
WARNING: FATAL AND SERIOUS TOXICITIES: HEPATIC, SEVERE DIARRHEA, COLITIS, PNEUMONITIS, INFECTIONS, and INTESTINAL PERFORATION
Fatal and/or serious hepatotoxicity occurred in 16% to 18% of Zydelig-treated patients…
Fatal and/or serious and severe diarrhea or colitis occurred in 14% to 20% of Zydelig-treated patients…
Fatal and/or serious infections occurred in 21% to 48% of Zydelig-treated patients…
AQUAMEPHYTON (PHYTONADIONE):
- Edited boxed warning, March 2018
WARNING: HYPERSENSITIVITY REACTIONS WITH INTRAVENOUS AND INTRAMUSCULAR USE
Fatal hypersensitivity reactions, including anaphylaxis, have occurred during and immediately after INTRAVENOUS and INTRAMUSCULAR injection of Aqua-MEPHYTON. Reactions have occurred despite dilution to avoid rapid infusion and upon first dose. Avoid the intravenous and intramuscular routes of administration unless the subcutaneous route is not feasible and the serious risk is justified.
FERAHEME (FERUMOXYTOL):
- Edited boxed warning, February 2018
WARNING: RISK FOR SERIOUS HYPERSENSITIVITY/ANAPHYLAXIS REACTIONS
Fatal and serious hypersensitivity reactions including anaphylaxis have occurred in patients receiving feraheme. Initial symptoms may
include hypotension, syncope, unresponsiveness, cardiac/cardiorespiratory arrest.
Only administer feraheme as an intravenous infusion over at least 15 minutes and only when personnel and therapies are immediately available for the treatment of anaphylaxis and other hypersensitivity reactions.
METHADONE HYDROCHLORIDE, METHADOSE (METHADONE HYDROCHLORIDE):
- Edited boxed warning, February 2018
RISKS FROM CONCOMITANT USE WITH BENZODIAZEPINES OR OTHER CNS DEPRESSANTS
Concomitant use with benzodiazepines or other central nervous system (CNS) depressants, including alcohol, is a risk factor for respiratory depression and death.
Reserve concomitant prescribing of benzodiazepines or other CNS depressants for use in patients for whom alternatives to benzodiazepines or other CNS depressants are inadequate.
Follow patients for signs and symptoms of respiratory depression and sedation. If the patient is visibly sedated, evaluate the cause of sedation, and consider delaying or omitting daily methadone dosing.
DOLOPHINE HYDROCHLORIDE (METHADONE HYDROCHLORIDE):
- Edited boxed warning, February 2018
RISKS FROM CONCOMITANT USE WITH BENZODIAZEPINES OR OTHER CNS DEPRESSANTS
Concomitant use of opioids with benzodiazepines or other central nervous system (CNS) depressants, including alcohol, may result in profound sedation, respiratory depression, coma, and death.
Reserve concomitant prescribing of DOLOPHINE Tablets and benzodiazepines or other CNS depressants for use in patients for whom alternatives to benzodiazepines or other CNS depressants are inadequate.
Limit dosages and durations to the minimum required for patients being treated for pain.
Follow patients for signs and symptoms of respiratory depression and sedation. If the patient is visibly sedated, evaluate the cause of sedation, and consider delaying or omitting the daily methadone dose.
PARNATE (TRANYLCYPROMINE SULFATE):
- Edited boxed warning, January 2018
WARNING: SUICIDAL THOUGHTS AND BEHAVIORS AND HYPERTENSIVE CRISIS WITH SIGNIFICANT TYRAMINE USE
SUICIDAL THOUGHTS AND BEHAVIORS
Antidepressants increased the risk of suicidal thoughts and behaviors in pediatric and young adult patients in short-term studies. Closely monitor all antidepressant-treated patients for clinical worsening, and for emergence of suicidal thoughts and behaviors. PARNATE is not approved for use in pediatric patients.
HYPERTENSIVE CRISIS WITH SIGNIFICANT TYRAMINE USE
Excessive consumption of foods or beverages with significant tyramine content or the use of certain drugs with PARNATE or after PARNATE discontinuation can precipitate hypertensive crisis. Monitor blood pressure and allow for medication-free intervals between administration of PARNATE and interacting drugs. Instruct patients to avoid ingestion of foods and beverages with high tyramine content.
OCALIVA (OBETICHOLIC ACID):
- New boxed warning/Newly added section, February 2018
WARNING: HEPATIC DECOMPENSATION AND FAILURE IN INCORRECTLY DOSED PBC PATIENTS WITH CHILD-PUGH CLASS B OR C OR DECOMPENSATED CIRRHOSIS
In postmarketing reports, hepatic decompensation and failure, in some cases fatal, have been reported in patients with primary biliary cholangitis (PBC) with decompensated cirrhosis or Child-Pugh Class B or C hepatic impairment when OCALIVA was dosed more frequently than recommended.
The recommended starting dosage of OCALIVA is 5 mg once weekly for patients with Child-Pugh Class B or C hepatic impairment or a prior decompensation event.
The FDA’s MedWatch program safety labeling changes for boxed warnings are compiled quarterly for drugs and therapeutic biologics where important changes have been made to the safety information. These and other label changes are searchable in the Drug Safety Labeling Changes (SLC) database, where data are available to the public in downloadable and searchable formats. Boxed warnings are ordinarily used to highlight either adverse reactions so serious in proportion to the potential benefi t from the drug that it is essential that it be considered in assessing the risks and benefi ts of using the drug; or serious adverse reactions that can be prevented/reduced in frequency or severity by appropriate use of the drug; or FDA approved the drug with restrictions to ensure safe use because FDA concluded that the drug can be safely used only if distribution or use is restricted. For complete FDA Drug Safety Labeling changes, please visit http://www.accessdata.fda.gov/scripts/cder/safetylabelingchanges.
VIDEX AND VIDEX EC (DIDANOSINE):
- Edited boxed warning, January 2018
WARNING: PANCREATITIS, LACTIC ACIDOSIS and HEPATOMEGALY with STEATOSIS
Coadministration of VIDEX or VIDEX EC and stavudine is contraindicated because of increased risk of serious and/or life-threatening events. Suspend treatment if clinical or laboratory findings suggestive of lactic acidosis or pronounced hepatotoxicity occurs.
ZYDELIG (IDELALISIB):
- Edited boxed warning, January 2018
WARNING: FATAL AND SERIOUS TOXICITIES: HEPATIC, SEVERE DIARRHEA, COLITIS, PNEUMONITIS, INFECTIONS, and INTESTINAL PERFORATION
Fatal and/or serious hepatotoxicity occurred in 16% to 18% of Zydelig-treated patients…
Fatal and/or serious and severe diarrhea or colitis occurred in 14% to 20% of Zydelig-treated patients…
Fatal and/or serious infections occurred in 21% to 48% of Zydelig-treated patients…
AQUAMEPHYTON (PHYTONADIONE):
- Edited boxed warning, March 2018
WARNING: HYPERSENSITIVITY REACTIONS WITH INTRAVENOUS AND INTRAMUSCULAR USE
Fatal hypersensitivity reactions, including anaphylaxis, have occurred during and immediately after INTRAVENOUS and INTRAMUSCULAR injection of Aqua-MEPHYTON. Reactions have occurred despite dilution to avoid rapid infusion and upon first dose. Avoid the intravenous and intramuscular routes of administration unless the subcutaneous route is not feasible and the serious risk is justified.
FERAHEME (FERUMOXYTOL):
- Edited boxed warning, February 2018
WARNING: RISK FOR SERIOUS HYPERSENSITIVITY/ANAPHYLAXIS REACTIONS
Fatal and serious hypersensitivity reactions including anaphylaxis have occurred in patients receiving feraheme. Initial symptoms may
include hypotension, syncope, unresponsiveness, cardiac/cardiorespiratory arrest.
Only administer feraheme as an intravenous infusion over at least 15 minutes and only when personnel and therapies are immediately available for the treatment of anaphylaxis and other hypersensitivity reactions.
METHADONE HYDROCHLORIDE, METHADOSE (METHADONE HYDROCHLORIDE):
- Edited boxed warning, February 2018
RISKS FROM CONCOMITANT USE WITH BENZODIAZEPINES OR OTHER CNS DEPRESSANTS
Concomitant use with benzodiazepines or other central nervous system (CNS) depressants, including alcohol, is a risk factor for respiratory depression and death.
Reserve concomitant prescribing of benzodiazepines or other CNS depressants for use in patients for whom alternatives to benzodiazepines or other CNS depressants are inadequate.
Follow patients for signs and symptoms of respiratory depression and sedation. If the patient is visibly sedated, evaluate the cause of sedation, and consider delaying or omitting daily methadone dosing.
DOLOPHINE HYDROCHLORIDE (METHADONE HYDROCHLORIDE):
- Edited boxed warning, February 2018
RISKS FROM CONCOMITANT USE WITH BENZODIAZEPINES OR OTHER CNS DEPRESSANTS
Concomitant use of opioids with benzodiazepines or other central nervous system (CNS) depressants, including alcohol, may result in profound sedation, respiratory depression, coma, and death.
Reserve concomitant prescribing of DOLOPHINE Tablets and benzodiazepines or other CNS depressants for use in patients for whom alternatives to benzodiazepines or other CNS depressants are inadequate.
Limit dosages and durations to the minimum required for patients being treated for pain.
Follow patients for signs and symptoms of respiratory depression and sedation. If the patient is visibly sedated, evaluate the cause of sedation, and consider delaying or omitting the daily methadone dose.
PARNATE (TRANYLCYPROMINE SULFATE):
- Edited boxed warning, January 2018
WARNING: SUICIDAL THOUGHTS AND BEHAVIORS AND HYPERTENSIVE CRISIS WITH SIGNIFICANT TYRAMINE USE
SUICIDAL THOUGHTS AND BEHAVIORS
Antidepressants increased the risk of suicidal thoughts and behaviors in pediatric and young adult patients in short-term studies. Closely monitor all antidepressant-treated patients for clinical worsening, and for emergence of suicidal thoughts and behaviors. PARNATE is not approved for use in pediatric patients.
HYPERTENSIVE CRISIS WITH SIGNIFICANT TYRAMINE USE
Excessive consumption of foods or beverages with significant tyramine content or the use of certain drugs with PARNATE or after PARNATE discontinuation can precipitate hypertensive crisis. Monitor blood pressure and allow for medication-free intervals between administration of PARNATE and interacting drugs. Instruct patients to avoid ingestion of foods and beverages with high tyramine content.
OCALIVA (OBETICHOLIC ACID):
- New boxed warning/Newly added section, February 2018
WARNING: HEPATIC DECOMPENSATION AND FAILURE IN INCORRECTLY DOSED PBC PATIENTS WITH CHILD-PUGH CLASS B OR C OR DECOMPENSATED CIRRHOSIS
In postmarketing reports, hepatic decompensation and failure, in some cases fatal, have been reported in patients with primary biliary cholangitis (PBC) with decompensated cirrhosis or Child-Pugh Class B or C hepatic impairment when OCALIVA was dosed more frequently than recommended.
The recommended starting dosage of OCALIVA is 5 mg once weekly for patients with Child-Pugh Class B or C hepatic impairment or a prior decompensation event.
The FDA’s MedWatch program safety labeling changes for boxed warnings are compiled quarterly for drugs and therapeutic biologics where important changes have been made to the safety information. These and other label changes are searchable in the Drug Safety Labeling Changes (SLC) database, where data are available to the public in downloadable and searchable formats. Boxed warnings are ordinarily used to highlight either adverse reactions so serious in proportion to the potential benefi t from the drug that it is essential that it be considered in assessing the risks and benefi ts of using the drug; or serious adverse reactions that can be prevented/reduced in frequency or severity by appropriate use of the drug; or FDA approved the drug with restrictions to ensure safe use because FDA concluded that the drug can be safely used only if distribution or use is restricted. For complete FDA Drug Safety Labeling changes, please visit http://www.accessdata.fda.gov/scripts/cder/safetylabelingchanges.
VIDEX AND VIDEX EC (DIDANOSINE):
- Edited boxed warning, January 2018
WARNING: PANCREATITIS, LACTIC ACIDOSIS and HEPATOMEGALY with STEATOSIS
Coadministration of VIDEX or VIDEX EC and stavudine is contraindicated because of increased risk of serious and/or life-threatening events. Suspend treatment if clinical or laboratory findings suggestive of lactic acidosis or pronounced hepatotoxicity occurs.
ZYDELIG (IDELALISIB):
- Edited boxed warning, January 2018
WARNING: FATAL AND SERIOUS TOXICITIES: HEPATIC, SEVERE DIARRHEA, COLITIS, PNEUMONITIS, INFECTIONS, and INTESTINAL PERFORATION
Fatal and/or serious hepatotoxicity occurred in 16% to 18% of Zydelig-treated patients…
Fatal and/or serious and severe diarrhea or colitis occurred in 14% to 20% of Zydelig-treated patients…
Fatal and/or serious infections occurred in 21% to 48% of Zydelig-treated patients…
AQUAMEPHYTON (PHYTONADIONE):
- Edited boxed warning, March 2018
WARNING: HYPERSENSITIVITY REACTIONS WITH INTRAVENOUS AND INTRAMUSCULAR USE
Fatal hypersensitivity reactions, including anaphylaxis, have occurred during and immediately after INTRAVENOUS and INTRAMUSCULAR injection of Aqua-MEPHYTON. Reactions have occurred despite dilution to avoid rapid infusion and upon first dose. Avoid the intravenous and intramuscular routes of administration unless the subcutaneous route is not feasible and the serious risk is justified.
FERAHEME (FERUMOXYTOL):
- Edited boxed warning, February 2018
WARNING: RISK FOR SERIOUS HYPERSENSITIVITY/ANAPHYLAXIS REACTIONS
Fatal and serious hypersensitivity reactions including anaphylaxis have occurred in patients receiving feraheme. Initial symptoms may
include hypotension, syncope, unresponsiveness, cardiac/cardiorespiratory arrest.
Only administer feraheme as an intravenous infusion over at least 15 minutes and only when personnel and therapies are immediately available for the treatment of anaphylaxis and other hypersensitivity reactions.
METHADONE HYDROCHLORIDE, METHADOSE (METHADONE HYDROCHLORIDE):
- Edited boxed warning, February 2018
RISKS FROM CONCOMITANT USE WITH BENZODIAZEPINES OR OTHER CNS DEPRESSANTS
Concomitant use with benzodiazepines or other central nervous system (CNS) depressants, including alcohol, is a risk factor for respiratory depression and death.
Reserve concomitant prescribing of benzodiazepines or other CNS depressants for use in patients for whom alternatives to benzodiazepines or other CNS depressants are inadequate.
Follow patients for signs and symptoms of respiratory depression and sedation. If the patient is visibly sedated, evaluate the cause of sedation, and consider delaying or omitting daily methadone dosing.
DOLOPHINE HYDROCHLORIDE (METHADONE HYDROCHLORIDE):
- Edited boxed warning, February 2018
RISKS FROM CONCOMITANT USE WITH BENZODIAZEPINES OR OTHER CNS DEPRESSANTS
Concomitant use of opioids with benzodiazepines or other central nervous system (CNS) depressants, including alcohol, may result in profound sedation, respiratory depression, coma, and death.
Reserve concomitant prescribing of DOLOPHINE Tablets and benzodiazepines or other CNS depressants for use in patients for whom alternatives to benzodiazepines or other CNS depressants are inadequate.
Limit dosages and durations to the minimum required for patients being treated for pain.
Follow patients for signs and symptoms of respiratory depression and sedation. If the patient is visibly sedated, evaluate the cause of sedation, and consider delaying or omitting the daily methadone dose.
PARNATE (TRANYLCYPROMINE SULFATE):
- Edited boxed warning, January 2018
WARNING: SUICIDAL THOUGHTS AND BEHAVIORS AND HYPERTENSIVE CRISIS WITH SIGNIFICANT TYRAMINE USE
SUICIDAL THOUGHTS AND BEHAVIORS
Antidepressants increased the risk of suicidal thoughts and behaviors in pediatric and young adult patients in short-term studies. Closely monitor all antidepressant-treated patients for clinical worsening, and for emergence of suicidal thoughts and behaviors. PARNATE is not approved for use in pediatric patients.
HYPERTENSIVE CRISIS WITH SIGNIFICANT TYRAMINE USE
Excessive consumption of foods or beverages with significant tyramine content or the use of certain drugs with PARNATE or after PARNATE discontinuation can precipitate hypertensive crisis. Monitor blood pressure and allow for medication-free intervals between administration of PARNATE and interacting drugs. Instruct patients to avoid ingestion of foods and beverages with high tyramine content.
OCALIVA (OBETICHOLIC ACID):
- New boxed warning/Newly added section, February 2018
WARNING: HEPATIC DECOMPENSATION AND FAILURE IN INCORRECTLY DOSED PBC PATIENTS WITH CHILD-PUGH CLASS B OR C OR DECOMPENSATED CIRRHOSIS
In postmarketing reports, hepatic decompensation and failure, in some cases fatal, have been reported in patients with primary biliary cholangitis (PBC) with decompensated cirrhosis or Child-Pugh Class B or C hepatic impairment when OCALIVA was dosed more frequently than recommended.
The recommended starting dosage of OCALIVA is 5 mg once weekly for patients with Child-Pugh Class B or C hepatic impairment or a prior decompensation event.
HIV Update: Which Single-Tablet Regimens, and When (FULL)
CASE: James G, age 43, recently had blood work performed for a life insurance policy, and his human immunodeficiency virus (HIV) test came back positive. At a follow-up office visit, Mr. G reports having anonymous male sexual partners when traveling to New York on business and rarely using condoms. His last HIV test was “about 4 years ago.” He is otherwise in good health, takes no regular medications, and is not married.
Having recently completed a primary care CME program on HIV disease, you order a CD4/T-cell count, an HIV RNA (viral load) test, and an HIV genotype drug resistance test on Mr. G, along with other baseline lab work, including a complete blood count, chemistry panel, and hepatitis panel. You schedule a follow-up visit with Mr. G in 2 weeks when all of the lab results will be available so that you can discuss his plan of care.
A diagnosis of HIV has moved from being a fatal disease to that of a chronic condition that can be effectively managed with combination antiretroviral therapy (ART) regimens over an almost normal lifespan. As a result, the role of the primary care practitioner in the ongoing care of patients with HIV has grown and will continue to do so, making knowledge of these drug combinations vital.
20 Years Have Changed Everything
Combination ART has existed since 1996 when the first protease inhibitors (PIs) were approved by the U.S. Food and Drug Administration (FDA). Prior to this, treatment was limited to mono or dual therapy with nucleoside reverse transcriptase inhibitors (NRTIs). These agents provided some short-term clinical benefit, but didn’t significantly improve patient survival and ultimately failed due to viral resistance.1
Since the approval of zidovudine (AZT) in 1987, the FDA has approved more than 25 drugs in 6 different classes for the treatment of HIV disease.2 These include the NRTIs, non-nucleoside reverse transcriptase inhibitors (NNRTIs), PIs, a fusion inhibitor (FI), a CCR5 antagonist, and, more recently, integrase strand transfer inhibitors (INSTIs). In addition, 2 drugs, cobicistat and ritonavir, are used solely to improve or “boost” the pharmacokinetic profiles of several antiretroviral drugs.2
Most of these newer agents are more potent, have a higher genetic barrier to resistance, and a longer halflife than their predecessors. Moreover, many are less toxic and thus more tolerable than older drugs. With the progressive development and approval of singletablet regimens (STRs) that contain 3 or 4 drugs, the majority of patients with HIV in the United States now take just one pill per day to treat their infection, facilitating far greater medication adherence.
Initiation of Antiretroviral Therapy
The U.S. Department of Health and Human Services (DHHS) guidelines now recommend that all people infected with HIV, regardless of CD4 cell count, begin ART.2 The evidence for this recommendation comes largely from the START3 and TEMPRANO4 trials, which found that early initiation of ART significantly reduces morbidity and mortality associated with HIV. In addition, the HPTN 052 study concluded that early ART is associated with a 93% lower risk of viral transmission in serodiscordant heterosexual couples.5 The DHHS guidelines do note that when initiating ART, it is important to appropriately educate patients on the benefits of treatment and address strategies to optimize adherence.2 (For more on factors to consider when selecting an initial HIV regimen, see Table 1.2) On a case-by-case basis, ART may be deferred because of clinical and/or psychosocial factors, but it should never be withheld unless the risks clearly outweigh the benefits. Ideally, ART should be initiated as soon as possible after the initial diagnosis of HIV.
The DHHS guidelines divide treatment options into 3 categories2:
- Recommended regimens are backed by randomized controlled trials that show optimal and durable virologic efficacy, they have favorable tolerability and toxicity profiles, and they are easy to use.
- Alternative regimens have less or lower quality supporting data than recommended regimens. Although they are effective and may be optimal for certain individual patients, they have potential disadvantages and/or limitations in certain populations.
- Other regimens have limited supporting data, reduced virologic activity, a higher pill burden, more drug interactions, and greater toxicity.
Currently Recommended First-Line Therapies
An antiretroviral regimen for a treatment-naive patient should consist of 2 NRTIs in combination with a third active antiretroviral drug from one of 3 drug classes. These include: an INSTI, a boosted PI, or, in some situations, an NNRTI. The DHHS guidelines panel currently recommends 6 different ART combinations as first-line treatment in treatment-naive patients (Table 2).2
INSTI-Based Regimens
Dolutegravir/abacavir/lamivudine (Triumeq). Approved by the FDA as a single-tablet regimen in 2014, the combination of dolutegravir/abacavir/lamivudine has proven to be highly effective and well-tolerated in many clinical trials.6-9 However, before this regimen is started, patients must be screened for the HLA-B*5701 allele, which predicts hypersensitivity to abacavir.10 Assessing patients’ risk for cardiovascular disease is also advised because some data suggest that abacavir may increase the risk of cardiovascular events, although this remains controversial.2
Dolutegravir is generally well-tolerated with minimal adverse effects (≥ 2% incidence of headache and insomnia) and toxicity.11 Dolutegravir/abacavir/lamivudine should be taken 2 hours before or 6 hours after taking antacids or laxatives, sucralfate, and oral supplements with iron or calcium. However, it may be taken with calcium or iron supplements if it is also taken with food.11 Dolutegravir increases levels of metformin about 2-fold, so patients should not take more than 1000 mg/d of this oral hypoglycemic agent.11
- Dolutegravir plus tenofovir disoproxil fumarate/ emtricitabine (Tivicay plus Truvada). The combination
of dolutegravir plus fixed-dose tenofovir disoproxil fumarate and emtricitabine is administered as 2 pills per day. Because tenofovir disoproxil fumarate can cause proximal renal tubular dysfunction, phosphate wasting, and decreased bone mineral density (BMD), avoid prescribing it for patients with underlying renal dysfunction (creatinine clearance [CrCl] <50 mL/min) and prescribe it cautiously for patients with hypertension or diabetes who are at increased risk of renal disease. Emtricitabine is generally safe and well tolerated, but the dose should be reduced in patients with renal insufficiency, which would preclude the use of this fixed-dose combination.12 - Elvitegravir/cobicistat/tenofovir alafenamide/emtricitabine (Genvoya). The newer 4-drug combination of elvitegravir/ cobicistat/tenofovir alafenamide/emtricitabinethat was approved by the FDA in November 2015,13 contains the more recently approved form of tenofovir, which can be used in patients who have a CrCl as low as 30 mL/min. Compared to formulations containing tenofovir disoproxil fumarate, the newer tenofovir alafenamide formulation achieves higher intracellular levels in CD4 lymphocytes (but not in renal tubular cells). This allows for a lower dose of the drug and a smaller tablet size with co-formulation. It does not appear to cause kidney problems or loss of BMD as can be seen with tenofovir disoproxil fumarate.14 This newer single-tablet regimen may be best suited for older patients with HIV or those with comorbidities such as hypertension or diabetes.
- Elvitegravir/cobicistat/tenofovir disoproxil fumarate/emtricitabine (Stribild). The FDA approved the combination of elvitegravir/cobicistat/tenofovir disoproxil fumarate/emtricitabine as a single-tablet regimen in 2012. The integrase inhibitor, elvitegravir, requires boosting with the CYP3A inhibitor, cobicistat, and should be taken with food.15 Two clinical trials demonstrated the superior efficacy of elvitegravir compared to a boosted PI and NNRTI-based regimen.16,17 Elvitegravir is generally well tolerated, but sometimes causes dyspepsia, nausea, or diarrhea.15 Similar to dolutegravir, it should not be taken concurrently with certain supplements—in this case, those containing aluminum, calcium, iron, magnesium, or zinc.15 Because it contains tenofovir disoproxil fumarate as an active agent, it should not be used in patients with a CrCl of <70 mL/min.15 Cobicistat inhibits tubular secretion of creatinine, so it may produce an elevation in serum creatinine without actually affecting glomerular function. Cobicistat may also cause drug-drug interactions with certain antiarrhythmics, sedative-hypnotics, and erectile dysfunction agents, and is contraindicated with some statins, anticonvulsants, and ergot derivatives.18
- Raltegravir plus tenofovir disoproxil fumarate/emtricitabine (Isentress plus Truvada). The combination of the integrase inhibitor raltegravir plus fixed-dose tenofovir disoproxil fumarate and emtricitabine has been recommended by the DHHS as first-line therapy for approximately 5 years. The recommendation is based mainly on data from the STARTMRK trial, a phase III non-inferiority trial that followed more than 500 patients for 5 years and concluded that raltegravir/ tenofovir/emtricitabine has superior efficacy with fewer drug-related adverse effects than efavirenz/tenofovir/emtricitabine.19 The overall pill burden with this regimen is 3 tablets per day. Although highly effective, the main drawbacks of raltegravir are that it must be dosed twice daily (which may be less preferable if adherence is a concern) and the genetic barrier to resistance is lower than that of the other 2 approved integrase inhibitors. In May 2017, FDA approved a new 1,200 mg once-daily version of raltegravir as an alternative to the twice daily regimen.20 Adverse effects and toxicities (except the renal and bone effects due to tenofovir disoproxil fumarate mentioned earlier) and drug interactions with this regimen are infrequent. Raltegravir can be taken with or without food. Concurrent use of antacids that contain aluminum or magnesium may reduce absorption of raltegravir and so should be avoided.21
PI-Based Regimen
Darunavir (Prezista) and ritonavir (Norvir) plus tenofovir disoproxil fumarate/emtricitabine (Truvada). PIs were once the key component of all ART regimens; however, boosted darunavir is now the only PI-based regimen currently recommended as first-line therapy. It is taken as 3 tablets once daily. If the co-formulation with cobicistat is used, just 2 tablets daily are required. One advantage with darunavir with either of the boosting agents is that it does not appear to cause insulin resistance or dyslipidemia as occurs with older PIs, such as indinavir and lopinavir.2 The boosting agents do, however, increase the likelihood of drug-drug interactions. As with all PIs, darunavir has a very high genetic barrier to resistance, which is important in patients for whom adherence is a concern.
Adverse effects of the PIs may include nausea, vomiting, and diarrhea, all of which are typically mild and selflimiting.22 Co-formulation of darunavir with cobicistat, tenofovir alafenamide, and emtricitabine is in phase III studies. Projected to be available in 2018, it will provide yet another daily STR option.23
The Addition of Fixed-Dose Tenofovir Alafenamide/Emtricitabine
In July 2016, the DHHS panel made some additions to their guidelines to reflect the FDA approval of 3 fixed-dose combination products that contain tenofovir alafenamide. Specifically, the combination of tenofovir alafenamide and emtricitabine is recommended for use with the integrase inhibitors—dolutegravir or raltegravir. It is also recommended in combination with ritonavir-boosted darunavir.
DHHS "Alternative" And "Other" Regimens
The DHHS guidelines also include “alternative” (Table 3) and “other” regimens (available at http://aidsinfo.nih.gov/guidelines) that may be used when first-line regimens may not.2 These second-line options are very effective, but have some possible clinical disadvantages or limitations. They are also less well supported by data from clinical trials. However, in certain situations, depending on an individual patient’s comorbidities, inability to tolerate one of the preferred regimens, or personal preferences, an alternative regimen may be the optimal choice.
Under the category of alternative regimens, the panel has included tenofovir alafenamide and emtricitabine in combination with the NNRTI efavirenz or with ritonavir or cobicistat-boosted atazanavir or darunavir.
The third group or “other” regimens have reduced virologic activity, increased toxicity, and even more limited data from clinical trials. Generally, medications from the DHHS “alternative” and “other” categories should be prescribed in consultation with an HIV specialist.
The Future of Art
The currently available drugs are highly effective in fully suppressing HIV and allowing for immune recovery and clinical stability for most patients. Life expectancy for patients living with HIV is estimated to be approaching that of uninfected adults—provided they remain on ART.24 As a way to further simplify ART, current clinical trials are looking at 2-drug regimens including an integrase inhibitor with an NRTI, an INSTI, or an NNRTI, or a PI with one NRTI.25,26 This approach could further reduce pill burden and toxicity and substantially decrease the cost of long-term treatment.27 Also on the horizon are long-acting injectable antiretroviral drugs that will likely be available for clinical use in the next 2 to 3 years.28,29
CASE: At the 2-week follow-up visit, you discuss with Mr. G that his CD4+ count is 390 cells/mm3, his HIV RNA level is 32,450 copies/mL, and his HIV genotype test showed no antiviral drug resistance. Explaining that all patients with HIV should be treated with antiviral therapy regardless of CD4+ count, you recommend that Mr. G begin taking fixed-dose tenofovir disoproxil fumarate/emtricitabine/elvitegravir/cobicistat (Stribild), noting that it is one of the regimens recommended by the DHHS national treatment guidelines. You provide a patient handout that discusses dosing and adverse effects, including nausea and headache. The patient’s pharmacy was contacted and it was determined that Mr. G’s co-pay for the drug would be $50, which he found acceptable.
In addition, you discuss the importance of good adherence to this medication, and instruct Mr. G to contact the office via phone or patient portal for any concerns or questions that arise after starting the medication. Lastly, you advise him to return in 4 weeks for follow-up blood testing, including viral load monitoring, and additional care, if needed, and strongly recommend that he begin using condoms regularly.
Click here to read the digital edition.
1. Concorde: MRC/ANRS randomised double-blind controlled trial of immediate and deferred zidovudine in symptom-free HIV infection. Concorde Coordinating Committee. Lancet. 1994;343:871-881.
2. Department of Health and Human Services. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Available at: http://www.aidsinfo.nih.gov/guidelines/html/1/adult-and-adolescent-treatment-guidelines/0. Accessed July 17, 2016.
3. The INSIGHT START Study Group. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med. 2015;373:795-807.
4. The TEMPRANO ANRS 12136 Study Group. A trial of early antiretrovirals and isoniazid preventive therapy in Africa. N Engl J Med. 2015;373:808-822.
5. Cohen MS, Chen YQ, McCauley M, et al. Antiretroviral therapy for the prevention of HIV-1 transmission. N Engl J Med. 2016;375:830-839.
6. Molina JM, Clotet B, van Lunzen J, et al. Once-daily dolutegravir versus darunavir plus ritonavir for treatment-naive adults with HIV-1 infection (FLAMINGO): 96 week results from a randomized, open-label, phase 3b study. Lancet HIV. 2015;2:e127-136.
7. Walmsley SL, Antela A, Clumeck N, et al. Dolutegravir plus abacavir-lamivudine for the treatment of HIV-1 infection. N Engl J Med. 2013;369:1807-1818.
8. Van Lunzen J, Maggiolo F, Arribas JR, et al. Once daily dolutegravir (S/GSK1349572) in combination therapy in antiretroviral-naïve adults with HIV: planned interim 48 week results from SPRING-1, a dose-ranging, randomized, phase 2b trial. Lancet Infect Dis. 2012;12:111-118.
9. Stellbrink HJ, Reynes J, Lazzarin A, et al. Dolutegravir in antiretroviral-naive adults with HIV-1: 96-week results from a randomized dose-ranging study. AIDS. 2013; 27:1771-1778.
10. Mallal S, Phillips E, Carosi G. HLA-B*5701 screening for hypersensitivity to abacavir. N Engl J Med. 2008;358:568-579.
11. AIDSinfo Drug Database. Dolutegravir. Available at: https://aidsinfo.nih.gov/drugs/509/dolutegravir/0/professional. Accessed July 17, 2016.
12. AIDSinfo Drug Database. Emtricitabine. Available at: https://aidsinfo.nih.gov/drugs/208/emtricitabine/0/patient. Accessed July 17, 2016.
13. AIDSinfo Drug Database. Elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide fumarate. Available at: https://aidsinfo.nih.gov/drugs/553/genvoya/0/professional. Accessed July 17, 2016.
14. Ray AS, Fordyce MW, Hitchcock, MJM. Tenofovir alafenamide: A novel prodrug of tenofovir for the treatment of human immunodeficiency virus. Antiviral Res. 2016;125:63-70.
15. AIDSinfo Drug Database. Elvitegravir. https://aidsinfo.nih.gov/drugs/421/elvitegravir/0/professional
16. Wohl DA, Cohen C, Gallant JE, et al. A randomized, double-blind comparison of single-tablet regimen elvitegravir/cobicistat/emtricitabine/tenofovir DF versus single-tablet regimen efavirenz/emtricitabine/tenofovir DF for initial treatment of HIV-1 infection: analysis of week 144 results. J Acquir Immune Defic Syndr. 2014;65:e118-120.
17. Clumeck N, Molina JM, Henry K, et al. A randomized, double-blind comparison of single- tablet regimen elvitegravir/cobicistat/emtricitabine/tenofovir DF vs ritonavir-boosted atazanavir plus emtricitabine/tenofovir for initial treatment of HIV-1 infection: analysis of week 144 results. J Acquir Immune Defic Syndr. 2014;65:e121-124.
18. AIDSinfo Drug Database. Cobicistat. Available at: https://aidsinfo.nih.gov/drug/537/evotaz/0/patient/. Accessed July 17, 2016.
19. Rockstroh JK, DeJesus E, Lennox JL, et al. Durable efficacy and safety of raltegravir versus efavirenz when combined with tenofovir/emtricitabine in treatmentnaïve HIV-1 infected patients: final 5-year results from STARTMRK. J Acquir Immune Defic Syndr. 2013;63:77-85.
20. Cahn P, Kaplan R, Sax P, et al. Raltegravir (RAL) 1200 mg once daily (QD) is non-inferior to RAL 400 mg twice daily (BID), in combination with tenofovir/emtricitabine, in treatment-naive HIV-1-infected subjects: week 48 results. Abstract FRAB0103LB presented at: 21st International AIDS Conference; July 18-22, 2016; Durban, South Africa.
21. Hicks C, Gulick RM. Raltegravir: the first HIV type 1 integrase inhibitor. Clin Infect Dis. 2009;48:931-939.
22. Prescriber’s Letter. HIV/AIDS Pharmacotherapy Review. Vol. 2015; Course no. 215. Available at: http://http://prescribersletter.therapeuticresearch.com/ce/documents/ce_15215-40.pdf. Accessed May 31, 2017.
23. AIDSinfo Drug Database. Tenofovir alafenamide. Available at: https://aidsinfo.nih.gov/drugs/514/tenofovir-alafenamide/0/patient. Accessed September 27, 2016.
24. Marcus JL, Chao C, Leyden W, et al. Narrowing the gap in life expectancy for HIV+ compared with HIV- individuals. Conference on Retroviruses and Opportunistic Infections. February 22-25, 2016, Boston. Abstract 54.
25. Gubavu C, Prazuck T, Niang M, et al. Dolutegravir-based monotherapy or dual therapy maintains a high proportion of viral suppression even in highly experienced HIV-1-infected patients. J Antimicrob Chemother. 2016;71:1046-1050.
26. Margolis DA, Brinson CC, Smith GHR. Cabotegravir plus rilpivirine, once a day, after induction with cabotegravir plus nucleoside reverse transcriptase inhibitors in antiretroviral naïve adults with HIV-1 infection (LATTE): a randomised, phase 2b, dose-ranging trial. Lancet Infect Dis. 2015;15:1145-1155.
27. Girouard MP, Sax PE, Parker RA, et al. The cost-effectiveness and budget impact of 2-drug dolutegravir-lamivudine regimens for the treatment of HIV infection in the United States. Clin Infect Dis. 2016; 62:784-791.
28. Margolis DA, Gonzalez-Garcia J, Stellbrink HJ, et al. Cabotegravir + rilpivirine as long-acting maintenance therapy: LATTE-2 week 32 results. Abstract number 31 LB. Conference on Retroviruses and Opportunistic Infections. February 22-25, 2016; Boston, MA.
29. Murray MI, Markowitz M, Frank I, et al. Tolerability and acceptability of cabotegravir LA injection: results from ECLAIR study. Abstract number 471. Conference on Retroviruses and Opportunistic Infections. February 22-25, 2016; Boston, MA.
CASE: James G, age 43, recently had blood work performed for a life insurance policy, and his human immunodeficiency virus (HIV) test came back positive. At a follow-up office visit, Mr. G reports having anonymous male sexual partners when traveling to New York on business and rarely using condoms. His last HIV test was “about 4 years ago.” He is otherwise in good health, takes no regular medications, and is not married.
Having recently completed a primary care CME program on HIV disease, you order a CD4/T-cell count, an HIV RNA (viral load) test, and an HIV genotype drug resistance test on Mr. G, along with other baseline lab work, including a complete blood count, chemistry panel, and hepatitis panel. You schedule a follow-up visit with Mr. G in 2 weeks when all of the lab results will be available so that you can discuss his plan of care.
A diagnosis of HIV has moved from being a fatal disease to that of a chronic condition that can be effectively managed with combination antiretroviral therapy (ART) regimens over an almost normal lifespan. As a result, the role of the primary care practitioner in the ongoing care of patients with HIV has grown and will continue to do so, making knowledge of these drug combinations vital.
20 Years Have Changed Everything
Combination ART has existed since 1996 when the first protease inhibitors (PIs) were approved by the U.S. Food and Drug Administration (FDA). Prior to this, treatment was limited to mono or dual therapy with nucleoside reverse transcriptase inhibitors (NRTIs). These agents provided some short-term clinical benefit, but didn’t significantly improve patient survival and ultimately failed due to viral resistance.1
Since the approval of zidovudine (AZT) in 1987, the FDA has approved more than 25 drugs in 6 different classes for the treatment of HIV disease.2 These include the NRTIs, non-nucleoside reverse transcriptase inhibitors (NNRTIs), PIs, a fusion inhibitor (FI), a CCR5 antagonist, and, more recently, integrase strand transfer inhibitors (INSTIs). In addition, 2 drugs, cobicistat and ritonavir, are used solely to improve or “boost” the pharmacokinetic profiles of several antiretroviral drugs.2
Most of these newer agents are more potent, have a higher genetic barrier to resistance, and a longer halflife than their predecessors. Moreover, many are less toxic and thus more tolerable than older drugs. With the progressive development and approval of singletablet regimens (STRs) that contain 3 or 4 drugs, the majority of patients with HIV in the United States now take just one pill per day to treat their infection, facilitating far greater medication adherence.
Initiation of Antiretroviral Therapy
The U.S. Department of Health and Human Services (DHHS) guidelines now recommend that all people infected with HIV, regardless of CD4 cell count, begin ART.2 The evidence for this recommendation comes largely from the START3 and TEMPRANO4 trials, which found that early initiation of ART significantly reduces morbidity and mortality associated with HIV. In addition, the HPTN 052 study concluded that early ART is associated with a 93% lower risk of viral transmission in serodiscordant heterosexual couples.5 The DHHS guidelines do note that when initiating ART, it is important to appropriately educate patients on the benefits of treatment and address strategies to optimize adherence.2 (For more on factors to consider when selecting an initial HIV regimen, see Table 1.2) On a case-by-case basis, ART may be deferred because of clinical and/or psychosocial factors, but it should never be withheld unless the risks clearly outweigh the benefits. Ideally, ART should be initiated as soon as possible after the initial diagnosis of HIV.
The DHHS guidelines divide treatment options into 3 categories2:
- Recommended regimens are backed by randomized controlled trials that show optimal and durable virologic efficacy, they have favorable tolerability and toxicity profiles, and they are easy to use.
- Alternative regimens have less or lower quality supporting data than recommended regimens. Although they are effective and may be optimal for certain individual patients, they have potential disadvantages and/or limitations in certain populations.
- Other regimens have limited supporting data, reduced virologic activity, a higher pill burden, more drug interactions, and greater toxicity.
Currently Recommended First-Line Therapies
An antiretroviral regimen for a treatment-naive patient should consist of 2 NRTIs in combination with a third active antiretroviral drug from one of 3 drug classes. These include: an INSTI, a boosted PI, or, in some situations, an NNRTI. The DHHS guidelines panel currently recommends 6 different ART combinations as first-line treatment in treatment-naive patients (Table 2).2
INSTI-Based Regimens
Dolutegravir/abacavir/lamivudine (Triumeq). Approved by the FDA as a single-tablet regimen in 2014, the combination of dolutegravir/abacavir/lamivudine has proven to be highly effective and well-tolerated in many clinical trials.6-9 However, before this regimen is started, patients must be screened for the HLA-B*5701 allele, which predicts hypersensitivity to abacavir.10 Assessing patients’ risk for cardiovascular disease is also advised because some data suggest that abacavir may increase the risk of cardiovascular events, although this remains controversial.2
Dolutegravir is generally well-tolerated with minimal adverse effects (≥ 2% incidence of headache and insomnia) and toxicity.11 Dolutegravir/abacavir/lamivudine should be taken 2 hours before or 6 hours after taking antacids or laxatives, sucralfate, and oral supplements with iron or calcium. However, it may be taken with calcium or iron supplements if it is also taken with food.11 Dolutegravir increases levels of metformin about 2-fold, so patients should not take more than 1000 mg/d of this oral hypoglycemic agent.11
- Dolutegravir plus tenofovir disoproxil fumarate/ emtricitabine (Tivicay plus Truvada). The combination
of dolutegravir plus fixed-dose tenofovir disoproxil fumarate and emtricitabine is administered as 2 pills per day. Because tenofovir disoproxil fumarate can cause proximal renal tubular dysfunction, phosphate wasting, and decreased bone mineral density (BMD), avoid prescribing it for patients with underlying renal dysfunction (creatinine clearance [CrCl] <50 mL/min) and prescribe it cautiously for patients with hypertension or diabetes who are at increased risk of renal disease. Emtricitabine is generally safe and well tolerated, but the dose should be reduced in patients with renal insufficiency, which would preclude the use of this fixed-dose combination.12 - Elvitegravir/cobicistat/tenofovir alafenamide/emtricitabine (Genvoya). The newer 4-drug combination of elvitegravir/ cobicistat/tenofovir alafenamide/emtricitabinethat was approved by the FDA in November 2015,13 contains the more recently approved form of tenofovir, which can be used in patients who have a CrCl as low as 30 mL/min. Compared to formulations containing tenofovir disoproxil fumarate, the newer tenofovir alafenamide formulation achieves higher intracellular levels in CD4 lymphocytes (but not in renal tubular cells). This allows for a lower dose of the drug and a smaller tablet size with co-formulation. It does not appear to cause kidney problems or loss of BMD as can be seen with tenofovir disoproxil fumarate.14 This newer single-tablet regimen may be best suited for older patients with HIV or those with comorbidities such as hypertension or diabetes.
- Elvitegravir/cobicistat/tenofovir disoproxil fumarate/emtricitabine (Stribild). The FDA approved the combination of elvitegravir/cobicistat/tenofovir disoproxil fumarate/emtricitabine as a single-tablet regimen in 2012. The integrase inhibitor, elvitegravir, requires boosting with the CYP3A inhibitor, cobicistat, and should be taken with food.15 Two clinical trials demonstrated the superior efficacy of elvitegravir compared to a boosted PI and NNRTI-based regimen.16,17 Elvitegravir is generally well tolerated, but sometimes causes dyspepsia, nausea, or diarrhea.15 Similar to dolutegravir, it should not be taken concurrently with certain supplements—in this case, those containing aluminum, calcium, iron, magnesium, or zinc.15 Because it contains tenofovir disoproxil fumarate as an active agent, it should not be used in patients with a CrCl of <70 mL/min.15 Cobicistat inhibits tubular secretion of creatinine, so it may produce an elevation in serum creatinine without actually affecting glomerular function. Cobicistat may also cause drug-drug interactions with certain antiarrhythmics, sedative-hypnotics, and erectile dysfunction agents, and is contraindicated with some statins, anticonvulsants, and ergot derivatives.18
- Raltegravir plus tenofovir disoproxil fumarate/emtricitabine (Isentress plus Truvada). The combination of the integrase inhibitor raltegravir plus fixed-dose tenofovir disoproxil fumarate and emtricitabine has been recommended by the DHHS as first-line therapy for approximately 5 years. The recommendation is based mainly on data from the STARTMRK trial, a phase III non-inferiority trial that followed more than 500 patients for 5 years and concluded that raltegravir/ tenofovir/emtricitabine has superior efficacy with fewer drug-related adverse effects than efavirenz/tenofovir/emtricitabine.19 The overall pill burden with this regimen is 3 tablets per day. Although highly effective, the main drawbacks of raltegravir are that it must be dosed twice daily (which may be less preferable if adherence is a concern) and the genetic barrier to resistance is lower than that of the other 2 approved integrase inhibitors. In May 2017, FDA approved a new 1,200 mg once-daily version of raltegravir as an alternative to the twice daily regimen.20 Adverse effects and toxicities (except the renal and bone effects due to tenofovir disoproxil fumarate mentioned earlier) and drug interactions with this regimen are infrequent. Raltegravir can be taken with or without food. Concurrent use of antacids that contain aluminum or magnesium may reduce absorption of raltegravir and so should be avoided.21
PI-Based Regimen
Darunavir (Prezista) and ritonavir (Norvir) plus tenofovir disoproxil fumarate/emtricitabine (Truvada). PIs were once the key component of all ART regimens; however, boosted darunavir is now the only PI-based regimen currently recommended as first-line therapy. It is taken as 3 tablets once daily. If the co-formulation with cobicistat is used, just 2 tablets daily are required. One advantage with darunavir with either of the boosting agents is that it does not appear to cause insulin resistance or dyslipidemia as occurs with older PIs, such as indinavir and lopinavir.2 The boosting agents do, however, increase the likelihood of drug-drug interactions. As with all PIs, darunavir has a very high genetic barrier to resistance, which is important in patients for whom adherence is a concern.
Adverse effects of the PIs may include nausea, vomiting, and diarrhea, all of which are typically mild and selflimiting.22 Co-formulation of darunavir with cobicistat, tenofovir alafenamide, and emtricitabine is in phase III studies. Projected to be available in 2018, it will provide yet another daily STR option.23
The Addition of Fixed-Dose Tenofovir Alafenamide/Emtricitabine
In July 2016, the DHHS panel made some additions to their guidelines to reflect the FDA approval of 3 fixed-dose combination products that contain tenofovir alafenamide. Specifically, the combination of tenofovir alafenamide and emtricitabine is recommended for use with the integrase inhibitors—dolutegravir or raltegravir. It is also recommended in combination with ritonavir-boosted darunavir.
DHHS "Alternative" And "Other" Regimens
The DHHS guidelines also include “alternative” (Table 3) and “other” regimens (available at http://aidsinfo.nih.gov/guidelines) that may be used when first-line regimens may not.2 These second-line options are very effective, but have some possible clinical disadvantages or limitations. They are also less well supported by data from clinical trials. However, in certain situations, depending on an individual patient’s comorbidities, inability to tolerate one of the preferred regimens, or personal preferences, an alternative regimen may be the optimal choice.
Under the category of alternative regimens, the panel has included tenofovir alafenamide and emtricitabine in combination with the NNRTI efavirenz or with ritonavir or cobicistat-boosted atazanavir or darunavir.
The third group or “other” regimens have reduced virologic activity, increased toxicity, and even more limited data from clinical trials. Generally, medications from the DHHS “alternative” and “other” categories should be prescribed in consultation with an HIV specialist.
The Future of Art
The currently available drugs are highly effective in fully suppressing HIV and allowing for immune recovery and clinical stability for most patients. Life expectancy for patients living with HIV is estimated to be approaching that of uninfected adults—provided they remain on ART.24 As a way to further simplify ART, current clinical trials are looking at 2-drug regimens including an integrase inhibitor with an NRTI, an INSTI, or an NNRTI, or a PI with one NRTI.25,26 This approach could further reduce pill burden and toxicity and substantially decrease the cost of long-term treatment.27 Also on the horizon are long-acting injectable antiretroviral drugs that will likely be available for clinical use in the next 2 to 3 years.28,29
CASE: At the 2-week follow-up visit, you discuss with Mr. G that his CD4+ count is 390 cells/mm3, his HIV RNA level is 32,450 copies/mL, and his HIV genotype test showed no antiviral drug resistance. Explaining that all patients with HIV should be treated with antiviral therapy regardless of CD4+ count, you recommend that Mr. G begin taking fixed-dose tenofovir disoproxil fumarate/emtricitabine/elvitegravir/cobicistat (Stribild), noting that it is one of the regimens recommended by the DHHS national treatment guidelines. You provide a patient handout that discusses dosing and adverse effects, including nausea and headache. The patient’s pharmacy was contacted and it was determined that Mr. G’s co-pay for the drug would be $50, which he found acceptable.
In addition, you discuss the importance of good adherence to this medication, and instruct Mr. G to contact the office via phone or patient portal for any concerns or questions that arise after starting the medication. Lastly, you advise him to return in 4 weeks for follow-up blood testing, including viral load monitoring, and additional care, if needed, and strongly recommend that he begin using condoms regularly.
Click here to read the digital edition.
CASE: James G, age 43, recently had blood work performed for a life insurance policy, and his human immunodeficiency virus (HIV) test came back positive. At a follow-up office visit, Mr. G reports having anonymous male sexual partners when traveling to New York on business and rarely using condoms. His last HIV test was “about 4 years ago.” He is otherwise in good health, takes no regular medications, and is not married.
Having recently completed a primary care CME program on HIV disease, you order a CD4/T-cell count, an HIV RNA (viral load) test, and an HIV genotype drug resistance test on Mr. G, along with other baseline lab work, including a complete blood count, chemistry panel, and hepatitis panel. You schedule a follow-up visit with Mr. G in 2 weeks when all of the lab results will be available so that you can discuss his plan of care.
A diagnosis of HIV has moved from being a fatal disease to that of a chronic condition that can be effectively managed with combination antiretroviral therapy (ART) regimens over an almost normal lifespan. As a result, the role of the primary care practitioner in the ongoing care of patients with HIV has grown and will continue to do so, making knowledge of these drug combinations vital.
20 Years Have Changed Everything
Combination ART has existed since 1996 when the first protease inhibitors (PIs) were approved by the U.S. Food and Drug Administration (FDA). Prior to this, treatment was limited to mono or dual therapy with nucleoside reverse transcriptase inhibitors (NRTIs). These agents provided some short-term clinical benefit, but didn’t significantly improve patient survival and ultimately failed due to viral resistance.1
Since the approval of zidovudine (AZT) in 1987, the FDA has approved more than 25 drugs in 6 different classes for the treatment of HIV disease.2 These include the NRTIs, non-nucleoside reverse transcriptase inhibitors (NNRTIs), PIs, a fusion inhibitor (FI), a CCR5 antagonist, and, more recently, integrase strand transfer inhibitors (INSTIs). In addition, 2 drugs, cobicistat and ritonavir, are used solely to improve or “boost” the pharmacokinetic profiles of several antiretroviral drugs.2
Most of these newer agents are more potent, have a higher genetic barrier to resistance, and a longer halflife than their predecessors. Moreover, many are less toxic and thus more tolerable than older drugs. With the progressive development and approval of singletablet regimens (STRs) that contain 3 or 4 drugs, the majority of patients with HIV in the United States now take just one pill per day to treat their infection, facilitating far greater medication adherence.
Initiation of Antiretroviral Therapy
The U.S. Department of Health and Human Services (DHHS) guidelines now recommend that all people infected with HIV, regardless of CD4 cell count, begin ART.2 The evidence for this recommendation comes largely from the START3 and TEMPRANO4 trials, which found that early initiation of ART significantly reduces morbidity and mortality associated with HIV. In addition, the HPTN 052 study concluded that early ART is associated with a 93% lower risk of viral transmission in serodiscordant heterosexual couples.5 The DHHS guidelines do note that when initiating ART, it is important to appropriately educate patients on the benefits of treatment and address strategies to optimize adherence.2 (For more on factors to consider when selecting an initial HIV regimen, see Table 1.2) On a case-by-case basis, ART may be deferred because of clinical and/or psychosocial factors, but it should never be withheld unless the risks clearly outweigh the benefits. Ideally, ART should be initiated as soon as possible after the initial diagnosis of HIV.
The DHHS guidelines divide treatment options into 3 categories2:
- Recommended regimens are backed by randomized controlled trials that show optimal and durable virologic efficacy, they have favorable tolerability and toxicity profiles, and they are easy to use.
- Alternative regimens have less or lower quality supporting data than recommended regimens. Although they are effective and may be optimal for certain individual patients, they have potential disadvantages and/or limitations in certain populations.
- Other regimens have limited supporting data, reduced virologic activity, a higher pill burden, more drug interactions, and greater toxicity.
Currently Recommended First-Line Therapies
An antiretroviral regimen for a treatment-naive patient should consist of 2 NRTIs in combination with a third active antiretroviral drug from one of 3 drug classes. These include: an INSTI, a boosted PI, or, in some situations, an NNRTI. The DHHS guidelines panel currently recommends 6 different ART combinations as first-line treatment in treatment-naive patients (Table 2).2
INSTI-Based Regimens
Dolutegravir/abacavir/lamivudine (Triumeq). Approved by the FDA as a single-tablet regimen in 2014, the combination of dolutegravir/abacavir/lamivudine has proven to be highly effective and well-tolerated in many clinical trials.6-9 However, before this regimen is started, patients must be screened for the HLA-B*5701 allele, which predicts hypersensitivity to abacavir.10 Assessing patients’ risk for cardiovascular disease is also advised because some data suggest that abacavir may increase the risk of cardiovascular events, although this remains controversial.2
Dolutegravir is generally well-tolerated with minimal adverse effects (≥ 2% incidence of headache and insomnia) and toxicity.11 Dolutegravir/abacavir/lamivudine should be taken 2 hours before or 6 hours after taking antacids or laxatives, sucralfate, and oral supplements with iron or calcium. However, it may be taken with calcium or iron supplements if it is also taken with food.11 Dolutegravir increases levels of metformin about 2-fold, so patients should not take more than 1000 mg/d of this oral hypoglycemic agent.11
- Dolutegravir plus tenofovir disoproxil fumarate/ emtricitabine (Tivicay plus Truvada). The combination
of dolutegravir plus fixed-dose tenofovir disoproxil fumarate and emtricitabine is administered as 2 pills per day. Because tenofovir disoproxil fumarate can cause proximal renal tubular dysfunction, phosphate wasting, and decreased bone mineral density (BMD), avoid prescribing it for patients with underlying renal dysfunction (creatinine clearance [CrCl] <50 mL/min) and prescribe it cautiously for patients with hypertension or diabetes who are at increased risk of renal disease. Emtricitabine is generally safe and well tolerated, but the dose should be reduced in patients with renal insufficiency, which would preclude the use of this fixed-dose combination.12 - Elvitegravir/cobicistat/tenofovir alafenamide/emtricitabine (Genvoya). The newer 4-drug combination of elvitegravir/ cobicistat/tenofovir alafenamide/emtricitabinethat was approved by the FDA in November 2015,13 contains the more recently approved form of tenofovir, which can be used in patients who have a CrCl as low as 30 mL/min. Compared to formulations containing tenofovir disoproxil fumarate, the newer tenofovir alafenamide formulation achieves higher intracellular levels in CD4 lymphocytes (but not in renal tubular cells). This allows for a lower dose of the drug and a smaller tablet size with co-formulation. It does not appear to cause kidney problems or loss of BMD as can be seen with tenofovir disoproxil fumarate.14 This newer single-tablet regimen may be best suited for older patients with HIV or those with comorbidities such as hypertension or diabetes.
- Elvitegravir/cobicistat/tenofovir disoproxil fumarate/emtricitabine (Stribild). The FDA approved the combination of elvitegravir/cobicistat/tenofovir disoproxil fumarate/emtricitabine as a single-tablet regimen in 2012. The integrase inhibitor, elvitegravir, requires boosting with the CYP3A inhibitor, cobicistat, and should be taken with food.15 Two clinical trials demonstrated the superior efficacy of elvitegravir compared to a boosted PI and NNRTI-based regimen.16,17 Elvitegravir is generally well tolerated, but sometimes causes dyspepsia, nausea, or diarrhea.15 Similar to dolutegravir, it should not be taken concurrently with certain supplements—in this case, those containing aluminum, calcium, iron, magnesium, or zinc.15 Because it contains tenofovir disoproxil fumarate as an active agent, it should not be used in patients with a CrCl of <70 mL/min.15 Cobicistat inhibits tubular secretion of creatinine, so it may produce an elevation in serum creatinine without actually affecting glomerular function. Cobicistat may also cause drug-drug interactions with certain antiarrhythmics, sedative-hypnotics, and erectile dysfunction agents, and is contraindicated with some statins, anticonvulsants, and ergot derivatives.18
- Raltegravir plus tenofovir disoproxil fumarate/emtricitabine (Isentress plus Truvada). The combination of the integrase inhibitor raltegravir plus fixed-dose tenofovir disoproxil fumarate and emtricitabine has been recommended by the DHHS as first-line therapy for approximately 5 years. The recommendation is based mainly on data from the STARTMRK trial, a phase III non-inferiority trial that followed more than 500 patients for 5 years and concluded that raltegravir/ tenofovir/emtricitabine has superior efficacy with fewer drug-related adverse effects than efavirenz/tenofovir/emtricitabine.19 The overall pill burden with this regimen is 3 tablets per day. Although highly effective, the main drawbacks of raltegravir are that it must be dosed twice daily (which may be less preferable if adherence is a concern) and the genetic barrier to resistance is lower than that of the other 2 approved integrase inhibitors. In May 2017, FDA approved a new 1,200 mg once-daily version of raltegravir as an alternative to the twice daily regimen.20 Adverse effects and toxicities (except the renal and bone effects due to tenofovir disoproxil fumarate mentioned earlier) and drug interactions with this regimen are infrequent. Raltegravir can be taken with or without food. Concurrent use of antacids that contain aluminum or magnesium may reduce absorption of raltegravir and so should be avoided.21
PI-Based Regimen
Darunavir (Prezista) and ritonavir (Norvir) plus tenofovir disoproxil fumarate/emtricitabine (Truvada). PIs were once the key component of all ART regimens; however, boosted darunavir is now the only PI-based regimen currently recommended as first-line therapy. It is taken as 3 tablets once daily. If the co-formulation with cobicistat is used, just 2 tablets daily are required. One advantage with darunavir with either of the boosting agents is that it does not appear to cause insulin resistance or dyslipidemia as occurs with older PIs, such as indinavir and lopinavir.2 The boosting agents do, however, increase the likelihood of drug-drug interactions. As with all PIs, darunavir has a very high genetic barrier to resistance, which is important in patients for whom adherence is a concern.
Adverse effects of the PIs may include nausea, vomiting, and diarrhea, all of which are typically mild and selflimiting.22 Co-formulation of darunavir with cobicistat, tenofovir alafenamide, and emtricitabine is in phase III studies. Projected to be available in 2018, it will provide yet another daily STR option.23
The Addition of Fixed-Dose Tenofovir Alafenamide/Emtricitabine
In July 2016, the DHHS panel made some additions to their guidelines to reflect the FDA approval of 3 fixed-dose combination products that contain tenofovir alafenamide. Specifically, the combination of tenofovir alafenamide and emtricitabine is recommended for use with the integrase inhibitors—dolutegravir or raltegravir. It is also recommended in combination with ritonavir-boosted darunavir.
DHHS "Alternative" And "Other" Regimens
The DHHS guidelines also include “alternative” (Table 3) and “other” regimens (available at http://aidsinfo.nih.gov/guidelines) that may be used when first-line regimens may not.2 These second-line options are very effective, but have some possible clinical disadvantages or limitations. They are also less well supported by data from clinical trials. However, in certain situations, depending on an individual patient’s comorbidities, inability to tolerate one of the preferred regimens, or personal preferences, an alternative regimen may be the optimal choice.
Under the category of alternative regimens, the panel has included tenofovir alafenamide and emtricitabine in combination with the NNRTI efavirenz or with ritonavir or cobicistat-boosted atazanavir or darunavir.
The third group or “other” regimens have reduced virologic activity, increased toxicity, and even more limited data from clinical trials. Generally, medications from the DHHS “alternative” and “other” categories should be prescribed in consultation with an HIV specialist.
The Future of Art
The currently available drugs are highly effective in fully suppressing HIV and allowing for immune recovery and clinical stability for most patients. Life expectancy for patients living with HIV is estimated to be approaching that of uninfected adults—provided they remain on ART.24 As a way to further simplify ART, current clinical trials are looking at 2-drug regimens including an integrase inhibitor with an NRTI, an INSTI, or an NNRTI, or a PI with one NRTI.25,26 This approach could further reduce pill burden and toxicity and substantially decrease the cost of long-term treatment.27 Also on the horizon are long-acting injectable antiretroviral drugs that will likely be available for clinical use in the next 2 to 3 years.28,29
CASE: At the 2-week follow-up visit, you discuss with Mr. G that his CD4+ count is 390 cells/mm3, his HIV RNA level is 32,450 copies/mL, and his HIV genotype test showed no antiviral drug resistance. Explaining that all patients with HIV should be treated with antiviral therapy regardless of CD4+ count, you recommend that Mr. G begin taking fixed-dose tenofovir disoproxil fumarate/emtricitabine/elvitegravir/cobicistat (Stribild), noting that it is one of the regimens recommended by the DHHS national treatment guidelines. You provide a patient handout that discusses dosing and adverse effects, including nausea and headache. The patient’s pharmacy was contacted and it was determined that Mr. G’s co-pay for the drug would be $50, which he found acceptable.
In addition, you discuss the importance of good adherence to this medication, and instruct Mr. G to contact the office via phone or patient portal for any concerns or questions that arise after starting the medication. Lastly, you advise him to return in 4 weeks for follow-up blood testing, including viral load monitoring, and additional care, if needed, and strongly recommend that he begin using condoms regularly.
Click here to read the digital edition.
1. Concorde: MRC/ANRS randomised double-blind controlled trial of immediate and deferred zidovudine in symptom-free HIV infection. Concorde Coordinating Committee. Lancet. 1994;343:871-881.
2. Department of Health and Human Services. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Available at: http://www.aidsinfo.nih.gov/guidelines/html/1/adult-and-adolescent-treatment-guidelines/0. Accessed July 17, 2016.
3. The INSIGHT START Study Group. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med. 2015;373:795-807.
4. The TEMPRANO ANRS 12136 Study Group. A trial of early antiretrovirals and isoniazid preventive therapy in Africa. N Engl J Med. 2015;373:808-822.
5. Cohen MS, Chen YQ, McCauley M, et al. Antiretroviral therapy for the prevention of HIV-1 transmission. N Engl J Med. 2016;375:830-839.
6. Molina JM, Clotet B, van Lunzen J, et al. Once-daily dolutegravir versus darunavir plus ritonavir for treatment-naive adults with HIV-1 infection (FLAMINGO): 96 week results from a randomized, open-label, phase 3b study. Lancet HIV. 2015;2:e127-136.
7. Walmsley SL, Antela A, Clumeck N, et al. Dolutegravir plus abacavir-lamivudine for the treatment of HIV-1 infection. N Engl J Med. 2013;369:1807-1818.
8. Van Lunzen J, Maggiolo F, Arribas JR, et al. Once daily dolutegravir (S/GSK1349572) in combination therapy in antiretroviral-naïve adults with HIV: planned interim 48 week results from SPRING-1, a dose-ranging, randomized, phase 2b trial. Lancet Infect Dis. 2012;12:111-118.
9. Stellbrink HJ, Reynes J, Lazzarin A, et al. Dolutegravir in antiretroviral-naive adults with HIV-1: 96-week results from a randomized dose-ranging study. AIDS. 2013; 27:1771-1778.
10. Mallal S, Phillips E, Carosi G. HLA-B*5701 screening for hypersensitivity to abacavir. N Engl J Med. 2008;358:568-579.
11. AIDSinfo Drug Database. Dolutegravir. Available at: https://aidsinfo.nih.gov/drugs/509/dolutegravir/0/professional. Accessed July 17, 2016.
12. AIDSinfo Drug Database. Emtricitabine. Available at: https://aidsinfo.nih.gov/drugs/208/emtricitabine/0/patient. Accessed July 17, 2016.
13. AIDSinfo Drug Database. Elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide fumarate. Available at: https://aidsinfo.nih.gov/drugs/553/genvoya/0/professional. Accessed July 17, 2016.
14. Ray AS, Fordyce MW, Hitchcock, MJM. Tenofovir alafenamide: A novel prodrug of tenofovir for the treatment of human immunodeficiency virus. Antiviral Res. 2016;125:63-70.
15. AIDSinfo Drug Database. Elvitegravir. https://aidsinfo.nih.gov/drugs/421/elvitegravir/0/professional
16. Wohl DA, Cohen C, Gallant JE, et al. A randomized, double-blind comparison of single-tablet regimen elvitegravir/cobicistat/emtricitabine/tenofovir DF versus single-tablet regimen efavirenz/emtricitabine/tenofovir DF for initial treatment of HIV-1 infection: analysis of week 144 results. J Acquir Immune Defic Syndr. 2014;65:e118-120.
17. Clumeck N, Molina JM, Henry K, et al. A randomized, double-blind comparison of single- tablet regimen elvitegravir/cobicistat/emtricitabine/tenofovir DF vs ritonavir-boosted atazanavir plus emtricitabine/tenofovir for initial treatment of HIV-1 infection: analysis of week 144 results. J Acquir Immune Defic Syndr. 2014;65:e121-124.
18. AIDSinfo Drug Database. Cobicistat. Available at: https://aidsinfo.nih.gov/drug/537/evotaz/0/patient/. Accessed July 17, 2016.
19. Rockstroh JK, DeJesus E, Lennox JL, et al. Durable efficacy and safety of raltegravir versus efavirenz when combined with tenofovir/emtricitabine in treatmentnaïve HIV-1 infected patients: final 5-year results from STARTMRK. J Acquir Immune Defic Syndr. 2013;63:77-85.
20. Cahn P, Kaplan R, Sax P, et al. Raltegravir (RAL) 1200 mg once daily (QD) is non-inferior to RAL 400 mg twice daily (BID), in combination with tenofovir/emtricitabine, in treatment-naive HIV-1-infected subjects: week 48 results. Abstract FRAB0103LB presented at: 21st International AIDS Conference; July 18-22, 2016; Durban, South Africa.
21. Hicks C, Gulick RM. Raltegravir: the first HIV type 1 integrase inhibitor. Clin Infect Dis. 2009;48:931-939.
22. Prescriber’s Letter. HIV/AIDS Pharmacotherapy Review. Vol. 2015; Course no. 215. Available at: http://http://prescribersletter.therapeuticresearch.com/ce/documents/ce_15215-40.pdf. Accessed May 31, 2017.
23. AIDSinfo Drug Database. Tenofovir alafenamide. Available at: https://aidsinfo.nih.gov/drugs/514/tenofovir-alafenamide/0/patient. Accessed September 27, 2016.
24. Marcus JL, Chao C, Leyden W, et al. Narrowing the gap in life expectancy for HIV+ compared with HIV- individuals. Conference on Retroviruses and Opportunistic Infections. February 22-25, 2016, Boston. Abstract 54.
25. Gubavu C, Prazuck T, Niang M, et al. Dolutegravir-based monotherapy or dual therapy maintains a high proportion of viral suppression even in highly experienced HIV-1-infected patients. J Antimicrob Chemother. 2016;71:1046-1050.
26. Margolis DA, Brinson CC, Smith GHR. Cabotegravir plus rilpivirine, once a day, after induction with cabotegravir plus nucleoside reverse transcriptase inhibitors in antiretroviral naïve adults with HIV-1 infection (LATTE): a randomised, phase 2b, dose-ranging trial. Lancet Infect Dis. 2015;15:1145-1155.
27. Girouard MP, Sax PE, Parker RA, et al. The cost-effectiveness and budget impact of 2-drug dolutegravir-lamivudine regimens for the treatment of HIV infection in the United States. Clin Infect Dis. 2016; 62:784-791.
28. Margolis DA, Gonzalez-Garcia J, Stellbrink HJ, et al. Cabotegravir + rilpivirine as long-acting maintenance therapy: LATTE-2 week 32 results. Abstract number 31 LB. Conference on Retroviruses and Opportunistic Infections. February 22-25, 2016; Boston, MA.
29. Murray MI, Markowitz M, Frank I, et al. Tolerability and acceptability of cabotegravir LA injection: results from ECLAIR study. Abstract number 471. Conference on Retroviruses and Opportunistic Infections. February 22-25, 2016; Boston, MA.
1. Concorde: MRC/ANRS randomised double-blind controlled trial of immediate and deferred zidovudine in symptom-free HIV infection. Concorde Coordinating Committee. Lancet. 1994;343:871-881.
2. Department of Health and Human Services. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Available at: http://www.aidsinfo.nih.gov/guidelines/html/1/adult-and-adolescent-treatment-guidelines/0. Accessed July 17, 2016.
3. The INSIGHT START Study Group. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med. 2015;373:795-807.
4. The TEMPRANO ANRS 12136 Study Group. A trial of early antiretrovirals and isoniazid preventive therapy in Africa. N Engl J Med. 2015;373:808-822.
5. Cohen MS, Chen YQ, McCauley M, et al. Antiretroviral therapy for the prevention of HIV-1 transmission. N Engl J Med. 2016;375:830-839.
6. Molina JM, Clotet B, van Lunzen J, et al. Once-daily dolutegravir versus darunavir plus ritonavir for treatment-naive adults with HIV-1 infection (FLAMINGO): 96 week results from a randomized, open-label, phase 3b study. Lancet HIV. 2015;2:e127-136.
7. Walmsley SL, Antela A, Clumeck N, et al. Dolutegravir plus abacavir-lamivudine for the treatment of HIV-1 infection. N Engl J Med. 2013;369:1807-1818.
8. Van Lunzen J, Maggiolo F, Arribas JR, et al. Once daily dolutegravir (S/GSK1349572) in combination therapy in antiretroviral-naïve adults with HIV: planned interim 48 week results from SPRING-1, a dose-ranging, randomized, phase 2b trial. Lancet Infect Dis. 2012;12:111-118.
9. Stellbrink HJ, Reynes J, Lazzarin A, et al. Dolutegravir in antiretroviral-naive adults with HIV-1: 96-week results from a randomized dose-ranging study. AIDS. 2013; 27:1771-1778.
10. Mallal S, Phillips E, Carosi G. HLA-B*5701 screening for hypersensitivity to abacavir. N Engl J Med. 2008;358:568-579.
11. AIDSinfo Drug Database. Dolutegravir. Available at: https://aidsinfo.nih.gov/drugs/509/dolutegravir/0/professional. Accessed July 17, 2016.
12. AIDSinfo Drug Database. Emtricitabine. Available at: https://aidsinfo.nih.gov/drugs/208/emtricitabine/0/patient. Accessed July 17, 2016.
13. AIDSinfo Drug Database. Elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide fumarate. Available at: https://aidsinfo.nih.gov/drugs/553/genvoya/0/professional. Accessed July 17, 2016.
14. Ray AS, Fordyce MW, Hitchcock, MJM. Tenofovir alafenamide: A novel prodrug of tenofovir for the treatment of human immunodeficiency virus. Antiviral Res. 2016;125:63-70.
15. AIDSinfo Drug Database. Elvitegravir. https://aidsinfo.nih.gov/drugs/421/elvitegravir/0/professional
16. Wohl DA, Cohen C, Gallant JE, et al. A randomized, double-blind comparison of single-tablet regimen elvitegravir/cobicistat/emtricitabine/tenofovir DF versus single-tablet regimen efavirenz/emtricitabine/tenofovir DF for initial treatment of HIV-1 infection: analysis of week 144 results. J Acquir Immune Defic Syndr. 2014;65:e118-120.
17. Clumeck N, Molina JM, Henry K, et al. A randomized, double-blind comparison of single- tablet regimen elvitegravir/cobicistat/emtricitabine/tenofovir DF vs ritonavir-boosted atazanavir plus emtricitabine/tenofovir for initial treatment of HIV-1 infection: analysis of week 144 results. J Acquir Immune Defic Syndr. 2014;65:e121-124.
18. AIDSinfo Drug Database. Cobicistat. Available at: https://aidsinfo.nih.gov/drug/537/evotaz/0/patient/. Accessed July 17, 2016.
19. Rockstroh JK, DeJesus E, Lennox JL, et al. Durable efficacy and safety of raltegravir versus efavirenz when combined with tenofovir/emtricitabine in treatmentnaïve HIV-1 infected patients: final 5-year results from STARTMRK. J Acquir Immune Defic Syndr. 2013;63:77-85.
20. Cahn P, Kaplan R, Sax P, et al. Raltegravir (RAL) 1200 mg once daily (QD) is non-inferior to RAL 400 mg twice daily (BID), in combination with tenofovir/emtricitabine, in treatment-naive HIV-1-infected subjects: week 48 results. Abstract FRAB0103LB presented at: 21st International AIDS Conference; July 18-22, 2016; Durban, South Africa.
21. Hicks C, Gulick RM. Raltegravir: the first HIV type 1 integrase inhibitor. Clin Infect Dis. 2009;48:931-939.
22. Prescriber’s Letter. HIV/AIDS Pharmacotherapy Review. Vol. 2015; Course no. 215. Available at: http://http://prescribersletter.therapeuticresearch.com/ce/documents/ce_15215-40.pdf. Accessed May 31, 2017.
23. AIDSinfo Drug Database. Tenofovir alafenamide. Available at: https://aidsinfo.nih.gov/drugs/514/tenofovir-alafenamide/0/patient. Accessed September 27, 2016.
24. Marcus JL, Chao C, Leyden W, et al. Narrowing the gap in life expectancy for HIV+ compared with HIV- individuals. Conference on Retroviruses and Opportunistic Infections. February 22-25, 2016, Boston. Abstract 54.
25. Gubavu C, Prazuck T, Niang M, et al. Dolutegravir-based monotherapy or dual therapy maintains a high proportion of viral suppression even in highly experienced HIV-1-infected patients. J Antimicrob Chemother. 2016;71:1046-1050.
26. Margolis DA, Brinson CC, Smith GHR. Cabotegravir plus rilpivirine, once a day, after induction with cabotegravir plus nucleoside reverse transcriptase inhibitors in antiretroviral naïve adults with HIV-1 infection (LATTE): a randomised, phase 2b, dose-ranging trial. Lancet Infect Dis. 2015;15:1145-1155.
27. Girouard MP, Sax PE, Parker RA, et al. The cost-effectiveness and budget impact of 2-drug dolutegravir-lamivudine regimens for the treatment of HIV infection in the United States. Clin Infect Dis. 2016; 62:784-791.
28. Margolis DA, Gonzalez-Garcia J, Stellbrink HJ, et al. Cabotegravir + rilpivirine as long-acting maintenance therapy: LATTE-2 week 32 results. Abstract number 31 LB. Conference on Retroviruses and Opportunistic Infections. February 22-25, 2016; Boston, MA.
29. Murray MI, Markowitz M, Frank I, et al. Tolerability and acceptability of cabotegravir LA injection: results from ECLAIR study. Abstract number 471. Conference on Retroviruses and Opportunistic Infections. February 22-25, 2016; Boston, MA.
Criteria for Use Updates for Enzalutamide, Daratumumab, Elotuzumab, Carfilzomib, and Ixazomib (FULL)
VA Pharmacy Benefit Management Service (PBM) continually issues or revises its guidances for hematology and oncology care providers on a number of cancer care medications. Below are excerpts from recently released Criteria for Use documents. The complete documents, including the inclusion criteria, dosage and administration guidance, monitoring information, and discontinuation criteria should be consulted and can be found at www.pbm.va.gov or vaww.cmopnational.va.gov/cmop/PBM/default.aspx.
ENZALUTAMIDE (XTANDI) Criteria for Use, January 2017
Exclusion Criteria: If the answer to ANY item below is met, then the patient should NOT receive enzalutamide.
- Brain metastases or active epidural disease
- Severe renal impairment (creatinine clearance < 30 mL/min)
- History of seizure (including febrile seizure, loss of consciousness, or transient ischemic attack within the previous 12 months, any condition predisposing to seizure: prior stroke, brain AV malformation, head trauma with loss of consciousness requiring hospitalization)
- ECOG Performance Status > 2
- Inability to swallow capsules
Issues for Consideration
- Enzalutamide is not indicated for use in women. Based on the mechanism of action, can cause fetal harm if used during pregnancy. Pregnancy Category X—use contraindicated during pregnancy. Exclude pregnancy before prescribing enzalutamide, discuss risks if pregnancy occurs, and provide contraceptive counseling.
- Use in patients taking concomitant medications that may lower the seizure threshold was not studied; caution patients about the risk of activities where the sudden loss of consciousness could cause serious harm if concomitant use cannot be avoided.
- Use in patients at risk for or with a strong history of falls: in the phase 3 clinical trial, falls or injuries from falls occurred in 4.6% of enzalutamide patients vs 1.3% of placebo patients.
- Avoid strong inhibitors of CYP2C8 (eg, gemfibrozil); if concomitant use of a strong CYP2C8 inhibitor cannot be avoided, reduce the dose of enzalutamide to 80 mg once daily according to the package insert.
- Co-administration with strong or moderate inducers of CYP3A4 (eg, carbamazepine, phenobarbital, phenytoin, rifampin, bosentan, efavirenz, modafinil, nafcillin, St. John’s Wort) or CYP2C8 (eg, rifampin) should be avoided if possible. If patient must be co-administered a strong CYP3A4 inducer, increase enzalutamide dose from 160 mg to 240 mg once daily.
- Drugs that are substrates of CYP3A4 (eg, alfentanil, cyclosporine, ergotamine, fentanyl, pimozide, quinidine, sirolimus, tacrolimus), CYP2C9 (eg, phenytoin, warfarin), or CYP2C19 (eg, S-mephenytoin) with a narrow therapeutic index should be avoided. If enzalutamide is co-administered with warfarin, additional INR testing should be conducted.
- Use in patients with hepatic impairment: Pharmacokinetics of enzalutamide and its metabolite were examined in volunteers with normal, Child-Pugh Class A, Child-Pugh Class B, and Child-Pugh Class C hepatic impairment. The composite AUC for enzalutamide and its metabolite after a single 160-mg dose was similar across all levels of hepatic impairment compared with normal volunteers.
- There have been postmarketing reports of posterior reversible encephalopathy syndrome (PRES) in patients receiving enzalutamide. PRES is a neurologic disorder presenting with rapidly evolving symptoms including seizure, headache, lethargy, confusion, blindness, and other visual/neurological disturbances with or without associated hypertension. Diagnosis of PRES requires brain imaging, preferably by MRI. Enzalutamide should be discontinued in patients developing PRES.
- Sequencing of enzalutamide and abiraterone has been evaluated in several small retrospective analyses; the majority of the analyses are in the post chemotherapy setting. From this limited observational data, it is unclear if there is a preferred sequencing of abiraterone and enzalutamide. There is some evidence for cross-resistance. There are ongoing investigations into mechanisms of resistance to enzalutamide and abiraterone.
DARATUMUMAB (DARZALEX) Criteria for Use, January 2017
Exclusion Criteria: If the answer to ANY item below is met, then the patient should NOT receive daratumumab.
- Patient is noncompliant with medication, follow-up, or laboratory appointments
- Patient unable or unwilling to be observed an extended period of time that may be necessary for first infusion (refer to Issues for Consideration)
- Hemoglobin < 8 g/dL; Must transfuse to hemoglobin above 8 gm/dL prior to therapy initiation
- Absolute neutrophil count (ANC) < 1,000/mm3
- Platelet count < 50,000/mm3 (< 30,000/mm3 if myeloma involvement in bone marrow > 50%)
- ECOG Performance Status > 2
- Total bilirubin > 3x the upper limit of the normal range (except for Gilbert syndrome: direct bilirubin 2x ULN) or ALT and AST > 3x ULN
- NYHA Class III or IV heart failure (refer to Issues for Consideration)
- Ongoing or active systemic infection, including active hepatitis B or C, or known HIV (refer to Issues for Consideration)
- Positive pregnancy test
Issues for Consideration
- Drug infusion time will be dependent upon patient tolerance and exposure to daratumumab. Median duration of the first infusion was ~ 7 hours in the SIRIUS trial, followed by infusion times of 4.2 and 3.4 hours, subsequently.
- Type and screen patients shortly prior to starting treatment. When the sample is provided to the blood bank, inform them that the patient will be receiving daratumumab.
- Those with NYHA Class III or IV heart failure, recent MI, conduction abnormalities, angina or arrhythmias uncontrolled by medications, were not eligible for clinical trials and may be at greater risk of cardiac complications.
- Patients with active hepatitis B, C, or HIV were excluded from clinical trials with daratumumab, therefore safety and efficacy data are unknown in these patient populations. The risk of infections was slightly higher in the daratumumab-treated arms of the comparative studies. Use of daratumumab should only be considered in those with well-controlled hepatitis B, hepatitis C, or HIV.
ELOTUZUMAB (EMPLICITI) Criteria for Use, January 2017
Exclusion Criteria: If the answer to ANY item below is met, then the patient should NOT receive elotuzumab.
- Patient is noncompliant with medication, follow-up, or laboratory appointments
- Patient is not a candidate for lenalidomide therapy (ie, is lenalidomide-refractory or possesses contraindications to therapy)
- Patient is not a candidate for high-dose dexamethasone therapy
- Hemoglobin < 8 g/dL; Must transfuse to hemoglobin above 8 gm/dL prior to therapy initiation
- Absolute neutrophil count (ANC) < 1,000/mm3
- Platelet count < 50,000/mm3 (< 30,000/mm3 if myeloma involvement in bone marrow > 50%)
- ECOG Performance Status > 2
- Total bilirubin > 2x the upper limit of the normal range (except for Gilbert syndrome: direct bilirubin > 2 mg/dL) or ALT and AST > 3x ULN
- NYHA Class III or IV heart failure (refer to Issues for Consideration)
- Ongoing or active systemic infection, including active hepatitis B or hepatitis C, or known HIV (refer to Issues for Consideration)
- Positive pregnancy test
- Patient intends to breastfeed during therapy
Issues for Consideration
- Those with NYHA Class III or IV heart failure, recent MI, conduction abnormalities, angina or arrhythmias uncontrolled by medications, were not eligible for clinical trials and may be at greater risk of cardiac complications.
- Patients with active hepatitis B, hepatitis C, or HIV were excluded from clinical trials with elotuzumab, therefore safety and efficacy data are unknown in these patient populations. The risk of infections (OI, fungal, viral) was greater in the elotuzumab arm vs control arm of the comparative clinical trial. Use of elotuzumab should only be considered in those with well-controlled hepatitis B, hepatitis C, or HIV.
- Disappointing response rates as monotherapy in the relapsed/refractory setting suggest that elotuzumab should be given in combination with lenalidomide and dexamethasone.
- Impact of elotuzumab/lenalidomide/dexamethasone on overall survival is not known as these data were not mature at the time ELOQUENT-2 was published.
CARFILZOMIB (KYPROLIS) Criteria for Use, December 2016
Exclusion Criteria: If the answer to ANY item below is met, then the patient should NOT receive carfilzomib.
- Care for the oncologic disease being treated not provided by a VA or VA purchased care (eg, Choice Program, Fee Basis) oncology provider
- Patient is noncompliant with medication, follow-up, or laboratory appointments
- Hemoglobin < 8 g/dL; Must transfuse to hemoglobin above 8 g/dL prior to therapy initiation
- Absolute neutrophil count (ANC) < 1,000/mm3
- Platelet count < 50,000/mm3 (< 30,000/mm3 if myeloma involvement in bone marrow > 50%)
- ECOG Performance Status > 2
- Total bilirubin > 1.5 x the upper limit of the normal range or ALT and AST > 3 x ULN
- NYHA Class III and IV heart failure (refer to Issues for Consideration; those at risk for cardiac failure and ischemia were also excluded from clinical trials)
- LVEF < 40%
- Uncontrolled hypertension
- Grade 3 or 4 peripheral neuropathy
- Ongoing or active systemic infection, including active hepatitis B or C, or known HIV
Issues for Consideration
- A significant percentage of patients on carfilzomib develop dyspnea. This drug should be used with caution in patients with underlying lung disease. Close monitoring for worsening of dyspnea is advised.
- Risk of cardiac failure increases in those aged > 75 years; those with NYHA Class III and IV heart failure, recent MI, conduction abnormalities, angina or arrhythmias uncontrolled by medications, were not eligible for clinical trials and may be at greater risk of cardiac complications. Refer to Prescribing Information for management recommendations.
- Those aged > 75 years experienced greater toxicity than their younger counterparts.
- Patients on dialysis: administer carfilzomib after the dialysis procedure.
- Phase III evidence in heavily pretreated relapsed/refractory patients (median 5 prior regimens) of carfilzomib vs low-dose steroids ± cyclophosphamide indicates that the median overall survival is not significantly different between these treatment arms.
IXAZOMIB (NILARO) Criteria for Use, December 2016
Exclusion Criteria: If the answer to ANY item below is met, then the patient should NOT receive ixazomib
- Care for the oncologic disease being treated not provided by a VA or VA purchased care (eg, Choice Program, Fee Basis) oncology provider
- Patient is not a candidate for lenalidomide or dexamethasone therapy
- Patient is refractory to lenalidomide or proteasomeinhibitor therapy (defined as disease progression while on treatment or within 60 days of last dose)
- Absolute neutrophil count (ANC) < 1,000/mm3
- Platelet count < 75,000/mm3
- ECOG Performance Status > 2
- Patient with CNS involvement
- Patient receiving concurrent therapy with a strong CYP3A inducer (ie, rifampin, phenytoin, carbamazepine) that cannot be discontinued
- Uncontrolled cardiovascular conditions, including uncontrolled hypertension, uncontrolled cardiac arrhythmias, symptomatic congestive heart failure, unstable angina or myocardial infarction within 6 months prior to start
- Ongoing or active systemic infection, including active hepatitis B, hepatitis C, or known HIV
Issues for Consideration
- Indirect comparisons of phase 3 data (KRd vs Rd and IRd vs Rd) show that in similar populations of pretreated relapsed, refractory myeloma patients, those receiving KRd experienced longer PFS (26.3 vs 21 months), greater CR (32% vs 12%), greater ORR (87% vs 78%) and longer duration of response (28.6 vs 20.5 months). Therefore, providers may want to consider using carfilzomib in those meeting its criteria for use.
- Ixazomib is cytotoxic. Capsules should not be opened or crushed. Waste should be considered hazardous.
- Avoid concomitant use of strong CYP3A inducers (rifampin, phenytoin, carbamazepine, and St. John’s Wort).
Click here to read the digital edition.
VA Pharmacy Benefit Management Service (PBM) continually issues or revises its guidances for hematology and oncology care providers on a number of cancer care medications. Below are excerpts from recently released Criteria for Use documents. The complete documents, including the inclusion criteria, dosage and administration guidance, monitoring information, and discontinuation criteria should be consulted and can be found at www.pbm.va.gov or vaww.cmopnational.va.gov/cmop/PBM/default.aspx.
ENZALUTAMIDE (XTANDI) Criteria for Use, January 2017
Exclusion Criteria: If the answer to ANY item below is met, then the patient should NOT receive enzalutamide.
- Brain metastases or active epidural disease
- Severe renal impairment (creatinine clearance < 30 mL/min)
- History of seizure (including febrile seizure, loss of consciousness, or transient ischemic attack within the previous 12 months, any condition predisposing to seizure: prior stroke, brain AV malformation, head trauma with loss of consciousness requiring hospitalization)
- ECOG Performance Status > 2
- Inability to swallow capsules
Issues for Consideration
- Enzalutamide is not indicated for use in women. Based on the mechanism of action, can cause fetal harm if used during pregnancy. Pregnancy Category X—use contraindicated during pregnancy. Exclude pregnancy before prescribing enzalutamide, discuss risks if pregnancy occurs, and provide contraceptive counseling.
- Use in patients taking concomitant medications that may lower the seizure threshold was not studied; caution patients about the risk of activities where the sudden loss of consciousness could cause serious harm if concomitant use cannot be avoided.
- Use in patients at risk for or with a strong history of falls: in the phase 3 clinical trial, falls or injuries from falls occurred in 4.6% of enzalutamide patients vs 1.3% of placebo patients.
- Avoid strong inhibitors of CYP2C8 (eg, gemfibrozil); if concomitant use of a strong CYP2C8 inhibitor cannot be avoided, reduce the dose of enzalutamide to 80 mg once daily according to the package insert.
- Co-administration with strong or moderate inducers of CYP3A4 (eg, carbamazepine, phenobarbital, phenytoin, rifampin, bosentan, efavirenz, modafinil, nafcillin, St. John’s Wort) or CYP2C8 (eg, rifampin) should be avoided if possible. If patient must be co-administered a strong CYP3A4 inducer, increase enzalutamide dose from 160 mg to 240 mg once daily.
- Drugs that are substrates of CYP3A4 (eg, alfentanil, cyclosporine, ergotamine, fentanyl, pimozide, quinidine, sirolimus, tacrolimus), CYP2C9 (eg, phenytoin, warfarin), or CYP2C19 (eg, S-mephenytoin) with a narrow therapeutic index should be avoided. If enzalutamide is co-administered with warfarin, additional INR testing should be conducted.
- Use in patients with hepatic impairment: Pharmacokinetics of enzalutamide and its metabolite were examined in volunteers with normal, Child-Pugh Class A, Child-Pugh Class B, and Child-Pugh Class C hepatic impairment. The composite AUC for enzalutamide and its metabolite after a single 160-mg dose was similar across all levels of hepatic impairment compared with normal volunteers.
- There have been postmarketing reports of posterior reversible encephalopathy syndrome (PRES) in patients receiving enzalutamide. PRES is a neurologic disorder presenting with rapidly evolving symptoms including seizure, headache, lethargy, confusion, blindness, and other visual/neurological disturbances with or without associated hypertension. Diagnosis of PRES requires brain imaging, preferably by MRI. Enzalutamide should be discontinued in patients developing PRES.
- Sequencing of enzalutamide and abiraterone has been evaluated in several small retrospective analyses; the majority of the analyses are in the post chemotherapy setting. From this limited observational data, it is unclear if there is a preferred sequencing of abiraterone and enzalutamide. There is some evidence for cross-resistance. There are ongoing investigations into mechanisms of resistance to enzalutamide and abiraterone.
DARATUMUMAB (DARZALEX) Criteria for Use, January 2017
Exclusion Criteria: If the answer to ANY item below is met, then the patient should NOT receive daratumumab.
- Patient is noncompliant with medication, follow-up, or laboratory appointments
- Patient unable or unwilling to be observed an extended period of time that may be necessary for first infusion (refer to Issues for Consideration)
- Hemoglobin < 8 g/dL; Must transfuse to hemoglobin above 8 gm/dL prior to therapy initiation
- Absolute neutrophil count (ANC) < 1,000/mm3
- Platelet count < 50,000/mm3 (< 30,000/mm3 if myeloma involvement in bone marrow > 50%)
- ECOG Performance Status > 2
- Total bilirubin > 3x the upper limit of the normal range (except for Gilbert syndrome: direct bilirubin 2x ULN) or ALT and AST > 3x ULN
- NYHA Class III or IV heart failure (refer to Issues for Consideration)
- Ongoing or active systemic infection, including active hepatitis B or C, or known HIV (refer to Issues for Consideration)
- Positive pregnancy test
Issues for Consideration
- Drug infusion time will be dependent upon patient tolerance and exposure to daratumumab. Median duration of the first infusion was ~ 7 hours in the SIRIUS trial, followed by infusion times of 4.2 and 3.4 hours, subsequently.
- Type and screen patients shortly prior to starting treatment. When the sample is provided to the blood bank, inform them that the patient will be receiving daratumumab.
- Those with NYHA Class III or IV heart failure, recent MI, conduction abnormalities, angina or arrhythmias uncontrolled by medications, were not eligible for clinical trials and may be at greater risk of cardiac complications.
- Patients with active hepatitis B, C, or HIV were excluded from clinical trials with daratumumab, therefore safety and efficacy data are unknown in these patient populations. The risk of infections was slightly higher in the daratumumab-treated arms of the comparative studies. Use of daratumumab should only be considered in those with well-controlled hepatitis B, hepatitis C, or HIV.
ELOTUZUMAB (EMPLICITI) Criteria for Use, January 2017
Exclusion Criteria: If the answer to ANY item below is met, then the patient should NOT receive elotuzumab.
- Patient is noncompliant with medication, follow-up, or laboratory appointments
- Patient is not a candidate for lenalidomide therapy (ie, is lenalidomide-refractory or possesses contraindications to therapy)
- Patient is not a candidate for high-dose dexamethasone therapy
- Hemoglobin < 8 g/dL; Must transfuse to hemoglobin above 8 gm/dL prior to therapy initiation
- Absolute neutrophil count (ANC) < 1,000/mm3
- Platelet count < 50,000/mm3 (< 30,000/mm3 if myeloma involvement in bone marrow > 50%)
- ECOG Performance Status > 2
- Total bilirubin > 2x the upper limit of the normal range (except for Gilbert syndrome: direct bilirubin > 2 mg/dL) or ALT and AST > 3x ULN
- NYHA Class III or IV heart failure (refer to Issues for Consideration)
- Ongoing or active systemic infection, including active hepatitis B or hepatitis C, or known HIV (refer to Issues for Consideration)
- Positive pregnancy test
- Patient intends to breastfeed during therapy
Issues for Consideration
- Those with NYHA Class III or IV heart failure, recent MI, conduction abnormalities, angina or arrhythmias uncontrolled by medications, were not eligible for clinical trials and may be at greater risk of cardiac complications.
- Patients with active hepatitis B, hepatitis C, or HIV were excluded from clinical trials with elotuzumab, therefore safety and efficacy data are unknown in these patient populations. The risk of infections (OI, fungal, viral) was greater in the elotuzumab arm vs control arm of the comparative clinical trial. Use of elotuzumab should only be considered in those with well-controlled hepatitis B, hepatitis C, or HIV.
- Disappointing response rates as monotherapy in the relapsed/refractory setting suggest that elotuzumab should be given in combination with lenalidomide and dexamethasone.
- Impact of elotuzumab/lenalidomide/dexamethasone on overall survival is not known as these data were not mature at the time ELOQUENT-2 was published.
CARFILZOMIB (KYPROLIS) Criteria for Use, December 2016
Exclusion Criteria: If the answer to ANY item below is met, then the patient should NOT receive carfilzomib.
- Care for the oncologic disease being treated not provided by a VA or VA purchased care (eg, Choice Program, Fee Basis) oncology provider
- Patient is noncompliant with medication, follow-up, or laboratory appointments
- Hemoglobin < 8 g/dL; Must transfuse to hemoglobin above 8 g/dL prior to therapy initiation
- Absolute neutrophil count (ANC) < 1,000/mm3
- Platelet count < 50,000/mm3 (< 30,000/mm3 if myeloma involvement in bone marrow > 50%)
- ECOG Performance Status > 2
- Total bilirubin > 1.5 x the upper limit of the normal range or ALT and AST > 3 x ULN
- NYHA Class III and IV heart failure (refer to Issues for Consideration; those at risk for cardiac failure and ischemia were also excluded from clinical trials)
- LVEF < 40%
- Uncontrolled hypertension
- Grade 3 or 4 peripheral neuropathy
- Ongoing or active systemic infection, including active hepatitis B or C, or known HIV
Issues for Consideration
- A significant percentage of patients on carfilzomib develop dyspnea. This drug should be used with caution in patients with underlying lung disease. Close monitoring for worsening of dyspnea is advised.
- Risk of cardiac failure increases in those aged > 75 years; those with NYHA Class III and IV heart failure, recent MI, conduction abnormalities, angina or arrhythmias uncontrolled by medications, were not eligible for clinical trials and may be at greater risk of cardiac complications. Refer to Prescribing Information for management recommendations.
- Those aged > 75 years experienced greater toxicity than their younger counterparts.
- Patients on dialysis: administer carfilzomib after the dialysis procedure.
- Phase III evidence in heavily pretreated relapsed/refractory patients (median 5 prior regimens) of carfilzomib vs low-dose steroids ± cyclophosphamide indicates that the median overall survival is not significantly different between these treatment arms.
IXAZOMIB (NILARO) Criteria for Use, December 2016
Exclusion Criteria: If the answer to ANY item below is met, then the patient should NOT receive ixazomib
- Care for the oncologic disease being treated not provided by a VA or VA purchased care (eg, Choice Program, Fee Basis) oncology provider
- Patient is not a candidate for lenalidomide or dexamethasone therapy
- Patient is refractory to lenalidomide or proteasomeinhibitor therapy (defined as disease progression while on treatment or within 60 days of last dose)
- Absolute neutrophil count (ANC) < 1,000/mm3
- Platelet count < 75,000/mm3
- ECOG Performance Status > 2
- Patient with CNS involvement
- Patient receiving concurrent therapy with a strong CYP3A inducer (ie, rifampin, phenytoin, carbamazepine) that cannot be discontinued
- Uncontrolled cardiovascular conditions, including uncontrolled hypertension, uncontrolled cardiac arrhythmias, symptomatic congestive heart failure, unstable angina or myocardial infarction within 6 months prior to start
- Ongoing or active systemic infection, including active hepatitis B, hepatitis C, or known HIV
Issues for Consideration
- Indirect comparisons of phase 3 data (KRd vs Rd and IRd vs Rd) show that in similar populations of pretreated relapsed, refractory myeloma patients, those receiving KRd experienced longer PFS (26.3 vs 21 months), greater CR (32% vs 12%), greater ORR (87% vs 78%) and longer duration of response (28.6 vs 20.5 months). Therefore, providers may want to consider using carfilzomib in those meeting its criteria for use.
- Ixazomib is cytotoxic. Capsules should not be opened or crushed. Waste should be considered hazardous.
- Avoid concomitant use of strong CYP3A inducers (rifampin, phenytoin, carbamazepine, and St. John’s Wort).
Click here to read the digital edition.
VA Pharmacy Benefit Management Service (PBM) continually issues or revises its guidances for hematology and oncology care providers on a number of cancer care medications. Below are excerpts from recently released Criteria for Use documents. The complete documents, including the inclusion criteria, dosage and administration guidance, monitoring information, and discontinuation criteria should be consulted and can be found at www.pbm.va.gov or vaww.cmopnational.va.gov/cmop/PBM/default.aspx.
ENZALUTAMIDE (XTANDI) Criteria for Use, January 2017
Exclusion Criteria: If the answer to ANY item below is met, then the patient should NOT receive enzalutamide.
- Brain metastases or active epidural disease
- Severe renal impairment (creatinine clearance < 30 mL/min)
- History of seizure (including febrile seizure, loss of consciousness, or transient ischemic attack within the previous 12 months, any condition predisposing to seizure: prior stroke, brain AV malformation, head trauma with loss of consciousness requiring hospitalization)
- ECOG Performance Status > 2
- Inability to swallow capsules
Issues for Consideration
- Enzalutamide is not indicated for use in women. Based on the mechanism of action, can cause fetal harm if used during pregnancy. Pregnancy Category X—use contraindicated during pregnancy. Exclude pregnancy before prescribing enzalutamide, discuss risks if pregnancy occurs, and provide contraceptive counseling.
- Use in patients taking concomitant medications that may lower the seizure threshold was not studied; caution patients about the risk of activities where the sudden loss of consciousness could cause serious harm if concomitant use cannot be avoided.
- Use in patients at risk for or with a strong history of falls: in the phase 3 clinical trial, falls or injuries from falls occurred in 4.6% of enzalutamide patients vs 1.3% of placebo patients.
- Avoid strong inhibitors of CYP2C8 (eg, gemfibrozil); if concomitant use of a strong CYP2C8 inhibitor cannot be avoided, reduce the dose of enzalutamide to 80 mg once daily according to the package insert.
- Co-administration with strong or moderate inducers of CYP3A4 (eg, carbamazepine, phenobarbital, phenytoin, rifampin, bosentan, efavirenz, modafinil, nafcillin, St. John’s Wort) or CYP2C8 (eg, rifampin) should be avoided if possible. If patient must be co-administered a strong CYP3A4 inducer, increase enzalutamide dose from 160 mg to 240 mg once daily.
- Drugs that are substrates of CYP3A4 (eg, alfentanil, cyclosporine, ergotamine, fentanyl, pimozide, quinidine, sirolimus, tacrolimus), CYP2C9 (eg, phenytoin, warfarin), or CYP2C19 (eg, S-mephenytoin) with a narrow therapeutic index should be avoided. If enzalutamide is co-administered with warfarin, additional INR testing should be conducted.
- Use in patients with hepatic impairment: Pharmacokinetics of enzalutamide and its metabolite were examined in volunteers with normal, Child-Pugh Class A, Child-Pugh Class B, and Child-Pugh Class C hepatic impairment. The composite AUC for enzalutamide and its metabolite after a single 160-mg dose was similar across all levels of hepatic impairment compared with normal volunteers.
- There have been postmarketing reports of posterior reversible encephalopathy syndrome (PRES) in patients receiving enzalutamide. PRES is a neurologic disorder presenting with rapidly evolving symptoms including seizure, headache, lethargy, confusion, blindness, and other visual/neurological disturbances with or without associated hypertension. Diagnosis of PRES requires brain imaging, preferably by MRI. Enzalutamide should be discontinued in patients developing PRES.
- Sequencing of enzalutamide and abiraterone has been evaluated in several small retrospective analyses; the majority of the analyses are in the post chemotherapy setting. From this limited observational data, it is unclear if there is a preferred sequencing of abiraterone and enzalutamide. There is some evidence for cross-resistance. There are ongoing investigations into mechanisms of resistance to enzalutamide and abiraterone.
DARATUMUMAB (DARZALEX) Criteria for Use, January 2017
Exclusion Criteria: If the answer to ANY item below is met, then the patient should NOT receive daratumumab.
- Patient is noncompliant with medication, follow-up, or laboratory appointments
- Patient unable or unwilling to be observed an extended period of time that may be necessary for first infusion (refer to Issues for Consideration)
- Hemoglobin < 8 g/dL; Must transfuse to hemoglobin above 8 gm/dL prior to therapy initiation
- Absolute neutrophil count (ANC) < 1,000/mm3
- Platelet count < 50,000/mm3 (< 30,000/mm3 if myeloma involvement in bone marrow > 50%)
- ECOG Performance Status > 2
- Total bilirubin > 3x the upper limit of the normal range (except for Gilbert syndrome: direct bilirubin 2x ULN) or ALT and AST > 3x ULN
- NYHA Class III or IV heart failure (refer to Issues for Consideration)
- Ongoing or active systemic infection, including active hepatitis B or C, or known HIV (refer to Issues for Consideration)
- Positive pregnancy test
Issues for Consideration
- Drug infusion time will be dependent upon patient tolerance and exposure to daratumumab. Median duration of the first infusion was ~ 7 hours in the SIRIUS trial, followed by infusion times of 4.2 and 3.4 hours, subsequently.
- Type and screen patients shortly prior to starting treatment. When the sample is provided to the blood bank, inform them that the patient will be receiving daratumumab.
- Those with NYHA Class III or IV heart failure, recent MI, conduction abnormalities, angina or arrhythmias uncontrolled by medications, were not eligible for clinical trials and may be at greater risk of cardiac complications.
- Patients with active hepatitis B, C, or HIV were excluded from clinical trials with daratumumab, therefore safety and efficacy data are unknown in these patient populations. The risk of infections was slightly higher in the daratumumab-treated arms of the comparative studies. Use of daratumumab should only be considered in those with well-controlled hepatitis B, hepatitis C, or HIV.
ELOTUZUMAB (EMPLICITI) Criteria for Use, January 2017
Exclusion Criteria: If the answer to ANY item below is met, then the patient should NOT receive elotuzumab.
- Patient is noncompliant with medication, follow-up, or laboratory appointments
- Patient is not a candidate for lenalidomide therapy (ie, is lenalidomide-refractory or possesses contraindications to therapy)
- Patient is not a candidate for high-dose dexamethasone therapy
- Hemoglobin < 8 g/dL; Must transfuse to hemoglobin above 8 gm/dL prior to therapy initiation
- Absolute neutrophil count (ANC) < 1,000/mm3
- Platelet count < 50,000/mm3 (< 30,000/mm3 if myeloma involvement in bone marrow > 50%)
- ECOG Performance Status > 2
- Total bilirubin > 2x the upper limit of the normal range (except for Gilbert syndrome: direct bilirubin > 2 mg/dL) or ALT and AST > 3x ULN
- NYHA Class III or IV heart failure (refer to Issues for Consideration)
- Ongoing or active systemic infection, including active hepatitis B or hepatitis C, or known HIV (refer to Issues for Consideration)
- Positive pregnancy test
- Patient intends to breastfeed during therapy
Issues for Consideration
- Those with NYHA Class III or IV heart failure, recent MI, conduction abnormalities, angina or arrhythmias uncontrolled by medications, were not eligible for clinical trials and may be at greater risk of cardiac complications.
- Patients with active hepatitis B, hepatitis C, or HIV were excluded from clinical trials with elotuzumab, therefore safety and efficacy data are unknown in these patient populations. The risk of infections (OI, fungal, viral) was greater in the elotuzumab arm vs control arm of the comparative clinical trial. Use of elotuzumab should only be considered in those with well-controlled hepatitis B, hepatitis C, or HIV.
- Disappointing response rates as monotherapy in the relapsed/refractory setting suggest that elotuzumab should be given in combination with lenalidomide and dexamethasone.
- Impact of elotuzumab/lenalidomide/dexamethasone on overall survival is not known as these data were not mature at the time ELOQUENT-2 was published.
CARFILZOMIB (KYPROLIS) Criteria for Use, December 2016
Exclusion Criteria: If the answer to ANY item below is met, then the patient should NOT receive carfilzomib.
- Care for the oncologic disease being treated not provided by a VA or VA purchased care (eg, Choice Program, Fee Basis) oncology provider
- Patient is noncompliant with medication, follow-up, or laboratory appointments
- Hemoglobin < 8 g/dL; Must transfuse to hemoglobin above 8 g/dL prior to therapy initiation
- Absolute neutrophil count (ANC) < 1,000/mm3
- Platelet count < 50,000/mm3 (< 30,000/mm3 if myeloma involvement in bone marrow > 50%)
- ECOG Performance Status > 2
- Total bilirubin > 1.5 x the upper limit of the normal range or ALT and AST > 3 x ULN
- NYHA Class III and IV heart failure (refer to Issues for Consideration; those at risk for cardiac failure and ischemia were also excluded from clinical trials)
- LVEF < 40%
- Uncontrolled hypertension
- Grade 3 or 4 peripheral neuropathy
- Ongoing or active systemic infection, including active hepatitis B or C, or known HIV
Issues for Consideration
- A significant percentage of patients on carfilzomib develop dyspnea. This drug should be used with caution in patients with underlying lung disease. Close monitoring for worsening of dyspnea is advised.
- Risk of cardiac failure increases in those aged > 75 years; those with NYHA Class III and IV heart failure, recent MI, conduction abnormalities, angina or arrhythmias uncontrolled by medications, were not eligible for clinical trials and may be at greater risk of cardiac complications. Refer to Prescribing Information for management recommendations.
- Those aged > 75 years experienced greater toxicity than their younger counterparts.
- Patients on dialysis: administer carfilzomib after the dialysis procedure.
- Phase III evidence in heavily pretreated relapsed/refractory patients (median 5 prior regimens) of carfilzomib vs low-dose steroids ± cyclophosphamide indicates that the median overall survival is not significantly different between these treatment arms.
IXAZOMIB (NILARO) Criteria for Use, December 2016
Exclusion Criteria: If the answer to ANY item below is met, then the patient should NOT receive ixazomib
- Care for the oncologic disease being treated not provided by a VA or VA purchased care (eg, Choice Program, Fee Basis) oncology provider
- Patient is not a candidate for lenalidomide or dexamethasone therapy
- Patient is refractory to lenalidomide or proteasomeinhibitor therapy (defined as disease progression while on treatment or within 60 days of last dose)
- Absolute neutrophil count (ANC) < 1,000/mm3
- Platelet count < 75,000/mm3
- ECOG Performance Status > 2
- Patient with CNS involvement
- Patient receiving concurrent therapy with a strong CYP3A inducer (ie, rifampin, phenytoin, carbamazepine) that cannot be discontinued
- Uncontrolled cardiovascular conditions, including uncontrolled hypertension, uncontrolled cardiac arrhythmias, symptomatic congestive heart failure, unstable angina or myocardial infarction within 6 months prior to start
- Ongoing or active systemic infection, including active hepatitis B, hepatitis C, or known HIV
Issues for Consideration
- Indirect comparisons of phase 3 data (KRd vs Rd and IRd vs Rd) show that in similar populations of pretreated relapsed, refractory myeloma patients, those receiving KRd experienced longer PFS (26.3 vs 21 months), greater CR (32% vs 12%), greater ORR (87% vs 78%) and longer duration of response (28.6 vs 20.5 months). Therefore, providers may want to consider using carfilzomib in those meeting its criteria for use.
- Ixazomib is cytotoxic. Capsules should not be opened or crushed. Waste should be considered hazardous.
- Avoid concomitant use of strong CYP3A inducers (rifampin, phenytoin, carbamazepine, and St. John’s Wort).
Click here to read the digital edition.
Reducing the Expenditures and Workload Associated With VA Partial-Fill Prescription Processing
According to the US Department of Veterans Affairs (VA) Pharmacy Benefits Management Service, about 80% of all outpatient prescriptions filled by the VA are sent to veterans by mail order, using the Centralized Mail Order Pharmacy (CMOP) network of highly automated pharmacies around the country.1 During fiscal year (FY) 2016, the 7 VA CMOP facilities throughout the US processed 119.7 million outpatient prescriptions. Each day, these CMOPs process nearly 470,000 prescriptions, an evidence of the efficiency provided through this mail-order service.1 The use of CMOP results in lower processing costs and increased convenience for veterans compared with filling prescriptions at pharmacies at individual VA facilities. Notably, VA CMOP has been rated “among the best” mail-order pharmacies in customer satisfaction according to the 2017 J.D. Power US Pharmacy Study.2
Background
Within the Fayetteville VA Medical Center (FVAMC) system in North Carolina, on-site patients receive a new prescription where an on-site pharmacy is available (at health care centers [HCC] and the medical center). For veterans seen at community-based outpatient clinics (CBOCs), emergent new prescriptions are filled through vouchers at contract community pharmacies, and nonemergent new prescriptions are filled by the CMOP (Figure 1).
The appropriate use of the VA CMOP for refills is intended to allow on-site pharmacy staff to focus on providing customer service for veterans requesting medication counseling from a clinical pharmacist, as well as those with new, changing, or urgent prescription needs. Filling prescriptions through the CMOP also can help control the VA facility pharmacy budget.
Despite the established mail-order process, FVAMC staff noted a high volume of medication refill and partial-fill prescriptions being requested at the on-site pharmacy. When a veteran presented to a pharmacy requesting a medication refill, pharmacy staff members ordered a refill to be filled by the CMOP and provided a limited quantity, otherwise known as a partial fill, to serve as an emergency supply to supplement the veteran until the full quantity of the prescription arrived by mail. Partial fills also were completed for new prescriptions that veterans requested to pick up at the on-site pharmacy. For new 90-day supply prescriptions, pharmacy staff often filled a 30-day supply in addition to submitting the entire 90-day prescription through the CMOP, which led to an unnecessary increase in material expenditures and workload.Preliminary data noted the most frequently used partial-fill days’ supply to be 10 days. Due to the lack of partial-fill criteria, prescriptions of all classes, quantities, and days’ supplies were provided as partial fills. Prior to the implementation of this quality improvement (QI) project, there was no standard approach for how to handle these requests.
Partial fills do not provide copay reimbursement to the facility filling the prescription. In an effort to steward the funds provided to the FVAMC pharmacy department wisely, an evaluation was performed with reference to partial fills. During FY 2016, about $350,000 was spent on medications, materials, and workload associated with the partial filling of FVAMC prescriptions. With respect to unique individuals who were provided care over the course of FY 2015 and 2016, the number of unique patients served by local comparator hospitals increased by only 2%, while the number of unique patients served by FVAMC increased by 12%. This substantial growth in the number of veterans served places further emphasis on the necessity of stewarding the allotted pharmacy budget. Moreover, the excessive number of medication refill and partial-fill prescriptions filled at on-site VA pharmacies can contribute to increased wait times for veterans with urgent prescription needs.
In November 2016, FVAMC implemented an updated partial-fill guidance. Partial-fill process and refill education was provided for VA staff and veterans in an effort to allow all parties involved to use pharmacy services efficiently. This analysis reviewed the reduction in partial-fill expenditures with a secondary focus on workload expenditures following the execution of this education.
Methods
This project was deemed to be a QI project and did not require institutional review board approval. Implementation for this QI project began in November 2016. Baseline raw drug cost, number, and class of prescriptions partialed were retrospectively collected for a 90-day period prior to implementation using all available data. Postintervention data were collected for 90 days following the implementation phase to compare partial-fill expenditures and workload expenditures with baseline data.
Calculations
Materials included in the partial-fill expenditure calculation were prescription vials, prescription vial caps, and prescription labeling. Material cost per partial fill was determined by using the facility’s wholesaler acquisition unit costs to estimate a summed cost for an individual prescription vial and prescription vial cap. The estimated acquisition price of 7 prescription-labeling pages was used in the material calculation, as this was the average number of pages used when performing test partial fills.
The following equation was used to calculate total partial-fill expenditures for any specified time frame:
Total partial-fill expenditure = total raw drug cost + (material cost × number of partial fills)
In addition, the average personnel cost per partial-fill prescription was determined. Average workload expenditure per partial fill was calculated by filling a subset of 10 test prescriptions and multiplying the average time spent by an average of the general station (GS) rate for pharmacists and technicians. The average hourly rate of a GS-12 pharmacist was calculated based on an average of the 10 available pay grades within the GS-12 ranking. The average hourly rate of a GS-6 pharmacy technician was calculated based on an average of the 10 available pay grades within the GS-6 ranking.3 The average workload expenditures were calculated using the following equations:
Average pharmacist workload expenditure per partial fill = time (in hours) × average hourly rate.
Average technician workload expenditure per partial fill = time (in hours) × average hourly rate.
Partial-Fill Guidance
Updated partial-fill guidance was drafted designating acceptable prescriptions to be limited to those responsible for preventing hospitalization and treatment of acute illness. This guidance provided generalized examples of medication classes that could be acceptable for partial filling, though it was not intended to be an all-inclusive list. The guidance also noted examples of classes or groups that should not be partial filled for nonemergent reasons (vitamins, nonprescription items, antilipemics), as well as controlled substances. The refill-process education was reiterated throughout the entirety of the guidance. Specifically, if a pharmacy staff member was to perform a partial fill, a review explaining the appropriate refill process to the veteran also must be provided. If the medication was determined to be of emergent need and not yet transmitted for filling via the CMOP, the directive recommended to fill the entire quantity locally as a onetime fill.
If a onetime on-site fill was determined infeasible, partial-fill quantities were recommended to be limited to only a 7-day supply, and the full quantity filled through the CMOP. Anticipated mail wait time for CMOP prescription delivery was estimated to be less than 7 days based on experience, local pending queues, and guidance from the regional CMOP; however, time could vary among VA and CMOP facilities. Original prescriptions were to be filled for the entire quantity for the first fill at the on-site pharmacy if requested by the veteran. If the pharmacy had an insufficient quantity for an entire initial supply, it could then be partial filled for a 7-day supply and then filled through the CMOP.
The final portion of the partial-fill guidance pertained to the use of partial-fill justification codes. Prior to the execution of the partial-fill guidance, free text was entered into the comments field when processing a partial-fill prescription, as the prescription-processing system used requires a comment to proceed with the partial fill. The use of these codes served to streamline data collection in the postintervention phase and helped identify areas for further education following the close of this project.
Education
Education to the pharmacy staff was disseminated by various modalities, including in-person sessions and written and electronic correspondences. This written guidance was distributed to pharmacy staff by e-mail, the pharmacy newsletter (Rx-tra), signage posted throughout the outpatient pharmacies, and on the facility’s pharmacy Microsoft SharePoint (Redmond, WA) site. In-services provided during pharmacy staff meetings detailed information on the updated partial-fill guidance. A FVAMC Talent Management System (TMS) training module was developed and assigned to all pharmacy service staff to reiterate key points regarding this QI initiative (eAppendix).
Nonpharmacy staff were educated through staff and in-service meetings and e-mail correspondences. These in-services emphasized how nurses, medical support assistants, and health care providers (HCPs) could assist veterans by knowing the correct refill process, ensuring sufficient refills remained until the next appointment, and providing continual refill-process education.
Following implementation, all veterans receiving prescriptions through the on-site pharmacies in the FVAMC were provided a copy of the refill-process handout with each trip to the pharmacy. Nonpharmacy staff and HCPs also were provided this handout to distribute to patients. The intent of this handout was to clearly detail the various ways in which refills could be ordered and the time frame in which they should be ordered. The pharmacy became involved in new patient orientation classes for all veterans new to FVAMC. Digital signage and messaging was created and circulated throughout several of the FVAMC facilities.
Results
The results of calculations for material cost, personnel time spent, hourly employee rates, and average workload expenditure per partial fill are summarized in Table 1.
Following the implementation of partial-fill and refill-process education, there was a 54.3% decrease in the total number of partial fills from 5,596 in the 90 days prior to implementation, to 2,555 partials completed over the 90 days postimplementation. Regarding the primary objective, total partial-fill expenditures decreased from $52,015.44 to $44,063.01 (-15.3%). When dissecting the individual components of partial-fill expenditures, material expenditures decreased from $1,454.96 to $664.28 (-54.3%), and raw drug cost expenditures decreased from $50,596.48 to $43,398.73 (-14.3%). Workload expenditures also decreased from $27,140.60 to $12,391.75 (-54.3%).
Several points of descriptive information also were collected. The average days’ supply trended down from a mode of 10 days to 7 days. This reduction in days’ supply likely was seen because staff became more aware of the customary amount required to bridge the veteran until the CMOP supply arrived by mail. Postintervention data showed a 70% utilization of partial-fill reasoning codes. The reasons for partial filling of prescriptions are summarized in Table 2.
Discussion
Following the implementation of the updated partial-fill guidance and provision of education to the FVAMC veterans and staff, a noteworthy cost savings was observed with respect to both material and workload expenditures. This large reduction in expenditure likely was not related to a reduction in the total prescription volume, as the number of total prescriptions filled by the CMOP and at FVAMC were similar in both the preliminary and postintervention periods. When the results of this 3-month QI project were extrapolated, the annual projected cost avoidance was $91,949.12.
Of note, there was no established process for adjudicating appeals to the partial-fill guidance. Any extenuating circumstances that fell outside the guidance were addressed by the outpatient pharmacy supervisor. There was no formal documentation for these disputed cases. Since there was no prespecified supervisory override code, the most appropriate partial-fill code was entered into the comments field for these scenarios. As such, there is no way to distinguish precisely how many of these partial fills were escalated to a supervisory level for a decision.
The positive fiscal impact noted from the implementation of this project should not be viewed as the only utility for such guidance. Though not directly measured within the confines of this project, a reduction in pharmacy staff time spent on partial-fill prescriptions will likely result in shorter pharmacy wait times, line lengths, and streamlining of pharmacy workflow. When the pharmacy staff is free to work on pressing issues rather than on continually educating veterans on the partial-fill or refill process, many will benefit. Veteran satisfaction was not directly measured during this project but could be an interesting topic to review as a future study.
Each VA facility is unique, with its own challenges for implementation of a project such as this. Nevertheless, the incorporation of a formal guidance and education process, perhaps adapted to the indi vidual facility’s needs may be considered for overall pharmacy operations QI.
Limitations
During the preliminary data collection period, FVAMC and its catchment area were impacted by a natural disaster, Hurricane Matthew. Based on a review of the text entered into the comments field for all partial fills, about 4% of the partial fills completed in the preliminary phase can be attributed to the hurricane. The effects of this hurricane may have potentially increased the number of partial fills completed in the preliminary phase compared with that in the postintervention phase, due to the number of veterans who were temporarily or permanently displaced from their homes. This increase in partial fills and associated expenses preintervention likely caused a slightly higher cost savings to be reflected in the postimplementation phase than what would have traditionally been observed without extenuating factors.
Several other limitations must be considered for this QI project. The implementation phase, during which all education and training was completed, was only 1 month. A longer implementation period and more opportunities to educate veterans and staff might have created a greater impact on the results. Additionally, because there were no data collected on New Patient Orientation attendance for this project, it is unclear exactly how many veterans received refill-process education through this outlet.
Though all staff members were trained on the appropriate process, it was discovered during interim analysis that several pharmacists were not following the partial-fill guidance, potentially negatively impacting the results. It is likely that staff would have benefited from continual reeducation of the process throughout the entirety of the project, as the restriction of partial filling was a novel concept to many. In addition to continual reeducation of current employees, any new hires would likely need this information as part of initial training.
Cost variance in the type of partial fills completed between the preliminary and postintervention phases also may have negatively impacted the results. The postintervention phase contained 2 high-cost classes of drugs (antivirals and immunoglobulins) that received multiple partial fills but were not partialed in the preliminary phase, which increased the raw drug cost in the postintervention phase.
Conclusion
The implementation of partial-fill and process education to FVAMC staff and veterans proved beneficial in reducing the expenditures and workload associated with partial-fill prescription processing. The continued use of the updated partial-fill guidance will provide a standardized approach for pharmacy staff when completing partial-fill prescriptions.
Facilities may consider annual reeducation on their guidance through a local TMS module, as well as occasional process reminders during staff meetings to improve staff adherence to the guidance. Moreover, the sustained incorporation of improved refill process education to new patients and with every prescription pickup could help guide the FVAMC veteran population to use pharmacy services more effectively. The adoption of such procedures may be useful for VA facilities’ health care system looking to maximize the use of funding provided for prescription services as well as improve veterans’ understanding of how to appropriately order prescription refills.
1. US Department of Veterans Affairs. Pharmacy benefits management services. https://www.pbm.va.gov/PBM/CMOP/VA_Mail_Order_Pharmacy.asp. Updated July 14, 2017. Accessed on February 26, 2018.
2. J.D. Power. Decline in pharmacy customer satisfaction driven by prescription drug costs, J. D. Power finds. [press release]. http://www.jdpower.com/press-releases/jd-power-2017-us-pharmacy-study. Published September 5, 2017. Accessed February 26, 2018.
3. US Office of Personnel Management. Pay and leave. https://www.opm.gov/policy-data-oversight/pay-leave/salaries-wages/. Accessed February 26, 2018.
4. Aragon BR, Pierce RA, Jones WN. VA CMOPs: producing a pattern of quality and efficiency in government. J Am Pharm Assoc (2003). 2012;52(6):810-815.
According to the US Department of Veterans Affairs (VA) Pharmacy Benefits Management Service, about 80% of all outpatient prescriptions filled by the VA are sent to veterans by mail order, using the Centralized Mail Order Pharmacy (CMOP) network of highly automated pharmacies around the country.1 During fiscal year (FY) 2016, the 7 VA CMOP facilities throughout the US processed 119.7 million outpatient prescriptions. Each day, these CMOPs process nearly 470,000 prescriptions, an evidence of the efficiency provided through this mail-order service.1 The use of CMOP results in lower processing costs and increased convenience for veterans compared with filling prescriptions at pharmacies at individual VA facilities. Notably, VA CMOP has been rated “among the best” mail-order pharmacies in customer satisfaction according to the 2017 J.D. Power US Pharmacy Study.2
Background
Within the Fayetteville VA Medical Center (FVAMC) system in North Carolina, on-site patients receive a new prescription where an on-site pharmacy is available (at health care centers [HCC] and the medical center). For veterans seen at community-based outpatient clinics (CBOCs), emergent new prescriptions are filled through vouchers at contract community pharmacies, and nonemergent new prescriptions are filled by the CMOP (Figure 1).
The appropriate use of the VA CMOP for refills is intended to allow on-site pharmacy staff to focus on providing customer service for veterans requesting medication counseling from a clinical pharmacist, as well as those with new, changing, or urgent prescription needs. Filling prescriptions through the CMOP also can help control the VA facility pharmacy budget.
Despite the established mail-order process, FVAMC staff noted a high volume of medication refill and partial-fill prescriptions being requested at the on-site pharmacy. When a veteran presented to a pharmacy requesting a medication refill, pharmacy staff members ordered a refill to be filled by the CMOP and provided a limited quantity, otherwise known as a partial fill, to serve as an emergency supply to supplement the veteran until the full quantity of the prescription arrived by mail. Partial fills also were completed for new prescriptions that veterans requested to pick up at the on-site pharmacy. For new 90-day supply prescriptions, pharmacy staff often filled a 30-day supply in addition to submitting the entire 90-day prescription through the CMOP, which led to an unnecessary increase in material expenditures and workload.Preliminary data noted the most frequently used partial-fill days’ supply to be 10 days. Due to the lack of partial-fill criteria, prescriptions of all classes, quantities, and days’ supplies were provided as partial fills. Prior to the implementation of this quality improvement (QI) project, there was no standard approach for how to handle these requests.
Partial fills do not provide copay reimbursement to the facility filling the prescription. In an effort to steward the funds provided to the FVAMC pharmacy department wisely, an evaluation was performed with reference to partial fills. During FY 2016, about $350,000 was spent on medications, materials, and workload associated with the partial filling of FVAMC prescriptions. With respect to unique individuals who were provided care over the course of FY 2015 and 2016, the number of unique patients served by local comparator hospitals increased by only 2%, while the number of unique patients served by FVAMC increased by 12%. This substantial growth in the number of veterans served places further emphasis on the necessity of stewarding the allotted pharmacy budget. Moreover, the excessive number of medication refill and partial-fill prescriptions filled at on-site VA pharmacies can contribute to increased wait times for veterans with urgent prescription needs.
In November 2016, FVAMC implemented an updated partial-fill guidance. Partial-fill process and refill education was provided for VA staff and veterans in an effort to allow all parties involved to use pharmacy services efficiently. This analysis reviewed the reduction in partial-fill expenditures with a secondary focus on workload expenditures following the execution of this education.
Methods
This project was deemed to be a QI project and did not require institutional review board approval. Implementation for this QI project began in November 2016. Baseline raw drug cost, number, and class of prescriptions partialed were retrospectively collected for a 90-day period prior to implementation using all available data. Postintervention data were collected for 90 days following the implementation phase to compare partial-fill expenditures and workload expenditures with baseline data.
Calculations
Materials included in the partial-fill expenditure calculation were prescription vials, prescription vial caps, and prescription labeling. Material cost per partial fill was determined by using the facility’s wholesaler acquisition unit costs to estimate a summed cost for an individual prescription vial and prescription vial cap. The estimated acquisition price of 7 prescription-labeling pages was used in the material calculation, as this was the average number of pages used when performing test partial fills.
The following equation was used to calculate total partial-fill expenditures for any specified time frame:
Total partial-fill expenditure = total raw drug cost + (material cost × number of partial fills)
In addition, the average personnel cost per partial-fill prescription was determined. Average workload expenditure per partial fill was calculated by filling a subset of 10 test prescriptions and multiplying the average time spent by an average of the general station (GS) rate for pharmacists and technicians. The average hourly rate of a GS-12 pharmacist was calculated based on an average of the 10 available pay grades within the GS-12 ranking. The average hourly rate of a GS-6 pharmacy technician was calculated based on an average of the 10 available pay grades within the GS-6 ranking.3 The average workload expenditures were calculated using the following equations:
Average pharmacist workload expenditure per partial fill = time (in hours) × average hourly rate.
Average technician workload expenditure per partial fill = time (in hours) × average hourly rate.
Partial-Fill Guidance
Updated partial-fill guidance was drafted designating acceptable prescriptions to be limited to those responsible for preventing hospitalization and treatment of acute illness. This guidance provided generalized examples of medication classes that could be acceptable for partial filling, though it was not intended to be an all-inclusive list. The guidance also noted examples of classes or groups that should not be partial filled for nonemergent reasons (vitamins, nonprescription items, antilipemics), as well as controlled substances. The refill-process education was reiterated throughout the entirety of the guidance. Specifically, if a pharmacy staff member was to perform a partial fill, a review explaining the appropriate refill process to the veteran also must be provided. If the medication was determined to be of emergent need and not yet transmitted for filling via the CMOP, the directive recommended to fill the entire quantity locally as a onetime fill.
If a onetime on-site fill was determined infeasible, partial-fill quantities were recommended to be limited to only a 7-day supply, and the full quantity filled through the CMOP. Anticipated mail wait time for CMOP prescription delivery was estimated to be less than 7 days based on experience, local pending queues, and guidance from the regional CMOP; however, time could vary among VA and CMOP facilities. Original prescriptions were to be filled for the entire quantity for the first fill at the on-site pharmacy if requested by the veteran. If the pharmacy had an insufficient quantity for an entire initial supply, it could then be partial filled for a 7-day supply and then filled through the CMOP.
The final portion of the partial-fill guidance pertained to the use of partial-fill justification codes. Prior to the execution of the partial-fill guidance, free text was entered into the comments field when processing a partial-fill prescription, as the prescription-processing system used requires a comment to proceed with the partial fill. The use of these codes served to streamline data collection in the postintervention phase and helped identify areas for further education following the close of this project.
Education
Education to the pharmacy staff was disseminated by various modalities, including in-person sessions and written and electronic correspondences. This written guidance was distributed to pharmacy staff by e-mail, the pharmacy newsletter (Rx-tra), signage posted throughout the outpatient pharmacies, and on the facility’s pharmacy Microsoft SharePoint (Redmond, WA) site. In-services provided during pharmacy staff meetings detailed information on the updated partial-fill guidance. A FVAMC Talent Management System (TMS) training module was developed and assigned to all pharmacy service staff to reiterate key points regarding this QI initiative (eAppendix).
Nonpharmacy staff were educated through staff and in-service meetings and e-mail correspondences. These in-services emphasized how nurses, medical support assistants, and health care providers (HCPs) could assist veterans by knowing the correct refill process, ensuring sufficient refills remained until the next appointment, and providing continual refill-process education.
Following implementation, all veterans receiving prescriptions through the on-site pharmacies in the FVAMC were provided a copy of the refill-process handout with each trip to the pharmacy. Nonpharmacy staff and HCPs also were provided this handout to distribute to patients. The intent of this handout was to clearly detail the various ways in which refills could be ordered and the time frame in which they should be ordered. The pharmacy became involved in new patient orientation classes for all veterans new to FVAMC. Digital signage and messaging was created and circulated throughout several of the FVAMC facilities.
Results
The results of calculations for material cost, personnel time spent, hourly employee rates, and average workload expenditure per partial fill are summarized in Table 1.
Following the implementation of partial-fill and refill-process education, there was a 54.3% decrease in the total number of partial fills from 5,596 in the 90 days prior to implementation, to 2,555 partials completed over the 90 days postimplementation. Regarding the primary objective, total partial-fill expenditures decreased from $52,015.44 to $44,063.01 (-15.3%). When dissecting the individual components of partial-fill expenditures, material expenditures decreased from $1,454.96 to $664.28 (-54.3%), and raw drug cost expenditures decreased from $50,596.48 to $43,398.73 (-14.3%). Workload expenditures also decreased from $27,140.60 to $12,391.75 (-54.3%).
Several points of descriptive information also were collected. The average days’ supply trended down from a mode of 10 days to 7 days. This reduction in days’ supply likely was seen because staff became more aware of the customary amount required to bridge the veteran until the CMOP supply arrived by mail. Postintervention data showed a 70% utilization of partial-fill reasoning codes. The reasons for partial filling of prescriptions are summarized in Table 2.
Discussion
Following the implementation of the updated partial-fill guidance and provision of education to the FVAMC veterans and staff, a noteworthy cost savings was observed with respect to both material and workload expenditures. This large reduction in expenditure likely was not related to a reduction in the total prescription volume, as the number of total prescriptions filled by the CMOP and at FVAMC were similar in both the preliminary and postintervention periods. When the results of this 3-month QI project were extrapolated, the annual projected cost avoidance was $91,949.12.
Of note, there was no established process for adjudicating appeals to the partial-fill guidance. Any extenuating circumstances that fell outside the guidance were addressed by the outpatient pharmacy supervisor. There was no formal documentation for these disputed cases. Since there was no prespecified supervisory override code, the most appropriate partial-fill code was entered into the comments field for these scenarios. As such, there is no way to distinguish precisely how many of these partial fills were escalated to a supervisory level for a decision.
The positive fiscal impact noted from the implementation of this project should not be viewed as the only utility for such guidance. Though not directly measured within the confines of this project, a reduction in pharmacy staff time spent on partial-fill prescriptions will likely result in shorter pharmacy wait times, line lengths, and streamlining of pharmacy workflow. When the pharmacy staff is free to work on pressing issues rather than on continually educating veterans on the partial-fill or refill process, many will benefit. Veteran satisfaction was not directly measured during this project but could be an interesting topic to review as a future study.
Each VA facility is unique, with its own challenges for implementation of a project such as this. Nevertheless, the incorporation of a formal guidance and education process, perhaps adapted to the indi vidual facility’s needs may be considered for overall pharmacy operations QI.
Limitations
During the preliminary data collection period, FVAMC and its catchment area were impacted by a natural disaster, Hurricane Matthew. Based on a review of the text entered into the comments field for all partial fills, about 4% of the partial fills completed in the preliminary phase can be attributed to the hurricane. The effects of this hurricane may have potentially increased the number of partial fills completed in the preliminary phase compared with that in the postintervention phase, due to the number of veterans who were temporarily or permanently displaced from their homes. This increase in partial fills and associated expenses preintervention likely caused a slightly higher cost savings to be reflected in the postimplementation phase than what would have traditionally been observed without extenuating factors.
Several other limitations must be considered for this QI project. The implementation phase, during which all education and training was completed, was only 1 month. A longer implementation period and more opportunities to educate veterans and staff might have created a greater impact on the results. Additionally, because there were no data collected on New Patient Orientation attendance for this project, it is unclear exactly how many veterans received refill-process education through this outlet.
Though all staff members were trained on the appropriate process, it was discovered during interim analysis that several pharmacists were not following the partial-fill guidance, potentially negatively impacting the results. It is likely that staff would have benefited from continual reeducation of the process throughout the entirety of the project, as the restriction of partial filling was a novel concept to many. In addition to continual reeducation of current employees, any new hires would likely need this information as part of initial training.
Cost variance in the type of partial fills completed between the preliminary and postintervention phases also may have negatively impacted the results. The postintervention phase contained 2 high-cost classes of drugs (antivirals and immunoglobulins) that received multiple partial fills but were not partialed in the preliminary phase, which increased the raw drug cost in the postintervention phase.
Conclusion
The implementation of partial-fill and process education to FVAMC staff and veterans proved beneficial in reducing the expenditures and workload associated with partial-fill prescription processing. The continued use of the updated partial-fill guidance will provide a standardized approach for pharmacy staff when completing partial-fill prescriptions.
Facilities may consider annual reeducation on their guidance through a local TMS module, as well as occasional process reminders during staff meetings to improve staff adherence to the guidance. Moreover, the sustained incorporation of improved refill process education to new patients and with every prescription pickup could help guide the FVAMC veteran population to use pharmacy services more effectively. The adoption of such procedures may be useful for VA facilities’ health care system looking to maximize the use of funding provided for prescription services as well as improve veterans’ understanding of how to appropriately order prescription refills.
According to the US Department of Veterans Affairs (VA) Pharmacy Benefits Management Service, about 80% of all outpatient prescriptions filled by the VA are sent to veterans by mail order, using the Centralized Mail Order Pharmacy (CMOP) network of highly automated pharmacies around the country.1 During fiscal year (FY) 2016, the 7 VA CMOP facilities throughout the US processed 119.7 million outpatient prescriptions. Each day, these CMOPs process nearly 470,000 prescriptions, an evidence of the efficiency provided through this mail-order service.1 The use of CMOP results in lower processing costs and increased convenience for veterans compared with filling prescriptions at pharmacies at individual VA facilities. Notably, VA CMOP has been rated “among the best” mail-order pharmacies in customer satisfaction according to the 2017 J.D. Power US Pharmacy Study.2
Background
Within the Fayetteville VA Medical Center (FVAMC) system in North Carolina, on-site patients receive a new prescription where an on-site pharmacy is available (at health care centers [HCC] and the medical center). For veterans seen at community-based outpatient clinics (CBOCs), emergent new prescriptions are filled through vouchers at contract community pharmacies, and nonemergent new prescriptions are filled by the CMOP (Figure 1).
The appropriate use of the VA CMOP for refills is intended to allow on-site pharmacy staff to focus on providing customer service for veterans requesting medication counseling from a clinical pharmacist, as well as those with new, changing, or urgent prescription needs. Filling prescriptions through the CMOP also can help control the VA facility pharmacy budget.
Despite the established mail-order process, FVAMC staff noted a high volume of medication refill and partial-fill prescriptions being requested at the on-site pharmacy. When a veteran presented to a pharmacy requesting a medication refill, pharmacy staff members ordered a refill to be filled by the CMOP and provided a limited quantity, otherwise known as a partial fill, to serve as an emergency supply to supplement the veteran until the full quantity of the prescription arrived by mail. Partial fills also were completed for new prescriptions that veterans requested to pick up at the on-site pharmacy. For new 90-day supply prescriptions, pharmacy staff often filled a 30-day supply in addition to submitting the entire 90-day prescription through the CMOP, which led to an unnecessary increase in material expenditures and workload.Preliminary data noted the most frequently used partial-fill days’ supply to be 10 days. Due to the lack of partial-fill criteria, prescriptions of all classes, quantities, and days’ supplies were provided as partial fills. Prior to the implementation of this quality improvement (QI) project, there was no standard approach for how to handle these requests.
Partial fills do not provide copay reimbursement to the facility filling the prescription. In an effort to steward the funds provided to the FVAMC pharmacy department wisely, an evaluation was performed with reference to partial fills. During FY 2016, about $350,000 was spent on medications, materials, and workload associated with the partial filling of FVAMC prescriptions. With respect to unique individuals who were provided care over the course of FY 2015 and 2016, the number of unique patients served by local comparator hospitals increased by only 2%, while the number of unique patients served by FVAMC increased by 12%. This substantial growth in the number of veterans served places further emphasis on the necessity of stewarding the allotted pharmacy budget. Moreover, the excessive number of medication refill and partial-fill prescriptions filled at on-site VA pharmacies can contribute to increased wait times for veterans with urgent prescription needs.
In November 2016, FVAMC implemented an updated partial-fill guidance. Partial-fill process and refill education was provided for VA staff and veterans in an effort to allow all parties involved to use pharmacy services efficiently. This analysis reviewed the reduction in partial-fill expenditures with a secondary focus on workload expenditures following the execution of this education.
Methods
This project was deemed to be a QI project and did not require institutional review board approval. Implementation for this QI project began in November 2016. Baseline raw drug cost, number, and class of prescriptions partialed were retrospectively collected for a 90-day period prior to implementation using all available data. Postintervention data were collected for 90 days following the implementation phase to compare partial-fill expenditures and workload expenditures with baseline data.
Calculations
Materials included in the partial-fill expenditure calculation were prescription vials, prescription vial caps, and prescription labeling. Material cost per partial fill was determined by using the facility’s wholesaler acquisition unit costs to estimate a summed cost for an individual prescription vial and prescription vial cap. The estimated acquisition price of 7 prescription-labeling pages was used in the material calculation, as this was the average number of pages used when performing test partial fills.
The following equation was used to calculate total partial-fill expenditures for any specified time frame:
Total partial-fill expenditure = total raw drug cost + (material cost × number of partial fills)
In addition, the average personnel cost per partial-fill prescription was determined. Average workload expenditure per partial fill was calculated by filling a subset of 10 test prescriptions and multiplying the average time spent by an average of the general station (GS) rate for pharmacists and technicians. The average hourly rate of a GS-12 pharmacist was calculated based on an average of the 10 available pay grades within the GS-12 ranking. The average hourly rate of a GS-6 pharmacy technician was calculated based on an average of the 10 available pay grades within the GS-6 ranking.3 The average workload expenditures were calculated using the following equations:
Average pharmacist workload expenditure per partial fill = time (in hours) × average hourly rate.
Average technician workload expenditure per partial fill = time (in hours) × average hourly rate.
Partial-Fill Guidance
Updated partial-fill guidance was drafted designating acceptable prescriptions to be limited to those responsible for preventing hospitalization and treatment of acute illness. This guidance provided generalized examples of medication classes that could be acceptable for partial filling, though it was not intended to be an all-inclusive list. The guidance also noted examples of classes or groups that should not be partial filled for nonemergent reasons (vitamins, nonprescription items, antilipemics), as well as controlled substances. The refill-process education was reiterated throughout the entirety of the guidance. Specifically, if a pharmacy staff member was to perform a partial fill, a review explaining the appropriate refill process to the veteran also must be provided. If the medication was determined to be of emergent need and not yet transmitted for filling via the CMOP, the directive recommended to fill the entire quantity locally as a onetime fill.
If a onetime on-site fill was determined infeasible, partial-fill quantities were recommended to be limited to only a 7-day supply, and the full quantity filled through the CMOP. Anticipated mail wait time for CMOP prescription delivery was estimated to be less than 7 days based on experience, local pending queues, and guidance from the regional CMOP; however, time could vary among VA and CMOP facilities. Original prescriptions were to be filled for the entire quantity for the first fill at the on-site pharmacy if requested by the veteran. If the pharmacy had an insufficient quantity for an entire initial supply, it could then be partial filled for a 7-day supply and then filled through the CMOP.
The final portion of the partial-fill guidance pertained to the use of partial-fill justification codes. Prior to the execution of the partial-fill guidance, free text was entered into the comments field when processing a partial-fill prescription, as the prescription-processing system used requires a comment to proceed with the partial fill. The use of these codes served to streamline data collection in the postintervention phase and helped identify areas for further education following the close of this project.
Education
Education to the pharmacy staff was disseminated by various modalities, including in-person sessions and written and electronic correspondences. This written guidance was distributed to pharmacy staff by e-mail, the pharmacy newsletter (Rx-tra), signage posted throughout the outpatient pharmacies, and on the facility’s pharmacy Microsoft SharePoint (Redmond, WA) site. In-services provided during pharmacy staff meetings detailed information on the updated partial-fill guidance. A FVAMC Talent Management System (TMS) training module was developed and assigned to all pharmacy service staff to reiterate key points regarding this QI initiative (eAppendix).
Nonpharmacy staff were educated through staff and in-service meetings and e-mail correspondences. These in-services emphasized how nurses, medical support assistants, and health care providers (HCPs) could assist veterans by knowing the correct refill process, ensuring sufficient refills remained until the next appointment, and providing continual refill-process education.
Following implementation, all veterans receiving prescriptions through the on-site pharmacies in the FVAMC were provided a copy of the refill-process handout with each trip to the pharmacy. Nonpharmacy staff and HCPs also were provided this handout to distribute to patients. The intent of this handout was to clearly detail the various ways in which refills could be ordered and the time frame in which they should be ordered. The pharmacy became involved in new patient orientation classes for all veterans new to FVAMC. Digital signage and messaging was created and circulated throughout several of the FVAMC facilities.
Results
The results of calculations for material cost, personnel time spent, hourly employee rates, and average workload expenditure per partial fill are summarized in Table 1.
Following the implementation of partial-fill and refill-process education, there was a 54.3% decrease in the total number of partial fills from 5,596 in the 90 days prior to implementation, to 2,555 partials completed over the 90 days postimplementation. Regarding the primary objective, total partial-fill expenditures decreased from $52,015.44 to $44,063.01 (-15.3%). When dissecting the individual components of partial-fill expenditures, material expenditures decreased from $1,454.96 to $664.28 (-54.3%), and raw drug cost expenditures decreased from $50,596.48 to $43,398.73 (-14.3%). Workload expenditures also decreased from $27,140.60 to $12,391.75 (-54.3%).
Several points of descriptive information also were collected. The average days’ supply trended down from a mode of 10 days to 7 days. This reduction in days’ supply likely was seen because staff became more aware of the customary amount required to bridge the veteran until the CMOP supply arrived by mail. Postintervention data showed a 70% utilization of partial-fill reasoning codes. The reasons for partial filling of prescriptions are summarized in Table 2.
Discussion
Following the implementation of the updated partial-fill guidance and provision of education to the FVAMC veterans and staff, a noteworthy cost savings was observed with respect to both material and workload expenditures. This large reduction in expenditure likely was not related to a reduction in the total prescription volume, as the number of total prescriptions filled by the CMOP and at FVAMC were similar in both the preliminary and postintervention periods. When the results of this 3-month QI project were extrapolated, the annual projected cost avoidance was $91,949.12.
Of note, there was no established process for adjudicating appeals to the partial-fill guidance. Any extenuating circumstances that fell outside the guidance were addressed by the outpatient pharmacy supervisor. There was no formal documentation for these disputed cases. Since there was no prespecified supervisory override code, the most appropriate partial-fill code was entered into the comments field for these scenarios. As such, there is no way to distinguish precisely how many of these partial fills were escalated to a supervisory level for a decision.
The positive fiscal impact noted from the implementation of this project should not be viewed as the only utility for such guidance. Though not directly measured within the confines of this project, a reduction in pharmacy staff time spent on partial-fill prescriptions will likely result in shorter pharmacy wait times, line lengths, and streamlining of pharmacy workflow. When the pharmacy staff is free to work on pressing issues rather than on continually educating veterans on the partial-fill or refill process, many will benefit. Veteran satisfaction was not directly measured during this project but could be an interesting topic to review as a future study.
Each VA facility is unique, with its own challenges for implementation of a project such as this. Nevertheless, the incorporation of a formal guidance and education process, perhaps adapted to the indi vidual facility’s needs may be considered for overall pharmacy operations QI.
Limitations
During the preliminary data collection period, FVAMC and its catchment area were impacted by a natural disaster, Hurricane Matthew. Based on a review of the text entered into the comments field for all partial fills, about 4% of the partial fills completed in the preliminary phase can be attributed to the hurricane. The effects of this hurricane may have potentially increased the number of partial fills completed in the preliminary phase compared with that in the postintervention phase, due to the number of veterans who were temporarily or permanently displaced from their homes. This increase in partial fills and associated expenses preintervention likely caused a slightly higher cost savings to be reflected in the postimplementation phase than what would have traditionally been observed without extenuating factors.
Several other limitations must be considered for this QI project. The implementation phase, during which all education and training was completed, was only 1 month. A longer implementation period and more opportunities to educate veterans and staff might have created a greater impact on the results. Additionally, because there were no data collected on New Patient Orientation attendance for this project, it is unclear exactly how many veterans received refill-process education through this outlet.
Though all staff members were trained on the appropriate process, it was discovered during interim analysis that several pharmacists were not following the partial-fill guidance, potentially negatively impacting the results. It is likely that staff would have benefited from continual reeducation of the process throughout the entirety of the project, as the restriction of partial filling was a novel concept to many. In addition to continual reeducation of current employees, any new hires would likely need this information as part of initial training.
Cost variance in the type of partial fills completed between the preliminary and postintervention phases also may have negatively impacted the results. The postintervention phase contained 2 high-cost classes of drugs (antivirals and immunoglobulins) that received multiple partial fills but were not partialed in the preliminary phase, which increased the raw drug cost in the postintervention phase.
Conclusion
The implementation of partial-fill and process education to FVAMC staff and veterans proved beneficial in reducing the expenditures and workload associated with partial-fill prescription processing. The continued use of the updated partial-fill guidance will provide a standardized approach for pharmacy staff when completing partial-fill prescriptions.
Facilities may consider annual reeducation on their guidance through a local TMS module, as well as occasional process reminders during staff meetings to improve staff adherence to the guidance. Moreover, the sustained incorporation of improved refill process education to new patients and with every prescription pickup could help guide the FVAMC veteran population to use pharmacy services more effectively. The adoption of such procedures may be useful for VA facilities’ health care system looking to maximize the use of funding provided for prescription services as well as improve veterans’ understanding of how to appropriately order prescription refills.
1. US Department of Veterans Affairs. Pharmacy benefits management services. https://www.pbm.va.gov/PBM/CMOP/VA_Mail_Order_Pharmacy.asp. Updated July 14, 2017. Accessed on February 26, 2018.
2. J.D. Power. Decline in pharmacy customer satisfaction driven by prescription drug costs, J. D. Power finds. [press release]. http://www.jdpower.com/press-releases/jd-power-2017-us-pharmacy-study. Published September 5, 2017. Accessed February 26, 2018.
3. US Office of Personnel Management. Pay and leave. https://www.opm.gov/policy-data-oversight/pay-leave/salaries-wages/. Accessed February 26, 2018.
4. Aragon BR, Pierce RA, Jones WN. VA CMOPs: producing a pattern of quality and efficiency in government. J Am Pharm Assoc (2003). 2012;52(6):810-815.
1. US Department of Veterans Affairs. Pharmacy benefits management services. https://www.pbm.va.gov/PBM/CMOP/VA_Mail_Order_Pharmacy.asp. Updated July 14, 2017. Accessed on February 26, 2018.
2. J.D. Power. Decline in pharmacy customer satisfaction driven by prescription drug costs, J. D. Power finds. [press release]. http://www.jdpower.com/press-releases/jd-power-2017-us-pharmacy-study. Published September 5, 2017. Accessed February 26, 2018.
3. US Office of Personnel Management. Pay and leave. https://www.opm.gov/policy-data-oversight/pay-leave/salaries-wages/. Accessed February 26, 2018.
4. Aragon BR, Pierce RA, Jones WN. VA CMOPs: producing a pattern of quality and efficiency in government. J Am Pharm Assoc (2003). 2012;52(6):810-815.
FDA Boxed Warning Updates: February 2018
The FDA’s MedWatch program safety labeling changes for boxed warnings are compiled quarterly for drugs and therapeutic biologics where important changes have been made to the safety information. These and other label changes are searchable in the Drug Safety Labeling Changes (SLC) database, where data are available to the public in downloadable and searchable formats. Boxed warnings are ordinarily used to highlight either adverse reactions so serious in proportion to the potential benefit from the drug that it is essential that it be considered in assessing the risks and benefits of using the drug; or serious adverse reactions that can be prevented/reduced in frequency or severity by appropriate use of the drug; or FDA approved the drug with restrictions to ensure safe use because FDA concluded that the drug can be safely used only if distribution or use is restricted. For complete FDA Drug Safety Labeling changes, please visit http://www.accessdata.fda.gov/scripts/cder/safetylabelingchanges.
CODEINE SULFATE: EPIVIR-HBV (LAMIVUDINE)
- Added to warning September 2017
WARNING: LACTIC ACIDOSIS AND SEVERE HEPATOMEGALY WITH STEATOSIS, EXACERBATIONS OF HEPATITIS B, and RISK OF HIV-1 RESISTANCE IF EPIVIR-HBV IS USED IN PATIENTS WITH UNRECOGNIZED OR UNTREATED HIV-1 INFECTION
Lactic acidosis and severe hepatomegaly with steatosis, including fatal cases, have been reported with the use of nucleoside analogues and other antiretrovirals. Discontinue EPIVIR-HBV if clinical or laboratory findings suggestive of lactic acidosis or pronounced hepatotoxicity occur.
Severe acute exacerbations of hepatitis B have been reported in patients who have discontinued anti-hepatitis B therapy (including EPIVIR-HBV). Hepatic function should be monitored closely with both clinical and laboratory followup for at least several months in patients who discontinue anti-hepatitis B therapy. If appropriate, initiation of anti-hepatitis B therapy may be warranted.
EPIVIR-HBV is not approved for the treatment of HIV-1 infection because the lamivudine dosage in EPIVIR-HBV is subtherapeutic and monotherapy is inappropriate for the treatment of HIV-1 infection. HIV-1 resistance may emerge in chronic hepatitis B-infected patients with unrecognized or untreated HIV-1 infection. HIV counseling and testing should be offered to all patients before beginning treatment with EPIVIR-HBV and periodically during treatment.
INVOKANA (CANAGLIFLOZIN)
- Added section to warning July 2017
WARNING: LOWER LIMB AMPUTATION
- An approximately 2-fold increased risk of lower limb amputations associated with INVOKANA use was observed in CANVAS and CANVAS-R, two large, randomized, placebo-controlled trials in patients with type 2 diabetes who had established cardiovascular disease (CVD) or were at risk for CVD.
- Amputations of the toe and midfoot were most frequent; however, amputations involving the leg were also observed. Some patients had multiple amputations, some involving both limbs.
- Before initiating, consider factors that may increase the risk of amputation, such as a history of prior amputation, peripheral vascular disease, neuropathy, and diabetic foot ulcers.
Monitor patients receiving INVOKANA for infection, new pain or tenderness, sores or ulcers involving the lower limbs, and discontinue if these complications occur.
INVOKAMET (CANAGLIFLOZIN; METFORMIN HYDROCHLORIDE)
- Edited warning August 2017
WARNING: LACTIC ACIDOSIS and LOWER LIMB AMPUTATION
Lactic Acidosis
- Risk factors for metformin-associated lactic acidosis include renal impairment, concomitant use of certain drugs (e.g., carbonic anhydrase inhibitors such as topiramate), age 65 years old or greater, having a radiological study with contrast, surgery and other procedures, hypoxic states (e.g., acute congestive heart failure), excessive alcohol intake, and hepatic impairment.
Risk of Lower Limb Amputation
- In patients with type 2 diabetes who have established cardiovascular disease (CVD) or at risk for CVD, canagliflozin, a component of INVOKAMET, has been associated with lower limb amputations, most frequently of the toe and midfoot; some also involved the leg.
INVOKAMET XR (CANAGLIFLOZIN; METFORMIN HYDROCHLORIDE)
- Edited warning August 2017
WARNING: LACTIC ACIDOSIS and LOWER LIMB AMPUTATION
Lactic Acidosis
- Post-marketing cases of metformin-associated lactic acidosis have resulted in death, hypothermia, hypotension, and resistant bradyarrhythmias. The onset of metformin-associated lactic acidosis is often subtle, accompanied only by nonspecific symptoms such as
malaise, myalgias, respiratory distress, somnolence, and abdominal pain. Metformin-associated lactic acidosis was characterized by elevated blood lactate levels (> 5 mmol/Liter), anion gap acidosis (without evidence of ketonuria or ketonemia), an increased lactate/ pyruvate ratio; and metformin plasma levels generally >5 mcg/mL. - Risk factors for metformin-associated lactic acidosis include renal impairment, concomitant use of certain drugs (eg, cationic drugs such as topiramate), age 65 years old or greater, having a radiological study with contrast, surgery and other procedures, hypoxic states (eg, acute congestive heart failure), excessive alcohol intake, and hepatic impairment.
- Steps to reduce the risk of and manage metforminassociated lactic acidosis in these high risk groups are provided in the full prescribing information.
- If metformin-associated lactic acidosis is suspected, immediately discontinue INVOKAMET and institute general supportive measures in a hospital setting. Prompt hemodialysis is recommended.
Risk of Lower Limb Amputation
- An approximately 2-fold increased risk of lower limb amputations associated with canagliflozin, a component of INVOKAMET, was observed in CANVAS and CANVAS-R, two large, randomized, placebo-controlled trials in patients with type 2 diabetes who had established cardiovascular disease (CVD) or were at risk for CVD.
- Amputations of the toe and midfoot were most frequent; however, amputations involving the leg were also observed. Some patients had multiple amputations, some involving both limbs.
- Before initiating, consider factors that may increase the risk of amputation, such as a history of prior amputation, peripheral vascular disease, neuropathy, and diabetic foot ulcers.
- Monitor patients receiving INVOKAMET for infection, new pain or tenderness, sores or ulcers involving the lower limbs, and discontinue if these complications occur.
JEVTANA KIT (CABAZITAXEL)
- Edited warning September 2017
WARNING: NEUTROPENIA AND HYPERSENSITIVITY
Neutropenia: Neutropenic deaths have been reported. Monitor for neutropenia with frequent blood cell counts. JEVTANA is contraindicated in patients with neutrophil counts of less than or equal to 1,500 cells/mm3. Primary prophylaxis with G-CSF is recommended in patients with high-risk clinical features.
THYRO-TABS (LEVOTHYROXINE SODIUM)
- Added section to warning August 2017
WARNING: NOT FOR TREATMENT OF OBESITY OR FOR WEIGHT LOSS
Thyroid hormones, including THYRO-TABS, either alone or with other therapeutic agents, should not be used for the treatment of obesity or for weight loss.
In euthyroid patients, doses within the range of daily hormonal requirements are ineffective for weight reduction.
Larger doses may produce serious or even life threatening manifestations of toxicity, particularly when given in association with sympathomimetic amines such as those used for their anorectic effects.
REGLAN (METOCLOPRAMIDE HYDROCHLORIDE)
- Edited warning August 2017
WARNING: TARDIVE DYSKINESIA
- Reglan can cause tardive dyskinesia (TD), a serious movement disorder that is often irreversible. There is no known treatment for TD. The risk of developing TD increases with duration of treatment and total cumulative dosage.
- Discontinue Reglan in patients who develop signs or symptoms of TD. In some patients, symptoms may lessen or resolve after Reglan is stopped.
- Avoid treatment with Reglan for longer than 12 weeks because of the increased risk of developing TD with longer-term use.
The FDA’s MedWatch program safety labeling changes for boxed warnings are compiled quarterly for drugs and therapeutic biologics where important changes have been made to the safety information. These and other label changes are searchable in the Drug Safety Labeling Changes (SLC) database, where data are available to the public in downloadable and searchable formats. Boxed warnings are ordinarily used to highlight either adverse reactions so serious in proportion to the potential benefit from the drug that it is essential that it be considered in assessing the risks and benefits of using the drug; or serious adverse reactions that can be prevented/reduced in frequency or severity by appropriate use of the drug; or FDA approved the drug with restrictions to ensure safe use because FDA concluded that the drug can be safely used only if distribution or use is restricted. For complete FDA Drug Safety Labeling changes, please visit http://www.accessdata.fda.gov/scripts/cder/safetylabelingchanges.
CODEINE SULFATE: EPIVIR-HBV (LAMIVUDINE)
- Added to warning September 2017
WARNING: LACTIC ACIDOSIS AND SEVERE HEPATOMEGALY WITH STEATOSIS, EXACERBATIONS OF HEPATITIS B, and RISK OF HIV-1 RESISTANCE IF EPIVIR-HBV IS USED IN PATIENTS WITH UNRECOGNIZED OR UNTREATED HIV-1 INFECTION
Lactic acidosis and severe hepatomegaly with steatosis, including fatal cases, have been reported with the use of nucleoside analogues and other antiretrovirals. Discontinue EPIVIR-HBV if clinical or laboratory findings suggestive of lactic acidosis or pronounced hepatotoxicity occur.
Severe acute exacerbations of hepatitis B have been reported in patients who have discontinued anti-hepatitis B therapy (including EPIVIR-HBV). Hepatic function should be monitored closely with both clinical and laboratory followup for at least several months in patients who discontinue anti-hepatitis B therapy. If appropriate, initiation of anti-hepatitis B therapy may be warranted.
EPIVIR-HBV is not approved for the treatment of HIV-1 infection because the lamivudine dosage in EPIVIR-HBV is subtherapeutic and monotherapy is inappropriate for the treatment of HIV-1 infection. HIV-1 resistance may emerge in chronic hepatitis B-infected patients with unrecognized or untreated HIV-1 infection. HIV counseling and testing should be offered to all patients before beginning treatment with EPIVIR-HBV and periodically during treatment.
INVOKANA (CANAGLIFLOZIN)
- Added section to warning July 2017
WARNING: LOWER LIMB AMPUTATION
- An approximately 2-fold increased risk of lower limb amputations associated with INVOKANA use was observed in CANVAS and CANVAS-R, two large, randomized, placebo-controlled trials in patients with type 2 diabetes who had established cardiovascular disease (CVD) or were at risk for CVD.
- Amputations of the toe and midfoot were most frequent; however, amputations involving the leg were also observed. Some patients had multiple amputations, some involving both limbs.
- Before initiating, consider factors that may increase the risk of amputation, such as a history of prior amputation, peripheral vascular disease, neuropathy, and diabetic foot ulcers.
Monitor patients receiving INVOKANA for infection, new pain or tenderness, sores or ulcers involving the lower limbs, and discontinue if these complications occur.
INVOKAMET (CANAGLIFLOZIN; METFORMIN HYDROCHLORIDE)
- Edited warning August 2017
WARNING: LACTIC ACIDOSIS and LOWER LIMB AMPUTATION
Lactic Acidosis
- Risk factors for metformin-associated lactic acidosis include renal impairment, concomitant use of certain drugs (e.g., carbonic anhydrase inhibitors such as topiramate), age 65 years old or greater, having a radiological study with contrast, surgery and other procedures, hypoxic states (e.g., acute congestive heart failure), excessive alcohol intake, and hepatic impairment.
Risk of Lower Limb Amputation
- In patients with type 2 diabetes who have established cardiovascular disease (CVD) or at risk for CVD, canagliflozin, a component of INVOKAMET, has been associated with lower limb amputations, most frequently of the toe and midfoot; some also involved the leg.
INVOKAMET XR (CANAGLIFLOZIN; METFORMIN HYDROCHLORIDE)
- Edited warning August 2017
WARNING: LACTIC ACIDOSIS and LOWER LIMB AMPUTATION
Lactic Acidosis
- Post-marketing cases of metformin-associated lactic acidosis have resulted in death, hypothermia, hypotension, and resistant bradyarrhythmias. The onset of metformin-associated lactic acidosis is often subtle, accompanied only by nonspecific symptoms such as
malaise, myalgias, respiratory distress, somnolence, and abdominal pain. Metformin-associated lactic acidosis was characterized by elevated blood lactate levels (> 5 mmol/Liter), anion gap acidosis (without evidence of ketonuria or ketonemia), an increased lactate/ pyruvate ratio; and metformin plasma levels generally >5 mcg/mL. - Risk factors for metformin-associated lactic acidosis include renal impairment, concomitant use of certain drugs (eg, cationic drugs such as topiramate), age 65 years old or greater, having a radiological study with contrast, surgery and other procedures, hypoxic states (eg, acute congestive heart failure), excessive alcohol intake, and hepatic impairment.
- Steps to reduce the risk of and manage metforminassociated lactic acidosis in these high risk groups are provided in the full prescribing information.
- If metformin-associated lactic acidosis is suspected, immediately discontinue INVOKAMET and institute general supportive measures in a hospital setting. Prompt hemodialysis is recommended.
Risk of Lower Limb Amputation
- An approximately 2-fold increased risk of lower limb amputations associated with canagliflozin, a component of INVOKAMET, was observed in CANVAS and CANVAS-R, two large, randomized, placebo-controlled trials in patients with type 2 diabetes who had established cardiovascular disease (CVD) or were at risk for CVD.
- Amputations of the toe and midfoot were most frequent; however, amputations involving the leg were also observed. Some patients had multiple amputations, some involving both limbs.
- Before initiating, consider factors that may increase the risk of amputation, such as a history of prior amputation, peripheral vascular disease, neuropathy, and diabetic foot ulcers.
- Monitor patients receiving INVOKAMET for infection, new pain or tenderness, sores or ulcers involving the lower limbs, and discontinue if these complications occur.
JEVTANA KIT (CABAZITAXEL)
- Edited warning September 2017
WARNING: NEUTROPENIA AND HYPERSENSITIVITY
Neutropenia: Neutropenic deaths have been reported. Monitor for neutropenia with frequent blood cell counts. JEVTANA is contraindicated in patients with neutrophil counts of less than or equal to 1,500 cells/mm3. Primary prophylaxis with G-CSF is recommended in patients with high-risk clinical features.
THYRO-TABS (LEVOTHYROXINE SODIUM)
- Added section to warning August 2017
WARNING: NOT FOR TREATMENT OF OBESITY OR FOR WEIGHT LOSS
Thyroid hormones, including THYRO-TABS, either alone or with other therapeutic agents, should not be used for the treatment of obesity or for weight loss.
In euthyroid patients, doses within the range of daily hormonal requirements are ineffective for weight reduction.
Larger doses may produce serious or even life threatening manifestations of toxicity, particularly when given in association with sympathomimetic amines such as those used for their anorectic effects.
REGLAN (METOCLOPRAMIDE HYDROCHLORIDE)
- Edited warning August 2017
WARNING: TARDIVE DYSKINESIA
- Reglan can cause tardive dyskinesia (TD), a serious movement disorder that is often irreversible. There is no known treatment for TD. The risk of developing TD increases with duration of treatment and total cumulative dosage.
- Discontinue Reglan in patients who develop signs or symptoms of TD. In some patients, symptoms may lessen or resolve after Reglan is stopped.
- Avoid treatment with Reglan for longer than 12 weeks because of the increased risk of developing TD with longer-term use.
The FDA’s MedWatch program safety labeling changes for boxed warnings are compiled quarterly for drugs and therapeutic biologics where important changes have been made to the safety information. These and other label changes are searchable in the Drug Safety Labeling Changes (SLC) database, where data are available to the public in downloadable and searchable formats. Boxed warnings are ordinarily used to highlight either adverse reactions so serious in proportion to the potential benefit from the drug that it is essential that it be considered in assessing the risks and benefits of using the drug; or serious adverse reactions that can be prevented/reduced in frequency or severity by appropriate use of the drug; or FDA approved the drug with restrictions to ensure safe use because FDA concluded that the drug can be safely used only if distribution or use is restricted. For complete FDA Drug Safety Labeling changes, please visit http://www.accessdata.fda.gov/scripts/cder/safetylabelingchanges.
CODEINE SULFATE: EPIVIR-HBV (LAMIVUDINE)
- Added to warning September 2017
WARNING: LACTIC ACIDOSIS AND SEVERE HEPATOMEGALY WITH STEATOSIS, EXACERBATIONS OF HEPATITIS B, and RISK OF HIV-1 RESISTANCE IF EPIVIR-HBV IS USED IN PATIENTS WITH UNRECOGNIZED OR UNTREATED HIV-1 INFECTION
Lactic acidosis and severe hepatomegaly with steatosis, including fatal cases, have been reported with the use of nucleoside analogues and other antiretrovirals. Discontinue EPIVIR-HBV if clinical or laboratory findings suggestive of lactic acidosis or pronounced hepatotoxicity occur.
Severe acute exacerbations of hepatitis B have been reported in patients who have discontinued anti-hepatitis B therapy (including EPIVIR-HBV). Hepatic function should be monitored closely with both clinical and laboratory followup for at least several months in patients who discontinue anti-hepatitis B therapy. If appropriate, initiation of anti-hepatitis B therapy may be warranted.
EPIVIR-HBV is not approved for the treatment of HIV-1 infection because the lamivudine dosage in EPIVIR-HBV is subtherapeutic and monotherapy is inappropriate for the treatment of HIV-1 infection. HIV-1 resistance may emerge in chronic hepatitis B-infected patients with unrecognized or untreated HIV-1 infection. HIV counseling and testing should be offered to all patients before beginning treatment with EPIVIR-HBV and periodically during treatment.
INVOKANA (CANAGLIFLOZIN)
- Added section to warning July 2017
WARNING: LOWER LIMB AMPUTATION
- An approximately 2-fold increased risk of lower limb amputations associated with INVOKANA use was observed in CANVAS and CANVAS-R, two large, randomized, placebo-controlled trials in patients with type 2 diabetes who had established cardiovascular disease (CVD) or were at risk for CVD.
- Amputations of the toe and midfoot were most frequent; however, amputations involving the leg were also observed. Some patients had multiple amputations, some involving both limbs.
- Before initiating, consider factors that may increase the risk of amputation, such as a history of prior amputation, peripheral vascular disease, neuropathy, and diabetic foot ulcers.
Monitor patients receiving INVOKANA for infection, new pain or tenderness, sores or ulcers involving the lower limbs, and discontinue if these complications occur.
INVOKAMET (CANAGLIFLOZIN; METFORMIN HYDROCHLORIDE)
- Edited warning August 2017
WARNING: LACTIC ACIDOSIS and LOWER LIMB AMPUTATION
Lactic Acidosis
- Risk factors for metformin-associated lactic acidosis include renal impairment, concomitant use of certain drugs (e.g., carbonic anhydrase inhibitors such as topiramate), age 65 years old or greater, having a radiological study with contrast, surgery and other procedures, hypoxic states (e.g., acute congestive heart failure), excessive alcohol intake, and hepatic impairment.
Risk of Lower Limb Amputation
- In patients with type 2 diabetes who have established cardiovascular disease (CVD) or at risk for CVD, canagliflozin, a component of INVOKAMET, has been associated with lower limb amputations, most frequently of the toe and midfoot; some also involved the leg.
INVOKAMET XR (CANAGLIFLOZIN; METFORMIN HYDROCHLORIDE)
- Edited warning August 2017
WARNING: LACTIC ACIDOSIS and LOWER LIMB AMPUTATION
Lactic Acidosis
- Post-marketing cases of metformin-associated lactic acidosis have resulted in death, hypothermia, hypotension, and resistant bradyarrhythmias. The onset of metformin-associated lactic acidosis is often subtle, accompanied only by nonspecific symptoms such as
malaise, myalgias, respiratory distress, somnolence, and abdominal pain. Metformin-associated lactic acidosis was characterized by elevated blood lactate levels (> 5 mmol/Liter), anion gap acidosis (without evidence of ketonuria or ketonemia), an increased lactate/ pyruvate ratio; and metformin plasma levels generally >5 mcg/mL. - Risk factors for metformin-associated lactic acidosis include renal impairment, concomitant use of certain drugs (eg, cationic drugs such as topiramate), age 65 years old or greater, having a radiological study with contrast, surgery and other procedures, hypoxic states (eg, acute congestive heart failure), excessive alcohol intake, and hepatic impairment.
- Steps to reduce the risk of and manage metforminassociated lactic acidosis in these high risk groups are provided in the full prescribing information.
- If metformin-associated lactic acidosis is suspected, immediately discontinue INVOKAMET and institute general supportive measures in a hospital setting. Prompt hemodialysis is recommended.
Risk of Lower Limb Amputation
- An approximately 2-fold increased risk of lower limb amputations associated with canagliflozin, a component of INVOKAMET, was observed in CANVAS and CANVAS-R, two large, randomized, placebo-controlled trials in patients with type 2 diabetes who had established cardiovascular disease (CVD) or were at risk for CVD.
- Amputations of the toe and midfoot were most frequent; however, amputations involving the leg were also observed. Some patients had multiple amputations, some involving both limbs.
- Before initiating, consider factors that may increase the risk of amputation, such as a history of prior amputation, peripheral vascular disease, neuropathy, and diabetic foot ulcers.
- Monitor patients receiving INVOKAMET for infection, new pain or tenderness, sores or ulcers involving the lower limbs, and discontinue if these complications occur.
JEVTANA KIT (CABAZITAXEL)
- Edited warning September 2017
WARNING: NEUTROPENIA AND HYPERSENSITIVITY
Neutropenia: Neutropenic deaths have been reported. Monitor for neutropenia with frequent blood cell counts. JEVTANA is contraindicated in patients with neutrophil counts of less than or equal to 1,500 cells/mm3. Primary prophylaxis with G-CSF is recommended in patients with high-risk clinical features.
THYRO-TABS (LEVOTHYROXINE SODIUM)
- Added section to warning August 2017
WARNING: NOT FOR TREATMENT OF OBESITY OR FOR WEIGHT LOSS
Thyroid hormones, including THYRO-TABS, either alone or with other therapeutic agents, should not be used for the treatment of obesity or for weight loss.
In euthyroid patients, doses within the range of daily hormonal requirements are ineffective for weight reduction.
Larger doses may produce serious or even life threatening manifestations of toxicity, particularly when given in association with sympathomimetic amines such as those used for their anorectic effects.
REGLAN (METOCLOPRAMIDE HYDROCHLORIDE)
- Edited warning August 2017
WARNING: TARDIVE DYSKINESIA
- Reglan can cause tardive dyskinesia (TD), a serious movement disorder that is often irreversible. There is no known treatment for TD. The risk of developing TD increases with duration of treatment and total cumulative dosage.
- Discontinue Reglan in patients who develop signs or symptoms of TD. In some patients, symptoms may lessen or resolve after Reglan is stopped.
- Avoid treatment with Reglan for longer than 12 weeks because of the increased risk of developing TD with longer-term use.
Risk Factors Associated With Multidrug-Resistant Pneumonia in Nonhospitalized Patients
Successful treatment of pneumonia depends on timely diagnosis and administration of antibiotics. Multidrug-resistant organisms (MDROs) complicate antibiotic therapies by rendering some antibiotic agents ineffective. Inappropriate initial therapy has been associated with a more than 2-fold increase in the risk of mortality.1 Because culture results are not available immediately, clinicians prescribe antibiotics empirically and must rely on guidelines and knowledge of risk factors associated with MDRO infection to make these selections.
Treatment guidelines exist for hospital-acquired and ventilator-associated pneumonia (HAP/VAP) and community-acquired pneumonia (CAP) to assist with empiric antibiotic selection. For HAP/VAP, 2 to 3 antibiotics with a broad-spectrum of activity are used due to increased prevalence of MDROs in hospitals, whereastreatment of CAP involves more narrow coverage because bacteria that cause this infection typically have fewer antibiotic resistances.2,3 The HAP/VAP guidelines stratify the risk of pneumonia due to the presence of a MDRO acquired during a hospitalization. However, neither the CAP nor HAP/VAP guidelines offer risk-stratification guidance for nonhospitalized patients who develop pneumonia but who may have become colonized with a MDRO during a previous hospitalization or from another exposure to a health care facility.
Health care-associated pneumonia (HCAP) was first described in the 2005 American Thoracic Society and the Infectious Diseases Society of America (ATS/IDSA) nosocomial pneumonia guidelines and was associated with criteria intended to aid clinician identification of nonhospitalized patients at risk for MDRO pneumonia, which warranted empiric broad-spectrum antibiotic therapy.2 According to these guidelines, patients were classified as having HCAP if they had been hospitalized for at least 48 hours in the past 90 days, admitted from a nursing home, received recent intravenous antibiotics, had hemodialysis in the past 30 days, had a history of home infusion therapy or wound care, received intravenous chemotherapy, or had a family member with MDRO colonization.
Since publication of the 2005 guidelines, HCAP has been criticized as being a poor predictor of MDRO infection. A 2014 meta-analysis of 24 studies investigated the discriminating ability of HCAP and reported that the specificity and sensitivity for MDRO infections was 71.2% and 53.7%, respectively.3 In 2016, the ATS/IDSA guidelines were updated to remove HCAP due to the risk of antibiotic overprescribing.4
Literature Review
Although criteria previously defining a patient as having HCAP have been shown to be a poor discriminator of MDRO pneumonia as a whole, MDRO infections still pose a threat to nonhospitalized patients who have exposure to the health care system. A literature review was performed to identify independent HCAP risk factors that may increase the risk of MDRO pneumonia infecting a nonhospitalized patient needing empiric broad-spectrum antibiotic therapy. All included studies were prospective or retrospective observational cohort studies that performed logistic regression analyses to assess the association between MDRO isolation and the previously defined HCAP risk factors (Table 1).
Five studies examined the risk of MDRO infection in patients with a previous hospital admission of 2 days or more in the past 90 days. Shindo and colleagues found a significant increase in MDRO infections by about 2-fold (adjusted odds ratio [AOR], 2.1; 95% confidence interval [CI], 1.2-3.4).5 Shorr and colleagues found a 4-fold increase in likelihood of identifying a MDRO in HCAP (AOR, 4.2; 95% CI, 2.9-6.3).6 Nseir and colleagues and Jung and colleagues found similar results (AOR 3.9, 95% CI 1.7-8.8; AOR 2.7, 95% CI 1.3-5.5, respectively).7,8 Conflicting results were reported by Gross and colleagues who did not find a significant relationship between previous hospitalization and MDRO isolation (AOR 1.2, 95% CI, 0.5-3.2).9
In patients with pneumonia admitted from a nursing home, MDRO infection risk also was evaluated in these 5 studies. Shorr and colleagues, Nseir and colleagues, and Gross and colleagues found significant AORs of 2.7 (95% CI 1.7-4.3), 2.0 (95% CI 1.1-3.7), and 4.2 (95% CI 1.6-11.3), respectively.6,7,9 Shindo and colleagues (AOR 1.1; 95% CI, 0.6-2.0) and Jung and colleagues (AOR 1.9, 95% CI, 0.5-6.9) found this risk factor not significant.5
Receipt of antibiotics within the previous 90 days was assessed in 3 studies. Shindo and colleagues, Nseir and colleagues, and Gross and colleagues all found significant AORs of 2.5 (95% CI 1.2-4.0), 2.3 (95% CI 1.2-4.3), and 2.9 (95% CI 1.1-7.5), respectively.5,7,9 Antibiotic therapy within the previous 90 days is an established risk factor for MDRO pneumonia, and the 2016 ATS/IDSA guidelines consider this a risk factor for HAP and VAP, including pneumonia caused by methicillin resistant Staphylococcus aureus and Pseudomonas aeruginosa.4
The impact of hemodialysis in the previous month on acquisition of MDRO pneumonia was investigated in 4 studies. Shindo and colleagues, Jung and colleagues, and Gross and colleagues concluded that this risk factor was not significantly related to MDRO infection, reporting AORs of 2.2 (95% CI 0.5-9.7), 2.8 (95% CI 0.9-9.2) and 0.7 (95% CI 0.1-5.1), respectively.5,8,9 Shorr and colleagues, however, found a significant AOR of 2.1 (95% CI 1.0-4.3).6
Shindo and colleagues investigated the impact of home infusion therapy on acquisition of pneumonia due to a MDRO and reported a nonsignificant AOR of 0.8 (95% CI 0.4-1.8).5 Gross and colleagues also found a nonsignificant AOR of 0 (P = .1).9 In the Shindo and colleagues study, resistance was found in 107 of 679 patients who did not receive infusion therapy, and 12 of 55 patients who were receiving infusion therapy.5 Gross and colleagues reported that home-infusion therapy was received by 0 of 20 patients with MDRO infection and 4 of the 501 patients without MDRO infection.9
Shindo and colleagues reported that home wound care was not found to be significantly related to MDRO pneumonia as well as did Gross and colleagues: AORs of 3.8 (0.8-18.4) and 1.4 (95% CI 0.5-4.4), respectively.5,9 Jung and colleagues examined IV chemotherapy in the past 30 days, and found this to not significantly impact the odds of MDRO isolation (AOR = 0.62, 95% CI 0.2-1.8).8 No data were available reflecting the risk of a family member with a MDRO.
Limitations
The variables on which logistic regression were performed differed among the studies. Therefore, results cannot be averaged or compared quantitatively, as AORs varied, depending on the variables included. In addition, data were drawn from multiple geographic locations that may impact MDRO prevalence within each patient population. Finally, this review examines the utility of the risk factors formerly included in HCAP. However, other risk factors for MDRO pneumonia outlined by the ATS/IDSA guidelines still should be considered when evaluating patient risk. The 2016 guidelines recommend local incidence of resistant strains be considered when initiating empiric therapy. Review of medical records for previous positive cultures and duration of current hospitalization also should be considered. Although the 2016 ATS/IDSA HAP guidelines are not intended for immunosuppressed patients, this risk factor also may be taken into account.
Conclusion
Review and synthesis of published literature found previous hospital admission (of ≥ 2 days in the past 90 days), admission from a nursing home, and IV antibiotic therapy in the last 90 days to be independent risk factors for identification of MDRO pneumonia in previously nonhospitalized patients (Table 2). Additionally, although no data were found to support this risk factor, existence of an in-home (close contact) source of MDROs would provide ample opportunity for transmission, so evaluation of known exposure to MDROs from contacts should be considered. When choosing empiric antibiotic therapy for patients admitted to the hospital for treatment of pneumonia, consideration of patient history and risk factors that may contribute to infection with a MDRO are recommended.
1. Kollef MH, Sherman G, Ward S, Fraser VJ. Inadequate antimicrobial treatment of infections: a risk factor for hospital mortality among critically ill patients. Chest. 1999;115(2):462-474.
2. American Thoracic Society; Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171(4):388-416.
3. Chalmers JD, Rother C, Salih W, Ewig S. Healthcare-associated pneumonia does not accurately identify potentially resistant pathogens: a systematic review and meta-analysis. Clin Infect Dis. 2014;58(3):330-339.
4. Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63(5):e61-e111.
5. Shindo Y, Ito R, Kobayashi D, et al. Risk factors for drug-resistant pathogens in community-acquired and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2013;188(8):985-995.
6. Shorr AF, Zilberberg MD, Micek ST, Kollef MH. Prediction of infection due to antibiotic-resistant bacteria by select risk factors for health care–associated pneumonia. Arch Intern Med. 2008;168(20):2205-2210.
7. Nseir S, Grailles G, Soury-Lavergne A, Minacori F, Alves I, Durocher A. Accuracy of American Thoracic Society/Infectious Diseases Society of America criteria in predicting infection or colonization with multidrug-resistant bacteria at intensive-care unit admission. Clin Microbiol Infect. 2010;16(7):902-908.
8. Jung JY, Park MS, Kim YS, et al. Healthcare-associated pneumonia among hospitalized patients in a Korean tertiary hospital. BMC Infectious Diseases. 2011;11:61.
9. Gross AE, Van Schooneveld TC, Olsen KM, et al. Epidemiology and predictors of multidrug-resistant community-acquired and health care-associated pneumonia. Antimicrob Agents Chemother. 2014;58(9):5262-5268.
Successful treatment of pneumonia depends on timely diagnosis and administration of antibiotics. Multidrug-resistant organisms (MDROs) complicate antibiotic therapies by rendering some antibiotic agents ineffective. Inappropriate initial therapy has been associated with a more than 2-fold increase in the risk of mortality.1 Because culture results are not available immediately, clinicians prescribe antibiotics empirically and must rely on guidelines and knowledge of risk factors associated with MDRO infection to make these selections.
Treatment guidelines exist for hospital-acquired and ventilator-associated pneumonia (HAP/VAP) and community-acquired pneumonia (CAP) to assist with empiric antibiotic selection. For HAP/VAP, 2 to 3 antibiotics with a broad-spectrum of activity are used due to increased prevalence of MDROs in hospitals, whereastreatment of CAP involves more narrow coverage because bacteria that cause this infection typically have fewer antibiotic resistances.2,3 The HAP/VAP guidelines stratify the risk of pneumonia due to the presence of a MDRO acquired during a hospitalization. However, neither the CAP nor HAP/VAP guidelines offer risk-stratification guidance for nonhospitalized patients who develop pneumonia but who may have become colonized with a MDRO during a previous hospitalization or from another exposure to a health care facility.
Health care-associated pneumonia (HCAP) was first described in the 2005 American Thoracic Society and the Infectious Diseases Society of America (ATS/IDSA) nosocomial pneumonia guidelines and was associated with criteria intended to aid clinician identification of nonhospitalized patients at risk for MDRO pneumonia, which warranted empiric broad-spectrum antibiotic therapy.2 According to these guidelines, patients were classified as having HCAP if they had been hospitalized for at least 48 hours in the past 90 days, admitted from a nursing home, received recent intravenous antibiotics, had hemodialysis in the past 30 days, had a history of home infusion therapy or wound care, received intravenous chemotherapy, or had a family member with MDRO colonization.
Since publication of the 2005 guidelines, HCAP has been criticized as being a poor predictor of MDRO infection. A 2014 meta-analysis of 24 studies investigated the discriminating ability of HCAP and reported that the specificity and sensitivity for MDRO infections was 71.2% and 53.7%, respectively.3 In 2016, the ATS/IDSA guidelines were updated to remove HCAP due to the risk of antibiotic overprescribing.4
Literature Review
Although criteria previously defining a patient as having HCAP have been shown to be a poor discriminator of MDRO pneumonia as a whole, MDRO infections still pose a threat to nonhospitalized patients who have exposure to the health care system. A literature review was performed to identify independent HCAP risk factors that may increase the risk of MDRO pneumonia infecting a nonhospitalized patient needing empiric broad-spectrum antibiotic therapy. All included studies were prospective or retrospective observational cohort studies that performed logistic regression analyses to assess the association between MDRO isolation and the previously defined HCAP risk factors (Table 1).
Five studies examined the risk of MDRO infection in patients with a previous hospital admission of 2 days or more in the past 90 days. Shindo and colleagues found a significant increase in MDRO infections by about 2-fold (adjusted odds ratio [AOR], 2.1; 95% confidence interval [CI], 1.2-3.4).5 Shorr and colleagues found a 4-fold increase in likelihood of identifying a MDRO in HCAP (AOR, 4.2; 95% CI, 2.9-6.3).6 Nseir and colleagues and Jung and colleagues found similar results (AOR 3.9, 95% CI 1.7-8.8; AOR 2.7, 95% CI 1.3-5.5, respectively).7,8 Conflicting results were reported by Gross and colleagues who did not find a significant relationship between previous hospitalization and MDRO isolation (AOR 1.2, 95% CI, 0.5-3.2).9
In patients with pneumonia admitted from a nursing home, MDRO infection risk also was evaluated in these 5 studies. Shorr and colleagues, Nseir and colleagues, and Gross and colleagues found significant AORs of 2.7 (95% CI 1.7-4.3), 2.0 (95% CI 1.1-3.7), and 4.2 (95% CI 1.6-11.3), respectively.6,7,9 Shindo and colleagues (AOR 1.1; 95% CI, 0.6-2.0) and Jung and colleagues (AOR 1.9, 95% CI, 0.5-6.9) found this risk factor not significant.5
Receipt of antibiotics within the previous 90 days was assessed in 3 studies. Shindo and colleagues, Nseir and colleagues, and Gross and colleagues all found significant AORs of 2.5 (95% CI 1.2-4.0), 2.3 (95% CI 1.2-4.3), and 2.9 (95% CI 1.1-7.5), respectively.5,7,9 Antibiotic therapy within the previous 90 days is an established risk factor for MDRO pneumonia, and the 2016 ATS/IDSA guidelines consider this a risk factor for HAP and VAP, including pneumonia caused by methicillin resistant Staphylococcus aureus and Pseudomonas aeruginosa.4
The impact of hemodialysis in the previous month on acquisition of MDRO pneumonia was investigated in 4 studies. Shindo and colleagues, Jung and colleagues, and Gross and colleagues concluded that this risk factor was not significantly related to MDRO infection, reporting AORs of 2.2 (95% CI 0.5-9.7), 2.8 (95% CI 0.9-9.2) and 0.7 (95% CI 0.1-5.1), respectively.5,8,9 Shorr and colleagues, however, found a significant AOR of 2.1 (95% CI 1.0-4.3).6
Shindo and colleagues investigated the impact of home infusion therapy on acquisition of pneumonia due to a MDRO and reported a nonsignificant AOR of 0.8 (95% CI 0.4-1.8).5 Gross and colleagues also found a nonsignificant AOR of 0 (P = .1).9 In the Shindo and colleagues study, resistance was found in 107 of 679 patients who did not receive infusion therapy, and 12 of 55 patients who were receiving infusion therapy.5 Gross and colleagues reported that home-infusion therapy was received by 0 of 20 patients with MDRO infection and 4 of the 501 patients without MDRO infection.9
Shindo and colleagues reported that home wound care was not found to be significantly related to MDRO pneumonia as well as did Gross and colleagues: AORs of 3.8 (0.8-18.4) and 1.4 (95% CI 0.5-4.4), respectively.5,9 Jung and colleagues examined IV chemotherapy in the past 30 days, and found this to not significantly impact the odds of MDRO isolation (AOR = 0.62, 95% CI 0.2-1.8).8 No data were available reflecting the risk of a family member with a MDRO.
Limitations
The variables on which logistic regression were performed differed among the studies. Therefore, results cannot be averaged or compared quantitatively, as AORs varied, depending on the variables included. In addition, data were drawn from multiple geographic locations that may impact MDRO prevalence within each patient population. Finally, this review examines the utility of the risk factors formerly included in HCAP. However, other risk factors for MDRO pneumonia outlined by the ATS/IDSA guidelines still should be considered when evaluating patient risk. The 2016 guidelines recommend local incidence of resistant strains be considered when initiating empiric therapy. Review of medical records for previous positive cultures and duration of current hospitalization also should be considered. Although the 2016 ATS/IDSA HAP guidelines are not intended for immunosuppressed patients, this risk factor also may be taken into account.
Conclusion
Review and synthesis of published literature found previous hospital admission (of ≥ 2 days in the past 90 days), admission from a nursing home, and IV antibiotic therapy in the last 90 days to be independent risk factors for identification of MDRO pneumonia in previously nonhospitalized patients (Table 2). Additionally, although no data were found to support this risk factor, existence of an in-home (close contact) source of MDROs would provide ample opportunity for transmission, so evaluation of known exposure to MDROs from contacts should be considered. When choosing empiric antibiotic therapy for patients admitted to the hospital for treatment of pneumonia, consideration of patient history and risk factors that may contribute to infection with a MDRO are recommended.
Successful treatment of pneumonia depends on timely diagnosis and administration of antibiotics. Multidrug-resistant organisms (MDROs) complicate antibiotic therapies by rendering some antibiotic agents ineffective. Inappropriate initial therapy has been associated with a more than 2-fold increase in the risk of mortality.1 Because culture results are not available immediately, clinicians prescribe antibiotics empirically and must rely on guidelines and knowledge of risk factors associated with MDRO infection to make these selections.
Treatment guidelines exist for hospital-acquired and ventilator-associated pneumonia (HAP/VAP) and community-acquired pneumonia (CAP) to assist with empiric antibiotic selection. For HAP/VAP, 2 to 3 antibiotics with a broad-spectrum of activity are used due to increased prevalence of MDROs in hospitals, whereastreatment of CAP involves more narrow coverage because bacteria that cause this infection typically have fewer antibiotic resistances.2,3 The HAP/VAP guidelines stratify the risk of pneumonia due to the presence of a MDRO acquired during a hospitalization. However, neither the CAP nor HAP/VAP guidelines offer risk-stratification guidance for nonhospitalized patients who develop pneumonia but who may have become colonized with a MDRO during a previous hospitalization or from another exposure to a health care facility.
Health care-associated pneumonia (HCAP) was first described in the 2005 American Thoracic Society and the Infectious Diseases Society of America (ATS/IDSA) nosocomial pneumonia guidelines and was associated with criteria intended to aid clinician identification of nonhospitalized patients at risk for MDRO pneumonia, which warranted empiric broad-spectrum antibiotic therapy.2 According to these guidelines, patients were classified as having HCAP if they had been hospitalized for at least 48 hours in the past 90 days, admitted from a nursing home, received recent intravenous antibiotics, had hemodialysis in the past 30 days, had a history of home infusion therapy or wound care, received intravenous chemotherapy, or had a family member with MDRO colonization.
Since publication of the 2005 guidelines, HCAP has been criticized as being a poor predictor of MDRO infection. A 2014 meta-analysis of 24 studies investigated the discriminating ability of HCAP and reported that the specificity and sensitivity for MDRO infections was 71.2% and 53.7%, respectively.3 In 2016, the ATS/IDSA guidelines were updated to remove HCAP due to the risk of antibiotic overprescribing.4
Literature Review
Although criteria previously defining a patient as having HCAP have been shown to be a poor discriminator of MDRO pneumonia as a whole, MDRO infections still pose a threat to nonhospitalized patients who have exposure to the health care system. A literature review was performed to identify independent HCAP risk factors that may increase the risk of MDRO pneumonia infecting a nonhospitalized patient needing empiric broad-spectrum antibiotic therapy. All included studies were prospective or retrospective observational cohort studies that performed logistic regression analyses to assess the association between MDRO isolation and the previously defined HCAP risk factors (Table 1).
Five studies examined the risk of MDRO infection in patients with a previous hospital admission of 2 days or more in the past 90 days. Shindo and colleagues found a significant increase in MDRO infections by about 2-fold (adjusted odds ratio [AOR], 2.1; 95% confidence interval [CI], 1.2-3.4).5 Shorr and colleagues found a 4-fold increase in likelihood of identifying a MDRO in HCAP (AOR, 4.2; 95% CI, 2.9-6.3).6 Nseir and colleagues and Jung and colleagues found similar results (AOR 3.9, 95% CI 1.7-8.8; AOR 2.7, 95% CI 1.3-5.5, respectively).7,8 Conflicting results were reported by Gross and colleagues who did not find a significant relationship between previous hospitalization and MDRO isolation (AOR 1.2, 95% CI, 0.5-3.2).9
In patients with pneumonia admitted from a nursing home, MDRO infection risk also was evaluated in these 5 studies. Shorr and colleagues, Nseir and colleagues, and Gross and colleagues found significant AORs of 2.7 (95% CI 1.7-4.3), 2.0 (95% CI 1.1-3.7), and 4.2 (95% CI 1.6-11.3), respectively.6,7,9 Shindo and colleagues (AOR 1.1; 95% CI, 0.6-2.0) and Jung and colleagues (AOR 1.9, 95% CI, 0.5-6.9) found this risk factor not significant.5
Receipt of antibiotics within the previous 90 days was assessed in 3 studies. Shindo and colleagues, Nseir and colleagues, and Gross and colleagues all found significant AORs of 2.5 (95% CI 1.2-4.0), 2.3 (95% CI 1.2-4.3), and 2.9 (95% CI 1.1-7.5), respectively.5,7,9 Antibiotic therapy within the previous 90 days is an established risk factor for MDRO pneumonia, and the 2016 ATS/IDSA guidelines consider this a risk factor for HAP and VAP, including pneumonia caused by methicillin resistant Staphylococcus aureus and Pseudomonas aeruginosa.4
The impact of hemodialysis in the previous month on acquisition of MDRO pneumonia was investigated in 4 studies. Shindo and colleagues, Jung and colleagues, and Gross and colleagues concluded that this risk factor was not significantly related to MDRO infection, reporting AORs of 2.2 (95% CI 0.5-9.7), 2.8 (95% CI 0.9-9.2) and 0.7 (95% CI 0.1-5.1), respectively.5,8,9 Shorr and colleagues, however, found a significant AOR of 2.1 (95% CI 1.0-4.3).6
Shindo and colleagues investigated the impact of home infusion therapy on acquisition of pneumonia due to a MDRO and reported a nonsignificant AOR of 0.8 (95% CI 0.4-1.8).5 Gross and colleagues also found a nonsignificant AOR of 0 (P = .1).9 In the Shindo and colleagues study, resistance was found in 107 of 679 patients who did not receive infusion therapy, and 12 of 55 patients who were receiving infusion therapy.5 Gross and colleagues reported that home-infusion therapy was received by 0 of 20 patients with MDRO infection and 4 of the 501 patients without MDRO infection.9
Shindo and colleagues reported that home wound care was not found to be significantly related to MDRO pneumonia as well as did Gross and colleagues: AORs of 3.8 (0.8-18.4) and 1.4 (95% CI 0.5-4.4), respectively.5,9 Jung and colleagues examined IV chemotherapy in the past 30 days, and found this to not significantly impact the odds of MDRO isolation (AOR = 0.62, 95% CI 0.2-1.8).8 No data were available reflecting the risk of a family member with a MDRO.
Limitations
The variables on which logistic regression were performed differed among the studies. Therefore, results cannot be averaged or compared quantitatively, as AORs varied, depending on the variables included. In addition, data were drawn from multiple geographic locations that may impact MDRO prevalence within each patient population. Finally, this review examines the utility of the risk factors formerly included in HCAP. However, other risk factors for MDRO pneumonia outlined by the ATS/IDSA guidelines still should be considered when evaluating patient risk. The 2016 guidelines recommend local incidence of resistant strains be considered when initiating empiric therapy. Review of medical records for previous positive cultures and duration of current hospitalization also should be considered. Although the 2016 ATS/IDSA HAP guidelines are not intended for immunosuppressed patients, this risk factor also may be taken into account.
Conclusion
Review and synthesis of published literature found previous hospital admission (of ≥ 2 days in the past 90 days), admission from a nursing home, and IV antibiotic therapy in the last 90 days to be independent risk factors for identification of MDRO pneumonia in previously nonhospitalized patients (Table 2). Additionally, although no data were found to support this risk factor, existence of an in-home (close contact) source of MDROs would provide ample opportunity for transmission, so evaluation of known exposure to MDROs from contacts should be considered. When choosing empiric antibiotic therapy for patients admitted to the hospital for treatment of pneumonia, consideration of patient history and risk factors that may contribute to infection with a MDRO are recommended.
1. Kollef MH, Sherman G, Ward S, Fraser VJ. Inadequate antimicrobial treatment of infections: a risk factor for hospital mortality among critically ill patients. Chest. 1999;115(2):462-474.
2. American Thoracic Society; Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171(4):388-416.
3. Chalmers JD, Rother C, Salih W, Ewig S. Healthcare-associated pneumonia does not accurately identify potentially resistant pathogens: a systematic review and meta-analysis. Clin Infect Dis. 2014;58(3):330-339.
4. Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63(5):e61-e111.
5. Shindo Y, Ito R, Kobayashi D, et al. Risk factors for drug-resistant pathogens in community-acquired and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2013;188(8):985-995.
6. Shorr AF, Zilberberg MD, Micek ST, Kollef MH. Prediction of infection due to antibiotic-resistant bacteria by select risk factors for health care–associated pneumonia. Arch Intern Med. 2008;168(20):2205-2210.
7. Nseir S, Grailles G, Soury-Lavergne A, Minacori F, Alves I, Durocher A. Accuracy of American Thoracic Society/Infectious Diseases Society of America criteria in predicting infection or colonization with multidrug-resistant bacteria at intensive-care unit admission. Clin Microbiol Infect. 2010;16(7):902-908.
8. Jung JY, Park MS, Kim YS, et al. Healthcare-associated pneumonia among hospitalized patients in a Korean tertiary hospital. BMC Infectious Diseases. 2011;11:61.
9. Gross AE, Van Schooneveld TC, Olsen KM, et al. Epidemiology and predictors of multidrug-resistant community-acquired and health care-associated pneumonia. Antimicrob Agents Chemother. 2014;58(9):5262-5268.
1. Kollef MH, Sherman G, Ward S, Fraser VJ. Inadequate antimicrobial treatment of infections: a risk factor for hospital mortality among critically ill patients. Chest. 1999;115(2):462-474.
2. American Thoracic Society; Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171(4):388-416.
3. Chalmers JD, Rother C, Salih W, Ewig S. Healthcare-associated pneumonia does not accurately identify potentially resistant pathogens: a systematic review and meta-analysis. Clin Infect Dis. 2014;58(3):330-339.
4. Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63(5):e61-e111.
5. Shindo Y, Ito R, Kobayashi D, et al. Risk factors for drug-resistant pathogens in community-acquired and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2013;188(8):985-995.
6. Shorr AF, Zilberberg MD, Micek ST, Kollef MH. Prediction of infection due to antibiotic-resistant bacteria by select risk factors for health care–associated pneumonia. Arch Intern Med. 2008;168(20):2205-2210.
7. Nseir S, Grailles G, Soury-Lavergne A, Minacori F, Alves I, Durocher A. Accuracy of American Thoracic Society/Infectious Diseases Society of America criteria in predicting infection or colonization with multidrug-resistant bacteria at intensive-care unit admission. Clin Microbiol Infect. 2010;16(7):902-908.
8. Jung JY, Park MS, Kim YS, et al. Healthcare-associated pneumonia among hospitalized patients in a Korean tertiary hospital. BMC Infectious Diseases. 2011;11:61.
9. Gross AE, Van Schooneveld TC, Olsen KM, et al. Epidemiology and predictors of multidrug-resistant community-acquired and health care-associated pneumonia. Antimicrob Agents Chemother. 2014;58(9):5262-5268.