User login
A white spot since birth
A 13-year-old Hispanic girl came into our skin clinic with her grandmother for evaluation of suspicious moles on her arms. The grandmother was also concerned about a hypopigmented lesion on the young woman’s chest.
The patient and her grandmother said that the chest lesion had been there since birth, but it had been slowly growing over the years. The lesion was asymptomatic—there was no pruritus, bleeding, or pain. The patient was otherwise healthy and was not taking any medications.
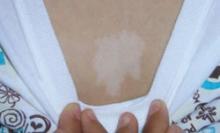
The patient and her grandmother indicated that no one in the family had a similar lesion. The patient had no fever or chills, nor any neurological, respiratory, cardiac, or gastrointestinal problems. The hypopigmented lesion on the patient’s chest had irregular borders and no scale (FIGURE 1).
There was no loss of sensation at the site and, upon applying pressure to the surrounding skin with a glass slide, the border between the lesion and normal skin disappeared.
FIGURE 1
Hypopigmented patch on chest
What is your diagnosis?
How would you manage this condition?
Diagnosis: Nevus anemicus
The patient had a nevus anemicus, which typically presents as an irregularly shaped hypopigmented patch on surrounding normal skin.1 Sometimes there are satellite macules, as well.2
Nevus anemicus is present at birth or appears shortly thereafter. It continues to grow with the child, and it remains asymptomatic. It is usually located on the trunk—primarily on the upper chest. However, there have been cases involving the extremities, head, and neck.3 The prevalence of this condition in the United States is unknown, but it is not considered rare. It is more common in females.4
Although nevus anemicus is an isolated finding in normal healthy individuals, it may occur in association with genodermatoses such as neurofibromatosis, and in conjunction with nevus flammeus and Mongolian spot in phakomatosis pigmentovascularis.1
Not a true nevus
This lesion is not a true nevus; rather, it is a congenital vascular anomaly with localized hypersensitivity to catecholamines. The vasoconstriction caused by this hypersensitivity results in skin pallor. When pressure is applied to the surrounding skin (diascopy), the border between the nevus and surrounding skin is lost due to blanching of surrounding skin.5
Intralesional injection of vasodilators, such as bradykinin, pilocarpine, acetylcholine, 5-hydroxytryptamine, nicotine, or histamine, fails to produce vasodilation in the affected areas.6 Axillary sympathetic block and intralesional injection of α-adrenergic blocking agents result in erythema.3 These findings support the conclusion that it is not a true nevus, but rather a vascular anomaly.
Differential Dx includes infectious diseases
The differential includes the following:
- Hansen’s disease (leprosy), caused by Mycobacterium leprae, presents with a loss of sensation at the site of hypopigmentation. This loss of sensation is due to nerve involvement. Histopathology yields a very specific pattern of epithelioid cell granulomas around the dermal nerves.7
- Tinea versicolor, caused by Malassezia furfur, has a fine scale on the hypopigmented patch. The lesion fluoresces under a Wood’s lamp, and a potassium hydroxide (KOH) preparation will be positive, revealing the well-known “spaghetti and meatballs” pattern. Hypopigmentation in tinea versicolor results from the inhibition of the enzyme tyrosinase in the melanocytes.7
- Vitiligo results from the complete absence of melanocytes. Vitiligo is rarely present at birth.
- Nevus depigmentosus, also known as nevus achromicus, is a well-demarcated patch of hypopigmentation that tends to occur on the trunk or proximal extremities in a dermatomal pattern (FIGURE 2). It is a true nevus and diascopy will not result in the loss of the border between the hypopigmented patch and surrounding skin.5
FIGURE 2
Don’t be fooled: This is not nevus anemicus
Make the diagnosis based on the exam
The diagnosis of nevus anemicus is made primarily based on the history and exam. A number of techniques aid in confirming the diagnosis and excluding some of the diagnoses mentioned above.
Diascopy results in the loss of the border between nevus anemicus and normal skin.4,5 Anatomic nevi do not demonstrate this loss in border. Shining a Wood’s lamp does not accentuate the lesion, helping to distinguish nevus anemicus from fungal infections that tend to fluoresce.
Neither friction (produced by scratching a line across both the lesion and normal surrounding skin), nor cold or heat application, induces erythema in the involved areas.2 And unlike leprosy, there is no loss of sensation at the site of the hypopigmentation. A biopsy of the lesion is not needed, but would reveal normal histology. Melanocytes are preserved and normally distributed. Electron microscopy, while not needed, would not detect any abnormalities in the vascular structure.4
Nothing to worry about for our patient
Our patient required no treatment. We simply provided her with some information on the benign nature of nevus anemicus. (In addition, we dealt with the moles on her arms that prompted her visit. They turned out to be normal melanocytic nevi.)
We told the patient that if the lesion on her chest bothered her, she could hide it with concealer make-up.1,2 Our patient and her grandmother were happy with the explanation and did not seek further care.
CORRESPONDENCE
Shehnaz Zaman, MD, 420 Elmington Avenue, #417, Nashville, TN 37205; [email protected]
1. Ahkami RN, Schwartz RA. Nevus anemicus. Dermatology. 1999;198:327-329.
2. Requena L, Sangueza OP. Cutaneous vascular anomalies. Part 1. Hamartomas, malformations, and dilation of preexisting vessels. J Am Acad Dermatol. 1997;37:523-549.
3. Mountcastle EA, Diestelmeier MR, Lupton GP. Nevus anemicus. J Am Acad Dermatol. 1986;14:628-632.
4. Knoepp TG, Davis L. Nevus anemicus. e Medicine Web site. Available at: http://www.emedicine.com/derm/topic292.htm. Accessed October 13, 2007.
5. Hsu S. Photo quiz: white patch on back. Am Fam Physician. 1999;60:1489-1490.
6. Greaves MW, Birkett D, Johnson C. Nevus anemicus: a unique catecholamine-dependent nevus. Arch Dermatol. 1970;102:172-176.
7. Wolff K, Johnson R, Suurmond R. Fitzpatrick’s Color Atlas & Synopsis of Clinical Dermatology. 5th ed. New York: McGraw Hill; 2005.
A 13-year-old Hispanic girl came into our skin clinic with her grandmother for evaluation of suspicious moles on her arms. The grandmother was also concerned about a hypopigmented lesion on the young woman’s chest.
The patient and her grandmother said that the chest lesion had been there since birth, but it had been slowly growing over the years. The lesion was asymptomatic—there was no pruritus, bleeding, or pain. The patient was otherwise healthy and was not taking any medications.
The patient and her grandmother indicated that no one in the family had a similar lesion. The patient had no fever or chills, nor any neurological, respiratory, cardiac, or gastrointestinal problems. The hypopigmented lesion on the patient’s chest had irregular borders and no scale (FIGURE 1).
There was no loss of sensation at the site and, upon applying pressure to the surrounding skin with a glass slide, the border between the lesion and normal skin disappeared.
FIGURE 1
Hypopigmented patch on chest
What is your diagnosis?
How would you manage this condition?
Diagnosis: Nevus anemicus
The patient had a nevus anemicus, which typically presents as an irregularly shaped hypopigmented patch on surrounding normal skin.1 Sometimes there are satellite macules, as well.2
Nevus anemicus is present at birth or appears shortly thereafter. It continues to grow with the child, and it remains asymptomatic. It is usually located on the trunk—primarily on the upper chest. However, there have been cases involving the extremities, head, and neck.3 The prevalence of this condition in the United States is unknown, but it is not considered rare. It is more common in females.4
Although nevus anemicus is an isolated finding in normal healthy individuals, it may occur in association with genodermatoses such as neurofibromatosis, and in conjunction with nevus flammeus and Mongolian spot in phakomatosis pigmentovascularis.1
Not a true nevus
This lesion is not a true nevus; rather, it is a congenital vascular anomaly with localized hypersensitivity to catecholamines. The vasoconstriction caused by this hypersensitivity results in skin pallor. When pressure is applied to the surrounding skin (diascopy), the border between the nevus and surrounding skin is lost due to blanching of surrounding skin.5
Intralesional injection of vasodilators, such as bradykinin, pilocarpine, acetylcholine, 5-hydroxytryptamine, nicotine, or histamine, fails to produce vasodilation in the affected areas.6 Axillary sympathetic block and intralesional injection of α-adrenergic blocking agents result in erythema.3 These findings support the conclusion that it is not a true nevus, but rather a vascular anomaly.
Differential Dx includes infectious diseases
The differential includes the following:
- Hansen’s disease (leprosy), caused by Mycobacterium leprae, presents with a loss of sensation at the site of hypopigmentation. This loss of sensation is due to nerve involvement. Histopathology yields a very specific pattern of epithelioid cell granulomas around the dermal nerves.7
- Tinea versicolor, caused by Malassezia furfur, has a fine scale on the hypopigmented patch. The lesion fluoresces under a Wood’s lamp, and a potassium hydroxide (KOH) preparation will be positive, revealing the well-known “spaghetti and meatballs” pattern. Hypopigmentation in tinea versicolor results from the inhibition of the enzyme tyrosinase in the melanocytes.7
- Vitiligo results from the complete absence of melanocytes. Vitiligo is rarely present at birth.
- Nevus depigmentosus, also known as nevus achromicus, is a well-demarcated patch of hypopigmentation that tends to occur on the trunk or proximal extremities in a dermatomal pattern (FIGURE 2). It is a true nevus and diascopy will not result in the loss of the border between the hypopigmented patch and surrounding skin.5
FIGURE 2
Don’t be fooled: This is not nevus anemicus
Make the diagnosis based on the exam
The diagnosis of nevus anemicus is made primarily based on the history and exam. A number of techniques aid in confirming the diagnosis and excluding some of the diagnoses mentioned above.
Diascopy results in the loss of the border between nevus anemicus and normal skin.4,5 Anatomic nevi do not demonstrate this loss in border. Shining a Wood’s lamp does not accentuate the lesion, helping to distinguish nevus anemicus from fungal infections that tend to fluoresce.
Neither friction (produced by scratching a line across both the lesion and normal surrounding skin), nor cold or heat application, induces erythema in the involved areas.2 And unlike leprosy, there is no loss of sensation at the site of the hypopigmentation. A biopsy of the lesion is not needed, but would reveal normal histology. Melanocytes are preserved and normally distributed. Electron microscopy, while not needed, would not detect any abnormalities in the vascular structure.4
Nothing to worry about for our patient
Our patient required no treatment. We simply provided her with some information on the benign nature of nevus anemicus. (In addition, we dealt with the moles on her arms that prompted her visit. They turned out to be normal melanocytic nevi.)
We told the patient that if the lesion on her chest bothered her, she could hide it with concealer make-up.1,2 Our patient and her grandmother were happy with the explanation and did not seek further care.
CORRESPONDENCE
Shehnaz Zaman, MD, 420 Elmington Avenue, #417, Nashville, TN 37205; [email protected]
A 13-year-old Hispanic girl came into our skin clinic with her grandmother for evaluation of suspicious moles on her arms. The grandmother was also concerned about a hypopigmented lesion on the young woman’s chest.
The patient and her grandmother said that the chest lesion had been there since birth, but it had been slowly growing over the years. The lesion was asymptomatic—there was no pruritus, bleeding, or pain. The patient was otherwise healthy and was not taking any medications.
The patient and her grandmother indicated that no one in the family had a similar lesion. The patient had no fever or chills, nor any neurological, respiratory, cardiac, or gastrointestinal problems. The hypopigmented lesion on the patient’s chest had irregular borders and no scale (FIGURE 1).
There was no loss of sensation at the site and, upon applying pressure to the surrounding skin with a glass slide, the border between the lesion and normal skin disappeared.
FIGURE 1
Hypopigmented patch on chest
What is your diagnosis?
How would you manage this condition?
Diagnosis: Nevus anemicus
The patient had a nevus anemicus, which typically presents as an irregularly shaped hypopigmented patch on surrounding normal skin.1 Sometimes there are satellite macules, as well.2
Nevus anemicus is present at birth or appears shortly thereafter. It continues to grow with the child, and it remains asymptomatic. It is usually located on the trunk—primarily on the upper chest. However, there have been cases involving the extremities, head, and neck.3 The prevalence of this condition in the United States is unknown, but it is not considered rare. It is more common in females.4
Although nevus anemicus is an isolated finding in normal healthy individuals, it may occur in association with genodermatoses such as neurofibromatosis, and in conjunction with nevus flammeus and Mongolian spot in phakomatosis pigmentovascularis.1
Not a true nevus
This lesion is not a true nevus; rather, it is a congenital vascular anomaly with localized hypersensitivity to catecholamines. The vasoconstriction caused by this hypersensitivity results in skin pallor. When pressure is applied to the surrounding skin (diascopy), the border between the nevus and surrounding skin is lost due to blanching of surrounding skin.5
Intralesional injection of vasodilators, such as bradykinin, pilocarpine, acetylcholine, 5-hydroxytryptamine, nicotine, or histamine, fails to produce vasodilation in the affected areas.6 Axillary sympathetic block and intralesional injection of α-adrenergic blocking agents result in erythema.3 These findings support the conclusion that it is not a true nevus, but rather a vascular anomaly.
Differential Dx includes infectious diseases
The differential includes the following:
- Hansen’s disease (leprosy), caused by Mycobacterium leprae, presents with a loss of sensation at the site of hypopigmentation. This loss of sensation is due to nerve involvement. Histopathology yields a very specific pattern of epithelioid cell granulomas around the dermal nerves.7
- Tinea versicolor, caused by Malassezia furfur, has a fine scale on the hypopigmented patch. The lesion fluoresces under a Wood’s lamp, and a potassium hydroxide (KOH) preparation will be positive, revealing the well-known “spaghetti and meatballs” pattern. Hypopigmentation in tinea versicolor results from the inhibition of the enzyme tyrosinase in the melanocytes.7
- Vitiligo results from the complete absence of melanocytes. Vitiligo is rarely present at birth.
- Nevus depigmentosus, also known as nevus achromicus, is a well-demarcated patch of hypopigmentation that tends to occur on the trunk or proximal extremities in a dermatomal pattern (FIGURE 2). It is a true nevus and diascopy will not result in the loss of the border between the hypopigmented patch and surrounding skin.5
FIGURE 2
Don’t be fooled: This is not nevus anemicus
Make the diagnosis based on the exam
The diagnosis of nevus anemicus is made primarily based on the history and exam. A number of techniques aid in confirming the diagnosis and excluding some of the diagnoses mentioned above.
Diascopy results in the loss of the border between nevus anemicus and normal skin.4,5 Anatomic nevi do not demonstrate this loss in border. Shining a Wood’s lamp does not accentuate the lesion, helping to distinguish nevus anemicus from fungal infections that tend to fluoresce.
Neither friction (produced by scratching a line across both the lesion and normal surrounding skin), nor cold or heat application, induces erythema in the involved areas.2 And unlike leprosy, there is no loss of sensation at the site of the hypopigmentation. A biopsy of the lesion is not needed, but would reveal normal histology. Melanocytes are preserved and normally distributed. Electron microscopy, while not needed, would not detect any abnormalities in the vascular structure.4
Nothing to worry about for our patient
Our patient required no treatment. We simply provided her with some information on the benign nature of nevus anemicus. (In addition, we dealt with the moles on her arms that prompted her visit. They turned out to be normal melanocytic nevi.)
We told the patient that if the lesion on her chest bothered her, she could hide it with concealer make-up.1,2 Our patient and her grandmother were happy with the explanation and did not seek further care.
CORRESPONDENCE
Shehnaz Zaman, MD, 420 Elmington Avenue, #417, Nashville, TN 37205; [email protected]
1. Ahkami RN, Schwartz RA. Nevus anemicus. Dermatology. 1999;198:327-329.
2. Requena L, Sangueza OP. Cutaneous vascular anomalies. Part 1. Hamartomas, malformations, and dilation of preexisting vessels. J Am Acad Dermatol. 1997;37:523-549.
3. Mountcastle EA, Diestelmeier MR, Lupton GP. Nevus anemicus. J Am Acad Dermatol. 1986;14:628-632.
4. Knoepp TG, Davis L. Nevus anemicus. e Medicine Web site. Available at: http://www.emedicine.com/derm/topic292.htm. Accessed October 13, 2007.
5. Hsu S. Photo quiz: white patch on back. Am Fam Physician. 1999;60:1489-1490.
6. Greaves MW, Birkett D, Johnson C. Nevus anemicus: a unique catecholamine-dependent nevus. Arch Dermatol. 1970;102:172-176.
7. Wolff K, Johnson R, Suurmond R. Fitzpatrick’s Color Atlas & Synopsis of Clinical Dermatology. 5th ed. New York: McGraw Hill; 2005.
1. Ahkami RN, Schwartz RA. Nevus anemicus. Dermatology. 1999;198:327-329.
2. Requena L, Sangueza OP. Cutaneous vascular anomalies. Part 1. Hamartomas, malformations, and dilation of preexisting vessels. J Am Acad Dermatol. 1997;37:523-549.
3. Mountcastle EA, Diestelmeier MR, Lupton GP. Nevus anemicus. J Am Acad Dermatol. 1986;14:628-632.
4. Knoepp TG, Davis L. Nevus anemicus. e Medicine Web site. Available at: http://www.emedicine.com/derm/topic292.htm. Accessed October 13, 2007.
5. Hsu S. Photo quiz: white patch on back. Am Fam Physician. 1999;60:1489-1490.
6. Greaves MW, Birkett D, Johnson C. Nevus anemicus: a unique catecholamine-dependent nevus. Arch Dermatol. 1970;102:172-176.
7. Wolff K, Johnson R, Suurmond R. Fitzpatrick’s Color Atlas & Synopsis of Clinical Dermatology. 5th ed. New York: McGraw Hill; 2005.
Lesions between breasts
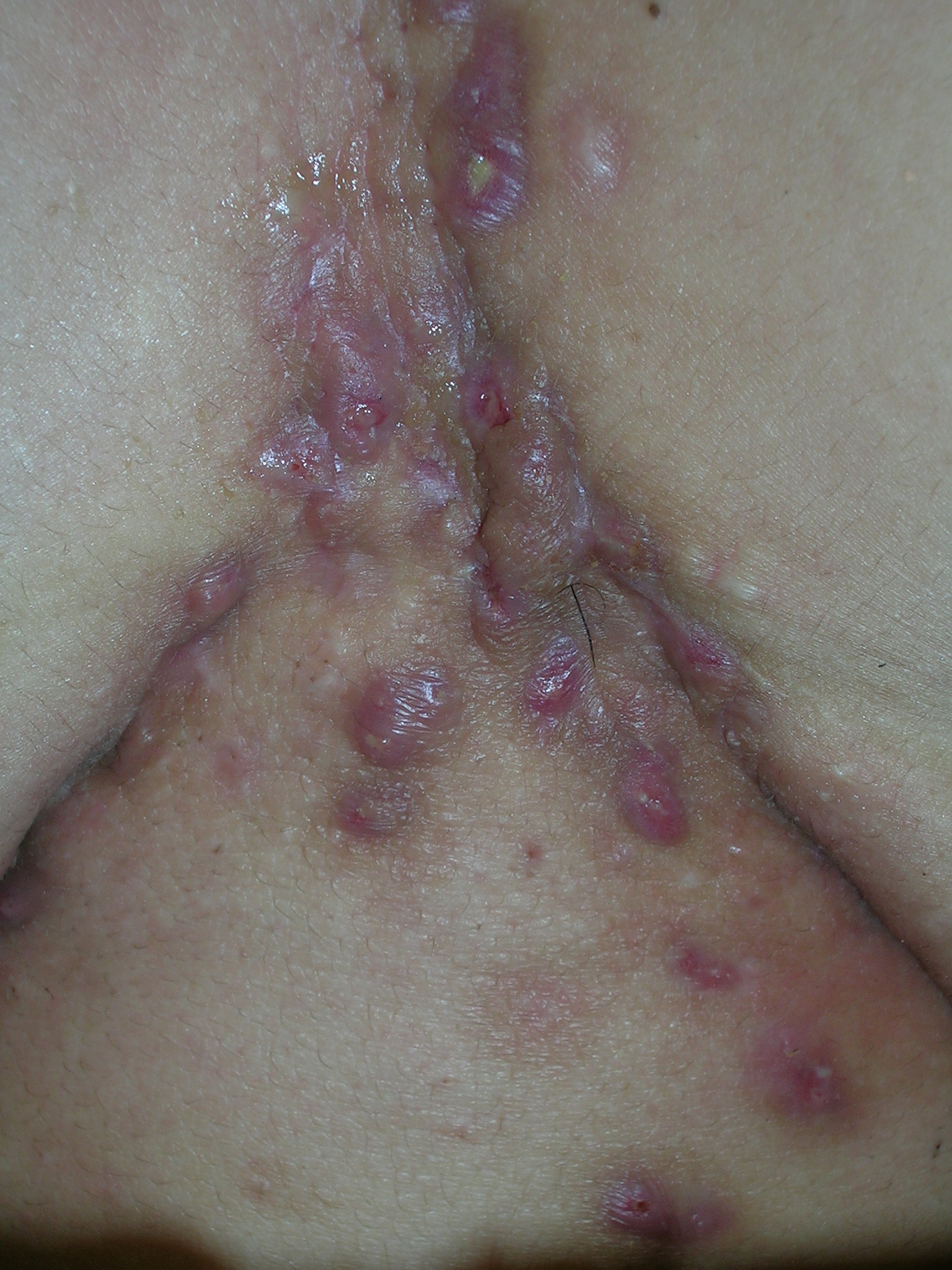
We diagnosed hidradenitis suppurativa, a disorder of the terminal follicular epithelium in the apocrine gland–bearing skin. It causes chronic relapsing inflammation with mucopurulent discharge. As seen in this case, it can lead to sinus tracts, draining fistulas, and progressive scarring. It is called acne inversa because it involves intertriginous localizations and not the regions affected by acne (face and back). The most common presentation is painful, tender, firm, nodular lesions in axillae. This patient had very little axillary involvement, but the diagnosis was still hidradenitis. Both obesity and smoking make the condition worse.
Hidradenitis suppurativa can cause disabling pain and social isolation. The patient was desperate for relief. She chose to have intralesional steroid injections for the 3 most painful nodules. We injected the nodules with 10 mg/cc triamcinolone and started the patient on doxycycline 100 mg bid. We stressed the importance of smoking cessation, but the patient did not think she could quit.
Two months later, the patient was still miserable, with multiple tender nodules between and under her breasts. We discussed the options of isotretinoin or surgery; she elected to receive a plastic surgery referral. Six months later the patient returned to the office with new painful nodules in the same area but now adjacent to the large surgical scars. Unfortunately, we still lack a good, long-lasting treatment for severe cases of hidradenitis suppurativa.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Usatine R. Hidradenitis suppurativa. In: Usatine R, Smith M, Mayeaux EJ, Chumley H, Tysinger J, eds. The Color Atlas of Family Medicine. New York, NY: McGraw-Hill; 2009:457-460.
To learn more about The Color Atlas of Family Medicine, see:
* http://www.amazon.com/Color-Atlas-Family-Medicine/dp/0071474641

We diagnosed hidradenitis suppurativa, a disorder of the terminal follicular epithelium in the apocrine gland–bearing skin. It causes chronic relapsing inflammation with mucopurulent discharge. As seen in this case, it can lead to sinus tracts, draining fistulas, and progressive scarring. It is called acne inversa because it involves intertriginous localizations and not the regions affected by acne (face and back). The most common presentation is painful, tender, firm, nodular lesions in axillae. This patient had very little axillary involvement, but the diagnosis was still hidradenitis. Both obesity and smoking make the condition worse.
Hidradenitis suppurativa can cause disabling pain and social isolation. The patient was desperate for relief. She chose to have intralesional steroid injections for the 3 most painful nodules. We injected the nodules with 10 mg/cc triamcinolone and started the patient on doxycycline 100 mg bid. We stressed the importance of smoking cessation, but the patient did not think she could quit.
Two months later, the patient was still miserable, with multiple tender nodules between and under her breasts. We discussed the options of isotretinoin or surgery; she elected to receive a plastic surgery referral. Six months later the patient returned to the office with new painful nodules in the same area but now adjacent to the large surgical scars. Unfortunately, we still lack a good, long-lasting treatment for severe cases of hidradenitis suppurativa.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Usatine R. Hidradenitis suppurativa. In: Usatine R, Smith M, Mayeaux EJ, Chumley H, Tysinger J, eds. The Color Atlas of Family Medicine. New York, NY: McGraw-Hill; 2009:457-460.
To learn more about The Color Atlas of Family Medicine, see:
* http://www.amazon.com/Color-Atlas-Family-Medicine/dp/0071474641

We diagnosed hidradenitis suppurativa, a disorder of the terminal follicular epithelium in the apocrine gland–bearing skin. It causes chronic relapsing inflammation with mucopurulent discharge. As seen in this case, it can lead to sinus tracts, draining fistulas, and progressive scarring. It is called acne inversa because it involves intertriginous localizations and not the regions affected by acne (face and back). The most common presentation is painful, tender, firm, nodular lesions in axillae. This patient had very little axillary involvement, but the diagnosis was still hidradenitis. Both obesity and smoking make the condition worse.
Hidradenitis suppurativa can cause disabling pain and social isolation. The patient was desperate for relief. She chose to have intralesional steroid injections for the 3 most painful nodules. We injected the nodules with 10 mg/cc triamcinolone and started the patient on doxycycline 100 mg bid. We stressed the importance of smoking cessation, but the patient did not think she could quit.
Two months later, the patient was still miserable, with multiple tender nodules between and under her breasts. We discussed the options of isotretinoin or surgery; she elected to receive a plastic surgery referral. Six months later the patient returned to the office with new painful nodules in the same area but now adjacent to the large surgical scars. Unfortunately, we still lack a good, long-lasting treatment for severe cases of hidradenitis suppurativa.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Usatine R. Hidradenitis suppurativa. In: Usatine R, Smith M, Mayeaux EJ, Chumley H, Tysinger J, eds. The Color Atlas of Family Medicine. New York, NY: McGraw-Hill; 2009:457-460.
To learn more about The Color Atlas of Family Medicine, see:
* http://www.amazon.com/Color-Atlas-Family-Medicine/dp/0071474641
Bumps on face
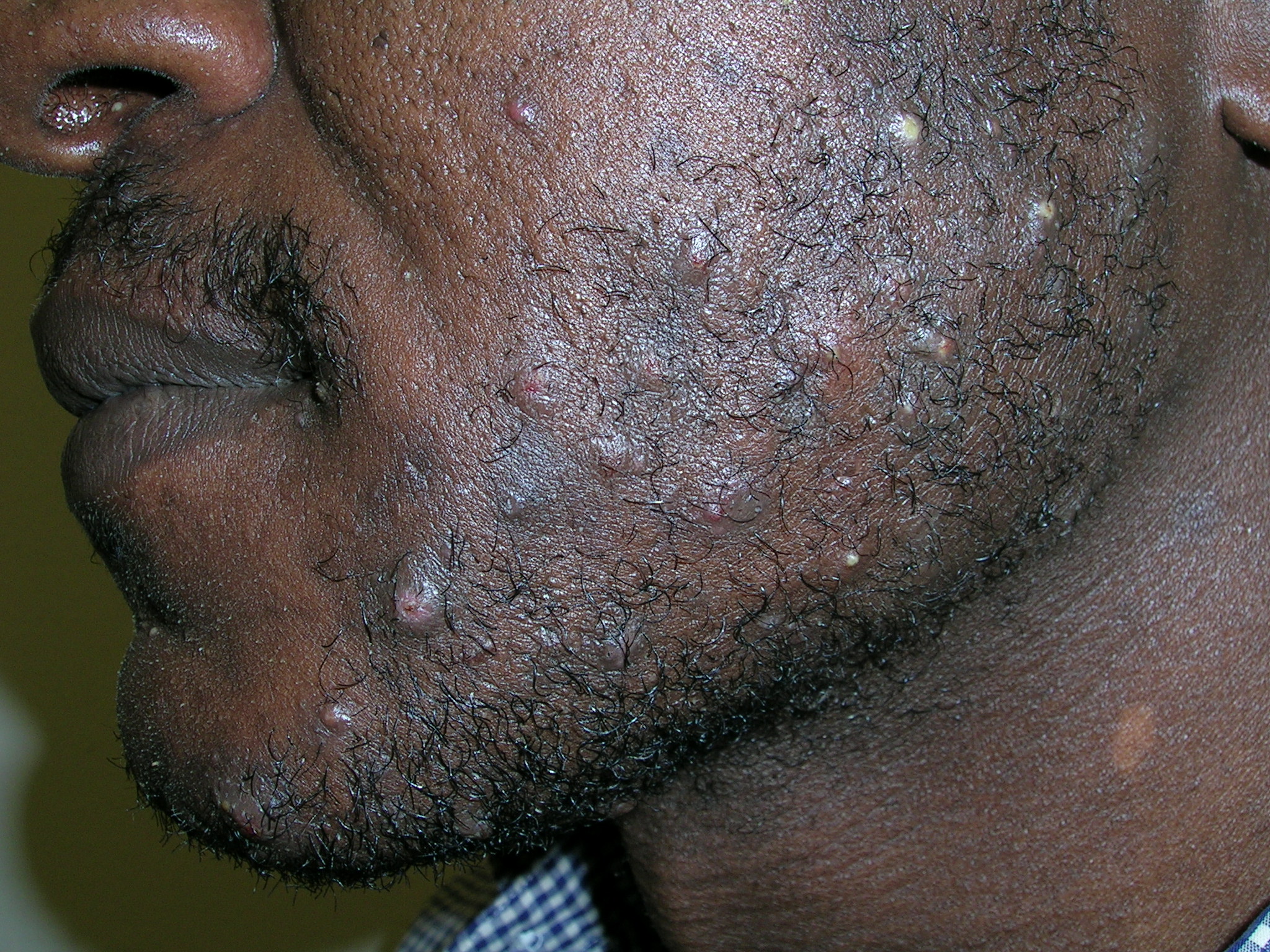
We diagnosed pseudofolliculitis (razor bumps or shave bumps).
Pseudofolliculitis is a common skin condition affecting the hair bearing areas of the body that are shaved. It is most common in black men; at least half of black men who shave are prone to the condition.
When this condition occurs in the beard area, it is called pseudofolliculitis barbae; when it occurs after pubic hair is shaved, it is called pseudofolliculitis pubis. Pseudofolliculitis develops when, after shaving, the free end of tightly coiled hair reenters the skin, causing a foreign-body-like inflammatory reaction. Shaving produces a sharp free end below the skin surface. Tightly curled hair has a greater tendency to pierce the follicle and the surface of the skin, explaining the relative predominance of this condition in patients of African descent.
We told the patient that the less he shaved the better his skin would become. We instructed him to search for ingrown hairs daily by using a magnifying mirror, and to release them gently with a sterilized needle or tweezers. We also treated him with nightly tretinoin cream to the beard area. We told him that he could safely use 1% hydrocortisone cream to the face as needed for inflammation and itching. On a follow-up visit 2 months later, his skin was significantly improved.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux, EJ. Pseudofolliculitis and acne keloidalis nuchae. In: Usatine R, Smith M, Mayeaux EJ, Chumley H, Tysinger J, eds. The Color Atlas of Family Medicine. New York, NY: McGraw-Hill; 2009:453-456.
To learn more about The Color Atlas of Family Medicine, see:
* http://www.amazon.com/Color-Atlas-Family-Medicine/dp/0071474641

We diagnosed pseudofolliculitis (razor bumps or shave bumps).
Pseudofolliculitis is a common skin condition affecting the hair bearing areas of the body that are shaved. It is most common in black men; at least half of black men who shave are prone to the condition.
When this condition occurs in the beard area, it is called pseudofolliculitis barbae; when it occurs after pubic hair is shaved, it is called pseudofolliculitis pubis. Pseudofolliculitis develops when, after shaving, the free end of tightly coiled hair reenters the skin, causing a foreign-body-like inflammatory reaction. Shaving produces a sharp free end below the skin surface. Tightly curled hair has a greater tendency to pierce the follicle and the surface of the skin, explaining the relative predominance of this condition in patients of African descent.
We told the patient that the less he shaved the better his skin would become. We instructed him to search for ingrown hairs daily by using a magnifying mirror, and to release them gently with a sterilized needle or tweezers. We also treated him with nightly tretinoin cream to the beard area. We told him that he could safely use 1% hydrocortisone cream to the face as needed for inflammation and itching. On a follow-up visit 2 months later, his skin was significantly improved.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux, EJ. Pseudofolliculitis and acne keloidalis nuchae. In: Usatine R, Smith M, Mayeaux EJ, Chumley H, Tysinger J, eds. The Color Atlas of Family Medicine. New York, NY: McGraw-Hill; 2009:453-456.
To learn more about The Color Atlas of Family Medicine, see:
* http://www.amazon.com/Color-Atlas-Family-Medicine/dp/0071474641

We diagnosed pseudofolliculitis (razor bumps or shave bumps).
Pseudofolliculitis is a common skin condition affecting the hair bearing areas of the body that are shaved. It is most common in black men; at least half of black men who shave are prone to the condition.
When this condition occurs in the beard area, it is called pseudofolliculitis barbae; when it occurs after pubic hair is shaved, it is called pseudofolliculitis pubis. Pseudofolliculitis develops when, after shaving, the free end of tightly coiled hair reenters the skin, causing a foreign-body-like inflammatory reaction. Shaving produces a sharp free end below the skin surface. Tightly curled hair has a greater tendency to pierce the follicle and the surface of the skin, explaining the relative predominance of this condition in patients of African descent.
We told the patient that the less he shaved the better his skin would become. We instructed him to search for ingrown hairs daily by using a magnifying mirror, and to release them gently with a sterilized needle or tweezers. We also treated him with nightly tretinoin cream to the beard area. We told him that he could safely use 1% hydrocortisone cream to the face as needed for inflammation and itching. On a follow-up visit 2 months later, his skin was significantly improved.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux, EJ. Pseudofolliculitis and acne keloidalis nuchae. In: Usatine R, Smith M, Mayeaux EJ, Chumley H, Tysinger J, eds. The Color Atlas of Family Medicine. New York, NY: McGraw-Hill; 2009:453-456.
To learn more about The Color Atlas of Family Medicine, see:
* http://www.amazon.com/Color-Atlas-Family-Medicine/dp/0071474641
Bumps on nose
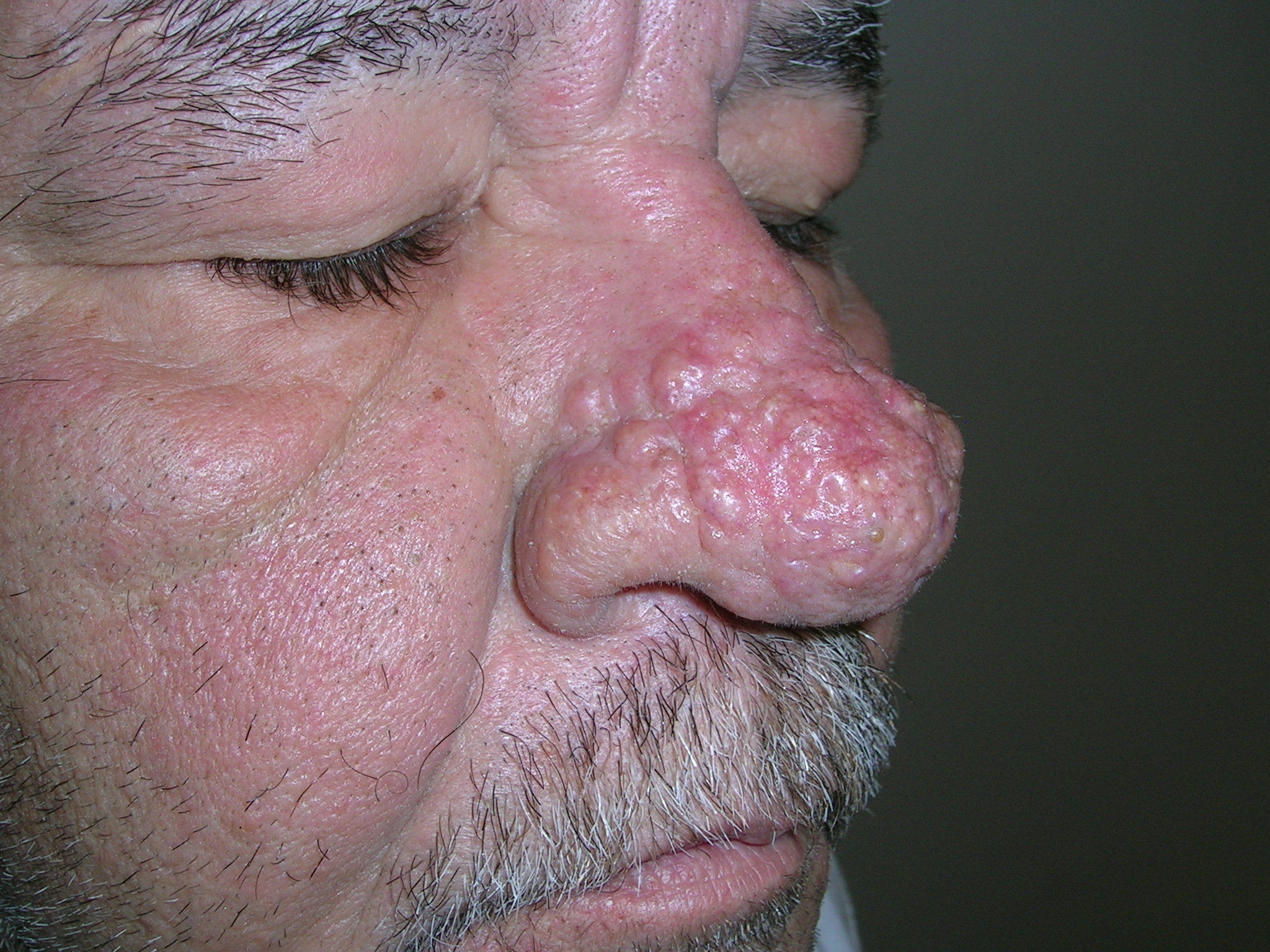
We diagnosed rhinophymatous rosacea causing hypertrophy of the sebaceous glands of the nose. In this case, the patient did not have other stigmata of rosacea such as erythema and telangiectasias on the cheeks. While this is often called a “W.C. Field's nose,” it is not necessarily related to heavy alcohol use.
We started the patient on oral tetracycline 500 mg PO bid and topical metronidazole gel 1% bid. The patient noted some improvement in the redness of his nose, but the enlarged appearance still bothered him. We discussed radiofrequency electrosurgery and he chose to go ahead with this treatment.
His nose was anesthetized with 1% lidocaine and epinephrine and an electrosurgical loop was used to pare down the hyperplastic sebaceous glands and skin. The patient tolerated the procedure well. He then applied petrolatum to the healing nose twice daily. The patient was pleased with the outcome.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Usatine R. Rosacea. In: Usatine R, Smith M, Mayeaux EJ, Chumley H, Tysinger J, eds. The Color Atlas of Family Medicine. New York, NY: McGraw-Hill; 2009:447-452.
To learn more about The Color Atlas of Family Medicine, see:
* http://www.amazon.com/Color-Atlas-Family-Medicine/dp/0071474641

We diagnosed rhinophymatous rosacea causing hypertrophy of the sebaceous glands of the nose. In this case, the patient did not have other stigmata of rosacea such as erythema and telangiectasias on the cheeks. While this is often called a “W.C. Field's nose,” it is not necessarily related to heavy alcohol use.
We started the patient on oral tetracycline 500 mg PO bid and topical metronidazole gel 1% bid. The patient noted some improvement in the redness of his nose, but the enlarged appearance still bothered him. We discussed radiofrequency electrosurgery and he chose to go ahead with this treatment.
His nose was anesthetized with 1% lidocaine and epinephrine and an electrosurgical loop was used to pare down the hyperplastic sebaceous glands and skin. The patient tolerated the procedure well. He then applied petrolatum to the healing nose twice daily. The patient was pleased with the outcome.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Usatine R. Rosacea. In: Usatine R, Smith M, Mayeaux EJ, Chumley H, Tysinger J, eds. The Color Atlas of Family Medicine. New York, NY: McGraw-Hill; 2009:447-452.
To learn more about The Color Atlas of Family Medicine, see:
* http://www.amazon.com/Color-Atlas-Family-Medicine/dp/0071474641

We diagnosed rhinophymatous rosacea causing hypertrophy of the sebaceous glands of the nose. In this case, the patient did not have other stigmata of rosacea such as erythema and telangiectasias on the cheeks. While this is often called a “W.C. Field's nose,” it is not necessarily related to heavy alcohol use.
We started the patient on oral tetracycline 500 mg PO bid and topical metronidazole gel 1% bid. The patient noted some improvement in the redness of his nose, but the enlarged appearance still bothered him. We discussed radiofrequency electrosurgery and he chose to go ahead with this treatment.
His nose was anesthetized with 1% lidocaine and epinephrine and an electrosurgical loop was used to pare down the hyperplastic sebaceous glands and skin. The patient tolerated the procedure well. He then applied petrolatum to the healing nose twice daily. The patient was pleased with the outcome.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Usatine R. Rosacea. In: Usatine R, Smith M, Mayeaux EJ, Chumley H, Tysinger J, eds. The Color Atlas of Family Medicine. New York, NY: McGraw-Hill; 2009:447-452.
To learn more about The Color Atlas of Family Medicine, see:
* http://www.amazon.com/Color-Atlas-Family-Medicine/dp/0071474641
Large red mass on face
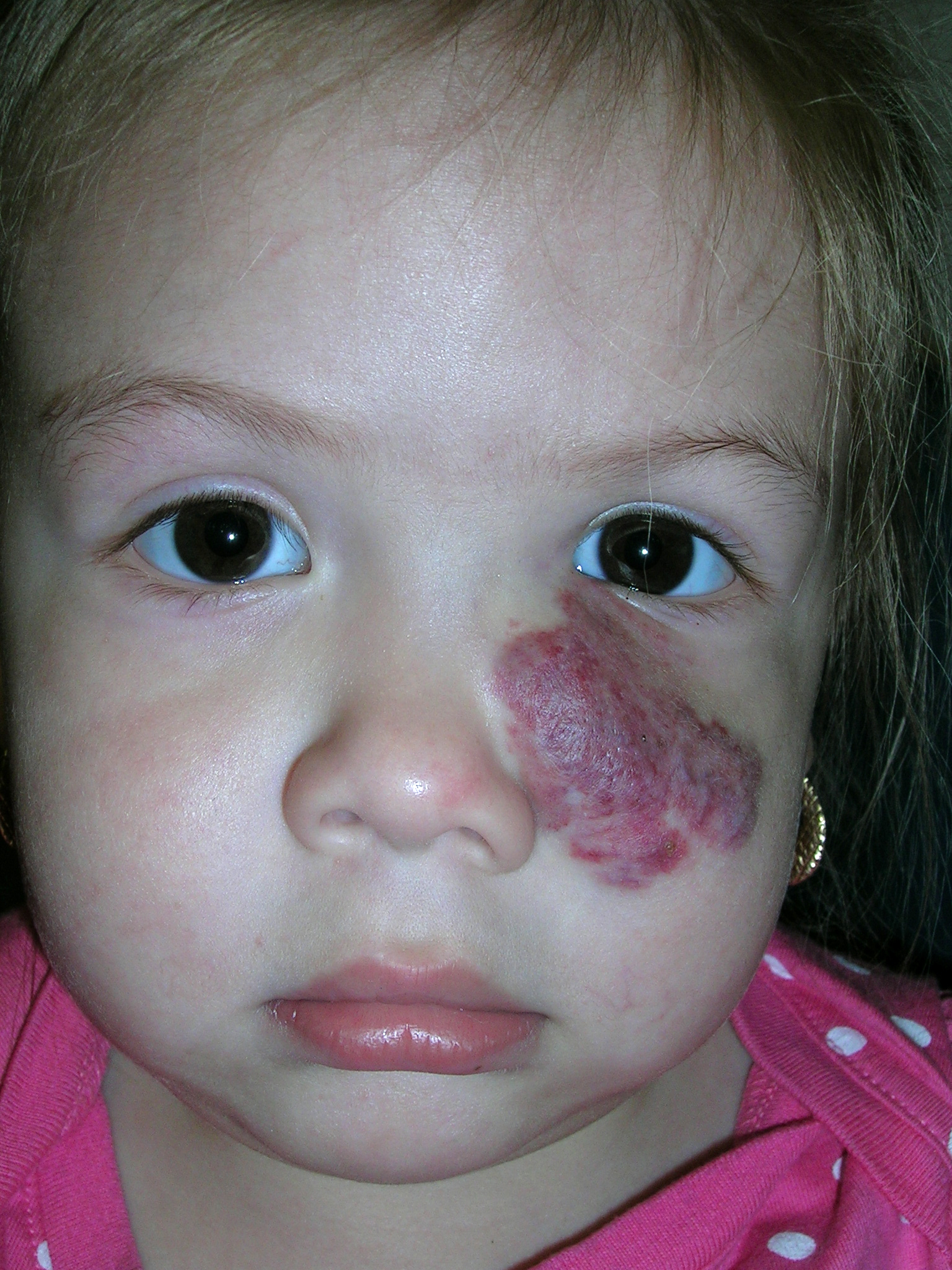
We diagnosed a strawberry hemangioma. Hemangiomas are the most common benign tumor of infancy and consist of an abnormally dense group of dilated blood vessels. While 30% are present at birth, the other 70% appear within the first few weeks of life. For some unknown reason, females are affected more often than males.
We told the mom that half of childhood hemangiomas completely involute by age 5, 70% by age 7, and the remainder take an additional 3 to 5 years to complete the process of involution. Of the lesions that have involuted by age 6, 38% will leave residual evidence of the hemangioma in the form of a scar, telangiectasia, or redundant, “bag-like” skin. The chance of a permanent scar increases the longer it takes to involute.
Fortunately the child had no problems with her vision. She had already been referred to an ophthalmologist, who confirmed that no treatment was needed despite the hemangioma’s proximity to the eye. For hemangiomas that block vision or other important functional organs, treatment options include oral propranolol, oral steroids, and laser therapy.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Madhukar M, Usatine R. Childhood hemangiomas. In: Usatine R, Smith M, Mayeaux EJ, Chumley H, Tysinger J, eds. The Color Atlas of Family Medicine. New York, NY: McGraw-Hill; 2009:427-431.
To learn more about The Color Atlas of Family Medicine, see:
* http://www.amazon.com/Color-Atlas-Family-Medicine/dp/0071474641

We diagnosed a strawberry hemangioma. Hemangiomas are the most common benign tumor of infancy and consist of an abnormally dense group of dilated blood vessels. While 30% are present at birth, the other 70% appear within the first few weeks of life. For some unknown reason, females are affected more often than males.
We told the mom that half of childhood hemangiomas completely involute by age 5, 70% by age 7, and the remainder take an additional 3 to 5 years to complete the process of involution. Of the lesions that have involuted by age 6, 38% will leave residual evidence of the hemangioma in the form of a scar, telangiectasia, or redundant, “bag-like” skin. The chance of a permanent scar increases the longer it takes to involute.
Fortunately the child had no problems with her vision. She had already been referred to an ophthalmologist, who confirmed that no treatment was needed despite the hemangioma’s proximity to the eye. For hemangiomas that block vision or other important functional organs, treatment options include oral propranolol, oral steroids, and laser therapy.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Madhukar M, Usatine R. Childhood hemangiomas. In: Usatine R, Smith M, Mayeaux EJ, Chumley H, Tysinger J, eds. The Color Atlas of Family Medicine. New York, NY: McGraw-Hill; 2009:427-431.
To learn more about The Color Atlas of Family Medicine, see:
* http://www.amazon.com/Color-Atlas-Family-Medicine/dp/0071474641

We diagnosed a strawberry hemangioma. Hemangiomas are the most common benign tumor of infancy and consist of an abnormally dense group of dilated blood vessels. While 30% are present at birth, the other 70% appear within the first few weeks of life. For some unknown reason, females are affected more often than males.
We told the mom that half of childhood hemangiomas completely involute by age 5, 70% by age 7, and the remainder take an additional 3 to 5 years to complete the process of involution. Of the lesions that have involuted by age 6, 38% will leave residual evidence of the hemangioma in the form of a scar, telangiectasia, or redundant, “bag-like” skin. The chance of a permanent scar increases the longer it takes to involute.
Fortunately the child had no problems with her vision. She had already been referred to an ophthalmologist, who confirmed that no treatment was needed despite the hemangioma’s proximity to the eye. For hemangiomas that block vision or other important functional organs, treatment options include oral propranolol, oral steroids, and laser therapy.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Madhukar M, Usatine R. Childhood hemangiomas. In: Usatine R, Smith M, Mayeaux EJ, Chumley H, Tysinger J, eds. The Color Atlas of Family Medicine. New York, NY: McGraw-Hill; 2009:427-431.
To learn more about The Color Atlas of Family Medicine, see:
* http://www.amazon.com/Color-Atlas-Family-Medicine/dp/0071474641
Verrucous nodules on the ankle
A 56-year-old woman came into our medical center complaining of multiple pruritic, slowly growing scaly nodules over her right ankle (FIGURE 1A AND 1B). She indicated that the lesions started as small pink “bumps” at the staple sites of an open reduction and internal fixation surgery of her talus that she’d had 8 years ago.
There were no lesions elsewhere on her body and her past medical history was otherwise unremarkable.
FIGURE 1
Multiple pruritic, scaly nodules
What is your diagnosis?
How would you manage this condition?
Diagnosis: Hypertrophic lichen planus
Hypertrophic lichen planus (HLP), a variant of lichen planus (LP), is a lesion that is usually found on the distal extremities. HLP plaques evolve from initial characteristic LP lesions (purple, planar, pruritic, polygonal papules or plaque) to form reddish-brown to violaceous, hypertrophic, verrucous round-to-elongated plaques. Primary lesions may be spread by scratching or other trauma and often develop dark brown hyperpigmentation over several years. Like other variants of LP, HLP most commonly affects adults 30 to 60 years of age, with a slight female predominance.1
HLP may be idiopathic, drug induced, or associated with a systemic disease. Although many drugs have been linked to this lesion, the most commonly reported medications are gold salts, beta-blockers, antimalarials, thiazides, furosemide, and penicillamines. If your patient has HLP and is taking one of these medications, you should consider discontinuing the medication.1 As with other forms of LP, HLP has been associated with hepatitis C. Consider transaminases and a hepatitis panel for all patients with HLP. Other HLP-associated conditions include venous insufficiency, herpes simplex virus, and varicella-zoster virus.1
When the history confuses the diagnosis
When there are surrounding classic LP lesions, the diagnosis of HLP is fairly straightforward. However, when the patient has a history of surgery or trauma preceding the lesions and no surrounding classic LP lesions, the diagnosis may be less clear-cut. In such cases, the differential diagnosis includes lichen simplex chronicus, mycetoma, chromoblastomycosis, and squamous cell carcinoma.
Lichen simplex chronicus can be distinguished from HLP by reviewing the patient’s history. Patients who describe habitual rubbing or scratching of the area are likely to have lichen simplex chronicus. On exam, lichen simplex chronicus lesions are slightly erythematous, scaly, well-demarcated, and firm. There are rough plaques with exaggerated skin lines (lichenification) rather than the verrucous surface typically seen with HLP lesions. Wickham’s striae (seen in LP) are not seen with lichen simplex chronicus, and the lesions are localized only to easily reached areas.2
Mycetoma is a tumor-like lesion produced by a fungus (eumycetoma) or bacteria (actinomycetoma), typically encountered in arid areas rather than humid environments.3 These chronic, localized, nonpainful subcutaneous nodules develop on the foot and lower extremity after traumatic inoculation with the bacteria or fungus. Mycetomas persist for many years and classically present with a triad of tumefaction, draining sinus tracts, and “sulfur grains” that distinguish it from the dry, hyperkeratotic lesions of HLP. Diagnosis requires biopsy for histologic examination and both fungal and bacterial culture in order to choose the appropriate therapy.
Chromoblastomycosis is a deep fungal infection most commonly caused by the pigmented fungus Phialophora verrucosa found in tropical climates.4 The fungi enter the skin of the lower extremity after minor trauma, resulting in a gradually expanding verrucous nodule or plaque. The nodular variant is often pedunculated with classic pigmented cauliflower-like florets. While the nodular variant is localized, the plaque variant may spread laterally, possibly metastasizing through lymphatic channels with a concomitant bacterial infection. There is also a characteristic unpleasant odor with lymph stasis.
On potassium hydroxide (KOH) mounts or histologic examination, the thick-walled cells (muriform bodies) of chromoblastomycosis are diagnostic. Patients with chromoblastomycosis have seen response rates >60% with 10 to 24 months of daily itraconazole (200 mg) therapy.5
Squamous cell carcinoma (SCC) is the second most common skin cancer and affects more than 250,000 Americans each year. While associated with sun exposure, it has also been linked to ionizing radiation, arsenic, human papilloma virus, cigarette smoking, and chronic nonhealing wounds and scars such as Marjolin’s ulcer.1
Marjolin’s ulcer usually appears as a triad of nodule formation, induration, and ulceration at a scar site and thus may be confused with HLP. It is more common than sun-induced SCC in Asian and dark-skinned individuals.6 Marjolin’s ulcer will usually present in the fifth decade, years after the initial insult. Diagnosis is supported by the clinical appearance and history of a preceding scar at the site. Marjolin’s ulcer has a higher rate of recurrence and metastasis than other forms of SCC, and thus should be treated aggressively.7,8
A biopsy may be needed
A drop of immersion oil can confirm your HLP suspicions by revealing the white, lacy reticular network of Wickham’s striae.1 Other clinical clues to the diagnosis of LP or one of its variants include a white reticular, erythematous, or ulcerative appearance on the buccal mucosa in addition to a dorsal pterygium and/or diffuse pitting on the nails.
A deep shave or punch biopsy may be necessary, however, when the clinical diagnosis is unclear. Histological findings demonstrate focal hyperorthokeratosis, saw-toothed rete ridges, vacuolar change at the basal layer, and a band-like lymphocytic infiltrate.
Corticosteroids are the treatment of choice
There have been few large-scale prospective studies exploring the treatment of HLP. However, treatment for HLP is similar to that of LP and typically begins with topical class I or II glucocorticoids or intralesional injections of triamcinolone. Narrow-band ultraviolet-B (UVB) markedly reduces pruritus and flattens plaques, and is considered second-line treatment (strength of recommendation [SOR]: C).9-11 The retinoid acitretin may be effective for severe HLP at oral dosages of 30 mg/d for 8 weeks (SOR: A).12 Azathioprine and cyclosporine have also been used successfully, but risk of renal dysfunction, hypertension, and increased viral and fungal infections make these agents third-line therapies (SOR: C).13-15
A good outcome for our patient
Our patient applied clobetasol ointment 0.05% to the affected areas twice daily until the lesions went away (approximately 2 months later).
CORRESPONDENCE
Oliver J. Wisco, Maj, USAF, MC, FS, Department of the Air Force, Wilford Hall Medical Center, 59 MDW/SGOMD/Dermatology, 2200 Bergquist Drive, Suite 1, Lackland AFB, TX 78236-9908; [email protected]
1. Bolognia JL, Jorizzo JL, Rapini RP. Dermatology. 2nd ed. St. Louis, Mo: Mosby Elsevier; 2008.
2. Habif TP. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 4th ed. Philadelphia, Pa: Mosby; 2004:65.
3. Fitzpatrick T, Eisen A, Wolff K, et al. Dermatology in General Medicine. 5th ed. New York, NY: McGraw-Hill; 1999:2301.
4. Fitzpatrick T, Eisen A, Wolff K, et al. Dermatology in General Medicine. 5th ed. New York, NY: McGraw-Hill; 1999:2375.
5. Restrepo A. Treatment of tropical mycoses. J Am Acad Dermatol. 1994;31(3 Pt 2):S91-S102.
6. Chuang TY, Reizner GT, Elpern DJ, et al. Nonmelanoma skin cancer in Japanese ethnic Hawaiians in Kauai, Hawaii: an incidence report. J Am Acad Dermatol. 1995;33:422-426.
7. Treves N, Pack GT. The development of cancer in burn scars. An analysis and report of thirty-four cases. Surg Gynecol Obstet. 1930;51:749.-
8. Lifeso RM, Rooney RJ, el-Shaker M. Post-traumatic squamous-cell carcinoma. J Bone Joint Surg. 1990;72:12-18.
9. Gambichler T, Breuckmann F, Boms S, et al. Narrow-band UVB phototherapy in skin conditions beyond psoriasis. J Am Acad Dermatol. 2005;52:660-670.
10. Saricaoglu H, Karadogan SK, Baskan EB. Narrowband UVB therapy in the treatment of lichen planus. Photodermatol Photoimmunol Photomed. 2003;19:265-267.
11. Taneja A, Taylor CR. Narrow-band UVB for lichen planus treatment. Int J Dermatol. 2002;41:282-283.
12. Laurberg G, Geiger JM, Hjorth N, et al. Treatment of lichen planus with acitretin: a double-blind, placebo-controlled study in 65 patients. J Am Acad Dermatol 1991;24:434-437.
13. Kossard S, Artemi P. Acitretin for hypertrophic lichen planus-like reaction in a burn scar. Arch Dermatol. 2000;136:591-594.
14. Lear JT, English JS. Erosive and generalized lichen planus responsive to azathioprine. Clin Exp Dermatol. 1996;21:56-57.
15. Ho VC, Gupta AK, Ellis CN, et al. Treatment of severe lichen planus with cyclosporine. J Am Acad Dermatol. 1990;22:64-68.
A 56-year-old woman came into our medical center complaining of multiple pruritic, slowly growing scaly nodules over her right ankle (FIGURE 1A AND 1B). She indicated that the lesions started as small pink “bumps” at the staple sites of an open reduction and internal fixation surgery of her talus that she’d had 8 years ago.
There were no lesions elsewhere on her body and her past medical history was otherwise unremarkable.
FIGURE 1
Multiple pruritic, scaly nodules
What is your diagnosis?
How would you manage this condition?
Diagnosis: Hypertrophic lichen planus
Hypertrophic lichen planus (HLP), a variant of lichen planus (LP), is a lesion that is usually found on the distal extremities. HLP plaques evolve from initial characteristic LP lesions (purple, planar, pruritic, polygonal papules or plaque) to form reddish-brown to violaceous, hypertrophic, verrucous round-to-elongated plaques. Primary lesions may be spread by scratching or other trauma and often develop dark brown hyperpigmentation over several years. Like other variants of LP, HLP most commonly affects adults 30 to 60 years of age, with a slight female predominance.1
HLP may be idiopathic, drug induced, or associated with a systemic disease. Although many drugs have been linked to this lesion, the most commonly reported medications are gold salts, beta-blockers, antimalarials, thiazides, furosemide, and penicillamines. If your patient has HLP and is taking one of these medications, you should consider discontinuing the medication.1 As with other forms of LP, HLP has been associated with hepatitis C. Consider transaminases and a hepatitis panel for all patients with HLP. Other HLP-associated conditions include venous insufficiency, herpes simplex virus, and varicella-zoster virus.1
When the history confuses the diagnosis
When there are surrounding classic LP lesions, the diagnosis of HLP is fairly straightforward. However, when the patient has a history of surgery or trauma preceding the lesions and no surrounding classic LP lesions, the diagnosis may be less clear-cut. In such cases, the differential diagnosis includes lichen simplex chronicus, mycetoma, chromoblastomycosis, and squamous cell carcinoma.
Lichen simplex chronicus can be distinguished from HLP by reviewing the patient’s history. Patients who describe habitual rubbing or scratching of the area are likely to have lichen simplex chronicus. On exam, lichen simplex chronicus lesions are slightly erythematous, scaly, well-demarcated, and firm. There are rough plaques with exaggerated skin lines (lichenification) rather than the verrucous surface typically seen with HLP lesions. Wickham’s striae (seen in LP) are not seen with lichen simplex chronicus, and the lesions are localized only to easily reached areas.2
Mycetoma is a tumor-like lesion produced by a fungus (eumycetoma) or bacteria (actinomycetoma), typically encountered in arid areas rather than humid environments.3 These chronic, localized, nonpainful subcutaneous nodules develop on the foot and lower extremity after traumatic inoculation with the bacteria or fungus. Mycetomas persist for many years and classically present with a triad of tumefaction, draining sinus tracts, and “sulfur grains” that distinguish it from the dry, hyperkeratotic lesions of HLP. Diagnosis requires biopsy for histologic examination and both fungal and bacterial culture in order to choose the appropriate therapy.
Chromoblastomycosis is a deep fungal infection most commonly caused by the pigmented fungus Phialophora verrucosa found in tropical climates.4 The fungi enter the skin of the lower extremity after minor trauma, resulting in a gradually expanding verrucous nodule or plaque. The nodular variant is often pedunculated with classic pigmented cauliflower-like florets. While the nodular variant is localized, the plaque variant may spread laterally, possibly metastasizing through lymphatic channels with a concomitant bacterial infection. There is also a characteristic unpleasant odor with lymph stasis.
On potassium hydroxide (KOH) mounts or histologic examination, the thick-walled cells (muriform bodies) of chromoblastomycosis are diagnostic. Patients with chromoblastomycosis have seen response rates >60% with 10 to 24 months of daily itraconazole (200 mg) therapy.5
Squamous cell carcinoma (SCC) is the second most common skin cancer and affects more than 250,000 Americans each year. While associated with sun exposure, it has also been linked to ionizing radiation, arsenic, human papilloma virus, cigarette smoking, and chronic nonhealing wounds and scars such as Marjolin’s ulcer.1
Marjolin’s ulcer usually appears as a triad of nodule formation, induration, and ulceration at a scar site and thus may be confused with HLP. It is more common than sun-induced SCC in Asian and dark-skinned individuals.6 Marjolin’s ulcer will usually present in the fifth decade, years after the initial insult. Diagnosis is supported by the clinical appearance and history of a preceding scar at the site. Marjolin’s ulcer has a higher rate of recurrence and metastasis than other forms of SCC, and thus should be treated aggressively.7,8
A biopsy may be needed
A drop of immersion oil can confirm your HLP suspicions by revealing the white, lacy reticular network of Wickham’s striae.1 Other clinical clues to the diagnosis of LP or one of its variants include a white reticular, erythematous, or ulcerative appearance on the buccal mucosa in addition to a dorsal pterygium and/or diffuse pitting on the nails.
A deep shave or punch biopsy may be necessary, however, when the clinical diagnosis is unclear. Histological findings demonstrate focal hyperorthokeratosis, saw-toothed rete ridges, vacuolar change at the basal layer, and a band-like lymphocytic infiltrate.
Corticosteroids are the treatment of choice
There have been few large-scale prospective studies exploring the treatment of HLP. However, treatment for HLP is similar to that of LP and typically begins with topical class I or II glucocorticoids or intralesional injections of triamcinolone. Narrow-band ultraviolet-B (UVB) markedly reduces pruritus and flattens plaques, and is considered second-line treatment (strength of recommendation [SOR]: C).9-11 The retinoid acitretin may be effective for severe HLP at oral dosages of 30 mg/d for 8 weeks (SOR: A).12 Azathioprine and cyclosporine have also been used successfully, but risk of renal dysfunction, hypertension, and increased viral and fungal infections make these agents third-line therapies (SOR: C).13-15
A good outcome for our patient
Our patient applied clobetasol ointment 0.05% to the affected areas twice daily until the lesions went away (approximately 2 months later).
CORRESPONDENCE
Oliver J. Wisco, Maj, USAF, MC, FS, Department of the Air Force, Wilford Hall Medical Center, 59 MDW/SGOMD/Dermatology, 2200 Bergquist Drive, Suite 1, Lackland AFB, TX 78236-9908; [email protected]
A 56-year-old woman came into our medical center complaining of multiple pruritic, slowly growing scaly nodules over her right ankle (FIGURE 1A AND 1B). She indicated that the lesions started as small pink “bumps” at the staple sites of an open reduction and internal fixation surgery of her talus that she’d had 8 years ago.
There were no lesions elsewhere on her body and her past medical history was otherwise unremarkable.
FIGURE 1
Multiple pruritic, scaly nodules
What is your diagnosis?
How would you manage this condition?
Diagnosis: Hypertrophic lichen planus
Hypertrophic lichen planus (HLP), a variant of lichen planus (LP), is a lesion that is usually found on the distal extremities. HLP plaques evolve from initial characteristic LP lesions (purple, planar, pruritic, polygonal papules or plaque) to form reddish-brown to violaceous, hypertrophic, verrucous round-to-elongated plaques. Primary lesions may be spread by scratching or other trauma and often develop dark brown hyperpigmentation over several years. Like other variants of LP, HLP most commonly affects adults 30 to 60 years of age, with a slight female predominance.1
HLP may be idiopathic, drug induced, or associated with a systemic disease. Although many drugs have been linked to this lesion, the most commonly reported medications are gold salts, beta-blockers, antimalarials, thiazides, furosemide, and penicillamines. If your patient has HLP and is taking one of these medications, you should consider discontinuing the medication.1 As with other forms of LP, HLP has been associated with hepatitis C. Consider transaminases and a hepatitis panel for all patients with HLP. Other HLP-associated conditions include venous insufficiency, herpes simplex virus, and varicella-zoster virus.1
When the history confuses the diagnosis
When there are surrounding classic LP lesions, the diagnosis of HLP is fairly straightforward. However, when the patient has a history of surgery or trauma preceding the lesions and no surrounding classic LP lesions, the diagnosis may be less clear-cut. In such cases, the differential diagnosis includes lichen simplex chronicus, mycetoma, chromoblastomycosis, and squamous cell carcinoma.
Lichen simplex chronicus can be distinguished from HLP by reviewing the patient’s history. Patients who describe habitual rubbing or scratching of the area are likely to have lichen simplex chronicus. On exam, lichen simplex chronicus lesions are slightly erythematous, scaly, well-demarcated, and firm. There are rough plaques with exaggerated skin lines (lichenification) rather than the verrucous surface typically seen with HLP lesions. Wickham’s striae (seen in LP) are not seen with lichen simplex chronicus, and the lesions are localized only to easily reached areas.2
Mycetoma is a tumor-like lesion produced by a fungus (eumycetoma) or bacteria (actinomycetoma), typically encountered in arid areas rather than humid environments.3 These chronic, localized, nonpainful subcutaneous nodules develop on the foot and lower extremity after traumatic inoculation with the bacteria or fungus. Mycetomas persist for many years and classically present with a triad of tumefaction, draining sinus tracts, and “sulfur grains” that distinguish it from the dry, hyperkeratotic lesions of HLP. Diagnosis requires biopsy for histologic examination and both fungal and bacterial culture in order to choose the appropriate therapy.
Chromoblastomycosis is a deep fungal infection most commonly caused by the pigmented fungus Phialophora verrucosa found in tropical climates.4 The fungi enter the skin of the lower extremity after minor trauma, resulting in a gradually expanding verrucous nodule or plaque. The nodular variant is often pedunculated with classic pigmented cauliflower-like florets. While the nodular variant is localized, the plaque variant may spread laterally, possibly metastasizing through lymphatic channels with a concomitant bacterial infection. There is also a characteristic unpleasant odor with lymph stasis.
On potassium hydroxide (KOH) mounts or histologic examination, the thick-walled cells (muriform bodies) of chromoblastomycosis are diagnostic. Patients with chromoblastomycosis have seen response rates >60% with 10 to 24 months of daily itraconazole (200 mg) therapy.5
Squamous cell carcinoma (SCC) is the second most common skin cancer and affects more than 250,000 Americans each year. While associated with sun exposure, it has also been linked to ionizing radiation, arsenic, human papilloma virus, cigarette smoking, and chronic nonhealing wounds and scars such as Marjolin’s ulcer.1
Marjolin’s ulcer usually appears as a triad of nodule formation, induration, and ulceration at a scar site and thus may be confused with HLP. It is more common than sun-induced SCC in Asian and dark-skinned individuals.6 Marjolin’s ulcer will usually present in the fifth decade, years after the initial insult. Diagnosis is supported by the clinical appearance and history of a preceding scar at the site. Marjolin’s ulcer has a higher rate of recurrence and metastasis than other forms of SCC, and thus should be treated aggressively.7,8
A biopsy may be needed
A drop of immersion oil can confirm your HLP suspicions by revealing the white, lacy reticular network of Wickham’s striae.1 Other clinical clues to the diagnosis of LP or one of its variants include a white reticular, erythematous, or ulcerative appearance on the buccal mucosa in addition to a dorsal pterygium and/or diffuse pitting on the nails.
A deep shave or punch biopsy may be necessary, however, when the clinical diagnosis is unclear. Histological findings demonstrate focal hyperorthokeratosis, saw-toothed rete ridges, vacuolar change at the basal layer, and a band-like lymphocytic infiltrate.
Corticosteroids are the treatment of choice
There have been few large-scale prospective studies exploring the treatment of HLP. However, treatment for HLP is similar to that of LP and typically begins with topical class I or II glucocorticoids or intralesional injections of triamcinolone. Narrow-band ultraviolet-B (UVB) markedly reduces pruritus and flattens plaques, and is considered second-line treatment (strength of recommendation [SOR]: C).9-11 The retinoid acitretin may be effective for severe HLP at oral dosages of 30 mg/d for 8 weeks (SOR: A).12 Azathioprine and cyclosporine have also been used successfully, but risk of renal dysfunction, hypertension, and increased viral and fungal infections make these agents third-line therapies (SOR: C).13-15
A good outcome for our patient
Our patient applied clobetasol ointment 0.05% to the affected areas twice daily until the lesions went away (approximately 2 months later).
CORRESPONDENCE
Oliver J. Wisco, Maj, USAF, MC, FS, Department of the Air Force, Wilford Hall Medical Center, 59 MDW/SGOMD/Dermatology, 2200 Bergquist Drive, Suite 1, Lackland AFB, TX 78236-9908; [email protected]
1. Bolognia JL, Jorizzo JL, Rapini RP. Dermatology. 2nd ed. St. Louis, Mo: Mosby Elsevier; 2008.
2. Habif TP. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 4th ed. Philadelphia, Pa: Mosby; 2004:65.
3. Fitzpatrick T, Eisen A, Wolff K, et al. Dermatology in General Medicine. 5th ed. New York, NY: McGraw-Hill; 1999:2301.
4. Fitzpatrick T, Eisen A, Wolff K, et al. Dermatology in General Medicine. 5th ed. New York, NY: McGraw-Hill; 1999:2375.
5. Restrepo A. Treatment of tropical mycoses. J Am Acad Dermatol. 1994;31(3 Pt 2):S91-S102.
6. Chuang TY, Reizner GT, Elpern DJ, et al. Nonmelanoma skin cancer in Japanese ethnic Hawaiians in Kauai, Hawaii: an incidence report. J Am Acad Dermatol. 1995;33:422-426.
7. Treves N, Pack GT. The development of cancer in burn scars. An analysis and report of thirty-four cases. Surg Gynecol Obstet. 1930;51:749.-
8. Lifeso RM, Rooney RJ, el-Shaker M. Post-traumatic squamous-cell carcinoma. J Bone Joint Surg. 1990;72:12-18.
9. Gambichler T, Breuckmann F, Boms S, et al. Narrow-band UVB phototherapy in skin conditions beyond psoriasis. J Am Acad Dermatol. 2005;52:660-670.
10. Saricaoglu H, Karadogan SK, Baskan EB. Narrowband UVB therapy in the treatment of lichen planus. Photodermatol Photoimmunol Photomed. 2003;19:265-267.
11. Taneja A, Taylor CR. Narrow-band UVB for lichen planus treatment. Int J Dermatol. 2002;41:282-283.
12. Laurberg G, Geiger JM, Hjorth N, et al. Treatment of lichen planus with acitretin: a double-blind, placebo-controlled study in 65 patients. J Am Acad Dermatol 1991;24:434-437.
13. Kossard S, Artemi P. Acitretin for hypertrophic lichen planus-like reaction in a burn scar. Arch Dermatol. 2000;136:591-594.
14. Lear JT, English JS. Erosive and generalized lichen planus responsive to azathioprine. Clin Exp Dermatol. 1996;21:56-57.
15. Ho VC, Gupta AK, Ellis CN, et al. Treatment of severe lichen planus with cyclosporine. J Am Acad Dermatol. 1990;22:64-68.
1. Bolognia JL, Jorizzo JL, Rapini RP. Dermatology. 2nd ed. St. Louis, Mo: Mosby Elsevier; 2008.
2. Habif TP. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 4th ed. Philadelphia, Pa: Mosby; 2004:65.
3. Fitzpatrick T, Eisen A, Wolff K, et al. Dermatology in General Medicine. 5th ed. New York, NY: McGraw-Hill; 1999:2301.
4. Fitzpatrick T, Eisen A, Wolff K, et al. Dermatology in General Medicine. 5th ed. New York, NY: McGraw-Hill; 1999:2375.
5. Restrepo A. Treatment of tropical mycoses. J Am Acad Dermatol. 1994;31(3 Pt 2):S91-S102.
6. Chuang TY, Reizner GT, Elpern DJ, et al. Nonmelanoma skin cancer in Japanese ethnic Hawaiians in Kauai, Hawaii: an incidence report. J Am Acad Dermatol. 1995;33:422-426.
7. Treves N, Pack GT. The development of cancer in burn scars. An analysis and report of thirty-four cases. Surg Gynecol Obstet. 1930;51:749.-
8. Lifeso RM, Rooney RJ, el-Shaker M. Post-traumatic squamous-cell carcinoma. J Bone Joint Surg. 1990;72:12-18.
9. Gambichler T, Breuckmann F, Boms S, et al. Narrow-band UVB phototherapy in skin conditions beyond psoriasis. J Am Acad Dermatol. 2005;52:660-670.
10. Saricaoglu H, Karadogan SK, Baskan EB. Narrowband UVB therapy in the treatment of lichen planus. Photodermatol Photoimmunol Photomed. 2003;19:265-267.
11. Taneja A, Taylor CR. Narrow-band UVB for lichen planus treatment. Int J Dermatol. 2002;41:282-283.
12. Laurberg G, Geiger JM, Hjorth N, et al. Treatment of lichen planus with acitretin: a double-blind, placebo-controlled study in 65 patients. J Am Acad Dermatol 1991;24:434-437.
13. Kossard S, Artemi P. Acitretin for hypertrophic lichen planus-like reaction in a burn scar. Arch Dermatol. 2000;136:591-594.
14. Lear JT, English JS. Erosive and generalized lichen planus responsive to azathioprine. Clin Exp Dermatol. 1996;21:56-57.
15. Ho VC, Gupta AK, Ellis CN, et al. Treatment of severe lichen planus with cyclosporine. J Am Acad Dermatol. 1990;22:64-68.
Blisters on back
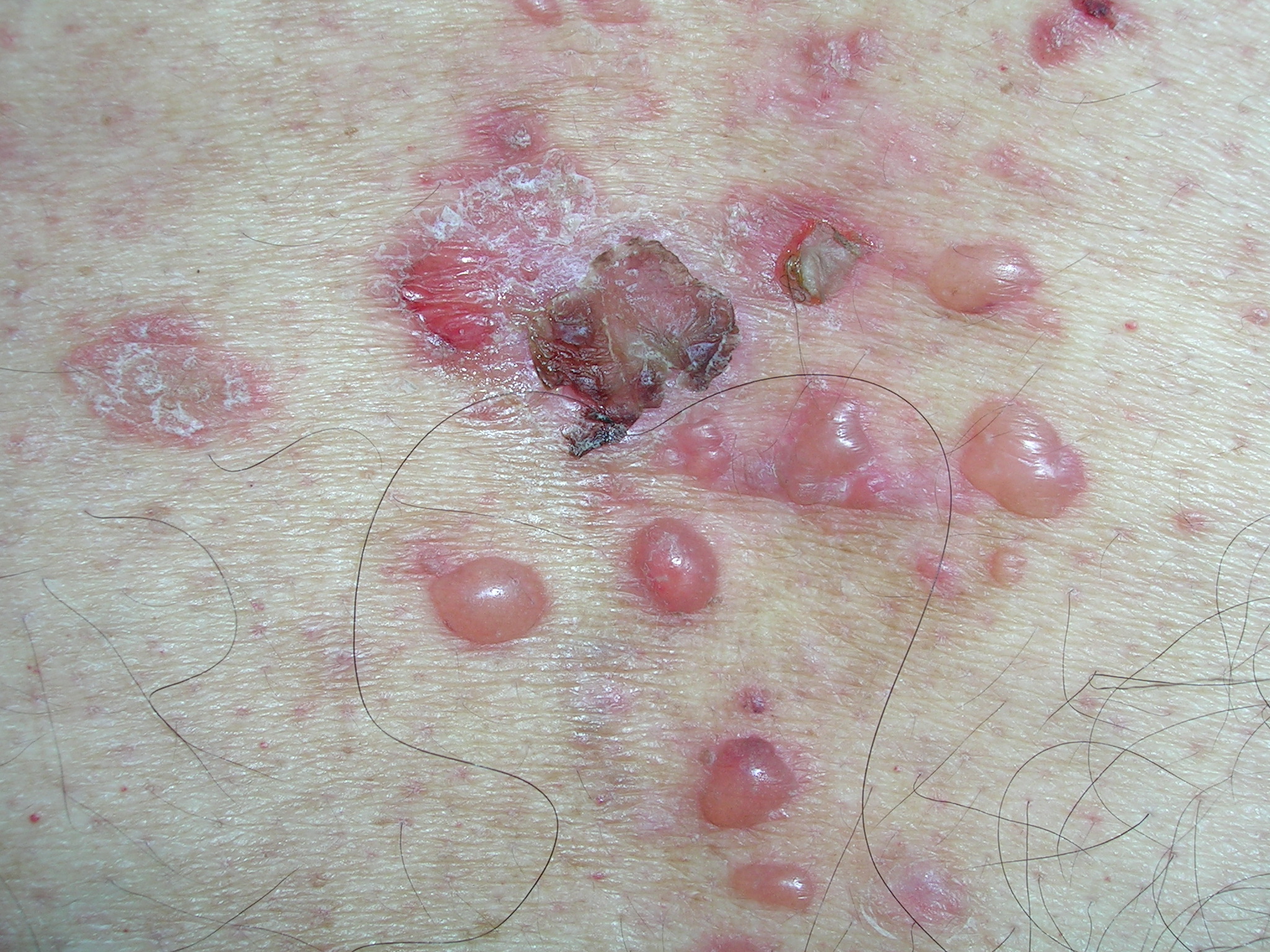
Bullous pemphigoid was suspected and a shave biopsy (ordered rush) was performed on the edge of an intact bulla. The physican started the patient on prednisone 60 mg daily while awaiting the biopsy result. In 2 days, the biopsy confirmed bullous pemphigoid and no new blisters formed during the time the patient was on the prednisone. The physician switched the patient to another antihypertensive agent just in case the metoprolol contributed to the new onset of the bullous pemphigoid.
After 2 weeks, the prednisone was dropped to 40 mg a day and tetracycline 500 mg 3 times a day was started. Later in the course, nicotinamide 500 mg 3 times a day was added while the prednisone dose was dropped even further. The bullae healed and the patient continued to do well as long as he took his medicine.
The differential diagnosis for bullous disease is long, but includes pemphigus, porphyria cutanea tarda, bullous impetigo, and pityriasis lichenoides.
Photos and text for Photo Rounds Friday courtesy of Richard Usatine, MD. This case was adapted from: Mohmand A. Bullous pemphigoid. In: Usatine R, Smith M, Mayeaux EJ, Chumley H, Tysinger J, eds. The Color Atlas of Family Medicine. New York, NY: McGraw-Hill; 2009:539-544.
To learn more about The Color Atlas of Family Medicine, see:
* http://www.amazon.com/Color-Atlas-Family-Medicine/dp/0071474641

Bullous pemphigoid was suspected and a shave biopsy (ordered rush) was performed on the edge of an intact bulla. The physican started the patient on prednisone 60 mg daily while awaiting the biopsy result. In 2 days, the biopsy confirmed bullous pemphigoid and no new blisters formed during the time the patient was on the prednisone. The physician switched the patient to another antihypertensive agent just in case the metoprolol contributed to the new onset of the bullous pemphigoid.
After 2 weeks, the prednisone was dropped to 40 mg a day and tetracycline 500 mg 3 times a day was started. Later in the course, nicotinamide 500 mg 3 times a day was added while the prednisone dose was dropped even further. The bullae healed and the patient continued to do well as long as he took his medicine.
The differential diagnosis for bullous disease is long, but includes pemphigus, porphyria cutanea tarda, bullous impetigo, and pityriasis lichenoides.
Photos and text for Photo Rounds Friday courtesy of Richard Usatine, MD. This case was adapted from: Mohmand A. Bullous pemphigoid. In: Usatine R, Smith M, Mayeaux EJ, Chumley H, Tysinger J, eds. The Color Atlas of Family Medicine. New York, NY: McGraw-Hill; 2009:539-544.
To learn more about The Color Atlas of Family Medicine, see:
* http://www.amazon.com/Color-Atlas-Family-Medicine/dp/0071474641

Bullous pemphigoid was suspected and a shave biopsy (ordered rush) was performed on the edge of an intact bulla. The physican started the patient on prednisone 60 mg daily while awaiting the biopsy result. In 2 days, the biopsy confirmed bullous pemphigoid and no new blisters formed during the time the patient was on the prednisone. The physician switched the patient to another antihypertensive agent just in case the metoprolol contributed to the new onset of the bullous pemphigoid.
After 2 weeks, the prednisone was dropped to 40 mg a day and tetracycline 500 mg 3 times a day was started. Later in the course, nicotinamide 500 mg 3 times a day was added while the prednisone dose was dropped even further. The bullae healed and the patient continued to do well as long as he took his medicine.
The differential diagnosis for bullous disease is long, but includes pemphigus, porphyria cutanea tarda, bullous impetigo, and pityriasis lichenoides.
Photos and text for Photo Rounds Friday courtesy of Richard Usatine, MD. This case was adapted from: Mohmand A. Bullous pemphigoid. In: Usatine R, Smith M, Mayeaux EJ, Chumley H, Tysinger J, eds. The Color Atlas of Family Medicine. New York, NY: McGraw-Hill; 2009:539-544.
To learn more about The Color Atlas of Family Medicine, see:
* http://www.amazon.com/Color-Atlas-Family-Medicine/dp/0071474641
Occasionally pruritic rash

We diagnosed pityriasis rosea by the clinical appearance, even though there was no obvious herald patch. The collarette scale was visible and the distribution was consistent with pityriasis rosea. The differential diagnosis for pityriasis rosea includes secondary syphilis and tinea versicolor.
The physician reassured the young woman and her mom that pityriasis rosea resolves spontaneously within 6 to 8 weeks. At a visit for a college physical 3 months later, her skin was clear and there was no scarring.
Photos and text for Photo Rounds Friday courtesy of Richard Usatine, MD. This case was adapted from: Henderson D, Usatine R. Pityriasis rosea. In: Usatine R, Smith M, Mayeaux EJ, Chumley H, Tysinger J, eds. The Color Atlas of Family Medicine. New York, NY: McGraw-Hill; 2009:539-544.
To learn more about The Color Atlas of Family Medicine, see:
* http://www.amazon.com/Color-Atlas-Family-Medicine/dp/0071474641

We diagnosed pityriasis rosea by the clinical appearance, even though there was no obvious herald patch. The collarette scale was visible and the distribution was consistent with pityriasis rosea. The differential diagnosis for pityriasis rosea includes secondary syphilis and tinea versicolor.
The physician reassured the young woman and her mom that pityriasis rosea resolves spontaneously within 6 to 8 weeks. At a visit for a college physical 3 months later, her skin was clear and there was no scarring.
Photos and text for Photo Rounds Friday courtesy of Richard Usatine, MD. This case was adapted from: Henderson D, Usatine R. Pityriasis rosea. In: Usatine R, Smith M, Mayeaux EJ, Chumley H, Tysinger J, eds. The Color Atlas of Family Medicine. New York, NY: McGraw-Hill; 2009:539-544.
To learn more about The Color Atlas of Family Medicine, see:
* http://www.amazon.com/Color-Atlas-Family-Medicine/dp/0071474641

We diagnosed pityriasis rosea by the clinical appearance, even though there was no obvious herald patch. The collarette scale was visible and the distribution was consistent with pityriasis rosea. The differential diagnosis for pityriasis rosea includes secondary syphilis and tinea versicolor.
The physician reassured the young woman and her mom that pityriasis rosea resolves spontaneously within 6 to 8 weeks. At a visit for a college physical 3 months later, her skin was clear and there was no scarring.
Photos and text for Photo Rounds Friday courtesy of Richard Usatine, MD. This case was adapted from: Henderson D, Usatine R. Pityriasis rosea. In: Usatine R, Smith M, Mayeaux EJ, Chumley H, Tysinger J, eds. The Color Atlas of Family Medicine. New York, NY: McGraw-Hill; 2009:539-544.
To learn more about The Color Atlas of Family Medicine, see:
* http://www.amazon.com/Color-Atlas-Family-Medicine/dp/0071474641
Red and swollen area on nose
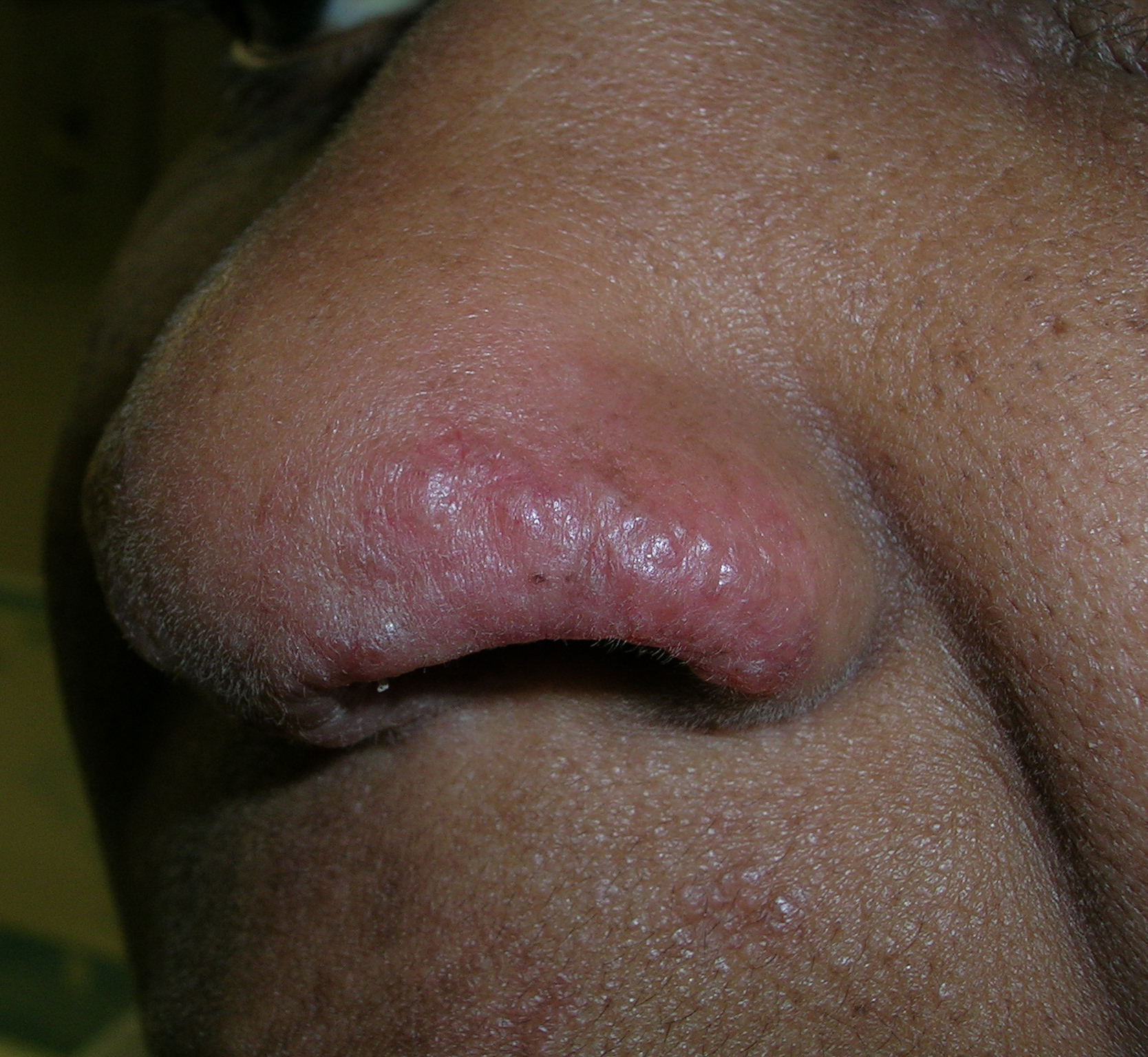
The patient’s physician diagnosed cutaneous sarcoidosis involving the nasal rim. This presentation of sarcoidosis is called “lupus pernio.” Lupus pernio type sarcoidosis presents with shiny pink to purplish lesions typically affecting the nose, cheeks, ears, and lips. The differential diagnosis for lupus pernio includes lupus erythematosus, granuloma annulare, and morphea. Sarcoidosis may involve the upper respiratory tract with pulmonary fibrosis. Less frequently, it may be associated with chronic uveitis and bone cysts. Sarcoidosis may present with erythema nodosum.
A shave biopsy confirmed the diagnosis in this patient. If the lesions are not on the nasal ala, a punch biopsy is generally preferred. This patient had a normal chest x-ray and no evidence of pulmonary involvement.
Treatment for localized sarcoidosis on the face includes topical or intralesional steroids. If the cutaneous sarcoidosis is more widespread, then treatment options include prednisone, methotrexate, and hydroxychloroquine. This patient’s physician started her on topical triamcinolone cream twice daily to the affected area.
Photos and text for Photo Rounds Friday courtesy of Richard Usatine, MD. This case was adapted from: Sarabi K, Khachemoune A. Sarcoidosis. In: Usatine R, Smith M, Mayeaux EJ, Chumley H, Tysinger J, eds. The Color Atlas of Family Medicine. New York, NY: McGraw-Hill; 2009:539-544.
To learn more about The Color Atlas of Family Medicine, see:
* http://www.amazon.com/Color-Atlas-Family-Medicine/dp/0071474641

The patient’s physician diagnosed cutaneous sarcoidosis involving the nasal rim. This presentation of sarcoidosis is called “lupus pernio.” Lupus pernio type sarcoidosis presents with shiny pink to purplish lesions typically affecting the nose, cheeks, ears, and lips. The differential diagnosis for lupus pernio includes lupus erythematosus, granuloma annulare, and morphea. Sarcoidosis may involve the upper respiratory tract with pulmonary fibrosis. Less frequently, it may be associated with chronic uveitis and bone cysts. Sarcoidosis may present with erythema nodosum.
A shave biopsy confirmed the diagnosis in this patient. If the lesions are not on the nasal ala, a punch biopsy is generally preferred. This patient had a normal chest x-ray and no evidence of pulmonary involvement.
Treatment for localized sarcoidosis on the face includes topical or intralesional steroids. If the cutaneous sarcoidosis is more widespread, then treatment options include prednisone, methotrexate, and hydroxychloroquine. This patient’s physician started her on topical triamcinolone cream twice daily to the affected area.
Photos and text for Photo Rounds Friday courtesy of Richard Usatine, MD. This case was adapted from: Sarabi K, Khachemoune A. Sarcoidosis. In: Usatine R, Smith M, Mayeaux EJ, Chumley H, Tysinger J, eds. The Color Atlas of Family Medicine. New York, NY: McGraw-Hill; 2009:539-544.
To learn more about The Color Atlas of Family Medicine, see:
* http://www.amazon.com/Color-Atlas-Family-Medicine/dp/0071474641

The patient’s physician diagnosed cutaneous sarcoidosis involving the nasal rim. This presentation of sarcoidosis is called “lupus pernio.” Lupus pernio type sarcoidosis presents with shiny pink to purplish lesions typically affecting the nose, cheeks, ears, and lips. The differential diagnosis for lupus pernio includes lupus erythematosus, granuloma annulare, and morphea. Sarcoidosis may involve the upper respiratory tract with pulmonary fibrosis. Less frequently, it may be associated with chronic uveitis and bone cysts. Sarcoidosis may present with erythema nodosum.
A shave biopsy confirmed the diagnosis in this patient. If the lesions are not on the nasal ala, a punch biopsy is generally preferred. This patient had a normal chest x-ray and no evidence of pulmonary involvement.
Treatment for localized sarcoidosis on the face includes topical or intralesional steroids. If the cutaneous sarcoidosis is more widespread, then treatment options include prednisone, methotrexate, and hydroxychloroquine. This patient’s physician started her on topical triamcinolone cream twice daily to the affected area.
Photos and text for Photo Rounds Friday courtesy of Richard Usatine, MD. This case was adapted from: Sarabi K, Khachemoune A. Sarcoidosis. In: Usatine R, Smith M, Mayeaux EJ, Chumley H, Tysinger J, eds. The Color Atlas of Family Medicine. New York, NY: McGraw-Hill; 2009:539-544.
To learn more about The Color Atlas of Family Medicine, see:
* http://www.amazon.com/Color-Atlas-Family-Medicine/dp/0071474641
Skin change on forehead

We diagnosed linear morphea. The pattern running down the patient’s forehead is called “en coup de sabre” meaning the blow of a sword. While the differential diagnosis could include lichen sclerosis and discoid lupus, her pattern was very specific for linear morphea. Linear morphea does not cause any health risks to the patient and is merely a cosmetic issue.
We told the patient that her treatment options included high-potency topical steroids and topical calcipotriol. Other options include ultraviolet-A light therapy and oral methotrexate. The combination of high-dose systemic steroids and low-dose methotrexate has also been used successfully in some cases. The patient took a prescription for topical calcipotriol and said she would fill it if her insurance covered the medication.
Photos and text for Photo Rounds Friday courtesy of Richard Usatine, MD. This case was adapted from: Mayeaux EJ. Scleroderma and morphea. In: Usatine R, Smith M, Mayeaux EJ, Chumley H, Tysinger J, eds. The Color Atlas of Family Medicine. New York, NY: McGraw-Hill; 2009:539-544.
To learn more about The Color Atlas of Family Medicine, see:
* http://www.amazon.com/Color-Atlas-Family-Medicine/dp/0071474641

We diagnosed linear morphea. The pattern running down the patient’s forehead is called “en coup de sabre” meaning the blow of a sword. While the differential diagnosis could include lichen sclerosis and discoid lupus, her pattern was very specific for linear morphea. Linear morphea does not cause any health risks to the patient and is merely a cosmetic issue.
We told the patient that her treatment options included high-potency topical steroids and topical calcipotriol. Other options include ultraviolet-A light therapy and oral methotrexate. The combination of high-dose systemic steroids and low-dose methotrexate has also been used successfully in some cases. The patient took a prescription for topical calcipotriol and said she would fill it if her insurance covered the medication.
Photos and text for Photo Rounds Friday courtesy of Richard Usatine, MD. This case was adapted from: Mayeaux EJ. Scleroderma and morphea. In: Usatine R, Smith M, Mayeaux EJ, Chumley H, Tysinger J, eds. The Color Atlas of Family Medicine. New York, NY: McGraw-Hill; 2009:539-544.
To learn more about The Color Atlas of Family Medicine, see:
* http://www.amazon.com/Color-Atlas-Family-Medicine/dp/0071474641

We diagnosed linear morphea. The pattern running down the patient’s forehead is called “en coup de sabre” meaning the blow of a sword. While the differential diagnosis could include lichen sclerosis and discoid lupus, her pattern was very specific for linear morphea. Linear morphea does not cause any health risks to the patient and is merely a cosmetic issue.
We told the patient that her treatment options included high-potency topical steroids and topical calcipotriol. Other options include ultraviolet-A light therapy and oral methotrexate. The combination of high-dose systemic steroids and low-dose methotrexate has also been used successfully in some cases. The patient took a prescription for topical calcipotriol and said she would fill it if her insurance covered the medication.
Photos and text for Photo Rounds Friday courtesy of Richard Usatine, MD. This case was adapted from: Mayeaux EJ. Scleroderma and morphea. In: Usatine R, Smith M, Mayeaux EJ, Chumley H, Tysinger J, eds. The Color Atlas of Family Medicine. New York, NY: McGraw-Hill; 2009:539-544.
To learn more about The Color Atlas of Family Medicine, see:
* http://www.amazon.com/Color-Atlas-Family-Medicine/dp/0071474641