User login
Evaluating Access to Full-Body Skin Examinations in Los Angeles County, California
Evaluating Access to Full-Body Skin Examinations in Los Angeles County, California
To the Editor:
Early skin cancer detection improves patient outcomes1; however, socioeconomic and racial disparities may impact access to dermatologic care.2 Although non-Hispanic White individuals have a high incidence of skin cancer, they experience higher melanoma-specific survival rates than non-White patients, who often receive later-stage diagnoses and experience higher mortality.2 Furthermore, racial/ ethnic minorities often face longer surgery wait times after diagnosis and have lower socioeconomic status (SES) and less favorable health insurance coverage, contributing to poorer outcomes.2,3
To examine access to full-body skin examinations (FBSEs) by board-certified dermatologists in Los Angeles (LA) County, California, we analyzed the availability of FBSEs based on racial demographics, income, and insurance type (Medicaid [Medi-Cal] vs private [Blue Cross Blue Shield (BCBS)]). Demographic data by zip code were obtained from the US Census Bureau.4 This validated metric highlights socioeconomic disparities and minimizes data gaps5,6 and was used to assess health care access among different population subgroups. Dermatologists’ contact information was obtained from the Find a Dermatologist page on the American Academy of Dermatology website and the listed phone numbers of their practice were used to contact them. Practices with board-certified dermatologists accepting new patients were included in the study; practices were not included if they had exclusive insurance plans; were pediatric, cosmetic, or research only; or were nonresponsive to calls. From August 2022 to September 2022, each practice was called twice within a 36-hour period—once by a simulated patient with Medi-Cal and once by a simulated patient with BCBS—and were asked about availability for new patient FBSE appointments and accepted insurance types. Data were analyzed using SAS software (SAS Institute Inc.).
Los Angeles County comprises 269 zip codes, of which 82 (30.5%) have dermatology practices. Of 213 total dermatologists in LA County listed on the American Academy of Dermatology website, 193 (90.6%) met preliminary criteria, and 169 (79.3%) were successfully contacted. Almost all (94.6% [160/169]) accepted new patients for FBSEs; of those, 63.1% (101/160) accepted only private insurance, 16.9% (27/160) accepted both private insurance and Medi-Cal, and 16.2% (26/160) did not accept any insurance. Racial predominance for each dermatology practice was analyzed by zip code (Table). Dermatologists included in our study were significantly more concentrated in predominantly non- Hispanic White areas of LA County vs predominantly Hispanic areas (P<.0001). Notably, the average income in predominantly non-Hispanic White zip codes ($114,757.74) was significantly higher than in predominantly Hispanic areas ($58,278.54)(P=.001)(Table).4
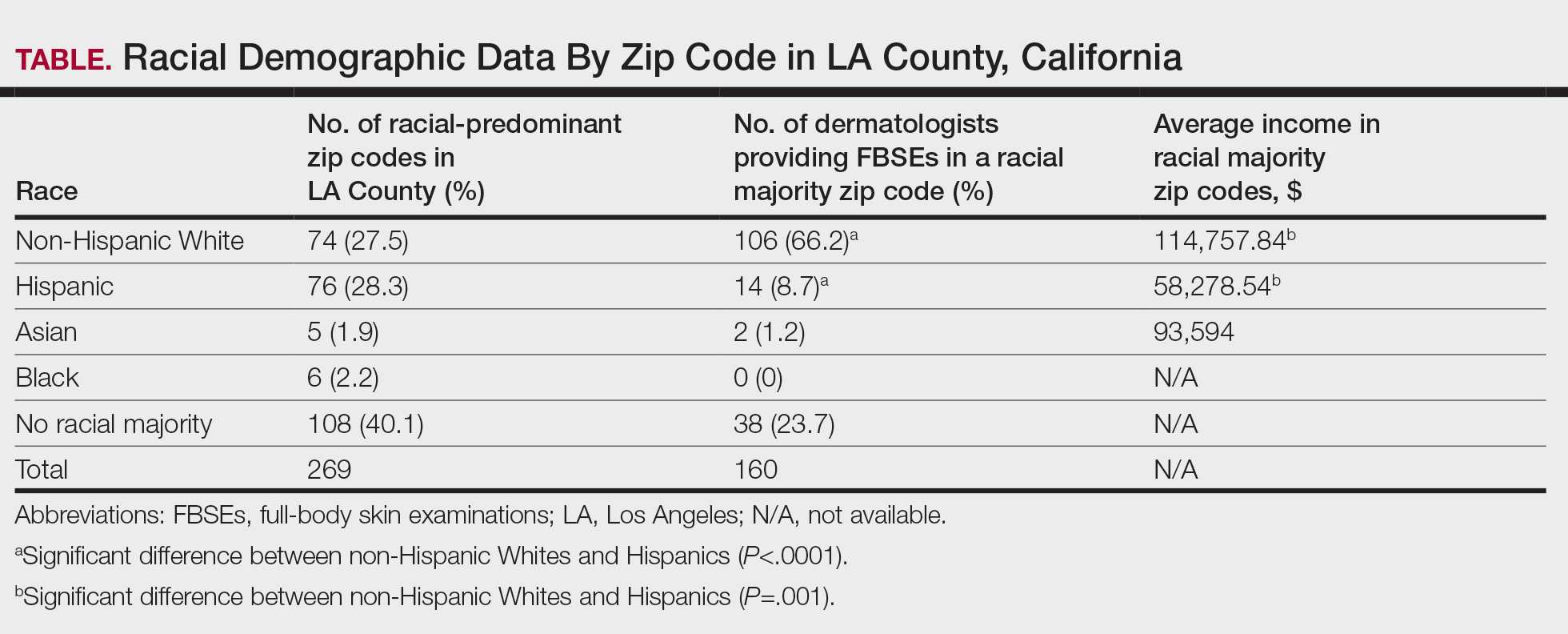
In LA County, 40.1% (108/269) of zip codes have no racial majority, 28.2% (76/269) are predominantly Hispanic, 27.5% (74/269) are predominantly non-Hispanic White, 2.2% (6/269) are predominantly Black, and 1.9% (5/269) are predominantly Asian.4 There are no dermatologists in predominantly Black zip codes, 2 in predominantly Asian zip codes, 14 in predominantly Hispanic zip codes, 38 in zip codes with no racial majority, and 106 in predominantly non-Hispanic White zip codes. There are significantly more dermatologists in predominantly non-Hispanic White zip codes compared to predominantly Hispanic zip codes (P<.0001). In LA County, the average income in predominantly Asian, non-Hispanic White, and Hispanic zip codes was $93,594, $114,757.84, and $58,278.54, respectively, in 2021.4 The average income in predominantly non-Hispanic White zip codes was significantly higher than in predominantly Hispanic zip codes (P=.001). There were no income data available for predominantly Black zip codes or zip codes with no racial majority.
The results from our study revealed potential barriers to FBSEs for racial and ethnic minorities in LA County, which supports previous research on the impact of SES, race, and insurance on access to dermatologic care.2,3 Predominantly Hispanic zip codes have significantly lower income (P<.0001) and fewer dermatologists (P=.001) compared to zip codes that are predominantly non-Hispanic White, reflecting how lower SES correlates with worse health outcomes and higher melanoma mortality. Conversely, predominantly non-Hispanic White areas with higher income have better access to dermatologists, which may contribute to the improved melanoma survival rates among White patients. Additionally, most dermatologists accept only private insurance, further highlighting the disparity in FBSE access for non-White patients across LA County. While our study focused on FBSE access, our findings may point to a wider barrier to dermatologic care, especially in zip codes with fewer dermatologists. Further studies are needed to determine whether these areas also face barriers to accessing primary care.
Our study was limited by the exclusion of nonphysician providers (eg, nurse practitioners, physician assistants), a small sample size, and lack of available economic data for predominantly Black zip codes.4 Additionally, the exclusion of practices with exclusive insurance plans (eg, Kaiser Permanente) limited the generalizability of our findings, as our results did not account for the populations served by these practices. Furthermore, our analysis did not account for variations in practice size or the proportion of care provided to patients with different insurance types, which could impact overall accessibility. Additional studies are needed to explore the impact of these factors on access to general dermatologic care and not just FBSEs.
Racial/ethnic minorities and lower SES populations face major barriers to FBSE access in LA County, such as difficulty finding a dermatologist in their area or one who accepts Medi-Cal. Addressing these disparities is crucial for improving skin cancer outcomes. Further research is needed to develop strategies to eliminate these barriers to dermatologic care, such as increasing access to teledermatology, offering mobile dermatology clinics, and improving insurance coverage.
- Chiaravalloti AJ, Laduca JR. Melanoma screening by means of complete skin exams for all patients in a dermatology practice reduces the thickness of primary melanomas at diagnosis. J Clin Aesthet Dermatol. 2014;7:18-22.
- Qian Y, Johannet P, Sawyers A, et al. The ongoing racial disparities in melanoma: an analysis of the Surveillance, Epidemiology, and End Results database (1975-2016). J Am Acad Dermatol. 2021;84:1585-1593.
- Baranowski MLH, Yeung H, Chen SC, et al. Factors associated with time to surgery in melanoma: an analysis of the National Cancer Database. J Am Acad Dermatol. 2019;81:908-916.
- United States Census Bureau. Explore census data. Accessed March 17, 2025. https://data.census.gov/all?q=los+angeles+county
- Berkowitz SA, Traore CY, Singer DE, et al. Evaluating area-based socioeconomic status indicators for monitoring disparities within health care systems: results from a primary care network. Health Serv Res. 2015;50:398-417.
- Jacobs B, Ir P, Bigdeli M, et al. Addressing access barriers to health services: an analytical framework for selecting appropriate interventions in lowincome Asian countries. Health Policy Plan. 2012;27:288-300.
To the Editor:
Early skin cancer detection improves patient outcomes1; however, socioeconomic and racial disparities may impact access to dermatologic care.2 Although non-Hispanic White individuals have a high incidence of skin cancer, they experience higher melanoma-specific survival rates than non-White patients, who often receive later-stage diagnoses and experience higher mortality.2 Furthermore, racial/ ethnic minorities often face longer surgery wait times after diagnosis and have lower socioeconomic status (SES) and less favorable health insurance coverage, contributing to poorer outcomes.2,3
To examine access to full-body skin examinations (FBSEs) by board-certified dermatologists in Los Angeles (LA) County, California, we analyzed the availability of FBSEs based on racial demographics, income, and insurance type (Medicaid [Medi-Cal] vs private [Blue Cross Blue Shield (BCBS)]). Demographic data by zip code were obtained from the US Census Bureau.4 This validated metric highlights socioeconomic disparities and minimizes data gaps5,6 and was used to assess health care access among different population subgroups. Dermatologists’ contact information was obtained from the Find a Dermatologist page on the American Academy of Dermatology website and the listed phone numbers of their practice were used to contact them. Practices with board-certified dermatologists accepting new patients were included in the study; practices were not included if they had exclusive insurance plans; were pediatric, cosmetic, or research only; or were nonresponsive to calls. From August 2022 to September 2022, each practice was called twice within a 36-hour period—once by a simulated patient with Medi-Cal and once by a simulated patient with BCBS—and were asked about availability for new patient FBSE appointments and accepted insurance types. Data were analyzed using SAS software (SAS Institute Inc.).
Los Angeles County comprises 269 zip codes, of which 82 (30.5%) have dermatology practices. Of 213 total dermatologists in LA County listed on the American Academy of Dermatology website, 193 (90.6%) met preliminary criteria, and 169 (79.3%) were successfully contacted. Almost all (94.6% [160/169]) accepted new patients for FBSEs; of those, 63.1% (101/160) accepted only private insurance, 16.9% (27/160) accepted both private insurance and Medi-Cal, and 16.2% (26/160) did not accept any insurance. Racial predominance for each dermatology practice was analyzed by zip code (Table). Dermatologists included in our study were significantly more concentrated in predominantly non- Hispanic White areas of LA County vs predominantly Hispanic areas (P<.0001). Notably, the average income in predominantly non-Hispanic White zip codes ($114,757.74) was significantly higher than in predominantly Hispanic areas ($58,278.54)(P=.001)(Table).4

In LA County, 40.1% (108/269) of zip codes have no racial majority, 28.2% (76/269) are predominantly Hispanic, 27.5% (74/269) are predominantly non-Hispanic White, 2.2% (6/269) are predominantly Black, and 1.9% (5/269) are predominantly Asian.4 There are no dermatologists in predominantly Black zip codes, 2 in predominantly Asian zip codes, 14 in predominantly Hispanic zip codes, 38 in zip codes with no racial majority, and 106 in predominantly non-Hispanic White zip codes. There are significantly more dermatologists in predominantly non-Hispanic White zip codes compared to predominantly Hispanic zip codes (P<.0001). In LA County, the average income in predominantly Asian, non-Hispanic White, and Hispanic zip codes was $93,594, $114,757.84, and $58,278.54, respectively, in 2021.4 The average income in predominantly non-Hispanic White zip codes was significantly higher than in predominantly Hispanic zip codes (P=.001). There were no income data available for predominantly Black zip codes or zip codes with no racial majority.
The results from our study revealed potential barriers to FBSEs for racial and ethnic minorities in LA County, which supports previous research on the impact of SES, race, and insurance on access to dermatologic care.2,3 Predominantly Hispanic zip codes have significantly lower income (P<.0001) and fewer dermatologists (P=.001) compared to zip codes that are predominantly non-Hispanic White, reflecting how lower SES correlates with worse health outcomes and higher melanoma mortality. Conversely, predominantly non-Hispanic White areas with higher income have better access to dermatologists, which may contribute to the improved melanoma survival rates among White patients. Additionally, most dermatologists accept only private insurance, further highlighting the disparity in FBSE access for non-White patients across LA County. While our study focused on FBSE access, our findings may point to a wider barrier to dermatologic care, especially in zip codes with fewer dermatologists. Further studies are needed to determine whether these areas also face barriers to accessing primary care.
Our study was limited by the exclusion of nonphysician providers (eg, nurse practitioners, physician assistants), a small sample size, and lack of available economic data for predominantly Black zip codes.4 Additionally, the exclusion of practices with exclusive insurance plans (eg, Kaiser Permanente) limited the generalizability of our findings, as our results did not account for the populations served by these practices. Furthermore, our analysis did not account for variations in practice size or the proportion of care provided to patients with different insurance types, which could impact overall accessibility. Additional studies are needed to explore the impact of these factors on access to general dermatologic care and not just FBSEs.
Racial/ethnic minorities and lower SES populations face major barriers to FBSE access in LA County, such as difficulty finding a dermatologist in their area or one who accepts Medi-Cal. Addressing these disparities is crucial for improving skin cancer outcomes. Further research is needed to develop strategies to eliminate these barriers to dermatologic care, such as increasing access to teledermatology, offering mobile dermatology clinics, and improving insurance coverage.
To the Editor:
Early skin cancer detection improves patient outcomes1; however, socioeconomic and racial disparities may impact access to dermatologic care.2 Although non-Hispanic White individuals have a high incidence of skin cancer, they experience higher melanoma-specific survival rates than non-White patients, who often receive later-stage diagnoses and experience higher mortality.2 Furthermore, racial/ ethnic minorities often face longer surgery wait times after diagnosis and have lower socioeconomic status (SES) and less favorable health insurance coverage, contributing to poorer outcomes.2,3
To examine access to full-body skin examinations (FBSEs) by board-certified dermatologists in Los Angeles (LA) County, California, we analyzed the availability of FBSEs based on racial demographics, income, and insurance type (Medicaid [Medi-Cal] vs private [Blue Cross Blue Shield (BCBS)]). Demographic data by zip code were obtained from the US Census Bureau.4 This validated metric highlights socioeconomic disparities and minimizes data gaps5,6 and was used to assess health care access among different population subgroups. Dermatologists’ contact information was obtained from the Find a Dermatologist page on the American Academy of Dermatology website and the listed phone numbers of their practice were used to contact them. Practices with board-certified dermatologists accepting new patients were included in the study; practices were not included if they had exclusive insurance plans; were pediatric, cosmetic, or research only; or were nonresponsive to calls. From August 2022 to September 2022, each practice was called twice within a 36-hour period—once by a simulated patient with Medi-Cal and once by a simulated patient with BCBS—and were asked about availability for new patient FBSE appointments and accepted insurance types. Data were analyzed using SAS software (SAS Institute Inc.).
Los Angeles County comprises 269 zip codes, of which 82 (30.5%) have dermatology practices. Of 213 total dermatologists in LA County listed on the American Academy of Dermatology website, 193 (90.6%) met preliminary criteria, and 169 (79.3%) were successfully contacted. Almost all (94.6% [160/169]) accepted new patients for FBSEs; of those, 63.1% (101/160) accepted only private insurance, 16.9% (27/160) accepted both private insurance and Medi-Cal, and 16.2% (26/160) did not accept any insurance. Racial predominance for each dermatology practice was analyzed by zip code (Table). Dermatologists included in our study were significantly more concentrated in predominantly non- Hispanic White areas of LA County vs predominantly Hispanic areas (P<.0001). Notably, the average income in predominantly non-Hispanic White zip codes ($114,757.74) was significantly higher than in predominantly Hispanic areas ($58,278.54)(P=.001)(Table).4

In LA County, 40.1% (108/269) of zip codes have no racial majority, 28.2% (76/269) are predominantly Hispanic, 27.5% (74/269) are predominantly non-Hispanic White, 2.2% (6/269) are predominantly Black, and 1.9% (5/269) are predominantly Asian.4 There are no dermatologists in predominantly Black zip codes, 2 in predominantly Asian zip codes, 14 in predominantly Hispanic zip codes, 38 in zip codes with no racial majority, and 106 in predominantly non-Hispanic White zip codes. There are significantly more dermatologists in predominantly non-Hispanic White zip codes compared to predominantly Hispanic zip codes (P<.0001). In LA County, the average income in predominantly Asian, non-Hispanic White, and Hispanic zip codes was $93,594, $114,757.84, and $58,278.54, respectively, in 2021.4 The average income in predominantly non-Hispanic White zip codes was significantly higher than in predominantly Hispanic zip codes (P=.001). There were no income data available for predominantly Black zip codes or zip codes with no racial majority.
The results from our study revealed potential barriers to FBSEs for racial and ethnic minorities in LA County, which supports previous research on the impact of SES, race, and insurance on access to dermatologic care.2,3 Predominantly Hispanic zip codes have significantly lower income (P<.0001) and fewer dermatologists (P=.001) compared to zip codes that are predominantly non-Hispanic White, reflecting how lower SES correlates with worse health outcomes and higher melanoma mortality. Conversely, predominantly non-Hispanic White areas with higher income have better access to dermatologists, which may contribute to the improved melanoma survival rates among White patients. Additionally, most dermatologists accept only private insurance, further highlighting the disparity in FBSE access for non-White patients across LA County. While our study focused on FBSE access, our findings may point to a wider barrier to dermatologic care, especially in zip codes with fewer dermatologists. Further studies are needed to determine whether these areas also face barriers to accessing primary care.
Our study was limited by the exclusion of nonphysician providers (eg, nurse practitioners, physician assistants), a small sample size, and lack of available economic data for predominantly Black zip codes.4 Additionally, the exclusion of practices with exclusive insurance plans (eg, Kaiser Permanente) limited the generalizability of our findings, as our results did not account for the populations served by these practices. Furthermore, our analysis did not account for variations in practice size or the proportion of care provided to patients with different insurance types, which could impact overall accessibility. Additional studies are needed to explore the impact of these factors on access to general dermatologic care and not just FBSEs.
Racial/ethnic minorities and lower SES populations face major barriers to FBSE access in LA County, such as difficulty finding a dermatologist in their area or one who accepts Medi-Cal. Addressing these disparities is crucial for improving skin cancer outcomes. Further research is needed to develop strategies to eliminate these barriers to dermatologic care, such as increasing access to teledermatology, offering mobile dermatology clinics, and improving insurance coverage.
- Chiaravalloti AJ, Laduca JR. Melanoma screening by means of complete skin exams for all patients in a dermatology practice reduces the thickness of primary melanomas at diagnosis. J Clin Aesthet Dermatol. 2014;7:18-22.
- Qian Y, Johannet P, Sawyers A, et al. The ongoing racial disparities in melanoma: an analysis of the Surveillance, Epidemiology, and End Results database (1975-2016). J Am Acad Dermatol. 2021;84:1585-1593.
- Baranowski MLH, Yeung H, Chen SC, et al. Factors associated with time to surgery in melanoma: an analysis of the National Cancer Database. J Am Acad Dermatol. 2019;81:908-916.
- United States Census Bureau. Explore census data. Accessed March 17, 2025. https://data.census.gov/all?q=los+angeles+county
- Berkowitz SA, Traore CY, Singer DE, et al. Evaluating area-based socioeconomic status indicators for monitoring disparities within health care systems: results from a primary care network. Health Serv Res. 2015;50:398-417.
- Jacobs B, Ir P, Bigdeli M, et al. Addressing access barriers to health services: an analytical framework for selecting appropriate interventions in lowincome Asian countries. Health Policy Plan. 2012;27:288-300.
- Chiaravalloti AJ, Laduca JR. Melanoma screening by means of complete skin exams for all patients in a dermatology practice reduces the thickness of primary melanomas at diagnosis. J Clin Aesthet Dermatol. 2014;7:18-22.
- Qian Y, Johannet P, Sawyers A, et al. The ongoing racial disparities in melanoma: an analysis of the Surveillance, Epidemiology, and End Results database (1975-2016). J Am Acad Dermatol. 2021;84:1585-1593.
- Baranowski MLH, Yeung H, Chen SC, et al. Factors associated with time to surgery in melanoma: an analysis of the National Cancer Database. J Am Acad Dermatol. 2019;81:908-916.
- United States Census Bureau. Explore census data. Accessed March 17, 2025. https://data.census.gov/all?q=los+angeles+county
- Berkowitz SA, Traore CY, Singer DE, et al. Evaluating area-based socioeconomic status indicators for monitoring disparities within health care systems: results from a primary care network. Health Serv Res. 2015;50:398-417.
- Jacobs B, Ir P, Bigdeli M, et al. Addressing access barriers to health services: an analytical framework for selecting appropriate interventions in lowincome Asian countries. Health Policy Plan. 2012;27:288-300.
Evaluating Access to Full-Body Skin Examinations in Los Angeles County, California
Evaluating Access to Full-Body Skin Examinations in Los Angeles County, California
PRACTICE POINTS
- Socioeconomic and racial disparities impact access to full-body skin examinations (FBSEs) in Los Angeles County.
- Most dermatologists included in this study were accepting new patients for a FBSE.
- There are significantly more dermatologists in predominantly non-Hispanic White zip codes than in predominantly Hispanic zip codes in Los Angeles County.
Exploring Skin Pigmentation Adaptation: A Systematic Review on the Vitamin D Adaptation Hypothesis
The risk for developing skin cancer can be somewhat attributed to variations in skin pigmentation. Historically, lighter skin pigmentation has been observed in populations living in higher latitudes and darker pigmentation in populations near the equator. Although skin pigmentation is a conglomeration of genetic and environmental factors, anthropologic studies have demonstrated an association of human skin lightening with historic human migratory patterns.1 It is postulated that migration to latitudes with less UVB light penetration has resulted in a compensatory natural selection of lighter skin types. Furthermore, the driving force behind this migration-associated skin lightening has remained unclear.1
The need for folate metabolism, vitamin D synthesis, and barrier protection, as well as cultural practices, has been postulated as driving factors for skin pigmentation variation. Synthesis of vitamin D is a UV radiation (UVR)–dependent process and has remained a prominent theoretical driver for the basis of evolutionary skin lightening. Vitamin D can be acquired both exogenously or endogenously via dietary supplementation or sunlight; however, historically it has been obtained through UVB exposure primarily. Once UVB is absorbed by the skin, it catalyzes conversion of 7-dehydrocholesterol to previtamin D3, which is converted to vitamin D in the kidneys.2,3 It is suggested that lighter skin tones have an advantage over darker skin tones in synthesizing vitamin D at higher latitudes where there is less UVB, thus leading to the adaptation process.1 In this systematic review, we analyzed the evolutionary vitamin D adaptation hypothesis and assessed the validity of evidence supporting this theory in the literature.
Methods
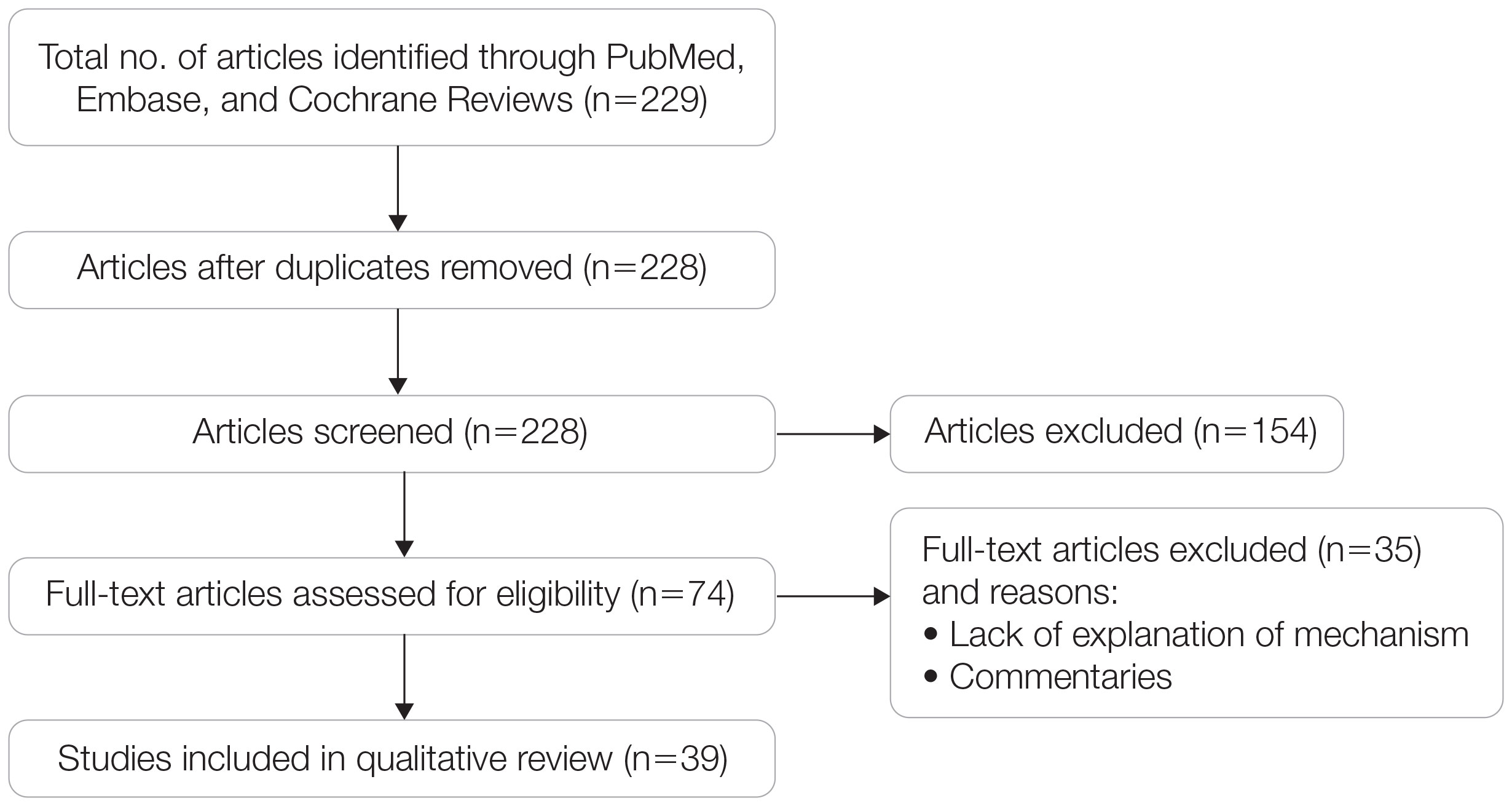
A search of PubMed, Embase, and the Cochrane Reviews database was conducted using the terms evolution, vitamin D, and skin to generate articles published from 2010 to 2022 that evaluated the influence of UVR-dependent production of vitamin D on skin pigmentation through historical migration patterns (Figure). Studies were excluded during an initial screening of abstracts followed by full-text assessment if they only had abstracts and if articles were inaccessible for review or in the form of case reports and commentaries.
The following data were extracted from each included study: reference citation, affiliated institutions of authors, author specialties, journal name, year of publication, study period, type of article, type of study, mechanism of adaptation, data concluding or supporting vitamin D as the driver, and data concluding or suggesting against vitamin D as the driver. Data concluding or supporting vitamin D as the driver were recorded from statistically significant results, study conclusions, and direct quotations. Data concluding or suggesting against vitamin D as the driver also were recorded from significant results, study conclusions, and direct quotes. The mechanism of adaptation was based on vitamin D synthesis modulation, melanin upregulation, genetic selections, genetic drift, mating patterns, increased vitamin D sensitivity, interbreeding, and diet.
Studies included in the analysis were placed into 1 of 3 categories: supporting, neutral, and against. Strength of Recommendation Taxonomy (SORT) criteria were used to classify the level of evidence of each article.4 Each article’s level of evidence was then graded (Table 1). The SORT grading levels were based on quality and evidence type: level 1 signified good-quality, patient-oriented evidence; level 2 signified limited-quality, patient-oriented evidence; and level 3 signified other evidence.4
Results
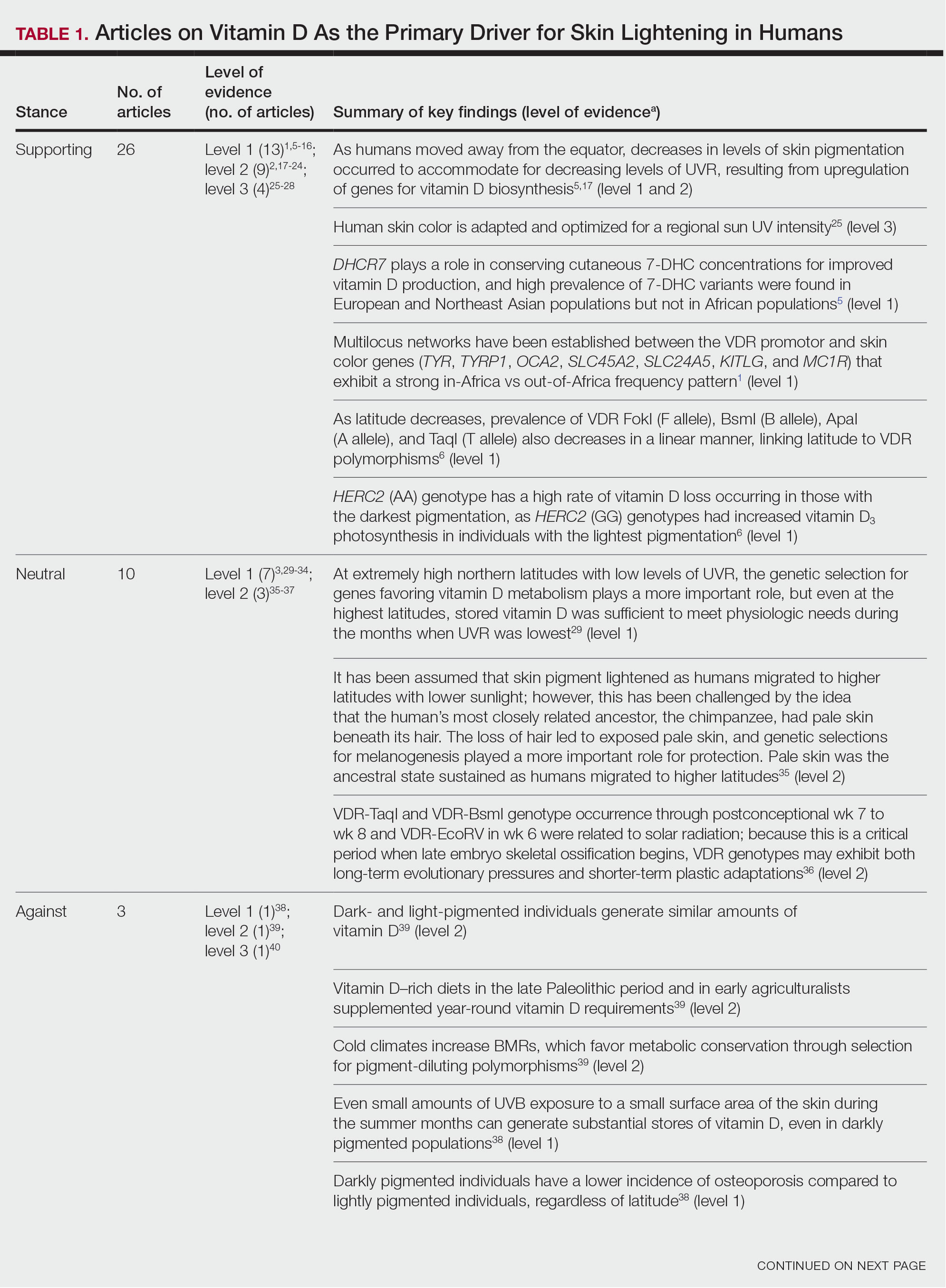
Article Selection—A total of 229 articles were identified for screening, and 39 studies met inclusion criteria.1-3,5-40 Systematic and retrospective reviews were the most common types of studies. Genomic analysis/sequencing/genome-wide association studies (GWAS) were the most common methods of analysis. Of these 39 articles, 26 were classified as supporting the evolutionary vitamin D adaptation hypothesis, 10 were classified as neutral, and 3 were classified as against (Table 1).
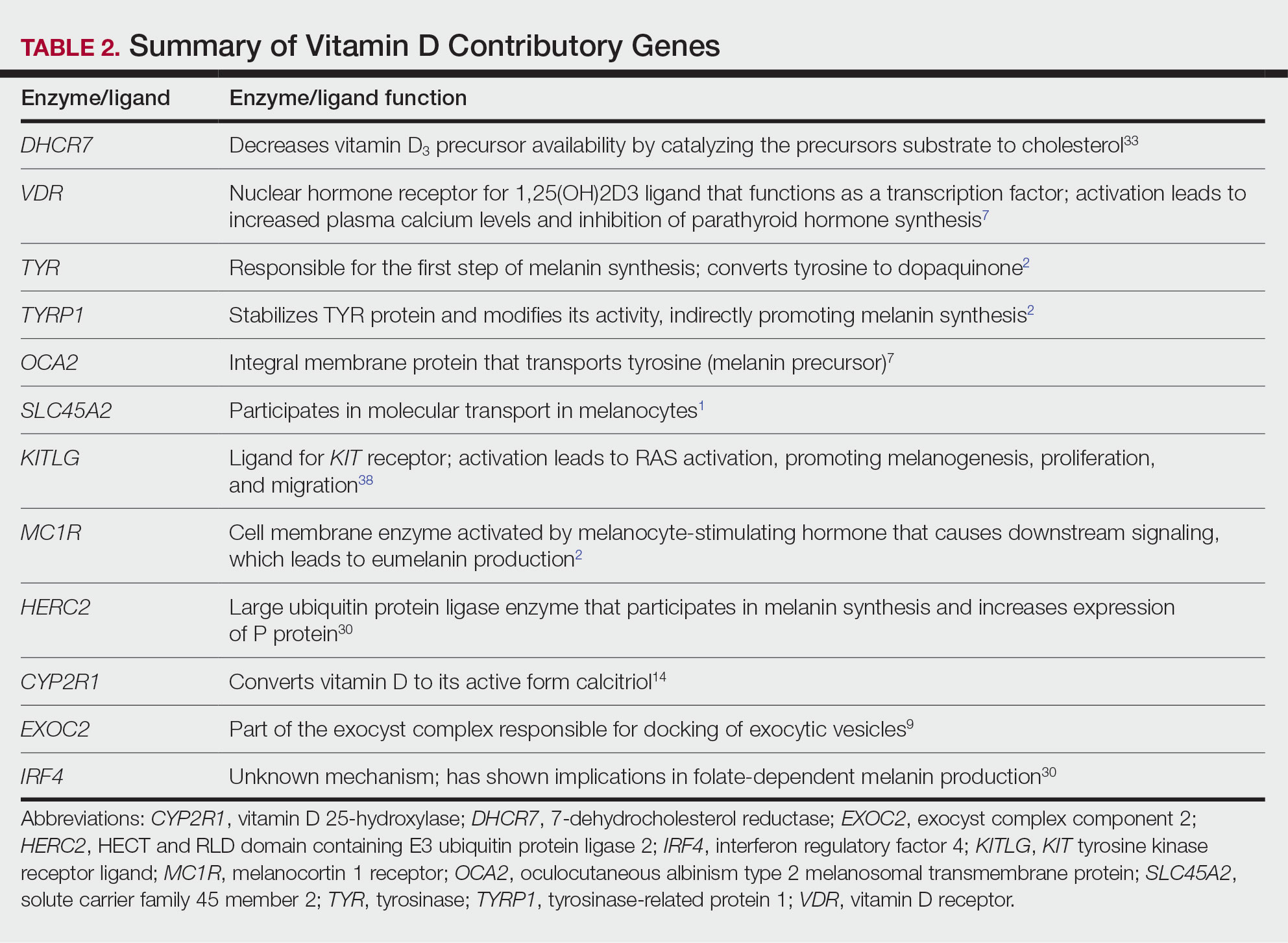
Of the articles classified as supporting the vitamin D hypothesis, 13 articles were level 1 evidence, 9 were level 2, and 4 were level 3. Key findings supporting the vitamin D hypothesis included genetic natural selection favoring vitamin D synthesis genes at higher latitudes with lower UVR and the skin lightening that occurred to protect against vitamin D deficiency (Table 1). Specific genes supporting these findings included 7-dehydrocholesterol reductase (DHCR7), vitamin D receptor (VDR), tyrosinase (TYR), tyrosinase-related protein 1 (TYRP1), oculocutaneous albinism type 2 melanosomal transmembrane protein (OCA2), solute carrier family 45 member 2 (SLC45A2), solute carrier family 4 member 5 (SLC24A5), Kit ligand (KITLG), melanocortin 1 receptor (MC1R), and HECT and RLD domain containing E3 ubiquitin protein ligase 2 (HERC2)(Table 2).
Of the articles classified as being against the vitamin D hypothesis, 1 article was level 1 evidence, 1 was level 2, and 1 was level 3. Key findings refuting the vitamin D hypothesis included similar amounts of vitamin D synthesis in contemporary dark- and light-pigmented individuals, vitamin D–rich diets in the late Paleolithic period and in early agriculturalists, and metabolic conservation being the primary driver (Table 1).
Of the articles classified as neutral to the hypothesis, 7 articles were level 1 evidence and 3 were level 2. Key findings of these articles included genetic selection favoring vitamin D synthesis only for populations at extremely northern latitudes, skin lightening that was sustained in northern latitudes from the neighboring human ancestor the chimpanzee, and evidence for long-term evolutionary pressures and short-term plastic adaptations in vitamin D genes (Table 1).
Comment
The importance of appropriate vitamin D levels is hypothesized as a potent driver in skin lightening because the vitamin is essential for many biochemical processes within the human body. Proper calcification of bones requires activated vitamin D to prevent rickets in childhood. Pelvic deformation in women with rickets can obstruct childbirth in primitive medical environments.15 This direct reproductive impairment suggests a strong selective pressure for skin lightening in populations that migrated northward to enhance vitamin D synthesis.
Of the 39 articles that we reviewed, the majority (n=26 [66.7%]) supported the hypothesis that vitamin D synthesis was the main driver behind skin lightening, whereas 3 (7.7%) did not support the hypothesis and 10 (25.6%) were neutral. Other leading theories explaining skin lightening included the idea that enhanced melanogenesis protected against folate degradation; genetic selection for light-skin alleles due to genetic drift; skin lightening being the result of sexual selection; and a combination of factors, including dietary choices, clothing preferences, and skin permeability barriers.
Articles With Supporting Evidence for the Vitamin D Theory—As Homo sapiens migrated out of Africa, migration patterns demonstrated the correlation between distance from the equator and skin pigmentation from natural selection. Individuals with darker skin pigment required higher levels of UVR to synthesize vitamin D. According to Beleza et al,1 as humans migrated to areas of higher latitudes with lower levels of UVR, natural selection favored the development of lighter skin to maximize vitamin D production. Vitamin D is linked to calcium metabolism, and its deficiency can lead to bone malformations and poor immune function.35 Several genes affecting melanogenesis and skin pigment have been found to have geospatial patterns that map to different geographic locations of various populations, indicating how human migration patterns out of Africa created this natural selection for skin lightening. The gene KITLG—associated with lighter skin pigmentation—has been found in high frequencies in both European and East Asian populations and is proposed to have increased in frequency after the migration out of Africa. However, the genes TYRP1, SLC24A5, and SLC45A2 were found at high frequencies only in European populations, and this selection occurred 11,000 to 19,000 years ago during the Last Glacial Maximum (15,000–20,000 years ago), demonstrating the selection for European over East Asian characteristics. During this period, seasonal changes increased the risk for vitamin D deficiency and provided an urgency for selection to a lighter skin pigment.1
The migration of H sapiens to northern latitudes prompted the selection of alleles that would increasevitamin D synthesis to counteract the reduced UV exposure. Genetic analysis studies have found key associations between genes encoding for the metabolism of vitamin D and pigmentation. Among this complex network are the essential downstream enzymes in the melanocortin receptor 1 pathway, including TYR and TYRP1. Forty-six of 960 single-nucleotide polymorphisms located in 29 different genes involved in skin pigmentation that were analyzed in a cohort of 2970 individuals were significantly associated with serum vitamin D levels (P<.05). The exocyst complex component 2 (EXOC2), TYR, and TYRP1 gene variants were shown to have the greatest influence on vitamin D status.9 These data reveal how pigment genotypes are predictive of vitamin D levels and the epistatic potential among many genes in this complex network.
Gene variation plays an important role in vitamin D status when comparing genetic polymorphisms in populations in northern latitudes to African populations. Vitamin D3 precursor availability is decreased by 7-DHCR catalyzing the precursors substrate to cholesterol. In a study using GWAS, it was found that “variations in DHCR7 may aid vitamin D production by conserving cutaneous 7-DHC levels. A high prevalence of DHCR7 variants were found in European and Northeast Asian populations but not in African populations, suggesting that selection occurred for these DHCR7 mutations in populations who migrated to more northern latitudes.5 Multilocus networks have been established between the VDR promotor and skin color genes (Table 2) that exhibit a strong in-Africa vs out-of-Africa frequency pattern. It also has been shown that genetic variation (suggesting a long-term evolutionary inclination) and epigenetic modification (indicative of short-term exposure) of VDR lends support to the vitamin D hypothesis. As latitude decreases, prevalence of VDR FokI (F allele), BsmI (B allele), ApaI (A allele), and TaqI (T allele) also decreases in a linear manner, linking latitude to VDR polymorphisms. Plasma vitamin D levels and photoperiod of conception—UV exposure during the periconceptional period—also were extrapolative of VDR methylation in a study involving 80 participants, where these 2 factors accounted for 17% of variance in methylation.6
Other noteworthy genes included HERC2, which has implications in the expression of OCA2 (melanocyte-specific transporter protein), and IRF4, which encodes for an important enzyme in folate-dependent melanin production. In an Australian cross-sectional study that analyzed vitamin D and pigmentation gene polymorphisms in conjunction with plasma vitamin D levels, the most notable rate of vitamin D loss occurred in individuals with the darkest pigmentation HERC2 (AA) genotype.31 In contrast, the lightest pigmentation HERC2 (GG) genotypes had increased vitamin D3 photosynthesis. Interestingly, the lightest interferon regulatory factor 4 (IRF4) TT genotype and the darkest HERC2 AA genotype, rendering the greatest folate loss and largest synthesis of vitamin D3, were not seen in combination in any of the participants.30 In addition to HERC2, derived alleles from pigment-associated genes SLC24A5*A and SLC45A2*G demonstrated greater frequencies in Europeans (>90%) compared to Africans and East Asians, where the allelic frequencies were either rare or absent.1 This evidence delineates not only the complexity but also the strong relationship between skin pigmentation, latitude, and vitamin D status. The GWAS also have supported this concept. In comparing European populations to African populations, there was a 4-fold increase in the frequencies of “derived alleles of the vitamin D transport protein (GC, rs3755967), the 25(OH)D3 synthesizing enzyme (CYP2R1, rs10741657), VDR (rs2228570 (commonly known as FokI polymorphism), rs1544410 (Bsm1), and rs731236 (Taq1) and the VDR target genes CYP24A1 (rs17216707), CD14 (rs2569190), and CARD9 (rs4077515).”32
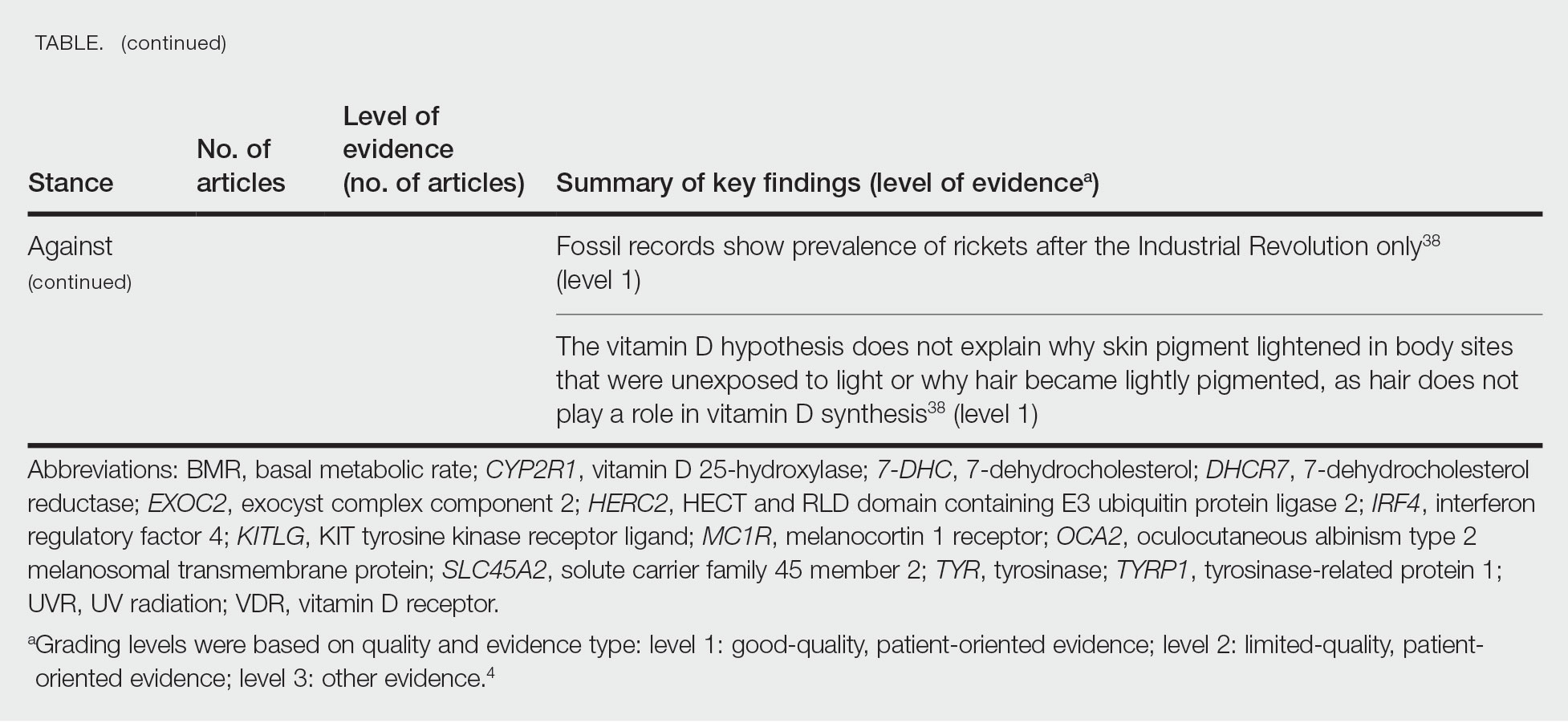
Articles With Evidence Against the Vitamin D Theory—This review analyzed the level of support for the theory that vitamin D was the main driver for skin lightening. Although most articles supported this theory, there were articles that listed other plausible counterarguments. Jablonski and Chaplin3 suggested that humans living in higher latitudes compensated for increased demand of vitamin D by placing cultural importance on a diet of vitamin D–rich foods and thus would not have experienced decreased vitamin D levels, which we hypothesize were the driver for skin lightening. Elias et al39 argued that initial pigment dilution may have instead served to improve metabolic conservation, as the authors found no evidence of rickets—the sequelae of vitamin D deficiency—in pre–industrial age human fossils. Elias and Williams38 proposed that differences in skin pigment are due to a more intact skin permeability barrier as “a requirement for life in a desiccating terrestrial environment,” which is seen in darker skin tones compared to lighter skin tones and thus can survive better in warmer climates with less risk of infections or dehydration.
Articles With Neutral Evidence for the Vitamin D Theory—Greaves41 argued against the idea that skin evolved to become lighter to protect against vitamin D deficiency. They proposed that the chimpanzee, which is the human’s most closely related species, had light skin covered by hair, and the loss of this hair led to exposed pale skin that created a need for increased melanin production for protection from UVR. Greaves41 stated that the MC1R gene (associated with darker pigmentation) was selected for in African populations, and those with pale skin retained their original pigment as they migrated to higher latitudes. Further research has demonstrated that the genetic natural selection for skin pigment is a complex process that involves multiple gene variants found throughout cultures across the globe.
Conclusion
Skin pigmentation has continuously evolved alongside humans. Genetic selection for lighter skin coincides with a favorable selection for genes involved in vitamin D synthesis as humans migrated to northern latitudes, which enabled humans to produce adequate levels of exogenous vitamin D in low-UVR areas and in turn promoted survival. Early humans without access to supplementation or foods rich in vitamin D acquired vitamin D primarily through sunlight. In comparison to modern society, where vitamin D supplementation is accessible and human lifespans are prolonged, lighter skin tone is now a risk factor for malignant cancers of the skin rather than being a protective adaptation. Current sun behavior recommendations conclude that the body’s need for vitamin D is satisfied by UV exposure to the arms, legs, hands, and/or face for only 5 to 30 minutes between 10
The hypothesis that skin lightening primarily was driven by the need for vitamin D can only be partially supported by our review. Studies have shown that there is a corresponding complex network of genes that determines skin pigmentation as well as vitamin D synthesis and conservation. However, there is sufficient evidence that skin lightening is multifactorial in nature, and vitamin D alone may not be the sole driver. The information in this review can be used by health care providers to educate patients on sun protection, given the lesser threat of severe vitamin D deficiency in developed communities today that have access to adequate nutrition and supplementation.
Skin lightening and its coinciding evolutionary drivers are a rather neglected area of research. Due to heterogeneous cohorts and conservative data analysis, GWAS studies run the risk of type II error, yielding a limitation in our data analysis.9 Furthermore, the data regarding specific time frames in evolutionary skin lightening as well as the intensity of gene polymorphisms are limited.1 Further studies are needed to determine the interconnectedness of the current skin-lightening theories to identify other important factors that may play a role in the process. Determining the key event can help us better understand skin-adaptation mechanisms and create a framework for understanding the vital process involved in adaptation, survival, and disease manifestation in different patient populations.
- Beleza S, Santos AM, McEvoy B, et al. The timing of pigmentation lightening in Europeans. Mol Biol Evol. 2013;30:24-35. doi:10.1093/molbev/mss207
- Carlberg C. Nutrigenomics of vitamin D. Nutrients. 2019;11:676. doi:10.3390/nu11030676
- Jablonski NG, Chaplin G. The roles of vitamin D and cutaneous vitamin D production in human evolution and health. Int J Paleopathol. 2018;23:54-59. doi:10.1016/j.ijpp.2018.01.005
- Weiss BD. SORT: strength of recommendation taxonomy. Fam Med. 2004;36:141-143.
- Wolf ST, Kenney WL. The vitamin D–folate hypothesis in human vascular health. Am J Physiol Regul Integr Comp Physiology. 2019;317:R491-R501. doi:10.1152/ajpregu.00136.2019
- Lucock M, Jones P, Martin C, et al. Photobiology of vitamins. Nutr Rev. 2018;76:512-525. doi:10.1093/nutrit/nuy013
- Hochberg Z, Hochberg I. Evolutionary perspective in rickets and vitamin D. Front Endocrinol (Lausanne). 2019;10:306. doi:10.3389/fendo.2019.00306
- Rossberg W, Saternus R, Wagenpfeil S, et al. Human pigmentation, cutaneous vitamin D synthesis and evolution: variants of genes (SNPs) involved in skin pigmentation are associated with 25(OH)D serum concentration. Anticancer Res. 2016;36:1429-1437.
- Saternus R, Pilz S, Gräber S, et al. A closer look at evolution: variants (SNPs) of genes involved in skin pigmentation, including EXOC2, TYR, TYRP1, and DCT, are associated with 25(OH)D serum concentration. Endocrinology. 2015;156:39-47. doi:10.1210/en.2014-1238
- López S, García Ó, Yurrebaso I, et al. The interplay between natural selection and susceptibility to melanoma on allele 374F of SLC45A2 gene in a south European population. PloS One. 2014;9:E104367. doi:1371/journal.pone.0104367
- Lucock M, Yates Z, Martin C, et al. Vitamin D, folate, and potential early lifecycle environmental origin of significant adult phenotypes. Evol Med Public Health. 2014;2014:69-91. doi:10.1093/emph/eou013
- Hudjashov G, Villems R, Kivisild T. Global patterns of diversity and selection in human tyrosinase gene. PloS One. 2013;8:E74307. doi:10.1371/journal.pone.0074307
- Khan R, Khan BSR. Diet, disease and pigment variation in humans. Med Hypotheses. 2010;75:363-367. doi:10.1016/j.mehy.2010.03.033
- Kuan V, Martineau AR, Griffiths CJ, et al. DHCR7 mutations linked to higher vitamin D status allowed early human migration to northern latitudes. BMC Evol Biol. 2013;13:144. doi:10.1186/1471-2148-13-144
- Omenn GS. Evolution and public health. Proc National Acad Sci. 2010;107(suppl 1):1702-1709. doi:10.1073/pnas.0906198106
- Yuen AWC, Jablonski NG. Vitamin D: in the evolution of human skin colour. Med Hypotheses. 2010;74:39-44. doi:10.1016/j.mehy.2009.08.007
- Vieth R. Weaker bones and white skin as adaptions to improve anthropological “fitness” for northern environments. Osteoporosis Int. 2020;31:617-624. doi:10.1007/s00198-019-05167-4
- Carlberg C. Vitamin D: a micronutrient regulating genes. Curr Pharm Des. 2019;25:1740-1746. doi:10.2174/1381612825666190705193227
- Haddadeen C, Lai C, Cho SY, et al. Variants of the melanocortin‐1 receptor: do they matter clinically? Exp Dermatol. 2015;1:5-9. doi:10.1111/exd.12540
- Yao S, Ambrosone CB. Associations between vitamin D deficiency and risk of aggressive breast cancer in African-American women. J Steroid Biochem Mol Biol. 2013;136:337-341. doi:10.1016/j.jsbmb.2012.09.010
- Jablonski N. The evolution of human skin colouration and its relevance to health in the modern world. J Royal Coll Physicians Edinb. 2012;42:58-63. doi:10.4997/jrcpe.2012.114
- Jablonski NG, Chaplin G. Human skin pigmentation as an adaptation to UV radiation. Proc National Acad Sci. 2010;107(suppl 2):8962-8968. doi:10.1073/pnas.0914628107
- Hochberg Z, Templeton AR. Evolutionary perspective in skin color, vitamin D and its receptor. Hormones. 2010;9:307-311. doi:10.14310/horm.2002.1281
- Jones P, Lucock M, Veysey M, et al. The vitamin D–folate hypothesis as an evolutionary model for skin pigmentation: an update and integration of current ideas. Nutrients. 2018;10:554. doi:10.3390/nu10050554
- Lindqvist PG, Epstein E, Landin-Olsson M, et al. Women with fair phenotypes seem to confer a survival advantage in a low UV milieu. a nested matched case control study. PloS One. 2020;15:E0228582. doi:10.1371/journal.pone.0228582
- Holick MF. Shedding new light on the role of the sunshine vitamin D for skin health: the lncRNA–skin cancer connection. Exp Dermatol. 2014;23:391-392. doi:10.1111/exd.12386
- Jablonski NG, Chaplin G. Epidermal pigmentation in the human lineage is an adaptation to ultraviolet radiation. J Hum Evol. 2013;65:671-675. doi:10.1016/j.jhevol.2013.06.004
- Jablonski NG, Chaplin G. The evolution of skin pigmentation and hair texture in people of African ancestry. Dermatol Clin. 2014;32:113-121. doi:10.1016/j.det.2013.11.003
- Jablonski NG. The evolution of human skin pigmentation involved the interactions of genetic, environmental, and cultural variables. Pigment Cell Melanoma Res. 2021;34:707-7 doi:10.1111/pcmr.12976
- Lucock MD, Jones PR, Veysey M, et al. Biophysical evidence to support and extend the vitamin D‐folate hypothesis as a paradigm for the evolution of human skin pigmentation. Am J Hum Biol. 2022;34:E23667. doi:10.1002/ajhb.23667
- Missaggia BO, Reales G, Cybis GB, et al. Adaptation and co‐adaptation of skin pigmentation and vitamin D genes in native Americans. Am J Med Genet C Semin Med Genet. 2020;184:1060-1077. doi:10.1002/ajmg.c.31873
- Hanel A, Carlberg C. Skin colour and vitamin D: an update. Exp Dermatol. 2020;29:864-875. doi:10.1111/exd.14142
- Hanel A, Carlberg C. Vitamin D and evolution: pharmacologic implications. Biochem Pharmacol. 2020;173:113595. doi:10.1016/j.bcp.2019.07.024
- Flegr J, Sýkorová K, Fiala V, et al. Increased 25(OH)D3 level in redheaded people: could redheadedness be an adaptation to temperate climate? Exp Dermatol. 2020;29:598-609. doi:10.1111/exd.14119
- James WPT, Johnson RJ, Speakman JR, et al. Nutrition and its role in human evolution. J Intern Med. 2019;285:533-549. doi:10.1111/joim.12878
- Lucock M, Jones P, Martin C, et al. Vitamin D: beyond metabolism. J Evid Based Complementary Altern Med. 2015;20:310-322. doi:10.1177/2156587215580491
- Jarrett P, Scragg R. Evolution, prehistory and vitamin D. Int J Environ Res Public Health. 2020;17:646. doi:10.3390/ijerph17020646
- Elias PM, Williams ML. Re-appraisal of current theories for thedevelopment and loss of epidermal pigmentation in hominins and modern humans. J Hum Evol. 2013;64:687-692. doi:10.1016/j.jhevol.2013.02.003
- Elias PM, Williams ML. Basis for the gain and subsequent dilution of epidermal pigmentation during human evolution: the barrier and metabolic conservation hypotheses revisited. Am J Phys Anthropol. 2016;161:189-207. doi:10.1002/ajpa.23030
- Williams JD, Jacobson EL, Kim H, et al. Water soluble vitamins, clinical research and future application. Subcell Biochem. 2011;56:181-197. doi:10.1007/978-94-007-2199-9_10
- Greaves M. Was skin cancer a selective force for black pigmentation in early hominin evolution [published online February 26, 2014]? Proc Biol Sci. 2014;281:20132955. doi:10.1098/rspb.2013.2955
- Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266-281. doi:10.1056/nejmra070553
- Bouillon R. Comparative analysis of nutritional guidelines for vitamin D. Nat Rev Endocrinol. 2017;13:466-479. doi:10.1038/nrendo.2017.31
- US Department of Health and Human Services. The Surgeon General’s Call to Action to Prevent Skin Cancer. US Dept of Health and Human Services, Office of the Surgeon General; 2014. Accessed April 29, 2024. https://www.hhs.gov/sites/default/files/call-to-action-prevent-skin-cancer.pdf
- Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium; Ross AC, Taylor CL, Yaktine AL, et al, eds. Dietary Reference Intakes for Calcium and Vitamin D. National Academies Press; 2011. https://www.ncbi.nlm.nih.gov/books/NBK56070/
The risk for developing skin cancer can be somewhat attributed to variations in skin pigmentation. Historically, lighter skin pigmentation has been observed in populations living in higher latitudes and darker pigmentation in populations near the equator. Although skin pigmentation is a conglomeration of genetic and environmental factors, anthropologic studies have demonstrated an association of human skin lightening with historic human migratory patterns.1 It is postulated that migration to latitudes with less UVB light penetration has resulted in a compensatory natural selection of lighter skin types. Furthermore, the driving force behind this migration-associated skin lightening has remained unclear.1
The need for folate metabolism, vitamin D synthesis, and barrier protection, as well as cultural practices, has been postulated as driving factors for skin pigmentation variation. Synthesis of vitamin D is a UV radiation (UVR)–dependent process and has remained a prominent theoretical driver for the basis of evolutionary skin lightening. Vitamin D can be acquired both exogenously or endogenously via dietary supplementation or sunlight; however, historically it has been obtained through UVB exposure primarily. Once UVB is absorbed by the skin, it catalyzes conversion of 7-dehydrocholesterol to previtamin D3, which is converted to vitamin D in the kidneys.2,3 It is suggested that lighter skin tones have an advantage over darker skin tones in synthesizing vitamin D at higher latitudes where there is less UVB, thus leading to the adaptation process.1 In this systematic review, we analyzed the evolutionary vitamin D adaptation hypothesis and assessed the validity of evidence supporting this theory in the literature.
Methods
A search of PubMed, Embase, and the Cochrane Reviews database was conducted using the terms evolution, vitamin D, and skin to generate articles published from 2010 to 2022 that evaluated the influence of UVR-dependent production of vitamin D on skin pigmentation through historical migration patterns (Figure). Studies were excluded during an initial screening of abstracts followed by full-text assessment if they only had abstracts and if articles were inaccessible for review or in the form of case reports and commentaries.
The following data were extracted from each included study: reference citation, affiliated institutions of authors, author specialties, journal name, year of publication, study period, type of article, type of study, mechanism of adaptation, data concluding or supporting vitamin D as the driver, and data concluding or suggesting against vitamin D as the driver. Data concluding or supporting vitamin D as the driver were recorded from statistically significant results, study conclusions, and direct quotations. Data concluding or suggesting against vitamin D as the driver also were recorded from significant results, study conclusions, and direct quotes. The mechanism of adaptation was based on vitamin D synthesis modulation, melanin upregulation, genetic selections, genetic drift, mating patterns, increased vitamin D sensitivity, interbreeding, and diet.
Studies included in the analysis were placed into 1 of 3 categories: supporting, neutral, and against. Strength of Recommendation Taxonomy (SORT) criteria were used to classify the level of evidence of each article.4 Each article’s level of evidence was then graded (Table 1). The SORT grading levels were based on quality and evidence type: level 1 signified good-quality, patient-oriented evidence; level 2 signified limited-quality, patient-oriented evidence; and level 3 signified other evidence.4
Results
Article Selection—A total of 229 articles were identified for screening, and 39 studies met inclusion criteria.1-3,5-40 Systematic and retrospective reviews were the most common types of studies. Genomic analysis/sequencing/genome-wide association studies (GWAS) were the most common methods of analysis. Of these 39 articles, 26 were classified as supporting the evolutionary vitamin D adaptation hypothesis, 10 were classified as neutral, and 3 were classified as against (Table 1).
Of the articles classified as supporting the vitamin D hypothesis, 13 articles were level 1 evidence, 9 were level 2, and 4 were level 3. Key findings supporting the vitamin D hypothesis included genetic natural selection favoring vitamin D synthesis genes at higher latitudes with lower UVR and the skin lightening that occurred to protect against vitamin D deficiency (Table 1). Specific genes supporting these findings included 7-dehydrocholesterol reductase (DHCR7), vitamin D receptor (VDR), tyrosinase (TYR), tyrosinase-related protein 1 (TYRP1), oculocutaneous albinism type 2 melanosomal transmembrane protein (OCA2), solute carrier family 45 member 2 (SLC45A2), solute carrier family 4 member 5 (SLC24A5), Kit ligand (KITLG), melanocortin 1 receptor (MC1R), and HECT and RLD domain containing E3 ubiquitin protein ligase 2 (HERC2)(Table 2).

Of the articles classified as being against the vitamin D hypothesis, 1 article was level 1 evidence, 1 was level 2, and 1 was level 3. Key findings refuting the vitamin D hypothesis included similar amounts of vitamin D synthesis in contemporary dark- and light-pigmented individuals, vitamin D–rich diets in the late Paleolithic period and in early agriculturalists, and metabolic conservation being the primary driver (Table 1).
Of the articles classified as neutral to the hypothesis, 7 articles were level 1 evidence and 3 were level 2. Key findings of these articles included genetic selection favoring vitamin D synthesis only for populations at extremely northern latitudes, skin lightening that was sustained in northern latitudes from the neighboring human ancestor the chimpanzee, and evidence for long-term evolutionary pressures and short-term plastic adaptations in vitamin D genes (Table 1).
Comment
The importance of appropriate vitamin D levels is hypothesized as a potent driver in skin lightening because the vitamin is essential for many biochemical processes within the human body. Proper calcification of bones requires activated vitamin D to prevent rickets in childhood. Pelvic deformation in women with rickets can obstruct childbirth in primitive medical environments.15 This direct reproductive impairment suggests a strong selective pressure for skin lightening in populations that migrated northward to enhance vitamin D synthesis.
Of the 39 articles that we reviewed, the majority (n=26 [66.7%]) supported the hypothesis that vitamin D synthesis was the main driver behind skin lightening, whereas 3 (7.7%) did not support the hypothesis and 10 (25.6%) were neutral. Other leading theories explaining skin lightening included the idea that enhanced melanogenesis protected against folate degradation; genetic selection for light-skin alleles due to genetic drift; skin lightening being the result of sexual selection; and a combination of factors, including dietary choices, clothing preferences, and skin permeability barriers.
Articles With Supporting Evidence for the Vitamin D Theory—As Homo sapiens migrated out of Africa, migration patterns demonstrated the correlation between distance from the equator and skin pigmentation from natural selection. Individuals with darker skin pigment required higher levels of UVR to synthesize vitamin D. According to Beleza et al,1 as humans migrated to areas of higher latitudes with lower levels of UVR, natural selection favored the development of lighter skin to maximize vitamin D production. Vitamin D is linked to calcium metabolism, and its deficiency can lead to bone malformations and poor immune function.35 Several genes affecting melanogenesis and skin pigment have been found to have geospatial patterns that map to different geographic locations of various populations, indicating how human migration patterns out of Africa created this natural selection for skin lightening. The gene KITLG—associated with lighter skin pigmentation—has been found in high frequencies in both European and East Asian populations and is proposed to have increased in frequency after the migration out of Africa. However, the genes TYRP1, SLC24A5, and SLC45A2 were found at high frequencies only in European populations, and this selection occurred 11,000 to 19,000 years ago during the Last Glacial Maximum (15,000–20,000 years ago), demonstrating the selection for European over East Asian characteristics. During this period, seasonal changes increased the risk for vitamin D deficiency and provided an urgency for selection to a lighter skin pigment.1
The migration of H sapiens to northern latitudes prompted the selection of alleles that would increasevitamin D synthesis to counteract the reduced UV exposure. Genetic analysis studies have found key associations between genes encoding for the metabolism of vitamin D and pigmentation. Among this complex network are the essential downstream enzymes in the melanocortin receptor 1 pathway, including TYR and TYRP1. Forty-six of 960 single-nucleotide polymorphisms located in 29 different genes involved in skin pigmentation that were analyzed in a cohort of 2970 individuals were significantly associated with serum vitamin D levels (P<.05). The exocyst complex component 2 (EXOC2), TYR, and TYRP1 gene variants were shown to have the greatest influence on vitamin D status.9 These data reveal how pigment genotypes are predictive of vitamin D levels and the epistatic potential among many genes in this complex network.
Gene variation plays an important role in vitamin D status when comparing genetic polymorphisms in populations in northern latitudes to African populations. Vitamin D3 precursor availability is decreased by 7-DHCR catalyzing the precursors substrate to cholesterol. In a study using GWAS, it was found that “variations in DHCR7 may aid vitamin D production by conserving cutaneous 7-DHC levels. A high prevalence of DHCR7 variants were found in European and Northeast Asian populations but not in African populations, suggesting that selection occurred for these DHCR7 mutations in populations who migrated to more northern latitudes.5 Multilocus networks have been established between the VDR promotor and skin color genes (Table 2) that exhibit a strong in-Africa vs out-of-Africa frequency pattern. It also has been shown that genetic variation (suggesting a long-term evolutionary inclination) and epigenetic modification (indicative of short-term exposure) of VDR lends support to the vitamin D hypothesis. As latitude decreases, prevalence of VDR FokI (F allele), BsmI (B allele), ApaI (A allele), and TaqI (T allele) also decreases in a linear manner, linking latitude to VDR polymorphisms. Plasma vitamin D levels and photoperiod of conception—UV exposure during the periconceptional period—also were extrapolative of VDR methylation in a study involving 80 participants, where these 2 factors accounted for 17% of variance in methylation.6


Other noteworthy genes included HERC2, which has implications in the expression of OCA2 (melanocyte-specific transporter protein), and IRF4, which encodes for an important enzyme in folate-dependent melanin production. In an Australian cross-sectional study that analyzed vitamin D and pigmentation gene polymorphisms in conjunction with plasma vitamin D levels, the most notable rate of vitamin D loss occurred in individuals with the darkest pigmentation HERC2 (AA) genotype.31 In contrast, the lightest pigmentation HERC2 (GG) genotypes had increased vitamin D3 photosynthesis. Interestingly, the lightest interferon regulatory factor 4 (IRF4) TT genotype and the darkest HERC2 AA genotype, rendering the greatest folate loss and largest synthesis of vitamin D3, were not seen in combination in any of the participants.30 In addition to HERC2, derived alleles from pigment-associated genes SLC24A5*A and SLC45A2*G demonstrated greater frequencies in Europeans (>90%) compared to Africans and East Asians, where the allelic frequencies were either rare or absent.1 This evidence delineates not only the complexity but also the strong relationship between skin pigmentation, latitude, and vitamin D status. The GWAS also have supported this concept. In comparing European populations to African populations, there was a 4-fold increase in the frequencies of “derived alleles of the vitamin D transport protein (GC, rs3755967), the 25(OH)D3 synthesizing enzyme (CYP2R1, rs10741657), VDR (rs2228570 (commonly known as FokI polymorphism), rs1544410 (Bsm1), and rs731236 (Taq1) and the VDR target genes CYP24A1 (rs17216707), CD14 (rs2569190), and CARD9 (rs4077515).”32
Articles With Evidence Against the Vitamin D Theory—This review analyzed the level of support for the theory that vitamin D was the main driver for skin lightening. Although most articles supported this theory, there were articles that listed other plausible counterarguments. Jablonski and Chaplin3 suggested that humans living in higher latitudes compensated for increased demand of vitamin D by placing cultural importance on a diet of vitamin D–rich foods and thus would not have experienced decreased vitamin D levels, which we hypothesize were the driver for skin lightening. Elias et al39 argued that initial pigment dilution may have instead served to improve metabolic conservation, as the authors found no evidence of rickets—the sequelae of vitamin D deficiency—in pre–industrial age human fossils. Elias and Williams38 proposed that differences in skin pigment are due to a more intact skin permeability barrier as “a requirement for life in a desiccating terrestrial environment,” which is seen in darker skin tones compared to lighter skin tones and thus can survive better in warmer climates with less risk of infections or dehydration.
Articles With Neutral Evidence for the Vitamin D Theory—Greaves41 argued against the idea that skin evolved to become lighter to protect against vitamin D deficiency. They proposed that the chimpanzee, which is the human’s most closely related species, had light skin covered by hair, and the loss of this hair led to exposed pale skin that created a need for increased melanin production for protection from UVR. Greaves41 stated that the MC1R gene (associated with darker pigmentation) was selected for in African populations, and those with pale skin retained their original pigment as they migrated to higher latitudes. Further research has demonstrated that the genetic natural selection for skin pigment is a complex process that involves multiple gene variants found throughout cultures across the globe.

Conclusion
Skin pigmentation has continuously evolved alongside humans. Genetic selection for lighter skin coincides with a favorable selection for genes involved in vitamin D synthesis as humans migrated to northern latitudes, which enabled humans to produce adequate levels of exogenous vitamin D in low-UVR areas and in turn promoted survival. Early humans without access to supplementation or foods rich in vitamin D acquired vitamin D primarily through sunlight. In comparison to modern society, where vitamin D supplementation is accessible and human lifespans are prolonged, lighter skin tone is now a risk factor for malignant cancers of the skin rather than being a protective adaptation. Current sun behavior recommendations conclude that the body’s need for vitamin D is satisfied by UV exposure to the arms, legs, hands, and/or face for only 5 to 30 minutes between 10
The hypothesis that skin lightening primarily was driven by the need for vitamin D can only be partially supported by our review. Studies have shown that there is a corresponding complex network of genes that determines skin pigmentation as well as vitamin D synthesis and conservation. However, there is sufficient evidence that skin lightening is multifactorial in nature, and vitamin D alone may not be the sole driver. The information in this review can be used by health care providers to educate patients on sun protection, given the lesser threat of severe vitamin D deficiency in developed communities today that have access to adequate nutrition and supplementation.
Skin lightening and its coinciding evolutionary drivers are a rather neglected area of research. Due to heterogeneous cohorts and conservative data analysis, GWAS studies run the risk of type II error, yielding a limitation in our data analysis.9 Furthermore, the data regarding specific time frames in evolutionary skin lightening as well as the intensity of gene polymorphisms are limited.1 Further studies are needed to determine the interconnectedness of the current skin-lightening theories to identify other important factors that may play a role in the process. Determining the key event can help us better understand skin-adaptation mechanisms and create a framework for understanding the vital process involved in adaptation, survival, and disease manifestation in different patient populations.
The risk for developing skin cancer can be somewhat attributed to variations in skin pigmentation. Historically, lighter skin pigmentation has been observed in populations living in higher latitudes and darker pigmentation in populations near the equator. Although skin pigmentation is a conglomeration of genetic and environmental factors, anthropologic studies have demonstrated an association of human skin lightening with historic human migratory patterns.1 It is postulated that migration to latitudes with less UVB light penetration has resulted in a compensatory natural selection of lighter skin types. Furthermore, the driving force behind this migration-associated skin lightening has remained unclear.1
The need for folate metabolism, vitamin D synthesis, and barrier protection, as well as cultural practices, has been postulated as driving factors for skin pigmentation variation. Synthesis of vitamin D is a UV radiation (UVR)–dependent process and has remained a prominent theoretical driver for the basis of evolutionary skin lightening. Vitamin D can be acquired both exogenously or endogenously via dietary supplementation or sunlight; however, historically it has been obtained through UVB exposure primarily. Once UVB is absorbed by the skin, it catalyzes conversion of 7-dehydrocholesterol to previtamin D3, which is converted to vitamin D in the kidneys.2,3 It is suggested that lighter skin tones have an advantage over darker skin tones in synthesizing vitamin D at higher latitudes where there is less UVB, thus leading to the adaptation process.1 In this systematic review, we analyzed the evolutionary vitamin D adaptation hypothesis and assessed the validity of evidence supporting this theory in the literature.
Methods
A search of PubMed, Embase, and the Cochrane Reviews database was conducted using the terms evolution, vitamin D, and skin to generate articles published from 2010 to 2022 that evaluated the influence of UVR-dependent production of vitamin D on skin pigmentation through historical migration patterns (Figure). Studies were excluded during an initial screening of abstracts followed by full-text assessment if they only had abstracts and if articles were inaccessible for review or in the form of case reports and commentaries.
The following data were extracted from each included study: reference citation, affiliated institutions of authors, author specialties, journal name, year of publication, study period, type of article, type of study, mechanism of adaptation, data concluding or supporting vitamin D as the driver, and data concluding or suggesting against vitamin D as the driver. Data concluding or supporting vitamin D as the driver were recorded from statistically significant results, study conclusions, and direct quotations. Data concluding or suggesting against vitamin D as the driver also were recorded from significant results, study conclusions, and direct quotes. The mechanism of adaptation was based on vitamin D synthesis modulation, melanin upregulation, genetic selections, genetic drift, mating patterns, increased vitamin D sensitivity, interbreeding, and diet.
Studies included in the analysis were placed into 1 of 3 categories: supporting, neutral, and against. Strength of Recommendation Taxonomy (SORT) criteria were used to classify the level of evidence of each article.4 Each article’s level of evidence was then graded (Table 1). The SORT grading levels were based on quality and evidence type: level 1 signified good-quality, patient-oriented evidence; level 2 signified limited-quality, patient-oriented evidence; and level 3 signified other evidence.4
Results
Article Selection—A total of 229 articles were identified for screening, and 39 studies met inclusion criteria.1-3,5-40 Systematic and retrospective reviews were the most common types of studies. Genomic analysis/sequencing/genome-wide association studies (GWAS) were the most common methods of analysis. Of these 39 articles, 26 were classified as supporting the evolutionary vitamin D adaptation hypothesis, 10 were classified as neutral, and 3 were classified as against (Table 1).
Of the articles classified as supporting the vitamin D hypothesis, 13 articles were level 1 evidence, 9 were level 2, and 4 were level 3. Key findings supporting the vitamin D hypothesis included genetic natural selection favoring vitamin D synthesis genes at higher latitudes with lower UVR and the skin lightening that occurred to protect against vitamin D deficiency (Table 1). Specific genes supporting these findings included 7-dehydrocholesterol reductase (DHCR7), vitamin D receptor (VDR), tyrosinase (TYR), tyrosinase-related protein 1 (TYRP1), oculocutaneous albinism type 2 melanosomal transmembrane protein (OCA2), solute carrier family 45 member 2 (SLC45A2), solute carrier family 4 member 5 (SLC24A5), Kit ligand (KITLG), melanocortin 1 receptor (MC1R), and HECT and RLD domain containing E3 ubiquitin protein ligase 2 (HERC2)(Table 2).

Of the articles classified as being against the vitamin D hypothesis, 1 article was level 1 evidence, 1 was level 2, and 1 was level 3. Key findings refuting the vitamin D hypothesis included similar amounts of vitamin D synthesis in contemporary dark- and light-pigmented individuals, vitamin D–rich diets in the late Paleolithic period and in early agriculturalists, and metabolic conservation being the primary driver (Table 1).
Of the articles classified as neutral to the hypothesis, 7 articles were level 1 evidence and 3 were level 2. Key findings of these articles included genetic selection favoring vitamin D synthesis only for populations at extremely northern latitudes, skin lightening that was sustained in northern latitudes from the neighboring human ancestor the chimpanzee, and evidence for long-term evolutionary pressures and short-term plastic adaptations in vitamin D genes (Table 1).
Comment
The importance of appropriate vitamin D levels is hypothesized as a potent driver in skin lightening because the vitamin is essential for many biochemical processes within the human body. Proper calcification of bones requires activated vitamin D to prevent rickets in childhood. Pelvic deformation in women with rickets can obstruct childbirth in primitive medical environments.15 This direct reproductive impairment suggests a strong selective pressure for skin lightening in populations that migrated northward to enhance vitamin D synthesis.
Of the 39 articles that we reviewed, the majority (n=26 [66.7%]) supported the hypothesis that vitamin D synthesis was the main driver behind skin lightening, whereas 3 (7.7%) did not support the hypothesis and 10 (25.6%) were neutral. Other leading theories explaining skin lightening included the idea that enhanced melanogenesis protected against folate degradation; genetic selection for light-skin alleles due to genetic drift; skin lightening being the result of sexual selection; and a combination of factors, including dietary choices, clothing preferences, and skin permeability barriers.
Articles With Supporting Evidence for the Vitamin D Theory—As Homo sapiens migrated out of Africa, migration patterns demonstrated the correlation between distance from the equator and skin pigmentation from natural selection. Individuals with darker skin pigment required higher levels of UVR to synthesize vitamin D. According to Beleza et al,1 as humans migrated to areas of higher latitudes with lower levels of UVR, natural selection favored the development of lighter skin to maximize vitamin D production. Vitamin D is linked to calcium metabolism, and its deficiency can lead to bone malformations and poor immune function.35 Several genes affecting melanogenesis and skin pigment have been found to have geospatial patterns that map to different geographic locations of various populations, indicating how human migration patterns out of Africa created this natural selection for skin lightening. The gene KITLG—associated with lighter skin pigmentation—has been found in high frequencies in both European and East Asian populations and is proposed to have increased in frequency after the migration out of Africa. However, the genes TYRP1, SLC24A5, and SLC45A2 were found at high frequencies only in European populations, and this selection occurred 11,000 to 19,000 years ago during the Last Glacial Maximum (15,000–20,000 years ago), demonstrating the selection for European over East Asian characteristics. During this period, seasonal changes increased the risk for vitamin D deficiency and provided an urgency for selection to a lighter skin pigment.1
The migration of H sapiens to northern latitudes prompted the selection of alleles that would increasevitamin D synthesis to counteract the reduced UV exposure. Genetic analysis studies have found key associations between genes encoding for the metabolism of vitamin D and pigmentation. Among this complex network are the essential downstream enzymes in the melanocortin receptor 1 pathway, including TYR and TYRP1. Forty-six of 960 single-nucleotide polymorphisms located in 29 different genes involved in skin pigmentation that were analyzed in a cohort of 2970 individuals were significantly associated with serum vitamin D levels (P<.05). The exocyst complex component 2 (EXOC2), TYR, and TYRP1 gene variants were shown to have the greatest influence on vitamin D status.9 These data reveal how pigment genotypes are predictive of vitamin D levels and the epistatic potential among many genes in this complex network.
Gene variation plays an important role in vitamin D status when comparing genetic polymorphisms in populations in northern latitudes to African populations. Vitamin D3 precursor availability is decreased by 7-DHCR catalyzing the precursors substrate to cholesterol. In a study using GWAS, it was found that “variations in DHCR7 may aid vitamin D production by conserving cutaneous 7-DHC levels. A high prevalence of DHCR7 variants were found in European and Northeast Asian populations but not in African populations, suggesting that selection occurred for these DHCR7 mutations in populations who migrated to more northern latitudes.5 Multilocus networks have been established between the VDR promotor and skin color genes (Table 2) that exhibit a strong in-Africa vs out-of-Africa frequency pattern. It also has been shown that genetic variation (suggesting a long-term evolutionary inclination) and epigenetic modification (indicative of short-term exposure) of VDR lends support to the vitamin D hypothesis. As latitude decreases, prevalence of VDR FokI (F allele), BsmI (B allele), ApaI (A allele), and TaqI (T allele) also decreases in a linear manner, linking latitude to VDR polymorphisms. Plasma vitamin D levels and photoperiod of conception—UV exposure during the periconceptional period—also were extrapolative of VDR methylation in a study involving 80 participants, where these 2 factors accounted for 17% of variance in methylation.6


Other noteworthy genes included HERC2, which has implications in the expression of OCA2 (melanocyte-specific transporter protein), and IRF4, which encodes for an important enzyme in folate-dependent melanin production. In an Australian cross-sectional study that analyzed vitamin D and pigmentation gene polymorphisms in conjunction with plasma vitamin D levels, the most notable rate of vitamin D loss occurred in individuals with the darkest pigmentation HERC2 (AA) genotype.31 In contrast, the lightest pigmentation HERC2 (GG) genotypes had increased vitamin D3 photosynthesis. Interestingly, the lightest interferon regulatory factor 4 (IRF4) TT genotype and the darkest HERC2 AA genotype, rendering the greatest folate loss and largest synthesis of vitamin D3, were not seen in combination in any of the participants.30 In addition to HERC2, derived alleles from pigment-associated genes SLC24A5*A and SLC45A2*G demonstrated greater frequencies in Europeans (>90%) compared to Africans and East Asians, where the allelic frequencies were either rare or absent.1 This evidence delineates not only the complexity but also the strong relationship between skin pigmentation, latitude, and vitamin D status. The GWAS also have supported this concept. In comparing European populations to African populations, there was a 4-fold increase in the frequencies of “derived alleles of the vitamin D transport protein (GC, rs3755967), the 25(OH)D3 synthesizing enzyme (CYP2R1, rs10741657), VDR (rs2228570 (commonly known as FokI polymorphism), rs1544410 (Bsm1), and rs731236 (Taq1) and the VDR target genes CYP24A1 (rs17216707), CD14 (rs2569190), and CARD9 (rs4077515).”32
Articles With Evidence Against the Vitamin D Theory—This review analyzed the level of support for the theory that vitamin D was the main driver for skin lightening. Although most articles supported this theory, there were articles that listed other plausible counterarguments. Jablonski and Chaplin3 suggested that humans living in higher latitudes compensated for increased demand of vitamin D by placing cultural importance on a diet of vitamin D–rich foods and thus would not have experienced decreased vitamin D levels, which we hypothesize were the driver for skin lightening. Elias et al39 argued that initial pigment dilution may have instead served to improve metabolic conservation, as the authors found no evidence of rickets—the sequelae of vitamin D deficiency—in pre–industrial age human fossils. Elias and Williams38 proposed that differences in skin pigment are due to a more intact skin permeability barrier as “a requirement for life in a desiccating terrestrial environment,” which is seen in darker skin tones compared to lighter skin tones and thus can survive better in warmer climates with less risk of infections or dehydration.
Articles With Neutral Evidence for the Vitamin D Theory—Greaves41 argued against the idea that skin evolved to become lighter to protect against vitamin D deficiency. They proposed that the chimpanzee, which is the human’s most closely related species, had light skin covered by hair, and the loss of this hair led to exposed pale skin that created a need for increased melanin production for protection from UVR. Greaves41 stated that the MC1R gene (associated with darker pigmentation) was selected for in African populations, and those with pale skin retained their original pigment as they migrated to higher latitudes. Further research has demonstrated that the genetic natural selection for skin pigment is a complex process that involves multiple gene variants found throughout cultures across the globe.

Conclusion
Skin pigmentation has continuously evolved alongside humans. Genetic selection for lighter skin coincides with a favorable selection for genes involved in vitamin D synthesis as humans migrated to northern latitudes, which enabled humans to produce adequate levels of exogenous vitamin D in low-UVR areas and in turn promoted survival. Early humans without access to supplementation or foods rich in vitamin D acquired vitamin D primarily through sunlight. In comparison to modern society, where vitamin D supplementation is accessible and human lifespans are prolonged, lighter skin tone is now a risk factor for malignant cancers of the skin rather than being a protective adaptation. Current sun behavior recommendations conclude that the body’s need for vitamin D is satisfied by UV exposure to the arms, legs, hands, and/or face for only 5 to 30 minutes between 10
The hypothesis that skin lightening primarily was driven by the need for vitamin D can only be partially supported by our review. Studies have shown that there is a corresponding complex network of genes that determines skin pigmentation as well as vitamin D synthesis and conservation. However, there is sufficient evidence that skin lightening is multifactorial in nature, and vitamin D alone may not be the sole driver. The information in this review can be used by health care providers to educate patients on sun protection, given the lesser threat of severe vitamin D deficiency in developed communities today that have access to adequate nutrition and supplementation.
Skin lightening and its coinciding evolutionary drivers are a rather neglected area of research. Due to heterogeneous cohorts and conservative data analysis, GWAS studies run the risk of type II error, yielding a limitation in our data analysis.9 Furthermore, the data regarding specific time frames in evolutionary skin lightening as well as the intensity of gene polymorphisms are limited.1 Further studies are needed to determine the interconnectedness of the current skin-lightening theories to identify other important factors that may play a role in the process. Determining the key event can help us better understand skin-adaptation mechanisms and create a framework for understanding the vital process involved in adaptation, survival, and disease manifestation in different patient populations.
- Beleza S, Santos AM, McEvoy B, et al. The timing of pigmentation lightening in Europeans. Mol Biol Evol. 2013;30:24-35. doi:10.1093/molbev/mss207
- Carlberg C. Nutrigenomics of vitamin D. Nutrients. 2019;11:676. doi:10.3390/nu11030676
- Jablonski NG, Chaplin G. The roles of vitamin D and cutaneous vitamin D production in human evolution and health. Int J Paleopathol. 2018;23:54-59. doi:10.1016/j.ijpp.2018.01.005
- Weiss BD. SORT: strength of recommendation taxonomy. Fam Med. 2004;36:141-143.
- Wolf ST, Kenney WL. The vitamin D–folate hypothesis in human vascular health. Am J Physiol Regul Integr Comp Physiology. 2019;317:R491-R501. doi:10.1152/ajpregu.00136.2019
- Lucock M, Jones P, Martin C, et al. Photobiology of vitamins. Nutr Rev. 2018;76:512-525. doi:10.1093/nutrit/nuy013
- Hochberg Z, Hochberg I. Evolutionary perspective in rickets and vitamin D. Front Endocrinol (Lausanne). 2019;10:306. doi:10.3389/fendo.2019.00306
- Rossberg W, Saternus R, Wagenpfeil S, et al. Human pigmentation, cutaneous vitamin D synthesis and evolution: variants of genes (SNPs) involved in skin pigmentation are associated with 25(OH)D serum concentration. Anticancer Res. 2016;36:1429-1437.
- Saternus R, Pilz S, Gräber S, et al. A closer look at evolution: variants (SNPs) of genes involved in skin pigmentation, including EXOC2, TYR, TYRP1, and DCT, are associated with 25(OH)D serum concentration. Endocrinology. 2015;156:39-47. doi:10.1210/en.2014-1238
- López S, García Ó, Yurrebaso I, et al. The interplay between natural selection and susceptibility to melanoma on allele 374F of SLC45A2 gene in a south European population. PloS One. 2014;9:E104367. doi:1371/journal.pone.0104367
- Lucock M, Yates Z, Martin C, et al. Vitamin D, folate, and potential early lifecycle environmental origin of significant adult phenotypes. Evol Med Public Health. 2014;2014:69-91. doi:10.1093/emph/eou013
- Hudjashov G, Villems R, Kivisild T. Global patterns of diversity and selection in human tyrosinase gene. PloS One. 2013;8:E74307. doi:10.1371/journal.pone.0074307
- Khan R, Khan BSR. Diet, disease and pigment variation in humans. Med Hypotheses. 2010;75:363-367. doi:10.1016/j.mehy.2010.03.033
- Kuan V, Martineau AR, Griffiths CJ, et al. DHCR7 mutations linked to higher vitamin D status allowed early human migration to northern latitudes. BMC Evol Biol. 2013;13:144. doi:10.1186/1471-2148-13-144
- Omenn GS. Evolution and public health. Proc National Acad Sci. 2010;107(suppl 1):1702-1709. doi:10.1073/pnas.0906198106
- Yuen AWC, Jablonski NG. Vitamin D: in the evolution of human skin colour. Med Hypotheses. 2010;74:39-44. doi:10.1016/j.mehy.2009.08.007
- Vieth R. Weaker bones and white skin as adaptions to improve anthropological “fitness” for northern environments. Osteoporosis Int. 2020;31:617-624. doi:10.1007/s00198-019-05167-4
- Carlberg C. Vitamin D: a micronutrient regulating genes. Curr Pharm Des. 2019;25:1740-1746. doi:10.2174/1381612825666190705193227
- Haddadeen C, Lai C, Cho SY, et al. Variants of the melanocortin‐1 receptor: do they matter clinically? Exp Dermatol. 2015;1:5-9. doi:10.1111/exd.12540
- Yao S, Ambrosone CB. Associations between vitamin D deficiency and risk of aggressive breast cancer in African-American women. J Steroid Biochem Mol Biol. 2013;136:337-341. doi:10.1016/j.jsbmb.2012.09.010
- Jablonski N. The evolution of human skin colouration and its relevance to health in the modern world. J Royal Coll Physicians Edinb. 2012;42:58-63. doi:10.4997/jrcpe.2012.114
- Jablonski NG, Chaplin G. Human skin pigmentation as an adaptation to UV radiation. Proc National Acad Sci. 2010;107(suppl 2):8962-8968. doi:10.1073/pnas.0914628107
- Hochberg Z, Templeton AR. Evolutionary perspective in skin color, vitamin D and its receptor. Hormones. 2010;9:307-311. doi:10.14310/horm.2002.1281
- Jones P, Lucock M, Veysey M, et al. The vitamin D–folate hypothesis as an evolutionary model for skin pigmentation: an update and integration of current ideas. Nutrients. 2018;10:554. doi:10.3390/nu10050554
- Lindqvist PG, Epstein E, Landin-Olsson M, et al. Women with fair phenotypes seem to confer a survival advantage in a low UV milieu. a nested matched case control study. PloS One. 2020;15:E0228582. doi:10.1371/journal.pone.0228582
- Holick MF. Shedding new light on the role of the sunshine vitamin D for skin health: the lncRNA–skin cancer connection. Exp Dermatol. 2014;23:391-392. doi:10.1111/exd.12386
- Jablonski NG, Chaplin G. Epidermal pigmentation in the human lineage is an adaptation to ultraviolet radiation. J Hum Evol. 2013;65:671-675. doi:10.1016/j.jhevol.2013.06.004
- Jablonski NG, Chaplin G. The evolution of skin pigmentation and hair texture in people of African ancestry. Dermatol Clin. 2014;32:113-121. doi:10.1016/j.det.2013.11.003
- Jablonski NG. The evolution of human skin pigmentation involved the interactions of genetic, environmental, and cultural variables. Pigment Cell Melanoma Res. 2021;34:707-7 doi:10.1111/pcmr.12976
- Lucock MD, Jones PR, Veysey M, et al. Biophysical evidence to support and extend the vitamin D‐folate hypothesis as a paradigm for the evolution of human skin pigmentation. Am J Hum Biol. 2022;34:E23667. doi:10.1002/ajhb.23667
- Missaggia BO, Reales G, Cybis GB, et al. Adaptation and co‐adaptation of skin pigmentation and vitamin D genes in native Americans. Am J Med Genet C Semin Med Genet. 2020;184:1060-1077. doi:10.1002/ajmg.c.31873
- Hanel A, Carlberg C. Skin colour and vitamin D: an update. Exp Dermatol. 2020;29:864-875. doi:10.1111/exd.14142
- Hanel A, Carlberg C. Vitamin D and evolution: pharmacologic implications. Biochem Pharmacol. 2020;173:113595. doi:10.1016/j.bcp.2019.07.024
- Flegr J, Sýkorová K, Fiala V, et al. Increased 25(OH)D3 level in redheaded people: could redheadedness be an adaptation to temperate climate? Exp Dermatol. 2020;29:598-609. doi:10.1111/exd.14119
- James WPT, Johnson RJ, Speakman JR, et al. Nutrition and its role in human evolution. J Intern Med. 2019;285:533-549. doi:10.1111/joim.12878
- Lucock M, Jones P, Martin C, et al. Vitamin D: beyond metabolism. J Evid Based Complementary Altern Med. 2015;20:310-322. doi:10.1177/2156587215580491
- Jarrett P, Scragg R. Evolution, prehistory and vitamin D. Int J Environ Res Public Health. 2020;17:646. doi:10.3390/ijerph17020646
- Elias PM, Williams ML. Re-appraisal of current theories for thedevelopment and loss of epidermal pigmentation in hominins and modern humans. J Hum Evol. 2013;64:687-692. doi:10.1016/j.jhevol.2013.02.003
- Elias PM, Williams ML. Basis for the gain and subsequent dilution of epidermal pigmentation during human evolution: the barrier and metabolic conservation hypotheses revisited. Am J Phys Anthropol. 2016;161:189-207. doi:10.1002/ajpa.23030
- Williams JD, Jacobson EL, Kim H, et al. Water soluble vitamins, clinical research and future application. Subcell Biochem. 2011;56:181-197. doi:10.1007/978-94-007-2199-9_10
- Greaves M. Was skin cancer a selective force for black pigmentation in early hominin evolution [published online February 26, 2014]? Proc Biol Sci. 2014;281:20132955. doi:10.1098/rspb.2013.2955
- Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266-281. doi:10.1056/nejmra070553
- Bouillon R. Comparative analysis of nutritional guidelines for vitamin D. Nat Rev Endocrinol. 2017;13:466-479. doi:10.1038/nrendo.2017.31
- US Department of Health and Human Services. The Surgeon General’s Call to Action to Prevent Skin Cancer. US Dept of Health and Human Services, Office of the Surgeon General; 2014. Accessed April 29, 2024. https://www.hhs.gov/sites/default/files/call-to-action-prevent-skin-cancer.pdf
- Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium; Ross AC, Taylor CL, Yaktine AL, et al, eds. Dietary Reference Intakes for Calcium and Vitamin D. National Academies Press; 2011. https://www.ncbi.nlm.nih.gov/books/NBK56070/
- Beleza S, Santos AM, McEvoy B, et al. The timing of pigmentation lightening in Europeans. Mol Biol Evol. 2013;30:24-35. doi:10.1093/molbev/mss207
- Carlberg C. Nutrigenomics of vitamin D. Nutrients. 2019;11:676. doi:10.3390/nu11030676
- Jablonski NG, Chaplin G. The roles of vitamin D and cutaneous vitamin D production in human evolution and health. Int J Paleopathol. 2018;23:54-59. doi:10.1016/j.ijpp.2018.01.005
- Weiss BD. SORT: strength of recommendation taxonomy. Fam Med. 2004;36:141-143.
- Wolf ST, Kenney WL. The vitamin D–folate hypothesis in human vascular health. Am J Physiol Regul Integr Comp Physiology. 2019;317:R491-R501. doi:10.1152/ajpregu.00136.2019
- Lucock M, Jones P, Martin C, et al. Photobiology of vitamins. Nutr Rev. 2018;76:512-525. doi:10.1093/nutrit/nuy013
- Hochberg Z, Hochberg I. Evolutionary perspective in rickets and vitamin D. Front Endocrinol (Lausanne). 2019;10:306. doi:10.3389/fendo.2019.00306
- Rossberg W, Saternus R, Wagenpfeil S, et al. Human pigmentation, cutaneous vitamin D synthesis and evolution: variants of genes (SNPs) involved in skin pigmentation are associated with 25(OH)D serum concentration. Anticancer Res. 2016;36:1429-1437.
- Saternus R, Pilz S, Gräber S, et al. A closer look at evolution: variants (SNPs) of genes involved in skin pigmentation, including EXOC2, TYR, TYRP1, and DCT, are associated with 25(OH)D serum concentration. Endocrinology. 2015;156:39-47. doi:10.1210/en.2014-1238
- López S, García Ó, Yurrebaso I, et al. The interplay between natural selection and susceptibility to melanoma on allele 374F of SLC45A2 gene in a south European population. PloS One. 2014;9:E104367. doi:1371/journal.pone.0104367
- Lucock M, Yates Z, Martin C, et al. Vitamin D, folate, and potential early lifecycle environmental origin of significant adult phenotypes. Evol Med Public Health. 2014;2014:69-91. doi:10.1093/emph/eou013
- Hudjashov G, Villems R, Kivisild T. Global patterns of diversity and selection in human tyrosinase gene. PloS One. 2013;8:E74307. doi:10.1371/journal.pone.0074307
- Khan R, Khan BSR. Diet, disease and pigment variation in humans. Med Hypotheses. 2010;75:363-367. doi:10.1016/j.mehy.2010.03.033
- Kuan V, Martineau AR, Griffiths CJ, et al. DHCR7 mutations linked to higher vitamin D status allowed early human migration to northern latitudes. BMC Evol Biol. 2013;13:144. doi:10.1186/1471-2148-13-144
- Omenn GS. Evolution and public health. Proc National Acad Sci. 2010;107(suppl 1):1702-1709. doi:10.1073/pnas.0906198106
- Yuen AWC, Jablonski NG. Vitamin D: in the evolution of human skin colour. Med Hypotheses. 2010;74:39-44. doi:10.1016/j.mehy.2009.08.007
- Vieth R. Weaker bones and white skin as adaptions to improve anthropological “fitness” for northern environments. Osteoporosis Int. 2020;31:617-624. doi:10.1007/s00198-019-05167-4
- Carlberg C. Vitamin D: a micronutrient regulating genes. Curr Pharm Des. 2019;25:1740-1746. doi:10.2174/1381612825666190705193227
- Haddadeen C, Lai C, Cho SY, et al. Variants of the melanocortin‐1 receptor: do they matter clinically? Exp Dermatol. 2015;1:5-9. doi:10.1111/exd.12540
- Yao S, Ambrosone CB. Associations between vitamin D deficiency and risk of aggressive breast cancer in African-American women. J Steroid Biochem Mol Biol. 2013;136:337-341. doi:10.1016/j.jsbmb.2012.09.010
- Jablonski N. The evolution of human skin colouration and its relevance to health in the modern world. J Royal Coll Physicians Edinb. 2012;42:58-63. doi:10.4997/jrcpe.2012.114
- Jablonski NG, Chaplin G. Human skin pigmentation as an adaptation to UV radiation. Proc National Acad Sci. 2010;107(suppl 2):8962-8968. doi:10.1073/pnas.0914628107
- Hochberg Z, Templeton AR. Evolutionary perspective in skin color, vitamin D and its receptor. Hormones. 2010;9:307-311. doi:10.14310/horm.2002.1281
- Jones P, Lucock M, Veysey M, et al. The vitamin D–folate hypothesis as an evolutionary model for skin pigmentation: an update and integration of current ideas. Nutrients. 2018;10:554. doi:10.3390/nu10050554
- Lindqvist PG, Epstein E, Landin-Olsson M, et al. Women with fair phenotypes seem to confer a survival advantage in a low UV milieu. a nested matched case control study. PloS One. 2020;15:E0228582. doi:10.1371/journal.pone.0228582
- Holick MF. Shedding new light on the role of the sunshine vitamin D for skin health: the lncRNA–skin cancer connection. Exp Dermatol. 2014;23:391-392. doi:10.1111/exd.12386
- Jablonski NG, Chaplin G. Epidermal pigmentation in the human lineage is an adaptation to ultraviolet radiation. J Hum Evol. 2013;65:671-675. doi:10.1016/j.jhevol.2013.06.004
- Jablonski NG, Chaplin G. The evolution of skin pigmentation and hair texture in people of African ancestry. Dermatol Clin. 2014;32:113-121. doi:10.1016/j.det.2013.11.003
- Jablonski NG. The evolution of human skin pigmentation involved the interactions of genetic, environmental, and cultural variables. Pigment Cell Melanoma Res. 2021;34:707-7 doi:10.1111/pcmr.12976
- Lucock MD, Jones PR, Veysey M, et al. Biophysical evidence to support and extend the vitamin D‐folate hypothesis as a paradigm for the evolution of human skin pigmentation. Am J Hum Biol. 2022;34:E23667. doi:10.1002/ajhb.23667
- Missaggia BO, Reales G, Cybis GB, et al. Adaptation and co‐adaptation of skin pigmentation and vitamin D genes in native Americans. Am J Med Genet C Semin Med Genet. 2020;184:1060-1077. doi:10.1002/ajmg.c.31873
- Hanel A, Carlberg C. Skin colour and vitamin D: an update. Exp Dermatol. 2020;29:864-875. doi:10.1111/exd.14142
- Hanel A, Carlberg C. Vitamin D and evolution: pharmacologic implications. Biochem Pharmacol. 2020;173:113595. doi:10.1016/j.bcp.2019.07.024
- Flegr J, Sýkorová K, Fiala V, et al. Increased 25(OH)D3 level in redheaded people: could redheadedness be an adaptation to temperate climate? Exp Dermatol. 2020;29:598-609. doi:10.1111/exd.14119
- James WPT, Johnson RJ, Speakman JR, et al. Nutrition and its role in human evolution. J Intern Med. 2019;285:533-549. doi:10.1111/joim.12878
- Lucock M, Jones P, Martin C, et al. Vitamin D: beyond metabolism. J Evid Based Complementary Altern Med. 2015;20:310-322. doi:10.1177/2156587215580491
- Jarrett P, Scragg R. Evolution, prehistory and vitamin D. Int J Environ Res Public Health. 2020;17:646. doi:10.3390/ijerph17020646
- Elias PM, Williams ML. Re-appraisal of current theories for thedevelopment and loss of epidermal pigmentation in hominins and modern humans. J Hum Evol. 2013;64:687-692. doi:10.1016/j.jhevol.2013.02.003
- Elias PM, Williams ML. Basis for the gain and subsequent dilution of epidermal pigmentation during human evolution: the barrier and metabolic conservation hypotheses revisited. Am J Phys Anthropol. 2016;161:189-207. doi:10.1002/ajpa.23030
- Williams JD, Jacobson EL, Kim H, et al. Water soluble vitamins, clinical research and future application. Subcell Biochem. 2011;56:181-197. doi:10.1007/978-94-007-2199-9_10
- Greaves M. Was skin cancer a selective force for black pigmentation in early hominin evolution [published online February 26, 2014]? Proc Biol Sci. 2014;281:20132955. doi:10.1098/rspb.2013.2955
- Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266-281. doi:10.1056/nejmra070553
- Bouillon R. Comparative analysis of nutritional guidelines for vitamin D. Nat Rev Endocrinol. 2017;13:466-479. doi:10.1038/nrendo.2017.31
- US Department of Health and Human Services. The Surgeon General’s Call to Action to Prevent Skin Cancer. US Dept of Health and Human Services, Office of the Surgeon General; 2014. Accessed April 29, 2024. https://www.hhs.gov/sites/default/files/call-to-action-prevent-skin-cancer.pdf
- Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium; Ross AC, Taylor CL, Yaktine AL, et al, eds. Dietary Reference Intakes for Calcium and Vitamin D. National Academies Press; 2011. https://www.ncbi.nlm.nih.gov/books/NBK56070/
Practice Points
- Sufficient UV radiation exposure is required to synthesize vitamin D, but excess exposure increases skin cancer risk.
- Genes associated with vitamin D production and melanin synthesis form an interconnected network that explains skin tone polymorphisms and their influence on healthy sun behaviors.
- Adaptations in genetics of skin pigmentation and vitamin D metabolism due to anthropologic patterns of migration to northern latitudes may help explain predisposition to dermatologic diseases such as skin cancer.
Alopecia with perifollicular papules and pustules
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
A 23-Year-Old African American Man sought care at our medical center because he had been losing hair over the vertex of his scalp for the past several years. He indicated that his father had early-onset male patterned alopecia. As a result, he considered his hair loss “genetic.” However, he described waxing and waning flares of painful pustules associated with occasional spontaneous bleeding and discharge of purulent material that occurred in the same area as the hair loss.
Physical examination revealed multiple perifollicular papules and pustules on the vertex of his scalp with interspersed patches of alopecia (FIGURE 1). There were no lesions elsewhere on his body and his past medical history was otherwise unremarkable.
FIGURE 1
Alopecia with a painful twist
This 23-year-old patient said that he had spontaneous bleeding and discharge of purulent material in the area of his hair loss.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU MANAGE THIS PATIENT?
Diagnosis: Folliculitis decalvans
Folliculitis decalvans (FD) is a highly inflammatory form of scarring alopecia characterized by inflammatory perifollicular papules and pustules. The term scarring alopecia refers to the fact that the follicular epithelium has been replaced by connective tissue, ultimately resulting in permanent hair loss. This manifests clinically as patches of skin without terminal or vellus hairs, whereas a nonscarring alopecia would demonstrate preservation of the vellus hairs. Left untreated, advancing permanent hair loss ensues and may result in an end-stage pattern of tufted folliculitis or polytrichia, where interspersed dilated follicular openings house multiple hairs.
Affected areas commonly include the vertex and occipital scalp. Common symptoms include pain, itching, burning, and occasionally spontaneous bleeding or discharge of purulent material.1
FD generally occurs in young and middle-aged African Americans with a slight predominance in males. It accounts for 11% of all primary scarring alopecias.2,3 The etiology of this inflammatory process is not fully understood; however, scalp colonization with Staphylococcus aureus has been implicated as a contributing factor.4 Other reports suggest patients may have an altered host immune response and/or genetic predisposition for this condition.2,3
The differential includes various scarring, nonscarring alopecias
Since clinical findings of FD can range from relatively nonspecific mild disease at its onset to the end stage described above, a detailed patient history is needed. The following scarring and nonscarring alopecias should be considered in the diff erential diagnosis: dissecting cellulitis of the scalp, central centrifugal cicatricial alopecia (CCCA), acne keloidalis nuchae, erosive pustular dermatosis, lichen planopilaris (LPP), inflammatory tinea capitis, and secondary syphilis.
Dissecting cellulitis of the scalp is a distinctive, often debilitating disease commonly seen in young adult African American men. It is considered part of the follicular occlusion tetrad that also includes hidradenitis suppurativa, acne conglobata, and pilonidal cysts. It presents as a scarring alopecia with firm scalp nodules that rapidly develop into boggy, fluctuant, oval to linear sinuses that may eventually discharge purulent material.
In contrast to FD, dissecting scalp cellulitis lesions interconnect via sinus tract formation so that pressure on one fluctuant area may result in purulent discharge from perfo-rations several centimeters away.5 Although both dissecting cellulitis and FD are considered primary neutrophilic scarring alopecias, the presence of true sinus tract formation can be a distinguishing finding.
CCCA is the most common form of scarring alopecia among African Americans and is particularly seen among African American women.5 It generally presents on the scalp vertex like FD, but it is much less inflamma-tory and typically causes only mild pruritus or tenderness of the involved areas.
Although numerous theories have been suggested, the etiology is unknown. The pathogenesis is thought to be associated with premature desquamation of the inner root sheath, which can be demonstrated on biopsy. Also seen histologically is lymphocytic perifollicular inflammation and polytrichia.6
Acne keloidalis nuchae is also a scarring alopecia. It is seen most commonly in African American men and presents as keloid-like papules and plaques with occasional pustules characteristically on the occipital scalp and posterior neck. In contrast to FD, acne keloidalis nuchae papules coalesce and may form firm, hairless, protuberant keloid-like plaques that may be painful and cosmetically disfiguring. The cause of acne keloidalis nuchae is unknown.
Shaving or cutting tight curly hair too short and subsequently having the new hair curve back and penetrate the skin may be the precipitating event. Thus, a history of close shaving should make one suspect this diagnosis. Histologic analysis reveals a chronic, predominantly lymphocytic folliculitis with eventual follicular destruction.
Erosive pustular dermatosis is a rare disorder that primarily aff ects the elderly. It is characterized by a chronic amicrobial pustular dermatosis with extensive boggy, crusted, erosive plaques on the scalp resulting in scarring alopecia. Most cases have an onset after the age of 40. Therefore, age of onset may help diff erentiate between erosive pustular dermatosis and FD.
The cause of erosive pustular dermatosis is unknown. It is thought to be related to local trauma, such as chronic sun exposure, occurring months to years prior to the onset of lesions or as an autoimmune process.6 Histologic specimens show nonspecific changes including parakeratosis or hyperkeratotic scale with atrophy or erosion of the epidermis, while an inflammatory infiltrate with lymphocytes and plasma cells is found in the dermis.
LPP is seen more commonly in women than men, and Caucasians are more often aff ected than African Americans. It presents with erythema, perifollicular scale, and scattered patches of scarring alopecia. Half of involved cases develop concomitant clinical features of lichen planus. When present, these characteristics may help distinguish it from FD and other scarring alopecias.6
The etiology of LPP is unknown, but is thought to be similar to the presumed cause of lichen planus: a T-cell?mediated autoimmune response that damages basal keratinocytes.5 Histologic findings include a band-like mononuclear cell infiltrate obscuring the interface between follicular epithelium and dermis at the superficial part of the follicle with occasional interfollicular epidermal changes consistent with lichen planus.
Inflammatory tinea capitis is a common dermatophyte infection of the scalp that aff ects children and adults alike. Typically, it is easily distinguished from FD. However, severe cases may result in a highly inflammatory pustular eruption with alopecia—with or without a kerion—which can make diff erentiation difficult.
In contrast to FD, the alopecia associated with tinea capitis is usually nonscarring, although this depends on the extent and depth of infection. Also, tinea capitis may present with either discrete patches or involve the entire scalp, whereas FD is usually localized to the vertex or occiput (as noted earlier). Correct diagnosis can be accomplished by means of light microscopy and fungal culture.
Secondary syphilis is usually a sexually transmitted disease, but it can also be acquired perinatally. It often presents with a “moth-eaten” alopecia and should be considered when examining patients with patchy alopecia such as that seen in FD. These lesions manifest 3 to 10 weeks after the onset of primary syphilis. Early in its course, the condition is reversible, but if it becomes chronic, the condition will cause a scarring alopecia.
The presence of other stigmata, including a generalized pruritic papulosquamous eruption with involvement of the palms and soles, mucosal lesions ranging from superficial ulcers to large gray plaques, and condylomata lata, should help to diff erentiate syphilis from FD.
Serologic tests such as rapid plasma reagin and venereal disease research laboratory assays are often preferred for routine screening. If the index of suspicion is high, confirmatory testing with direct antibody as-says such as a microhemagglutination assay or fluorescent treponemal antibody absorption test is indicated.
Biopsy is needed for the diagnosis
Two scalp biopsies should be performed to make the diagnosis. Recommended guidelines for sampling the scalp include performance of 4-mm punch biopsies extending into the fat at 2 diff erent clinically active sites.7 One biopsy should be processed for standard horizontal sectioning, but the second biopsy should be bisected vertically, with half sent for histologic examination and the other half for tissue culture (fungal and bacterial). An additional subsequent biopsy for direct immunofluorescence may also be considered if the initial biopsies are nondiagnostic.
Bacterial and fungal cultures collected from an intact pustule on the scalp with a standard culture swab should also be undertaken with pustular disease. If scale is present, a potassium hydroxide examination can help establish the diagnosis of a fungal etiology.
Doxycycline, intralesional corticosteroids are the first line of Tx
Management of FD can be difficult, and long-term treatment is often necessary. You’ll need to explain to patients that their current hair loss is permanent and that the goal of treatment is to decrease inflammation and prevent further balding.
After initial bacterial cultures and sensitivities are obtained, primary treatment is aimed at eliminating S aureus colonization. Often, this requires oral antibiotic therapy, most commonly doxycycline 100 mg twice daily5(strength of recommendation [SOR]: C). Topical antibiotics, however, may be used in mild cases; options include 2% mupirocin, 1% clindamycin, 1.5% fusidic acid, or 2% erythromycin applied twice daily1(SOR: C). In recalcitrant cases, a common treatment regimen includes oral rifampin 300 mg and clindamycin 300 mg twice daily for 10 weeks4(SOR: C).
Adjunctive topical and intralesional corticosteroids may help reduce inflammation and provide symptomatic relief from itching, burning, and pain. Topical class I or II corticosteroids can be used twice daily, whereas intralesional triamcinolone acetonide (combined with topical and/or oral antibiotics) may be administered every 4 to 6 weeks, starting at a concentration of 10 mg/mL1(SOR: C). Oral corticosteroids should only be considered for highly active and rapidly progressive symptoms.
Dapsone may also be considered as a treatment option for FD due to its antimicrobial activity and anti-inflammatory action directed toward neutrophil metabolism. Relapse, however, is frequent after treatment withdrawal1(SOR: C).
Improvement, but anticipated chronicity
We prescribed oral doxycycline 100 mg twice daily for our patient, as well as clobetasol 0.05% topical solution, to be applied to the affected area in the morning and evening.
We told our patient that FD is a chronic relapsing disorder and that while we could not make the condition go away completely, we could control it. We advised the patient to follow up every 2 months for the next 6 months, then every 6 months to ensure there was no progression or need to change the treatment regimen.
The patient’s symptoms improved after the first 2 months. After weaning the patient off doxycycline over a 6-month period, we planned to transition the patient to topical clindamycin solution twice daily.
In some cases, the patient can be weaned off oral antibiotics once the condition is controlled, but for most patients, continuous systemic therapy is needed.
CORRESPONDENCE Oliver J. Wisco, Maj, USAF, MC, FS, Department of the Air Force, Wilford Hall Medical Center, 59 MDW/ SG05D/Dermatology, 2200 Bergquist Drive, Suite 1, Lackland AFB, TX 78236-9908; [email protected]
1. Otberg N, Kang H, Alzolibani AA, et al. Folliculitis decalvans. Dermatol Ther. 2008;21:238-244.
2. Douwes KE, Landthaler M, Szeimies RM. Simultaneous occur-rence of folliculitis decalvans capillitii in identical twins. Br J Dermatol. 2000;143:195-197.
3. Chandrawansa PH, Giam YC. Folliculitis decalvans-a retrospective study in a tertiary referred center, over five years. Singapore Med J. 2003;44:84-87.
4. Powell JJ, Dawber RP, Gatter K. Folliculitis decalvans including tufted folliculitis: clinical histological and therapeutic findings. Br J Dermatol. 1999;140:328-333.
5. Bolognia JL, Jorizzo JL, Rapini RP. Dermatology. 2nd ed. St. Louis, Mo: Mosby Elsevier; 2008.
6. Somani N, Bergfeld WF. Cicatricial alopecia: classification and histopathology. Dermatol Ther. 2008;21:221-237.
7. Olsen EA, Bergfeld WF, Cotsarelis G, et al. Summary of North American Hair Research Society (NAHRS)-sponsored workshop on cicatricial alopecia, Duke University Medical Center, February 10 and 11, 2001. J Am Acad Dermatol. 2003;48:103-110.
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
A 23-Year-Old African American Man sought care at our medical center because he had been losing hair over the vertex of his scalp for the past several years. He indicated that his father had early-onset male patterned alopecia. As a result, he considered his hair loss “genetic.” However, he described waxing and waning flares of painful pustules associated with occasional spontaneous bleeding and discharge of purulent material that occurred in the same area as the hair loss.
Physical examination revealed multiple perifollicular papules and pustules on the vertex of his scalp with interspersed patches of alopecia (FIGURE 1). There were no lesions elsewhere on his body and his past medical history was otherwise unremarkable.
FIGURE 1
Alopecia with a painful twist
This 23-year-old patient said that he had spontaneous bleeding and discharge of purulent material in the area of his hair loss.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU MANAGE THIS PATIENT?
Diagnosis: Folliculitis decalvans
Folliculitis decalvans (FD) is a highly inflammatory form of scarring alopecia characterized by inflammatory perifollicular papules and pustules. The term scarring alopecia refers to the fact that the follicular epithelium has been replaced by connective tissue, ultimately resulting in permanent hair loss. This manifests clinically as patches of skin without terminal or vellus hairs, whereas a nonscarring alopecia would demonstrate preservation of the vellus hairs. Left untreated, advancing permanent hair loss ensues and may result in an end-stage pattern of tufted folliculitis or polytrichia, where interspersed dilated follicular openings house multiple hairs.
Affected areas commonly include the vertex and occipital scalp. Common symptoms include pain, itching, burning, and occasionally spontaneous bleeding or discharge of purulent material.1
FD generally occurs in young and middle-aged African Americans with a slight predominance in males. It accounts for 11% of all primary scarring alopecias.2,3 The etiology of this inflammatory process is not fully understood; however, scalp colonization with Staphylococcus aureus has been implicated as a contributing factor.4 Other reports suggest patients may have an altered host immune response and/or genetic predisposition for this condition.2,3
The differential includes various scarring, nonscarring alopecias
Since clinical findings of FD can range from relatively nonspecific mild disease at its onset to the end stage described above, a detailed patient history is needed. The following scarring and nonscarring alopecias should be considered in the diff erential diagnosis: dissecting cellulitis of the scalp, central centrifugal cicatricial alopecia (CCCA), acne keloidalis nuchae, erosive pustular dermatosis, lichen planopilaris (LPP), inflammatory tinea capitis, and secondary syphilis.
Dissecting cellulitis of the scalp is a distinctive, often debilitating disease commonly seen in young adult African American men. It is considered part of the follicular occlusion tetrad that also includes hidradenitis suppurativa, acne conglobata, and pilonidal cysts. It presents as a scarring alopecia with firm scalp nodules that rapidly develop into boggy, fluctuant, oval to linear sinuses that may eventually discharge purulent material.
In contrast to FD, dissecting scalp cellulitis lesions interconnect via sinus tract formation so that pressure on one fluctuant area may result in purulent discharge from perfo-rations several centimeters away.5 Although both dissecting cellulitis and FD are considered primary neutrophilic scarring alopecias, the presence of true sinus tract formation can be a distinguishing finding.
CCCA is the most common form of scarring alopecia among African Americans and is particularly seen among African American women.5 It generally presents on the scalp vertex like FD, but it is much less inflamma-tory and typically causes only mild pruritus or tenderness of the involved areas.
Although numerous theories have been suggested, the etiology is unknown. The pathogenesis is thought to be associated with premature desquamation of the inner root sheath, which can be demonstrated on biopsy. Also seen histologically is lymphocytic perifollicular inflammation and polytrichia.6
Acne keloidalis nuchae is also a scarring alopecia. It is seen most commonly in African American men and presents as keloid-like papules and plaques with occasional pustules characteristically on the occipital scalp and posterior neck. In contrast to FD, acne keloidalis nuchae papules coalesce and may form firm, hairless, protuberant keloid-like plaques that may be painful and cosmetically disfiguring. The cause of acne keloidalis nuchae is unknown.
Shaving or cutting tight curly hair too short and subsequently having the new hair curve back and penetrate the skin may be the precipitating event. Thus, a history of close shaving should make one suspect this diagnosis. Histologic analysis reveals a chronic, predominantly lymphocytic folliculitis with eventual follicular destruction.
Erosive pustular dermatosis is a rare disorder that primarily aff ects the elderly. It is characterized by a chronic amicrobial pustular dermatosis with extensive boggy, crusted, erosive plaques on the scalp resulting in scarring alopecia. Most cases have an onset after the age of 40. Therefore, age of onset may help diff erentiate between erosive pustular dermatosis and FD.
The cause of erosive pustular dermatosis is unknown. It is thought to be related to local trauma, such as chronic sun exposure, occurring months to years prior to the onset of lesions or as an autoimmune process.6 Histologic specimens show nonspecific changes including parakeratosis or hyperkeratotic scale with atrophy or erosion of the epidermis, while an inflammatory infiltrate with lymphocytes and plasma cells is found in the dermis.
LPP is seen more commonly in women than men, and Caucasians are more often aff ected than African Americans. It presents with erythema, perifollicular scale, and scattered patches of scarring alopecia. Half of involved cases develop concomitant clinical features of lichen planus. When present, these characteristics may help distinguish it from FD and other scarring alopecias.6
The etiology of LPP is unknown, but is thought to be similar to the presumed cause of lichen planus: a T-cell?mediated autoimmune response that damages basal keratinocytes.5 Histologic findings include a band-like mononuclear cell infiltrate obscuring the interface between follicular epithelium and dermis at the superficial part of the follicle with occasional interfollicular epidermal changes consistent with lichen planus.
Inflammatory tinea capitis is a common dermatophyte infection of the scalp that aff ects children and adults alike. Typically, it is easily distinguished from FD. However, severe cases may result in a highly inflammatory pustular eruption with alopecia—with or without a kerion—which can make diff erentiation difficult.
In contrast to FD, the alopecia associated with tinea capitis is usually nonscarring, although this depends on the extent and depth of infection. Also, tinea capitis may present with either discrete patches or involve the entire scalp, whereas FD is usually localized to the vertex or occiput (as noted earlier). Correct diagnosis can be accomplished by means of light microscopy and fungal culture.
Secondary syphilis is usually a sexually transmitted disease, but it can also be acquired perinatally. It often presents with a “moth-eaten” alopecia and should be considered when examining patients with patchy alopecia such as that seen in FD. These lesions manifest 3 to 10 weeks after the onset of primary syphilis. Early in its course, the condition is reversible, but if it becomes chronic, the condition will cause a scarring alopecia.
The presence of other stigmata, including a generalized pruritic papulosquamous eruption with involvement of the palms and soles, mucosal lesions ranging from superficial ulcers to large gray plaques, and condylomata lata, should help to diff erentiate syphilis from FD.
Serologic tests such as rapid plasma reagin and venereal disease research laboratory assays are often preferred for routine screening. If the index of suspicion is high, confirmatory testing with direct antibody as-says such as a microhemagglutination assay or fluorescent treponemal antibody absorption test is indicated.
Biopsy is needed for the diagnosis
Two scalp biopsies should be performed to make the diagnosis. Recommended guidelines for sampling the scalp include performance of 4-mm punch biopsies extending into the fat at 2 diff erent clinically active sites.7 One biopsy should be processed for standard horizontal sectioning, but the second biopsy should be bisected vertically, with half sent for histologic examination and the other half for tissue culture (fungal and bacterial). An additional subsequent biopsy for direct immunofluorescence may also be considered if the initial biopsies are nondiagnostic.
Bacterial and fungal cultures collected from an intact pustule on the scalp with a standard culture swab should also be undertaken with pustular disease. If scale is present, a potassium hydroxide examination can help establish the diagnosis of a fungal etiology.
Doxycycline, intralesional corticosteroids are the first line of Tx
Management of FD can be difficult, and long-term treatment is often necessary. You’ll need to explain to patients that their current hair loss is permanent and that the goal of treatment is to decrease inflammation and prevent further balding.
After initial bacterial cultures and sensitivities are obtained, primary treatment is aimed at eliminating S aureus colonization. Often, this requires oral antibiotic therapy, most commonly doxycycline 100 mg twice daily5(strength of recommendation [SOR]: C). Topical antibiotics, however, may be used in mild cases; options include 2% mupirocin, 1% clindamycin, 1.5% fusidic acid, or 2% erythromycin applied twice daily1(SOR: C). In recalcitrant cases, a common treatment regimen includes oral rifampin 300 mg and clindamycin 300 mg twice daily for 10 weeks4(SOR: C).
Adjunctive topical and intralesional corticosteroids may help reduce inflammation and provide symptomatic relief from itching, burning, and pain. Topical class I or II corticosteroids can be used twice daily, whereas intralesional triamcinolone acetonide (combined with topical and/or oral antibiotics) may be administered every 4 to 6 weeks, starting at a concentration of 10 mg/mL1(SOR: C). Oral corticosteroids should only be considered for highly active and rapidly progressive symptoms.
Dapsone may also be considered as a treatment option for FD due to its antimicrobial activity and anti-inflammatory action directed toward neutrophil metabolism. Relapse, however, is frequent after treatment withdrawal1(SOR: C).
Improvement, but anticipated chronicity
We prescribed oral doxycycline 100 mg twice daily for our patient, as well as clobetasol 0.05% topical solution, to be applied to the affected area in the morning and evening.
We told our patient that FD is a chronic relapsing disorder and that while we could not make the condition go away completely, we could control it. We advised the patient to follow up every 2 months for the next 6 months, then every 6 months to ensure there was no progression or need to change the treatment regimen.
The patient’s symptoms improved after the first 2 months. After weaning the patient off doxycycline over a 6-month period, we planned to transition the patient to topical clindamycin solution twice daily.
In some cases, the patient can be weaned off oral antibiotics once the condition is controlled, but for most patients, continuous systemic therapy is needed.
CORRESPONDENCE Oliver J. Wisco, Maj, USAF, MC, FS, Department of the Air Force, Wilford Hall Medical Center, 59 MDW/ SG05D/Dermatology, 2200 Bergquist Drive, Suite 1, Lackland AFB, TX 78236-9908; [email protected]
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
A 23-Year-Old African American Man sought care at our medical center because he had been losing hair over the vertex of his scalp for the past several years. He indicated that his father had early-onset male patterned alopecia. As a result, he considered his hair loss “genetic.” However, he described waxing and waning flares of painful pustules associated with occasional spontaneous bleeding and discharge of purulent material that occurred in the same area as the hair loss.
Physical examination revealed multiple perifollicular papules and pustules on the vertex of his scalp with interspersed patches of alopecia (FIGURE 1). There were no lesions elsewhere on his body and his past medical history was otherwise unremarkable.
FIGURE 1
Alopecia with a painful twist
This 23-year-old patient said that he had spontaneous bleeding and discharge of purulent material in the area of his hair loss.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU MANAGE THIS PATIENT?
Diagnosis: Folliculitis decalvans
Folliculitis decalvans (FD) is a highly inflammatory form of scarring alopecia characterized by inflammatory perifollicular papules and pustules. The term scarring alopecia refers to the fact that the follicular epithelium has been replaced by connective tissue, ultimately resulting in permanent hair loss. This manifests clinically as patches of skin without terminal or vellus hairs, whereas a nonscarring alopecia would demonstrate preservation of the vellus hairs. Left untreated, advancing permanent hair loss ensues and may result in an end-stage pattern of tufted folliculitis or polytrichia, where interspersed dilated follicular openings house multiple hairs.
Affected areas commonly include the vertex and occipital scalp. Common symptoms include pain, itching, burning, and occasionally spontaneous bleeding or discharge of purulent material.1
FD generally occurs in young and middle-aged African Americans with a slight predominance in males. It accounts for 11% of all primary scarring alopecias.2,3 The etiology of this inflammatory process is not fully understood; however, scalp colonization with Staphylococcus aureus has been implicated as a contributing factor.4 Other reports suggest patients may have an altered host immune response and/or genetic predisposition for this condition.2,3
The differential includes various scarring, nonscarring alopecias
Since clinical findings of FD can range from relatively nonspecific mild disease at its onset to the end stage described above, a detailed patient history is needed. The following scarring and nonscarring alopecias should be considered in the diff erential diagnosis: dissecting cellulitis of the scalp, central centrifugal cicatricial alopecia (CCCA), acne keloidalis nuchae, erosive pustular dermatosis, lichen planopilaris (LPP), inflammatory tinea capitis, and secondary syphilis.
Dissecting cellulitis of the scalp is a distinctive, often debilitating disease commonly seen in young adult African American men. It is considered part of the follicular occlusion tetrad that also includes hidradenitis suppurativa, acne conglobata, and pilonidal cysts. It presents as a scarring alopecia with firm scalp nodules that rapidly develop into boggy, fluctuant, oval to linear sinuses that may eventually discharge purulent material.
In contrast to FD, dissecting scalp cellulitis lesions interconnect via sinus tract formation so that pressure on one fluctuant area may result in purulent discharge from perfo-rations several centimeters away.5 Although both dissecting cellulitis and FD are considered primary neutrophilic scarring alopecias, the presence of true sinus tract formation can be a distinguishing finding.
CCCA is the most common form of scarring alopecia among African Americans and is particularly seen among African American women.5 It generally presents on the scalp vertex like FD, but it is much less inflamma-tory and typically causes only mild pruritus or tenderness of the involved areas.
Although numerous theories have been suggested, the etiology is unknown. The pathogenesis is thought to be associated with premature desquamation of the inner root sheath, which can be demonstrated on biopsy. Also seen histologically is lymphocytic perifollicular inflammation and polytrichia.6
Acne keloidalis nuchae is also a scarring alopecia. It is seen most commonly in African American men and presents as keloid-like papules and plaques with occasional pustules characteristically on the occipital scalp and posterior neck. In contrast to FD, acne keloidalis nuchae papules coalesce and may form firm, hairless, protuberant keloid-like plaques that may be painful and cosmetically disfiguring. The cause of acne keloidalis nuchae is unknown.
Shaving or cutting tight curly hair too short and subsequently having the new hair curve back and penetrate the skin may be the precipitating event. Thus, a history of close shaving should make one suspect this diagnosis. Histologic analysis reveals a chronic, predominantly lymphocytic folliculitis with eventual follicular destruction.
Erosive pustular dermatosis is a rare disorder that primarily aff ects the elderly. It is characterized by a chronic amicrobial pustular dermatosis with extensive boggy, crusted, erosive plaques on the scalp resulting in scarring alopecia. Most cases have an onset after the age of 40. Therefore, age of onset may help diff erentiate between erosive pustular dermatosis and FD.
The cause of erosive pustular dermatosis is unknown. It is thought to be related to local trauma, such as chronic sun exposure, occurring months to years prior to the onset of lesions or as an autoimmune process.6 Histologic specimens show nonspecific changes including parakeratosis or hyperkeratotic scale with atrophy or erosion of the epidermis, while an inflammatory infiltrate with lymphocytes and plasma cells is found in the dermis.
LPP is seen more commonly in women than men, and Caucasians are more often aff ected than African Americans. It presents with erythema, perifollicular scale, and scattered patches of scarring alopecia. Half of involved cases develop concomitant clinical features of lichen planus. When present, these characteristics may help distinguish it from FD and other scarring alopecias.6
The etiology of LPP is unknown, but is thought to be similar to the presumed cause of lichen planus: a T-cell?mediated autoimmune response that damages basal keratinocytes.5 Histologic findings include a band-like mononuclear cell infiltrate obscuring the interface between follicular epithelium and dermis at the superficial part of the follicle with occasional interfollicular epidermal changes consistent with lichen planus.
Inflammatory tinea capitis is a common dermatophyte infection of the scalp that aff ects children and adults alike. Typically, it is easily distinguished from FD. However, severe cases may result in a highly inflammatory pustular eruption with alopecia—with or without a kerion—which can make diff erentiation difficult.
In contrast to FD, the alopecia associated with tinea capitis is usually nonscarring, although this depends on the extent and depth of infection. Also, tinea capitis may present with either discrete patches or involve the entire scalp, whereas FD is usually localized to the vertex or occiput (as noted earlier). Correct diagnosis can be accomplished by means of light microscopy and fungal culture.
Secondary syphilis is usually a sexually transmitted disease, but it can also be acquired perinatally. It often presents with a “moth-eaten” alopecia and should be considered when examining patients with patchy alopecia such as that seen in FD. These lesions manifest 3 to 10 weeks after the onset of primary syphilis. Early in its course, the condition is reversible, but if it becomes chronic, the condition will cause a scarring alopecia.
The presence of other stigmata, including a generalized pruritic papulosquamous eruption with involvement of the palms and soles, mucosal lesions ranging from superficial ulcers to large gray plaques, and condylomata lata, should help to diff erentiate syphilis from FD.
Serologic tests such as rapid plasma reagin and venereal disease research laboratory assays are often preferred for routine screening. If the index of suspicion is high, confirmatory testing with direct antibody as-says such as a microhemagglutination assay or fluorescent treponemal antibody absorption test is indicated.
Biopsy is needed for the diagnosis
Two scalp biopsies should be performed to make the diagnosis. Recommended guidelines for sampling the scalp include performance of 4-mm punch biopsies extending into the fat at 2 diff erent clinically active sites.7 One biopsy should be processed for standard horizontal sectioning, but the second biopsy should be bisected vertically, with half sent for histologic examination and the other half for tissue culture (fungal and bacterial). An additional subsequent biopsy for direct immunofluorescence may also be considered if the initial biopsies are nondiagnostic.
Bacterial and fungal cultures collected from an intact pustule on the scalp with a standard culture swab should also be undertaken with pustular disease. If scale is present, a potassium hydroxide examination can help establish the diagnosis of a fungal etiology.
Doxycycline, intralesional corticosteroids are the first line of Tx
Management of FD can be difficult, and long-term treatment is often necessary. You’ll need to explain to patients that their current hair loss is permanent and that the goal of treatment is to decrease inflammation and prevent further balding.
After initial bacterial cultures and sensitivities are obtained, primary treatment is aimed at eliminating S aureus colonization. Often, this requires oral antibiotic therapy, most commonly doxycycline 100 mg twice daily5(strength of recommendation [SOR]: C). Topical antibiotics, however, may be used in mild cases; options include 2% mupirocin, 1% clindamycin, 1.5% fusidic acid, or 2% erythromycin applied twice daily1(SOR: C). In recalcitrant cases, a common treatment regimen includes oral rifampin 300 mg and clindamycin 300 mg twice daily for 10 weeks4(SOR: C).
Adjunctive topical and intralesional corticosteroids may help reduce inflammation and provide symptomatic relief from itching, burning, and pain. Topical class I or II corticosteroids can be used twice daily, whereas intralesional triamcinolone acetonide (combined with topical and/or oral antibiotics) may be administered every 4 to 6 weeks, starting at a concentration of 10 mg/mL1(SOR: C). Oral corticosteroids should only be considered for highly active and rapidly progressive symptoms.
Dapsone may also be considered as a treatment option for FD due to its antimicrobial activity and anti-inflammatory action directed toward neutrophil metabolism. Relapse, however, is frequent after treatment withdrawal1(SOR: C).
Improvement, but anticipated chronicity
We prescribed oral doxycycline 100 mg twice daily for our patient, as well as clobetasol 0.05% topical solution, to be applied to the affected area in the morning and evening.
We told our patient that FD is a chronic relapsing disorder and that while we could not make the condition go away completely, we could control it. We advised the patient to follow up every 2 months for the next 6 months, then every 6 months to ensure there was no progression or need to change the treatment regimen.
The patient’s symptoms improved after the first 2 months. After weaning the patient off doxycycline over a 6-month period, we planned to transition the patient to topical clindamycin solution twice daily.
In some cases, the patient can be weaned off oral antibiotics once the condition is controlled, but for most patients, continuous systemic therapy is needed.
CORRESPONDENCE Oliver J. Wisco, Maj, USAF, MC, FS, Department of the Air Force, Wilford Hall Medical Center, 59 MDW/ SG05D/Dermatology, 2200 Bergquist Drive, Suite 1, Lackland AFB, TX 78236-9908; [email protected]
1. Otberg N, Kang H, Alzolibani AA, et al. Folliculitis decalvans. Dermatol Ther. 2008;21:238-244.
2. Douwes KE, Landthaler M, Szeimies RM. Simultaneous occur-rence of folliculitis decalvans capillitii in identical twins. Br J Dermatol. 2000;143:195-197.
3. Chandrawansa PH, Giam YC. Folliculitis decalvans-a retrospective study in a tertiary referred center, over five years. Singapore Med J. 2003;44:84-87.
4. Powell JJ, Dawber RP, Gatter K. Folliculitis decalvans including tufted folliculitis: clinical histological and therapeutic findings. Br J Dermatol. 1999;140:328-333.
5. Bolognia JL, Jorizzo JL, Rapini RP. Dermatology. 2nd ed. St. Louis, Mo: Mosby Elsevier; 2008.
6. Somani N, Bergfeld WF. Cicatricial alopecia: classification and histopathology. Dermatol Ther. 2008;21:221-237.
7. Olsen EA, Bergfeld WF, Cotsarelis G, et al. Summary of North American Hair Research Society (NAHRS)-sponsored workshop on cicatricial alopecia, Duke University Medical Center, February 10 and 11, 2001. J Am Acad Dermatol. 2003;48:103-110.
1. Otberg N, Kang H, Alzolibani AA, et al. Folliculitis decalvans. Dermatol Ther. 2008;21:238-244.
2. Douwes KE, Landthaler M, Szeimies RM. Simultaneous occur-rence of folliculitis decalvans capillitii in identical twins. Br J Dermatol. 2000;143:195-197.
3. Chandrawansa PH, Giam YC. Folliculitis decalvans-a retrospective study in a tertiary referred center, over five years. Singapore Med J. 2003;44:84-87.
4. Powell JJ, Dawber RP, Gatter K. Folliculitis decalvans including tufted folliculitis: clinical histological and therapeutic findings. Br J Dermatol. 1999;140:328-333.
5. Bolognia JL, Jorizzo JL, Rapini RP. Dermatology. 2nd ed. St. Louis, Mo: Mosby Elsevier; 2008.
6. Somani N, Bergfeld WF. Cicatricial alopecia: classification and histopathology. Dermatol Ther. 2008;21:221-237.
7. Olsen EA, Bergfeld WF, Cotsarelis G, et al. Summary of North American Hair Research Society (NAHRS)-sponsored workshop on cicatricial alopecia, Duke University Medical Center, February 10 and 11, 2001. J Am Acad Dermatol. 2003;48:103-110.
Verrucous nodules on the ankle
A 56-year-old woman came into our medical center complaining of multiple pruritic, slowly growing scaly nodules over her right ankle (FIGURE 1A AND 1B). She indicated that the lesions started as small pink “bumps” at the staple sites of an open reduction and internal fixation surgery of her talus that she’d had 8 years ago.
There were no lesions elsewhere on her body and her past medical history was otherwise unremarkable.
FIGURE 1
Multiple pruritic, scaly nodules
What is your diagnosis?
How would you manage this condition?
Diagnosis: Hypertrophic lichen planus
Hypertrophic lichen planus (HLP), a variant of lichen planus (LP), is a lesion that is usually found on the distal extremities. HLP plaques evolve from initial characteristic LP lesions (purple, planar, pruritic, polygonal papules or plaque) to form reddish-brown to violaceous, hypertrophic, verrucous round-to-elongated plaques. Primary lesions may be spread by scratching or other trauma and often develop dark brown hyperpigmentation over several years. Like other variants of LP, HLP most commonly affects adults 30 to 60 years of age, with a slight female predominance.1
HLP may be idiopathic, drug induced, or associated with a systemic disease. Although many drugs have been linked to this lesion, the most commonly reported medications are gold salts, beta-blockers, antimalarials, thiazides, furosemide, and penicillamines. If your patient has HLP and is taking one of these medications, you should consider discontinuing the medication.1 As with other forms of LP, HLP has been associated with hepatitis C. Consider transaminases and a hepatitis panel for all patients with HLP. Other HLP-associated conditions include venous insufficiency, herpes simplex virus, and varicella-zoster virus.1
When the history confuses the diagnosis
When there are surrounding classic LP lesions, the diagnosis of HLP is fairly straightforward. However, when the patient has a history of surgery or trauma preceding the lesions and no surrounding classic LP lesions, the diagnosis may be less clear-cut. In such cases, the differential diagnosis includes lichen simplex chronicus, mycetoma, chromoblastomycosis, and squamous cell carcinoma.
Lichen simplex chronicus can be distinguished from HLP by reviewing the patient’s history. Patients who describe habitual rubbing or scratching of the area are likely to have lichen simplex chronicus. On exam, lichen simplex chronicus lesions are slightly erythematous, scaly, well-demarcated, and firm. There are rough plaques with exaggerated skin lines (lichenification) rather than the verrucous surface typically seen with HLP lesions. Wickham’s striae (seen in LP) are not seen with lichen simplex chronicus, and the lesions are localized only to easily reached areas.2
Mycetoma is a tumor-like lesion produced by a fungus (eumycetoma) or bacteria (actinomycetoma), typically encountered in arid areas rather than humid environments.3 These chronic, localized, nonpainful subcutaneous nodules develop on the foot and lower extremity after traumatic inoculation with the bacteria or fungus. Mycetomas persist for many years and classically present with a triad of tumefaction, draining sinus tracts, and “sulfur grains” that distinguish it from the dry, hyperkeratotic lesions of HLP. Diagnosis requires biopsy for histologic examination and both fungal and bacterial culture in order to choose the appropriate therapy.
Chromoblastomycosis is a deep fungal infection most commonly caused by the pigmented fungus Phialophora verrucosa found in tropical climates.4 The fungi enter the skin of the lower extremity after minor trauma, resulting in a gradually expanding verrucous nodule or plaque. The nodular variant is often pedunculated with classic pigmented cauliflower-like florets. While the nodular variant is localized, the plaque variant may spread laterally, possibly metastasizing through lymphatic channels with a concomitant bacterial infection. There is also a characteristic unpleasant odor with lymph stasis.
On potassium hydroxide (KOH) mounts or histologic examination, the thick-walled cells (muriform bodies) of chromoblastomycosis are diagnostic. Patients with chromoblastomycosis have seen response rates >60% with 10 to 24 months of daily itraconazole (200 mg) therapy.5
Squamous cell carcinoma (SCC) is the second most common skin cancer and affects more than 250,000 Americans each year. While associated with sun exposure, it has also been linked to ionizing radiation, arsenic, human papilloma virus, cigarette smoking, and chronic nonhealing wounds and scars such as Marjolin’s ulcer.1
Marjolin’s ulcer usually appears as a triad of nodule formation, induration, and ulceration at a scar site and thus may be confused with HLP. It is more common than sun-induced SCC in Asian and dark-skinned individuals.6 Marjolin’s ulcer will usually present in the fifth decade, years after the initial insult. Diagnosis is supported by the clinical appearance and history of a preceding scar at the site. Marjolin’s ulcer has a higher rate of recurrence and metastasis than other forms of SCC, and thus should be treated aggressively.7,8
A biopsy may be needed
A drop of immersion oil can confirm your HLP suspicions by revealing the white, lacy reticular network of Wickham’s striae.1 Other clinical clues to the diagnosis of LP or one of its variants include a white reticular, erythematous, or ulcerative appearance on the buccal mucosa in addition to a dorsal pterygium and/or diffuse pitting on the nails.
A deep shave or punch biopsy may be necessary, however, when the clinical diagnosis is unclear. Histological findings demonstrate focal hyperorthokeratosis, saw-toothed rete ridges, vacuolar change at the basal layer, and a band-like lymphocytic infiltrate.
Corticosteroids are the treatment of choice
There have been few large-scale prospective studies exploring the treatment of HLP. However, treatment for HLP is similar to that of LP and typically begins with topical class I or II glucocorticoids or intralesional injections of triamcinolone. Narrow-band ultraviolet-B (UVB) markedly reduces pruritus and flattens plaques, and is considered second-line treatment (strength of recommendation [SOR]: C).9-11 The retinoid acitretin may be effective for severe HLP at oral dosages of 30 mg/d for 8 weeks (SOR: A).12 Azathioprine and cyclosporine have also been used successfully, but risk of renal dysfunction, hypertension, and increased viral and fungal infections make these agents third-line therapies (SOR: C).13-15
A good outcome for our patient
Our patient applied clobetasol ointment 0.05% to the affected areas twice daily until the lesions went away (approximately 2 months later).
CORRESPONDENCE
Oliver J. Wisco, Maj, USAF, MC, FS, Department of the Air Force, Wilford Hall Medical Center, 59 MDW/SGOMD/Dermatology, 2200 Bergquist Drive, Suite 1, Lackland AFB, TX 78236-9908; [email protected]
1. Bolognia JL, Jorizzo JL, Rapini RP. Dermatology. 2nd ed. St. Louis, Mo: Mosby Elsevier; 2008.
2. Habif TP. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 4th ed. Philadelphia, Pa: Mosby; 2004:65.
3. Fitzpatrick T, Eisen A, Wolff K, et al. Dermatology in General Medicine. 5th ed. New York, NY: McGraw-Hill; 1999:2301.
4. Fitzpatrick T, Eisen A, Wolff K, et al. Dermatology in General Medicine. 5th ed. New York, NY: McGraw-Hill; 1999:2375.
5. Restrepo A. Treatment of tropical mycoses. J Am Acad Dermatol. 1994;31(3 Pt 2):S91-S102.
6. Chuang TY, Reizner GT, Elpern DJ, et al. Nonmelanoma skin cancer in Japanese ethnic Hawaiians in Kauai, Hawaii: an incidence report. J Am Acad Dermatol. 1995;33:422-426.
7. Treves N, Pack GT. The development of cancer in burn scars. An analysis and report of thirty-four cases. Surg Gynecol Obstet. 1930;51:749.-
8. Lifeso RM, Rooney RJ, el-Shaker M. Post-traumatic squamous-cell carcinoma. J Bone Joint Surg. 1990;72:12-18.
9. Gambichler T, Breuckmann F, Boms S, et al. Narrow-band UVB phototherapy in skin conditions beyond psoriasis. J Am Acad Dermatol. 2005;52:660-670.
10. Saricaoglu H, Karadogan SK, Baskan EB. Narrowband UVB therapy in the treatment of lichen planus. Photodermatol Photoimmunol Photomed. 2003;19:265-267.
11. Taneja A, Taylor CR. Narrow-band UVB for lichen planus treatment. Int J Dermatol. 2002;41:282-283.
12. Laurberg G, Geiger JM, Hjorth N, et al. Treatment of lichen planus with acitretin: a double-blind, placebo-controlled study in 65 patients. J Am Acad Dermatol 1991;24:434-437.
13. Kossard S, Artemi P. Acitretin for hypertrophic lichen planus-like reaction in a burn scar. Arch Dermatol. 2000;136:591-594.
14. Lear JT, English JS. Erosive and generalized lichen planus responsive to azathioprine. Clin Exp Dermatol. 1996;21:56-57.
15. Ho VC, Gupta AK, Ellis CN, et al. Treatment of severe lichen planus with cyclosporine. J Am Acad Dermatol. 1990;22:64-68.
A 56-year-old woman came into our medical center complaining of multiple pruritic, slowly growing scaly nodules over her right ankle (FIGURE 1A AND 1B). She indicated that the lesions started as small pink “bumps” at the staple sites of an open reduction and internal fixation surgery of her talus that she’d had 8 years ago.
There were no lesions elsewhere on her body and her past medical history was otherwise unremarkable.
FIGURE 1
Multiple pruritic, scaly nodules
What is your diagnosis?
How would you manage this condition?
Diagnosis: Hypertrophic lichen planus
Hypertrophic lichen planus (HLP), a variant of lichen planus (LP), is a lesion that is usually found on the distal extremities. HLP plaques evolve from initial characteristic LP lesions (purple, planar, pruritic, polygonal papules or plaque) to form reddish-brown to violaceous, hypertrophic, verrucous round-to-elongated plaques. Primary lesions may be spread by scratching or other trauma and often develop dark brown hyperpigmentation over several years. Like other variants of LP, HLP most commonly affects adults 30 to 60 years of age, with a slight female predominance.1
HLP may be idiopathic, drug induced, or associated with a systemic disease. Although many drugs have been linked to this lesion, the most commonly reported medications are gold salts, beta-blockers, antimalarials, thiazides, furosemide, and penicillamines. If your patient has HLP and is taking one of these medications, you should consider discontinuing the medication.1 As with other forms of LP, HLP has been associated with hepatitis C. Consider transaminases and a hepatitis panel for all patients with HLP. Other HLP-associated conditions include venous insufficiency, herpes simplex virus, and varicella-zoster virus.1
When the history confuses the diagnosis
When there are surrounding classic LP lesions, the diagnosis of HLP is fairly straightforward. However, when the patient has a history of surgery or trauma preceding the lesions and no surrounding classic LP lesions, the diagnosis may be less clear-cut. In such cases, the differential diagnosis includes lichen simplex chronicus, mycetoma, chromoblastomycosis, and squamous cell carcinoma.
Lichen simplex chronicus can be distinguished from HLP by reviewing the patient’s history. Patients who describe habitual rubbing or scratching of the area are likely to have lichen simplex chronicus. On exam, lichen simplex chronicus lesions are slightly erythematous, scaly, well-demarcated, and firm. There are rough plaques with exaggerated skin lines (lichenification) rather than the verrucous surface typically seen with HLP lesions. Wickham’s striae (seen in LP) are not seen with lichen simplex chronicus, and the lesions are localized only to easily reached areas.2
Mycetoma is a tumor-like lesion produced by a fungus (eumycetoma) or bacteria (actinomycetoma), typically encountered in arid areas rather than humid environments.3 These chronic, localized, nonpainful subcutaneous nodules develop on the foot and lower extremity after traumatic inoculation with the bacteria or fungus. Mycetomas persist for many years and classically present with a triad of tumefaction, draining sinus tracts, and “sulfur grains” that distinguish it from the dry, hyperkeratotic lesions of HLP. Diagnosis requires biopsy for histologic examination and both fungal and bacterial culture in order to choose the appropriate therapy.
Chromoblastomycosis is a deep fungal infection most commonly caused by the pigmented fungus Phialophora verrucosa found in tropical climates.4 The fungi enter the skin of the lower extremity after minor trauma, resulting in a gradually expanding verrucous nodule or plaque. The nodular variant is often pedunculated with classic pigmented cauliflower-like florets. While the nodular variant is localized, the plaque variant may spread laterally, possibly metastasizing through lymphatic channels with a concomitant bacterial infection. There is also a characteristic unpleasant odor with lymph stasis.
On potassium hydroxide (KOH) mounts or histologic examination, the thick-walled cells (muriform bodies) of chromoblastomycosis are diagnostic. Patients with chromoblastomycosis have seen response rates >60% with 10 to 24 months of daily itraconazole (200 mg) therapy.5
Squamous cell carcinoma (SCC) is the second most common skin cancer and affects more than 250,000 Americans each year. While associated with sun exposure, it has also been linked to ionizing radiation, arsenic, human papilloma virus, cigarette smoking, and chronic nonhealing wounds and scars such as Marjolin’s ulcer.1
Marjolin’s ulcer usually appears as a triad of nodule formation, induration, and ulceration at a scar site and thus may be confused with HLP. It is more common than sun-induced SCC in Asian and dark-skinned individuals.6 Marjolin’s ulcer will usually present in the fifth decade, years after the initial insult. Diagnosis is supported by the clinical appearance and history of a preceding scar at the site. Marjolin’s ulcer has a higher rate of recurrence and metastasis than other forms of SCC, and thus should be treated aggressively.7,8
A biopsy may be needed
A drop of immersion oil can confirm your HLP suspicions by revealing the white, lacy reticular network of Wickham’s striae.1 Other clinical clues to the diagnosis of LP or one of its variants include a white reticular, erythematous, or ulcerative appearance on the buccal mucosa in addition to a dorsal pterygium and/or diffuse pitting on the nails.
A deep shave or punch biopsy may be necessary, however, when the clinical diagnosis is unclear. Histological findings demonstrate focal hyperorthokeratosis, saw-toothed rete ridges, vacuolar change at the basal layer, and a band-like lymphocytic infiltrate.
Corticosteroids are the treatment of choice
There have been few large-scale prospective studies exploring the treatment of HLP. However, treatment for HLP is similar to that of LP and typically begins with topical class I or II glucocorticoids or intralesional injections of triamcinolone. Narrow-band ultraviolet-B (UVB) markedly reduces pruritus and flattens plaques, and is considered second-line treatment (strength of recommendation [SOR]: C).9-11 The retinoid acitretin may be effective for severe HLP at oral dosages of 30 mg/d for 8 weeks (SOR: A).12 Azathioprine and cyclosporine have also been used successfully, but risk of renal dysfunction, hypertension, and increased viral and fungal infections make these agents third-line therapies (SOR: C).13-15
A good outcome for our patient
Our patient applied clobetasol ointment 0.05% to the affected areas twice daily until the lesions went away (approximately 2 months later).
CORRESPONDENCE
Oliver J. Wisco, Maj, USAF, MC, FS, Department of the Air Force, Wilford Hall Medical Center, 59 MDW/SGOMD/Dermatology, 2200 Bergquist Drive, Suite 1, Lackland AFB, TX 78236-9908; [email protected]
A 56-year-old woman came into our medical center complaining of multiple pruritic, slowly growing scaly nodules over her right ankle (FIGURE 1A AND 1B). She indicated that the lesions started as small pink “bumps” at the staple sites of an open reduction and internal fixation surgery of her talus that she’d had 8 years ago.
There were no lesions elsewhere on her body and her past medical history was otherwise unremarkable.
FIGURE 1
Multiple pruritic, scaly nodules
What is your diagnosis?
How would you manage this condition?
Diagnosis: Hypertrophic lichen planus
Hypertrophic lichen planus (HLP), a variant of lichen planus (LP), is a lesion that is usually found on the distal extremities. HLP plaques evolve from initial characteristic LP lesions (purple, planar, pruritic, polygonal papules or plaque) to form reddish-brown to violaceous, hypertrophic, verrucous round-to-elongated plaques. Primary lesions may be spread by scratching or other trauma and often develop dark brown hyperpigmentation over several years. Like other variants of LP, HLP most commonly affects adults 30 to 60 years of age, with a slight female predominance.1
HLP may be idiopathic, drug induced, or associated with a systemic disease. Although many drugs have been linked to this lesion, the most commonly reported medications are gold salts, beta-blockers, antimalarials, thiazides, furosemide, and penicillamines. If your patient has HLP and is taking one of these medications, you should consider discontinuing the medication.1 As with other forms of LP, HLP has been associated with hepatitis C. Consider transaminases and a hepatitis panel for all patients with HLP. Other HLP-associated conditions include venous insufficiency, herpes simplex virus, and varicella-zoster virus.1
When the history confuses the diagnosis
When there are surrounding classic LP lesions, the diagnosis of HLP is fairly straightforward. However, when the patient has a history of surgery or trauma preceding the lesions and no surrounding classic LP lesions, the diagnosis may be less clear-cut. In such cases, the differential diagnosis includes lichen simplex chronicus, mycetoma, chromoblastomycosis, and squamous cell carcinoma.
Lichen simplex chronicus can be distinguished from HLP by reviewing the patient’s history. Patients who describe habitual rubbing or scratching of the area are likely to have lichen simplex chronicus. On exam, lichen simplex chronicus lesions are slightly erythematous, scaly, well-demarcated, and firm. There are rough plaques with exaggerated skin lines (lichenification) rather than the verrucous surface typically seen with HLP lesions. Wickham’s striae (seen in LP) are not seen with lichen simplex chronicus, and the lesions are localized only to easily reached areas.2
Mycetoma is a tumor-like lesion produced by a fungus (eumycetoma) or bacteria (actinomycetoma), typically encountered in arid areas rather than humid environments.3 These chronic, localized, nonpainful subcutaneous nodules develop on the foot and lower extremity after traumatic inoculation with the bacteria or fungus. Mycetomas persist for many years and classically present with a triad of tumefaction, draining sinus tracts, and “sulfur grains” that distinguish it from the dry, hyperkeratotic lesions of HLP. Diagnosis requires biopsy for histologic examination and both fungal and bacterial culture in order to choose the appropriate therapy.
Chromoblastomycosis is a deep fungal infection most commonly caused by the pigmented fungus Phialophora verrucosa found in tropical climates.4 The fungi enter the skin of the lower extremity after minor trauma, resulting in a gradually expanding verrucous nodule or plaque. The nodular variant is often pedunculated with classic pigmented cauliflower-like florets. While the nodular variant is localized, the plaque variant may spread laterally, possibly metastasizing through lymphatic channels with a concomitant bacterial infection. There is also a characteristic unpleasant odor with lymph stasis.
On potassium hydroxide (KOH) mounts or histologic examination, the thick-walled cells (muriform bodies) of chromoblastomycosis are diagnostic. Patients with chromoblastomycosis have seen response rates >60% with 10 to 24 months of daily itraconazole (200 mg) therapy.5
Squamous cell carcinoma (SCC) is the second most common skin cancer and affects more than 250,000 Americans each year. While associated with sun exposure, it has also been linked to ionizing radiation, arsenic, human papilloma virus, cigarette smoking, and chronic nonhealing wounds and scars such as Marjolin’s ulcer.1
Marjolin’s ulcer usually appears as a triad of nodule formation, induration, and ulceration at a scar site and thus may be confused with HLP. It is more common than sun-induced SCC in Asian and dark-skinned individuals.6 Marjolin’s ulcer will usually present in the fifth decade, years after the initial insult. Diagnosis is supported by the clinical appearance and history of a preceding scar at the site. Marjolin’s ulcer has a higher rate of recurrence and metastasis than other forms of SCC, and thus should be treated aggressively.7,8
A biopsy may be needed
A drop of immersion oil can confirm your HLP suspicions by revealing the white, lacy reticular network of Wickham’s striae.1 Other clinical clues to the diagnosis of LP or one of its variants include a white reticular, erythematous, or ulcerative appearance on the buccal mucosa in addition to a dorsal pterygium and/or diffuse pitting on the nails.
A deep shave or punch biopsy may be necessary, however, when the clinical diagnosis is unclear. Histological findings demonstrate focal hyperorthokeratosis, saw-toothed rete ridges, vacuolar change at the basal layer, and a band-like lymphocytic infiltrate.
Corticosteroids are the treatment of choice
There have been few large-scale prospective studies exploring the treatment of HLP. However, treatment for HLP is similar to that of LP and typically begins with topical class I or II glucocorticoids or intralesional injections of triamcinolone. Narrow-band ultraviolet-B (UVB) markedly reduces pruritus and flattens plaques, and is considered second-line treatment (strength of recommendation [SOR]: C).9-11 The retinoid acitretin may be effective for severe HLP at oral dosages of 30 mg/d for 8 weeks (SOR: A).12 Azathioprine and cyclosporine have also been used successfully, but risk of renal dysfunction, hypertension, and increased viral and fungal infections make these agents third-line therapies (SOR: C).13-15
A good outcome for our patient
Our patient applied clobetasol ointment 0.05% to the affected areas twice daily until the lesions went away (approximately 2 months later).
CORRESPONDENCE
Oliver J. Wisco, Maj, USAF, MC, FS, Department of the Air Force, Wilford Hall Medical Center, 59 MDW/SGOMD/Dermatology, 2200 Bergquist Drive, Suite 1, Lackland AFB, TX 78236-9908; [email protected]
1. Bolognia JL, Jorizzo JL, Rapini RP. Dermatology. 2nd ed. St. Louis, Mo: Mosby Elsevier; 2008.
2. Habif TP. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 4th ed. Philadelphia, Pa: Mosby; 2004:65.
3. Fitzpatrick T, Eisen A, Wolff K, et al. Dermatology in General Medicine. 5th ed. New York, NY: McGraw-Hill; 1999:2301.
4. Fitzpatrick T, Eisen A, Wolff K, et al. Dermatology in General Medicine. 5th ed. New York, NY: McGraw-Hill; 1999:2375.
5. Restrepo A. Treatment of tropical mycoses. J Am Acad Dermatol. 1994;31(3 Pt 2):S91-S102.
6. Chuang TY, Reizner GT, Elpern DJ, et al. Nonmelanoma skin cancer in Japanese ethnic Hawaiians in Kauai, Hawaii: an incidence report. J Am Acad Dermatol. 1995;33:422-426.
7. Treves N, Pack GT. The development of cancer in burn scars. An analysis and report of thirty-four cases. Surg Gynecol Obstet. 1930;51:749.-
8. Lifeso RM, Rooney RJ, el-Shaker M. Post-traumatic squamous-cell carcinoma. J Bone Joint Surg. 1990;72:12-18.
9. Gambichler T, Breuckmann F, Boms S, et al. Narrow-band UVB phototherapy in skin conditions beyond psoriasis. J Am Acad Dermatol. 2005;52:660-670.
10. Saricaoglu H, Karadogan SK, Baskan EB. Narrowband UVB therapy in the treatment of lichen planus. Photodermatol Photoimmunol Photomed. 2003;19:265-267.
11. Taneja A, Taylor CR. Narrow-band UVB for lichen planus treatment. Int J Dermatol. 2002;41:282-283.
12. Laurberg G, Geiger JM, Hjorth N, et al. Treatment of lichen planus with acitretin: a double-blind, placebo-controlled study in 65 patients. J Am Acad Dermatol 1991;24:434-437.
13. Kossard S, Artemi P. Acitretin for hypertrophic lichen planus-like reaction in a burn scar. Arch Dermatol. 2000;136:591-594.
14. Lear JT, English JS. Erosive and generalized lichen planus responsive to azathioprine. Clin Exp Dermatol. 1996;21:56-57.
15. Ho VC, Gupta AK, Ellis CN, et al. Treatment of severe lichen planus with cyclosporine. J Am Acad Dermatol. 1990;22:64-68.
1. Bolognia JL, Jorizzo JL, Rapini RP. Dermatology. 2nd ed. St. Louis, Mo: Mosby Elsevier; 2008.
2. Habif TP. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 4th ed. Philadelphia, Pa: Mosby; 2004:65.
3. Fitzpatrick T, Eisen A, Wolff K, et al. Dermatology in General Medicine. 5th ed. New York, NY: McGraw-Hill; 1999:2301.
4. Fitzpatrick T, Eisen A, Wolff K, et al. Dermatology in General Medicine. 5th ed. New York, NY: McGraw-Hill; 1999:2375.
5. Restrepo A. Treatment of tropical mycoses. J Am Acad Dermatol. 1994;31(3 Pt 2):S91-S102.
6. Chuang TY, Reizner GT, Elpern DJ, et al. Nonmelanoma skin cancer in Japanese ethnic Hawaiians in Kauai, Hawaii: an incidence report. J Am Acad Dermatol. 1995;33:422-426.
7. Treves N, Pack GT. The development of cancer in burn scars. An analysis and report of thirty-four cases. Surg Gynecol Obstet. 1930;51:749.-
8. Lifeso RM, Rooney RJ, el-Shaker M. Post-traumatic squamous-cell carcinoma. J Bone Joint Surg. 1990;72:12-18.
9. Gambichler T, Breuckmann F, Boms S, et al. Narrow-band UVB phototherapy in skin conditions beyond psoriasis. J Am Acad Dermatol. 2005;52:660-670.
10. Saricaoglu H, Karadogan SK, Baskan EB. Narrowband UVB therapy in the treatment of lichen planus. Photodermatol Photoimmunol Photomed. 2003;19:265-267.
11. Taneja A, Taylor CR. Narrow-band UVB for lichen planus treatment. Int J Dermatol. 2002;41:282-283.
12. Laurberg G, Geiger JM, Hjorth N, et al. Treatment of lichen planus with acitretin: a double-blind, placebo-controlled study in 65 patients. J Am Acad Dermatol 1991;24:434-437.
13. Kossard S, Artemi P. Acitretin for hypertrophic lichen planus-like reaction in a burn scar. Arch Dermatol. 2000;136:591-594.
14. Lear JT, English JS. Erosive and generalized lichen planus responsive to azathioprine. Clin Exp Dermatol. 1996;21:56-57.
15. Ho VC, Gupta AK, Ellis CN, et al. Treatment of severe lichen planus with cyclosporine. J Am Acad Dermatol. 1990;22:64-68.
