User login
ACIP weighs in on meningococcal B vaccines
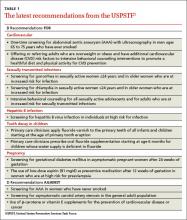
The Advisory Committee on Immunization Practices (ACIP) voted at its June 2015 meeting to make a “B” recommendation for the use of meningococcal B vaccine for individuals 16 through 23 years of age. The Committee felt that the vaccine can be used if one desires it, but at this time it should not be included in the category of a routinely recommended vaccine.
Meningococcal meningitis caused by serogroup B is a serious disease, but it is rare. From 2009 to 2013, the annual number of meningococcal B cases in individuals ages 11 to 24 years ranged from 54 to 67, with 5 to 10 deaths and 5 to 13 serious sequelae.1 Since 2009, there have been outbreaks on 7 university campuses with cases-per-outbreak numbering 2 to 13.1 These well publicized outbreaks created much disruption and an impression of increased risk among college students. But the surveillance system of the Centers for Disease Control and Prevention (CDC) demonstrates that the rate of infection among college students is actually lower than it is among individuals the same age who are not in college (TABLE 1).1
The combined incidence of 0.14/100,000 means that to prevent one case, 714,000 individuals need to be vaccinated; 5 million need to be vaccinated to prevent one death.1 These numbers are subject to yearly variation and would be more favorable should the incidence of the disease increase. (For a look at the historical incidence of meningococcal meningitis from all serotypes, see the FIGURE.1) The question facing ACIP was whether the current very low levels of meningococcal B disease merit widespread, routinely-recommended use of the vaccine.
A look at the 2 meningococcal B vaccines
Two meningococcal B vaccines are now licensed for use in the United States. MenB-FHbp (Trumenba, Pfizer) was licensed in October 2014 as a 3-dose series given at 0, 2, and 6 months.2 MenB-4C (Bexsero, Novartis/GSK) was licensed in January 2015 and requires 2 doses at 0 and ≥1 month.3 Both vaccines induce a level of antibody production that is considered immunogenic in a high proportion of those vaccinated, but the level of immunity wanes after 6 to 24 months. The clinical significance of this drop in immunity is unknown and cannot be tested currently because of the rarity of the disease. Unfortunately, the rate of asymptomatic carriage of meningococcal B does not appear to be affected by vaccination.1
Both vaccines produce local and systemic reactions at rates higher than other recommended vaccines for this age group: pain at the injection site (83%-85%), headache (33%-35%), myalgia (30%-48%), fatigue (35%-40%), induration (28%), nausea (18%), chills (15%), and arthralgia (13%).2,3 There is some theoretical concern about the potential for autoimmune disease from the use of meningococcal B vaccines that will be studied as the vaccines are used more widely.1 In addition, the CDC estimates that serious anaphylactic reactions can occur after administration of any vaccine, estimated at about one per every million doses.1
Meningococcal serotype B bacteria consist of different strains. The 2 approved vaccines cover today’s most frequently found strains in the United States, but it’s uncertain if this will hold true in the future.
USPSTF: Screen obese/overweight adults for type 2 diabetes
The United States Preventive Services Task Force (USPSTF) recently updated its recommendation for screening for type 2 diabetes in adults. USPSTF recommends screening adults, ages 40 to 70 years, who are obese or overweight and referring those who have abnormal blood glucose to intensive behavioral counseling to promote a healthful diet and physical activity.
The Task Force gave this recommendation a grade of B, meaning that it is likely to result in a moderate level of benefit from a reduction in progression to diabetes. The Task Force also emphasized that lifestyle modifications have a greater risk-reducing effect than metformin and other medications.
The recommendation rationale points out that screening might also benefit those at high risk of diabetes based on family history or race/ethnicity and does not apply to those with signs and symptoms of diabetes; testing in this latter group is considered diagnostic testing, not screening.
Screening can be done by measuring glycated hemoglobin A1c or fasting glucose or with a glucose tolerance test. The recommendation includes tables that list the cutoffs for abnormal glucose levels for impaired fasting glucose, impaired glucose tolerance, and increased average glucose level. Obesity is defined as a body mass index ≥30 kg/m2 and overweight as >25 kg/m2.
This new recommendation expands the list of those at risk and those who should be screened compared to the previous recommendation, but the Task Force found no evidence to support universal screening in adults as advocated by other organizations.
Source: USPSTF. Final recommendation statement. Abnormal blood glucose and type 2 diabetes mellitus: screening. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/screening-for-abnormal-blood-glucose-and-type-2-diabetes. Accessed November 13, 2015.
Recommendation considerations that came into play
A number of factors affected ACIP’s recommendation decision: the low incidence of the meningococcal B disease; the large number-needed-to-vaccinate to prevent a case and a death; uncertainties regarding the duration of protection; cost, lack of effect on carriage rates, and limited safety data with the potential for serious reactions to exceed the number of cases prevented; and the severity of the disease and the concern it elicits.
ACIP has multiple options when considering a vaccine: recommend it routinely for everyone or everyone in a defined group (A recommendation), recommend for individual decision making (B recommendation), recommend against use, and make no recommendation at all. Given that 2 meningococcal B vaccines are licensed in the United States and can be used by those who want them—and the Committee’s opinion that these vaccines should not (at this time) be included in the schedule of routinely-recommended vaccines—ACIP chose to make a B recommendation on their use (TABLE 2).1 Vaccines recommended by ACIP (both A and B recommendations) are mandated in the Affordable Care Act to be provided by commercial health insurance at no out-of-pocket expense to the patient.
A word about high-risk populations
At its February 2015 meeting, ACIP voted to recommend meningococcal B vaccine for use in high-risk populations and during outbreaks (TABLE 3).4 This recommendation—plus the most recent B recommendation for general use—comprise the totality of current recommendations for the prevention of meningococcal B disease in the United States.
1. MacNeil J. Considerations for the use of serogroup B meningococcal (MenB) vaccines in adolescents. Presented at: Advisory Committee on Immunization Practices; June 24, 2015; Atlanta, GA. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2015-06/mening-03-macneil.pdf. Accessed October 14, 2015.
2. Trumenba [package insert]. Philadelphia, PA: Wyeth Pharmaceuticals Inc. (Pfizer); 2014. Available at: http://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM421139.pdf. Accessed October 14, 2015.
3. Bexsero [package insert]. Cambridge, MA: Novartis Vaccines and Diagnostics Inc; 2015. Available at: http://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM431447.pdf. Accessed October 14, 2015.
4. Folaranmi T, Rubin L, Martin SW, et al. Use of serogroup B meningococcal vaccines in persons aged ≥10 years at increased risk for serogroup B meningococcal disease: recommendations of the Advisory Committee on Immunization Practices, 2015. MMWR Morb Mortal Wkly Rep. 2015;64:608-612.
The Advisory Committee on Immunization Practices (ACIP) voted at its June 2015 meeting to make a “B” recommendation for the use of meningococcal B vaccine for individuals 16 through 23 years of age. The Committee felt that the vaccine can be used if one desires it, but at this time it should not be included in the category of a routinely recommended vaccine.
Meningococcal meningitis caused by serogroup B is a serious disease, but it is rare. From 2009 to 2013, the annual number of meningococcal B cases in individuals ages 11 to 24 years ranged from 54 to 67, with 5 to 10 deaths and 5 to 13 serious sequelae.1 Since 2009, there have been outbreaks on 7 university campuses with cases-per-outbreak numbering 2 to 13.1 These well publicized outbreaks created much disruption and an impression of increased risk among college students. But the surveillance system of the Centers for Disease Control and Prevention (CDC) demonstrates that the rate of infection among college students is actually lower than it is among individuals the same age who are not in college (TABLE 1).1
The combined incidence of 0.14/100,000 means that to prevent one case, 714,000 individuals need to be vaccinated; 5 million need to be vaccinated to prevent one death.1 These numbers are subject to yearly variation and would be more favorable should the incidence of the disease increase. (For a look at the historical incidence of meningococcal meningitis from all serotypes, see the FIGURE.1) The question facing ACIP was whether the current very low levels of meningococcal B disease merit widespread, routinely-recommended use of the vaccine.
A look at the 2 meningococcal B vaccines
Two meningococcal B vaccines are now licensed for use in the United States. MenB-FHbp (Trumenba, Pfizer) was licensed in October 2014 as a 3-dose series given at 0, 2, and 6 months.2 MenB-4C (Bexsero, Novartis/GSK) was licensed in January 2015 and requires 2 doses at 0 and ≥1 month.3 Both vaccines induce a level of antibody production that is considered immunogenic in a high proportion of those vaccinated, but the level of immunity wanes after 6 to 24 months. The clinical significance of this drop in immunity is unknown and cannot be tested currently because of the rarity of the disease. Unfortunately, the rate of asymptomatic carriage of meningococcal B does not appear to be affected by vaccination.1
Both vaccines produce local and systemic reactions at rates higher than other recommended vaccines for this age group: pain at the injection site (83%-85%), headache (33%-35%), myalgia (30%-48%), fatigue (35%-40%), induration (28%), nausea (18%), chills (15%), and arthralgia (13%).2,3 There is some theoretical concern about the potential for autoimmune disease from the use of meningococcal B vaccines that will be studied as the vaccines are used more widely.1 In addition, the CDC estimates that serious anaphylactic reactions can occur after administration of any vaccine, estimated at about one per every million doses.1
Meningococcal serotype B bacteria consist of different strains. The 2 approved vaccines cover today’s most frequently found strains in the United States, but it’s uncertain if this will hold true in the future.
USPSTF: Screen obese/overweight adults for type 2 diabetes
The United States Preventive Services Task Force (USPSTF) recently updated its recommendation for screening for type 2 diabetes in adults. USPSTF recommends screening adults, ages 40 to 70 years, who are obese or overweight and referring those who have abnormal blood glucose to intensive behavioral counseling to promote a healthful diet and physical activity.
The Task Force gave this recommendation a grade of B, meaning that it is likely to result in a moderate level of benefit from a reduction in progression to diabetes. The Task Force also emphasized that lifestyle modifications have a greater risk-reducing effect than metformin and other medications.
The recommendation rationale points out that screening might also benefit those at high risk of diabetes based on family history or race/ethnicity and does not apply to those with signs and symptoms of diabetes; testing in this latter group is considered diagnostic testing, not screening.
Screening can be done by measuring glycated hemoglobin A1c or fasting glucose or with a glucose tolerance test. The recommendation includes tables that list the cutoffs for abnormal glucose levels for impaired fasting glucose, impaired glucose tolerance, and increased average glucose level. Obesity is defined as a body mass index ≥30 kg/m2 and overweight as >25 kg/m2.
This new recommendation expands the list of those at risk and those who should be screened compared to the previous recommendation, but the Task Force found no evidence to support universal screening in adults as advocated by other organizations.
Source: USPSTF. Final recommendation statement. Abnormal blood glucose and type 2 diabetes mellitus: screening. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/screening-for-abnormal-blood-glucose-and-type-2-diabetes. Accessed November 13, 2015.
Recommendation considerations that came into play
A number of factors affected ACIP’s recommendation decision: the low incidence of the meningococcal B disease; the large number-needed-to-vaccinate to prevent a case and a death; uncertainties regarding the duration of protection; cost, lack of effect on carriage rates, and limited safety data with the potential for serious reactions to exceed the number of cases prevented; and the severity of the disease and the concern it elicits.
ACIP has multiple options when considering a vaccine: recommend it routinely for everyone or everyone in a defined group (A recommendation), recommend for individual decision making (B recommendation), recommend against use, and make no recommendation at all. Given that 2 meningococcal B vaccines are licensed in the United States and can be used by those who want them—and the Committee’s opinion that these vaccines should not (at this time) be included in the schedule of routinely-recommended vaccines—ACIP chose to make a B recommendation on their use (TABLE 2).1 Vaccines recommended by ACIP (both A and B recommendations) are mandated in the Affordable Care Act to be provided by commercial health insurance at no out-of-pocket expense to the patient.
A word about high-risk populations
At its February 2015 meeting, ACIP voted to recommend meningococcal B vaccine for use in high-risk populations and during outbreaks (TABLE 3).4 This recommendation—plus the most recent B recommendation for general use—comprise the totality of current recommendations for the prevention of meningococcal B disease in the United States.
The Advisory Committee on Immunization Practices (ACIP) voted at its June 2015 meeting to make a “B” recommendation for the use of meningococcal B vaccine for individuals 16 through 23 years of age. The Committee felt that the vaccine can be used if one desires it, but at this time it should not be included in the category of a routinely recommended vaccine.
Meningococcal meningitis caused by serogroup B is a serious disease, but it is rare. From 2009 to 2013, the annual number of meningococcal B cases in individuals ages 11 to 24 years ranged from 54 to 67, with 5 to 10 deaths and 5 to 13 serious sequelae.1 Since 2009, there have been outbreaks on 7 university campuses with cases-per-outbreak numbering 2 to 13.1 These well publicized outbreaks created much disruption and an impression of increased risk among college students. But the surveillance system of the Centers for Disease Control and Prevention (CDC) demonstrates that the rate of infection among college students is actually lower than it is among individuals the same age who are not in college (TABLE 1).1
The combined incidence of 0.14/100,000 means that to prevent one case, 714,000 individuals need to be vaccinated; 5 million need to be vaccinated to prevent one death.1 These numbers are subject to yearly variation and would be more favorable should the incidence of the disease increase. (For a look at the historical incidence of meningococcal meningitis from all serotypes, see the FIGURE.1) The question facing ACIP was whether the current very low levels of meningococcal B disease merit widespread, routinely-recommended use of the vaccine.
A look at the 2 meningococcal B vaccines
Two meningococcal B vaccines are now licensed for use in the United States. MenB-FHbp (Trumenba, Pfizer) was licensed in October 2014 as a 3-dose series given at 0, 2, and 6 months.2 MenB-4C (Bexsero, Novartis/GSK) was licensed in January 2015 and requires 2 doses at 0 and ≥1 month.3 Both vaccines induce a level of antibody production that is considered immunogenic in a high proportion of those vaccinated, but the level of immunity wanes after 6 to 24 months. The clinical significance of this drop in immunity is unknown and cannot be tested currently because of the rarity of the disease. Unfortunately, the rate of asymptomatic carriage of meningococcal B does not appear to be affected by vaccination.1
Both vaccines produce local and systemic reactions at rates higher than other recommended vaccines for this age group: pain at the injection site (83%-85%), headache (33%-35%), myalgia (30%-48%), fatigue (35%-40%), induration (28%), nausea (18%), chills (15%), and arthralgia (13%).2,3 There is some theoretical concern about the potential for autoimmune disease from the use of meningococcal B vaccines that will be studied as the vaccines are used more widely.1 In addition, the CDC estimates that serious anaphylactic reactions can occur after administration of any vaccine, estimated at about one per every million doses.1
Meningococcal serotype B bacteria consist of different strains. The 2 approved vaccines cover today’s most frequently found strains in the United States, but it’s uncertain if this will hold true in the future.
USPSTF: Screen obese/overweight adults for type 2 diabetes
The United States Preventive Services Task Force (USPSTF) recently updated its recommendation for screening for type 2 diabetes in adults. USPSTF recommends screening adults, ages 40 to 70 years, who are obese or overweight and referring those who have abnormal blood glucose to intensive behavioral counseling to promote a healthful diet and physical activity.
The Task Force gave this recommendation a grade of B, meaning that it is likely to result in a moderate level of benefit from a reduction in progression to diabetes. The Task Force also emphasized that lifestyle modifications have a greater risk-reducing effect than metformin and other medications.
The recommendation rationale points out that screening might also benefit those at high risk of diabetes based on family history or race/ethnicity and does not apply to those with signs and symptoms of diabetes; testing in this latter group is considered diagnostic testing, not screening.
Screening can be done by measuring glycated hemoglobin A1c or fasting glucose or with a glucose tolerance test. The recommendation includes tables that list the cutoffs for abnormal glucose levels for impaired fasting glucose, impaired glucose tolerance, and increased average glucose level. Obesity is defined as a body mass index ≥30 kg/m2 and overweight as >25 kg/m2.
This new recommendation expands the list of those at risk and those who should be screened compared to the previous recommendation, but the Task Force found no evidence to support universal screening in adults as advocated by other organizations.
Source: USPSTF. Final recommendation statement. Abnormal blood glucose and type 2 diabetes mellitus: screening. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/screening-for-abnormal-blood-glucose-and-type-2-diabetes. Accessed November 13, 2015.
Recommendation considerations that came into play
A number of factors affected ACIP’s recommendation decision: the low incidence of the meningococcal B disease; the large number-needed-to-vaccinate to prevent a case and a death; uncertainties regarding the duration of protection; cost, lack of effect on carriage rates, and limited safety data with the potential for serious reactions to exceed the number of cases prevented; and the severity of the disease and the concern it elicits.
ACIP has multiple options when considering a vaccine: recommend it routinely for everyone or everyone in a defined group (A recommendation), recommend for individual decision making (B recommendation), recommend against use, and make no recommendation at all. Given that 2 meningococcal B vaccines are licensed in the United States and can be used by those who want them—and the Committee’s opinion that these vaccines should not (at this time) be included in the schedule of routinely-recommended vaccines—ACIP chose to make a B recommendation on their use (TABLE 2).1 Vaccines recommended by ACIP (both A and B recommendations) are mandated in the Affordable Care Act to be provided by commercial health insurance at no out-of-pocket expense to the patient.
A word about high-risk populations
At its February 2015 meeting, ACIP voted to recommend meningococcal B vaccine for use in high-risk populations and during outbreaks (TABLE 3).4 This recommendation—plus the most recent B recommendation for general use—comprise the totality of current recommendations for the prevention of meningococcal B disease in the United States.
1. MacNeil J. Considerations for the use of serogroup B meningococcal (MenB) vaccines in adolescents. Presented at: Advisory Committee on Immunization Practices; June 24, 2015; Atlanta, GA. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2015-06/mening-03-macneil.pdf. Accessed October 14, 2015.
2. Trumenba [package insert]. Philadelphia, PA: Wyeth Pharmaceuticals Inc. (Pfizer); 2014. Available at: http://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM421139.pdf. Accessed October 14, 2015.
3. Bexsero [package insert]. Cambridge, MA: Novartis Vaccines and Diagnostics Inc; 2015. Available at: http://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM431447.pdf. Accessed October 14, 2015.
4. Folaranmi T, Rubin L, Martin SW, et al. Use of serogroup B meningococcal vaccines in persons aged ≥10 years at increased risk for serogroup B meningococcal disease: recommendations of the Advisory Committee on Immunization Practices, 2015. MMWR Morb Mortal Wkly Rep. 2015;64:608-612.
1. MacNeil J. Considerations for the use of serogroup B meningococcal (MenB) vaccines in adolescents. Presented at: Advisory Committee on Immunization Practices; June 24, 2015; Atlanta, GA. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2015-06/mening-03-macneil.pdf. Accessed October 14, 2015.
2. Trumenba [package insert]. Philadelphia, PA: Wyeth Pharmaceuticals Inc. (Pfizer); 2014. Available at: http://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM421139.pdf. Accessed October 14, 2015.
3. Bexsero [package insert]. Cambridge, MA: Novartis Vaccines and Diagnostics Inc; 2015. Available at: http://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM431447.pdf. Accessed October 14, 2015.
4. Folaranmi T, Rubin L, Martin SW, et al. Use of serogroup B meningococcal vaccines in persons aged ≥10 years at increased risk for serogroup B meningococcal disease: recommendations of the Advisory Committee on Immunization Practices, 2015. MMWR Morb Mortal Wkly Rep. 2015;64:608-612.
Influenza vaccination: What’s new this season
The Centers for Disease Control and Prevention (CDC) has published its recommendations for the use of influenza vaccines for the 2015-2016 influenza season.1 (See the CDC’s Web site at http://www.cdc.gov/flu/professionals/vaccination/index.htm.) This Practice Alert describes recent changes in vaccine products, discusses the timing of vaccination, reviews a new algorithm for deciding on the number of doses for children ages 6 months through 8 years, and raises issues to consider when thinking about specific products for individual patients.
Vaccine product modifications for 2015-2016
Influenza vaccines contain either 3 or 4 antigens (trivalent or quadrivalent) and either inactivated or modified live viruses (inactivated influenza vaccine [IIV]) or live attenuated influenza vaccine [LAIV]). These vaccines are produced in eggs, by cell cultures, or with recombinant technology. The vaccine products for this influenza season will contain antigens of one H1N1 virus, one H3N2 virus, and one B virus (trivalent products) or 2 B viruses (quadrivalent products).
The viruses selected for the vaccine are based on the most prevalent types in circulation globally, and vaccine effectiveness in the United States will be directly proportional to how well these strains match those circulating here during the influenza season. In the 2014-2015 influenza season, an antigenic drift in the circulating H3N2 virus rendered influenza vaccines only 23% effective in preventing laboratory-confirmed influenza.2
One product, Afluria, an IIV trivalent product, has been approved for administration using a needle-free jet injector in individuals ages 18 to 64 years.1 Additionally, intramuscular injection of Afluria is still available for those 18 years and older. All other IIV products are administered via needle and syringe. Vaccination with a jet injector achieves protection equivalent to needle injection, but its use is associated with higher rates of local reactions.
Flublok, another trivalent IIV product, is produced using a recombinant egg-free process. It was originally approved for individuals 18 to 49 years; there is now no upper age limit, providing an egg-free option for an expanded age group. An intradermal option, Fluzone, was a trivalent product last year and will be replaced by Fluzone Intradermal Quadrivalent this season.
A complete list of all influenza products and their respective patient-specific recommendations can be found on the CDC influenza Web site (http://www.cdc.gov/flu/professionals/vaccination/index.htm).
Timing of vaccine administration
Start providing influenza vaccine by the beginning of October and continue offering it throughout the influenza season to those who are unimmunized. In the past, the recommendation was to begin vaccination as soon as vaccine was available. But studies have shown that vaccine effectiveness declines after 6 months, especially among those over age 65 years, which can result in inadequate protection late in the influenza season.3,4 Administering the vaccine later in the fall may confer greater protection later in the season, but, as a strategy, it could also lead to missed opportunities to vaccinate.
Annual vaccination for the entire population is a public health challenge and the October start date is appropriate middle ground. However, children who need 2 doses should receive the first dose as soon as vaccine is available, and the second dose 4 weeks later.1
One or two doses in children?
Children ages 6 months through 8 years who are receiving influenza vaccine for the first or second time need 2 doses for maximum immune response. Past algorithms that aided in deciding which children needed 2 doses instead of one considered not only the number and timing of previous doses but also whether the product had contained pandemic H1N1 antigen. The algorithm for the coming year asks just one question: How many doses of influenza vaccine has the child received previously? This is without regard to when or to the specific products. If the answer is 2 or more doses (not necessarily given in the same season or even in consecutive seasons), only one dose is needed this season. If the answer is “one dose” or “none,” 2 doses are recommended this season, separated by at least 4 weeks.
Considerations for individual patients
The Advisory Committee on Immunization Practices (ACIP) recommends annual influenza vaccination for everyone ages 6 months and older who do not have a contraindication. It states no preference for any product for any age. Last year’s preference for LAIV over IIV for children through age 8 years has been changed; either LAIV or IIV is appropriate for this age group.1 Although quadrivalent vaccines offer some added protection with an additional B virus, do not delay vaccination if only a trivalent product is available.
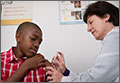
Use the LAIV only for individuals ages 2 years to 49 years who do not have a contraindication listed in the TABLE.1 There are other conditions that pose a theoretical increased risk of complications with the use of LAIV (TABLE), but they do not preclude the use of the vaccine. Additionally, anyone providing care for a severely immunosuppressed individual should avoid being vaccinated with LAIV or, if vaccinated with the live virus, avoid contact with the individual for 7 days following vaccination.
Recommendations for use of influenza vaccines in those who say they are allergic to eggs remain unchanged from last year (FIGURE1). The amount of egg protein in influenza vaccines is very low and serious allergic reactions are rare. The availability of trivalent recombinant vaccine provides an egg-free option for those ages 18 and older.
Vaccines are not all we have to protect the public
Remember that while influenza vaccines are recommended and are the most effective intervention to prevent influenza morbidity and mortality, they are imperfect. Their effectiveness varies from year to year, and it wanes with time after administration. The proportion of the population vaccinated is also suboptimal, which makes other prevention interventions important to implement. These include good infection control practices in all health care facilities, social distancing of those who are infectious, infection control practices in homes with an infected person, vaccination of all health care workers, and judicious use of pre- and post-exposure chemoprevention when indicated. These have all been discussed in a previous Practice Alert.5
1. Grohskopf LA, Sokolow LZ, Olsen SJ, et al. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices, United States, 2015-16 Influenza Season. MMWR Morb Mortal Wkly Rep. 2015;64:818-825.
2. Flannery B, Clippard J. End-of-season influenza vaccine effectiveness estimates for the 2014-15 season. Presented at: Meeting of the Advisory Committee on Immunization Practices; June 24, 2015; Atlanta, GA. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2015-06/flu-02-flannery.pdf. Accessed August 11, 2015.
3. Song JY, Cheong HJ, Hwang IS, et al. Long-term immunogenicity of influenza vaccine among the elderly: Risk factors for poor immune response and persistence. Vaccine. 2010;28:3929-3935.
4. Castilla J, Martinez-Baz I, Martinez-Artola V, et al. Decline in influenza vaccine effectiveness with time after vaccination, Navarre, Spain, season 2011/12. Euro Surveill. 2013;18(5). pii:20388.
5. Campos-Outcalt D. Influenza: Update for the 2013-2014 season. J Fam Prac. 2013;62:494-498.
The Centers for Disease Control and Prevention (CDC) has published its recommendations for the use of influenza vaccines for the 2015-2016 influenza season.1 (See the CDC’s Web site at http://www.cdc.gov/flu/professionals/vaccination/index.htm.) This Practice Alert describes recent changes in vaccine products, discusses the timing of vaccination, reviews a new algorithm for deciding on the number of doses for children ages 6 months through 8 years, and raises issues to consider when thinking about specific products for individual patients.
Vaccine product modifications for 2015-2016
Influenza vaccines contain either 3 or 4 antigens (trivalent or quadrivalent) and either inactivated or modified live viruses (inactivated influenza vaccine [IIV]) or live attenuated influenza vaccine [LAIV]). These vaccines are produced in eggs, by cell cultures, or with recombinant technology. The vaccine products for this influenza season will contain antigens of one H1N1 virus, one H3N2 virus, and one B virus (trivalent products) or 2 B viruses (quadrivalent products).
The viruses selected for the vaccine are based on the most prevalent types in circulation globally, and vaccine effectiveness in the United States will be directly proportional to how well these strains match those circulating here during the influenza season. In the 2014-2015 influenza season, an antigenic drift in the circulating H3N2 virus rendered influenza vaccines only 23% effective in preventing laboratory-confirmed influenza.2
One product, Afluria, an IIV trivalent product, has been approved for administration using a needle-free jet injector in individuals ages 18 to 64 years.1 Additionally, intramuscular injection of Afluria is still available for those 18 years and older. All other IIV products are administered via needle and syringe. Vaccination with a jet injector achieves protection equivalent to needle injection, but its use is associated with higher rates of local reactions.
Flublok, another trivalent IIV product, is produced using a recombinant egg-free process. It was originally approved for individuals 18 to 49 years; there is now no upper age limit, providing an egg-free option for an expanded age group. An intradermal option, Fluzone, was a trivalent product last year and will be replaced by Fluzone Intradermal Quadrivalent this season.
A complete list of all influenza products and their respective patient-specific recommendations can be found on the CDC influenza Web site (http://www.cdc.gov/flu/professionals/vaccination/index.htm).
Timing of vaccine administration
Start providing influenza vaccine by the beginning of October and continue offering it throughout the influenza season to those who are unimmunized. In the past, the recommendation was to begin vaccination as soon as vaccine was available. But studies have shown that vaccine effectiveness declines after 6 months, especially among those over age 65 years, which can result in inadequate protection late in the influenza season.3,4 Administering the vaccine later in the fall may confer greater protection later in the season, but, as a strategy, it could also lead to missed opportunities to vaccinate.
Annual vaccination for the entire population is a public health challenge and the October start date is appropriate middle ground. However, children who need 2 doses should receive the first dose as soon as vaccine is available, and the second dose 4 weeks later.1
One or two doses in children?
Children ages 6 months through 8 years who are receiving influenza vaccine for the first or second time need 2 doses for maximum immune response. Past algorithms that aided in deciding which children needed 2 doses instead of one considered not only the number and timing of previous doses but also whether the product had contained pandemic H1N1 antigen. The algorithm for the coming year asks just one question: How many doses of influenza vaccine has the child received previously? This is without regard to when or to the specific products. If the answer is 2 or more doses (not necessarily given in the same season or even in consecutive seasons), only one dose is needed this season. If the answer is “one dose” or “none,” 2 doses are recommended this season, separated by at least 4 weeks.
Considerations for individual patients
The Advisory Committee on Immunization Practices (ACIP) recommends annual influenza vaccination for everyone ages 6 months and older who do not have a contraindication. It states no preference for any product for any age. Last year’s preference for LAIV over IIV for children through age 8 years has been changed; either LAIV or IIV is appropriate for this age group.1 Although quadrivalent vaccines offer some added protection with an additional B virus, do not delay vaccination if only a trivalent product is available.
Use the LAIV only for individuals ages 2 years to 49 years who do not have a contraindication listed in the TABLE.1 There are other conditions that pose a theoretical increased risk of complications with the use of LAIV (TABLE), but they do not preclude the use of the vaccine. Additionally, anyone providing care for a severely immunosuppressed individual should avoid being vaccinated with LAIV or, if vaccinated with the live virus, avoid contact with the individual for 7 days following vaccination.
Recommendations for use of influenza vaccines in those who say they are allergic to eggs remain unchanged from last year (FIGURE1). The amount of egg protein in influenza vaccines is very low and serious allergic reactions are rare. The availability of trivalent recombinant vaccine provides an egg-free option for those ages 18 and older.
Vaccines are not all we have to protect the public
Remember that while influenza vaccines are recommended and are the most effective intervention to prevent influenza morbidity and mortality, they are imperfect. Their effectiveness varies from year to year, and it wanes with time after administration. The proportion of the population vaccinated is also suboptimal, which makes other prevention interventions important to implement. These include good infection control practices in all health care facilities, social distancing of those who are infectious, infection control practices in homes with an infected person, vaccination of all health care workers, and judicious use of pre- and post-exposure chemoprevention when indicated. These have all been discussed in a previous Practice Alert.5
The Centers for Disease Control and Prevention (CDC) has published its recommendations for the use of influenza vaccines for the 2015-2016 influenza season.1 (See the CDC’s Web site at http://www.cdc.gov/flu/professionals/vaccination/index.htm.) This Practice Alert describes recent changes in vaccine products, discusses the timing of vaccination, reviews a new algorithm for deciding on the number of doses for children ages 6 months through 8 years, and raises issues to consider when thinking about specific products for individual patients.
Vaccine product modifications for 2015-2016
Influenza vaccines contain either 3 or 4 antigens (trivalent or quadrivalent) and either inactivated or modified live viruses (inactivated influenza vaccine [IIV]) or live attenuated influenza vaccine [LAIV]). These vaccines are produced in eggs, by cell cultures, or with recombinant technology. The vaccine products for this influenza season will contain antigens of one H1N1 virus, one H3N2 virus, and one B virus (trivalent products) or 2 B viruses (quadrivalent products).
The viruses selected for the vaccine are based on the most prevalent types in circulation globally, and vaccine effectiveness in the United States will be directly proportional to how well these strains match those circulating here during the influenza season. In the 2014-2015 influenza season, an antigenic drift in the circulating H3N2 virus rendered influenza vaccines only 23% effective in preventing laboratory-confirmed influenza.2
One product, Afluria, an IIV trivalent product, has been approved for administration using a needle-free jet injector in individuals ages 18 to 64 years.1 Additionally, intramuscular injection of Afluria is still available for those 18 years and older. All other IIV products are administered via needle and syringe. Vaccination with a jet injector achieves protection equivalent to needle injection, but its use is associated with higher rates of local reactions.
Flublok, another trivalent IIV product, is produced using a recombinant egg-free process. It was originally approved for individuals 18 to 49 years; there is now no upper age limit, providing an egg-free option for an expanded age group. An intradermal option, Fluzone, was a trivalent product last year and will be replaced by Fluzone Intradermal Quadrivalent this season.
A complete list of all influenza products and their respective patient-specific recommendations can be found on the CDC influenza Web site (http://www.cdc.gov/flu/professionals/vaccination/index.htm).
Timing of vaccine administration
Start providing influenza vaccine by the beginning of October and continue offering it throughout the influenza season to those who are unimmunized. In the past, the recommendation was to begin vaccination as soon as vaccine was available. But studies have shown that vaccine effectiveness declines after 6 months, especially among those over age 65 years, which can result in inadequate protection late in the influenza season.3,4 Administering the vaccine later in the fall may confer greater protection later in the season, but, as a strategy, it could also lead to missed opportunities to vaccinate.
Annual vaccination for the entire population is a public health challenge and the October start date is appropriate middle ground. However, children who need 2 doses should receive the first dose as soon as vaccine is available, and the second dose 4 weeks later.1
One or two doses in children?
Children ages 6 months through 8 years who are receiving influenza vaccine for the first or second time need 2 doses for maximum immune response. Past algorithms that aided in deciding which children needed 2 doses instead of one considered not only the number and timing of previous doses but also whether the product had contained pandemic H1N1 antigen. The algorithm for the coming year asks just one question: How many doses of influenza vaccine has the child received previously? This is without regard to when or to the specific products. If the answer is 2 or more doses (not necessarily given in the same season or even in consecutive seasons), only one dose is needed this season. If the answer is “one dose” or “none,” 2 doses are recommended this season, separated by at least 4 weeks.
Considerations for individual patients
The Advisory Committee on Immunization Practices (ACIP) recommends annual influenza vaccination for everyone ages 6 months and older who do not have a contraindication. It states no preference for any product for any age. Last year’s preference for LAIV over IIV for children through age 8 years has been changed; either LAIV or IIV is appropriate for this age group.1 Although quadrivalent vaccines offer some added protection with an additional B virus, do not delay vaccination if only a trivalent product is available.
Use the LAIV only for individuals ages 2 years to 49 years who do not have a contraindication listed in the TABLE.1 There are other conditions that pose a theoretical increased risk of complications with the use of LAIV (TABLE), but they do not preclude the use of the vaccine. Additionally, anyone providing care for a severely immunosuppressed individual should avoid being vaccinated with LAIV or, if vaccinated with the live virus, avoid contact with the individual for 7 days following vaccination.
Recommendations for use of influenza vaccines in those who say they are allergic to eggs remain unchanged from last year (FIGURE1). The amount of egg protein in influenza vaccines is very low and serious allergic reactions are rare. The availability of trivalent recombinant vaccine provides an egg-free option for those ages 18 and older.
Vaccines are not all we have to protect the public
Remember that while influenza vaccines are recommended and are the most effective intervention to prevent influenza morbidity and mortality, they are imperfect. Their effectiveness varies from year to year, and it wanes with time after administration. The proportion of the population vaccinated is also suboptimal, which makes other prevention interventions important to implement. These include good infection control practices in all health care facilities, social distancing of those who are infectious, infection control practices in homes with an infected person, vaccination of all health care workers, and judicious use of pre- and post-exposure chemoprevention when indicated. These have all been discussed in a previous Practice Alert.5
1. Grohskopf LA, Sokolow LZ, Olsen SJ, et al. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices, United States, 2015-16 Influenza Season. MMWR Morb Mortal Wkly Rep. 2015;64:818-825.
2. Flannery B, Clippard J. End-of-season influenza vaccine effectiveness estimates for the 2014-15 season. Presented at: Meeting of the Advisory Committee on Immunization Practices; June 24, 2015; Atlanta, GA. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2015-06/flu-02-flannery.pdf. Accessed August 11, 2015.
3. Song JY, Cheong HJ, Hwang IS, et al. Long-term immunogenicity of influenza vaccine among the elderly: Risk factors for poor immune response and persistence. Vaccine. 2010;28:3929-3935.
4. Castilla J, Martinez-Baz I, Martinez-Artola V, et al. Decline in influenza vaccine effectiveness with time after vaccination, Navarre, Spain, season 2011/12. Euro Surveill. 2013;18(5). pii:20388.
5. Campos-Outcalt D. Influenza: Update for the 2013-2014 season. J Fam Prac. 2013;62:494-498.
1. Grohskopf LA, Sokolow LZ, Olsen SJ, et al. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices, United States, 2015-16 Influenza Season. MMWR Morb Mortal Wkly Rep. 2015;64:818-825.
2. Flannery B, Clippard J. End-of-season influenza vaccine effectiveness estimates for the 2014-15 season. Presented at: Meeting of the Advisory Committee on Immunization Practices; June 24, 2015; Atlanta, GA. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2015-06/flu-02-flannery.pdf. Accessed August 11, 2015.
3. Song JY, Cheong HJ, Hwang IS, et al. Long-term immunogenicity of influenza vaccine among the elderly: Risk factors for poor immune response and persistence. Vaccine. 2010;28:3929-3935.
4. Castilla J, Martinez-Baz I, Martinez-Artola V, et al. Decline in influenza vaccine effectiveness with time after vaccination, Navarre, Spain, season 2011/12. Euro Surveill. 2013;18(5). pii:20388.
5. Campos-Outcalt D. Influenza: Update for the 2013-2014 season. J Fam Prac. 2013;62:494-498.
Breast cancer screening: The latest from the USPSTF
The United States Preventive Services Task Force (USPSTF) recently released draft recommendations on breast cancer screening, which could be finalized within the next few months.1 The last time the Task Force (TF) weighed in on this topic was in 2009, just as the Affordable Care Act (ACA) was being debated. At that time, the TF recommendations were so controversial that Congress specified in the ACA that they should not be used to determine insurance coverage (more on this later).
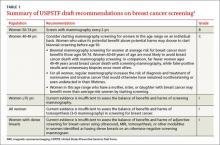
The draft recommendations (TABLE 1)1 carry a C grade for women ages 40 to 49 years (ie, offer or provide screening mammography for selected patients depending on individual circumstances) and a B grade for biennial screening of women ages 50 to 74. The proposed recommendations are basically the same as the ones made in 2009, with more detailed wording to explain the rationale for the C recommendation, and to address 2 new issues: tomosynthesis (3-D mammography) and adjunctive screening for women with dense breasts. The previous D recommendation against self breast examination was left unchanged.
Benefit of mammography screening varies by decade of life
Breast cancer is the leading cause of non-skin cancers in women and, after lung cancer, the second leading cause of cancer deaths in women. In 2014 there were 233,000 new cases diagnosed and 40,000 breast cancer deaths.1,2 While the TF found that mammography reduces deaths from breast cancer in women between the ages of 40 and 74, women ages 40 to 49 benefit the least; those ages 60 to 69 benefit the most.1,3
If 10,000 women are screened routinely for 10 years, 4 breast cancer deaths will be prevented in those ages 40 to 49, 8 in those 50 to 59, and 21 in those 60 to 69.1 And harms appear to be higher in the younger age group. TABLE 21,3 shows some of the harms resulting from one-time mammography screening of 10,000 women in each age group. Notice the benefits listed previously are from repeated screenings over a 10-year period and the harms in TABLE 21,3 are from a single mammogram.
The total benefits and harms of biennial screening in 1000 women starting at age 40 (vs age 50) include 8 cancer deaths prevented (vs 7) with a cost of 1529 false positive tests (vs 953); 204 unnecessary breast biopsies (vs 146); and 20 overdiagnoses (vs 18). However, the confidence intervals on these estimates are wide, and in each case, they overlap between the 2 groups.1
The TF recommended biennial screening for women between the ages of 50 and 74 because observational studies and modeling show no clear benefit with annual screening vs every 2 years, while annual screening results in more false positives and biopsies.
Overdiagnosis may occur in nearly 20% of cases
The potential for overdiagnosis and overtreatment is increasingly recognized as a harm of cancer screening. Overdiagnosis results from detecting a tumor during screening that would not have been detected otherwise and that would not have caused death or disease but is treated anyway. This sometimes occurs with the detection of early tumors that would not have progressed or would have progressed slowly, not causing health problems before the woman dies of other causes.
The TF is one of the only organizations that considers the potential harmful effects of this problem. While it is not possible to know for certain the rate of overdiagnosis that occurs with cancer screening, high-quality studies indicate it is close to 20% for breast cancer.3
Guidance regarding women ages 40 to 49
The new draft recommendations carefully point out that, while the overall benefit of screening women ages 40 to 49 is small, the decision to begin screening before age 50 should be an individual one, and an informed one. They state that women who value the small potential benefit over the potential for harm may choose to be screened, as might women who have a family history of breast cancer. And the recommendations do not apply to women who have a genotype that places them at increased risk for breast cancer.
Tomosynthesis: Evidence of benefit is insufficient
Tomosynthesis as a primary breast cancer screening tool was studied in a separate evidence report commissioned by the TF.4 While tomosynthesis, compared with routine mammography, appears to have increased sensitivity and specificity in detecting breast cancer, no studies looked at this technology as a primary screening tool and its effect on breast cancer mortality, overall mortality, and quality of life. Sticking to its nationally-recognized methodological rigor, the TF states that information at this time is insufficient to make a recommendation on the use of tomosynthesis.
Dense breasts: Usefulness of adjunctive screening modalities
Breast density is categorized into 4 groups, from category a (breasts are almost all fatty with little fibro nodular tissue) to category d (breasts are extremely dense).1 About 43% of women ages 40 to 74 are in categories c and d.1 Dense breasts adversely affect the accuracy of mammography, decreasing sensitivity and specificity. In one study, sensitivity was 87% in category a and 63% in category d; specificities were 97% and 89%, respectively.5
Tomosynthesis, magnetic resonance imaging, and ultrasound, when used in addition to mammography, all appear to detect more cancers, but they also yield more false-positive results.6 The long-term outcome of detecting more tumors is not known. For an individual, there are 3 possibilities when a tumor is detected earlier: a better outcome, no difference in outcome, or a worse outcome resulting from overdiagnosis and overtreatment. The TF felt that the available data are insufficient to judge benefits and harms of an increased frequency of screening or the use of adjunctive screening methods in women with dense breasts.
Benefit for women ≥75 years is inconclusive
There are limited data on the impact of mammography on outcomes for women older than 70. The TF feels that, since women ages 60 to 69 benefit the most from mammography, this benefit is likely to carry over into the next decade. Modeling also predicts this.
However, women ages 70 to 74 who have chronic illnesses are unlikely to benefit from mammography. The conditions specifically mentioned are cardiovascular disease, diabetes, lung disease, liver disease, renal failure, acquired immunodeficiency syndrome, and dementia.
For all women ages 75 and older, the TF feels the evidence is insufficient to make a recommendation.
Insurance coverage
The ACA mandates that 4 sets of preventive services be included in commercial health insurance plans with no out-of-pocket expenses to the patient: immunizations recommended by the Advisory Committee on Immunization Practices; children’s preventive services recommended by the Health Resources and Services Administration (HRSA); women’s preventive services recommended by HRSA; and recommendations with an A or B rating from the USPSTF.7
For children, HRSA opted to use those preventive services listed by the American Academy of Pediatrics in Bright Futures, the society’s national initiative providing recommendations on prevention screenings and well-child visits.8 For women, HRSA asked the Institute of Medicine to form a panel to construct a list of recommended preventive services.
At the time the ACA was passed, the TF had just made new recommendations on breast cancer screening, which were very similar to the current draft recommendations. Due to the resulting controversy, Congress mandated that the new recommendations not be used to determine first-dollar insurance coverage, and it cited the TF’s pre-2009 recommendations as the applicable standard.
Those earlier recommendations included annual mammography starting at age 40. The wording of the law, however, was not clear as to future mammography recommendations. One interpretation is that the TF recommendations in place before 2009 are the basis for first-dollar coverage until changed by Congress. Another interpretation is that the ACA special provision trumped only the 2009 recommendations and the 2015 recommendations will become the standard. If the latter turns out to be true, it is not clear if commercial insurance plans will begin to charge co-payments for mammography before age 50 or for mammograms ordered more frequently than every 2 years for women ages 50 to 74.
The issue of insurance coverage is important because of the lack of uniformity in recommendations regarding mammography. The American Congress of Obstetricians and Gynecologists,9 the American Cancer Society,10 and the American College of Radiology11 all recommend annual mammography starting at age 40. The American Academy of Family Physicians recommendations12 mirror those of the USPSTF, and the Canadian Task Force on Preventive Health Care recommends against routine screening for women ages 40 to 49 and recommends mammography every 2 to 3 years for women ages 50 to 74.13
USPSTF rationale is informed and accessible for review
Breast cancer screening remains a highly controversial and emotional topic. The USPSTF has made a set of recommendations based on extensive and rigorous evidence reports that consider both benefits and harms. There will be those who vigorously disagree. The evidence reports, recommendations, and rationale behind them are easily accessible on the TF Web site (www.uspreventiveservicestaskforce.org) for those who want to read them.1
1. USPSTF. Draft recommendation statement. Breast cancer: screening. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementDraft/breast-cancer-screening1#tab1. Accessed May 25, 2015.
2. National Cancer Institute. SEER Stat Fact Sheets: Breast Cancer. Available at: http://seer.cancer.gov/statfacts/html/breast.html. Accessed June 11, 2015.
3. Nelson HD, Cantor A, Humphrey L, et al. Screening for breast cancer; a systematic review to update the 2009 U.S. Preventive Services Task Force recommendation. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/draftevidence-review-screening-for-breast-cancer/breast-cancerscreening1. Accessed May 25, 2015.
4. Melnikow J, Fenton JJ, Miglioretti D, et al. Screening for Breast Cancer with Digital Tomosynthesis. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/draft-evidence-review-screening-for-breast-cancer-with-digit/breastcancer-screening1. Accessed May 25, 2015.
5. Carney PA, Miglioretti D, Yaankaskas BC, et al. Individual and combined effects of age, breast density, and hormone replacement therapy use on the accuracy of screening mammography. Ann Intern Med. 2003;138:168-175.
6. Melnikow J, Fenton JJ, Whitlock EP, et al. Adjunctive screening for breast cancer in women with dense breasts: a systematic review for the U.S. Preventive Services Task Force. AHRQ Publication No. 14-05201-EF-2.
7. 111th Congress Public Law 111-148, section 2713. Available at: http://www.gpo.gov/fdsys/pkg/PLAW-111publ148/html/PLAW-111publ148.htm. Accessed May 25, 2015.
8. American Academy of Pediatrics. Bright Futures. Available at: https://brightfutures.aap.org/Pages/default.aspx. Accessed May 25, 2015.
9. American Congress of Obstetricians and Gynecologists. ACOG statement on breast cancer screening. Available at: http://www.acog.org/About-ACOG/News-Room/Statements/2015/ACOGStatement-on-Breast-Cancer-Screening. Accessed May 25, 2015.
10. Smith RA, Manassaram-Baptiste D, Brooks D, et al. Cancer screening in the United States, 2015: a review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J Clin. 2015;65:30-54.
11. Lee CH, Dershaw DD, Kopans D, et al. Breast cancer screening with imaging: recommendations from the Society of Breast Imaging and the ACR on the use of mammography, breast MRI, breast ultrasound, and other technologies for the detection of clinically occult breast cancer. J Am Coll Radiol. 2010;7:18-27.
12. American Academy of Family Physicians. Breast cancer. Available at: http://www.aafp.org/patient-care/clinical-recommendations/all/breast-cancer.html. Accessed May 25, 2015.
13. Canadian Task Force on Preventive Health Care. Screening for breast cancer. Available at: http://canadiantaskforce.ca/ctfphcguidelines/2011-breast-cancer. Accessed May 25, 2015.
The United States Preventive Services Task Force (USPSTF) recently released draft recommendations on breast cancer screening, which could be finalized within the next few months.1 The last time the Task Force (TF) weighed in on this topic was in 2009, just as the Affordable Care Act (ACA) was being debated. At that time, the TF recommendations were so controversial that Congress specified in the ACA that they should not be used to determine insurance coverage (more on this later).
The draft recommendations (TABLE 1)1 carry a C grade for women ages 40 to 49 years (ie, offer or provide screening mammography for selected patients depending on individual circumstances) and a B grade for biennial screening of women ages 50 to 74. The proposed recommendations are basically the same as the ones made in 2009, with more detailed wording to explain the rationale for the C recommendation, and to address 2 new issues: tomosynthesis (3-D mammography) and adjunctive screening for women with dense breasts. The previous D recommendation against self breast examination was left unchanged.
Benefit of mammography screening varies by decade of life
Breast cancer is the leading cause of non-skin cancers in women and, after lung cancer, the second leading cause of cancer deaths in women. In 2014 there were 233,000 new cases diagnosed and 40,000 breast cancer deaths.1,2 While the TF found that mammography reduces deaths from breast cancer in women between the ages of 40 and 74, women ages 40 to 49 benefit the least; those ages 60 to 69 benefit the most.1,3
If 10,000 women are screened routinely for 10 years, 4 breast cancer deaths will be prevented in those ages 40 to 49, 8 in those 50 to 59, and 21 in those 60 to 69.1 And harms appear to be higher in the younger age group. TABLE 21,3 shows some of the harms resulting from one-time mammography screening of 10,000 women in each age group. Notice the benefits listed previously are from repeated screenings over a 10-year period and the harms in TABLE 21,3 are from a single mammogram.
The total benefits and harms of biennial screening in 1000 women starting at age 40 (vs age 50) include 8 cancer deaths prevented (vs 7) with a cost of 1529 false positive tests (vs 953); 204 unnecessary breast biopsies (vs 146); and 20 overdiagnoses (vs 18). However, the confidence intervals on these estimates are wide, and in each case, they overlap between the 2 groups.1
The TF recommended biennial screening for women between the ages of 50 and 74 because observational studies and modeling show no clear benefit with annual screening vs every 2 years, while annual screening results in more false positives and biopsies.
Overdiagnosis may occur in nearly 20% of cases
The potential for overdiagnosis and overtreatment is increasingly recognized as a harm of cancer screening. Overdiagnosis results from detecting a tumor during screening that would not have been detected otherwise and that would not have caused death or disease but is treated anyway. This sometimes occurs with the detection of early tumors that would not have progressed or would have progressed slowly, not causing health problems before the woman dies of other causes.
The TF is one of the only organizations that considers the potential harmful effects of this problem. While it is not possible to know for certain the rate of overdiagnosis that occurs with cancer screening, high-quality studies indicate it is close to 20% for breast cancer.3
Guidance regarding women ages 40 to 49
The new draft recommendations carefully point out that, while the overall benefit of screening women ages 40 to 49 is small, the decision to begin screening before age 50 should be an individual one, and an informed one. They state that women who value the small potential benefit over the potential for harm may choose to be screened, as might women who have a family history of breast cancer. And the recommendations do not apply to women who have a genotype that places them at increased risk for breast cancer.
Tomosynthesis: Evidence of benefit is insufficient
Tomosynthesis as a primary breast cancer screening tool was studied in a separate evidence report commissioned by the TF.4 While tomosynthesis, compared with routine mammography, appears to have increased sensitivity and specificity in detecting breast cancer, no studies looked at this technology as a primary screening tool and its effect on breast cancer mortality, overall mortality, and quality of life. Sticking to its nationally-recognized methodological rigor, the TF states that information at this time is insufficient to make a recommendation on the use of tomosynthesis.
Dense breasts: Usefulness of adjunctive screening modalities
Breast density is categorized into 4 groups, from category a (breasts are almost all fatty with little fibro nodular tissue) to category d (breasts are extremely dense).1 About 43% of women ages 40 to 74 are in categories c and d.1 Dense breasts adversely affect the accuracy of mammography, decreasing sensitivity and specificity. In one study, sensitivity was 87% in category a and 63% in category d; specificities were 97% and 89%, respectively.5
Tomosynthesis, magnetic resonance imaging, and ultrasound, when used in addition to mammography, all appear to detect more cancers, but they also yield more false-positive results.6 The long-term outcome of detecting more tumors is not known. For an individual, there are 3 possibilities when a tumor is detected earlier: a better outcome, no difference in outcome, or a worse outcome resulting from overdiagnosis and overtreatment. The TF felt that the available data are insufficient to judge benefits and harms of an increased frequency of screening or the use of adjunctive screening methods in women with dense breasts.
Benefit for women ≥75 years is inconclusive
There are limited data on the impact of mammography on outcomes for women older than 70. The TF feels that, since women ages 60 to 69 benefit the most from mammography, this benefit is likely to carry over into the next decade. Modeling also predicts this.
However, women ages 70 to 74 who have chronic illnesses are unlikely to benefit from mammography. The conditions specifically mentioned are cardiovascular disease, diabetes, lung disease, liver disease, renal failure, acquired immunodeficiency syndrome, and dementia.
For all women ages 75 and older, the TF feels the evidence is insufficient to make a recommendation.
Insurance coverage
The ACA mandates that 4 sets of preventive services be included in commercial health insurance plans with no out-of-pocket expenses to the patient: immunizations recommended by the Advisory Committee on Immunization Practices; children’s preventive services recommended by the Health Resources and Services Administration (HRSA); women’s preventive services recommended by HRSA; and recommendations with an A or B rating from the USPSTF.7
For children, HRSA opted to use those preventive services listed by the American Academy of Pediatrics in Bright Futures, the society’s national initiative providing recommendations on prevention screenings and well-child visits.8 For women, HRSA asked the Institute of Medicine to form a panel to construct a list of recommended preventive services.
At the time the ACA was passed, the TF had just made new recommendations on breast cancer screening, which were very similar to the current draft recommendations. Due to the resulting controversy, Congress mandated that the new recommendations not be used to determine first-dollar insurance coverage, and it cited the TF’s pre-2009 recommendations as the applicable standard.
Those earlier recommendations included annual mammography starting at age 40. The wording of the law, however, was not clear as to future mammography recommendations. One interpretation is that the TF recommendations in place before 2009 are the basis for first-dollar coverage until changed by Congress. Another interpretation is that the ACA special provision trumped only the 2009 recommendations and the 2015 recommendations will become the standard. If the latter turns out to be true, it is not clear if commercial insurance plans will begin to charge co-payments for mammography before age 50 or for mammograms ordered more frequently than every 2 years for women ages 50 to 74.
The issue of insurance coverage is important because of the lack of uniformity in recommendations regarding mammography. The American Congress of Obstetricians and Gynecologists,9 the American Cancer Society,10 and the American College of Radiology11 all recommend annual mammography starting at age 40. The American Academy of Family Physicians recommendations12 mirror those of the USPSTF, and the Canadian Task Force on Preventive Health Care recommends against routine screening for women ages 40 to 49 and recommends mammography every 2 to 3 years for women ages 50 to 74.13
USPSTF rationale is informed and accessible for review
Breast cancer screening remains a highly controversial and emotional topic. The USPSTF has made a set of recommendations based on extensive and rigorous evidence reports that consider both benefits and harms. There will be those who vigorously disagree. The evidence reports, recommendations, and rationale behind them are easily accessible on the TF Web site (www.uspreventiveservicestaskforce.org) for those who want to read them.1
The United States Preventive Services Task Force (USPSTF) recently released draft recommendations on breast cancer screening, which could be finalized within the next few months.1 The last time the Task Force (TF) weighed in on this topic was in 2009, just as the Affordable Care Act (ACA) was being debated. At that time, the TF recommendations were so controversial that Congress specified in the ACA that they should not be used to determine insurance coverage (more on this later).
The draft recommendations (TABLE 1)1 carry a C grade for women ages 40 to 49 years (ie, offer or provide screening mammography for selected patients depending on individual circumstances) and a B grade for biennial screening of women ages 50 to 74. The proposed recommendations are basically the same as the ones made in 2009, with more detailed wording to explain the rationale for the C recommendation, and to address 2 new issues: tomosynthesis (3-D mammography) and adjunctive screening for women with dense breasts. The previous D recommendation against self breast examination was left unchanged.
Benefit of mammography screening varies by decade of life
Breast cancer is the leading cause of non-skin cancers in women and, after lung cancer, the second leading cause of cancer deaths in women. In 2014 there were 233,000 new cases diagnosed and 40,000 breast cancer deaths.1,2 While the TF found that mammography reduces deaths from breast cancer in women between the ages of 40 and 74, women ages 40 to 49 benefit the least; those ages 60 to 69 benefit the most.1,3
If 10,000 women are screened routinely for 10 years, 4 breast cancer deaths will be prevented in those ages 40 to 49, 8 in those 50 to 59, and 21 in those 60 to 69.1 And harms appear to be higher in the younger age group. TABLE 21,3 shows some of the harms resulting from one-time mammography screening of 10,000 women in each age group. Notice the benefits listed previously are from repeated screenings over a 10-year period and the harms in TABLE 21,3 are from a single mammogram.
The total benefits and harms of biennial screening in 1000 women starting at age 40 (vs age 50) include 8 cancer deaths prevented (vs 7) with a cost of 1529 false positive tests (vs 953); 204 unnecessary breast biopsies (vs 146); and 20 overdiagnoses (vs 18). However, the confidence intervals on these estimates are wide, and in each case, they overlap between the 2 groups.1
The TF recommended biennial screening for women between the ages of 50 and 74 because observational studies and modeling show no clear benefit with annual screening vs every 2 years, while annual screening results in more false positives and biopsies.
Overdiagnosis may occur in nearly 20% of cases
The potential for overdiagnosis and overtreatment is increasingly recognized as a harm of cancer screening. Overdiagnosis results from detecting a tumor during screening that would not have been detected otherwise and that would not have caused death or disease but is treated anyway. This sometimes occurs with the detection of early tumors that would not have progressed or would have progressed slowly, not causing health problems before the woman dies of other causes.
The TF is one of the only organizations that considers the potential harmful effects of this problem. While it is not possible to know for certain the rate of overdiagnosis that occurs with cancer screening, high-quality studies indicate it is close to 20% for breast cancer.3
Guidance regarding women ages 40 to 49
The new draft recommendations carefully point out that, while the overall benefit of screening women ages 40 to 49 is small, the decision to begin screening before age 50 should be an individual one, and an informed one. They state that women who value the small potential benefit over the potential for harm may choose to be screened, as might women who have a family history of breast cancer. And the recommendations do not apply to women who have a genotype that places them at increased risk for breast cancer.
Tomosynthesis: Evidence of benefit is insufficient
Tomosynthesis as a primary breast cancer screening tool was studied in a separate evidence report commissioned by the TF.4 While tomosynthesis, compared with routine mammography, appears to have increased sensitivity and specificity in detecting breast cancer, no studies looked at this technology as a primary screening tool and its effect on breast cancer mortality, overall mortality, and quality of life. Sticking to its nationally-recognized methodological rigor, the TF states that information at this time is insufficient to make a recommendation on the use of tomosynthesis.
Dense breasts: Usefulness of adjunctive screening modalities
Breast density is categorized into 4 groups, from category a (breasts are almost all fatty with little fibro nodular tissue) to category d (breasts are extremely dense).1 About 43% of women ages 40 to 74 are in categories c and d.1 Dense breasts adversely affect the accuracy of mammography, decreasing sensitivity and specificity. In one study, sensitivity was 87% in category a and 63% in category d; specificities were 97% and 89%, respectively.5
Tomosynthesis, magnetic resonance imaging, and ultrasound, when used in addition to mammography, all appear to detect more cancers, but they also yield more false-positive results.6 The long-term outcome of detecting more tumors is not known. For an individual, there are 3 possibilities when a tumor is detected earlier: a better outcome, no difference in outcome, or a worse outcome resulting from overdiagnosis and overtreatment. The TF felt that the available data are insufficient to judge benefits and harms of an increased frequency of screening or the use of adjunctive screening methods in women with dense breasts.
Benefit for women ≥75 years is inconclusive
There are limited data on the impact of mammography on outcomes for women older than 70. The TF feels that, since women ages 60 to 69 benefit the most from mammography, this benefit is likely to carry over into the next decade. Modeling also predicts this.
However, women ages 70 to 74 who have chronic illnesses are unlikely to benefit from mammography. The conditions specifically mentioned are cardiovascular disease, diabetes, lung disease, liver disease, renal failure, acquired immunodeficiency syndrome, and dementia.
For all women ages 75 and older, the TF feels the evidence is insufficient to make a recommendation.
Insurance coverage
The ACA mandates that 4 sets of preventive services be included in commercial health insurance plans with no out-of-pocket expenses to the patient: immunizations recommended by the Advisory Committee on Immunization Practices; children’s preventive services recommended by the Health Resources and Services Administration (HRSA); women’s preventive services recommended by HRSA; and recommendations with an A or B rating from the USPSTF.7
For children, HRSA opted to use those preventive services listed by the American Academy of Pediatrics in Bright Futures, the society’s national initiative providing recommendations on prevention screenings and well-child visits.8 For women, HRSA asked the Institute of Medicine to form a panel to construct a list of recommended preventive services.
At the time the ACA was passed, the TF had just made new recommendations on breast cancer screening, which were very similar to the current draft recommendations. Due to the resulting controversy, Congress mandated that the new recommendations not be used to determine first-dollar insurance coverage, and it cited the TF’s pre-2009 recommendations as the applicable standard.
Those earlier recommendations included annual mammography starting at age 40. The wording of the law, however, was not clear as to future mammography recommendations. One interpretation is that the TF recommendations in place before 2009 are the basis for first-dollar coverage until changed by Congress. Another interpretation is that the ACA special provision trumped only the 2009 recommendations and the 2015 recommendations will become the standard. If the latter turns out to be true, it is not clear if commercial insurance plans will begin to charge co-payments for mammography before age 50 or for mammograms ordered more frequently than every 2 years for women ages 50 to 74.
The issue of insurance coverage is important because of the lack of uniformity in recommendations regarding mammography. The American Congress of Obstetricians and Gynecologists,9 the American Cancer Society,10 and the American College of Radiology11 all recommend annual mammography starting at age 40. The American Academy of Family Physicians recommendations12 mirror those of the USPSTF, and the Canadian Task Force on Preventive Health Care recommends against routine screening for women ages 40 to 49 and recommends mammography every 2 to 3 years for women ages 50 to 74.13
USPSTF rationale is informed and accessible for review
Breast cancer screening remains a highly controversial and emotional topic. The USPSTF has made a set of recommendations based on extensive and rigorous evidence reports that consider both benefits and harms. There will be those who vigorously disagree. The evidence reports, recommendations, and rationale behind them are easily accessible on the TF Web site (www.uspreventiveservicestaskforce.org) for those who want to read them.1
1. USPSTF. Draft recommendation statement. Breast cancer: screening. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementDraft/breast-cancer-screening1#tab1. Accessed May 25, 2015.
2. National Cancer Institute. SEER Stat Fact Sheets: Breast Cancer. Available at: http://seer.cancer.gov/statfacts/html/breast.html. Accessed June 11, 2015.
3. Nelson HD, Cantor A, Humphrey L, et al. Screening for breast cancer; a systematic review to update the 2009 U.S. Preventive Services Task Force recommendation. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/draftevidence-review-screening-for-breast-cancer/breast-cancerscreening1. Accessed May 25, 2015.
4. Melnikow J, Fenton JJ, Miglioretti D, et al. Screening for Breast Cancer with Digital Tomosynthesis. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/draft-evidence-review-screening-for-breast-cancer-with-digit/breastcancer-screening1. Accessed May 25, 2015.
5. Carney PA, Miglioretti D, Yaankaskas BC, et al. Individual and combined effects of age, breast density, and hormone replacement therapy use on the accuracy of screening mammography. Ann Intern Med. 2003;138:168-175.
6. Melnikow J, Fenton JJ, Whitlock EP, et al. Adjunctive screening for breast cancer in women with dense breasts: a systematic review for the U.S. Preventive Services Task Force. AHRQ Publication No. 14-05201-EF-2.
7. 111th Congress Public Law 111-148, section 2713. Available at: http://www.gpo.gov/fdsys/pkg/PLAW-111publ148/html/PLAW-111publ148.htm. Accessed May 25, 2015.
8. American Academy of Pediatrics. Bright Futures. Available at: https://brightfutures.aap.org/Pages/default.aspx. Accessed May 25, 2015.
9. American Congress of Obstetricians and Gynecologists. ACOG statement on breast cancer screening. Available at: http://www.acog.org/About-ACOG/News-Room/Statements/2015/ACOGStatement-on-Breast-Cancer-Screening. Accessed May 25, 2015.
10. Smith RA, Manassaram-Baptiste D, Brooks D, et al. Cancer screening in the United States, 2015: a review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J Clin. 2015;65:30-54.
11. Lee CH, Dershaw DD, Kopans D, et al. Breast cancer screening with imaging: recommendations from the Society of Breast Imaging and the ACR on the use of mammography, breast MRI, breast ultrasound, and other technologies for the detection of clinically occult breast cancer. J Am Coll Radiol. 2010;7:18-27.
12. American Academy of Family Physicians. Breast cancer. Available at: http://www.aafp.org/patient-care/clinical-recommendations/all/breast-cancer.html. Accessed May 25, 2015.
13. Canadian Task Force on Preventive Health Care. Screening for breast cancer. Available at: http://canadiantaskforce.ca/ctfphcguidelines/2011-breast-cancer. Accessed May 25, 2015.
1. USPSTF. Draft recommendation statement. Breast cancer: screening. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementDraft/breast-cancer-screening1#tab1. Accessed May 25, 2015.
2. National Cancer Institute. SEER Stat Fact Sheets: Breast Cancer. Available at: http://seer.cancer.gov/statfacts/html/breast.html. Accessed June 11, 2015.
3. Nelson HD, Cantor A, Humphrey L, et al. Screening for breast cancer; a systematic review to update the 2009 U.S. Preventive Services Task Force recommendation. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/draftevidence-review-screening-for-breast-cancer/breast-cancerscreening1. Accessed May 25, 2015.
4. Melnikow J, Fenton JJ, Miglioretti D, et al. Screening for Breast Cancer with Digital Tomosynthesis. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/draft-evidence-review-screening-for-breast-cancer-with-digit/breastcancer-screening1. Accessed May 25, 2015.
5. Carney PA, Miglioretti D, Yaankaskas BC, et al. Individual and combined effects of age, breast density, and hormone replacement therapy use on the accuracy of screening mammography. Ann Intern Med. 2003;138:168-175.
6. Melnikow J, Fenton JJ, Whitlock EP, et al. Adjunctive screening for breast cancer in women with dense breasts: a systematic review for the U.S. Preventive Services Task Force. AHRQ Publication No. 14-05201-EF-2.
7. 111th Congress Public Law 111-148, section 2713. Available at: http://www.gpo.gov/fdsys/pkg/PLAW-111publ148/html/PLAW-111publ148.htm. Accessed May 25, 2015.
8. American Academy of Pediatrics. Bright Futures. Available at: https://brightfutures.aap.org/Pages/default.aspx. Accessed May 25, 2015.
9. American Congress of Obstetricians and Gynecologists. ACOG statement on breast cancer screening. Available at: http://www.acog.org/About-ACOG/News-Room/Statements/2015/ACOGStatement-on-Breast-Cancer-Screening. Accessed May 25, 2015.
10. Smith RA, Manassaram-Baptiste D, Brooks D, et al. Cancer screening in the United States, 2015: a review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J Clin. 2015;65:30-54.
11. Lee CH, Dershaw DD, Kopans D, et al. Breast cancer screening with imaging: recommendations from the Society of Breast Imaging and the ACR on the use of mammography, breast MRI, breast ultrasound, and other technologies for the detection of clinically occult breast cancer. J Am Coll Radiol. 2010;7:18-27.
12. American Academy of Family Physicians. Breast cancer. Available at: http://www.aafp.org/patient-care/clinical-recommendations/all/breast-cancer.html. Accessed May 25, 2015.
13. Canadian Task Force on Preventive Health Care. Screening for breast cancer. Available at: http://canadiantaskforce.ca/ctfphcguidelines/2011-breast-cancer. Accessed May 25, 2015.
Catching up on the latest USPSTF recommendations
In 2014, the United States Preventive Services Task Force released 24 recommendations on 14 topics.1 There were no level A recommendations, 10 B recommendations, 1 C recommendation, 3 D recommendations, and 10 I statements. A and B recommendations require that commercial insurance plans offer the recommended services at no cost to patients. This Practice Alert focuses on last year’s B and D recommendations (TABLE 11).
Cardiovascular disease
When to screen for abdominal aortic aneurism. The Task Force (TF) reaffirmed a previous B recommendation for a one-time abdominal ultrasound (US) screening for abdominal aortic aneurism (AAA) in men ages 65 to 75 years who have ever smoked. This screening and follow-up of abnormal findings results in decreased AAA rupture and AAA-related mortality, although it appears to have no effect on all-cause mortality.2 The value of screening men who have never smoked is very small and should be considered selectively for men who have a family history of AAA, or a personal history of cardiovascular risk factors or disease. The prevalence of AAA in men in the target age group is 6% to 7% (it is 0.8% for women overall in the same age range).2
The recommended screening modality, abdominal US, matches the sensitivity and specificity of abdominal CT but at lower cost and with no radiation exposure. Refer patients with AAAs ≥5.5 cm for surgical repair.2
Patients with smaller aneurysms (3.0 to 5.4 cm) can be managed conservatively with repeated US every 3 to 12 months. Patients with AAAs <3 cm that exhibit rapid growth (>1 cm/year) or that cross the threshold of 5.5 cm on repeated US should undergo surgical consultation.2
The TF also looked at the value of AAA screening for women in the same age group who have ever smoked, and it could not find enough evidence to make a recommendation. However, in women who have never smoked, the TF concluded that, largely due to the low prevalence of AAA, potential harms of screening outweigh its benefits.2
General screening for carotid artery stenosis is unhelpful. For asymptomatic adults, the TF gave a thumbs-down D recommendation on screening for carotid artery stenosis.3 Carotid artery screening is conducted with US, followed by, if findings indicate the need, confirmatory testing with angiography. US has reasonable sensitivity (90%) for finding the most significant lesions, but the specificity of 94% often leads to false-positive results that can bring about unnecessary surgery and serious harms, including death, stroke, and myocardial infarction. There is no evidence of any benefit from screening by auscultation of the neck.
The TF believes it is better to focus on primary prevention of stroke, including screening for hypertension and dyslipidemia, counseling on smoking cessation, encouraging healthful diet and physical activity, and recommending aspirin use for those at increased risk for cardiovascular disease.3
Focus on CVD prevention. For adults who are overweight or obese and have additional cardiovascular disease (CVD) risk factors, the TF recommends offering, or referring patients for, intensive behavioral counseling interventions to promote a healthy diet and increased physical activity. A previous Practice Alert discussed the rationale behind this selective intensive approach to CVD prevention, as well as the lack of endorsement of vitamins to prevent CVD or cancer.4
Sexually transmitted infections
When to screen for gonorrhea and chlamydia. The TF recommends screening for chlamydial and gonorrheal infections in all sexually active women ages 24 years and younger, and for women older than 24 years who are at high risk.5 The TF could not find adequate evidence to make a recommendation for or against screening men for either disease.
Risk is defined rather broadly to include having a new sex partner, more than one sex partner, or a sex partner with concurrent partners or a sexually transmitted infection (STI); inconsistent condom use among individuals who are not in mutually monogamous relationships; having a previous or coexisting STI; and exchanging sex for money or drugs. The TF also points out that physicians should know the prevalence of these infections in their community and be aware of particular groups that are at higher risk.
Chlamydia and gonorrhea are the most commonly reported STIs in the United States. In 2012, more than 1.4 million cases of chlamydial infection were reported to the Centers for Disease Control and Prevention (CDC).5 This is an underestimate of true prevalence because most infections are asymptomatic and not detected. The rate of chlamydial infection in females was 643.3 cases per 100,000 (more than twice that seen in males—262.6 cases per 100,000), with most infections occurring in females ages 15 to 24 years.5
In 2012, more than 330,000 cases of gonococcal infection were reported to the CDC. The rate of gonorrhea infection was similar for females and males (108.7 vs. 105.8 cases per 100,000, respectively), but while most infections in females occurred between the ages of 15 and 24 years, men most often affected were ages 20 to 24 years.5
Chlamydial and gonococcal infections can be diagnosed by nucleic acid amplification tests conducted on specimens collected in a number of ways: urine; endocervical, vaginal, and male urethral specimens; and self-collected vaginal specimens in clinical settings. Treatment recommendations for both infections can be found on the CDC STI treatment Web site.6
Intensive behavioral counseling as a means of preventing STIs is recommended for all sexually active adolescents and adults at elevated risk—ie, those with current STIs or infected within the past year, those who have multiple sex partners, and those who do not consistently use condoms.7
Intensive intervention ranges from 30 minutes to 2 hours or more of contact time. All counseling within this range is beneficial, with more time being more effective.7 These interventions can be delivered by primary care clinicians or behavioral counselors. The most successful approaches provide basic information about STIs (and STI transmission) and train patients in important skills, such as condom use, communication about safe sex, problem solving, and goal setting.
Hepatitis B screening: A change
The TF changed its previous position on screening for chronic hepatitis B virus (HBV) in those at high risk from an I statement to a B recommendation. Previously, the TF opposed screening of low-risk populations; the new recommendation is silent on this issue. Those at high risk for HBV include:8
• individuals born in countries and regions with a prevalence of HBV infection ≥2%
• US-born individuals not vaccinated as infants, whose parents are from regions with a very high prevalence of HBV infection (≥8%)—eg, sub-Saharan Africa or southeast or central Asia
• HIV-positive individuals
• injection drug users
• men who have sex with men
• household contacts or sexual partners of individuals with HBV infection.
Information on countries and regions with a high prevalence of HBV infection can be found at: www.cdc.gov/mmwr/preview/mmwrhtml/rr5708a1.htm.
The TF notes that approximately 700,000 to 2.2 million individuals in the United States have chronic HBV infection.8 However, HBV vaccine has been a recommended child vaccine for more than 20 years and the pool of those at risk shrinks annually.
Chronic HBV infection can lead to cirrhosis, hepatic failure, and hepatocellular carcinoma. An estimated 15% to 25% of individuals with chronic HBV infection die of cirrhosis or hepatocellular carcinoma.8 Those with chronic infection can also infect others. Screening for HBV infection could identify chronically infected people who may benefit from treatment and be counseled to prevent transmission.
In screening, test for hepatitis B surface antigen (HBsAg), which has a reported sensitivity and specificity of >98%.8 While the TF did not find direct evidence of screening benefits on mortality, it found convincing evidence that antiviral treatment in patients with chronic HBV infection improves intermediate outcomes (virologic or histologic improvement or clearance of hepatitis B e antigen [HBeAg]) and adequate evidence that antiviral regimens improve health outcomes (such as reduced risk for hepatocellular carcinoma).8
Prevention of tooth decay in kids
The TF recommends that primary care physicians implement 2 interventions to prevent tooth decay in infants and children: prescribing oral fluoride supplementation starting at age 6 months in areas where the local water supply is deficient in fluoride (defined as <0.6 ppm F); and periodically applying fluoride varnish to primary teeth starting at the age of tooth eruption through age 5 years. The TF emphasizes, however, that the most effective way to prevent dental decay in children is to maintain recommended levels of fluoride in community water supplies.9
Both recommended interventions are supported by good evidence, although no study directly assessed the appropriate ages at which to start and stop the application of fluoride varnish or the optimal frequency of applications. Most studies looked at children ages 3 to 5 years, but the TF believes that benefits are likely to begin at the time of primary tooth eruption.
Limited evidence found no clear difference in benefit between performing a single fluoride varnish once every 6 months vs once a year or between a single application every 6 months vs multiple applications once a year or every 6 months.9
Pregnancy
Screen for gestational diabetes. The previous TF statement on gestational diabetes mellitus (GDM) found insufficient evidence to screen for this condition. The new recommendation advises screening starting at 24 weeks gestation using the 50-g oral glucose challenge test.10 Other screening options, such as the use of fasting plasma glucose testing or basing decisions to screen on risk factors, have not been studied as extensively. The USPSTF found inadequate evidence to compare the effectiveness of different screening tests or thresholds in determining positive screen results.
Treating those with GDM with diet, glucose monitoring, and insulin (if needed) can significantly reduce the risk of preeclampsia, fetal macrosomia, and shoulder dystocia, which, according to the TF, adds up to a moderate net benefit for both mother and infant. There is no evidence that treatment will improve long-term metabolic outcomes in women.
The TF found inadequate evidence to determine whether there are benefits to screening for GDM in women before 24 weeks of gestation.
Give low-dose aspirin to prevent preeclampsia. In a new recommendation, the TF endorses low-dose aspirin (81 mg/d) to reduce rates of preeclampsia, preterm birth, and intrauterine growth restriction (IUGR) in women at increased risk for preeclampsia—defined as those with kidney disease, diabetes (type 1 or 2), hypertension, autoimmune disease, a history of preeclampsia, or a current multifetal pregnancy.11
Aspirin should be started after 12 weeks and before 28 weeks of gestation, which has been shown to reduce the risk of preeclampsia by 24%, preterm birth by 14%, and IUGR by 20%.11 The number needed to treat to prevent one case of preeclampsia was 42; 71 for IUGR, and 65 for preterm birth.11 (For more on the evidence behind this recommendation, see “Another good reason to recommend lowdose aspirin” on page 301.)
TABLE 211 lists risk factors for preeclampsia and recommendations for those in high-, moderate-, and low-risk groups.
Screenings/interventions with insufficient supporting evidence
Three conditions that cause significant morbidity or mortality were looked at by the TF last year, and insufficient evidence was found to make a recommendation—screening for cognitive impairment (early Alzheimer’s); primary care interventions to prevent or reduce illicit drug or nonmedical pharmaceutical use in children and adolescents; and screening for suicide risk in adolescents, adults, and older adults in primary care. In addition, no evidence could be found for the benefit of screening for vitamin D deficiency in adults.
1. US Preventive Services Task Force. Published recommendations. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/BrowseRec/Index. Accessed March 24, 2015.
2. US Preventive Services Task Force. Abdominal aortic aneurism: screening. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/abdominal-aortic-aneurysm-screening. Accessed March 24, 2015.
3. US Preventive Services Task Force. Carotid artery stenosis: screening. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/carotid-artery-stenosis-screening. Accessed March 24, 2015.
4. Campos-Outcalt D. Diet, exercise, and CVD: When counseling makes the most sense. J Fam Pract. 2014;63:458-460.
5. US Preventive Services Task Force. Chlamydia and gonorrhea screening. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/chlamydia-and-gonorrhea-screening. Accessed March 24, 2015.
6. Centers for Disease Control and Prevention. 2010 STD treatment guidelines. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/std/treatment/2010/default.htm. Accessed March 24, 2015.
7. US Preventive Services Task Force. Sexually transmitted infections: behavioral counseling. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/sexually-transmitted-infections-behavioral-counseling1. Accessed March 24, 2015.
8. US Preventive Services Task Force. Hepatitis B virus infection: screening, 2014. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/hepatitis-bvirus-infection-screening-2014. Accessed March 24, 2015.
9. US Preventive Services Task Force. Dental caries in children from birth through age 5 years: screening. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/dental-caries-in-children-from-birth-through-age-5-years-screening. Accessed March 24, 2015.
10. US Preventive Services Task Force. Gestational diabetes mellitus, screening. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/gestational-diabetesmellitus-screening. Accessed March 24, 2015.
11. US Preventive Services Task Force. Low-dose aspirin use for the prevention of morbidity and mortality from preeclampsia: preventive medication. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/low-dose-aspirin-use-for-the-prevention-of-morbidity-and-mortality-frompreeclampsia-preventive-medication. Accessed March 24, 2015.
In 2014, the United States Preventive Services Task Force released 24 recommendations on 14 topics.1 There were no level A recommendations, 10 B recommendations, 1 C recommendation, 3 D recommendations, and 10 I statements. A and B recommendations require that commercial insurance plans offer the recommended services at no cost to patients. This Practice Alert focuses on last year’s B and D recommendations (TABLE 11).
Cardiovascular disease
When to screen for abdominal aortic aneurism. The Task Force (TF) reaffirmed a previous B recommendation for a one-time abdominal ultrasound (US) screening for abdominal aortic aneurism (AAA) in men ages 65 to 75 years who have ever smoked. This screening and follow-up of abnormal findings results in decreased AAA rupture and AAA-related mortality, although it appears to have no effect on all-cause mortality.2 The value of screening men who have never smoked is very small and should be considered selectively for men who have a family history of AAA, or a personal history of cardiovascular risk factors or disease. The prevalence of AAA in men in the target age group is 6% to 7% (it is 0.8% for women overall in the same age range).2
The recommended screening modality, abdominal US, matches the sensitivity and specificity of abdominal CT but at lower cost and with no radiation exposure. Refer patients with AAAs ≥5.5 cm for surgical repair.2
Patients with smaller aneurysms (3.0 to 5.4 cm) can be managed conservatively with repeated US every 3 to 12 months. Patients with AAAs <3 cm that exhibit rapid growth (>1 cm/year) or that cross the threshold of 5.5 cm on repeated US should undergo surgical consultation.2
The TF also looked at the value of AAA screening for women in the same age group who have ever smoked, and it could not find enough evidence to make a recommendation. However, in women who have never smoked, the TF concluded that, largely due to the low prevalence of AAA, potential harms of screening outweigh its benefits.2
General screening for carotid artery stenosis is unhelpful. For asymptomatic adults, the TF gave a thumbs-down D recommendation on screening for carotid artery stenosis.3 Carotid artery screening is conducted with US, followed by, if findings indicate the need, confirmatory testing with angiography. US has reasonable sensitivity (90%) for finding the most significant lesions, but the specificity of 94% often leads to false-positive results that can bring about unnecessary surgery and serious harms, including death, stroke, and myocardial infarction. There is no evidence of any benefit from screening by auscultation of the neck.
The TF believes it is better to focus on primary prevention of stroke, including screening for hypertension and dyslipidemia, counseling on smoking cessation, encouraging healthful diet and physical activity, and recommending aspirin use for those at increased risk for cardiovascular disease.3
Focus on CVD prevention. For adults who are overweight or obese and have additional cardiovascular disease (CVD) risk factors, the TF recommends offering, or referring patients for, intensive behavioral counseling interventions to promote a healthy diet and increased physical activity. A previous Practice Alert discussed the rationale behind this selective intensive approach to CVD prevention, as well as the lack of endorsement of vitamins to prevent CVD or cancer.4
Sexually transmitted infections
When to screen for gonorrhea and chlamydia. The TF recommends screening for chlamydial and gonorrheal infections in all sexually active women ages 24 years and younger, and for women older than 24 years who are at high risk.5 The TF could not find adequate evidence to make a recommendation for or against screening men for either disease.
Risk is defined rather broadly to include having a new sex partner, more than one sex partner, or a sex partner with concurrent partners or a sexually transmitted infection (STI); inconsistent condom use among individuals who are not in mutually monogamous relationships; having a previous or coexisting STI; and exchanging sex for money or drugs. The TF also points out that physicians should know the prevalence of these infections in their community and be aware of particular groups that are at higher risk.
Chlamydia and gonorrhea are the most commonly reported STIs in the United States. In 2012, more than 1.4 million cases of chlamydial infection were reported to the Centers for Disease Control and Prevention (CDC).5 This is an underestimate of true prevalence because most infections are asymptomatic and not detected. The rate of chlamydial infection in females was 643.3 cases per 100,000 (more than twice that seen in males—262.6 cases per 100,000), with most infections occurring in females ages 15 to 24 years.5
In 2012, more than 330,000 cases of gonococcal infection were reported to the CDC. The rate of gonorrhea infection was similar for females and males (108.7 vs. 105.8 cases per 100,000, respectively), but while most infections in females occurred between the ages of 15 and 24 years, men most often affected were ages 20 to 24 years.5
Chlamydial and gonococcal infections can be diagnosed by nucleic acid amplification tests conducted on specimens collected in a number of ways: urine; endocervical, vaginal, and male urethral specimens; and self-collected vaginal specimens in clinical settings. Treatment recommendations for both infections can be found on the CDC STI treatment Web site.6
Intensive behavioral counseling as a means of preventing STIs is recommended for all sexually active adolescents and adults at elevated risk—ie, those with current STIs or infected within the past year, those who have multiple sex partners, and those who do not consistently use condoms.7
Intensive intervention ranges from 30 minutes to 2 hours or more of contact time. All counseling within this range is beneficial, with more time being more effective.7 These interventions can be delivered by primary care clinicians or behavioral counselors. The most successful approaches provide basic information about STIs (and STI transmission) and train patients in important skills, such as condom use, communication about safe sex, problem solving, and goal setting.
Hepatitis B screening: A change
The TF changed its previous position on screening for chronic hepatitis B virus (HBV) in those at high risk from an I statement to a B recommendation. Previously, the TF opposed screening of low-risk populations; the new recommendation is silent on this issue. Those at high risk for HBV include:8
• individuals born in countries and regions with a prevalence of HBV infection ≥2%
• US-born individuals not vaccinated as infants, whose parents are from regions with a very high prevalence of HBV infection (≥8%)—eg, sub-Saharan Africa or southeast or central Asia
• HIV-positive individuals
• injection drug users
• men who have sex with men
• household contacts or sexual partners of individuals with HBV infection.
Information on countries and regions with a high prevalence of HBV infection can be found at: www.cdc.gov/mmwr/preview/mmwrhtml/rr5708a1.htm.
The TF notes that approximately 700,000 to 2.2 million individuals in the United States have chronic HBV infection.8 However, HBV vaccine has been a recommended child vaccine for more than 20 years and the pool of those at risk shrinks annually.
Chronic HBV infection can lead to cirrhosis, hepatic failure, and hepatocellular carcinoma. An estimated 15% to 25% of individuals with chronic HBV infection die of cirrhosis or hepatocellular carcinoma.8 Those with chronic infection can also infect others. Screening for HBV infection could identify chronically infected people who may benefit from treatment and be counseled to prevent transmission.
In screening, test for hepatitis B surface antigen (HBsAg), which has a reported sensitivity and specificity of >98%.8 While the TF did not find direct evidence of screening benefits on mortality, it found convincing evidence that antiviral treatment in patients with chronic HBV infection improves intermediate outcomes (virologic or histologic improvement or clearance of hepatitis B e antigen [HBeAg]) and adequate evidence that antiviral regimens improve health outcomes (such as reduced risk for hepatocellular carcinoma).8
Prevention of tooth decay in kids
The TF recommends that primary care physicians implement 2 interventions to prevent tooth decay in infants and children: prescribing oral fluoride supplementation starting at age 6 months in areas where the local water supply is deficient in fluoride (defined as <0.6 ppm F); and periodically applying fluoride varnish to primary teeth starting at the age of tooth eruption through age 5 years. The TF emphasizes, however, that the most effective way to prevent dental decay in children is to maintain recommended levels of fluoride in community water supplies.9
Both recommended interventions are supported by good evidence, although no study directly assessed the appropriate ages at which to start and stop the application of fluoride varnish or the optimal frequency of applications. Most studies looked at children ages 3 to 5 years, but the TF believes that benefits are likely to begin at the time of primary tooth eruption.
Limited evidence found no clear difference in benefit between performing a single fluoride varnish once every 6 months vs once a year or between a single application every 6 months vs multiple applications once a year or every 6 months.9
Pregnancy
Screen for gestational diabetes. The previous TF statement on gestational diabetes mellitus (GDM) found insufficient evidence to screen for this condition. The new recommendation advises screening starting at 24 weeks gestation using the 50-g oral glucose challenge test.10 Other screening options, such as the use of fasting plasma glucose testing or basing decisions to screen on risk factors, have not been studied as extensively. The USPSTF found inadequate evidence to compare the effectiveness of different screening tests or thresholds in determining positive screen results.
Treating those with GDM with diet, glucose monitoring, and insulin (if needed) can significantly reduce the risk of preeclampsia, fetal macrosomia, and shoulder dystocia, which, according to the TF, adds up to a moderate net benefit for both mother and infant. There is no evidence that treatment will improve long-term metabolic outcomes in women.
The TF found inadequate evidence to determine whether there are benefits to screening for GDM in women before 24 weeks of gestation.
Give low-dose aspirin to prevent preeclampsia. In a new recommendation, the TF endorses low-dose aspirin (81 mg/d) to reduce rates of preeclampsia, preterm birth, and intrauterine growth restriction (IUGR) in women at increased risk for preeclampsia—defined as those with kidney disease, diabetes (type 1 or 2), hypertension, autoimmune disease, a history of preeclampsia, or a current multifetal pregnancy.11
Aspirin should be started after 12 weeks and before 28 weeks of gestation, which has been shown to reduce the risk of preeclampsia by 24%, preterm birth by 14%, and IUGR by 20%.11 The number needed to treat to prevent one case of preeclampsia was 42; 71 for IUGR, and 65 for preterm birth.11 (For more on the evidence behind this recommendation, see “Another good reason to recommend lowdose aspirin” on page 301.)
TABLE 211 lists risk factors for preeclampsia and recommendations for those in high-, moderate-, and low-risk groups.
Screenings/interventions with insufficient supporting evidence
Three conditions that cause significant morbidity or mortality were looked at by the TF last year, and insufficient evidence was found to make a recommendation—screening for cognitive impairment (early Alzheimer’s); primary care interventions to prevent or reduce illicit drug or nonmedical pharmaceutical use in children and adolescents; and screening for suicide risk in adolescents, adults, and older adults in primary care. In addition, no evidence could be found for the benefit of screening for vitamin D deficiency in adults.
In 2014, the United States Preventive Services Task Force released 24 recommendations on 14 topics.1 There were no level A recommendations, 10 B recommendations, 1 C recommendation, 3 D recommendations, and 10 I statements. A and B recommendations require that commercial insurance plans offer the recommended services at no cost to patients. This Practice Alert focuses on last year’s B and D recommendations (TABLE 11).
Cardiovascular disease
When to screen for abdominal aortic aneurism. The Task Force (TF) reaffirmed a previous B recommendation for a one-time abdominal ultrasound (US) screening for abdominal aortic aneurism (AAA) in men ages 65 to 75 years who have ever smoked. This screening and follow-up of abnormal findings results in decreased AAA rupture and AAA-related mortality, although it appears to have no effect on all-cause mortality.2 The value of screening men who have never smoked is very small and should be considered selectively for men who have a family history of AAA, or a personal history of cardiovascular risk factors or disease. The prevalence of AAA in men in the target age group is 6% to 7% (it is 0.8% for women overall in the same age range).2
The recommended screening modality, abdominal US, matches the sensitivity and specificity of abdominal CT but at lower cost and with no radiation exposure. Refer patients with AAAs ≥5.5 cm for surgical repair.2
Patients with smaller aneurysms (3.0 to 5.4 cm) can be managed conservatively with repeated US every 3 to 12 months. Patients with AAAs <3 cm that exhibit rapid growth (>1 cm/year) or that cross the threshold of 5.5 cm on repeated US should undergo surgical consultation.2
The TF also looked at the value of AAA screening for women in the same age group who have ever smoked, and it could not find enough evidence to make a recommendation. However, in women who have never smoked, the TF concluded that, largely due to the low prevalence of AAA, potential harms of screening outweigh its benefits.2
General screening for carotid artery stenosis is unhelpful. For asymptomatic adults, the TF gave a thumbs-down D recommendation on screening for carotid artery stenosis.3 Carotid artery screening is conducted with US, followed by, if findings indicate the need, confirmatory testing with angiography. US has reasonable sensitivity (90%) for finding the most significant lesions, but the specificity of 94% often leads to false-positive results that can bring about unnecessary surgery and serious harms, including death, stroke, and myocardial infarction. There is no evidence of any benefit from screening by auscultation of the neck.
The TF believes it is better to focus on primary prevention of stroke, including screening for hypertension and dyslipidemia, counseling on smoking cessation, encouraging healthful diet and physical activity, and recommending aspirin use for those at increased risk for cardiovascular disease.3
Focus on CVD prevention. For adults who are overweight or obese and have additional cardiovascular disease (CVD) risk factors, the TF recommends offering, or referring patients for, intensive behavioral counseling interventions to promote a healthy diet and increased physical activity. A previous Practice Alert discussed the rationale behind this selective intensive approach to CVD prevention, as well as the lack of endorsement of vitamins to prevent CVD or cancer.4
Sexually transmitted infections
When to screen for gonorrhea and chlamydia. The TF recommends screening for chlamydial and gonorrheal infections in all sexually active women ages 24 years and younger, and for women older than 24 years who are at high risk.5 The TF could not find adequate evidence to make a recommendation for or against screening men for either disease.
Risk is defined rather broadly to include having a new sex partner, more than one sex partner, or a sex partner with concurrent partners or a sexually transmitted infection (STI); inconsistent condom use among individuals who are not in mutually monogamous relationships; having a previous or coexisting STI; and exchanging sex for money or drugs. The TF also points out that physicians should know the prevalence of these infections in their community and be aware of particular groups that are at higher risk.
Chlamydia and gonorrhea are the most commonly reported STIs in the United States. In 2012, more than 1.4 million cases of chlamydial infection were reported to the Centers for Disease Control and Prevention (CDC).5 This is an underestimate of true prevalence because most infections are asymptomatic and not detected. The rate of chlamydial infection in females was 643.3 cases per 100,000 (more than twice that seen in males—262.6 cases per 100,000), with most infections occurring in females ages 15 to 24 years.5
In 2012, more than 330,000 cases of gonococcal infection were reported to the CDC. The rate of gonorrhea infection was similar for females and males (108.7 vs. 105.8 cases per 100,000, respectively), but while most infections in females occurred between the ages of 15 and 24 years, men most often affected were ages 20 to 24 years.5
Chlamydial and gonococcal infections can be diagnosed by nucleic acid amplification tests conducted on specimens collected in a number of ways: urine; endocervical, vaginal, and male urethral specimens; and self-collected vaginal specimens in clinical settings. Treatment recommendations for both infections can be found on the CDC STI treatment Web site.6
Intensive behavioral counseling as a means of preventing STIs is recommended for all sexually active adolescents and adults at elevated risk—ie, those with current STIs or infected within the past year, those who have multiple sex partners, and those who do not consistently use condoms.7
Intensive intervention ranges from 30 minutes to 2 hours or more of contact time. All counseling within this range is beneficial, with more time being more effective.7 These interventions can be delivered by primary care clinicians or behavioral counselors. The most successful approaches provide basic information about STIs (and STI transmission) and train patients in important skills, such as condom use, communication about safe sex, problem solving, and goal setting.
Hepatitis B screening: A change
The TF changed its previous position on screening for chronic hepatitis B virus (HBV) in those at high risk from an I statement to a B recommendation. Previously, the TF opposed screening of low-risk populations; the new recommendation is silent on this issue. Those at high risk for HBV include:8
• individuals born in countries and regions with a prevalence of HBV infection ≥2%
• US-born individuals not vaccinated as infants, whose parents are from regions with a very high prevalence of HBV infection (≥8%)—eg, sub-Saharan Africa or southeast or central Asia
• HIV-positive individuals
• injection drug users
• men who have sex with men
• household contacts or sexual partners of individuals with HBV infection.
Information on countries and regions with a high prevalence of HBV infection can be found at: www.cdc.gov/mmwr/preview/mmwrhtml/rr5708a1.htm.
The TF notes that approximately 700,000 to 2.2 million individuals in the United States have chronic HBV infection.8 However, HBV vaccine has been a recommended child vaccine for more than 20 years and the pool of those at risk shrinks annually.
Chronic HBV infection can lead to cirrhosis, hepatic failure, and hepatocellular carcinoma. An estimated 15% to 25% of individuals with chronic HBV infection die of cirrhosis or hepatocellular carcinoma.8 Those with chronic infection can also infect others. Screening for HBV infection could identify chronically infected people who may benefit from treatment and be counseled to prevent transmission.
In screening, test for hepatitis B surface antigen (HBsAg), which has a reported sensitivity and specificity of >98%.8 While the TF did not find direct evidence of screening benefits on mortality, it found convincing evidence that antiviral treatment in patients with chronic HBV infection improves intermediate outcomes (virologic or histologic improvement or clearance of hepatitis B e antigen [HBeAg]) and adequate evidence that antiviral regimens improve health outcomes (such as reduced risk for hepatocellular carcinoma).8
Prevention of tooth decay in kids
The TF recommends that primary care physicians implement 2 interventions to prevent tooth decay in infants and children: prescribing oral fluoride supplementation starting at age 6 months in areas where the local water supply is deficient in fluoride (defined as <0.6 ppm F); and periodically applying fluoride varnish to primary teeth starting at the age of tooth eruption through age 5 years. The TF emphasizes, however, that the most effective way to prevent dental decay in children is to maintain recommended levels of fluoride in community water supplies.9
Both recommended interventions are supported by good evidence, although no study directly assessed the appropriate ages at which to start and stop the application of fluoride varnish or the optimal frequency of applications. Most studies looked at children ages 3 to 5 years, but the TF believes that benefits are likely to begin at the time of primary tooth eruption.
Limited evidence found no clear difference in benefit between performing a single fluoride varnish once every 6 months vs once a year or between a single application every 6 months vs multiple applications once a year or every 6 months.9
Pregnancy
Screen for gestational diabetes. The previous TF statement on gestational diabetes mellitus (GDM) found insufficient evidence to screen for this condition. The new recommendation advises screening starting at 24 weeks gestation using the 50-g oral glucose challenge test.10 Other screening options, such as the use of fasting plasma glucose testing or basing decisions to screen on risk factors, have not been studied as extensively. The USPSTF found inadequate evidence to compare the effectiveness of different screening tests or thresholds in determining positive screen results.
Treating those with GDM with diet, glucose monitoring, and insulin (if needed) can significantly reduce the risk of preeclampsia, fetal macrosomia, and shoulder dystocia, which, according to the TF, adds up to a moderate net benefit for both mother and infant. There is no evidence that treatment will improve long-term metabolic outcomes in women.
The TF found inadequate evidence to determine whether there are benefits to screening for GDM in women before 24 weeks of gestation.
Give low-dose aspirin to prevent preeclampsia. In a new recommendation, the TF endorses low-dose aspirin (81 mg/d) to reduce rates of preeclampsia, preterm birth, and intrauterine growth restriction (IUGR) in women at increased risk for preeclampsia—defined as those with kidney disease, diabetes (type 1 or 2), hypertension, autoimmune disease, a history of preeclampsia, or a current multifetal pregnancy.11
Aspirin should be started after 12 weeks and before 28 weeks of gestation, which has been shown to reduce the risk of preeclampsia by 24%, preterm birth by 14%, and IUGR by 20%.11 The number needed to treat to prevent one case of preeclampsia was 42; 71 for IUGR, and 65 for preterm birth.11 (For more on the evidence behind this recommendation, see “Another good reason to recommend lowdose aspirin” on page 301.)
TABLE 211 lists risk factors for preeclampsia and recommendations for those in high-, moderate-, and low-risk groups.
Screenings/interventions with insufficient supporting evidence
Three conditions that cause significant morbidity or mortality were looked at by the TF last year, and insufficient evidence was found to make a recommendation—screening for cognitive impairment (early Alzheimer’s); primary care interventions to prevent or reduce illicit drug or nonmedical pharmaceutical use in children and adolescents; and screening for suicide risk in adolescents, adults, and older adults in primary care. In addition, no evidence could be found for the benefit of screening for vitamin D deficiency in adults.
1. US Preventive Services Task Force. Published recommendations. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/BrowseRec/Index. Accessed March 24, 2015.
2. US Preventive Services Task Force. Abdominal aortic aneurism: screening. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/abdominal-aortic-aneurysm-screening. Accessed March 24, 2015.
3. US Preventive Services Task Force. Carotid artery stenosis: screening. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/carotid-artery-stenosis-screening. Accessed March 24, 2015.
4. Campos-Outcalt D. Diet, exercise, and CVD: When counseling makes the most sense. J Fam Pract. 2014;63:458-460.
5. US Preventive Services Task Force. Chlamydia and gonorrhea screening. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/chlamydia-and-gonorrhea-screening. Accessed March 24, 2015.
6. Centers for Disease Control and Prevention. 2010 STD treatment guidelines. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/std/treatment/2010/default.htm. Accessed March 24, 2015.
7. US Preventive Services Task Force. Sexually transmitted infections: behavioral counseling. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/sexually-transmitted-infections-behavioral-counseling1. Accessed March 24, 2015.
8. US Preventive Services Task Force. Hepatitis B virus infection: screening, 2014. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/hepatitis-bvirus-infection-screening-2014. Accessed March 24, 2015.
9. US Preventive Services Task Force. Dental caries in children from birth through age 5 years: screening. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/dental-caries-in-children-from-birth-through-age-5-years-screening. Accessed March 24, 2015.
10. US Preventive Services Task Force. Gestational diabetes mellitus, screening. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/gestational-diabetesmellitus-screening. Accessed March 24, 2015.
11. US Preventive Services Task Force. Low-dose aspirin use for the prevention of morbidity and mortality from preeclampsia: preventive medication. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/low-dose-aspirin-use-for-the-prevention-of-morbidity-and-mortality-frompreeclampsia-preventive-medication. Accessed March 24, 2015.
1. US Preventive Services Task Force. Published recommendations. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/BrowseRec/Index. Accessed March 24, 2015.
2. US Preventive Services Task Force. Abdominal aortic aneurism: screening. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/abdominal-aortic-aneurysm-screening. Accessed March 24, 2015.
3. US Preventive Services Task Force. Carotid artery stenosis: screening. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/carotid-artery-stenosis-screening. Accessed March 24, 2015.
4. Campos-Outcalt D. Diet, exercise, and CVD: When counseling makes the most sense. J Fam Pract. 2014;63:458-460.
5. US Preventive Services Task Force. Chlamydia and gonorrhea screening. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/chlamydia-and-gonorrhea-screening. Accessed March 24, 2015.
6. Centers for Disease Control and Prevention. 2010 STD treatment guidelines. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/std/treatment/2010/default.htm. Accessed March 24, 2015.
7. US Preventive Services Task Force. Sexually transmitted infections: behavioral counseling. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/sexually-transmitted-infections-behavioral-counseling1. Accessed March 24, 2015.
8. US Preventive Services Task Force. Hepatitis B virus infection: screening, 2014. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/hepatitis-bvirus-infection-screening-2014. Accessed March 24, 2015.
9. US Preventive Services Task Force. Dental caries in children from birth through age 5 years: screening. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/dental-caries-in-children-from-birth-through-age-5-years-screening. Accessed March 24, 2015.
10. US Preventive Services Task Force. Gestational diabetes mellitus, screening. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/gestational-diabetesmellitus-screening. Accessed March 24, 2015.
11. US Preventive Services Task Force. Low-dose aspirin use for the prevention of morbidity and mortality from preeclampsia: preventive medication. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/low-dose-aspirin-use-for-the-prevention-of-morbidity-and-mortality-frompreeclampsia-preventive-medication. Accessed March 24, 2015.
Immunization update: What’s changed, what’s on the way
The Centers for Disease Control and Prevention (CDC) has published its 2015 immunization schedules for adults and for children and adolescents.1,2 There are very few changes from 2014 recommendations; most are alterations in the footnotes to clarify complex and confusing catch-up schedules. The 2 substantive changes have been discussed in previous Practice Alerts:
- the addition of the 13-valent pneumococcal conjugate vaccine (PCV13) to the 23-valent pneumococcal polysaccharide vaccine (PPSV23) in the routine older-adult recommendations;3
- a stated preference for live attenuated influenza vaccine (LAIV) for children ages 2 through 8 years.4
The LAIV statement came under criticism at the recent meeting of the Advisory Committee on Immunization Practices (ACIP). A prospective case-control study conducted at 5 sites in the US Flu Vaccine Effectiveness Network looked at the effectiveness of LAIV and inactivated influenza vaccine (IIV) against medically-attended influenza in 3 flu seasons: 2011-2012, 2012-2013, and 2013-2014.5 The results differed by age.
In patients ages 9 to 18 years, the vaccines were equally effective in all 3 seasons, with effectiveness ranging between 32% and 67% depending on the year and the vaccine. In children ages 2 to 8 years, LAIV appeared to be more effective than IIV in the 2011-2012 and 2012-2013 seasons, with odds ratios of .54 and .74, respectively (although not statistically significant). In the 2013-2014 season, however, IIV was the more effective vaccine, with a statistically significant odds ratio of 5.17.
In the immediate past season, the predominant influenza strain circulating was H1N1 pdm09, against which the LAIV appeared to be minimally, if at all, effective. These results were replicated in a study conducted by the LAIV producer, MedImmune, and in a study conducted by the United States Air Force.5 Based on the predominant circulating strains in the 2014-2015 flu season, ACIP has not changed its preference for LAIV for ages 2 through 8 years.
Typhoid fever vaccines
Late last year, ACIP updated its recommendations on the use of typhoid vaccines. They had last been reviewed with the recommendations in 1994, and surprisingly few changes were needed. Roughly 400 cases of typhoid fever occur in the United States each year, mostly in travelers returning from India, Bangladesh, or Pakistan. Each year, worldwide, there are an estimated 20 million cases of typhoid and 200,000 related deaths.6
ACIP recommends typhoid vaccine for travelers to areas within Asia, Africa, and Latin America that present a risk of exposure to Salmonella typhi. Country-specific recommendations can be found on the CDC travel Web site (http://wwwnc.cdc.gov/travel). Others for whom the vaccine is recommended: those who have a household contact with S. typhi or who have had other intimate exposure to a chronic S. typhi carrier (eg, someone who has excreted S. typhi in stool or urine for a year or more); and microbiologists and lab workers who might be exposed to S. typhi.
Two typhoid vaccines are available and neither is listed as preferred. One is a live vaccine (Ty21a) taken orally in 4 doses, one dose every other day over 7 days. The other is a killed vaccine (Vi capsular polysaccharide vaccine [ViCPS]), given intramuscularly in a single dose (TABLE).6,7 Ty21a is approved for individuals ages 6 years and older; ViCPS for ages 2 years and older.
Anticipated changes this year
HPV vaccine
Two human papillomavirus (HPV) vaccines are available in the United States: Gardasil, a quadrivalent vaccine (HPV4) that protects against types 6, 11, 16, and 18, and Cervarix, a bivalent product (HPV2) protecting against types 16 and 18. Both vaccines contain antigens of HPV subtypes 16 and 18, which cause 70% of cervical cancers in the United States and the rest of the world. The HPV4 is soon to be replaced with a 9-valent product that will contain antigens for types 6, 11, 16, 18, 31, 33, 45, 52, and 58, which are responsible for 90% of cervical cancers worldwide.8
Many countries now allow a 2-dose schedule for both HPV2 and HPV4. For girls younger than 15 years, the World Health Organization recommends a 2-dose schedule for HPV vaccines, 6 to 12 months apart.9 A 3-dose schedule is still recommended for those ages 15 years or older and for those who are immunocompromised.
ACIP will assess studies on the effectiveness of 2-dose schedules of HPV2, HPV4, and HPV9, and will make recommendations within the next year. Although the manufacturers of the HPV vaccines have not applied to the US Food and Drug Administration (FDA) for approval of a 2-dose schedule, ACIP will still consider the possibility of recommending it. The current 3-dose schedule is seen as a barrier to HPV vaccination and one reason why the rate of vaccination in girls in the United States remains at a disappointing 37.6% for 3 doses, 47.7% for at least 2 doses, and 57.3% for 1 dose.10
ACIP will attempt to address multiple issues in the next year regarding HPV vaccination: HPV9 use in men and women, including the possibility of catch-up schedules for those who have received HPV4 or HPV2; the possibility of using a 2-dose schedule for all HPV vaccines; and ways to increase uptake of this cancer-preventing vaccine.
Meningococcus type B
With the widespread use of quadrivalent meningococcal vaccines (MCV4), meningococcal meningitis has declined markedly in all age groups. The incidence of disease caused by meningococcal serotype B, which MCV4 does not protect against, has also declined from 0.3 to less than 0.1 cases per 100,000 between 1994 and 2013.11 The highest incidence occurs in infants under the age of 1 year, at 1.5/100,000, with 67% of cases attributable to serotype B. A slight bump in risk is seen with those ages 19 to 22 years (0.2/100,000) compared with other adolescents and adults.
While serotype B accounts for a larger proportion of all meningococcal disease than it did before, it is still relatively rare. In the United States between 2010 and 2012, annual cases totaled 48 to 56.11 Groups that are at higher risk of infection include those with complement deficiencies or asplenia (functional or anatomical), microbiologists and lab personnel who work with the organism, and those who have close contact with infected individuals.
In the past few years, well-publicized outbreaks of meningococcus B have occurred on some university campuses. Princeton had 9 cases, and the University of California at Santa Barbara had 4.11 This led to the use of meningococcal B vaccine as an outbreak control measure, with permission from the FDA before the vaccine was licensed. While these outbreaks created an impression of increased risks on college campuses, college students are actually at lower risk of type B meningococcal disease than others of the same age.11
This year, 2 meningococcal B vaccines will be available in the United States. The first, rLP2086, Trumenba (Pfizer) is a 3-dose series that was licensed in late 2014. The second, 4CMenB, Bexsero (Novartis) is a 2-dose series that received FDA approval in January 2015. Both are licensed for individuals ages 10 to 25 years. Formulating a recommendation for the use of these vaccines will be challenging because of several factors: the multiple dose schedules, the low rate of meningococcal B disease, and the age group for whom the vaccines are licensed.
1. Centers for Disease Control and Prevention. Recommended adult immunization schedule: United States - 2015. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/vaccines/schedules/downloads/adult/adult-combined-schedule.pdf. Accessed February 11, 2015.
2. Centers for Disease Control and Prevention. Birth – 18 years & “Catch-up” immunization schedules. United States, 2015. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/vaccines/schedules/hcp/child-adolescent.html. Accessed January 27, 2015.
3. Campos-Outcalt D. Pneumococcal vaccines for older adults: getting the timing right. J Fam Pract. 2014;63:730-733.
4. Campos-Outcalt D. The 2014-2015 influenza season: what you need to know. J Fam Pract. 2014;63:532-533.
5. Flannery B. Update on effectiveness of live-attenuated versus inactivated influenza vaccine in children and adolescents aged 2-18 years. Presented at: Advisory Committee on Immunization Practices; October 29, 2014; Atlanta, GA. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2014-10/flu-03-flannery.pdf. Accessed January 27, 2015.
6. Jackson BR. Typhoid and typhoid vaccines. Presented at: Advisory Committee on Immunization Practices; October 30, 2014; Atlanta, GA. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2014-10/Typhoid-02-Jackson.pdf. Accessed January 27, 2015.
7. Centers for Disease Control and Prevention. Typhoid immunization—recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 1994:43:1-7.
8. Luxembourg A. 9-valent HPV vaccine program key results--Part III. Presented at: Advisory Committee on Immunization Practices; October 30, 2014; Atlanta, GA. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2014-10/HPV-02-Luxembourg.pdf. Accessed January 27, 2015.
9. Markowitz L. 2-dose HPV vaccination schedules. Presented at: Advisory Committee on Immunization Practices; June 25, 2014; Atlanta, GA. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2014-06/HPV-04-Markowitz.pdf. Accessed January 27, 2015.
10. Stokley S, Jeyarajah J, Yankey D, et al; Immunization Services Division, National Center for Immunization and Respiratory Diseases, CDC; Centers for Disease Control and Prevention (CDC). Human papillomavirus vaccination coverage among adolescents, 2007-2013, and postlicensure safety monitoring, 2006-2014—United States. MMWR Morb Mortal Wkly Rep. 2014;63:620-624.
11. MacNeil J. Epidemiology of serogroup B meningococcal disease, United States. Presented at: Advisory Committee on Immunization Practices; October 30, 2014; Atlanta, GA. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2014-10/mening-02-MacNeil.pdf. Accessed January 27, 2015.
The Centers for Disease Control and Prevention (CDC) has published its 2015 immunization schedules for adults and for children and adolescents.1,2 There are very few changes from 2014 recommendations; most are alterations in the footnotes to clarify complex and confusing catch-up schedules. The 2 substantive changes have been discussed in previous Practice Alerts:
- the addition of the 13-valent pneumococcal conjugate vaccine (PCV13) to the 23-valent pneumococcal polysaccharide vaccine (PPSV23) in the routine older-adult recommendations;3
- a stated preference for live attenuated influenza vaccine (LAIV) for children ages 2 through 8 years.4
The LAIV statement came under criticism at the recent meeting of the Advisory Committee on Immunization Practices (ACIP). A prospective case-control study conducted at 5 sites in the US Flu Vaccine Effectiveness Network looked at the effectiveness of LAIV and inactivated influenza vaccine (IIV) against medically-attended influenza in 3 flu seasons: 2011-2012, 2012-2013, and 2013-2014.5 The results differed by age.
In patients ages 9 to 18 years, the vaccines were equally effective in all 3 seasons, with effectiveness ranging between 32% and 67% depending on the year and the vaccine. In children ages 2 to 8 years, LAIV appeared to be more effective than IIV in the 2011-2012 and 2012-2013 seasons, with odds ratios of .54 and .74, respectively (although not statistically significant). In the 2013-2014 season, however, IIV was the more effective vaccine, with a statistically significant odds ratio of 5.17.
In the immediate past season, the predominant influenza strain circulating was H1N1 pdm09, against which the LAIV appeared to be minimally, if at all, effective. These results were replicated in a study conducted by the LAIV producer, MedImmune, and in a study conducted by the United States Air Force.5 Based on the predominant circulating strains in the 2014-2015 flu season, ACIP has not changed its preference for LAIV for ages 2 through 8 years.
Typhoid fever vaccines
Late last year, ACIP updated its recommendations on the use of typhoid vaccines. They had last been reviewed with the recommendations in 1994, and surprisingly few changes were needed. Roughly 400 cases of typhoid fever occur in the United States each year, mostly in travelers returning from India, Bangladesh, or Pakistan. Each year, worldwide, there are an estimated 20 million cases of typhoid and 200,000 related deaths.6
ACIP recommends typhoid vaccine for travelers to areas within Asia, Africa, and Latin America that present a risk of exposure to Salmonella typhi. Country-specific recommendations can be found on the CDC travel Web site (http://wwwnc.cdc.gov/travel). Others for whom the vaccine is recommended: those who have a household contact with S. typhi or who have had other intimate exposure to a chronic S. typhi carrier (eg, someone who has excreted S. typhi in stool or urine for a year or more); and microbiologists and lab workers who might be exposed to S. typhi.
Two typhoid vaccines are available and neither is listed as preferred. One is a live vaccine (Ty21a) taken orally in 4 doses, one dose every other day over 7 days. The other is a killed vaccine (Vi capsular polysaccharide vaccine [ViCPS]), given intramuscularly in a single dose (TABLE).6,7 Ty21a is approved for individuals ages 6 years and older; ViCPS for ages 2 years and older.
Anticipated changes this year
HPV vaccine
Two human papillomavirus (HPV) vaccines are available in the United States: Gardasil, a quadrivalent vaccine (HPV4) that protects against types 6, 11, 16, and 18, and Cervarix, a bivalent product (HPV2) protecting against types 16 and 18. Both vaccines contain antigens of HPV subtypes 16 and 18, which cause 70% of cervical cancers in the United States and the rest of the world. The HPV4 is soon to be replaced with a 9-valent product that will contain antigens for types 6, 11, 16, 18, 31, 33, 45, 52, and 58, which are responsible for 90% of cervical cancers worldwide.8
Many countries now allow a 2-dose schedule for both HPV2 and HPV4. For girls younger than 15 years, the World Health Organization recommends a 2-dose schedule for HPV vaccines, 6 to 12 months apart.9 A 3-dose schedule is still recommended for those ages 15 years or older and for those who are immunocompromised.
ACIP will assess studies on the effectiveness of 2-dose schedules of HPV2, HPV4, and HPV9, and will make recommendations within the next year. Although the manufacturers of the HPV vaccines have not applied to the US Food and Drug Administration (FDA) for approval of a 2-dose schedule, ACIP will still consider the possibility of recommending it. The current 3-dose schedule is seen as a barrier to HPV vaccination and one reason why the rate of vaccination in girls in the United States remains at a disappointing 37.6% for 3 doses, 47.7% for at least 2 doses, and 57.3% for 1 dose.10
ACIP will attempt to address multiple issues in the next year regarding HPV vaccination: HPV9 use in men and women, including the possibility of catch-up schedules for those who have received HPV4 or HPV2; the possibility of using a 2-dose schedule for all HPV vaccines; and ways to increase uptake of this cancer-preventing vaccine.
Meningococcus type B
With the widespread use of quadrivalent meningococcal vaccines (MCV4), meningococcal meningitis has declined markedly in all age groups. The incidence of disease caused by meningococcal serotype B, which MCV4 does not protect against, has also declined from 0.3 to less than 0.1 cases per 100,000 between 1994 and 2013.11 The highest incidence occurs in infants under the age of 1 year, at 1.5/100,000, with 67% of cases attributable to serotype B. A slight bump in risk is seen with those ages 19 to 22 years (0.2/100,000) compared with other adolescents and adults.
While serotype B accounts for a larger proportion of all meningococcal disease than it did before, it is still relatively rare. In the United States between 2010 and 2012, annual cases totaled 48 to 56.11 Groups that are at higher risk of infection include those with complement deficiencies or asplenia (functional or anatomical), microbiologists and lab personnel who work with the organism, and those who have close contact with infected individuals.
In the past few years, well-publicized outbreaks of meningococcus B have occurred on some university campuses. Princeton had 9 cases, and the University of California at Santa Barbara had 4.11 This led to the use of meningococcal B vaccine as an outbreak control measure, with permission from the FDA before the vaccine was licensed. While these outbreaks created an impression of increased risks on college campuses, college students are actually at lower risk of type B meningococcal disease than others of the same age.11
This year, 2 meningococcal B vaccines will be available in the United States. The first, rLP2086, Trumenba (Pfizer) is a 3-dose series that was licensed in late 2014. The second, 4CMenB, Bexsero (Novartis) is a 2-dose series that received FDA approval in January 2015. Both are licensed for individuals ages 10 to 25 years. Formulating a recommendation for the use of these vaccines will be challenging because of several factors: the multiple dose schedules, the low rate of meningococcal B disease, and the age group for whom the vaccines are licensed.
The Centers for Disease Control and Prevention (CDC) has published its 2015 immunization schedules for adults and for children and adolescents.1,2 There are very few changes from 2014 recommendations; most are alterations in the footnotes to clarify complex and confusing catch-up schedules. The 2 substantive changes have been discussed in previous Practice Alerts:
- the addition of the 13-valent pneumococcal conjugate vaccine (PCV13) to the 23-valent pneumococcal polysaccharide vaccine (PPSV23) in the routine older-adult recommendations;3
- a stated preference for live attenuated influenza vaccine (LAIV) for children ages 2 through 8 years.4
The LAIV statement came under criticism at the recent meeting of the Advisory Committee on Immunization Practices (ACIP). A prospective case-control study conducted at 5 sites in the US Flu Vaccine Effectiveness Network looked at the effectiveness of LAIV and inactivated influenza vaccine (IIV) against medically-attended influenza in 3 flu seasons: 2011-2012, 2012-2013, and 2013-2014.5 The results differed by age.
In patients ages 9 to 18 years, the vaccines were equally effective in all 3 seasons, with effectiveness ranging between 32% and 67% depending on the year and the vaccine. In children ages 2 to 8 years, LAIV appeared to be more effective than IIV in the 2011-2012 and 2012-2013 seasons, with odds ratios of .54 and .74, respectively (although not statistically significant). In the 2013-2014 season, however, IIV was the more effective vaccine, with a statistically significant odds ratio of 5.17.
In the immediate past season, the predominant influenza strain circulating was H1N1 pdm09, against which the LAIV appeared to be minimally, if at all, effective. These results were replicated in a study conducted by the LAIV producer, MedImmune, and in a study conducted by the United States Air Force.5 Based on the predominant circulating strains in the 2014-2015 flu season, ACIP has not changed its preference for LAIV for ages 2 through 8 years.
Typhoid fever vaccines
Late last year, ACIP updated its recommendations on the use of typhoid vaccines. They had last been reviewed with the recommendations in 1994, and surprisingly few changes were needed. Roughly 400 cases of typhoid fever occur in the United States each year, mostly in travelers returning from India, Bangladesh, or Pakistan. Each year, worldwide, there are an estimated 20 million cases of typhoid and 200,000 related deaths.6
ACIP recommends typhoid vaccine for travelers to areas within Asia, Africa, and Latin America that present a risk of exposure to Salmonella typhi. Country-specific recommendations can be found on the CDC travel Web site (http://wwwnc.cdc.gov/travel). Others for whom the vaccine is recommended: those who have a household contact with S. typhi or who have had other intimate exposure to a chronic S. typhi carrier (eg, someone who has excreted S. typhi in stool or urine for a year or more); and microbiologists and lab workers who might be exposed to S. typhi.
Two typhoid vaccines are available and neither is listed as preferred. One is a live vaccine (Ty21a) taken orally in 4 doses, one dose every other day over 7 days. The other is a killed vaccine (Vi capsular polysaccharide vaccine [ViCPS]), given intramuscularly in a single dose (TABLE).6,7 Ty21a is approved for individuals ages 6 years and older; ViCPS for ages 2 years and older.
Anticipated changes this year
HPV vaccine
Two human papillomavirus (HPV) vaccines are available in the United States: Gardasil, a quadrivalent vaccine (HPV4) that protects against types 6, 11, 16, and 18, and Cervarix, a bivalent product (HPV2) protecting against types 16 and 18. Both vaccines contain antigens of HPV subtypes 16 and 18, which cause 70% of cervical cancers in the United States and the rest of the world. The HPV4 is soon to be replaced with a 9-valent product that will contain antigens for types 6, 11, 16, 18, 31, 33, 45, 52, and 58, which are responsible for 90% of cervical cancers worldwide.8
Many countries now allow a 2-dose schedule for both HPV2 and HPV4. For girls younger than 15 years, the World Health Organization recommends a 2-dose schedule for HPV vaccines, 6 to 12 months apart.9 A 3-dose schedule is still recommended for those ages 15 years or older and for those who are immunocompromised.
ACIP will assess studies on the effectiveness of 2-dose schedules of HPV2, HPV4, and HPV9, and will make recommendations within the next year. Although the manufacturers of the HPV vaccines have not applied to the US Food and Drug Administration (FDA) for approval of a 2-dose schedule, ACIP will still consider the possibility of recommending it. The current 3-dose schedule is seen as a barrier to HPV vaccination and one reason why the rate of vaccination in girls in the United States remains at a disappointing 37.6% for 3 doses, 47.7% for at least 2 doses, and 57.3% for 1 dose.10
ACIP will attempt to address multiple issues in the next year regarding HPV vaccination: HPV9 use in men and women, including the possibility of catch-up schedules for those who have received HPV4 or HPV2; the possibility of using a 2-dose schedule for all HPV vaccines; and ways to increase uptake of this cancer-preventing vaccine.
Meningococcus type B
With the widespread use of quadrivalent meningococcal vaccines (MCV4), meningococcal meningitis has declined markedly in all age groups. The incidence of disease caused by meningococcal serotype B, which MCV4 does not protect against, has also declined from 0.3 to less than 0.1 cases per 100,000 between 1994 and 2013.11 The highest incidence occurs in infants under the age of 1 year, at 1.5/100,000, with 67% of cases attributable to serotype B. A slight bump in risk is seen with those ages 19 to 22 years (0.2/100,000) compared with other adolescents and adults.
While serotype B accounts for a larger proportion of all meningococcal disease than it did before, it is still relatively rare. In the United States between 2010 and 2012, annual cases totaled 48 to 56.11 Groups that are at higher risk of infection include those with complement deficiencies or asplenia (functional or anatomical), microbiologists and lab personnel who work with the organism, and those who have close contact with infected individuals.
In the past few years, well-publicized outbreaks of meningococcus B have occurred on some university campuses. Princeton had 9 cases, and the University of California at Santa Barbara had 4.11 This led to the use of meningococcal B vaccine as an outbreak control measure, with permission from the FDA before the vaccine was licensed. While these outbreaks created an impression of increased risks on college campuses, college students are actually at lower risk of type B meningococcal disease than others of the same age.11
This year, 2 meningococcal B vaccines will be available in the United States. The first, rLP2086, Trumenba (Pfizer) is a 3-dose series that was licensed in late 2014. The second, 4CMenB, Bexsero (Novartis) is a 2-dose series that received FDA approval in January 2015. Both are licensed for individuals ages 10 to 25 years. Formulating a recommendation for the use of these vaccines will be challenging because of several factors: the multiple dose schedules, the low rate of meningococcal B disease, and the age group for whom the vaccines are licensed.
1. Centers for Disease Control and Prevention. Recommended adult immunization schedule: United States - 2015. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/vaccines/schedules/downloads/adult/adult-combined-schedule.pdf. Accessed February 11, 2015.
2. Centers for Disease Control and Prevention. Birth – 18 years & “Catch-up” immunization schedules. United States, 2015. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/vaccines/schedules/hcp/child-adolescent.html. Accessed January 27, 2015.
3. Campos-Outcalt D. Pneumococcal vaccines for older adults: getting the timing right. J Fam Pract. 2014;63:730-733.
4. Campos-Outcalt D. The 2014-2015 influenza season: what you need to know. J Fam Pract. 2014;63:532-533.
5. Flannery B. Update on effectiveness of live-attenuated versus inactivated influenza vaccine in children and adolescents aged 2-18 years. Presented at: Advisory Committee on Immunization Practices; October 29, 2014; Atlanta, GA. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2014-10/flu-03-flannery.pdf. Accessed January 27, 2015.
6. Jackson BR. Typhoid and typhoid vaccines. Presented at: Advisory Committee on Immunization Practices; October 30, 2014; Atlanta, GA. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2014-10/Typhoid-02-Jackson.pdf. Accessed January 27, 2015.
7. Centers for Disease Control and Prevention. Typhoid immunization—recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 1994:43:1-7.
8. Luxembourg A. 9-valent HPV vaccine program key results--Part III. Presented at: Advisory Committee on Immunization Practices; October 30, 2014; Atlanta, GA. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2014-10/HPV-02-Luxembourg.pdf. Accessed January 27, 2015.
9. Markowitz L. 2-dose HPV vaccination schedules. Presented at: Advisory Committee on Immunization Practices; June 25, 2014; Atlanta, GA. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2014-06/HPV-04-Markowitz.pdf. Accessed January 27, 2015.
10. Stokley S, Jeyarajah J, Yankey D, et al; Immunization Services Division, National Center for Immunization and Respiratory Diseases, CDC; Centers for Disease Control and Prevention (CDC). Human papillomavirus vaccination coverage among adolescents, 2007-2013, and postlicensure safety monitoring, 2006-2014—United States. MMWR Morb Mortal Wkly Rep. 2014;63:620-624.
11. MacNeil J. Epidemiology of serogroup B meningococcal disease, United States. Presented at: Advisory Committee on Immunization Practices; October 30, 2014; Atlanta, GA. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2014-10/mening-02-MacNeil.pdf. Accessed January 27, 2015.
1. Centers for Disease Control and Prevention. Recommended adult immunization schedule: United States - 2015. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/vaccines/schedules/downloads/adult/adult-combined-schedule.pdf. Accessed February 11, 2015.
2. Centers for Disease Control and Prevention. Birth – 18 years & “Catch-up” immunization schedules. United States, 2015. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/vaccines/schedules/hcp/child-adolescent.html. Accessed January 27, 2015.
3. Campos-Outcalt D. Pneumococcal vaccines for older adults: getting the timing right. J Fam Pract. 2014;63:730-733.
4. Campos-Outcalt D. The 2014-2015 influenza season: what you need to know. J Fam Pract. 2014;63:532-533.
5. Flannery B. Update on effectiveness of live-attenuated versus inactivated influenza vaccine in children and adolescents aged 2-18 years. Presented at: Advisory Committee on Immunization Practices; October 29, 2014; Atlanta, GA. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2014-10/flu-03-flannery.pdf. Accessed January 27, 2015.
6. Jackson BR. Typhoid and typhoid vaccines. Presented at: Advisory Committee on Immunization Practices; October 30, 2014; Atlanta, GA. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2014-10/Typhoid-02-Jackson.pdf. Accessed January 27, 2015.
7. Centers for Disease Control and Prevention. Typhoid immunization—recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 1994:43:1-7.
8. Luxembourg A. 9-valent HPV vaccine program key results--Part III. Presented at: Advisory Committee on Immunization Practices; October 30, 2014; Atlanta, GA. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2014-10/HPV-02-Luxembourg.pdf. Accessed January 27, 2015.
9. Markowitz L. 2-dose HPV vaccination schedules. Presented at: Advisory Committee on Immunization Practices; June 25, 2014; Atlanta, GA. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2014-06/HPV-04-Markowitz.pdf. Accessed January 27, 2015.
10. Stokley S, Jeyarajah J, Yankey D, et al; Immunization Services Division, National Center for Immunization and Respiratory Diseases, CDC; Centers for Disease Control and Prevention (CDC). Human papillomavirus vaccination coverage among adolescents, 2007-2013, and postlicensure safety monitoring, 2006-2014—United States. MMWR Morb Mortal Wkly Rep. 2014;63:620-624.
11. MacNeil J. Epidemiology of serogroup B meningococcal disease, United States. Presented at: Advisory Committee on Immunization Practices; October 30, 2014; Atlanta, GA. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2014-10/mening-02-MacNeil.pdf. Accessed January 27, 2015.
Pneumococcal vaccines for older adults: Getting the timing right
In August 2014, the Advisory Committee on Immunization Practices (ACIP) decided to add the 13-valent pneumococcal conjugate vaccine (PCV13) to the routine immunization schedule for adults ages 65 years and older; previously, it had recommended that these patients receive only the 23-valent pneumococcal polysaccharide vaccine (PPSV23).1 The US Food and Drug Administration (FDA) had approved PCV13 for use in adults ages 50 years and older in late 2011. The delay between FDA approval and this new ACIP recommendation occurred for 2 reasons: The epidemiology of pneumococcal disease (pneumonia, meningitis, and bacteremia) in older adults is evolving due to the widespread use of PCV13 in children, and a large clinical trial looking at the efficacy of this vaccine in individuals 65 and older was still underway.
Pneumococcal disease in older adults remains a problem
Routine use of the 7-valent pneumococcal conjugate vaccine (PCV7) in children began in 2000. In 2010, the vaccine was expanded to include 6 more antigens (PCV13). The routine use of this vaccine has markedly reduced pneumococcal disease in children and, by way of indirect protection, in adults. Between 2010 and 2013, the incidence of invasive pneumococcal disease (eg, meningitis and bacteremia) caused by the 13 serotypes in the vaccine had decreased by 50% in adults ages 65 years and older.1 However, in this age group, there are still more than 13,000 cases of invasive pneumococcal disease each year.1 Approximately 20% of these cases—and 10% of cases community-acquired pneumonia (CAP) in this age group—are still caused by one of the PCV13 serotypes. This epidemiology left ACIP to consider whether to recommend PCV13 for older adults even though the incidence of pneumococcal disease was declining without the use of the vaccine. ACIP took a middle-of-the-road position on August 13, 2014 by recommending the vaccine now but agreeing to reexamine the issue again in 2018.1
PCV13 substantially cuts the rate of pneumococcal disease
In June 2014, ACIP reviewed the results of a large randomized, placebo-controlled clinical trial of PCV13 in 85,000 adults ages 65 years and older that was conducted in the Netherlands from 2008 to 2013.1 PCV13 reduced the rate of disease caused by the vaccine serotypes by 45.6% for pneumonia and 75% for invasive pneumococcal disease.
Because the population in this study was PPSV23-naïve, the added advantage of PCV13 in patients who have been vaccinated with PPSV23 has not been determined. Twelve of the 13 serotypes in PCV13 are in PPSV23. And while PPSV23 can protect against invasive pneumococcal disease, its effectiveness against CAP is less well proven.
Using modeling that took into consideration anticipated rates of vaccination with both PCV13 and PPSV23 in adults and children, the Centers for Disease Control and Prevention estimated that adding PCV13 to the adult immunization schedule would prevent 230 cases of invasive pneumococcal disease and 12,000 cases of CAP over the lifetime of a cohort of 65 year olds.1 With time, however, and the increasing indirect protection from routine use of PCV13 in children, these numbers would decline.
Timing of administration depends on patients’ vaccine history
Adults 65 years of age and older should receive both PCV13 and PPSV23, but not at the same time. In those who have not received any pneumococcal vaccine, the preferred sequence is to first administer PCV13 and then PPSV23 6 to 12 months later (FIGURE); the minimum acceptable interval between PCV13 and PPSV23 is 8 weeks.1 If PPSV23 is administered first, PCV13 should not be given until at least 12 months after the PPSV23 dose. This is because the immune response to PCV13 is not as robust when PCV13 follows PPSV23.
For patients who have been vaccinated with PPSV23 before age 65, PCV13 should be administered at least 12 months after PPSV23, followed by another dose of PPSV23 that should be administered 6 to 12 months after PCV13, but no sooner than 5 years since the previous PPSV23 (FIGURE).
Coadministration of PCV13 with trivalent influenza vaccine results in a slight decrease in the immune response to each vaccine;1 this is unlikely to be clinically important. Coadministration with other vaccines has not been studied.
Who’ll reimburse for the PCV13 vaccine? One issue that could delay the use of both vaccines in older adults is that currently, Medicare pays for only one pneumococcal vaccine in patients who are 65 and older. The Centers for Medicare and Medicaid Services will attempt to amend this policy, but how quickly this will occur is unknown.
Different recommendations for patients at higher risk
There are 2 sets of recommendations for use of pneumococcal vaccines: one for routine use for most patients, and a separate set of recommendations for those with conditions that put them at higher risk of infections and/or complications from pneumococcal disease.1-4 PPSV23 is recommended for children (starting at age 2 years) and adults with certain high-risk medical conditions, such as chronic heart, lung, or liver disease, and diabetes; functional or anatomical asplenia; or immunocompromising conditions such as human immunodeficiency virus infection, chronic renal failure, leukemia, or lymphoma.3 PPSV23 should be repeated 5 years after the first dose in patients with asplenia, those who are immunocompromised, and for everyone age 65 and older who received it before age 65. No more than 3 doses of PPSV23 should be given to anyone.
PCV13 is recommended for previously unvaccinated children and adults who have cochlear implants, cerebrospinal fluid leaks, functional or anatomical asplenia, or are immunocompromised.
1. Tomczyk S, Bennett NM, Stoecker C, et al; Centers for Disease Control and Prevention (CDC). Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥65 years: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2014;63:822-825.
2. Centers for Disease Control and Prevention (CDC). Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2012;61:816-819.
3. Centers for Disease Control and Prevention (CDC). Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among children aged 6-18 years with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2013;62:521-524.
4. Nuorti JP, Whitney CG; Centers for Disease Control and Prevention (CDC). Prevention of pneumococcal disease among infants and children - Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine - Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2010;59:1-18.
In August 2014, the Advisory Committee on Immunization Practices (ACIP) decided to add the 13-valent pneumococcal conjugate vaccine (PCV13) to the routine immunization schedule for adults ages 65 years and older; previously, it had recommended that these patients receive only the 23-valent pneumococcal polysaccharide vaccine (PPSV23).1 The US Food and Drug Administration (FDA) had approved PCV13 for use in adults ages 50 years and older in late 2011. The delay between FDA approval and this new ACIP recommendation occurred for 2 reasons: The epidemiology of pneumococcal disease (pneumonia, meningitis, and bacteremia) in older adults is evolving due to the widespread use of PCV13 in children, and a large clinical trial looking at the efficacy of this vaccine in individuals 65 and older was still underway.
Pneumococcal disease in older adults remains a problem
Routine use of the 7-valent pneumococcal conjugate vaccine (PCV7) in children began in 2000. In 2010, the vaccine was expanded to include 6 more antigens (PCV13). The routine use of this vaccine has markedly reduced pneumococcal disease in children and, by way of indirect protection, in adults. Between 2010 and 2013, the incidence of invasive pneumococcal disease (eg, meningitis and bacteremia) caused by the 13 serotypes in the vaccine had decreased by 50% in adults ages 65 years and older.1 However, in this age group, there are still more than 13,000 cases of invasive pneumococcal disease each year.1 Approximately 20% of these cases—and 10% of cases community-acquired pneumonia (CAP) in this age group—are still caused by one of the PCV13 serotypes. This epidemiology left ACIP to consider whether to recommend PCV13 for older adults even though the incidence of pneumococcal disease was declining without the use of the vaccine. ACIP took a middle-of-the-road position on August 13, 2014 by recommending the vaccine now but agreeing to reexamine the issue again in 2018.1
PCV13 substantially cuts the rate of pneumococcal disease
In June 2014, ACIP reviewed the results of a large randomized, placebo-controlled clinical trial of PCV13 in 85,000 adults ages 65 years and older that was conducted in the Netherlands from 2008 to 2013.1 PCV13 reduced the rate of disease caused by the vaccine serotypes by 45.6% for pneumonia and 75% for invasive pneumococcal disease.
Because the population in this study was PPSV23-naïve, the added advantage of PCV13 in patients who have been vaccinated with PPSV23 has not been determined. Twelve of the 13 serotypes in PCV13 are in PPSV23. And while PPSV23 can protect against invasive pneumococcal disease, its effectiveness against CAP is less well proven.
Using modeling that took into consideration anticipated rates of vaccination with both PCV13 and PPSV23 in adults and children, the Centers for Disease Control and Prevention estimated that adding PCV13 to the adult immunization schedule would prevent 230 cases of invasive pneumococcal disease and 12,000 cases of CAP over the lifetime of a cohort of 65 year olds.1 With time, however, and the increasing indirect protection from routine use of PCV13 in children, these numbers would decline.
Timing of administration depends on patients’ vaccine history
Adults 65 years of age and older should receive both PCV13 and PPSV23, but not at the same time. In those who have not received any pneumococcal vaccine, the preferred sequence is to first administer PCV13 and then PPSV23 6 to 12 months later (FIGURE); the minimum acceptable interval between PCV13 and PPSV23 is 8 weeks.1 If PPSV23 is administered first, PCV13 should not be given until at least 12 months after the PPSV23 dose. This is because the immune response to PCV13 is not as robust when PCV13 follows PPSV23.
For patients who have been vaccinated with PPSV23 before age 65, PCV13 should be administered at least 12 months after PPSV23, followed by another dose of PPSV23 that should be administered 6 to 12 months after PCV13, but no sooner than 5 years since the previous PPSV23 (FIGURE).
Coadministration of PCV13 with trivalent influenza vaccine results in a slight decrease in the immune response to each vaccine;1 this is unlikely to be clinically important. Coadministration with other vaccines has not been studied.
Who’ll reimburse for the PCV13 vaccine? One issue that could delay the use of both vaccines in older adults is that currently, Medicare pays for only one pneumococcal vaccine in patients who are 65 and older. The Centers for Medicare and Medicaid Services will attempt to amend this policy, but how quickly this will occur is unknown.
Different recommendations for patients at higher risk
There are 2 sets of recommendations for use of pneumococcal vaccines: one for routine use for most patients, and a separate set of recommendations for those with conditions that put them at higher risk of infections and/or complications from pneumococcal disease.1-4 PPSV23 is recommended for children (starting at age 2 years) and adults with certain high-risk medical conditions, such as chronic heart, lung, or liver disease, and diabetes; functional or anatomical asplenia; or immunocompromising conditions such as human immunodeficiency virus infection, chronic renal failure, leukemia, or lymphoma.3 PPSV23 should be repeated 5 years after the first dose in patients with asplenia, those who are immunocompromised, and for everyone age 65 and older who received it before age 65. No more than 3 doses of PPSV23 should be given to anyone.
PCV13 is recommended for previously unvaccinated children and adults who have cochlear implants, cerebrospinal fluid leaks, functional or anatomical asplenia, or are immunocompromised.
In August 2014, the Advisory Committee on Immunization Practices (ACIP) decided to add the 13-valent pneumococcal conjugate vaccine (PCV13) to the routine immunization schedule for adults ages 65 years and older; previously, it had recommended that these patients receive only the 23-valent pneumococcal polysaccharide vaccine (PPSV23).1 The US Food and Drug Administration (FDA) had approved PCV13 for use in adults ages 50 years and older in late 2011. The delay between FDA approval and this new ACIP recommendation occurred for 2 reasons: The epidemiology of pneumococcal disease (pneumonia, meningitis, and bacteremia) in older adults is evolving due to the widespread use of PCV13 in children, and a large clinical trial looking at the efficacy of this vaccine in individuals 65 and older was still underway.
Pneumococcal disease in older adults remains a problem
Routine use of the 7-valent pneumococcal conjugate vaccine (PCV7) in children began in 2000. In 2010, the vaccine was expanded to include 6 more antigens (PCV13). The routine use of this vaccine has markedly reduced pneumococcal disease in children and, by way of indirect protection, in adults. Between 2010 and 2013, the incidence of invasive pneumococcal disease (eg, meningitis and bacteremia) caused by the 13 serotypes in the vaccine had decreased by 50% in adults ages 65 years and older.1 However, in this age group, there are still more than 13,000 cases of invasive pneumococcal disease each year.1 Approximately 20% of these cases—and 10% of cases community-acquired pneumonia (CAP) in this age group—are still caused by one of the PCV13 serotypes. This epidemiology left ACIP to consider whether to recommend PCV13 for older adults even though the incidence of pneumococcal disease was declining without the use of the vaccine. ACIP took a middle-of-the-road position on August 13, 2014 by recommending the vaccine now but agreeing to reexamine the issue again in 2018.1
PCV13 substantially cuts the rate of pneumococcal disease
In June 2014, ACIP reviewed the results of a large randomized, placebo-controlled clinical trial of PCV13 in 85,000 adults ages 65 years and older that was conducted in the Netherlands from 2008 to 2013.1 PCV13 reduced the rate of disease caused by the vaccine serotypes by 45.6% for pneumonia and 75% for invasive pneumococcal disease.
Because the population in this study was PPSV23-naïve, the added advantage of PCV13 in patients who have been vaccinated with PPSV23 has not been determined. Twelve of the 13 serotypes in PCV13 are in PPSV23. And while PPSV23 can protect against invasive pneumococcal disease, its effectiveness against CAP is less well proven.
Using modeling that took into consideration anticipated rates of vaccination with both PCV13 and PPSV23 in adults and children, the Centers for Disease Control and Prevention estimated that adding PCV13 to the adult immunization schedule would prevent 230 cases of invasive pneumococcal disease and 12,000 cases of CAP over the lifetime of a cohort of 65 year olds.1 With time, however, and the increasing indirect protection from routine use of PCV13 in children, these numbers would decline.
Timing of administration depends on patients’ vaccine history
Adults 65 years of age and older should receive both PCV13 and PPSV23, but not at the same time. In those who have not received any pneumococcal vaccine, the preferred sequence is to first administer PCV13 and then PPSV23 6 to 12 months later (FIGURE); the minimum acceptable interval between PCV13 and PPSV23 is 8 weeks.1 If PPSV23 is administered first, PCV13 should not be given until at least 12 months after the PPSV23 dose. This is because the immune response to PCV13 is not as robust when PCV13 follows PPSV23.
For patients who have been vaccinated with PPSV23 before age 65, PCV13 should be administered at least 12 months after PPSV23, followed by another dose of PPSV23 that should be administered 6 to 12 months after PCV13, but no sooner than 5 years since the previous PPSV23 (FIGURE).
Coadministration of PCV13 with trivalent influenza vaccine results in a slight decrease in the immune response to each vaccine;1 this is unlikely to be clinically important. Coadministration with other vaccines has not been studied.
Who’ll reimburse for the PCV13 vaccine? One issue that could delay the use of both vaccines in older adults is that currently, Medicare pays for only one pneumococcal vaccine in patients who are 65 and older. The Centers for Medicare and Medicaid Services will attempt to amend this policy, but how quickly this will occur is unknown.
Different recommendations for patients at higher risk
There are 2 sets of recommendations for use of pneumococcal vaccines: one for routine use for most patients, and a separate set of recommendations for those with conditions that put them at higher risk of infections and/or complications from pneumococcal disease.1-4 PPSV23 is recommended for children (starting at age 2 years) and adults with certain high-risk medical conditions, such as chronic heart, lung, or liver disease, and diabetes; functional or anatomical asplenia; or immunocompromising conditions such as human immunodeficiency virus infection, chronic renal failure, leukemia, or lymphoma.3 PPSV23 should be repeated 5 years after the first dose in patients with asplenia, those who are immunocompromised, and for everyone age 65 and older who received it before age 65. No more than 3 doses of PPSV23 should be given to anyone.
PCV13 is recommended for previously unvaccinated children and adults who have cochlear implants, cerebrospinal fluid leaks, functional or anatomical asplenia, or are immunocompromised.
1. Tomczyk S, Bennett NM, Stoecker C, et al; Centers for Disease Control and Prevention (CDC). Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥65 years: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2014;63:822-825.
2. Centers for Disease Control and Prevention (CDC). Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2012;61:816-819.
3. Centers for Disease Control and Prevention (CDC). Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among children aged 6-18 years with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2013;62:521-524.
4. Nuorti JP, Whitney CG; Centers for Disease Control and Prevention (CDC). Prevention of pneumococcal disease among infants and children - Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine - Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2010;59:1-18.
1. Tomczyk S, Bennett NM, Stoecker C, et al; Centers for Disease Control and Prevention (CDC). Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥65 years: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2014;63:822-825.
2. Centers for Disease Control and Prevention (CDC). Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2012;61:816-819.
3. Centers for Disease Control and Prevention (CDC). Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among children aged 6-18 years with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2013;62:521-524.
4. Nuorti JP, Whitney CG; Centers for Disease Control and Prevention (CDC). Prevention of pneumococcal disease among infants and children - Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine - Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2010;59:1-18.
The 2014-2015 influenza season: What you need to know
As physicians and the Centers for Disease Control and Prevention (CDC) prepare for the upcoming influenza season, many of the recommendations remain unchanged from last season. Vaccination continues to be recommended for everyone 6 months of age and older. However, for the first time, a specific vaccine is preferred for children ages 2 through 8 years. Here’s what you need to know about this change, as well as how to handle vaccination in patients who are, or might be, allergic to eggs.
Use LAIV for kids ages 2 through 8 (if available)
For the first time, the CDC’s Advisory Committee on Immunization Practices (ACIP) has stated a preference for a specific influenza vaccine for a specific age group. It recommends using the live attenuated influenza vaccine (LAIV), which is a nasal spray, for children ages 2 through 8 years.1
A systematic review found evidence of increased efficacy of LAIV compared to inactivated influenza vaccine (IIV) in this age group; both types of vaccine have similar rates of adverse reactions.2 This increased effectiveness results in 46 fewer cases of confirmed influenza per 1000 children vaccinated (number needed to treat=24). Although the evidence of LAIV’s increased effectiveness was found for children ages 2 to 6 years, ACIP extended this recommendation through age 8 because this is the age through which physicians need to consider 2 doses of vaccine for a child previously unvaccinated with the influenza vaccine. Children younger than age 2 should receive IIV3 or IIV4.3
ACIP realizes that due to programmatic constraints it would be difficult to vaccinate all children with LAIV this year and is emphasizing that it should be implemented when feasible this year but no later than the 2015 to 2016 influenza season. IIV is effective in children and should be given if LAIV is not available or is contraindicated. Vaccine should not be delayed in the hopes of receiving LAIV if IIV is available.1
LAIV should not be used in children <2 years or adults >49. This vaccine is contraindicated in children and adolescents who are taking chronic aspirin therapy, pregnant women, those who are immunosuppressed, those with a history of egg allergy, or those who have taken influenza antiviral medications in the past 48 hours.1 LAIV also is not recommended for children ages 2 through 4 years who have asthma or had a wheezing episode in the past 12 months.1
There are precautions for the use of LAIV in patients with chronic medical conditions that can place them at high risk for complications from influenza, such as chronic lung, heart, renal, neurologic, liver, blood, or metabolic disorders, including asthma and diabetes.1
Which vaccine for patients who are allergic to eggs?
Two influenza vaccines are now available that are not prepared in embryonated eggs: recombinant influenza vaccine (RIV3) and cell culture-based inactivated influenza vaccine (ccIIV3). Both are trivalent products that contain antigens from 2 influenza A viruses and one influenza B virus and were introduced in time for the 2013 to 2014 flu season. The RIV3 is considered egg-free but ccIIV3 is not, although the amount of egg protein in it is miniscule, estimated at 5 × 10-8 mcg/0.5 mL dose.1 Neither product is licensed for children younger than 18 years and RIV3 is licensed only for those ages 18 through 49 years.
Patients who experience only hives after egg exposure can receive any of the flu vaccines except LAIV, and only because of a lack of data on this product, not because it has been shown to be less safe than the other vaccines. Patients who are unsure if they have an egg allergy or only get hives when they eat eggs should be observed for at least 30 minutes1 following injection as a precaution. Those ages 18 through 49 who have a history of severe reactions to eggs should receive RIV3. Patients younger than 18 years of age and older than 49 years of age can receive IIV vaccines approved for their specific age group. Any patient who is severely allergic and who cannot receive an egg-free vaccine should be vaccinated by a physician with experience managing severe allergic conditions.
Although severe, anaphylactic reactions to influenza vaccine are very rare, physicians should be equipped and prepared to respond to a severe allergic reaction after providing influenza vaccine to anyone with a history of an egg allergy.
Additional tips and resources
In addition to the LAIV, RIV3, and ccIIV3 vaccines described here, 10 other vaccines are available, including 5 egg-based IIV3 products in standard-dose form, 1 IIV3 vaccine for intradermal use, 1 high-dose IIV3 product for patients ages 65 or older, and 3 standard-dose IIV4 products. More details on each of these vaccines are available at http://www.cdc.gov/mmwr/preview/mmwrhtml/rr6207a1.htm?_%20cid=rr6207a1_w#Tab1.
Regardless of which type of flu vaccine they receive, children 6 months through 8 years should receive 2 doses, at least 4 weeks apart, unless they received:
- 1 dose in 2013 to 2014, or
- 2 or more doses of seasonal influenza vaccine since July 2010, or
- 2 or more doses of seasonal influenza vaccine before July 2010 and ≥1 dose of monovalent H1N1 vaccine, or
- at least 1 dose of seasonal influenza vaccine prior to July 2010 and ≥1 after.
Vaccine effectiveness. The CDC estimated that vaccine effectiveness during the 2013 to 2014 flu season was 66%.3 While this degree of effectiveness is important for minimizing the morbidity and mortality from influenza each year, it’s important to appreciate the limitations of the vaccine and not rely on it as the only prevention intervention.
Other forms of prevention. We need to advise and practice good respiratory hygiene, frequent hand washing, self-isolation when sick, effective infection control practices at health care facilities, targeted early treatment with antivirals, and targeted pre- and post-exposure antiviral chemoprevention. Details on each of these interventions, including recommendations on the use of antiviral medications, can be found on the CDC Web site at http://www.cdc.gov/flu.
1. Grohskopf LA, Olsen SJ, Sokolow LZ, et al; Influenza Division, National Center for Immunization and Respiratory Diseases, CDC. Prevention and control of seasonal influenza with vaccines: Recommendations of the Advisory Committee on Immunization Practices (ACIP)—United States 2014-2015 influenza season. MMWR Morb Mortal Wkly Rep. 2014;63:691-697.
2. Grohskopf L, Olsen S, Sokolow L. Effectiveness of live-attenuated vs inactivated influenza vaccines for healthy children. PowerPoint presented at: Meeting of the Advisory Committee on Immunization Practices; February 26, 2014; Atlanta, GA. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2014-02/05-Flu-Grohskopf.pdf. Accessed August 6, 2014.
3. Flannery B. Interim estimates of 2013-14 seasonal influenza vaccine effectiveness. PowerPoint presented at: Meeting of the Advisory Committee on Immunization Practices; February 26, 2014; Atlanta, GA. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2014-02/04-Flu-Flannery.pdf. Accessed August 6, 2014.
As physicians and the Centers for Disease Control and Prevention (CDC) prepare for the upcoming influenza season, many of the recommendations remain unchanged from last season. Vaccination continues to be recommended for everyone 6 months of age and older. However, for the first time, a specific vaccine is preferred for children ages 2 through 8 years. Here’s what you need to know about this change, as well as how to handle vaccination in patients who are, or might be, allergic to eggs.
Use LAIV for kids ages 2 through 8 (if available)
For the first time, the CDC’s Advisory Committee on Immunization Practices (ACIP) has stated a preference for a specific influenza vaccine for a specific age group. It recommends using the live attenuated influenza vaccine (LAIV), which is a nasal spray, for children ages 2 through 8 years.1
A systematic review found evidence of increased efficacy of LAIV compared to inactivated influenza vaccine (IIV) in this age group; both types of vaccine have similar rates of adverse reactions.2 This increased effectiveness results in 46 fewer cases of confirmed influenza per 1000 children vaccinated (number needed to treat=24). Although the evidence of LAIV’s increased effectiveness was found for children ages 2 to 6 years, ACIP extended this recommendation through age 8 because this is the age through which physicians need to consider 2 doses of vaccine for a child previously unvaccinated with the influenza vaccine. Children younger than age 2 should receive IIV3 or IIV4.3
ACIP realizes that due to programmatic constraints it would be difficult to vaccinate all children with LAIV this year and is emphasizing that it should be implemented when feasible this year but no later than the 2015 to 2016 influenza season. IIV is effective in children and should be given if LAIV is not available or is contraindicated. Vaccine should not be delayed in the hopes of receiving LAIV if IIV is available.1
LAIV should not be used in children <2 years or adults >49. This vaccine is contraindicated in children and adolescents who are taking chronic aspirin therapy, pregnant women, those who are immunosuppressed, those with a history of egg allergy, or those who have taken influenza antiviral medications in the past 48 hours.1 LAIV also is not recommended for children ages 2 through 4 years who have asthma or had a wheezing episode in the past 12 months.1
There are precautions for the use of LAIV in patients with chronic medical conditions that can place them at high risk for complications from influenza, such as chronic lung, heart, renal, neurologic, liver, blood, or metabolic disorders, including asthma and diabetes.1
Which vaccine for patients who are allergic to eggs?
Two influenza vaccines are now available that are not prepared in embryonated eggs: recombinant influenza vaccine (RIV3) and cell culture-based inactivated influenza vaccine (ccIIV3). Both are trivalent products that contain antigens from 2 influenza A viruses and one influenza B virus and were introduced in time for the 2013 to 2014 flu season. The RIV3 is considered egg-free but ccIIV3 is not, although the amount of egg protein in it is miniscule, estimated at 5 × 10-8 mcg/0.5 mL dose.1 Neither product is licensed for children younger than 18 years and RIV3 is licensed only for those ages 18 through 49 years.
Patients who experience only hives after egg exposure can receive any of the flu vaccines except LAIV, and only because of a lack of data on this product, not because it has been shown to be less safe than the other vaccines. Patients who are unsure if they have an egg allergy or only get hives when they eat eggs should be observed for at least 30 minutes1 following injection as a precaution. Those ages 18 through 49 who have a history of severe reactions to eggs should receive RIV3. Patients younger than 18 years of age and older than 49 years of age can receive IIV vaccines approved for their specific age group. Any patient who is severely allergic and who cannot receive an egg-free vaccine should be vaccinated by a physician with experience managing severe allergic conditions.
Although severe, anaphylactic reactions to influenza vaccine are very rare, physicians should be equipped and prepared to respond to a severe allergic reaction after providing influenza vaccine to anyone with a history of an egg allergy.
Additional tips and resources
In addition to the LAIV, RIV3, and ccIIV3 vaccines described here, 10 other vaccines are available, including 5 egg-based IIV3 products in standard-dose form, 1 IIV3 vaccine for intradermal use, 1 high-dose IIV3 product for patients ages 65 or older, and 3 standard-dose IIV4 products. More details on each of these vaccines are available at http://www.cdc.gov/mmwr/preview/mmwrhtml/rr6207a1.htm?_%20cid=rr6207a1_w#Tab1.
Regardless of which type of flu vaccine they receive, children 6 months through 8 years should receive 2 doses, at least 4 weeks apart, unless they received:
- 1 dose in 2013 to 2014, or
- 2 or more doses of seasonal influenza vaccine since July 2010, or
- 2 or more doses of seasonal influenza vaccine before July 2010 and ≥1 dose of monovalent H1N1 vaccine, or
- at least 1 dose of seasonal influenza vaccine prior to July 2010 and ≥1 after.
Vaccine effectiveness. The CDC estimated that vaccine effectiveness during the 2013 to 2014 flu season was 66%.3 While this degree of effectiveness is important for minimizing the morbidity and mortality from influenza each year, it’s important to appreciate the limitations of the vaccine and not rely on it as the only prevention intervention.
Other forms of prevention. We need to advise and practice good respiratory hygiene, frequent hand washing, self-isolation when sick, effective infection control practices at health care facilities, targeted early treatment with antivirals, and targeted pre- and post-exposure antiviral chemoprevention. Details on each of these interventions, including recommendations on the use of antiviral medications, can be found on the CDC Web site at http://www.cdc.gov/flu.
As physicians and the Centers for Disease Control and Prevention (CDC) prepare for the upcoming influenza season, many of the recommendations remain unchanged from last season. Vaccination continues to be recommended for everyone 6 months of age and older. However, for the first time, a specific vaccine is preferred for children ages 2 through 8 years. Here’s what you need to know about this change, as well as how to handle vaccination in patients who are, or might be, allergic to eggs.
Use LAIV for kids ages 2 through 8 (if available)
For the first time, the CDC’s Advisory Committee on Immunization Practices (ACIP) has stated a preference for a specific influenza vaccine for a specific age group. It recommends using the live attenuated influenza vaccine (LAIV), which is a nasal spray, for children ages 2 through 8 years.1
A systematic review found evidence of increased efficacy of LAIV compared to inactivated influenza vaccine (IIV) in this age group; both types of vaccine have similar rates of adverse reactions.2 This increased effectiveness results in 46 fewer cases of confirmed influenza per 1000 children vaccinated (number needed to treat=24). Although the evidence of LAIV’s increased effectiveness was found for children ages 2 to 6 years, ACIP extended this recommendation through age 8 because this is the age through which physicians need to consider 2 doses of vaccine for a child previously unvaccinated with the influenza vaccine. Children younger than age 2 should receive IIV3 or IIV4.3
ACIP realizes that due to programmatic constraints it would be difficult to vaccinate all children with LAIV this year and is emphasizing that it should be implemented when feasible this year but no later than the 2015 to 2016 influenza season. IIV is effective in children and should be given if LAIV is not available or is contraindicated. Vaccine should not be delayed in the hopes of receiving LAIV if IIV is available.1
LAIV should not be used in children <2 years or adults >49. This vaccine is contraindicated in children and adolescents who are taking chronic aspirin therapy, pregnant women, those who are immunosuppressed, those with a history of egg allergy, or those who have taken influenza antiviral medications in the past 48 hours.1 LAIV also is not recommended for children ages 2 through 4 years who have asthma or had a wheezing episode in the past 12 months.1
There are precautions for the use of LAIV in patients with chronic medical conditions that can place them at high risk for complications from influenza, such as chronic lung, heart, renal, neurologic, liver, blood, or metabolic disorders, including asthma and diabetes.1
Which vaccine for patients who are allergic to eggs?
Two influenza vaccines are now available that are not prepared in embryonated eggs: recombinant influenza vaccine (RIV3) and cell culture-based inactivated influenza vaccine (ccIIV3). Both are trivalent products that contain antigens from 2 influenza A viruses and one influenza B virus and were introduced in time for the 2013 to 2014 flu season. The RIV3 is considered egg-free but ccIIV3 is not, although the amount of egg protein in it is miniscule, estimated at 5 × 10-8 mcg/0.5 mL dose.1 Neither product is licensed for children younger than 18 years and RIV3 is licensed only for those ages 18 through 49 years.
Patients who experience only hives after egg exposure can receive any of the flu vaccines except LAIV, and only because of a lack of data on this product, not because it has been shown to be less safe than the other vaccines. Patients who are unsure if they have an egg allergy or only get hives when they eat eggs should be observed for at least 30 minutes1 following injection as a precaution. Those ages 18 through 49 who have a history of severe reactions to eggs should receive RIV3. Patients younger than 18 years of age and older than 49 years of age can receive IIV vaccines approved for their specific age group. Any patient who is severely allergic and who cannot receive an egg-free vaccine should be vaccinated by a physician with experience managing severe allergic conditions.
Although severe, anaphylactic reactions to influenza vaccine are very rare, physicians should be equipped and prepared to respond to a severe allergic reaction after providing influenza vaccine to anyone with a history of an egg allergy.
Additional tips and resources
In addition to the LAIV, RIV3, and ccIIV3 vaccines described here, 10 other vaccines are available, including 5 egg-based IIV3 products in standard-dose form, 1 IIV3 vaccine for intradermal use, 1 high-dose IIV3 product for patients ages 65 or older, and 3 standard-dose IIV4 products. More details on each of these vaccines are available at http://www.cdc.gov/mmwr/preview/mmwrhtml/rr6207a1.htm?_%20cid=rr6207a1_w#Tab1.
Regardless of which type of flu vaccine they receive, children 6 months through 8 years should receive 2 doses, at least 4 weeks apart, unless they received:
- 1 dose in 2013 to 2014, or
- 2 or more doses of seasonal influenza vaccine since July 2010, or
- 2 or more doses of seasonal influenza vaccine before July 2010 and ≥1 dose of monovalent H1N1 vaccine, or
- at least 1 dose of seasonal influenza vaccine prior to July 2010 and ≥1 after.
Vaccine effectiveness. The CDC estimated that vaccine effectiveness during the 2013 to 2014 flu season was 66%.3 While this degree of effectiveness is important for minimizing the morbidity and mortality from influenza each year, it’s important to appreciate the limitations of the vaccine and not rely on it as the only prevention intervention.
Other forms of prevention. We need to advise and practice good respiratory hygiene, frequent hand washing, self-isolation when sick, effective infection control practices at health care facilities, targeted early treatment with antivirals, and targeted pre- and post-exposure antiviral chemoprevention. Details on each of these interventions, including recommendations on the use of antiviral medications, can be found on the CDC Web site at http://www.cdc.gov/flu.
1. Grohskopf LA, Olsen SJ, Sokolow LZ, et al; Influenza Division, National Center for Immunization and Respiratory Diseases, CDC. Prevention and control of seasonal influenza with vaccines: Recommendations of the Advisory Committee on Immunization Practices (ACIP)—United States 2014-2015 influenza season. MMWR Morb Mortal Wkly Rep. 2014;63:691-697.
2. Grohskopf L, Olsen S, Sokolow L. Effectiveness of live-attenuated vs inactivated influenza vaccines for healthy children. PowerPoint presented at: Meeting of the Advisory Committee on Immunization Practices; February 26, 2014; Atlanta, GA. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2014-02/05-Flu-Grohskopf.pdf. Accessed August 6, 2014.
3. Flannery B. Interim estimates of 2013-14 seasonal influenza vaccine effectiveness. PowerPoint presented at: Meeting of the Advisory Committee on Immunization Practices; February 26, 2014; Atlanta, GA. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2014-02/04-Flu-Flannery.pdf. Accessed August 6, 2014.
1. Grohskopf LA, Olsen SJ, Sokolow LZ, et al; Influenza Division, National Center for Immunization and Respiratory Diseases, CDC. Prevention and control of seasonal influenza with vaccines: Recommendations of the Advisory Committee on Immunization Practices (ACIP)—United States 2014-2015 influenza season. MMWR Morb Mortal Wkly Rep. 2014;63:691-697.
2. Grohskopf L, Olsen S, Sokolow L. Effectiveness of live-attenuated vs inactivated influenza vaccines for healthy children. PowerPoint presented at: Meeting of the Advisory Committee on Immunization Practices; February 26, 2014; Atlanta, GA. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2014-02/05-Flu-Grohskopf.pdf. Accessed August 6, 2014.
3. Flannery B. Interim estimates of 2013-14 seasonal influenza vaccine effectiveness. PowerPoint presented at: Meeting of the Advisory Committee on Immunization Practices; February 26, 2014; Atlanta, GA. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2014-02/04-Flu-Flannery.pdf. Accessed August 6, 2014.
Diet, exercise, and CVD: When counseling makes the most sense
In the past 2 years, the US Preventive Services Task Force (USPSTF) has released 2 recommendations on the primary prevention of cardiovascular disease (CVD). And it is proposing a third. The first recommendation, released in 2012, covered behavioral counseling on diet and physical activity to prevent CVD in individuals without documented CVD risks.1 The second recommendation, released earlier this year, covered the use of vitamins and mineral supplements to prevent CVD.2 A draft of the proposed third recommendation, which was posted for public review until early June, covers behavioral counseling to help adults with known CVD risk factors improve their diet and physical activity (TABLE).1-3
Counseling can influence behavior, but does it affect outcomes?
CVD is the leading cause of death in the United States, accounting for >596,000 deaths per year with an age-adjusted rate of 191.4 per 100,000.4 Age-adjusted CVD mortality has been declining for decades thanks to improved medical care and a reduction in smoking and other risk factors. It is well documented that adults who follow national recommendations for a healthy diet and levels of physical activity have lower rates of CVD and CVD mortality.1 The USPSTF agrees with the American Heart Association (AHA) and the American College of Cardiology (ACC) that everyone would benefit from a healthier diet and more exercise.5 However, the Task Force reviewed the evidence on behavioral counseling in the primary care setting and found that, for adults who do not have known CVD, hypertension, hyperlipidemia, or diabetes, even high-intensity behavioral counseling resulted in only a small benefit in intermediate outcomes, which would translate into very small population-wide improvements.
In the evidence report prepared by the Task Force, the intensity of counseling intervention was defined as low, medium, or high if it lasted, respectively, 1 to 30 minutes, 31 to 360 minutes, or ≥361 minutes. Low-intensity interventions involved brief counseling sessions performed by primary care clinicians or mailing educational materials to patients or both. Medium- and high-intensity interventions usually were conducted by health educators, nutritionists, or other professionals instead of primary care clinicians. These interventions improved patients’ consumption of a healthier diet and participation in physical activity, but yielded only modest reductions in body mass index (BMI), blood pressure (BP), and lipid levels. Moreover, no direct evidence exists for improved CVD outcomes with these interventions.
The recent AHA/ACC guideline on lifestyle modifications recommends that clinicians advise all adults on healthy dietary choices and exercise, based on the known benefits of these behaviors. The guideline developers recognized that the evidence for benefits appears in the highest risk groups, and they did not assess the evidence for effectiveness of behavioral counseling itself.6
The Task Force rationale for recommending counseling
In the draft of its third recommendation addressing those at highest risk for CVD, the Task Force does advise high-intensity behavioral counseling for those who are overweight or obese and who have other CVD risk factors such as hypertension, hyperlipidemia, or impaired fasting glucose levels. This proposed new recommendation replaces one from 2003 that advised intensive dietary counseling for those with CVD risks including hyperlipidemia. The draft focuses attention in primary care on those who are overweight or obese. It complements another Task Force recommendation to provide or to refer patients for intensive multicomponent behavioral interventions if they are obese, defined as a BMI ≥30 kg/m2.7
The Task Force cited 2 examples of behavioral interventions that can improve outcomes in those with CVD risks—the Diabetes Prevention Program and PREMIER, a set of interventions to lower BP.8,9 These programs have improved intermediate outcomes after 12 to 24 months, decreasing total cholesterol by 3 to 6 mg/dL and low-density lipoprotein cholesterol by 1.5 to 5 mg/dL; systolic and diastolic BP by 1 to 3 mm Hg and 1 to 2 mm Hg, respectively; fasting glucose by 1 to 3 mg/dL; and weight by approximately 3 kg. The Task Force felt that while hard evidence is lacking for reducing CVD with counseling, epidemiologic studies demonstrate that, in those at high risk, reductions in CVD rates generally reflect the magnitude of improvement in intermediate measures.
Half of all adults in the United States have at least one documented CVD risk factor. But the potential benefit of behavioral counseling for those without documented CVD risks is relatively small. Rather than expending effort for only modest gain in the lower risk group, the Task Force recommends focusing on those with highest CVD risk. Thus the non-high risk group received a “C” recommendation, while the group of overweight and obese patients with other CVD risks received a “B” recommendation for essentially the same interventions. (For more on the grade definitions, see http://www.uspreventiveservicestaskforce.org/uspstf/grades.htm.)
In addition to counseling...
The Task Force also recommends other interventions for the primary prevention of CVD:
- screening for and treating hypertension
- selectively screening for hyperlipidemia
- using aspirin to prevent CVD in those at high risk
- intensive counseling on weight management for those who are obese
- advising children and adolescents to avoid tobacco, and using brief interventions for tobacco cessation for smokers.
The recent Task Force recommendation on the use of vitamins, minerals, and multivitamins2 states that, while many adults take vitamin and mineral supplements in the belief that they prevent both heart disease and cancer, there is no evidence to support that belief. And there is good evidence that both β-carotene and vitamin E do not prevent disease. For other vitamins and minerals, singly or in combination, there is insufficient evidence to recommend for or against their use.2
The Community Preventive Services Task Force—a separate expert panel established by the US Department of Health and Human Services to complement the USPSTF—makes recommendations on population-level interventions and has a series of recommendations on ways to improve the population’s nutrition and physical activity.10 These community-based interventions, if widely implemented, would probably yield greater improvements in healthy eating and increased activity levels than resource-intense clinical interventions based on individual patients with low risk.
1. USPSTF. Behavioral counseling to promote a healthful diet and physical activity for cardiovascular disease prevention in adults. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspsphys.htm. Accessed May 21, 2014.
2. USPSTF. Vitamin, mineral, and multivitamin supplements for the primary prevention of cardiovascular disease and cancer. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/uspstf14/vitasupp/vitasuppfinalrs.htm. Accessed May 21, 2014.
3. USPSTF. Behavioral counseling to promote a healthy diet and physical activity for cardiovascular disease prevention in adults with known risk factors: US Preventive Services Task Force Recommendation Statement (Draft). US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/uspstf13/cvdhighrisk/cvdhighriskdraftrec.htm. Accessed July 22, 2014.
4. Centers for Disease Control and Prevention. Hoyert DL, Xu J. Deaths: preliminary data for 2011. Natl Vital Stat Report. 2012;61:1-51.
5. Eckel RH, Jakicic JM, Ard, JD, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2013 Nov 12. [Epub ahead of print].
6. Stone NJ, Robinson JG, Lichtenstein AH, et al; 2013 ACC/AHA Cholesterol Guideline Panel. Treatment of blood cholesterol to reduce atherosclerotic cardiovascular disease risk in adults: synopsis of the 2013 American College of Cardiology/American Heart Association cholesterol guideline. Ann Intern Med. 2014;160:339-343.
7. USPSTF. Screening for and management of obesity in adults. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspsobes.htm. Accessed May 21, 2014.
8. Knowler WC, Barrett-Connor E, Fowler SE, et al; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393-403.
9. Elmer PJ, Obarzanek E, Vollmer WM, et al; PREMIER Collaborative Research Group. Effects of comprehensive lifestyle modification on diet, weight, physical fitness, and blood pressure control: 18-month results of a randomized trial. Ann Intern Med. 2006;144:485-495.
10. USPSTF. The Guide to Community Preventive Services. Community Preventive Services Task Force Web site. Available at: http://www.thecommunityguide.org/index.html. Accessed May 21, 2014.
In the past 2 years, the US Preventive Services Task Force (USPSTF) has released 2 recommendations on the primary prevention of cardiovascular disease (CVD). And it is proposing a third. The first recommendation, released in 2012, covered behavioral counseling on diet and physical activity to prevent CVD in individuals without documented CVD risks.1 The second recommendation, released earlier this year, covered the use of vitamins and mineral supplements to prevent CVD.2 A draft of the proposed third recommendation, which was posted for public review until early June, covers behavioral counseling to help adults with known CVD risk factors improve their diet and physical activity (TABLE).1-3
Counseling can influence behavior, but does it affect outcomes?
CVD is the leading cause of death in the United States, accounting for >596,000 deaths per year with an age-adjusted rate of 191.4 per 100,000.4 Age-adjusted CVD mortality has been declining for decades thanks to improved medical care and a reduction in smoking and other risk factors. It is well documented that adults who follow national recommendations for a healthy diet and levels of physical activity have lower rates of CVD and CVD mortality.1 The USPSTF agrees with the American Heart Association (AHA) and the American College of Cardiology (ACC) that everyone would benefit from a healthier diet and more exercise.5 However, the Task Force reviewed the evidence on behavioral counseling in the primary care setting and found that, for adults who do not have known CVD, hypertension, hyperlipidemia, or diabetes, even high-intensity behavioral counseling resulted in only a small benefit in intermediate outcomes, which would translate into very small population-wide improvements.
In the evidence report prepared by the Task Force, the intensity of counseling intervention was defined as low, medium, or high if it lasted, respectively, 1 to 30 minutes, 31 to 360 minutes, or ≥361 minutes. Low-intensity interventions involved brief counseling sessions performed by primary care clinicians or mailing educational materials to patients or both. Medium- and high-intensity interventions usually were conducted by health educators, nutritionists, or other professionals instead of primary care clinicians. These interventions improved patients’ consumption of a healthier diet and participation in physical activity, but yielded only modest reductions in body mass index (BMI), blood pressure (BP), and lipid levels. Moreover, no direct evidence exists for improved CVD outcomes with these interventions.
The recent AHA/ACC guideline on lifestyle modifications recommends that clinicians advise all adults on healthy dietary choices and exercise, based on the known benefits of these behaviors. The guideline developers recognized that the evidence for benefits appears in the highest risk groups, and they did not assess the evidence for effectiveness of behavioral counseling itself.6
The Task Force rationale for recommending counseling
In the draft of its third recommendation addressing those at highest risk for CVD, the Task Force does advise high-intensity behavioral counseling for those who are overweight or obese and who have other CVD risk factors such as hypertension, hyperlipidemia, or impaired fasting glucose levels. This proposed new recommendation replaces one from 2003 that advised intensive dietary counseling for those with CVD risks including hyperlipidemia. The draft focuses attention in primary care on those who are overweight or obese. It complements another Task Force recommendation to provide or to refer patients for intensive multicomponent behavioral interventions if they are obese, defined as a BMI ≥30 kg/m2.7
The Task Force cited 2 examples of behavioral interventions that can improve outcomes in those with CVD risks—the Diabetes Prevention Program and PREMIER, a set of interventions to lower BP.8,9 These programs have improved intermediate outcomes after 12 to 24 months, decreasing total cholesterol by 3 to 6 mg/dL and low-density lipoprotein cholesterol by 1.5 to 5 mg/dL; systolic and diastolic BP by 1 to 3 mm Hg and 1 to 2 mm Hg, respectively; fasting glucose by 1 to 3 mg/dL; and weight by approximately 3 kg. The Task Force felt that while hard evidence is lacking for reducing CVD with counseling, epidemiologic studies demonstrate that, in those at high risk, reductions in CVD rates generally reflect the magnitude of improvement in intermediate measures.
Half of all adults in the United States have at least one documented CVD risk factor. But the potential benefit of behavioral counseling for those without documented CVD risks is relatively small. Rather than expending effort for only modest gain in the lower risk group, the Task Force recommends focusing on those with highest CVD risk. Thus the non-high risk group received a “C” recommendation, while the group of overweight and obese patients with other CVD risks received a “B” recommendation for essentially the same interventions. (For more on the grade definitions, see http://www.uspreventiveservicestaskforce.org/uspstf/grades.htm.)
In addition to counseling...
The Task Force also recommends other interventions for the primary prevention of CVD:
- screening for and treating hypertension
- selectively screening for hyperlipidemia
- using aspirin to prevent CVD in those at high risk
- intensive counseling on weight management for those who are obese
- advising children and adolescents to avoid tobacco, and using brief interventions for tobacco cessation for smokers.
The recent Task Force recommendation on the use of vitamins, minerals, and multivitamins2 states that, while many adults take vitamin and mineral supplements in the belief that they prevent both heart disease and cancer, there is no evidence to support that belief. And there is good evidence that both β-carotene and vitamin E do not prevent disease. For other vitamins and minerals, singly or in combination, there is insufficient evidence to recommend for or against their use.2
The Community Preventive Services Task Force—a separate expert panel established by the US Department of Health and Human Services to complement the USPSTF—makes recommendations on population-level interventions and has a series of recommendations on ways to improve the population’s nutrition and physical activity.10 These community-based interventions, if widely implemented, would probably yield greater improvements in healthy eating and increased activity levels than resource-intense clinical interventions based on individual patients with low risk.
In the past 2 years, the US Preventive Services Task Force (USPSTF) has released 2 recommendations on the primary prevention of cardiovascular disease (CVD). And it is proposing a third. The first recommendation, released in 2012, covered behavioral counseling on diet and physical activity to prevent CVD in individuals without documented CVD risks.1 The second recommendation, released earlier this year, covered the use of vitamins and mineral supplements to prevent CVD.2 A draft of the proposed third recommendation, which was posted for public review until early June, covers behavioral counseling to help adults with known CVD risk factors improve their diet and physical activity (TABLE).1-3
Counseling can influence behavior, but does it affect outcomes?
CVD is the leading cause of death in the United States, accounting for >596,000 deaths per year with an age-adjusted rate of 191.4 per 100,000.4 Age-adjusted CVD mortality has been declining for decades thanks to improved medical care and a reduction in smoking and other risk factors. It is well documented that adults who follow national recommendations for a healthy diet and levels of physical activity have lower rates of CVD and CVD mortality.1 The USPSTF agrees with the American Heart Association (AHA) and the American College of Cardiology (ACC) that everyone would benefit from a healthier diet and more exercise.5 However, the Task Force reviewed the evidence on behavioral counseling in the primary care setting and found that, for adults who do not have known CVD, hypertension, hyperlipidemia, or diabetes, even high-intensity behavioral counseling resulted in only a small benefit in intermediate outcomes, which would translate into very small population-wide improvements.
In the evidence report prepared by the Task Force, the intensity of counseling intervention was defined as low, medium, or high if it lasted, respectively, 1 to 30 minutes, 31 to 360 minutes, or ≥361 minutes. Low-intensity interventions involved brief counseling sessions performed by primary care clinicians or mailing educational materials to patients or both. Medium- and high-intensity interventions usually were conducted by health educators, nutritionists, or other professionals instead of primary care clinicians. These interventions improved patients’ consumption of a healthier diet and participation in physical activity, but yielded only modest reductions in body mass index (BMI), blood pressure (BP), and lipid levels. Moreover, no direct evidence exists for improved CVD outcomes with these interventions.
The recent AHA/ACC guideline on lifestyle modifications recommends that clinicians advise all adults on healthy dietary choices and exercise, based on the known benefits of these behaviors. The guideline developers recognized that the evidence for benefits appears in the highest risk groups, and they did not assess the evidence for effectiveness of behavioral counseling itself.6
The Task Force rationale for recommending counseling
In the draft of its third recommendation addressing those at highest risk for CVD, the Task Force does advise high-intensity behavioral counseling for those who are overweight or obese and who have other CVD risk factors such as hypertension, hyperlipidemia, or impaired fasting glucose levels. This proposed new recommendation replaces one from 2003 that advised intensive dietary counseling for those with CVD risks including hyperlipidemia. The draft focuses attention in primary care on those who are overweight or obese. It complements another Task Force recommendation to provide or to refer patients for intensive multicomponent behavioral interventions if they are obese, defined as a BMI ≥30 kg/m2.7
The Task Force cited 2 examples of behavioral interventions that can improve outcomes in those with CVD risks—the Diabetes Prevention Program and PREMIER, a set of interventions to lower BP.8,9 These programs have improved intermediate outcomes after 12 to 24 months, decreasing total cholesterol by 3 to 6 mg/dL and low-density lipoprotein cholesterol by 1.5 to 5 mg/dL; systolic and diastolic BP by 1 to 3 mm Hg and 1 to 2 mm Hg, respectively; fasting glucose by 1 to 3 mg/dL; and weight by approximately 3 kg. The Task Force felt that while hard evidence is lacking for reducing CVD with counseling, epidemiologic studies demonstrate that, in those at high risk, reductions in CVD rates generally reflect the magnitude of improvement in intermediate measures.
Half of all adults in the United States have at least one documented CVD risk factor. But the potential benefit of behavioral counseling for those without documented CVD risks is relatively small. Rather than expending effort for only modest gain in the lower risk group, the Task Force recommends focusing on those with highest CVD risk. Thus the non-high risk group received a “C” recommendation, while the group of overweight and obese patients with other CVD risks received a “B” recommendation for essentially the same interventions. (For more on the grade definitions, see http://www.uspreventiveservicestaskforce.org/uspstf/grades.htm.)
In addition to counseling...
The Task Force also recommends other interventions for the primary prevention of CVD:
- screening for and treating hypertension
- selectively screening for hyperlipidemia
- using aspirin to prevent CVD in those at high risk
- intensive counseling on weight management for those who are obese
- advising children and adolescents to avoid tobacco, and using brief interventions for tobacco cessation for smokers.
The recent Task Force recommendation on the use of vitamins, minerals, and multivitamins2 states that, while many adults take vitamin and mineral supplements in the belief that they prevent both heart disease and cancer, there is no evidence to support that belief. And there is good evidence that both β-carotene and vitamin E do not prevent disease. For other vitamins and minerals, singly or in combination, there is insufficient evidence to recommend for or against their use.2
The Community Preventive Services Task Force—a separate expert panel established by the US Department of Health and Human Services to complement the USPSTF—makes recommendations on population-level interventions and has a series of recommendations on ways to improve the population’s nutrition and physical activity.10 These community-based interventions, if widely implemented, would probably yield greater improvements in healthy eating and increased activity levels than resource-intense clinical interventions based on individual patients with low risk.
1. USPSTF. Behavioral counseling to promote a healthful diet and physical activity for cardiovascular disease prevention in adults. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspsphys.htm. Accessed May 21, 2014.
2. USPSTF. Vitamin, mineral, and multivitamin supplements for the primary prevention of cardiovascular disease and cancer. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/uspstf14/vitasupp/vitasuppfinalrs.htm. Accessed May 21, 2014.
3. USPSTF. Behavioral counseling to promote a healthy diet and physical activity for cardiovascular disease prevention in adults with known risk factors: US Preventive Services Task Force Recommendation Statement (Draft). US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/uspstf13/cvdhighrisk/cvdhighriskdraftrec.htm. Accessed July 22, 2014.
4. Centers for Disease Control and Prevention. Hoyert DL, Xu J. Deaths: preliminary data for 2011. Natl Vital Stat Report. 2012;61:1-51.
5. Eckel RH, Jakicic JM, Ard, JD, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2013 Nov 12. [Epub ahead of print].
6. Stone NJ, Robinson JG, Lichtenstein AH, et al; 2013 ACC/AHA Cholesterol Guideline Panel. Treatment of blood cholesterol to reduce atherosclerotic cardiovascular disease risk in adults: synopsis of the 2013 American College of Cardiology/American Heart Association cholesterol guideline. Ann Intern Med. 2014;160:339-343.
7. USPSTF. Screening for and management of obesity in adults. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspsobes.htm. Accessed May 21, 2014.
8. Knowler WC, Barrett-Connor E, Fowler SE, et al; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393-403.
9. Elmer PJ, Obarzanek E, Vollmer WM, et al; PREMIER Collaborative Research Group. Effects of comprehensive lifestyle modification on diet, weight, physical fitness, and blood pressure control: 18-month results of a randomized trial. Ann Intern Med. 2006;144:485-495.
10. USPSTF. The Guide to Community Preventive Services. Community Preventive Services Task Force Web site. Available at: http://www.thecommunityguide.org/index.html. Accessed May 21, 2014.
1. USPSTF. Behavioral counseling to promote a healthful diet and physical activity for cardiovascular disease prevention in adults. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspsphys.htm. Accessed May 21, 2014.
2. USPSTF. Vitamin, mineral, and multivitamin supplements for the primary prevention of cardiovascular disease and cancer. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/uspstf14/vitasupp/vitasuppfinalrs.htm. Accessed May 21, 2014.
3. USPSTF. Behavioral counseling to promote a healthy diet and physical activity for cardiovascular disease prevention in adults with known risk factors: US Preventive Services Task Force Recommendation Statement (Draft). US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/uspstf13/cvdhighrisk/cvdhighriskdraftrec.htm. Accessed July 22, 2014.
4. Centers for Disease Control and Prevention. Hoyert DL, Xu J. Deaths: preliminary data for 2011. Natl Vital Stat Report. 2012;61:1-51.
5. Eckel RH, Jakicic JM, Ard, JD, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2013 Nov 12. [Epub ahead of print].
6. Stone NJ, Robinson JG, Lichtenstein AH, et al; 2013 ACC/AHA Cholesterol Guideline Panel. Treatment of blood cholesterol to reduce atherosclerotic cardiovascular disease risk in adults: synopsis of the 2013 American College of Cardiology/American Heart Association cholesterol guideline. Ann Intern Med. 2014;160:339-343.
7. USPSTF. Screening for and management of obesity in adults. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspsobes.htm. Accessed May 21, 2014.
8. Knowler WC, Barrett-Connor E, Fowler SE, et al; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393-403.
9. Elmer PJ, Obarzanek E, Vollmer WM, et al; PREMIER Collaborative Research Group. Effects of comprehensive lifestyle modification on diet, weight, physical fitness, and blood pressure control: 18-month results of a randomized trial. Ann Intern Med. 2006;144:485-495.
10. USPSTF. The Guide to Community Preventive Services. Community Preventive Services Task Force Web site. Available at: http://www.thecommunityguide.org/index.html. Accessed May 21, 2014.
USPSTF: What’s recommended, what’s not
The United States Preventive Services Task Force (USPSTF) was busy in 2013, issuing 26 recommendations on 16 topics (TABLES 1-3). We have covered some of these topics previously in Practice Alerts or audiocasts—vitamin D for bone health and fall prevention,1 screening for lung cancer,2 human immunodeficiency virus infection,3 and the use of multivitamins to prevent cancer and cardiovascular disease (CVD).4 Another Practice Alert on chronic hepatitis C virus infection reviewed recommendations of the Centers for Disease Control and Prevention,5 which agree with those of the USPSTF. This Practice Alert discusses the remaining USPSTF recommendations.
Alcohol and tobacco
The Task Force (TF) reports that 30% of adults are affected by alcohol-related problems and that alcohol causes 85,000 deaths per year, making it the third leading cause of preventable death.6 The TF reviewed evidence on screening and counseling and now recommends screening adults ≥18 years for alcohol misuse and providing brief counseling to reduce alcohol use for those who engage in risky or hazardous drinking.6 The TF recommends any of 3 screening tools: using either the Alcohol Use Disorders Identification Test (AUDIT) or the abbreviated AUDIT-Consumption (AUDIT-C), or asking a single-question, such as “How many times in the past year have you had 5 (for men) or 4 (for women and all adults >65 years) or more drinks in a day?”6
Counseling for 5 to 15 minutes during the initial clinical encounter and then at subsequent visits is more effective than very brief (<5 minutes) or single-episode counseling. Counseling can include action plans, drinking diaries, stress management, or problem solving, and it can be done face-to-face or with written self-help materials, computer- or Web-based programs, or telephone support. Despite the importance of alcohol misuse as a health problem, the TF could find no evidence that screening and behavioral counseling is effective for adolescents.
For tobacco use, however, the TF now recommends providing prevention advice to school-age children and adolescents,7 presented in person or through written materials, videos, or other media. Over 8% of middle school children and close to 24% of high school students use tobacco.7 Tobacco is the leading cause of preventable deaths in the United States, and most smokers start before they are adults.7
Cancer screening and prevention
In addition to the recommendation for lung cancer screening, 2 other cancer screening/prevention recommendations were made in 2013. One is a modification of the previous recommendation on the use of BRCA gene testing to detect increased risk of breast and ovarian cancer. The recommendation now states that if a woman has a family member with breast, ovarian, tubal, or peritoneal cancer, her physician should use a screening tool to determine if her family history suggests high risk for having either BRCA1 or BRCA2. With a positive screening result, referral for genetic counseling is warranted. After counseling, the patient may choose to undergo BRCA testing. Screening tools reviewed by the TF are the Ontario Family History Assessment Tool, the Manchester Scoring System, the Referral Screening Tool, the Pedigree Assessment Tool, and the Family History Screening-7 instrument.8
The second recommendation is complex and concerns whether to prescribe tamoxifen or raloxifene to prevent breast cancer in women at high risk—ie, a 5-year risk ≥3%.9 One tool for estimating risk can be found at http://www.cancer.gov/bcrisktool/. It calculates risk based on age, race, genetic profile, age at menopause and menarche, family history of breast cancer, and personal history of breast cancer and biopsies. The TF recommends that physicians share decision making with women who are at high risk of breast cancer and offer medication to those at low risk of complications (those who have had a hysterectomy). Use of tamoxifen or raloxifene can reduce risk of the invasive cancer by 7 to 9 cases per 1000 women over 5 years. However, the risk of venous thromboembolism increases by 4 to 7 cases per 1000 over 5 years, and tamoxifen increases the risk of endometrial cancer by 4 in 1000. Both medications can cause hot flashes.9
Gestational diabetes
For a number of years the TF has assigned an “I” statement (insufficient evidence to assess benefits and harms) to screening for gestational diabetes. It recently changed that to a “B” recommendation for all pregnant women after the 24th week of pregnancy. Screening before 24 weeks is still listed as an I. Possible screening tools include a fasting blood glucose test, a 50-g oral glucose challenge test, or an assessment of risk factors. The TF did not find evidence of superiority with any of these methods. The TF found that diet modifications, glucose monitoring, and use of insulin can, in some cases, moderately reduce the incidence of preeclampsia, macrosomia, and shoulder dystocia.10
Intimate partner violence
Another change from a previous “I” statement pertains to intimate partner violence (IPV). The TF now recommends screening women of childbearing age for IPV and either providing intervention services for those who screen positive for IPV or referring for services. Reproductive age is defined as 14 to 46 years, although the TF admits that most studies have looked at women ≥18 years.11 Most of the benefits from screening and counseling have been demonstrated in pregnant women.
IPV can include physical, sexual, or psychological harm by a current or former partner or spouse, and it is not limited to opposite sex couples.11 Screening tools with the highest sensitivity and specificity include the Hurt, Insult, Threaten, and Scream (HITS) scale. Potential interventions include counseling, home visits, information cards, referrals to community services, and mentoring support.
While the TF acknowledges that both child abuse and elder abuse are prominent problems, there is not enough evidence to assess and recommend interventions.11,12
D recommendations
There were 4 “D” recommendations (recommend against) in 2013: testing for BRCA or using tamoxifen or raloxifene in women at low risk of breast cancer; using β-carotene or vitamin E to prevent CVD and cancer; and using low doses of vitamin D and calcium to prevent fractures in noninstitutionalized postmenopausal women (TABLE 2). In each instance the harms of the intervention were deemed to exceed potential benefits.
I statements
The TF still finds little evidence to support some common practices (TABLE 3). Physicians who use these interventions should realize that the TF, after thorough systematic reviews of the available evidence, does not find enough evidence to assess their relative benefits and harms. A description of the evidence on each condition can be found in the recommendations section of the USPSTF Web site (http://www.uspreventiveservicestaskforce.org/uspstopics.htm).
1. Campos-Outcalt D. Vitamin D: when it helps, when it harms. J Fam Pract. 2013;62:368-370.
2. Campos-Outcalt D. Lung cancer screening: USPSTF revises its recommendation. J Fam Pract. 2013;62:733-740.
3. Campos-Outcalt D. HIV screening: what the USPSTF says now. [Audiocast]. Parsippany, NJ; The Journal of Family Practice: 2013. Available at: http://www.jfponline.com/multimedia/audio/article/hiv-screening-what-the-uspstf-says-now/a1c4bc0fc9405f18820bb19fe971f743.html. Accessed March 14, 2014.
4. Campos-Outcalt D. Does your patient really need a supplement? [Audiocast]. Parsippany, NJ: The Journal of Family Practice; 2013. Available at: http://www.jfponline.com/index.php?id=21643&cHash=071010&tx_ttnews[tt_news]=226385. Accessed March 14, 2014.
5. Campos-Outcalt D. Hepatitis C: new CDC screening recommendations. J Fam Pract. 2012;61:744-746.
6. US Preventive Services Task Force Web site. Screening and behavioral counseling interventions in primary care to reduce alcohol misuse. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspsdrin.htm. Accessed March 14, 2014.
7. US Preventive Services Task Force Web site. Primary care interventions to prevent tobacco use in children and adolescents. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspstbac.htm. Accessed March 14, 2014.
8. US Preventive Services Task Force Web site. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer in women. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspsbrgen.htm. Accessed March 14, 2014.
9. US Preventive Services Task Force Web site. Medication for risk reduction of primary breast cancer in women. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspsbrpv.htm. Accessed March 14, 2014.
10. US Preventive Services Task Force Web site. Screening for gestational diabetes mellitius. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspsgdm.htm. Accessed March 14, 2014.
11. US Preventive Services Task Force Web site. Screening for intimate partner violence and abuse of elderly and vulnerable adults. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspsipv.htm. Accessed March 14, 2014.
12. US Preventive Services Task Force Web site. Primary interventions to prevent child maltreatment. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspsfamv.htm. Accessed March 14, 2014.
The United States Preventive Services Task Force (USPSTF) was busy in 2013, issuing 26 recommendations on 16 topics (TABLES 1-3). We have covered some of these topics previously in Practice Alerts or audiocasts—vitamin D for bone health and fall prevention,1 screening for lung cancer,2 human immunodeficiency virus infection,3 and the use of multivitamins to prevent cancer and cardiovascular disease (CVD).4 Another Practice Alert on chronic hepatitis C virus infection reviewed recommendations of the Centers for Disease Control and Prevention,5 which agree with those of the USPSTF. This Practice Alert discusses the remaining USPSTF recommendations.
Alcohol and tobacco
The Task Force (TF) reports that 30% of adults are affected by alcohol-related problems and that alcohol causes 85,000 deaths per year, making it the third leading cause of preventable death.6 The TF reviewed evidence on screening and counseling and now recommends screening adults ≥18 years for alcohol misuse and providing brief counseling to reduce alcohol use for those who engage in risky or hazardous drinking.6 The TF recommends any of 3 screening tools: using either the Alcohol Use Disorders Identification Test (AUDIT) or the abbreviated AUDIT-Consumption (AUDIT-C), or asking a single-question, such as “How many times in the past year have you had 5 (for men) or 4 (for women and all adults >65 years) or more drinks in a day?”6
Counseling for 5 to 15 minutes during the initial clinical encounter and then at subsequent visits is more effective than very brief (<5 minutes) or single-episode counseling. Counseling can include action plans, drinking diaries, stress management, or problem solving, and it can be done face-to-face or with written self-help materials, computer- or Web-based programs, or telephone support. Despite the importance of alcohol misuse as a health problem, the TF could find no evidence that screening and behavioral counseling is effective for adolescents.
For tobacco use, however, the TF now recommends providing prevention advice to school-age children and adolescents,7 presented in person or through written materials, videos, or other media. Over 8% of middle school children and close to 24% of high school students use tobacco.7 Tobacco is the leading cause of preventable deaths in the United States, and most smokers start before they are adults.7
Cancer screening and prevention
In addition to the recommendation for lung cancer screening, 2 other cancer screening/prevention recommendations were made in 2013. One is a modification of the previous recommendation on the use of BRCA gene testing to detect increased risk of breast and ovarian cancer. The recommendation now states that if a woman has a family member with breast, ovarian, tubal, or peritoneal cancer, her physician should use a screening tool to determine if her family history suggests high risk for having either BRCA1 or BRCA2. With a positive screening result, referral for genetic counseling is warranted. After counseling, the patient may choose to undergo BRCA testing. Screening tools reviewed by the TF are the Ontario Family History Assessment Tool, the Manchester Scoring System, the Referral Screening Tool, the Pedigree Assessment Tool, and the Family History Screening-7 instrument.8
The second recommendation is complex and concerns whether to prescribe tamoxifen or raloxifene to prevent breast cancer in women at high risk—ie, a 5-year risk ≥3%.9 One tool for estimating risk can be found at http://www.cancer.gov/bcrisktool/. It calculates risk based on age, race, genetic profile, age at menopause and menarche, family history of breast cancer, and personal history of breast cancer and biopsies. The TF recommends that physicians share decision making with women who are at high risk of breast cancer and offer medication to those at low risk of complications (those who have had a hysterectomy). Use of tamoxifen or raloxifene can reduce risk of the invasive cancer by 7 to 9 cases per 1000 women over 5 years. However, the risk of venous thromboembolism increases by 4 to 7 cases per 1000 over 5 years, and tamoxifen increases the risk of endometrial cancer by 4 in 1000. Both medications can cause hot flashes.9
Gestational diabetes
For a number of years the TF has assigned an “I” statement (insufficient evidence to assess benefits and harms) to screening for gestational diabetes. It recently changed that to a “B” recommendation for all pregnant women after the 24th week of pregnancy. Screening before 24 weeks is still listed as an I. Possible screening tools include a fasting blood glucose test, a 50-g oral glucose challenge test, or an assessment of risk factors. The TF did not find evidence of superiority with any of these methods. The TF found that diet modifications, glucose monitoring, and use of insulin can, in some cases, moderately reduce the incidence of preeclampsia, macrosomia, and shoulder dystocia.10
Intimate partner violence
Another change from a previous “I” statement pertains to intimate partner violence (IPV). The TF now recommends screening women of childbearing age for IPV and either providing intervention services for those who screen positive for IPV or referring for services. Reproductive age is defined as 14 to 46 years, although the TF admits that most studies have looked at women ≥18 years.11 Most of the benefits from screening and counseling have been demonstrated in pregnant women.
IPV can include physical, sexual, or psychological harm by a current or former partner or spouse, and it is not limited to opposite sex couples.11 Screening tools with the highest sensitivity and specificity include the Hurt, Insult, Threaten, and Scream (HITS) scale. Potential interventions include counseling, home visits, information cards, referrals to community services, and mentoring support.
While the TF acknowledges that both child abuse and elder abuse are prominent problems, there is not enough evidence to assess and recommend interventions.11,12
D recommendations
There were 4 “D” recommendations (recommend against) in 2013: testing for BRCA or using tamoxifen or raloxifene in women at low risk of breast cancer; using β-carotene or vitamin E to prevent CVD and cancer; and using low doses of vitamin D and calcium to prevent fractures in noninstitutionalized postmenopausal women (TABLE 2). In each instance the harms of the intervention were deemed to exceed potential benefits.
I statements
The TF still finds little evidence to support some common practices (TABLE 3). Physicians who use these interventions should realize that the TF, after thorough systematic reviews of the available evidence, does not find enough evidence to assess their relative benefits and harms. A description of the evidence on each condition can be found in the recommendations section of the USPSTF Web site (http://www.uspreventiveservicestaskforce.org/uspstopics.htm).
The United States Preventive Services Task Force (USPSTF) was busy in 2013, issuing 26 recommendations on 16 topics (TABLES 1-3). We have covered some of these topics previously in Practice Alerts or audiocasts—vitamin D for bone health and fall prevention,1 screening for lung cancer,2 human immunodeficiency virus infection,3 and the use of multivitamins to prevent cancer and cardiovascular disease (CVD).4 Another Practice Alert on chronic hepatitis C virus infection reviewed recommendations of the Centers for Disease Control and Prevention,5 which agree with those of the USPSTF. This Practice Alert discusses the remaining USPSTF recommendations.
Alcohol and tobacco
The Task Force (TF) reports that 30% of adults are affected by alcohol-related problems and that alcohol causes 85,000 deaths per year, making it the third leading cause of preventable death.6 The TF reviewed evidence on screening and counseling and now recommends screening adults ≥18 years for alcohol misuse and providing brief counseling to reduce alcohol use for those who engage in risky or hazardous drinking.6 The TF recommends any of 3 screening tools: using either the Alcohol Use Disorders Identification Test (AUDIT) or the abbreviated AUDIT-Consumption (AUDIT-C), or asking a single-question, such as “How many times in the past year have you had 5 (for men) or 4 (for women and all adults >65 years) or more drinks in a day?”6
Counseling for 5 to 15 minutes during the initial clinical encounter and then at subsequent visits is more effective than very brief (<5 minutes) or single-episode counseling. Counseling can include action plans, drinking diaries, stress management, or problem solving, and it can be done face-to-face or with written self-help materials, computer- or Web-based programs, or telephone support. Despite the importance of alcohol misuse as a health problem, the TF could find no evidence that screening and behavioral counseling is effective for adolescents.
For tobacco use, however, the TF now recommends providing prevention advice to school-age children and adolescents,7 presented in person or through written materials, videos, or other media. Over 8% of middle school children and close to 24% of high school students use tobacco.7 Tobacco is the leading cause of preventable deaths in the United States, and most smokers start before they are adults.7
Cancer screening and prevention
In addition to the recommendation for lung cancer screening, 2 other cancer screening/prevention recommendations were made in 2013. One is a modification of the previous recommendation on the use of BRCA gene testing to detect increased risk of breast and ovarian cancer. The recommendation now states that if a woman has a family member with breast, ovarian, tubal, or peritoneal cancer, her physician should use a screening tool to determine if her family history suggests high risk for having either BRCA1 or BRCA2. With a positive screening result, referral for genetic counseling is warranted. After counseling, the patient may choose to undergo BRCA testing. Screening tools reviewed by the TF are the Ontario Family History Assessment Tool, the Manchester Scoring System, the Referral Screening Tool, the Pedigree Assessment Tool, and the Family History Screening-7 instrument.8
The second recommendation is complex and concerns whether to prescribe tamoxifen or raloxifene to prevent breast cancer in women at high risk—ie, a 5-year risk ≥3%.9 One tool for estimating risk can be found at http://www.cancer.gov/bcrisktool/. It calculates risk based on age, race, genetic profile, age at menopause and menarche, family history of breast cancer, and personal history of breast cancer and biopsies. The TF recommends that physicians share decision making with women who are at high risk of breast cancer and offer medication to those at low risk of complications (those who have had a hysterectomy). Use of tamoxifen or raloxifene can reduce risk of the invasive cancer by 7 to 9 cases per 1000 women over 5 years. However, the risk of venous thromboembolism increases by 4 to 7 cases per 1000 over 5 years, and tamoxifen increases the risk of endometrial cancer by 4 in 1000. Both medications can cause hot flashes.9
Gestational diabetes
For a number of years the TF has assigned an “I” statement (insufficient evidence to assess benefits and harms) to screening for gestational diabetes. It recently changed that to a “B” recommendation for all pregnant women after the 24th week of pregnancy. Screening before 24 weeks is still listed as an I. Possible screening tools include a fasting blood glucose test, a 50-g oral glucose challenge test, or an assessment of risk factors. The TF did not find evidence of superiority with any of these methods. The TF found that diet modifications, glucose monitoring, and use of insulin can, in some cases, moderately reduce the incidence of preeclampsia, macrosomia, and shoulder dystocia.10
Intimate partner violence
Another change from a previous “I” statement pertains to intimate partner violence (IPV). The TF now recommends screening women of childbearing age for IPV and either providing intervention services for those who screen positive for IPV or referring for services. Reproductive age is defined as 14 to 46 years, although the TF admits that most studies have looked at women ≥18 years.11 Most of the benefits from screening and counseling have been demonstrated in pregnant women.
IPV can include physical, sexual, or psychological harm by a current or former partner or spouse, and it is not limited to opposite sex couples.11 Screening tools with the highest sensitivity and specificity include the Hurt, Insult, Threaten, and Scream (HITS) scale. Potential interventions include counseling, home visits, information cards, referrals to community services, and mentoring support.
While the TF acknowledges that both child abuse and elder abuse are prominent problems, there is not enough evidence to assess and recommend interventions.11,12
D recommendations
There were 4 “D” recommendations (recommend against) in 2013: testing for BRCA or using tamoxifen or raloxifene in women at low risk of breast cancer; using β-carotene or vitamin E to prevent CVD and cancer; and using low doses of vitamin D and calcium to prevent fractures in noninstitutionalized postmenopausal women (TABLE 2). In each instance the harms of the intervention were deemed to exceed potential benefits.
I statements
The TF still finds little evidence to support some common practices (TABLE 3). Physicians who use these interventions should realize that the TF, after thorough systematic reviews of the available evidence, does not find enough evidence to assess their relative benefits and harms. A description of the evidence on each condition can be found in the recommendations section of the USPSTF Web site (http://www.uspreventiveservicestaskforce.org/uspstopics.htm).
1. Campos-Outcalt D. Vitamin D: when it helps, when it harms. J Fam Pract. 2013;62:368-370.
2. Campos-Outcalt D. Lung cancer screening: USPSTF revises its recommendation. J Fam Pract. 2013;62:733-740.
3. Campos-Outcalt D. HIV screening: what the USPSTF says now. [Audiocast]. Parsippany, NJ; The Journal of Family Practice: 2013. Available at: http://www.jfponline.com/multimedia/audio/article/hiv-screening-what-the-uspstf-says-now/a1c4bc0fc9405f18820bb19fe971f743.html. Accessed March 14, 2014.
4. Campos-Outcalt D. Does your patient really need a supplement? [Audiocast]. Parsippany, NJ: The Journal of Family Practice; 2013. Available at: http://www.jfponline.com/index.php?id=21643&cHash=071010&tx_ttnews[tt_news]=226385. Accessed March 14, 2014.
5. Campos-Outcalt D. Hepatitis C: new CDC screening recommendations. J Fam Pract. 2012;61:744-746.
6. US Preventive Services Task Force Web site. Screening and behavioral counseling interventions in primary care to reduce alcohol misuse. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspsdrin.htm. Accessed March 14, 2014.
7. US Preventive Services Task Force Web site. Primary care interventions to prevent tobacco use in children and adolescents. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspstbac.htm. Accessed March 14, 2014.
8. US Preventive Services Task Force Web site. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer in women. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspsbrgen.htm. Accessed March 14, 2014.
9. US Preventive Services Task Force Web site. Medication for risk reduction of primary breast cancer in women. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspsbrpv.htm. Accessed March 14, 2014.
10. US Preventive Services Task Force Web site. Screening for gestational diabetes mellitius. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspsgdm.htm. Accessed March 14, 2014.
11. US Preventive Services Task Force Web site. Screening for intimate partner violence and abuse of elderly and vulnerable adults. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspsipv.htm. Accessed March 14, 2014.
12. US Preventive Services Task Force Web site. Primary interventions to prevent child maltreatment. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspsfamv.htm. Accessed March 14, 2014.
1. Campos-Outcalt D. Vitamin D: when it helps, when it harms. J Fam Pract. 2013;62:368-370.
2. Campos-Outcalt D. Lung cancer screening: USPSTF revises its recommendation. J Fam Pract. 2013;62:733-740.
3. Campos-Outcalt D. HIV screening: what the USPSTF says now. [Audiocast]. Parsippany, NJ; The Journal of Family Practice: 2013. Available at: http://www.jfponline.com/multimedia/audio/article/hiv-screening-what-the-uspstf-says-now/a1c4bc0fc9405f18820bb19fe971f743.html. Accessed March 14, 2014.
4. Campos-Outcalt D. Does your patient really need a supplement? [Audiocast]. Parsippany, NJ: The Journal of Family Practice; 2013. Available at: http://www.jfponline.com/index.php?id=21643&cHash=071010&tx_ttnews[tt_news]=226385. Accessed March 14, 2014.
5. Campos-Outcalt D. Hepatitis C: new CDC screening recommendations. J Fam Pract. 2012;61:744-746.
6. US Preventive Services Task Force Web site. Screening and behavioral counseling interventions in primary care to reduce alcohol misuse. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspsdrin.htm. Accessed March 14, 2014.
7. US Preventive Services Task Force Web site. Primary care interventions to prevent tobacco use in children and adolescents. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspstbac.htm. Accessed March 14, 2014.
8. US Preventive Services Task Force Web site. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer in women. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspsbrgen.htm. Accessed March 14, 2014.
9. US Preventive Services Task Force Web site. Medication for risk reduction of primary breast cancer in women. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspsbrpv.htm. Accessed March 14, 2014.
10. US Preventive Services Task Force Web site. Screening for gestational diabetes mellitius. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspsgdm.htm. Accessed March 14, 2014.
11. US Preventive Services Task Force Web site. Screening for intimate partner violence and abuse of elderly and vulnerable adults. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspsipv.htm. Accessed March 14, 2014.
12. US Preventive Services Task Force Web site. Primary interventions to prevent child maltreatment. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspsfamv.htm. Accessed March 14, 2014.
Immunization update: The latest ACIP recommendations
The Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC) released several new immunization recommendations in 2013, and they pertain to special populations: infants at high risk for meningococcal disease who could benefit from a new vaccine; children ages 2 to 16 years who may now receive the Japanese encephalitis (JE) vaccine if traveling to Asia; and children and adolescents at high risk for Streptococcus pneumoniae infections who should receive the 13-valent pneumococcal conjugate vaccine (PCV13).
New meningococcal vaccine for high-risk infants
A third vaccine against meningococcal disease, Menveo (Novartis), has been approved for use in high-risk infants ages 2 to 23 months—those with complement deficiencies or functional or anatomic asplenia, those involved in an institutional or community outbreak of meningococcal disease, and those traveling to a meningococcal-endemic area (eg, the meningitis belt of Africa). The 3 vaccines approved for use in infants (TABLE 1) vary in indications and use for travel.1 Keep in mind that vaccination against meningococcal disease is not recommended for routine use in infants because of the low prevalence of disease and a predominance of meningococcal type B, which is not prevented by any of the current vaccines.2
Japanese encephalitis vaccine approved for children, adolescents
Since 2009, a vaccine of inactivated JE virus derived from Vero cell culture (Ixiaro, manufactured by Intercell Biomedical and distributed by Norvartis) has been available in the United States for adults ≥17 years who travel during JE virus transmission season to parts of Asia where the virus is endemic. In May 2013, the US Food and Drug Administration licensed the JE vaccine for use in children ages 2 to 16 years, and in June 2013, ACIP recommended it for those in this age group for the same indications as adults.3
Individuals at high risk are those likely to visit rural areas where the virus is prevalent. The vaccine is not recommended for short-term travelers who will confine their visit to urban areas or who will refrain from traveling during the JE transmission season. The CDC travel Web site describes the geographic areas where the JE virus can be found and the timing of the JE season (http://wwwnc.cdc.gov/travel/diseases/japanese-encephalitis; see “Who is at risk?”). Use of the vaccine will be uncommon, and most of those who will receive it will need to visit a travel clinic or local health department. A primary series is 2 doses, given 28 days apart.
Pneumococcal vaccine for high-risk children up to age 18
ACIP now recommends the use of both PCV13 and 23-valent pneumococcal polysaccharide vaccine (PPSV23) in children ages 6 to 18 years who have not been vaccinated with these products and who have certain immunocompromising conditions, functional or anatomic asplenia (including those with sickle cell disease), cerebrospinal fluid leaks, or cochlear implants (TABLE 2).4 PPSV23 alone is recommended for those in this age group with chronic heart, lung, or liver disease or diabetes, and for those who smoke. The recommendation is complicated because those with asplenia or immunocompromising conditions should receive 2 doses of PPSV23 at least 5 years apart (TABLE 2). PCV13 is indicated even if the patient was previously fully immunized with 7-valent pneumococcal conjugate vaccine.
Ideally, all children for whom PCV13 is indicated would receive a full vaccine series as infants, and at 2, 4, 6, and 12 to 15 months of age. Those with chronic medical conditions and immunocompromising conditions should additionally receive PPSV23 at age 2 years, and again 5 years later if they have asplenia or an immunocompromising condition.5 However, some of these children will remain unimmunized; others can become underimmunized if they acquire a condition that heightens their risk for pneumococcal disease. Both situations make necessary the new recommendation for those ages 6 to 18 years. For those who have received neither vaccine, the recommended sequence is to receive PCV13 first, followed by PPSV23 at least 8 weeks later.
Pneumococcal vaccine recommendations are now uniform for those ages 6 to 64 years with chronic medical conditions and immunocompromising conditions.2,6 All those ≥65 should receive PPSV23, even if they have received 2 previous doses. At least 5 years should have passed from a previous PPSV23 dose. This is the only scenario in which a third dose of PPSV23 is recommended.
Universal use of pneumococcal vaccines has markedly reduced invasive pneumococcal disease nationally in vaccinated and unvaccinated individuals.5 While invasive pneumococcal disease rates have fallen overall, patients with high-risk conditions still have a high relative risk. The incidence rates (cases per 100,000) for children with hematologic malignancies were estimated at 1282 (rate ratio [RR], compared with children without this condition, of 822); 197 for those with HIV infection (RR=122), and 56 for those with sickle cell disease (RR=27).4
As the incidence of antibiotic resistance increases, the primary prevention of bacterial infections becomes more significant. It is of both public health and individual importance that those who develop conditions placing them at increased risk for pneumococcal disease or complications from pneumococcal infection receive recommended pneumococcal vaccines.
1. MacNeil J. Recommendations for use of MenACWY-CRM (Menveo) in infants at increased risk for meningococcal disease. Presented at: meeting of the Advisory Committee on Immunization Practices (ACIP); October 23, 2013; Atlanta, Ga. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-oct-2013/04-MCV-MacNeil.pdf. Accessed February 12, 2014.
2. Centers for Disease Control and Prevention (CDC). Infant meningococcal vaccination: Advisory Committee on Immunization Practices (ACIP) recommendations and rationale. MMWR Morb Mortal Wkly Rep. 2013;62:52-54.
3. Centers for Disease Control and Prevention (CDC). Use of Japanese encephalitis vaccine in children: recommendations of the advisory committee on immunization practices, 2013. MMWR Morb Mortal Wkly Rep. 2013;62:898-900.
4. Centers for Disease Control and Prevention (CDC). Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among children aged 6-18 years with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2013;62:521-524.
5. Nuorti JP, Whitney CG; Centers for Disease Control and Prevention (CDC). Prevention of pneumococcal disease among infants and children - use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine - recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2010;59(RR-11):1-18.
6. Centers for Disease Control and Prevention (CDC). Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2012;61:816-819.
The Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC) released several new immunization recommendations in 2013, and they pertain to special populations: infants at high risk for meningococcal disease who could benefit from a new vaccine; children ages 2 to 16 years who may now receive the Japanese encephalitis (JE) vaccine if traveling to Asia; and children and adolescents at high risk for Streptococcus pneumoniae infections who should receive the 13-valent pneumococcal conjugate vaccine (PCV13).
New meningococcal vaccine for high-risk infants
A third vaccine against meningococcal disease, Menveo (Novartis), has been approved for use in high-risk infants ages 2 to 23 months—those with complement deficiencies or functional or anatomic asplenia, those involved in an institutional or community outbreak of meningococcal disease, and those traveling to a meningococcal-endemic area (eg, the meningitis belt of Africa). The 3 vaccines approved for use in infants (TABLE 1) vary in indications and use for travel.1 Keep in mind that vaccination against meningococcal disease is not recommended for routine use in infants because of the low prevalence of disease and a predominance of meningococcal type B, which is not prevented by any of the current vaccines.2
Japanese encephalitis vaccine approved for children, adolescents
Since 2009, a vaccine of inactivated JE virus derived from Vero cell culture (Ixiaro, manufactured by Intercell Biomedical and distributed by Norvartis) has been available in the United States for adults ≥17 years who travel during JE virus transmission season to parts of Asia where the virus is endemic. In May 2013, the US Food and Drug Administration licensed the JE vaccine for use in children ages 2 to 16 years, and in June 2013, ACIP recommended it for those in this age group for the same indications as adults.3
Individuals at high risk are those likely to visit rural areas where the virus is prevalent. The vaccine is not recommended for short-term travelers who will confine their visit to urban areas or who will refrain from traveling during the JE transmission season. The CDC travel Web site describes the geographic areas where the JE virus can be found and the timing of the JE season (http://wwwnc.cdc.gov/travel/diseases/japanese-encephalitis; see “Who is at risk?”). Use of the vaccine will be uncommon, and most of those who will receive it will need to visit a travel clinic or local health department. A primary series is 2 doses, given 28 days apart.
Pneumococcal vaccine for high-risk children up to age 18
ACIP now recommends the use of both PCV13 and 23-valent pneumococcal polysaccharide vaccine (PPSV23) in children ages 6 to 18 years who have not been vaccinated with these products and who have certain immunocompromising conditions, functional or anatomic asplenia (including those with sickle cell disease), cerebrospinal fluid leaks, or cochlear implants (TABLE 2).4 PPSV23 alone is recommended for those in this age group with chronic heart, lung, or liver disease or diabetes, and for those who smoke. The recommendation is complicated because those with asplenia or immunocompromising conditions should receive 2 doses of PPSV23 at least 5 years apart (TABLE 2). PCV13 is indicated even if the patient was previously fully immunized with 7-valent pneumococcal conjugate vaccine.
Ideally, all children for whom PCV13 is indicated would receive a full vaccine series as infants, and at 2, 4, 6, and 12 to 15 months of age. Those with chronic medical conditions and immunocompromising conditions should additionally receive PPSV23 at age 2 years, and again 5 years later if they have asplenia or an immunocompromising condition.5 However, some of these children will remain unimmunized; others can become underimmunized if they acquire a condition that heightens their risk for pneumococcal disease. Both situations make necessary the new recommendation for those ages 6 to 18 years. For those who have received neither vaccine, the recommended sequence is to receive PCV13 first, followed by PPSV23 at least 8 weeks later.
Pneumococcal vaccine recommendations are now uniform for those ages 6 to 64 years with chronic medical conditions and immunocompromising conditions.2,6 All those ≥65 should receive PPSV23, even if they have received 2 previous doses. At least 5 years should have passed from a previous PPSV23 dose. This is the only scenario in which a third dose of PPSV23 is recommended.
Universal use of pneumococcal vaccines has markedly reduced invasive pneumococcal disease nationally in vaccinated and unvaccinated individuals.5 While invasive pneumococcal disease rates have fallen overall, patients with high-risk conditions still have a high relative risk. The incidence rates (cases per 100,000) for children with hematologic malignancies were estimated at 1282 (rate ratio [RR], compared with children without this condition, of 822); 197 for those with HIV infection (RR=122), and 56 for those with sickle cell disease (RR=27).4
As the incidence of antibiotic resistance increases, the primary prevention of bacterial infections becomes more significant. It is of both public health and individual importance that those who develop conditions placing them at increased risk for pneumococcal disease or complications from pneumococcal infection receive recommended pneumococcal vaccines.
The Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC) released several new immunization recommendations in 2013, and they pertain to special populations: infants at high risk for meningococcal disease who could benefit from a new vaccine; children ages 2 to 16 years who may now receive the Japanese encephalitis (JE) vaccine if traveling to Asia; and children and adolescents at high risk for Streptococcus pneumoniae infections who should receive the 13-valent pneumococcal conjugate vaccine (PCV13).
New meningococcal vaccine for high-risk infants
A third vaccine against meningococcal disease, Menveo (Novartis), has been approved for use in high-risk infants ages 2 to 23 months—those with complement deficiencies or functional or anatomic asplenia, those involved in an institutional or community outbreak of meningococcal disease, and those traveling to a meningococcal-endemic area (eg, the meningitis belt of Africa). The 3 vaccines approved for use in infants (TABLE 1) vary in indications and use for travel.1 Keep in mind that vaccination against meningococcal disease is not recommended for routine use in infants because of the low prevalence of disease and a predominance of meningococcal type B, which is not prevented by any of the current vaccines.2
Japanese encephalitis vaccine approved for children, adolescents
Since 2009, a vaccine of inactivated JE virus derived from Vero cell culture (Ixiaro, manufactured by Intercell Biomedical and distributed by Norvartis) has been available in the United States for adults ≥17 years who travel during JE virus transmission season to parts of Asia where the virus is endemic. In May 2013, the US Food and Drug Administration licensed the JE vaccine for use in children ages 2 to 16 years, and in June 2013, ACIP recommended it for those in this age group for the same indications as adults.3
Individuals at high risk are those likely to visit rural areas where the virus is prevalent. The vaccine is not recommended for short-term travelers who will confine their visit to urban areas or who will refrain from traveling during the JE transmission season. The CDC travel Web site describes the geographic areas where the JE virus can be found and the timing of the JE season (http://wwwnc.cdc.gov/travel/diseases/japanese-encephalitis; see “Who is at risk?”). Use of the vaccine will be uncommon, and most of those who will receive it will need to visit a travel clinic or local health department. A primary series is 2 doses, given 28 days apart.
Pneumococcal vaccine for high-risk children up to age 18
ACIP now recommends the use of both PCV13 and 23-valent pneumococcal polysaccharide vaccine (PPSV23) in children ages 6 to 18 years who have not been vaccinated with these products and who have certain immunocompromising conditions, functional or anatomic asplenia (including those with sickle cell disease), cerebrospinal fluid leaks, or cochlear implants (TABLE 2).4 PPSV23 alone is recommended for those in this age group with chronic heart, lung, or liver disease or diabetes, and for those who smoke. The recommendation is complicated because those with asplenia or immunocompromising conditions should receive 2 doses of PPSV23 at least 5 years apart (TABLE 2). PCV13 is indicated even if the patient was previously fully immunized with 7-valent pneumococcal conjugate vaccine.
Ideally, all children for whom PCV13 is indicated would receive a full vaccine series as infants, and at 2, 4, 6, and 12 to 15 months of age. Those with chronic medical conditions and immunocompromising conditions should additionally receive PPSV23 at age 2 years, and again 5 years later if they have asplenia or an immunocompromising condition.5 However, some of these children will remain unimmunized; others can become underimmunized if they acquire a condition that heightens their risk for pneumococcal disease. Both situations make necessary the new recommendation for those ages 6 to 18 years. For those who have received neither vaccine, the recommended sequence is to receive PCV13 first, followed by PPSV23 at least 8 weeks later.
Pneumococcal vaccine recommendations are now uniform for those ages 6 to 64 years with chronic medical conditions and immunocompromising conditions.2,6 All those ≥65 should receive PPSV23, even if they have received 2 previous doses. At least 5 years should have passed from a previous PPSV23 dose. This is the only scenario in which a third dose of PPSV23 is recommended.
Universal use of pneumococcal vaccines has markedly reduced invasive pneumococcal disease nationally in vaccinated and unvaccinated individuals.5 While invasive pneumococcal disease rates have fallen overall, patients with high-risk conditions still have a high relative risk. The incidence rates (cases per 100,000) for children with hematologic malignancies were estimated at 1282 (rate ratio [RR], compared with children without this condition, of 822); 197 for those with HIV infection (RR=122), and 56 for those with sickle cell disease (RR=27).4
As the incidence of antibiotic resistance increases, the primary prevention of bacterial infections becomes more significant. It is of both public health and individual importance that those who develop conditions placing them at increased risk for pneumococcal disease or complications from pneumococcal infection receive recommended pneumococcal vaccines.
1. MacNeil J. Recommendations for use of MenACWY-CRM (Menveo) in infants at increased risk for meningococcal disease. Presented at: meeting of the Advisory Committee on Immunization Practices (ACIP); October 23, 2013; Atlanta, Ga. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-oct-2013/04-MCV-MacNeil.pdf. Accessed February 12, 2014.
2. Centers for Disease Control and Prevention (CDC). Infant meningococcal vaccination: Advisory Committee on Immunization Practices (ACIP) recommendations and rationale. MMWR Morb Mortal Wkly Rep. 2013;62:52-54.
3. Centers for Disease Control and Prevention (CDC). Use of Japanese encephalitis vaccine in children: recommendations of the advisory committee on immunization practices, 2013. MMWR Morb Mortal Wkly Rep. 2013;62:898-900.
4. Centers for Disease Control and Prevention (CDC). Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among children aged 6-18 years with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2013;62:521-524.
5. Nuorti JP, Whitney CG; Centers for Disease Control and Prevention (CDC). Prevention of pneumococcal disease among infants and children - use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine - recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2010;59(RR-11):1-18.
6. Centers for Disease Control and Prevention (CDC). Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2012;61:816-819.
1. MacNeil J. Recommendations for use of MenACWY-CRM (Menveo) in infants at increased risk for meningococcal disease. Presented at: meeting of the Advisory Committee on Immunization Practices (ACIP); October 23, 2013; Atlanta, Ga. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-oct-2013/04-MCV-MacNeil.pdf. Accessed February 12, 2014.
2. Centers for Disease Control and Prevention (CDC). Infant meningococcal vaccination: Advisory Committee on Immunization Practices (ACIP) recommendations and rationale. MMWR Morb Mortal Wkly Rep. 2013;62:52-54.
3. Centers for Disease Control and Prevention (CDC). Use of Japanese encephalitis vaccine in children: recommendations of the advisory committee on immunization practices, 2013. MMWR Morb Mortal Wkly Rep. 2013;62:898-900.
4. Centers for Disease Control and Prevention (CDC). Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among children aged 6-18 years with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2013;62:521-524.
5. Nuorti JP, Whitney CG; Centers for Disease Control and Prevention (CDC). Prevention of pneumococcal disease among infants and children - use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine - recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2010;59(RR-11):1-18.
6. Centers for Disease Control and Prevention (CDC). Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2012;61:816-819.