User login
The new cardiovascular disease prevention guidelines: What you need to know
A significant milestone in evidence-based practice was reached in November 2013, when the American Heart Association and American College of Cardiology (AHA/ACC) published 4 clinical practice guidelines on the prevention of cardiovascular disease.1-4 These guidelines—on obesity, lifestyle management, cardiovascular disease (CVD) risk assessment, and cholesterol—were developed under the auspices of the National Heart, Lung, and Blood Institute (NHLBI) to update its prior guidelines on the treatment of hypertension, high cholesterol, and obesity that were published more than a decade ago.5-7 After the NHLBI had organized the respective guideline panels and progressed through most of the guideline development process (which lasted several years each), it arranged for the AHA/ACC to assume sponsorship and publication of the guidelines. The NHLBI decided its role should be to develop evidence reports, leaving the development of guidelines to professional organizations.
While the prior guidelines on hypertension and hypercholesterolemia were influential and widely cited as the standard of care, they were heavily influenced by expert opinion and were not strictly evidence based. The NHLBI sought to develop the new guidelines using more contemporary and rigorous evidence-based processes to meet standards set by the Institute of Medicine (IOM). The group started with key clinical questions, conducted comprehensive systematic reviews of the evidence, and then rated the quality of the evidence and assigned strength of recommendation ratings.8 The guidelines and evidence reports are lengthy, and are summarized below.
In December 2013, the Eighth Joint National Committee (the 5th panel organized by the NHLBI to address CVD prevention) published its updated guideline on the treatment of hypertension, which has also generated controversy. Visit www.jfponline.com to listen to an audiocast summary of these recommendations.9
Obesity and overweight
The guideline on managing obesity and overweight adults has 17 recommendations, only 3 of which are based on expert opinion.1 (TABLE 1 summarizes the strong [A] and moderate [B] recommendations.) The recommendations stress screening, diagnosis, and treatment using diet, exercise, and lifestyle modification. They also address bariatric surgery for those with a body mass index (BMI) ≥40 or a persisting BMI ≥35 despite weight loss interventions. This set of recommendations, like those of the United States Preventive Services Task Force, advises intensive interventions for weight management and additionally offers much more detail on recommended diet and exercise.
Lifestyle management
The 10 recommendations on lifestyle management to reduce cardiovascular risk, all evidence based, are limited to diet and exercise as a means to control hypertension and hypercholesterolemia.2 They do not cover other important lifestyle modifications for preventing CVD, such as smoking cessation. The guideline panel acknowledged that the interventions are aimed at those with high blood pressure and elevated cholesterol, but they encourage all adults to follow them. Although these recommendations are not particularly controversial, the 2 recommendations to reduce sodium intake are said to be based on strong or moderate strength evidence, in contrast to a recent IOM report that concluded evidence for the health benefits of salt intake <2.3 g/d is weak.10 This illustrates how separate authoritative groups can rate the strength of the same evidence differently.
Summary highlights:
• Encourage adults who would benefit from lowering either blood pressure (BP) or low-density lipoprotein cholesterol (LDL-C) to eat a diet that emphasizes vegetables, fruits, whole grains, low-fat dairy products, and other notably healthful foods, and to cut down on products high in sugar content and on red meats.
• Review, as appropriate, such options as the DASH (dietary approaches to stop hypertension) eating plan, US Department of Agriculture Food Patterns, or the American Heart Association’s diet.
• Establish a dietary plan that also incorporates nutritional requirements for an existing comorbidity, such as type 2 diabetes mellitus (T2DM).
• Lower saturated-fat intake to 5% to 6% of total calories, and reduce trans fats.
• Advise patients with high BP to reduce sodium consumption to ≤2400 mg/d; or, at the very least, to reduce daily consumption by 1000 mg.
• Promote aerobic activity to reduce either LDL-C or BP, at moderate or vigorous intensity 3 to 4 times a week with 40-minute sessions.
CVD risk assessment
The CVD risk assessment guideline3 has generated a lot of controversy. It proposes a new tool for assessing an individual’s 10-year risk of developing an atherosclerotic cardiovascular disease (ASCVD) event, defined as a fatal or nonfatal heart attack or stroke. While the tool is new, the risk factor categories it uses have been known for decades: age, gender, race, lipid levels, diabetes, smoking status, and BP. It has not performed better in validation studies than other existing tools (all of which are suboptimal), and it may be worse.11,12 Moreover, this new tool has been tested only in African Americans and non-Hispanic whites. Using it could classify 33 million adults age 40 to 79 years as having a 10-year risk of 7.5%, and 13 million a risk between 5% and 7.5%.12 The significance of this is discussed in the next section on the management of high cholesterol levels.
Summary highlights:
• Use race- and sex-specific Pooled Cohort Equations to predict 10-year risk for a first hard ASCVD event (nonfatalmyocardial infarction, coronary death, or nonfatal or fatal stroke) in non-Hispanic African Americans and non-Hispanic Whites, 40 to 79 years of age.
• Consider assessing a patient’s family history, high-sensitivity C-reactive protein, coronary artery calcium, or anklebrachial index to help guide treatment decisions if quantitative risk assessment has led to uncertainty. (This recommendation is based on expert opinion.)
• Consider evaluating ASCVD risk factors every 4 to 6 years in individuals 20 to 79 years of age who do not have ASCVD, and calculating the 10-year risk of an ASCVD event in those 40 to 79 years of age.
• Consider evaluating 30-year or lifetime ASCVD risk using traditional risk factors in individuals 20 to 59 years of age who do not have ASCVD and have no high short-term risk. (This is based on low-level evidence.)
Cholesterol management
The guideline on lowering blood cholesterol4 is a significant departure from the previous one.6 It contains 54 recommendations, 21 based on expert opinion. Using an unusual methodology that considered only randomized controlled trials in the evidence report, the guideline panel stated that the evidence demonstrates that 4 groups will benefit from treatment with statins:
• patients with established ASCVD
• individuals whose LDL-C is ≥190 mg/dL
• patients with diabetes and no established ASCVD who are 40 to 75 years of age and have an LDL-C between 70 and 189 mg/dL
• anyone with an estimated 10-year ASCVD risk of ≥7.5% (based on the new risk-assessment tool) and an LDL-C of 70 to 189 mg/dL.
The major departure from the old guideline is an abandonment of “treating to target” that attempts to lower LDL-C to a specified level. The panel concluded that the evidence does not show any benefit in achieving a specified level of LDL-C and that this approach can lead to either over- or under-treatment. The proposed new approach is to use high-, moderate-, or low-intensity statin treatment based on a patient’s age and reason for treatment, and the dose that they can tolerate (TABLE 2).4
Absent any contraindications, high-intensity treatment is indicated for:
• patients ≤75 years old with established ASCVD
• patients with an LDL-C level ≥190mg/dL
• patients 40 to 75 years old with diabetes and a ≥7.5% 10-year risk of ASCVD. z
Moderate-intensity treatment is indicated for those who cannot tolerate a high-intensity regimen, and for those ages 40 to 75 with diabetes and <7.5% 10-year ASCVD risk.
Low-intensity treatment is recommended for those who should receive moderate-intensity treatment but cannot tolerate it.
For those >75 years of age, the guideline makes only 2 recommendations:
• Prescribe a statin at the highest tolerable intensity for an LDL-C ≥190mg/dL.
• Assess those with established ASCVD for potential benefits and risks of moderate to high-intensity statin treatment. (It is reasonable to continue statin therapy for those already on it and tolerating it.)
Value of nonstatin drugs is questionable. In another significant departure from the previous guideline, the panel said that other cholesterol-lowering drugs can be considered when LDL-C remains high after statin treatment, but the benefit of these agents in preventing ASCVD is not proven.
Several objections to the new guideline have been raised in the short time since its release. Criticisms center on the large number of adults who would now qualify for statin treatment based on the new risk-assessment tool. Using the 7.5% 10-year risk cutoff, the number needed to treat to prevent one ASCVD event over 10 years would be 67. Also of concern to many is the fact that 7 out of 16 members of the guideline panel had financial ties to the pharmaceutical industry.12
Commentary
The new guidelines reflect a more rigorous evidence-based approach than those of the past. That some of them diverge significantly from previous recommendations that relied heavily on expert opinion reveals the pitfalls of making authoritative recommendations based on weak evidence. Such recommendations, especially those emerging from the National Institutes of Health, are used as national and international standards and serve as the basis of performance measures. When they do not stand the test of time because of a weak evidence base, medicine’s reputation is damaged. Notably, the new set of cholesterol recommendations, while an improvement from an evidentiary perspective, is founded partly on a questionable risk-assessment tool, and it is possible it will suffer the same long-term fate as its predecessor. (For more on these guidelines, see “The new cholesterol guideline: Beyond the headlines,” [J Fam Pract. 2013;62:730.])
1. Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS Guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2013 Nov 12. [Epub ahead of print].
2. Eckel RH, Jakicic JM, Ard JD, et al. 2013 AHA/ACC Guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2013 Nov 12. [Epub ahead of print].
3. Goff DC Jr, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA Guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2013 Nov 12. [Epub ahead of print].
4. Stone NJ, Robinson J, Lichtenstein AH, et al. 2013 ACC/AHA Guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2013 Nov 12. [Epub ahead of print].
5. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143-3421.
6. Chobanian AV, Bakris GL, Black HR, et al; Joint National Committee on Prevention Detection, Evaluation, and Treatment of High Blood Pressure. National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC 7 report. JAMA. 2003;289:2560-2572.
7. Clinical guidelines on the identification, evaluation and treatment of overweight and obesity in adults—the evidence report. National Institutes of Health. Obes Res. 1998;6(Suppl 2):51S-209S.
8. Institute of Medicine. Clinical Practice Guidelines we can trust. Washington, DC: National Academy of Sciences; 2011.
9. James PA, Oparil S, Carter BL, et al. 2014 Evidence-based guideline for the management of high blood pressure in adults. Report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2013 Dec 18. [Epub ahead of print].
10. Institute of Medicine. Sodium intake in populations: assessment of evidence. Washington, DC: National Academy of Sciences; 2013.
11. Siontis GC, Tzoulaki I, Siontis KC, et al. Comparisons of established risk prediction models for cardiovascular disease: systematic review. BMJ. 2012;344:e3318.
12. Ioannidis JP. More than a billion people taking statins? Potential political implications of the new cardiovascular guidelines. JAMA. 2013 Dec 2. [Epub ahead of print].
A significant milestone in evidence-based practice was reached in November 2013, when the American Heart Association and American College of Cardiology (AHA/ACC) published 4 clinical practice guidelines on the prevention of cardiovascular disease.1-4 These guidelines—on obesity, lifestyle management, cardiovascular disease (CVD) risk assessment, and cholesterol—were developed under the auspices of the National Heart, Lung, and Blood Institute (NHLBI) to update its prior guidelines on the treatment of hypertension, high cholesterol, and obesity that were published more than a decade ago.5-7 After the NHLBI had organized the respective guideline panels and progressed through most of the guideline development process (which lasted several years each), it arranged for the AHA/ACC to assume sponsorship and publication of the guidelines. The NHLBI decided its role should be to develop evidence reports, leaving the development of guidelines to professional organizations.
While the prior guidelines on hypertension and hypercholesterolemia were influential and widely cited as the standard of care, they were heavily influenced by expert opinion and were not strictly evidence based. The NHLBI sought to develop the new guidelines using more contemporary and rigorous evidence-based processes to meet standards set by the Institute of Medicine (IOM). The group started with key clinical questions, conducted comprehensive systematic reviews of the evidence, and then rated the quality of the evidence and assigned strength of recommendation ratings.8 The guidelines and evidence reports are lengthy, and are summarized below.
In December 2013, the Eighth Joint National Committee (the 5th panel organized by the NHLBI to address CVD prevention) published its updated guideline on the treatment of hypertension, which has also generated controversy. Visit www.jfponline.com to listen to an audiocast summary of these recommendations.9
Obesity and overweight
The guideline on managing obesity and overweight adults has 17 recommendations, only 3 of which are based on expert opinion.1 (TABLE 1 summarizes the strong [A] and moderate [B] recommendations.) The recommendations stress screening, diagnosis, and treatment using diet, exercise, and lifestyle modification. They also address bariatric surgery for those with a body mass index (BMI) ≥40 or a persisting BMI ≥35 despite weight loss interventions. This set of recommendations, like those of the United States Preventive Services Task Force, advises intensive interventions for weight management and additionally offers much more detail on recommended diet and exercise.
Lifestyle management
The 10 recommendations on lifestyle management to reduce cardiovascular risk, all evidence based, are limited to diet and exercise as a means to control hypertension and hypercholesterolemia.2 They do not cover other important lifestyle modifications for preventing CVD, such as smoking cessation. The guideline panel acknowledged that the interventions are aimed at those with high blood pressure and elevated cholesterol, but they encourage all adults to follow them. Although these recommendations are not particularly controversial, the 2 recommendations to reduce sodium intake are said to be based on strong or moderate strength evidence, in contrast to a recent IOM report that concluded evidence for the health benefits of salt intake <2.3 g/d is weak.10 This illustrates how separate authoritative groups can rate the strength of the same evidence differently.
Summary highlights:
• Encourage adults who would benefit from lowering either blood pressure (BP) or low-density lipoprotein cholesterol (LDL-C) to eat a diet that emphasizes vegetables, fruits, whole grains, low-fat dairy products, and other notably healthful foods, and to cut down on products high in sugar content and on red meats.
• Review, as appropriate, such options as the DASH (dietary approaches to stop hypertension) eating plan, US Department of Agriculture Food Patterns, or the American Heart Association’s diet.
• Establish a dietary plan that also incorporates nutritional requirements for an existing comorbidity, such as type 2 diabetes mellitus (T2DM).
• Lower saturated-fat intake to 5% to 6% of total calories, and reduce trans fats.
• Advise patients with high BP to reduce sodium consumption to ≤2400 mg/d; or, at the very least, to reduce daily consumption by 1000 mg.
• Promote aerobic activity to reduce either LDL-C or BP, at moderate or vigorous intensity 3 to 4 times a week with 40-minute sessions.
CVD risk assessment
The CVD risk assessment guideline3 has generated a lot of controversy. It proposes a new tool for assessing an individual’s 10-year risk of developing an atherosclerotic cardiovascular disease (ASCVD) event, defined as a fatal or nonfatal heart attack or stroke. While the tool is new, the risk factor categories it uses have been known for decades: age, gender, race, lipid levels, diabetes, smoking status, and BP. It has not performed better in validation studies than other existing tools (all of which are suboptimal), and it may be worse.11,12 Moreover, this new tool has been tested only in African Americans and non-Hispanic whites. Using it could classify 33 million adults age 40 to 79 years as having a 10-year risk of 7.5%, and 13 million a risk between 5% and 7.5%.12 The significance of this is discussed in the next section on the management of high cholesterol levels.
Summary highlights:
• Use race- and sex-specific Pooled Cohort Equations to predict 10-year risk for a first hard ASCVD event (nonfatalmyocardial infarction, coronary death, or nonfatal or fatal stroke) in non-Hispanic African Americans and non-Hispanic Whites, 40 to 79 years of age.
• Consider assessing a patient’s family history, high-sensitivity C-reactive protein, coronary artery calcium, or anklebrachial index to help guide treatment decisions if quantitative risk assessment has led to uncertainty. (This recommendation is based on expert opinion.)
• Consider evaluating ASCVD risk factors every 4 to 6 years in individuals 20 to 79 years of age who do not have ASCVD, and calculating the 10-year risk of an ASCVD event in those 40 to 79 years of age.
• Consider evaluating 30-year or lifetime ASCVD risk using traditional risk factors in individuals 20 to 59 years of age who do not have ASCVD and have no high short-term risk. (This is based on low-level evidence.)
Cholesterol management
The guideline on lowering blood cholesterol4 is a significant departure from the previous one.6 It contains 54 recommendations, 21 based on expert opinion. Using an unusual methodology that considered only randomized controlled trials in the evidence report, the guideline panel stated that the evidence demonstrates that 4 groups will benefit from treatment with statins:
• patients with established ASCVD
• individuals whose LDL-C is ≥190 mg/dL
• patients with diabetes and no established ASCVD who are 40 to 75 years of age and have an LDL-C between 70 and 189 mg/dL
• anyone with an estimated 10-year ASCVD risk of ≥7.5% (based on the new risk-assessment tool) and an LDL-C of 70 to 189 mg/dL.
The major departure from the old guideline is an abandonment of “treating to target” that attempts to lower LDL-C to a specified level. The panel concluded that the evidence does not show any benefit in achieving a specified level of LDL-C and that this approach can lead to either over- or under-treatment. The proposed new approach is to use high-, moderate-, or low-intensity statin treatment based on a patient’s age and reason for treatment, and the dose that they can tolerate (TABLE 2).4
Absent any contraindications, high-intensity treatment is indicated for:
• patients ≤75 years old with established ASCVD
• patients with an LDL-C level ≥190mg/dL
• patients 40 to 75 years old with diabetes and a ≥7.5% 10-year risk of ASCVD. z
Moderate-intensity treatment is indicated for those who cannot tolerate a high-intensity regimen, and for those ages 40 to 75 with diabetes and <7.5% 10-year ASCVD risk.
Low-intensity treatment is recommended for those who should receive moderate-intensity treatment but cannot tolerate it.
For those >75 years of age, the guideline makes only 2 recommendations:
• Prescribe a statin at the highest tolerable intensity for an LDL-C ≥190mg/dL.
• Assess those with established ASCVD for potential benefits and risks of moderate to high-intensity statin treatment. (It is reasonable to continue statin therapy for those already on it and tolerating it.)
Value of nonstatin drugs is questionable. In another significant departure from the previous guideline, the panel said that other cholesterol-lowering drugs can be considered when LDL-C remains high after statin treatment, but the benefit of these agents in preventing ASCVD is not proven.
Several objections to the new guideline have been raised in the short time since its release. Criticisms center on the large number of adults who would now qualify for statin treatment based on the new risk-assessment tool. Using the 7.5% 10-year risk cutoff, the number needed to treat to prevent one ASCVD event over 10 years would be 67. Also of concern to many is the fact that 7 out of 16 members of the guideline panel had financial ties to the pharmaceutical industry.12
Commentary
The new guidelines reflect a more rigorous evidence-based approach than those of the past. That some of them diverge significantly from previous recommendations that relied heavily on expert opinion reveals the pitfalls of making authoritative recommendations based on weak evidence. Such recommendations, especially those emerging from the National Institutes of Health, are used as national and international standards and serve as the basis of performance measures. When they do not stand the test of time because of a weak evidence base, medicine’s reputation is damaged. Notably, the new set of cholesterol recommendations, while an improvement from an evidentiary perspective, is founded partly on a questionable risk-assessment tool, and it is possible it will suffer the same long-term fate as its predecessor. (For more on these guidelines, see “The new cholesterol guideline: Beyond the headlines,” [J Fam Pract. 2013;62:730.])
A significant milestone in evidence-based practice was reached in November 2013, when the American Heart Association and American College of Cardiology (AHA/ACC) published 4 clinical practice guidelines on the prevention of cardiovascular disease.1-4 These guidelines—on obesity, lifestyle management, cardiovascular disease (CVD) risk assessment, and cholesterol—were developed under the auspices of the National Heart, Lung, and Blood Institute (NHLBI) to update its prior guidelines on the treatment of hypertension, high cholesterol, and obesity that were published more than a decade ago.5-7 After the NHLBI had organized the respective guideline panels and progressed through most of the guideline development process (which lasted several years each), it arranged for the AHA/ACC to assume sponsorship and publication of the guidelines. The NHLBI decided its role should be to develop evidence reports, leaving the development of guidelines to professional organizations.
While the prior guidelines on hypertension and hypercholesterolemia were influential and widely cited as the standard of care, they were heavily influenced by expert opinion and were not strictly evidence based. The NHLBI sought to develop the new guidelines using more contemporary and rigorous evidence-based processes to meet standards set by the Institute of Medicine (IOM). The group started with key clinical questions, conducted comprehensive systematic reviews of the evidence, and then rated the quality of the evidence and assigned strength of recommendation ratings.8 The guidelines and evidence reports are lengthy, and are summarized below.
In December 2013, the Eighth Joint National Committee (the 5th panel organized by the NHLBI to address CVD prevention) published its updated guideline on the treatment of hypertension, which has also generated controversy. Visit www.jfponline.com to listen to an audiocast summary of these recommendations.9
Obesity and overweight
The guideline on managing obesity and overweight adults has 17 recommendations, only 3 of which are based on expert opinion.1 (TABLE 1 summarizes the strong [A] and moderate [B] recommendations.) The recommendations stress screening, diagnosis, and treatment using diet, exercise, and lifestyle modification. They also address bariatric surgery for those with a body mass index (BMI) ≥40 or a persisting BMI ≥35 despite weight loss interventions. This set of recommendations, like those of the United States Preventive Services Task Force, advises intensive interventions for weight management and additionally offers much more detail on recommended diet and exercise.
Lifestyle management
The 10 recommendations on lifestyle management to reduce cardiovascular risk, all evidence based, are limited to diet and exercise as a means to control hypertension and hypercholesterolemia.2 They do not cover other important lifestyle modifications for preventing CVD, such as smoking cessation. The guideline panel acknowledged that the interventions are aimed at those with high blood pressure and elevated cholesterol, but they encourage all adults to follow them. Although these recommendations are not particularly controversial, the 2 recommendations to reduce sodium intake are said to be based on strong or moderate strength evidence, in contrast to a recent IOM report that concluded evidence for the health benefits of salt intake <2.3 g/d is weak.10 This illustrates how separate authoritative groups can rate the strength of the same evidence differently.
Summary highlights:
• Encourage adults who would benefit from lowering either blood pressure (BP) or low-density lipoprotein cholesterol (LDL-C) to eat a diet that emphasizes vegetables, fruits, whole grains, low-fat dairy products, and other notably healthful foods, and to cut down on products high in sugar content and on red meats.
• Review, as appropriate, such options as the DASH (dietary approaches to stop hypertension) eating plan, US Department of Agriculture Food Patterns, or the American Heart Association’s diet.
• Establish a dietary plan that also incorporates nutritional requirements for an existing comorbidity, such as type 2 diabetes mellitus (T2DM).
• Lower saturated-fat intake to 5% to 6% of total calories, and reduce trans fats.
• Advise patients with high BP to reduce sodium consumption to ≤2400 mg/d; or, at the very least, to reduce daily consumption by 1000 mg.
• Promote aerobic activity to reduce either LDL-C or BP, at moderate or vigorous intensity 3 to 4 times a week with 40-minute sessions.
CVD risk assessment
The CVD risk assessment guideline3 has generated a lot of controversy. It proposes a new tool for assessing an individual’s 10-year risk of developing an atherosclerotic cardiovascular disease (ASCVD) event, defined as a fatal or nonfatal heart attack or stroke. While the tool is new, the risk factor categories it uses have been known for decades: age, gender, race, lipid levels, diabetes, smoking status, and BP. It has not performed better in validation studies than other existing tools (all of which are suboptimal), and it may be worse.11,12 Moreover, this new tool has been tested only in African Americans and non-Hispanic whites. Using it could classify 33 million adults age 40 to 79 years as having a 10-year risk of 7.5%, and 13 million a risk between 5% and 7.5%.12 The significance of this is discussed in the next section on the management of high cholesterol levels.
Summary highlights:
• Use race- and sex-specific Pooled Cohort Equations to predict 10-year risk for a first hard ASCVD event (nonfatalmyocardial infarction, coronary death, or nonfatal or fatal stroke) in non-Hispanic African Americans and non-Hispanic Whites, 40 to 79 years of age.
• Consider assessing a patient’s family history, high-sensitivity C-reactive protein, coronary artery calcium, or anklebrachial index to help guide treatment decisions if quantitative risk assessment has led to uncertainty. (This recommendation is based on expert opinion.)
• Consider evaluating ASCVD risk factors every 4 to 6 years in individuals 20 to 79 years of age who do not have ASCVD, and calculating the 10-year risk of an ASCVD event in those 40 to 79 years of age.
• Consider evaluating 30-year or lifetime ASCVD risk using traditional risk factors in individuals 20 to 59 years of age who do not have ASCVD and have no high short-term risk. (This is based on low-level evidence.)
Cholesterol management
The guideline on lowering blood cholesterol4 is a significant departure from the previous one.6 It contains 54 recommendations, 21 based on expert opinion. Using an unusual methodology that considered only randomized controlled trials in the evidence report, the guideline panel stated that the evidence demonstrates that 4 groups will benefit from treatment with statins:
• patients with established ASCVD
• individuals whose LDL-C is ≥190 mg/dL
• patients with diabetes and no established ASCVD who are 40 to 75 years of age and have an LDL-C between 70 and 189 mg/dL
• anyone with an estimated 10-year ASCVD risk of ≥7.5% (based on the new risk-assessment tool) and an LDL-C of 70 to 189 mg/dL.
The major departure from the old guideline is an abandonment of “treating to target” that attempts to lower LDL-C to a specified level. The panel concluded that the evidence does not show any benefit in achieving a specified level of LDL-C and that this approach can lead to either over- or under-treatment. The proposed new approach is to use high-, moderate-, or low-intensity statin treatment based on a patient’s age and reason for treatment, and the dose that they can tolerate (TABLE 2).4
Absent any contraindications, high-intensity treatment is indicated for:
• patients ≤75 years old with established ASCVD
• patients with an LDL-C level ≥190mg/dL
• patients 40 to 75 years old with diabetes and a ≥7.5% 10-year risk of ASCVD. z
Moderate-intensity treatment is indicated for those who cannot tolerate a high-intensity regimen, and for those ages 40 to 75 with diabetes and <7.5% 10-year ASCVD risk.
Low-intensity treatment is recommended for those who should receive moderate-intensity treatment but cannot tolerate it.
For those >75 years of age, the guideline makes only 2 recommendations:
• Prescribe a statin at the highest tolerable intensity for an LDL-C ≥190mg/dL.
• Assess those with established ASCVD for potential benefits and risks of moderate to high-intensity statin treatment. (It is reasonable to continue statin therapy for those already on it and tolerating it.)
Value of nonstatin drugs is questionable. In another significant departure from the previous guideline, the panel said that other cholesterol-lowering drugs can be considered when LDL-C remains high after statin treatment, but the benefit of these agents in preventing ASCVD is not proven.
Several objections to the new guideline have been raised in the short time since its release. Criticisms center on the large number of adults who would now qualify for statin treatment based on the new risk-assessment tool. Using the 7.5% 10-year risk cutoff, the number needed to treat to prevent one ASCVD event over 10 years would be 67. Also of concern to many is the fact that 7 out of 16 members of the guideline panel had financial ties to the pharmaceutical industry.12
Commentary
The new guidelines reflect a more rigorous evidence-based approach than those of the past. That some of them diverge significantly from previous recommendations that relied heavily on expert opinion reveals the pitfalls of making authoritative recommendations based on weak evidence. Such recommendations, especially those emerging from the National Institutes of Health, are used as national and international standards and serve as the basis of performance measures. When they do not stand the test of time because of a weak evidence base, medicine’s reputation is damaged. Notably, the new set of cholesterol recommendations, while an improvement from an evidentiary perspective, is founded partly on a questionable risk-assessment tool, and it is possible it will suffer the same long-term fate as its predecessor. (For more on these guidelines, see “The new cholesterol guideline: Beyond the headlines,” [J Fam Pract. 2013;62:730.])
1. Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS Guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2013 Nov 12. [Epub ahead of print].
2. Eckel RH, Jakicic JM, Ard JD, et al. 2013 AHA/ACC Guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2013 Nov 12. [Epub ahead of print].
3. Goff DC Jr, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA Guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2013 Nov 12. [Epub ahead of print].
4. Stone NJ, Robinson J, Lichtenstein AH, et al. 2013 ACC/AHA Guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2013 Nov 12. [Epub ahead of print].
5. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143-3421.
6. Chobanian AV, Bakris GL, Black HR, et al; Joint National Committee on Prevention Detection, Evaluation, and Treatment of High Blood Pressure. National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC 7 report. JAMA. 2003;289:2560-2572.
7. Clinical guidelines on the identification, evaluation and treatment of overweight and obesity in adults—the evidence report. National Institutes of Health. Obes Res. 1998;6(Suppl 2):51S-209S.
8. Institute of Medicine. Clinical Practice Guidelines we can trust. Washington, DC: National Academy of Sciences; 2011.
9. James PA, Oparil S, Carter BL, et al. 2014 Evidence-based guideline for the management of high blood pressure in adults. Report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2013 Dec 18. [Epub ahead of print].
10. Institute of Medicine. Sodium intake in populations: assessment of evidence. Washington, DC: National Academy of Sciences; 2013.
11. Siontis GC, Tzoulaki I, Siontis KC, et al. Comparisons of established risk prediction models for cardiovascular disease: systematic review. BMJ. 2012;344:e3318.
12. Ioannidis JP. More than a billion people taking statins? Potential political implications of the new cardiovascular guidelines. JAMA. 2013 Dec 2. [Epub ahead of print].
1. Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS Guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2013 Nov 12. [Epub ahead of print].
2. Eckel RH, Jakicic JM, Ard JD, et al. 2013 AHA/ACC Guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2013 Nov 12. [Epub ahead of print].
3. Goff DC Jr, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA Guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2013 Nov 12. [Epub ahead of print].
4. Stone NJ, Robinson J, Lichtenstein AH, et al. 2013 ACC/AHA Guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2013 Nov 12. [Epub ahead of print].
5. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143-3421.
6. Chobanian AV, Bakris GL, Black HR, et al; Joint National Committee on Prevention Detection, Evaluation, and Treatment of High Blood Pressure. National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC 7 report. JAMA. 2003;289:2560-2572.
7. Clinical guidelines on the identification, evaluation and treatment of overweight and obesity in adults—the evidence report. National Institutes of Health. Obes Res. 1998;6(Suppl 2):51S-209S.
8. Institute of Medicine. Clinical Practice Guidelines we can trust. Washington, DC: National Academy of Sciences; 2011.
9. James PA, Oparil S, Carter BL, et al. 2014 Evidence-based guideline for the management of high blood pressure in adults. Report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2013 Dec 18. [Epub ahead of print].
10. Institute of Medicine. Sodium intake in populations: assessment of evidence. Washington, DC: National Academy of Sciences; 2013.
11. Siontis GC, Tzoulaki I, Siontis KC, et al. Comparisons of established risk prediction models for cardiovascular disease: systematic review. BMJ. 2012;344:e3318.
12. Ioannidis JP. More than a billion people taking statins? Potential political implications of the new cardiovascular guidelines. JAMA. 2013 Dec 2. [Epub ahead of print].
Lung cancer screening: USPSTF revises its recommendation
The US Preventive Services Task Force (USPSTF) recently released a draft recommendation on lung cancer screen- ing, advising annual screening with low-dose computed tomography (LDCT) for individuals at high risk for lung cancer based on age and smoking history. Once finalized, this recommendation will replace its “I” rating, which indicated that evidence was insufficient to recommend for or against screening for lung cancer.
While the wording of the new recommendation is nonspecific regarding who should be screened, the Task Force elaborates in its follow-on commentary: Screening should start at age 55 and continue through age 79 for those who have ≥30 pack-year history of smoking and are either current smokers or past smokers who quit <15 years earlier.1 The draft recommendation advises caution in screening those with significant comorbidities, as well as individuals in their late 70s. Examples of how these specifications would work in practice are included in TABLE 1.
Lung cancer epidemiology
Lung cancer is the second most common cancer in both men and women and the leading cause of cancer deaths in the United States, accounting for more than 158,000 deaths in 2010.2 Lung cancer is highly lethal, with >90% mortality rate and a 5-year survival rate <20%.1 However, non-small cell lung cancer (NSCLC), which can be cured with surgical resection if caught early, is responsible for 80% of cases.3 The incidence of lung cancer increases markedly after age 50, with >80% of cases occurring in those 60 years or older.3
Smoking causes >90% of lung cancers,2 which are preventable with avoidance of smoking and smoking cessation programs. Currently, 19% of Americans smoke and 37% are current or former smokers.1
Evidence report
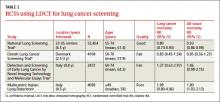
The systematic review4 that the new draft rec- ommendation was based on found 4 clinical trials of LDCT screening that met inclusion criteria (TABLE 2). One, the National Lung Screening Trial (NLST), was a large study involving 33 centers in the United States and 53,454 current and former smokers ages 55 to 74 years. Participants had a mean age of 61.4 years and ≥30 pack-year history of smoking, with a mean of 56 pack-years.5
The study population was relatively young and healthy; only 8.8% of participants were older than 70. The researchers excluded anyone with a significant comorbidity that would make it unlikely that they would undergo surgery if cancer were detected.
Participants were randomized to either LDCT or chest x-ray, given 3 annual screens, and followed for a mean of 6.5 years. In the LDCT group, there was a 20% reduction in lung cancer mortality and a 7% decrease in overall mortality. This translates to a number needed to screen (NNS) of 320 to prevent one lung cancer death, which compares favorably with other cancer screening tests. Mammography has an NNS of about 1339 for women ages 50 to 59, for example, and colon cancer screening using flexible sigmoidoscopy has an NNS of 817 among individuals ages 55 to 74 years.4
The other 3 studies in the systematic review were conducted in other countries, and were smaller, of shorter duration, and of lower quality.6-8 None demonstrated a reduction in either lung cancer or all-cause mortality, and one showed a small increase in all-cause mortality.8 A Forest plot of all 4 studies raises questions about the significance of the decline in all-cause or lung-cancer mortality.4 However, a meta-analysis that deletes the one poor quality study did demonstrate a 19% decrease in lung cancer mortality, but no decline in all- cause mortality.9
The evidence report included an assessment of 15 studies on the accuracy of LDCT screening. Sensitivity varied from 80% to 100% and specificity ranged from 28% to 100%. The positive predictive value (PPV) for lung cancer ranged from 2% to 42%; however, most abnormal findings resolved with further imaging. As a result, the PPV for those who had a biopsy or surgery after retesting was 50% to 92%.4
Potential harms in the recommendation
Radiation exposure from an LDCT is slightly greater than that of a mammogram. The long-term effects of annual LDCT plus follow-up of abnormal findings is not fully known. There is some concern about the potential for lung cancer screening to have a negative effect on smoking cessation efforts. However, evidence suggests that the use of LDCT as a lung cancer screening tool has no influence on smoking cessation.4
Extrapolating results. The NLST was a well-controlled trial conducted at academic health centers, with strict procedures for conservative follow-up of suspicious lesions. A potential for harm exists in extrapolating results from such a study to the community at large, where work-ups may be more aggressive and include biopsy.
Overdiagnosis. Routine LDCT will likely result in some degree of overdiagnosis—eg, detection of low-grade cancers that would either regress on their own or simply not progress—and overtreatment, with the potential for complications.
Full impact is unknown
The ultimate balance of benefits and harms of the USPSTF’s lung cancer screening draft recommendation rests on some unknowns. Widespread screening is unlikely to achieve the same results as did the NLST. As already noted, those enrolled in the NLST were relatively young and had large pack-year smoking histories. The Task Force acknowledges that the 20% reduction in lung cancer mortality achieved in the NLST is unlikely to be duplicated in older patients and individuals with less significant smoking histories. Additional harms will likely accrue if suspicious findings are more aggressively pursued than they were in this study. The potential harms, as well as benefits, from incidental findings on chest LDCT scans are also unknown.
The number of screenings. The potential for benefits beyond 3 screenings is also unknown, as the USPSTF’s projections in such cases are based on modeling. The degree of overdiagnosis is not fully understood, nor is the harm that could result from the accumulated radiation of what could be an annual LDCT for 25 years. The harm/benefit ratio will become clearer with time and can then be compared with other medical interventions.
Financial burden. While it may appear to some that the draft recommendation would unfairly benefit smokers by allowing them to undergo free annual CT screening, patients are likely to incur significant financial obligations as a result of doing so. The Affordable Care Act mandates that the annual LDCT screening would have to be offered with no patient cost sharing, but follow-up CTs for questionable findings, biopsies, and treatment will all be subject to deductibles and copayments.
Recommendations of others
Other organizations have adopted recommendations on lung cancer screening similar to the USPSTF proposal. These include the American Association for Thoracic Surgery, American Cancer Society, American College of Chest Physicians, American Lung Association, American Society of Clinical Oncology, and American Thoracic Society. Most apply to those ages 55 to 74 years and use other inclusion criteria of the NLST. Some stipulate that patients should be in good enough health to benefit from early detection, and most include a reference to the quality of the centers at which screening should occur. The American Academy of Family Physicians is currently considering what its recommendation on lung cancer screening will be.
Final USPSTF recommendation expected soon
Noticeably absent from the news coverage of the proposed USPSTF recommendation was the word “draft.” The Task Force has now collected public comments about its proposed recommendation and will be considering potential changes to the wording. Publication of the final recommendation is expected in December—shortly after press time.
1. Screening for Lung Cancer: US Preventive Services Task Force Recommendation Statement Draft. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservices- taskforce.org/uspstf13/lungcan/lungcandraftrec.htm. Accessed October 2, 2013.
2. Lung Cancer Statistics. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/cancer/lung/ statistics/. Updated October 23, 2013. Accessed November 15, 2013.
3. Lung Cancer Fact Sheet. American Lung Association Web site. Available at: http://www.lung.org/lung-disease/lung-cancer/resources/facts-figures/lung-cancer-fact-sheet.html#Prevalence_ and_Incidence. Accessed October 2, 2013.
4. Humphrey LL, Deffeback M, Pappas M, et al. Screening for lung cancer using low-dose computed tomography. a systematic review to update the US Preventive Services Task Force Recom- mendation. Ann Intern Med. 2013;159:411-420.
5. National Lung Screening Trial Research Team; Aberle DR, Adams AM, Berg CD, et al. Reduced lung cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395-409.
6. Saghir Z, Dirksen A, Ashraf H, et al. CT screening for lung cancer brings forward early disease. The randomised Danish Lung Cancer Screening Trial: status after five annual screening rounds with low-dose CT. Thorax. 2012;67:296-301.
7. Infante M, Cavuto S, Lutman FR, et al; DANTE Study Group. A randomized study of lung cancer screening with spiral computed tomography: three-year results from the DANTE trial. Am J Respir Crit Care Med. 2009;180:445-453.
8. Pastorino U, Rossi M, Rosato V, et al. Annual or biennial CT screening versus observation in heavy smokers: 5-year results of the MILD trial. Eur J Cancer Prev. 2012;21: 308-315.
9. Humphrey L, Deffebach M, Pappas M, et al. Screening for lung cancer: systematic review to update the US Preventive Services Task Force Recommendation. Evidence Synthesis No. 105. AHRQ Publication No. 13-05188-EF-1. Rockville, MD: Agency for Health- care Research and Quality; 2013. Available at: http://www.uspre- ventiveservicestaskforce.org/uspstf13/lungcan/lungcanes105. pdf. Accessed October 2, 2013.
The US Preventive Services Task Force (USPSTF) recently released a draft recommendation on lung cancer screen- ing, advising annual screening with low-dose computed tomography (LDCT) for individuals at high risk for lung cancer based on age and smoking history. Once finalized, this recommendation will replace its “I” rating, which indicated that evidence was insufficient to recommend for or against screening for lung cancer.
While the wording of the new recommendation is nonspecific regarding who should be screened, the Task Force elaborates in its follow-on commentary: Screening should start at age 55 and continue through age 79 for those who have ≥30 pack-year history of smoking and are either current smokers or past smokers who quit <15 years earlier.1 The draft recommendation advises caution in screening those with significant comorbidities, as well as individuals in their late 70s. Examples of how these specifications would work in practice are included in TABLE 1.
Lung cancer epidemiology
Lung cancer is the second most common cancer in both men and women and the leading cause of cancer deaths in the United States, accounting for more than 158,000 deaths in 2010.2 Lung cancer is highly lethal, with >90% mortality rate and a 5-year survival rate <20%.1 However, non-small cell lung cancer (NSCLC), which can be cured with surgical resection if caught early, is responsible for 80% of cases.3 The incidence of lung cancer increases markedly after age 50, with >80% of cases occurring in those 60 years or older.3
Smoking causes >90% of lung cancers,2 which are preventable with avoidance of smoking and smoking cessation programs. Currently, 19% of Americans smoke and 37% are current or former smokers.1
Evidence report
The systematic review4 that the new draft rec- ommendation was based on found 4 clinical trials of LDCT screening that met inclusion criteria (TABLE 2). One, the National Lung Screening Trial (NLST), was a large study involving 33 centers in the United States and 53,454 current and former smokers ages 55 to 74 years. Participants had a mean age of 61.4 years and ≥30 pack-year history of smoking, with a mean of 56 pack-years.5
The study population was relatively young and healthy; only 8.8% of participants were older than 70. The researchers excluded anyone with a significant comorbidity that would make it unlikely that they would undergo surgery if cancer were detected.
Participants were randomized to either LDCT or chest x-ray, given 3 annual screens, and followed for a mean of 6.5 years. In the LDCT group, there was a 20% reduction in lung cancer mortality and a 7% decrease in overall mortality. This translates to a number needed to screen (NNS) of 320 to prevent one lung cancer death, which compares favorably with other cancer screening tests. Mammography has an NNS of about 1339 for women ages 50 to 59, for example, and colon cancer screening using flexible sigmoidoscopy has an NNS of 817 among individuals ages 55 to 74 years.4
The other 3 studies in the systematic review were conducted in other countries, and were smaller, of shorter duration, and of lower quality.6-8 None demonstrated a reduction in either lung cancer or all-cause mortality, and one showed a small increase in all-cause mortality.8 A Forest plot of all 4 studies raises questions about the significance of the decline in all-cause or lung-cancer mortality.4 However, a meta-analysis that deletes the one poor quality study did demonstrate a 19% decrease in lung cancer mortality, but no decline in all- cause mortality.9
The evidence report included an assessment of 15 studies on the accuracy of LDCT screening. Sensitivity varied from 80% to 100% and specificity ranged from 28% to 100%. The positive predictive value (PPV) for lung cancer ranged from 2% to 42%; however, most abnormal findings resolved with further imaging. As a result, the PPV for those who had a biopsy or surgery after retesting was 50% to 92%.4
Potential harms in the recommendation
Radiation exposure from an LDCT is slightly greater than that of a mammogram. The long-term effects of annual LDCT plus follow-up of abnormal findings is not fully known. There is some concern about the potential for lung cancer screening to have a negative effect on smoking cessation efforts. However, evidence suggests that the use of LDCT as a lung cancer screening tool has no influence on smoking cessation.4
Extrapolating results. The NLST was a well-controlled trial conducted at academic health centers, with strict procedures for conservative follow-up of suspicious lesions. A potential for harm exists in extrapolating results from such a study to the community at large, where work-ups may be more aggressive and include biopsy.
Overdiagnosis. Routine LDCT will likely result in some degree of overdiagnosis—eg, detection of low-grade cancers that would either regress on their own or simply not progress—and overtreatment, with the potential for complications.
Full impact is unknown
The ultimate balance of benefits and harms of the USPSTF’s lung cancer screening draft recommendation rests on some unknowns. Widespread screening is unlikely to achieve the same results as did the NLST. As already noted, those enrolled in the NLST were relatively young and had large pack-year smoking histories. The Task Force acknowledges that the 20% reduction in lung cancer mortality achieved in the NLST is unlikely to be duplicated in older patients and individuals with less significant smoking histories. Additional harms will likely accrue if suspicious findings are more aggressively pursued than they were in this study. The potential harms, as well as benefits, from incidental findings on chest LDCT scans are also unknown.
The number of screenings. The potential for benefits beyond 3 screenings is also unknown, as the USPSTF’s projections in such cases are based on modeling. The degree of overdiagnosis is not fully understood, nor is the harm that could result from the accumulated radiation of what could be an annual LDCT for 25 years. The harm/benefit ratio will become clearer with time and can then be compared with other medical interventions.
Financial burden. While it may appear to some that the draft recommendation would unfairly benefit smokers by allowing them to undergo free annual CT screening, patients are likely to incur significant financial obligations as a result of doing so. The Affordable Care Act mandates that the annual LDCT screening would have to be offered with no patient cost sharing, but follow-up CTs for questionable findings, biopsies, and treatment will all be subject to deductibles and copayments.
Recommendations of others
Other organizations have adopted recommendations on lung cancer screening similar to the USPSTF proposal. These include the American Association for Thoracic Surgery, American Cancer Society, American College of Chest Physicians, American Lung Association, American Society of Clinical Oncology, and American Thoracic Society. Most apply to those ages 55 to 74 years and use other inclusion criteria of the NLST. Some stipulate that patients should be in good enough health to benefit from early detection, and most include a reference to the quality of the centers at which screening should occur. The American Academy of Family Physicians is currently considering what its recommendation on lung cancer screening will be.
Final USPSTF recommendation expected soon
Noticeably absent from the news coverage of the proposed USPSTF recommendation was the word “draft.” The Task Force has now collected public comments about its proposed recommendation and will be considering potential changes to the wording. Publication of the final recommendation is expected in December—shortly after press time.
The US Preventive Services Task Force (USPSTF) recently released a draft recommendation on lung cancer screen- ing, advising annual screening with low-dose computed tomography (LDCT) for individuals at high risk for lung cancer based on age and smoking history. Once finalized, this recommendation will replace its “I” rating, which indicated that evidence was insufficient to recommend for or against screening for lung cancer.
While the wording of the new recommendation is nonspecific regarding who should be screened, the Task Force elaborates in its follow-on commentary: Screening should start at age 55 and continue through age 79 for those who have ≥30 pack-year history of smoking and are either current smokers or past smokers who quit <15 years earlier.1 The draft recommendation advises caution in screening those with significant comorbidities, as well as individuals in their late 70s. Examples of how these specifications would work in practice are included in TABLE 1.
Lung cancer epidemiology
Lung cancer is the second most common cancer in both men and women and the leading cause of cancer deaths in the United States, accounting for more than 158,000 deaths in 2010.2 Lung cancer is highly lethal, with >90% mortality rate and a 5-year survival rate <20%.1 However, non-small cell lung cancer (NSCLC), which can be cured with surgical resection if caught early, is responsible for 80% of cases.3 The incidence of lung cancer increases markedly after age 50, with >80% of cases occurring in those 60 years or older.3
Smoking causes >90% of lung cancers,2 which are preventable with avoidance of smoking and smoking cessation programs. Currently, 19% of Americans smoke and 37% are current or former smokers.1
Evidence report
The systematic review4 that the new draft rec- ommendation was based on found 4 clinical trials of LDCT screening that met inclusion criteria (TABLE 2). One, the National Lung Screening Trial (NLST), was a large study involving 33 centers in the United States and 53,454 current and former smokers ages 55 to 74 years. Participants had a mean age of 61.4 years and ≥30 pack-year history of smoking, with a mean of 56 pack-years.5
The study population was relatively young and healthy; only 8.8% of participants were older than 70. The researchers excluded anyone with a significant comorbidity that would make it unlikely that they would undergo surgery if cancer were detected.
Participants were randomized to either LDCT or chest x-ray, given 3 annual screens, and followed for a mean of 6.5 years. In the LDCT group, there was a 20% reduction in lung cancer mortality and a 7% decrease in overall mortality. This translates to a number needed to screen (NNS) of 320 to prevent one lung cancer death, which compares favorably with other cancer screening tests. Mammography has an NNS of about 1339 for women ages 50 to 59, for example, and colon cancer screening using flexible sigmoidoscopy has an NNS of 817 among individuals ages 55 to 74 years.4
The other 3 studies in the systematic review were conducted in other countries, and were smaller, of shorter duration, and of lower quality.6-8 None demonstrated a reduction in either lung cancer or all-cause mortality, and one showed a small increase in all-cause mortality.8 A Forest plot of all 4 studies raises questions about the significance of the decline in all-cause or lung-cancer mortality.4 However, a meta-analysis that deletes the one poor quality study did demonstrate a 19% decrease in lung cancer mortality, but no decline in all- cause mortality.9
The evidence report included an assessment of 15 studies on the accuracy of LDCT screening. Sensitivity varied from 80% to 100% and specificity ranged from 28% to 100%. The positive predictive value (PPV) for lung cancer ranged from 2% to 42%; however, most abnormal findings resolved with further imaging. As a result, the PPV for those who had a biopsy or surgery after retesting was 50% to 92%.4
Potential harms in the recommendation
Radiation exposure from an LDCT is slightly greater than that of a mammogram. The long-term effects of annual LDCT plus follow-up of abnormal findings is not fully known. There is some concern about the potential for lung cancer screening to have a negative effect on smoking cessation efforts. However, evidence suggests that the use of LDCT as a lung cancer screening tool has no influence on smoking cessation.4
Extrapolating results. The NLST was a well-controlled trial conducted at academic health centers, with strict procedures for conservative follow-up of suspicious lesions. A potential for harm exists in extrapolating results from such a study to the community at large, where work-ups may be more aggressive and include biopsy.
Overdiagnosis. Routine LDCT will likely result in some degree of overdiagnosis—eg, detection of low-grade cancers that would either regress on their own or simply not progress—and overtreatment, with the potential for complications.
Full impact is unknown
The ultimate balance of benefits and harms of the USPSTF’s lung cancer screening draft recommendation rests on some unknowns. Widespread screening is unlikely to achieve the same results as did the NLST. As already noted, those enrolled in the NLST were relatively young and had large pack-year smoking histories. The Task Force acknowledges that the 20% reduction in lung cancer mortality achieved in the NLST is unlikely to be duplicated in older patients and individuals with less significant smoking histories. Additional harms will likely accrue if suspicious findings are more aggressively pursued than they were in this study. The potential harms, as well as benefits, from incidental findings on chest LDCT scans are also unknown.
The number of screenings. The potential for benefits beyond 3 screenings is also unknown, as the USPSTF’s projections in such cases are based on modeling. The degree of overdiagnosis is not fully understood, nor is the harm that could result from the accumulated radiation of what could be an annual LDCT for 25 years. The harm/benefit ratio will become clearer with time and can then be compared with other medical interventions.
Financial burden. While it may appear to some that the draft recommendation would unfairly benefit smokers by allowing them to undergo free annual CT screening, patients are likely to incur significant financial obligations as a result of doing so. The Affordable Care Act mandates that the annual LDCT screening would have to be offered with no patient cost sharing, but follow-up CTs for questionable findings, biopsies, and treatment will all be subject to deductibles and copayments.
Recommendations of others
Other organizations have adopted recommendations on lung cancer screening similar to the USPSTF proposal. These include the American Association for Thoracic Surgery, American Cancer Society, American College of Chest Physicians, American Lung Association, American Society of Clinical Oncology, and American Thoracic Society. Most apply to those ages 55 to 74 years and use other inclusion criteria of the NLST. Some stipulate that patients should be in good enough health to benefit from early detection, and most include a reference to the quality of the centers at which screening should occur. The American Academy of Family Physicians is currently considering what its recommendation on lung cancer screening will be.
Final USPSTF recommendation expected soon
Noticeably absent from the news coverage of the proposed USPSTF recommendation was the word “draft.” The Task Force has now collected public comments about its proposed recommendation and will be considering potential changes to the wording. Publication of the final recommendation is expected in December—shortly after press time.
1. Screening for Lung Cancer: US Preventive Services Task Force Recommendation Statement Draft. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservices- taskforce.org/uspstf13/lungcan/lungcandraftrec.htm. Accessed October 2, 2013.
2. Lung Cancer Statistics. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/cancer/lung/ statistics/. Updated October 23, 2013. Accessed November 15, 2013.
3. Lung Cancer Fact Sheet. American Lung Association Web site. Available at: http://www.lung.org/lung-disease/lung-cancer/resources/facts-figures/lung-cancer-fact-sheet.html#Prevalence_ and_Incidence. Accessed October 2, 2013.
4. Humphrey LL, Deffeback M, Pappas M, et al. Screening for lung cancer using low-dose computed tomography. a systematic review to update the US Preventive Services Task Force Recom- mendation. Ann Intern Med. 2013;159:411-420.
5. National Lung Screening Trial Research Team; Aberle DR, Adams AM, Berg CD, et al. Reduced lung cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395-409.
6. Saghir Z, Dirksen A, Ashraf H, et al. CT screening for lung cancer brings forward early disease. The randomised Danish Lung Cancer Screening Trial: status after five annual screening rounds with low-dose CT. Thorax. 2012;67:296-301.
7. Infante M, Cavuto S, Lutman FR, et al; DANTE Study Group. A randomized study of lung cancer screening with spiral computed tomography: three-year results from the DANTE trial. Am J Respir Crit Care Med. 2009;180:445-453.
8. Pastorino U, Rossi M, Rosato V, et al. Annual or biennial CT screening versus observation in heavy smokers: 5-year results of the MILD trial. Eur J Cancer Prev. 2012;21: 308-315.
9. Humphrey L, Deffebach M, Pappas M, et al. Screening for lung cancer: systematic review to update the US Preventive Services Task Force Recommendation. Evidence Synthesis No. 105. AHRQ Publication No. 13-05188-EF-1. Rockville, MD: Agency for Health- care Research and Quality; 2013. Available at: http://www.uspre- ventiveservicestaskforce.org/uspstf13/lungcan/lungcanes105. pdf. Accessed October 2, 2013.
1. Screening for Lung Cancer: US Preventive Services Task Force Recommendation Statement Draft. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservices- taskforce.org/uspstf13/lungcan/lungcandraftrec.htm. Accessed October 2, 2013.
2. Lung Cancer Statistics. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/cancer/lung/ statistics/. Updated October 23, 2013. Accessed November 15, 2013.
3. Lung Cancer Fact Sheet. American Lung Association Web site. Available at: http://www.lung.org/lung-disease/lung-cancer/resources/facts-figures/lung-cancer-fact-sheet.html#Prevalence_ and_Incidence. Accessed October 2, 2013.
4. Humphrey LL, Deffeback M, Pappas M, et al. Screening for lung cancer using low-dose computed tomography. a systematic review to update the US Preventive Services Task Force Recom- mendation. Ann Intern Med. 2013;159:411-420.
5. National Lung Screening Trial Research Team; Aberle DR, Adams AM, Berg CD, et al. Reduced lung cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395-409.
6. Saghir Z, Dirksen A, Ashraf H, et al. CT screening for lung cancer brings forward early disease. The randomised Danish Lung Cancer Screening Trial: status after five annual screening rounds with low-dose CT. Thorax. 2012;67:296-301.
7. Infante M, Cavuto S, Lutman FR, et al; DANTE Study Group. A randomized study of lung cancer screening with spiral computed tomography: three-year results from the DANTE trial. Am J Respir Crit Care Med. 2009;180:445-453.
8. Pastorino U, Rossi M, Rosato V, et al. Annual or biennial CT screening versus observation in heavy smokers: 5-year results of the MILD trial. Eur J Cancer Prev. 2012;21: 308-315.
9. Humphrey L, Deffebach M, Pappas M, et al. Screening for lung cancer: systematic review to update the US Preventive Services Task Force Recommendation. Evidence Synthesis No. 105. AHRQ Publication No. 13-05188-EF-1. Rockville, MD: Agency for Health- care Research and Quality; 2013. Available at: http://www.uspre- ventiveservicestaskforce.org/uspstf13/lungcan/lungcanes105. pdf. Accessed October 2, 2013.
Influenza: Update for the 2013-2014 season
Each year in late summer, the Centers for Disease Control and Prevention (CDC) publishes its recommendations for the prevention of influenza for the upcoming season. The severity of each influenza season varies and is difficult to predict, which underscores the need to provide maximal vaccine coverage for at-risk patient populations.
Hoping for the best, planning for the worst. Over the past several decades the annual number of influenza-related hospitalizations has varied from approximately 55,000 to 431,000,1 and the number of deaths from influenza has been as low as 3349 and as high as 48,614.2 Infection rates are usually highest in children. Complications, hospitalizations, and deaths are highest in those ≥65 years, children<2 years, and patients with medical conditions known to increase risk for influenza complications. Those at high risk of complications appear in TABLE 1.3 The main recommendations for this coming year are the same as last year, including vaccinating everyone ≥6 months of age without a contraindication, starting vaccinations as soon as vaccine is available, and continuing throughout the influenza season for those who need it.
What’s new this year
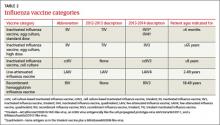
An increasing number of influenza vaccine products are available; although to date, their effectiveness (which was determined to be 56% for all vaccines used last influenza season) 4 remains below what we would hope for. The CDC’s recommendations address these new types of vaccines, including ones that have 4 antigens instead of 3, and use new terminology to describe the vaccines.3
New terminology reflects changing vaccine formulations. Last influenza season there were 2 major categories of influenza vaccines: live-attenuated influenza vaccine (LAIV) and trivalent inactivated influenza vaccine (TIV). All products were produced using egg-culture methods and contained 2 influenza A antigen subtypes and 1 B subtype. Several products this year include 4 antigens (2 A subtypes and 2 B subtypes), and some are now produced with non–eggculture methods. This has led to a new system of classification, with the term inactivated influenza vaccine (IIV) replacing TIV. TABLE 2 lists the influenza vaccine categories and abbreviations. TABLE 3 lists the contraindications for the different vaccine types.3
The new products include Flumist Quadrivalent (MedImmune), a quadrivalent LAIV (LAIV4); Fluarix Quadrivalent (GlaxoSmithKline), a quadrivalent IIV (IIV4); Flucelvax (Novartis Vaccines and Diagnostics), a cell culture-based trivalent IIV (ccIIV3); and FluBlok (Protein Sciences), a trivalent recombinant hemagglutinin influenza vaccine (RIV3). Fluzone (Sanofi Pasteur), introduced last season in a trivalent formulation, is also available this season as a quadrivalent IIV (IIV4). As a group, influenza vaccine products now offer 3 routes of administration: intramuscular, subcutaneous, and intranasal. There is currently no evidence that any route offers an advantage over another, and the CDC states no preference for any particular product or route of administration.
Mercury content is not a problem
Even though there is no scientific controversy over the safety of the mercury-containing preservative thimerosal, some patients still have doubts and may ask for a thimerosalfree product. The only influenza products that contain any thimerosal are those that come in multidose vials. A description of each influenza vaccine product, including thimerosal content, indicated ages, and routes of administration, can be found on the CDC’s Web site3 (http://www.cdc.gov/flu/professionals/acip/2013-summary-recommendations.htm).
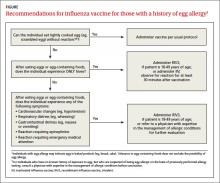
Options for those with egg allergy
There is now a product, RIV3 (FluBlok), that is manufactured without the use of eggs. It can be used in those 18 to 49 years of age with a history of egg allergy of any severity. Since 2011, the Advisory Committee on Immunization Practices (ACIP) has recommended that individuals with a history of mild egg allergy (those who experience only hives after egg exposure) may receive IIV, with additional safety precautions. Do not delay vaccination for these individuals if RIV is unavailable. Because of a lack of data demonstrating safety of LAIV for individuals with egg allergy, those allergic to eggs should receive RIV or IIV rather than LAIV.
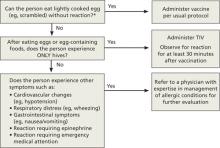
Though the new ccIIV product, Flucelvax, is manufactured without the use of eggs, the seed viruses used to create the vaccine have been processed in eggs. The egg protein content in the vaccine is extremely low (<50 femtograms [5 × 10-14 g] per 0.5-mL dose), but the CDC does not consider it egg free. The FIGURE depicts the recommendations for those with a history of egg allergy.3
Other interventions for influenza prevention
Vaccination is only one tool available to prevent morbidity and mortality from influenza. Antiviral chemoprevention and treatment, and infection control practices can also be effective.
Antiviral chemoprevention is available for both pre- and post-exposure administration. In the past few years, the CDC has de-emphasized such use of antivirals for these indications out of concern for the supply of these agents and for the possibility that their use might lead to increased rates of viral resistance. Consider antiviral chemoprevention for those who have conditions that place them at risk for complications, and for those who are unvaccinated if they are at high risk for exposure to influenza (preexposure prophylaxis) or have been exposed (postexposure prophylaxis), if the medication can be started within 48 hours of exposure. Another option for unvaccinated high-risk patients is vigilant symptom monitoring with early treatment for influenza symptoms. Chemoprophylaxis is recommended in addition to vaccination to control influenza outbreaks at institutions that house patients at high risk for complications of influenza. Details on recommended antivirals including doses and duration of treatment can be found in a 2011 issue of Morbidity and Mortality Weekly Report.5
Antiviral treatment. The CDC recommends antiviral treatment for anyone with suspected or confirmed influenza who has progressive, severe, or complicated illness or is hospitalized for their illness.5 Treatment is also recommended for outpatients with suspected or confirmed influenza who are at higher risk for influenza complications. This latter group includes those in TABLE 1, particularly children 6 to 59 months and adults ≥50 years. Start antiviral treatment within 48 hours of the first symptoms. For hospitalized patients, however, begin treatment at any point regardless of duration of illness.
Infection control practices can prevent the spread of influenza in the health care setting and in the homes of those with influenza. These practices are also described on the CDC influenza Web site.6
1. Thompson WW, Shay DK, Weintraub E. Influenza-associated hospitalizations in the United States. JAMA. 2004;292:1333-1340.
2. CDC. Estimates of deaths associated with seasonal influenza–United States, 1976-2007. MMWR Morb Mortal Wkly Rep. 2010;59:1057-1062.
3. CDC. Summary* recommendations: prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—(ACIP)—United States, 2013-14. Available at: http://www.cdc.gov/flu/professionals/ acip/2013-summary-recommendations.htm. Accessed August 9, 2013.
4. CDC. Interim adjusted estimates of seasonal influenza vaccine effectiveness—United States, February 2013. MMWR Morb Mortal Wkly Rep. 2013;62:119-123.
5. CDC. Antiviral agents for the treatment and chemoprophylaxis of influenza. MMWR Recomm Rep. 2011;60(RR01):1-24. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/rr6001a1. htm. Accessed July 2, 2013.
6. CDC. Infection control in health care facilities. Available at:http://www.cdc.gov/flu/professionals/infectioncontrol/index.htm. Accessed July 2, 2013.
Each year in late summer, the Centers for Disease Control and Prevention (CDC) publishes its recommendations for the prevention of influenza for the upcoming season. The severity of each influenza season varies and is difficult to predict, which underscores the need to provide maximal vaccine coverage for at-risk patient populations.
Hoping for the best, planning for the worst. Over the past several decades the annual number of influenza-related hospitalizations has varied from approximately 55,000 to 431,000,1 and the number of deaths from influenza has been as low as 3349 and as high as 48,614.2 Infection rates are usually highest in children. Complications, hospitalizations, and deaths are highest in those ≥65 years, children<2 years, and patients with medical conditions known to increase risk for influenza complications. Those at high risk of complications appear in TABLE 1.3 The main recommendations for this coming year are the same as last year, including vaccinating everyone ≥6 months of age without a contraindication, starting vaccinations as soon as vaccine is available, and continuing throughout the influenza season for those who need it.
What’s new this year
An increasing number of influenza vaccine products are available; although to date, their effectiveness (which was determined to be 56% for all vaccines used last influenza season) 4 remains below what we would hope for. The CDC’s recommendations address these new types of vaccines, including ones that have 4 antigens instead of 3, and use new terminology to describe the vaccines.3
New terminology reflects changing vaccine formulations. Last influenza season there were 2 major categories of influenza vaccines: live-attenuated influenza vaccine (LAIV) and trivalent inactivated influenza vaccine (TIV). All products were produced using egg-culture methods and contained 2 influenza A antigen subtypes and 1 B subtype. Several products this year include 4 antigens (2 A subtypes and 2 B subtypes), and some are now produced with non–eggculture methods. This has led to a new system of classification, with the term inactivated influenza vaccine (IIV) replacing TIV. TABLE 2 lists the influenza vaccine categories and abbreviations. TABLE 3 lists the contraindications for the different vaccine types.3
The new products include Flumist Quadrivalent (MedImmune), a quadrivalent LAIV (LAIV4); Fluarix Quadrivalent (GlaxoSmithKline), a quadrivalent IIV (IIV4); Flucelvax (Novartis Vaccines and Diagnostics), a cell culture-based trivalent IIV (ccIIV3); and FluBlok (Protein Sciences), a trivalent recombinant hemagglutinin influenza vaccine (RIV3). Fluzone (Sanofi Pasteur), introduced last season in a trivalent formulation, is also available this season as a quadrivalent IIV (IIV4). As a group, influenza vaccine products now offer 3 routes of administration: intramuscular, subcutaneous, and intranasal. There is currently no evidence that any route offers an advantage over another, and the CDC states no preference for any particular product or route of administration.
Mercury content is not a problem
Even though there is no scientific controversy over the safety of the mercury-containing preservative thimerosal, some patients still have doubts and may ask for a thimerosalfree product. The only influenza products that contain any thimerosal are those that come in multidose vials. A description of each influenza vaccine product, including thimerosal content, indicated ages, and routes of administration, can be found on the CDC’s Web site3 (http://www.cdc.gov/flu/professionals/acip/2013-summary-recommendations.htm).
Options for those with egg allergy
There is now a product, RIV3 (FluBlok), that is manufactured without the use of eggs. It can be used in those 18 to 49 years of age with a history of egg allergy of any severity. Since 2011, the Advisory Committee on Immunization Practices (ACIP) has recommended that individuals with a history of mild egg allergy (those who experience only hives after egg exposure) may receive IIV, with additional safety precautions. Do not delay vaccination for these individuals if RIV is unavailable. Because of a lack of data demonstrating safety of LAIV for individuals with egg allergy, those allergic to eggs should receive RIV or IIV rather than LAIV.
Though the new ccIIV product, Flucelvax, is manufactured without the use of eggs, the seed viruses used to create the vaccine have been processed in eggs. The egg protein content in the vaccine is extremely low (<50 femtograms [5 × 10-14 g] per 0.5-mL dose), but the CDC does not consider it egg free. The FIGURE depicts the recommendations for those with a history of egg allergy.3
Other interventions for influenza prevention
Vaccination is only one tool available to prevent morbidity and mortality from influenza. Antiviral chemoprevention and treatment, and infection control practices can also be effective.
Antiviral chemoprevention is available for both pre- and post-exposure administration. In the past few years, the CDC has de-emphasized such use of antivirals for these indications out of concern for the supply of these agents and for the possibility that their use might lead to increased rates of viral resistance. Consider antiviral chemoprevention for those who have conditions that place them at risk for complications, and for those who are unvaccinated if they are at high risk for exposure to influenza (preexposure prophylaxis) or have been exposed (postexposure prophylaxis), if the medication can be started within 48 hours of exposure. Another option for unvaccinated high-risk patients is vigilant symptom monitoring with early treatment for influenza symptoms. Chemoprophylaxis is recommended in addition to vaccination to control influenza outbreaks at institutions that house patients at high risk for complications of influenza. Details on recommended antivirals including doses and duration of treatment can be found in a 2011 issue of Morbidity and Mortality Weekly Report.5
Antiviral treatment. The CDC recommends antiviral treatment for anyone with suspected or confirmed influenza who has progressive, severe, or complicated illness or is hospitalized for their illness.5 Treatment is also recommended for outpatients with suspected or confirmed influenza who are at higher risk for influenza complications. This latter group includes those in TABLE 1, particularly children 6 to 59 months and adults ≥50 years. Start antiviral treatment within 48 hours of the first symptoms. For hospitalized patients, however, begin treatment at any point regardless of duration of illness.
Infection control practices can prevent the spread of influenza in the health care setting and in the homes of those with influenza. These practices are also described on the CDC influenza Web site.6
Each year in late summer, the Centers for Disease Control and Prevention (CDC) publishes its recommendations for the prevention of influenza for the upcoming season. The severity of each influenza season varies and is difficult to predict, which underscores the need to provide maximal vaccine coverage for at-risk patient populations.
Hoping for the best, planning for the worst. Over the past several decades the annual number of influenza-related hospitalizations has varied from approximately 55,000 to 431,000,1 and the number of deaths from influenza has been as low as 3349 and as high as 48,614.2 Infection rates are usually highest in children. Complications, hospitalizations, and deaths are highest in those ≥65 years, children<2 years, and patients with medical conditions known to increase risk for influenza complications. Those at high risk of complications appear in TABLE 1.3 The main recommendations for this coming year are the same as last year, including vaccinating everyone ≥6 months of age without a contraindication, starting vaccinations as soon as vaccine is available, and continuing throughout the influenza season for those who need it.
What’s new this year
An increasing number of influenza vaccine products are available; although to date, their effectiveness (which was determined to be 56% for all vaccines used last influenza season) 4 remains below what we would hope for. The CDC’s recommendations address these new types of vaccines, including ones that have 4 antigens instead of 3, and use new terminology to describe the vaccines.3
New terminology reflects changing vaccine formulations. Last influenza season there were 2 major categories of influenza vaccines: live-attenuated influenza vaccine (LAIV) and trivalent inactivated influenza vaccine (TIV). All products were produced using egg-culture methods and contained 2 influenza A antigen subtypes and 1 B subtype. Several products this year include 4 antigens (2 A subtypes and 2 B subtypes), and some are now produced with non–eggculture methods. This has led to a new system of classification, with the term inactivated influenza vaccine (IIV) replacing TIV. TABLE 2 lists the influenza vaccine categories and abbreviations. TABLE 3 lists the contraindications for the different vaccine types.3
The new products include Flumist Quadrivalent (MedImmune), a quadrivalent LAIV (LAIV4); Fluarix Quadrivalent (GlaxoSmithKline), a quadrivalent IIV (IIV4); Flucelvax (Novartis Vaccines and Diagnostics), a cell culture-based trivalent IIV (ccIIV3); and FluBlok (Protein Sciences), a trivalent recombinant hemagglutinin influenza vaccine (RIV3). Fluzone (Sanofi Pasteur), introduced last season in a trivalent formulation, is also available this season as a quadrivalent IIV (IIV4). As a group, influenza vaccine products now offer 3 routes of administration: intramuscular, subcutaneous, and intranasal. There is currently no evidence that any route offers an advantage over another, and the CDC states no preference for any particular product or route of administration.
Mercury content is not a problem
Even though there is no scientific controversy over the safety of the mercury-containing preservative thimerosal, some patients still have doubts and may ask for a thimerosalfree product. The only influenza products that contain any thimerosal are those that come in multidose vials. A description of each influenza vaccine product, including thimerosal content, indicated ages, and routes of administration, can be found on the CDC’s Web site3 (http://www.cdc.gov/flu/professionals/acip/2013-summary-recommendations.htm).
Options for those with egg allergy
There is now a product, RIV3 (FluBlok), that is manufactured without the use of eggs. It can be used in those 18 to 49 years of age with a history of egg allergy of any severity. Since 2011, the Advisory Committee on Immunization Practices (ACIP) has recommended that individuals with a history of mild egg allergy (those who experience only hives after egg exposure) may receive IIV, with additional safety precautions. Do not delay vaccination for these individuals if RIV is unavailable. Because of a lack of data demonstrating safety of LAIV for individuals with egg allergy, those allergic to eggs should receive RIV or IIV rather than LAIV.
Though the new ccIIV product, Flucelvax, is manufactured without the use of eggs, the seed viruses used to create the vaccine have been processed in eggs. The egg protein content in the vaccine is extremely low (<50 femtograms [5 × 10-14 g] per 0.5-mL dose), but the CDC does not consider it egg free. The FIGURE depicts the recommendations for those with a history of egg allergy.3
Other interventions for influenza prevention
Vaccination is only one tool available to prevent morbidity and mortality from influenza. Antiviral chemoprevention and treatment, and infection control practices can also be effective.
Antiviral chemoprevention is available for both pre- and post-exposure administration. In the past few years, the CDC has de-emphasized such use of antivirals for these indications out of concern for the supply of these agents and for the possibility that their use might lead to increased rates of viral resistance. Consider antiviral chemoprevention for those who have conditions that place them at risk for complications, and for those who are unvaccinated if they are at high risk for exposure to influenza (preexposure prophylaxis) or have been exposed (postexposure prophylaxis), if the medication can be started within 48 hours of exposure. Another option for unvaccinated high-risk patients is vigilant symptom monitoring with early treatment for influenza symptoms. Chemoprophylaxis is recommended in addition to vaccination to control influenza outbreaks at institutions that house patients at high risk for complications of influenza. Details on recommended antivirals including doses and duration of treatment can be found in a 2011 issue of Morbidity and Mortality Weekly Report.5
Antiviral treatment. The CDC recommends antiviral treatment for anyone with suspected or confirmed influenza who has progressive, severe, or complicated illness or is hospitalized for their illness.5 Treatment is also recommended for outpatients with suspected or confirmed influenza who are at higher risk for influenza complications. This latter group includes those in TABLE 1, particularly children 6 to 59 months and adults ≥50 years. Start antiviral treatment within 48 hours of the first symptoms. For hospitalized patients, however, begin treatment at any point regardless of duration of illness.
Infection control practices can prevent the spread of influenza in the health care setting and in the homes of those with influenza. These practices are also described on the CDC influenza Web site.6
1. Thompson WW, Shay DK, Weintraub E. Influenza-associated hospitalizations in the United States. JAMA. 2004;292:1333-1340.
2. CDC. Estimates of deaths associated with seasonal influenza–United States, 1976-2007. MMWR Morb Mortal Wkly Rep. 2010;59:1057-1062.
3. CDC. Summary* recommendations: prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—(ACIP)—United States, 2013-14. Available at: http://www.cdc.gov/flu/professionals/ acip/2013-summary-recommendations.htm. Accessed August 9, 2013.
4. CDC. Interim adjusted estimates of seasonal influenza vaccine effectiveness—United States, February 2013. MMWR Morb Mortal Wkly Rep. 2013;62:119-123.
5. CDC. Antiviral agents for the treatment and chemoprophylaxis of influenza. MMWR Recomm Rep. 2011;60(RR01):1-24. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/rr6001a1. htm. Accessed July 2, 2013.
6. CDC. Infection control in health care facilities. Available at:http://www.cdc.gov/flu/professionals/infectioncontrol/index.htm. Accessed July 2, 2013.
1. Thompson WW, Shay DK, Weintraub E. Influenza-associated hospitalizations in the United States. JAMA. 2004;292:1333-1340.
2. CDC. Estimates of deaths associated with seasonal influenza–United States, 1976-2007. MMWR Morb Mortal Wkly Rep. 2010;59:1057-1062.
3. CDC. Summary* recommendations: prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—(ACIP)—United States, 2013-14. Available at: http://www.cdc.gov/flu/professionals/ acip/2013-summary-recommendations.htm. Accessed August 9, 2013.
4. CDC. Interim adjusted estimates of seasonal influenza vaccine effectiveness—United States, February 2013. MMWR Morb Mortal Wkly Rep. 2013;62:119-123.
5. CDC. Antiviral agents for the treatment and chemoprophylaxis of influenza. MMWR Recomm Rep. 2011;60(RR01):1-24. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/rr6001a1. htm. Accessed July 2, 2013.
6. CDC. Infection control in health care facilities. Available at:http://www.cdc.gov/flu/professionals/infectioncontrol/index.htm. Accessed July 2, 2013.
Vitamin D: When it helps, when it harms
Vitamin D is the new wonder cure and preventive for all kinds of ailments and chronic diseases. Or so it would seem from the popular press and Internet.1
But what do we actually know about the health benefits of vitamin D? Should we be screening patients for vitamin D deficiency? How much vitamin D should our patients consume daily? This Practice Alert answers these questions.
Vitamin D basics
Vitamin D is synthesized in the skin from cholesterol through sun exposure (vitamin D3) and consumed in food fortified with vitamin D2, such as milk, yogurt, and orange juice, or food that contains vitamin D3 (fatty fish and eggs). Both forms of vitamin D are inactive until metabolized in the liver to 25(OH)D (TABLE 1), which is further metabolized in the kidney to the biologically active calcitriol. The 25(OH)D circulates in the blood with a vitamin D–binding protein and is the basis of measurement of serum vitamin D levels.
The terminology of vitamin D
The metabolic actions of calcitriol include regulation of calcium and phosphate levels and maintenance of bone health. It also has a role in regulating cell proliferation and immune system functions. These last 2 activities are not well understood; still, they have led to the hypothesis that vitamin D may help prevent cancer, autoimmune conditions, and cardiovascular disease. Promotion of calcitriol for these purposes has yet to be supported by well-controlled clinical trials.
One more source…Vitamin D can also be found in multivitamin preparations at various dosages and is sold as a single vitamin supplement—sometimes in megadoses of up to 50,000 international units (IU).
How much vitamin D is enough?
There is universal agreement that vitamin D and calcium are important for bone health. The Institute of Medicine (IOM) recently revised the recommended dietary allowance (RDA) of vitamin D, by age (TABLE 2).2 The IOM calculated the newer RDAs under the assumption that, in the United States and Canada, little or no vitamin D is obtained from sun exposure, particularly given anticancer campaigns that stress sun avoidance. The IOM committee also expressed concern, however, about high levels of vitamin D intake that have not been linked to any proven benefits but have been linked to harms.2 Excess vitamin D from oral intake (not from sun exposure, which is subject to autoregulatory mechanisms) can cause vitamin D intoxication, hypercalcemia, and kidney stones.
Daily dietary reference intakes for calcium and vitamin D
Recent systematic reviews and recommendations
The IOM reviewed the medical literature on the effects of vitamin D to prevent or treat cancer, cardiovascular disease, hypertension, diabetes, metabolic syndrome, falls, and preeclampsia; and to boost immune response, neuropsychological function, physical performance, and reproductive outcomes. The panel found that the evidence for all of these effects is mixed and inconclusive, even though the media often report a beneficial effect.2
Several Cochrane systematic reviews have yielded similar results. One looked at overall mortality in adults and found that vitamin D3 seems to decrease mortality, but mostly in elderly women in institutions and dependent-care settings. Vitamin D2 had no effect on mortality. Vitamin D3 and calcium significantly increased the incidence of kidney stones.3Another review examined the effect of vitamin D on chronic pain and concluded that there were only low-quality observational studies insufficient for drawing conclusions.4
The United States Preventive Services Task Force (USPSTF) recently released 2 recommendations related to vitamin D supplementation.5,6 It first recommends exercise or physical therapy and vitamin D supplementation (800 IU daily) to prevent falls in community-dwelling adults ≥65 years at increased risk for falls (described in a previous Practice Alert7). The second recommendation pertains to primary prevention of fractures and advises against daily supplementation with vitamin D and calcium at doses ≤400 IU and 1000 mg, respectively, for noninstitutionalized postmenopausal women. At these doses, supplementation with vitamin D and calcium does not prevent fractures but does cause kidney stones, with a number needed to harm of 273 over 7 years.6 The USPSTF concluded that the evidence is insufficient to assess the value of either vitamin D or calcium in men and premenopausal women at any dose, or daily supplementation with >400 IU of vitamin D3 and >1000 mg of calcium for the primary prevention of fractures in noninstitutionalized postmenopausal women.
What about screening for vitamin D deficiency?
The Endocrine Society recommends screening for vitamin D deficiency in individuals at risk FAST TRACK. The USPSTF recommends vitamin D supplementation at 800 IU/d to prevent falls in community-dwelling adults >65 years at increased risk for falls. for deficiency—ie, those who have darkly pigmented skin, live in northern latitudes, or receive little exposure to sun. It does not recommend population screening for vitamin D deficiency in individuals not at risk. It defines vitamin D deficiency as a 25(OH)D level <20 ng/mL (50 nmol/L) and vitamin D insufficiency as a 25(OH)D level of 21 to 29 ng/mL (52.5-72.5 nmol/L).8
The IOM expresses concern about testing for vitamin D levels because there is no validated cutoff, and some labs report cutoffs above what the IOM considers a deficient level, leading to inflated numbers of those labeled as deficient.2 The USPSTF is about to weigh in on this issue. It has posted a draft research plan that will guide its evidence report and recommendation considerations.9
Take-home message
Information on the health benefits of vitamin D is difficult to sort out. Evidence for anything other than bone health and fall prevention is problematic. Consider vitamin D supplements along with calcium for the frail elderly at risk for falls10and for those who have osteoporosis. Screening for vitamin D deficiency is of questionable value and the USPSTF will be producing an evidence-based report on this topic, which should be available in about a year. The IOM RDA tables are available to guide dietary advice.
1. Vitamin D Council. Available at: http://www.vitamindcouncil.org/health-conditions. Accessed May 7, 2013.
2. Institute of Medicine of the National Academies. Dietary reference intakes for calcium and vitamin D. Washington, DC: The National Academies Press; 2011. Available at: http://www.nap.edu/catalog.php?record_id=13050. Accessed June 3, 2013.
3. Bjelakovic G, Gluud LL, Whitfield K, et al. Vitamin D supplementation for prevention of mortality in adults. Cochrane Database Syst Rev. 2011;(7):CD007470.
4. Straube S, Derry S, Moore RA, et al. Vitamin D for the treatment of chronic painful conditions in adults. Cochrane Database Syst Rev. 2010;(1):CD007771.
5. USPSTF. Prevention of falls in community-dwelling older adults. Available at: http://www.uspreventiveservicestaskforce.org/
uspstf/uspsfalls.htm. Accessed May 7, 2013.
6. USPSTF. Vitamin D and calcium supplementation to prevent fractures in adults. Available at: http://www.uspreventive
servicestaskforce.org/uspstf/uspsvitd.htm. Accessed May 7, 2013.
7. Campos-Outcalt D. The latest recommendations from the
USPSTF. J Fam Pract. 2013;62:249-252.
8. Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency. J Clin Endocrinol Metab. 2011;96:1911-1930.
9. USPSTF. Draft research plan. Screening for vitamin D deficiency. Available at: http://www.uspreventiveservicestaskforce.org/
uspstf13/vitddefic/vitddeficdraftresplan.htm. Accessed May 7, 2013.
10. Avenell A, Gillespie WJ, Gillespie LD, et al. Vitamin D and vitamin D analogues for preventing fractures associated with involutional and post-menopausal osteoporosis. Cochrane Database Syst Rev. 2009;(2):CD000227.
Vitamin D is the new wonder cure and preventive for all kinds of ailments and chronic diseases. Or so it would seem from the popular press and Internet.1
But what do we actually know about the health benefits of vitamin D? Should we be screening patients for vitamin D deficiency? How much vitamin D should our patients consume daily? This Practice Alert answers these questions.
Vitamin D basics
Vitamin D is synthesized in the skin from cholesterol through sun exposure (vitamin D3) and consumed in food fortified with vitamin D2, such as milk, yogurt, and orange juice, or food that contains vitamin D3 (fatty fish and eggs). Both forms of vitamin D are inactive until metabolized in the liver to 25(OH)D (TABLE 1), which is further metabolized in the kidney to the biologically active calcitriol. The 25(OH)D circulates in the blood with a vitamin D–binding protein and is the basis of measurement of serum vitamin D levels.
The terminology of vitamin D
The metabolic actions of calcitriol include regulation of calcium and phosphate levels and maintenance of bone health. It also has a role in regulating cell proliferation and immune system functions. These last 2 activities are not well understood; still, they have led to the hypothesis that vitamin D may help prevent cancer, autoimmune conditions, and cardiovascular disease. Promotion of calcitriol for these purposes has yet to be supported by well-controlled clinical trials.
One more source…Vitamin D can also be found in multivitamin preparations at various dosages and is sold as a single vitamin supplement—sometimes in megadoses of up to 50,000 international units (IU).
How much vitamin D is enough?
There is universal agreement that vitamin D and calcium are important for bone health. The Institute of Medicine (IOM) recently revised the recommended dietary allowance (RDA) of vitamin D, by age (TABLE 2).2 The IOM calculated the newer RDAs under the assumption that, in the United States and Canada, little or no vitamin D is obtained from sun exposure, particularly given anticancer campaigns that stress sun avoidance. The IOM committee also expressed concern, however, about high levels of vitamin D intake that have not been linked to any proven benefits but have been linked to harms.2 Excess vitamin D from oral intake (not from sun exposure, which is subject to autoregulatory mechanisms) can cause vitamin D intoxication, hypercalcemia, and kidney stones.
Daily dietary reference intakes for calcium and vitamin D
Recent systematic reviews and recommendations
The IOM reviewed the medical literature on the effects of vitamin D to prevent or treat cancer, cardiovascular disease, hypertension, diabetes, metabolic syndrome, falls, and preeclampsia; and to boost immune response, neuropsychological function, physical performance, and reproductive outcomes. The panel found that the evidence for all of these effects is mixed and inconclusive, even though the media often report a beneficial effect.2
Several Cochrane systematic reviews have yielded similar results. One looked at overall mortality in adults and found that vitamin D3 seems to decrease mortality, but mostly in elderly women in institutions and dependent-care settings. Vitamin D2 had no effect on mortality. Vitamin D3 and calcium significantly increased the incidence of kidney stones.3Another review examined the effect of vitamin D on chronic pain and concluded that there were only low-quality observational studies insufficient for drawing conclusions.4
The United States Preventive Services Task Force (USPSTF) recently released 2 recommendations related to vitamin D supplementation.5,6 It first recommends exercise or physical therapy and vitamin D supplementation (800 IU daily) to prevent falls in community-dwelling adults ≥65 years at increased risk for falls (described in a previous Practice Alert7). The second recommendation pertains to primary prevention of fractures and advises against daily supplementation with vitamin D and calcium at doses ≤400 IU and 1000 mg, respectively, for noninstitutionalized postmenopausal women. At these doses, supplementation with vitamin D and calcium does not prevent fractures but does cause kidney stones, with a number needed to harm of 273 over 7 years.6 The USPSTF concluded that the evidence is insufficient to assess the value of either vitamin D or calcium in men and premenopausal women at any dose, or daily supplementation with >400 IU of vitamin D3 and >1000 mg of calcium for the primary prevention of fractures in noninstitutionalized postmenopausal women.
What about screening for vitamin D deficiency?
The Endocrine Society recommends screening for vitamin D deficiency in individuals at risk FAST TRACK. The USPSTF recommends vitamin D supplementation at 800 IU/d to prevent falls in community-dwelling adults >65 years at increased risk for falls. for deficiency—ie, those who have darkly pigmented skin, live in northern latitudes, or receive little exposure to sun. It does not recommend population screening for vitamin D deficiency in individuals not at risk. It defines vitamin D deficiency as a 25(OH)D level <20 ng/mL (50 nmol/L) and vitamin D insufficiency as a 25(OH)D level of 21 to 29 ng/mL (52.5-72.5 nmol/L).8
The IOM expresses concern about testing for vitamin D levels because there is no validated cutoff, and some labs report cutoffs above what the IOM considers a deficient level, leading to inflated numbers of those labeled as deficient.2 The USPSTF is about to weigh in on this issue. It has posted a draft research plan that will guide its evidence report and recommendation considerations.9
Take-home message
Information on the health benefits of vitamin D is difficult to sort out. Evidence for anything other than bone health and fall prevention is problematic. Consider vitamin D supplements along with calcium for the frail elderly at risk for falls10and for those who have osteoporosis. Screening for vitamin D deficiency is of questionable value and the USPSTF will be producing an evidence-based report on this topic, which should be available in about a year. The IOM RDA tables are available to guide dietary advice.
Vitamin D is the new wonder cure and preventive for all kinds of ailments and chronic diseases. Or so it would seem from the popular press and Internet.1
But what do we actually know about the health benefits of vitamin D? Should we be screening patients for vitamin D deficiency? How much vitamin D should our patients consume daily? This Practice Alert answers these questions.
Vitamin D basics
Vitamin D is synthesized in the skin from cholesterol through sun exposure (vitamin D3) and consumed in food fortified with vitamin D2, such as milk, yogurt, and orange juice, or food that contains vitamin D3 (fatty fish and eggs). Both forms of vitamin D are inactive until metabolized in the liver to 25(OH)D (TABLE 1), which is further metabolized in the kidney to the biologically active calcitriol. The 25(OH)D circulates in the blood with a vitamin D–binding protein and is the basis of measurement of serum vitamin D levels.
The terminology of vitamin D
The metabolic actions of calcitriol include regulation of calcium and phosphate levels and maintenance of bone health. It also has a role in regulating cell proliferation and immune system functions. These last 2 activities are not well understood; still, they have led to the hypothesis that vitamin D may help prevent cancer, autoimmune conditions, and cardiovascular disease. Promotion of calcitriol for these purposes has yet to be supported by well-controlled clinical trials.
One more source…Vitamin D can also be found in multivitamin preparations at various dosages and is sold as a single vitamin supplement—sometimes in megadoses of up to 50,000 international units (IU).
How much vitamin D is enough?
There is universal agreement that vitamin D and calcium are important for bone health. The Institute of Medicine (IOM) recently revised the recommended dietary allowance (RDA) of vitamin D, by age (TABLE 2).2 The IOM calculated the newer RDAs under the assumption that, in the United States and Canada, little or no vitamin D is obtained from sun exposure, particularly given anticancer campaigns that stress sun avoidance. The IOM committee also expressed concern, however, about high levels of vitamin D intake that have not been linked to any proven benefits but have been linked to harms.2 Excess vitamin D from oral intake (not from sun exposure, which is subject to autoregulatory mechanisms) can cause vitamin D intoxication, hypercalcemia, and kidney stones.
Daily dietary reference intakes for calcium and vitamin D
Recent systematic reviews and recommendations
The IOM reviewed the medical literature on the effects of vitamin D to prevent or treat cancer, cardiovascular disease, hypertension, diabetes, metabolic syndrome, falls, and preeclampsia; and to boost immune response, neuropsychological function, physical performance, and reproductive outcomes. The panel found that the evidence for all of these effects is mixed and inconclusive, even though the media often report a beneficial effect.2
Several Cochrane systematic reviews have yielded similar results. One looked at overall mortality in adults and found that vitamin D3 seems to decrease mortality, but mostly in elderly women in institutions and dependent-care settings. Vitamin D2 had no effect on mortality. Vitamin D3 and calcium significantly increased the incidence of kidney stones.3Another review examined the effect of vitamin D on chronic pain and concluded that there were only low-quality observational studies insufficient for drawing conclusions.4
The United States Preventive Services Task Force (USPSTF) recently released 2 recommendations related to vitamin D supplementation.5,6 It first recommends exercise or physical therapy and vitamin D supplementation (800 IU daily) to prevent falls in community-dwelling adults ≥65 years at increased risk for falls (described in a previous Practice Alert7). The second recommendation pertains to primary prevention of fractures and advises against daily supplementation with vitamin D and calcium at doses ≤400 IU and 1000 mg, respectively, for noninstitutionalized postmenopausal women. At these doses, supplementation with vitamin D and calcium does not prevent fractures but does cause kidney stones, with a number needed to harm of 273 over 7 years.6 The USPSTF concluded that the evidence is insufficient to assess the value of either vitamin D or calcium in men and premenopausal women at any dose, or daily supplementation with >400 IU of vitamin D3 and >1000 mg of calcium for the primary prevention of fractures in noninstitutionalized postmenopausal women.
What about screening for vitamin D deficiency?
The Endocrine Society recommends screening for vitamin D deficiency in individuals at risk FAST TRACK. The USPSTF recommends vitamin D supplementation at 800 IU/d to prevent falls in community-dwelling adults >65 years at increased risk for falls. for deficiency—ie, those who have darkly pigmented skin, live in northern latitudes, or receive little exposure to sun. It does not recommend population screening for vitamin D deficiency in individuals not at risk. It defines vitamin D deficiency as a 25(OH)D level <20 ng/mL (50 nmol/L) and vitamin D insufficiency as a 25(OH)D level of 21 to 29 ng/mL (52.5-72.5 nmol/L).8
The IOM expresses concern about testing for vitamin D levels because there is no validated cutoff, and some labs report cutoffs above what the IOM considers a deficient level, leading to inflated numbers of those labeled as deficient.2 The USPSTF is about to weigh in on this issue. It has posted a draft research plan that will guide its evidence report and recommendation considerations.9
Take-home message
Information on the health benefits of vitamin D is difficult to sort out. Evidence for anything other than bone health and fall prevention is problematic. Consider vitamin D supplements along with calcium for the frail elderly at risk for falls10and for those who have osteoporosis. Screening for vitamin D deficiency is of questionable value and the USPSTF will be producing an evidence-based report on this topic, which should be available in about a year. The IOM RDA tables are available to guide dietary advice.
1. Vitamin D Council. Available at: http://www.vitamindcouncil.org/health-conditions. Accessed May 7, 2013.
2. Institute of Medicine of the National Academies. Dietary reference intakes for calcium and vitamin D. Washington, DC: The National Academies Press; 2011. Available at: http://www.nap.edu/catalog.php?record_id=13050. Accessed June 3, 2013.
3. Bjelakovic G, Gluud LL, Whitfield K, et al. Vitamin D supplementation for prevention of mortality in adults. Cochrane Database Syst Rev. 2011;(7):CD007470.
4. Straube S, Derry S, Moore RA, et al. Vitamin D for the treatment of chronic painful conditions in adults. Cochrane Database Syst Rev. 2010;(1):CD007771.
5. USPSTF. Prevention of falls in community-dwelling older adults. Available at: http://www.uspreventiveservicestaskforce.org/
uspstf/uspsfalls.htm. Accessed May 7, 2013.
6. USPSTF. Vitamin D and calcium supplementation to prevent fractures in adults. Available at: http://www.uspreventive
servicestaskforce.org/uspstf/uspsvitd.htm. Accessed May 7, 2013.
7. Campos-Outcalt D. The latest recommendations from the
USPSTF. J Fam Pract. 2013;62:249-252.
8. Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency. J Clin Endocrinol Metab. 2011;96:1911-1930.
9. USPSTF. Draft research plan. Screening for vitamin D deficiency. Available at: http://www.uspreventiveservicestaskforce.org/
uspstf13/vitddefic/vitddeficdraftresplan.htm. Accessed May 7, 2013.
10. Avenell A, Gillespie WJ, Gillespie LD, et al. Vitamin D and vitamin D analogues for preventing fractures associated with involutional and post-menopausal osteoporosis. Cochrane Database Syst Rev. 2009;(2):CD000227.
1. Vitamin D Council. Available at: http://www.vitamindcouncil.org/health-conditions. Accessed May 7, 2013.
2. Institute of Medicine of the National Academies. Dietary reference intakes for calcium and vitamin D. Washington, DC: The National Academies Press; 2011. Available at: http://www.nap.edu/catalog.php?record_id=13050. Accessed June 3, 2013.
3. Bjelakovic G, Gluud LL, Whitfield K, et al. Vitamin D supplementation for prevention of mortality in adults. Cochrane Database Syst Rev. 2011;(7):CD007470.
4. Straube S, Derry S, Moore RA, et al. Vitamin D for the treatment of chronic painful conditions in adults. Cochrane Database Syst Rev. 2010;(1):CD007771.
5. USPSTF. Prevention of falls in community-dwelling older adults. Available at: http://www.uspreventiveservicestaskforce.org/
uspstf/uspsfalls.htm. Accessed May 7, 2013.
6. USPSTF. Vitamin D and calcium supplementation to prevent fractures in adults. Available at: http://www.uspreventive
servicestaskforce.org/uspstf/uspsvitd.htm. Accessed May 7, 2013.
7. Campos-Outcalt D. The latest recommendations from the
USPSTF. J Fam Pract. 2013;62:249-252.
8. Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency. J Clin Endocrinol Metab. 2011;96:1911-1930.
9. USPSTF. Draft research plan. Screening for vitamin D deficiency. Available at: http://www.uspreventiveservicestaskforce.org/
uspstf13/vitddefic/vitddeficdraftresplan.htm. Accessed May 7, 2013.
10. Avenell A, Gillespie WJ, Gillespie LD, et al. Vitamin D and vitamin D analogues for preventing fractures associated with involutional and post-menopausal osteoporosis. Cochrane Database Syst Rev. 2009;(2):CD000227.
The latest recommendations from the USPSTF
Since the last Practice Alert update on the US Preventive Services Task Force (USPSTF) recommendations,1 the Task Force released 16 final recommendations, through January of this year (TABLE).2 However, none of these were level A recommendations and only 4 were level B. This is significant in that USPSTF level A and B recommendations must now be covered by health insurance plans without patient cost sharing as a result of a clause in the Affordable Care Act. There were 5 D recommendations (recommend against), and some of the tests that fell into this category are in common use. I discuss the B and D recommendations below.
TABLE
Recent recommendations from the USPSTF2
| B recommendations |
The USPSTF recommends:
|
| C recommendations |
The USPSTF recommends against automatically:
|
| D recommendations |
The USPSTF recommends against:
|
| I statements |
The USPSTF concludes that the current evidence is insufficient to assess the balance of benefits and harms of:
|
| For more on the USPSTF’s grade definitions, see http://www.uspreventiveservicestaskforce.org/uspstf/grades.htm. |
B recommendations
Encourage vitamin D supplementation and regular exercise to prevent falls in elderly
Falls in the elderly are a significant cause of morbidity and mortality. The Task Force found that between 30% and 40% of community-dwelling adults ≥65 years fall each year, and 5% to 10% of those who fall will sustain a fracture, head injury, or laceration.3 Those at highest risk have a history of falls, report mobility problems, have chronic diseases, use psychotropic medications, or have difficulty on a “get up and go” test, which involves rising from a sitting position in an arm chair, walking 10 feet, turning, walking back, and sitting down. If this activity takes more than 10 seconds, the risk of a fall is increased.3
Two interventions were found to be effective in preventing falls: vitamin D supplementation and regular exercise or physical therapy. Vitamin D enhances muscular strength and balance, and supplementation of 800 IU daily for 12 months can decrease the risk of a fall by 17%, with a number needed to treat (NNT) of 10 to prevent one fall.3 Exercise or physical therapy that focuses on gait and balance, strength or resistance training, or general fitness can reduce the risk of falls with an NNT of 16. Individuals who benefit the most are those at higher risk.3
As for multifactorial risk assessment and comprehensive management of risks to prevent falls, a pooled analysis of studies showed that these interventions do little to reduce falls and do not warrant routine use. The Task Force evaluated other interventions—vision correction, medication discontinuation, protein supplementation, education or counseling, and home hazard modification—but could not find sufficient evidence to recommend for or against them.
Screen for obesity in adults
The Task Force reaffirmed its recommendation to screen all adults for obesity and to offer intensive behavioral interventions to those with a body mass index of ≥30 kg/m2. Helpful interventions include multiple behavioral management activities in group or individual sessions; setting weight-loss goals; improving diet or nutrition; physical activity sessions; addressing barriers to change; active use of self-monitoring; and strategizing ways to maintain lifestyle changes. High-intensity programs involve 12 to 26 sessions a year and result, on average, in a reduction of 6% of body weight.4
Counsel fair-skinned patients to minimize sun exposure
The Task Force now recommends counseling fair-skinned children, adolescents, and young adults (10-24 years of age) about reducing their exposure to ultraviolet (UV) radiation. UV radiation exposure occurs when outdoors in the sun, especially in the middle of the day; and when using artificial sources of UV light, such as an indoor tanning bed. Unprotected UV light exposure is a cause of skin cancer, especially when this exposure occurs in childhood or young adulthood.
Behaviors that protect from UV radiation exposure include using broad-spectrum sunscreen with a sun-protection factor of at least 15, wearing hats and protective clothing, avoiding the outdoors during midday hours (10 am-3 pm), and avoiding indoor tanning. Brief counseling offered in a primary care setting can increase protective behaviors in the targeted age group.
UV light exposure in adults is also linked to skin cancer, but the effectiveness of counseling in this population is less certain and the benefit from protective behaviors is less. In addition, almost all studies of skin cancer prevention have been conducted with fair-skinned subjects, so the Task Force limited this recommendation to those who have fair skin and are between the ages of 10 and 24.5
Screen for intimate partner violence
The USPSTF has changed its recommendation on screening women for intimate partner violence (IPV). Previously it said that the evidence was insufficient to make a recommendation. New evidence has since been published and the Task Force recommends that women of childbearing age (14-46 years, with most evidence for those over age 18) be screened using one of 6 screening tools found to have satisfactory performance characteristics.6 IPV means physical, sexual, or psychological abuse by a current or former partner or spouse, among heterosexual or same-sex couples. To learn more, see “Time to routinely screen for intimate partner violence?” (J Fam Pract. 2013;62:90-92).
Services found to be effective in preventing IPV include counseling, home visits, information cards, referrals to community services, and mentoring support provided by physicians or other health professionals.6
The evidence on screening for the prevention of elder abuse and abuse of vulnerable adults still remains insufficient for a recommendation.
D recommendations
No need for prostate cancer screening, or these other interventions
The list of new D recommendations (interventions that have no benefit or that cause more harm than benefit) includes:
- screening for ovarian and prostate cancer
- using estrogen or estrogen combined with progestin in postmenopausal women for the prevention of chronic conditions
- screening with resting or exercise electrocardiography for the prediction of coronary heart disease events in asymptomatic adults at low risk for such events.
The most controversial D recommendation is to avoid measuring prostate-specific antigen (PSA) to screen for prostate cancer. The Task Force has never endorsed use of the PSA test, previously stating that evidence was not of sufficient strength to recommend for or against it in men <75 years and recommending against it for older men. The evidence report conducted for the reconsideration of this topic provided sufficient evidence that the PSA test results in far more harm than benefit.
In February, the USPSTF finalized a recommendation on “Vitamin D and Calcium Supplementation to Prevent Fractures in Adults.” For more information, go to:
http://www.uspreventiveservicestaskforce.org/announcements.htm
The troublesome C recommendation
Proceed with caution with these 2 interventions
The wording of level C recommendations has undergone revision once again. In recognition that some preventive services may benefit select patients—although the overall benefit in the population is small—the USPSTF now states that a C recommendation means that the Task Force “recommends selectively offering or providing this service to individual patients based on professional judgment and patient preferences.” This past year, 2 interventions fell into this category: multifactorial risk assessment and management to prevent falls in community dwelling elders, and counseling adults about a healthy diet and exercise to prevent cardiovascular disease (TABLE).2
1. Campos-Outcalt D. The latest recommendations from the USPSTF. J Fam Pract. 2012;61:278-282.
2. USPSTF. Announcements. Available at: http://www.uspreventiveservicestaskforce.org/announcements.htm. Accessed March 6, 2013.
3. USPSTF. Prevention of falls in community dwelling older adults. Available at: http://www.uspreventiveservicestaskforce.org/uspstf11/fallsprevention/fallsprevrs.htm. Accessed March 6, 2013.
4. USPSTF. Screening for and management of obesity in adults. Available at: http://www.uspreventiveservicestaskforce.org/uspstf11/obeseadult/obesers.htm. Accessed March 6, 2013.
5. USPSTF. Behavioral counseling to prevent skin cancer. Available at: http://www.uspreventiveservicestaskforce.org/uspstf11/skincancouns/skincancounsrs.htm. Accessed March 6, 2013.
6. USPSTF. Screening for intimate partner violence and abuse of elderly and vulnerable adults. Available at: http://www.uspreventiveservicestaskforce.org/uspstf12/ipvelder/ipvelderfinalrs.htm. Accessed March 6, 2013.
Since the last Practice Alert update on the US Preventive Services Task Force (USPSTF) recommendations,1 the Task Force released 16 final recommendations, through January of this year (TABLE).2 However, none of these were level A recommendations and only 4 were level B. This is significant in that USPSTF level A and B recommendations must now be covered by health insurance plans without patient cost sharing as a result of a clause in the Affordable Care Act. There were 5 D recommendations (recommend against), and some of the tests that fell into this category are in common use. I discuss the B and D recommendations below.
TABLE
Recent recommendations from the USPSTF2
| B recommendations |
The USPSTF recommends:
|
| C recommendations |
The USPSTF recommends against automatically:
|
| D recommendations |
The USPSTF recommends against:
|
| I statements |
The USPSTF concludes that the current evidence is insufficient to assess the balance of benefits and harms of:
|
| For more on the USPSTF’s grade definitions, see http://www.uspreventiveservicestaskforce.org/uspstf/grades.htm. |
B recommendations
Encourage vitamin D supplementation and regular exercise to prevent falls in elderly
Falls in the elderly are a significant cause of morbidity and mortality. The Task Force found that between 30% and 40% of community-dwelling adults ≥65 years fall each year, and 5% to 10% of those who fall will sustain a fracture, head injury, or laceration.3 Those at highest risk have a history of falls, report mobility problems, have chronic diseases, use psychotropic medications, or have difficulty on a “get up and go” test, which involves rising from a sitting position in an arm chair, walking 10 feet, turning, walking back, and sitting down. If this activity takes more than 10 seconds, the risk of a fall is increased.3
Two interventions were found to be effective in preventing falls: vitamin D supplementation and regular exercise or physical therapy. Vitamin D enhances muscular strength and balance, and supplementation of 800 IU daily for 12 months can decrease the risk of a fall by 17%, with a number needed to treat (NNT) of 10 to prevent one fall.3 Exercise or physical therapy that focuses on gait and balance, strength or resistance training, or general fitness can reduce the risk of falls with an NNT of 16. Individuals who benefit the most are those at higher risk.3
As for multifactorial risk assessment and comprehensive management of risks to prevent falls, a pooled analysis of studies showed that these interventions do little to reduce falls and do not warrant routine use. The Task Force evaluated other interventions—vision correction, medication discontinuation, protein supplementation, education or counseling, and home hazard modification—but could not find sufficient evidence to recommend for or against them.
Screen for obesity in adults
The Task Force reaffirmed its recommendation to screen all adults for obesity and to offer intensive behavioral interventions to those with a body mass index of ≥30 kg/m2. Helpful interventions include multiple behavioral management activities in group or individual sessions; setting weight-loss goals; improving diet or nutrition; physical activity sessions; addressing barriers to change; active use of self-monitoring; and strategizing ways to maintain lifestyle changes. High-intensity programs involve 12 to 26 sessions a year and result, on average, in a reduction of 6% of body weight.4
Counsel fair-skinned patients to minimize sun exposure
The Task Force now recommends counseling fair-skinned children, adolescents, and young adults (10-24 years of age) about reducing their exposure to ultraviolet (UV) radiation. UV radiation exposure occurs when outdoors in the sun, especially in the middle of the day; and when using artificial sources of UV light, such as an indoor tanning bed. Unprotected UV light exposure is a cause of skin cancer, especially when this exposure occurs in childhood or young adulthood.
Behaviors that protect from UV radiation exposure include using broad-spectrum sunscreen with a sun-protection factor of at least 15, wearing hats and protective clothing, avoiding the outdoors during midday hours (10 am-3 pm), and avoiding indoor tanning. Brief counseling offered in a primary care setting can increase protective behaviors in the targeted age group.
UV light exposure in adults is also linked to skin cancer, but the effectiveness of counseling in this population is less certain and the benefit from protective behaviors is less. In addition, almost all studies of skin cancer prevention have been conducted with fair-skinned subjects, so the Task Force limited this recommendation to those who have fair skin and are between the ages of 10 and 24.5
Screen for intimate partner violence
The USPSTF has changed its recommendation on screening women for intimate partner violence (IPV). Previously it said that the evidence was insufficient to make a recommendation. New evidence has since been published and the Task Force recommends that women of childbearing age (14-46 years, with most evidence for those over age 18) be screened using one of 6 screening tools found to have satisfactory performance characteristics.6 IPV means physical, sexual, or psychological abuse by a current or former partner or spouse, among heterosexual or same-sex couples. To learn more, see “Time to routinely screen for intimate partner violence?” (J Fam Pract. 2013;62:90-92).
Services found to be effective in preventing IPV include counseling, home visits, information cards, referrals to community services, and mentoring support provided by physicians or other health professionals.6
The evidence on screening for the prevention of elder abuse and abuse of vulnerable adults still remains insufficient for a recommendation.
D recommendations
No need for prostate cancer screening, or these other interventions
The list of new D recommendations (interventions that have no benefit or that cause more harm than benefit) includes:
- screening for ovarian and prostate cancer
- using estrogen or estrogen combined with progestin in postmenopausal women for the prevention of chronic conditions
- screening with resting or exercise electrocardiography for the prediction of coronary heart disease events in asymptomatic adults at low risk for such events.
The most controversial D recommendation is to avoid measuring prostate-specific antigen (PSA) to screen for prostate cancer. The Task Force has never endorsed use of the PSA test, previously stating that evidence was not of sufficient strength to recommend for or against it in men <75 years and recommending against it for older men. The evidence report conducted for the reconsideration of this topic provided sufficient evidence that the PSA test results in far more harm than benefit.
In February, the USPSTF finalized a recommendation on “Vitamin D and Calcium Supplementation to Prevent Fractures in Adults.” For more information, go to:
http://www.uspreventiveservicestaskforce.org/announcements.htm
The troublesome C recommendation
Proceed with caution with these 2 interventions
The wording of level C recommendations has undergone revision once again. In recognition that some preventive services may benefit select patients—although the overall benefit in the population is small—the USPSTF now states that a C recommendation means that the Task Force “recommends selectively offering or providing this service to individual patients based on professional judgment and patient preferences.” This past year, 2 interventions fell into this category: multifactorial risk assessment and management to prevent falls in community dwelling elders, and counseling adults about a healthy diet and exercise to prevent cardiovascular disease (TABLE).2
Since the last Practice Alert update on the US Preventive Services Task Force (USPSTF) recommendations,1 the Task Force released 16 final recommendations, through January of this year (TABLE).2 However, none of these were level A recommendations and only 4 were level B. This is significant in that USPSTF level A and B recommendations must now be covered by health insurance plans without patient cost sharing as a result of a clause in the Affordable Care Act. There were 5 D recommendations (recommend against), and some of the tests that fell into this category are in common use. I discuss the B and D recommendations below.
TABLE
Recent recommendations from the USPSTF2
| B recommendations |
The USPSTF recommends:
|
| C recommendations |
The USPSTF recommends against automatically:
|
| D recommendations |
The USPSTF recommends against:
|
| I statements |
The USPSTF concludes that the current evidence is insufficient to assess the balance of benefits and harms of:
|
| For more on the USPSTF’s grade definitions, see http://www.uspreventiveservicestaskforce.org/uspstf/grades.htm. |
B recommendations
Encourage vitamin D supplementation and regular exercise to prevent falls in elderly
Falls in the elderly are a significant cause of morbidity and mortality. The Task Force found that between 30% and 40% of community-dwelling adults ≥65 years fall each year, and 5% to 10% of those who fall will sustain a fracture, head injury, or laceration.3 Those at highest risk have a history of falls, report mobility problems, have chronic diseases, use psychotropic medications, or have difficulty on a “get up and go” test, which involves rising from a sitting position in an arm chair, walking 10 feet, turning, walking back, and sitting down. If this activity takes more than 10 seconds, the risk of a fall is increased.3
Two interventions were found to be effective in preventing falls: vitamin D supplementation and regular exercise or physical therapy. Vitamin D enhances muscular strength and balance, and supplementation of 800 IU daily for 12 months can decrease the risk of a fall by 17%, with a number needed to treat (NNT) of 10 to prevent one fall.3 Exercise or physical therapy that focuses on gait and balance, strength or resistance training, or general fitness can reduce the risk of falls with an NNT of 16. Individuals who benefit the most are those at higher risk.3
As for multifactorial risk assessment and comprehensive management of risks to prevent falls, a pooled analysis of studies showed that these interventions do little to reduce falls and do not warrant routine use. The Task Force evaluated other interventions—vision correction, medication discontinuation, protein supplementation, education or counseling, and home hazard modification—but could not find sufficient evidence to recommend for or against them.
Screen for obesity in adults
The Task Force reaffirmed its recommendation to screen all adults for obesity and to offer intensive behavioral interventions to those with a body mass index of ≥30 kg/m2. Helpful interventions include multiple behavioral management activities in group or individual sessions; setting weight-loss goals; improving diet or nutrition; physical activity sessions; addressing barriers to change; active use of self-monitoring; and strategizing ways to maintain lifestyle changes. High-intensity programs involve 12 to 26 sessions a year and result, on average, in a reduction of 6% of body weight.4
Counsel fair-skinned patients to minimize sun exposure
The Task Force now recommends counseling fair-skinned children, adolescents, and young adults (10-24 years of age) about reducing their exposure to ultraviolet (UV) radiation. UV radiation exposure occurs when outdoors in the sun, especially in the middle of the day; and when using artificial sources of UV light, such as an indoor tanning bed. Unprotected UV light exposure is a cause of skin cancer, especially when this exposure occurs in childhood or young adulthood.
Behaviors that protect from UV radiation exposure include using broad-spectrum sunscreen with a sun-protection factor of at least 15, wearing hats and protective clothing, avoiding the outdoors during midday hours (10 am-3 pm), and avoiding indoor tanning. Brief counseling offered in a primary care setting can increase protective behaviors in the targeted age group.
UV light exposure in adults is also linked to skin cancer, but the effectiveness of counseling in this population is less certain and the benefit from protective behaviors is less. In addition, almost all studies of skin cancer prevention have been conducted with fair-skinned subjects, so the Task Force limited this recommendation to those who have fair skin and are between the ages of 10 and 24.5
Screen for intimate partner violence
The USPSTF has changed its recommendation on screening women for intimate partner violence (IPV). Previously it said that the evidence was insufficient to make a recommendation. New evidence has since been published and the Task Force recommends that women of childbearing age (14-46 years, with most evidence for those over age 18) be screened using one of 6 screening tools found to have satisfactory performance characteristics.6 IPV means physical, sexual, or psychological abuse by a current or former partner or spouse, among heterosexual or same-sex couples. To learn more, see “Time to routinely screen for intimate partner violence?” (J Fam Pract. 2013;62:90-92).
Services found to be effective in preventing IPV include counseling, home visits, information cards, referrals to community services, and mentoring support provided by physicians or other health professionals.6
The evidence on screening for the prevention of elder abuse and abuse of vulnerable adults still remains insufficient for a recommendation.
D recommendations
No need for prostate cancer screening, or these other interventions
The list of new D recommendations (interventions that have no benefit or that cause more harm than benefit) includes:
- screening for ovarian and prostate cancer
- using estrogen or estrogen combined with progestin in postmenopausal women for the prevention of chronic conditions
- screening with resting or exercise electrocardiography for the prediction of coronary heart disease events in asymptomatic adults at low risk for such events.
The most controversial D recommendation is to avoid measuring prostate-specific antigen (PSA) to screen for prostate cancer. The Task Force has never endorsed use of the PSA test, previously stating that evidence was not of sufficient strength to recommend for or against it in men <75 years and recommending against it for older men. The evidence report conducted for the reconsideration of this topic provided sufficient evidence that the PSA test results in far more harm than benefit.
In February, the USPSTF finalized a recommendation on “Vitamin D and Calcium Supplementation to Prevent Fractures in Adults.” For more information, go to:
http://www.uspreventiveservicestaskforce.org/announcements.htm
The troublesome C recommendation
Proceed with caution with these 2 interventions
The wording of level C recommendations has undergone revision once again. In recognition that some preventive services may benefit select patients—although the overall benefit in the population is small—the USPSTF now states that a C recommendation means that the Task Force “recommends selectively offering or providing this service to individual patients based on professional judgment and patient preferences.” This past year, 2 interventions fell into this category: multifactorial risk assessment and management to prevent falls in community dwelling elders, and counseling adults about a healthy diet and exercise to prevent cardiovascular disease (TABLE).2
1. Campos-Outcalt D. The latest recommendations from the USPSTF. J Fam Pract. 2012;61:278-282.
2. USPSTF. Announcements. Available at: http://www.uspreventiveservicestaskforce.org/announcements.htm. Accessed March 6, 2013.
3. USPSTF. Prevention of falls in community dwelling older adults. Available at: http://www.uspreventiveservicestaskforce.org/uspstf11/fallsprevention/fallsprevrs.htm. Accessed March 6, 2013.
4. USPSTF. Screening for and management of obesity in adults. Available at: http://www.uspreventiveservicestaskforce.org/uspstf11/obeseadult/obesers.htm. Accessed March 6, 2013.
5. USPSTF. Behavioral counseling to prevent skin cancer. Available at: http://www.uspreventiveservicestaskforce.org/uspstf11/skincancouns/skincancounsrs.htm. Accessed March 6, 2013.
6. USPSTF. Screening for intimate partner violence and abuse of elderly and vulnerable adults. Available at: http://www.uspreventiveservicestaskforce.org/uspstf12/ipvelder/ipvelderfinalrs.htm. Accessed March 6, 2013.
1. Campos-Outcalt D. The latest recommendations from the USPSTF. J Fam Pract. 2012;61:278-282.
2. USPSTF. Announcements. Available at: http://www.uspreventiveservicestaskforce.org/announcements.htm. Accessed March 6, 2013.
3. USPSTF. Prevention of falls in community dwelling older adults. Available at: http://www.uspreventiveservicestaskforce.org/uspstf11/fallsprevention/fallsprevrs.htm. Accessed March 6, 2013.
4. USPSTF. Screening for and management of obesity in adults. Available at: http://www.uspreventiveservicestaskforce.org/uspstf11/obeseadult/obesers.htm. Accessed March 6, 2013.
5. USPSTF. Behavioral counseling to prevent skin cancer. Available at: http://www.uspreventiveservicestaskforce.org/uspstf11/skincancouns/skincancounsrs.htm. Accessed March 6, 2013.
6. USPSTF. Screening for intimate partner violence and abuse of elderly and vulnerable adults. Available at: http://www.uspreventiveservicestaskforce.org/uspstf12/ipvelder/ipvelderfinalrs.htm. Accessed March 6, 2013.
Vaccine update: The latest from ACIP
The 2013 immunization schedules have been published by the Centers for Disease Control and Prevention (CDC)’s Advisory Committee on Immunization Practices (ACIP).1,2 Perhaps the most noticeable change is a single schedule for infants, children, and adolescents, instead of the previous 2 schedules (for those ages 0-6 years, and for those ages 7-18 years). Other major new recommendations include the following:
- tetanus-diphtheria-pertussis (Tdap) vaccine for individuals ≥65 years of age
- Tdap for pregnant women during every pregnancy
- meningococcal conjugate vaccine for high-risk infants and children
- pneumococcal conjugate vaccine for high-risk adults.
There are also minor changes in recommendations for the use of measles, mumps, and rubella (MMR) vaccine among those with human immunodeficiency virus (HIV) infection. The new immunization schedules can be found on the CDC’s immunization Web site, at http://www.cdc.gov/vaccines/schedules/index.html.
Previous Practice Alerts have reported on recommendation changes made throughout 2012, including removal of egg allergy as a contraindication for influenza vaccine for those who experience only hives after eating eggs,3 the addition of a simplified algorithm for deciding whether children younger than 9 years need one or 2 doses of influenza vaccine,3 and the addition of human papillomavirus vaccine as a routine recommendation for males ages 11 to 21 years.4
Tdap: Some recommendations are off label
Given the continuing elevated rates of pertussis in the United States and our understanding about the duration of protection and safety of the Tdap vaccine, ACIP has made new recommendations for the use of Tdap, including some off-label uses. Two Tdap products are available: Boostrix, approved for individuals ≥10 years, and Adacel, approved for individuals 11 to 64 years (TABLE 1).5 ACIP states that those ≥65 years may be vaccinated with Tdap, and that an opportunity for vaccination should not be missed; Adacel can be substituted if it is the only product available. To control the spread of pertussis to the most vulnerable, it is especially important to immunize grandparents, childcare providers, and those who are around infants.
TABLE 1
Available tetanus-diphtheria-pertussis vaccines5
| Trade name | Manufacturer | FDA-approved age for use* (y) | Pertussis antigens (mcg) | Diphtheria toxoid (Lf) | Tetanus toxoid (Lf) | |||
|---|---|---|---|---|---|---|---|---|
| PT | FHA | PRN | FIM | |||||
| Boostrix | GlaxoSmithKline Biologicals | ≥10 | 8 | 8 | 2.5 | — | 2.5 | 5 |
| Adacel | Sanofi Pasteur | 11-64 | 2.5 | 5 | 3 | 5† | 2 | 5 |
| FDA, Food and Drug Administration; FHA, filamentous hemagglutinin; FIM, fimbriae; Lf, limit of flocculation units; PRN, pertactin; PT, pertussis toxin. *Indicated as a single dose. †Types 2 and 3. | ||||||||
Wound management. If a tetanus booster is indicated for wound management in an individual ≥19 years who has never received Tdap, this product is preferred to Td.5 There is now no suggested minimum time interval for administering Tdap after Td. Currently only one dose of Tdap is recommended for adults (except for pregnant women, as described in the next section). But this may change as time passes and we learn more about the duration of protection from the acellular pertussis antigen in the vaccine.
Pregnancy. ACIP first recommended the use of Tdap during pregnancy in October 2011, in an attempt to provide protection for newborns through the transfer of maternal antibodies to the fetus.6 Recent evidence indicates that the duration of protective antibody levels wanes between pregnancies and may not be high enough to protect a newborn in subsequent pregnancies.7 ACIP voted in October 2012 to recommend Tdap for pregnant women during each pregnancy, at the gestational age of 27 through 36 weeks. If a mother does not receive Tdap during pregnancy and has never received it, she should be vaccinated soon after delivery.
The safety data for serial vaccination with Tdap in pregnant women is sparse, and ACIP considered this concern. In the opinion of ACIP, the potential benefits to the newborn, coupled with the high rate of pertussis, outweigh this concern, and efforts will be made to monitor for safety issues. If the rate of pertussis declines, ACIP will likely revisit this recommendation.
Meningococcal vaccine: No routine immunization for infants
A previous Practice Alert described 3 new products to protect infants and children against meningococcal disease, and identified issues that make recommendations about their use difficult at a time when rates of meningococcal disease in this age group are very low.8 At its October 2012 meeting, ACIP considered one of these products, HibMenCY (MenHibrix), which contains antigens against meningococcal serogroups C and Y and Haemophilus influenzae B (Hib).
ACIP voted not to recommend routine immunization against meningococcal disease in infants. However, HibMenCY was recommended for high-risk infants, and it was noted that it can be used as an Hib vaccine. The details of the recommendation appear in “When should you use HibMenCY in infants?”.9 The current recommendation also includes vaccinating high-risk infants ages 9 through 23 months with 2 doses of MenACWY-D (Menactra) with at least 8 weeks between doses. Only one of these products should be used, and ACIP does not cite a preference between them.
Vaccinate infants at increased risk for meningococcal disease with 4 doses of HibMenCY at 2, 4, 6, and 12-15 months. Candidates for vaccination are infants with recognized persistent complement pathway deficiencies and infants who have anatomic or functional asplenia (including sickle cell disease).
HibMenCY can also be used for infants ages 2-18 months in communities with serogroup C and Y meningococcal disease outbreaks for which vaccination is recommended.
ACIP does not recommend routine meningococcal vaccination for infants.
HibMenCY is safe and immunogenic and may be administered to infants to complete the routine Hib vaccination series. If HibMenCY is used to achieve protection against serogroups C and Y, HibMenCY should be used for all 4 doses of Hib vaccine.
Pneumococcal conjugate vaccine recommended for high-risk adults
There are now 2 products that provide protection for adults against pneumococcal disease: a 23-valent polysaccharide product (PPSV23) and a 13-valent conjugate product (PCV13). PPSV23 is recommended for all adults ≥65 years and for those <65 who are at high risk for pneumococcal disease or complications from pneumococcal disease. While PCV13 is approved by the FDA for all adults ≥50 years, ACIP recommends it only for those at higher risk for pneumococcal disease.10
ACIP also recommends that those at risk should receive both PCV13 and PPSV23. Give PCV13 first, followed by PPSV23 2 months later.10 However, if PPSV23 is given first, administer PCV13 12 months later. To complicate matters, for some risk categories it is recommended that patients receive a second dose of PPSV23 5 years after the first one. No more than 2 doses of PPSV23 should be given prior to age 65. This complicated set of recommendations is summarized in TABLE 2.10
TABLE 2
Indications for using pneumococcal vaccines in adults ≥19 years*10
| Risk group | Underlying medical conditions | PCV13 | PPSV23 | |
|---|---|---|---|---|
| Recommended | Recommended | Revaccination 5 years after first dose | ||
| Immunocompetent individuals | Chronic heart disease† | √ | ||
| Chronic lung disease‡ | √ | |||
| Diabetes mellitus | √ | |||
| Cerebrospinal fluid leak | √ | √ | ||
| Cochlear implant | √ | √ | ||
| Alcoholism | √ | |||
| Chronic liver disease, cirrhosis | √ | |||
| Cigarette smoking | √ | |||
| Individuals with functional or anatomic asplenia | Sickle cell disease/other hemoglobinopathy | √ | √ | √ |
| Congenital or acquired asplenia | √ | √ | √ | |
| Immunocompromised individuals | Congenital or acquired immunodeficiency§ | √ | √ | √ |
| Human immunodeficiency virus infection | √ | √ | √ | |
| Chronic renal failure | √ | √ | √ | |
| Nephrotic syndrome | √ | √ | √ | |
| Leukemia | √ | √ | √ | |
| Lymphoma | √ | √ | √ | |
| Hodgkin disease | √ | √ | √ | |
| Generalized malignancy | √ | √ | √ | |
| Iatrogenic immunosuppression|| | √ | √ | √ | |
| Solid organ transplant | √ | √ | √ | |
| Multiple myeloma | √ | √ | √ | |
| PCV13, 13-valent pneumococcal conjugate vaccine; PPSV23, 23-valent pneumococcal polysaccharide vaccine. *All adults ≥65 years should receive a dose of PPSV23, regardless of previous history of vaccination with pneumococcal vaccine. †Including congestive heart failure and cardiomyopathies; excluding hypertension. ‡Including chronic obstructive pulmonary disease, emphysema, and asthma. §Including B- (humoral) or T-lymphocyte deficiency, complement deficiencies (particularly C1, C2, C3, and C4 deficiencies), and phagocytic disorders (excluding chronic granulomatous disease). ||Diseases requiring treatment with immunosuppressive drugs, including long-term systemic corticosteroids and radiation therapy. | ||||
MMR for those with HIV and use of IG for measles prevention
The last set of significant changes to the schedules are updated recommendations for the use of MMR vaccine in those who have HIV infection, and the use of immune globulin to prevent measles in those previously unvaccinated who are exposed to the disease. Details of these recommendations can be found at http://www.cdc.gov/vaccines/recs/provisional/downloads/mmr-Oct-2012.pdf.
1. CDC. Advisory Committee on Immunization Practices (ACIP) recommended immunization schedules for persons aged 0 through 18 years—United States, 2013. MMWR Morb Mortal Wkly Rep. 2013;62:2-8.
2. CDC. Advisory Committee on Immunization Practices (ACIP) recommended immunization schedule for adults aged 19 years and older—United States, 2013. MMWR Morb Mortal Wkly Rep. 2013;62:9-19.
3. Campos-Outcalt D. Battling influenza: changes for the 2012-2013 season. J Fam Pract. 2012;61:606-609.
4. Campos-Outcalt D. HPV is now routinely recommended for males. J Fam Pract. 2012;61:38-40.
5. CDC. Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap) vaccine in adults aged 65 years and older—Advisory Committee on Immunization Practices (ACIP), 2012. MMWR Morb Mortal Wkly Rep. 2012;61:468-470.
6. CDC. Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine (Tdap) in pregnant women and persons who have or anticipate having close contact with an infant aged <12 months—Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep. 2011;60:1424-1426.
7. Liang JL. Review of evidence considered for pregnancy Tdap recommendation. Presented at: meeting of the Advisory Committee on Immunization Practices (ACIP); October 24, 2012; Atlanta, Ga. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-oct-2012/02-pertussis-Liang.pdf. Accessed December 15, 2012.
8. Campos-Outcalt D. Meningococcal vaccine for infants? J Fam Pract. 2012;61:482-484.
9. Cohn A. Considerations for use of meningococcal conjugate vaccines in infants. Presented at: meeting of the Advisory Committee on Immunization Practices (ACIP); October 24, 2012; Atlanta, Ga. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-oct-2012/04-MCV-Cohn.pdf. Accessed February 8, 2013.
10. CDC. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2012;61:816-819.
The 2013 immunization schedules have been published by the Centers for Disease Control and Prevention (CDC)’s Advisory Committee on Immunization Practices (ACIP).1,2 Perhaps the most noticeable change is a single schedule for infants, children, and adolescents, instead of the previous 2 schedules (for those ages 0-6 years, and for those ages 7-18 years). Other major new recommendations include the following:
- tetanus-diphtheria-pertussis (Tdap) vaccine for individuals ≥65 years of age
- Tdap for pregnant women during every pregnancy
- meningococcal conjugate vaccine for high-risk infants and children
- pneumococcal conjugate vaccine for high-risk adults.
There are also minor changes in recommendations for the use of measles, mumps, and rubella (MMR) vaccine among those with human immunodeficiency virus (HIV) infection. The new immunization schedules can be found on the CDC’s immunization Web site, at http://www.cdc.gov/vaccines/schedules/index.html.
Previous Practice Alerts have reported on recommendation changes made throughout 2012, including removal of egg allergy as a contraindication for influenza vaccine for those who experience only hives after eating eggs,3 the addition of a simplified algorithm for deciding whether children younger than 9 years need one or 2 doses of influenza vaccine,3 and the addition of human papillomavirus vaccine as a routine recommendation for males ages 11 to 21 years.4
Tdap: Some recommendations are off label
Given the continuing elevated rates of pertussis in the United States and our understanding about the duration of protection and safety of the Tdap vaccine, ACIP has made new recommendations for the use of Tdap, including some off-label uses. Two Tdap products are available: Boostrix, approved for individuals ≥10 years, and Adacel, approved for individuals 11 to 64 years (TABLE 1).5 ACIP states that those ≥65 years may be vaccinated with Tdap, and that an opportunity for vaccination should not be missed; Adacel can be substituted if it is the only product available. To control the spread of pertussis to the most vulnerable, it is especially important to immunize grandparents, childcare providers, and those who are around infants.
TABLE 1
Available tetanus-diphtheria-pertussis vaccines5
| Trade name | Manufacturer | FDA-approved age for use* (y) | Pertussis antigens (mcg) | Diphtheria toxoid (Lf) | Tetanus toxoid (Lf) | |||
|---|---|---|---|---|---|---|---|---|
| PT | FHA | PRN | FIM | |||||
| Boostrix | GlaxoSmithKline Biologicals | ≥10 | 8 | 8 | 2.5 | — | 2.5 | 5 |
| Adacel | Sanofi Pasteur | 11-64 | 2.5 | 5 | 3 | 5† | 2 | 5 |
| FDA, Food and Drug Administration; FHA, filamentous hemagglutinin; FIM, fimbriae; Lf, limit of flocculation units; PRN, pertactin; PT, pertussis toxin. *Indicated as a single dose. †Types 2 and 3. | ||||||||
Wound management. If a tetanus booster is indicated for wound management in an individual ≥19 years who has never received Tdap, this product is preferred to Td.5 There is now no suggested minimum time interval for administering Tdap after Td. Currently only one dose of Tdap is recommended for adults (except for pregnant women, as described in the next section). But this may change as time passes and we learn more about the duration of protection from the acellular pertussis antigen in the vaccine.
Pregnancy. ACIP first recommended the use of Tdap during pregnancy in October 2011, in an attempt to provide protection for newborns through the transfer of maternal antibodies to the fetus.6 Recent evidence indicates that the duration of protective antibody levels wanes between pregnancies and may not be high enough to protect a newborn in subsequent pregnancies.7 ACIP voted in October 2012 to recommend Tdap for pregnant women during each pregnancy, at the gestational age of 27 through 36 weeks. If a mother does not receive Tdap during pregnancy and has never received it, she should be vaccinated soon after delivery.
The safety data for serial vaccination with Tdap in pregnant women is sparse, and ACIP considered this concern. In the opinion of ACIP, the potential benefits to the newborn, coupled with the high rate of pertussis, outweigh this concern, and efforts will be made to monitor for safety issues. If the rate of pertussis declines, ACIP will likely revisit this recommendation.
Meningococcal vaccine: No routine immunization for infants
A previous Practice Alert described 3 new products to protect infants and children against meningococcal disease, and identified issues that make recommendations about their use difficult at a time when rates of meningococcal disease in this age group are very low.8 At its October 2012 meeting, ACIP considered one of these products, HibMenCY (MenHibrix), which contains antigens against meningococcal serogroups C and Y and Haemophilus influenzae B (Hib).
ACIP voted not to recommend routine immunization against meningococcal disease in infants. However, HibMenCY was recommended for high-risk infants, and it was noted that it can be used as an Hib vaccine. The details of the recommendation appear in “When should you use HibMenCY in infants?”.9 The current recommendation also includes vaccinating high-risk infants ages 9 through 23 months with 2 doses of MenACWY-D (Menactra) with at least 8 weeks between doses. Only one of these products should be used, and ACIP does not cite a preference between them.
Vaccinate infants at increased risk for meningococcal disease with 4 doses of HibMenCY at 2, 4, 6, and 12-15 months. Candidates for vaccination are infants with recognized persistent complement pathway deficiencies and infants who have anatomic or functional asplenia (including sickle cell disease).
HibMenCY can also be used for infants ages 2-18 months in communities with serogroup C and Y meningococcal disease outbreaks for which vaccination is recommended.
ACIP does not recommend routine meningococcal vaccination for infants.
HibMenCY is safe and immunogenic and may be administered to infants to complete the routine Hib vaccination series. If HibMenCY is used to achieve protection against serogroups C and Y, HibMenCY should be used for all 4 doses of Hib vaccine.
Pneumococcal conjugate vaccine recommended for high-risk adults
There are now 2 products that provide protection for adults against pneumococcal disease: a 23-valent polysaccharide product (PPSV23) and a 13-valent conjugate product (PCV13). PPSV23 is recommended for all adults ≥65 years and for those <65 who are at high risk for pneumococcal disease or complications from pneumococcal disease. While PCV13 is approved by the FDA for all adults ≥50 years, ACIP recommends it only for those at higher risk for pneumococcal disease.10
ACIP also recommends that those at risk should receive both PCV13 and PPSV23. Give PCV13 first, followed by PPSV23 2 months later.10 However, if PPSV23 is given first, administer PCV13 12 months later. To complicate matters, for some risk categories it is recommended that patients receive a second dose of PPSV23 5 years after the first one. No more than 2 doses of PPSV23 should be given prior to age 65. This complicated set of recommendations is summarized in TABLE 2.10
TABLE 2
Indications for using pneumococcal vaccines in adults ≥19 years*10
| Risk group | Underlying medical conditions | PCV13 | PPSV23 | |
|---|---|---|---|---|
| Recommended | Recommended | Revaccination 5 years after first dose | ||
| Immunocompetent individuals | Chronic heart disease† | √ | ||
| Chronic lung disease‡ | √ | |||
| Diabetes mellitus | √ | |||
| Cerebrospinal fluid leak | √ | √ | ||
| Cochlear implant | √ | √ | ||
| Alcoholism | √ | |||
| Chronic liver disease, cirrhosis | √ | |||
| Cigarette smoking | √ | |||
| Individuals with functional or anatomic asplenia | Sickle cell disease/other hemoglobinopathy | √ | √ | √ |
| Congenital or acquired asplenia | √ | √ | √ | |
| Immunocompromised individuals | Congenital or acquired immunodeficiency§ | √ | √ | √ |
| Human immunodeficiency virus infection | √ | √ | √ | |
| Chronic renal failure | √ | √ | √ | |
| Nephrotic syndrome | √ | √ | √ | |
| Leukemia | √ | √ | √ | |
| Lymphoma | √ | √ | √ | |
| Hodgkin disease | √ | √ | √ | |
| Generalized malignancy | √ | √ | √ | |
| Iatrogenic immunosuppression|| | √ | √ | √ | |
| Solid organ transplant | √ | √ | √ | |
| Multiple myeloma | √ | √ | √ | |
| PCV13, 13-valent pneumococcal conjugate vaccine; PPSV23, 23-valent pneumococcal polysaccharide vaccine. *All adults ≥65 years should receive a dose of PPSV23, regardless of previous history of vaccination with pneumococcal vaccine. †Including congestive heart failure and cardiomyopathies; excluding hypertension. ‡Including chronic obstructive pulmonary disease, emphysema, and asthma. §Including B- (humoral) or T-lymphocyte deficiency, complement deficiencies (particularly C1, C2, C3, and C4 deficiencies), and phagocytic disorders (excluding chronic granulomatous disease). ||Diseases requiring treatment with immunosuppressive drugs, including long-term systemic corticosteroids and radiation therapy. | ||||
MMR for those with HIV and use of IG for measles prevention
The last set of significant changes to the schedules are updated recommendations for the use of MMR vaccine in those who have HIV infection, and the use of immune globulin to prevent measles in those previously unvaccinated who are exposed to the disease. Details of these recommendations can be found at http://www.cdc.gov/vaccines/recs/provisional/downloads/mmr-Oct-2012.pdf.
The 2013 immunization schedules have been published by the Centers for Disease Control and Prevention (CDC)’s Advisory Committee on Immunization Practices (ACIP).1,2 Perhaps the most noticeable change is a single schedule for infants, children, and adolescents, instead of the previous 2 schedules (for those ages 0-6 years, and for those ages 7-18 years). Other major new recommendations include the following:
- tetanus-diphtheria-pertussis (Tdap) vaccine for individuals ≥65 years of age
- Tdap for pregnant women during every pregnancy
- meningococcal conjugate vaccine for high-risk infants and children
- pneumococcal conjugate vaccine for high-risk adults.
There are also minor changes in recommendations for the use of measles, mumps, and rubella (MMR) vaccine among those with human immunodeficiency virus (HIV) infection. The new immunization schedules can be found on the CDC’s immunization Web site, at http://www.cdc.gov/vaccines/schedules/index.html.
Previous Practice Alerts have reported on recommendation changes made throughout 2012, including removal of egg allergy as a contraindication for influenza vaccine for those who experience only hives after eating eggs,3 the addition of a simplified algorithm for deciding whether children younger than 9 years need one or 2 doses of influenza vaccine,3 and the addition of human papillomavirus vaccine as a routine recommendation for males ages 11 to 21 years.4
Tdap: Some recommendations are off label
Given the continuing elevated rates of pertussis in the United States and our understanding about the duration of protection and safety of the Tdap vaccine, ACIP has made new recommendations for the use of Tdap, including some off-label uses. Two Tdap products are available: Boostrix, approved for individuals ≥10 years, and Adacel, approved for individuals 11 to 64 years (TABLE 1).5 ACIP states that those ≥65 years may be vaccinated with Tdap, and that an opportunity for vaccination should not be missed; Adacel can be substituted if it is the only product available. To control the spread of pertussis to the most vulnerable, it is especially important to immunize grandparents, childcare providers, and those who are around infants.
TABLE 1
Available tetanus-diphtheria-pertussis vaccines5
| Trade name | Manufacturer | FDA-approved age for use* (y) | Pertussis antigens (mcg) | Diphtheria toxoid (Lf) | Tetanus toxoid (Lf) | |||
|---|---|---|---|---|---|---|---|---|
| PT | FHA | PRN | FIM | |||||
| Boostrix | GlaxoSmithKline Biologicals | ≥10 | 8 | 8 | 2.5 | — | 2.5 | 5 |
| Adacel | Sanofi Pasteur | 11-64 | 2.5 | 5 | 3 | 5† | 2 | 5 |
| FDA, Food and Drug Administration; FHA, filamentous hemagglutinin; FIM, fimbriae; Lf, limit of flocculation units; PRN, pertactin; PT, pertussis toxin. *Indicated as a single dose. †Types 2 and 3. | ||||||||
Wound management. If a tetanus booster is indicated for wound management in an individual ≥19 years who has never received Tdap, this product is preferred to Td.5 There is now no suggested minimum time interval for administering Tdap after Td. Currently only one dose of Tdap is recommended for adults (except for pregnant women, as described in the next section). But this may change as time passes and we learn more about the duration of protection from the acellular pertussis antigen in the vaccine.
Pregnancy. ACIP first recommended the use of Tdap during pregnancy in October 2011, in an attempt to provide protection for newborns through the transfer of maternal antibodies to the fetus.6 Recent evidence indicates that the duration of protective antibody levels wanes between pregnancies and may not be high enough to protect a newborn in subsequent pregnancies.7 ACIP voted in October 2012 to recommend Tdap for pregnant women during each pregnancy, at the gestational age of 27 through 36 weeks. If a mother does not receive Tdap during pregnancy and has never received it, she should be vaccinated soon after delivery.
The safety data for serial vaccination with Tdap in pregnant women is sparse, and ACIP considered this concern. In the opinion of ACIP, the potential benefits to the newborn, coupled with the high rate of pertussis, outweigh this concern, and efforts will be made to monitor for safety issues. If the rate of pertussis declines, ACIP will likely revisit this recommendation.
Meningococcal vaccine: No routine immunization for infants
A previous Practice Alert described 3 new products to protect infants and children against meningococcal disease, and identified issues that make recommendations about their use difficult at a time when rates of meningococcal disease in this age group are very low.8 At its October 2012 meeting, ACIP considered one of these products, HibMenCY (MenHibrix), which contains antigens against meningococcal serogroups C and Y and Haemophilus influenzae B (Hib).
ACIP voted not to recommend routine immunization against meningococcal disease in infants. However, HibMenCY was recommended for high-risk infants, and it was noted that it can be used as an Hib vaccine. The details of the recommendation appear in “When should you use HibMenCY in infants?”.9 The current recommendation also includes vaccinating high-risk infants ages 9 through 23 months with 2 doses of MenACWY-D (Menactra) with at least 8 weeks between doses. Only one of these products should be used, and ACIP does not cite a preference between them.
Vaccinate infants at increased risk for meningococcal disease with 4 doses of HibMenCY at 2, 4, 6, and 12-15 months. Candidates for vaccination are infants with recognized persistent complement pathway deficiencies and infants who have anatomic or functional asplenia (including sickle cell disease).
HibMenCY can also be used for infants ages 2-18 months in communities with serogroup C and Y meningococcal disease outbreaks for which vaccination is recommended.
ACIP does not recommend routine meningococcal vaccination for infants.
HibMenCY is safe and immunogenic and may be administered to infants to complete the routine Hib vaccination series. If HibMenCY is used to achieve protection against serogroups C and Y, HibMenCY should be used for all 4 doses of Hib vaccine.
Pneumococcal conjugate vaccine recommended for high-risk adults
There are now 2 products that provide protection for adults against pneumococcal disease: a 23-valent polysaccharide product (PPSV23) and a 13-valent conjugate product (PCV13). PPSV23 is recommended for all adults ≥65 years and for those <65 who are at high risk for pneumococcal disease or complications from pneumococcal disease. While PCV13 is approved by the FDA for all adults ≥50 years, ACIP recommends it only for those at higher risk for pneumococcal disease.10
ACIP also recommends that those at risk should receive both PCV13 and PPSV23. Give PCV13 first, followed by PPSV23 2 months later.10 However, if PPSV23 is given first, administer PCV13 12 months later. To complicate matters, for some risk categories it is recommended that patients receive a second dose of PPSV23 5 years after the first one. No more than 2 doses of PPSV23 should be given prior to age 65. This complicated set of recommendations is summarized in TABLE 2.10
TABLE 2
Indications for using pneumococcal vaccines in adults ≥19 years*10
| Risk group | Underlying medical conditions | PCV13 | PPSV23 | |
|---|---|---|---|---|
| Recommended | Recommended | Revaccination 5 years after first dose | ||
| Immunocompetent individuals | Chronic heart disease† | √ | ||
| Chronic lung disease‡ | √ | |||
| Diabetes mellitus | √ | |||
| Cerebrospinal fluid leak | √ | √ | ||
| Cochlear implant | √ | √ | ||
| Alcoholism | √ | |||
| Chronic liver disease, cirrhosis | √ | |||
| Cigarette smoking | √ | |||
| Individuals with functional or anatomic asplenia | Sickle cell disease/other hemoglobinopathy | √ | √ | √ |
| Congenital or acquired asplenia | √ | √ | √ | |
| Immunocompromised individuals | Congenital or acquired immunodeficiency§ | √ | √ | √ |
| Human immunodeficiency virus infection | √ | √ | √ | |
| Chronic renal failure | √ | √ | √ | |
| Nephrotic syndrome | √ | √ | √ | |
| Leukemia | √ | √ | √ | |
| Lymphoma | √ | √ | √ | |
| Hodgkin disease | √ | √ | √ | |
| Generalized malignancy | √ | √ | √ | |
| Iatrogenic immunosuppression|| | √ | √ | √ | |
| Solid organ transplant | √ | √ | √ | |
| Multiple myeloma | √ | √ | √ | |
| PCV13, 13-valent pneumococcal conjugate vaccine; PPSV23, 23-valent pneumococcal polysaccharide vaccine. *All adults ≥65 years should receive a dose of PPSV23, regardless of previous history of vaccination with pneumococcal vaccine. †Including congestive heart failure and cardiomyopathies; excluding hypertension. ‡Including chronic obstructive pulmonary disease, emphysema, and asthma. §Including B- (humoral) or T-lymphocyte deficiency, complement deficiencies (particularly C1, C2, C3, and C4 deficiencies), and phagocytic disorders (excluding chronic granulomatous disease). ||Diseases requiring treatment with immunosuppressive drugs, including long-term systemic corticosteroids and radiation therapy. | ||||
MMR for those with HIV and use of IG for measles prevention
The last set of significant changes to the schedules are updated recommendations for the use of MMR vaccine in those who have HIV infection, and the use of immune globulin to prevent measles in those previously unvaccinated who are exposed to the disease. Details of these recommendations can be found at http://www.cdc.gov/vaccines/recs/provisional/downloads/mmr-Oct-2012.pdf.
1. CDC. Advisory Committee on Immunization Practices (ACIP) recommended immunization schedules for persons aged 0 through 18 years—United States, 2013. MMWR Morb Mortal Wkly Rep. 2013;62:2-8.
2. CDC. Advisory Committee on Immunization Practices (ACIP) recommended immunization schedule for adults aged 19 years and older—United States, 2013. MMWR Morb Mortal Wkly Rep. 2013;62:9-19.
3. Campos-Outcalt D. Battling influenza: changes for the 2012-2013 season. J Fam Pract. 2012;61:606-609.
4. Campos-Outcalt D. HPV is now routinely recommended for males. J Fam Pract. 2012;61:38-40.
5. CDC. Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap) vaccine in adults aged 65 years and older—Advisory Committee on Immunization Practices (ACIP), 2012. MMWR Morb Mortal Wkly Rep. 2012;61:468-470.
6. CDC. Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine (Tdap) in pregnant women and persons who have or anticipate having close contact with an infant aged <12 months—Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep. 2011;60:1424-1426.
7. Liang JL. Review of evidence considered for pregnancy Tdap recommendation. Presented at: meeting of the Advisory Committee on Immunization Practices (ACIP); October 24, 2012; Atlanta, Ga. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-oct-2012/02-pertussis-Liang.pdf. Accessed December 15, 2012.
8. Campos-Outcalt D. Meningococcal vaccine for infants? J Fam Pract. 2012;61:482-484.
9. Cohn A. Considerations for use of meningococcal conjugate vaccines in infants. Presented at: meeting of the Advisory Committee on Immunization Practices (ACIP); October 24, 2012; Atlanta, Ga. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-oct-2012/04-MCV-Cohn.pdf. Accessed February 8, 2013.
10. CDC. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2012;61:816-819.
1. CDC. Advisory Committee on Immunization Practices (ACIP) recommended immunization schedules for persons aged 0 through 18 years—United States, 2013. MMWR Morb Mortal Wkly Rep. 2013;62:2-8.
2. CDC. Advisory Committee on Immunization Practices (ACIP) recommended immunization schedule for adults aged 19 years and older—United States, 2013. MMWR Morb Mortal Wkly Rep. 2013;62:9-19.
3. Campos-Outcalt D. Battling influenza: changes for the 2012-2013 season. J Fam Pract. 2012;61:606-609.
4. Campos-Outcalt D. HPV is now routinely recommended for males. J Fam Pract. 2012;61:38-40.
5. CDC. Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap) vaccine in adults aged 65 years and older—Advisory Committee on Immunization Practices (ACIP), 2012. MMWR Morb Mortal Wkly Rep. 2012;61:468-470.
6. CDC. Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine (Tdap) in pregnant women and persons who have or anticipate having close contact with an infant aged <12 months—Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep. 2011;60:1424-1426.
7. Liang JL. Review of evidence considered for pregnancy Tdap recommendation. Presented at: meeting of the Advisory Committee on Immunization Practices (ACIP); October 24, 2012; Atlanta, Ga. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-oct-2012/02-pertussis-Liang.pdf. Accessed December 15, 2012.
8. Campos-Outcalt D. Meningococcal vaccine for infants? J Fam Pract. 2012;61:482-484.
9. Cohn A. Considerations for use of meningococcal conjugate vaccines in infants. Presented at: meeting of the Advisory Committee on Immunization Practices (ACIP); October 24, 2012; Atlanta, Ga. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-oct-2012/04-MCV-Cohn.pdf. Accessed February 8, 2013.
10. CDC. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2012;61:816-819.
Hepatitis C: New CDC screening recommendations
The Centers for Disease Control and Prevention (CDC) recently released new recommendations for screening for hepatitis C virus (HCV) infection that include a one-time screening for everyone in the United States born between 1945 and 1965, regardless of risk.1 These new recommendations are an enhancement of, but not a replacement for, the recommendations for HCV screening made in 1998, which called for screening those at high risk.2
HCV causes considerable morbidity and mortality in this country. Approximately 17,000 new infections occurred in 2010.1 Between 2.7 and 3.9 million Americans (1%-1.5% of the population) are living with chronic HCV infection, and many do not know they are infected.1 This lack of awareness appears to be due to a failure of both health care providers to offer testing to those at known risk and patients to either acknowledge or recall past high-risk behaviors.
Those at highest risk for HCV infection are current or past users of illegal injected drugs and recipients of a blood transfusion before 1992 (when HCV screening of the blood supply was instituted). Other risk factors are listed in TABLE 1.1 Many of those with HCV infection do not report injection drug use or having received a transfusion prior to 1992, and they are not detected by current risk-based testing.
TABLE 1
Risk factors for HCV infection1
| Most common risks |
|
| Less common risks |
|
| HCV, hepatitis C virus |
Approximately three-fourths of those who acquire HCV are unable to clear the virus and become chronically infected.1 Twenty percent of these individuals will develop cirrhosis and 5% will die from an HCV-related liver disease, such as decompensated cirrhosis or hepatocellular carcinoma (HCC).3
New treatments. In 2011, 2 protease inhibitor drugs, telaprevir and boceprevir, were approved for the treatment of HCV geno-type 1. These are the first generation of a class of drugs called direct-acting antiviral agents (DAAs). When a DAA is added to the standard therapy of ribavirin and pegylated interferon, the rate of viral clearance increases (from 44% to 75% for telaprevir and from 38% to 63% for boceprevir).1 However, the adverse reactions caused by these new drugs can lead to a 34% rise in the rate at which patients stop treatment.1 Twenty potential new HCV antivirals are in clinical trials, and it is expected that treatment recommendations will change rapidly as some of these are approved.
Does treatment improve long-term outcomes?
Clinical guidelines recommend antiviral treatment for anyone with HCV infection and biopsy evidence of bridging fibrosis, septal fibrosis, or cirrhosis.4
A look at Tx and all-cause mortality. Studies looking at patient-oriented outcomes such as all-cause mortality and incidence of HCC have been conducted with pegylated interferon and ribavirin, without the newer DAAs. The most commonly cited study assessed all-cause mortality in a large sample of veterans with multiple comorbidities.
Those who achieved a sustained virological response after treatment exhibited a reduction in all-cause mortality >50% compared with nonresponders. This endpoint included substantially lower rates of liver-related deaths and cirrhosis complicated by ascites, variceal bleeding, or encephalopathy.5 However, in such a nonrandom clinical trial, an improved outcome for responders could be due to their relatively good health, with fewer comorbid conditions, and other undetected biases. While this study attempted to control for such biases, it didn’t provide evidence that treating infection detected by screening a low-risk population would improve intermediate or long-term outcomes.
Observational studies look at carcinoma incidence. Twelve observational studies have addressed treatment effects on HCC incidence. They showed a 75% reduction in HCC rates in those who achieved viral clearance compared with those who did not.1 Again, these studies did not compare treated and untreated patients in a controlled clinical trial; they looked only at treated individuals and compared the outcomes of responders and nonresponders.
HCV transmission research is lacking. The CDC found no studies in its evidence review that addressed the issue of HCV transmission. Nevertheless, the new recommendations state that HCV transmission was a critical factor in determining the strength of the recommendation for age cohort screening. It is expected that those who have a sustained viral response will be less likely to transmit the virus to others.
Why the 1945-1965 birth cohort?
The prevalence of HCV infection in those born between 1945 and 1965 is 3.25%, and three-fourths of all those with HCV infection in the United States are in this cohort. The FIGURE depicts the large difference in prevalence between this age group and others. Within 3 cohorts defined by date of birth (1945-1965, 1950-1970, and 1945-1970), the prevalence of HCV infection is twice as high in men than in women, and in black non-Hispanics than in white non-Hispanics and Mexican Americans. However, extending the birth cohort to those born through 1970 yields only a marginal difference in prevalence figures. The CDC justifies restricting the new universal screening recommendation to the 1945-1965 age group mainly on the results of focus groups in which the public identified this cohort as “baby boomers” who would likely adopt the recommendation.
FIGURE
Prevalence of hepatitis C virus antibody by year of birth1
*National Health and Nutrition Examination Survey, United States, 1988–1994 and 1999–2002.
Two-step screening process
Screen individuals using a test for antibodies to HCV (anti-HCV). If the anti-HCV test result is positive, order a test for HCV nucleic acid that gives either a quantitative measure of viral load or a qualitative assessment of presence or absence of virus. If the confirmatory nucleic acid test result is negative, the individual does not have chronic HCV infection and is among the approximately 25% who clear the virus on their own. They do not need further testing or treatment.
What to tell infected patients
If the confirmatory test result is positive, presume the patient has HCV infection and offer the advice contained in TABLE 2.1 Patients should undergo further assessment for possible chronic liver disease and, with the counsel of their physician, decide whether to initiate treatment. They should also take measures to protect the liver from further damage, such as reducing alcohol consumption, avoiding medication and herbal products that can damage the liver, maintaining an optimal weight, and receiving vaccines against hepatitis A and B, if still susceptible to these viruses. Finally, encourage patients to take steps to avoid transmission of HCV to others.
TABLE 2
Advice for your patients with HCV infection1
Consult a health care provider (either a primary care physician or specialist [eg, in hepatology, gastroenterology, or infectious disease]) for:
|
Protect the liver from further harm by:
|
Maintain optimal weight by:
|
Minimize the risk of infecting others by:
|
| BMI, body mass index; HCV, hepatitis C virus; HIV, human immunodeficiency virus. |
The decision on whether to begin treatment immediately is complicated by the large number of new antivirals in development, which will be available in the near future and may be more effective with fewer adverse effects.
Lingering controversies
Given the lack of evidence of improved outcomes with HCV screening in the general population, it will be interesting to see how widely accepted the new CDC recommendations will be. The US Preventive Services Task Force is in the process of revising its HCV screening recommendations. Given the Task Force’s evidence-based methodology and the lack of evidence on the benefits and harms of screening those with no reported risks, there may be some differences with the new CDC recommendations.
If the CDC’s assumption proves correct—ie, that the benefits of treating high-risk populations will also occur with treating detected infection in the general population—and if the age cohort screening recommendation is fully implemented, 47,000 cases of HCC and 15,000 liver transplants will be prevented.1
1. CDC. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945-1965. MMWR Morb Mortal Wkly Rep. 2012;61:1-18.Available at: http://www.cdc.gov/mmwr/pdf/rr/rr6104.pdf. Accessed October 5, 2012.
2. CDC. Recommendations for prevention and control of hepatitis C virus infection and HCV-related chronic disease. MMWR Morb Mortal Wkly Rep. 1998;47(RR-19):1-54.
3. Alter HJ, Seeff LB. Recovery, persistence, and sequelae in hepatitis C virus infection: a perspective on long-term outcome. Semin Liver Dis. 2000;20:17-35.
4. Ghany MG, Strader DB, Thomas DL, et al. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49:1335-1374.
5. Backus LI, Boothroyd DB, Phillips BR, et al. A sustained virological response reduces risk of all-cause mortality in patients with hepatitis C. Clin Gastroenterol Hepatol. 2011;9:509-516.
The Centers for Disease Control and Prevention (CDC) recently released new recommendations for screening for hepatitis C virus (HCV) infection that include a one-time screening for everyone in the United States born between 1945 and 1965, regardless of risk.1 These new recommendations are an enhancement of, but not a replacement for, the recommendations for HCV screening made in 1998, which called for screening those at high risk.2
HCV causes considerable morbidity and mortality in this country. Approximately 17,000 new infections occurred in 2010.1 Between 2.7 and 3.9 million Americans (1%-1.5% of the population) are living with chronic HCV infection, and many do not know they are infected.1 This lack of awareness appears to be due to a failure of both health care providers to offer testing to those at known risk and patients to either acknowledge or recall past high-risk behaviors.
Those at highest risk for HCV infection are current or past users of illegal injected drugs and recipients of a blood transfusion before 1992 (when HCV screening of the blood supply was instituted). Other risk factors are listed in TABLE 1.1 Many of those with HCV infection do not report injection drug use or having received a transfusion prior to 1992, and they are not detected by current risk-based testing.
TABLE 1
Risk factors for HCV infection1
| Most common risks |
|
| Less common risks |
|
| HCV, hepatitis C virus |
Approximately three-fourths of those who acquire HCV are unable to clear the virus and become chronically infected.1 Twenty percent of these individuals will develop cirrhosis and 5% will die from an HCV-related liver disease, such as decompensated cirrhosis or hepatocellular carcinoma (HCC).3
New treatments. In 2011, 2 protease inhibitor drugs, telaprevir and boceprevir, were approved for the treatment of HCV geno-type 1. These are the first generation of a class of drugs called direct-acting antiviral agents (DAAs). When a DAA is added to the standard therapy of ribavirin and pegylated interferon, the rate of viral clearance increases (from 44% to 75% for telaprevir and from 38% to 63% for boceprevir).1 However, the adverse reactions caused by these new drugs can lead to a 34% rise in the rate at which patients stop treatment.1 Twenty potential new HCV antivirals are in clinical trials, and it is expected that treatment recommendations will change rapidly as some of these are approved.
Does treatment improve long-term outcomes?
Clinical guidelines recommend antiviral treatment for anyone with HCV infection and biopsy evidence of bridging fibrosis, septal fibrosis, or cirrhosis.4
A look at Tx and all-cause mortality. Studies looking at patient-oriented outcomes such as all-cause mortality and incidence of HCC have been conducted with pegylated interferon and ribavirin, without the newer DAAs. The most commonly cited study assessed all-cause mortality in a large sample of veterans with multiple comorbidities.
Those who achieved a sustained virological response after treatment exhibited a reduction in all-cause mortality >50% compared with nonresponders. This endpoint included substantially lower rates of liver-related deaths and cirrhosis complicated by ascites, variceal bleeding, or encephalopathy.5 However, in such a nonrandom clinical trial, an improved outcome for responders could be due to their relatively good health, with fewer comorbid conditions, and other undetected biases. While this study attempted to control for such biases, it didn’t provide evidence that treating infection detected by screening a low-risk population would improve intermediate or long-term outcomes.
Observational studies look at carcinoma incidence. Twelve observational studies have addressed treatment effects on HCC incidence. They showed a 75% reduction in HCC rates in those who achieved viral clearance compared with those who did not.1 Again, these studies did not compare treated and untreated patients in a controlled clinical trial; they looked only at treated individuals and compared the outcomes of responders and nonresponders.
HCV transmission research is lacking. The CDC found no studies in its evidence review that addressed the issue of HCV transmission. Nevertheless, the new recommendations state that HCV transmission was a critical factor in determining the strength of the recommendation for age cohort screening. It is expected that those who have a sustained viral response will be less likely to transmit the virus to others.
Why the 1945-1965 birth cohort?
The prevalence of HCV infection in those born between 1945 and 1965 is 3.25%, and three-fourths of all those with HCV infection in the United States are in this cohort. The FIGURE depicts the large difference in prevalence between this age group and others. Within 3 cohorts defined by date of birth (1945-1965, 1950-1970, and 1945-1970), the prevalence of HCV infection is twice as high in men than in women, and in black non-Hispanics than in white non-Hispanics and Mexican Americans. However, extending the birth cohort to those born through 1970 yields only a marginal difference in prevalence figures. The CDC justifies restricting the new universal screening recommendation to the 1945-1965 age group mainly on the results of focus groups in which the public identified this cohort as “baby boomers” who would likely adopt the recommendation.
FIGURE
Prevalence of hepatitis C virus antibody by year of birth1
*National Health and Nutrition Examination Survey, United States, 1988–1994 and 1999–2002.
Two-step screening process
Screen individuals using a test for antibodies to HCV (anti-HCV). If the anti-HCV test result is positive, order a test for HCV nucleic acid that gives either a quantitative measure of viral load or a qualitative assessment of presence or absence of virus. If the confirmatory nucleic acid test result is negative, the individual does not have chronic HCV infection and is among the approximately 25% who clear the virus on their own. They do not need further testing or treatment.
What to tell infected patients
If the confirmatory test result is positive, presume the patient has HCV infection and offer the advice contained in TABLE 2.1 Patients should undergo further assessment for possible chronic liver disease and, with the counsel of their physician, decide whether to initiate treatment. They should also take measures to protect the liver from further damage, such as reducing alcohol consumption, avoiding medication and herbal products that can damage the liver, maintaining an optimal weight, and receiving vaccines against hepatitis A and B, if still susceptible to these viruses. Finally, encourage patients to take steps to avoid transmission of HCV to others.
TABLE 2
Advice for your patients with HCV infection1
Consult a health care provider (either a primary care physician or specialist [eg, in hepatology, gastroenterology, or infectious disease]) for:
|
Protect the liver from further harm by:
|
Maintain optimal weight by:
|
Minimize the risk of infecting others by:
|
| BMI, body mass index; HCV, hepatitis C virus; HIV, human immunodeficiency virus. |
The decision on whether to begin treatment immediately is complicated by the large number of new antivirals in development, which will be available in the near future and may be more effective with fewer adverse effects.
Lingering controversies
Given the lack of evidence of improved outcomes with HCV screening in the general population, it will be interesting to see how widely accepted the new CDC recommendations will be. The US Preventive Services Task Force is in the process of revising its HCV screening recommendations. Given the Task Force’s evidence-based methodology and the lack of evidence on the benefits and harms of screening those with no reported risks, there may be some differences with the new CDC recommendations.
If the CDC’s assumption proves correct—ie, that the benefits of treating high-risk populations will also occur with treating detected infection in the general population—and if the age cohort screening recommendation is fully implemented, 47,000 cases of HCC and 15,000 liver transplants will be prevented.1
The Centers for Disease Control and Prevention (CDC) recently released new recommendations for screening for hepatitis C virus (HCV) infection that include a one-time screening for everyone in the United States born between 1945 and 1965, regardless of risk.1 These new recommendations are an enhancement of, but not a replacement for, the recommendations for HCV screening made in 1998, which called for screening those at high risk.2
HCV causes considerable morbidity and mortality in this country. Approximately 17,000 new infections occurred in 2010.1 Between 2.7 and 3.9 million Americans (1%-1.5% of the population) are living with chronic HCV infection, and many do not know they are infected.1 This lack of awareness appears to be due to a failure of both health care providers to offer testing to those at known risk and patients to either acknowledge or recall past high-risk behaviors.
Those at highest risk for HCV infection are current or past users of illegal injected drugs and recipients of a blood transfusion before 1992 (when HCV screening of the blood supply was instituted). Other risk factors are listed in TABLE 1.1 Many of those with HCV infection do not report injection drug use or having received a transfusion prior to 1992, and they are not detected by current risk-based testing.
TABLE 1
Risk factors for HCV infection1
| Most common risks |
|
| Less common risks |
|
| HCV, hepatitis C virus |
Approximately three-fourths of those who acquire HCV are unable to clear the virus and become chronically infected.1 Twenty percent of these individuals will develop cirrhosis and 5% will die from an HCV-related liver disease, such as decompensated cirrhosis or hepatocellular carcinoma (HCC).3
New treatments. In 2011, 2 protease inhibitor drugs, telaprevir and boceprevir, were approved for the treatment of HCV geno-type 1. These are the first generation of a class of drugs called direct-acting antiviral agents (DAAs). When a DAA is added to the standard therapy of ribavirin and pegylated interferon, the rate of viral clearance increases (from 44% to 75% for telaprevir and from 38% to 63% for boceprevir).1 However, the adverse reactions caused by these new drugs can lead to a 34% rise in the rate at which patients stop treatment.1 Twenty potential new HCV antivirals are in clinical trials, and it is expected that treatment recommendations will change rapidly as some of these are approved.
Does treatment improve long-term outcomes?
Clinical guidelines recommend antiviral treatment for anyone with HCV infection and biopsy evidence of bridging fibrosis, septal fibrosis, or cirrhosis.4
A look at Tx and all-cause mortality. Studies looking at patient-oriented outcomes such as all-cause mortality and incidence of HCC have been conducted with pegylated interferon and ribavirin, without the newer DAAs. The most commonly cited study assessed all-cause mortality in a large sample of veterans with multiple comorbidities.
Those who achieved a sustained virological response after treatment exhibited a reduction in all-cause mortality >50% compared with nonresponders. This endpoint included substantially lower rates of liver-related deaths and cirrhosis complicated by ascites, variceal bleeding, or encephalopathy.5 However, in such a nonrandom clinical trial, an improved outcome for responders could be due to their relatively good health, with fewer comorbid conditions, and other undetected biases. While this study attempted to control for such biases, it didn’t provide evidence that treating infection detected by screening a low-risk population would improve intermediate or long-term outcomes.
Observational studies look at carcinoma incidence. Twelve observational studies have addressed treatment effects on HCC incidence. They showed a 75% reduction in HCC rates in those who achieved viral clearance compared with those who did not.1 Again, these studies did not compare treated and untreated patients in a controlled clinical trial; they looked only at treated individuals and compared the outcomes of responders and nonresponders.
HCV transmission research is lacking. The CDC found no studies in its evidence review that addressed the issue of HCV transmission. Nevertheless, the new recommendations state that HCV transmission was a critical factor in determining the strength of the recommendation for age cohort screening. It is expected that those who have a sustained viral response will be less likely to transmit the virus to others.
Why the 1945-1965 birth cohort?
The prevalence of HCV infection in those born between 1945 and 1965 is 3.25%, and three-fourths of all those with HCV infection in the United States are in this cohort. The FIGURE depicts the large difference in prevalence between this age group and others. Within 3 cohorts defined by date of birth (1945-1965, 1950-1970, and 1945-1970), the prevalence of HCV infection is twice as high in men than in women, and in black non-Hispanics than in white non-Hispanics and Mexican Americans. However, extending the birth cohort to those born through 1970 yields only a marginal difference in prevalence figures. The CDC justifies restricting the new universal screening recommendation to the 1945-1965 age group mainly on the results of focus groups in which the public identified this cohort as “baby boomers” who would likely adopt the recommendation.
FIGURE
Prevalence of hepatitis C virus antibody by year of birth1
*National Health and Nutrition Examination Survey, United States, 1988–1994 and 1999–2002.
Two-step screening process
Screen individuals using a test for antibodies to HCV (anti-HCV). If the anti-HCV test result is positive, order a test for HCV nucleic acid that gives either a quantitative measure of viral load or a qualitative assessment of presence or absence of virus. If the confirmatory nucleic acid test result is negative, the individual does not have chronic HCV infection and is among the approximately 25% who clear the virus on their own. They do not need further testing or treatment.
What to tell infected patients
If the confirmatory test result is positive, presume the patient has HCV infection and offer the advice contained in TABLE 2.1 Patients should undergo further assessment for possible chronic liver disease and, with the counsel of their physician, decide whether to initiate treatment. They should also take measures to protect the liver from further damage, such as reducing alcohol consumption, avoiding medication and herbal products that can damage the liver, maintaining an optimal weight, and receiving vaccines against hepatitis A and B, if still susceptible to these viruses. Finally, encourage patients to take steps to avoid transmission of HCV to others.
TABLE 2
Advice for your patients with HCV infection1
Consult a health care provider (either a primary care physician or specialist [eg, in hepatology, gastroenterology, or infectious disease]) for:
|
Protect the liver from further harm by:
|
Maintain optimal weight by:
|
Minimize the risk of infecting others by:
|
| BMI, body mass index; HCV, hepatitis C virus; HIV, human immunodeficiency virus. |
The decision on whether to begin treatment immediately is complicated by the large number of new antivirals in development, which will be available in the near future and may be more effective with fewer adverse effects.
Lingering controversies
Given the lack of evidence of improved outcomes with HCV screening in the general population, it will be interesting to see how widely accepted the new CDC recommendations will be. The US Preventive Services Task Force is in the process of revising its HCV screening recommendations. Given the Task Force’s evidence-based methodology and the lack of evidence on the benefits and harms of screening those with no reported risks, there may be some differences with the new CDC recommendations.
If the CDC’s assumption proves correct—ie, that the benefits of treating high-risk populations will also occur with treating detected infection in the general population—and if the age cohort screening recommendation is fully implemented, 47,000 cases of HCC and 15,000 liver transplants will be prevented.1
1. CDC. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945-1965. MMWR Morb Mortal Wkly Rep. 2012;61:1-18.Available at: http://www.cdc.gov/mmwr/pdf/rr/rr6104.pdf. Accessed October 5, 2012.
2. CDC. Recommendations for prevention and control of hepatitis C virus infection and HCV-related chronic disease. MMWR Morb Mortal Wkly Rep. 1998;47(RR-19):1-54.
3. Alter HJ, Seeff LB. Recovery, persistence, and sequelae in hepatitis C virus infection: a perspective on long-term outcome. Semin Liver Dis. 2000;20:17-35.
4. Ghany MG, Strader DB, Thomas DL, et al. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49:1335-1374.
5. Backus LI, Boothroyd DB, Phillips BR, et al. A sustained virological response reduces risk of all-cause mortality in patients with hepatitis C. Clin Gastroenterol Hepatol. 2011;9:509-516.
1. CDC. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945-1965. MMWR Morb Mortal Wkly Rep. 2012;61:1-18.Available at: http://www.cdc.gov/mmwr/pdf/rr/rr6104.pdf. Accessed October 5, 2012.
2. CDC. Recommendations for prevention and control of hepatitis C virus infection and HCV-related chronic disease. MMWR Morb Mortal Wkly Rep. 1998;47(RR-19):1-54.
3. Alter HJ, Seeff LB. Recovery, persistence, and sequelae in hepatitis C virus infection: a perspective on long-term outcome. Semin Liver Dis. 2000;20:17-35.
4. Ghany MG, Strader DB, Thomas DL, et al. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49:1335-1374.
5. Backus LI, Boothroyd DB, Phillips BR, et al. A sustained virological response reduces risk of all-cause mortality in patients with hepatitis C. Clin Gastroenterol Hepatol. 2011;9:509-516.
Battling influenza: Changes for the 2012-2013 season
By all measures, last year’s flu season was a mild one that peaked in early March. The number of cases reported, the total number of pneumonia/influenza deaths, and the number of pediatric deaths were all comparatively low.1 The main circulating strains were the same as the previous 2 years, leaving much of the population protected by immunity gained from natural infections. And there was a good match between the vaccine and the circulating strains, leading to higher vaccine effectiveness.
So what’s new—and what remains the same—for the 2012-2013 season? Annual flu vaccination continues to be recommended for everyone ≥6 months of age, starting as soon as the vaccine is available and continuing through the influenza season. This season’s vaccine contains 2 new strains in addition to the A/California/7/2009 (H1N1) strain that has been in the vaccine the past 2 seasons and was used for a monovalent vaccine in 2009. The new strains are A/Victoria/361/2011 (H3N2) and B/Wisconsin/1/2010.2
Each year, influenza experts decide which strains to include in the vaccine based on the ones currently circulating and the ones expected to circulate into the next season. The degree to which vaccine strains match circulating strains will determine the effectiveness of the vaccine.
A variety of products
The number of influenza vaccine products has increased, and they differ in the ages for which they are approved.2 All trivalent inactivated vaccine (TIV) products are given intramuscularly, with the exception of a relatively new product, Fluzone Intradermal (Sanofi Pasteur). There is also FluMist (MedImmune), a live-attenuated influenza vaccine (LAIV), administered as a nasal spray. And, for adults ≥65 years, the product Fluzone High-Dose contains 4 times the antigen content of other vaccines for this age group. The Centers for Disease Control and Prevention (CDC) does not express a preference for any one of the TIV products over the others.
A new algorithm for children <9 years
To mount an adequate immune response in children <9 years, 2 doses of vaccine are needed in the first year of receiving vaccine. The time between doses should be at least 4 weeks; longer intervals do not appear to matter. This leads to a complex algorithm when taking into consideration vaccines given in past years. To simplify the issue, the CDC has approved an algorithm for this season that involves only 2 questions (FIGURE 1).
However, strict adherence to this algorithm will result in some children receiving 2 doses when one would suffice. If a child has received at least 2 doses of seasonal influenza vaccine in any prior season, and at least one dose of A/California/7/2009 (H1N1), then just a single dose of vaccine is needed this year. The A/California antigen could have been in the monovalent product in 2009 or the regular trivalent products in the past 2 seasons. If any doubt or confusion exists, administer 2 doses, 4 weeks apart. There is no harm in receiving 2 doses if only one is needed.
FIGURE 1
The CDC’s new dosing algorithm for children 6 months–8 years receiving influenza vaccine in the 2012-2013 flu season2
*Doses should be administered at least 4 weeks apart.
†For simplicity, this algorithm takes into consideration only doses of seasonal influenza vaccine received since July 1, 2010. However, if a child 6 months through 8 years of age is known to have received at least 2 seasonal influenza vaccines during any prior season, and at least one dose of a 2009 (H1N1)-containing vaccine-—ie, either 2010-2011 or 2011-2012 seasonal vaccine or the monovalent 2009 (H1N1) vaccine—then the child needs only one dose for 2012-2013.
Egg allergy precautions
While all influenza vaccines are manufactured by injecting virus into chicken eggs, the amount of egg protein in the vaccine is extremely low. Those who react to egg products only with hives can receive TIV (not LAIV) under the care of a clinician who is familiar with egg allergy manifestations. (Patients should be observed for 30 minutes after vaccine administration.) Those who have more severe reactions to eggs (FIGURE 2) require assessment by a physician with allergy expertise before receiving an influenza vaccine. All facilities that administer vaccines should be equipped to respond to anaphylaxis, and all providers who administer vaccines should be adequately trained in anaphylaxis management. The CDC made these recommendations last year, and there was no increase in egg allergy adverse events following vaccination reported during the 2011-2012 flu season.3
FIGURE 2
ACIP 2012-2013 recommendations regarding influenza vaccination for those with egg allergy2
TIV, trivalent inactivated vaccine.
*Individuals with egg allergy may tolerate egg in baked products (eg, bread, cake). Tolerance to egg-containing foods does not exclude the possibility of egg allergy.
Febrile seizures and vaccine safety
Last flu season, vaccine safety surveillance detected an increased risk for febrile seizures among children 6 months to 4 years during the 24 hours after receiving TIV, when it was given at the same time as 13-valent pneumococcal conjugate vaccine (PCV13).3 This increased risk was <1 per 1000 children vaccinated, and the Advisory Committee on Immunization Practices (ACIP) did not consider it significant enough to warrant any changes in TIV or PCV13 recommendations.
No other safety concerns arose for influenza vaccines last year. The vaccine safety monitoring system looks specifically at Guillain-Barré syndrome (GBS), and it detected no increased risk for GBS related to influenza vaccine.3
Newer quadrivalent vaccines
Historically, influenza vaccines have contained 3 antigens: 2 type A and 1 type B. A newly approved quadrivalent LAIV (FluMist Quadrivalent, MedImmune) contains 2 antigenically different B strains. The inclusion of 2 B strains is expected to increase the likelihood of the vaccine matching the circulating B influenza strains and thereby increase vaccine effectiveness. This new product will probably not be available this coming flu season, but will be marketed for 2013-2014. Other, inactivated, quadrivalent vaccines are also in development and should be available in future flu seasons.
Improving influenza vaccine coverage
In 2011, only 36.3% of people ≥6 months of age had received influenza vaccination by the first week in November (36.7% of children 6 months to 17 years and 36.2% of adults ≥18 years).4 The Community Preventive Services Task Force (a nonfederal group whose members are appointed by the director of the CDC) recommends a number of evidence-based interventions to increase vaccine rates, including patient reminder and recall systems, provider quality assessment and feedback, and standing orders.5 Increased coverage is important if we are to lower the annual morbidity and mortality associated with influenza.
The role of antivirals
All influenza A and B strains currently circulating have low or zero rates of resistance to the neuraminidase inhibitors, oseltamivir (Tamiflu) and zanamivir (Relenza). The circulating A strains continue to have high levels of resistance to the adamantanes (amantadine and rimantadine). Therefore, use only the neuraminidase inhibitors to treat influenza, and for pre- and post exposure chemoprevention.1
Those who should receive treatment include anyone with suspected or confirmed influenza who is hospitalized or who meets specific criteria (TABLE). Details regarding influenza antivirals, doses, and duration of treatment—as well as indications for chemoprevention—are on the CDC influenza Web site (http://www.cdc.gov/flu/professionals/antivirals/antiviral-use-influenza.htm#indications).
Table
Indications for treatment of influenza with antivirals
|
| BMI, body mass index; HIV, human immunodeficiency virus. Source: CDC. Antiviral agents for the treatment and chemoprophylaxis of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2011;60:1-24. |
1. Finelli L. Influenza surveillance season summary. Presented at: meeting of the Advisory Committee on Immunization Practices (ACIP); June 20, 2012; Atlanta, GA. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-jun-2012/02-influenza-finelli.pdf. Accessed July 15, 2012.
2. CDC. Prevention and control of influenza with vaccines: Recommendations of the Advisory Committee on Immunization Practices (ACIP)—United States, 2012-13 influenza season. MMWR Morb Mortal Wkly Rep. 2012;61:613–618.
3. Shimabukuro T. Update on influenza vaccine safety monitoring. Presented at: meeting of the Advisory Committee on Immunization Practices (ACIP); June 20, 2012; Atlanta, GA. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-jun-2012/03-influenza-Shimabukuro.pdf. Accessed July 15, 2012.
4. CDC. National mid-season flu vaccination coverage. Available at: http://www.cdc.gov/flu/professionals/vaccination/national-flu-survey.htm. Accessed July 15, 2012.
5. Community Preventive Services Task Force. Vaccines to prevent diseases: universally recommended vaccines. Available at: http://www.thecommunityguide.org/vaccines/universally/index.html. Accessed July 15, 2012.
By all measures, last year’s flu season was a mild one that peaked in early March. The number of cases reported, the total number of pneumonia/influenza deaths, and the number of pediatric deaths were all comparatively low.1 The main circulating strains were the same as the previous 2 years, leaving much of the population protected by immunity gained from natural infections. And there was a good match between the vaccine and the circulating strains, leading to higher vaccine effectiveness.
So what’s new—and what remains the same—for the 2012-2013 season? Annual flu vaccination continues to be recommended for everyone ≥6 months of age, starting as soon as the vaccine is available and continuing through the influenza season. This season’s vaccine contains 2 new strains in addition to the A/California/7/2009 (H1N1) strain that has been in the vaccine the past 2 seasons and was used for a monovalent vaccine in 2009. The new strains are A/Victoria/361/2011 (H3N2) and B/Wisconsin/1/2010.2
Each year, influenza experts decide which strains to include in the vaccine based on the ones currently circulating and the ones expected to circulate into the next season. The degree to which vaccine strains match circulating strains will determine the effectiveness of the vaccine.
A variety of products
The number of influenza vaccine products has increased, and they differ in the ages for which they are approved.2 All trivalent inactivated vaccine (TIV) products are given intramuscularly, with the exception of a relatively new product, Fluzone Intradermal (Sanofi Pasteur). There is also FluMist (MedImmune), a live-attenuated influenza vaccine (LAIV), administered as a nasal spray. And, for adults ≥65 years, the product Fluzone High-Dose contains 4 times the antigen content of other vaccines for this age group. The Centers for Disease Control and Prevention (CDC) does not express a preference for any one of the TIV products over the others.
A new algorithm for children <9 years
To mount an adequate immune response in children <9 years, 2 doses of vaccine are needed in the first year of receiving vaccine. The time between doses should be at least 4 weeks; longer intervals do not appear to matter. This leads to a complex algorithm when taking into consideration vaccines given in past years. To simplify the issue, the CDC has approved an algorithm for this season that involves only 2 questions (FIGURE 1).
However, strict adherence to this algorithm will result in some children receiving 2 doses when one would suffice. If a child has received at least 2 doses of seasonal influenza vaccine in any prior season, and at least one dose of A/California/7/2009 (H1N1), then just a single dose of vaccine is needed this year. The A/California antigen could have been in the monovalent product in 2009 or the regular trivalent products in the past 2 seasons. If any doubt or confusion exists, administer 2 doses, 4 weeks apart. There is no harm in receiving 2 doses if only one is needed.
FIGURE 1
The CDC’s new dosing algorithm for children 6 months–8 years receiving influenza vaccine in the 2012-2013 flu season2
*Doses should be administered at least 4 weeks apart.
†For simplicity, this algorithm takes into consideration only doses of seasonal influenza vaccine received since July 1, 2010. However, if a child 6 months through 8 years of age is known to have received at least 2 seasonal influenza vaccines during any prior season, and at least one dose of a 2009 (H1N1)-containing vaccine-—ie, either 2010-2011 or 2011-2012 seasonal vaccine or the monovalent 2009 (H1N1) vaccine—then the child needs only one dose for 2012-2013.
Egg allergy precautions
While all influenza vaccines are manufactured by injecting virus into chicken eggs, the amount of egg protein in the vaccine is extremely low. Those who react to egg products only with hives can receive TIV (not LAIV) under the care of a clinician who is familiar with egg allergy manifestations. (Patients should be observed for 30 minutes after vaccine administration.) Those who have more severe reactions to eggs (FIGURE 2) require assessment by a physician with allergy expertise before receiving an influenza vaccine. All facilities that administer vaccines should be equipped to respond to anaphylaxis, and all providers who administer vaccines should be adequately trained in anaphylaxis management. The CDC made these recommendations last year, and there was no increase in egg allergy adverse events following vaccination reported during the 2011-2012 flu season.3
FIGURE 2
ACIP 2012-2013 recommendations regarding influenza vaccination for those with egg allergy2
TIV, trivalent inactivated vaccine.
*Individuals with egg allergy may tolerate egg in baked products (eg, bread, cake). Tolerance to egg-containing foods does not exclude the possibility of egg allergy.
Febrile seizures and vaccine safety
Last flu season, vaccine safety surveillance detected an increased risk for febrile seizures among children 6 months to 4 years during the 24 hours after receiving TIV, when it was given at the same time as 13-valent pneumococcal conjugate vaccine (PCV13).3 This increased risk was <1 per 1000 children vaccinated, and the Advisory Committee on Immunization Practices (ACIP) did not consider it significant enough to warrant any changes in TIV or PCV13 recommendations.
No other safety concerns arose for influenza vaccines last year. The vaccine safety monitoring system looks specifically at Guillain-Barré syndrome (GBS), and it detected no increased risk for GBS related to influenza vaccine.3
Newer quadrivalent vaccines
Historically, influenza vaccines have contained 3 antigens: 2 type A and 1 type B. A newly approved quadrivalent LAIV (FluMist Quadrivalent, MedImmune) contains 2 antigenically different B strains. The inclusion of 2 B strains is expected to increase the likelihood of the vaccine matching the circulating B influenza strains and thereby increase vaccine effectiveness. This new product will probably not be available this coming flu season, but will be marketed for 2013-2014. Other, inactivated, quadrivalent vaccines are also in development and should be available in future flu seasons.
Improving influenza vaccine coverage
In 2011, only 36.3% of people ≥6 months of age had received influenza vaccination by the first week in November (36.7% of children 6 months to 17 years and 36.2% of adults ≥18 years).4 The Community Preventive Services Task Force (a nonfederal group whose members are appointed by the director of the CDC) recommends a number of evidence-based interventions to increase vaccine rates, including patient reminder and recall systems, provider quality assessment and feedback, and standing orders.5 Increased coverage is important if we are to lower the annual morbidity and mortality associated with influenza.
The role of antivirals
All influenza A and B strains currently circulating have low or zero rates of resistance to the neuraminidase inhibitors, oseltamivir (Tamiflu) and zanamivir (Relenza). The circulating A strains continue to have high levels of resistance to the adamantanes (amantadine and rimantadine). Therefore, use only the neuraminidase inhibitors to treat influenza, and for pre- and post exposure chemoprevention.1
Those who should receive treatment include anyone with suspected or confirmed influenza who is hospitalized or who meets specific criteria (TABLE). Details regarding influenza antivirals, doses, and duration of treatment—as well as indications for chemoprevention—are on the CDC influenza Web site (http://www.cdc.gov/flu/professionals/antivirals/antiviral-use-influenza.htm#indications).
Table
Indications for treatment of influenza with antivirals
|
| BMI, body mass index; HIV, human immunodeficiency virus. Source: CDC. Antiviral agents for the treatment and chemoprophylaxis of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2011;60:1-24. |
By all measures, last year’s flu season was a mild one that peaked in early March. The number of cases reported, the total number of pneumonia/influenza deaths, and the number of pediatric deaths were all comparatively low.1 The main circulating strains were the same as the previous 2 years, leaving much of the population protected by immunity gained from natural infections. And there was a good match between the vaccine and the circulating strains, leading to higher vaccine effectiveness.
So what’s new—and what remains the same—for the 2012-2013 season? Annual flu vaccination continues to be recommended for everyone ≥6 months of age, starting as soon as the vaccine is available and continuing through the influenza season. This season’s vaccine contains 2 new strains in addition to the A/California/7/2009 (H1N1) strain that has been in the vaccine the past 2 seasons and was used for a monovalent vaccine in 2009. The new strains are A/Victoria/361/2011 (H3N2) and B/Wisconsin/1/2010.2
Each year, influenza experts decide which strains to include in the vaccine based on the ones currently circulating and the ones expected to circulate into the next season. The degree to which vaccine strains match circulating strains will determine the effectiveness of the vaccine.
A variety of products
The number of influenza vaccine products has increased, and they differ in the ages for which they are approved.2 All trivalent inactivated vaccine (TIV) products are given intramuscularly, with the exception of a relatively new product, Fluzone Intradermal (Sanofi Pasteur). There is also FluMist (MedImmune), a live-attenuated influenza vaccine (LAIV), administered as a nasal spray. And, for adults ≥65 years, the product Fluzone High-Dose contains 4 times the antigen content of other vaccines for this age group. The Centers for Disease Control and Prevention (CDC) does not express a preference for any one of the TIV products over the others.
A new algorithm for children <9 years
To mount an adequate immune response in children <9 years, 2 doses of vaccine are needed in the first year of receiving vaccine. The time between doses should be at least 4 weeks; longer intervals do not appear to matter. This leads to a complex algorithm when taking into consideration vaccines given in past years. To simplify the issue, the CDC has approved an algorithm for this season that involves only 2 questions (FIGURE 1).
However, strict adherence to this algorithm will result in some children receiving 2 doses when one would suffice. If a child has received at least 2 doses of seasonal influenza vaccine in any prior season, and at least one dose of A/California/7/2009 (H1N1), then just a single dose of vaccine is needed this year. The A/California antigen could have been in the monovalent product in 2009 or the regular trivalent products in the past 2 seasons. If any doubt or confusion exists, administer 2 doses, 4 weeks apart. There is no harm in receiving 2 doses if only one is needed.
FIGURE 1
The CDC’s new dosing algorithm for children 6 months–8 years receiving influenza vaccine in the 2012-2013 flu season2
*Doses should be administered at least 4 weeks apart.
†For simplicity, this algorithm takes into consideration only doses of seasonal influenza vaccine received since July 1, 2010. However, if a child 6 months through 8 years of age is known to have received at least 2 seasonal influenza vaccines during any prior season, and at least one dose of a 2009 (H1N1)-containing vaccine-—ie, either 2010-2011 or 2011-2012 seasonal vaccine or the monovalent 2009 (H1N1) vaccine—then the child needs only one dose for 2012-2013.
Egg allergy precautions
While all influenza vaccines are manufactured by injecting virus into chicken eggs, the amount of egg protein in the vaccine is extremely low. Those who react to egg products only with hives can receive TIV (not LAIV) under the care of a clinician who is familiar with egg allergy manifestations. (Patients should be observed for 30 minutes after vaccine administration.) Those who have more severe reactions to eggs (FIGURE 2) require assessment by a physician with allergy expertise before receiving an influenza vaccine. All facilities that administer vaccines should be equipped to respond to anaphylaxis, and all providers who administer vaccines should be adequately trained in anaphylaxis management. The CDC made these recommendations last year, and there was no increase in egg allergy adverse events following vaccination reported during the 2011-2012 flu season.3
FIGURE 2
ACIP 2012-2013 recommendations regarding influenza vaccination for those with egg allergy2
TIV, trivalent inactivated vaccine.
*Individuals with egg allergy may tolerate egg in baked products (eg, bread, cake). Tolerance to egg-containing foods does not exclude the possibility of egg allergy.
Febrile seizures and vaccine safety
Last flu season, vaccine safety surveillance detected an increased risk for febrile seizures among children 6 months to 4 years during the 24 hours after receiving TIV, when it was given at the same time as 13-valent pneumococcal conjugate vaccine (PCV13).3 This increased risk was <1 per 1000 children vaccinated, and the Advisory Committee on Immunization Practices (ACIP) did not consider it significant enough to warrant any changes in TIV or PCV13 recommendations.
No other safety concerns arose for influenza vaccines last year. The vaccine safety monitoring system looks specifically at Guillain-Barré syndrome (GBS), and it detected no increased risk for GBS related to influenza vaccine.3
Newer quadrivalent vaccines
Historically, influenza vaccines have contained 3 antigens: 2 type A and 1 type B. A newly approved quadrivalent LAIV (FluMist Quadrivalent, MedImmune) contains 2 antigenically different B strains. The inclusion of 2 B strains is expected to increase the likelihood of the vaccine matching the circulating B influenza strains and thereby increase vaccine effectiveness. This new product will probably not be available this coming flu season, but will be marketed for 2013-2014. Other, inactivated, quadrivalent vaccines are also in development and should be available in future flu seasons.
Improving influenza vaccine coverage
In 2011, only 36.3% of people ≥6 months of age had received influenza vaccination by the first week in November (36.7% of children 6 months to 17 years and 36.2% of adults ≥18 years).4 The Community Preventive Services Task Force (a nonfederal group whose members are appointed by the director of the CDC) recommends a number of evidence-based interventions to increase vaccine rates, including patient reminder and recall systems, provider quality assessment and feedback, and standing orders.5 Increased coverage is important if we are to lower the annual morbidity and mortality associated with influenza.
The role of antivirals
All influenza A and B strains currently circulating have low or zero rates of resistance to the neuraminidase inhibitors, oseltamivir (Tamiflu) and zanamivir (Relenza). The circulating A strains continue to have high levels of resistance to the adamantanes (amantadine and rimantadine). Therefore, use only the neuraminidase inhibitors to treat influenza, and for pre- and post exposure chemoprevention.1
Those who should receive treatment include anyone with suspected or confirmed influenza who is hospitalized or who meets specific criteria (TABLE). Details regarding influenza antivirals, doses, and duration of treatment—as well as indications for chemoprevention—are on the CDC influenza Web site (http://www.cdc.gov/flu/professionals/antivirals/antiviral-use-influenza.htm#indications).
Table
Indications for treatment of influenza with antivirals
|
| BMI, body mass index; HIV, human immunodeficiency virus. Source: CDC. Antiviral agents for the treatment and chemoprophylaxis of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2011;60:1-24. |
1. Finelli L. Influenza surveillance season summary. Presented at: meeting of the Advisory Committee on Immunization Practices (ACIP); June 20, 2012; Atlanta, GA. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-jun-2012/02-influenza-finelli.pdf. Accessed July 15, 2012.
2. CDC. Prevention and control of influenza with vaccines: Recommendations of the Advisory Committee on Immunization Practices (ACIP)—United States, 2012-13 influenza season. MMWR Morb Mortal Wkly Rep. 2012;61:613–618.
3. Shimabukuro T. Update on influenza vaccine safety monitoring. Presented at: meeting of the Advisory Committee on Immunization Practices (ACIP); June 20, 2012; Atlanta, GA. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-jun-2012/03-influenza-Shimabukuro.pdf. Accessed July 15, 2012.
4. CDC. National mid-season flu vaccination coverage. Available at: http://www.cdc.gov/flu/professionals/vaccination/national-flu-survey.htm. Accessed July 15, 2012.
5. Community Preventive Services Task Force. Vaccines to prevent diseases: universally recommended vaccines. Available at: http://www.thecommunityguide.org/vaccines/universally/index.html. Accessed July 15, 2012.
1. Finelli L. Influenza surveillance season summary. Presented at: meeting of the Advisory Committee on Immunization Practices (ACIP); June 20, 2012; Atlanta, GA. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-jun-2012/02-influenza-finelli.pdf. Accessed July 15, 2012.
2. CDC. Prevention and control of influenza with vaccines: Recommendations of the Advisory Committee on Immunization Practices (ACIP)—United States, 2012-13 influenza season. MMWR Morb Mortal Wkly Rep. 2012;61:613–618.
3. Shimabukuro T. Update on influenza vaccine safety monitoring. Presented at: meeting of the Advisory Committee on Immunization Practices (ACIP); June 20, 2012; Atlanta, GA. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-jun-2012/03-influenza-Shimabukuro.pdf. Accessed July 15, 2012.
4. CDC. National mid-season flu vaccination coverage. Available at: http://www.cdc.gov/flu/professionals/vaccination/national-flu-survey.htm. Accessed July 15, 2012.
5. Community Preventive Services Task Force. Vaccines to prevent diseases: universally recommended vaccines. Available at: http://www.thecommunityguide.org/vaccines/universally/index.html. Accessed July 15, 2012.
Meningococcal vaccine for infants?
The Advisory Committee on Immunization Practices (ACIP) is evaluating whether to recommend the use of meningococcal vaccines for infants and children <2 years.1 The decision may be made within the next 4 to 8 months. In its deliberation, ACIP must consider several issues, which I review here.
Current and impending vaccine options. Two quadrivalent meningococcal conjugate vaccines (MCV4) are licensed by the US Food and Drug Administration (FDA) for use in the United States: Men ACWY-CRM (Menveo, Novartis) and Men ACWY-D (Menactra, Sanofi Pasteur).2 Both vaccines protect against 4 meningococcal serogroups (A, C, Y, and W-135) and are approved for use among those ages 2 to 55 years. In addition, Menactra was recently licensed as a 2-dose series for children ages 9 to 23 months. ACIP recommends routine use of MCV4 for adolescents ages 11 to 18 years, with a preference for the first dose at ages 11 to 12 years; and for all individuals between the ages of 2 and 55 years who are at increased risk for meningococcal disease ( TABLE ).
Complicating matters is the pending availability of more formulations. In addition to the 2-dose Menactra option for children 9 to 23 months, Novartis has an application before the FDA for a 4-dose schedule with Menveo, given at ages 2, 4, 6, and 12 months. GlaxoSmithKline has just received approval from the FDA for MenHibrix, a combination vaccine that contains antigens against Haemophilus influenzae type b (Hib) and 2 meningococcal serogroups, C and Y, licensed as a 4-dose series given at ages 2, 4, 6, and 12 months.
These vaccines have proven to be immunogenic in infants without diminishing the effectiveness of other, co-administered vaccines in normal infant populations. They also appear to be safe, although the studies to date have not been sufficiently large to detect uncommon adverse events.3-7
Table
Patients at high risk for meningococcal disease
|
| Source: CDC. Updated recommendations for use of meningococcal conjugate vaccines—Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Morb Mortal Wkly Rep. 2011;60:72-76. |
Meningococcal disease incidence and prevalence are declining
One major consideration for ACIP is the changing epidemiology of meningococcal disease and the low prevalence of disease in all age groups, including infants. The incidence of meningococcal disease has declined in the United States since 1980, with a marked and sustained decline since 2000 ( FIGURE 1 ).8 This decline has occurred in all age groups including infants, who have the highest rate of infection ( FIGURE 2 ).8 This decline in incidence occurred for all serogroups, including serogroup B.8
Serogroup B. Among children <5 years, including infants, half of meningococcal disease is caused by serogroup B,8 and these infections would not be prevented by any of the currently licensed vaccines or by those under review. Furthermore, half of all infections occur before age 9 months8 —an age range for which Menactra in not approved.
Serogroup C and Y. One-third of infections with serogroups C and Y occur before the age of 6 months8 and would not be prevented by any of the new products. Also of note: From 2007 to 2009, the mean number of cases of serotype A or C infection occurring each year in children <5 years was 77.8
The impact on children vs adults. Meningococcal disease in children is generally less severe than that occurring in older age groups. Overall case fatality in children is 6%; 10% in those with serogroup B and 1% in those with serogroup Y.8 The disease in children does result in significant sequelae, however, with 10% suffering hearing loss and 1% to 2% requiring amputation. From 2007 to 2009, there were 4 to 8 deaths per year among children under age 5, and 8 to 12 children per year experienced serious sequelae.8
FIGURE 1
The incidence of meningococcal disease has declined steadily since 20008
1970-1996 National Notifiable Diseases Surveillance System data. 1997-2009 Active Bacterial Core surveillance data estimated to the US population.
FIGURE 2
Meningococcal disease has declined among infants and other age groups8
Active Bacterial Core surveillance cases from 1993-2009 estimated to the US population with 18% correction for underreporting.
ACIP’s dilemma
The low morbidity and mortality associated with meningococcal disease is one issue to consider when deciding whether to recommend new vaccines as part of the routine infant and child immunization schedule. The vaccine schedule is already crowded and complex, and parents increasingly are questioning the need for additional antigens.
In addition, the cost of vaccines for children has escalated over the past decade due mainly to the new, more expensive formulations.
The reason for a declining incidence of meningococcal disease is not fully known. It may be partly explained by increasing rates of vaccination among adolescents. However, the overall low rate of disease in the population makes assessing herd immunity difficult.
If ACIP decides to recommend vaccinating infants against meningococcal disease, it is unclear how long immunity will last, potentially necessitating a booster dose before the currently recommended adolescent dose.
Finally, in children at high risk, it is not fully known how meningococcal vaccines will affect the immune response to pneumococcal conjugate vaccine. This is an important consideration because the incidence of pneumococcal disease among these children is much higher than that of meningococcal disease.
1. CDC. Report from the Advisory Committee on Immunization Practices (ACIP): decision not to recommend routine vaccination of all children aged 2-10 years with quadrivalent meningococcal conjugate vaccine (MCV4). MMWR Morb Mortal Wkly Rep. 2008;57:462-465.
2. CDC. Licensure of a meningococcal conjugate vaccine for children aged 2 through 10 years and updated booster dose guidance for adolescents and other persons at increased risk for meningococcal disease—Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep. 2011;60:1018-1019.
3. Marshall GS, Marchant CD, Blatter M, et al. Immune response and one-year antibody persistence after a fourth dose of a novel Haemophilus influenzae type B and Neisseria meningitides serogroups C and T-tetanus toxoid conjugate vaccine (HibMenCY) at 12 to 15 months of age. Pediatr Infect Dis J. 2010;29:469-471.
4. Nolan T, Richmond P, Marshall H, et al. Immunogenicity and safety of an investigational combined Haemophilus influenzae type B-Neisseria meningitides serogroups C and Y-tetanus toxoid conjugate vaccine. Pediatr Infect Dis J. 2011;30:190-196.
5. Bryant KA, Marshall GS, Marchant CD, et al. Immunogenicity and safety of H influenzae type b-N meningitides C/Y conjugate vaccine in infants. Pediatrics. 2011;127:e1375-e1385.
6. Marshall GS, Marchant CD, Blatter M, et al. Co-administration of a novel Haemophilus influenzae type b and Neisseria meningitides serogroups C and Y-tetanus toxoid conjugate vaccine does not interfere with the immune response to antigens contained in infant vaccines routinely used in the United States. Hum Vaccin. 2011;7:258-264.
7. Perrett KP, Snape MD, Ford KJ, et al. Immunogenicity and immune memory of a nonadjuvanted quadrivalent meningococcal glycoconjugate vaccine in infants. Pediatr Infect Dis J. 2009;28:186-193.
8. MacNeil J. Epidemiology of meningococcal diseases in infants and young children. Paper presented at: Meeting of the Advisory Committee on Immunization Practices; October 25, 2011; Atlanta, GA.
The Advisory Committee on Immunization Practices (ACIP) is evaluating whether to recommend the use of meningococcal vaccines for infants and children <2 years.1 The decision may be made within the next 4 to 8 months. In its deliberation, ACIP must consider several issues, which I review here.
Current and impending vaccine options. Two quadrivalent meningococcal conjugate vaccines (MCV4) are licensed by the US Food and Drug Administration (FDA) for use in the United States: Men ACWY-CRM (Menveo, Novartis) and Men ACWY-D (Menactra, Sanofi Pasteur).2 Both vaccines protect against 4 meningococcal serogroups (A, C, Y, and W-135) and are approved for use among those ages 2 to 55 years. In addition, Menactra was recently licensed as a 2-dose series for children ages 9 to 23 months. ACIP recommends routine use of MCV4 for adolescents ages 11 to 18 years, with a preference for the first dose at ages 11 to 12 years; and for all individuals between the ages of 2 and 55 years who are at increased risk for meningococcal disease ( TABLE ).
Complicating matters is the pending availability of more formulations. In addition to the 2-dose Menactra option for children 9 to 23 months, Novartis has an application before the FDA for a 4-dose schedule with Menveo, given at ages 2, 4, 6, and 12 months. GlaxoSmithKline has just received approval from the FDA for MenHibrix, a combination vaccine that contains antigens against Haemophilus influenzae type b (Hib) and 2 meningococcal serogroups, C and Y, licensed as a 4-dose series given at ages 2, 4, 6, and 12 months.
These vaccines have proven to be immunogenic in infants without diminishing the effectiveness of other, co-administered vaccines in normal infant populations. They also appear to be safe, although the studies to date have not been sufficiently large to detect uncommon adverse events.3-7
Table
Patients at high risk for meningococcal disease
|
| Source: CDC. Updated recommendations for use of meningococcal conjugate vaccines—Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Morb Mortal Wkly Rep. 2011;60:72-76. |
Meningococcal disease incidence and prevalence are declining
One major consideration for ACIP is the changing epidemiology of meningococcal disease and the low prevalence of disease in all age groups, including infants. The incidence of meningococcal disease has declined in the United States since 1980, with a marked and sustained decline since 2000 ( FIGURE 1 ).8 This decline has occurred in all age groups including infants, who have the highest rate of infection ( FIGURE 2 ).8 This decline in incidence occurred for all serogroups, including serogroup B.8
Serogroup B. Among children <5 years, including infants, half of meningococcal disease is caused by serogroup B,8 and these infections would not be prevented by any of the currently licensed vaccines or by those under review. Furthermore, half of all infections occur before age 9 months8 —an age range for which Menactra in not approved.
Serogroup C and Y. One-third of infections with serogroups C and Y occur before the age of 6 months8 and would not be prevented by any of the new products. Also of note: From 2007 to 2009, the mean number of cases of serotype A or C infection occurring each year in children <5 years was 77.8
The impact on children vs adults. Meningococcal disease in children is generally less severe than that occurring in older age groups. Overall case fatality in children is 6%; 10% in those with serogroup B and 1% in those with serogroup Y.8 The disease in children does result in significant sequelae, however, with 10% suffering hearing loss and 1% to 2% requiring amputation. From 2007 to 2009, there were 4 to 8 deaths per year among children under age 5, and 8 to 12 children per year experienced serious sequelae.8
FIGURE 1
The incidence of meningococcal disease has declined steadily since 20008
1970-1996 National Notifiable Diseases Surveillance System data. 1997-2009 Active Bacterial Core surveillance data estimated to the US population.
FIGURE 2
Meningococcal disease has declined among infants and other age groups8
Active Bacterial Core surveillance cases from 1993-2009 estimated to the US population with 18% correction for underreporting.
ACIP’s dilemma
The low morbidity and mortality associated with meningococcal disease is one issue to consider when deciding whether to recommend new vaccines as part of the routine infant and child immunization schedule. The vaccine schedule is already crowded and complex, and parents increasingly are questioning the need for additional antigens.
In addition, the cost of vaccines for children has escalated over the past decade due mainly to the new, more expensive formulations.
The reason for a declining incidence of meningococcal disease is not fully known. It may be partly explained by increasing rates of vaccination among adolescents. However, the overall low rate of disease in the population makes assessing herd immunity difficult.
If ACIP decides to recommend vaccinating infants against meningococcal disease, it is unclear how long immunity will last, potentially necessitating a booster dose before the currently recommended adolescent dose.
Finally, in children at high risk, it is not fully known how meningococcal vaccines will affect the immune response to pneumococcal conjugate vaccine. This is an important consideration because the incidence of pneumococcal disease among these children is much higher than that of meningococcal disease.
The Advisory Committee on Immunization Practices (ACIP) is evaluating whether to recommend the use of meningococcal vaccines for infants and children <2 years.1 The decision may be made within the next 4 to 8 months. In its deliberation, ACIP must consider several issues, which I review here.
Current and impending vaccine options. Two quadrivalent meningococcal conjugate vaccines (MCV4) are licensed by the US Food and Drug Administration (FDA) for use in the United States: Men ACWY-CRM (Menveo, Novartis) and Men ACWY-D (Menactra, Sanofi Pasteur).2 Both vaccines protect against 4 meningococcal serogroups (A, C, Y, and W-135) and are approved for use among those ages 2 to 55 years. In addition, Menactra was recently licensed as a 2-dose series for children ages 9 to 23 months. ACIP recommends routine use of MCV4 for adolescents ages 11 to 18 years, with a preference for the first dose at ages 11 to 12 years; and for all individuals between the ages of 2 and 55 years who are at increased risk for meningococcal disease ( TABLE ).
Complicating matters is the pending availability of more formulations. In addition to the 2-dose Menactra option for children 9 to 23 months, Novartis has an application before the FDA for a 4-dose schedule with Menveo, given at ages 2, 4, 6, and 12 months. GlaxoSmithKline has just received approval from the FDA for MenHibrix, a combination vaccine that contains antigens against Haemophilus influenzae type b (Hib) and 2 meningococcal serogroups, C and Y, licensed as a 4-dose series given at ages 2, 4, 6, and 12 months.
These vaccines have proven to be immunogenic in infants without diminishing the effectiveness of other, co-administered vaccines in normal infant populations. They also appear to be safe, although the studies to date have not been sufficiently large to detect uncommon adverse events.3-7
Table
Patients at high risk for meningococcal disease
|
| Source: CDC. Updated recommendations for use of meningococcal conjugate vaccines—Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Morb Mortal Wkly Rep. 2011;60:72-76. |
Meningococcal disease incidence and prevalence are declining
One major consideration for ACIP is the changing epidemiology of meningococcal disease and the low prevalence of disease in all age groups, including infants. The incidence of meningococcal disease has declined in the United States since 1980, with a marked and sustained decline since 2000 ( FIGURE 1 ).8 This decline has occurred in all age groups including infants, who have the highest rate of infection ( FIGURE 2 ).8 This decline in incidence occurred for all serogroups, including serogroup B.8
Serogroup B. Among children <5 years, including infants, half of meningococcal disease is caused by serogroup B,8 and these infections would not be prevented by any of the currently licensed vaccines or by those under review. Furthermore, half of all infections occur before age 9 months8 —an age range for which Menactra in not approved.
Serogroup C and Y. One-third of infections with serogroups C and Y occur before the age of 6 months8 and would not be prevented by any of the new products. Also of note: From 2007 to 2009, the mean number of cases of serotype A or C infection occurring each year in children <5 years was 77.8
The impact on children vs adults. Meningococcal disease in children is generally less severe than that occurring in older age groups. Overall case fatality in children is 6%; 10% in those with serogroup B and 1% in those with serogroup Y.8 The disease in children does result in significant sequelae, however, with 10% suffering hearing loss and 1% to 2% requiring amputation. From 2007 to 2009, there were 4 to 8 deaths per year among children under age 5, and 8 to 12 children per year experienced serious sequelae.8
FIGURE 1
The incidence of meningococcal disease has declined steadily since 20008
1970-1996 National Notifiable Diseases Surveillance System data. 1997-2009 Active Bacterial Core surveillance data estimated to the US population.
FIGURE 2
Meningococcal disease has declined among infants and other age groups8
Active Bacterial Core surveillance cases from 1993-2009 estimated to the US population with 18% correction for underreporting.
ACIP’s dilemma
The low morbidity and mortality associated with meningococcal disease is one issue to consider when deciding whether to recommend new vaccines as part of the routine infant and child immunization schedule. The vaccine schedule is already crowded and complex, and parents increasingly are questioning the need for additional antigens.
In addition, the cost of vaccines for children has escalated over the past decade due mainly to the new, more expensive formulations.
The reason for a declining incidence of meningococcal disease is not fully known. It may be partly explained by increasing rates of vaccination among adolescents. However, the overall low rate of disease in the population makes assessing herd immunity difficult.
If ACIP decides to recommend vaccinating infants against meningococcal disease, it is unclear how long immunity will last, potentially necessitating a booster dose before the currently recommended adolescent dose.
Finally, in children at high risk, it is not fully known how meningococcal vaccines will affect the immune response to pneumococcal conjugate vaccine. This is an important consideration because the incidence of pneumococcal disease among these children is much higher than that of meningococcal disease.
1. CDC. Report from the Advisory Committee on Immunization Practices (ACIP): decision not to recommend routine vaccination of all children aged 2-10 years with quadrivalent meningococcal conjugate vaccine (MCV4). MMWR Morb Mortal Wkly Rep. 2008;57:462-465.
2. CDC. Licensure of a meningococcal conjugate vaccine for children aged 2 through 10 years and updated booster dose guidance for adolescents and other persons at increased risk for meningococcal disease—Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep. 2011;60:1018-1019.
3. Marshall GS, Marchant CD, Blatter M, et al. Immune response and one-year antibody persistence after a fourth dose of a novel Haemophilus influenzae type B and Neisseria meningitides serogroups C and T-tetanus toxoid conjugate vaccine (HibMenCY) at 12 to 15 months of age. Pediatr Infect Dis J. 2010;29:469-471.
4. Nolan T, Richmond P, Marshall H, et al. Immunogenicity and safety of an investigational combined Haemophilus influenzae type B-Neisseria meningitides serogroups C and Y-tetanus toxoid conjugate vaccine. Pediatr Infect Dis J. 2011;30:190-196.
5. Bryant KA, Marshall GS, Marchant CD, et al. Immunogenicity and safety of H influenzae type b-N meningitides C/Y conjugate vaccine in infants. Pediatrics. 2011;127:e1375-e1385.
6. Marshall GS, Marchant CD, Blatter M, et al. Co-administration of a novel Haemophilus influenzae type b and Neisseria meningitides serogroups C and Y-tetanus toxoid conjugate vaccine does not interfere with the immune response to antigens contained in infant vaccines routinely used in the United States. Hum Vaccin. 2011;7:258-264.
7. Perrett KP, Snape MD, Ford KJ, et al. Immunogenicity and immune memory of a nonadjuvanted quadrivalent meningococcal glycoconjugate vaccine in infants. Pediatr Infect Dis J. 2009;28:186-193.
8. MacNeil J. Epidemiology of meningococcal diseases in infants and young children. Paper presented at: Meeting of the Advisory Committee on Immunization Practices; October 25, 2011; Atlanta, GA.
1. CDC. Report from the Advisory Committee on Immunization Practices (ACIP): decision not to recommend routine vaccination of all children aged 2-10 years with quadrivalent meningococcal conjugate vaccine (MCV4). MMWR Morb Mortal Wkly Rep. 2008;57:462-465.
2. CDC. Licensure of a meningococcal conjugate vaccine for children aged 2 through 10 years and updated booster dose guidance for adolescents and other persons at increased risk for meningococcal disease—Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep. 2011;60:1018-1019.
3. Marshall GS, Marchant CD, Blatter M, et al. Immune response and one-year antibody persistence after a fourth dose of a novel Haemophilus influenzae type B and Neisseria meningitides serogroups C and T-tetanus toxoid conjugate vaccine (HibMenCY) at 12 to 15 months of age. Pediatr Infect Dis J. 2010;29:469-471.
4. Nolan T, Richmond P, Marshall H, et al. Immunogenicity and safety of an investigational combined Haemophilus influenzae type B-Neisseria meningitides serogroups C and Y-tetanus toxoid conjugate vaccine. Pediatr Infect Dis J. 2011;30:190-196.
5. Bryant KA, Marshall GS, Marchant CD, et al. Immunogenicity and safety of H influenzae type b-N meningitides C/Y conjugate vaccine in infants. Pediatrics. 2011;127:e1375-e1385.
6. Marshall GS, Marchant CD, Blatter M, et al. Co-administration of a novel Haemophilus influenzae type b and Neisseria meningitides serogroups C and Y-tetanus toxoid conjugate vaccine does not interfere with the immune response to antigens contained in infant vaccines routinely used in the United States. Hum Vaccin. 2011;7:258-264.
7. Perrett KP, Snape MD, Ford KJ, et al. Immunogenicity and immune memory of a nonadjuvanted quadrivalent meningococcal glycoconjugate vaccine in infants. Pediatr Infect Dis J. 2009;28:186-193.
8. MacNeil J. Epidemiology of meningococcal diseases in infants and young children. Paper presented at: Meeting of the Advisory Committee on Immunization Practices; October 25, 2011; Atlanta, GA.
Meningococcal vaccine for infants?
The Advisory Committee on Immunization Practices (ACIP) is evaluating whether to recommend the use of meningococcal vaccines for infants and children <2 years.1 The decision may be made within the next 4 to 8 months. In its deliberation, ACIP must consider several issues, which I review here.
Current and impending vaccine options. Two quadrivalent meningococcal conjugate vaccines (MCV4) are licensed by the US Food and Drug Administration (FDA) for use in the United States: Men ACWY-CRM (Menveo, Novartis) and Men ACWY-D (Menactra, Sanofi Pasteur).2 Both vaccines protect against 4 meningococcal serogroups (A, C, Y, and W-135) and are approved for use among those ages 2 to 55 years. In addition, Menactra was recently licensed as a 2-dose series for children ages 9 to 23 months. ACIP recommends routine use of MCV4 for adolescents ages 11 to 18 years, with a preference for the first dose at ages 11 to 12 years; and for all individuals between the ages of 2 and 55 years who are at increased risk for meningococcal disease ( TABLE ).
Complicating matters is the pending availability of more formulations. In addition to the 2-dose Menactra option for children 9 to 23 months, Novartis has an application before the FDA for a 4-dose schedule with Menveo, given at ages 2, 4, 6, and 12 months. GlaxoSmithKline has just received approval from the FDA for MenHibrix, a combination vaccine that contains antigens against Haemophilus influenzae type b (Hib) and 2 meningococcal serogroups, C and Y, licensed as a 4-dose series given at ages 2, 4, 6, and 12 months.
These vaccines have proven to be immunogenic in infants without diminishing the effectiveness of other, co-administered vaccines in normal infant populations. They also appear to be safe, although the studies to date have not been sufficiently large to detect uncommon adverse events.3-7
Table
Patients at high risk for meningococcal disease
|
| Source: CDC. Updated recommendations for use of meningococcal conjugate vaccines—Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Morb Mortal Wkly Rep. 2011;60:72-76. |
Meningococcal disease incidence and prevalence are declining
One major consideration for ACIP is the changing epidemiology of meningococcal disease and the low prevalence of disease in all age groups, including infants. The incidence of meningococcal disease has declined in the United States since 1980, with a marked and sustained decline since 2000 ( FIGURE 1 ).8 This decline has occurred in all age groups including infants, who have the highest rate of infection ( FIGURE 2 ).8 This decline in incidence occurred for all serogroups, including serogroup B.8
Serogroup B. Among children <5 years, including infants, half of meningococcal disease is caused by serogroup B,8 and these infections would not be prevented by any of the currently licensed vaccines or by those under review. Furthermore, half of all infections occur before age 9 months8 —an age range for which Menactra in not approved.
Serogroup C and Y. One-third of infections with serogroups C and Y occur before the age of 6 months8 and would not be prevented by any of the new products. Also of note: From 2007 to 2009, the mean number of cases of serotype A or C infection occurring each year in children <5 years was 77.8
The impact on children vs adults. Meningococcal disease in children is generally less severe than that occurring in older age groups. Overall case fatality in children is 6%; 10% in those with serogroup B and 1% in those with serogroup Y.8 The disease in children does result in significant sequelae, however, with 10% suffering hearing loss and 1% to 2% requiring amputation. From 2007 to 2009, there were 4 to 8 deaths per year among children under age 5, and 8 to 12 children per year experienced serious sequelae.8
FIGURE 1
The incidence of meningococcal disease has declined steadily since 20008
1970-1996 National Notifiable Diseases Surveillance System data. 1997-2009 Active Bacterial Core surveillance data estimated to the US population.
FIGURE 2
Meningococcal disease has declined among infants and other age groups8
Active Bacterial Core surveillance cases from 1993-2009 estimated to the US population with 18% correction for underreporting.
ACIP’s dilemma
The low morbidity and mortality associated with meningococcal disease is one issue to consider when deciding whether to recommend new vaccines as part of the routine infant and child immunization schedule. The vaccine schedule is already crowded and complex, and parents increasingly are questioning the need for additional antigens.
In addition, the cost of vaccines for children has escalated over the past decade due mainly to the new, more expensive formulations.
The reason for a declining incidence of meningococcal disease is not fully known. It may be partly explained by increasing rates of vaccination among adolescents. However, the overall low rate of disease in the population makes assessing herd immunity difficult.
If ACIP decides to recommend vaccinating infants against meningococcal disease, it is unclear how long immunity will last, potentially necessitating a booster dose before the currently recommended adolescent dose.
Finally, in children at high risk, it is not fully known how meningococcal vaccines will affect the immune response to pneumococcal conjugate vaccine. This is an important consideration because the incidence of pneumococcal disease among these children is much higher than that of meningococcal disease.
1. CDC. Report from the Advisory Committee on Immunization Practices (ACIP): decision not to recommend routine vaccination of all children aged 2-10 years with quadrivalent meningococcal conjugate vaccine (MCV4). MMWR Morb Mortal Wkly Rep. 2008;57:462-465.
2. CDC. Licensure of a meningococcal conjugate vaccine for children aged 2 through 10 years and updated booster dose guidance for adolescents and other persons at increased risk for meningococcal disease—Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep. 2011;60:1018-1019.
3. Marshall GS, Marchant CD, Blatter M, et al. Immune response and one-year antibody persistence after a fourth dose of a novel Haemophilus influenzae type B and Neisseria meningitides serogroups C and T-tetanus toxoid conjugate vaccine (HibMenCY) at 12 to 15 months of age. Pediatr Infect Dis J. 2010;29:469-471.
4. Nolan T, Richmond P, Marshall H, et al. Immunogenicity and safety of an investigational combined Haemophilus influenzae type B-Neisseria meningitides serogroups C and Y-tetanus toxoid conjugate vaccine. Pediatr Infect Dis J. 2011;30:190-196.
5. Bryant KA, Marshall GS, Marchant CD, et al. Immunogenicity and safety of H influenzae type b-N meningitides C/Y conjugate vaccine in infants. Pediatrics. 2011;127:e1375-e1385.
6. Marshall GS, Marchant CD, Blatter M, et al. Co-administration of a novel Haemophilus influenzae type b and Neisseria meningitides serogroups C and Y-tetanus toxoid conjugate vaccine does not interfere with the immune response to antigens contained in infant vaccines routinely used in the United States. Hum Vaccin. 2011;7:258-264.
7. Perrett KP, Snape MD, Ford KJ, et al. Immunogenicity and immune memory of a nonadjuvanted quadrivalent meningococcal glycoconjugate vaccine in infants. Pediatr Infect Dis J. 2009;28:186-193.
8. MacNeil J. Epidemiology of meningococcal diseases in infants and young children. Paper presented at: Meeting of the Advisory Committee on Immunization Practices; October 25, 2011; Atlanta, GA.
The Advisory Committee on Immunization Practices (ACIP) is evaluating whether to recommend the use of meningococcal vaccines for infants and children <2 years.1 The decision may be made within the next 4 to 8 months. In its deliberation, ACIP must consider several issues, which I review here.
Current and impending vaccine options. Two quadrivalent meningococcal conjugate vaccines (MCV4) are licensed by the US Food and Drug Administration (FDA) for use in the United States: Men ACWY-CRM (Menveo, Novartis) and Men ACWY-D (Menactra, Sanofi Pasteur).2 Both vaccines protect against 4 meningococcal serogroups (A, C, Y, and W-135) and are approved for use among those ages 2 to 55 years. In addition, Menactra was recently licensed as a 2-dose series for children ages 9 to 23 months. ACIP recommends routine use of MCV4 for adolescents ages 11 to 18 years, with a preference for the first dose at ages 11 to 12 years; and for all individuals between the ages of 2 and 55 years who are at increased risk for meningococcal disease ( TABLE ).
Complicating matters is the pending availability of more formulations. In addition to the 2-dose Menactra option for children 9 to 23 months, Novartis has an application before the FDA for a 4-dose schedule with Menveo, given at ages 2, 4, 6, and 12 months. GlaxoSmithKline has just received approval from the FDA for MenHibrix, a combination vaccine that contains antigens against Haemophilus influenzae type b (Hib) and 2 meningococcal serogroups, C and Y, licensed as a 4-dose series given at ages 2, 4, 6, and 12 months.
These vaccines have proven to be immunogenic in infants without diminishing the effectiveness of other, co-administered vaccines in normal infant populations. They also appear to be safe, although the studies to date have not been sufficiently large to detect uncommon adverse events.3-7
Table
Patients at high risk for meningococcal disease
|
| Source: CDC. Updated recommendations for use of meningococcal conjugate vaccines—Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Morb Mortal Wkly Rep. 2011;60:72-76. |
Meningococcal disease incidence and prevalence are declining
One major consideration for ACIP is the changing epidemiology of meningococcal disease and the low prevalence of disease in all age groups, including infants. The incidence of meningococcal disease has declined in the United States since 1980, with a marked and sustained decline since 2000 ( FIGURE 1 ).8 This decline has occurred in all age groups including infants, who have the highest rate of infection ( FIGURE 2 ).8 This decline in incidence occurred for all serogroups, including serogroup B.8
Serogroup B. Among children <5 years, including infants, half of meningococcal disease is caused by serogroup B,8 and these infections would not be prevented by any of the currently licensed vaccines or by those under review. Furthermore, half of all infections occur before age 9 months8 —an age range for which Menactra in not approved.
Serogroup C and Y. One-third of infections with serogroups C and Y occur before the age of 6 months8 and would not be prevented by any of the new products. Also of note: From 2007 to 2009, the mean number of cases of serotype A or C infection occurring each year in children <5 years was 77.8
The impact on children vs adults. Meningococcal disease in children is generally less severe than that occurring in older age groups. Overall case fatality in children is 6%; 10% in those with serogroup B and 1% in those with serogroup Y.8 The disease in children does result in significant sequelae, however, with 10% suffering hearing loss and 1% to 2% requiring amputation. From 2007 to 2009, there were 4 to 8 deaths per year among children under age 5, and 8 to 12 children per year experienced serious sequelae.8
FIGURE 1
The incidence of meningococcal disease has declined steadily since 20008
1970-1996 National Notifiable Diseases Surveillance System data. 1997-2009 Active Bacterial Core surveillance data estimated to the US population.
FIGURE 2
Meningococcal disease has declined among infants and other age groups8
Active Bacterial Core surveillance cases from 1993-2009 estimated to the US population with 18% correction for underreporting.
ACIP’s dilemma
The low morbidity and mortality associated with meningococcal disease is one issue to consider when deciding whether to recommend new vaccines as part of the routine infant and child immunization schedule. The vaccine schedule is already crowded and complex, and parents increasingly are questioning the need for additional antigens.
In addition, the cost of vaccines for children has escalated over the past decade due mainly to the new, more expensive formulations.
The reason for a declining incidence of meningococcal disease is not fully known. It may be partly explained by increasing rates of vaccination among adolescents. However, the overall low rate of disease in the population makes assessing herd immunity difficult.
If ACIP decides to recommend vaccinating infants against meningococcal disease, it is unclear how long immunity will last, potentially necessitating a booster dose before the currently recommended adolescent dose.
Finally, in children at high risk, it is not fully known how meningococcal vaccines will affect the immune response to pneumococcal conjugate vaccine. This is an important consideration because the incidence of pneumococcal disease among these children is much higher than that of meningococcal disease.
The Advisory Committee on Immunization Practices (ACIP) is evaluating whether to recommend the use of meningococcal vaccines for infants and children <2 years.1 The decision may be made within the next 4 to 8 months. In its deliberation, ACIP must consider several issues, which I review here.
Current and impending vaccine options. Two quadrivalent meningococcal conjugate vaccines (MCV4) are licensed by the US Food and Drug Administration (FDA) for use in the United States: Men ACWY-CRM (Menveo, Novartis) and Men ACWY-D (Menactra, Sanofi Pasteur).2 Both vaccines protect against 4 meningococcal serogroups (A, C, Y, and W-135) and are approved for use among those ages 2 to 55 years. In addition, Menactra was recently licensed as a 2-dose series for children ages 9 to 23 months. ACIP recommends routine use of MCV4 for adolescents ages 11 to 18 years, with a preference for the first dose at ages 11 to 12 years; and for all individuals between the ages of 2 and 55 years who are at increased risk for meningococcal disease ( TABLE ).
Complicating matters is the pending availability of more formulations. In addition to the 2-dose Menactra option for children 9 to 23 months, Novartis has an application before the FDA for a 4-dose schedule with Menveo, given at ages 2, 4, 6, and 12 months. GlaxoSmithKline has just received approval from the FDA for MenHibrix, a combination vaccine that contains antigens against Haemophilus influenzae type b (Hib) and 2 meningococcal serogroups, C and Y, licensed as a 4-dose series given at ages 2, 4, 6, and 12 months.
These vaccines have proven to be immunogenic in infants without diminishing the effectiveness of other, co-administered vaccines in normal infant populations. They also appear to be safe, although the studies to date have not been sufficiently large to detect uncommon adverse events.3-7
Table
Patients at high risk for meningococcal disease
|
| Source: CDC. Updated recommendations for use of meningococcal conjugate vaccines—Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Morb Mortal Wkly Rep. 2011;60:72-76. |
Meningococcal disease incidence and prevalence are declining
One major consideration for ACIP is the changing epidemiology of meningococcal disease and the low prevalence of disease in all age groups, including infants. The incidence of meningococcal disease has declined in the United States since 1980, with a marked and sustained decline since 2000 ( FIGURE 1 ).8 This decline has occurred in all age groups including infants, who have the highest rate of infection ( FIGURE 2 ).8 This decline in incidence occurred for all serogroups, including serogroup B.8
Serogroup B. Among children <5 years, including infants, half of meningococcal disease is caused by serogroup B,8 and these infections would not be prevented by any of the currently licensed vaccines or by those under review. Furthermore, half of all infections occur before age 9 months8 —an age range for which Menactra in not approved.
Serogroup C and Y. One-third of infections with serogroups C and Y occur before the age of 6 months8 and would not be prevented by any of the new products. Also of note: From 2007 to 2009, the mean number of cases of serotype A or C infection occurring each year in children <5 years was 77.8
The impact on children vs adults. Meningococcal disease in children is generally less severe than that occurring in older age groups. Overall case fatality in children is 6%; 10% in those with serogroup B and 1% in those with serogroup Y.8 The disease in children does result in significant sequelae, however, with 10% suffering hearing loss and 1% to 2% requiring amputation. From 2007 to 2009, there were 4 to 8 deaths per year among children under age 5, and 8 to 12 children per year experienced serious sequelae.8
FIGURE 1
The incidence of meningococcal disease has declined steadily since 20008
1970-1996 National Notifiable Diseases Surveillance System data. 1997-2009 Active Bacterial Core surveillance data estimated to the US population.
FIGURE 2
Meningococcal disease has declined among infants and other age groups8
Active Bacterial Core surveillance cases from 1993-2009 estimated to the US population with 18% correction for underreporting.
ACIP’s dilemma
The low morbidity and mortality associated with meningococcal disease is one issue to consider when deciding whether to recommend new vaccines as part of the routine infant and child immunization schedule. The vaccine schedule is already crowded and complex, and parents increasingly are questioning the need for additional antigens.
In addition, the cost of vaccines for children has escalated over the past decade due mainly to the new, more expensive formulations.
The reason for a declining incidence of meningococcal disease is not fully known. It may be partly explained by increasing rates of vaccination among adolescents. However, the overall low rate of disease in the population makes assessing herd immunity difficult.
If ACIP decides to recommend vaccinating infants against meningococcal disease, it is unclear how long immunity will last, potentially necessitating a booster dose before the currently recommended adolescent dose.
Finally, in children at high risk, it is not fully known how meningococcal vaccines will affect the immune response to pneumococcal conjugate vaccine. This is an important consideration because the incidence of pneumococcal disease among these children is much higher than that of meningococcal disease.
1. CDC. Report from the Advisory Committee on Immunization Practices (ACIP): decision not to recommend routine vaccination of all children aged 2-10 years with quadrivalent meningococcal conjugate vaccine (MCV4). MMWR Morb Mortal Wkly Rep. 2008;57:462-465.
2. CDC. Licensure of a meningococcal conjugate vaccine for children aged 2 through 10 years and updated booster dose guidance for adolescents and other persons at increased risk for meningococcal disease—Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep. 2011;60:1018-1019.
3. Marshall GS, Marchant CD, Blatter M, et al. Immune response and one-year antibody persistence after a fourth dose of a novel Haemophilus influenzae type B and Neisseria meningitides serogroups C and T-tetanus toxoid conjugate vaccine (HibMenCY) at 12 to 15 months of age. Pediatr Infect Dis J. 2010;29:469-471.
4. Nolan T, Richmond P, Marshall H, et al. Immunogenicity and safety of an investigational combined Haemophilus influenzae type B-Neisseria meningitides serogroups C and Y-tetanus toxoid conjugate vaccine. Pediatr Infect Dis J. 2011;30:190-196.
5. Bryant KA, Marshall GS, Marchant CD, et al. Immunogenicity and safety of H influenzae type b-N meningitides C/Y conjugate vaccine in infants. Pediatrics. 2011;127:e1375-e1385.
6. Marshall GS, Marchant CD, Blatter M, et al. Co-administration of a novel Haemophilus influenzae type b and Neisseria meningitides serogroups C and Y-tetanus toxoid conjugate vaccine does not interfere with the immune response to antigens contained in infant vaccines routinely used in the United States. Hum Vaccin. 2011;7:258-264.
7. Perrett KP, Snape MD, Ford KJ, et al. Immunogenicity and immune memory of a nonadjuvanted quadrivalent meningococcal glycoconjugate vaccine in infants. Pediatr Infect Dis J. 2009;28:186-193.
8. MacNeil J. Epidemiology of meningococcal diseases in infants and young children. Paper presented at: Meeting of the Advisory Committee on Immunization Practices; October 25, 2011; Atlanta, GA.
1. CDC. Report from the Advisory Committee on Immunization Practices (ACIP): decision not to recommend routine vaccination of all children aged 2-10 years with quadrivalent meningococcal conjugate vaccine (MCV4). MMWR Morb Mortal Wkly Rep. 2008;57:462-465.
2. CDC. Licensure of a meningococcal conjugate vaccine for children aged 2 through 10 years and updated booster dose guidance for adolescents and other persons at increased risk for meningococcal disease—Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep. 2011;60:1018-1019.
3. Marshall GS, Marchant CD, Blatter M, et al. Immune response and one-year antibody persistence after a fourth dose of a novel Haemophilus influenzae type B and Neisseria meningitides serogroups C and T-tetanus toxoid conjugate vaccine (HibMenCY) at 12 to 15 months of age. Pediatr Infect Dis J. 2010;29:469-471.
4. Nolan T, Richmond P, Marshall H, et al. Immunogenicity and safety of an investigational combined Haemophilus influenzae type B-Neisseria meningitides serogroups C and Y-tetanus toxoid conjugate vaccine. Pediatr Infect Dis J. 2011;30:190-196.
5. Bryant KA, Marshall GS, Marchant CD, et al. Immunogenicity and safety of H influenzae type b-N meningitides C/Y conjugate vaccine in infants. Pediatrics. 2011;127:e1375-e1385.
6. Marshall GS, Marchant CD, Blatter M, et al. Co-administration of a novel Haemophilus influenzae type b and Neisseria meningitides serogroups C and Y-tetanus toxoid conjugate vaccine does not interfere with the immune response to antigens contained in infant vaccines routinely used in the United States. Hum Vaccin. 2011;7:258-264.
7. Perrett KP, Snape MD, Ford KJ, et al. Immunogenicity and immune memory of a nonadjuvanted quadrivalent meningococcal glycoconjugate vaccine in infants. Pediatr Infect Dis J. 2009;28:186-193.
8. MacNeil J. Epidemiology of meningococcal diseases in infants and young children. Paper presented at: Meeting of the Advisory Committee on Immunization Practices; October 25, 2011; Atlanta, GA.