User login
ACIP vaccine update
The Advisory Committee on Immunization Practices (ACIP) made relatively few new vaccine recommendations in 2017. One pertained to prevention of hepatitis B virus (HBV) infection in infants born to HBV-infected mothers. Another recommended a new vaccine to prevent shingles. A third advised considering an additional dose of mumps vaccine during an outbreak. This year’s recommendations pertaining to influenza vaccines were covered in a previous Practice Alert.1
Perinatal HBV prevention: New strategy if revaccination is required
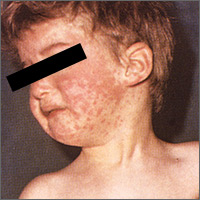
Hepatitis B prevention programs in the United States have decreased the incidence of HBV infections from 9.6 cases per 100,000 population in 1982 (the year the hepatitis B [HepB] vaccine was first available) to 1.1 cases per 100,000 population in 2015 (FIGURE 1).2 One major route of HBV dissemination worldwide is perinatal transmission to infants by HBV-infected mothers. However, this route of infection has been greatly diminished in the United States because of widespread screening of pregnant women and because newborns of mothers with known active HBV infection receive prophylaxis with hepatitis B immune globulin and HBV vaccine.
Each year in the United States an estimated 25,000 infants are born to mothers who are positive for hepatitis B surface antigen (HBsAg).3 Without post-exposure prophylaxis, 85% of these infants would develop HBV infection if the mother is also hepatitis B e antigen (HBeAg) positive; 30% would develop HBV infection if the mother is HBeAg negative.2 Eighty percent to 90% of infected infants develop chronic HBV infection and are at increased risk of chronic liver disease.2 Of all infants receiving the recommended post-exposure prophylaxis, only about 1% develop infection.2
Available HepB vaccines. HepB vaccine consists of HBsAg derived from yeast using recombinant DNA technology, which is then purified by biochemical separation techniques. Three vaccine products are available for newborns and infants in the United States. Two are single-antigen vaccines—Engerix-B (GlaxoSmithKline Biologicals) and Recombivax HB (Merck & Co.)—and both can be used starting at birth. One combination vaccine, Pediarix (GlaxoSmithKline Biologicals) is used for children ages 6 weeks to 6 years. It contains HBsAg as do the other 2 vaccines, as well as diphtheria and tetanus toxoids, acellular pertussis adsorbed, and inactivated poliovirus (DTaP-HepB-IPV).
Until December 31, 2014, a vaccine combining HBsAg and haemophilus-B antigen, Comvax (Merck and Co.), was available for infants 6 weeks or older. Comvax is no longer produced.
Factors affecting the dosing schedule. For infants born to HBsAg-positive mothers, the final dose of the HepB series should be completed at age 6 months with either one of the monovalent HepB vaccines or the DTaP-HepB-IPV vaccine. When the now-discontinued Comvax was used to complete the series, the final dose was administered at 12 to 15 months. The timing of HepB vaccine at birth and at subsequent intervals, and a decision on whether to give hepatitis B immune globulin, depend on the baby’s birth weight, the mother’s HBsAg status, and type of vaccine used.2
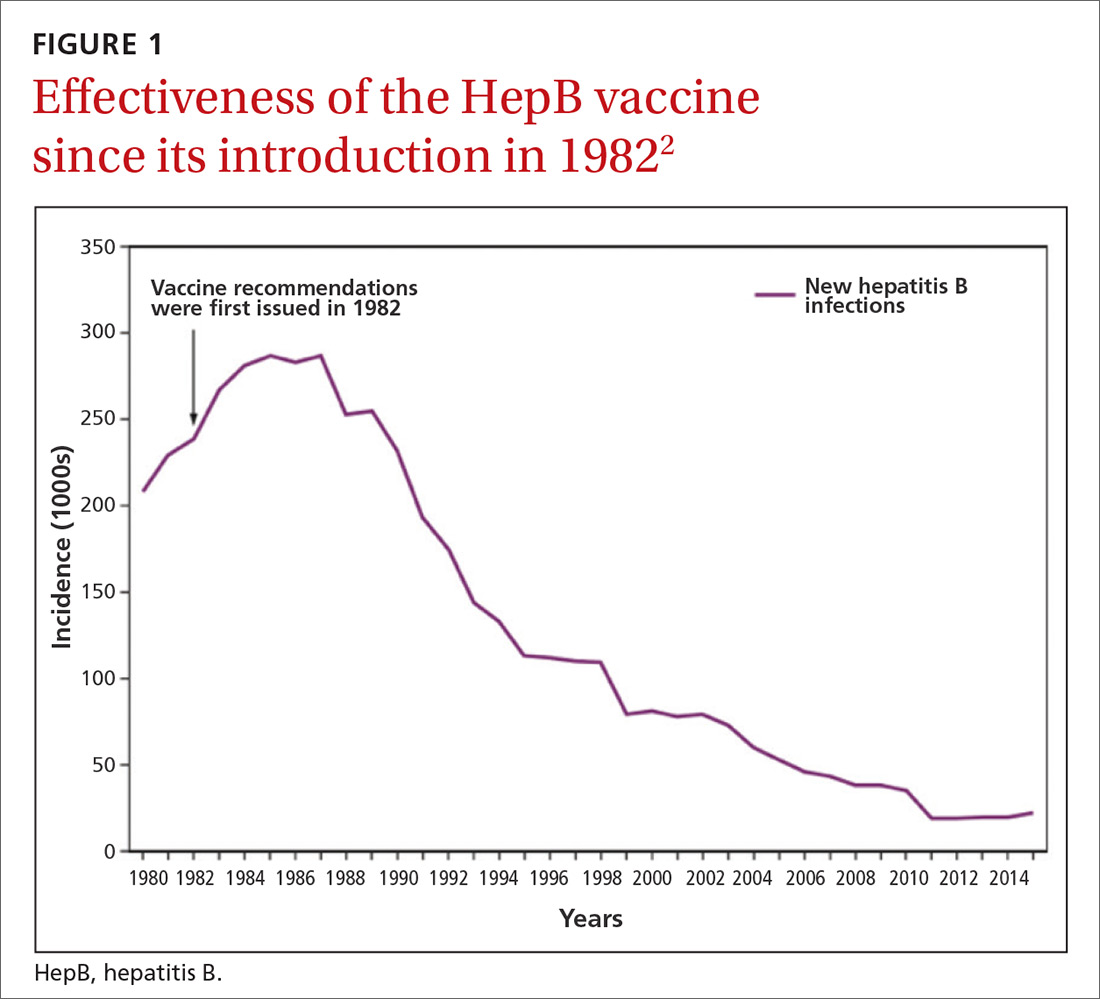
Post-vaccination assessment. ACIP recommends that babies born to HBsAg-positive mothers and having received the final dose of the vaccine series be serologically tested for immunity to HBV at age 9 to 12 months; or if the series is delayed, at one to 2 months after the final dose.4 Infants without evidence of active infection (ie, HBsAg negative) and with levels of antibody to HBsAg ≥10 mIU/mL are considered protected and need no further vaccinations.4 Revaccination is advised for those with antibody levels <10 mIU/mL—who account for only about 2% of infants having received the recommended schedule.4
New revaccination strategy. The previous recommendation on revaccination advised a second 3-dose series with repeat serologic testing one to 2 months after the final dose of vaccine. Although this strategy is still acceptable, the new recommendation for infants with antibody levels <10 mIU/mL favors (for cost savings and convenience) administration of a single dose of HepB vaccine with retesting one to 2 months later.2
Several studies presented at the ACIP meeting in February 2017 showed that more than 90% of infants revaccinated with the single dose will develop a protective antibody level.4 Infants whose anti-HBs remain <10 mIU/mL following the single-dose re-vaccination should receive 2 additional doses of HepB vaccine, followed by testing one to 2 months after the last dose4 (FIGURE 22).
(A new HepB vaccine, HEPLISAV-B [Dynavax Technologies Corp]), has been approved for use in adults. More on this in a bit.)
Herpes zoster vaccine: Data guidance on product selection
In 2017, the US Food and Drug Administration (FDA) approved a new vaccine against shingles, an adjuvanted herpes zoster subunit (HZ/su) vaccine, Shingrix (GlaxoSmithKline Biologicals). It is now an alternative to the live attenuated virus (ZVL) vaccine, Zostavax (Merck & Co.), licensed in 2006. ZVL is approved for use in adults ages 50 to 59 years, but ACIP recommends it only for adults 60 and older.5 It is given as a single dose, while HZ/su is given as a 2-dose series at 0 and at 2 to 6 months. By ACIP’s analysis, HZ/su is more effective than ZVL. In a comparison model looking at health outcomes over a lifetime among one million patients 60 to 69 years of age, HZ/su would prevent 53,000 more cases of shingles and 4000 more cases of postherpetic neuralgia than would ZVL.6
Additional mumps vaccine is warranted in an outbreak
While use of mumps-containing vaccine in the United States has led to markedly lower disease incidence rates than existed in the pre-vaccine era, in recent years there have been large mumps outbreaks among young adults at universities and other close-knit communities. These groups have had relatively high rates of completion of 2 doses of measles, mumps, and rubella (MMR) vaccine, and the cause of the outbreaks is not fully understood. Potential contributors include waning immunity following vaccination and antigenic differences between the virus strains circulating and those in the vaccine.
ACIP considered whether a third dose of MMR should be recommended to those fully vaccinated if they are at high risk due to an outbreak. Although the evidence to support the effectiveness of a third dose was scant and of very low quality, the evidence for vaccine safety was reassuring and ACIP voted to recommend the use of a third dose in outbreaks.9
One new vaccine and others on the horizon
ACIP is evaluating a new HepB vaccine, HEPLISAV-B, which was approved by the FDA in November 2017 for use in adults.10,11 The vaccine contains the same antigen as other available HepB vaccines but a different adjuvant. It is administered in 2 doses one month apart, which is preferable to the current 3-dose, 6-month schedule. There is, however, some indication that it causes increased rates of cardiovascular complications.10 ACIP is evaluating the relative effectiveness and safety of HEPLISAV-B and other HepB vaccines, and recommendations are expected this spring.
Other vaccines in various stages of development, but not ready for ACIP evaluation, include those against Zika virus, norovirus, respiratory syncytial virus, and dengue virus.
ACIP is also retrospectively assessing whether adding the 13 valent pneumococcal conjugate vaccine to the schedule for those over the age of 65 has led to improved pneumonia outcomes. It will reconsider the previous recommendation based on the results of its assessment.
1. Campos-Outcalt D. Latest recommendations for the 2017-2018 flu season. J Fam Pract. 2017;66:570-572.
2. Schillie S, Vellozzi C, Reingold A, et al. Prevention of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2018;67:1-31. Available at: https://www.cdc.gov/mmwr/volumes/67/rr/rr6701a1.htm. Accessed January 19, 2018.
3. CDC. Postvaccination serologic testing results for infants aged ≤24 months exposed to hepatitis B virus at birth: United States, 2008-2011. MMWR Morb Mortal Wkly Rep. 2012;61:768-771. Available at: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6138a4.htm. Accessed February 14, 2018.
4. Nelson N. Revaccination for infants born to hepatitis B virus (HBV)-infected mothers. Presented at: Advisory Committee on Immunization Practices. February 22, 2017; Atlanta, GA. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-02/hepatitis-02-background-nelson.pdf. Accessed January 19, 2017.
5. Hales CM, Harpaz R, Ortega-Sanchez I, et al. Update on recommendations for use of herpes zoster vaccine. MMWR Morb Mortal Wkly Rep. 2014;63:729-731. Available at: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6333a3.htm?s_cid=mm6333a3_w. Accessed January 23, 2018.
6. Dooling KL. Considerations for the use of herpes zoster vaccines. Presented at: Advisory Committee on Immunization Practices. October 25, 2017; Atlanta, GA. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-10/zoster-04-dooling.pdf. Accessed January 19, 2018.
7. Dooling KL, Guo A, Patel M, et al. Recommendations of the Advisory Committee on Immunization Practices for use of herpes zoster vaccines. MMWR Morb Mortal Wkly Rep. 2018;67:103-108.
8. Campos-Outcalt D. The new shingles vaccine: what PCPs need to know. J Fam Pract. 2017;66:audio. Available at: https://www.mdedge.com/jfponline/article/153168/vaccines/new-shingles-vaccine-what-pcps-need-know. Accessed January 19, 2018.
9. Marlow M. Grading of recommendations assessment, development and evaluation (GRADE): third dose of MMR vaccine. Presented at: Advisory Committee on Immunization Practices. October 25, 2017; Atlanta, GA. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-10/mumps-03-marlow-508.pdf. Accessed January 19, 2018.
10. HEPLISAV-B [package insert]. Berkeley, CA: Dynavax Technology Corporation; 2017. Available at: https://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM584762.pdf. Accessed January 23, 2018.
11. Janssen R. HEPLISAV-B. Presented at: Advisory Committee on Immunization Practices. October 25, 2017; Atlanta, GA. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-10/hepatitis-02-janssen.pdf. Accessed January 19, 2018.
The Advisory Committee on Immunization Practices (ACIP) made relatively few new vaccine recommendations in 2017. One pertained to prevention of hepatitis B virus (HBV) infection in infants born to HBV-infected mothers. Another recommended a new vaccine to prevent shingles. A third advised considering an additional dose of mumps vaccine during an outbreak. This year’s recommendations pertaining to influenza vaccines were covered in a previous Practice Alert.1
Perinatal HBV prevention: New strategy if revaccination is required
Hepatitis B prevention programs in the United States have decreased the incidence of HBV infections from 9.6 cases per 100,000 population in 1982 (the year the hepatitis B [HepB] vaccine was first available) to 1.1 cases per 100,000 population in 2015 (FIGURE 1).2 One major route of HBV dissemination worldwide is perinatal transmission to infants by HBV-infected mothers. However, this route of infection has been greatly diminished in the United States because of widespread screening of pregnant women and because newborns of mothers with known active HBV infection receive prophylaxis with hepatitis B immune globulin and HBV vaccine.

Each year in the United States an estimated 25,000 infants are born to mothers who are positive for hepatitis B surface antigen (HBsAg).3 Without post-exposure prophylaxis, 85% of these infants would develop HBV infection if the mother is also hepatitis B e antigen (HBeAg) positive; 30% would develop HBV infection if the mother is HBeAg negative.2 Eighty percent to 90% of infected infants develop chronic HBV infection and are at increased risk of chronic liver disease.2 Of all infants receiving the recommended post-exposure prophylaxis, only about 1% develop infection.2
Available HepB vaccines. HepB vaccine consists of HBsAg derived from yeast using recombinant DNA technology, which is then purified by biochemical separation techniques. Three vaccine products are available for newborns and infants in the United States. Two are single-antigen vaccines—Engerix-B (GlaxoSmithKline Biologicals) and Recombivax HB (Merck & Co.)—and both can be used starting at birth. One combination vaccine, Pediarix (GlaxoSmithKline Biologicals) is used for children ages 6 weeks to 6 years. It contains HBsAg as do the other 2 vaccines, as well as diphtheria and tetanus toxoids, acellular pertussis adsorbed, and inactivated poliovirus (DTaP-HepB-IPV).
Until December 31, 2014, a vaccine combining HBsAg and haemophilus-B antigen, Comvax (Merck and Co.), was available for infants 6 weeks or older. Comvax is no longer produced.
Factors affecting the dosing schedule. For infants born to HBsAg-positive mothers, the final dose of the HepB series should be completed at age 6 months with either one of the monovalent HepB vaccines or the DTaP-HepB-IPV vaccine. When the now-discontinued Comvax was used to complete the series, the final dose was administered at 12 to 15 months. The timing of HepB vaccine at birth and at subsequent intervals, and a decision on whether to give hepatitis B immune globulin, depend on the baby’s birth weight, the mother’s HBsAg status, and type of vaccine used.2
Post-vaccination assessment. ACIP recommends that babies born to HBsAg-positive mothers and having received the final dose of the vaccine series be serologically tested for immunity to HBV at age 9 to 12 months; or if the series is delayed, at one to 2 months after the final dose.4 Infants without evidence of active infection (ie, HBsAg negative) and with levels of antibody to HBsAg ≥10 mIU/mL are considered protected and need no further vaccinations.4 Revaccination is advised for those with antibody levels <10 mIU/mL—who account for only about 2% of infants having received the recommended schedule.4
New revaccination strategy. The previous recommendation on revaccination advised a second 3-dose series with repeat serologic testing one to 2 months after the final dose of vaccine. Although this strategy is still acceptable, the new recommendation for infants with antibody levels <10 mIU/mL favors (for cost savings and convenience) administration of a single dose of HepB vaccine with retesting one to 2 months later.2
Several studies presented at the ACIP meeting in February 2017 showed that more than 90% of infants revaccinated with the single dose will develop a protective antibody level.4 Infants whose anti-HBs remain <10 mIU/mL following the single-dose re-vaccination should receive 2 additional doses of HepB vaccine, followed by testing one to 2 months after the last dose4 (FIGURE 22).

(A new HepB vaccine, HEPLISAV-B [Dynavax Technologies Corp]), has been approved for use in adults. More on this in a bit.)
Herpes zoster vaccine: Data guidance on product selection
In 2017, the US Food and Drug Administration (FDA) approved a new vaccine against shingles, an adjuvanted herpes zoster subunit (HZ/su) vaccine, Shingrix (GlaxoSmithKline Biologicals). It is now an alternative to the live attenuated virus (ZVL) vaccine, Zostavax (Merck & Co.), licensed in 2006. ZVL is approved for use in adults ages 50 to 59 years, but ACIP recommends it only for adults 60 and older.5 It is given as a single dose, while HZ/su is given as a 2-dose series at 0 and at 2 to 6 months. By ACIP’s analysis, HZ/su is more effective than ZVL. In a comparison model looking at health outcomes over a lifetime among one million patients 60 to 69 years of age, HZ/su would prevent 53,000 more cases of shingles and 4000 more cases of postherpetic neuralgia than would ZVL.6
Additional mumps vaccine is warranted in an outbreak
While use of mumps-containing vaccine in the United States has led to markedly lower disease incidence rates than existed in the pre-vaccine era, in recent years there have been large mumps outbreaks among young adults at universities and other close-knit communities. These groups have had relatively high rates of completion of 2 doses of measles, mumps, and rubella (MMR) vaccine, and the cause of the outbreaks is not fully understood. Potential contributors include waning immunity following vaccination and antigenic differences between the virus strains circulating and those in the vaccine.
ACIP considered whether a third dose of MMR should be recommended to those fully vaccinated if they are at high risk due to an outbreak. Although the evidence to support the effectiveness of a third dose was scant and of very low quality, the evidence for vaccine safety was reassuring and ACIP voted to recommend the use of a third dose in outbreaks.9
One new vaccine and others on the horizon
ACIP is evaluating a new HepB vaccine, HEPLISAV-B, which was approved by the FDA in November 2017 for use in adults.10,11 The vaccine contains the same antigen as other available HepB vaccines but a different adjuvant. It is administered in 2 doses one month apart, which is preferable to the current 3-dose, 6-month schedule. There is, however, some indication that it causes increased rates of cardiovascular complications.10 ACIP is evaluating the relative effectiveness and safety of HEPLISAV-B and other HepB vaccines, and recommendations are expected this spring.
Other vaccines in various stages of development, but not ready for ACIP evaluation, include those against Zika virus, norovirus, respiratory syncytial virus, and dengue virus.
ACIP is also retrospectively assessing whether adding the 13 valent pneumococcal conjugate vaccine to the schedule for those over the age of 65 has led to improved pneumonia outcomes. It will reconsider the previous recommendation based on the results of its assessment.
The Advisory Committee on Immunization Practices (ACIP) made relatively few new vaccine recommendations in 2017. One pertained to prevention of hepatitis B virus (HBV) infection in infants born to HBV-infected mothers. Another recommended a new vaccine to prevent shingles. A third advised considering an additional dose of mumps vaccine during an outbreak. This year’s recommendations pertaining to influenza vaccines were covered in a previous Practice Alert.1
Perinatal HBV prevention: New strategy if revaccination is required
Hepatitis B prevention programs in the United States have decreased the incidence of HBV infections from 9.6 cases per 100,000 population in 1982 (the year the hepatitis B [HepB] vaccine was first available) to 1.1 cases per 100,000 population in 2015 (FIGURE 1).2 One major route of HBV dissemination worldwide is perinatal transmission to infants by HBV-infected mothers. However, this route of infection has been greatly diminished in the United States because of widespread screening of pregnant women and because newborns of mothers with known active HBV infection receive prophylaxis with hepatitis B immune globulin and HBV vaccine.

Each year in the United States an estimated 25,000 infants are born to mothers who are positive for hepatitis B surface antigen (HBsAg).3 Without post-exposure prophylaxis, 85% of these infants would develop HBV infection if the mother is also hepatitis B e antigen (HBeAg) positive; 30% would develop HBV infection if the mother is HBeAg negative.2 Eighty percent to 90% of infected infants develop chronic HBV infection and are at increased risk of chronic liver disease.2 Of all infants receiving the recommended post-exposure prophylaxis, only about 1% develop infection.2
Available HepB vaccines. HepB vaccine consists of HBsAg derived from yeast using recombinant DNA technology, which is then purified by biochemical separation techniques. Three vaccine products are available for newborns and infants in the United States. Two are single-antigen vaccines—Engerix-B (GlaxoSmithKline Biologicals) and Recombivax HB (Merck & Co.)—and both can be used starting at birth. One combination vaccine, Pediarix (GlaxoSmithKline Biologicals) is used for children ages 6 weeks to 6 years. It contains HBsAg as do the other 2 vaccines, as well as diphtheria and tetanus toxoids, acellular pertussis adsorbed, and inactivated poliovirus (DTaP-HepB-IPV).
Until December 31, 2014, a vaccine combining HBsAg and haemophilus-B antigen, Comvax (Merck and Co.), was available for infants 6 weeks or older. Comvax is no longer produced.
Factors affecting the dosing schedule. For infants born to HBsAg-positive mothers, the final dose of the HepB series should be completed at age 6 months with either one of the monovalent HepB vaccines or the DTaP-HepB-IPV vaccine. When the now-discontinued Comvax was used to complete the series, the final dose was administered at 12 to 15 months. The timing of HepB vaccine at birth and at subsequent intervals, and a decision on whether to give hepatitis B immune globulin, depend on the baby’s birth weight, the mother’s HBsAg status, and type of vaccine used.2
Post-vaccination assessment. ACIP recommends that babies born to HBsAg-positive mothers and having received the final dose of the vaccine series be serologically tested for immunity to HBV at age 9 to 12 months; or if the series is delayed, at one to 2 months after the final dose.4 Infants without evidence of active infection (ie, HBsAg negative) and with levels of antibody to HBsAg ≥10 mIU/mL are considered protected and need no further vaccinations.4 Revaccination is advised for those with antibody levels <10 mIU/mL—who account for only about 2% of infants having received the recommended schedule.4
New revaccination strategy. The previous recommendation on revaccination advised a second 3-dose series with repeat serologic testing one to 2 months after the final dose of vaccine. Although this strategy is still acceptable, the new recommendation for infants with antibody levels <10 mIU/mL favors (for cost savings and convenience) administration of a single dose of HepB vaccine with retesting one to 2 months later.2
Several studies presented at the ACIP meeting in February 2017 showed that more than 90% of infants revaccinated with the single dose will develop a protective antibody level.4 Infants whose anti-HBs remain <10 mIU/mL following the single-dose re-vaccination should receive 2 additional doses of HepB vaccine, followed by testing one to 2 months after the last dose4 (FIGURE 22).

(A new HepB vaccine, HEPLISAV-B [Dynavax Technologies Corp]), has been approved for use in adults. More on this in a bit.)
Herpes zoster vaccine: Data guidance on product selection
In 2017, the US Food and Drug Administration (FDA) approved a new vaccine against shingles, an adjuvanted herpes zoster subunit (HZ/su) vaccine, Shingrix (GlaxoSmithKline Biologicals). It is now an alternative to the live attenuated virus (ZVL) vaccine, Zostavax (Merck & Co.), licensed in 2006. ZVL is approved for use in adults ages 50 to 59 years, but ACIP recommends it only for adults 60 and older.5 It is given as a single dose, while HZ/su is given as a 2-dose series at 0 and at 2 to 6 months. By ACIP’s analysis, HZ/su is more effective than ZVL. In a comparison model looking at health outcomes over a lifetime among one million patients 60 to 69 years of age, HZ/su would prevent 53,000 more cases of shingles and 4000 more cases of postherpetic neuralgia than would ZVL.6
Additional mumps vaccine is warranted in an outbreak
While use of mumps-containing vaccine in the United States has led to markedly lower disease incidence rates than existed in the pre-vaccine era, in recent years there have been large mumps outbreaks among young adults at universities and other close-knit communities. These groups have had relatively high rates of completion of 2 doses of measles, mumps, and rubella (MMR) vaccine, and the cause of the outbreaks is not fully understood. Potential contributors include waning immunity following vaccination and antigenic differences between the virus strains circulating and those in the vaccine.
ACIP considered whether a third dose of MMR should be recommended to those fully vaccinated if they are at high risk due to an outbreak. Although the evidence to support the effectiveness of a third dose was scant and of very low quality, the evidence for vaccine safety was reassuring and ACIP voted to recommend the use of a third dose in outbreaks.9
One new vaccine and others on the horizon
ACIP is evaluating a new HepB vaccine, HEPLISAV-B, which was approved by the FDA in November 2017 for use in adults.10,11 The vaccine contains the same antigen as other available HepB vaccines but a different adjuvant. It is administered in 2 doses one month apart, which is preferable to the current 3-dose, 6-month schedule. There is, however, some indication that it causes increased rates of cardiovascular complications.10 ACIP is evaluating the relative effectiveness and safety of HEPLISAV-B and other HepB vaccines, and recommendations are expected this spring.
Other vaccines in various stages of development, but not ready for ACIP evaluation, include those against Zika virus, norovirus, respiratory syncytial virus, and dengue virus.
ACIP is also retrospectively assessing whether adding the 13 valent pneumococcal conjugate vaccine to the schedule for those over the age of 65 has led to improved pneumonia outcomes. It will reconsider the previous recommendation based on the results of its assessment.
1. Campos-Outcalt D. Latest recommendations for the 2017-2018 flu season. J Fam Pract. 2017;66:570-572.
2. Schillie S, Vellozzi C, Reingold A, et al. Prevention of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2018;67:1-31. Available at: https://www.cdc.gov/mmwr/volumes/67/rr/rr6701a1.htm. Accessed January 19, 2018.
3. CDC. Postvaccination serologic testing results for infants aged ≤24 months exposed to hepatitis B virus at birth: United States, 2008-2011. MMWR Morb Mortal Wkly Rep. 2012;61:768-771. Available at: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6138a4.htm. Accessed February 14, 2018.
4. Nelson N. Revaccination for infants born to hepatitis B virus (HBV)-infected mothers. Presented at: Advisory Committee on Immunization Practices. February 22, 2017; Atlanta, GA. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-02/hepatitis-02-background-nelson.pdf. Accessed January 19, 2017.
5. Hales CM, Harpaz R, Ortega-Sanchez I, et al. Update on recommendations for use of herpes zoster vaccine. MMWR Morb Mortal Wkly Rep. 2014;63:729-731. Available at: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6333a3.htm?s_cid=mm6333a3_w. Accessed January 23, 2018.
6. Dooling KL. Considerations for the use of herpes zoster vaccines. Presented at: Advisory Committee on Immunization Practices. October 25, 2017; Atlanta, GA. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-10/zoster-04-dooling.pdf. Accessed January 19, 2018.
7. Dooling KL, Guo A, Patel M, et al. Recommendations of the Advisory Committee on Immunization Practices for use of herpes zoster vaccines. MMWR Morb Mortal Wkly Rep. 2018;67:103-108.
8. Campos-Outcalt D. The new shingles vaccine: what PCPs need to know. J Fam Pract. 2017;66:audio. Available at: https://www.mdedge.com/jfponline/article/153168/vaccines/new-shingles-vaccine-what-pcps-need-know. Accessed January 19, 2018.
9. Marlow M. Grading of recommendations assessment, development and evaluation (GRADE): third dose of MMR vaccine. Presented at: Advisory Committee on Immunization Practices. October 25, 2017; Atlanta, GA. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-10/mumps-03-marlow-508.pdf. Accessed January 19, 2018.
10. HEPLISAV-B [package insert]. Berkeley, CA: Dynavax Technology Corporation; 2017. Available at: https://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM584762.pdf. Accessed January 23, 2018.
11. Janssen R. HEPLISAV-B. Presented at: Advisory Committee on Immunization Practices. October 25, 2017; Atlanta, GA. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-10/hepatitis-02-janssen.pdf. Accessed January 19, 2018.
1. Campos-Outcalt D. Latest recommendations for the 2017-2018 flu season. J Fam Pract. 2017;66:570-572.
2. Schillie S, Vellozzi C, Reingold A, et al. Prevention of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2018;67:1-31. Available at: https://www.cdc.gov/mmwr/volumes/67/rr/rr6701a1.htm. Accessed January 19, 2018.
3. CDC. Postvaccination serologic testing results for infants aged ≤24 months exposed to hepatitis B virus at birth: United States, 2008-2011. MMWR Morb Mortal Wkly Rep. 2012;61:768-771. Available at: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6138a4.htm. Accessed February 14, 2018.
4. Nelson N. Revaccination for infants born to hepatitis B virus (HBV)-infected mothers. Presented at: Advisory Committee on Immunization Practices. February 22, 2017; Atlanta, GA. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-02/hepatitis-02-background-nelson.pdf. Accessed January 19, 2017.
5. Hales CM, Harpaz R, Ortega-Sanchez I, et al. Update on recommendations for use of herpes zoster vaccine. MMWR Morb Mortal Wkly Rep. 2014;63:729-731. Available at: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6333a3.htm?s_cid=mm6333a3_w. Accessed January 23, 2018.
6. Dooling KL. Considerations for the use of herpes zoster vaccines. Presented at: Advisory Committee on Immunization Practices. October 25, 2017; Atlanta, GA. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-10/zoster-04-dooling.pdf. Accessed January 19, 2018.
7. Dooling KL, Guo A, Patel M, et al. Recommendations of the Advisory Committee on Immunization Practices for use of herpes zoster vaccines. MMWR Morb Mortal Wkly Rep. 2018;67:103-108.
8. Campos-Outcalt D. The new shingles vaccine: what PCPs need to know. J Fam Pract. 2017;66:audio. Available at: https://www.mdedge.com/jfponline/article/153168/vaccines/new-shingles-vaccine-what-pcps-need-know. Accessed January 19, 2018.
9. Marlow M. Grading of recommendations assessment, development and evaluation (GRADE): third dose of MMR vaccine. Presented at: Advisory Committee on Immunization Practices. October 25, 2017; Atlanta, GA. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-10/mumps-03-marlow-508.pdf. Accessed January 19, 2018.
10. HEPLISAV-B [package insert]. Berkeley, CA: Dynavax Technology Corporation; 2017. Available at: https://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM584762.pdf. Accessed January 23, 2018.
11. Janssen R. HEPLISAV-B. Presented at: Advisory Committee on Immunization Practices. October 25, 2017; Atlanta, GA. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-10/hepatitis-02-janssen.pdf. Accessed January 19, 2018.
CDC provides advice on recent hepatitis A outbreaks
The epidemiology of hepatitis A virus (HAV) disease has changed. Since July 2016, there have been 5 large outbreaks of infection involving more than 1600 cases,1 with affected states requiring assistance from the Centers for Disease Control and Prevention (CDC). Two of these outbreaks were foodborne, and 3 involved person-to-person transmission.1
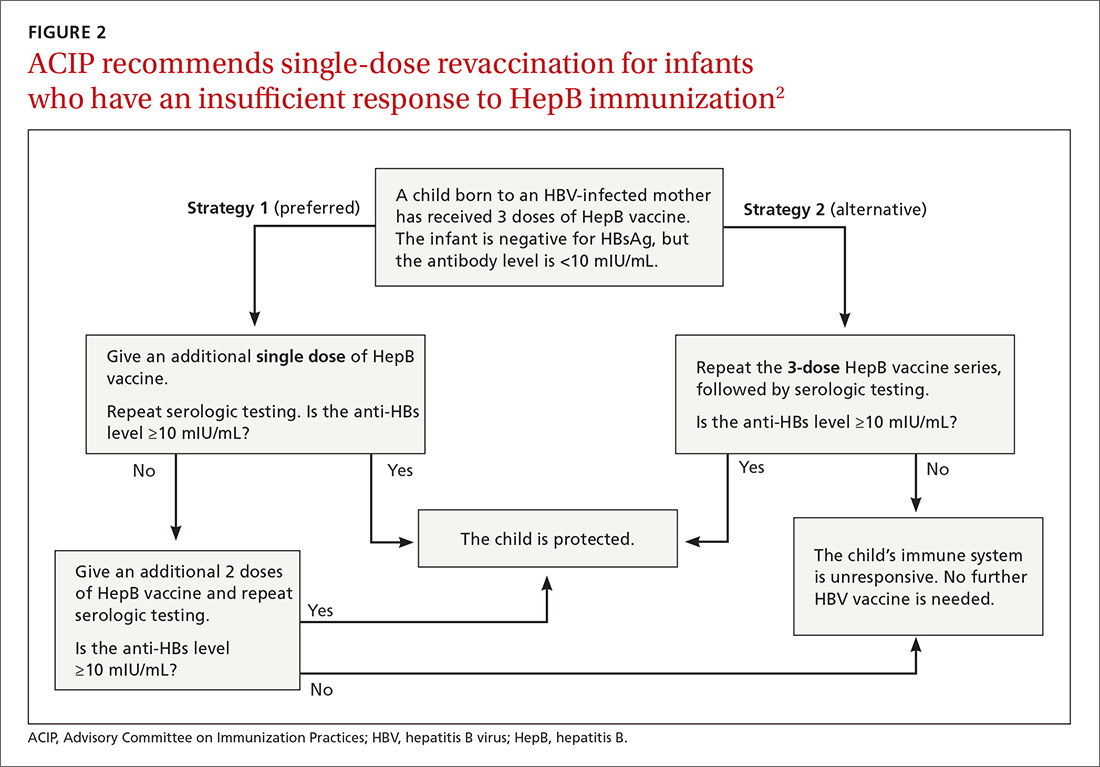
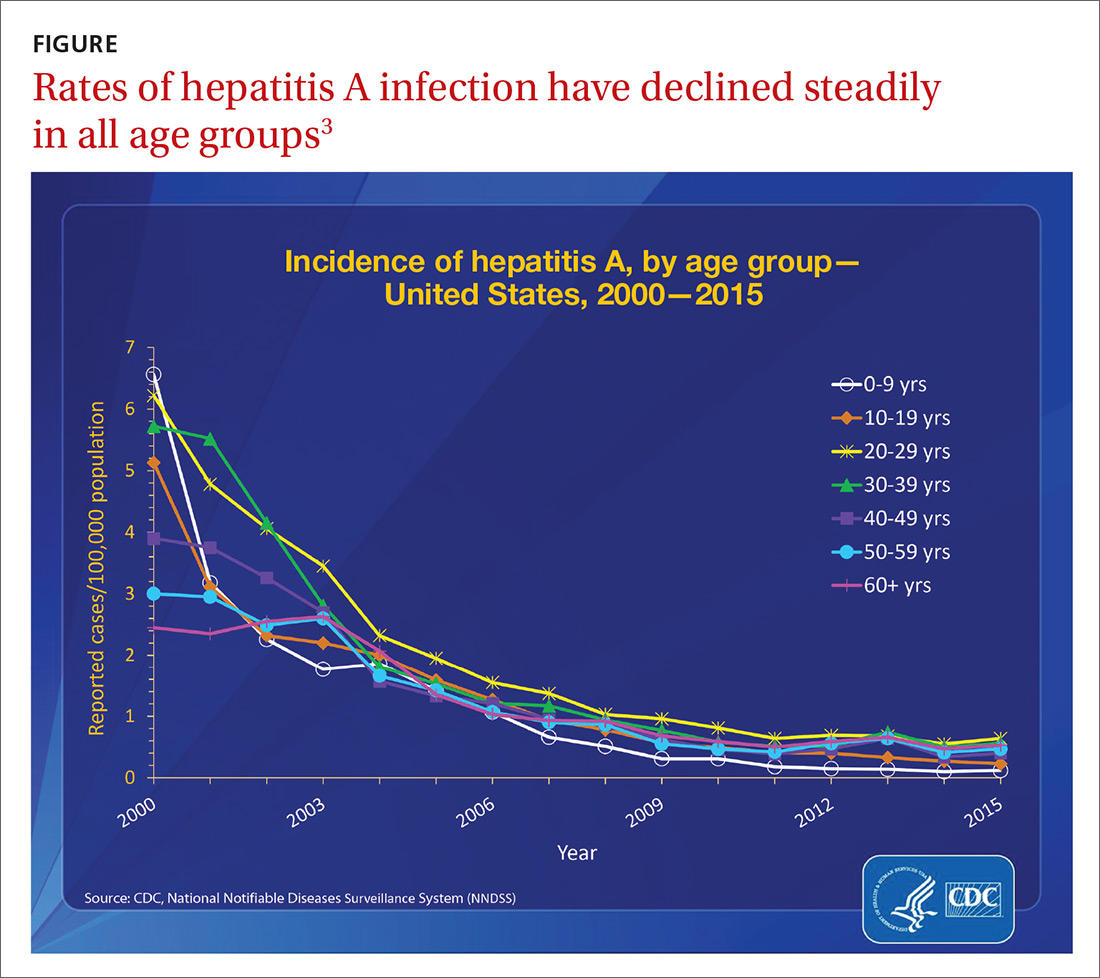
Before 2016, the number of outbreaks had been very low, and were predominantly associated with contaminated food, infected food handlers, and other food service-related exposures. Total annual cases of HAV infection had been declining steadily in all age groups since 1995 when HAV vaccine became available, from an estimated 271,000 cases resulting in 100 deaths2 to an estimated 2800 cases (with 1390 reported) resulting in 67 deaths in 2015 (FIGURE).3
Extent of the outbreaks
The largest hepatitis A outbreak involving person-to-person transmission in the United States in the past 20 years is occurring now in California. Predominantly affected are the homeless and users of illicit drugs, whose risk of infection is compounded by exposure to fecally-contaminated environments. As of December 1, the largest number of cases were recorded in San Diego (567), Santa Cruz (76), and Los Angeles (11).4 Adding 18 cases from other locations, the total has reached 672, resulting in 430 hospitalizations (64%) and 21 deaths (3%).4 In San Diego, 20% of those infected also had chronic hepatitis C and 5% had chronic hepatitis B.1
In southeastern Michigan, 555 cases have been reported, with 457 hospitalizations (82%) and 20 deaths (4%).5 In Utah, 91 cases and 53 hospitalizations (58%) have been documented.6 In these regions, the predominant risk factors have been homelessness and illicit drug use. And many of those infected have had chronic hepatitis C (27.5%), hepatitis B (13.2%), or both (9.9%).6 In 2 of the 3 states just described, the outbreaks have involved HAV genotype 1B.1
In New York City, an outbreak starting in January 2017 resulted in 51 cases. The epidemiology of this outbreak has been different from the others, involving men who have sex with men (MSM) and the HAV genotype 1A that matches a strain circulating among MSM in Europe.7
Low adult immunity is behind the outbreaks
These outbreaks have occurred in an adult US population that has low levels of immunity to HAV. In 2012 only 12.2% of adults ages 19 to 49 years had received 2 doses of HAV vaccine8 and only 24.2% of adults had antibodies to HAV,9 showing that most adults had never been infected with the virus or vaccinated. The reduction in HAV incidence previously described is due to the introduction of targeted, and then universal, child HAV vaccination recommendations by the Advisory Committee on Immunization Practices.
As the incidence of HAV disease declined, fewer individuals became infected as children, leading later to a susceptible pool of adults who had not been infected as children and who did not receive the vaccine in adulthood. Most of these adults will not be exposed to HAV due to decreased rates of infection in children, which, historically, has been the predominant means of adult exposure. The high hospitalization and death rates encountered in the recent and ongoing large outbreaks are explained by the multiple comorbidities of those infected.
Who should be vaccinated against HAV
The CDC recommends giving HAV vaccine to all children at age one year, and to the following groups:2,10,11
- residents of a community that has a high rate of hepatitis A infection
- household members or other close personal contacts (eg, regular babysitters) of adopted children newly arrived from countries with high or intermediate hepatitis A endemicity
- men who have sex with other men
- users of illicit injection and noninjection drugs
- workers in, or travelers to, countries with high rates of hepatitis A infection
- individuals with chronic liver disease
- individuals who work with HAV-infected animals or with HAV in a research setting.
Outbreak-specific vaccine recommendations
The CDC has additionally recommended that, during outbreaks, health care providers should consider taking the following 4 steps:12,13
- Increase the availability of HAV vaccine to the homeless and to those who use illicit drugs; to anyone who has ongoing, close contact with people who are homeless or who use injection and non-injection drugs; and as post-exposure prophylaxis for unvaccinated people who have been exposed to HAV in the previous 2 weeks.
- Defer the second dose of HAV vaccine if it is in short supply.
- Perform pre-vaccination serologic testing to identify those who are immune, thereby preserving vaccine and reducing costs.
- Use TWINRIX if other HAV vaccines are unavailable, keeping in mind that a single dose of TWINRIX achieves 94% protection against HAV but only 31% against hepatitis B virus (HBV). Three doses of TWINRIX are needed for full protection against HBV.
Available vaccines
Three vaccines are available for protection against HAV (TABLE2,14). Post-exposure prevention of HAV can be achieved with HAV vaccine or immune globulin.15 Vaccine is preferred for individuals up to age 40 years and can be used for older individuals if immune globulin is unavailable.
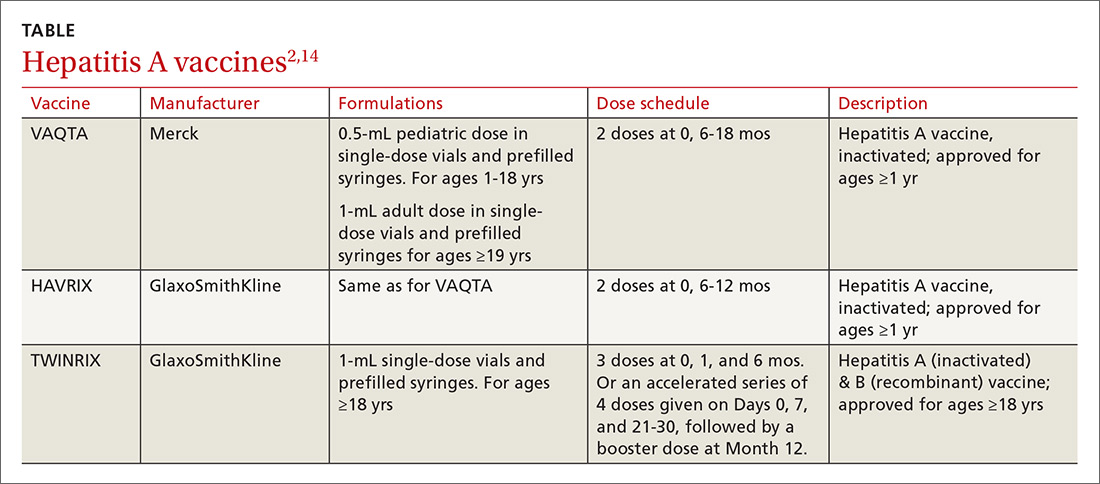
The CDC reports that the supply of adult HAV vaccine is being strained by these large outbreaks.16 Physicians will need to stay in touch with their local public health departments regarding vaccine availability in the community and any local recommendations being made regarding vaccine administration, as well as to the status of any local HAV outbreaks.
1. Nelson N. Hepatitis A outbreaks. Presented at: Advisory Committee on Immunization Practices; October 25, 2017; Atlanta, GA. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-10/hepatitis-04-nelson.pdf. Accessed December 5, 2017.
2. CDC. Prevention of hepatitis A through passive or active immunization. Recommendations of the Advisory Committee on Immunization Practices. Available at: https://www.cdc.gov/mmwr/preview/mmwrhtml/rr5507a1.htm. Accessed November 28, 2017.
3. CDC. Viral hepatitis surveillance—United States, 2015. Available at: https://www.cdc.gov/hepatitis/statistics/2015surveillance/pdfs/2015HepSurveillanceRpt.pdf. Accessed November 28, 2017.
4. California Department of Public Health. Hepatitis A outbreak in California. Available at: https://www.cdph.ca.gov/Programs/CID/DCDC/Pages/Immunization/Hepatitis-A-Outbreak.aspx. Accessed November 28, 2017.
5. Michigan Department of Health & Human Services. Hepatitis A southeast Michigan outbreak. Available at: http://www.michigan.gov/mdhhs/0,5885,7-339-71550_2955_2976_82305_82310-447907--,00.html. Accessed November 28, 2017.
6. Utah Department of Health. Hepatitis A outbreak. Available at: http://health.utah.gov/epi/diseases/hepatitisA/HAVoutbreak_2017. Accessed November 28, 2017.
7. Latash J, Dorsinville M, Del Rosso P, et al. Notes from the field: increase in reported hepatitis A infections among men who have sex with men–New York City, January-August 2017. MMWR Morb Mortal Wkly Rep. 2017;66:999-1000.
8. CDC. Murphy TV, Denniston MM, Hill HA, et al. Progress toward eliminating hepatitis A disease in the United States. MMWR Morb Mortal Wkly Rep. 2016;65:29-41.
9. Klevens RM, Denniston MM, Jiles-Chapman RB, et al. Decreasing immunity to hepatitis A virus infection among US adults: findings from the National Health and Nutrition Examination Survey (NHANES), 1999-2012. Vaccine. 2015;33:6192-6198.
10. CDC. Vaccines and preventable diseases. Hepatitis A in-short. Available at: https://www.cdc.gov/vaccines/vpd/hepa/public/in-short-adult.html#who. Accessed November 20, 2017.
11. CDC. Updated recommendations from the Advisory Committee on Immunization Practices (ACIP) for use of hepatitis A vaccine in close contacts of newly arriving international adoptees. MMWR Morb Mortal Wkly Rep. 2009;58:1006-1007.
12. CDC. Interim outbreak-specific guidance on hepatitis A vaccine administration. Available at: https://www.cdc.gov/hepatitis/outbreaks/InterimOutbreakGuidance-HAV-VaccineAdmin.htm. Accessed November 20, 2017.
13. CDC. 2017–Outbreaks of hepatitis A in multiple states among people who are homeless and people who use drugs. Available at: https://www.cdc.gov/hepatitis/outbreaks/2017March-HepatitisA.htm. Accessed December 11, 2017.
14. CDC. Notice to readers: FDA approval of an alternate dosing schedule for a combined hepatitis A and B vaccine (Twinrix). Available at: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5640a5.htm. Accessed December 8, 2017.
15. CDC. Update: prevention of hepatitis A after exposure to hepatitis A virus and in International Travelers. Updated recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2007;56:1080-1084.
16. CDC. Current vaccine shortages and delays. Available at: https://www.cdc.gov/vaccines/hcp/clinical-resources/shortages.html. Accessed November 28, 2017.
The epidemiology of hepatitis A virus (HAV) disease has changed. Since July 2016, there have been 5 large outbreaks of infection involving more than 1600 cases,1 with affected states requiring assistance from the Centers for Disease Control and Prevention (CDC). Two of these outbreaks were foodborne, and 3 involved person-to-person transmission.1
Before 2016, the number of outbreaks had been very low, and were predominantly associated with contaminated food, infected food handlers, and other food service-related exposures. Total annual cases of HAV infection had been declining steadily in all age groups since 1995 when HAV vaccine became available, from an estimated 271,000 cases resulting in 100 deaths2 to an estimated 2800 cases (with 1390 reported) resulting in 67 deaths in 2015 (FIGURE).3

Extent of the outbreaks
The largest hepatitis A outbreak involving person-to-person transmission in the United States in the past 20 years is occurring now in California. Predominantly affected are the homeless and users of illicit drugs, whose risk of infection is compounded by exposure to fecally-contaminated environments. As of December 1, the largest number of cases were recorded in San Diego (567), Santa Cruz (76), and Los Angeles (11).4 Adding 18 cases from other locations, the total has reached 672, resulting in 430 hospitalizations (64%) and 21 deaths (3%).4 In San Diego, 20% of those infected also had chronic hepatitis C and 5% had chronic hepatitis B.1
In southeastern Michigan, 555 cases have been reported, with 457 hospitalizations (82%) and 20 deaths (4%).5 In Utah, 91 cases and 53 hospitalizations (58%) have been documented.6 In these regions, the predominant risk factors have been homelessness and illicit drug use. And many of those infected have had chronic hepatitis C (27.5%), hepatitis B (13.2%), or both (9.9%).6 In 2 of the 3 states just described, the outbreaks have involved HAV genotype 1B.1
In New York City, an outbreak starting in January 2017 resulted in 51 cases. The epidemiology of this outbreak has been different from the others, involving men who have sex with men (MSM) and the HAV genotype 1A that matches a strain circulating among MSM in Europe.7
Low adult immunity is behind the outbreaks
These outbreaks have occurred in an adult US population that has low levels of immunity to HAV. In 2012 only 12.2% of adults ages 19 to 49 years had received 2 doses of HAV vaccine8 and only 24.2% of adults had antibodies to HAV,9 showing that most adults had never been infected with the virus or vaccinated. The reduction in HAV incidence previously described is due to the introduction of targeted, and then universal, child HAV vaccination recommendations by the Advisory Committee on Immunization Practices.
As the incidence of HAV disease declined, fewer individuals became infected as children, leading later to a susceptible pool of adults who had not been infected as children and who did not receive the vaccine in adulthood. Most of these adults will not be exposed to HAV due to decreased rates of infection in children, which, historically, has been the predominant means of adult exposure. The high hospitalization and death rates encountered in the recent and ongoing large outbreaks are explained by the multiple comorbidities of those infected.
Who should be vaccinated against HAV
The CDC recommends giving HAV vaccine to all children at age one year, and to the following groups:2,10,11
- residents of a community that has a high rate of hepatitis A infection
- household members or other close personal contacts (eg, regular babysitters) of adopted children newly arrived from countries with high or intermediate hepatitis A endemicity
- men who have sex with other men
- users of illicit injection and noninjection drugs
- workers in, or travelers to, countries with high rates of hepatitis A infection
- individuals with chronic liver disease
- individuals who work with HAV-infected animals or with HAV in a research setting.
Outbreak-specific vaccine recommendations
The CDC has additionally recommended that, during outbreaks, health care providers should consider taking the following 4 steps:12,13
- Increase the availability of HAV vaccine to the homeless and to those who use illicit drugs; to anyone who has ongoing, close contact with people who are homeless or who use injection and non-injection drugs; and as post-exposure prophylaxis for unvaccinated people who have been exposed to HAV in the previous 2 weeks.
- Defer the second dose of HAV vaccine if it is in short supply.
- Perform pre-vaccination serologic testing to identify those who are immune, thereby preserving vaccine and reducing costs.
- Use TWINRIX if other HAV vaccines are unavailable, keeping in mind that a single dose of TWINRIX achieves 94% protection against HAV but only 31% against hepatitis B virus (HBV). Three doses of TWINRIX are needed for full protection against HBV.
Available vaccines
Three vaccines are available for protection against HAV (TABLE2,14). Post-exposure prevention of HAV can be achieved with HAV vaccine or immune globulin.15 Vaccine is preferred for individuals up to age 40 years and can be used for older individuals if immune globulin is unavailable.

The CDC reports that the supply of adult HAV vaccine is being strained by these large outbreaks.16 Physicians will need to stay in touch with their local public health departments regarding vaccine availability in the community and any local recommendations being made regarding vaccine administration, as well as to the status of any local HAV outbreaks.
The epidemiology of hepatitis A virus (HAV) disease has changed. Since July 2016, there have been 5 large outbreaks of infection involving more than 1600 cases,1 with affected states requiring assistance from the Centers for Disease Control and Prevention (CDC). Two of these outbreaks were foodborne, and 3 involved person-to-person transmission.1
Before 2016, the number of outbreaks had been very low, and were predominantly associated with contaminated food, infected food handlers, and other food service-related exposures. Total annual cases of HAV infection had been declining steadily in all age groups since 1995 when HAV vaccine became available, from an estimated 271,000 cases resulting in 100 deaths2 to an estimated 2800 cases (with 1390 reported) resulting in 67 deaths in 2015 (FIGURE).3

Extent of the outbreaks
The largest hepatitis A outbreak involving person-to-person transmission in the United States in the past 20 years is occurring now in California. Predominantly affected are the homeless and users of illicit drugs, whose risk of infection is compounded by exposure to fecally-contaminated environments. As of December 1, the largest number of cases were recorded in San Diego (567), Santa Cruz (76), and Los Angeles (11).4 Adding 18 cases from other locations, the total has reached 672, resulting in 430 hospitalizations (64%) and 21 deaths (3%).4 In San Diego, 20% of those infected also had chronic hepatitis C and 5% had chronic hepatitis B.1
In southeastern Michigan, 555 cases have been reported, with 457 hospitalizations (82%) and 20 deaths (4%).5 In Utah, 91 cases and 53 hospitalizations (58%) have been documented.6 In these regions, the predominant risk factors have been homelessness and illicit drug use. And many of those infected have had chronic hepatitis C (27.5%), hepatitis B (13.2%), or both (9.9%).6 In 2 of the 3 states just described, the outbreaks have involved HAV genotype 1B.1
In New York City, an outbreak starting in January 2017 resulted in 51 cases. The epidemiology of this outbreak has been different from the others, involving men who have sex with men (MSM) and the HAV genotype 1A that matches a strain circulating among MSM in Europe.7
Low adult immunity is behind the outbreaks
These outbreaks have occurred in an adult US population that has low levels of immunity to HAV. In 2012 only 12.2% of adults ages 19 to 49 years had received 2 doses of HAV vaccine8 and only 24.2% of adults had antibodies to HAV,9 showing that most adults had never been infected with the virus or vaccinated. The reduction in HAV incidence previously described is due to the introduction of targeted, and then universal, child HAV vaccination recommendations by the Advisory Committee on Immunization Practices.
As the incidence of HAV disease declined, fewer individuals became infected as children, leading later to a susceptible pool of adults who had not been infected as children and who did not receive the vaccine in adulthood. Most of these adults will not be exposed to HAV due to decreased rates of infection in children, which, historically, has been the predominant means of adult exposure. The high hospitalization and death rates encountered in the recent and ongoing large outbreaks are explained by the multiple comorbidities of those infected.
Who should be vaccinated against HAV
The CDC recommends giving HAV vaccine to all children at age one year, and to the following groups:2,10,11
- residents of a community that has a high rate of hepatitis A infection
- household members or other close personal contacts (eg, regular babysitters) of adopted children newly arrived from countries with high or intermediate hepatitis A endemicity
- men who have sex with other men
- users of illicit injection and noninjection drugs
- workers in, or travelers to, countries with high rates of hepatitis A infection
- individuals with chronic liver disease
- individuals who work with HAV-infected animals or with HAV in a research setting.
Outbreak-specific vaccine recommendations
The CDC has additionally recommended that, during outbreaks, health care providers should consider taking the following 4 steps:12,13
- Increase the availability of HAV vaccine to the homeless and to those who use illicit drugs; to anyone who has ongoing, close contact with people who are homeless or who use injection and non-injection drugs; and as post-exposure prophylaxis for unvaccinated people who have been exposed to HAV in the previous 2 weeks.
- Defer the second dose of HAV vaccine if it is in short supply.
- Perform pre-vaccination serologic testing to identify those who are immune, thereby preserving vaccine and reducing costs.
- Use TWINRIX if other HAV vaccines are unavailable, keeping in mind that a single dose of TWINRIX achieves 94% protection against HAV but only 31% against hepatitis B virus (HBV). Three doses of TWINRIX are needed for full protection against HBV.
Available vaccines
Three vaccines are available for protection against HAV (TABLE2,14). Post-exposure prevention of HAV can be achieved with HAV vaccine or immune globulin.15 Vaccine is preferred for individuals up to age 40 years and can be used for older individuals if immune globulin is unavailable.

The CDC reports that the supply of adult HAV vaccine is being strained by these large outbreaks.16 Physicians will need to stay in touch with their local public health departments regarding vaccine availability in the community and any local recommendations being made regarding vaccine administration, as well as to the status of any local HAV outbreaks.
1. Nelson N. Hepatitis A outbreaks. Presented at: Advisory Committee on Immunization Practices; October 25, 2017; Atlanta, GA. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-10/hepatitis-04-nelson.pdf. Accessed December 5, 2017.
2. CDC. Prevention of hepatitis A through passive or active immunization. Recommendations of the Advisory Committee on Immunization Practices. Available at: https://www.cdc.gov/mmwr/preview/mmwrhtml/rr5507a1.htm. Accessed November 28, 2017.
3. CDC. Viral hepatitis surveillance—United States, 2015. Available at: https://www.cdc.gov/hepatitis/statistics/2015surveillance/pdfs/2015HepSurveillanceRpt.pdf. Accessed November 28, 2017.
4. California Department of Public Health. Hepatitis A outbreak in California. Available at: https://www.cdph.ca.gov/Programs/CID/DCDC/Pages/Immunization/Hepatitis-A-Outbreak.aspx. Accessed November 28, 2017.
5. Michigan Department of Health & Human Services. Hepatitis A southeast Michigan outbreak. Available at: http://www.michigan.gov/mdhhs/0,5885,7-339-71550_2955_2976_82305_82310-447907--,00.html. Accessed November 28, 2017.
6. Utah Department of Health. Hepatitis A outbreak. Available at: http://health.utah.gov/epi/diseases/hepatitisA/HAVoutbreak_2017. Accessed November 28, 2017.
7. Latash J, Dorsinville M, Del Rosso P, et al. Notes from the field: increase in reported hepatitis A infections among men who have sex with men–New York City, January-August 2017. MMWR Morb Mortal Wkly Rep. 2017;66:999-1000.
8. CDC. Murphy TV, Denniston MM, Hill HA, et al. Progress toward eliminating hepatitis A disease in the United States. MMWR Morb Mortal Wkly Rep. 2016;65:29-41.
9. Klevens RM, Denniston MM, Jiles-Chapman RB, et al. Decreasing immunity to hepatitis A virus infection among US adults: findings from the National Health and Nutrition Examination Survey (NHANES), 1999-2012. Vaccine. 2015;33:6192-6198.
10. CDC. Vaccines and preventable diseases. Hepatitis A in-short. Available at: https://www.cdc.gov/vaccines/vpd/hepa/public/in-short-adult.html#who. Accessed November 20, 2017.
11. CDC. Updated recommendations from the Advisory Committee on Immunization Practices (ACIP) for use of hepatitis A vaccine in close contacts of newly arriving international adoptees. MMWR Morb Mortal Wkly Rep. 2009;58:1006-1007.
12. CDC. Interim outbreak-specific guidance on hepatitis A vaccine administration. Available at: https://www.cdc.gov/hepatitis/outbreaks/InterimOutbreakGuidance-HAV-VaccineAdmin.htm. Accessed November 20, 2017.
13. CDC. 2017–Outbreaks of hepatitis A in multiple states among people who are homeless and people who use drugs. Available at: https://www.cdc.gov/hepatitis/outbreaks/2017March-HepatitisA.htm. Accessed December 11, 2017.
14. CDC. Notice to readers: FDA approval of an alternate dosing schedule for a combined hepatitis A and B vaccine (Twinrix). Available at: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5640a5.htm. Accessed December 8, 2017.
15. CDC. Update: prevention of hepatitis A after exposure to hepatitis A virus and in International Travelers. Updated recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2007;56:1080-1084.
16. CDC. Current vaccine shortages and delays. Available at: https://www.cdc.gov/vaccines/hcp/clinical-resources/shortages.html. Accessed November 28, 2017.
1. Nelson N. Hepatitis A outbreaks. Presented at: Advisory Committee on Immunization Practices; October 25, 2017; Atlanta, GA. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-10/hepatitis-04-nelson.pdf. Accessed December 5, 2017.
2. CDC. Prevention of hepatitis A through passive or active immunization. Recommendations of the Advisory Committee on Immunization Practices. Available at: https://www.cdc.gov/mmwr/preview/mmwrhtml/rr5507a1.htm. Accessed November 28, 2017.
3. CDC. Viral hepatitis surveillance—United States, 2015. Available at: https://www.cdc.gov/hepatitis/statistics/2015surveillance/pdfs/2015HepSurveillanceRpt.pdf. Accessed November 28, 2017.
4. California Department of Public Health. Hepatitis A outbreak in California. Available at: https://www.cdph.ca.gov/Programs/CID/DCDC/Pages/Immunization/Hepatitis-A-Outbreak.aspx. Accessed November 28, 2017.
5. Michigan Department of Health & Human Services. Hepatitis A southeast Michigan outbreak. Available at: http://www.michigan.gov/mdhhs/0,5885,7-339-71550_2955_2976_82305_82310-447907--,00.html. Accessed November 28, 2017.
6. Utah Department of Health. Hepatitis A outbreak. Available at: http://health.utah.gov/epi/diseases/hepatitisA/HAVoutbreak_2017. Accessed November 28, 2017.
7. Latash J, Dorsinville M, Del Rosso P, et al. Notes from the field: increase in reported hepatitis A infections among men who have sex with men–New York City, January-August 2017. MMWR Morb Mortal Wkly Rep. 2017;66:999-1000.
8. CDC. Murphy TV, Denniston MM, Hill HA, et al. Progress toward eliminating hepatitis A disease in the United States. MMWR Morb Mortal Wkly Rep. 2016;65:29-41.
9. Klevens RM, Denniston MM, Jiles-Chapman RB, et al. Decreasing immunity to hepatitis A virus infection among US adults: findings from the National Health and Nutrition Examination Survey (NHANES), 1999-2012. Vaccine. 2015;33:6192-6198.
10. CDC. Vaccines and preventable diseases. Hepatitis A in-short. Available at: https://www.cdc.gov/vaccines/vpd/hepa/public/in-short-adult.html#who. Accessed November 20, 2017.
11. CDC. Updated recommendations from the Advisory Committee on Immunization Practices (ACIP) for use of hepatitis A vaccine in close contacts of newly arriving international adoptees. MMWR Morb Mortal Wkly Rep. 2009;58:1006-1007.
12. CDC. Interim outbreak-specific guidance on hepatitis A vaccine administration. Available at: https://www.cdc.gov/hepatitis/outbreaks/InterimOutbreakGuidance-HAV-VaccineAdmin.htm. Accessed November 20, 2017.
13. CDC. 2017–Outbreaks of hepatitis A in multiple states among people who are homeless and people who use drugs. Available at: https://www.cdc.gov/hepatitis/outbreaks/2017March-HepatitisA.htm. Accessed December 11, 2017.
14. CDC. Notice to readers: FDA approval of an alternate dosing schedule for a combined hepatitis A and B vaccine (Twinrix). Available at: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5640a5.htm. Accessed December 8, 2017.
15. CDC. Update: prevention of hepatitis A after exposure to hepatitis A virus and in International Travelers. Updated recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2007;56:1080-1084.
16. CDC. Current vaccine shortages and delays. Available at: https://www.cdc.gov/vaccines/hcp/clinical-resources/shortages.html. Accessed November 28, 2017.
Screening for tuberculosis: Updated recommendations
Tuberculosis (TB) remains a significant public health problem worldwide with an estimated 10.4 million new cases and 1.7 million deaths having occurred in 2016.1 In that same year, there were 9287 new cases in the United States—the lowest number of TB cases on record.2
TB appears in one of 2 forms: active disease, which causes symptoms, morbidity, and mortality and is a source of transmission to others; and latent TB infection (LTBI), which is asymptomatic and noninfectious but can progress to active disease. The estimated prevalence of LTBI worldwide is 23%,3 although in the United States it is only about 5%.4 The proportion of those with LTBI who will develop active disease is estimated at 5% to 10% and is highly variable depending on risks.4
In the United States, about two-thirds of active TB cases occur among those who are foreign born, whose rate of active disease is 14.6/100,000.2 Five countries account for more than half of foreign-born cases: Mexico, the Philippines, India, Vietnam, and China.2
Who should be tested?
A major public health strategy for controlling TB in the United States is targeted screening for LTBI and treatment to prevent progression to active disease. The US Preventive Services Task Force (USPSTF) recommends screening for LTBI in adults age 18 and older who are at high risk of TB infection.4 This is consistent with recommendations from the Centers for Disease Control and Prevention (CDC), although the CDC also recommends testing infants and children
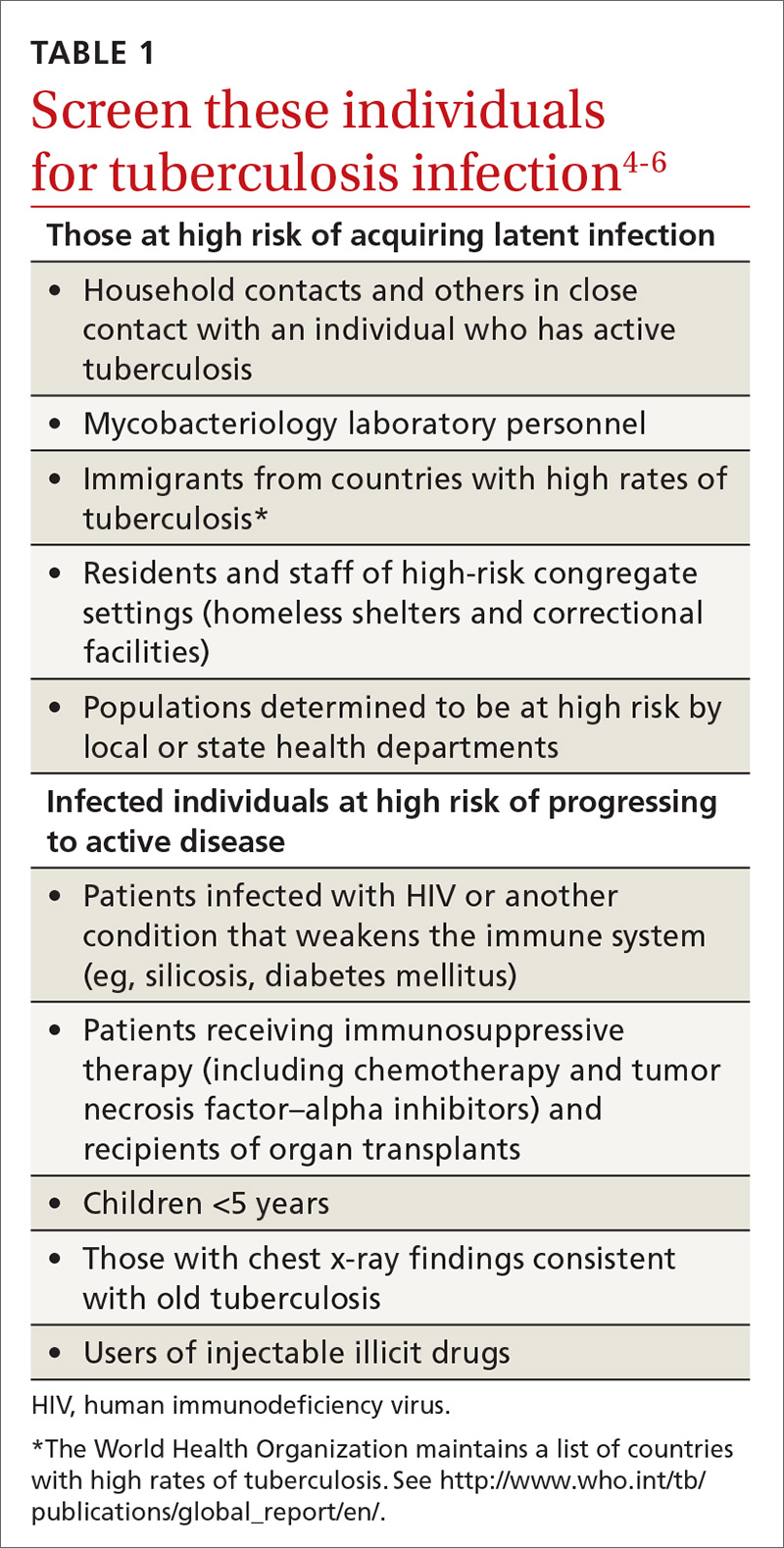
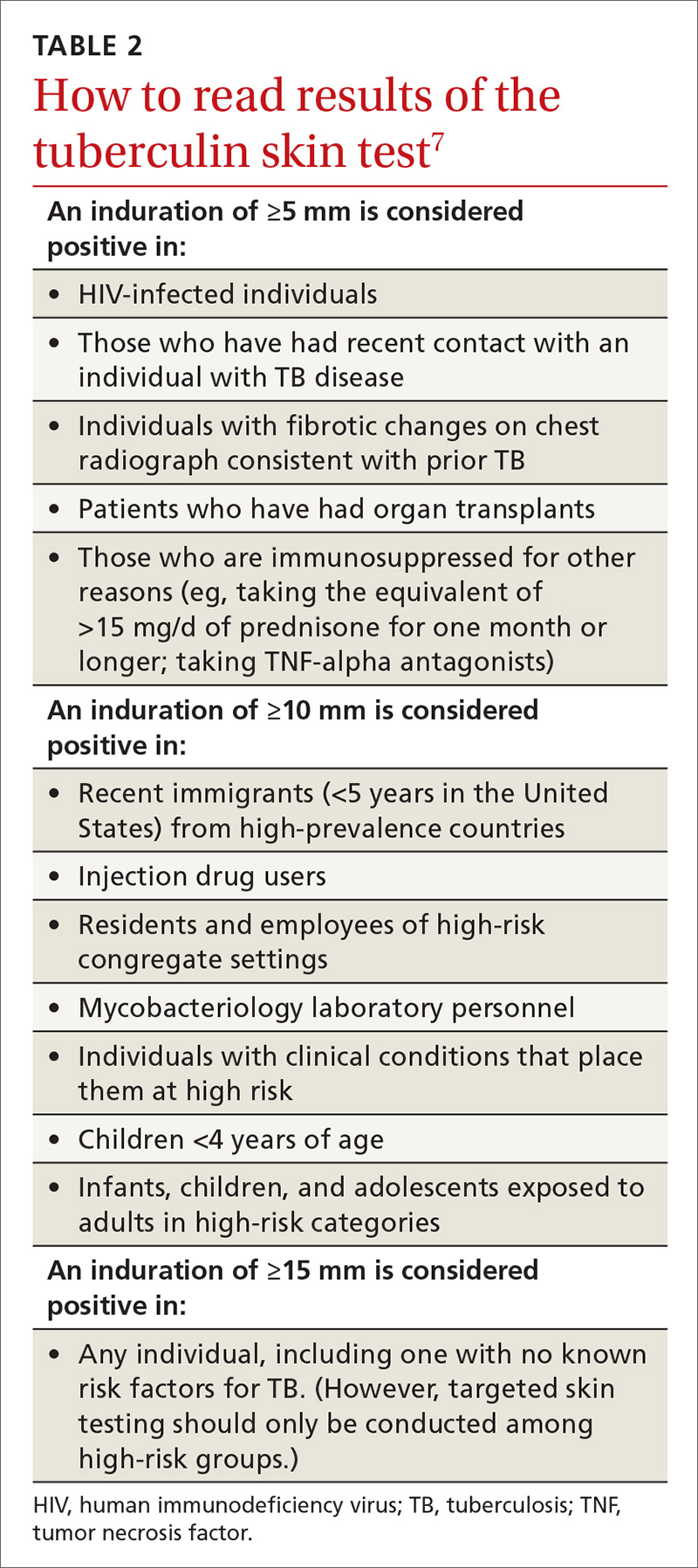
Two types of testing are available for TB screening: the TB skin test (TST) and the interferon-gamma release assay (IGRA). There are 2 IGRA test options: T-SPOT. TB (Oxford Immunotec) and QuantiFERON-TB Gold (Qiagen). The TST and IGRA each has advantages and disadvantages. The TST must be placed intradermally and read correctly, and the patient must return for the interpretation 48 to 72 hours after placement. Test interpretation depends on the patient’s risk category, with either a 5-mm, 10-mm, or 15-mm induration being classified as a positive result (TABLE 27).
IGRA is a blood test that needs to be processed within a limited time frame and is more expensive than the TST. The USPSTF lists the sensitivity and specificity of each option as follows: TST, using a 10-mm cutoff, 79%, 97%; T-SPOT, 90%, 95%; QuantiFERON-TB Gold In-Tube, 80%, 97%.4
Which test to use?
Recently the CDC, the American Thoracic Society, and the Infectious Diseases Society of America jointly published revised recommendations on TB testing:8
- For children younger than 5 years, TST is the preferred option, although IGRA is acceptable in children older than 3 years of age.
- For individuals at high risk of infection but not at high risk of disease progression, IGRA is recommended if they have received a bacille Calmette-Guerin vaccine or are unlikely to return for TST interpretation.
- For others at high risk of infection but not at high risk of disease progression, IGRA is preferred but TST is acceptable.
- For those who have both a high risk of infection and a high risk of disease progression, evidence is insufficient to recommend one test over another; either type is acceptable.
- For those with neither high risk of infection nor high risk of disease progression, testing is not recommended. However, it may be required by law or for credentialing of some kind (eg, for some health professionals or those who work in schools or nursing homes). If this is the case, IGRA is suggested as the preferred test. If the test result is positive, performing a second test is advised (either TST or an alternative type of IGRA). Consider the individual to be infected only if the second test result is also positive.
If a TB screening result is positive, confirm or rule out active TB by asking about symptoms (cough, fever, weight loss) and performing a chest x-ray. If the radiograph shows signs of active TB, collect 3 sputum samples by induction for analysis by smear microscopy, culture, and, possibly, nucleic acid amplification and rifampin susceptibility testing. Consider consulting your local public health department for advice on, or assistance with, sample collection. Report LTBI to the local health department and seek advice on the appropriate tests and treatments.
Expanded treatment selections
With LTBI there are now 4 treatment options for patients and physicians to consider:9 isoniazid given daily or twice weekly for either 6 or 9 months; isoniazid and rifapentine given once weekly for 3 months; or rifampin given daily for 4 months. Factors influencing treatment selection include a patient’s age, concomitant conditions, and the likelihood of bacterial resistance. Free treatment for LTBI may be available; again, check with your local health department.
1. WHO. Global tuberculosis report 2017. Geneva, Switzerland: World Health Organization. Available at: http://www.who.int/tb/publications/global_report/en/. Accessed November 8, 2017.
2. Schmit KM, Wansaula Z, Pratt R, et al. Tuberculosis—United States, 2016. MMWR Morb Mortal Wkly Rep. 2017;66:289-294.
3. Houben RMGJ, Dodd PJ. The global burden of latent tuberculosis infection: a re-estimation using mathematical modelling. PLoS Med. 2016;13(10):e1002152. Available at: http://journals.plos.org/plosmedicine/article?id=10.1371/journal.pmed.1002152. Accessed November 10, 2017.
4. USPSTF. Screening for latent tuberculosis infection in adults: US Preventive Services Task Force recommendation statement. JAMA. 2016;316:962-969.
5. CDC. Tuberculosis. Who should be tested. Available at: https://www.cdc.gov/tb/topic/testing/whobetested.htm. Accessed November 8, 2017.
6. CDC. Latent tuberculosis infection: a guide for primary health care providers. Targeted testing for tuberculosis. Available at: https://www.cdc.gov/tb/publications/ltbi/targetedtesting.htm#identifyingTBDisease. Accessed November 8, 2017.
7. CDC. TB elimination. Tuberculin skin testing. Available at: https://www.cdc.gov/tb/publications/factsheets/testing/skintesting.pdf. Accessed November 8, 2017.
8. Lewinsohn DM, Leonard MK, LoBue PA, el al. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention Clinical Practice Guidelines: Diagnosis of tuberculosis in adults and children. Clin Infect Dis. 2017;64:111-115.
9. CDC. Treatment regimens for latent TB infection (LTBI). Available at: https://www.cdc.gov/tb/topic/treatment/ltbi.htm. Accessed November 8, 2017.
Tuberculosis (TB) remains a significant public health problem worldwide with an estimated 10.4 million new cases and 1.7 million deaths having occurred in 2016.1 In that same year, there were 9287 new cases in the United States—the lowest number of TB cases on record.2
TB appears in one of 2 forms: active disease, which causes symptoms, morbidity, and mortality and is a source of transmission to others; and latent TB infection (LTBI), which is asymptomatic and noninfectious but can progress to active disease. The estimated prevalence of LTBI worldwide is 23%,3 although in the United States it is only about 5%.4 The proportion of those with LTBI who will develop active disease is estimated at 5% to 10% and is highly variable depending on risks.4
In the United States, about two-thirds of active TB cases occur among those who are foreign born, whose rate of active disease is 14.6/100,000.2 Five countries account for more than half of foreign-born cases: Mexico, the Philippines, India, Vietnam, and China.2
Who should be tested?
A major public health strategy for controlling TB in the United States is targeted screening for LTBI and treatment to prevent progression to active disease. The US Preventive Services Task Force (USPSTF) recommends screening for LTBI in adults age 18 and older who are at high risk of TB infection.4 This is consistent with recommendations from the Centers for Disease Control and Prevention (CDC), although the CDC also recommends testing infants and children

Two types of testing are available for TB screening: the TB skin test (TST) and the interferon-gamma release assay (IGRA). There are 2 IGRA test options: T-SPOT. TB (Oxford Immunotec) and QuantiFERON-TB Gold (Qiagen). The TST and IGRA each has advantages and disadvantages. The TST must be placed intradermally and read correctly, and the patient must return for the interpretation 48 to 72 hours after placement. Test interpretation depends on the patient’s risk category, with either a 5-mm, 10-mm, or 15-mm induration being classified as a positive result (TABLE 27).

IGRA is a blood test that needs to be processed within a limited time frame and is more expensive than the TST. The USPSTF lists the sensitivity and specificity of each option as follows: TST, using a 10-mm cutoff, 79%, 97%; T-SPOT, 90%, 95%; QuantiFERON-TB Gold In-Tube, 80%, 97%.4
Which test to use?
Recently the CDC, the American Thoracic Society, and the Infectious Diseases Society of America jointly published revised recommendations on TB testing:8
- For children younger than 5 years, TST is the preferred option, although IGRA is acceptable in children older than 3 years of age.
- For individuals at high risk of infection but not at high risk of disease progression, IGRA is recommended if they have received a bacille Calmette-Guerin vaccine or are unlikely to return for TST interpretation.
- For others at high risk of infection but not at high risk of disease progression, IGRA is preferred but TST is acceptable.
- For those who have both a high risk of infection and a high risk of disease progression, evidence is insufficient to recommend one test over another; either type is acceptable.
- For those with neither high risk of infection nor high risk of disease progression, testing is not recommended. However, it may be required by law or for credentialing of some kind (eg, for some health professionals or those who work in schools or nursing homes). If this is the case, IGRA is suggested as the preferred test. If the test result is positive, performing a second test is advised (either TST or an alternative type of IGRA). Consider the individual to be infected only if the second test result is also positive.
If a TB screening result is positive, confirm or rule out active TB by asking about symptoms (cough, fever, weight loss) and performing a chest x-ray. If the radiograph shows signs of active TB, collect 3 sputum samples by induction for analysis by smear microscopy, culture, and, possibly, nucleic acid amplification and rifampin susceptibility testing. Consider consulting your local public health department for advice on, or assistance with, sample collection. Report LTBI to the local health department and seek advice on the appropriate tests and treatments.
Expanded treatment selections
With LTBI there are now 4 treatment options for patients and physicians to consider:9 isoniazid given daily or twice weekly for either 6 or 9 months; isoniazid and rifapentine given once weekly for 3 months; or rifampin given daily for 4 months. Factors influencing treatment selection include a patient’s age, concomitant conditions, and the likelihood of bacterial resistance. Free treatment for LTBI may be available; again, check with your local health department.
Tuberculosis (TB) remains a significant public health problem worldwide with an estimated 10.4 million new cases and 1.7 million deaths having occurred in 2016.1 In that same year, there were 9287 new cases in the United States—the lowest number of TB cases on record.2
TB appears in one of 2 forms: active disease, which causes symptoms, morbidity, and mortality and is a source of transmission to others; and latent TB infection (LTBI), which is asymptomatic and noninfectious but can progress to active disease. The estimated prevalence of LTBI worldwide is 23%,3 although in the United States it is only about 5%.4 The proportion of those with LTBI who will develop active disease is estimated at 5% to 10% and is highly variable depending on risks.4
In the United States, about two-thirds of active TB cases occur among those who are foreign born, whose rate of active disease is 14.6/100,000.2 Five countries account for more than half of foreign-born cases: Mexico, the Philippines, India, Vietnam, and China.2
Who should be tested?
A major public health strategy for controlling TB in the United States is targeted screening for LTBI and treatment to prevent progression to active disease. The US Preventive Services Task Force (USPSTF) recommends screening for LTBI in adults age 18 and older who are at high risk of TB infection.4 This is consistent with recommendations from the Centers for Disease Control and Prevention (CDC), although the CDC also recommends testing infants and children

Two types of testing are available for TB screening: the TB skin test (TST) and the interferon-gamma release assay (IGRA). There are 2 IGRA test options: T-SPOT. TB (Oxford Immunotec) and QuantiFERON-TB Gold (Qiagen). The TST and IGRA each has advantages and disadvantages. The TST must be placed intradermally and read correctly, and the patient must return for the interpretation 48 to 72 hours after placement. Test interpretation depends on the patient’s risk category, with either a 5-mm, 10-mm, or 15-mm induration being classified as a positive result (TABLE 27).

IGRA is a blood test that needs to be processed within a limited time frame and is more expensive than the TST. The USPSTF lists the sensitivity and specificity of each option as follows: TST, using a 10-mm cutoff, 79%, 97%; T-SPOT, 90%, 95%; QuantiFERON-TB Gold In-Tube, 80%, 97%.4
Which test to use?
Recently the CDC, the American Thoracic Society, and the Infectious Diseases Society of America jointly published revised recommendations on TB testing:8
- For children younger than 5 years, TST is the preferred option, although IGRA is acceptable in children older than 3 years of age.
- For individuals at high risk of infection but not at high risk of disease progression, IGRA is recommended if they have received a bacille Calmette-Guerin vaccine or are unlikely to return for TST interpretation.
- For others at high risk of infection but not at high risk of disease progression, IGRA is preferred but TST is acceptable.
- For those who have both a high risk of infection and a high risk of disease progression, evidence is insufficient to recommend one test over another; either type is acceptable.
- For those with neither high risk of infection nor high risk of disease progression, testing is not recommended. However, it may be required by law or for credentialing of some kind (eg, for some health professionals or those who work in schools or nursing homes). If this is the case, IGRA is suggested as the preferred test. If the test result is positive, performing a second test is advised (either TST or an alternative type of IGRA). Consider the individual to be infected only if the second test result is also positive.
If a TB screening result is positive, confirm or rule out active TB by asking about symptoms (cough, fever, weight loss) and performing a chest x-ray. If the radiograph shows signs of active TB, collect 3 sputum samples by induction for analysis by smear microscopy, culture, and, possibly, nucleic acid amplification and rifampin susceptibility testing. Consider consulting your local public health department for advice on, or assistance with, sample collection. Report LTBI to the local health department and seek advice on the appropriate tests and treatments.
Expanded treatment selections
With LTBI there are now 4 treatment options for patients and physicians to consider:9 isoniazid given daily or twice weekly for either 6 or 9 months; isoniazid and rifapentine given once weekly for 3 months; or rifampin given daily for 4 months. Factors influencing treatment selection include a patient’s age, concomitant conditions, and the likelihood of bacterial resistance. Free treatment for LTBI may be available; again, check with your local health department.
1. WHO. Global tuberculosis report 2017. Geneva, Switzerland: World Health Organization. Available at: http://www.who.int/tb/publications/global_report/en/. Accessed November 8, 2017.
2. Schmit KM, Wansaula Z, Pratt R, et al. Tuberculosis—United States, 2016. MMWR Morb Mortal Wkly Rep. 2017;66:289-294.
3. Houben RMGJ, Dodd PJ. The global burden of latent tuberculosis infection: a re-estimation using mathematical modelling. PLoS Med. 2016;13(10):e1002152. Available at: http://journals.plos.org/plosmedicine/article?id=10.1371/journal.pmed.1002152. Accessed November 10, 2017.
4. USPSTF. Screening for latent tuberculosis infection in adults: US Preventive Services Task Force recommendation statement. JAMA. 2016;316:962-969.
5. CDC. Tuberculosis. Who should be tested. Available at: https://www.cdc.gov/tb/topic/testing/whobetested.htm. Accessed November 8, 2017.
6. CDC. Latent tuberculosis infection: a guide for primary health care providers. Targeted testing for tuberculosis. Available at: https://www.cdc.gov/tb/publications/ltbi/targetedtesting.htm#identifyingTBDisease. Accessed November 8, 2017.
7. CDC. TB elimination. Tuberculin skin testing. Available at: https://www.cdc.gov/tb/publications/factsheets/testing/skintesting.pdf. Accessed November 8, 2017.
8. Lewinsohn DM, Leonard MK, LoBue PA, el al. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention Clinical Practice Guidelines: Diagnosis of tuberculosis in adults and children. Clin Infect Dis. 2017;64:111-115.
9. CDC. Treatment regimens for latent TB infection (LTBI). Available at: https://www.cdc.gov/tb/topic/treatment/ltbi.htm. Accessed November 8, 2017.
1. WHO. Global tuberculosis report 2017. Geneva, Switzerland: World Health Organization. Available at: http://www.who.int/tb/publications/global_report/en/. Accessed November 8, 2017.
2. Schmit KM, Wansaula Z, Pratt R, et al. Tuberculosis—United States, 2016. MMWR Morb Mortal Wkly Rep. 2017;66:289-294.
3. Houben RMGJ, Dodd PJ. The global burden of latent tuberculosis infection: a re-estimation using mathematical modelling. PLoS Med. 2016;13(10):e1002152. Available at: http://journals.plos.org/plosmedicine/article?id=10.1371/journal.pmed.1002152. Accessed November 10, 2017.
4. USPSTF. Screening for latent tuberculosis infection in adults: US Preventive Services Task Force recommendation statement. JAMA. 2016;316:962-969.
5. CDC. Tuberculosis. Who should be tested. Available at: https://www.cdc.gov/tb/topic/testing/whobetested.htm. Accessed November 8, 2017.
6. CDC. Latent tuberculosis infection: a guide for primary health care providers. Targeted testing for tuberculosis. Available at: https://www.cdc.gov/tb/publications/ltbi/targetedtesting.htm#identifyingTBDisease. Accessed November 8, 2017.
7. CDC. TB elimination. Tuberculin skin testing. Available at: https://www.cdc.gov/tb/publications/factsheets/testing/skintesting.pdf. Accessed November 8, 2017.
8. Lewinsohn DM, Leonard MK, LoBue PA, el al. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention Clinical Practice Guidelines: Diagnosis of tuberculosis in adults and children. Clin Infect Dis. 2017;64:111-115.
9. CDC. Treatment regimens for latent TB infection (LTBI). Available at: https://www.cdc.gov/tb/topic/treatment/ltbi.htm. Accessed November 8, 2017.
From The Journal of Family Practice | 2017;66(12):755-757.
Latest recommendations for the 2017-2018 flu season
The Centers for Disease Control and Prevention (CDC) recently reported details of the 2016-2017 influenza season in Morbidity and Mortality Weekly Report1 and at the June meeting of the Advisory Committee on Immunization Practices. The CDC monitors influenza activity using several systems, and last flu season was shown to be moderately severe, starting in December in the Western United States, moving east, and peaking in February.
During the peak, 5.1% of outpatient visits were attributed to influenza-like illnesses, and 8.2% of reported deaths were due to pneumonia and influenza. For the whole influenza season, there were more than 18,000 confirmed influenza-related hospitalizations, with 60% of these occurring among those ≥65 years.1 Confirmed influenza-associated pediatric deaths totaled 98.1
The predominant influenza strain last year was type A (H3N2), accounting for about 76% of positive tests in public health laboratories (FIGURE).1 This was followed by influenza B (all lineages) at 22%, and influenza A (H1N1), accounting for only 2%. However, in early April, the predominant strain changed from A (H3N2) to influenza B. Importantly, all viruses tested last year were sensitive to oseltamivir, zanamivir, and peramivir. No antiviral resistance was detected to these neuraminidase inhibitors.
Good news and bad news on vaccine effectiveness. The good news: Circulating viruses were a close match to those contained in the vaccine. The bad news: Vaccine effectiveness at preventing illness was estimated to be just 34% against A (H3N2) and 56% against influenza B viruses.1 There has been no analysis of the relative effectiveness of different vaccines and vaccine types.
The past 6 influenza seasons have revealed a pattern of lower vaccine effectiveness against A (H3N2) compared with effectiveness against A (H1N1) and influenza B viruses. While vaccine effectiveness is not optimal, routine universal use still prevents a great deal of mortality and morbidity. It’s estimated that in 2012-2013, vaccine effectiveness (comparable to that in 2016-2017) prevented 5.6 million illnesses, 2.7 million medical visits, 61,500 hospitalizations, and 1800 deaths.1
More good news: Vaccine safety studies are reassuring
The CDC monitors influenza vaccine safety by using several sources, including the Vaccine Adverse Event Reporting System and the Vaccine Safety Datalink.2
Changes for the 2017-2018 influenza season
The composition of influenza vaccine products for the 2017-2018 season will differ slightly from last year’s formulation in the H1N1 component. Viral antigens to be included in the trivalent products are A/Michigan (H1N1), A/Hong Kong (H3N2), and B/Brisbane.3 Quadrivalent products will add B/Phuket to the other 3 antigens.3 A wide array of influenza vaccine products is available. Each one is described on the CDC Web site.4
Two minor changes in the recommendations were made at the June ACIP meeting.5 Afluria is approved by the FDA for use in children starting at age 5 years. ACIP had recommended that its use be reserved for children 9 years and older because previous influenza seasons had raised concerns about increased rates of febrile seizures in children younger than age 9. These concerns have been resolved, however, and the ACIP recommendations are now in concert with those of the FDA for this product.
Influenza immunization with an inactivated influenza vaccine product has been recommended for all pregnant women. Safety data are increasingly available for other product options as well, and ACIP now recommends vaccination in pregnancy with any age-appropriate product except for live attenuated influenza vaccine. 5
Antivirals: Give as needed, even before lab confirmation
The CDC recommends antiviral medication for individuals with confirmed or suspected influenza who have severe, complicated, or progressive illness, who require hospitalization, or who are at high risk of complications from influenza (TABLE6). Start treatment without waiting for laboratory confirmation for those with suspected influenza who are seriously ill. Outcomes are best when antivirals are started within 48 hours of illness onset, but they can be started even after this “window” has passed.
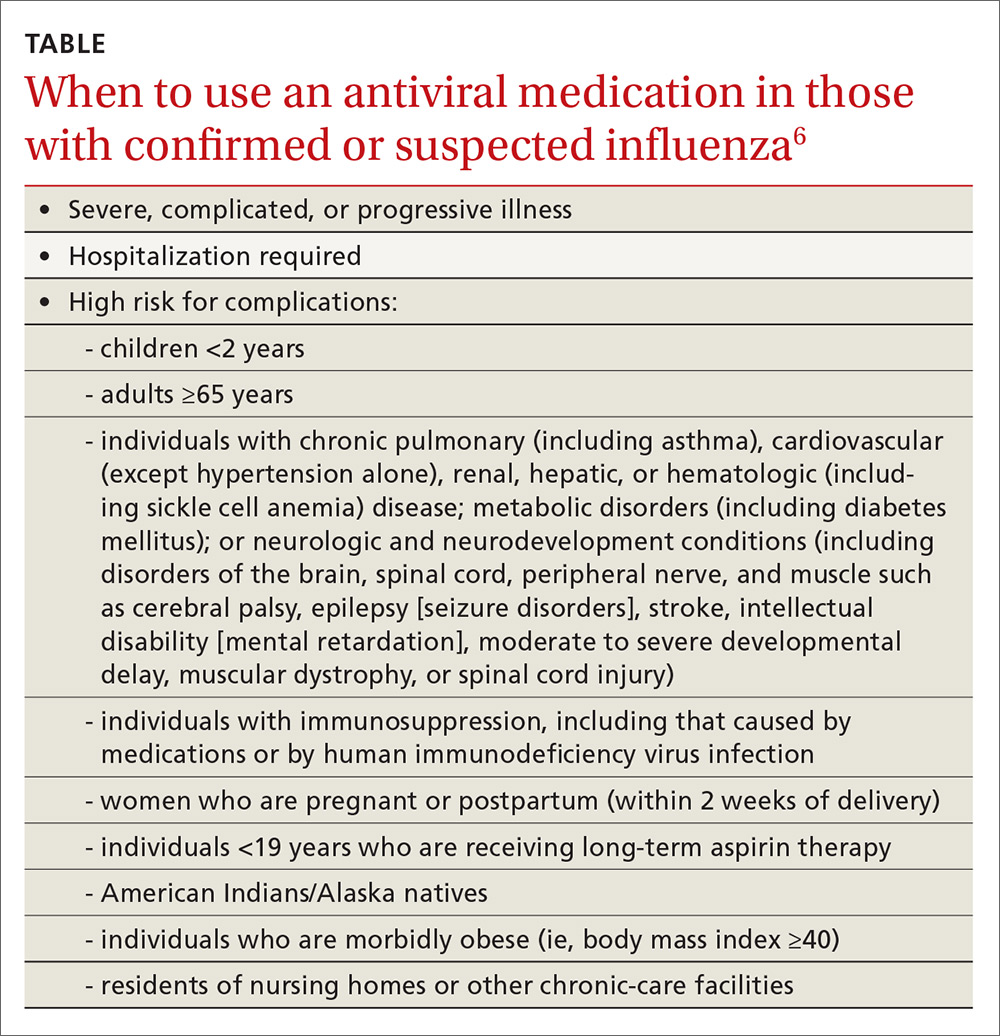
Once antiviral treatment has begun, make sure the full 5-day course is completed regardless of culture or rapid-test results.6 Use only neuraminidase inhibitors, as there is widespread resistance to adamantanes among influenza A viruses.
Influenza can occur year round
Rates of influenza infection are low in the summer, but cases do occur. Be especially alert if patients with influenza-like illness have been exposed to swine or poultry; they may have contracted a novel influenza A virus. Report such cases to the state or local health department so that staff can facilitate laboratory testing of viral subtypes. Follow the same protocol for patients with influenza symptoms who have traveled to areas where avian influenza viruses have been detected. The CDC is interested in detecting novel influenza viruses, which can start a pandemic.
Prepare for the 2017-2018 influenza season
Family physicians can help prevent influenza and its associated morbidity and mortality in several ways. Offer immunization to all patients, and immunize all health care personnel in your offices and clinics. Treat with antivirals those for whom they are recommended. Prepare office triage policies that prevent patients with flu symptoms from mixing with other patients, ensure that clinic infection control practices are enforced, and advise ill patients to avoid exposing others.7 Finally, stay current on influenza epidemiology and changes in recommendations for treatment and vaccination.
1. Blanton L, Alabi N, Mustaquim D, et al. Update: Influenza activity in the United States during the 2016-2017 season and composition of the 2017-2018 influenza vaccine. MMWR Morb Mortal Wkly Rep. 2017;66:668-676.
2. Shimabukuro T. End-of-season update: 2016-2017 influenza vaccine safety monitoring. Presented at: meeting of the Advisory Committee on Immunization Practices; June 21, 2017; Atlanta, Ga. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-06/flu-04-shimabukuro.pdf. Accessed August 1, 2017.
3. CDC. Frequently asked flu questions 2017-2018 influenza season. Available at: https://www.cdc.gov/flu/about/season/flu-season-2017-2018.htm. Accessed July 17, 2017.
4. CDC. Influenza vaccines — United States, 2016-17 influenza season. Available at: https://www.cdc.gov/flu/protect/vaccine/vaccines.htm. Accessed July 17, 2017.
5. Grohskopf L. Influenza WG considerations and proposed recommendations. Presented at: meeting of the Advisory Committee on Immunization Practices; June 21, 2017; Atlanta, Ga. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-06/flu-06-grohskopf.pdf. Accessed August 1, 2017.
6. CDC. Use of antivirals. Available at: https://www.cdc.gov/flu/professionals/antivirals/antiviral-use-influenza.htm#Box. Accessed July 17, 2017.
7. CDC. Prevention strategies for seasonal influenza in healthcare settings. Available at: https://www.cdc.gov/flu/professionals/infectioncontrol/healthcaresettings.htm. Accessed July 17, 2017.
The Centers for Disease Control and Prevention (CDC) recently reported details of the 2016-2017 influenza season in Morbidity and Mortality Weekly Report1 and at the June meeting of the Advisory Committee on Immunization Practices. The CDC monitors influenza activity using several systems, and last flu season was shown to be moderately severe, starting in December in the Western United States, moving east, and peaking in February.
During the peak, 5.1% of outpatient visits were attributed to influenza-like illnesses, and 8.2% of reported deaths were due to pneumonia and influenza. For the whole influenza season, there were more than 18,000 confirmed influenza-related hospitalizations, with 60% of these occurring among those ≥65 years.1 Confirmed influenza-associated pediatric deaths totaled 98.1
The predominant influenza strain last year was type A (H3N2), accounting for about 76% of positive tests in public health laboratories (FIGURE).1 This was followed by influenza B (all lineages) at 22%, and influenza A (H1N1), accounting for only 2%. However, in early April, the predominant strain changed from A (H3N2) to influenza B. Importantly, all viruses tested last year were sensitive to oseltamivir, zanamivir, and peramivir. No antiviral resistance was detected to these neuraminidase inhibitors.

Good news and bad news on vaccine effectiveness. The good news: Circulating viruses were a close match to those contained in the vaccine. The bad news: Vaccine effectiveness at preventing illness was estimated to be just 34% against A (H3N2) and 56% against influenza B viruses.1 There has been no analysis of the relative effectiveness of different vaccines and vaccine types.
The past 6 influenza seasons have revealed a pattern of lower vaccine effectiveness against A (H3N2) compared with effectiveness against A (H1N1) and influenza B viruses. While vaccine effectiveness is not optimal, routine universal use still prevents a great deal of mortality and morbidity. It’s estimated that in 2012-2013, vaccine effectiveness (comparable to that in 2016-2017) prevented 5.6 million illnesses, 2.7 million medical visits, 61,500 hospitalizations, and 1800 deaths.1
More good news: Vaccine safety studies are reassuring
The CDC monitors influenza vaccine safety by using several sources, including the Vaccine Adverse Event Reporting System and the Vaccine Safety Datalink.2
Changes for the 2017-2018 influenza season
The composition of influenza vaccine products for the 2017-2018 season will differ slightly from last year’s formulation in the H1N1 component. Viral antigens to be included in the trivalent products are A/Michigan (H1N1), A/Hong Kong (H3N2), and B/Brisbane.3 Quadrivalent products will add B/Phuket to the other 3 antigens.3 A wide array of influenza vaccine products is available. Each one is described on the CDC Web site.4
Two minor changes in the recommendations were made at the June ACIP meeting.5 Afluria is approved by the FDA for use in children starting at age 5 years. ACIP had recommended that its use be reserved for children 9 years and older because previous influenza seasons had raised concerns about increased rates of febrile seizures in children younger than age 9. These concerns have been resolved, however, and the ACIP recommendations are now in concert with those of the FDA for this product.
Influenza immunization with an inactivated influenza vaccine product has been recommended for all pregnant women. Safety data are increasingly available for other product options as well, and ACIP now recommends vaccination in pregnancy with any age-appropriate product except for live attenuated influenza vaccine. 5
Antivirals: Give as needed, even before lab confirmation
The CDC recommends antiviral medication for individuals with confirmed or suspected influenza who have severe, complicated, or progressive illness, who require hospitalization, or who are at high risk of complications from influenza (TABLE6). Start treatment without waiting for laboratory confirmation for those with suspected influenza who are seriously ill. Outcomes are best when antivirals are started within 48 hours of illness onset, but they can be started even after this “window” has passed.

Once antiviral treatment has begun, make sure the full 5-day course is completed regardless of culture or rapid-test results.6 Use only neuraminidase inhibitors, as there is widespread resistance to adamantanes among influenza A viruses.
Influenza can occur year round
Rates of influenza infection are low in the summer, but cases do occur. Be especially alert if patients with influenza-like illness have been exposed to swine or poultry; they may have contracted a novel influenza A virus. Report such cases to the state or local health department so that staff can facilitate laboratory testing of viral subtypes. Follow the same protocol for patients with influenza symptoms who have traveled to areas where avian influenza viruses have been detected. The CDC is interested in detecting novel influenza viruses, which can start a pandemic.
Prepare for the 2017-2018 influenza season
Family physicians can help prevent influenza and its associated morbidity and mortality in several ways. Offer immunization to all patients, and immunize all health care personnel in your offices and clinics. Treat with antivirals those for whom they are recommended. Prepare office triage policies that prevent patients with flu symptoms from mixing with other patients, ensure that clinic infection control practices are enforced, and advise ill patients to avoid exposing others.7 Finally, stay current on influenza epidemiology and changes in recommendations for treatment and vaccination.
The Centers for Disease Control and Prevention (CDC) recently reported details of the 2016-2017 influenza season in Morbidity and Mortality Weekly Report1 and at the June meeting of the Advisory Committee on Immunization Practices. The CDC monitors influenza activity using several systems, and last flu season was shown to be moderately severe, starting in December in the Western United States, moving east, and peaking in February.
During the peak, 5.1% of outpatient visits were attributed to influenza-like illnesses, and 8.2% of reported deaths were due to pneumonia and influenza. For the whole influenza season, there were more than 18,000 confirmed influenza-related hospitalizations, with 60% of these occurring among those ≥65 years.1 Confirmed influenza-associated pediatric deaths totaled 98.1
The predominant influenza strain last year was type A (H3N2), accounting for about 76% of positive tests in public health laboratories (FIGURE).1 This was followed by influenza B (all lineages) at 22%, and influenza A (H1N1), accounting for only 2%. However, in early April, the predominant strain changed from A (H3N2) to influenza B. Importantly, all viruses tested last year were sensitive to oseltamivir, zanamivir, and peramivir. No antiviral resistance was detected to these neuraminidase inhibitors.

Good news and bad news on vaccine effectiveness. The good news: Circulating viruses were a close match to those contained in the vaccine. The bad news: Vaccine effectiveness at preventing illness was estimated to be just 34% against A (H3N2) and 56% against influenza B viruses.1 There has been no analysis of the relative effectiveness of different vaccines and vaccine types.
The past 6 influenza seasons have revealed a pattern of lower vaccine effectiveness against A (H3N2) compared with effectiveness against A (H1N1) and influenza B viruses. While vaccine effectiveness is not optimal, routine universal use still prevents a great deal of mortality and morbidity. It’s estimated that in 2012-2013, vaccine effectiveness (comparable to that in 2016-2017) prevented 5.6 million illnesses, 2.7 million medical visits, 61,500 hospitalizations, and 1800 deaths.1
More good news: Vaccine safety studies are reassuring
The CDC monitors influenza vaccine safety by using several sources, including the Vaccine Adverse Event Reporting System and the Vaccine Safety Datalink.2
Changes for the 2017-2018 influenza season
The composition of influenza vaccine products for the 2017-2018 season will differ slightly from last year’s formulation in the H1N1 component. Viral antigens to be included in the trivalent products are A/Michigan (H1N1), A/Hong Kong (H3N2), and B/Brisbane.3 Quadrivalent products will add B/Phuket to the other 3 antigens.3 A wide array of influenza vaccine products is available. Each one is described on the CDC Web site.4
Two minor changes in the recommendations were made at the June ACIP meeting.5 Afluria is approved by the FDA for use in children starting at age 5 years. ACIP had recommended that its use be reserved for children 9 years and older because previous influenza seasons had raised concerns about increased rates of febrile seizures in children younger than age 9. These concerns have been resolved, however, and the ACIP recommendations are now in concert with those of the FDA for this product.
Influenza immunization with an inactivated influenza vaccine product has been recommended for all pregnant women. Safety data are increasingly available for other product options as well, and ACIP now recommends vaccination in pregnancy with any age-appropriate product except for live attenuated influenza vaccine. 5
Antivirals: Give as needed, even before lab confirmation
The CDC recommends antiviral medication for individuals with confirmed or suspected influenza who have severe, complicated, or progressive illness, who require hospitalization, or who are at high risk of complications from influenza (TABLE6). Start treatment without waiting for laboratory confirmation for those with suspected influenza who are seriously ill. Outcomes are best when antivirals are started within 48 hours of illness onset, but they can be started even after this “window” has passed.

Once antiviral treatment has begun, make sure the full 5-day course is completed regardless of culture or rapid-test results.6 Use only neuraminidase inhibitors, as there is widespread resistance to adamantanes among influenza A viruses.
Influenza can occur year round
Rates of influenza infection are low in the summer, but cases do occur. Be especially alert if patients with influenza-like illness have been exposed to swine or poultry; they may have contracted a novel influenza A virus. Report such cases to the state or local health department so that staff can facilitate laboratory testing of viral subtypes. Follow the same protocol for patients with influenza symptoms who have traveled to areas where avian influenza viruses have been detected. The CDC is interested in detecting novel influenza viruses, which can start a pandemic.
Prepare for the 2017-2018 influenza season
Family physicians can help prevent influenza and its associated morbidity and mortality in several ways. Offer immunization to all patients, and immunize all health care personnel in your offices and clinics. Treat with antivirals those for whom they are recommended. Prepare office triage policies that prevent patients with flu symptoms from mixing with other patients, ensure that clinic infection control practices are enforced, and advise ill patients to avoid exposing others.7 Finally, stay current on influenza epidemiology and changes in recommendations for treatment and vaccination.
1. Blanton L, Alabi N, Mustaquim D, et al. Update: Influenza activity in the United States during the 2016-2017 season and composition of the 2017-2018 influenza vaccine. MMWR Morb Mortal Wkly Rep. 2017;66:668-676.
2. Shimabukuro T. End-of-season update: 2016-2017 influenza vaccine safety monitoring. Presented at: meeting of the Advisory Committee on Immunization Practices; June 21, 2017; Atlanta, Ga. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-06/flu-04-shimabukuro.pdf. Accessed August 1, 2017.
3. CDC. Frequently asked flu questions 2017-2018 influenza season. Available at: https://www.cdc.gov/flu/about/season/flu-season-2017-2018.htm. Accessed July 17, 2017.
4. CDC. Influenza vaccines — United States, 2016-17 influenza season. Available at: https://www.cdc.gov/flu/protect/vaccine/vaccines.htm. Accessed July 17, 2017.
5. Grohskopf L. Influenza WG considerations and proposed recommendations. Presented at: meeting of the Advisory Committee on Immunization Practices; June 21, 2017; Atlanta, Ga. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-06/flu-06-grohskopf.pdf. Accessed August 1, 2017.
6. CDC. Use of antivirals. Available at: https://www.cdc.gov/flu/professionals/antivirals/antiviral-use-influenza.htm#Box. Accessed July 17, 2017.
7. CDC. Prevention strategies for seasonal influenza in healthcare settings. Available at: https://www.cdc.gov/flu/professionals/infectioncontrol/healthcaresettings.htm. Accessed July 17, 2017.
1. Blanton L, Alabi N, Mustaquim D, et al. Update: Influenza activity in the United States during the 2016-2017 season and composition of the 2017-2018 influenza vaccine. MMWR Morb Mortal Wkly Rep. 2017;66:668-676.
2. Shimabukuro T. End-of-season update: 2016-2017 influenza vaccine safety monitoring. Presented at: meeting of the Advisory Committee on Immunization Practices; June 21, 2017; Atlanta, Ga. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-06/flu-04-shimabukuro.pdf. Accessed August 1, 2017.
3. CDC. Frequently asked flu questions 2017-2018 influenza season. Available at: https://www.cdc.gov/flu/about/season/flu-season-2017-2018.htm. Accessed July 17, 2017.
4. CDC. Influenza vaccines — United States, 2016-17 influenza season. Available at: https://www.cdc.gov/flu/protect/vaccine/vaccines.htm. Accessed July 17, 2017.
5. Grohskopf L. Influenza WG considerations and proposed recommendations. Presented at: meeting of the Advisory Committee on Immunization Practices; June 21, 2017; Atlanta, Ga. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-06/flu-06-grohskopf.pdf. Accessed August 1, 2017.
6. CDC. Use of antivirals. Available at: https://www.cdc.gov/flu/professionals/antivirals/antiviral-use-influenza.htm#Box. Accessed July 17, 2017.
7. CDC. Prevention strategies for seasonal influenza in healthcare settings. Available at: https://www.cdc.gov/flu/professionals/infectioncontrol/healthcaresettings.htm. Accessed July 17, 2017.
Measles: Why it’s still a threat
In April of this year, 3 counties in Minnesota reported a measles outbreak, illustrating the danger of vaccine hesitancy that exists in some communities, resulting in low rates of childhood immunization. Fifty people—mostly children under the age of 5 and almost all unimmunized—have been diagnosed with measles since this outbreak began. As of early May, 11 had been hospitalized. Most of those infected have been American-born children of Somali immigrants.1,2
At the time of the outbreak, only 42% of the Somali children had been immunized against measles, compared with 88.5% of non-Somalis in Minnesota.2 Because of concern about the number of Somali children being diagnosed with autism, a condition apparently not recognized in Somalia, Somali parents living in Minnesota began questioning why this was occurring.
High profile anti-vaccine advocates reportedly visited the community and advised these parents that the measles-mumps-rubella (MMR) vaccine was the cause of this rise in autism incidence and encouraged them to avoid the vaccine.2 This series of events led to low vaccination rates in what was once a well-vaccinated community. The outbreak appears to have started with a Somali child who visited Africa and then returned to his community while incubating measles.
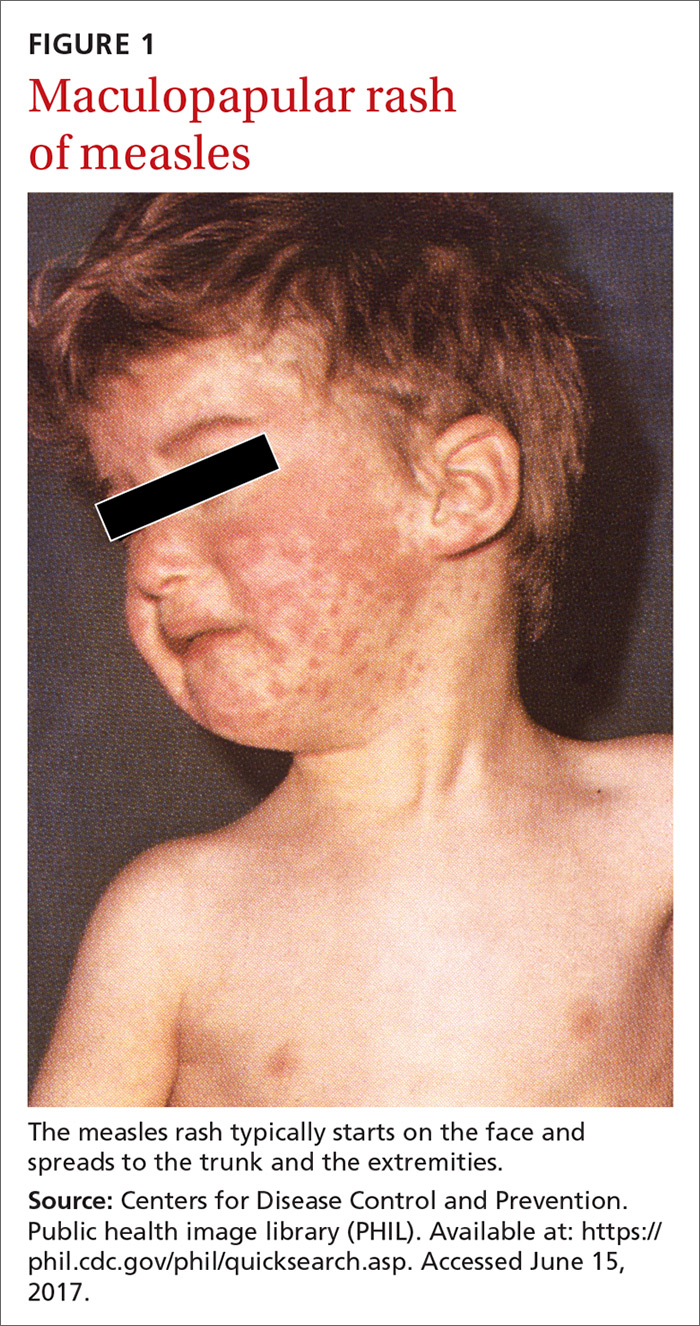
The clinical course of measles. Measles is an acute viral respiratory illness, which, after an incubation period of
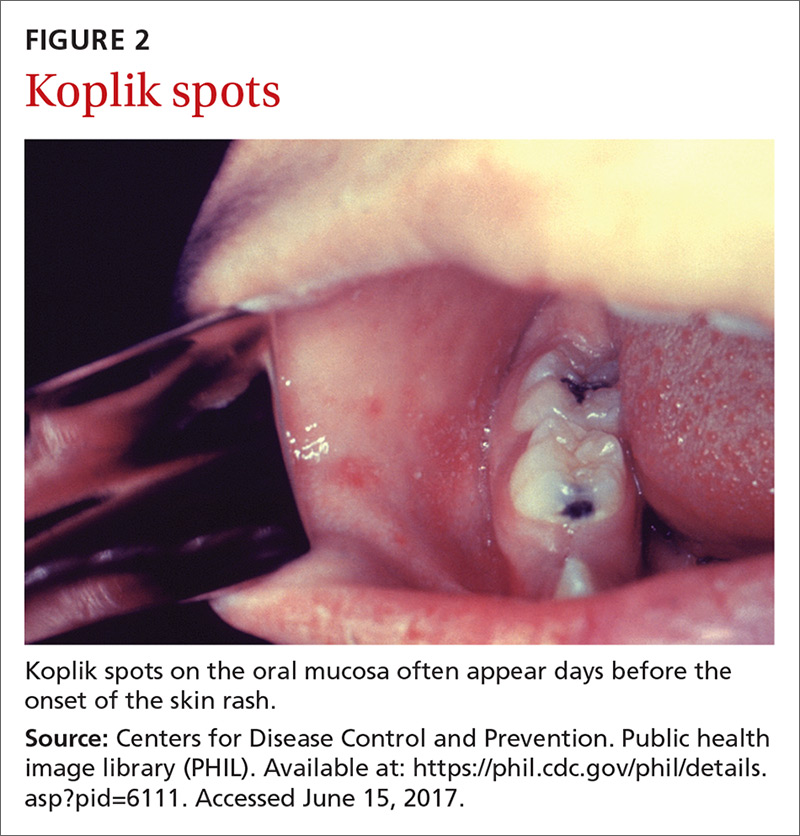
Measles is not a benign childhood illness. Before the licensure of live measles vaccine in 1963, an average of 549,000 measles cases were reported in the United States each year.3 That number is likely an underestimate due to inconsistent reporting, with a more plausible number of infections annually being 3 to 4 million.3 These regular epidemics led each year to about 48,000 people being hospitalized from complications, 1000 developing chronic disability from acute measles encephalitis, and about 500 dying from measles-related complications. Today, worldwide, an estimated 134,200 individuals die from measles each year.3
Where the risk is greatest. In the year 2000, measles was declared eliminated from the United States, meaning that endemic transmission was no longer occurring. Since then, the annual number of cases has ranged from a low of 37 in 2004 to a high of 667 in 2014.3 Most measles cases have occurred in unvaccinated individuals and primarily through importation by people infected in other countries who then transmit the infection upon entry or reentry to this country. In the United States, measles is more likely to spread and cause outbreaks in communities where large groups of people are unvaccinated.
Laboratory confirmation of measles is important to establish a correct clinical diagnosis, as well as to verify the infection for public health purposes. Confirmation is achieved by detecting in a patient’s blood sample the measles-specific IgM antibody or measles RNA by real-time polymerase chain reaction (RT-PCR). Obtain both a serum sample and a throat swab (or nasopharyngeal swab) from patients you suspect may have measles. Urine samples may also contain virus, and can be useful. The local health department can offer advice on how to collect and process these laboratory specimens.
Measles is a preventable infection
The Centers for Disease Control and Prevention (CDC) recommends routine childhood immunization with MMR vaccine, with the first dose given at age 12 through 15 months, and the second dose at 4 through 6 years of age (or at least 28 days following the first dose).3,5 Others for whom the vaccine is recommended are included in the TABLE.3

Because the MMR vaccine is a modified live-virus vaccine, it is contraindicated for pregnant women and those with severe immune deficiencies. It is also contraindicated for individuals who have ever had a life-threatening allergic reaction to the antibiotic neomycin or to any other MMR vaccine component.4 That these high-risk groups cannot receive protection from the vaccine underscores the importance of maintaining community herd immunity at a high level to prevent the spread of infection.
In response to this latest outbreak, the Minnesota Department of Health (MDH) has augmented its routine recommendations regarding measles vaccine,1 including advising that:
- All children 12 months and older who have not received the MMR vaccine and all adults born in 1957 (or later) who have not received the vaccine or ever had the measles should get the first dose as soon as possible.
- Children who live in counties where measles cases have occurred and who have received their first dose of the MMR vaccine at least 28 days ago should get their second dose as soon as possible.
- All Somali Minnesotan children statewide who received their first dose of the vaccine at least 28 days ago should get their second as soon as possible.
- Health care providers statewide may recommend an early (before age 4 years) second dose of the vaccine during routine appointments for children.
Preventing measles outbreaks and minimizing community impact
Measures family physicians can take to protect their staff, patients, and community from measles (and other infectious diseases) include ensuring that all staff are fully immunized as recommended by the CDC,6 vaccinating all patients according to the recommended immunization schedules, implementing and enforcing good infection control practices in the clinical setting, and taking appropriate measures to diagnose and manage individuals with suspected measles. These measures are described on the CDC Web site.7
Measles virus, commonly believed to be the most infectious agent known, is often transmitted in medical facilities. An individual can become infected simply by entering a closed space that had been occupied by someone with measles several hours earlier. In your facility, physically separate those with fever and rash from other patients as soon as possible and, if measles is suspected, care for them in an isolation room or one that can be kept unused afterwards.
Any time you suspect that a patient has measles, immediately inform the local public health department. The health department should conduct an investigation to find susceptible individuals, provide immunizations for case contacts (and immune globulin for unvaccinated pregnant women and those who are severely immunosuppressed), and implement isolation and quarantine measures as indicated by the situation.
There is no antiviral medication for measles. Aim treatment at controlling symptoms and addressing any complicating bacterial infections. Children who have severe illness should receive vitamin A at recommended doses.3
Outbreaks such as the one in Minnesota demonstrate the importance of family physicians working in collaboration with public health officials to minimize the effect of infectious illnesses on the community.
1. Minnesota Department of Health. MDH expands advice for measles vaccination to make sure more children in state are protected. Available at: http://www.health.state.mn.us/news/pressrel/2017/measles050417.html. Accessed May 24, 2017.
2. Offit PA. Did Anti-Vaxxers Spark a Measles Outbreak in an Immigrant Community? Daily Beast. Available at: http://www.thedailybeast.com/articles/2017/05/13/did-anti-vaxxers-spark-a-measles-outbreak-in-an-immigrant-community. Accessed May 24, 2017.
3. CDC. Measles. For healthcare professionals. Available at: https://www.cdc.gov/measles/hcp/index.html. Accessed May 24, 2017.
4. CDC. Prevention of measles, rubella, congenital rubella syndrome, and mumps, 2013: Summary recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2013;62:1-34. Available at: https://www.cdc.gov/mmwr/preview/mmwrhtml/rr6204a1.htm. Accessed May 24, 2017.
5. CDC. Immunization schedules. Available at: https://www.cdc.gov/vaccines/schedules/hcp/child-adolescent.html. Accessed May 24, 2017.
6. CDC. Recommended vaccines for health care workers. Available at: https://www.cdc.gov/vaccines/adults/rec-vac/hcw.html. Accessed May 24, 2017.
7. CDC. Infection control in health care facilities. Available at: https://www.cdc.gov/flu/professionals/infectioncontrol/index.htm. Accessed May 24, 2017.
In April of this year, 3 counties in Minnesota reported a measles outbreak, illustrating the danger of vaccine hesitancy that exists in some communities, resulting in low rates of childhood immunization. Fifty people—mostly children under the age of 5 and almost all unimmunized—have been diagnosed with measles since this outbreak began. As of early May, 11 had been hospitalized. Most of those infected have been American-born children of Somali immigrants.1,2
At the time of the outbreak, only 42% of the Somali children had been immunized against measles, compared with 88.5% of non-Somalis in Minnesota.2 Because of concern about the number of Somali children being diagnosed with autism, a condition apparently not recognized in Somalia, Somali parents living in Minnesota began questioning why this was occurring.
High profile anti-vaccine advocates reportedly visited the community and advised these parents that the measles-mumps-rubella (MMR) vaccine was the cause of this rise in autism incidence and encouraged them to avoid the vaccine.2 This series of events led to low vaccination rates in what was once a well-vaccinated community. The outbreak appears to have started with a Somali child who visited Africa and then returned to his community while incubating measles.

The clinical course of measles. Measles is an acute viral respiratory illness, which, after an incubation period of

Measles is not a benign childhood illness. Before the licensure of live measles vaccine in 1963, an average of 549,000 measles cases were reported in the United States each year.3 That number is likely an underestimate due to inconsistent reporting, with a more plausible number of infections annually being 3 to 4 million.3 These regular epidemics led each year to about 48,000 people being hospitalized from complications, 1000 developing chronic disability from acute measles encephalitis, and about 500 dying from measles-related complications. Today, worldwide, an estimated 134,200 individuals die from measles each year.3
Where the risk is greatest. In the year 2000, measles was declared eliminated from the United States, meaning that endemic transmission was no longer occurring. Since then, the annual number of cases has ranged from a low of 37 in 2004 to a high of 667 in 2014.3 Most measles cases have occurred in unvaccinated individuals and primarily through importation by people infected in other countries who then transmit the infection upon entry or reentry to this country. In the United States, measles is more likely to spread and cause outbreaks in communities where large groups of people are unvaccinated.
Laboratory confirmation of measles is important to establish a correct clinical diagnosis, as well as to verify the infection for public health purposes. Confirmation is achieved by detecting in a patient’s blood sample the measles-specific IgM antibody or measles RNA by real-time polymerase chain reaction (RT-PCR). Obtain both a serum sample and a throat swab (or nasopharyngeal swab) from patients you suspect may have measles. Urine samples may also contain virus, and can be useful. The local health department can offer advice on how to collect and process these laboratory specimens.
Measles is a preventable infection
The Centers for Disease Control and Prevention (CDC) recommends routine childhood immunization with MMR vaccine, with the first dose given at age 12 through 15 months, and the second dose at 4 through 6 years of age (or at least 28 days following the first dose).3,5 Others for whom the vaccine is recommended are included in the TABLE.3

Because the MMR vaccine is a modified live-virus vaccine, it is contraindicated for pregnant women and those with severe immune deficiencies. It is also contraindicated for individuals who have ever had a life-threatening allergic reaction to the antibiotic neomycin or to any other MMR vaccine component.4 That these high-risk groups cannot receive protection from the vaccine underscores the importance of maintaining community herd immunity at a high level to prevent the spread of infection.
In response to this latest outbreak, the Minnesota Department of Health (MDH) has augmented its routine recommendations regarding measles vaccine,1 including advising that:
- All children 12 months and older who have not received the MMR vaccine and all adults born in 1957 (or later) who have not received the vaccine or ever had the measles should get the first dose as soon as possible.
- Children who live in counties where measles cases have occurred and who have received their first dose of the MMR vaccine at least 28 days ago should get their second dose as soon as possible.
- All Somali Minnesotan children statewide who received their first dose of the vaccine at least 28 days ago should get their second as soon as possible.
- Health care providers statewide may recommend an early (before age 4 years) second dose of the vaccine during routine appointments for children.
Preventing measles outbreaks and minimizing community impact
Measures family physicians can take to protect their staff, patients, and community from measles (and other infectious diseases) include ensuring that all staff are fully immunized as recommended by the CDC,6 vaccinating all patients according to the recommended immunization schedules, implementing and enforcing good infection control practices in the clinical setting, and taking appropriate measures to diagnose and manage individuals with suspected measles. These measures are described on the CDC Web site.7
Measles virus, commonly believed to be the most infectious agent known, is often transmitted in medical facilities. An individual can become infected simply by entering a closed space that had been occupied by someone with measles several hours earlier. In your facility, physically separate those with fever and rash from other patients as soon as possible and, if measles is suspected, care for them in an isolation room or one that can be kept unused afterwards.
Any time you suspect that a patient has measles, immediately inform the local public health department. The health department should conduct an investigation to find susceptible individuals, provide immunizations for case contacts (and immune globulin for unvaccinated pregnant women and those who are severely immunosuppressed), and implement isolation and quarantine measures as indicated by the situation.
There is no antiviral medication for measles. Aim treatment at controlling symptoms and addressing any complicating bacterial infections. Children who have severe illness should receive vitamin A at recommended doses.3
Outbreaks such as the one in Minnesota demonstrate the importance of family physicians working in collaboration with public health officials to minimize the effect of infectious illnesses on the community.
In April of this year, 3 counties in Minnesota reported a measles outbreak, illustrating the danger of vaccine hesitancy that exists in some communities, resulting in low rates of childhood immunization. Fifty people—mostly children under the age of 5 and almost all unimmunized—have been diagnosed with measles since this outbreak began. As of early May, 11 had been hospitalized. Most of those infected have been American-born children of Somali immigrants.1,2
At the time of the outbreak, only 42% of the Somali children had been immunized against measles, compared with 88.5% of non-Somalis in Minnesota.2 Because of concern about the number of Somali children being diagnosed with autism, a condition apparently not recognized in Somalia, Somali parents living in Minnesota began questioning why this was occurring.
High profile anti-vaccine advocates reportedly visited the community and advised these parents that the measles-mumps-rubella (MMR) vaccine was the cause of this rise in autism incidence and encouraged them to avoid the vaccine.2 This series of events led to low vaccination rates in what was once a well-vaccinated community. The outbreak appears to have started with a Somali child who visited Africa and then returned to his community while incubating measles.

The clinical course of measles. Measles is an acute viral respiratory illness, which, after an incubation period of

Measles is not a benign childhood illness. Before the licensure of live measles vaccine in 1963, an average of 549,000 measles cases were reported in the United States each year.3 That number is likely an underestimate due to inconsistent reporting, with a more plausible number of infections annually being 3 to 4 million.3 These regular epidemics led each year to about 48,000 people being hospitalized from complications, 1000 developing chronic disability from acute measles encephalitis, and about 500 dying from measles-related complications. Today, worldwide, an estimated 134,200 individuals die from measles each year.3
Where the risk is greatest. In the year 2000, measles was declared eliminated from the United States, meaning that endemic transmission was no longer occurring. Since then, the annual number of cases has ranged from a low of 37 in 2004 to a high of 667 in 2014.3 Most measles cases have occurred in unvaccinated individuals and primarily through importation by people infected in other countries who then transmit the infection upon entry or reentry to this country. In the United States, measles is more likely to spread and cause outbreaks in communities where large groups of people are unvaccinated.
Laboratory confirmation of measles is important to establish a correct clinical diagnosis, as well as to verify the infection for public health purposes. Confirmation is achieved by detecting in a patient’s blood sample the measles-specific IgM antibody or measles RNA by real-time polymerase chain reaction (RT-PCR). Obtain both a serum sample and a throat swab (or nasopharyngeal swab) from patients you suspect may have measles. Urine samples may also contain virus, and can be useful. The local health department can offer advice on how to collect and process these laboratory specimens.
Measles is a preventable infection
The Centers for Disease Control and Prevention (CDC) recommends routine childhood immunization with MMR vaccine, with the first dose given at age 12 through 15 months, and the second dose at 4 through 6 years of age (or at least 28 days following the first dose).3,5 Others for whom the vaccine is recommended are included in the TABLE.3

Because the MMR vaccine is a modified live-virus vaccine, it is contraindicated for pregnant women and those with severe immune deficiencies. It is also contraindicated for individuals who have ever had a life-threatening allergic reaction to the antibiotic neomycin or to any other MMR vaccine component.4 That these high-risk groups cannot receive protection from the vaccine underscores the importance of maintaining community herd immunity at a high level to prevent the spread of infection.
In response to this latest outbreak, the Minnesota Department of Health (MDH) has augmented its routine recommendations regarding measles vaccine,1 including advising that:
- All children 12 months and older who have not received the MMR vaccine and all adults born in 1957 (or later) who have not received the vaccine or ever had the measles should get the first dose as soon as possible.
- Children who live in counties where measles cases have occurred and who have received their first dose of the MMR vaccine at least 28 days ago should get their second dose as soon as possible.
- All Somali Minnesotan children statewide who received their first dose of the vaccine at least 28 days ago should get their second as soon as possible.
- Health care providers statewide may recommend an early (before age 4 years) second dose of the vaccine during routine appointments for children.
Preventing measles outbreaks and minimizing community impact
Measures family physicians can take to protect their staff, patients, and community from measles (and other infectious diseases) include ensuring that all staff are fully immunized as recommended by the CDC,6 vaccinating all patients according to the recommended immunization schedules, implementing and enforcing good infection control practices in the clinical setting, and taking appropriate measures to diagnose and manage individuals with suspected measles. These measures are described on the CDC Web site.7
Measles virus, commonly believed to be the most infectious agent known, is often transmitted in medical facilities. An individual can become infected simply by entering a closed space that had been occupied by someone with measles several hours earlier. In your facility, physically separate those with fever and rash from other patients as soon as possible and, if measles is suspected, care for them in an isolation room or one that can be kept unused afterwards.
Any time you suspect that a patient has measles, immediately inform the local public health department. The health department should conduct an investigation to find susceptible individuals, provide immunizations for case contacts (and immune globulin for unvaccinated pregnant women and those who are severely immunosuppressed), and implement isolation and quarantine measures as indicated by the situation.
There is no antiviral medication for measles. Aim treatment at controlling symptoms and addressing any complicating bacterial infections. Children who have severe illness should receive vitamin A at recommended doses.3
Outbreaks such as the one in Minnesota demonstrate the importance of family physicians working in collaboration with public health officials to minimize the effect of infectious illnesses on the community.
1. Minnesota Department of Health. MDH expands advice for measles vaccination to make sure more children in state are protected. Available at: http://www.health.state.mn.us/news/pressrel/2017/measles050417.html. Accessed May 24, 2017.
2. Offit PA. Did Anti-Vaxxers Spark a Measles Outbreak in an Immigrant Community? Daily Beast. Available at: http://www.thedailybeast.com/articles/2017/05/13/did-anti-vaxxers-spark-a-measles-outbreak-in-an-immigrant-community. Accessed May 24, 2017.
3. CDC. Measles. For healthcare professionals. Available at: https://www.cdc.gov/measles/hcp/index.html. Accessed May 24, 2017.
4. CDC. Prevention of measles, rubella, congenital rubella syndrome, and mumps, 2013: Summary recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2013;62:1-34. Available at: https://www.cdc.gov/mmwr/preview/mmwrhtml/rr6204a1.htm. Accessed May 24, 2017.
5. CDC. Immunization schedules. Available at: https://www.cdc.gov/vaccines/schedules/hcp/child-adolescent.html. Accessed May 24, 2017.
6. CDC. Recommended vaccines for health care workers. Available at: https://www.cdc.gov/vaccines/adults/rec-vac/hcw.html. Accessed May 24, 2017.
7. CDC. Infection control in health care facilities. Available at: https://www.cdc.gov/flu/professionals/infectioncontrol/index.htm. Accessed May 24, 2017.
1. Minnesota Department of Health. MDH expands advice for measles vaccination to make sure more children in state are protected. Available at: http://www.health.state.mn.us/news/pressrel/2017/measles050417.html. Accessed May 24, 2017.
2. Offit PA. Did Anti-Vaxxers Spark a Measles Outbreak in an Immigrant Community? Daily Beast. Available at: http://www.thedailybeast.com/articles/2017/05/13/did-anti-vaxxers-spark-a-measles-outbreak-in-an-immigrant-community. Accessed May 24, 2017.
3. CDC. Measles. For healthcare professionals. Available at: https://www.cdc.gov/measles/hcp/index.html. Accessed May 24, 2017.
4. CDC. Prevention of measles, rubella, congenital rubella syndrome, and mumps, 2013: Summary recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2013;62:1-34. Available at: https://www.cdc.gov/mmwr/preview/mmwrhtml/rr6204a1.htm. Accessed May 24, 2017.
5. CDC. Immunization schedules. Available at: https://www.cdc.gov/vaccines/schedules/hcp/child-adolescent.html. Accessed May 24, 2017.
6. CDC. Recommended vaccines for health care workers. Available at: https://www.cdc.gov/vaccines/adults/rec-vac/hcw.html. Accessed May 24, 2017.
7. CDC. Infection control in health care facilities. Available at: https://www.cdc.gov/flu/professionals/infectioncontrol/index.htm. Accessed May 24, 2017.