User login
“The Physical Activity Guidelines for Americans”— a summary with tips
Resources
US Department of Health and Human Services. Physical Activity Guidelines for Americans. 2nd ed. Office of Disease Prevention and Health Promotion Web site. Published 2018. https://health.gov/paguidelines/second-edition/. Accessed January 7, 2019.
Piercy KL, Troiano RP, Ballard RM, et al. The Physical Activity Guidelines for Americans. JAMA. 2018;320:2020-2028.
Resources
US Department of Health and Human Services. Physical Activity Guidelines for Americans. 2nd ed. Office of Disease Prevention and Health Promotion Web site. Published 2018. https://health.gov/paguidelines/second-edition/. Accessed January 7, 2019.
Piercy KL, Troiano RP, Ballard RM, et al. The Physical Activity Guidelines for Americans. JAMA. 2018;320:2020-2028.
Resources
US Department of Health and Human Services. Physical Activity Guidelines for Americans. 2nd ed. Office of Disease Prevention and Health Promotion Web site. Published 2018. https://health.gov/paguidelines/second-edition/. Accessed January 7, 2019.
Piercy KL, Troiano RP, Ballard RM, et al. The Physical Activity Guidelines for Americans. JAMA. 2018;320:2020-2028.
A look at new guidelines for HIV treatment and prevention
An International Antiviral Society-USA Panel recently published an updated set of recommendations on using antiviral drugs to treat and prevent human immunodeficiency virus (HIV) infection1—a rapidly changing and complex topic. This new guideline updates the society’s 2016 publication.2 It contains recommendations on when to start antiretroviral therapy for those who are HIV positive and advice on suitable combinations of antiretroviral drugs. It also details pre- and post-exposure prophylaxis strategies for preventing HIV infection in those at risk.
This Practice Alert highlights the most important recommendations on treating those newly diagnosed as HIV positive and on preventing infection. Physicians who provide care for those who are HIV positive should familiarize themselves with the entire guideline.
Initiating treatment in those newly diagnosed as HIV positive
The panel now recommends starting antiretroviral therapy (ART) as soon as possible after HIV infection is confirmed; immediately if a patient is ready to commit to starting and continuing treatment. Any patient with an opportunistic infection should begin ART within 2 weeks of its diagnosis. Patients being treated for tuberculosis (TB) should begin ART within 2 weeks of starting TB treatment if their CD4 cell count is <50/mcL; those whose count is ≥50/mcL should begin ART within 2 to 8 weeks.
The panel recommends one of 3 ART combinations (TABLE 11), all of which contain an integrase strand transfer inhibitor (INSTI). ART started immediately should not include a nonnucleoside reverse transcriptase inhibitor (NNRTI) because of possible viral resistance. The guideline recommends 6 other ART combinations if none of the first 3 options can be used.1

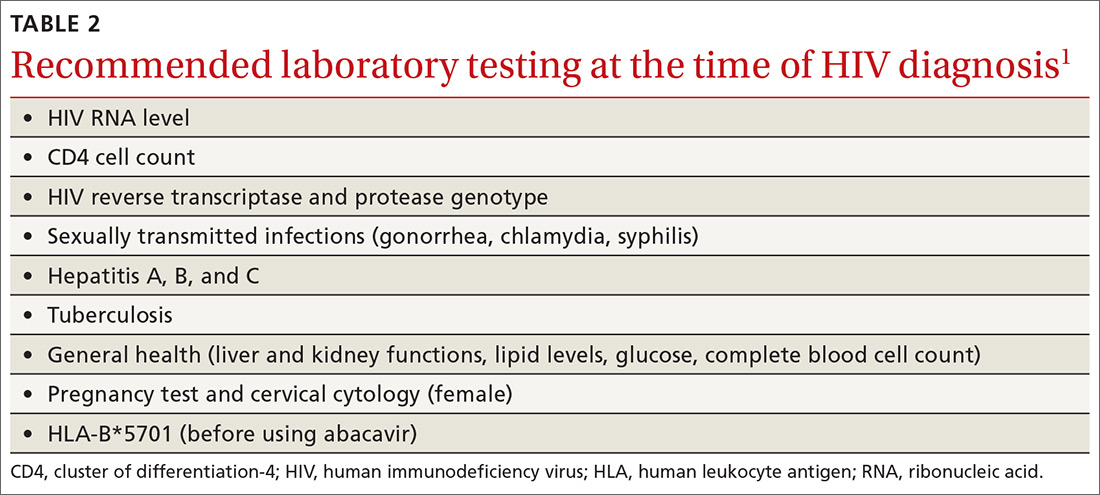
An initial set of laboratory tests (TABLE 21) should be conducted on each individual receiving ART, although treatment can start before the results are returned. Ongoing laboratory monitoring, described in detail in the guideline, depends on the ART regimen chosen and the patient’s response to therapy. The only routinely recommended prophylaxis for opportunistic infections is for Pneumocystis pneumonia if the CD4 count is <200/mcL.
Preventing HIV with prEP
Consider prescribing daily pre-exposure prophylaxis (PrEP) with emtricitabine/tenofovir disoproxil fumarate (Truvada) for men and women who are at risk from sexual exposure to HIV or who inject illicit drugs. It takes about 1 week for protective tissue levels to be achieved. Testing to rule out HIV infection is recommended before starting PrEP, as is testing for serum creatinine level, estimated glomerular filtration rate, and hepatitis B surface antigen. Tenofovir disoproxil fumarate is not recommended for those with creatinine clearance of less than 60 mL/min/1.73 m2. For patients taking PrEP, emphasize other preventive measures such as using condoms to protect against both HIV and other sexually-transmitted diseases (STDs), using clean needles and syringes when injecting drugs, or entering a drug rehabilitation program. After initiating PrEP, schedule the first follow-up visit for 30 days later to repeat the HIV test and to assess adverse reactions and PrEP adherence.
For men who have sex with men (MSM), there is an alternative form of PrEP when sexual exposure is infrequent. “On-demand” or “event-driven” PrEP involves 4 doses of emtricitabine/tenofovir disoproxil fumarate; 2 doses given with food 2 to 24 hours before sex (the closer to 24 the better), one dose 24 hours after the first and one 24 hours after the second. This is referred to as 2-1-1 dosing. This option has only been tested in MSM with sexual exposure. It is not recommended at this time for others at risk for HIV or for MSM with chronic or active hepatitis B infection.
Continue to: Preventing HIV infection with post-exposure prophylaxis
Preventing HIV infection with post-exposure prophylaxis
Post-exposure prophylaxis (PEP) for HIV infection is divided into 2 categories: occupational PEP (oPEP) and non-occupational PEP (nPEP). Recommendations for oPEP are described elsewhere3 and are not covered in this Practice Alert. Summarized below are the recommendations for nPEP after sex, injection drug use, and other nonoccupational exposures, which are also described on the Centers for Disease Control and Prevention (CDC) Web site.4
Assess the need for nPEP if high-risk exposure (TABLE 34) occurred ≤72 hours earlier. Before starting nPEP, perform a rapid HIV blood test. If rapid testing is unavailable, start nPEP, which can be discontinued if the patient is later determined to have HIV infection. Repeat HIV testing at 4 to 6 weeks and 3 months following initiation of nPEP. Approved HIV tests are described on the CDC Web site at http://www.cdc.gov/hiv/testing/laboratorytests.html. Oral HIV tests are not recommended for HIV testing before initiating nPEP.
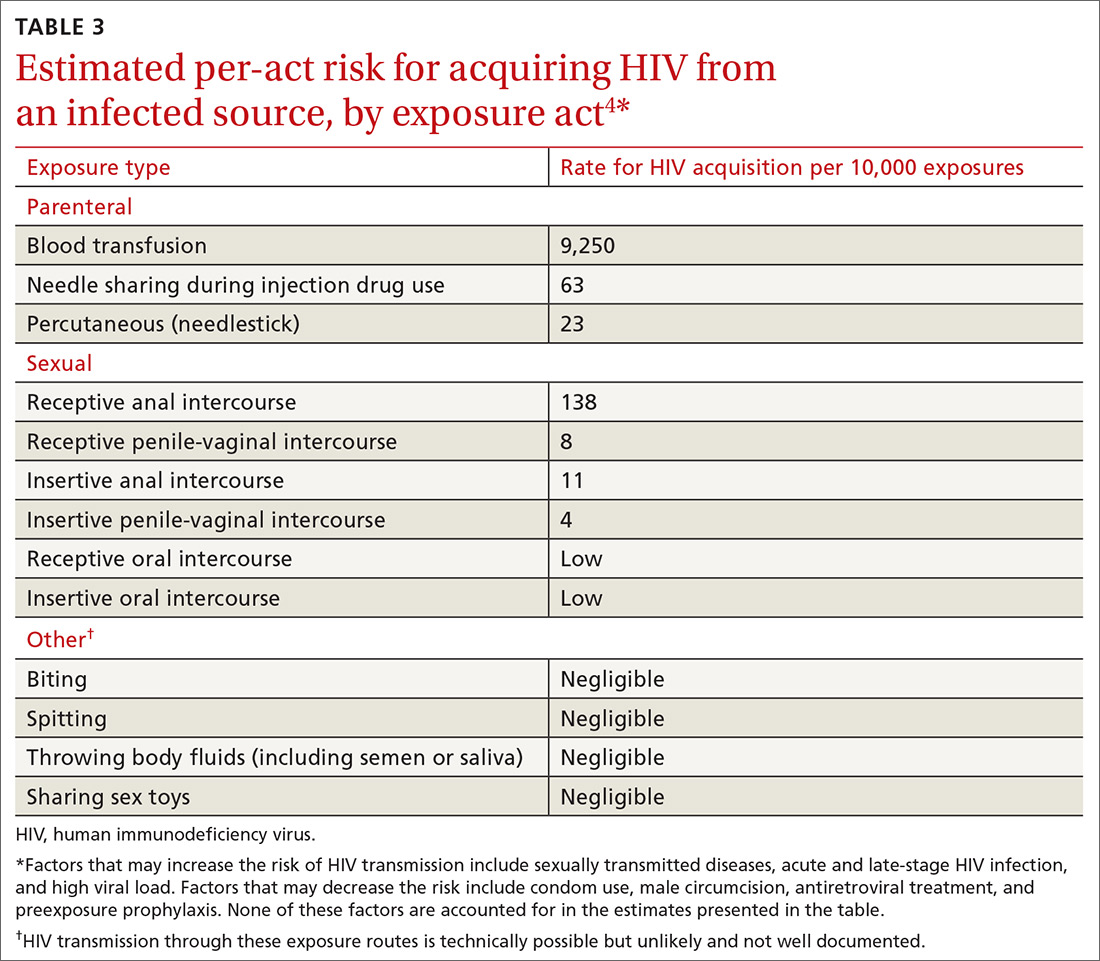
nPEP is not recommended when an individual’s risk of exposure to HIV is not high, or if the exposure occurred more than 72 hours before presentation. An algorithm is available to assist with assessing whether nPEP is recommended (FIGURE4).
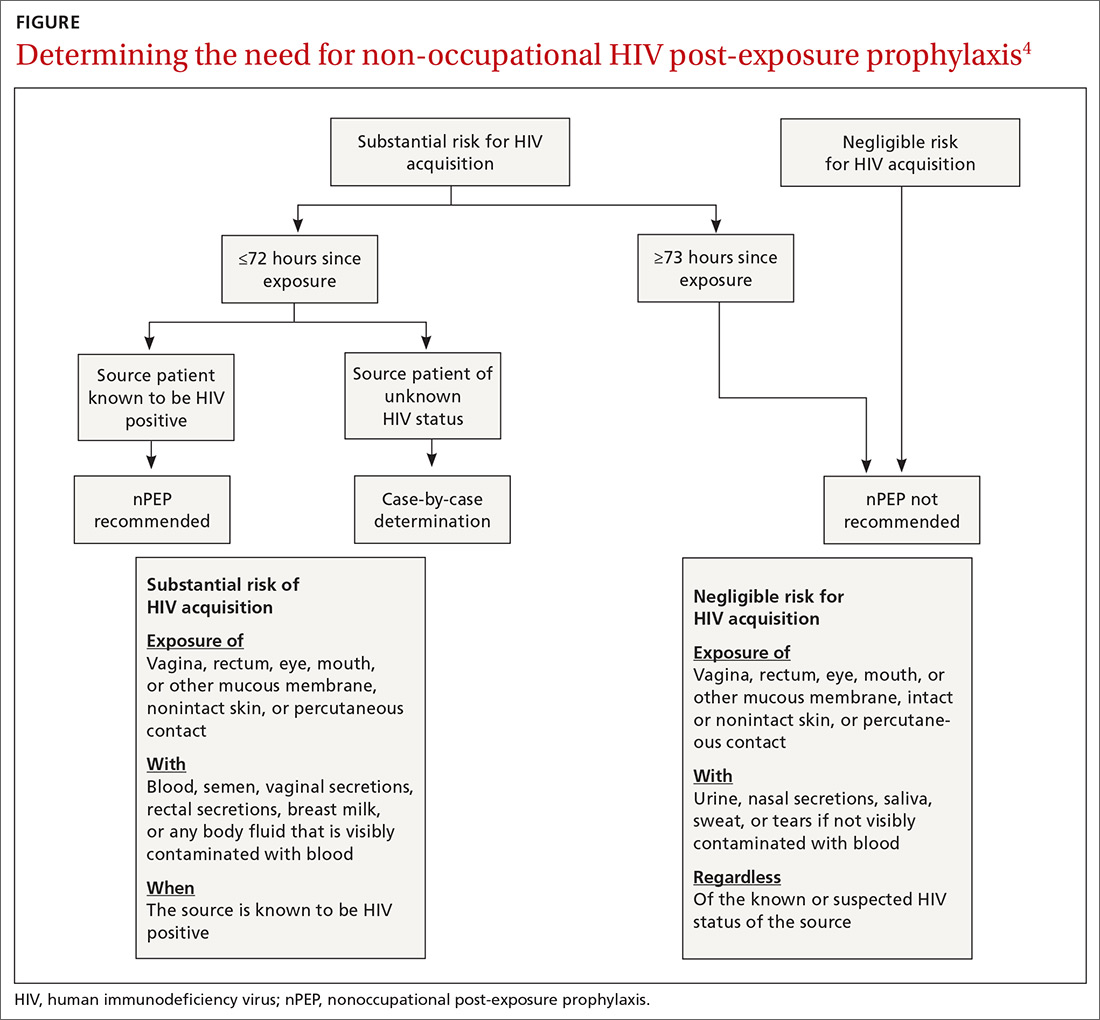
Specific nPEP regimens. For otherwise healthy adults and adolescents, preferred nPEP consists of a 28-day course of a 3-drug combination: tenofovir disoproxil fumarate 300 mg once daily; emtricitabine 200 mg once daily; and raltegravir, 400 mg twice daily, or dolutegravir 50 mg once daily. Alternative regimens for adults and adolescents are described in the guideline, as are options for children, those with decreased renal function, and pregnant women. Those who receive more than one course of nPEP within a 12-month period should consider PrEP.
When additional vaccination is needed. For victims of sexual assault, offer prophylaxis against STD (TABLE 44) and hepatitis B virus (HBV). Those who have not been vaccinated against HBV should receive the first dose at the initial visit. If the exposure source is known to be HBsAg-positive, give the unvaccinated patient both hepatitis B vaccine and hepatitis B immune globulin at the first visit. The full hepatitis B vaccine series should then be completed according to the recommended schedule and the vaccine product used. Those who have completed hepatitis B vaccination but who were not tested with a post-vaccine titer should receive a single dose of hepatitis B vaccine.

Continue to: Victims of sexual assault...
Victims of sexual assault can benefit from referral to professionals with expertise in post-assault counseling. Sexual Assault Nurse Examiner programs are listed at http://www.sane-sart.com.
Financial assistance for patients. Anti-retroviral drugs are expensive, and those who need nPEP may not have a payer source. Many pharmaceutical manufacturers offer medication assistance programs, and processes are set up to handle time-sensitive requests. Information for specific medications can be found at http://www.pparx.org/en/prescription_assistance_programs/list_of_participating_programs. Those who are prescribed nPEP after a sexual assault can receive reimbursement for medications and health care costs through state Crime Victim Compensation Programs funded by the Department of Justice. State-specific contact information is available at http://www.nacvcb.org/index.asp?sid=6.
1. Saag MS, Benson CA, Gandhi RT, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2018 recommendations of the International Antiviral Society-USA Panel. JAMA. 2018;320:379-396.
2. Günthard HF, Saag MS, Benson CA, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2016 recommendations of the International Antiviral Society-USA Panel. JAMA. 2016;316:191-210.
3. Kuhar DT, Henderson DK, Struble KA, et al; US Public Health Service Working Group. Updated US Public Health Service guidelines for the management of occupational exposures to human immunodeficiency virus and recommendations for postexposure prophylaxis. Infect Control Hosp Epidemiol. 2013;34:875-892.
4. CDC. Updated guidelines for antiretroviral postexposure prophylaxis after sexual, injection drug use, or other nonoccupational exposure to HIV—United States, 2016. https://www-cdc-gov.ezproxy3.library.arizona.edu/hiv/pdf/programresources/cdc-hiv-npep-guidelines.pdf. Accessed October 11, 2018.
An International Antiviral Society-USA Panel recently published an updated set of recommendations on using antiviral drugs to treat and prevent human immunodeficiency virus (HIV) infection1—a rapidly changing and complex topic. This new guideline updates the society’s 2016 publication.2 It contains recommendations on when to start antiretroviral therapy for those who are HIV positive and advice on suitable combinations of antiretroviral drugs. It also details pre- and post-exposure prophylaxis strategies for preventing HIV infection in those at risk.
This Practice Alert highlights the most important recommendations on treating those newly diagnosed as HIV positive and on preventing infection. Physicians who provide care for those who are HIV positive should familiarize themselves with the entire guideline.
Initiating treatment in those newly diagnosed as HIV positive
The panel now recommends starting antiretroviral therapy (ART) as soon as possible after HIV infection is confirmed; immediately if a patient is ready to commit to starting and continuing treatment. Any patient with an opportunistic infection should begin ART within 2 weeks of its diagnosis. Patients being treated for tuberculosis (TB) should begin ART within 2 weeks of starting TB treatment if their CD4 cell count is <50/mcL; those whose count is ≥50/mcL should begin ART within 2 to 8 weeks.
The panel recommends one of 3 ART combinations (TABLE 11), all of which contain an integrase strand transfer inhibitor (INSTI). ART started immediately should not include a nonnucleoside reverse transcriptase inhibitor (NNRTI) because of possible viral resistance. The guideline recommends 6 other ART combinations if none of the first 3 options can be used.1

An initial set of laboratory tests (TABLE 21) should be conducted on each individual receiving ART, although treatment can start before the results are returned. Ongoing laboratory monitoring, described in detail in the guideline, depends on the ART regimen chosen and the patient’s response to therapy. The only routinely recommended prophylaxis for opportunistic infections is for Pneumocystis pneumonia if the CD4 count is <200/mcL.

Preventing HIV with prEP
Consider prescribing daily pre-exposure prophylaxis (PrEP) with emtricitabine/tenofovir disoproxil fumarate (Truvada) for men and women who are at risk from sexual exposure to HIV or who inject illicit drugs. It takes about 1 week for protective tissue levels to be achieved. Testing to rule out HIV infection is recommended before starting PrEP, as is testing for serum creatinine level, estimated glomerular filtration rate, and hepatitis B surface antigen. Tenofovir disoproxil fumarate is not recommended for those with creatinine clearance of less than 60 mL/min/1.73 m2. For patients taking PrEP, emphasize other preventive measures such as using condoms to protect against both HIV and other sexually-transmitted diseases (STDs), using clean needles and syringes when injecting drugs, or entering a drug rehabilitation program. After initiating PrEP, schedule the first follow-up visit for 30 days later to repeat the HIV test and to assess adverse reactions and PrEP adherence.
For men who have sex with men (MSM), there is an alternative form of PrEP when sexual exposure is infrequent. “On-demand” or “event-driven” PrEP involves 4 doses of emtricitabine/tenofovir disoproxil fumarate; 2 doses given with food 2 to 24 hours before sex (the closer to 24 the better), one dose 24 hours after the first and one 24 hours after the second. This is referred to as 2-1-1 dosing. This option has only been tested in MSM with sexual exposure. It is not recommended at this time for others at risk for HIV or for MSM with chronic or active hepatitis B infection.
Continue to: Preventing HIV infection with post-exposure prophylaxis
Preventing HIV infection with post-exposure prophylaxis
Post-exposure prophylaxis (PEP) for HIV infection is divided into 2 categories: occupational PEP (oPEP) and non-occupational PEP (nPEP). Recommendations for oPEP are described elsewhere3 and are not covered in this Practice Alert. Summarized below are the recommendations for nPEP after sex, injection drug use, and other nonoccupational exposures, which are also described on the Centers for Disease Control and Prevention (CDC) Web site.4
Assess the need for nPEP if high-risk exposure (TABLE 34) occurred ≤72 hours earlier. Before starting nPEP, perform a rapid HIV blood test. If rapid testing is unavailable, start nPEP, which can be discontinued if the patient is later determined to have HIV infection. Repeat HIV testing at 4 to 6 weeks and 3 months following initiation of nPEP. Approved HIV tests are described on the CDC Web site at http://www.cdc.gov/hiv/testing/laboratorytests.html. Oral HIV tests are not recommended for HIV testing before initiating nPEP.

nPEP is not recommended when an individual’s risk of exposure to HIV is not high, or if the exposure occurred more than 72 hours before presentation. An algorithm is available to assist with assessing whether nPEP is recommended (FIGURE4).

Specific nPEP regimens. For otherwise healthy adults and adolescents, preferred nPEP consists of a 28-day course of a 3-drug combination: tenofovir disoproxil fumarate 300 mg once daily; emtricitabine 200 mg once daily; and raltegravir, 400 mg twice daily, or dolutegravir 50 mg once daily. Alternative regimens for adults and adolescents are described in the guideline, as are options for children, those with decreased renal function, and pregnant women. Those who receive more than one course of nPEP within a 12-month period should consider PrEP.
When additional vaccination is needed. For victims of sexual assault, offer prophylaxis against STD (TABLE 44) and hepatitis B virus (HBV). Those who have not been vaccinated against HBV should receive the first dose at the initial visit. If the exposure source is known to be HBsAg-positive, give the unvaccinated patient both hepatitis B vaccine and hepatitis B immune globulin at the first visit. The full hepatitis B vaccine series should then be completed according to the recommended schedule and the vaccine product used. Those who have completed hepatitis B vaccination but who were not tested with a post-vaccine titer should receive a single dose of hepatitis B vaccine.

Continue to: Victims of sexual assault...
Victims of sexual assault can benefit from referral to professionals with expertise in post-assault counseling. Sexual Assault Nurse Examiner programs are listed at http://www.sane-sart.com.
Financial assistance for patients. Anti-retroviral drugs are expensive, and those who need nPEP may not have a payer source. Many pharmaceutical manufacturers offer medication assistance programs, and processes are set up to handle time-sensitive requests. Information for specific medications can be found at http://www.pparx.org/en/prescription_assistance_programs/list_of_participating_programs. Those who are prescribed nPEP after a sexual assault can receive reimbursement for medications and health care costs through state Crime Victim Compensation Programs funded by the Department of Justice. State-specific contact information is available at http://www.nacvcb.org/index.asp?sid=6.
An International Antiviral Society-USA Panel recently published an updated set of recommendations on using antiviral drugs to treat and prevent human immunodeficiency virus (HIV) infection1—a rapidly changing and complex topic. This new guideline updates the society’s 2016 publication.2 It contains recommendations on when to start antiretroviral therapy for those who are HIV positive and advice on suitable combinations of antiretroviral drugs. It also details pre- and post-exposure prophylaxis strategies for preventing HIV infection in those at risk.
This Practice Alert highlights the most important recommendations on treating those newly diagnosed as HIV positive and on preventing infection. Physicians who provide care for those who are HIV positive should familiarize themselves with the entire guideline.
Initiating treatment in those newly diagnosed as HIV positive
The panel now recommends starting antiretroviral therapy (ART) as soon as possible after HIV infection is confirmed; immediately if a patient is ready to commit to starting and continuing treatment. Any patient with an opportunistic infection should begin ART within 2 weeks of its diagnosis. Patients being treated for tuberculosis (TB) should begin ART within 2 weeks of starting TB treatment if their CD4 cell count is <50/mcL; those whose count is ≥50/mcL should begin ART within 2 to 8 weeks.
The panel recommends one of 3 ART combinations (TABLE 11), all of which contain an integrase strand transfer inhibitor (INSTI). ART started immediately should not include a nonnucleoside reverse transcriptase inhibitor (NNRTI) because of possible viral resistance. The guideline recommends 6 other ART combinations if none of the first 3 options can be used.1

An initial set of laboratory tests (TABLE 21) should be conducted on each individual receiving ART, although treatment can start before the results are returned. Ongoing laboratory monitoring, described in detail in the guideline, depends on the ART regimen chosen and the patient’s response to therapy. The only routinely recommended prophylaxis for opportunistic infections is for Pneumocystis pneumonia if the CD4 count is <200/mcL.

Preventing HIV with prEP
Consider prescribing daily pre-exposure prophylaxis (PrEP) with emtricitabine/tenofovir disoproxil fumarate (Truvada) for men and women who are at risk from sexual exposure to HIV or who inject illicit drugs. It takes about 1 week for protective tissue levels to be achieved. Testing to rule out HIV infection is recommended before starting PrEP, as is testing for serum creatinine level, estimated glomerular filtration rate, and hepatitis B surface antigen. Tenofovir disoproxil fumarate is not recommended for those with creatinine clearance of less than 60 mL/min/1.73 m2. For patients taking PrEP, emphasize other preventive measures such as using condoms to protect against both HIV and other sexually-transmitted diseases (STDs), using clean needles and syringes when injecting drugs, or entering a drug rehabilitation program. After initiating PrEP, schedule the first follow-up visit for 30 days later to repeat the HIV test and to assess adverse reactions and PrEP adherence.
For men who have sex with men (MSM), there is an alternative form of PrEP when sexual exposure is infrequent. “On-demand” or “event-driven” PrEP involves 4 doses of emtricitabine/tenofovir disoproxil fumarate; 2 doses given with food 2 to 24 hours before sex (the closer to 24 the better), one dose 24 hours after the first and one 24 hours after the second. This is referred to as 2-1-1 dosing. This option has only been tested in MSM with sexual exposure. It is not recommended at this time for others at risk for HIV or for MSM with chronic or active hepatitis B infection.
Continue to: Preventing HIV infection with post-exposure prophylaxis
Preventing HIV infection with post-exposure prophylaxis
Post-exposure prophylaxis (PEP) for HIV infection is divided into 2 categories: occupational PEP (oPEP) and non-occupational PEP (nPEP). Recommendations for oPEP are described elsewhere3 and are not covered in this Practice Alert. Summarized below are the recommendations for nPEP after sex, injection drug use, and other nonoccupational exposures, which are also described on the Centers for Disease Control and Prevention (CDC) Web site.4
Assess the need for nPEP if high-risk exposure (TABLE 34) occurred ≤72 hours earlier. Before starting nPEP, perform a rapid HIV blood test. If rapid testing is unavailable, start nPEP, which can be discontinued if the patient is later determined to have HIV infection. Repeat HIV testing at 4 to 6 weeks and 3 months following initiation of nPEP. Approved HIV tests are described on the CDC Web site at http://www.cdc.gov/hiv/testing/laboratorytests.html. Oral HIV tests are not recommended for HIV testing before initiating nPEP.

nPEP is not recommended when an individual’s risk of exposure to HIV is not high, or if the exposure occurred more than 72 hours before presentation. An algorithm is available to assist with assessing whether nPEP is recommended (FIGURE4).

Specific nPEP regimens. For otherwise healthy adults and adolescents, preferred nPEP consists of a 28-day course of a 3-drug combination: tenofovir disoproxil fumarate 300 mg once daily; emtricitabine 200 mg once daily; and raltegravir, 400 mg twice daily, or dolutegravir 50 mg once daily. Alternative regimens for adults and adolescents are described in the guideline, as are options for children, those with decreased renal function, and pregnant women. Those who receive more than one course of nPEP within a 12-month period should consider PrEP.
When additional vaccination is needed. For victims of sexual assault, offer prophylaxis against STD (TABLE 44) and hepatitis B virus (HBV). Those who have not been vaccinated against HBV should receive the first dose at the initial visit. If the exposure source is known to be HBsAg-positive, give the unvaccinated patient both hepatitis B vaccine and hepatitis B immune globulin at the first visit. The full hepatitis B vaccine series should then be completed according to the recommended schedule and the vaccine product used. Those who have completed hepatitis B vaccination but who were not tested with a post-vaccine titer should receive a single dose of hepatitis B vaccine.

Continue to: Victims of sexual assault...
Victims of sexual assault can benefit from referral to professionals with expertise in post-assault counseling. Sexual Assault Nurse Examiner programs are listed at http://www.sane-sart.com.
Financial assistance for patients. Anti-retroviral drugs are expensive, and those who need nPEP may not have a payer source. Many pharmaceutical manufacturers offer medication assistance programs, and processes are set up to handle time-sensitive requests. Information for specific medications can be found at http://www.pparx.org/en/prescription_assistance_programs/list_of_participating_programs. Those who are prescribed nPEP after a sexual assault can receive reimbursement for medications and health care costs through state Crime Victim Compensation Programs funded by the Department of Justice. State-specific contact information is available at http://www.nacvcb.org/index.asp?sid=6.
1. Saag MS, Benson CA, Gandhi RT, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2018 recommendations of the International Antiviral Society-USA Panel. JAMA. 2018;320:379-396.
2. Günthard HF, Saag MS, Benson CA, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2016 recommendations of the International Antiviral Society-USA Panel. JAMA. 2016;316:191-210.
3. Kuhar DT, Henderson DK, Struble KA, et al; US Public Health Service Working Group. Updated US Public Health Service guidelines for the management of occupational exposures to human immunodeficiency virus and recommendations for postexposure prophylaxis. Infect Control Hosp Epidemiol. 2013;34:875-892.
4. CDC. Updated guidelines for antiretroviral postexposure prophylaxis after sexual, injection drug use, or other nonoccupational exposure to HIV—United States, 2016. https://www-cdc-gov.ezproxy3.library.arizona.edu/hiv/pdf/programresources/cdc-hiv-npep-guidelines.pdf. Accessed October 11, 2018.
1. Saag MS, Benson CA, Gandhi RT, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2018 recommendations of the International Antiviral Society-USA Panel. JAMA. 2018;320:379-396.
2. Günthard HF, Saag MS, Benson CA, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2016 recommendations of the International Antiviral Society-USA Panel. JAMA. 2016;316:191-210.
3. Kuhar DT, Henderson DK, Struble KA, et al; US Public Health Service Working Group. Updated US Public Health Service guidelines for the management of occupational exposures to human immunodeficiency virus and recommendations for postexposure prophylaxis. Infect Control Hosp Epidemiol. 2013;34:875-892.
4. CDC. Updated guidelines for antiretroviral postexposure prophylaxis after sexual, injection drug use, or other nonoccupational exposure to HIV—United States, 2016. https://www-cdc-gov.ezproxy3.library.arizona.edu/hiv/pdf/programresources/cdc-hiv-npep-guidelines.pdf. Accessed October 11, 2018.
CDC recommendations for the 2018-2019 influenza season
The 2017-2018 influenza season was one of the most severe in this century, according to every indicator measured by the Centers for Disease Control and Prevention (CDC). The proportion of outpatient visits due to influenza-like illness (ILI) was elevated nationally above a baseline of 2.2% for 19 straight weeks, and for 3 weeks it was over 7%.1 High ILI activity was widespread and included all 50 states in January.
From October 2017 through April 2018, the CDC estimates that the influenza-related hospitalization rate was 106.6 per 100,000 population, with the highest rates among children 0 to 4 years (74.3/100,000), adults 50 to 64 years (115.7/100,000), and adults 65 years and older (460.9/100,000). More than 90% of adults hospitalized had a chronic condition, such as heart or lung disease, diabetes, or obesity, placing them at high risk for influenza complications.1
Influenza severity is also measured as the proportion of deaths due to pneumonia and influenza, which was above the epidemic threshold for 16 weeks in 2017-2018 and was above 10% for 4 weeks in January.1 Based on all of these indicators, the 2017-2018 influenza season was classified as high severity overall and for all age groups, the first time this has happened since the 2003-2004 season. There were 171 pediatric deaths attributed to influenza, and more than three-quarters of vaccine-eligible children who died from influenza this season had not received influenza vaccine.1
The type of influenza predominating last season was influenza A from early- through mid-season, and was influenza B later in the season (see https://stacks.cdc.gov/view/cdc/54974).1 For the entire season, 71.2% of specimens that tested positive for influenza in public health labs were Influenza A and 84.9% of these were H3N2.1
Effectiveness of influenza vaccine last season. As measured by preventing respiratory illness needing medical attention, vaccine effectiveness was 36% overall: 25% against influenza A (H3N2), 67% against influenza A (H1N1), and 42% against influenza B.1 Effectiveness varied by age, being the highest in those 8 years and younger.2 Effectiveness was questionable in those older than 65, with an estimated effectiveness of 23% but confidence intervals including 0.2
While the effectiveness of influenza vaccines remains suboptimal, the morbidity and mortality they prevent is still considerable. The CDC estimates that in 2016-2017, more than 5 million influenza illnesses, 2.6 million medical visits, and 84,700 hospitalizations were prevented.3 And effectiveness last season was similar to, or better than, what has been seen in each of the past 10 years (FIGURE).4

Three drugs were recommended for use to treat influenza in 2017-1018 (oseltamivir, peramivir, and zanamivir), and no resistance was found except in 1% of influenza A (H1N1) tested.1 No resistance was found in other A or any B viruses tested.1
Continue to: Safety
Safety
The safety of influenza vaccines is studied each year by both the CDC and US Food and Drug Administration (FDA). This past year, studies were conducted using the CDC-supported Safety Datalink System, looking for increased rates of acute disseminated encephalomyelitis, anaphylaxis, Bell’s palsy, encephalitis, Guillain-Barré syndrome (GBS), seizures, and transverse myelitis.5 No safety signals were detected. However, for some of the newer vaccines, the numbers of vaccinated individuals studied were small. The FDA studied the incidence of GBS using Medicare data and found no increased rates in those vaccinated.5
2018-2019 Recommendations
There are only a few changes to the recommendations for the upcoming influenza season. The Advisory Committee on Immunization Practices (ACIP) still recommends universal vaccination for anyone age 6 months and older who does not have a contraindication (TABLE 16). Two of the antigens in the vaccines for this coming season are slightly different from last season (TABLE 27).
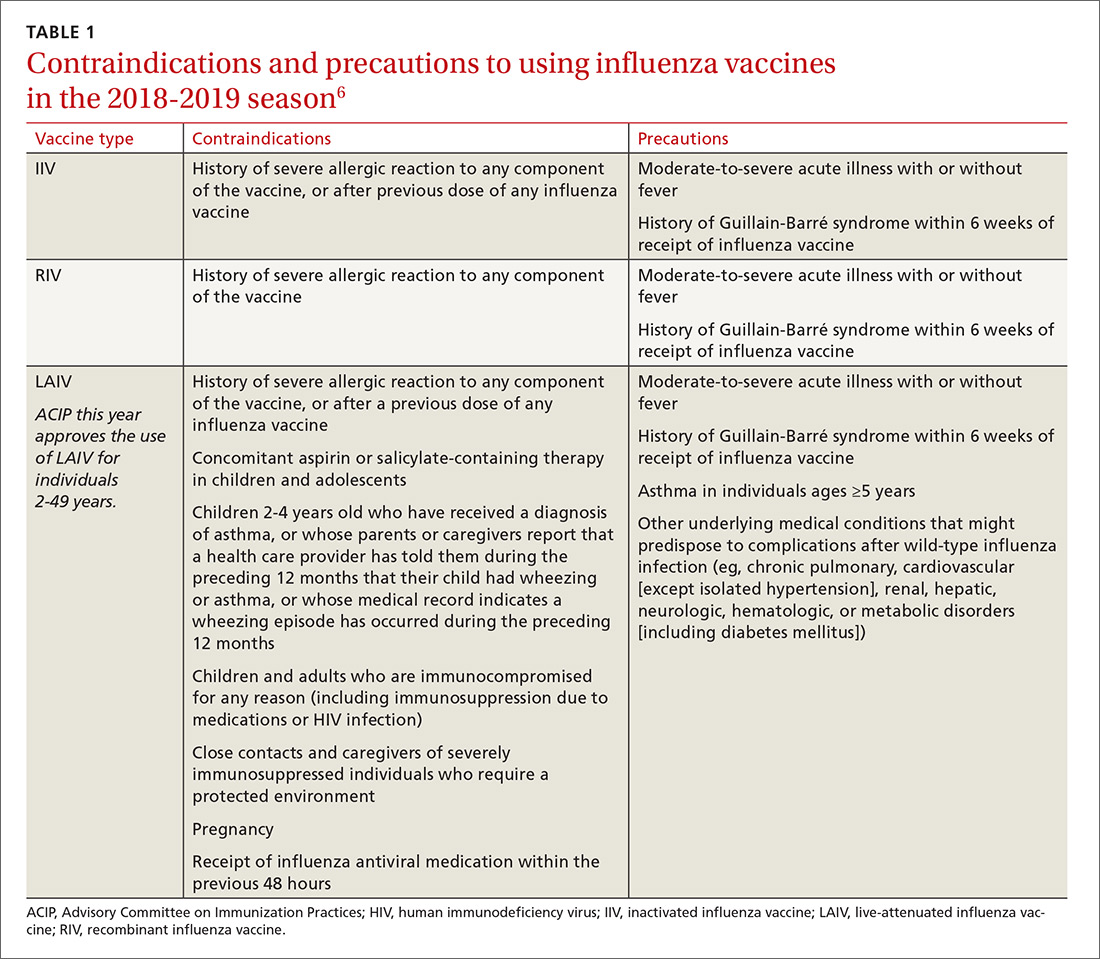
After 2 years of recommending against the use of live attenuated influenza vaccine (LAIV) because of its low effectiveness in children against influenza A (H1N1), ACIP now includes it as an option for the upcoming season in individuals ages 2 through 49 years.8 The basis of this revised recommendation was 2-fold: 1) evidence of LAIV effectiveness comparable to that of inactivated products against A (H3N2) and B viruses; and 2) evidence that a new strain of A (H1N1) now used to produce the vaccine (A/Slovenia) produces a significantly higher antibody response than the strain (A/Bolivia) used in the years when the vaccine was not effective against A (H1N1).

However, the new formulation’s clinical effectiveness against A (H1N1) has not been demonstrated, leading the American Academy of Pediatrics to recommend that LAIV should be used in children only if other options are not available or if injectable vaccine is refused.9 Contraindications to the use of LAIV remain the same as the previous version of the vaccine (TABLE 16).
Individuals with non-severe egg allergies can receive any licensed, recommended age-appropriate influenza vaccine and no longer have to be monitored for 30 minutes after receiving the vaccine. People who have severe egg allergies should be vaccinated with an egg-free product or in a medical setting and be supervised by a health care provider who is able to recognize and manage severe allergic conditions.
Continue to: Children 6 months through 8 years...
Children 6 months through 8 years who have previously received an influenza vaccine, either trivalent or quadrivalent, need only 1 dose; those who have not received vaccination need 2 doses separated by at least 4 weeks.
Available vaccine products
A table found on the CDC influenza Web site lists the vaccine products available in the United States and the ages for which they are approved.6 The options now include 2 standard-dose trivalent inactivated influenza vaccines (IIV3), 4 standard-dose quadrivalent inactivated influenza vaccines (IIV4), one cell culture-based IIV4 (ccIIV4), one standard dose IIV4 intradermal option, a trivalent and a quadrivalent recombinant influenza vaccine (RIV3, RIV4), one LAIV, and 2 products for those 65 years and older—an adjuvanted IIV3 (aIIV3) and a high dose IIV3. Three of these products do not depend on egg-based technology: RIV3, RIV4, and ccIIV4.
Comparative effectiveness studies of these vaccine options, including those available for the elderly, are being conducted. Studies presented at the June 2018 ACIP meeting show comparable effectiveness of egg-based and non–egg-based products.6 At this time, ACIP does not make a preferential recommendation for any influenza vaccine product for any age group.
1. Garten R, Blanton L, Elal AIA, eta al. Update: Influenza activity in the United States during the 2017-18 season and composition of the 2018-2019 influenza vaccine. MMWR Morb Mortal Wkly Rep. 2018;67;634-642.
2. Flannery B, Chung JR, Belongia EA, et al. Interim estimates of 2017-18 seasonal influenza vaccine effectiveness – United States, February 2018. MMWR Morb Mortal Wkly Rep. 2018;67:180-185.
3. Flannery B, Chung J, Ferdinands J. Preliminary estimates of 2017-2018 seasonal influenza vaccine effectiveness against laboratory-confirmed influenza from the US Flu VE and HAIVEN network. Meeting of the Advisory Committee on Immunization Practices; June 20, 2018; Atlanta, Ga. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2018-06/flu-02-Flannery-508.pdf. Accessed August 11, 2018.
4. CDC. Seasonal influenza vaccine effectiveness, 2005-2018. Available at: https://www.cdc.gov/flu/professionals/vaccination/effectiveness-studies.htm. Accessed July 27, 2018.
5. Shimabukuro T. End-of-season update: 2017-2018 influenza vaccine safety monitoring. Presented at: meeting of the Advisory Committee on Immunization Practices; June 20, 2018; Atlanta, Ga. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2018-06/flu-04-Shimabukuro-508.pdf. Accessed August 11, 2018.
6. CDC. Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices—United States, 2018–19 Influenza Season. Available at: https://www.cdc.gov/mmwr/volumes/67/rr/rr6703a1.htm?s_cid=rr6703a1_w. Accessed August 23, 2018.
7. CDC. Update: Influenza activity in the United States during the 2017-18 season and composition of the 2018-19 influenza vaccine. Available at: https://www.cdc.gov/mmwr/volumes/67/wr/mm6722a4.htm. Accessed July 27, 2018.
8. Grohskopf LA, Sokolow LZ, Fry AM, et al. Update: ACIP recommendations for the use of quadrivalent live attenuated influenza vaccine (LAIV4) — United States, 2018–19 influenza season. MMWR Morb Mortal Wkly Rep. 2018;67:643-645.
9. Jenco M. AAP: Give children IIV flu shot; use LAIV as last resort. Available at: http://www.aappublications.org/news/2018/05/21/fluvaccine051818. Accessed August 1, 2018.
The 2017-2018 influenza season was one of the most severe in this century, according to every indicator measured by the Centers for Disease Control and Prevention (CDC). The proportion of outpatient visits due to influenza-like illness (ILI) was elevated nationally above a baseline of 2.2% for 19 straight weeks, and for 3 weeks it was over 7%.1 High ILI activity was widespread and included all 50 states in January.
From October 2017 through April 2018, the CDC estimates that the influenza-related hospitalization rate was 106.6 per 100,000 population, with the highest rates among children 0 to 4 years (74.3/100,000), adults 50 to 64 years (115.7/100,000), and adults 65 years and older (460.9/100,000). More than 90% of adults hospitalized had a chronic condition, such as heart or lung disease, diabetes, or obesity, placing them at high risk for influenza complications.1
Influenza severity is also measured as the proportion of deaths due to pneumonia and influenza, which was above the epidemic threshold for 16 weeks in 2017-2018 and was above 10% for 4 weeks in January.1 Based on all of these indicators, the 2017-2018 influenza season was classified as high severity overall and for all age groups, the first time this has happened since the 2003-2004 season. There were 171 pediatric deaths attributed to influenza, and more than three-quarters of vaccine-eligible children who died from influenza this season had not received influenza vaccine.1
The type of influenza predominating last season was influenza A from early- through mid-season, and was influenza B later in the season (see https://stacks.cdc.gov/view/cdc/54974).1 For the entire season, 71.2% of specimens that tested positive for influenza in public health labs were Influenza A and 84.9% of these were H3N2.1
Effectiveness of influenza vaccine last season. As measured by preventing respiratory illness needing medical attention, vaccine effectiveness was 36% overall: 25% against influenza A (H3N2), 67% against influenza A (H1N1), and 42% against influenza B.1 Effectiveness varied by age, being the highest in those 8 years and younger.2 Effectiveness was questionable in those older than 65, with an estimated effectiveness of 23% but confidence intervals including 0.2
While the effectiveness of influenza vaccines remains suboptimal, the morbidity and mortality they prevent is still considerable. The CDC estimates that in 2016-2017, more than 5 million influenza illnesses, 2.6 million medical visits, and 84,700 hospitalizations were prevented.3 And effectiveness last season was similar to, or better than, what has been seen in each of the past 10 years (FIGURE).4

Three drugs were recommended for use to treat influenza in 2017-1018 (oseltamivir, peramivir, and zanamivir), and no resistance was found except in 1% of influenza A (H1N1) tested.1 No resistance was found in other A or any B viruses tested.1
Continue to: Safety
Safety
The safety of influenza vaccines is studied each year by both the CDC and US Food and Drug Administration (FDA). This past year, studies were conducted using the CDC-supported Safety Datalink System, looking for increased rates of acute disseminated encephalomyelitis, anaphylaxis, Bell’s palsy, encephalitis, Guillain-Barré syndrome (GBS), seizures, and transverse myelitis.5 No safety signals were detected. However, for some of the newer vaccines, the numbers of vaccinated individuals studied were small. The FDA studied the incidence of GBS using Medicare data and found no increased rates in those vaccinated.5
2018-2019 Recommendations
There are only a few changes to the recommendations for the upcoming influenza season. The Advisory Committee on Immunization Practices (ACIP) still recommends universal vaccination for anyone age 6 months and older who does not have a contraindication (TABLE 16). Two of the antigens in the vaccines for this coming season are slightly different from last season (TABLE 27).

After 2 years of recommending against the use of live attenuated influenza vaccine (LAIV) because of its low effectiveness in children against influenza A (H1N1), ACIP now includes it as an option for the upcoming season in individuals ages 2 through 49 years.8 The basis of this revised recommendation was 2-fold: 1) evidence of LAIV effectiveness comparable to that of inactivated products against A (H3N2) and B viruses; and 2) evidence that a new strain of A (H1N1) now used to produce the vaccine (A/Slovenia) produces a significantly higher antibody response than the strain (A/Bolivia) used in the years when the vaccine was not effective against A (H1N1).

However, the new formulation’s clinical effectiveness against A (H1N1) has not been demonstrated, leading the American Academy of Pediatrics to recommend that LAIV should be used in children only if other options are not available or if injectable vaccine is refused.9 Contraindications to the use of LAIV remain the same as the previous version of the vaccine (TABLE 16).
Individuals with non-severe egg allergies can receive any licensed, recommended age-appropriate influenza vaccine and no longer have to be monitored for 30 minutes after receiving the vaccine. People who have severe egg allergies should be vaccinated with an egg-free product or in a medical setting and be supervised by a health care provider who is able to recognize and manage severe allergic conditions.
Continue to: Children 6 months through 8 years...
Children 6 months through 8 years who have previously received an influenza vaccine, either trivalent or quadrivalent, need only 1 dose; those who have not received vaccination need 2 doses separated by at least 4 weeks.
Available vaccine products
A table found on the CDC influenza Web site lists the vaccine products available in the United States and the ages for which they are approved.6 The options now include 2 standard-dose trivalent inactivated influenza vaccines (IIV3), 4 standard-dose quadrivalent inactivated influenza vaccines (IIV4), one cell culture-based IIV4 (ccIIV4), one standard dose IIV4 intradermal option, a trivalent and a quadrivalent recombinant influenza vaccine (RIV3, RIV4), one LAIV, and 2 products for those 65 years and older—an adjuvanted IIV3 (aIIV3) and a high dose IIV3. Three of these products do not depend on egg-based technology: RIV3, RIV4, and ccIIV4.
Comparative effectiveness studies of these vaccine options, including those available for the elderly, are being conducted. Studies presented at the June 2018 ACIP meeting show comparable effectiveness of egg-based and non–egg-based products.6 At this time, ACIP does not make a preferential recommendation for any influenza vaccine product for any age group.
The 2017-2018 influenza season was one of the most severe in this century, according to every indicator measured by the Centers for Disease Control and Prevention (CDC). The proportion of outpatient visits due to influenza-like illness (ILI) was elevated nationally above a baseline of 2.2% for 19 straight weeks, and for 3 weeks it was over 7%.1 High ILI activity was widespread and included all 50 states in January.
From October 2017 through April 2018, the CDC estimates that the influenza-related hospitalization rate was 106.6 per 100,000 population, with the highest rates among children 0 to 4 years (74.3/100,000), adults 50 to 64 years (115.7/100,000), and adults 65 years and older (460.9/100,000). More than 90% of adults hospitalized had a chronic condition, such as heart or lung disease, diabetes, or obesity, placing them at high risk for influenza complications.1
Influenza severity is also measured as the proportion of deaths due to pneumonia and influenza, which was above the epidemic threshold for 16 weeks in 2017-2018 and was above 10% for 4 weeks in January.1 Based on all of these indicators, the 2017-2018 influenza season was classified as high severity overall and for all age groups, the first time this has happened since the 2003-2004 season. There were 171 pediatric deaths attributed to influenza, and more than three-quarters of vaccine-eligible children who died from influenza this season had not received influenza vaccine.1
The type of influenza predominating last season was influenza A from early- through mid-season, and was influenza B later in the season (see https://stacks.cdc.gov/view/cdc/54974).1 For the entire season, 71.2% of specimens that tested positive for influenza in public health labs were Influenza A and 84.9% of these were H3N2.1
Effectiveness of influenza vaccine last season. As measured by preventing respiratory illness needing medical attention, vaccine effectiveness was 36% overall: 25% against influenza A (H3N2), 67% against influenza A (H1N1), and 42% against influenza B.1 Effectiveness varied by age, being the highest in those 8 years and younger.2 Effectiveness was questionable in those older than 65, with an estimated effectiveness of 23% but confidence intervals including 0.2
While the effectiveness of influenza vaccines remains suboptimal, the morbidity and mortality they prevent is still considerable. The CDC estimates that in 2016-2017, more than 5 million influenza illnesses, 2.6 million medical visits, and 84,700 hospitalizations were prevented.3 And effectiveness last season was similar to, or better than, what has been seen in each of the past 10 years (FIGURE).4

Three drugs were recommended for use to treat influenza in 2017-1018 (oseltamivir, peramivir, and zanamivir), and no resistance was found except in 1% of influenza A (H1N1) tested.1 No resistance was found in other A or any B viruses tested.1
Continue to: Safety
Safety
The safety of influenza vaccines is studied each year by both the CDC and US Food and Drug Administration (FDA). This past year, studies were conducted using the CDC-supported Safety Datalink System, looking for increased rates of acute disseminated encephalomyelitis, anaphylaxis, Bell’s palsy, encephalitis, Guillain-Barré syndrome (GBS), seizures, and transverse myelitis.5 No safety signals were detected. However, for some of the newer vaccines, the numbers of vaccinated individuals studied were small. The FDA studied the incidence of GBS using Medicare data and found no increased rates in those vaccinated.5
2018-2019 Recommendations
There are only a few changes to the recommendations for the upcoming influenza season. The Advisory Committee on Immunization Practices (ACIP) still recommends universal vaccination for anyone age 6 months and older who does not have a contraindication (TABLE 16). Two of the antigens in the vaccines for this coming season are slightly different from last season (TABLE 27).

After 2 years of recommending against the use of live attenuated influenza vaccine (LAIV) because of its low effectiveness in children against influenza A (H1N1), ACIP now includes it as an option for the upcoming season in individuals ages 2 through 49 years.8 The basis of this revised recommendation was 2-fold: 1) evidence of LAIV effectiveness comparable to that of inactivated products against A (H3N2) and B viruses; and 2) evidence that a new strain of A (H1N1) now used to produce the vaccine (A/Slovenia) produces a significantly higher antibody response than the strain (A/Bolivia) used in the years when the vaccine was not effective against A (H1N1).

However, the new formulation’s clinical effectiveness against A (H1N1) has not been demonstrated, leading the American Academy of Pediatrics to recommend that LAIV should be used in children only if other options are not available or if injectable vaccine is refused.9 Contraindications to the use of LAIV remain the same as the previous version of the vaccine (TABLE 16).
Individuals with non-severe egg allergies can receive any licensed, recommended age-appropriate influenza vaccine and no longer have to be monitored for 30 minutes after receiving the vaccine. People who have severe egg allergies should be vaccinated with an egg-free product or in a medical setting and be supervised by a health care provider who is able to recognize and manage severe allergic conditions.
Continue to: Children 6 months through 8 years...
Children 6 months through 8 years who have previously received an influenza vaccine, either trivalent or quadrivalent, need only 1 dose; those who have not received vaccination need 2 doses separated by at least 4 weeks.
Available vaccine products
A table found on the CDC influenza Web site lists the vaccine products available in the United States and the ages for which they are approved.6 The options now include 2 standard-dose trivalent inactivated influenza vaccines (IIV3), 4 standard-dose quadrivalent inactivated influenza vaccines (IIV4), one cell culture-based IIV4 (ccIIV4), one standard dose IIV4 intradermal option, a trivalent and a quadrivalent recombinant influenza vaccine (RIV3, RIV4), one LAIV, and 2 products for those 65 years and older—an adjuvanted IIV3 (aIIV3) and a high dose IIV3. Three of these products do not depend on egg-based technology: RIV3, RIV4, and ccIIV4.
Comparative effectiveness studies of these vaccine options, including those available for the elderly, are being conducted. Studies presented at the June 2018 ACIP meeting show comparable effectiveness of egg-based and non–egg-based products.6 At this time, ACIP does not make a preferential recommendation for any influenza vaccine product for any age group.
1. Garten R, Blanton L, Elal AIA, eta al. Update: Influenza activity in the United States during the 2017-18 season and composition of the 2018-2019 influenza vaccine. MMWR Morb Mortal Wkly Rep. 2018;67;634-642.
2. Flannery B, Chung JR, Belongia EA, et al. Interim estimates of 2017-18 seasonal influenza vaccine effectiveness – United States, February 2018. MMWR Morb Mortal Wkly Rep. 2018;67:180-185.
3. Flannery B, Chung J, Ferdinands J. Preliminary estimates of 2017-2018 seasonal influenza vaccine effectiveness against laboratory-confirmed influenza from the US Flu VE and HAIVEN network. Meeting of the Advisory Committee on Immunization Practices; June 20, 2018; Atlanta, Ga. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2018-06/flu-02-Flannery-508.pdf. Accessed August 11, 2018.
4. CDC. Seasonal influenza vaccine effectiveness, 2005-2018. Available at: https://www.cdc.gov/flu/professionals/vaccination/effectiveness-studies.htm. Accessed July 27, 2018.
5. Shimabukuro T. End-of-season update: 2017-2018 influenza vaccine safety monitoring. Presented at: meeting of the Advisory Committee on Immunization Practices; June 20, 2018; Atlanta, Ga. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2018-06/flu-04-Shimabukuro-508.pdf. Accessed August 11, 2018.
6. CDC. Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices—United States, 2018–19 Influenza Season. Available at: https://www.cdc.gov/mmwr/volumes/67/rr/rr6703a1.htm?s_cid=rr6703a1_w. Accessed August 23, 2018.
7. CDC. Update: Influenza activity in the United States during the 2017-18 season and composition of the 2018-19 influenza vaccine. Available at: https://www.cdc.gov/mmwr/volumes/67/wr/mm6722a4.htm. Accessed July 27, 2018.
8. Grohskopf LA, Sokolow LZ, Fry AM, et al. Update: ACIP recommendations for the use of quadrivalent live attenuated influenza vaccine (LAIV4) — United States, 2018–19 influenza season. MMWR Morb Mortal Wkly Rep. 2018;67:643-645.
9. Jenco M. AAP: Give children IIV flu shot; use LAIV as last resort. Available at: http://www.aappublications.org/news/2018/05/21/fluvaccine051818. Accessed August 1, 2018.
1. Garten R, Blanton L, Elal AIA, eta al. Update: Influenza activity in the United States during the 2017-18 season and composition of the 2018-2019 influenza vaccine. MMWR Morb Mortal Wkly Rep. 2018;67;634-642.
2. Flannery B, Chung JR, Belongia EA, et al. Interim estimates of 2017-18 seasonal influenza vaccine effectiveness – United States, February 2018. MMWR Morb Mortal Wkly Rep. 2018;67:180-185.
3. Flannery B, Chung J, Ferdinands J. Preliminary estimates of 2017-2018 seasonal influenza vaccine effectiveness against laboratory-confirmed influenza from the US Flu VE and HAIVEN network. Meeting of the Advisory Committee on Immunization Practices; June 20, 2018; Atlanta, Ga. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2018-06/flu-02-Flannery-508.pdf. Accessed August 11, 2018.
4. CDC. Seasonal influenza vaccine effectiveness, 2005-2018. Available at: https://www.cdc.gov/flu/professionals/vaccination/effectiveness-studies.htm. Accessed July 27, 2018.
5. Shimabukuro T. End-of-season update: 2017-2018 influenza vaccine safety monitoring. Presented at: meeting of the Advisory Committee on Immunization Practices; June 20, 2018; Atlanta, Ga. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2018-06/flu-04-Shimabukuro-508.pdf. Accessed August 11, 2018.
6. CDC. Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices—United States, 2018–19 Influenza Season. Available at: https://www.cdc.gov/mmwr/volumes/67/rr/rr6703a1.htm?s_cid=rr6703a1_w. Accessed August 23, 2018.
7. CDC. Update: Influenza activity in the United States during the 2017-18 season and composition of the 2018-19 influenza vaccine. Available at: https://www.cdc.gov/mmwr/volumes/67/wr/mm6722a4.htm. Accessed July 27, 2018.
8. Grohskopf LA, Sokolow LZ, Fry AM, et al. Update: ACIP recommendations for the use of quadrivalent live attenuated influenza vaccine (LAIV4) — United States, 2018–19 influenza season. MMWR Morb Mortal Wkly Rep. 2018;67:643-645.
9. Jenco M. AAP: Give children IIV flu shot; use LAIV as last resort. Available at: http://www.aappublications.org/news/2018/05/21/fluvaccine051818. Accessed August 1, 2018.
Dietary recommendations for patients with diabetes
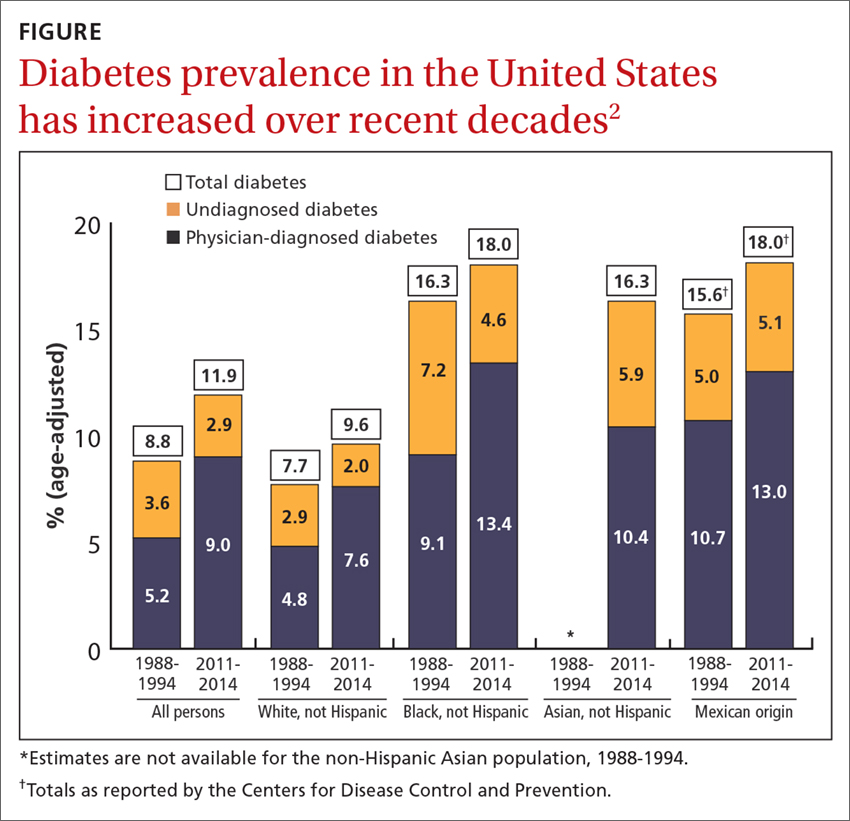
Diabetes affects approximately 9.4% of the US population (more than 30 million people),1 and it is one of the most common conditions treated by family physicians. Additionally, more than 80 million Americans meet the criteria for prediabetes.1 The prevalence of diabetes has increased in adults between the time periods 1988-1994 and 2011-2014, and it varies by race and ethnicity, with the highest prevalence, 18%, among African Americans and Mexican Americans, and the lowest, 9.6%, among non-Hispanic whites (FIGURE).2
Diet is the cornerstone of diabetes treatment
The foundation of a comprehensive management plan for type 2 diabetes mellitus (T2DM) is an appropriate diet. A growing body of evidence shows that a well-structured diet is important in controlling diabetes, delaying or preventing the onset of diabetes, and, in some instances, contributing to its remission. Diabetes UK, the United Kingdom’s equivalent of the American Diabetes Association (ADA), recently updated its clinical guideline for physicians and patients on the role of nutrition in managing and preventing diabetes, and it is consistent with one published by the ADA in 2013.3,4
The Diabetes UK guideline is the result of an evidence-based process that meets the standards recommended by the National Academy of Medicine (previously the Institute of Medicine): a systematic review and formal assessment of the quality of the evidence, and recommendations based on the highest quality evidence available, with the level of evidence stated for each recommendation.5 Assessing the level of evidence and determining the strengths of recommendations were done using the Grades of Recommendation Assessment, Development, and Evaluation (GRADE) system, which uses an approach similar to that of the Strength of Recommendation Taxonomy (SORT).
What, and what not, to focus on. The first set of recommendations states that everyone with, or at risk for, diabetes should receive structured, personalized, and ongoing nutritional advice from a dietician who is coordinated with their clinical care. Nutritional advice should focus on the quality and quantity of food, not on specific nutrients (fat and carbohydrates), since there is no good evidence on what proportion of such nutrients is optimal. And it should be tailored to the culture and eating preferences of the patient.
The type of diet with the strongest evidence base for preventing T2DM is a Mediterranean diet, which is supported by level-4, high-quality evidence. Important aspects of a Mediterranean diet are the regular consumption of nuts, whole grains, fruits, and vegetables; use of olive oil instead of butter; and favoring fish over red meat.6 Other dietary patterns associated with reduced risk but supported only by level-2, low-quality evidence, include Dietary Approaches to Stop Hypertension (DASH), vegetarian, vegan, and Nordic healthy diets. Moderate carbohydrate restriction is supported only by level-1, very low-quality evidence.
The UK guideline, too, recommends preferentially eating whole grains, fruits, and green leafy vegetables, as well as yogurt, cheese, tea, and coffee. And it advises reducing consumption of red processed meats, potatoes (especially French fries), sugar-sweetened beverages, and refined carbohydrates. However, these specific food preferences are supported only by low-level evidence.
Plant stanols and plant sterols are found in a variety of plant foods such as cereals, vegetable oils, seeds, and nuts, and are now being added to some food products. (For more on plant stanols and plant sterols.) They have a chemical structure similar to cholesterol and reduce the intestinal absorption of cholesterol, thereby lowering total serum cholesterol and LDL-cholesterol. Both Diabetes UK and the ADA recommend 2 to 3 grams of stanols/sterols per day.
Continue to: Alcohol intake
Alcohol intake. And what about alcohol intake in those with T2DM? Once again, both guidelines are in concert by stating that alcohol use in those with diabetes should be moderate, defined by the ADA as one or fewer drinks/d for women and 2 or fewer for men.
Weight loss and exercise are important, too. Those who are overweight or obese with T2DM can improve glycemic control with a 5% weight loss achieved by reducing caloric intake and by increasing energy expenditure with 150 minutes of moderate physical activity per week over at least 3 days.3 This recommendation is supported by high-quality evidence.
A 15-kg weight loss is recommended for those attempting diabetes remission (supported by moderate-level evidence).3 One small study in the United Kingdom found that more than half of those with T2DM could achieve remission with weight loss of 10 kg or more; 86% with weight loss of 15 kg or more.7 The Diabetes UK guideline panel rated this as having moderate-level evidence.
The bottom line. Diet and exercise are key interventions for the prevention and treatment of diabetes and can lead to remission if sufficient weight loss is achieved. To achieve and maintain an optimal diet, patients need individualized professional advice and followup. The evidence base for nutritional advice is growing and can be used to improve the quality of these patient-provider interactions.
1. America Diabetes Association. Statistics About Diabetes. http://www.diabetes.org/diabetes-basics/statistics/. Accessed May 13, 2018.
2. CDC. National Center for Health Statistics. Health, United States, 2016. Available at: https://www.cdc.gov/nchs/data/hus/hus16.pdf. Accessed May 21, 2018.
3. Dyson PA, Twenefour D, Breen C, et al. Diabetes UK evidence-based nutrition guidelines for the prevention and management of diabetes. Diabet Med. 2018;35:541-547.
4. Evert AB, Boucher JL, Cypress M, et al. Nutrition therapy recommendations for the management of adults with diabetes. Diabetes Care. 2013;36:3821-3842.
5. IOM (Institute of Medicine). 2011. Clinical Practice Guidelines We Can Trust. Washington, DC: The National Academies Press.
6. Romagnolo DF, Selmin OI. Mediterranean diet and prevention of chronic diseases. Nutr Today. 2017;52:208-222.
7. Lean ME, Leslie WS, Barnes AC, et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trial. Lancet. 2018;391:541-551.
Diabetes affects approximately 9.4% of the US population (more than 30 million people),1 and it is one of the most common conditions treated by family physicians. Additionally, more than 80 million Americans meet the criteria for prediabetes.1 The prevalence of diabetes has increased in adults between the time periods 1988-1994 and 2011-2014, and it varies by race and ethnicity, with the highest prevalence, 18%, among African Americans and Mexican Americans, and the lowest, 9.6%, among non-Hispanic whites (FIGURE).2

Diet is the cornerstone of diabetes treatment
The foundation of a comprehensive management plan for type 2 diabetes mellitus (T2DM) is an appropriate diet. A growing body of evidence shows that a well-structured diet is important in controlling diabetes, delaying or preventing the onset of diabetes, and, in some instances, contributing to its remission. Diabetes UK, the United Kingdom’s equivalent of the American Diabetes Association (ADA), recently updated its clinical guideline for physicians and patients on the role of nutrition in managing and preventing diabetes, and it is consistent with one published by the ADA in 2013.3,4
The Diabetes UK guideline is the result of an evidence-based process that meets the standards recommended by the National Academy of Medicine (previously the Institute of Medicine): a systematic review and formal assessment of the quality of the evidence, and recommendations based on the highest quality evidence available, with the level of evidence stated for each recommendation.5 Assessing the level of evidence and determining the strengths of recommendations were done using the Grades of Recommendation Assessment, Development, and Evaluation (GRADE) system, which uses an approach similar to that of the Strength of Recommendation Taxonomy (SORT).
What, and what not, to focus on. The first set of recommendations states that everyone with, or at risk for, diabetes should receive structured, personalized, and ongoing nutritional advice from a dietician who is coordinated with their clinical care. Nutritional advice should focus on the quality and quantity of food, not on specific nutrients (fat and carbohydrates), since there is no good evidence on what proportion of such nutrients is optimal. And it should be tailored to the culture and eating preferences of the patient.
The type of diet with the strongest evidence base for preventing T2DM is a Mediterranean diet, which is supported by level-4, high-quality evidence. Important aspects of a Mediterranean diet are the regular consumption of nuts, whole grains, fruits, and vegetables; use of olive oil instead of butter; and favoring fish over red meat.6 Other dietary patterns associated with reduced risk but supported only by level-2, low-quality evidence, include Dietary Approaches to Stop Hypertension (DASH), vegetarian, vegan, and Nordic healthy diets. Moderate carbohydrate restriction is supported only by level-1, very low-quality evidence.
The UK guideline, too, recommends preferentially eating whole grains, fruits, and green leafy vegetables, as well as yogurt, cheese, tea, and coffee. And it advises reducing consumption of red processed meats, potatoes (especially French fries), sugar-sweetened beverages, and refined carbohydrates. However, these specific food preferences are supported only by low-level evidence.
Plant stanols and plant sterols are found in a variety of plant foods such as cereals, vegetable oils, seeds, and nuts, and are now being added to some food products. (For more on plant stanols and plant sterols.) They have a chemical structure similar to cholesterol and reduce the intestinal absorption of cholesterol, thereby lowering total serum cholesterol and LDL-cholesterol. Both Diabetes UK and the ADA recommend 2 to 3 grams of stanols/sterols per day.
Continue to: Alcohol intake
Alcohol intake. And what about alcohol intake in those with T2DM? Once again, both guidelines are in concert by stating that alcohol use in those with diabetes should be moderate, defined by the ADA as one or fewer drinks/d for women and 2 or fewer for men.
Weight loss and exercise are important, too. Those who are overweight or obese with T2DM can improve glycemic control with a 5% weight loss achieved by reducing caloric intake and by increasing energy expenditure with 150 minutes of moderate physical activity per week over at least 3 days.3 This recommendation is supported by high-quality evidence.
A 15-kg weight loss is recommended for those attempting diabetes remission (supported by moderate-level evidence).3 One small study in the United Kingdom found that more than half of those with T2DM could achieve remission with weight loss of 10 kg or more; 86% with weight loss of 15 kg or more.7 The Diabetes UK guideline panel rated this as having moderate-level evidence.
The bottom line. Diet and exercise are key interventions for the prevention and treatment of diabetes and can lead to remission if sufficient weight loss is achieved. To achieve and maintain an optimal diet, patients need individualized professional advice and followup. The evidence base for nutritional advice is growing and can be used to improve the quality of these patient-provider interactions.
Diabetes affects approximately 9.4% of the US population (more than 30 million people),1 and it is one of the most common conditions treated by family physicians. Additionally, more than 80 million Americans meet the criteria for prediabetes.1 The prevalence of diabetes has increased in adults between the time periods 1988-1994 and 2011-2014, and it varies by race and ethnicity, with the highest prevalence, 18%, among African Americans and Mexican Americans, and the lowest, 9.6%, among non-Hispanic whites (FIGURE).2

Diet is the cornerstone of diabetes treatment
The foundation of a comprehensive management plan for type 2 diabetes mellitus (T2DM) is an appropriate diet. A growing body of evidence shows that a well-structured diet is important in controlling diabetes, delaying or preventing the onset of diabetes, and, in some instances, contributing to its remission. Diabetes UK, the United Kingdom’s equivalent of the American Diabetes Association (ADA), recently updated its clinical guideline for physicians and patients on the role of nutrition in managing and preventing diabetes, and it is consistent with one published by the ADA in 2013.3,4
The Diabetes UK guideline is the result of an evidence-based process that meets the standards recommended by the National Academy of Medicine (previously the Institute of Medicine): a systematic review and formal assessment of the quality of the evidence, and recommendations based on the highest quality evidence available, with the level of evidence stated for each recommendation.5 Assessing the level of evidence and determining the strengths of recommendations were done using the Grades of Recommendation Assessment, Development, and Evaluation (GRADE) system, which uses an approach similar to that of the Strength of Recommendation Taxonomy (SORT).
What, and what not, to focus on. The first set of recommendations states that everyone with, or at risk for, diabetes should receive structured, personalized, and ongoing nutritional advice from a dietician who is coordinated with their clinical care. Nutritional advice should focus on the quality and quantity of food, not on specific nutrients (fat and carbohydrates), since there is no good evidence on what proportion of such nutrients is optimal. And it should be tailored to the culture and eating preferences of the patient.
The type of diet with the strongest evidence base for preventing T2DM is a Mediterranean diet, which is supported by level-4, high-quality evidence. Important aspects of a Mediterranean diet are the regular consumption of nuts, whole grains, fruits, and vegetables; use of olive oil instead of butter; and favoring fish over red meat.6 Other dietary patterns associated with reduced risk but supported only by level-2, low-quality evidence, include Dietary Approaches to Stop Hypertension (DASH), vegetarian, vegan, and Nordic healthy diets. Moderate carbohydrate restriction is supported only by level-1, very low-quality evidence.
The UK guideline, too, recommends preferentially eating whole grains, fruits, and green leafy vegetables, as well as yogurt, cheese, tea, and coffee. And it advises reducing consumption of red processed meats, potatoes (especially French fries), sugar-sweetened beverages, and refined carbohydrates. However, these specific food preferences are supported only by low-level evidence.
Plant stanols and plant sterols are found in a variety of plant foods such as cereals, vegetable oils, seeds, and nuts, and are now being added to some food products. (For more on plant stanols and plant sterols.) They have a chemical structure similar to cholesterol and reduce the intestinal absorption of cholesterol, thereby lowering total serum cholesterol and LDL-cholesterol. Both Diabetes UK and the ADA recommend 2 to 3 grams of stanols/sterols per day.
Continue to: Alcohol intake
Alcohol intake. And what about alcohol intake in those with T2DM? Once again, both guidelines are in concert by stating that alcohol use in those with diabetes should be moderate, defined by the ADA as one or fewer drinks/d for women and 2 or fewer for men.
Weight loss and exercise are important, too. Those who are overweight or obese with T2DM can improve glycemic control with a 5% weight loss achieved by reducing caloric intake and by increasing energy expenditure with 150 minutes of moderate physical activity per week over at least 3 days.3 This recommendation is supported by high-quality evidence.
A 15-kg weight loss is recommended for those attempting diabetes remission (supported by moderate-level evidence).3 One small study in the United Kingdom found that more than half of those with T2DM could achieve remission with weight loss of 10 kg or more; 86% with weight loss of 15 kg or more.7 The Diabetes UK guideline panel rated this as having moderate-level evidence.
The bottom line. Diet and exercise are key interventions for the prevention and treatment of diabetes and can lead to remission if sufficient weight loss is achieved. To achieve and maintain an optimal diet, patients need individualized professional advice and followup. The evidence base for nutritional advice is growing and can be used to improve the quality of these patient-provider interactions.
1. America Diabetes Association. Statistics About Diabetes. http://www.diabetes.org/diabetes-basics/statistics/. Accessed May 13, 2018.
2. CDC. National Center for Health Statistics. Health, United States, 2016. Available at: https://www.cdc.gov/nchs/data/hus/hus16.pdf. Accessed May 21, 2018.
3. Dyson PA, Twenefour D, Breen C, et al. Diabetes UK evidence-based nutrition guidelines for the prevention and management of diabetes. Diabet Med. 2018;35:541-547.
4. Evert AB, Boucher JL, Cypress M, et al. Nutrition therapy recommendations for the management of adults with diabetes. Diabetes Care. 2013;36:3821-3842.
5. IOM (Institute of Medicine). 2011. Clinical Practice Guidelines We Can Trust. Washington, DC: The National Academies Press.
6. Romagnolo DF, Selmin OI. Mediterranean diet and prevention of chronic diseases. Nutr Today. 2017;52:208-222.
7. Lean ME, Leslie WS, Barnes AC, et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trial. Lancet. 2018;391:541-551.
1. America Diabetes Association. Statistics About Diabetes. http://www.diabetes.org/diabetes-basics/statistics/. Accessed May 13, 2018.
2. CDC. National Center for Health Statistics. Health, United States, 2016. Available at: https://www.cdc.gov/nchs/data/hus/hus16.pdf. Accessed May 21, 2018.
3. Dyson PA, Twenefour D, Breen C, et al. Diabetes UK evidence-based nutrition guidelines for the prevention and management of diabetes. Diabet Med. 2018;35:541-547.
4. Evert AB, Boucher JL, Cypress M, et al. Nutrition therapy recommendations for the management of adults with diabetes. Diabetes Care. 2013;36:3821-3842.
5. IOM (Institute of Medicine). 2011. Clinical Practice Guidelines We Can Trust. Washington, DC: The National Academies Press.
6. Romagnolo DF, Selmin OI. Mediterranean diet and prevention of chronic diseases. Nutr Today. 2017;52:208-222.
7. Lean ME, Leslie WS, Barnes AC, et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trial. Lancet. 2018;391:541-551.
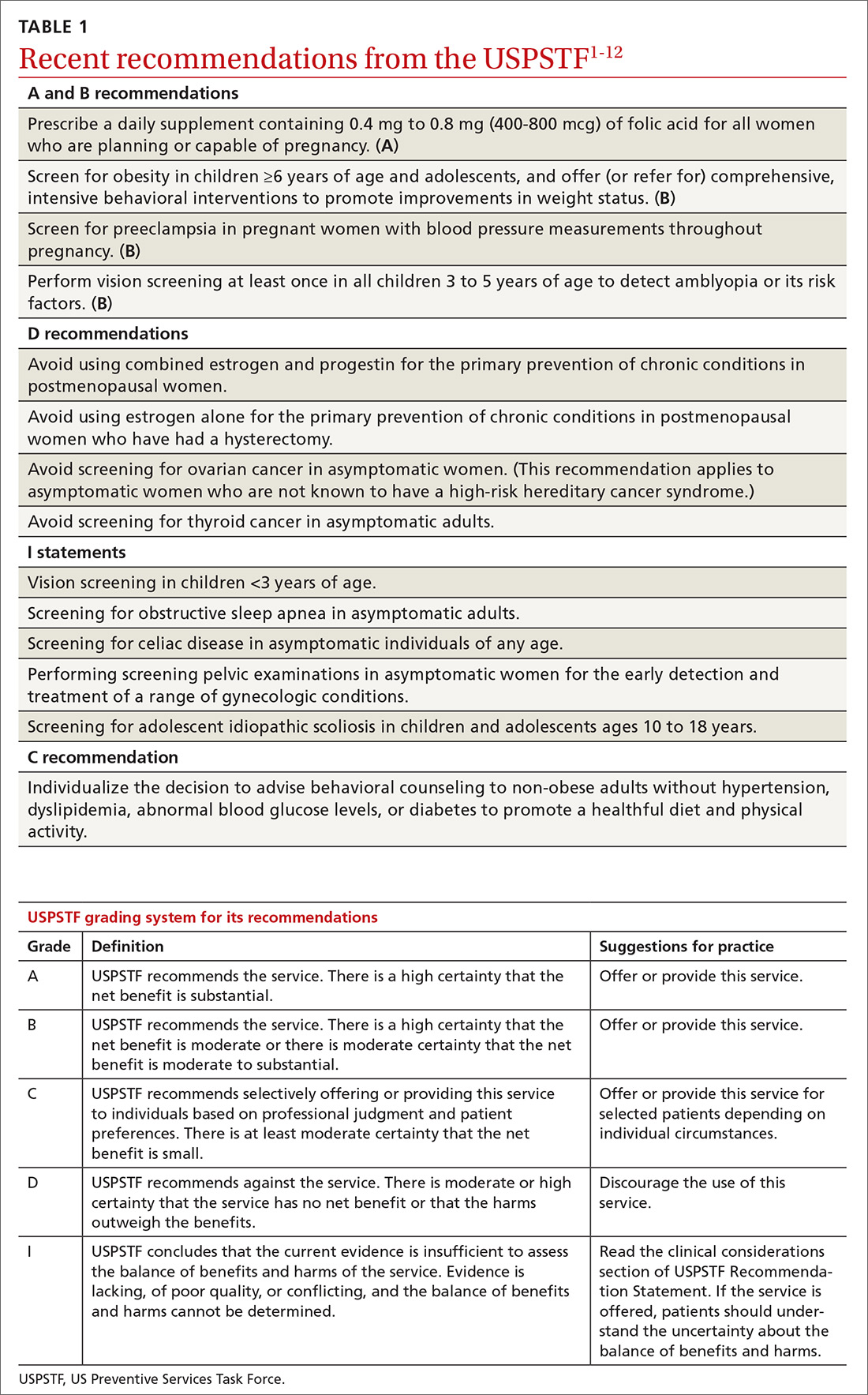
USPSTF update: New and revised recommendations
Over the past year the US Preventive Services Task Force made 14 recommendations on 12 conditions (TABLE 11-12). One of these pronouncements was the unusual reversal of a previous “D” recommendation against screening for scoliosis in adolescents, changing it to an “I” statement (insufficient evidence).
Affirmative recommendations
Four interventions were given an “A” or “B” recommendation this past year. Both grades signify a recommendation to perform the service, with “A” reflecting a higher level of certainty or a higher level of net benefit than “B.”
Recommend folic acid to prevent neural tube defects (A)
The evidence is very strong that folic acid intake prevents neural tube defects. In 2009 the Task Force recommended folic acid supplementation for women of childbearing age. In 2017 this recommendation was updated and slightly reworded to advise that all women who are planning a pregnancy or capable of becoming pregnant take a daily supplement containing 0.4 mg to 0.8 mg (400-800 mcg) of folic acid.
In the United States many grain products have been fortified with folic acid since 1996. This step has reduced the prevalence of neural tube defects from 10.7 cases per 10,000 live births to 7 cases per 10,000 live births in 2011.1 However, in spite of food fortification, most women in the United States do not consume the recommended daily amount of 0.4 mg (400 mcg) of folic acid. This supplementation is most important from one month before conception through the first 3 months of pregnancy.
Screen for obesity in children and adolescents (B)
Nearly 17% of children and adolescents ages 2 to 19 years in the United States are obese, and almost 32% are overweight or obese.2 Obesity is defined as a body mass index (BMI) ≥95th percentile, based on year-2000 growth charts published by the Centers for Disease Control and Prevention. Overweight is defined as a BMI between the 85th and 94th percentiles.
Obesity in children and adolescents is associated with many physical problems, including obstructive sleep apnea, orthopedic problems, high blood pressure, hyperlipidemia, and diabetes, as well as psychological harms from being teased and bullied. Obesity that continues into adulthood is associated with diabetes, cardiovascular disease, and orthopedic problems.
The Task Force found that intensive behavioral interventions for obesity in children ≥6 years of age and in adolescents can lead to moderate improvements in weight status for up to 12 months. Intensive behavioral interventions need to include at least 26 contact hours over 2 to 12 months. The recommendation statement includes a more detailed description of the types of programs that have evidence to support them.2
The Task Force did not recommend the use of either metformin or orlistat because of inadequate evidence on the harmful effects of metformin and because of sound evidence that orlistat causes moderate harms, such as abdominal pain, cramping, incontinence, and flatus.
Screen for preeclampsia (B), but dipstick testing is unreliable
Preeclampsia occurs in a little more than 3% of pregnancies in the United States.13 For the mother, this condition can lead to stroke, eclampsia, organ failure, and death; for the fetus, intrauterine growth retardation, preterm birth, low birth weight, and still birth. Preeclampsia is a leading cause of maternal mortality worldwide. Adverse health outcomes can be prevented by early detection of preeclampsia and by managing it appropriately.3
In 1996 the Task Force recommended screening for preeclampsia during pregnancy, and it reaffirmed that recommendation last year. The Task Force recommends taking blood pressure measurements at every prenatal visit, but does not recommend testing for urine protein with a dipstick because of the technique’s low accuracy.
Since 2014 the Task Force has also recommended using low-dose aspirin after Week 12 of pregnancy to prevent preeclampsia in women who are at high risk.14
Conduct vision screening in all children ages 3 to 5 years (B)
One of the more nuanced recommendations involves vision screening in children. The Task Force recently reaffirmed its 2011 recommendation to perform vision screening at least once in all children ages 3 to 5 years to detect amblyopia or its risk factors. But it found insufficient evidence to test children <3 years of age.
Amblyopia is a “functional reduction in visual acuity characterized by abnormal processing of visual images; [it is] established by the brain during a critical period of vision development.”4 Risk factors associated with the development of amblyopia include strabismus (ocular misalignment); vision loss caused by cataracts; refractive errors such as near and far sightedness, astigmatism (“blurred vision at any distance due to abnormal curvature of the cornea or lens”); and anisometropia (“asymmetric refractive error between the … eyes that causes image suppression in the eye with the larger error”). 4
Physical exam- and machine-based screening tests are available in the primary care setting (TABLE 2).4
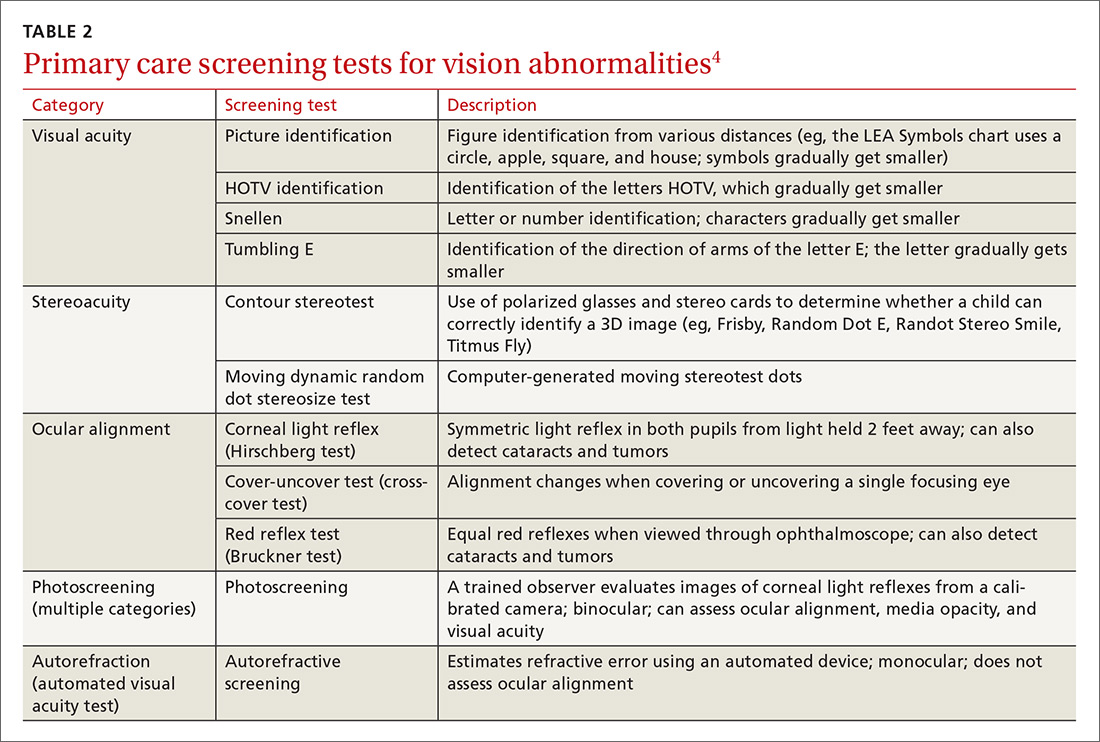
At first glance it appears that the Task Force recommends screening only for amblyopia, but the addition of “risk factors” implies a more comprehensive vision evaluation that would include visual acuity. This interpretation more closely aligns the Task Force recommendation with that of a joint report by the American Academy of Pediatrics, American Association for Pediatric Ophthalmology and Strabismus, American Academy of Certified Orthoptists, and American Academy of Ophthalmology, which recommends testing for a variety of vision problems in children.15 Nevertheless, the Task Force maintains that the evidence of benefit in testing more extensively before age 3 is insufficient, while the other organizations recommend starting testing at age 6 months.
Negative “D” recommendations
Equally as important as affirmative recommendations for effective interventions are the “D” recommendations advising against interventions that are ineffective or cause more harm than benefits. This past year, the Task Force recommended against 4 interventions. Two pertain to the use of estrogen or combined estrogen and progestin for the primary prevention of chronic conditions in postmenopausal women.5 This topic has been discussed in a recent JFP audiocast. Also receiving “D” recommendations were screening for ovarian cancer in asymptomatic women,6 discussed in another JFP audiocast, and screening for thyroid cancer in asymptomatic adults.7
The “D” recommendation for thyroid cancer screening was based on the low incidence of thyroid cancer, the evidence showing no change in mortality after the introduction of population-based screening, and the likelihood of overdiagnosis and overtreatment that would result from screening. The screening tests considered by the Task Force included neck palpation and ultrasound.7
Insufficient evidence
In addition to the previously mentioned “I” statement on vision screening for children <3 years of age,4 4 other interventions lacked sufficient evidence that the Task Force could use in determining relative levels of harms and benefits. These interventions were screening for obstructive sleep apnea in asymptomatic adults,8 screening for celiac disease in asymptomatic patients of all ages,9 screening with a pelvic examination in asymptomatic women,10 and screening for adolescent idiopathic scoliosis in children and adolescents ages 10 to 18 years.11
The lack of evidence regarding the value of a routine pelvic exam for asymptomatic women is surprising given how often this procedure is performed. The Task Force defined a pelvic exam as an “assessment of the external genitalia, internal speculum examination, bimanual palpation, and rectovaginal examination.”10 The Task Force found very little evidence on the accuracy and effectiveness of this exam for a range of gynecologic conditions other than cervical cancer, gonorrhea, and chlamydia, for which screening is recommended.10
The “I” statement on screening for adolescent idiopathic scoliosis in children and adolescents is an unusual revision of a “D” recommendation from 2004. At that time, the Task Force found that treatment of adolescent idiopathic scoliosis leads to health benefits in only a small proportion of individuals and that there are harms of treatment such as unnecessary bracing and referral to specialty care. For the most recent evidence report, the Task Force used a new methodology to assess treatment harms and concluded that the evidence is now inadequate. That finding, along with new evidence that “suggests that brace treatment can interrupt or slow scoliosis progression” led the Task Force to move away from a “D” recommendation.11
The enigmatic “C” recommendation
Perhaps the most difficult recommendation category to understand and implement is the “C” recommendation. With a “C” intervention, there is moderate certainty that the net benefit of universal implementation would be very small; but there are some individuals who might benefit from it, and physicians should offer it selectively.
The Task Force made one “C” recommendation over the past year: for adults who are not obese and who do not have other cardiovascular disease (CVD) risks, the net gain in referring them to behavioral counseling to promote a healthful diet and physical activity is small. However, the harms from such referrals are also small. Counseling interventions can result in healthier habits and in small improvements in intermediate outcomes, such as blood pressure, cholesterol levels, and weight. The effect on overall CVD mortality, though, has been minimal.12 The Task Force concluded that “[those] who are interested and ready to make behavioral changes may be most likely to benefit from behavioral counseling.”
1. USPSTF. Folic acid for the prevention of neural tube defects: preventive medication. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/folic-acid-for-the-prevention-of-neural-tube-defects-preventive-medication. Accessed March 22, 2018.
2. USPSTF. Obesity in children and adolescents: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/obesity-in-children-and-adolescents-screening1. Accessed March 22, 2018.
3. USPSTF. Preeclampsia: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/preeclampsia-screening1. Accessed March 22, 2018.
4. USPSTF. Vision in children ages 6 months to 5 years: Screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/vision-in-children-ages-6-months-to-5-years-screening. Accessed March 22, 2018.
5. USPSTF. Hormone therapy in postmenopausal women: primary prevention of chronic conditions. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/menopausal-hormone-therapy-preventive-medication1. Accessed March 24, 2018.
6. USPSTF. Ovarian cancer: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/ovarian-cancer-screening1. Accessed March 24, 2018.
7. USPSTF. Thyroid cancer: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/thyroid-cancer-screening1. Accessed March 22, 2018.
8. USPSTF. Obstructive sleep apnea in adults: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/obstructive-sleep-apnea-in-adults-screening. Accessed March 22, 2018.
9. USPSTF. Celiac disease: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/celiac-disease-screening. Accessed March 24, 2018.
10. USPSTF. Gynecological conditions: periodic screening with the pelvic examination. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/gynecological-conditions-screening-with-the-pelvic-examination. Accessed March 22, 2018.
11. USPSTF. Adolescent idiopathic scoliosis: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/adolescent-idiopathic-scoliosis-screening1. Accessed March 22, 2018.
12. USPSTF. Healthful diet and physical activity for cardiovascular disease prevention in adults without known risk factors: behavioral counseling. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/healthful-diet-and-physical-activity-for-cardiovascular-disease-prevention-in-adults-without-known-risk-factors-behavioral-counseling. Accessed March 22, 2018.
13. Ananth CV, Keyes KM, Wapner RJ. Pre-eclampsia rates in the United States, 1980-2010: age-period-cohort analysis. BMJ. 2013;347:f6564.
14. USPSTF. Low-dose aspirin use for the prevention of morbidity and mortality from preeclampsia: preventive medication. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/low-dose-aspirin-use-for-the-prevention-of-morbidity-and-mortality-from-preeclampsia-preventive-medication. Accessed March 22, 2018.
15. Donahue SP, Baker CN; Committee on Practice and Ambulatory Medicine, American Academy of Pediatrics; Section on Ophthalmology, American Academy of Pediatrics; American Association of Certified Orthoptists; American Association for Pediatric Ophthalmology and Strabismus; American Academy of Ophthalmology. Procedures for the evaluation of the visual system by pediatricians. Pediatrics. 2016;137.2015-3597.
Over the past year the US Preventive Services Task Force made 14 recommendations on 12 conditions (TABLE 11-12). One of these pronouncements was the unusual reversal of a previous “D” recommendation against screening for scoliosis in adolescents, changing it to an “I” statement (insufficient evidence).

Affirmative recommendations
Four interventions were given an “A” or “B” recommendation this past year. Both grades signify a recommendation to perform the service, with “A” reflecting a higher level of certainty or a higher level of net benefit than “B.”
Recommend folic acid to prevent neural tube defects (A)
The evidence is very strong that folic acid intake prevents neural tube defects. In 2009 the Task Force recommended folic acid supplementation for women of childbearing age. In 2017 this recommendation was updated and slightly reworded to advise that all women who are planning a pregnancy or capable of becoming pregnant take a daily supplement containing 0.4 mg to 0.8 mg (400-800 mcg) of folic acid.
In the United States many grain products have been fortified with folic acid since 1996. This step has reduced the prevalence of neural tube defects from 10.7 cases per 10,000 live births to 7 cases per 10,000 live births in 2011.1 However, in spite of food fortification, most women in the United States do not consume the recommended daily amount of 0.4 mg (400 mcg) of folic acid. This supplementation is most important from one month before conception through the first 3 months of pregnancy.
Screen for obesity in children and adolescents (B)
Nearly 17% of children and adolescents ages 2 to 19 years in the United States are obese, and almost 32% are overweight or obese.2 Obesity is defined as a body mass index (BMI) ≥95th percentile, based on year-2000 growth charts published by the Centers for Disease Control and Prevention. Overweight is defined as a BMI between the 85th and 94th percentiles.
Obesity in children and adolescents is associated with many physical problems, including obstructive sleep apnea, orthopedic problems, high blood pressure, hyperlipidemia, and diabetes, as well as psychological harms from being teased and bullied. Obesity that continues into adulthood is associated with diabetes, cardiovascular disease, and orthopedic problems.
The Task Force found that intensive behavioral interventions for obesity in children ≥6 years of age and in adolescents can lead to moderate improvements in weight status for up to 12 months. Intensive behavioral interventions need to include at least 26 contact hours over 2 to 12 months. The recommendation statement includes a more detailed description of the types of programs that have evidence to support them.2
The Task Force did not recommend the use of either metformin or orlistat because of inadequate evidence on the harmful effects of metformin and because of sound evidence that orlistat causes moderate harms, such as abdominal pain, cramping, incontinence, and flatus.
Screen for preeclampsia (B), but dipstick testing is unreliable
Preeclampsia occurs in a little more than 3% of pregnancies in the United States.13 For the mother, this condition can lead to stroke, eclampsia, organ failure, and death; for the fetus, intrauterine growth retardation, preterm birth, low birth weight, and still birth. Preeclampsia is a leading cause of maternal mortality worldwide. Adverse health outcomes can be prevented by early detection of preeclampsia and by managing it appropriately.3
In 1996 the Task Force recommended screening for preeclampsia during pregnancy, and it reaffirmed that recommendation last year. The Task Force recommends taking blood pressure measurements at every prenatal visit, but does not recommend testing for urine protein with a dipstick because of the technique’s low accuracy.
Since 2014 the Task Force has also recommended using low-dose aspirin after Week 12 of pregnancy to prevent preeclampsia in women who are at high risk.14
Conduct vision screening in all children ages 3 to 5 years (B)
One of the more nuanced recommendations involves vision screening in children. The Task Force recently reaffirmed its 2011 recommendation to perform vision screening at least once in all children ages 3 to 5 years to detect amblyopia or its risk factors. But it found insufficient evidence to test children <3 years of age.
Amblyopia is a “functional reduction in visual acuity characterized by abnormal processing of visual images; [it is] established by the brain during a critical period of vision development.”4 Risk factors associated with the development of amblyopia include strabismus (ocular misalignment); vision loss caused by cataracts; refractive errors such as near and far sightedness, astigmatism (“blurred vision at any distance due to abnormal curvature of the cornea or lens”); and anisometropia (“asymmetric refractive error between the … eyes that causes image suppression in the eye with the larger error”). 4
Physical exam- and machine-based screening tests are available in the primary care setting (TABLE 2).4

At first glance it appears that the Task Force recommends screening only for amblyopia, but the addition of “risk factors” implies a more comprehensive vision evaluation that would include visual acuity. This interpretation more closely aligns the Task Force recommendation with that of a joint report by the American Academy of Pediatrics, American Association for Pediatric Ophthalmology and Strabismus, American Academy of Certified Orthoptists, and American Academy of Ophthalmology, which recommends testing for a variety of vision problems in children.15 Nevertheless, the Task Force maintains that the evidence of benefit in testing more extensively before age 3 is insufficient, while the other organizations recommend starting testing at age 6 months.
Negative “D” recommendations
Equally as important as affirmative recommendations for effective interventions are the “D” recommendations advising against interventions that are ineffective or cause more harm than benefits. This past year, the Task Force recommended against 4 interventions. Two pertain to the use of estrogen or combined estrogen and progestin for the primary prevention of chronic conditions in postmenopausal women.5 This topic has been discussed in a recent JFP audiocast. Also receiving “D” recommendations were screening for ovarian cancer in asymptomatic women,6 discussed in another JFP audiocast, and screening for thyroid cancer in asymptomatic adults.7
The “D” recommendation for thyroid cancer screening was based on the low incidence of thyroid cancer, the evidence showing no change in mortality after the introduction of population-based screening, and the likelihood of overdiagnosis and overtreatment that would result from screening. The screening tests considered by the Task Force included neck palpation and ultrasound.7
Insufficient evidence
In addition to the previously mentioned “I” statement on vision screening for children <3 years of age,4 4 other interventions lacked sufficient evidence that the Task Force could use in determining relative levels of harms and benefits. These interventions were screening for obstructive sleep apnea in asymptomatic adults,8 screening for celiac disease in asymptomatic patients of all ages,9 screening with a pelvic examination in asymptomatic women,10 and screening for adolescent idiopathic scoliosis in children and adolescents ages 10 to 18 years.11
The lack of evidence regarding the value of a routine pelvic exam for asymptomatic women is surprising given how often this procedure is performed. The Task Force defined a pelvic exam as an “assessment of the external genitalia, internal speculum examination, bimanual palpation, and rectovaginal examination.”10 The Task Force found very little evidence on the accuracy and effectiveness of this exam for a range of gynecologic conditions other than cervical cancer, gonorrhea, and chlamydia, for which screening is recommended.10
The “I” statement on screening for adolescent idiopathic scoliosis in children and adolescents is an unusual revision of a “D” recommendation from 2004. At that time, the Task Force found that treatment of adolescent idiopathic scoliosis leads to health benefits in only a small proportion of individuals and that there are harms of treatment such as unnecessary bracing and referral to specialty care. For the most recent evidence report, the Task Force used a new methodology to assess treatment harms and concluded that the evidence is now inadequate. That finding, along with new evidence that “suggests that brace treatment can interrupt or slow scoliosis progression” led the Task Force to move away from a “D” recommendation.11
The enigmatic “C” recommendation
Perhaps the most difficult recommendation category to understand and implement is the “C” recommendation. With a “C” intervention, there is moderate certainty that the net benefit of universal implementation would be very small; but there are some individuals who might benefit from it, and physicians should offer it selectively.
The Task Force made one “C” recommendation over the past year: for adults who are not obese and who do not have other cardiovascular disease (CVD) risks, the net gain in referring them to behavioral counseling to promote a healthful diet and physical activity is small. However, the harms from such referrals are also small. Counseling interventions can result in healthier habits and in small improvements in intermediate outcomes, such as blood pressure, cholesterol levels, and weight. The effect on overall CVD mortality, though, has been minimal.12 The Task Force concluded that “[those] who are interested and ready to make behavioral changes may be most likely to benefit from behavioral counseling.”
Over the past year the US Preventive Services Task Force made 14 recommendations on 12 conditions (TABLE 11-12). One of these pronouncements was the unusual reversal of a previous “D” recommendation against screening for scoliosis in adolescents, changing it to an “I” statement (insufficient evidence).

Affirmative recommendations
Four interventions were given an “A” or “B” recommendation this past year. Both grades signify a recommendation to perform the service, with “A” reflecting a higher level of certainty or a higher level of net benefit than “B.”
Recommend folic acid to prevent neural tube defects (A)
The evidence is very strong that folic acid intake prevents neural tube defects. In 2009 the Task Force recommended folic acid supplementation for women of childbearing age. In 2017 this recommendation was updated and slightly reworded to advise that all women who are planning a pregnancy or capable of becoming pregnant take a daily supplement containing 0.4 mg to 0.8 mg (400-800 mcg) of folic acid.
In the United States many grain products have been fortified with folic acid since 1996. This step has reduced the prevalence of neural tube defects from 10.7 cases per 10,000 live births to 7 cases per 10,000 live births in 2011.1 However, in spite of food fortification, most women in the United States do not consume the recommended daily amount of 0.4 mg (400 mcg) of folic acid. This supplementation is most important from one month before conception through the first 3 months of pregnancy.
Screen for obesity in children and adolescents (B)
Nearly 17% of children and adolescents ages 2 to 19 years in the United States are obese, and almost 32% are overweight or obese.2 Obesity is defined as a body mass index (BMI) ≥95th percentile, based on year-2000 growth charts published by the Centers for Disease Control and Prevention. Overweight is defined as a BMI between the 85th and 94th percentiles.
Obesity in children and adolescents is associated with many physical problems, including obstructive sleep apnea, orthopedic problems, high blood pressure, hyperlipidemia, and diabetes, as well as psychological harms from being teased and bullied. Obesity that continues into adulthood is associated with diabetes, cardiovascular disease, and orthopedic problems.
The Task Force found that intensive behavioral interventions for obesity in children ≥6 years of age and in adolescents can lead to moderate improvements in weight status for up to 12 months. Intensive behavioral interventions need to include at least 26 contact hours over 2 to 12 months. The recommendation statement includes a more detailed description of the types of programs that have evidence to support them.2
The Task Force did not recommend the use of either metformin or orlistat because of inadequate evidence on the harmful effects of metformin and because of sound evidence that orlistat causes moderate harms, such as abdominal pain, cramping, incontinence, and flatus.
Screen for preeclampsia (B), but dipstick testing is unreliable
Preeclampsia occurs in a little more than 3% of pregnancies in the United States.13 For the mother, this condition can lead to stroke, eclampsia, organ failure, and death; for the fetus, intrauterine growth retardation, preterm birth, low birth weight, and still birth. Preeclampsia is a leading cause of maternal mortality worldwide. Adverse health outcomes can be prevented by early detection of preeclampsia and by managing it appropriately.3
In 1996 the Task Force recommended screening for preeclampsia during pregnancy, and it reaffirmed that recommendation last year. The Task Force recommends taking blood pressure measurements at every prenatal visit, but does not recommend testing for urine protein with a dipstick because of the technique’s low accuracy.
Since 2014 the Task Force has also recommended using low-dose aspirin after Week 12 of pregnancy to prevent preeclampsia in women who are at high risk.14
Conduct vision screening in all children ages 3 to 5 years (B)
One of the more nuanced recommendations involves vision screening in children. The Task Force recently reaffirmed its 2011 recommendation to perform vision screening at least once in all children ages 3 to 5 years to detect amblyopia or its risk factors. But it found insufficient evidence to test children <3 years of age.
Amblyopia is a “functional reduction in visual acuity characterized by abnormal processing of visual images; [it is] established by the brain during a critical period of vision development.”4 Risk factors associated with the development of amblyopia include strabismus (ocular misalignment); vision loss caused by cataracts; refractive errors such as near and far sightedness, astigmatism (“blurred vision at any distance due to abnormal curvature of the cornea or lens”); and anisometropia (“asymmetric refractive error between the … eyes that causes image suppression in the eye with the larger error”). 4
Physical exam- and machine-based screening tests are available in the primary care setting (TABLE 2).4

At first glance it appears that the Task Force recommends screening only for amblyopia, but the addition of “risk factors” implies a more comprehensive vision evaluation that would include visual acuity. This interpretation more closely aligns the Task Force recommendation with that of a joint report by the American Academy of Pediatrics, American Association for Pediatric Ophthalmology and Strabismus, American Academy of Certified Orthoptists, and American Academy of Ophthalmology, which recommends testing for a variety of vision problems in children.15 Nevertheless, the Task Force maintains that the evidence of benefit in testing more extensively before age 3 is insufficient, while the other organizations recommend starting testing at age 6 months.
Negative “D” recommendations
Equally as important as affirmative recommendations for effective interventions are the “D” recommendations advising against interventions that are ineffective or cause more harm than benefits. This past year, the Task Force recommended against 4 interventions. Two pertain to the use of estrogen or combined estrogen and progestin for the primary prevention of chronic conditions in postmenopausal women.5 This topic has been discussed in a recent JFP audiocast. Also receiving “D” recommendations were screening for ovarian cancer in asymptomatic women,6 discussed in another JFP audiocast, and screening for thyroid cancer in asymptomatic adults.7
The “D” recommendation for thyroid cancer screening was based on the low incidence of thyroid cancer, the evidence showing no change in mortality after the introduction of population-based screening, and the likelihood of overdiagnosis and overtreatment that would result from screening. The screening tests considered by the Task Force included neck palpation and ultrasound.7
Insufficient evidence
In addition to the previously mentioned “I” statement on vision screening for children <3 years of age,4 4 other interventions lacked sufficient evidence that the Task Force could use in determining relative levels of harms and benefits. These interventions were screening for obstructive sleep apnea in asymptomatic adults,8 screening for celiac disease in asymptomatic patients of all ages,9 screening with a pelvic examination in asymptomatic women,10 and screening for adolescent idiopathic scoliosis in children and adolescents ages 10 to 18 years.11
The lack of evidence regarding the value of a routine pelvic exam for asymptomatic women is surprising given how often this procedure is performed. The Task Force defined a pelvic exam as an “assessment of the external genitalia, internal speculum examination, bimanual palpation, and rectovaginal examination.”10 The Task Force found very little evidence on the accuracy and effectiveness of this exam for a range of gynecologic conditions other than cervical cancer, gonorrhea, and chlamydia, for which screening is recommended.10
The “I” statement on screening for adolescent idiopathic scoliosis in children and adolescents is an unusual revision of a “D” recommendation from 2004. At that time, the Task Force found that treatment of adolescent idiopathic scoliosis leads to health benefits in only a small proportion of individuals and that there are harms of treatment such as unnecessary bracing and referral to specialty care. For the most recent evidence report, the Task Force used a new methodology to assess treatment harms and concluded that the evidence is now inadequate. That finding, along with new evidence that “suggests that brace treatment can interrupt or slow scoliosis progression” led the Task Force to move away from a “D” recommendation.11
The enigmatic “C” recommendation
Perhaps the most difficult recommendation category to understand and implement is the “C” recommendation. With a “C” intervention, there is moderate certainty that the net benefit of universal implementation would be very small; but there are some individuals who might benefit from it, and physicians should offer it selectively.
The Task Force made one “C” recommendation over the past year: for adults who are not obese and who do not have other cardiovascular disease (CVD) risks, the net gain in referring them to behavioral counseling to promote a healthful diet and physical activity is small. However, the harms from such referrals are also small. Counseling interventions can result in healthier habits and in small improvements in intermediate outcomes, such as blood pressure, cholesterol levels, and weight. The effect on overall CVD mortality, though, has been minimal.12 The Task Force concluded that “[those] who are interested and ready to make behavioral changes may be most likely to benefit from behavioral counseling.”
1. USPSTF. Folic acid for the prevention of neural tube defects: preventive medication. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/folic-acid-for-the-prevention-of-neural-tube-defects-preventive-medication. Accessed March 22, 2018.
2. USPSTF. Obesity in children and adolescents: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/obesity-in-children-and-adolescents-screening1. Accessed March 22, 2018.
3. USPSTF. Preeclampsia: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/preeclampsia-screening1. Accessed March 22, 2018.
4. USPSTF. Vision in children ages 6 months to 5 years: Screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/vision-in-children-ages-6-months-to-5-years-screening. Accessed March 22, 2018.
5. USPSTF. Hormone therapy in postmenopausal women: primary prevention of chronic conditions. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/menopausal-hormone-therapy-preventive-medication1. Accessed March 24, 2018.
6. USPSTF. Ovarian cancer: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/ovarian-cancer-screening1. Accessed March 24, 2018.
7. USPSTF. Thyroid cancer: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/thyroid-cancer-screening1. Accessed March 22, 2018.
8. USPSTF. Obstructive sleep apnea in adults: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/obstructive-sleep-apnea-in-adults-screening. Accessed March 22, 2018.
9. USPSTF. Celiac disease: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/celiac-disease-screening. Accessed March 24, 2018.
10. USPSTF. Gynecological conditions: periodic screening with the pelvic examination. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/gynecological-conditions-screening-with-the-pelvic-examination. Accessed March 22, 2018.
11. USPSTF. Adolescent idiopathic scoliosis: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/adolescent-idiopathic-scoliosis-screening1. Accessed March 22, 2018.
12. USPSTF. Healthful diet and physical activity for cardiovascular disease prevention in adults without known risk factors: behavioral counseling. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/healthful-diet-and-physical-activity-for-cardiovascular-disease-prevention-in-adults-without-known-risk-factors-behavioral-counseling. Accessed March 22, 2018.
13. Ananth CV, Keyes KM, Wapner RJ. Pre-eclampsia rates in the United States, 1980-2010: age-period-cohort analysis. BMJ. 2013;347:f6564.
14. USPSTF. Low-dose aspirin use for the prevention of morbidity and mortality from preeclampsia: preventive medication. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/low-dose-aspirin-use-for-the-prevention-of-morbidity-and-mortality-from-preeclampsia-preventive-medication. Accessed March 22, 2018.
15. Donahue SP, Baker CN; Committee on Practice and Ambulatory Medicine, American Academy of Pediatrics; Section on Ophthalmology, American Academy of Pediatrics; American Association of Certified Orthoptists; American Association for Pediatric Ophthalmology and Strabismus; American Academy of Ophthalmology. Procedures for the evaluation of the visual system by pediatricians. Pediatrics. 2016;137.2015-3597.
1. USPSTF. Folic acid for the prevention of neural tube defects: preventive medication. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/folic-acid-for-the-prevention-of-neural-tube-defects-preventive-medication. Accessed March 22, 2018.
2. USPSTF. Obesity in children and adolescents: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/obesity-in-children-and-adolescents-screening1. Accessed March 22, 2018.
3. USPSTF. Preeclampsia: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/preeclampsia-screening1. Accessed March 22, 2018.
4. USPSTF. Vision in children ages 6 months to 5 years: Screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/vision-in-children-ages-6-months-to-5-years-screening. Accessed March 22, 2018.
5. USPSTF. Hormone therapy in postmenopausal women: primary prevention of chronic conditions. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/menopausal-hormone-therapy-preventive-medication1. Accessed March 24, 2018.
6. USPSTF. Ovarian cancer: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/ovarian-cancer-screening1. Accessed March 24, 2018.
7. USPSTF. Thyroid cancer: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/thyroid-cancer-screening1. Accessed March 22, 2018.
8. USPSTF. Obstructive sleep apnea in adults: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/obstructive-sleep-apnea-in-adults-screening. Accessed March 22, 2018.
9. USPSTF. Celiac disease: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/celiac-disease-screening. Accessed March 24, 2018.
10. USPSTF. Gynecological conditions: periodic screening with the pelvic examination. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/gynecological-conditions-screening-with-the-pelvic-examination. Accessed March 22, 2018.
11. USPSTF. Adolescent idiopathic scoliosis: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/adolescent-idiopathic-scoliosis-screening1. Accessed March 22, 2018.
12. USPSTF. Healthful diet and physical activity for cardiovascular disease prevention in adults without known risk factors: behavioral counseling. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/healthful-diet-and-physical-activity-for-cardiovascular-disease-prevention-in-adults-without-known-risk-factors-behavioral-counseling. Accessed March 22, 2018.
13. Ananth CV, Keyes KM, Wapner RJ. Pre-eclampsia rates in the United States, 1980-2010: age-period-cohort analysis. BMJ. 2013;347:f6564.
14. USPSTF. Low-dose aspirin use for the prevention of morbidity and mortality from preeclampsia: preventive medication. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/low-dose-aspirin-use-for-the-prevention-of-morbidity-and-mortality-from-preeclampsia-preventive-medication. Accessed March 22, 2018.
15. Donahue SP, Baker CN; Committee on Practice and Ambulatory Medicine, American Academy of Pediatrics; Section on Ophthalmology, American Academy of Pediatrics; American Association of Certified Orthoptists; American Association for Pediatric Ophthalmology and Strabismus; American Academy of Ophthalmology. Procedures for the evaluation of the visual system by pediatricians. Pediatrics. 2016;137.2015-3597.
From The Journal of Family Practice | 2018;67(5):294-296,298-299.