User login
USPSTF recommendations: A 2017 roundup
Since the last Practice Alert update on the United States Preventive Services Task Force in May of 2016,1 the Task Force has released 19 recommendations on 13 topics that include: the use of aspirin and statins for the prevention of cardiovascular disease (CVD); support for breastfeeding; use of folic acid during pregnancy; and screening for syphilis, latent tuberculosis (TB), herpes, chronic obstructive pulmonary disease (COPD), colorectal cancer (CRC), obstructive sleep apnea (OSA), celiac disease, and skin cancer. The Task Force also released a draft recommendation regarding prostate cancer screening in asymptomatic men (see “A change for prostate cancer screening?”) and addressed screening pelvic examinations in asymptomatic women, the subject of this month’s audiocast. (To listen, go to: http://bit.ly/2nIVoD5.)
Recommendations to implement
Recommendations from the past year that family physicians should put into practice are detailed below and in TABLE 1.2-8

CRC: Screen all individuals ages 50 to 75, but 76 to 85 selectively. The Task Force reaffirmed its 2008 finding that screening for CRC in adults ages 50 to 75 years is substantially beneficial.2 In contrast to the previous recommendation, however, the new one does not state which screening tests are preferred. The tests considered were 3 stool tests (fecal immunochemical test [FIT], FIT-tumor DNA testing [FIT-DNA], and guaiac-based fecal occult blood test [gFOBT]), as well as 3 direct visualization tests (colonoscopy, sigmoidoscopy, and CT colonoscopy). The Task Force assessed various testing frequencies of each test and some test combinations. While the Task Force does not recommend any one screening strategy, there are still significant unknowns about FIT-DNA and CT colonoscopy. The American Academy of Family Physicians does not recommend using these 2 tests for screening purposes at this time.9
CRC screening for adults ages 76 to 85 was given a “C” recommendation, which means the value of the service to the population overall is small, but that certain individuals may benefit from it. The Task Force advises selectively offering a “C” service to individuals based on professional judgment and patient preferences. Regarding CRC screening in individuals 76 years or older, the ones most likely to benefit are those who have never been screened and those without significant comorbidities that could limit life expectancy. All “C” recommendations from the past year are listed in TABLE 2.2-4

CVD prevention: When aspirin or a statin is indicated. The Task Force released 2 recommendations for the prevention of CVD this past year. One pertained to the use of low-dose aspirin3 (which also helps to prevent CRC), and the other addressed the use of low- to moderate-dose statins.4 Each recommendation is fairly complicated and nuanced in terms of age and risk for CVD. A decision to use low-dose aspirin must also consider the risk of bleeding.
To calculate a patient’s risk for CVD, the Task Force recommends using the risk calculator developed by the American College of Cardiology and the American Heart Association (http://www.cvriskcalculator.com/).
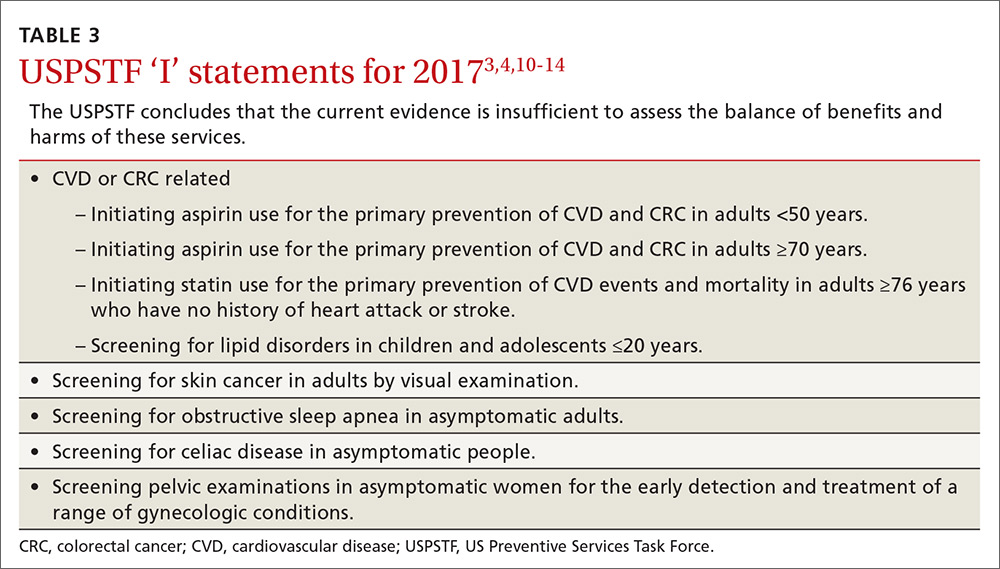
Adults for whom low-dose aspirin and low- to moderate-dose statins are recommended are described in TABLE 1.2-8 Patients for whom individual decision making is advised, rather than a generalized recommendation, are reviewed in TABLE 2.2-4 There is insufficient evidence to make a recommendation for the use of aspirin before age 50 or at age 70 and older,3 and for the use of statins in adults age 76 and older who do not have a history of CVD4 (TABLE 33,4,10-14). The use of low-dose aspirin and low-to-moderate dose statins have been the subject of JFP audiocasts in May 2016 and January 2017. (See http://bit.ly/2oiun8d and http://bit.ly/2oqkohR.)
2 pregnancy-related recommendations. To prevent neural tube defects in newborns, the Task Force now recommends daily folic acid, 0.4 to 0.8 mg (400 to 800 mcg), for all women who are planning on or are capable of becoming pregnant.5 This is an update of a 2009 recommendation that was worded slightly differently, recommending the supplement for all women of childbearing age.
A new recommendation on breastfeeding recognizes its benefits for both mother and baby and finds that interventions to encourage breastfeeding increase the prevalence of this practice and its duration.6 Interventions—provided to individuals or groups by professionals or peers or through formal education—include promoting the benefits of breastfeeding, giving practical advice and direct support on how to breastfeed, and offering psychological support.
Latent TB: Advantages of newer testing method. The recommendation on screening for latent tuberculosis (TB) is an update from the one made in 1996.7 At that time, screening for latent infection was performed using a tuberculin skin test (TST). Now a TST or interferon-gamma release assay (IGRA) can be used. Testing with IGRA may be the best option for those who have received a bacille Calmette–Guérin vaccination (because it can cause a false-positive TST) or for those who are not likely to return to have their TST read.
Those at high risk for latent TB include people who were born or have resided in countries with a high TB prevalence, those who have lived in a correctional institution or homeless shelter, and anyone in a high-risk group based on local epidemiology of the disease. (Read more on TB in this month’s Case Report.) Others at high risk are those who are immune suppressed because of infection or medications, and those who work in health care or correctional facilities. Screening of these groups is usually conducted as part of occupational health or is considered part of routine health care.
Syphilis: Screen high-risk individuals in 2 steps. The recommendation on syphilis screening basically reaffirms the one from 2004.8 Those at high risk for syphilis include men who have sex with men (who now account for 90% of new cases), those who are HIV positive, and those who engage in commercial sex work. Other racial and demographic groups can be at high risk depending on the local epidemiology of the disease. In a separate recommendation, the Task Force advises screening all pregnant women for syphilis.
Screening for syphilis infection involves 2 steps: first, a nontreponemal test (Venereal Disease Research Laboratory [VDRL] or rapid plasma reagin [RPR] test); second, a confirmatory treponemal antibody detection test (fluorescent treponemal antibody absorption [FTA-ABS] or Treponema pallidum particle agglutination [TPPA] test). Treatment for syphilis remains benzathine penicillin with the number of injections depending on the stage of infection. The Centers for Disease Control and Prevention is the best source for current recommendations for treatment of all sexually transmitted infections.15
Screening tests to avoid
TABLE 416,17 lists screening tests the Task Force recommends against. While chronic obstructive pulmonary disease afflicts 14% of US adults ages 40 to 79 years and is the third leading cause of death in the country, the Task Force found that early detection in asymptomatic adults does not affect the course of the illness and is of no benefit.16

Genital herpes, also prevalent, infects an estimated one out of 6 individuals, ages 14 to 49. It causes little mortality, except in neonates, but those infected can have recurrent flares and suffer psychological harms from stigmatization. Most genital herpes is caused by herpes simplex virus-2, and there is a serological test to detect it. However, the Task Force recommends against using the test to screen asymptomatic adults and adolescents, including those who are pregnant. This recommendation is based on the test’s high false-positive rate, which can cause emotional harm, and on the lack of evidence that detection through screening improves outcomes.17
The evidence is lacking for these practices
The Task Force is one of only a few organizations that will not make a recommendation if evidence is lacking on benefits and harms. In addition to the ‘I’ statements regarding CVD and CRC mentioned earlier, the Task Force found insufficient evidence to recommend screening for lipid disorders in individuals ages 20 years or younger,10 performing a visual skin exam as a screening tool for skin cancer,11 screening for celiac disease,12 performing a periodic pelvic examination in asymptomatic women,13 and screening for obstructive sleep apnea using screening questionnaires14 (TABLE 33,4,10-14).
SIDEBAR
A change for prostate cancer screening?The USPSTF recently issued new draft recommendations regarding prostate cancer screening in asymptomatic men (available at: https://screeningforprostatecancer.org/).
The draft now divides men into 2 age groups, stating that the decision to screen for prostate cancer using a prostate specific antigen (PSA) test should be individualized for men ages 55 to 69 years (a C recommendation, meaning that there is at least moderate certainty that the net benefit is small), and that men ages 70 and older (lowered from age 75 in the previous 2012 recommendation1) should not be screened (a D recommendation, meaning that there is moderate or high certainty that the service has no net benefit or that the harms outweigh the benefits).
The USPSTF believes that clinicians should explain to men ages 55 to 69 years that screening offers a small potential benefit of reducing the chance of dying from prostate cancer, but also comes with potential harms, including false-positive results requiring additional testing/procedures, overdiagnosis and overtreatment, and treatment complications such as incontinence and impotence. In this way, each man has the chance to incorporate his values and preferences into the decision.
For men ages 70 and older, the potential benefits simply do not outweigh the potential harms, according to the USPSTF.
1. USPSTF. Final recommendation statement. Prostate cancer: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/prostate-cancer-screening#Pod1. Accessed April 11, 2017.
1. Campos-Outcalt D. Eight USPSTF recommendations FPs need to know about. J Fam Pract. 2016;65:338-341.
2. USPSTF. Colorectal cancer: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/colorectal-cancer-screening2. Accessed March 22, 2017.
3. USPSTF. Aspirin use to prevent cardiovascular disease and colorectal cancer: preventive medications. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/aspirin-to-prevent-cardiovascular-disease-and-cancer. Accessed March 22, 2017.
4. USPSTF. Statin use for the prevention of cardiovascular disease in adults: preventive medication. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/statin-use-in-adults-preventive-medication1. Accessed March 22, 2017.
5. USPSTF. Folic acid for the prevention of neural tube defects: preventive medication. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/folic-acid-for-the-prevention-of-neural-tube-defects-preventive-medication. Accessed March 22, 2017.
6. USPSTF. Breastfeeding: primary care interventions. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/breastfeeding-primary-care-interventions. Accessed March 22, 2017.
7. USPSTF. Latent tuberculosis infection: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/latent-tuberculosis-infection-screening. Accessed March 22, 2017.
8. USPSTF. Syphilis infection in nonpregnant adults and adolescents: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/syphilis-infection-in-nonpregnant-adults-and-adolescents. Accessed March 22, 2017.
9. AAFP. Colorectal cancer screening, adults. Available at: http://www.aafp.org/patient-care/clinical-recommendations/all/colorectal-cancer.html. Accessed March 22, 2017.
10. USPSTF. Lipid disorders in children and adolescents: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/lipid-disorders-in-children-screening1. Accessed March 22, 2017.
11. USPSTF. Skin cancer: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/skin-cancer-screening2. Accessed March 22, 2017.
12. USPSTF. Celiac disease: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/celiac-disease-screening. Accessed March 22, 2017.
13. USPSTF. Gynecological conditions: periodic screening with the pelvic examination. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/gynecological-conditions-screening-with-the-pelvic-examination. Accessed March 22, 2017.
14. USPSTF. Obstructive sleep apnea in adults: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/obstructive-sleep-apnea-in-adults-screening. Accessed March 22, 2017.
15. CDC. 2015 sexually transmitted diseases treatment guidelines. Available at: https://www.cdc.gov/std/tg2015/default.htm. Accessed March 22, 2017.
16. USPSTF. Chronic obstructive pulmonary disease: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/chronic-obstructive-pulmonary-disease-screening. Accessed March 22, 2017.
17. USPSTF. Genital herpes infection: serologic screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/genital-herpes-screening1. Accessed March 22, 2017.
Since the last Practice Alert update on the United States Preventive Services Task Force in May of 2016,1 the Task Force has released 19 recommendations on 13 topics that include: the use of aspirin and statins for the prevention of cardiovascular disease (CVD); support for breastfeeding; use of folic acid during pregnancy; and screening for syphilis, latent tuberculosis (TB), herpes, chronic obstructive pulmonary disease (COPD), colorectal cancer (CRC), obstructive sleep apnea (OSA), celiac disease, and skin cancer. The Task Force also released a draft recommendation regarding prostate cancer screening in asymptomatic men (see “A change for prostate cancer screening?”) and addressed screening pelvic examinations in asymptomatic women, the subject of this month’s audiocast. (To listen, go to: http://bit.ly/2nIVoD5.)
Recommendations to implement
Recommendations from the past year that family physicians should put into practice are detailed below and in TABLE 1.2-8

CRC: Screen all individuals ages 50 to 75, but 76 to 85 selectively. The Task Force reaffirmed its 2008 finding that screening for CRC in adults ages 50 to 75 years is substantially beneficial.2 In contrast to the previous recommendation, however, the new one does not state which screening tests are preferred. The tests considered were 3 stool tests (fecal immunochemical test [FIT], FIT-tumor DNA testing [FIT-DNA], and guaiac-based fecal occult blood test [gFOBT]), as well as 3 direct visualization tests (colonoscopy, sigmoidoscopy, and CT colonoscopy). The Task Force assessed various testing frequencies of each test and some test combinations. While the Task Force does not recommend any one screening strategy, there are still significant unknowns about FIT-DNA and CT colonoscopy. The American Academy of Family Physicians does not recommend using these 2 tests for screening purposes at this time.9
CRC screening for adults ages 76 to 85 was given a “C” recommendation, which means the value of the service to the population overall is small, but that certain individuals may benefit from it. The Task Force advises selectively offering a “C” service to individuals based on professional judgment and patient preferences. Regarding CRC screening in individuals 76 years or older, the ones most likely to benefit are those who have never been screened and those without significant comorbidities that could limit life expectancy. All “C” recommendations from the past year are listed in TABLE 2.2-4

CVD prevention: When aspirin or a statin is indicated. The Task Force released 2 recommendations for the prevention of CVD this past year. One pertained to the use of low-dose aspirin3 (which also helps to prevent CRC), and the other addressed the use of low- to moderate-dose statins.4 Each recommendation is fairly complicated and nuanced in terms of age and risk for CVD. A decision to use low-dose aspirin must also consider the risk of bleeding.
To calculate a patient’s risk for CVD, the Task Force recommends using the risk calculator developed by the American College of Cardiology and the American Heart Association (http://www.cvriskcalculator.com/).
Adults for whom low-dose aspirin and low- to moderate-dose statins are recommended are described in TABLE 1.2-8 Patients for whom individual decision making is advised, rather than a generalized recommendation, are reviewed in TABLE 2.2-4 There is insufficient evidence to make a recommendation for the use of aspirin before age 50 or at age 70 and older,3 and for the use of statins in adults age 76 and older who do not have a history of CVD4 (TABLE 33,4,10-14). The use of low-dose aspirin and low-to-moderate dose statins have been the subject of JFP audiocasts in May 2016 and January 2017. (See http://bit.ly/2oiun8d and http://bit.ly/2oqkohR.)

2 pregnancy-related recommendations. To prevent neural tube defects in newborns, the Task Force now recommends daily folic acid, 0.4 to 0.8 mg (400 to 800 mcg), for all women who are planning on or are capable of becoming pregnant.5 This is an update of a 2009 recommendation that was worded slightly differently, recommending the supplement for all women of childbearing age.
A new recommendation on breastfeeding recognizes its benefits for both mother and baby and finds that interventions to encourage breastfeeding increase the prevalence of this practice and its duration.6 Interventions—provided to individuals or groups by professionals or peers or through formal education—include promoting the benefits of breastfeeding, giving practical advice and direct support on how to breastfeed, and offering psychological support.
Latent TB: Advantages of newer testing method. The recommendation on screening for latent tuberculosis (TB) is an update from the one made in 1996.7 At that time, screening for latent infection was performed using a tuberculin skin test (TST). Now a TST or interferon-gamma release assay (IGRA) can be used. Testing with IGRA may be the best option for those who have received a bacille Calmette–Guérin vaccination (because it can cause a false-positive TST) or for those who are not likely to return to have their TST read.
Those at high risk for latent TB include people who were born or have resided in countries with a high TB prevalence, those who have lived in a correctional institution or homeless shelter, and anyone in a high-risk group based on local epidemiology of the disease. (Read more on TB in this month’s Case Report.) Others at high risk are those who are immune suppressed because of infection or medications, and those who work in health care or correctional facilities. Screening of these groups is usually conducted as part of occupational health or is considered part of routine health care.
Syphilis: Screen high-risk individuals in 2 steps. The recommendation on syphilis screening basically reaffirms the one from 2004.8 Those at high risk for syphilis include men who have sex with men (who now account for 90% of new cases), those who are HIV positive, and those who engage in commercial sex work. Other racial and demographic groups can be at high risk depending on the local epidemiology of the disease. In a separate recommendation, the Task Force advises screening all pregnant women for syphilis.
Screening for syphilis infection involves 2 steps: first, a nontreponemal test (Venereal Disease Research Laboratory [VDRL] or rapid plasma reagin [RPR] test); second, a confirmatory treponemal antibody detection test (fluorescent treponemal antibody absorption [FTA-ABS] or Treponema pallidum particle agglutination [TPPA] test). Treatment for syphilis remains benzathine penicillin with the number of injections depending on the stage of infection. The Centers for Disease Control and Prevention is the best source for current recommendations for treatment of all sexually transmitted infections.15
Screening tests to avoid
TABLE 416,17 lists screening tests the Task Force recommends against. While chronic obstructive pulmonary disease afflicts 14% of US adults ages 40 to 79 years and is the third leading cause of death in the country, the Task Force found that early detection in asymptomatic adults does not affect the course of the illness and is of no benefit.16

Genital herpes, also prevalent, infects an estimated one out of 6 individuals, ages 14 to 49. It causes little mortality, except in neonates, but those infected can have recurrent flares and suffer psychological harms from stigmatization. Most genital herpes is caused by herpes simplex virus-2, and there is a serological test to detect it. However, the Task Force recommends against using the test to screen asymptomatic adults and adolescents, including those who are pregnant. This recommendation is based on the test’s high false-positive rate, which can cause emotional harm, and on the lack of evidence that detection through screening improves outcomes.17
The evidence is lacking for these practices
The Task Force is one of only a few organizations that will not make a recommendation if evidence is lacking on benefits and harms. In addition to the ‘I’ statements regarding CVD and CRC mentioned earlier, the Task Force found insufficient evidence to recommend screening for lipid disorders in individuals ages 20 years or younger,10 performing a visual skin exam as a screening tool for skin cancer,11 screening for celiac disease,12 performing a periodic pelvic examination in asymptomatic women,13 and screening for obstructive sleep apnea using screening questionnaires14 (TABLE 33,4,10-14).
SIDEBAR
A change for prostate cancer screening?The USPSTF recently issued new draft recommendations regarding prostate cancer screening in asymptomatic men (available at: https://screeningforprostatecancer.org/).
The draft now divides men into 2 age groups, stating that the decision to screen for prostate cancer using a prostate specific antigen (PSA) test should be individualized for men ages 55 to 69 years (a C recommendation, meaning that there is at least moderate certainty that the net benefit is small), and that men ages 70 and older (lowered from age 75 in the previous 2012 recommendation1) should not be screened (a D recommendation, meaning that there is moderate or high certainty that the service has no net benefit or that the harms outweigh the benefits).
The USPSTF believes that clinicians should explain to men ages 55 to 69 years that screening offers a small potential benefit of reducing the chance of dying from prostate cancer, but also comes with potential harms, including false-positive results requiring additional testing/procedures, overdiagnosis and overtreatment, and treatment complications such as incontinence and impotence. In this way, each man has the chance to incorporate his values and preferences into the decision.
For men ages 70 and older, the potential benefits simply do not outweigh the potential harms, according to the USPSTF.
1. USPSTF. Final recommendation statement. Prostate cancer: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/prostate-cancer-screening#Pod1. Accessed April 11, 2017.
Since the last Practice Alert update on the United States Preventive Services Task Force in May of 2016,1 the Task Force has released 19 recommendations on 13 topics that include: the use of aspirin and statins for the prevention of cardiovascular disease (CVD); support for breastfeeding; use of folic acid during pregnancy; and screening for syphilis, latent tuberculosis (TB), herpes, chronic obstructive pulmonary disease (COPD), colorectal cancer (CRC), obstructive sleep apnea (OSA), celiac disease, and skin cancer. The Task Force also released a draft recommendation regarding prostate cancer screening in asymptomatic men (see “A change for prostate cancer screening?”) and addressed screening pelvic examinations in asymptomatic women, the subject of this month’s audiocast. (To listen, go to: http://bit.ly/2nIVoD5.)
Recommendations to implement
Recommendations from the past year that family physicians should put into practice are detailed below and in TABLE 1.2-8

CRC: Screen all individuals ages 50 to 75, but 76 to 85 selectively. The Task Force reaffirmed its 2008 finding that screening for CRC in adults ages 50 to 75 years is substantially beneficial.2 In contrast to the previous recommendation, however, the new one does not state which screening tests are preferred. The tests considered were 3 stool tests (fecal immunochemical test [FIT], FIT-tumor DNA testing [FIT-DNA], and guaiac-based fecal occult blood test [gFOBT]), as well as 3 direct visualization tests (colonoscopy, sigmoidoscopy, and CT colonoscopy). The Task Force assessed various testing frequencies of each test and some test combinations. While the Task Force does not recommend any one screening strategy, there are still significant unknowns about FIT-DNA and CT colonoscopy. The American Academy of Family Physicians does not recommend using these 2 tests for screening purposes at this time.9
CRC screening for adults ages 76 to 85 was given a “C” recommendation, which means the value of the service to the population overall is small, but that certain individuals may benefit from it. The Task Force advises selectively offering a “C” service to individuals based on professional judgment and patient preferences. Regarding CRC screening in individuals 76 years or older, the ones most likely to benefit are those who have never been screened and those without significant comorbidities that could limit life expectancy. All “C” recommendations from the past year are listed in TABLE 2.2-4

CVD prevention: When aspirin or a statin is indicated. The Task Force released 2 recommendations for the prevention of CVD this past year. One pertained to the use of low-dose aspirin3 (which also helps to prevent CRC), and the other addressed the use of low- to moderate-dose statins.4 Each recommendation is fairly complicated and nuanced in terms of age and risk for CVD. A decision to use low-dose aspirin must also consider the risk of bleeding.
To calculate a patient’s risk for CVD, the Task Force recommends using the risk calculator developed by the American College of Cardiology and the American Heart Association (http://www.cvriskcalculator.com/).
Adults for whom low-dose aspirin and low- to moderate-dose statins are recommended are described in TABLE 1.2-8 Patients for whom individual decision making is advised, rather than a generalized recommendation, are reviewed in TABLE 2.2-4 There is insufficient evidence to make a recommendation for the use of aspirin before age 50 or at age 70 and older,3 and for the use of statins in adults age 76 and older who do not have a history of CVD4 (TABLE 33,4,10-14). The use of low-dose aspirin and low-to-moderate dose statins have been the subject of JFP audiocasts in May 2016 and January 2017. (See http://bit.ly/2oiun8d and http://bit.ly/2oqkohR.)

2 pregnancy-related recommendations. To prevent neural tube defects in newborns, the Task Force now recommends daily folic acid, 0.4 to 0.8 mg (400 to 800 mcg), for all women who are planning on or are capable of becoming pregnant.5 This is an update of a 2009 recommendation that was worded slightly differently, recommending the supplement for all women of childbearing age.
A new recommendation on breastfeeding recognizes its benefits for both mother and baby and finds that interventions to encourage breastfeeding increase the prevalence of this practice and its duration.6 Interventions—provided to individuals or groups by professionals or peers or through formal education—include promoting the benefits of breastfeeding, giving practical advice and direct support on how to breastfeed, and offering psychological support.
Latent TB: Advantages of newer testing method. The recommendation on screening for latent tuberculosis (TB) is an update from the one made in 1996.7 At that time, screening for latent infection was performed using a tuberculin skin test (TST). Now a TST or interferon-gamma release assay (IGRA) can be used. Testing with IGRA may be the best option for those who have received a bacille Calmette–Guérin vaccination (because it can cause a false-positive TST) or for those who are not likely to return to have their TST read.
Those at high risk for latent TB include people who were born or have resided in countries with a high TB prevalence, those who have lived in a correctional institution or homeless shelter, and anyone in a high-risk group based on local epidemiology of the disease. (Read more on TB in this month’s Case Report.) Others at high risk are those who are immune suppressed because of infection or medications, and those who work in health care or correctional facilities. Screening of these groups is usually conducted as part of occupational health or is considered part of routine health care.
Syphilis: Screen high-risk individuals in 2 steps. The recommendation on syphilis screening basically reaffirms the one from 2004.8 Those at high risk for syphilis include men who have sex with men (who now account for 90% of new cases), those who are HIV positive, and those who engage in commercial sex work. Other racial and demographic groups can be at high risk depending on the local epidemiology of the disease. In a separate recommendation, the Task Force advises screening all pregnant women for syphilis.
Screening for syphilis infection involves 2 steps: first, a nontreponemal test (Venereal Disease Research Laboratory [VDRL] or rapid plasma reagin [RPR] test); second, a confirmatory treponemal antibody detection test (fluorescent treponemal antibody absorption [FTA-ABS] or Treponema pallidum particle agglutination [TPPA] test). Treatment for syphilis remains benzathine penicillin with the number of injections depending on the stage of infection. The Centers for Disease Control and Prevention is the best source for current recommendations for treatment of all sexually transmitted infections.15
Screening tests to avoid
TABLE 416,17 lists screening tests the Task Force recommends against. While chronic obstructive pulmonary disease afflicts 14% of US adults ages 40 to 79 years and is the third leading cause of death in the country, the Task Force found that early detection in asymptomatic adults does not affect the course of the illness and is of no benefit.16

Genital herpes, also prevalent, infects an estimated one out of 6 individuals, ages 14 to 49. It causes little mortality, except in neonates, but those infected can have recurrent flares and suffer psychological harms from stigmatization. Most genital herpes is caused by herpes simplex virus-2, and there is a serological test to detect it. However, the Task Force recommends against using the test to screen asymptomatic adults and adolescents, including those who are pregnant. This recommendation is based on the test’s high false-positive rate, which can cause emotional harm, and on the lack of evidence that detection through screening improves outcomes.17
The evidence is lacking for these practices
The Task Force is one of only a few organizations that will not make a recommendation if evidence is lacking on benefits and harms. In addition to the ‘I’ statements regarding CVD and CRC mentioned earlier, the Task Force found insufficient evidence to recommend screening for lipid disorders in individuals ages 20 years or younger,10 performing a visual skin exam as a screening tool for skin cancer,11 screening for celiac disease,12 performing a periodic pelvic examination in asymptomatic women,13 and screening for obstructive sleep apnea using screening questionnaires14 (TABLE 33,4,10-14).
SIDEBAR
A change for prostate cancer screening?The USPSTF recently issued new draft recommendations regarding prostate cancer screening in asymptomatic men (available at: https://screeningforprostatecancer.org/).
The draft now divides men into 2 age groups, stating that the decision to screen for prostate cancer using a prostate specific antigen (PSA) test should be individualized for men ages 55 to 69 years (a C recommendation, meaning that there is at least moderate certainty that the net benefit is small), and that men ages 70 and older (lowered from age 75 in the previous 2012 recommendation1) should not be screened (a D recommendation, meaning that there is moderate or high certainty that the service has no net benefit or that the harms outweigh the benefits).
The USPSTF believes that clinicians should explain to men ages 55 to 69 years that screening offers a small potential benefit of reducing the chance of dying from prostate cancer, but also comes with potential harms, including false-positive results requiring additional testing/procedures, overdiagnosis and overtreatment, and treatment complications such as incontinence and impotence. In this way, each man has the chance to incorporate his values and preferences into the decision.
For men ages 70 and older, the potential benefits simply do not outweigh the potential harms, according to the USPSTF.
1. USPSTF. Final recommendation statement. Prostate cancer: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/prostate-cancer-screening#Pod1. Accessed April 11, 2017.
1. Campos-Outcalt D. Eight USPSTF recommendations FPs need to know about. J Fam Pract. 2016;65:338-341.
2. USPSTF. Colorectal cancer: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/colorectal-cancer-screening2. Accessed March 22, 2017.
3. USPSTF. Aspirin use to prevent cardiovascular disease and colorectal cancer: preventive medications. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/aspirin-to-prevent-cardiovascular-disease-and-cancer. Accessed March 22, 2017.
4. USPSTF. Statin use for the prevention of cardiovascular disease in adults: preventive medication. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/statin-use-in-adults-preventive-medication1. Accessed March 22, 2017.
5. USPSTF. Folic acid for the prevention of neural tube defects: preventive medication. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/folic-acid-for-the-prevention-of-neural-tube-defects-preventive-medication. Accessed March 22, 2017.
6. USPSTF. Breastfeeding: primary care interventions. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/breastfeeding-primary-care-interventions. Accessed March 22, 2017.
7. USPSTF. Latent tuberculosis infection: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/latent-tuberculosis-infection-screening. Accessed March 22, 2017.
8. USPSTF. Syphilis infection in nonpregnant adults and adolescents: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/syphilis-infection-in-nonpregnant-adults-and-adolescents. Accessed March 22, 2017.
9. AAFP. Colorectal cancer screening, adults. Available at: http://www.aafp.org/patient-care/clinical-recommendations/all/colorectal-cancer.html. Accessed March 22, 2017.
10. USPSTF. Lipid disorders in children and adolescents: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/lipid-disorders-in-children-screening1. Accessed March 22, 2017.
11. USPSTF. Skin cancer: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/skin-cancer-screening2. Accessed March 22, 2017.
12. USPSTF. Celiac disease: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/celiac-disease-screening. Accessed March 22, 2017.
13. USPSTF. Gynecological conditions: periodic screening with the pelvic examination. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/gynecological-conditions-screening-with-the-pelvic-examination. Accessed March 22, 2017.
14. USPSTF. Obstructive sleep apnea in adults: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/obstructive-sleep-apnea-in-adults-screening. Accessed March 22, 2017.
15. CDC. 2015 sexually transmitted diseases treatment guidelines. Available at: https://www.cdc.gov/std/tg2015/default.htm. Accessed March 22, 2017.
16. USPSTF. Chronic obstructive pulmonary disease: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/chronic-obstructive-pulmonary-disease-screening. Accessed March 22, 2017.
17. USPSTF. Genital herpes infection: serologic screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/genital-herpes-screening1. Accessed March 22, 2017.
1. Campos-Outcalt D. Eight USPSTF recommendations FPs need to know about. J Fam Pract. 2016;65:338-341.
2. USPSTF. Colorectal cancer: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/colorectal-cancer-screening2. Accessed March 22, 2017.
3. USPSTF. Aspirin use to prevent cardiovascular disease and colorectal cancer: preventive medications. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/aspirin-to-prevent-cardiovascular-disease-and-cancer. Accessed March 22, 2017.
4. USPSTF. Statin use for the prevention of cardiovascular disease in adults: preventive medication. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/statin-use-in-adults-preventive-medication1. Accessed March 22, 2017.
5. USPSTF. Folic acid for the prevention of neural tube defects: preventive medication. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/folic-acid-for-the-prevention-of-neural-tube-defects-preventive-medication. Accessed March 22, 2017.
6. USPSTF. Breastfeeding: primary care interventions. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/breastfeeding-primary-care-interventions. Accessed March 22, 2017.
7. USPSTF. Latent tuberculosis infection: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/latent-tuberculosis-infection-screening. Accessed March 22, 2017.
8. USPSTF. Syphilis infection in nonpregnant adults and adolescents: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/syphilis-infection-in-nonpregnant-adults-and-adolescents. Accessed March 22, 2017.
9. AAFP. Colorectal cancer screening, adults. Available at: http://www.aafp.org/patient-care/clinical-recommendations/all/colorectal-cancer.html. Accessed March 22, 2017.
10. USPSTF. Lipid disorders in children and adolescents: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/lipid-disorders-in-children-screening1. Accessed March 22, 2017.
11. USPSTF. Skin cancer: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/skin-cancer-screening2. Accessed March 22, 2017.
12. USPSTF. Celiac disease: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/celiac-disease-screening. Accessed March 22, 2017.
13. USPSTF. Gynecological conditions: periodic screening with the pelvic examination. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/gynecological-conditions-screening-with-the-pelvic-examination. Accessed March 22, 2017.
14. USPSTF. Obstructive sleep apnea in adults: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/obstructive-sleep-apnea-in-adults-screening. Accessed March 22, 2017.
15. CDC. 2015 sexually transmitted diseases treatment guidelines. Available at: https://www.cdc.gov/std/tg2015/default.htm. Accessed March 22, 2017.
16. USPSTF. Chronic obstructive pulmonary disease: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/chronic-obstructive-pulmonary-disease-screening. Accessed March 22, 2017.
17. USPSTF. Genital herpes infection: serologic screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/genital-herpes-screening1. Accessed March 22, 2017.
ACIP vaccine update, 2017
The Advisory Committee on Immunization Practices (ACIP) met 3 times in 2016 and introduced or revised recommendations on influenza, meningococcal, human papillomavirus (HPV), cholera, and hepatitis B vaccines. This Practice Alert highlights the most important new recommendations, except those for influenza vaccines, which were described in a previous Practice Alert.1 (See the summary of how this year’s flu season compares to last year’s.)
SIDEBAR
PRACTICE ALERT UPDATE
How this year's flu season compares to last yearThe 2016-2017 influenza season has been relatively mild, with activity nationwide picking up in late January and continuing to increase in February. As of February 16, 90% of the infections typed were type A, and most of those cases (more than 90%) were H3N1. Not surprisingly, the age group most heavily affected has been the elderly.
The hospitalization rate among those ≥65 years as of early February was 113.5/100,000, which is about half the rate of the same week during the 2014-2015 flu season. The hospitalization rate among those ages 50 to 64 years was 23.5/100,000—about 40% lower than the rate during the same week last flu season. At press time, 20 pediatric deaths had occurred, which is less than one-quarter of the number that occurred during the same time last year, and resistance to oseltamivir had not yet been detected in any isolates.
Source: Centers for Disease Control and Prevention. Situation update: summary of weekly FluView report. Available at: https://www.cdc.gov/flu/weekly/summary.htm. Accessed February 16, 2017.
Meningococcal vaccine: Now recommended for HIV-positive patients
Meningococcal conjugate vaccine (serogroups A, C, W, and Y) is recommended for all adolescents ages 11 to 12 as a single dose with a booster at age 16.2 It is also recommended for adults and for children (starting at age 2 months) who have high-risk conditions such as functional or anatomic asplenia or complement deficiencies. Others at high risk include microbiologists routinely exposed to isolates of Neisseria meningitidis and those traveling to areas of high meningococcal incidence. ACIP recently added human immunodeficiency virus (HIV) infection to the list of high-risk conditions.3
Two meningococcal conjugate vaccines are available in the United States: Menactra, (Sanofi Pasteur), licensed for use in individuals ages 9 months to 55 years; and Menveo (GlaxoSmithKline), licensed for use in individuals ages 2 months to 55 years. Menveo is the preferred vaccine for children younger than 2 years infected with HIV. However, if Menactra is used, give it at least 4 weeks after completing all pneumococcal conjugate vaccine doses and either before or concomitantly with diphtheria and tetanus toxoid and acellular pertussis vaccine (DTaP). All individuals who are HIV positive should receive a multi-dose primary series and booster doses. The number of primary doses and timing of boosters depends on the product used and the ages of those vaccinated (TABLE3).
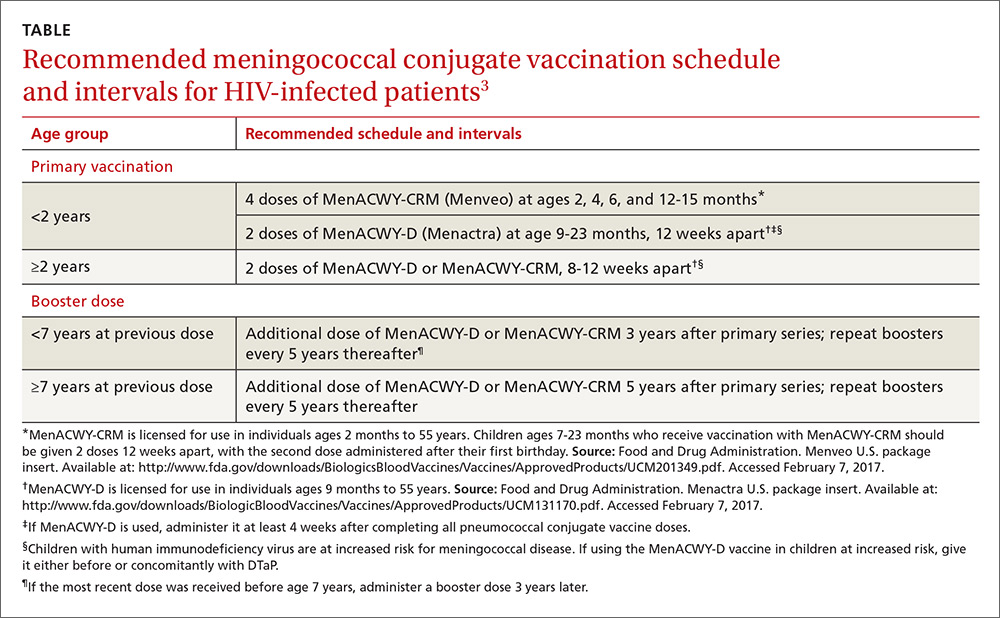
Although neither meningococcal conjugate vaccine product is licensed for use in individuals 56 years or older, ACIP recommends using one of the products for HIV-infected individuals in this age group because the only meningococcal vaccine licensed for use in adults 56 or older, meningococcal polysaccharide vaccine (MPSV4, Menomune, Sanofi Pasteur), has not been studied in patients with HIV infection.
Serogroup B. Two vaccine products provide short-term protection against meningococcal serogroup B: MenB-FHbp (Trumenba, Wyeth Pharmaceuticals, Inc.) and MenB-4C (Bexsero, GlaxoSmithKline). In 2015, ACIP made a “B” recommendation for the use of these vaccines in individuals 16 to 23 years of age, with the preferred age range being 16 to 18.4 A “B” recommendation means that while ACIP does not advise routine use of the vaccines in this age group, the vaccines can be administered to those who desire them. ACIP has recommended routine use of these products only for individuals 10 years and older who are at high risk for meningococcal disease.5
Trumenba was approved as a 3-dose vaccine, administered at 0, 2, and 6 months. Bexsero requires 2 doses given at least one month apart. At its October 2016 meeting, ACIP approved a 2-dose Trumenba schedule, at 0 and 6 months, when administered to those not at risk for meningococcal disease.6 However, during an outbreak, and for those at high risk for meningococcal disease, adhere to the original 3-dose schedule.
HPV vaccine: Now a 2-dose schedule for younger patients
The only HPV vaccine available in the United States is the 9-valent HPV vaccine (9vHPV), Gardasil 9. It is approved for both males and females ages 9 to 26 years. ACIP recommends it for both sexes at ages 11 or 12, and advises catch-up doses for men through age 21 and women through age 26. It also recommends vaccination through age 26 for men who have sex with men and men
The HPV vaccine is approved for a 3-dose schedule at 0, 1 to 2, and 6 months. At its October 2016 meeting, ACIP approved a 2-dose schedule (0, 6-12 months) for those starting the vaccine before their 15th birthday.7 Those starting the vaccine after their 15th birthday, and individuals at any age with an immune-compromising condition, should receive 3 doses. It is hoped that a 2-dose schedule will help to increase the uptake of this safe, effective, and underused vaccine.
Cholera: A new vaccine is available
In June 2016, the FDA approved a live, attenuated, single-dose, oral vaccine (Vaxchora, PaxVax, Inc.) for the prevention of cholera in adults ages 18 to 64 years. It is the only cholera vaccine approved in the United States.
Cholera occurs at low rates among travelers to areas where the disease is endemic. The key to prevention is food and water precautions, and thus the vaccine is not recommended for most travelers—only for those who are at increased risk of exposure to cholera or who have a medical condition that predisposes them to a poor response to medical care if cholera is contracted.8 Risk increases with long-term or frequent travel to endemic areas where safe food and water is not always available. Examples of compromising medical conditions include a blood type O, low gastric acidity, and heart or kidney disease.
Duration of the vaccine’s effectiveness is unknown, given a lack of data beyond 6 months. No recommendation for revaccination has been made, and this issue will be assessed as more data are collected. Other unknowns about the vaccine include its effectiveness among immune-suppressed individuals and pregnant women, as well as for those who live in cholera endemic areas or were previously vaccinated with another cholera vaccine.
Hepatitis B: Vaccinate newborns sooner
The incidence of hepatitis B virus (HBV) infection has declined by more than 90% since the introduction of a vaccine in 1982.9
Current recommendations for the prevention of HBV include:9
- Screen all pregnant women for hepatitis B surface antigen (HBsAg), and use HBIG and hepatitis B vaccines within 12 hours of birth for all newborns whose mothers are HBsAg positive or have an unknown HBsAg status.
- Administer the 3-dose hepatitis B vaccine to all other infants.
- Routinely vaccinate previously unvaccinated children and adolescents.
- Routinely vaccinate adults who are non-immune and at risk for HBV infection.
At its October 2016 meeting, ACIP adopted a comprehensive update of all HBV prevention recommendations. (This will be the subject of a future Practice Alert.) Included was a revision of a previously permissive recommendation that allowed the first dose of hepatitis B vaccine for newborns to be given within 2 months of hospital discharge. The new recommendation9 states that newborns of mothers known to be HBsAg negative should be vaccinated within 24 hours (if weight is ≥2000 g) or at age one month or at hospital discharge (if weight is <2000 g).
The first dose should be given within 12 hours of birth to all newborns whose mothers are HBsAg positive or have an unknown HBsAg status.9
Immunization schedules
Every year ACIP updates the adult and child immunization schedules to incorporate the changes from the previous year. These can be found on the ACIP Web site at https://www.cdc.gov/vaccines/schedules/hcp/index.html. This Web site remains the most authoritative and accurate source of information on vaccines and immunizations for both professionals and the public.
1. Campos-Outcalt D. Need-to-know information for the 2016-2017 flu season. J Fam Pract. 2016;65:613-617.
2. Cohn AC, MacNeil JR, Clark TA, et al. Prevention and control of meningococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2013;62:1-28.
3. MacNeil JR, Rubin LG, Patton M, et al. Recommendations for use of meningococcal conjugate vaccines in HIV-infected persons— Advisory Committee on Immunization Practices, 2016. MMWR Morb Mortal Wkly Rep. 2016;65:1189-1194.
4. MacNeil JR, Rubin LG, Folaranmi T, et al. Use of serogroup B meningococcal vaccines in adolescents and young adults: recommendations of the Advisory Committee on Immunization Practices, 2015. MMWR Morb Mortal Wkly Rep. 2015;64:1171-1176.
5. Folaranmi T, Rubin L, Martin SW, et al. Use of serogroup B meningococcal vaccines in persons aged ≥10 years at increased risk for serogroup B meningococcal disease: recommendations of the Advisory Committee on Immunization Practices, 2015. MMWR Morb Mortal Wkly Rep. 2015;64:608-612.
6. MacNeil J. Considerations for Use of 2- and 3-Dose Schedules of MenB-FHbp (Trumenba). Presentation at: Advisory Committee on Immunization Practices; October 19, 2016; Atlanta, GA. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2016-10/meningococcal-05-macneil.pdf. Accessed February 6, 2017.
7. Meites E, Kempe A, Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination—updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2016;65:1405-1408.
8. Wong KW. Cholera vaccine update and proposed recommendations. Presentation at: Advisory Committee on Immunization Practices; June 22, 2016; Atlanta, GA. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2016-06/cholera-02-wong.pdf. Accessed January 27, 2017.
9. Schillie S. Revised ACIP Hepatitis B (HepB) vaccine recommendations. Presentation at: Advisory Committee on Immunization Practices; October 19, 2016. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2016-10/hepatitis-02-schillie-october-2016.pdf. Accessed January 27, 2017.
10.
11. Ko SC, Fan L, Smith EA, et al. Estimated annual perinatal hepatitis B virus infections in the United States, 2000-2009. J Pediatric Infect Dis Soc. 2016;5:114-121.
12. Mast EE, Weinbaum CM, Fiore AE, et al. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States. MMWR Morb Mortal Wkly Rep. 2006;55:1-25.
13. Beasley RP, Hwang LY, Lee GC, et al. Prevention of perinatally transmitted hepatitis B virus infections with hepatitis B immune globulin and hepatitis B vaccine. Lancet. 1983;2:1099-1102.
The Advisory Committee on Immunization Practices (ACIP) met 3 times in 2016 and introduced or revised recommendations on influenza, meningococcal, human papillomavirus (HPV), cholera, and hepatitis B vaccines. This Practice Alert highlights the most important new recommendations, except those for influenza vaccines, which were described in a previous Practice Alert.1 (See the summary of how this year’s flu season compares to last year’s.)
SIDEBAR
PRACTICE ALERT UPDATE
How this year's flu season compares to last yearThe 2016-2017 influenza season has been relatively mild, with activity nationwide picking up in late January and continuing to increase in February. As of February 16, 90% of the infections typed were type A, and most of those cases (more than 90%) were H3N1. Not surprisingly, the age group most heavily affected has been the elderly.
The hospitalization rate among those ≥65 years as of early February was 113.5/100,000, which is about half the rate of the same week during the 2014-2015 flu season. The hospitalization rate among those ages 50 to 64 years was 23.5/100,000—about 40% lower than the rate during the same week last flu season. At press time, 20 pediatric deaths had occurred, which is less than one-quarter of the number that occurred during the same time last year, and resistance to oseltamivir had not yet been detected in any isolates.
Source: Centers for Disease Control and Prevention. Situation update: summary of weekly FluView report. Available at: https://www.cdc.gov/flu/weekly/summary.htm. Accessed February 16, 2017.
Meningococcal vaccine: Now recommended for HIV-positive patients
Meningococcal conjugate vaccine (serogroups A, C, W, and Y) is recommended for all adolescents ages 11 to 12 as a single dose with a booster at age 16.2 It is also recommended for adults and for children (starting at age 2 months) who have high-risk conditions such as functional or anatomic asplenia or complement deficiencies. Others at high risk include microbiologists routinely exposed to isolates of Neisseria meningitidis and those traveling to areas of high meningococcal incidence. ACIP recently added human immunodeficiency virus (HIV) infection to the list of high-risk conditions.3
Two meningococcal conjugate vaccines are available in the United States: Menactra, (Sanofi Pasteur), licensed for use in individuals ages 9 months to 55 years; and Menveo (GlaxoSmithKline), licensed for use in individuals ages 2 months to 55 years. Menveo is the preferred vaccine for children younger than 2 years infected with HIV. However, if Menactra is used, give it at least 4 weeks after completing all pneumococcal conjugate vaccine doses and either before or concomitantly with diphtheria and tetanus toxoid and acellular pertussis vaccine (DTaP). All individuals who are HIV positive should receive a multi-dose primary series and booster doses. The number of primary doses and timing of boosters depends on the product used and the ages of those vaccinated (TABLE3).

Although neither meningococcal conjugate vaccine product is licensed for use in individuals 56 years or older, ACIP recommends using one of the products for HIV-infected individuals in this age group because the only meningococcal vaccine licensed for use in adults 56 or older, meningococcal polysaccharide vaccine (MPSV4, Menomune, Sanofi Pasteur), has not been studied in patients with HIV infection.
Serogroup B. Two vaccine products provide short-term protection against meningococcal serogroup B: MenB-FHbp (Trumenba, Wyeth Pharmaceuticals, Inc.) and MenB-4C (Bexsero, GlaxoSmithKline). In 2015, ACIP made a “B” recommendation for the use of these vaccines in individuals 16 to 23 years of age, with the preferred age range being 16 to 18.4 A “B” recommendation means that while ACIP does not advise routine use of the vaccines in this age group, the vaccines can be administered to those who desire them. ACIP has recommended routine use of these products only for individuals 10 years and older who are at high risk for meningococcal disease.5
Trumenba was approved as a 3-dose vaccine, administered at 0, 2, and 6 months. Bexsero requires 2 doses given at least one month apart. At its October 2016 meeting, ACIP approved a 2-dose Trumenba schedule, at 0 and 6 months, when administered to those not at risk for meningococcal disease.6 However, during an outbreak, and for those at high risk for meningococcal disease, adhere to the original 3-dose schedule.
HPV vaccine: Now a 2-dose schedule for younger patients
The only HPV vaccine available in the United States is the 9-valent HPV vaccine (9vHPV), Gardasil 9. It is approved for both males and females ages 9 to 26 years. ACIP recommends it for both sexes at ages 11 or 12, and advises catch-up doses for men through age 21 and women through age 26. It also recommends vaccination through age 26 for men who have sex with men and men
The HPV vaccine is approved for a 3-dose schedule at 0, 1 to 2, and 6 months. At its October 2016 meeting, ACIP approved a 2-dose schedule (0, 6-12 months) for those starting the vaccine before their 15th birthday.7 Those starting the vaccine after their 15th birthday, and individuals at any age with an immune-compromising condition, should receive 3 doses. It is hoped that a 2-dose schedule will help to increase the uptake of this safe, effective, and underused vaccine.
Cholera: A new vaccine is available
In June 2016, the FDA approved a live, attenuated, single-dose, oral vaccine (Vaxchora, PaxVax, Inc.) for the prevention of cholera in adults ages 18 to 64 years. It is the only cholera vaccine approved in the United States.
Cholera occurs at low rates among travelers to areas where the disease is endemic. The key to prevention is food and water precautions, and thus the vaccine is not recommended for most travelers—only for those who are at increased risk of exposure to cholera or who have a medical condition that predisposes them to a poor response to medical care if cholera is contracted.8 Risk increases with long-term or frequent travel to endemic areas where safe food and water is not always available. Examples of compromising medical conditions include a blood type O, low gastric acidity, and heart or kidney disease.
Duration of the vaccine’s effectiveness is unknown, given a lack of data beyond 6 months. No recommendation for revaccination has been made, and this issue will be assessed as more data are collected. Other unknowns about the vaccine include its effectiveness among immune-suppressed individuals and pregnant women, as well as for those who live in cholera endemic areas or were previously vaccinated with another cholera vaccine.
Hepatitis B: Vaccinate newborns sooner
The incidence of hepatitis B virus (HBV) infection has declined by more than 90% since the introduction of a vaccine in 1982.9
Current recommendations for the prevention of HBV include:9
- Screen all pregnant women for hepatitis B surface antigen (HBsAg), and use HBIG and hepatitis B vaccines within 12 hours of birth for all newborns whose mothers are HBsAg positive or have an unknown HBsAg status.
- Administer the 3-dose hepatitis B vaccine to all other infants.
- Routinely vaccinate previously unvaccinated children and adolescents.
- Routinely vaccinate adults who are non-immune and at risk for HBV infection.
At its October 2016 meeting, ACIP adopted a comprehensive update of all HBV prevention recommendations. (This will be the subject of a future Practice Alert.) Included was a revision of a previously permissive recommendation that allowed the first dose of hepatitis B vaccine for newborns to be given within 2 months of hospital discharge. The new recommendation9 states that newborns of mothers known to be HBsAg negative should be vaccinated within 24 hours (if weight is ≥2000 g) or at age one month or at hospital discharge (if weight is <2000 g).
The first dose should be given within 12 hours of birth to all newborns whose mothers are HBsAg positive or have an unknown HBsAg status.9
Immunization schedules
Every year ACIP updates the adult and child immunization schedules to incorporate the changes from the previous year. These can be found on the ACIP Web site at https://www.cdc.gov/vaccines/schedules/hcp/index.html. This Web site remains the most authoritative and accurate source of information on vaccines and immunizations for both professionals and the public.
The Advisory Committee on Immunization Practices (ACIP) met 3 times in 2016 and introduced or revised recommendations on influenza, meningococcal, human papillomavirus (HPV), cholera, and hepatitis B vaccines. This Practice Alert highlights the most important new recommendations, except those for influenza vaccines, which were described in a previous Practice Alert.1 (See the summary of how this year’s flu season compares to last year’s.)
SIDEBAR
PRACTICE ALERT UPDATE
How this year's flu season compares to last yearThe 2016-2017 influenza season has been relatively mild, with activity nationwide picking up in late January and continuing to increase in February. As of February 16, 90% of the infections typed were type A, and most of those cases (more than 90%) were H3N1. Not surprisingly, the age group most heavily affected has been the elderly.
The hospitalization rate among those ≥65 years as of early February was 113.5/100,000, which is about half the rate of the same week during the 2014-2015 flu season. The hospitalization rate among those ages 50 to 64 years was 23.5/100,000—about 40% lower than the rate during the same week last flu season. At press time, 20 pediatric deaths had occurred, which is less than one-quarter of the number that occurred during the same time last year, and resistance to oseltamivir had not yet been detected in any isolates.
Source: Centers for Disease Control and Prevention. Situation update: summary of weekly FluView report. Available at: https://www.cdc.gov/flu/weekly/summary.htm. Accessed February 16, 2017.
Meningococcal vaccine: Now recommended for HIV-positive patients
Meningococcal conjugate vaccine (serogroups A, C, W, and Y) is recommended for all adolescents ages 11 to 12 as a single dose with a booster at age 16.2 It is also recommended for adults and for children (starting at age 2 months) who have high-risk conditions such as functional or anatomic asplenia or complement deficiencies. Others at high risk include microbiologists routinely exposed to isolates of Neisseria meningitidis and those traveling to areas of high meningococcal incidence. ACIP recently added human immunodeficiency virus (HIV) infection to the list of high-risk conditions.3
Two meningococcal conjugate vaccines are available in the United States: Menactra, (Sanofi Pasteur), licensed for use in individuals ages 9 months to 55 years; and Menveo (GlaxoSmithKline), licensed for use in individuals ages 2 months to 55 years. Menveo is the preferred vaccine for children younger than 2 years infected with HIV. However, if Menactra is used, give it at least 4 weeks after completing all pneumococcal conjugate vaccine doses and either before or concomitantly with diphtheria and tetanus toxoid and acellular pertussis vaccine (DTaP). All individuals who are HIV positive should receive a multi-dose primary series and booster doses. The number of primary doses and timing of boosters depends on the product used and the ages of those vaccinated (TABLE3).

Although neither meningococcal conjugate vaccine product is licensed for use in individuals 56 years or older, ACIP recommends using one of the products for HIV-infected individuals in this age group because the only meningococcal vaccine licensed for use in adults 56 or older, meningococcal polysaccharide vaccine (MPSV4, Menomune, Sanofi Pasteur), has not been studied in patients with HIV infection.
Serogroup B. Two vaccine products provide short-term protection against meningococcal serogroup B: MenB-FHbp (Trumenba, Wyeth Pharmaceuticals, Inc.) and MenB-4C (Bexsero, GlaxoSmithKline). In 2015, ACIP made a “B” recommendation for the use of these vaccines in individuals 16 to 23 years of age, with the preferred age range being 16 to 18.4 A “B” recommendation means that while ACIP does not advise routine use of the vaccines in this age group, the vaccines can be administered to those who desire them. ACIP has recommended routine use of these products only for individuals 10 years and older who are at high risk for meningococcal disease.5
Trumenba was approved as a 3-dose vaccine, administered at 0, 2, and 6 months. Bexsero requires 2 doses given at least one month apart. At its October 2016 meeting, ACIP approved a 2-dose Trumenba schedule, at 0 and 6 months, when administered to those not at risk for meningococcal disease.6 However, during an outbreak, and for those at high risk for meningococcal disease, adhere to the original 3-dose schedule.
HPV vaccine: Now a 2-dose schedule for younger patients
The only HPV vaccine available in the United States is the 9-valent HPV vaccine (9vHPV), Gardasil 9. It is approved for both males and females ages 9 to 26 years. ACIP recommends it for both sexes at ages 11 or 12, and advises catch-up doses for men through age 21 and women through age 26. It also recommends vaccination through age 26 for men who have sex with men and men
The HPV vaccine is approved for a 3-dose schedule at 0, 1 to 2, and 6 months. At its October 2016 meeting, ACIP approved a 2-dose schedule (0, 6-12 months) for those starting the vaccine before their 15th birthday.7 Those starting the vaccine after their 15th birthday, and individuals at any age with an immune-compromising condition, should receive 3 doses. It is hoped that a 2-dose schedule will help to increase the uptake of this safe, effective, and underused vaccine.
Cholera: A new vaccine is available
In June 2016, the FDA approved a live, attenuated, single-dose, oral vaccine (Vaxchora, PaxVax, Inc.) for the prevention of cholera in adults ages 18 to 64 years. It is the only cholera vaccine approved in the United States.
Cholera occurs at low rates among travelers to areas where the disease is endemic. The key to prevention is food and water precautions, and thus the vaccine is not recommended for most travelers—only for those who are at increased risk of exposure to cholera or who have a medical condition that predisposes them to a poor response to medical care if cholera is contracted.8 Risk increases with long-term or frequent travel to endemic areas where safe food and water is not always available. Examples of compromising medical conditions include a blood type O, low gastric acidity, and heart or kidney disease.
Duration of the vaccine’s effectiveness is unknown, given a lack of data beyond 6 months. No recommendation for revaccination has been made, and this issue will be assessed as more data are collected. Other unknowns about the vaccine include its effectiveness among immune-suppressed individuals and pregnant women, as well as for those who live in cholera endemic areas or were previously vaccinated with another cholera vaccine.
Hepatitis B: Vaccinate newborns sooner
The incidence of hepatitis B virus (HBV) infection has declined by more than 90% since the introduction of a vaccine in 1982.9
Current recommendations for the prevention of HBV include:9
- Screen all pregnant women for hepatitis B surface antigen (HBsAg), and use HBIG and hepatitis B vaccines within 12 hours of birth for all newborns whose mothers are HBsAg positive or have an unknown HBsAg status.
- Administer the 3-dose hepatitis B vaccine to all other infants.
- Routinely vaccinate previously unvaccinated children and adolescents.
- Routinely vaccinate adults who are non-immune and at risk for HBV infection.
At its October 2016 meeting, ACIP adopted a comprehensive update of all HBV prevention recommendations. (This will be the subject of a future Practice Alert.) Included was a revision of a previously permissive recommendation that allowed the first dose of hepatitis B vaccine for newborns to be given within 2 months of hospital discharge. The new recommendation9 states that newborns of mothers known to be HBsAg negative should be vaccinated within 24 hours (if weight is ≥2000 g) or at age one month or at hospital discharge (if weight is <2000 g).
The first dose should be given within 12 hours of birth to all newborns whose mothers are HBsAg positive or have an unknown HBsAg status.9
Immunization schedules
Every year ACIP updates the adult and child immunization schedules to incorporate the changes from the previous year. These can be found on the ACIP Web site at https://www.cdc.gov/vaccines/schedules/hcp/index.html. This Web site remains the most authoritative and accurate source of information on vaccines and immunizations for both professionals and the public.
1. Campos-Outcalt D. Need-to-know information for the 2016-2017 flu season. J Fam Pract. 2016;65:613-617.
2. Cohn AC, MacNeil JR, Clark TA, et al. Prevention and control of meningococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2013;62:1-28.
3. MacNeil JR, Rubin LG, Patton M, et al. Recommendations for use of meningococcal conjugate vaccines in HIV-infected persons— Advisory Committee on Immunization Practices, 2016. MMWR Morb Mortal Wkly Rep. 2016;65:1189-1194.
4. MacNeil JR, Rubin LG, Folaranmi T, et al. Use of serogroup B meningococcal vaccines in adolescents and young adults: recommendations of the Advisory Committee on Immunization Practices, 2015. MMWR Morb Mortal Wkly Rep. 2015;64:1171-1176.
5. Folaranmi T, Rubin L, Martin SW, et al. Use of serogroup B meningococcal vaccines in persons aged ≥10 years at increased risk for serogroup B meningococcal disease: recommendations of the Advisory Committee on Immunization Practices, 2015. MMWR Morb Mortal Wkly Rep. 2015;64:608-612.
6. MacNeil J. Considerations for Use of 2- and 3-Dose Schedules of MenB-FHbp (Trumenba). Presentation at: Advisory Committee on Immunization Practices; October 19, 2016; Atlanta, GA. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2016-10/meningococcal-05-macneil.pdf. Accessed February 6, 2017.
7. Meites E, Kempe A, Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination—updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2016;65:1405-1408.
8. Wong KW. Cholera vaccine update and proposed recommendations. Presentation at: Advisory Committee on Immunization Practices; June 22, 2016; Atlanta, GA. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2016-06/cholera-02-wong.pdf. Accessed January 27, 2017.
9. Schillie S. Revised ACIP Hepatitis B (HepB) vaccine recommendations. Presentation at: Advisory Committee on Immunization Practices; October 19, 2016. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2016-10/hepatitis-02-schillie-october-2016.pdf. Accessed January 27, 2017.
10.
11. Ko SC, Fan L, Smith EA, et al. Estimated annual perinatal hepatitis B virus infections in the United States, 2000-2009. J Pediatric Infect Dis Soc. 2016;5:114-121.
12. Mast EE, Weinbaum CM, Fiore AE, et al. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States. MMWR Morb Mortal Wkly Rep. 2006;55:1-25.
13. Beasley RP, Hwang LY, Lee GC, et al. Prevention of perinatally transmitted hepatitis B virus infections with hepatitis B immune globulin and hepatitis B vaccine. Lancet. 1983;2:1099-1102.
1. Campos-Outcalt D. Need-to-know information for the 2016-2017 flu season. J Fam Pract. 2016;65:613-617.
2. Cohn AC, MacNeil JR, Clark TA, et al. Prevention and control of meningococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2013;62:1-28.
3. MacNeil JR, Rubin LG, Patton M, et al. Recommendations for use of meningococcal conjugate vaccines in HIV-infected persons— Advisory Committee on Immunization Practices, 2016. MMWR Morb Mortal Wkly Rep. 2016;65:1189-1194.
4. MacNeil JR, Rubin LG, Folaranmi T, et al. Use of serogroup B meningococcal vaccines in adolescents and young adults: recommendations of the Advisory Committee on Immunization Practices, 2015. MMWR Morb Mortal Wkly Rep. 2015;64:1171-1176.
5. Folaranmi T, Rubin L, Martin SW, et al. Use of serogroup B meningococcal vaccines in persons aged ≥10 years at increased risk for serogroup B meningococcal disease: recommendations of the Advisory Committee on Immunization Practices, 2015. MMWR Morb Mortal Wkly Rep. 2015;64:608-612.
6. MacNeil J. Considerations for Use of 2- and 3-Dose Schedules of MenB-FHbp (Trumenba). Presentation at: Advisory Committee on Immunization Practices; October 19, 2016; Atlanta, GA. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2016-10/meningococcal-05-macneil.pdf. Accessed February 6, 2017.
7. Meites E, Kempe A, Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination—updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2016;65:1405-1408.
8. Wong KW. Cholera vaccine update and proposed recommendations. Presentation at: Advisory Committee on Immunization Practices; June 22, 2016; Atlanta, GA. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2016-06/cholera-02-wong.pdf. Accessed January 27, 2017.
9. Schillie S. Revised ACIP Hepatitis B (HepB) vaccine recommendations. Presentation at: Advisory Committee on Immunization Practices; October 19, 2016. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2016-10/hepatitis-02-schillie-october-2016.pdf. Accessed January 27, 2017.
10.
11. Ko SC, Fan L, Smith EA, et al. Estimated annual perinatal hepatitis B virus infections in the United States, 2000-2009. J Pediatric Infect Dis Soc. 2016;5:114-121.
12. Mast EE, Weinbaum CM, Fiore AE, et al. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States. MMWR Morb Mortal Wkly Rep. 2006;55:1-25.
13. Beasley RP, Hwang LY, Lee GC, et al. Prevention of perinatally transmitted hepatitis B virus infections with hepatitis B immune globulin and hepatitis B vaccine. Lancet. 1983;2:1099-1102.
CPSTF: A lesser known, but valuable, resource for FPs
Family physicians have come to rely on the US Preventive Services Task Force (USPSTF) for rigorous, evidence-based recommendations on the use of clinical preventive services. Still, many such services reach too few individuals who need them. And that’s where the less well known Community Preventive Services Task Force comes in. The CPSTF makes recommendations regarding public health interventions and ways to increase the use of preventive services in the clinical setting—eg, means of improving childhood immunization rates or increasing screening for cervical, breast, and colon cancer.
To better understand how the CPSTF can serve as a resource to busy family physicians, it’s helpful to first understand a bit about the inner-workings of the CPSTF itself.
How CPSTF figures out what works
Formed in 1996, the CPSTF consists of 15 independent, nonfederal members with expertise in public health and preventive medicine, appointed by the Director of the Centers for Disease Control and Prevention (CDC). The Task Force makes recommendations and develops guidance on which community-based health promotion and disease-prevention interventions work and which do not, based on available scientific evidence. The Task Force uses an evidence-based methodology similar to that of the USPSTF—ie, assessing systematic reviews of the evidence and tying recommendations to the strength of the evidence. However, the Task Force has only 3 levels of recommendations: recommend for, recommend against, and insufficient evidence to recommend.
Three CPSTF meetings are held each year, and a representative from the American Academy of Family Physicians (AAFP) attends as a liaison, along with liaisons from other organizations with an interest in the methods and recommendations. The CDC provides the CPSTF with technical and administrative support. However, the recommendations developed do not undergo review or approval by the CDC and are the sole responsibility of the Task Force.
The recommendations made are contained in the Guide to Community Preventive Services, often called The Community Guide, which is available on the Task Force’s Web site at www.thecommunityguide.org/index.html. The topics on which the CPSTF currently has recommendations are listed in TABLE 1. (Since community-wide recommendations are rarely subjected to controlled clinical trials, methods of assessing and ranking other forms of evidence are required. To learn more about how the CPSTF approaches this, see: https://www.thecommunityguide.org/about/our-methodology.)

Improving immunization rates
The topic of immunizations is an example of how synergistic the CPSTF recommendations can be with those from clinical organizations. The Advisory Committee on Immunization Practices (ACIP) makes recommendations on the use of vaccines.1 The CPSTF has developed a set of recommendations on how to increase the uptake of vaccines to improve rates of immunization.2 Interventions they recommend include vaccine requirements for attendance at preschool, primary and secondary school, and college; patient reminder and recall systems; patient and family incentives and rewards; providing vaccines at Women, Infants, and Children clinics, schools, work sites, and homes; standing orders for vaccine administration; physician reminders; physician assessments and feedback; reducing out-of-pocket expenses for vaccines; and using immunization registries. Just as important, the CPSTF identifies interventions that lack hard evidence to support their effectiveness.
Cancer screening works, but patient buy-in lags
The USPSTF recommends screening for breast, cervical, and colorectal cancer. And yet, despite the proven effectiveness of these screening tests in decreasing cancer mortality, many people do not get screened. The CPSTF has developed a set of implementation recommendations that are proven to increase the uptake of recommended cancer screening tests.3 These include:
- sending reminders to patients when screening tests are due
- providing one-on-one or group educational sessions
- providing videos and printed materials that describe screening tests and recommendations
- offering testing at locations and times that are convenient for patients
- offering on-site translation, transportation, patient navigators, and other administrative services to facilitate screening
- assessing provider performance and providing feedback.
CPSTF’s range of resources
Resources provided by the CPSTF (TABLE 2) also include the following materials for physicians, patients, and policy makers:
- tools to assist communities in performing a community health assessment and in prioritizing health needs
- fact sheets on what works for specific populations or conditions. (One recently added fact sheet is a description of interventions to address the leading health problems that affect women.4)
- examples of how communities have used CPSTF recommendations to address a major health concern in their populations. (See “An immunization ‘success story’ from the field.”)

Tackling controversial social issues
Public health interventions are often politically charged, and the CPSTF at times makes recommendations that, while supported by evidence, raise objections from certain groups. One example is a recommendation for “comprehensive risk reduction interventions to promote behaviors that prevent or reduce the risk of pregnancy, human immunodeficiency virus (HIV), and other sexually transmitted infections (STIs).”5 These interventions may include a hierarchy of recommended behaviors that identifies abstinence as the best or preferred method, but also provides information about sexual risk reduction strategies. Abstinence-only education initiatives were rated as having insufficient evidence for effectiveness.6
Another example that falls in the controversial realm is a recommendation against “policies facilitating the transfer of juveniles from juvenile to adult criminal justice systems for the purpose of reducing violence, based on strong evidence that these laws and policies are associated with increased subsequent violent behavior among transferred youth.”7
And a third example is a recommendation for “the use of regulatory authority (eg, through licensing and zoning) to limit alcohol outlet density on the basis of sufficient evidence of a positive association between outlet density and excessive alcohol consumption and related harms.”8 The CPSTF also recommends increasing taxes on alcohol products to reduce excess alcohol consumption.9
SIDEBAR
An immunization “success story” from the field
Before 2009, the vaccination completion rates for 2-year-olds in Duval County, Florida, consistently ranked below the national target of 90%, with particularly low rates in Jacksonville. With the aim of improving vaccination rates—and not wanting to waste time “reinventing the wheel”—the Duval County Health Department (DCHD) turned to The Community Guide for interventions proven to work synergistically: system-based efforts (eg, client reminders, standing orders, clinic-based education) and community-based efforts (eg, staff outreach to clients, educational activities).
Checking the Florida Shots Registry, clinic staff identified infants and toddlers who were due for, or had missed, vaccinations. They sent monthly reminders to parents, urging them to make appointments. DCHD also provided parents with educational materials, vaccination schedules, and safety evidence to reinforce awareness of the need for immunizations.
At local clinics, DCHD trained staff to administer vaccines and established standing orders authorizing them to do so even in the absence of a physician or other approving practitioner.
DCHD also formed an immunization task force of community stakeholders that worked with hospitals to send nurses and physicians each week to immunize children at churches and other convenient locations.
Within one year, the rate of complete immunization for 2-year-olds rose from 75% to 90%—the national target. DCHD is now applying interventions from The Community Guide to discourage tobacco use and to prevent sexually transmitted infections.
Read the full story at: https://www.thecommunityguide.org/stories/good-shot-reaching-immunization-targets-duval-county.
Reducing health disparities
The CPSTF places a high priority on interventions that can reduce health disparities. Many of their topics of interest focus on interventions to reduce health inequities among racial and ethnic minorities and low-income populations. For instance, the Task Force recommends early childhood education, all-day kindergarten, and after-school academic programs as ways to improve health and decrease health disparities.10
Social determinants of health for individuals and populations are increasingly appreciated as issues to be addressed by physicians and health systems. The CPSTF can serve as a valuable evidence-based resource in these efforts, and their recommendations complement and build on those of other authoritative groups such as the USPSTF, ACIP, and AAFP.
1. Centers for Disease Control and Prevention. ACIP vaccine recommendations. Available at: http://www.cdc.gov/vaccines/hcp/acip-recs/index.html. Accessed December 6, 2016.
2. Community Preventive Services Task Force. The Community Guide. Vaccination. Available at: https://www.thecommunityguide.org/topic/vaccination. Accessed December 6, 2016.
3. Community Preventive Services Task Force. The Community Guide. Cancer prevention and control: cancer screening [fact sheet]. Available at: https://www.thecommunityguide.org/sites/default/files/assets/What-Works-Cancer-Screening-factsheet-and-insert.pdf. Accessed December 6, 2016.
4. Community Preventive Services Task Fo
5. Community Preventive Services Task Force. The Community Guide. HIV/AIDS, other STIs, and teen pregnancy: group-based comprehensive risk reduction interventions for adolescents. Available at: https://www.thecommunityguide.org/findings/hivaids-other-stis-and-teen-pregnancy-group-based-comprehensive-risk-reduction-interventions. Accessed December 6, 2016.
6. Community Preventive Services Task Force. The Community Guide. HIV/AIDS, STIs and pregnancy. Available at: https://www.thecommunityguide.org/topic/hivaids-stis-and-pregnancy. Accessed December 6, 2016.
7. Community Preventive Services Task Force. The Community Guide. Violence: policies facilitating the transfer of juveniles to adult justice systems. Available at: https://www.thecommunityguide.org/findings/violence-policies-facilitating-transfer-juveniles-adult-justice-systems. Accessed December 6, 2016.
8. Community Preventive Services Task Force. The Community Guide. Alcohol – excessive consumption: regulation of alcohol outlet density. Available at: https://www.thecommunityguide.org/findings/alcohol-excessive-consumption-regulation-alcohol-outlet-density. Accessed December 6, 2016.
9. Community Preventive Services Task Force. The Community Guide. Excessive alcohol consumption. Available at: https://www.thecommunityguide.org/topic/excessive-alcohol-consumption. Accessed December 6, 2016.
10. Community Preventive Services Task Force. The Community Guide. Health equity. Available at: https://www.thecommunityguide.org/topic/health-equity. Accessed December 6, 2016.
Family physicians have come to rely on the US Preventive Services Task Force (USPSTF) for rigorous, evidence-based recommendations on the use of clinical preventive services. Still, many such services reach too few individuals who need them. And that’s where the less well known Community Preventive Services Task Force comes in. The CPSTF makes recommendations regarding public health interventions and ways to increase the use of preventive services in the clinical setting—eg, means of improving childhood immunization rates or increasing screening for cervical, breast, and colon cancer.
To better understand how the CPSTF can serve as a resource to busy family physicians, it’s helpful to first understand a bit about the inner-workings of the CPSTF itself.
How CPSTF figures out what works
Formed in 1996, the CPSTF consists of 15 independent, nonfederal members with expertise in public health and preventive medicine, appointed by the Director of the Centers for Disease Control and Prevention (CDC). The Task Force makes recommendations and develops guidance on which community-based health promotion and disease-prevention interventions work and which do not, based on available scientific evidence. The Task Force uses an evidence-based methodology similar to that of the USPSTF—ie, assessing systematic reviews of the evidence and tying recommendations to the strength of the evidence. However, the Task Force has only 3 levels of recommendations: recommend for, recommend against, and insufficient evidence to recommend.
Three CPSTF meetings are held each year, and a representative from the American Academy of Family Physicians (AAFP) attends as a liaison, along with liaisons from other organizations with an interest in the methods and recommendations. The CDC provides the CPSTF with technical and administrative support. However, the recommendations developed do not undergo review or approval by the CDC and are the sole responsibility of the Task Force.
The recommendations made are contained in the Guide to Community Preventive Services, often called The Community Guide, which is available on the Task Force’s Web site at www.thecommunityguide.org/index.html. The topics on which the CPSTF currently has recommendations are listed in TABLE 1. (Since community-wide recommendations are rarely subjected to controlled clinical trials, methods of assessing and ranking other forms of evidence are required. To learn more about how the CPSTF approaches this, see: https://www.thecommunityguide.org/about/our-methodology.)

Improving immunization rates
The topic of immunizations is an example of how synergistic the CPSTF recommendations can be with those from clinical organizations. The Advisory Committee on Immunization Practices (ACIP) makes recommendations on the use of vaccines.1 The CPSTF has developed a set of recommendations on how to increase the uptake of vaccines to improve rates of immunization.2 Interventions they recommend include vaccine requirements for attendance at preschool, primary and secondary school, and college; patient reminder and recall systems; patient and family incentives and rewards; providing vaccines at Women, Infants, and Children clinics, schools, work sites, and homes; standing orders for vaccine administration; physician reminders; physician assessments and feedback; reducing out-of-pocket expenses for vaccines; and using immunization registries. Just as important, the CPSTF identifies interventions that lack hard evidence to support their effectiveness.
Cancer screening works, but patient buy-in lags
The USPSTF recommends screening for breast, cervical, and colorectal cancer. And yet, despite the proven effectiveness of these screening tests in decreasing cancer mortality, many people do not get screened. The CPSTF has developed a set of implementation recommendations that are proven to increase the uptake of recommended cancer screening tests.3 These include:
- sending reminders to patients when screening tests are due
- providing one-on-one or group educational sessions
- providing videos and printed materials that describe screening tests and recommendations
- offering testing at locations and times that are convenient for patients
- offering on-site translation, transportation, patient navigators, and other administrative services to facilitate screening
- assessing provider performance and providing feedback.
CPSTF’s range of resources
Resources provided by the CPSTF (TABLE 2) also include the following materials for physicians, patients, and policy makers:
- tools to assist communities in performing a community health assessment and in prioritizing health needs
- fact sheets on what works for specific populations or conditions. (One recently added fact sheet is a description of interventions to address the leading health problems that affect women.4)
- examples of how communities have used CPSTF recommendations to address a major health concern in their populations. (See “An immunization ‘success story’ from the field.”)

Tackling controversial social issues
Public health interventions are often politically charged, and the CPSTF at times makes recommendations that, while supported by evidence, raise objections from certain groups. One example is a recommendation for “comprehensive risk reduction interventions to promote behaviors that prevent or reduce the risk of pregnancy, human immunodeficiency virus (HIV), and other sexually transmitted infections (STIs).”5 These interventions may include a hierarchy of recommended behaviors that identifies abstinence as the best or preferred method, but also provides information about sexual risk reduction strategies. Abstinence-only education initiatives were rated as having insufficient evidence for effectiveness.6
Another example that falls in the controversial realm is a recommendation against “policies facilitating the transfer of juveniles from juvenile to adult criminal justice systems for the purpose of reducing violence, based on strong evidence that these laws and policies are associated with increased subsequent violent behavior among transferred youth.”7
And a third example is a recommendation for “the use of regulatory authority (eg, through licensing and zoning) to limit alcohol outlet density on the basis of sufficient evidence of a positive association between outlet density and excessive alcohol consumption and related harms.”8 The CPSTF also recommends increasing taxes on alcohol products to reduce excess alcohol consumption.9
SIDEBAR
An immunization “success story” from the field
Before 2009, the vaccination completion rates for 2-year-olds in Duval County, Florida, consistently ranked below the national target of 90%, with particularly low rates in Jacksonville. With the aim of improving vaccination rates—and not wanting to waste time “reinventing the wheel”—the Duval County Health Department (DCHD) turned to The Community Guide for interventions proven to work synergistically: system-based efforts (eg, client reminders, standing orders, clinic-based education) and community-based efforts (eg, staff outreach to clients, educational activities).
Checking the Florida Shots Registry, clinic staff identified infants and toddlers who were due for, or had missed, vaccinations. They sent monthly reminders to parents, urging them to make appointments. DCHD also provided parents with educational materials, vaccination schedules, and safety evidence to reinforce awareness of the need for immunizations.
At local clinics, DCHD trained staff to administer vaccines and established standing orders authorizing them to do so even in the absence of a physician or other approving practitioner.
DCHD also formed an immunization task force of community stakeholders that worked with hospitals to send nurses and physicians each week to immunize children at churches and other convenient locations.
Within one year, the rate of complete immunization for 2-year-olds rose from 75% to 90%—the national target. DCHD is now applying interventions from The Community Guide to discourage tobacco use and to prevent sexually transmitted infections.
Read the full story at: https://www.thecommunityguide.org/stories/good-shot-reaching-immunization-targets-duval-county.
Reducing health disparities
The CPSTF places a high priority on interventions that can reduce health disparities. Many of their topics of interest focus on interventions to reduce health inequities among racial and ethnic minorities and low-income populations. For instance, the Task Force recommends early childhood education, all-day kindergarten, and after-school academic programs as ways to improve health and decrease health disparities.10
Social determinants of health for individuals and populations are increasingly appreciated as issues to be addressed by physicians and health systems. The CPSTF can serve as a valuable evidence-based resource in these efforts, and their recommendations complement and build on those of other authoritative groups such as the USPSTF, ACIP, and AAFP.
Family physicians have come to rely on the US Preventive Services Task Force (USPSTF) for rigorous, evidence-based recommendations on the use of clinical preventive services. Still, many such services reach too few individuals who need them. And that’s where the less well known Community Preventive Services Task Force comes in. The CPSTF makes recommendations regarding public health interventions and ways to increase the use of preventive services in the clinical setting—eg, means of improving childhood immunization rates or increasing screening for cervical, breast, and colon cancer.
To better understand how the CPSTF can serve as a resource to busy family physicians, it’s helpful to first understand a bit about the inner-workings of the CPSTF itself.
How CPSTF figures out what works
Formed in 1996, the CPSTF consists of 15 independent, nonfederal members with expertise in public health and preventive medicine, appointed by the Director of the Centers for Disease Control and Prevention (CDC). The Task Force makes recommendations and develops guidance on which community-based health promotion and disease-prevention interventions work and which do not, based on available scientific evidence. The Task Force uses an evidence-based methodology similar to that of the USPSTF—ie, assessing systematic reviews of the evidence and tying recommendations to the strength of the evidence. However, the Task Force has only 3 levels of recommendations: recommend for, recommend against, and insufficient evidence to recommend.
Three CPSTF meetings are held each year, and a representative from the American Academy of Family Physicians (AAFP) attends as a liaison, along with liaisons from other organizations with an interest in the methods and recommendations. The CDC provides the CPSTF with technical and administrative support. However, the recommendations developed do not undergo review or approval by the CDC and are the sole responsibility of the Task Force.
The recommendations made are contained in the Guide to Community Preventive Services, often called The Community Guide, which is available on the Task Force’s Web site at www.thecommunityguide.org/index.html. The topics on which the CPSTF currently has recommendations are listed in TABLE 1. (Since community-wide recommendations are rarely subjected to controlled clinical trials, methods of assessing and ranking other forms of evidence are required. To learn more about how the CPSTF approaches this, see: https://www.thecommunityguide.org/about/our-methodology.)

Improving immunization rates
The topic of immunizations is an example of how synergistic the CPSTF recommendations can be with those from clinical organizations. The Advisory Committee on Immunization Practices (ACIP) makes recommendations on the use of vaccines.1 The CPSTF has developed a set of recommendations on how to increase the uptake of vaccines to improve rates of immunization.2 Interventions they recommend include vaccine requirements for attendance at preschool, primary and secondary school, and college; patient reminder and recall systems; patient and family incentives and rewards; providing vaccines at Women, Infants, and Children clinics, schools, work sites, and homes; standing orders for vaccine administration; physician reminders; physician assessments and feedback; reducing out-of-pocket expenses for vaccines; and using immunization registries. Just as important, the CPSTF identifies interventions that lack hard evidence to support their effectiveness.
Cancer screening works, but patient buy-in lags
The USPSTF recommends screening for breast, cervical, and colorectal cancer. And yet, despite the proven effectiveness of these screening tests in decreasing cancer mortality, many people do not get screened. The CPSTF has developed a set of implementation recommendations that are proven to increase the uptake of recommended cancer screening tests.3 These include:
- sending reminders to patients when screening tests are due
- providing one-on-one or group educational sessions
- providing videos and printed materials that describe screening tests and recommendations
- offering testing at locations and times that are convenient for patients
- offering on-site translation, transportation, patient navigators, and other administrative services to facilitate screening
- assessing provider performance and providing feedback.
CPSTF’s range of resources
Resources provided by the CPSTF (TABLE 2) also include the following materials for physicians, patients, and policy makers:
- tools to assist communities in performing a community health assessment and in prioritizing health needs
- fact sheets on what works for specific populations or conditions. (One recently added fact sheet is a description of interventions to address the leading health problems that affect women.4)
- examples of how communities have used CPSTF recommendations to address a major health concern in their populations. (See “An immunization ‘success story’ from the field.”)

Tackling controversial social issues
Public health interventions are often politically charged, and the CPSTF at times makes recommendations that, while supported by evidence, raise objections from certain groups. One example is a recommendation for “comprehensive risk reduction interventions to promote behaviors that prevent or reduce the risk of pregnancy, human immunodeficiency virus (HIV), and other sexually transmitted infections (STIs).”5 These interventions may include a hierarchy of recommended behaviors that identifies abstinence as the best or preferred method, but also provides information about sexual risk reduction strategies. Abstinence-only education initiatives were rated as having insufficient evidence for effectiveness.6
Another example that falls in the controversial realm is a recommendation against “policies facilitating the transfer of juveniles from juvenile to adult criminal justice systems for the purpose of reducing violence, based on strong evidence that these laws and policies are associated with increased subsequent violent behavior among transferred youth.”7
And a third example is a recommendation for “the use of regulatory authority (eg, through licensing and zoning) to limit alcohol outlet density on the basis of sufficient evidence of a positive association between outlet density and excessive alcohol consumption and related harms.”8 The CPSTF also recommends increasing taxes on alcohol products to reduce excess alcohol consumption.9
SIDEBAR
An immunization “success story” from the field
Before 2009, the vaccination completion rates for 2-year-olds in Duval County, Florida, consistently ranked below the national target of 90%, with particularly low rates in Jacksonville. With the aim of improving vaccination rates—and not wanting to waste time “reinventing the wheel”—the Duval County Health Department (DCHD) turned to The Community Guide for interventions proven to work synergistically: system-based efforts (eg, client reminders, standing orders, clinic-based education) and community-based efforts (eg, staff outreach to clients, educational activities).
Checking the Florida Shots Registry, clinic staff identified infants and toddlers who were due for, or had missed, vaccinations. They sent monthly reminders to parents, urging them to make appointments. DCHD also provided parents with educational materials, vaccination schedules, and safety evidence to reinforce awareness of the need for immunizations.
At local clinics, DCHD trained staff to administer vaccines and established standing orders authorizing them to do so even in the absence of a physician or other approving practitioner.
DCHD also formed an immunization task force of community stakeholders that worked with hospitals to send nurses and physicians each week to immunize children at churches and other convenient locations.
Within one year, the rate of complete immunization for 2-year-olds rose from 75% to 90%—the national target. DCHD is now applying interventions from The Community Guide to discourage tobacco use and to prevent sexually transmitted infections.
Read the full story at: https://www.thecommunityguide.org/stories/good-shot-reaching-immunization-targets-duval-county.
Reducing health disparities
The CPSTF places a high priority on interventions that can reduce health disparities. Many of their topics of interest focus on interventions to reduce health inequities among racial and ethnic minorities and low-income populations. For instance, the Task Force recommends early childhood education, all-day kindergarten, and after-school academic programs as ways to improve health and decrease health disparities.10
Social determinants of health for individuals and populations are increasingly appreciated as issues to be addressed by physicians and health systems. The CPSTF can serve as a valuable evidence-based resource in these efforts, and their recommendations complement and build on those of other authoritative groups such as the USPSTF, ACIP, and AAFP.
1. Centers for Disease Control and Prevention. ACIP vaccine recommendations. Available at: http://www.cdc.gov/vaccines/hcp/acip-recs/index.html. Accessed December 6, 2016.
2. Community Preventive Services Task Force. The Community Guide. Vaccination. Available at: https://www.thecommunityguide.org/topic/vaccination. Accessed December 6, 2016.
3. Community Preventive Services Task Force. The Community Guide. Cancer prevention and control: cancer screening [fact sheet]. Available at: https://www.thecommunityguide.org/sites/default/files/assets/What-Works-Cancer-Screening-factsheet-and-insert.pdf. Accessed December 6, 2016.
4. Community Preventive Services Task Fo
5. Community Preventive Services Task Force. The Community Guide. HIV/AIDS, other STIs, and teen pregnancy: group-based comprehensive risk reduction interventions for adolescents. Available at: https://www.thecommunityguide.org/findings/hivaids-other-stis-and-teen-pregnancy-group-based-comprehensive-risk-reduction-interventions. Accessed December 6, 2016.
6. Community Preventive Services Task Force. The Community Guide. HIV/AIDS, STIs and pregnancy. Available at: https://www.thecommunityguide.org/topic/hivaids-stis-and-pregnancy. Accessed December 6, 2016.
7. Community Preventive Services Task Force. The Community Guide. Violence: policies facilitating the transfer of juveniles to adult justice systems. Available at: https://www.thecommunityguide.org/findings/violence-policies-facilitating-transfer-juveniles-adult-justice-systems. Accessed December 6, 2016.
8. Community Preventive Services Task Force. The Community Guide. Alcohol – excessive consumption: regulation of alcohol outlet density. Available at: https://www.thecommunityguide.org/findings/alcohol-excessive-consumption-regulation-alcohol-outlet-density. Accessed December 6, 2016.
9. Community Preventive Services Task Force. The Community Guide. Excessive alcohol consumption. Available at: https://www.thecommunityguide.org/topic/excessive-alcohol-consumption. Accessed December 6, 2016.
10. Community Preventive Services Task Force. The Community Guide. Health equity. Available at: https://www.thecommunityguide.org/topic/health-equity. Accessed December 6, 2016.
1. Centers for Disease Control and Prevention. ACIP vaccine recommendations. Available at: http://www.cdc.gov/vaccines/hcp/acip-recs/index.html. Accessed December 6, 2016.
2. Community Preventive Services Task Force. The Community Guide. Vaccination. Available at: https://www.thecommunityguide.org/topic/vaccination. Accessed December 6, 2016.
3. Community Preventive Services Task Force. The Community Guide. Cancer prevention and control: cancer screening [fact sheet]. Available at: https://www.thecommunityguide.org/sites/default/files/assets/What-Works-Cancer-Screening-factsheet-and-insert.pdf. Accessed December 6, 2016.
4. Community Preventive Services Task Fo
5. Community Preventive Services Task Force. The Community Guide. HIV/AIDS, other STIs, and teen pregnancy: group-based comprehensive risk reduction interventions for adolescents. Available at: https://www.thecommunityguide.org/findings/hivaids-other-stis-and-teen-pregnancy-group-based-comprehensive-risk-reduction-interventions. Accessed December 6, 2016.
6. Community Preventive Services Task Force. The Community Guide. HIV/AIDS, STIs and pregnancy. Available at: https://www.thecommunityguide.org/topic/hivaids-stis-and-pregnancy. Accessed December 6, 2016.
7. Community Preventive Services Task Force. The Community Guide. Violence: policies facilitating the transfer of juveniles to adult justice systems. Available at: https://www.thecommunityguide.org/findings/violence-policies-facilitating-transfer-juveniles-adult-justice-systems. Accessed December 6, 2016.
8. Community Preventive Services Task Force. The Community Guide. Alcohol – excessive consumption: regulation of alcohol outlet density. Available at: https://www.thecommunityguide.org/findings/alcohol-excessive-consumption-regulation-alcohol-outlet-density. Accessed December 6, 2016.
9. Community Preventive Services Task Force. The Community Guide. Excessive alcohol consumption. Available at: https://www.thecommunityguide.org/topic/excessive-alcohol-consumption. Accessed December 6, 2016.
10. Community Preventive Services Task Force. The Community Guide. Health equity. Available at: https://www.thecommunityguide.org/topic/health-equity. Accessed December 6, 2016.
Opioids for chronic pain: The CDC’s 12 recommendations
Earlier this year, the Centers for Disease Control and Prevention (CDC) published a clinical practice guideline aimed at decreasing opioid use in the treatment of chronic pain.1 It developed this guideline in response to the increasing problem of opioid abuse and opioid-related mortality in the United States.
The CDC notes that an estimated 1.9 million people abused or were dependent on prescription opioid pain medication in 2013.1 Between 1999 and 2014, more than 165,000 people in the United States died from an overdose of opioid pain medication, with that rate increasing markedly in the past decade.1 In 2011, an estimated 420,000 emergency department visits were related to the abuse of narcotic pain relievers.2
While the problem of increasing opioid-related abuse and deaths has been apparent for some time, effective interventions have been elusive. Evidence remains sparse on the benefits and harms of long-term opioid therapy for chronic pain, except for those at the end of life. Evidence has been insufficient to determine long-term benefits of opioid therapy vs no opioid therapy, although the potential for harms from high doses of opioids are documented. There is not much evidence comparing nonpharmacologic and non-opioid pharmacologic treatments with long-term opioid therapy.
This lack of an evidence base is reflected in the CDC guideline. Of the guideline’s 12 recommendations, not one has high-level supporting evidence and only one has even moderate-level evidence behind it. Four recommendations are supported by low-level evidence, and 7 by very-low-level evidence. Yet 11 of the 12 are given an A recommendation, meaning that the guideline panel feels that most patients should receive this course of action.
Methodology used to create the guideline
The guideline committee used a modified GRADE approach (Grading of Recommendations Assessment, Development, and Evaluation) to develop the guideline. It is the same system the Advisory Committee on Immunization Practices adopted to assess and make recommendations on vaccines.3 The system’s classification of levels of evidence and recommendation categories are described in FIGURE 1.1
The committee started by assessing evidence with a report on the long-term effectiveness of opioids for chronic pain, produced by the Agency for Health Care Research and Quality in 2014;4 it then augmented that report by performing an updated search for new evidence published since the report came out.5 The committee then conducted a “contextual evidence review”6 on the following 4 areas:
- the effectiveness of nonpharmacologic (cognitive behavioral therapy, exercise therapy, interventional treatments, multimodal pain treatment) and non-opioid pharmacologic treatments (acetaminophen, nonsteroidal anti-inflammatory drugs, antidepressants, anticonvulsants)
- the benefits and harms of opioid therapy
- clinician and patient values and preferences related to opioids and medication risks, benefits, and use
- resource allocation, including costs and economic analyses.
The guideline wording indicates that, for this contextual analysis, the committee used a rapid systematic review methodology, in part because of time constraints given the imperative to produce a guideline to address a pressing problem, and because of a recognition that evidence on the questions would be scant and not of high quality.1 The 12 recommendations are categorized under 3 main headings.
Determining when to initiate or continue opioids for chronic pain
1. Nonpharmacologic therapy and non-opioid pharmacologic therapy are preferred for chronic pain. Consider opioid therapy only if you anticipate that benefits for both pain and function will outweigh risks to the patient. If opioids are used, combine them as appropriate with nonpharmacologic therapy and non-opioid pharmacologic therapy. (Recommendation category: A; evidence type: 3)
2. Before starting opioid therapy for chronic pain, establish treatment goals with the patient, including realistic goals for pain and function, and consider how therapy will be discontinued if the benefits do not outweigh the risks. Continue opioid therapy only if there is clinically meaningful improvement in pain and function that outweighs risks to patient safety. (Recommendation category: A; evidence type: 4)
3. Before starting opioid therapy, and periodically during its course, discuss with patients known risks and realistic benefits of opioid therapy and patient and clinician responsibilities for managing therapy. (Recommendation category: A; evidence type: 3)
Opioid selection, dosage, duration, follow-up, and discontinuation
4. When starting opioid therapy for chronic pain, prescribe immediate-release opioids instead of extended-release/long-acting (ER/LA) agents. (Recommendation category: A; evidence type: 4)
5. When starting opioids, prescribe the lowest effective dosage. Use caution when prescribing opioids at any dosage; carefully reassess the evidence for individual benefits and risks when increasing the dosage to ≥50 morphine milligram equivalents (MME)/d; and avoid increasing the dosage to ≥90 MME/d (or carefully justify such a decision, if made). (Recommendation category: A; evidence type: 3)
6. Long-term opioid use often begins with treatment of acute pain. When opioids are used for acute pain, prescribe the lowest effective dose of immediate-release opioids at a quantity no greater than is needed for the expected duration of pain severe enough to require opioids. Three days or less will often be sufficient; more than 7 days will rarely be needed. (Recommendation category: A; evidence type: 4)
7. In monitoring opioid therapy for chronic pain, reevaluate benefits and harms with patients within one to 4 weeks of starting opioid therapy or escalating the dose. Also, evaluate the benefits and harms of continued therapy with patients every 3 months or more frequently. If the benefits of continued opioid therapy do not outweigh the harms, optimize other therapies and work with patients to taper opioids to lower dosages or to taper and discontinue them. (Recommendation category: A; evidence type: 4)
Assessing risk and addressing harms of opioid use
8. Before starting opioid therapy, and periodically during its continuation, evaluate risk factors for opioid-related harms. Incorporate strategies into the management plan to mitigate risk; consider offering naloxone when factors are present that increase the risk for opioid overdose—eg, a history of overdose, history of substance use disorder, higher opioid dosages (≥50 MME/d), or concurrent benzodiazepine use. (Recommendation category: A; evidence type: 4)
9. Review the patient’s history of controlled substance prescriptions. Use data from the state prescription drug monitoring program (PDMP) to determine whether the patient is receiving opioid dosages or dangerous combinations that put him or her at high risk for overdose. (State Web sites are available at http://www.pdmpassist.org/content/state-pdmp-websites.) Review PDMP data when starting opioid therapy for chronic pain and periodically during its continuation, at least every 3 months and with each new prescription. (Recommendation category: A; evidence type: 4)
10. Before prescribing opioids for chronic pain, use urine drug testing to assess for prescribed medications, as well as other controlled prescription drugs and illicit drugs, and consider urine drug testing at least annually. (Recommendation category: B; evidence type: 4)
11. Avoid prescribing opioid pain medication and benzodiazepines concurrently whenever possible. (Recommendation category: A; evidence type: 3)
12. For patients with opioid use disorder, offer or arrange for evidence-based treatment (usually medication-assisted treatment with buprenorphine or methadone in combination with behavioral therapies). (Recommendation category: A; evidence type: 2)
Aids for guideline implementation
The CDC has produced materials to assist physicians in implementing this guideline, including checklists for prescribing or continuing opioids. The checklist for initiation of opioids is reproduced in FIGURE 2.7
The CDC is addressing a severe public health problem and doing so by using contemporary evidence-based methodology and guideline development processes. The lack of high-quality evidence on the topic and the use of a less-than-optimal evidence review process for some key questions may hamper this effort. However, given the prominence of the CDC, this clinical guideline will likely be considered the standard of care for family physicians.
1. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain — United States, 2016. MMWR Recomm Rep. 2016;65:1–49. Available at: https://www.cdc.gov/mmwr/volumes/65/rr/rr6501e1.htm. Accessed October 17, 20
2. Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality. The DAWN Report: Highlights of the 2011 Drug Abuse Warning Network (DAWN) Findings on Drug-Related Emergency Department Visits. 20
3. Ahmed F, Temte JL, Campos-Outcalt D, et al; for the ACIP Evidence Based Recommendations Work Group (EBRWG). Methods for developing evidence-based recommendations by the Advisory Committee on Immunization Practices (ACIP) of the U.S. Centers for Disease Control and Prevention (CDC). Vaccine. 2011;29:9171-9176.
4. Chou R, Deyo R, Devine B, et al. The effectiveness and risks of long-term opioid treatment of chronic pain. Evidence Report/Technology Assessment No. 218. AHRQ Publication No. 14-E005-EF. Rockville, MD: Agency for Healthcare Research and Quality; 2014. Available at: http://www.effectivehealthcare.ahrq.gov/ehc/products/557/1971/chronic-pain-opioid-treatment-report-141205.pdf. Accessed October 17, 2016.
5. Centers for Disease Control and Prevention. Clinical evidence review for the CDC Guideline for Prescribing Opioids for Chronic Pain-United States, 2016. Available at: https://stacks.cdc.gov/view/cdc/38026. Accessed October 17, 2016.
6. Centers for Disease Control and Prevention. Contextual evidence review for the CDC Guideline for Prescribing Opioids for Chronic Pain – United States, 2016. Available at: https://stacks.cdc.gov/view/cdc/38027. Accessed October 17, 2016.
7. Centers for Disease Control and Prevention. Checklist for prescribing opioids for chronic pain. Available at: https://stacks.cdc.gov/view/cdc/38025. Accessed October 17, 2016.
Earlier this year, the Centers for Disease Control and Prevention (CDC) published a clinical practice guideline aimed at decreasing opioid use in the treatment of chronic pain.1 It developed this guideline in response to the increasing problem of opioid abuse and opioid-related mortality in the United States.
The CDC notes that an estimated 1.9 million people abused or were dependent on prescription opioid pain medication in 2013.1 Between 1999 and 2014, more than 165,000 people in the United States died from an overdose of opioid pain medication, with that rate increasing markedly in the past decade.1 In 2011, an estimated 420,000 emergency department visits were related to the abuse of narcotic pain relievers.2
While the problem of increasing opioid-related abuse and deaths has been apparent for some time, effective interventions have been elusive. Evidence remains sparse on the benefits and harms of long-term opioid therapy for chronic pain, except for those at the end of life. Evidence has been insufficient to determine long-term benefits of opioid therapy vs no opioid therapy, although the potential for harms from high doses of opioids are documented. There is not much evidence comparing nonpharmacologic and non-opioid pharmacologic treatments with long-term opioid therapy.
This lack of an evidence base is reflected in the CDC guideline. Of the guideline’s 12 recommendations, not one has high-level supporting evidence and only one has even moderate-level evidence behind it. Four recommendations are supported by low-level evidence, and 7 by very-low-level evidence. Yet 11 of the 12 are given an A recommendation, meaning that the guideline panel feels that most patients should receive this course of action.
Methodology used to create the guideline
The guideline committee used a modified GRADE approach (Grading of Recommendations Assessment, Development, and Evaluation) to develop the guideline. It is the same system the Advisory Committee on Immunization Practices adopted to assess and make recommendations on vaccines.3 The system’s classification of levels of evidence and recommendation categories are described in FIGURE 1.1

The committee started by assessing evidence with a report on the long-term effectiveness of opioids for chronic pain, produced by the Agency for Health Care Research and Quality in 2014;4 it then augmented that report by performing an updated search for new evidence published since the report came out.5 The committee then conducted a “contextual evidence review”6 on the following 4 areas:
- the effectiveness of nonpharmacologic (cognitive behavioral therapy, exercise therapy, interventional treatments, multimodal pain treatment) and non-opioid pharmacologic treatments (acetaminophen, nonsteroidal anti-inflammatory drugs, antidepressants, anticonvulsants)
- the benefits and harms of opioid therapy
- clinician and patient values and preferences related to opioids and medication risks, benefits, and use
- resource allocation, including costs and economic analyses.
The guideline wording indicates that, for this contextual analysis, the committee used a rapid systematic review methodology, in part because of time constraints given the imperative to produce a guideline to address a pressing problem, and because of a recognition that evidence on the questions would be scant and not of high quality.1 The 12 recommendations are categorized under 3 main headings.
Determining when to initiate or continue opioids for chronic pain
1. Nonpharmacologic therapy and non-opioid pharmacologic therapy are preferred for chronic pain. Consider opioid therapy only if you anticipate that benefits for both pain and function will outweigh risks to the patient. If opioids are used, combine them as appropriate with nonpharmacologic therapy and non-opioid pharmacologic therapy. (Recommendation category: A; evidence type: 3)
2. Before starting opioid therapy for chronic pain, establish treatment goals with the patient, including realistic goals for pain and function, and consider how therapy will be discontinued if the benefits do not outweigh the risks. Continue opioid therapy only if there is clinically meaningful improvement in pain and function that outweighs risks to patient safety. (Recommendation category: A; evidence type: 4)
3. Before starting opioid therapy, and periodically during its course, discuss with patients known risks and realistic benefits of opioid therapy and patient and clinician responsibilities for managing therapy. (Recommendation category: A; evidence type: 3)
Opioid selection, dosage, duration, follow-up, and discontinuation
4. When starting opioid therapy for chronic pain, prescribe immediate-release opioids instead of extended-release/long-acting (ER/LA) agents. (Recommendation category: A; evidence type: 4)
5. When starting opioids, prescribe the lowest effective dosage. Use caution when prescribing opioids at any dosage; carefully reassess the evidence for individual benefits and risks when increasing the dosage to ≥50 morphine milligram equivalents (MME)/d; and avoid increasing the dosage to ≥90 MME/d (or carefully justify such a decision, if made). (Recommendation category: A; evidence type: 3)
6. Long-term opioid use often begins with treatment of acute pain. When opioids are used for acute pain, prescribe the lowest effective dose of immediate-release opioids at a quantity no greater than is needed for the expected duration of pain severe enough to require opioids. Three days or less will often be sufficient; more than 7 days will rarely be needed. (Recommendation category: A; evidence type: 4)
7. In monitoring opioid therapy for chronic pain, reevaluate benefits and harms with patients within one to 4 weeks of starting opioid therapy or escalating the dose. Also, evaluate the benefits and harms of continued therapy with patients every 3 months or more frequently. If the benefits of continued opioid therapy do not outweigh the harms, optimize other therapies and work with patients to taper opioids to lower dosages or to taper and discontinue them. (Recommendation category: A; evidence type: 4)
Assessing risk and addressing harms of opioid use
8. Before starting opioid therapy, and periodically during its continuation, evaluate risk factors for opioid-related harms. Incorporate strategies into the management plan to mitigate risk; consider offering naloxone when factors are present that increase the risk for opioid overdose—eg, a history of overdose, history of substance use disorder, higher opioid dosages (≥50 MME/d), or concurrent benzodiazepine use. (Recommendation category: A; evidence type: 4)
9. Review the patient’s history of controlled substance prescriptions. Use data from the state prescription drug monitoring program (PDMP) to determine whether the patient is receiving opioid dosages or dangerous combinations that put him or her at high risk for overdose. (State Web sites are available at http://www.pdmpassist.org/content/state-pdmp-websites.) Review PDMP data when starting opioid therapy for chronic pain and periodically during its continuation, at least every 3 months and with each new prescription. (Recommendation category: A; evidence type: 4)
10. Before prescribing opioids for chronic pain, use urine drug testing to assess for prescribed medications, as well as other controlled prescription drugs and illicit drugs, and consider urine drug testing at least annually. (Recommendation category: B; evidence type: 4)
11. Avoid prescribing opioid pain medication and benzodiazepines concurrently whenever possible. (Recommendation category: A; evidence type: 3)
12. For patients with opioid use disorder, offer or arrange for evidence-based treatment (usually medication-assisted treatment with buprenorphine or methadone in combination with behavioral therapies). (Recommendation category: A; evidence type: 2)
Aids for guideline implementation
The CDC has produced materials to assist physicians in implementing this guideline, including checklists for prescribing or continuing opioids. The checklist for initiation of opioids is reproduced in FIGURE 2.7

The CDC is addressing a severe public health problem and doing so by using contemporary evidence-based methodology and guideline development processes. The lack of high-quality evidence on the topic and the use of a less-than-optimal evidence review process for some key questions may hamper this effort. However, given the prominence of the CDC, this clinical guideline will likely be considered the standard of care for family physicians.
Earlier this year, the Centers for Disease Control and Prevention (CDC) published a clinical practice guideline aimed at decreasing opioid use in the treatment of chronic pain.1 It developed this guideline in response to the increasing problem of opioid abuse and opioid-related mortality in the United States.
The CDC notes that an estimated 1.9 million people abused or were dependent on prescription opioid pain medication in 2013.1 Between 1999 and 2014, more than 165,000 people in the United States died from an overdose of opioid pain medication, with that rate increasing markedly in the past decade.1 In 2011, an estimated 420,000 emergency department visits were related to the abuse of narcotic pain relievers.2
While the problem of increasing opioid-related abuse and deaths has been apparent for some time, effective interventions have been elusive. Evidence remains sparse on the benefits and harms of long-term opioid therapy for chronic pain, except for those at the end of life. Evidence has been insufficient to determine long-term benefits of opioid therapy vs no opioid therapy, although the potential for harms from high doses of opioids are documented. There is not much evidence comparing nonpharmacologic and non-opioid pharmacologic treatments with long-term opioid therapy.
This lack of an evidence base is reflected in the CDC guideline. Of the guideline’s 12 recommendations, not one has high-level supporting evidence and only one has even moderate-level evidence behind it. Four recommendations are supported by low-level evidence, and 7 by very-low-level evidence. Yet 11 of the 12 are given an A recommendation, meaning that the guideline panel feels that most patients should receive this course of action.
Methodology used to create the guideline
The guideline committee used a modified GRADE approach (Grading of Recommendations Assessment, Development, and Evaluation) to develop the guideline. It is the same system the Advisory Committee on Immunization Practices adopted to assess and make recommendations on vaccines.3 The system’s classification of levels of evidence and recommendation categories are described in FIGURE 1.1

The committee started by assessing evidence with a report on the long-term effectiveness of opioids for chronic pain, produced by the Agency for Health Care Research and Quality in 2014;4 it then augmented that report by performing an updated search for new evidence published since the report came out.5 The committee then conducted a “contextual evidence review”6 on the following 4 areas:
- the effectiveness of nonpharmacologic (cognitive behavioral therapy, exercise therapy, interventional treatments, multimodal pain treatment) and non-opioid pharmacologic treatments (acetaminophen, nonsteroidal anti-inflammatory drugs, antidepressants, anticonvulsants)
- the benefits and harms of opioid therapy
- clinician and patient values and preferences related to opioids and medication risks, benefits, and use
- resource allocation, including costs and economic analyses.
The guideline wording indicates that, for this contextual analysis, the committee used a rapid systematic review methodology, in part because of time constraints given the imperative to produce a guideline to address a pressing problem, and because of a recognition that evidence on the questions would be scant and not of high quality.1 The 12 recommendations are categorized under 3 main headings.
Determining when to initiate or continue opioids for chronic pain
1. Nonpharmacologic therapy and non-opioid pharmacologic therapy are preferred for chronic pain. Consider opioid therapy only if you anticipate that benefits for both pain and function will outweigh risks to the patient. If opioids are used, combine them as appropriate with nonpharmacologic therapy and non-opioid pharmacologic therapy. (Recommendation category: A; evidence type: 3)
2. Before starting opioid therapy for chronic pain, establish treatment goals with the patient, including realistic goals for pain and function, and consider how therapy will be discontinued if the benefits do not outweigh the risks. Continue opioid therapy only if there is clinically meaningful improvement in pain and function that outweighs risks to patient safety. (Recommendation category: A; evidence type: 4)
3. Before starting opioid therapy, and periodically during its course, discuss with patients known risks and realistic benefits of opioid therapy and patient and clinician responsibilities for managing therapy. (Recommendation category: A; evidence type: 3)
Opioid selection, dosage, duration, follow-up, and discontinuation
4. When starting opioid therapy for chronic pain, prescribe immediate-release opioids instead of extended-release/long-acting (ER/LA) agents. (Recommendation category: A; evidence type: 4)
5. When starting opioids, prescribe the lowest effective dosage. Use caution when prescribing opioids at any dosage; carefully reassess the evidence for individual benefits and risks when increasing the dosage to ≥50 morphine milligram equivalents (MME)/d; and avoid increasing the dosage to ≥90 MME/d (or carefully justify such a decision, if made). (Recommendation category: A; evidence type: 3)
6. Long-term opioid use often begins with treatment of acute pain. When opioids are used for acute pain, prescribe the lowest effective dose of immediate-release opioids at a quantity no greater than is needed for the expected duration of pain severe enough to require opioids. Three days or less will often be sufficient; more than 7 days will rarely be needed. (Recommendation category: A; evidence type: 4)
7. In monitoring opioid therapy for chronic pain, reevaluate benefits and harms with patients within one to 4 weeks of starting opioid therapy or escalating the dose. Also, evaluate the benefits and harms of continued therapy with patients every 3 months or more frequently. If the benefits of continued opioid therapy do not outweigh the harms, optimize other therapies and work with patients to taper opioids to lower dosages or to taper and discontinue them. (Recommendation category: A; evidence type: 4)
Assessing risk and addressing harms of opioid use
8. Before starting opioid therapy, and periodically during its continuation, evaluate risk factors for opioid-related harms. Incorporate strategies into the management plan to mitigate risk; consider offering naloxone when factors are present that increase the risk for opioid overdose—eg, a history of overdose, history of substance use disorder, higher opioid dosages (≥50 MME/d), or concurrent benzodiazepine use. (Recommendation category: A; evidence type: 4)
9. Review the patient’s history of controlled substance prescriptions. Use data from the state prescription drug monitoring program (PDMP) to determine whether the patient is receiving opioid dosages or dangerous combinations that put him or her at high risk for overdose. (State Web sites are available at http://www.pdmpassist.org/content/state-pdmp-websites.) Review PDMP data when starting opioid therapy for chronic pain and periodically during its continuation, at least every 3 months and with each new prescription. (Recommendation category: A; evidence type: 4)
10. Before prescribing opioids for chronic pain, use urine drug testing to assess for prescribed medications, as well as other controlled prescription drugs and illicit drugs, and consider urine drug testing at least annually. (Recommendation category: B; evidence type: 4)
11. Avoid prescribing opioid pain medication and benzodiazepines concurrently whenever possible. (Recommendation category: A; evidence type: 3)
12. For patients with opioid use disorder, offer or arrange for evidence-based treatment (usually medication-assisted treatment with buprenorphine or methadone in combination with behavioral therapies). (Recommendation category: A; evidence type: 2)
Aids for guideline implementation
The CDC has produced materials to assist physicians in implementing this guideline, including checklists for prescribing or continuing opioids. The checklist for initiation of opioids is reproduced in FIGURE 2.7

The CDC is addressing a severe public health problem and doing so by using contemporary evidence-based methodology and guideline development processes. The lack of high-quality evidence on the topic and the use of a less-than-optimal evidence review process for some key questions may hamper this effort. However, given the prominence of the CDC, this clinical guideline will likely be considered the standard of care for family physicians.
1. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain — United States, 2016. MMWR Recomm Rep. 2016;65:1–49. Available at: https://www.cdc.gov/mmwr/volumes/65/rr/rr6501e1.htm. Accessed October 17, 20
2. Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality. The DAWN Report: Highlights of the 2011 Drug Abuse Warning Network (DAWN) Findings on Drug-Related Emergency Department Visits. 20
3. Ahmed F, Temte JL, Campos-Outcalt D, et al; for the ACIP Evidence Based Recommendations Work Group (EBRWG). Methods for developing evidence-based recommendations by the Advisory Committee on Immunization Practices (ACIP) of the U.S. Centers for Disease Control and Prevention (CDC). Vaccine. 2011;29:9171-9176.
4. Chou R, Deyo R, Devine B, et al. The effectiveness and risks of long-term opioid treatment of chronic pain. Evidence Report/Technology Assessment No. 218. AHRQ Publication No. 14-E005-EF. Rockville, MD: Agency for Healthcare Research and Quality; 2014. Available at: http://www.effectivehealthcare.ahrq.gov/ehc/products/557/1971/chronic-pain-opioid-treatment-report-141205.pdf. Accessed October 17, 2016.
5. Centers for Disease Control and Prevention. Clinical evidence review for the CDC Guideline for Prescribing Opioids for Chronic Pain-United States, 2016. Available at: https://stacks.cdc.gov/view/cdc/38026. Accessed October 17, 2016.
6. Centers for Disease Control and Prevention. Contextual evidence review for the CDC Guideline for Prescribing Opioids for Chronic Pain – United States, 2016. Available at: https://stacks.cdc.gov/view/cdc/38027. Accessed October 17, 2016.
7. Centers for Disease Control and Prevention. Checklist for prescribing opioids for chronic pain. Available at: https://stacks.cdc.gov/view/cdc/38025. Accessed October 17, 2016.
1. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain — United States, 2016. MMWR Recomm Rep. 2016;65:1–49. Available at: https://www.cdc.gov/mmwr/volumes/65/rr/rr6501e1.htm. Accessed October 17, 20
2. Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality. The DAWN Report: Highlights of the 2011 Drug Abuse Warning Network (DAWN) Findings on Drug-Related Emergency Department Visits. 20
3. Ahmed F, Temte JL, Campos-Outcalt D, et al; for the ACIP Evidence Based Recommendations Work Group (EBRWG). Methods for developing evidence-based recommendations by the Advisory Committee on Immunization Practices (ACIP) of the U.S. Centers for Disease Control and Prevention (CDC). Vaccine. 2011;29:9171-9176.
4. Chou R, Deyo R, Devine B, et al. The effectiveness and risks of long-term opioid treatment of chronic pain. Evidence Report/Technology Assessment No. 218. AHRQ Publication No. 14-E005-EF. Rockville, MD: Agency for Healthcare Research and Quality; 2014. Available at: http://www.effectivehealthcare.ahrq.gov/ehc/products/557/1971/chronic-pain-opioid-treatment-report-141205.pdf. Accessed October 17, 2016.
5. Centers for Disease Control and Prevention. Clinical evidence review for the CDC Guideline for Prescribing Opioids for Chronic Pain-United States, 2016. Available at: https://stacks.cdc.gov/view/cdc/38026. Accessed October 17, 2016.
6. Centers for Disease Control and Prevention. Contextual evidence review for the CDC Guideline for Prescribing Opioids for Chronic Pain – United States, 2016. Available at: https://stacks.cdc.gov/view/cdc/38027. Accessed October 17, 2016.
7. Centers for Disease Control and Prevention. Checklist for prescribing opioids for chronic pain. Available at: https://stacks.cdc.gov/view/cdc/38025. Accessed October 17, 2016.
Need-to-know information for the 2016-2017 flu season
The Advisory Committee on Immunization Practices (ACIP) took the unusual step at its June 2016 meeting of recommending against using a currently licensed vaccine, live attenuated influenza vaccine (LAIV), in the 2016-2017 influenza season.1 ACIP based its recommendation on surveillance data collected by the US Influenza Vaccine Effectiveness Network of the Centers for Disease Control and Prevention (CDC), which showed poor effectiveness by the LAIV vaccine among children and adolescents during the past 3 years.
The US Food and Drug Administration (FDA), however, has chosen not to take any action on this matter, saying on its Web site it “has determined that specific regulatory action is not warranted at this time. This determination is based on FDA’s review of manufacturing and clinical data supporting licensure … the totality of the evidence presented at the ACIP meeting, taking into account the inherent limitations of observational studies conducted to evaluate influenza vaccine effectiveness, as well as the well-known variability of influenza vaccine effectiveness across influenza seasons.”2
CDC data for the 2015-2016 flu season showed the effectiveness of LAIV to be just 3% among children 2 years through 17 years of age.3 The reason for this apparent lack of effectiveness is unknown. Other LAIV-effectiveness studies conducted in the 2015-2016 season—one each, in the United States, United Kingdom, and Finland—had results that differed from the CDC surveillance data, with effectiveness ranging from 46% to 58% against all strains combined.2 These results are comparable to vaccine effectiveness found in observational studies in children for both LAIV and inactivated influenza vaccines (IIV) in prior seasons.2
Vaccine manufacturers had projected that 171 to 176 million doses of flu vaccine, in all forms, would be available in the United States during the 2016-2017 season.3 LAIV accounts for about 8% of the total supply of influenza vaccine in the United States,3 and ACIP’s recommendation is not expected to create shortages of other options for the upcoming season. However, the LAIV accounts for one-third of flu vaccines administered to children, and clinicians who provide vaccinations to children have already ordered their vaccine supplies for the upcoming season. Also, it is not clear if children who have previously received the LAIV product will now accept other options for influenza vaccination—all of which involve an injection.
Whether the recommendation against LAIV will continue after this season is also unknown.
What happened during the 2015-2016 influenza season?
The 2015-2016 influenza season was relatively mild with the peak activity occurring in March, somewhat later than in previous years. The circulating influenza strains matched closely to those in the vaccine, making it more effective than the previous year’s vaccine. The predominant circulating strain was A (H1N1), accounting for 58% of illness; A (H3N2) caused 6% of cases and all B types together accounted for 34%.4 The hospitalization rate for all ages was 31.3/100,000 compared with 64.1 the year before.5 There were 85 pediatric deaths compared with 148 in 2014-2015.6
Vaccine effectiveness among all age groups and against all circulating strains was 47%.4 No major vaccine safety concerns were detected. Among those who received IIV3, there was a slight increase in the incidence of Guillain-Barré syndrome of 2.6 cases per one million vaccines.7
Other recommendations for 2016-2017
Once again, ACIP recommends influenza vaccine for all individuals 6 months and older.8 The CDC additionally specifies particular groups that should not skip vaccination given that they are at high risk of complications from influenza infection or because they could expose high-risk individuals to infection (TABLE 1).9
There will continue to be a selection of trivalent and quadrivalent influenza vaccine products in 2016-2017. Trivalent products will contain 3 viral strains: A/California/7/2009 (H1N1), A/Hong Kong/4801/2014 (H3N2) and B/Brisbane/60/2008.10 The quadrivalent products will contain those 3 antigens plus B/Phuket/3073/2013.10 The H3N2 strain is different from the one in last year’s vaccine. Each year, influenza experts analyze surveillance data to predict which circulating strains will predominate in North America, and these antigens constitute the vaccine formulation. The accuracy of this prediction in large part determines how effective the vaccine will be that season.
Two new vaccines have been approved for use in the United States. A quadrivalent cell culture inactivated vaccine (CCIV4), Flucelvax, was licensed in May 2016. It is prepared from virus propagated in canine kidney cells, not with an egg-based production process. It is approved for use in individuals 4 years of age and older.8 Fluad, an adjuvanted trivalent inactivated influenza vaccine, was licensed in late 2015 for individuals 65 years of age and older.8 This is the first adjuvanted influenza vaccine licensed in the United States and will compete with high-dose quadrivalent vaccine for use in older adults. ACIP does not express a preference for any vaccine in this age group.
Two other vaccines should also be available by this fall: Flublok, a quadrivalent recombinant influenza vaccine for individuals 18 years and older, and Flulaval, a quadrivalent inactivated influenza vaccine, for individuals 6 months of age and older. TABLE 211 lists approved influenza vaccines.
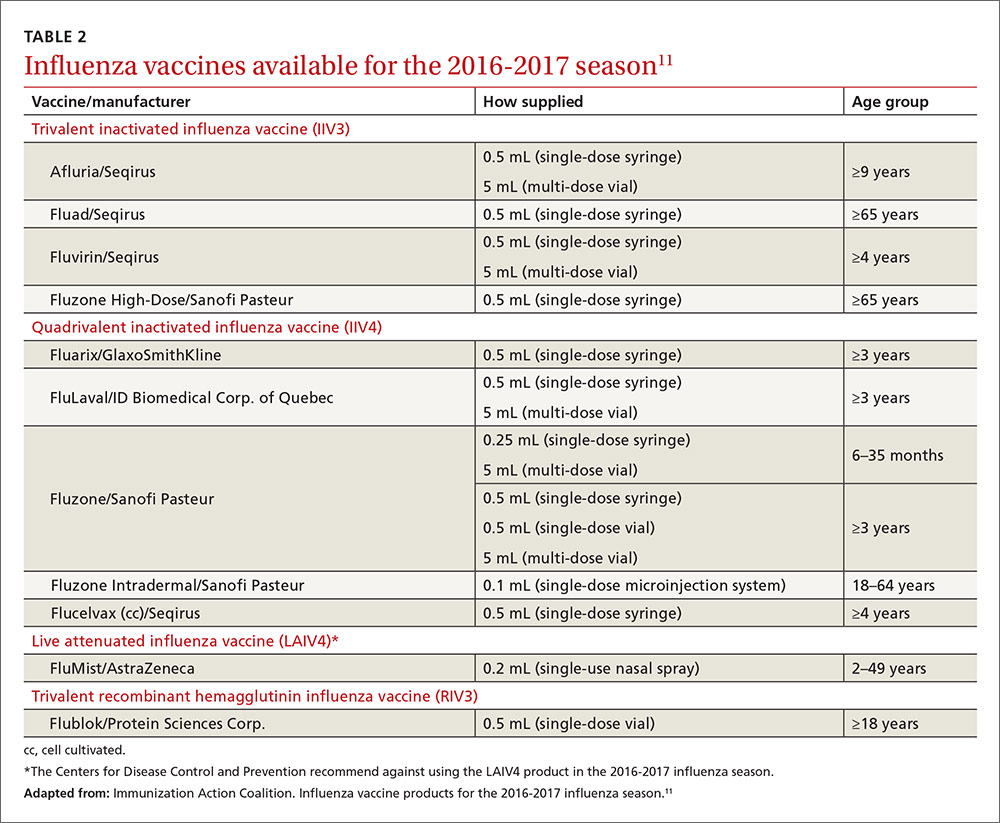
Issues specific to children
Deciding how many vaccine doses children need has been further simplified. Children younger than 9 years need 2 doses if they have received fewer than 2 doses of trivalent or quadrivalent influenza vaccine before July 1, 2016. The interval between the 2 doses should be at least 4 weeks. The 2 doses do not have to be the same product; importantly, do not delay a second dose just to obtain the same product used for the first dose. Also, one dose can be trivalent and the other one quadrivalent, although this offers less-than-optimal protection against the B-virus that is only in the quadrivalent product.
Children younger than 9 years require only one dose if they have received 2 or more total doses of trivalent or quadrivalent influenza vaccine before July 1, 2016. The 2 previous doses need not have been received during the same influenza season or consecutive influenza seasons.
In children ages 6 through 23 months there is a slight increased risk of febrile seizure if the influenza vaccine is co-administered with other vaccines, specifically pneumococcal conjugate vaccine (PCV 13) and diphtheria-tetanus-acellular-pertussis (DTaP). The 3 vaccines administered at the same time result in 30 febrile seizures per 100,000 children;12 the rate is lower when influenza vaccine is co-administered with only one of the others. ACIP believes that the risk of a febrile seizure, which does no long-term harm, does not warrant delaying vaccines that could be co-administered.13
Egg allergy requires no special precautions
Evidence continues to grow that influenza vaccine products do not contain enough egg protein to cause significant problems in those with a history of egg allergies. This year’s recommendations state that no special precautions are needed regarding the anatomic site of immunization or the length of observation after administering influenza vaccine in those with a history of allergies to eggs, no matter how severe. All vaccine-administration facilities should be able to respond to any hypersensitivity reaction, and the standard waiting time for observation after all vaccinations is 15 minutes.
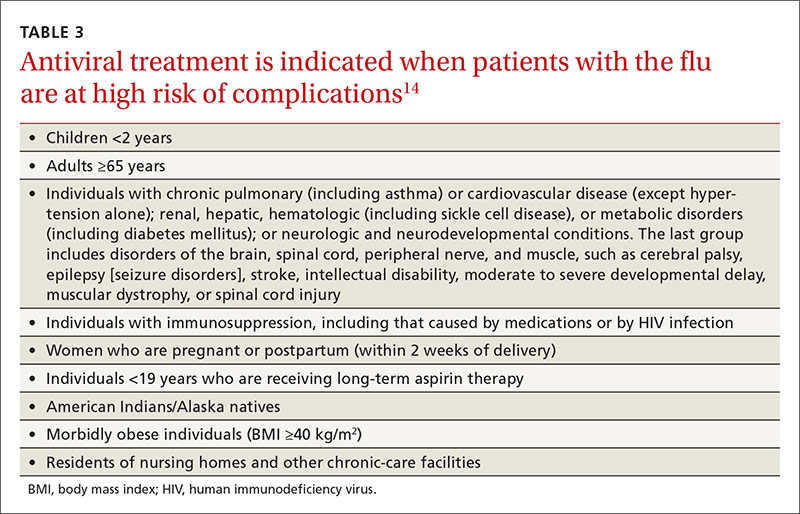
Antiviral medications for treatment or prevention
Most influenza strains circulating in 2016-2017 are expected to remain sensitive to oseltamivir and zanamivir, which can be used for treatment or disease prevention. A third neuraminidase inhibitor, peramivir, is available for intravenous use in adults 18 and older. Treatment is recommended for those who have confirmed or suspected influenza and are at high risk for complications (TABLE 3).14 Consideration of antiviral chemoprevention is recommended under certain circumstances (TABLE 4).15,16 The CDC influenza Web site lists recommended doses and duration for each antiviral for treatment and chemoprevention.15

1. Grohskopf LA, Sokolow LZ, Broder KR, et al. Prevention and control of seasonal influenza with vaccines: Recommendations of the Advisory Committee on Immunization Practices—United States, 2016-17 influenza season. MMWR Recomm Rep. 2016;65:1-54.
2. U.S. Food and Drug Administration. FDA information regarding FluMist quadrivalent vaccine. Available at: http://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm508761.htm. Accessed July 13, 2016.
3. Centers for Disease Control and Prevention. ACIP votes down use of LAIV for 2016-2017 flu season. Available at: http://www.cdc.gov/media/releases/2016/s0622-laiv-flu.html. Accessed July 13, 2016.
4. Flannery B, Chung J. Influenza vaccine effectiveness, including LAIV vs IIV in children and adolescents, US Flu VE Network, 2015-2016. Presented at: meeting of the Advisory Committee on Immunization Practices; June 22, 2016; Atlanta, GA. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2016-06/influenza-05-flannery.pdf. Accessed July 22, 2016.
5. Centers for Disease Control and Prevention. FluView. Laboratory-confirmed influenza hospitalizations. Available at: http://gis.cdc.gov/GRASP/Fluview/FluHospRates.html. Accessed July 25, 2016.
6. Centers for Disease Control and Prevention. FluView. Number of influenza-associated pediatric deaths by week of death. Available at: http://gis.cdc.gov/GRASP/Fluview/PedFluDeath.html. Accessed July 25, 2016.
7. Shimabukuro T. End-of-season update: 2015-2016 influenza vaccine safety monitoring. Presented at: meeting of the Advisory Committee on Immunization Practices; June 22, 2016; Atlanta, GA. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2016-06/influenza-04-shimabukuro.pdf. Accessed July 22, 2016.
8. Grohskopf L. Proposed recommendations 2016-2017 influenza season. Presented at: meeting of the Advisory Committee on Immunization Practices; June 22, 2016; Atlanta, GA. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2016-06/influenza-08-grohskopf.pdf. Accessed July 22, 2016.
9. Centers for Disease Control and Prevention. Influenza vaccination: a summary for clinicians. Available at: http://www.cdc.gov/flu/professionals/vaccination/vax-summary.htm. Accessed July 13, 2016.
10. Centers for Disease Control and Prevention. What you should know for the 2016-2017 influenza season. Available at: http://www.cdc.gov/flu/about/season/flu-season-2016-2017.htm. Accessed July 13, 2016.
11. Immunization Action Coalition. Influenza vaccine products for the 2016-2017 influenza season. Available at: http://www.immunize.org/catg.d/p4072.pdf. Accessed July 13, 2016.
12. Duffy J, Weintraub E, Hambidge SJ, et al. Febrile seizure risk after vaccination in children 6 to 23 months. Pediatrics. 2016;138.
13. Centers for Disease Control and Prevention. Childhood vaccines and febrile seizures. Available at: http://www.cdc.gov/vaccinesafety/concerns/febrile-seizures.html. Accessed August 11, 2016.
14. Centers for Disease Control and Prevention. Use of antivirals. Background and guidance on the use of influenza antiviral agents. Available at: http://www.cdc.gov/flu/professionals/antivirals/antiviral-use-influenza.htm. Accessed July 13, 2016.
15. Centers for Disease Control and Prevention. Influenza antiviral medications: summary for clinicians. Available at: http://www.cdc.gov/flu/professionals/antivirals/summary-clinicians.htm. Accessed July 13, 2016.
16. American Academy of Pediatrics. Recommendations for prevention and control of influenza in children, 2015-2016. Pediatrics. 2015;136:792-808.
The Advisory Committee on Immunization Practices (ACIP) took the unusual step at its June 2016 meeting of recommending against using a currently licensed vaccine, live attenuated influenza vaccine (LAIV), in the 2016-2017 influenza season.1 ACIP based its recommendation on surveillance data collected by the US Influenza Vaccine Effectiveness Network of the Centers for Disease Control and Prevention (CDC), which showed poor effectiveness by the LAIV vaccine among children and adolescents during the past 3 years.
The US Food and Drug Administration (FDA), however, has chosen not to take any action on this matter, saying on its Web site it “has determined that specific regulatory action is not warranted at this time. This determination is based on FDA’s review of manufacturing and clinical data supporting licensure … the totality of the evidence presented at the ACIP meeting, taking into account the inherent limitations of observational studies conducted to evaluate influenza vaccine effectiveness, as well as the well-known variability of influenza vaccine effectiveness across influenza seasons.”2
CDC data for the 2015-2016 flu season showed the effectiveness of LAIV to be just 3% among children 2 years through 17 years of age.3 The reason for this apparent lack of effectiveness is unknown. Other LAIV-effectiveness studies conducted in the 2015-2016 season—one each, in the United States, United Kingdom, and Finland—had results that differed from the CDC surveillance data, with effectiveness ranging from 46% to 58% against all strains combined.2 These results are comparable to vaccine effectiveness found in observational studies in children for both LAIV and inactivated influenza vaccines (IIV) in prior seasons.2
Vaccine manufacturers had projected that 171 to 176 million doses of flu vaccine, in all forms, would be available in the United States during the 2016-2017 season.3 LAIV accounts for about 8% of the total supply of influenza vaccine in the United States,3 and ACIP’s recommendation is not expected to create shortages of other options for the upcoming season. However, the LAIV accounts for one-third of flu vaccines administered to children, and clinicians who provide vaccinations to children have already ordered their vaccine supplies for the upcoming season. Also, it is not clear if children who have previously received the LAIV product will now accept other options for influenza vaccination—all of which involve an injection.
Whether the recommendation against LAIV will continue after this season is also unknown.
What happened during the 2015-2016 influenza season?
The 2015-2016 influenza season was relatively mild with the peak activity occurring in March, somewhat later than in previous years. The circulating influenza strains matched closely to those in the vaccine, making it more effective than the previous year’s vaccine. The predominant circulating strain was A (H1N1), accounting for 58% of illness; A (H3N2) caused 6% of cases and all B types together accounted for 34%.4 The hospitalization rate for all ages was 31.3/100,000 compared with 64.1 the year before.5 There were 85 pediatric deaths compared with 148 in 2014-2015.6
Vaccine effectiveness among all age groups and against all circulating strains was 47%.4 No major vaccine safety concerns were detected. Among those who received IIV3, there was a slight increase in the incidence of Guillain-Barré syndrome of 2.6 cases per one million vaccines.7
Other recommendations for 2016-2017
Once again, ACIP recommends influenza vaccine for all individuals 6 months and older.8 The CDC additionally specifies particular groups that should not skip vaccination given that they are at high risk of complications from influenza infection or because they could expose high-risk individuals to infection (TABLE 1).9

There will continue to be a selection of trivalent and quadrivalent influenza vaccine products in 2016-2017. Trivalent products will contain 3 viral strains: A/California/7/2009 (H1N1), A/Hong Kong/4801/2014 (H3N2) and B/Brisbane/60/2008.10 The quadrivalent products will contain those 3 antigens plus B/Phuket/3073/2013.10 The H3N2 strain is different from the one in last year’s vaccine. Each year, influenza experts analyze surveillance data to predict which circulating strains will predominate in North America, and these antigens constitute the vaccine formulation. The accuracy of this prediction in large part determines how effective the vaccine will be that season.
Two new vaccines have been approved for use in the United States. A quadrivalent cell culture inactivated vaccine (CCIV4), Flucelvax, was licensed in May 2016. It is prepared from virus propagated in canine kidney cells, not with an egg-based production process. It is approved for use in individuals 4 years of age and older.8 Fluad, an adjuvanted trivalent inactivated influenza vaccine, was licensed in late 2015 for individuals 65 years of age and older.8 This is the first adjuvanted influenza vaccine licensed in the United States and will compete with high-dose quadrivalent vaccine for use in older adults. ACIP does not express a preference for any vaccine in this age group.
Two other vaccines should also be available by this fall: Flublok, a quadrivalent recombinant influenza vaccine for individuals 18 years and older, and Flulaval, a quadrivalent inactivated influenza vaccine, for individuals 6 months of age and older. TABLE 211 lists approved influenza vaccines.

Issues specific to children
Deciding how many vaccine doses children need has been further simplified. Children younger than 9 years need 2 doses if they have received fewer than 2 doses of trivalent or quadrivalent influenza vaccine before July 1, 2016. The interval between the 2 doses should be at least 4 weeks. The 2 doses do not have to be the same product; importantly, do not delay a second dose just to obtain the same product used for the first dose. Also, one dose can be trivalent and the other one quadrivalent, although this offers less-than-optimal protection against the B-virus that is only in the quadrivalent product.
Children younger than 9 years require only one dose if they have received 2 or more total doses of trivalent or quadrivalent influenza vaccine before July 1, 2016. The 2 previous doses need not have been received during the same influenza season or consecutive influenza seasons.
In children ages 6 through 23 months there is a slight increased risk of febrile seizure if the influenza vaccine is co-administered with other vaccines, specifically pneumococcal conjugate vaccine (PCV 13) and diphtheria-tetanus-acellular-pertussis (DTaP). The 3 vaccines administered at the same time result in 30 febrile seizures per 100,000 children;12 the rate is lower when influenza vaccine is co-administered with only one of the others. ACIP believes that the risk of a febrile seizure, which does no long-term harm, does not warrant delaying vaccines that could be co-administered.13
Egg allergy requires no special precautions
Evidence continues to grow that influenza vaccine products do not contain enough egg protein to cause significant problems in those with a history of egg allergies. This year’s recommendations state that no special precautions are needed regarding the anatomic site of immunization or the length of observation after administering influenza vaccine in those with a history of allergies to eggs, no matter how severe. All vaccine-administration facilities should be able to respond to any hypersensitivity reaction, and the standard waiting time for observation after all vaccinations is 15 minutes.

Antiviral medications for treatment or prevention
Most influenza strains circulating in 2016-2017 are expected to remain sensitive to oseltamivir and zanamivir, which can be used for treatment or disease prevention. A third neuraminidase inhibitor, peramivir, is available for intravenous use in adults 18 and older. Treatment is recommended for those who have confirmed or suspected influenza and are at high risk for complications (TABLE 3).14 Consideration of antiviral chemoprevention is recommended under certain circumstances (TABLE 4).15,16 The CDC influenza Web site lists recommended doses and duration for each antiviral for treatment and chemoprevention.15

The Advisory Committee on Immunization Practices (ACIP) took the unusual step at its June 2016 meeting of recommending against using a currently licensed vaccine, live attenuated influenza vaccine (LAIV), in the 2016-2017 influenza season.1 ACIP based its recommendation on surveillance data collected by the US Influenza Vaccine Effectiveness Network of the Centers for Disease Control and Prevention (CDC), which showed poor effectiveness by the LAIV vaccine among children and adolescents during the past 3 years.
The US Food and Drug Administration (FDA), however, has chosen not to take any action on this matter, saying on its Web site it “has determined that specific regulatory action is not warranted at this time. This determination is based on FDA’s review of manufacturing and clinical data supporting licensure … the totality of the evidence presented at the ACIP meeting, taking into account the inherent limitations of observational studies conducted to evaluate influenza vaccine effectiveness, as well as the well-known variability of influenza vaccine effectiveness across influenza seasons.”2
CDC data for the 2015-2016 flu season showed the effectiveness of LAIV to be just 3% among children 2 years through 17 years of age.3 The reason for this apparent lack of effectiveness is unknown. Other LAIV-effectiveness studies conducted in the 2015-2016 season—one each, in the United States, United Kingdom, and Finland—had results that differed from the CDC surveillance data, with effectiveness ranging from 46% to 58% against all strains combined.2 These results are comparable to vaccine effectiveness found in observational studies in children for both LAIV and inactivated influenza vaccines (IIV) in prior seasons.2
Vaccine manufacturers had projected that 171 to 176 million doses of flu vaccine, in all forms, would be available in the United States during the 2016-2017 season.3 LAIV accounts for about 8% of the total supply of influenza vaccine in the United States,3 and ACIP’s recommendation is not expected to create shortages of other options for the upcoming season. However, the LAIV accounts for one-third of flu vaccines administered to children, and clinicians who provide vaccinations to children have already ordered their vaccine supplies for the upcoming season. Also, it is not clear if children who have previously received the LAIV product will now accept other options for influenza vaccination—all of which involve an injection.
Whether the recommendation against LAIV will continue after this season is also unknown.
What happened during the 2015-2016 influenza season?
The 2015-2016 influenza season was relatively mild with the peak activity occurring in March, somewhat later than in previous years. The circulating influenza strains matched closely to those in the vaccine, making it more effective than the previous year’s vaccine. The predominant circulating strain was A (H1N1), accounting for 58% of illness; A (H3N2) caused 6% of cases and all B types together accounted for 34%.4 The hospitalization rate for all ages was 31.3/100,000 compared with 64.1 the year before.5 There were 85 pediatric deaths compared with 148 in 2014-2015.6
Vaccine effectiveness among all age groups and against all circulating strains was 47%.4 No major vaccine safety concerns were detected. Among those who received IIV3, there was a slight increase in the incidence of Guillain-Barré syndrome of 2.6 cases per one million vaccines.7
Other recommendations for 2016-2017
Once again, ACIP recommends influenza vaccine for all individuals 6 months and older.8 The CDC additionally specifies particular groups that should not skip vaccination given that they are at high risk of complications from influenza infection or because they could expose high-risk individuals to infection (TABLE 1).9

There will continue to be a selection of trivalent and quadrivalent influenza vaccine products in 2016-2017. Trivalent products will contain 3 viral strains: A/California/7/2009 (H1N1), A/Hong Kong/4801/2014 (H3N2) and B/Brisbane/60/2008.10 The quadrivalent products will contain those 3 antigens plus B/Phuket/3073/2013.10 The H3N2 strain is different from the one in last year’s vaccine. Each year, influenza experts analyze surveillance data to predict which circulating strains will predominate in North America, and these antigens constitute the vaccine formulation. The accuracy of this prediction in large part determines how effective the vaccine will be that season.
Two new vaccines have been approved for use in the United States. A quadrivalent cell culture inactivated vaccine (CCIV4), Flucelvax, was licensed in May 2016. It is prepared from virus propagated in canine kidney cells, not with an egg-based production process. It is approved for use in individuals 4 years of age and older.8 Fluad, an adjuvanted trivalent inactivated influenza vaccine, was licensed in late 2015 for individuals 65 years of age and older.8 This is the first adjuvanted influenza vaccine licensed in the United States and will compete with high-dose quadrivalent vaccine for use in older adults. ACIP does not express a preference for any vaccine in this age group.
Two other vaccines should also be available by this fall: Flublok, a quadrivalent recombinant influenza vaccine for individuals 18 years and older, and Flulaval, a quadrivalent inactivated influenza vaccine, for individuals 6 months of age and older. TABLE 211 lists approved influenza vaccines.

Issues specific to children
Deciding how many vaccine doses children need has been further simplified. Children younger than 9 years need 2 doses if they have received fewer than 2 doses of trivalent or quadrivalent influenza vaccine before July 1, 2016. The interval between the 2 doses should be at least 4 weeks. The 2 doses do not have to be the same product; importantly, do not delay a second dose just to obtain the same product used for the first dose. Also, one dose can be trivalent and the other one quadrivalent, although this offers less-than-optimal protection against the B-virus that is only in the quadrivalent product.
Children younger than 9 years require only one dose if they have received 2 or more total doses of trivalent or quadrivalent influenza vaccine before July 1, 2016. The 2 previous doses need not have been received during the same influenza season or consecutive influenza seasons.
In children ages 6 through 23 months there is a slight increased risk of febrile seizure if the influenza vaccine is co-administered with other vaccines, specifically pneumococcal conjugate vaccine (PCV 13) and diphtheria-tetanus-acellular-pertussis (DTaP). The 3 vaccines administered at the same time result in 30 febrile seizures per 100,000 children;12 the rate is lower when influenza vaccine is co-administered with only one of the others. ACIP believes that the risk of a febrile seizure, which does no long-term harm, does not warrant delaying vaccines that could be co-administered.13
Egg allergy requires no special precautions
Evidence continues to grow that influenza vaccine products do not contain enough egg protein to cause significant problems in those with a history of egg allergies. This year’s recommendations state that no special precautions are needed regarding the anatomic site of immunization or the length of observation after administering influenza vaccine in those with a history of allergies to eggs, no matter how severe. All vaccine-administration facilities should be able to respond to any hypersensitivity reaction, and the standard waiting time for observation after all vaccinations is 15 minutes.

Antiviral medications for treatment or prevention
Most influenza strains circulating in 2016-2017 are expected to remain sensitive to oseltamivir and zanamivir, which can be used for treatment or disease prevention. A third neuraminidase inhibitor, peramivir, is available for intravenous use in adults 18 and older. Treatment is recommended for those who have confirmed or suspected influenza and are at high risk for complications (TABLE 3).14 Consideration of antiviral chemoprevention is recommended under certain circumstances (TABLE 4).15,16 The CDC influenza Web site lists recommended doses and duration for each antiviral for treatment and chemoprevention.15

1. Grohskopf LA, Sokolow LZ, Broder KR, et al. Prevention and control of seasonal influenza with vaccines: Recommendations of the Advisory Committee on Immunization Practices—United States, 2016-17 influenza season. MMWR Recomm Rep. 2016;65:1-54.
2. U.S. Food and Drug Administration. FDA information regarding FluMist quadrivalent vaccine. Available at: http://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm508761.htm. Accessed July 13, 2016.
3. Centers for Disease Control and Prevention. ACIP votes down use of LAIV for 2016-2017 flu season. Available at: http://www.cdc.gov/media/releases/2016/s0622-laiv-flu.html. Accessed July 13, 2016.
4. Flannery B, Chung J. Influenza vaccine effectiveness, including LAIV vs IIV in children and adolescents, US Flu VE Network, 2015-2016. Presented at: meeting of the Advisory Committee on Immunization Practices; June 22, 2016; Atlanta, GA. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2016-06/influenza-05-flannery.pdf. Accessed July 22, 2016.
5. Centers for Disease Control and Prevention. FluView. Laboratory-confirmed influenza hospitalizations. Available at: http://gis.cdc.gov/GRASP/Fluview/FluHospRates.html. Accessed July 25, 2016.
6. Centers for Disease Control and Prevention. FluView. Number of influenza-associated pediatric deaths by week of death. Available at: http://gis.cdc.gov/GRASP/Fluview/PedFluDeath.html. Accessed July 25, 2016.
7. Shimabukuro T. End-of-season update: 2015-2016 influenza vaccine safety monitoring. Presented at: meeting of the Advisory Committee on Immunization Practices; June 22, 2016; Atlanta, GA. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2016-06/influenza-04-shimabukuro.pdf. Accessed July 22, 2016.
8. Grohskopf L. Proposed recommendations 2016-2017 influenza season. Presented at: meeting of the Advisory Committee on Immunization Practices; June 22, 2016; Atlanta, GA. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2016-06/influenza-08-grohskopf.pdf. Accessed July 22, 2016.
9. Centers for Disease Control and Prevention. Influenza vaccination: a summary for clinicians. Available at: http://www.cdc.gov/flu/professionals/vaccination/vax-summary.htm. Accessed July 13, 2016.
10. Centers for Disease Control and Prevention. What you should know for the 2016-2017 influenza season. Available at: http://www.cdc.gov/flu/about/season/flu-season-2016-2017.htm. Accessed July 13, 2016.
11. Immunization Action Coalition. Influenza vaccine products for the 2016-2017 influenza season. Available at: http://www.immunize.org/catg.d/p4072.pdf. Accessed July 13, 2016.
12. Duffy J, Weintraub E, Hambidge SJ, et al. Febrile seizure risk after vaccination in children 6 to 23 months. Pediatrics. 2016;138.
13. Centers for Disease Control and Prevention. Childhood vaccines and febrile seizures. Available at: http://www.cdc.gov/vaccinesafety/concerns/febrile-seizures.html. Accessed August 11, 2016.
14. Centers for Disease Control and Prevention. Use of antivirals. Background and guidance on the use of influenza antiviral agents. Available at: http://www.cdc.gov/flu/professionals/antivirals/antiviral-use-influenza.htm. Accessed July 13, 2016.
15. Centers for Disease Control and Prevention. Influenza antiviral medications: summary for clinicians. Available at: http://www.cdc.gov/flu/professionals/antivirals/summary-clinicians.htm. Accessed July 13, 2016.
16. American Academy of Pediatrics. Recommendations for prevention and control of influenza in children, 2015-2016. Pediatrics. 2015;136:792-808.
1. Grohskopf LA, Sokolow LZ, Broder KR, et al. Prevention and control of seasonal influenza with vaccines: Recommendations of the Advisory Committee on Immunization Practices—United States, 2016-17 influenza season. MMWR Recomm Rep. 2016;65:1-54.
2. U.S. Food and Drug Administration. FDA information regarding FluMist quadrivalent vaccine. Available at: http://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm508761.htm. Accessed July 13, 2016.
3. Centers for Disease Control and Prevention. ACIP votes down use of LAIV for 2016-2017 flu season. Available at: http://www.cdc.gov/media/releases/2016/s0622-laiv-flu.html. Accessed July 13, 2016.
4. Flannery B, Chung J. Influenza vaccine effectiveness, including LAIV vs IIV in children and adolescents, US Flu VE Network, 2015-2016. Presented at: meeting of the Advisory Committee on Immunization Practices; June 22, 2016; Atlanta, GA. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2016-06/influenza-05-flannery.pdf. Accessed July 22, 2016.
5. Centers for Disease Control and Prevention. FluView. Laboratory-confirmed influenza hospitalizations. Available at: http://gis.cdc.gov/GRASP/Fluview/FluHospRates.html. Accessed July 25, 2016.
6. Centers for Disease Control and Prevention. FluView. Number of influenza-associated pediatric deaths by week of death. Available at: http://gis.cdc.gov/GRASP/Fluview/PedFluDeath.html. Accessed July 25, 2016.
7. Shimabukuro T. End-of-season update: 2015-2016 influenza vaccine safety monitoring. Presented at: meeting of the Advisory Committee on Immunization Practices; June 22, 2016; Atlanta, GA. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2016-06/influenza-04-shimabukuro.pdf. Accessed July 22, 2016.
8. Grohskopf L. Proposed recommendations 2016-2017 influenza season. Presented at: meeting of the Advisory Committee on Immunization Practices; June 22, 2016; Atlanta, GA. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2016-06/influenza-08-grohskopf.pdf. Accessed July 22, 2016.
9. Centers for Disease Control and Prevention. Influenza vaccination: a summary for clinicians. Available at: http://www.cdc.gov/flu/professionals/vaccination/vax-summary.htm. Accessed July 13, 2016.
10. Centers for Disease Control and Prevention. What you should know for the 2016-2017 influenza season. Available at: http://www.cdc.gov/flu/about/season/flu-season-2016-2017.htm. Accessed July 13, 2016.
11. Immunization Action Coalition. Influenza vaccine products for the 2016-2017 influenza season. Available at: http://www.immunize.org/catg.d/p4072.pdf. Accessed July 13, 2016.
12. Duffy J, Weintraub E, Hambidge SJ, et al. Febrile seizure risk after vaccination in children 6 to 23 months. Pediatrics. 2016;138.
13. Centers for Disease Control and Prevention. Childhood vaccines and febrile seizures. Available at: http://www.cdc.gov/vaccinesafety/concerns/febrile-seizures.html. Accessed August 11, 2016.
14. Centers for Disease Control and Prevention. Use of antivirals. Background and guidance on the use of influenza antiviral agents. Available at: http://www.cdc.gov/flu/professionals/antivirals/antiviral-use-influenza.htm. Accessed July 13, 2016.
15. Centers for Disease Control and Prevention. Influenza antiviral medications: summary for clinicians. Available at: http://www.cdc.gov/flu/professionals/antivirals/summary-clinicians.htm. Accessed July 13, 2016.
16. American Academy of Pediatrics. Recommendations for prevention and control of influenza in children, 2015-2016. Pediatrics. 2015;136:792-808.