User login
Experts see larger role for CDC in preventing hospital deaths
The Centers for Disease Control and Prevention, which has lately been under attack for mishandling infectious disease samples, got a vote of confidence from patient safety experts.
At a July 17 hearing of the Primary Health and Aging Subcommittee of the Senate Health, Education, Labor and Pensions Committee, patient safety advocates and physicians called on Congress to get the CDC more involved in tracking and reporting on all forms of preventable harm that occur in the hospital.
The agency already monitors and reports on health care–associated infections such as catheter-associated urinary tract infections and surgical site infections. But the CDC also should be enlisted to help hospitals in other areas where they currently lack a good surveillance system, including venous thromboembolism and medication errors, experts testified.
"We need to expand the efforts of the CDC," said Dr. Ashish Jha, professor of health policy and management at the Harvard School of Public Health. "There is no reason to think that what they have been able to do around health care associated infections, they can’t do in other areas."
As many as 440,000 patients die of preventable errors in U.S. hospitals, according to most recent estimates (J. Pat. Safety 2013;9:122-8 [doi: 10.1097/PTS.0b013e3182948a69]).
About 180,000 Medicare patients likely die each year from preventable adverse events in the hospital, according to a 2010 report from the U.S. Department of Health & Human Services.
Those numbers may seem daunting, but the experts testified that there are plenty of policy steps the government can take to aid physicians and hospitals in tackling the problem.
Creating standards for reporting health care quality and cost measures, similar to the standards for the financial industry created under the Security and Exchange Act, would be a good place to start, said Dr. Peter Pronovost, senior vice president for patient safety and quality at Johns Hopkins Medicine in Baltimore.
"Right now we have no guarantee that the measures that we’re reporting are accurate," said Dr. Pronovost, who spearheaded a checklist protocol that led has resulted in significantly lower rates of central line associated bloodstream infections. "Johns Hopkins Hospital was both congratulated and criticized for its performance on the exact same measure for the exact same time period for bloodstream infections. And when we looked, the one we’re paid on, using administrative data, got it right 13% of the time."
Billing data isn’t adequate for this type of reporting, he said.
Experts at the hearing also called on Congress to help make safety a priority for hospitals by beefing up penalties for errors.
Lisa McGiffert, director of the Safe Patient Project at Consumers Union, said Medicare needs to put more financial pressure on hospitals. Under Medicare’s Hospital-Acquired Conditions payment program, the government withholds payment for hospitalizations in which certain preventable errors occur. But Ms. McGiffert said that policy doesn’t go far enough. The hospital should be responsible for the full range of follow-up care that results from the original adverse event, including physician visits, rehospitalizations, and medications, she said.
Dr. Pronovost said Medicare and the Joint Commission could also use their existing authority to sanction hospitals that have infection rates that are consistently above the national average. But he cautioned that regulators should only exercise this authority in areas where there is good data and validated measures, such as central line associated bloodstream infections.
CEO compensation also plays a role, said Dr. Jha, who has researched this issue among nonprofit hospitals. Right now, patient safety is typically not one of the factors influencing how CEOs at nonprofit hospital are paid, he said.
"Until we get to a point where the CEO of the hospital is lying awake at night worrying about patient safety, I don’t think we’re going to really meaningfully move the needle," Dr. Jha said.
On Twitter @maryellenny
The Centers for Disease Control and Prevention, which has lately been under attack for mishandling infectious disease samples, got a vote of confidence from patient safety experts.
At a July 17 hearing of the Primary Health and Aging Subcommittee of the Senate Health, Education, Labor and Pensions Committee, patient safety advocates and physicians called on Congress to get the CDC more involved in tracking and reporting on all forms of preventable harm that occur in the hospital.
The agency already monitors and reports on health care–associated infections such as catheter-associated urinary tract infections and surgical site infections. But the CDC also should be enlisted to help hospitals in other areas where they currently lack a good surveillance system, including venous thromboembolism and medication errors, experts testified.
"We need to expand the efforts of the CDC," said Dr. Ashish Jha, professor of health policy and management at the Harvard School of Public Health. "There is no reason to think that what they have been able to do around health care associated infections, they can’t do in other areas."
As many as 440,000 patients die of preventable errors in U.S. hospitals, according to most recent estimates (J. Pat. Safety 2013;9:122-8 [doi: 10.1097/PTS.0b013e3182948a69]).
About 180,000 Medicare patients likely die each year from preventable adverse events in the hospital, according to a 2010 report from the U.S. Department of Health & Human Services.
Those numbers may seem daunting, but the experts testified that there are plenty of policy steps the government can take to aid physicians and hospitals in tackling the problem.
Creating standards for reporting health care quality and cost measures, similar to the standards for the financial industry created under the Security and Exchange Act, would be a good place to start, said Dr. Peter Pronovost, senior vice president for patient safety and quality at Johns Hopkins Medicine in Baltimore.
"Right now we have no guarantee that the measures that we’re reporting are accurate," said Dr. Pronovost, who spearheaded a checklist protocol that led has resulted in significantly lower rates of central line associated bloodstream infections. "Johns Hopkins Hospital was both congratulated and criticized for its performance on the exact same measure for the exact same time period for bloodstream infections. And when we looked, the one we’re paid on, using administrative data, got it right 13% of the time."
Billing data isn’t adequate for this type of reporting, he said.
Experts at the hearing also called on Congress to help make safety a priority for hospitals by beefing up penalties for errors.
Lisa McGiffert, director of the Safe Patient Project at Consumers Union, said Medicare needs to put more financial pressure on hospitals. Under Medicare’s Hospital-Acquired Conditions payment program, the government withholds payment for hospitalizations in which certain preventable errors occur. But Ms. McGiffert said that policy doesn’t go far enough. The hospital should be responsible for the full range of follow-up care that results from the original adverse event, including physician visits, rehospitalizations, and medications, she said.
Dr. Pronovost said Medicare and the Joint Commission could also use their existing authority to sanction hospitals that have infection rates that are consistently above the national average. But he cautioned that regulators should only exercise this authority in areas where there is good data and validated measures, such as central line associated bloodstream infections.
CEO compensation also plays a role, said Dr. Jha, who has researched this issue among nonprofit hospitals. Right now, patient safety is typically not one of the factors influencing how CEOs at nonprofit hospital are paid, he said.
"Until we get to a point where the CEO of the hospital is lying awake at night worrying about patient safety, I don’t think we’re going to really meaningfully move the needle," Dr. Jha said.
On Twitter @maryellenny
The Centers for Disease Control and Prevention, which has lately been under attack for mishandling infectious disease samples, got a vote of confidence from patient safety experts.
At a July 17 hearing of the Primary Health and Aging Subcommittee of the Senate Health, Education, Labor and Pensions Committee, patient safety advocates and physicians called on Congress to get the CDC more involved in tracking and reporting on all forms of preventable harm that occur in the hospital.
The agency already monitors and reports on health care–associated infections such as catheter-associated urinary tract infections and surgical site infections. But the CDC also should be enlisted to help hospitals in other areas where they currently lack a good surveillance system, including venous thromboembolism and medication errors, experts testified.
"We need to expand the efforts of the CDC," said Dr. Ashish Jha, professor of health policy and management at the Harvard School of Public Health. "There is no reason to think that what they have been able to do around health care associated infections, they can’t do in other areas."
As many as 440,000 patients die of preventable errors in U.S. hospitals, according to most recent estimates (J. Pat. Safety 2013;9:122-8 [doi: 10.1097/PTS.0b013e3182948a69]).
About 180,000 Medicare patients likely die each year from preventable adverse events in the hospital, according to a 2010 report from the U.S. Department of Health & Human Services.
Those numbers may seem daunting, but the experts testified that there are plenty of policy steps the government can take to aid physicians and hospitals in tackling the problem.
Creating standards for reporting health care quality and cost measures, similar to the standards for the financial industry created under the Security and Exchange Act, would be a good place to start, said Dr. Peter Pronovost, senior vice president for patient safety and quality at Johns Hopkins Medicine in Baltimore.
"Right now we have no guarantee that the measures that we’re reporting are accurate," said Dr. Pronovost, who spearheaded a checklist protocol that led has resulted in significantly lower rates of central line associated bloodstream infections. "Johns Hopkins Hospital was both congratulated and criticized for its performance on the exact same measure for the exact same time period for bloodstream infections. And when we looked, the one we’re paid on, using administrative data, got it right 13% of the time."
Billing data isn’t adequate for this type of reporting, he said.
Experts at the hearing also called on Congress to help make safety a priority for hospitals by beefing up penalties for errors.
Lisa McGiffert, director of the Safe Patient Project at Consumers Union, said Medicare needs to put more financial pressure on hospitals. Under Medicare’s Hospital-Acquired Conditions payment program, the government withholds payment for hospitalizations in which certain preventable errors occur. But Ms. McGiffert said that policy doesn’t go far enough. The hospital should be responsible for the full range of follow-up care that results from the original adverse event, including physician visits, rehospitalizations, and medications, she said.
Dr. Pronovost said Medicare and the Joint Commission could also use their existing authority to sanction hospitals that have infection rates that are consistently above the national average. But he cautioned that regulators should only exercise this authority in areas where there is good data and validated measures, such as central line associated bloodstream infections.
CEO compensation also plays a role, said Dr. Jha, who has researched this issue among nonprofit hospitals. Right now, patient safety is typically not one of the factors influencing how CEOs at nonprofit hospital are paid, he said.
"Until we get to a point where the CEO of the hospital is lying awake at night worrying about patient safety, I don’t think we’re going to really meaningfully move the needle," Dr. Jha said.
On Twitter @maryellenny
FROM A SENATE COMMITTEE HEARING
ACA: Uninsured patient numbers dropped after first enrollment
The uninsured rate of working adults in the United States declined from 20% to 15% after the first Affordable Care Act open enrollment period.
Sara Collins, Ph.D., of the Commonwealth Fund and her associates also found that 6 in 10 newly covered patients have used their health plan to visit a doctor or hospital, or to fill a prescription.
The Commonwealth Fund tracking survey examined data from 4,425 adults about health and insurance trends from April 9 to June 2 and compared the findings with those from a 2013 Commonwealth Fund survey.
The uninsured rate among patients aged 19-64 years went from 20% for July to September 2013 to 15% for April to June 2014. For young adults (19-34 years), the uninsured rate dropped from 28% to 18% over the same time period. Among the Hispanic community, uninsured patients declined from 36% to 23%, the survey found. The findings were published July 10.
In states that expanded Medicaid, the uninsured rate fell from 28% to 17% for patients at the poverty level. However, in states that chose not to expand Medicaid, the rate of uninsured poor patients changed from 38% to 36%.
The survey findings provide early evidence that the ACA’s coverage provisions are helping Americans most at risk for lacking health insurance, Dr. Collins and associates noted. Evidence also shows that the new insurance coverage is helping people gain new access to the health care system. Nearly three-fourths of previously uninsured adults who used their new plan reported they would not have received the same care prior to gaining coverage.
On Twitter @legal_med
The uninsured rate of working adults in the United States declined from 20% to 15% after the first Affordable Care Act open enrollment period.
Sara Collins, Ph.D., of the Commonwealth Fund and her associates also found that 6 in 10 newly covered patients have used their health plan to visit a doctor or hospital, or to fill a prescription.
The Commonwealth Fund tracking survey examined data from 4,425 adults about health and insurance trends from April 9 to June 2 and compared the findings with those from a 2013 Commonwealth Fund survey.
The uninsured rate among patients aged 19-64 years went from 20% for July to September 2013 to 15% for April to June 2014. For young adults (19-34 years), the uninsured rate dropped from 28% to 18% over the same time period. Among the Hispanic community, uninsured patients declined from 36% to 23%, the survey found. The findings were published July 10.
In states that expanded Medicaid, the uninsured rate fell from 28% to 17% for patients at the poverty level. However, in states that chose not to expand Medicaid, the rate of uninsured poor patients changed from 38% to 36%.
The survey findings provide early evidence that the ACA’s coverage provisions are helping Americans most at risk for lacking health insurance, Dr. Collins and associates noted. Evidence also shows that the new insurance coverage is helping people gain new access to the health care system. Nearly three-fourths of previously uninsured adults who used their new plan reported they would not have received the same care prior to gaining coverage.
On Twitter @legal_med
The uninsured rate of working adults in the United States declined from 20% to 15% after the first Affordable Care Act open enrollment period.
Sara Collins, Ph.D., of the Commonwealth Fund and her associates also found that 6 in 10 newly covered patients have used their health plan to visit a doctor or hospital, or to fill a prescription.
The Commonwealth Fund tracking survey examined data from 4,425 adults about health and insurance trends from April 9 to June 2 and compared the findings with those from a 2013 Commonwealth Fund survey.
The uninsured rate among patients aged 19-64 years went from 20% for July to September 2013 to 15% for April to June 2014. For young adults (19-34 years), the uninsured rate dropped from 28% to 18% over the same time period. Among the Hispanic community, uninsured patients declined from 36% to 23%, the survey found. The findings were published July 10.
In states that expanded Medicaid, the uninsured rate fell from 28% to 17% for patients at the poverty level. However, in states that chose not to expand Medicaid, the rate of uninsured poor patients changed from 38% to 36%.
The survey findings provide early evidence that the ACA’s coverage provisions are helping Americans most at risk for lacking health insurance, Dr. Collins and associates noted. Evidence also shows that the new insurance coverage is helping people gain new access to the health care system. Nearly three-fourths of previously uninsured adults who used their new plan reported they would not have received the same care prior to gaining coverage.
On Twitter @legal_med
Key clinical point: Early evidence shows ACA coverage provisions are helping residents most at risk for lacking health insurance.
Major finding: After the first enrollment period, the uninsured rate among working adults declined from 20% to 15%; the uninsured rate for young adults aged 19-34 declined by 10%.
Data source: Phone interviews of 4,425 adults.
Disclosures: The investigators reported no relevant financial disclosures.
Seventy percent of docs using EHRs to e-prescribe
About 70% of U.S. physicians were using an electronic health record system to electronically transmit prescriptions to pharmacies as of April 2014, according to an analysis by the Office of the National Coordinator for Health Information Technology.
This represents a rapid scale-up in the use of EHRs with the passage of MIPPA [Medicare Improvements for Patients and Providers Act] in December 2008 (7%) and when the Medicare and Medicaid EHR Incentive Programs began (24%) in 2009, according to the ONCHIT report.
The figures are based on an analysis of data from Surescripts, which provides IT network infrastructure that transmits electronic prescriptions and other health-related data.
Nearly all community pharmacies across the United States are enabled to accept e-prescriptions, according to the report.
"The growth of physicians and pharmacies e-prescribing has corresponded with a 14-fold increase in the growth of new and renewal prescriptions sent electronically," the report states, growing from 4% in 2008 to 57% in 2013. For 2013, this translates to 1 billion of the total 1.8 billion new and renewal prescriptions being sent electronically.
About 70% of U.S. physicians were using an electronic health record system to electronically transmit prescriptions to pharmacies as of April 2014, according to an analysis by the Office of the National Coordinator for Health Information Technology.
This represents a rapid scale-up in the use of EHRs with the passage of MIPPA [Medicare Improvements for Patients and Providers Act] in December 2008 (7%) and when the Medicare and Medicaid EHR Incentive Programs began (24%) in 2009, according to the ONCHIT report.
The figures are based on an analysis of data from Surescripts, which provides IT network infrastructure that transmits electronic prescriptions and other health-related data.
Nearly all community pharmacies across the United States are enabled to accept e-prescriptions, according to the report.
"The growth of physicians and pharmacies e-prescribing has corresponded with a 14-fold increase in the growth of new and renewal prescriptions sent electronically," the report states, growing from 4% in 2008 to 57% in 2013. For 2013, this translates to 1 billion of the total 1.8 billion new and renewal prescriptions being sent electronically.
About 70% of U.S. physicians were using an electronic health record system to electronically transmit prescriptions to pharmacies as of April 2014, according to an analysis by the Office of the National Coordinator for Health Information Technology.
This represents a rapid scale-up in the use of EHRs with the passage of MIPPA [Medicare Improvements for Patients and Providers Act] in December 2008 (7%) and when the Medicare and Medicaid EHR Incentive Programs began (24%) in 2009, according to the ONCHIT report.
The figures are based on an analysis of data from Surescripts, which provides IT network infrastructure that transmits electronic prescriptions and other health-related data.
Nearly all community pharmacies across the United States are enabled to accept e-prescriptions, according to the report.
"The growth of physicians and pharmacies e-prescribing has corresponded with a 14-fold increase in the growth of new and renewal prescriptions sent electronically," the report states, growing from 4% in 2008 to 57% in 2013. For 2013, this translates to 1 billion of the total 1.8 billion new and renewal prescriptions being sent electronically.
EHR use hasn’t sent Medicare payments soaring
Concerns that electronic health records will result in an uptick in Medicare payments to hospitals – for legitimate or fraudulent reasons – appear to be unfounded.
Anecdotal reports have surfaced in the wake of a federal incentive program to push for the adoption of EHRs that hospitals might be using the systems to boost payments by more accurately capturing, and more accurately charging, for services rendered than previously recorded, by "upcoding" or selecting billing codes that reflect more intensive procedures or reflect a sicker patient population, or by simply "cloning" entries into an EHR to provide higher billing to multiple patients that might not reflect the care provided. These reports have led the Department of Health & Human Services Office of Inspector General to call for Medicare administrative and program integrity contractors to do more to detect potential fraud from the use of EHRs.
However, research by Julia Adler-Milstein, Ph.D., of the University of Michigan, Ann Arbor, and Dr. Ashish Jha of the Harvard School of Public Health, Boston, suggests that this kind of fraud has not been an issue.
"We found that hospitals that adopted EHRs increased billing to Medicare, but at a rate comparable to that of matched controls of non-EHR adopters," the researchers wrote in the article appearing in the July issue of Health Affairs (July 2014 [doi:10.1377/hlthaff.2014.0023]).
Researchers looked at 393 hospitals that had newly adopted a basic EHR (181 that adopted between 2008 and 2009 and 212 that adopted between 2009 and 2010) and compared them to 782 control hospitals that did not adopt during the same periods. New adopters were predominantly nonteaching (61%), for-profit (70%), and medium-size (47%) hospitals.
In the research models, "we found no significant relationship between EHR adoption and patient acuity," the report states. Also, "adopters and controls had indistinguishable changes in Medicare payments. Between the pre- and postadoption periods, payment per discharge to adopters grew by $849 and to controls by $945. This $96 difference in difference was not in the predicted direction, but it was not significant, either (P = .673)," the researchers wrote.
They suggested that the results were due to hospitals heavily investing in optimizing coding before adopting EHRs.
"Hospitals operate on thin financial margins and therefore likely work hard to maximize reimbursement," the researchers wrote. "Thus, simply having more electronic data or better documentation may not provide as much of an opportunity to increase coding as has been postulated."
They concluded that while there will always be outliers that engage in fraudulent behavior, "our findings suggest that a large-scale policy effort targeting EHR-driven fraudulent coding, such as the one recently recommended by the HHS Office of Inspector General, is not likely to be useful. Substantial savings are unlikely to result even from a policy targeting certain subgroups of hospitals that might be expected to experience the greatest pressure to increase billing after investing in an EHR system."
Concerns that electronic health records will result in an uptick in Medicare payments to hospitals – for legitimate or fraudulent reasons – appear to be unfounded.
Anecdotal reports have surfaced in the wake of a federal incentive program to push for the adoption of EHRs that hospitals might be using the systems to boost payments by more accurately capturing, and more accurately charging, for services rendered than previously recorded, by "upcoding" or selecting billing codes that reflect more intensive procedures or reflect a sicker patient population, or by simply "cloning" entries into an EHR to provide higher billing to multiple patients that might not reflect the care provided. These reports have led the Department of Health & Human Services Office of Inspector General to call for Medicare administrative and program integrity contractors to do more to detect potential fraud from the use of EHRs.
However, research by Julia Adler-Milstein, Ph.D., of the University of Michigan, Ann Arbor, and Dr. Ashish Jha of the Harvard School of Public Health, Boston, suggests that this kind of fraud has not been an issue.
"We found that hospitals that adopted EHRs increased billing to Medicare, but at a rate comparable to that of matched controls of non-EHR adopters," the researchers wrote in the article appearing in the July issue of Health Affairs (July 2014 [doi:10.1377/hlthaff.2014.0023]).
Researchers looked at 393 hospitals that had newly adopted a basic EHR (181 that adopted between 2008 and 2009 and 212 that adopted between 2009 and 2010) and compared them to 782 control hospitals that did not adopt during the same periods. New adopters were predominantly nonteaching (61%), for-profit (70%), and medium-size (47%) hospitals.
In the research models, "we found no significant relationship between EHR adoption and patient acuity," the report states. Also, "adopters and controls had indistinguishable changes in Medicare payments. Between the pre- and postadoption periods, payment per discharge to adopters grew by $849 and to controls by $945. This $96 difference in difference was not in the predicted direction, but it was not significant, either (P = .673)," the researchers wrote.
They suggested that the results were due to hospitals heavily investing in optimizing coding before adopting EHRs.
"Hospitals operate on thin financial margins and therefore likely work hard to maximize reimbursement," the researchers wrote. "Thus, simply having more electronic data or better documentation may not provide as much of an opportunity to increase coding as has been postulated."
They concluded that while there will always be outliers that engage in fraudulent behavior, "our findings suggest that a large-scale policy effort targeting EHR-driven fraudulent coding, such as the one recently recommended by the HHS Office of Inspector General, is not likely to be useful. Substantial savings are unlikely to result even from a policy targeting certain subgroups of hospitals that might be expected to experience the greatest pressure to increase billing after investing in an EHR system."
Concerns that electronic health records will result in an uptick in Medicare payments to hospitals – for legitimate or fraudulent reasons – appear to be unfounded.
Anecdotal reports have surfaced in the wake of a federal incentive program to push for the adoption of EHRs that hospitals might be using the systems to boost payments by more accurately capturing, and more accurately charging, for services rendered than previously recorded, by "upcoding" or selecting billing codes that reflect more intensive procedures or reflect a sicker patient population, or by simply "cloning" entries into an EHR to provide higher billing to multiple patients that might not reflect the care provided. These reports have led the Department of Health & Human Services Office of Inspector General to call for Medicare administrative and program integrity contractors to do more to detect potential fraud from the use of EHRs.
However, research by Julia Adler-Milstein, Ph.D., of the University of Michigan, Ann Arbor, and Dr. Ashish Jha of the Harvard School of Public Health, Boston, suggests that this kind of fraud has not been an issue.
"We found that hospitals that adopted EHRs increased billing to Medicare, but at a rate comparable to that of matched controls of non-EHR adopters," the researchers wrote in the article appearing in the July issue of Health Affairs (July 2014 [doi:10.1377/hlthaff.2014.0023]).
Researchers looked at 393 hospitals that had newly adopted a basic EHR (181 that adopted between 2008 and 2009 and 212 that adopted between 2009 and 2010) and compared them to 782 control hospitals that did not adopt during the same periods. New adopters were predominantly nonteaching (61%), for-profit (70%), and medium-size (47%) hospitals.
In the research models, "we found no significant relationship between EHR adoption and patient acuity," the report states. Also, "adopters and controls had indistinguishable changes in Medicare payments. Between the pre- and postadoption periods, payment per discharge to adopters grew by $849 and to controls by $945. This $96 difference in difference was not in the predicted direction, but it was not significant, either (P = .673)," the researchers wrote.
They suggested that the results were due to hospitals heavily investing in optimizing coding before adopting EHRs.
"Hospitals operate on thin financial margins and therefore likely work hard to maximize reimbursement," the researchers wrote. "Thus, simply having more electronic data or better documentation may not provide as much of an opportunity to increase coding as has been postulated."
They concluded that while there will always be outliers that engage in fraudulent behavior, "our findings suggest that a large-scale policy effort targeting EHR-driven fraudulent coding, such as the one recently recommended by the HHS Office of Inspector General, is not likely to be useful. Substantial savings are unlikely to result even from a policy targeting certain subgroups of hospitals that might be expected to experience the greatest pressure to increase billing after investing in an EHR system."
FROM HEALTH AFFAIRS
Major finding: Hospitals adopting EHRs are not showing greater increases in Medicare payments or patient acuity.
Data source: Analysis of data provided by the Centers for Medicare & Medicaid Services and the American Hospital Association.
Disclosures: Article was submitted to Health Affairs, no outside sources of financing were used to fund the research. Authors reported no financial disclosures.
HHS awards more money for innovative care models
The federal government has announced another round of awards to health care organizations that have designed innovative ways to provide better-quality care at a lower cost.
The latest group includes 27 health care organizations, receiving awards of $2 million to $24 million over 3 years.
"The Health Care Innovation Awards support our ongoing work to drive down health care costs while providing high-quality care," Sylvia Mathews Burwell, secretary of the Department of Health & Human Services (HHS), said in a statement. "These awards advance innovative solutions in delivering and improving care from all across our nation."
The awards are funded with up to $1 billion in money authorized by the Affordable Care Act. HHS began seeking awardees in late 2011, soliciting a broad range of proposals. The Centers for Medicare & Medicaid Services Innovation Center ultimately gave out 107 3-year awards in May and June 2012.
HHS started soliciting applicants for a second round of awards last year, this time looking for innovations that would focus on four areas: rapidly reducing costs in the outpatient and postacute setting for patients with Medicare, Medicaid, and/or the Children’s Health Insurance Program; improving care for populations with specialized needs; testing improved financial and clinical models for specific types of providers, including specialists; and models that improve the health of populations – such as a community, or those with specific diseases – through prevention, wellness, and comprehensive care that extends beyond the clinical setting.
The 27 new awardees named on July 9 include:
• $6 million for a care coordination project at the Boston Medical Center that will pair a complex care nurse, a care coordinator, and pediatricians in the community in Boston and Springfield, with the goal of providing a medical home for children with complex conditions.
• $10 million for the Detroit Medical Center, which will establish patient-centered medical home clinics next to emergency departments (EDs) at four inner-city hospitals, in an attempt to make primary care immediately available to patients seeking nonurgent care. The initial focus will be on improving diabetes or asthma, and targeting so-called ED superutilizers, who have 10 or more visits a year.
• $15 million for the University of New Mexico Health Sciences Center, Albuquerque, which will expand its 11-hospital telehealth system to 30 hospitals, to test providing remote emergency neurological consultation using inexpensive audiovisual equipment and software.
HHS does not anticipate making any further innovation awards, according to the agency.
Awardees still have to meet certain criteria to receive their funds. Final award notices will be sent in a few months.
On Twitter @aliciaault
The federal government has announced another round of awards to health care organizations that have designed innovative ways to provide better-quality care at a lower cost.
The latest group includes 27 health care organizations, receiving awards of $2 million to $24 million over 3 years.
"The Health Care Innovation Awards support our ongoing work to drive down health care costs while providing high-quality care," Sylvia Mathews Burwell, secretary of the Department of Health & Human Services (HHS), said in a statement. "These awards advance innovative solutions in delivering and improving care from all across our nation."
The awards are funded with up to $1 billion in money authorized by the Affordable Care Act. HHS began seeking awardees in late 2011, soliciting a broad range of proposals. The Centers for Medicare & Medicaid Services Innovation Center ultimately gave out 107 3-year awards in May and June 2012.
HHS started soliciting applicants for a second round of awards last year, this time looking for innovations that would focus on four areas: rapidly reducing costs in the outpatient and postacute setting for patients with Medicare, Medicaid, and/or the Children’s Health Insurance Program; improving care for populations with specialized needs; testing improved financial and clinical models for specific types of providers, including specialists; and models that improve the health of populations – such as a community, or those with specific diseases – through prevention, wellness, and comprehensive care that extends beyond the clinical setting.
The 27 new awardees named on July 9 include:
• $6 million for a care coordination project at the Boston Medical Center that will pair a complex care nurse, a care coordinator, and pediatricians in the community in Boston and Springfield, with the goal of providing a medical home for children with complex conditions.
• $10 million for the Detroit Medical Center, which will establish patient-centered medical home clinics next to emergency departments (EDs) at four inner-city hospitals, in an attempt to make primary care immediately available to patients seeking nonurgent care. The initial focus will be on improving diabetes or asthma, and targeting so-called ED superutilizers, who have 10 or more visits a year.
• $15 million for the University of New Mexico Health Sciences Center, Albuquerque, which will expand its 11-hospital telehealth system to 30 hospitals, to test providing remote emergency neurological consultation using inexpensive audiovisual equipment and software.
HHS does not anticipate making any further innovation awards, according to the agency.
Awardees still have to meet certain criteria to receive their funds. Final award notices will be sent in a few months.
On Twitter @aliciaault
The federal government has announced another round of awards to health care organizations that have designed innovative ways to provide better-quality care at a lower cost.
The latest group includes 27 health care organizations, receiving awards of $2 million to $24 million over 3 years.
"The Health Care Innovation Awards support our ongoing work to drive down health care costs while providing high-quality care," Sylvia Mathews Burwell, secretary of the Department of Health & Human Services (HHS), said in a statement. "These awards advance innovative solutions in delivering and improving care from all across our nation."
The awards are funded with up to $1 billion in money authorized by the Affordable Care Act. HHS began seeking awardees in late 2011, soliciting a broad range of proposals. The Centers for Medicare & Medicaid Services Innovation Center ultimately gave out 107 3-year awards in May and June 2012.
HHS started soliciting applicants for a second round of awards last year, this time looking for innovations that would focus on four areas: rapidly reducing costs in the outpatient and postacute setting for patients with Medicare, Medicaid, and/or the Children’s Health Insurance Program; improving care for populations with specialized needs; testing improved financial and clinical models for specific types of providers, including specialists; and models that improve the health of populations – such as a community, or those with specific diseases – through prevention, wellness, and comprehensive care that extends beyond the clinical setting.
The 27 new awardees named on July 9 include:
• $6 million for a care coordination project at the Boston Medical Center that will pair a complex care nurse, a care coordinator, and pediatricians in the community in Boston and Springfield, with the goal of providing a medical home for children with complex conditions.
• $10 million for the Detroit Medical Center, which will establish patient-centered medical home clinics next to emergency departments (EDs) at four inner-city hospitals, in an attempt to make primary care immediately available to patients seeking nonurgent care. The initial focus will be on improving diabetes or asthma, and targeting so-called ED superutilizers, who have 10 or more visits a year.
• $15 million for the University of New Mexico Health Sciences Center, Albuquerque, which will expand its 11-hospital telehealth system to 30 hospitals, to test providing remote emergency neurological consultation using inexpensive audiovisual equipment and software.
HHS does not anticipate making any further innovation awards, according to the agency.
Awardees still have to meet certain criteria to receive their funds. Final award notices will be sent in a few months.
On Twitter @aliciaault
Heroin-related drug-poisoning deaths rose 110% from 2002 to 2011
The annual number of drug-poisoning deaths involving heroin more than doubled from 2002 to 2011, increasing from 2,089 to 4,397, the National Center for Health Statistics reported.
Over that period, the rate of heroin-related drug-poisoning deaths increased 171% among non-Hispanic whites aged 18-44 years – going from 1.4 per 100,000 population to 3.8 – and by 100% among non-Hispanic whites aged 45-64 years – from 0.7 to 1.4 per 100,000, according to the NCHS (MMWR 2014;63:595).
The rates for non-Hispanic blacks and Hispanics, by comparison, were stable. Non-Hispanic blacks were at 1.0 per 100,000 for younger adults and 2.2 per 100,000 for those aged 45-64 years in 2002 and remained there in 2011. The rate for Hispanics aged 18-44 years rose from 1.3 to 1.5, but the rate for 45- to 64-year-olds dropped from 2 to 1.9 per 100,000, according to mortality data from the National Vital Statistics System.

The annual number of drug-poisoning deaths involving heroin more than doubled from 2002 to 2011, increasing from 2,089 to 4,397, the National Center for Health Statistics reported.
Over that period, the rate of heroin-related drug-poisoning deaths increased 171% among non-Hispanic whites aged 18-44 years – going from 1.4 per 100,000 population to 3.8 – and by 100% among non-Hispanic whites aged 45-64 years – from 0.7 to 1.4 per 100,000, according to the NCHS (MMWR 2014;63:595).
The rates for non-Hispanic blacks and Hispanics, by comparison, were stable. Non-Hispanic blacks were at 1.0 per 100,000 for younger adults and 2.2 per 100,000 for those aged 45-64 years in 2002 and remained there in 2011. The rate for Hispanics aged 18-44 years rose from 1.3 to 1.5, but the rate for 45- to 64-year-olds dropped from 2 to 1.9 per 100,000, according to mortality data from the National Vital Statistics System.

The annual number of drug-poisoning deaths involving heroin more than doubled from 2002 to 2011, increasing from 2,089 to 4,397, the National Center for Health Statistics reported.
Over that period, the rate of heroin-related drug-poisoning deaths increased 171% among non-Hispanic whites aged 18-44 years – going from 1.4 per 100,000 population to 3.8 – and by 100% among non-Hispanic whites aged 45-64 years – from 0.7 to 1.4 per 100,000, according to the NCHS (MMWR 2014;63:595).
The rates for non-Hispanic blacks and Hispanics, by comparison, were stable. Non-Hispanic blacks were at 1.0 per 100,000 for younger adults and 2.2 per 100,000 for those aged 45-64 years in 2002 and remained there in 2011. The rate for Hispanics aged 18-44 years rose from 1.3 to 1.5, but the rate for 45- to 64-year-olds dropped from 2 to 1.9 per 100,000, according to mortality data from the National Vital Statistics System.

FROM MMWR
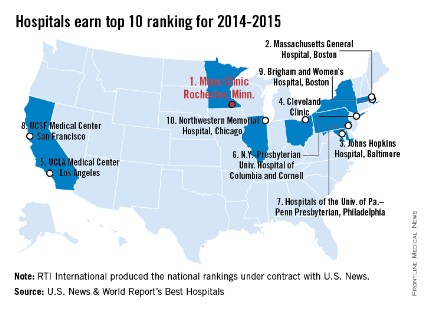
Mayo Clinic tops hospital rankings for 2014-2015
The Mayo Clinic in Rochester, Minn., is the best hospital in the United States, according to the U.S. News & World Report Best Hospitals rankings for 2014-2015.
This is the first time that the Mayo Clinic has taken the top spot in the Best Hospitals Honor Roll. This year’s second-place finisher was Massachusetts General Hospital in Boston, followed by Johns Hopkins Hospital in Baltimore – which won last year – the Cleveland Clinic, and UCLA Medical Center in Los Angeles, U.S. News announced.
For 2014-2015, there were 17 hospitals in the Honor Roll, which is reserved for those institutions that finish at or near the top in 6 or more of the 16 specialties included in the U.S. News rankings. This year, only 144 hospitals did well enough to be nationally ranked in one or more specialties. The 16 ranked specialties are cancer, cardiology and heart surgery; diabetes and endocrinology; otolaryngology; gastroenterology and gastrointestinal surgery; geriatrics; gynecology; nephrology; neurology and neurosurgery; ophthalmology; orthopedics; psychiatry; pulmonology; rehabilitation; rheumatology; and urology.
The ranking process initially comprised 4,743 nonfederal community hospitals, which were rated in the 16 specialties. In the 12 data-driven specialties, scores were based on four elements: reputation with specialists (27.5%), survival (32.5%), patient safety (10%), and other care-related indicators (30%). Four specialties were rated by reputation only. The research organization RTI International conducted the physician survey and produced the Best Hospitals methodology and national rankings under contract with U.S. News.
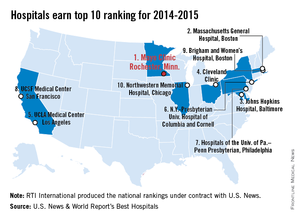
The Mayo Clinic in Rochester, Minn., is the best hospital in the United States, according to the U.S. News & World Report Best Hospitals rankings for 2014-2015.
This is the first time that the Mayo Clinic has taken the top spot in the Best Hospitals Honor Roll. This year’s second-place finisher was Massachusetts General Hospital in Boston, followed by Johns Hopkins Hospital in Baltimore – which won last year – the Cleveland Clinic, and UCLA Medical Center in Los Angeles, U.S. News announced.
For 2014-2015, there were 17 hospitals in the Honor Roll, which is reserved for those institutions that finish at or near the top in 6 or more of the 16 specialties included in the U.S. News rankings. This year, only 144 hospitals did well enough to be nationally ranked in one or more specialties. The 16 ranked specialties are cancer, cardiology and heart surgery; diabetes and endocrinology; otolaryngology; gastroenterology and gastrointestinal surgery; geriatrics; gynecology; nephrology; neurology and neurosurgery; ophthalmology; orthopedics; psychiatry; pulmonology; rehabilitation; rheumatology; and urology.
The ranking process initially comprised 4,743 nonfederal community hospitals, which were rated in the 16 specialties. In the 12 data-driven specialties, scores were based on four elements: reputation with specialists (27.5%), survival (32.5%), patient safety (10%), and other care-related indicators (30%). Four specialties were rated by reputation only. The research organization RTI International conducted the physician survey and produced the Best Hospitals methodology and national rankings under contract with U.S. News.

The Mayo Clinic in Rochester, Minn., is the best hospital in the United States, according to the U.S. News & World Report Best Hospitals rankings for 2014-2015.
This is the first time that the Mayo Clinic has taken the top spot in the Best Hospitals Honor Roll. This year’s second-place finisher was Massachusetts General Hospital in Boston, followed by Johns Hopkins Hospital in Baltimore – which won last year – the Cleveland Clinic, and UCLA Medical Center in Los Angeles, U.S. News announced.
For 2014-2015, there were 17 hospitals in the Honor Roll, which is reserved for those institutions that finish at or near the top in 6 or more of the 16 specialties included in the U.S. News rankings. This year, only 144 hospitals did well enough to be nationally ranked in one or more specialties. The 16 ranked specialties are cancer, cardiology and heart surgery; diabetes and endocrinology; otolaryngology; gastroenterology and gastrointestinal surgery; geriatrics; gynecology; nephrology; neurology and neurosurgery; ophthalmology; orthopedics; psychiatry; pulmonology; rehabilitation; rheumatology; and urology.
The ranking process initially comprised 4,743 nonfederal community hospitals, which were rated in the 16 specialties. In the 12 data-driven specialties, scores were based on four elements: reputation with specialists (27.5%), survival (32.5%), patient safety (10%), and other care-related indicators (30%). Four specialties were rated by reputation only. The research organization RTI International conducted the physician survey and produced the Best Hospitals methodology and national rankings under contract with U.S. News.

ICD-10: Dual coding is only for testing, claims backlog
There’s plenty to be confused about when it comes to the transition to the new ICD-10 coding system in October 2015. But the government has issued some clarification about at least one issue: when to use dual coding.
Dual coding, also called dual processing, generally means using both ICD-9 and ICD-10 at the same time when submitting claims.
In an e-mail July 10, officials at the Centers for Medicare & Medicaid Services (CMS) said physicians and coders may engage in dual coding as a way to test their ICD-10 readiness before the compliance date. They may also code in both systems after the compliance date, if they have a backlog of claims.
Here’s how it works: Before ICD-10 goes into effect on Oct. 1, 2015, physicians and coders can practice by coding current claims in both systems to see if they have the right level of documentation for the new system. ICD-10 codes can also be used to test whether payers and clearinghouses are ready to receive and process the new codes. However, only the ICD-9 code can be sent to payers as part of a "live" transaction before the compliance date.
Physicians may also find that they will use both coding sets for a short period of time after the compliance date, if they need to submit claims with a date of service that occurred before Oct. 1, 2015.
But the physicians and coders don’t get to choose which system to code in. The date of service determines whether ICD-9 or ICD-10 is used, according to CMS, with services performed before Oct. 1 getting an ICD-9 code and services on and after Oct. 1 receiving an ICD-10 code.
CMS is expected to release a regulation soon with more details on the rollout of ICD-10.
On Twitter @maryellenny
There’s plenty to be confused about when it comes to the transition to the new ICD-10 coding system in October 2015. But the government has issued some clarification about at least one issue: when to use dual coding.
Dual coding, also called dual processing, generally means using both ICD-9 and ICD-10 at the same time when submitting claims.
In an e-mail July 10, officials at the Centers for Medicare & Medicaid Services (CMS) said physicians and coders may engage in dual coding as a way to test their ICD-10 readiness before the compliance date. They may also code in both systems after the compliance date, if they have a backlog of claims.
Here’s how it works: Before ICD-10 goes into effect on Oct. 1, 2015, physicians and coders can practice by coding current claims in both systems to see if they have the right level of documentation for the new system. ICD-10 codes can also be used to test whether payers and clearinghouses are ready to receive and process the new codes. However, only the ICD-9 code can be sent to payers as part of a "live" transaction before the compliance date.
Physicians may also find that they will use both coding sets for a short period of time after the compliance date, if they need to submit claims with a date of service that occurred before Oct. 1, 2015.
But the physicians and coders don’t get to choose which system to code in. The date of service determines whether ICD-9 or ICD-10 is used, according to CMS, with services performed before Oct. 1 getting an ICD-9 code and services on and after Oct. 1 receiving an ICD-10 code.
CMS is expected to release a regulation soon with more details on the rollout of ICD-10.
On Twitter @maryellenny
There’s plenty to be confused about when it comes to the transition to the new ICD-10 coding system in October 2015. But the government has issued some clarification about at least one issue: when to use dual coding.
Dual coding, also called dual processing, generally means using both ICD-9 and ICD-10 at the same time when submitting claims.
In an e-mail July 10, officials at the Centers for Medicare & Medicaid Services (CMS) said physicians and coders may engage in dual coding as a way to test their ICD-10 readiness before the compliance date. They may also code in both systems after the compliance date, if they have a backlog of claims.
Here’s how it works: Before ICD-10 goes into effect on Oct. 1, 2015, physicians and coders can practice by coding current claims in both systems to see if they have the right level of documentation for the new system. ICD-10 codes can also be used to test whether payers and clearinghouses are ready to receive and process the new codes. However, only the ICD-9 code can be sent to payers as part of a "live" transaction before the compliance date.
Physicians may also find that they will use both coding sets for a short period of time after the compliance date, if they need to submit claims with a date of service that occurred before Oct. 1, 2015.
But the physicians and coders don’t get to choose which system to code in. The date of service determines whether ICD-9 or ICD-10 is used, according to CMS, with services performed before Oct. 1 getting an ICD-9 code and services on and after Oct. 1 receiving an ICD-10 code.
CMS is expected to release a regulation soon with more details on the rollout of ICD-10.
On Twitter @maryellenny
Medicare’s 2015 outpatient proposal continues focus on bundled pay
The Centers for Medicare and Medicaid Services’ proposed rule on outpatient department and ambulatory surgery center payment for 2015 expands the agency’s focus on bundling pay for device-related procedures, largely in cardiology, neurology, oncology, and gynecology.
The Hospital Outpatient Prospective Payment System (OPPS) rule also continues the same payment rate for outpatient drug delivery such as chemotherapy. That payment rate has been a source of disappointment for oncologists. The American Society of Clinical Oncology has said in the past that, with the additional impact of budget sequestration, the actual payment for delivering chemotherapy drugs falls by 28%.
The agency is proposing again in 2015 to continue paying average sales price plus 6% for non–pass through drugs and biologicals that are administered under Part B of Medicare.
Proposed on July 3, the rule will be published on July 14. It covers payment for 4,000 hospitals, including general acute care hospitals, inpatient rehabilitation facilities, inpatient psychiatric facilities, long-term acute care hospitals, children’s hospitals, and cancer hospitals. It also applies to 5,300 ambulatory surgery centers (ASCs) that participate in Medicare.
Overall, the government is proposing to increase payments to outpatient departments by 2%. The CMS expects to pay out some $57 billion for outpatient services in 2015. Payments to ASCs will increase just over 1% to $4 billion in 2015.
The agency is proposing to expand its Comprehensive Ambulatory Payment Classification (APC) policy, which was first discussed in its 2014 rule. The idea is to give a single Medicare payment and require a single beneficiary copayment for the entire hospital stay for a group of 28 procedures, including pacemaker insertion, implantation of neurostimulators, and stereotactic radiosurgery. It also would cover implantable cardioverter defibrillators.
The single, bundled payments would begin in 2015.
The proposed rule also contains several adjustments to both the Hospital Outpatient Quality Reporting Program and the ASC Quality Reporting Program. On the hospital side, the CMS is proposing to remove three quality measures, stating that performance has been uniformly high among reporting facilities. Those measures are aspirin at arrival (cardiac care), timing of prophylaxis antibiotics, and prophylactic antibiotic selection for surgical patients. The agency is proposing to add a claims-based measure – facility 7-day risk-standardized hospital visit rate after outpatient colonoscopy – for 2017 and beyond.
For ASCs, the agency is proposing to continue its effort to align measures with the hospital program. In 2015, ASCs will be required to report on the 7-day risk-standardized visit rate after outpatient colonoscopy measure.
The CMS is accepting comments on the proposed rule until Sept. 2, 2014. A final rule will be issued by Nov. 1.
On Twitter @aliciaault
The Centers for Medicare and Medicaid Services’ proposed rule on outpatient department and ambulatory surgery center payment for 2015 expands the agency’s focus on bundling pay for device-related procedures, largely in cardiology, neurology, oncology, and gynecology.
The Hospital Outpatient Prospective Payment System (OPPS) rule also continues the same payment rate for outpatient drug delivery such as chemotherapy. That payment rate has been a source of disappointment for oncologists. The American Society of Clinical Oncology has said in the past that, with the additional impact of budget sequestration, the actual payment for delivering chemotherapy drugs falls by 28%.
The agency is proposing again in 2015 to continue paying average sales price plus 6% for non–pass through drugs and biologicals that are administered under Part B of Medicare.
Proposed on July 3, the rule will be published on July 14. It covers payment for 4,000 hospitals, including general acute care hospitals, inpatient rehabilitation facilities, inpatient psychiatric facilities, long-term acute care hospitals, children’s hospitals, and cancer hospitals. It also applies to 5,300 ambulatory surgery centers (ASCs) that participate in Medicare.
Overall, the government is proposing to increase payments to outpatient departments by 2%. The CMS expects to pay out some $57 billion for outpatient services in 2015. Payments to ASCs will increase just over 1% to $4 billion in 2015.
The agency is proposing to expand its Comprehensive Ambulatory Payment Classification (APC) policy, which was first discussed in its 2014 rule. The idea is to give a single Medicare payment and require a single beneficiary copayment for the entire hospital stay for a group of 28 procedures, including pacemaker insertion, implantation of neurostimulators, and stereotactic radiosurgery. It also would cover implantable cardioverter defibrillators.
The single, bundled payments would begin in 2015.
The proposed rule also contains several adjustments to both the Hospital Outpatient Quality Reporting Program and the ASC Quality Reporting Program. On the hospital side, the CMS is proposing to remove three quality measures, stating that performance has been uniformly high among reporting facilities. Those measures are aspirin at arrival (cardiac care), timing of prophylaxis antibiotics, and prophylactic antibiotic selection for surgical patients. The agency is proposing to add a claims-based measure – facility 7-day risk-standardized hospital visit rate after outpatient colonoscopy – for 2017 and beyond.
For ASCs, the agency is proposing to continue its effort to align measures with the hospital program. In 2015, ASCs will be required to report on the 7-day risk-standardized visit rate after outpatient colonoscopy measure.
The CMS is accepting comments on the proposed rule until Sept. 2, 2014. A final rule will be issued by Nov. 1.
On Twitter @aliciaault
The Centers for Medicare and Medicaid Services’ proposed rule on outpatient department and ambulatory surgery center payment for 2015 expands the agency’s focus on bundling pay for device-related procedures, largely in cardiology, neurology, oncology, and gynecology.
The Hospital Outpatient Prospective Payment System (OPPS) rule also continues the same payment rate for outpatient drug delivery such as chemotherapy. That payment rate has been a source of disappointment for oncologists. The American Society of Clinical Oncology has said in the past that, with the additional impact of budget sequestration, the actual payment for delivering chemotherapy drugs falls by 28%.
The agency is proposing again in 2015 to continue paying average sales price plus 6% for non–pass through drugs and biologicals that are administered under Part B of Medicare.
Proposed on July 3, the rule will be published on July 14. It covers payment for 4,000 hospitals, including general acute care hospitals, inpatient rehabilitation facilities, inpatient psychiatric facilities, long-term acute care hospitals, children’s hospitals, and cancer hospitals. It also applies to 5,300 ambulatory surgery centers (ASCs) that participate in Medicare.
Overall, the government is proposing to increase payments to outpatient departments by 2%. The CMS expects to pay out some $57 billion for outpatient services in 2015. Payments to ASCs will increase just over 1% to $4 billion in 2015.
The agency is proposing to expand its Comprehensive Ambulatory Payment Classification (APC) policy, which was first discussed in its 2014 rule. The idea is to give a single Medicare payment and require a single beneficiary copayment for the entire hospital stay for a group of 28 procedures, including pacemaker insertion, implantation of neurostimulators, and stereotactic radiosurgery. It also would cover implantable cardioverter defibrillators.
The single, bundled payments would begin in 2015.
The proposed rule also contains several adjustments to both the Hospital Outpatient Quality Reporting Program and the ASC Quality Reporting Program. On the hospital side, the CMS is proposing to remove three quality measures, stating that performance has been uniformly high among reporting facilities. Those measures are aspirin at arrival (cardiac care), timing of prophylaxis antibiotics, and prophylactic antibiotic selection for surgical patients. The agency is proposing to add a claims-based measure – facility 7-day risk-standardized hospital visit rate after outpatient colonoscopy – for 2017 and beyond.
For ASCs, the agency is proposing to continue its effort to align measures with the hospital program. In 2015, ASCs will be required to report on the 7-day risk-standardized visit rate after outpatient colonoscopy measure.
The CMS is accepting comments on the proposed rule until Sept. 2, 2014. A final rule will be issued by Nov. 1.
On Twitter @aliciaault
Accountable care organizations may fuel new litigation theories
The aim of accountable care organizations is to improve health care quality, enhance care coordination, and reduce unnecessary costs. But the new health care delivery models are raising questions about possible hidden legal dangers for participating physicians.
"We’re talking about unchartered territory," said Christopher E. DiGiacinto, a medical liability defense attorney and partner in a New York law firm that focuses on the defense of professional liability claims including those brought against health care professionals and others. "There’s been a lot of uncertainty about how [ACOs] will affect the landscape of litigation. It could go any number of ways."
Mr. DiGiacinto cowrote an article in the 2013 summer issue of Risk Management Quarterly, the journal of the Association for Healthcare Risk Management of New York, detailing malpractice risks doctors may face within ACOs (RMQ Summer 2013). The liability dangers stem primarily from federal guidelines that outline how ACOs should operate and how doctors can enhance their practices.
For example, the Affordance Care Act requires that ACOs share medical information across multiple health care environments to improve knowledge among providers and to eliminate duplication of treatment across the care continuum. But such enhanced record maintenance could expose physicians to increased liability, Mr. DiGiacinto said. A plaintiff’s attorney could claim a doctor’s failure to access a patient’s prior medical records led to a subsequent poor medical outcome.
"There’s going to be a lot more data in this model, which is great for patients and allowing physicians to track patients," he said. "The downside for physicians is, where in the past, they might only be responsible for their own record and knowledge of the patient from their own perspective, now, they’re being responsible for knowing the [patient’s] history from other doctors. There’s going to be a wealth of information that could be used against them."
ACOs also create the potential for a heightened duty of informed consent for physicians, said Julian D. "Bo" Bobbitt Jr., senior partner and head of a health law group at a law firm in Raleigh, N.C. Federal guidelines call for ACOs to promote patient engagement during individualized treatment by involving patients and their families in making medical decisions.
"Under Medicare ACO regulations, there has to be a patient care plan and there has to be significant commitment to patient and family engagement and joint decision making," Mr. Bobbitt said. "What happens if you did a care plan, but you didn’t follow it? You were supposed to engage the family, but you didn’t?"
In such an instance, it’s possible a family member could sue, claiming he or she was not involved enough in the medical decision–making process, said Mr. Bobbitt.
Physicians who help create ACOs or hold administrative positions within the organizations may also be more at risk for being sued, say liability experts, whether or not they were directly involved in patient care.
In the past, entities such as HMOs were rarely sued for the actions of participants because such corporate structures are not generally responsible for the rendering of care, Mr. DiGiacinto said. However, federal guidelines recommend that medical professionals be involved in the corporate structure of ACOs, and that the organizations be accountable for the care they provide. This framework could fuel vicarious liability or corporate negligence claims in which the ACO itself is said to be liable for care provided to patients, according to the Accountable Care Legal Guide and RMQ article. In addition, physician leaders could potentially be sued for alleged negligent credentialing of other health professionals in the ACO, said legal experts.
But some, such as Christi J. Braun, believe suggested ACO litigation dangers are being overblown. Clinically integrated networks are designed to improve quality across all care providers, said Ms. Braun, a Washington-based health care antitrust attorney and cochair of the American Health Lawyers Association’s Accountable Care Organization Task Force.
"Even if you may not be following the protocols all the time, just the fact that you’re looking at best practices and trying to apply best practices makes it more likely that you’re going to provide better care on a more consistent basis," she said. "That actually reduces liability."
At the same time, physicians should not be so focused on following federal guidelines that they allow metrics and benchmarks to override quality medical judgment, said Brandy A. Boone, an Alabama-based senior risk management consultant for a national medical liability insurer.
"I think the biggest risk associated with ACOs or any other arrangement where physicians are incentivized to keep costs down by the prospect of making more money is the allegation that necessary tests or treatments were not offered or recommended because of the effect on reimbursement," Ms. Boone said. "We always caution our insured physicians that treatment recommendations should never be based on the patient’s ability to pay. While the majority of physicians would never actually let reimbursement sway their clinical decisions, avoiding that perception is also very important."
Only time will tell how ACO guidelines will affect malpractice cases. Often, it takes years for case law and legal precedents to develop around new issues and more clearly define boundaries, Mr. Bobbitt said.
In the meantime, litigation experts recommend that physicians joining ACOs protect themselves from lawsuits by thoroughly documenting patient interactions and clinical decision making. Mr. Bobbitt suggests also that physicians participating in ACOs become involved in developing best practice guidelines and ensuring those guidelines are clinically valid. Having a strong voice will empower physicians and assure ACO guidelines act as a lawsuit shield, rather than a sword.
"It can be a legal minefield, but it is navigable," Mr. Bobbitt said. "As an attorney and health care adviser, I try to convey that yes, there are legal issues – novel legal issues – but at the same time, this is such a positive improvement to health care, it is navigable if done right."
The aim of accountable care organizations is to improve health care quality, enhance care coordination, and reduce unnecessary costs. But the new health care delivery models are raising questions about possible hidden legal dangers for participating physicians.
"We’re talking about unchartered territory," said Christopher E. DiGiacinto, a medical liability defense attorney and partner in a New York law firm that focuses on the defense of professional liability claims including those brought against health care professionals and others. "There’s been a lot of uncertainty about how [ACOs] will affect the landscape of litigation. It could go any number of ways."
Mr. DiGiacinto cowrote an article in the 2013 summer issue of Risk Management Quarterly, the journal of the Association for Healthcare Risk Management of New York, detailing malpractice risks doctors may face within ACOs (RMQ Summer 2013). The liability dangers stem primarily from federal guidelines that outline how ACOs should operate and how doctors can enhance their practices.
For example, the Affordance Care Act requires that ACOs share medical information across multiple health care environments to improve knowledge among providers and to eliminate duplication of treatment across the care continuum. But such enhanced record maintenance could expose physicians to increased liability, Mr. DiGiacinto said. A plaintiff’s attorney could claim a doctor’s failure to access a patient’s prior medical records led to a subsequent poor medical outcome.
"There’s going to be a lot more data in this model, which is great for patients and allowing physicians to track patients," he said. "The downside for physicians is, where in the past, they might only be responsible for their own record and knowledge of the patient from their own perspective, now, they’re being responsible for knowing the [patient’s] history from other doctors. There’s going to be a wealth of information that could be used against them."
ACOs also create the potential for a heightened duty of informed consent for physicians, said Julian D. "Bo" Bobbitt Jr., senior partner and head of a health law group at a law firm in Raleigh, N.C. Federal guidelines call for ACOs to promote patient engagement during individualized treatment by involving patients and their families in making medical decisions.
"Under Medicare ACO regulations, there has to be a patient care plan and there has to be significant commitment to patient and family engagement and joint decision making," Mr. Bobbitt said. "What happens if you did a care plan, but you didn’t follow it? You were supposed to engage the family, but you didn’t?"
In such an instance, it’s possible a family member could sue, claiming he or she was not involved enough in the medical decision–making process, said Mr. Bobbitt.
Physicians who help create ACOs or hold administrative positions within the organizations may also be more at risk for being sued, say liability experts, whether or not they were directly involved in patient care.
In the past, entities such as HMOs were rarely sued for the actions of participants because such corporate structures are not generally responsible for the rendering of care, Mr. DiGiacinto said. However, federal guidelines recommend that medical professionals be involved in the corporate structure of ACOs, and that the organizations be accountable for the care they provide. This framework could fuel vicarious liability or corporate negligence claims in which the ACO itself is said to be liable for care provided to patients, according to the Accountable Care Legal Guide and RMQ article. In addition, physician leaders could potentially be sued for alleged negligent credentialing of other health professionals in the ACO, said legal experts.
But some, such as Christi J. Braun, believe suggested ACO litigation dangers are being overblown. Clinically integrated networks are designed to improve quality across all care providers, said Ms. Braun, a Washington-based health care antitrust attorney and cochair of the American Health Lawyers Association’s Accountable Care Organization Task Force.
"Even if you may not be following the protocols all the time, just the fact that you’re looking at best practices and trying to apply best practices makes it more likely that you’re going to provide better care on a more consistent basis," she said. "That actually reduces liability."
At the same time, physicians should not be so focused on following federal guidelines that they allow metrics and benchmarks to override quality medical judgment, said Brandy A. Boone, an Alabama-based senior risk management consultant for a national medical liability insurer.
"I think the biggest risk associated with ACOs or any other arrangement where physicians are incentivized to keep costs down by the prospect of making more money is the allegation that necessary tests or treatments were not offered or recommended because of the effect on reimbursement," Ms. Boone said. "We always caution our insured physicians that treatment recommendations should never be based on the patient’s ability to pay. While the majority of physicians would never actually let reimbursement sway their clinical decisions, avoiding that perception is also very important."
Only time will tell how ACO guidelines will affect malpractice cases. Often, it takes years for case law and legal precedents to develop around new issues and more clearly define boundaries, Mr. Bobbitt said.
In the meantime, litigation experts recommend that physicians joining ACOs protect themselves from lawsuits by thoroughly documenting patient interactions and clinical decision making. Mr. Bobbitt suggests also that physicians participating in ACOs become involved in developing best practice guidelines and ensuring those guidelines are clinically valid. Having a strong voice will empower physicians and assure ACO guidelines act as a lawsuit shield, rather than a sword.
"It can be a legal minefield, but it is navigable," Mr. Bobbitt said. "As an attorney and health care adviser, I try to convey that yes, there are legal issues – novel legal issues – but at the same time, this is such a positive improvement to health care, it is navigable if done right."
The aim of accountable care organizations is to improve health care quality, enhance care coordination, and reduce unnecessary costs. But the new health care delivery models are raising questions about possible hidden legal dangers for participating physicians.
"We’re talking about unchartered territory," said Christopher E. DiGiacinto, a medical liability defense attorney and partner in a New York law firm that focuses on the defense of professional liability claims including those brought against health care professionals and others. "There’s been a lot of uncertainty about how [ACOs] will affect the landscape of litigation. It could go any number of ways."
Mr. DiGiacinto cowrote an article in the 2013 summer issue of Risk Management Quarterly, the journal of the Association for Healthcare Risk Management of New York, detailing malpractice risks doctors may face within ACOs (RMQ Summer 2013). The liability dangers stem primarily from federal guidelines that outline how ACOs should operate and how doctors can enhance their practices.
For example, the Affordance Care Act requires that ACOs share medical information across multiple health care environments to improve knowledge among providers and to eliminate duplication of treatment across the care continuum. But such enhanced record maintenance could expose physicians to increased liability, Mr. DiGiacinto said. A plaintiff’s attorney could claim a doctor’s failure to access a patient’s prior medical records led to a subsequent poor medical outcome.
"There’s going to be a lot more data in this model, which is great for patients and allowing physicians to track patients," he said. "The downside for physicians is, where in the past, they might only be responsible for their own record and knowledge of the patient from their own perspective, now, they’re being responsible for knowing the [patient’s] history from other doctors. There’s going to be a wealth of information that could be used against them."
ACOs also create the potential for a heightened duty of informed consent for physicians, said Julian D. "Bo" Bobbitt Jr., senior partner and head of a health law group at a law firm in Raleigh, N.C. Federal guidelines call for ACOs to promote patient engagement during individualized treatment by involving patients and their families in making medical decisions.
"Under Medicare ACO regulations, there has to be a patient care plan and there has to be significant commitment to patient and family engagement and joint decision making," Mr. Bobbitt said. "What happens if you did a care plan, but you didn’t follow it? You were supposed to engage the family, but you didn’t?"
In such an instance, it’s possible a family member could sue, claiming he or she was not involved enough in the medical decision–making process, said Mr. Bobbitt.
Physicians who help create ACOs or hold administrative positions within the organizations may also be more at risk for being sued, say liability experts, whether or not they were directly involved in patient care.
In the past, entities such as HMOs were rarely sued for the actions of participants because such corporate structures are not generally responsible for the rendering of care, Mr. DiGiacinto said. However, federal guidelines recommend that medical professionals be involved in the corporate structure of ACOs, and that the organizations be accountable for the care they provide. This framework could fuel vicarious liability or corporate negligence claims in which the ACO itself is said to be liable for care provided to patients, according to the Accountable Care Legal Guide and RMQ article. In addition, physician leaders could potentially be sued for alleged negligent credentialing of other health professionals in the ACO, said legal experts.
But some, such as Christi J. Braun, believe suggested ACO litigation dangers are being overblown. Clinically integrated networks are designed to improve quality across all care providers, said Ms. Braun, a Washington-based health care antitrust attorney and cochair of the American Health Lawyers Association’s Accountable Care Organization Task Force.
"Even if you may not be following the protocols all the time, just the fact that you’re looking at best practices and trying to apply best practices makes it more likely that you’re going to provide better care on a more consistent basis," she said. "That actually reduces liability."
At the same time, physicians should not be so focused on following federal guidelines that they allow metrics and benchmarks to override quality medical judgment, said Brandy A. Boone, an Alabama-based senior risk management consultant for a national medical liability insurer.
"I think the biggest risk associated with ACOs or any other arrangement where physicians are incentivized to keep costs down by the prospect of making more money is the allegation that necessary tests or treatments were not offered or recommended because of the effect on reimbursement," Ms. Boone said. "We always caution our insured physicians that treatment recommendations should never be based on the patient’s ability to pay. While the majority of physicians would never actually let reimbursement sway their clinical decisions, avoiding that perception is also very important."
Only time will tell how ACO guidelines will affect malpractice cases. Often, it takes years for case law and legal precedents to develop around new issues and more clearly define boundaries, Mr. Bobbitt said.
In the meantime, litigation experts recommend that physicians joining ACOs protect themselves from lawsuits by thoroughly documenting patient interactions and clinical decision making. Mr. Bobbitt suggests also that physicians participating in ACOs become involved in developing best practice guidelines and ensuring those guidelines are clinically valid. Having a strong voice will empower physicians and assure ACO guidelines act as a lawsuit shield, rather than a sword.
"It can be a legal minefield, but it is navigable," Mr. Bobbitt said. "As an attorney and health care adviser, I try to convey that yes, there are legal issues – novel legal issues – but at the same time, this is such a positive improvement to health care, it is navigable if done right."