User login
Product News: 04 2018
Avène Mineral Light Mattifying Sunscreen Lotion
Pierre Fabre Dermo-Cosmetique introduces the Avène Mineral Light Mattifying Sunscreen Lotion with SPF 50+. This sunscreen offers broad-spectrum sun protection without irritation while delivering oil control and providing a natural mattifying finish for oily and acne-prone skin. This product absorbs quickly into the skin and can be worn under makeup. Avène Mineral Light Mattifying Sunscreen Lotion should be applied to the face 15 minutes prior to sun exposure and reapplied after 80 minutes of swimming or sweating, immediately after towel drying, or every 2 hours. For more information, visit www.aveneusa.com.
Ducray Anacaps Activ+ Dietary Supplement
Pierre Fabre Dermo-Cosmetique introduces Ducray Anacaps Activ+ Dietary Supplement, a once-daily capsule that contains zinc, molybdenum, iron, and selenium. This supplement targets factors that trigger sudden hair loss, including seasonal changes, stress, and diet. It also targets chronic hair loss with genetic, hormonal, and vascular causes. This formula provides essential nutrients needed to promote healthy hair growth from within, preserve hair density, and maintain the strength and vitality of hair. This supplement also is used for weak, devitalized nails and has a vegan formula with good digestive tolerance. For more information, visit www.ducray.com/en-us/.
Luzu
Ortho Dermatologics receives US Food and Drug Administration approval of the Supplemental New Drug Application to expand the use of Luzu (luliconazole) Cream 1% to pediatric patients. This new indication is for the topical treatment of interdigital tinea pedis and tinea cruris in patients 12 years of age and older and for tinea corporis in patients 2 years of age and older. Luzu is a topical azole antifungal agent with a 1-week, once-daily treatment regimen with results available 3 weeks post-treatment. Luzu previously was approved for use in adult patients. For more information, visit www.luzurx.com/HCP.
Xeljanz and Xeljanz XR
Pfizer Inc announces US Food and Drug Administration approval of twice-daily Xeljanz 5 mg and once-daily Xeljanz XR extended release 11 mg (tofacitinib) for the treatment of adult patients with active psoriatic arthritis who have had an inadequate response or intolerance to methotrexate or other disease-modifying antirheumatic drugs. Xeljanz and Xeljanz XR are Janus kinase inhibitors that previously were approved for the treatment of rheumatoid arthritis. For more information, visit www.xeljanz.com.
If you would like your product included in Product News, please email a press release to the Editorial Office at [email protected].
Avène Mineral Light Mattifying Sunscreen Lotion
Pierre Fabre Dermo-Cosmetique introduces the Avène Mineral Light Mattifying Sunscreen Lotion with SPF 50+. This sunscreen offers broad-spectrum sun protection without irritation while delivering oil control and providing a natural mattifying finish for oily and acne-prone skin. This product absorbs quickly into the skin and can be worn under makeup. Avène Mineral Light Mattifying Sunscreen Lotion should be applied to the face 15 minutes prior to sun exposure and reapplied after 80 minutes of swimming or sweating, immediately after towel drying, or every 2 hours. For more information, visit www.aveneusa.com.
Ducray Anacaps Activ+ Dietary Supplement
Pierre Fabre Dermo-Cosmetique introduces Ducray Anacaps Activ+ Dietary Supplement, a once-daily capsule that contains zinc, molybdenum, iron, and selenium. This supplement targets factors that trigger sudden hair loss, including seasonal changes, stress, and diet. It also targets chronic hair loss with genetic, hormonal, and vascular causes. This formula provides essential nutrients needed to promote healthy hair growth from within, preserve hair density, and maintain the strength and vitality of hair. This supplement also is used for weak, devitalized nails and has a vegan formula with good digestive tolerance. For more information, visit www.ducray.com/en-us/.
Luzu
Ortho Dermatologics receives US Food and Drug Administration approval of the Supplemental New Drug Application to expand the use of Luzu (luliconazole) Cream 1% to pediatric patients. This new indication is for the topical treatment of interdigital tinea pedis and tinea cruris in patients 12 years of age and older and for tinea corporis in patients 2 years of age and older. Luzu is a topical azole antifungal agent with a 1-week, once-daily treatment regimen with results available 3 weeks post-treatment. Luzu previously was approved for use in adult patients. For more information, visit www.luzurx.com/HCP.
Xeljanz and Xeljanz XR
Pfizer Inc announces US Food and Drug Administration approval of twice-daily Xeljanz 5 mg and once-daily Xeljanz XR extended release 11 mg (tofacitinib) for the treatment of adult patients with active psoriatic arthritis who have had an inadequate response or intolerance to methotrexate or other disease-modifying antirheumatic drugs. Xeljanz and Xeljanz XR are Janus kinase inhibitors that previously were approved for the treatment of rheumatoid arthritis. For more information, visit www.xeljanz.com.
If you would like your product included in Product News, please email a press release to the Editorial Office at [email protected].
Avène Mineral Light Mattifying Sunscreen Lotion
Pierre Fabre Dermo-Cosmetique introduces the Avène Mineral Light Mattifying Sunscreen Lotion with SPF 50+. This sunscreen offers broad-spectrum sun protection without irritation while delivering oil control and providing a natural mattifying finish for oily and acne-prone skin. This product absorbs quickly into the skin and can be worn under makeup. Avène Mineral Light Mattifying Sunscreen Lotion should be applied to the face 15 minutes prior to sun exposure and reapplied after 80 minutes of swimming or sweating, immediately after towel drying, or every 2 hours. For more information, visit www.aveneusa.com.
Ducray Anacaps Activ+ Dietary Supplement
Pierre Fabre Dermo-Cosmetique introduces Ducray Anacaps Activ+ Dietary Supplement, a once-daily capsule that contains zinc, molybdenum, iron, and selenium. This supplement targets factors that trigger sudden hair loss, including seasonal changes, stress, and diet. It also targets chronic hair loss with genetic, hormonal, and vascular causes. This formula provides essential nutrients needed to promote healthy hair growth from within, preserve hair density, and maintain the strength and vitality of hair. This supplement also is used for weak, devitalized nails and has a vegan formula with good digestive tolerance. For more information, visit www.ducray.com/en-us/.
Luzu
Ortho Dermatologics receives US Food and Drug Administration approval of the Supplemental New Drug Application to expand the use of Luzu (luliconazole) Cream 1% to pediatric patients. This new indication is for the topical treatment of interdigital tinea pedis and tinea cruris in patients 12 years of age and older and for tinea corporis in patients 2 years of age and older. Luzu is a topical azole antifungal agent with a 1-week, once-daily treatment regimen with results available 3 weeks post-treatment. Luzu previously was approved for use in adult patients. For more information, visit www.luzurx.com/HCP.
Xeljanz and Xeljanz XR
Pfizer Inc announces US Food and Drug Administration approval of twice-daily Xeljanz 5 mg and once-daily Xeljanz XR extended release 11 mg (tofacitinib) for the treatment of adult patients with active psoriatic arthritis who have had an inadequate response or intolerance to methotrexate or other disease-modifying antirheumatic drugs. Xeljanz and Xeljanz XR are Janus kinase inhibitors that previously were approved for the treatment of rheumatoid arthritis. For more information, visit www.xeljanz.com.
If you would like your product included in Product News, please email a press release to the Editorial Office at [email protected].
Product News: 03 2018
Avène TriXera
Pierre Fabre Dermo-Cosmetique launches the Avène TriXera line consisting of 3 products. The TriXera Nutrition Nutri-fluid Cleanser gently cleanses and nourishes dry to very dry, sensitive skin. TriXera Nutrition Nutri-fluid Lotion offers 48-hour hydration for dry, sensitive skin. These nourishing effects last up to 6 hours after application. TriXera Nutrition Nutri-fluid Balm has a fluidlike texture and also offers 48-hour hydration. All 3 products contain Avène Thermal Spring Water to soothe and soften as it restores the skin’s balance, as well as evening primrose oil and soy extract to rebuild the skin barrier and prevent moisture loss. For more information, visit www.aveneusa.com.
CoolSculpting
Allergan plc announces US Food and Drug Administration clearance for the CoolSculpting treatment, a nonsurgical fat reduction technology for improved appearance of lax tissue in conjunction with submental fat. CoolSculpting works by gently cooling targeted fat cells in the body to induce natural, controlled elimination of fat cells without affecting surrounding tissue. For more information, visit www.coolsculpting.com.
Discoloration Defense
SkinCeuticals introduces Discoloration Defense, a high-potency treatment serum that prevents and corrects multiple types of discoloration. This product contains tranexamic acid and niacinamide for pigmentation, hepes for hydration, and kojic acid to brighten skin and help to reduce the amount of melanin produced. These ingredients work together to help break up existing melanin clusters, inhibit the formation of melanocytes, and deactivate inflammatory mediators. The serum provides a reduction in visible and stubborn pigmentation for a revitalized and even complexion with refined texture and clarity. For more information, visit www.skinceuticals.com.
Eskata
Aclaris Therapeutics, Inc, announces US Food and Drug Administration approval of Eskata (hydrogen peroxide) topical solution 40% for the treatment of raised seborrheic keratoses. This product is a targeted, in-office treatment applied directly to raised seborrheic keratoses using a penlike applicator. Clinical studies showed clearing of seborrheic keratoses after 2 treatments. Eskata is expected to be commercially available in the spring of 2018. For more information, visit www.eskata.com.
Ixifi
Pfizer Inc announces US Food and Drug Administration approval of Ixifi (infliximab-qbtx), a chimeric human-murine monoclonal antibody against tumor necrosis factor, as a biosimilar to Remicade (infliximab) for all eligible indications of the reference product. Ixifi has been approved in the United States as a treatment for rheumatoid arthritis, Crohn disease, ulcerative colitis, psoriatic arthritis, and plaque psoriasis. For more information, visit www.pfizer.com.
Jemdel
Ortho Dermatologics announces US Food and Drug Administration acceptance of the New Drug Application for Jemdel (halobetasol propionate 0.01%), a high-potency topical steroid for the treatment of plaque psoriasis with dosing for as long as 8 weeks. Jemdel has a Prescription Drug User Fee Act action date of October 5, 2018. For more information, visit www.ortho-dermatologics.com.
Retin-A Micro
Ortho Dermatologics announces that Retin-A Micro (tretinoin) gel microsphere 0.06%, a topical treatment for acne vulgaris, will be available commercially to health care professionals. The US Food and Drug Administration previously approved the Supplemental New Drug Application for this product in October 2017. Retin-A Micro features a microsponge delivery system technology that helps control the release of tretinoin and improves photostability, even when used in conjunction with benzoyl peroxide. This product also features a pump delivery system for controlled dispensing and consistent dosing. Caution should be exercised when prescribing to eczema patients and nursing mothers. For more information, visit www.retinamicro.com.
If you would like your product included in Product News, please email a press release to the Editorial Office at [email protected].
Avène TriXera
Pierre Fabre Dermo-Cosmetique launches the Avène TriXera line consisting of 3 products. The TriXera Nutrition Nutri-fluid Cleanser gently cleanses and nourishes dry to very dry, sensitive skin. TriXera Nutrition Nutri-fluid Lotion offers 48-hour hydration for dry, sensitive skin. These nourishing effects last up to 6 hours after application. TriXera Nutrition Nutri-fluid Balm has a fluidlike texture and also offers 48-hour hydration. All 3 products contain Avène Thermal Spring Water to soothe and soften as it restores the skin’s balance, as well as evening primrose oil and soy extract to rebuild the skin barrier and prevent moisture loss. For more information, visit www.aveneusa.com.
CoolSculpting
Allergan plc announces US Food and Drug Administration clearance for the CoolSculpting treatment, a nonsurgical fat reduction technology for improved appearance of lax tissue in conjunction with submental fat. CoolSculpting works by gently cooling targeted fat cells in the body to induce natural, controlled elimination of fat cells without affecting surrounding tissue. For more information, visit www.coolsculpting.com.
Discoloration Defense
SkinCeuticals introduces Discoloration Defense, a high-potency treatment serum that prevents and corrects multiple types of discoloration. This product contains tranexamic acid and niacinamide for pigmentation, hepes for hydration, and kojic acid to brighten skin and help to reduce the amount of melanin produced. These ingredients work together to help break up existing melanin clusters, inhibit the formation of melanocytes, and deactivate inflammatory mediators. The serum provides a reduction in visible and stubborn pigmentation for a revitalized and even complexion with refined texture and clarity. For more information, visit www.skinceuticals.com.
Eskata
Aclaris Therapeutics, Inc, announces US Food and Drug Administration approval of Eskata (hydrogen peroxide) topical solution 40% for the treatment of raised seborrheic keratoses. This product is a targeted, in-office treatment applied directly to raised seborrheic keratoses using a penlike applicator. Clinical studies showed clearing of seborrheic keratoses after 2 treatments. Eskata is expected to be commercially available in the spring of 2018. For more information, visit www.eskata.com.
Ixifi
Pfizer Inc announces US Food and Drug Administration approval of Ixifi (infliximab-qbtx), a chimeric human-murine monoclonal antibody against tumor necrosis factor, as a biosimilar to Remicade (infliximab) for all eligible indications of the reference product. Ixifi has been approved in the United States as a treatment for rheumatoid arthritis, Crohn disease, ulcerative colitis, psoriatic arthritis, and plaque psoriasis. For more information, visit www.pfizer.com.
Jemdel
Ortho Dermatologics announces US Food and Drug Administration acceptance of the New Drug Application for Jemdel (halobetasol propionate 0.01%), a high-potency topical steroid for the treatment of plaque psoriasis with dosing for as long as 8 weeks. Jemdel has a Prescription Drug User Fee Act action date of October 5, 2018. For more information, visit www.ortho-dermatologics.com.
Retin-A Micro
Ortho Dermatologics announces that Retin-A Micro (tretinoin) gel microsphere 0.06%, a topical treatment for acne vulgaris, will be available commercially to health care professionals. The US Food and Drug Administration previously approved the Supplemental New Drug Application for this product in October 2017. Retin-A Micro features a microsponge delivery system technology that helps control the release of tretinoin and improves photostability, even when used in conjunction with benzoyl peroxide. This product also features a pump delivery system for controlled dispensing and consistent dosing. Caution should be exercised when prescribing to eczema patients and nursing mothers. For more information, visit www.retinamicro.com.
If you would like your product included in Product News, please email a press release to the Editorial Office at [email protected].
Avène TriXera
Pierre Fabre Dermo-Cosmetique launches the Avène TriXera line consisting of 3 products. The TriXera Nutrition Nutri-fluid Cleanser gently cleanses and nourishes dry to very dry, sensitive skin. TriXera Nutrition Nutri-fluid Lotion offers 48-hour hydration for dry, sensitive skin. These nourishing effects last up to 6 hours after application. TriXera Nutrition Nutri-fluid Balm has a fluidlike texture and also offers 48-hour hydration. All 3 products contain Avène Thermal Spring Water to soothe and soften as it restores the skin’s balance, as well as evening primrose oil and soy extract to rebuild the skin barrier and prevent moisture loss. For more information, visit www.aveneusa.com.
CoolSculpting
Allergan plc announces US Food and Drug Administration clearance for the CoolSculpting treatment, a nonsurgical fat reduction technology for improved appearance of lax tissue in conjunction with submental fat. CoolSculpting works by gently cooling targeted fat cells in the body to induce natural, controlled elimination of fat cells without affecting surrounding tissue. For more information, visit www.coolsculpting.com.
Discoloration Defense
SkinCeuticals introduces Discoloration Defense, a high-potency treatment serum that prevents and corrects multiple types of discoloration. This product contains tranexamic acid and niacinamide for pigmentation, hepes for hydration, and kojic acid to brighten skin and help to reduce the amount of melanin produced. These ingredients work together to help break up existing melanin clusters, inhibit the formation of melanocytes, and deactivate inflammatory mediators. The serum provides a reduction in visible and stubborn pigmentation for a revitalized and even complexion with refined texture and clarity. For more information, visit www.skinceuticals.com.
Eskata
Aclaris Therapeutics, Inc, announces US Food and Drug Administration approval of Eskata (hydrogen peroxide) topical solution 40% for the treatment of raised seborrheic keratoses. This product is a targeted, in-office treatment applied directly to raised seborrheic keratoses using a penlike applicator. Clinical studies showed clearing of seborrheic keratoses after 2 treatments. Eskata is expected to be commercially available in the spring of 2018. For more information, visit www.eskata.com.
Ixifi
Pfizer Inc announces US Food and Drug Administration approval of Ixifi (infliximab-qbtx), a chimeric human-murine monoclonal antibody against tumor necrosis factor, as a biosimilar to Remicade (infliximab) for all eligible indications of the reference product. Ixifi has been approved in the United States as a treatment for rheumatoid arthritis, Crohn disease, ulcerative colitis, psoriatic arthritis, and plaque psoriasis. For more information, visit www.pfizer.com.
Jemdel
Ortho Dermatologics announces US Food and Drug Administration acceptance of the New Drug Application for Jemdel (halobetasol propionate 0.01%), a high-potency topical steroid for the treatment of plaque psoriasis with dosing for as long as 8 weeks. Jemdel has a Prescription Drug User Fee Act action date of October 5, 2018. For more information, visit www.ortho-dermatologics.com.
Retin-A Micro
Ortho Dermatologics announces that Retin-A Micro (tretinoin) gel microsphere 0.06%, a topical treatment for acne vulgaris, will be available commercially to health care professionals. The US Food and Drug Administration previously approved the Supplemental New Drug Application for this product in October 2017. Retin-A Micro features a microsponge delivery system technology that helps control the release of tretinoin and improves photostability, even when used in conjunction with benzoyl peroxide. This product also features a pump delivery system for controlled dispensing and consistent dosing. Caution should be exercised when prescribing to eczema patients and nursing mothers. For more information, visit www.retinamicro.com.
If you would like your product included in Product News, please email a press release to the Editorial Office at [email protected].
Product Update: Vistara; Ultravision trocar; CompuFlo Epidural; and Philips ultrasound
PRENATAL SCREENING FOR SINGLE-GENE DISORDERS
Vistara®, a non-invasive prenatal test (NIPT) from Natera, Inc, screens for single-gene disorders after 9 weeks’ gestation. Complementing the Panorama® NIPT, Vistara tests for major anatomic abnormalities and chromosome imbalances that have a combined incidence rate of 1 in 600 (higher than Down syndrome). These mutations can cause severe conditions affecting skeletal, cardiac, and neurologic systems, such as Noonan syndrome, osteogenesis imperfecta, craniosynostosis syndromes, achondroplasia, and Rett syndrome. Standard NIPT commonly cannot detect these de novo (not inherited) mutations. Ultrasound exams may either completely miss the disorders or identify nonspecific findings later in pregnancy.
Natera says that Vistara has a combined analytical sensitivity of >99% and a combined analytical specificity of >99% in validation studies.
FOR MORE INFORMATION, VISIT: https://www.natera.com/vistara
ELECTROSTATIC SURGICAL SMOKE REMOVAL
The UltravisionTM Trocar device from Alesi Surgical Technologies uses a low-energy electrostatic charge to eliminate the surgical smoke generated by cutting instruments during laparoscopic surgery. Electrostatic precipitation accelerates the natural process of sedimentation; Ultravision creates negatively charged gas ions that draw water vapor and particulate matter away from the surgical site toward “positive” patient tissue.
Alesi says that bench studies comparing Ultravision with a vacuum-system when using monopolar, bipolar, and ultrasonic instruments show that its device is faster and more efficient than smoke evacuation. When switched on before cutting, Ultravision precipitates 99% of particles within 30 seconds. After 1 minute of continuous use, Ultravision precipitates 99.9% of particles, independent of particle size, from 7 nm to 10 µm. Smoke evacuation removes 30.2% of particles after 1 minute, according to Alesi.
FOR MORE INFORMATION, VISIT: http://www.alesi-surgical.com
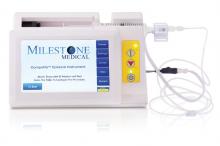
PRESSURE-SENSING TECHNOLOGY FOR EPIDURALS
The CompuFlo®Epidural from Milestone Scientific uses pressure-sensing technology to identify the epidural space, and provides a computer-controlled drug delivery system.
Knowing the precise needle location during an epidural injection procedure provides a measure of safety not available to physicians who use conventional syringes. Milestone says that its CompuFlo Epidural allows anesthesiologists to use both hands to advance and direct the needle, and to confirm the epidural space with 99% accuracy on the first attempt.
CompuFlo Epidural differentiates tissue types for the medical professional via visual and audio feedback, leading to precise location guidance as the needle advances toward the intended area. It also allows for controlled needle exit pressure, precise flow rate and drug volumes, and patient treatment documentation.
FOR MORE INFORMATION, VISIT: https://www.milestonescientific.com/products/compuflo-epidural
OBGYN ULTRASOUND INNOVATIONS
Philips recently announced enhancements to its EPIQ 7 and 5 and Affiniti 70 ultrasound systems. According to Philips, the eL18-4 transducer provides high-detail resolution and image uniformity with penetration for enhanced diagnostic quality in 1st- and 2nd-trimester obstetric exams. aBiometry AssistAI, with anatomical intelligence of fetal anatomy, streamlines fetal measurement by preplacing measurement cursors on selected structures. The new TouchVue control-panel interface on TrueVue allows practitioners to interact with finger gestures and to direct 3D-volume rotation and internal light-source position. The 2D Tilt feature offered on the 3D9-v3 transducer provides lateral scanning of anatomic structures that are off-axis without having to manually angle the transducer.
These new features complement the existing suite of Philips ObGyn ultrasound visualization tools: TrueVue, GlassVue, aRevealAI, and MaxVue.
FOR MORE INFORMATION, VISIT: https://www.usa.philips.com/healthcare/resources/feature-detail/ultrasound-truevue-imaging
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
PRENATAL SCREENING FOR SINGLE-GENE DISORDERS
Vistara®, a non-invasive prenatal test (NIPT) from Natera, Inc, screens for single-gene disorders after 9 weeks’ gestation. Complementing the Panorama® NIPT, Vistara tests for major anatomic abnormalities and chromosome imbalances that have a combined incidence rate of 1 in 600 (higher than Down syndrome). These mutations can cause severe conditions affecting skeletal, cardiac, and neurologic systems, such as Noonan syndrome, osteogenesis imperfecta, craniosynostosis syndromes, achondroplasia, and Rett syndrome. Standard NIPT commonly cannot detect these de novo (not inherited) mutations. Ultrasound exams may either completely miss the disorders or identify nonspecific findings later in pregnancy.
Natera says that Vistara has a combined analytical sensitivity of >99% and a combined analytical specificity of >99% in validation studies.
FOR MORE INFORMATION, VISIT: https://www.natera.com/vistara
ELECTROSTATIC SURGICAL SMOKE REMOVAL
The UltravisionTM Trocar device from Alesi Surgical Technologies uses a low-energy electrostatic charge to eliminate the surgical smoke generated by cutting instruments during laparoscopic surgery. Electrostatic precipitation accelerates the natural process of sedimentation; Ultravision creates negatively charged gas ions that draw water vapor and particulate matter away from the surgical site toward “positive” patient tissue.
Alesi says that bench studies comparing Ultravision with a vacuum-system when using monopolar, bipolar, and ultrasonic instruments show that its device is faster and more efficient than smoke evacuation. When switched on before cutting, Ultravision precipitates 99% of particles within 30 seconds. After 1 minute of continuous use, Ultravision precipitates 99.9% of particles, independent of particle size, from 7 nm to 10 µm. Smoke evacuation removes 30.2% of particles after 1 minute, according to Alesi.
FOR MORE INFORMATION, VISIT: http://www.alesi-surgical.com
PRESSURE-SENSING TECHNOLOGY FOR EPIDURALS
The CompuFlo®Epidural from Milestone Scientific uses pressure-sensing technology to identify the epidural space, and provides a computer-controlled drug delivery system.
Knowing the precise needle location during an epidural injection procedure provides a measure of safety not available to physicians who use conventional syringes. Milestone says that its CompuFlo Epidural allows anesthesiologists to use both hands to advance and direct the needle, and to confirm the epidural space with 99% accuracy on the first attempt.
CompuFlo Epidural differentiates tissue types for the medical professional via visual and audio feedback, leading to precise location guidance as the needle advances toward the intended area. It also allows for controlled needle exit pressure, precise flow rate and drug volumes, and patient treatment documentation.
FOR MORE INFORMATION, VISIT: https://www.milestonescientific.com/products/compuflo-epidural
OBGYN ULTRASOUND INNOVATIONS
Philips recently announced enhancements to its EPIQ 7 and 5 and Affiniti 70 ultrasound systems. According to Philips, the eL18-4 transducer provides high-detail resolution and image uniformity with penetration for enhanced diagnostic quality in 1st- and 2nd-trimester obstetric exams. aBiometry AssistAI, with anatomical intelligence of fetal anatomy, streamlines fetal measurement by preplacing measurement cursors on selected structures. The new TouchVue control-panel interface on TrueVue allows practitioners to interact with finger gestures and to direct 3D-volume rotation and internal light-source position. The 2D Tilt feature offered on the 3D9-v3 transducer provides lateral scanning of anatomic structures that are off-axis without having to manually angle the transducer.
These new features complement the existing suite of Philips ObGyn ultrasound visualization tools: TrueVue, GlassVue, aRevealAI, and MaxVue.
FOR MORE INFORMATION, VISIT: https://www.usa.philips.com/healthcare/resources/feature-detail/ultrasound-truevue-imaging
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
PRENATAL SCREENING FOR SINGLE-GENE DISORDERS
Vistara®, a non-invasive prenatal test (NIPT) from Natera, Inc, screens for single-gene disorders after 9 weeks’ gestation. Complementing the Panorama® NIPT, Vistara tests for major anatomic abnormalities and chromosome imbalances that have a combined incidence rate of 1 in 600 (higher than Down syndrome). These mutations can cause severe conditions affecting skeletal, cardiac, and neurologic systems, such as Noonan syndrome, osteogenesis imperfecta, craniosynostosis syndromes, achondroplasia, and Rett syndrome. Standard NIPT commonly cannot detect these de novo (not inherited) mutations. Ultrasound exams may either completely miss the disorders or identify nonspecific findings later in pregnancy.
Natera says that Vistara has a combined analytical sensitivity of >99% and a combined analytical specificity of >99% in validation studies.
FOR MORE INFORMATION, VISIT: https://www.natera.com/vistara
ELECTROSTATIC SURGICAL SMOKE REMOVAL
The UltravisionTM Trocar device from Alesi Surgical Technologies uses a low-energy electrostatic charge to eliminate the surgical smoke generated by cutting instruments during laparoscopic surgery. Electrostatic precipitation accelerates the natural process of sedimentation; Ultravision creates negatively charged gas ions that draw water vapor and particulate matter away from the surgical site toward “positive” patient tissue.
Alesi says that bench studies comparing Ultravision with a vacuum-system when using monopolar, bipolar, and ultrasonic instruments show that its device is faster and more efficient than smoke evacuation. When switched on before cutting, Ultravision precipitates 99% of particles within 30 seconds. After 1 minute of continuous use, Ultravision precipitates 99.9% of particles, independent of particle size, from 7 nm to 10 µm. Smoke evacuation removes 30.2% of particles after 1 minute, according to Alesi.
FOR MORE INFORMATION, VISIT: http://www.alesi-surgical.com
PRESSURE-SENSING TECHNOLOGY FOR EPIDURALS
The CompuFlo®Epidural from Milestone Scientific uses pressure-sensing technology to identify the epidural space, and provides a computer-controlled drug delivery system.
Knowing the precise needle location during an epidural injection procedure provides a measure of safety not available to physicians who use conventional syringes. Milestone says that its CompuFlo Epidural allows anesthesiologists to use both hands to advance and direct the needle, and to confirm the epidural space with 99% accuracy on the first attempt.
CompuFlo Epidural differentiates tissue types for the medical professional via visual and audio feedback, leading to precise location guidance as the needle advances toward the intended area. It also allows for controlled needle exit pressure, precise flow rate and drug volumes, and patient treatment documentation.
FOR MORE INFORMATION, VISIT: https://www.milestonescientific.com/products/compuflo-epidural
OBGYN ULTRASOUND INNOVATIONS
Philips recently announced enhancements to its EPIQ 7 and 5 and Affiniti 70 ultrasound systems. According to Philips, the eL18-4 transducer provides high-detail resolution and image uniformity with penetration for enhanced diagnostic quality in 1st- and 2nd-trimester obstetric exams. aBiometry AssistAI, with anatomical intelligence of fetal anatomy, streamlines fetal measurement by preplacing measurement cursors on selected structures. The new TouchVue control-panel interface on TrueVue allows practitioners to interact with finger gestures and to direct 3D-volume rotation and internal light-source position. The 2D Tilt feature offered on the 3D9-v3 transducer provides lateral scanning of anatomic structures that are off-axis without having to manually angle the transducer.
These new features complement the existing suite of Philips ObGyn ultrasound visualization tools: TrueVue, GlassVue, aRevealAI, and MaxVue.
FOR MORE INFORMATION, VISIT: https://www.usa.philips.com/healthcare/resources/feature-detail/ultrasound-truevue-imaging
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Product Update: MyoSure MANUAL; Rivanna's Accuro 3D; PeriGen; Instavit
HYSTEROSCOPY TISSUE REMOVAL DEVICE

Hologic, Inc, has introduced the MyoSure® MANUAL Tissue Removal Device for resecting and removing tissue during in-office hysteroscopic intrauterine procedures. When used with the MyoSure hysteroscope, the MyoSure MANUAL device has a fully integrated vacuum that does not require external suction and can be operated using a 1-L saline bag. The clear tissue trap allows for visual co
This new Hologic product joins the MyoSure suite of gynecologic surgical products that includes the MyoSure, MyoSure REACH, MyoSure XL, and MyoSure LITE devices.
FOR MORE INFORMATION, VISIT: http://myosure.com/
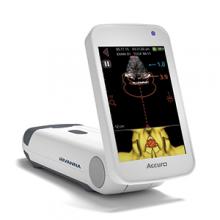
SPINAL NAVIGATION TECHNOLOGY FOR EPIDURAL ANESTHESIA
The Accuro® 3D image-guided spinal navigation technology by Rivanna Medical® is a handheld, lightweight, untethered ultrasound-based system designed to help apply spinal and epidural anesthesia. Ultrasonography is the imaging modality of choice for epidurals in expectant mothers who must avoid the radiation involved in other imaging procedures, according to Rivanna Medical.
In a recent trial, Accuro identified the appropriate epidural injection sites along the lower spine and calculated the depth to the epidural space. Actual epidural depth was confirmed by measuring needle penetration during successful epidural delivery by anesthesia providers. Accuro predicted this depth within an average of 0.61 cm, reports Rivanna Medical. In addition, Accuro identified the appropriate spinal interspace for needle insertion in 94% of patients and enabled 87% success in first-attempt epidural administration.
FOR MORE INFORMATION, VISIT: https://rivannamedical.com/
SOFTWARE AND HUB HELP IDENTIFY CRITICALLY ILL L&D PATIENTS
PeriGen, Inc, a software-solutions company, has launched PeriWatch™ HUB™, new perinatal software and a dashboard for labor and delivery (L&D) units.
PeriGen says that its PeriWatch modules provide state-of-the-art L&D documentation and fetal surveillance coupled with analytics and an electronic critical-condition dashboard for hospital maternity units.
HUB is an intelligent perinatal dashboard designed to facilitate the timely recognition of maternity patients who develop critical illness. Using PeriGen’s proprietary algorithms, it prioritizes patients based on physician-chosen threshold settings for vital signs, labor progress, and fetal heart rate patterns, and consolidates that data into an easy-to-read interactive dashboard.
FOR MORE INFORMATION, VISIT: http://perigen.com/
ORAL SPRAY VITAMINS: ALTERNATIVE TO PILLS

Instavit® Spray Vitamins offer an alternative to patients who have difficulty swallowing pills. Instavit says that its oral vitamins, sprayed directly into the mouth, are sugar-free, tasty, gluten-free, and contain zero calories. The sprays are manufactured to the highest standard in cGMP, FDA approved facilities in the United States. Each Instavit spray provides an exact and measured amount of liquid, allowing for correct dosing and also permitting individualization of intake. A 14-oz spray bottle contains about 28 doses.
The Instavit line includes: “Prenatal Care,” “Vitamin B12,” “Instant Energy,” “Vitamin D,” “Daily Health,” “Sweet Dreams,” “Immune Strength,” “Clearer Thinking,” and “Instavit for Kids.” Instavit products are available in retail stores in North America.
FOR MORE INFORMATION, VISIT: http://www.instavit.com/
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
HYSTEROSCOPY TISSUE REMOVAL DEVICE

Hologic, Inc, has introduced the MyoSure® MANUAL Tissue Removal Device for resecting and removing tissue during in-office hysteroscopic intrauterine procedures. When used with the MyoSure hysteroscope, the MyoSure MANUAL device has a fully integrated vacuum that does not require external suction and can be operated using a 1-L saline bag. The clear tissue trap allows for visual co
This new Hologic product joins the MyoSure suite of gynecologic surgical products that includes the MyoSure, MyoSure REACH, MyoSure XL, and MyoSure LITE devices.
FOR MORE INFORMATION, VISIT: http://myosure.com/
SPINAL NAVIGATION TECHNOLOGY FOR EPIDURAL ANESTHESIA
The Accuro® 3D image-guided spinal navigation technology by Rivanna Medical® is a handheld, lightweight, untethered ultrasound-based system designed to help apply spinal and epidural anesthesia. Ultrasonography is the imaging modality of choice for epidurals in expectant mothers who must avoid the radiation involved in other imaging procedures, according to Rivanna Medical.
In a recent trial, Accuro identified the appropriate epidural injection sites along the lower spine and calculated the depth to the epidural space. Actual epidural depth was confirmed by measuring needle penetration during successful epidural delivery by anesthesia providers. Accuro predicted this depth within an average of 0.61 cm, reports Rivanna Medical. In addition, Accuro identified the appropriate spinal interspace for needle insertion in 94% of patients and enabled 87% success in first-attempt epidural administration.
FOR MORE INFORMATION, VISIT: https://rivannamedical.com/
SOFTWARE AND HUB HELP IDENTIFY CRITICALLY ILL L&D PATIENTS
PeriGen, Inc, a software-solutions company, has launched PeriWatch™ HUB™, new perinatal software and a dashboard for labor and delivery (L&D) units.
PeriGen says that its PeriWatch modules provide state-of-the-art L&D documentation and fetal surveillance coupled with analytics and an electronic critical-condition dashboard for hospital maternity units.
HUB is an intelligent perinatal dashboard designed to facilitate the timely recognition of maternity patients who develop critical illness. Using PeriGen’s proprietary algorithms, it prioritizes patients based on physician-chosen threshold settings for vital signs, labor progress, and fetal heart rate patterns, and consolidates that data into an easy-to-read interactive dashboard.
FOR MORE INFORMATION, VISIT: http://perigen.com/
ORAL SPRAY VITAMINS: ALTERNATIVE TO PILLS

Instavit® Spray Vitamins offer an alternative to patients who have difficulty swallowing pills. Instavit says that its oral vitamins, sprayed directly into the mouth, are sugar-free, tasty, gluten-free, and contain zero calories. The sprays are manufactured to the highest standard in cGMP, FDA approved facilities in the United States. Each Instavit spray provides an exact and measured amount of liquid, allowing for correct dosing and also permitting individualization of intake. A 14-oz spray bottle contains about 28 doses.
The Instavit line includes: “Prenatal Care,” “Vitamin B12,” “Instant Energy,” “Vitamin D,” “Daily Health,” “Sweet Dreams,” “Immune Strength,” “Clearer Thinking,” and “Instavit for Kids.” Instavit products are available in retail stores in North America.
FOR MORE INFORMATION, VISIT: http://www.instavit.com/
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
HYSTEROSCOPY TISSUE REMOVAL DEVICE

Hologic, Inc, has introduced the MyoSure® MANUAL Tissue Removal Device for resecting and removing tissue during in-office hysteroscopic intrauterine procedures. When used with the MyoSure hysteroscope, the MyoSure MANUAL device has a fully integrated vacuum that does not require external suction and can be operated using a 1-L saline bag. The clear tissue trap allows for visual co
This new Hologic product joins the MyoSure suite of gynecologic surgical products that includes the MyoSure, MyoSure REACH, MyoSure XL, and MyoSure LITE devices.
FOR MORE INFORMATION, VISIT: http://myosure.com/
SPINAL NAVIGATION TECHNOLOGY FOR EPIDURAL ANESTHESIA
The Accuro® 3D image-guided spinal navigation technology by Rivanna Medical® is a handheld, lightweight, untethered ultrasound-based system designed to help apply spinal and epidural anesthesia. Ultrasonography is the imaging modality of choice for epidurals in expectant mothers who must avoid the radiation involved in other imaging procedures, according to Rivanna Medical.
In a recent trial, Accuro identified the appropriate epidural injection sites along the lower spine and calculated the depth to the epidural space. Actual epidural depth was confirmed by measuring needle penetration during successful epidural delivery by anesthesia providers. Accuro predicted this depth within an average of 0.61 cm, reports Rivanna Medical. In addition, Accuro identified the appropriate spinal interspace for needle insertion in 94% of patients and enabled 87% success in first-attempt epidural administration.
FOR MORE INFORMATION, VISIT: https://rivannamedical.com/
SOFTWARE AND HUB HELP IDENTIFY CRITICALLY ILL L&D PATIENTS
PeriGen, Inc, a software-solutions company, has launched PeriWatch™ HUB™, new perinatal software and a dashboard for labor and delivery (L&D) units.
PeriGen says that its PeriWatch modules provide state-of-the-art L&D documentation and fetal surveillance coupled with analytics and an electronic critical-condition dashboard for hospital maternity units.
HUB is an intelligent perinatal dashboard designed to facilitate the timely recognition of maternity patients who develop critical illness. Using PeriGen’s proprietary algorithms, it prioritizes patients based on physician-chosen threshold settings for vital signs, labor progress, and fetal heart rate patterns, and consolidates that data into an easy-to-read interactive dashboard.
FOR MORE INFORMATION, VISIT: http://perigen.com/
ORAL SPRAY VITAMINS: ALTERNATIVE TO PILLS

Instavit® Spray Vitamins offer an alternative to patients who have difficulty swallowing pills. Instavit says that its oral vitamins, sprayed directly into the mouth, are sugar-free, tasty, gluten-free, and contain zero calories. The sprays are manufactured to the highest standard in cGMP, FDA approved facilities in the United States. Each Instavit spray provides an exact and measured amount of liquid, allowing for correct dosing and also permitting individualization of intake. A 14-oz spray bottle contains about 28 doses.
The Instavit line includes: “Prenatal Care,” “Vitamin B12,” “Instant Energy,” “Vitamin D,” “Daily Health,” “Sweet Dreams,” “Immune Strength,” “Clearer Thinking,” and “Instavit for Kids.” Instavit products are available in retail stores in North America.
FOR MORE INFORMATION, VISIT: http://www.instavit.com/
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.