User login
Product Update: Bijuva; Liletta; Aegea Vapor System; Natural Cycles
TREATMENT FOR VASOMOTOR SYMPTOMS
BIJUVA™ has been US Food and Drug Administration (FDA)–approved as the first oral treatment for moderate-to-severe vasomotor symptoms due to menopause in women with a uterus. BIJUVA offers a combination of bioidentical estradiol to reduce moderate-to-severe hot flashes and bioidentical progesterone to reduce the risk for endometrial hyperplasia.
TherapeuticsMD says that BIJUVA will be available Spring 2019 and is a proven treatment option for women who are experiencing bothersome symptoms of menopause, with clinical trial data demonstrating a statistically significant reduction in both the frequency and severity of moderate-to-severe vasomotor symptoms. The manufacturer also says that BIJUVA is developed to be identical in molecular structure to the hormones already produced by the body and is designed to help women restore what is lost during menopause.
BIJUVA estradiol and progesterone combination (1 mg/100 mg) will be available in capsule form.
FOR MORE INFORMATION, VISIT:https://bijuva.com/discover/
LILETTA USE EXTENDED
The FDA has approved LILETTA® (levonorgestrel-releasing intrauterine system) 52 mg for 5-year use. This approval is based on efficacy and safety data from ACCESS IUS, the largest ongoing intrauterine device (IUD) Phase 3 clinical trial in the United States. Previously, LILETTA was indicated for use up to 4 years.
LILETTA continues to be greater than 99% effective in preventing pregnancy in a broad range of women, regardless of age, race, body mass index, or parity, according to Allergan and Medicines360. The extended duration and proven efficacy across a diverse population enables more women in the United States to obtain effective birth control, as the IUD is now available for a low cost at public health clinics.
FOR MORE INFORMATION, VISIT: https://www.liletta.com
Continue to: ENDOMETRIAL ABLATION TECHNOLOGY
ENDOMETRIAL ABLATION TECHNOLOGY
AEGEA Medical introduces the AEGEA Vapor SystemTM, an innovative solution for endometrial ablation to treat menorrhagia.
The system uses Adaptive Vapor Ablation and is the first endometrial ablation system specifically designed for use in the doctor’s office, allowing minimal anesthesia/analgesia and rapid recovery, says AEGEA Medical.
AEGEA Medical describes the AEGEA Vapor System as a fully automated safety monitoring and vapor delivery system that uses a slender, flexible Vapor Probe with SmartSealTM technology and the Integrity ProTM safety feature, for an added level of confidence. The 4-minute procedure time includes 2 minutes of vapor treatment and can be performed in patients with a wider range of uterine anatomies than indicated for use with currently available treatments, says AEGEA Medical.
FOR MORE INFORMATION, VISIT: http://aegeamedical.com/
NATURAL CYCLES
The FDA has cleared Natural Cycles as the first digital method of birth control in the United States. Delivered in the form of an app, Natural Cycles is a fertility awareness–based contraceptive that uses a sophisticated algorithm to accurately and conveniently determine a woman’s daily fertility based on basal body temperature.
That data builds into a personalized fertility indicator that informs her when she needs to use protection to minimize the chance of conception. The app also can be used to help plan a pregnancy when the time is right, according to Natural Cycles.
A clinical study showed that the efficacy of a contraceptive mobile application is higher than usually reported for traditional fertility awareness–based methods. The application may contribute to reducing the unmet need for contraception, says Natural Cycles.
FOR MORE INFORMATION, VISIT: https://www.naturalcycles.com/en/hcp
TREATMENT FOR VASOMOTOR SYMPTOMS
BIJUVA™ has been US Food and Drug Administration (FDA)–approved as the first oral treatment for moderate-to-severe vasomotor symptoms due to menopause in women with a uterus. BIJUVA offers a combination of bioidentical estradiol to reduce moderate-to-severe hot flashes and bioidentical progesterone to reduce the risk for endometrial hyperplasia.
TherapeuticsMD says that BIJUVA will be available Spring 2019 and is a proven treatment option for women who are experiencing bothersome symptoms of menopause, with clinical trial data demonstrating a statistically significant reduction in both the frequency and severity of moderate-to-severe vasomotor symptoms. The manufacturer also says that BIJUVA is developed to be identical in molecular structure to the hormones already produced by the body and is designed to help women restore what is lost during menopause.
BIJUVA estradiol and progesterone combination (1 mg/100 mg) will be available in capsule form.
FOR MORE INFORMATION, VISIT:https://bijuva.com/discover/
LILETTA USE EXTENDED
The FDA has approved LILETTA® (levonorgestrel-releasing intrauterine system) 52 mg for 5-year use. This approval is based on efficacy and safety data from ACCESS IUS, the largest ongoing intrauterine device (IUD) Phase 3 clinical trial in the United States. Previously, LILETTA was indicated for use up to 4 years.
LILETTA continues to be greater than 99% effective in preventing pregnancy in a broad range of women, regardless of age, race, body mass index, or parity, according to Allergan and Medicines360. The extended duration and proven efficacy across a diverse population enables more women in the United States to obtain effective birth control, as the IUD is now available for a low cost at public health clinics.
FOR MORE INFORMATION, VISIT: https://www.liletta.com
Continue to: ENDOMETRIAL ABLATION TECHNOLOGY
ENDOMETRIAL ABLATION TECHNOLOGY
AEGEA Medical introduces the AEGEA Vapor SystemTM, an innovative solution for endometrial ablation to treat menorrhagia.
The system uses Adaptive Vapor Ablation and is the first endometrial ablation system specifically designed for use in the doctor’s office, allowing minimal anesthesia/analgesia and rapid recovery, says AEGEA Medical.
AEGEA Medical describes the AEGEA Vapor System as a fully automated safety monitoring and vapor delivery system that uses a slender, flexible Vapor Probe with SmartSealTM technology and the Integrity ProTM safety feature, for an added level of confidence. The 4-minute procedure time includes 2 minutes of vapor treatment and can be performed in patients with a wider range of uterine anatomies than indicated for use with currently available treatments, says AEGEA Medical.
FOR MORE INFORMATION, VISIT: http://aegeamedical.com/
NATURAL CYCLES
The FDA has cleared Natural Cycles as the first digital method of birth control in the United States. Delivered in the form of an app, Natural Cycles is a fertility awareness–based contraceptive that uses a sophisticated algorithm to accurately and conveniently determine a woman’s daily fertility based on basal body temperature.
That data builds into a personalized fertility indicator that informs her when she needs to use protection to minimize the chance of conception. The app also can be used to help plan a pregnancy when the time is right, according to Natural Cycles.
A clinical study showed that the efficacy of a contraceptive mobile application is higher than usually reported for traditional fertility awareness–based methods. The application may contribute to reducing the unmet need for contraception, says Natural Cycles.
FOR MORE INFORMATION, VISIT: https://www.naturalcycles.com/en/hcp
TREATMENT FOR VASOMOTOR SYMPTOMS
BIJUVA™ has been US Food and Drug Administration (FDA)–approved as the first oral treatment for moderate-to-severe vasomotor symptoms due to menopause in women with a uterus. BIJUVA offers a combination of bioidentical estradiol to reduce moderate-to-severe hot flashes and bioidentical progesterone to reduce the risk for endometrial hyperplasia.
TherapeuticsMD says that BIJUVA will be available Spring 2019 and is a proven treatment option for women who are experiencing bothersome symptoms of menopause, with clinical trial data demonstrating a statistically significant reduction in both the frequency and severity of moderate-to-severe vasomotor symptoms. The manufacturer also says that BIJUVA is developed to be identical in molecular structure to the hormones already produced by the body and is designed to help women restore what is lost during menopause.
BIJUVA estradiol and progesterone combination (1 mg/100 mg) will be available in capsule form.
FOR MORE INFORMATION, VISIT:https://bijuva.com/discover/
LILETTA USE EXTENDED
The FDA has approved LILETTA® (levonorgestrel-releasing intrauterine system) 52 mg for 5-year use. This approval is based on efficacy and safety data from ACCESS IUS, the largest ongoing intrauterine device (IUD) Phase 3 clinical trial in the United States. Previously, LILETTA was indicated for use up to 4 years.
LILETTA continues to be greater than 99% effective in preventing pregnancy in a broad range of women, regardless of age, race, body mass index, or parity, according to Allergan and Medicines360. The extended duration and proven efficacy across a diverse population enables more women in the United States to obtain effective birth control, as the IUD is now available for a low cost at public health clinics.
FOR MORE INFORMATION, VISIT: https://www.liletta.com
Continue to: ENDOMETRIAL ABLATION TECHNOLOGY
ENDOMETRIAL ABLATION TECHNOLOGY
AEGEA Medical introduces the AEGEA Vapor SystemTM, an innovative solution for endometrial ablation to treat menorrhagia.
The system uses Adaptive Vapor Ablation and is the first endometrial ablation system specifically designed for use in the doctor’s office, allowing minimal anesthesia/analgesia and rapid recovery, says AEGEA Medical.
AEGEA Medical describes the AEGEA Vapor System as a fully automated safety monitoring and vapor delivery system that uses a slender, flexible Vapor Probe with SmartSealTM technology and the Integrity ProTM safety feature, for an added level of confidence. The 4-minute procedure time includes 2 minutes of vapor treatment and can be performed in patients with a wider range of uterine anatomies than indicated for use with currently available treatments, says AEGEA Medical.
FOR MORE INFORMATION, VISIT: http://aegeamedical.com/
NATURAL CYCLES
The FDA has cleared Natural Cycles as the first digital method of birth control in the United States. Delivered in the form of an app, Natural Cycles is a fertility awareness–based contraceptive that uses a sophisticated algorithm to accurately and conveniently determine a woman’s daily fertility based on basal body temperature.
That data builds into a personalized fertility indicator that informs her when she needs to use protection to minimize the chance of conception. The app also can be used to help plan a pregnancy when the time is right, according to Natural Cycles.
A clinical study showed that the efficacy of a contraceptive mobile application is higher than usually reported for traditional fertility awareness–based methods. The application may contribute to reducing the unmet need for contraception, says Natural Cycles.
FOR MORE INFORMATION, VISIT: https://www.naturalcycles.com/en/hcp
Product Update: Spectrum, Viera, YO, and SmartCurve
PREIMPLANTATION GENETIC TESTING
In a retrospective study published in Fertility and Sterility, Spectrum, a single-nucleotide polymorphism (SNP)-based PGT-A technology, was successful in screening all 24 chromosomes to provide comprehensive embryo aneuploidy results. Natera says that the study results showed that use of Spectrum PGT-A during IVF led to excellent implantation (70%), clinical pregnancy (71%), and live birth (65%) rates during single embryo transfer.
Spectrum evaluates the number of chromosomes in embryos to detect extra or missing chromosomes and screens for inherited genetic disorders to help provide the best chance of transferring a healthy embryo with the correct number of chromosomes.
FOR MORE INFORMATION, VISIT: https://www.natera.com/spectrum
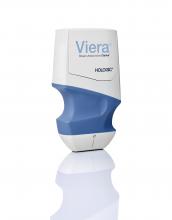
PORTABLE BREAST ULTRASOUND
Viera is a wireless, handheld breast ultrasound scanner that Hologic says produces exceptional image quality. The scanner uses a 14-4 MHz linear transducer, contains 192 elements, and has 4 parallel software beamformers. It utilizes spatial compounding to reduce image noise and speckle. Presets are available for breast, dense breast, and interventional procedures with B, M, power Doppler, color Doppler, and needle enhancement modes. On-demand high-resolution images are transmitted wirelessly to smart devices and patient archive systems (PACS) in the office, exam room, or surgical suite, or to the Cloud for efficient documentation. Smart device platforms include iOS and Android devices using WiFi and Bluetooth connectivity. The system includes a 1.2-lb scanner, 2 rechargeable batteries, and a charger with global AC adapter.
FOR MORE INFORMATION, VISIT: https://www.vieraportableultrasound.com
HOME SPERM TEST
The customer downloads the smart-phone app and acquires the YO Kit. After collecting a semen sample, he uses a pipette from the kit to place semen on a slide, which is slipped into the Yo Clip. The clip slides onto the smartphone, which uses its camera to take a high-resolution video. Test results and the sperm video appear in about 2 minutes.
At $59.95, the YO Kit includes 2 tests, in case a second sample is desired.
FOR MORE INFORMATION, VISIT:
https://www.yospermtest.com
Continue to Improving the Mammography Experience…
IMPROVING THE MAMMOGRAPHY EXPERIENCE
SmartCurve is standard on Hologic’s new 3Dimensions™ mammography system and as an enhancement option to existing Hologic Selenia®Dimensions® systems.
FOR MORE INFORMATION, VISIT:https://www.smartcurvesystem.com
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
PREIMPLANTATION GENETIC TESTING
In a retrospective study published in Fertility and Sterility, Spectrum, a single-nucleotide polymorphism (SNP)-based PGT-A technology, was successful in screening all 24 chromosomes to provide comprehensive embryo aneuploidy results. Natera says that the study results showed that use of Spectrum PGT-A during IVF led to excellent implantation (70%), clinical pregnancy (71%), and live birth (65%) rates during single embryo transfer.
Spectrum evaluates the number of chromosomes in embryos to detect extra or missing chromosomes and screens for inherited genetic disorders to help provide the best chance of transferring a healthy embryo with the correct number of chromosomes.
FOR MORE INFORMATION, VISIT: https://www.natera.com/spectrum
PORTABLE BREAST ULTRASOUND
Viera is a wireless, handheld breast ultrasound scanner that Hologic says produces exceptional image quality. The scanner uses a 14-4 MHz linear transducer, contains 192 elements, and has 4 parallel software beamformers. It utilizes spatial compounding to reduce image noise and speckle. Presets are available for breast, dense breast, and interventional procedures with B, M, power Doppler, color Doppler, and needle enhancement modes. On-demand high-resolution images are transmitted wirelessly to smart devices and patient archive systems (PACS) in the office, exam room, or surgical suite, or to the Cloud for efficient documentation. Smart device platforms include iOS and Android devices using WiFi and Bluetooth connectivity. The system includes a 1.2-lb scanner, 2 rechargeable batteries, and a charger with global AC adapter.
FOR MORE INFORMATION, VISIT: https://www.vieraportableultrasound.com
HOME SPERM TEST
The customer downloads the smart-phone app and acquires the YO Kit. After collecting a semen sample, he uses a pipette from the kit to place semen on a slide, which is slipped into the Yo Clip. The clip slides onto the smartphone, which uses its camera to take a high-resolution video. Test results and the sperm video appear in about 2 minutes.
At $59.95, the YO Kit includes 2 tests, in case a second sample is desired.
FOR MORE INFORMATION, VISIT:
https://www.yospermtest.com
Continue to Improving the Mammography Experience…
IMPROVING THE MAMMOGRAPHY EXPERIENCE
SmartCurve is standard on Hologic’s new 3Dimensions™ mammography system and as an enhancement option to existing Hologic Selenia®Dimensions® systems.
FOR MORE INFORMATION, VISIT:https://www.smartcurvesystem.com
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
PREIMPLANTATION GENETIC TESTING
In a retrospective study published in Fertility and Sterility, Spectrum, a single-nucleotide polymorphism (SNP)-based PGT-A technology, was successful in screening all 24 chromosomes to provide comprehensive embryo aneuploidy results. Natera says that the study results showed that use of Spectrum PGT-A during IVF led to excellent implantation (70%), clinical pregnancy (71%), and live birth (65%) rates during single embryo transfer.
Spectrum evaluates the number of chromosomes in embryos to detect extra or missing chromosomes and screens for inherited genetic disorders to help provide the best chance of transferring a healthy embryo with the correct number of chromosomes.
FOR MORE INFORMATION, VISIT: https://www.natera.com/spectrum
PORTABLE BREAST ULTRASOUND
Viera is a wireless, handheld breast ultrasound scanner that Hologic says produces exceptional image quality. The scanner uses a 14-4 MHz linear transducer, contains 192 elements, and has 4 parallel software beamformers. It utilizes spatial compounding to reduce image noise and speckle. Presets are available for breast, dense breast, and interventional procedures with B, M, power Doppler, color Doppler, and needle enhancement modes. On-demand high-resolution images are transmitted wirelessly to smart devices and patient archive systems (PACS) in the office, exam room, or surgical suite, or to the Cloud for efficient documentation. Smart device platforms include iOS and Android devices using WiFi and Bluetooth connectivity. The system includes a 1.2-lb scanner, 2 rechargeable batteries, and a charger with global AC adapter.
FOR MORE INFORMATION, VISIT: https://www.vieraportableultrasound.com
HOME SPERM TEST
The customer downloads the smart-phone app and acquires the YO Kit. After collecting a semen sample, he uses a pipette from the kit to place semen on a slide, which is slipped into the Yo Clip. The clip slides onto the smartphone, which uses its camera to take a high-resolution video. Test results and the sperm video appear in about 2 minutes.
At $59.95, the YO Kit includes 2 tests, in case a second sample is desired.
FOR MORE INFORMATION, VISIT:
https://www.yospermtest.com
Continue to Improving the Mammography Experience…
IMPROVING THE MAMMOGRAPHY EXPERIENCE
SmartCurve is standard on Hologic’s new 3Dimensions™ mammography system and as an enhancement option to existing Hologic Selenia®Dimensions® systems.
FOR MORE INFORMATION, VISIT:https://www.smartcurvesystem.com
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Product News: 12 2018
Altreno Lotion Now Available for Acne Vulgaris
Ortho Dermatologics launches Altreno (tretinoin) Lotion 0.05% for the treatment of acne vulgaris in patients 9 years and older. It was approved by the US Food and Drug Administration in August 2018, providing patients with the efficacy of tretinoin and the tolerability of a lotion formulation containing hyaluronic acid, glycerin, and collagen to help hydrate and moisturize the skin. For more information, visit www.ortho-dermatologics.com.
Bryhali Approved for Plaque Psoriasis in Adults
Ortho Dermatologics announces US Food and Drug Administration approval of Bryhali (halobetasol propionate) Lotion 0.01% for the treatment of plaque psoriasis in adults. Bryhali is a corticosteroid in a novel vehicle lotion with safety established for dosing up to 8 weeks, offering patients a longer duration of use than other topical steroids. For more information, visit www.bryhali.com.
CoolSculpting Cleared for the Submandibular Area
Zeltiq Aesthetics, Inc, an Allergan affiliate, announces US Food and Drug Administration (FDA) clearance of CoolSculpting to treat the submandibular area. The FDA clearance also was expanded to include patients with a body mass index of up to 46.2 when treating the submental and submandibular areas. CoolSculpting is a nonsurgical treatment that works by gently cooling targeted fat cells in the body to induce natural controlled elimination of fat cells without affecting surrounding tissue. CoolSculpting also is cleared for treatment of visible fat bulges on the thighs, abdomen, and flanks. For more information, visit www.coolsculpting.com.
Glytone Age-Defying Vitamin C+E Serum Reduces Signs of Aging
Pierre Fabre Dermo-Cosmetique introduces Glytone Age-Defying Vitamin C+E Serum, a layering serum with time-released, high concentrations of stabilized vitamins C and E combined with red tea flavonoids to deliver antioxidant protection and antiaging benefits. It is indicated for premature aging caused by environmental damage and oxidative stress as well as postprocedure relief. For more information, visit www.glytone-usa.com.
Hyrimoz Biosimilar Approved for Psoriasis
Sandoz, a Novartis Division, announces US Food and Drug Administration (FDA) approval of Hyrimoz (adalimumab-adaz), a biosimilar indicated for the treatment of psoriatic arthritis and plaque psoriasis, as well as rheumatoid arthritis, juvenile idiopathic arthritis in patients 4 years and older, ankylosing spondylitis, adult Crohn disease, and ulcerative colitis. Hyrimoz is the third FDA-approved biosimilar from Sandoz. For more information, visit www.sandoz.com.
Libtayo Approved for SCC
Regeneron Pharmaceuticals, Inc, and sanofi-aventis US LLC, announce US Food and Drug Administration (FDA) approval of Libtayo (cemiplimab-rwlc) for the treatment of patients with metastatic cutaneous squamous cell carcinoma (SCC) or locally advanced cutaneous SCC who are not candidates for curative surgery or radiation. Libtayo is a monoclonal antibody targeting the programmed death receptor 1.
Restylane Lyft Now Approved for Hand Rejuvenation
Nestlé Skin Health announces US Food and Drug Administration approval of Restylane Lyft with Lidocaine, a hyaluronic acid dermal filler, for the correction of age-related volume loss in the back of the hands for patients older than 21 years. Restylane Lyft with Lidocaine also is indicated for correction of moderate to severe facial wrinkles and folds such as the nasolabial folds, cheek augmentation, and age-related midface contour deficiencies. For more information, visit www.RestylaneUSA.com.
Xepi Launches for Impetigo
Cutanea Life Sciences launches Xepi (ozenoxacin) Cream 1%, a quinolone antimicrobial, for the treatment of impetigo in adult and pediatric patients 2 months or older. Xepi is applied twice daily for 5 days and has been shown to be active against most isolates of Staphylococcus aureus (including methicillin-resistant isolates) and Streptococcus pyogenes, both in vitro and in clinical infections. For more information, visit www.XepiCream.com.
If you would like your product included in Product News, please email a press release to the Editorial Office at [email protected].
Altreno Lotion Now Available for Acne Vulgaris
Ortho Dermatologics launches Altreno (tretinoin) Lotion 0.05% for the treatment of acne vulgaris in patients 9 years and older. It was approved by the US Food and Drug Administration in August 2018, providing patients with the efficacy of tretinoin and the tolerability of a lotion formulation containing hyaluronic acid, glycerin, and collagen to help hydrate and moisturize the skin. For more information, visit www.ortho-dermatologics.com.
Bryhali Approved for Plaque Psoriasis in Adults
Ortho Dermatologics announces US Food and Drug Administration approval of Bryhali (halobetasol propionate) Lotion 0.01% for the treatment of plaque psoriasis in adults. Bryhali is a corticosteroid in a novel vehicle lotion with safety established for dosing up to 8 weeks, offering patients a longer duration of use than other topical steroids. For more information, visit www.bryhali.com.
CoolSculpting Cleared for the Submandibular Area
Zeltiq Aesthetics, Inc, an Allergan affiliate, announces US Food and Drug Administration (FDA) clearance of CoolSculpting to treat the submandibular area. The FDA clearance also was expanded to include patients with a body mass index of up to 46.2 when treating the submental and submandibular areas. CoolSculpting is a nonsurgical treatment that works by gently cooling targeted fat cells in the body to induce natural controlled elimination of fat cells without affecting surrounding tissue. CoolSculpting also is cleared for treatment of visible fat bulges on the thighs, abdomen, and flanks. For more information, visit www.coolsculpting.com.
Glytone Age-Defying Vitamin C+E Serum Reduces Signs of Aging
Pierre Fabre Dermo-Cosmetique introduces Glytone Age-Defying Vitamin C+E Serum, a layering serum with time-released, high concentrations of stabilized vitamins C and E combined with red tea flavonoids to deliver antioxidant protection and antiaging benefits. It is indicated for premature aging caused by environmental damage and oxidative stress as well as postprocedure relief. For more information, visit www.glytone-usa.com.
Hyrimoz Biosimilar Approved for Psoriasis
Sandoz, a Novartis Division, announces US Food and Drug Administration (FDA) approval of Hyrimoz (adalimumab-adaz), a biosimilar indicated for the treatment of psoriatic arthritis and plaque psoriasis, as well as rheumatoid arthritis, juvenile idiopathic arthritis in patients 4 years and older, ankylosing spondylitis, adult Crohn disease, and ulcerative colitis. Hyrimoz is the third FDA-approved biosimilar from Sandoz. For more information, visit www.sandoz.com.
Libtayo Approved for SCC
Regeneron Pharmaceuticals, Inc, and sanofi-aventis US LLC, announce US Food and Drug Administration (FDA) approval of Libtayo (cemiplimab-rwlc) for the treatment of patients with metastatic cutaneous squamous cell carcinoma (SCC) or locally advanced cutaneous SCC who are not candidates for curative surgery or radiation. Libtayo is a monoclonal antibody targeting the programmed death receptor 1.
Restylane Lyft Now Approved for Hand Rejuvenation
Nestlé Skin Health announces US Food and Drug Administration approval of Restylane Lyft with Lidocaine, a hyaluronic acid dermal filler, for the correction of age-related volume loss in the back of the hands for patients older than 21 years. Restylane Lyft with Lidocaine also is indicated for correction of moderate to severe facial wrinkles and folds such as the nasolabial folds, cheek augmentation, and age-related midface contour deficiencies. For more information, visit www.RestylaneUSA.com.
Xepi Launches for Impetigo
Cutanea Life Sciences launches Xepi (ozenoxacin) Cream 1%, a quinolone antimicrobial, for the treatment of impetigo in adult and pediatric patients 2 months or older. Xepi is applied twice daily for 5 days and has been shown to be active against most isolates of Staphylococcus aureus (including methicillin-resistant isolates) and Streptococcus pyogenes, both in vitro and in clinical infections. For more information, visit www.XepiCream.com.
If you would like your product included in Product News, please email a press release to the Editorial Office at [email protected].
Altreno Lotion Now Available for Acne Vulgaris
Ortho Dermatologics launches Altreno (tretinoin) Lotion 0.05% for the treatment of acne vulgaris in patients 9 years and older. It was approved by the US Food and Drug Administration in August 2018, providing patients with the efficacy of tretinoin and the tolerability of a lotion formulation containing hyaluronic acid, glycerin, and collagen to help hydrate and moisturize the skin. For more information, visit www.ortho-dermatologics.com.
Bryhali Approved for Plaque Psoriasis in Adults
Ortho Dermatologics announces US Food and Drug Administration approval of Bryhali (halobetasol propionate) Lotion 0.01% for the treatment of plaque psoriasis in adults. Bryhali is a corticosteroid in a novel vehicle lotion with safety established for dosing up to 8 weeks, offering patients a longer duration of use than other topical steroids. For more information, visit www.bryhali.com.
CoolSculpting Cleared for the Submandibular Area
Zeltiq Aesthetics, Inc, an Allergan affiliate, announces US Food and Drug Administration (FDA) clearance of CoolSculpting to treat the submandibular area. The FDA clearance also was expanded to include patients with a body mass index of up to 46.2 when treating the submental and submandibular areas. CoolSculpting is a nonsurgical treatment that works by gently cooling targeted fat cells in the body to induce natural controlled elimination of fat cells without affecting surrounding tissue. CoolSculpting also is cleared for treatment of visible fat bulges on the thighs, abdomen, and flanks. For more information, visit www.coolsculpting.com.
Glytone Age-Defying Vitamin C+E Serum Reduces Signs of Aging
Pierre Fabre Dermo-Cosmetique introduces Glytone Age-Defying Vitamin C+E Serum, a layering serum with time-released, high concentrations of stabilized vitamins C and E combined with red tea flavonoids to deliver antioxidant protection and antiaging benefits. It is indicated for premature aging caused by environmental damage and oxidative stress as well as postprocedure relief. For more information, visit www.glytone-usa.com.
Hyrimoz Biosimilar Approved for Psoriasis
Sandoz, a Novartis Division, announces US Food and Drug Administration (FDA) approval of Hyrimoz (adalimumab-adaz), a biosimilar indicated for the treatment of psoriatic arthritis and plaque psoriasis, as well as rheumatoid arthritis, juvenile idiopathic arthritis in patients 4 years and older, ankylosing spondylitis, adult Crohn disease, and ulcerative colitis. Hyrimoz is the third FDA-approved biosimilar from Sandoz. For more information, visit www.sandoz.com.
Libtayo Approved for SCC
Regeneron Pharmaceuticals, Inc, and sanofi-aventis US LLC, announce US Food and Drug Administration (FDA) approval of Libtayo (cemiplimab-rwlc) for the treatment of patients with metastatic cutaneous squamous cell carcinoma (SCC) or locally advanced cutaneous SCC who are not candidates for curative surgery or radiation. Libtayo is a monoclonal antibody targeting the programmed death receptor 1.
Restylane Lyft Now Approved for Hand Rejuvenation
Nestlé Skin Health announces US Food and Drug Administration approval of Restylane Lyft with Lidocaine, a hyaluronic acid dermal filler, for the correction of age-related volume loss in the back of the hands for patients older than 21 years. Restylane Lyft with Lidocaine also is indicated for correction of moderate to severe facial wrinkles and folds such as the nasolabial folds, cheek augmentation, and age-related midface contour deficiencies. For more information, visit www.RestylaneUSA.com.
Xepi Launches for Impetigo
Cutanea Life Sciences launches Xepi (ozenoxacin) Cream 1%, a quinolone antimicrobial, for the treatment of impetigo in adult and pediatric patients 2 months or older. Xepi is applied twice daily for 5 days and has been shown to be active against most isolates of Staphylococcus aureus (including methicillin-resistant isolates) and Streptococcus pyogenes, both in vitro and in clinical infections. For more information, visit www.XepiCream.com.
If you would like your product included in Product News, please email a press release to the Editorial Office at [email protected].
Product Update: PICO NPWT; Encision; TimerCap; AMA
SURGICAL SITE WOUND THERAPY
PICO NPWT is a negative-pressure wound therapy device to treat surgical site infection (SSI). According to Smith & Nephew, a new meta-analysis demonstrates that the prophylactic application of PICO with AIRLOCK™ Technology significantly reduces surgical site complications by 58%, the rate of dehiscence by 26%, and length of stay by one-half day when compared with standard care.
The PICO System is canister-free and disposable. Patients can be discharged safely with PICO in place. Seven days of therapy are provided in each kit, with 1 pump, 2 dressings, and fixation strips to allow for a dressing change.
PICO uses a 4-layer multifunction dressing design in which the layers work together to ensure that negative pressure is delivered to the wound bed and exudate is removed through absorption and evaporation. Approximately 20% of fluid still remains in the dressing. The top film layer has a high-moisture vapor transmission rate to transpire as much as 80% of the exudate, says Smith & Nephew.
FOR MORE INFORMATION, VISIT: http://www.smith-nephew.com/
SHIELDED LAPAROSCOPIC INSTRUMENTS PREVENT BURNS
Encision’s patented Active Electrode Monitoring (AEM®) Shielded Laparoscopic Instruments eliminate patient burns and the associated complications.
Every 90 minutes in the United States, a patient is severely injured from a stray energy burn during laparoscopic surgery, according to Encision. The AEM® Shielded Instruments are designed to eliminate burns caused by monopolar energy insulation failure and capacitive coupling, reducing complications and re-admissions.
In addition to helping health care professionals improve patient safety in line with a recent FDA safety communication, Active Electrode Monitoring is a recommended practice of AORN and AAGL.
Encision offers a complete line of premium laparoscopic monopolar surgical instruments with integrated AEM® technology as well as complimentary products to improve clinical effectiveness and patient safety, including bipolar and cold instrumentation.
FOR MORE INFORMATION, VISIT: https://www.encision.com/
iSORT: 7-DAY BLUETOOTH PILLBOX
TimerCap has a new Bluetooth-enabled 7-day pill box called the iSort that sends reminders to take medication to a patient’s phone using a free TimerCap App found at the AppStore and Android Market.
The iSort automatically records and stores the times when each door/slot is opened and closed. It knows which door has been used and seamlessly updates the TimerCap App. The app will notify the patient and, if designated, a caregiver, whenever a dose is due or missed using pictures to show what and how many meds are scheduled. More than one iSort box can be used with the app.
iSort provides reminders that help improve adherence to medication dosing instructions and eliminates annoying false alarms, double entries, and unnecessary reminders when pills already have been taken. The portable iSort uses 2 AA batteries that need to be changed about once per year.
FOR MORE INFORMATION, VISIT: https://www.timercap.com/isort
PLATFORM TO COORDINATE HEALTH AND TECHNOLOGY
The American Medical Association (AMA) recently has established a new initiative that introduces a solution to improve, organize, and share health care information. The Integrated Health Model Initiative (IHMI) is a platform that coordinates the health and technology sectors around a common data model. IHMI fills the national imperative to pioneer a shared framework for organizing health data, emphasizing patient-centric information, and refining data elements to those most predictive of better outcomes. The AMA says that evolving available health data to depict a complete picture of a patient’s journey from wellness to illness to treatment and beyond allows health care delivery to fully focus on patient outcomes, goals, and wellness. Participation in IHMI is open to all health care and technology stakeholders.
FOR MORE INFORMATION, VISIT: www.ama-assn.org/ihmi
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
SURGICAL SITE WOUND THERAPY
PICO NPWT is a negative-pressure wound therapy device to treat surgical site infection (SSI). According to Smith & Nephew, a new meta-analysis demonstrates that the prophylactic application of PICO with AIRLOCK™ Technology significantly reduces surgical site complications by 58%, the rate of dehiscence by 26%, and length of stay by one-half day when compared with standard care.
The PICO System is canister-free and disposable. Patients can be discharged safely with PICO in place. Seven days of therapy are provided in each kit, with 1 pump, 2 dressings, and fixation strips to allow for a dressing change.
PICO uses a 4-layer multifunction dressing design in which the layers work together to ensure that negative pressure is delivered to the wound bed and exudate is removed through absorption and evaporation. Approximately 20% of fluid still remains in the dressing. The top film layer has a high-moisture vapor transmission rate to transpire as much as 80% of the exudate, says Smith & Nephew.
FOR MORE INFORMATION, VISIT: http://www.smith-nephew.com/
SHIELDED LAPAROSCOPIC INSTRUMENTS PREVENT BURNS
Encision’s patented Active Electrode Monitoring (AEM®) Shielded Laparoscopic Instruments eliminate patient burns and the associated complications.
Every 90 minutes in the United States, a patient is severely injured from a stray energy burn during laparoscopic surgery, according to Encision. The AEM® Shielded Instruments are designed to eliminate burns caused by monopolar energy insulation failure and capacitive coupling, reducing complications and re-admissions.
In addition to helping health care professionals improve patient safety in line with a recent FDA safety communication, Active Electrode Monitoring is a recommended practice of AORN and AAGL.
Encision offers a complete line of premium laparoscopic monopolar surgical instruments with integrated AEM® technology as well as complimentary products to improve clinical effectiveness and patient safety, including bipolar and cold instrumentation.
FOR MORE INFORMATION, VISIT: https://www.encision.com/
iSORT: 7-DAY BLUETOOTH PILLBOX
TimerCap has a new Bluetooth-enabled 7-day pill box called the iSort that sends reminders to take medication to a patient’s phone using a free TimerCap App found at the AppStore and Android Market.
The iSort automatically records and stores the times when each door/slot is opened and closed. It knows which door has been used and seamlessly updates the TimerCap App. The app will notify the patient and, if designated, a caregiver, whenever a dose is due or missed using pictures to show what and how many meds are scheduled. More than one iSort box can be used with the app.
iSort provides reminders that help improve adherence to medication dosing instructions and eliminates annoying false alarms, double entries, and unnecessary reminders when pills already have been taken. The portable iSort uses 2 AA batteries that need to be changed about once per year.
FOR MORE INFORMATION, VISIT: https://www.timercap.com/isort
PLATFORM TO COORDINATE HEALTH AND TECHNOLOGY
The American Medical Association (AMA) recently has established a new initiative that introduces a solution to improve, organize, and share health care information. The Integrated Health Model Initiative (IHMI) is a platform that coordinates the health and technology sectors around a common data model. IHMI fills the national imperative to pioneer a shared framework for organizing health data, emphasizing patient-centric information, and refining data elements to those most predictive of better outcomes. The AMA says that evolving available health data to depict a complete picture of a patient’s journey from wellness to illness to treatment and beyond allows health care delivery to fully focus on patient outcomes, goals, and wellness. Participation in IHMI is open to all health care and technology stakeholders.
FOR MORE INFORMATION, VISIT: www.ama-assn.org/ihmi
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
SURGICAL SITE WOUND THERAPY
PICO NPWT is a negative-pressure wound therapy device to treat surgical site infection (SSI). According to Smith & Nephew, a new meta-analysis demonstrates that the prophylactic application of PICO with AIRLOCK™ Technology significantly reduces surgical site complications by 58%, the rate of dehiscence by 26%, and length of stay by one-half day when compared with standard care.
The PICO System is canister-free and disposable. Patients can be discharged safely with PICO in place. Seven days of therapy are provided in each kit, with 1 pump, 2 dressings, and fixation strips to allow for a dressing change.
PICO uses a 4-layer multifunction dressing design in which the layers work together to ensure that negative pressure is delivered to the wound bed and exudate is removed through absorption and evaporation. Approximately 20% of fluid still remains in the dressing. The top film layer has a high-moisture vapor transmission rate to transpire as much as 80% of the exudate, says Smith & Nephew.
FOR MORE INFORMATION, VISIT: http://www.smith-nephew.com/
SHIELDED LAPAROSCOPIC INSTRUMENTS PREVENT BURNS
Encision’s patented Active Electrode Monitoring (AEM®) Shielded Laparoscopic Instruments eliminate patient burns and the associated complications.
Every 90 minutes in the United States, a patient is severely injured from a stray energy burn during laparoscopic surgery, according to Encision. The AEM® Shielded Instruments are designed to eliminate burns caused by monopolar energy insulation failure and capacitive coupling, reducing complications and re-admissions.
In addition to helping health care professionals improve patient safety in line with a recent FDA safety communication, Active Electrode Monitoring is a recommended practice of AORN and AAGL.
Encision offers a complete line of premium laparoscopic monopolar surgical instruments with integrated AEM® technology as well as complimentary products to improve clinical effectiveness and patient safety, including bipolar and cold instrumentation.
FOR MORE INFORMATION, VISIT: https://www.encision.com/
iSORT: 7-DAY BLUETOOTH PILLBOX
TimerCap has a new Bluetooth-enabled 7-day pill box called the iSort that sends reminders to take medication to a patient’s phone using a free TimerCap App found at the AppStore and Android Market.
The iSort automatically records and stores the times when each door/slot is opened and closed. It knows which door has been used and seamlessly updates the TimerCap App. The app will notify the patient and, if designated, a caregiver, whenever a dose is due or missed using pictures to show what and how many meds are scheduled. More than one iSort box can be used with the app.
iSort provides reminders that help improve adherence to medication dosing instructions and eliminates annoying false alarms, double entries, and unnecessary reminders when pills already have been taken. The portable iSort uses 2 AA batteries that need to be changed about once per year.
FOR MORE INFORMATION, VISIT: https://www.timercap.com/isort
PLATFORM TO COORDINATE HEALTH AND TECHNOLOGY
The American Medical Association (AMA) recently has established a new initiative that introduces a solution to improve, organize, and share health care information. The Integrated Health Model Initiative (IHMI) is a platform that coordinates the health and technology sectors around a common data model. IHMI fills the national imperative to pioneer a shared framework for organizing health data, emphasizing patient-centric information, and refining data elements to those most predictive of better outcomes. The AMA says that evolving available health data to depict a complete picture of a patient’s journey from wellness to illness to treatment and beyond allows health care delivery to fully focus on patient outcomes, goals, and wellness. Participation in IHMI is open to all health care and technology stakeholders.
FOR MORE INFORMATION, VISIT: www.ama-assn.org/ihmi
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Product News: 09 2018
Almirall Acquires 5 Products From Allergan
Almirall, SA, announces the acquisition of 5 products from Allergan: Aczone (dapsone), Tazorac (tazarotene), Azelex (azelaic acid), Cordran Tape (flurandrenolide), and Seysara (sarecycline). Seysara is a tetracycline-derived antibiotic with anti-inflammatory properties for the treatment of acne vulgaris in patients aged 9 years and older. Approval for Seysara is anticipated later in 2018. For more information, visit www.almirall.com.
Aspire Higher Scholarship Honorees Selected
Ortho Dermatologics announces the 2018 honorees of the Aspire Higher scholarship program. Nine students—3 pursuing undergraduate degrees, 3 pursuing graduate degrees, and 3 who are mothers pursuing either degree—will receive scholarships of $10,000 each. The 2018 honorees were selected from nearly 1200 applicants who shared their experience of living with a dermatologic condition, as well as the role that a dermatologist, physician assistant, or nurse practitioner has played in helping treat it. The applications were judged by an independent panel of dermatologists from across the country. Since 2013 the program has granted more than $450,000 in scholarships to students who have been affected by dermatologic conditions. For more information, visit www.aspirehigherscholarships.com.
Duobrii NDA Resubmitted to FDA
Ortho Dermatologics resubmits a New Drug Application to the US Food and Drug Administration (FDA) for Duobrii (halobetasol propionate and tazarotene) for plaque psoriasis with additional pharmacokinetic data as requested by the FDA in June 2018. The unique formulation will allow for a potentially expanded duration of use if approved. For more information, visit www.ortho-dermatologics.com.
JubliApp Now Available for Onychomycosis Patients
Ortho Dermatologics launches JubliApp, a mobile application (app) designed to encourage patient adherence to long-term treatment with Jublia (efinaconazole topical solution 10%) for onychomycosis. The app offers treatment and refill reminders, as well as a game (Mission Plu-Toe) to keep patients engaged while the daily application is drying, helping to make the 48-week long therapy less intimidating. The app also includes efficacy tracking with side-by-side photographs that can be shared with health care providers to monitor progress. It is available in the Apple App Store and the Google Play store. For more information, visit www.jubliarx.com.
Qbrexza Receives FDA Approval for Hyperhidrosis
Dermira, Inc, receives US Food and Drug Administration approval of Qbrexza (glycopyrronium cloth 2.4%) for the topical treatment of primary axillary hyperhidrosis in patients 9 years and older. Qbrexza works by blocking receptors responsible for sweat gland activation and can be used once daily. Qbrexza is expected to be available in October 2018. For more information, visit www.qbrexza.com/hcp.
Rituxan Announces FDA Approval for Pemphigus Vulgaris
Genentech USA, Inc, announces US Food and Drug Administration approval of Rituxan (rituximab) for the treatment of adults with moderate to severe pemphigus vulgaris, making it the first approved treatment for the condition in more than 60 years. Rituxan also is indicated for the treatment of rheumatoid arthritis, granulomatosis with polyangiitis, and microscopic polyangiitis. Clinical trials indicated th
Verrica Is Developing a Topical Treatment for Molluscum Contagiosum
Verrica Pharmaceuticals, a clinical-stage medical dermatology company committed to identifying, developing, and commercializing pharmaceutical products for underserved patients, is working on a novel therapy for molluscum contagiosum and verruca vulgaris (common warts). Verrica’s lead product VP-102 is a proprietary drug-device combination of a topical solution of cantharidin administered through a single-use precision applicator. Top-line phase 3 trial results are expected in 2019. For more information, visit www.verrica.com.
If you would like your product included in Product News, please email a press release to the Editorial Office at [email protected].
Almirall Acquires 5 Products From Allergan
Almirall, SA, announces the acquisition of 5 products from Allergan: Aczone (dapsone), Tazorac (tazarotene), Azelex (azelaic acid), Cordran Tape (flurandrenolide), and Seysara (sarecycline). Seysara is a tetracycline-derived antibiotic with anti-inflammatory properties for the treatment of acne vulgaris in patients aged 9 years and older. Approval for Seysara is anticipated later in 2018. For more information, visit www.almirall.com.
Aspire Higher Scholarship Honorees Selected
Ortho Dermatologics announces the 2018 honorees of the Aspire Higher scholarship program. Nine students—3 pursuing undergraduate degrees, 3 pursuing graduate degrees, and 3 who are mothers pursuing either degree—will receive scholarships of $10,000 each. The 2018 honorees were selected from nearly 1200 applicants who shared their experience of living with a dermatologic condition, as well as the role that a dermatologist, physician assistant, or nurse practitioner has played in helping treat it. The applications were judged by an independent panel of dermatologists from across the country. Since 2013 the program has granted more than $450,000 in scholarships to students who have been affected by dermatologic conditions. For more information, visit www.aspirehigherscholarships.com.
Duobrii NDA Resubmitted to FDA
Ortho Dermatologics resubmits a New Drug Application to the US Food and Drug Administration (FDA) for Duobrii (halobetasol propionate and tazarotene) for plaque psoriasis with additional pharmacokinetic data as requested by the FDA in June 2018. The unique formulation will allow for a potentially expanded duration of use if approved. For more information, visit www.ortho-dermatologics.com.
JubliApp Now Available for Onychomycosis Patients
Ortho Dermatologics launches JubliApp, a mobile application (app) designed to encourage patient adherence to long-term treatment with Jublia (efinaconazole topical solution 10%) for onychomycosis. The app offers treatment and refill reminders, as well as a game (Mission Plu-Toe) to keep patients engaged while the daily application is drying, helping to make the 48-week long therapy less intimidating. The app also includes efficacy tracking with side-by-side photographs that can be shared with health care providers to monitor progress. It is available in the Apple App Store and the Google Play store. For more information, visit www.jubliarx.com.
Qbrexza Receives FDA Approval for Hyperhidrosis
Dermira, Inc, receives US Food and Drug Administration approval of Qbrexza (glycopyrronium cloth 2.4%) for the topical treatment of primary axillary hyperhidrosis in patients 9 years and older. Qbrexza works by blocking receptors responsible for sweat gland activation and can be used once daily. Qbrexza is expected to be available in October 2018. For more information, visit www.qbrexza.com/hcp.
Rituxan Announces FDA Approval for Pemphigus Vulgaris
Genentech USA, Inc, announces US Food and Drug Administration approval of Rituxan (rituximab) for the treatment of adults with moderate to severe pemphigus vulgaris, making it the first approved treatment for the condition in more than 60 years. Rituxan also is indicated for the treatment of rheumatoid arthritis, granulomatosis with polyangiitis, and microscopic polyangiitis. Clinical trials indicated th
Verrica Is Developing a Topical Treatment for Molluscum Contagiosum
Verrica Pharmaceuticals, a clinical-stage medical dermatology company committed to identifying, developing, and commercializing pharmaceutical products for underserved patients, is working on a novel therapy for molluscum contagiosum and verruca vulgaris (common warts). Verrica’s lead product VP-102 is a proprietary drug-device combination of a topical solution of cantharidin administered through a single-use precision applicator. Top-line phase 3 trial results are expected in 2019. For more information, visit www.verrica.com.
If you would like your product included in Product News, please email a press release to the Editorial Office at [email protected].
Almirall Acquires 5 Products From Allergan
Almirall, SA, announces the acquisition of 5 products from Allergan: Aczone (dapsone), Tazorac (tazarotene), Azelex (azelaic acid), Cordran Tape (flurandrenolide), and Seysara (sarecycline). Seysara is a tetracycline-derived antibiotic with anti-inflammatory properties for the treatment of acne vulgaris in patients aged 9 years and older. Approval for Seysara is anticipated later in 2018. For more information, visit www.almirall.com.
Aspire Higher Scholarship Honorees Selected
Ortho Dermatologics announces the 2018 honorees of the Aspire Higher scholarship program. Nine students—3 pursuing undergraduate degrees, 3 pursuing graduate degrees, and 3 who are mothers pursuing either degree—will receive scholarships of $10,000 each. The 2018 honorees were selected from nearly 1200 applicants who shared their experience of living with a dermatologic condition, as well as the role that a dermatologist, physician assistant, or nurse practitioner has played in helping treat it. The applications were judged by an independent panel of dermatologists from across the country. Since 2013 the program has granted more than $450,000 in scholarships to students who have been affected by dermatologic conditions. For more information, visit www.aspirehigherscholarships.com.
Duobrii NDA Resubmitted to FDA
Ortho Dermatologics resubmits a New Drug Application to the US Food and Drug Administration (FDA) for Duobrii (halobetasol propionate and tazarotene) for plaque psoriasis with additional pharmacokinetic data as requested by the FDA in June 2018. The unique formulation will allow for a potentially expanded duration of use if approved. For more information, visit www.ortho-dermatologics.com.
JubliApp Now Available for Onychomycosis Patients
Ortho Dermatologics launches JubliApp, a mobile application (app) designed to encourage patient adherence to long-term treatment with Jublia (efinaconazole topical solution 10%) for onychomycosis. The app offers treatment and refill reminders, as well as a game (Mission Plu-Toe) to keep patients engaged while the daily application is drying, helping to make the 48-week long therapy less intimidating. The app also includes efficacy tracking with side-by-side photographs that can be shared with health care providers to monitor progress. It is available in the Apple App Store and the Google Play store. For more information, visit www.jubliarx.com.
Qbrexza Receives FDA Approval for Hyperhidrosis
Dermira, Inc, receives US Food and Drug Administration approval of Qbrexza (glycopyrronium cloth 2.4%) for the topical treatment of primary axillary hyperhidrosis in patients 9 years and older. Qbrexza works by blocking receptors responsible for sweat gland activation and can be used once daily. Qbrexza is expected to be available in October 2018. For more information, visit www.qbrexza.com/hcp.
Rituxan Announces FDA Approval for Pemphigus Vulgaris
Genentech USA, Inc, announces US Food and Drug Administration approval of Rituxan (rituximab) for the treatment of adults with moderate to severe pemphigus vulgaris, making it the first approved treatment for the condition in more than 60 years. Rituxan also is indicated for the treatment of rheumatoid arthritis, granulomatosis with polyangiitis, and microscopic polyangiitis. Clinical trials indicated th
Verrica Is Developing a Topical Treatment for Molluscum Contagiosum
Verrica Pharmaceuticals, a clinical-stage medical dermatology company committed to identifying, developing, and commercializing pharmaceutical products for underserved patients, is working on a novel therapy for molluscum contagiosum and verruca vulgaris (common warts). Verrica’s lead product VP-102 is a proprietary drug-device combination of a topical solution of cantharidin administered through a single-use precision applicator. Top-line phase 3 trial results are expected in 2019. For more information, visit www.verrica.com.
If you would like your product included in Product News, please email a press release to the Editorial Office at [email protected].
Product Update: FUJIFILM; Freemie, Preventeza, and C-Panty
NEW VISUALIZATION SYSTEMS FROM FUJIFILM
FUJIFILM New Development, USA, has introduced 2 visualization systems for minimally invasive surgery. Using proprietary technology, the Ultra-Slim Video Laparoscope System (EL-580FN) delivers enhanced image resolution, color fidelity, and display quality, says FUJIFILM. The product features include “Chip on the Tip” high-definition digital imaging processing, less fogging, autoclave sterilization reprocessing, and a low profile, lightweight ergonomic handle. The 3.8-mm-diameter distal end was designed to improve workflow, reduce physician fatigue, and potentially reduce the size of incisions. The accompanying Digital Video Processor System is used for endoscopic procedures with automatic light control, an anti-blur function for motion images, and digital zoom.
FUJIFILM reports that the Full High Definition Surgical Visualization System is designed for a wide variety of surgical applications and offers edge enhancement, automatic gain control, dynamic contrast function, selective color enhancement, smoke reduction, and grid removal features. It includes a portfolio of rigid scopes, cameras, and video processing systems.
FOR MORE INFORMATION, VISIT: http://www.fujifilmusa.com
FREEMIE BREAST MILK COLLECTION SYSTEM
Freemie® offers a hands-free breast-milk collection system with the Freemie Liberty Mobile Hands Free Breast Pump System and Next Generation Freemie Closed System Collection Cups.
The concealable pump has a rechargeable battery and hospital-power suction for single or double pumping. Programmable memory buttons allow the mother to preset or adjust speed and suction functions. Tubing lengths can be changed so that the pump can be placed on a desk, worn with a detachable belt clip, or carried in a bag.
Freemie says the cups are lower-profile and more compact than other pump system cups, and when placed on the breast under the mother’s bra, can be easily removed so that milk can be transferred to storage. Each cup, with a 25 mm or 28 mm funnel and valve, holds 8 oz of milk.
FOR MORE INFORMATION, VISIT: http://www.freemie.com
PREVENTEZA: EMERGENCY CONTRACEPTIVE
Combe, Inc, the maker of Vagisil®, has launched Preventeza™ (levonorgestrel tablet, 1.5 mg), an emergency contraceptive for the prevention of pregnancy if unprotected sex or failed birth control occurs.
Available online or over-the-counter as a single tablet, Preventeza is a proven option to help women prevent pregnancy before it starts by using a higher dose of levonorgestrel than most birth control pills. It must be used within 72 hours of unprotected intercourse, and is not intended to be used as regular birth control. Combe says that Preventeza works mainly by stopping the release of an egg from the ovary and may also prevent fertilization of an egg or prevent a fertilized egg from implanting in the uterus. Combe also says that levonorgestrel 1.5 mg will not work if the woman is already pregnant and will not affect an existing pregnancy.
FOR MORE INFORMATION, VISIT: https://www.vagisil.com/products/preventeza-emergency-contraceptive
UPSPRING’S C-PANTY FOR POSTCESAREAN RECOVERY
UpSpring® says that its patented C-Panty® undergarment provides medical-grade compression and speeds recovery after cesarean delivery. C-Panty helps to reduce swelling and discomfort, supports weakened muscles, and reduces the incision bulge without hooks, straps, or Velcro that might irritate the incision area.
C-Panty’s medical-grade silicone panel suppresses the formation of excess or improperly formed collagen, which can contribute to scarring, says UpSpring. The silicone may help reduce itchiness, and can lessen the chance of infection at the incision area. The silicone is durable, washable, and integrated into the panty, eliminating the need for scar gel or scar gel pads.
The C-Panty can be worn immediately after birth and for up to 12 months. If worn when the incision is not healed, the silicone panel should be covered with a panty liner or pad. Once the incision has healed, the covering can be discontinued.
FOR MORE INFORMATION, VISIT: https://www.upspringbaby.com/cpanty
NEW VISUALIZATION SYSTEMS FROM FUJIFILM
FUJIFILM New Development, USA, has introduced 2 visualization systems for minimally invasive surgery. Using proprietary technology, the Ultra-Slim Video Laparoscope System (EL-580FN) delivers enhanced image resolution, color fidelity, and display quality, says FUJIFILM. The product features include “Chip on the Tip” high-definition digital imaging processing, less fogging, autoclave sterilization reprocessing, and a low profile, lightweight ergonomic handle. The 3.8-mm-diameter distal end was designed to improve workflow, reduce physician fatigue, and potentially reduce the size of incisions. The accompanying Digital Video Processor System is used for endoscopic procedures with automatic light control, an anti-blur function for motion images, and digital zoom.
FUJIFILM reports that the Full High Definition Surgical Visualization System is designed for a wide variety of surgical applications and offers edge enhancement, automatic gain control, dynamic contrast function, selective color enhancement, smoke reduction, and grid removal features. It includes a portfolio of rigid scopes, cameras, and video processing systems.
FOR MORE INFORMATION, VISIT: http://www.fujifilmusa.com
FREEMIE BREAST MILK COLLECTION SYSTEM
Freemie® offers a hands-free breast-milk collection system with the Freemie Liberty Mobile Hands Free Breast Pump System and Next Generation Freemie Closed System Collection Cups.
The concealable pump has a rechargeable battery and hospital-power suction for single or double pumping. Programmable memory buttons allow the mother to preset or adjust speed and suction functions. Tubing lengths can be changed so that the pump can be placed on a desk, worn with a detachable belt clip, or carried in a bag.
Freemie says the cups are lower-profile and more compact than other pump system cups, and when placed on the breast under the mother’s bra, can be easily removed so that milk can be transferred to storage. Each cup, with a 25 mm or 28 mm funnel and valve, holds 8 oz of milk.
FOR MORE INFORMATION, VISIT: http://www.freemie.com
PREVENTEZA: EMERGENCY CONTRACEPTIVE
Combe, Inc, the maker of Vagisil®, has launched Preventeza™ (levonorgestrel tablet, 1.5 mg), an emergency contraceptive for the prevention of pregnancy if unprotected sex or failed birth control occurs.
Available online or over-the-counter as a single tablet, Preventeza is a proven option to help women prevent pregnancy before it starts by using a higher dose of levonorgestrel than most birth control pills. It must be used within 72 hours of unprotected intercourse, and is not intended to be used as regular birth control. Combe says that Preventeza works mainly by stopping the release of an egg from the ovary and may also prevent fertilization of an egg or prevent a fertilized egg from implanting in the uterus. Combe also says that levonorgestrel 1.5 mg will not work if the woman is already pregnant and will not affect an existing pregnancy.
FOR MORE INFORMATION, VISIT: https://www.vagisil.com/products/preventeza-emergency-contraceptive
UPSPRING’S C-PANTY FOR POSTCESAREAN RECOVERY
UpSpring® says that its patented C-Panty® undergarment provides medical-grade compression and speeds recovery after cesarean delivery. C-Panty helps to reduce swelling and discomfort, supports weakened muscles, and reduces the incision bulge without hooks, straps, or Velcro that might irritate the incision area.
C-Panty’s medical-grade silicone panel suppresses the formation of excess or improperly formed collagen, which can contribute to scarring, says UpSpring. The silicone may help reduce itchiness, and can lessen the chance of infection at the incision area. The silicone is durable, washable, and integrated into the panty, eliminating the need for scar gel or scar gel pads.
The C-Panty can be worn immediately after birth and for up to 12 months. If worn when the incision is not healed, the silicone panel should be covered with a panty liner or pad. Once the incision has healed, the covering can be discontinued.
FOR MORE INFORMATION, VISIT: https://www.upspringbaby.com/cpanty
NEW VISUALIZATION SYSTEMS FROM FUJIFILM
FUJIFILM New Development, USA, has introduced 2 visualization systems for minimally invasive surgery. Using proprietary technology, the Ultra-Slim Video Laparoscope System (EL-580FN) delivers enhanced image resolution, color fidelity, and display quality, says FUJIFILM. The product features include “Chip on the Tip” high-definition digital imaging processing, less fogging, autoclave sterilization reprocessing, and a low profile, lightweight ergonomic handle. The 3.8-mm-diameter distal end was designed to improve workflow, reduce physician fatigue, and potentially reduce the size of incisions. The accompanying Digital Video Processor System is used for endoscopic procedures with automatic light control, an anti-blur function for motion images, and digital zoom.
FUJIFILM reports that the Full High Definition Surgical Visualization System is designed for a wide variety of surgical applications and offers edge enhancement, automatic gain control, dynamic contrast function, selective color enhancement, smoke reduction, and grid removal features. It includes a portfolio of rigid scopes, cameras, and video processing systems.
FOR MORE INFORMATION, VISIT: http://www.fujifilmusa.com
FREEMIE BREAST MILK COLLECTION SYSTEM
Freemie® offers a hands-free breast-milk collection system with the Freemie Liberty Mobile Hands Free Breast Pump System and Next Generation Freemie Closed System Collection Cups.
The concealable pump has a rechargeable battery and hospital-power suction for single or double pumping. Programmable memory buttons allow the mother to preset or adjust speed and suction functions. Tubing lengths can be changed so that the pump can be placed on a desk, worn with a detachable belt clip, or carried in a bag.
Freemie says the cups are lower-profile and more compact than other pump system cups, and when placed on the breast under the mother’s bra, can be easily removed so that milk can be transferred to storage. Each cup, with a 25 mm or 28 mm funnel and valve, holds 8 oz of milk.
FOR MORE INFORMATION, VISIT: http://www.freemie.com
PREVENTEZA: EMERGENCY CONTRACEPTIVE
Combe, Inc, the maker of Vagisil®, has launched Preventeza™ (levonorgestrel tablet, 1.5 mg), an emergency contraceptive for the prevention of pregnancy if unprotected sex or failed birth control occurs.
Available online or over-the-counter as a single tablet, Preventeza is a proven option to help women prevent pregnancy before it starts by using a higher dose of levonorgestrel than most birth control pills. It must be used within 72 hours of unprotected intercourse, and is not intended to be used as regular birth control. Combe says that Preventeza works mainly by stopping the release of an egg from the ovary and may also prevent fertilization of an egg or prevent a fertilized egg from implanting in the uterus. Combe also says that levonorgestrel 1.5 mg will not work if the woman is already pregnant and will not affect an existing pregnancy.
FOR MORE INFORMATION, VISIT: https://www.vagisil.com/products/preventeza-emergency-contraceptive
UPSPRING’S C-PANTY FOR POSTCESAREAN RECOVERY
UpSpring® says that its patented C-Panty® undergarment provides medical-grade compression and speeds recovery after cesarean delivery. C-Panty helps to reduce swelling and discomfort, supports weakened muscles, and reduces the incision bulge without hooks, straps, or Velcro that might irritate the incision area.
C-Panty’s medical-grade silicone panel suppresses the formation of excess or improperly formed collagen, which can contribute to scarring, says UpSpring. The silicone may help reduce itchiness, and can lessen the chance of infection at the incision area. The silicone is durable, washable, and integrated into the panty, eliminating the need for scar gel or scar gel pads.
The C-Panty can be worn immediately after birth and for up to 12 months. If worn when the incision is not healed, the silicone panel should be covered with a panty liner or pad. Once the incision has healed, the covering can be discontinued.
FOR MORE INFORMATION, VISIT: https://www.upspringbaby.com/cpanty
Criteria for Use Updates for Enzalutamide, Daratumumab, Elotuzumab, Carfilzomib, and Ixazomib (FULL)
VA Pharmacy Benefit Management Service (PBM) continually issues or revises its guidances for hematology and oncology care providers on a number of cancer care medications. Below are excerpts from recently released Criteria for Use documents. The complete documents, including the inclusion criteria, dosage and administration guidance, monitoring information, and discontinuation criteria should be consulted and can be found at www.pbm.va.gov or vaww.cmopnational.va.gov/cmop/PBM/default.aspx.
ENZALUTAMIDE (XTANDI) Criteria for Use, January 2017
Exclusion Criteria: If the answer to ANY item below is met, then the patient should NOT receive enzalutamide.
- Brain metastases or active epidural disease
- Severe renal impairment (creatinine clearance < 30 mL/min)
- History of seizure (including febrile seizure, loss of consciousness, or transient ischemic attack within the previous 12 months, any condition predisposing to seizure: prior stroke, brain AV malformation, head trauma with loss of consciousness requiring hospitalization)
- ECOG Performance Status > 2
- Inability to swallow capsules
Issues for Consideration
- Enzalutamide is not indicated for use in women. Based on the mechanism of action, can cause fetal harm if used during pregnancy. Pregnancy Category X—use contraindicated during pregnancy. Exclude pregnancy before prescribing enzalutamide, discuss risks if pregnancy occurs, and provide contraceptive counseling.
- Use in patients taking concomitant medications that may lower the seizure threshold was not studied; caution patients about the risk of activities where the sudden loss of consciousness could cause serious harm if concomitant use cannot be avoided.
- Use in patients at risk for or with a strong history of falls: in the phase 3 clinical trial, falls or injuries from falls occurred in 4.6% of enzalutamide patients vs 1.3% of placebo patients.
- Avoid strong inhibitors of CYP2C8 (eg, gemfibrozil); if concomitant use of a strong CYP2C8 inhibitor cannot be avoided, reduce the dose of enzalutamide to 80 mg once daily according to the package insert.
- Co-administration with strong or moderate inducers of CYP3A4 (eg, carbamazepine, phenobarbital, phenytoin, rifampin, bosentan, efavirenz, modafinil, nafcillin, St. John’s Wort) or CYP2C8 (eg, rifampin) should be avoided if possible. If patient must be co-administered a strong CYP3A4 inducer, increase enzalutamide dose from 160 mg to 240 mg once daily.
- Drugs that are substrates of CYP3A4 (eg, alfentanil, cyclosporine, ergotamine, fentanyl, pimozide, quinidine, sirolimus, tacrolimus), CYP2C9 (eg, phenytoin, warfarin), or CYP2C19 (eg, S-mephenytoin) with a narrow therapeutic index should be avoided. If enzalutamide is co-administered with warfarin, additional INR testing should be conducted.
- Use in patients with hepatic impairment: Pharmacokinetics of enzalutamide and its metabolite were examined in volunteers with normal, Child-Pugh Class A, Child-Pugh Class B, and Child-Pugh Class C hepatic impairment. The composite AUC for enzalutamide and its metabolite after a single 160-mg dose was similar across all levels of hepatic impairment compared with normal volunteers.
- There have been postmarketing reports of posterior reversible encephalopathy syndrome (PRES) in patients receiving enzalutamide. PRES is a neurologic disorder presenting with rapidly evolving symptoms including seizure, headache, lethargy, confusion, blindness, and other visual/neurological disturbances with or without associated hypertension. Diagnosis of PRES requires brain imaging, preferably by MRI. Enzalutamide should be discontinued in patients developing PRES.
- Sequencing of enzalutamide and abiraterone has been evaluated in several small retrospective analyses; the majority of the analyses are in the post chemotherapy setting. From this limited observational data, it is unclear if there is a preferred sequencing of abiraterone and enzalutamide. There is some evidence for cross-resistance. There are ongoing investigations into mechanisms of resistance to enzalutamide and abiraterone.
DARATUMUMAB (DARZALEX) Criteria for Use, January 2017
Exclusion Criteria: If the answer to ANY item below is met, then the patient should NOT receive daratumumab.
- Patient is noncompliant with medication, follow-up, or laboratory appointments
- Patient unable or unwilling to be observed an extended period of time that may be necessary for first infusion (refer to Issues for Consideration)
- Hemoglobin < 8 g/dL; Must transfuse to hemoglobin above 8 gm/dL prior to therapy initiation
- Absolute neutrophil count (ANC) < 1,000/mm3
- Platelet count < 50,000/mm3 (< 30,000/mm3 if myeloma involvement in bone marrow > 50%)
- ECOG Performance Status > 2
- Total bilirubin > 3x the upper limit of the normal range (except for Gilbert syndrome: direct bilirubin 2x ULN) or ALT and AST > 3x ULN
- NYHA Class III or IV heart failure (refer to Issues for Consideration)
- Ongoing or active systemic infection, including active hepatitis B or C, or known HIV (refer to Issues for Consideration)
- Positive pregnancy test
Issues for Consideration
- Drug infusion time will be dependent upon patient tolerance and exposure to daratumumab. Median duration of the first infusion was ~ 7 hours in the SIRIUS trial, followed by infusion times of 4.2 and 3.4 hours, subsequently.
- Type and screen patients shortly prior to starting treatment. When the sample is provided to the blood bank, inform them that the patient will be receiving daratumumab.
- Those with NYHA Class III or IV heart failure, recent MI, conduction abnormalities, angina or arrhythmias uncontrolled by medications, were not eligible for clinical trials and may be at greater risk of cardiac complications.
- Patients with active hepatitis B, C, or HIV were excluded from clinical trials with daratumumab, therefore safety and efficacy data are unknown in these patient populations. The risk of infections was slightly higher in the daratumumab-treated arms of the comparative studies. Use of daratumumab should only be considered in those with well-controlled hepatitis B, hepatitis C, or HIV.
ELOTUZUMAB (EMPLICITI) Criteria for Use, January 2017
Exclusion Criteria: If the answer to ANY item below is met, then the patient should NOT receive elotuzumab.
- Patient is noncompliant with medication, follow-up, or laboratory appointments
- Patient is not a candidate for lenalidomide therapy (ie, is lenalidomide-refractory or possesses contraindications to therapy)
- Patient is not a candidate for high-dose dexamethasone therapy
- Hemoglobin < 8 g/dL; Must transfuse to hemoglobin above 8 gm/dL prior to therapy initiation
- Absolute neutrophil count (ANC) < 1,000/mm3
- Platelet count < 50,000/mm3 (< 30,000/mm3 if myeloma involvement in bone marrow > 50%)
- ECOG Performance Status > 2
- Total bilirubin > 2x the upper limit of the normal range (except for Gilbert syndrome: direct bilirubin > 2 mg/dL) or ALT and AST > 3x ULN
- NYHA Class III or IV heart failure (refer to Issues for Consideration)
- Ongoing or active systemic infection, including active hepatitis B or hepatitis C, or known HIV (refer to Issues for Consideration)
- Positive pregnancy test
- Patient intends to breastfeed during therapy
Issues for Consideration
- Those with NYHA Class III or IV heart failure, recent MI, conduction abnormalities, angina or arrhythmias uncontrolled by medications, were not eligible for clinical trials and may be at greater risk of cardiac complications.
- Patients with active hepatitis B, hepatitis C, or HIV were excluded from clinical trials with elotuzumab, therefore safety and efficacy data are unknown in these patient populations. The risk of infections (OI, fungal, viral) was greater in the elotuzumab arm vs control arm of the comparative clinical trial. Use of elotuzumab should only be considered in those with well-controlled hepatitis B, hepatitis C, or HIV.
- Disappointing response rates as monotherapy in the relapsed/refractory setting suggest that elotuzumab should be given in combination with lenalidomide and dexamethasone.
- Impact of elotuzumab/lenalidomide/dexamethasone on overall survival is not known as these data were not mature at the time ELOQUENT-2 was published.
CARFILZOMIB (KYPROLIS) Criteria for Use, December 2016
Exclusion Criteria: If the answer to ANY item below is met, then the patient should NOT receive carfilzomib.
- Care for the oncologic disease being treated not provided by a VA or VA purchased care (eg, Choice Program, Fee Basis) oncology provider
- Patient is noncompliant with medication, follow-up, or laboratory appointments
- Hemoglobin < 8 g/dL; Must transfuse to hemoglobin above 8 g/dL prior to therapy initiation
- Absolute neutrophil count (ANC) < 1,000/mm3
- Platelet count < 50,000/mm3 (< 30,000/mm3 if myeloma involvement in bone marrow > 50%)
- ECOG Performance Status > 2
- Total bilirubin > 1.5 x the upper limit of the normal range or ALT and AST > 3 x ULN
- NYHA Class III and IV heart failure (refer to Issues for Consideration; those at risk for cardiac failure and ischemia were also excluded from clinical trials)
- LVEF < 40%
- Uncontrolled hypertension
- Grade 3 or 4 peripheral neuropathy
- Ongoing or active systemic infection, including active hepatitis B or C, or known HIV
Issues for Consideration
- A significant percentage of patients on carfilzomib develop dyspnea. This drug should be used with caution in patients with underlying lung disease. Close monitoring for worsening of dyspnea is advised.
- Risk of cardiac failure increases in those aged > 75 years; those with NYHA Class III and IV heart failure, recent MI, conduction abnormalities, angina or arrhythmias uncontrolled by medications, were not eligible for clinical trials and may be at greater risk of cardiac complications. Refer to Prescribing Information for management recommendations.
- Those aged > 75 years experienced greater toxicity than their younger counterparts.
- Patients on dialysis: administer carfilzomib after the dialysis procedure.
- Phase III evidence in heavily pretreated relapsed/refractory patients (median 5 prior regimens) of carfilzomib vs low-dose steroids ± cyclophosphamide indicates that the median overall survival is not significantly different between these treatment arms.
IXAZOMIB (NILARO) Criteria for Use, December 2016
Exclusion Criteria: If the answer to ANY item below is met, then the patient should NOT receive ixazomib
- Care for the oncologic disease being treated not provided by a VA or VA purchased care (eg, Choice Program, Fee Basis) oncology provider
- Patient is not a candidate for lenalidomide or dexamethasone therapy
- Patient is refractory to lenalidomide or proteasomeinhibitor therapy (defined as disease progression while on treatment or within 60 days of last dose)
- Absolute neutrophil count (ANC) < 1,000/mm3
- Platelet count < 75,000/mm3
- ECOG Performance Status > 2
- Patient with CNS involvement
- Patient receiving concurrent therapy with a strong CYP3A inducer (ie, rifampin, phenytoin, carbamazepine) that cannot be discontinued
- Uncontrolled cardiovascular conditions, including uncontrolled hypertension, uncontrolled cardiac arrhythmias, symptomatic congestive heart failure, unstable angina or myocardial infarction within 6 months prior to start
- Ongoing or active systemic infection, including active hepatitis B, hepatitis C, or known HIV
Issues for Consideration
- Indirect comparisons of phase 3 data (KRd vs Rd and IRd vs Rd) show that in similar populations of pretreated relapsed, refractory myeloma patients, those receiving KRd experienced longer PFS (26.3 vs 21 months), greater CR (32% vs 12%), greater ORR (87% vs 78%) and longer duration of response (28.6 vs 20.5 months). Therefore, providers may want to consider using carfilzomib in those meeting its criteria for use.
- Ixazomib is cytotoxic. Capsules should not be opened or crushed. Waste should be considered hazardous.
- Avoid concomitant use of strong CYP3A inducers (rifampin, phenytoin, carbamazepine, and St. John’s Wort).
Click here to read the digital edition.
VA Pharmacy Benefit Management Service (PBM) continually issues or revises its guidances for hematology and oncology care providers on a number of cancer care medications. Below are excerpts from recently released Criteria for Use documents. The complete documents, including the inclusion criteria, dosage and administration guidance, monitoring information, and discontinuation criteria should be consulted and can be found at www.pbm.va.gov or vaww.cmopnational.va.gov/cmop/PBM/default.aspx.
ENZALUTAMIDE (XTANDI) Criteria for Use, January 2017
Exclusion Criteria: If the answer to ANY item below is met, then the patient should NOT receive enzalutamide.
- Brain metastases or active epidural disease
- Severe renal impairment (creatinine clearance < 30 mL/min)
- History of seizure (including febrile seizure, loss of consciousness, or transient ischemic attack within the previous 12 months, any condition predisposing to seizure: prior stroke, brain AV malformation, head trauma with loss of consciousness requiring hospitalization)
- ECOG Performance Status > 2
- Inability to swallow capsules
Issues for Consideration
- Enzalutamide is not indicated for use in women. Based on the mechanism of action, can cause fetal harm if used during pregnancy. Pregnancy Category X—use contraindicated during pregnancy. Exclude pregnancy before prescribing enzalutamide, discuss risks if pregnancy occurs, and provide contraceptive counseling.
- Use in patients taking concomitant medications that may lower the seizure threshold was not studied; caution patients about the risk of activities where the sudden loss of consciousness could cause serious harm if concomitant use cannot be avoided.
- Use in patients at risk for or with a strong history of falls: in the phase 3 clinical trial, falls or injuries from falls occurred in 4.6% of enzalutamide patients vs 1.3% of placebo patients.
- Avoid strong inhibitors of CYP2C8 (eg, gemfibrozil); if concomitant use of a strong CYP2C8 inhibitor cannot be avoided, reduce the dose of enzalutamide to 80 mg once daily according to the package insert.
- Co-administration with strong or moderate inducers of CYP3A4 (eg, carbamazepine, phenobarbital, phenytoin, rifampin, bosentan, efavirenz, modafinil, nafcillin, St. John’s Wort) or CYP2C8 (eg, rifampin) should be avoided if possible. If patient must be co-administered a strong CYP3A4 inducer, increase enzalutamide dose from 160 mg to 240 mg once daily.
- Drugs that are substrates of CYP3A4 (eg, alfentanil, cyclosporine, ergotamine, fentanyl, pimozide, quinidine, sirolimus, tacrolimus), CYP2C9 (eg, phenytoin, warfarin), or CYP2C19 (eg, S-mephenytoin) with a narrow therapeutic index should be avoided. If enzalutamide is co-administered with warfarin, additional INR testing should be conducted.
- Use in patients with hepatic impairment: Pharmacokinetics of enzalutamide and its metabolite were examined in volunteers with normal, Child-Pugh Class A, Child-Pugh Class B, and Child-Pugh Class C hepatic impairment. The composite AUC for enzalutamide and its metabolite after a single 160-mg dose was similar across all levels of hepatic impairment compared with normal volunteers.
- There have been postmarketing reports of posterior reversible encephalopathy syndrome (PRES) in patients receiving enzalutamide. PRES is a neurologic disorder presenting with rapidly evolving symptoms including seizure, headache, lethargy, confusion, blindness, and other visual/neurological disturbances with or without associated hypertension. Diagnosis of PRES requires brain imaging, preferably by MRI. Enzalutamide should be discontinued in patients developing PRES.
- Sequencing of enzalutamide and abiraterone has been evaluated in several small retrospective analyses; the majority of the analyses are in the post chemotherapy setting. From this limited observational data, it is unclear if there is a preferred sequencing of abiraterone and enzalutamide. There is some evidence for cross-resistance. There are ongoing investigations into mechanisms of resistance to enzalutamide and abiraterone.
DARATUMUMAB (DARZALEX) Criteria for Use, January 2017
Exclusion Criteria: If the answer to ANY item below is met, then the patient should NOT receive daratumumab.
- Patient is noncompliant with medication, follow-up, or laboratory appointments
- Patient unable or unwilling to be observed an extended period of time that may be necessary for first infusion (refer to Issues for Consideration)
- Hemoglobin < 8 g/dL; Must transfuse to hemoglobin above 8 gm/dL prior to therapy initiation
- Absolute neutrophil count (ANC) < 1,000/mm3
- Platelet count < 50,000/mm3 (< 30,000/mm3 if myeloma involvement in bone marrow > 50%)
- ECOG Performance Status > 2
- Total bilirubin > 3x the upper limit of the normal range (except for Gilbert syndrome: direct bilirubin 2x ULN) or ALT and AST > 3x ULN
- NYHA Class III or IV heart failure (refer to Issues for Consideration)
- Ongoing or active systemic infection, including active hepatitis B or C, or known HIV (refer to Issues for Consideration)
- Positive pregnancy test
Issues for Consideration
- Drug infusion time will be dependent upon patient tolerance and exposure to daratumumab. Median duration of the first infusion was ~ 7 hours in the SIRIUS trial, followed by infusion times of 4.2 and 3.4 hours, subsequently.
- Type and screen patients shortly prior to starting treatment. When the sample is provided to the blood bank, inform them that the patient will be receiving daratumumab.
- Those with NYHA Class III or IV heart failure, recent MI, conduction abnormalities, angina or arrhythmias uncontrolled by medications, were not eligible for clinical trials and may be at greater risk of cardiac complications.
- Patients with active hepatitis B, C, or HIV were excluded from clinical trials with daratumumab, therefore safety and efficacy data are unknown in these patient populations. The risk of infections was slightly higher in the daratumumab-treated arms of the comparative studies. Use of daratumumab should only be considered in those with well-controlled hepatitis B, hepatitis C, or HIV.
ELOTUZUMAB (EMPLICITI) Criteria for Use, January 2017
Exclusion Criteria: If the answer to ANY item below is met, then the patient should NOT receive elotuzumab.
- Patient is noncompliant with medication, follow-up, or laboratory appointments
- Patient is not a candidate for lenalidomide therapy (ie, is lenalidomide-refractory or possesses contraindications to therapy)
- Patient is not a candidate for high-dose dexamethasone therapy
- Hemoglobin < 8 g/dL; Must transfuse to hemoglobin above 8 gm/dL prior to therapy initiation
- Absolute neutrophil count (ANC) < 1,000/mm3
- Platelet count < 50,000/mm3 (< 30,000/mm3 if myeloma involvement in bone marrow > 50%)
- ECOG Performance Status > 2
- Total bilirubin > 2x the upper limit of the normal range (except for Gilbert syndrome: direct bilirubin > 2 mg/dL) or ALT and AST > 3x ULN
- NYHA Class III or IV heart failure (refer to Issues for Consideration)
- Ongoing or active systemic infection, including active hepatitis B or hepatitis C, or known HIV (refer to Issues for Consideration)
- Positive pregnancy test
- Patient intends to breastfeed during therapy
Issues for Consideration
- Those with NYHA Class III or IV heart failure, recent MI, conduction abnormalities, angina or arrhythmias uncontrolled by medications, were not eligible for clinical trials and may be at greater risk of cardiac complications.
- Patients with active hepatitis B, hepatitis C, or HIV were excluded from clinical trials with elotuzumab, therefore safety and efficacy data are unknown in these patient populations. The risk of infections (OI, fungal, viral) was greater in the elotuzumab arm vs control arm of the comparative clinical trial. Use of elotuzumab should only be considered in those with well-controlled hepatitis B, hepatitis C, or HIV.
- Disappointing response rates as monotherapy in the relapsed/refractory setting suggest that elotuzumab should be given in combination with lenalidomide and dexamethasone.
- Impact of elotuzumab/lenalidomide/dexamethasone on overall survival is not known as these data were not mature at the time ELOQUENT-2 was published.
CARFILZOMIB (KYPROLIS) Criteria for Use, December 2016
Exclusion Criteria: If the answer to ANY item below is met, then the patient should NOT receive carfilzomib.
- Care for the oncologic disease being treated not provided by a VA or VA purchased care (eg, Choice Program, Fee Basis) oncology provider
- Patient is noncompliant with medication, follow-up, or laboratory appointments
- Hemoglobin < 8 g/dL; Must transfuse to hemoglobin above 8 g/dL prior to therapy initiation
- Absolute neutrophil count (ANC) < 1,000/mm3
- Platelet count < 50,000/mm3 (< 30,000/mm3 if myeloma involvement in bone marrow > 50%)
- ECOG Performance Status > 2
- Total bilirubin > 1.5 x the upper limit of the normal range or ALT and AST > 3 x ULN
- NYHA Class III and IV heart failure (refer to Issues for Consideration; those at risk for cardiac failure and ischemia were also excluded from clinical trials)
- LVEF < 40%
- Uncontrolled hypertension
- Grade 3 or 4 peripheral neuropathy
- Ongoing or active systemic infection, including active hepatitis B or C, or known HIV
Issues for Consideration
- A significant percentage of patients on carfilzomib develop dyspnea. This drug should be used with caution in patients with underlying lung disease. Close monitoring for worsening of dyspnea is advised.
- Risk of cardiac failure increases in those aged > 75 years; those with NYHA Class III and IV heart failure, recent MI, conduction abnormalities, angina or arrhythmias uncontrolled by medications, were not eligible for clinical trials and may be at greater risk of cardiac complications. Refer to Prescribing Information for management recommendations.
- Those aged > 75 years experienced greater toxicity than their younger counterparts.
- Patients on dialysis: administer carfilzomib after the dialysis procedure.
- Phase III evidence in heavily pretreated relapsed/refractory patients (median 5 prior regimens) of carfilzomib vs low-dose steroids ± cyclophosphamide indicates that the median overall survival is not significantly different between these treatment arms.
IXAZOMIB (NILARO) Criteria for Use, December 2016
Exclusion Criteria: If the answer to ANY item below is met, then the patient should NOT receive ixazomib
- Care for the oncologic disease being treated not provided by a VA or VA purchased care (eg, Choice Program, Fee Basis) oncology provider
- Patient is not a candidate for lenalidomide or dexamethasone therapy
- Patient is refractory to lenalidomide or proteasomeinhibitor therapy (defined as disease progression while on treatment or within 60 days of last dose)
- Absolute neutrophil count (ANC) < 1,000/mm3
- Platelet count < 75,000/mm3
- ECOG Performance Status > 2
- Patient with CNS involvement
- Patient receiving concurrent therapy with a strong CYP3A inducer (ie, rifampin, phenytoin, carbamazepine) that cannot be discontinued
- Uncontrolled cardiovascular conditions, including uncontrolled hypertension, uncontrolled cardiac arrhythmias, symptomatic congestive heart failure, unstable angina or myocardial infarction within 6 months prior to start
- Ongoing or active systemic infection, including active hepatitis B, hepatitis C, or known HIV
Issues for Consideration
- Indirect comparisons of phase 3 data (KRd vs Rd and IRd vs Rd) show that in similar populations of pretreated relapsed, refractory myeloma patients, those receiving KRd experienced longer PFS (26.3 vs 21 months), greater CR (32% vs 12%), greater ORR (87% vs 78%) and longer duration of response (28.6 vs 20.5 months). Therefore, providers may want to consider using carfilzomib in those meeting its criteria for use.
- Ixazomib is cytotoxic. Capsules should not be opened or crushed. Waste should be considered hazardous.
- Avoid concomitant use of strong CYP3A inducers (rifampin, phenytoin, carbamazepine, and St. John’s Wort).
Click here to read the digital edition.
VA Pharmacy Benefit Management Service (PBM) continually issues or revises its guidances for hematology and oncology care providers on a number of cancer care medications. Below are excerpts from recently released Criteria for Use documents. The complete documents, including the inclusion criteria, dosage and administration guidance, monitoring information, and discontinuation criteria should be consulted and can be found at www.pbm.va.gov or vaww.cmopnational.va.gov/cmop/PBM/default.aspx.
ENZALUTAMIDE (XTANDI) Criteria for Use, January 2017
Exclusion Criteria: If the answer to ANY item below is met, then the patient should NOT receive enzalutamide.
- Brain metastases or active epidural disease
- Severe renal impairment (creatinine clearance < 30 mL/min)
- History of seizure (including febrile seizure, loss of consciousness, or transient ischemic attack within the previous 12 months, any condition predisposing to seizure: prior stroke, brain AV malformation, head trauma with loss of consciousness requiring hospitalization)
- ECOG Performance Status > 2
- Inability to swallow capsules
Issues for Consideration
- Enzalutamide is not indicated for use in women. Based on the mechanism of action, can cause fetal harm if used during pregnancy. Pregnancy Category X—use contraindicated during pregnancy. Exclude pregnancy before prescribing enzalutamide, discuss risks if pregnancy occurs, and provide contraceptive counseling.
- Use in patients taking concomitant medications that may lower the seizure threshold was not studied; caution patients about the risk of activities where the sudden loss of consciousness could cause serious harm if concomitant use cannot be avoided.
- Use in patients at risk for or with a strong history of falls: in the phase 3 clinical trial, falls or injuries from falls occurred in 4.6% of enzalutamide patients vs 1.3% of placebo patients.
- Avoid strong inhibitors of CYP2C8 (eg, gemfibrozil); if concomitant use of a strong CYP2C8 inhibitor cannot be avoided, reduce the dose of enzalutamide to 80 mg once daily according to the package insert.
- Co-administration with strong or moderate inducers of CYP3A4 (eg, carbamazepine, phenobarbital, phenytoin, rifampin, bosentan, efavirenz, modafinil, nafcillin, St. John’s Wort) or CYP2C8 (eg, rifampin) should be avoided if possible. If patient must be co-administered a strong CYP3A4 inducer, increase enzalutamide dose from 160 mg to 240 mg once daily.
- Drugs that are substrates of CYP3A4 (eg, alfentanil, cyclosporine, ergotamine, fentanyl, pimozide, quinidine, sirolimus, tacrolimus), CYP2C9 (eg, phenytoin, warfarin), or CYP2C19 (eg, S-mephenytoin) with a narrow therapeutic index should be avoided. If enzalutamide is co-administered with warfarin, additional INR testing should be conducted.
- Use in patients with hepatic impairment: Pharmacokinetics of enzalutamide and its metabolite were examined in volunteers with normal, Child-Pugh Class A, Child-Pugh Class B, and Child-Pugh Class C hepatic impairment. The composite AUC for enzalutamide and its metabolite after a single 160-mg dose was similar across all levels of hepatic impairment compared with normal volunteers.
- There have been postmarketing reports of posterior reversible encephalopathy syndrome (PRES) in patients receiving enzalutamide. PRES is a neurologic disorder presenting with rapidly evolving symptoms including seizure, headache, lethargy, confusion, blindness, and other visual/neurological disturbances with or without associated hypertension. Diagnosis of PRES requires brain imaging, preferably by MRI. Enzalutamide should be discontinued in patients developing PRES.
- Sequencing of enzalutamide and abiraterone has been evaluated in several small retrospective analyses; the majority of the analyses are in the post chemotherapy setting. From this limited observational data, it is unclear if there is a preferred sequencing of abiraterone and enzalutamide. There is some evidence for cross-resistance. There are ongoing investigations into mechanisms of resistance to enzalutamide and abiraterone.
DARATUMUMAB (DARZALEX) Criteria for Use, January 2017
Exclusion Criteria: If the answer to ANY item below is met, then the patient should NOT receive daratumumab.
- Patient is noncompliant with medication, follow-up, or laboratory appointments
- Patient unable or unwilling to be observed an extended period of time that may be necessary for first infusion (refer to Issues for Consideration)
- Hemoglobin < 8 g/dL; Must transfuse to hemoglobin above 8 gm/dL prior to therapy initiation
- Absolute neutrophil count (ANC) < 1,000/mm3
- Platelet count < 50,000/mm3 (< 30,000/mm3 if myeloma involvement in bone marrow > 50%)
- ECOG Performance Status > 2
- Total bilirubin > 3x the upper limit of the normal range (except for Gilbert syndrome: direct bilirubin 2x ULN) or ALT and AST > 3x ULN
- NYHA Class III or IV heart failure (refer to Issues for Consideration)
- Ongoing or active systemic infection, including active hepatitis B or C, or known HIV (refer to Issues for Consideration)
- Positive pregnancy test
Issues for Consideration
- Drug infusion time will be dependent upon patient tolerance and exposure to daratumumab. Median duration of the first infusion was ~ 7 hours in the SIRIUS trial, followed by infusion times of 4.2 and 3.4 hours, subsequently.
- Type and screen patients shortly prior to starting treatment. When the sample is provided to the blood bank, inform them that the patient will be receiving daratumumab.
- Those with NYHA Class III or IV heart failure, recent MI, conduction abnormalities, angina or arrhythmias uncontrolled by medications, were not eligible for clinical trials and may be at greater risk of cardiac complications.
- Patients with active hepatitis B, C, or HIV were excluded from clinical trials with daratumumab, therefore safety and efficacy data are unknown in these patient populations. The risk of infections was slightly higher in the daratumumab-treated arms of the comparative studies. Use of daratumumab should only be considered in those with well-controlled hepatitis B, hepatitis C, or HIV.
ELOTUZUMAB (EMPLICITI) Criteria for Use, January 2017
Exclusion Criteria: If the answer to ANY item below is met, then the patient should NOT receive elotuzumab.
- Patient is noncompliant with medication, follow-up, or laboratory appointments
- Patient is not a candidate for lenalidomide therapy (ie, is lenalidomide-refractory or possesses contraindications to therapy)
- Patient is not a candidate for high-dose dexamethasone therapy
- Hemoglobin < 8 g/dL; Must transfuse to hemoglobin above 8 gm/dL prior to therapy initiation
- Absolute neutrophil count (ANC) < 1,000/mm3
- Platelet count < 50,000/mm3 (< 30,000/mm3 if myeloma involvement in bone marrow > 50%)
- ECOG Performance Status > 2
- Total bilirubin > 2x the upper limit of the normal range (except for Gilbert syndrome: direct bilirubin > 2 mg/dL) or ALT and AST > 3x ULN
- NYHA Class III or IV heart failure (refer to Issues for Consideration)
- Ongoing or active systemic infection, including active hepatitis B or hepatitis C, or known HIV (refer to Issues for Consideration)
- Positive pregnancy test
- Patient intends to breastfeed during therapy
Issues for Consideration
- Those with NYHA Class III or IV heart failure, recent MI, conduction abnormalities, angina or arrhythmias uncontrolled by medications, were not eligible for clinical trials and may be at greater risk of cardiac complications.
- Patients with active hepatitis B, hepatitis C, or HIV were excluded from clinical trials with elotuzumab, therefore safety and efficacy data are unknown in these patient populations. The risk of infections (OI, fungal, viral) was greater in the elotuzumab arm vs control arm of the comparative clinical trial. Use of elotuzumab should only be considered in those with well-controlled hepatitis B, hepatitis C, or HIV.
- Disappointing response rates as monotherapy in the relapsed/refractory setting suggest that elotuzumab should be given in combination with lenalidomide and dexamethasone.
- Impact of elotuzumab/lenalidomide/dexamethasone on overall survival is not known as these data were not mature at the time ELOQUENT-2 was published.
CARFILZOMIB (KYPROLIS) Criteria for Use, December 2016
Exclusion Criteria: If the answer to ANY item below is met, then the patient should NOT receive carfilzomib.
- Care for the oncologic disease being treated not provided by a VA or VA purchased care (eg, Choice Program, Fee Basis) oncology provider
- Patient is noncompliant with medication, follow-up, or laboratory appointments
- Hemoglobin < 8 g/dL; Must transfuse to hemoglobin above 8 g/dL prior to therapy initiation
- Absolute neutrophil count (ANC) < 1,000/mm3
- Platelet count < 50,000/mm3 (< 30,000/mm3 if myeloma involvement in bone marrow > 50%)
- ECOG Performance Status > 2
- Total bilirubin > 1.5 x the upper limit of the normal range or ALT and AST > 3 x ULN
- NYHA Class III and IV heart failure (refer to Issues for Consideration; those at risk for cardiac failure and ischemia were also excluded from clinical trials)
- LVEF < 40%
- Uncontrolled hypertension
- Grade 3 or 4 peripheral neuropathy
- Ongoing or active systemic infection, including active hepatitis B or C, or known HIV
Issues for Consideration
- A significant percentage of patients on carfilzomib develop dyspnea. This drug should be used with caution in patients with underlying lung disease. Close monitoring for worsening of dyspnea is advised.
- Risk of cardiac failure increases in those aged > 75 years; those with NYHA Class III and IV heart failure, recent MI, conduction abnormalities, angina or arrhythmias uncontrolled by medications, were not eligible for clinical trials and may be at greater risk of cardiac complications. Refer to Prescribing Information for management recommendations.
- Those aged > 75 years experienced greater toxicity than their younger counterparts.
- Patients on dialysis: administer carfilzomib after the dialysis procedure.
- Phase III evidence in heavily pretreated relapsed/refractory patients (median 5 prior regimens) of carfilzomib vs low-dose steroids ± cyclophosphamide indicates that the median overall survival is not significantly different between these treatment arms.
IXAZOMIB (NILARO) Criteria for Use, December 2016
Exclusion Criteria: If the answer to ANY item below is met, then the patient should NOT receive ixazomib
- Care for the oncologic disease being treated not provided by a VA or VA purchased care (eg, Choice Program, Fee Basis) oncology provider
- Patient is not a candidate for lenalidomide or dexamethasone therapy
- Patient is refractory to lenalidomide or proteasomeinhibitor therapy (defined as disease progression while on treatment or within 60 days of last dose)
- Absolute neutrophil count (ANC) < 1,000/mm3
- Platelet count < 75,000/mm3
- ECOG Performance Status > 2
- Patient with CNS involvement
- Patient receiving concurrent therapy with a strong CYP3A inducer (ie, rifampin, phenytoin, carbamazepine) that cannot be discontinued
- Uncontrolled cardiovascular conditions, including uncontrolled hypertension, uncontrolled cardiac arrhythmias, symptomatic congestive heart failure, unstable angina or myocardial infarction within 6 months prior to start
- Ongoing or active systemic infection, including active hepatitis B, hepatitis C, or known HIV
Issues for Consideration
- Indirect comparisons of phase 3 data (KRd vs Rd and IRd vs Rd) show that in similar populations of pretreated relapsed, refractory myeloma patients, those receiving KRd experienced longer PFS (26.3 vs 21 months), greater CR (32% vs 12%), greater ORR (87% vs 78%) and longer duration of response (28.6 vs 20.5 months). Therefore, providers may want to consider using carfilzomib in those meeting its criteria for use.
- Ixazomib is cytotoxic. Capsules should not be opened or crushed. Waste should be considered hazardous.
- Avoid concomitant use of strong CYP3A inducers (rifampin, phenytoin, carbamazepine, and St. John’s Wort).