User login
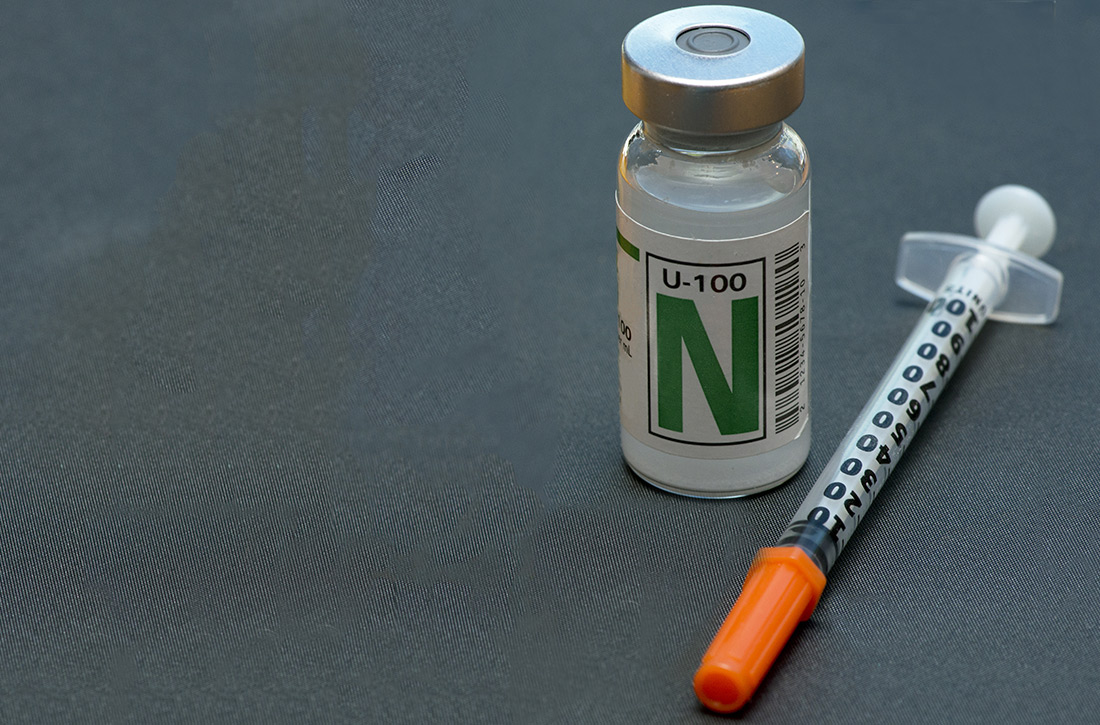
NPH insulin: It remains a good option
ILLUSTRATIVE CASE
Blanche is a 54-year-old overweight woman who has had type 2 diabetes mellitus (T2DM) for 5 years. She has been optimized on both metformin (1000 mg bid) and exenatide (2 mg weekly). While taking these medications, her hemoglobin A1C (HbA1C) has dropped from 11.2 to 8.4, and her body mass index (BMI) has declined from 35 to 31. However, she is still not at goal. You decide to start her on long-acting basal insulin. She has limited income, and she currently spends $75/month for her metformin, exenatide, atorvastatin, and lisinopril. What insulin do you prescribe?
The Centers for Disease Control and Prevention (CDC) reported that the prevalence of diabetes in the United States was 9.4% (30.3 million people) in 2015.2 Among those affected, approximately 95.8% had T2DM.2 The same report estimated that 1.5 million new cases of diabetes (6.7 per 1000 persons) were diagnosed annually among US adults ≥ 18 years of age, and that about $7900 of annual medical expenses for patients diagnosed with diabetes was directly attributable to diabetes.2
In the United States, neutral protamine Hagedorn (NPH) insulin was the most commonly used intermediate- to long-acting insulin until the introduction of the long-acting insulin analogs (insulin glargine in 2000 and insulin detemir in 2005).3 Despite being considerably more expensive than NPH insulin, long-acting insulin analogs had captured more than 80% of the total long-acting insulin market by 2010.4 The market share for NPH insulin dropped from 81.9% in 2001 to 16.2% in 2010.4
While the newer insulin analogs are significantly more expensive than NPH insulin, with higher corresponding out-of-pocket costs to patients, researchers have had a difficult time demonstrating greater effectiveness or any definitive differences in any long-term outcomes between NPH and the insulin analogs. A 2007 Cochrane review comparing NPH insulin to both glargine and detemir showed little difference in metabolic control (as measured by HbA1C) or in the rate of severe hypoglycemia. However, the rates of symptomatic, overall, and nocturnal hypoglycemia were statistically lower with the insulin analogs.5
A 2015 retrospective observational study from the Veterans Health Administration (N = 142,940) covering a 10-year period from 2000 to 2010 found no consistent differences in long-term health outcomes when comparing the use of long-acting insulin analogs to that of NPH insulin.3,6
STUDY SUMMARY
Study compares performance of basal insulin analogs to that of NPH
This retrospective, observational study included 25,489 adult patients with T2DM who were enrolled in Kaiser Permanente of Northern California, had full medical and prescription coverage, and initiated basal insulin therapy with either NPH or an insulin analog between 2006 and 2015.
The primary outcome was the time from basal insulin therapy initiation to a hypoglycemia-related emergency department (ED) visit or hospital admission. The secondary outcome was the change in HbA1C level within 1 year of initiation of basal insulin therapy.
Continue to: Per 1000 person-years...
Per 1000 person-years, there was no significant difference in hypoglycemia-related ED visits or hospital admissions between the analog and NPH groups (11.9 events vs 8.8 events, respectively; between-group difference, 3.1 events; 95% confidence interval [CI], –1.5 to 7.7). HbA1C reduction was statistically greater with NPH, but most likely not clinically significant between insulin analogs and NPH (1.26 vs 1.48 percentage points; between group difference, –0.22%; 95% CI, –0.09% to –0.37%).
WHAT’S NEW?
No clinically relevant differences between insulin analogs and NPH
This study revealed that there is no clinically relevant difference in HbA1C levels and no difference in patient-focused outcomes of hypoglycemia-related ED visits or hospital admissions between NPH insulin and the more expensive insulin analogs. This makes a strong case for a different approach to initial basal insulin therapy for patients with T2DM who need insulin for glucose control.
CAVEATS
Demographics and less severe hypoglycemia might be at issue
This retrospective, observational study has broad demographics (but moderate under-representation of African-Americans), minimal patient health care disparities, and good access to medications. But generalizability outside of an integrated health delivery system may be limited. The study design also is subject to confounding, as not all potential impacts on the results can be corrected for or controlled in an observational study. Also, less profound hypoglycemia that did not require an ED visit or hospital admission was not captured.
CHALLENGES TO IMPLEMENTATION
Convenience and marketing factors may hinder change
Insulin analogs may have a number of convenience and marketing factors that may make it hard for providers and systems to change and use more NPH. However, the easy-to-use insulin analog pens are matched in availability and convenience by the much less advertised NPH insulin pens produced by at least 3 major pharmaceutical companies. In addition, while the overall cost for the insulin analogs continues to be 2 to 3 times that of non-human NPH insulin, insurance often covers up to, or more than, 80% of the cost of the insulin analogs, making the difference in the patient’s copay between the 2 not as severe. For example, patients may pay $30 to $40 per month for insulin analogs vs $10 to $25 per month for cheaper versions of NPH.7,8
ACKNOWLEDGMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Lipska KJ, Parker MM, Moffet HH, et al. Association of initiation of basal insulin analogs vs neutral protamine Hagedorn insulin with hypoglycemia-related emergency department visits or hospital admissions and with glycemic control in patients with type 2 diabetes. JAMA. 2018;320:53-62.
2. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, U.S. Dept of Health and Human Services; 2017. www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed January 15, 2020.
3. Prentice JC, Conlin PR, Gellad WF, et al. Long-term outcomes of analogue insulin compared with NPH for patients with type 2 diabetes mellitus. Am J Manag Care. 2015;21:e235-e243.
4. Turner LW, Nartey D, Stafford RS, et al. Ambulatory treatment of type 2 diabetes in the U.S., 1997-2012. Diabetes Care. 2014;37:985-992.
5. Horvath K, Jeitler K, Berghold A, et al. Long-acting insulin analogues versus NPH insulin (human isophane insulin) for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2007;(2):CD005613.
6. Chamberlain JJ, Herman WH, Leal S, et al. Pharmacologic therapy for type 2 diabetes: synopsis of the 2017 American Diabetes Association standards of medical care in diabetes. Ann Intern Med. 2017;166:572-578.
7. GoodRx.com. Insulins. www.goodrx.com/insulins. Accessed January 20, 2020. 8. Cefalu WT, Dawes DE, Gavlak G, et al. Insulin access and affordability working group: conclusions and recommendations. Diabetes Care. 2018;41:1299-1311.
ILLUSTRATIVE CASE
Blanche is a 54-year-old overweight woman who has had type 2 diabetes mellitus (T2DM) for 5 years. She has been optimized on both metformin (1000 mg bid) and exenatide (2 mg weekly). While taking these medications, her hemoglobin A1C (HbA1C) has dropped from 11.2 to 8.4, and her body mass index (BMI) has declined from 35 to 31. However, she is still not at goal. You decide to start her on long-acting basal insulin. She has limited income, and she currently spends $75/month for her metformin, exenatide, atorvastatin, and lisinopril. What insulin do you prescribe?
The Centers for Disease Control and Prevention (CDC) reported that the prevalence of diabetes in the United States was 9.4% (30.3 million people) in 2015.2 Among those affected, approximately 95.8% had T2DM.2 The same report estimated that 1.5 million new cases of diabetes (6.7 per 1000 persons) were diagnosed annually among US adults ≥ 18 years of age, and that about $7900 of annual medical expenses for patients diagnosed with diabetes was directly attributable to diabetes.2
In the United States, neutral protamine Hagedorn (NPH) insulin was the most commonly used intermediate- to long-acting insulin until the introduction of the long-acting insulin analogs (insulin glargine in 2000 and insulin detemir in 2005).3 Despite being considerably more expensive than NPH insulin, long-acting insulin analogs had captured more than 80% of the total long-acting insulin market by 2010.4 The market share for NPH insulin dropped from 81.9% in 2001 to 16.2% in 2010.4
While the newer insulin analogs are significantly more expensive than NPH insulin, with higher corresponding out-of-pocket costs to patients, researchers have had a difficult time demonstrating greater effectiveness or any definitive differences in any long-term outcomes between NPH and the insulin analogs. A 2007 Cochrane review comparing NPH insulin to both glargine and detemir showed little difference in metabolic control (as measured by HbA1C) or in the rate of severe hypoglycemia. However, the rates of symptomatic, overall, and nocturnal hypoglycemia were statistically lower with the insulin analogs.5
A 2015 retrospective observational study from the Veterans Health Administration (N = 142,940) covering a 10-year period from 2000 to 2010 found no consistent differences in long-term health outcomes when comparing the use of long-acting insulin analogs to that of NPH insulin.3,6
STUDY SUMMARY
Study compares performance of basal insulin analogs to that of NPH
This retrospective, observational study included 25,489 adult patients with T2DM who were enrolled in Kaiser Permanente of Northern California, had full medical and prescription coverage, and initiated basal insulin therapy with either NPH or an insulin analog between 2006 and 2015.
The primary outcome was the time from basal insulin therapy initiation to a hypoglycemia-related emergency department (ED) visit or hospital admission. The secondary outcome was the change in HbA1C level within 1 year of initiation of basal insulin therapy.
Continue to: Per 1000 person-years...
Per 1000 person-years, there was no significant difference in hypoglycemia-related ED visits or hospital admissions between the analog and NPH groups (11.9 events vs 8.8 events, respectively; between-group difference, 3.1 events; 95% confidence interval [CI], –1.5 to 7.7). HbA1C reduction was statistically greater with NPH, but most likely not clinically significant between insulin analogs and NPH (1.26 vs 1.48 percentage points; between group difference, –0.22%; 95% CI, –0.09% to –0.37%).
WHAT’S NEW?
No clinically relevant differences between insulin analogs and NPH
This study revealed that there is no clinically relevant difference in HbA1C levels and no difference in patient-focused outcomes of hypoglycemia-related ED visits or hospital admissions between NPH insulin and the more expensive insulin analogs. This makes a strong case for a different approach to initial basal insulin therapy for patients with T2DM who need insulin for glucose control.
CAVEATS
Demographics and less severe hypoglycemia might be at issue
This retrospective, observational study has broad demographics (but moderate under-representation of African-Americans), minimal patient health care disparities, and good access to medications. But generalizability outside of an integrated health delivery system may be limited. The study design also is subject to confounding, as not all potential impacts on the results can be corrected for or controlled in an observational study. Also, less profound hypoglycemia that did not require an ED visit or hospital admission was not captured.
CHALLENGES TO IMPLEMENTATION
Convenience and marketing factors may hinder change
Insulin analogs may have a number of convenience and marketing factors that may make it hard for providers and systems to change and use more NPH. However, the easy-to-use insulin analog pens are matched in availability and convenience by the much less advertised NPH insulin pens produced by at least 3 major pharmaceutical companies. In addition, while the overall cost for the insulin analogs continues to be 2 to 3 times that of non-human NPH insulin, insurance often covers up to, or more than, 80% of the cost of the insulin analogs, making the difference in the patient’s copay between the 2 not as severe. For example, patients may pay $30 to $40 per month for insulin analogs vs $10 to $25 per month for cheaper versions of NPH.7,8
ACKNOWLEDGMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
Blanche is a 54-year-old overweight woman who has had type 2 diabetes mellitus (T2DM) for 5 years. She has been optimized on both metformin (1000 mg bid) and exenatide (2 mg weekly). While taking these medications, her hemoglobin A1C (HbA1C) has dropped from 11.2 to 8.4, and her body mass index (BMI) has declined from 35 to 31. However, she is still not at goal. You decide to start her on long-acting basal insulin. She has limited income, and she currently spends $75/month for her metformin, exenatide, atorvastatin, and lisinopril. What insulin do you prescribe?
The Centers for Disease Control and Prevention (CDC) reported that the prevalence of diabetes in the United States was 9.4% (30.3 million people) in 2015.2 Among those affected, approximately 95.8% had T2DM.2 The same report estimated that 1.5 million new cases of diabetes (6.7 per 1000 persons) were diagnosed annually among US adults ≥ 18 years of age, and that about $7900 of annual medical expenses for patients diagnosed with diabetes was directly attributable to diabetes.2
In the United States, neutral protamine Hagedorn (NPH) insulin was the most commonly used intermediate- to long-acting insulin until the introduction of the long-acting insulin analogs (insulin glargine in 2000 and insulin detemir in 2005).3 Despite being considerably more expensive than NPH insulin, long-acting insulin analogs had captured more than 80% of the total long-acting insulin market by 2010.4 The market share for NPH insulin dropped from 81.9% in 2001 to 16.2% in 2010.4
While the newer insulin analogs are significantly more expensive than NPH insulin, with higher corresponding out-of-pocket costs to patients, researchers have had a difficult time demonstrating greater effectiveness or any definitive differences in any long-term outcomes between NPH and the insulin analogs. A 2007 Cochrane review comparing NPH insulin to both glargine and detemir showed little difference in metabolic control (as measured by HbA1C) or in the rate of severe hypoglycemia. However, the rates of symptomatic, overall, and nocturnal hypoglycemia were statistically lower with the insulin analogs.5
A 2015 retrospective observational study from the Veterans Health Administration (N = 142,940) covering a 10-year period from 2000 to 2010 found no consistent differences in long-term health outcomes when comparing the use of long-acting insulin analogs to that of NPH insulin.3,6
STUDY SUMMARY
Study compares performance of basal insulin analogs to that of NPH
This retrospective, observational study included 25,489 adult patients with T2DM who were enrolled in Kaiser Permanente of Northern California, had full medical and prescription coverage, and initiated basal insulin therapy with either NPH or an insulin analog between 2006 and 2015.
The primary outcome was the time from basal insulin therapy initiation to a hypoglycemia-related emergency department (ED) visit or hospital admission. The secondary outcome was the change in HbA1C level within 1 year of initiation of basal insulin therapy.
Continue to: Per 1000 person-years...
Per 1000 person-years, there was no significant difference in hypoglycemia-related ED visits or hospital admissions between the analog and NPH groups (11.9 events vs 8.8 events, respectively; between-group difference, 3.1 events; 95% confidence interval [CI], –1.5 to 7.7). HbA1C reduction was statistically greater with NPH, but most likely not clinically significant between insulin analogs and NPH (1.26 vs 1.48 percentage points; between group difference, –0.22%; 95% CI, –0.09% to –0.37%).
WHAT’S NEW?
No clinically relevant differences between insulin analogs and NPH
This study revealed that there is no clinically relevant difference in HbA1C levels and no difference in patient-focused outcomes of hypoglycemia-related ED visits or hospital admissions between NPH insulin and the more expensive insulin analogs. This makes a strong case for a different approach to initial basal insulin therapy for patients with T2DM who need insulin for glucose control.
CAVEATS
Demographics and less severe hypoglycemia might be at issue
This retrospective, observational study has broad demographics (but moderate under-representation of African-Americans), minimal patient health care disparities, and good access to medications. But generalizability outside of an integrated health delivery system may be limited. The study design also is subject to confounding, as not all potential impacts on the results can be corrected for or controlled in an observational study. Also, less profound hypoglycemia that did not require an ED visit or hospital admission was not captured.
CHALLENGES TO IMPLEMENTATION
Convenience and marketing factors may hinder change
Insulin analogs may have a number of convenience and marketing factors that may make it hard for providers and systems to change and use more NPH. However, the easy-to-use insulin analog pens are matched in availability and convenience by the much less advertised NPH insulin pens produced by at least 3 major pharmaceutical companies. In addition, while the overall cost for the insulin analogs continues to be 2 to 3 times that of non-human NPH insulin, insurance often covers up to, or more than, 80% of the cost of the insulin analogs, making the difference in the patient’s copay between the 2 not as severe. For example, patients may pay $30 to $40 per month for insulin analogs vs $10 to $25 per month for cheaper versions of NPH.7,8
ACKNOWLEDGMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Lipska KJ, Parker MM, Moffet HH, et al. Association of initiation of basal insulin analogs vs neutral protamine Hagedorn insulin with hypoglycemia-related emergency department visits or hospital admissions and with glycemic control in patients with type 2 diabetes. JAMA. 2018;320:53-62.
2. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, U.S. Dept of Health and Human Services; 2017. www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed January 15, 2020.
3. Prentice JC, Conlin PR, Gellad WF, et al. Long-term outcomes of analogue insulin compared with NPH for patients with type 2 diabetes mellitus. Am J Manag Care. 2015;21:e235-e243.
4. Turner LW, Nartey D, Stafford RS, et al. Ambulatory treatment of type 2 diabetes in the U.S., 1997-2012. Diabetes Care. 2014;37:985-992.
5. Horvath K, Jeitler K, Berghold A, et al. Long-acting insulin analogues versus NPH insulin (human isophane insulin) for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2007;(2):CD005613.
6. Chamberlain JJ, Herman WH, Leal S, et al. Pharmacologic therapy for type 2 diabetes: synopsis of the 2017 American Diabetes Association standards of medical care in diabetes. Ann Intern Med. 2017;166:572-578.
7. GoodRx.com. Insulins. www.goodrx.com/insulins. Accessed January 20, 2020. 8. Cefalu WT, Dawes DE, Gavlak G, et al. Insulin access and affordability working group: conclusions and recommendations. Diabetes Care. 2018;41:1299-1311.
1. Lipska KJ, Parker MM, Moffet HH, et al. Association of initiation of basal insulin analogs vs neutral protamine Hagedorn insulin with hypoglycemia-related emergency department visits or hospital admissions and with glycemic control in patients with type 2 diabetes. JAMA. 2018;320:53-62.
2. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, U.S. Dept of Health and Human Services; 2017. www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed January 15, 2020.
3. Prentice JC, Conlin PR, Gellad WF, et al. Long-term outcomes of analogue insulin compared with NPH for patients with type 2 diabetes mellitus. Am J Manag Care. 2015;21:e235-e243.
4. Turner LW, Nartey D, Stafford RS, et al. Ambulatory treatment of type 2 diabetes in the U.S., 1997-2012. Diabetes Care. 2014;37:985-992.
5. Horvath K, Jeitler K, Berghold A, et al. Long-acting insulin analogues versus NPH insulin (human isophane insulin) for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2007;(2):CD005613.
6. Chamberlain JJ, Herman WH, Leal S, et al. Pharmacologic therapy for type 2 diabetes: synopsis of the 2017 American Diabetes Association standards of medical care in diabetes. Ann Intern Med. 2017;166:572-578.
7. GoodRx.com. Insulins. www.goodrx.com/insulins. Accessed January 20, 2020. 8. Cefalu WT, Dawes DE, Gavlak G, et al. Insulin access and affordability working group: conclusions and recommendations. Diabetes Care. 2018;41:1299-1311.
PRACTICE CHANGER
Consider NPH insulin for patients who require initiation of long-acting insulin therapy because it is as safe as, and more cost-effective than, basal insulin analogs.
STRENGTH OF RECOMMENDATION
B: Based on a single, large, retrospective, observational study.
Lipska KJ, Parker MM, Moffet HH, et al. Association of initiation of basal insulin analogs vs neutral protamine Hagedorn insulin with hypoglycemia-related emergency department visits or hospital admissions and with glycemic control in patients with type 2 diabetes. JAMA. 2018;320:53-62.1
A better approach to preventing active TB?
ILLUSTRATIVE CASE
A 27-year-old daycare worker was tested for tuberculosis (TB) as part of a recent work physical. She presents to your office for follow-up for her positive purified protein derivative (PPD) skin test. You confirm the result with a quantiferon gold test and ensure she does not have active TB. What medication should you prescribe to treat her latent TB infection (LTBI)?
In 2017, there were 9093 cases of new active TB in the United States.2 It’s estimated that one-fourth of the world’s population has latent TB.3 Identifying and treating latent TB infection is vital to achieving TB’s elimination.4,5
Primary care clinicians are at the forefront of screening high-risk populations for TB. Once identified, treating LTBI can be challenging for providers and patients. Treatment guidelines recommend 4 to 9 months of daily isoniazid.5-8 Shorter treatment regimens were recommended previously; they tended to be rigorous, to involve multiple drugs, and to require high adherence rates. As such, they included directly observed therapy, which prevented widespread adoption.
Consequently, the mainstay for treating LTBI has been 9 months of daily isoniazid. However, isoniazid use is limited by hepatoxicity and by suboptimal treatment completion rates. A 2018 retrospective analysis of patients treated for LTBI reported a completion rate of only 49% for 9 months of isoniazid.9 Additionally, a Cochrane review last updated in 2013 suggests that shorter courses of rifampin are similar in efficacy to isoniazid (although with a wide confidence interval [CI]), and likely have higher adherence rates.10
STUDY SUMMARY
Rifampin is as effective as isoniazid with fewer adverse effects
The study by Menzies et al1 was a multisite, 9-country, open-label, randomized controlled trial (RCT) that compared 4 months of daily rifampin to 9 months of daily isoniazid for the treatment of LTBI in adults. Participants were eligible if they had a positive tuberculin skin test or interferon-gamma-release assay, were ≥ 18 years of age, had an increased risk for reactivation of active TB, and if their health care provider had recommended treatment with isoniazid. Exclusion criteria included current pregnancy or plans to become pregnant, exposure to a patient with TB whose isolates were resistant to either trial drug, an allergy to either of the trial drugs, use of a medication with serious potential interactions with the trial drugs, or current active TB.
Method, outcomes, patient characteristics. Patients received either isoniazid 5 mg/kg body weight (maximum dose 300 mg) daily for 9 months or rifampin 10 mg/kg (maximum dose 600 mg) daily for 4 months and were followed for 28 months. Patients in the isoniazid group also received pyridoxine (vitamin B6) if they were at risk for neuropathy. The primary outcome was the rate of active TB. Secondary outcomes included adverse events, medication regimen completion rate, and drug resistance, among others.
A total of 2989 patients were treated with isoniazid; 3023 patients were treated with rifampin. The mean age of the participants was 38.4 years, 41% of the population was male, and 71% of the groups had confirmed active TB in close contacts.
Continue to: Results
Results. Overall, rates of active TB were low with 9 cases in the isoniazid group and 8 in the rifampin group. In the intention-to-treat analysis, the rate difference for confirmed active TB was < 0.01 cases per 100 person-years (95% CI; −0.14 to 0.16). This met the prespecified noninferiority endpoint, but did not show superiority. A total of 79% of patients treated with rifampin vs 63% treated with isoniazid completed their respective medication courses (difference of 15.1 percentage points; 95% CI, 12.7-17.4; P < .001). Compared with patients in the isoniazid group, those taking rifampin had fewer adverse events, leading to discontinuation (5.6% vs 2.8%).
WHAT’S NEW?
First high-quality study to show that less is more
This is the first large, high-quality study to show that a shorter (4 month) rifampin-based regimen is not inferior to a longer (9 months) isoniazid-based regimen for the treatment of LTBI, and that rifampin is associated with improved adherence and fewer adverse events.
CAVEATS
Low rate of active TB infection and potential bias
The current study had lower-than-anticipated rates of active TB infection, which made the study’s conclusions less compelling. This may have been because of a small number of patients with human immunodeficiency virus enrolled in the study and/or that even participants who discontinued treatment received a median of 3 months of partial treatment.
In addition, the study was an open-label RCT, subjecting it to potential bias. However, the diagnosis of active TB and attribution of adverse events were made by an independent, blinded review panel.
CHALLENGES TO IMPLEMENTATION
No challenges to speak of
We see no challenges to implementing this recommendation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Menzies D, Adjobimey M, Ruslami R, et al. Four months of rifampin or nine months of isoniazid for latent tuberculosis in adults. N Engl J Med. 2018;379:440-453.
2. Stewart RJ, Tsang CA, Pratt RH, et al. Tuberculosis — United States, 2017. MMWR Morb Mortal Wkly Rep. 2018;67:317-323.
3. Houben RM, Dodd PJ. The global burden of latent tuberculosis infection: a re-estimation using mathematical modeling. PLoS Med. 2016;13:e1002152.
4. Lönnroth K, Migliori GB, Abubakar I, et al. Towards tuberculosis elimination: an action framework for low-incidence countries. Eur Respir J. 2015;45:928-952.
5. Uplekar M, Weil D, Lonnroth K, et al. WHO’s new end TB strategy. Lancet. 2015;385:1799-1801.
6. Centers for Disease Control and Prevention. Treatment regimens for latent TB infection (LTBI). Last reviewed April 5, 2016. https://www.cdc.gov/tb/topic/treatment/ltbi.htm. Accessed January 15, 2020.
7. World Health Organization. Latent TB infection: updated and consolidated guidelines for programmatic management. 2018. Publication no. WHO/CDS/TB/2018.4. https://www.who.int/tb/publications/2018/latent-tuberculosis-infection/en/. Accessed January 15, 2020.
8. Borisov AS, Bamrah Morris S, Njie GJ, et al. Update of recommendations for use of once-weekly isoniazid-rifapentine regimen to treat latent Mycobacterium tuberculosis infection. MMWR Morb Mortal Wkly Rep. 2018;67:723-726.
9. Macaraig MM, Jalees M, Lam C, et al. Improved treatment completion with shorter treatment regimens for latent tuberculous infection. Int J Tuber Lung Dis. 2018;22:1344-1349. 10. Sharma SK, Sharma A, Kadhiravan T, et al. Rifamycins (rifampicin, rifabutin and rifapentine) compared to isoniazid for preventing tuberculosis in HIV-negative people at risk of active TB. Cochrane Database Syst Rev. 2013;(7):CD007545.
ILLUSTRATIVE CASE
A 27-year-old daycare worker was tested for tuberculosis (TB) as part of a recent work physical. She presents to your office for follow-up for her positive purified protein derivative (PPD) skin test. You confirm the result with a quantiferon gold test and ensure she does not have active TB. What medication should you prescribe to treat her latent TB infection (LTBI)?
In 2017, there were 9093 cases of new active TB in the United States.2 It’s estimated that one-fourth of the world’s population has latent TB.3 Identifying and treating latent TB infection is vital to achieving TB’s elimination.4,5
Primary care clinicians are at the forefront of screening high-risk populations for TB. Once identified, treating LTBI can be challenging for providers and patients. Treatment guidelines recommend 4 to 9 months of daily isoniazid.5-8 Shorter treatment regimens were recommended previously; they tended to be rigorous, to involve multiple drugs, and to require high adherence rates. As such, they included directly observed therapy, which prevented widespread adoption.
Consequently, the mainstay for treating LTBI has been 9 months of daily isoniazid. However, isoniazid use is limited by hepatoxicity and by suboptimal treatment completion rates. A 2018 retrospective analysis of patients treated for LTBI reported a completion rate of only 49% for 9 months of isoniazid.9 Additionally, a Cochrane review last updated in 2013 suggests that shorter courses of rifampin are similar in efficacy to isoniazid (although with a wide confidence interval [CI]), and likely have higher adherence rates.10
STUDY SUMMARY
Rifampin is as effective as isoniazid with fewer adverse effects
The study by Menzies et al1 was a multisite, 9-country, open-label, randomized controlled trial (RCT) that compared 4 months of daily rifampin to 9 months of daily isoniazid for the treatment of LTBI in adults. Participants were eligible if they had a positive tuberculin skin test or interferon-gamma-release assay, were ≥ 18 years of age, had an increased risk for reactivation of active TB, and if their health care provider had recommended treatment with isoniazid. Exclusion criteria included current pregnancy or plans to become pregnant, exposure to a patient with TB whose isolates were resistant to either trial drug, an allergy to either of the trial drugs, use of a medication with serious potential interactions with the trial drugs, or current active TB.
Method, outcomes, patient characteristics. Patients received either isoniazid 5 mg/kg body weight (maximum dose 300 mg) daily for 9 months or rifampin 10 mg/kg (maximum dose 600 mg) daily for 4 months and were followed for 28 months. Patients in the isoniazid group also received pyridoxine (vitamin B6) if they were at risk for neuropathy. The primary outcome was the rate of active TB. Secondary outcomes included adverse events, medication regimen completion rate, and drug resistance, among others.
A total of 2989 patients were treated with isoniazid; 3023 patients were treated with rifampin. The mean age of the participants was 38.4 years, 41% of the population was male, and 71% of the groups had confirmed active TB in close contacts.
Continue to: Results
Results. Overall, rates of active TB were low with 9 cases in the isoniazid group and 8 in the rifampin group. In the intention-to-treat analysis, the rate difference for confirmed active TB was < 0.01 cases per 100 person-years (95% CI; −0.14 to 0.16). This met the prespecified noninferiority endpoint, but did not show superiority. A total of 79% of patients treated with rifampin vs 63% treated with isoniazid completed their respective medication courses (difference of 15.1 percentage points; 95% CI, 12.7-17.4; P < .001). Compared with patients in the isoniazid group, those taking rifampin had fewer adverse events, leading to discontinuation (5.6% vs 2.8%).
WHAT’S NEW?
First high-quality study to show that less is more
This is the first large, high-quality study to show that a shorter (4 month) rifampin-based regimen is not inferior to a longer (9 months) isoniazid-based regimen for the treatment of LTBI, and that rifampin is associated with improved adherence and fewer adverse events.
CAVEATS
Low rate of active TB infection and potential bias
The current study had lower-than-anticipated rates of active TB infection, which made the study’s conclusions less compelling. This may have been because of a small number of patients with human immunodeficiency virus enrolled in the study and/or that even participants who discontinued treatment received a median of 3 months of partial treatment.
In addition, the study was an open-label RCT, subjecting it to potential bias. However, the diagnosis of active TB and attribution of adverse events were made by an independent, blinded review panel.
CHALLENGES TO IMPLEMENTATION
No challenges to speak of
We see no challenges to implementing this recommendation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 27-year-old daycare worker was tested for tuberculosis (TB) as part of a recent work physical. She presents to your office for follow-up for her positive purified protein derivative (PPD) skin test. You confirm the result with a quantiferon gold test and ensure she does not have active TB. What medication should you prescribe to treat her latent TB infection (LTBI)?
In 2017, there were 9093 cases of new active TB in the United States.2 It’s estimated that one-fourth of the world’s population has latent TB.3 Identifying and treating latent TB infection is vital to achieving TB’s elimination.4,5
Primary care clinicians are at the forefront of screening high-risk populations for TB. Once identified, treating LTBI can be challenging for providers and patients. Treatment guidelines recommend 4 to 9 months of daily isoniazid.5-8 Shorter treatment regimens were recommended previously; they tended to be rigorous, to involve multiple drugs, and to require high adherence rates. As such, they included directly observed therapy, which prevented widespread adoption.
Consequently, the mainstay for treating LTBI has been 9 months of daily isoniazid. However, isoniazid use is limited by hepatoxicity and by suboptimal treatment completion rates. A 2018 retrospective analysis of patients treated for LTBI reported a completion rate of only 49% for 9 months of isoniazid.9 Additionally, a Cochrane review last updated in 2013 suggests that shorter courses of rifampin are similar in efficacy to isoniazid (although with a wide confidence interval [CI]), and likely have higher adherence rates.10
STUDY SUMMARY
Rifampin is as effective as isoniazid with fewer adverse effects
The study by Menzies et al1 was a multisite, 9-country, open-label, randomized controlled trial (RCT) that compared 4 months of daily rifampin to 9 months of daily isoniazid for the treatment of LTBI in adults. Participants were eligible if they had a positive tuberculin skin test or interferon-gamma-release assay, were ≥ 18 years of age, had an increased risk for reactivation of active TB, and if their health care provider had recommended treatment with isoniazid. Exclusion criteria included current pregnancy or plans to become pregnant, exposure to a patient with TB whose isolates were resistant to either trial drug, an allergy to either of the trial drugs, use of a medication with serious potential interactions with the trial drugs, or current active TB.
Method, outcomes, patient characteristics. Patients received either isoniazid 5 mg/kg body weight (maximum dose 300 mg) daily for 9 months or rifampin 10 mg/kg (maximum dose 600 mg) daily for 4 months and were followed for 28 months. Patients in the isoniazid group also received pyridoxine (vitamin B6) if they were at risk for neuropathy. The primary outcome was the rate of active TB. Secondary outcomes included adverse events, medication regimen completion rate, and drug resistance, among others.
A total of 2989 patients were treated with isoniazid; 3023 patients were treated with rifampin. The mean age of the participants was 38.4 years, 41% of the population was male, and 71% of the groups had confirmed active TB in close contacts.
Continue to: Results
Results. Overall, rates of active TB were low with 9 cases in the isoniazid group and 8 in the rifampin group. In the intention-to-treat analysis, the rate difference for confirmed active TB was < 0.01 cases per 100 person-years (95% CI; −0.14 to 0.16). This met the prespecified noninferiority endpoint, but did not show superiority. A total of 79% of patients treated with rifampin vs 63% treated with isoniazid completed their respective medication courses (difference of 15.1 percentage points; 95% CI, 12.7-17.4; P < .001). Compared with patients in the isoniazid group, those taking rifampin had fewer adverse events, leading to discontinuation (5.6% vs 2.8%).
WHAT’S NEW?
First high-quality study to show that less is more
This is the first large, high-quality study to show that a shorter (4 month) rifampin-based regimen is not inferior to a longer (9 months) isoniazid-based regimen for the treatment of LTBI, and that rifampin is associated with improved adherence and fewer adverse events.
CAVEATS
Low rate of active TB infection and potential bias
The current study had lower-than-anticipated rates of active TB infection, which made the study’s conclusions less compelling. This may have been because of a small number of patients with human immunodeficiency virus enrolled in the study and/or that even participants who discontinued treatment received a median of 3 months of partial treatment.
In addition, the study was an open-label RCT, subjecting it to potential bias. However, the diagnosis of active TB and attribution of adverse events were made by an independent, blinded review panel.
CHALLENGES TO IMPLEMENTATION
No challenges to speak of
We see no challenges to implementing this recommendation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Menzies D, Adjobimey M, Ruslami R, et al. Four months of rifampin or nine months of isoniazid for latent tuberculosis in adults. N Engl J Med. 2018;379:440-453.
2. Stewart RJ, Tsang CA, Pratt RH, et al. Tuberculosis — United States, 2017. MMWR Morb Mortal Wkly Rep. 2018;67:317-323.
3. Houben RM, Dodd PJ. The global burden of latent tuberculosis infection: a re-estimation using mathematical modeling. PLoS Med. 2016;13:e1002152.
4. Lönnroth K, Migliori GB, Abubakar I, et al. Towards tuberculosis elimination: an action framework for low-incidence countries. Eur Respir J. 2015;45:928-952.
5. Uplekar M, Weil D, Lonnroth K, et al. WHO’s new end TB strategy. Lancet. 2015;385:1799-1801.
6. Centers for Disease Control and Prevention. Treatment regimens for latent TB infection (LTBI). Last reviewed April 5, 2016. https://www.cdc.gov/tb/topic/treatment/ltbi.htm. Accessed January 15, 2020.
7. World Health Organization. Latent TB infection: updated and consolidated guidelines for programmatic management. 2018. Publication no. WHO/CDS/TB/2018.4. https://www.who.int/tb/publications/2018/latent-tuberculosis-infection/en/. Accessed January 15, 2020.
8. Borisov AS, Bamrah Morris S, Njie GJ, et al. Update of recommendations for use of once-weekly isoniazid-rifapentine regimen to treat latent Mycobacterium tuberculosis infection. MMWR Morb Mortal Wkly Rep. 2018;67:723-726.
9. Macaraig MM, Jalees M, Lam C, et al. Improved treatment completion with shorter treatment regimens for latent tuberculous infection. Int J Tuber Lung Dis. 2018;22:1344-1349. 10. Sharma SK, Sharma A, Kadhiravan T, et al. Rifamycins (rifampicin, rifabutin and rifapentine) compared to isoniazid for preventing tuberculosis in HIV-negative people at risk of active TB. Cochrane Database Syst Rev. 2013;(7):CD007545.
1. Menzies D, Adjobimey M, Ruslami R, et al. Four months of rifampin or nine months of isoniazid for latent tuberculosis in adults. N Engl J Med. 2018;379:440-453.
2. Stewart RJ, Tsang CA, Pratt RH, et al. Tuberculosis — United States, 2017. MMWR Morb Mortal Wkly Rep. 2018;67:317-323.
3. Houben RM, Dodd PJ. The global burden of latent tuberculosis infection: a re-estimation using mathematical modeling. PLoS Med. 2016;13:e1002152.
4. Lönnroth K, Migliori GB, Abubakar I, et al. Towards tuberculosis elimination: an action framework for low-incidence countries. Eur Respir J. 2015;45:928-952.
5. Uplekar M, Weil D, Lonnroth K, et al. WHO’s new end TB strategy. Lancet. 2015;385:1799-1801.
6. Centers for Disease Control and Prevention. Treatment regimens for latent TB infection (LTBI). Last reviewed April 5, 2016. https://www.cdc.gov/tb/topic/treatment/ltbi.htm. Accessed January 15, 2020.
7. World Health Organization. Latent TB infection: updated and consolidated guidelines for programmatic management. 2018. Publication no. WHO/CDS/TB/2018.4. https://www.who.int/tb/publications/2018/latent-tuberculosis-infection/en/. Accessed January 15, 2020.
8. Borisov AS, Bamrah Morris S, Njie GJ, et al. Update of recommendations for use of once-weekly isoniazid-rifapentine regimen to treat latent Mycobacterium tuberculosis infection. MMWR Morb Mortal Wkly Rep. 2018;67:723-726.
9. Macaraig MM, Jalees M, Lam C, et al. Improved treatment completion with shorter treatment regimens for latent tuberculous infection. Int J Tuber Lung Dis. 2018;22:1344-1349. 10. Sharma SK, Sharma A, Kadhiravan T, et al. Rifamycins (rifampicin, rifabutin and rifapentine) compared to isoniazid for preventing tuberculosis in HIV-negative people at risk of active TB. Cochrane Database Syst Rev. 2013;(7):CD007545.
PRACTICE CHANGER
Use 4 months of rifampin instead of 9 months of isoniazid to treat adults with latent tuberculosis; rifampin is associated with fewer adverse events and higher completion rates.
STRENGTH OF RECOMMENDATION
A: Based on a randomized controlled trial and a previous Cochrane review.
Menzies D, Adjobimey M, Ruslami R, et al. Four months of rifampin or nine months of isoniazid for latent tuberculosis in adults. N Engl J Med. 2018;379:440-453.
Early pregnancy loss: Pretreat with mifepristone?
ILLUSTRATIVE CASE
Jenny is a 29-year-old G2P1001 woman who presents to your clinic for a missed period. Her last menstrual period was about 10 weeks ago. She is found to have a positive pregnancy test in the office. On examination, her uterus is nontender and consistent in size with gestation of 7 weeks. She denies any bleeding or cramping. On ultrasound, you see a gestational sac measuring 28 mm and no embryo. You confirm early pregnancy loss. Jenny is sad about this diagnosis. She does not wish to proceed with expectant management and is hopeful to avoid a surgical procedure. How do you counsel her regarding medical management?
Early pregnancy loss or first trimester miscarriage is estimated to occur in about 1 million women in the United States annually and is the most common complication of early pregnancy.2,3 Early pregnancy loss is defined as a nonviable, intrauterine pregnancy with either an empty gestational sac or a gestational sac containing an embryo or fetus without fetal heart activity within the first 12 weeks 6 days of gestation.4
Once early pregnancy loss is confirmed by ultrasound, expectant management with no intervention is an acceptable treatment option. Women generally prefer active management, either medically or with surgical evacuation.5,6 Misoprostol 800 mcg administered vaginally or orally has been the accepted medication regimen for medical management.5 However, failure rates with misoprostol have been reported to be as high as 40%, particularly among women with a closed cervical os, who then require repeat dosing of misoprostol or surgical evacuation.6
STUDY SUMMARY
Mifepristone before misoprostol improves efficacy for early pregnancy loss
The PreFaiR (Comparative Effectiveness of Pregnancy Failure Management Regimens) study was a randomized trial that took place at 3 US centers. The study was designed to assess the safety and efficacy of pretreatment with oral mifepristone prior to use of vaginal misoprostol for the medical management of early pregnancy loss.1
Three hundred women, ≥ 18 years and undergoing medical management for early pregnancy loss, were randomized to receive misoprostol 800 mcg vaginally alone or mifepristone 200 mg orally followed by misoprostol 800 mcg vaginally 24 hours later.
Inclusion and exclusion criteria. Women who showed a nonviable intrauterine pregnancy at 5 to 12 weeks’ gestation by ultrasound were eligible for the study. Exclusion criteria included incomplete or inevitable abortion, contraindications to either study drug, viable or ectopic pregnancy, hemoglobin < 9.5 g/dL, current use of anticoagulants or the presence of a clotting disorder, and pregnancy with an intrauterine device in place.
Outcomes. The primary outcome was gestational sac expulsion by the first follow-up visit and no additional interventions within 30 days of treatment. Secondary outcomes included acceptability of treatment, adverse events, and clinical characteristics associated with successful expulsion.
Continue to: Demographics
Demographics. The mean age of the study participants in both groups was ~30 years, and there was a similar percentage of participants by self-reported race and ethnicity in both groups (~44% black, ~35% white, and ~25% Hispanic). The majority of participants in both groups were at 6 to 8 weeks’ gestation and had been pregnant at least 3 times.
Results. Researchers were able to evaluate 297 women at the initial follow-up. Of the women who received mifepristone and misoprostol, 83.8% (124 of 148 women; 95% confidence interval [CI], 76.8-89.3) had complete expulsion within 1 to 3 days, compared to 67.1% (100 of 149 women; 95% CI, 59-74.6) in the misoprostol alone group. The number needed to treat with mifepristone and misoprostol to achieve complete expulsion at the first follow-up visit was 6. The percentage of patients receiving uterine aspiration was lower in the mifepristone and misoprostol group (8.8%) than in the misoprostol alone group (23.5%; relative risk = 0.37; 95% CI, 0.21-0.68). There were no significant differences in adverse events including bleeding intensity, pelvic infection, or pain.
WHAT’S NEW
A high-quality RCT demonstrates improved efficacy
Prior studies that have looked at combined mifepristone and misoprostol treatment for early pregnancy loss had heterogeneity in outcome definitions and study designs leading to variable reports of effectiveness.1,5 This is the first high-quality, randomized trial to demonstrate the safety and efficacy of oral mifepristone pretreatment prior to misoprostol vaginal administration in the medical management of early pregnancy loss.
CAVEATS
Would a placebo group—or other forms of misoprostol—change the results?
The study did not include a placebo group; however, an investigator who was blinded to the treatment group allocation determined the primary outcome, and the lack of placebo did not introduce bias related to the outcomes.
Intravaginal misoprostol was used in this study, rather than oral, rectal, buccal, or sublingual misoprostol.7 It is not clear from this study if the results of pretreatment with mifepristone would be different if misoprostol was administered via one of these other routes.
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
FDA restrictions limit availability of mifepristone
The main challenge to implementation is the availability of mifepristone. Mifepristone was approved by the US Food and Drug Administration in 2000. The approval included Risk Evaluation and Mitigation Strategy (REMS) restrictions, stipulating that a health provider be specially certified for prescribing; dispensing must occur in clinics, medical offices, or hospitals; and patients must sign a patient agreement form prior to obtaining the agent.8
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Schreiber CA, Creinin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:2161-2170.
2. Ventura SJ, Curtin SC, Abma JC, et al. Estimated pregnancy rates and rates of pregnancy outcomes for the United States, 1990-2008. Natl Vital Stat Rep. 2012;60:1-21.
3. The American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 200. Early pregnancy loss. Obstet Gynecol. 2018;132:e197-e207.
4. National Institute for Health and Clinical Excellence. Ectopic pregnancy and miscarriage: diagnosis and initial management. Clinical guideline 154. www.nice.org.uk/guidance/cg154/resources/guidance-ectopic-pregnancy-and-miscarriage-pdf. Published December 2012. Accessed December 5, 2019.
5. Neilson JP, Hickey M, Vazquez JC. Medical treatment for early fetal death (less than 24 weeks). Cochrane Database Syst Rev. 2006;CD002253.
6. Schreiber CA, Chavez V, Whittaker PG, et al. Treatment decisions at the time of miscarriage diagnosis. Obstet Gynecol. 2016;128:1347-1356.
7. Ngoc NT, Blum J, Westheimer E, et al. Medical treatment of missed abortion using misoprostol. Int J Gynaecol Obstet. 2004;87:138-142.
8. US Food and Drug Administration. Mifeprex (mifepristone) information. www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/mifeprex-mifepristone-information. Updated February 5, 2018. Accessed December 5, 2019.
ILLUSTRATIVE CASE
Jenny is a 29-year-old G2P1001 woman who presents to your clinic for a missed period. Her last menstrual period was about 10 weeks ago. She is found to have a positive pregnancy test in the office. On examination, her uterus is nontender and consistent in size with gestation of 7 weeks. She denies any bleeding or cramping. On ultrasound, you see a gestational sac measuring 28 mm and no embryo. You confirm early pregnancy loss. Jenny is sad about this diagnosis. She does not wish to proceed with expectant management and is hopeful to avoid a surgical procedure. How do you counsel her regarding medical management?
Early pregnancy loss or first trimester miscarriage is estimated to occur in about 1 million women in the United States annually and is the most common complication of early pregnancy.2,3 Early pregnancy loss is defined as a nonviable, intrauterine pregnancy with either an empty gestational sac or a gestational sac containing an embryo or fetus without fetal heart activity within the first 12 weeks 6 days of gestation.4
Once early pregnancy loss is confirmed by ultrasound, expectant management with no intervention is an acceptable treatment option. Women generally prefer active management, either medically or with surgical evacuation.5,6 Misoprostol 800 mcg administered vaginally or orally has been the accepted medication regimen for medical management.5 However, failure rates with misoprostol have been reported to be as high as 40%, particularly among women with a closed cervical os, who then require repeat dosing of misoprostol or surgical evacuation.6
STUDY SUMMARY
Mifepristone before misoprostol improves efficacy for early pregnancy loss
The PreFaiR (Comparative Effectiveness of Pregnancy Failure Management Regimens) study was a randomized trial that took place at 3 US centers. The study was designed to assess the safety and efficacy of pretreatment with oral mifepristone prior to use of vaginal misoprostol for the medical management of early pregnancy loss.1
Three hundred women, ≥ 18 years and undergoing medical management for early pregnancy loss, were randomized to receive misoprostol 800 mcg vaginally alone or mifepristone 200 mg orally followed by misoprostol 800 mcg vaginally 24 hours later.
Inclusion and exclusion criteria. Women who showed a nonviable intrauterine pregnancy at 5 to 12 weeks’ gestation by ultrasound were eligible for the study. Exclusion criteria included incomplete or inevitable abortion, contraindications to either study drug, viable or ectopic pregnancy, hemoglobin < 9.5 g/dL, current use of anticoagulants or the presence of a clotting disorder, and pregnancy with an intrauterine device in place.
Outcomes. The primary outcome was gestational sac expulsion by the first follow-up visit and no additional interventions within 30 days of treatment. Secondary outcomes included acceptability of treatment, adverse events, and clinical characteristics associated with successful expulsion.
Continue to: Demographics
Demographics. The mean age of the study participants in both groups was ~30 years, and there was a similar percentage of participants by self-reported race and ethnicity in both groups (~44% black, ~35% white, and ~25% Hispanic). The majority of participants in both groups were at 6 to 8 weeks’ gestation and had been pregnant at least 3 times.
Results. Researchers were able to evaluate 297 women at the initial follow-up. Of the women who received mifepristone and misoprostol, 83.8% (124 of 148 women; 95% confidence interval [CI], 76.8-89.3) had complete expulsion within 1 to 3 days, compared to 67.1% (100 of 149 women; 95% CI, 59-74.6) in the misoprostol alone group. The number needed to treat with mifepristone and misoprostol to achieve complete expulsion at the first follow-up visit was 6. The percentage of patients receiving uterine aspiration was lower in the mifepristone and misoprostol group (8.8%) than in the misoprostol alone group (23.5%; relative risk = 0.37; 95% CI, 0.21-0.68). There were no significant differences in adverse events including bleeding intensity, pelvic infection, or pain.
WHAT’S NEW
A high-quality RCT demonstrates improved efficacy
Prior studies that have looked at combined mifepristone and misoprostol treatment for early pregnancy loss had heterogeneity in outcome definitions and study designs leading to variable reports of effectiveness.1,5 This is the first high-quality, randomized trial to demonstrate the safety and efficacy of oral mifepristone pretreatment prior to misoprostol vaginal administration in the medical management of early pregnancy loss.
CAVEATS
Would a placebo group—or other forms of misoprostol—change the results?
The study did not include a placebo group; however, an investigator who was blinded to the treatment group allocation determined the primary outcome, and the lack of placebo did not introduce bias related to the outcomes.
Intravaginal misoprostol was used in this study, rather than oral, rectal, buccal, or sublingual misoprostol.7 It is not clear from this study if the results of pretreatment with mifepristone would be different if misoprostol was administered via one of these other routes.
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
FDA restrictions limit availability of mifepristone
The main challenge to implementation is the availability of mifepristone. Mifepristone was approved by the US Food and Drug Administration in 2000. The approval included Risk Evaluation and Mitigation Strategy (REMS) restrictions, stipulating that a health provider be specially certified for prescribing; dispensing must occur in clinics, medical offices, or hospitals; and patients must sign a patient agreement form prior to obtaining the agent.8
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
Jenny is a 29-year-old G2P1001 woman who presents to your clinic for a missed period. Her last menstrual period was about 10 weeks ago. She is found to have a positive pregnancy test in the office. On examination, her uterus is nontender and consistent in size with gestation of 7 weeks. She denies any bleeding or cramping. On ultrasound, you see a gestational sac measuring 28 mm and no embryo. You confirm early pregnancy loss. Jenny is sad about this diagnosis. She does not wish to proceed with expectant management and is hopeful to avoid a surgical procedure. How do you counsel her regarding medical management?
Early pregnancy loss or first trimester miscarriage is estimated to occur in about 1 million women in the United States annually and is the most common complication of early pregnancy.2,3 Early pregnancy loss is defined as a nonviable, intrauterine pregnancy with either an empty gestational sac or a gestational sac containing an embryo or fetus without fetal heart activity within the first 12 weeks 6 days of gestation.4
Once early pregnancy loss is confirmed by ultrasound, expectant management with no intervention is an acceptable treatment option. Women generally prefer active management, either medically or with surgical evacuation.5,6 Misoprostol 800 mcg administered vaginally or orally has been the accepted medication regimen for medical management.5 However, failure rates with misoprostol have been reported to be as high as 40%, particularly among women with a closed cervical os, who then require repeat dosing of misoprostol or surgical evacuation.6
STUDY SUMMARY
Mifepristone before misoprostol improves efficacy for early pregnancy loss
The PreFaiR (Comparative Effectiveness of Pregnancy Failure Management Regimens) study was a randomized trial that took place at 3 US centers. The study was designed to assess the safety and efficacy of pretreatment with oral mifepristone prior to use of vaginal misoprostol for the medical management of early pregnancy loss.1
Three hundred women, ≥ 18 years and undergoing medical management for early pregnancy loss, were randomized to receive misoprostol 800 mcg vaginally alone or mifepristone 200 mg orally followed by misoprostol 800 mcg vaginally 24 hours later.
Inclusion and exclusion criteria. Women who showed a nonviable intrauterine pregnancy at 5 to 12 weeks’ gestation by ultrasound were eligible for the study. Exclusion criteria included incomplete or inevitable abortion, contraindications to either study drug, viable or ectopic pregnancy, hemoglobin < 9.5 g/dL, current use of anticoagulants or the presence of a clotting disorder, and pregnancy with an intrauterine device in place.
Outcomes. The primary outcome was gestational sac expulsion by the first follow-up visit and no additional interventions within 30 days of treatment. Secondary outcomes included acceptability of treatment, adverse events, and clinical characteristics associated with successful expulsion.
Continue to: Demographics
Demographics. The mean age of the study participants in both groups was ~30 years, and there was a similar percentage of participants by self-reported race and ethnicity in both groups (~44% black, ~35% white, and ~25% Hispanic). The majority of participants in both groups were at 6 to 8 weeks’ gestation and had been pregnant at least 3 times.
Results. Researchers were able to evaluate 297 women at the initial follow-up. Of the women who received mifepristone and misoprostol, 83.8% (124 of 148 women; 95% confidence interval [CI], 76.8-89.3) had complete expulsion within 1 to 3 days, compared to 67.1% (100 of 149 women; 95% CI, 59-74.6) in the misoprostol alone group. The number needed to treat with mifepristone and misoprostol to achieve complete expulsion at the first follow-up visit was 6. The percentage of patients receiving uterine aspiration was lower in the mifepristone and misoprostol group (8.8%) than in the misoprostol alone group (23.5%; relative risk = 0.37; 95% CI, 0.21-0.68). There were no significant differences in adverse events including bleeding intensity, pelvic infection, or pain.
WHAT’S NEW
A high-quality RCT demonstrates improved efficacy
Prior studies that have looked at combined mifepristone and misoprostol treatment for early pregnancy loss had heterogeneity in outcome definitions and study designs leading to variable reports of effectiveness.1,5 This is the first high-quality, randomized trial to demonstrate the safety and efficacy of oral mifepristone pretreatment prior to misoprostol vaginal administration in the medical management of early pregnancy loss.
CAVEATS
Would a placebo group—or other forms of misoprostol—change the results?
The study did not include a placebo group; however, an investigator who was blinded to the treatment group allocation determined the primary outcome, and the lack of placebo did not introduce bias related to the outcomes.
Intravaginal misoprostol was used in this study, rather than oral, rectal, buccal, or sublingual misoprostol.7 It is not clear from this study if the results of pretreatment with mifepristone would be different if misoprostol was administered via one of these other routes.
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
FDA restrictions limit availability of mifepristone
The main challenge to implementation is the availability of mifepristone. Mifepristone was approved by the US Food and Drug Administration in 2000. The approval included Risk Evaluation and Mitigation Strategy (REMS) restrictions, stipulating that a health provider be specially certified for prescribing; dispensing must occur in clinics, medical offices, or hospitals; and patients must sign a patient agreement form prior to obtaining the agent.8
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Schreiber CA, Creinin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:2161-2170.
2. Ventura SJ, Curtin SC, Abma JC, et al. Estimated pregnancy rates and rates of pregnancy outcomes for the United States, 1990-2008. Natl Vital Stat Rep. 2012;60:1-21.
3. The American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 200. Early pregnancy loss. Obstet Gynecol. 2018;132:e197-e207.
4. National Institute for Health and Clinical Excellence. Ectopic pregnancy and miscarriage: diagnosis and initial management. Clinical guideline 154. www.nice.org.uk/guidance/cg154/resources/guidance-ectopic-pregnancy-and-miscarriage-pdf. Published December 2012. Accessed December 5, 2019.
5. Neilson JP, Hickey M, Vazquez JC. Medical treatment for early fetal death (less than 24 weeks). Cochrane Database Syst Rev. 2006;CD002253.
6. Schreiber CA, Chavez V, Whittaker PG, et al. Treatment decisions at the time of miscarriage diagnosis. Obstet Gynecol. 2016;128:1347-1356.
7. Ngoc NT, Blum J, Westheimer E, et al. Medical treatment of missed abortion using misoprostol. Int J Gynaecol Obstet. 2004;87:138-142.
8. US Food and Drug Administration. Mifeprex (mifepristone) information. www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/mifeprex-mifepristone-information. Updated February 5, 2018. Accessed December 5, 2019.
1. Schreiber CA, Creinin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:2161-2170.
2. Ventura SJ, Curtin SC, Abma JC, et al. Estimated pregnancy rates and rates of pregnancy outcomes for the United States, 1990-2008. Natl Vital Stat Rep. 2012;60:1-21.
3. The American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 200. Early pregnancy loss. Obstet Gynecol. 2018;132:e197-e207.
4. National Institute for Health and Clinical Excellence. Ectopic pregnancy and miscarriage: diagnosis and initial management. Clinical guideline 154. www.nice.org.uk/guidance/cg154/resources/guidance-ectopic-pregnancy-and-miscarriage-pdf. Published December 2012. Accessed December 5, 2019.
5. Neilson JP, Hickey M, Vazquez JC. Medical treatment for early fetal death (less than 24 weeks). Cochrane Database Syst Rev. 2006;CD002253.
6. Schreiber CA, Chavez V, Whittaker PG, et al. Treatment decisions at the time of miscarriage diagnosis. Obstet Gynecol. 2016;128:1347-1356.
7. Ngoc NT, Blum J, Westheimer E, et al. Medical treatment of missed abortion using misoprostol. Int J Gynaecol Obstet. 2004;87:138-142.
8. US Food and Drug Administration. Mifeprex (mifepristone) information. www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/mifeprex-mifepristone-information. Updated February 5, 2018. Accessed December 5, 2019.
PRACTICE CHANGER
Pretreat patients with oral mifepristone prior to using vaginal misoprostol to increase the efficacy of medical management of early pregnancy loss over that with misoprostol alone.
STRENGTH OF RECOMMENDATION
B: Based on a single, well-executed, randomized controlled trial.1
Schreiber CA, Creinin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:2161-2170.
Antidepressant Tx for Anxiety Disorders: How Long?
PRACTICE CHANGER
Keep patients on antidepressant therapy for anxiety disorders for a year or longer before considering a taper.1
STRENGTH OF RECOMMENDATION
A: Based on a systematic review/meta-analysis of several good-quality randomized controlled trials.
A 42-year-old woman with generalized anxiety disorder (GAD) and panic attacks has been treated with sertraline (100 mg/d) for the past 8 months. She has also engaged in cognitive behavioral therapy (CBT) for 6 months. Her Generalized Anxiety Disorder-7 score has decreased from 19 prior to treatment to 5 at present. Now she would like to stop her antidepressant medication because she feels better. Would you recommend that she discontinue her medication at this point?
Anxiety disorders are common and often chronic and can cause significant morbidity and impairment.2,3 Firstline treatments for anxiety disorders include CBT and antidepressants, particularly selective serotonin reuptake inhibitors and serotonin-norepinephrine reuptake inhibitors.4-6
There is limited evidence regarding duration of antidepressant therapy for anxiety disorders. Previous studies have shown a high risk for relapse after discontinuation of antidepressants.6 A review of current practice patterns regarding pharmacologic treatment of depression and anxiety indicates an uptick in longer term antidepressant use for up to 2 years.7 However, long-term studies to guide treatment decisions are lacking.
STUDY SUMMARY
Clear benefit of continuing treatment
This systematic review and meta-analysis evaluated studies that looked at relapse rates and time to relapse in patients treated for anxiety disorders.1 The authors used PubMed, Cochrane, and Embase to identify studies involving patients treated for a variety of psychiatric disorders, including GAD, posttraumatic stress disorder (PTSD), panic disorder (PD), obsessive compulsive disorder (OCD), and social phobia. Eligible studies enrolled patients with anxiety disorders who had a positive response to an antidepressant and then randomized them in a double-blind fashion to either discontinuation of antidepressants and commencement of placebo (stopping group) or continuation of antidepressants (continuation group) for a duration of 8 to 52 weeks. The primary outcomes were relapse rate and time to relapse.
Twenty-eight studies met the inclusion criteria for the meta-analysis, with a total of 5233 patients (2625 patients in the continuation group and 2608 patients in the stopping group). A breakdown of the trials by indication included OCD (7), PD (6), GAD (6), social phobia (5), and PTSD (4). The authors graded the overall risk for bias to be low but noted that attrition bias was present in most studies.
Continue to: Results
Results. Relapse was more likely in the stopping group (odds ratio [OR], 3.11; n = 28 studies). Heterogeneity for relapse rate was low (I2 = 8.07%). Subgroup analyses by type of antidepressant, mode of discontinuation, and exclusion of patient comorbidities yielded similar results. Relapse prevalence was 16.4% in the continuation group and 36.4% in the stopping group. Additionally, time to relapse was shorter when antidepressants were discontinued (hazard ratio [HR], 3.63; n = 11 studies). Again, the heterogeneity for relapse rate was low (I2 = 0%). The original publications did not consistently report medication tolerance or withdrawal symptoms, preventing analysis of these. Dropout rates were higher in the stopping group (OR, 1.31; n = 27 studies).
WHAT’S NEW
No more guessing about how long to treat
Previously, there was limited evidence to guide decisions about the duration of antidepressant treatment for anxiety disorders. This study provides evidence that stopping antidepressants before completing 1 year of treatment increases the risk for relapse.
CAVEATS
In a word: Bias
While the authors used standard and appropriate methodologies for this type of study, some significant threats to validity remained. All but 2 studies in the analysis were industry funded. Publication bias is another potential issue, even though the authors identified and included 6 unpublished studies, 4 of which had negative results.
Additionally, the authors graded 11 of 28 trials as having a high likelihood of selective reporting bias, meaning that important portions of the original studies’ results may not have been published. Most studies were at high risk for attrition bias, resulting in loss of information when patients dropped out of the study. While this happened more often in the stopping groups, it is still possible that there are unidentified harms or unexpected outcomes in the medication groups.
While PTSD and OCD are no longer considered anxiety disorders, subgroup analyses found no difference in relapse rates between these diagnoses and the others included in the studies. Finally, a treatment duration longer than 52 weeks has not been studied, so the optimal treatment duration is unknown.
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
Patient resistance to continuing treatment
Some patients may want to discontinue antidepressant treatment if their anxiety symptoms improve before completing 1 year. It may be difficult to convince them that continuing treatment will prevent relapse of their condition. Providing patients with information about the increased relapse rate associated with stopping their antidepressant early (with an estimated number needed to treat of 5) may help patients make a more informed decision.
ACKNOWLEDGMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2019. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2019;68[7]:409-410).
1. Batelaan NM, Bosman RC, Muntingh A, et al. Risk of relapse after antidepressant discontinuation in anxiety disorders, obsessive-compulsive disorder, and post-traumatic stress disorder: systematic review and meta-analysis of relapse prevention trials. BMJ. 2017;358:j3927. Erratum in: BMJ. 2017;358:j4461.
2. National Institute of Mental Health. Prevalence of any anxiety disorder among adults. www.nimh.nih.gov/health/statistics/any-anxiety-disorder.shtml#part_155094. Updated November 2017. Accessed November 26, 2019.
3. Kessler RC, Petukhova M, Sampson NA, et al. Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. Int J Methods Psychiatr Res. 2012;21:169-184.
4. Bandelow B, Sher L, Bunevicius R, et al. Guidelines for the pharmacological treatment of anxiety disorders, obsessive-compulsive disorder and posttraumatic stress disorder in primary care. Int J Psychiatry Clin Pract. 2012;16:77-84.
5. Kaczkurkin AN, Foa EB. Cognitive-behavioral therapy for anxiety disorders: an update on the empirical evidence. Dialogues Clin Neurosci. 2015;17:337-346.
6. Donovan MR, Glue P, Kolluri S, et al. Comparative efficacy of antidepressants in preventing relapse in anxiety disorders—a meta-analysis. J Affect Disord. 2010;123:9-16.
7. Mojtabai R, Olfson M. National trends in long-term use of antidepressant medications: results from the U.S. National Health and Nutrition Examination Survey. J Clin Psychiatry. 2014;75:169-177.
PRACTICE CHANGER
Keep patients on antidepressant therapy for anxiety disorders for a year or longer before considering a taper.1
STRENGTH OF RECOMMENDATION
A: Based on a systematic review/meta-analysis of several good-quality randomized controlled trials.
A 42-year-old woman with generalized anxiety disorder (GAD) and panic attacks has been treated with sertraline (100 mg/d) for the past 8 months. She has also engaged in cognitive behavioral therapy (CBT) for 6 months. Her Generalized Anxiety Disorder-7 score has decreased from 19 prior to treatment to 5 at present. Now she would like to stop her antidepressant medication because she feels better. Would you recommend that she discontinue her medication at this point?
Anxiety disorders are common and often chronic and can cause significant morbidity and impairment.2,3 Firstline treatments for anxiety disorders include CBT and antidepressants, particularly selective serotonin reuptake inhibitors and serotonin-norepinephrine reuptake inhibitors.4-6
There is limited evidence regarding duration of antidepressant therapy for anxiety disorders. Previous studies have shown a high risk for relapse after discontinuation of antidepressants.6 A review of current practice patterns regarding pharmacologic treatment of depression and anxiety indicates an uptick in longer term antidepressant use for up to 2 years.7 However, long-term studies to guide treatment decisions are lacking.
STUDY SUMMARY
Clear benefit of continuing treatment
This systematic review and meta-analysis evaluated studies that looked at relapse rates and time to relapse in patients treated for anxiety disorders.1 The authors used PubMed, Cochrane, and Embase to identify studies involving patients treated for a variety of psychiatric disorders, including GAD, posttraumatic stress disorder (PTSD), panic disorder (PD), obsessive compulsive disorder (OCD), and social phobia. Eligible studies enrolled patients with anxiety disorders who had a positive response to an antidepressant and then randomized them in a double-blind fashion to either discontinuation of antidepressants and commencement of placebo (stopping group) or continuation of antidepressants (continuation group) for a duration of 8 to 52 weeks. The primary outcomes were relapse rate and time to relapse.
Twenty-eight studies met the inclusion criteria for the meta-analysis, with a total of 5233 patients (2625 patients in the continuation group and 2608 patients in the stopping group). A breakdown of the trials by indication included OCD (7), PD (6), GAD (6), social phobia (5), and PTSD (4). The authors graded the overall risk for bias to be low but noted that attrition bias was present in most studies.
Continue to: Results
Results. Relapse was more likely in the stopping group (odds ratio [OR], 3.11; n = 28 studies). Heterogeneity for relapse rate was low (I2 = 8.07%). Subgroup analyses by type of antidepressant, mode of discontinuation, and exclusion of patient comorbidities yielded similar results. Relapse prevalence was 16.4% in the continuation group and 36.4% in the stopping group. Additionally, time to relapse was shorter when antidepressants were discontinued (hazard ratio [HR], 3.63; n = 11 studies). Again, the heterogeneity for relapse rate was low (I2 = 0%). The original publications did not consistently report medication tolerance or withdrawal symptoms, preventing analysis of these. Dropout rates were higher in the stopping group (OR, 1.31; n = 27 studies).
WHAT’S NEW
No more guessing about how long to treat
Previously, there was limited evidence to guide decisions about the duration of antidepressant treatment for anxiety disorders. This study provides evidence that stopping antidepressants before completing 1 year of treatment increases the risk for relapse.
CAVEATS
In a word: Bias
While the authors used standard and appropriate methodologies for this type of study, some significant threats to validity remained. All but 2 studies in the analysis were industry funded. Publication bias is another potential issue, even though the authors identified and included 6 unpublished studies, 4 of which had negative results.
Additionally, the authors graded 11 of 28 trials as having a high likelihood of selective reporting bias, meaning that important portions of the original studies’ results may not have been published. Most studies were at high risk for attrition bias, resulting in loss of information when patients dropped out of the study. While this happened more often in the stopping groups, it is still possible that there are unidentified harms or unexpected outcomes in the medication groups.
While PTSD and OCD are no longer considered anxiety disorders, subgroup analyses found no difference in relapse rates between these diagnoses and the others included in the studies. Finally, a treatment duration longer than 52 weeks has not been studied, so the optimal treatment duration is unknown.
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
Patient resistance to continuing treatment
Some patients may want to discontinue antidepressant treatment if their anxiety symptoms improve before completing 1 year. It may be difficult to convince them that continuing treatment will prevent relapse of their condition. Providing patients with information about the increased relapse rate associated with stopping their antidepressant early (with an estimated number needed to treat of 5) may help patients make a more informed decision.
ACKNOWLEDGMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2019. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2019;68[7]:409-410).
PRACTICE CHANGER
Keep patients on antidepressant therapy for anxiety disorders for a year or longer before considering a taper.1
STRENGTH OF RECOMMENDATION
A: Based on a systematic review/meta-analysis of several good-quality randomized controlled trials.
A 42-year-old woman with generalized anxiety disorder (GAD) and panic attacks has been treated with sertraline (100 mg/d) for the past 8 months. She has also engaged in cognitive behavioral therapy (CBT) for 6 months. Her Generalized Anxiety Disorder-7 score has decreased from 19 prior to treatment to 5 at present. Now she would like to stop her antidepressant medication because she feels better. Would you recommend that she discontinue her medication at this point?
Anxiety disorders are common and often chronic and can cause significant morbidity and impairment.2,3 Firstline treatments for anxiety disorders include CBT and antidepressants, particularly selective serotonin reuptake inhibitors and serotonin-norepinephrine reuptake inhibitors.4-6
There is limited evidence regarding duration of antidepressant therapy for anxiety disorders. Previous studies have shown a high risk for relapse after discontinuation of antidepressants.6 A review of current practice patterns regarding pharmacologic treatment of depression and anxiety indicates an uptick in longer term antidepressant use for up to 2 years.7 However, long-term studies to guide treatment decisions are lacking.
STUDY SUMMARY
Clear benefit of continuing treatment
This systematic review and meta-analysis evaluated studies that looked at relapse rates and time to relapse in patients treated for anxiety disorders.1 The authors used PubMed, Cochrane, and Embase to identify studies involving patients treated for a variety of psychiatric disorders, including GAD, posttraumatic stress disorder (PTSD), panic disorder (PD), obsessive compulsive disorder (OCD), and social phobia. Eligible studies enrolled patients with anxiety disorders who had a positive response to an antidepressant and then randomized them in a double-blind fashion to either discontinuation of antidepressants and commencement of placebo (stopping group) or continuation of antidepressants (continuation group) for a duration of 8 to 52 weeks. The primary outcomes were relapse rate and time to relapse.
Twenty-eight studies met the inclusion criteria for the meta-analysis, with a total of 5233 patients (2625 patients in the continuation group and 2608 patients in the stopping group). A breakdown of the trials by indication included OCD (7), PD (6), GAD (6), social phobia (5), and PTSD (4). The authors graded the overall risk for bias to be low but noted that attrition bias was present in most studies.
Continue to: Results
Results. Relapse was more likely in the stopping group (odds ratio [OR], 3.11; n = 28 studies). Heterogeneity for relapse rate was low (I2 = 8.07%). Subgroup analyses by type of antidepressant, mode of discontinuation, and exclusion of patient comorbidities yielded similar results. Relapse prevalence was 16.4% in the continuation group and 36.4% in the stopping group. Additionally, time to relapse was shorter when antidepressants were discontinued (hazard ratio [HR], 3.63; n = 11 studies). Again, the heterogeneity for relapse rate was low (I2 = 0%). The original publications did not consistently report medication tolerance or withdrawal symptoms, preventing analysis of these. Dropout rates were higher in the stopping group (OR, 1.31; n = 27 studies).
WHAT’S NEW
No more guessing about how long to treat
Previously, there was limited evidence to guide decisions about the duration of antidepressant treatment for anxiety disorders. This study provides evidence that stopping antidepressants before completing 1 year of treatment increases the risk for relapse.
CAVEATS
In a word: Bias
While the authors used standard and appropriate methodologies for this type of study, some significant threats to validity remained. All but 2 studies in the analysis were industry funded. Publication bias is another potential issue, even though the authors identified and included 6 unpublished studies, 4 of which had negative results.
Additionally, the authors graded 11 of 28 trials as having a high likelihood of selective reporting bias, meaning that important portions of the original studies’ results may not have been published. Most studies were at high risk for attrition bias, resulting in loss of information when patients dropped out of the study. While this happened more often in the stopping groups, it is still possible that there are unidentified harms or unexpected outcomes in the medication groups.
While PTSD and OCD are no longer considered anxiety disorders, subgroup analyses found no difference in relapse rates between these diagnoses and the others included in the studies. Finally, a treatment duration longer than 52 weeks has not been studied, so the optimal treatment duration is unknown.
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
Patient resistance to continuing treatment
Some patients may want to discontinue antidepressant treatment if their anxiety symptoms improve before completing 1 year. It may be difficult to convince them that continuing treatment will prevent relapse of their condition. Providing patients with information about the increased relapse rate associated with stopping their antidepressant early (with an estimated number needed to treat of 5) may help patients make a more informed decision.
ACKNOWLEDGMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2019. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2019;68[7]:409-410).
1. Batelaan NM, Bosman RC, Muntingh A, et al. Risk of relapse after antidepressant discontinuation in anxiety disorders, obsessive-compulsive disorder, and post-traumatic stress disorder: systematic review and meta-analysis of relapse prevention trials. BMJ. 2017;358:j3927. Erratum in: BMJ. 2017;358:j4461.
2. National Institute of Mental Health. Prevalence of any anxiety disorder among adults. www.nimh.nih.gov/health/statistics/any-anxiety-disorder.shtml#part_155094. Updated November 2017. Accessed November 26, 2019.
3. Kessler RC, Petukhova M, Sampson NA, et al. Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. Int J Methods Psychiatr Res. 2012;21:169-184.
4. Bandelow B, Sher L, Bunevicius R, et al. Guidelines for the pharmacological treatment of anxiety disorders, obsessive-compulsive disorder and posttraumatic stress disorder in primary care. Int J Psychiatry Clin Pract. 2012;16:77-84.
5. Kaczkurkin AN, Foa EB. Cognitive-behavioral therapy for anxiety disorders: an update on the empirical evidence. Dialogues Clin Neurosci. 2015;17:337-346.
6. Donovan MR, Glue P, Kolluri S, et al. Comparative efficacy of antidepressants in preventing relapse in anxiety disorders—a meta-analysis. J Affect Disord. 2010;123:9-16.
7. Mojtabai R, Olfson M. National trends in long-term use of antidepressant medications: results from the U.S. National Health and Nutrition Examination Survey. J Clin Psychiatry. 2014;75:169-177.
1. Batelaan NM, Bosman RC, Muntingh A, et al. Risk of relapse after antidepressant discontinuation in anxiety disorders, obsessive-compulsive disorder, and post-traumatic stress disorder: systematic review and meta-analysis of relapse prevention trials. BMJ. 2017;358:j3927. Erratum in: BMJ. 2017;358:j4461.
2. National Institute of Mental Health. Prevalence of any anxiety disorder among adults. www.nimh.nih.gov/health/statistics/any-anxiety-disorder.shtml#part_155094. Updated November 2017. Accessed November 26, 2019.
3. Kessler RC, Petukhova M, Sampson NA, et al. Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. Int J Methods Psychiatr Res. 2012;21:169-184.
4. Bandelow B, Sher L, Bunevicius R, et al. Guidelines for the pharmacological treatment of anxiety disorders, obsessive-compulsive disorder and posttraumatic stress disorder in primary care. Int J Psychiatry Clin Pract. 2012;16:77-84.
5. Kaczkurkin AN, Foa EB. Cognitive-behavioral therapy for anxiety disorders: an update on the empirical evidence. Dialogues Clin Neurosci. 2015;17:337-346.
6. Donovan MR, Glue P, Kolluri S, et al. Comparative efficacy of antidepressants in preventing relapse in anxiety disorders—a meta-analysis. J Affect Disord. 2010;123:9-16.
7. Mojtabai R, Olfson M. National trends in long-term use of antidepressant medications: results from the U.S. National Health and Nutrition Examination Survey. J Clin Psychiatry. 2014;75:169-177.
Supplemental oxygen: More isn’t always better
ILLUSTRATIVE CASE
A 60-year-old woman who is generally healthy except for a history of recurrent urinary tract infections presents to the emergency department with fever, hypotension, and altered mental status, meeting criteria for septic shock. During her resuscitation, supplemental oxygen is administered. Standard treatment calls for a minimum SpO2 (saturation of peripheral oxygen) > 90%. What should your SpO2 goal be?
Use of supplemental oxygen in the acute care of the critically ill adult is a common practice in pre-hospital, emergency department (ED), and hospitalized settings.2,3 Despite their prevalence, guidelines about appropriate oxygen concentration and target SpO2 levels are often conflicting or vague.3-5
Excessive oxygen supplementation in acute illness may be harmful and cause increased risk of hypercapnic respiratory failure, delayed recognition of clinical deterioration, and oxygen toxicity.2,6 The perception of oxygen safety persists despite these findings, and it likely contributes to the ongoing practice of liberal oxygen supplementation in the acutely ill adult.2,7,8
STUDY SUMMARY
Liberal supplemental O2 linked to increased mortality
The Improving Oxygen Therapy in Acute illness (IOTA) study was a systematic review and meta-analysis of 25 randomized controlled trials (RCTs) that compared liberal vs conservative oxygen strategies for acutely ill adults (N = 16,037; median age = 64 years; range = 28-76 years). Patients with sepsis, critical illness, stroke, trauma, myocardial infarction, or cardiac arrest, and patients who had emergency surgery were included. Studies were excluded if they involved patients who had chronic respiratory illness or psychiatric diseases, were receiving extracorporeal membrane oxygenation, were undergoing elective surgeries, were being treated with hyperbaric oxygen therapy, or were pregnant.
The outcomes studied were mortality (in-hospital, at 30 days, and at the longest follow-up) and morbidity (disability measured by the modified Rankin Scale at longest follow-up, risk of hospital-acquired pneumonia, risk of any hospital-acquired infection, and hospital length of stay).
Liberal supplemental oxygen, above an SpO2 range of 94% to 96%, increased mortality during inpatient stays (relative risk [RR] = 1.21; 95% confidence interval [CI], 1.03-1.43; N = 15,071), at 30 days (RR = 1.14; 95% CI, 1.01-1.29; N = 15,053), and at longest follow-up (RR = 1.10; 95% CI, 1.00-1.20; N = 15,754; median = 90 days; range = 14,365 days). There was no difference in morbidity outcomes between groups.
While it’s difficult to define a specific target SpO2 range, the number needed to harm when using a liberal oxygen approach (SpO2 > 96%) resulting in 1 death was 71 (95% CI, 37-1000).
Continue to: WHAT'S NEW
WHAT’S NEW
High-quality evidence points to the dangers of liberal O2 therapy
This comprehensive meta-analysis is the first high-quality evidence to suggest that liberal use of oxygen in acutely ill adults above a specific SpO2 level increases all-cause mortality. Previous small RCTs and observational studies have examined the effect of liberal oxygen only on specific presenting conditions, thus making more generalizable conclusions challenging.9-12
CAVEATS
Varied definitions of “liberal” and “conservative”
This review included studies with variable ranges of SpO2 defined as liberal vs conservative supplementation. However, in all of these, SpO2 above 96% was correlated with unfavorable outcomes.
The study excluded 2 potentially important patient groups: patients with chronic respiratory diseases and pregnant patients. Increased oxygen supplementation in patients with chronic respiratory diseases in noncritical settings has been shown to be deleterious.13-15 While this study does not address the issue of oxygen supplementation in acutely ill patients with chronic respiratory disease, use should be considered with caution. The results from this study may not be generalizable to women who are pregnant.
CHALLENGES TO IMPLEMENTATION
Reversing the tide
Liberal oxygen administration continues to be practiced in many health care settings. The main challenges to implementing the conclusions of this study are these pervasive practices.
ACKNOWLEDGMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Chu DK, Kim LH, Young PJ, et al. Mortality and morbidity in acutely ill adults treated with liberal versus conservative oxygen therapy (IOTA): a systematic review and meta-analysis. Lancet. 2018;391:1693-1705.
2. Hale KE, Gavin C, O’Driscoll BR. Audit of oxygen use in emergency ambulances and in a hospital emergency department. Emerg Med J. 2008;25:773-776.
3. O’Driscoll BR, Howard LS, Earis J, et al. BTS guideline for oxygen use in adults in healthcare and emergency settings. Thorax. 2017;72(suppl 1):ii1-ii90.
4. Kallstrom TJ, American Association for Respiratory Care. AARC Clinical Practice Guideline: oxygen therapy for adults in the acute care facility—2002 revision and update. Respir Care. 2002;47:717-720.
5. Henry TD, Torbati S. Oxygen for ACS: too much, too little, or just right? May 15, 2017. https://www.acc.org/latest-in-cardiology/articles/2017/05/15/08/34/oxygen-for-acs. Accessed October 1, 2019.
6. Hafner S, Beloncle F, Koch A, et al. Hyperoxia in intensive care, emergency, and peri-operative medicine: Dr. Jekyll or Mr. Hyde? A 2015 update. Ann Intensive Care. 2015;5:42.
7. Helmerhorst HJ, Schultz MJ, van der Voort PH, et al. Self-reported attitudes versus actual practice of oxygen therapy by ICU physicians and nurses. Ann Intensive Care. 2014;4:23.
8. Kelly CA, Lynes D, O’Brien MR, et al. A wolf in sheep’s clothing? Patients’ and healthcare professionals’ perceptions of oxygen therapy: an interpretative phenomenological analysis. Clin Respir J. 2018;12:616-632.
9. Meyhoff CS, Wetterslev J, Jorgensen LN, et al. Effect of high perioperative oxygen fraction on surgical site infection and pulmonary complications after abdominal surgery: the PROXI randomized clinical trial. JAMA. 2009;302:1543-1550.
10. Stub D, Smith K, Bernard S, et al. A randomized controlled trial on oxygen therapy in acute myocardial infarction Air Verses Oxygen in Myocardial infarction study (AVOID Study). Am Heart J. 2012;163:339-345.E1.
11. Girardis M, Busani S, Damiani E, et al. Effect of conservative vs conventional oxygen therapy on mortality among patients in an intensive care unit: the oxygen-ICU randomized clinical trial. JAMA. 2016;316:1583-1589.
12. Helmerhorst HJ, Roos-Blom MJ, van Westerloo DJ, et al. Association between arterial hyperoxia and outcome in subsets of critical illness: a systematic review, meta-analysis, and meta-regression of cohort studies. Crit Care Med. 2015;43:1508-1519.
13. Pope JV, Jones AE, Gaieski DF, et al. Multicenter study of central venous oxygen saturation (ScvO(2)) as a predictor of mortality in patients with sepsis. Ann Emerg Med. 2010;55:40-46.E1.
14. Kim V, Benditt JO, Wise RA, et al. Oxygen therapy in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2008;5:513-518.
15. Austin MA, Wills KE, Blizzard L, et al. Effect of high flow oxygen on mortality in chronic obstructive pulmonary disease patients in prehospital setting: randomised controlled trial. BMJ. 2010;341:C5462.
ILLUSTRATIVE CASE
A 60-year-old woman who is generally healthy except for a history of recurrent urinary tract infections presents to the emergency department with fever, hypotension, and altered mental status, meeting criteria for septic shock. During her resuscitation, supplemental oxygen is administered. Standard treatment calls for a minimum SpO2 (saturation of peripheral oxygen) > 90%. What should your SpO2 goal be?
Use of supplemental oxygen in the acute care of the critically ill adult is a common practice in pre-hospital, emergency department (ED), and hospitalized settings.2,3 Despite their prevalence, guidelines about appropriate oxygen concentration and target SpO2 levels are often conflicting or vague.3-5
Excessive oxygen supplementation in acute illness may be harmful and cause increased risk of hypercapnic respiratory failure, delayed recognition of clinical deterioration, and oxygen toxicity.2,6 The perception of oxygen safety persists despite these findings, and it likely contributes to the ongoing practice of liberal oxygen supplementation in the acutely ill adult.2,7,8
STUDY SUMMARY
Liberal supplemental O2 linked to increased mortality
The Improving Oxygen Therapy in Acute illness (IOTA) study was a systematic review and meta-analysis of 25 randomized controlled trials (RCTs) that compared liberal vs conservative oxygen strategies for acutely ill adults (N = 16,037; median age = 64 years; range = 28-76 years). Patients with sepsis, critical illness, stroke, trauma, myocardial infarction, or cardiac arrest, and patients who had emergency surgery were included. Studies were excluded if they involved patients who had chronic respiratory illness or psychiatric diseases, were receiving extracorporeal membrane oxygenation, were undergoing elective surgeries, were being treated with hyperbaric oxygen therapy, or were pregnant.
The outcomes studied were mortality (in-hospital, at 30 days, and at the longest follow-up) and morbidity (disability measured by the modified Rankin Scale at longest follow-up, risk of hospital-acquired pneumonia, risk of any hospital-acquired infection, and hospital length of stay).
Liberal supplemental oxygen, above an SpO2 range of 94% to 96%, increased mortality during inpatient stays (relative risk [RR] = 1.21; 95% confidence interval [CI], 1.03-1.43; N = 15,071), at 30 days (RR = 1.14; 95% CI, 1.01-1.29; N = 15,053), and at longest follow-up (RR = 1.10; 95% CI, 1.00-1.20; N = 15,754; median = 90 days; range = 14,365 days). There was no difference in morbidity outcomes between groups.
While it’s difficult to define a specific target SpO2 range, the number needed to harm when using a liberal oxygen approach (SpO2 > 96%) resulting in 1 death was 71 (95% CI, 37-1000).
Continue to: WHAT'S NEW
WHAT’S NEW
High-quality evidence points to the dangers of liberal O2 therapy
This comprehensive meta-analysis is the first high-quality evidence to suggest that liberal use of oxygen in acutely ill adults above a specific SpO2 level increases all-cause mortality. Previous small RCTs and observational studies have examined the effect of liberal oxygen only on specific presenting conditions, thus making more generalizable conclusions challenging.9-12
CAVEATS
Varied definitions of “liberal” and “conservative”
This review included studies with variable ranges of SpO2 defined as liberal vs conservative supplementation. However, in all of these, SpO2 above 96% was correlated with unfavorable outcomes.
The study excluded 2 potentially important patient groups: patients with chronic respiratory diseases and pregnant patients. Increased oxygen supplementation in patients with chronic respiratory diseases in noncritical settings has been shown to be deleterious.13-15 While this study does not address the issue of oxygen supplementation in acutely ill patients with chronic respiratory disease, use should be considered with caution. The results from this study may not be generalizable to women who are pregnant.
CHALLENGES TO IMPLEMENTATION
Reversing the tide
Liberal oxygen administration continues to be practiced in many health care settings. The main challenges to implementing the conclusions of this study are these pervasive practices.
ACKNOWLEDGMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 60-year-old woman who is generally healthy except for a history of recurrent urinary tract infections presents to the emergency department with fever, hypotension, and altered mental status, meeting criteria for septic shock. During her resuscitation, supplemental oxygen is administered. Standard treatment calls for a minimum SpO2 (saturation of peripheral oxygen) > 90%. What should your SpO2 goal be?
Use of supplemental oxygen in the acute care of the critically ill adult is a common practice in pre-hospital, emergency department (ED), and hospitalized settings.2,3 Despite their prevalence, guidelines about appropriate oxygen concentration and target SpO2 levels are often conflicting or vague.3-5
Excessive oxygen supplementation in acute illness may be harmful and cause increased risk of hypercapnic respiratory failure, delayed recognition of clinical deterioration, and oxygen toxicity.2,6 The perception of oxygen safety persists despite these findings, and it likely contributes to the ongoing practice of liberal oxygen supplementation in the acutely ill adult.2,7,8
STUDY SUMMARY
Liberal supplemental O2 linked to increased mortality
The Improving Oxygen Therapy in Acute illness (IOTA) study was a systematic review and meta-analysis of 25 randomized controlled trials (RCTs) that compared liberal vs conservative oxygen strategies for acutely ill adults (N = 16,037; median age = 64 years; range = 28-76 years). Patients with sepsis, critical illness, stroke, trauma, myocardial infarction, or cardiac arrest, and patients who had emergency surgery were included. Studies were excluded if they involved patients who had chronic respiratory illness or psychiatric diseases, were receiving extracorporeal membrane oxygenation, were undergoing elective surgeries, were being treated with hyperbaric oxygen therapy, or were pregnant.
The outcomes studied were mortality (in-hospital, at 30 days, and at the longest follow-up) and morbidity (disability measured by the modified Rankin Scale at longest follow-up, risk of hospital-acquired pneumonia, risk of any hospital-acquired infection, and hospital length of stay).
Liberal supplemental oxygen, above an SpO2 range of 94% to 96%, increased mortality during inpatient stays (relative risk [RR] = 1.21; 95% confidence interval [CI], 1.03-1.43; N = 15,071), at 30 days (RR = 1.14; 95% CI, 1.01-1.29; N = 15,053), and at longest follow-up (RR = 1.10; 95% CI, 1.00-1.20; N = 15,754; median = 90 days; range = 14,365 days). There was no difference in morbidity outcomes between groups.
While it’s difficult to define a specific target SpO2 range, the number needed to harm when using a liberal oxygen approach (SpO2 > 96%) resulting in 1 death was 71 (95% CI, 37-1000).
Continue to: WHAT'S NEW
WHAT’S NEW
High-quality evidence points to the dangers of liberal O2 therapy
This comprehensive meta-analysis is the first high-quality evidence to suggest that liberal use of oxygen in acutely ill adults above a specific SpO2 level increases all-cause mortality. Previous small RCTs and observational studies have examined the effect of liberal oxygen only on specific presenting conditions, thus making more generalizable conclusions challenging.9-12
CAVEATS
Varied definitions of “liberal” and “conservative”
This review included studies with variable ranges of SpO2 defined as liberal vs conservative supplementation. However, in all of these, SpO2 above 96% was correlated with unfavorable outcomes.
The study excluded 2 potentially important patient groups: patients with chronic respiratory diseases and pregnant patients. Increased oxygen supplementation in patients with chronic respiratory diseases in noncritical settings has been shown to be deleterious.13-15 While this study does not address the issue of oxygen supplementation in acutely ill patients with chronic respiratory disease, use should be considered with caution. The results from this study may not be generalizable to women who are pregnant.
CHALLENGES TO IMPLEMENTATION
Reversing the tide
Liberal oxygen administration continues to be practiced in many health care settings. The main challenges to implementing the conclusions of this study are these pervasive practices.
ACKNOWLEDGMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Chu DK, Kim LH, Young PJ, et al. Mortality and morbidity in acutely ill adults treated with liberal versus conservative oxygen therapy (IOTA): a systematic review and meta-analysis. Lancet. 2018;391:1693-1705.
2. Hale KE, Gavin C, O’Driscoll BR. Audit of oxygen use in emergency ambulances and in a hospital emergency department. Emerg Med J. 2008;25:773-776.
3. O’Driscoll BR, Howard LS, Earis J, et al. BTS guideline for oxygen use in adults in healthcare and emergency settings. Thorax. 2017;72(suppl 1):ii1-ii90.
4. Kallstrom TJ, American Association for Respiratory Care. AARC Clinical Practice Guideline: oxygen therapy for adults in the acute care facility—2002 revision and update. Respir Care. 2002;47:717-720.
5. Henry TD, Torbati S. Oxygen for ACS: too much, too little, or just right? May 15, 2017. https://www.acc.org/latest-in-cardiology/articles/2017/05/15/08/34/oxygen-for-acs. Accessed October 1, 2019.
6. Hafner S, Beloncle F, Koch A, et al. Hyperoxia in intensive care, emergency, and peri-operative medicine: Dr. Jekyll or Mr. Hyde? A 2015 update. Ann Intensive Care. 2015;5:42.
7. Helmerhorst HJ, Schultz MJ, van der Voort PH, et al. Self-reported attitudes versus actual practice of oxygen therapy by ICU physicians and nurses. Ann Intensive Care. 2014;4:23.
8. Kelly CA, Lynes D, O’Brien MR, et al. A wolf in sheep’s clothing? Patients’ and healthcare professionals’ perceptions of oxygen therapy: an interpretative phenomenological analysis. Clin Respir J. 2018;12:616-632.
9. Meyhoff CS, Wetterslev J, Jorgensen LN, et al. Effect of high perioperative oxygen fraction on surgical site infection and pulmonary complications after abdominal surgery: the PROXI randomized clinical trial. JAMA. 2009;302:1543-1550.
10. Stub D, Smith K, Bernard S, et al. A randomized controlled trial on oxygen therapy in acute myocardial infarction Air Verses Oxygen in Myocardial infarction study (AVOID Study). Am Heart J. 2012;163:339-345.E1.
11. Girardis M, Busani S, Damiani E, et al. Effect of conservative vs conventional oxygen therapy on mortality among patients in an intensive care unit: the oxygen-ICU randomized clinical trial. JAMA. 2016;316:1583-1589.
12. Helmerhorst HJ, Roos-Blom MJ, van Westerloo DJ, et al. Association between arterial hyperoxia and outcome in subsets of critical illness: a systematic review, meta-analysis, and meta-regression of cohort studies. Crit Care Med. 2015;43:1508-1519.
13. Pope JV, Jones AE, Gaieski DF, et al. Multicenter study of central venous oxygen saturation (ScvO(2)) as a predictor of mortality in patients with sepsis. Ann Emerg Med. 2010;55:40-46.E1.
14. Kim V, Benditt JO, Wise RA, et al. Oxygen therapy in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2008;5:513-518.
15. Austin MA, Wills KE, Blizzard L, et al. Effect of high flow oxygen on mortality in chronic obstructive pulmonary disease patients in prehospital setting: randomised controlled trial. BMJ. 2010;341:C5462.
1. Chu DK, Kim LH, Young PJ, et al. Mortality and morbidity in acutely ill adults treated with liberal versus conservative oxygen therapy (IOTA): a systematic review and meta-analysis. Lancet. 2018;391:1693-1705.
2. Hale KE, Gavin C, O’Driscoll BR. Audit of oxygen use in emergency ambulances and in a hospital emergency department. Emerg Med J. 2008;25:773-776.
3. O’Driscoll BR, Howard LS, Earis J, et al. BTS guideline for oxygen use in adults in healthcare and emergency settings. Thorax. 2017;72(suppl 1):ii1-ii90.
4. Kallstrom TJ, American Association for Respiratory Care. AARC Clinical Practice Guideline: oxygen therapy for adults in the acute care facility—2002 revision and update. Respir Care. 2002;47:717-720.
5. Henry TD, Torbati S. Oxygen for ACS: too much, too little, or just right? May 15, 2017. https://www.acc.org/latest-in-cardiology/articles/2017/05/15/08/34/oxygen-for-acs. Accessed October 1, 2019.
6. Hafner S, Beloncle F, Koch A, et al. Hyperoxia in intensive care, emergency, and peri-operative medicine: Dr. Jekyll or Mr. Hyde? A 2015 update. Ann Intensive Care. 2015;5:42.
7. Helmerhorst HJ, Schultz MJ, van der Voort PH, et al. Self-reported attitudes versus actual practice of oxygen therapy by ICU physicians and nurses. Ann Intensive Care. 2014;4:23.
8. Kelly CA, Lynes D, O’Brien MR, et al. A wolf in sheep’s clothing? Patients’ and healthcare professionals’ perceptions of oxygen therapy: an interpretative phenomenological analysis. Clin Respir J. 2018;12:616-632.
9. Meyhoff CS, Wetterslev J, Jorgensen LN, et al. Effect of high perioperative oxygen fraction on surgical site infection and pulmonary complications after abdominal surgery: the PROXI randomized clinical trial. JAMA. 2009;302:1543-1550.
10. Stub D, Smith K, Bernard S, et al. A randomized controlled trial on oxygen therapy in acute myocardial infarction Air Verses Oxygen in Myocardial infarction study (AVOID Study). Am Heart J. 2012;163:339-345.E1.
11. Girardis M, Busani S, Damiani E, et al. Effect of conservative vs conventional oxygen therapy on mortality among patients in an intensive care unit: the oxygen-ICU randomized clinical trial. JAMA. 2016;316:1583-1589.
12. Helmerhorst HJ, Roos-Blom MJ, van Westerloo DJ, et al. Association between arterial hyperoxia and outcome in subsets of critical illness: a systematic review, meta-analysis, and meta-regression of cohort studies. Crit Care Med. 2015;43:1508-1519.
13. Pope JV, Jones AE, Gaieski DF, et al. Multicenter study of central venous oxygen saturation (ScvO(2)) as a predictor of mortality in patients with sepsis. Ann Emerg Med. 2010;55:40-46.E1.
14. Kim V, Benditt JO, Wise RA, et al. Oxygen therapy in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2008;5:513-518.
15. Austin MA, Wills KE, Blizzard L, et al. Effect of high flow oxygen on mortality in chronic obstructive pulmonary disease patients in prehospital setting: randomised controlled trial. BMJ. 2010;341:C5462.
PRACTICE CHANGER
Do not use liberal oxygen therapy (SpO2 > 96%) in acutely ill adults, as it is associated with increased all-cause mortality.1
STRENGTH OF RECOMMENDATION
A: Based on a systematic review and meta-analysis of 25 randomized controlled trials.
Chu DK, Kim LH, Young PJ, et al. Mortality and morbidity in acutely ill adults treated with liberal versus conservative oxygen therapy (IOTA): a systematic review and meta-analysis. Lancet. 2018;391:1693-1705.
Best timing for measuring orthostatic vital signs?
ILLUSTRATIVE CASE
A 54-year-old woman with a history of hypertension presents with a chief complaint of dizziness. You require an assessment of orthostatic vital signs to proceed. In your busy clinical practice, when should assessment take place to be most useful?
Orthostatic hypotension (OH) is defined as a postural reduction in systolic blood pressure (BP) of ≥ 20 mm Hg or diastolic BP of ≥ 10 mm Hg, measured within 3 minutes of rising from supine to standing. This definition is based on consensus guidelines from the American Academy of Neurology and the American Autonomic Society2 and has been upheld by European guidelines.3
The prevalence of OH is approximately 6% in the general population, with estimates ranging from 10% to 55% in older adults.4 Etiology is often multifactorial; causes may be neurogenic (mediated by autonomic failure as in Parkinson’s disease, multiple system atrophy, or diabetic neuropathy), non-neurogenic (related to medications or hypovolemia), or idiopathic.
It’s important to identify OH because of its associated increase in morbidities, such as an increased risk of falls (hazard ratio [HR] = 1.5),5 coronary heart disease (HR = 1.3), stroke (HR = 1.2), and all-cause mortality (HR = 1.4).6 Treatments include physical maneuvers (getting up slowly, leg crossing, and muscle clenching), increased salt and water intake, compression stockings, the addition of medications (such as fludrocortisone or midodrine), and the avoidance of other medications (such as benzodiazepines and diuretics).
The guideline-recommended 3-minute delay in assessment can be impractical in a busy clinical setting. Using data from the Atherosclerosis Risk in Communities (ARIC) study, investigators correlated the timing of measurements of postural change in BP with long-term adverse outcomes.1
STUDY SUMMARY
Early vs late OH assessment in middle-aged adults
The ARIC study is a longitudinal, prospective, cohort study of almost 16,000 adults followed since 1987. Juraschek et al1 assessed the optimal time to identify OH and its association with the adverse clinical outcomes of fall, fracture, syncope, motor vehicle crash, and mortality. The researchers sought to discover whether BP measurements determined immediately after standing predict adverse events as well as BP measurements taken closer to 3 minutes.
Study participants were between the ages of 45 and 64 years (mean 54 years), and 26% were black and 54% were female. They lived in 4 different US communities. The researchers excluded patients with missing OH assessments or other relevant cohort or historical data, leaving a cohort of 11,429 subjects.
Continue to: As part of their...
As part of their enrollment into the ARIC study, subjects had their BP measurements taken 2 to 5 times in the lying position (90% of participants had ≥ 4 measurements) and after standing (91% participants had ≥ 4 measurements) using a programmable automatic BP cuff. All 5 standing BP measurements (taken at a mean of 28, 53, 76, 100, and 116 seconds after standing) were measured for 7385 out of 11,429 (64.6%) participants. Subjects were asked if he or she “usually gets dizzy on standing up.”
Researchers determined the association between OH and postural change in systolic BP or postural change in diastolic BP with history of dizziness after standing. They also determined the incidence of falls, fracture, syncope, motor vehicle crash, and mortality via a review of hospitalizations and billing for Medicaid and Medicare services. Subjects were followed for a median of 23 years.
Results
Of the entire cohort, 1138 (10%) reported dizziness on standing. Only OH identified at the first BP measurement (mean 28 secs) was associated with a history of dizziness upon standing (odds ratio [OR] = 1.49; 95% confidence interval [CI], 1.18-1.89). Also, it was associated with the highest incidence of fracture, syncope, and death (18.9, 17, and 31.4 per 1000 person-years, respectively).
After adjusting for age, sex, and multiple other cardiovascular risk factors, the risk of falls was significantly associated with OH at BP measurements 1 to 4, but was most strongly associated with BP measurement 2 (taken at a mean of 53 secs after standing) (HR = 1.29; 95% CI, 1.12-1.49), which translates to 13.2 falls per 1000 patient-years. Fracture was associated with OH at measurements 1 (HR = 1.16; 95% CI, 1.01-1.34) and 2 (HR = 1.14; 95% CI, 1.01-1.29). Motor vehicle crashes were associated only with BP measurement 2 (HR = 1.43; 95% CI, 1.04-1.96). Finally, risk of syncope and risk of death were statistically associated with the presence of OH at all 5 BP measurements.
WHAT’S NEW
Earlier OH assessments are more informative than late ones
This study found OH identified within 1 minute of standing to be more clinically meaningful than OH identified after 1 minute. Also, the findings reinforce the relationship between OH and adverse events, including injury and overall mortality. Evaluation for OH performed only at 3 minutes may miss symptomatic OH.
Continue to: CAVEATS
CAVEATS
Could a healthy population skew the results?
The population in this study was relatively healthy, with a lower prevalence of diabetes and coronary artery disease than the general population. While there is no reason to expect detection of OH to differ in a population with more comorbidities, the possibility exists.
If OH is not identified in < 1 minute of standing, standard OH evaluation within 3 minutes after standing should be performed, as OH identified at any time point after standing is associated with adverse events and increased mortality.
This study did not address the effects of medical intervention for OH on injury or mortality. Also, whether OH is the direct cause of the adverse outcomes or a marker for other disease is unknown.
CHALLENGES TO IMPLEMENTATION
A change to protocols and guidelines
Although none were noted, any change in practice requires updating clinical protocols and guidelines, which can take time.
ACKNOWLEDGMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Juraschek SP, Daya N, Rawlings AM, et al. Association of history of dizziness and long-term adverse outcomes with early vs later orthostatic hypotension assessment times in middle-aged adults. JAMA Internal Med. 2017;177:1316-1323.
2. The Consensus Committee of the American Autonomic Society and the American Academy of Neurology. Consensus statement on the definition of orthostatic hypotension, pure autonomic failure, and multiple system atrophy. Neurology. 1996;46:1470.
3. Lahrmann H, Cortelli P, Hilz M, et al. EFNS guidelines on the diagnosis and management of orthostatic hypotension. Eur J Neurol. 2006;13:930-936.
4. Freeman R, Wieling W, Axelrod FB, et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res. 2011;21:69-72.
5. Rutan GH, Hermanson B, Bild DE, et al. Orthostatic hypotension in older adults: the Cardiovascular Health Study. Hypertension. 1992;19(6 Pt 1):508-519.
6. Xin W, Lin Z, Mi S. Orthostatic hypotension and mortality risk: a meta-analysis of cohort studies. Heart. 2014;100:406-413.
ILLUSTRATIVE CASE
A 54-year-old woman with a history of hypertension presents with a chief complaint of dizziness. You require an assessment of orthostatic vital signs to proceed. In your busy clinical practice, when should assessment take place to be most useful?
Orthostatic hypotension (OH) is defined as a postural reduction in systolic blood pressure (BP) of ≥ 20 mm Hg or diastolic BP of ≥ 10 mm Hg, measured within 3 minutes of rising from supine to standing. This definition is based on consensus guidelines from the American Academy of Neurology and the American Autonomic Society2 and has been upheld by European guidelines.3
The prevalence of OH is approximately 6% in the general population, with estimates ranging from 10% to 55% in older adults.4 Etiology is often multifactorial; causes may be neurogenic (mediated by autonomic failure as in Parkinson’s disease, multiple system atrophy, or diabetic neuropathy), non-neurogenic (related to medications or hypovolemia), or idiopathic.
It’s important to identify OH because of its associated increase in morbidities, such as an increased risk of falls (hazard ratio [HR] = 1.5),5 coronary heart disease (HR = 1.3), stroke (HR = 1.2), and all-cause mortality (HR = 1.4).6 Treatments include physical maneuvers (getting up slowly, leg crossing, and muscle clenching), increased salt and water intake, compression stockings, the addition of medications (such as fludrocortisone or midodrine), and the avoidance of other medications (such as benzodiazepines and diuretics).
The guideline-recommended 3-minute delay in assessment can be impractical in a busy clinical setting. Using data from the Atherosclerosis Risk in Communities (ARIC) study, investigators correlated the timing of measurements of postural change in BP with long-term adverse outcomes.1
STUDY SUMMARY
Early vs late OH assessment in middle-aged adults
The ARIC study is a longitudinal, prospective, cohort study of almost 16,000 adults followed since 1987. Juraschek et al1 assessed the optimal time to identify OH and its association with the adverse clinical outcomes of fall, fracture, syncope, motor vehicle crash, and mortality. The researchers sought to discover whether BP measurements determined immediately after standing predict adverse events as well as BP measurements taken closer to 3 minutes.
Study participants were between the ages of 45 and 64 years (mean 54 years), and 26% were black and 54% were female. They lived in 4 different US communities. The researchers excluded patients with missing OH assessments or other relevant cohort or historical data, leaving a cohort of 11,429 subjects.
Continue to: As part of their...
As part of their enrollment into the ARIC study, subjects had their BP measurements taken 2 to 5 times in the lying position (90% of participants had ≥ 4 measurements) and after standing (91% participants had ≥ 4 measurements) using a programmable automatic BP cuff. All 5 standing BP measurements (taken at a mean of 28, 53, 76, 100, and 116 seconds after standing) were measured for 7385 out of 11,429 (64.6%) participants. Subjects were asked if he or she “usually gets dizzy on standing up.”
Researchers determined the association between OH and postural change in systolic BP or postural change in diastolic BP with history of dizziness after standing. They also determined the incidence of falls, fracture, syncope, motor vehicle crash, and mortality via a review of hospitalizations and billing for Medicaid and Medicare services. Subjects were followed for a median of 23 years.
Results
Of the entire cohort, 1138 (10%) reported dizziness on standing. Only OH identified at the first BP measurement (mean 28 secs) was associated with a history of dizziness upon standing (odds ratio [OR] = 1.49; 95% confidence interval [CI], 1.18-1.89). Also, it was associated with the highest incidence of fracture, syncope, and death (18.9, 17, and 31.4 per 1000 person-years, respectively).
After adjusting for age, sex, and multiple other cardiovascular risk factors, the risk of falls was significantly associated with OH at BP measurements 1 to 4, but was most strongly associated with BP measurement 2 (taken at a mean of 53 secs after standing) (HR = 1.29; 95% CI, 1.12-1.49), which translates to 13.2 falls per 1000 patient-years. Fracture was associated with OH at measurements 1 (HR = 1.16; 95% CI, 1.01-1.34) and 2 (HR = 1.14; 95% CI, 1.01-1.29). Motor vehicle crashes were associated only with BP measurement 2 (HR = 1.43; 95% CI, 1.04-1.96). Finally, risk of syncope and risk of death were statistically associated with the presence of OH at all 5 BP measurements.
WHAT’S NEW
Earlier OH assessments are more informative than late ones
This study found OH identified within 1 minute of standing to be more clinically meaningful than OH identified after 1 minute. Also, the findings reinforce the relationship between OH and adverse events, including injury and overall mortality. Evaluation for OH performed only at 3 minutes may miss symptomatic OH.
Continue to: CAVEATS
CAVEATS
Could a healthy population skew the results?
The population in this study was relatively healthy, with a lower prevalence of diabetes and coronary artery disease than the general population. While there is no reason to expect detection of OH to differ in a population with more comorbidities, the possibility exists.
If OH is not identified in < 1 minute of standing, standard OH evaluation within 3 minutes after standing should be performed, as OH identified at any time point after standing is associated with adverse events and increased mortality.
This study did not address the effects of medical intervention for OH on injury or mortality. Also, whether OH is the direct cause of the adverse outcomes or a marker for other disease is unknown.
CHALLENGES TO IMPLEMENTATION
A change to protocols and guidelines
Although none were noted, any change in practice requires updating clinical protocols and guidelines, which can take time.
ACKNOWLEDGMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 54-year-old woman with a history of hypertension presents with a chief complaint of dizziness. You require an assessment of orthostatic vital signs to proceed. In your busy clinical practice, when should assessment take place to be most useful?
Orthostatic hypotension (OH) is defined as a postural reduction in systolic blood pressure (BP) of ≥ 20 mm Hg or diastolic BP of ≥ 10 mm Hg, measured within 3 minutes of rising from supine to standing. This definition is based on consensus guidelines from the American Academy of Neurology and the American Autonomic Society2 and has been upheld by European guidelines.3
The prevalence of OH is approximately 6% in the general population, with estimates ranging from 10% to 55% in older adults.4 Etiology is often multifactorial; causes may be neurogenic (mediated by autonomic failure as in Parkinson’s disease, multiple system atrophy, or diabetic neuropathy), non-neurogenic (related to medications or hypovolemia), or idiopathic.
It’s important to identify OH because of its associated increase in morbidities, such as an increased risk of falls (hazard ratio [HR] = 1.5),5 coronary heart disease (HR = 1.3), stroke (HR = 1.2), and all-cause mortality (HR = 1.4).6 Treatments include physical maneuvers (getting up slowly, leg crossing, and muscle clenching), increased salt and water intake, compression stockings, the addition of medications (such as fludrocortisone or midodrine), and the avoidance of other medications (such as benzodiazepines and diuretics).
The guideline-recommended 3-minute delay in assessment can be impractical in a busy clinical setting. Using data from the Atherosclerosis Risk in Communities (ARIC) study, investigators correlated the timing of measurements of postural change in BP with long-term adverse outcomes.1
STUDY SUMMARY
Early vs late OH assessment in middle-aged adults
The ARIC study is a longitudinal, prospective, cohort study of almost 16,000 adults followed since 1987. Juraschek et al1 assessed the optimal time to identify OH and its association with the adverse clinical outcomes of fall, fracture, syncope, motor vehicle crash, and mortality. The researchers sought to discover whether BP measurements determined immediately after standing predict adverse events as well as BP measurements taken closer to 3 minutes.
Study participants were between the ages of 45 and 64 years (mean 54 years), and 26% were black and 54% were female. They lived in 4 different US communities. The researchers excluded patients with missing OH assessments or other relevant cohort or historical data, leaving a cohort of 11,429 subjects.
Continue to: As part of their...
As part of their enrollment into the ARIC study, subjects had their BP measurements taken 2 to 5 times in the lying position (90% of participants had ≥ 4 measurements) and after standing (91% participants had ≥ 4 measurements) using a programmable automatic BP cuff. All 5 standing BP measurements (taken at a mean of 28, 53, 76, 100, and 116 seconds after standing) were measured for 7385 out of 11,429 (64.6%) participants. Subjects were asked if he or she “usually gets dizzy on standing up.”
Researchers determined the association between OH and postural change in systolic BP or postural change in diastolic BP with history of dizziness after standing. They also determined the incidence of falls, fracture, syncope, motor vehicle crash, and mortality via a review of hospitalizations and billing for Medicaid and Medicare services. Subjects were followed for a median of 23 years.
Results
Of the entire cohort, 1138 (10%) reported dizziness on standing. Only OH identified at the first BP measurement (mean 28 secs) was associated with a history of dizziness upon standing (odds ratio [OR] = 1.49; 95% confidence interval [CI], 1.18-1.89). Also, it was associated with the highest incidence of fracture, syncope, and death (18.9, 17, and 31.4 per 1000 person-years, respectively).
After adjusting for age, sex, and multiple other cardiovascular risk factors, the risk of falls was significantly associated with OH at BP measurements 1 to 4, but was most strongly associated with BP measurement 2 (taken at a mean of 53 secs after standing) (HR = 1.29; 95% CI, 1.12-1.49), which translates to 13.2 falls per 1000 patient-years. Fracture was associated with OH at measurements 1 (HR = 1.16; 95% CI, 1.01-1.34) and 2 (HR = 1.14; 95% CI, 1.01-1.29). Motor vehicle crashes were associated only with BP measurement 2 (HR = 1.43; 95% CI, 1.04-1.96). Finally, risk of syncope and risk of death were statistically associated with the presence of OH at all 5 BP measurements.
WHAT’S NEW
Earlier OH assessments are more informative than late ones
This study found OH identified within 1 minute of standing to be more clinically meaningful than OH identified after 1 minute. Also, the findings reinforce the relationship between OH and adverse events, including injury and overall mortality. Evaluation for OH performed only at 3 minutes may miss symptomatic OH.
Continue to: CAVEATS
CAVEATS
Could a healthy population skew the results?
The population in this study was relatively healthy, with a lower prevalence of diabetes and coronary artery disease than the general population. While there is no reason to expect detection of OH to differ in a population with more comorbidities, the possibility exists.
If OH is not identified in < 1 minute of standing, standard OH evaluation within 3 minutes after standing should be performed, as OH identified at any time point after standing is associated with adverse events and increased mortality.
This study did not address the effects of medical intervention for OH on injury or mortality. Also, whether OH is the direct cause of the adverse outcomes or a marker for other disease is unknown.
CHALLENGES TO IMPLEMENTATION
A change to protocols and guidelines
Although none were noted, any change in practice requires updating clinical protocols and guidelines, which can take time.
ACKNOWLEDGMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Juraschek SP, Daya N, Rawlings AM, et al. Association of history of dizziness and long-term adverse outcomes with early vs later orthostatic hypotension assessment times in middle-aged adults. JAMA Internal Med. 2017;177:1316-1323.
2. The Consensus Committee of the American Autonomic Society and the American Academy of Neurology. Consensus statement on the definition of orthostatic hypotension, pure autonomic failure, and multiple system atrophy. Neurology. 1996;46:1470.
3. Lahrmann H, Cortelli P, Hilz M, et al. EFNS guidelines on the diagnosis and management of orthostatic hypotension. Eur J Neurol. 2006;13:930-936.
4. Freeman R, Wieling W, Axelrod FB, et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res. 2011;21:69-72.
5. Rutan GH, Hermanson B, Bild DE, et al. Orthostatic hypotension in older adults: the Cardiovascular Health Study. Hypertension. 1992;19(6 Pt 1):508-519.
6. Xin W, Lin Z, Mi S. Orthostatic hypotension and mortality risk: a meta-analysis of cohort studies. Heart. 2014;100:406-413.
1. Juraschek SP, Daya N, Rawlings AM, et al. Association of history of dizziness and long-term adverse outcomes with early vs later orthostatic hypotension assessment times in middle-aged adults. JAMA Internal Med. 2017;177:1316-1323.
2. The Consensus Committee of the American Autonomic Society and the American Academy of Neurology. Consensus statement on the definition of orthostatic hypotension, pure autonomic failure, and multiple system atrophy. Neurology. 1996;46:1470.
3. Lahrmann H, Cortelli P, Hilz M, et al. EFNS guidelines on the diagnosis and management of orthostatic hypotension. Eur J Neurol. 2006;13:930-936.
4. Freeman R, Wieling W, Axelrod FB, et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res. 2011;21:69-72.
5. Rutan GH, Hermanson B, Bild DE, et al. Orthostatic hypotension in older adults: the Cardiovascular Health Study. Hypertension. 1992;19(6 Pt 1):508-519.
6. Xin W, Lin Z, Mi S. Orthostatic hypotension and mortality risk: a meta-analysis of cohort studies. Heart. 2014;100:406-413.
PRACTICE CHANGER
Measure orthostatic vital signs within 1 minute of standing to most accurately correlate dizziness with long-term adverse outcomes. 1
STRENGTH OF RECOMMENDATION
B: Based on a single, high-quality, prospective cohort study with patient-oriented outcomes and good follow-up.
Juraschek SP, Daya N, Rawlings AM, et al. Association of history of dizziness and long-term adverse outcomes with early vs later orthostatic hypotension assessment times in middle-aged adults. JAMA Intern Med. 2017;177:1316-1323.