User login
Hypertriglyceridemia: Identifying Secondary Causes
Screening for cardiovascular (CV) risk often includes a routine serum fasting lipid profile. However, with the focus on LDL cholesterol, triglyceride measurement is frequently overlooked. Yet this element of the lipid profile is particularly important, given its strong association with not only atherosclerotic coronary heart disease but also pancreatitis.
Hypertriglyceridemia is defined as a serum triglyceride level that exceeds 150 mg/dL. In the US, an estimated 25% of patients have hypertriglyceridemia.1 Of these, 33.1% have “borderline high” triglyceride levels (150 to 199 mg/dL), 17.8% have “high” levels (200 to 499 mg/dL), and 1.7% have “very high” levels (> 500 mg/dL).1,2
Most of the time, hypertriglyceridemia is caused (or at least exacerbated) by underlying etiology. The best way to identify and manage these secondary causes is through a systematic approach.
CONSIDER THE EVIDENCE
For mild to moderately elevated (borderline high) triglyceride levels, our reflex reaction may be to recommend a triglyceride-lowering medication, such as fenofibrate. But this may not be the best answer. Although there is increasing evidence of an independent association between elevated triglyceride levels and CV risk, it remains unclear whether targeting them specifically can reduce that risk.3
In well-designed, peer-reviewed clinical trials, statins have been shown to reduce CV risk in patients with known cardiovascular disease (CVD) and those at high risk for CVD, as well as in primary prevention. However, these trials also suggest that significant residual CV risk remains after statin therapy.4
Several trials have attempted to prove residual risk reduction following combination therapy including statins—with inconclusive results:
ACCORD: Fenofibrate showed no overall macrovascular benefit when added to a statin in patients with type 2 diabetes and a triglyceride level < 204 mg/dL.3,5
AIM-HIGH: There was a 25% reduction in triglyceride levels when niacin was added to a regimen of a statin +/- ezetimibe, with an aggressive LDL treatment target (40 to 80 mg/dL). But the study was stopped early due to the lack of expected reduction in CVD events.4,6
JELIS: A reduction in major CV events was seen with 1,800 mg/d of eicosapentaenoic acid (EPA) supplementation plus a low-dose statin, compared to statin monotherapy. However, there was minimal change in triglyceride levels, leading the researchers to hypothesize that multiple mechanisms—such as decreasing oxidative stress, platelet aggregation, plaque formation and stabilization—contributed to the outcome.4,7
Informed by the JELIS results, the much-anticipated REDUCE-IT trial is currently in progress to address the lingering question of whether combination therapy can reduce residual CV risk. In this trial, EPA omega-3 fatty acid is being added to the regimen of statin-treated patients with persistently elevated triglycerides. Results are expected in 2017 to 2018.8
Remember that a triglyceride level of 150 mg/dL is a parameter—it does not represent a therapeutic target. There is insufficient evidence that treating to this level improves CV risk beyond LDL target recommendations.7
The National Lipid Association Expert Panel’s consensus view is that non-HDL is a better primary target than triglycerides alone or LDL. Using non-HDL as a target for intervention also simplifies the management of patients with high triglycerides (200 to 499 mg/dL). The non-HDL goal is considered to be 30 mg/dL greater than the LDL target. For patients with diabetes and those with CVD, the individualized non-HDL targets are 130 mg/dL and 100 mg/dL, respectively.9
REVIEW THE MEDICATION LIST
Several commonly used medications, including ß-blockers and thiazide diuretics, can increase triglyceride levels.10 Other medications with exacerbating effects on triglycerides include corticosteroids, retrovirals, immunosuppressants, retinoids, and some antipsychotics.10 Bile acid sequestrants (eg, colesevelam) should be avoided in patients with elevated triglycerides (> 200 mg/dL).7
In women, oral estrogen (ie, menopausal hormone replacement and oral birth control) can greatly exacerbate triglyceride levels, making transdermal delivery a better option. Tamoxifen, the hormonal medication used for breast cancer prophylaxis, can also increase triglyceride levels.11
LOOK FOR UNDERLYING CONDITIONS
Among those to consider: Hypothyroidism is common and easily ruled out by a simple blood test. Nephrotic syndrome should be ruled out, particularly in patients with concomitant renal dysfunction and peripheral edema, by checking a random urine protein-to-creatinine ratio or 24-hour urine for protein. Other factors that should be explored because of their potential effect on lipid metabolism include obesity and excessive intake of sugary beverages (ie, soda, fruit juice) and alcohol.11
High triglyceride levels occurring with low HDL are characteristic of insulin resistance and concerning for metabolic syndrome and/or polycystic ovarian syndrome.3,12 Often, patients will have underlying prediabetes (fasting glucose ≥ 100 mg/dL or random glucose ≥ 140 mg/dL with an A1C > 5.7%13) or covert type 2 diabetes. Another underdiagnosed but very common condition, obstructive sleep apnea, can greatly affect insulin sensitivity and has been associated with lipid abnormalities and metabolic syndrome.14
EXAMINE YOUR PATIENT
The physical exam is an essential component of assessment for patients with high triglycerides. As discussed, elevated triglycerides and low HDL are hallmarks for insulin resistance. As triglyceride levels are affected by obesity and body fat distribution, measuring BMI and assessing for visceral adiposity are an important part of the physical exam.4
The physical exam may also yield dermatologic clues, such as skin tags or acanthosis nigricans, a dark, velvety lesion usually found on the posterior and lateral neck creases, axillae, groin, and elbows.13 In rare cases—usually those with genetic involvement from a familial lipid metabolism disorder—patients may exhibit xanthomas. These cutaneous, lipid-rich lesions can appear as flat, yellowish plaques on various parts of the body, such as the eyelids (xanthelasma) or tendons of the hands, feet, and heels. Widespread, eruptive xanthomas, which manifest as pruritic pink papules with creamy centers, are associated with severe emergent triglyceride elevation and pancreatitis.10
CONSIDER NONPHARMACOLOGIC MANAGEMENT
In mild to moderate hypertriglyceridemia, intensive lifestyle changes are considered firstline therapy. Weight loss is recommended in obese patients; a 5% to 10% reduction in body weight can lower triglycerides by 20%.15
A quick 24-hour diet recall, including beverages, is helpful for identifying key issues. The goal should be to reduce carbohydrates—in particular, simple, high glycemic index, processed foods—as well as total and saturated fats. A substantial problem in our population is the consumption of high-fructose beverages and fruit juices. Referral to a dietitian can be very helpful, not only for initial meal planning but also for continuing counseling on successful long-term weight loss and maintenance.
Exercise is also very helpful for improving lipid parameters. A daily minimum of 30 to 60 minutes of intermittent aerobic exercise or mild resistance exercise has been shown to reduce triglyceride levels.10
PRESCRIBE APPROPRIATELY
The most important indication for treatment of hypertriglyceridemia is reduction of CVD risk. However, in patients with very high triglyceride levels (> 500 mg/dL), the goal is to decrease risk for life-threatening pancreatitis.15 Lipid-lowering medications and dietary restrictions should be promptly employed.
There are medications, as discussed earlier, that specifically lower triglycerides. Fibrates offer the most robust decrease, with a 20% to 50% reduction in triglyceride levels. Fenofibrate is considered a safer option when used in combination with a statin, due to the risk for significant muscle toxicity with gemfibrozil. There is some evidence that adding a fibrate may actually increase risk for pancreatitis; since this risk is otherwise low in patients with mild to moderate triglyceride elevation, the addition of a fibrate to their regimen should be avoided.3
Statins are the drug of choice when CV risk reduction is the goal (for patients with hypertriglyceridemia < 500 mg/dL). In addition to lowering LDL, statins can reduce triglycerides by 7% to 30%, depending on the dose.15
Other triglyceride-lowering medications include omega-3 fatty acids and niacin preparations. Prescription-strength omega-3 fatty acids have been found to lower serum triglyceride levels by 50% or more; the newest preparation, icosapent ethyl, demonstrated up to 45% reduction without significant effect on LDL levels.3 (Other preparations have been shown to substantially increase LDL in many cases.) Niacin (1,500 to 2,000 mg/d) can decrease triglycerides by 15% to 25%. However, it is no longer recommended for CV risk reduction; recent data indicate it may increase stroke risk when used in combination with statins.3,10 In April 2016, the FDA revoked its approval of the co-administration of niacin and fenofibrate with statin therapy, due to a lack of CV benefit.16
Other secondline options to consider for patients with insulin resistance or diabetes are metformin and pioglitazone. These medications have been shown to improve insulin sensitivity and decrease LDL and triglycerides in patients with prediabetes. Pioglitazone has proven beneficial in the treatment of steatohepatitis.17 Insulin is an excellent rapid triglyceride-lowering agent for patients with diabetes. It is important to reinforce that reduction of glucose is a key component in reduction of triglyceride levels.3
CONCLUSION
Hypertriglyceridemia is a complex condition that requires individualized and comprehensive management strategies. Clinicians must be able to identify and address secondary causes. Treatment options should be tailored to decrease CV and pancreatitis risk, and medication recommendations should be evidenced based and carefully selected to mitigate potential adverse effects. Patients should receive education and lifestyle management support to help motivate and equip them to employ strategies to improve their health.
1. CDC. Trends in elevated triglyceride in adults: United States, 2001-2012. www.cdc.gov/nchs/data/databriefs/db198.pdf. Accessed December 27, 2016.
2. Maki KC, Bays HE, Dicklin MR. Treatment options for the management of hypertriglyceridemia: strategies based on the best-available evidence. J Clin Lipidol. 2012;6(5):413-426.
3. Rosenson RS. Approach to the patient with hypertriglyceridemia. www.uptodate.com/contents/approach-to-the-patient-with-hypertriglyceridemia. Accessed December 28, 2016.
4. Talayero BG, Sacks FM. The role of triglycerides in atherosclerosis. Curr Cardiol Rep. 2011;13(6): 544-552.
5. Ginsberg HN, Elam MB, Lovato LC, et al; ACCORD Study Group. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010; 362(17):1563-1574.
6. AIM-HIGH Investigators. The role of niacin in raising high-density lipoprotein cholesterol to reduce cardiovascular events in patients with atherosclerotic cardiovascular disease and optimally treated low-density lipoprotein cholesterol. Rationale and study design. The Atherothrombosis Intervention in Metabolic syndrome with low HDL/high triglycerides: impact on Global Health outcomes (AIM-HIGH). Am Heart J. 2011;161(3):471-477.
7. Miller M, Stone NJ, Ballantyne C, et al. Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2011;123(20):2292-2333.
8. Borow KM, Nelson JR, Mason RP. Biologic plausibility, cellular effects, and molecular mechanisms of eicosapentaenoic acid (EPA) in atherosclerosis. Atherosclerosis. 2015;242(1):357-366.
9. Jacobson TA, Ito MK, Maki KC, et al. National Lipid Association recommendations for patient-centered management of dyslipidemia. J Clin Lipidol. 2015;9(2):129-169.
10. Berglund L, Brunzell JD, Goldberg AC, et al. Evaluation and treatment of hypertriglyceridemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012; 97(9):2969-2989.
11. Stone NJ, Robinson JG, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 pt B):2889-2934.
12. Amini L, Sadeghi MR, Oskuie F, Maleki H. Lipid profile in women with polycystic ovary syndrome. Crescent J Med Biol Sci. 2014;1(4):147-150.
13. Mantzoros C. Insulin resistance: definition and clinical spectrum. www.uptodate.com/contents/insulin-resistance-definition-and-clinical-spectrum. Accessed December 28, 2016.
14. Lin M, Lin H, Lee P, et al. Beneficial effect of continuous positive airway pressure on lipid profiles in obstructive sleep apnea: a meta-analysis. Sleep Breath. 2015;19(3):809-817.
15. Kaur J. A comprehensive review on metabolic syndrome. Cardiol Res Pract. 2014;2014:943162.
16. FDA. Withdrawal of approval of indications related to the coadministration with statins in applications for niacin extended-release tablets and fenofibric acid delayed-release capsules. https://s3.amazonaws.com/public-inspection.federalregister.gov/2016-08887.pdf. Accessed December 28, 2016.
17. Mazza A, Fruci B, Garinis GA, et al. The role of metformin in the management of NAFLD. Exp Diabetes Res. 2012;2012: 716404.
Screening for cardiovascular (CV) risk often includes a routine serum fasting lipid profile. However, with the focus on LDL cholesterol, triglyceride measurement is frequently overlooked. Yet this element of the lipid profile is particularly important, given its strong association with not only atherosclerotic coronary heart disease but also pancreatitis.
Hypertriglyceridemia is defined as a serum triglyceride level that exceeds 150 mg/dL. In the US, an estimated 25% of patients have hypertriglyceridemia.1 Of these, 33.1% have “borderline high” triglyceride levels (150 to 199 mg/dL), 17.8% have “high” levels (200 to 499 mg/dL), and 1.7% have “very high” levels (> 500 mg/dL).1,2
Most of the time, hypertriglyceridemia is caused (or at least exacerbated) by underlying etiology. The best way to identify and manage these secondary causes is through a systematic approach.
CONSIDER THE EVIDENCE
For mild to moderately elevated (borderline high) triglyceride levels, our reflex reaction may be to recommend a triglyceride-lowering medication, such as fenofibrate. But this may not be the best answer. Although there is increasing evidence of an independent association between elevated triglyceride levels and CV risk, it remains unclear whether targeting them specifically can reduce that risk.3
In well-designed, peer-reviewed clinical trials, statins have been shown to reduce CV risk in patients with known cardiovascular disease (CVD) and those at high risk for CVD, as well as in primary prevention. However, these trials also suggest that significant residual CV risk remains after statin therapy.4
Several trials have attempted to prove residual risk reduction following combination therapy including statins—with inconclusive results:
ACCORD: Fenofibrate showed no overall macrovascular benefit when added to a statin in patients with type 2 diabetes and a triglyceride level < 204 mg/dL.3,5
AIM-HIGH: There was a 25% reduction in triglyceride levels when niacin was added to a regimen of a statin +/- ezetimibe, with an aggressive LDL treatment target (40 to 80 mg/dL). But the study was stopped early due to the lack of expected reduction in CVD events.4,6
JELIS: A reduction in major CV events was seen with 1,800 mg/d of eicosapentaenoic acid (EPA) supplementation plus a low-dose statin, compared to statin monotherapy. However, there was minimal change in triglyceride levels, leading the researchers to hypothesize that multiple mechanisms—such as decreasing oxidative stress, platelet aggregation, plaque formation and stabilization—contributed to the outcome.4,7
Informed by the JELIS results, the much-anticipated REDUCE-IT trial is currently in progress to address the lingering question of whether combination therapy can reduce residual CV risk. In this trial, EPA omega-3 fatty acid is being added to the regimen of statin-treated patients with persistently elevated triglycerides. Results are expected in 2017 to 2018.8
Remember that a triglyceride level of 150 mg/dL is a parameter—it does not represent a therapeutic target. There is insufficient evidence that treating to this level improves CV risk beyond LDL target recommendations.7
The National Lipid Association Expert Panel’s consensus view is that non-HDL is a better primary target than triglycerides alone or LDL. Using non-HDL as a target for intervention also simplifies the management of patients with high triglycerides (200 to 499 mg/dL). The non-HDL goal is considered to be 30 mg/dL greater than the LDL target. For patients with diabetes and those with CVD, the individualized non-HDL targets are 130 mg/dL and 100 mg/dL, respectively.9
REVIEW THE MEDICATION LIST
Several commonly used medications, including ß-blockers and thiazide diuretics, can increase triglyceride levels.10 Other medications with exacerbating effects on triglycerides include corticosteroids, retrovirals, immunosuppressants, retinoids, and some antipsychotics.10 Bile acid sequestrants (eg, colesevelam) should be avoided in patients with elevated triglycerides (> 200 mg/dL).7
In women, oral estrogen (ie, menopausal hormone replacement and oral birth control) can greatly exacerbate triglyceride levels, making transdermal delivery a better option. Tamoxifen, the hormonal medication used for breast cancer prophylaxis, can also increase triglyceride levels.11
LOOK FOR UNDERLYING CONDITIONS
Among those to consider: Hypothyroidism is common and easily ruled out by a simple blood test. Nephrotic syndrome should be ruled out, particularly in patients with concomitant renal dysfunction and peripheral edema, by checking a random urine protein-to-creatinine ratio or 24-hour urine for protein. Other factors that should be explored because of their potential effect on lipid metabolism include obesity and excessive intake of sugary beverages (ie, soda, fruit juice) and alcohol.11
High triglyceride levels occurring with low HDL are characteristic of insulin resistance and concerning for metabolic syndrome and/or polycystic ovarian syndrome.3,12 Often, patients will have underlying prediabetes (fasting glucose ≥ 100 mg/dL or random glucose ≥ 140 mg/dL with an A1C > 5.7%13) or covert type 2 diabetes. Another underdiagnosed but very common condition, obstructive sleep apnea, can greatly affect insulin sensitivity and has been associated with lipid abnormalities and metabolic syndrome.14
EXAMINE YOUR PATIENT
The physical exam is an essential component of assessment for patients with high triglycerides. As discussed, elevated triglycerides and low HDL are hallmarks for insulin resistance. As triglyceride levels are affected by obesity and body fat distribution, measuring BMI and assessing for visceral adiposity are an important part of the physical exam.4
The physical exam may also yield dermatologic clues, such as skin tags or acanthosis nigricans, a dark, velvety lesion usually found on the posterior and lateral neck creases, axillae, groin, and elbows.13 In rare cases—usually those with genetic involvement from a familial lipid metabolism disorder—patients may exhibit xanthomas. These cutaneous, lipid-rich lesions can appear as flat, yellowish plaques on various parts of the body, such as the eyelids (xanthelasma) or tendons of the hands, feet, and heels. Widespread, eruptive xanthomas, which manifest as pruritic pink papules with creamy centers, are associated with severe emergent triglyceride elevation and pancreatitis.10
CONSIDER NONPHARMACOLOGIC MANAGEMENT
In mild to moderate hypertriglyceridemia, intensive lifestyle changes are considered firstline therapy. Weight loss is recommended in obese patients; a 5% to 10% reduction in body weight can lower triglycerides by 20%.15
A quick 24-hour diet recall, including beverages, is helpful for identifying key issues. The goal should be to reduce carbohydrates—in particular, simple, high glycemic index, processed foods—as well as total and saturated fats. A substantial problem in our population is the consumption of high-fructose beverages and fruit juices. Referral to a dietitian can be very helpful, not only for initial meal planning but also for continuing counseling on successful long-term weight loss and maintenance.
Exercise is also very helpful for improving lipid parameters. A daily minimum of 30 to 60 minutes of intermittent aerobic exercise or mild resistance exercise has been shown to reduce triglyceride levels.10
PRESCRIBE APPROPRIATELY
The most important indication for treatment of hypertriglyceridemia is reduction of CVD risk. However, in patients with very high triglyceride levels (> 500 mg/dL), the goal is to decrease risk for life-threatening pancreatitis.15 Lipid-lowering medications and dietary restrictions should be promptly employed.
There are medications, as discussed earlier, that specifically lower triglycerides. Fibrates offer the most robust decrease, with a 20% to 50% reduction in triglyceride levels. Fenofibrate is considered a safer option when used in combination with a statin, due to the risk for significant muscle toxicity with gemfibrozil. There is some evidence that adding a fibrate may actually increase risk for pancreatitis; since this risk is otherwise low in patients with mild to moderate triglyceride elevation, the addition of a fibrate to their regimen should be avoided.3
Statins are the drug of choice when CV risk reduction is the goal (for patients with hypertriglyceridemia < 500 mg/dL). In addition to lowering LDL, statins can reduce triglycerides by 7% to 30%, depending on the dose.15
Other triglyceride-lowering medications include omega-3 fatty acids and niacin preparations. Prescription-strength omega-3 fatty acids have been found to lower serum triglyceride levels by 50% or more; the newest preparation, icosapent ethyl, demonstrated up to 45% reduction without significant effect on LDL levels.3 (Other preparations have been shown to substantially increase LDL in many cases.) Niacin (1,500 to 2,000 mg/d) can decrease triglycerides by 15% to 25%. However, it is no longer recommended for CV risk reduction; recent data indicate it may increase stroke risk when used in combination with statins.3,10 In April 2016, the FDA revoked its approval of the co-administration of niacin and fenofibrate with statin therapy, due to a lack of CV benefit.16
Other secondline options to consider for patients with insulin resistance or diabetes are metformin and pioglitazone. These medications have been shown to improve insulin sensitivity and decrease LDL and triglycerides in patients with prediabetes. Pioglitazone has proven beneficial in the treatment of steatohepatitis.17 Insulin is an excellent rapid triglyceride-lowering agent for patients with diabetes. It is important to reinforce that reduction of glucose is a key component in reduction of triglyceride levels.3
CONCLUSION
Hypertriglyceridemia is a complex condition that requires individualized and comprehensive management strategies. Clinicians must be able to identify and address secondary causes. Treatment options should be tailored to decrease CV and pancreatitis risk, and medication recommendations should be evidenced based and carefully selected to mitigate potential adverse effects. Patients should receive education and lifestyle management support to help motivate and equip them to employ strategies to improve their health.
Screening for cardiovascular (CV) risk often includes a routine serum fasting lipid profile. However, with the focus on LDL cholesterol, triglyceride measurement is frequently overlooked. Yet this element of the lipid profile is particularly important, given its strong association with not only atherosclerotic coronary heart disease but also pancreatitis.
Hypertriglyceridemia is defined as a serum triglyceride level that exceeds 150 mg/dL. In the US, an estimated 25% of patients have hypertriglyceridemia.1 Of these, 33.1% have “borderline high” triglyceride levels (150 to 199 mg/dL), 17.8% have “high” levels (200 to 499 mg/dL), and 1.7% have “very high” levels (> 500 mg/dL).1,2
Most of the time, hypertriglyceridemia is caused (or at least exacerbated) by underlying etiology. The best way to identify and manage these secondary causes is through a systematic approach.
CONSIDER THE EVIDENCE
For mild to moderately elevated (borderline high) triglyceride levels, our reflex reaction may be to recommend a triglyceride-lowering medication, such as fenofibrate. But this may not be the best answer. Although there is increasing evidence of an independent association between elevated triglyceride levels and CV risk, it remains unclear whether targeting them specifically can reduce that risk.3
In well-designed, peer-reviewed clinical trials, statins have been shown to reduce CV risk in patients with known cardiovascular disease (CVD) and those at high risk for CVD, as well as in primary prevention. However, these trials also suggest that significant residual CV risk remains after statin therapy.4
Several trials have attempted to prove residual risk reduction following combination therapy including statins—with inconclusive results:
ACCORD: Fenofibrate showed no overall macrovascular benefit when added to a statin in patients with type 2 diabetes and a triglyceride level < 204 mg/dL.3,5
AIM-HIGH: There was a 25% reduction in triglyceride levels when niacin was added to a regimen of a statin +/- ezetimibe, with an aggressive LDL treatment target (40 to 80 mg/dL). But the study was stopped early due to the lack of expected reduction in CVD events.4,6
JELIS: A reduction in major CV events was seen with 1,800 mg/d of eicosapentaenoic acid (EPA) supplementation plus a low-dose statin, compared to statin monotherapy. However, there was minimal change in triglyceride levels, leading the researchers to hypothesize that multiple mechanisms—such as decreasing oxidative stress, platelet aggregation, plaque formation and stabilization—contributed to the outcome.4,7
Informed by the JELIS results, the much-anticipated REDUCE-IT trial is currently in progress to address the lingering question of whether combination therapy can reduce residual CV risk. In this trial, EPA omega-3 fatty acid is being added to the regimen of statin-treated patients with persistently elevated triglycerides. Results are expected in 2017 to 2018.8
Remember that a triglyceride level of 150 mg/dL is a parameter—it does not represent a therapeutic target. There is insufficient evidence that treating to this level improves CV risk beyond LDL target recommendations.7
The National Lipid Association Expert Panel’s consensus view is that non-HDL is a better primary target than triglycerides alone or LDL. Using non-HDL as a target for intervention also simplifies the management of patients with high triglycerides (200 to 499 mg/dL). The non-HDL goal is considered to be 30 mg/dL greater than the LDL target. For patients with diabetes and those with CVD, the individualized non-HDL targets are 130 mg/dL and 100 mg/dL, respectively.9
REVIEW THE MEDICATION LIST
Several commonly used medications, including ß-blockers and thiazide diuretics, can increase triglyceride levels.10 Other medications with exacerbating effects on triglycerides include corticosteroids, retrovirals, immunosuppressants, retinoids, and some antipsychotics.10 Bile acid sequestrants (eg, colesevelam) should be avoided in patients with elevated triglycerides (> 200 mg/dL).7
In women, oral estrogen (ie, menopausal hormone replacement and oral birth control) can greatly exacerbate triglyceride levels, making transdermal delivery a better option. Tamoxifen, the hormonal medication used for breast cancer prophylaxis, can also increase triglyceride levels.11
LOOK FOR UNDERLYING CONDITIONS
Among those to consider: Hypothyroidism is common and easily ruled out by a simple blood test. Nephrotic syndrome should be ruled out, particularly in patients with concomitant renal dysfunction and peripheral edema, by checking a random urine protein-to-creatinine ratio or 24-hour urine for protein. Other factors that should be explored because of their potential effect on lipid metabolism include obesity and excessive intake of sugary beverages (ie, soda, fruit juice) and alcohol.11
High triglyceride levels occurring with low HDL are characteristic of insulin resistance and concerning for metabolic syndrome and/or polycystic ovarian syndrome.3,12 Often, patients will have underlying prediabetes (fasting glucose ≥ 100 mg/dL or random glucose ≥ 140 mg/dL with an A1C > 5.7%13) or covert type 2 diabetes. Another underdiagnosed but very common condition, obstructive sleep apnea, can greatly affect insulin sensitivity and has been associated with lipid abnormalities and metabolic syndrome.14
EXAMINE YOUR PATIENT
The physical exam is an essential component of assessment for patients with high triglycerides. As discussed, elevated triglycerides and low HDL are hallmarks for insulin resistance. As triglyceride levels are affected by obesity and body fat distribution, measuring BMI and assessing for visceral adiposity are an important part of the physical exam.4
The physical exam may also yield dermatologic clues, such as skin tags or acanthosis nigricans, a dark, velvety lesion usually found on the posterior and lateral neck creases, axillae, groin, and elbows.13 In rare cases—usually those with genetic involvement from a familial lipid metabolism disorder—patients may exhibit xanthomas. These cutaneous, lipid-rich lesions can appear as flat, yellowish plaques on various parts of the body, such as the eyelids (xanthelasma) or tendons of the hands, feet, and heels. Widespread, eruptive xanthomas, which manifest as pruritic pink papules with creamy centers, are associated with severe emergent triglyceride elevation and pancreatitis.10
CONSIDER NONPHARMACOLOGIC MANAGEMENT
In mild to moderate hypertriglyceridemia, intensive lifestyle changes are considered firstline therapy. Weight loss is recommended in obese patients; a 5% to 10% reduction in body weight can lower triglycerides by 20%.15
A quick 24-hour diet recall, including beverages, is helpful for identifying key issues. The goal should be to reduce carbohydrates—in particular, simple, high glycemic index, processed foods—as well as total and saturated fats. A substantial problem in our population is the consumption of high-fructose beverages and fruit juices. Referral to a dietitian can be very helpful, not only for initial meal planning but also for continuing counseling on successful long-term weight loss and maintenance.
Exercise is also very helpful for improving lipid parameters. A daily minimum of 30 to 60 minutes of intermittent aerobic exercise or mild resistance exercise has been shown to reduce triglyceride levels.10
PRESCRIBE APPROPRIATELY
The most important indication for treatment of hypertriglyceridemia is reduction of CVD risk. However, in patients with very high triglyceride levels (> 500 mg/dL), the goal is to decrease risk for life-threatening pancreatitis.15 Lipid-lowering medications and dietary restrictions should be promptly employed.
There are medications, as discussed earlier, that specifically lower triglycerides. Fibrates offer the most robust decrease, with a 20% to 50% reduction in triglyceride levels. Fenofibrate is considered a safer option when used in combination with a statin, due to the risk for significant muscle toxicity with gemfibrozil. There is some evidence that adding a fibrate may actually increase risk for pancreatitis; since this risk is otherwise low in patients with mild to moderate triglyceride elevation, the addition of a fibrate to their regimen should be avoided.3
Statins are the drug of choice when CV risk reduction is the goal (for patients with hypertriglyceridemia < 500 mg/dL). In addition to lowering LDL, statins can reduce triglycerides by 7% to 30%, depending on the dose.15
Other triglyceride-lowering medications include omega-3 fatty acids and niacin preparations. Prescription-strength omega-3 fatty acids have been found to lower serum triglyceride levels by 50% or more; the newest preparation, icosapent ethyl, demonstrated up to 45% reduction without significant effect on LDL levels.3 (Other preparations have been shown to substantially increase LDL in many cases.) Niacin (1,500 to 2,000 mg/d) can decrease triglycerides by 15% to 25%. However, it is no longer recommended for CV risk reduction; recent data indicate it may increase stroke risk when used in combination with statins.3,10 In April 2016, the FDA revoked its approval of the co-administration of niacin and fenofibrate with statin therapy, due to a lack of CV benefit.16
Other secondline options to consider for patients with insulin resistance or diabetes are metformin and pioglitazone. These medications have been shown to improve insulin sensitivity and decrease LDL and triglycerides in patients with prediabetes. Pioglitazone has proven beneficial in the treatment of steatohepatitis.17 Insulin is an excellent rapid triglyceride-lowering agent for patients with diabetes. It is important to reinforce that reduction of glucose is a key component in reduction of triglyceride levels.3
CONCLUSION
Hypertriglyceridemia is a complex condition that requires individualized and comprehensive management strategies. Clinicians must be able to identify and address secondary causes. Treatment options should be tailored to decrease CV and pancreatitis risk, and medication recommendations should be evidenced based and carefully selected to mitigate potential adverse effects. Patients should receive education and lifestyle management support to help motivate and equip them to employ strategies to improve their health.
1. CDC. Trends in elevated triglyceride in adults: United States, 2001-2012. www.cdc.gov/nchs/data/databriefs/db198.pdf. Accessed December 27, 2016.
2. Maki KC, Bays HE, Dicklin MR. Treatment options for the management of hypertriglyceridemia: strategies based on the best-available evidence. J Clin Lipidol. 2012;6(5):413-426.
3. Rosenson RS. Approach to the patient with hypertriglyceridemia. www.uptodate.com/contents/approach-to-the-patient-with-hypertriglyceridemia. Accessed December 28, 2016.
4. Talayero BG, Sacks FM. The role of triglycerides in atherosclerosis. Curr Cardiol Rep. 2011;13(6): 544-552.
5. Ginsberg HN, Elam MB, Lovato LC, et al; ACCORD Study Group. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010; 362(17):1563-1574.
6. AIM-HIGH Investigators. The role of niacin in raising high-density lipoprotein cholesterol to reduce cardiovascular events in patients with atherosclerotic cardiovascular disease and optimally treated low-density lipoprotein cholesterol. Rationale and study design. The Atherothrombosis Intervention in Metabolic syndrome with low HDL/high triglycerides: impact on Global Health outcomes (AIM-HIGH). Am Heart J. 2011;161(3):471-477.
7. Miller M, Stone NJ, Ballantyne C, et al. Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2011;123(20):2292-2333.
8. Borow KM, Nelson JR, Mason RP. Biologic plausibility, cellular effects, and molecular mechanisms of eicosapentaenoic acid (EPA) in atherosclerosis. Atherosclerosis. 2015;242(1):357-366.
9. Jacobson TA, Ito MK, Maki KC, et al. National Lipid Association recommendations for patient-centered management of dyslipidemia. J Clin Lipidol. 2015;9(2):129-169.
10. Berglund L, Brunzell JD, Goldberg AC, et al. Evaluation and treatment of hypertriglyceridemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012; 97(9):2969-2989.
11. Stone NJ, Robinson JG, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 pt B):2889-2934.
12. Amini L, Sadeghi MR, Oskuie F, Maleki H. Lipid profile in women with polycystic ovary syndrome. Crescent J Med Biol Sci. 2014;1(4):147-150.
13. Mantzoros C. Insulin resistance: definition and clinical spectrum. www.uptodate.com/contents/insulin-resistance-definition-and-clinical-spectrum. Accessed December 28, 2016.
14. Lin M, Lin H, Lee P, et al. Beneficial effect of continuous positive airway pressure on lipid profiles in obstructive sleep apnea: a meta-analysis. Sleep Breath. 2015;19(3):809-817.
15. Kaur J. A comprehensive review on metabolic syndrome. Cardiol Res Pract. 2014;2014:943162.
16. FDA. Withdrawal of approval of indications related to the coadministration with statins in applications for niacin extended-release tablets and fenofibric acid delayed-release capsules. https://s3.amazonaws.com/public-inspection.federalregister.gov/2016-08887.pdf. Accessed December 28, 2016.
17. Mazza A, Fruci B, Garinis GA, et al. The role of metformin in the management of NAFLD. Exp Diabetes Res. 2012;2012: 716404.
1. CDC. Trends in elevated triglyceride in adults: United States, 2001-2012. www.cdc.gov/nchs/data/databriefs/db198.pdf. Accessed December 27, 2016.
2. Maki KC, Bays HE, Dicklin MR. Treatment options for the management of hypertriglyceridemia: strategies based on the best-available evidence. J Clin Lipidol. 2012;6(5):413-426.
3. Rosenson RS. Approach to the patient with hypertriglyceridemia. www.uptodate.com/contents/approach-to-the-patient-with-hypertriglyceridemia. Accessed December 28, 2016.
4. Talayero BG, Sacks FM. The role of triglycerides in atherosclerosis. Curr Cardiol Rep. 2011;13(6): 544-552.
5. Ginsberg HN, Elam MB, Lovato LC, et al; ACCORD Study Group. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010; 362(17):1563-1574.
6. AIM-HIGH Investigators. The role of niacin in raising high-density lipoprotein cholesterol to reduce cardiovascular events in patients with atherosclerotic cardiovascular disease and optimally treated low-density lipoprotein cholesterol. Rationale and study design. The Atherothrombosis Intervention in Metabolic syndrome with low HDL/high triglycerides: impact on Global Health outcomes (AIM-HIGH). Am Heart J. 2011;161(3):471-477.
7. Miller M, Stone NJ, Ballantyne C, et al. Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2011;123(20):2292-2333.
8. Borow KM, Nelson JR, Mason RP. Biologic plausibility, cellular effects, and molecular mechanisms of eicosapentaenoic acid (EPA) in atherosclerosis. Atherosclerosis. 2015;242(1):357-366.
9. Jacobson TA, Ito MK, Maki KC, et al. National Lipid Association recommendations for patient-centered management of dyslipidemia. J Clin Lipidol. 2015;9(2):129-169.
10. Berglund L, Brunzell JD, Goldberg AC, et al. Evaluation and treatment of hypertriglyceridemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012; 97(9):2969-2989.
11. Stone NJ, Robinson JG, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 pt B):2889-2934.
12. Amini L, Sadeghi MR, Oskuie F, Maleki H. Lipid profile in women with polycystic ovary syndrome. Crescent J Med Biol Sci. 2014;1(4):147-150.
13. Mantzoros C. Insulin resistance: definition and clinical spectrum. www.uptodate.com/contents/insulin-resistance-definition-and-clinical-spectrum. Accessed December 28, 2016.
14. Lin M, Lin H, Lee P, et al. Beneficial effect of continuous positive airway pressure on lipid profiles in obstructive sleep apnea: a meta-analysis. Sleep Breath. 2015;19(3):809-817.
15. Kaur J. A comprehensive review on metabolic syndrome. Cardiol Res Pract. 2014;2014:943162.
16. FDA. Withdrawal of approval of indications related to the coadministration with statins in applications for niacin extended-release tablets and fenofibric acid delayed-release capsules. https://s3.amazonaws.com/public-inspection.federalregister.gov/2016-08887.pdf. Accessed December 28, 2016.
17. Mazza A, Fruci B, Garinis GA, et al. The role of metformin in the management of NAFLD. Exp Diabetes Res. 2012;2012: 716404.
Kidney Disease Progression: How to Attenuate Risk
Q)I overheard a conversation at the hospital in which one of the nephrologists told an internist that allopurinol is better than other medications for treating gout because it slows the progression of chronic kidney disease (CKD). What does the data say?
CKD is a growing problem in America; the number of adults with CKD doubled from 2000 to 2008.1 Gout is considered an independent risk factor for CKD progression.2 Some randomized contr
A recent large retrospective review of Medicare charts assessed the correlation between use and dose of allopurinol and incidence of renal failure in patients older than 65.1 The researchers found that, compared with lower doses, allopurinol doses of 200 to 299 mg/d and > 300 mg/d were associated with a significantly lower hazard ratio for kidney failure, in a multivariate-adjusted model. The findings therefore suggest that doses > 199 mg may slow progression to kidney failure in the elderly.
Despite the strengths of this study, it is worth noting that it did not consider stage of kidney disease, nor did it distinguish comorbidities of the patients. The retrospective chart review format did not allow for identification of concurrent medication use (including OTC and herbal products).
The National Institute of Diabetes and Digestive and Kidney Diseases is currently conducting an RCT to investigate the renoprotective effects of allopurinol versus placebo in diabetic patients. (Clinical Trials.gov identifier: NCT02017171). Enrollment was completed in 2014, and results are expected in June 2019.
One important proviso about allopurinol: While it is inexpensive and generally well tolerated, prescribers should be aware of rare sensitivity reactions, particularly Stevens-Johnson syndrome. —MRS
Mary Rogers Sorey, MSN
Division of Nephrology and Hypertension at Vanderbilt University Medical Center, Nashville
1. Singh JA, Yu S. Are allopurinol dose and duration of use nephroprotective in the elderly? A Medicare claims study of allopurinol use and incident renal failure. Ann Rheum Dis. 2016 Jun 13. [Epub ahead of print]
2. Roughley MJ, Belcher J, Mallen CD, Roddy E. Gout and risk of chronic kidney disease and nephrolithiasis: meta-analysis of observational studies. Arthritis Res Ther. 2015;17:90.
3. Kanbay M, Huddam B, Azak A, et al. A randomized study of allopurinol on endothelial function and estimated glomerular filtration rate in asymptomatic hyperuricemic subjects with normal renal function. Clin J Am Soc Nephrol. 2011;6(8):1887-1894.
Q)I overheard a conversation at the hospital in which one of the nephrologists told an internist that allopurinol is better than other medications for treating gout because it slows the progression of chronic kidney disease (CKD). What does the data say?
CKD is a growing problem in America; the number of adults with CKD doubled from 2000 to 2008.1 Gout is considered an independent risk factor for CKD progression.2 Some randomized contr
A recent large retrospective review of Medicare charts assessed the correlation between use and dose of allopurinol and incidence of renal failure in patients older than 65.1 The researchers found that, compared with lower doses, allopurinol doses of 200 to 299 mg/d and > 300 mg/d were associated with a significantly lower hazard ratio for kidney failure, in a multivariate-adjusted model. The findings therefore suggest that doses > 199 mg may slow progression to kidney failure in the elderly.
Despite the strengths of this study, it is worth noting that it did not consider stage of kidney disease, nor did it distinguish comorbidities of the patients. The retrospective chart review format did not allow for identification of concurrent medication use (including OTC and herbal products).
The National Institute of Diabetes and Digestive and Kidney Diseases is currently conducting an RCT to investigate the renoprotective effects of allopurinol versus placebo in diabetic patients. (Clinical Trials.gov identifier: NCT02017171). Enrollment was completed in 2014, and results are expected in June 2019.
One important proviso about allopurinol: While it is inexpensive and generally well tolerated, prescribers should be aware of rare sensitivity reactions, particularly Stevens-Johnson syndrome. —MRS
Mary Rogers Sorey, MSN
Division of Nephrology and Hypertension at Vanderbilt University Medical Center, Nashville
Q)I overheard a conversation at the hospital in which one of the nephrologists told an internist that allopurinol is better than other medications for treating gout because it slows the progression of chronic kidney disease (CKD). What does the data say?
CKD is a growing problem in America; the number of adults with CKD doubled from 2000 to 2008.1 Gout is considered an independent risk factor for CKD progression.2 Some randomized contr
A recent large retrospective review of Medicare charts assessed the correlation between use and dose of allopurinol and incidence of renal failure in patients older than 65.1 The researchers found that, compared with lower doses, allopurinol doses of 200 to 299 mg/d and > 300 mg/d were associated with a significantly lower hazard ratio for kidney failure, in a multivariate-adjusted model. The findings therefore suggest that doses > 199 mg may slow progression to kidney failure in the elderly.
Despite the strengths of this study, it is worth noting that it did not consider stage of kidney disease, nor did it distinguish comorbidities of the patients. The retrospective chart review format did not allow for identification of concurrent medication use (including OTC and herbal products).
The National Institute of Diabetes and Digestive and Kidney Diseases is currently conducting an RCT to investigate the renoprotective effects of allopurinol versus placebo in diabetic patients. (Clinical Trials.gov identifier: NCT02017171). Enrollment was completed in 2014, and results are expected in June 2019.
One important proviso about allopurinol: While it is inexpensive and generally well tolerated, prescribers should be aware of rare sensitivity reactions, particularly Stevens-Johnson syndrome. —MRS
Mary Rogers Sorey, MSN
Division of Nephrology and Hypertension at Vanderbilt University Medical Center, Nashville
1. Singh JA, Yu S. Are allopurinol dose and duration of use nephroprotective in the elderly? A Medicare claims study of allopurinol use and incident renal failure. Ann Rheum Dis. 2016 Jun 13. [Epub ahead of print]
2. Roughley MJ, Belcher J, Mallen CD, Roddy E. Gout and risk of chronic kidney disease and nephrolithiasis: meta-analysis of observational studies. Arthritis Res Ther. 2015;17:90.
3. Kanbay M, Huddam B, Azak A, et al. A randomized study of allopurinol on endothelial function and estimated glomerular filtration rate in asymptomatic hyperuricemic subjects with normal renal function. Clin J Am Soc Nephrol. 2011;6(8):1887-1894.
1. Singh JA, Yu S. Are allopurinol dose and duration of use nephroprotective in the elderly? A Medicare claims study of allopurinol use and incident renal failure. Ann Rheum Dis. 2016 Jun 13. [Epub ahead of print]
2. Roughley MJ, Belcher J, Mallen CD, Roddy E. Gout and risk of chronic kidney disease and nephrolithiasis: meta-analysis of observational studies. Arthritis Res Ther. 2015;17:90.
3. Kanbay M, Huddam B, Azak A, et al. A randomized study of allopurinol on endothelial function and estimated glomerular filtration rate in asymptomatic hyperuricemic subjects with normal renal function. Clin J Am Soc Nephrol. 2011;6(8):1887-1894.
Kidney Disease Progression: How to Calculate Risk
Q)When I diagnose patients with minor kidney disease, they often ask if they will require dialysis. I know it is unlikely, but I wish I could give them a better answer. Can you help me?
The diagnosis of chronic kidney disease (CKD) is understandably concerning for many patients. Being able to estimate CKD progression helps patients gain a better understanding of their condition while allowing clinicians to develop more personalized care plans. Tangri and colleagues developed a model that can be used to predict risk for kidney failure requiring dialysis or transplantation in patients with stage III to V CKD. This model has been validated in multiple diverse populations in North America and worldwide.1
The Kidney Failure Risk Equation (found at www.kidneyfailurerisk.com) uses four variables—age, gender, glomerular filtration rate (GFR), and urine albumin-to-creatinine ratio (ACR)—to assess two- and five-year risk for kidney failure.1,2 For example
- A 63-year-old woman with a GFR of 45 mL/min and an ACR of 30 mg/g has a 0.4% two-year risk and a 1.3% five-year risk for progression to kidney failure requiring dialysis or transplant.1
- Alternatively, a 55-year-old man with a GFR of 38 mL/min and an ACR of 150 mg/g has a 2.9% two-year risk and a 9% five-year risk for progression to end-stage renal disease (ESRD).1
Per proposed thresholds, patients with a score < 5% would be deemed “low risk”; with scores of 5% to 15%, “intermediate risk”; and with scores > 15%, “high risk.”1,2
The Kidney Failure Risk Equation can be incorporated into clinic visits to provide context for lab results. For patients with low risk for progression, optimal care and lifestyle measures can be reinforced. For those with intermediate or high risk, more intensive treatments and appropriate referrals can be initiated. (The National Kidney Foundation advises referral when a patient’s estimated GFR is 20 mL/min or the urine ACR is ≥ 300 mg/g.3) Providing a numeric risk for progression can help alleviate the patient’s uncertainty surrounding the diagnosis of CKD. —NDM
Nicole D. McCormick, MS, MBA, NP-C, CCTC
University of Colorado Renal Transplant Clinic, Aurora, Colorado
1. Tangri N, Grams ME, Levey AS, et al. Multinational assessment of accuracy of equations for predicting risk of kidney failure: a meta-analysis. JAMA. 2016;315(2):164-174.
2. Tangri N, Stevens LA, Griffith J, et al. A predictive model for progression of chronic kidney disease to kidney failure. JAMA. 2011;305(15):1553-1559.
3. National Kidney Foundation. Renal Replacement Therapy: What the PCP Needs to Know. www.kidney.org/sites/default/files/PCP%20in%20a%20Box%20-%20Module%203.pptx. Accessed December 5, 2016.
Q)When I diagnose patients with minor kidney disease, they often ask if they will require dialysis. I know it is unlikely, but I wish I could give them a better answer. Can you help me?
The diagnosis of chronic kidney disease (CKD) is understandably concerning for many patients. Being able to estimate CKD progression helps patients gain a better understanding of their condition while allowing clinicians to develop more personalized care plans. Tangri and colleagues developed a model that can be used to predict risk for kidney failure requiring dialysis or transplantation in patients with stage III to V CKD. This model has been validated in multiple diverse populations in North America and worldwide.1
The Kidney Failure Risk Equation (found at www.kidneyfailurerisk.com) uses four variables—age, gender, glomerular filtration rate (GFR), and urine albumin-to-creatinine ratio (ACR)—to assess two- and five-year risk for kidney failure.1,2 For example
- A 63-year-old woman with a GFR of 45 mL/min and an ACR of 30 mg/g has a 0.4% two-year risk and a 1.3% five-year risk for progression to kidney failure requiring dialysis or transplant.1
- Alternatively, a 55-year-old man with a GFR of 38 mL/min and an ACR of 150 mg/g has a 2.9% two-year risk and a 9% five-year risk for progression to end-stage renal disease (ESRD).1
Per proposed thresholds, patients with a score < 5% would be deemed “low risk”; with scores of 5% to 15%, “intermediate risk”; and with scores > 15%, “high risk.”1,2
The Kidney Failure Risk Equation can be incorporated into clinic visits to provide context for lab results. For patients with low risk for progression, optimal care and lifestyle measures can be reinforced. For those with intermediate or high risk, more intensive treatments and appropriate referrals can be initiated. (The National Kidney Foundation advises referral when a patient’s estimated GFR is 20 mL/min or the urine ACR is ≥ 300 mg/g.3) Providing a numeric risk for progression can help alleviate the patient’s uncertainty surrounding the diagnosis of CKD. —NDM
Nicole D. McCormick, MS, MBA, NP-C, CCTC
University of Colorado Renal Transplant Clinic, Aurora, Colorado
Q)When I diagnose patients with minor kidney disease, they often ask if they will require dialysis. I know it is unlikely, but I wish I could give them a better answer. Can you help me?
The diagnosis of chronic kidney disease (CKD) is understandably concerning for many patients. Being able to estimate CKD progression helps patients gain a better understanding of their condition while allowing clinicians to develop more personalized care plans. Tangri and colleagues developed a model that can be used to predict risk for kidney failure requiring dialysis or transplantation in patients with stage III to V CKD. This model has been validated in multiple diverse populations in North America and worldwide.1
The Kidney Failure Risk Equation (found at www.kidneyfailurerisk.com) uses four variables—age, gender, glomerular filtration rate (GFR), and urine albumin-to-creatinine ratio (ACR)—to assess two- and five-year risk for kidney failure.1,2 For example
- A 63-year-old woman with a GFR of 45 mL/min and an ACR of 30 mg/g has a 0.4% two-year risk and a 1.3% five-year risk for progression to kidney failure requiring dialysis or transplant.1
- Alternatively, a 55-year-old man with a GFR of 38 mL/min and an ACR of 150 mg/g has a 2.9% two-year risk and a 9% five-year risk for progression to end-stage renal disease (ESRD).1
Per proposed thresholds, patients with a score < 5% would be deemed “low risk”; with scores of 5% to 15%, “intermediate risk”; and with scores > 15%, “high risk.”1,2
The Kidney Failure Risk Equation can be incorporated into clinic visits to provide context for lab results. For patients with low risk for progression, optimal care and lifestyle measures can be reinforced. For those with intermediate or high risk, more intensive treatments and appropriate referrals can be initiated. (The National Kidney Foundation advises referral when a patient’s estimated GFR is 20 mL/min or the urine ACR is ≥ 300 mg/g.3) Providing a numeric risk for progression can help alleviate the patient’s uncertainty surrounding the diagnosis of CKD. —NDM
Nicole D. McCormick, MS, MBA, NP-C, CCTC
University of Colorado Renal Transplant Clinic, Aurora, Colorado
1. Tangri N, Grams ME, Levey AS, et al. Multinational assessment of accuracy of equations for predicting risk of kidney failure: a meta-analysis. JAMA. 2016;315(2):164-174.
2. Tangri N, Stevens LA, Griffith J, et al. A predictive model for progression of chronic kidney disease to kidney failure. JAMA. 2011;305(15):1553-1559.
3. National Kidney Foundation. Renal Replacement Therapy: What the PCP Needs to Know. www.kidney.org/sites/default/files/PCP%20in%20a%20Box%20-%20Module%203.pptx. Accessed December 5, 2016.
1. Tangri N, Grams ME, Levey AS, et al. Multinational assessment of accuracy of equations for predicting risk of kidney failure: a meta-analysis. JAMA. 2016;315(2):164-174.
2. Tangri N, Stevens LA, Griffith J, et al. A predictive model for progression of chronic kidney disease to kidney failure. JAMA. 2011;305(15):1553-1559.
3. National Kidney Foundation. Renal Replacement Therapy: What the PCP Needs to Know. www.kidney.org/sites/default/files/PCP%20in%20a%20Box%20-%20Module%203.pptx. Accessed December 5, 2016.
MS & Pregnancy: What's Safe?
Q)What are the considerations and recommendations for pregnancy and breastfeeding in women with multiple sclerosis? When should women discontinue their disease-modifying therapies?
Multiple sclerosis (MS) is an inflammatory, demyelinating, degenerative disease. Three times more common in women than men, it may affect pregnancy planning and childbearing experiences.6 Evidence demonstrates a reduction in annualized relapse rate during pregnancy and the postpartum period with exclusive breastfeeding. Therefore, pregnancy and exclusive breastfeeding provide a favorable immunomodulatory effect in women with MS, which combats the increased relapse risk associated with the postpartum period.7,8
Reproductive education—including conception, pregnancy, and breastfeeding—is critical for patients with MS and their partners during a woman’s childbearing years. However, women with MS do not require special considerations during pregnancy unless they have remarkable disability. As soon as these women and their partners decide upon pregnancy, a plan should be established that includes a discussion about potential risks to the fetus due to drug exposure, as well as risks to the mother. The goal should be to minimize risk for disease activity and optimize the health of the baby.
Use of DMTs during conception, pregnancy, and breastfeeding. All disease-modifying therapies (DMTs) are usually discontinued during pregnancy and breastfeeding. Common practice among MS experts is to discontinue DMTs prior to conception, with a few exceptions. There is no consensus about timing of discontinuation and washout period for each DMT. The decision is based on the half-life of each DMT, the opinions of the woman and her partner, and risk tolerance. Note: For the purposes of this discussion, the old pregnancy category designations are used, since they are familiar to clinicians. New guidelines took effect in June 2015; Table 3 outlines the change in format.

Injectables. Glatiramer acetate (GA) is the safest drug in relation to pregnancy and breastfeeding (category B). There is no evidence of congenital malformation or spontaneous abortion. The common recommendation is to discontinue the drug one to two months before conception, although some clinicians allow continuation of the injections throughout pregnancy and into breastfeeding.
Interferon-ßs are category C and therefore pose minimal risk for the fetus. The washout period before conception is two to three months, varying among clinicians. Although there is no evidence of spontaneous abortion or birth defects in humans, animal data show increased risk for abortion.9
Both GA and interferon-ßs are large molecules; there is a very minimal chance that the medication will transmit to the baby via breast milk. Thus, both DMTs are considered safe during lactation.7
Oral MS medications. The three approved MS oral medications are fingolimod, dimethyl fumarate (DMF), and teriflunomide. Fingolimod and DMF are both category C. Women on DMF must discontinue use of the medication one month prior to conception due to its short half-life. There are no reports of birth defects or spontaneous abortion in women taking DMF. Fingolimod needs to be discontinued two months prior to conception. Animal data show evidence for teratogenicity and embryolethality at lower doses of fingolimod than those used in humans.7
Teriflunomide is category X, posing high risk for the health of the fetus. It stays in the blood for approximately eight months after discontinuation of use. Animal data show teratogenicity and embryotoxicity; therefore, teriflunomide is contraindicated in pregnancy. Women on teriflunomide who plan to become pregnant need to undergo an elimination procedure with cholestyramine or charcoal.
Infusions and injections (monoclonal antibodies). The approved monoclonal antibodies include natalizumab, alemtuzumab, and daclizumab. (Currently, use of rituximab in MS is off label, and the FDA is reviewing the efficacy and safety data for ocrelizumab.) The monoclonal antibodies are category C. The recommendation is to discontinue natalizumab one to two months prior to conception and discontinue alemtuzumab four months preconception.10 There is no evidence of spontaneous abortion or birth defects in women on alemtuzumab, but there is potentially increased risk for spontaneous abortion in those on natalizumab.
The spontaneous abortion rate in daclizumab-exposed women is consistent with early pregnancy loss in the general population (12% to 26%). Data on a small number of pregnancies exposed to daclizumab did not suggest an increased risk for adverse fetal or maternal outcomes.11 However, the recommendation is to discontinue daclizumab four months prior to conception. Rituximab should be discontinued 12 months prior to conception, based on the manufacturer recommendation, although it is potentially safe to conceive when the B cell counts return to normal.
Chemotherapy agents. Mitoxantrone is the only FDA-approved chemotherapeutic drug used in MS. However, a few chemotherapy drugs—among them, azathioprine, methotrexate, and cyclophosphamide—are used off label. Chemotherapeutic agents are category D, except methotrexate (category X). Women on category D medications must use a method of birth control for three months after stopping the DMT. Often, clinicians will recommend women initiate GA or interferon during this period, in the hope of minimizing disease activity. Women on mitoxantrone and methotrexate need to use birth control for six months after stopping these immunosuppressive medications, before conceiving.12 These women likely need to switch to a safer pregnancy DMT during the long washout period.
Pregnancy and breastfeeding among women with MS require planning and decision making. The recommendations differ among clinicians and MS experts since there is no definitive evidence about the risks of the DMTs on the mother and/or the fetus. Clinicians should discuss the potential risks with women based on their knowledge and experience, and the data available based on animal research and the pregnancy registries. —ABB-Z
Aliza Bitton Ben-Zacharia, DNP, ANP
President-Elect of IOMSN
Neurology Faculty, Icahn School of Medicine, Mount Sinai Hospital System, New York, New York
6. Tullman MJ. Overview of the epidemiology, diagnosis, and disease progression associated with multiple sclerosis. Am J Manag Care. 2013;19(2):S15-S20.
7. Fabian M. Pregnancy in the setting of multiple sclerosis. Continuum (Minneap Minn). 2016; 22(3):837-850.
8. Vukusic S, Hutchinson M, Hours M, et al. Pregnancy and multiple sclerosis (the PRIMS study): clinical predictors of post-partum relapse. Brain. 2004;127(6):1353-1360.
9. Sandberg-Wollheim M, Alteri E, Moraga MS, Kornmann G. Pregnancy outcomes in multiple sclerosis following subcutaneous interferon beta-1a therapy. Mult Scler. 2011; 17(4):423-430.
10. Coyle PK. Multiple sclerosis in pregnancy. Continuum (Minneap Minn). 2014;20(1):42-59.
11. Gold SM, Voskuhl RR. Pregnancy and multiple sclerosis: from molecular mechanisms to clinical application. Semin Immunopathol. 2016;38:709.
12. Vukusic S, Marignier R. Multiple sclerosis and pregnancy in the ‘treatment era’. Nat Rev Neurol. 2015;11(5):280-289.
13. FDA. Pregnancy and lactation labeling (drugs) final rule. www.fda.gov/Drugs/DevelopmentApprovalProcess/DevelopmentResources/Labeling/ucm093307.htm. Accessed November 2, 2016.
14. Whyte J. FDA implements new labeling for medications used during pregnancy and lactation. Am Fam Physician. 2016;94(1):12-13.
Q)What are the considerations and recommendations for pregnancy and breastfeeding in women with multiple sclerosis? When should women discontinue their disease-modifying therapies?
Multiple sclerosis (MS) is an inflammatory, demyelinating, degenerative disease. Three times more common in women than men, it may affect pregnancy planning and childbearing experiences.6 Evidence demonstrates a reduction in annualized relapse rate during pregnancy and the postpartum period with exclusive breastfeeding. Therefore, pregnancy and exclusive breastfeeding provide a favorable immunomodulatory effect in women with MS, which combats the increased relapse risk associated with the postpartum period.7,8
Reproductive education—including conception, pregnancy, and breastfeeding—is critical for patients with MS and their partners during a woman’s childbearing years. However, women with MS do not require special considerations during pregnancy unless they have remarkable disability. As soon as these women and their partners decide upon pregnancy, a plan should be established that includes a discussion about potential risks to the fetus due to drug exposure, as well as risks to the mother. The goal should be to minimize risk for disease activity and optimize the health of the baby.
Use of DMTs during conception, pregnancy, and breastfeeding. All disease-modifying therapies (DMTs) are usually discontinued during pregnancy and breastfeeding. Common practice among MS experts is to discontinue DMTs prior to conception, with a few exceptions. There is no consensus about timing of discontinuation and washout period for each DMT. The decision is based on the half-life of each DMT, the opinions of the woman and her partner, and risk tolerance. Note: For the purposes of this discussion, the old pregnancy category designations are used, since they are familiar to clinicians. New guidelines took effect in June 2015; Table 3 outlines the change in format.

Injectables. Glatiramer acetate (GA) is the safest drug in relation to pregnancy and breastfeeding (category B). There is no evidence of congenital malformation or spontaneous abortion. The common recommendation is to discontinue the drug one to two months before conception, although some clinicians allow continuation of the injections throughout pregnancy and into breastfeeding.
Interferon-ßs are category C and therefore pose minimal risk for the fetus. The washout period before conception is two to three months, varying among clinicians. Although there is no evidence of spontaneous abortion or birth defects in humans, animal data show increased risk for abortion.9
Both GA and interferon-ßs are large molecules; there is a very minimal chance that the medication will transmit to the baby via breast milk. Thus, both DMTs are considered safe during lactation.7
Oral MS medications. The three approved MS oral medications are fingolimod, dimethyl fumarate (DMF), and teriflunomide. Fingolimod and DMF are both category C. Women on DMF must discontinue use of the medication one month prior to conception due to its short half-life. There are no reports of birth defects or spontaneous abortion in women taking DMF. Fingolimod needs to be discontinued two months prior to conception. Animal data show evidence for teratogenicity and embryolethality at lower doses of fingolimod than those used in humans.7
Teriflunomide is category X, posing high risk for the health of the fetus. It stays in the blood for approximately eight months after discontinuation of use. Animal data show teratogenicity and embryotoxicity; therefore, teriflunomide is contraindicated in pregnancy. Women on teriflunomide who plan to become pregnant need to undergo an elimination procedure with cholestyramine or charcoal.
Infusions and injections (monoclonal antibodies). The approved monoclonal antibodies include natalizumab, alemtuzumab, and daclizumab. (Currently, use of rituximab in MS is off label, and the FDA is reviewing the efficacy and safety data for ocrelizumab.) The monoclonal antibodies are category C. The recommendation is to discontinue natalizumab one to two months prior to conception and discontinue alemtuzumab four months preconception.10 There is no evidence of spontaneous abortion or birth defects in women on alemtuzumab, but there is potentially increased risk for spontaneous abortion in those on natalizumab.
The spontaneous abortion rate in daclizumab-exposed women is consistent with early pregnancy loss in the general population (12% to 26%). Data on a small number of pregnancies exposed to daclizumab did not suggest an increased risk for adverse fetal or maternal outcomes.11 However, the recommendation is to discontinue daclizumab four months prior to conception. Rituximab should be discontinued 12 months prior to conception, based on the manufacturer recommendation, although it is potentially safe to conceive when the B cell counts return to normal.
Chemotherapy agents. Mitoxantrone is the only FDA-approved chemotherapeutic drug used in MS. However, a few chemotherapy drugs—among them, azathioprine, methotrexate, and cyclophosphamide—are used off label. Chemotherapeutic agents are category D, except methotrexate (category X). Women on category D medications must use a method of birth control for three months after stopping the DMT. Often, clinicians will recommend women initiate GA or interferon during this period, in the hope of minimizing disease activity. Women on mitoxantrone and methotrexate need to use birth control for six months after stopping these immunosuppressive medications, before conceiving.12 These women likely need to switch to a safer pregnancy DMT during the long washout period.
Pregnancy and breastfeeding among women with MS require planning and decision making. The recommendations differ among clinicians and MS experts since there is no definitive evidence about the risks of the DMTs on the mother and/or the fetus. Clinicians should discuss the potential risks with women based on their knowledge and experience, and the data available based on animal research and the pregnancy registries. —ABB-Z
Aliza Bitton Ben-Zacharia, DNP, ANP
President-Elect of IOMSN
Neurology Faculty, Icahn School of Medicine, Mount Sinai Hospital System, New York, New York
Q)What are the considerations and recommendations for pregnancy and breastfeeding in women with multiple sclerosis? When should women discontinue their disease-modifying therapies?
Multiple sclerosis (MS) is an inflammatory, demyelinating, degenerative disease. Three times more common in women than men, it may affect pregnancy planning and childbearing experiences.6 Evidence demonstrates a reduction in annualized relapse rate during pregnancy and the postpartum period with exclusive breastfeeding. Therefore, pregnancy and exclusive breastfeeding provide a favorable immunomodulatory effect in women with MS, which combats the increased relapse risk associated with the postpartum period.7,8
Reproductive education—including conception, pregnancy, and breastfeeding—is critical for patients with MS and their partners during a woman’s childbearing years. However, women with MS do not require special considerations during pregnancy unless they have remarkable disability. As soon as these women and their partners decide upon pregnancy, a plan should be established that includes a discussion about potential risks to the fetus due to drug exposure, as well as risks to the mother. The goal should be to minimize risk for disease activity and optimize the health of the baby.
Use of DMTs during conception, pregnancy, and breastfeeding. All disease-modifying therapies (DMTs) are usually discontinued during pregnancy and breastfeeding. Common practice among MS experts is to discontinue DMTs prior to conception, with a few exceptions. There is no consensus about timing of discontinuation and washout period for each DMT. The decision is based on the half-life of each DMT, the opinions of the woman and her partner, and risk tolerance. Note: For the purposes of this discussion, the old pregnancy category designations are used, since they are familiar to clinicians. New guidelines took effect in June 2015; Table 3 outlines the change in format.

Injectables. Glatiramer acetate (GA) is the safest drug in relation to pregnancy and breastfeeding (category B). There is no evidence of congenital malformation or spontaneous abortion. The common recommendation is to discontinue the drug one to two months before conception, although some clinicians allow continuation of the injections throughout pregnancy and into breastfeeding.
Interferon-ßs are category C and therefore pose minimal risk for the fetus. The washout period before conception is two to three months, varying among clinicians. Although there is no evidence of spontaneous abortion or birth defects in humans, animal data show increased risk for abortion.9
Both GA and interferon-ßs are large molecules; there is a very minimal chance that the medication will transmit to the baby via breast milk. Thus, both DMTs are considered safe during lactation.7
Oral MS medications. The three approved MS oral medications are fingolimod, dimethyl fumarate (DMF), and teriflunomide. Fingolimod and DMF are both category C. Women on DMF must discontinue use of the medication one month prior to conception due to its short half-life. There are no reports of birth defects or spontaneous abortion in women taking DMF. Fingolimod needs to be discontinued two months prior to conception. Animal data show evidence for teratogenicity and embryolethality at lower doses of fingolimod than those used in humans.7
Teriflunomide is category X, posing high risk for the health of the fetus. It stays in the blood for approximately eight months after discontinuation of use. Animal data show teratogenicity and embryotoxicity; therefore, teriflunomide is contraindicated in pregnancy. Women on teriflunomide who plan to become pregnant need to undergo an elimination procedure with cholestyramine or charcoal.
Infusions and injections (monoclonal antibodies). The approved monoclonal antibodies include natalizumab, alemtuzumab, and daclizumab. (Currently, use of rituximab in MS is off label, and the FDA is reviewing the efficacy and safety data for ocrelizumab.) The monoclonal antibodies are category C. The recommendation is to discontinue natalizumab one to two months prior to conception and discontinue alemtuzumab four months preconception.10 There is no evidence of spontaneous abortion or birth defects in women on alemtuzumab, but there is potentially increased risk for spontaneous abortion in those on natalizumab.
The spontaneous abortion rate in daclizumab-exposed women is consistent with early pregnancy loss in the general population (12% to 26%). Data on a small number of pregnancies exposed to daclizumab did not suggest an increased risk for adverse fetal or maternal outcomes.11 However, the recommendation is to discontinue daclizumab four months prior to conception. Rituximab should be discontinued 12 months prior to conception, based on the manufacturer recommendation, although it is potentially safe to conceive when the B cell counts return to normal.
Chemotherapy agents. Mitoxantrone is the only FDA-approved chemotherapeutic drug used in MS. However, a few chemotherapy drugs—among them, azathioprine, methotrexate, and cyclophosphamide—are used off label. Chemotherapeutic agents are category D, except methotrexate (category X). Women on category D medications must use a method of birth control for three months after stopping the DMT. Often, clinicians will recommend women initiate GA or interferon during this period, in the hope of minimizing disease activity. Women on mitoxantrone and methotrexate need to use birth control for six months after stopping these immunosuppressive medications, before conceiving.12 These women likely need to switch to a safer pregnancy DMT during the long washout period.
Pregnancy and breastfeeding among women with MS require planning and decision making. The recommendations differ among clinicians and MS experts since there is no definitive evidence about the risks of the DMTs on the mother and/or the fetus. Clinicians should discuss the potential risks with women based on their knowledge and experience, and the data available based on animal research and the pregnancy registries. —ABB-Z
Aliza Bitton Ben-Zacharia, DNP, ANP
President-Elect of IOMSN
Neurology Faculty, Icahn School of Medicine, Mount Sinai Hospital System, New York, New York
6. Tullman MJ. Overview of the epidemiology, diagnosis, and disease progression associated with multiple sclerosis. Am J Manag Care. 2013;19(2):S15-S20.
7. Fabian M. Pregnancy in the setting of multiple sclerosis. Continuum (Minneap Minn). 2016; 22(3):837-850.
8. Vukusic S, Hutchinson M, Hours M, et al. Pregnancy and multiple sclerosis (the PRIMS study): clinical predictors of post-partum relapse. Brain. 2004;127(6):1353-1360.
9. Sandberg-Wollheim M, Alteri E, Moraga MS, Kornmann G. Pregnancy outcomes in multiple sclerosis following subcutaneous interferon beta-1a therapy. Mult Scler. 2011; 17(4):423-430.
10. Coyle PK. Multiple sclerosis in pregnancy. Continuum (Minneap Minn). 2014;20(1):42-59.
11. Gold SM, Voskuhl RR. Pregnancy and multiple sclerosis: from molecular mechanisms to clinical application. Semin Immunopathol. 2016;38:709.
12. Vukusic S, Marignier R. Multiple sclerosis and pregnancy in the ‘treatment era’. Nat Rev Neurol. 2015;11(5):280-289.
13. FDA. Pregnancy and lactation labeling (drugs) final rule. www.fda.gov/Drugs/DevelopmentApprovalProcess/DevelopmentResources/Labeling/ucm093307.htm. Accessed November 2, 2016.
14. Whyte J. FDA implements new labeling for medications used during pregnancy and lactation. Am Fam Physician. 2016;94(1):12-13.
6. Tullman MJ. Overview of the epidemiology, diagnosis, and disease progression associated with multiple sclerosis. Am J Manag Care. 2013;19(2):S15-S20.
7. Fabian M. Pregnancy in the setting of multiple sclerosis. Continuum (Minneap Minn). 2016; 22(3):837-850.
8. Vukusic S, Hutchinson M, Hours M, et al. Pregnancy and multiple sclerosis (the PRIMS study): clinical predictors of post-partum relapse. Brain. 2004;127(6):1353-1360.
9. Sandberg-Wollheim M, Alteri E, Moraga MS, Kornmann G. Pregnancy outcomes in multiple sclerosis following subcutaneous interferon beta-1a therapy. Mult Scler. 2011; 17(4):423-430.
10. Coyle PK. Multiple sclerosis in pregnancy. Continuum (Minneap Minn). 2014;20(1):42-59.
11. Gold SM, Voskuhl RR. Pregnancy and multiple sclerosis: from molecular mechanisms to clinical application. Semin Immunopathol. 2016;38:709.
12. Vukusic S, Marignier R. Multiple sclerosis and pregnancy in the ‘treatment era’. Nat Rev Neurol. 2015;11(5):280-289.
13. FDA. Pregnancy and lactation labeling (drugs) final rule. www.fda.gov/Drugs/DevelopmentApprovalProcess/DevelopmentResources/Labeling/ucm093307.htm. Accessed November 2, 2016.
14. Whyte J. FDA implements new labeling for medications used during pregnancy and lactation. Am Fam Physician. 2016;94(1):12-13.
CAM in MS: What Works?
Q)How is complementary and alternative medicine used in multiple sclerosis, and how can I safely recommend it to my patients?
Complementary and alternative medicine (CAM) is a non-mainstream practice used in conjunction with conventional medicine.1 Its use is prevalent among people with and without chronic illnesses, including those living with multiple sclerosis (MS). Up to 70% of Americans with MS have used some type of CAM therapy, compared with 36% of the general population.1,2 CAM use is higher in women than in men and is highest among persons ages 35 to 49—two demographics also associated with MS.3
CAM practices include a myriad of therapies from different disciplines (see Table 1).3 Because most people who use CAM do not discuss it with their health care providers, it is important that providers inquire about patient use and are armed with basic safety and efficacy information.
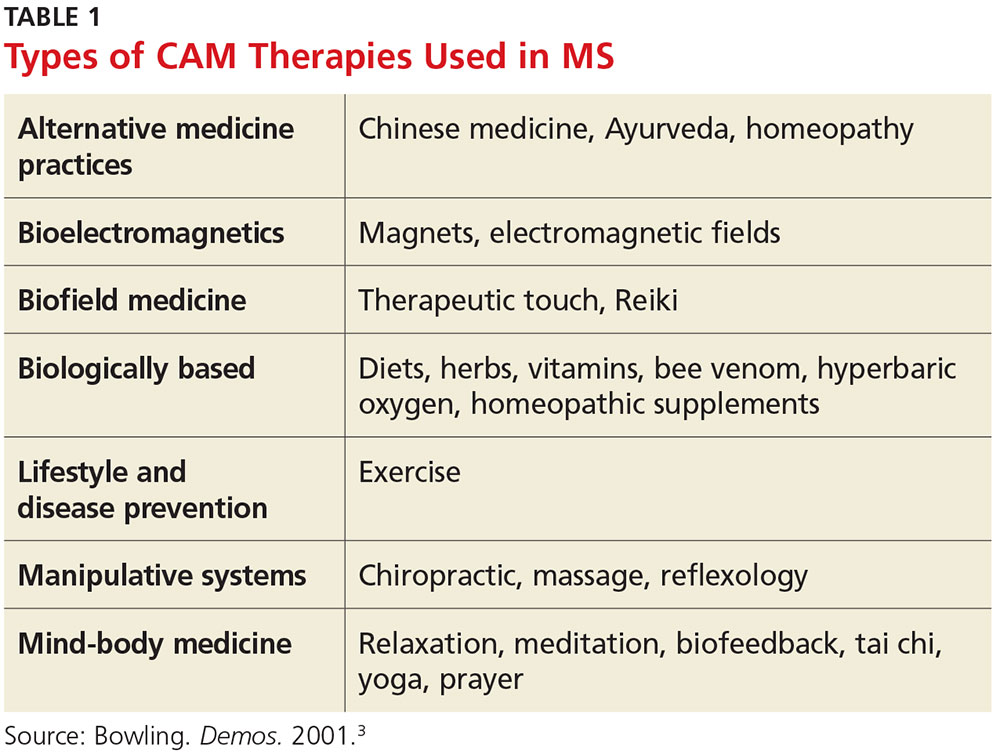
Office visits for MS should include safety and efficacy discussions about all therapeutic treatments (disease modifying, relapse, and symptom management). Some issues—such as adverse effects—are obvious, while others, such as cost, are less so. A patient with MS may pursue an extremely expensive CAM therapy that lacks substantial evidence for the condition. Providers should therefore consider cost as part of the safety equation and be aware that while some CAM therapies have been studied in MS, most have not (or the research has been of poor quality).4
For many commonly used therapies, there is insufficient scientific evidence to support their usefulness in MS. These include acupuncture, biofeedback, Chinese medicine, chiropractic care, replacing amalgam dental fillings, equine therapy, hyperbaric oxygen treatment, low-dose naltrexone, massage therapy, tai chi, and yoga. While many of these practices are relatively safe and inexpensive, others may cause financial harm. Conversely, something considered safe and inexpensive (eg, a low-fat diet with omega-3 supplementation) may be found to be ineffective. Although recommending this type of diet for a person with MS is safe, realistic expectations must be discussed regarding its effect (or lack thereof) on the condition.4
The effectiveness of medical marijuana for MS is another popular deliberation. While data suggest that several administration methods of oral cannabinoids may be effective for spasticity and pain reduction, there is inadequate evidence to support the use of smoked cannabis. The deleterious effects of cannabis on cognition also need to be considered.5
Dietary supplements (eg, vitamins, minerals, botanicals, dietary substances) are often regarded as safe by patients because they are “natural.” As clinicians, we must be direct in asking patients about everything they are taking—many dietary supplements have drug interactions and/or toxic effects and may adversely stimulate the immune system. Vitamin D, for example, is one supplement that has been heavily studied in MS; lower levels of vitamin D have been shown to increase the risk for MS, and higher levels may be associated with lower relapse and disability rates. Therefore, standard of practice is to monitor vitamin D levels and supplement accordingly.
CAM can be safely and effectively recommended to people living with MS with due diligence. A question guide to aid recommendations is listed in Table 2.

Currently, no CAM therapies have been shown to modify MS, and CAM should not be recommended in place of disease-modifying treatment. However, if the proper questions are addressed, many CAM therapies can be safely recommended for common MS symptoms. Insurance coverage varies significantly among policies, but some treatments (eg, acupuncture and chiropractic care) are gaining coverage.
Finally, it is safe and a good standard of care to recommend a healthy anti-inflammatory diet, such as the Mediterranean diet, to people living with MS in order to improve general health. —MW
Megan Weigel, DNP, ARNP-C, MSCN
President of IOMSN
Baptist Neurology, Jacksonville Beach, Florida
1. CDC. Complementary and alternative medicine use among adults: United States, 2002. http://nccih.nih.gov/sites/nccam.nih.gov/files/news/camstats/2002/report.pdf. Accessed October 28, 2016.
2. Yadav V, Shinto L, Bourdette D. Complementary and alternative medicine for the treatment of multiple sclerosis. Expert Rev Clin Immunol. 2010;6(3):381-395.
3. Bowling AC. Alternative Medicine and Multiple Sclerosis. New York: Demos; 2001.
4. Bowling AC. Complementary and alternative medicine in MS. www.nationalmssociety.org/nationalmssociety/media/msnationalfiles/brochures/clinical_bulletin_complementary-and-alternative-medicine-in-ms.pdf. Accessed November 2, 2016.
5. Honarmand K, Tierney MC, O’Connor P, Feinstein A. Effects of cannabis on cognitive function in patients with multiple sclerosis. Neurology. 2011;76(13):1153-1160.
Q)How is complementary and alternative medicine used in multiple sclerosis, and how can I safely recommend it to my patients?
Complementary and alternative medicine (CAM) is a non-mainstream practice used in conjunction with conventional medicine.1 Its use is prevalent among people with and without chronic illnesses, including those living with multiple sclerosis (MS). Up to 70% of Americans with MS have used some type of CAM therapy, compared with 36% of the general population.1,2 CAM use is higher in women than in men and is highest among persons ages 35 to 49—two demographics also associated with MS.3
CAM practices include a myriad of therapies from different disciplines (see Table 1).3 Because most people who use CAM do not discuss it with their health care providers, it is important that providers inquire about patient use and are armed with basic safety and efficacy information.

Office visits for MS should include safety and efficacy discussions about all therapeutic treatments (disease modifying, relapse, and symptom management). Some issues—such as adverse effects—are obvious, while others, such as cost, are less so. A patient with MS may pursue an extremely expensive CAM therapy that lacks substantial evidence for the condition. Providers should therefore consider cost as part of the safety equation and be aware that while some CAM therapies have been studied in MS, most have not (or the research has been of poor quality).4
For many commonly used therapies, there is insufficient scientific evidence to support their usefulness in MS. These include acupuncture, biofeedback, Chinese medicine, chiropractic care, replacing amalgam dental fillings, equine therapy, hyperbaric oxygen treatment, low-dose naltrexone, massage therapy, tai chi, and yoga. While many of these practices are relatively safe and inexpensive, others may cause financial harm. Conversely, something considered safe and inexpensive (eg, a low-fat diet with omega-3 supplementation) may be found to be ineffective. Although recommending this type of diet for a person with MS is safe, realistic expectations must be discussed regarding its effect (or lack thereof) on the condition.4
The effectiveness of medical marijuana for MS is another popular deliberation. While data suggest that several administration methods of oral cannabinoids may be effective for spasticity and pain reduction, there is inadequate evidence to support the use of smoked cannabis. The deleterious effects of cannabis on cognition also need to be considered.5
Dietary supplements (eg, vitamins, minerals, botanicals, dietary substances) are often regarded as safe by patients because they are “natural.” As clinicians, we must be direct in asking patients about everything they are taking—many dietary supplements have drug interactions and/or toxic effects and may adversely stimulate the immune system. Vitamin D, for example, is one supplement that has been heavily studied in MS; lower levels of vitamin D have been shown to increase the risk for MS, and higher levels may be associated with lower relapse and disability rates. Therefore, standard of practice is to monitor vitamin D levels and supplement accordingly.
CAM can be safely and effectively recommended to people living with MS with due diligence. A question guide to aid recommendations is listed in Table 2.

Currently, no CAM therapies have been shown to modify MS, and CAM should not be recommended in place of disease-modifying treatment. However, if the proper questions are addressed, many CAM therapies can be safely recommended for common MS symptoms. Insurance coverage varies significantly among policies, but some treatments (eg, acupuncture and chiropractic care) are gaining coverage.
Finally, it is safe and a good standard of care to recommend a healthy anti-inflammatory diet, such as the Mediterranean diet, to people living with MS in order to improve general health. —MW
Megan Weigel, DNP, ARNP-C, MSCN
President of IOMSN
Baptist Neurology, Jacksonville Beach, Florida
Q)How is complementary and alternative medicine used in multiple sclerosis, and how can I safely recommend it to my patients?
Complementary and alternative medicine (CAM) is a non-mainstream practice used in conjunction with conventional medicine.1 Its use is prevalent among people with and without chronic illnesses, including those living with multiple sclerosis (MS). Up to 70% of Americans with MS have used some type of CAM therapy, compared with 36% of the general population.1,2 CAM use is higher in women than in men and is highest among persons ages 35 to 49—two demographics also associated with MS.3
CAM practices include a myriad of therapies from different disciplines (see Table 1).3 Because most people who use CAM do not discuss it with their health care providers, it is important that providers inquire about patient use and are armed with basic safety and efficacy information.

Office visits for MS should include safety and efficacy discussions about all therapeutic treatments (disease modifying, relapse, and symptom management). Some issues—such as adverse effects—are obvious, while others, such as cost, are less so. A patient with MS may pursue an extremely expensive CAM therapy that lacks substantial evidence for the condition. Providers should therefore consider cost as part of the safety equation and be aware that while some CAM therapies have been studied in MS, most have not (or the research has been of poor quality).4
For many commonly used therapies, there is insufficient scientific evidence to support their usefulness in MS. These include acupuncture, biofeedback, Chinese medicine, chiropractic care, replacing amalgam dental fillings, equine therapy, hyperbaric oxygen treatment, low-dose naltrexone, massage therapy, tai chi, and yoga. While many of these practices are relatively safe and inexpensive, others may cause financial harm. Conversely, something considered safe and inexpensive (eg, a low-fat diet with omega-3 supplementation) may be found to be ineffective. Although recommending this type of diet for a person with MS is safe, realistic expectations must be discussed regarding its effect (or lack thereof) on the condition.4
The effectiveness of medical marijuana for MS is another popular deliberation. While data suggest that several administration methods of oral cannabinoids may be effective for spasticity and pain reduction, there is inadequate evidence to support the use of smoked cannabis. The deleterious effects of cannabis on cognition also need to be considered.5
Dietary supplements (eg, vitamins, minerals, botanicals, dietary substances) are often regarded as safe by patients because they are “natural.” As clinicians, we must be direct in asking patients about everything they are taking—many dietary supplements have drug interactions and/or toxic effects and may adversely stimulate the immune system. Vitamin D, for example, is one supplement that has been heavily studied in MS; lower levels of vitamin D have been shown to increase the risk for MS, and higher levels may be associated with lower relapse and disability rates. Therefore, standard of practice is to monitor vitamin D levels and supplement accordingly.
CAM can be safely and effectively recommended to people living with MS with due diligence. A question guide to aid recommendations is listed in Table 2.

Currently, no CAM therapies have been shown to modify MS, and CAM should not be recommended in place of disease-modifying treatment. However, if the proper questions are addressed, many CAM therapies can be safely recommended for common MS symptoms. Insurance coverage varies significantly among policies, but some treatments (eg, acupuncture and chiropractic care) are gaining coverage.
Finally, it is safe and a good standard of care to recommend a healthy anti-inflammatory diet, such as the Mediterranean diet, to people living with MS in order to improve general health. —MW
Megan Weigel, DNP, ARNP-C, MSCN
President of IOMSN
Baptist Neurology, Jacksonville Beach, Florida
1. CDC. Complementary and alternative medicine use among adults: United States, 2002. http://nccih.nih.gov/sites/nccam.nih.gov/files/news/camstats/2002/report.pdf. Accessed October 28, 2016.
2. Yadav V, Shinto L, Bourdette D. Complementary and alternative medicine for the treatment of multiple sclerosis. Expert Rev Clin Immunol. 2010;6(3):381-395.
3. Bowling AC. Alternative Medicine and Multiple Sclerosis. New York: Demos; 2001.
4. Bowling AC. Complementary and alternative medicine in MS. www.nationalmssociety.org/nationalmssociety/media/msnationalfiles/brochures/clinical_bulletin_complementary-and-alternative-medicine-in-ms.pdf. Accessed November 2, 2016.
5. Honarmand K, Tierney MC, O’Connor P, Feinstein A. Effects of cannabis on cognitive function in patients with multiple sclerosis. Neurology. 2011;76(13):1153-1160.
1. CDC. Complementary and alternative medicine use among adults: United States, 2002. http://nccih.nih.gov/sites/nccam.nih.gov/files/news/camstats/2002/report.pdf. Accessed October 28, 2016.
2. Yadav V, Shinto L, Bourdette D. Complementary and alternative medicine for the treatment of multiple sclerosis. Expert Rev Clin Immunol. 2010;6(3):381-395.
3. Bowling AC. Alternative Medicine and Multiple Sclerosis. New York: Demos; 2001.
4. Bowling AC. Complementary and alternative medicine in MS. www.nationalmssociety.org/nationalmssociety/media/msnationalfiles/brochures/clinical_bulletin_complementary-and-alternative-medicine-in-ms.pdf. Accessed November 2, 2016.
5. Honarmand K, Tierney MC, O’Connor P, Feinstein A. Effects of cannabis on cognitive function in patients with multiple sclerosis. Neurology. 2011;76(13):1153-1160.
Kidney Disease & “Bad Teeth”
Q)Someone at a conference I attended said kidney disease and bad teeth go hand in hand. Is this true? What does that mean for my patients?
“Bad teeth” can refer to periodontitis, a chronic inflammation of the tissue and structures around the teeth. The sixth most common disease in the world, periodontitis often leads to shrinkage of the gums, infection, and subsequent loosening or loss of teeth.3
Patients with chronic kidney disease (CKD) are predisposed to oral lesions and tooth decay related to dryness of the mouth; alterations in taste; malnutrition; and low albumin. Certain medications—such as ß-blockers, diuretics, anticholinergics, anticonvulsants, and serotonin reuptake inhibitors—can increase the risk for dry mouth and negatively affect oral structures.4
Compared with community-dwelling adults, those with CKD have higher rates of periodontitis, which increase with disease progression.5 A systematic review found that periodontitis increases the risk for CKD; evidence was inconclusive for the impact of periodontal treatment on estimated glomerular filtration rates (eGFR) but suggested positive improvements in eGFR.6
There is growing evidence of a multifaceted relationship between CKD, diabetes, periodontitis, and cardiovascular disease (CVD), the leading cause of mortality in patients with CKD.7 Studies have shown that periodontitis can contribute to systemic inflammation, inhibiting glycemic control and elevating the risk for conditions such as CVD.8-10
Diabetes, the most common cause of CKD, is associated with adverse dental outcomes and poor glycemic control. Vice versa, severe periodontitis increases risk for diabetes and worsening glucose control. Mechanical periodontal treatment has been shown to improve glycemic control.8
A recent study showed an increased risk for both CVD events and all-cause mortality in those with stage III to stage V CKD (eGFR < 60 mL/min/1.73 m2). The study also found that periodontitis increased 10-year all-cause mortality in this population (see Figure).11
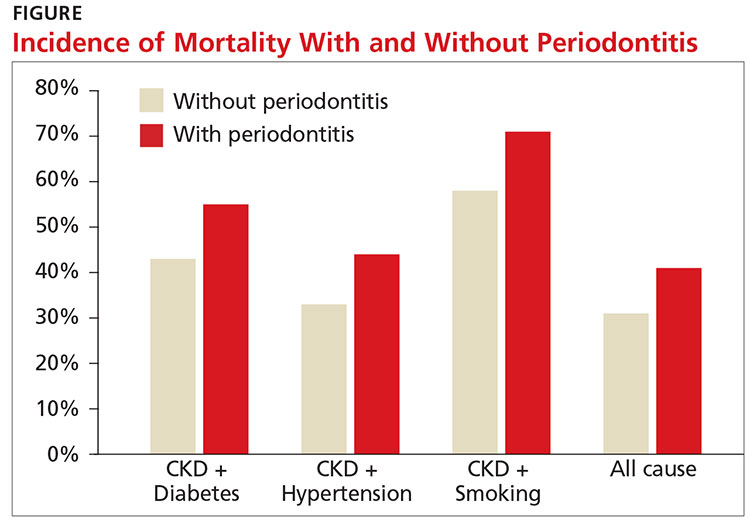
Research is ongoing regarding the complex relationship between CKD and oral health. For patients with CKD at any stage, evidence promotes the benefits of good oral health habits. Encourage smoking cessation, daily flossing and tooth brushing, regular dental cleanings, and prompt evaluation and treatment of any oral issues.12 —CS
Cynthia Smith, DNP, CNN-NP, FNP-BC, APRN
Renal Consultants PLLC, South Charleston, West Virginia
3. Page RC, Eke PI. Case definitions for use in population-based surveillance of periodontitis. J Periodontol. 2007;78(7):1387-1399.
4. Akar H, Akar GC, Carrero JJ, et al. Systemic consequences of poor oral health in chronic kidney disease patients. Clin J Am Soc Nephrol. 2011;6(1):218-226.
5. Borawski J, Wilczyn´ska-Borawska M, Stokowska W, Mys´liwiec M. The periodontal status of pre-dialysis chronic kidney disease and maintenance dialysis patients. Nephrol Dial Transplant. 2007;22(2):457-464.
6. Chambrone L, Foz AM, Guglielmetti MR, et al. Periodontitis and chronic kidney disease: a systematic review of the association of diseases and the effect of periodontal treatment on estimated glomerular filtration rate. J Clin Periodontol. 2013;40(5):443-456.
7. Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296-1305.
8. Kassebaum NJ, Bernabé E, Dahiya M, et al. Global burden of severe periodontitis in 1990-2010: a systematic review and meta-regression. J Dent Res. 2014;93(11):1045-1053.
9. Chapple IL, Genco R; Working Group 2 of Joint EFP/AAP Workshop. Diabetes and periodontal diseases: consensus report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. J Clin Periodontol. 2013; 40(14):S106-S112.
10. Menon V, Greene T, Wang X, et al. C-reactive protein and albumin as predictors of all-cause and cardiovascular mortality in chronic kidney disease. Kidney Int. 2005;68(2):766-772.
11. Sharma P, Dietrich T, Ferro CJ, et al. Association between periodontitis and mortality in stages 3-5 chronic kidney disease: NHANES III and linked mortality study. J Clin Periodontol. 2016;43(2):104-113.
12. Ariyamuthu VK, Nolph KD, Ringdahl BE. Periodontal disease in chronic kidney disease and end-stage renal disease patients: a review. Cardiorenal Med. 2013;3(1):71-78.
Q)Someone at a conference I attended said kidney disease and bad teeth go hand in hand. Is this true? What does that mean for my patients?
“Bad teeth” can refer to periodontitis, a chronic inflammation of the tissue and structures around the teeth. The sixth most common disease in the world, periodontitis often leads to shrinkage of the gums, infection, and subsequent loosening or loss of teeth.3
Patients with chronic kidney disease (CKD) are predisposed to oral lesions and tooth decay related to dryness of the mouth; alterations in taste; malnutrition; and low albumin. Certain medications—such as ß-blockers, diuretics, anticholinergics, anticonvulsants, and serotonin reuptake inhibitors—can increase the risk for dry mouth and negatively affect oral structures.4
Compared with community-dwelling adults, those with CKD have higher rates of periodontitis, which increase with disease progression.5 A systematic review found that periodontitis increases the risk for CKD; evidence was inconclusive for the impact of periodontal treatment on estimated glomerular filtration rates (eGFR) but suggested positive improvements in eGFR.6
There is growing evidence of a multifaceted relationship between CKD, diabetes, periodontitis, and cardiovascular disease (CVD), the leading cause of mortality in patients with CKD.7 Studies have shown that periodontitis can contribute to systemic inflammation, inhibiting glycemic control and elevating the risk for conditions such as CVD.8-10
Diabetes, the most common cause of CKD, is associated with adverse dental outcomes and poor glycemic control. Vice versa, severe periodontitis increases risk for diabetes and worsening glucose control. Mechanical periodontal treatment has been shown to improve glycemic control.8
A recent study showed an increased risk for both CVD events and all-cause mortality in those with stage III to stage V CKD (eGFR < 60 mL/min/1.73 m2). The study also found that periodontitis increased 10-year all-cause mortality in this population (see Figure).11

Research is ongoing regarding the complex relationship between CKD and oral health. For patients with CKD at any stage, evidence promotes the benefits of good oral health habits. Encourage smoking cessation, daily flossing and tooth brushing, regular dental cleanings, and prompt evaluation and treatment of any oral issues.12 —CS
Cynthia Smith, DNP, CNN-NP, FNP-BC, APRN
Renal Consultants PLLC, South Charleston, West Virginia
Q)Someone at a conference I attended said kidney disease and bad teeth go hand in hand. Is this true? What does that mean for my patients?
“Bad teeth” can refer to periodontitis, a chronic inflammation of the tissue and structures around the teeth. The sixth most common disease in the world, periodontitis often leads to shrinkage of the gums, infection, and subsequent loosening or loss of teeth.3
Patients with chronic kidney disease (CKD) are predisposed to oral lesions and tooth decay related to dryness of the mouth; alterations in taste; malnutrition; and low albumin. Certain medications—such as ß-blockers, diuretics, anticholinergics, anticonvulsants, and serotonin reuptake inhibitors—can increase the risk for dry mouth and negatively affect oral structures.4
Compared with community-dwelling adults, those with CKD have higher rates of periodontitis, which increase with disease progression.5 A systematic review found that periodontitis increases the risk for CKD; evidence was inconclusive for the impact of periodontal treatment on estimated glomerular filtration rates (eGFR) but suggested positive improvements in eGFR.6
There is growing evidence of a multifaceted relationship between CKD, diabetes, periodontitis, and cardiovascular disease (CVD), the leading cause of mortality in patients with CKD.7 Studies have shown that periodontitis can contribute to systemic inflammation, inhibiting glycemic control and elevating the risk for conditions such as CVD.8-10
Diabetes, the most common cause of CKD, is associated with adverse dental outcomes and poor glycemic control. Vice versa, severe periodontitis increases risk for diabetes and worsening glucose control. Mechanical periodontal treatment has been shown to improve glycemic control.8
A recent study showed an increased risk for both CVD events and all-cause mortality in those with stage III to stage V CKD (eGFR < 60 mL/min/1.73 m2). The study also found that periodontitis increased 10-year all-cause mortality in this population (see Figure).11

Research is ongoing regarding the complex relationship between CKD and oral health. For patients with CKD at any stage, evidence promotes the benefits of good oral health habits. Encourage smoking cessation, daily flossing and tooth brushing, regular dental cleanings, and prompt evaluation and treatment of any oral issues.12 —CS
Cynthia Smith, DNP, CNN-NP, FNP-BC, APRN
Renal Consultants PLLC, South Charleston, West Virginia
3. Page RC, Eke PI. Case definitions for use in population-based surveillance of periodontitis. J Periodontol. 2007;78(7):1387-1399.
4. Akar H, Akar GC, Carrero JJ, et al. Systemic consequences of poor oral health in chronic kidney disease patients. Clin J Am Soc Nephrol. 2011;6(1):218-226.
5. Borawski J, Wilczyn´ska-Borawska M, Stokowska W, Mys´liwiec M. The periodontal status of pre-dialysis chronic kidney disease and maintenance dialysis patients. Nephrol Dial Transplant. 2007;22(2):457-464.
6. Chambrone L, Foz AM, Guglielmetti MR, et al. Periodontitis and chronic kidney disease: a systematic review of the association of diseases and the effect of periodontal treatment on estimated glomerular filtration rate. J Clin Periodontol. 2013;40(5):443-456.
7. Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296-1305.
8. Kassebaum NJ, Bernabé E, Dahiya M, et al. Global burden of severe periodontitis in 1990-2010: a systematic review and meta-regression. J Dent Res. 2014;93(11):1045-1053.
9. Chapple IL, Genco R; Working Group 2 of Joint EFP/AAP Workshop. Diabetes and periodontal diseases: consensus report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. J Clin Periodontol. 2013; 40(14):S106-S112.
10. Menon V, Greene T, Wang X, et al. C-reactive protein and albumin as predictors of all-cause and cardiovascular mortality in chronic kidney disease. Kidney Int. 2005;68(2):766-772.
11. Sharma P, Dietrich T, Ferro CJ, et al. Association between periodontitis and mortality in stages 3-5 chronic kidney disease: NHANES III and linked mortality study. J Clin Periodontol. 2016;43(2):104-113.
12. Ariyamuthu VK, Nolph KD, Ringdahl BE. Periodontal disease in chronic kidney disease and end-stage renal disease patients: a review. Cardiorenal Med. 2013;3(1):71-78.
3. Page RC, Eke PI. Case definitions for use in population-based surveillance of periodontitis. J Periodontol. 2007;78(7):1387-1399.
4. Akar H, Akar GC, Carrero JJ, et al. Systemic consequences of poor oral health in chronic kidney disease patients. Clin J Am Soc Nephrol. 2011;6(1):218-226.
5. Borawski J, Wilczyn´ska-Borawska M, Stokowska W, Mys´liwiec M. The periodontal status of pre-dialysis chronic kidney disease and maintenance dialysis patients. Nephrol Dial Transplant. 2007;22(2):457-464.
6. Chambrone L, Foz AM, Guglielmetti MR, et al. Periodontitis and chronic kidney disease: a systematic review of the association of diseases and the effect of periodontal treatment on estimated glomerular filtration rate. J Clin Periodontol. 2013;40(5):443-456.
7. Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296-1305.
8. Kassebaum NJ, Bernabé E, Dahiya M, et al. Global burden of severe periodontitis in 1990-2010: a systematic review and meta-regression. J Dent Res. 2014;93(11):1045-1053.
9. Chapple IL, Genco R; Working Group 2 of Joint EFP/AAP Workshop. Diabetes and periodontal diseases: consensus report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. J Clin Periodontol. 2013; 40(14):S106-S112.
10. Menon V, Greene T, Wang X, et al. C-reactive protein and albumin as predictors of all-cause and cardiovascular mortality in chronic kidney disease. Kidney Int. 2005;68(2):766-772.
11. Sharma P, Dietrich T, Ferro CJ, et al. Association between periodontitis and mortality in stages 3-5 chronic kidney disease: NHANES III and linked mortality study. J Clin Periodontol. 2016;43(2):104-113.
12. Ariyamuthu VK, Nolph KD, Ringdahl BE. Periodontal disease in chronic kidney disease and end-stage renal disease patients: a review. Cardiorenal Med. 2013;3(1):71-78.
Innovative Approaches to Substance Use Disorder Treatment at the VA
While much of private health care in the U.S. is only beginning to confront the burgeoning opioid epidemic, the VA has a long-standing commitment to providing treatment to veterans struggling with substance use disorders. To understand the scope of the challenge and the VA’s approach, Federal Practitioner Editor-in-Chief Cynthia M.A. Geppert, MD, talked with Karen Drexler, MD, national mental health program director, substance use disorders. Dr. Drexler served in the U.S. Air Force for 8 years before joining the Atlanta VAMC in Georgia, where she directed the substance abuse treatment program. She is an associate professor of psychiatry and behavioral sciences at Emory University. In 2014, Dr. Drexler was named deputy mental health program director, addictive disorders.
Editor-in-Chief Cynthia M.A. Geppert, MD. As national mental health program director for substance use disorders, what are the challenges facing VA substance use programs?
Karen Drexler, MD. The biggest challenge is the increasing demand for services. Veterans have been coming to the VA for mental health care in general and for substance use disorder treatment in particular. The most common substance use disorder that we treat in addition to tobacco is alcohol use disorder, and the demand for alcohol use disorder treatment continues to grow.
Also, with the opioid crisis, there’s increasing demand for opioid use disorder treatments, including what we recommend as first-line treatment, which is medication-assisted treatment using buprenorphine or methadone, or injectable naltrexone as a second-line treatment. Gearing up with medication-assisted treatment for both alcohol and opioid use disorders is probably our biggest challenge.
Dr. Geppert. What were the most important accomplishments of the VA substance abuse programs over the past 5 years?
Dr. Drexler. There have been a lot! First of all, one of the things I love about practicing within the VA is that we are an integrated health care system. When I talk with colleagues in the Emory system here in Atlanta and across the country, oftentimes mental health and substance use disorders are isolated. The funding streams through the public sector come in different ways. Third-party payers have carve-outs for behavioral health.
It’s really wonderful to be able to collaborate with my colleagues in primary care, medical specialty care, and general mental health care, to provide a holistic and team-based approach to our patients who have both medical and substance use disorder problems and often co-occurring mental illness. As we’ve moved from a hospital-based system to more outpatient [care], the VA continues to be able to collaborate through our electronic health records as well as just being able to pick up the phone or send a [Microsoft] Lync message or an Outlook encrypted e-mail to help facilitate that coordinated care.
Another accomplishment has been to [develop] policy. It’s one of our challenges, but it’s also an accomplishment even at this early stage. In 2008, we issued the Uniform Mental Health Services benefits package as part of VHA Handbook on Mental Health Services. From that very beginning, we have included medication-assisted therapy when indicated for veterans with substance use disorders.
Now, we also know there’s a lot of variability in the system, so not every facility is providing these indicated treatments yet; but at least it’s part of our policy, and it’s been a focus of ongoing quality improvement to make these treatments available for veterans when they need them.
Dr. Geppert. We’ve heard a lot lately about the CDC guidelines for prescribing opiates for chronic pain that were published earlier this year. How do you see these guidelines affecting the VA Opiate Safety Initiative? Do you see any important contradictions between current VA policy and these new guidelines?
Dr. Drexler. Yes—not necessarily with policy but with our VA/DoD Clinical Practice Guidelines from 2010 and the new CDC guidelines. There’s a shift in emphasis. The 2010 VA/DoD guidelines were maybe a little too optimistic about the safety of opioids for chronic, noncancer pain. As time goes on, more evidence is mounting of the potential harms, including from my perspective, the risk of developing an opioid use disorder when taking opioid analgesics as prescribed.
In the 1990s and the early 2000s, experts in the field reassured us. They told us all—patients and providers alike—that if opioid analgesics were taken as prescribed for legitimate pain by people who didn’t have a previous history of a substance use disorder, they could take them and would not be at risk for becoming addicted. We now know that’s simply not true. Patients who take these medicines as prescribed by their providers end up developing tolerance, developing hyperalgesia, needing more and more medication to get the desired effect, and crossing that line from just physiologic tolerance to developing an opioid use disorder.
We now have better guidelines about where that risk increases. We have some data from observational studies that patients who are maintained on opioid analgesics that are < 50 mg of daily morphine equivalent dose (MED) tend not to have as high a risk of overdose as those who are maintained on more, but the risk of developing an opioid use disorder actually is not insignificant even below 50 mg MED.
The key for developing an opioid use disorder is how long the patient is on the treatment. For patients who take opioid analgesics for < 90 days—again, these are observational studies—very few went on to develop opioid use disorder. However, those who took it for > 90 days, even those who were maintained on < 36 mg MED, had a significantly increased risk of developing an opioid use disorder. And for those who were on the higher dose, say, > 120 mg MED, the risk went up to 122-fold.
We just don’t see those kinds of odds ratios elsewhere in medicine. You know, we address risk factors when it increases the risk by 50%, but this is 122-fold.
Dr. Geppert. Pain as the fifth vital sign had a dark side there. Is there anything else you want to say to us about the Opiate Safety Initiative?
Dr. Drexler. I think it’s been incredibly successful; but like any change, there are unintended consequences and challenges with any change, even a good change. One of the things that has been a good change is raising awareness that opioids are not the answer for chronic pain. We have better evidence that self-care strategies (movement, exercise, physical therapy, and cognitive behavioral therapy for pain) help folks learn strategies for addressing automatic alarming thoughts about their pain—that it’s signaling something terrible when, really, it’s not. There are things that people can do to manage pain on their own. Those [strategies] are safer, and we have better evidence that they are effective than we have for opioids taken past 12 weeks.
Dr. Geppert. It’s really a paradigm shift in understanding. The Comprehensive Addiction and Recovery Act of 2016 has proposed to allow some nurse practitioners (NPs) and physician assistants (PAs) to obtain a waiver to prescribe buprenorphine. What role does the VA see for PAs and NPs in patient care for veterans with substance use disorders with medication-assisted therapy, and do you think that’ll involve prescribing buprenorphine?
Dr. Drexler. One of the things that I love about practicing within the VA is working within an interdisciplinary team and having that team-based approach. When we work together, our results far outshine anything that any one of us could do separately. I am very much looking forward to being able to provide or collaborate with other federal partners like the Substance Abuse and Mental Health Services Administration [SAMHSA] to provide the training for VA NPs and PAs who are able to prescribe controlled substances with their state licensure to provide buprenorphine for our patients.
Even without that prescriptive authority, NPs and PAs are already important partners and members of the interdisciplinary team who are managing patients and providing the evidence-based counseling, the monitoring, following up urine drug screen results, and doing a lot of the management, but not signing the prescription that’s being provided now by the Drug Addiction Treatment Act-waivered physicians. But SAMHSA has yet to define what the 24 hours of training will be that NPs and PAs will need to complete in order to apply for their U.S. Drug Enforcement Administration waiver, but we’re very much looking forward to it. And I am in contact with colleagues at SAMHSA, and they promise me they will keep me informed when they have the regulations set out for us to follow.
Dr. Geppert. The new VA/DoD Clinical Practice Guidelines for Management of Substance Use Disorders were published earlier this year. Could you give us an overview of the guidelines and what’s significant for most of our providers in the field?
Dr. Drexler. I’m very excited about the guidelines. One of the first things you should know is that they are very evidence based. If you’ve been practicing medicine as long as I have, you’ll remember, when the first clinical practice guidelines came out, they were expert opinion. They got some of the leading practitioners and researchers in the country together, put them in a room, and said, “What do you think people should do to treat this disorder?” Over time, clinical practice guidelines have become more evidence based; and the VA/DoD collaborative follows a highly respected methodology called the GRADE methodology.
Let me explain that just briefly. It means that our recommendations are based on systematic reviews of the literature. We all have our own screens and filters and our favorite studies. And so, if you get a bunch of experts together, they may not have as dispassionate a view of the entire literature as when you do a systematic review; and that’s what we started with.
We followed a specific methodology as well that took into account not only the strength of the evidence where the gold standard is a randomized, controlled clinical trial and, even better than that, a meta-analysis of multiple clinical trials. And for our recommendations, the strength of the evidence was, by far, supported mostly by clinical trials or meta-analyses. So, when you look at the VA/DoD Clinical Practice Guidelines, and I hope you will, you can feel confident that what we’re recommending is not just pulled out of the sky or some idiosyncratic favorite thing that Karen Drexler likes. It’s really based on good, strong clinical evidence.
We went from the previous version that had over 160 recommendations. We distilled them down to 36, and those 36 recommendations cover everything from prevention in primary care settings to stabilization and withdrawal management for alcohol and sedatives and opioids, and then treatment or rehabilitation of the 4 most common substance use disorders and then some general principles (Table 1).
There’s good evidence that what we’re doing in VA with screening in primary care every year using the AUDIT-C [Alcohol Use Disorders Identification Test-Consumption] is making a difference (Table 2). That folks who screen positive for at-risk alcohol use are very likely to respond to a brief intervention by their health care provider and reduce their alcohol consumption to safe levels, where they’re not likely to go on to develop an addiction to the alcohol or to develop medical complications from heavy alcohol use.
After screening, then, if someone does develop a substance use disorder, we have evidence-based treatments, both medications for 2 of our most common disorders and psychosocial interventions for all 4 most common disorders after tobacco. And I don’t exclude tobacco because it’s not important; it’s incredibly important, but it gets its own clinical practice guidelines. So, with our limited budget, we just addressed the other 10 substances….
For alcohol we have great evidence for 5 different medications and for 6 different psychosocial approaches. It is the most common substance use disorder, but we also have a whole menu of different treatments that folks can choose from, everything from naltrexone—oral naltrexone is the most common medication that we prescribe—but also acamprosate; disulfiram; extended-release injectable naltrexone. Topiramate, interestingly, is not FDA approved for that indication, but we have found good evidence from clinical trials supporting topiramate, so we strongly recommend it. If any of those are not acceptable or not tolerated or ineffective, then we also have some evidence to support gabapentin as well. So we have a strong recommendation for the first ones and a suggestion for gabapentin if it’s clinically indicated.
In terms of psychosocial approaches, [we recommend] everything from motivation enhancement therapy or motivational interviewing; cognitive-behavioral therapy; 12-step facilitation, which is a structured way of getting folks to get more active in Alcoholics Anonymous; behavioral approaches like community reinforcement approach or contingency management.
For opioid use disorder, interestingly, we found good, strong evidence that medication-assisted treatments work but no evidence for any psychosocial intervention without medication. Let me say that again because, nationwide, the most common treatment that’s provided for opioid use disorder, for any substance use disorder, is a psychosocial intervention—talking therapy alone.
For all of the medicines for alcohol use disorder, the medicine improved upon talking therapy alone. For opioid use disorder, medication helps people stay sober; it retains them in treatment; it saves lives. But for talking therapy without medication, we have found insufficient evidence to recommend any particular treatment.
Dr. Geppert. Incredible. That leads to my next question: One of the most challenging issues facing primary care practitioners is how to manage patients who are on chronic opiate therapy but also use medical marijuana in the increasing number of states where it’s legal. Do you have some advice on how to approach these situations?
Dr. Drexler. It’s a great question, and it’s one I’m sure more and more VA practitioners are grappling with. By our policy, we do not exclude veterans who are taking medical marijuana from any VA services, and we encourage the veterans to speak up about it so that it can be considered as part of their treatment plan.
By policy, however, VA practitioners do not fill out forms for state-approved marijuana. Marijuana is still a Schedule I controlled substance by federal law, so it’s a very interesting legal situation where it’s illegal federally; but some states have passed laws making it legal by state law. It will be interesting to see how this plays out over the next few years.
But in that situation—and really, this is our policy, in general for substance use disorders—a patient having a substance use disorder should not be precluded or excluded from getting treatment for other medical conditions. Similarly, other medical conditions shouldn’t exclude patients from getting treatment for their substance use disorder. However, the best treatment comes when we look at the whole person and try to take both things into account.
In the same way that patients can become addicted to their opioid pain medicines, patients can become addicted to medical marijuana. So the marijuana use needs to be evaluated objectively; and whether or not they have a form, just like whether or not someone has a prescription for their opioid that may be causing them problems, that needs to be taken into account in the treatment plan; and the treatment needs to be individualized.
Dr. Geppert. What in VA substance use disorder programs and treatment do you think is especially innovative when compared to the community?
Dr. Drexler. One of the places where I think the VA is well ahead of our colleagues elsewhere in health care is…from SAIL [Strategic Analytics for Improvement and Learning] metrics. About 34% or 35% of veterans who are diagnosed clinically within our system with opioid use disorders are receiving medication-assisted therapy either with buprenorphine, methadone, or extended-release injectable naltrexone. When you look at the SAMHSA survey, the National Survey on Drug Use and Health, the percentage of patients in the community who meet criteria for a substance use disorder [who] actually receive treatment is more like 12%. We have no numbers from SAMHSA about what percentage are receiving medication-assisted treatment. They don’t ask. In that way, even though we have a long ways to go, we’re ahead of the curve when it comes to medication-assisted treatment. But the demand is growing, we’re going to have to keep working hard to keep up and to get ahead at this. When I think about it compared to other chronic illnesses, only 35% of our patients are getting the first-line recommended treatment. We still have a long ways to go.
Another place where we’re innovative is with contingency management. I mentioned psychosocial treatments. Behavioral treatment is very effective and yet is not often used in treatment of substance use disorders; but it works. And even better than that, it is fun.
Let me try to explain what this means. It’s based on the idea that the benefits of using drugs are immediate, and the benefits of staying sober take a long time to realize. And it is that difference that makes early recovery so challenging because, if someone has a craving for alcohol or another drug, they can satisfy that craving immediately and then have to pay the price, though, of health consequences or financial consequences or legal consequences from that alcohol or drug use.
On the other hand, staying sober is tough. But it takes a long time to put one’s life back together, to get one’s chronic health conditions that have been caused or made worse by the drug use back together. And the idea behind contingency management is to give immediate rewards early in recovery to keep folks engaged long enough to start realizing some of the longer-term benefits of recovery.
So the way this works, we use what we call the fishbowl model of contingency management. And we started with stimulant use disorders, which is one of the substance use disorders for which there’s no strong evidence for a medication that works; but this particular psychosocial intervention works very well. So patients come to the clinic twice a week, give a urine specimen for drug screen; and, based on those test results, if their test is negative and they haven’t used any stimulants, then they get a draw from the fishbowl, which is filled with slips of paper. Half of the slips of paper say, “Good job! Keep up the good work,” or something encouraging like that. And half of the slips of paper have a prize associated with them, and it could be a small prize like $1 or $5 for exchange at the VA Canteen store, or it could be a larger prize like $100 or $200. Of course, the less expensive prizes are more common than the more expensive prizes; but just that immediate reward can make such a difference.
With the first negative urine drug screen, you get 1 draw from the fishbowl. The second one in a row, you get 2; and so it goes that the longer you stay sober, the more chances you have for winning a prize. And this treatment can go on for as long as the veteran wants to continue for up to 12 weeks. Usually by 12 weeks, folks’ health and their lives are coming back together; and so there’s many more rewards for staying sober than just the draws from the fishbowl. And what we find is folks who get a good start with contingency management tend to stay on that trajectory towards recovery.
And this has been a wonderful partnership between the Center of Excellence for Substance Abuse Treatment and Education in Philadelphia and the VA Canteen Service, who has generously donated canteen coupons. So it doesn’t cost the medical center anything to offer these prizes, and we’ve had just some amazing results.
To listen to the unedited interview here
While much of private health care in the U.S. is only beginning to confront the burgeoning opioid epidemic, the VA has a long-standing commitment to providing treatment to veterans struggling with substance use disorders. To understand the scope of the challenge and the VA’s approach, Federal Practitioner Editor-in-Chief Cynthia M.A. Geppert, MD, talked with Karen Drexler, MD, national mental health program director, substance use disorders. Dr. Drexler served in the U.S. Air Force for 8 years before joining the Atlanta VAMC in Georgia, where she directed the substance abuse treatment program. She is an associate professor of psychiatry and behavioral sciences at Emory University. In 2014, Dr. Drexler was named deputy mental health program director, addictive disorders.
Editor-in-Chief Cynthia M.A. Geppert, MD. As national mental health program director for substance use disorders, what are the challenges facing VA substance use programs?
Karen Drexler, MD. The biggest challenge is the increasing demand for services. Veterans have been coming to the VA for mental health care in general and for substance use disorder treatment in particular. The most common substance use disorder that we treat in addition to tobacco is alcohol use disorder, and the demand for alcohol use disorder treatment continues to grow.
Also, with the opioid crisis, there’s increasing demand for opioid use disorder treatments, including what we recommend as first-line treatment, which is medication-assisted treatment using buprenorphine or methadone, or injectable naltrexone as a second-line treatment. Gearing up with medication-assisted treatment for both alcohol and opioid use disorders is probably our biggest challenge.
Dr. Geppert. What were the most important accomplishments of the VA substance abuse programs over the past 5 years?
Dr. Drexler. There have been a lot! First of all, one of the things I love about practicing within the VA is that we are an integrated health care system. When I talk with colleagues in the Emory system here in Atlanta and across the country, oftentimes mental health and substance use disorders are isolated. The funding streams through the public sector come in different ways. Third-party payers have carve-outs for behavioral health.
It’s really wonderful to be able to collaborate with my colleagues in primary care, medical specialty care, and general mental health care, to provide a holistic and team-based approach to our patients who have both medical and substance use disorder problems and often co-occurring mental illness. As we’ve moved from a hospital-based system to more outpatient [care], the VA continues to be able to collaborate through our electronic health records as well as just being able to pick up the phone or send a [Microsoft] Lync message or an Outlook encrypted e-mail to help facilitate that coordinated care.
Another accomplishment has been to [develop] policy. It’s one of our challenges, but it’s also an accomplishment even at this early stage. In 2008, we issued the Uniform Mental Health Services benefits package as part of VHA Handbook on Mental Health Services. From that very beginning, we have included medication-assisted therapy when indicated for veterans with substance use disorders.
Now, we also know there’s a lot of variability in the system, so not every facility is providing these indicated treatments yet; but at least it’s part of our policy, and it’s been a focus of ongoing quality improvement to make these treatments available for veterans when they need them.
Dr. Geppert. We’ve heard a lot lately about the CDC guidelines for prescribing opiates for chronic pain that were published earlier this year. How do you see these guidelines affecting the VA Opiate Safety Initiative? Do you see any important contradictions between current VA policy and these new guidelines?
Dr. Drexler. Yes—not necessarily with policy but with our VA/DoD Clinical Practice Guidelines from 2010 and the new CDC guidelines. There’s a shift in emphasis. The 2010 VA/DoD guidelines were maybe a little too optimistic about the safety of opioids for chronic, noncancer pain. As time goes on, more evidence is mounting of the potential harms, including from my perspective, the risk of developing an opioid use disorder when taking opioid analgesics as prescribed.
In the 1990s and the early 2000s, experts in the field reassured us. They told us all—patients and providers alike—that if opioid analgesics were taken as prescribed for legitimate pain by people who didn’t have a previous history of a substance use disorder, they could take them and would not be at risk for becoming addicted. We now know that’s simply not true. Patients who take these medicines as prescribed by their providers end up developing tolerance, developing hyperalgesia, needing more and more medication to get the desired effect, and crossing that line from just physiologic tolerance to developing an opioid use disorder.
We now have better guidelines about where that risk increases. We have some data from observational studies that patients who are maintained on opioid analgesics that are < 50 mg of daily morphine equivalent dose (MED) tend not to have as high a risk of overdose as those who are maintained on more, but the risk of developing an opioid use disorder actually is not insignificant even below 50 mg MED.
The key for developing an opioid use disorder is how long the patient is on the treatment. For patients who take opioid analgesics for < 90 days—again, these are observational studies—very few went on to develop opioid use disorder. However, those who took it for > 90 days, even those who were maintained on < 36 mg MED, had a significantly increased risk of developing an opioid use disorder. And for those who were on the higher dose, say, > 120 mg MED, the risk went up to 122-fold.
We just don’t see those kinds of odds ratios elsewhere in medicine. You know, we address risk factors when it increases the risk by 50%, but this is 122-fold.
Dr. Geppert. Pain as the fifth vital sign had a dark side there. Is there anything else you want to say to us about the Opiate Safety Initiative?
Dr. Drexler. I think it’s been incredibly successful; but like any change, there are unintended consequences and challenges with any change, even a good change. One of the things that has been a good change is raising awareness that opioids are not the answer for chronic pain. We have better evidence that self-care strategies (movement, exercise, physical therapy, and cognitive behavioral therapy for pain) help folks learn strategies for addressing automatic alarming thoughts about their pain—that it’s signaling something terrible when, really, it’s not. There are things that people can do to manage pain on their own. Those [strategies] are safer, and we have better evidence that they are effective than we have for opioids taken past 12 weeks.
Dr. Geppert. It’s really a paradigm shift in understanding. The Comprehensive Addiction and Recovery Act of 2016 has proposed to allow some nurse practitioners (NPs) and physician assistants (PAs) to obtain a waiver to prescribe buprenorphine. What role does the VA see for PAs and NPs in patient care for veterans with substance use disorders with medication-assisted therapy, and do you think that’ll involve prescribing buprenorphine?
Dr. Drexler. One of the things that I love about practicing within the VA is working within an interdisciplinary team and having that team-based approach. When we work together, our results far outshine anything that any one of us could do separately. I am very much looking forward to being able to provide or collaborate with other federal partners like the Substance Abuse and Mental Health Services Administration [SAMHSA] to provide the training for VA NPs and PAs who are able to prescribe controlled substances with their state licensure to provide buprenorphine for our patients.
Even without that prescriptive authority, NPs and PAs are already important partners and members of the interdisciplinary team who are managing patients and providing the evidence-based counseling, the monitoring, following up urine drug screen results, and doing a lot of the management, but not signing the prescription that’s being provided now by the Drug Addiction Treatment Act-waivered physicians. But SAMHSA has yet to define what the 24 hours of training will be that NPs and PAs will need to complete in order to apply for their U.S. Drug Enforcement Administration waiver, but we’re very much looking forward to it. And I am in contact with colleagues at SAMHSA, and they promise me they will keep me informed when they have the regulations set out for us to follow.
Dr. Geppert. The new VA/DoD Clinical Practice Guidelines for Management of Substance Use Disorders were published earlier this year. Could you give us an overview of the guidelines and what’s significant for most of our providers in the field?
Dr. Drexler. I’m very excited about the guidelines. One of the first things you should know is that they are very evidence based. If you’ve been practicing medicine as long as I have, you’ll remember, when the first clinical practice guidelines came out, they were expert opinion. They got some of the leading practitioners and researchers in the country together, put them in a room, and said, “What do you think people should do to treat this disorder?” Over time, clinical practice guidelines have become more evidence based; and the VA/DoD collaborative follows a highly respected methodology called the GRADE methodology.
Let me explain that just briefly. It means that our recommendations are based on systematic reviews of the literature. We all have our own screens and filters and our favorite studies. And so, if you get a bunch of experts together, they may not have as dispassionate a view of the entire literature as when you do a systematic review; and that’s what we started with.
We followed a specific methodology as well that took into account not only the strength of the evidence where the gold standard is a randomized, controlled clinical trial and, even better than that, a meta-analysis of multiple clinical trials. And for our recommendations, the strength of the evidence was, by far, supported mostly by clinical trials or meta-analyses. So, when you look at the VA/DoD Clinical Practice Guidelines, and I hope you will, you can feel confident that what we’re recommending is not just pulled out of the sky or some idiosyncratic favorite thing that Karen Drexler likes. It’s really based on good, strong clinical evidence.
We went from the previous version that had over 160 recommendations. We distilled them down to 36, and those 36 recommendations cover everything from prevention in primary care settings to stabilization and withdrawal management for alcohol and sedatives and opioids, and then treatment or rehabilitation of the 4 most common substance use disorders and then some general principles (Table 1).
There’s good evidence that what we’re doing in VA with screening in primary care every year using the AUDIT-C [Alcohol Use Disorders Identification Test-Consumption] is making a difference (Table 2). That folks who screen positive for at-risk alcohol use are very likely to respond to a brief intervention by their health care provider and reduce their alcohol consumption to safe levels, where they’re not likely to go on to develop an addiction to the alcohol or to develop medical complications from heavy alcohol use.
After screening, then, if someone does develop a substance use disorder, we have evidence-based treatments, both medications for 2 of our most common disorders and psychosocial interventions for all 4 most common disorders after tobacco. And I don’t exclude tobacco because it’s not important; it’s incredibly important, but it gets its own clinical practice guidelines. So, with our limited budget, we just addressed the other 10 substances….
For alcohol we have great evidence for 5 different medications and for 6 different psychosocial approaches. It is the most common substance use disorder, but we also have a whole menu of different treatments that folks can choose from, everything from naltrexone—oral naltrexone is the most common medication that we prescribe—but also acamprosate; disulfiram; extended-release injectable naltrexone. Topiramate, interestingly, is not FDA approved for that indication, but we have found good evidence from clinical trials supporting topiramate, so we strongly recommend it. If any of those are not acceptable or not tolerated or ineffective, then we also have some evidence to support gabapentin as well. So we have a strong recommendation for the first ones and a suggestion for gabapentin if it’s clinically indicated.
In terms of psychosocial approaches, [we recommend] everything from motivation enhancement therapy or motivational interviewing; cognitive-behavioral therapy; 12-step facilitation, which is a structured way of getting folks to get more active in Alcoholics Anonymous; behavioral approaches like community reinforcement approach or contingency management.
For opioid use disorder, interestingly, we found good, strong evidence that medication-assisted treatments work but no evidence for any psychosocial intervention without medication. Let me say that again because, nationwide, the most common treatment that’s provided for opioid use disorder, for any substance use disorder, is a psychosocial intervention—talking therapy alone.
For all of the medicines for alcohol use disorder, the medicine improved upon talking therapy alone. For opioid use disorder, medication helps people stay sober; it retains them in treatment; it saves lives. But for talking therapy without medication, we have found insufficient evidence to recommend any particular treatment.
Dr. Geppert. Incredible. That leads to my next question: One of the most challenging issues facing primary care practitioners is how to manage patients who are on chronic opiate therapy but also use medical marijuana in the increasing number of states where it’s legal. Do you have some advice on how to approach these situations?
Dr. Drexler. It’s a great question, and it’s one I’m sure more and more VA practitioners are grappling with. By our policy, we do not exclude veterans who are taking medical marijuana from any VA services, and we encourage the veterans to speak up about it so that it can be considered as part of their treatment plan.
By policy, however, VA practitioners do not fill out forms for state-approved marijuana. Marijuana is still a Schedule I controlled substance by federal law, so it’s a very interesting legal situation where it’s illegal federally; but some states have passed laws making it legal by state law. It will be interesting to see how this plays out over the next few years.
But in that situation—and really, this is our policy, in general for substance use disorders—a patient having a substance use disorder should not be precluded or excluded from getting treatment for other medical conditions. Similarly, other medical conditions shouldn’t exclude patients from getting treatment for their substance use disorder. However, the best treatment comes when we look at the whole person and try to take both things into account.
In the same way that patients can become addicted to their opioid pain medicines, patients can become addicted to medical marijuana. So the marijuana use needs to be evaluated objectively; and whether or not they have a form, just like whether or not someone has a prescription for their opioid that may be causing them problems, that needs to be taken into account in the treatment plan; and the treatment needs to be individualized.
Dr. Geppert. What in VA substance use disorder programs and treatment do you think is especially innovative when compared to the community?
Dr. Drexler. One of the places where I think the VA is well ahead of our colleagues elsewhere in health care is…from SAIL [Strategic Analytics for Improvement and Learning] metrics. About 34% or 35% of veterans who are diagnosed clinically within our system with opioid use disorders are receiving medication-assisted therapy either with buprenorphine, methadone, or extended-release injectable naltrexone. When you look at the SAMHSA survey, the National Survey on Drug Use and Health, the percentage of patients in the community who meet criteria for a substance use disorder [who] actually receive treatment is more like 12%. We have no numbers from SAMHSA about what percentage are receiving medication-assisted treatment. They don’t ask. In that way, even though we have a long ways to go, we’re ahead of the curve when it comes to medication-assisted treatment. But the demand is growing, we’re going to have to keep working hard to keep up and to get ahead at this. When I think about it compared to other chronic illnesses, only 35% of our patients are getting the first-line recommended treatment. We still have a long ways to go.
Another place where we’re innovative is with contingency management. I mentioned psychosocial treatments. Behavioral treatment is very effective and yet is not often used in treatment of substance use disorders; but it works. And even better than that, it is fun.
Let me try to explain what this means. It’s based on the idea that the benefits of using drugs are immediate, and the benefits of staying sober take a long time to realize. And it is that difference that makes early recovery so challenging because, if someone has a craving for alcohol or another drug, they can satisfy that craving immediately and then have to pay the price, though, of health consequences or financial consequences or legal consequences from that alcohol or drug use.
On the other hand, staying sober is tough. But it takes a long time to put one’s life back together, to get one’s chronic health conditions that have been caused or made worse by the drug use back together. And the idea behind contingency management is to give immediate rewards early in recovery to keep folks engaged long enough to start realizing some of the longer-term benefits of recovery.
So the way this works, we use what we call the fishbowl model of contingency management. And we started with stimulant use disorders, which is one of the substance use disorders for which there’s no strong evidence for a medication that works; but this particular psychosocial intervention works very well. So patients come to the clinic twice a week, give a urine specimen for drug screen; and, based on those test results, if their test is negative and they haven’t used any stimulants, then they get a draw from the fishbowl, which is filled with slips of paper. Half of the slips of paper say, “Good job! Keep up the good work,” or something encouraging like that. And half of the slips of paper have a prize associated with them, and it could be a small prize like $1 or $5 for exchange at the VA Canteen store, or it could be a larger prize like $100 or $200. Of course, the less expensive prizes are more common than the more expensive prizes; but just that immediate reward can make such a difference.
With the first negative urine drug screen, you get 1 draw from the fishbowl. The second one in a row, you get 2; and so it goes that the longer you stay sober, the more chances you have for winning a prize. And this treatment can go on for as long as the veteran wants to continue for up to 12 weeks. Usually by 12 weeks, folks’ health and their lives are coming back together; and so there’s many more rewards for staying sober than just the draws from the fishbowl. And what we find is folks who get a good start with contingency management tend to stay on that trajectory towards recovery.
And this has been a wonderful partnership between the Center of Excellence for Substance Abuse Treatment and Education in Philadelphia and the VA Canteen Service, who has generously donated canteen coupons. So it doesn’t cost the medical center anything to offer these prizes, and we’ve had just some amazing results.
To listen to the unedited interview here
While much of private health care in the U.S. is only beginning to confront the burgeoning opioid epidemic, the VA has a long-standing commitment to providing treatment to veterans struggling with substance use disorders. To understand the scope of the challenge and the VA’s approach, Federal Practitioner Editor-in-Chief Cynthia M.A. Geppert, MD, talked with Karen Drexler, MD, national mental health program director, substance use disorders. Dr. Drexler served in the U.S. Air Force for 8 years before joining the Atlanta VAMC in Georgia, where she directed the substance abuse treatment program. She is an associate professor of psychiatry and behavioral sciences at Emory University. In 2014, Dr. Drexler was named deputy mental health program director, addictive disorders.
Editor-in-Chief Cynthia M.A. Geppert, MD. As national mental health program director for substance use disorders, what are the challenges facing VA substance use programs?
Karen Drexler, MD. The biggest challenge is the increasing demand for services. Veterans have been coming to the VA for mental health care in general and for substance use disorder treatment in particular. The most common substance use disorder that we treat in addition to tobacco is alcohol use disorder, and the demand for alcohol use disorder treatment continues to grow.
Also, with the opioid crisis, there’s increasing demand for opioid use disorder treatments, including what we recommend as first-line treatment, which is medication-assisted treatment using buprenorphine or methadone, or injectable naltrexone as a second-line treatment. Gearing up with medication-assisted treatment for both alcohol and opioid use disorders is probably our biggest challenge.
Dr. Geppert. What were the most important accomplishments of the VA substance abuse programs over the past 5 years?
Dr. Drexler. There have been a lot! First of all, one of the things I love about practicing within the VA is that we are an integrated health care system. When I talk with colleagues in the Emory system here in Atlanta and across the country, oftentimes mental health and substance use disorders are isolated. The funding streams through the public sector come in different ways. Third-party payers have carve-outs for behavioral health.
It’s really wonderful to be able to collaborate with my colleagues in primary care, medical specialty care, and general mental health care, to provide a holistic and team-based approach to our patients who have both medical and substance use disorder problems and often co-occurring mental illness. As we’ve moved from a hospital-based system to more outpatient [care], the VA continues to be able to collaborate through our electronic health records as well as just being able to pick up the phone or send a [Microsoft] Lync message or an Outlook encrypted e-mail to help facilitate that coordinated care.
Another accomplishment has been to [develop] policy. It’s one of our challenges, but it’s also an accomplishment even at this early stage. In 2008, we issued the Uniform Mental Health Services benefits package as part of VHA Handbook on Mental Health Services. From that very beginning, we have included medication-assisted therapy when indicated for veterans with substance use disorders.
Now, we also know there’s a lot of variability in the system, so not every facility is providing these indicated treatments yet; but at least it’s part of our policy, and it’s been a focus of ongoing quality improvement to make these treatments available for veterans when they need them.
Dr. Geppert. We’ve heard a lot lately about the CDC guidelines for prescribing opiates for chronic pain that were published earlier this year. How do you see these guidelines affecting the VA Opiate Safety Initiative? Do you see any important contradictions between current VA policy and these new guidelines?
Dr. Drexler. Yes—not necessarily with policy but with our VA/DoD Clinical Practice Guidelines from 2010 and the new CDC guidelines. There’s a shift in emphasis. The 2010 VA/DoD guidelines were maybe a little too optimistic about the safety of opioids for chronic, noncancer pain. As time goes on, more evidence is mounting of the potential harms, including from my perspective, the risk of developing an opioid use disorder when taking opioid analgesics as prescribed.
In the 1990s and the early 2000s, experts in the field reassured us. They told us all—patients and providers alike—that if opioid analgesics were taken as prescribed for legitimate pain by people who didn’t have a previous history of a substance use disorder, they could take them and would not be at risk for becoming addicted. We now know that’s simply not true. Patients who take these medicines as prescribed by their providers end up developing tolerance, developing hyperalgesia, needing more and more medication to get the desired effect, and crossing that line from just physiologic tolerance to developing an opioid use disorder.
We now have better guidelines about where that risk increases. We have some data from observational studies that patients who are maintained on opioid analgesics that are < 50 mg of daily morphine equivalent dose (MED) tend not to have as high a risk of overdose as those who are maintained on more, but the risk of developing an opioid use disorder actually is not insignificant even below 50 mg MED.
The key for developing an opioid use disorder is how long the patient is on the treatment. For patients who take opioid analgesics for < 90 days—again, these are observational studies—very few went on to develop opioid use disorder. However, those who took it for > 90 days, even those who were maintained on < 36 mg MED, had a significantly increased risk of developing an opioid use disorder. And for those who were on the higher dose, say, > 120 mg MED, the risk went up to 122-fold.
We just don’t see those kinds of odds ratios elsewhere in medicine. You know, we address risk factors when it increases the risk by 50%, but this is 122-fold.
Dr. Geppert. Pain as the fifth vital sign had a dark side there. Is there anything else you want to say to us about the Opiate Safety Initiative?
Dr. Drexler. I think it’s been incredibly successful; but like any change, there are unintended consequences and challenges with any change, even a good change. One of the things that has been a good change is raising awareness that opioids are not the answer for chronic pain. We have better evidence that self-care strategies (movement, exercise, physical therapy, and cognitive behavioral therapy for pain) help folks learn strategies for addressing automatic alarming thoughts about their pain—that it’s signaling something terrible when, really, it’s not. There are things that people can do to manage pain on their own. Those [strategies] are safer, and we have better evidence that they are effective than we have for opioids taken past 12 weeks.
Dr. Geppert. It’s really a paradigm shift in understanding. The Comprehensive Addiction and Recovery Act of 2016 has proposed to allow some nurse practitioners (NPs) and physician assistants (PAs) to obtain a waiver to prescribe buprenorphine. What role does the VA see for PAs and NPs in patient care for veterans with substance use disorders with medication-assisted therapy, and do you think that’ll involve prescribing buprenorphine?
Dr. Drexler. One of the things that I love about practicing within the VA is working within an interdisciplinary team and having that team-based approach. When we work together, our results far outshine anything that any one of us could do separately. I am very much looking forward to being able to provide or collaborate with other federal partners like the Substance Abuse and Mental Health Services Administration [SAMHSA] to provide the training for VA NPs and PAs who are able to prescribe controlled substances with their state licensure to provide buprenorphine for our patients.
Even without that prescriptive authority, NPs and PAs are already important partners and members of the interdisciplinary team who are managing patients and providing the evidence-based counseling, the monitoring, following up urine drug screen results, and doing a lot of the management, but not signing the prescription that’s being provided now by the Drug Addiction Treatment Act-waivered physicians. But SAMHSA has yet to define what the 24 hours of training will be that NPs and PAs will need to complete in order to apply for their U.S. Drug Enforcement Administration waiver, but we’re very much looking forward to it. And I am in contact with colleagues at SAMHSA, and they promise me they will keep me informed when they have the regulations set out for us to follow.
Dr. Geppert. The new VA/DoD Clinical Practice Guidelines for Management of Substance Use Disorders were published earlier this year. Could you give us an overview of the guidelines and what’s significant for most of our providers in the field?
Dr. Drexler. I’m very excited about the guidelines. One of the first things you should know is that they are very evidence based. If you’ve been practicing medicine as long as I have, you’ll remember, when the first clinical practice guidelines came out, they were expert opinion. They got some of the leading practitioners and researchers in the country together, put them in a room, and said, “What do you think people should do to treat this disorder?” Over time, clinical practice guidelines have become more evidence based; and the VA/DoD collaborative follows a highly respected methodology called the GRADE methodology.
Let me explain that just briefly. It means that our recommendations are based on systematic reviews of the literature. We all have our own screens and filters and our favorite studies. And so, if you get a bunch of experts together, they may not have as dispassionate a view of the entire literature as when you do a systematic review; and that’s what we started with.
We followed a specific methodology as well that took into account not only the strength of the evidence where the gold standard is a randomized, controlled clinical trial and, even better than that, a meta-analysis of multiple clinical trials. And for our recommendations, the strength of the evidence was, by far, supported mostly by clinical trials or meta-analyses. So, when you look at the VA/DoD Clinical Practice Guidelines, and I hope you will, you can feel confident that what we’re recommending is not just pulled out of the sky or some idiosyncratic favorite thing that Karen Drexler likes. It’s really based on good, strong clinical evidence.
We went from the previous version that had over 160 recommendations. We distilled them down to 36, and those 36 recommendations cover everything from prevention in primary care settings to stabilization and withdrawal management for alcohol and sedatives and opioids, and then treatment or rehabilitation of the 4 most common substance use disorders and then some general principles (Table 1).
There’s good evidence that what we’re doing in VA with screening in primary care every year using the AUDIT-C [Alcohol Use Disorders Identification Test-Consumption] is making a difference (Table 2). That folks who screen positive for at-risk alcohol use are very likely to respond to a brief intervention by their health care provider and reduce their alcohol consumption to safe levels, where they’re not likely to go on to develop an addiction to the alcohol or to develop medical complications from heavy alcohol use.
After screening, then, if someone does develop a substance use disorder, we have evidence-based treatments, both medications for 2 of our most common disorders and psychosocial interventions for all 4 most common disorders after tobacco. And I don’t exclude tobacco because it’s not important; it’s incredibly important, but it gets its own clinical practice guidelines. So, with our limited budget, we just addressed the other 10 substances….
For alcohol we have great evidence for 5 different medications and for 6 different psychosocial approaches. It is the most common substance use disorder, but we also have a whole menu of different treatments that folks can choose from, everything from naltrexone—oral naltrexone is the most common medication that we prescribe—but also acamprosate; disulfiram; extended-release injectable naltrexone. Topiramate, interestingly, is not FDA approved for that indication, but we have found good evidence from clinical trials supporting topiramate, so we strongly recommend it. If any of those are not acceptable or not tolerated or ineffective, then we also have some evidence to support gabapentin as well. So we have a strong recommendation for the first ones and a suggestion for gabapentin if it’s clinically indicated.
In terms of psychosocial approaches, [we recommend] everything from motivation enhancement therapy or motivational interviewing; cognitive-behavioral therapy; 12-step facilitation, which is a structured way of getting folks to get more active in Alcoholics Anonymous; behavioral approaches like community reinforcement approach or contingency management.
For opioid use disorder, interestingly, we found good, strong evidence that medication-assisted treatments work but no evidence for any psychosocial intervention without medication. Let me say that again because, nationwide, the most common treatment that’s provided for opioid use disorder, for any substance use disorder, is a psychosocial intervention—talking therapy alone.
For all of the medicines for alcohol use disorder, the medicine improved upon talking therapy alone. For opioid use disorder, medication helps people stay sober; it retains them in treatment; it saves lives. But for talking therapy without medication, we have found insufficient evidence to recommend any particular treatment.
Dr. Geppert. Incredible. That leads to my next question: One of the most challenging issues facing primary care practitioners is how to manage patients who are on chronic opiate therapy but also use medical marijuana in the increasing number of states where it’s legal. Do you have some advice on how to approach these situations?
Dr. Drexler. It’s a great question, and it’s one I’m sure more and more VA practitioners are grappling with. By our policy, we do not exclude veterans who are taking medical marijuana from any VA services, and we encourage the veterans to speak up about it so that it can be considered as part of their treatment plan.
By policy, however, VA practitioners do not fill out forms for state-approved marijuana. Marijuana is still a Schedule I controlled substance by federal law, so it’s a very interesting legal situation where it’s illegal federally; but some states have passed laws making it legal by state law. It will be interesting to see how this plays out over the next few years.
But in that situation—and really, this is our policy, in general for substance use disorders—a patient having a substance use disorder should not be precluded or excluded from getting treatment for other medical conditions. Similarly, other medical conditions shouldn’t exclude patients from getting treatment for their substance use disorder. However, the best treatment comes when we look at the whole person and try to take both things into account.
In the same way that patients can become addicted to their opioid pain medicines, patients can become addicted to medical marijuana. So the marijuana use needs to be evaluated objectively; and whether or not they have a form, just like whether or not someone has a prescription for their opioid that may be causing them problems, that needs to be taken into account in the treatment plan; and the treatment needs to be individualized.
Dr. Geppert. What in VA substance use disorder programs and treatment do you think is especially innovative when compared to the community?
Dr. Drexler. One of the places where I think the VA is well ahead of our colleagues elsewhere in health care is…from SAIL [Strategic Analytics for Improvement and Learning] metrics. About 34% or 35% of veterans who are diagnosed clinically within our system with opioid use disorders are receiving medication-assisted therapy either with buprenorphine, methadone, or extended-release injectable naltrexone. When you look at the SAMHSA survey, the National Survey on Drug Use and Health, the percentage of patients in the community who meet criteria for a substance use disorder [who] actually receive treatment is more like 12%. We have no numbers from SAMHSA about what percentage are receiving medication-assisted treatment. They don’t ask. In that way, even though we have a long ways to go, we’re ahead of the curve when it comes to medication-assisted treatment. But the demand is growing, we’re going to have to keep working hard to keep up and to get ahead at this. When I think about it compared to other chronic illnesses, only 35% of our patients are getting the first-line recommended treatment. We still have a long ways to go.
Another place where we’re innovative is with contingency management. I mentioned psychosocial treatments. Behavioral treatment is very effective and yet is not often used in treatment of substance use disorders; but it works. And even better than that, it is fun.
Let me try to explain what this means. It’s based on the idea that the benefits of using drugs are immediate, and the benefits of staying sober take a long time to realize. And it is that difference that makes early recovery so challenging because, if someone has a craving for alcohol or another drug, they can satisfy that craving immediately and then have to pay the price, though, of health consequences or financial consequences or legal consequences from that alcohol or drug use.
On the other hand, staying sober is tough. But it takes a long time to put one’s life back together, to get one’s chronic health conditions that have been caused or made worse by the drug use back together. And the idea behind contingency management is to give immediate rewards early in recovery to keep folks engaged long enough to start realizing some of the longer-term benefits of recovery.
So the way this works, we use what we call the fishbowl model of contingency management. And we started with stimulant use disorders, which is one of the substance use disorders for which there’s no strong evidence for a medication that works; but this particular psychosocial intervention works very well. So patients come to the clinic twice a week, give a urine specimen for drug screen; and, based on those test results, if their test is negative and they haven’t used any stimulants, then they get a draw from the fishbowl, which is filled with slips of paper. Half of the slips of paper say, “Good job! Keep up the good work,” or something encouraging like that. And half of the slips of paper have a prize associated with them, and it could be a small prize like $1 or $5 for exchange at the VA Canteen store, or it could be a larger prize like $100 or $200. Of course, the less expensive prizes are more common than the more expensive prizes; but just that immediate reward can make such a difference.
With the first negative urine drug screen, you get 1 draw from the fishbowl. The second one in a row, you get 2; and so it goes that the longer you stay sober, the more chances you have for winning a prize. And this treatment can go on for as long as the veteran wants to continue for up to 12 weeks. Usually by 12 weeks, folks’ health and their lives are coming back together; and so there’s many more rewards for staying sober than just the draws from the fishbowl. And what we find is folks who get a good start with contingency management tend to stay on that trajectory towards recovery.
And this has been a wonderful partnership between the Center of Excellence for Substance Abuse Treatment and Education in Philadelphia and the VA Canteen Service, who has generously donated canteen coupons. So it doesn’t cost the medical center anything to offer these prizes, and we’ve had just some amazing results.
To listen to the unedited interview here
Do Kidney Patients Know an App From a Nap?
Q)It seems that at every conference I attend, a tech/marketing rep stands up to rave about “this” app or “that” online program. My average patient is older than 60 (physiologically 80), has vision issues related to diabetes, hypertension, or cataracts, can’t afford a smartphone, wouldn’t know an app from a nap, and has trouble just managing to eat correctly. What makes these reps think that patients can use technology?
We are often encouraged to incorporate technology into our daily interactions with patients. Meaningful use has us signing up 70-year-old patients for our practice’s patient portal and counting on them to write a message to us so we can receive credit. Our initial response is to groan and ask if the government knows what kind of patients we see.
However, a new article in the Clinical Journal of the American Society of Nephrology suggests that our patients may be more tech savvy than we think.1 The study found that patients with chronic kidney disease (CKD) not only know how to use a smartphone application but also find its implementation useful.
Patients included in the study were, on average, 59 and had stage IV to stage V CKD and an estimated glomerular filtration rate (eGFR) of ≥ 30 mL/min/1.73 m2. The study assessed knowledge of blood pressure, medications, CKD-related symptoms, and CKD-related laboratory tests.
Although 60% of the study cohort had never used a smartphone before, monthly adherence rates were higher than 80%. Outcomes included a statistically significant reduction in blood pressure, which was attributed to patients’ ability to better monitor their health and reduce their anxiety. The smartphone data sets also helped to identify cases of masked hypertension and more than 100 medication errors, 60% of which required intervention. Subsequent visits with providers were found to be more useful as a result, since both patients and providers had better quality information.
An accompanying editorial cautioned, however, that despite these positive findings, we must be mindful that smartphone ownership is less common among lower income patients. Fifty percent of those making less than $30,000 per year own a smartphone, compared with 84% of patients with an annual income of $75,000 or more.2 CKD patients are of varying socioeconomic status, with lower eGFR often corresponding to lower socioeconomic status.
So while medical apps have a future with CKD (and by implication, all) patients, they are not unlike much else in medicine: We must tailor our practice to meet the needs of our patient population. These findings are encouraging for use of smartphone technology, but it is not a “one size fits all” solution. —SM
Sherry Mathes, NP-C
Georgia Nephrology LLC, Lawrenceville, Georgia
1. Ong SW, Jassal SV, Miller JA, et al. Integrating a smartphone-based self-management system into usual care of advanced CKD. Clin J Am Soc Nephrol. 2016;11(6):1054-1062.
2. Desai T, Yee J, Soman S. Smartphone apps: a patient’s new best friend? Clin J Am Soc Nephrol. 2016;11(6):935-937.
Q)It seems that at every conference I attend, a tech/marketing rep stands up to rave about “this” app or “that” online program. My average patient is older than 60 (physiologically 80), has vision issues related to diabetes, hypertension, or cataracts, can’t afford a smartphone, wouldn’t know an app from a nap, and has trouble just managing to eat correctly. What makes these reps think that patients can use technology?
We are often encouraged to incorporate technology into our daily interactions with patients. Meaningful use has us signing up 70-year-old patients for our practice’s patient portal and counting on them to write a message to us so we can receive credit. Our initial response is to groan and ask if the government knows what kind of patients we see.
However, a new article in the Clinical Journal of the American Society of Nephrology suggests that our patients may be more tech savvy than we think.1 The study found that patients with chronic kidney disease (CKD) not only know how to use a smartphone application but also find its implementation useful.
Patients included in the study were, on average, 59 and had stage IV to stage V CKD and an estimated glomerular filtration rate (eGFR) of ≥ 30 mL/min/1.73 m2. The study assessed knowledge of blood pressure, medications, CKD-related symptoms, and CKD-related laboratory tests.
Although 60% of the study cohort had never used a smartphone before, monthly adherence rates were higher than 80%. Outcomes included a statistically significant reduction in blood pressure, which was attributed to patients’ ability to better monitor their health and reduce their anxiety. The smartphone data sets also helped to identify cases of masked hypertension and more than 100 medication errors, 60% of which required intervention. Subsequent visits with providers were found to be more useful as a result, since both patients and providers had better quality information.
An accompanying editorial cautioned, however, that despite these positive findings, we must be mindful that smartphone ownership is less common among lower income patients. Fifty percent of those making less than $30,000 per year own a smartphone, compared with 84% of patients with an annual income of $75,000 or more.2 CKD patients are of varying socioeconomic status, with lower eGFR often corresponding to lower socioeconomic status.
So while medical apps have a future with CKD (and by implication, all) patients, they are not unlike much else in medicine: We must tailor our practice to meet the needs of our patient population. These findings are encouraging for use of smartphone technology, but it is not a “one size fits all” solution. —SM
Sherry Mathes, NP-C
Georgia Nephrology LLC, Lawrenceville, Georgia
Q)It seems that at every conference I attend, a tech/marketing rep stands up to rave about “this” app or “that” online program. My average patient is older than 60 (physiologically 80), has vision issues related to diabetes, hypertension, or cataracts, can’t afford a smartphone, wouldn’t know an app from a nap, and has trouble just managing to eat correctly. What makes these reps think that patients can use technology?
We are often encouraged to incorporate technology into our daily interactions with patients. Meaningful use has us signing up 70-year-old patients for our practice’s patient portal and counting on them to write a message to us so we can receive credit. Our initial response is to groan and ask if the government knows what kind of patients we see.
However, a new article in the Clinical Journal of the American Society of Nephrology suggests that our patients may be more tech savvy than we think.1 The study found that patients with chronic kidney disease (CKD) not only know how to use a smartphone application but also find its implementation useful.
Patients included in the study were, on average, 59 and had stage IV to stage V CKD and an estimated glomerular filtration rate (eGFR) of ≥ 30 mL/min/1.73 m2. The study assessed knowledge of blood pressure, medications, CKD-related symptoms, and CKD-related laboratory tests.
Although 60% of the study cohort had never used a smartphone before, monthly adherence rates were higher than 80%. Outcomes included a statistically significant reduction in blood pressure, which was attributed to patients’ ability to better monitor their health and reduce their anxiety. The smartphone data sets also helped to identify cases of masked hypertension and more than 100 medication errors, 60% of which required intervention. Subsequent visits with providers were found to be more useful as a result, since both patients and providers had better quality information.
An accompanying editorial cautioned, however, that despite these positive findings, we must be mindful that smartphone ownership is less common among lower income patients. Fifty percent of those making less than $30,000 per year own a smartphone, compared with 84% of patients with an annual income of $75,000 or more.2 CKD patients are of varying socioeconomic status, with lower eGFR often corresponding to lower socioeconomic status.
So while medical apps have a future with CKD (and by implication, all) patients, they are not unlike much else in medicine: We must tailor our practice to meet the needs of our patient population. These findings are encouraging for use of smartphone technology, but it is not a “one size fits all” solution. —SM
Sherry Mathes, NP-C
Georgia Nephrology LLC, Lawrenceville, Georgia
1. Ong SW, Jassal SV, Miller JA, et al. Integrating a smartphone-based self-management system into usual care of advanced CKD. Clin J Am Soc Nephrol. 2016;11(6):1054-1062.
2. Desai T, Yee J, Soman S. Smartphone apps: a patient’s new best friend? Clin J Am Soc Nephrol. 2016;11(6):935-937.
1. Ong SW, Jassal SV, Miller JA, et al. Integrating a smartphone-based self-management system into usual care of advanced CKD. Clin J Am Soc Nephrol. 2016;11(6):1054-1062.
2. Desai T, Yee J, Soman S. Smartphone apps: a patient’s new best friend? Clin J Am Soc Nephrol. 2016;11(6):935-937.
Thyroid Cancer: Incidence on the Rise
Detection of thyroid cancer is widespread, increasing by about 4.5% annually. In the past year, approximately 64,300 new cases were identified. An estimated one in 100 people will be diagnosed with thyroid cancer during their lifetime, making it the eighth most common cancer in the United States.1
Incidental thyroid nodules found on carotid ultrasounds and other neck imaging may account for much of the increase; evaluation of these “incidentalomas” may account for the doubling incidence of thyroid cancer cases. (For more on thyroid nodules, see “To Cut or Not to Cut?” Clinician Reviews. 2016;26[8]:34-36.) If this pace continues, thyroid cancer may become the third most common cancer among women in the US by 2019.2
RISK FACTORS
Generally, women are diagnosed with thyroid cancer more frequently than men.3 Other risk factors include
- Age (40 to 60 in women; 60 to 80 in men; median age at diagnosis, 51)
- Inherited conditions, such as multiple endocrine neoplasia (MEN) or familial medullary and nonmedullary thyroid carcinoma
- Other cancers, including breast cancer and familial adenomatous polyposis
- Iodine deficiency
- Radiation exposure, particularly head and neck radiation in childhood. This can be through treatment of acne, tinea capitis, enlarged tonsils, or adenoids (usually prior to 1960); treatment of lymphoma, Wilms tumor, or neuroblastoma; or proximity to Chernobyl in 1986.1,2
BIOPSY RECOMMENDATIONS
While thyroid nodules are fairly common, only 7% to 15% of nodules are found to be malignant.2 However, all patients presenting with a palpable thyroid nodule should undergo thyroid ultrasound for further evaluation.
According to American Thyroid Association guidelines, all nodules 2 cm or larger should be evaluated with fine needle aspiration (FNA) due to a concern for metastatic thyroid cancer in larger nodules.2 Some clinicians prefer to aspirate nodules 1 cm or larger. Nodules that are smaller than 2 cm with sonographic features suspicious for thyroid cancer (see Table 1) should be biopsied.
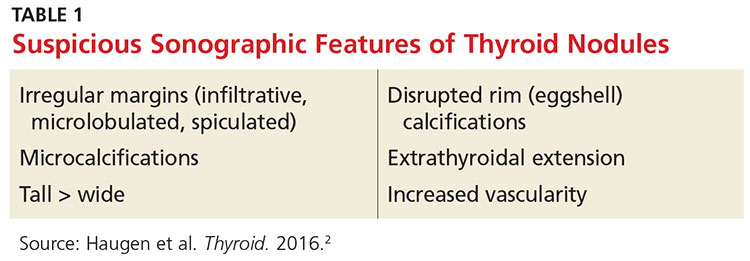
Nodules that are spongiform in appearance or are completely cystic with no solid components may be monitored without FNA.2
The FNA is typically performed by an endocrinologist under ultrasound guidance. No anesthetic is required, but a topical ethyl chloride spray can assist with patient comfort. Three to four passes are made into the nodule with a 27-gauge needle; most patients describe pressure or a pinching sensation, rather than pain, during the procedure. After the procedure, ice applied to the FNA area may help with patient comfort.
TYPES OF THYROID CANCER
Four possible types of thyroid cancer are identified on pathology after FNA: papillary, follicular, medullary, and anaplastic. Differentiated thyroid cancers, which encompass papillary and follicular cancers, are the most commonly diagnosed. Approximately 90% of thyroid cancers fall into this category.2
In most cases of differentiated thyroid cancer, patients can be treated with thyroidectomy alone if the cancer remains confined to the thyroid.2 Just over two-thirds of differentiated thyroid cancer cases are localized in the thyroid. The five-year survival rate for these patients is nearly 100%.1
About 27% of differentiated thyroid cancer is also found in neck lymph nodes; these patients may be treated with thyroidectomy and radioactive iodine.2 The five-year survival rate in these cases is nearly 98%.1 Chemotherapy is generally not needed for differentiated thyroid cancers.
Medullary thyroid cancer (MTC) is diagnosed in up to 4% of thyroid cancer patients. Characterized by high levels of calcitonin, MTC can be genetically mediated or sporadic. MTC is associated with a variety of RET oncogene mutations; genetic testing of family members is recommended, as well as prophylactic thyroidectomy when high-risk RET oncogenes are detected.3
The 10-year survival prognosis for MTC patients varies according to stage at diagnosis (see Table 2). Up to 70% of patients with a palpable MTC nodule present with metastasis consistent with stage III or IV disease.3
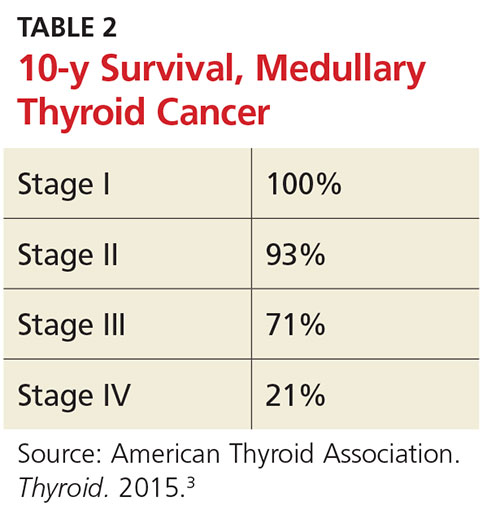
Medullary thyroid cancer is treated with total thyroidectomy and cervical lymph node dissection. Radioactive iodine has not been proven effective for MTC patients, unless there is also papillary or follicular thyroid cancer present.3
Anaplastic thyroid cancer has the highest mortality rate of all types of thyroid cancer. Fortunately, it is relatively rare, occurring in only 1.7% of thyroid cancer patients. The one-year survival rate is 20%, with a median postdiagnosis survival prognosis of approximately five months. Anaplastic thyroid cancer is treated with total thyroidectomy and radical neck dissection when it is considered resectable. Metastatic lesions in the brain or spine are often indicators of unresectable disease. In some cases, external beam radiation therapy is used as palliative treatment.4
PEDIATRIC INCIDENCE
Thyroid cancer in children is rare, making up only 1.8% of all pediatric cancers diagnosed in the US annually. Patients are most often between ages 15 and 19, but it is possible for thyroid cancer to manifest in younger patients. Thyroid nodules are more likely to be malignant in children, with a greater incidence of metastatic disease at diagnosis. Prognosis is generally better in children than in adults, however, even with extensive disease.5
Children with prior history of other types of cancer treated with radiation, such as Hodgkin lymphoma or leukemia, are at increased risk for thyroid cancer and should be monitored.5 Children with a family history of MEN or MTC and evidence of RET oncogenes should be monitored starting as early as age 3 with thyroid exam, ultrasound, and measurement of calcitonin levels.3 Prophylactic thyroidectomy is an option in the first few months of life, depending on the presence of specific RET oncogenes.3
CHEMOTHERAPY
Chemotherapy may be helpful for metastatic medullary or anaplastic thyroid cancer, particularly in patients with unresectable disease. Though not usually curative, it may increase progression-free survival time. New chemotherapy agents approved for use in metastatic MTC include cabozantinib and vandetanib.3 Carboplatin, docetaxel, doxorubicin, and paclitaxel are used in treatment of anaplastic thyroid cancer.4
LONG-TERM PATIENT MANAGEMENT
After thyroidectomy and radioactive iodine treatment, follicular cell cancers (eg, papillary, follicular, anaplastic) are managed by following patients’ thyroid-stimulating hormone (TSH), thyroglobulin, and antithyroglobulin antibody levels. A cervical ultrasound is performed to detect possible disease in lymph nodes.2
Levothyroxine is dosed to suppress TSH below the recommended levels for hypothyroid patients in order to prevent disease recurrence. Low-risk patients may have TSH suppression below 1 to 2 mU/L, while high-risk patients may be managed with TSH levels below 0.1 mU/L.2
Lab levels should be checked annually and a cervical ultrasound performed at six to 12 months, then periodically thereafter depending on patient risk status.2 Patients with long-term TSH suppression must be monitored for atrial fibrillation and osteoporosis.
Patients who have been treated for medullary thyroid cancer require a different long-term management strategy. Patients should have ultrasound and measurement of TSH as well as calcitonin and carcinoembryonic antigen levels every six to 12 months.3 TSH suppression is not required; TSH may be maintained at typical euthyroid levels.
A FINAL THOUGHT
For clinicians, it’s easy to attempt to minimize thyroid cancer, since the disease is curable for most patients without the burden of chemotherapy and external radiation. However, for a patient, this is still a cancer diagnosis, with the accompanying surgery and required lifelong monitoring. It can be very disruptive to the lives of both patients and their families.
Support groups are available to help patients navigate their new reality. The Thyroid Cancer Survivors’ Association (www.thyca.org) has resources that may be beneficial to patients (and caregivers) as they learn how to live as a thyroid cancer survivor.
1. National Cancer Institute Surveillance, Epidemiology, and End Results Program. SEER stat fact sheets: thyroid cancer. http://seer.cancer.gov/statfacts/html/thyro.html. Accessed September 16, 2016.
2. Haugen BR, Alexander EK, Bible KC, et al; American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. 2015 American Thyroid Association guidelines for adult patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26(1):1-133.
3. Wells SA Jr, Asa SL, Dralle H, et al; American Thyroid Association Guidelines Task Force on Medullary Thyroid Carcinoma. Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid. 2015;25(6):567-610.
4. Smallridge RC, Ain KB, Asa SL, et al; American Thyroid Association Anaplastic Thyroid Cancer Guidelines Taskforce. American Thyroid Association guidelines for the management of patients with anaplastic thyroid cancer. Thyroid. 2012;22(11):1104-1139.
5. Francis GL, Waguespack SG, Bauer AJ, et al; American Thyroid Association Guidelines Task Force. Management guidelines for children with thyroid nodules and differentiated thyroid cancer. Thyroid. 2015;25(7):716-759.
Detection of thyroid cancer is widespread, increasing by about 4.5% annually. In the past year, approximately 64,300 new cases were identified. An estimated one in 100 people will be diagnosed with thyroid cancer during their lifetime, making it the eighth most common cancer in the United States.1
Incidental thyroid nodules found on carotid ultrasounds and other neck imaging may account for much of the increase; evaluation of these “incidentalomas” may account for the doubling incidence of thyroid cancer cases. (For more on thyroid nodules, see “To Cut or Not to Cut?” Clinician Reviews. 2016;26[8]:34-36.) If this pace continues, thyroid cancer may become the third most common cancer among women in the US by 2019.2

RISK FACTORS
Generally, women are diagnosed with thyroid cancer more frequently than men.3 Other risk factors include
- Age (40 to 60 in women; 60 to 80 in men; median age at diagnosis, 51)
- Inherited conditions, such as multiple endocrine neoplasia (MEN) or familial medullary and nonmedullary thyroid carcinoma
- Other cancers, including breast cancer and familial adenomatous polyposis
- Iodine deficiency
- Radiation exposure, particularly head and neck radiation in childhood. This can be through treatment of acne, tinea capitis, enlarged tonsils, or adenoids (usually prior to 1960); treatment of lymphoma, Wilms tumor, or neuroblastoma; or proximity to Chernobyl in 1986.1,2
BIOPSY RECOMMENDATIONS
While thyroid nodules are fairly common, only 7% to 15% of nodules are found to be malignant.2 However, all patients presenting with a palpable thyroid nodule should undergo thyroid ultrasound for further evaluation.
According to American Thyroid Association guidelines, all nodules 2 cm or larger should be evaluated with fine needle aspiration (FNA) due to a concern for metastatic thyroid cancer in larger nodules.2 Some clinicians prefer to aspirate nodules 1 cm or larger. Nodules that are smaller than 2 cm with sonographic features suspicious for thyroid cancer (see Table 1) should be biopsied.

Nodules that are spongiform in appearance or are completely cystic with no solid components may be monitored without FNA.2
The FNA is typically performed by an endocrinologist under ultrasound guidance. No anesthetic is required, but a topical ethyl chloride spray can assist with patient comfort. Three to four passes are made into the nodule with a 27-gauge needle; most patients describe pressure or a pinching sensation, rather than pain, during the procedure. After the procedure, ice applied to the FNA area may help with patient comfort.
TYPES OF THYROID CANCER
Four possible types of thyroid cancer are identified on pathology after FNA: papillary, follicular, medullary, and anaplastic. Differentiated thyroid cancers, which encompass papillary and follicular cancers, are the most commonly diagnosed. Approximately 90% of thyroid cancers fall into this category.2
In most cases of differentiated thyroid cancer, patients can be treated with thyroidectomy alone if the cancer remains confined to the thyroid.2 Just over two-thirds of differentiated thyroid cancer cases are localized in the thyroid. The five-year survival rate for these patients is nearly 100%.1
About 27% of differentiated thyroid cancer is also found in neck lymph nodes; these patients may be treated with thyroidectomy and radioactive iodine.2 The five-year survival rate in these cases is nearly 98%.1 Chemotherapy is generally not needed for differentiated thyroid cancers.
Medullary thyroid cancer (MTC) is diagnosed in up to 4% of thyroid cancer patients. Characterized by high levels of calcitonin, MTC can be genetically mediated or sporadic. MTC is associated with a variety of RET oncogene mutations; genetic testing of family members is recommended, as well as prophylactic thyroidectomy when high-risk RET oncogenes are detected.3
The 10-year survival prognosis for MTC patients varies according to stage at diagnosis (see Table 2). Up to 70% of patients with a palpable MTC nodule present with metastasis consistent with stage III or IV disease.3

Medullary thyroid cancer is treated with total thyroidectomy and cervical lymph node dissection. Radioactive iodine has not been proven effective for MTC patients, unless there is also papillary or follicular thyroid cancer present.3
Anaplastic thyroid cancer has the highest mortality rate of all types of thyroid cancer. Fortunately, it is relatively rare, occurring in only 1.7% of thyroid cancer patients. The one-year survival rate is 20%, with a median postdiagnosis survival prognosis of approximately five months. Anaplastic thyroid cancer is treated with total thyroidectomy and radical neck dissection when it is considered resectable. Metastatic lesions in the brain or spine are often indicators of unresectable disease. In some cases, external beam radiation therapy is used as palliative treatment.4
PEDIATRIC INCIDENCE
Thyroid cancer in children is rare, making up only 1.8% of all pediatric cancers diagnosed in the US annually. Patients are most often between ages 15 and 19, but it is possible for thyroid cancer to manifest in younger patients. Thyroid nodules are more likely to be malignant in children, with a greater incidence of metastatic disease at diagnosis. Prognosis is generally better in children than in adults, however, even with extensive disease.5
Children with prior history of other types of cancer treated with radiation, such as Hodgkin lymphoma or leukemia, are at increased risk for thyroid cancer and should be monitored.5 Children with a family history of MEN or MTC and evidence of RET oncogenes should be monitored starting as early as age 3 with thyroid exam, ultrasound, and measurement of calcitonin levels.3 Prophylactic thyroidectomy is an option in the first few months of life, depending on the presence of specific RET oncogenes.3
CHEMOTHERAPY
Chemotherapy may be helpful for metastatic medullary or anaplastic thyroid cancer, particularly in patients with unresectable disease. Though not usually curative, it may increase progression-free survival time. New chemotherapy agents approved for use in metastatic MTC include cabozantinib and vandetanib.3 Carboplatin, docetaxel, doxorubicin, and paclitaxel are used in treatment of anaplastic thyroid cancer.4
LONG-TERM PATIENT MANAGEMENT
After thyroidectomy and radioactive iodine treatment, follicular cell cancers (eg, papillary, follicular, anaplastic) are managed by following patients’ thyroid-stimulating hormone (TSH), thyroglobulin, and antithyroglobulin antibody levels. A cervical ultrasound is performed to detect possible disease in lymph nodes.2
Levothyroxine is dosed to suppress TSH below the recommended levels for hypothyroid patients in order to prevent disease recurrence. Low-risk patients may have TSH suppression below 1 to 2 mU/L, while high-risk patients may be managed with TSH levels below 0.1 mU/L.2
Lab levels should be checked annually and a cervical ultrasound performed at six to 12 months, then periodically thereafter depending on patient risk status.2 Patients with long-term TSH suppression must be monitored for atrial fibrillation and osteoporosis.
Patients who have been treated for medullary thyroid cancer require a different long-term management strategy. Patients should have ultrasound and measurement of TSH as well as calcitonin and carcinoembryonic antigen levels every six to 12 months.3 TSH suppression is not required; TSH may be maintained at typical euthyroid levels.
A FINAL THOUGHT
For clinicians, it’s easy to attempt to minimize thyroid cancer, since the disease is curable for most patients without the burden of chemotherapy and external radiation. However, for a patient, this is still a cancer diagnosis, with the accompanying surgery and required lifelong monitoring. It can be very disruptive to the lives of both patients and their families.
Support groups are available to help patients navigate their new reality. The Thyroid Cancer Survivors’ Association (www.thyca.org) has resources that may be beneficial to patients (and caregivers) as they learn how to live as a thyroid cancer survivor.
Detection of thyroid cancer is widespread, increasing by about 4.5% annually. In the past year, approximately 64,300 new cases were identified. An estimated one in 100 people will be diagnosed with thyroid cancer during their lifetime, making it the eighth most common cancer in the United States.1
Incidental thyroid nodules found on carotid ultrasounds and other neck imaging may account for much of the increase; evaluation of these “incidentalomas” may account for the doubling incidence of thyroid cancer cases. (For more on thyroid nodules, see “To Cut or Not to Cut?” Clinician Reviews. 2016;26[8]:34-36.) If this pace continues, thyroid cancer may become the third most common cancer among women in the US by 2019.2

RISK FACTORS
Generally, women are diagnosed with thyroid cancer more frequently than men.3 Other risk factors include
- Age (40 to 60 in women; 60 to 80 in men; median age at diagnosis, 51)
- Inherited conditions, such as multiple endocrine neoplasia (MEN) or familial medullary and nonmedullary thyroid carcinoma
- Other cancers, including breast cancer and familial adenomatous polyposis
- Iodine deficiency
- Radiation exposure, particularly head and neck radiation in childhood. This can be through treatment of acne, tinea capitis, enlarged tonsils, or adenoids (usually prior to 1960); treatment of lymphoma, Wilms tumor, or neuroblastoma; or proximity to Chernobyl in 1986.1,2
BIOPSY RECOMMENDATIONS
While thyroid nodules are fairly common, only 7% to 15% of nodules are found to be malignant.2 However, all patients presenting with a palpable thyroid nodule should undergo thyroid ultrasound for further evaluation.
According to American Thyroid Association guidelines, all nodules 2 cm or larger should be evaluated with fine needle aspiration (FNA) due to a concern for metastatic thyroid cancer in larger nodules.2 Some clinicians prefer to aspirate nodules 1 cm or larger. Nodules that are smaller than 2 cm with sonographic features suspicious for thyroid cancer (see Table 1) should be biopsied.

Nodules that are spongiform in appearance or are completely cystic with no solid components may be monitored without FNA.2
The FNA is typically performed by an endocrinologist under ultrasound guidance. No anesthetic is required, but a topical ethyl chloride spray can assist with patient comfort. Three to four passes are made into the nodule with a 27-gauge needle; most patients describe pressure or a pinching sensation, rather than pain, during the procedure. After the procedure, ice applied to the FNA area may help with patient comfort.
TYPES OF THYROID CANCER
Four possible types of thyroid cancer are identified on pathology after FNA: papillary, follicular, medullary, and anaplastic. Differentiated thyroid cancers, which encompass papillary and follicular cancers, are the most commonly diagnosed. Approximately 90% of thyroid cancers fall into this category.2
In most cases of differentiated thyroid cancer, patients can be treated with thyroidectomy alone if the cancer remains confined to the thyroid.2 Just over two-thirds of differentiated thyroid cancer cases are localized in the thyroid. The five-year survival rate for these patients is nearly 100%.1
About 27% of differentiated thyroid cancer is also found in neck lymph nodes; these patients may be treated with thyroidectomy and radioactive iodine.2 The five-year survival rate in these cases is nearly 98%.1 Chemotherapy is generally not needed for differentiated thyroid cancers.
Medullary thyroid cancer (MTC) is diagnosed in up to 4% of thyroid cancer patients. Characterized by high levels of calcitonin, MTC can be genetically mediated or sporadic. MTC is associated with a variety of RET oncogene mutations; genetic testing of family members is recommended, as well as prophylactic thyroidectomy when high-risk RET oncogenes are detected.3
The 10-year survival prognosis for MTC patients varies according to stage at diagnosis (see Table 2). Up to 70% of patients with a palpable MTC nodule present with metastasis consistent with stage III or IV disease.3

Medullary thyroid cancer is treated with total thyroidectomy and cervical lymph node dissection. Radioactive iodine has not been proven effective for MTC patients, unless there is also papillary or follicular thyroid cancer present.3
Anaplastic thyroid cancer has the highest mortality rate of all types of thyroid cancer. Fortunately, it is relatively rare, occurring in only 1.7% of thyroid cancer patients. The one-year survival rate is 20%, with a median postdiagnosis survival prognosis of approximately five months. Anaplastic thyroid cancer is treated with total thyroidectomy and radical neck dissection when it is considered resectable. Metastatic lesions in the brain or spine are often indicators of unresectable disease. In some cases, external beam radiation therapy is used as palliative treatment.4
PEDIATRIC INCIDENCE
Thyroid cancer in children is rare, making up only 1.8% of all pediatric cancers diagnosed in the US annually. Patients are most often between ages 15 and 19, but it is possible for thyroid cancer to manifest in younger patients. Thyroid nodules are more likely to be malignant in children, with a greater incidence of metastatic disease at diagnosis. Prognosis is generally better in children than in adults, however, even with extensive disease.5
Children with prior history of other types of cancer treated with radiation, such as Hodgkin lymphoma or leukemia, are at increased risk for thyroid cancer and should be monitored.5 Children with a family history of MEN or MTC and evidence of RET oncogenes should be monitored starting as early as age 3 with thyroid exam, ultrasound, and measurement of calcitonin levels.3 Prophylactic thyroidectomy is an option in the first few months of life, depending on the presence of specific RET oncogenes.3
CHEMOTHERAPY
Chemotherapy may be helpful for metastatic medullary or anaplastic thyroid cancer, particularly in patients with unresectable disease. Though not usually curative, it may increase progression-free survival time. New chemotherapy agents approved for use in metastatic MTC include cabozantinib and vandetanib.3 Carboplatin, docetaxel, doxorubicin, and paclitaxel are used in treatment of anaplastic thyroid cancer.4
LONG-TERM PATIENT MANAGEMENT
After thyroidectomy and radioactive iodine treatment, follicular cell cancers (eg, papillary, follicular, anaplastic) are managed by following patients’ thyroid-stimulating hormone (TSH), thyroglobulin, and antithyroglobulin antibody levels. A cervical ultrasound is performed to detect possible disease in lymph nodes.2
Levothyroxine is dosed to suppress TSH below the recommended levels for hypothyroid patients in order to prevent disease recurrence. Low-risk patients may have TSH suppression below 1 to 2 mU/L, while high-risk patients may be managed with TSH levels below 0.1 mU/L.2
Lab levels should be checked annually and a cervical ultrasound performed at six to 12 months, then periodically thereafter depending on patient risk status.2 Patients with long-term TSH suppression must be monitored for atrial fibrillation and osteoporosis.
Patients who have been treated for medullary thyroid cancer require a different long-term management strategy. Patients should have ultrasound and measurement of TSH as well as calcitonin and carcinoembryonic antigen levels every six to 12 months.3 TSH suppression is not required; TSH may be maintained at typical euthyroid levels.
A FINAL THOUGHT
For clinicians, it’s easy to attempt to minimize thyroid cancer, since the disease is curable for most patients without the burden of chemotherapy and external radiation. However, for a patient, this is still a cancer diagnosis, with the accompanying surgery and required lifelong monitoring. It can be very disruptive to the lives of both patients and their families.
Support groups are available to help patients navigate their new reality. The Thyroid Cancer Survivors’ Association (www.thyca.org) has resources that may be beneficial to patients (and caregivers) as they learn how to live as a thyroid cancer survivor.
1. National Cancer Institute Surveillance, Epidemiology, and End Results Program. SEER stat fact sheets: thyroid cancer. http://seer.cancer.gov/statfacts/html/thyro.html. Accessed September 16, 2016.
2. Haugen BR, Alexander EK, Bible KC, et al; American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. 2015 American Thyroid Association guidelines for adult patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26(1):1-133.
3. Wells SA Jr, Asa SL, Dralle H, et al; American Thyroid Association Guidelines Task Force on Medullary Thyroid Carcinoma. Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid. 2015;25(6):567-610.
4. Smallridge RC, Ain KB, Asa SL, et al; American Thyroid Association Anaplastic Thyroid Cancer Guidelines Taskforce. American Thyroid Association guidelines for the management of patients with anaplastic thyroid cancer. Thyroid. 2012;22(11):1104-1139.
5. Francis GL, Waguespack SG, Bauer AJ, et al; American Thyroid Association Guidelines Task Force. Management guidelines for children with thyroid nodules and differentiated thyroid cancer. Thyroid. 2015;25(7):716-759.
1. National Cancer Institute Surveillance, Epidemiology, and End Results Program. SEER stat fact sheets: thyroid cancer. http://seer.cancer.gov/statfacts/html/thyro.html. Accessed September 16, 2016.
2. Haugen BR, Alexander EK, Bible KC, et al; American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. 2015 American Thyroid Association guidelines for adult patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26(1):1-133.
3. Wells SA Jr, Asa SL, Dralle H, et al; American Thyroid Association Guidelines Task Force on Medullary Thyroid Carcinoma. Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid. 2015;25(6):567-610.
4. Smallridge RC, Ain KB, Asa SL, et al; American Thyroid Association Anaplastic Thyroid Cancer Guidelines Taskforce. American Thyroid Association guidelines for the management of patients with anaplastic thyroid cancer. Thyroid. 2012;22(11):1104-1139.
5. Francis GL, Waguespack SG, Bauer AJ, et al; American Thyroid Association Guidelines Task Force. Management guidelines for children with thyroid nodules and differentiated thyroid cancer. Thyroid. 2015;25(7):716-759.
Data-based Recommendations for Dialysis
Q) I work in a cardiology practice. Recently, a patient on dialysis mentioned that her nephrology practitioner recommended either home therapy or nocturnal dialysis. Why would someone recommend these, and what are the differences between home, nocturnal, and regular daytime dialysis?
Patients usually require dialysis when 90% or more of their renal function is lost.5 This can happen acutely or result from a chronic process. Dialysis performs many of the functions of a kidney, such as removing waste and fluid buildup that damaged kidneys cannot. It also helps maintain electrolyte balance.
There are several forms of hemodialysis including home, incenter, and nocturnal; the most frequently used is in-center hemodialysis.5 Patients on in-center hemodialysis visit the center three times a week, and their treatments last from three to five hours; the nationwide average is four hours. These patients have very restricted schedules and must maintain their appointments with limited flexibility. Food, drinks, and nonmedical personnel may not be allowed in the treatment area. Between treatments, patients must follow a diet that restricts fluid, sodium, and potassium intake.
Home dialysis has become a popular alternative, since it may be done in a location and at a time that is convenient for the patient. With more flexibility, many patients are able to continue working and feel like they have a more “normal” life. Types of home dialysis include home hemodialysis (HHD) or peritoneal dialysis (PD). A relative or friend may need to assist the patient during HHD, which is undergone more frequently (between five and seven days per week) and for a shorter duration of time than in-center dialysis. PD is done every day, either at night or multiple times throughout the day. Although no partner is needed for PD, a medical provider is available by phone to address any concerns that may arise during treatment.
Nocturnal hemodialysis is similar to daytime in-center hemodialysis, but it occurs while the patient is asleep. The treatment duration is longer (an average of eight hours per treatment). The slower blood flow allows for gentler dialysis. Patients who undergo nocturnal hemodialysis have higher survival and lower hospitalization rates, with better phosphorus control and blood pressure.6 This is attributed to the slower removal of excess fluid and more effective clearance of toxins.
So, why is your patient being encouraged to consider home or nocturnal dialysis? Studies have shown that for the cardiac patient, slower, gentler dialysis is preferable.7 The clinician who recommended it has the patient’s best interest in mind. —TAH
Tricia A. Howard, MHS, PA-C, DFAAPA
PA Program, South University, Savannah, Georgia
5. Gilbert S, Weiner DE. National Kidney Foundation Primer on Kidney Diseases. 6th ed. Philadelphia, PA: Elsevier; 2014.
6. Lacson E, Wang W, Lester K, et al. Outcomes associated with in-center nocturnal hemodialysis from a large multicenter program. Clin J Am Soc Nephrol. 2010;5(2):220-226.
7. Lin J, Berns JS. Is hemodialysis bad for the heart? Semin Dial. 2012;25(1):86-87.
Q) I work in a cardiology practice. Recently, a patient on dialysis mentioned that her nephrology practitioner recommended either home therapy or nocturnal dialysis. Why would someone recommend these, and what are the differences between home, nocturnal, and regular daytime dialysis?
Patients usually require dialysis when 90% or more of their renal function is lost.5 This can happen acutely or result from a chronic process. Dialysis performs many of the functions of a kidney, such as removing waste and fluid buildup that damaged kidneys cannot. It also helps maintain electrolyte balance.
There are several forms of hemodialysis including home, incenter, and nocturnal; the most frequently used is in-center hemodialysis.5 Patients on in-center hemodialysis visit the center three times a week, and their treatments last from three to five hours; the nationwide average is four hours. These patients have very restricted schedules and must maintain their appointments with limited flexibility. Food, drinks, and nonmedical personnel may not be allowed in the treatment area. Between treatments, patients must follow a diet that restricts fluid, sodium, and potassium intake.
Home dialysis has become a popular alternative, since it may be done in a location and at a time that is convenient for the patient. With more flexibility, many patients are able to continue working and feel like they have a more “normal” life. Types of home dialysis include home hemodialysis (HHD) or peritoneal dialysis (PD). A relative or friend may need to assist the patient during HHD, which is undergone more frequently (between five and seven days per week) and for a shorter duration of time than in-center dialysis. PD is done every day, either at night or multiple times throughout the day. Although no partner is needed for PD, a medical provider is available by phone to address any concerns that may arise during treatment.
Nocturnal hemodialysis is similar to daytime in-center hemodialysis, but it occurs while the patient is asleep. The treatment duration is longer (an average of eight hours per treatment). The slower blood flow allows for gentler dialysis. Patients who undergo nocturnal hemodialysis have higher survival and lower hospitalization rates, with better phosphorus control and blood pressure.6 This is attributed to the slower removal of excess fluid and more effective clearance of toxins.
So, why is your patient being encouraged to consider home or nocturnal dialysis? Studies have shown that for the cardiac patient, slower, gentler dialysis is preferable.7 The clinician who recommended it has the patient’s best interest in mind. —TAH
Tricia A. Howard, MHS, PA-C, DFAAPA
PA Program, South University, Savannah, Georgia
Q) I work in a cardiology practice. Recently, a patient on dialysis mentioned that her nephrology practitioner recommended either home therapy or nocturnal dialysis. Why would someone recommend these, and what are the differences between home, nocturnal, and regular daytime dialysis?
Patients usually require dialysis when 90% or more of their renal function is lost.5 This can happen acutely or result from a chronic process. Dialysis performs many of the functions of a kidney, such as removing waste and fluid buildup that damaged kidneys cannot. It also helps maintain electrolyte balance.
There are several forms of hemodialysis including home, incenter, and nocturnal; the most frequently used is in-center hemodialysis.5 Patients on in-center hemodialysis visit the center three times a week, and their treatments last from three to five hours; the nationwide average is four hours. These patients have very restricted schedules and must maintain their appointments with limited flexibility. Food, drinks, and nonmedical personnel may not be allowed in the treatment area. Between treatments, patients must follow a diet that restricts fluid, sodium, and potassium intake.
Home dialysis has become a popular alternative, since it may be done in a location and at a time that is convenient for the patient. With more flexibility, many patients are able to continue working and feel like they have a more “normal” life. Types of home dialysis include home hemodialysis (HHD) or peritoneal dialysis (PD). A relative or friend may need to assist the patient during HHD, which is undergone more frequently (between five and seven days per week) and for a shorter duration of time than in-center dialysis. PD is done every day, either at night or multiple times throughout the day. Although no partner is needed for PD, a medical provider is available by phone to address any concerns that may arise during treatment.
Nocturnal hemodialysis is similar to daytime in-center hemodialysis, but it occurs while the patient is asleep. The treatment duration is longer (an average of eight hours per treatment). The slower blood flow allows for gentler dialysis. Patients who undergo nocturnal hemodialysis have higher survival and lower hospitalization rates, with better phosphorus control and blood pressure.6 This is attributed to the slower removal of excess fluid and more effective clearance of toxins.
So, why is your patient being encouraged to consider home or nocturnal dialysis? Studies have shown that for the cardiac patient, slower, gentler dialysis is preferable.7 The clinician who recommended it has the patient’s best interest in mind. —TAH
Tricia A. Howard, MHS, PA-C, DFAAPA
PA Program, South University, Savannah, Georgia
5. Gilbert S, Weiner DE. National Kidney Foundation Primer on Kidney Diseases. 6th ed. Philadelphia, PA: Elsevier; 2014.
6. Lacson E, Wang W, Lester K, et al. Outcomes associated with in-center nocturnal hemodialysis from a large multicenter program. Clin J Am Soc Nephrol. 2010;5(2):220-226.
7. Lin J, Berns JS. Is hemodialysis bad for the heart? Semin Dial. 2012;25(1):86-87.
5. Gilbert S, Weiner DE. National Kidney Foundation Primer on Kidney Diseases. 6th ed. Philadelphia, PA: Elsevier; 2014.
6. Lacson E, Wang W, Lester K, et al. Outcomes associated with in-center nocturnal hemodialysis from a large multicenter program. Clin J Am Soc Nephrol. 2010;5(2):220-226.
7. Lin J, Berns JS. Is hemodialysis bad for the heart? Semin Dial. 2012;25(1):86-87.