User login
Anemia, A1C, and Rhabdomyolysis
Q) Does anemia in CKD patients affect their A1C? Is A1C accurate in CKD patients?
Tight glycemic control is imperative for patients with chronic kidney disease (CKD), but the management of diabetes in CKD can be complex due to factors including anemia and changes in glucose and insulin homeostasis.
A1C is directly proportionate to the ambient blood glucose concentration and in the general diabetic population has proven to be a reliable marker.1 However, it may not be valid in patients with diabetes and CKD. Reduced red blood cell (RBC) lifespan, rapid hemolysis, and iron deficiency may lead to falsely decreased results.2 Decreased RBC survival results from an increase in hemoglobin turnover, which decreases glycemic exposure time.1 This process then lowers the amount of nonenzymatic glucose binding to hemoglobin.1 Folate deficiency caused by impaired intestinal absorption in CKD also affects RBC survival.3 Falsely increased results may be related to carbamylation of the hemoglobin and acidosis, both of which are influenced by uremia.2
Special considerations should be made for dialysis patients with diabetes. In hemodialysis patients, A1C may be falsely decreased due to blood loss, RBC transfusion, and erythropoietin therapy.3 Observational studies have shown that erythropoietin therapy is associated with lower A1C due to the increased number of immature RBCs that have a decreased glycemic exposure time.1 In peritoneal dialysis patients, A1C may increase after the start of therapy as a result of dialysate absorption.3
Research suggests that glycated albumin (GA) provides a short-term index of glycemic control (typically two to three weeks) and is not influenced by albumin concentration, RBC lifespan, or erythropoietin administration.1 A clear consensus on optimal levels of GA has not been established, but GA may be a more reliable marker of glycemic control in patients with diabetes and CKD. Further research is needed to establish a target GA level that predicts the best prognosis for patients with both conditions.1
A1C is the most reliable marker at this time, but special considerations should be made for the patient with CKD. Rather than focus on a single measurement, clinicians need to consider the patient’s symptoms and results from all labwork, along with A1C, to best evaluate glycemic control.4 Further research is needed in patients with diabetes and CKD to explore other reliable markers to help maintain tight glycemic control.
Continued on next page >>
Q) One of my patients developed severe leg cramps while taking statins. I felt it was questionable rhabdomyolysis and stopped the medication; the leg pain went away. Is there a way to know if the rhabdomyolysis is progressive?
Rhabdomyolysis is a serious condition caused by the breakdown of muscle tissue that leads to the release of myoglobin into the bloodstream. This condition can lead to severe kidney failure and death.
Previously, there has been no easy method to predict progressive rhabdomyolysis. But researchers from Brigham and Women’s Hospital recently developed the Rhabdomyolysis Risk Calculator, a prediction score that can help determine whether a patient with rhabdomyolysis is at risk for severe kidney failure or death.
The researchers conducted a retrospective cohort study of 2,371 patients admitted between 2000 and 2011 and examined variables that may be associated with kidney failure and death.5 They identified independent predictors for these outcomes, including age; gender; initial levels of phosphate, calcium, creatinine, carbon dioxide, and creatine kinase; and etiology of rhabdomyolysis (myositis, exercise, statin use, or seizure).5
This tool can assist providers in developing a patient-specific treatment plan. However, further research is needed to validate the current variables, verify the risk prediction score in other populations, and examine its ability to guide individualized treatment plans.
The Rhabdomyolysis Risk Calculator is available at www.brighamandwomens.org/research/rhabdo/default.aspx
Kristy Washinger, MSN, CRNP
Nephrology Associates of Central PA
Camp Hill, PA
REFERENCES
1. Vos FE, Schollum JB, Walker RJ. Glycated albumin is the preferred marker for assessing glycaemic control in advanced chronic kidney disease. Nephrol Dial Transplant Plus. 2011; 4(6):368-375.
2. National Kidney Foundation Kidney Disease Outcomes Quality Initiative. Clinical practice guidelines and clinical practice recommendations for diabetes and chronic kidney disease. Guideline 2: management of hyperglycemia and general diabetes care in chronic kidney disease. www.kidney.org/professionals/kdoqi/guideline_diabetes/guide2.htm. Accessed April 15, 2014.
3. Sharif A, Baboolal K. Diagnostic application of the A1c assay in renal disease. J Am Soc Nephrol. 2010;21(3):383-385.
4. American Diabetes Association. Standards of medical care in diabetes—2013. Diabetes Care. 2013;36(suppl 1):S11-S66.
5. McMahon GM, Zeng X, Walker SS. A risk prediction score for kidney failure or mortality in rhabdomyolysis. JAMA Intern Med. 2013;173(19):1821-1828.
Q) Does anemia in CKD patients affect their A1C? Is A1C accurate in CKD patients?
Tight glycemic control is imperative for patients with chronic kidney disease (CKD), but the management of diabetes in CKD can be complex due to factors including anemia and changes in glucose and insulin homeostasis.
A1C is directly proportionate to the ambient blood glucose concentration and in the general diabetic population has proven to be a reliable marker.1 However, it may not be valid in patients with diabetes and CKD. Reduced red blood cell (RBC) lifespan, rapid hemolysis, and iron deficiency may lead to falsely decreased results.2 Decreased RBC survival results from an increase in hemoglobin turnover, which decreases glycemic exposure time.1 This process then lowers the amount of nonenzymatic glucose binding to hemoglobin.1 Folate deficiency caused by impaired intestinal absorption in CKD also affects RBC survival.3 Falsely increased results may be related to carbamylation of the hemoglobin and acidosis, both of which are influenced by uremia.2
Special considerations should be made for dialysis patients with diabetes. In hemodialysis patients, A1C may be falsely decreased due to blood loss, RBC transfusion, and erythropoietin therapy.3 Observational studies have shown that erythropoietin therapy is associated with lower A1C due to the increased number of immature RBCs that have a decreased glycemic exposure time.1 In peritoneal dialysis patients, A1C may increase after the start of therapy as a result of dialysate absorption.3
Research suggests that glycated albumin (GA) provides a short-term index of glycemic control (typically two to three weeks) and is not influenced by albumin concentration, RBC lifespan, or erythropoietin administration.1 A clear consensus on optimal levels of GA has not been established, but GA may be a more reliable marker of glycemic control in patients with diabetes and CKD. Further research is needed to establish a target GA level that predicts the best prognosis for patients with both conditions.1
A1C is the most reliable marker at this time, but special considerations should be made for the patient with CKD. Rather than focus on a single measurement, clinicians need to consider the patient’s symptoms and results from all labwork, along with A1C, to best evaluate glycemic control.4 Further research is needed in patients with diabetes and CKD to explore other reliable markers to help maintain tight glycemic control.
Continued on next page >>
Q) One of my patients developed severe leg cramps while taking statins. I felt it was questionable rhabdomyolysis and stopped the medication; the leg pain went away. Is there a way to know if the rhabdomyolysis is progressive?
Rhabdomyolysis is a serious condition caused by the breakdown of muscle tissue that leads to the release of myoglobin into the bloodstream. This condition can lead to severe kidney failure and death.
Previously, there has been no easy method to predict progressive rhabdomyolysis. But researchers from Brigham and Women’s Hospital recently developed the Rhabdomyolysis Risk Calculator, a prediction score that can help determine whether a patient with rhabdomyolysis is at risk for severe kidney failure or death.
The researchers conducted a retrospective cohort study of 2,371 patients admitted between 2000 and 2011 and examined variables that may be associated with kidney failure and death.5 They identified independent predictors for these outcomes, including age; gender; initial levels of phosphate, calcium, creatinine, carbon dioxide, and creatine kinase; and etiology of rhabdomyolysis (myositis, exercise, statin use, or seizure).5
This tool can assist providers in developing a patient-specific treatment plan. However, further research is needed to validate the current variables, verify the risk prediction score in other populations, and examine its ability to guide individualized treatment plans.
The Rhabdomyolysis Risk Calculator is available at www.brighamandwomens.org/research/rhabdo/default.aspx
Kristy Washinger, MSN, CRNP
Nephrology Associates of Central PA
Camp Hill, PA
REFERENCES
1. Vos FE, Schollum JB, Walker RJ. Glycated albumin is the preferred marker for assessing glycaemic control in advanced chronic kidney disease. Nephrol Dial Transplant Plus. 2011; 4(6):368-375.
2. National Kidney Foundation Kidney Disease Outcomes Quality Initiative. Clinical practice guidelines and clinical practice recommendations for diabetes and chronic kidney disease. Guideline 2: management of hyperglycemia and general diabetes care in chronic kidney disease. www.kidney.org/professionals/kdoqi/guideline_diabetes/guide2.htm. Accessed April 15, 2014.
3. Sharif A, Baboolal K. Diagnostic application of the A1c assay in renal disease. J Am Soc Nephrol. 2010;21(3):383-385.
4. American Diabetes Association. Standards of medical care in diabetes—2013. Diabetes Care. 2013;36(suppl 1):S11-S66.
5. McMahon GM, Zeng X, Walker SS. A risk prediction score for kidney failure or mortality in rhabdomyolysis. JAMA Intern Med. 2013;173(19):1821-1828.
Q) Does anemia in CKD patients affect their A1C? Is A1C accurate in CKD patients?
Tight glycemic control is imperative for patients with chronic kidney disease (CKD), but the management of diabetes in CKD can be complex due to factors including anemia and changes in glucose and insulin homeostasis.
A1C is directly proportionate to the ambient blood glucose concentration and in the general diabetic population has proven to be a reliable marker.1 However, it may not be valid in patients with diabetes and CKD. Reduced red blood cell (RBC) lifespan, rapid hemolysis, and iron deficiency may lead to falsely decreased results.2 Decreased RBC survival results from an increase in hemoglobin turnover, which decreases glycemic exposure time.1 This process then lowers the amount of nonenzymatic glucose binding to hemoglobin.1 Folate deficiency caused by impaired intestinal absorption in CKD also affects RBC survival.3 Falsely increased results may be related to carbamylation of the hemoglobin and acidosis, both of which are influenced by uremia.2
Special considerations should be made for dialysis patients with diabetes. In hemodialysis patients, A1C may be falsely decreased due to blood loss, RBC transfusion, and erythropoietin therapy.3 Observational studies have shown that erythropoietin therapy is associated with lower A1C due to the increased number of immature RBCs that have a decreased glycemic exposure time.1 In peritoneal dialysis patients, A1C may increase after the start of therapy as a result of dialysate absorption.3
Research suggests that glycated albumin (GA) provides a short-term index of glycemic control (typically two to three weeks) and is not influenced by albumin concentration, RBC lifespan, or erythropoietin administration.1 A clear consensus on optimal levels of GA has not been established, but GA may be a more reliable marker of glycemic control in patients with diabetes and CKD. Further research is needed to establish a target GA level that predicts the best prognosis for patients with both conditions.1
A1C is the most reliable marker at this time, but special considerations should be made for the patient with CKD. Rather than focus on a single measurement, clinicians need to consider the patient’s symptoms and results from all labwork, along with A1C, to best evaluate glycemic control.4 Further research is needed in patients with diabetes and CKD to explore other reliable markers to help maintain tight glycemic control.
Continued on next page >>
Q) One of my patients developed severe leg cramps while taking statins. I felt it was questionable rhabdomyolysis and stopped the medication; the leg pain went away. Is there a way to know if the rhabdomyolysis is progressive?
Rhabdomyolysis is a serious condition caused by the breakdown of muscle tissue that leads to the release of myoglobin into the bloodstream. This condition can lead to severe kidney failure and death.
Previously, there has been no easy method to predict progressive rhabdomyolysis. But researchers from Brigham and Women’s Hospital recently developed the Rhabdomyolysis Risk Calculator, a prediction score that can help determine whether a patient with rhabdomyolysis is at risk for severe kidney failure or death.
The researchers conducted a retrospective cohort study of 2,371 patients admitted between 2000 and 2011 and examined variables that may be associated with kidney failure and death.5 They identified independent predictors for these outcomes, including age; gender; initial levels of phosphate, calcium, creatinine, carbon dioxide, and creatine kinase; and etiology of rhabdomyolysis (myositis, exercise, statin use, or seizure).5
This tool can assist providers in developing a patient-specific treatment plan. However, further research is needed to validate the current variables, verify the risk prediction score in other populations, and examine its ability to guide individualized treatment plans.
The Rhabdomyolysis Risk Calculator is available at www.brighamandwomens.org/research/rhabdo/default.aspx
Kristy Washinger, MSN, CRNP
Nephrology Associates of Central PA
Camp Hill, PA
REFERENCES
1. Vos FE, Schollum JB, Walker RJ. Glycated albumin is the preferred marker for assessing glycaemic control in advanced chronic kidney disease. Nephrol Dial Transplant Plus. 2011; 4(6):368-375.
2. National Kidney Foundation Kidney Disease Outcomes Quality Initiative. Clinical practice guidelines and clinical practice recommendations for diabetes and chronic kidney disease. Guideline 2: management of hyperglycemia and general diabetes care in chronic kidney disease. www.kidney.org/professionals/kdoqi/guideline_diabetes/guide2.htm. Accessed April 15, 2014.
3. Sharif A, Baboolal K. Diagnostic application of the A1c assay in renal disease. J Am Soc Nephrol. 2010;21(3):383-385.
4. American Diabetes Association. Standards of medical care in diabetes—2013. Diabetes Care. 2013;36(suppl 1):S11-S66.
5. McMahon GM, Zeng X, Walker SS. A risk prediction score for kidney failure or mortality in rhabdomyolysis. JAMA Intern Med. 2013;173(19):1821-1828.
Is DXA Valid for Kidney Patients?
Q) A 53-year-old dialysis patient in my clinic says her nephrologist told her she did not need a DXA scan because it was not valid for kidney patients. Why not?
Osteoporosis is a condition of reduced bone mass, causing decreased bone strength and leading to increased risk for fractures. The World Health Organization definition of osteoporosis is based on bone mineral density (BMD). While there is some overlap between idiopathic osteoporosis and chronic kidney disease–mineral bone disorder (CKD-MBD), both conditions have different pathophysiologic backgrounds and require different treatments.1
There is not an accurate correlation between BMD as measured by DXA and the type of CKD-associated bone disease.2 Patients with CKD typically have lower bone density than the general population, making the interpretation of DXA (dual x-ray absorptiometry) scans more complicated. This is due to focal areas of osteosclerosis, the presence of extraskeletal calcifications, and the variable presence of osteomalacia.
The gold standard for assessment and diagnosis of bone disease in CKD patients is the iliac crest bone biopsy. However, it is not frequently performed due to the painful and invasive nature of the procedure and the limitations in access to centers where it is performed and to experienced pathologists.
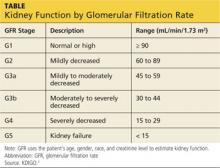
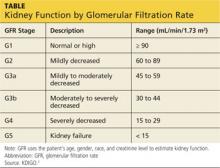
KDIGO (Kidney Disease Improving Global Outcomes) guidelines3 recommend that in patients with CKD stages 3 to 5D (see chart for explanation), measurements of serum parathyroid hormone or bone-specific alkaline phosphatase be used to evaluate bone disease, because markedly high or low values predict underlying bone turnover.
Shelly Levinstein, MSN, CRNP
Nephrology Associates of York
York, PA
REFERENCES
1. Toussaint N, Elder G, Kerr P. Bisphosphonates in chronic kidney disease; balancing potential benefits and adverse effects on bone and soft tissue. Clin J Am Soc Nephrol. 2009;4:221-233.
2. Tanenbaum N, Quarles LD. Bone disorders in chronic kidney disease. In: Greenberg A, Cheung AK, eds. Primer on Kidney Diseases. 5th ed. Philadelphia, PA: Elsevier Saunders; 2009:487-498.
3. Moe S, Drueke T, Cunningham J, et al; Kidney Disease: Improving Global Outcomes (KDIGO). Definition, evaluation, and classification of renal osteodystrophy: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2006;69:1945-1953.
4. Gilbert S, Weiner DE. National Kidney Foundation’s Primer on Kidney Diseases. 6th ed. Philadelphia, PA: Elsevier Saunders; 2013:330.
Q) A 53-year-old dialysis patient in my clinic says her nephrologist told her she did not need a DXA scan because it was not valid for kidney patients. Why not?
Osteoporosis is a condition of reduced bone mass, causing decreased bone strength and leading to increased risk for fractures. The World Health Organization definition of osteoporosis is based on bone mineral density (BMD). While there is some overlap between idiopathic osteoporosis and chronic kidney disease–mineral bone disorder (CKD-MBD), both conditions have different pathophysiologic backgrounds and require different treatments.1
There is not an accurate correlation between BMD as measured by DXA and the type of CKD-associated bone disease.2 Patients with CKD typically have lower bone density than the general population, making the interpretation of DXA (dual x-ray absorptiometry) scans more complicated. This is due to focal areas of osteosclerosis, the presence of extraskeletal calcifications, and the variable presence of osteomalacia.
The gold standard for assessment and diagnosis of bone disease in CKD patients is the iliac crest bone biopsy. However, it is not frequently performed due to the painful and invasive nature of the procedure and the limitations in access to centers where it is performed and to experienced pathologists.
KDIGO (Kidney Disease Improving Global Outcomes) guidelines3 recommend that in patients with CKD stages 3 to 5D (see chart for explanation), measurements of serum parathyroid hormone or bone-specific alkaline phosphatase be used to evaluate bone disease, because markedly high or low values predict underlying bone turnover.
Shelly Levinstein, MSN, CRNP
Nephrology Associates of York
York, PA
REFERENCES
1. Toussaint N, Elder G, Kerr P. Bisphosphonates in chronic kidney disease; balancing potential benefits and adverse effects on bone and soft tissue. Clin J Am Soc Nephrol. 2009;4:221-233.
2. Tanenbaum N, Quarles LD. Bone disorders in chronic kidney disease. In: Greenberg A, Cheung AK, eds. Primer on Kidney Diseases. 5th ed. Philadelphia, PA: Elsevier Saunders; 2009:487-498.
3. Moe S, Drueke T, Cunningham J, et al; Kidney Disease: Improving Global Outcomes (KDIGO). Definition, evaluation, and classification of renal osteodystrophy: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2006;69:1945-1953.
4. Gilbert S, Weiner DE. National Kidney Foundation’s Primer on Kidney Diseases. 6th ed. Philadelphia, PA: Elsevier Saunders; 2013:330.
Q) A 53-year-old dialysis patient in my clinic says her nephrologist told her she did not need a DXA scan because it was not valid for kidney patients. Why not?
Osteoporosis is a condition of reduced bone mass, causing decreased bone strength and leading to increased risk for fractures. The World Health Organization definition of osteoporosis is based on bone mineral density (BMD). While there is some overlap between idiopathic osteoporosis and chronic kidney disease–mineral bone disorder (CKD-MBD), both conditions have different pathophysiologic backgrounds and require different treatments.1
There is not an accurate correlation between BMD as measured by DXA and the type of CKD-associated bone disease.2 Patients with CKD typically have lower bone density than the general population, making the interpretation of DXA (dual x-ray absorptiometry) scans more complicated. This is due to focal areas of osteosclerosis, the presence of extraskeletal calcifications, and the variable presence of osteomalacia.
The gold standard for assessment and diagnosis of bone disease in CKD patients is the iliac crest bone biopsy. However, it is not frequently performed due to the painful and invasive nature of the procedure and the limitations in access to centers where it is performed and to experienced pathologists.
KDIGO (Kidney Disease Improving Global Outcomes) guidelines3 recommend that in patients with CKD stages 3 to 5D (see chart for explanation), measurements of serum parathyroid hormone or bone-specific alkaline phosphatase be used to evaluate bone disease, because markedly high or low values predict underlying bone turnover.
Shelly Levinstein, MSN, CRNP
Nephrology Associates of York
York, PA
REFERENCES
1. Toussaint N, Elder G, Kerr P. Bisphosphonates in chronic kidney disease; balancing potential benefits and adverse effects on bone and soft tissue. Clin J Am Soc Nephrol. 2009;4:221-233.
2. Tanenbaum N, Quarles LD. Bone disorders in chronic kidney disease. In: Greenberg A, Cheung AK, eds. Primer on Kidney Diseases. 5th ed. Philadelphia, PA: Elsevier Saunders; 2009:487-498.
3. Moe S, Drueke T, Cunningham J, et al; Kidney Disease: Improving Global Outcomes (KDIGO). Definition, evaluation, and classification of renal osteodystrophy: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2006;69:1945-1953.
4. Gilbert S, Weiner DE. National Kidney Foundation’s Primer on Kidney Diseases. 6th ed. Philadelphia, PA: Elsevier Saunders; 2013:330.
How to Treat Bone Loss in Kidney Patients
Q) Since bone loss is common in patients with kidney disease, how should it be treated?
In elderly CKD patients, it is recognized that osteoporosis and CKD-MBD may co-exist—which only makes the bone-loss issue more problematic. Therefore, diagnosis and management are crucial to effective treatment.
Although osteoporosis is recognized and treated in CKD stages 1 to 3a, the interpretation of BMD levels in the osteoporotic range is controversial in advanced kidney disease (GFR < 35).1 There are limited data for treatment of patients with CKD-MBD.
Studies of bisphosphonate use in postmenopausal women and in patients with glucocorticoid-induced osteoporosis have generally excluded those with renal impairment. For these patients, treatment of low BMD using standard therapies for osteoporosis is not without potential for harm, due to the possibility of worsening low bone turnover, osteomalacia, mixed uremic osteodystrophy, and exacerbated hyperparathyroidism.
The choice of pharmaceutical treatment should be based on whether you are treating CKD-MBD or osteoporosis. A large percentage of CKD patients have adynamic bone disease with low bone resorption. In patients with low bone resorption, treatment entails choosing a drug that stimulates bone formation and not those that inhibit bone resorption. The benefit of bisphosphonate therapy is seen in patients with high bone resorption.4
In CKD stages 1 to 3, one must evaluate laboratory features of low BMD, including serum levels of calcium, phosphate, parathyroid hormone, alkaline phosphatase, and vitamin D. If all are found to be normal, bisphosphonate use in CKD stages 1 to 3 is usually safe.1
A bone biopsy is recommended for patients with advanced CKD stages 4 to 5/5D, with therapy individualized per disease process.1
Shelly Levinstein, MSN, CRNP
Nephrology Associates of York
York, PA
REFERENCES
1. Toussaint N, Elder G, Kerr P. Bisphosphonates in chronic kidney disease; balancing potential benefits and adverse effects on bone and soft tissue. Clin J Am Soc Nephrol. 2009;4:221-233.
2. Tanenbaum N, Quarles LD. Bone disorders in chronic kidney disease. In: Greenberg A, Cheung AK, eds. Primer on Kidney Diseases. 5th ed. Philadelphia, PA: Elsevier Saunders; 2009:487-498.
3. Moe S, Drueke T, Cunningham J, et al; Kidney Disease: Improving Global Outcomes (KDIGO). Definition, evaluation, and classification of renal osteodystrophy: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2006;69:1945-1953.
4. Gilbert S, Weiner DE. National Kidney Foundation’s Primer on Kidney Diseases. 6th ed. Philadelphia, PA: Elsevier Saunders; 2013:330.
Q) Since bone loss is common in patients with kidney disease, how should it be treated?
In elderly CKD patients, it is recognized that osteoporosis and CKD-MBD may co-exist—which only makes the bone-loss issue more problematic. Therefore, diagnosis and management are crucial to effective treatment.
Although osteoporosis is recognized and treated in CKD stages 1 to 3a, the interpretation of BMD levels in the osteoporotic range is controversial in advanced kidney disease (GFR < 35).1 There are limited data for treatment of patients with CKD-MBD.
Studies of bisphosphonate use in postmenopausal women and in patients with glucocorticoid-induced osteoporosis have generally excluded those with renal impairment. For these patients, treatment of low BMD using standard therapies for osteoporosis is not without potential for harm, due to the possibility of worsening low bone turnover, osteomalacia, mixed uremic osteodystrophy, and exacerbated hyperparathyroidism.
The choice of pharmaceutical treatment should be based on whether you are treating CKD-MBD or osteoporosis. A large percentage of CKD patients have adynamic bone disease with low bone resorption. In patients with low bone resorption, treatment entails choosing a drug that stimulates bone formation and not those that inhibit bone resorption. The benefit of bisphosphonate therapy is seen in patients with high bone resorption.4
In CKD stages 1 to 3, one must evaluate laboratory features of low BMD, including serum levels of calcium, phosphate, parathyroid hormone, alkaline phosphatase, and vitamin D. If all are found to be normal, bisphosphonate use in CKD stages 1 to 3 is usually safe.1
A bone biopsy is recommended for patients with advanced CKD stages 4 to 5/5D, with therapy individualized per disease process.1
Shelly Levinstein, MSN, CRNP
Nephrology Associates of York
York, PA
REFERENCES
1. Toussaint N, Elder G, Kerr P. Bisphosphonates in chronic kidney disease; balancing potential benefits and adverse effects on bone and soft tissue. Clin J Am Soc Nephrol. 2009;4:221-233.
2. Tanenbaum N, Quarles LD. Bone disorders in chronic kidney disease. In: Greenberg A, Cheung AK, eds. Primer on Kidney Diseases. 5th ed. Philadelphia, PA: Elsevier Saunders; 2009:487-498.
3. Moe S, Drueke T, Cunningham J, et al; Kidney Disease: Improving Global Outcomes (KDIGO). Definition, evaluation, and classification of renal osteodystrophy: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2006;69:1945-1953.
4. Gilbert S, Weiner DE. National Kidney Foundation’s Primer on Kidney Diseases. 6th ed. Philadelphia, PA: Elsevier Saunders; 2013:330.
Q) Since bone loss is common in patients with kidney disease, how should it be treated?
In elderly CKD patients, it is recognized that osteoporosis and CKD-MBD may co-exist—which only makes the bone-loss issue more problematic. Therefore, diagnosis and management are crucial to effective treatment.
Although osteoporosis is recognized and treated in CKD stages 1 to 3a, the interpretation of BMD levels in the osteoporotic range is controversial in advanced kidney disease (GFR < 35).1 There are limited data for treatment of patients with CKD-MBD.
Studies of bisphosphonate use in postmenopausal women and in patients with glucocorticoid-induced osteoporosis have generally excluded those with renal impairment. For these patients, treatment of low BMD using standard therapies for osteoporosis is not without potential for harm, due to the possibility of worsening low bone turnover, osteomalacia, mixed uremic osteodystrophy, and exacerbated hyperparathyroidism.
The choice of pharmaceutical treatment should be based on whether you are treating CKD-MBD or osteoporosis. A large percentage of CKD patients have adynamic bone disease with low bone resorption. In patients with low bone resorption, treatment entails choosing a drug that stimulates bone formation and not those that inhibit bone resorption. The benefit of bisphosphonate therapy is seen in patients with high bone resorption.4
In CKD stages 1 to 3, one must evaluate laboratory features of low BMD, including serum levels of calcium, phosphate, parathyroid hormone, alkaline phosphatase, and vitamin D. If all are found to be normal, bisphosphonate use in CKD stages 1 to 3 is usually safe.1
A bone biopsy is recommended for patients with advanced CKD stages 4 to 5/5D, with therapy individualized per disease process.1
Shelly Levinstein, MSN, CRNP
Nephrology Associates of York
York, PA
REFERENCES
1. Toussaint N, Elder G, Kerr P. Bisphosphonates in chronic kidney disease; balancing potential benefits and adverse effects on bone and soft tissue. Clin J Am Soc Nephrol. 2009;4:221-233.
2. Tanenbaum N, Quarles LD. Bone disorders in chronic kidney disease. In: Greenberg A, Cheung AK, eds. Primer on Kidney Diseases. 5th ed. Philadelphia, PA: Elsevier Saunders; 2009:487-498.
3. Moe S, Drueke T, Cunningham J, et al; Kidney Disease: Improving Global Outcomes (KDIGO). Definition, evaluation, and classification of renal osteodystrophy: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2006;69:1945-1953.
4. Gilbert S, Weiner DE. National Kidney Foundation’s Primer on Kidney Diseases. 6th ed. Philadelphia, PA: Elsevier Saunders; 2013:330.
The Modern Realities of Kidney Stones: Diagnosis
Q) I am new to practice and working in urgent care. I was discussing the diagnosis of kidney stones with my supervising physician. He said he does an intravenous pyelogram (IVP) to diagnose stones. He is a little “old school,” and I’m not sure he is right. What is “state of the art” in the work-up and acute treatment for kidney stones?
An IVP involves taking a series of x-rays following the injection of dye into a patient’s vein. As the dye moves through the bloodstream, the anatomy of the urinary system can be better visualized and the stone location identified, as the dye tends to accumulate at areas of obstruction. The downside to this test is that contrast can cause allergic reactions in some patients and can only be used in those with normal renal function. Also, a radiologist is required to be present during the procedure, and the test can take a long time to complete if a severe blockage is present.4
Today, noncontrast CT is considered the gold standard for imaging renal calculi because it is fast, safe (no worries for those with contrast allergy or renal impairment), and nearly 100% accurate.5
There are multiple options for treating kidney stones, though some are more invasive than others. For stones 2 cm or less identified in the upper or middle calyx and renal pelvis, extracorporeal shock wave lithotripsy (ESWL) is the treatment of choice.5,6 The goal of ESWL is to break the stone into small particles that can then be expelled through the urinary system. Adjunct measures, including mechanical percussion, diuresis, and inversion therapy, are often used following lithotripsy to facilitate passage of stone fragments. Medications such as calcium blockers and α-receptor blockers are also used to improve outcomes after lithotripsy. In the past, obese patients had less success with lithotripsy; however, technological advances have improved outcomes in depths up to 17 cm from skin to stone.6
For stones in the lower pole of the kidney (and depending on the size of the stone), ESWL, percutaneous nephrolitholapaxy, retrograde flexible ureteronephroscopy, and partial nephrectomy are options for treatment.3,7 Using an intravenous urogram, measurements and angles are calculated to help determine which procedure would be best for a particular patient.7 In simple terms, narrow angles and longer tube distances make it more difficult for stone particles to exit the urinary system. Therefore, if these problems are identified, an invasive approach may be needed to remove a stone. Other important considerations include stone size, patient symptoms, evidence of infection, or obstruction.7
ESWL is an attractive option for stone removal because it is noninvasive, has a reasonable safety profile, and is less costly than other, more invasive measures. However, advances in endoscopic instrument design are reducing complications previously associated with more invasive approaches, while improving long-term stone-free outcome rates. There may be increased utilization of procedures such as percutaneous nephrolitholapaxy and retrograde flexible ureteronephroscopy in the future.
Kristina Unterseher, CNN-NP
PeaceHealth
St. John Medical Group
Longview, WA
REFERENCES
1. Fink HA, Wilt TJ, Eidman KE, et al. Medical management to prevent recurrent nephrolithiasis in adults: a systematic review for an American College of Physicians Clinical Guideline. Ann Intern Med. 2013;158(7):535-543.
2. Hiatt RA, Ettinger B, Caan B, et al. Randomized controlled trial of a low animal protein, high fiber diet in the prevention of recurrent calcium oxalate kidney stones. Am J Epidemiol. 1996;144(1):25-33.
3. Moe OW. Kidney stones: pathophysiology and medical management. Lancet. 2006; 367(9507):333-344.
4. American College of Radiology and Radiological Society of North America. Intravenous pyelogram (2013). www.radiologyinfo.org/en/info.cfm?pg=ivp. Accessed December 16, 2013.
5. Boyce CJ, Pickhardt PJ, Lawrence EM, et al. Prevalence of urolithiasis in asymptomatic adults: objective determination using low dose noncontrast computerized tomography. J Urol. 2010;183(3):1017-1021.
6. Christian C, Thorsten B. The preferred treatment for upper tract stones is extracorporeal shock wave lithotripsy (ESWL) or ureteroscopic: pro ESWL. Urology. 2009;74(2):259-262.
7. Bourdoumis A, Papatsoris AG, Chrisofos M, Deliveliotis C. Lower pole stone management. Med Surg Urol. 2012. www.omicsonline.org/lower-pole-stone-management%20-2168-9857.S4-004.php?aid=7058?abstract _id=7058. Accessed December 16, 2013.
Q) I am new to practice and working in urgent care. I was discussing the diagnosis of kidney stones with my supervising physician. He said he does an intravenous pyelogram (IVP) to diagnose stones. He is a little “old school,” and I’m not sure he is right. What is “state of the art” in the work-up and acute treatment for kidney stones?
An IVP involves taking a series of x-rays following the injection of dye into a patient’s vein. As the dye moves through the bloodstream, the anatomy of the urinary system can be better visualized and the stone location identified, as the dye tends to accumulate at areas of obstruction. The downside to this test is that contrast can cause allergic reactions in some patients and can only be used in those with normal renal function. Also, a radiologist is required to be present during the procedure, and the test can take a long time to complete if a severe blockage is present.4
Today, noncontrast CT is considered the gold standard for imaging renal calculi because it is fast, safe (no worries for those with contrast allergy or renal impairment), and nearly 100% accurate.5
There are multiple options for treating kidney stones, though some are more invasive than others. For stones 2 cm or less identified in the upper or middle calyx and renal pelvis, extracorporeal shock wave lithotripsy (ESWL) is the treatment of choice.5,6 The goal of ESWL is to break the stone into small particles that can then be expelled through the urinary system. Adjunct measures, including mechanical percussion, diuresis, and inversion therapy, are often used following lithotripsy to facilitate passage of stone fragments. Medications such as calcium blockers and α-receptor blockers are also used to improve outcomes after lithotripsy. In the past, obese patients had less success with lithotripsy; however, technological advances have improved outcomes in depths up to 17 cm from skin to stone.6
For stones in the lower pole of the kidney (and depending on the size of the stone), ESWL, percutaneous nephrolitholapaxy, retrograde flexible ureteronephroscopy, and partial nephrectomy are options for treatment.3,7 Using an intravenous urogram, measurements and angles are calculated to help determine which procedure would be best for a particular patient.7 In simple terms, narrow angles and longer tube distances make it more difficult for stone particles to exit the urinary system. Therefore, if these problems are identified, an invasive approach may be needed to remove a stone. Other important considerations include stone size, patient symptoms, evidence of infection, or obstruction.7
ESWL is an attractive option for stone removal because it is noninvasive, has a reasonable safety profile, and is less costly than other, more invasive measures. However, advances in endoscopic instrument design are reducing complications previously associated with more invasive approaches, while improving long-term stone-free outcome rates. There may be increased utilization of procedures such as percutaneous nephrolitholapaxy and retrograde flexible ureteronephroscopy in the future.
Kristina Unterseher, CNN-NP
PeaceHealth
St. John Medical Group
Longview, WA
REFERENCES
1. Fink HA, Wilt TJ, Eidman KE, et al. Medical management to prevent recurrent nephrolithiasis in adults: a systematic review for an American College of Physicians Clinical Guideline. Ann Intern Med. 2013;158(7):535-543.
2. Hiatt RA, Ettinger B, Caan B, et al. Randomized controlled trial of a low animal protein, high fiber diet in the prevention of recurrent calcium oxalate kidney stones. Am J Epidemiol. 1996;144(1):25-33.
3. Moe OW. Kidney stones: pathophysiology and medical management. Lancet. 2006; 367(9507):333-344.
4. American College of Radiology and Radiological Society of North America. Intravenous pyelogram (2013). www.radiologyinfo.org/en/info.cfm?pg=ivp. Accessed December 16, 2013.
5. Boyce CJ, Pickhardt PJ, Lawrence EM, et al. Prevalence of urolithiasis in asymptomatic adults: objective determination using low dose noncontrast computerized tomography. J Urol. 2010;183(3):1017-1021.
6. Christian C, Thorsten B. The preferred treatment for upper tract stones is extracorporeal shock wave lithotripsy (ESWL) or ureteroscopic: pro ESWL. Urology. 2009;74(2):259-262.
7. Bourdoumis A, Papatsoris AG, Chrisofos M, Deliveliotis C. Lower pole stone management. Med Surg Urol. 2012. www.omicsonline.org/lower-pole-stone-management%20-2168-9857.S4-004.php?aid=7058?abstract _id=7058. Accessed December 16, 2013.
Q) I am new to practice and working in urgent care. I was discussing the diagnosis of kidney stones with my supervising physician. He said he does an intravenous pyelogram (IVP) to diagnose stones. He is a little “old school,” and I’m not sure he is right. What is “state of the art” in the work-up and acute treatment for kidney stones?
An IVP involves taking a series of x-rays following the injection of dye into a patient’s vein. As the dye moves through the bloodstream, the anatomy of the urinary system can be better visualized and the stone location identified, as the dye tends to accumulate at areas of obstruction. The downside to this test is that contrast can cause allergic reactions in some patients and can only be used in those with normal renal function. Also, a radiologist is required to be present during the procedure, and the test can take a long time to complete if a severe blockage is present.4
Today, noncontrast CT is considered the gold standard for imaging renal calculi because it is fast, safe (no worries for those with contrast allergy or renal impairment), and nearly 100% accurate.5
There are multiple options for treating kidney stones, though some are more invasive than others. For stones 2 cm or less identified in the upper or middle calyx and renal pelvis, extracorporeal shock wave lithotripsy (ESWL) is the treatment of choice.5,6 The goal of ESWL is to break the stone into small particles that can then be expelled through the urinary system. Adjunct measures, including mechanical percussion, diuresis, and inversion therapy, are often used following lithotripsy to facilitate passage of stone fragments. Medications such as calcium blockers and α-receptor blockers are also used to improve outcomes after lithotripsy. In the past, obese patients had less success with lithotripsy; however, technological advances have improved outcomes in depths up to 17 cm from skin to stone.6
For stones in the lower pole of the kidney (and depending on the size of the stone), ESWL, percutaneous nephrolitholapaxy, retrograde flexible ureteronephroscopy, and partial nephrectomy are options for treatment.3,7 Using an intravenous urogram, measurements and angles are calculated to help determine which procedure would be best for a particular patient.7 In simple terms, narrow angles and longer tube distances make it more difficult for stone particles to exit the urinary system. Therefore, if these problems are identified, an invasive approach may be needed to remove a stone. Other important considerations include stone size, patient symptoms, evidence of infection, or obstruction.7
ESWL is an attractive option for stone removal because it is noninvasive, has a reasonable safety profile, and is less costly than other, more invasive measures. However, advances in endoscopic instrument design are reducing complications previously associated with more invasive approaches, while improving long-term stone-free outcome rates. There may be increased utilization of procedures such as percutaneous nephrolitholapaxy and retrograde flexible ureteronephroscopy in the future.
Kristina Unterseher, CNN-NP
PeaceHealth
St. John Medical Group
Longview, WA
REFERENCES
1. Fink HA, Wilt TJ, Eidman KE, et al. Medical management to prevent recurrent nephrolithiasis in adults: a systematic review for an American College of Physicians Clinical Guideline. Ann Intern Med. 2013;158(7):535-543.
2. Hiatt RA, Ettinger B, Caan B, et al. Randomized controlled trial of a low animal protein, high fiber diet in the prevention of recurrent calcium oxalate kidney stones. Am J Epidemiol. 1996;144(1):25-33.
3. Moe OW. Kidney stones: pathophysiology and medical management. Lancet. 2006; 367(9507):333-344.
4. American College of Radiology and Radiological Society of North America. Intravenous pyelogram (2013). www.radiologyinfo.org/en/info.cfm?pg=ivp. Accessed December 16, 2013.
5. Boyce CJ, Pickhardt PJ, Lawrence EM, et al. Prevalence of urolithiasis in asymptomatic adults: objective determination using low dose noncontrast computerized tomography. J Urol. 2010;183(3):1017-1021.
6. Christian C, Thorsten B. The preferred treatment for upper tract stones is extracorporeal shock wave lithotripsy (ESWL) or ureteroscopic: pro ESWL. Urology. 2009;74(2):259-262.
7. Bourdoumis A, Papatsoris AG, Chrisofos M, Deliveliotis C. Lower pole stone management. Med Surg Urol. 2012. www.omicsonline.org/lower-pole-stone-management%20-2168-9857.S4-004.php?aid=7058?abstract _id=7058. Accessed December 16, 2013.
The Modern Realities of Kidney Stones: Preventing Reoccurrence
Q) A patient recently came in after an episode of kidney stones. He said he had never experienced such pain before (and this is a former Army Ranger!) and asked if there was anything he could do to keep it from happening again. I told him what I had learned in school (lots of fluids, no organ meats), but is there anything new?
Your patient has some reason for concern. For people who have had a symptomatic kidney stone, the likelihood of developing another within five years is 35% to 50% if no preventive action is taken.1 Certain factors—including family history, younger age at onset, and predisposing medical conditions (eg, hyperparathyroidism, diabetes, obesity, gout)—increase risk for recurrence.2,3
In the past, patients were often advised to restrict their dietary calcium intake to prevent calcium oxalate and/or calcium phosphate stones. However, more recent research has proven the opposite to be true: People with lower dietary calcium intake can be at greater risk for kidney stones.1-3 Therefore, encourage patients to consume about 800 to 1,200 mg/d of dietary calcium. Oral supplementation does not seem to yield the same protective benefits as dietary calcium. This may be related to absorption.1
Diets high in oxalates (eg, chocolate, nuts, spinach) can increase risk for stone formation, particularly in patients who have bowel diseases that cause inflammation or a history of a bowel resection.2 Animal protein in the diet can cause hypercalcinuria and increased uric acid levels, which is particularly problematic for individuals with gout or inflammatory arthritis. High-sodium diets can cause higher urinary calcium oxalate levels, while diets high in phosphorus (particularly dark cola soft drinks) can increase risk for stone formation. Advise your patient to avoid foods high in oxalates, animal proteins, sodium, and phosphorus.2
Dehydration, either due to exercise or poor fluid intake, can result in concentrated urine, which facilitates stone formation. While opinions differ on the benefits of certain dietary restrictions, most research supports the idea that generous fluid intake is the most successful intervention in preventing recurrence of stone formation (regardless of underlying cause). Diluting the urine decreases the concentration of solutes responsible for stone formation.1-3
If the conservative measures of dietary restriction and adequate hydration fail, medications may be beneficial, depending on stone type or underlying metabolic condition. Thiazide diuretics can help lower urinary calcium by enhancing reabsorption of calcium from the distal convoluted tubule and sodium excretion; however, they should be used cautiously due to the risk for adverse effects such as dizziness and lightheadedness.1,3 Allopurinol can lower uric acid levels, decreasing recurrence of both uric acid and calcium oxalate stones. Hypocitraturia is prevalent in 20% to 60% of persons with stones; prescribing potassium citrate can inhibit crystal growth of calcium phosphate and calcium oxalate in urine.2
To help patients prevent stone recurrence, perform a comprehensive assessment of their dietary and lifestyle habits and medical history to identify possible contributing factors. Educate patients on adequate dietary calcium intake, generous water intake to keep urine dilute, and avoidance of dietary triggers.
Nephrolithiasis should be considered a manifestation of another underlying problem. If a patient presents with a kidney stone, attempt to identify the cause—not only to try to prevent recurrence, but also to identify a previously unrecognized disease process.
Kristina Unterseher, CNN-NP
PeaceHealth
St. John Medical Group
Longview, WA
REFERENCES
1. Fink HA, Wilt TJ, Eidman KE, et al. Medical management to prevent recurrent nephrolithiasis in adults: a systematic review for an American College of Physicians Clinical Guideline. Ann Intern Med. 2013;158(7):535-543.
2. Hiatt RA, Ettinger B, Caan B, et al. Randomized controlled trial of a low animal protein, high fiber diet in the prevention of recurrent calcium oxalate kidney stones. Am J Epidemiol. 1996;144(1):25-33.
3. Moe OW. Kidney stones: pathophysiology and medical management. Lancet. 2006; 367(9507):333-344.
4. American College of Radiology and Radiological Society of North America. Intravenous pyelogram (2013). www.radiologyinfo.org/en/info.cfm?pg=ivp. Accessed December 16, 2013.
5. Boyce CJ, Pickhardt PJ, Lawrence EM, et al. Prevalence of urolithiasis in asymptomatic adults: objective determination using low dose noncontrast computerized tomography. J Urol. 2010;183(3):1017-1021.
6. Christian C, Thorsten B. The preferred treatment for upper tract stones is extracorporeal shock wave lithotripsy (ESWL) or ureteroscopic: pro ESWL. Urology. 2009;74(2):259-262.
7. Bourdoumis A, Papatsoris AG, Chrisofos M, Deliveliotis C. Lower pole stone management. Med Surg Urol. 2012. www.omicsonline.org/lower-pole-stone-management%20-2168-9857.S4-004.php?aid=7058?abstract _id=7058. Accessed December 16, 2013.
Q) A patient recently came in after an episode of kidney stones. He said he had never experienced such pain before (and this is a former Army Ranger!) and asked if there was anything he could do to keep it from happening again. I told him what I had learned in school (lots of fluids, no organ meats), but is there anything new?
Your patient has some reason for concern. For people who have had a symptomatic kidney stone, the likelihood of developing another within five years is 35% to 50% if no preventive action is taken.1 Certain factors—including family history, younger age at onset, and predisposing medical conditions (eg, hyperparathyroidism, diabetes, obesity, gout)—increase risk for recurrence.2,3
In the past, patients were often advised to restrict their dietary calcium intake to prevent calcium oxalate and/or calcium phosphate stones. However, more recent research has proven the opposite to be true: People with lower dietary calcium intake can be at greater risk for kidney stones.1-3 Therefore, encourage patients to consume about 800 to 1,200 mg/d of dietary calcium. Oral supplementation does not seem to yield the same protective benefits as dietary calcium. This may be related to absorption.1
Diets high in oxalates (eg, chocolate, nuts, spinach) can increase risk for stone formation, particularly in patients who have bowel diseases that cause inflammation or a history of a bowel resection.2 Animal protein in the diet can cause hypercalcinuria and increased uric acid levels, which is particularly problematic for individuals with gout or inflammatory arthritis. High-sodium diets can cause higher urinary calcium oxalate levels, while diets high in phosphorus (particularly dark cola soft drinks) can increase risk for stone formation. Advise your patient to avoid foods high in oxalates, animal proteins, sodium, and phosphorus.2
Dehydration, either due to exercise or poor fluid intake, can result in concentrated urine, which facilitates stone formation. While opinions differ on the benefits of certain dietary restrictions, most research supports the idea that generous fluid intake is the most successful intervention in preventing recurrence of stone formation (regardless of underlying cause). Diluting the urine decreases the concentration of solutes responsible for stone formation.1-3
If the conservative measures of dietary restriction and adequate hydration fail, medications may be beneficial, depending on stone type or underlying metabolic condition. Thiazide diuretics can help lower urinary calcium by enhancing reabsorption of calcium from the distal convoluted tubule and sodium excretion; however, they should be used cautiously due to the risk for adverse effects such as dizziness and lightheadedness.1,3 Allopurinol can lower uric acid levels, decreasing recurrence of both uric acid and calcium oxalate stones. Hypocitraturia is prevalent in 20% to 60% of persons with stones; prescribing potassium citrate can inhibit crystal growth of calcium phosphate and calcium oxalate in urine.2
To help patients prevent stone recurrence, perform a comprehensive assessment of their dietary and lifestyle habits and medical history to identify possible contributing factors. Educate patients on adequate dietary calcium intake, generous water intake to keep urine dilute, and avoidance of dietary triggers.
Nephrolithiasis should be considered a manifestation of another underlying problem. If a patient presents with a kidney stone, attempt to identify the cause—not only to try to prevent recurrence, but also to identify a previously unrecognized disease process.
Kristina Unterseher, CNN-NP
PeaceHealth
St. John Medical Group
Longview, WA
REFERENCES
1. Fink HA, Wilt TJ, Eidman KE, et al. Medical management to prevent recurrent nephrolithiasis in adults: a systematic review for an American College of Physicians Clinical Guideline. Ann Intern Med. 2013;158(7):535-543.
2. Hiatt RA, Ettinger B, Caan B, et al. Randomized controlled trial of a low animal protein, high fiber diet in the prevention of recurrent calcium oxalate kidney stones. Am J Epidemiol. 1996;144(1):25-33.
3. Moe OW. Kidney stones: pathophysiology and medical management. Lancet. 2006; 367(9507):333-344.
4. American College of Radiology and Radiological Society of North America. Intravenous pyelogram (2013). www.radiologyinfo.org/en/info.cfm?pg=ivp. Accessed December 16, 2013.
5. Boyce CJ, Pickhardt PJ, Lawrence EM, et al. Prevalence of urolithiasis in asymptomatic adults: objective determination using low dose noncontrast computerized tomography. J Urol. 2010;183(3):1017-1021.
6. Christian C, Thorsten B. The preferred treatment for upper tract stones is extracorporeal shock wave lithotripsy (ESWL) or ureteroscopic: pro ESWL. Urology. 2009;74(2):259-262.
7. Bourdoumis A, Papatsoris AG, Chrisofos M, Deliveliotis C. Lower pole stone management. Med Surg Urol. 2012. www.omicsonline.org/lower-pole-stone-management%20-2168-9857.S4-004.php?aid=7058?abstract _id=7058. Accessed December 16, 2013.
Q) A patient recently came in after an episode of kidney stones. He said he had never experienced such pain before (and this is a former Army Ranger!) and asked if there was anything he could do to keep it from happening again. I told him what I had learned in school (lots of fluids, no organ meats), but is there anything new?
Your patient has some reason for concern. For people who have had a symptomatic kidney stone, the likelihood of developing another within five years is 35% to 50% if no preventive action is taken.1 Certain factors—including family history, younger age at onset, and predisposing medical conditions (eg, hyperparathyroidism, diabetes, obesity, gout)—increase risk for recurrence.2,3
In the past, patients were often advised to restrict their dietary calcium intake to prevent calcium oxalate and/or calcium phosphate stones. However, more recent research has proven the opposite to be true: People with lower dietary calcium intake can be at greater risk for kidney stones.1-3 Therefore, encourage patients to consume about 800 to 1,200 mg/d of dietary calcium. Oral supplementation does not seem to yield the same protective benefits as dietary calcium. This may be related to absorption.1
Diets high in oxalates (eg, chocolate, nuts, spinach) can increase risk for stone formation, particularly in patients who have bowel diseases that cause inflammation or a history of a bowel resection.2 Animal protein in the diet can cause hypercalcinuria and increased uric acid levels, which is particularly problematic for individuals with gout or inflammatory arthritis. High-sodium diets can cause higher urinary calcium oxalate levels, while diets high in phosphorus (particularly dark cola soft drinks) can increase risk for stone formation. Advise your patient to avoid foods high in oxalates, animal proteins, sodium, and phosphorus.2
Dehydration, either due to exercise or poor fluid intake, can result in concentrated urine, which facilitates stone formation. While opinions differ on the benefits of certain dietary restrictions, most research supports the idea that generous fluid intake is the most successful intervention in preventing recurrence of stone formation (regardless of underlying cause). Diluting the urine decreases the concentration of solutes responsible for stone formation.1-3
If the conservative measures of dietary restriction and adequate hydration fail, medications may be beneficial, depending on stone type or underlying metabolic condition. Thiazide diuretics can help lower urinary calcium by enhancing reabsorption of calcium from the distal convoluted tubule and sodium excretion; however, they should be used cautiously due to the risk for adverse effects such as dizziness and lightheadedness.1,3 Allopurinol can lower uric acid levels, decreasing recurrence of both uric acid and calcium oxalate stones. Hypocitraturia is prevalent in 20% to 60% of persons with stones; prescribing potassium citrate can inhibit crystal growth of calcium phosphate and calcium oxalate in urine.2
To help patients prevent stone recurrence, perform a comprehensive assessment of their dietary and lifestyle habits and medical history to identify possible contributing factors. Educate patients on adequate dietary calcium intake, generous water intake to keep urine dilute, and avoidance of dietary triggers.
Nephrolithiasis should be considered a manifestation of another underlying problem. If a patient presents with a kidney stone, attempt to identify the cause—not only to try to prevent recurrence, but also to identify a previously unrecognized disease process.
Kristina Unterseher, CNN-NP
PeaceHealth
St. John Medical Group
Longview, WA
REFERENCES
1. Fink HA, Wilt TJ, Eidman KE, et al. Medical management to prevent recurrent nephrolithiasis in adults: a systematic review for an American College of Physicians Clinical Guideline. Ann Intern Med. 2013;158(7):535-543.
2. Hiatt RA, Ettinger B, Caan B, et al. Randomized controlled trial of a low animal protein, high fiber diet in the prevention of recurrent calcium oxalate kidney stones. Am J Epidemiol. 1996;144(1):25-33.
3. Moe OW. Kidney stones: pathophysiology and medical management. Lancet. 2006; 367(9507):333-344.
4. American College of Radiology and Radiological Society of North America. Intravenous pyelogram (2013). www.radiologyinfo.org/en/info.cfm?pg=ivp. Accessed December 16, 2013.
5. Boyce CJ, Pickhardt PJ, Lawrence EM, et al. Prevalence of urolithiasis in asymptomatic adults: objective determination using low dose noncontrast computerized tomography. J Urol. 2010;183(3):1017-1021.
6. Christian C, Thorsten B. The preferred treatment for upper tract stones is extracorporeal shock wave lithotripsy (ESWL) or ureteroscopic: pro ESWL. Urology. 2009;74(2):259-262.
7. Bourdoumis A, Papatsoris AG, Chrisofos M, Deliveliotis C. Lower pole stone management. Med Surg Urol. 2012. www.omicsonline.org/lower-pole-stone-management%20-2168-9857.S4-004.php?aid=7058?abstract _id=7058. Accessed December 16, 2013.
Pregnancy: Hypertension and Risk for Kidney Disease
Q If a pregnant woman has mild hypertension (eg, 140/90 mm Hg) but no albuminuria, is she at higher risk for kidney disease as she ages?
Gestational hypertension and preeclampsia are two types of hypertension that occur during pregnancy. Both occur after 20 weeks’ gestation and include a blood pressure reading greater than 140/90 mm Hg and no maternal history of hypertension. Proteinuria does not occur in gestational hypertension as it does in preeclampsia (proteinuria ≥ 300 mg in a 24-h urine collection).
One study evaluated more than 26,000 Taiwanese women with hypertension during pregnancy and compared them to more than 200,000 women without hypertension during pregnancy. It was found that hypertension during pregnancy increased the risk for chronic kidney disease (CKD) and end-stage renal disease (ESRD) later in life. Preeclampsia and eclampsia were even more likely to increase the risk for ESRD, compared with gestational hypertension.1
Preeclampsia occurs after 20 weeks’ gestation and includes elevated blood pressure (≥ 140 mm Hg systolic or ≥ 90 mm Hg diastolic) and proteinuria (≥ 0.3 g in 24 h). Severe preeclampsia develops with the addition of worsening hypertension and proteinuria, oliguria less than 500 mL in 24 h, thrombocytopenia, hemolysis, elevated liver enzymes, low platelets, pulmonary edema, and fetal growth restriction. Eclampsia is when seizures occur in addition to preeclampsia.2 Gestational hypertension has also been linked with a higher risk for ischemic heart disease, myocardial infarcts and death, heart failure, ischem-
ic stroke, kidney disease, and diabetes.3
Because of the association between hypertension during pregnancy and subsequent development of CKD, it is very important that mothers who have hypertension during pregnancy continue to have their renal function monitored after delivery.
Mandy Trolinger, MS, RD, PA-C
Denver Nephrology
Denver, CO
Personal Note: Mandy is a two-time kidney transplant recipient. She delivered a healthy baby boy in 2012.
REFERENCES
1. Wang I-K, Muo C-H, Liang C-C, et al. Association between hypertensive disorders during pregnancy and end-stage renal disease: a population-based study. CMAJ. 2013;85: 207-213.
2. McPhee SJ, Papadakis MA, Tierney LM, et al. Current Medical Diagnosis and Treatment. 47th ed. New York, NY: McGraw-Hill/Lange; 2008.
3. Männistö T, Mendola P, Vääräsmäki M, et al. Elevated blood pressure in pregnancy and subsequent chronic disease risk. Circulation. 2013;127:681-690.
4. Nevis IF, Reitsma A, Dominic A, et al. Pregnancy outcomes in women with chronic kidney disease: a systematic review. Clin J Am Soc Nephrol. 2011;6:2587-2598.
5. Hou S. Pregnancy in chronic renal insufficiency and end-stage renal disease. Am J Kidney Dis. 1999;33:235-252.
6. Davison JM. Dialysis, transplantation, and pregnancy. Am J Kidney Dis. 1991;17:127-132.
7. Asamiya Y, Otsubo S, Matsuda Y, et al. The importance of low blood urea nitrogen levels in pregnant patients undergoing hemodialysis to optimize birth weight and gestational age. Kidney Int. 2009;75:1217-1222.
8. Giatras I, Levy DP, Malone FD, et al. Pregnancy during dialysis: case report and management guidelines. Nephrol Dial Transplant. 1998;13:3266-3272.
9. McKay DB, Josephson MA. Pregnancy in recipients of solid organs—effects on mother and child. N Engl J Med. 2006;354:1281-1293.
10. Sifontis NM, Coscia LA, Constantinescu S, et al. Pregnancy outcomes in solid organ transplant recipients with exposure to mycophenolate mofetil or sirolimus. Transplantation. 2006;82:1698-1702.
11. Kainz A, Harabacz I, Cowlrick IS, et al. Review of the course and outcome of 100 pregnancies in 84 women treated with tacrolimus. Transplantation. 2000;70:1718-1721.
12. Josephson MA. Pregnancy in renal transplant recipients: more questions answered, still more asked. Clin J Am Soc Nephrol. 2013;8: 182-183.
Q If a pregnant woman has mild hypertension (eg, 140/90 mm Hg) but no albuminuria, is she at higher risk for kidney disease as she ages?
Gestational hypertension and preeclampsia are two types of hypertension that occur during pregnancy. Both occur after 20 weeks’ gestation and include a blood pressure reading greater than 140/90 mm Hg and no maternal history of hypertension. Proteinuria does not occur in gestational hypertension as it does in preeclampsia (proteinuria ≥ 300 mg in a 24-h urine collection).
One study evaluated more than 26,000 Taiwanese women with hypertension during pregnancy and compared them to more than 200,000 women without hypertension during pregnancy. It was found that hypertension during pregnancy increased the risk for chronic kidney disease (CKD) and end-stage renal disease (ESRD) later in life. Preeclampsia and eclampsia were even more likely to increase the risk for ESRD, compared with gestational hypertension.1
Preeclampsia occurs after 20 weeks’ gestation and includes elevated blood pressure (≥ 140 mm Hg systolic or ≥ 90 mm Hg diastolic) and proteinuria (≥ 0.3 g in 24 h). Severe preeclampsia develops with the addition of worsening hypertension and proteinuria, oliguria less than 500 mL in 24 h, thrombocytopenia, hemolysis, elevated liver enzymes, low platelets, pulmonary edema, and fetal growth restriction. Eclampsia is when seizures occur in addition to preeclampsia.2 Gestational hypertension has also been linked with a higher risk for ischemic heart disease, myocardial infarcts and death, heart failure, ischem-
ic stroke, kidney disease, and diabetes.3
Because of the association between hypertension during pregnancy and subsequent development of CKD, it is very important that mothers who have hypertension during pregnancy continue to have their renal function monitored after delivery.
Mandy Trolinger, MS, RD, PA-C
Denver Nephrology
Denver, CO
Personal Note: Mandy is a two-time kidney transplant recipient. She delivered a healthy baby boy in 2012.
REFERENCES
1. Wang I-K, Muo C-H, Liang C-C, et al. Association between hypertensive disorders during pregnancy and end-stage renal disease: a population-based study. CMAJ. 2013;85: 207-213.
2. McPhee SJ, Papadakis MA, Tierney LM, et al. Current Medical Diagnosis and Treatment. 47th ed. New York, NY: McGraw-Hill/Lange; 2008.
3. Männistö T, Mendola P, Vääräsmäki M, et al. Elevated blood pressure in pregnancy and subsequent chronic disease risk. Circulation. 2013;127:681-690.
4. Nevis IF, Reitsma A, Dominic A, et al. Pregnancy outcomes in women with chronic kidney disease: a systematic review. Clin J Am Soc Nephrol. 2011;6:2587-2598.
5. Hou S. Pregnancy in chronic renal insufficiency and end-stage renal disease. Am J Kidney Dis. 1999;33:235-252.
6. Davison JM. Dialysis, transplantation, and pregnancy. Am J Kidney Dis. 1991;17:127-132.
7. Asamiya Y, Otsubo S, Matsuda Y, et al. The importance of low blood urea nitrogen levels in pregnant patients undergoing hemodialysis to optimize birth weight and gestational age. Kidney Int. 2009;75:1217-1222.
8. Giatras I, Levy DP, Malone FD, et al. Pregnancy during dialysis: case report and management guidelines. Nephrol Dial Transplant. 1998;13:3266-3272.
9. McKay DB, Josephson MA. Pregnancy in recipients of solid organs—effects on mother and child. N Engl J Med. 2006;354:1281-1293.
10. Sifontis NM, Coscia LA, Constantinescu S, et al. Pregnancy outcomes in solid organ transplant recipients with exposure to mycophenolate mofetil or sirolimus. Transplantation. 2006;82:1698-1702.
11. Kainz A, Harabacz I, Cowlrick IS, et al. Review of the course and outcome of 100 pregnancies in 84 women treated with tacrolimus. Transplantation. 2000;70:1718-1721.
12. Josephson MA. Pregnancy in renal transplant recipients: more questions answered, still more asked. Clin J Am Soc Nephrol. 2013;8: 182-183.
Q If a pregnant woman has mild hypertension (eg, 140/90 mm Hg) but no albuminuria, is she at higher risk for kidney disease as she ages?
Gestational hypertension and preeclampsia are two types of hypertension that occur during pregnancy. Both occur after 20 weeks’ gestation and include a blood pressure reading greater than 140/90 mm Hg and no maternal history of hypertension. Proteinuria does not occur in gestational hypertension as it does in preeclampsia (proteinuria ≥ 300 mg in a 24-h urine collection).
One study evaluated more than 26,000 Taiwanese women with hypertension during pregnancy and compared them to more than 200,000 women without hypertension during pregnancy. It was found that hypertension during pregnancy increased the risk for chronic kidney disease (CKD) and end-stage renal disease (ESRD) later in life. Preeclampsia and eclampsia were even more likely to increase the risk for ESRD, compared with gestational hypertension.1
Preeclampsia occurs after 20 weeks’ gestation and includes elevated blood pressure (≥ 140 mm Hg systolic or ≥ 90 mm Hg diastolic) and proteinuria (≥ 0.3 g in 24 h). Severe preeclampsia develops with the addition of worsening hypertension and proteinuria, oliguria less than 500 mL in 24 h, thrombocytopenia, hemolysis, elevated liver enzymes, low platelets, pulmonary edema, and fetal growth restriction. Eclampsia is when seizures occur in addition to preeclampsia.2 Gestational hypertension has also been linked with a higher risk for ischemic heart disease, myocardial infarcts and death, heart failure, ischem-
ic stroke, kidney disease, and diabetes.3
Because of the association between hypertension during pregnancy and subsequent development of CKD, it is very important that mothers who have hypertension during pregnancy continue to have their renal function monitored after delivery.
Mandy Trolinger, MS, RD, PA-C
Denver Nephrology
Denver, CO
Personal Note: Mandy is a two-time kidney transplant recipient. She delivered a healthy baby boy in 2012.
REFERENCES
1. Wang I-K, Muo C-H, Liang C-C, et al. Association between hypertensive disorders during pregnancy and end-stage renal disease: a population-based study. CMAJ. 2013;85: 207-213.
2. McPhee SJ, Papadakis MA, Tierney LM, et al. Current Medical Diagnosis and Treatment. 47th ed. New York, NY: McGraw-Hill/Lange; 2008.
3. Männistö T, Mendola P, Vääräsmäki M, et al. Elevated blood pressure in pregnancy and subsequent chronic disease risk. Circulation. 2013;127:681-690.
4. Nevis IF, Reitsma A, Dominic A, et al. Pregnancy outcomes in women with chronic kidney disease: a systematic review. Clin J Am Soc Nephrol. 2011;6:2587-2598.
5. Hou S. Pregnancy in chronic renal insufficiency and end-stage renal disease. Am J Kidney Dis. 1999;33:235-252.
6. Davison JM. Dialysis, transplantation, and pregnancy. Am J Kidney Dis. 1991;17:127-132.
7. Asamiya Y, Otsubo S, Matsuda Y, et al. The importance of low blood urea nitrogen levels in pregnant patients undergoing hemodialysis to optimize birth weight and gestational age. Kidney Int. 2009;75:1217-1222.
8. Giatras I, Levy DP, Malone FD, et al. Pregnancy during dialysis: case report and management guidelines. Nephrol Dial Transplant. 1998;13:3266-3272.
9. McKay DB, Josephson MA. Pregnancy in recipients of solid organs—effects on mother and child. N Engl J Med. 2006;354:1281-1293.
10. Sifontis NM, Coscia LA, Constantinescu S, et al. Pregnancy outcomes in solid organ transplant recipients with exposure to mycophenolate mofetil or sirolimus. Transplantation. 2006;82:1698-1702.
11. Kainz A, Harabacz I, Cowlrick IS, et al. Review of the course and outcome of 100 pregnancies in 84 women treated with tacrolimus. Transplantation. 2000;70:1718-1721.
12. Josephson MA. Pregnancy in renal transplant recipients: more questions answered, still more asked. Clin J Am Soc Nephrol. 2013;8: 182-183.
Pregnancy: CKD, Dialysis, and Transplant Patients
Q) I was having a “discussion” over lunch about CKD, pregnancy, and transplant. I said that dialysis patients cannot get pregnant, but someone said I was wrong. A friend said that transplant patients should not get pregnant because of the toxicity of the immunosuppressant medications they take, but another practitioner said that was in the “olden days.” What is the current state of the CKD, dialysis, and transplant patient and pregnancy?
The first healthy baby delivered by a pregnant kidney transplant patient was born in 1958. With the advances in treatment of kidney disease, we are now seeing more pregnancies in these patients. However, they are still considered high risk and should be monitored by a transplant nephrologist and a high-risk obstetrician.4
CKD patients (not on dialysis) are at increased risk for pregnancy complications. Maternal risks include gestational hypertension, preeclampsia/eclampsia, ESRD, or death. Fetal complications include prematurity, small-for-gestational-age babies, and stillbirth. It is very important to control hypertension because it is directly linked to fetal outcome; however, not all blood pressure medications are safe in pregnancy. ACE inhibitors, for example, are teratogenic and absolutely contraindicated.4
Fertility is decreased in dialysis patients; however, pregnancy occurs in 0.3% to 1.5% of women of childbearing age on dialysis. Pregnancy should be confirmed with an ultrasound because serum β-human chorionic gonadotropin can be falsely elevated in ESRD.5
It also can be difficult to monitor pregnancy weight gain due to fluid gains between dialysis treatments. Dialysis prescriptions should be increased either in time or frequency (or both), with a goal of keeping the blood urea nitrogen concentration below 50 mg/dL to improve maternal and fetal outcomes.6,7 Other important factors to control are metabolic acidosis, hypocalcemia, and anemia (increased erythropoietin-stimulating agents may be needed). Frequent uterine and fetal monitoring is also indicated to prevent preterm labor due to the dialysis process. Preeclampsia, prematurity, low birth weight, and hypertension are the most common risks in these pregnancies.8
Renal transplantation often returns fertility to normal and allows pregnancy to occur; however, it is recommended that female patients wait until one year post-transplant if the transplanted kidney is from a living related donor (two years if from a deceased donor), have a serum creatinine level less than 1.5 mg/dL, and a urinary protein level less than 500 mg/d.9 The immunosuppression regimen usually needs adjustment because certain immunosuppressants are contraindicated in pregnancy and have been linked to teratogenic effects; however, data is still limited in this area.10,11 Pregnant transplant recipients are at higher risk for preeclampsia, gestational diabetes, preterm delivery, small-for-gestational-age babies, miscarriage, stillbirth, neonatal death, and congenital abnormalities.12
The National Transplantation Pregnancy Registry (www.ntpr.giftoflifeinstitute.org/) is an ongoing registry in the United States that reports on transplant pregnancies and their outcomes. Data collected by the registry show that there have been many healthy pregnancies without any adverse maternal or fetal outcomes among transplant recipients.
Mandy Trolinger, MS, RD, PA-C
Denver Nephrology
Denver, CO
Personal Note: Mandy is a two-time kidney transplant recipient. She delivered a healthy baby boy in 2012.
REFERENCES
1. Wang I-K, Muo C-H, Liang C-C, et al. Association between hypertensive disorders during pregnancy and end-stage renal disease: a population-based study. CMAJ. 2013;85: 207-213.
2. McPhee SJ, Papadakis MA, Tierney LM, et al. Current Medical Diagnosis and Treatment. 47th ed. New York, NY: McGraw-Hill/Lange; 2008.
3. Männistö T, Mendola P, Vääräsmäki M, et al. Elevated blood pressure in pregnancy and subsequent chronic disease risk. Circulation. 2013;127:681-690.
4. Nevis IF, Reitsma A, Dominic A, et al. Pregnancy outcomes in women with chronic kidney disease: a systematic review. Clin J Am Soc Nephrol. 2011;6:2587-2598.
5. Hou S. Pregnancy in chronic renal insufficiency and end-stage renal disease. Am J Kidney Dis. 1999;33:235-252.
6. Davison JM. Dialysis, transplantation, and pregnancy. Am J Kidney Dis. 1991;17:127-132.
7. Asamiya Y, Otsubo S, Matsuda Y, et al. The importance of low blood urea nitrogen levels in pregnant patients undergoing hemodialysis to optimize birth weight and gestational age. Kidney Int. 2009;75:1217-1222.
8. Giatras I, Levy DP, Malone FD, et al. Pregnancy during dialysis: case report and management guidelines. Nephrol Dial Transplant. 1998;13:3266-3272.
9. McKay DB, Josephson MA. Pregnancy in recipients of solid organs—effects on mother and child. N Engl J Med. 2006;354:1281-1293.
10. Sifontis NM, Coscia LA, Constantinescu S, et al. Pregnancy outcomes in solid organ transplant recipients with exposure to mycophenolate mofetil or sirolimus. Transplantation. 2006;82:1698-1702.
11. Kainz A, Harabacz I, Cowlrick IS, et al. Review of the course and outcome of 100 pregnancies in 84 women treated with tacrolimus. Transplantation. 2000;70:1718-1721.
12. Josephson MA. Pregnancy in renal transplant recipients: more questions answered, still more asked. Clin J Am Soc Nephrol. 2013;8: 182-183.
Q) I was having a “discussion” over lunch about CKD, pregnancy, and transplant. I said that dialysis patients cannot get pregnant, but someone said I was wrong. A friend said that transplant patients should not get pregnant because of the toxicity of the immunosuppressant medications they take, but another practitioner said that was in the “olden days.” What is the current state of the CKD, dialysis, and transplant patient and pregnancy?
The first healthy baby delivered by a pregnant kidney transplant patient was born in 1958. With the advances in treatment of kidney disease, we are now seeing more pregnancies in these patients. However, they are still considered high risk and should be monitored by a transplant nephrologist and a high-risk obstetrician.4
CKD patients (not on dialysis) are at increased risk for pregnancy complications. Maternal risks include gestational hypertension, preeclampsia/eclampsia, ESRD, or death. Fetal complications include prematurity, small-for-gestational-age babies, and stillbirth. It is very important to control hypertension because it is directly linked to fetal outcome; however, not all blood pressure medications are safe in pregnancy. ACE inhibitors, for example, are teratogenic and absolutely contraindicated.4
Fertility is decreased in dialysis patients; however, pregnancy occurs in 0.3% to 1.5% of women of childbearing age on dialysis. Pregnancy should be confirmed with an ultrasound because serum β-human chorionic gonadotropin can be falsely elevated in ESRD.5
It also can be difficult to monitor pregnancy weight gain due to fluid gains between dialysis treatments. Dialysis prescriptions should be increased either in time or frequency (or both), with a goal of keeping the blood urea nitrogen concentration below 50 mg/dL to improve maternal and fetal outcomes.6,7 Other important factors to control are metabolic acidosis, hypocalcemia, and anemia (increased erythropoietin-stimulating agents may be needed). Frequent uterine and fetal monitoring is also indicated to prevent preterm labor due to the dialysis process. Preeclampsia, prematurity, low birth weight, and hypertension are the most common risks in these pregnancies.8
Renal transplantation often returns fertility to normal and allows pregnancy to occur; however, it is recommended that female patients wait until one year post-transplant if the transplanted kidney is from a living related donor (two years if from a deceased donor), have a serum creatinine level less than 1.5 mg/dL, and a urinary protein level less than 500 mg/d.9 The immunosuppression regimen usually needs adjustment because certain immunosuppressants are contraindicated in pregnancy and have been linked to teratogenic effects; however, data is still limited in this area.10,11 Pregnant transplant recipients are at higher risk for preeclampsia, gestational diabetes, preterm delivery, small-for-gestational-age babies, miscarriage, stillbirth, neonatal death, and congenital abnormalities.12
The National Transplantation Pregnancy Registry (www.ntpr.giftoflifeinstitute.org/) is an ongoing registry in the United States that reports on transplant pregnancies and their outcomes. Data collected by the registry show that there have been many healthy pregnancies without any adverse maternal or fetal outcomes among transplant recipients.
Mandy Trolinger, MS, RD, PA-C
Denver Nephrology
Denver, CO
Personal Note: Mandy is a two-time kidney transplant recipient. She delivered a healthy baby boy in 2012.
REFERENCES
1. Wang I-K, Muo C-H, Liang C-C, et al. Association between hypertensive disorders during pregnancy and end-stage renal disease: a population-based study. CMAJ. 2013;85: 207-213.
2. McPhee SJ, Papadakis MA, Tierney LM, et al. Current Medical Diagnosis and Treatment. 47th ed. New York, NY: McGraw-Hill/Lange; 2008.
3. Männistö T, Mendola P, Vääräsmäki M, et al. Elevated blood pressure in pregnancy and subsequent chronic disease risk. Circulation. 2013;127:681-690.
4. Nevis IF, Reitsma A, Dominic A, et al. Pregnancy outcomes in women with chronic kidney disease: a systematic review. Clin J Am Soc Nephrol. 2011;6:2587-2598.
5. Hou S. Pregnancy in chronic renal insufficiency and end-stage renal disease. Am J Kidney Dis. 1999;33:235-252.
6. Davison JM. Dialysis, transplantation, and pregnancy. Am J Kidney Dis. 1991;17:127-132.
7. Asamiya Y, Otsubo S, Matsuda Y, et al. The importance of low blood urea nitrogen levels in pregnant patients undergoing hemodialysis to optimize birth weight and gestational age. Kidney Int. 2009;75:1217-1222.
8. Giatras I, Levy DP, Malone FD, et al. Pregnancy during dialysis: case report and management guidelines. Nephrol Dial Transplant. 1998;13:3266-3272.
9. McKay DB, Josephson MA. Pregnancy in recipients of solid organs—effects on mother and child. N Engl J Med. 2006;354:1281-1293.
10. Sifontis NM, Coscia LA, Constantinescu S, et al. Pregnancy outcomes in solid organ transplant recipients with exposure to mycophenolate mofetil or sirolimus. Transplantation. 2006;82:1698-1702.
11. Kainz A, Harabacz I, Cowlrick IS, et al. Review of the course and outcome of 100 pregnancies in 84 women treated with tacrolimus. Transplantation. 2000;70:1718-1721.
12. Josephson MA. Pregnancy in renal transplant recipients: more questions answered, still more asked. Clin J Am Soc Nephrol. 2013;8: 182-183.
Q) I was having a “discussion” over lunch about CKD, pregnancy, and transplant. I said that dialysis patients cannot get pregnant, but someone said I was wrong. A friend said that transplant patients should not get pregnant because of the toxicity of the immunosuppressant medications they take, but another practitioner said that was in the “olden days.” What is the current state of the CKD, dialysis, and transplant patient and pregnancy?
The first healthy baby delivered by a pregnant kidney transplant patient was born in 1958. With the advances in treatment of kidney disease, we are now seeing more pregnancies in these patients. However, they are still considered high risk and should be monitored by a transplant nephrologist and a high-risk obstetrician.4
CKD patients (not on dialysis) are at increased risk for pregnancy complications. Maternal risks include gestational hypertension, preeclampsia/eclampsia, ESRD, or death. Fetal complications include prematurity, small-for-gestational-age babies, and stillbirth. It is very important to control hypertension because it is directly linked to fetal outcome; however, not all blood pressure medications are safe in pregnancy. ACE inhibitors, for example, are teratogenic and absolutely contraindicated.4
Fertility is decreased in dialysis patients; however, pregnancy occurs in 0.3% to 1.5% of women of childbearing age on dialysis. Pregnancy should be confirmed with an ultrasound because serum β-human chorionic gonadotropin can be falsely elevated in ESRD.5
It also can be difficult to monitor pregnancy weight gain due to fluid gains between dialysis treatments. Dialysis prescriptions should be increased either in time or frequency (or both), with a goal of keeping the blood urea nitrogen concentration below 50 mg/dL to improve maternal and fetal outcomes.6,7 Other important factors to control are metabolic acidosis, hypocalcemia, and anemia (increased erythropoietin-stimulating agents may be needed). Frequent uterine and fetal monitoring is also indicated to prevent preterm labor due to the dialysis process. Preeclampsia, prematurity, low birth weight, and hypertension are the most common risks in these pregnancies.8
Renal transplantation often returns fertility to normal and allows pregnancy to occur; however, it is recommended that female patients wait until one year post-transplant if the transplanted kidney is from a living related donor (two years if from a deceased donor), have a serum creatinine level less than 1.5 mg/dL, and a urinary protein level less than 500 mg/d.9 The immunosuppression regimen usually needs adjustment because certain immunosuppressants are contraindicated in pregnancy and have been linked to teratogenic effects; however, data is still limited in this area.10,11 Pregnant transplant recipients are at higher risk for preeclampsia, gestational diabetes, preterm delivery, small-for-gestational-age babies, miscarriage, stillbirth, neonatal death, and congenital abnormalities.12
The National Transplantation Pregnancy Registry (www.ntpr.giftoflifeinstitute.org/) is an ongoing registry in the United States that reports on transplant pregnancies and their outcomes. Data collected by the registry show that there have been many healthy pregnancies without any adverse maternal or fetal outcomes among transplant recipients.
Mandy Trolinger, MS, RD, PA-C
Denver Nephrology
Denver, CO
Personal Note: Mandy is a two-time kidney transplant recipient. She delivered a healthy baby boy in 2012.
REFERENCES
1. Wang I-K, Muo C-H, Liang C-C, et al. Association between hypertensive disorders during pregnancy and end-stage renal disease: a population-based study. CMAJ. 2013;85: 207-213.
2. McPhee SJ, Papadakis MA, Tierney LM, et al. Current Medical Diagnosis and Treatment. 47th ed. New York, NY: McGraw-Hill/Lange; 2008.
3. Männistö T, Mendola P, Vääräsmäki M, et al. Elevated blood pressure in pregnancy and subsequent chronic disease risk. Circulation. 2013;127:681-690.
4. Nevis IF, Reitsma A, Dominic A, et al. Pregnancy outcomes in women with chronic kidney disease: a systematic review. Clin J Am Soc Nephrol. 2011;6:2587-2598.
5. Hou S. Pregnancy in chronic renal insufficiency and end-stage renal disease. Am J Kidney Dis. 1999;33:235-252.
6. Davison JM. Dialysis, transplantation, and pregnancy. Am J Kidney Dis. 1991;17:127-132.
7. Asamiya Y, Otsubo S, Matsuda Y, et al. The importance of low blood urea nitrogen levels in pregnant patients undergoing hemodialysis to optimize birth weight and gestational age. Kidney Int. 2009;75:1217-1222.
8. Giatras I, Levy DP, Malone FD, et al. Pregnancy during dialysis: case report and management guidelines. Nephrol Dial Transplant. 1998;13:3266-3272.
9. McKay DB, Josephson MA. Pregnancy in recipients of solid organs—effects on mother and child. N Engl J Med. 2006;354:1281-1293.
10. Sifontis NM, Coscia LA, Constantinescu S, et al. Pregnancy outcomes in solid organ transplant recipients with exposure to mycophenolate mofetil or sirolimus. Transplantation. 2006;82:1698-1702.
11. Kainz A, Harabacz I, Cowlrick IS, et al. Review of the course and outcome of 100 pregnancies in 84 women treated with tacrolimus. Transplantation. 2000;70:1718-1721.
12. Josephson MA. Pregnancy in renal transplant recipients: more questions answered, still more asked. Clin J Am Soc Nephrol. 2013;8: 182-183.
Determining Renal Function: What Those Test Results Mean
Q: Even though you suggested a random urine ACR (albumin-to-creatinine ratio), the internal medicine group ordered a 24-hour urine test for protein. As you can see from the results (see Table 2), the PCR (protein-to-creatinine ratio) is high. What does this mean? Does my patient have more severe kidney disease than I thought for her age?
Advanced age is a risk factor for CKD, and the patient has also had weight loss that can affect her serum creatinine. Because of her femur fracture, she has likely been in pain and probably has been taking nephrotoxic analgesics, such as NSAIDs or a ketorolac injection, commonly given postoperatively.
The patient’s weight does not appear to be stable, and she may have a degree of malnutrition. Both malnutrition and reduced muscle mass are known to decrease serum creatinine, which can mask worsening kidney disease. Thus she may have a lower true GFR than predicted by CG, which tends to overestimate renal function in the case of lower levels of creatinine production.6
Looking at all of these factors, it is likely that she has some degree of renal disease; however, it is important to determine if this is an acute change or a chronic issue. Looking closely at the higher-than-normal urinary protein result requires some out-of-the-box thinking.
Proteinuria has four types; each indicates a particular disorder.5 Table 3 provides examples of causative factors for each type.
Based on the data provided (Table 2), you have a high urinary protein result and are unsure if it is albumin. It is important to determine if this is albumin—and therefore pathognomonic for progressive kidney disease—or if the protein is of a nonalbumin type that will require further evaluation. What started as just an elderly female with a femur fracture and decreased GFR can turn into a diagnosis of multiple myeloma (which is more common in this age-group), kidney damage from postoperative medications, or another form of kidney disease. Only by looking at urinary protein type can one “tease out” what this might be.
In conclusion, there are many different ways to determine renal function, either by creatinine clearance or by using an estimation formula. Each one, used correctly, can offer advantages in certain populations. It is extremely important to determine whether an individual has diminished kidney function in order to be able to delay the progression of CKD.
Catherine B. York, MSN, APRN-BC
Springfield Nephrology
Associates, Springfield, MO
References
1. CDC. National chronic kidney disease fact sheet: general information and national estimates on chronic kidney disease in the United States, 2010. Atlanta, GA: US Department of Health and Human Services, CDC; 2010.
2. US Renal Data System. USRDS 2012 annual data report: atlas of end-stage renal disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2012.
3. US Renal Data System. USRDS 2012 annual data report: atlas of end-stage renal disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2011.
4. Clarkson MR, Brenner BM. Clinical assessment of the patient with kidney disease. In: Clarkson MR, Brenner BM. Pocket Companion to Brenner & Rector’s The Kidney. 7th ed. Philadelphia, PA: Elsevier Saunders; 2005: 3-19.5.
5. Hsu C. Clinical evaluation of kidney function. In: Greenberg A, Cheung A, Coffman T, et al, eds. Primer on Kidney Diseases, 5th ed. Philadelphia, PA; Saunders Elsevier; 2009:19-237.
6. Kidney Disease Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1-150.
7. National Kidney Foundation. Guideline 5: assessment of proteinuria. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification; 2000.
8. Stevens LA, Coresh J, Greene T, et al. Assessing kidney function-measured and estimated glomerular filtration rate. N Engl J Med. 2006;354:2473-2483.
Q: Even though you suggested a random urine ACR (albumin-to-creatinine ratio), the internal medicine group ordered a 24-hour urine test for protein. As you can see from the results (see Table 2), the PCR (protein-to-creatinine ratio) is high. What does this mean? Does my patient have more severe kidney disease than I thought for her age?
Advanced age is a risk factor for CKD, and the patient has also had weight loss that can affect her serum creatinine. Because of her femur fracture, she has likely been in pain and probably has been taking nephrotoxic analgesics, such as NSAIDs or a ketorolac injection, commonly given postoperatively.
The patient’s weight does not appear to be stable, and she may have a degree of malnutrition. Both malnutrition and reduced muscle mass are known to decrease serum creatinine, which can mask worsening kidney disease. Thus she may have a lower true GFR than predicted by CG, which tends to overestimate renal function in the case of lower levels of creatinine production.6
Looking at all of these factors, it is likely that she has some degree of renal disease; however, it is important to determine if this is an acute change or a chronic issue. Looking closely at the higher-than-normal urinary protein result requires some out-of-the-box thinking.
Proteinuria has four types; each indicates a particular disorder.5 Table 3 provides examples of causative factors for each type.
Based on the data provided (Table 2), you have a high urinary protein result and are unsure if it is albumin. It is important to determine if this is albumin—and therefore pathognomonic for progressive kidney disease—or if the protein is of a nonalbumin type that will require further evaluation. What started as just an elderly female with a femur fracture and decreased GFR can turn into a diagnosis of multiple myeloma (which is more common in this age-group), kidney damage from postoperative medications, or another form of kidney disease. Only by looking at urinary protein type can one “tease out” what this might be.
In conclusion, there are many different ways to determine renal function, either by creatinine clearance or by using an estimation formula. Each one, used correctly, can offer advantages in certain populations. It is extremely important to determine whether an individual has diminished kidney function in order to be able to delay the progression of CKD.
Catherine B. York, MSN, APRN-BC
Springfield Nephrology
Associates, Springfield, MO
References
1. CDC. National chronic kidney disease fact sheet: general information and national estimates on chronic kidney disease in the United States, 2010. Atlanta, GA: US Department of Health and Human Services, CDC; 2010.
2. US Renal Data System. USRDS 2012 annual data report: atlas of end-stage renal disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2012.
3. US Renal Data System. USRDS 2012 annual data report: atlas of end-stage renal disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2011.
4. Clarkson MR, Brenner BM. Clinical assessment of the patient with kidney disease. In: Clarkson MR, Brenner BM. Pocket Companion to Brenner & Rector’s The Kidney. 7th ed. Philadelphia, PA: Elsevier Saunders; 2005: 3-19.5.
5. Hsu C. Clinical evaluation of kidney function. In: Greenberg A, Cheung A, Coffman T, et al, eds. Primer on Kidney Diseases, 5th ed. Philadelphia, PA; Saunders Elsevier; 2009:19-237.
6. Kidney Disease Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1-150.
7. National Kidney Foundation. Guideline 5: assessment of proteinuria. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification; 2000.
8. Stevens LA, Coresh J, Greene T, et al. Assessing kidney function-measured and estimated glomerular filtration rate. N Engl J Med. 2006;354:2473-2483.
Q: Even though you suggested a random urine ACR (albumin-to-creatinine ratio), the internal medicine group ordered a 24-hour urine test for protein. As you can see from the results (see Table 2), the PCR (protein-to-creatinine ratio) is high. What does this mean? Does my patient have more severe kidney disease than I thought for her age?
Advanced age is a risk factor for CKD, and the patient has also had weight loss that can affect her serum creatinine. Because of her femur fracture, she has likely been in pain and probably has been taking nephrotoxic analgesics, such as NSAIDs or a ketorolac injection, commonly given postoperatively.
The patient’s weight does not appear to be stable, and she may have a degree of malnutrition. Both malnutrition and reduced muscle mass are known to decrease serum creatinine, which can mask worsening kidney disease. Thus she may have a lower true GFR than predicted by CG, which tends to overestimate renal function in the case of lower levels of creatinine production.6
Looking at all of these factors, it is likely that she has some degree of renal disease; however, it is important to determine if this is an acute change or a chronic issue. Looking closely at the higher-than-normal urinary protein result requires some out-of-the-box thinking.
Proteinuria has four types; each indicates a particular disorder.5 Table 3 provides examples of causative factors for each type.
Based on the data provided (Table 2), you have a high urinary protein result and are unsure if it is albumin. It is important to determine if this is albumin—and therefore pathognomonic for progressive kidney disease—or if the protein is of a nonalbumin type that will require further evaluation. What started as just an elderly female with a femur fracture and decreased GFR can turn into a diagnosis of multiple myeloma (which is more common in this age-group), kidney damage from postoperative medications, or another form of kidney disease. Only by looking at urinary protein type can one “tease out” what this might be.
In conclusion, there are many different ways to determine renal function, either by creatinine clearance or by using an estimation formula. Each one, used correctly, can offer advantages in certain populations. It is extremely important to determine whether an individual has diminished kidney function in order to be able to delay the progression of CKD.
Catherine B. York, MSN, APRN-BC
Springfield Nephrology
Associates, Springfield, MO
References
1. CDC. National chronic kidney disease fact sheet: general information and national estimates on chronic kidney disease in the United States, 2010. Atlanta, GA: US Department of Health and Human Services, CDC; 2010.
2. US Renal Data System. USRDS 2012 annual data report: atlas of end-stage renal disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2012.
3. US Renal Data System. USRDS 2012 annual data report: atlas of end-stage renal disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2011.
4. Clarkson MR, Brenner BM. Clinical assessment of the patient with kidney disease. In: Clarkson MR, Brenner BM. Pocket Companion to Brenner & Rector’s The Kidney. 7th ed. Philadelphia, PA: Elsevier Saunders; 2005: 3-19.5.
5. Hsu C. Clinical evaluation of kidney function. In: Greenberg A, Cheung A, Coffman T, et al, eds. Primer on Kidney Diseases, 5th ed. Philadelphia, PA; Saunders Elsevier; 2009:19-237.
6. Kidney Disease Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1-150.
7. National Kidney Foundation. Guideline 5: assessment of proteinuria. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification; 2000.
8. Stevens LA, Coresh J, Greene T, et al. Assessing kidney function-measured and estimated glomerular filtration rate. N Engl J Med. 2006;354:2473-2483.
Determining Renal Function: What’s the Best Way to Evaluate?
Q: One of my patients is a 72-year-old woman who weighs 59 kg. Her creatinine clearance by Cockcroft-Gault (CG) came back low (49 mL/min). Is this due to her age, gender, and weight loss during the past five months (subsequent to a femur fracture), or does she have underlying kidney disease? Would a 24-hour urine creatinine test be the best way to determine her level of kidney function—and would it be appropriate for someone her age? Is there a better way to evaluate her kidney function?
Accurate measurement of renal function is vital for any patient suspected of having chronic kidney disease (CKD). More than 20 million adults in the United States, or more than 10% of the adult population, have CKD.1 The 2012 US Renal Data System (USRDS) Annual Data Report states that the prevalence of chronic kidney disease in the Medicare population alone rose more than three-fold between 2000 and 2010, from 2.7% to 9.2%.2
CKD consumes a large proportion of Medicare dollars: more than $23,000 per person per year (PPPY) annually. For end-stage renal disease (ESRD) patients on hemodialysis, the cost is an astounding $88,000 PPPY.2 The cost of treating 871,000 ESRD patients was more than $40 billion in both public and private funds in 2009.3
Risk factors for CKD include but are not limited to: advancing age, male sex, race, hypertension, diabetes mellitus, smoking, family history of kidney disease, proteinuria, exposure to nephrotoxins, and atherosclerosis.4
In the US, the most common methods used to estimate renal function are the CG (Cockcroft-Gault) equation, Modification of Diet in Renal Disease (MDRD) study equations, and the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation. It is often difficult to determine which test is best suited for a patient, because there are pros and cons to each formula and no one test is perfectly suited for every clinical application.4
Since we know this patient’s renal function is low via CG (49 mL/min), the next important question to ask is, “Is it progressive?” I would recommend obtaining a urinalysis to look for hematuria and albuminuria. Proteinuria is an all-encompassing term. Albumin is only one type of protein and is the single most predictive risk factor for kidney disease progression. Persistent albuminuria alone is diagnostic of renal disease.5 The recommended test is a random urine albumin-to-creatinine ratio (ACR; see Table 1).6
You asked if a 24-hour urine creatinine clearance might evaluate her renal function better. Creatinine clearance can be determined by a 24-hour urine test and a serum blood sample in a steady state. However, this test should be interpreted with caution due to both collection errors and the fact that creatinine clearance overestimates true glomerular filtration rate (GFR) due to tubular secretion of creatinine.7,8 Thus, this test is no longer routinely recommended to determine kidney function.8
Catherine B. York, MSN, APRN-BC
Springfield Nephrology
Associates, Springfield, MO
References
1. CDC. National chronic kidney disease fact sheet: general information and national estimates on chronic kidney disease in the United States, 2010. Atlanta, GA: US Department of Health and Human Services, CDC; 2010.
2. US Renal Data System. USRDS 2012 annual data report: atlas of end-stage renal disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2012.
3. US Renal Data System. USRDS 2012 annual data report: atlas of end-stage renal disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2011.
4. Clarkson MR, Brenner BM. Clinical assessment of the patient with kidney disease. In: Clarkson MR, Brenner BM. Pocket Companion to Brenner & Rector’s The Kidney. 7th ed. Philadelphia, PA: Elsevier Saunders; 2005: 3-19.5.
5. Hsu C. Clinical evaluation of kidney function. In: Greenberg A, Cheung A, Coffman T, et al, eds. Primer on Kidney Diseases, 5th ed. Philadelphia, PA; Saunders Elsevier; 2009:19-237.
6. Kidney Disease Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1-150.
7. National Kidney Foundation. Guideline 5: assessment of proteinuria. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification; 2000.
8. Stevens LA, Coresh J, Greene T, et al. Assessing kidney function-measured and estimated glomerular filtration rate. N Engl J Med. 2006;354:2473-2483.
Q: One of my patients is a 72-year-old woman who weighs 59 kg. Her creatinine clearance by Cockcroft-Gault (CG) came back low (49 mL/min). Is this due to her age, gender, and weight loss during the past five months (subsequent to a femur fracture), or does she have underlying kidney disease? Would a 24-hour urine creatinine test be the best way to determine her level of kidney function—and would it be appropriate for someone her age? Is there a better way to evaluate her kidney function?
Accurate measurement of renal function is vital for any patient suspected of having chronic kidney disease (CKD). More than 20 million adults in the United States, or more than 10% of the adult population, have CKD.1 The 2012 US Renal Data System (USRDS) Annual Data Report states that the prevalence of chronic kidney disease in the Medicare population alone rose more than three-fold between 2000 and 2010, from 2.7% to 9.2%.2
CKD consumes a large proportion of Medicare dollars: more than $23,000 per person per year (PPPY) annually. For end-stage renal disease (ESRD) patients on hemodialysis, the cost is an astounding $88,000 PPPY.2 The cost of treating 871,000 ESRD patients was more than $40 billion in both public and private funds in 2009.3
Risk factors for CKD include but are not limited to: advancing age, male sex, race, hypertension, diabetes mellitus, smoking, family history of kidney disease, proteinuria, exposure to nephrotoxins, and atherosclerosis.4
In the US, the most common methods used to estimate renal function are the CG (Cockcroft-Gault) equation, Modification of Diet in Renal Disease (MDRD) study equations, and the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation. It is often difficult to determine which test is best suited for a patient, because there are pros and cons to each formula and no one test is perfectly suited for every clinical application.4
Since we know this patient’s renal function is low via CG (49 mL/min), the next important question to ask is, “Is it progressive?” I would recommend obtaining a urinalysis to look for hematuria and albuminuria. Proteinuria is an all-encompassing term. Albumin is only one type of protein and is the single most predictive risk factor for kidney disease progression. Persistent albuminuria alone is diagnostic of renal disease.5 The recommended test is a random urine albumin-to-creatinine ratio (ACR; see Table 1).6
You asked if a 24-hour urine creatinine clearance might evaluate her renal function better. Creatinine clearance can be determined by a 24-hour urine test and a serum blood sample in a steady state. However, this test should be interpreted with caution due to both collection errors and the fact that creatinine clearance overestimates true glomerular filtration rate (GFR) due to tubular secretion of creatinine.7,8 Thus, this test is no longer routinely recommended to determine kidney function.8
Catherine B. York, MSN, APRN-BC
Springfield Nephrology
Associates, Springfield, MO
References
1. CDC. National chronic kidney disease fact sheet: general information and national estimates on chronic kidney disease in the United States, 2010. Atlanta, GA: US Department of Health and Human Services, CDC; 2010.
2. US Renal Data System. USRDS 2012 annual data report: atlas of end-stage renal disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2012.
3. US Renal Data System. USRDS 2012 annual data report: atlas of end-stage renal disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2011.
4. Clarkson MR, Brenner BM. Clinical assessment of the patient with kidney disease. In: Clarkson MR, Brenner BM. Pocket Companion to Brenner & Rector’s The Kidney. 7th ed. Philadelphia, PA: Elsevier Saunders; 2005: 3-19.5.
5. Hsu C. Clinical evaluation of kidney function. In: Greenberg A, Cheung A, Coffman T, et al, eds. Primer on Kidney Diseases, 5th ed. Philadelphia, PA; Saunders Elsevier; 2009:19-237.
6. Kidney Disease Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1-150.
7. National Kidney Foundation. Guideline 5: assessment of proteinuria. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification; 2000.
8. Stevens LA, Coresh J, Greene T, et al. Assessing kidney function-measured and estimated glomerular filtration rate. N Engl J Med. 2006;354:2473-2483.
Q: One of my patients is a 72-year-old woman who weighs 59 kg. Her creatinine clearance by Cockcroft-Gault (CG) came back low (49 mL/min). Is this due to her age, gender, and weight loss during the past five months (subsequent to a femur fracture), or does she have underlying kidney disease? Would a 24-hour urine creatinine test be the best way to determine her level of kidney function—and would it be appropriate for someone her age? Is there a better way to evaluate her kidney function?
Accurate measurement of renal function is vital for any patient suspected of having chronic kidney disease (CKD). More than 20 million adults in the United States, or more than 10% of the adult population, have CKD.1 The 2012 US Renal Data System (USRDS) Annual Data Report states that the prevalence of chronic kidney disease in the Medicare population alone rose more than three-fold between 2000 and 2010, from 2.7% to 9.2%.2
CKD consumes a large proportion of Medicare dollars: more than $23,000 per person per year (PPPY) annually. For end-stage renal disease (ESRD) patients on hemodialysis, the cost is an astounding $88,000 PPPY.2 The cost of treating 871,000 ESRD patients was more than $40 billion in both public and private funds in 2009.3
Risk factors for CKD include but are not limited to: advancing age, male sex, race, hypertension, diabetes mellitus, smoking, family history of kidney disease, proteinuria, exposure to nephrotoxins, and atherosclerosis.4
In the US, the most common methods used to estimate renal function are the CG (Cockcroft-Gault) equation, Modification of Diet in Renal Disease (MDRD) study equations, and the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation. It is often difficult to determine which test is best suited for a patient, because there are pros and cons to each formula and no one test is perfectly suited for every clinical application.4
Since we know this patient’s renal function is low via CG (49 mL/min), the next important question to ask is, “Is it progressive?” I would recommend obtaining a urinalysis to look for hematuria and albuminuria. Proteinuria is an all-encompassing term. Albumin is only one type of protein and is the single most predictive risk factor for kidney disease progression. Persistent albuminuria alone is diagnostic of renal disease.5 The recommended test is a random urine albumin-to-creatinine ratio (ACR; see Table 1).6
You asked if a 24-hour urine creatinine clearance might evaluate her renal function better. Creatinine clearance can be determined by a 24-hour urine test and a serum blood sample in a steady state. However, this test should be interpreted with caution due to both collection errors and the fact that creatinine clearance overestimates true glomerular filtration rate (GFR) due to tubular secretion of creatinine.7,8 Thus, this test is no longer routinely recommended to determine kidney function.8
Catherine B. York, MSN, APRN-BC
Springfield Nephrology
Associates, Springfield, MO
References
1. CDC. National chronic kidney disease fact sheet: general information and national estimates on chronic kidney disease in the United States, 2010. Atlanta, GA: US Department of Health and Human Services, CDC; 2010.
2. US Renal Data System. USRDS 2012 annual data report: atlas of end-stage renal disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2012.
3. US Renal Data System. USRDS 2012 annual data report: atlas of end-stage renal disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2011.
4. Clarkson MR, Brenner BM. Clinical assessment of the patient with kidney disease. In: Clarkson MR, Brenner BM. Pocket Companion to Brenner & Rector’s The Kidney. 7th ed. Philadelphia, PA: Elsevier Saunders; 2005: 3-19.5.
5. Hsu C. Clinical evaluation of kidney function. In: Greenberg A, Cheung A, Coffman T, et al, eds. Primer on Kidney Diseases, 5th ed. Philadelphia, PA; Saunders Elsevier; 2009:19-237.
6. Kidney Disease Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1-150.
7. National Kidney Foundation. Guideline 5: assessment of proteinuria. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification; 2000.
8. Stevens LA, Coresh J, Greene T, et al. Assessing kidney function-measured and estimated glomerular filtration rate. N Engl J Med. 2006;354:2473-2483.
Why Take This Patient Off Her ACEI?
Q: I sent a 68-year-old, hypertensive, diabetic woman with stage 4 kidney disease (estimated glomerular filtration rate [eGFR], 25 mL/min/1.73 m2; serum creatinine [SCr], 2 mg/mL) to a local nephrology group. Since she was diabetic, we had had her taking an angiotensin-converting enzyme inhibitor (ACEI) for years. The first thing the nephrology group did was take her off the ACEI. Why would they do that? I thought the hypertensive drug of choice for all diabetic patients is an ACEI or an angiotensin receptor blocker (ARB). Am I wrong?
A: The renin-angiotensin-aldosterone system (RAAS) plays an important role in the regulation of blood pressure and intravascular volume through its effects on renin, angiotensin, and aldosterone production. Activation of RAAS causes an increase in blood pressure through vasoconstriction (angiotensin II effects) and the fluid retention associated with reabsorption of sodium and water (aldosterone effects). As such, the physiologic effects of RAAS have been implicated in the pathophysiology of cardiovascular diseases, such as heart failure, kidney disease, and hypertension.1
The JNC 7 guidelines2 (Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure) recommend the use of ACEIs or ARBs for the treatment of hypertension in patients with chronic kidney disease (CKD) or diabetes to slow progression of kidney disease. Given the benefits of ACEI or ARB therapy in reducing mortality in cardiovascular disease (for which patients with CKD are at increased risk3), these medications are indicated in patients with CKD.
Angiotensin II causes systemic vasoconstriction and also acts on the efferent arterioles of the glomerulus. ACEI/ARB therapy causes vasodilation of the efferent arterioles, thus lowering the intraglomerular capillary pressure.1 This mechanism accounts for the antiproteinuric effect and the subsequent decrease in GFR when these agents are initiated.
Proteinuria is a marker that may indicate nephropathy and may lead to further kidney damage.1 Slowing renal disease progression depends on controlling blood pressure and proteinuria.3
To date, a number of trials have been conducted to explore the benefit of the antiproteinuric effects of ACEI/ARB therapy. The antiproteinuric effect is more pronounced in patients with more severe proteinuria. Studies of these agents in patients with diabetic and nondiabetic nephropathy have shown that besides reducing proteinuria, they slow progression to end-stage renal disease (ESRD), thus delaying the need for renal replacement therapy.4 As such, ACEI/ARB therapy is widely used to reduce proteinuria, independent of the blood pressure–lowering effects.
Initiation of ACEI/ARB therapy is associated with an increase in SCr due to the drugs’ effects on the efferent arterioles, resulting in a decrease in intraglomerular pressure.3 However, discontinuing therapy is not warranted unless the SCr rises to more than 30% above baseline. According to findings from one meta-analysis, the degree of loss in renal function when ACEI therapy was initiated was inversely related to the rate of annual decline in renal function.5 Thus, patients with higher SCr levels at the start of therapy had poorer renal function initially, but they received the greatest benefit in long-term renal preservation.
The rise in SCr typically occurs within a few days of therapy initiation; thus, SCr should be measured within the first seven days of therapy.3 The SCr level is expected to stabilize within six to eight weeks of therapy. Patients whose SCr level continues to rise more than 30% to 35% above baseline may need to discontinue ACEI/ARB therapy; this rise may be attributed to kidney hypoperfusion. Kidney hypoperfusion can also occur when diuretics are initiated or their dosage increased, when NSAIDs are used, or in patients with bilateral renal artery stenosis or volume depletion resulting from gastroenteritis.3
Hyperkalemia due to decreased urinary excretion of potassium (K+) may present yet another reason to discontinue ACEI/ARB therapy in patients with CKD. Incidence of hyperkalemia in those with CKD stages 3 through 5 who receive either an ACEI or an ARB ranges from 5% to 50%.3 Hyperkalemia can occur when a long-acting ACEI is prescribed or when ACEI/ARB therapy is used concurrently with NSAIDs or potassium-sparing diuretics; thiazides or loop diuretics, by contrast, can reduce the risk for hyperkalemia.
Risk factors for moderate hyperkalemia (serum K+ ≥ 5.6 mmol/L) include age older than 65, congestive heart failure, SCr level greater than 1.6 mg/dL, and a blood urea nitrogen level exceeding 18 mg/dL.5
A dosing reduction or discontinuation of ACEI/ARB therapy should be considered when serum K+ levels are 5.6 mmol/L or greater.3 Patients who are older than 70 or whose serum urea nitrogen level exceeds 25 mg/dL are at increased risk for severe hyperkalemia (K+ > 6.0 mmol/L). Termination of an ACEI or an ARB may be warranted in patients with a serum K+ level exceeding 6.0 mmol/L or in those considered at increased risk for severe hyperkalemia.3
Other patients may also benefit from discontinuing ACEI/ARB therapy. Few data exist to support their use in patients with kidney function at 25% or less, or those with a GFR below 30 mL/min/1.73 m2.3 Onuigbo6 suggests that ESRD in patients with CKD can either progress gradually and steadily or rapidly as a result of acute kidney injury (AKI). Patients with CKD who are at risk for ESRD because of AKI include those currently taking an ACEI or an ARB, older patients, and those who experience unexplained decreases in GFR.
To avoid any worsening of renal function, Onuigbo6 suggests temporarily discontinuing ACEI/ARB therapy in the following patients:
• Those older than 65 who are scheduled for colonoscopy, administration of IV radiocontrast, or surgery (especially cardiovascular surgery); or
• Patients hospitalized for an acute ailment.
Discontinuing these agents may prevent progression to ESRD in such patients.
In summary, use of ACEIs or ARBs to delay progression of renal disease may be continued if a clear therapeutic benefit exists. However, discontinuation should be considered if a patient with CKD is at risk for hyperkalemia or if the K+ level remains higher than 5.6 mmol/L, if SCr levels increase more than 30% above baseline, or if patients (especially those older than 65) are at risk for AKI.
References
1. Remuzzi G, Perico N, Macia M, Ruggenenti P. The role of renin-angiotensin-aldosterone system in the progression of chronic kidney disease. Kidney Int Suppl. 2005;99:S57-S65.
2. Chobanian AV, Bakris GL, Black HR, et al; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560-2572.
3. Mangrum AJ, Bakris GL. Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers in chronic renal disease: safety issues. Semin Nephrol. 2004;24:168-175.
4. St Peter WL, Odum LE, Whaley-Connell AT. To RAS or not to RAS? The evidence for and cautions with renin-angiotensin system inhibition in patients with diabetic kidney disease. Pharmacotherapy. 2013 Apr 9.
5. Bakris GL, Weir MR. Angiotensin-converting enzyme inhibitor–associated elevations in serum creatinine: is this a cause for concern? Arch Intern Med. 2000;160:685-693.
6. Onuigbo MA. Is renoprotection with RAAS blockade a failed paradigm? Have we learnt any lessons so far? Int J Clin Pract. 2010;64:1341-1346.
Q: I sent a 68-year-old, hypertensive, diabetic woman with stage 4 kidney disease (estimated glomerular filtration rate [eGFR], 25 mL/min/1.73 m2; serum creatinine [SCr], 2 mg/mL) to a local nephrology group. Since she was diabetic, we had had her taking an angiotensin-converting enzyme inhibitor (ACEI) for years. The first thing the nephrology group did was take her off the ACEI. Why would they do that? I thought the hypertensive drug of choice for all diabetic patients is an ACEI or an angiotensin receptor blocker (ARB). Am I wrong?
A: The renin-angiotensin-aldosterone system (RAAS) plays an important role in the regulation of blood pressure and intravascular volume through its effects on renin, angiotensin, and aldosterone production. Activation of RAAS causes an increase in blood pressure through vasoconstriction (angiotensin II effects) and the fluid retention associated with reabsorption of sodium and water (aldosterone effects). As such, the physiologic effects of RAAS have been implicated in the pathophysiology of cardiovascular diseases, such as heart failure, kidney disease, and hypertension.1
The JNC 7 guidelines2 (Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure) recommend the use of ACEIs or ARBs for the treatment of hypertension in patients with chronic kidney disease (CKD) or diabetes to slow progression of kidney disease. Given the benefits of ACEI or ARB therapy in reducing mortality in cardiovascular disease (for which patients with CKD are at increased risk3), these medications are indicated in patients with CKD.
Angiotensin II causes systemic vasoconstriction and also acts on the efferent arterioles of the glomerulus. ACEI/ARB therapy causes vasodilation of the efferent arterioles, thus lowering the intraglomerular capillary pressure.1 This mechanism accounts for the antiproteinuric effect and the subsequent decrease in GFR when these agents are initiated.
Proteinuria is a marker that may indicate nephropathy and may lead to further kidney damage.1 Slowing renal disease progression depends on controlling blood pressure and proteinuria.3
To date, a number of trials have been conducted to explore the benefit of the antiproteinuric effects of ACEI/ARB therapy. The antiproteinuric effect is more pronounced in patients with more severe proteinuria. Studies of these agents in patients with diabetic and nondiabetic nephropathy have shown that besides reducing proteinuria, they slow progression to end-stage renal disease (ESRD), thus delaying the need for renal replacement therapy.4 As such, ACEI/ARB therapy is widely used to reduce proteinuria, independent of the blood pressure–lowering effects.
Initiation of ACEI/ARB therapy is associated with an increase in SCr due to the drugs’ effects on the efferent arterioles, resulting in a decrease in intraglomerular pressure.3 However, discontinuing therapy is not warranted unless the SCr rises to more than 30% above baseline. According to findings from one meta-analysis, the degree of loss in renal function when ACEI therapy was initiated was inversely related to the rate of annual decline in renal function.5 Thus, patients with higher SCr levels at the start of therapy had poorer renal function initially, but they received the greatest benefit in long-term renal preservation.
The rise in SCr typically occurs within a few days of therapy initiation; thus, SCr should be measured within the first seven days of therapy.3 The SCr level is expected to stabilize within six to eight weeks of therapy. Patients whose SCr level continues to rise more than 30% to 35% above baseline may need to discontinue ACEI/ARB therapy; this rise may be attributed to kidney hypoperfusion. Kidney hypoperfusion can also occur when diuretics are initiated or their dosage increased, when NSAIDs are used, or in patients with bilateral renal artery stenosis or volume depletion resulting from gastroenteritis.3
Hyperkalemia due to decreased urinary excretion of potassium (K+) may present yet another reason to discontinue ACEI/ARB therapy in patients with CKD. Incidence of hyperkalemia in those with CKD stages 3 through 5 who receive either an ACEI or an ARB ranges from 5% to 50%.3 Hyperkalemia can occur when a long-acting ACEI is prescribed or when ACEI/ARB therapy is used concurrently with NSAIDs or potassium-sparing diuretics; thiazides or loop diuretics, by contrast, can reduce the risk for hyperkalemia.
Risk factors for moderate hyperkalemia (serum K+ ≥ 5.6 mmol/L) include age older than 65, congestive heart failure, SCr level greater than 1.6 mg/dL, and a blood urea nitrogen level exceeding 18 mg/dL.5
A dosing reduction or discontinuation of ACEI/ARB therapy should be considered when serum K+ levels are 5.6 mmol/L or greater.3 Patients who are older than 70 or whose serum urea nitrogen level exceeds 25 mg/dL are at increased risk for severe hyperkalemia (K+ > 6.0 mmol/L). Termination of an ACEI or an ARB may be warranted in patients with a serum K+ level exceeding 6.0 mmol/L or in those considered at increased risk for severe hyperkalemia.3
Other patients may also benefit from discontinuing ACEI/ARB therapy. Few data exist to support their use in patients with kidney function at 25% or less, or those with a GFR below 30 mL/min/1.73 m2.3 Onuigbo6 suggests that ESRD in patients with CKD can either progress gradually and steadily or rapidly as a result of acute kidney injury (AKI). Patients with CKD who are at risk for ESRD because of AKI include those currently taking an ACEI or an ARB, older patients, and those who experience unexplained decreases in GFR.
To avoid any worsening of renal function, Onuigbo6 suggests temporarily discontinuing ACEI/ARB therapy in the following patients:
• Those older than 65 who are scheduled for colonoscopy, administration of IV radiocontrast, or surgery (especially cardiovascular surgery); or
• Patients hospitalized for an acute ailment.
Discontinuing these agents may prevent progression to ESRD in such patients.
In summary, use of ACEIs or ARBs to delay progression of renal disease may be continued if a clear therapeutic benefit exists. However, discontinuation should be considered if a patient with CKD is at risk for hyperkalemia or if the K+ level remains higher than 5.6 mmol/L, if SCr levels increase more than 30% above baseline, or if patients (especially those older than 65) are at risk for AKI.
References
1. Remuzzi G, Perico N, Macia M, Ruggenenti P. The role of renin-angiotensin-aldosterone system in the progression of chronic kidney disease. Kidney Int Suppl. 2005;99:S57-S65.
2. Chobanian AV, Bakris GL, Black HR, et al; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560-2572.
3. Mangrum AJ, Bakris GL. Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers in chronic renal disease: safety issues. Semin Nephrol. 2004;24:168-175.
4. St Peter WL, Odum LE, Whaley-Connell AT. To RAS or not to RAS? The evidence for and cautions with renin-angiotensin system inhibition in patients with diabetic kidney disease. Pharmacotherapy. 2013 Apr 9.
5. Bakris GL, Weir MR. Angiotensin-converting enzyme inhibitor–associated elevations in serum creatinine: is this a cause for concern? Arch Intern Med. 2000;160:685-693.
6. Onuigbo MA. Is renoprotection with RAAS blockade a failed paradigm? Have we learnt any lessons so far? Int J Clin Pract. 2010;64:1341-1346.
Q: I sent a 68-year-old, hypertensive, diabetic woman with stage 4 kidney disease (estimated glomerular filtration rate [eGFR], 25 mL/min/1.73 m2; serum creatinine [SCr], 2 mg/mL) to a local nephrology group. Since she was diabetic, we had had her taking an angiotensin-converting enzyme inhibitor (ACEI) for years. The first thing the nephrology group did was take her off the ACEI. Why would they do that? I thought the hypertensive drug of choice for all diabetic patients is an ACEI or an angiotensin receptor blocker (ARB). Am I wrong?
A: The renin-angiotensin-aldosterone system (RAAS) plays an important role in the regulation of blood pressure and intravascular volume through its effects on renin, angiotensin, and aldosterone production. Activation of RAAS causes an increase in blood pressure through vasoconstriction (angiotensin II effects) and the fluid retention associated with reabsorption of sodium and water (aldosterone effects). As such, the physiologic effects of RAAS have been implicated in the pathophysiology of cardiovascular diseases, such as heart failure, kidney disease, and hypertension.1
The JNC 7 guidelines2 (Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure) recommend the use of ACEIs or ARBs for the treatment of hypertension in patients with chronic kidney disease (CKD) or diabetes to slow progression of kidney disease. Given the benefits of ACEI or ARB therapy in reducing mortality in cardiovascular disease (for which patients with CKD are at increased risk3), these medications are indicated in patients with CKD.
Angiotensin II causes systemic vasoconstriction and also acts on the efferent arterioles of the glomerulus. ACEI/ARB therapy causes vasodilation of the efferent arterioles, thus lowering the intraglomerular capillary pressure.1 This mechanism accounts for the antiproteinuric effect and the subsequent decrease in GFR when these agents are initiated.
Proteinuria is a marker that may indicate nephropathy and may lead to further kidney damage.1 Slowing renal disease progression depends on controlling blood pressure and proteinuria.3
To date, a number of trials have been conducted to explore the benefit of the antiproteinuric effects of ACEI/ARB therapy. The antiproteinuric effect is more pronounced in patients with more severe proteinuria. Studies of these agents in patients with diabetic and nondiabetic nephropathy have shown that besides reducing proteinuria, they slow progression to end-stage renal disease (ESRD), thus delaying the need for renal replacement therapy.4 As such, ACEI/ARB therapy is widely used to reduce proteinuria, independent of the blood pressure–lowering effects.
Initiation of ACEI/ARB therapy is associated with an increase in SCr due to the drugs’ effects on the efferent arterioles, resulting in a decrease in intraglomerular pressure.3 However, discontinuing therapy is not warranted unless the SCr rises to more than 30% above baseline. According to findings from one meta-analysis, the degree of loss in renal function when ACEI therapy was initiated was inversely related to the rate of annual decline in renal function.5 Thus, patients with higher SCr levels at the start of therapy had poorer renal function initially, but they received the greatest benefit in long-term renal preservation.
The rise in SCr typically occurs within a few days of therapy initiation; thus, SCr should be measured within the first seven days of therapy.3 The SCr level is expected to stabilize within six to eight weeks of therapy. Patients whose SCr level continues to rise more than 30% to 35% above baseline may need to discontinue ACEI/ARB therapy; this rise may be attributed to kidney hypoperfusion. Kidney hypoperfusion can also occur when diuretics are initiated or their dosage increased, when NSAIDs are used, or in patients with bilateral renal artery stenosis or volume depletion resulting from gastroenteritis.3
Hyperkalemia due to decreased urinary excretion of potassium (K+) may present yet another reason to discontinue ACEI/ARB therapy in patients with CKD. Incidence of hyperkalemia in those with CKD stages 3 through 5 who receive either an ACEI or an ARB ranges from 5% to 50%.3 Hyperkalemia can occur when a long-acting ACEI is prescribed or when ACEI/ARB therapy is used concurrently with NSAIDs or potassium-sparing diuretics; thiazides or loop diuretics, by contrast, can reduce the risk for hyperkalemia.
Risk factors for moderate hyperkalemia (serum K+ ≥ 5.6 mmol/L) include age older than 65, congestive heart failure, SCr level greater than 1.6 mg/dL, and a blood urea nitrogen level exceeding 18 mg/dL.5
A dosing reduction or discontinuation of ACEI/ARB therapy should be considered when serum K+ levels are 5.6 mmol/L or greater.3 Patients who are older than 70 or whose serum urea nitrogen level exceeds 25 mg/dL are at increased risk for severe hyperkalemia (K+ > 6.0 mmol/L). Termination of an ACEI or an ARB may be warranted in patients with a serum K+ level exceeding 6.0 mmol/L or in those considered at increased risk for severe hyperkalemia.3
Other patients may also benefit from discontinuing ACEI/ARB therapy. Few data exist to support their use in patients with kidney function at 25% or less, or those with a GFR below 30 mL/min/1.73 m2.3 Onuigbo6 suggests that ESRD in patients with CKD can either progress gradually and steadily or rapidly as a result of acute kidney injury (AKI). Patients with CKD who are at risk for ESRD because of AKI include those currently taking an ACEI or an ARB, older patients, and those who experience unexplained decreases in GFR.
To avoid any worsening of renal function, Onuigbo6 suggests temporarily discontinuing ACEI/ARB therapy in the following patients:
• Those older than 65 who are scheduled for colonoscopy, administration of IV radiocontrast, or surgery (especially cardiovascular surgery); or
• Patients hospitalized for an acute ailment.
Discontinuing these agents may prevent progression to ESRD in such patients.
In summary, use of ACEIs or ARBs to delay progression of renal disease may be continued if a clear therapeutic benefit exists. However, discontinuation should be considered if a patient with CKD is at risk for hyperkalemia or if the K+ level remains higher than 5.6 mmol/L, if SCr levels increase more than 30% above baseline, or if patients (especially those older than 65) are at risk for AKI.
References
1. Remuzzi G, Perico N, Macia M, Ruggenenti P. The role of renin-angiotensin-aldosterone system in the progression of chronic kidney disease. Kidney Int Suppl. 2005;99:S57-S65.
2. Chobanian AV, Bakris GL, Black HR, et al; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560-2572.
3. Mangrum AJ, Bakris GL. Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers in chronic renal disease: safety issues. Semin Nephrol. 2004;24:168-175.
4. St Peter WL, Odum LE, Whaley-Connell AT. To RAS or not to RAS? The evidence for and cautions with renin-angiotensin system inhibition in patients with diabetic kidney disease. Pharmacotherapy. 2013 Apr 9.
5. Bakris GL, Weir MR. Angiotensin-converting enzyme inhibitor–associated elevations in serum creatinine: is this a cause for concern? Arch Intern Med. 2000;160:685-693.
6. Onuigbo MA. Is renoprotection with RAAS blockade a failed paradigm? Have we learnt any lessons so far? Int J Clin Pract. 2010;64:1341-1346.