User login
Losing a patient to suicide: What we know
Studies have found that 1 in 2 psychiatrists,1-4 and 1 in 5 psychologists, clinical social workers, and other mental health professionals,5 will lose a patient to suicide in the course of their career. This statistic suggests that losing a patient to suicide constitutes a clear occupational hazard.6,7 Despite this, most mental health professionals continue to view suicide loss as an aberration. Consequently, there is often a lack of preparedness for such an event when it does occur.
This 2-part article summarizes what is currently known about the unique personal and professional issues experienced by clinician-survivors (clinicians who have lost patients and/or loved ones to suicide). In Part 1, I cover:
- the impact of losing a patient to suicide
- confidentiality-related constraints on the ability to discuss and process the loss
- legal and ethical issues
- colleagues’ reactions and stigma
- the effects of a suicide loss on one’s clinical work.
Part 2 will discuss the opportunities for personal growth that can result from experiencing a suicide loss, guidelines for optimal postventions, and steps clinicians can take to help support colleagues who have lost a patient to suicide.
A neglected topic
For psychiatrists and other mental health professionals, the loss of a patient to suicide is certainly not uncommon.1-5 Despite this, coping with a patient’s suicide is a “neglected topic”8 in residency and general mental health training.
There are many published articles on clinicians experiencing suicide loss (for a comprehensive bibliography, see McIntosh9), and several authors10-19 have developed suggestions, guidelines, and detailed postvention protocols to help clinicians navigate the often-complicated sequelae to such a loss. However, these resources have generally not been integrated into clinical training, and tend to be poorly disseminated. In a national survey of chief residents, Melton and Coverdale20 found that only 25% of residency training programs covered topics related to postvention, and 72% of chief residents felt this topic needed more attention. Thus, despite the existence of guidelines for optimal postvention and support, clinicians are often left to cope with the consequences of this difficult loss on their own, and under less-than-optimal conditions.
A patient’s suicide typically affects clinicians on multiple levels, both personally and professionally. In this article, I highlight the range of normative responses, as well as the factors that may facilitate or inhibit subsequent healing and growth, with the hope that this knowledge may be utilized to help current and future generations of clinician-survivors obtain optimal support, and that institutions who treat potentially suicidal individuals will develop optimal postvention responses following a suicide loss. Many aspects of what this article discusses also apply to clinicians who have experienced a suicide loss in their personal or family life, as this also tends to “spill over” into one’s professional roles and identity.
Grief and other emotional effects
In many ways, clinicians’ responses after a patient’s suicide are similar to those of other survivors after the loss of a loved one to suicide.21 Chemtob et al2 found that approximately one-half of psychiatrists who lost a patient to suicide had scores on the Impact of an Event Scale that were comparable to those of a clinical population seeking treatment after the death of a parent.
Continue to: Jordan and McIntosh have detailed...
Jordan and McIntosh22 have detailed several elements and themes that differentiate suicide loss and its associated reactions from other types of loss and grief. In general, suicide loss is considered traumatic, and is often accompanied by intense confusion and existential questioning, reflecting a negative impact on one’s core beliefs and assumptive world. The subsequent need to address the myriad of “why” questions left in its wake are often tinted with what Jordan and Baugher23 term the “tyranny of hindsight,” and take the form of implicit guilt for “sins of omission or commission” in relation to the lost individual.
Responses to suicide loss typically include initial shock, denial and numbness, intense sadness, anxiety, anger, and intense distress. Consistent with the traumatic nature of the loss, survivors are also likely to experience posttraumatic stress disorder symptoms such as intrusive thoughts, avoidance, and dissociation. Survivors also commonly experience significant guilt and shame, and this is likely to be socially reinforced by the general stigma associated with suicide as well as the actual blaming and avoidance responses of others.24-27
Clinicians’ unique reactions
For clinicians, there are additional components that may further complicate or exacerbate these reactions and extend their duration. First and foremost, such a loss affects clinicians on both personal and professional levels, a phenomenon that Plakun and Tillman13 have termed a “twin bereavement.” Thus, in addition to the personal grief and trauma reactions entailed in losing a patient to suicide, this loss is likely to impact clinicians’ professional identities, their relationships with colleagues, and their clinical work.
Clinicians’ professional identities are often predicated on generally shared assumptions and beliefs that, as trained professionals, they should have the power, aptitude, and competence to heal, or at least improve, the lives of patients, to reduce their distress, and to provide safety. In addition, such assumptions about clinicians’ responsibility and ability to prevent suicide are often reinforced in the clinical literature.28,29
These assumptions are often challenged, if not shattered, when patients take their own lives. A clinician’s sense of professional responsibility, the guilt and self-blame that may accompany this, self-doubts about one’s skills and clinical competence, the fear of (and actual) blame of colleagues and family members, and the real or imagined threat of litigation may all greatly exacerbate a clinician’s distress.11
Continue to: Hendin et al found...
Hendin et al30 found that mental health therapists have described losing a patient as “the most profoundly disturbing event of their professional careers,” noting that one-third of these clinicians experienced severe distress that lasted at least 1 year beyond the initial loss. In a 2004 study, Ruskin et al4 similarly found that one-quarter of psychiatrists and psychiatric trainees noted that losing a patient had a “profound and enduring effect on them.” In her article on surviving a patient’s suicide, Rycroft31 describes a “professional void” following the loss of her patient, in which “the world had changed, nothing was predictable any more, and it was no longer safe to assume anything.” Additionally, many clinicians experience an “acute sense of aloneness and isolation” subsequent to the loss.32
Many clinicians have noted that they considered leaving the field after such a loss,33,34 and it is hypothesized that many may have done so.35-37 Others have noted that, at least temporarily, they stopped treating patients who were potentially suicidal.29,35
Box 1
Several authors have proposed general models for describing the suicide grief trajectories of clinicians after a suicide loss. Tillman38 identified distinct groups of responses to this event: traumatic, affective, those related to the treatment, those related to interactions with colleagues, liability concerns, and the impact on one’s professional philosophy. She also found that Erikson’s stages of identity39 provided an uncannily similar trajectory to the ways in which those who participated in her research—clinicians at a mental hospital—had attempted to cope with their patients’ deaths, noting that the “suicide of a patient may provoke a revisiting of Erikson’s psychosocial crises in a telescoped and accelerated fashion.”38
Maltsberger40 offered a detailed psychoanalytic analysis of the responses clinicians may manifest in relation to a suicide loss, including the initial narcissistic injury sustained in relation to their patient’s actions; the subsequent potential for melancholic, atonement, or avoidance reactions; and the eventual capacity for the resolution of these reactions.
Al-Mateen et al33 described 3 phases of the clinician’s reaction after losing a patient who was a child to suicide:
- initial, which includes trauma and shock
- turmoil, which includes emotional flooding and functional impairments
- new growth, in which clinicians are able to reflect on their experiences and implications for training and policy.
For each phase, they also described staff activities that would foster forward movement through the trajectory.
In a 1981 study, Bissell41 found that psychiatric nurses who had experienced patient completed suicides progressed through several developmental stages (naïveté, recognition, responsibility, individual choice) that enabled them to come to terms with their personal reactions and place the ultimate responsibility for the suicide with the patient.
After losing a patient to suicide, a clinician may experience grief that proceeds through specific stages (Box 133,38-41). Box 22-4,6,16,24,29,30,33,34,40,42-45 describes a wide range of factors that affect each clinician’s unique response to losing a patient to suicide.
Box 2
There are many factors that make the experience of losing a patient to suicide unique and variable for individual clinicians. These include the amount of a clinician’s professional training and experience, both in general and in working with potentially suicidal individuals. Chemtob et al2 found that trainees were more likely to experience patient suicide loss than more seasoned clinicians, and to experience more distress.4,30,42 Brown24 noted that many training programs were likely to assign the most “extraordinarily sick patients to inexperienced trainees.” He noted that because the skill level of trainees has not yet tempered their personal aspirations, they are likely to experience a patient’s suicide as a personal failure. However, in contrast to the findings of Kleespies,42 Hendin,30 Ruskin et al,4 and Brown24 suggested that the overall impact of a patient’s suicide may be greater for seasoned clinicians, when the “protective advantage” or “explanation” of being in training is no longer applicable. This appears consistent with Munson’s study,43 which found that a greater number of years of clinical experience prior to a suicide loss was negatively correlated with posttraumatic growth.
Other factors affecting a clinician’s grief response include the context in which the treatment occurred, such as inpatient, outpatient, clinic, private practice, etc.44; the presence and involvement of supportive mentors or supervisors16; the length and intensity of the clinical relationship6,29; countertransference issues40; whether the patient was a child33; and the time elapsed since the suicide occurred.
In addition, each clinician’s set of personal and life experiences can affect the way he/ she moves through the grieving process. Any previous trauma or losses, particularly prior exposure to suicide, will likely impact a clinician’s reaction to his/her current loss, as will any susceptibility to anxiety or depression. Gorkin45 has suggested that the degree of omnipotence in the clinician’s therapeutic strivings will affect his/her ability to accept the inherent ambiguity involved in suicide loss. Gender may also play a role: Henry et al34 found that female clinicians had higher levels of stress reactions, and Grad et al3 found that female clinicians felt more shame and guilt and professed more doubts about their professional competence than male clinicians, and were more than twice as likely as men to identify talking with colleagues as an effective coping strategy.
Continue to: Implications of confidentiality restrictions
Implications of confidentiality restrictions
Confidentiality issues, as well as advice from attorneys to limit the disclosure of information about a patient, are likely to preclude a clinician’s ability to talk freely about the patient, the therapeutic relationship, and his/her reactions to the loss, all of which are known to facilitate movement through the grief process.46
The development of trust and the sharing of pain are just 2 factors that can make the clinical encounter an intense emotional experience for both parties. Recent trends in the psychodynamic literature acknowledge the profundity and depth of the personal impact that patients have on the clinician, an impact that is neither pathological nor an indication of poor boundaries in the therapy dyad, but instead a recognition of how all aspects of the clinician’s person, whether consciously or not, are used within the context of a therapeutic relationship. Yet when clinicians lose a patient, confidentiality restrictions often leave them wondering if and where any aspects of their experiences can be shared. Legal counsel may advise a clinician against speaking to consultants or supervisors or even surviving family members for fear that these non-privileged communications are subject to discovery should any legal proceedings ensue. Furthermore, the usual grief rituals that facilitate the healing of loss and the processing of grief (eg, gathering with others who knew the deceased, sharing feelings and memories, attending memorials) are usually denied to the clinician, and are often compounded by the reactions of one’s professional colleagues, who tend not to view the therapist’s grief as “legitimate.” Thus, clinician-survivors, despite having experienced a profound and traumatic loss, have very few places where this may be processed or even validated. As one clinician in a clinician-survivors support group stated, “I felt like I was grieving in a vacuum, that I wasn’t allowed to talk about how much my patient meant to me or how I’m feeling about it.” The isolation of grieving alone is likely to be compounded by the general lack of resources for supporting clinicians after such a loss. In contrast to the general suicide “survivor” network of support groups for family members who have experienced a suicide loss, there is an almost complete lack of supportive resources for clinicians following such a loss, and most clinicians are not aware of the resources that are available, such as the Clinician Survivor Task Force of the American Association of Suicidology (Box 312).
Box 3
Frank Jones and Judy Meade founded the Clinician Survivor Task Force (CSTF) of the American Association of Suicidology (AAS) in 1987. As Jones noted, “clinicians who have lost patients to suicide need a place to acknowledge and carry forward their personal loss … to benefit both personally and professionally from the opportunity to talk with other therapists who have survived the loss of a patient through suicide.”12
Nina Gutin, PhD, and Vanessa McGann, PhD, have co-chaired the CSTF since 2003. It now supports clinicians who have lost patients and/or loved ones, with the recognition that both types of losses carry implications within clinical and professional domains. The CSTF provides a listserve, opportunities to participate in video support groups, and a web site (www. cliniciansurvivor.org) that provides information about the clinician-survivor experience, the opportunity to read and post narratives about one’s experience with suicide loss, an updated bibliography maintained by John McIntosh, PhD, a list of clinical contacts, and a link to several excellent postvention protocols. In addition, Drs. Gutin and McGann conduct clinician-survivor support activities at the annual AAS conference, and in their respective geographic areas.
Continue to: Doka has described...
Doka47 has described “disenfranchised grief” in which the bereaved person does not receive the type and quality of support accorded to other bereaved persons, and thus is likely to internalize the view that his/her grief is not legitimate, and to believe that sharing related distress is a shame-ridden liability. This clearly relates to the sense of profound isolation and distress often described by clinician-survivors.
Other legal/ethical issues
The clinician-survivor’s concern about litigation, or an actual lawsuit, is likely to produce intense anxiety. This common fear is both understandable and credible. According to Bongar,48 the most common malpractice lawsuits filed against clinicians are those that involve a patient’s suicide. Peterson et al49 found that 34% of surviving family members considered bringing a lawsuit against the clinician, and of these, 57% consulted a lawyer.
In addition, an institution’s concern about protecting itself from liability may compromise its ability to support the clinician or trainee who sustained the loss. As noted above, the potential prohibitions around discussing the case can compromise the grief process. Additionally, the fear of (or actual) legal reprisals against supervisors and the larger institution may engender angry and blaming responses toward the treating clinician. In a personal communication (April 2008), Quinnett described an incident in which a supervising psychologist stomped into the grieving therapist’s office unannounced and shouted, “Now look what you’ve done! You’re going to get me sued!”
Other studies29,50,51 note that clinician-survivors fear losing their job, and that their colleagues and supervisors will be reluctant to assign new patients to them. Spiegleman and Werth17 also note that trainees grapple with additional concerns over negative evaluations, suspension or termination from clinical sites or training programs, and a potential interruption of obtaining a degree. Such supervisory and institutional reactions are likely to intensify a clinician’s sense of shame and distress, and are antithetical to postvention responses that promote optimal personal and professional growth. Such negative reactions are also likely to contribute to a clinician or trainee’s subsequent reluctance to work with suicidal individuals, or their decision to discontinue their clinical work altogether. Lastly, other ethical issues, such as contact with the patient’s family following the suicide, attending the funeral, etc., are likely to be a source of additional anxiety and distress, particularly if the clinician needs to address these issues in isolation.
Professional relationships/colleagues’ reactions
Many clinician-survivors have described reactions from colleagues and supervisors that are hurtful and unsupportive. According to Jobes and Maltsberger,52 “the suicide death of a patient in active treatment is commonly taken as prima facie evidence that the therapist, somehow or another, has mismanaged the case,” and thus the clinician often faces unwarranted blame and censure from colleagues and supervisors. Hendin et al30 noted that many trainees found reactions by their institutions to be insensitive and unsupportive, one noting that the department’s review of the case “felt more like a tribunal or inquest.” In a personal communication (April 2008), Quinnett noted that many clinicians he interviewed following a suicide loss reported a pattern of isolation and interpersonal discomfort with their colleagues, who implicitly or explicitly expressed concerns about their competence. He described how a respected colleague received “no understanding, no support, only abuse” from her supervisors. Such responses, while perhaps surprising from mental health professionals, probably reflect the long-standing cultural attitude of social condemnation of suicide, and of those who are associated with it.
Continue to: Negative reactions from professional colleagues...
Negative reactions from professional colleagues are most likely to occur immediately after the suicide loss and/or during the course of a subsequent investigation or psychological autopsy. Castelli-Dransart et al53 found that the lack of institutional support after a clinician experiences a suicide loss contributed to significantly higher stress responses for impacted clinicians, and may lead to a well-founded ambivalence about disclosure to colleagues, and consequent resistance to seeking out optimal supervision/consultation or even personal therapy that could help the clinician gain clarity on the effects of these issues. Many mental health professionals have described how, after the distressing experience of losing a patient to suicide, they moved through this process in relative isolation and loneliness, feeling abandoned by their colleagues and by their own hopes and expectations for support.
Stigmatization. In clinical settings, when a patient in treatment completes suicide, the treating clinician becomes an easy scapegoat for family members and colleagues. To the extent that mental health professionals are not immune from the effects and imposition of stigma, this might also affect their previously mentioned tendency to project judgment, overtly or covertly, onto the treating clinician.
Stigma around suicide is well documented.25 In The Surgeon General’s Call to Action to Prevent Suicide,54 former Surgeon General David Satcher specifically described stigma around suicide as one of the biggest barriers to prevention. Studies have shown that individuals bereaved by suicide are also stigmatized, and that those who were in caregiving roles (parents, clinicians) are believed to be more psychologically disturbed, less likable, more blameworthy, and less worthy of receiving support than other bereaved individuals.25,55-63 These judgments often mirror survivors’ self-punitive assessments, which then become exacerbated by and intertwined with both externally imposed and internalized stigma. Hence, it is not uncommon for suicide survivors to question their own right to grieve, to report low expectations of social support, and to feel compelled to deny or hide the mode of death. Feigelman et al26 found that stigmatization after a suicide loss was specifically associated with ongoing grief difficulties, depression, and suicidal thinking.
In my long-term work with clinician-survivors, I’ve come to believe that in addition to stigma around suicide, there may also be stigma projected by colleagues in relation to a clinician’s perceived emotional vulnerability. A traumatized clinician potentially challenges the notion of the implicit dichotomy/power imbalance between professionals and the patients we treat: “Us”—the professional, competent, healthy, and benevolent clinicians who have the care to offer, and “Them”—our patients, being needy, pathological, looking to us for care. This “us/them” distinction may serve to bolster a clinician’s professional esteem and identity. But when one of “us” becomes one of “them”—when a professional colleague is perceived as being emotionally vulnerable—this can be threatening to the predicates of this distinction, leading to the need to put the affected clinician firmly into the “them” camp. Thus, unwarranted condemnations of the clinician-survivor’s handling of the case, and/or the pathologizing of their normative grief reactions after the suicide loss, can seem justified.
Stigma associated both with suicide and with professional vulnerability is likely to be internalized and to have a profound effect on the clinician’s decisions about disclosure, asking for support, and ultimately on one’s ability to integrate the loss. When this occurs, it is likely to lead to even more isolation, shame, and self-blame. It is not surprising that many clinicians consider leaving the profession after this type of experience.
Continue to: Effects on clinical work
Effects on clinical work
A suicide loss is also likely to affect a clinician’s therapeutic work. Many authors12,52,64-67 have found that this commonly leads therapists to question their abilities as clinicians, and to experience a sharp loss of confidence in their work with patients. The shattered beliefs and assumptions around the efficacy of the therapeutic process, a sense of guilt or self-blame, and any perceived or actual negative judgment from colleagues can dramatically compromise a clinician’s sense of competence. Hendin et al30 noted that even the most experienced therapists expressed difficulty in trusting their own clinical judgment, or accurately assessing risk after a suicide loss.
In addition, the common grief and trauma-related responses to a suicide loss (including shock, numbness, sadness, anxiety, and generalized distress) are likely to result in at least some temporary disruption of a clinician’s optimal functioning. If trauma-related symptoms are more pronounced, the effect and longevity of such impairment may be exacerbated, and are likely to “impair clinical response and therapeutic judgment.”15 In addition, because such symptoms and states may be triggered by exposure to other potentially suicidal patients, they are more likely to impact clinical functioning when the clinician works with suicidal individuals. Thus, the normative responses to a suicide loss are likely to impact a clinician’s work, just as they are likely to impact the personal and occupational functioning of any survivor of suicide loss.
In clinician-survivor discussions and support groups I’ve led, participants have identified many common areas of clinical impact. Perhaps one of the most common early responses reported by clinician-survivors who continued to work with potentially suicidal individuals was to become hypervigilant in relation to any perceived suicide risk, to interpret such risk in such a way as to warrant more conservative interventions than are necessary, and to consequently minimize the patient’s own capacities for self-care.68 Conversely, others reported a tendency to minimize or deny suicidal potential by, for example, avoiding asking patients directly about suicidal ideation, even when they later realized that such questioning was indicated.69
Suicide loss may also lead to more subtle clinical reactions that have been observed not only with suicidal patients, but also in relation to patients who struggle with loss or grief. These include avoidant or even dissociative reactions in relation to their patient’s pain, which in turn can impact the clinician’s ability to “be fully present” or empathic in clinical encounters.50,69 Still, other clinicians noted that they tended to project residual feelings of anger onto their current suicidal patients, or envied patients who seemed to have mastered their grief. Consistent with Maltsberger’s description of “atonement reactions,”40 some clinicians found themselves doing more than should be expected for their patients, even losing their sense of professional boundaries in the process. Anderson70 noted that in pushing herself beyond what she knew were her optimal clinical boundaries, she was “punishing herself” for failing to prevent her patient’s suicide because, as she realized, “doing ‘penance’ was better than feeling helpless and powerless.” And Schultz16 described how therapists may have subsequent difficulty in trusting other patients, especially if patients who completed suicide did not disclose or denied their suicidal intent.
Working toward a supportive solution
In summary, unless clinicians who lose a patient to suicide have more supportive experiences, the combination of confidentiality-related restrictions, confusion about legal/ethical repercussions, unsupportive reactions from colleagues, and unexpected impairments in clinical work are likely to lead to intensified distress, isolation, the perceived need to “hide” the impact in professional settings, and consideration of leaving the profession. However, as I will describe in Part 2 (
Bottom Line
For mental health clinicians, losing a patient to suicide is a clear occupational hazard. After a suicide loss, clinicians often experience unique personal and professional challenges, including the impact of the loss on clinical work and professional identity, legal/ethical issues, and confidentiality-related constraints on the ability to discuss and process the loss.
Related Resources
- American Association of Suicidology Clinician Survivor Task Force. www.cliniciansurvivor.org.
- Gutin N. Helping survivors in the aftermath of suicide loss. Current Psychiatry. 2018;17(8):27-33.
1. Alexander D, Klein S, Gray NM, et al. Suicide by patients: questionnaire study of its effect on consultant psychiatrists. BMJ. 2000;320(7249):1571-1574.
2. Chemtob CM, Hamada RS, Bauer G, et al. Patients’ suicides: frequency and impact on psychiatrists. Am J Psychiatry. 1988;145(2):224-228.
3. Grad OT, Zavasnik A, Groleger U. Suicide of a patient: gender differences in bereavement reactions of therapists. Suicide Life Threat Behav. 1997;27(4):379-386.
4. Ruskin R, Sakinofsky I, Bagby RM, et al. Impact of patient suicide on psychiatrists and psychiatric trainees. Acad Psychiatry. 2004;28(2):104-110.
5. Bersoff DN. Ethical conflicts in psychology, 2nd ed. Washington, DC: American Psychological Association; 1999.
6. Chemtob CM, Bauer GB, Hamada RS, et al. Patient suicide: occupational hazard for psychologists and psychiatrists. Prof Psychol Res Pr. 1989;20(5):294-300.
7. Rubin HL. Surviving a suicide in your practice. In: Blumenthal SJ, Kupfer DJ, eds. Suicide over the life cycle: risk factors, assessment, and treatment of suicidal patients. Washington, DC: American Psychiatric Press; 1990:619-636.
8. Kaye NS, Soreff SM. The psychiatrist’s role, responses, and responsibilities when a patient commits suicide. Am J Psychiatry. 1991;148(6):739-743.
9. McIntosh JL. Clinicians as survivors of suicide: bibliography. American Association of Suicidology Clinician Survivor Task Force. http://pages.iu.edu/~jmcintos/Surv.Ther.bib.htm. Updated May 19, 2019. Accessed August 26, 2019.
10. Douglas J, Brown HN. Suicide: understanding and responding: Harvard Medical School perspectives. Madison, CT: International Universities Press; 1989.
11. Farberow NL. The mental health professional as suicide survivor. Clin Neuropsychiatry. 2005;2(1):13-20.
12. Jones FA Jr. Therapists as survivors of patient suicide. In: Dunne EJ, McIntosh JL, Dunne-Maxim K, eds. Suicide and its aftermath: understanding and counseling the survivors. New York, NY: W.W. Norton; 1987;126-141.
13. Plakun EM, Tillman JG. Responding to clinicians after loss of a patient to suicide. Dir Psychiatry. 2005;25:301-310.
14. Prabhakar D, Anzia JM, Balon R, et al. “Collateral damages”: preparing residents for coping with patient suicide. Acad Psychiatry. 2013;37(6):429-30.
15. Quinnett P. QPR: for suicide prevention. QPR Institute, Inc. http://pages.iu.edu/~jmcintos/postvention.htm. Published September 21, 2009. Accessed August 26, 2019.
16. Schultz, D. Suggestions for supervisors when a therapist experiences a client’s suicide. Women Ther. 2005;28(1):59-69.
17. Spiegelman JS Jr, Werth JL Jr. Don’t forget about me: the experiences of therapists-in-training after a patient has attempted or died by suicide. Women Ther. 2005;28(1):35-57.
18. American Association of Suicidology. Clinician Survivor Task Force. Clinicians as survivors of suicide: postvention information.
19. Whitmore CA, Cook J, Salg L. Supporting residents in the wake of patient suicide. The American Journal of Psychiatry Residents’ Journal. 2017;12(1):5-7.
20. Melton B, Coverdale J. What do we teach psychiatric residents about suicide? A national survey of chief residents. Acad Psychiatry. 2009;33(1):47-50.
21. Valente SM. Psychotherapist reactions to the suicide of a patient. Am J Orthopsychiatry. 1994;64(4):614-621.
22. Jordan JR, McIntosh JL. Is suicide bereavement different? A framework for rethinking the question. In: Jordan JR, McIntosh JL, eds. Grief after suicide: understanding the consequences and caring for the survivors. New York, NY: Routledge; 2011:19-42.
23. Jordan JR, Baugher B. After suicide loss: coping with your grief, 2nd ed. Newcastle, WA: Caring People Press; 2016.
24. Brown HB. The impact of suicide on therapists in training. Compr Psychiatry. 1987;28(2):101-112.
25. Cvinar JG. Do suicide survivors suffer social stigma: a review of the literature. Perspect Psychiatr Care. 2005;41(1):14-21.
26. Feigelman W, Gorman BS, Jordan JR. Stigmatization and suicide bereavement. Death Stud. 2009;33(7):591-608.
27. Goffman E. Stigma: notes on the management of spoiled identity. New York, NY: Simon & Schuster; 1963.
28. Goldney RD. The privilege and responsibility of suicide prevention. Crisis. 2000;21(1):8-15.
29. Litman RE. When patients commit suicide. Am J Psychother. 1965;19(4):570-576.
30. Hendin H, Lipschitz A, Maltsberger JT, et al. Therapists’ reactions to patients’ suicides. Am J Psychiatry. 2000;157(12):2022-2027.
31. Rycroft P. Touching the heart and soul of therapy: surviving client suicide. Women Ther. 2004;28(1):83-94.
32. Ellis TE, Patel AB. Client suicide: what now? Cogn Behav Pract. 2012;19(2):277-287.
33. Al-Mateen CS, Jones K, Linker J, et al. Clinician response to a child who completes suicide. Child Adolesc Psychiatric Clin N Am. 2018;27(4):621-635.
34. Henry M, Séguin M, Drouin M-S. Mental health professionals’ response to the suicide of their patients [in French]. Revue Québécoise de Psychologie. 2004;25:241-257.
35. Carter RE. Some effects of client suicide on the therapist. Psychother Theory Res Practice. 1971;8(4):287-289.
36. Dewar I, Eagles J, Klein S, et al. Psychiatric trainees’ experiences of, and reactions to, patient suicide. Psychiatr Bull. 2000;24(1):20-23.
37. Gitlin M. Aftermath of a tragedy: reaction of psychiatrists to patient suicides. Psychiatr Ann. 2007;37(10):684-687.
38. Tillman JG. When a patient commits suicide: an empirical study of psychoanalytic clinicians. Inter J Psychoanal. 2006;87(1):159-177.
39. Erikson EH. Identity and the life cycle. New York, NY: International Universities Press, Inc.; 1959.
40. Maltsberger JT. The implications of patient suicide for the surviving psychotherapist. In: Jacobs D, ed. Suicide and clinical practice. Washington, DC: American Psychiatric Press; 1992:169-182.
41. Bissell BPH. The experience of the nurse therapist working with suicidal cases: a developmental study [dissertation]. Boston, MA: Boston University School of Education; 1981.
42. Kleespies PM. The stress of patient suicidal behavior: Implications for interns and training programs in psychology. Prof Psychol Res Pract. 1993;24(4):477-482.
43. Munson JS. Impact of client suicide on practitioner posttraumatic growth [dissertation]. Gainsville, Florida: University of Florida; 2009.
44. Hodgkinson PE. Responding to in-patient suicide. Br J Med Psychol. 1987;60(4):387-392.
45. Gorkin M. On the suicide of one’s patient. Bull Menninger Clin. 1985;49(1):1-9.
46. Fuentes MA, Cruz D. Posttraumatic growth: positive psychological changes after trauma. Mental Health News. 2009;11(1):31,37.
47. Doka KJ. Disenfranchised grief: new Directions, challenges, and strategies for practice. Champaign, IL: Research Press; 2002.
48. Bongar B. The suicidal patient: clinical and legal standards of care, 2nd ed. Washington, DC: American Psychological Association; 2002.
49. Peterson EM, Luoma JB, Dunne E. Suicide survivors’ perceptions of the treating clinician. Suicide Life Threat Behav. 2002;32(2):158-166.
50. Kolodny S, Binder RL, Bronstein AA, et al. The working through of patients’ suicides by four therapists. Suicide Life Threat Behav. 1979;9(1):33-46.
51. Marshall KA. When a patient commits suicide. Suicide Life Threat Behav. 1980;10(1):29-40.
52. Jobes DA, Maltsberger JT. The hazards of treating suicidal patients. In: Sussman MB, ed. A perilous calling: the hazards of psychotherapy practice. New York, NY: Wiley & Sons; 1995:200-214.
53. Castelli-Dransart DA, Gutjahr E, Gulfi A, et al. Patient suicide in institutions: emotional responses and traumatic impact on Swiss mental health professionals. Death Stud. 2014;38(1-5):315-321.
54. US Public Health Service. The Surgeon General’s call to action to prevent suicide. Washington, DC: Department of Health and Human Services; 1999.
55. Armour M. Violent death: understanding the context of traumatic and stigmatized grief. J Hum Behav Soc Environ. 2006;14(4):53-90.
56. Calhoun, LG, Allen BG. Social reactions to the survivor of a suicide in the family: a review of the literature. Omega (Westport). 1991;23(2):95-107.
57. Dunne EJ, McIntosh JL, Dunne-Maxim K, eds. Suicide and its aftermath: understanding and counseling the survivors. New York, NY: WW Norton & Co; 1987.
58. Harwood D, Hawton K, Hope J, et al. The grief experiences and needs of bereaved relatives and friends of older people dying through suicide: a descriptive and case-control study. J Affect Disord. 2002;72(2):185-194.
59. Jordan JR. Is suicide bereavement different? A reassessment of the literature. Suicide Life Threat Behav. 2001;31(1):91-102.
60. McIntosh JL. Control group studies of suicide survivors: a review and critique. Suicide Life Threat Behav. 2003;23(2):146-161.
61. Range LM. When a loss is due to suicide: unique aspects of bereavement. In: Harvey JH, ed. Perspectives on loss: a sourcebook. Philadelphia, PA: Brunner/Mazel; 1998:213-220.
62. Sveen CA, Walby FA. Suicide survivors’ mental health and grief reactions: a systematic review of controlled studies. Suicide Life Threat Behav. 2008;38(1):13-29.
63. Van Dongen CJ. Social context of postsuicide bereavement. Death Stud. 1993;17(2):125-141.
64. Bultema JK. The healing process for the multidisciplinary team: recovering post-inpatient suicide. J Psychosoc Nurs. 1994;32(2):19-24.
65. Cooper C. Patient suicide and assault: their impact on psychiatric hospital staff. J Psychosoc Nurs Ment Health Serv. 1995;33(6):26-29.
66. Foster VA, McAdams CR III. The impact of client suicide in counselor training: Implications for counselor education and supervision. Counselor Educ Supervision. 1999;39(1):22-33.
67. Little JD. Staff response to inpatient and outpatient suicide: what happened and what do we do? Aust N Z J Psychiatry. 1992;26(2):162-167.
68. Horn PJ. Therapists’ psychological adaptation to client suicidal behavior. Chicago, IL: Loyola University of Chicago; 1995.
69. Gutin N, McGann VM, Jordan JR. The impact of suicide on professional caregivers. In: Jordan J, McIntosh J, eds. Grief after suicide: understanding the consequences and caring for the survivors. New York, NY: Routledge; 2011:93-111.
70. Anderson GO. Who, what, when, where, how, and mostly why? A therapist’s grief over the suicide of a client. Women Ther. 2004;28(1):25-34.
Studies have found that 1 in 2 psychiatrists,1-4 and 1 in 5 psychologists, clinical social workers, and other mental health professionals,5 will lose a patient to suicide in the course of their career. This statistic suggests that losing a patient to suicide constitutes a clear occupational hazard.6,7 Despite this, most mental health professionals continue to view suicide loss as an aberration. Consequently, there is often a lack of preparedness for such an event when it does occur.
This 2-part article summarizes what is currently known about the unique personal and professional issues experienced by clinician-survivors (clinicians who have lost patients and/or loved ones to suicide). In Part 1, I cover:
- the impact of losing a patient to suicide
- confidentiality-related constraints on the ability to discuss and process the loss
- legal and ethical issues
- colleagues’ reactions and stigma
- the effects of a suicide loss on one’s clinical work.
Part 2 will discuss the opportunities for personal growth that can result from experiencing a suicide loss, guidelines for optimal postventions, and steps clinicians can take to help support colleagues who have lost a patient to suicide.
A neglected topic
For psychiatrists and other mental health professionals, the loss of a patient to suicide is certainly not uncommon.1-5 Despite this, coping with a patient’s suicide is a “neglected topic”8 in residency and general mental health training.
There are many published articles on clinicians experiencing suicide loss (for a comprehensive bibliography, see McIntosh9), and several authors10-19 have developed suggestions, guidelines, and detailed postvention protocols to help clinicians navigate the often-complicated sequelae to such a loss. However, these resources have generally not been integrated into clinical training, and tend to be poorly disseminated. In a national survey of chief residents, Melton and Coverdale20 found that only 25% of residency training programs covered topics related to postvention, and 72% of chief residents felt this topic needed more attention. Thus, despite the existence of guidelines for optimal postvention and support, clinicians are often left to cope with the consequences of this difficult loss on their own, and under less-than-optimal conditions.
A patient’s suicide typically affects clinicians on multiple levels, both personally and professionally. In this article, I highlight the range of normative responses, as well as the factors that may facilitate or inhibit subsequent healing and growth, with the hope that this knowledge may be utilized to help current and future generations of clinician-survivors obtain optimal support, and that institutions who treat potentially suicidal individuals will develop optimal postvention responses following a suicide loss. Many aspects of what this article discusses also apply to clinicians who have experienced a suicide loss in their personal or family life, as this also tends to “spill over” into one’s professional roles and identity.
Grief and other emotional effects
In many ways, clinicians’ responses after a patient’s suicide are similar to those of other survivors after the loss of a loved one to suicide.21 Chemtob et al2 found that approximately one-half of psychiatrists who lost a patient to suicide had scores on the Impact of an Event Scale that were comparable to those of a clinical population seeking treatment after the death of a parent.
Continue to: Jordan and McIntosh have detailed...
Jordan and McIntosh22 have detailed several elements and themes that differentiate suicide loss and its associated reactions from other types of loss and grief. In general, suicide loss is considered traumatic, and is often accompanied by intense confusion and existential questioning, reflecting a negative impact on one’s core beliefs and assumptive world. The subsequent need to address the myriad of “why” questions left in its wake are often tinted with what Jordan and Baugher23 term the “tyranny of hindsight,” and take the form of implicit guilt for “sins of omission or commission” in relation to the lost individual.
Responses to suicide loss typically include initial shock, denial and numbness, intense sadness, anxiety, anger, and intense distress. Consistent with the traumatic nature of the loss, survivors are also likely to experience posttraumatic stress disorder symptoms such as intrusive thoughts, avoidance, and dissociation. Survivors also commonly experience significant guilt and shame, and this is likely to be socially reinforced by the general stigma associated with suicide as well as the actual blaming and avoidance responses of others.24-27
Clinicians’ unique reactions
For clinicians, there are additional components that may further complicate or exacerbate these reactions and extend their duration. First and foremost, such a loss affects clinicians on both personal and professional levels, a phenomenon that Plakun and Tillman13 have termed a “twin bereavement.” Thus, in addition to the personal grief and trauma reactions entailed in losing a patient to suicide, this loss is likely to impact clinicians’ professional identities, their relationships with colleagues, and their clinical work.
Clinicians’ professional identities are often predicated on generally shared assumptions and beliefs that, as trained professionals, they should have the power, aptitude, and competence to heal, or at least improve, the lives of patients, to reduce their distress, and to provide safety. In addition, such assumptions about clinicians’ responsibility and ability to prevent suicide are often reinforced in the clinical literature.28,29
These assumptions are often challenged, if not shattered, when patients take their own lives. A clinician’s sense of professional responsibility, the guilt and self-blame that may accompany this, self-doubts about one’s skills and clinical competence, the fear of (and actual) blame of colleagues and family members, and the real or imagined threat of litigation may all greatly exacerbate a clinician’s distress.11
Continue to: Hendin et al found...
Hendin et al30 found that mental health therapists have described losing a patient as “the most profoundly disturbing event of their professional careers,” noting that one-third of these clinicians experienced severe distress that lasted at least 1 year beyond the initial loss. In a 2004 study, Ruskin et al4 similarly found that one-quarter of psychiatrists and psychiatric trainees noted that losing a patient had a “profound and enduring effect on them.” In her article on surviving a patient’s suicide, Rycroft31 describes a “professional void” following the loss of her patient, in which “the world had changed, nothing was predictable any more, and it was no longer safe to assume anything.” Additionally, many clinicians experience an “acute sense of aloneness and isolation” subsequent to the loss.32
Many clinicians have noted that they considered leaving the field after such a loss,33,34 and it is hypothesized that many may have done so.35-37 Others have noted that, at least temporarily, they stopped treating patients who were potentially suicidal.29,35
Box 1
Several authors have proposed general models for describing the suicide grief trajectories of clinicians after a suicide loss. Tillman38 identified distinct groups of responses to this event: traumatic, affective, those related to the treatment, those related to interactions with colleagues, liability concerns, and the impact on one’s professional philosophy. She also found that Erikson’s stages of identity39 provided an uncannily similar trajectory to the ways in which those who participated in her research—clinicians at a mental hospital—had attempted to cope with their patients’ deaths, noting that the “suicide of a patient may provoke a revisiting of Erikson’s psychosocial crises in a telescoped and accelerated fashion.”38
Maltsberger40 offered a detailed psychoanalytic analysis of the responses clinicians may manifest in relation to a suicide loss, including the initial narcissistic injury sustained in relation to their patient’s actions; the subsequent potential for melancholic, atonement, or avoidance reactions; and the eventual capacity for the resolution of these reactions.
Al-Mateen et al33 described 3 phases of the clinician’s reaction after losing a patient who was a child to suicide:
- initial, which includes trauma and shock
- turmoil, which includes emotional flooding and functional impairments
- new growth, in which clinicians are able to reflect on their experiences and implications for training and policy.
For each phase, they also described staff activities that would foster forward movement through the trajectory.
In a 1981 study, Bissell41 found that psychiatric nurses who had experienced patient completed suicides progressed through several developmental stages (naïveté, recognition, responsibility, individual choice) that enabled them to come to terms with their personal reactions and place the ultimate responsibility for the suicide with the patient.
After losing a patient to suicide, a clinician may experience grief that proceeds through specific stages (Box 133,38-41). Box 22-4,6,16,24,29,30,33,34,40,42-45 describes a wide range of factors that affect each clinician’s unique response to losing a patient to suicide.
Box 2
There are many factors that make the experience of losing a patient to suicide unique and variable for individual clinicians. These include the amount of a clinician’s professional training and experience, both in general and in working with potentially suicidal individuals. Chemtob et al2 found that trainees were more likely to experience patient suicide loss than more seasoned clinicians, and to experience more distress.4,30,42 Brown24 noted that many training programs were likely to assign the most “extraordinarily sick patients to inexperienced trainees.” He noted that because the skill level of trainees has not yet tempered their personal aspirations, they are likely to experience a patient’s suicide as a personal failure. However, in contrast to the findings of Kleespies,42 Hendin,30 Ruskin et al,4 and Brown24 suggested that the overall impact of a patient’s suicide may be greater for seasoned clinicians, when the “protective advantage” or “explanation” of being in training is no longer applicable. This appears consistent with Munson’s study,43 which found that a greater number of years of clinical experience prior to a suicide loss was negatively correlated with posttraumatic growth.
Other factors affecting a clinician’s grief response include the context in which the treatment occurred, such as inpatient, outpatient, clinic, private practice, etc.44; the presence and involvement of supportive mentors or supervisors16; the length and intensity of the clinical relationship6,29; countertransference issues40; whether the patient was a child33; and the time elapsed since the suicide occurred.
In addition, each clinician’s set of personal and life experiences can affect the way he/ she moves through the grieving process. Any previous trauma or losses, particularly prior exposure to suicide, will likely impact a clinician’s reaction to his/her current loss, as will any susceptibility to anxiety or depression. Gorkin45 has suggested that the degree of omnipotence in the clinician’s therapeutic strivings will affect his/her ability to accept the inherent ambiguity involved in suicide loss. Gender may also play a role: Henry et al34 found that female clinicians had higher levels of stress reactions, and Grad et al3 found that female clinicians felt more shame and guilt and professed more doubts about their professional competence than male clinicians, and were more than twice as likely as men to identify talking with colleagues as an effective coping strategy.
Continue to: Implications of confidentiality restrictions
Implications of confidentiality restrictions
Confidentiality issues, as well as advice from attorneys to limit the disclosure of information about a patient, are likely to preclude a clinician’s ability to talk freely about the patient, the therapeutic relationship, and his/her reactions to the loss, all of which are known to facilitate movement through the grief process.46
The development of trust and the sharing of pain are just 2 factors that can make the clinical encounter an intense emotional experience for both parties. Recent trends in the psychodynamic literature acknowledge the profundity and depth of the personal impact that patients have on the clinician, an impact that is neither pathological nor an indication of poor boundaries in the therapy dyad, but instead a recognition of how all aspects of the clinician’s person, whether consciously or not, are used within the context of a therapeutic relationship. Yet when clinicians lose a patient, confidentiality restrictions often leave them wondering if and where any aspects of their experiences can be shared. Legal counsel may advise a clinician against speaking to consultants or supervisors or even surviving family members for fear that these non-privileged communications are subject to discovery should any legal proceedings ensue. Furthermore, the usual grief rituals that facilitate the healing of loss and the processing of grief (eg, gathering with others who knew the deceased, sharing feelings and memories, attending memorials) are usually denied to the clinician, and are often compounded by the reactions of one’s professional colleagues, who tend not to view the therapist’s grief as “legitimate.” Thus, clinician-survivors, despite having experienced a profound and traumatic loss, have very few places where this may be processed or even validated. As one clinician in a clinician-survivors support group stated, “I felt like I was grieving in a vacuum, that I wasn’t allowed to talk about how much my patient meant to me or how I’m feeling about it.” The isolation of grieving alone is likely to be compounded by the general lack of resources for supporting clinicians after such a loss. In contrast to the general suicide “survivor” network of support groups for family members who have experienced a suicide loss, there is an almost complete lack of supportive resources for clinicians following such a loss, and most clinicians are not aware of the resources that are available, such as the Clinician Survivor Task Force of the American Association of Suicidology (Box 312).
Box 3
Frank Jones and Judy Meade founded the Clinician Survivor Task Force (CSTF) of the American Association of Suicidology (AAS) in 1987. As Jones noted, “clinicians who have lost patients to suicide need a place to acknowledge and carry forward their personal loss … to benefit both personally and professionally from the opportunity to talk with other therapists who have survived the loss of a patient through suicide.”12
Nina Gutin, PhD, and Vanessa McGann, PhD, have co-chaired the CSTF since 2003. It now supports clinicians who have lost patients and/or loved ones, with the recognition that both types of losses carry implications within clinical and professional domains. The CSTF provides a listserve, opportunities to participate in video support groups, and a web site (www. cliniciansurvivor.org) that provides information about the clinician-survivor experience, the opportunity to read and post narratives about one’s experience with suicide loss, an updated bibliography maintained by John McIntosh, PhD, a list of clinical contacts, and a link to several excellent postvention protocols. In addition, Drs. Gutin and McGann conduct clinician-survivor support activities at the annual AAS conference, and in their respective geographic areas.
Continue to: Doka has described...
Doka47 has described “disenfranchised grief” in which the bereaved person does not receive the type and quality of support accorded to other bereaved persons, and thus is likely to internalize the view that his/her grief is not legitimate, and to believe that sharing related distress is a shame-ridden liability. This clearly relates to the sense of profound isolation and distress often described by clinician-survivors.
Other legal/ethical issues
The clinician-survivor’s concern about litigation, or an actual lawsuit, is likely to produce intense anxiety. This common fear is both understandable and credible. According to Bongar,48 the most common malpractice lawsuits filed against clinicians are those that involve a patient’s suicide. Peterson et al49 found that 34% of surviving family members considered bringing a lawsuit against the clinician, and of these, 57% consulted a lawyer.
In addition, an institution’s concern about protecting itself from liability may compromise its ability to support the clinician or trainee who sustained the loss. As noted above, the potential prohibitions around discussing the case can compromise the grief process. Additionally, the fear of (or actual) legal reprisals against supervisors and the larger institution may engender angry and blaming responses toward the treating clinician. In a personal communication (April 2008), Quinnett described an incident in which a supervising psychologist stomped into the grieving therapist’s office unannounced and shouted, “Now look what you’ve done! You’re going to get me sued!”
Other studies29,50,51 note that clinician-survivors fear losing their job, and that their colleagues and supervisors will be reluctant to assign new patients to them. Spiegleman and Werth17 also note that trainees grapple with additional concerns over negative evaluations, suspension or termination from clinical sites or training programs, and a potential interruption of obtaining a degree. Such supervisory and institutional reactions are likely to intensify a clinician’s sense of shame and distress, and are antithetical to postvention responses that promote optimal personal and professional growth. Such negative reactions are also likely to contribute to a clinician or trainee’s subsequent reluctance to work with suicidal individuals, or their decision to discontinue their clinical work altogether. Lastly, other ethical issues, such as contact with the patient’s family following the suicide, attending the funeral, etc., are likely to be a source of additional anxiety and distress, particularly if the clinician needs to address these issues in isolation.
Professional relationships/colleagues’ reactions
Many clinician-survivors have described reactions from colleagues and supervisors that are hurtful and unsupportive. According to Jobes and Maltsberger,52 “the suicide death of a patient in active treatment is commonly taken as prima facie evidence that the therapist, somehow or another, has mismanaged the case,” and thus the clinician often faces unwarranted blame and censure from colleagues and supervisors. Hendin et al30 noted that many trainees found reactions by their institutions to be insensitive and unsupportive, one noting that the department’s review of the case “felt more like a tribunal or inquest.” In a personal communication (April 2008), Quinnett noted that many clinicians he interviewed following a suicide loss reported a pattern of isolation and interpersonal discomfort with their colleagues, who implicitly or explicitly expressed concerns about their competence. He described how a respected colleague received “no understanding, no support, only abuse” from her supervisors. Such responses, while perhaps surprising from mental health professionals, probably reflect the long-standing cultural attitude of social condemnation of suicide, and of those who are associated with it.
Continue to: Negative reactions from professional colleagues...
Negative reactions from professional colleagues are most likely to occur immediately after the suicide loss and/or during the course of a subsequent investigation or psychological autopsy. Castelli-Dransart et al53 found that the lack of institutional support after a clinician experiences a suicide loss contributed to significantly higher stress responses for impacted clinicians, and may lead to a well-founded ambivalence about disclosure to colleagues, and consequent resistance to seeking out optimal supervision/consultation or even personal therapy that could help the clinician gain clarity on the effects of these issues. Many mental health professionals have described how, after the distressing experience of losing a patient to suicide, they moved through this process in relative isolation and loneliness, feeling abandoned by their colleagues and by their own hopes and expectations for support.
Stigmatization. In clinical settings, when a patient in treatment completes suicide, the treating clinician becomes an easy scapegoat for family members and colleagues. To the extent that mental health professionals are not immune from the effects and imposition of stigma, this might also affect their previously mentioned tendency to project judgment, overtly or covertly, onto the treating clinician.
Stigma around suicide is well documented.25 In The Surgeon General’s Call to Action to Prevent Suicide,54 former Surgeon General David Satcher specifically described stigma around suicide as one of the biggest barriers to prevention. Studies have shown that individuals bereaved by suicide are also stigmatized, and that those who were in caregiving roles (parents, clinicians) are believed to be more psychologically disturbed, less likable, more blameworthy, and less worthy of receiving support than other bereaved individuals.25,55-63 These judgments often mirror survivors’ self-punitive assessments, which then become exacerbated by and intertwined with both externally imposed and internalized stigma. Hence, it is not uncommon for suicide survivors to question their own right to grieve, to report low expectations of social support, and to feel compelled to deny or hide the mode of death. Feigelman et al26 found that stigmatization after a suicide loss was specifically associated with ongoing grief difficulties, depression, and suicidal thinking.
In my long-term work with clinician-survivors, I’ve come to believe that in addition to stigma around suicide, there may also be stigma projected by colleagues in relation to a clinician’s perceived emotional vulnerability. A traumatized clinician potentially challenges the notion of the implicit dichotomy/power imbalance between professionals and the patients we treat: “Us”—the professional, competent, healthy, and benevolent clinicians who have the care to offer, and “Them”—our patients, being needy, pathological, looking to us for care. This “us/them” distinction may serve to bolster a clinician’s professional esteem and identity. But when one of “us” becomes one of “them”—when a professional colleague is perceived as being emotionally vulnerable—this can be threatening to the predicates of this distinction, leading to the need to put the affected clinician firmly into the “them” camp. Thus, unwarranted condemnations of the clinician-survivor’s handling of the case, and/or the pathologizing of their normative grief reactions after the suicide loss, can seem justified.
Stigma associated both with suicide and with professional vulnerability is likely to be internalized and to have a profound effect on the clinician’s decisions about disclosure, asking for support, and ultimately on one’s ability to integrate the loss. When this occurs, it is likely to lead to even more isolation, shame, and self-blame. It is not surprising that many clinicians consider leaving the profession after this type of experience.
Continue to: Effects on clinical work
Effects on clinical work
A suicide loss is also likely to affect a clinician’s therapeutic work. Many authors12,52,64-67 have found that this commonly leads therapists to question their abilities as clinicians, and to experience a sharp loss of confidence in their work with patients. The shattered beliefs and assumptions around the efficacy of the therapeutic process, a sense of guilt or self-blame, and any perceived or actual negative judgment from colleagues can dramatically compromise a clinician’s sense of competence. Hendin et al30 noted that even the most experienced therapists expressed difficulty in trusting their own clinical judgment, or accurately assessing risk after a suicide loss.
In addition, the common grief and trauma-related responses to a suicide loss (including shock, numbness, sadness, anxiety, and generalized distress) are likely to result in at least some temporary disruption of a clinician’s optimal functioning. If trauma-related symptoms are more pronounced, the effect and longevity of such impairment may be exacerbated, and are likely to “impair clinical response and therapeutic judgment.”15 In addition, because such symptoms and states may be triggered by exposure to other potentially suicidal patients, they are more likely to impact clinical functioning when the clinician works with suicidal individuals. Thus, the normative responses to a suicide loss are likely to impact a clinician’s work, just as they are likely to impact the personal and occupational functioning of any survivor of suicide loss.
In clinician-survivor discussions and support groups I’ve led, participants have identified many common areas of clinical impact. Perhaps one of the most common early responses reported by clinician-survivors who continued to work with potentially suicidal individuals was to become hypervigilant in relation to any perceived suicide risk, to interpret such risk in such a way as to warrant more conservative interventions than are necessary, and to consequently minimize the patient’s own capacities for self-care.68 Conversely, others reported a tendency to minimize or deny suicidal potential by, for example, avoiding asking patients directly about suicidal ideation, even when they later realized that such questioning was indicated.69
Suicide loss may also lead to more subtle clinical reactions that have been observed not only with suicidal patients, but also in relation to patients who struggle with loss or grief. These include avoidant or even dissociative reactions in relation to their patient’s pain, which in turn can impact the clinician’s ability to “be fully present” or empathic in clinical encounters.50,69 Still, other clinicians noted that they tended to project residual feelings of anger onto their current suicidal patients, or envied patients who seemed to have mastered their grief. Consistent with Maltsberger’s description of “atonement reactions,”40 some clinicians found themselves doing more than should be expected for their patients, even losing their sense of professional boundaries in the process. Anderson70 noted that in pushing herself beyond what she knew were her optimal clinical boundaries, she was “punishing herself” for failing to prevent her patient’s suicide because, as she realized, “doing ‘penance’ was better than feeling helpless and powerless.” And Schultz16 described how therapists may have subsequent difficulty in trusting other patients, especially if patients who completed suicide did not disclose or denied their suicidal intent.
Working toward a supportive solution
In summary, unless clinicians who lose a patient to suicide have more supportive experiences, the combination of confidentiality-related restrictions, confusion about legal/ethical repercussions, unsupportive reactions from colleagues, and unexpected impairments in clinical work are likely to lead to intensified distress, isolation, the perceived need to “hide” the impact in professional settings, and consideration of leaving the profession. However, as I will describe in Part 2 (
Bottom Line
For mental health clinicians, losing a patient to suicide is a clear occupational hazard. After a suicide loss, clinicians often experience unique personal and professional challenges, including the impact of the loss on clinical work and professional identity, legal/ethical issues, and confidentiality-related constraints on the ability to discuss and process the loss.
Related Resources
- American Association of Suicidology Clinician Survivor Task Force. www.cliniciansurvivor.org.
- Gutin N. Helping survivors in the aftermath of suicide loss. Current Psychiatry. 2018;17(8):27-33.
Studies have found that 1 in 2 psychiatrists,1-4 and 1 in 5 psychologists, clinical social workers, and other mental health professionals,5 will lose a patient to suicide in the course of their career. This statistic suggests that losing a patient to suicide constitutes a clear occupational hazard.6,7 Despite this, most mental health professionals continue to view suicide loss as an aberration. Consequently, there is often a lack of preparedness for such an event when it does occur.
This 2-part article summarizes what is currently known about the unique personal and professional issues experienced by clinician-survivors (clinicians who have lost patients and/or loved ones to suicide). In Part 1, I cover:
- the impact of losing a patient to suicide
- confidentiality-related constraints on the ability to discuss and process the loss
- legal and ethical issues
- colleagues’ reactions and stigma
- the effects of a suicide loss on one’s clinical work.
Part 2 will discuss the opportunities for personal growth that can result from experiencing a suicide loss, guidelines for optimal postventions, and steps clinicians can take to help support colleagues who have lost a patient to suicide.
A neglected topic
For psychiatrists and other mental health professionals, the loss of a patient to suicide is certainly not uncommon.1-5 Despite this, coping with a patient’s suicide is a “neglected topic”8 in residency and general mental health training.
There are many published articles on clinicians experiencing suicide loss (for a comprehensive bibliography, see McIntosh9), and several authors10-19 have developed suggestions, guidelines, and detailed postvention protocols to help clinicians navigate the often-complicated sequelae to such a loss. However, these resources have generally not been integrated into clinical training, and tend to be poorly disseminated. In a national survey of chief residents, Melton and Coverdale20 found that only 25% of residency training programs covered topics related to postvention, and 72% of chief residents felt this topic needed more attention. Thus, despite the existence of guidelines for optimal postvention and support, clinicians are often left to cope with the consequences of this difficult loss on their own, and under less-than-optimal conditions.
A patient’s suicide typically affects clinicians on multiple levels, both personally and professionally. In this article, I highlight the range of normative responses, as well as the factors that may facilitate or inhibit subsequent healing and growth, with the hope that this knowledge may be utilized to help current and future generations of clinician-survivors obtain optimal support, and that institutions who treat potentially suicidal individuals will develop optimal postvention responses following a suicide loss. Many aspects of what this article discusses also apply to clinicians who have experienced a suicide loss in their personal or family life, as this also tends to “spill over” into one’s professional roles and identity.
Grief and other emotional effects
In many ways, clinicians’ responses after a patient’s suicide are similar to those of other survivors after the loss of a loved one to suicide.21 Chemtob et al2 found that approximately one-half of psychiatrists who lost a patient to suicide had scores on the Impact of an Event Scale that were comparable to those of a clinical population seeking treatment after the death of a parent.
Continue to: Jordan and McIntosh have detailed...
Jordan and McIntosh22 have detailed several elements and themes that differentiate suicide loss and its associated reactions from other types of loss and grief. In general, suicide loss is considered traumatic, and is often accompanied by intense confusion and existential questioning, reflecting a negative impact on one’s core beliefs and assumptive world. The subsequent need to address the myriad of “why” questions left in its wake are often tinted with what Jordan and Baugher23 term the “tyranny of hindsight,” and take the form of implicit guilt for “sins of omission or commission” in relation to the lost individual.
Responses to suicide loss typically include initial shock, denial and numbness, intense sadness, anxiety, anger, and intense distress. Consistent with the traumatic nature of the loss, survivors are also likely to experience posttraumatic stress disorder symptoms such as intrusive thoughts, avoidance, and dissociation. Survivors also commonly experience significant guilt and shame, and this is likely to be socially reinforced by the general stigma associated with suicide as well as the actual blaming and avoidance responses of others.24-27
Clinicians’ unique reactions
For clinicians, there are additional components that may further complicate or exacerbate these reactions and extend their duration. First and foremost, such a loss affects clinicians on both personal and professional levels, a phenomenon that Plakun and Tillman13 have termed a “twin bereavement.” Thus, in addition to the personal grief and trauma reactions entailed in losing a patient to suicide, this loss is likely to impact clinicians’ professional identities, their relationships with colleagues, and their clinical work.
Clinicians’ professional identities are often predicated on generally shared assumptions and beliefs that, as trained professionals, they should have the power, aptitude, and competence to heal, or at least improve, the lives of patients, to reduce their distress, and to provide safety. In addition, such assumptions about clinicians’ responsibility and ability to prevent suicide are often reinforced in the clinical literature.28,29
These assumptions are often challenged, if not shattered, when patients take their own lives. A clinician’s sense of professional responsibility, the guilt and self-blame that may accompany this, self-doubts about one’s skills and clinical competence, the fear of (and actual) blame of colleagues and family members, and the real or imagined threat of litigation may all greatly exacerbate a clinician’s distress.11
Continue to: Hendin et al found...
Hendin et al30 found that mental health therapists have described losing a patient as “the most profoundly disturbing event of their professional careers,” noting that one-third of these clinicians experienced severe distress that lasted at least 1 year beyond the initial loss. In a 2004 study, Ruskin et al4 similarly found that one-quarter of psychiatrists and psychiatric trainees noted that losing a patient had a “profound and enduring effect on them.” In her article on surviving a patient’s suicide, Rycroft31 describes a “professional void” following the loss of her patient, in which “the world had changed, nothing was predictable any more, and it was no longer safe to assume anything.” Additionally, many clinicians experience an “acute sense of aloneness and isolation” subsequent to the loss.32
Many clinicians have noted that they considered leaving the field after such a loss,33,34 and it is hypothesized that many may have done so.35-37 Others have noted that, at least temporarily, they stopped treating patients who were potentially suicidal.29,35
Box 1
Several authors have proposed general models for describing the suicide grief trajectories of clinicians after a suicide loss. Tillman38 identified distinct groups of responses to this event: traumatic, affective, those related to the treatment, those related to interactions with colleagues, liability concerns, and the impact on one’s professional philosophy. She also found that Erikson’s stages of identity39 provided an uncannily similar trajectory to the ways in which those who participated in her research—clinicians at a mental hospital—had attempted to cope with their patients’ deaths, noting that the “suicide of a patient may provoke a revisiting of Erikson’s psychosocial crises in a telescoped and accelerated fashion.”38
Maltsberger40 offered a detailed psychoanalytic analysis of the responses clinicians may manifest in relation to a suicide loss, including the initial narcissistic injury sustained in relation to their patient’s actions; the subsequent potential for melancholic, atonement, or avoidance reactions; and the eventual capacity for the resolution of these reactions.
Al-Mateen et al33 described 3 phases of the clinician’s reaction after losing a patient who was a child to suicide:
- initial, which includes trauma and shock
- turmoil, which includes emotional flooding and functional impairments
- new growth, in which clinicians are able to reflect on their experiences and implications for training and policy.
For each phase, they also described staff activities that would foster forward movement through the trajectory.
In a 1981 study, Bissell41 found that psychiatric nurses who had experienced patient completed suicides progressed through several developmental stages (naïveté, recognition, responsibility, individual choice) that enabled them to come to terms with their personal reactions and place the ultimate responsibility for the suicide with the patient.
After losing a patient to suicide, a clinician may experience grief that proceeds through specific stages (Box 133,38-41). Box 22-4,6,16,24,29,30,33,34,40,42-45 describes a wide range of factors that affect each clinician’s unique response to losing a patient to suicide.
Box 2
There are many factors that make the experience of losing a patient to suicide unique and variable for individual clinicians. These include the amount of a clinician’s professional training and experience, both in general and in working with potentially suicidal individuals. Chemtob et al2 found that trainees were more likely to experience patient suicide loss than more seasoned clinicians, and to experience more distress.4,30,42 Brown24 noted that many training programs were likely to assign the most “extraordinarily sick patients to inexperienced trainees.” He noted that because the skill level of trainees has not yet tempered their personal aspirations, they are likely to experience a patient’s suicide as a personal failure. However, in contrast to the findings of Kleespies,42 Hendin,30 Ruskin et al,4 and Brown24 suggested that the overall impact of a patient’s suicide may be greater for seasoned clinicians, when the “protective advantage” or “explanation” of being in training is no longer applicable. This appears consistent with Munson’s study,43 which found that a greater number of years of clinical experience prior to a suicide loss was negatively correlated with posttraumatic growth.
Other factors affecting a clinician’s grief response include the context in which the treatment occurred, such as inpatient, outpatient, clinic, private practice, etc.44; the presence and involvement of supportive mentors or supervisors16; the length and intensity of the clinical relationship6,29; countertransference issues40; whether the patient was a child33; and the time elapsed since the suicide occurred.
In addition, each clinician’s set of personal and life experiences can affect the way he/ she moves through the grieving process. Any previous trauma or losses, particularly prior exposure to suicide, will likely impact a clinician’s reaction to his/her current loss, as will any susceptibility to anxiety or depression. Gorkin45 has suggested that the degree of omnipotence in the clinician’s therapeutic strivings will affect his/her ability to accept the inherent ambiguity involved in suicide loss. Gender may also play a role: Henry et al34 found that female clinicians had higher levels of stress reactions, and Grad et al3 found that female clinicians felt more shame and guilt and professed more doubts about their professional competence than male clinicians, and were more than twice as likely as men to identify talking with colleagues as an effective coping strategy.
Continue to: Implications of confidentiality restrictions
Implications of confidentiality restrictions
Confidentiality issues, as well as advice from attorneys to limit the disclosure of information about a patient, are likely to preclude a clinician’s ability to talk freely about the patient, the therapeutic relationship, and his/her reactions to the loss, all of which are known to facilitate movement through the grief process.46
The development of trust and the sharing of pain are just 2 factors that can make the clinical encounter an intense emotional experience for both parties. Recent trends in the psychodynamic literature acknowledge the profundity and depth of the personal impact that patients have on the clinician, an impact that is neither pathological nor an indication of poor boundaries in the therapy dyad, but instead a recognition of how all aspects of the clinician’s person, whether consciously or not, are used within the context of a therapeutic relationship. Yet when clinicians lose a patient, confidentiality restrictions often leave them wondering if and where any aspects of their experiences can be shared. Legal counsel may advise a clinician against speaking to consultants or supervisors or even surviving family members for fear that these non-privileged communications are subject to discovery should any legal proceedings ensue. Furthermore, the usual grief rituals that facilitate the healing of loss and the processing of grief (eg, gathering with others who knew the deceased, sharing feelings and memories, attending memorials) are usually denied to the clinician, and are often compounded by the reactions of one’s professional colleagues, who tend not to view the therapist’s grief as “legitimate.” Thus, clinician-survivors, despite having experienced a profound and traumatic loss, have very few places where this may be processed or even validated. As one clinician in a clinician-survivors support group stated, “I felt like I was grieving in a vacuum, that I wasn’t allowed to talk about how much my patient meant to me or how I’m feeling about it.” The isolation of grieving alone is likely to be compounded by the general lack of resources for supporting clinicians after such a loss. In contrast to the general suicide “survivor” network of support groups for family members who have experienced a suicide loss, there is an almost complete lack of supportive resources for clinicians following such a loss, and most clinicians are not aware of the resources that are available, such as the Clinician Survivor Task Force of the American Association of Suicidology (Box 312).
Box 3
Frank Jones and Judy Meade founded the Clinician Survivor Task Force (CSTF) of the American Association of Suicidology (AAS) in 1987. As Jones noted, “clinicians who have lost patients to suicide need a place to acknowledge and carry forward their personal loss … to benefit both personally and professionally from the opportunity to talk with other therapists who have survived the loss of a patient through suicide.”12
Nina Gutin, PhD, and Vanessa McGann, PhD, have co-chaired the CSTF since 2003. It now supports clinicians who have lost patients and/or loved ones, with the recognition that both types of losses carry implications within clinical and professional domains. The CSTF provides a listserve, opportunities to participate in video support groups, and a web site (www. cliniciansurvivor.org) that provides information about the clinician-survivor experience, the opportunity to read and post narratives about one’s experience with suicide loss, an updated bibliography maintained by John McIntosh, PhD, a list of clinical contacts, and a link to several excellent postvention protocols. In addition, Drs. Gutin and McGann conduct clinician-survivor support activities at the annual AAS conference, and in their respective geographic areas.
Continue to: Doka has described...
Doka47 has described “disenfranchised grief” in which the bereaved person does not receive the type and quality of support accorded to other bereaved persons, and thus is likely to internalize the view that his/her grief is not legitimate, and to believe that sharing related distress is a shame-ridden liability. This clearly relates to the sense of profound isolation and distress often described by clinician-survivors.
Other legal/ethical issues
The clinician-survivor’s concern about litigation, or an actual lawsuit, is likely to produce intense anxiety. This common fear is both understandable and credible. According to Bongar,48 the most common malpractice lawsuits filed against clinicians are those that involve a patient’s suicide. Peterson et al49 found that 34% of surviving family members considered bringing a lawsuit against the clinician, and of these, 57% consulted a lawyer.
In addition, an institution’s concern about protecting itself from liability may compromise its ability to support the clinician or trainee who sustained the loss. As noted above, the potential prohibitions around discussing the case can compromise the grief process. Additionally, the fear of (or actual) legal reprisals against supervisors and the larger institution may engender angry and blaming responses toward the treating clinician. In a personal communication (April 2008), Quinnett described an incident in which a supervising psychologist stomped into the grieving therapist’s office unannounced and shouted, “Now look what you’ve done! You’re going to get me sued!”
Other studies29,50,51 note that clinician-survivors fear losing their job, and that their colleagues and supervisors will be reluctant to assign new patients to them. Spiegleman and Werth17 also note that trainees grapple with additional concerns over negative evaluations, suspension or termination from clinical sites or training programs, and a potential interruption of obtaining a degree. Such supervisory and institutional reactions are likely to intensify a clinician’s sense of shame and distress, and are antithetical to postvention responses that promote optimal personal and professional growth. Such negative reactions are also likely to contribute to a clinician or trainee’s subsequent reluctance to work with suicidal individuals, or their decision to discontinue their clinical work altogether. Lastly, other ethical issues, such as contact with the patient’s family following the suicide, attending the funeral, etc., are likely to be a source of additional anxiety and distress, particularly if the clinician needs to address these issues in isolation.
Professional relationships/colleagues’ reactions
Many clinician-survivors have described reactions from colleagues and supervisors that are hurtful and unsupportive. According to Jobes and Maltsberger,52 “the suicide death of a patient in active treatment is commonly taken as prima facie evidence that the therapist, somehow or another, has mismanaged the case,” and thus the clinician often faces unwarranted blame and censure from colleagues and supervisors. Hendin et al30 noted that many trainees found reactions by their institutions to be insensitive and unsupportive, one noting that the department’s review of the case “felt more like a tribunal or inquest.” In a personal communication (April 2008), Quinnett noted that many clinicians he interviewed following a suicide loss reported a pattern of isolation and interpersonal discomfort with their colleagues, who implicitly or explicitly expressed concerns about their competence. He described how a respected colleague received “no understanding, no support, only abuse” from her supervisors. Such responses, while perhaps surprising from mental health professionals, probably reflect the long-standing cultural attitude of social condemnation of suicide, and of those who are associated with it.
Continue to: Negative reactions from professional colleagues...
Negative reactions from professional colleagues are most likely to occur immediately after the suicide loss and/or during the course of a subsequent investigation or psychological autopsy. Castelli-Dransart et al53 found that the lack of institutional support after a clinician experiences a suicide loss contributed to significantly higher stress responses for impacted clinicians, and may lead to a well-founded ambivalence about disclosure to colleagues, and consequent resistance to seeking out optimal supervision/consultation or even personal therapy that could help the clinician gain clarity on the effects of these issues. Many mental health professionals have described how, after the distressing experience of losing a patient to suicide, they moved through this process in relative isolation and loneliness, feeling abandoned by their colleagues and by their own hopes and expectations for support.
Stigmatization. In clinical settings, when a patient in treatment completes suicide, the treating clinician becomes an easy scapegoat for family members and colleagues. To the extent that mental health professionals are not immune from the effects and imposition of stigma, this might also affect their previously mentioned tendency to project judgment, overtly or covertly, onto the treating clinician.
Stigma around suicide is well documented.25 In The Surgeon General’s Call to Action to Prevent Suicide,54 former Surgeon General David Satcher specifically described stigma around suicide as one of the biggest barriers to prevention. Studies have shown that individuals bereaved by suicide are also stigmatized, and that those who were in caregiving roles (parents, clinicians) are believed to be more psychologically disturbed, less likable, more blameworthy, and less worthy of receiving support than other bereaved individuals.25,55-63 These judgments often mirror survivors’ self-punitive assessments, which then become exacerbated by and intertwined with both externally imposed and internalized stigma. Hence, it is not uncommon for suicide survivors to question their own right to grieve, to report low expectations of social support, and to feel compelled to deny or hide the mode of death. Feigelman et al26 found that stigmatization after a suicide loss was specifically associated with ongoing grief difficulties, depression, and suicidal thinking.
In my long-term work with clinician-survivors, I’ve come to believe that in addition to stigma around suicide, there may also be stigma projected by colleagues in relation to a clinician’s perceived emotional vulnerability. A traumatized clinician potentially challenges the notion of the implicit dichotomy/power imbalance between professionals and the patients we treat: “Us”—the professional, competent, healthy, and benevolent clinicians who have the care to offer, and “Them”—our patients, being needy, pathological, looking to us for care. This “us/them” distinction may serve to bolster a clinician’s professional esteem and identity. But when one of “us” becomes one of “them”—when a professional colleague is perceived as being emotionally vulnerable—this can be threatening to the predicates of this distinction, leading to the need to put the affected clinician firmly into the “them” camp. Thus, unwarranted condemnations of the clinician-survivor’s handling of the case, and/or the pathologizing of their normative grief reactions after the suicide loss, can seem justified.
Stigma associated both with suicide and with professional vulnerability is likely to be internalized and to have a profound effect on the clinician’s decisions about disclosure, asking for support, and ultimately on one’s ability to integrate the loss. When this occurs, it is likely to lead to even more isolation, shame, and self-blame. It is not surprising that many clinicians consider leaving the profession after this type of experience.
Continue to: Effects on clinical work
Effects on clinical work
A suicide loss is also likely to affect a clinician’s therapeutic work. Many authors12,52,64-67 have found that this commonly leads therapists to question their abilities as clinicians, and to experience a sharp loss of confidence in their work with patients. The shattered beliefs and assumptions around the efficacy of the therapeutic process, a sense of guilt or self-blame, and any perceived or actual negative judgment from colleagues can dramatically compromise a clinician’s sense of competence. Hendin et al30 noted that even the most experienced therapists expressed difficulty in trusting their own clinical judgment, or accurately assessing risk after a suicide loss.
In addition, the common grief and trauma-related responses to a suicide loss (including shock, numbness, sadness, anxiety, and generalized distress) are likely to result in at least some temporary disruption of a clinician’s optimal functioning. If trauma-related symptoms are more pronounced, the effect and longevity of such impairment may be exacerbated, and are likely to “impair clinical response and therapeutic judgment.”15 In addition, because such symptoms and states may be triggered by exposure to other potentially suicidal patients, they are more likely to impact clinical functioning when the clinician works with suicidal individuals. Thus, the normative responses to a suicide loss are likely to impact a clinician’s work, just as they are likely to impact the personal and occupational functioning of any survivor of suicide loss.
In clinician-survivor discussions and support groups I’ve led, participants have identified many common areas of clinical impact. Perhaps one of the most common early responses reported by clinician-survivors who continued to work with potentially suicidal individuals was to become hypervigilant in relation to any perceived suicide risk, to interpret such risk in such a way as to warrant more conservative interventions than are necessary, and to consequently minimize the patient’s own capacities for self-care.68 Conversely, others reported a tendency to minimize or deny suicidal potential by, for example, avoiding asking patients directly about suicidal ideation, even when they later realized that such questioning was indicated.69
Suicide loss may also lead to more subtle clinical reactions that have been observed not only with suicidal patients, but also in relation to patients who struggle with loss or grief. These include avoidant or even dissociative reactions in relation to their patient’s pain, which in turn can impact the clinician’s ability to “be fully present” or empathic in clinical encounters.50,69 Still, other clinicians noted that they tended to project residual feelings of anger onto their current suicidal patients, or envied patients who seemed to have mastered their grief. Consistent with Maltsberger’s description of “atonement reactions,”40 some clinicians found themselves doing more than should be expected for their patients, even losing their sense of professional boundaries in the process. Anderson70 noted that in pushing herself beyond what she knew were her optimal clinical boundaries, she was “punishing herself” for failing to prevent her patient’s suicide because, as she realized, “doing ‘penance’ was better than feeling helpless and powerless.” And Schultz16 described how therapists may have subsequent difficulty in trusting other patients, especially if patients who completed suicide did not disclose or denied their suicidal intent.
Working toward a supportive solution
In summary, unless clinicians who lose a patient to suicide have more supportive experiences, the combination of confidentiality-related restrictions, confusion about legal/ethical repercussions, unsupportive reactions from colleagues, and unexpected impairments in clinical work are likely to lead to intensified distress, isolation, the perceived need to “hide” the impact in professional settings, and consideration of leaving the profession. However, as I will describe in Part 2 (
Bottom Line
For mental health clinicians, losing a patient to suicide is a clear occupational hazard. After a suicide loss, clinicians often experience unique personal and professional challenges, including the impact of the loss on clinical work and professional identity, legal/ethical issues, and confidentiality-related constraints on the ability to discuss and process the loss.
Related Resources
- American Association of Suicidology Clinician Survivor Task Force. www.cliniciansurvivor.org.
- Gutin N. Helping survivors in the aftermath of suicide loss. Current Psychiatry. 2018;17(8):27-33.
1. Alexander D, Klein S, Gray NM, et al. Suicide by patients: questionnaire study of its effect on consultant psychiatrists. BMJ. 2000;320(7249):1571-1574.
2. Chemtob CM, Hamada RS, Bauer G, et al. Patients’ suicides: frequency and impact on psychiatrists. Am J Psychiatry. 1988;145(2):224-228.
3. Grad OT, Zavasnik A, Groleger U. Suicide of a patient: gender differences in bereavement reactions of therapists. Suicide Life Threat Behav. 1997;27(4):379-386.
4. Ruskin R, Sakinofsky I, Bagby RM, et al. Impact of patient suicide on psychiatrists and psychiatric trainees. Acad Psychiatry. 2004;28(2):104-110.
5. Bersoff DN. Ethical conflicts in psychology, 2nd ed. Washington, DC: American Psychological Association; 1999.
6. Chemtob CM, Bauer GB, Hamada RS, et al. Patient suicide: occupational hazard for psychologists and psychiatrists. Prof Psychol Res Pr. 1989;20(5):294-300.
7. Rubin HL. Surviving a suicide in your practice. In: Blumenthal SJ, Kupfer DJ, eds. Suicide over the life cycle: risk factors, assessment, and treatment of suicidal patients. Washington, DC: American Psychiatric Press; 1990:619-636.
8. Kaye NS, Soreff SM. The psychiatrist’s role, responses, and responsibilities when a patient commits suicide. Am J Psychiatry. 1991;148(6):739-743.
9. McIntosh JL. Clinicians as survivors of suicide: bibliography. American Association of Suicidology Clinician Survivor Task Force. http://pages.iu.edu/~jmcintos/Surv.Ther.bib.htm. Updated May 19, 2019. Accessed August 26, 2019.
10. Douglas J, Brown HN. Suicide: understanding and responding: Harvard Medical School perspectives. Madison, CT: International Universities Press; 1989.
11. Farberow NL. The mental health professional as suicide survivor. Clin Neuropsychiatry. 2005;2(1):13-20.
12. Jones FA Jr. Therapists as survivors of patient suicide. In: Dunne EJ, McIntosh JL, Dunne-Maxim K, eds. Suicide and its aftermath: understanding and counseling the survivors. New York, NY: W.W. Norton; 1987;126-141.
13. Plakun EM, Tillman JG. Responding to clinicians after loss of a patient to suicide. Dir Psychiatry. 2005;25:301-310.
14. Prabhakar D, Anzia JM, Balon R, et al. “Collateral damages”: preparing residents for coping with patient suicide. Acad Psychiatry. 2013;37(6):429-30.
15. Quinnett P. QPR: for suicide prevention. QPR Institute, Inc. http://pages.iu.edu/~jmcintos/postvention.htm. Published September 21, 2009. Accessed August 26, 2019.
16. Schultz, D. Suggestions for supervisors when a therapist experiences a client’s suicide. Women Ther. 2005;28(1):59-69.
17. Spiegelman JS Jr, Werth JL Jr. Don’t forget about me: the experiences of therapists-in-training after a patient has attempted or died by suicide. Women Ther. 2005;28(1):35-57.
18. American Association of Suicidology. Clinician Survivor Task Force. Clinicians as survivors of suicide: postvention information.
19. Whitmore CA, Cook J, Salg L. Supporting residents in the wake of patient suicide. The American Journal of Psychiatry Residents’ Journal. 2017;12(1):5-7.
20. Melton B, Coverdale J. What do we teach psychiatric residents about suicide? A national survey of chief residents. Acad Psychiatry. 2009;33(1):47-50.
21. Valente SM. Psychotherapist reactions to the suicide of a patient. Am J Orthopsychiatry. 1994;64(4):614-621.
22. Jordan JR, McIntosh JL. Is suicide bereavement different? A framework for rethinking the question. In: Jordan JR, McIntosh JL, eds. Grief after suicide: understanding the consequences and caring for the survivors. New York, NY: Routledge; 2011:19-42.
23. Jordan JR, Baugher B. After suicide loss: coping with your grief, 2nd ed. Newcastle, WA: Caring People Press; 2016.
24. Brown HB. The impact of suicide on therapists in training. Compr Psychiatry. 1987;28(2):101-112.
25. Cvinar JG. Do suicide survivors suffer social stigma: a review of the literature. Perspect Psychiatr Care. 2005;41(1):14-21.
26. Feigelman W, Gorman BS, Jordan JR. Stigmatization and suicide bereavement. Death Stud. 2009;33(7):591-608.
27. Goffman E. Stigma: notes on the management of spoiled identity. New York, NY: Simon & Schuster; 1963.
28. Goldney RD. The privilege and responsibility of suicide prevention. Crisis. 2000;21(1):8-15.
29. Litman RE. When patients commit suicide. Am J Psychother. 1965;19(4):570-576.
30. Hendin H, Lipschitz A, Maltsberger JT, et al. Therapists’ reactions to patients’ suicides. Am J Psychiatry. 2000;157(12):2022-2027.
31. Rycroft P. Touching the heart and soul of therapy: surviving client suicide. Women Ther. 2004;28(1):83-94.
32. Ellis TE, Patel AB. Client suicide: what now? Cogn Behav Pract. 2012;19(2):277-287.
33. Al-Mateen CS, Jones K, Linker J, et al. Clinician response to a child who completes suicide. Child Adolesc Psychiatric Clin N Am. 2018;27(4):621-635.
34. Henry M, Séguin M, Drouin M-S. Mental health professionals’ response to the suicide of their patients [in French]. Revue Québécoise de Psychologie. 2004;25:241-257.
35. Carter RE. Some effects of client suicide on the therapist. Psychother Theory Res Practice. 1971;8(4):287-289.
36. Dewar I, Eagles J, Klein S, et al. Psychiatric trainees’ experiences of, and reactions to, patient suicide. Psychiatr Bull. 2000;24(1):20-23.
37. Gitlin M. Aftermath of a tragedy: reaction of psychiatrists to patient suicides. Psychiatr Ann. 2007;37(10):684-687.
38. Tillman JG. When a patient commits suicide: an empirical study of psychoanalytic clinicians. Inter J Psychoanal. 2006;87(1):159-177.
39. Erikson EH. Identity and the life cycle. New York, NY: International Universities Press, Inc.; 1959.
40. Maltsberger JT. The implications of patient suicide for the surviving psychotherapist. In: Jacobs D, ed. Suicide and clinical practice. Washington, DC: American Psychiatric Press; 1992:169-182.
41. Bissell BPH. The experience of the nurse therapist working with suicidal cases: a developmental study [dissertation]. Boston, MA: Boston University School of Education; 1981.
42. Kleespies PM. The stress of patient suicidal behavior: Implications for interns and training programs in psychology. Prof Psychol Res Pract. 1993;24(4):477-482.
43. Munson JS. Impact of client suicide on practitioner posttraumatic growth [dissertation]. Gainsville, Florida: University of Florida; 2009.
44. Hodgkinson PE. Responding to in-patient suicide. Br J Med Psychol. 1987;60(4):387-392.
45. Gorkin M. On the suicide of one’s patient. Bull Menninger Clin. 1985;49(1):1-9.
46. Fuentes MA, Cruz D. Posttraumatic growth: positive psychological changes after trauma. Mental Health News. 2009;11(1):31,37.
47. Doka KJ. Disenfranchised grief: new Directions, challenges, and strategies for practice. Champaign, IL: Research Press; 2002.
48. Bongar B. The suicidal patient: clinical and legal standards of care, 2nd ed. Washington, DC: American Psychological Association; 2002.
49. Peterson EM, Luoma JB, Dunne E. Suicide survivors’ perceptions of the treating clinician. Suicide Life Threat Behav. 2002;32(2):158-166.
50. Kolodny S, Binder RL, Bronstein AA, et al. The working through of patients’ suicides by four therapists. Suicide Life Threat Behav. 1979;9(1):33-46.
51. Marshall KA. When a patient commits suicide. Suicide Life Threat Behav. 1980;10(1):29-40.
52. Jobes DA, Maltsberger JT. The hazards of treating suicidal patients. In: Sussman MB, ed. A perilous calling: the hazards of psychotherapy practice. New York, NY: Wiley & Sons; 1995:200-214.
53. Castelli-Dransart DA, Gutjahr E, Gulfi A, et al. Patient suicide in institutions: emotional responses and traumatic impact on Swiss mental health professionals. Death Stud. 2014;38(1-5):315-321.
54. US Public Health Service. The Surgeon General’s call to action to prevent suicide. Washington, DC: Department of Health and Human Services; 1999.
55. Armour M. Violent death: understanding the context of traumatic and stigmatized grief. J Hum Behav Soc Environ. 2006;14(4):53-90.
56. Calhoun, LG, Allen BG. Social reactions to the survivor of a suicide in the family: a review of the literature. Omega (Westport). 1991;23(2):95-107.
57. Dunne EJ, McIntosh JL, Dunne-Maxim K, eds. Suicide and its aftermath: understanding and counseling the survivors. New York, NY: WW Norton & Co; 1987.
58. Harwood D, Hawton K, Hope J, et al. The grief experiences and needs of bereaved relatives and friends of older people dying through suicide: a descriptive and case-control study. J Affect Disord. 2002;72(2):185-194.
59. Jordan JR. Is suicide bereavement different? A reassessment of the literature. Suicide Life Threat Behav. 2001;31(1):91-102.
60. McIntosh JL. Control group studies of suicide survivors: a review and critique. Suicide Life Threat Behav. 2003;23(2):146-161.
61. Range LM. When a loss is due to suicide: unique aspects of bereavement. In: Harvey JH, ed. Perspectives on loss: a sourcebook. Philadelphia, PA: Brunner/Mazel; 1998:213-220.
62. Sveen CA, Walby FA. Suicide survivors’ mental health and grief reactions: a systematic review of controlled studies. Suicide Life Threat Behav. 2008;38(1):13-29.
63. Van Dongen CJ. Social context of postsuicide bereavement. Death Stud. 1993;17(2):125-141.
64. Bultema JK. The healing process for the multidisciplinary team: recovering post-inpatient suicide. J Psychosoc Nurs. 1994;32(2):19-24.
65. Cooper C. Patient suicide and assault: their impact on psychiatric hospital staff. J Psychosoc Nurs Ment Health Serv. 1995;33(6):26-29.
66. Foster VA, McAdams CR III. The impact of client suicide in counselor training: Implications for counselor education and supervision. Counselor Educ Supervision. 1999;39(1):22-33.
67. Little JD. Staff response to inpatient and outpatient suicide: what happened and what do we do? Aust N Z J Psychiatry. 1992;26(2):162-167.
68. Horn PJ. Therapists’ psychological adaptation to client suicidal behavior. Chicago, IL: Loyola University of Chicago; 1995.
69. Gutin N, McGann VM, Jordan JR. The impact of suicide on professional caregivers. In: Jordan J, McIntosh J, eds. Grief after suicide: understanding the consequences and caring for the survivors. New York, NY: Routledge; 2011:93-111.
70. Anderson GO. Who, what, when, where, how, and mostly why? A therapist’s grief over the suicide of a client. Women Ther. 2004;28(1):25-34.
1. Alexander D, Klein S, Gray NM, et al. Suicide by patients: questionnaire study of its effect on consultant psychiatrists. BMJ. 2000;320(7249):1571-1574.
2. Chemtob CM, Hamada RS, Bauer G, et al. Patients’ suicides: frequency and impact on psychiatrists. Am J Psychiatry. 1988;145(2):224-228.
3. Grad OT, Zavasnik A, Groleger U. Suicide of a patient: gender differences in bereavement reactions of therapists. Suicide Life Threat Behav. 1997;27(4):379-386.
4. Ruskin R, Sakinofsky I, Bagby RM, et al. Impact of patient suicide on psychiatrists and psychiatric trainees. Acad Psychiatry. 2004;28(2):104-110.
5. Bersoff DN. Ethical conflicts in psychology, 2nd ed. Washington, DC: American Psychological Association; 1999.
6. Chemtob CM, Bauer GB, Hamada RS, et al. Patient suicide: occupational hazard for psychologists and psychiatrists. Prof Psychol Res Pr. 1989;20(5):294-300.
7. Rubin HL. Surviving a suicide in your practice. In: Blumenthal SJ, Kupfer DJ, eds. Suicide over the life cycle: risk factors, assessment, and treatment of suicidal patients. Washington, DC: American Psychiatric Press; 1990:619-636.
8. Kaye NS, Soreff SM. The psychiatrist’s role, responses, and responsibilities when a patient commits suicide. Am J Psychiatry. 1991;148(6):739-743.
9. McIntosh JL. Clinicians as survivors of suicide: bibliography. American Association of Suicidology Clinician Survivor Task Force. http://pages.iu.edu/~jmcintos/Surv.Ther.bib.htm. Updated May 19, 2019. Accessed August 26, 2019.
10. Douglas J, Brown HN. Suicide: understanding and responding: Harvard Medical School perspectives. Madison, CT: International Universities Press; 1989.
11. Farberow NL. The mental health professional as suicide survivor. Clin Neuropsychiatry. 2005;2(1):13-20.
12. Jones FA Jr. Therapists as survivors of patient suicide. In: Dunne EJ, McIntosh JL, Dunne-Maxim K, eds. Suicide and its aftermath: understanding and counseling the survivors. New York, NY: W.W. Norton; 1987;126-141.
13. Plakun EM, Tillman JG. Responding to clinicians after loss of a patient to suicide. Dir Psychiatry. 2005;25:301-310.
14. Prabhakar D, Anzia JM, Balon R, et al. “Collateral damages”: preparing residents for coping with patient suicide. Acad Psychiatry. 2013;37(6):429-30.
15. Quinnett P. QPR: for suicide prevention. QPR Institute, Inc. http://pages.iu.edu/~jmcintos/postvention.htm. Published September 21, 2009. Accessed August 26, 2019.
16. Schultz, D. Suggestions for supervisors when a therapist experiences a client’s suicide. Women Ther. 2005;28(1):59-69.
17. Spiegelman JS Jr, Werth JL Jr. Don’t forget about me: the experiences of therapists-in-training after a patient has attempted or died by suicide. Women Ther. 2005;28(1):35-57.
18. American Association of Suicidology. Clinician Survivor Task Force. Clinicians as survivors of suicide: postvention information.
19. Whitmore CA, Cook J, Salg L. Supporting residents in the wake of patient suicide. The American Journal of Psychiatry Residents’ Journal. 2017;12(1):5-7.
20. Melton B, Coverdale J. What do we teach psychiatric residents about suicide? A national survey of chief residents. Acad Psychiatry. 2009;33(1):47-50.
21. Valente SM. Psychotherapist reactions to the suicide of a patient. Am J Orthopsychiatry. 1994;64(4):614-621.
22. Jordan JR, McIntosh JL. Is suicide bereavement different? A framework for rethinking the question. In: Jordan JR, McIntosh JL, eds. Grief after suicide: understanding the consequences and caring for the survivors. New York, NY: Routledge; 2011:19-42.
23. Jordan JR, Baugher B. After suicide loss: coping with your grief, 2nd ed. Newcastle, WA: Caring People Press; 2016.
24. Brown HB. The impact of suicide on therapists in training. Compr Psychiatry. 1987;28(2):101-112.
25. Cvinar JG. Do suicide survivors suffer social stigma: a review of the literature. Perspect Psychiatr Care. 2005;41(1):14-21.
26. Feigelman W, Gorman BS, Jordan JR. Stigmatization and suicide bereavement. Death Stud. 2009;33(7):591-608.
27. Goffman E. Stigma: notes on the management of spoiled identity. New York, NY: Simon & Schuster; 1963.
28. Goldney RD. The privilege and responsibility of suicide prevention. Crisis. 2000;21(1):8-15.
29. Litman RE. When patients commit suicide. Am J Psychother. 1965;19(4):570-576.
30. Hendin H, Lipschitz A, Maltsberger JT, et al. Therapists’ reactions to patients’ suicides. Am J Psychiatry. 2000;157(12):2022-2027.
31. Rycroft P. Touching the heart and soul of therapy: surviving client suicide. Women Ther. 2004;28(1):83-94.
32. Ellis TE, Patel AB. Client suicide: what now? Cogn Behav Pract. 2012;19(2):277-287.
33. Al-Mateen CS, Jones K, Linker J, et al. Clinician response to a child who completes suicide. Child Adolesc Psychiatric Clin N Am. 2018;27(4):621-635.
34. Henry M, Séguin M, Drouin M-S. Mental health professionals’ response to the suicide of their patients [in French]. Revue Québécoise de Psychologie. 2004;25:241-257.
35. Carter RE. Some effects of client suicide on the therapist. Psychother Theory Res Practice. 1971;8(4):287-289.
36. Dewar I, Eagles J, Klein S, et al. Psychiatric trainees’ experiences of, and reactions to, patient suicide. Psychiatr Bull. 2000;24(1):20-23.
37. Gitlin M. Aftermath of a tragedy: reaction of psychiatrists to patient suicides. Psychiatr Ann. 2007;37(10):684-687.
38. Tillman JG. When a patient commits suicide: an empirical study of psychoanalytic clinicians. Inter J Psychoanal. 2006;87(1):159-177.
39. Erikson EH. Identity and the life cycle. New York, NY: International Universities Press, Inc.; 1959.
40. Maltsberger JT. The implications of patient suicide for the surviving psychotherapist. In: Jacobs D, ed. Suicide and clinical practice. Washington, DC: American Psychiatric Press; 1992:169-182.
41. Bissell BPH. The experience of the nurse therapist working with suicidal cases: a developmental study [dissertation]. Boston, MA: Boston University School of Education; 1981.
42. Kleespies PM. The stress of patient suicidal behavior: Implications for interns and training programs in psychology. Prof Psychol Res Pract. 1993;24(4):477-482.
43. Munson JS. Impact of client suicide on practitioner posttraumatic growth [dissertation]. Gainsville, Florida: University of Florida; 2009.
44. Hodgkinson PE. Responding to in-patient suicide. Br J Med Psychol. 1987;60(4):387-392.
45. Gorkin M. On the suicide of one’s patient. Bull Menninger Clin. 1985;49(1):1-9.
46. Fuentes MA, Cruz D. Posttraumatic growth: positive psychological changes after trauma. Mental Health News. 2009;11(1):31,37.
47. Doka KJ. Disenfranchised grief: new Directions, challenges, and strategies for practice. Champaign, IL: Research Press; 2002.
48. Bongar B. The suicidal patient: clinical and legal standards of care, 2nd ed. Washington, DC: American Psychological Association; 2002.
49. Peterson EM, Luoma JB, Dunne E. Suicide survivors’ perceptions of the treating clinician. Suicide Life Threat Behav. 2002;32(2):158-166.
50. Kolodny S, Binder RL, Bronstein AA, et al. The working through of patients’ suicides by four therapists. Suicide Life Threat Behav. 1979;9(1):33-46.
51. Marshall KA. When a patient commits suicide. Suicide Life Threat Behav. 1980;10(1):29-40.
52. Jobes DA, Maltsberger JT. The hazards of treating suicidal patients. In: Sussman MB, ed. A perilous calling: the hazards of psychotherapy practice. New York, NY: Wiley & Sons; 1995:200-214.
53. Castelli-Dransart DA, Gutjahr E, Gulfi A, et al. Patient suicide in institutions: emotional responses and traumatic impact on Swiss mental health professionals. Death Stud. 2014;38(1-5):315-321.
54. US Public Health Service. The Surgeon General’s call to action to prevent suicide. Washington, DC: Department of Health and Human Services; 1999.
55. Armour M. Violent death: understanding the context of traumatic and stigmatized grief. J Hum Behav Soc Environ. 2006;14(4):53-90.
56. Calhoun, LG, Allen BG. Social reactions to the survivor of a suicide in the family: a review of the literature. Omega (Westport). 1991;23(2):95-107.
57. Dunne EJ, McIntosh JL, Dunne-Maxim K, eds. Suicide and its aftermath: understanding and counseling the survivors. New York, NY: WW Norton & Co; 1987.
58. Harwood D, Hawton K, Hope J, et al. The grief experiences and needs of bereaved relatives and friends of older people dying through suicide: a descriptive and case-control study. J Affect Disord. 2002;72(2):185-194.
59. Jordan JR. Is suicide bereavement different? A reassessment of the literature. Suicide Life Threat Behav. 2001;31(1):91-102.
60. McIntosh JL. Control group studies of suicide survivors: a review and critique. Suicide Life Threat Behav. 2003;23(2):146-161.
61. Range LM. When a loss is due to suicide: unique aspects of bereavement. In: Harvey JH, ed. Perspectives on loss: a sourcebook. Philadelphia, PA: Brunner/Mazel; 1998:213-220.
62. Sveen CA, Walby FA. Suicide survivors’ mental health and grief reactions: a systematic review of controlled studies. Suicide Life Threat Behav. 2008;38(1):13-29.
63. Van Dongen CJ. Social context of postsuicide bereavement. Death Stud. 1993;17(2):125-141.
64. Bultema JK. The healing process for the multidisciplinary team: recovering post-inpatient suicide. J Psychosoc Nurs. 1994;32(2):19-24.
65. Cooper C. Patient suicide and assault: their impact on psychiatric hospital staff. J Psychosoc Nurs Ment Health Serv. 1995;33(6):26-29.
66. Foster VA, McAdams CR III. The impact of client suicide in counselor training: Implications for counselor education and supervision. Counselor Educ Supervision. 1999;39(1):22-33.
67. Little JD. Staff response to inpatient and outpatient suicide: what happened and what do we do? Aust N Z J Psychiatry. 1992;26(2):162-167.
68. Horn PJ. Therapists’ psychological adaptation to client suicidal behavior. Chicago, IL: Loyola University of Chicago; 1995.
69. Gutin N, McGann VM, Jordan JR. The impact of suicide on professional caregivers. In: Jordan J, McIntosh J, eds. Grief after suicide: understanding the consequences and caring for the survivors. New York, NY: Routledge; 2011:93-111.
70. Anderson GO. Who, what, when, where, how, and mostly why? A therapist’s grief over the suicide of a client. Women Ther. 2004;28(1):25-34.
Assessing decisional capacity in patients with substance use disorders
Ms. B, age 31, is brought to the emergency department (ED) via ambulance after emergency medical technicians used naloxone nasal spray to revive her following an overdose on heroin. She reports daily IV heroin use for the last 4 years as well as frequent use of other illicit substances, including marijuana and alprazolam, for which she does not have
How can you determine if Ms. B has the capacity to make decisions regarding her care?
Decisional capacity is defined as a patient’s ability to use information about an illness and the proposed treatment options to make a choice that is congruent with one’s own values and preferences.1 Determining whether a patient has adequate capacity to make decisions regarding their care is an inherent aspect of all clinician-patient interactions.
Published reports have focused on the challenges clinicians face when assessing decisional capacity in patients with psychiatric and cognitive disorders. However, there is little evidence about assessing decisional capacity in patients with substance use disorders (SUDs), even though increasing numbers of patients with SUDs are presenting to EDs2 and being admitted as inpatients in general hospitals.3 In this article, I discuss:
- the biologic basis for impaired decision-making in patients with SUDs
- common substance use–related conditions that may impact a patient’s decisional capacity
- the clinical challenges and legal considerations clinicians face when assessing decisional capacity in patients with SUDs
- how to assess decisional capacity in such patients.
Decisional capacity vs competence
“Capacity” and “competence” are not the same. Decisional capacity, which refers to the ability to make decisions, is a clinical construct that is determined by clinicians and is generally used in the acute clinical setting. Because cognition is the main determinant of capacity, conditions or treatments that affect cognition can impair an individual’s decision-making capacity.1 Decisional capacity is not a global concept but a decision-specific one, subject to fluctuations depending on the time and the nature of the decision at hand. Therefore, requests for determination of decisional capacity in the clinical setting should be specific to an individual decision or set of decisions.
In contrast, competence is an enduring legal determination of incapacitation, typically made by a probate judge. It refers to the ability of an individual to perform actions needed to put decisions into effect. Decisional capacity as assessed by a clinician often serves as the basis for petitions submitted for the purpose of competency adjudication by the judicial system.
A biologic basis for impaired decision-making?
Jeste and Saks4 suggested that addiction itself is characterized by impaired decision-making because individuals keep using a substance despite experiencing recurrent physical, psychologic, or social problems caused or worsened by the substance. Several studies suggest there may be a biologic basis for impaired decision-making in these patients, even in the absence of severe psychiatric or cognitive disorders.
Continue to: Bechara and Damasio found...
Bechara and Damasio5 found that the decision-making impairment seen in some patients with SUDs was similar to that observed in patients who have lesions of the ventromedial prefrontal cortex. In both groups of patients, the impaired decision-making was characterized by a preference to opt for high immediate reward despite even higher future losses.
These deficits were also observed by Grant et al.6 In this study, patients with SUDs displayed markedly impaired performance on the Gambling Task, which examines decisions that result in long-term losses that exceed short-term gains. However, patients with SUDs performed similarly to controls on the Wisconsin Card Sorting Test, which evaluates the ability to form abstract concepts and to shift from established response sets.
MacDonald et al7 used a laboratory experiment and 2 field studies to test the hypothesis that alcohol affects attitudes and intentions toward drinking and driving. Their findings support the concept that alcohol intoxication decreases cognitive capacity such that people are more likely to attend to only the most salient cues.7
Whether the impairment documented in such studies is a contributing factor in addiction or is a result of addiction remains uncertain. While individuals with SUDs may have some level of impairment in decision-making in general, particularly in regard to their substance use, their decisional capacity on specific clinical decisions should be assessed carefully. In a study of 300 consecutive psychiatric consultations for decisional capacity at an urban hospital, Boettger et al8 found that 41% were related to SUDs. Of these, 37% were found to have impaired decisional capacity.
Impaired decision-making in patients with SUDs may specifically pertain to choices related to their addiction, including9:
- consent for addiction treatment
- consistency in maintaining a choice of recovery
- changing values regarding treatment over time
- capacity to participate in addiction research involving the use of addictive substances.
Continue to: It is important to recognize...
It is important to recognize that this impairment may not necessarily translate into altered decisional capacity regarding other health care decisions, such as consenting to surgery or other necessary medical interventions.9
Substance-related disorders that affect decisional capacity
Substance-related syndromes can affect mood, reality testing, and/or cognitive function, thereby directly impacting a patient’s decisional capacity. Substance-related syndromes can be divided into 2 categories: 1) disorders resulting from the direct effects of the substance, and 2) secondary disorders resulting from/or associated with substance use.
Disorders resulting from the direct effects of the substance
Temporary/reversible incapacitation
- Acute intoxication or intoxication delirium may be the most frequent type of temporary incapacitation. It can result from toxic levels of licit or illicit substances; alcohol is likely the most frequent offending agent. Although some individuals who are intoxicated may appear to be alert, oriented, and able to engage in lengthy conversations, the majority do not possess adequate decisional capacity.10
- Withdrawal delirium, associated with longstanding alcohol, sedative-hypnotic, or barbiturate dependence, is typically prolonged, but usually resolves, either spontaneously or with treatment. Although most deliria resolve once the underlying etiology is corrected, vulnerable individuals may experience irreversible cognitive impairment and permanent decisional incapacitation.11,12
- Severe substance-induced depressive disorders, especially if accompanied by frank psychotic symptoms or severe depressive distortions of reality, may result in decisional incapacity. Substance abuse treatment that incorporates multiple strategies, sometimes in conjunction with pharmacotherapy to manage depression, should lead to sufficient recovery and restoration of decisional capacity.
- Transient psychotic disorders such as those associated with the use of stimulants are often treatable. Patients may recover decisional capacity spontaneously or with treatment.
Permanent incapacitation
- Dementia is associated with substance use, particularly alcohol use.13 For a patient who develops dementia, no appreciable recovery can be expected, even with prolonged abstinence.
- Persistent amnestic disorders (eg, Korsakoff syndrome) resulting from undiagnosed or untreated severe thiamine deficiency (Wernicke’s encephalopathy). Although an isolated Korsakoff syndrome consists primarily of anterograde amnesia, these patients may experience additional cognitive impairment resulting from years of alcohol consumption or associated with other neurodegenerative processes, and therefore are sufficiently impaired and lack decisional capacity. Even in the absence of such concomitant cognitive deficits, a very severe anterograde amnestic disorder directly impacts a patient’s capacity to perform the necessary tasks required to give informed consent. The inability to consolidate information about new medical developments, treatments, and procedures, even when they are thoroughly explained by the medical team, can pose serious challenges. For example, a patient may protest to being taken to surgery because he/she does not recall signing a consent form the previous day.
- Enduring severe and treatment-refractory psychotic disorders associated with drug use, specifically stimulants, can result in permanent incapacitation similar to that seen in severe primary psychotic disorders (such as treatment-resistant schizophrenia).
- Hepatic encephalopathy may be seen in patients with advanced cirrhosis of the liver (due to hepatitis C resulting from IV drug use, and/or alcohol use). In late stages of cirrhosis, the confusional state patients experience may become severe and may no longer be reversible unless liver transplantation is available and successful. This would therefore constitute a basis for permanent decisional incapacitation.
- Human immunodeficiency virus encephalitis or dementia can result from IV drug use.
Continue to: Clinical challenges
Clinical challenges
In intensive care settings, where a patient with a SUD may be treated for acute life-threatening intoxication or severe withdrawal delirium, an assumption of decisional incapacitation often exists as a result of medical acuity and impaired mentation. In these situations, treatment usually proceeds with consent obtained from next-of-kin, a guardian, or an administrative (hospital) authority when other substitute decision makers are unavailable or unwilling. In such cases, psychiatric consultation can play a dual role in documenting the patient’s decisional capacity and also in contributing to the care of patients with SUDs.
It is critical to perform a cognitive evaluation and mental status examination in a medically compromised patient with an SUD. Unfortunately, serious cognitive disorders can often be concealed by a superficially jovial or verbally skilled patient, or by an uncooperative individual who refuses to engage in a thorough conversation with his/her clinicians. These scenarios present significant challenges and may result in missed opportunities for care or premature discharges. Negative countertransference by clinicians toward patients with SUDs may also promote poor outcomes. For difficult cases, legal and ethical consultations may help mitigate risk and guide management approaches (Box14).
Box
The legal system rarely views patients with substance use disorders (SUDs) as lacking decisional capacity in the absence of overt psychiatric or cognitive deficits. The penal system offers little if any mitigation of liability on account of addiction in civil or criminal cases. On the contrary, intoxication is an aggravating factor in such settings. Despite extensive literature that questions the “free will,” accountability, and responsibility of patients with SUDs, the legal system takes an “all-or-none” approach to this issue. It assumes free choice and accountability for patients with SUDs, except when a clear superimposed psychiatric or cognitive disorder (such as psychosis or dementia) exists. Rarely, some jurisdictions may allow for mental health commitments on account of severe and persistent addictive behaviors that clearly pose a risk to the individual or to society, implicitly recognizing that incapacitation can result from severe addiction. Nevertheless, a finding of imminent or impending dangerousness is generally required for such commitments to be justified.
In other situations, individual health care settings may resort to local hospital policies that allow impaired patients with SUDs with a clearly altered mental status to be detained for the purpose of completing medical treatment. Presumably, discharge would occur when the medical and psychiatric acuity has resolved (often under the umbrella of a “Medical Hold” policy). Jain et al14 suggested that although such commitment laws for patients with SUDs may be appealing to some people, especially family members, specific statutes and their implementation are highly variable; the deprivation of liberty raises ethical concerns; and outcome data are limited. Conversely, most states either do not have such legislation, or rarely enforce it.
How to assess decisional capacity
A direct conclusion of incapacity in an individual cannot be determined solely on the knowledge of the patient having a SUD-related clinical condition. (The possible exception to this may be a patient with severe dementia.)
- understand the decision at hand
- discuss its benefits and risks
- describe alternatives
- demonstrate an appreciation of the implications of treatment or lack thereof
- communicate a clear and consistent choice.
Continue to: While most clinicians...
While most clinicians rely on a psychiatric interview (with or without a cognitive examination) to make these determinations, several instruments have been developed to aid these evaluations, such as the MacArthur Competence Assessment Tool for Treatment (Mac-CAT-T).15 In patients with potentially reversible incapacitating conditions, serial examinations over time, especially re-evaluation when a patient has achieved and maintained sobriety, may be necessary and helpful.
The Table offers a guide to assessing decisional capacity in a patient with an SUD.
Who should conduct the assessment?
Mental health professionals—usually psychiatrists or psychologists—are consulted when there is uncertainty about a patient’s decisional capacity, and when a more thorough mental status examination is warranted to formulate an informed opinion.16 Unfortunately, this typically occurs only if a patient refuses treatment or demands to be discharged before treatment has been completed, or there is a high level of risk to the patient or others after discharge.
In acute settings, when a patient consents to treatment, a psychiatric consultation regarding decisional capacity is rarely requested. While it is often tempting for medical or surgical teams to proceed with an intervention in a cooperative patient who willingly signs a consent form without a formal assessment of his/her decisional capacity, doing so raises challenging ethical and legal questions in the event of an adverse outcome. It is therefore prudent to strongly recommend that medical and surgical colleagues obtain a psychiatric consultation when an individual’s decisional capacity is uncertain, especially when a patient is known to have a psychiatric or neurocognitive disorder, or exhibits evidence of recent mental status changes. In cases of potentially reversible impairment (eg, delirium, psychosis, or acute anxiety), targeted interventions may help restore capacity and allow treatment to proceed.
No jurisdictions mandate that the determination of decisional capacity should be made exclusively by a mental health professional. Any treating health care professional (usually the attending physician) can make a determination of decisional capacity in scenarios where there is no overt evidence the patient has a mental or cognitive disorder and the patient is communicating clear and reasoned choices, or when a patient is profoundly impaired and no meaningful communication can take place.
Continue to: CASE CONTINUED
CASE CONTINUED
The emergency physician requests a psychiatric consultation. You assess Ms. B’s decisional capacity using the Mac-CAT-T along with a standard psychiatric evaluation. Her score of 14 reflects that she is able to understand the risks associated with her opioid use, and although irritated by engaging in such a discussion, is capable of reasoning through the various medical and psychosocial aspects of her addiction, and shows moderate appreciation of the impact of her choices on her future and that of significant others. The psychiatric evaluation fails to elicit any substantial mood, anxiety, or psychotic disorders associated with/or resulting from her addiction, and her cognitive examination is within normal limits. She does not exhibit severe withdrawal and is not delirious on examination. Finally, she did not harbor thoughts of intentional harm to self or others and is not deemed imminently dangerous.
You document that in your opinion, despite Ms. B’s unfortunate choices and questionable judgment, she does have the capacity to make informed decisions regarding her care and could be released against medical advice if she so chooses, while providing her with information about available resources should she decide to seek rehabilitation in the future.
An increasingly common scenario
Decisional capacity assessment in patients with SUDs is an increasingly common reason for psychiatric consultations. Primary and secondary conditions related to substance use can affect a patient’s decisional capacity on a temporary or permanent basis. The same principles that guide the assessment of decisional capacity in patients with other psychiatric or cognitive disorders should be applied to compromised individuals with SUDs. In challenging cases, a skilled psychiatric evaluation that is supported by a thorough cognitive examination and, when required, complemented by a legal or ethical consultation, can help clinicians make safe and judicious decisions.
Bottom Line
Assessing the decisional capacity of a patient with a substance use disorder can be challenging. Primary or secondary conditions related to substance use can affect a patient’s decisional capacity on a temporary or permanent basis. A skilled psychiatric evaluation that includes a thorough cognitive examination and is complemented by legal or ethical consultation can help in making judicious decisions.
Related Resources
- Tan SY. Determining patients’ decisional capacity. Clinical Psychiatry News. https://www.mdedge.com/psychiatry/article/137939/practice-management/determining-patients-decisional-capacity. Published May 10, 2017.
- Sorrentino R. Performing capacity evaluations: What’s expected from your consult. Current Psychiatry. 2014;13(1):41-44.
Drug Brand Names
Alprazolam • Xanax
Naloxone nasal spray • Narcan
1. Karlawish K. Assessment of decision-making capacity in adults. UpToDate. https://www.uptodate.com/contents/assessment-of-decision-making-capacity-in-adults. Updated July 2019. Accessed August 19, 2019.
2. Owens PL, Mutter R, Stocks C. Mental health and substance abuse-related emergency department visits among adults, 2007. HCUP Statistical Brief #92. https://www.ncbi.nlm.nih.gov/books/NBK52659/pdf/Bookshelf_NBK52659.pdf. Published July 2010. Accessed August 19, 2019.
3. Smothers BA, Yahr HT. Alcohol use disorder and illicit drug use in admissions to general hospitals in the United States. Am J Addict. 2005;14(3):256-267.
4. Jeste DV, Saks E. Decisional capacity in mental illness and substance use disorders: empirical database and policy implications. Behav Sci Law. 2006;24(4):607-628.
5. Bechara A, Damasio H. Decision-making and addiction (part I): impaired activation of somatic states in substance dependent individuals when pondering decisions with negative future consequences. Neuropsychologia. 2002;40(10):1675-1689.
6. Grant S, Contoreggi C, London ED. Drug abusers show impaired performance in a laboratory test of decision making. Neuropsychologia. 2000;38(8):1180-1187.
7. MacDonald TK, Zanna MP, Fong GT. Decision making in altered states: effects of alcohol on attitudes toward drinking and driving. J Pers Soc Psychol. 1995;68(6):973-985.
8. Boettger S, Bergman M, Jenewein J, et al. Assessment of decisional capacity: prevalence of medical illness and psychiatric comorbidities. Palliat Support Care. 2015;13(5):1275-1281.
9. Charland LC. Chapter 6: Decision-making capacity and responsibility in addiction. In: Poland J, Graham G. Addiction and responsibility. Cambridge, MA: MIT Press Scholarship Online; 2011:139-158.
10. Martel ML, Klein LR, Miner JR, et al. A brief assessment of capacity to consent instrument in acutely intoxicated emergency department patients. Am J Emerg Med. 2018;36(1):18-23.
11. MacLullich AM, Beaglehole A, Hall RJ, et al. Delirium and long-term cognitive impairment. Int Rev Psychiatry. 2009;21(1):30-42.
12. Pandharipande PP, Girard TD, Jackson JC, et al. Long-term cognitive impairment after critical illness. N Engl J Med. 2013;369(14):1306-1316.
13. Rehm J, Hasan OSM, Black SE, et al. Alcohol use and dementia: a systematic scoping review. Alzheimers Res Ther. 2019;11(1):1.
14. Jain A, Christopher P, Appelbaum PS. Civil commitment for opioid and other substance use disorders: does it work? Psychiatr Serv. 2018;69(4):374-376.
15. Grisso T, Appelbaum PS. Chapter 6: Using the MacArthur competence assessment tool – treatment. In: Grisso T, Appelbaum PS. Assessing competence to consent to treatment: a guide for physicians and other health professionals. New York, NY: Oxford University Press; 1998:101-126.
16. Hazelton LD, Sterns GL, Chisholm T. Decision-making capacity and alcohol abuse: clinical and ethical considerations in personal care choices. Gen Hosp Psychiatry. 2003;25(2):130-135.
Ms. B, age 31, is brought to the emergency department (ED) via ambulance after emergency medical technicians used naloxone nasal spray to revive her following an overdose on heroin. She reports daily IV heroin use for the last 4 years as well as frequent use of other illicit substances, including marijuana and alprazolam, for which she does not have
How can you determine if Ms. B has the capacity to make decisions regarding her care?
Decisional capacity is defined as a patient’s ability to use information about an illness and the proposed treatment options to make a choice that is congruent with one’s own values and preferences.1 Determining whether a patient has adequate capacity to make decisions regarding their care is an inherent aspect of all clinician-patient interactions.
Published reports have focused on the challenges clinicians face when assessing decisional capacity in patients with psychiatric and cognitive disorders. However, there is little evidence about assessing decisional capacity in patients with substance use disorders (SUDs), even though increasing numbers of patients with SUDs are presenting to EDs2 and being admitted as inpatients in general hospitals.3 In this article, I discuss:
- the biologic basis for impaired decision-making in patients with SUDs
- common substance use–related conditions that may impact a patient’s decisional capacity
- the clinical challenges and legal considerations clinicians face when assessing decisional capacity in patients with SUDs
- how to assess decisional capacity in such patients.
Decisional capacity vs competence
“Capacity” and “competence” are not the same. Decisional capacity, which refers to the ability to make decisions, is a clinical construct that is determined by clinicians and is generally used in the acute clinical setting. Because cognition is the main determinant of capacity, conditions or treatments that affect cognition can impair an individual’s decision-making capacity.1 Decisional capacity is not a global concept but a decision-specific one, subject to fluctuations depending on the time and the nature of the decision at hand. Therefore, requests for determination of decisional capacity in the clinical setting should be specific to an individual decision or set of decisions.
In contrast, competence is an enduring legal determination of incapacitation, typically made by a probate judge. It refers to the ability of an individual to perform actions needed to put decisions into effect. Decisional capacity as assessed by a clinician often serves as the basis for petitions submitted for the purpose of competency adjudication by the judicial system.
A biologic basis for impaired decision-making?
Jeste and Saks4 suggested that addiction itself is characterized by impaired decision-making because individuals keep using a substance despite experiencing recurrent physical, psychologic, or social problems caused or worsened by the substance. Several studies suggest there may be a biologic basis for impaired decision-making in these patients, even in the absence of severe psychiatric or cognitive disorders.
Continue to: Bechara and Damasio found...
Bechara and Damasio5 found that the decision-making impairment seen in some patients with SUDs was similar to that observed in patients who have lesions of the ventromedial prefrontal cortex. In both groups of patients, the impaired decision-making was characterized by a preference to opt for high immediate reward despite even higher future losses.
These deficits were also observed by Grant et al.6 In this study, patients with SUDs displayed markedly impaired performance on the Gambling Task, which examines decisions that result in long-term losses that exceed short-term gains. However, patients with SUDs performed similarly to controls on the Wisconsin Card Sorting Test, which evaluates the ability to form abstract concepts and to shift from established response sets.
MacDonald et al7 used a laboratory experiment and 2 field studies to test the hypothesis that alcohol affects attitudes and intentions toward drinking and driving. Their findings support the concept that alcohol intoxication decreases cognitive capacity such that people are more likely to attend to only the most salient cues.7
Whether the impairment documented in such studies is a contributing factor in addiction or is a result of addiction remains uncertain. While individuals with SUDs may have some level of impairment in decision-making in general, particularly in regard to their substance use, their decisional capacity on specific clinical decisions should be assessed carefully. In a study of 300 consecutive psychiatric consultations for decisional capacity at an urban hospital, Boettger et al8 found that 41% were related to SUDs. Of these, 37% were found to have impaired decisional capacity.
Impaired decision-making in patients with SUDs may specifically pertain to choices related to their addiction, including9:
- consent for addiction treatment
- consistency in maintaining a choice of recovery
- changing values regarding treatment over time
- capacity to participate in addiction research involving the use of addictive substances.
Continue to: It is important to recognize...
It is important to recognize that this impairment may not necessarily translate into altered decisional capacity regarding other health care decisions, such as consenting to surgery or other necessary medical interventions.9
Substance-related disorders that affect decisional capacity
Substance-related syndromes can affect mood, reality testing, and/or cognitive function, thereby directly impacting a patient’s decisional capacity. Substance-related syndromes can be divided into 2 categories: 1) disorders resulting from the direct effects of the substance, and 2) secondary disorders resulting from/or associated with substance use.
Disorders resulting from the direct effects of the substance
Temporary/reversible incapacitation
- Acute intoxication or intoxication delirium may be the most frequent type of temporary incapacitation. It can result from toxic levels of licit or illicit substances; alcohol is likely the most frequent offending agent. Although some individuals who are intoxicated may appear to be alert, oriented, and able to engage in lengthy conversations, the majority do not possess adequate decisional capacity.10
- Withdrawal delirium, associated with longstanding alcohol, sedative-hypnotic, or barbiturate dependence, is typically prolonged, but usually resolves, either spontaneously or with treatment. Although most deliria resolve once the underlying etiology is corrected, vulnerable individuals may experience irreversible cognitive impairment and permanent decisional incapacitation.11,12
- Severe substance-induced depressive disorders, especially if accompanied by frank psychotic symptoms or severe depressive distortions of reality, may result in decisional incapacity. Substance abuse treatment that incorporates multiple strategies, sometimes in conjunction with pharmacotherapy to manage depression, should lead to sufficient recovery and restoration of decisional capacity.
- Transient psychotic disorders such as those associated with the use of stimulants are often treatable. Patients may recover decisional capacity spontaneously or with treatment.
Permanent incapacitation
- Dementia is associated with substance use, particularly alcohol use.13 For a patient who develops dementia, no appreciable recovery can be expected, even with prolonged abstinence.
- Persistent amnestic disorders (eg, Korsakoff syndrome) resulting from undiagnosed or untreated severe thiamine deficiency (Wernicke’s encephalopathy). Although an isolated Korsakoff syndrome consists primarily of anterograde amnesia, these patients may experience additional cognitive impairment resulting from years of alcohol consumption or associated with other neurodegenerative processes, and therefore are sufficiently impaired and lack decisional capacity. Even in the absence of such concomitant cognitive deficits, a very severe anterograde amnestic disorder directly impacts a patient’s capacity to perform the necessary tasks required to give informed consent. The inability to consolidate information about new medical developments, treatments, and procedures, even when they are thoroughly explained by the medical team, can pose serious challenges. For example, a patient may protest to being taken to surgery because he/she does not recall signing a consent form the previous day.
- Enduring severe and treatment-refractory psychotic disorders associated with drug use, specifically stimulants, can result in permanent incapacitation similar to that seen in severe primary psychotic disorders (such as treatment-resistant schizophrenia).
- Hepatic encephalopathy may be seen in patients with advanced cirrhosis of the liver (due to hepatitis C resulting from IV drug use, and/or alcohol use). In late stages of cirrhosis, the confusional state patients experience may become severe and may no longer be reversible unless liver transplantation is available and successful. This would therefore constitute a basis for permanent decisional incapacitation.
- Human immunodeficiency virus encephalitis or dementia can result from IV drug use.
Continue to: Clinical challenges
Clinical challenges
In intensive care settings, where a patient with a SUD may be treated for acute life-threatening intoxication or severe withdrawal delirium, an assumption of decisional incapacitation often exists as a result of medical acuity and impaired mentation. In these situations, treatment usually proceeds with consent obtained from next-of-kin, a guardian, or an administrative (hospital) authority when other substitute decision makers are unavailable or unwilling. In such cases, psychiatric consultation can play a dual role in documenting the patient’s decisional capacity and also in contributing to the care of patients with SUDs.
It is critical to perform a cognitive evaluation and mental status examination in a medically compromised patient with an SUD. Unfortunately, serious cognitive disorders can often be concealed by a superficially jovial or verbally skilled patient, or by an uncooperative individual who refuses to engage in a thorough conversation with his/her clinicians. These scenarios present significant challenges and may result in missed opportunities for care or premature discharges. Negative countertransference by clinicians toward patients with SUDs may also promote poor outcomes. For difficult cases, legal and ethical consultations may help mitigate risk and guide management approaches (Box14).
Box
The legal system rarely views patients with substance use disorders (SUDs) as lacking decisional capacity in the absence of overt psychiatric or cognitive deficits. The penal system offers little if any mitigation of liability on account of addiction in civil or criminal cases. On the contrary, intoxication is an aggravating factor in such settings. Despite extensive literature that questions the “free will,” accountability, and responsibility of patients with SUDs, the legal system takes an “all-or-none” approach to this issue. It assumes free choice and accountability for patients with SUDs, except when a clear superimposed psychiatric or cognitive disorder (such as psychosis or dementia) exists. Rarely, some jurisdictions may allow for mental health commitments on account of severe and persistent addictive behaviors that clearly pose a risk to the individual or to society, implicitly recognizing that incapacitation can result from severe addiction. Nevertheless, a finding of imminent or impending dangerousness is generally required for such commitments to be justified.
In other situations, individual health care settings may resort to local hospital policies that allow impaired patients with SUDs with a clearly altered mental status to be detained for the purpose of completing medical treatment. Presumably, discharge would occur when the medical and psychiatric acuity has resolved (often under the umbrella of a “Medical Hold” policy). Jain et al14 suggested that although such commitment laws for patients with SUDs may be appealing to some people, especially family members, specific statutes and their implementation are highly variable; the deprivation of liberty raises ethical concerns; and outcome data are limited. Conversely, most states either do not have such legislation, or rarely enforce it.
How to assess decisional capacity
A direct conclusion of incapacity in an individual cannot be determined solely on the knowledge of the patient having a SUD-related clinical condition. (The possible exception to this may be a patient with severe dementia.)
- understand the decision at hand
- discuss its benefits and risks
- describe alternatives
- demonstrate an appreciation of the implications of treatment or lack thereof
- communicate a clear and consistent choice.
Continue to: While most clinicians...
While most clinicians rely on a psychiatric interview (with or without a cognitive examination) to make these determinations, several instruments have been developed to aid these evaluations, such as the MacArthur Competence Assessment Tool for Treatment (Mac-CAT-T).15 In patients with potentially reversible incapacitating conditions, serial examinations over time, especially re-evaluation when a patient has achieved and maintained sobriety, may be necessary and helpful.
The Table offers a guide to assessing decisional capacity in a patient with an SUD.
Who should conduct the assessment?
Mental health professionals—usually psychiatrists or psychologists—are consulted when there is uncertainty about a patient’s decisional capacity, and when a more thorough mental status examination is warranted to formulate an informed opinion.16 Unfortunately, this typically occurs only if a patient refuses treatment or demands to be discharged before treatment has been completed, or there is a high level of risk to the patient or others after discharge.
In acute settings, when a patient consents to treatment, a psychiatric consultation regarding decisional capacity is rarely requested. While it is often tempting for medical or surgical teams to proceed with an intervention in a cooperative patient who willingly signs a consent form without a formal assessment of his/her decisional capacity, doing so raises challenging ethical and legal questions in the event of an adverse outcome. It is therefore prudent to strongly recommend that medical and surgical colleagues obtain a psychiatric consultation when an individual’s decisional capacity is uncertain, especially when a patient is known to have a psychiatric or neurocognitive disorder, or exhibits evidence of recent mental status changes. In cases of potentially reversible impairment (eg, delirium, psychosis, or acute anxiety), targeted interventions may help restore capacity and allow treatment to proceed.
No jurisdictions mandate that the determination of decisional capacity should be made exclusively by a mental health professional. Any treating health care professional (usually the attending physician) can make a determination of decisional capacity in scenarios where there is no overt evidence the patient has a mental or cognitive disorder and the patient is communicating clear and reasoned choices, or when a patient is profoundly impaired and no meaningful communication can take place.
Continue to: CASE CONTINUED
CASE CONTINUED
The emergency physician requests a psychiatric consultation. You assess Ms. B’s decisional capacity using the Mac-CAT-T along with a standard psychiatric evaluation. Her score of 14 reflects that she is able to understand the risks associated with her opioid use, and although irritated by engaging in such a discussion, is capable of reasoning through the various medical and psychosocial aspects of her addiction, and shows moderate appreciation of the impact of her choices on her future and that of significant others. The psychiatric evaluation fails to elicit any substantial mood, anxiety, or psychotic disorders associated with/or resulting from her addiction, and her cognitive examination is within normal limits. She does not exhibit severe withdrawal and is not delirious on examination. Finally, she did not harbor thoughts of intentional harm to self or others and is not deemed imminently dangerous.
You document that in your opinion, despite Ms. B’s unfortunate choices and questionable judgment, she does have the capacity to make informed decisions regarding her care and could be released against medical advice if she so chooses, while providing her with information about available resources should she decide to seek rehabilitation in the future.
An increasingly common scenario
Decisional capacity assessment in patients with SUDs is an increasingly common reason for psychiatric consultations. Primary and secondary conditions related to substance use can affect a patient’s decisional capacity on a temporary or permanent basis. The same principles that guide the assessment of decisional capacity in patients with other psychiatric or cognitive disorders should be applied to compromised individuals with SUDs. In challenging cases, a skilled psychiatric evaluation that is supported by a thorough cognitive examination and, when required, complemented by a legal or ethical consultation, can help clinicians make safe and judicious decisions.
Bottom Line
Assessing the decisional capacity of a patient with a substance use disorder can be challenging. Primary or secondary conditions related to substance use can affect a patient’s decisional capacity on a temporary or permanent basis. A skilled psychiatric evaluation that includes a thorough cognitive examination and is complemented by legal or ethical consultation can help in making judicious decisions.
Related Resources
- Tan SY. Determining patients’ decisional capacity. Clinical Psychiatry News. https://www.mdedge.com/psychiatry/article/137939/practice-management/determining-patients-decisional-capacity. Published May 10, 2017.
- Sorrentino R. Performing capacity evaluations: What’s expected from your consult. Current Psychiatry. 2014;13(1):41-44.
Drug Brand Names
Alprazolam • Xanax
Naloxone nasal spray • Narcan
Ms. B, age 31, is brought to the emergency department (ED) via ambulance after emergency medical technicians used naloxone nasal spray to revive her following an overdose on heroin. She reports daily IV heroin use for the last 4 years as well as frequent use of other illicit substances, including marijuana and alprazolam, for which she does not have
How can you determine if Ms. B has the capacity to make decisions regarding her care?
Decisional capacity is defined as a patient’s ability to use information about an illness and the proposed treatment options to make a choice that is congruent with one’s own values and preferences.1 Determining whether a patient has adequate capacity to make decisions regarding their care is an inherent aspect of all clinician-patient interactions.
Published reports have focused on the challenges clinicians face when assessing decisional capacity in patients with psychiatric and cognitive disorders. However, there is little evidence about assessing decisional capacity in patients with substance use disorders (SUDs), even though increasing numbers of patients with SUDs are presenting to EDs2 and being admitted as inpatients in general hospitals.3 In this article, I discuss:
- the biologic basis for impaired decision-making in patients with SUDs
- common substance use–related conditions that may impact a patient’s decisional capacity
- the clinical challenges and legal considerations clinicians face when assessing decisional capacity in patients with SUDs
- how to assess decisional capacity in such patients.
Decisional capacity vs competence
“Capacity” and “competence” are not the same. Decisional capacity, which refers to the ability to make decisions, is a clinical construct that is determined by clinicians and is generally used in the acute clinical setting. Because cognition is the main determinant of capacity, conditions or treatments that affect cognition can impair an individual’s decision-making capacity.1 Decisional capacity is not a global concept but a decision-specific one, subject to fluctuations depending on the time and the nature of the decision at hand. Therefore, requests for determination of decisional capacity in the clinical setting should be specific to an individual decision or set of decisions.
In contrast, competence is an enduring legal determination of incapacitation, typically made by a probate judge. It refers to the ability of an individual to perform actions needed to put decisions into effect. Decisional capacity as assessed by a clinician often serves as the basis for petitions submitted for the purpose of competency adjudication by the judicial system.
A biologic basis for impaired decision-making?
Jeste and Saks4 suggested that addiction itself is characterized by impaired decision-making because individuals keep using a substance despite experiencing recurrent physical, psychologic, or social problems caused or worsened by the substance. Several studies suggest there may be a biologic basis for impaired decision-making in these patients, even in the absence of severe psychiatric or cognitive disorders.
Continue to: Bechara and Damasio found...
Bechara and Damasio5 found that the decision-making impairment seen in some patients with SUDs was similar to that observed in patients who have lesions of the ventromedial prefrontal cortex. In both groups of patients, the impaired decision-making was characterized by a preference to opt for high immediate reward despite even higher future losses.
These deficits were also observed by Grant et al.6 In this study, patients with SUDs displayed markedly impaired performance on the Gambling Task, which examines decisions that result in long-term losses that exceed short-term gains. However, patients with SUDs performed similarly to controls on the Wisconsin Card Sorting Test, which evaluates the ability to form abstract concepts and to shift from established response sets.
MacDonald et al7 used a laboratory experiment and 2 field studies to test the hypothesis that alcohol affects attitudes and intentions toward drinking and driving. Their findings support the concept that alcohol intoxication decreases cognitive capacity such that people are more likely to attend to only the most salient cues.7
Whether the impairment documented in such studies is a contributing factor in addiction or is a result of addiction remains uncertain. While individuals with SUDs may have some level of impairment in decision-making in general, particularly in regard to their substance use, their decisional capacity on specific clinical decisions should be assessed carefully. In a study of 300 consecutive psychiatric consultations for decisional capacity at an urban hospital, Boettger et al8 found that 41% were related to SUDs. Of these, 37% were found to have impaired decisional capacity.
Impaired decision-making in patients with SUDs may specifically pertain to choices related to their addiction, including9:
- consent for addiction treatment
- consistency in maintaining a choice of recovery
- changing values regarding treatment over time
- capacity to participate in addiction research involving the use of addictive substances.
Continue to: It is important to recognize...
It is important to recognize that this impairment may not necessarily translate into altered decisional capacity regarding other health care decisions, such as consenting to surgery or other necessary medical interventions.9
Substance-related disorders that affect decisional capacity
Substance-related syndromes can affect mood, reality testing, and/or cognitive function, thereby directly impacting a patient’s decisional capacity. Substance-related syndromes can be divided into 2 categories: 1) disorders resulting from the direct effects of the substance, and 2) secondary disorders resulting from/or associated with substance use.
Disorders resulting from the direct effects of the substance
Temporary/reversible incapacitation
- Acute intoxication or intoxication delirium may be the most frequent type of temporary incapacitation. It can result from toxic levels of licit or illicit substances; alcohol is likely the most frequent offending agent. Although some individuals who are intoxicated may appear to be alert, oriented, and able to engage in lengthy conversations, the majority do not possess adequate decisional capacity.10
- Withdrawal delirium, associated with longstanding alcohol, sedative-hypnotic, or barbiturate dependence, is typically prolonged, but usually resolves, either spontaneously or with treatment. Although most deliria resolve once the underlying etiology is corrected, vulnerable individuals may experience irreversible cognitive impairment and permanent decisional incapacitation.11,12
- Severe substance-induced depressive disorders, especially if accompanied by frank psychotic symptoms or severe depressive distortions of reality, may result in decisional incapacity. Substance abuse treatment that incorporates multiple strategies, sometimes in conjunction with pharmacotherapy to manage depression, should lead to sufficient recovery and restoration of decisional capacity.
- Transient psychotic disorders such as those associated with the use of stimulants are often treatable. Patients may recover decisional capacity spontaneously or with treatment.
Permanent incapacitation
- Dementia is associated with substance use, particularly alcohol use.13 For a patient who develops dementia, no appreciable recovery can be expected, even with prolonged abstinence.
- Persistent amnestic disorders (eg, Korsakoff syndrome) resulting from undiagnosed or untreated severe thiamine deficiency (Wernicke’s encephalopathy). Although an isolated Korsakoff syndrome consists primarily of anterograde amnesia, these patients may experience additional cognitive impairment resulting from years of alcohol consumption or associated with other neurodegenerative processes, and therefore are sufficiently impaired and lack decisional capacity. Even in the absence of such concomitant cognitive deficits, a very severe anterograde amnestic disorder directly impacts a patient’s capacity to perform the necessary tasks required to give informed consent. The inability to consolidate information about new medical developments, treatments, and procedures, even when they are thoroughly explained by the medical team, can pose serious challenges. For example, a patient may protest to being taken to surgery because he/she does not recall signing a consent form the previous day.
- Enduring severe and treatment-refractory psychotic disorders associated with drug use, specifically stimulants, can result in permanent incapacitation similar to that seen in severe primary psychotic disorders (such as treatment-resistant schizophrenia).
- Hepatic encephalopathy may be seen in patients with advanced cirrhosis of the liver (due to hepatitis C resulting from IV drug use, and/or alcohol use). In late stages of cirrhosis, the confusional state patients experience may become severe and may no longer be reversible unless liver transplantation is available and successful. This would therefore constitute a basis for permanent decisional incapacitation.
- Human immunodeficiency virus encephalitis or dementia can result from IV drug use.
Continue to: Clinical challenges
Clinical challenges
In intensive care settings, where a patient with a SUD may be treated for acute life-threatening intoxication or severe withdrawal delirium, an assumption of decisional incapacitation often exists as a result of medical acuity and impaired mentation. In these situations, treatment usually proceeds with consent obtained from next-of-kin, a guardian, or an administrative (hospital) authority when other substitute decision makers are unavailable or unwilling. In such cases, psychiatric consultation can play a dual role in documenting the patient’s decisional capacity and also in contributing to the care of patients with SUDs.
It is critical to perform a cognitive evaluation and mental status examination in a medically compromised patient with an SUD. Unfortunately, serious cognitive disorders can often be concealed by a superficially jovial or verbally skilled patient, or by an uncooperative individual who refuses to engage in a thorough conversation with his/her clinicians. These scenarios present significant challenges and may result in missed opportunities for care or premature discharges. Negative countertransference by clinicians toward patients with SUDs may also promote poor outcomes. For difficult cases, legal and ethical consultations may help mitigate risk and guide management approaches (Box14).
Box
The legal system rarely views patients with substance use disorders (SUDs) as lacking decisional capacity in the absence of overt psychiatric or cognitive deficits. The penal system offers little if any mitigation of liability on account of addiction in civil or criminal cases. On the contrary, intoxication is an aggravating factor in such settings. Despite extensive literature that questions the “free will,” accountability, and responsibility of patients with SUDs, the legal system takes an “all-or-none” approach to this issue. It assumes free choice and accountability for patients with SUDs, except when a clear superimposed psychiatric or cognitive disorder (such as psychosis or dementia) exists. Rarely, some jurisdictions may allow for mental health commitments on account of severe and persistent addictive behaviors that clearly pose a risk to the individual or to society, implicitly recognizing that incapacitation can result from severe addiction. Nevertheless, a finding of imminent or impending dangerousness is generally required for such commitments to be justified.
In other situations, individual health care settings may resort to local hospital policies that allow impaired patients with SUDs with a clearly altered mental status to be detained for the purpose of completing medical treatment. Presumably, discharge would occur when the medical and psychiatric acuity has resolved (often under the umbrella of a “Medical Hold” policy). Jain et al14 suggested that although such commitment laws for patients with SUDs may be appealing to some people, especially family members, specific statutes and their implementation are highly variable; the deprivation of liberty raises ethical concerns; and outcome data are limited. Conversely, most states either do not have such legislation, or rarely enforce it.
How to assess decisional capacity
A direct conclusion of incapacity in an individual cannot be determined solely on the knowledge of the patient having a SUD-related clinical condition. (The possible exception to this may be a patient with severe dementia.)
- understand the decision at hand
- discuss its benefits and risks
- describe alternatives
- demonstrate an appreciation of the implications of treatment or lack thereof
- communicate a clear and consistent choice.
Continue to: While most clinicians...
While most clinicians rely on a psychiatric interview (with or without a cognitive examination) to make these determinations, several instruments have been developed to aid these evaluations, such as the MacArthur Competence Assessment Tool for Treatment (Mac-CAT-T).15 In patients with potentially reversible incapacitating conditions, serial examinations over time, especially re-evaluation when a patient has achieved and maintained sobriety, may be necessary and helpful.
The Table offers a guide to assessing decisional capacity in a patient with an SUD.
Who should conduct the assessment?
Mental health professionals—usually psychiatrists or psychologists—are consulted when there is uncertainty about a patient’s decisional capacity, and when a more thorough mental status examination is warranted to formulate an informed opinion.16 Unfortunately, this typically occurs only if a patient refuses treatment or demands to be discharged before treatment has been completed, or there is a high level of risk to the patient or others after discharge.
In acute settings, when a patient consents to treatment, a psychiatric consultation regarding decisional capacity is rarely requested. While it is often tempting for medical or surgical teams to proceed with an intervention in a cooperative patient who willingly signs a consent form without a formal assessment of his/her decisional capacity, doing so raises challenging ethical and legal questions in the event of an adverse outcome. It is therefore prudent to strongly recommend that medical and surgical colleagues obtain a psychiatric consultation when an individual’s decisional capacity is uncertain, especially when a patient is known to have a psychiatric or neurocognitive disorder, or exhibits evidence of recent mental status changes. In cases of potentially reversible impairment (eg, delirium, psychosis, or acute anxiety), targeted interventions may help restore capacity and allow treatment to proceed.
No jurisdictions mandate that the determination of decisional capacity should be made exclusively by a mental health professional. Any treating health care professional (usually the attending physician) can make a determination of decisional capacity in scenarios where there is no overt evidence the patient has a mental or cognitive disorder and the patient is communicating clear and reasoned choices, or when a patient is profoundly impaired and no meaningful communication can take place.
Continue to: CASE CONTINUED
CASE CONTINUED
The emergency physician requests a psychiatric consultation. You assess Ms. B’s decisional capacity using the Mac-CAT-T along with a standard psychiatric evaluation. Her score of 14 reflects that she is able to understand the risks associated with her opioid use, and although irritated by engaging in such a discussion, is capable of reasoning through the various medical and psychosocial aspects of her addiction, and shows moderate appreciation of the impact of her choices on her future and that of significant others. The psychiatric evaluation fails to elicit any substantial mood, anxiety, or psychotic disorders associated with/or resulting from her addiction, and her cognitive examination is within normal limits. She does not exhibit severe withdrawal and is not delirious on examination. Finally, she did not harbor thoughts of intentional harm to self or others and is not deemed imminently dangerous.
You document that in your opinion, despite Ms. B’s unfortunate choices and questionable judgment, she does have the capacity to make informed decisions regarding her care and could be released against medical advice if she so chooses, while providing her with information about available resources should she decide to seek rehabilitation in the future.
An increasingly common scenario
Decisional capacity assessment in patients with SUDs is an increasingly common reason for psychiatric consultations. Primary and secondary conditions related to substance use can affect a patient’s decisional capacity on a temporary or permanent basis. The same principles that guide the assessment of decisional capacity in patients with other psychiatric or cognitive disorders should be applied to compromised individuals with SUDs. In challenging cases, a skilled psychiatric evaluation that is supported by a thorough cognitive examination and, when required, complemented by a legal or ethical consultation, can help clinicians make safe and judicious decisions.
Bottom Line
Assessing the decisional capacity of a patient with a substance use disorder can be challenging. Primary or secondary conditions related to substance use can affect a patient’s decisional capacity on a temporary or permanent basis. A skilled psychiatric evaluation that includes a thorough cognitive examination and is complemented by legal or ethical consultation can help in making judicious decisions.
Related Resources
- Tan SY. Determining patients’ decisional capacity. Clinical Psychiatry News. https://www.mdedge.com/psychiatry/article/137939/practice-management/determining-patients-decisional-capacity. Published May 10, 2017.
- Sorrentino R. Performing capacity evaluations: What’s expected from your consult. Current Psychiatry. 2014;13(1):41-44.
Drug Brand Names
Alprazolam • Xanax
Naloxone nasal spray • Narcan
1. Karlawish K. Assessment of decision-making capacity in adults. UpToDate. https://www.uptodate.com/contents/assessment-of-decision-making-capacity-in-adults. Updated July 2019. Accessed August 19, 2019.
2. Owens PL, Mutter R, Stocks C. Mental health and substance abuse-related emergency department visits among adults, 2007. HCUP Statistical Brief #92. https://www.ncbi.nlm.nih.gov/books/NBK52659/pdf/Bookshelf_NBK52659.pdf. Published July 2010. Accessed August 19, 2019.
3. Smothers BA, Yahr HT. Alcohol use disorder and illicit drug use in admissions to general hospitals in the United States. Am J Addict. 2005;14(3):256-267.
4. Jeste DV, Saks E. Decisional capacity in mental illness and substance use disorders: empirical database and policy implications. Behav Sci Law. 2006;24(4):607-628.
5. Bechara A, Damasio H. Decision-making and addiction (part I): impaired activation of somatic states in substance dependent individuals when pondering decisions with negative future consequences. Neuropsychologia. 2002;40(10):1675-1689.
6. Grant S, Contoreggi C, London ED. Drug abusers show impaired performance in a laboratory test of decision making. Neuropsychologia. 2000;38(8):1180-1187.
7. MacDonald TK, Zanna MP, Fong GT. Decision making in altered states: effects of alcohol on attitudes toward drinking and driving. J Pers Soc Psychol. 1995;68(6):973-985.
8. Boettger S, Bergman M, Jenewein J, et al. Assessment of decisional capacity: prevalence of medical illness and psychiatric comorbidities. Palliat Support Care. 2015;13(5):1275-1281.
9. Charland LC. Chapter 6: Decision-making capacity and responsibility in addiction. In: Poland J, Graham G. Addiction and responsibility. Cambridge, MA: MIT Press Scholarship Online; 2011:139-158.
10. Martel ML, Klein LR, Miner JR, et al. A brief assessment of capacity to consent instrument in acutely intoxicated emergency department patients. Am J Emerg Med. 2018;36(1):18-23.
11. MacLullich AM, Beaglehole A, Hall RJ, et al. Delirium and long-term cognitive impairment. Int Rev Psychiatry. 2009;21(1):30-42.
12. Pandharipande PP, Girard TD, Jackson JC, et al. Long-term cognitive impairment after critical illness. N Engl J Med. 2013;369(14):1306-1316.
13. Rehm J, Hasan OSM, Black SE, et al. Alcohol use and dementia: a systematic scoping review. Alzheimers Res Ther. 2019;11(1):1.
14. Jain A, Christopher P, Appelbaum PS. Civil commitment for opioid and other substance use disorders: does it work? Psychiatr Serv. 2018;69(4):374-376.
15. Grisso T, Appelbaum PS. Chapter 6: Using the MacArthur competence assessment tool – treatment. In: Grisso T, Appelbaum PS. Assessing competence to consent to treatment: a guide for physicians and other health professionals. New York, NY: Oxford University Press; 1998:101-126.
16. Hazelton LD, Sterns GL, Chisholm T. Decision-making capacity and alcohol abuse: clinical and ethical considerations in personal care choices. Gen Hosp Psychiatry. 2003;25(2):130-135.
1. Karlawish K. Assessment of decision-making capacity in adults. UpToDate. https://www.uptodate.com/contents/assessment-of-decision-making-capacity-in-adults. Updated July 2019. Accessed August 19, 2019.
2. Owens PL, Mutter R, Stocks C. Mental health and substance abuse-related emergency department visits among adults, 2007. HCUP Statistical Brief #92. https://www.ncbi.nlm.nih.gov/books/NBK52659/pdf/Bookshelf_NBK52659.pdf. Published July 2010. Accessed August 19, 2019.
3. Smothers BA, Yahr HT. Alcohol use disorder and illicit drug use in admissions to general hospitals in the United States. Am J Addict. 2005;14(3):256-267.
4. Jeste DV, Saks E. Decisional capacity in mental illness and substance use disorders: empirical database and policy implications. Behav Sci Law. 2006;24(4):607-628.
5. Bechara A, Damasio H. Decision-making and addiction (part I): impaired activation of somatic states in substance dependent individuals when pondering decisions with negative future consequences. Neuropsychologia. 2002;40(10):1675-1689.
6. Grant S, Contoreggi C, London ED. Drug abusers show impaired performance in a laboratory test of decision making. Neuropsychologia. 2000;38(8):1180-1187.
7. MacDonald TK, Zanna MP, Fong GT. Decision making in altered states: effects of alcohol on attitudes toward drinking and driving. J Pers Soc Psychol. 1995;68(6):973-985.
8. Boettger S, Bergman M, Jenewein J, et al. Assessment of decisional capacity: prevalence of medical illness and psychiatric comorbidities. Palliat Support Care. 2015;13(5):1275-1281.
9. Charland LC. Chapter 6: Decision-making capacity and responsibility in addiction. In: Poland J, Graham G. Addiction and responsibility. Cambridge, MA: MIT Press Scholarship Online; 2011:139-158.
10. Martel ML, Klein LR, Miner JR, et al. A brief assessment of capacity to consent instrument in acutely intoxicated emergency department patients. Am J Emerg Med. 2018;36(1):18-23.
11. MacLullich AM, Beaglehole A, Hall RJ, et al. Delirium and long-term cognitive impairment. Int Rev Psychiatry. 2009;21(1):30-42.
12. Pandharipande PP, Girard TD, Jackson JC, et al. Long-term cognitive impairment after critical illness. N Engl J Med. 2013;369(14):1306-1316.
13. Rehm J, Hasan OSM, Black SE, et al. Alcohol use and dementia: a systematic scoping review. Alzheimers Res Ther. 2019;11(1):1.
14. Jain A, Christopher P, Appelbaum PS. Civil commitment for opioid and other substance use disorders: does it work? Psychiatr Serv. 2018;69(4):374-376.
15. Grisso T, Appelbaum PS. Chapter 6: Using the MacArthur competence assessment tool – treatment. In: Grisso T, Appelbaum PS. Assessing competence to consent to treatment: a guide for physicians and other health professionals. New York, NY: Oxford University Press; 1998:101-126.
16. Hazelton LD, Sterns GL, Chisholm T. Decision-making capacity and alcohol abuse: clinical and ethical considerations in personal care choices. Gen Hosp Psychiatry. 2003;25(2):130-135.
Psychotherapy for psychiatric disorders: A review of 4 studies
Psychotherapy is among the evidence-based treatment options for treating various psychiatric disorders. How we approach psychiatric disorders via psychotherapy has been shaped by numerous theories of personality and psychopathology, including psychodynamic, behavioral, cognitive, systems, and existential-humanistic approaches. Whether used as primary treatment or in conjunction with medication, psychotherapy has played a pivotal role in shaping psychiatric disease management and treatment. Several evidence-based therapy modalities have been used throughout the years and continue to significantly improve and impact our patients’ lives. In the armamentarium of treatment modalities, therapy takes the leading role for several conditions. Here we review 4 studies from current psychotherapy literature; these studies are summarized in the Table.1-4

1.
Panic disorder has a lifetime prevalence of 3.7% in the general population. Three treatment modalities recommended for patients with panic disorder are psychological therapy, pharmacologic therapy, and self-help. Among the psychological therapies, cognitive-behavioral therapy (CBT) is one of the most widely used.1
Cognitive-behavioral therapy for panic disorder has been proven to be an efficacious and impactful treatment. For panic disorder, CBT may consist of different combinations of several therapeutic components, such as relaxation, breathing retraining, cognitive restructuring, interoceptive exposure, and/or in vivo exposure. It is therefore important, both theoretically and clinically, to examine whether specific components of CBT or their combinations are superior to others for treating panic disorder.1
Pompoli et al1 conducted a component network meta-analysis (NMA) of 72 studies in order to determine which CBT components were the most efficacious in treating patients with panic disorder. Component NMA is an extension of standard NMA; it is used to disentangle the treatment effects of different components included in composite interventions.1
The aim of this study was to determine which specific component or combination of components was superior to others when treating panic disorder.1
Study design
- Researchers reviewed 2,526 references from Medline, EMBASE, PsycINFO, and Cochrane Central and selected 72 studies that included 4,064 patients with panic disorder.1
- The primary outcome was remission of panic disorder with or without agoraphobia in the short term (3 to 6 months). Remission was defined as achieving a score of ≤7 on the Panic Disorder Severity Scale (PDSS).1
- Secondary outcomes included response (≥40% reduction in PDSS score from baseline) and dropout for any reason in the short term.1
Continue to: Outcomes
Outcomes
- Using component NMA, researchers determined that interoceptive exposure and face-to-face setting (administration of therapeutic components in a face-to-face setting rather than through self-help means) led to better efficacy and acceptability. Muscle relaxation and virtual reality exposure corresponded to lower efficacy. Breathing retraining and in vivo exposure improved treatment acceptability, but had small effects on efficacy.1
- Based on an analysis of remission rates, the most efficacious CBT incorporated cognitive restructuring and interoceptive exposure. The least efficacious CBT incorporated breathing retraining, muscle relaxation, in vivo exposure, and virtual reality exposure.1
- Application of cognitive and behavioral therapeutic elements was superior to administration of behavioral elements alone. When administering CBT, face-to-face therapy led to better outcomes in response and remission rates. Dropout rates occurred at a lower frequency when CBT was administered face-to-face when compared with self-help groups. The placebo effect was associated with the highest dropout rate.1
Conclusion
- Findings from this meta-analysis have high practical utility. Which CBT components are used can significantly alter CBT’s efficacy and acceptability in patients with panic disorder.1
- The “most efficacious CBT” would include cognitive restructuring and interoceptive exposure delivered in a face-to-face setting. Breathing retraining, muscle relaxation, and virtual reality may have a minimal or even negative impact.1
- Limitations of this meta-analysis include the high number of studies used for the data analysis, complex statistical analysis, inability to include unpublished studies, and limited relevant studies. A future implication of this study is the consideration of formal methodology based on the clinical application of efficacious CBT components when treating patients with panic disorder.1
2. Sloan DM, Marx BP, Lee DJ, et al. A brief exposure-based treatment vs cognitive processing therapy for posttraumatic stress disorder: a randomized noninferiority clinical trial. JAMA Psychiatry. 2018;75(3):233-239.
Psychotherapy is also a useful modality for treating posttraumatic stress disorder (PTSD). Sloan et al2 compared brief exposure-based treatment with cognitive processing therapy (CPT) for PTSD.
Clinical practice guidelines for the management of PTSD and acute stress disorder recommend the use of individual, trauma-focused therapies that focus on exposure and cognitive restructuring, such as prolonged exposure, CPT, and written narrative exposure.5
Continue to: One type of written narrative...
One type of written narrative exposure treatment is written exposure therapy (WET), which consists of 5 sessions during which patients write about their trauma. The first session is comprised of psychoeducation about PTSD and a review of treatment reasoning, followed by 30 minutes of writing. The therapist provides feedback and instructions. Written exposure therapy requires less therapist training and less supervision than prolonged exposure or CPT. Prior studies have suggested that WET can significantly reduce PTSD symptoms in various trauma survivors.2
Although efficacious for PTSD, WET had not been compared with CPT, which is the most commonly used first-line treatment of PTSD. The aim of this study was to determine whether WET is noninferior to CPT.2
Study design
- In this randomized noninferiority clinical trial conducted in Boston, Massachusetts from February 28, 2013 to November 6, 2016, 126 veterans and non-veteran adults were randomized to WET or CPT. Participants met DSM-5 criteria for PTSD and were taking stable doses of their medications for at least 4 weeks.2
- Participants assigned to CPT (n = 63) underwent 12 sessions, and participants assigned to WET (n = 63) received 5 sessions. Cognitive processing therapy was conducted over 60-minute weekly sessions. Written exposure therapy consisted of an initial session that was 60 minutes long and four 40-minute follow-up sessions.2
- Interviews were conducted by 4 independent evaluators at baseline and 6, 12, 24, and 36 weeks. During the WET sessions, participants wrote about a traumatic event while focusing on details, thoughts, and feelings associated with the event.2
- Cognitive processing therapy involved 12 trauma-focused therapy sessions during which participants learn how to become aware of and address problematic cognitions about the trauma as well as thoughts about themselves and others. Between sessions, participants were required to write 2 trauma accounts and complete other assignments.2
Outcomes
- The primary outcome was change in total score on the Clinician-Administered PTSD Scale for DSM-5 (CAPS-5). The CAPS-5 scores for participants in the WET group were noninferior to those for participants in the CPT group at all assessment points.2
- Participants did not significantly differ in age, education, income, or PTSD severity. Participants in the 2 groups did not differ in treatment expectations or level of satisfaction with treatment. Individuals assigned to CPT were more likely to drop out of the study: 20 participants in the CPT group dropped out in the first 5 sessions, whereas only 4 dropped out of the WET group. The dropout rate in the CPT group was 39.7%. Improvements in PTSD symptoms in the WET group were noninferior to improvements in the CPT group.2
- Written exposure therapy showed no difference compared with CPT in decreasing PTSD symptoms. Furthermore, this study demonstrated that PTSD symptoms can decrease with a smaller number of shorter therapeutic sessions.2
Conclusion
- This study demonstrated noninferiority between an established, commonly used PTSD therapy (CPT) and a version of exposure therapy that is briefer, simpler, and requires less homework and less therapist training and expertise. This “lower-dose” approach may improve access for the expanding number of patients who require treatment for PTSD, especially in the Veterans Affairs system.2
- In summary, WET is well tolerated and time-efficient. Although it requires fewer sessions, WET was noninferior to CPT.2
Continue to: Multisystemic therapy versus management as usual...
3. Fonagy P, Butler S, Cottrell D, et al. Multisystemic therapy versus management as usual in the treatment of adolescent antisocial behaviour (START): a pragmatic, randomised controlled, superiority trial. Lancet Psychiatry. 2018;5(2):119-133.
Multisystemic therapy (MST) is an intensive, family-based, home-based intervention for young people with serious antisocial behavior. It has been found effective for childhood conduct disorders in the United States. However, previous studies that supported its efficacy were conducted by the therapy’s developers and used noncomprehensive comparators, such as individual therapy. Fonagy et al3 assessed the effectiveness and cost-effectiveness of MST vs management as usual for treating adolescent antisocial behavior. This is the first study that was performed by independent investigators and used a comprehensive control.3
Study design
- This 18-month, multisite, pragmatic, randomized controlled superiority trial was conducted in England.3
- Participants were age 11 to 17, with moderate to severe antisocial behavior. They had at least 3 severity criteria indicating difficulties across several settings and at least one of the 5 inclusion criteria for antisocial behavior. Six hundred eighty-four families were randomly assigned to MST or management as usual, and 491 families completed the study.3
- For the MST intervention, therapists worked with the adolescent’s caregiver 3 times a week for 3 to 5 months to improve parenting skills, enhance family relationships, increase support from social networks, develop skills and resources, address communication problems, increase school attendance and achievement, and reduce the adolescent’s association with delinquent peers.3
- For the management as usual intervention, management was based on local services for young people and was designed to be in line with current community practice.3
Outcomes
- The primary outcome was the proportion of participants in out-of-home placements at 18 months. The secondary outcomes were time to first criminal offense and the total number of offenses.3
- In terms of the risk of out-of-home placement, MST had no effect: 13% of participants in the MST group had out-of-home placement at 18 months, compared with 11% in the management-as-usual group.3
- Multisystemic therapy also did not significantly delay the time to first offense (hazard ratio, 1.06; 95% confidence interval, 0.84 to 1.33). Also, at 18-month follow-up, participants in the MST group had committed more offenses than those in the management-as-usual group, although the difference was not statistically significant.3
- Parents in the MST group reported increased parental support and involvement and reduced problems at 6 months, but the adolescents’ reports of parenting behavior indicated no significant effect for MST vs management as usual at any time point.3
Conclusion
- Multisystemic therapy was not superior to management as usual in reducing out-of-home placements. Although the parents believed that MST brought about a rapid and effective change, this was not reflected in objective indicators of antisocial behavior. These results are contrary to previous studies in the United States. The substantial improvements observed in both groups reflected the effectiveness of routinely offered interventions for this group of young people, at least when observed in clinical trials.3
Continue to: Mindfulness-based cognitive therapy...
4. Janssen L, Kan CC, Carpentier PJ, et al. Mindfulness-based cognitive therapy v. treatment as usual in adults with ADHD: a multicentre, single-blind, randomised controlled trial. Psychol Med. 2019;49(1):55-65.
There is empirical support for using psychotherapy to treat attention-deficit/hyperactivity disorder (ADHD). Although medication management plays a leading role in treating ADHD, Janssen et al4 conducted a multicenter, single-blind trial comparing mindfulness-based cognitive therapy (MBCT) vs treatment as usual (TAU) for ADHD.
The aim of this study was to determine the efficacy of MBCT plus TAU vs TAU only in decreasing symptoms of adults with ADHD.4
Study design
- This multicenter, single-blind randomized controlled trial was conducted in the Netherlands. Participants (N = 120) met criteria for ADHD and were age ≥18. Patients were randomly assigned to MBCT plus TAU (n = 60) or TAU only (n = 60). Patients in the MBCT plus TAU group received weekly group therapy sessions, meditation exercises, psychoeducation, and group discussions. Patients in the TAU-only group received pharmacotherapy and psychoeducation.4
- Blinded clinicians used the Connors’ Adult ADHD Rating Scale to assess ADHD symptoms.4
- Secondary outcomes were determined by self-reported questionnaires that patients completed online.4
- All statistical analyses were performed on an intention-to-treat sample as well as the per protocol sample.4
Outcomes
- The primary outcome was ADHD symptoms rated by clinicians. Secondary outcomes included self-reported ADHD symptoms, executive functioning, mindfulness skills, positive mental health, and general functioning. Outcomes were examined at baseline and then at post treatment and 3- and 6-month follow-up.4
- Patients in the MBCT plus TAU group had a significant decrease in clinician-rated ADHD symptoms that was maintained at 6-month follow-up. More patients in the MBCT plus TAU group (27%) vs patients in the TAU group (4%) showed a ≥30% reduction in ADHD symptoms. Compared with patients in the TAU group, patients in the MBCT plus TAU group had significant improvements in ADHD symptoms, mindfulness skills, and positive mental health at post treatment and at 6-month follow-up. Compared with those receiving TAU only, patients treated with MBCT plus TAU reported no improvement in executive functioning at post treatment, but did improve at 6-month follow-up.4
Continue to: Conclusion
Conclusion
- Compared with TAU only, MBCT plus TAU is more effective in reducing ADHD symptoms, with a lasting effect at 6-month follow-up. In terms of secondary outcomes, MBCT plus TAU proved to be effective in improving mindfulness, self-compassion, positive mental health, and executive functioning. The results of this trial demonstrate that psychosocial treatments can be effective in addition to TAU in patients with ADHD, and MBCT holds promise for adult ADHD.4
1. Pompoli A, Furukawa TA, Efthimiou O, et al. Dismantling cognitive-behaviour therapy for panic disorder: a systematic review and component network meta-analysis. Psychol Med. 2018;48(12):1945-1953.
2. Sloan DM, Marx BP, Lee DJ, et al. A brief exposure-based treatment vs cognitive processing therapy for posttraumatic stress disorder: a randomized noninferiority clinical trial. JAMA Psychiatry. 2018;75(3):233-239.
3. Fonagy P, Butler S, Cottrell D, et al. Multisystemic therapy versus management as usual in the treatment of adolescent antisocial behaviour (START): a pragmatic, randomised controlled, superiority trial. Lancet Psychiatry. 2018;5(2):119-133.
4. Janssen L, Kan CC, Carpentier PJ, et al. Mindfulness-based cognitive therapy v. treatment as usual in adults with ADHD: a multicentre, single-blind, randomised controlled trial. Psychol Med. 2019;49(1):55-65.
5. US Department of Veterans Affairs and Department of Defense. VA/DoD clinical practice guideline for the management of posttraumatic stress disorder and acute stress disorder . https://www.healthquality.va.gov/guidelines/MH/ptsd/VADoDPTSDCPGFinal082917.pdf. Published June 2017. Accessed September 8, 2019.
Psychotherapy is among the evidence-based treatment options for treating various psychiatric disorders. How we approach psychiatric disorders via psychotherapy has been shaped by numerous theories of personality and psychopathology, including psychodynamic, behavioral, cognitive, systems, and existential-humanistic approaches. Whether used as primary treatment or in conjunction with medication, psychotherapy has played a pivotal role in shaping psychiatric disease management and treatment. Several evidence-based therapy modalities have been used throughout the years and continue to significantly improve and impact our patients’ lives. In the armamentarium of treatment modalities, therapy takes the leading role for several conditions. Here we review 4 studies from current psychotherapy literature; these studies are summarized in the Table.1-4

1.
Panic disorder has a lifetime prevalence of 3.7% in the general population. Three treatment modalities recommended for patients with panic disorder are psychological therapy, pharmacologic therapy, and self-help. Among the psychological therapies, cognitive-behavioral therapy (CBT) is one of the most widely used.1
Cognitive-behavioral therapy for panic disorder has been proven to be an efficacious and impactful treatment. For panic disorder, CBT may consist of different combinations of several therapeutic components, such as relaxation, breathing retraining, cognitive restructuring, interoceptive exposure, and/or in vivo exposure. It is therefore important, both theoretically and clinically, to examine whether specific components of CBT or their combinations are superior to others for treating panic disorder.1
Pompoli et al1 conducted a component network meta-analysis (NMA) of 72 studies in order to determine which CBT components were the most efficacious in treating patients with panic disorder. Component NMA is an extension of standard NMA; it is used to disentangle the treatment effects of different components included in composite interventions.1
The aim of this study was to determine which specific component or combination of components was superior to others when treating panic disorder.1
Study design
- Researchers reviewed 2,526 references from Medline, EMBASE, PsycINFO, and Cochrane Central and selected 72 studies that included 4,064 patients with panic disorder.1
- The primary outcome was remission of panic disorder with or without agoraphobia in the short term (3 to 6 months). Remission was defined as achieving a score of ≤7 on the Panic Disorder Severity Scale (PDSS).1
- Secondary outcomes included response (≥40% reduction in PDSS score from baseline) and dropout for any reason in the short term.1
Continue to: Outcomes
Outcomes
- Using component NMA, researchers determined that interoceptive exposure and face-to-face setting (administration of therapeutic components in a face-to-face setting rather than through self-help means) led to better efficacy and acceptability. Muscle relaxation and virtual reality exposure corresponded to lower efficacy. Breathing retraining and in vivo exposure improved treatment acceptability, but had small effects on efficacy.1
- Based on an analysis of remission rates, the most efficacious CBT incorporated cognitive restructuring and interoceptive exposure. The least efficacious CBT incorporated breathing retraining, muscle relaxation, in vivo exposure, and virtual reality exposure.1
- Application of cognitive and behavioral therapeutic elements was superior to administration of behavioral elements alone. When administering CBT, face-to-face therapy led to better outcomes in response and remission rates. Dropout rates occurred at a lower frequency when CBT was administered face-to-face when compared with self-help groups. The placebo effect was associated with the highest dropout rate.1
Conclusion
- Findings from this meta-analysis have high practical utility. Which CBT components are used can significantly alter CBT’s efficacy and acceptability in patients with panic disorder.1
- The “most efficacious CBT” would include cognitive restructuring and interoceptive exposure delivered in a face-to-face setting. Breathing retraining, muscle relaxation, and virtual reality may have a minimal or even negative impact.1
- Limitations of this meta-analysis include the high number of studies used for the data analysis, complex statistical analysis, inability to include unpublished studies, and limited relevant studies. A future implication of this study is the consideration of formal methodology based on the clinical application of efficacious CBT components when treating patients with panic disorder.1
2. Sloan DM, Marx BP, Lee DJ, et al. A brief exposure-based treatment vs cognitive processing therapy for posttraumatic stress disorder: a randomized noninferiority clinical trial. JAMA Psychiatry. 2018;75(3):233-239.
Psychotherapy is also a useful modality for treating posttraumatic stress disorder (PTSD). Sloan et al2 compared brief exposure-based treatment with cognitive processing therapy (CPT) for PTSD.
Clinical practice guidelines for the management of PTSD and acute stress disorder recommend the use of individual, trauma-focused therapies that focus on exposure and cognitive restructuring, such as prolonged exposure, CPT, and written narrative exposure.5
Continue to: One type of written narrative...
One type of written narrative exposure treatment is written exposure therapy (WET), which consists of 5 sessions during which patients write about their trauma. The first session is comprised of psychoeducation about PTSD and a review of treatment reasoning, followed by 30 minutes of writing. The therapist provides feedback and instructions. Written exposure therapy requires less therapist training and less supervision than prolonged exposure or CPT. Prior studies have suggested that WET can significantly reduce PTSD symptoms in various trauma survivors.2
Although efficacious for PTSD, WET had not been compared with CPT, which is the most commonly used first-line treatment of PTSD. The aim of this study was to determine whether WET is noninferior to CPT.2
Study design
- In this randomized noninferiority clinical trial conducted in Boston, Massachusetts from February 28, 2013 to November 6, 2016, 126 veterans and non-veteran adults were randomized to WET or CPT. Participants met DSM-5 criteria for PTSD and were taking stable doses of their medications for at least 4 weeks.2
- Participants assigned to CPT (n = 63) underwent 12 sessions, and participants assigned to WET (n = 63) received 5 sessions. Cognitive processing therapy was conducted over 60-minute weekly sessions. Written exposure therapy consisted of an initial session that was 60 minutes long and four 40-minute follow-up sessions.2
- Interviews were conducted by 4 independent evaluators at baseline and 6, 12, 24, and 36 weeks. During the WET sessions, participants wrote about a traumatic event while focusing on details, thoughts, and feelings associated with the event.2
- Cognitive processing therapy involved 12 trauma-focused therapy sessions during which participants learn how to become aware of and address problematic cognitions about the trauma as well as thoughts about themselves and others. Between sessions, participants were required to write 2 trauma accounts and complete other assignments.2
Outcomes
- The primary outcome was change in total score on the Clinician-Administered PTSD Scale for DSM-5 (CAPS-5). The CAPS-5 scores for participants in the WET group were noninferior to those for participants in the CPT group at all assessment points.2
- Participants did not significantly differ in age, education, income, or PTSD severity. Participants in the 2 groups did not differ in treatment expectations or level of satisfaction with treatment. Individuals assigned to CPT were more likely to drop out of the study: 20 participants in the CPT group dropped out in the first 5 sessions, whereas only 4 dropped out of the WET group. The dropout rate in the CPT group was 39.7%. Improvements in PTSD symptoms in the WET group were noninferior to improvements in the CPT group.2
- Written exposure therapy showed no difference compared with CPT in decreasing PTSD symptoms. Furthermore, this study demonstrated that PTSD symptoms can decrease with a smaller number of shorter therapeutic sessions.2
Conclusion
- This study demonstrated noninferiority between an established, commonly used PTSD therapy (CPT) and a version of exposure therapy that is briefer, simpler, and requires less homework and less therapist training and expertise. This “lower-dose” approach may improve access for the expanding number of patients who require treatment for PTSD, especially in the Veterans Affairs system.2
- In summary, WET is well tolerated and time-efficient. Although it requires fewer sessions, WET was noninferior to CPT.2
Continue to: Multisystemic therapy versus management as usual...
3. Fonagy P, Butler S, Cottrell D, et al. Multisystemic therapy versus management as usual in the treatment of adolescent antisocial behaviour (START): a pragmatic, randomised controlled, superiority trial. Lancet Psychiatry. 2018;5(2):119-133.
Multisystemic therapy (MST) is an intensive, family-based, home-based intervention for young people with serious antisocial behavior. It has been found effective for childhood conduct disorders in the United States. However, previous studies that supported its efficacy were conducted by the therapy’s developers and used noncomprehensive comparators, such as individual therapy. Fonagy et al3 assessed the effectiveness and cost-effectiveness of MST vs management as usual for treating adolescent antisocial behavior. This is the first study that was performed by independent investigators and used a comprehensive control.3
Study design
- This 18-month, multisite, pragmatic, randomized controlled superiority trial was conducted in England.3
- Participants were age 11 to 17, with moderate to severe antisocial behavior. They had at least 3 severity criteria indicating difficulties across several settings and at least one of the 5 inclusion criteria for antisocial behavior. Six hundred eighty-four families were randomly assigned to MST or management as usual, and 491 families completed the study.3
- For the MST intervention, therapists worked with the adolescent’s caregiver 3 times a week for 3 to 5 months to improve parenting skills, enhance family relationships, increase support from social networks, develop skills and resources, address communication problems, increase school attendance and achievement, and reduce the adolescent’s association with delinquent peers.3
- For the management as usual intervention, management was based on local services for young people and was designed to be in line with current community practice.3
Outcomes
- The primary outcome was the proportion of participants in out-of-home placements at 18 months. The secondary outcomes were time to first criminal offense and the total number of offenses.3
- In terms of the risk of out-of-home placement, MST had no effect: 13% of participants in the MST group had out-of-home placement at 18 months, compared with 11% in the management-as-usual group.3
- Multisystemic therapy also did not significantly delay the time to first offense (hazard ratio, 1.06; 95% confidence interval, 0.84 to 1.33). Also, at 18-month follow-up, participants in the MST group had committed more offenses than those in the management-as-usual group, although the difference was not statistically significant.3
- Parents in the MST group reported increased parental support and involvement and reduced problems at 6 months, but the adolescents’ reports of parenting behavior indicated no significant effect for MST vs management as usual at any time point.3
Conclusion
- Multisystemic therapy was not superior to management as usual in reducing out-of-home placements. Although the parents believed that MST brought about a rapid and effective change, this was not reflected in objective indicators of antisocial behavior. These results are contrary to previous studies in the United States. The substantial improvements observed in both groups reflected the effectiveness of routinely offered interventions for this group of young people, at least when observed in clinical trials.3
Continue to: Mindfulness-based cognitive therapy...
4. Janssen L, Kan CC, Carpentier PJ, et al. Mindfulness-based cognitive therapy v. treatment as usual in adults with ADHD: a multicentre, single-blind, randomised controlled trial. Psychol Med. 2019;49(1):55-65.
There is empirical support for using psychotherapy to treat attention-deficit/hyperactivity disorder (ADHD). Although medication management plays a leading role in treating ADHD, Janssen et al4 conducted a multicenter, single-blind trial comparing mindfulness-based cognitive therapy (MBCT) vs treatment as usual (TAU) for ADHD.
The aim of this study was to determine the efficacy of MBCT plus TAU vs TAU only in decreasing symptoms of adults with ADHD.4
Study design
- This multicenter, single-blind randomized controlled trial was conducted in the Netherlands. Participants (N = 120) met criteria for ADHD and were age ≥18. Patients were randomly assigned to MBCT plus TAU (n = 60) or TAU only (n = 60). Patients in the MBCT plus TAU group received weekly group therapy sessions, meditation exercises, psychoeducation, and group discussions. Patients in the TAU-only group received pharmacotherapy and psychoeducation.4
- Blinded clinicians used the Connors’ Adult ADHD Rating Scale to assess ADHD symptoms.4
- Secondary outcomes were determined by self-reported questionnaires that patients completed online.4
- All statistical analyses were performed on an intention-to-treat sample as well as the per protocol sample.4
Outcomes
- The primary outcome was ADHD symptoms rated by clinicians. Secondary outcomes included self-reported ADHD symptoms, executive functioning, mindfulness skills, positive mental health, and general functioning. Outcomes were examined at baseline and then at post treatment and 3- and 6-month follow-up.4
- Patients in the MBCT plus TAU group had a significant decrease in clinician-rated ADHD symptoms that was maintained at 6-month follow-up. More patients in the MBCT plus TAU group (27%) vs patients in the TAU group (4%) showed a ≥30% reduction in ADHD symptoms. Compared with patients in the TAU group, patients in the MBCT plus TAU group had significant improvements in ADHD symptoms, mindfulness skills, and positive mental health at post treatment and at 6-month follow-up. Compared with those receiving TAU only, patients treated with MBCT plus TAU reported no improvement in executive functioning at post treatment, but did improve at 6-month follow-up.4
Continue to: Conclusion
Conclusion
- Compared with TAU only, MBCT plus TAU is more effective in reducing ADHD symptoms, with a lasting effect at 6-month follow-up. In terms of secondary outcomes, MBCT plus TAU proved to be effective in improving mindfulness, self-compassion, positive mental health, and executive functioning. The results of this trial demonstrate that psychosocial treatments can be effective in addition to TAU in patients with ADHD, and MBCT holds promise for adult ADHD.4
Psychotherapy is among the evidence-based treatment options for treating various psychiatric disorders. How we approach psychiatric disorders via psychotherapy has been shaped by numerous theories of personality and psychopathology, including psychodynamic, behavioral, cognitive, systems, and existential-humanistic approaches. Whether used as primary treatment or in conjunction with medication, psychotherapy has played a pivotal role in shaping psychiatric disease management and treatment. Several evidence-based therapy modalities have been used throughout the years and continue to significantly improve and impact our patients’ lives. In the armamentarium of treatment modalities, therapy takes the leading role for several conditions. Here we review 4 studies from current psychotherapy literature; these studies are summarized in the Table.1-4

1.
Panic disorder has a lifetime prevalence of 3.7% in the general population. Three treatment modalities recommended for patients with panic disorder are psychological therapy, pharmacologic therapy, and self-help. Among the psychological therapies, cognitive-behavioral therapy (CBT) is one of the most widely used.1
Cognitive-behavioral therapy for panic disorder has been proven to be an efficacious and impactful treatment. For panic disorder, CBT may consist of different combinations of several therapeutic components, such as relaxation, breathing retraining, cognitive restructuring, interoceptive exposure, and/or in vivo exposure. It is therefore important, both theoretically and clinically, to examine whether specific components of CBT or their combinations are superior to others for treating panic disorder.1
Pompoli et al1 conducted a component network meta-analysis (NMA) of 72 studies in order to determine which CBT components were the most efficacious in treating patients with panic disorder. Component NMA is an extension of standard NMA; it is used to disentangle the treatment effects of different components included in composite interventions.1
The aim of this study was to determine which specific component or combination of components was superior to others when treating panic disorder.1
Study design
- Researchers reviewed 2,526 references from Medline, EMBASE, PsycINFO, and Cochrane Central and selected 72 studies that included 4,064 patients with panic disorder.1
- The primary outcome was remission of panic disorder with or without agoraphobia in the short term (3 to 6 months). Remission was defined as achieving a score of ≤7 on the Panic Disorder Severity Scale (PDSS).1
- Secondary outcomes included response (≥40% reduction in PDSS score from baseline) and dropout for any reason in the short term.1
Continue to: Outcomes
Outcomes
- Using component NMA, researchers determined that interoceptive exposure and face-to-face setting (administration of therapeutic components in a face-to-face setting rather than through self-help means) led to better efficacy and acceptability. Muscle relaxation and virtual reality exposure corresponded to lower efficacy. Breathing retraining and in vivo exposure improved treatment acceptability, but had small effects on efficacy.1
- Based on an analysis of remission rates, the most efficacious CBT incorporated cognitive restructuring and interoceptive exposure. The least efficacious CBT incorporated breathing retraining, muscle relaxation, in vivo exposure, and virtual reality exposure.1
- Application of cognitive and behavioral therapeutic elements was superior to administration of behavioral elements alone. When administering CBT, face-to-face therapy led to better outcomes in response and remission rates. Dropout rates occurred at a lower frequency when CBT was administered face-to-face when compared with self-help groups. The placebo effect was associated with the highest dropout rate.1
Conclusion
- Findings from this meta-analysis have high practical utility. Which CBT components are used can significantly alter CBT’s efficacy and acceptability in patients with panic disorder.1
- The “most efficacious CBT” would include cognitive restructuring and interoceptive exposure delivered in a face-to-face setting. Breathing retraining, muscle relaxation, and virtual reality may have a minimal or even negative impact.1
- Limitations of this meta-analysis include the high number of studies used for the data analysis, complex statistical analysis, inability to include unpublished studies, and limited relevant studies. A future implication of this study is the consideration of formal methodology based on the clinical application of efficacious CBT components when treating patients with panic disorder.1
2. Sloan DM, Marx BP, Lee DJ, et al. A brief exposure-based treatment vs cognitive processing therapy for posttraumatic stress disorder: a randomized noninferiority clinical trial. JAMA Psychiatry. 2018;75(3):233-239.
Psychotherapy is also a useful modality for treating posttraumatic stress disorder (PTSD). Sloan et al2 compared brief exposure-based treatment with cognitive processing therapy (CPT) for PTSD.
Clinical practice guidelines for the management of PTSD and acute stress disorder recommend the use of individual, trauma-focused therapies that focus on exposure and cognitive restructuring, such as prolonged exposure, CPT, and written narrative exposure.5
Continue to: One type of written narrative...
One type of written narrative exposure treatment is written exposure therapy (WET), which consists of 5 sessions during which patients write about their trauma. The first session is comprised of psychoeducation about PTSD and a review of treatment reasoning, followed by 30 minutes of writing. The therapist provides feedback and instructions. Written exposure therapy requires less therapist training and less supervision than prolonged exposure or CPT. Prior studies have suggested that WET can significantly reduce PTSD symptoms in various trauma survivors.2
Although efficacious for PTSD, WET had not been compared with CPT, which is the most commonly used first-line treatment of PTSD. The aim of this study was to determine whether WET is noninferior to CPT.2
Study design
- In this randomized noninferiority clinical trial conducted in Boston, Massachusetts from February 28, 2013 to November 6, 2016, 126 veterans and non-veteran adults were randomized to WET or CPT. Participants met DSM-5 criteria for PTSD and were taking stable doses of their medications for at least 4 weeks.2
- Participants assigned to CPT (n = 63) underwent 12 sessions, and participants assigned to WET (n = 63) received 5 sessions. Cognitive processing therapy was conducted over 60-minute weekly sessions. Written exposure therapy consisted of an initial session that was 60 minutes long and four 40-minute follow-up sessions.2
- Interviews were conducted by 4 independent evaluators at baseline and 6, 12, 24, and 36 weeks. During the WET sessions, participants wrote about a traumatic event while focusing on details, thoughts, and feelings associated with the event.2
- Cognitive processing therapy involved 12 trauma-focused therapy sessions during which participants learn how to become aware of and address problematic cognitions about the trauma as well as thoughts about themselves and others. Between sessions, participants were required to write 2 trauma accounts and complete other assignments.2
Outcomes
- The primary outcome was change in total score on the Clinician-Administered PTSD Scale for DSM-5 (CAPS-5). The CAPS-5 scores for participants in the WET group were noninferior to those for participants in the CPT group at all assessment points.2
- Participants did not significantly differ in age, education, income, or PTSD severity. Participants in the 2 groups did not differ in treatment expectations or level of satisfaction with treatment. Individuals assigned to CPT were more likely to drop out of the study: 20 participants in the CPT group dropped out in the first 5 sessions, whereas only 4 dropped out of the WET group. The dropout rate in the CPT group was 39.7%. Improvements in PTSD symptoms in the WET group were noninferior to improvements in the CPT group.2
- Written exposure therapy showed no difference compared with CPT in decreasing PTSD symptoms. Furthermore, this study demonstrated that PTSD symptoms can decrease with a smaller number of shorter therapeutic sessions.2
Conclusion
- This study demonstrated noninferiority between an established, commonly used PTSD therapy (CPT) and a version of exposure therapy that is briefer, simpler, and requires less homework and less therapist training and expertise. This “lower-dose” approach may improve access for the expanding number of patients who require treatment for PTSD, especially in the Veterans Affairs system.2
- In summary, WET is well tolerated and time-efficient. Although it requires fewer sessions, WET was noninferior to CPT.2
Continue to: Multisystemic therapy versus management as usual...
3. Fonagy P, Butler S, Cottrell D, et al. Multisystemic therapy versus management as usual in the treatment of adolescent antisocial behaviour (START): a pragmatic, randomised controlled, superiority trial. Lancet Psychiatry. 2018;5(2):119-133.
Multisystemic therapy (MST) is an intensive, family-based, home-based intervention for young people with serious antisocial behavior. It has been found effective for childhood conduct disorders in the United States. However, previous studies that supported its efficacy were conducted by the therapy’s developers and used noncomprehensive comparators, such as individual therapy. Fonagy et al3 assessed the effectiveness and cost-effectiveness of MST vs management as usual for treating adolescent antisocial behavior. This is the first study that was performed by independent investigators and used a comprehensive control.3
Study design
- This 18-month, multisite, pragmatic, randomized controlled superiority trial was conducted in England.3
- Participants were age 11 to 17, with moderate to severe antisocial behavior. They had at least 3 severity criteria indicating difficulties across several settings and at least one of the 5 inclusion criteria for antisocial behavior. Six hundred eighty-four families were randomly assigned to MST or management as usual, and 491 families completed the study.3
- For the MST intervention, therapists worked with the adolescent’s caregiver 3 times a week for 3 to 5 months to improve parenting skills, enhance family relationships, increase support from social networks, develop skills and resources, address communication problems, increase school attendance and achievement, and reduce the adolescent’s association with delinquent peers.3
- For the management as usual intervention, management was based on local services for young people and was designed to be in line with current community practice.3
Outcomes
- The primary outcome was the proportion of participants in out-of-home placements at 18 months. The secondary outcomes were time to first criminal offense and the total number of offenses.3
- In terms of the risk of out-of-home placement, MST had no effect: 13% of participants in the MST group had out-of-home placement at 18 months, compared with 11% in the management-as-usual group.3
- Multisystemic therapy also did not significantly delay the time to first offense (hazard ratio, 1.06; 95% confidence interval, 0.84 to 1.33). Also, at 18-month follow-up, participants in the MST group had committed more offenses than those in the management-as-usual group, although the difference was not statistically significant.3
- Parents in the MST group reported increased parental support and involvement and reduced problems at 6 months, but the adolescents’ reports of parenting behavior indicated no significant effect for MST vs management as usual at any time point.3
Conclusion
- Multisystemic therapy was not superior to management as usual in reducing out-of-home placements. Although the parents believed that MST brought about a rapid and effective change, this was not reflected in objective indicators of antisocial behavior. These results are contrary to previous studies in the United States. The substantial improvements observed in both groups reflected the effectiveness of routinely offered interventions for this group of young people, at least when observed in clinical trials.3
Continue to: Mindfulness-based cognitive therapy...
4. Janssen L, Kan CC, Carpentier PJ, et al. Mindfulness-based cognitive therapy v. treatment as usual in adults with ADHD: a multicentre, single-blind, randomised controlled trial. Psychol Med. 2019;49(1):55-65.
There is empirical support for using psychotherapy to treat attention-deficit/hyperactivity disorder (ADHD). Although medication management plays a leading role in treating ADHD, Janssen et al4 conducted a multicenter, single-blind trial comparing mindfulness-based cognitive therapy (MBCT) vs treatment as usual (TAU) for ADHD.
The aim of this study was to determine the efficacy of MBCT plus TAU vs TAU only in decreasing symptoms of adults with ADHD.4
Study design
- This multicenter, single-blind randomized controlled trial was conducted in the Netherlands. Participants (N = 120) met criteria for ADHD and were age ≥18. Patients were randomly assigned to MBCT plus TAU (n = 60) or TAU only (n = 60). Patients in the MBCT plus TAU group received weekly group therapy sessions, meditation exercises, psychoeducation, and group discussions. Patients in the TAU-only group received pharmacotherapy and psychoeducation.4
- Blinded clinicians used the Connors’ Adult ADHD Rating Scale to assess ADHD symptoms.4
- Secondary outcomes were determined by self-reported questionnaires that patients completed online.4
- All statistical analyses were performed on an intention-to-treat sample as well as the per protocol sample.4
Outcomes
- The primary outcome was ADHD symptoms rated by clinicians. Secondary outcomes included self-reported ADHD symptoms, executive functioning, mindfulness skills, positive mental health, and general functioning. Outcomes were examined at baseline and then at post treatment and 3- and 6-month follow-up.4
- Patients in the MBCT plus TAU group had a significant decrease in clinician-rated ADHD symptoms that was maintained at 6-month follow-up. More patients in the MBCT plus TAU group (27%) vs patients in the TAU group (4%) showed a ≥30% reduction in ADHD symptoms. Compared with patients in the TAU group, patients in the MBCT plus TAU group had significant improvements in ADHD symptoms, mindfulness skills, and positive mental health at post treatment and at 6-month follow-up. Compared with those receiving TAU only, patients treated with MBCT plus TAU reported no improvement in executive functioning at post treatment, but did improve at 6-month follow-up.4
Continue to: Conclusion
Conclusion
- Compared with TAU only, MBCT plus TAU is more effective in reducing ADHD symptoms, with a lasting effect at 6-month follow-up. In terms of secondary outcomes, MBCT plus TAU proved to be effective in improving mindfulness, self-compassion, positive mental health, and executive functioning. The results of this trial demonstrate that psychosocial treatments can be effective in addition to TAU in patients with ADHD, and MBCT holds promise for adult ADHD.4
1. Pompoli A, Furukawa TA, Efthimiou O, et al. Dismantling cognitive-behaviour therapy for panic disorder: a systematic review and component network meta-analysis. Psychol Med. 2018;48(12):1945-1953.
2. Sloan DM, Marx BP, Lee DJ, et al. A brief exposure-based treatment vs cognitive processing therapy for posttraumatic stress disorder: a randomized noninferiority clinical trial. JAMA Psychiatry. 2018;75(3):233-239.
3. Fonagy P, Butler S, Cottrell D, et al. Multisystemic therapy versus management as usual in the treatment of adolescent antisocial behaviour (START): a pragmatic, randomised controlled, superiority trial. Lancet Psychiatry. 2018;5(2):119-133.
4. Janssen L, Kan CC, Carpentier PJ, et al. Mindfulness-based cognitive therapy v. treatment as usual in adults with ADHD: a multicentre, single-blind, randomised controlled trial. Psychol Med. 2019;49(1):55-65.
5. US Department of Veterans Affairs and Department of Defense. VA/DoD clinical practice guideline for the management of posttraumatic stress disorder and acute stress disorder . https://www.healthquality.va.gov/guidelines/MH/ptsd/VADoDPTSDCPGFinal082917.pdf. Published June 2017. Accessed September 8, 2019.
1. Pompoli A, Furukawa TA, Efthimiou O, et al. Dismantling cognitive-behaviour therapy for panic disorder: a systematic review and component network meta-analysis. Psychol Med. 2018;48(12):1945-1953.
2. Sloan DM, Marx BP, Lee DJ, et al. A brief exposure-based treatment vs cognitive processing therapy for posttraumatic stress disorder: a randomized noninferiority clinical trial. JAMA Psychiatry. 2018;75(3):233-239.
3. Fonagy P, Butler S, Cottrell D, et al. Multisystemic therapy versus management as usual in the treatment of adolescent antisocial behaviour (START): a pragmatic, randomised controlled, superiority trial. Lancet Psychiatry. 2018;5(2):119-133.
4. Janssen L, Kan CC, Carpentier PJ, et al. Mindfulness-based cognitive therapy v. treatment as usual in adults with ADHD: a multicentre, single-blind, randomised controlled trial. Psychol Med. 2019;49(1):55-65.
5. US Department of Veterans Affairs and Department of Defense. VA/DoD clinical practice guideline for the management of posttraumatic stress disorder and acute stress disorder . https://www.healthquality.va.gov/guidelines/MH/ptsd/VADoDPTSDCPGFinal082917.pdf. Published June 2017. Accessed September 8, 2019.
Would you recognize this ‘invisible’ encephalopathy?
Mr. Z, an obese adult with a history of portal hypertension and cirrhosis from alcoholism, visits your clinic because he is having difficulty sleeping and concentrating at work. He recently reduced his alcohol use and has improved support from his spouse. He walks into your office with an unremarkable gait before stopping to jot down a note in crisp, neat handwriting. He sits facing you, making good eye contact and exhibiting no involuntary movements. As has been the case at previous visits, Mr. Z is fully oriented to person, place, and time. You can follow one another’s train of thought and collaborate on treatment decisions. You’ve ruled out hepatic encephalopathy. Could you be missing something?
Hepatic encephalopathy is a neuropsychiatric condition caused by metabolic changes secondary to liver dysfunction and/or by blood flow bypassing the portal venous system. Signs and symptoms of hepatic encephalopathy range from subtle changes in cognition and affect to coma.Pathophysiologic mechanisms involved in hepatic encephalopathy include inflammation, neurotoxins, oxidative stress, permeability changes in the blood-brain barrier, and impaired brain energy metabolism.1
Patients with poor liver function commonly have psychometrically detectable cognitive and psychomotor deficits that can substantially affect their lives. When such deficits are undetectable by
Approximately 22% to 74% of patients with liver dysfunction develop MHE.2 Prevalence estimates vary widely because of the poor standardization of diagnostic criteria and potential underdiagnosis due to a lack of obvious symptoms.2
How is MHE diagnosed?
The most commonly administered psychometric test to assess for MHE is the Psychometric Hepatic Encephalopathy Score, a written test that measures motor speed and accuracy, concentration, attention, visual perception, visual-spatial orientation, visual construction, and memory.3,4 Other methods for evaluating MHE, including EEG, MRI, single-photon emission CT, positron emission tomography, and determining a patient’s frequency threshold of perceiving a flickering light, have predictive power, but they do not have a well-defined, standardized role in the diagnosis of MHE.2 Although ammonia levels can correlate with severity of impairment in episodic hepatic encephalopathy, they are not well correlated with the deficits in MHE, and often it is not feasible to properly measure ammonia concentrations in outpatient settings.2
Limited treatment options
Few studies have investigated interventions specifically for MHE. The beststudied treatments are lactulose5 and rifaximin.6 Lactulose reduces the formation of ammonia and the absorption of both ammonia and glutamine in the colonic lumen.5 In addition to improving MHE, lactulose helps prevent the recurrence of episodic overt hepatic encephalopathy.5 The antibiotic rifaximin kills ammonia-producing gut bacteria because it is minimally absorbed in the digestive system. No studies investigating rifaximin have observed antibiotic resistance, even with prolonged use. Rifaximin improves cognitive ability, driving ability, and quality of life in patients with MHE. Adding rifaximin to a treatment regimen that includes lactulose also can reduce the recurrence of overt hepatic encephalopathy.6 Branched chain amino acids, L-carnitine, L-ornithine aspartate, treating a comorbid zinc deficiency, probiotics, and increasing vegetable protein intake relative to animal protein intake may also have roles in treating MHE.2
1. Hadjihambi A, Arias N, Sheikh M, et al. Hepatic encephalopathy: a critical current review. Hepatol Int. 2018;12(suppl 1):S135-S147.
2. Zhan T, Stremmel W. The diagnosis and treatment of minimal hepatic encephalopathy. Dtsch Arztebl Int. 2012;109(10):180-1877.
3. Weissenborn K, Ennen JC, Schomerus H, et al. Neuropsychological characterization of hepatic encephalopathy. J Hepatol. 2001;34(5):768-773.
4. Nabi E, Bajaj J. Useful tests for hepatic encephalopathy in clinical practice. Curr Gastroenterol Rep. 2014;16(1):362.
5. Sharma BC, Sharma P, Agrawal A, et al. Secondary prophylaxis of hepatic encephalopathy: an open-label randomized controlled trial of lactulose versus placebo. Gastroenterology. 2009;137(3):885-891.
6. Bass NM, Mullen KD, Sanyal A et al. Rifaximin treatment in hepatic encephalopathy. N Engl J Med. 2010;362:1071-1081.
Mr. Z, an obese adult with a history of portal hypertension and cirrhosis from alcoholism, visits your clinic because he is having difficulty sleeping and concentrating at work. He recently reduced his alcohol use and has improved support from his spouse. He walks into your office with an unremarkable gait before stopping to jot down a note in crisp, neat handwriting. He sits facing you, making good eye contact and exhibiting no involuntary movements. As has been the case at previous visits, Mr. Z is fully oriented to person, place, and time. You can follow one another’s train of thought and collaborate on treatment decisions. You’ve ruled out hepatic encephalopathy. Could you be missing something?
Hepatic encephalopathy is a neuropsychiatric condition caused by metabolic changes secondary to liver dysfunction and/or by blood flow bypassing the portal venous system. Signs and symptoms of hepatic encephalopathy range from subtle changes in cognition and affect to coma.Pathophysiologic mechanisms involved in hepatic encephalopathy include inflammation, neurotoxins, oxidative stress, permeability changes in the blood-brain barrier, and impaired brain energy metabolism.1
Patients with poor liver function commonly have psychometrically detectable cognitive and psychomotor deficits that can substantially affect their lives. When such deficits are undetectable by
Approximately 22% to 74% of patients with liver dysfunction develop MHE.2 Prevalence estimates vary widely because of the poor standardization of diagnostic criteria and potential underdiagnosis due to a lack of obvious symptoms.2
How is MHE diagnosed?
The most commonly administered psychometric test to assess for MHE is the Psychometric Hepatic Encephalopathy Score, a written test that measures motor speed and accuracy, concentration, attention, visual perception, visual-spatial orientation, visual construction, and memory.3,4 Other methods for evaluating MHE, including EEG, MRI, single-photon emission CT, positron emission tomography, and determining a patient’s frequency threshold of perceiving a flickering light, have predictive power, but they do not have a well-defined, standardized role in the diagnosis of MHE.2 Although ammonia levels can correlate with severity of impairment in episodic hepatic encephalopathy, they are not well correlated with the deficits in MHE, and often it is not feasible to properly measure ammonia concentrations in outpatient settings.2
Limited treatment options
Few studies have investigated interventions specifically for MHE. The beststudied treatments are lactulose5 and rifaximin.6 Lactulose reduces the formation of ammonia and the absorption of both ammonia and glutamine in the colonic lumen.5 In addition to improving MHE, lactulose helps prevent the recurrence of episodic overt hepatic encephalopathy.5 The antibiotic rifaximin kills ammonia-producing gut bacteria because it is minimally absorbed in the digestive system. No studies investigating rifaximin have observed antibiotic resistance, even with prolonged use. Rifaximin improves cognitive ability, driving ability, and quality of life in patients with MHE. Adding rifaximin to a treatment regimen that includes lactulose also can reduce the recurrence of overt hepatic encephalopathy.6 Branched chain amino acids, L-carnitine, L-ornithine aspartate, treating a comorbid zinc deficiency, probiotics, and increasing vegetable protein intake relative to animal protein intake may also have roles in treating MHE.2
Mr. Z, an obese adult with a history of portal hypertension and cirrhosis from alcoholism, visits your clinic because he is having difficulty sleeping and concentrating at work. He recently reduced his alcohol use and has improved support from his spouse. He walks into your office with an unremarkable gait before stopping to jot down a note in crisp, neat handwriting. He sits facing you, making good eye contact and exhibiting no involuntary movements. As has been the case at previous visits, Mr. Z is fully oriented to person, place, and time. You can follow one another’s train of thought and collaborate on treatment decisions. You’ve ruled out hepatic encephalopathy. Could you be missing something?
Hepatic encephalopathy is a neuropsychiatric condition caused by metabolic changes secondary to liver dysfunction and/or by blood flow bypassing the portal venous system. Signs and symptoms of hepatic encephalopathy range from subtle changes in cognition and affect to coma.Pathophysiologic mechanisms involved in hepatic encephalopathy include inflammation, neurotoxins, oxidative stress, permeability changes in the blood-brain barrier, and impaired brain energy metabolism.1
Patients with poor liver function commonly have psychometrically detectable cognitive and psychomotor deficits that can substantially affect their lives. When such deficits are undetectable by
Approximately 22% to 74% of patients with liver dysfunction develop MHE.2 Prevalence estimates vary widely because of the poor standardization of diagnostic criteria and potential underdiagnosis due to a lack of obvious symptoms.2
How is MHE diagnosed?
The most commonly administered psychometric test to assess for MHE is the Psychometric Hepatic Encephalopathy Score, a written test that measures motor speed and accuracy, concentration, attention, visual perception, visual-spatial orientation, visual construction, and memory.3,4 Other methods for evaluating MHE, including EEG, MRI, single-photon emission CT, positron emission tomography, and determining a patient’s frequency threshold of perceiving a flickering light, have predictive power, but they do not have a well-defined, standardized role in the diagnosis of MHE.2 Although ammonia levels can correlate with severity of impairment in episodic hepatic encephalopathy, they are not well correlated with the deficits in MHE, and often it is not feasible to properly measure ammonia concentrations in outpatient settings.2
Limited treatment options
Few studies have investigated interventions specifically for MHE. The beststudied treatments are lactulose5 and rifaximin.6 Lactulose reduces the formation of ammonia and the absorption of both ammonia and glutamine in the colonic lumen.5 In addition to improving MHE, lactulose helps prevent the recurrence of episodic overt hepatic encephalopathy.5 The antibiotic rifaximin kills ammonia-producing gut bacteria because it is minimally absorbed in the digestive system. No studies investigating rifaximin have observed antibiotic resistance, even with prolonged use. Rifaximin improves cognitive ability, driving ability, and quality of life in patients with MHE. Adding rifaximin to a treatment regimen that includes lactulose also can reduce the recurrence of overt hepatic encephalopathy.6 Branched chain amino acids, L-carnitine, L-ornithine aspartate, treating a comorbid zinc deficiency, probiotics, and increasing vegetable protein intake relative to animal protein intake may also have roles in treating MHE.2
1. Hadjihambi A, Arias N, Sheikh M, et al. Hepatic encephalopathy: a critical current review. Hepatol Int. 2018;12(suppl 1):S135-S147.
2. Zhan T, Stremmel W. The diagnosis and treatment of minimal hepatic encephalopathy. Dtsch Arztebl Int. 2012;109(10):180-1877.
3. Weissenborn K, Ennen JC, Schomerus H, et al. Neuropsychological characterization of hepatic encephalopathy. J Hepatol. 2001;34(5):768-773.
4. Nabi E, Bajaj J. Useful tests for hepatic encephalopathy in clinical practice. Curr Gastroenterol Rep. 2014;16(1):362.
5. Sharma BC, Sharma P, Agrawal A, et al. Secondary prophylaxis of hepatic encephalopathy: an open-label randomized controlled trial of lactulose versus placebo. Gastroenterology. 2009;137(3):885-891.
6. Bass NM, Mullen KD, Sanyal A et al. Rifaximin treatment in hepatic encephalopathy. N Engl J Med. 2010;362:1071-1081.
1. Hadjihambi A, Arias N, Sheikh M, et al. Hepatic encephalopathy: a critical current review. Hepatol Int. 2018;12(suppl 1):S135-S147.
2. Zhan T, Stremmel W. The diagnosis and treatment of minimal hepatic encephalopathy. Dtsch Arztebl Int. 2012;109(10):180-1877.
3. Weissenborn K, Ennen JC, Schomerus H, et al. Neuropsychological characterization of hepatic encephalopathy. J Hepatol. 2001;34(5):768-773.
4. Nabi E, Bajaj J. Useful tests for hepatic encephalopathy in clinical practice. Curr Gastroenterol Rep. 2014;16(1):362.
5. Sharma BC, Sharma P, Agrawal A, et al. Secondary prophylaxis of hepatic encephalopathy: an open-label randomized controlled trial of lactulose versus placebo. Gastroenterology. 2009;137(3):885-891.
6. Bass NM, Mullen KD, Sanyal A et al. Rifaximin treatment in hepatic encephalopathy. N Engl J Med. 2010;362:1071-1081.
Physician burnout vs depression: Recognize the signs
Although all health care professionals are at risk for burnout, physicians have especially high rates of self-reported burnout—which is commonly understood as a work-related syndrome of emotional exhaustion, depersonalization, and a decreased sense of accomplishment that develops over time.1 In a 2019 report investigating burnout in approximately 15,000 physicians, 39% of psychiatrists and nearly 50% of physicians from multiple other specialities described themselves as “burned out.”2 In addition, 15% reported symptoms of clinical depression (4%) or subclinical depression (11%). In comparison, in 2017, 7.1% of US adults experienced at least 1 major depressive episode.3 Because physician burnout and depression can be associated with adverse outcomes in patient care and personal health, rapid identification and differentiation of the 2 conditions is paramount.
Differentiating burnout and depression
Burnout and depression are distinct but overlapping entities. Although burnout can be difficult to recognize and is not currently a DSM diagnosis, physicians can learn to identify the signs with reference to the more familiar features of depression (Table4,5). Many features of burnout are work-related, while the negative feelings and thoughts of depression pertain to all areas of life. Furthermore, a major depressive episode often includes hopelessness, suicidality, or mood-congruent delusions; burnout does not. Shared symptoms of burnout and depression include extreme exhaustion, feeling unhappy, and reduced performance.
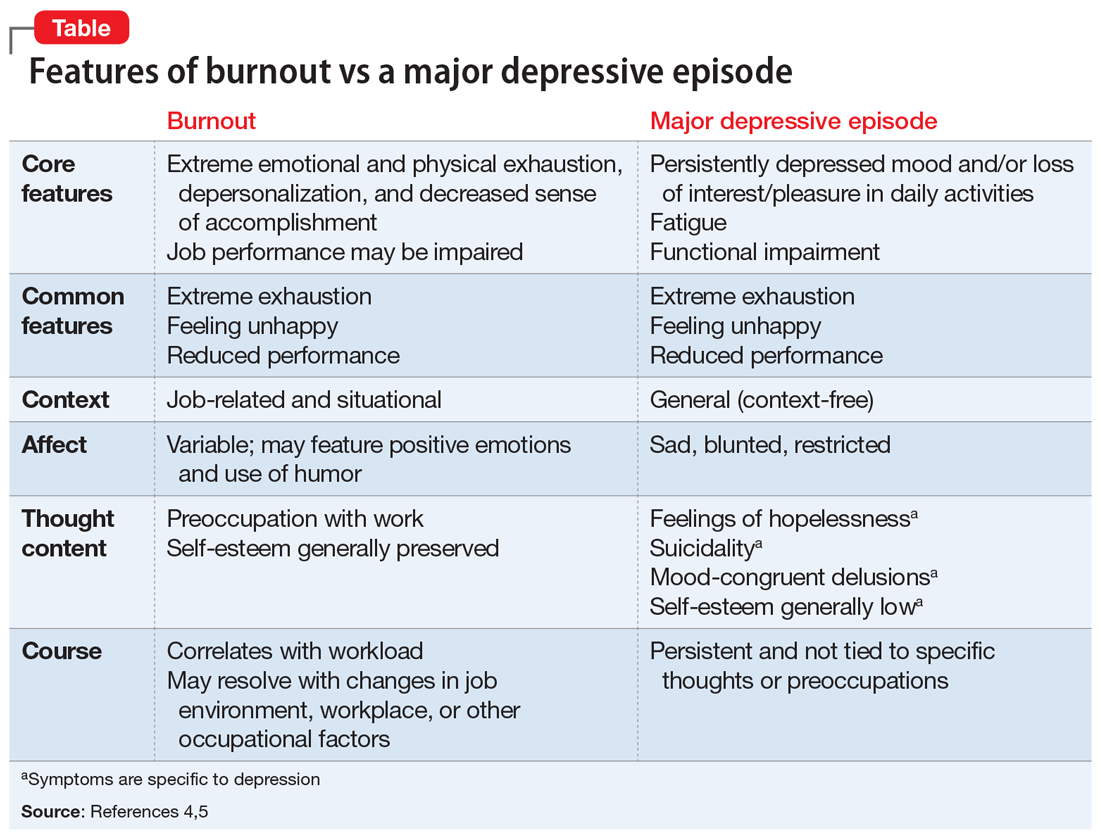
Surprisingly, there is no universally accepted definition of burnout.4,5 Some researchers have proposed that physicians who are categorized as “burned out” may actually have underlying anxiety or depressive disorders that have been misdiagnosed and not appropriately treated.4,5 Others claim that burnout is best formulated as a depressive condition in need of formal diagnostic criteria.4,5 Because the definition of burnout is in question,4,5 strategies to prevent and detect burnout in individual clinicians remain elusive.
Key areas that contribute to vulnerability to burnout include one’s sense of community, fairness, and control in the workplace; personal and organization values; and work-life balance. We propose the mnemonic WORK to help clinicians quickly assess their vulnerability to burnout in these areas.
Workload. Outside of working hours, are you satisfied with the amount of time you devote to self-care, recreation, and other activities that are important to you? Do you honor your “down time”?
Oversight. Are you satisfied with the flexibility and autonomy in your professional life? Are you able to cope with the systemic demands of your practice while upholding your priorities within these restrictions?
Reward. Are the mechanisms for feedback, opportunities for advancement, and financial compensation in your workplace fair? Do you find positive meaning in the work that you do?
Continue to: Kinship
Kinship. Does your place of work support cooperation and collaboration, rather than competition and isolation? Do you approach and receive support from your colleagues when you need assistance?
Persistent dissatisfaction in any of these aspects should prompt clinicians to further develop strategies that promote workplace engagement, job satisfaction, and resilience. We hope this mnemonic helps clinicians to take responsibility for their own well-being and ultimately reap the rewards of a fulfilling professional life.
1. Brindley P. Psychological burnout and the intensive care practitioner: a practical and candid review for those who care. J Inten Care Soc. 2017;18(4):270-275.
2. Kane L. Medscape national physician b urnout & depression report 2019. https://www.medscape.com/slideshow/2019-lifestyle-burnout-depression-6011056#1. Published January 16, 2019. Accessed September 17, 2019.
3. National Institute of Mental Health. Prevalence of major depressive episode among adults. https://www.nimh.nih.gov/health/statistics/major-depression.shtml. Updated February 2019. Accessed September 17, 2019.
4. Messias E, Flynn V. The tired, retired, and recovered physician: professional burnout versus major depressive disorder. Am J Psychiatry. 2018;175(8):716-719.
5. Melnick ER, Powsner SM, Shanafelt TD. In reply—defining physician burnout, and differentiating between burnout and depression. Mayo Clinic Proc. 2017;92(9):1456-1458.
Although all health care professionals are at risk for burnout, physicians have especially high rates of self-reported burnout—which is commonly understood as a work-related syndrome of emotional exhaustion, depersonalization, and a decreased sense of accomplishment that develops over time.1 In a 2019 report investigating burnout in approximately 15,000 physicians, 39% of psychiatrists and nearly 50% of physicians from multiple other specialities described themselves as “burned out.”2 In addition, 15% reported symptoms of clinical depression (4%) or subclinical depression (11%). In comparison, in 2017, 7.1% of US adults experienced at least 1 major depressive episode.3 Because physician burnout and depression can be associated with adverse outcomes in patient care and personal health, rapid identification and differentiation of the 2 conditions is paramount.
Differentiating burnout and depression
Burnout and depression are distinct but overlapping entities. Although burnout can be difficult to recognize and is not currently a DSM diagnosis, physicians can learn to identify the signs with reference to the more familiar features of depression (Table4,5). Many features of burnout are work-related, while the negative feelings and thoughts of depression pertain to all areas of life. Furthermore, a major depressive episode often includes hopelessness, suicidality, or mood-congruent delusions; burnout does not. Shared symptoms of burnout and depression include extreme exhaustion, feeling unhappy, and reduced performance.

Surprisingly, there is no universally accepted definition of burnout.4,5 Some researchers have proposed that physicians who are categorized as “burned out” may actually have underlying anxiety or depressive disorders that have been misdiagnosed and not appropriately treated.4,5 Others claim that burnout is best formulated as a depressive condition in need of formal diagnostic criteria.4,5 Because the definition of burnout is in question,4,5 strategies to prevent and detect burnout in individual clinicians remain elusive.
Key areas that contribute to vulnerability to burnout include one’s sense of community, fairness, and control in the workplace; personal and organization values; and work-life balance. We propose the mnemonic WORK to help clinicians quickly assess their vulnerability to burnout in these areas.
Workload. Outside of working hours, are you satisfied with the amount of time you devote to self-care, recreation, and other activities that are important to you? Do you honor your “down time”?
Oversight. Are you satisfied with the flexibility and autonomy in your professional life? Are you able to cope with the systemic demands of your practice while upholding your priorities within these restrictions?
Reward. Are the mechanisms for feedback, opportunities for advancement, and financial compensation in your workplace fair? Do you find positive meaning in the work that you do?
Continue to: Kinship
Kinship. Does your place of work support cooperation and collaboration, rather than competition and isolation? Do you approach and receive support from your colleagues when you need assistance?
Persistent dissatisfaction in any of these aspects should prompt clinicians to further develop strategies that promote workplace engagement, job satisfaction, and resilience. We hope this mnemonic helps clinicians to take responsibility for their own well-being and ultimately reap the rewards of a fulfilling professional life.
Although all health care professionals are at risk for burnout, physicians have especially high rates of self-reported burnout—which is commonly understood as a work-related syndrome of emotional exhaustion, depersonalization, and a decreased sense of accomplishment that develops over time.1 In a 2019 report investigating burnout in approximately 15,000 physicians, 39% of psychiatrists and nearly 50% of physicians from multiple other specialities described themselves as “burned out.”2 In addition, 15% reported symptoms of clinical depression (4%) or subclinical depression (11%). In comparison, in 2017, 7.1% of US adults experienced at least 1 major depressive episode.3 Because physician burnout and depression can be associated with adverse outcomes in patient care and personal health, rapid identification and differentiation of the 2 conditions is paramount.
Differentiating burnout and depression
Burnout and depression are distinct but overlapping entities. Although burnout can be difficult to recognize and is not currently a DSM diagnosis, physicians can learn to identify the signs with reference to the more familiar features of depression (Table4,5). Many features of burnout are work-related, while the negative feelings and thoughts of depression pertain to all areas of life. Furthermore, a major depressive episode often includes hopelessness, suicidality, or mood-congruent delusions; burnout does not. Shared symptoms of burnout and depression include extreme exhaustion, feeling unhappy, and reduced performance.

Surprisingly, there is no universally accepted definition of burnout.4,5 Some researchers have proposed that physicians who are categorized as “burned out” may actually have underlying anxiety or depressive disorders that have been misdiagnosed and not appropriately treated.4,5 Others claim that burnout is best formulated as a depressive condition in need of formal diagnostic criteria.4,5 Because the definition of burnout is in question,4,5 strategies to prevent and detect burnout in individual clinicians remain elusive.
Key areas that contribute to vulnerability to burnout include one’s sense of community, fairness, and control in the workplace; personal and organization values; and work-life balance. We propose the mnemonic WORK to help clinicians quickly assess their vulnerability to burnout in these areas.
Workload. Outside of working hours, are you satisfied with the amount of time you devote to self-care, recreation, and other activities that are important to you? Do you honor your “down time”?
Oversight. Are you satisfied with the flexibility and autonomy in your professional life? Are you able to cope with the systemic demands of your practice while upholding your priorities within these restrictions?
Reward. Are the mechanisms for feedback, opportunities for advancement, and financial compensation in your workplace fair? Do you find positive meaning in the work that you do?
Continue to: Kinship
Kinship. Does your place of work support cooperation and collaboration, rather than competition and isolation? Do you approach and receive support from your colleagues when you need assistance?
Persistent dissatisfaction in any of these aspects should prompt clinicians to further develop strategies that promote workplace engagement, job satisfaction, and resilience. We hope this mnemonic helps clinicians to take responsibility for their own well-being and ultimately reap the rewards of a fulfilling professional life.
1. Brindley P. Psychological burnout and the intensive care practitioner: a practical and candid review for those who care. J Inten Care Soc. 2017;18(4):270-275.
2. Kane L. Medscape national physician b urnout & depression report 2019. https://www.medscape.com/slideshow/2019-lifestyle-burnout-depression-6011056#1. Published January 16, 2019. Accessed September 17, 2019.
3. National Institute of Mental Health. Prevalence of major depressive episode among adults. https://www.nimh.nih.gov/health/statistics/major-depression.shtml. Updated February 2019. Accessed September 17, 2019.
4. Messias E, Flynn V. The tired, retired, and recovered physician: professional burnout versus major depressive disorder. Am J Psychiatry. 2018;175(8):716-719.
5. Melnick ER, Powsner SM, Shanafelt TD. In reply—defining physician burnout, and differentiating between burnout and depression. Mayo Clinic Proc. 2017;92(9):1456-1458.
1. Brindley P. Psychological burnout and the intensive care practitioner: a practical and candid review for those who care. J Inten Care Soc. 2017;18(4):270-275.
2. Kane L. Medscape national physician b urnout & depression report 2019. https://www.medscape.com/slideshow/2019-lifestyle-burnout-depression-6011056#1. Published January 16, 2019. Accessed September 17, 2019.
3. National Institute of Mental Health. Prevalence of major depressive episode among adults. https://www.nimh.nih.gov/health/statistics/major-depression.shtml. Updated February 2019. Accessed September 17, 2019.
4. Messias E, Flynn V. The tired, retired, and recovered physician: professional burnout versus major depressive disorder. Am J Psychiatry. 2018;175(8):716-719.
5. Melnick ER, Powsner SM, Shanafelt TD. In reply—defining physician burnout, and differentiating between burnout and depression. Mayo Clinic Proc. 2017;92(9):1456-1458.
Quality and Safety of Pediatric Inpatient Care in Community Hospitals: A Scoping Review
Despite efforts to provide high-quality healthcare, Americans die from medical errors each year and many patients do not receive recommended medical care. Risk is particularly acute during times of hospitalization.1-4 In response, the Institute of Medicine (IOM, now the Academy of Medicine) has released “Crossing the Quality Chasm: A New Health System for the 21st Century,” providing a framework to guide delivery and measurement of high-quality healthcare.5
Although the IOM framework has motivated the development of quality improvement (QI) and quality measurement initiatives, relatively few resources have been allocated to improving the quality of pediatric inpatient care.6,7 The resultant gap in our knowledge of quality and safety of pediatric hospital-based care is further widened by the variability of settings in which children are hospitalized. These settings include freestanding children’s hospitals, children’s hospitals nested within larger hospitals, and community hospitals, defined as general, nonuniversity, and nonchildren’s hospitals.8
Although almost three-quarters of children needing hospitalization are cared for outside of freestanding children’s hospitals, we know particularly little about the quality and safety of pediatric hospital-based care outside of these settings.6,9 Therefore, our scoping review aims to summarize literature regarding the quality and safety of pediatric inpatient care within community hospitals.
METHODS
We used a scoping review approach because this methodology, by design, is utilized to synthesize evidence and map existing literature and is particularly useful when a body of literature is heterogeneous, rendering a more targeted systematic review approach infeasible.10 This methodology thereby provided an organized approach to answer our broad research question, “What evidence exists regarding the quality and safety of pediatric inpatient care in United States community hospitals?“ We followed the scoping review guidelines put forth by the Joanna Briggs Institute and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) reporting guideline Extension for Scoping Reviews.10,11
Data Sources and Search Strategies
We searched Medline, Medline-In-Process, Embase, the Cochrane Database of Systematic Reviews, the Cumulative Index to Nursing and Allied Health Literature (CINAHL), PsycINFO, and Scopus for studies that reported at least one outcome related to healthcare quality or patient safety and involved pediatric patients (aged <18 years) receiving inpatient care at a community hospital. Outcomes included measures from the IOM-defined aims of quality healthcare: (1) safety, (2) effectiveness, (3) efficiency, (4) timeliness, (5) patient-centeredness, and (6) equity (Appendix Table 1). Terms were searched as controlled vocabulary in applicable databases (Medline, Embase, CINAHL, PsycINFO) and as keywords in all databases. Search strategies tailored to each database were developed, tested, and refined in collaboration with a reference librarian. Date parameters for retrieval were set from 1989 to the search date, with the start date chosen to correspond with the establishment of the Agency for Health Care Policy and Research, currently known as the Agency for Healthcare Research and Quality (AHRQ), targeting literature produced in the wake of the AHRQ emphasis on quality in healthcare. Results were limited to articles published in English. Searches were conducted on October 24 and 25, 2016. Complete search strategies can be found in Appendix Methods.
Independent authors performed handsearches of Academic Pediatrics, BMJ Quality & Safety, Hospital Pediatrics, JAMA Pediatrics, and Pediatrics Quality Reports for the five years preceding the search date (July 2011-October 2016).
Study Selection and Definitions
To identify studies conducted in community hospitals, we operationalized the definition of community hospital proposed by Percelay.8 We used “community”, “general”, “nonuniversity”, and “nonchildren’s” as search terms and operationalized these through the addition of qualifying descriptors (Table 1).
Studies were excluded if they (1) were performed outside of the United States, as community hospital definitions may differ by country; (2) included participants aged >18 years and did not report any pediatric-specific results; (3) were performed exclusively in tertiary hospitals; (4) evaluated only outpatient or emergency department care; (5) did not report any results specific to community hospitals; (6) did not report any data-driven or parent/patient-reported measures of safety, effectiveness, efficiency, timeliness, patient-centeredness, or equity in their results; and (7) were case reports, case series, editorials, or abstracts without an associated full-text article. We read commentaries and literature reviews related to our objectives and reviewed their references to identify additional articles, but did not include these manuscripts.
Two authors independently reviewed each abstract, and full-text articles were reviewed if one or more authors determined that the abstract met the inclusion criteria. Two authors then independently reviewed each full-text article to determine whether the article met the criteria for the final review. Disagreements were resolved through consensus after discussion and review with the entire research team, and reasons for exclusion were recorded.
Charting the Results and Data Synthesis
We used a standardized charting form to collect information regarding study design, community hospital terms, population descriptors, IOM aims of quality healthcare, and outcome measures. To minimize bias in data collection, information from each full-text article was extracted independently by two authors, and differences in extraction were resolved through discussion and re-review among the same two authors. To evaluate the quality of included evidence, two authors (JCL and JKL) independently assessed the risk of bias for each study using modified Newcastle-Ottawa Quality Assessment Scales (NOS), with disagreements resolved through discussion and re-review among the same two authors. For cohort studies, five of the eight NOS domains were relevant and applied to all included studies (maximum score 6). Using the NOS adapted for cross-sectional studies, six of the seven domains were relevant and applied (maximum score 9). For cross-sectional studies, we defined the risk of bias to be low for scores ≥8, moderate for scores 5-7, and high for scores ≤4, consistent with previous work.12 For cohort studies, we similarly defined these strata by scores ≥5, 3-4, and ≤2, given the lower maximum score for this study type.
We categorized studies as either observational, defined as cohort or cross-sectional studies of usual healthcare delivery, or interventional, defined as studies evaluating the development and/or implementation of an intervention designed to improve healthcare quality. We further categorized the studies into the following four overarching medical domains: (1) neonatal, (2) pediatric medicine, (3) surgery, or (4) radiology.
RESULTS
After removal of duplicates, our search identified 2,068 abstracts for screening (Figure). Of these, 1,777 did not meet the inclusion criteria, leaving 291 articles for full-text review. Of these, 43 articles met all the inclusion criteria, and one additional article was included from search of references, resulting in a total of 44 articles.
Study designs, patient populations, and quality outcome measures were heterogeneous. We identified only one randomized controlled trial (RCT). A total of 30 articles were observational studies, 27 of which used retrospective cohort or cross-sectional designs; the remaining three used prospective cohort designs (Table 2). Of these studies, 20 involved multiple hospitals, whereas 10 were conducted at a single community hospital. Sample sizes ranged from 29 (single-site) to 107,727 (multisite) patients. Twenty-two studies aimed to compare quality outcomes at community hospitals with other hospital types, of which 16 performed risk-adjusted analyses (detailed findings of observational studies are summarized in Appendix Table 2). The remaining 14 articles were interventional studies (Table 2). Of these, 12 (86%) reported improvement in quality outcomes after implementation (detailed findings of interventional studies are summarized in Appendix Table 3).
The included studies evaluated quality outcomes addressing all six of the IOM aims of quality healthcare, with safety, effectiveness, and efficiency being the most predominant (Table 3). Patient-centeredness and timeliness were infrequently addressed, and only one study assessed equity.
Risk of bias was moderate or high for 27 (69%) of the observational and interventional cohort studies and four (100%) of the included cross-sectional studies (Table 2), with the median NOS score being 4 (range: 0-6) for cohort studies (Appendix Table 4) and 4 (range: 3-6) for cross-sectional studies (Appendix Table 5). The higher risk of bias was largely driven by low comparability scores due to inadequate risk adjustment or statistical reporting. Of the 12 studies with low risk of bias, 11 (92%) were multisite, 9 (75%) used large regional or national databases, and half reported quality outcomes limited to hospital charges and/or mortality.
Observational Studies
Neonatal Medicine
Five multisite studies focused on neonatal care,13,14,16,19,20 of which four examined outcomes associated with the transfer of neonates to or from community hospitals to tertiary care hospitals, such as neonatal morbidity, readmission, completion of preventative health measures and screening, parent satisfaction, and hospital charges.14,16,19,20 For example, in a study of extremely premature very low birth weight infants born in Hawaii, Kuo et al. demonstrated that the odds of retinopathy of prematurity was 2.9 times higher for infants born at a community hospital and transported to their tertiary center compared with those inborn at the tertiary center (P = .02).16 The fifth study examined neonatal mortality by site of birth, demonstrating that, among infants with birth weights less than 2,000 g, birth at a hospital with a community neonatal intensive care unit (NICU) was associated with 1.4 times higher odds of risk-adjusted mortality compared with birth at a regional NICU (P < .001).13
Three studies evaluated the quality of neonatal care at a single community hospital.15,17,18 Quality outcomes were heterogeneous, including utility of rebound bilirubin levels for infants with jaundice, morbidity of neonates requiring mechanical ventilation, and provision of breastfeeding advice to mothers of breastfeeding infants. For instance, Meadow et al. attempted to determine the quality of care for ventilated neonates at one community NICU compared with a tertiary hospital.18 They found no difference in days on ventilation or need for home oxygen therapy between the community hospital and the tertiary center, although P values and effect sizes were not reported for these outcomes and analyses were not adjusted beyond matching on birthdate and birth weight.
Pediatric Medicine
Nine multisite studies explored the quality and safety of pediatric medical care across hospital types.21-23,25-30 Of these, four were conducted using the Kids’ Inpatient Database (KID)28-30 or the National Inpatient Sample (NIS),27 two were conducted using other national databases,21,25 and two were conducted using electronic medical record data.22,26 All of the KID and NIS studies examined hospital charges, either alone or in conjunction with mortality. Two of these studies found no differences in risk-adjusted hospital charges between children’s hospitals and community hospitals (for burn injuries),28,29 whereas two found that hospitalization at community hospitals was associated with lower risk-adjusted charges (for asthma and sepsis).27,30 The remaining five studies evaluated diverse quality outcomes such as medication errors, therapeutic drug monitoring, practice guideline compliance, antibiotic prescribing, or hospital-to-home transition summary scores.21-23,25,26
Three single-site studies examined quality outcomes for pediatric medical patients, including mortality, outcome ratings for dehydration, and measures of peripherally inserted central catheter (PICC) safety and effectiveness.24,31,32 For example, Frank et al. evaluated safety of care in one community hospital pediatric intensive care unit (PICU) compared with a tertiary hospital using the Pediatric Risk of Mortality (PRISM) score.24 They reported that the observed number of deaths in their community PICU did not differ significantly from the number of deaths predicted in a tertiary center (23 vs 33, respectively, P > .2).
Surgery
Three multisite studies examined quality outcomes among children with surgical conditions, including surgical complications, readmission, and hospital charges.34,35,37 For example, Kelley-Quon et al. examined outcomes following surgery in infants with hypertrophic pyloric stenosis and found that infants who received their surgery at community hospitals had twice the odds of a surgical complication compared with those at children’s hospitals (P = .027).34 They also examined how the risk of appendiceal perforation differed by hospital type, finding that black children who received their surgery at children’s hospitals had twice the odds of appendiceal perforation compared with those who received care at community hospitals.35
In addition, two studies evaluated quality and safety outcomes for pediatric surgical care in a single community hospital.33,36 For example, Beaty et al. prospectively evaluated the incidence of missed injuries in hospitalized pediatric trauma patients, reporting that the incidence of missed injury was 33% when admission evaluation was performed by a trauma surgeon alone compared to 11% when performed by a pediatric doctor or a trauma surgeon and a pediatric doctor together (P < .001).33
Radiology
Three multisite studies examined quality and safety outcomes associated with radiographic imaging in community hospitals, including radiation dosing and frequency of preoperative imaging modalities and accuracy.38,39,41 For instance, Marin et al. found substantial variation in radiation dose across hospital types, with children’s hospitals delivering lower median radiation doses than academic and community hospitals.39 Similarly, Saito et al. demonstrated increased use of radiating modalities when evaluation was performed at community hospitals, with four times higher odds of computed tomography (CT) and five times lower odds of ultrasound use compared to a children’s hospital.41
Two single-site studies also evaluated quality and safety outcomes associated with the use of radiographic imaging for pediatric appendicitis in a community hospital.40,42 For example, York et al. demonstrated that, compared with nonimaged patients, patients who underwent diagnostic imaging for appendicitis experienced a significant time delay from initial evaluation to surgery and incurred significantly higher hospital charges, whereas there were no significant differences in intraoperative findings, antibiotic requirements, and surgical complications between the groups.42
Interventional Studies
Neonatal Medicine
We identified six studies that evaluated interventions to improve healthcare quality for neonates in community hospitals; two involved telemedicine.43-48 Hall et al. described “Telenursery,” a program linking regional perinatal centers with a large academic neonatal practice through real-time teleconferencing, in addition to providing weekly educational conferences.45 After its implementation, there was an increase in the state-recommended delivery of very low birth weight infants at the regional perinatal center from 24% to 33% (P < .05); clinical outcomes were not discussed. In a similar study, Sable et al. found that a videoconferencing system for cardiologists from an academic center to guide care in a community setting provided diagnostic services more quickly (28 minutes vs 12 hours) and had high diagnostic accuracy.47 The remaining four studies evaluated heterogeneous interventions such as a maternal education program to reduce shaking injuries to infants or implementation of evidence-based order sets to reduce early onset group B streptococcal disease in neonates.43,44,46,48 Five of the six neonatal studies demonstrated improved quality outcomes after intervention.43-47
Pediatric Medicine
Seven studies evaluated QI interventions for children at community hospitals,49-55 three of which described interventions to improve quality of management of respiratory diseases.49,51,53 Dayal et al. evaluated the implementation of respiratory illness order sets and an asthma pathway, demonstrating a 41% reduction in asthma hospitalization cost per patient (P < .05), reduced bronchodilator use for all respiratory illnesses, and no change in readmission rates.49 Similarly, Nkoy et al. evaluated an asthma “Evidence-Based Care Practice Model” implemented at seven community hospitals and demonstrated a nonsignificant reduction in readmissions (P = .12), as well as lower hospitalization costs (P = .05).53 Using QI methods, Kuhlmann et al. demonstrated improved compliance from 43% to 97% with the Asthma Home Management Plan of Care measure.51 The remaining four studies evaluated heterogeneous interventions, including telemedicine critical care consultations, use of I-PASS, and consolidation of pediatric care onto one hospital unit.50,52,54,55 Six of the seven pediatric studies (86%) demonstrated improved inpatient quality outcomes after intervention,49,51-55 but only one study was multisite.53
Surgery
We identified only one study evaluating a surgical intervention aimed at improving the quality of pediatric care.56 Kelley-Quon evaluated the impact of a community hospital partnering with an Academic Medical Pediatric Trauma Center to become a Level II Pediatric Trauma Center (PTC). After achieving Level II PTC designation, they reported that children treated at the community hospital had reduced rates of CT use, transfers, and in-hospital mortality (from 81% to 51%, 8.5% to 2.5%, and 2% to 0.4%, respectively, P < .05 for all) compared to those treated predesignation.
Radiology
Within the domain of radiology, our review identified no interventional studies.
DISCUSSION
In this scoping review of the quality and safety of pediatric inpatient care in community hospitals, we identified 44 studies applying heterogeneous study designs and evaluating diverse patient populations and quality outcomes. We identified only one RCT in our search; all the remaining studies applied observational designs.
We found only three clinical areas that were explored in multiple studies, with consistent directionality of results: (1) perinatal regionalization, (2) telemedicine, and (3) imaging radiation. The limited evidence identified in our review suggests that delivery of early premature infants at community hospitals, rather than at tertiary hospitals, may increase risk of neonatal morbidity and/or mortality,13,16,46 that use of telemedicine may improve the effectiveness and efficiency of intensive or specialized care in community settings,45,47,52,55 and that CT use and radiation doses may be higher in community hospitals compared with other settings.38,39,41 However, even within these clinical domains, the literature was limited in amount and heterogeneous; additional research is needed to systematically review the effect of individual interventions or particular community hospital quality outcomes compared with other hospital types.
Our search identified only 14 studies evaluating QI interventions within community hospitals over the almost 30-year review period. Although limited in number, 86% of these demonstrated improvements in healthcare quality and safety, providing a positive “proof of concept” that pediatric care in community hospitals can be improved by multidisciplinary efforts and be sustained over time.43-47,49,51-56 As pediatric departments within community hospitals may have limited resources, aligning pediatric QI efforts with adult initiatives within the same hospitals could prove advantageous. However, we did not identify any studies meeting our inclusion criteria that took this approach. Alternatively, QI collaboratives across structurally diverse hospitals may provide valuable infrastructure for QI in community hospitals. For example, the Value in Inpatient Pediatrics Network has conducted several multisite QI initiatives that have engaged both children’s and community hospitals.57-60 However, none of these studies have reported community hospital-specific quality outcomes, resulting in their exclusion from this review. In future study, researchers may consider separating community hospital results from those of pediatric hospitals to highlight the effect of community hospital-specific QI efforts and to allow valuable direct comparisons between hospital types.
Many studies identified in our scoping review were conducted at single hospitals. Sample sizes were often very small and power calculations were rarely reported, raising questions about the validity of the “nonsignificant” differences reported by some. Although such single-center studies provide valuable information for local improvement of inpatient care, by design the findings from these studies are unlikely generalizable to other community hospital systems. Without inclusion of another hospital type and/or quality measures with clear national benchmark for comparison, the additional conclusions that can be drawn from these studies are limited. In comparison, many of the multicenter studies had large study samples, but more than half used data registries with limited data to evaluate outcomes, clinical context, or important covariates.13,20,21,25,27-30,34,35,37,39 The most frequently reported outcomes in these data sets—hospital charges and lengths of stay—are of limited utility in understanding healthcare quality without clear benchmarks. As a result, the evidence-base from which to draw conclusions about quality and safety in community hospitals is very limited.
Therefore, our review highlights a great need for additional research in community hospital medicine and the need for high-quality evidence generation. Risk of bias was moderate or high for the majority of included studies because of inadequate risk adjustment or statistical analysis. In the future, multicenter collaborations may help to connect research methodologists with community hospital teams to aid in the application of robust study designs and analytic techniques. Multisite collaboration may also overcome the limitation of small sample sizes that are a reality at many community hospitals.
Our study findings must be considered in the setting of several methodological limitations. The lack of a standard definition for a community hospital has led to inconsistent terms and hospital definitions used in the literature. It is possible that, while following our systematic approach to define a community hospital, we inadvertently missed relevant studies that used different terms. We also excluded unpublished articles. Given that publication bias tends to favor studies with significant associations, it is possible that some studies with insignificant changes in quality outcomes were missed. Finally, in our exclusion of all non-US studies, we may have unknowingly missed literature from countries with community hospital definitions similar to those in the United States.
CONCLUSIONS
Recognizing that more than half of all children admitted to hospitals in the US receive their care at community hospitals, understanding healthcare quality in community hospitals is important. This scoping review underscores the need for additional research and higher quality evidence to determine the quality of pediatric inpatient care in these settings and identifies some particularly wide gaps that could be targeted in future research. Acknowledging that further research is necessary to address all aims of quality healthcare, markedly few studies have examined timeliness, equity, or patient-centeredness. Collaborations between academic medical centers and community hospitals may be an effective means to connect researchers with community hospital clinical teams to facilitate the application of robust study designs and analytic approaches and to facilitate multisite investigations. Research in this field would benefit from a standardized definition of a community hospital that could be consistently applied in research and QI endeavors.
Disclosures
The authors have no potential conflicts of interest to disclose.
Funding
Jana Leary was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, Grant Number 5TL1TR0001062-03. JoAnna Leyenaar was supported by the Agency for Healthcare Research and Quality (K08HS024133).
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the AHRQ.
1. McGlynn EA, Asch SM, Adams J, et al. The quality of health care delivered to adults in the United States. N Engl J Med. 2003;348(26):2635-2645. https://doi.org/10.1056/NEJMsa022615.
2. Richardson WC, Berwick DM, Bisgard C, et al. To err is human: building a safer health system-Institute of Medicine. Medscape. http://www.iom.edu/Reports/1999/To-Err-is-Human-Building-A-Safer-Health-System.aspx; 2000. Accessed October 7, 2016.
3. Classen DC, Resar R, Griffin F, et al. ‘Global Trigger Tool’ shows that adverse events in hospitals may be ten times greater than previously measured. Health Aff. 2011;30(4):581-589. https://doi.org/10.1377/hlthaff.2011.0190.
4. Stockwell DC, Landrigan CP, Schuster MA, et al. Using a pediatric trigger tool to estimate total harm burden hospital-acquired conditions represent. Pediatr Qual Saf. 2018;3(3):e081. https://doi.org/10.1097/pq9.0000000000000081.
5. Institute of Medicine (US) Committee on Quality of Health Care in America. Crossing the Quality Chasm. Washington (DC): National Academies Press (US); 2001. https://doi.org/10.17226/10027.
6. Rauch DA, Lye PS, Carlson D, et al. Pediatric hospital medicine: a strategic planning roundtable to chart the future. J Hosp Med. 2012;7(4):329-334. https://doi.org/10.1002/jhm.950.
7. Simpson L, Fairbrother G, Hale S et al. Reauthorizing SCHIP: Opportunities for Promoting Effective Health Coverage and HighQuality Care for Children and Adolescents. (Publication 1051). Commonw Fund; 2007.
8. Percelay JM. Pediatric hospitalists working in community hospitals. Pediatr Clin North Am. 2014;61(4):681-691. https://doi.org/10.1016/j.pcl.2014.04.005.
9. Leyenaar JK, Ralston SL, Shieh MS et al. Epidemiology of pediatric hospitalizations at general hospitals and freestanding children’s hospitals in the United States. J Hosp Med. 2016;11(11):743-749. https://doi.org/10.1002/jhm.2624.
10. Peters MDJ, Godfrey CM, Khalil H et al. Guidance for conducting systematic scoping reviews. Int J Evid Based Healthc. 2015;13(3):141-146. https://doi.org/10.1097/XEB.0000000000000050.
11. Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467-473. https://doi.org/10.7326/M18-0850.
12. Alobaidi R, Morgan C, Basu RK, et al. Association between fluid balance and outcomes in critically ill children: a systematic review and meta-analysis. JAMA Pediatr. 2018;172(3):257-268. https://doi.org/10.1001/jamapediatrics.2017.4540.
13. Cifuentes J, Bronstein J, Phibbs CS et al. Mortality in low birth weight infants according to level of neonatal care at hospital of birth. Pediatrics. 2002;109(5):745-751. https://doi.org/10.1542/peds.109.5.745.
14. Donohue PK, Hussey-Gardner B, Sulpar LJ, Fox R, Aucott SW. Convalescent care of infants in the neonatal intensive care unit in community hospitals: risk or benefit? Pediatrics. 2009;124(1):105-111. https://doi.org/10.1542/peds.2008-0880.
15. Izatt SD. Breastfeeding counseling by health care providers. J Hum Lact. 1997;13(2):109-113. https://doi.org/10.1177/089033449701300210.
16. Kuo S, Kimata C, Akamine K, Young B, Balaraman V. Outcomes of inborn and transported extremely premature very-low-birthweight infants in Hawai’i. Pediatr Int. 2012;54(3):365-369. https://doi.org/10.1111/j.1442-200X.2012.03561.x.
17. Maisels MJ, Kring E. Rebound in serum bilirubin level following intensive phototherapy. Arch Pediatr Adolesc Med. 2002;156(7):669-672. https://doi.org/10.1001/archpedi.156.7.669.
18. Meadow W, Mendez D, Makela J et al. Can and should level II nurseries care for newborns who require mechanical ventilation? Clin Perinatol. 1996;23(3):551-561. https://doi.org/10.1016/S0095-5108(18)30227-6.
19. Phibbs CS, Mortensen L. Back transporting infants from neonatal intensive care units to community hospitals for recovery care: effect on total hospital charges. Pediatrics. 1992;90(1):22-26.
20. Wall SN, Handler AS, Park CG. Hospital factors and nontransfer of small babies: A marker of deregionalized perinatal care? J Perinatol. 2004;24(6):351-359. https://doi.org/10.1038/sj.jp.7211101.
21. Alexander DC, Bundy DG, Shore AD et al. Cardiovascular medication errors in children. Pediatrics. 2009;124(1):324-332. https://doi.org/10.1542/peds.2008-2073.
22. Balch AH, Constance JE, Thorell EA, et al. Pediatric vancomycin dosing: trends over time and the impact of therapeutic drug monitoring. J Clin Pharmacol. 2015;55(2):212-220. https://doi.org/10.1002/jcph.402.
23. Conway PH, Edwards S, Stucky ER et al. Variations in management of common inpatient pediatric illnesses: hospitalists and community pediatricians. Pediatrics. 2006;118(2):441-447. https://doi.org/10.1542/peds.2006-0484.
24. Frank BS, Pollack MM. Quantitative quality assurance in a community hospital pediatric intensive care unit. West J Med. 1992;157(2):149-151.
25. Leyenaar JK, Lagu T, Shieh MS, Pekow PS, Lindenauer PK. Variation in resource utilization for the management of uncomplicated community-acquired pneumonia across community and children’s hospitals. J Pediatr. 2014;165(3):585-591. https://doi.org/10.1016/j.jpeds.2014.04.062.
26. Leyenaar JK, Desai AD, Burkhart Q, et al. Quality measures to assess care transitions for hospitalized children. Pediatrics. 2016;138(2):e20160906. https://doi.org/10.1542/peds.2016-0906.
27. Meurer JR, Kuhn EM, George V, Yauck JS, Layde PM. Charges for childhood asthma by hospital characteristics. Pediatrics. 1998;102(6):E70. https://doi.org/10.1542/peds.102.6.e70.
28. Myers J, Lehna C. Where are lengths of stay longer and total charges higher for pediatric burn patients? J Burn Care Res. 2014;35(5):382-387. https://doi.org/10.1097/BCR.0000000000000012.
29. Myers J, Smith M, Woods C, Espinosa C, Lehna C. The effect of transfers between health care facilities on costs and length of stay for pediatric burn patients. J Burn Care Res. 2015;36(1):178-183. https://doi.org/10.1097/BCR.0000000000000206.
30. Odetola FO, Gebremariam A, Freed GL. Patient and hospital correlates of clinical outcomes and resource utilization in severe pediatric sepsis. Pediatrics. 2007;119(3):487-494. https://doi.org/10.1542/peds.2006-2353.
31. Scherb CA, Stevens MS, Busman C. Outcomes related to dehydration in the pediatric population. J Pediatr Nurs. 2007;22(5):376-382. https://doi.org/10.1016/j.pedn.2006.10.004.
32. Van Winkle P, Whiffen T, Liu IL. Experience using peripherally inserted central venous catheters for outpatient parenteral antibiotic therapy in children at a community hospital. ediatr Infect Dis J. 2008;27(12):1069-1072. https://doi.org/10.1097/INF.0b013e31817d32f2.
33. Beaty JS, Chendrasekhar A, Hopkins J, Gruelke L. Missed injuries in pediatric trauma patients. J Appl Res. 2003;3(1):84-88. https://jrnlappliedresearch.com/articles/Vol3Iss1/CHENDRASEKHAR.htm. Accessed July 8, 2019.
34. Kelley-Quon LI, Tseng CH, Jen HC, Shew SB. Hospital type predicts surgical complications for infants with hypertrophic pyloric stenosis. Am Surg. 2012;78(10):1079-1082.
35. Kelley-Quon LI, Tseng CH, Jen HC, Lee SL, Shew SB. Hospital type as a metric for racial disparities in pediatric appendicitis. J Am Coll Surg. 2013;216(1):74-82. https://doi.org/10.1016/j.jamcollsurg.2012.09.018.
36. Pokala N, Sadhasivam S, Kiran RP, Parithivel V. Complicated appendicitis—is the laparoscopic approach appropriate? A comparative study with the open approach: outcome in a community hospital setting. Am Surg. 2007;73(8):732-737.
37. Smith JT, Price C, Stevens PM, Masters KS, Young M. Does pediatric orthopedic subspecialization affect hospital utilization and charges? J Pediatr Orthop. 1999;19(4):553-555. https://doi.org/10.1097/01241398-199907000-00027.
38. Calvert C, Strauss KJ, Mooney DP. Variation in computed tomography radiation dose in community hospitals. J Pediatr Surg. 2012;47(6):1167-1169. https://doi.org/10.1016/j.jpedsurg.2012.03.021.
39. Marin JR, Sengupta D, Bhargavan-Chatfield M et al. Variation in pediatric cervical spine computed tomography radiation dose index. Acad Emerg Med. 2015;22(12):1499-1505. https://doi.org/10.1111/acem.12822.
40. Reich JD, Brogdon B, Ray WE, Eckert J, Gorell H. Use of CT scan in the diagnosis of pediatric acute appendicitis. Pediatr Emerg Care. 2000;16(4):241-243. https://doi.org/10.1097/00006565-200008000-00006.
41. Saito JM, Yan Y, Evashwick TW, Warner BW, Tarr PI. Use and accuracy of diagnostic imaging by hospital type in pediatric appendicitis. Pediatrics. 2013;131(1):e37-e44. https://doi.org/10.1542/peds.2012-1665.
42. York D, Smith A, Phillips JD, Von Allmen D. The influence of advanced radiographic imaging on the treatment of pediatric appendicitis. J Pediatr Surg. 2005;40(12):1908-1911. https://doi.org/10.1016/j.jpedsurg.2005.08.004.
43. Altman RL, Canter J, Patrick PA et al. Parent education by maternity nurses and prevention of abusive head trauma. Pediatrics. 2011;128(5):e1164-e1172. https://doi.org/10.1542/peds.2010-3260.
44. Clemens CJ, Gable EK. The development of a group B streptococcus prevention policy at a community hospital. J Perinatol. 2002;22(7):523-525. https://doi.org/10.1038/sj.jp.7210794.
45. Hall RW, Hall-Barrow J, Garcia-Rill E. Neonatal regionalization through telemedicine using a community-based research and education core facility. Ethn Dis. 2010;20(1 Suppl 1):S136-S140.
46. Hulsey TC, Pittard WB 3rd, Ebeling M. Regionalized perinatal transport systems: association with changes in location of birth, neonatal transport, and survival of very low birth weight deliveries. J S C Med Assoc. 1991;87(12):581-584.
47. Sable CA, Cummings SD, Pearson GD, et al. Impact of telemedicine on the practice of pediatric cardiology in community hospitals. Pediatrics. 2002;109(1):E3. https://doi.org/10.1542/peds.109.1.e3.
48. Wexelblatt SL, Ward LP, Torok K et al. Universal maternal drug testing in a high-prevalence region of prescription opiate abuse. J Pediatr. 2015;166(3):582-586. https://doi.org/10.1016/j.jpeds.2014.10.004.
49. Dayal A, Alvarez F. The effect of implementation of standardized, evidence-based order sets on efficiency and quality measures for pediatric respiratory illnesses in a community hospital. Hosp Pediatr. 2015;5(12):624-629. https://doi.org/10.1542/hpeds.2015-0140.
50. Krugman SD, Suggs A, Photowala HY, Beck A. Redefining the community pediatric hospitalist: the combined Pediatric ED/Inpatient Unit. Pediatr Emerg Care. 2007;23(1):33-37. https://doi.org/10.1097/01.pec.0000248685.94647.01.
51. Kuhlmann S, Mason B, Ahlers-Schmidt CR. A quality improvement project to improve compliance with the joint commission children’s asthma care-3 measure. Hosp Pediatr. 2013;3(1):45-51. https://doi.org/10.1542/hpeds.2012-0015.
52. Labarbera JM, Ellenby MS, Bouressa P et al. The impact of telemedicine intensivist support and a pediatric hospitalist program on a community hospital. Telemed J E Health. 2013;19(10):760-766. https://doi.org/10.1089/tmj.2012.0303.
53. Nkoy F, Fassl B, Stone B, et al. Improving pediatric asthma care and outcomes Across multiple hospitals. Pediatrics. 2015;136(6):e1602-e1610. https://doi.org/10.1542/peds.2015-0285.
54. Walia J, Qayumi Z, Khawar N, et al. Physician transition of care: benefits of I-PASS and an electronic handoff system in a community pediatric residency program. Acad Pediatr. 2016;16(6):519-523. https://doi.org/10.1016/j.acap.2016.04.001.
55. Yang CP, Hunt EA, Shilkofski N et al. Can telemedicine improve adherence to resuscitation guidelines for critically ill children at community hospitals? A randomized controlled trial using high-fidelity simulation. Pediatr Emerg Care. 2017;33(7):474-479. https://doi.org/10.1097/PEC.0000000000000653.
56. Kelley-Quon LI, Crowley MA, Applebaum H, et al. Academic-community partnerships improve outcomes in pediatric trauma care. J Pediatr Surg. 2015;50(6):1032-1036. https://doi.org/10.1016/j.jpedsurg.2015.03.033.
57. Ralston S, Garber M, Narang S, et al. Decreasing unnecessary utilization in acute bronchiolitis care: results from the value in inpatient pediatrics network. J Hosp Med. 2013;8(1):25-30. https://doi.org/10.1002/jhm.1982.
58. Ralston SL, Garber MD, Rice-Conboy E, et al. A multicenter collaborative to reduce unnecessary care in inpatient bronchiolitis. Pediatrics. 2016;137(1):e20150851. https://doi.org/10.1542/peds.2015-0851.
59. Parikh K, Biondi E, Nazif J, et al. A multicenter collaborative to improve care of community acquired pneumonia in hospitalized children. Pediatrics. 2017;139(3):e20161411. https://doi.org/10.1542/peds.2016-1411.
60. Leyenaar JK, Bergert L, Mallory LA, et al. Pediatric primary care providers’ perspectives regarding hospital discharge communication: a mixed methods analysis. Acad Pediatr. 2015;15(1):61-68. https://doi.org/10.1016/j.acap.2014.07.004.
Despite efforts to provide high-quality healthcare, Americans die from medical errors each year and many patients do not receive recommended medical care. Risk is particularly acute during times of hospitalization.1-4 In response, the Institute of Medicine (IOM, now the Academy of Medicine) has released “Crossing the Quality Chasm: A New Health System for the 21st Century,” providing a framework to guide delivery and measurement of high-quality healthcare.5
Although the IOM framework has motivated the development of quality improvement (QI) and quality measurement initiatives, relatively few resources have been allocated to improving the quality of pediatric inpatient care.6,7 The resultant gap in our knowledge of quality and safety of pediatric hospital-based care is further widened by the variability of settings in which children are hospitalized. These settings include freestanding children’s hospitals, children’s hospitals nested within larger hospitals, and community hospitals, defined as general, nonuniversity, and nonchildren’s hospitals.8
Although almost three-quarters of children needing hospitalization are cared for outside of freestanding children’s hospitals, we know particularly little about the quality and safety of pediatric hospital-based care outside of these settings.6,9 Therefore, our scoping review aims to summarize literature regarding the quality and safety of pediatric inpatient care within community hospitals.
METHODS
We used a scoping review approach because this methodology, by design, is utilized to synthesize evidence and map existing literature and is particularly useful when a body of literature is heterogeneous, rendering a more targeted systematic review approach infeasible.10 This methodology thereby provided an organized approach to answer our broad research question, “What evidence exists regarding the quality and safety of pediatric inpatient care in United States community hospitals?“ We followed the scoping review guidelines put forth by the Joanna Briggs Institute and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) reporting guideline Extension for Scoping Reviews.10,11
Data Sources and Search Strategies
We searched Medline, Medline-In-Process, Embase, the Cochrane Database of Systematic Reviews, the Cumulative Index to Nursing and Allied Health Literature (CINAHL), PsycINFO, and Scopus for studies that reported at least one outcome related to healthcare quality or patient safety and involved pediatric patients (aged <18 years) receiving inpatient care at a community hospital. Outcomes included measures from the IOM-defined aims of quality healthcare: (1) safety, (2) effectiveness, (3) efficiency, (4) timeliness, (5) patient-centeredness, and (6) equity (Appendix Table 1). Terms were searched as controlled vocabulary in applicable databases (Medline, Embase, CINAHL, PsycINFO) and as keywords in all databases. Search strategies tailored to each database were developed, tested, and refined in collaboration with a reference librarian. Date parameters for retrieval were set from 1989 to the search date, with the start date chosen to correspond with the establishment of the Agency for Health Care Policy and Research, currently known as the Agency for Healthcare Research and Quality (AHRQ), targeting literature produced in the wake of the AHRQ emphasis on quality in healthcare. Results were limited to articles published in English. Searches were conducted on October 24 and 25, 2016. Complete search strategies can be found in Appendix Methods.
Independent authors performed handsearches of Academic Pediatrics, BMJ Quality & Safety, Hospital Pediatrics, JAMA Pediatrics, and Pediatrics Quality Reports for the five years preceding the search date (July 2011-October 2016).
Study Selection and Definitions
To identify studies conducted in community hospitals, we operationalized the definition of community hospital proposed by Percelay.8 We used “community”, “general”, “nonuniversity”, and “nonchildren’s” as search terms and operationalized these through the addition of qualifying descriptors (Table 1).
Studies were excluded if they (1) were performed outside of the United States, as community hospital definitions may differ by country; (2) included participants aged >18 years and did not report any pediatric-specific results; (3) were performed exclusively in tertiary hospitals; (4) evaluated only outpatient or emergency department care; (5) did not report any results specific to community hospitals; (6) did not report any data-driven or parent/patient-reported measures of safety, effectiveness, efficiency, timeliness, patient-centeredness, or equity in their results; and (7) were case reports, case series, editorials, or abstracts without an associated full-text article. We read commentaries and literature reviews related to our objectives and reviewed their references to identify additional articles, but did not include these manuscripts.
Two authors independently reviewed each abstract, and full-text articles were reviewed if one or more authors determined that the abstract met the inclusion criteria. Two authors then independently reviewed each full-text article to determine whether the article met the criteria for the final review. Disagreements were resolved through consensus after discussion and review with the entire research team, and reasons for exclusion were recorded.
Charting the Results and Data Synthesis
We used a standardized charting form to collect information regarding study design, community hospital terms, population descriptors, IOM aims of quality healthcare, and outcome measures. To minimize bias in data collection, information from each full-text article was extracted independently by two authors, and differences in extraction were resolved through discussion and re-review among the same two authors. To evaluate the quality of included evidence, two authors (JCL and JKL) independently assessed the risk of bias for each study using modified Newcastle-Ottawa Quality Assessment Scales (NOS), with disagreements resolved through discussion and re-review among the same two authors. For cohort studies, five of the eight NOS domains were relevant and applied to all included studies (maximum score 6). Using the NOS adapted for cross-sectional studies, six of the seven domains were relevant and applied (maximum score 9). For cross-sectional studies, we defined the risk of bias to be low for scores ≥8, moderate for scores 5-7, and high for scores ≤4, consistent with previous work.12 For cohort studies, we similarly defined these strata by scores ≥5, 3-4, and ≤2, given the lower maximum score for this study type.
We categorized studies as either observational, defined as cohort or cross-sectional studies of usual healthcare delivery, or interventional, defined as studies evaluating the development and/or implementation of an intervention designed to improve healthcare quality. We further categorized the studies into the following four overarching medical domains: (1) neonatal, (2) pediatric medicine, (3) surgery, or (4) radiology.
RESULTS
After removal of duplicates, our search identified 2,068 abstracts for screening (Figure). Of these, 1,777 did not meet the inclusion criteria, leaving 291 articles for full-text review. Of these, 43 articles met all the inclusion criteria, and one additional article was included from search of references, resulting in a total of 44 articles.
Study designs, patient populations, and quality outcome measures were heterogeneous. We identified only one randomized controlled trial (RCT). A total of 30 articles were observational studies, 27 of which used retrospective cohort or cross-sectional designs; the remaining three used prospective cohort designs (Table 2). Of these studies, 20 involved multiple hospitals, whereas 10 were conducted at a single community hospital. Sample sizes ranged from 29 (single-site) to 107,727 (multisite) patients. Twenty-two studies aimed to compare quality outcomes at community hospitals with other hospital types, of which 16 performed risk-adjusted analyses (detailed findings of observational studies are summarized in Appendix Table 2). The remaining 14 articles were interventional studies (Table 2). Of these, 12 (86%) reported improvement in quality outcomes after implementation (detailed findings of interventional studies are summarized in Appendix Table 3).
The included studies evaluated quality outcomes addressing all six of the IOM aims of quality healthcare, with safety, effectiveness, and efficiency being the most predominant (Table 3). Patient-centeredness and timeliness were infrequently addressed, and only one study assessed equity.
Risk of bias was moderate or high for 27 (69%) of the observational and interventional cohort studies and four (100%) of the included cross-sectional studies (Table 2), with the median NOS score being 4 (range: 0-6) for cohort studies (Appendix Table 4) and 4 (range: 3-6) for cross-sectional studies (Appendix Table 5). The higher risk of bias was largely driven by low comparability scores due to inadequate risk adjustment or statistical reporting. Of the 12 studies with low risk of bias, 11 (92%) were multisite, 9 (75%) used large regional or national databases, and half reported quality outcomes limited to hospital charges and/or mortality.
Observational Studies
Neonatal Medicine
Five multisite studies focused on neonatal care,13,14,16,19,20 of which four examined outcomes associated with the transfer of neonates to or from community hospitals to tertiary care hospitals, such as neonatal morbidity, readmission, completion of preventative health measures and screening, parent satisfaction, and hospital charges.14,16,19,20 For example, in a study of extremely premature very low birth weight infants born in Hawaii, Kuo et al. demonstrated that the odds of retinopathy of prematurity was 2.9 times higher for infants born at a community hospital and transported to their tertiary center compared with those inborn at the tertiary center (P = .02).16 The fifth study examined neonatal mortality by site of birth, demonstrating that, among infants with birth weights less than 2,000 g, birth at a hospital with a community neonatal intensive care unit (NICU) was associated with 1.4 times higher odds of risk-adjusted mortality compared with birth at a regional NICU (P < .001).13
Three studies evaluated the quality of neonatal care at a single community hospital.15,17,18 Quality outcomes were heterogeneous, including utility of rebound bilirubin levels for infants with jaundice, morbidity of neonates requiring mechanical ventilation, and provision of breastfeeding advice to mothers of breastfeeding infants. For instance, Meadow et al. attempted to determine the quality of care for ventilated neonates at one community NICU compared with a tertiary hospital.18 They found no difference in days on ventilation or need for home oxygen therapy between the community hospital and the tertiary center, although P values and effect sizes were not reported for these outcomes and analyses were not adjusted beyond matching on birthdate and birth weight.
Pediatric Medicine
Nine multisite studies explored the quality and safety of pediatric medical care across hospital types.21-23,25-30 Of these, four were conducted using the Kids’ Inpatient Database (KID)28-30 or the National Inpatient Sample (NIS),27 two were conducted using other national databases,21,25 and two were conducted using electronic medical record data.22,26 All of the KID and NIS studies examined hospital charges, either alone or in conjunction with mortality. Two of these studies found no differences in risk-adjusted hospital charges between children’s hospitals and community hospitals (for burn injuries),28,29 whereas two found that hospitalization at community hospitals was associated with lower risk-adjusted charges (for asthma and sepsis).27,30 The remaining five studies evaluated diverse quality outcomes such as medication errors, therapeutic drug monitoring, practice guideline compliance, antibiotic prescribing, or hospital-to-home transition summary scores.21-23,25,26
Three single-site studies examined quality outcomes for pediatric medical patients, including mortality, outcome ratings for dehydration, and measures of peripherally inserted central catheter (PICC) safety and effectiveness.24,31,32 For example, Frank et al. evaluated safety of care in one community hospital pediatric intensive care unit (PICU) compared with a tertiary hospital using the Pediatric Risk of Mortality (PRISM) score.24 They reported that the observed number of deaths in their community PICU did not differ significantly from the number of deaths predicted in a tertiary center (23 vs 33, respectively, P > .2).
Surgery
Three multisite studies examined quality outcomes among children with surgical conditions, including surgical complications, readmission, and hospital charges.34,35,37 For example, Kelley-Quon et al. examined outcomes following surgery in infants with hypertrophic pyloric stenosis and found that infants who received their surgery at community hospitals had twice the odds of a surgical complication compared with those at children’s hospitals (P = .027).34 They also examined how the risk of appendiceal perforation differed by hospital type, finding that black children who received their surgery at children’s hospitals had twice the odds of appendiceal perforation compared with those who received care at community hospitals.35
In addition, two studies evaluated quality and safety outcomes for pediatric surgical care in a single community hospital.33,36 For example, Beaty et al. prospectively evaluated the incidence of missed injuries in hospitalized pediatric trauma patients, reporting that the incidence of missed injury was 33% when admission evaluation was performed by a trauma surgeon alone compared to 11% when performed by a pediatric doctor or a trauma surgeon and a pediatric doctor together (P < .001).33
Radiology
Three multisite studies examined quality and safety outcomes associated with radiographic imaging in community hospitals, including radiation dosing and frequency of preoperative imaging modalities and accuracy.38,39,41 For instance, Marin et al. found substantial variation in radiation dose across hospital types, with children’s hospitals delivering lower median radiation doses than academic and community hospitals.39 Similarly, Saito et al. demonstrated increased use of radiating modalities when evaluation was performed at community hospitals, with four times higher odds of computed tomography (CT) and five times lower odds of ultrasound use compared to a children’s hospital.41
Two single-site studies also evaluated quality and safety outcomes associated with the use of radiographic imaging for pediatric appendicitis in a community hospital.40,42 For example, York et al. demonstrated that, compared with nonimaged patients, patients who underwent diagnostic imaging for appendicitis experienced a significant time delay from initial evaluation to surgery and incurred significantly higher hospital charges, whereas there were no significant differences in intraoperative findings, antibiotic requirements, and surgical complications between the groups.42
Interventional Studies
Neonatal Medicine
We identified six studies that evaluated interventions to improve healthcare quality for neonates in community hospitals; two involved telemedicine.43-48 Hall et al. described “Telenursery,” a program linking regional perinatal centers with a large academic neonatal practice through real-time teleconferencing, in addition to providing weekly educational conferences.45 After its implementation, there was an increase in the state-recommended delivery of very low birth weight infants at the regional perinatal center from 24% to 33% (P < .05); clinical outcomes were not discussed. In a similar study, Sable et al. found that a videoconferencing system for cardiologists from an academic center to guide care in a community setting provided diagnostic services more quickly (28 minutes vs 12 hours) and had high diagnostic accuracy.47 The remaining four studies evaluated heterogeneous interventions such as a maternal education program to reduce shaking injuries to infants or implementation of evidence-based order sets to reduce early onset group B streptococcal disease in neonates.43,44,46,48 Five of the six neonatal studies demonstrated improved quality outcomes after intervention.43-47
Pediatric Medicine
Seven studies evaluated QI interventions for children at community hospitals,49-55 three of which described interventions to improve quality of management of respiratory diseases.49,51,53 Dayal et al. evaluated the implementation of respiratory illness order sets and an asthma pathway, demonstrating a 41% reduction in asthma hospitalization cost per patient (P < .05), reduced bronchodilator use for all respiratory illnesses, and no change in readmission rates.49 Similarly, Nkoy et al. evaluated an asthma “Evidence-Based Care Practice Model” implemented at seven community hospitals and demonstrated a nonsignificant reduction in readmissions (P = .12), as well as lower hospitalization costs (P = .05).53 Using QI methods, Kuhlmann et al. demonstrated improved compliance from 43% to 97% with the Asthma Home Management Plan of Care measure.51 The remaining four studies evaluated heterogeneous interventions, including telemedicine critical care consultations, use of I-PASS, and consolidation of pediatric care onto one hospital unit.50,52,54,55 Six of the seven pediatric studies (86%) demonstrated improved inpatient quality outcomes after intervention,49,51-55 but only one study was multisite.53
Surgery
We identified only one study evaluating a surgical intervention aimed at improving the quality of pediatric care.56 Kelley-Quon evaluated the impact of a community hospital partnering with an Academic Medical Pediatric Trauma Center to become a Level II Pediatric Trauma Center (PTC). After achieving Level II PTC designation, they reported that children treated at the community hospital had reduced rates of CT use, transfers, and in-hospital mortality (from 81% to 51%, 8.5% to 2.5%, and 2% to 0.4%, respectively, P < .05 for all) compared to those treated predesignation.
Radiology
Within the domain of radiology, our review identified no interventional studies.
DISCUSSION
In this scoping review of the quality and safety of pediatric inpatient care in community hospitals, we identified 44 studies applying heterogeneous study designs and evaluating diverse patient populations and quality outcomes. We identified only one RCT in our search; all the remaining studies applied observational designs.
We found only three clinical areas that were explored in multiple studies, with consistent directionality of results: (1) perinatal regionalization, (2) telemedicine, and (3) imaging radiation. The limited evidence identified in our review suggests that delivery of early premature infants at community hospitals, rather than at tertiary hospitals, may increase risk of neonatal morbidity and/or mortality,13,16,46 that use of telemedicine may improve the effectiveness and efficiency of intensive or specialized care in community settings,45,47,52,55 and that CT use and radiation doses may be higher in community hospitals compared with other settings.38,39,41 However, even within these clinical domains, the literature was limited in amount and heterogeneous; additional research is needed to systematically review the effect of individual interventions or particular community hospital quality outcomes compared with other hospital types.
Our search identified only 14 studies evaluating QI interventions within community hospitals over the almost 30-year review period. Although limited in number, 86% of these demonstrated improvements in healthcare quality and safety, providing a positive “proof of concept” that pediatric care in community hospitals can be improved by multidisciplinary efforts and be sustained over time.43-47,49,51-56 As pediatric departments within community hospitals may have limited resources, aligning pediatric QI efforts with adult initiatives within the same hospitals could prove advantageous. However, we did not identify any studies meeting our inclusion criteria that took this approach. Alternatively, QI collaboratives across structurally diverse hospitals may provide valuable infrastructure for QI in community hospitals. For example, the Value in Inpatient Pediatrics Network has conducted several multisite QI initiatives that have engaged both children’s and community hospitals.57-60 However, none of these studies have reported community hospital-specific quality outcomes, resulting in their exclusion from this review. In future study, researchers may consider separating community hospital results from those of pediatric hospitals to highlight the effect of community hospital-specific QI efforts and to allow valuable direct comparisons between hospital types.
Many studies identified in our scoping review were conducted at single hospitals. Sample sizes were often very small and power calculations were rarely reported, raising questions about the validity of the “nonsignificant” differences reported by some. Although such single-center studies provide valuable information for local improvement of inpatient care, by design the findings from these studies are unlikely generalizable to other community hospital systems. Without inclusion of another hospital type and/or quality measures with clear national benchmark for comparison, the additional conclusions that can be drawn from these studies are limited. In comparison, many of the multicenter studies had large study samples, but more than half used data registries with limited data to evaluate outcomes, clinical context, or important covariates.13,20,21,25,27-30,34,35,37,39 The most frequently reported outcomes in these data sets—hospital charges and lengths of stay—are of limited utility in understanding healthcare quality without clear benchmarks. As a result, the evidence-base from which to draw conclusions about quality and safety in community hospitals is very limited.
Therefore, our review highlights a great need for additional research in community hospital medicine and the need for high-quality evidence generation. Risk of bias was moderate or high for the majority of included studies because of inadequate risk adjustment or statistical analysis. In the future, multicenter collaborations may help to connect research methodologists with community hospital teams to aid in the application of robust study designs and analytic techniques. Multisite collaboration may also overcome the limitation of small sample sizes that are a reality at many community hospitals.
Our study findings must be considered in the setting of several methodological limitations. The lack of a standard definition for a community hospital has led to inconsistent terms and hospital definitions used in the literature. It is possible that, while following our systematic approach to define a community hospital, we inadvertently missed relevant studies that used different terms. We also excluded unpublished articles. Given that publication bias tends to favor studies with significant associations, it is possible that some studies with insignificant changes in quality outcomes were missed. Finally, in our exclusion of all non-US studies, we may have unknowingly missed literature from countries with community hospital definitions similar to those in the United States.
CONCLUSIONS
Recognizing that more than half of all children admitted to hospitals in the US receive their care at community hospitals, understanding healthcare quality in community hospitals is important. This scoping review underscores the need for additional research and higher quality evidence to determine the quality of pediatric inpatient care in these settings and identifies some particularly wide gaps that could be targeted in future research. Acknowledging that further research is necessary to address all aims of quality healthcare, markedly few studies have examined timeliness, equity, or patient-centeredness. Collaborations between academic medical centers and community hospitals may be an effective means to connect researchers with community hospital clinical teams to facilitate the application of robust study designs and analytic approaches and to facilitate multisite investigations. Research in this field would benefit from a standardized definition of a community hospital that could be consistently applied in research and QI endeavors.
Disclosures
The authors have no potential conflicts of interest to disclose.
Funding
Jana Leary was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, Grant Number 5TL1TR0001062-03. JoAnna Leyenaar was supported by the Agency for Healthcare Research and Quality (K08HS024133).
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the AHRQ.
Despite efforts to provide high-quality healthcare, Americans die from medical errors each year and many patients do not receive recommended medical care. Risk is particularly acute during times of hospitalization.1-4 In response, the Institute of Medicine (IOM, now the Academy of Medicine) has released “Crossing the Quality Chasm: A New Health System for the 21st Century,” providing a framework to guide delivery and measurement of high-quality healthcare.5
Although the IOM framework has motivated the development of quality improvement (QI) and quality measurement initiatives, relatively few resources have been allocated to improving the quality of pediatric inpatient care.6,7 The resultant gap in our knowledge of quality and safety of pediatric hospital-based care is further widened by the variability of settings in which children are hospitalized. These settings include freestanding children’s hospitals, children’s hospitals nested within larger hospitals, and community hospitals, defined as general, nonuniversity, and nonchildren’s hospitals.8
Although almost three-quarters of children needing hospitalization are cared for outside of freestanding children’s hospitals, we know particularly little about the quality and safety of pediatric hospital-based care outside of these settings.6,9 Therefore, our scoping review aims to summarize literature regarding the quality and safety of pediatric inpatient care within community hospitals.
METHODS
We used a scoping review approach because this methodology, by design, is utilized to synthesize evidence and map existing literature and is particularly useful when a body of literature is heterogeneous, rendering a more targeted systematic review approach infeasible.10 This methodology thereby provided an organized approach to answer our broad research question, “What evidence exists regarding the quality and safety of pediatric inpatient care in United States community hospitals?“ We followed the scoping review guidelines put forth by the Joanna Briggs Institute and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) reporting guideline Extension for Scoping Reviews.10,11
Data Sources and Search Strategies
We searched Medline, Medline-In-Process, Embase, the Cochrane Database of Systematic Reviews, the Cumulative Index to Nursing and Allied Health Literature (CINAHL), PsycINFO, and Scopus for studies that reported at least one outcome related to healthcare quality or patient safety and involved pediatric patients (aged <18 years) receiving inpatient care at a community hospital. Outcomes included measures from the IOM-defined aims of quality healthcare: (1) safety, (2) effectiveness, (3) efficiency, (4) timeliness, (5) patient-centeredness, and (6) equity (Appendix Table 1). Terms were searched as controlled vocabulary in applicable databases (Medline, Embase, CINAHL, PsycINFO) and as keywords in all databases. Search strategies tailored to each database were developed, tested, and refined in collaboration with a reference librarian. Date parameters for retrieval were set from 1989 to the search date, with the start date chosen to correspond with the establishment of the Agency for Health Care Policy and Research, currently known as the Agency for Healthcare Research and Quality (AHRQ), targeting literature produced in the wake of the AHRQ emphasis on quality in healthcare. Results were limited to articles published in English. Searches were conducted on October 24 and 25, 2016. Complete search strategies can be found in Appendix Methods.
Independent authors performed handsearches of Academic Pediatrics, BMJ Quality & Safety, Hospital Pediatrics, JAMA Pediatrics, and Pediatrics Quality Reports for the five years preceding the search date (July 2011-October 2016).
Study Selection and Definitions
To identify studies conducted in community hospitals, we operationalized the definition of community hospital proposed by Percelay.8 We used “community”, “general”, “nonuniversity”, and “nonchildren’s” as search terms and operationalized these through the addition of qualifying descriptors (Table 1).
Studies were excluded if they (1) were performed outside of the United States, as community hospital definitions may differ by country; (2) included participants aged >18 years and did not report any pediatric-specific results; (3) were performed exclusively in tertiary hospitals; (4) evaluated only outpatient or emergency department care; (5) did not report any results specific to community hospitals; (6) did not report any data-driven or parent/patient-reported measures of safety, effectiveness, efficiency, timeliness, patient-centeredness, or equity in their results; and (7) were case reports, case series, editorials, or abstracts without an associated full-text article. We read commentaries and literature reviews related to our objectives and reviewed their references to identify additional articles, but did not include these manuscripts.
Two authors independently reviewed each abstract, and full-text articles were reviewed if one or more authors determined that the abstract met the inclusion criteria. Two authors then independently reviewed each full-text article to determine whether the article met the criteria for the final review. Disagreements were resolved through consensus after discussion and review with the entire research team, and reasons for exclusion were recorded.
Charting the Results and Data Synthesis
We used a standardized charting form to collect information regarding study design, community hospital terms, population descriptors, IOM aims of quality healthcare, and outcome measures. To minimize bias in data collection, information from each full-text article was extracted independently by two authors, and differences in extraction were resolved through discussion and re-review among the same two authors. To evaluate the quality of included evidence, two authors (JCL and JKL) independently assessed the risk of bias for each study using modified Newcastle-Ottawa Quality Assessment Scales (NOS), with disagreements resolved through discussion and re-review among the same two authors. For cohort studies, five of the eight NOS domains were relevant and applied to all included studies (maximum score 6). Using the NOS adapted for cross-sectional studies, six of the seven domains were relevant and applied (maximum score 9). For cross-sectional studies, we defined the risk of bias to be low for scores ≥8, moderate for scores 5-7, and high for scores ≤4, consistent with previous work.12 For cohort studies, we similarly defined these strata by scores ≥5, 3-4, and ≤2, given the lower maximum score for this study type.
We categorized studies as either observational, defined as cohort or cross-sectional studies of usual healthcare delivery, or interventional, defined as studies evaluating the development and/or implementation of an intervention designed to improve healthcare quality. We further categorized the studies into the following four overarching medical domains: (1) neonatal, (2) pediatric medicine, (3) surgery, or (4) radiology.
RESULTS
After removal of duplicates, our search identified 2,068 abstracts for screening (Figure). Of these, 1,777 did not meet the inclusion criteria, leaving 291 articles for full-text review. Of these, 43 articles met all the inclusion criteria, and one additional article was included from search of references, resulting in a total of 44 articles.
Study designs, patient populations, and quality outcome measures were heterogeneous. We identified only one randomized controlled trial (RCT). A total of 30 articles were observational studies, 27 of which used retrospective cohort or cross-sectional designs; the remaining three used prospective cohort designs (Table 2). Of these studies, 20 involved multiple hospitals, whereas 10 were conducted at a single community hospital. Sample sizes ranged from 29 (single-site) to 107,727 (multisite) patients. Twenty-two studies aimed to compare quality outcomes at community hospitals with other hospital types, of which 16 performed risk-adjusted analyses (detailed findings of observational studies are summarized in Appendix Table 2). The remaining 14 articles were interventional studies (Table 2). Of these, 12 (86%) reported improvement in quality outcomes after implementation (detailed findings of interventional studies are summarized in Appendix Table 3).
The included studies evaluated quality outcomes addressing all six of the IOM aims of quality healthcare, with safety, effectiveness, and efficiency being the most predominant (Table 3). Patient-centeredness and timeliness were infrequently addressed, and only one study assessed equity.
Risk of bias was moderate or high for 27 (69%) of the observational and interventional cohort studies and four (100%) of the included cross-sectional studies (Table 2), with the median NOS score being 4 (range: 0-6) for cohort studies (Appendix Table 4) and 4 (range: 3-6) for cross-sectional studies (Appendix Table 5). The higher risk of bias was largely driven by low comparability scores due to inadequate risk adjustment or statistical reporting. Of the 12 studies with low risk of bias, 11 (92%) were multisite, 9 (75%) used large regional or national databases, and half reported quality outcomes limited to hospital charges and/or mortality.
Observational Studies
Neonatal Medicine
Five multisite studies focused on neonatal care,13,14,16,19,20 of which four examined outcomes associated with the transfer of neonates to or from community hospitals to tertiary care hospitals, such as neonatal morbidity, readmission, completion of preventative health measures and screening, parent satisfaction, and hospital charges.14,16,19,20 For example, in a study of extremely premature very low birth weight infants born in Hawaii, Kuo et al. demonstrated that the odds of retinopathy of prematurity was 2.9 times higher for infants born at a community hospital and transported to their tertiary center compared with those inborn at the tertiary center (P = .02).16 The fifth study examined neonatal mortality by site of birth, demonstrating that, among infants with birth weights less than 2,000 g, birth at a hospital with a community neonatal intensive care unit (NICU) was associated with 1.4 times higher odds of risk-adjusted mortality compared with birth at a regional NICU (P < .001).13
Three studies evaluated the quality of neonatal care at a single community hospital.15,17,18 Quality outcomes were heterogeneous, including utility of rebound bilirubin levels for infants with jaundice, morbidity of neonates requiring mechanical ventilation, and provision of breastfeeding advice to mothers of breastfeeding infants. For instance, Meadow et al. attempted to determine the quality of care for ventilated neonates at one community NICU compared with a tertiary hospital.18 They found no difference in days on ventilation or need for home oxygen therapy between the community hospital and the tertiary center, although P values and effect sizes were not reported for these outcomes and analyses were not adjusted beyond matching on birthdate and birth weight.
Pediatric Medicine
Nine multisite studies explored the quality and safety of pediatric medical care across hospital types.21-23,25-30 Of these, four were conducted using the Kids’ Inpatient Database (KID)28-30 or the National Inpatient Sample (NIS),27 two were conducted using other national databases,21,25 and two were conducted using electronic medical record data.22,26 All of the KID and NIS studies examined hospital charges, either alone or in conjunction with mortality. Two of these studies found no differences in risk-adjusted hospital charges between children’s hospitals and community hospitals (for burn injuries),28,29 whereas two found that hospitalization at community hospitals was associated with lower risk-adjusted charges (for asthma and sepsis).27,30 The remaining five studies evaluated diverse quality outcomes such as medication errors, therapeutic drug monitoring, practice guideline compliance, antibiotic prescribing, or hospital-to-home transition summary scores.21-23,25,26
Three single-site studies examined quality outcomes for pediatric medical patients, including mortality, outcome ratings for dehydration, and measures of peripherally inserted central catheter (PICC) safety and effectiveness.24,31,32 For example, Frank et al. evaluated safety of care in one community hospital pediatric intensive care unit (PICU) compared with a tertiary hospital using the Pediatric Risk of Mortality (PRISM) score.24 They reported that the observed number of deaths in their community PICU did not differ significantly from the number of deaths predicted in a tertiary center (23 vs 33, respectively, P > .2).
Surgery
Three multisite studies examined quality outcomes among children with surgical conditions, including surgical complications, readmission, and hospital charges.34,35,37 For example, Kelley-Quon et al. examined outcomes following surgery in infants with hypertrophic pyloric stenosis and found that infants who received their surgery at community hospitals had twice the odds of a surgical complication compared with those at children’s hospitals (P = .027).34 They also examined how the risk of appendiceal perforation differed by hospital type, finding that black children who received their surgery at children’s hospitals had twice the odds of appendiceal perforation compared with those who received care at community hospitals.35
In addition, two studies evaluated quality and safety outcomes for pediatric surgical care in a single community hospital.33,36 For example, Beaty et al. prospectively evaluated the incidence of missed injuries in hospitalized pediatric trauma patients, reporting that the incidence of missed injury was 33% when admission evaluation was performed by a trauma surgeon alone compared to 11% when performed by a pediatric doctor or a trauma surgeon and a pediatric doctor together (P < .001).33
Radiology
Three multisite studies examined quality and safety outcomes associated with radiographic imaging in community hospitals, including radiation dosing and frequency of preoperative imaging modalities and accuracy.38,39,41 For instance, Marin et al. found substantial variation in radiation dose across hospital types, with children’s hospitals delivering lower median radiation doses than academic and community hospitals.39 Similarly, Saito et al. demonstrated increased use of radiating modalities when evaluation was performed at community hospitals, with four times higher odds of computed tomography (CT) and five times lower odds of ultrasound use compared to a children’s hospital.41
Two single-site studies also evaluated quality and safety outcomes associated with the use of radiographic imaging for pediatric appendicitis in a community hospital.40,42 For example, York et al. demonstrated that, compared with nonimaged patients, patients who underwent diagnostic imaging for appendicitis experienced a significant time delay from initial evaluation to surgery and incurred significantly higher hospital charges, whereas there were no significant differences in intraoperative findings, antibiotic requirements, and surgical complications between the groups.42
Interventional Studies
Neonatal Medicine
We identified six studies that evaluated interventions to improve healthcare quality for neonates in community hospitals; two involved telemedicine.43-48 Hall et al. described “Telenursery,” a program linking regional perinatal centers with a large academic neonatal practice through real-time teleconferencing, in addition to providing weekly educational conferences.45 After its implementation, there was an increase in the state-recommended delivery of very low birth weight infants at the regional perinatal center from 24% to 33% (P < .05); clinical outcomes were not discussed. In a similar study, Sable et al. found that a videoconferencing system for cardiologists from an academic center to guide care in a community setting provided diagnostic services more quickly (28 minutes vs 12 hours) and had high diagnostic accuracy.47 The remaining four studies evaluated heterogeneous interventions such as a maternal education program to reduce shaking injuries to infants or implementation of evidence-based order sets to reduce early onset group B streptococcal disease in neonates.43,44,46,48 Five of the six neonatal studies demonstrated improved quality outcomes after intervention.43-47
Pediatric Medicine
Seven studies evaluated QI interventions for children at community hospitals,49-55 three of which described interventions to improve quality of management of respiratory diseases.49,51,53 Dayal et al. evaluated the implementation of respiratory illness order sets and an asthma pathway, demonstrating a 41% reduction in asthma hospitalization cost per patient (P < .05), reduced bronchodilator use for all respiratory illnesses, and no change in readmission rates.49 Similarly, Nkoy et al. evaluated an asthma “Evidence-Based Care Practice Model” implemented at seven community hospitals and demonstrated a nonsignificant reduction in readmissions (P = .12), as well as lower hospitalization costs (P = .05).53 Using QI methods, Kuhlmann et al. demonstrated improved compliance from 43% to 97% with the Asthma Home Management Plan of Care measure.51 The remaining four studies evaluated heterogeneous interventions, including telemedicine critical care consultations, use of I-PASS, and consolidation of pediatric care onto one hospital unit.50,52,54,55 Six of the seven pediatric studies (86%) demonstrated improved inpatient quality outcomes after intervention,49,51-55 but only one study was multisite.53
Surgery
We identified only one study evaluating a surgical intervention aimed at improving the quality of pediatric care.56 Kelley-Quon evaluated the impact of a community hospital partnering with an Academic Medical Pediatric Trauma Center to become a Level II Pediatric Trauma Center (PTC). After achieving Level II PTC designation, they reported that children treated at the community hospital had reduced rates of CT use, transfers, and in-hospital mortality (from 81% to 51%, 8.5% to 2.5%, and 2% to 0.4%, respectively, P < .05 for all) compared to those treated predesignation.
Radiology
Within the domain of radiology, our review identified no interventional studies.
DISCUSSION
In this scoping review of the quality and safety of pediatric inpatient care in community hospitals, we identified 44 studies applying heterogeneous study designs and evaluating diverse patient populations and quality outcomes. We identified only one RCT in our search; all the remaining studies applied observational designs.
We found only three clinical areas that were explored in multiple studies, with consistent directionality of results: (1) perinatal regionalization, (2) telemedicine, and (3) imaging radiation. The limited evidence identified in our review suggests that delivery of early premature infants at community hospitals, rather than at tertiary hospitals, may increase risk of neonatal morbidity and/or mortality,13,16,46 that use of telemedicine may improve the effectiveness and efficiency of intensive or specialized care in community settings,45,47,52,55 and that CT use and radiation doses may be higher in community hospitals compared with other settings.38,39,41 However, even within these clinical domains, the literature was limited in amount and heterogeneous; additional research is needed to systematically review the effect of individual interventions or particular community hospital quality outcomes compared with other hospital types.
Our search identified only 14 studies evaluating QI interventions within community hospitals over the almost 30-year review period. Although limited in number, 86% of these demonstrated improvements in healthcare quality and safety, providing a positive “proof of concept” that pediatric care in community hospitals can be improved by multidisciplinary efforts and be sustained over time.43-47,49,51-56 As pediatric departments within community hospitals may have limited resources, aligning pediatric QI efforts with adult initiatives within the same hospitals could prove advantageous. However, we did not identify any studies meeting our inclusion criteria that took this approach. Alternatively, QI collaboratives across structurally diverse hospitals may provide valuable infrastructure for QI in community hospitals. For example, the Value in Inpatient Pediatrics Network has conducted several multisite QI initiatives that have engaged both children’s and community hospitals.57-60 However, none of these studies have reported community hospital-specific quality outcomes, resulting in their exclusion from this review. In future study, researchers may consider separating community hospital results from those of pediatric hospitals to highlight the effect of community hospital-specific QI efforts and to allow valuable direct comparisons between hospital types.
Many studies identified in our scoping review were conducted at single hospitals. Sample sizes were often very small and power calculations were rarely reported, raising questions about the validity of the “nonsignificant” differences reported by some. Although such single-center studies provide valuable information for local improvement of inpatient care, by design the findings from these studies are unlikely generalizable to other community hospital systems. Without inclusion of another hospital type and/or quality measures with clear national benchmark for comparison, the additional conclusions that can be drawn from these studies are limited. In comparison, many of the multicenter studies had large study samples, but more than half used data registries with limited data to evaluate outcomes, clinical context, or important covariates.13,20,21,25,27-30,34,35,37,39 The most frequently reported outcomes in these data sets—hospital charges and lengths of stay—are of limited utility in understanding healthcare quality without clear benchmarks. As a result, the evidence-base from which to draw conclusions about quality and safety in community hospitals is very limited.
Therefore, our review highlights a great need for additional research in community hospital medicine and the need for high-quality evidence generation. Risk of bias was moderate or high for the majority of included studies because of inadequate risk adjustment or statistical analysis. In the future, multicenter collaborations may help to connect research methodologists with community hospital teams to aid in the application of robust study designs and analytic techniques. Multisite collaboration may also overcome the limitation of small sample sizes that are a reality at many community hospitals.
Our study findings must be considered in the setting of several methodological limitations. The lack of a standard definition for a community hospital has led to inconsistent terms and hospital definitions used in the literature. It is possible that, while following our systematic approach to define a community hospital, we inadvertently missed relevant studies that used different terms. We also excluded unpublished articles. Given that publication bias tends to favor studies with significant associations, it is possible that some studies with insignificant changes in quality outcomes were missed. Finally, in our exclusion of all non-US studies, we may have unknowingly missed literature from countries with community hospital definitions similar to those in the United States.
CONCLUSIONS
Recognizing that more than half of all children admitted to hospitals in the US receive their care at community hospitals, understanding healthcare quality in community hospitals is important. This scoping review underscores the need for additional research and higher quality evidence to determine the quality of pediatric inpatient care in these settings and identifies some particularly wide gaps that could be targeted in future research. Acknowledging that further research is necessary to address all aims of quality healthcare, markedly few studies have examined timeliness, equity, or patient-centeredness. Collaborations between academic medical centers and community hospitals may be an effective means to connect researchers with community hospital clinical teams to facilitate the application of robust study designs and analytic approaches and to facilitate multisite investigations. Research in this field would benefit from a standardized definition of a community hospital that could be consistently applied in research and QI endeavors.
Disclosures
The authors have no potential conflicts of interest to disclose.
Funding
Jana Leary was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, Grant Number 5TL1TR0001062-03. JoAnna Leyenaar was supported by the Agency for Healthcare Research and Quality (K08HS024133).
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the AHRQ.
1. McGlynn EA, Asch SM, Adams J, et al. The quality of health care delivered to adults in the United States. N Engl J Med. 2003;348(26):2635-2645. https://doi.org/10.1056/NEJMsa022615.
2. Richardson WC, Berwick DM, Bisgard C, et al. To err is human: building a safer health system-Institute of Medicine. Medscape. http://www.iom.edu/Reports/1999/To-Err-is-Human-Building-A-Safer-Health-System.aspx; 2000. Accessed October 7, 2016.
3. Classen DC, Resar R, Griffin F, et al. ‘Global Trigger Tool’ shows that adverse events in hospitals may be ten times greater than previously measured. Health Aff. 2011;30(4):581-589. https://doi.org/10.1377/hlthaff.2011.0190.
4. Stockwell DC, Landrigan CP, Schuster MA, et al. Using a pediatric trigger tool to estimate total harm burden hospital-acquired conditions represent. Pediatr Qual Saf. 2018;3(3):e081. https://doi.org/10.1097/pq9.0000000000000081.
5. Institute of Medicine (US) Committee on Quality of Health Care in America. Crossing the Quality Chasm. Washington (DC): National Academies Press (US); 2001. https://doi.org/10.17226/10027.
6. Rauch DA, Lye PS, Carlson D, et al. Pediatric hospital medicine: a strategic planning roundtable to chart the future. J Hosp Med. 2012;7(4):329-334. https://doi.org/10.1002/jhm.950.
7. Simpson L, Fairbrother G, Hale S et al. Reauthorizing SCHIP: Opportunities for Promoting Effective Health Coverage and HighQuality Care for Children and Adolescents. (Publication 1051). Commonw Fund; 2007.
8. Percelay JM. Pediatric hospitalists working in community hospitals. Pediatr Clin North Am. 2014;61(4):681-691. https://doi.org/10.1016/j.pcl.2014.04.005.
9. Leyenaar JK, Ralston SL, Shieh MS et al. Epidemiology of pediatric hospitalizations at general hospitals and freestanding children’s hospitals in the United States. J Hosp Med. 2016;11(11):743-749. https://doi.org/10.1002/jhm.2624.
10. Peters MDJ, Godfrey CM, Khalil H et al. Guidance for conducting systematic scoping reviews. Int J Evid Based Healthc. 2015;13(3):141-146. https://doi.org/10.1097/XEB.0000000000000050.
11. Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467-473. https://doi.org/10.7326/M18-0850.
12. Alobaidi R, Morgan C, Basu RK, et al. Association between fluid balance and outcomes in critically ill children: a systematic review and meta-analysis. JAMA Pediatr. 2018;172(3):257-268. https://doi.org/10.1001/jamapediatrics.2017.4540.
13. Cifuentes J, Bronstein J, Phibbs CS et al. Mortality in low birth weight infants according to level of neonatal care at hospital of birth. Pediatrics. 2002;109(5):745-751. https://doi.org/10.1542/peds.109.5.745.
14. Donohue PK, Hussey-Gardner B, Sulpar LJ, Fox R, Aucott SW. Convalescent care of infants in the neonatal intensive care unit in community hospitals: risk or benefit? Pediatrics. 2009;124(1):105-111. https://doi.org/10.1542/peds.2008-0880.
15. Izatt SD. Breastfeeding counseling by health care providers. J Hum Lact. 1997;13(2):109-113. https://doi.org/10.1177/089033449701300210.
16. Kuo S, Kimata C, Akamine K, Young B, Balaraman V. Outcomes of inborn and transported extremely premature very-low-birthweight infants in Hawai’i. Pediatr Int. 2012;54(3):365-369. https://doi.org/10.1111/j.1442-200X.2012.03561.x.
17. Maisels MJ, Kring E. Rebound in serum bilirubin level following intensive phototherapy. Arch Pediatr Adolesc Med. 2002;156(7):669-672. https://doi.org/10.1001/archpedi.156.7.669.
18. Meadow W, Mendez D, Makela J et al. Can and should level II nurseries care for newborns who require mechanical ventilation? Clin Perinatol. 1996;23(3):551-561. https://doi.org/10.1016/S0095-5108(18)30227-6.
19. Phibbs CS, Mortensen L. Back transporting infants from neonatal intensive care units to community hospitals for recovery care: effect on total hospital charges. Pediatrics. 1992;90(1):22-26.
20. Wall SN, Handler AS, Park CG. Hospital factors and nontransfer of small babies: A marker of deregionalized perinatal care? J Perinatol. 2004;24(6):351-359. https://doi.org/10.1038/sj.jp.7211101.
21. Alexander DC, Bundy DG, Shore AD et al. Cardiovascular medication errors in children. Pediatrics. 2009;124(1):324-332. https://doi.org/10.1542/peds.2008-2073.
22. Balch AH, Constance JE, Thorell EA, et al. Pediatric vancomycin dosing: trends over time and the impact of therapeutic drug monitoring. J Clin Pharmacol. 2015;55(2):212-220. https://doi.org/10.1002/jcph.402.
23. Conway PH, Edwards S, Stucky ER et al. Variations in management of common inpatient pediatric illnesses: hospitalists and community pediatricians. Pediatrics. 2006;118(2):441-447. https://doi.org/10.1542/peds.2006-0484.
24. Frank BS, Pollack MM. Quantitative quality assurance in a community hospital pediatric intensive care unit. West J Med. 1992;157(2):149-151.
25. Leyenaar JK, Lagu T, Shieh MS, Pekow PS, Lindenauer PK. Variation in resource utilization for the management of uncomplicated community-acquired pneumonia across community and children’s hospitals. J Pediatr. 2014;165(3):585-591. https://doi.org/10.1016/j.jpeds.2014.04.062.
26. Leyenaar JK, Desai AD, Burkhart Q, et al. Quality measures to assess care transitions for hospitalized children. Pediatrics. 2016;138(2):e20160906. https://doi.org/10.1542/peds.2016-0906.
27. Meurer JR, Kuhn EM, George V, Yauck JS, Layde PM. Charges for childhood asthma by hospital characteristics. Pediatrics. 1998;102(6):E70. https://doi.org/10.1542/peds.102.6.e70.
28. Myers J, Lehna C. Where are lengths of stay longer and total charges higher for pediatric burn patients? J Burn Care Res. 2014;35(5):382-387. https://doi.org/10.1097/BCR.0000000000000012.
29. Myers J, Smith M, Woods C, Espinosa C, Lehna C. The effect of transfers between health care facilities on costs and length of stay for pediatric burn patients. J Burn Care Res. 2015;36(1):178-183. https://doi.org/10.1097/BCR.0000000000000206.
30. Odetola FO, Gebremariam A, Freed GL. Patient and hospital correlates of clinical outcomes and resource utilization in severe pediatric sepsis. Pediatrics. 2007;119(3):487-494. https://doi.org/10.1542/peds.2006-2353.
31. Scherb CA, Stevens MS, Busman C. Outcomes related to dehydration in the pediatric population. J Pediatr Nurs. 2007;22(5):376-382. https://doi.org/10.1016/j.pedn.2006.10.004.
32. Van Winkle P, Whiffen T, Liu IL. Experience using peripherally inserted central venous catheters for outpatient parenteral antibiotic therapy in children at a community hospital. ediatr Infect Dis J. 2008;27(12):1069-1072. https://doi.org/10.1097/INF.0b013e31817d32f2.
33. Beaty JS, Chendrasekhar A, Hopkins J, Gruelke L. Missed injuries in pediatric trauma patients. J Appl Res. 2003;3(1):84-88. https://jrnlappliedresearch.com/articles/Vol3Iss1/CHENDRASEKHAR.htm. Accessed July 8, 2019.
34. Kelley-Quon LI, Tseng CH, Jen HC, Shew SB. Hospital type predicts surgical complications for infants with hypertrophic pyloric stenosis. Am Surg. 2012;78(10):1079-1082.
35. Kelley-Quon LI, Tseng CH, Jen HC, Lee SL, Shew SB. Hospital type as a metric for racial disparities in pediatric appendicitis. J Am Coll Surg. 2013;216(1):74-82. https://doi.org/10.1016/j.jamcollsurg.2012.09.018.
36. Pokala N, Sadhasivam S, Kiran RP, Parithivel V. Complicated appendicitis—is the laparoscopic approach appropriate? A comparative study with the open approach: outcome in a community hospital setting. Am Surg. 2007;73(8):732-737.
37. Smith JT, Price C, Stevens PM, Masters KS, Young M. Does pediatric orthopedic subspecialization affect hospital utilization and charges? J Pediatr Orthop. 1999;19(4):553-555. https://doi.org/10.1097/01241398-199907000-00027.
38. Calvert C, Strauss KJ, Mooney DP. Variation in computed tomography radiation dose in community hospitals. J Pediatr Surg. 2012;47(6):1167-1169. https://doi.org/10.1016/j.jpedsurg.2012.03.021.
39. Marin JR, Sengupta D, Bhargavan-Chatfield M et al. Variation in pediatric cervical spine computed tomography radiation dose index. Acad Emerg Med. 2015;22(12):1499-1505. https://doi.org/10.1111/acem.12822.
40. Reich JD, Brogdon B, Ray WE, Eckert J, Gorell H. Use of CT scan in the diagnosis of pediatric acute appendicitis. Pediatr Emerg Care. 2000;16(4):241-243. https://doi.org/10.1097/00006565-200008000-00006.
41. Saito JM, Yan Y, Evashwick TW, Warner BW, Tarr PI. Use and accuracy of diagnostic imaging by hospital type in pediatric appendicitis. Pediatrics. 2013;131(1):e37-e44. https://doi.org/10.1542/peds.2012-1665.
42. York D, Smith A, Phillips JD, Von Allmen D. The influence of advanced radiographic imaging on the treatment of pediatric appendicitis. J Pediatr Surg. 2005;40(12):1908-1911. https://doi.org/10.1016/j.jpedsurg.2005.08.004.
43. Altman RL, Canter J, Patrick PA et al. Parent education by maternity nurses and prevention of abusive head trauma. Pediatrics. 2011;128(5):e1164-e1172. https://doi.org/10.1542/peds.2010-3260.
44. Clemens CJ, Gable EK. The development of a group B streptococcus prevention policy at a community hospital. J Perinatol. 2002;22(7):523-525. https://doi.org/10.1038/sj.jp.7210794.
45. Hall RW, Hall-Barrow J, Garcia-Rill E. Neonatal regionalization through telemedicine using a community-based research and education core facility. Ethn Dis. 2010;20(1 Suppl 1):S136-S140.
46. Hulsey TC, Pittard WB 3rd, Ebeling M. Regionalized perinatal transport systems: association with changes in location of birth, neonatal transport, and survival of very low birth weight deliveries. J S C Med Assoc. 1991;87(12):581-584.
47. Sable CA, Cummings SD, Pearson GD, et al. Impact of telemedicine on the practice of pediatric cardiology in community hospitals. Pediatrics. 2002;109(1):E3. https://doi.org/10.1542/peds.109.1.e3.
48. Wexelblatt SL, Ward LP, Torok K et al. Universal maternal drug testing in a high-prevalence region of prescription opiate abuse. J Pediatr. 2015;166(3):582-586. https://doi.org/10.1016/j.jpeds.2014.10.004.
49. Dayal A, Alvarez F. The effect of implementation of standardized, evidence-based order sets on efficiency and quality measures for pediatric respiratory illnesses in a community hospital. Hosp Pediatr. 2015;5(12):624-629. https://doi.org/10.1542/hpeds.2015-0140.
50. Krugman SD, Suggs A, Photowala HY, Beck A. Redefining the community pediatric hospitalist: the combined Pediatric ED/Inpatient Unit. Pediatr Emerg Care. 2007;23(1):33-37. https://doi.org/10.1097/01.pec.0000248685.94647.01.
51. Kuhlmann S, Mason B, Ahlers-Schmidt CR. A quality improvement project to improve compliance with the joint commission children’s asthma care-3 measure. Hosp Pediatr. 2013;3(1):45-51. https://doi.org/10.1542/hpeds.2012-0015.
52. Labarbera JM, Ellenby MS, Bouressa P et al. The impact of telemedicine intensivist support and a pediatric hospitalist program on a community hospital. Telemed J E Health. 2013;19(10):760-766. https://doi.org/10.1089/tmj.2012.0303.
53. Nkoy F, Fassl B, Stone B, et al. Improving pediatric asthma care and outcomes Across multiple hospitals. Pediatrics. 2015;136(6):e1602-e1610. https://doi.org/10.1542/peds.2015-0285.
54. Walia J, Qayumi Z, Khawar N, et al. Physician transition of care: benefits of I-PASS and an electronic handoff system in a community pediatric residency program. Acad Pediatr. 2016;16(6):519-523. https://doi.org/10.1016/j.acap.2016.04.001.
55. Yang CP, Hunt EA, Shilkofski N et al. Can telemedicine improve adherence to resuscitation guidelines for critically ill children at community hospitals? A randomized controlled trial using high-fidelity simulation. Pediatr Emerg Care. 2017;33(7):474-479. https://doi.org/10.1097/PEC.0000000000000653.
56. Kelley-Quon LI, Crowley MA, Applebaum H, et al. Academic-community partnerships improve outcomes in pediatric trauma care. J Pediatr Surg. 2015;50(6):1032-1036. https://doi.org/10.1016/j.jpedsurg.2015.03.033.
57. Ralston S, Garber M, Narang S, et al. Decreasing unnecessary utilization in acute bronchiolitis care: results from the value in inpatient pediatrics network. J Hosp Med. 2013;8(1):25-30. https://doi.org/10.1002/jhm.1982.
58. Ralston SL, Garber MD, Rice-Conboy E, et al. A multicenter collaborative to reduce unnecessary care in inpatient bronchiolitis. Pediatrics. 2016;137(1):e20150851. https://doi.org/10.1542/peds.2015-0851.
59. Parikh K, Biondi E, Nazif J, et al. A multicenter collaborative to improve care of community acquired pneumonia in hospitalized children. Pediatrics. 2017;139(3):e20161411. https://doi.org/10.1542/peds.2016-1411.
60. Leyenaar JK, Bergert L, Mallory LA, et al. Pediatric primary care providers’ perspectives regarding hospital discharge communication: a mixed methods analysis. Acad Pediatr. 2015;15(1):61-68. https://doi.org/10.1016/j.acap.2014.07.004.
1. McGlynn EA, Asch SM, Adams J, et al. The quality of health care delivered to adults in the United States. N Engl J Med. 2003;348(26):2635-2645. https://doi.org/10.1056/NEJMsa022615.
2. Richardson WC, Berwick DM, Bisgard C, et al. To err is human: building a safer health system-Institute of Medicine. Medscape. http://www.iom.edu/Reports/1999/To-Err-is-Human-Building-A-Safer-Health-System.aspx; 2000. Accessed October 7, 2016.
3. Classen DC, Resar R, Griffin F, et al. ‘Global Trigger Tool’ shows that adverse events in hospitals may be ten times greater than previously measured. Health Aff. 2011;30(4):581-589. https://doi.org/10.1377/hlthaff.2011.0190.
4. Stockwell DC, Landrigan CP, Schuster MA, et al. Using a pediatric trigger tool to estimate total harm burden hospital-acquired conditions represent. Pediatr Qual Saf. 2018;3(3):e081. https://doi.org/10.1097/pq9.0000000000000081.
5. Institute of Medicine (US) Committee on Quality of Health Care in America. Crossing the Quality Chasm. Washington (DC): National Academies Press (US); 2001. https://doi.org/10.17226/10027.
6. Rauch DA, Lye PS, Carlson D, et al. Pediatric hospital medicine: a strategic planning roundtable to chart the future. J Hosp Med. 2012;7(4):329-334. https://doi.org/10.1002/jhm.950.
7. Simpson L, Fairbrother G, Hale S et al. Reauthorizing SCHIP: Opportunities for Promoting Effective Health Coverage and HighQuality Care for Children and Adolescents. (Publication 1051). Commonw Fund; 2007.
8. Percelay JM. Pediatric hospitalists working in community hospitals. Pediatr Clin North Am. 2014;61(4):681-691. https://doi.org/10.1016/j.pcl.2014.04.005.
9. Leyenaar JK, Ralston SL, Shieh MS et al. Epidemiology of pediatric hospitalizations at general hospitals and freestanding children’s hospitals in the United States. J Hosp Med. 2016;11(11):743-749. https://doi.org/10.1002/jhm.2624.
10. Peters MDJ, Godfrey CM, Khalil H et al. Guidance for conducting systematic scoping reviews. Int J Evid Based Healthc. 2015;13(3):141-146. https://doi.org/10.1097/XEB.0000000000000050.
11. Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467-473. https://doi.org/10.7326/M18-0850.
12. Alobaidi R, Morgan C, Basu RK, et al. Association between fluid balance and outcomes in critically ill children: a systematic review and meta-analysis. JAMA Pediatr. 2018;172(3):257-268. https://doi.org/10.1001/jamapediatrics.2017.4540.
13. Cifuentes J, Bronstein J, Phibbs CS et al. Mortality in low birth weight infants according to level of neonatal care at hospital of birth. Pediatrics. 2002;109(5):745-751. https://doi.org/10.1542/peds.109.5.745.
14. Donohue PK, Hussey-Gardner B, Sulpar LJ, Fox R, Aucott SW. Convalescent care of infants in the neonatal intensive care unit in community hospitals: risk or benefit? Pediatrics. 2009;124(1):105-111. https://doi.org/10.1542/peds.2008-0880.
15. Izatt SD. Breastfeeding counseling by health care providers. J Hum Lact. 1997;13(2):109-113. https://doi.org/10.1177/089033449701300210.
16. Kuo S, Kimata C, Akamine K, Young B, Balaraman V. Outcomes of inborn and transported extremely premature very-low-birthweight infants in Hawai’i. Pediatr Int. 2012;54(3):365-369. https://doi.org/10.1111/j.1442-200X.2012.03561.x.
17. Maisels MJ, Kring E. Rebound in serum bilirubin level following intensive phototherapy. Arch Pediatr Adolesc Med. 2002;156(7):669-672. https://doi.org/10.1001/archpedi.156.7.669.
18. Meadow W, Mendez D, Makela J et al. Can and should level II nurseries care for newborns who require mechanical ventilation? Clin Perinatol. 1996;23(3):551-561. https://doi.org/10.1016/S0095-5108(18)30227-6.
19. Phibbs CS, Mortensen L. Back transporting infants from neonatal intensive care units to community hospitals for recovery care: effect on total hospital charges. Pediatrics. 1992;90(1):22-26.
20. Wall SN, Handler AS, Park CG. Hospital factors and nontransfer of small babies: A marker of deregionalized perinatal care? J Perinatol. 2004;24(6):351-359. https://doi.org/10.1038/sj.jp.7211101.
21. Alexander DC, Bundy DG, Shore AD et al. Cardiovascular medication errors in children. Pediatrics. 2009;124(1):324-332. https://doi.org/10.1542/peds.2008-2073.
22. Balch AH, Constance JE, Thorell EA, et al. Pediatric vancomycin dosing: trends over time and the impact of therapeutic drug monitoring. J Clin Pharmacol. 2015;55(2):212-220. https://doi.org/10.1002/jcph.402.
23. Conway PH, Edwards S, Stucky ER et al. Variations in management of common inpatient pediatric illnesses: hospitalists and community pediatricians. Pediatrics. 2006;118(2):441-447. https://doi.org/10.1542/peds.2006-0484.
24. Frank BS, Pollack MM. Quantitative quality assurance in a community hospital pediatric intensive care unit. West J Med. 1992;157(2):149-151.
25. Leyenaar JK, Lagu T, Shieh MS, Pekow PS, Lindenauer PK. Variation in resource utilization for the management of uncomplicated community-acquired pneumonia across community and children’s hospitals. J Pediatr. 2014;165(3):585-591. https://doi.org/10.1016/j.jpeds.2014.04.062.
26. Leyenaar JK, Desai AD, Burkhart Q, et al. Quality measures to assess care transitions for hospitalized children. Pediatrics. 2016;138(2):e20160906. https://doi.org/10.1542/peds.2016-0906.
27. Meurer JR, Kuhn EM, George V, Yauck JS, Layde PM. Charges for childhood asthma by hospital characteristics. Pediatrics. 1998;102(6):E70. https://doi.org/10.1542/peds.102.6.e70.
28. Myers J, Lehna C. Where are lengths of stay longer and total charges higher for pediatric burn patients? J Burn Care Res. 2014;35(5):382-387. https://doi.org/10.1097/BCR.0000000000000012.
29. Myers J, Smith M, Woods C, Espinosa C, Lehna C. The effect of transfers between health care facilities on costs and length of stay for pediatric burn patients. J Burn Care Res. 2015;36(1):178-183. https://doi.org/10.1097/BCR.0000000000000206.
30. Odetola FO, Gebremariam A, Freed GL. Patient and hospital correlates of clinical outcomes and resource utilization in severe pediatric sepsis. Pediatrics. 2007;119(3):487-494. https://doi.org/10.1542/peds.2006-2353.
31. Scherb CA, Stevens MS, Busman C. Outcomes related to dehydration in the pediatric population. J Pediatr Nurs. 2007;22(5):376-382. https://doi.org/10.1016/j.pedn.2006.10.004.
32. Van Winkle P, Whiffen T, Liu IL. Experience using peripherally inserted central venous catheters for outpatient parenteral antibiotic therapy in children at a community hospital. ediatr Infect Dis J. 2008;27(12):1069-1072. https://doi.org/10.1097/INF.0b013e31817d32f2.
33. Beaty JS, Chendrasekhar A, Hopkins J, Gruelke L. Missed injuries in pediatric trauma patients. J Appl Res. 2003;3(1):84-88. https://jrnlappliedresearch.com/articles/Vol3Iss1/CHENDRASEKHAR.htm. Accessed July 8, 2019.
34. Kelley-Quon LI, Tseng CH, Jen HC, Shew SB. Hospital type predicts surgical complications for infants with hypertrophic pyloric stenosis. Am Surg. 2012;78(10):1079-1082.
35. Kelley-Quon LI, Tseng CH, Jen HC, Lee SL, Shew SB. Hospital type as a metric for racial disparities in pediatric appendicitis. J Am Coll Surg. 2013;216(1):74-82. https://doi.org/10.1016/j.jamcollsurg.2012.09.018.
36. Pokala N, Sadhasivam S, Kiran RP, Parithivel V. Complicated appendicitis—is the laparoscopic approach appropriate? A comparative study with the open approach: outcome in a community hospital setting. Am Surg. 2007;73(8):732-737.
37. Smith JT, Price C, Stevens PM, Masters KS, Young M. Does pediatric orthopedic subspecialization affect hospital utilization and charges? J Pediatr Orthop. 1999;19(4):553-555. https://doi.org/10.1097/01241398-199907000-00027.
38. Calvert C, Strauss KJ, Mooney DP. Variation in computed tomography radiation dose in community hospitals. J Pediatr Surg. 2012;47(6):1167-1169. https://doi.org/10.1016/j.jpedsurg.2012.03.021.
39. Marin JR, Sengupta D, Bhargavan-Chatfield M et al. Variation in pediatric cervical spine computed tomography radiation dose index. Acad Emerg Med. 2015;22(12):1499-1505. https://doi.org/10.1111/acem.12822.
40. Reich JD, Brogdon B, Ray WE, Eckert J, Gorell H. Use of CT scan in the diagnosis of pediatric acute appendicitis. Pediatr Emerg Care. 2000;16(4):241-243. https://doi.org/10.1097/00006565-200008000-00006.
41. Saito JM, Yan Y, Evashwick TW, Warner BW, Tarr PI. Use and accuracy of diagnostic imaging by hospital type in pediatric appendicitis. Pediatrics. 2013;131(1):e37-e44. https://doi.org/10.1542/peds.2012-1665.
42. York D, Smith A, Phillips JD, Von Allmen D. The influence of advanced radiographic imaging on the treatment of pediatric appendicitis. J Pediatr Surg. 2005;40(12):1908-1911. https://doi.org/10.1016/j.jpedsurg.2005.08.004.
43. Altman RL, Canter J, Patrick PA et al. Parent education by maternity nurses and prevention of abusive head trauma. Pediatrics. 2011;128(5):e1164-e1172. https://doi.org/10.1542/peds.2010-3260.
44. Clemens CJ, Gable EK. The development of a group B streptococcus prevention policy at a community hospital. J Perinatol. 2002;22(7):523-525. https://doi.org/10.1038/sj.jp.7210794.
45. Hall RW, Hall-Barrow J, Garcia-Rill E. Neonatal regionalization through telemedicine using a community-based research and education core facility. Ethn Dis. 2010;20(1 Suppl 1):S136-S140.
46. Hulsey TC, Pittard WB 3rd, Ebeling M. Regionalized perinatal transport systems: association with changes in location of birth, neonatal transport, and survival of very low birth weight deliveries. J S C Med Assoc. 1991;87(12):581-584.
47. Sable CA, Cummings SD, Pearson GD, et al. Impact of telemedicine on the practice of pediatric cardiology in community hospitals. Pediatrics. 2002;109(1):E3. https://doi.org/10.1542/peds.109.1.e3.
48. Wexelblatt SL, Ward LP, Torok K et al. Universal maternal drug testing in a high-prevalence region of prescription opiate abuse. J Pediatr. 2015;166(3):582-586. https://doi.org/10.1016/j.jpeds.2014.10.004.
49. Dayal A, Alvarez F. The effect of implementation of standardized, evidence-based order sets on efficiency and quality measures for pediatric respiratory illnesses in a community hospital. Hosp Pediatr. 2015;5(12):624-629. https://doi.org/10.1542/hpeds.2015-0140.
50. Krugman SD, Suggs A, Photowala HY, Beck A. Redefining the community pediatric hospitalist: the combined Pediatric ED/Inpatient Unit. Pediatr Emerg Care. 2007;23(1):33-37. https://doi.org/10.1097/01.pec.0000248685.94647.01.
51. Kuhlmann S, Mason B, Ahlers-Schmidt CR. A quality improvement project to improve compliance with the joint commission children’s asthma care-3 measure. Hosp Pediatr. 2013;3(1):45-51. https://doi.org/10.1542/hpeds.2012-0015.
52. Labarbera JM, Ellenby MS, Bouressa P et al. The impact of telemedicine intensivist support and a pediatric hospitalist program on a community hospital. Telemed J E Health. 2013;19(10):760-766. https://doi.org/10.1089/tmj.2012.0303.
53. Nkoy F, Fassl B, Stone B, et al. Improving pediatric asthma care and outcomes Across multiple hospitals. Pediatrics. 2015;136(6):e1602-e1610. https://doi.org/10.1542/peds.2015-0285.
54. Walia J, Qayumi Z, Khawar N, et al. Physician transition of care: benefits of I-PASS and an electronic handoff system in a community pediatric residency program. Acad Pediatr. 2016;16(6):519-523. https://doi.org/10.1016/j.acap.2016.04.001.
55. Yang CP, Hunt EA, Shilkofski N et al. Can telemedicine improve adherence to resuscitation guidelines for critically ill children at community hospitals? A randomized controlled trial using high-fidelity simulation. Pediatr Emerg Care. 2017;33(7):474-479. https://doi.org/10.1097/PEC.0000000000000653.
56. Kelley-Quon LI, Crowley MA, Applebaum H, et al. Academic-community partnerships improve outcomes in pediatric trauma care. J Pediatr Surg. 2015;50(6):1032-1036. https://doi.org/10.1016/j.jpedsurg.2015.03.033.
57. Ralston S, Garber M, Narang S, et al. Decreasing unnecessary utilization in acute bronchiolitis care: results from the value in inpatient pediatrics network. J Hosp Med. 2013;8(1):25-30. https://doi.org/10.1002/jhm.1982.
58. Ralston SL, Garber MD, Rice-Conboy E, et al. A multicenter collaborative to reduce unnecessary care in inpatient bronchiolitis. Pediatrics. 2016;137(1):e20150851. https://doi.org/10.1542/peds.2015-0851.
59. Parikh K, Biondi E, Nazif J, et al. A multicenter collaborative to improve care of community acquired pneumonia in hospitalized children. Pediatrics. 2017;139(3):e20161411. https://doi.org/10.1542/peds.2016-1411.
60. Leyenaar JK, Bergert L, Mallory LA, et al. Pediatric primary care providers’ perspectives regarding hospital discharge communication: a mixed methods analysis. Acad Pediatr. 2015;15(1):61-68. https://doi.org/10.1016/j.acap.2014.07.004.
© 2019 Society of Hospital Medicine
Catheter-Associated Urinary Tract Infections in Adults: Diagnosis, Treatment, and Prevention
Every day in the United States, approximately 4% of patients in acute care hospitals have at least one hospital-acquired infection (HAI).1,2 Among the top 10 causes of death in the United States, HAIs are associated with increased morbidity, mortality, and hospital length of stay (LOS).2 The direct medical cost of treating HAIs is substantial for both hospitals and patients.3,4 Urinary tract infections (UTIs) are a leading cause of HAI, and 70%-80% of these are catheter-associated urinary tract infections (CAUTIs).5,6 In 2016, 26,983 CAUTIs occurred in acute care hospitals.7 The high incidence of CAUTI can substantially contribute to morbidity, length of stay, and mortality.8-11
The recognition that a substantial proportion of HAIs may be preventable, including 55%-70% of CAUTIs,12 has resulted in implementing multiple strategies to reduce CAUTI rates.13-17 These include simple prevention interventions such as avoiding placement of unnecessary indwelling urinary catheters and early removal of urinary catheters when they are no longer clinically indicated. Hospitalists are responsible for the care of many, if not most, inpatients with indwelling urinary catheters and are integral in antimicrobial stewardship efforts surrounding CAUTIs.18 Diagnostic stewardship, including appropriate urine specimen ordering, collection, processing, and reporting, works synergistically with antimicrobial stewardship and allows for appropriate antibiotic prescribing in symptomatic patients.19
DEFINITIONS
CAUTIs can be defined using either clinical or surveillance definitions. Clinical definitions are used at the bedside and take individual clinical characteristics into consideration, but vary among clinicians since there is no gold standard. Abnormal laboratory urinary findings in the absence of symptoms are not sufficient for the diagnosis of UTI, including CAUTI. Surveillance definitions, such as those used by the Centers for Disease Control and Prevention,20 are designed to be simple, easily applicable in any healthcare setting, and standardized to all patients. Surveillance definitions generally include at least one systemic or local symptom (such as fever or dysuria) and positive urine culture in a patient with an indwelling urinary catheter (or within 48 hours after its removal).
Pyuria is leukocytes or white blood cells (WBCs) in a urine specimen, with a threshold of >10 WBCs/high-power field using urine microscopy. The predictive value of different thresholds of pyuria for UTI is unclear.
Bacteriuria denotes the presence (on microscopy or culture) of bacteria in the urine. In a patient without signs or symptoms of a UTI, this is termed asymptomatic bacteriuria (ASB). A full discussion of bacteriuria, a major reason for inappropriate antibiotic use, is beyond the scope of this article but is discussed in a recent guideline.21
Urinary tract infections are usually characterized by a clinical syndrome along with evidence of pyuria and/or bacteriuria. The two major clinical syndromes that are observed are lower UTI (cystitis or bladder infections) and upper UTI (pyelonephritis or kidney infections). Rarely, patients may develop asymptomatic bacteremic UTI, where blood and urine cultures grow the same pathogen in the absence of clinical symptoms. (Table 1 summarizes the key points for these definitions).
CAUSES AND RISK FACTORS FOR CAUTI
Bacterial biofilm can form on the inner and outer surfaces of an indwelling urinary catheter following its insertion and can be associated with bacteriuria and CAUTI.22,23 The biofilm comprises bacteria from the periurethral area that migrate upwards from a colonized drainage system. Bacteria present in the biofilm tend to exhibit slow growth, are protected from antibiotic exposure, and have less susceptibility to these agents.22-24 When a mature biofilm has formed, catheter removal may be necessary for source control and to facilitate effective antimicrobial treatment. The pathogens that most commonly cause CAUTIs are Escherichia coli (23.9%), Pseudomonas aeruginosa (10.3%), and Klebsiella pneumoniae/oxytoca (10.1%).25 Although urine cultures often grow yeast, particularly Candida spp., nonbacterial pathogens rarely cause UTI.
The risk of developing a CAUTI is directly related to catheter dwell time.26,27 For catheterized patients, the rate of development of catheter-associated bacteriuria is approximately 3% to 7% per day14,28 and is more common in the elderly and females. The likelihood of bacteriuria approaches 100% if a patient has an indwelling urinary catheter for ≥30 days,27,29,30 which is part of the rationale for why a urine culture alone is not sufficient to diagnose a CAUTI. While bacteriuria is a risk factor for UTI, the frequency of progression from bacteriuria to CAUTI is low and treating ASB does not decrease the risk of future CAUTI. Other risk factors for the development of CAUTI include urinary tract instrumentation, diabetes mellitus, and malnutrition.31,32
The two most important factors that lead to the development of CAUTIs and have been the main focus of quality improvement areas are unnecessary urinary catheter placement and inappropriate delay in removing a catheter when it is no longer needed.26,33 Unfortunately, 38% of attending physicians are unaware that their patients have a urinary catheter in place.34 Furthermore, in 20% to 50% of cases, there is no clear indication for catheter placement.2,34
DIAGNOSIS OF CAUTI
A CAUTI diagnosis is typically one of exclusion, as most patients present with fever and no apparent alternative source.14,29 Since catheterized patients may not exhibit common cystitis symptoms,29 most who develop CAUTI present with fever alone. However, most fevers in patients with bacteriuria and catheters are not CAUTIs and can be attributed to other sources. If a patient with an indwelling urinary catheter develops a fever and there is a suspicion of a CAUTI, careful evaluation is warranted for alternative sources of infection. This particularly applies to patients with severe systemic illness, such as hypotension or systemic inflammatory response syndrome, since these are unusual manifestations of CAUTI. The presence of either cloudy or malodorous urine does not indicate a UTI, and should not be the sole rationale for obtaining a urine culture.
Diagnostic workup of fever should include a clinical assessment of the patient. Indeed, professional guidelines recommend against obtaining a urine culture routinely for fever, unless invasive UTI risk is elevated, such as in patients with neutropenia, history of renal transplantation, or recent genitourinary surgery.35 Diagnostic stewardship, focusing on the appropriate use of urine cultures, can reduce CAUTI rates.36 For catheterized patients, hospitals are increasingly adopting reflex urine culture, where urine is simultaneously collected for a urinalysis and urine culture, but a urine culture is performed only if the urinalysis is positive for a predetermined threshold for pyuria, leukocyte esterase, or both. However, the use of reflex urine cultures remains an area of debate.37 In addition, the Infectious Diseases Society of America recommends against screening for or treating ASB in patients with either short-term (<30 days) or long-term indwelling urethral catheters.21
Ideally, a urine culture should be obtained by collecting a midstream sample. In catheterized patients, a sample should be obtained after removal of the catheter; or, in patients with a clinical indication for ongoing catheterization, a sample should be obtained after a new catheter has been placed.14 If an indwelling urinary catheter must be continued, the recommendation is to disinfect the drainage system’s aspiration port and then obtain a urine culture. Urine should never be obtained from a catheter collection bag. (Table 2 summarizes best practices for diagnosis of CAUTI).
TREATMENT OF CAUTI
For all CAUTIs, an indwelling urinary catheter should be removed as soon as possible. If an indwelling urinary catheter remains necessary, but the existing catheter has been in place longer than two weeks, a new catheter should be placed before initiating antibiotic therapy14 to accelerate symptom resolution and reduce the likelihood of relapse or recurrence.32
Urinary tract agents such as fosfomycin and nitrofurantoin are recommended as first-line agents for simple cystitis in women and can be used in patients with lower UTI and sufficient renal function to achieve adequate drug concentration in urine. Upper UTIs require antibiotics with good penetration into renal parenchyma such as ceftriaxone. If empiric antimicrobial therapy is needed before culture results are available, then previous urine culture results, local antibiograms, or practice guidelines can guide selection. Definitive antimicrobial therapy should be based on urine culture results. It is important to narrow empiric therapy14 to reduce risk of Clostridioides difficile infection and emergence of other resistant bacteria. Fluoroquinolones should be avoided for lower UTIs because of these risks and multiple United States Food and Drug Administration warnings.38-40
The optimal duration of antimicrobial therapy for a CAUTI is unclear;14 however, most patients can be treated with a relatively short duration of therapy (≤7 days) if they respond promptly to therapy. Patients with a slow response to therapy may require 10-14 days of treatment.14 (Table 2 summarizes best practices for the treatment of CAUTI).
STRATEGIES FOR CAUTI PREVENTION
Since CAUTI is predicated on the presence of an indwelling urinary catheter, the simplest way to reduce CAUTI is to avoid placing or retaining unnecessary catheters. Some examples of appropriate indications9 for placement and maintenance of an indwelling urinary catheter are listed below:
1. Accurate measurement of urinary output in severely ill patients;
2. Improved comfort for patients receiving end-of-life care;
3. Acute urinary retention or bladder outlet obstruction;
4. Need for a period of prolonged immobilization (eg, potentially unstable lumbar or thoracic spine, or has multiple traumatic injuries);
5. Selected surgical procedures, such as urologic procedures and those that are expected to have a prolonged duration, require intraoperative monitoring of urine output, require the administration of either large volumes of intravenous infusions or diuretics;
6. To promote healing of open perineal or sacral wounds in patients with incontinence;
7. Neurogenic bladder; and
8. Hematuria with clots.
To increase the timely removal of urinary catheters that are no longer indicated, daily assessment of catheter necessity must be an integral part of clinicians’ workflow.32,33 Alternatives, such as external catheters or intermittent catheterization, should be considered before indwelling urinary catheter placement since both options are associated with a reduced CAUTI risk.41-44 Although indwelling urinary catheters can be seen as being more convenient for both patients and healthcare providers, many patients have expressed a preference for the use of intermittent catheterization compared with indwelling urinary catheterization.43
For urinary retention, bladder scanning can noninvasively assess the amount of residual urine in a patient’s bladder and can avoid unnecessary insertion of an indwelling urinary catheter. However, if indwelling urinary catheters are ultimately needed, they must be inserted and maintained appropriately. Of note, the use of antibiotic-impregnated catheters has not been shown to reduce CAUTI rates significantly.45
CAUTI prevention requires a multidisciplinary collaborative approach. Nurse-driven protocols and checklists to remove indwelling urinary catheters that are no longer indicated can be very effective.46,47 Automatic stop orders and catheter removal reminders are useful for reducing the duration of catheter placement.26,48 Both of these approaches require appropriate, consistent documentation with input from bedside nurses, physicians, advanced practice providers, and information technology. (Table 3 summarizes best practices for the prevention of CAUTI).
Importantly, CAUTI prevention supports broader antimicrobial stewardship. Over 55% of inpatients receive at least one dose of an antibiotic during their hospital stay.48,49 In 2015, the White House released the National Action Plan for Combating Antibiotic-Resistant Bacteria with the goals of slowing the emergence of resistant bacteria, preventing the spread of antibiotic-resistant infections, and setting a target of a 20% reduction in the inappropriate use of antibiotics for hospitalized patients.50 Hospitalists care for a substantial number of inpatients and, in turn, can drive actions to decrease CAUTIs, and promote stewardship efforts. Through actions to decrease CAUTIs, hospitalists can promote these stewardship efforts.
CONCLUSIONS
CAUTI is one of the most common types of HAI and is associated with increased morbidity, hospital length of stay, and patient costs. Most CAUTIs are preventable by limiting the placement of unnecessary catheters to instances of true necessity and removing catheters when they are no longer clinically indicated. Proper technique for the insertion and maintenance of catheters is also important for reducing CAUTI rates. Hospitalists care for a substantial number of inpatients and can make major contributions to the appropriate diagnosis, treatment, and prevention of CAUTIs.
1. Centers for Disease Control and Prevention. Estimates of healthcare-associated infections. http://www.cdc.gov/ncidod/dhqp/hai.html. Accessed September 27, 2018.
2. Calfee D. Crisis in hospital-acquired, healthcare-associated infections. Annu Rev Med. 2012;63(1):359-371. https://doi.org/10.1146/annurev-med-081210-144458.
3. Scott R. 2009. The direct medical costs of healthcare-associated infections in U.S. hospitals and the benefits of prevention. Centers for Disease Control and Prevention, Atlanta. http://www.cdc.gov/ncidod/dhqp/pdf/Scott_CostPaper.pdf. Accessed September 27, 2018.
4. Zimlachman E, Henderson D, Tamir O, et al. Health care associated infections. A meta-analysis of costs and financial impact on the US health care system. JAMA Intern Med. 2013;173(22):2039-2046. https://doi.org/10.1001/jamainternmed.2013.9763.
5. Nicolle LE. Catheter-acquired urinary tract infection: the once and future guidelines. Infect Control Hosp Epidemiol. 2010;31(4):327-329. https://doi.org/10.1086/651092.
6. Weber DJ, Sickbert-Bennett EE, Gould CV, et al. Incidence of catheter-associated and noncatheter-associated urinary tract infections in a healthcare system. Infect Control Hosp Epidemiol. 2011;32(8):822-823. https://doi.org/10.1086/661107.
7. Healthcare-associated infections. National and state HAI progress reports or SIR reports. https://www.cdc.gov/hai/data/archive/archive.html. Accessed May 6, 2019.
8. Saint S. Clinical and economic consequences of nosocomial catheter-related bacteriuria. Am J Infect Control. 2000;28(1):68-75. https://doi.org/10.1016/S0196-6553(00)90015-4.
9. Platt R, Polk BF, Murdock B, Rosner B. Mortality associated with nosocomial urinary-tract infection. N Engl J Med. 1982;307(11):637-642. https://doi.org/10.1056/NEJM198209093071101.
10. Tambyah PA, Knasinski V, Maki DG. The direct costs of nosocomial catheter-associated urinary tract infection in the era of managed care. Infect Control Hosp Epidemiol. 2002;23(1):27-31.
11. Chant C, Smith OM, Marshall JC, Friedrich JO. Relationship of catheter-associated urinary tract infection to mortality and length of stay in critically ill patients: a systematic review and meta-analysis of observational studies. Crit Care Med. 2011;39(5):1167-1173. https://doi.org/10.1097/CCM.0b013e31820a8581.
12. Umscheid CA, Mitchell MD, Doshi JA, et al. Estimating the proportion of healthcare-associated infections that are reasonably preventable and the related mortality and costs. Infect Control Hosp Epidemiol. 2011;32(2):101-114. https://doi.org/10.1086/657912.
13. Gould C, Umscheid C, Agarwal R, et al. Healthcare Infection Control Practices Advisory Committee (HICPAC): guideline for the prevention of catheter-associated urinary tract infections, 2009. http://www.cdc.gov/hicpac/pdf/CAUTI/CAUTIguideline2009final.pdf. Accessed November 1, 2018.
14. Hooton TM, Bradley SF, Cardenas DD, et al. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 International Clinical Practice Guidelines from the Infectious Diseases Society of America. Clin Infect Dis. 2010;50(5):625-663. https://doi.org/10.1086/650482.
15. Conway LJ, Larson EL. Guidelines to prevent catheter-associated urinary tract infection: 1980 to 2010. Heart Lung. 2011;41(3):271-283. https://doi.org/10.1016/j.hrtlng.2011.08.001.
16. Greene L, Marx J, Oriola S. APIC elimination guide: guide to the elimination of catheter-associated urinary tract infections (CAUTIs). http://www.apic.org/Content/NavigationMenu/PracticeGuidance/APICEliminationGuides/CAUTI_Guide_0609.pdf. Accessed October 15, 2018.
17. Yokoe DS, Mermel LA, Anderson DJ, et al. A compendium of strategies to prevent healthcare-associated infections in acute care hospitals. Infect Control Hosp Epidemiol. 2008;29(S1):S12-S21.
18. Wiley Z, Kobaidze K, Sexton ME, Jacob JT. Hospitalists as integral stakeholders in antimicrobial stewardship. Curr Treat Options Infect Dis. 2018;10(2):240-248. https://doi.org/10.1007/s40506-018-0162-z.
19. Claeys KC, Blanco N, Morgan DJ, Leekha S, Sullivan KV. Advances and challenges in the diagnosis and treatment of urinary tract infections: the need for diagnostic stewardship. Curr Infect Dis Rep. 2019;21(4):11. https://doi.org/10.1007/s11908-019-0668-7.
20. Horan T, Andrus M, Dudeck M. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36(5):309-332. https://doi.org/10.1016/j.ajic.2008.03.002.
21. Nicolle LE, Gupta K, Bradley S, et al. Clinical practice guideline for the management of asymptomatic bacteriuria: 2019 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2019;68(10):e83-e110.
22. Donlan R. Biofilms and device-associated infections. Emerg Infect Dis. 2001;7(2):277-281. https://doi.org/10.3201/eid0702.010226.
23. Choe HS, Son SW, Choi HA, et al. Analysis of the distribution of bacteria within urinary catheter biofilms using four different molecular techniques. Am J Infect Control. 2012;40(9):e249-e254. https://doi.org/10.1016/j.ajic.2012.05.010.
24. Mohajer M, Darouiche R. Prevention and treatment of urinary catheter-associated infections. Curr Infect Dis Rep. 2013;15(2):116-123. https://doi.org/10.1007/s11908-013-0316-6.
25. Weiner LM, Webb AK, Limbago B, et al. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2011-2014. Infect Control Hosp Epidemiol. 2016;37(11):1288-1301. https://doi.org/10.1017/ice.2016.174.
26. Tambyah PA, Oon J. Catheter-associated urinary tract infection. Curr Opin Infect Dis. 2012;25(4):365-370. https://doi.org/10.1097/QCO.0b013e32835565cc.
27. Warren JW, Tenney JH, Hoopes JM, Muncie HL, Anthony WC. A prospective microbiologic study of bacteriuria in patients with chronic indwelling urethral catheters. J Infect Dis. 1982;146(6):719-723. https://doi.org/10.1093/infdis/146.6.719.
28. Lo E, Nicolle L, Coffin S, et al. Strategies to prevent catheter-associated urinary tract infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35(5):464-479. https://doi.org/10.1086/675718.
29. Nicolle LE. Urinary catheter-associated infections. Infect Dis Clin N Am. 2012;26(1):13-27. https://doi.org/10.1016/j.idc.2011.09.009.
30. Titsworth WL, Hester J, Correia T, et al. Reduction of catheter-associated urinary tract infections among patients in a neurological intensive care unit: a single institution’s success. J Neurosurg. 2012;116(4):911-920. https://doi.org/10.3171/2011.11.JNS11974.
31. Lobdell KW, Stamou S, Sanchez JA. Hospital-acquired infections. Surg Clin N Am. 2012;92(1):65-77. https://doi.org/10.1016/j.suc.2011.11.003.
32. Gray M. Reducing catheter-associated urinary tract infection in the critical care unit. AACN Adv Crit Care. 2010;21(3):247-257. 33. Fakih MG, Watson SR, Greene T, et al. Reducing inappropriate urinary catheter use. Arch Intern Med. 2012;172(3):255-260. https://doi.org/10.1001/archinternmed.2011.627.
33. Fakih MG, Watson SR, Greene T, et al. Reducing inappropriate urinary catheter use. Arch Intern Med. 2012;172(3):255-260. https://doi.org/10.1001/archinternmed.2011.627.
34. Saint S, Wiese J, Amory JK, Bernstein ML, et al. Are physicians aware of which of their patients have an indwelling urinary catheters? Am J Med. 2000;109(6):476-480. https://doi.org/10.1016/s0002-9343(00)00531-3.
35. O’Grady NP, Barie PS, Bartlett JG, et al. Guidelines for evaluation of new fever in critically ill adult patients: 2008 update from the American College of Critical Care Medicine and the Infectious Diseases Society of America. Crit Care Med. 2008;36(4):1330-1349. https://doi.org/10.1097/CCM.0b013e318169eda9.
36. Mullin K, Kovacs C, Fatica C, et al. A multifaceted approach to reduction of catheter-associated urinary tract infections in the intensive care unit with an emphasis on “stewardship of culturing.” Infect Control Hosp Epidemiol. 2017;38(2):186-188. https://doi.org/10.1017/ice.2016.266.
37. Humphries R, Bard J. Point-counterpoint: reflex cultures reduce laboratory workload and improve antimicrobial stewardship in patients suspected of having urinary tract infections. J Clin Microbiol. 2016;54(2):254-258. https://doi.org/10.1128/JCM.03021-15.
38. Voelker R. New fluoroquinolone warning. JAMA. 2016;315(23):2514. https://doi.org/10.1001/jama.2016.7290.
39. Tillotson S. FDA and the safe and appropriate antibiotic use of fluoroquinolones. Lancet Infect Dis. 2016;16(3):PE11-E12.
40. Tanne J. FDA adds “black box” warning label to fluoroquinolone antibiotics. BMJ 2008;337:a816. https://doi.org/10.1136/bmj.a816.
41. Wyndaele JJ, Brauner A, Geerlings SE, et al. Clean intermittent catheterization and urinary tract infection: review and guide for future research. BJU Intern. 2012;110(11c):E910-E917. https://doi.org/10.1111/j.1464-410X.2012.11549.x.
42. Hakwoort RA, Thijs SD, Bouwmeester FW, et al. Comparing clean intermittent catheterization and transurethral indwelling catheterization for incomplete voiding after vaginal prolapse surgery: a multicenter randomized trial. BJOG. 2011;118(9):1055-1060. https://doi.org/10.1111/j.1471-0528.2011.02935.x.
43. Hakvoort RA, Nieuwkerk PT, Burger MP, et al. Patient preferences for clean intermittent catheterization and transurethral indwelling catheterization for treatment of abnormal post-void residual bladder volume after vaginal prolapsed surgery. BJOG. 2011;118(11):1324-1328. https://doi.org/10.1111/j.1471-0528.2011.03056.x.
44. Saint S, Kaufman SR, Roger M, et al. Condom versus indwelling urinary catheters: a randomized trial. JAGS. 2006;54(7):1055-1061. https://doi.org/10.1111/j.1532-5415.2006.00785.x.
45. Pickard R, Lam T, MacLennan G, et al. Antimicrobial catheters for reduction of symptomatic urinary tract infection in adults requiring short-term catheterization in hospital: a multicenter randomized controlled trial. Lancet. 2012;380(9857):1927-1935. https://doi.org/10.1016/S0140-6736(12)61380-4.
46. Parry M, Grant B, Sestovic M. Successful reduction in catheter-associated urinary tract infections: focus on nurse-directed catheter removal. Am J Infect Control. 2013;41(12):1178-1181. https://doi.org/10.1016/j.ajic.2013.03.296.
47. Fuchs MA, Sexton DJ, Thornlow DK, Champagne MT. Evaluation of an evidence-based, nurse-driven checklist to prevent hospital-acquired catheter-associated urinary tract infections in intensive care units. J Nurs Care Qual. 2011;26(2):101-109. https://doi.org/10.1097/NCQ.0b013e3181fb7847.
48. Meddings J, Rogers MA, Macy M, Saint S. Systematic review and meta-analysis: reminder systems to reduce catheter-associated urinary tract infections and urinary catheter use in hospitalized patients. Clin Infect Dis. 2010;51(5):550-560. https://doi.org/10.1086/655133.
49. Baggs J, Fridkin SK, Pollack LA, Srinivasan A, Jernigan JA. Estimating national trends in inpatient antibiotic use among US hospitals from 2006 to 2012. JAMA Intern Med. 2016;176(11):1639-1648. https://doi.org/10.1001/jamainternmed.2016.5651.
50. National Action Plan for Combating Antibiotic-resistant Bacteria. 2015. https://obamawhitehouse.archives.gov/sites/default/files/docs/national_action_plan_for_combatin
Every day in the United States, approximately 4% of patients in acute care hospitals have at least one hospital-acquired infection (HAI).1,2 Among the top 10 causes of death in the United States, HAIs are associated with increased morbidity, mortality, and hospital length of stay (LOS).2 The direct medical cost of treating HAIs is substantial for both hospitals and patients.3,4 Urinary tract infections (UTIs) are a leading cause of HAI, and 70%-80% of these are catheter-associated urinary tract infections (CAUTIs).5,6 In 2016, 26,983 CAUTIs occurred in acute care hospitals.7 The high incidence of CAUTI can substantially contribute to morbidity, length of stay, and mortality.8-11
The recognition that a substantial proportion of HAIs may be preventable, including 55%-70% of CAUTIs,12 has resulted in implementing multiple strategies to reduce CAUTI rates.13-17 These include simple prevention interventions such as avoiding placement of unnecessary indwelling urinary catheters and early removal of urinary catheters when they are no longer clinically indicated. Hospitalists are responsible for the care of many, if not most, inpatients with indwelling urinary catheters and are integral in antimicrobial stewardship efforts surrounding CAUTIs.18 Diagnostic stewardship, including appropriate urine specimen ordering, collection, processing, and reporting, works synergistically with antimicrobial stewardship and allows for appropriate antibiotic prescribing in symptomatic patients.19
DEFINITIONS
CAUTIs can be defined using either clinical or surveillance definitions. Clinical definitions are used at the bedside and take individual clinical characteristics into consideration, but vary among clinicians since there is no gold standard. Abnormal laboratory urinary findings in the absence of symptoms are not sufficient for the diagnosis of UTI, including CAUTI. Surveillance definitions, such as those used by the Centers for Disease Control and Prevention,20 are designed to be simple, easily applicable in any healthcare setting, and standardized to all patients. Surveillance definitions generally include at least one systemic or local symptom (such as fever or dysuria) and positive urine culture in a patient with an indwelling urinary catheter (or within 48 hours after its removal).
Pyuria is leukocytes or white blood cells (WBCs) in a urine specimen, with a threshold of >10 WBCs/high-power field using urine microscopy. The predictive value of different thresholds of pyuria for UTI is unclear.
Bacteriuria denotes the presence (on microscopy or culture) of bacteria in the urine. In a patient without signs or symptoms of a UTI, this is termed asymptomatic bacteriuria (ASB). A full discussion of bacteriuria, a major reason for inappropriate antibiotic use, is beyond the scope of this article but is discussed in a recent guideline.21
Urinary tract infections are usually characterized by a clinical syndrome along with evidence of pyuria and/or bacteriuria. The two major clinical syndromes that are observed are lower UTI (cystitis or bladder infections) and upper UTI (pyelonephritis or kidney infections). Rarely, patients may develop asymptomatic bacteremic UTI, where blood and urine cultures grow the same pathogen in the absence of clinical symptoms. (Table 1 summarizes the key points for these definitions).
CAUSES AND RISK FACTORS FOR CAUTI
Bacterial biofilm can form on the inner and outer surfaces of an indwelling urinary catheter following its insertion and can be associated with bacteriuria and CAUTI.22,23 The biofilm comprises bacteria from the periurethral area that migrate upwards from a colonized drainage system. Bacteria present in the biofilm tend to exhibit slow growth, are protected from antibiotic exposure, and have less susceptibility to these agents.22-24 When a mature biofilm has formed, catheter removal may be necessary for source control and to facilitate effective antimicrobial treatment. The pathogens that most commonly cause CAUTIs are Escherichia coli (23.9%), Pseudomonas aeruginosa (10.3%), and Klebsiella pneumoniae/oxytoca (10.1%).25 Although urine cultures often grow yeast, particularly Candida spp., nonbacterial pathogens rarely cause UTI.
The risk of developing a CAUTI is directly related to catheter dwell time.26,27 For catheterized patients, the rate of development of catheter-associated bacteriuria is approximately 3% to 7% per day14,28 and is more common in the elderly and females. The likelihood of bacteriuria approaches 100% if a patient has an indwelling urinary catheter for ≥30 days,27,29,30 which is part of the rationale for why a urine culture alone is not sufficient to diagnose a CAUTI. While bacteriuria is a risk factor for UTI, the frequency of progression from bacteriuria to CAUTI is low and treating ASB does not decrease the risk of future CAUTI. Other risk factors for the development of CAUTI include urinary tract instrumentation, diabetes mellitus, and malnutrition.31,32
The two most important factors that lead to the development of CAUTIs and have been the main focus of quality improvement areas are unnecessary urinary catheter placement and inappropriate delay in removing a catheter when it is no longer needed.26,33 Unfortunately, 38% of attending physicians are unaware that their patients have a urinary catheter in place.34 Furthermore, in 20% to 50% of cases, there is no clear indication for catheter placement.2,34
DIAGNOSIS OF CAUTI
A CAUTI diagnosis is typically one of exclusion, as most patients present with fever and no apparent alternative source.14,29 Since catheterized patients may not exhibit common cystitis symptoms,29 most who develop CAUTI present with fever alone. However, most fevers in patients with bacteriuria and catheters are not CAUTIs and can be attributed to other sources. If a patient with an indwelling urinary catheter develops a fever and there is a suspicion of a CAUTI, careful evaluation is warranted for alternative sources of infection. This particularly applies to patients with severe systemic illness, such as hypotension or systemic inflammatory response syndrome, since these are unusual manifestations of CAUTI. The presence of either cloudy or malodorous urine does not indicate a UTI, and should not be the sole rationale for obtaining a urine culture.
Diagnostic workup of fever should include a clinical assessment of the patient. Indeed, professional guidelines recommend against obtaining a urine culture routinely for fever, unless invasive UTI risk is elevated, such as in patients with neutropenia, history of renal transplantation, or recent genitourinary surgery.35 Diagnostic stewardship, focusing on the appropriate use of urine cultures, can reduce CAUTI rates.36 For catheterized patients, hospitals are increasingly adopting reflex urine culture, where urine is simultaneously collected for a urinalysis and urine culture, but a urine culture is performed only if the urinalysis is positive for a predetermined threshold for pyuria, leukocyte esterase, or both. However, the use of reflex urine cultures remains an area of debate.37 In addition, the Infectious Diseases Society of America recommends against screening for or treating ASB in patients with either short-term (<30 days) or long-term indwelling urethral catheters.21
Ideally, a urine culture should be obtained by collecting a midstream sample. In catheterized patients, a sample should be obtained after removal of the catheter; or, in patients with a clinical indication for ongoing catheterization, a sample should be obtained after a new catheter has been placed.14 If an indwelling urinary catheter must be continued, the recommendation is to disinfect the drainage system’s aspiration port and then obtain a urine culture. Urine should never be obtained from a catheter collection bag. (Table 2 summarizes best practices for diagnosis of CAUTI).
TREATMENT OF CAUTI
For all CAUTIs, an indwelling urinary catheter should be removed as soon as possible. If an indwelling urinary catheter remains necessary, but the existing catheter has been in place longer than two weeks, a new catheter should be placed before initiating antibiotic therapy14 to accelerate symptom resolution and reduce the likelihood of relapse or recurrence.32
Urinary tract agents such as fosfomycin and nitrofurantoin are recommended as first-line agents for simple cystitis in women and can be used in patients with lower UTI and sufficient renal function to achieve adequate drug concentration in urine. Upper UTIs require antibiotics with good penetration into renal parenchyma such as ceftriaxone. If empiric antimicrobial therapy is needed before culture results are available, then previous urine culture results, local antibiograms, or practice guidelines can guide selection. Definitive antimicrobial therapy should be based on urine culture results. It is important to narrow empiric therapy14 to reduce risk of Clostridioides difficile infection and emergence of other resistant bacteria. Fluoroquinolones should be avoided for lower UTIs because of these risks and multiple United States Food and Drug Administration warnings.38-40
The optimal duration of antimicrobial therapy for a CAUTI is unclear;14 however, most patients can be treated with a relatively short duration of therapy (≤7 days) if they respond promptly to therapy. Patients with a slow response to therapy may require 10-14 days of treatment.14 (Table 2 summarizes best practices for the treatment of CAUTI).
STRATEGIES FOR CAUTI PREVENTION
Since CAUTI is predicated on the presence of an indwelling urinary catheter, the simplest way to reduce CAUTI is to avoid placing or retaining unnecessary catheters. Some examples of appropriate indications9 for placement and maintenance of an indwelling urinary catheter are listed below:
1. Accurate measurement of urinary output in severely ill patients;
2. Improved comfort for patients receiving end-of-life care;
3. Acute urinary retention or bladder outlet obstruction;
4. Need for a period of prolonged immobilization (eg, potentially unstable lumbar or thoracic spine, or has multiple traumatic injuries);
5. Selected surgical procedures, such as urologic procedures and those that are expected to have a prolonged duration, require intraoperative monitoring of urine output, require the administration of either large volumes of intravenous infusions or diuretics;
6. To promote healing of open perineal or sacral wounds in patients with incontinence;
7. Neurogenic bladder; and
8. Hematuria with clots.
To increase the timely removal of urinary catheters that are no longer indicated, daily assessment of catheter necessity must be an integral part of clinicians’ workflow.32,33 Alternatives, such as external catheters or intermittent catheterization, should be considered before indwelling urinary catheter placement since both options are associated with a reduced CAUTI risk.41-44 Although indwelling urinary catheters can be seen as being more convenient for both patients and healthcare providers, many patients have expressed a preference for the use of intermittent catheterization compared with indwelling urinary catheterization.43
For urinary retention, bladder scanning can noninvasively assess the amount of residual urine in a patient’s bladder and can avoid unnecessary insertion of an indwelling urinary catheter. However, if indwelling urinary catheters are ultimately needed, they must be inserted and maintained appropriately. Of note, the use of antibiotic-impregnated catheters has not been shown to reduce CAUTI rates significantly.45
CAUTI prevention requires a multidisciplinary collaborative approach. Nurse-driven protocols and checklists to remove indwelling urinary catheters that are no longer indicated can be very effective.46,47 Automatic stop orders and catheter removal reminders are useful for reducing the duration of catheter placement.26,48 Both of these approaches require appropriate, consistent documentation with input from bedside nurses, physicians, advanced practice providers, and information technology. (Table 3 summarizes best practices for the prevention of CAUTI).
Importantly, CAUTI prevention supports broader antimicrobial stewardship. Over 55% of inpatients receive at least one dose of an antibiotic during their hospital stay.48,49 In 2015, the White House released the National Action Plan for Combating Antibiotic-Resistant Bacteria with the goals of slowing the emergence of resistant bacteria, preventing the spread of antibiotic-resistant infections, and setting a target of a 20% reduction in the inappropriate use of antibiotics for hospitalized patients.50 Hospitalists care for a substantial number of inpatients and, in turn, can drive actions to decrease CAUTIs, and promote stewardship efforts. Through actions to decrease CAUTIs, hospitalists can promote these stewardship efforts.
CONCLUSIONS
CAUTI is one of the most common types of HAI and is associated with increased morbidity, hospital length of stay, and patient costs. Most CAUTIs are preventable by limiting the placement of unnecessary catheters to instances of true necessity and removing catheters when they are no longer clinically indicated. Proper technique for the insertion and maintenance of catheters is also important for reducing CAUTI rates. Hospitalists care for a substantial number of inpatients and can make major contributions to the appropriate diagnosis, treatment, and prevention of CAUTIs.
Every day in the United States, approximately 4% of patients in acute care hospitals have at least one hospital-acquired infection (HAI).1,2 Among the top 10 causes of death in the United States, HAIs are associated with increased morbidity, mortality, and hospital length of stay (LOS).2 The direct medical cost of treating HAIs is substantial for both hospitals and patients.3,4 Urinary tract infections (UTIs) are a leading cause of HAI, and 70%-80% of these are catheter-associated urinary tract infections (CAUTIs).5,6 In 2016, 26,983 CAUTIs occurred in acute care hospitals.7 The high incidence of CAUTI can substantially contribute to morbidity, length of stay, and mortality.8-11
The recognition that a substantial proportion of HAIs may be preventable, including 55%-70% of CAUTIs,12 has resulted in implementing multiple strategies to reduce CAUTI rates.13-17 These include simple prevention interventions such as avoiding placement of unnecessary indwelling urinary catheters and early removal of urinary catheters when they are no longer clinically indicated. Hospitalists are responsible for the care of many, if not most, inpatients with indwelling urinary catheters and are integral in antimicrobial stewardship efforts surrounding CAUTIs.18 Diagnostic stewardship, including appropriate urine specimen ordering, collection, processing, and reporting, works synergistically with antimicrobial stewardship and allows for appropriate antibiotic prescribing in symptomatic patients.19
DEFINITIONS
CAUTIs can be defined using either clinical or surveillance definitions. Clinical definitions are used at the bedside and take individual clinical characteristics into consideration, but vary among clinicians since there is no gold standard. Abnormal laboratory urinary findings in the absence of symptoms are not sufficient for the diagnosis of UTI, including CAUTI. Surveillance definitions, such as those used by the Centers for Disease Control and Prevention,20 are designed to be simple, easily applicable in any healthcare setting, and standardized to all patients. Surveillance definitions generally include at least one systemic or local symptom (such as fever or dysuria) and positive urine culture in a patient with an indwelling urinary catheter (or within 48 hours after its removal).
Pyuria is leukocytes or white blood cells (WBCs) in a urine specimen, with a threshold of >10 WBCs/high-power field using urine microscopy. The predictive value of different thresholds of pyuria for UTI is unclear.
Bacteriuria denotes the presence (on microscopy or culture) of bacteria in the urine. In a patient without signs or symptoms of a UTI, this is termed asymptomatic bacteriuria (ASB). A full discussion of bacteriuria, a major reason for inappropriate antibiotic use, is beyond the scope of this article but is discussed in a recent guideline.21
Urinary tract infections are usually characterized by a clinical syndrome along with evidence of pyuria and/or bacteriuria. The two major clinical syndromes that are observed are lower UTI (cystitis or bladder infections) and upper UTI (pyelonephritis or kidney infections). Rarely, patients may develop asymptomatic bacteremic UTI, where blood and urine cultures grow the same pathogen in the absence of clinical symptoms. (Table 1 summarizes the key points for these definitions).
CAUSES AND RISK FACTORS FOR CAUTI
Bacterial biofilm can form on the inner and outer surfaces of an indwelling urinary catheter following its insertion and can be associated with bacteriuria and CAUTI.22,23 The biofilm comprises bacteria from the periurethral area that migrate upwards from a colonized drainage system. Bacteria present in the biofilm tend to exhibit slow growth, are protected from antibiotic exposure, and have less susceptibility to these agents.22-24 When a mature biofilm has formed, catheter removal may be necessary for source control and to facilitate effective antimicrobial treatment. The pathogens that most commonly cause CAUTIs are Escherichia coli (23.9%), Pseudomonas aeruginosa (10.3%), and Klebsiella pneumoniae/oxytoca (10.1%).25 Although urine cultures often grow yeast, particularly Candida spp., nonbacterial pathogens rarely cause UTI.
The risk of developing a CAUTI is directly related to catheter dwell time.26,27 For catheterized patients, the rate of development of catheter-associated bacteriuria is approximately 3% to 7% per day14,28 and is more common in the elderly and females. The likelihood of bacteriuria approaches 100% if a patient has an indwelling urinary catheter for ≥30 days,27,29,30 which is part of the rationale for why a urine culture alone is not sufficient to diagnose a CAUTI. While bacteriuria is a risk factor for UTI, the frequency of progression from bacteriuria to CAUTI is low and treating ASB does not decrease the risk of future CAUTI. Other risk factors for the development of CAUTI include urinary tract instrumentation, diabetes mellitus, and malnutrition.31,32
The two most important factors that lead to the development of CAUTIs and have been the main focus of quality improvement areas are unnecessary urinary catheter placement and inappropriate delay in removing a catheter when it is no longer needed.26,33 Unfortunately, 38% of attending physicians are unaware that their patients have a urinary catheter in place.34 Furthermore, in 20% to 50% of cases, there is no clear indication for catheter placement.2,34
DIAGNOSIS OF CAUTI
A CAUTI diagnosis is typically one of exclusion, as most patients present with fever and no apparent alternative source.14,29 Since catheterized patients may not exhibit common cystitis symptoms,29 most who develop CAUTI present with fever alone. However, most fevers in patients with bacteriuria and catheters are not CAUTIs and can be attributed to other sources. If a patient with an indwelling urinary catheter develops a fever and there is a suspicion of a CAUTI, careful evaluation is warranted for alternative sources of infection. This particularly applies to patients with severe systemic illness, such as hypotension or systemic inflammatory response syndrome, since these are unusual manifestations of CAUTI. The presence of either cloudy or malodorous urine does not indicate a UTI, and should not be the sole rationale for obtaining a urine culture.
Diagnostic workup of fever should include a clinical assessment of the patient. Indeed, professional guidelines recommend against obtaining a urine culture routinely for fever, unless invasive UTI risk is elevated, such as in patients with neutropenia, history of renal transplantation, or recent genitourinary surgery.35 Diagnostic stewardship, focusing on the appropriate use of urine cultures, can reduce CAUTI rates.36 For catheterized patients, hospitals are increasingly adopting reflex urine culture, where urine is simultaneously collected for a urinalysis and urine culture, but a urine culture is performed only if the urinalysis is positive for a predetermined threshold for pyuria, leukocyte esterase, or both. However, the use of reflex urine cultures remains an area of debate.37 In addition, the Infectious Diseases Society of America recommends against screening for or treating ASB in patients with either short-term (<30 days) or long-term indwelling urethral catheters.21
Ideally, a urine culture should be obtained by collecting a midstream sample. In catheterized patients, a sample should be obtained after removal of the catheter; or, in patients with a clinical indication for ongoing catheterization, a sample should be obtained after a new catheter has been placed.14 If an indwelling urinary catheter must be continued, the recommendation is to disinfect the drainage system’s aspiration port and then obtain a urine culture. Urine should never be obtained from a catheter collection bag. (Table 2 summarizes best practices for diagnosis of CAUTI).
TREATMENT OF CAUTI
For all CAUTIs, an indwelling urinary catheter should be removed as soon as possible. If an indwelling urinary catheter remains necessary, but the existing catheter has been in place longer than two weeks, a new catheter should be placed before initiating antibiotic therapy14 to accelerate symptom resolution and reduce the likelihood of relapse or recurrence.32
Urinary tract agents such as fosfomycin and nitrofurantoin are recommended as first-line agents for simple cystitis in women and can be used in patients with lower UTI and sufficient renal function to achieve adequate drug concentration in urine. Upper UTIs require antibiotics with good penetration into renal parenchyma such as ceftriaxone. If empiric antimicrobial therapy is needed before culture results are available, then previous urine culture results, local antibiograms, or practice guidelines can guide selection. Definitive antimicrobial therapy should be based on urine culture results. It is important to narrow empiric therapy14 to reduce risk of Clostridioides difficile infection and emergence of other resistant bacteria. Fluoroquinolones should be avoided for lower UTIs because of these risks and multiple United States Food and Drug Administration warnings.38-40
The optimal duration of antimicrobial therapy for a CAUTI is unclear;14 however, most patients can be treated with a relatively short duration of therapy (≤7 days) if they respond promptly to therapy. Patients with a slow response to therapy may require 10-14 days of treatment.14 (Table 2 summarizes best practices for the treatment of CAUTI).
STRATEGIES FOR CAUTI PREVENTION
Since CAUTI is predicated on the presence of an indwelling urinary catheter, the simplest way to reduce CAUTI is to avoid placing or retaining unnecessary catheters. Some examples of appropriate indications9 for placement and maintenance of an indwelling urinary catheter are listed below:
1. Accurate measurement of urinary output in severely ill patients;
2. Improved comfort for patients receiving end-of-life care;
3. Acute urinary retention or bladder outlet obstruction;
4. Need for a period of prolonged immobilization (eg, potentially unstable lumbar or thoracic spine, or has multiple traumatic injuries);
5. Selected surgical procedures, such as urologic procedures and those that are expected to have a prolonged duration, require intraoperative monitoring of urine output, require the administration of either large volumes of intravenous infusions or diuretics;
6. To promote healing of open perineal or sacral wounds in patients with incontinence;
7. Neurogenic bladder; and
8. Hematuria with clots.
To increase the timely removal of urinary catheters that are no longer indicated, daily assessment of catheter necessity must be an integral part of clinicians’ workflow.32,33 Alternatives, such as external catheters or intermittent catheterization, should be considered before indwelling urinary catheter placement since both options are associated with a reduced CAUTI risk.41-44 Although indwelling urinary catheters can be seen as being more convenient for both patients and healthcare providers, many patients have expressed a preference for the use of intermittent catheterization compared with indwelling urinary catheterization.43
For urinary retention, bladder scanning can noninvasively assess the amount of residual urine in a patient’s bladder and can avoid unnecessary insertion of an indwelling urinary catheter. However, if indwelling urinary catheters are ultimately needed, they must be inserted and maintained appropriately. Of note, the use of antibiotic-impregnated catheters has not been shown to reduce CAUTI rates significantly.45
CAUTI prevention requires a multidisciplinary collaborative approach. Nurse-driven protocols and checklists to remove indwelling urinary catheters that are no longer indicated can be very effective.46,47 Automatic stop orders and catheter removal reminders are useful for reducing the duration of catheter placement.26,48 Both of these approaches require appropriate, consistent documentation with input from bedside nurses, physicians, advanced practice providers, and information technology. (Table 3 summarizes best practices for the prevention of CAUTI).
Importantly, CAUTI prevention supports broader antimicrobial stewardship. Over 55% of inpatients receive at least one dose of an antibiotic during their hospital stay.48,49 In 2015, the White House released the National Action Plan for Combating Antibiotic-Resistant Bacteria with the goals of slowing the emergence of resistant bacteria, preventing the spread of antibiotic-resistant infections, and setting a target of a 20% reduction in the inappropriate use of antibiotics for hospitalized patients.50 Hospitalists care for a substantial number of inpatients and, in turn, can drive actions to decrease CAUTIs, and promote stewardship efforts. Through actions to decrease CAUTIs, hospitalists can promote these stewardship efforts.
CONCLUSIONS
CAUTI is one of the most common types of HAI and is associated with increased morbidity, hospital length of stay, and patient costs. Most CAUTIs are preventable by limiting the placement of unnecessary catheters to instances of true necessity and removing catheters when they are no longer clinically indicated. Proper technique for the insertion and maintenance of catheters is also important for reducing CAUTI rates. Hospitalists care for a substantial number of inpatients and can make major contributions to the appropriate diagnosis, treatment, and prevention of CAUTIs.
1. Centers for Disease Control and Prevention. Estimates of healthcare-associated infections. http://www.cdc.gov/ncidod/dhqp/hai.html. Accessed September 27, 2018.
2. Calfee D. Crisis in hospital-acquired, healthcare-associated infections. Annu Rev Med. 2012;63(1):359-371. https://doi.org/10.1146/annurev-med-081210-144458.
3. Scott R. 2009. The direct medical costs of healthcare-associated infections in U.S. hospitals and the benefits of prevention. Centers for Disease Control and Prevention, Atlanta. http://www.cdc.gov/ncidod/dhqp/pdf/Scott_CostPaper.pdf. Accessed September 27, 2018.
4. Zimlachman E, Henderson D, Tamir O, et al. Health care associated infections. A meta-analysis of costs and financial impact on the US health care system. JAMA Intern Med. 2013;173(22):2039-2046. https://doi.org/10.1001/jamainternmed.2013.9763.
5. Nicolle LE. Catheter-acquired urinary tract infection: the once and future guidelines. Infect Control Hosp Epidemiol. 2010;31(4):327-329. https://doi.org/10.1086/651092.
6. Weber DJ, Sickbert-Bennett EE, Gould CV, et al. Incidence of catheter-associated and noncatheter-associated urinary tract infections in a healthcare system. Infect Control Hosp Epidemiol. 2011;32(8):822-823. https://doi.org/10.1086/661107.
7. Healthcare-associated infections. National and state HAI progress reports or SIR reports. https://www.cdc.gov/hai/data/archive/archive.html. Accessed May 6, 2019.
8. Saint S. Clinical and economic consequences of nosocomial catheter-related bacteriuria. Am J Infect Control. 2000;28(1):68-75. https://doi.org/10.1016/S0196-6553(00)90015-4.
9. Platt R, Polk BF, Murdock B, Rosner B. Mortality associated with nosocomial urinary-tract infection. N Engl J Med. 1982;307(11):637-642. https://doi.org/10.1056/NEJM198209093071101.
10. Tambyah PA, Knasinski V, Maki DG. The direct costs of nosocomial catheter-associated urinary tract infection in the era of managed care. Infect Control Hosp Epidemiol. 2002;23(1):27-31.
11. Chant C, Smith OM, Marshall JC, Friedrich JO. Relationship of catheter-associated urinary tract infection to mortality and length of stay in critically ill patients: a systematic review and meta-analysis of observational studies. Crit Care Med. 2011;39(5):1167-1173. https://doi.org/10.1097/CCM.0b013e31820a8581.
12. Umscheid CA, Mitchell MD, Doshi JA, et al. Estimating the proportion of healthcare-associated infections that are reasonably preventable and the related mortality and costs. Infect Control Hosp Epidemiol. 2011;32(2):101-114. https://doi.org/10.1086/657912.
13. Gould C, Umscheid C, Agarwal R, et al. Healthcare Infection Control Practices Advisory Committee (HICPAC): guideline for the prevention of catheter-associated urinary tract infections, 2009. http://www.cdc.gov/hicpac/pdf/CAUTI/CAUTIguideline2009final.pdf. Accessed November 1, 2018.
14. Hooton TM, Bradley SF, Cardenas DD, et al. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 International Clinical Practice Guidelines from the Infectious Diseases Society of America. Clin Infect Dis. 2010;50(5):625-663. https://doi.org/10.1086/650482.
15. Conway LJ, Larson EL. Guidelines to prevent catheter-associated urinary tract infection: 1980 to 2010. Heart Lung. 2011;41(3):271-283. https://doi.org/10.1016/j.hrtlng.2011.08.001.
16. Greene L, Marx J, Oriola S. APIC elimination guide: guide to the elimination of catheter-associated urinary tract infections (CAUTIs). http://www.apic.org/Content/NavigationMenu/PracticeGuidance/APICEliminationGuides/CAUTI_Guide_0609.pdf. Accessed October 15, 2018.
17. Yokoe DS, Mermel LA, Anderson DJ, et al. A compendium of strategies to prevent healthcare-associated infections in acute care hospitals. Infect Control Hosp Epidemiol. 2008;29(S1):S12-S21.
18. Wiley Z, Kobaidze K, Sexton ME, Jacob JT. Hospitalists as integral stakeholders in antimicrobial stewardship. Curr Treat Options Infect Dis. 2018;10(2):240-248. https://doi.org/10.1007/s40506-018-0162-z.
19. Claeys KC, Blanco N, Morgan DJ, Leekha S, Sullivan KV. Advances and challenges in the diagnosis and treatment of urinary tract infections: the need for diagnostic stewardship. Curr Infect Dis Rep. 2019;21(4):11. https://doi.org/10.1007/s11908-019-0668-7.
20. Horan T, Andrus M, Dudeck M. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36(5):309-332. https://doi.org/10.1016/j.ajic.2008.03.002.
21. Nicolle LE, Gupta K, Bradley S, et al. Clinical practice guideline for the management of asymptomatic bacteriuria: 2019 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2019;68(10):e83-e110.
22. Donlan R. Biofilms and device-associated infections. Emerg Infect Dis. 2001;7(2):277-281. https://doi.org/10.3201/eid0702.010226.
23. Choe HS, Son SW, Choi HA, et al. Analysis of the distribution of bacteria within urinary catheter biofilms using four different molecular techniques. Am J Infect Control. 2012;40(9):e249-e254. https://doi.org/10.1016/j.ajic.2012.05.010.
24. Mohajer M, Darouiche R. Prevention and treatment of urinary catheter-associated infections. Curr Infect Dis Rep. 2013;15(2):116-123. https://doi.org/10.1007/s11908-013-0316-6.
25. Weiner LM, Webb AK, Limbago B, et al. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2011-2014. Infect Control Hosp Epidemiol. 2016;37(11):1288-1301. https://doi.org/10.1017/ice.2016.174.
26. Tambyah PA, Oon J. Catheter-associated urinary tract infection. Curr Opin Infect Dis. 2012;25(4):365-370. https://doi.org/10.1097/QCO.0b013e32835565cc.
27. Warren JW, Tenney JH, Hoopes JM, Muncie HL, Anthony WC. A prospective microbiologic study of bacteriuria in patients with chronic indwelling urethral catheters. J Infect Dis. 1982;146(6):719-723. https://doi.org/10.1093/infdis/146.6.719.
28. Lo E, Nicolle L, Coffin S, et al. Strategies to prevent catheter-associated urinary tract infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35(5):464-479. https://doi.org/10.1086/675718.
29. Nicolle LE. Urinary catheter-associated infections. Infect Dis Clin N Am. 2012;26(1):13-27. https://doi.org/10.1016/j.idc.2011.09.009.
30. Titsworth WL, Hester J, Correia T, et al. Reduction of catheter-associated urinary tract infections among patients in a neurological intensive care unit: a single institution’s success. J Neurosurg. 2012;116(4):911-920. https://doi.org/10.3171/2011.11.JNS11974.
31. Lobdell KW, Stamou S, Sanchez JA. Hospital-acquired infections. Surg Clin N Am. 2012;92(1):65-77. https://doi.org/10.1016/j.suc.2011.11.003.
32. Gray M. Reducing catheter-associated urinary tract infection in the critical care unit. AACN Adv Crit Care. 2010;21(3):247-257. 33. Fakih MG, Watson SR, Greene T, et al. Reducing inappropriate urinary catheter use. Arch Intern Med. 2012;172(3):255-260. https://doi.org/10.1001/archinternmed.2011.627.
33. Fakih MG, Watson SR, Greene T, et al. Reducing inappropriate urinary catheter use. Arch Intern Med. 2012;172(3):255-260. https://doi.org/10.1001/archinternmed.2011.627.
34. Saint S, Wiese J, Amory JK, Bernstein ML, et al. Are physicians aware of which of their patients have an indwelling urinary catheters? Am J Med. 2000;109(6):476-480. https://doi.org/10.1016/s0002-9343(00)00531-3.
35. O’Grady NP, Barie PS, Bartlett JG, et al. Guidelines for evaluation of new fever in critically ill adult patients: 2008 update from the American College of Critical Care Medicine and the Infectious Diseases Society of America. Crit Care Med. 2008;36(4):1330-1349. https://doi.org/10.1097/CCM.0b013e318169eda9.
36. Mullin K, Kovacs C, Fatica C, et al. A multifaceted approach to reduction of catheter-associated urinary tract infections in the intensive care unit with an emphasis on “stewardship of culturing.” Infect Control Hosp Epidemiol. 2017;38(2):186-188. https://doi.org/10.1017/ice.2016.266.
37. Humphries R, Bard J. Point-counterpoint: reflex cultures reduce laboratory workload and improve antimicrobial stewardship in patients suspected of having urinary tract infections. J Clin Microbiol. 2016;54(2):254-258. https://doi.org/10.1128/JCM.03021-15.
38. Voelker R. New fluoroquinolone warning. JAMA. 2016;315(23):2514. https://doi.org/10.1001/jama.2016.7290.
39. Tillotson S. FDA and the safe and appropriate antibiotic use of fluoroquinolones. Lancet Infect Dis. 2016;16(3):PE11-E12.
40. Tanne J. FDA adds “black box” warning label to fluoroquinolone antibiotics. BMJ 2008;337:a816. https://doi.org/10.1136/bmj.a816.
41. Wyndaele JJ, Brauner A, Geerlings SE, et al. Clean intermittent catheterization and urinary tract infection: review and guide for future research. BJU Intern. 2012;110(11c):E910-E917. https://doi.org/10.1111/j.1464-410X.2012.11549.x.
42. Hakwoort RA, Thijs SD, Bouwmeester FW, et al. Comparing clean intermittent catheterization and transurethral indwelling catheterization for incomplete voiding after vaginal prolapse surgery: a multicenter randomized trial. BJOG. 2011;118(9):1055-1060. https://doi.org/10.1111/j.1471-0528.2011.02935.x.
43. Hakvoort RA, Nieuwkerk PT, Burger MP, et al. Patient preferences for clean intermittent catheterization and transurethral indwelling catheterization for treatment of abnormal post-void residual bladder volume after vaginal prolapsed surgery. BJOG. 2011;118(11):1324-1328. https://doi.org/10.1111/j.1471-0528.2011.03056.x.
44. Saint S, Kaufman SR, Roger M, et al. Condom versus indwelling urinary catheters: a randomized trial. JAGS. 2006;54(7):1055-1061. https://doi.org/10.1111/j.1532-5415.2006.00785.x.
45. Pickard R, Lam T, MacLennan G, et al. Antimicrobial catheters for reduction of symptomatic urinary tract infection in adults requiring short-term catheterization in hospital: a multicenter randomized controlled trial. Lancet. 2012;380(9857):1927-1935. https://doi.org/10.1016/S0140-6736(12)61380-4.
46. Parry M, Grant B, Sestovic M. Successful reduction in catheter-associated urinary tract infections: focus on nurse-directed catheter removal. Am J Infect Control. 2013;41(12):1178-1181. https://doi.org/10.1016/j.ajic.2013.03.296.
47. Fuchs MA, Sexton DJ, Thornlow DK, Champagne MT. Evaluation of an evidence-based, nurse-driven checklist to prevent hospital-acquired catheter-associated urinary tract infections in intensive care units. J Nurs Care Qual. 2011;26(2):101-109. https://doi.org/10.1097/NCQ.0b013e3181fb7847.
48. Meddings J, Rogers MA, Macy M, Saint S. Systematic review and meta-analysis: reminder systems to reduce catheter-associated urinary tract infections and urinary catheter use in hospitalized patients. Clin Infect Dis. 2010;51(5):550-560. https://doi.org/10.1086/655133.
49. Baggs J, Fridkin SK, Pollack LA, Srinivasan A, Jernigan JA. Estimating national trends in inpatient antibiotic use among US hospitals from 2006 to 2012. JAMA Intern Med. 2016;176(11):1639-1648. https://doi.org/10.1001/jamainternmed.2016.5651.
50. National Action Plan for Combating Antibiotic-resistant Bacteria. 2015. https://obamawhitehouse.archives.gov/sites/default/files/docs/national_action_plan_for_combatin
1. Centers for Disease Control and Prevention. Estimates of healthcare-associated infections. http://www.cdc.gov/ncidod/dhqp/hai.html. Accessed September 27, 2018.
2. Calfee D. Crisis in hospital-acquired, healthcare-associated infections. Annu Rev Med. 2012;63(1):359-371. https://doi.org/10.1146/annurev-med-081210-144458.
3. Scott R. 2009. The direct medical costs of healthcare-associated infections in U.S. hospitals and the benefits of prevention. Centers for Disease Control and Prevention, Atlanta. http://www.cdc.gov/ncidod/dhqp/pdf/Scott_CostPaper.pdf. Accessed September 27, 2018.
4. Zimlachman E, Henderson D, Tamir O, et al. Health care associated infections. A meta-analysis of costs and financial impact on the US health care system. JAMA Intern Med. 2013;173(22):2039-2046. https://doi.org/10.1001/jamainternmed.2013.9763.
5. Nicolle LE. Catheter-acquired urinary tract infection: the once and future guidelines. Infect Control Hosp Epidemiol. 2010;31(4):327-329. https://doi.org/10.1086/651092.
6. Weber DJ, Sickbert-Bennett EE, Gould CV, et al. Incidence of catheter-associated and noncatheter-associated urinary tract infections in a healthcare system. Infect Control Hosp Epidemiol. 2011;32(8):822-823. https://doi.org/10.1086/661107.
7. Healthcare-associated infections. National and state HAI progress reports or SIR reports. https://www.cdc.gov/hai/data/archive/archive.html. Accessed May 6, 2019.
8. Saint S. Clinical and economic consequences of nosocomial catheter-related bacteriuria. Am J Infect Control. 2000;28(1):68-75. https://doi.org/10.1016/S0196-6553(00)90015-4.
9. Platt R, Polk BF, Murdock B, Rosner B. Mortality associated with nosocomial urinary-tract infection. N Engl J Med. 1982;307(11):637-642. https://doi.org/10.1056/NEJM198209093071101.
10. Tambyah PA, Knasinski V, Maki DG. The direct costs of nosocomial catheter-associated urinary tract infection in the era of managed care. Infect Control Hosp Epidemiol. 2002;23(1):27-31.
11. Chant C, Smith OM, Marshall JC, Friedrich JO. Relationship of catheter-associated urinary tract infection to mortality and length of stay in critically ill patients: a systematic review and meta-analysis of observational studies. Crit Care Med. 2011;39(5):1167-1173. https://doi.org/10.1097/CCM.0b013e31820a8581.
12. Umscheid CA, Mitchell MD, Doshi JA, et al. Estimating the proportion of healthcare-associated infections that are reasonably preventable and the related mortality and costs. Infect Control Hosp Epidemiol. 2011;32(2):101-114. https://doi.org/10.1086/657912.
13. Gould C, Umscheid C, Agarwal R, et al. Healthcare Infection Control Practices Advisory Committee (HICPAC): guideline for the prevention of catheter-associated urinary tract infections, 2009. http://www.cdc.gov/hicpac/pdf/CAUTI/CAUTIguideline2009final.pdf. Accessed November 1, 2018.
14. Hooton TM, Bradley SF, Cardenas DD, et al. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 International Clinical Practice Guidelines from the Infectious Diseases Society of America. Clin Infect Dis. 2010;50(5):625-663. https://doi.org/10.1086/650482.
15. Conway LJ, Larson EL. Guidelines to prevent catheter-associated urinary tract infection: 1980 to 2010. Heart Lung. 2011;41(3):271-283. https://doi.org/10.1016/j.hrtlng.2011.08.001.
16. Greene L, Marx J, Oriola S. APIC elimination guide: guide to the elimination of catheter-associated urinary tract infections (CAUTIs). http://www.apic.org/Content/NavigationMenu/PracticeGuidance/APICEliminationGuides/CAUTI_Guide_0609.pdf. Accessed October 15, 2018.
17. Yokoe DS, Mermel LA, Anderson DJ, et al. A compendium of strategies to prevent healthcare-associated infections in acute care hospitals. Infect Control Hosp Epidemiol. 2008;29(S1):S12-S21.
18. Wiley Z, Kobaidze K, Sexton ME, Jacob JT. Hospitalists as integral stakeholders in antimicrobial stewardship. Curr Treat Options Infect Dis. 2018;10(2):240-248. https://doi.org/10.1007/s40506-018-0162-z.
19. Claeys KC, Blanco N, Morgan DJ, Leekha S, Sullivan KV. Advances and challenges in the diagnosis and treatment of urinary tract infections: the need for diagnostic stewardship. Curr Infect Dis Rep. 2019;21(4):11. https://doi.org/10.1007/s11908-019-0668-7.
20. Horan T, Andrus M, Dudeck M. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36(5):309-332. https://doi.org/10.1016/j.ajic.2008.03.002.
21. Nicolle LE, Gupta K, Bradley S, et al. Clinical practice guideline for the management of asymptomatic bacteriuria: 2019 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2019;68(10):e83-e110.
22. Donlan R. Biofilms and device-associated infections. Emerg Infect Dis. 2001;7(2):277-281. https://doi.org/10.3201/eid0702.010226.
23. Choe HS, Son SW, Choi HA, et al. Analysis of the distribution of bacteria within urinary catheter biofilms using four different molecular techniques. Am J Infect Control. 2012;40(9):e249-e254. https://doi.org/10.1016/j.ajic.2012.05.010.
24. Mohajer M, Darouiche R. Prevention and treatment of urinary catheter-associated infections. Curr Infect Dis Rep. 2013;15(2):116-123. https://doi.org/10.1007/s11908-013-0316-6.
25. Weiner LM, Webb AK, Limbago B, et al. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2011-2014. Infect Control Hosp Epidemiol. 2016;37(11):1288-1301. https://doi.org/10.1017/ice.2016.174.
26. Tambyah PA, Oon J. Catheter-associated urinary tract infection. Curr Opin Infect Dis. 2012;25(4):365-370. https://doi.org/10.1097/QCO.0b013e32835565cc.
27. Warren JW, Tenney JH, Hoopes JM, Muncie HL, Anthony WC. A prospective microbiologic study of bacteriuria in patients with chronic indwelling urethral catheters. J Infect Dis. 1982;146(6):719-723. https://doi.org/10.1093/infdis/146.6.719.
28. Lo E, Nicolle L, Coffin S, et al. Strategies to prevent catheter-associated urinary tract infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35(5):464-479. https://doi.org/10.1086/675718.
29. Nicolle LE. Urinary catheter-associated infections. Infect Dis Clin N Am. 2012;26(1):13-27. https://doi.org/10.1016/j.idc.2011.09.009.
30. Titsworth WL, Hester J, Correia T, et al. Reduction of catheter-associated urinary tract infections among patients in a neurological intensive care unit: a single institution’s success. J Neurosurg. 2012;116(4):911-920. https://doi.org/10.3171/2011.11.JNS11974.
31. Lobdell KW, Stamou S, Sanchez JA. Hospital-acquired infections. Surg Clin N Am. 2012;92(1):65-77. https://doi.org/10.1016/j.suc.2011.11.003.
32. Gray M. Reducing catheter-associated urinary tract infection in the critical care unit. AACN Adv Crit Care. 2010;21(3):247-257. 33. Fakih MG, Watson SR, Greene T, et al. Reducing inappropriate urinary catheter use. Arch Intern Med. 2012;172(3):255-260. https://doi.org/10.1001/archinternmed.2011.627.
33. Fakih MG, Watson SR, Greene T, et al. Reducing inappropriate urinary catheter use. Arch Intern Med. 2012;172(3):255-260. https://doi.org/10.1001/archinternmed.2011.627.
34. Saint S, Wiese J, Amory JK, Bernstein ML, et al. Are physicians aware of which of their patients have an indwelling urinary catheters? Am J Med. 2000;109(6):476-480. https://doi.org/10.1016/s0002-9343(00)00531-3.
35. O’Grady NP, Barie PS, Bartlett JG, et al. Guidelines for evaluation of new fever in critically ill adult patients: 2008 update from the American College of Critical Care Medicine and the Infectious Diseases Society of America. Crit Care Med. 2008;36(4):1330-1349. https://doi.org/10.1097/CCM.0b013e318169eda9.
36. Mullin K, Kovacs C, Fatica C, et al. A multifaceted approach to reduction of catheter-associated urinary tract infections in the intensive care unit with an emphasis on “stewardship of culturing.” Infect Control Hosp Epidemiol. 2017;38(2):186-188. https://doi.org/10.1017/ice.2016.266.
37. Humphries R, Bard J. Point-counterpoint: reflex cultures reduce laboratory workload and improve antimicrobial stewardship in patients suspected of having urinary tract infections. J Clin Microbiol. 2016;54(2):254-258. https://doi.org/10.1128/JCM.03021-15.
38. Voelker R. New fluoroquinolone warning. JAMA. 2016;315(23):2514. https://doi.org/10.1001/jama.2016.7290.
39. Tillotson S. FDA and the safe and appropriate antibiotic use of fluoroquinolones. Lancet Infect Dis. 2016;16(3):PE11-E12.
40. Tanne J. FDA adds “black box” warning label to fluoroquinolone antibiotics. BMJ 2008;337:a816. https://doi.org/10.1136/bmj.a816.
41. Wyndaele JJ, Brauner A, Geerlings SE, et al. Clean intermittent catheterization and urinary tract infection: review and guide for future research. BJU Intern. 2012;110(11c):E910-E917. https://doi.org/10.1111/j.1464-410X.2012.11549.x.
42. Hakwoort RA, Thijs SD, Bouwmeester FW, et al. Comparing clean intermittent catheterization and transurethral indwelling catheterization for incomplete voiding after vaginal prolapse surgery: a multicenter randomized trial. BJOG. 2011;118(9):1055-1060. https://doi.org/10.1111/j.1471-0528.2011.02935.x.
43. Hakvoort RA, Nieuwkerk PT, Burger MP, et al. Patient preferences for clean intermittent catheterization and transurethral indwelling catheterization for treatment of abnormal post-void residual bladder volume after vaginal prolapsed surgery. BJOG. 2011;118(11):1324-1328. https://doi.org/10.1111/j.1471-0528.2011.03056.x.
44. Saint S, Kaufman SR, Roger M, et al. Condom versus indwelling urinary catheters: a randomized trial. JAGS. 2006;54(7):1055-1061. https://doi.org/10.1111/j.1532-5415.2006.00785.x.
45. Pickard R, Lam T, MacLennan G, et al. Antimicrobial catheters for reduction of symptomatic urinary tract infection in adults requiring short-term catheterization in hospital: a multicenter randomized controlled trial. Lancet. 2012;380(9857):1927-1935. https://doi.org/10.1016/S0140-6736(12)61380-4.
46. Parry M, Grant B, Sestovic M. Successful reduction in catheter-associated urinary tract infections: focus on nurse-directed catheter removal. Am J Infect Control. 2013;41(12):1178-1181. https://doi.org/10.1016/j.ajic.2013.03.296.
47. Fuchs MA, Sexton DJ, Thornlow DK, Champagne MT. Evaluation of an evidence-based, nurse-driven checklist to prevent hospital-acquired catheter-associated urinary tract infections in intensive care units. J Nurs Care Qual. 2011;26(2):101-109. https://doi.org/10.1097/NCQ.0b013e3181fb7847.
48. Meddings J, Rogers MA, Macy M, Saint S. Systematic review and meta-analysis: reminder systems to reduce catheter-associated urinary tract infections and urinary catheter use in hospitalized patients. Clin Infect Dis. 2010;51(5):550-560. https://doi.org/10.1086/655133.
49. Baggs J, Fridkin SK, Pollack LA, Srinivasan A, Jernigan JA. Estimating national trends in inpatient antibiotic use among US hospitals from 2006 to 2012. JAMA Intern Med. 2016;176(11):1639-1648. https://doi.org/10.1001/jamainternmed.2016.5651.
50. National Action Plan for Combating Antibiotic-resistant Bacteria. 2015. https://obamawhitehouse.archives.gov/sites/default/files/docs/national_action_plan_for_combatin
© 2019 Society of Hospital Medicine
Hemorrhoids: A range of treatments
Aspects of modern life that may promote hemorrhoids include increased consumption of processed foods, a sedentary lifestyle, and using cell phones while defecating, which translates to much more time spent on the toilet.
Hemorrhoids accounted for more than 3.5 million US outpatient visits in 2010, and they were the third leading cause of hospital admissions related to gastrointestinal disease.1
Here, we review the process for diagnosing and grading hemorrhoids, as well as for selecting the appropriate medical or surgical treatment based on the most recent clinical evidence.
DIAGNOSING AND CLASSIFYING HEMORRHOIDS
Hemorrhoids are the distal prolapse of the arteriovenous bundle, muscle fibers, and surrounding connective tissue as an envelope below the dentate line in the anal canal. They usually present with painless rectal bleeding.2
The diagnosis of hemorrhoids relies on the history and physical examination rather than on laboratory testing or imaging studies. Typically, the presenting symptom is painless rectal bleeding associated with bowel movements, usually appearing as bright red blood on the toilet paper or coating the stool. Severe itching and anal discomfort are also common, especially with chronic hemorrhoids.
Detailed patient history
A detailed patient history is important. It should include the extent, severity, and duration of symptoms, frequency of bowel movements, associated symptoms (eg, constipation, fecal incontinence), daily dietary habits, and details of bowel movements (eg, time spent during each bowel movement and concomitant cell phone use).3
Regarding bowel habits, some patients experience lifelong constipation or diarrhea. Therefore, what a patient considers a normal bowel habit may not be normal and should be investigated.4 Also, it is important to exclude external thrombosed hemorrhoids, anal fissure, anal abscess, and Crohn disease.5
Physical examination
A digital rectal examination is the second step. During the examination, look for skin tags, sphincter tone, perianal hygiene, and synchronous anal lesions.3 Of note, the Valsalva maneuver can be performed during the digital rectal examination.
Red flags for colorectal cancer on the digital rectal examination include a mass with or without presence of hemorrhoidal sacs and a bleeding source above the level of internal hemorrhoids.
Endoscopy
Since rectal bleeding can be a sign of several diseases, including colorectal cancer, it is important to review any previous endoscopic results. Patients at high risk of colon cancer should undergo rigid proctoscopy, flexible sigmoidoscopy, or colonoscopy.3,4 In our practice, we recommend endoscopic evaluation for patients over age 40 with rectal bleeding, especially if they have a family history of colorectal cancer.
External or internal (grades I–IV)
Hemorrhoids can be categorized as either external or internal.
External hemorrhoids are distinguished by their outer covering with perianal skin and anoderm and their location inferior to the dentate line. They are painful if the hemorrhoidal sac is occluded by a thrombotic clot.
Internal hemorrhoids are above the dentate line and covered with rectal columnar and transitional mucosa. They are further graded on a 4-point scale3:
- Grade I—Visible hemorrhoids that do not prolapse
- Grade II—Hemorrhoids that prolapse during the Valsalva maneuver but spontaneously reduce
- Grade III—Hemorrhoids that prolapse during the Valsalva maneuver and need manual reduction
- Grade IV—Nonreducible hemorrhoids.
A RANGE OF TREATMENTS
In choosing the treatment for hemorrhoids, one should consider the disease grade and severity, its impact on the quality of life, the degree of pain it causes, the patient’s likelihood of adhering to treatment, and the patient’s personal preference.
Regardless of severity, treatment almost always starts with a high-fiber diet and other lifestyle modifications that include bowel movement behaviors. This requires practitioners to spend significant time on patient education regardless of the type or the severity of the disease.
Treatments can be grouped in 3 categories: conservative, office-based, and surgical. Practitioners should thoroughly discuss the options with the patient, emphasizing the pros and cons of each.
CONSERVATIVE MEASURES
Conservative measures are aimed at softening the stool, relieving pain, and correcting bad toileting habits. In most cases, the primary precipitating factor is lifestyle, and unless patients change it, they are more likely to have recurrent symptoms in the long term.
No phone in the bathroom
People take their phones into the bathroom, and this habit is blamed for increasing the time on the toilet and leading to increased pressure on the anal region and straining during defecation. Some research points to a direct correlation between the time spent on the toilet and hemorrhoidal disease, although the exact cause-and-effect relationship with cell phone use has not been determined. In general, spending excessive time on the commode, including reading, should be discouraged.
Less time in the bathroom
Johannsson et al6 reported that patients with hemorrhoids spent more time on the toilet and had to strain harder and more often than controls in the community and hospital.
Garg and Singh7 and Garg8 use the mnemonic “TONE” for appropriate defecation habits:
- Three minutes during defecation
- Once-daily defecation
- No straining and no cell phone use during defecation
- Enough fiber.
More fiber
Fiber draws water into the lumen of the colon, increasing the water content of the stool. Recommended daily fiber intake is about 28 g for women and 38 g for men.9 This high level of intake is hard to achieve without supplements for someone who consumes a classic American diet with a lot of fast food.
Fiber supplements are strongly recommended in the American Society of Colon and Rectal Surgeons (ASCRS) practice guidelines3 based on a Cochrane review.10 In this meta-analysis, with fiber supplements, the relative risk of persisting or nonimproving symptoms was 0.53 (95% confidence interval [CI] 0.38–0.73) and the relative risk of bleeding was 0.50 (95% CI 028–0.89). Psyllium husk is an inexpensive bulk-forming fiber supplement; the optimal daily dosage is not known.
We recommend at least 28 g of daily fiber intake for women and 38 g for men, for which psyllium husk can be used to complement the diet.
Laxatives for some
Laxatives such as docusate are used to change the stool consistency when there is an organic bowel problem rather than a dietary issue. They can be used as a complementary treatment to enhance the effect of the fiber treatment.
Other measures
Topical anesthesia (eg, 5% lidocaine) is commonly used to treat pain from low-grade lesions, but no reliable data have been published. As most cases of hemorrhoids tend to progress over time, one should not expect long-term improvement with topical anesthesia. Nevertheless, it can be used as an ancillary treatment in select cases when short-term improvement is the main goal, and we recommend it based on our own experience.
Hygiene. Bidet use or cleaning the perianal area with water is recommended.
Phlebotonics contain a variety of ingredients including natural plant extracts such as flavonoids and synthetic products. Even though the exact mechanism of action is not known, phlebotonics are thought to increase venous and lymphatic drainage, normalize capillary permeability, and decrease inflammation in the hemorrhoidal cushions.4,11–13
In a Cochrane review of 24 randomized controlled trials, Perera et al14 found that phlebotonics improved the outcomes of:
- Bleeding (odds ratio [OR] 0.21, number needed to treat [NNT] 4.8, P = .0002)
- Pruritus (OR 0.23, NNT 9.1, P = .02)
- Discharge or leakage (OR 0.12, NNT 5, P = .0008)
- Overall symptoms (OR 15.99, NNT 2.7, P < .00001). Overall symptoms were also improved in the subgroup of pregnant patients.
Although phlebotonics give better results than placebo in the short-term management of hemorrhoids, there is a paucity of long-term data. Thus, the ASCRS clinical practice guidelines gives the regular use of these agents only a weak recommendation.3
Flavonoids (diosmin, hesperidin, rutoside), in a meta-analysis vs placebo in 1,514 patients, showed a beneficial response in terms of bleeding (relative risk [RR] 0.33), pruritus (relative risk [RR] 0.65), and recurrences (RR 0.53).15
Although Preparation H is commonly used as an over-the-counter medication, there are no good data on it, and it is not considered a phlebotonic.
OFFICE-BASED TREATMENTS
Office-based treatments—rubber band ligation, infrared photocoagulation, and sclerotherapy—are commonly used for grade I, II, and III hemorrhoids that have not responded to conservative management. The primary goal of these treatments is to decrease blood flow into the hemorrhoidal sac.
Even though office-based treatments are highly effective and major complications are uncommon, recurrence rates can be high, requiring patients to undergo additional treatments. Moreover, septic complications can occur, so patients should be closely observed for fever and urinary problems. Pain is a common symptom after office-based treatments, and bleeding may also occur.
The ASCRS guidelines strongly recommend office-based treatments for patients with grade I and II hemorrhoids, and for some with grade III hemorrhoids.3
Rubber band ligation
Iyer et al18 reported that patients on warfarin therapy had up to a 9 times higher risk of postprocedural bleeding, and patients on aspirin had a risk up to 3 times higher. Therefore, whether patients on anticoagulant therapy should undergo this procedure is unclear.
A Cochrane database review19 found this technique effective for hemorrhoid grades I through III, although some patients with grade III hemorrhoids may benefit more from excisional hemorrhoidectomy, which is associated with a lower recurrence rate than rubber band ligation.
Brown et al20 performed a randomized controlled trial comparing hemorrhoidal artery ligation and rubber band ligation for symptomatic hemorrhoids in 372 patients with grade II and III hemorrhoids. Postprocedural pain scores on days 1 and 7 were significantly lower with rubber band ligation, but recurrences were more common (49% vs 30%, P = .0005, respectively).
Overall, rubber band ligation is an excellent option for grade II hemorrhoids, as it is easy to perform, is associated with low pain scores, and can be used to treat recurrences.
Infrared photocoagulation
In this procedure, an infrared probe produces heat to induce coagulation, fibrosis, and ultimately necrosis of the protruding tissue in the hemorrhoidal cushions.21 Even though its use was initially directed at grade I and II hemorrhoids, recent reports showed acceptable results for grades III and IV.22,23 A randomized controlled trial comparing infrared photocoagulation and rubber band ligation in 94 patients found that both procedures were well accepted and highly effective; however, patients had better pain scores with photocoagulation in the first 24 hours after the procedure (P < .05).24
Sclerotherapy
The injection can cause prostatic abscess and sepsis, although this is rare.25 Nevertheless, high fever and postprocedural pain should be carefully evaluated.
There have been few randomized trials of sclerotherapy, but success rates so far have been higher for grade I hemorrhoids than for grades II and III.26–28 It is the preferred method for patients who have bleeding abnormalities caused by medications or other diseases (eg, cirrhosis).
SURGERY
Although nonsurgical treatments have substantially improved, surgery is the most effective and strongly recommended treatment for patients with high-grade internal hemorrhoids (grades III and IV), external and mixed hemorrhoids, and recurrent hemorrhoids.
The most popular surgical options are open or closed hemorrhoidectomy, stapled hemorrhoidopexy, and Doppler-guided hemorrhoidal artery ligation. Each has different success rates and different complication profiles, which need to be discussed with the patient.
Overall, surgery is associated with more adverse effects than office-based treatments or medical management. Postoperative pain is the most common complaint, but anal stricture (rare) or incontinence may occur due to excessive tissue excision and damage to the sphincter muscles. These can be avoided by maintaining the normal anoderm between excisions, by not excising all hemorrhoid sacs at once if the patient has extensive lesions, and by performing a careful dissection in the submucosal plane.
Patients with profuse bleeding or an underlying bleeding abnormality are best managed with surgical approaches performed in an operating room.
Excisional surgical hemorrhoidectomy
Excision of the hemorrhoidal sac, the most conventional surgical technique, is generally reserved for prolapsing disease. The recurrence rate after excisional hemorrhoidectomy is significantly lower than with any other approach.29
Excisional hemorrhoidectomy can be performed using either an open approach, in which the edges of the mucosal defect are not reapproximated, or a closed approach, in which they are. In a systematic review, Bhatti et al30 compared open vs closed techniques and found that the closed technique resulted in less postoperative pain, better wound healing, and less bleeding. Rates of recurrence, postoperative complications, and surgical site infection and lengths of stay were comparable with either procedure.
Overall, excisional hemorrhoidectomy is associated with higher pain scores than any other surgical method.29 Recently, the use of electrodiathermy energy devices, also described as electrosurgical vessel-sealing devices, have further improved overall patient satisfaction.31
Multiple painful hemorrhoidal sacs require a careful surgical approach, as extensive resection may cause widespread fibrosis and stricture. As with anal stricture, fecal incontinence can be prevented by careful dissection. However, already existing incontinence is not a contraindication for the surgery.
Doppler-guided hemorrhoidal artery ligation
Doppler-guided hemorrhoidal artery ligation involves using a Doppler probe to find and ligate individual hemorrhoidal arteries. Additionally, mucopexy (transanal rectoanal repair) is performed to relocate the prolapsing tissue. Avital et al32 reported that at 1 year after this procedure, recurrence rates were 5.3% for grade II hemorrhoids and 13% for grade III hemorrhoids. At 5 years, recurrence rates were 12% for grade II and 31% for grade III.
To date, this procedure appears to be suitable for grade I, II, and III hemorrhoids, especially for grade II, but more studies are needed to prove its efficacy and recurrence rates for more advanced lesions. Although this technique has a high morbidity rate (18%), primarily pain or tenesmus, it causes less postoperative pain than other surgical methods.33 Overall, it has the potential to become a favored treatment.
Stapled hemorrhoidopexy
Although pain scores are lower with stapled hemorrhoidopexy than with excisional hemorrhoidectomy, this procedure is not superior in terms of recurrences.34,35 Also, practitioners should be careful about specific complications of stapled hemorrhoidopexy, such as rectovaginal fistula, anal stenosis, or sphincter injuries. These specific complications should be clearly explained to patients, and necessary information should be given to patients upon discharge. The primary care physician should also be careful about fistulas and stenoses in this particular patient population.
NO ‘BEST’ TREATMENT
There is no best treatment for hemorrhoids. Every patient is different, and the physician and patient need to understand each other’s expectations, weigh the risks and benefits, and arrive at a mutual decision. A good patient-doctor relationship is essential.
Given the variety of available treatments, head-to-head comparisons are difficult. Moreover, the efficacy and applicability of each technique changes with the grade of the lesion or lesions and the skill of the practitioner. Lacking comprehensive studies comparing conservative, office-based, and surgical management, no decisive statements can be made based on current evidence.
Patients with compounding conditions
Pregnant patients often develop hemorrhoids as intra-abdominal pressure increases, particularly during the third trimester.36 Also, acute episodes of pain and bleeding are common in pregnant women with preexisting hemorrhoids.
Conservative treatment is the main approach in pregnant patients because most hemorrhoids regress after childbirth. This includes increased dietary fiber, stool softeners, and sitz baths, which are safe to use for external hemorrhoids. Any office-based or surgical intervention should be postponed until after childbirth, if possible. Kegel exercises and lying on the left side are also recommended to relieve symptoms. In cases of severe bleeding, anal packing appears to be useful.
Immunosuppressed patients and those on anticoagulant therapy are more prone to serious complications such as sepsis and profuse bleeding. Thus, conservative management should be used in these patients as well. Injection sclerotherapy may be beneficial, as it has been shown to decrease bleeding. Of note, patients on immunosuppressive agents should stop taking them and start taking an antibiotic, and patients on anticoagulant or antiplatelet medications should be instructed to stop them 1 week before any intervention.
Crohn disease. Some patients with Crohn disease may have hemorrhoids, though this is rare. Eglinton et al,37 in a series of 715 patients with Crohn disease, reported that 190 (26.6%) had symptomatic perianal disease. Of these, only 3 (1.6%) had hemorrhoids. Treatment is always conservative and directed at the Crohn disease rather than the hemorrhoids.
Patients with portal hypertension (eg, due to cirrhosis) are prone to have anorectal varices that may resemble hemorrhoids. Anorectal varices can be treated with vascular ligation, whereas sclerotherapy is the preferred method for hemorrhoids in this group, in whom coagulopathy is common.
TAKE-HOME MESSAGES
Hemorrhoidal disease is common in the United States, and with our diet and lifestyle, the incidence is likely to increase. (A national survey found that overall dietary quality improved modestly in children and adolescents in the United States from 1999 to 2012 but remained far below optimal.38) Practitioners need to carefully assess hemorrhoidal symptoms and complete any necessary screening tests before establishing a diagnosis. This helps to avoid missing any underlying disease.
Fiber supplements along with dietary and lifestyle changes constitute the baseline of the management regardless of the disease grade. Office-based interventions are beneficial for grade I and II hemorrhoids and for some grade III hemorrhoids. Repeated interventions can increase the success rate. In patients with high-grade, symptomatic hemorrhoids, surgical hemorrhoidectomy is the most effective modality with the lowest recurrence rates, although it causes more pain than conservative methods.
- Peery AF, Crockett SD, Barritt AS, et al. Burden of gastrointestinal, liver, and pancreatic diseases in the United States. Gastroenterology 2015; 149(7):1731–1741.e3. doi:10.1053/j.gastro.2015.08.045
- Thomson WH. The nature and cause of haemorrhoids. Proc R Soc Med 1975; 68(9):574–575. pmid:1197343
- Davis BR, Lee-Kong SA, Migaly J, Feingold DL, Steele SR. The American Society of Colon and Rectal Surgeons clinical practice guidelines for the management of hemorrhoids. Dis Colon Rectum 2018; 61(3):284–292. doi:10.1097/DCR.0000000000001030
- Lohsiriwat V. Treatment of hemorrhoids: a coloproctologist’s view. World J Gastroenterol 2015; 21(31):9245–9252. doi:10.3748/wjg.v21.i31.9245
- Wolf AMD, Fontham ETH, Church TR, et al. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J Clin 2018; 68(4):250–281. doi:10.3322/caac.21457
- Johannsson HO, Graf W, Påhlman L. Bowel habits in hemorrhoid patients and normal subjects. Am J Gastroenterol 2005; 100(2):401–406. doi:10.1111/j.1572-0241.2005.40195.x
- Garg P, Singh P. Adequate dietary fiber supplement and TONE can help avoid surgery in most patients with advanced hemorrhoids. Minerva Gastroenterol Dietol 2017; 63(2):92–96. doi:10.23736/S1121-421X.17.02364-9
- Garg P. Conservative treatment of hemorrhoids deserves more attention in guidelines and clinical practice [letter]. Dis Colon Rectum 2018; 61(7):e348. doi:10.1097/DCR.0000000000001127
- Rakinic J, Poola VP. Hemorrhoids and fistulas: new solutions to old problems. Curr Probl Surg 2014; 51(3):98–137. doi:10.1067/j.cpsurg.2013.11.002
- Alonso-Coello P, Guyatt G, Heels-Ansdell D, et al. Laxatives for the treatment of hemorrhoids. Cochrane Database Syst Rev 2005; (4):CD004649. doi:10.1002/14651858.CD004649.pub2
- Struckmann JR. Clinical efficacy of micronized purified flavonoid fraction: an overview. J Vasc Res 1999; 36(suppl 1):37–41. doi:10.1159/000054072
- Shoab SS, Porter J, Scurr JH, Coleridge-Smith PD. Endothelial activation response to oral micronised flavonoid therapy in patients with chronic venous disease—a prospective study. Eur J Vasc Endovasc Surg 1999; 17(4):313–318. doi:10.1053/ejvs.1998.0751
- Meyer OC. Safety and security of Daflon 500 mg in venous insufficiency and in hemorrhoidal disease. Angiology 1994; 45(6 pt 2):579–584. pmid:8203791
- Perera N, Liolitsa D, Iype S, et al. Phlebotonics for haemorrhoids. Cochrane Database Syst Rev 2012;(8):CD004322. doi:10.1002/14651858.CD004322.pub3
- Alonso-Coello P, Zhou Q, Martinez-Zapata MJ, et al. Meta-analysis of flavonoids for the treatment of haemorrhoids. Br J Surg 2006; 93(8):909–920. doi:10.1002/bjs.5378
- Lee HH, Spencer RJ, Beart RW Jr. Multiple hemorrhoidal bandings in a single session. Dis Colon Rectum 1994; 37(1):37–41. pmid:8287745
- Law WL, Chu KW. Triple rubber band ligation for hemorrhoids: prospective, randomized trial of use of local anesthetic injection. Dis Colon Rectum 1999; 42(3):363–366. pmid:10223757
- Iyer VS, Shrier I, Gordon PH. Long-term outcome of rubber band ligation for symptomatic primary and recurrent internal hemorrhoids. Dis Colon Rectum 2004; 47(8):1364–1370. pmid:15484351
- Shanmugam V, Thaha MA, Rabindranath KS, Campbell KL, Steele RJ, Loudon MA. Rubber band ligation versus excisional haemorrhoidectomy for haemorrhoids. Cochrane Database Syst Rev 2005; (3):CD005034. doi:10.1002/14651858.CD005034.pub2
- Brown SR, Tiernan JP, Watson AJM, et al; HubBLe Study team. Haemorrhoidal artery ligation versus rubber band ligation for the management of symptomatic second-degree and third-degree haemorrhoids (HubBLe): a multicentre, open-label, randomised controlled trial. Lancet 2016; 388(10042):356–364. doi:10.1016/S0140-6736(16)30584-0
- ASGE Technology Committee; Siddiqui UD, Barth BA, Banerjee S, et al. Devices for the endoscopic treatment of hemorrhoids. Gastrointest Endosc 2014; 79(1):8–14. doi:10.1016/j.gie.2013.07.021
- Ahmad A, Kant R, Gupta A. Comparative analysis of Doppler guided hemorrhoidal artery ligation (DG-HAL) & infrared coagulation (IRC) in management of hemorrhoids. Indian J Surg 2013; 75(4):274–277. doi:10.1007/s12262-012-0444-5
- Poen AC, Felt-Bersma RJ, Cuesta MA, Devillé W, Meuwissen SG. A randomized controlled trial of rubber band ligation versus infra-red coagulation in the treatment of internal haemorrhoids. Eur J Gastroenterol Hepatol 2000; 12(5):535–539. pmid:10833097
- Marques CF, Nahas SC, Nahas CS, Sobrado CW Jr, Habr-Gama A, Kiss DR. Early results of the treatment of internal hemorrhoid disease by infrared coagulation and elastic banding: a prospective randomized cross-over trial. Tech Coloproctol 2006; 10(4):312–317. doi:10.1007/s10151-006-0299-5
- Madoff RD, Fleshman JW; Clinical Practice Committee, American Gastroenterological Association. American Gastroenterological Association technical review on the diagnosis and treatment of hemorrhoids. Gastroenterology 2004; 126(5):1463–1473. pmid:15131807
- Yano T, Yano K. Comparison of injection sclerotherapy between 5% phenol in almond oil and aluminum potassium sulfate and tannic acid for grade 3 hemorrhoids. Ann Coloproctol 2015; 31(3):103–105. doi:10.3393/ac.2015.31.3.103
- Kanellos I, Goulimaris I, Vakalis I, Dadoukis I. Long-term evaluation of sclerotherapy for haemorrhoids. A prospective study. Int J Surg Investig 2000; 2(4):295–298. pmid:12678531
- Moser KH, Mosch C, Walgenbach M, et al. Efficacy and safety of sclerotherapy with polidocanol foam in comparison with fluid sclerosant in the treatment of first-grade haemorrhoidal disease: a randomised, controlled, single-blind, multicentre trial. Int J Colorectal Dis 2013; 28(10):1439–1447. doi:10.1007/s00384-013-1729-2
- MacRae HM, McLeod RS. Comparison of hemorrhoidal treatments: a meta-analysis. Can J Surg 1997; 40(1):14–7. pmid:9030078
- Bhatti MI, Sajid MS, Baig MK. Milligan-Morgan (open) versus Ferguson haemorrhoidectomy (closed): a systematic review and meta-analysis of published randomized, controlled trials. World J Surg 2016; 40(6):1509–1519. doi:10.1007/s00268-016-3419-z
- Nienhuijs S, de Hingh I. Conventional versus LigaSure hemorrhoidectomy for patients with symptomatic hemorrhoids. Cochrane Database Syst Rev 2009; (1):CD006761. doi:10.1002/14651858.CD006761.pub2
- Avital S, Inbar R, Karin E, Greenberg R. Five-year follow-up of Doppler-guided hemorrhoidal artery ligation. Tech Coloproctol 2012; 16(1):61–65. doi:10.1007/s10151-011-0801-6
- Ratto C, Parello A, Veronese E, et al. Doppler-guided transanal haemorrhoidal dearterialization for haemorrhoids: results from a multicentre trial. Colorectal Dis 2015; 17(1):010–019. doi:10.1111/codi.12779
- Senagore AJ, Singer M, Abcarian H, et al; Procedure for Prolapse and Hemmorrhoids (PPH) Multicenter Study Group. A prospective, randomized, controlled multicenter trial comparing stapled hemorrhoidopexy and Ferguson hemorrhoidectomy: perioperative and one-year results. Dis Colon Rectum 2004; 47(11):1824–1836. pmid:15622574
- Jayaraman S, Colquhoun PH, Malthaner RA. Stapled versus conventional surgery for hemorrhoids. Cochrane Database Syst Rev 2006; (4):CD005393.
- Poskus T, Buzinskiene D, Drasutiene G, et al. Haemorrhoids and anal fissures during pregnancy and after childbirth: a prospective cohort study. BJOG 2014; 121(13):1666–1671. doi:10.1111/1471-0528.12838
- Eglinton TW, Barclay ML, Gearry RB, Frizelle FA. The spectrum of perianal Crohn’s disease in a population-based cohort. Dis Colon Rectum 2012; 55(7):773–777. doi:10.1097/DCR.0b013e31825228b0
- Gu X, Tucker KL. Dietary quality of the US child and adolescent population: trends from 1999 to 2012 and associations with the use of federal nutrition assistance programs. Am J Clin Nutr 2017; 105(1):194–202. doi:10.3945/ajcn.116.135095
Aspects of modern life that may promote hemorrhoids include increased consumption of processed foods, a sedentary lifestyle, and using cell phones while defecating, which translates to much more time spent on the toilet.
Hemorrhoids accounted for more than 3.5 million US outpatient visits in 2010, and they were the third leading cause of hospital admissions related to gastrointestinal disease.1
Here, we review the process for diagnosing and grading hemorrhoids, as well as for selecting the appropriate medical or surgical treatment based on the most recent clinical evidence.
DIAGNOSING AND CLASSIFYING HEMORRHOIDS
Hemorrhoids are the distal prolapse of the arteriovenous bundle, muscle fibers, and surrounding connective tissue as an envelope below the dentate line in the anal canal. They usually present with painless rectal bleeding.2
The diagnosis of hemorrhoids relies on the history and physical examination rather than on laboratory testing or imaging studies. Typically, the presenting symptom is painless rectal bleeding associated with bowel movements, usually appearing as bright red blood on the toilet paper or coating the stool. Severe itching and anal discomfort are also common, especially with chronic hemorrhoids.
Detailed patient history
A detailed patient history is important. It should include the extent, severity, and duration of symptoms, frequency of bowel movements, associated symptoms (eg, constipation, fecal incontinence), daily dietary habits, and details of bowel movements (eg, time spent during each bowel movement and concomitant cell phone use).3
Regarding bowel habits, some patients experience lifelong constipation or diarrhea. Therefore, what a patient considers a normal bowel habit may not be normal and should be investigated.4 Also, it is important to exclude external thrombosed hemorrhoids, anal fissure, anal abscess, and Crohn disease.5
Physical examination
A digital rectal examination is the second step. During the examination, look for skin tags, sphincter tone, perianal hygiene, and synchronous anal lesions.3 Of note, the Valsalva maneuver can be performed during the digital rectal examination.
Red flags for colorectal cancer on the digital rectal examination include a mass with or without presence of hemorrhoidal sacs and a bleeding source above the level of internal hemorrhoids.
Endoscopy
Since rectal bleeding can be a sign of several diseases, including colorectal cancer, it is important to review any previous endoscopic results. Patients at high risk of colon cancer should undergo rigid proctoscopy, flexible sigmoidoscopy, or colonoscopy.3,4 In our practice, we recommend endoscopic evaluation for patients over age 40 with rectal bleeding, especially if they have a family history of colorectal cancer.
External or internal (grades I–IV)
Hemorrhoids can be categorized as either external or internal.
External hemorrhoids are distinguished by their outer covering with perianal skin and anoderm and their location inferior to the dentate line. They are painful if the hemorrhoidal sac is occluded by a thrombotic clot.
Internal hemorrhoids are above the dentate line and covered with rectal columnar and transitional mucosa. They are further graded on a 4-point scale3:
- Grade I—Visible hemorrhoids that do not prolapse
- Grade II—Hemorrhoids that prolapse during the Valsalva maneuver but spontaneously reduce
- Grade III—Hemorrhoids that prolapse during the Valsalva maneuver and need manual reduction
- Grade IV—Nonreducible hemorrhoids.
A RANGE OF TREATMENTS
In choosing the treatment for hemorrhoids, one should consider the disease grade and severity, its impact on the quality of life, the degree of pain it causes, the patient’s likelihood of adhering to treatment, and the patient’s personal preference.
Regardless of severity, treatment almost always starts with a high-fiber diet and other lifestyle modifications that include bowel movement behaviors. This requires practitioners to spend significant time on patient education regardless of the type or the severity of the disease.
Treatments can be grouped in 3 categories: conservative, office-based, and surgical. Practitioners should thoroughly discuss the options with the patient, emphasizing the pros and cons of each.
CONSERVATIVE MEASURES
Conservative measures are aimed at softening the stool, relieving pain, and correcting bad toileting habits. In most cases, the primary precipitating factor is lifestyle, and unless patients change it, they are more likely to have recurrent symptoms in the long term.
No phone in the bathroom
People take their phones into the bathroom, and this habit is blamed for increasing the time on the toilet and leading to increased pressure on the anal region and straining during defecation. Some research points to a direct correlation between the time spent on the toilet and hemorrhoidal disease, although the exact cause-and-effect relationship with cell phone use has not been determined. In general, spending excessive time on the commode, including reading, should be discouraged.
Less time in the bathroom
Johannsson et al6 reported that patients with hemorrhoids spent more time on the toilet and had to strain harder and more often than controls in the community and hospital.
Garg and Singh7 and Garg8 use the mnemonic “TONE” for appropriate defecation habits:
- Three minutes during defecation
- Once-daily defecation
- No straining and no cell phone use during defecation
- Enough fiber.
More fiber
Fiber draws water into the lumen of the colon, increasing the water content of the stool. Recommended daily fiber intake is about 28 g for women and 38 g for men.9 This high level of intake is hard to achieve without supplements for someone who consumes a classic American diet with a lot of fast food.
Fiber supplements are strongly recommended in the American Society of Colon and Rectal Surgeons (ASCRS) practice guidelines3 based on a Cochrane review.10 In this meta-analysis, with fiber supplements, the relative risk of persisting or nonimproving symptoms was 0.53 (95% confidence interval [CI] 0.38–0.73) and the relative risk of bleeding was 0.50 (95% CI 028–0.89). Psyllium husk is an inexpensive bulk-forming fiber supplement; the optimal daily dosage is not known.
We recommend at least 28 g of daily fiber intake for women and 38 g for men, for which psyllium husk can be used to complement the diet.
Laxatives for some
Laxatives such as docusate are used to change the stool consistency when there is an organic bowel problem rather than a dietary issue. They can be used as a complementary treatment to enhance the effect of the fiber treatment.
Other measures
Topical anesthesia (eg, 5% lidocaine) is commonly used to treat pain from low-grade lesions, but no reliable data have been published. As most cases of hemorrhoids tend to progress over time, one should not expect long-term improvement with topical anesthesia. Nevertheless, it can be used as an ancillary treatment in select cases when short-term improvement is the main goal, and we recommend it based on our own experience.
Hygiene. Bidet use or cleaning the perianal area with water is recommended.
Phlebotonics contain a variety of ingredients including natural plant extracts such as flavonoids and synthetic products. Even though the exact mechanism of action is not known, phlebotonics are thought to increase venous and lymphatic drainage, normalize capillary permeability, and decrease inflammation in the hemorrhoidal cushions.4,11–13
In a Cochrane review of 24 randomized controlled trials, Perera et al14 found that phlebotonics improved the outcomes of:
- Bleeding (odds ratio [OR] 0.21, number needed to treat [NNT] 4.8, P = .0002)
- Pruritus (OR 0.23, NNT 9.1, P = .02)
- Discharge or leakage (OR 0.12, NNT 5, P = .0008)
- Overall symptoms (OR 15.99, NNT 2.7, P < .00001). Overall symptoms were also improved in the subgroup of pregnant patients.
Although phlebotonics give better results than placebo in the short-term management of hemorrhoids, there is a paucity of long-term data. Thus, the ASCRS clinical practice guidelines gives the regular use of these agents only a weak recommendation.3
Flavonoids (diosmin, hesperidin, rutoside), in a meta-analysis vs placebo in 1,514 patients, showed a beneficial response in terms of bleeding (relative risk [RR] 0.33), pruritus (relative risk [RR] 0.65), and recurrences (RR 0.53).15
Although Preparation H is commonly used as an over-the-counter medication, there are no good data on it, and it is not considered a phlebotonic.
OFFICE-BASED TREATMENTS
Office-based treatments—rubber band ligation, infrared photocoagulation, and sclerotherapy—are commonly used for grade I, II, and III hemorrhoids that have not responded to conservative management. The primary goal of these treatments is to decrease blood flow into the hemorrhoidal sac.
Even though office-based treatments are highly effective and major complications are uncommon, recurrence rates can be high, requiring patients to undergo additional treatments. Moreover, septic complications can occur, so patients should be closely observed for fever and urinary problems. Pain is a common symptom after office-based treatments, and bleeding may also occur.
The ASCRS guidelines strongly recommend office-based treatments for patients with grade I and II hemorrhoids, and for some with grade III hemorrhoids.3
Rubber band ligation
Iyer et al18 reported that patients on warfarin therapy had up to a 9 times higher risk of postprocedural bleeding, and patients on aspirin had a risk up to 3 times higher. Therefore, whether patients on anticoagulant therapy should undergo this procedure is unclear.
A Cochrane database review19 found this technique effective for hemorrhoid grades I through III, although some patients with grade III hemorrhoids may benefit more from excisional hemorrhoidectomy, which is associated with a lower recurrence rate than rubber band ligation.
Brown et al20 performed a randomized controlled trial comparing hemorrhoidal artery ligation and rubber band ligation for symptomatic hemorrhoids in 372 patients with grade II and III hemorrhoids. Postprocedural pain scores on days 1 and 7 were significantly lower with rubber band ligation, but recurrences were more common (49% vs 30%, P = .0005, respectively).
Overall, rubber band ligation is an excellent option for grade II hemorrhoids, as it is easy to perform, is associated with low pain scores, and can be used to treat recurrences.
Infrared photocoagulation
In this procedure, an infrared probe produces heat to induce coagulation, fibrosis, and ultimately necrosis of the protruding tissue in the hemorrhoidal cushions.21 Even though its use was initially directed at grade I and II hemorrhoids, recent reports showed acceptable results for grades III and IV.22,23 A randomized controlled trial comparing infrared photocoagulation and rubber band ligation in 94 patients found that both procedures were well accepted and highly effective; however, patients had better pain scores with photocoagulation in the first 24 hours after the procedure (P < .05).24
Sclerotherapy
The injection can cause prostatic abscess and sepsis, although this is rare.25 Nevertheless, high fever and postprocedural pain should be carefully evaluated.
There have been few randomized trials of sclerotherapy, but success rates so far have been higher for grade I hemorrhoids than for grades II and III.26–28 It is the preferred method for patients who have bleeding abnormalities caused by medications or other diseases (eg, cirrhosis).
SURGERY
Although nonsurgical treatments have substantially improved, surgery is the most effective and strongly recommended treatment for patients with high-grade internal hemorrhoids (grades III and IV), external and mixed hemorrhoids, and recurrent hemorrhoids.
The most popular surgical options are open or closed hemorrhoidectomy, stapled hemorrhoidopexy, and Doppler-guided hemorrhoidal artery ligation. Each has different success rates and different complication profiles, which need to be discussed with the patient.
Overall, surgery is associated with more adverse effects than office-based treatments or medical management. Postoperative pain is the most common complaint, but anal stricture (rare) or incontinence may occur due to excessive tissue excision and damage to the sphincter muscles. These can be avoided by maintaining the normal anoderm between excisions, by not excising all hemorrhoid sacs at once if the patient has extensive lesions, and by performing a careful dissection in the submucosal plane.
Patients with profuse bleeding or an underlying bleeding abnormality are best managed with surgical approaches performed in an operating room.
Excisional surgical hemorrhoidectomy
Excision of the hemorrhoidal sac, the most conventional surgical technique, is generally reserved for prolapsing disease. The recurrence rate after excisional hemorrhoidectomy is significantly lower than with any other approach.29
Excisional hemorrhoidectomy can be performed using either an open approach, in which the edges of the mucosal defect are not reapproximated, or a closed approach, in which they are. In a systematic review, Bhatti et al30 compared open vs closed techniques and found that the closed technique resulted in less postoperative pain, better wound healing, and less bleeding. Rates of recurrence, postoperative complications, and surgical site infection and lengths of stay were comparable with either procedure.
Overall, excisional hemorrhoidectomy is associated with higher pain scores than any other surgical method.29 Recently, the use of electrodiathermy energy devices, also described as electrosurgical vessel-sealing devices, have further improved overall patient satisfaction.31
Multiple painful hemorrhoidal sacs require a careful surgical approach, as extensive resection may cause widespread fibrosis and stricture. As with anal stricture, fecal incontinence can be prevented by careful dissection. However, already existing incontinence is not a contraindication for the surgery.
Doppler-guided hemorrhoidal artery ligation
Doppler-guided hemorrhoidal artery ligation involves using a Doppler probe to find and ligate individual hemorrhoidal arteries. Additionally, mucopexy (transanal rectoanal repair) is performed to relocate the prolapsing tissue. Avital et al32 reported that at 1 year after this procedure, recurrence rates were 5.3% for grade II hemorrhoids and 13% for grade III hemorrhoids. At 5 years, recurrence rates were 12% for grade II and 31% for grade III.
To date, this procedure appears to be suitable for grade I, II, and III hemorrhoids, especially for grade II, but more studies are needed to prove its efficacy and recurrence rates for more advanced lesions. Although this technique has a high morbidity rate (18%), primarily pain or tenesmus, it causes less postoperative pain than other surgical methods.33 Overall, it has the potential to become a favored treatment.
Stapled hemorrhoidopexy
Although pain scores are lower with stapled hemorrhoidopexy than with excisional hemorrhoidectomy, this procedure is not superior in terms of recurrences.34,35 Also, practitioners should be careful about specific complications of stapled hemorrhoidopexy, such as rectovaginal fistula, anal stenosis, or sphincter injuries. These specific complications should be clearly explained to patients, and necessary information should be given to patients upon discharge. The primary care physician should also be careful about fistulas and stenoses in this particular patient population.
NO ‘BEST’ TREATMENT
There is no best treatment for hemorrhoids. Every patient is different, and the physician and patient need to understand each other’s expectations, weigh the risks and benefits, and arrive at a mutual decision. A good patient-doctor relationship is essential.
Given the variety of available treatments, head-to-head comparisons are difficult. Moreover, the efficacy and applicability of each technique changes with the grade of the lesion or lesions and the skill of the practitioner. Lacking comprehensive studies comparing conservative, office-based, and surgical management, no decisive statements can be made based on current evidence.
Patients with compounding conditions
Pregnant patients often develop hemorrhoids as intra-abdominal pressure increases, particularly during the third trimester.36 Also, acute episodes of pain and bleeding are common in pregnant women with preexisting hemorrhoids.
Conservative treatment is the main approach in pregnant patients because most hemorrhoids regress after childbirth. This includes increased dietary fiber, stool softeners, and sitz baths, which are safe to use for external hemorrhoids. Any office-based or surgical intervention should be postponed until after childbirth, if possible. Kegel exercises and lying on the left side are also recommended to relieve symptoms. In cases of severe bleeding, anal packing appears to be useful.
Immunosuppressed patients and those on anticoagulant therapy are more prone to serious complications such as sepsis and profuse bleeding. Thus, conservative management should be used in these patients as well. Injection sclerotherapy may be beneficial, as it has been shown to decrease bleeding. Of note, patients on immunosuppressive agents should stop taking them and start taking an antibiotic, and patients on anticoagulant or antiplatelet medications should be instructed to stop them 1 week before any intervention.
Crohn disease. Some patients with Crohn disease may have hemorrhoids, though this is rare. Eglinton et al,37 in a series of 715 patients with Crohn disease, reported that 190 (26.6%) had symptomatic perianal disease. Of these, only 3 (1.6%) had hemorrhoids. Treatment is always conservative and directed at the Crohn disease rather than the hemorrhoids.
Patients with portal hypertension (eg, due to cirrhosis) are prone to have anorectal varices that may resemble hemorrhoids. Anorectal varices can be treated with vascular ligation, whereas sclerotherapy is the preferred method for hemorrhoids in this group, in whom coagulopathy is common.
TAKE-HOME MESSAGES
Hemorrhoidal disease is common in the United States, and with our diet and lifestyle, the incidence is likely to increase. (A national survey found that overall dietary quality improved modestly in children and adolescents in the United States from 1999 to 2012 but remained far below optimal.38) Practitioners need to carefully assess hemorrhoidal symptoms and complete any necessary screening tests before establishing a diagnosis. This helps to avoid missing any underlying disease.
Fiber supplements along with dietary and lifestyle changes constitute the baseline of the management regardless of the disease grade. Office-based interventions are beneficial for grade I and II hemorrhoids and for some grade III hemorrhoids. Repeated interventions can increase the success rate. In patients with high-grade, symptomatic hemorrhoids, surgical hemorrhoidectomy is the most effective modality with the lowest recurrence rates, although it causes more pain than conservative methods.
Aspects of modern life that may promote hemorrhoids include increased consumption of processed foods, a sedentary lifestyle, and using cell phones while defecating, which translates to much more time spent on the toilet.
Hemorrhoids accounted for more than 3.5 million US outpatient visits in 2010, and they were the third leading cause of hospital admissions related to gastrointestinal disease.1
Here, we review the process for diagnosing and grading hemorrhoids, as well as for selecting the appropriate medical or surgical treatment based on the most recent clinical evidence.
DIAGNOSING AND CLASSIFYING HEMORRHOIDS
Hemorrhoids are the distal prolapse of the arteriovenous bundle, muscle fibers, and surrounding connective tissue as an envelope below the dentate line in the anal canal. They usually present with painless rectal bleeding.2
The diagnosis of hemorrhoids relies on the history and physical examination rather than on laboratory testing or imaging studies. Typically, the presenting symptom is painless rectal bleeding associated with bowel movements, usually appearing as bright red blood on the toilet paper or coating the stool. Severe itching and anal discomfort are also common, especially with chronic hemorrhoids.
Detailed patient history
A detailed patient history is important. It should include the extent, severity, and duration of symptoms, frequency of bowel movements, associated symptoms (eg, constipation, fecal incontinence), daily dietary habits, and details of bowel movements (eg, time spent during each bowel movement and concomitant cell phone use).3
Regarding bowel habits, some patients experience lifelong constipation or diarrhea. Therefore, what a patient considers a normal bowel habit may not be normal and should be investigated.4 Also, it is important to exclude external thrombosed hemorrhoids, anal fissure, anal abscess, and Crohn disease.5
Physical examination
A digital rectal examination is the second step. During the examination, look for skin tags, sphincter tone, perianal hygiene, and synchronous anal lesions.3 Of note, the Valsalva maneuver can be performed during the digital rectal examination.
Red flags for colorectal cancer on the digital rectal examination include a mass with or without presence of hemorrhoidal sacs and a bleeding source above the level of internal hemorrhoids.
Endoscopy
Since rectal bleeding can be a sign of several diseases, including colorectal cancer, it is important to review any previous endoscopic results. Patients at high risk of colon cancer should undergo rigid proctoscopy, flexible sigmoidoscopy, or colonoscopy.3,4 In our practice, we recommend endoscopic evaluation for patients over age 40 with rectal bleeding, especially if they have a family history of colorectal cancer.
External or internal (grades I–IV)
Hemorrhoids can be categorized as either external or internal.
External hemorrhoids are distinguished by their outer covering with perianal skin and anoderm and their location inferior to the dentate line. They are painful if the hemorrhoidal sac is occluded by a thrombotic clot.
Internal hemorrhoids are above the dentate line and covered with rectal columnar and transitional mucosa. They are further graded on a 4-point scale3:
- Grade I—Visible hemorrhoids that do not prolapse
- Grade II—Hemorrhoids that prolapse during the Valsalva maneuver but spontaneously reduce
- Grade III—Hemorrhoids that prolapse during the Valsalva maneuver and need manual reduction
- Grade IV—Nonreducible hemorrhoids.
A RANGE OF TREATMENTS
In choosing the treatment for hemorrhoids, one should consider the disease grade and severity, its impact on the quality of life, the degree of pain it causes, the patient’s likelihood of adhering to treatment, and the patient’s personal preference.
Regardless of severity, treatment almost always starts with a high-fiber diet and other lifestyle modifications that include bowel movement behaviors. This requires practitioners to spend significant time on patient education regardless of the type or the severity of the disease.
Treatments can be grouped in 3 categories: conservative, office-based, and surgical. Practitioners should thoroughly discuss the options with the patient, emphasizing the pros and cons of each.
CONSERVATIVE MEASURES
Conservative measures are aimed at softening the stool, relieving pain, and correcting bad toileting habits. In most cases, the primary precipitating factor is lifestyle, and unless patients change it, they are more likely to have recurrent symptoms in the long term.
No phone in the bathroom
People take their phones into the bathroom, and this habit is blamed for increasing the time on the toilet and leading to increased pressure on the anal region and straining during defecation. Some research points to a direct correlation between the time spent on the toilet and hemorrhoidal disease, although the exact cause-and-effect relationship with cell phone use has not been determined. In general, spending excessive time on the commode, including reading, should be discouraged.
Less time in the bathroom
Johannsson et al6 reported that patients with hemorrhoids spent more time on the toilet and had to strain harder and more often than controls in the community and hospital.
Garg and Singh7 and Garg8 use the mnemonic “TONE” for appropriate defecation habits:
- Three minutes during defecation
- Once-daily defecation
- No straining and no cell phone use during defecation
- Enough fiber.
More fiber
Fiber draws water into the lumen of the colon, increasing the water content of the stool. Recommended daily fiber intake is about 28 g for women and 38 g for men.9 This high level of intake is hard to achieve without supplements for someone who consumes a classic American diet with a lot of fast food.
Fiber supplements are strongly recommended in the American Society of Colon and Rectal Surgeons (ASCRS) practice guidelines3 based on a Cochrane review.10 In this meta-analysis, with fiber supplements, the relative risk of persisting or nonimproving symptoms was 0.53 (95% confidence interval [CI] 0.38–0.73) and the relative risk of bleeding was 0.50 (95% CI 028–0.89). Psyllium husk is an inexpensive bulk-forming fiber supplement; the optimal daily dosage is not known.
We recommend at least 28 g of daily fiber intake for women and 38 g for men, for which psyllium husk can be used to complement the diet.
Laxatives for some
Laxatives such as docusate are used to change the stool consistency when there is an organic bowel problem rather than a dietary issue. They can be used as a complementary treatment to enhance the effect of the fiber treatment.
Other measures
Topical anesthesia (eg, 5% lidocaine) is commonly used to treat pain from low-grade lesions, but no reliable data have been published. As most cases of hemorrhoids tend to progress over time, one should not expect long-term improvement with topical anesthesia. Nevertheless, it can be used as an ancillary treatment in select cases when short-term improvement is the main goal, and we recommend it based on our own experience.
Hygiene. Bidet use or cleaning the perianal area with water is recommended.
Phlebotonics contain a variety of ingredients including natural plant extracts such as flavonoids and synthetic products. Even though the exact mechanism of action is not known, phlebotonics are thought to increase venous and lymphatic drainage, normalize capillary permeability, and decrease inflammation in the hemorrhoidal cushions.4,11–13
In a Cochrane review of 24 randomized controlled trials, Perera et al14 found that phlebotonics improved the outcomes of:
- Bleeding (odds ratio [OR] 0.21, number needed to treat [NNT] 4.8, P = .0002)
- Pruritus (OR 0.23, NNT 9.1, P = .02)
- Discharge or leakage (OR 0.12, NNT 5, P = .0008)
- Overall symptoms (OR 15.99, NNT 2.7, P < .00001). Overall symptoms were also improved in the subgroup of pregnant patients.
Although phlebotonics give better results than placebo in the short-term management of hemorrhoids, there is a paucity of long-term data. Thus, the ASCRS clinical practice guidelines gives the regular use of these agents only a weak recommendation.3
Flavonoids (diosmin, hesperidin, rutoside), in a meta-analysis vs placebo in 1,514 patients, showed a beneficial response in terms of bleeding (relative risk [RR] 0.33), pruritus (relative risk [RR] 0.65), and recurrences (RR 0.53).15
Although Preparation H is commonly used as an over-the-counter medication, there are no good data on it, and it is not considered a phlebotonic.
OFFICE-BASED TREATMENTS
Office-based treatments—rubber band ligation, infrared photocoagulation, and sclerotherapy—are commonly used for grade I, II, and III hemorrhoids that have not responded to conservative management. The primary goal of these treatments is to decrease blood flow into the hemorrhoidal sac.
Even though office-based treatments are highly effective and major complications are uncommon, recurrence rates can be high, requiring patients to undergo additional treatments. Moreover, septic complications can occur, so patients should be closely observed for fever and urinary problems. Pain is a common symptom after office-based treatments, and bleeding may also occur.
The ASCRS guidelines strongly recommend office-based treatments for patients with grade I and II hemorrhoids, and for some with grade III hemorrhoids.3
Rubber band ligation
Iyer et al18 reported that patients on warfarin therapy had up to a 9 times higher risk of postprocedural bleeding, and patients on aspirin had a risk up to 3 times higher. Therefore, whether patients on anticoagulant therapy should undergo this procedure is unclear.
A Cochrane database review19 found this technique effective for hemorrhoid grades I through III, although some patients with grade III hemorrhoids may benefit more from excisional hemorrhoidectomy, which is associated with a lower recurrence rate than rubber band ligation.
Brown et al20 performed a randomized controlled trial comparing hemorrhoidal artery ligation and rubber band ligation for symptomatic hemorrhoids in 372 patients with grade II and III hemorrhoids. Postprocedural pain scores on days 1 and 7 were significantly lower with rubber band ligation, but recurrences were more common (49% vs 30%, P = .0005, respectively).
Overall, rubber band ligation is an excellent option for grade II hemorrhoids, as it is easy to perform, is associated with low pain scores, and can be used to treat recurrences.
Infrared photocoagulation
In this procedure, an infrared probe produces heat to induce coagulation, fibrosis, and ultimately necrosis of the protruding tissue in the hemorrhoidal cushions.21 Even though its use was initially directed at grade I and II hemorrhoids, recent reports showed acceptable results for grades III and IV.22,23 A randomized controlled trial comparing infrared photocoagulation and rubber band ligation in 94 patients found that both procedures were well accepted and highly effective; however, patients had better pain scores with photocoagulation in the first 24 hours after the procedure (P < .05).24
Sclerotherapy
The injection can cause prostatic abscess and sepsis, although this is rare.25 Nevertheless, high fever and postprocedural pain should be carefully evaluated.
There have been few randomized trials of sclerotherapy, but success rates so far have been higher for grade I hemorrhoids than for grades II and III.26–28 It is the preferred method for patients who have bleeding abnormalities caused by medications or other diseases (eg, cirrhosis).
SURGERY
Although nonsurgical treatments have substantially improved, surgery is the most effective and strongly recommended treatment for patients with high-grade internal hemorrhoids (grades III and IV), external and mixed hemorrhoids, and recurrent hemorrhoids.
The most popular surgical options are open or closed hemorrhoidectomy, stapled hemorrhoidopexy, and Doppler-guided hemorrhoidal artery ligation. Each has different success rates and different complication profiles, which need to be discussed with the patient.
Overall, surgery is associated with more adverse effects than office-based treatments or medical management. Postoperative pain is the most common complaint, but anal stricture (rare) or incontinence may occur due to excessive tissue excision and damage to the sphincter muscles. These can be avoided by maintaining the normal anoderm between excisions, by not excising all hemorrhoid sacs at once if the patient has extensive lesions, and by performing a careful dissection in the submucosal plane.
Patients with profuse bleeding or an underlying bleeding abnormality are best managed with surgical approaches performed in an operating room.
Excisional surgical hemorrhoidectomy
Excision of the hemorrhoidal sac, the most conventional surgical technique, is generally reserved for prolapsing disease. The recurrence rate after excisional hemorrhoidectomy is significantly lower than with any other approach.29
Excisional hemorrhoidectomy can be performed using either an open approach, in which the edges of the mucosal defect are not reapproximated, or a closed approach, in which they are. In a systematic review, Bhatti et al30 compared open vs closed techniques and found that the closed technique resulted in less postoperative pain, better wound healing, and less bleeding. Rates of recurrence, postoperative complications, and surgical site infection and lengths of stay were comparable with either procedure.
Overall, excisional hemorrhoidectomy is associated with higher pain scores than any other surgical method.29 Recently, the use of electrodiathermy energy devices, also described as electrosurgical vessel-sealing devices, have further improved overall patient satisfaction.31
Multiple painful hemorrhoidal sacs require a careful surgical approach, as extensive resection may cause widespread fibrosis and stricture. As with anal stricture, fecal incontinence can be prevented by careful dissection. However, already existing incontinence is not a contraindication for the surgery.
Doppler-guided hemorrhoidal artery ligation
Doppler-guided hemorrhoidal artery ligation involves using a Doppler probe to find and ligate individual hemorrhoidal arteries. Additionally, mucopexy (transanal rectoanal repair) is performed to relocate the prolapsing tissue. Avital et al32 reported that at 1 year after this procedure, recurrence rates were 5.3% for grade II hemorrhoids and 13% for grade III hemorrhoids. At 5 years, recurrence rates were 12% for grade II and 31% for grade III.
To date, this procedure appears to be suitable for grade I, II, and III hemorrhoids, especially for grade II, but more studies are needed to prove its efficacy and recurrence rates for more advanced lesions. Although this technique has a high morbidity rate (18%), primarily pain or tenesmus, it causes less postoperative pain than other surgical methods.33 Overall, it has the potential to become a favored treatment.
Stapled hemorrhoidopexy
Although pain scores are lower with stapled hemorrhoidopexy than with excisional hemorrhoidectomy, this procedure is not superior in terms of recurrences.34,35 Also, practitioners should be careful about specific complications of stapled hemorrhoidopexy, such as rectovaginal fistula, anal stenosis, or sphincter injuries. These specific complications should be clearly explained to patients, and necessary information should be given to patients upon discharge. The primary care physician should also be careful about fistulas and stenoses in this particular patient population.
NO ‘BEST’ TREATMENT
There is no best treatment for hemorrhoids. Every patient is different, and the physician and patient need to understand each other’s expectations, weigh the risks and benefits, and arrive at a mutual decision. A good patient-doctor relationship is essential.
Given the variety of available treatments, head-to-head comparisons are difficult. Moreover, the efficacy and applicability of each technique changes with the grade of the lesion or lesions and the skill of the practitioner. Lacking comprehensive studies comparing conservative, office-based, and surgical management, no decisive statements can be made based on current evidence.
Patients with compounding conditions
Pregnant patients often develop hemorrhoids as intra-abdominal pressure increases, particularly during the third trimester.36 Also, acute episodes of pain and bleeding are common in pregnant women with preexisting hemorrhoids.
Conservative treatment is the main approach in pregnant patients because most hemorrhoids regress after childbirth. This includes increased dietary fiber, stool softeners, and sitz baths, which are safe to use for external hemorrhoids. Any office-based or surgical intervention should be postponed until after childbirth, if possible. Kegel exercises and lying on the left side are also recommended to relieve symptoms. In cases of severe bleeding, anal packing appears to be useful.
Immunosuppressed patients and those on anticoagulant therapy are more prone to serious complications such as sepsis and profuse bleeding. Thus, conservative management should be used in these patients as well. Injection sclerotherapy may be beneficial, as it has been shown to decrease bleeding. Of note, patients on immunosuppressive agents should stop taking them and start taking an antibiotic, and patients on anticoagulant or antiplatelet medications should be instructed to stop them 1 week before any intervention.
Crohn disease. Some patients with Crohn disease may have hemorrhoids, though this is rare. Eglinton et al,37 in a series of 715 patients with Crohn disease, reported that 190 (26.6%) had symptomatic perianal disease. Of these, only 3 (1.6%) had hemorrhoids. Treatment is always conservative and directed at the Crohn disease rather than the hemorrhoids.
Patients with portal hypertension (eg, due to cirrhosis) are prone to have anorectal varices that may resemble hemorrhoids. Anorectal varices can be treated with vascular ligation, whereas sclerotherapy is the preferred method for hemorrhoids in this group, in whom coagulopathy is common.
TAKE-HOME MESSAGES
Hemorrhoidal disease is common in the United States, and with our diet and lifestyle, the incidence is likely to increase. (A national survey found that overall dietary quality improved modestly in children and adolescents in the United States from 1999 to 2012 but remained far below optimal.38) Practitioners need to carefully assess hemorrhoidal symptoms and complete any necessary screening tests before establishing a diagnosis. This helps to avoid missing any underlying disease.
Fiber supplements along with dietary and lifestyle changes constitute the baseline of the management regardless of the disease grade. Office-based interventions are beneficial for grade I and II hemorrhoids and for some grade III hemorrhoids. Repeated interventions can increase the success rate. In patients with high-grade, symptomatic hemorrhoids, surgical hemorrhoidectomy is the most effective modality with the lowest recurrence rates, although it causes more pain than conservative methods.
- Peery AF, Crockett SD, Barritt AS, et al. Burden of gastrointestinal, liver, and pancreatic diseases in the United States. Gastroenterology 2015; 149(7):1731–1741.e3. doi:10.1053/j.gastro.2015.08.045
- Thomson WH. The nature and cause of haemorrhoids. Proc R Soc Med 1975; 68(9):574–575. pmid:1197343
- Davis BR, Lee-Kong SA, Migaly J, Feingold DL, Steele SR. The American Society of Colon and Rectal Surgeons clinical practice guidelines for the management of hemorrhoids. Dis Colon Rectum 2018; 61(3):284–292. doi:10.1097/DCR.0000000000001030
- Lohsiriwat V. Treatment of hemorrhoids: a coloproctologist’s view. World J Gastroenterol 2015; 21(31):9245–9252. doi:10.3748/wjg.v21.i31.9245
- Wolf AMD, Fontham ETH, Church TR, et al. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J Clin 2018; 68(4):250–281. doi:10.3322/caac.21457
- Johannsson HO, Graf W, Påhlman L. Bowel habits in hemorrhoid patients and normal subjects. Am J Gastroenterol 2005; 100(2):401–406. doi:10.1111/j.1572-0241.2005.40195.x
- Garg P, Singh P. Adequate dietary fiber supplement and TONE can help avoid surgery in most patients with advanced hemorrhoids. Minerva Gastroenterol Dietol 2017; 63(2):92–96. doi:10.23736/S1121-421X.17.02364-9
- Garg P. Conservative treatment of hemorrhoids deserves more attention in guidelines and clinical practice [letter]. Dis Colon Rectum 2018; 61(7):e348. doi:10.1097/DCR.0000000000001127
- Rakinic J, Poola VP. Hemorrhoids and fistulas: new solutions to old problems. Curr Probl Surg 2014; 51(3):98–137. doi:10.1067/j.cpsurg.2013.11.002
- Alonso-Coello P, Guyatt G, Heels-Ansdell D, et al. Laxatives for the treatment of hemorrhoids. Cochrane Database Syst Rev 2005; (4):CD004649. doi:10.1002/14651858.CD004649.pub2
- Struckmann JR. Clinical efficacy of micronized purified flavonoid fraction: an overview. J Vasc Res 1999; 36(suppl 1):37–41. doi:10.1159/000054072
- Shoab SS, Porter J, Scurr JH, Coleridge-Smith PD. Endothelial activation response to oral micronised flavonoid therapy in patients with chronic venous disease—a prospective study. Eur J Vasc Endovasc Surg 1999; 17(4):313–318. doi:10.1053/ejvs.1998.0751
- Meyer OC. Safety and security of Daflon 500 mg in venous insufficiency and in hemorrhoidal disease. Angiology 1994; 45(6 pt 2):579–584. pmid:8203791
- Perera N, Liolitsa D, Iype S, et al. Phlebotonics for haemorrhoids. Cochrane Database Syst Rev 2012;(8):CD004322. doi:10.1002/14651858.CD004322.pub3
- Alonso-Coello P, Zhou Q, Martinez-Zapata MJ, et al. Meta-analysis of flavonoids for the treatment of haemorrhoids. Br J Surg 2006; 93(8):909–920. doi:10.1002/bjs.5378
- Lee HH, Spencer RJ, Beart RW Jr. Multiple hemorrhoidal bandings in a single session. Dis Colon Rectum 1994; 37(1):37–41. pmid:8287745
- Law WL, Chu KW. Triple rubber band ligation for hemorrhoids: prospective, randomized trial of use of local anesthetic injection. Dis Colon Rectum 1999; 42(3):363–366. pmid:10223757
- Iyer VS, Shrier I, Gordon PH. Long-term outcome of rubber band ligation for symptomatic primary and recurrent internal hemorrhoids. Dis Colon Rectum 2004; 47(8):1364–1370. pmid:15484351
- Shanmugam V, Thaha MA, Rabindranath KS, Campbell KL, Steele RJ, Loudon MA. Rubber band ligation versus excisional haemorrhoidectomy for haemorrhoids. Cochrane Database Syst Rev 2005; (3):CD005034. doi:10.1002/14651858.CD005034.pub2
- Brown SR, Tiernan JP, Watson AJM, et al; HubBLe Study team. Haemorrhoidal artery ligation versus rubber band ligation for the management of symptomatic second-degree and third-degree haemorrhoids (HubBLe): a multicentre, open-label, randomised controlled trial. Lancet 2016; 388(10042):356–364. doi:10.1016/S0140-6736(16)30584-0
- ASGE Technology Committee; Siddiqui UD, Barth BA, Banerjee S, et al. Devices for the endoscopic treatment of hemorrhoids. Gastrointest Endosc 2014; 79(1):8–14. doi:10.1016/j.gie.2013.07.021
- Ahmad A, Kant R, Gupta A. Comparative analysis of Doppler guided hemorrhoidal artery ligation (DG-HAL) & infrared coagulation (IRC) in management of hemorrhoids. Indian J Surg 2013; 75(4):274–277. doi:10.1007/s12262-012-0444-5
- Poen AC, Felt-Bersma RJ, Cuesta MA, Devillé W, Meuwissen SG. A randomized controlled trial of rubber band ligation versus infra-red coagulation in the treatment of internal haemorrhoids. Eur J Gastroenterol Hepatol 2000; 12(5):535–539. pmid:10833097
- Marques CF, Nahas SC, Nahas CS, Sobrado CW Jr, Habr-Gama A, Kiss DR. Early results of the treatment of internal hemorrhoid disease by infrared coagulation and elastic banding: a prospective randomized cross-over trial. Tech Coloproctol 2006; 10(4):312–317. doi:10.1007/s10151-006-0299-5
- Madoff RD, Fleshman JW; Clinical Practice Committee, American Gastroenterological Association. American Gastroenterological Association technical review on the diagnosis and treatment of hemorrhoids. Gastroenterology 2004; 126(5):1463–1473. pmid:15131807
- Yano T, Yano K. Comparison of injection sclerotherapy between 5% phenol in almond oil and aluminum potassium sulfate and tannic acid for grade 3 hemorrhoids. Ann Coloproctol 2015; 31(3):103–105. doi:10.3393/ac.2015.31.3.103
- Kanellos I, Goulimaris I, Vakalis I, Dadoukis I. Long-term evaluation of sclerotherapy for haemorrhoids. A prospective study. Int J Surg Investig 2000; 2(4):295–298. pmid:12678531
- Moser KH, Mosch C, Walgenbach M, et al. Efficacy and safety of sclerotherapy with polidocanol foam in comparison with fluid sclerosant in the treatment of first-grade haemorrhoidal disease: a randomised, controlled, single-blind, multicentre trial. Int J Colorectal Dis 2013; 28(10):1439–1447. doi:10.1007/s00384-013-1729-2
- MacRae HM, McLeod RS. Comparison of hemorrhoidal treatments: a meta-analysis. Can J Surg 1997; 40(1):14–7. pmid:9030078
- Bhatti MI, Sajid MS, Baig MK. Milligan-Morgan (open) versus Ferguson haemorrhoidectomy (closed): a systematic review and meta-analysis of published randomized, controlled trials. World J Surg 2016; 40(6):1509–1519. doi:10.1007/s00268-016-3419-z
- Nienhuijs S, de Hingh I. Conventional versus LigaSure hemorrhoidectomy for patients with symptomatic hemorrhoids. Cochrane Database Syst Rev 2009; (1):CD006761. doi:10.1002/14651858.CD006761.pub2
- Avital S, Inbar R, Karin E, Greenberg R. Five-year follow-up of Doppler-guided hemorrhoidal artery ligation. Tech Coloproctol 2012; 16(1):61–65. doi:10.1007/s10151-011-0801-6
- Ratto C, Parello A, Veronese E, et al. Doppler-guided transanal haemorrhoidal dearterialization for haemorrhoids: results from a multicentre trial. Colorectal Dis 2015; 17(1):010–019. doi:10.1111/codi.12779
- Senagore AJ, Singer M, Abcarian H, et al; Procedure for Prolapse and Hemmorrhoids (PPH) Multicenter Study Group. A prospective, randomized, controlled multicenter trial comparing stapled hemorrhoidopexy and Ferguson hemorrhoidectomy: perioperative and one-year results. Dis Colon Rectum 2004; 47(11):1824–1836. pmid:15622574
- Jayaraman S, Colquhoun PH, Malthaner RA. Stapled versus conventional surgery for hemorrhoids. Cochrane Database Syst Rev 2006; (4):CD005393.
- Poskus T, Buzinskiene D, Drasutiene G, et al. Haemorrhoids and anal fissures during pregnancy and after childbirth: a prospective cohort study. BJOG 2014; 121(13):1666–1671. doi:10.1111/1471-0528.12838
- Eglinton TW, Barclay ML, Gearry RB, Frizelle FA. The spectrum of perianal Crohn’s disease in a population-based cohort. Dis Colon Rectum 2012; 55(7):773–777. doi:10.1097/DCR.0b013e31825228b0
- Gu X, Tucker KL. Dietary quality of the US child and adolescent population: trends from 1999 to 2012 and associations with the use of federal nutrition assistance programs. Am J Clin Nutr 2017; 105(1):194–202. doi:10.3945/ajcn.116.135095
- Peery AF, Crockett SD, Barritt AS, et al. Burden of gastrointestinal, liver, and pancreatic diseases in the United States. Gastroenterology 2015; 149(7):1731–1741.e3. doi:10.1053/j.gastro.2015.08.045
- Thomson WH. The nature and cause of haemorrhoids. Proc R Soc Med 1975; 68(9):574–575. pmid:1197343
- Davis BR, Lee-Kong SA, Migaly J, Feingold DL, Steele SR. The American Society of Colon and Rectal Surgeons clinical practice guidelines for the management of hemorrhoids. Dis Colon Rectum 2018; 61(3):284–292. doi:10.1097/DCR.0000000000001030
- Lohsiriwat V. Treatment of hemorrhoids: a coloproctologist’s view. World J Gastroenterol 2015; 21(31):9245–9252. doi:10.3748/wjg.v21.i31.9245
- Wolf AMD, Fontham ETH, Church TR, et al. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J Clin 2018; 68(4):250–281. doi:10.3322/caac.21457
- Johannsson HO, Graf W, Påhlman L. Bowel habits in hemorrhoid patients and normal subjects. Am J Gastroenterol 2005; 100(2):401–406. doi:10.1111/j.1572-0241.2005.40195.x
- Garg P, Singh P. Adequate dietary fiber supplement and TONE can help avoid surgery in most patients with advanced hemorrhoids. Minerva Gastroenterol Dietol 2017; 63(2):92–96. doi:10.23736/S1121-421X.17.02364-9
- Garg P. Conservative treatment of hemorrhoids deserves more attention in guidelines and clinical practice [letter]. Dis Colon Rectum 2018; 61(7):e348. doi:10.1097/DCR.0000000000001127
- Rakinic J, Poola VP. Hemorrhoids and fistulas: new solutions to old problems. Curr Probl Surg 2014; 51(3):98–137. doi:10.1067/j.cpsurg.2013.11.002
- Alonso-Coello P, Guyatt G, Heels-Ansdell D, et al. Laxatives for the treatment of hemorrhoids. Cochrane Database Syst Rev 2005; (4):CD004649. doi:10.1002/14651858.CD004649.pub2
- Struckmann JR. Clinical efficacy of micronized purified flavonoid fraction: an overview. J Vasc Res 1999; 36(suppl 1):37–41. doi:10.1159/000054072
- Shoab SS, Porter J, Scurr JH, Coleridge-Smith PD. Endothelial activation response to oral micronised flavonoid therapy in patients with chronic venous disease—a prospective study. Eur J Vasc Endovasc Surg 1999; 17(4):313–318. doi:10.1053/ejvs.1998.0751
- Meyer OC. Safety and security of Daflon 500 mg in venous insufficiency and in hemorrhoidal disease. Angiology 1994; 45(6 pt 2):579–584. pmid:8203791
- Perera N, Liolitsa D, Iype S, et al. Phlebotonics for haemorrhoids. Cochrane Database Syst Rev 2012;(8):CD004322. doi:10.1002/14651858.CD004322.pub3
- Alonso-Coello P, Zhou Q, Martinez-Zapata MJ, et al. Meta-analysis of flavonoids for the treatment of haemorrhoids. Br J Surg 2006; 93(8):909–920. doi:10.1002/bjs.5378
- Lee HH, Spencer RJ, Beart RW Jr. Multiple hemorrhoidal bandings in a single session. Dis Colon Rectum 1994; 37(1):37–41. pmid:8287745
- Law WL, Chu KW. Triple rubber band ligation for hemorrhoids: prospective, randomized trial of use of local anesthetic injection. Dis Colon Rectum 1999; 42(3):363–366. pmid:10223757
- Iyer VS, Shrier I, Gordon PH. Long-term outcome of rubber band ligation for symptomatic primary and recurrent internal hemorrhoids. Dis Colon Rectum 2004; 47(8):1364–1370. pmid:15484351
- Shanmugam V, Thaha MA, Rabindranath KS, Campbell KL, Steele RJ, Loudon MA. Rubber band ligation versus excisional haemorrhoidectomy for haemorrhoids. Cochrane Database Syst Rev 2005; (3):CD005034. doi:10.1002/14651858.CD005034.pub2
- Brown SR, Tiernan JP, Watson AJM, et al; HubBLe Study team. Haemorrhoidal artery ligation versus rubber band ligation for the management of symptomatic second-degree and third-degree haemorrhoids (HubBLe): a multicentre, open-label, randomised controlled trial. Lancet 2016; 388(10042):356–364. doi:10.1016/S0140-6736(16)30584-0
- ASGE Technology Committee; Siddiqui UD, Barth BA, Banerjee S, et al. Devices for the endoscopic treatment of hemorrhoids. Gastrointest Endosc 2014; 79(1):8–14. doi:10.1016/j.gie.2013.07.021
- Ahmad A, Kant R, Gupta A. Comparative analysis of Doppler guided hemorrhoidal artery ligation (DG-HAL) & infrared coagulation (IRC) in management of hemorrhoids. Indian J Surg 2013; 75(4):274–277. doi:10.1007/s12262-012-0444-5
- Poen AC, Felt-Bersma RJ, Cuesta MA, Devillé W, Meuwissen SG. A randomized controlled trial of rubber band ligation versus infra-red coagulation in the treatment of internal haemorrhoids. Eur J Gastroenterol Hepatol 2000; 12(5):535–539. pmid:10833097
- Marques CF, Nahas SC, Nahas CS, Sobrado CW Jr, Habr-Gama A, Kiss DR. Early results of the treatment of internal hemorrhoid disease by infrared coagulation and elastic banding: a prospective randomized cross-over trial. Tech Coloproctol 2006; 10(4):312–317. doi:10.1007/s10151-006-0299-5
- Madoff RD, Fleshman JW; Clinical Practice Committee, American Gastroenterological Association. American Gastroenterological Association technical review on the diagnosis and treatment of hemorrhoids. Gastroenterology 2004; 126(5):1463–1473. pmid:15131807
- Yano T, Yano K. Comparison of injection sclerotherapy between 5% phenol in almond oil and aluminum potassium sulfate and tannic acid for grade 3 hemorrhoids. Ann Coloproctol 2015; 31(3):103–105. doi:10.3393/ac.2015.31.3.103
- Kanellos I, Goulimaris I, Vakalis I, Dadoukis I. Long-term evaluation of sclerotherapy for haemorrhoids. A prospective study. Int J Surg Investig 2000; 2(4):295–298. pmid:12678531
- Moser KH, Mosch C, Walgenbach M, et al. Efficacy and safety of sclerotherapy with polidocanol foam in comparison with fluid sclerosant in the treatment of first-grade haemorrhoidal disease: a randomised, controlled, single-blind, multicentre trial. Int J Colorectal Dis 2013; 28(10):1439–1447. doi:10.1007/s00384-013-1729-2
- MacRae HM, McLeod RS. Comparison of hemorrhoidal treatments: a meta-analysis. Can J Surg 1997; 40(1):14–7. pmid:9030078
- Bhatti MI, Sajid MS, Baig MK. Milligan-Morgan (open) versus Ferguson haemorrhoidectomy (closed): a systematic review and meta-analysis of published randomized, controlled trials. World J Surg 2016; 40(6):1509–1519. doi:10.1007/s00268-016-3419-z
- Nienhuijs S, de Hingh I. Conventional versus LigaSure hemorrhoidectomy for patients with symptomatic hemorrhoids. Cochrane Database Syst Rev 2009; (1):CD006761. doi:10.1002/14651858.CD006761.pub2
- Avital S, Inbar R, Karin E, Greenberg R. Five-year follow-up of Doppler-guided hemorrhoidal artery ligation. Tech Coloproctol 2012; 16(1):61–65. doi:10.1007/s10151-011-0801-6
- Ratto C, Parello A, Veronese E, et al. Doppler-guided transanal haemorrhoidal dearterialization for haemorrhoids: results from a multicentre trial. Colorectal Dis 2015; 17(1):010–019. doi:10.1111/codi.12779
- Senagore AJ, Singer M, Abcarian H, et al; Procedure for Prolapse and Hemmorrhoids (PPH) Multicenter Study Group. A prospective, randomized, controlled multicenter trial comparing stapled hemorrhoidopexy and Ferguson hemorrhoidectomy: perioperative and one-year results. Dis Colon Rectum 2004; 47(11):1824–1836. pmid:15622574
- Jayaraman S, Colquhoun PH, Malthaner RA. Stapled versus conventional surgery for hemorrhoids. Cochrane Database Syst Rev 2006; (4):CD005393.
- Poskus T, Buzinskiene D, Drasutiene G, et al. Haemorrhoids and anal fissures during pregnancy and after childbirth: a prospective cohort study. BJOG 2014; 121(13):1666–1671. doi:10.1111/1471-0528.12838
- Eglinton TW, Barclay ML, Gearry RB, Frizelle FA. The spectrum of perianal Crohn’s disease in a population-based cohort. Dis Colon Rectum 2012; 55(7):773–777. doi:10.1097/DCR.0b013e31825228b0
- Gu X, Tucker KL. Dietary quality of the US child and adolescent population: trends from 1999 to 2012 and associations with the use of federal nutrition assistance programs. Am J Clin Nutr 2017; 105(1):194–202. doi:10.3945/ajcn.116.135095
KEY POINTS
- Hemorrhoids account for more than 3.5 million office visits annually.
- Most patients present with painless rectal bleeding, but this can also be a sign of colorectal cancer, which needs to be ruled out.
- Fiber supplements along with dietary and lifestyle changes are recommended for all patients with hemorrhoids regardless of symptom severity.
- Hemorrhoids are graded on a scale of I (least severe) through IV (most severe). Office-based treatments are effective for grades I, II, and some grade III hemorrhoids. Surgical excision is the standard for high-grade hemorrhoids.
Brexanolone injection for postpartum depression
Postpartum depression (PPD) is one of the most prevalent complications associated with pregnancy and childbirth in the United States, affecting more than 400,000 women annually.1 Postpartum depression is most commonly treated with psychotherapy and antidepressants approved for the treatment of major depressive disorder. Until recently, there was no pharmacologic therapy approved by the FDA specifically for the treatment of PPD. Considering the adverse outcomes associated with untreated or inadequately treated PPD, and the limitations of existing therapies, there is a significant unmet need for pharmacologic treatment options for PPD.2 To help address this need, the FDA recently approved brexanolone injection (brand name: ZULRESSO™) (Table 13) as a first-in-class therapy for the treatment of adults with PPD.3
Clinical implications
Postpartum depression can result in adverse outcomes for the patient, baby, and family when under- or untreated, and the need for rapid resolution of symptoms cannot be overstated.2 Suicide is strongly associated with depression and is a leading cause of pregnancy-related deaths.4 Additionally, PPD can impact the health, safety, and well-being of the child, with both short- and long-term consequences, including greater rates of psychological or behavioral difficulties among children of patients with PPD.5 Postpartum depression can also have negative effects on the patient’s partner, with 24% to 50% of partners experiencing depression.6 Current PPD management strategies include the use of psychotherapy and pharmacologic interventions for major depressive disorder that may take up to 4 to 6 weeks for some patients, and may not achieve remission for all patients.7-9
Brexanolone injection is a first-in-class medication with a novel mechanism of action. In clinical studies, it achieved rapid (by Hour 60) and sustained (through Day 30) reductions in depressive symptoms and could provide a meaningful new treatment option for adult women with PPD.10,11
How it works
Animal and human studies have established the re
Brexanolone is a neuroactive steroid that is chemically identical to endogenous allopregnanolone produced in the CNS. Brexanolone potentiates GABA-mediated currents from recombinant human GABAARs in mammalian cells expressing α1β2γ2 receptor subunits, α4β3δ receptor subunits, and α6β3δ receptor subunits.3 Positive allosteric modulation of both synaptic and extrasynaptic GABAARs differentiates brexanolone from other GABAAR modulators, such as benzodiazepines.10,11
Brexanolone’s mechanism of action in the treatment of PPD is not fully understood, but it is thought to be related to GABAAR PAM activity.3
Supporting evidence
The FDA approval of brexanolone injection was based on the efficacy demonstrated in 2 Phase III multicenter, randomized, double-blind, placebo-controlled studies in adult women (age 18 to 45) with PPD (defined by DSM-IV criteria for a major depressive episode, with onset of symptoms in the third trimester or within 4 weeks of delivery). Exclusion criteria included the presence of bipolar disorder or psychosis. In these studies, 60-hour continuous IV infusions of brexanolone or placebo were given, followed by 4 weeks of observation. Study 1 (202B) enrolled patients with severe PPD (Hamilton Rating Scale for Depression [HAM-D] total score ≥26), and Study 2 (202C) enrolled patients with moderate PPD (HAM-D score 20 to 25). A titration to the recommended target dosage of 90 μg/kg/hour was evaluated in both studies. BRX90 patients received 30 μg/kg/hour for 4 hours, 60 μg/kg/hour for 20 hours, 90 μg/kg/hour for 28 hours, followed by a taper to 60 μg/kg/hour for 4 hours and then 30 μg/kg/hour for 4 hours. The primary endpoint in both studies was the mean change from baseline in depressive symptoms as measured by HAM-D total score at the end of the 60-hour infusion. A pre-specified secondary efficacy endpoint was the mean change from baseline in HAM-D total score at Day 30.
Continue to: Efficacy
Efficacy. In both placebo-controlled studies, titration to a target dose of brexanolone 90 μg/kg/hour was superior to placebo in improvement of depressive symptoms (Table 33).
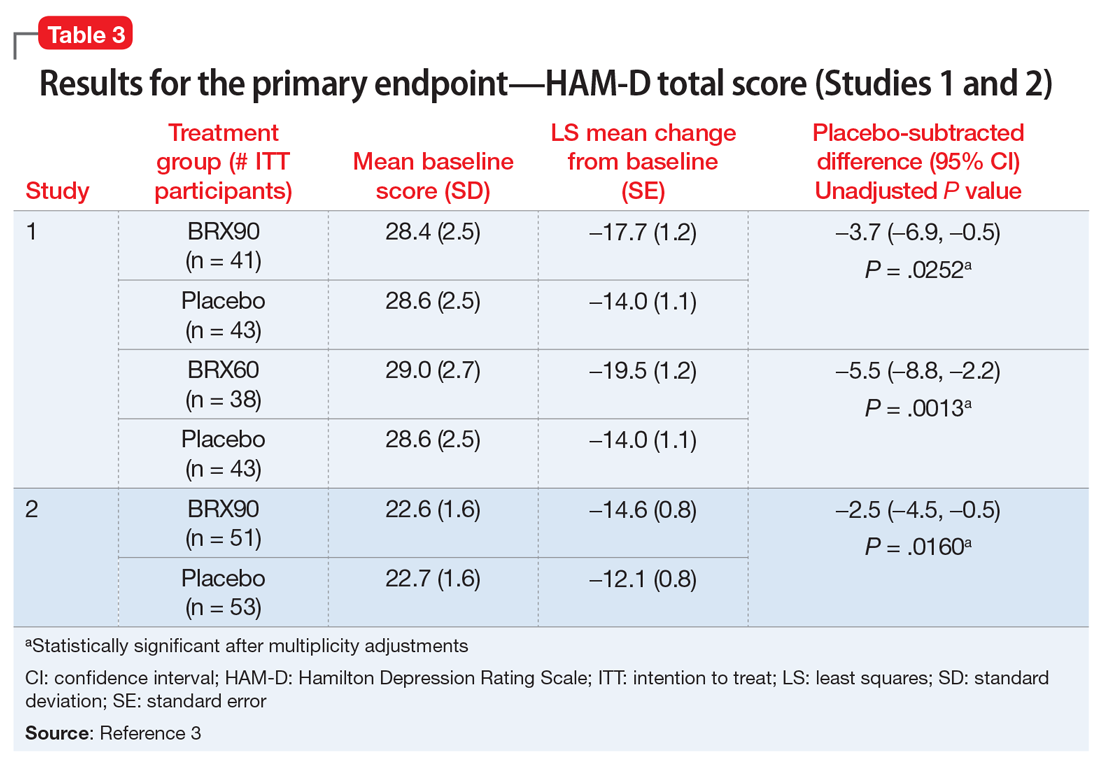
Pharmacological profile
Brexanolone exposure-response relationships and the time course of pharmacodynamic response are unknown.3
Adverse reactions. Safety was evaluated from all patients receiving brexanolone injection, regardless of dosing regimen (N = 140, including patients from a Phase IIb study, 202A).3,11
The most common adverse reactions (incidence ≥5% and at least twice the rate of placebo) were sedation/somnolence, dry mouth, loss of consciousness, and flushing/hot flush.3 The incidence of patients discontinuing due to any adverse reaction was 2% for brexanolone vs 1% for placebo.3
Sedation, somnolence, and loss of consciousness. In clinical studies, brexanolone caused sedation and somnolence that required dose interruption or reduction in some patients during the infusion (5% of brexanolone-treated patients compared with 0% of placebo-treated patients).3 Some patients were also reported to have loss of consciousness or altered state of consciousness during the brexanolone infusion (4% of patients treated with brexanolone compared with 0% of patients treated with placebo).3 All patients with loss of or altered state of consciousness recovered fully 15 to 60 minutes after dose interruption.3 There was no clear association between loss or alteration of consciousness and pattern or timing of dose, and not all patients who experienced a loss or alteration of consciousness reported sedation or somnolence before the episode.
Continue to: Suicidality
Suicidality. The risk of developing suicidal thoughts and behaviors with brexanolone is unknown, due to the relatively low number of exposures to brexanolone injection during clinical development and a mechanism of action distinct from that of existing antidepressant medications.3
Pharmacokinetics
In clinical trials, brexanolone exhibited dose-proportional pharmacokinetics, and the terminal half-life is approximately 9 hours (Table 43). Brexanolone is metabolized by non-cytochrome P450 (CYP)-based pathways, including keto-reduction, glucuronidation, and sulfation.3 No clinically significant differences in the pharmacokinetics of brexanolone were observed based on renal or hepatic impairment, and no studies were conducted to evaluate the effects of other drugs on brexanolone.3
Lactation. A population pharmacokinetics model constructed from studies in the clinical development program calculated the maximum relative infant dose for brexanolone during infusion as 1.3%.3 Given the low oral bioavailability of brexanolone (<5%) in adults, the potential for breastfed infant exposure is considered low.3
Clinical considerations
Risk Evaluation and Mitigation Strategies (REMS) requirements. Brexanolone injection is a Schedule IV controlled substance. It has a “black-box” warning regarding excessive sedation and sudden loss of consciousness, which has been taken into account within the REMS drug safety program. Health care facilities and pharmacies must enroll in the REMS program and ensure that brexanolone is administered only to patients who are enrolled in the REMS program. Staff must be trained on the processes and procedures to administer brexanolone, and the facility must have a fall precautions protocol in place and be equipped with a programmable peristaltic IV infusion pump and continuous pulse oximetry with alarms.3
Monitoring. A REMS-trained clinician must be available continuously on-site to oversee each patient for the duration of the continuous IV infusion, which lasts 60 hours (2.5 days) and should be initiated early enough in the day to allow for recognition of excessive sedation. Patients must be monitored for hypoxia using continuous pulse oximetry equipped with an alarm and should also be assessed for excessive sedation every 2 hours during planned, non-sleep periods. If excessive sedation occurs, the infusion should be stopped until symptoms resolve, after which the infusion may be resumed at the same or a lower dose as clinically appropriate. In case of overdosage, the infusion should be stopped immediately and supportive measures initiated as necessary. Patients must not be the primary caregiver of dependents, and must be accompanied during interactions with their child(ren).
Continue to: Contraindications
Contraindications. There are no contraindications for the use of brexanolone in adults with PPD.
End-stage renal disease (ESRD). Avoid using brexanolone in patients with ESRD because of the potential accumulation of the solubilizing agent, betadex sulfobutyl ether sodium.
Pregnancy. Brexanolone has not been studied in pregnant patients. Pregnant women and women of reproductive age should be informed of the potential risk to a fetus based on data from other drugs that enhance GABAergic inhibition.
Breastfeeding. There are no data on the effects of brexanolone on a breastfed infant. Breastfeeding should be a discussion of risk and benefit between the patient and her doctor. The developmental and health benefits of breastfeeding should be considered, along with the mother’s clinical need for brexanolone and any potential adverse effects on the breastfed child from brexanolone or from the underlying maternal condition. However, based on the low relative infant dose (<2%) and the low oral bioavailability in adults, the risk to breastfed infants is thought to be low.16
Potential for abuse. Brexanolone injection is a Schedule IV controlled substance. Although it was not possible to assess physical dependency in the registrational trials due to dose tapering at the end of treatment, clinicians should advise patients about the theoretical possibility for brexanolone to be abused or lead to dependence based on other medications with similar primary pharmacology.
Continue to: Concomitant medications
Concomitant medications. Caution patients that taking opioids or other CNS depressants, such as benzodiazepines, in combination with brexanolone may increase the severity of sedative effects.
Suicidal thoughts and behaviors. Advise patients and caregivers to look for the emergence of suicidal thoughts and behavior and instruct them to report such symptoms to their clinician. Consider changing the therapeutic regimen, including discontinuing brexanolone, in patients whose depression becomes worse or who experience emergent suicidal thoughts and behaviors.
Why Rx?
Postpartum depression is a common and often devastating medical complication of childbirth that can result in adverse outcomes for the patient, baby, and family when left undertreated or untreated. There is a great need to identify and treat women who develop PPD. Rapid and sustained resolution of symptoms in women who experience PPD should be the goal of treatment, and consequently, brexanolone injection presents an important new tool in available treatment options for PPD.
Bottom Line
Brexanolone injection is a neuroactive steroid gamma-aminobutyric acid (GABA) A receptor positive allosteric modulator that’s been FDA-approved for the treatment of postpartum depression (PPD). It is administered as a continuous IV infusion over 60 hours. The rapid and sustained improvement of PPD observed in clinical trials with brexanolone injection may support a new treatment paradigm for women with PPD.
1. Ko JY, Rockhill KM, Tong VT, et al. Trends in postpartum depressive symptoms - 27 states, 2004, 2008, and 2012. MMWR Morb Mortal Wkly Rep. 2017;66(6):153-158.
2. Frieder A, Fersh M, Hainline R, et al. Pharmacotherapy of postpartum depression: current approaches and novel drug development. CNS Drugs. 2019;33(3):265-282.
3. Brexanolone injection [package insert]. Cambridge, MA: Sage Therapeutics, Inc.; 2019.
4. Bodnar-Deren S, Klipstein K, Fersh M, et al. Suicidal ideation during the postpartum period. J Womens Health (Larchmt). 2016;25(12):1219-1224.
5. Netsi E, Pearson RM, Murray L, et al. Association of persistent and severe postnatal depression with child outcomes. JAMA Psychiatry. 2018;75(3):247-253.
6. Goodman JH. Paternal postpartum depression, its relationship to maternal postpartum depression, and implications for family health. J Adv Nurs. 2004;45(1):26-35.
7. Gelenberg AJ, Freeman MP, Markowitz JC, et al; American Psychiatric Association Work Group on Major Depressive Disorder. Practice guidelines for the treatment of patients with major depressive disorder. 3rd ed. Washington, DC: American Psychiatric Association; 2010.
8. Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163(11):1905-1917.
9. Molyneaux E, Telesia LA, Henshaw C, et al. Antidepressants for preventing postnatal depression. Cochrane Database Syst Rev. 2018;4:CD004363.
10. Kanes S, Colquhoun H, Gunduz-Bruce H, et al. Brexanolone (SAGE-547 injection) in post-partum depression: a randomised controlled trial. Lancet. 2017;390(10093):480-489.
11. Meltzer-Brody S, Colquhoun H, Riesenberg R, et al. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet. 2018;392(10152):1058-1070.
12. Melon LC, Hooper A, Yang X, et al. Inability to suppress the stress-induced activation of the HPA axis during the peripartum period engenders deficits in postpartum behaviors in mice. Psychoneuroendocrinology. 2018;90:182-193.
13. Deligiannidis KM, Fales CL, Kroll-Desrosiers AR, et al. Resting-state functional connectivity, cortical GABA, and neuroactive steroids in peripartum and peripartum depressed women: a functional magnetic resonance imaging and spectroscopy study. Neuropsychopharmacology. 2019;44(3):546-554.
14. Licheri V, Talani G, Gorule AA, et al. Plasticity of GABAA receptors during pregnancy and postpartum period: from gene to function. Neural Plast. 2015;2015:170435. doi: 10.1155/2015/170435.
15. Luisi S, Petraglia F, Benedetto C, et al. Serum allopregnanolone levels in pregnant women: changes during pregnancy, at delivery, and in hypertensive patients. J Clin Endocrinol Metab. 2000;85(7):2429-2433.
16. Hoffmann E, Wald J, Dray D, et al. Brexanolone injection administration to lactating women: breast milk allopregnanolone levels [30J]. Obstetrics & Gynecology. 2019;133:115S.
Postpartum depression (PPD) is one of the most prevalent complications associated with pregnancy and childbirth in the United States, affecting more than 400,000 women annually.1 Postpartum depression is most commonly treated with psychotherapy and antidepressants approved for the treatment of major depressive disorder. Until recently, there was no pharmacologic therapy approved by the FDA specifically for the treatment of PPD. Considering the adverse outcomes associated with untreated or inadequately treated PPD, and the limitations of existing therapies, there is a significant unmet need for pharmacologic treatment options for PPD.2 To help address this need, the FDA recently approved brexanolone injection (brand name: ZULRESSO™) (Table 13) as a first-in-class therapy for the treatment of adults with PPD.3
Clinical implications
Postpartum depression can result in adverse outcomes for the patient, baby, and family when under- or untreated, and the need for rapid resolution of symptoms cannot be overstated.2 Suicide is strongly associated with depression and is a leading cause of pregnancy-related deaths.4 Additionally, PPD can impact the health, safety, and well-being of the child, with both short- and long-term consequences, including greater rates of psychological or behavioral difficulties among children of patients with PPD.5 Postpartum depression can also have negative effects on the patient’s partner, with 24% to 50% of partners experiencing depression.6 Current PPD management strategies include the use of psychotherapy and pharmacologic interventions for major depressive disorder that may take up to 4 to 6 weeks for some patients, and may not achieve remission for all patients.7-9
Brexanolone injection is a first-in-class medication with a novel mechanism of action. In clinical studies, it achieved rapid (by Hour 60) and sustained (through Day 30) reductions in depressive symptoms and could provide a meaningful new treatment option for adult women with PPD.10,11
How it works
Animal and human studies have established the re
Brexanolone is a neuroactive steroid that is chemically identical to endogenous allopregnanolone produced in the CNS. Brexanolone potentiates GABA-mediated currents from recombinant human GABAARs in mammalian cells expressing α1β2γ2 receptor subunits, α4β3δ receptor subunits, and α6β3δ receptor subunits.3 Positive allosteric modulation of both synaptic and extrasynaptic GABAARs differentiates brexanolone from other GABAAR modulators, such as benzodiazepines.10,11
Brexanolone’s mechanism of action in the treatment of PPD is not fully understood, but it is thought to be related to GABAAR PAM activity.3
Supporting evidence
The FDA approval of brexanolone injection was based on the efficacy demonstrated in 2 Phase III multicenter, randomized, double-blind, placebo-controlled studies in adult women (age 18 to 45) with PPD (defined by DSM-IV criteria for a major depressive episode, with onset of symptoms in the third trimester or within 4 weeks of delivery). Exclusion criteria included the presence of bipolar disorder or psychosis. In these studies, 60-hour continuous IV infusions of brexanolone or placebo were given, followed by 4 weeks of observation. Study 1 (202B) enrolled patients with severe PPD (Hamilton Rating Scale for Depression [HAM-D] total score ≥26), and Study 2 (202C) enrolled patients with moderate PPD (HAM-D score 20 to 25). A titration to the recommended target dosage of 90 μg/kg/hour was evaluated in both studies. BRX90 patients received 30 μg/kg/hour for 4 hours, 60 μg/kg/hour for 20 hours, 90 μg/kg/hour for 28 hours, followed by a taper to 60 μg/kg/hour for 4 hours and then 30 μg/kg/hour for 4 hours. The primary endpoint in both studies was the mean change from baseline in depressive symptoms as measured by HAM-D total score at the end of the 60-hour infusion. A pre-specified secondary efficacy endpoint was the mean change from baseline in HAM-D total score at Day 30.
Continue to: Efficacy
Efficacy. In both placebo-controlled studies, titration to a target dose of brexanolone 90 μg/kg/hour was superior to placebo in improvement of depressive symptoms (Table 33).

Pharmacological profile
Brexanolone exposure-response relationships and the time course of pharmacodynamic response are unknown.3
Adverse reactions. Safety was evaluated from all patients receiving brexanolone injection, regardless of dosing regimen (N = 140, including patients from a Phase IIb study, 202A).3,11
The most common adverse reactions (incidence ≥5% and at least twice the rate of placebo) were sedation/somnolence, dry mouth, loss of consciousness, and flushing/hot flush.3 The incidence of patients discontinuing due to any adverse reaction was 2% for brexanolone vs 1% for placebo.3
Sedation, somnolence, and loss of consciousness. In clinical studies, brexanolone caused sedation and somnolence that required dose interruption or reduction in some patients during the infusion (5% of brexanolone-treated patients compared with 0% of placebo-treated patients).3 Some patients were also reported to have loss of consciousness or altered state of consciousness during the brexanolone infusion (4% of patients treated with brexanolone compared with 0% of patients treated with placebo).3 All patients with loss of or altered state of consciousness recovered fully 15 to 60 minutes after dose interruption.3 There was no clear association between loss or alteration of consciousness and pattern or timing of dose, and not all patients who experienced a loss or alteration of consciousness reported sedation or somnolence before the episode.
Continue to: Suicidality
Suicidality. The risk of developing suicidal thoughts and behaviors with brexanolone is unknown, due to the relatively low number of exposures to brexanolone injection during clinical development and a mechanism of action distinct from that of existing antidepressant medications.3
Pharmacokinetics
In clinical trials, brexanolone exhibited dose-proportional pharmacokinetics, and the terminal half-life is approximately 9 hours (Table 43). Brexanolone is metabolized by non-cytochrome P450 (CYP)-based pathways, including keto-reduction, glucuronidation, and sulfation.3 No clinically significant differences in the pharmacokinetics of brexanolone were observed based on renal or hepatic impairment, and no studies were conducted to evaluate the effects of other drugs on brexanolone.3
Lactation. A population pharmacokinetics model constructed from studies in the clinical development program calculated the maximum relative infant dose for brexanolone during infusion as 1.3%.3 Given the low oral bioavailability of brexanolone (<5%) in adults, the potential for breastfed infant exposure is considered low.3
Clinical considerations
Risk Evaluation and Mitigation Strategies (REMS) requirements. Brexanolone injection is a Schedule IV controlled substance. It has a “black-box” warning regarding excessive sedation and sudden loss of consciousness, which has been taken into account within the REMS drug safety program. Health care facilities and pharmacies must enroll in the REMS program and ensure that brexanolone is administered only to patients who are enrolled in the REMS program. Staff must be trained on the processes and procedures to administer brexanolone, and the facility must have a fall precautions protocol in place and be equipped with a programmable peristaltic IV infusion pump and continuous pulse oximetry with alarms.3
Monitoring. A REMS-trained clinician must be available continuously on-site to oversee each patient for the duration of the continuous IV infusion, which lasts 60 hours (2.5 days) and should be initiated early enough in the day to allow for recognition of excessive sedation. Patients must be monitored for hypoxia using continuous pulse oximetry equipped with an alarm and should also be assessed for excessive sedation every 2 hours during planned, non-sleep periods. If excessive sedation occurs, the infusion should be stopped until symptoms resolve, after which the infusion may be resumed at the same or a lower dose as clinically appropriate. In case of overdosage, the infusion should be stopped immediately and supportive measures initiated as necessary. Patients must not be the primary caregiver of dependents, and must be accompanied during interactions with their child(ren).
Continue to: Contraindications
Contraindications. There are no contraindications for the use of brexanolone in adults with PPD.
End-stage renal disease (ESRD). Avoid using brexanolone in patients with ESRD because of the potential accumulation of the solubilizing agent, betadex sulfobutyl ether sodium.
Pregnancy. Brexanolone has not been studied in pregnant patients. Pregnant women and women of reproductive age should be informed of the potential risk to a fetus based on data from other drugs that enhance GABAergic inhibition.
Breastfeeding. There are no data on the effects of brexanolone on a breastfed infant. Breastfeeding should be a discussion of risk and benefit between the patient and her doctor. The developmental and health benefits of breastfeeding should be considered, along with the mother’s clinical need for brexanolone and any potential adverse effects on the breastfed child from brexanolone or from the underlying maternal condition. However, based on the low relative infant dose (<2%) and the low oral bioavailability in adults, the risk to breastfed infants is thought to be low.16
Potential for abuse. Brexanolone injection is a Schedule IV controlled substance. Although it was not possible to assess physical dependency in the registrational trials due to dose tapering at the end of treatment, clinicians should advise patients about the theoretical possibility for brexanolone to be abused or lead to dependence based on other medications with similar primary pharmacology.
Continue to: Concomitant medications
Concomitant medications. Caution patients that taking opioids or other CNS depressants, such as benzodiazepines, in combination with brexanolone may increase the severity of sedative effects.
Suicidal thoughts and behaviors. Advise patients and caregivers to look for the emergence of suicidal thoughts and behavior and instruct them to report such symptoms to their clinician. Consider changing the therapeutic regimen, including discontinuing brexanolone, in patients whose depression becomes worse or who experience emergent suicidal thoughts and behaviors.
Why Rx?
Postpartum depression is a common and often devastating medical complication of childbirth that can result in adverse outcomes for the patient, baby, and family when left undertreated or untreated. There is a great need to identify and treat women who develop PPD. Rapid and sustained resolution of symptoms in women who experience PPD should be the goal of treatment, and consequently, brexanolone injection presents an important new tool in available treatment options for PPD.
Bottom Line
Brexanolone injection is a neuroactive steroid gamma-aminobutyric acid (GABA) A receptor positive allosteric modulator that’s been FDA-approved for the treatment of postpartum depression (PPD). It is administered as a continuous IV infusion over 60 hours. The rapid and sustained improvement of PPD observed in clinical trials with brexanolone injection may support a new treatment paradigm for women with PPD.
Postpartum depression (PPD) is one of the most prevalent complications associated with pregnancy and childbirth in the United States, affecting more than 400,000 women annually.1 Postpartum depression is most commonly treated with psychotherapy and antidepressants approved for the treatment of major depressive disorder. Until recently, there was no pharmacologic therapy approved by the FDA specifically for the treatment of PPD. Considering the adverse outcomes associated with untreated or inadequately treated PPD, and the limitations of existing therapies, there is a significant unmet need for pharmacologic treatment options for PPD.2 To help address this need, the FDA recently approved brexanolone injection (brand name: ZULRESSO™) (Table 13) as a first-in-class therapy for the treatment of adults with PPD.3
Clinical implications
Postpartum depression can result in adverse outcomes for the patient, baby, and family when under- or untreated, and the need for rapid resolution of symptoms cannot be overstated.2 Suicide is strongly associated with depression and is a leading cause of pregnancy-related deaths.4 Additionally, PPD can impact the health, safety, and well-being of the child, with both short- and long-term consequences, including greater rates of psychological or behavioral difficulties among children of patients with PPD.5 Postpartum depression can also have negative effects on the patient’s partner, with 24% to 50% of partners experiencing depression.6 Current PPD management strategies include the use of psychotherapy and pharmacologic interventions for major depressive disorder that may take up to 4 to 6 weeks for some patients, and may not achieve remission for all patients.7-9
Brexanolone injection is a first-in-class medication with a novel mechanism of action. In clinical studies, it achieved rapid (by Hour 60) and sustained (through Day 30) reductions in depressive symptoms and could provide a meaningful new treatment option for adult women with PPD.10,11
How it works
Animal and human studies have established the re
Brexanolone is a neuroactive steroid that is chemically identical to endogenous allopregnanolone produced in the CNS. Brexanolone potentiates GABA-mediated currents from recombinant human GABAARs in mammalian cells expressing α1β2γ2 receptor subunits, α4β3δ receptor subunits, and α6β3δ receptor subunits.3 Positive allosteric modulation of both synaptic and extrasynaptic GABAARs differentiates brexanolone from other GABAAR modulators, such as benzodiazepines.10,11
Brexanolone’s mechanism of action in the treatment of PPD is not fully understood, but it is thought to be related to GABAAR PAM activity.3
Supporting evidence
The FDA approval of brexanolone injection was based on the efficacy demonstrated in 2 Phase III multicenter, randomized, double-blind, placebo-controlled studies in adult women (age 18 to 45) with PPD (defined by DSM-IV criteria for a major depressive episode, with onset of symptoms in the third trimester or within 4 weeks of delivery). Exclusion criteria included the presence of bipolar disorder or psychosis. In these studies, 60-hour continuous IV infusions of brexanolone or placebo were given, followed by 4 weeks of observation. Study 1 (202B) enrolled patients with severe PPD (Hamilton Rating Scale for Depression [HAM-D] total score ≥26), and Study 2 (202C) enrolled patients with moderate PPD (HAM-D score 20 to 25). A titration to the recommended target dosage of 90 μg/kg/hour was evaluated in both studies. BRX90 patients received 30 μg/kg/hour for 4 hours, 60 μg/kg/hour for 20 hours, 90 μg/kg/hour for 28 hours, followed by a taper to 60 μg/kg/hour for 4 hours and then 30 μg/kg/hour for 4 hours. The primary endpoint in both studies was the mean change from baseline in depressive symptoms as measured by HAM-D total score at the end of the 60-hour infusion. A pre-specified secondary efficacy endpoint was the mean change from baseline in HAM-D total score at Day 30.
Continue to: Efficacy
Efficacy. In both placebo-controlled studies, titration to a target dose of brexanolone 90 μg/kg/hour was superior to placebo in improvement of depressive symptoms (Table 33).

Pharmacological profile
Brexanolone exposure-response relationships and the time course of pharmacodynamic response are unknown.3
Adverse reactions. Safety was evaluated from all patients receiving brexanolone injection, regardless of dosing regimen (N = 140, including patients from a Phase IIb study, 202A).3,11
The most common adverse reactions (incidence ≥5% and at least twice the rate of placebo) were sedation/somnolence, dry mouth, loss of consciousness, and flushing/hot flush.3 The incidence of patients discontinuing due to any adverse reaction was 2% for brexanolone vs 1% for placebo.3
Sedation, somnolence, and loss of consciousness. In clinical studies, brexanolone caused sedation and somnolence that required dose interruption or reduction in some patients during the infusion (5% of brexanolone-treated patients compared with 0% of placebo-treated patients).3 Some patients were also reported to have loss of consciousness or altered state of consciousness during the brexanolone infusion (4% of patients treated with brexanolone compared with 0% of patients treated with placebo).3 All patients with loss of or altered state of consciousness recovered fully 15 to 60 minutes after dose interruption.3 There was no clear association between loss or alteration of consciousness and pattern or timing of dose, and not all patients who experienced a loss or alteration of consciousness reported sedation or somnolence before the episode.
Continue to: Suicidality
Suicidality. The risk of developing suicidal thoughts and behaviors with brexanolone is unknown, due to the relatively low number of exposures to brexanolone injection during clinical development and a mechanism of action distinct from that of existing antidepressant medications.3
Pharmacokinetics
In clinical trials, brexanolone exhibited dose-proportional pharmacokinetics, and the terminal half-life is approximately 9 hours (Table 43). Brexanolone is metabolized by non-cytochrome P450 (CYP)-based pathways, including keto-reduction, glucuronidation, and sulfation.3 No clinically significant differences in the pharmacokinetics of brexanolone were observed based on renal or hepatic impairment, and no studies were conducted to evaluate the effects of other drugs on brexanolone.3
Lactation. A population pharmacokinetics model constructed from studies in the clinical development program calculated the maximum relative infant dose for brexanolone during infusion as 1.3%.3 Given the low oral bioavailability of brexanolone (<5%) in adults, the potential for breastfed infant exposure is considered low.3
Clinical considerations
Risk Evaluation and Mitigation Strategies (REMS) requirements. Brexanolone injection is a Schedule IV controlled substance. It has a “black-box” warning regarding excessive sedation and sudden loss of consciousness, which has been taken into account within the REMS drug safety program. Health care facilities and pharmacies must enroll in the REMS program and ensure that brexanolone is administered only to patients who are enrolled in the REMS program. Staff must be trained on the processes and procedures to administer brexanolone, and the facility must have a fall precautions protocol in place and be equipped with a programmable peristaltic IV infusion pump and continuous pulse oximetry with alarms.3
Monitoring. A REMS-trained clinician must be available continuously on-site to oversee each patient for the duration of the continuous IV infusion, which lasts 60 hours (2.5 days) and should be initiated early enough in the day to allow for recognition of excessive sedation. Patients must be monitored for hypoxia using continuous pulse oximetry equipped with an alarm and should also be assessed for excessive sedation every 2 hours during planned, non-sleep periods. If excessive sedation occurs, the infusion should be stopped until symptoms resolve, after which the infusion may be resumed at the same or a lower dose as clinically appropriate. In case of overdosage, the infusion should be stopped immediately and supportive measures initiated as necessary. Patients must not be the primary caregiver of dependents, and must be accompanied during interactions with their child(ren).
Continue to: Contraindications
Contraindications. There are no contraindications for the use of brexanolone in adults with PPD.
End-stage renal disease (ESRD). Avoid using brexanolone in patients with ESRD because of the potential accumulation of the solubilizing agent, betadex sulfobutyl ether sodium.
Pregnancy. Brexanolone has not been studied in pregnant patients. Pregnant women and women of reproductive age should be informed of the potential risk to a fetus based on data from other drugs that enhance GABAergic inhibition.
Breastfeeding. There are no data on the effects of brexanolone on a breastfed infant. Breastfeeding should be a discussion of risk and benefit between the patient and her doctor. The developmental and health benefits of breastfeeding should be considered, along with the mother’s clinical need for brexanolone and any potential adverse effects on the breastfed child from brexanolone or from the underlying maternal condition. However, based on the low relative infant dose (<2%) and the low oral bioavailability in adults, the risk to breastfed infants is thought to be low.16
Potential for abuse. Brexanolone injection is a Schedule IV controlled substance. Although it was not possible to assess physical dependency in the registrational trials due to dose tapering at the end of treatment, clinicians should advise patients about the theoretical possibility for brexanolone to be abused or lead to dependence based on other medications with similar primary pharmacology.
Continue to: Concomitant medications
Concomitant medications. Caution patients that taking opioids or other CNS depressants, such as benzodiazepines, in combination with brexanolone may increase the severity of sedative effects.
Suicidal thoughts and behaviors. Advise patients and caregivers to look for the emergence of suicidal thoughts and behavior and instruct them to report such symptoms to their clinician. Consider changing the therapeutic regimen, including discontinuing brexanolone, in patients whose depression becomes worse or who experience emergent suicidal thoughts and behaviors.
Why Rx?
Postpartum depression is a common and often devastating medical complication of childbirth that can result in adverse outcomes for the patient, baby, and family when left undertreated or untreated. There is a great need to identify and treat women who develop PPD. Rapid and sustained resolution of symptoms in women who experience PPD should be the goal of treatment, and consequently, brexanolone injection presents an important new tool in available treatment options for PPD.
Bottom Line
Brexanolone injection is a neuroactive steroid gamma-aminobutyric acid (GABA) A receptor positive allosteric modulator that’s been FDA-approved for the treatment of postpartum depression (PPD). It is administered as a continuous IV infusion over 60 hours. The rapid and sustained improvement of PPD observed in clinical trials with brexanolone injection may support a new treatment paradigm for women with PPD.
1. Ko JY, Rockhill KM, Tong VT, et al. Trends in postpartum depressive symptoms - 27 states, 2004, 2008, and 2012. MMWR Morb Mortal Wkly Rep. 2017;66(6):153-158.
2. Frieder A, Fersh M, Hainline R, et al. Pharmacotherapy of postpartum depression: current approaches and novel drug development. CNS Drugs. 2019;33(3):265-282.
3. Brexanolone injection [package insert]. Cambridge, MA: Sage Therapeutics, Inc.; 2019.
4. Bodnar-Deren S, Klipstein K, Fersh M, et al. Suicidal ideation during the postpartum period. J Womens Health (Larchmt). 2016;25(12):1219-1224.
5. Netsi E, Pearson RM, Murray L, et al. Association of persistent and severe postnatal depression with child outcomes. JAMA Psychiatry. 2018;75(3):247-253.
6. Goodman JH. Paternal postpartum depression, its relationship to maternal postpartum depression, and implications for family health. J Adv Nurs. 2004;45(1):26-35.
7. Gelenberg AJ, Freeman MP, Markowitz JC, et al; American Psychiatric Association Work Group on Major Depressive Disorder. Practice guidelines for the treatment of patients with major depressive disorder. 3rd ed. Washington, DC: American Psychiatric Association; 2010.
8. Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163(11):1905-1917.
9. Molyneaux E, Telesia LA, Henshaw C, et al. Antidepressants for preventing postnatal depression. Cochrane Database Syst Rev. 2018;4:CD004363.
10. Kanes S, Colquhoun H, Gunduz-Bruce H, et al. Brexanolone (SAGE-547 injection) in post-partum depression: a randomised controlled trial. Lancet. 2017;390(10093):480-489.
11. Meltzer-Brody S, Colquhoun H, Riesenberg R, et al. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet. 2018;392(10152):1058-1070.
12. Melon LC, Hooper A, Yang X, et al. Inability to suppress the stress-induced activation of the HPA axis during the peripartum period engenders deficits in postpartum behaviors in mice. Psychoneuroendocrinology. 2018;90:182-193.
13. Deligiannidis KM, Fales CL, Kroll-Desrosiers AR, et al. Resting-state functional connectivity, cortical GABA, and neuroactive steroids in peripartum and peripartum depressed women: a functional magnetic resonance imaging and spectroscopy study. Neuropsychopharmacology. 2019;44(3):546-554.
14. Licheri V, Talani G, Gorule AA, et al. Plasticity of GABAA receptors during pregnancy and postpartum period: from gene to function. Neural Plast. 2015;2015:170435. doi: 10.1155/2015/170435.
15. Luisi S, Petraglia F, Benedetto C, et al. Serum allopregnanolone levels in pregnant women: changes during pregnancy, at delivery, and in hypertensive patients. J Clin Endocrinol Metab. 2000;85(7):2429-2433.
16. Hoffmann E, Wald J, Dray D, et al. Brexanolone injection administration to lactating women: breast milk allopregnanolone levels [30J]. Obstetrics & Gynecology. 2019;133:115S.
1. Ko JY, Rockhill KM, Tong VT, et al. Trends in postpartum depressive symptoms - 27 states, 2004, 2008, and 2012. MMWR Morb Mortal Wkly Rep. 2017;66(6):153-158.
2. Frieder A, Fersh M, Hainline R, et al. Pharmacotherapy of postpartum depression: current approaches and novel drug development. CNS Drugs. 2019;33(3):265-282.
3. Brexanolone injection [package insert]. Cambridge, MA: Sage Therapeutics, Inc.; 2019.
4. Bodnar-Deren S, Klipstein K, Fersh M, et al. Suicidal ideation during the postpartum period. J Womens Health (Larchmt). 2016;25(12):1219-1224.
5. Netsi E, Pearson RM, Murray L, et al. Association of persistent and severe postnatal depression with child outcomes. JAMA Psychiatry. 2018;75(3):247-253.
6. Goodman JH. Paternal postpartum depression, its relationship to maternal postpartum depression, and implications for family health. J Adv Nurs. 2004;45(1):26-35.
7. Gelenberg AJ, Freeman MP, Markowitz JC, et al; American Psychiatric Association Work Group on Major Depressive Disorder. Practice guidelines for the treatment of patients with major depressive disorder. 3rd ed. Washington, DC: American Psychiatric Association; 2010.
8. Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163(11):1905-1917.
9. Molyneaux E, Telesia LA, Henshaw C, et al. Antidepressants for preventing postnatal depression. Cochrane Database Syst Rev. 2018;4:CD004363.
10. Kanes S, Colquhoun H, Gunduz-Bruce H, et al. Brexanolone (SAGE-547 injection) in post-partum depression: a randomised controlled trial. Lancet. 2017;390(10093):480-489.
11. Meltzer-Brody S, Colquhoun H, Riesenberg R, et al. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet. 2018;392(10152):1058-1070.
12. Melon LC, Hooper A, Yang X, et al. Inability to suppress the stress-induced activation of the HPA axis during the peripartum period engenders deficits in postpartum behaviors in mice. Psychoneuroendocrinology. 2018;90:182-193.
13. Deligiannidis KM, Fales CL, Kroll-Desrosiers AR, et al. Resting-state functional connectivity, cortical GABA, and neuroactive steroids in peripartum and peripartum depressed women: a functional magnetic resonance imaging and spectroscopy study. Neuropsychopharmacology. 2019;44(3):546-554.
14. Licheri V, Talani G, Gorule AA, et al. Plasticity of GABAA receptors during pregnancy and postpartum period: from gene to function. Neural Plast. 2015;2015:170435. doi: 10.1155/2015/170435.
15. Luisi S, Petraglia F, Benedetto C, et al. Serum allopregnanolone levels in pregnant women: changes during pregnancy, at delivery, and in hypertensive patients. J Clin Endocrinol Metab. 2000;85(7):2429-2433.
16. Hoffmann E, Wald J, Dray D, et al. Brexanolone injection administration to lactating women: breast milk allopregnanolone levels [30J]. Obstetrics & Gynecology. 2019;133:115S.