User login
Neuropsychological testing: A useful but underutilized resource
We have all treated a patient for whom you know you had the diagnosis correct, the medication regimen was working, and the patient adhered to treatment, but something was still “off.” There was something cognitively that wasn’t right, and you had identified subtle (and some overt) errors in the standard psychiatric cognitive assessment that didn’t seem amenable to psychotropic medications. Perhaps what was needed was neuropsychological testing, one of the most useful but underutilized resources available to help fine-tune diagnosis and treatment. Finding a neuropsychologist who is sensitive to the unique needs of patients with psychiatric disorders, and knowing what and how to communicate the clinical picture and need for the referral, can be challenging due to the limited availability, time, and cost of a full battery of standardized tests.
This article describes the purpose of neuropsychological testing, why it is an important part of psychiatry, and how to make the best use of it.
What is neuropsychological testing?
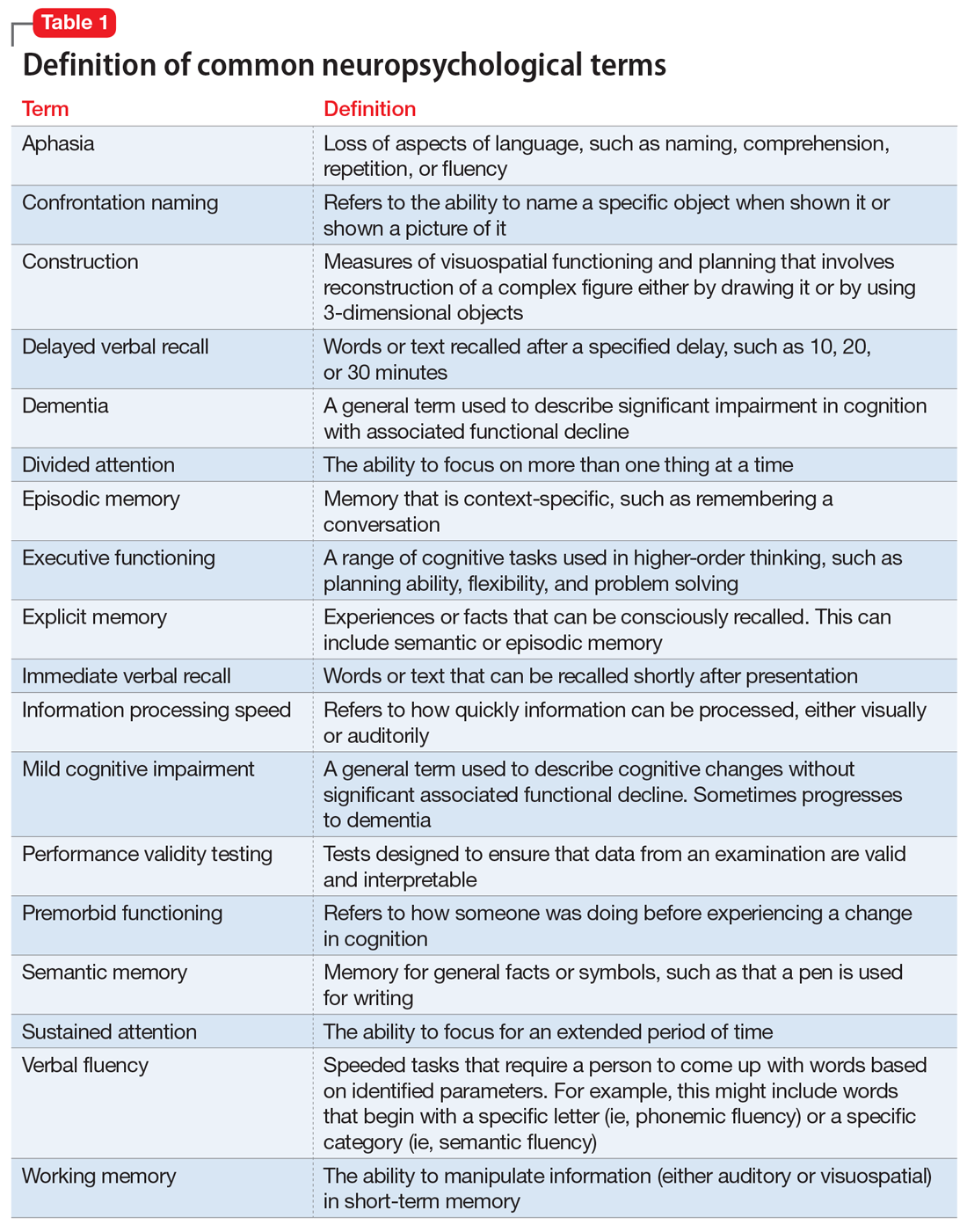
Neuropsychological testing is a comprehensive evaluation designed to assess cognitive functioning, such as attention, language, learning, memory, and visuospatial and executive functioning. Neuropsychology has its own vocabulary and lexicon that are important for psychiatric clinicians to understand. Some terms, such as aphasia, working memory, and dementia, are familiar to many clinicians. However, others, such as information processing speed, performance validity testing, and semantic memory, might not be. Common neuropsychological terms are defined in Table 1.
The neuropsychologist’s role
A neuropsychologist is a psychologist with advanced training in brain-behavior relationships who can help determine if cognitive problems are related to neurologic, medical, or psychiatric factors. A neuropsychological evaluation can identify the etiology of a patient’s cognitive difficulties, such as stroke, poorly controlled diabetes, or mental health symptoms, to help guide treatment. It can be difficult to determine if a patient who is experiencing significant cognitive, functional, or behavioral changes has an underlying cognitive disorder (eg, dementia or major neurocognitive disorder) or something else, such as a psychiatric condition. Indeed, many psychiatric conditions, including schizophrenia, bipolar disorder, posttraumatic stress disorder (PTSD), and major depressive disorder (MDD), can present with significant cognitive difficulties. Thus, when patients report an increase in forgetfulness or changes in their ability to care for themselves, neuropsychological testing can help determine the cause.
How to refer to a neuropsychologist
Developing a referral network with a neuropsychologist should be a component of establishing a psychiatric practice. A neuropsychologist can help identify deficits that may interfere with the patient’s ability to adhere to a treatment plan, monitor medications, or actively participate in treatment and therapy. When making a referral for neuropsychological testing, it is important to be clear about the specific concerns so the neuropsychologist knows how to best evaluate the patient. A psychiatric clinician does not order specific neuropsychological tests, but thoroughly describes the problem so the neuropsychologist can determine the appropriate tests after interviewing the patient. For example, if a patient reports memory problems, it is essential to give the neuropsychologist specific clinical data so he/she can determine if the symptoms are due to a neurodegenerative or psychiatric condition. Then, after interviewing the patient (and, possibly, a family member), the neuropsychologist can construct a battery of tests to best answer the question.
Which neuropsychological tests are available?
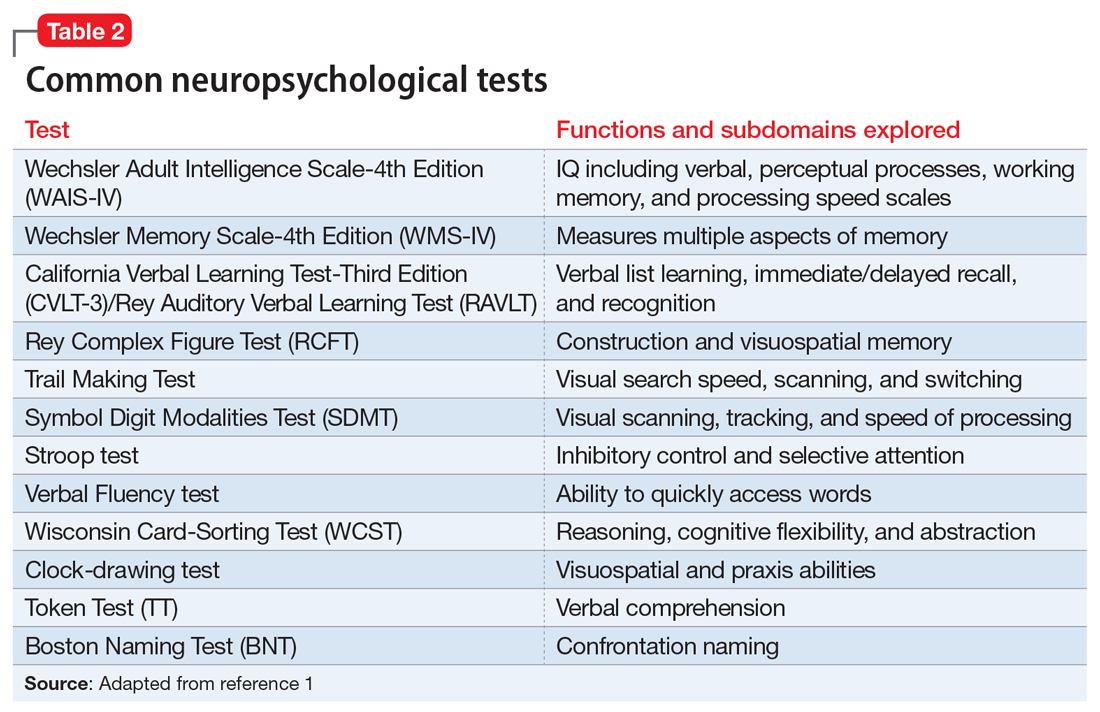
There is a large battery of neuropsychological tests that require a licensed psychologist to administer and interpret.1 Those commonly used in research and practice to differentiate neurologically-based cognitive deficits associated with psychiatric disorders include the Wechsler Adult Intelligence Scale-4th edition (WAIS-IV) for assessing intelligence, the California Verbal Learning Test-Third Edition (CVLT-3) for verbal memory and learning, the Brief Visuospatial Memory Test-Revised for visual memory, the Wisconsin Card Sorting Test (WCST) for executive functions, and the Ruff 2&7 Selective Attention Test for sustained attention.2 These and other commonly used tests are described in Table 2.1
Neuropsychological testing vs psychological testing
The neuropsychologist will use psychometric properties (such as the validity and reliability of the test) and available normative data to pick the most appropriate tests. To date, there are no specific tests that clearly delineate psychiatric from nonpsychiatric etiologies, although the Screen for Cognitive Impairment in Psychiatry (SCIP)3 was developed in 2013 to explore cognitive abilities in the functional psychoses; it is beginning to be used in other studies.4,5 The neuropsychologist will consider the patient’s current concerns, the onset and progression of these concerns, and the pattern in testing behavior to help determine if psychiatric conditions are the most likely etiology.
Continue to: In addition to cognitive tests...
In addition to cognitive tests, the neuropsychologist might also administer psychological tests. These might include commonly used screening tools such as the Patient Health Questionnaire-9 (PHQ-9)6 or Geriatric Depression Scale (GDS),7 or more comprehensive objective personality measures, such as the Minnesota Multiphasic Personality Inventory-2-Restructured Format (MMPI-2-RF)8 or Personality Assessment Inventory (PAI).9 These tests, along with a thorough clinical history, can help identify if a psychiatric condition is present. In addition, for the more extensive tests such as the MMPI-2-RF or PAI, there are certain neuropsychological profiles that are consistent with a psychiatric etiology for cognitive difficulties. These profiles are formulated based on specific test scores in combination with complex patient variables.
Understanding the report
While there will be stylistic differences in reports depending on the neuropsychologist’s setting, referral source, and personal preferences, most will include discussion of why the patient was referred for evaluation and a description of the onset and progression of the problem.10 Reports often also include pertinent medical and psychiatric history, substance use history, and family medical history. A section on social history is important to help establish premorbid functioning, and might include information about prenatal/birth complications, developmental milestones, educational history, and occupational history. Information about current psychosocial support or stressors, including marital status or current/past legal issues, can be helpful. In addition to this history, there is often a section on behavioral observations, especially if anything stood out or might have affected the validity of the data.
There are also objective measures of validity that the neuropsychologist might administer to evaluate whether the results are valid. Issues of validity are monitored through the evaluation, and are used to determine if the results are consistent with known neurologic patterns. If the results are deemed not valid, then low scores cannot be reliably interpreted as evidence of impairment. This is akin to an arm moving during an X-ray, thereby blurring the results. If valid, the results of objective testing are include in the neuropsychologist’s report; this can range from providing raw scores, standard scores, and/or percentiles to a general description of how the patient did on testing.
The section that is usually of most interest to psychiatric clinicians is the summary, which explains the results, might offer a diagnosis, and discusses possible etiologies. This might be where the neuropsychologist discusses if the findings are due to a neurologic or psychiatric condition. From this comes the neuropsychologist’s recommendations. When a psychiatric condition is determined to be the underlying etiology, the neuropsychologist might recommend psychotherapy or some other psychiatric treatment.
Why is neuropsychological testing important?
Schizophrenia, MDD, bipolar disorder, and PTSD produce significant neurobiologic changes that often result in deterioration of a patient’s global cognitive function. Increased emphasis and attention in psychiatric research have yielded more clues to the neurobiology of cognition. However, even though many psychiatric clinicians are trained in cognitive assessments, such as the “clock test,” “serial sevens,” “numbers forward and backward,” “proverb,” and “word recall,” and common scenarios to evaluate judgment and insight, such as “mailing a letter” and “smoke in a movie theatre,” most of these components are not completed during a standard psychiatric evaluation. Because the time allotted to completing a psychiatric evaluation continues to be shortened, it is sometimes difficult to complete the “6 bullets” required by the Centers for Medicare & Medicaid Services as part of the mental status exam (Table 311).
Continue to: To date, the best evidence...
To date, the best evidence for neuropsychological deficits exists for patients with schizophrenia, bipolar disorder, MDD, and PTSD.12,13 The Box2,14-24 describes the findings of studies of neuropsychological deficits in patients with schizophrenia and bipolar disorder.
Box
Patients with schizophrenia have been the subjects of neuropsychological testing for decades. The results have shown deficits on many standardized tests, including those of attention, memory, and executive functioning, although some patients might perform within normal limits.15
A federal initiative through the National Institute of Mental Health (NIMH) known as MATRICS (Measurement and Treatment Research to Improve Cognition in Schizophrenia) was developed in the late 1990s to develop consensus on the underlying cognitive deficits in schizophrenia. MATRICS was created with the hopes that it would allow the FDA to approve treatments for those cognitive deficits independent of psychosis because current psychotropic medications have minimal efficacy on cognition.16,17 The MATRICS group identified working memory, attention/vigilance, verbal learning and memory, visual learning and memory, speed of processing, reasoning and problem solving, and social cognition as the key cognitive domains most affected in schizophrenia.14 The initial program has since evolved into 3 distinct NIMH programs: CNTRICS18 (Cognitive Neuroscience Treatment Research to Improve Cognition in Schizophrenia), TURNS19 (Treatment Units for Research on Neurocognition in Schizophrenia), and TENETS20 (Treatment and Evaluation Network for Trials in Schizophrenia). The combination of neuropsychological testing and neuroimaging has led to the conceptualization of schizophrenia as a neurodevelopmental disorder.
Individuals at risk for psychosis
As clinicians, we have long heard from parents of children with schizophrenia a standard trajectory of functional decline: early premorbid changes, a fairly measurable prodromal period marked by subtle deterioration in cognitive functioning, followed by the actual illness trajectory. In a recent meta-analysis, researchers compared the results of 60 neuropsychological tests comprising 9 domains in people who were at clinical high risk for psychosis who eventually converted to a psychotic disorder (CHR-P), those at clinical high risk who did not convert to psychosis (CHR-NP), and healthy controls.21 They found that neuropsychological performance deficits were greater in CHR-P individuals than in those in the CHR-NP group, and both had greater deficits than healthy controls.
For many patients with schizophrenia, full cognitive maturation is never reached.22 In general, decreased motivation in schizophrenia has been correlated with neurocognitive deficits.23
Schizophrenia vs bipolar disorder
In a study comparing neuropsychological functioning in patients with schizophrenia and bipolar disorder with psychotic features (BP-P), researchers found greater deficits in schizophrenia, including immediate verbal recall, working memory, processing speed, and verbal fluency.22 Patients with BP-P demonstrated impairment consistent with generalized impairment in verbal learning and memory, working memory, and processing speed.22
Children/adolescents
In a recent study comparing child and adolescent offspring of patients with schizophrenia (n = 41) and bipolar disorder (n = 90), researchers identified neuropsychological deficits in visual memory for both groups, suggesting common genetic linkages. The schizophrenia offspring scored lower in verbal memory and word memory, while bipolar offspring scored lower on the processing speed index and visual memory.2
Information processing
Another study compared the results of neuropsychological testing and the P300 component of auditory event-related potential (an electrophysiological measure) in 30 patients with schizophrenia, siblings without illness, and normal controls.24 The battery of neuropsychological tests included the Digit Symbol Substitution Test, Digit Vigilance Test, Trail Making Test-B, and Stroop test. The P300 is well correlated with information processing. Researchers found decreased P300 amplitude and latency in the patients and normal levels in the controls; siblings scored somewhere in between.24 Scores on the neuropsychological tests were consistently below normal in both patients and their siblings, with patients scoring the lowest.24
Continue to: Neuropsychological testing
Neuropsychological testing: 2 Case studies
The following 2 cases illustrate the pivotal role of neuropsychological testing in formulating an accurate differential diagnosis, and facilitating improved outcomes.
Case 1
A veteran with PTSD and memory complaints
Mr. J, age 70, is a married man who spent his career in the military, including combat service in the Vietnam War. His service in Vietnam included an event in which he couldn’t save platoon members from an ambush and death in a firefight, after which he developed PTSD. He retired after 25 years of service.
Mr. J’s psychiatrist refers him to a neuropsychologist for complaints of memory difficulties, including a fear that he’s developing Alzheimer’s disease (AD). Because of the concern for AD, he undergoes tests of learning and memory, such as the CVLT-3, the Brief Visuospatial Memory Test-Revised, and the Logical Memory subtest from the Wechsler Memory Scale–4th Edition. Other tests include a measure of confrontation naming, verbal fluency (phonemic and semantic fluency), construction, attention, processing speed, and problem solving. In addition, a measure of psychiatric and emotional functioning is also administered (the MMPI-2-RF).
The results determined that Mr. J’s subjective experience of recall deficits is better explained by anxiety resulting from the cumulative impact of day-to-day emotional stress in the setting of chronic PTSD.25 Mr. J was experiencing cognitive sequelae from a complicated emotional dynamic, comprised of situational stress, amplified by coping difficulties that were rooted in older posttraumatic symptoms. These emotions, and the cognitive load they generated, interfered with the normal processes of attention and organization necessary for the encoding of information to be remembered.26 He described being visibly angered by the clutter in his home (the result of multiple people living there, including a young grandchild), having his efforts to get things done interrupted by the needs of others, and a perceived loss of control gradually generalized to even mundane circumstances, as often occurs with traumatic responses. In short, he was chronically overwhelmed and not experiencing the beginnings of dementia.
For Mr. J, neuropsychological testing helped define the focus and course of therapy. If he had been diagnosed with a major neurocognitive disorder, therapy might have taken a more acceptance and grief-based approach, to help him adjust to a chronic, potentially life-limiting condition. Because this diagnosis was ruled out, and his cognitive complaints were determined to be secondary to a core diagnosis of PTSD, therapy instead focused on treating PTSD.
Continue to: Case 2
Case 2
A 55-year-old with bipolar I disorder
Mr. S, age 55, is taken to the emergency department (ED) because of his complaints of a severe headache. While undergoing brain MRI, Mr. S becomes highly agitated and aggressive to the radiology staff and is transferred to the psychiatric inpatient unit. He has a history of bipolar disorder that was treated with lithium approximately 20 years ago. Due to continued agitation, he is transferred to the state hospital and prescribed multiple medications, including an unspecified first-generation antipsychotic (FGA) that results in drooling and causes him to stoop and shuffle.
Mr. S’s wife contacts a community psychiatrist after becoming frustrated by her inability to communicate with the staff at the state hospital. During a 1-hour consult, she reveals that Mr. S was a competitive speedboat racer and had suffered numerous concussions due to accidents; at least 3 of these concussions that occurred when he was in his 20s and 30s had included a loss of consciousness. Mr. S had always been treated in the ED, and never required hospitalization. He had a previous marriage, was estranged from his ex-wife and 3 children, and has a history of alcohol abuse.
The MRI taken in the ED reveals numerous patches of scar tissue throughout the cortex, most notably in the striatum areas. The psychiatrist suspects that Mr. S’s agitation and irritation were related to focal seizure activity. He encourages Mr. S’s wife to speak with the attending psychiatrist at the state hospital and ask for him to be discharged home under her care.
Eventually, Mr. S is referred for a neurologic consult and neuropsychological testing. The testing included measures of attention and working, learning and memory, and executive functioning. The results reveal numerous deficits that Mr. S had been able to compensate for when he was younger, including problems with recall of newly learned information and difficulty modifying his behavior according to feedback. Mr. S is weaned from high doses of the FGA and is stabilized on 2 antiepileptic agents, sertraline, and low-dose olanzapine. A rehabilitation plan is developed, and Mr. S remains out of the hospital.
A team-based approach
Psychiatric clinicians need to recognize the subtle as well as overt cognitive deficits present in patients with many of the illnesses that we treat on a daily basis. In this era of performance- and value-based care, it is important to understand the common neuropsychological tests available to assist in providing patient-centered care tailored to specific cognitive deficits. Including a neuropsychologist is essential to implementing a team-based approach.
Continue to: Bottom Line
Bottom Line
Neuropsychological testing can help pinpoint key cognitive deficits that interfere with the potential for optimal patient outcomes. Psychiatric clinicians need to be knowledgeable about the common tests used and how to incorporate the results into their diagnosis and treatment plans.
Related Resources
- The American Academy of Clinical Neuropsychology. www.theaacn.org.
- Schwarz L. Answers to 7 questions about using neuropsychological testing in your practice. Current Psychiatry. 2014;13(3):33-39.
Drug Brand Names
Lithium • Eskalith, Lithobid
Olanzapine • Zyprexa
Sertraline • Zoloft
1. Zucchella C, Federico A, Martini A, et al. Neuropsychological testing: how to understand it. Practical Neurology. 2018;18(3):227-237.
2. de la Serna E, Sugranyes G, Sanchez-Gistau V, et al. Neuropsychological characteristics of child and adolescent offspring of patients with schizophrenia or bipolar disorder. Schizophr Res. 2017;183:110-115.
3. Gómez-Benito J, Guilera G, Pino Ó, et al. The screen for cognitive impairment in psychiatry: diagnostic-specific standardization in psychiatric ill patients. BMC Psychiatry. 2013;13:127.
4. Fuente-Tomas L, Arranz B, Safont G, et al. Classification of patients with bipolar disorder using k-means clustering. PLoS One. 2019;14(1):e0210314.
5. Kronbichler L, Stelzig-Schöler R, Pearce BG, et al. Schizophrenia and category-selectivity in the brain: Normal for faces but abnormal for houses. Front Psychiatry. 2018;9:47.
6. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613.
7. Yesavage A, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1983;17(1):37-49.
8. Ben-Porath YS, Tellegen A. Minnesota multi-phasic personality inventory-2 restructured form: MMPI-2-RF. San Antonio, TX: NCS Pearson; 2008.
9. Morey LC. Personality assessment inventory. Odessa, FL: Psychological Assessment Resources; 1991.
10. Donder J, ed. Neuropsychological report writing. New York, NY: The Guilford Press; 2016.
11. Department of Health and Human Services, Centers for Medicare and Medicaid Services. Evaluation and management services. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/Downloads/eval-mgmt-serv-guide-ICN006764.pdf. Published August 2017. Accessed October 10, 2019.
12. Hunt S, Root JC, Bascetta BL. Effort testing in schizophrenia and schizoaffective disorder: validity indicator profile and test of memory malingering performance characteristics. Arch Clin Neuropsychol. 2014;29(2):164-172.
13. Gorlyn M, Keilp J, Burke A, et al. Treatment-related improvement in neuropsychological functioning in suicidal depressed patients: paroxetine vs. bupropion. Psychiatry Res. 2015;225(3):407-412.
14. Pettersson R, Söderström S, Nilsson KW. Diagnosing ADHD in adults: an examination of the discriminative validity of neuropsychological tests and diagnostic assessment instruments. J Atten Disord. 2018;22(11):1019-1031.
15. Urfer-Parnas, A, Mortensen EL, Parnas J. Core of schizophrenia: estrangement, dementia or neurocognitive disorder? Psychopathology. 2010;43(5):300-311.
16. Green MF, Nuechterlein KH, Gold JM, et al. Approaching a consensus cognitive battery for clinical trials in schizophrenia: the NIMH-MATRICS conference to select cognitive domains and test criteria. Biolog Psych. 2004;56(5):301-307.
17. Green MF, Nuechterlein KH. The MATRICS initiative: developing a consensus cognitive battery for clinical trials. Schizophr Res. 2004;72(1):1-3.
18. Kern RS, Green MF, Nuechterlein KH, et al. NIMH-MATRICS survey on assessment of neurocognition in schizophrenia. Schizophr Res. 2004;72(1):11-19.
19. Carter CS, Barch DM. Cognitive neuroscience-based approaches to measuring and improving treatment effects on cognition in schizophrenia: the CNTRICS initiative. Schizophr Bull. 2007;33(5):1131-1137.
20. Geyer M. New opportunities in the treatment of cognitive impairments associated with schizophrenia. Curr Dir Psych Sci. 2010;19(4):264-269.
21. Hauser M, Zhang JP, Sheridan EM, et al. Neuropsychological test performance to enhance identification of subjects at clinical high risk for psychosis and to be most promising for predictive algorithms for conversion to psychosis: a meta-analysis. J Clin Psych. 2017;78(1):e28-e40. doi: 10.4088/JCP.15r10197.
22. Menkes MW, Armstrong K, Blackford JU, et al. Neuropsychological functioning in early and chronic stages of schizophrenia and psychotic bipolar disorder. Schizophr Res. 2019;206:413-419.
23. Najas-Garcia A, Gomez-Benito J, Hueda-Medina T. The relationship of motivation and neurocognition with functionality in schizophrenia: a meta-analytic review. Community Ment Health J. 2018;54(7):1019-1049.
24. Raghavan DV, Shanmugiah A, Bharathi P, et al. P300 and neuropsychological measurements in patients with schizophrenia and their healthy biological siblings. Indian J Psychiatry. 2016;58(4):454-458.
25. Mozzambani A, Fuso S, Malta S, et al. Long-term follow-up of attentional and executive functions of PTSD patients. Psychol Neurosci. 2017;10(2):215-224.
26. Woon F, Farrer T, Braman C, et al A meta-analysis of the relationship between symptom severity of posttraumatic stress disorder and executive function. Cogn Neuropsychiatry. 2017;22(1):1-16.
We have all treated a patient for whom you know you had the diagnosis correct, the medication regimen was working, and the patient adhered to treatment, but something was still “off.” There was something cognitively that wasn’t right, and you had identified subtle (and some overt) errors in the standard psychiatric cognitive assessment that didn’t seem amenable to psychotropic medications. Perhaps what was needed was neuropsychological testing, one of the most useful but underutilized resources available to help fine-tune diagnosis and treatment. Finding a neuropsychologist who is sensitive to the unique needs of patients with psychiatric disorders, and knowing what and how to communicate the clinical picture and need for the referral, can be challenging due to the limited availability, time, and cost of a full battery of standardized tests.
This article describes the purpose of neuropsychological testing, why it is an important part of psychiatry, and how to make the best use of it.
What is neuropsychological testing?
Neuropsychological testing is a comprehensive evaluation designed to assess cognitive functioning, such as attention, language, learning, memory, and visuospatial and executive functioning. Neuropsychology has its own vocabulary and lexicon that are important for psychiatric clinicians to understand. Some terms, such as aphasia, working memory, and dementia, are familiar to many clinicians. However, others, such as information processing speed, performance validity testing, and semantic memory, might not be. Common neuropsychological terms are defined in Table 1.
The neuropsychologist’s role
A neuropsychologist is a psychologist with advanced training in brain-behavior relationships who can help determine if cognitive problems are related to neurologic, medical, or psychiatric factors. A neuropsychological evaluation can identify the etiology of a patient’s cognitive difficulties, such as stroke, poorly controlled diabetes, or mental health symptoms, to help guide treatment. It can be difficult to determine if a patient who is experiencing significant cognitive, functional, or behavioral changes has an underlying cognitive disorder (eg, dementia or major neurocognitive disorder) or something else, such as a psychiatric condition. Indeed, many psychiatric conditions, including schizophrenia, bipolar disorder, posttraumatic stress disorder (PTSD), and major depressive disorder (MDD), can present with significant cognitive difficulties. Thus, when patients report an increase in forgetfulness or changes in their ability to care for themselves, neuropsychological testing can help determine the cause.
How to refer to a neuropsychologist
Developing a referral network with a neuropsychologist should be a component of establishing a psychiatric practice. A neuropsychologist can help identify deficits that may interfere with the patient’s ability to adhere to a treatment plan, monitor medications, or actively participate in treatment and therapy. When making a referral for neuropsychological testing, it is important to be clear about the specific concerns so the neuropsychologist knows how to best evaluate the patient. A psychiatric clinician does not order specific neuropsychological tests, but thoroughly describes the problem so the neuropsychologist can determine the appropriate tests after interviewing the patient. For example, if a patient reports memory problems, it is essential to give the neuropsychologist specific clinical data so he/she can determine if the symptoms are due to a neurodegenerative or psychiatric condition. Then, after interviewing the patient (and, possibly, a family member), the neuropsychologist can construct a battery of tests to best answer the question.
Which neuropsychological tests are available?
There is a large battery of neuropsychological tests that require a licensed psychologist to administer and interpret.1 Those commonly used in research and practice to differentiate neurologically-based cognitive deficits associated with psychiatric disorders include the Wechsler Adult Intelligence Scale-4th edition (WAIS-IV) for assessing intelligence, the California Verbal Learning Test-Third Edition (CVLT-3) for verbal memory and learning, the Brief Visuospatial Memory Test-Revised for visual memory, the Wisconsin Card Sorting Test (WCST) for executive functions, and the Ruff 2&7 Selective Attention Test for sustained attention.2 These and other commonly used tests are described in Table 2.1
Neuropsychological testing vs psychological testing
The neuropsychologist will use psychometric properties (such as the validity and reliability of the test) and available normative data to pick the most appropriate tests. To date, there are no specific tests that clearly delineate psychiatric from nonpsychiatric etiologies, although the Screen for Cognitive Impairment in Psychiatry (SCIP)3 was developed in 2013 to explore cognitive abilities in the functional psychoses; it is beginning to be used in other studies.4,5 The neuropsychologist will consider the patient’s current concerns, the onset and progression of these concerns, and the pattern in testing behavior to help determine if psychiatric conditions are the most likely etiology.
Continue to: In addition to cognitive tests...
In addition to cognitive tests, the neuropsychologist might also administer psychological tests. These might include commonly used screening tools such as the Patient Health Questionnaire-9 (PHQ-9)6 or Geriatric Depression Scale (GDS),7 or more comprehensive objective personality measures, such as the Minnesota Multiphasic Personality Inventory-2-Restructured Format (MMPI-2-RF)8 or Personality Assessment Inventory (PAI).9 These tests, along with a thorough clinical history, can help identify if a psychiatric condition is present. In addition, for the more extensive tests such as the MMPI-2-RF or PAI, there are certain neuropsychological profiles that are consistent with a psychiatric etiology for cognitive difficulties. These profiles are formulated based on specific test scores in combination with complex patient variables.
Understanding the report
While there will be stylistic differences in reports depending on the neuropsychologist’s setting, referral source, and personal preferences, most will include discussion of why the patient was referred for evaluation and a description of the onset and progression of the problem.10 Reports often also include pertinent medical and psychiatric history, substance use history, and family medical history. A section on social history is important to help establish premorbid functioning, and might include information about prenatal/birth complications, developmental milestones, educational history, and occupational history. Information about current psychosocial support or stressors, including marital status or current/past legal issues, can be helpful. In addition to this history, there is often a section on behavioral observations, especially if anything stood out or might have affected the validity of the data.
There are also objective measures of validity that the neuropsychologist might administer to evaluate whether the results are valid. Issues of validity are monitored through the evaluation, and are used to determine if the results are consistent with known neurologic patterns. If the results are deemed not valid, then low scores cannot be reliably interpreted as evidence of impairment. This is akin to an arm moving during an X-ray, thereby blurring the results. If valid, the results of objective testing are include in the neuropsychologist’s report; this can range from providing raw scores, standard scores, and/or percentiles to a general description of how the patient did on testing.
The section that is usually of most interest to psychiatric clinicians is the summary, which explains the results, might offer a diagnosis, and discusses possible etiologies. This might be where the neuropsychologist discusses if the findings are due to a neurologic or psychiatric condition. From this comes the neuropsychologist’s recommendations. When a psychiatric condition is determined to be the underlying etiology, the neuropsychologist might recommend psychotherapy or some other psychiatric treatment.
Why is neuropsychological testing important?
Schizophrenia, MDD, bipolar disorder, and PTSD produce significant neurobiologic changes that often result in deterioration of a patient’s global cognitive function. Increased emphasis and attention in psychiatric research have yielded more clues to the neurobiology of cognition. However, even though many psychiatric clinicians are trained in cognitive assessments, such as the “clock test,” “serial sevens,” “numbers forward and backward,” “proverb,” and “word recall,” and common scenarios to evaluate judgment and insight, such as “mailing a letter” and “smoke in a movie theatre,” most of these components are not completed during a standard psychiatric evaluation. Because the time allotted to completing a psychiatric evaluation continues to be shortened, it is sometimes difficult to complete the “6 bullets” required by the Centers for Medicare & Medicaid Services as part of the mental status exam (Table 311).
Continue to: To date, the best evidence...
To date, the best evidence for neuropsychological deficits exists for patients with schizophrenia, bipolar disorder, MDD, and PTSD.12,13 The Box2,14-24 describes the findings of studies of neuropsychological deficits in patients with schizophrenia and bipolar disorder.
Box
Patients with schizophrenia have been the subjects of neuropsychological testing for decades. The results have shown deficits on many standardized tests, including those of attention, memory, and executive functioning, although some patients might perform within normal limits.15
A federal initiative through the National Institute of Mental Health (NIMH) known as MATRICS (Measurement and Treatment Research to Improve Cognition in Schizophrenia) was developed in the late 1990s to develop consensus on the underlying cognitive deficits in schizophrenia. MATRICS was created with the hopes that it would allow the FDA to approve treatments for those cognitive deficits independent of psychosis because current psychotropic medications have minimal efficacy on cognition.16,17 The MATRICS group identified working memory, attention/vigilance, verbal learning and memory, visual learning and memory, speed of processing, reasoning and problem solving, and social cognition as the key cognitive domains most affected in schizophrenia.14 The initial program has since evolved into 3 distinct NIMH programs: CNTRICS18 (Cognitive Neuroscience Treatment Research to Improve Cognition in Schizophrenia), TURNS19 (Treatment Units for Research on Neurocognition in Schizophrenia), and TENETS20 (Treatment and Evaluation Network for Trials in Schizophrenia). The combination of neuropsychological testing and neuroimaging has led to the conceptualization of schizophrenia as a neurodevelopmental disorder.
Individuals at risk for psychosis
As clinicians, we have long heard from parents of children with schizophrenia a standard trajectory of functional decline: early premorbid changes, a fairly measurable prodromal period marked by subtle deterioration in cognitive functioning, followed by the actual illness trajectory. In a recent meta-analysis, researchers compared the results of 60 neuropsychological tests comprising 9 domains in people who were at clinical high risk for psychosis who eventually converted to a psychotic disorder (CHR-P), those at clinical high risk who did not convert to psychosis (CHR-NP), and healthy controls.21 They found that neuropsychological performance deficits were greater in CHR-P individuals than in those in the CHR-NP group, and both had greater deficits than healthy controls.
For many patients with schizophrenia, full cognitive maturation is never reached.22 In general, decreased motivation in schizophrenia has been correlated with neurocognitive deficits.23
Schizophrenia vs bipolar disorder
In a study comparing neuropsychological functioning in patients with schizophrenia and bipolar disorder with psychotic features (BP-P), researchers found greater deficits in schizophrenia, including immediate verbal recall, working memory, processing speed, and verbal fluency.22 Patients with BP-P demonstrated impairment consistent with generalized impairment in verbal learning and memory, working memory, and processing speed.22
Children/adolescents
In a recent study comparing child and adolescent offspring of patients with schizophrenia (n = 41) and bipolar disorder (n = 90), researchers identified neuropsychological deficits in visual memory for both groups, suggesting common genetic linkages. The schizophrenia offspring scored lower in verbal memory and word memory, while bipolar offspring scored lower on the processing speed index and visual memory.2
Information processing
Another study compared the results of neuropsychological testing and the P300 component of auditory event-related potential (an electrophysiological measure) in 30 patients with schizophrenia, siblings without illness, and normal controls.24 The battery of neuropsychological tests included the Digit Symbol Substitution Test, Digit Vigilance Test, Trail Making Test-B, and Stroop test. The P300 is well correlated with information processing. Researchers found decreased P300 amplitude and latency in the patients and normal levels in the controls; siblings scored somewhere in between.24 Scores on the neuropsychological tests were consistently below normal in both patients and their siblings, with patients scoring the lowest.24
Continue to: Neuropsychological testing
Neuropsychological testing: 2 Case studies
The following 2 cases illustrate the pivotal role of neuropsychological testing in formulating an accurate differential diagnosis, and facilitating improved outcomes.
Case 1
A veteran with PTSD and memory complaints
Mr. J, age 70, is a married man who spent his career in the military, including combat service in the Vietnam War. His service in Vietnam included an event in which he couldn’t save platoon members from an ambush and death in a firefight, after which he developed PTSD. He retired after 25 years of service.
Mr. J’s psychiatrist refers him to a neuropsychologist for complaints of memory difficulties, including a fear that he’s developing Alzheimer’s disease (AD). Because of the concern for AD, he undergoes tests of learning and memory, such as the CVLT-3, the Brief Visuospatial Memory Test-Revised, and the Logical Memory subtest from the Wechsler Memory Scale–4th Edition. Other tests include a measure of confrontation naming, verbal fluency (phonemic and semantic fluency), construction, attention, processing speed, and problem solving. In addition, a measure of psychiatric and emotional functioning is also administered (the MMPI-2-RF).
The results determined that Mr. J’s subjective experience of recall deficits is better explained by anxiety resulting from the cumulative impact of day-to-day emotional stress in the setting of chronic PTSD.25 Mr. J was experiencing cognitive sequelae from a complicated emotional dynamic, comprised of situational stress, amplified by coping difficulties that were rooted in older posttraumatic symptoms. These emotions, and the cognitive load they generated, interfered with the normal processes of attention and organization necessary for the encoding of information to be remembered.26 He described being visibly angered by the clutter in his home (the result of multiple people living there, including a young grandchild), having his efforts to get things done interrupted by the needs of others, and a perceived loss of control gradually generalized to even mundane circumstances, as often occurs with traumatic responses. In short, he was chronically overwhelmed and not experiencing the beginnings of dementia.
For Mr. J, neuropsychological testing helped define the focus and course of therapy. If he had been diagnosed with a major neurocognitive disorder, therapy might have taken a more acceptance and grief-based approach, to help him adjust to a chronic, potentially life-limiting condition. Because this diagnosis was ruled out, and his cognitive complaints were determined to be secondary to a core diagnosis of PTSD, therapy instead focused on treating PTSD.
Continue to: Case 2
Case 2
A 55-year-old with bipolar I disorder
Mr. S, age 55, is taken to the emergency department (ED) because of his complaints of a severe headache. While undergoing brain MRI, Mr. S becomes highly agitated and aggressive to the radiology staff and is transferred to the psychiatric inpatient unit. He has a history of bipolar disorder that was treated with lithium approximately 20 years ago. Due to continued agitation, he is transferred to the state hospital and prescribed multiple medications, including an unspecified first-generation antipsychotic (FGA) that results in drooling and causes him to stoop and shuffle.
Mr. S’s wife contacts a community psychiatrist after becoming frustrated by her inability to communicate with the staff at the state hospital. During a 1-hour consult, she reveals that Mr. S was a competitive speedboat racer and had suffered numerous concussions due to accidents; at least 3 of these concussions that occurred when he was in his 20s and 30s had included a loss of consciousness. Mr. S had always been treated in the ED, and never required hospitalization. He had a previous marriage, was estranged from his ex-wife and 3 children, and has a history of alcohol abuse.
The MRI taken in the ED reveals numerous patches of scar tissue throughout the cortex, most notably in the striatum areas. The psychiatrist suspects that Mr. S’s agitation and irritation were related to focal seizure activity. He encourages Mr. S’s wife to speak with the attending psychiatrist at the state hospital and ask for him to be discharged home under her care.
Eventually, Mr. S is referred for a neurologic consult and neuropsychological testing. The testing included measures of attention and working, learning and memory, and executive functioning. The results reveal numerous deficits that Mr. S had been able to compensate for when he was younger, including problems with recall of newly learned information and difficulty modifying his behavior according to feedback. Mr. S is weaned from high doses of the FGA and is stabilized on 2 antiepileptic agents, sertraline, and low-dose olanzapine. A rehabilitation plan is developed, and Mr. S remains out of the hospital.
A team-based approach
Psychiatric clinicians need to recognize the subtle as well as overt cognitive deficits present in patients with many of the illnesses that we treat on a daily basis. In this era of performance- and value-based care, it is important to understand the common neuropsychological tests available to assist in providing patient-centered care tailored to specific cognitive deficits. Including a neuropsychologist is essential to implementing a team-based approach.
Continue to: Bottom Line
Bottom Line
Neuropsychological testing can help pinpoint key cognitive deficits that interfere with the potential for optimal patient outcomes. Psychiatric clinicians need to be knowledgeable about the common tests used and how to incorporate the results into their diagnosis and treatment plans.
Related Resources
- The American Academy of Clinical Neuropsychology. www.theaacn.org.
- Schwarz L. Answers to 7 questions about using neuropsychological testing in your practice. Current Psychiatry. 2014;13(3):33-39.
Drug Brand Names
Lithium • Eskalith, Lithobid
Olanzapine • Zyprexa
Sertraline • Zoloft
We have all treated a patient for whom you know you had the diagnosis correct, the medication regimen was working, and the patient adhered to treatment, but something was still “off.” There was something cognitively that wasn’t right, and you had identified subtle (and some overt) errors in the standard psychiatric cognitive assessment that didn’t seem amenable to psychotropic medications. Perhaps what was needed was neuropsychological testing, one of the most useful but underutilized resources available to help fine-tune diagnosis and treatment. Finding a neuropsychologist who is sensitive to the unique needs of patients with psychiatric disorders, and knowing what and how to communicate the clinical picture and need for the referral, can be challenging due to the limited availability, time, and cost of a full battery of standardized tests.
This article describes the purpose of neuropsychological testing, why it is an important part of psychiatry, and how to make the best use of it.
What is neuropsychological testing?
Neuropsychological testing is a comprehensive evaluation designed to assess cognitive functioning, such as attention, language, learning, memory, and visuospatial and executive functioning. Neuropsychology has its own vocabulary and lexicon that are important for psychiatric clinicians to understand. Some terms, such as aphasia, working memory, and dementia, are familiar to many clinicians. However, others, such as information processing speed, performance validity testing, and semantic memory, might not be. Common neuropsychological terms are defined in Table 1.
The neuropsychologist’s role
A neuropsychologist is a psychologist with advanced training in brain-behavior relationships who can help determine if cognitive problems are related to neurologic, medical, or psychiatric factors. A neuropsychological evaluation can identify the etiology of a patient’s cognitive difficulties, such as stroke, poorly controlled diabetes, or mental health symptoms, to help guide treatment. It can be difficult to determine if a patient who is experiencing significant cognitive, functional, or behavioral changes has an underlying cognitive disorder (eg, dementia or major neurocognitive disorder) or something else, such as a psychiatric condition. Indeed, many psychiatric conditions, including schizophrenia, bipolar disorder, posttraumatic stress disorder (PTSD), and major depressive disorder (MDD), can present with significant cognitive difficulties. Thus, when patients report an increase in forgetfulness or changes in their ability to care for themselves, neuropsychological testing can help determine the cause.
How to refer to a neuropsychologist
Developing a referral network with a neuropsychologist should be a component of establishing a psychiatric practice. A neuropsychologist can help identify deficits that may interfere with the patient’s ability to adhere to a treatment plan, monitor medications, or actively participate in treatment and therapy. When making a referral for neuropsychological testing, it is important to be clear about the specific concerns so the neuropsychologist knows how to best evaluate the patient. A psychiatric clinician does not order specific neuropsychological tests, but thoroughly describes the problem so the neuropsychologist can determine the appropriate tests after interviewing the patient. For example, if a patient reports memory problems, it is essential to give the neuropsychologist specific clinical data so he/she can determine if the symptoms are due to a neurodegenerative or psychiatric condition. Then, after interviewing the patient (and, possibly, a family member), the neuropsychologist can construct a battery of tests to best answer the question.
Which neuropsychological tests are available?
There is a large battery of neuropsychological tests that require a licensed psychologist to administer and interpret.1 Those commonly used in research and practice to differentiate neurologically-based cognitive deficits associated with psychiatric disorders include the Wechsler Adult Intelligence Scale-4th edition (WAIS-IV) for assessing intelligence, the California Verbal Learning Test-Third Edition (CVLT-3) for verbal memory and learning, the Brief Visuospatial Memory Test-Revised for visual memory, the Wisconsin Card Sorting Test (WCST) for executive functions, and the Ruff 2&7 Selective Attention Test for sustained attention.2 These and other commonly used tests are described in Table 2.1
Neuropsychological testing vs psychological testing
The neuropsychologist will use psychometric properties (such as the validity and reliability of the test) and available normative data to pick the most appropriate tests. To date, there are no specific tests that clearly delineate psychiatric from nonpsychiatric etiologies, although the Screen for Cognitive Impairment in Psychiatry (SCIP)3 was developed in 2013 to explore cognitive abilities in the functional psychoses; it is beginning to be used in other studies.4,5 The neuropsychologist will consider the patient’s current concerns, the onset and progression of these concerns, and the pattern in testing behavior to help determine if psychiatric conditions are the most likely etiology.
Continue to: In addition to cognitive tests...
In addition to cognitive tests, the neuropsychologist might also administer psychological tests. These might include commonly used screening tools such as the Patient Health Questionnaire-9 (PHQ-9)6 or Geriatric Depression Scale (GDS),7 or more comprehensive objective personality measures, such as the Minnesota Multiphasic Personality Inventory-2-Restructured Format (MMPI-2-RF)8 or Personality Assessment Inventory (PAI).9 These tests, along with a thorough clinical history, can help identify if a psychiatric condition is present. In addition, for the more extensive tests such as the MMPI-2-RF or PAI, there are certain neuropsychological profiles that are consistent with a psychiatric etiology for cognitive difficulties. These profiles are formulated based on specific test scores in combination with complex patient variables.
Understanding the report
While there will be stylistic differences in reports depending on the neuropsychologist’s setting, referral source, and personal preferences, most will include discussion of why the patient was referred for evaluation and a description of the onset and progression of the problem.10 Reports often also include pertinent medical and psychiatric history, substance use history, and family medical history. A section on social history is important to help establish premorbid functioning, and might include information about prenatal/birth complications, developmental milestones, educational history, and occupational history. Information about current psychosocial support or stressors, including marital status or current/past legal issues, can be helpful. In addition to this history, there is often a section on behavioral observations, especially if anything stood out or might have affected the validity of the data.
There are also objective measures of validity that the neuropsychologist might administer to evaluate whether the results are valid. Issues of validity are monitored through the evaluation, and are used to determine if the results are consistent with known neurologic patterns. If the results are deemed not valid, then low scores cannot be reliably interpreted as evidence of impairment. This is akin to an arm moving during an X-ray, thereby blurring the results. If valid, the results of objective testing are include in the neuropsychologist’s report; this can range from providing raw scores, standard scores, and/or percentiles to a general description of how the patient did on testing.
The section that is usually of most interest to psychiatric clinicians is the summary, which explains the results, might offer a diagnosis, and discusses possible etiologies. This might be where the neuropsychologist discusses if the findings are due to a neurologic or psychiatric condition. From this comes the neuropsychologist’s recommendations. When a psychiatric condition is determined to be the underlying etiology, the neuropsychologist might recommend psychotherapy or some other psychiatric treatment.
Why is neuropsychological testing important?
Schizophrenia, MDD, bipolar disorder, and PTSD produce significant neurobiologic changes that often result in deterioration of a patient’s global cognitive function. Increased emphasis and attention in psychiatric research have yielded more clues to the neurobiology of cognition. However, even though many psychiatric clinicians are trained in cognitive assessments, such as the “clock test,” “serial sevens,” “numbers forward and backward,” “proverb,” and “word recall,” and common scenarios to evaluate judgment and insight, such as “mailing a letter” and “smoke in a movie theatre,” most of these components are not completed during a standard psychiatric evaluation. Because the time allotted to completing a psychiatric evaluation continues to be shortened, it is sometimes difficult to complete the “6 bullets” required by the Centers for Medicare & Medicaid Services as part of the mental status exam (Table 311).
Continue to: To date, the best evidence...
To date, the best evidence for neuropsychological deficits exists for patients with schizophrenia, bipolar disorder, MDD, and PTSD.12,13 The Box2,14-24 describes the findings of studies of neuropsychological deficits in patients with schizophrenia and bipolar disorder.
Box
Patients with schizophrenia have been the subjects of neuropsychological testing for decades. The results have shown deficits on many standardized tests, including those of attention, memory, and executive functioning, although some patients might perform within normal limits.15
A federal initiative through the National Institute of Mental Health (NIMH) known as MATRICS (Measurement and Treatment Research to Improve Cognition in Schizophrenia) was developed in the late 1990s to develop consensus on the underlying cognitive deficits in schizophrenia. MATRICS was created with the hopes that it would allow the FDA to approve treatments for those cognitive deficits independent of psychosis because current psychotropic medications have minimal efficacy on cognition.16,17 The MATRICS group identified working memory, attention/vigilance, verbal learning and memory, visual learning and memory, speed of processing, reasoning and problem solving, and social cognition as the key cognitive domains most affected in schizophrenia.14 The initial program has since evolved into 3 distinct NIMH programs: CNTRICS18 (Cognitive Neuroscience Treatment Research to Improve Cognition in Schizophrenia), TURNS19 (Treatment Units for Research on Neurocognition in Schizophrenia), and TENETS20 (Treatment and Evaluation Network for Trials in Schizophrenia). The combination of neuropsychological testing and neuroimaging has led to the conceptualization of schizophrenia as a neurodevelopmental disorder.
Individuals at risk for psychosis
As clinicians, we have long heard from parents of children with schizophrenia a standard trajectory of functional decline: early premorbid changes, a fairly measurable prodromal period marked by subtle deterioration in cognitive functioning, followed by the actual illness trajectory. In a recent meta-analysis, researchers compared the results of 60 neuropsychological tests comprising 9 domains in people who were at clinical high risk for psychosis who eventually converted to a psychotic disorder (CHR-P), those at clinical high risk who did not convert to psychosis (CHR-NP), and healthy controls.21 They found that neuropsychological performance deficits were greater in CHR-P individuals than in those in the CHR-NP group, and both had greater deficits than healthy controls.
For many patients with schizophrenia, full cognitive maturation is never reached.22 In general, decreased motivation in schizophrenia has been correlated with neurocognitive deficits.23
Schizophrenia vs bipolar disorder
In a study comparing neuropsychological functioning in patients with schizophrenia and bipolar disorder with psychotic features (BP-P), researchers found greater deficits in schizophrenia, including immediate verbal recall, working memory, processing speed, and verbal fluency.22 Patients with BP-P demonstrated impairment consistent with generalized impairment in verbal learning and memory, working memory, and processing speed.22
Children/adolescents
In a recent study comparing child and adolescent offspring of patients with schizophrenia (n = 41) and bipolar disorder (n = 90), researchers identified neuropsychological deficits in visual memory for both groups, suggesting common genetic linkages. The schizophrenia offspring scored lower in verbal memory and word memory, while bipolar offspring scored lower on the processing speed index and visual memory.2
Information processing
Another study compared the results of neuropsychological testing and the P300 component of auditory event-related potential (an electrophysiological measure) in 30 patients with schizophrenia, siblings without illness, and normal controls.24 The battery of neuropsychological tests included the Digit Symbol Substitution Test, Digit Vigilance Test, Trail Making Test-B, and Stroop test. The P300 is well correlated with information processing. Researchers found decreased P300 amplitude and latency in the patients and normal levels in the controls; siblings scored somewhere in between.24 Scores on the neuropsychological tests were consistently below normal in both patients and their siblings, with patients scoring the lowest.24
Continue to: Neuropsychological testing
Neuropsychological testing: 2 Case studies
The following 2 cases illustrate the pivotal role of neuropsychological testing in formulating an accurate differential diagnosis, and facilitating improved outcomes.
Case 1
A veteran with PTSD and memory complaints
Mr. J, age 70, is a married man who spent his career in the military, including combat service in the Vietnam War. His service in Vietnam included an event in which he couldn’t save platoon members from an ambush and death in a firefight, after which he developed PTSD. He retired after 25 years of service.
Mr. J’s psychiatrist refers him to a neuropsychologist for complaints of memory difficulties, including a fear that he’s developing Alzheimer’s disease (AD). Because of the concern for AD, he undergoes tests of learning and memory, such as the CVLT-3, the Brief Visuospatial Memory Test-Revised, and the Logical Memory subtest from the Wechsler Memory Scale–4th Edition. Other tests include a measure of confrontation naming, verbal fluency (phonemic and semantic fluency), construction, attention, processing speed, and problem solving. In addition, a measure of psychiatric and emotional functioning is also administered (the MMPI-2-RF).
The results determined that Mr. J’s subjective experience of recall deficits is better explained by anxiety resulting from the cumulative impact of day-to-day emotional stress in the setting of chronic PTSD.25 Mr. J was experiencing cognitive sequelae from a complicated emotional dynamic, comprised of situational stress, amplified by coping difficulties that were rooted in older posttraumatic symptoms. These emotions, and the cognitive load they generated, interfered with the normal processes of attention and organization necessary for the encoding of information to be remembered.26 He described being visibly angered by the clutter in his home (the result of multiple people living there, including a young grandchild), having his efforts to get things done interrupted by the needs of others, and a perceived loss of control gradually generalized to even mundane circumstances, as often occurs with traumatic responses. In short, he was chronically overwhelmed and not experiencing the beginnings of dementia.
For Mr. J, neuropsychological testing helped define the focus and course of therapy. If he had been diagnosed with a major neurocognitive disorder, therapy might have taken a more acceptance and grief-based approach, to help him adjust to a chronic, potentially life-limiting condition. Because this diagnosis was ruled out, and his cognitive complaints were determined to be secondary to a core diagnosis of PTSD, therapy instead focused on treating PTSD.
Continue to: Case 2
Case 2
A 55-year-old with bipolar I disorder
Mr. S, age 55, is taken to the emergency department (ED) because of his complaints of a severe headache. While undergoing brain MRI, Mr. S becomes highly agitated and aggressive to the radiology staff and is transferred to the psychiatric inpatient unit. He has a history of bipolar disorder that was treated with lithium approximately 20 years ago. Due to continued agitation, he is transferred to the state hospital and prescribed multiple medications, including an unspecified first-generation antipsychotic (FGA) that results in drooling and causes him to stoop and shuffle.
Mr. S’s wife contacts a community psychiatrist after becoming frustrated by her inability to communicate with the staff at the state hospital. During a 1-hour consult, she reveals that Mr. S was a competitive speedboat racer and had suffered numerous concussions due to accidents; at least 3 of these concussions that occurred when he was in his 20s and 30s had included a loss of consciousness. Mr. S had always been treated in the ED, and never required hospitalization. He had a previous marriage, was estranged from his ex-wife and 3 children, and has a history of alcohol abuse.
The MRI taken in the ED reveals numerous patches of scar tissue throughout the cortex, most notably in the striatum areas. The psychiatrist suspects that Mr. S’s agitation and irritation were related to focal seizure activity. He encourages Mr. S’s wife to speak with the attending psychiatrist at the state hospital and ask for him to be discharged home under her care.
Eventually, Mr. S is referred for a neurologic consult and neuropsychological testing. The testing included measures of attention and working, learning and memory, and executive functioning. The results reveal numerous deficits that Mr. S had been able to compensate for when he was younger, including problems with recall of newly learned information and difficulty modifying his behavior according to feedback. Mr. S is weaned from high doses of the FGA and is stabilized on 2 antiepileptic agents, sertraline, and low-dose olanzapine. A rehabilitation plan is developed, and Mr. S remains out of the hospital.
A team-based approach
Psychiatric clinicians need to recognize the subtle as well as overt cognitive deficits present in patients with many of the illnesses that we treat on a daily basis. In this era of performance- and value-based care, it is important to understand the common neuropsychological tests available to assist in providing patient-centered care tailored to specific cognitive deficits. Including a neuropsychologist is essential to implementing a team-based approach.
Continue to: Bottom Line
Bottom Line
Neuropsychological testing can help pinpoint key cognitive deficits that interfere with the potential for optimal patient outcomes. Psychiatric clinicians need to be knowledgeable about the common tests used and how to incorporate the results into their diagnosis and treatment plans.
Related Resources
- The American Academy of Clinical Neuropsychology. www.theaacn.org.
- Schwarz L. Answers to 7 questions about using neuropsychological testing in your practice. Current Psychiatry. 2014;13(3):33-39.
Drug Brand Names
Lithium • Eskalith, Lithobid
Olanzapine • Zyprexa
Sertraline • Zoloft
1. Zucchella C, Federico A, Martini A, et al. Neuropsychological testing: how to understand it. Practical Neurology. 2018;18(3):227-237.
2. de la Serna E, Sugranyes G, Sanchez-Gistau V, et al. Neuropsychological characteristics of child and adolescent offspring of patients with schizophrenia or bipolar disorder. Schizophr Res. 2017;183:110-115.
3. Gómez-Benito J, Guilera G, Pino Ó, et al. The screen for cognitive impairment in psychiatry: diagnostic-specific standardization in psychiatric ill patients. BMC Psychiatry. 2013;13:127.
4. Fuente-Tomas L, Arranz B, Safont G, et al. Classification of patients with bipolar disorder using k-means clustering. PLoS One. 2019;14(1):e0210314.
5. Kronbichler L, Stelzig-Schöler R, Pearce BG, et al. Schizophrenia and category-selectivity in the brain: Normal for faces but abnormal for houses. Front Psychiatry. 2018;9:47.
6. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613.
7. Yesavage A, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1983;17(1):37-49.
8. Ben-Porath YS, Tellegen A. Minnesota multi-phasic personality inventory-2 restructured form: MMPI-2-RF. San Antonio, TX: NCS Pearson; 2008.
9. Morey LC. Personality assessment inventory. Odessa, FL: Psychological Assessment Resources; 1991.
10. Donder J, ed. Neuropsychological report writing. New York, NY: The Guilford Press; 2016.
11. Department of Health and Human Services, Centers for Medicare and Medicaid Services. Evaluation and management services. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/Downloads/eval-mgmt-serv-guide-ICN006764.pdf. Published August 2017. Accessed October 10, 2019.
12. Hunt S, Root JC, Bascetta BL. Effort testing in schizophrenia and schizoaffective disorder: validity indicator profile and test of memory malingering performance characteristics. Arch Clin Neuropsychol. 2014;29(2):164-172.
13. Gorlyn M, Keilp J, Burke A, et al. Treatment-related improvement in neuropsychological functioning in suicidal depressed patients: paroxetine vs. bupropion. Psychiatry Res. 2015;225(3):407-412.
14. Pettersson R, Söderström S, Nilsson KW. Diagnosing ADHD in adults: an examination of the discriminative validity of neuropsychological tests and diagnostic assessment instruments. J Atten Disord. 2018;22(11):1019-1031.
15. Urfer-Parnas, A, Mortensen EL, Parnas J. Core of schizophrenia: estrangement, dementia or neurocognitive disorder? Psychopathology. 2010;43(5):300-311.
16. Green MF, Nuechterlein KH, Gold JM, et al. Approaching a consensus cognitive battery for clinical trials in schizophrenia: the NIMH-MATRICS conference to select cognitive domains and test criteria. Biolog Psych. 2004;56(5):301-307.
17. Green MF, Nuechterlein KH. The MATRICS initiative: developing a consensus cognitive battery for clinical trials. Schizophr Res. 2004;72(1):1-3.
18. Kern RS, Green MF, Nuechterlein KH, et al. NIMH-MATRICS survey on assessment of neurocognition in schizophrenia. Schizophr Res. 2004;72(1):11-19.
19. Carter CS, Barch DM. Cognitive neuroscience-based approaches to measuring and improving treatment effects on cognition in schizophrenia: the CNTRICS initiative. Schizophr Bull. 2007;33(5):1131-1137.
20. Geyer M. New opportunities in the treatment of cognitive impairments associated with schizophrenia. Curr Dir Psych Sci. 2010;19(4):264-269.
21. Hauser M, Zhang JP, Sheridan EM, et al. Neuropsychological test performance to enhance identification of subjects at clinical high risk for psychosis and to be most promising for predictive algorithms for conversion to psychosis: a meta-analysis. J Clin Psych. 2017;78(1):e28-e40. doi: 10.4088/JCP.15r10197.
22. Menkes MW, Armstrong K, Blackford JU, et al. Neuropsychological functioning in early and chronic stages of schizophrenia and psychotic bipolar disorder. Schizophr Res. 2019;206:413-419.
23. Najas-Garcia A, Gomez-Benito J, Hueda-Medina T. The relationship of motivation and neurocognition with functionality in schizophrenia: a meta-analytic review. Community Ment Health J. 2018;54(7):1019-1049.
24. Raghavan DV, Shanmugiah A, Bharathi P, et al. P300 and neuropsychological measurements in patients with schizophrenia and their healthy biological siblings. Indian J Psychiatry. 2016;58(4):454-458.
25. Mozzambani A, Fuso S, Malta S, et al. Long-term follow-up of attentional and executive functions of PTSD patients. Psychol Neurosci. 2017;10(2):215-224.
26. Woon F, Farrer T, Braman C, et al A meta-analysis of the relationship between symptom severity of posttraumatic stress disorder and executive function. Cogn Neuropsychiatry. 2017;22(1):1-16.
1. Zucchella C, Federico A, Martini A, et al. Neuropsychological testing: how to understand it. Practical Neurology. 2018;18(3):227-237.
2. de la Serna E, Sugranyes G, Sanchez-Gistau V, et al. Neuropsychological characteristics of child and adolescent offspring of patients with schizophrenia or bipolar disorder. Schizophr Res. 2017;183:110-115.
3. Gómez-Benito J, Guilera G, Pino Ó, et al. The screen for cognitive impairment in psychiatry: diagnostic-specific standardization in psychiatric ill patients. BMC Psychiatry. 2013;13:127.
4. Fuente-Tomas L, Arranz B, Safont G, et al. Classification of patients with bipolar disorder using k-means clustering. PLoS One. 2019;14(1):e0210314.
5. Kronbichler L, Stelzig-Schöler R, Pearce BG, et al. Schizophrenia and category-selectivity in the brain: Normal for faces but abnormal for houses. Front Psychiatry. 2018;9:47.
6. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613.
7. Yesavage A, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1983;17(1):37-49.
8. Ben-Porath YS, Tellegen A. Minnesota multi-phasic personality inventory-2 restructured form: MMPI-2-RF. San Antonio, TX: NCS Pearson; 2008.
9. Morey LC. Personality assessment inventory. Odessa, FL: Psychological Assessment Resources; 1991.
10. Donder J, ed. Neuropsychological report writing. New York, NY: The Guilford Press; 2016.
11. Department of Health and Human Services, Centers for Medicare and Medicaid Services. Evaluation and management services. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/Downloads/eval-mgmt-serv-guide-ICN006764.pdf. Published August 2017. Accessed October 10, 2019.
12. Hunt S, Root JC, Bascetta BL. Effort testing in schizophrenia and schizoaffective disorder: validity indicator profile and test of memory malingering performance characteristics. Arch Clin Neuropsychol. 2014;29(2):164-172.
13. Gorlyn M, Keilp J, Burke A, et al. Treatment-related improvement in neuropsychological functioning in suicidal depressed patients: paroxetine vs. bupropion. Psychiatry Res. 2015;225(3):407-412.
14. Pettersson R, Söderström S, Nilsson KW. Diagnosing ADHD in adults: an examination of the discriminative validity of neuropsychological tests and diagnostic assessment instruments. J Atten Disord. 2018;22(11):1019-1031.
15. Urfer-Parnas, A, Mortensen EL, Parnas J. Core of schizophrenia: estrangement, dementia or neurocognitive disorder? Psychopathology. 2010;43(5):300-311.
16. Green MF, Nuechterlein KH, Gold JM, et al. Approaching a consensus cognitive battery for clinical trials in schizophrenia: the NIMH-MATRICS conference to select cognitive domains and test criteria. Biolog Psych. 2004;56(5):301-307.
17. Green MF, Nuechterlein KH. The MATRICS initiative: developing a consensus cognitive battery for clinical trials. Schizophr Res. 2004;72(1):1-3.
18. Kern RS, Green MF, Nuechterlein KH, et al. NIMH-MATRICS survey on assessment of neurocognition in schizophrenia. Schizophr Res. 2004;72(1):11-19.
19. Carter CS, Barch DM. Cognitive neuroscience-based approaches to measuring and improving treatment effects on cognition in schizophrenia: the CNTRICS initiative. Schizophr Bull. 2007;33(5):1131-1137.
20. Geyer M. New opportunities in the treatment of cognitive impairments associated with schizophrenia. Curr Dir Psych Sci. 2010;19(4):264-269.
21. Hauser M, Zhang JP, Sheridan EM, et al. Neuropsychological test performance to enhance identification of subjects at clinical high risk for psychosis and to be most promising for predictive algorithms for conversion to psychosis: a meta-analysis. J Clin Psych. 2017;78(1):e28-e40. doi: 10.4088/JCP.15r10197.
22. Menkes MW, Armstrong K, Blackford JU, et al. Neuropsychological functioning in early and chronic stages of schizophrenia and psychotic bipolar disorder. Schizophr Res. 2019;206:413-419.
23. Najas-Garcia A, Gomez-Benito J, Hueda-Medina T. The relationship of motivation and neurocognition with functionality in schizophrenia: a meta-analytic review. Community Ment Health J. 2018;54(7):1019-1049.
24. Raghavan DV, Shanmugiah A, Bharathi P, et al. P300 and neuropsychological measurements in patients with schizophrenia and their healthy biological siblings. Indian J Psychiatry. 2016;58(4):454-458.
25. Mozzambani A, Fuso S, Malta S, et al. Long-term follow-up of attentional and executive functions of PTSD patients. Psychol Neurosci. 2017;10(2):215-224.
26. Woon F, Farrer T, Braman C, et al A meta-analysis of the relationship between symptom severity of posttraumatic stress disorder and executive function. Cogn Neuropsychiatry. 2017;22(1):1-16.
Bipolar disorder or borderline personality disorder?
Although evidence suggests that bipolar disorder (BD) and borderline personality disorder (BPD) are distinct entities, their differential diagnosis is often challenging as a result of considerable overlap of phenotypical features. Moreover, BD and BPD frequently co-occur, which makes it even more difficult to differentiate these 2 conditions. Strategies for improving diagnostic accuracy are critical to optimizing patients’ clinical outcomes and long-term prognosis. Misdiagnosing these 2 conditions can be particularly deleterious, and failure to recognize their co-occurrence can result in additional burden to typically complex and severe clinical presentations.
This article describes key aspects of the differential diagnosis between BD and BPD, emphasizing core features and major dissimilarities between these 2 conditions, and discusses the implications of misdiagnosis. The goal is to highlight the clinical and psychopathological aspects of BD and BPD to help clinicians properly distinguish these 2 disorders.
Psychopathological and sociodemographic correlates
Bipolar disorder is a chronic and severe mental illness that is classified based on clusters of symptoms—manic, hypomanic, and depressive.1 It is among the 10 leading causes of disability worldwide, with significant morbidity arising from acute affective episodes and subacute states.2 Data suggest the lifetime prevalence of BPD is 2.1%, and subthreshold forms may affect an additional 2.4% of the US population.3 The onset of symptoms typically occurs during late adolescence or early adulthood, and mood lability and cyclothymic temperament are the most common prodromal features.4
In contrast, personality disorders, such as BPD, are characteristically pervasive and maladaptive patterns of emotional responses that usually deviate from an individual’s stage of development and cultural background.1 These disorders tend to cause significant impairment, particularly in personal, occupational, and social domains. Environmental factors, such as early childhood trauma, seem to play an important role in the genesis of personality disorders, which may be particularly relevant in BPD, a disorder characterized by marked impulsivity and a pattern of instability in personal relationships, self-image, and affect.1,5,6 Similarly to BD, BPD is also chronic and highly disabling.
According to the National Survey on Alcohol and Related Conditions (NESARC), approximately 15% of US adults were found to have at least one type of personality disorder, and 6% met criteria for a cluster B personality disorder (antisocial, borderline, narcissistic, and histrionic).7 The lifetime prevalence of BPD is nearly 2%, with higher estimates observed in psychiatric settings.7,8
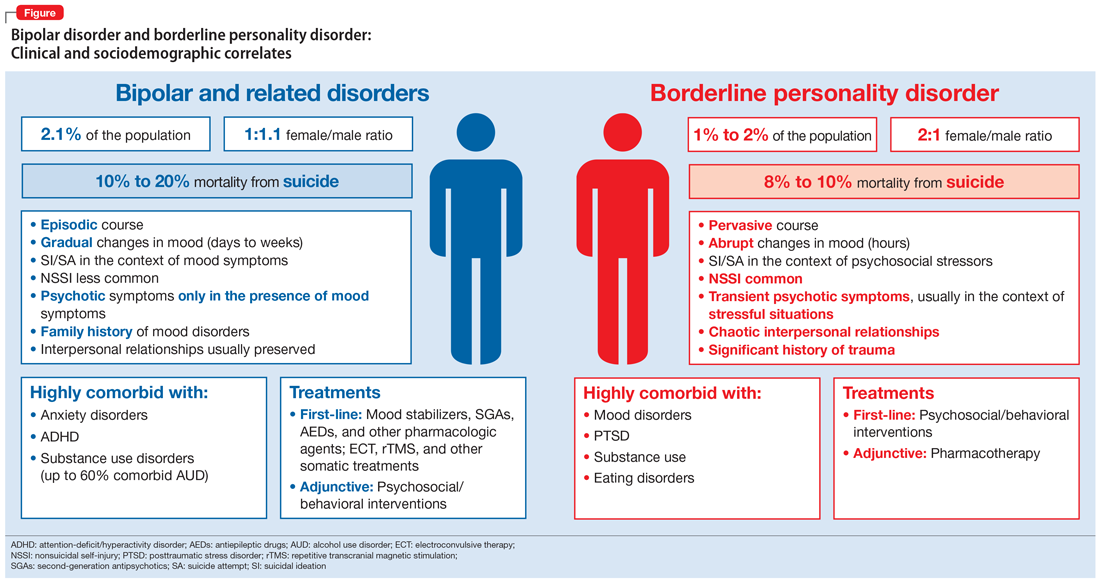
As a result of the phenotypical resemblance between BD and BPD (Figure), the differential diagnosis is often difficult. Recent studies suggest that co-occurrence of BD and BPD is common, with rates of comorbid BPD as high as 29% in BD I and 24% in BD II.8,9 On the other hand, nearly 20% of individuals with BPD seem to have comorbid BD.8,9 Several studies suggest that comorbid personality disorders represent a negative prognostic factor in the course of mood disorders, and the presence of BPD in patients with BD seems to be associated with more severe clinical presentations, greater treatment complexity, a higher number of depressive episodes, poor inter-episode functioning, and higher rates of other comorbidities, such as substance use disorders (SUDs).8-11 The effect of BD on the course of BPD is unclear and fairly unexplored, although it has been suggested that better control of mood symptoms may lead to more stable psychosocial functioning in BPD.9
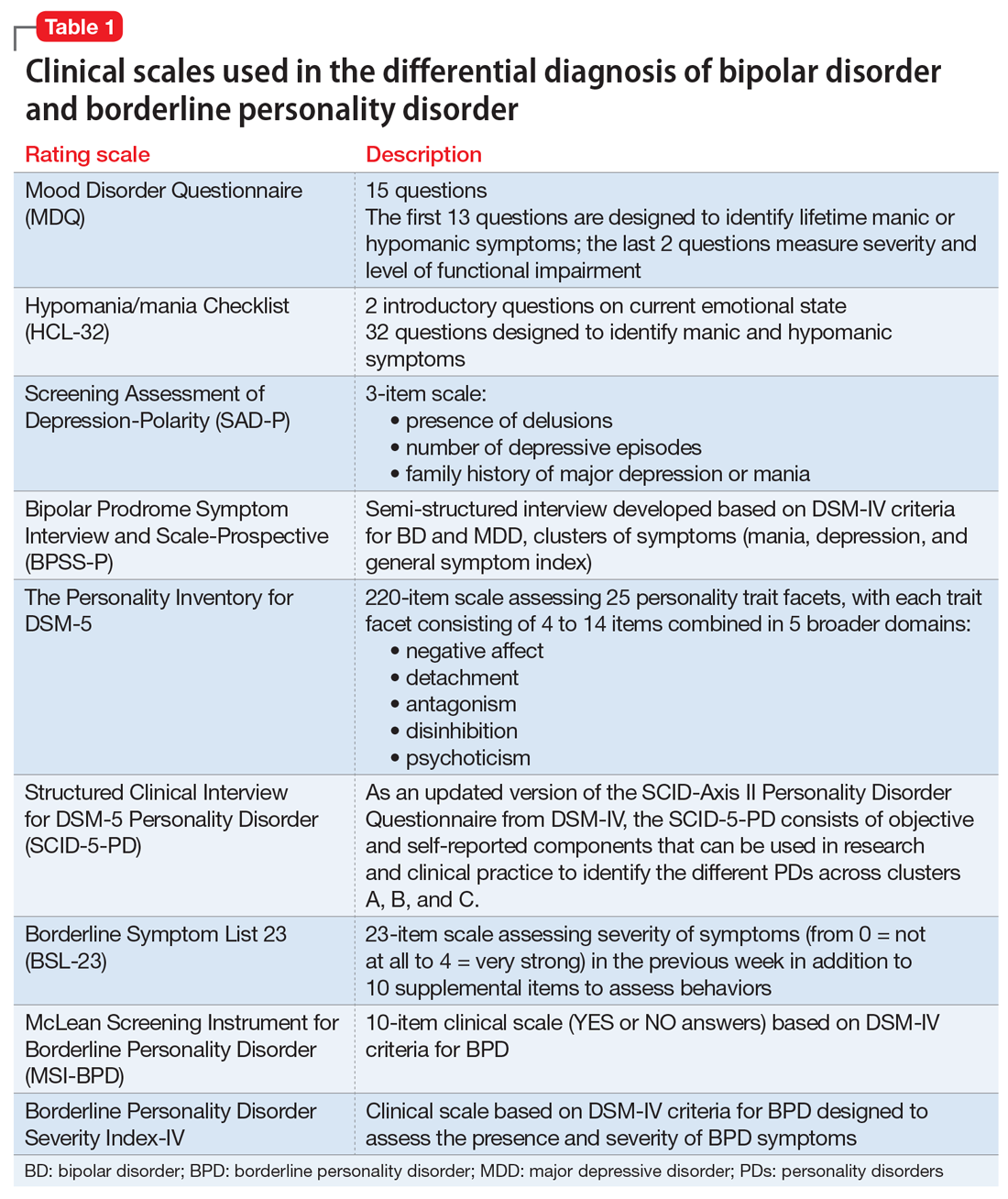
Whether BD and BPD are part of the same spectrum is a matter for debate.12-14 Multidimensional approaches have been proposed to better characterize these disorders in at-risk populations, based on structured interviews, self-administered and clinician-rated clinical scales (Table 1), neuroimaging studies, biological markers, and machine-learning models.15,16 Compelling evidence suggests that BD and BPD have distinct underlying neurobiological and psychopathological mechanisms12,13; however, the differential diagnosis still relies on phenotypical features, since the search for biological markers has not yet identified specific biomarkers that can be used in clinical practice.
Continue to: Core features of BPD...
Core features of BPD, such as mood lability, impulsivity, and risk-taking behaviors, are also part of the diagnostic criteria for BD (Table 2).1 Similarly, depressive symptoms prevail in the course of BD.17,18 This adds complexity to the differential because “depressivity” is also part of the diagnostic criteria for BPD.1 Therefore, comprehensive psychiatric assessments and longitudinal observations are critical to diagnostic accuracy and treatment planning. Further characterization of symptoms, such as onset patterns, clinical course, phenomenology of symptoms (eg, timing, frequency, duration, triggers), and personality traits, will provide information to properly distinguish these 2 syndromes when, for example, it is unclear if the “mood swings” and impulsivity are part of a mood or a personality disorder (Table 3).
Clinical features: A closer look
Borderline personality disorder. Affect dysregulation, emotional instability, impoverished and unstable self-image, and chronic feelings of emptiness are core features of BPD.1,5,19 These characteristics, when combined with a fear of abandonment or rejection, a compromised ability to recognize the feelings and needs of others, and extremes of idealization-devaluation, tend to culminate in problematic and chaotic relationships.6,19 Individuals with BPD may become suspicious or paranoid under stressful situations. Under these circumstances, individuals with BPD may also experience depersonalization and other dissociative symptoms.6,20 The mood lability and emotional instability observed in patients with BPD usually are in response to environmental factors, and although generally intense and out of proportion, they tend to be ephemeral and short-lived, typically lasting a few hours.1,5 The anxiety and depressive symptoms reported by patients with BPD frequently are associated with feelings of “falling apart” or “losing control,” pessimism, shame, and low self-esteem. Coping strategies tend to be poorly developed and/or maladaptive, and individuals with BPD usually display a hostile and antagonistic demeanor and engage in suicidal or nonsuicidal self-injury (NSSI) behaviors as means to alleviate overwhelming emotional distress. Impulsivity, disinhibition, poor tolerance to frustration, and risk-taking behaviors are also characteristic of BPD.1,5 As a result, BPD is usually associated with significant impairment in functioning, multiple hospitalizations, and high rates of comorbid mood disorders, posttraumatic stress disorder (PTSD), SUDs, and death by suicide.
Bipolar disorder. Conversely, the fluctuations in mood and affect observed in patients with BD are usually episodic rather than pervasive, and tend to last longer (typically days to weeks) compared with the transient mood shifts observed in patients with BPD.4,17,18 The impulsivity, psychomotor agitation, and increased goal-directed activity reported by patients with BD are usually seen in the context of an acute affective episode, and are far less common during periods of stability or euthymic affect.4,17,18 Grandiosity and inflated self-esteem—hallmarks of a manic or hypomanic state—seem to oppose the unstable self-image observed in BPD, although indecisiveness and low self-worth may be observed in individuals with BD during depressive episodes. Antidepressant-induced mania or hypomania, atypical depressive episodes, and disruptions in sleep and circadian rhythms may be predictors of BD.4,21 Furthermore, although psychosocial stressors may be associated with acute affective episodes in early stages of bipolar illness, over time minimal stressors are necessary to ignite new affective episodes.22,23 Although BD is associated with high rates of suicide, suicide attempts are usually seen in the context of an acute depressive episode, and NSSI behaviors are less common among patients with BD.24
Lastly, other biographical data, such as a history of early life trauma, comorbidity, and a family history of psychiatric illnesses, can be particularly helpful in establishing the differential diagnosis between BD and BPD.25 For instance, evidence suggests that the heritability of BD may be as high as 70%, which usually translates into an extensive family history of bipolar and related disorders.26 In addition, studies suggest a high co-occurrence of anxiety disorders, attention-deficit/hyperactivity disorder, and SUDs in patients with BD, whereas PTSD, SUDs, and eating disorders tend to be highly comorbid with BPD.27 Childhood adversity (ie, a history of physical, sexual, or emotional abuse, or neglect) seems to be pivotal in the genesis of BPD and may predispose these individuals to psychotic and dissociative symptoms, particularly those with a history of sexual abuse, while playing a more secondary role in BD.28-31
Implications of misdiagnosis
In the view of the limitations of the existing models, multidimensional approaches are necessary to improve diagnostic accuracy. Presently, the differential diagnosis of BD and BPD continues to rely on clinical findings and syndromic classifications. Misdiagnosing BD and BPD has adverse therapeutic and prognostic implications.32 For instance, while psychotropic medications and neuromodulatory therapies (eg, electroconvulsive therapy, repetitive transcranial magnetic stimulation) are considered first-line treatments for patients with BD, psychosocial interventions tend to be adjunctive treatments in BD.33 Conversely, although pharmacotherapy might be helpful for patients with BPD, psychosocial and behavioral interventions are the mainstay treatment for this disorder, with the strongest evidence supporting cognitive-behavioral therapy, dialectical behavioral therapy, mentalization-based therapy, and transference-focused therapy.34-36 Thus, misdiagnosing BD as BPD with comorbid depression may result in the use of antidepressants, which can be detrimental in BD. Antidepressant treatment of BD, particularly as monotherapy, has been associated with manic or hypomanic switch, mixed states, and frequent cycling.21 Moreover, delays in diagnosis and proper treatment of BD may result in protracted mood symptoms, prolonged affective episodes, higher rates of disability, functional impairment, and overall worse clinical outcomes.24 In addition, because behavioral and psychosocial interventions are usually adjunctive therapies rather than first-line interventions for patients with BD, misdiagnosing BPD as BD may ultimately prevent these individuals from receiving proper treatment, likely resulting in more severe functional impairment, multiple hospitalizations, self-inflicted injuries, and suicide attempts, since psychotropic medications are not particularly effective for improving self-efficacy and coping strategies, nor for correcting cognitive distortions, particularly in self-image, and pathological personality traits, all of which are critical aspects of BPD treatment.
Continue to: Several factors might...
Several factors might make clinicians reluctant to diagnose BPD, or bias them to diagnose BD more frequently. These include a lack of familiarity with the diagnostic criteria for BPD, the phenotypical resemblance between BP and BPD, or even concerns about the stigma and negative implications that are associated with a BPD diagnosis.32,37,38
Whereas BD is currently perceived as a condition with a strong biological basis, there are considerable misconceptions regarding BPD and its nature.4-6,22,26 As a consequence, individuals with BPD tend to be perceived as “difficult-to-treat,” “uncooperative,” or “attention-seeking.” These misconceptions may result in poor clinician-patient relationships, unmet clinical and psychiatric needs, and frustration for both clinicians and patients.37
Through advances in biological psychiatry, precision medicine may someday be a part of psychiatric practice. Biological “signatures” may eventually help clinicians in diagnosing and treating psychiatric disorders. Presently, however, rigorous history-taking and comprehensive clinical assessments are still the most powerful tools a clinician can use to accomplish these goals. Finally, destigmatizing psychiatric disorders and educating patients and clinicians are also critical to improving clinical outcomes and promoting mental health in a compassionate and empathetic fashion.
Bottom Line
Despite the phenotypical resemblance between bipolar disorder (BP) and borderline personality disorder (BPD), the 2 are independent conditions with distinct neurobiological and psychopathological underpinnings. Clinicians can use a rigorous assessment of pathological personality traits and characterization of symptoms, such as onset patterns, clinical course, and phenomenology, to properly distinguish between BP and BPD.
Related Resources
- Fiedorowicz JG, Black DW. Borderline, bipolar, or both? Frame your diagnosis on the patient history. Current Psychiatry. 2010; 9(1):21-24,29-32.
- Zimmerman M. Improving the recognition of borderline personality disorder. Current Psychiatry. 2017;16(10):13-19.
- National Institute of Mental Health. Overview on bipolar disorder. www.nimh.nih.gov/health/publications/bipolar-disorder/index.shtml. Revised October 2018.
- National Institute of Mental Health. Overview on borderline personality disorder. www.nimh.nih.gov/health/publications/borderline-personality-disorder-fact-sheet/index.shtml.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Whiteford HA, Degenhardt L, Rehm J, et al. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet. 2013;382(9904):1575-1586.
3. Merikangas KR, Akiskal HS, Angst J, et al. Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey replication. Arch Gen Psychiatry. 2007;64(5):543-552.
4. Malhi GS, Bargh DM, Coulston CM, et al. Predicting bipolar disorder on the basis of phenomenology: implications for prevention and early intervention. Bipolar Disord. 2014;16(5):455-470.
5. Skodol AE, Gunderson JG, Pfohl B, et al. The borderline diagnosis I: psychopathology. Biol Psychiatry. 2002;51(12):936-950.
6. Skodol AE, Siever LJ, Livesley WJ, et al. The borderline diagnosis II: biology, genetics, and clinical course. Biol Psychiatry. 2002;51(12):951-963.
7. Hasin DS, Grant BF. The National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) Waves 1 and 2: review and summary of findings. Soc Psychiatry Psychiatr Epidemiol. 2015;50(11):1609-1640.
8. McDermid J, Sareen J, El-Gabalawy R, et al. Co-morbidity of bipolar disorder and borderline personality disorder: findings from the National Epidemiologic Survey on Alcohol and Related Conditions. Compr Psychiatry. 2015;58:18-28.
9. Gunderson JG, Weinberg I, Daversa MT, et al. Descriptive and longitudinal observations on the relationship of borderline personality disorder and bipolar disorder. Am J Psychiatry. 2006;163(7):1173-1178.
10. Swartz HA, Pilkonis PA, Frank E, et al. Acute treatment outcomes in patients with bipolar I disorder and co-morbid borderline personality disorder receiving medication and psychotherapy. Bipolar Disord. 2005;7(2):192-197.
11. Riemann G, Weisscher N, Post RM, et al. The relationship between self-reported borderline personality features and prospective illness course in bipolar disorder. Int J Bipolar Disord. 2017;5(1):31.
12. de la Rosa I, Oquendo MA, García G, et al. Determining if borderline personality disorder and bipolar disorder are alternative expressions of the same disorder. J Clin Psychiatry. 2017;778(8):e994-e999. doi: 10.4088/JCP.16m11190.
13. di Giacomo E, Aspesi F, Fotiadou M, et al. Unblending borderline personality and bipolar disorders. J Psychiatr Res. 2017;91:90-97.
14. Parker G, Bayes A, McClure G, et al. Clinical status of comorbid bipolar disorder and borderline personality disorder. Br J Psychiatry. 2016;209(3):209-215.
15. Perez Arribas I, Goodwin GM, Geddes JR, et al. A signature-based machine learning model for distinguishing bipolar disorder and borderline personality disorder. Transl Psychiatry. 2018;8(1):274.
16. Insel T, Cuthbert B, Garvey M, et al. Research Domain Criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167(7):748-751.
17. Judd LL, Akiskal HS, Schettler PJ, et al. The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry. 2002;59(6):530-537.
18. Judd LL, Akiskal HS, Schettler PJ, et al. A prospective investigation of the natural history of the long-term weekly symptomatic status of bipolar II disorder. Arch Gen Psychiatry. 2003;60(3):261-269.
19. Oldham JM, Skodol AE, Bender DS. A current integrative perspective on personality disorders. American Psychiatric Publishing, Inc. 2005.
20. Herzog JI, Schmahl C. Adverse childhood experiences and the consequences on neurobiological, psychosocial, and somatic conditions across the lifespan. Front Psychiatry. 2018;9:420.
21. Barbuti M, Pacchiarotti I, Vieta E, et al. Antidepressant-induced hypomania/mania in patients with major depression: evidence from the BRIDGE-II-MIX study. J Affect Disord. 2017;219:187-192.
22. Post RM. Mechanisms of illness progression in the recurrent affective disorders. Neurotox Res. 2010;18(3-4):256-271.
23. da Costa SC, Passos IC, Lowri C, et al. Refractory bipolar disorder and neuroprogression. Prog Neuro-Psychopharmacology Biol Psychiatry. 2016;70:103-110.
24. Crump C, Sundquist K, Winkleby MA, et al. Comorbidities and mortality in bipolar disorder: a Swedish national cohort study. JAMA Psychiatry. 2013;70(9):931-939.
25. Zimmerman M, Martinez JH, Morgan TA, et al. Distinguishing bipolar II depression from major depressive disorder with comorbid borderline personality disorder: demographic, clinical, and family history differences. J Clin Psychiatry. 2013;74(9):880-886.
26. Hasler G, Drevets WC, Gould TD, et al. Toward constructing an endophenotype strategy for bipolar disorders. Biol Psychiatry. 2006;60(2):93-105.
27. Brieger P, Ehrt U, Marneros A. Frequency of comorbid personality disorders in bipolar and unipolar affective disorders. Compr Psychiatry. 2003;44(1):28-34.
28. Leverich GS, McElroy SL, Suppes T, et al. Early physical and sexual abuse associated with an adverse course of bipolar illness. Biol Psychiatry. 2002;51(4):288-297.
29. Leverich GS, Post RM. Course of bipolar illness after history of childhood trauma. Lancet. 2006;367(9516):1040-1042.
30. Golier JA, Yehuda R, Bierer LM, et al. The relationship of borderline personality disorder to posttraumatic stress disorder and traumatic events. Am J Psychiatry. 2003;160(11):2018-2024.
31. Nicol K, Pope M, Romaniuk L, et al. Childhood trauma, midbrain activation and psychotic symptoms in borderline personality disorder. Transl Psychiatry. 2015;5:e559. doi:10.1038/tp.2015.53.
32. Ruggero CJ, Zimmerman M, Chelminski I, et al. Borderline personality disorder and the misdiagnosis of bipolar disorder. J Psychiatr Res. 2010;44(6):405-408.
33. Geddes JR, Miklowitz DJ. Treatment of bipolar disorder. Lancet. 2013;381(9878):1672-1682.
34. McMain S, Korman LM, Dimeff L. Dialectical behavior therapy and the treatment of emotion dysregulation. J Clin Psychol. 2001;57(2):183-196.
35. Cristea IA, Gentili C, Cotet CD, et al. Efficacy of psychotherapies for borderline personality disorder: a systematic review and meta-analysis. JAMA Psychiatry. 2017;74(4):319-328.
36. Linehan MM, Korslund KE, Harned MS, et al. Dialectical behavior therapy for high suicide risk in individuals with borderline personality disorder. JAMA Psychiatry. 2015;72(75);475-482.
37. LeQuesne ER, Hersh RG. Disclosure of a diagnosis of borderline personality disorder. J Psychiatr Pract. 2004:10(3):170-176.
38. Young AH. Bipolar disorder: diagnostic conundrums and associated comorbidities. J Clin Psychiatry. 2009;70(8):e26. doi:10.4088/jcp.7067br6c.
Although evidence suggests that bipolar disorder (BD) and borderline personality disorder (BPD) are distinct entities, their differential diagnosis is often challenging as a result of considerable overlap of phenotypical features. Moreover, BD and BPD frequently co-occur, which makes it even more difficult to differentiate these 2 conditions. Strategies for improving diagnostic accuracy are critical to optimizing patients’ clinical outcomes and long-term prognosis. Misdiagnosing these 2 conditions can be particularly deleterious, and failure to recognize their co-occurrence can result in additional burden to typically complex and severe clinical presentations.
This article describes key aspects of the differential diagnosis between BD and BPD, emphasizing core features and major dissimilarities between these 2 conditions, and discusses the implications of misdiagnosis. The goal is to highlight the clinical and psychopathological aspects of BD and BPD to help clinicians properly distinguish these 2 disorders.
Psychopathological and sociodemographic correlates
Bipolar disorder is a chronic and severe mental illness that is classified based on clusters of symptoms—manic, hypomanic, and depressive.1 It is among the 10 leading causes of disability worldwide, with significant morbidity arising from acute affective episodes and subacute states.2 Data suggest the lifetime prevalence of BPD is 2.1%, and subthreshold forms may affect an additional 2.4% of the US population.3 The onset of symptoms typically occurs during late adolescence or early adulthood, and mood lability and cyclothymic temperament are the most common prodromal features.4
In contrast, personality disorders, such as BPD, are characteristically pervasive and maladaptive patterns of emotional responses that usually deviate from an individual’s stage of development and cultural background.1 These disorders tend to cause significant impairment, particularly in personal, occupational, and social domains. Environmental factors, such as early childhood trauma, seem to play an important role in the genesis of personality disorders, which may be particularly relevant in BPD, a disorder characterized by marked impulsivity and a pattern of instability in personal relationships, self-image, and affect.1,5,6 Similarly to BD, BPD is also chronic and highly disabling.
According to the National Survey on Alcohol and Related Conditions (NESARC), approximately 15% of US adults were found to have at least one type of personality disorder, and 6% met criteria for a cluster B personality disorder (antisocial, borderline, narcissistic, and histrionic).7 The lifetime prevalence of BPD is nearly 2%, with higher estimates observed in psychiatric settings.7,8
As a result of the phenotypical resemblance between BD and BPD (Figure), the differential diagnosis is often difficult. Recent studies suggest that co-occurrence of BD and BPD is common, with rates of comorbid BPD as high as 29% in BD I and 24% in BD II.8,9 On the other hand, nearly 20% of individuals with BPD seem to have comorbid BD.8,9 Several studies suggest that comorbid personality disorders represent a negative prognostic factor in the course of mood disorders, and the presence of BPD in patients with BD seems to be associated with more severe clinical presentations, greater treatment complexity, a higher number of depressive episodes, poor inter-episode functioning, and higher rates of other comorbidities, such as substance use disorders (SUDs).8-11 The effect of BD on the course of BPD is unclear and fairly unexplored, although it has been suggested that better control of mood symptoms may lead to more stable psychosocial functioning in BPD.9
Whether BD and BPD are part of the same spectrum is a matter for debate.12-14 Multidimensional approaches have been proposed to better characterize these disorders in at-risk populations, based on structured interviews, self-administered and clinician-rated clinical scales (Table 1), neuroimaging studies, biological markers, and machine-learning models.15,16 Compelling evidence suggests that BD and BPD have distinct underlying neurobiological and psychopathological mechanisms12,13; however, the differential diagnosis still relies on phenotypical features, since the search for biological markers has not yet identified specific biomarkers that can be used in clinical practice.
Continue to: Core features of BPD...
Core features of BPD, such as mood lability, impulsivity, and risk-taking behaviors, are also part of the diagnostic criteria for BD (Table 2).1 Similarly, depressive symptoms prevail in the course of BD.17,18 This adds complexity to the differential because “depressivity” is also part of the diagnostic criteria for BPD.1 Therefore, comprehensive psychiatric assessments and longitudinal observations are critical to diagnostic accuracy and treatment planning. Further characterization of symptoms, such as onset patterns, clinical course, phenomenology of symptoms (eg, timing, frequency, duration, triggers), and personality traits, will provide information to properly distinguish these 2 syndromes when, for example, it is unclear if the “mood swings” and impulsivity are part of a mood or a personality disorder (Table 3).
Clinical features: A closer look
Borderline personality disorder. Affect dysregulation, emotional instability, impoverished and unstable self-image, and chronic feelings of emptiness are core features of BPD.1,5,19 These characteristics, when combined with a fear of abandonment or rejection, a compromised ability to recognize the feelings and needs of others, and extremes of idealization-devaluation, tend to culminate in problematic and chaotic relationships.6,19 Individuals with BPD may become suspicious or paranoid under stressful situations. Under these circumstances, individuals with BPD may also experience depersonalization and other dissociative symptoms.6,20 The mood lability and emotional instability observed in patients with BPD usually are in response to environmental factors, and although generally intense and out of proportion, they tend to be ephemeral and short-lived, typically lasting a few hours.1,5 The anxiety and depressive symptoms reported by patients with BPD frequently are associated with feelings of “falling apart” or “losing control,” pessimism, shame, and low self-esteem. Coping strategies tend to be poorly developed and/or maladaptive, and individuals with BPD usually display a hostile and antagonistic demeanor and engage in suicidal or nonsuicidal self-injury (NSSI) behaviors as means to alleviate overwhelming emotional distress. Impulsivity, disinhibition, poor tolerance to frustration, and risk-taking behaviors are also characteristic of BPD.1,5 As a result, BPD is usually associated with significant impairment in functioning, multiple hospitalizations, and high rates of comorbid mood disorders, posttraumatic stress disorder (PTSD), SUDs, and death by suicide.
Bipolar disorder. Conversely, the fluctuations in mood and affect observed in patients with BD are usually episodic rather than pervasive, and tend to last longer (typically days to weeks) compared with the transient mood shifts observed in patients with BPD.4,17,18 The impulsivity, psychomotor agitation, and increased goal-directed activity reported by patients with BD are usually seen in the context of an acute affective episode, and are far less common during periods of stability or euthymic affect.4,17,18 Grandiosity and inflated self-esteem—hallmarks of a manic or hypomanic state—seem to oppose the unstable self-image observed in BPD, although indecisiveness and low self-worth may be observed in individuals with BD during depressive episodes. Antidepressant-induced mania or hypomania, atypical depressive episodes, and disruptions in sleep and circadian rhythms may be predictors of BD.4,21 Furthermore, although psychosocial stressors may be associated with acute affective episodes in early stages of bipolar illness, over time minimal stressors are necessary to ignite new affective episodes.22,23 Although BD is associated with high rates of suicide, suicide attempts are usually seen in the context of an acute depressive episode, and NSSI behaviors are less common among patients with BD.24
Lastly, other biographical data, such as a history of early life trauma, comorbidity, and a family history of psychiatric illnesses, can be particularly helpful in establishing the differential diagnosis between BD and BPD.25 For instance, evidence suggests that the heritability of BD may be as high as 70%, which usually translates into an extensive family history of bipolar and related disorders.26 In addition, studies suggest a high co-occurrence of anxiety disorders, attention-deficit/hyperactivity disorder, and SUDs in patients with BD, whereas PTSD, SUDs, and eating disorders tend to be highly comorbid with BPD.27 Childhood adversity (ie, a history of physical, sexual, or emotional abuse, or neglect) seems to be pivotal in the genesis of BPD and may predispose these individuals to psychotic and dissociative symptoms, particularly those with a history of sexual abuse, while playing a more secondary role in BD.28-31
Implications of misdiagnosis
In the view of the limitations of the existing models, multidimensional approaches are necessary to improve diagnostic accuracy. Presently, the differential diagnosis of BD and BPD continues to rely on clinical findings and syndromic classifications. Misdiagnosing BD and BPD has adverse therapeutic and prognostic implications.32 For instance, while psychotropic medications and neuromodulatory therapies (eg, electroconvulsive therapy, repetitive transcranial magnetic stimulation) are considered first-line treatments for patients with BD, psychosocial interventions tend to be adjunctive treatments in BD.33 Conversely, although pharmacotherapy might be helpful for patients with BPD, psychosocial and behavioral interventions are the mainstay treatment for this disorder, with the strongest evidence supporting cognitive-behavioral therapy, dialectical behavioral therapy, mentalization-based therapy, and transference-focused therapy.34-36 Thus, misdiagnosing BD as BPD with comorbid depression may result in the use of antidepressants, which can be detrimental in BD. Antidepressant treatment of BD, particularly as monotherapy, has been associated with manic or hypomanic switch, mixed states, and frequent cycling.21 Moreover, delays in diagnosis and proper treatment of BD may result in protracted mood symptoms, prolonged affective episodes, higher rates of disability, functional impairment, and overall worse clinical outcomes.24 In addition, because behavioral and psychosocial interventions are usually adjunctive therapies rather than first-line interventions for patients with BD, misdiagnosing BPD as BD may ultimately prevent these individuals from receiving proper treatment, likely resulting in more severe functional impairment, multiple hospitalizations, self-inflicted injuries, and suicide attempts, since psychotropic medications are not particularly effective for improving self-efficacy and coping strategies, nor for correcting cognitive distortions, particularly in self-image, and pathological personality traits, all of which are critical aspects of BPD treatment.
Continue to: Several factors might...
Several factors might make clinicians reluctant to diagnose BPD, or bias them to diagnose BD more frequently. These include a lack of familiarity with the diagnostic criteria for BPD, the phenotypical resemblance between BP and BPD, or even concerns about the stigma and negative implications that are associated with a BPD diagnosis.32,37,38
Whereas BD is currently perceived as a condition with a strong biological basis, there are considerable misconceptions regarding BPD and its nature.4-6,22,26 As a consequence, individuals with BPD tend to be perceived as “difficult-to-treat,” “uncooperative,” or “attention-seeking.” These misconceptions may result in poor clinician-patient relationships, unmet clinical and psychiatric needs, and frustration for both clinicians and patients.37
Through advances in biological psychiatry, precision medicine may someday be a part of psychiatric practice. Biological “signatures” may eventually help clinicians in diagnosing and treating psychiatric disorders. Presently, however, rigorous history-taking and comprehensive clinical assessments are still the most powerful tools a clinician can use to accomplish these goals. Finally, destigmatizing psychiatric disorders and educating patients and clinicians are also critical to improving clinical outcomes and promoting mental health in a compassionate and empathetic fashion.
Bottom Line
Despite the phenotypical resemblance between bipolar disorder (BP) and borderline personality disorder (BPD), the 2 are independent conditions with distinct neurobiological and psychopathological underpinnings. Clinicians can use a rigorous assessment of pathological personality traits and characterization of symptoms, such as onset patterns, clinical course, and phenomenology, to properly distinguish between BP and BPD.
Related Resources
- Fiedorowicz JG, Black DW. Borderline, bipolar, or both? Frame your diagnosis on the patient history. Current Psychiatry. 2010; 9(1):21-24,29-32.
- Zimmerman M. Improving the recognition of borderline personality disorder. Current Psychiatry. 2017;16(10):13-19.
- National Institute of Mental Health. Overview on bipolar disorder. www.nimh.nih.gov/health/publications/bipolar-disorder/index.shtml. Revised October 2018.
- National Institute of Mental Health. Overview on borderline personality disorder. www.nimh.nih.gov/health/publications/borderline-personality-disorder-fact-sheet/index.shtml.
Although evidence suggests that bipolar disorder (BD) and borderline personality disorder (BPD) are distinct entities, their differential diagnosis is often challenging as a result of considerable overlap of phenotypical features. Moreover, BD and BPD frequently co-occur, which makes it even more difficult to differentiate these 2 conditions. Strategies for improving diagnostic accuracy are critical to optimizing patients’ clinical outcomes and long-term prognosis. Misdiagnosing these 2 conditions can be particularly deleterious, and failure to recognize their co-occurrence can result in additional burden to typically complex and severe clinical presentations.
This article describes key aspects of the differential diagnosis between BD and BPD, emphasizing core features and major dissimilarities between these 2 conditions, and discusses the implications of misdiagnosis. The goal is to highlight the clinical and psychopathological aspects of BD and BPD to help clinicians properly distinguish these 2 disorders.
Psychopathological and sociodemographic correlates
Bipolar disorder is a chronic and severe mental illness that is classified based on clusters of symptoms—manic, hypomanic, and depressive.1 It is among the 10 leading causes of disability worldwide, with significant morbidity arising from acute affective episodes and subacute states.2 Data suggest the lifetime prevalence of BPD is 2.1%, and subthreshold forms may affect an additional 2.4% of the US population.3 The onset of symptoms typically occurs during late adolescence or early adulthood, and mood lability and cyclothymic temperament are the most common prodromal features.4
In contrast, personality disorders, such as BPD, are characteristically pervasive and maladaptive patterns of emotional responses that usually deviate from an individual’s stage of development and cultural background.1 These disorders tend to cause significant impairment, particularly in personal, occupational, and social domains. Environmental factors, such as early childhood trauma, seem to play an important role in the genesis of personality disorders, which may be particularly relevant in BPD, a disorder characterized by marked impulsivity and a pattern of instability in personal relationships, self-image, and affect.1,5,6 Similarly to BD, BPD is also chronic and highly disabling.
According to the National Survey on Alcohol and Related Conditions (NESARC), approximately 15% of US adults were found to have at least one type of personality disorder, and 6% met criteria for a cluster B personality disorder (antisocial, borderline, narcissistic, and histrionic).7 The lifetime prevalence of BPD is nearly 2%, with higher estimates observed in psychiatric settings.7,8
As a result of the phenotypical resemblance between BD and BPD (Figure), the differential diagnosis is often difficult. Recent studies suggest that co-occurrence of BD and BPD is common, with rates of comorbid BPD as high as 29% in BD I and 24% in BD II.8,9 On the other hand, nearly 20% of individuals with BPD seem to have comorbid BD.8,9 Several studies suggest that comorbid personality disorders represent a negative prognostic factor in the course of mood disorders, and the presence of BPD in patients with BD seems to be associated with more severe clinical presentations, greater treatment complexity, a higher number of depressive episodes, poor inter-episode functioning, and higher rates of other comorbidities, such as substance use disorders (SUDs).8-11 The effect of BD on the course of BPD is unclear and fairly unexplored, although it has been suggested that better control of mood symptoms may lead to more stable psychosocial functioning in BPD.9
Whether BD and BPD are part of the same spectrum is a matter for debate.12-14 Multidimensional approaches have been proposed to better characterize these disorders in at-risk populations, based on structured interviews, self-administered and clinician-rated clinical scales (Table 1), neuroimaging studies, biological markers, and machine-learning models.15,16 Compelling evidence suggests that BD and BPD have distinct underlying neurobiological and psychopathological mechanisms12,13; however, the differential diagnosis still relies on phenotypical features, since the search for biological markers has not yet identified specific biomarkers that can be used in clinical practice.
Continue to: Core features of BPD...
Core features of BPD, such as mood lability, impulsivity, and risk-taking behaviors, are also part of the diagnostic criteria for BD (Table 2).1 Similarly, depressive symptoms prevail in the course of BD.17,18 This adds complexity to the differential because “depressivity” is also part of the diagnostic criteria for BPD.1 Therefore, comprehensive psychiatric assessments and longitudinal observations are critical to diagnostic accuracy and treatment planning. Further characterization of symptoms, such as onset patterns, clinical course, phenomenology of symptoms (eg, timing, frequency, duration, triggers), and personality traits, will provide information to properly distinguish these 2 syndromes when, for example, it is unclear if the “mood swings” and impulsivity are part of a mood or a personality disorder (Table 3).
Clinical features: A closer look
Borderline personality disorder. Affect dysregulation, emotional instability, impoverished and unstable self-image, and chronic feelings of emptiness are core features of BPD.1,5,19 These characteristics, when combined with a fear of abandonment or rejection, a compromised ability to recognize the feelings and needs of others, and extremes of idealization-devaluation, tend to culminate in problematic and chaotic relationships.6,19 Individuals with BPD may become suspicious or paranoid under stressful situations. Under these circumstances, individuals with BPD may also experience depersonalization and other dissociative symptoms.6,20 The mood lability and emotional instability observed in patients with BPD usually are in response to environmental factors, and although generally intense and out of proportion, they tend to be ephemeral and short-lived, typically lasting a few hours.1,5 The anxiety and depressive symptoms reported by patients with BPD frequently are associated with feelings of “falling apart” or “losing control,” pessimism, shame, and low self-esteem. Coping strategies tend to be poorly developed and/or maladaptive, and individuals with BPD usually display a hostile and antagonistic demeanor and engage in suicidal or nonsuicidal self-injury (NSSI) behaviors as means to alleviate overwhelming emotional distress. Impulsivity, disinhibition, poor tolerance to frustration, and risk-taking behaviors are also characteristic of BPD.1,5 As a result, BPD is usually associated with significant impairment in functioning, multiple hospitalizations, and high rates of comorbid mood disorders, posttraumatic stress disorder (PTSD), SUDs, and death by suicide.
Bipolar disorder. Conversely, the fluctuations in mood and affect observed in patients with BD are usually episodic rather than pervasive, and tend to last longer (typically days to weeks) compared with the transient mood shifts observed in patients with BPD.4,17,18 The impulsivity, psychomotor agitation, and increased goal-directed activity reported by patients with BD are usually seen in the context of an acute affective episode, and are far less common during periods of stability or euthymic affect.4,17,18 Grandiosity and inflated self-esteem—hallmarks of a manic or hypomanic state—seem to oppose the unstable self-image observed in BPD, although indecisiveness and low self-worth may be observed in individuals with BD during depressive episodes. Antidepressant-induced mania or hypomania, atypical depressive episodes, and disruptions in sleep and circadian rhythms may be predictors of BD.4,21 Furthermore, although psychosocial stressors may be associated with acute affective episodes in early stages of bipolar illness, over time minimal stressors are necessary to ignite new affective episodes.22,23 Although BD is associated with high rates of suicide, suicide attempts are usually seen in the context of an acute depressive episode, and NSSI behaviors are less common among patients with BD.24
Lastly, other biographical data, such as a history of early life trauma, comorbidity, and a family history of psychiatric illnesses, can be particularly helpful in establishing the differential diagnosis between BD and BPD.25 For instance, evidence suggests that the heritability of BD may be as high as 70%, which usually translates into an extensive family history of bipolar and related disorders.26 In addition, studies suggest a high co-occurrence of anxiety disorders, attention-deficit/hyperactivity disorder, and SUDs in patients with BD, whereas PTSD, SUDs, and eating disorders tend to be highly comorbid with BPD.27 Childhood adversity (ie, a history of physical, sexual, or emotional abuse, or neglect) seems to be pivotal in the genesis of BPD and may predispose these individuals to psychotic and dissociative symptoms, particularly those with a history of sexual abuse, while playing a more secondary role in BD.28-31
Implications of misdiagnosis
In the view of the limitations of the existing models, multidimensional approaches are necessary to improve diagnostic accuracy. Presently, the differential diagnosis of BD and BPD continues to rely on clinical findings and syndromic classifications. Misdiagnosing BD and BPD has adverse therapeutic and prognostic implications.32 For instance, while psychotropic medications and neuromodulatory therapies (eg, electroconvulsive therapy, repetitive transcranial magnetic stimulation) are considered first-line treatments for patients with BD, psychosocial interventions tend to be adjunctive treatments in BD.33 Conversely, although pharmacotherapy might be helpful for patients with BPD, psychosocial and behavioral interventions are the mainstay treatment for this disorder, with the strongest evidence supporting cognitive-behavioral therapy, dialectical behavioral therapy, mentalization-based therapy, and transference-focused therapy.34-36 Thus, misdiagnosing BD as BPD with comorbid depression may result in the use of antidepressants, which can be detrimental in BD. Antidepressant treatment of BD, particularly as monotherapy, has been associated with manic or hypomanic switch, mixed states, and frequent cycling.21 Moreover, delays in diagnosis and proper treatment of BD may result in protracted mood symptoms, prolonged affective episodes, higher rates of disability, functional impairment, and overall worse clinical outcomes.24 In addition, because behavioral and psychosocial interventions are usually adjunctive therapies rather than first-line interventions for patients with BD, misdiagnosing BPD as BD may ultimately prevent these individuals from receiving proper treatment, likely resulting in more severe functional impairment, multiple hospitalizations, self-inflicted injuries, and suicide attempts, since psychotropic medications are not particularly effective for improving self-efficacy and coping strategies, nor for correcting cognitive distortions, particularly in self-image, and pathological personality traits, all of which are critical aspects of BPD treatment.
Continue to: Several factors might...
Several factors might make clinicians reluctant to diagnose BPD, or bias them to diagnose BD more frequently. These include a lack of familiarity with the diagnostic criteria for BPD, the phenotypical resemblance between BP and BPD, or even concerns about the stigma and negative implications that are associated with a BPD diagnosis.32,37,38
Whereas BD is currently perceived as a condition with a strong biological basis, there are considerable misconceptions regarding BPD and its nature.4-6,22,26 As a consequence, individuals with BPD tend to be perceived as “difficult-to-treat,” “uncooperative,” or “attention-seeking.” These misconceptions may result in poor clinician-patient relationships, unmet clinical and psychiatric needs, and frustration for both clinicians and patients.37
Through advances in biological psychiatry, precision medicine may someday be a part of psychiatric practice. Biological “signatures” may eventually help clinicians in diagnosing and treating psychiatric disorders. Presently, however, rigorous history-taking and comprehensive clinical assessments are still the most powerful tools a clinician can use to accomplish these goals. Finally, destigmatizing psychiatric disorders and educating patients and clinicians are also critical to improving clinical outcomes and promoting mental health in a compassionate and empathetic fashion.
Bottom Line
Despite the phenotypical resemblance between bipolar disorder (BP) and borderline personality disorder (BPD), the 2 are independent conditions with distinct neurobiological and psychopathological underpinnings. Clinicians can use a rigorous assessment of pathological personality traits and characterization of symptoms, such as onset patterns, clinical course, and phenomenology, to properly distinguish between BP and BPD.
Related Resources
- Fiedorowicz JG, Black DW. Borderline, bipolar, or both? Frame your diagnosis on the patient history. Current Psychiatry. 2010; 9(1):21-24,29-32.
- Zimmerman M. Improving the recognition of borderline personality disorder. Current Psychiatry. 2017;16(10):13-19.
- National Institute of Mental Health. Overview on bipolar disorder. www.nimh.nih.gov/health/publications/bipolar-disorder/index.shtml. Revised October 2018.
- National Institute of Mental Health. Overview on borderline personality disorder. www.nimh.nih.gov/health/publications/borderline-personality-disorder-fact-sheet/index.shtml.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Whiteford HA, Degenhardt L, Rehm J, et al. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet. 2013;382(9904):1575-1586.
3. Merikangas KR, Akiskal HS, Angst J, et al. Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey replication. Arch Gen Psychiatry. 2007;64(5):543-552.
4. Malhi GS, Bargh DM, Coulston CM, et al. Predicting bipolar disorder on the basis of phenomenology: implications for prevention and early intervention. Bipolar Disord. 2014;16(5):455-470.
5. Skodol AE, Gunderson JG, Pfohl B, et al. The borderline diagnosis I: psychopathology. Biol Psychiatry. 2002;51(12):936-950.
6. Skodol AE, Siever LJ, Livesley WJ, et al. The borderline diagnosis II: biology, genetics, and clinical course. Biol Psychiatry. 2002;51(12):951-963.
7. Hasin DS, Grant BF. The National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) Waves 1 and 2: review and summary of findings. Soc Psychiatry Psychiatr Epidemiol. 2015;50(11):1609-1640.
8. McDermid J, Sareen J, El-Gabalawy R, et al. Co-morbidity of bipolar disorder and borderline personality disorder: findings from the National Epidemiologic Survey on Alcohol and Related Conditions. Compr Psychiatry. 2015;58:18-28.
9. Gunderson JG, Weinberg I, Daversa MT, et al. Descriptive and longitudinal observations on the relationship of borderline personality disorder and bipolar disorder. Am J Psychiatry. 2006;163(7):1173-1178.
10. Swartz HA, Pilkonis PA, Frank E, et al. Acute treatment outcomes in patients with bipolar I disorder and co-morbid borderline personality disorder receiving medication and psychotherapy. Bipolar Disord. 2005;7(2):192-197.
11. Riemann G, Weisscher N, Post RM, et al. The relationship between self-reported borderline personality features and prospective illness course in bipolar disorder. Int J Bipolar Disord. 2017;5(1):31.
12. de la Rosa I, Oquendo MA, García G, et al. Determining if borderline personality disorder and bipolar disorder are alternative expressions of the same disorder. J Clin Psychiatry. 2017;778(8):e994-e999. doi: 10.4088/JCP.16m11190.
13. di Giacomo E, Aspesi F, Fotiadou M, et al. Unblending borderline personality and bipolar disorders. J Psychiatr Res. 2017;91:90-97.
14. Parker G, Bayes A, McClure G, et al. Clinical status of comorbid bipolar disorder and borderline personality disorder. Br J Psychiatry. 2016;209(3):209-215.
15. Perez Arribas I, Goodwin GM, Geddes JR, et al. A signature-based machine learning model for distinguishing bipolar disorder and borderline personality disorder. Transl Psychiatry. 2018;8(1):274.
16. Insel T, Cuthbert B, Garvey M, et al. Research Domain Criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167(7):748-751.
17. Judd LL, Akiskal HS, Schettler PJ, et al. The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry. 2002;59(6):530-537.
18. Judd LL, Akiskal HS, Schettler PJ, et al. A prospective investigation of the natural history of the long-term weekly symptomatic status of bipolar II disorder. Arch Gen Psychiatry. 2003;60(3):261-269.
19. Oldham JM, Skodol AE, Bender DS. A current integrative perspective on personality disorders. American Psychiatric Publishing, Inc. 2005.
20. Herzog JI, Schmahl C. Adverse childhood experiences and the consequences on neurobiological, psychosocial, and somatic conditions across the lifespan. Front Psychiatry. 2018;9:420.
21. Barbuti M, Pacchiarotti I, Vieta E, et al. Antidepressant-induced hypomania/mania in patients with major depression: evidence from the BRIDGE-II-MIX study. J Affect Disord. 2017;219:187-192.
22. Post RM. Mechanisms of illness progression in the recurrent affective disorders. Neurotox Res. 2010;18(3-4):256-271.
23. da Costa SC, Passos IC, Lowri C, et al. Refractory bipolar disorder and neuroprogression. Prog Neuro-Psychopharmacology Biol Psychiatry. 2016;70:103-110.
24. Crump C, Sundquist K, Winkleby MA, et al. Comorbidities and mortality in bipolar disorder: a Swedish national cohort study. JAMA Psychiatry. 2013;70(9):931-939.
25. Zimmerman M, Martinez JH, Morgan TA, et al. Distinguishing bipolar II depression from major depressive disorder with comorbid borderline personality disorder: demographic, clinical, and family history differences. J Clin Psychiatry. 2013;74(9):880-886.
26. Hasler G, Drevets WC, Gould TD, et al. Toward constructing an endophenotype strategy for bipolar disorders. Biol Psychiatry. 2006;60(2):93-105.
27. Brieger P, Ehrt U, Marneros A. Frequency of comorbid personality disorders in bipolar and unipolar affective disorders. Compr Psychiatry. 2003;44(1):28-34.
28. Leverich GS, McElroy SL, Suppes T, et al. Early physical and sexual abuse associated with an adverse course of bipolar illness. Biol Psychiatry. 2002;51(4):288-297.
29. Leverich GS, Post RM. Course of bipolar illness after history of childhood trauma. Lancet. 2006;367(9516):1040-1042.
30. Golier JA, Yehuda R, Bierer LM, et al. The relationship of borderline personality disorder to posttraumatic stress disorder and traumatic events. Am J Psychiatry. 2003;160(11):2018-2024.
31. Nicol K, Pope M, Romaniuk L, et al. Childhood trauma, midbrain activation and psychotic symptoms in borderline personality disorder. Transl Psychiatry. 2015;5:e559. doi:10.1038/tp.2015.53.
32. Ruggero CJ, Zimmerman M, Chelminski I, et al. Borderline personality disorder and the misdiagnosis of bipolar disorder. J Psychiatr Res. 2010;44(6):405-408.
33. Geddes JR, Miklowitz DJ. Treatment of bipolar disorder. Lancet. 2013;381(9878):1672-1682.
34. McMain S, Korman LM, Dimeff L. Dialectical behavior therapy and the treatment of emotion dysregulation. J Clin Psychol. 2001;57(2):183-196.
35. Cristea IA, Gentili C, Cotet CD, et al. Efficacy of psychotherapies for borderline personality disorder: a systematic review and meta-analysis. JAMA Psychiatry. 2017;74(4):319-328.
36. Linehan MM, Korslund KE, Harned MS, et al. Dialectical behavior therapy for high suicide risk in individuals with borderline personality disorder. JAMA Psychiatry. 2015;72(75);475-482.
37. LeQuesne ER, Hersh RG. Disclosure of a diagnosis of borderline personality disorder. J Psychiatr Pract. 2004:10(3):170-176.
38. Young AH. Bipolar disorder: diagnostic conundrums and associated comorbidities. J Clin Psychiatry. 2009;70(8):e26. doi:10.4088/jcp.7067br6c.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Whiteford HA, Degenhardt L, Rehm J, et al. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet. 2013;382(9904):1575-1586.
3. Merikangas KR, Akiskal HS, Angst J, et al. Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey replication. Arch Gen Psychiatry. 2007;64(5):543-552.
4. Malhi GS, Bargh DM, Coulston CM, et al. Predicting bipolar disorder on the basis of phenomenology: implications for prevention and early intervention. Bipolar Disord. 2014;16(5):455-470.
5. Skodol AE, Gunderson JG, Pfohl B, et al. The borderline diagnosis I: psychopathology. Biol Psychiatry. 2002;51(12):936-950.
6. Skodol AE, Siever LJ, Livesley WJ, et al. The borderline diagnosis II: biology, genetics, and clinical course. Biol Psychiatry. 2002;51(12):951-963.
7. Hasin DS, Grant BF. The National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) Waves 1 and 2: review and summary of findings. Soc Psychiatry Psychiatr Epidemiol. 2015;50(11):1609-1640.
8. McDermid J, Sareen J, El-Gabalawy R, et al. Co-morbidity of bipolar disorder and borderline personality disorder: findings from the National Epidemiologic Survey on Alcohol and Related Conditions. Compr Psychiatry. 2015;58:18-28.
9. Gunderson JG, Weinberg I, Daversa MT, et al. Descriptive and longitudinal observations on the relationship of borderline personality disorder and bipolar disorder. Am J Psychiatry. 2006;163(7):1173-1178.
10. Swartz HA, Pilkonis PA, Frank E, et al. Acute treatment outcomes in patients with bipolar I disorder and co-morbid borderline personality disorder receiving medication and psychotherapy. Bipolar Disord. 2005;7(2):192-197.
11. Riemann G, Weisscher N, Post RM, et al. The relationship between self-reported borderline personality features and prospective illness course in bipolar disorder. Int J Bipolar Disord. 2017;5(1):31.
12. de la Rosa I, Oquendo MA, García G, et al. Determining if borderline personality disorder and bipolar disorder are alternative expressions of the same disorder. J Clin Psychiatry. 2017;778(8):e994-e999. doi: 10.4088/JCP.16m11190.
13. di Giacomo E, Aspesi F, Fotiadou M, et al. Unblending borderline personality and bipolar disorders. J Psychiatr Res. 2017;91:90-97.
14. Parker G, Bayes A, McClure G, et al. Clinical status of comorbid bipolar disorder and borderline personality disorder. Br J Psychiatry. 2016;209(3):209-215.
15. Perez Arribas I, Goodwin GM, Geddes JR, et al. A signature-based machine learning model for distinguishing bipolar disorder and borderline personality disorder. Transl Psychiatry. 2018;8(1):274.
16. Insel T, Cuthbert B, Garvey M, et al. Research Domain Criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167(7):748-751.
17. Judd LL, Akiskal HS, Schettler PJ, et al. The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry. 2002;59(6):530-537.
18. Judd LL, Akiskal HS, Schettler PJ, et al. A prospective investigation of the natural history of the long-term weekly symptomatic status of bipolar II disorder. Arch Gen Psychiatry. 2003;60(3):261-269.
19. Oldham JM, Skodol AE, Bender DS. A current integrative perspective on personality disorders. American Psychiatric Publishing, Inc. 2005.
20. Herzog JI, Schmahl C. Adverse childhood experiences and the consequences on neurobiological, psychosocial, and somatic conditions across the lifespan. Front Psychiatry. 2018;9:420.
21. Barbuti M, Pacchiarotti I, Vieta E, et al. Antidepressant-induced hypomania/mania in patients with major depression: evidence from the BRIDGE-II-MIX study. J Affect Disord. 2017;219:187-192.
22. Post RM. Mechanisms of illness progression in the recurrent affective disorders. Neurotox Res. 2010;18(3-4):256-271.
23. da Costa SC, Passos IC, Lowri C, et al. Refractory bipolar disorder and neuroprogression. Prog Neuro-Psychopharmacology Biol Psychiatry. 2016;70:103-110.
24. Crump C, Sundquist K, Winkleby MA, et al. Comorbidities and mortality in bipolar disorder: a Swedish national cohort study. JAMA Psychiatry. 2013;70(9):931-939.
25. Zimmerman M, Martinez JH, Morgan TA, et al. Distinguishing bipolar II depression from major depressive disorder with comorbid borderline personality disorder: demographic, clinical, and family history differences. J Clin Psychiatry. 2013;74(9):880-886.
26. Hasler G, Drevets WC, Gould TD, et al. Toward constructing an endophenotype strategy for bipolar disorders. Biol Psychiatry. 2006;60(2):93-105.
27. Brieger P, Ehrt U, Marneros A. Frequency of comorbid personality disorders in bipolar and unipolar affective disorders. Compr Psychiatry. 2003;44(1):28-34.
28. Leverich GS, McElroy SL, Suppes T, et al. Early physical and sexual abuse associated with an adverse course of bipolar illness. Biol Psychiatry. 2002;51(4):288-297.
29. Leverich GS, Post RM. Course of bipolar illness after history of childhood trauma. Lancet. 2006;367(9516):1040-1042.
30. Golier JA, Yehuda R, Bierer LM, et al. The relationship of borderline personality disorder to posttraumatic stress disorder and traumatic events. Am J Psychiatry. 2003;160(11):2018-2024.
31. Nicol K, Pope M, Romaniuk L, et al. Childhood trauma, midbrain activation and psychotic symptoms in borderline personality disorder. Transl Psychiatry. 2015;5:e559. doi:10.1038/tp.2015.53.
32. Ruggero CJ, Zimmerman M, Chelminski I, et al. Borderline personality disorder and the misdiagnosis of bipolar disorder. J Psychiatr Res. 2010;44(6):405-408.
33. Geddes JR, Miklowitz DJ. Treatment of bipolar disorder. Lancet. 2013;381(9878):1672-1682.
34. McMain S, Korman LM, Dimeff L. Dialectical behavior therapy and the treatment of emotion dysregulation. J Clin Psychol. 2001;57(2):183-196.
35. Cristea IA, Gentili C, Cotet CD, et al. Efficacy of psychotherapies for borderline personality disorder: a systematic review and meta-analysis. JAMA Psychiatry. 2017;74(4):319-328.
36. Linehan MM, Korslund KE, Harned MS, et al. Dialectical behavior therapy for high suicide risk in individuals with borderline personality disorder. JAMA Psychiatry. 2015;72(75);475-482.
37. LeQuesne ER, Hersh RG. Disclosure of a diagnosis of borderline personality disorder. J Psychiatr Pract. 2004:10(3):170-176.
38. Young AH. Bipolar disorder: diagnostic conundrums and associated comorbidities. J Clin Psychiatry. 2009;70(8):e26. doi:10.4088/jcp.7067br6c.
Losing a patient to suicide: Navigating the aftermath
At some point during their career, many mental health professionals will lose a patient to suicide, but few will be prepared for the experience and its aftermath. As I described in Part 1 of this article (
A chance for growth
Traumatic experiences such as a suicide loss can paradoxically present a multitude of opportunities for new growth and profound personal transformation.1 Such transformation is primarily fostered by social support in the aftermath of the trauma.2
Virtually all of the models of the clinician’s suicide grief trajectory I described in Part 1 not only assume the eventual resolution of the distressing reactions accompanying the original loss, but also suggest that mastery of these reactions can be a catalyst for both personal and professional growth. Clearly, not everyone who experiences such a loss will experience subsequent growth; there are many reports of clinicians leaving the field3 or becoming “burned out” after this occurs. Yet most clinicians who have described this loss in the literature and in discussion groups (including those I’ve conducted) have reported more positive eventual outcomes. It is difficult to establish whether this is due to a cohort effect—clinicians who are most likely to write about their experiences, be interviewed for research studies, and/or to seek out and participate in discussion/support groups may be more prone to find benefits in this experience, either by virtue of their nature or through the subsequent process of sharing these experiences in a supportive atmosphere.
The literature on patient suicide loss, as well as anecdotal reports, confirms that clinicians who experience optimal support are able to identify many retrospective benefits of their experience.4-6 Clinicians generally report that they are better able to identify potential risk and protective factors for suicide, and are more knowledgeable about optimal interventions with individuals who are suicidal. They also describe an increased sensitivity towards patients who are suicidal and those bereaved by suicide. In addition, clinicians report a reduction in therapeutic grandiosity/omnipotence, and more realistic appraisals and expectations in relation to their clinical competence. In their effort to understand the “whys” of their patient’s suicide, they are likely to retrospectively identify errors in treatment, “missed cues,” or things they might subsequently do differently,7 and to learn from these mistakes. Optimally, clinicians become more aware of their own therapeutic limitations, both in the short- and the long-term, and can use this knowledge to better determine how they will continue their clinical work. They also become much more aware of the issues involved in the aftermath of a patient suicide, including perceived gaps in the clinical and institutional systems that could optimally offer support to families and clinicians.
In addition to the positive changes related to knowledge and clinical skills, many clinicians also note deeper personal changes subsequent to their patient’s suicide, consistent with the literature on posttraumatic growth.1 Munson8 explored internal changes in clinicians following a patient suicide and found that in the aftermath, clinicians experienced both posttraumatic growth and compassion fatigue. He also found that the amount of time that elapsed since the patient’s suicide predicted posttraumatic growth, and the seemingly counterintuitive result that the number of years of clinical experience prior to the suicide was negatively correlated with posttraumatic growth.
Huhra et al4 described some of the existential issues that a clinician is likely to confront following a patient suicide. A clinician’s attempt to find a way to meaningfully understand the circumstances around this loss often prompts reflection on mortality, freedom, choice and personal autonomy, and the scope and limits of one’s responsibility toward others. The suicide challenges one’s previous conceptions and expectations around these professional issues, and the clinician must construct new paradigms that serve to integrate these new experiences and perspectives in a coherent way.
One of the most notable sequelae of this (and to other traumatic) experience is a subsequent desire to make use of the learning inherent in these experiences and to “give back.” Once they feel that they have resolved their own grief process, many clinicians express the desire to support others with similar experiences. Even when their experiences have been quite distressing, many clinicians are able to view the suicide as an opportunity to learn about ongoing limitations in the systems of support, and to work toward changing these in a way that ensures that future clinician-survivors will have more supportive experiences. Many view these new perspectives, and their consequent ability to be more helpful, as “unexpected gifts.” They often express gratitude toward the people and resources that have allowed them to make these transformations. Jones5 noted “the tragedy of patient suicide can also be an opportunity for us as therapists to grow in our skills at assessing and intervening in a suicidal crisis, to broaden and deepen the support we give and receive, to grow in our appreciation of the precious gift that life is, and to help each other live it more fully.”
Continue to: Guidelines for postvention
Guidelines for postvention
When a patient suicide occurs in the context of an agency setting, Grad9 recommends prompt responses on 4 levels:
- administrative
- institutional
- educational
- emotional.
Numerous authors5,10-21 have developed suggestions, guidelines, and detailed postvention protocols to help agencies and clinicians in various mental health settings navigate the often-complicated sequelae to a patient’s suicide. The Table highlights a few of these. Most emphasize that information about suicide loss, including both its statistical likelihood and its potential aftermath, should integrated into clinicians’ general education and training. They also suggest that suicide postvention policies and protocols be in place from the outset, and that such information be incorporated into institutional policy and procedure manuals. In addition, they stress that legal, institutional, and administrative needs be balanced with the emotional needs of affected clinicians and staff, as well as those of the surviving family.
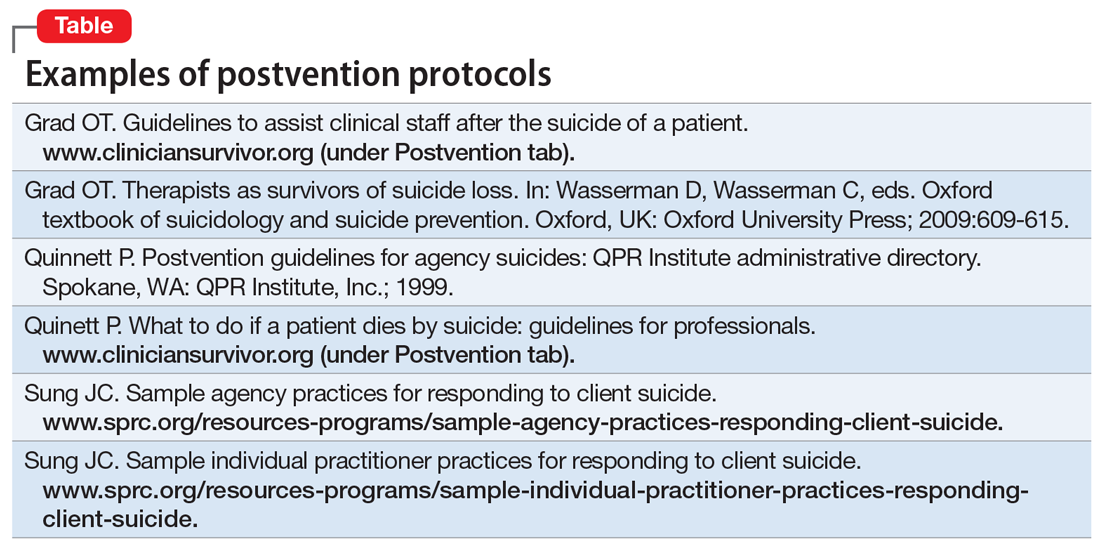
Institutional and administrative procedures
The following are some of the recommended procedures that should take place following a suicide loss. The postvention protocols listed in the Table provide more detailed recommendations.
Legal/ethical. It is essential to consult with a legal representative/risk management specialist associated with the affected agency (ideally, one with specific expertise in suicide litigation.). It is also crucial to clarify who holds privilege after a patient’s death (varies by state), what may and may not be shared under the restrictions of confidentiality and Health Insurance Portability and Accountability Act (HIPAA) laws, and to clarify procedures for chart completion and review. It is also important to clarify the specific information to be shared both within and outside of the agency, and how to address the needs of current patients in the agency settings.
Case review. The optimal purpose of the case review (also known as a psychological autopsy) is to facilitate learning, identify gaps in agency procedures and training, improve pre- and postvention procedures, and help clinicians cope with the loss.22 Again, the legal and administrative needs of the agency need to be balanced with the attention to the emotional impact on the treating clinician.17 Ellis and Patel18 recommend delaying this procedure until the treating clinician is no longer in the “shock” phase of the loss, and is able to think and process events more objectively.
Continue to: Family contact
Family contact. Most authors have recommended that clinicians and/or agencies reach out to surviving families. Although some legal representatives will advise against this, experts in the field of suicide litigation have noted that compassionate family contact reduces liability and facilitates healing for both parties. In a personal communication (May 2008), Eric Harris, of the American Psychological Association Trust, recommended “compassion over caution” when considering these issues. Again, it is important to clarify who holds privilege after a patient’s death in determining when and with whom the patient’s confidential information may be shared. When confidentiality may be broken, clinical judgment should be used to determine how best to present the information to grieving family members.
Even if surviving family members do not hold privilege, there are many things that clinicians can do to be helpful.23 Inevitably, families will want any information that will help them make sense of the loss, and general psychoeducation about mental illness and suicide can be helpful in this regard. In addition, providing information about “Survivors After Suicide” support groups, reading materials, etc., can be helpful. Both support groups and survivor-related bibliographies are available on the web sites of the American Association of Suicidology (www.suicidology.org) and The American Foundation for Suicide Prevention (www.afsp.org).
In addition, clinicians should ask the family if it would be helpful if they were to attend the funeral/memorial services, and how to introduce themselves if asked by other attendees.
Patients in clinics/hospitals. When a patient suicide occurs in a clinic or hospital setting, it is likely to impact other patients in that setting to the extent that they have heard, about the event, even from outside sources.According to Hodgkinson,24 in addition to being overwhelmed with intense feelings about the suicide loss (particularly if they had known the patient), affected patients are likely to be at increased risk for suicidal behaviors. This is consistent with the considerable literature on suicide contagion.
Thus, it is important to clarify information to be shared with patients; however, avoid describing details of the method, because this can foster contagion and “copycat” suicides. In addition, Kaye and Soreff22 noted that these patients may now be concerned about the staff’s ability to be helpful to them, because they were unable to help the deceased. In light of this, take extra care to attend to the impact of the suicide on current patients, and to monitor both pre-existing and new suicidality.
Continue to: Helping affected clinicians
Helping affected clinicians
Suggestions for optimally supporting affected clinicians include:
- clear communication about the nature of upcoming administrative procedures (including chart and institutional reviews)
- consultation from supervisors and/or colleagues that is supportive and reassuring, rather than blaming
- opportunities for the clinician to talk openly about the experience of the loss, either individually or in group settings, without fear of judgment or censure
- recognition that the loss is likely to impact clinical work, support in monitoring this impact, and the provision of medical leaves and/or modified caseloads (ie, fewer high-risk patients) as necessary.
Box 1
Frank Jones and Judy Meade founded the Clinical Survivor Task Force (CSTF) of the American Association of Suicidology (AAS) in 1987. As Jones noted, “clinicians who have lost patients to suicide need a place to acknowledge and carry forward their personal loss…to benefit both personally and professionally from the opportunity to talk with other therapists who have survived the loss of a patient through suicide.”7 Nina Gutin, PhD, and Vanessa McGann, PhD, have co-chaired the CSTF since 2003. It supports clinicians who have lost patients and/or loved ones, with the recognition that both types of losses carry implications within clinical and professional domains. The CSTF provides a listserve, opportunities to participate in video support groups, and a web site (www.cliniciansurvivor.org) that provides information about the clinician-survivor experience, the opportunity to read and post narratives about one’s experience with suicide loss, an updated bibliography maintained by John McIntosh, PhD, a list of clinical contacts, and links to several excellent postvention protocols. In addition, Drs. Gutin and McGann conduct clinician-survivor support activities at the annual AAS conference, and in their respective geographic areas.
Both researchers and clinician-survivors in my practice and support groups have noted that speaking with other clinicians who have experienced suicide loss can be particularly reassuring and validating. If none are available on staff, the listserve and online support groups of the American Association of Suicidology’s Clinician Survivor Task Force may be helpful (Box 17). In addition, the film “Collateral Damages: The Impact of Patient Suicide on the Physician” features physicians describing their experience of losing a patient to suicide (Box 2).
Box 2
“Collateral Damages: The Impact of Patient Suicide on the Physician” is a film that features several physicians speaking about their experience of losing a patient to suicide, as well as a group discussion. Psychiatrists in this educational film include Drs. Glen Gabbard, Sidney Zisook, and Jim Lomax. This resource can be used to facilitate an educational session for physicians, psychologists, residents, or other trainees. Please contact [email protected] to request a DVD of this film and a copy of a related article, Prabhakar D, Anzia JM, Balon R, et al. “Collateral damages”: preparing residents for coping with patient suicide. Acad Psychiatry. 2013;37(6):429-430.
Schultz14 offered suggestions for staff in supervisory positions, noting that they may bear at least some clinical and legal responsibility for the treatments that they supervise. She encouraged supervisors to take an active stance in advocating for trainees, to encourage colleagues to express their support, and to discourage rumors and other stigmatizing reactions. Schultz also urges supervisors to14:
- allow extra time for the clinician to engage in the normative exploration of the “whys” that are unique to suicide survivors
- use education about suicide to help the clinician gain a more realistic perspective on their relative culpability
- become aware of and provide education about normative grief reactions following a suicide.
Continue to: Because a suicide loss...
Because a suicide loss is likely to affect a clinician’s subsequent clinical activity, Schultz encourages supervisors to help clinicians monitor this impact on their work.14
A supportive environment is key
Losing a patient to suicide is a complicated, potentially traumatic process that many mental health clinicians will face. Yet with comprehensive and supportive postvention policies in place, clinicians who are impacted are more likely to experience healing and posttraumatic growth in both personal and professional domains.
Bottom Line
Although often traumatic, losing a patient to suicide presents clinicians with an opportunity for personal and professional growth. Following established postvention protocols can help ensure that legal, institutional, and administrative needs are balanced with the emotional needs of affected clinicians and staff, as well as those of the surviving family.
Related Resources
- American Association of Suicidology Clinician Survivor Task Force. www.cliniciansurvivor.org.
- Dotinga R. Coping when a patient commits suicide. July 21, 2017. www.mdedge.com/psychiatry/article/142975/depression/coping-when-patient-commits-suicide.
- Gutin N. Losing a patent to suicide: What we know. Part 1. 2019;18(10):14-16,19-22,30-32.
1. Tedeschi RG, Calhoun LG. Beyond the concept of recovery: Growth and the experience of loss. Death Stud. 2008;32(1):27-39.
2. Fuentes MA, Cruz D. Posttraumatic growth: positive psychological changes after trauma. Mental Health News. 2009;11(1):31,37.
3. Gitlin M. Aftermath of a tragedy: reaction of psychiatrists to patient suicides. Psychiatr Ann. 2007;37(10):684-687.
4. Huhra R, Hunka N, Rogers J, et al. Finding meaning: theoretical perspectives on patient suicide. Paper presented at: 2004 Annual Conference of the American Association of Suicidology; April 2004; Miami, FL.
5. Jones FA Jr. Therapists as survivors of patient suicide. In: Dunne EJ, McIntosh JL, Dunne-Maxim K, eds. Suicide and its aftermath: understanding and counseling the survivors. New York, NY: W.W. Norton; 1987;126-141.
6. Gutin N, McGann VM, Jordan JR. The impact of suicide on professional caregivers. In: Jordan J, McIntosh J, eds. Grief after suicide: understanding the consequences and caring for the survivors. New York, NY: Routledge; 2011:93-111.
7. Hendin H, Lipschitz A, Maltsberger JT, et al. Therapists’ reactions to patients’ suicides. Am J Psychiatry. 2000;157(12):2022-2027.
8. Munson JS. Impact of client suicide on practitioner posttraumatic growth [dissertation]. Gainesville, Florida: University of Florida; 2009.
9. Grad OT. Therapists as survivors of suicide loss. In: Wasserman D, Wasserman C, eds. Oxford textbook of suicidology and suicide prevention. Oxford, UK: Oxford University Press; 2009:609-615.
10. Douglas J, Brown HN. Suicide: understanding and responding: Harvard Medical School perspectives. Madison, CT: International Universities Press; 1989.
11. Farberow NL. The mental health professional as suicide survivor. Clin Neuropsychiatry. 2005;2(1):13-20.
12. Plakun EM, Tillman JG. Responding to clinicians after loss of a patient to suicide. Dir Psychiatry. 2005;25:301-310.
13. Quinnett P. QPR: for suicide prevention. QPR Institute, Inc. www.cliniciansurvivor.org (under Postvention tab). Published September 21, 2009. Accessed August 26, 2019.
14. Schultz, D. Suggestions for supervisors when a therapist experiences a client’s suicide. Women Ther. 2005;28(1):59-69.
15. Spiegelman JS Jr, Werth JL Jr. Don’t forget about me: the experiences of therapists-in-training after a patient has attempted or died by suicide. Women Ther. 2005;28(1):35-57.
16. American Association of Suicidology. Clinician Survivor Task Force. Clinicians as survivors of suicide: postvention information. http://cliniciansurvivor.org. Published May 16, 2016. Accessed January 13, 2019.
17. Whitmore CA, Cook J, Salg L. Supporting residents in the wake of patient suicide. The American Journal of Psychiatry Residents’ Journal. 2017;12(1):5-7.
18. Ellis TE, Patel AB. Client suicide: what now? Cogn Behav Pract. 2012;19(2):277-287.
19. Figueroa S, Dalack GW. Exploring the impact of suicide on clinicians: a multidisciplinary retreat model. J Psychiatr Pract. 2013;19(1):72-77.
20. Lerner U, Brooks, K, McNeil DE, et al. Coping with a patient’s suicide: a curriculum for psychiatry residency training programs. Acad Psychiatry. 2012;36(1):29-33.
21. Prabhakar D, Balon R, Anzia J, et al. Helping psychiatry residents cope with patient suicide. Acad Psychiatry. 2014;38(5):593-597.
22. Kaye NS, Soreff SM. The psychiatrist’s role, responses, and responsibilities when a patient commits suicide. Am J Psychiatry. 1991;148(6):739-743.
23. McGann VL, Gutin N, Jordan JR. Guidelines for postvention care with survivor families after the suicide of a client. In: Jordan JR, McIntosh JL, eds. Grief after suicide: understanding the consequences and caring for the survivors. New York, NY: Routledge; 2011:133-155.
24. Hodgkinson PE. Responding to in-patient suicide. Br J Med Psychol. 1987;60(4):387-392.
At some point during their career, many mental health professionals will lose a patient to suicide, but few will be prepared for the experience and its aftermath. As I described in Part 1 of this article (
A chance for growth
Traumatic experiences such as a suicide loss can paradoxically present a multitude of opportunities for new growth and profound personal transformation.1 Such transformation is primarily fostered by social support in the aftermath of the trauma.2
Virtually all of the models of the clinician’s suicide grief trajectory I described in Part 1 not only assume the eventual resolution of the distressing reactions accompanying the original loss, but also suggest that mastery of these reactions can be a catalyst for both personal and professional growth. Clearly, not everyone who experiences such a loss will experience subsequent growth; there are many reports of clinicians leaving the field3 or becoming “burned out” after this occurs. Yet most clinicians who have described this loss in the literature and in discussion groups (including those I’ve conducted) have reported more positive eventual outcomes. It is difficult to establish whether this is due to a cohort effect—clinicians who are most likely to write about their experiences, be interviewed for research studies, and/or to seek out and participate in discussion/support groups may be more prone to find benefits in this experience, either by virtue of their nature or through the subsequent process of sharing these experiences in a supportive atmosphere.
The literature on patient suicide loss, as well as anecdotal reports, confirms that clinicians who experience optimal support are able to identify many retrospective benefits of their experience.4-6 Clinicians generally report that they are better able to identify potential risk and protective factors for suicide, and are more knowledgeable about optimal interventions with individuals who are suicidal. They also describe an increased sensitivity towards patients who are suicidal and those bereaved by suicide. In addition, clinicians report a reduction in therapeutic grandiosity/omnipotence, and more realistic appraisals and expectations in relation to their clinical competence. In their effort to understand the “whys” of their patient’s suicide, they are likely to retrospectively identify errors in treatment, “missed cues,” or things they might subsequently do differently,7 and to learn from these mistakes. Optimally, clinicians become more aware of their own therapeutic limitations, both in the short- and the long-term, and can use this knowledge to better determine how they will continue their clinical work. They also become much more aware of the issues involved in the aftermath of a patient suicide, including perceived gaps in the clinical and institutional systems that could optimally offer support to families and clinicians.
In addition to the positive changes related to knowledge and clinical skills, many clinicians also note deeper personal changes subsequent to their patient’s suicide, consistent with the literature on posttraumatic growth.1 Munson8 explored internal changes in clinicians following a patient suicide and found that in the aftermath, clinicians experienced both posttraumatic growth and compassion fatigue. He also found that the amount of time that elapsed since the patient’s suicide predicted posttraumatic growth, and the seemingly counterintuitive result that the number of years of clinical experience prior to the suicide was negatively correlated with posttraumatic growth.
Huhra et al4 described some of the existential issues that a clinician is likely to confront following a patient suicide. A clinician’s attempt to find a way to meaningfully understand the circumstances around this loss often prompts reflection on mortality, freedom, choice and personal autonomy, and the scope and limits of one’s responsibility toward others. The suicide challenges one’s previous conceptions and expectations around these professional issues, and the clinician must construct new paradigms that serve to integrate these new experiences and perspectives in a coherent way.
One of the most notable sequelae of this (and to other traumatic) experience is a subsequent desire to make use of the learning inherent in these experiences and to “give back.” Once they feel that they have resolved their own grief process, many clinicians express the desire to support others with similar experiences. Even when their experiences have been quite distressing, many clinicians are able to view the suicide as an opportunity to learn about ongoing limitations in the systems of support, and to work toward changing these in a way that ensures that future clinician-survivors will have more supportive experiences. Many view these new perspectives, and their consequent ability to be more helpful, as “unexpected gifts.” They often express gratitude toward the people and resources that have allowed them to make these transformations. Jones5 noted “the tragedy of patient suicide can also be an opportunity for us as therapists to grow in our skills at assessing and intervening in a suicidal crisis, to broaden and deepen the support we give and receive, to grow in our appreciation of the precious gift that life is, and to help each other live it more fully.”
Continue to: Guidelines for postvention
Guidelines for postvention
When a patient suicide occurs in the context of an agency setting, Grad9 recommends prompt responses on 4 levels:
- administrative
- institutional
- educational
- emotional.
Numerous authors5,10-21 have developed suggestions, guidelines, and detailed postvention protocols to help agencies and clinicians in various mental health settings navigate the often-complicated sequelae to a patient’s suicide. The Table highlights a few of these. Most emphasize that information about suicide loss, including both its statistical likelihood and its potential aftermath, should integrated into clinicians’ general education and training. They also suggest that suicide postvention policies and protocols be in place from the outset, and that such information be incorporated into institutional policy and procedure manuals. In addition, they stress that legal, institutional, and administrative needs be balanced with the emotional needs of affected clinicians and staff, as well as those of the surviving family.

Institutional and administrative procedures
The following are some of the recommended procedures that should take place following a suicide loss. The postvention protocols listed in the Table provide more detailed recommendations.
Legal/ethical. It is essential to consult with a legal representative/risk management specialist associated with the affected agency (ideally, one with specific expertise in suicide litigation.). It is also crucial to clarify who holds privilege after a patient’s death (varies by state), what may and may not be shared under the restrictions of confidentiality and Health Insurance Portability and Accountability Act (HIPAA) laws, and to clarify procedures for chart completion and review. It is also important to clarify the specific information to be shared both within and outside of the agency, and how to address the needs of current patients in the agency settings.
Case review. The optimal purpose of the case review (also known as a psychological autopsy) is to facilitate learning, identify gaps in agency procedures and training, improve pre- and postvention procedures, and help clinicians cope with the loss.22 Again, the legal and administrative needs of the agency need to be balanced with the attention to the emotional impact on the treating clinician.17 Ellis and Patel18 recommend delaying this procedure until the treating clinician is no longer in the “shock” phase of the loss, and is able to think and process events more objectively.
Continue to: Family contact
Family contact. Most authors have recommended that clinicians and/or agencies reach out to surviving families. Although some legal representatives will advise against this, experts in the field of suicide litigation have noted that compassionate family contact reduces liability and facilitates healing for both parties. In a personal communication (May 2008), Eric Harris, of the American Psychological Association Trust, recommended “compassion over caution” when considering these issues. Again, it is important to clarify who holds privilege after a patient’s death in determining when and with whom the patient’s confidential information may be shared. When confidentiality may be broken, clinical judgment should be used to determine how best to present the information to grieving family members.
Even if surviving family members do not hold privilege, there are many things that clinicians can do to be helpful.23 Inevitably, families will want any information that will help them make sense of the loss, and general psychoeducation about mental illness and suicide can be helpful in this regard. In addition, providing information about “Survivors After Suicide” support groups, reading materials, etc., can be helpful. Both support groups and survivor-related bibliographies are available on the web sites of the American Association of Suicidology (www.suicidology.org) and The American Foundation for Suicide Prevention (www.afsp.org).
In addition, clinicians should ask the family if it would be helpful if they were to attend the funeral/memorial services, and how to introduce themselves if asked by other attendees.
Patients in clinics/hospitals. When a patient suicide occurs in a clinic or hospital setting, it is likely to impact other patients in that setting to the extent that they have heard, about the event, even from outside sources.According to Hodgkinson,24 in addition to being overwhelmed with intense feelings about the suicide loss (particularly if they had known the patient), affected patients are likely to be at increased risk for suicidal behaviors. This is consistent with the considerable literature on suicide contagion.
Thus, it is important to clarify information to be shared with patients; however, avoid describing details of the method, because this can foster contagion and “copycat” suicides. In addition, Kaye and Soreff22 noted that these patients may now be concerned about the staff’s ability to be helpful to them, because they were unable to help the deceased. In light of this, take extra care to attend to the impact of the suicide on current patients, and to monitor both pre-existing and new suicidality.
Continue to: Helping affected clinicians
Helping affected clinicians
Suggestions for optimally supporting affected clinicians include:
- clear communication about the nature of upcoming administrative procedures (including chart and institutional reviews)
- consultation from supervisors and/or colleagues that is supportive and reassuring, rather than blaming
- opportunities for the clinician to talk openly about the experience of the loss, either individually or in group settings, without fear of judgment or censure
- recognition that the loss is likely to impact clinical work, support in monitoring this impact, and the provision of medical leaves and/or modified caseloads (ie, fewer high-risk patients) as necessary.
Box 1
Frank Jones and Judy Meade founded the Clinical Survivor Task Force (CSTF) of the American Association of Suicidology (AAS) in 1987. As Jones noted, “clinicians who have lost patients to suicide need a place to acknowledge and carry forward their personal loss…to benefit both personally and professionally from the opportunity to talk with other therapists who have survived the loss of a patient through suicide.”7 Nina Gutin, PhD, and Vanessa McGann, PhD, have co-chaired the CSTF since 2003. It supports clinicians who have lost patients and/or loved ones, with the recognition that both types of losses carry implications within clinical and professional domains. The CSTF provides a listserve, opportunities to participate in video support groups, and a web site (www.cliniciansurvivor.org) that provides information about the clinician-survivor experience, the opportunity to read and post narratives about one’s experience with suicide loss, an updated bibliography maintained by John McIntosh, PhD, a list of clinical contacts, and links to several excellent postvention protocols. In addition, Drs. Gutin and McGann conduct clinician-survivor support activities at the annual AAS conference, and in their respective geographic areas.
Both researchers and clinician-survivors in my practice and support groups have noted that speaking with other clinicians who have experienced suicide loss can be particularly reassuring and validating. If none are available on staff, the listserve and online support groups of the American Association of Suicidology’s Clinician Survivor Task Force may be helpful (Box 17). In addition, the film “Collateral Damages: The Impact of Patient Suicide on the Physician” features physicians describing their experience of losing a patient to suicide (Box 2).
Box 2
“Collateral Damages: The Impact of Patient Suicide on the Physician” is a film that features several physicians speaking about their experience of losing a patient to suicide, as well as a group discussion. Psychiatrists in this educational film include Drs. Glen Gabbard, Sidney Zisook, and Jim Lomax. This resource can be used to facilitate an educational session for physicians, psychologists, residents, or other trainees. Please contact [email protected] to request a DVD of this film and a copy of a related article, Prabhakar D, Anzia JM, Balon R, et al. “Collateral damages”: preparing residents for coping with patient suicide. Acad Psychiatry. 2013;37(6):429-430.
Schultz14 offered suggestions for staff in supervisory positions, noting that they may bear at least some clinical and legal responsibility for the treatments that they supervise. She encouraged supervisors to take an active stance in advocating for trainees, to encourage colleagues to express their support, and to discourage rumors and other stigmatizing reactions. Schultz also urges supervisors to14:
- allow extra time for the clinician to engage in the normative exploration of the “whys” that are unique to suicide survivors
- use education about suicide to help the clinician gain a more realistic perspective on their relative culpability
- become aware of and provide education about normative grief reactions following a suicide.
Continue to: Because a suicide loss...
Because a suicide loss is likely to affect a clinician’s subsequent clinical activity, Schultz encourages supervisors to help clinicians monitor this impact on their work.14
A supportive environment is key
Losing a patient to suicide is a complicated, potentially traumatic process that many mental health clinicians will face. Yet with comprehensive and supportive postvention policies in place, clinicians who are impacted are more likely to experience healing and posttraumatic growth in both personal and professional domains.
Bottom Line
Although often traumatic, losing a patient to suicide presents clinicians with an opportunity for personal and professional growth. Following established postvention protocols can help ensure that legal, institutional, and administrative needs are balanced with the emotional needs of affected clinicians and staff, as well as those of the surviving family.
Related Resources
- American Association of Suicidology Clinician Survivor Task Force. www.cliniciansurvivor.org.
- Dotinga R. Coping when a patient commits suicide. July 21, 2017. www.mdedge.com/psychiatry/article/142975/depression/coping-when-patient-commits-suicide.
- Gutin N. Losing a patent to suicide: What we know. Part 1. 2019;18(10):14-16,19-22,30-32.
At some point during their career, many mental health professionals will lose a patient to suicide, but few will be prepared for the experience and its aftermath. As I described in Part 1 of this article (
A chance for growth
Traumatic experiences such as a suicide loss can paradoxically present a multitude of opportunities for new growth and profound personal transformation.1 Such transformation is primarily fostered by social support in the aftermath of the trauma.2
Virtually all of the models of the clinician’s suicide grief trajectory I described in Part 1 not only assume the eventual resolution of the distressing reactions accompanying the original loss, but also suggest that mastery of these reactions can be a catalyst for both personal and professional growth. Clearly, not everyone who experiences such a loss will experience subsequent growth; there are many reports of clinicians leaving the field3 or becoming “burned out” after this occurs. Yet most clinicians who have described this loss in the literature and in discussion groups (including those I’ve conducted) have reported more positive eventual outcomes. It is difficult to establish whether this is due to a cohort effect—clinicians who are most likely to write about their experiences, be interviewed for research studies, and/or to seek out and participate in discussion/support groups may be more prone to find benefits in this experience, either by virtue of their nature or through the subsequent process of sharing these experiences in a supportive atmosphere.
The literature on patient suicide loss, as well as anecdotal reports, confirms that clinicians who experience optimal support are able to identify many retrospective benefits of their experience.4-6 Clinicians generally report that they are better able to identify potential risk and protective factors for suicide, and are more knowledgeable about optimal interventions with individuals who are suicidal. They also describe an increased sensitivity towards patients who are suicidal and those bereaved by suicide. In addition, clinicians report a reduction in therapeutic grandiosity/omnipotence, and more realistic appraisals and expectations in relation to their clinical competence. In their effort to understand the “whys” of their patient’s suicide, they are likely to retrospectively identify errors in treatment, “missed cues,” or things they might subsequently do differently,7 and to learn from these mistakes. Optimally, clinicians become more aware of their own therapeutic limitations, both in the short- and the long-term, and can use this knowledge to better determine how they will continue their clinical work. They also become much more aware of the issues involved in the aftermath of a patient suicide, including perceived gaps in the clinical and institutional systems that could optimally offer support to families and clinicians.
In addition to the positive changes related to knowledge and clinical skills, many clinicians also note deeper personal changes subsequent to their patient’s suicide, consistent with the literature on posttraumatic growth.1 Munson8 explored internal changes in clinicians following a patient suicide and found that in the aftermath, clinicians experienced both posttraumatic growth and compassion fatigue. He also found that the amount of time that elapsed since the patient’s suicide predicted posttraumatic growth, and the seemingly counterintuitive result that the number of years of clinical experience prior to the suicide was negatively correlated with posttraumatic growth.
Huhra et al4 described some of the existential issues that a clinician is likely to confront following a patient suicide. A clinician’s attempt to find a way to meaningfully understand the circumstances around this loss often prompts reflection on mortality, freedom, choice and personal autonomy, and the scope and limits of one’s responsibility toward others. The suicide challenges one’s previous conceptions and expectations around these professional issues, and the clinician must construct new paradigms that serve to integrate these new experiences and perspectives in a coherent way.
One of the most notable sequelae of this (and to other traumatic) experience is a subsequent desire to make use of the learning inherent in these experiences and to “give back.” Once they feel that they have resolved their own grief process, many clinicians express the desire to support others with similar experiences. Even when their experiences have been quite distressing, many clinicians are able to view the suicide as an opportunity to learn about ongoing limitations in the systems of support, and to work toward changing these in a way that ensures that future clinician-survivors will have more supportive experiences. Many view these new perspectives, and their consequent ability to be more helpful, as “unexpected gifts.” They often express gratitude toward the people and resources that have allowed them to make these transformations. Jones5 noted “the tragedy of patient suicide can also be an opportunity for us as therapists to grow in our skills at assessing and intervening in a suicidal crisis, to broaden and deepen the support we give and receive, to grow in our appreciation of the precious gift that life is, and to help each other live it more fully.”
Continue to: Guidelines for postvention
Guidelines for postvention
When a patient suicide occurs in the context of an agency setting, Grad9 recommends prompt responses on 4 levels:
- administrative
- institutional
- educational
- emotional.
Numerous authors5,10-21 have developed suggestions, guidelines, and detailed postvention protocols to help agencies and clinicians in various mental health settings navigate the often-complicated sequelae to a patient’s suicide. The Table highlights a few of these. Most emphasize that information about suicide loss, including both its statistical likelihood and its potential aftermath, should integrated into clinicians’ general education and training. They also suggest that suicide postvention policies and protocols be in place from the outset, and that such information be incorporated into institutional policy and procedure manuals. In addition, they stress that legal, institutional, and administrative needs be balanced with the emotional needs of affected clinicians and staff, as well as those of the surviving family.

Institutional and administrative procedures
The following are some of the recommended procedures that should take place following a suicide loss. The postvention protocols listed in the Table provide more detailed recommendations.
Legal/ethical. It is essential to consult with a legal representative/risk management specialist associated with the affected agency (ideally, one with specific expertise in suicide litigation.). It is also crucial to clarify who holds privilege after a patient’s death (varies by state), what may and may not be shared under the restrictions of confidentiality and Health Insurance Portability and Accountability Act (HIPAA) laws, and to clarify procedures for chart completion and review. It is also important to clarify the specific information to be shared both within and outside of the agency, and how to address the needs of current patients in the agency settings.
Case review. The optimal purpose of the case review (also known as a psychological autopsy) is to facilitate learning, identify gaps in agency procedures and training, improve pre- and postvention procedures, and help clinicians cope with the loss.22 Again, the legal and administrative needs of the agency need to be balanced with the attention to the emotional impact on the treating clinician.17 Ellis and Patel18 recommend delaying this procedure until the treating clinician is no longer in the “shock” phase of the loss, and is able to think and process events more objectively.
Continue to: Family contact
Family contact. Most authors have recommended that clinicians and/or agencies reach out to surviving families. Although some legal representatives will advise against this, experts in the field of suicide litigation have noted that compassionate family contact reduces liability and facilitates healing for both parties. In a personal communication (May 2008), Eric Harris, of the American Psychological Association Trust, recommended “compassion over caution” when considering these issues. Again, it is important to clarify who holds privilege after a patient’s death in determining when and with whom the patient’s confidential information may be shared. When confidentiality may be broken, clinical judgment should be used to determine how best to present the information to grieving family members.
Even if surviving family members do not hold privilege, there are many things that clinicians can do to be helpful.23 Inevitably, families will want any information that will help them make sense of the loss, and general psychoeducation about mental illness and suicide can be helpful in this regard. In addition, providing information about “Survivors After Suicide” support groups, reading materials, etc., can be helpful. Both support groups and survivor-related bibliographies are available on the web sites of the American Association of Suicidology (www.suicidology.org) and The American Foundation for Suicide Prevention (www.afsp.org).
In addition, clinicians should ask the family if it would be helpful if they were to attend the funeral/memorial services, and how to introduce themselves if asked by other attendees.
Patients in clinics/hospitals. When a patient suicide occurs in a clinic or hospital setting, it is likely to impact other patients in that setting to the extent that they have heard, about the event, even from outside sources.According to Hodgkinson,24 in addition to being overwhelmed with intense feelings about the suicide loss (particularly if they had known the patient), affected patients are likely to be at increased risk for suicidal behaviors. This is consistent with the considerable literature on suicide contagion.
Thus, it is important to clarify information to be shared with patients; however, avoid describing details of the method, because this can foster contagion and “copycat” suicides. In addition, Kaye and Soreff22 noted that these patients may now be concerned about the staff’s ability to be helpful to them, because they were unable to help the deceased. In light of this, take extra care to attend to the impact of the suicide on current patients, and to monitor both pre-existing and new suicidality.
Continue to: Helping affected clinicians
Helping affected clinicians
Suggestions for optimally supporting affected clinicians include:
- clear communication about the nature of upcoming administrative procedures (including chart and institutional reviews)
- consultation from supervisors and/or colleagues that is supportive and reassuring, rather than blaming
- opportunities for the clinician to talk openly about the experience of the loss, either individually or in group settings, without fear of judgment or censure
- recognition that the loss is likely to impact clinical work, support in monitoring this impact, and the provision of medical leaves and/or modified caseloads (ie, fewer high-risk patients) as necessary.
Box 1
Frank Jones and Judy Meade founded the Clinical Survivor Task Force (CSTF) of the American Association of Suicidology (AAS) in 1987. As Jones noted, “clinicians who have lost patients to suicide need a place to acknowledge and carry forward their personal loss…to benefit both personally and professionally from the opportunity to talk with other therapists who have survived the loss of a patient through suicide.”7 Nina Gutin, PhD, and Vanessa McGann, PhD, have co-chaired the CSTF since 2003. It supports clinicians who have lost patients and/or loved ones, with the recognition that both types of losses carry implications within clinical and professional domains. The CSTF provides a listserve, opportunities to participate in video support groups, and a web site (www.cliniciansurvivor.org) that provides information about the clinician-survivor experience, the opportunity to read and post narratives about one’s experience with suicide loss, an updated bibliography maintained by John McIntosh, PhD, a list of clinical contacts, and links to several excellent postvention protocols. In addition, Drs. Gutin and McGann conduct clinician-survivor support activities at the annual AAS conference, and in their respective geographic areas.
Both researchers and clinician-survivors in my practice and support groups have noted that speaking with other clinicians who have experienced suicide loss can be particularly reassuring and validating. If none are available on staff, the listserve and online support groups of the American Association of Suicidology’s Clinician Survivor Task Force may be helpful (Box 17). In addition, the film “Collateral Damages: The Impact of Patient Suicide on the Physician” features physicians describing their experience of losing a patient to suicide (Box 2).
Box 2
“Collateral Damages: The Impact of Patient Suicide on the Physician” is a film that features several physicians speaking about their experience of losing a patient to suicide, as well as a group discussion. Psychiatrists in this educational film include Drs. Glen Gabbard, Sidney Zisook, and Jim Lomax. This resource can be used to facilitate an educational session for physicians, psychologists, residents, or other trainees. Please contact [email protected] to request a DVD of this film and a copy of a related article, Prabhakar D, Anzia JM, Balon R, et al. “Collateral damages”: preparing residents for coping with patient suicide. Acad Psychiatry. 2013;37(6):429-430.
Schultz14 offered suggestions for staff in supervisory positions, noting that they may bear at least some clinical and legal responsibility for the treatments that they supervise. She encouraged supervisors to take an active stance in advocating for trainees, to encourage colleagues to express their support, and to discourage rumors and other stigmatizing reactions. Schultz also urges supervisors to14:
- allow extra time for the clinician to engage in the normative exploration of the “whys” that are unique to suicide survivors
- use education about suicide to help the clinician gain a more realistic perspective on their relative culpability
- become aware of and provide education about normative grief reactions following a suicide.
Continue to: Because a suicide loss...
Because a suicide loss is likely to affect a clinician’s subsequent clinical activity, Schultz encourages supervisors to help clinicians monitor this impact on their work.14
A supportive environment is key
Losing a patient to suicide is a complicated, potentially traumatic process that many mental health clinicians will face. Yet with comprehensive and supportive postvention policies in place, clinicians who are impacted are more likely to experience healing and posttraumatic growth in both personal and professional domains.
Bottom Line
Although often traumatic, losing a patient to suicide presents clinicians with an opportunity for personal and professional growth. Following established postvention protocols can help ensure that legal, institutional, and administrative needs are balanced with the emotional needs of affected clinicians and staff, as well as those of the surviving family.
Related Resources
- American Association of Suicidology Clinician Survivor Task Force. www.cliniciansurvivor.org.
- Dotinga R. Coping when a patient commits suicide. July 21, 2017. www.mdedge.com/psychiatry/article/142975/depression/coping-when-patient-commits-suicide.
- Gutin N. Losing a patent to suicide: What we know. Part 1. 2019;18(10):14-16,19-22,30-32.
1. Tedeschi RG, Calhoun LG. Beyond the concept of recovery: Growth and the experience of loss. Death Stud. 2008;32(1):27-39.
2. Fuentes MA, Cruz D. Posttraumatic growth: positive psychological changes after trauma. Mental Health News. 2009;11(1):31,37.
3. Gitlin M. Aftermath of a tragedy: reaction of psychiatrists to patient suicides. Psychiatr Ann. 2007;37(10):684-687.
4. Huhra R, Hunka N, Rogers J, et al. Finding meaning: theoretical perspectives on patient suicide. Paper presented at: 2004 Annual Conference of the American Association of Suicidology; April 2004; Miami, FL.
5. Jones FA Jr. Therapists as survivors of patient suicide. In: Dunne EJ, McIntosh JL, Dunne-Maxim K, eds. Suicide and its aftermath: understanding and counseling the survivors. New York, NY: W.W. Norton; 1987;126-141.
6. Gutin N, McGann VM, Jordan JR. The impact of suicide on professional caregivers. In: Jordan J, McIntosh J, eds. Grief after suicide: understanding the consequences and caring for the survivors. New York, NY: Routledge; 2011:93-111.
7. Hendin H, Lipschitz A, Maltsberger JT, et al. Therapists’ reactions to patients’ suicides. Am J Psychiatry. 2000;157(12):2022-2027.
8. Munson JS. Impact of client suicide on practitioner posttraumatic growth [dissertation]. Gainesville, Florida: University of Florida; 2009.
9. Grad OT. Therapists as survivors of suicide loss. In: Wasserman D, Wasserman C, eds. Oxford textbook of suicidology and suicide prevention. Oxford, UK: Oxford University Press; 2009:609-615.
10. Douglas J, Brown HN. Suicide: understanding and responding: Harvard Medical School perspectives. Madison, CT: International Universities Press; 1989.
11. Farberow NL. The mental health professional as suicide survivor. Clin Neuropsychiatry. 2005;2(1):13-20.
12. Plakun EM, Tillman JG. Responding to clinicians after loss of a patient to suicide. Dir Psychiatry. 2005;25:301-310.
13. Quinnett P. QPR: for suicide prevention. QPR Institute, Inc. www.cliniciansurvivor.org (under Postvention tab). Published September 21, 2009. Accessed August 26, 2019.
14. Schultz, D. Suggestions for supervisors when a therapist experiences a client’s suicide. Women Ther. 2005;28(1):59-69.
15. Spiegelman JS Jr, Werth JL Jr. Don’t forget about me: the experiences of therapists-in-training after a patient has attempted or died by suicide. Women Ther. 2005;28(1):35-57.
16. American Association of Suicidology. Clinician Survivor Task Force. Clinicians as survivors of suicide: postvention information. http://cliniciansurvivor.org. Published May 16, 2016. Accessed January 13, 2019.
17. Whitmore CA, Cook J, Salg L. Supporting residents in the wake of patient suicide. The American Journal of Psychiatry Residents’ Journal. 2017;12(1):5-7.
18. Ellis TE, Patel AB. Client suicide: what now? Cogn Behav Pract. 2012;19(2):277-287.
19. Figueroa S, Dalack GW. Exploring the impact of suicide on clinicians: a multidisciplinary retreat model. J Psychiatr Pract. 2013;19(1):72-77.
20. Lerner U, Brooks, K, McNeil DE, et al. Coping with a patient’s suicide: a curriculum for psychiatry residency training programs. Acad Psychiatry. 2012;36(1):29-33.
21. Prabhakar D, Balon R, Anzia J, et al. Helping psychiatry residents cope with patient suicide. Acad Psychiatry. 2014;38(5):593-597.
22. Kaye NS, Soreff SM. The psychiatrist’s role, responses, and responsibilities when a patient commits suicide. Am J Psychiatry. 1991;148(6):739-743.
23. McGann VL, Gutin N, Jordan JR. Guidelines for postvention care with survivor families after the suicide of a client. In: Jordan JR, McIntosh JL, eds. Grief after suicide: understanding the consequences and caring for the survivors. New York, NY: Routledge; 2011:133-155.
24. Hodgkinson PE. Responding to in-patient suicide. Br J Med Psychol. 1987;60(4):387-392.
1. Tedeschi RG, Calhoun LG. Beyond the concept of recovery: Growth and the experience of loss. Death Stud. 2008;32(1):27-39.
2. Fuentes MA, Cruz D. Posttraumatic growth: positive psychological changes after trauma. Mental Health News. 2009;11(1):31,37.
3. Gitlin M. Aftermath of a tragedy: reaction of psychiatrists to patient suicides. Psychiatr Ann. 2007;37(10):684-687.
4. Huhra R, Hunka N, Rogers J, et al. Finding meaning: theoretical perspectives on patient suicide. Paper presented at: 2004 Annual Conference of the American Association of Suicidology; April 2004; Miami, FL.
5. Jones FA Jr. Therapists as survivors of patient suicide. In: Dunne EJ, McIntosh JL, Dunne-Maxim K, eds. Suicide and its aftermath: understanding and counseling the survivors. New York, NY: W.W. Norton; 1987;126-141.
6. Gutin N, McGann VM, Jordan JR. The impact of suicide on professional caregivers. In: Jordan J, McIntosh J, eds. Grief after suicide: understanding the consequences and caring for the survivors. New York, NY: Routledge; 2011:93-111.
7. Hendin H, Lipschitz A, Maltsberger JT, et al. Therapists’ reactions to patients’ suicides. Am J Psychiatry. 2000;157(12):2022-2027.
8. Munson JS. Impact of client suicide on practitioner posttraumatic growth [dissertation]. Gainesville, Florida: University of Florida; 2009.
9. Grad OT. Therapists as survivors of suicide loss. In: Wasserman D, Wasserman C, eds. Oxford textbook of suicidology and suicide prevention. Oxford, UK: Oxford University Press; 2009:609-615.
10. Douglas J, Brown HN. Suicide: understanding and responding: Harvard Medical School perspectives. Madison, CT: International Universities Press; 1989.
11. Farberow NL. The mental health professional as suicide survivor. Clin Neuropsychiatry. 2005;2(1):13-20.
12. Plakun EM, Tillman JG. Responding to clinicians after loss of a patient to suicide. Dir Psychiatry. 2005;25:301-310.
13. Quinnett P. QPR: for suicide prevention. QPR Institute, Inc. www.cliniciansurvivor.org (under Postvention tab). Published September 21, 2009. Accessed August 26, 2019.
14. Schultz, D. Suggestions for supervisors when a therapist experiences a client’s suicide. Women Ther. 2005;28(1):59-69.
15. Spiegelman JS Jr, Werth JL Jr. Don’t forget about me: the experiences of therapists-in-training after a patient has attempted or died by suicide. Women Ther. 2005;28(1):35-57.
16. American Association of Suicidology. Clinician Survivor Task Force. Clinicians as survivors of suicide: postvention information. http://cliniciansurvivor.org. Published May 16, 2016. Accessed January 13, 2019.
17. Whitmore CA, Cook J, Salg L. Supporting residents in the wake of patient suicide. The American Journal of Psychiatry Residents’ Journal. 2017;12(1):5-7.
18. Ellis TE, Patel AB. Client suicide: what now? Cogn Behav Pract. 2012;19(2):277-287.
19. Figueroa S, Dalack GW. Exploring the impact of suicide on clinicians: a multidisciplinary retreat model. J Psychiatr Pract. 2013;19(1):72-77.
20. Lerner U, Brooks, K, McNeil DE, et al. Coping with a patient’s suicide: a curriculum for psychiatry residency training programs. Acad Psychiatry. 2012;36(1):29-33.
21. Prabhakar D, Balon R, Anzia J, et al. Helping psychiatry residents cope with patient suicide. Acad Psychiatry. 2014;38(5):593-597.
22. Kaye NS, Soreff SM. The psychiatrist’s role, responses, and responsibilities when a patient commits suicide. Am J Psychiatry. 1991;148(6):739-743.
23. McGann VL, Gutin N, Jordan JR. Guidelines for postvention care with survivor families after the suicide of a client. In: Jordan JR, McIntosh JL, eds. Grief after suicide: understanding the consequences and caring for the survivors. New York, NY: Routledge; 2011:133-155.
24. Hodgkinson PE. Responding to in-patient suicide. Br J Med Psychol. 1987;60(4):387-392.
Hospital Medicine Update: High-Impact Literature from March 2018 to April 2019
Given the breadth and depth of patients cared for by hospital medicine providers, it is challenging to remain current with the literature. The authors critically appraised the literature from March 2018 to April 2019 for high-quality studies relevant to hospital medicine. Articles were selected based on methodologic rigor and likelihood to impact clinical practice. Thirty articles were selected by the presenting authors for the Hospital Medicine Updates at the 2019 Society of Hospital Medicine (CH, CM) and Society of General Internal Medicine Annual Meetings (BS, AB). After two sequential rounds of voting and group discussion to adjudicate voting discrepancies, the authors selected the 10 most impactful articles for this review. Each article is described below with the key points summarized in the Table.
ESSENTIAL PUBLICATIONS
Clinical Practice Guidelines for Clostridium difficile Infection in Adults and Children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). McDonald LC, et al. Clin Infect Dis. 2018;66(7):e1–e48.1
Background. In the United States, approximately 500,000 Clostridioides difficile infections (CDI) occur annually with 15,000-30,000 deaths. CDI has become a marker of hospital quality and has been placed under numerous “pay for performance” metrics. The Infectious Diseases Society of America/Society of Healthcare Epidemiology of America updated their guidelines from 2010 regarding hospital surveillance, diagnostic testing, treatment, and infection precautions and control.
Findings. The panel included 14 multidisciplinary experts in epidemiology, diagnosis, infection control, and clinical management of adult and pediatric CDI. They used problem intervention comparison-outcome (PICO)-formatted, evidence-based questions. The selection of data and final recommendations were made in accordance with the GRADE criteria. A total of 35 recommendations were made.
Key clinical recommendations for hospitalists caring for adults: (1) Prescribe vancomycin or fidaxomicin over metronidazole for the initial treatment of CDI (strong recommendation, high quality of evidence); (2) Limit testing to the patients with unexplained new onset diarrhea, which is defined as greater than or equal to 3 unformed stools in 24 hours (weak recommendation, very low-quality evidence); (3) Avoid routine repeat testing within seven days, and only test asymptomatic patients for epidemiologic reasons (strong recommendation, moderate-quality evidence); (4) Minimize the frequency and duration of high-risk antibiotic therapy and the number of antibiotic agents prescribed (strong recommendation, moderate quality of evidence); (5) Discontinue therapy with the inciting antibiotic agent as soon as possible (strong recommendation, moderate quality of evidence).
Caveats. As with the clinical application of any guidelines, individual case adjustments may be required.
Implications. Vancomycin or fidaxomicin should be used for the initial episode of CDI instead of metronidazole.
Mortality and Morbidity in Acutely Ill Adults Treated with Liberal versus Conservative Oxygen Therapy (IOTA): a Systematic Review and Meta-analysis. Chu DK, et al. Lancet. 2018;391(10131):1693-1705.2
Background. Supplemental oxygen is often given to acutely ill hospitalized adults, even when they are not hypoxic or dyspneic. The safety and efficacy of this practice is unknown.
Findings. This systematic review and meta-analysis evaluated 25 randomized controlled trials enrolling 16,037 patients. Patients presented with several conditions, including sepsis, critical illness, stroke, myocardial infarction, and emergency surgery. The fraction of inspired oxygen in the liberal arms varied from 30% to 100%. Most patients randomized to the conservative arm received no supplemental oxygen. Delivery of liberal oxygen to acutely ill adults was associated with increased in-hospital mortality (relative risk [RR]: 1.21; 95% CI: 1.03-1.43), 30-day mortality (RR: 1.14; 95% CI: 1.01-1.29), and 90-day mortality (RR: 1.10; 95% CI: 1.00-1.20). The results were believed to be of high quality and were robust across multiple sensitivity analyses. It seemed that the mortality began to increase when supplemental oxygen raised the peripheral oxygen saturation (Sp02) above a range of 94%-96%.
Caveats. Heterogeneity was observed in the study settings and oxygen delivery. In addition, the cause for increased mortality could not be determined.
Implications. In hospitalized acutely ill adults, “liberal” supplemental oxygen was associated with increased in-hospital and longer-term mortality. The study authors postulated that this finding resulted from the direct toxic effects of oxygen or that oxygen delivery may “mask” illness and lead to delays in diagnosis and treatment. A subsequent clinical practice guideline recommends (1) a target SpO2 of less than 96% for patients receiving oxygen therapy; (2) a target SpO2 range of 90%-94% seems appropriate for most hospitalized adults.3
Do Words Matter? Stigmatizing Language and the Transmission of Bias in the Medical Record. P Goddu A, et al. J Gen Intern Med. 2018;33(5):68-91.4
Background. Previous work has shown that clinician bias affects health outcomes, often worsening health disparities. It is unknown whether clinicians’ language in medical records biases other clinicians and whether this affects patients.
Findings. The investigators randomized medical students and residents in internal and emergency medicine at one academic medical center to review one of two vignettes in the format of notes on the same hypothetical patient with sickle cell disease (SCD) admitted with a pain crisis. One vignette contained stigmatizing language, and the other contained neutral language. The trainees exposed to the vignettes with stigmatizing language showed a more negative attitude toward the patient, as measured by a previously validated scale of attitudes toward patients with SCD (20.6 stigmatizing vs 25.6 neutral, with a total score range of 7-35 for the instrument; higher scores indicate more positive attitudes; P < .001). Furthermore, the intensity of pain treatment was assessed in the resident group and was less aggressive when residents were exposed to stigmatizing language (5.56 stigmatizing vs 6.22 neutral on a scale of 2-7, with higher scores indicating more aggressive pain treatment; P = .003).
Cautions. This research was a single-center study of residents and medical students in two departments. Additionally, the study used vignettes on a hypothetical patient so trainees in the study group might have witnessed stronger stigmatizing language than what is typically observed in an actual patients’ notes.
Implications. Stigmatizing language used in medical records possibly contributed to health disparities by negatively impacting other physicians’ biases and prescribing practices toward patients with SCD at an academic medical center. Clinicians should avoid stigmatizing language in medical records.
Catheter Ablation for Atrial Fibrillation with Heart Failure. Marrouche, NF et al. New Engl J Med. 2018;378:417-427.5
Background. Atrial fibrillation (AF) in patients with heart failure is associated with increased mortality and morbidity. Small-scale studies have suggested that ablation of AF may benefit patients with heart failure.
Findings. This multicenter trial included 398 patients with heart failure and symptomatic AF. Patients had New York Heart Association Class II-IV heart failure, an ejection fraction (EF) of 35% or less, and an internal cardiac defibrillator (ICD). Patients were randomized to either ablation or medical therapy. All enrolled patients either refused, failed, or showed poor tolerance to antiarrhythmic therapy for AF. The primary outcome was death from any cause or hospitalization for heart failure.
The composite endpoint occurred in 28.5% of the ablation group versus 44.6% of patients in the medical therapy group (hazard ratio [HR]: 0.62; 95% CI: 0.43-0.87). Fewer patients in the ablation group died (13% vs 25%; HR: 0.53; 95% CI: 0.32-0.86) or were hospitalized for heart failure (21% vs 36%; HR: 0.56; 95% CI: 0.37-0.83). The patients in the ablation group had higher EF increases above baseline and a greater proportion were in sinus rhythm at the 60-month follow-up visit.
Cautions. The trial was terminated early due to slow recruitment and lower than expected events. Over twice as many patients were lost to follow-up in the ablation group versus the medical therapy group, and by 60 months, AF recurred in 50% of patients who underwent ablation. The sample size was small, and the trial was unblinded.
Implications. Ablation should be considered for AF in patients with heart failure. Additional studies to evaluate ablation versus medical therapy for patients with heart failure and AF are underway.
Medication for Opioid Use Disorder after Nonfatal Opioid Overdose and Association with Mortality. Larochelle MR, et al. Ann Intern Med. 2018;169(3):137-145.6
Background. More than 70,000 Americans died of drug overdose in 2017; this number is higher than the deaths resulting from human immunodeficiency virus, car crash, or gun violence at their peaks.7 Methadone, buprenorphine, and naltrexone are approved by the Federal Drug Administration for the treatment of opioid use disorder (OUD). These medications increase treatment retention; methadone and buprenorphine have been associated with significant decreases in all-cause and overdose mortality.8 However, whether receipt of these medications following a nonfatal opioid overdose reduces mortality is unknown.
Findings. This retrospective cohort study included 17,568 opioid overdose survivors from the Massachusetts’s Public Health Dataset between 2012 and 2014. Only three in 10 of these patients received any medications for OUD over 12 months following overdose. All-cause mortality was 4.7 deaths (95% CI: 4.4-5.0 deaths) per 100 person-years. The relative risk for all-cause mortality was 53% lower with methadone (adjusted hazard ratio [aHR]: 0.47; 95% CI: 0.32-0.71) and 37% lower with buprenorphine (aHR: 0.63; 95% CI: 0.46-0.87).
Caveats. This cohort study may have missed confounders explaining why certain patients received medications for OUD. As a result, association cannot be interpreted as causation.
Implications. Methadone and buprenorphine are associated with a reduction in preventable deaths in patients with OUD who have survived an overdose. All patients with OUD should be considered for therapy.
Outcomes Associated with Apixaban Use in Patients with End-Stage Kidney Disease and Atrial Fibrillation in the United States. Siontis, KC, et al. Circulation. 2018;138:1519–1529.9
Background. Patients with end-stage kidney disease (ESKD) have poor outcomes when treated with warfarin for AF. These patients were excluded from clinical trials of direct oral anticoagulants. The goal of this study was to determine the outcomes of the use of apixaban in patients with ESKD and AF.
Findings. This retrospective cohort study included 25,523 Medicare patients with ESKD and AF on anticoagulants. A 3:1 propensity score match was performed between patients on warfarin and apixaban. Time without stroke/systemic embolism, bleeding (major, gastrointestinal, and intracranial), and death were assessed. A total of 2,351 patients were on apixaban, and 23,172 patients were on warfarin. No difference was observed in the risk of stroke/systemic embolism between apixaban and warfarin (HR 0.88; 95% CI: 0.69-1.12). Apixaban was associated with a lower risk of major bleeding (HR: 0.72; 95% CI: 0.59-0.87). Standard-dose apixaban (5 mg twice a day) was associated with lower risks of stroke/systemic embolism and death compared with reduced-dose apixaban (2.5 mg twice a day; n = 1,317; HR: 0.61; 95% CI: 0.37-0.98; P = .04 for stroke/systemic embolism; HR: 0.64; 95% CI: 0.45-0.92; P = .01 for death) or warfarin (HR: 0.64; 95% CI: 0.42-0.97; P = .04 for stroke/systemic embolism; HR: 0.63; 95% CI: 0.46-0.85; P = .003 for death).
Cautions. There may be unique patient factors that led providers to prescribe apixaban to patients with ESKD.
Implications. The use of standard-dose apixaban appears safe and potentially preferable in patients with ESKD and AF due to reductions in major bleeding, thromboembolism, and mortality risk compared with warfarin. Several additional studies are pending to evaluate the use and dose of apixaban in patients with ESKD and AF.
Outcomes Associated with De-escalating Therapy for Methicillin-Resistant Staphylococcus aureus in Culture-Negative Nosocomial Pneumonia. Cowley MC, et al. Chest. 2019;155(1):53-59.10
Background. Patients diagnosed with hospital-acquired pneumonia (HAP) are often treated empirically with broad-spectrum antibiotics. In many patients with HAP, cultures remain negative, and providers must decide if antibiotics can safely be narrowed. Specifically, the safety of deciding to “de-escalate” and discontinue the coverage for methicillin-resistant Staphylococcus aureus (MRSA) if cultures remain negative is unclear.
Findings. In this single-center retrospective cohort study, 279 patients who were (1) diagnosed with HAP and (2) had negative sputum cultures were enrolled. The patients in whom MRSA coverage was de-escalated by day four were compared with those with continued anti-MRSA coverage. No difference was observed between the two groups in terms of degree of illness or comorbidities. The patients who were de-escalated received five fewer days of anti-MRSA coverage than patients who were not. No difference was noted in the 28-day mortality between the two groups (de-escalation: 23% vs no de-escalation: 28%; 95% CI: −16.1%-6.5%). The incidence of acute kidney injury (AKI) was significantly lower in the de-escalation group (36% vs 50%; 95% CI: −26.9- 0.04), and the overall length of stay was five days shorter in the de-escalation group (95% CI: 0.1-6.4 days).
Caveats. Given the retrospective nature, unmeasured confounders may have impacted the decision to de-escalate anti-MRSA coverage. The observed lower risk of AKI in the de-escalation group may be due to the simultaneous de-escalation of anti-Pseudomonas antibiotic agents in addition to the de-escalation of anti-MRSA coverage, as opposed to de-escalation of the anti-MRSA coverage alone.
Implications. De-escalation of anti-MRSA coverage in patients with HAP with negative cultures is associated with fewer antibiotic days, less AKI, and possibly shorter length of stay.
Partial Oral versus Intravenous Antibiotic Treatment for Endocarditis (POET). Iversen K et al. New Engl J Med. 2019;380(5):415-424.11
Background. Patients with left-sided infective endocarditis are typically treated with up to six weeks of intravenous (IV) antibiotics. The investigators studied the effectiveness and safety of switching to oral antibiotics after at least 10 days of IV therapy.
Findings. This randomized, multicenter, noninferiority trial at cardiac centers across Denmark included 400 adults with left-sided endocarditis who were clinically stable after at least 10 days of IV antibiotics. Half of the patients were randomized to continue IV therapy, whereas the other half was switched to oral antibiotics to complete the treatment course. Six months after therapy, no significant difference was observed between the two groups in terms of the primary composite outcomes, including all-cause mortality, unplanned cardiac surgery, embolic events, or relapse of bacteremia with the primary pathogen (IV-treated group: 12.1%; orally treated group: 9.0% [between-group difference: 3.1%; P = .40]).
Caveats. A total of 20% of the screened population (1,954 adults) was randomized, and about 1% (5/400) of patients used injection drugs. None of the patients had MRSA. Patients in the oral group were assessed two to three times per week as outpatients, which may not be feasible in most settings.
Implications. Switching to oral antibiotics after at least 10 days of IV therapy appears to be safe and effective in selected patients with left-sided endocarditis. However, this study largely excluded patients with injection drug use and/or MRSA infections.
Oral versus Intravenous Antibiotics for Bone and Joint Infection (OVIVA). Li HK, et al. New Engl J Med. 2019;380(5):425-436.12
Background. Most complex orthopedic infections are treated with several weeks of IV antibiotics. This study sought to determine whether oral antibiotics are noninferior to IV antibiotics for bone and joint infections.
Findings. This randomized, multicenter, noninferiority, open-label trial of 1,054 adults with bone and joint infections in the United Kingdom included patients with prosthetic joints, other indwelling joint hardware, and native joint infections. Within seven days of antibiotic medication or within seven days of surgery (if performed), the patients received either IV or oral antibiotics for six weeks with a primary endpoint of treatment failure one year after the study randomization. The choice and duration of antibiotic treatment were determined by the involved infectious disease physician. A majority (77%) of patients received greater than six weeks of therapy. Treatment failure was defined by clinical, microbiologic, or histologic criteria. Most enrolled patients were infected with Staphylococcus aureus, with 10% having methicillin-resistant S. aureus. Treatment failure was more frequent in the IV group than the oral group (14.6% vs 13.2%), and these findings were consistent across all subgroups. More patients discontinued treatment in the IV group than the oral group.
Cautions. This study included a heterogenous population of patients with bone and joint infections, with or without hardware, and with different species of bacteria. Patients with bacteremia, endocarditis, or another indication for IV therapy were excluded. Limited injection drug use history was available for the enrolled patients. Most patients had lower limb infections. Thus, these findings are less applicable to vertebral osteomyelitis. Additionally, the study offered no comparison of specific antibiotics.
Implications. With appropriate oversight from infectious disease specialists, targeted oral therapy may be appropriate for the treatment of osteomyelitis. This shift in practice likely requires more study before broad implementation.
Prognostic Accuracy of the HEART Score for Prediction of Major Adverse Cardiac Events in Patients Presenting with Chest Pain: A Systematic Review and Meta‐analysis. Fernando S, et al. Acad Emerg Med. 2019;26(2):140-151.13
Background. Chest pain accounts for over eight million emergency department (ED) visits yearly in the United States. Of those presenting with chest pain, 10%-20% will experience acute coronary syndrome (ACS) requiring further medical treatment. Given the fear of missing ACS, many low-risk patients are hospitalized. The American Heart Association has advocated using validated predictive scoring models to identify patients with chest pain who are at low risk for short-term major cardiovascular adverse event (MACE) for potential discharge without further testing. The authors evaluated the prognostic accuracy of higher risk scores to predict MACE in adult ED patients presenting with chest pain.
Findings. This study was a systematic review and meta-analysis of 30 prospective and retrospective studies evaluating the history–electrocardiogram–age–risk factors–troponin (HEART) score through May 1, 2018. Meta-analysis compared the sensitivity, specificity, positive likelihood ratios, negative likelihood ratios, and diagnostic odds ratios of the HEART score and the Thrombolysis in Myocardial Infarction (TIMI) score when reported. An intermediate HEART score of 4-6 had a sensitivity of 95.9% and a specificity of 44.6%. A high HEART score of greater than or equal to 7 had a sensitivity of 39.5% and a specificity of 95.0%. Similarly, a high TIMI score of great than or equal to 6 had a sensitivity of only 2.8% and a specificity of 99.6%. The authors concluded that a HEART score of greater than or equal to 4 best identifies patients at risk of MACE who need greater consideration for additional testing.
Caveats. This meta-analysis failed to assess the potential adverse effects of false positive downstream testing. Additionally, no study compared the HEART score with the experienced clinician gestalt, which has often been equivalent to decision rules.
Implication. A HEART score greater than or equal to 4 risk stratifies ED patients with chest pain requiring further consideration for evaluation versus those that can be discharged with low risk for short-term MACE.
1. McDonald LC, Gerding DN, Johnson S, et al. Clinical practice guidelines for clostridium difficile infection in adults and children: 2017 update by the infectious diseases society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018;66(7):e1-e48. https://doi.org/10.1093/cid/cix1085.
2. Chu DK, Kim LH, Young PJ, et al. Mortality and morbidity in acutely ill adults treated with liberal versus conservative oxygen therapy (IOTA): a systematic review and meta-analysis. Lancet. 2018;391(10131):1693-1705. https://doi.org/10.1016/S0140-6736(18)30479-3.
3. Siemieniuk RAC, Chu DK, Kim LH, et al. Oxygen therapy for acutely ill medical patients: a clinical practice guideline. BMJ. 2018;363:k4169. https://doi.org/https://doi.org/10.1136/bmj.k4169
4. A PG, O’Conor KJ, Lanzkron S, et al. Do words matter? Stigmatizing language and the transmission of bias in the medical record. J Gen Intern Med. 2018;33(5):685-691. https://doi.org/10.1007/s11606-017-4289-2.
5. Marrouche NF, Kheirkhahan M, Brachmann J. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med. 2018;379(5):492. https://doi.org/10.1056/NEJMoa1707855.
6. Larochelle MR, Bernson D, Land T, et al. Medication for opioid use disorder after nonfatal opioid overdose and association with mortality: a cohort study. Ann Intern Med. 2018;169(3):137-145. https://doi.org/10.7326/M17-3107.
7. Hedegaard HM, A; Warner, M. Drug Overdose Deaths in the United States, 1999-2017. 2018; https://www.cdc.gov/nchs/products/databriefs/db329.htm. Accessed March 07, 2019.
8. Medications for Opioid Use Disorder Save Lives. 2019; http://www.nationalacademies.org/hmd/Reports/2019/medications-for-opioid-use-disorder-save-lives.aspx. Accessed March 07, 2019.
9. Siontis KC, Zhang X, Eckard A, et al. Outcomes associated with apixaban use in patients with end-stage kidney disease and atrial fibrillation in the United States. Circulation. 2018;138(15):1519-1529. https://doi.org/10.1161/CIRCULATIONAHA.118.035418.
10. Cowley MC, Ritchie DJ, Hampton N, Kollef MH, Micek ST. Outcomes Associated With De-escalating Therapy for Methicillin-Resistant Staphylococcus aureus in Culture-Negative Nosocomial Pneumonia. Chest. 2019;155(1):53-59. https://doi.org/10.1016/j.chest.2018.10.014
11. Iversen K, Ihlemann N, Gill SU, et al. Partial oral versus intravenous antibiotic treatment of endocarditis. N Engl J Med. 2019;380(5):415-424. https://doi.org/10.1056/NEJMoa1808312
12. Li HK, Rombach I, Zambellas R, et al. Oral versus Intravenous Antibiotics for Bone and Joint Infection. N Engl J Med. 2019;380(5):425-436. https://doi.org/10.1056/NEJMoa1710926
13. Fernando SM, Tran A, Cheng W, et al. Prognostic accuracy of the HEART score for prediction of major adverse cardiac events in patients presenting with chest pain: a systematic review and meta-analysis. Acad Emerg Med. 2019;26(2):140-151. https://doi.org/10.1111/acem.13649.
Given the breadth and depth of patients cared for by hospital medicine providers, it is challenging to remain current with the literature. The authors critically appraised the literature from March 2018 to April 2019 for high-quality studies relevant to hospital medicine. Articles were selected based on methodologic rigor and likelihood to impact clinical practice. Thirty articles were selected by the presenting authors for the Hospital Medicine Updates at the 2019 Society of Hospital Medicine (CH, CM) and Society of General Internal Medicine Annual Meetings (BS, AB). After two sequential rounds of voting and group discussion to adjudicate voting discrepancies, the authors selected the 10 most impactful articles for this review. Each article is described below with the key points summarized in the Table.
ESSENTIAL PUBLICATIONS
Clinical Practice Guidelines for Clostridium difficile Infection in Adults and Children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). McDonald LC, et al. Clin Infect Dis. 2018;66(7):e1–e48.1
Background. In the United States, approximately 500,000 Clostridioides difficile infections (CDI) occur annually with 15,000-30,000 deaths. CDI has become a marker of hospital quality and has been placed under numerous “pay for performance” metrics. The Infectious Diseases Society of America/Society of Healthcare Epidemiology of America updated their guidelines from 2010 regarding hospital surveillance, diagnostic testing, treatment, and infection precautions and control.
Findings. The panel included 14 multidisciplinary experts in epidemiology, diagnosis, infection control, and clinical management of adult and pediatric CDI. They used problem intervention comparison-outcome (PICO)-formatted, evidence-based questions. The selection of data and final recommendations were made in accordance with the GRADE criteria. A total of 35 recommendations were made.
Key clinical recommendations for hospitalists caring for adults: (1) Prescribe vancomycin or fidaxomicin over metronidazole for the initial treatment of CDI (strong recommendation, high quality of evidence); (2) Limit testing to the patients with unexplained new onset diarrhea, which is defined as greater than or equal to 3 unformed stools in 24 hours (weak recommendation, very low-quality evidence); (3) Avoid routine repeat testing within seven days, and only test asymptomatic patients for epidemiologic reasons (strong recommendation, moderate-quality evidence); (4) Minimize the frequency and duration of high-risk antibiotic therapy and the number of antibiotic agents prescribed (strong recommendation, moderate quality of evidence); (5) Discontinue therapy with the inciting antibiotic agent as soon as possible (strong recommendation, moderate quality of evidence).
Caveats. As with the clinical application of any guidelines, individual case adjustments may be required.
Implications. Vancomycin or fidaxomicin should be used for the initial episode of CDI instead of metronidazole.
Mortality and Morbidity in Acutely Ill Adults Treated with Liberal versus Conservative Oxygen Therapy (IOTA): a Systematic Review and Meta-analysis. Chu DK, et al. Lancet. 2018;391(10131):1693-1705.2
Background. Supplemental oxygen is often given to acutely ill hospitalized adults, even when they are not hypoxic or dyspneic. The safety and efficacy of this practice is unknown.
Findings. This systematic review and meta-analysis evaluated 25 randomized controlled trials enrolling 16,037 patients. Patients presented with several conditions, including sepsis, critical illness, stroke, myocardial infarction, and emergency surgery. The fraction of inspired oxygen in the liberal arms varied from 30% to 100%. Most patients randomized to the conservative arm received no supplemental oxygen. Delivery of liberal oxygen to acutely ill adults was associated with increased in-hospital mortality (relative risk [RR]: 1.21; 95% CI: 1.03-1.43), 30-day mortality (RR: 1.14; 95% CI: 1.01-1.29), and 90-day mortality (RR: 1.10; 95% CI: 1.00-1.20). The results were believed to be of high quality and were robust across multiple sensitivity analyses. It seemed that the mortality began to increase when supplemental oxygen raised the peripheral oxygen saturation (Sp02) above a range of 94%-96%.
Caveats. Heterogeneity was observed in the study settings and oxygen delivery. In addition, the cause for increased mortality could not be determined.
Implications. In hospitalized acutely ill adults, “liberal” supplemental oxygen was associated with increased in-hospital and longer-term mortality. The study authors postulated that this finding resulted from the direct toxic effects of oxygen or that oxygen delivery may “mask” illness and lead to delays in diagnosis and treatment. A subsequent clinical practice guideline recommends (1) a target SpO2 of less than 96% for patients receiving oxygen therapy; (2) a target SpO2 range of 90%-94% seems appropriate for most hospitalized adults.3
Do Words Matter? Stigmatizing Language and the Transmission of Bias in the Medical Record. P Goddu A, et al. J Gen Intern Med. 2018;33(5):68-91.4
Background. Previous work has shown that clinician bias affects health outcomes, often worsening health disparities. It is unknown whether clinicians’ language in medical records biases other clinicians and whether this affects patients.
Findings. The investigators randomized medical students and residents in internal and emergency medicine at one academic medical center to review one of two vignettes in the format of notes on the same hypothetical patient with sickle cell disease (SCD) admitted with a pain crisis. One vignette contained stigmatizing language, and the other contained neutral language. The trainees exposed to the vignettes with stigmatizing language showed a more negative attitude toward the patient, as measured by a previously validated scale of attitudes toward patients with SCD (20.6 stigmatizing vs 25.6 neutral, with a total score range of 7-35 for the instrument; higher scores indicate more positive attitudes; P < .001). Furthermore, the intensity of pain treatment was assessed in the resident group and was less aggressive when residents were exposed to stigmatizing language (5.56 stigmatizing vs 6.22 neutral on a scale of 2-7, with higher scores indicating more aggressive pain treatment; P = .003).
Cautions. This research was a single-center study of residents and medical students in two departments. Additionally, the study used vignettes on a hypothetical patient so trainees in the study group might have witnessed stronger stigmatizing language than what is typically observed in an actual patients’ notes.
Implications. Stigmatizing language used in medical records possibly contributed to health disparities by negatively impacting other physicians’ biases and prescribing practices toward patients with SCD at an academic medical center. Clinicians should avoid stigmatizing language in medical records.
Catheter Ablation for Atrial Fibrillation with Heart Failure. Marrouche, NF et al. New Engl J Med. 2018;378:417-427.5
Background. Atrial fibrillation (AF) in patients with heart failure is associated with increased mortality and morbidity. Small-scale studies have suggested that ablation of AF may benefit patients with heart failure.
Findings. This multicenter trial included 398 patients with heart failure and symptomatic AF. Patients had New York Heart Association Class II-IV heart failure, an ejection fraction (EF) of 35% or less, and an internal cardiac defibrillator (ICD). Patients were randomized to either ablation or medical therapy. All enrolled patients either refused, failed, or showed poor tolerance to antiarrhythmic therapy for AF. The primary outcome was death from any cause or hospitalization for heart failure.
The composite endpoint occurred in 28.5% of the ablation group versus 44.6% of patients in the medical therapy group (hazard ratio [HR]: 0.62; 95% CI: 0.43-0.87). Fewer patients in the ablation group died (13% vs 25%; HR: 0.53; 95% CI: 0.32-0.86) or were hospitalized for heart failure (21% vs 36%; HR: 0.56; 95% CI: 0.37-0.83). The patients in the ablation group had higher EF increases above baseline and a greater proportion were in sinus rhythm at the 60-month follow-up visit.
Cautions. The trial was terminated early due to slow recruitment and lower than expected events. Over twice as many patients were lost to follow-up in the ablation group versus the medical therapy group, and by 60 months, AF recurred in 50% of patients who underwent ablation. The sample size was small, and the trial was unblinded.
Implications. Ablation should be considered for AF in patients with heart failure. Additional studies to evaluate ablation versus medical therapy for patients with heart failure and AF are underway.
Medication for Opioid Use Disorder after Nonfatal Opioid Overdose and Association with Mortality. Larochelle MR, et al. Ann Intern Med. 2018;169(3):137-145.6
Background. More than 70,000 Americans died of drug overdose in 2017; this number is higher than the deaths resulting from human immunodeficiency virus, car crash, or gun violence at their peaks.7 Methadone, buprenorphine, and naltrexone are approved by the Federal Drug Administration for the treatment of opioid use disorder (OUD). These medications increase treatment retention; methadone and buprenorphine have been associated with significant decreases in all-cause and overdose mortality.8 However, whether receipt of these medications following a nonfatal opioid overdose reduces mortality is unknown.
Findings. This retrospective cohort study included 17,568 opioid overdose survivors from the Massachusetts’s Public Health Dataset between 2012 and 2014. Only three in 10 of these patients received any medications for OUD over 12 months following overdose. All-cause mortality was 4.7 deaths (95% CI: 4.4-5.0 deaths) per 100 person-years. The relative risk for all-cause mortality was 53% lower with methadone (adjusted hazard ratio [aHR]: 0.47; 95% CI: 0.32-0.71) and 37% lower with buprenorphine (aHR: 0.63; 95% CI: 0.46-0.87).
Caveats. This cohort study may have missed confounders explaining why certain patients received medications for OUD. As a result, association cannot be interpreted as causation.
Implications. Methadone and buprenorphine are associated with a reduction in preventable deaths in patients with OUD who have survived an overdose. All patients with OUD should be considered for therapy.
Outcomes Associated with Apixaban Use in Patients with End-Stage Kidney Disease and Atrial Fibrillation in the United States. Siontis, KC, et al. Circulation. 2018;138:1519–1529.9
Background. Patients with end-stage kidney disease (ESKD) have poor outcomes when treated with warfarin for AF. These patients were excluded from clinical trials of direct oral anticoagulants. The goal of this study was to determine the outcomes of the use of apixaban in patients with ESKD and AF.
Findings. This retrospective cohort study included 25,523 Medicare patients with ESKD and AF on anticoagulants. A 3:1 propensity score match was performed between patients on warfarin and apixaban. Time without stroke/systemic embolism, bleeding (major, gastrointestinal, and intracranial), and death were assessed. A total of 2,351 patients were on apixaban, and 23,172 patients were on warfarin. No difference was observed in the risk of stroke/systemic embolism between apixaban and warfarin (HR 0.88; 95% CI: 0.69-1.12). Apixaban was associated with a lower risk of major bleeding (HR: 0.72; 95% CI: 0.59-0.87). Standard-dose apixaban (5 mg twice a day) was associated with lower risks of stroke/systemic embolism and death compared with reduced-dose apixaban (2.5 mg twice a day; n = 1,317; HR: 0.61; 95% CI: 0.37-0.98; P = .04 for stroke/systemic embolism; HR: 0.64; 95% CI: 0.45-0.92; P = .01 for death) or warfarin (HR: 0.64; 95% CI: 0.42-0.97; P = .04 for stroke/systemic embolism; HR: 0.63; 95% CI: 0.46-0.85; P = .003 for death).
Cautions. There may be unique patient factors that led providers to prescribe apixaban to patients with ESKD.
Implications. The use of standard-dose apixaban appears safe and potentially preferable in patients with ESKD and AF due to reductions in major bleeding, thromboembolism, and mortality risk compared with warfarin. Several additional studies are pending to evaluate the use and dose of apixaban in patients with ESKD and AF.
Outcomes Associated with De-escalating Therapy for Methicillin-Resistant Staphylococcus aureus in Culture-Negative Nosocomial Pneumonia. Cowley MC, et al. Chest. 2019;155(1):53-59.10
Background. Patients diagnosed with hospital-acquired pneumonia (HAP) are often treated empirically with broad-spectrum antibiotics. In many patients with HAP, cultures remain negative, and providers must decide if antibiotics can safely be narrowed. Specifically, the safety of deciding to “de-escalate” and discontinue the coverage for methicillin-resistant Staphylococcus aureus (MRSA) if cultures remain negative is unclear.
Findings. In this single-center retrospective cohort study, 279 patients who were (1) diagnosed with HAP and (2) had negative sputum cultures were enrolled. The patients in whom MRSA coverage was de-escalated by day four were compared with those with continued anti-MRSA coverage. No difference was observed between the two groups in terms of degree of illness or comorbidities. The patients who were de-escalated received five fewer days of anti-MRSA coverage than patients who were not. No difference was noted in the 28-day mortality between the two groups (de-escalation: 23% vs no de-escalation: 28%; 95% CI: −16.1%-6.5%). The incidence of acute kidney injury (AKI) was significantly lower in the de-escalation group (36% vs 50%; 95% CI: −26.9- 0.04), and the overall length of stay was five days shorter in the de-escalation group (95% CI: 0.1-6.4 days).
Caveats. Given the retrospective nature, unmeasured confounders may have impacted the decision to de-escalate anti-MRSA coverage. The observed lower risk of AKI in the de-escalation group may be due to the simultaneous de-escalation of anti-Pseudomonas antibiotic agents in addition to the de-escalation of anti-MRSA coverage, as opposed to de-escalation of the anti-MRSA coverage alone.
Implications. De-escalation of anti-MRSA coverage in patients with HAP with negative cultures is associated with fewer antibiotic days, less AKI, and possibly shorter length of stay.
Partial Oral versus Intravenous Antibiotic Treatment for Endocarditis (POET). Iversen K et al. New Engl J Med. 2019;380(5):415-424.11
Background. Patients with left-sided infective endocarditis are typically treated with up to six weeks of intravenous (IV) antibiotics. The investigators studied the effectiveness and safety of switching to oral antibiotics after at least 10 days of IV therapy.
Findings. This randomized, multicenter, noninferiority trial at cardiac centers across Denmark included 400 adults with left-sided endocarditis who were clinically stable after at least 10 days of IV antibiotics. Half of the patients were randomized to continue IV therapy, whereas the other half was switched to oral antibiotics to complete the treatment course. Six months after therapy, no significant difference was observed between the two groups in terms of the primary composite outcomes, including all-cause mortality, unplanned cardiac surgery, embolic events, or relapse of bacteremia with the primary pathogen (IV-treated group: 12.1%; orally treated group: 9.0% [between-group difference: 3.1%; P = .40]).
Caveats. A total of 20% of the screened population (1,954 adults) was randomized, and about 1% (5/400) of patients used injection drugs. None of the patients had MRSA. Patients in the oral group were assessed two to three times per week as outpatients, which may not be feasible in most settings.
Implications. Switching to oral antibiotics after at least 10 days of IV therapy appears to be safe and effective in selected patients with left-sided endocarditis. However, this study largely excluded patients with injection drug use and/or MRSA infections.
Oral versus Intravenous Antibiotics for Bone and Joint Infection (OVIVA). Li HK, et al. New Engl J Med. 2019;380(5):425-436.12
Background. Most complex orthopedic infections are treated with several weeks of IV antibiotics. This study sought to determine whether oral antibiotics are noninferior to IV antibiotics for bone and joint infections.
Findings. This randomized, multicenter, noninferiority, open-label trial of 1,054 adults with bone and joint infections in the United Kingdom included patients with prosthetic joints, other indwelling joint hardware, and native joint infections. Within seven days of antibiotic medication or within seven days of surgery (if performed), the patients received either IV or oral antibiotics for six weeks with a primary endpoint of treatment failure one year after the study randomization. The choice and duration of antibiotic treatment were determined by the involved infectious disease physician. A majority (77%) of patients received greater than six weeks of therapy. Treatment failure was defined by clinical, microbiologic, or histologic criteria. Most enrolled patients were infected with Staphylococcus aureus, with 10% having methicillin-resistant S. aureus. Treatment failure was more frequent in the IV group than the oral group (14.6% vs 13.2%), and these findings were consistent across all subgroups. More patients discontinued treatment in the IV group than the oral group.
Cautions. This study included a heterogenous population of patients with bone and joint infections, with or without hardware, and with different species of bacteria. Patients with bacteremia, endocarditis, or another indication for IV therapy were excluded. Limited injection drug use history was available for the enrolled patients. Most patients had lower limb infections. Thus, these findings are less applicable to vertebral osteomyelitis. Additionally, the study offered no comparison of specific antibiotics.
Implications. With appropriate oversight from infectious disease specialists, targeted oral therapy may be appropriate for the treatment of osteomyelitis. This shift in practice likely requires more study before broad implementation.
Prognostic Accuracy of the HEART Score for Prediction of Major Adverse Cardiac Events in Patients Presenting with Chest Pain: A Systematic Review and Meta‐analysis. Fernando S, et al. Acad Emerg Med. 2019;26(2):140-151.13
Background. Chest pain accounts for over eight million emergency department (ED) visits yearly in the United States. Of those presenting with chest pain, 10%-20% will experience acute coronary syndrome (ACS) requiring further medical treatment. Given the fear of missing ACS, many low-risk patients are hospitalized. The American Heart Association has advocated using validated predictive scoring models to identify patients with chest pain who are at low risk for short-term major cardiovascular adverse event (MACE) for potential discharge without further testing. The authors evaluated the prognostic accuracy of higher risk scores to predict MACE in adult ED patients presenting with chest pain.
Findings. This study was a systematic review and meta-analysis of 30 prospective and retrospective studies evaluating the history–electrocardiogram–age–risk factors–troponin (HEART) score through May 1, 2018. Meta-analysis compared the sensitivity, specificity, positive likelihood ratios, negative likelihood ratios, and diagnostic odds ratios of the HEART score and the Thrombolysis in Myocardial Infarction (TIMI) score when reported. An intermediate HEART score of 4-6 had a sensitivity of 95.9% and a specificity of 44.6%. A high HEART score of greater than or equal to 7 had a sensitivity of 39.5% and a specificity of 95.0%. Similarly, a high TIMI score of great than or equal to 6 had a sensitivity of only 2.8% and a specificity of 99.6%. The authors concluded that a HEART score of greater than or equal to 4 best identifies patients at risk of MACE who need greater consideration for additional testing.
Caveats. This meta-analysis failed to assess the potential adverse effects of false positive downstream testing. Additionally, no study compared the HEART score with the experienced clinician gestalt, which has often been equivalent to decision rules.
Implication. A HEART score greater than or equal to 4 risk stratifies ED patients with chest pain requiring further consideration for evaluation versus those that can be discharged with low risk for short-term MACE.
Given the breadth and depth of patients cared for by hospital medicine providers, it is challenging to remain current with the literature. The authors critically appraised the literature from March 2018 to April 2019 for high-quality studies relevant to hospital medicine. Articles were selected based on methodologic rigor and likelihood to impact clinical practice. Thirty articles were selected by the presenting authors for the Hospital Medicine Updates at the 2019 Society of Hospital Medicine (CH, CM) and Society of General Internal Medicine Annual Meetings (BS, AB). After two sequential rounds of voting and group discussion to adjudicate voting discrepancies, the authors selected the 10 most impactful articles for this review. Each article is described below with the key points summarized in the Table.
ESSENTIAL PUBLICATIONS
Clinical Practice Guidelines for Clostridium difficile Infection in Adults and Children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). McDonald LC, et al. Clin Infect Dis. 2018;66(7):e1–e48.1
Background. In the United States, approximately 500,000 Clostridioides difficile infections (CDI) occur annually with 15,000-30,000 deaths. CDI has become a marker of hospital quality and has been placed under numerous “pay for performance” metrics. The Infectious Diseases Society of America/Society of Healthcare Epidemiology of America updated their guidelines from 2010 regarding hospital surveillance, diagnostic testing, treatment, and infection precautions and control.
Findings. The panel included 14 multidisciplinary experts in epidemiology, diagnosis, infection control, and clinical management of adult and pediatric CDI. They used problem intervention comparison-outcome (PICO)-formatted, evidence-based questions. The selection of data and final recommendations were made in accordance with the GRADE criteria. A total of 35 recommendations were made.
Key clinical recommendations for hospitalists caring for adults: (1) Prescribe vancomycin or fidaxomicin over metronidazole for the initial treatment of CDI (strong recommendation, high quality of evidence); (2) Limit testing to the patients with unexplained new onset diarrhea, which is defined as greater than or equal to 3 unformed stools in 24 hours (weak recommendation, very low-quality evidence); (3) Avoid routine repeat testing within seven days, and only test asymptomatic patients for epidemiologic reasons (strong recommendation, moderate-quality evidence); (4) Minimize the frequency and duration of high-risk antibiotic therapy and the number of antibiotic agents prescribed (strong recommendation, moderate quality of evidence); (5) Discontinue therapy with the inciting antibiotic agent as soon as possible (strong recommendation, moderate quality of evidence).
Caveats. As with the clinical application of any guidelines, individual case adjustments may be required.
Implications. Vancomycin or fidaxomicin should be used for the initial episode of CDI instead of metronidazole.
Mortality and Morbidity in Acutely Ill Adults Treated with Liberal versus Conservative Oxygen Therapy (IOTA): a Systematic Review and Meta-analysis. Chu DK, et al. Lancet. 2018;391(10131):1693-1705.2
Background. Supplemental oxygen is often given to acutely ill hospitalized adults, even when they are not hypoxic or dyspneic. The safety and efficacy of this practice is unknown.
Findings. This systematic review and meta-analysis evaluated 25 randomized controlled trials enrolling 16,037 patients. Patients presented with several conditions, including sepsis, critical illness, stroke, myocardial infarction, and emergency surgery. The fraction of inspired oxygen in the liberal arms varied from 30% to 100%. Most patients randomized to the conservative arm received no supplemental oxygen. Delivery of liberal oxygen to acutely ill adults was associated with increased in-hospital mortality (relative risk [RR]: 1.21; 95% CI: 1.03-1.43), 30-day mortality (RR: 1.14; 95% CI: 1.01-1.29), and 90-day mortality (RR: 1.10; 95% CI: 1.00-1.20). The results were believed to be of high quality and were robust across multiple sensitivity analyses. It seemed that the mortality began to increase when supplemental oxygen raised the peripheral oxygen saturation (Sp02) above a range of 94%-96%.
Caveats. Heterogeneity was observed in the study settings and oxygen delivery. In addition, the cause for increased mortality could not be determined.
Implications. In hospitalized acutely ill adults, “liberal” supplemental oxygen was associated with increased in-hospital and longer-term mortality. The study authors postulated that this finding resulted from the direct toxic effects of oxygen or that oxygen delivery may “mask” illness and lead to delays in diagnosis and treatment. A subsequent clinical practice guideline recommends (1) a target SpO2 of less than 96% for patients receiving oxygen therapy; (2) a target SpO2 range of 90%-94% seems appropriate for most hospitalized adults.3
Do Words Matter? Stigmatizing Language and the Transmission of Bias in the Medical Record. P Goddu A, et al. J Gen Intern Med. 2018;33(5):68-91.4
Background. Previous work has shown that clinician bias affects health outcomes, often worsening health disparities. It is unknown whether clinicians’ language in medical records biases other clinicians and whether this affects patients.
Findings. The investigators randomized medical students and residents in internal and emergency medicine at one academic medical center to review one of two vignettes in the format of notes on the same hypothetical patient with sickle cell disease (SCD) admitted with a pain crisis. One vignette contained stigmatizing language, and the other contained neutral language. The trainees exposed to the vignettes with stigmatizing language showed a more negative attitude toward the patient, as measured by a previously validated scale of attitudes toward patients with SCD (20.6 stigmatizing vs 25.6 neutral, with a total score range of 7-35 for the instrument; higher scores indicate more positive attitudes; P < .001). Furthermore, the intensity of pain treatment was assessed in the resident group and was less aggressive when residents were exposed to stigmatizing language (5.56 stigmatizing vs 6.22 neutral on a scale of 2-7, with higher scores indicating more aggressive pain treatment; P = .003).
Cautions. This research was a single-center study of residents and medical students in two departments. Additionally, the study used vignettes on a hypothetical patient so trainees in the study group might have witnessed stronger stigmatizing language than what is typically observed in an actual patients’ notes.
Implications. Stigmatizing language used in medical records possibly contributed to health disparities by negatively impacting other physicians’ biases and prescribing practices toward patients with SCD at an academic medical center. Clinicians should avoid stigmatizing language in medical records.
Catheter Ablation for Atrial Fibrillation with Heart Failure. Marrouche, NF et al. New Engl J Med. 2018;378:417-427.5
Background. Atrial fibrillation (AF) in patients with heart failure is associated with increased mortality and morbidity. Small-scale studies have suggested that ablation of AF may benefit patients with heart failure.
Findings. This multicenter trial included 398 patients with heart failure and symptomatic AF. Patients had New York Heart Association Class II-IV heart failure, an ejection fraction (EF) of 35% or less, and an internal cardiac defibrillator (ICD). Patients were randomized to either ablation or medical therapy. All enrolled patients either refused, failed, or showed poor tolerance to antiarrhythmic therapy for AF. The primary outcome was death from any cause or hospitalization for heart failure.
The composite endpoint occurred in 28.5% of the ablation group versus 44.6% of patients in the medical therapy group (hazard ratio [HR]: 0.62; 95% CI: 0.43-0.87). Fewer patients in the ablation group died (13% vs 25%; HR: 0.53; 95% CI: 0.32-0.86) or were hospitalized for heart failure (21% vs 36%; HR: 0.56; 95% CI: 0.37-0.83). The patients in the ablation group had higher EF increases above baseline and a greater proportion were in sinus rhythm at the 60-month follow-up visit.
Cautions. The trial was terminated early due to slow recruitment and lower than expected events. Over twice as many patients were lost to follow-up in the ablation group versus the medical therapy group, and by 60 months, AF recurred in 50% of patients who underwent ablation. The sample size was small, and the trial was unblinded.
Implications. Ablation should be considered for AF in patients with heart failure. Additional studies to evaluate ablation versus medical therapy for patients with heart failure and AF are underway.
Medication for Opioid Use Disorder after Nonfatal Opioid Overdose and Association with Mortality. Larochelle MR, et al. Ann Intern Med. 2018;169(3):137-145.6
Background. More than 70,000 Americans died of drug overdose in 2017; this number is higher than the deaths resulting from human immunodeficiency virus, car crash, or gun violence at their peaks.7 Methadone, buprenorphine, and naltrexone are approved by the Federal Drug Administration for the treatment of opioid use disorder (OUD). These medications increase treatment retention; methadone and buprenorphine have been associated with significant decreases in all-cause and overdose mortality.8 However, whether receipt of these medications following a nonfatal opioid overdose reduces mortality is unknown.
Findings. This retrospective cohort study included 17,568 opioid overdose survivors from the Massachusetts’s Public Health Dataset between 2012 and 2014. Only three in 10 of these patients received any medications for OUD over 12 months following overdose. All-cause mortality was 4.7 deaths (95% CI: 4.4-5.0 deaths) per 100 person-years. The relative risk for all-cause mortality was 53% lower with methadone (adjusted hazard ratio [aHR]: 0.47; 95% CI: 0.32-0.71) and 37% lower with buprenorphine (aHR: 0.63; 95% CI: 0.46-0.87).
Caveats. This cohort study may have missed confounders explaining why certain patients received medications for OUD. As a result, association cannot be interpreted as causation.
Implications. Methadone and buprenorphine are associated with a reduction in preventable deaths in patients with OUD who have survived an overdose. All patients with OUD should be considered for therapy.
Outcomes Associated with Apixaban Use in Patients with End-Stage Kidney Disease and Atrial Fibrillation in the United States. Siontis, KC, et al. Circulation. 2018;138:1519–1529.9
Background. Patients with end-stage kidney disease (ESKD) have poor outcomes when treated with warfarin for AF. These patients were excluded from clinical trials of direct oral anticoagulants. The goal of this study was to determine the outcomes of the use of apixaban in patients with ESKD and AF.
Findings. This retrospective cohort study included 25,523 Medicare patients with ESKD and AF on anticoagulants. A 3:1 propensity score match was performed between patients on warfarin and apixaban. Time without stroke/systemic embolism, bleeding (major, gastrointestinal, and intracranial), and death were assessed. A total of 2,351 patients were on apixaban, and 23,172 patients were on warfarin. No difference was observed in the risk of stroke/systemic embolism between apixaban and warfarin (HR 0.88; 95% CI: 0.69-1.12). Apixaban was associated with a lower risk of major bleeding (HR: 0.72; 95% CI: 0.59-0.87). Standard-dose apixaban (5 mg twice a day) was associated with lower risks of stroke/systemic embolism and death compared with reduced-dose apixaban (2.5 mg twice a day; n = 1,317; HR: 0.61; 95% CI: 0.37-0.98; P = .04 for stroke/systemic embolism; HR: 0.64; 95% CI: 0.45-0.92; P = .01 for death) or warfarin (HR: 0.64; 95% CI: 0.42-0.97; P = .04 for stroke/systemic embolism; HR: 0.63; 95% CI: 0.46-0.85; P = .003 for death).
Cautions. There may be unique patient factors that led providers to prescribe apixaban to patients with ESKD.
Implications. The use of standard-dose apixaban appears safe and potentially preferable in patients with ESKD and AF due to reductions in major bleeding, thromboembolism, and mortality risk compared with warfarin. Several additional studies are pending to evaluate the use and dose of apixaban in patients with ESKD and AF.
Outcomes Associated with De-escalating Therapy for Methicillin-Resistant Staphylococcus aureus in Culture-Negative Nosocomial Pneumonia. Cowley MC, et al. Chest. 2019;155(1):53-59.10
Background. Patients diagnosed with hospital-acquired pneumonia (HAP) are often treated empirically with broad-spectrum antibiotics. In many patients with HAP, cultures remain negative, and providers must decide if antibiotics can safely be narrowed. Specifically, the safety of deciding to “de-escalate” and discontinue the coverage for methicillin-resistant Staphylococcus aureus (MRSA) if cultures remain negative is unclear.
Findings. In this single-center retrospective cohort study, 279 patients who were (1) diagnosed with HAP and (2) had negative sputum cultures were enrolled. The patients in whom MRSA coverage was de-escalated by day four were compared with those with continued anti-MRSA coverage. No difference was observed between the two groups in terms of degree of illness or comorbidities. The patients who were de-escalated received five fewer days of anti-MRSA coverage than patients who were not. No difference was noted in the 28-day mortality between the two groups (de-escalation: 23% vs no de-escalation: 28%; 95% CI: −16.1%-6.5%). The incidence of acute kidney injury (AKI) was significantly lower in the de-escalation group (36% vs 50%; 95% CI: −26.9- 0.04), and the overall length of stay was five days shorter in the de-escalation group (95% CI: 0.1-6.4 days).
Caveats. Given the retrospective nature, unmeasured confounders may have impacted the decision to de-escalate anti-MRSA coverage. The observed lower risk of AKI in the de-escalation group may be due to the simultaneous de-escalation of anti-Pseudomonas antibiotic agents in addition to the de-escalation of anti-MRSA coverage, as opposed to de-escalation of the anti-MRSA coverage alone.
Implications. De-escalation of anti-MRSA coverage in patients with HAP with negative cultures is associated with fewer antibiotic days, less AKI, and possibly shorter length of stay.
Partial Oral versus Intravenous Antibiotic Treatment for Endocarditis (POET). Iversen K et al. New Engl J Med. 2019;380(5):415-424.11
Background. Patients with left-sided infective endocarditis are typically treated with up to six weeks of intravenous (IV) antibiotics. The investigators studied the effectiveness and safety of switching to oral antibiotics after at least 10 days of IV therapy.
Findings. This randomized, multicenter, noninferiority trial at cardiac centers across Denmark included 400 adults with left-sided endocarditis who were clinically stable after at least 10 days of IV antibiotics. Half of the patients were randomized to continue IV therapy, whereas the other half was switched to oral antibiotics to complete the treatment course. Six months after therapy, no significant difference was observed between the two groups in terms of the primary composite outcomes, including all-cause mortality, unplanned cardiac surgery, embolic events, or relapse of bacteremia with the primary pathogen (IV-treated group: 12.1%; orally treated group: 9.0% [between-group difference: 3.1%; P = .40]).
Caveats. A total of 20% of the screened population (1,954 adults) was randomized, and about 1% (5/400) of patients used injection drugs. None of the patients had MRSA. Patients in the oral group were assessed two to three times per week as outpatients, which may not be feasible in most settings.
Implications. Switching to oral antibiotics after at least 10 days of IV therapy appears to be safe and effective in selected patients with left-sided endocarditis. However, this study largely excluded patients with injection drug use and/or MRSA infections.
Oral versus Intravenous Antibiotics for Bone and Joint Infection (OVIVA). Li HK, et al. New Engl J Med. 2019;380(5):425-436.12
Background. Most complex orthopedic infections are treated with several weeks of IV antibiotics. This study sought to determine whether oral antibiotics are noninferior to IV antibiotics for bone and joint infections.
Findings. This randomized, multicenter, noninferiority, open-label trial of 1,054 adults with bone and joint infections in the United Kingdom included patients with prosthetic joints, other indwelling joint hardware, and native joint infections. Within seven days of antibiotic medication or within seven days of surgery (if performed), the patients received either IV or oral antibiotics for six weeks with a primary endpoint of treatment failure one year after the study randomization. The choice and duration of antibiotic treatment were determined by the involved infectious disease physician. A majority (77%) of patients received greater than six weeks of therapy. Treatment failure was defined by clinical, microbiologic, or histologic criteria. Most enrolled patients were infected with Staphylococcus aureus, with 10% having methicillin-resistant S. aureus. Treatment failure was more frequent in the IV group than the oral group (14.6% vs 13.2%), and these findings were consistent across all subgroups. More patients discontinued treatment in the IV group than the oral group.
Cautions. This study included a heterogenous population of patients with bone and joint infections, with or without hardware, and with different species of bacteria. Patients with bacteremia, endocarditis, or another indication for IV therapy were excluded. Limited injection drug use history was available for the enrolled patients. Most patients had lower limb infections. Thus, these findings are less applicable to vertebral osteomyelitis. Additionally, the study offered no comparison of specific antibiotics.
Implications. With appropriate oversight from infectious disease specialists, targeted oral therapy may be appropriate for the treatment of osteomyelitis. This shift in practice likely requires more study before broad implementation.
Prognostic Accuracy of the HEART Score for Prediction of Major Adverse Cardiac Events in Patients Presenting with Chest Pain: A Systematic Review and Meta‐analysis. Fernando S, et al. Acad Emerg Med. 2019;26(2):140-151.13
Background. Chest pain accounts for over eight million emergency department (ED) visits yearly in the United States. Of those presenting with chest pain, 10%-20% will experience acute coronary syndrome (ACS) requiring further medical treatment. Given the fear of missing ACS, many low-risk patients are hospitalized. The American Heart Association has advocated using validated predictive scoring models to identify patients with chest pain who are at low risk for short-term major cardiovascular adverse event (MACE) for potential discharge without further testing. The authors evaluated the prognostic accuracy of higher risk scores to predict MACE in adult ED patients presenting with chest pain.
Findings. This study was a systematic review and meta-analysis of 30 prospective and retrospective studies evaluating the history–electrocardiogram–age–risk factors–troponin (HEART) score through May 1, 2018. Meta-analysis compared the sensitivity, specificity, positive likelihood ratios, negative likelihood ratios, and diagnostic odds ratios of the HEART score and the Thrombolysis in Myocardial Infarction (TIMI) score when reported. An intermediate HEART score of 4-6 had a sensitivity of 95.9% and a specificity of 44.6%. A high HEART score of greater than or equal to 7 had a sensitivity of 39.5% and a specificity of 95.0%. Similarly, a high TIMI score of great than or equal to 6 had a sensitivity of only 2.8% and a specificity of 99.6%. The authors concluded that a HEART score of greater than or equal to 4 best identifies patients at risk of MACE who need greater consideration for additional testing.
Caveats. This meta-analysis failed to assess the potential adverse effects of false positive downstream testing. Additionally, no study compared the HEART score with the experienced clinician gestalt, which has often been equivalent to decision rules.
Implication. A HEART score greater than or equal to 4 risk stratifies ED patients with chest pain requiring further consideration for evaluation versus those that can be discharged with low risk for short-term MACE.
1. McDonald LC, Gerding DN, Johnson S, et al. Clinical practice guidelines for clostridium difficile infection in adults and children: 2017 update by the infectious diseases society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018;66(7):e1-e48. https://doi.org/10.1093/cid/cix1085.
2. Chu DK, Kim LH, Young PJ, et al. Mortality and morbidity in acutely ill adults treated with liberal versus conservative oxygen therapy (IOTA): a systematic review and meta-analysis. Lancet. 2018;391(10131):1693-1705. https://doi.org/10.1016/S0140-6736(18)30479-3.
3. Siemieniuk RAC, Chu DK, Kim LH, et al. Oxygen therapy for acutely ill medical patients: a clinical practice guideline. BMJ. 2018;363:k4169. https://doi.org/https://doi.org/10.1136/bmj.k4169
4. A PG, O’Conor KJ, Lanzkron S, et al. Do words matter? Stigmatizing language and the transmission of bias in the medical record. J Gen Intern Med. 2018;33(5):685-691. https://doi.org/10.1007/s11606-017-4289-2.
5. Marrouche NF, Kheirkhahan M, Brachmann J. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med. 2018;379(5):492. https://doi.org/10.1056/NEJMoa1707855.
6. Larochelle MR, Bernson D, Land T, et al. Medication for opioid use disorder after nonfatal opioid overdose and association with mortality: a cohort study. Ann Intern Med. 2018;169(3):137-145. https://doi.org/10.7326/M17-3107.
7. Hedegaard HM, A; Warner, M. Drug Overdose Deaths in the United States, 1999-2017. 2018; https://www.cdc.gov/nchs/products/databriefs/db329.htm. Accessed March 07, 2019.
8. Medications for Opioid Use Disorder Save Lives. 2019; http://www.nationalacademies.org/hmd/Reports/2019/medications-for-opioid-use-disorder-save-lives.aspx. Accessed March 07, 2019.
9. Siontis KC, Zhang X, Eckard A, et al. Outcomes associated with apixaban use in patients with end-stage kidney disease and atrial fibrillation in the United States. Circulation. 2018;138(15):1519-1529. https://doi.org/10.1161/CIRCULATIONAHA.118.035418.
10. Cowley MC, Ritchie DJ, Hampton N, Kollef MH, Micek ST. Outcomes Associated With De-escalating Therapy for Methicillin-Resistant Staphylococcus aureus in Culture-Negative Nosocomial Pneumonia. Chest. 2019;155(1):53-59. https://doi.org/10.1016/j.chest.2018.10.014
11. Iversen K, Ihlemann N, Gill SU, et al. Partial oral versus intravenous antibiotic treatment of endocarditis. N Engl J Med. 2019;380(5):415-424. https://doi.org/10.1056/NEJMoa1808312
12. Li HK, Rombach I, Zambellas R, et al. Oral versus Intravenous Antibiotics for Bone and Joint Infection. N Engl J Med. 2019;380(5):425-436. https://doi.org/10.1056/NEJMoa1710926
13. Fernando SM, Tran A, Cheng W, et al. Prognostic accuracy of the HEART score for prediction of major adverse cardiac events in patients presenting with chest pain: a systematic review and meta-analysis. Acad Emerg Med. 2019;26(2):140-151. https://doi.org/10.1111/acem.13649.
1. McDonald LC, Gerding DN, Johnson S, et al. Clinical practice guidelines for clostridium difficile infection in adults and children: 2017 update by the infectious diseases society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018;66(7):e1-e48. https://doi.org/10.1093/cid/cix1085.
2. Chu DK, Kim LH, Young PJ, et al. Mortality and morbidity in acutely ill adults treated with liberal versus conservative oxygen therapy (IOTA): a systematic review and meta-analysis. Lancet. 2018;391(10131):1693-1705. https://doi.org/10.1016/S0140-6736(18)30479-3.
3. Siemieniuk RAC, Chu DK, Kim LH, et al. Oxygen therapy for acutely ill medical patients: a clinical practice guideline. BMJ. 2018;363:k4169. https://doi.org/https://doi.org/10.1136/bmj.k4169
4. A PG, O’Conor KJ, Lanzkron S, et al. Do words matter? Stigmatizing language and the transmission of bias in the medical record. J Gen Intern Med. 2018;33(5):685-691. https://doi.org/10.1007/s11606-017-4289-2.
5. Marrouche NF, Kheirkhahan M, Brachmann J. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med. 2018;379(5):492. https://doi.org/10.1056/NEJMoa1707855.
6. Larochelle MR, Bernson D, Land T, et al. Medication for opioid use disorder after nonfatal opioid overdose and association with mortality: a cohort study. Ann Intern Med. 2018;169(3):137-145. https://doi.org/10.7326/M17-3107.
7. Hedegaard HM, A; Warner, M. Drug Overdose Deaths in the United States, 1999-2017. 2018; https://www.cdc.gov/nchs/products/databriefs/db329.htm. Accessed March 07, 2019.
8. Medications for Opioid Use Disorder Save Lives. 2019; http://www.nationalacademies.org/hmd/Reports/2019/medications-for-opioid-use-disorder-save-lives.aspx. Accessed March 07, 2019.
9. Siontis KC, Zhang X, Eckard A, et al. Outcomes associated with apixaban use in patients with end-stage kidney disease and atrial fibrillation in the United States. Circulation. 2018;138(15):1519-1529. https://doi.org/10.1161/CIRCULATIONAHA.118.035418.
10. Cowley MC, Ritchie DJ, Hampton N, Kollef MH, Micek ST. Outcomes Associated With De-escalating Therapy for Methicillin-Resistant Staphylococcus aureus in Culture-Negative Nosocomial Pneumonia. Chest. 2019;155(1):53-59. https://doi.org/10.1016/j.chest.2018.10.014
11. Iversen K, Ihlemann N, Gill SU, et al. Partial oral versus intravenous antibiotic treatment of endocarditis. N Engl J Med. 2019;380(5):415-424. https://doi.org/10.1056/NEJMoa1808312
12. Li HK, Rombach I, Zambellas R, et al. Oral versus Intravenous Antibiotics for Bone and Joint Infection. N Engl J Med. 2019;380(5):425-436. https://doi.org/10.1056/NEJMoa1710926
13. Fernando SM, Tran A, Cheng W, et al. Prognostic accuracy of the HEART score for prediction of major adverse cardiac events in patients presenting with chest pain: a systematic review and meta-analysis. Acad Emerg Med. 2019;26(2):140-151. https://doi.org/10.1111/acem.13649.
© 2019 Society of Hospital Medicine
The electronic medical record’s role in ObGyn burnout and patient care

Physician burnout has been labeled a public health crisis by the Harvard School of Public Health and other institutions.1 A 2018 Physician’s Foundation survey found that 78% of physicians had symptoms of burnout,2 which result from chronic workplace stress and include feeling depleted of energy or exhausted, mentally distanced from or cynical about one’s job, and problems getting one’s job done successfully.3 Among ObGyns, almost half (46%) report burnout.4 One-third of ObGyns responded on a recent Medscape Burnout Report that the computerization of practice is contributing to their burnout, and 54% said too many bureaucratic tasks, including charting, were adding to their burnout.5
Inefficient electronic medical records (EMRs) have been implicated as one reason for burnout, with improvements in efficiency cited as one of several potential resolutions to the problem. About 96% of hospitals have adopted EMRs today, compared with only 9% in 2008,6 and many physicians report recognizing value in the technology. For instance, 60% of participants in Stanford Medicine’s 2018 National Physician Poll said EMRs had led to improved patient care. At the same time, however, about as many (59%) said EMRs needed a “complete overhaul” and that the systems had detracted from their professional satisfaction (54%) as well as from their clinical effectiveness (49%).7
With this roundtable, we explore the concerns with hours spent on the EMR with several experts, and whether it is a problem that has been contributing to burnout among staff at their institutions. In addition, are there solutions that their institutions have implemented that they can share to help to cope with the problem?
John J. Dougherty, MD, MBA: Yes, absolutely. There is not a day that goes by that I don’t hear about or experience “Epic Fails.” (We use Epic’s EMR product at our institution.) Too many clicks are needed to navigate even the simplest tasks—finding notes or results, documenting visits, and billing for services are all unnecessarily complex. In addition, we are being held accountable for achieving a long and growing list of “metrics” measures, education projects (HealthStream), and productivity goals. When do we have time to treat patients? And it is not just practicing physicians and clinicians. Our resident physicians spend an inordinate amount of time in front of the computer documenting, placing orders, and transferring patients using a system with a very inefficient user interface, to say the least.
Megan L. Evans, MD, MPH: I absolutely agree. Over the years, my institution has created a conglomerate of EMRs, requiring physicians across the hospital to be fluent in a multitude of systems. For example, you finish your clinic notes in one system, sign off on discharge summaries in another, and complete your operative notes in an entirely different system. As busy attendings, it is hard to keep ahead of all of these tasks, especially when the systems do not talk to one another. Fortunately, my hospital is changing our EMR to a single system within the next year. Until then, however, we will work in this piecemeal system.
Mark Woodland, MS, MD: EMR and computerization of medicine is the number 1 issue relating to dissatisfaction by ObGyn providers in our institution. Providers are earnest in their attempt to be compliant with EMR requirements, but the reality is that they are dealing with an automated system that does not have realistic expectations for management of results, follow-up tasks, and patient communications for a human provider. The actual charting, ordering of tests and consults, and communication between providers has been enhanced. However, the “in-basket” of tasks to be accomplished are extraordinary and much of it relies on the provider, which requires an inordinate amount of time. Additionally, while other members of the medical staff are stationary at a desk, physicians and other providers are not. They are mobile between inpatient units, labor and delivery, operating rooms, and emergency rooms. Time management does not always allow for providers to access computers from all of these areas to facilitate their managing the expectations of the EMR. This requires providers to access the EMR at off hours, extending their workload. Finally, the EMR is neither personal nor friendly. It is not designed with the clinician in mind, and it is not fun or engaging for a provider.
EMRs are not just inefficient and contributing to physician burnout, according to a joint report from Kaiser Health News (KHN) and Fortune magazine, they are inadequate and contributing to patient safety concerns.1 This was not the intended goal of the HITECH Act, signed into law in 2009 as part of the stimulus bill. HITECH was intended to promote the adoption of meaningful use of health information technology by providing financial incentives to clinicians to adopt electronic medical records (EMRs). It also intended to increase security for health care data--achieved through larger penalties for HIPAA violations.2
Ten years later, however, "America has little to show" for its $36 billion investment, according to KHN and Fortune. Yes, 96% of hospitals have one of the currently available EMRs, among thousands, but they are disconnected. And they are "glitchy." At least 2 EMR vendors have reached settlements with the federal government over egregious patient errors. At least 7 deaths have resulted from errors related to the EMR, according to the firm Quantros, reports KHN and Fortune, and the number of EMR-related safety events tops 18,000. The problem is that information, critical to a patient's well-being, may get buried in the EMR. Clinicians may not have been aware of, because they did not see, a critical medication allergy or piece of patient history.1
The problems with health information technology usability do have solutions, however, asserts Raj M. Ratwani, MD, and colleagues. In a recent article published in the Journal of the American Medical Association, the researchers propose 5 priorities for achieving progress3:
- Establishment of a national database of usability and safety issues. This database should allow sharing of safety information among EMR vendors, hospitals, and clinicians, and make the public aware of any technology risks.
- Establishment of basic design standards, which should promote innovation and be regulated by a board composed of all stakeholders: EMR vendors, researchers, clinicians, and health care organizations.
- Addressing unintended harms. Causes of harm could include "vendor design and development, vendor and health care organization implementation, and customization by the health care organization." Along with shared responsibility and collaboration comes shared liability for harms caused by inadequate usability.
- Simplification of mandated documentation requirements that affect usability. Reducing clinician's "busy work" would go a long way toward simplifying documentation requirements.
- Development of standard usability and safety measures so that progress can be tracked and the market can react. EMR vendors cannot be directly compared currently, since no standards for usability are in place.
Ratwani and colleagues cite shared responsibility and commitment among all of the parties invested in EMR usability success as keys to solving the current challenges affecting health information technology, with policy makers at the helm.3 The federal government is attempting to respond: As part of the 2016 21st Century Cures Act and with an aim toward alleviating physician time spent on the EMR, the Department of Health and Human Services is required to recommend reductions to current EMR burdens required under the HITECH Act. It plans to revise E&M codes, lessening documentation. And the Centers for Medicare and Medicaid Services aims to make meaningful use requirements more flexible, require information exchange between providers and patients, and provide incentive to clinicians to allow patient access to EMRs.4,5
References
- Fry E, Schulte F. Death by a thousand clicks. Fortune. March 18, 2019. http://fortune.com/longform/medical-records/. Accessed September 9, 2019.
- Burde H. The HITECH Act: an overview. AMA J Ethics. March 2011. https://journalofethics.ama-assn.org/article/hitech-act-overview/2011-03. Accessed September 9, 2019.
- Ratwani R, Reider J, Singh H. A decade of health information technology usability challenges and the path forward. JAMA. 2019;321:743-744.
- Hoffman S. Healing the healers: legal remedies for physician burnout. Case Western Reserve University School of Law. September 2018.
- Morris G, Anthony ES. 21st Century Cures Act overview for states. Office of the National Coordinator for Health Information Technology. https://www.healthit.gov/sites/default/files/curesactlearningsession_1_v6_10818.pdf. Accessed September 11, 2019.
Continue to:
Dr. Dougherty: When our institution compared EMR offerings, EMR companies put their best collective marketing feet forward. The general notion, at least with the Epic EMR, was that “you can customize Epic to your liking.” It did not take long for a bunch of motivated Epic users to create “smart” stuff (lists, phrases, and texts) in an effort to customize workflows and create fancy-looking electronic notes. Shortly thereafter, it was obvious that, as an institution, our reporting efforts kept coming up short—our reports lacked accuracy and meaning. Everyone was documenting in different ways and in different areas. Considering that reports are currently generated using (mostly) discrete data entries (data placed in specific fields within the EMR), it became obvious that our data entry paradigm needed to change. Therefore, standardization became the leading buzzword. Our institution recently initiated a project aimed at standardizing our workflows and documentation habits. In addition, we have incorporated a third-party information exchange product into our health system data aggregation and analysis workflow. Much more needs to be done, but it is a start.
Dr. Evans: At my institution, as a group, we have created templates for routine procedures and visits that also auto populate billing codes. I know that some departments have used scribes. From the hospital side, there has been improved access to the EMR from home. Some of my colleagues like this feature; however, others, like myself, believe this contributes to some of our burnout. I like to leave work at work. Having the ability to continue working at home is not a solution in my mind.
Dr. Woodland: At our institution, we have engaged our chaperones and medical assistants to help facilitate completion of the medical records during the office visit. Providers work with their assistants to accommodate documentation of history and physical findings while also listening to the provider as they are speaking in order to document patient care plans and orders. This saves the clinicians time in reviewing and editing the record as well as making sure the appropriate care plan is instituted. Our EMR provider recently has begun experimenting with personalization of color themes as well as pictures as part of the interface. Having said this, I still ask, “Why have medical professionals allowed non–clinical agencies and information technology groups to run this show?” It is also inconceivable to me that this unfunded mandate—that has increased cost, decreased clinical efficiency, and decreased clinician satisfaction—has not been addressed by national and international medical communities.
Dr. Woodland: I feel that we need to appropriately manage expectations of the EMR and the institution with relation to EMR and providers. By this I mean that we need to make the EMR more user-friendly and appropriate for different clinicians as well as patients. We also need to manage expectations of our patients. In a digital age where immediate contact is the norm, we need to address the issue that the EMR is not social media but rather a communication tool for routine contact and information transmission. Emergencies are not typically addressed well through the EMR platform; they are better handled with a more appropriate communication interface.
Dr. Dougherty: I feel that the biggest change needed is a competent, simple, and standard user-interface. Our old charting methods were great on a number of levels. For instance, if I wanted to add an order, I flipped to the ”Orders” tab and entered an order. If I needed to document a note, I flipped to the “Notes” tab and started writing, etc. Obviously, manual charting had its downsides—like trying to decipher handwriting art! EMRs could easily adopt the stuff that worked from our old methods of documentation, while leveraging the advantages that computerized workflows can bring to practitioners, including efficient transfer of records, meaningful reporting, simple electronic ordering, and interprofessional communication portals.
Dr. Evans: Our systems need to better communicate with one another. I am in an academic practice, and I should be able to see labs, consultant notes, imaging, all in one spot to improve efficiency and ease with patient visits. Minimizing clicks would be helpful as well. I try to write as much as I can while in the room with a patient to avoid after-hours note writing, but it takes away from my interaction with each patient.
Continue to:
Dr. Evans: When I first started as a new attending, it would take me hours to finish my notes, partly because of the level of detail I would write in my history of present illness (HPI) and assessment and plan. One great piece of advice I received was to be satisfied with good notes, not perfect notes. I worked to consolidate my thoughts and use preconstructed phrases/paragraphs on common problems I saw. This saved time to focus on other aspects of my academic job.
Dr. Dougherty: We need to refocus on the patient first, and mold our systems to meet that priority. Much too often, we have our backs to the patients or spend too much time interfacing with our EMR systems, and our patients are not happy about it (as many surveys have demonstrated). More importantly, a renewed focus on patient care, not EMR care, would allow our practitioners to do what they signed up for—treating patients. In the meantime, I would suggest that practitioners stay away from EMR gimmicks and go back to old-style documentation practices (like those established by the Centers for Medicare and Medicaid Services in 1997 and 1998), and ask the IT folks to help with molding the EMR systems to meet your own standards, not the standards established by EMR companies. I am also very hopeful that the consumer will drive most of the health care-related data collection in the near future, thereby marginalizing the current generation of EMR systems.
Dr. Woodland: I would add that providers need to manage the EMR and not let the EMR manage them. Set up task reminders at point times to handle results and communications from the EMR and set up time in your schedule where you can facilitate meeting these tasks. When providers are out on vacation, make sure to have an out-of-office reminder built into their EMR so that patients and others know timing of potential responses. Try to make the EMR as enjoyable as possible and focus on the good points of the EMR, such as legibility, order verification, safety, and documentation.
1. Engage the computer in your patient encounter, says Rey Wuerth and colleagues. Share the screen, and any test results you are highlighting, with your patient by turning it toward her during your discussion. This can increase patient satisfaction.1
2. Go mobile at the point of care, suggests Tom Giannulli, MD, MS, Chief Medical Information Officer at Kareo. By using a tablet or mobile device, you can enter data while facing a patient or on the go.2
3. Use templates when documenting data, advises Wuerth and colleagues, as pre-filled templates, that are provided through the EMR or that you create within the EMR, can reduce the time required to enter patient visits, findings, and referrals.1
4. Delegate responsibility for routing documents, says Brian Anderson, MD. Hand off to staff administrative duties, such as patient forms and routine negative test results.3
5. Involve medical assistants (MAs) in the process. Make the MA feel part of the team, says R. Scott Eden, and assign them history-taking responsibilities, utilizing your EMR's templates. Assign them other tasks as well, including medication reconciliation, referrals, refills, routine screening, and patient education.4
6. Employ physical or virtual scribes who are specifically assigned to EMR duty. Although drawbacks can include patient privacy concerns and reduced practice income due to salary requirements, employing a scribe (often a pre-medical or graduate student), who trails you on patient visits, or who is connected virtually, can leave the clinician free to interact with patients.5,6
References
- Wuerth R, Campbell C, Peng MD, et al. Top 10 tips for effective use of electronic health records. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3959973/. Paediatr Child Health. 2014;19:138.
- Giannulli T. 7 time-saving EHR use tips to boost physician productivity. April 28, 2016. https://ehrintelligence.com/news/7-time-saving-emr-use-tips-to-boost-physician-productivity. Accessed September 9, 2019.
- Anderson B. 5 ways to increase your EMR efficiency. October 28, 2014. https://www.kevinmd.com/blog/2014/10/5-ways-increase-emr-efficiency.html. Accessed September 9, 2019.
- Eden RS. Maximizing your medical assistant's role. Fam Pract Manag. 2016;23:5-7. https://www.aafp.org/fpm/2016/0500/p5.html.
- Hoffman S. Healing the healers: legal remedies for physician burnout. Case Western Reserve University School of Law. September 2018.
- Caliri A. The case for virtual scribes. January 2, 2019. Becker's Hospital Review. https://www.beckershospitalreview.com/hospital-physician-relationships/the-case-for-virtual-scribes.html. Accessed September 20, 2019.
Dr. Evans: Yes and no. Yes, in that it can be much easier to follow a patient’s health care history from other provider notes or prior surgeries. Information is searchable and legible. If an EMR is built correctly, it can save time for providers, through smart phrases and templates, and it can help providers with proper billing codes and documentation requirements. No, in that it can take away from important patient interaction. We are required to see more patients in less time all while using, at times, a cumbersome EMR system.
Dr. Woodland: This is a tricky question because the EMR has both positive and negative attributes. Certainly, the legibility and order verification has improved, but the ease of accessing information in the EMR has changed. Additionally, there has been a drastic increase in provider dissatisfaction that has not been addressed. Provider dissatisfaction can lead to problems in patient care. If there was a clear-cut increased value for the cost, I do not think the EMR would be such a huge focus of negative attention. Providers need to take back control of their EMR and their profession so that they can utilize the EMR as the tool it was supposed to be and not the dissatisfier that it has become.
Dr. Dougherty: I do not believe patient care has been improved by EMR systems, for all of the reasons we have discussed, and then some. But there is an enormous amount of potential, if we get the interface between humans and EMR systems right!
- A crisis in health care: a call to action on physician burnout. Massachusetts Health and Hospital Association. Massachusetts Medical Society. Harvard T.H. Chan School of Public Health. https://cdn1.sph.harvard.edu/wp-content/uploads/sites/21/2019/01/PhysicianBurnoutReport2018FINAL.pdf. Accessed September 9, 2019.
- Physician’s Foundation. 2018 survey of America’s physicians practice patterns and perspectives. https://physiciansfoundation.org/wp-content/uploads/2018/09/physicians-survey-results-final-2018.pdf. Accessed September 9, 2019.
- Burn-out. ICD-11 for Mortality and Morbidity Statistics. Version 04/2019. https://icd.who.int/browse11/l-m/en#/http://id.who.int/icd/entity/129180281. Accessed September 11, 2019.
- Peckham C. Medscape National Physician Burnout & Depression Report 2018. January 17, 2018. https://www.medscape.com/slideshow/2018-lifestyle-burnout-depression-6009235#3. Accessed September 9, 2019.
- Kane L. Medscape National Physician Burnout, Depression & Suicide Report 2019. January 16, 2019. https://www.medscape.com/slideshow/2019-lifestyle-burnout-depression-6011056#5. Accessed September 9, 2019.
- Fry E, Schulte F. Death by a thousand clicks: where electronic health records went wrong. Fortune. March 18, 2019. http://fortune.com/longform/medical-records/. Accessed September 9, 2019.
- How doctors feel about electronic health records: National Physician Poll by The Harris Poll. https://med.stanford.edu/content/dam/sm/ehr/documents/EHR-Poll-Presentation.pdf. Accessed September 9, 2019.

Physician burnout has been labeled a public health crisis by the Harvard School of Public Health and other institutions.1 A 2018 Physician’s Foundation survey found that 78% of physicians had symptoms of burnout,2 which result from chronic workplace stress and include feeling depleted of energy or exhausted, mentally distanced from or cynical about one’s job, and problems getting one’s job done successfully.3 Among ObGyns, almost half (46%) report burnout.4 One-third of ObGyns responded on a recent Medscape Burnout Report that the computerization of practice is contributing to their burnout, and 54% said too many bureaucratic tasks, including charting, were adding to their burnout.5
Inefficient electronic medical records (EMRs) have been implicated as one reason for burnout, with improvements in efficiency cited as one of several potential resolutions to the problem. About 96% of hospitals have adopted EMRs today, compared with only 9% in 2008,6 and many physicians report recognizing value in the technology. For instance, 60% of participants in Stanford Medicine’s 2018 National Physician Poll said EMRs had led to improved patient care. At the same time, however, about as many (59%) said EMRs needed a “complete overhaul” and that the systems had detracted from their professional satisfaction (54%) as well as from their clinical effectiveness (49%).7
With this roundtable, we explore the concerns with hours spent on the EMR with several experts, and whether it is a problem that has been contributing to burnout among staff at their institutions. In addition, are there solutions that their institutions have implemented that they can share to help to cope with the problem?
John J. Dougherty, MD, MBA: Yes, absolutely. There is not a day that goes by that I don’t hear about or experience “Epic Fails.” (We use Epic’s EMR product at our institution.) Too many clicks are needed to navigate even the simplest tasks—finding notes or results, documenting visits, and billing for services are all unnecessarily complex. In addition, we are being held accountable for achieving a long and growing list of “metrics” measures, education projects (HealthStream), and productivity goals. When do we have time to treat patients? And it is not just practicing physicians and clinicians. Our resident physicians spend an inordinate amount of time in front of the computer documenting, placing orders, and transferring patients using a system with a very inefficient user interface, to say the least.
Megan L. Evans, MD, MPH: I absolutely agree. Over the years, my institution has created a conglomerate of EMRs, requiring physicians across the hospital to be fluent in a multitude of systems. For example, you finish your clinic notes in one system, sign off on discharge summaries in another, and complete your operative notes in an entirely different system. As busy attendings, it is hard to keep ahead of all of these tasks, especially when the systems do not talk to one another. Fortunately, my hospital is changing our EMR to a single system within the next year. Until then, however, we will work in this piecemeal system.
Mark Woodland, MS, MD: EMR and computerization of medicine is the number 1 issue relating to dissatisfaction by ObGyn providers in our institution. Providers are earnest in their attempt to be compliant with EMR requirements, but the reality is that they are dealing with an automated system that does not have realistic expectations for management of results, follow-up tasks, and patient communications for a human provider. The actual charting, ordering of tests and consults, and communication between providers has been enhanced. However, the “in-basket” of tasks to be accomplished are extraordinary and much of it relies on the provider, which requires an inordinate amount of time. Additionally, while other members of the medical staff are stationary at a desk, physicians and other providers are not. They are mobile between inpatient units, labor and delivery, operating rooms, and emergency rooms. Time management does not always allow for providers to access computers from all of these areas to facilitate their managing the expectations of the EMR. This requires providers to access the EMR at off hours, extending their workload. Finally, the EMR is neither personal nor friendly. It is not designed with the clinician in mind, and it is not fun or engaging for a provider.
EMRs are not just inefficient and contributing to physician burnout, according to a joint report from Kaiser Health News (KHN) and Fortune magazine, they are inadequate and contributing to patient safety concerns.1 This was not the intended goal of the HITECH Act, signed into law in 2009 as part of the stimulus bill. HITECH was intended to promote the adoption of meaningful use of health information technology by providing financial incentives to clinicians to adopt electronic medical records (EMRs). It also intended to increase security for health care data--achieved through larger penalties for HIPAA violations.2
Ten years later, however, "America has little to show" for its $36 billion investment, according to KHN and Fortune. Yes, 96% of hospitals have one of the currently available EMRs, among thousands, but they are disconnected. And they are "glitchy." At least 2 EMR vendors have reached settlements with the federal government over egregious patient errors. At least 7 deaths have resulted from errors related to the EMR, according to the firm Quantros, reports KHN and Fortune, and the number of EMR-related safety events tops 18,000. The problem is that information, critical to a patient's well-being, may get buried in the EMR. Clinicians may not have been aware of, because they did not see, a critical medication allergy or piece of patient history.1
The problems with health information technology usability do have solutions, however, asserts Raj M. Ratwani, MD, and colleagues. In a recent article published in the Journal of the American Medical Association, the researchers propose 5 priorities for achieving progress3:
- Establishment of a national database of usability and safety issues. This database should allow sharing of safety information among EMR vendors, hospitals, and clinicians, and make the public aware of any technology risks.
- Establishment of basic design standards, which should promote innovation and be regulated by a board composed of all stakeholders: EMR vendors, researchers, clinicians, and health care organizations.
- Addressing unintended harms. Causes of harm could include "vendor design and development, vendor and health care organization implementation, and customization by the health care organization." Along with shared responsibility and collaboration comes shared liability for harms caused by inadequate usability.
- Simplification of mandated documentation requirements that affect usability. Reducing clinician's "busy work" would go a long way toward simplifying documentation requirements.
- Development of standard usability and safety measures so that progress can be tracked and the market can react. EMR vendors cannot be directly compared currently, since no standards for usability are in place.
Ratwani and colleagues cite shared responsibility and commitment among all of the parties invested in EMR usability success as keys to solving the current challenges affecting health information technology, with policy makers at the helm.3 The federal government is attempting to respond: As part of the 2016 21st Century Cures Act and with an aim toward alleviating physician time spent on the EMR, the Department of Health and Human Services is required to recommend reductions to current EMR burdens required under the HITECH Act. It plans to revise E&M codes, lessening documentation. And the Centers for Medicare and Medicaid Services aims to make meaningful use requirements more flexible, require information exchange between providers and patients, and provide incentive to clinicians to allow patient access to EMRs.4,5
References
- Fry E, Schulte F. Death by a thousand clicks. Fortune. March 18, 2019. http://fortune.com/longform/medical-records/. Accessed September 9, 2019.
- Burde H. The HITECH Act: an overview. AMA J Ethics. March 2011. https://journalofethics.ama-assn.org/article/hitech-act-overview/2011-03. Accessed September 9, 2019.
- Ratwani R, Reider J, Singh H. A decade of health information technology usability challenges and the path forward. JAMA. 2019;321:743-744.
- Hoffman S. Healing the healers: legal remedies for physician burnout. Case Western Reserve University School of Law. September 2018.
- Morris G, Anthony ES. 21st Century Cures Act overview for states. Office of the National Coordinator for Health Information Technology. https://www.healthit.gov/sites/default/files/curesactlearningsession_1_v6_10818.pdf. Accessed September 11, 2019.
Continue to:
Dr. Dougherty: When our institution compared EMR offerings, EMR companies put their best collective marketing feet forward. The general notion, at least with the Epic EMR, was that “you can customize Epic to your liking.” It did not take long for a bunch of motivated Epic users to create “smart” stuff (lists, phrases, and texts) in an effort to customize workflows and create fancy-looking electronic notes. Shortly thereafter, it was obvious that, as an institution, our reporting efforts kept coming up short—our reports lacked accuracy and meaning. Everyone was documenting in different ways and in different areas. Considering that reports are currently generated using (mostly) discrete data entries (data placed in specific fields within the EMR), it became obvious that our data entry paradigm needed to change. Therefore, standardization became the leading buzzword. Our institution recently initiated a project aimed at standardizing our workflows and documentation habits. In addition, we have incorporated a third-party information exchange product into our health system data aggregation and analysis workflow. Much more needs to be done, but it is a start.
Dr. Evans: At my institution, as a group, we have created templates for routine procedures and visits that also auto populate billing codes. I know that some departments have used scribes. From the hospital side, there has been improved access to the EMR from home. Some of my colleagues like this feature; however, others, like myself, believe this contributes to some of our burnout. I like to leave work at work. Having the ability to continue working at home is not a solution in my mind.
Dr. Woodland: At our institution, we have engaged our chaperones and medical assistants to help facilitate completion of the medical records during the office visit. Providers work with their assistants to accommodate documentation of history and physical findings while also listening to the provider as they are speaking in order to document patient care plans and orders. This saves the clinicians time in reviewing and editing the record as well as making sure the appropriate care plan is instituted. Our EMR provider recently has begun experimenting with personalization of color themes as well as pictures as part of the interface. Having said this, I still ask, “Why have medical professionals allowed non–clinical agencies and information technology groups to run this show?” It is also inconceivable to me that this unfunded mandate—that has increased cost, decreased clinical efficiency, and decreased clinician satisfaction—has not been addressed by national and international medical communities.
Dr. Woodland: I feel that we need to appropriately manage expectations of the EMR and the institution with relation to EMR and providers. By this I mean that we need to make the EMR more user-friendly and appropriate for different clinicians as well as patients. We also need to manage expectations of our patients. In a digital age where immediate contact is the norm, we need to address the issue that the EMR is not social media but rather a communication tool for routine contact and information transmission. Emergencies are not typically addressed well through the EMR platform; they are better handled with a more appropriate communication interface.
Dr. Dougherty: I feel that the biggest change needed is a competent, simple, and standard user-interface. Our old charting methods were great on a number of levels. For instance, if I wanted to add an order, I flipped to the ”Orders” tab and entered an order. If I needed to document a note, I flipped to the “Notes” tab and started writing, etc. Obviously, manual charting had its downsides—like trying to decipher handwriting art! EMRs could easily adopt the stuff that worked from our old methods of documentation, while leveraging the advantages that computerized workflows can bring to practitioners, including efficient transfer of records, meaningful reporting, simple electronic ordering, and interprofessional communication portals.
Dr. Evans: Our systems need to better communicate with one another. I am in an academic practice, and I should be able to see labs, consultant notes, imaging, all in one spot to improve efficiency and ease with patient visits. Minimizing clicks would be helpful as well. I try to write as much as I can while in the room with a patient to avoid after-hours note writing, but it takes away from my interaction with each patient.
Continue to:
Dr. Evans: When I first started as a new attending, it would take me hours to finish my notes, partly because of the level of detail I would write in my history of present illness (HPI) and assessment and plan. One great piece of advice I received was to be satisfied with good notes, not perfect notes. I worked to consolidate my thoughts and use preconstructed phrases/paragraphs on common problems I saw. This saved time to focus on other aspects of my academic job.
Dr. Dougherty: We need to refocus on the patient first, and mold our systems to meet that priority. Much too often, we have our backs to the patients or spend too much time interfacing with our EMR systems, and our patients are not happy about it (as many surveys have demonstrated). More importantly, a renewed focus on patient care, not EMR care, would allow our practitioners to do what they signed up for—treating patients. In the meantime, I would suggest that practitioners stay away from EMR gimmicks and go back to old-style documentation practices (like those established by the Centers for Medicare and Medicaid Services in 1997 and 1998), and ask the IT folks to help with molding the EMR systems to meet your own standards, not the standards established by EMR companies. I am also very hopeful that the consumer will drive most of the health care-related data collection in the near future, thereby marginalizing the current generation of EMR systems.
Dr. Woodland: I would add that providers need to manage the EMR and not let the EMR manage them. Set up task reminders at point times to handle results and communications from the EMR and set up time in your schedule where you can facilitate meeting these tasks. When providers are out on vacation, make sure to have an out-of-office reminder built into their EMR so that patients and others know timing of potential responses. Try to make the EMR as enjoyable as possible and focus on the good points of the EMR, such as legibility, order verification, safety, and documentation.
1. Engage the computer in your patient encounter, says Rey Wuerth and colleagues. Share the screen, and any test results you are highlighting, with your patient by turning it toward her during your discussion. This can increase patient satisfaction.1
2. Go mobile at the point of care, suggests Tom Giannulli, MD, MS, Chief Medical Information Officer at Kareo. By using a tablet or mobile device, you can enter data while facing a patient or on the go.2
3. Use templates when documenting data, advises Wuerth and colleagues, as pre-filled templates, that are provided through the EMR or that you create within the EMR, can reduce the time required to enter patient visits, findings, and referrals.1
4. Delegate responsibility for routing documents, says Brian Anderson, MD. Hand off to staff administrative duties, such as patient forms and routine negative test results.3
5. Involve medical assistants (MAs) in the process. Make the MA feel part of the team, says R. Scott Eden, and assign them history-taking responsibilities, utilizing your EMR's templates. Assign them other tasks as well, including medication reconciliation, referrals, refills, routine screening, and patient education.4
6. Employ physical or virtual scribes who are specifically assigned to EMR duty. Although drawbacks can include patient privacy concerns and reduced practice income due to salary requirements, employing a scribe (often a pre-medical or graduate student), who trails you on patient visits, or who is connected virtually, can leave the clinician free to interact with patients.5,6
References
- Wuerth R, Campbell C, Peng MD, et al. Top 10 tips for effective use of electronic health records. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3959973/. Paediatr Child Health. 2014;19:138.
- Giannulli T. 7 time-saving EHR use tips to boost physician productivity. April 28, 2016. https://ehrintelligence.com/news/7-time-saving-emr-use-tips-to-boost-physician-productivity. Accessed September 9, 2019.
- Anderson B. 5 ways to increase your EMR efficiency. October 28, 2014. https://www.kevinmd.com/blog/2014/10/5-ways-increase-emr-efficiency.html. Accessed September 9, 2019.
- Eden RS. Maximizing your medical assistant's role. Fam Pract Manag. 2016;23:5-7. https://www.aafp.org/fpm/2016/0500/p5.html.
- Hoffman S. Healing the healers: legal remedies for physician burnout. Case Western Reserve University School of Law. September 2018.
- Caliri A. The case for virtual scribes. January 2, 2019. Becker's Hospital Review. https://www.beckershospitalreview.com/hospital-physician-relationships/the-case-for-virtual-scribes.html. Accessed September 20, 2019.
Dr. Evans: Yes and no. Yes, in that it can be much easier to follow a patient’s health care history from other provider notes or prior surgeries. Information is searchable and legible. If an EMR is built correctly, it can save time for providers, through smart phrases and templates, and it can help providers with proper billing codes and documentation requirements. No, in that it can take away from important patient interaction. We are required to see more patients in less time all while using, at times, a cumbersome EMR system.
Dr. Woodland: This is a tricky question because the EMR has both positive and negative attributes. Certainly, the legibility and order verification has improved, but the ease of accessing information in the EMR has changed. Additionally, there has been a drastic increase in provider dissatisfaction that has not been addressed. Provider dissatisfaction can lead to problems in patient care. If there was a clear-cut increased value for the cost, I do not think the EMR would be such a huge focus of negative attention. Providers need to take back control of their EMR and their profession so that they can utilize the EMR as the tool it was supposed to be and not the dissatisfier that it has become.
Dr. Dougherty: I do not believe patient care has been improved by EMR systems, for all of the reasons we have discussed, and then some. But there is an enormous amount of potential, if we get the interface between humans and EMR systems right!

Physician burnout has been labeled a public health crisis by the Harvard School of Public Health and other institutions.1 A 2018 Physician’s Foundation survey found that 78% of physicians had symptoms of burnout,2 which result from chronic workplace stress and include feeling depleted of energy or exhausted, mentally distanced from or cynical about one’s job, and problems getting one’s job done successfully.3 Among ObGyns, almost half (46%) report burnout.4 One-third of ObGyns responded on a recent Medscape Burnout Report that the computerization of practice is contributing to their burnout, and 54% said too many bureaucratic tasks, including charting, were adding to their burnout.5
Inefficient electronic medical records (EMRs) have been implicated as one reason for burnout, with improvements in efficiency cited as one of several potential resolutions to the problem. About 96% of hospitals have adopted EMRs today, compared with only 9% in 2008,6 and many physicians report recognizing value in the technology. For instance, 60% of participants in Stanford Medicine’s 2018 National Physician Poll said EMRs had led to improved patient care. At the same time, however, about as many (59%) said EMRs needed a “complete overhaul” and that the systems had detracted from their professional satisfaction (54%) as well as from their clinical effectiveness (49%).7
With this roundtable, we explore the concerns with hours spent on the EMR with several experts, and whether it is a problem that has been contributing to burnout among staff at their institutions. In addition, are there solutions that their institutions have implemented that they can share to help to cope with the problem?
John J. Dougherty, MD, MBA: Yes, absolutely. There is not a day that goes by that I don’t hear about or experience “Epic Fails.” (We use Epic’s EMR product at our institution.) Too many clicks are needed to navigate even the simplest tasks—finding notes or results, documenting visits, and billing for services are all unnecessarily complex. In addition, we are being held accountable for achieving a long and growing list of “metrics” measures, education projects (HealthStream), and productivity goals. When do we have time to treat patients? And it is not just practicing physicians and clinicians. Our resident physicians spend an inordinate amount of time in front of the computer documenting, placing orders, and transferring patients using a system with a very inefficient user interface, to say the least.
Megan L. Evans, MD, MPH: I absolutely agree. Over the years, my institution has created a conglomerate of EMRs, requiring physicians across the hospital to be fluent in a multitude of systems. For example, you finish your clinic notes in one system, sign off on discharge summaries in another, and complete your operative notes in an entirely different system. As busy attendings, it is hard to keep ahead of all of these tasks, especially when the systems do not talk to one another. Fortunately, my hospital is changing our EMR to a single system within the next year. Until then, however, we will work in this piecemeal system.
Mark Woodland, MS, MD: EMR and computerization of medicine is the number 1 issue relating to dissatisfaction by ObGyn providers in our institution. Providers are earnest in their attempt to be compliant with EMR requirements, but the reality is that they are dealing with an automated system that does not have realistic expectations for management of results, follow-up tasks, and patient communications for a human provider. The actual charting, ordering of tests and consults, and communication between providers has been enhanced. However, the “in-basket” of tasks to be accomplished are extraordinary and much of it relies on the provider, which requires an inordinate amount of time. Additionally, while other members of the medical staff are stationary at a desk, physicians and other providers are not. They are mobile between inpatient units, labor and delivery, operating rooms, and emergency rooms. Time management does not always allow for providers to access computers from all of these areas to facilitate their managing the expectations of the EMR. This requires providers to access the EMR at off hours, extending their workload. Finally, the EMR is neither personal nor friendly. It is not designed with the clinician in mind, and it is not fun or engaging for a provider.
EMRs are not just inefficient and contributing to physician burnout, according to a joint report from Kaiser Health News (KHN) and Fortune magazine, they are inadequate and contributing to patient safety concerns.1 This was not the intended goal of the HITECH Act, signed into law in 2009 as part of the stimulus bill. HITECH was intended to promote the adoption of meaningful use of health information technology by providing financial incentives to clinicians to adopt electronic medical records (EMRs). It also intended to increase security for health care data--achieved through larger penalties for HIPAA violations.2
Ten years later, however, "America has little to show" for its $36 billion investment, according to KHN and Fortune. Yes, 96% of hospitals have one of the currently available EMRs, among thousands, but they are disconnected. And they are "glitchy." At least 2 EMR vendors have reached settlements with the federal government over egregious patient errors. At least 7 deaths have resulted from errors related to the EMR, according to the firm Quantros, reports KHN and Fortune, and the number of EMR-related safety events tops 18,000. The problem is that information, critical to a patient's well-being, may get buried in the EMR. Clinicians may not have been aware of, because they did not see, a critical medication allergy or piece of patient history.1
The problems with health information technology usability do have solutions, however, asserts Raj M. Ratwani, MD, and colleagues. In a recent article published in the Journal of the American Medical Association, the researchers propose 5 priorities for achieving progress3:
- Establishment of a national database of usability and safety issues. This database should allow sharing of safety information among EMR vendors, hospitals, and clinicians, and make the public aware of any technology risks.
- Establishment of basic design standards, which should promote innovation and be regulated by a board composed of all stakeholders: EMR vendors, researchers, clinicians, and health care organizations.
- Addressing unintended harms. Causes of harm could include "vendor design and development, vendor and health care organization implementation, and customization by the health care organization." Along with shared responsibility and collaboration comes shared liability for harms caused by inadequate usability.
- Simplification of mandated documentation requirements that affect usability. Reducing clinician's "busy work" would go a long way toward simplifying documentation requirements.
- Development of standard usability and safety measures so that progress can be tracked and the market can react. EMR vendors cannot be directly compared currently, since no standards for usability are in place.
Ratwani and colleagues cite shared responsibility and commitment among all of the parties invested in EMR usability success as keys to solving the current challenges affecting health information technology, with policy makers at the helm.3 The federal government is attempting to respond: As part of the 2016 21st Century Cures Act and with an aim toward alleviating physician time spent on the EMR, the Department of Health and Human Services is required to recommend reductions to current EMR burdens required under the HITECH Act. It plans to revise E&M codes, lessening documentation. And the Centers for Medicare and Medicaid Services aims to make meaningful use requirements more flexible, require information exchange between providers and patients, and provide incentive to clinicians to allow patient access to EMRs.4,5
References
- Fry E, Schulte F. Death by a thousand clicks. Fortune. March 18, 2019. http://fortune.com/longform/medical-records/. Accessed September 9, 2019.
- Burde H. The HITECH Act: an overview. AMA J Ethics. March 2011. https://journalofethics.ama-assn.org/article/hitech-act-overview/2011-03. Accessed September 9, 2019.
- Ratwani R, Reider J, Singh H. A decade of health information technology usability challenges and the path forward. JAMA. 2019;321:743-744.
- Hoffman S. Healing the healers: legal remedies for physician burnout. Case Western Reserve University School of Law. September 2018.
- Morris G, Anthony ES. 21st Century Cures Act overview for states. Office of the National Coordinator for Health Information Technology. https://www.healthit.gov/sites/default/files/curesactlearningsession_1_v6_10818.pdf. Accessed September 11, 2019.
Continue to:
Dr. Dougherty: When our institution compared EMR offerings, EMR companies put their best collective marketing feet forward. The general notion, at least with the Epic EMR, was that “you can customize Epic to your liking.” It did not take long for a bunch of motivated Epic users to create “smart” stuff (lists, phrases, and texts) in an effort to customize workflows and create fancy-looking electronic notes. Shortly thereafter, it was obvious that, as an institution, our reporting efforts kept coming up short—our reports lacked accuracy and meaning. Everyone was documenting in different ways and in different areas. Considering that reports are currently generated using (mostly) discrete data entries (data placed in specific fields within the EMR), it became obvious that our data entry paradigm needed to change. Therefore, standardization became the leading buzzword. Our institution recently initiated a project aimed at standardizing our workflows and documentation habits. In addition, we have incorporated a third-party information exchange product into our health system data aggregation and analysis workflow. Much more needs to be done, but it is a start.
Dr. Evans: At my institution, as a group, we have created templates for routine procedures and visits that also auto populate billing codes. I know that some departments have used scribes. From the hospital side, there has been improved access to the EMR from home. Some of my colleagues like this feature; however, others, like myself, believe this contributes to some of our burnout. I like to leave work at work. Having the ability to continue working at home is not a solution in my mind.
Dr. Woodland: At our institution, we have engaged our chaperones and medical assistants to help facilitate completion of the medical records during the office visit. Providers work with their assistants to accommodate documentation of history and physical findings while also listening to the provider as they are speaking in order to document patient care plans and orders. This saves the clinicians time in reviewing and editing the record as well as making sure the appropriate care plan is instituted. Our EMR provider recently has begun experimenting with personalization of color themes as well as pictures as part of the interface. Having said this, I still ask, “Why have medical professionals allowed non–clinical agencies and information technology groups to run this show?” It is also inconceivable to me that this unfunded mandate—that has increased cost, decreased clinical efficiency, and decreased clinician satisfaction—has not been addressed by national and international medical communities.
Dr. Woodland: I feel that we need to appropriately manage expectations of the EMR and the institution with relation to EMR and providers. By this I mean that we need to make the EMR more user-friendly and appropriate for different clinicians as well as patients. We also need to manage expectations of our patients. In a digital age where immediate contact is the norm, we need to address the issue that the EMR is not social media but rather a communication tool for routine contact and information transmission. Emergencies are not typically addressed well through the EMR platform; they are better handled with a more appropriate communication interface.
Dr. Dougherty: I feel that the biggest change needed is a competent, simple, and standard user-interface. Our old charting methods were great on a number of levels. For instance, if I wanted to add an order, I flipped to the ”Orders” tab and entered an order. If I needed to document a note, I flipped to the “Notes” tab and started writing, etc. Obviously, manual charting had its downsides—like trying to decipher handwriting art! EMRs could easily adopt the stuff that worked from our old methods of documentation, while leveraging the advantages that computerized workflows can bring to practitioners, including efficient transfer of records, meaningful reporting, simple electronic ordering, and interprofessional communication portals.
Dr. Evans: Our systems need to better communicate with one another. I am in an academic practice, and I should be able to see labs, consultant notes, imaging, all in one spot to improve efficiency and ease with patient visits. Minimizing clicks would be helpful as well. I try to write as much as I can while in the room with a patient to avoid after-hours note writing, but it takes away from my interaction with each patient.
Continue to:
Dr. Evans: When I first started as a new attending, it would take me hours to finish my notes, partly because of the level of detail I would write in my history of present illness (HPI) and assessment and plan. One great piece of advice I received was to be satisfied with good notes, not perfect notes. I worked to consolidate my thoughts and use preconstructed phrases/paragraphs on common problems I saw. This saved time to focus on other aspects of my academic job.
Dr. Dougherty: We need to refocus on the patient first, and mold our systems to meet that priority. Much too often, we have our backs to the patients or spend too much time interfacing with our EMR systems, and our patients are not happy about it (as many surveys have demonstrated). More importantly, a renewed focus on patient care, not EMR care, would allow our practitioners to do what they signed up for—treating patients. In the meantime, I would suggest that practitioners stay away from EMR gimmicks and go back to old-style documentation practices (like those established by the Centers for Medicare and Medicaid Services in 1997 and 1998), and ask the IT folks to help with molding the EMR systems to meet your own standards, not the standards established by EMR companies. I am also very hopeful that the consumer will drive most of the health care-related data collection in the near future, thereby marginalizing the current generation of EMR systems.
Dr. Woodland: I would add that providers need to manage the EMR and not let the EMR manage them. Set up task reminders at point times to handle results and communications from the EMR and set up time in your schedule where you can facilitate meeting these tasks. When providers are out on vacation, make sure to have an out-of-office reminder built into their EMR so that patients and others know timing of potential responses. Try to make the EMR as enjoyable as possible and focus on the good points of the EMR, such as legibility, order verification, safety, and documentation.
1. Engage the computer in your patient encounter, says Rey Wuerth and colleagues. Share the screen, and any test results you are highlighting, with your patient by turning it toward her during your discussion. This can increase patient satisfaction.1
2. Go mobile at the point of care, suggests Tom Giannulli, MD, MS, Chief Medical Information Officer at Kareo. By using a tablet or mobile device, you can enter data while facing a patient or on the go.2
3. Use templates when documenting data, advises Wuerth and colleagues, as pre-filled templates, that are provided through the EMR or that you create within the EMR, can reduce the time required to enter patient visits, findings, and referrals.1
4. Delegate responsibility for routing documents, says Brian Anderson, MD. Hand off to staff administrative duties, such as patient forms and routine negative test results.3
5. Involve medical assistants (MAs) in the process. Make the MA feel part of the team, says R. Scott Eden, and assign them history-taking responsibilities, utilizing your EMR's templates. Assign them other tasks as well, including medication reconciliation, referrals, refills, routine screening, and patient education.4
6. Employ physical or virtual scribes who are specifically assigned to EMR duty. Although drawbacks can include patient privacy concerns and reduced practice income due to salary requirements, employing a scribe (often a pre-medical or graduate student), who trails you on patient visits, or who is connected virtually, can leave the clinician free to interact with patients.5,6
References
- Wuerth R, Campbell C, Peng MD, et al. Top 10 tips for effective use of electronic health records. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3959973/. Paediatr Child Health. 2014;19:138.
- Giannulli T. 7 time-saving EHR use tips to boost physician productivity. April 28, 2016. https://ehrintelligence.com/news/7-time-saving-emr-use-tips-to-boost-physician-productivity. Accessed September 9, 2019.
- Anderson B. 5 ways to increase your EMR efficiency. October 28, 2014. https://www.kevinmd.com/blog/2014/10/5-ways-increase-emr-efficiency.html. Accessed September 9, 2019.
- Eden RS. Maximizing your medical assistant's role. Fam Pract Manag. 2016;23:5-7. https://www.aafp.org/fpm/2016/0500/p5.html.
- Hoffman S. Healing the healers: legal remedies for physician burnout. Case Western Reserve University School of Law. September 2018.
- Caliri A. The case for virtual scribes. January 2, 2019. Becker's Hospital Review. https://www.beckershospitalreview.com/hospital-physician-relationships/the-case-for-virtual-scribes.html. Accessed September 20, 2019.
Dr. Evans: Yes and no. Yes, in that it can be much easier to follow a patient’s health care history from other provider notes or prior surgeries. Information is searchable and legible. If an EMR is built correctly, it can save time for providers, through smart phrases and templates, and it can help providers with proper billing codes and documentation requirements. No, in that it can take away from important patient interaction. We are required to see more patients in less time all while using, at times, a cumbersome EMR system.
Dr. Woodland: This is a tricky question because the EMR has both positive and negative attributes. Certainly, the legibility and order verification has improved, but the ease of accessing information in the EMR has changed. Additionally, there has been a drastic increase in provider dissatisfaction that has not been addressed. Provider dissatisfaction can lead to problems in patient care. If there was a clear-cut increased value for the cost, I do not think the EMR would be such a huge focus of negative attention. Providers need to take back control of their EMR and their profession so that they can utilize the EMR as the tool it was supposed to be and not the dissatisfier that it has become.
Dr. Dougherty: I do not believe patient care has been improved by EMR systems, for all of the reasons we have discussed, and then some. But there is an enormous amount of potential, if we get the interface between humans and EMR systems right!
- A crisis in health care: a call to action on physician burnout. Massachusetts Health and Hospital Association. Massachusetts Medical Society. Harvard T.H. Chan School of Public Health. https://cdn1.sph.harvard.edu/wp-content/uploads/sites/21/2019/01/PhysicianBurnoutReport2018FINAL.pdf. Accessed September 9, 2019.
- Physician’s Foundation. 2018 survey of America’s physicians practice patterns and perspectives. https://physiciansfoundation.org/wp-content/uploads/2018/09/physicians-survey-results-final-2018.pdf. Accessed September 9, 2019.
- Burn-out. ICD-11 for Mortality and Morbidity Statistics. Version 04/2019. https://icd.who.int/browse11/l-m/en#/http://id.who.int/icd/entity/129180281. Accessed September 11, 2019.
- Peckham C. Medscape National Physician Burnout & Depression Report 2018. January 17, 2018. https://www.medscape.com/slideshow/2018-lifestyle-burnout-depression-6009235#3. Accessed September 9, 2019.
- Kane L. Medscape National Physician Burnout, Depression & Suicide Report 2019. January 16, 2019. https://www.medscape.com/slideshow/2019-lifestyle-burnout-depression-6011056#5. Accessed September 9, 2019.
- Fry E, Schulte F. Death by a thousand clicks: where electronic health records went wrong. Fortune. March 18, 2019. http://fortune.com/longform/medical-records/. Accessed September 9, 2019.
- How doctors feel about electronic health records: National Physician Poll by The Harris Poll. https://med.stanford.edu/content/dam/sm/ehr/documents/EHR-Poll-Presentation.pdf. Accessed September 9, 2019.
- A crisis in health care: a call to action on physician burnout. Massachusetts Health and Hospital Association. Massachusetts Medical Society. Harvard T.H. Chan School of Public Health. https://cdn1.sph.harvard.edu/wp-content/uploads/sites/21/2019/01/PhysicianBurnoutReport2018FINAL.pdf. Accessed September 9, 2019.
- Physician’s Foundation. 2018 survey of America’s physicians practice patterns and perspectives. https://physiciansfoundation.org/wp-content/uploads/2018/09/physicians-survey-results-final-2018.pdf. Accessed September 9, 2019.
- Burn-out. ICD-11 for Mortality and Morbidity Statistics. Version 04/2019. https://icd.who.int/browse11/l-m/en#/http://id.who.int/icd/entity/129180281. Accessed September 11, 2019.
- Peckham C. Medscape National Physician Burnout & Depression Report 2018. January 17, 2018. https://www.medscape.com/slideshow/2018-lifestyle-burnout-depression-6009235#3. Accessed September 9, 2019.
- Kane L. Medscape National Physician Burnout, Depression & Suicide Report 2019. January 16, 2019. https://www.medscape.com/slideshow/2019-lifestyle-burnout-depression-6011056#5. Accessed September 9, 2019.
- Fry E, Schulte F. Death by a thousand clicks: where electronic health records went wrong. Fortune. March 18, 2019. http://fortune.com/longform/medical-records/. Accessed September 9, 2019.
- How doctors feel about electronic health records: National Physician Poll by The Harris Poll. https://med.stanford.edu/content/dam/sm/ehr/documents/EHR-Poll-Presentation.pdf. Accessed September 9, 2019.
Cardiovascular complications of systemic sclerosis: What to look for
Autoimmune rheumatic diseases increase the risk of cardiovascular disease. In rheumatoid arthritis and systemic lupus erythematosus, the risk is driven primarily by the inflammatory milieu, leading to accelerated coronary and cerebrovascular atherosclerosis independent of traditional atherosclerotic risk factors.1–3 The extent of cardiovascular involvement in other rheumatologic diseases has been less well characterized but is an area of growing interest.
In this review, we focus on the cardiovascular complications of systemic sclerosis and review recommendations for monitoring these patients in clinical practice.
SYSTEMIC SCLEROSIS, AN AUTOIMMUNE RHEUMATIC DISEASE
Systemic sclerosis is an autoimmune rheumatic disease characterized by excessive extracellular matrix deposition leading to diffuse fibrosis, endothelial dysfunction, and microvascular injury. It is most common in North America, Southern Europe, and Australia,4,5 and it affects women more than men in ratios ranging from 3:1 to 14:1.6 The mean age at diagnosis is around 50.
The disease can affect the lungs (interstitial lung disease and pulmonary hypertension), the heart, the kidneys, and the gastrointestinal tract.
Systemic sclerosis has 2 main subtypes: limited cutaneous systemic sclerosis, formerly called CREST syndrome) and diffuse cutaneous systemic sclerosis. The limited cutaneous subtype is characterized by tightening of the skin of the distal extremities (below the elbows and knees) and face, while diffuse cutaneous systemic sclerosis can manifest as more extensive skin tightening also involving proximal extremities and the trunk. Both subtypes can have an effect on the cardiovascular system.
Some cardiovascular risk factors such as dyslipidemia, diabetes mellitus, and high body mass index are less common in patients with systemic sclerosis than in patients with rheumatoid arthritis, while the rates of arterial hypertension, smoking, chronic obstructive pulmonary disease, osteoporosis, and neoplasms are similar between the 2 groups.7
HEART INVOLVEMENT HAS SERIOUS CONSEQUENCES
Overt cardiac involvement in systemic sclerosis is associated with a mortality rate of up to 70% over 5 years,8,9 and about one-fourth of deaths in patients with systemic sclerosis are from cardiac causes.10,11 Studies in Europe10,12 showed that many patients with systemic sclerosis have cardiac involvement detectable by magnetic resonance imaging even if they do not have clinical disease. Pulmonary arterial hypertension (PAH) is a complication of both subtypes of systemic sclerosis and portends a higher risk of death.8
Thus, it is critical for clinicians to understand the potential comorbid conditions associated with systemic sclerosis, particularly the cardiovascular ones, and to work closely with cardiologists to help optimize the evaluation and management.
MECHANISMS OF CARDIAC DISEASE IN SYSTEMIC SCLEROSIS
Abnormal vasoreactivity, a consequence of an imbalance between endothelium-derived vasoconstrictors and vasodilators, defective angiogenesis, and endothelial injury, leads to tissue ischemia and vascular endothelial growth factor expression, which initiates injury and fibrosis in the myocardium and in other organs.14–17 Fibrosis involves the myocardium, pericardium, and conduction system.13,18
Myocardial involvement in systemic sclerosis is thought to be due mainly to abnormal vasoreactivity and microvascular abnormalities such as transient coronary artery spasm leading to repeated focal ischemia.19,20 Abnormal vasoreactivity has been demonstrated during cardiac catheterization21: while mean coronary sinus blood flow in systemic sclerosis patients was normal at rest, vasodilator reserve was significantly reduced in patients with diffuse cutaneous systemic sclerosis after maximal vasodilation with dipyridamole. Additionally, endomyocardial biopsy showed fibrosis and concentric intimal hypertrophy with normal epicardial coronary arteries.21
More research into other mechanisms of cardiovascular disease in systemic sclerosis is needed to allow for better preventive care for these patients.
PULMONARY ARTERIAL HYPERTENSION
Systemic sclerosis can be associated with World Health Organization (WHO) groups 1, 2, 3, and 4 pulmonary hypertension. WHO group 1, called pulmonary arterial hypertension or PAH, is one of the most common cardiac complications of systemic sclerosis, with a reported prevalence as high as 12%.22 Systemic sclerosis-associated PAH carries a high mortality rate, with a mean survival of only 3 years.23
With advances in treatments for other complications of systemic sclerosis, the percentage of systemic sclerosis patients who die of PAH has increased from 6% to 33%.24
Compared with patients with idiopathic PAH, those with systemic sclerosis get less of a response from therapy and have poorer outcomes despite lower mean pulmonary artery pressures and similar reductions in cardiac index. However, recent studies have suggested that with aggressive treatment, patients with systemic sclerosis-related PAH can achieve outcomes similar to those with idiopathic PAH.25 Thus, recognizing this condition early is imperative.
Pulmonary arterial hypertension defined
PAH is defined as the combination of all of the following26:
- Mean pulmonary artery pressure > 20 mm Hg at rest
- Normal pulmonary capillary wedge pressure (≤ 15 mm Hg)
- Pulmonary vascular resistance ≥ 3 Wood units on right heart catheterization.
Other causes of pulmonary hypertension such as interstitial lung disease, chronic pulmonary thromboembolic disease, and left heart disease must be excluded.24,27
Remodeling in the pulmonary arteries
The events that lead to PAH in systemic sclerosis remain unclear but are believed to involve initial inflammation or endothelial injury that leads to a dysequilibrium between proliferative mediators and antiproliferative vasodilators. This dysequilibrium, along with endothelial dysfunction, causes an obliterative vasculopathy in the pulmonary artery branches and arterioles. Sympathetic overactivity, hypoxemia, and ischemia-reperfusion injury additionally promote vascular proliferation, fibrosis, and remodeling, leading to increased pulmonary vascular resistance, PAH, and increased right ventricular pressures.23,27
The subtype of systemic sclerosis is an important factor in the development and progression of PAH. PAH appears to be the major cause of death in limited cutaneous systemic sclerosis, while interstitial lung disease is the major cause of death in diffuse cutaneous systemic sclerosis.28
Pulmonary arterial hypertension is a late complication of systemic sclerosis
Data from the South Australian Scleroderma Registry29 revealed that PAH tends to be a late complication of systemic sclerosis, occurring around 20 years after disease onset. In this study of 608 patients, no patient with diffuse cutaneous systemic sclerosis developed PAH.
Systemic sclerosis-related PAH initially follows an indolent course with few symptoms until right ventricular function deteriorates. Early in the disease, patients may experience nonspecific symptoms of fatigue, lightheadedness, and dyspnea on exertion.23 As it progresses, they tend to have worsening dyspnea and may experience exertional syncope, palpitations, and chest pain.
Physical findings may suggest elevated right ventricular pressure and right ventricular failure; these include a loud P2, a prominent jugular a wave, a tricuspid regurgitant murmur, jugular venous distention, and lower-extremity edema.27
Screening for pulmonary arterial hypertension in systemic sclerosis
Significant signs and symptoms usually occur late in the disease; thus, it is important to appropriately screen patients who are at risk so that they can begin aggressive treatment.
Doppler echocardiography is recommended by European and American guidelines to screen for PAH in patients who have systemic sclerosis, and most agree that screening is appropriate even if the patient has no symptoms.30 European consensus documents recommend that transthoracic echocardiography be done annually for the first 5 years of disease and be continued every year in patients at high risk, ie, those with anticentromere antibodies, anti-Th/To antibodies, or interstitial lung disease. Patients not at high risk of developing pulmonary hypertension should also have regular transthoracic echocardiography, though the exact timing is not defined.31 While American societies have not issued corresponding recommendations, many experts follow the European recommendations.
Worrisome features on echocardiography in asymptomatic patients should be followed up with right heart catheterization to assess mean right ventricular pressure. These include:
- Estimated right ventricular systolic pressure ≥ 40 mm Hg
- Tricuspid regurgitant jet velocity > 2.8 m/s
- Right atrial enlargement > 53 mm
- Right ventricular enlargement (mid-cavity dimension > 35 mm).32
Although echocardiography is the most common form of screening, it gives only an estimate of right ventricular systolic pressure, which is imprecise. Other noninvasive markers are helpful and necessary to appropriately screen this population.
Diffusion capacity. The Itinerair study33 found that a diffusing capacity for carbon monoxide (DLCO) of 60% or higher has a high specificity in excluding PAH.
Uric acid has been found to be elevated in patients with systemic sclerosis-related PAH, and levels inversely correlate with 6-minute walking distance.34
Other predictors. N-terminal pro-B-type natriuretic peptide (NT-proBNP), left atrial volume, and the right ventricular myocardial performance index have also been shown to be independent predictors of PAH in patients with systemic sclerosis.35
An algorithm. The DETECT study36 enrolled patients at increased risk who had had systemic sclerosis longer than 3 years and a DLCO less than 60%. The investigators developed a 2-step algorithm to determine which patients should be referred for right heart catheterization to try to detect PAH earlier while minimizing the number of missed diagnoses and optimizing the use of invasive diagnostic right heart catheterization.
The first step was to assess serum values of anticentromere antibodies, NT-proBNP, and urate, and clinical features (telangiectasias), forced vital capacity, and electrocardiographic changes of right axis deviation to derive a prediction score. The second step was to assess surface echocardiographic features of the right atrial area and tricuspid regurgitation velocity.
This approach led to right heart catheterization in 62% of patients and was associated with a false-negative rate of 4%. Importantly, of the patients with PAH, 1 in 5 had no symptoms, and 33% had tricuspid regurgitation velocity less than 2.8 m/s. No single measurement performed well in isolation in this study.37
Thus, we recommend that, in addition to routine surface echocardiography, a multimodal approach be used that includes laboratory testing, clinical features, and electrocardiographic findings when screening this high-risk patient population.
ATHEROSCLEROTIC DISEASES
Although macrovascular disease has not typically been regarded as a significant systemic feature in systemic sclerosis, myocardial infarction and stroke are more common in patients with systemic sclerosis than in controls.38,39
Coronary artery disease in systemic sclerosis
Man et al38 reported that the incidence of myocardial infarction in patients with systemic sclerosis was 4.4 per 1,000 persons per year, and the incidence of stroke was 4.8 per 1,000 persons per year, compared with 2.5 per 1,000 persons per year for both myocardial infarction and stroke in healthy controls matched for age, sex, and time of entry.
The Australian Scleroderma Cohort Study39 found a 3-fold higher prevalence of coronary artery disease in systemic sclerosis patients than in controls after factoring in traditional risk factors.
Aviña-Zubieta et al,40 in a cohort of 1,239 systemic sclerosis patients, estimated a hazard ratio (HR) of 3.49 for myocardial infarction and 2.35 for stroke compared with age- and sex-matched controls. Not all of these events were related to macrovascular atherosclerosis—vasospasm and microvascular ischemia may have played significant roles in the etiology of clinical manifestations.
Studies of coronary atherosclerosis in systemic sclerosis are limited. An autopsy study41 of 58 patients with systemic sclerosis and 58 controls matched for age, sex, and ethnicity found that the prevalence of atherosclerosis of small coronary arteries and arterioles was significantly higher in systemic sclerosis patients than in controls (17% vs 2%, P < .01). However, the prevalence of medium-vessel coronary atherosclerosis was similar (48% vs 43%).
Why patients with systemic sclerosis develop atherosclerosis has not yet been determined. Traditional risk factors such as hypertension, dyslipidemia, diabetes mellitus, and obesity are typically no more prevalent in systemic sclerosis patients than in controls,38,42 and thus do not explain the increased risk of atherosclerotic cardiovascular disease. There is some evidence that novel markers of atherosclerotic risk such as homocysteine,43 lipoprotein[a],44 and oxidized low-density lipoprotein45 are more prevalent in systemic sclerosis, but these results have not been substantiated in more extensive studies.
Peripheral artery disease
It remains unclear whether peripheral artery disease is more prevalent in systemic sclerosis patients than in controls.
Individual studies have shown mixed results in comparing carotid artery stenosis between systemic sclerosis patients and controls using carotid duplex ultrasonography,46 the ankle-brachial index,46–48 carotid intima-media thickness,49–54 and brachial flow-mediated dilation.51,53,55–58 A meta-analysis found that the carotid intima and media are significantly thicker in systemic sclerosis patients than in controls,59 and the magnitude of difference is similar to that in other groups at increased cardiovascular risk, such as those with rheumatoid arthritis, diabetes, and familial hypercholesterolemia.60–63
A meta-analysis of brachial artery findings showed significantly lower flow-mediated dilation in systemic sclerosis patients than in controls.64
Overall, given the inconsistency of study results, systemic sclerosis patients should be screened and managed as in other patients with peripheral artery disease, but the clinician should be aware that there may be a higher risk of peripheral artery disease in these patients.
RIGHT AND LEFT VENTRICULAR DYSFUNCTION
Many patients with systemic sclerosis have right ventricular dysfunction as a consequence of PAH.65 It is important to detect diastolic dysfunction in this population, as it may be an even stronger predictor of death than pulmonary hypertension on right heart catheterization (HR 3.7 vs 2.0).66
Fewer patients have left ventricular dysfunction. In a multicenter study of 570 systemic sclerosis patients, only 1.4% had left ventricular systolic dysfunction on echocardiography, though 22.6% had left ventricular hypertrophy and 17.7% had left ventricular diastolic dysfunction.67 In the European League Against Rheumatism (EULAR) database, the prevalence of reduced left ventricular ejection fraction was 5.4%.68
Though traditional echocardiographic screening suggests the prevalence of left ventricular dysfunction in systemic sclerosis patients is low, cardiac magnetic resonance imaging (MRI) may be more sensitive than echocardiography for detecting subclinical myocardial involvement. Cardiac MRI has been shown to detect evidence of myocardial pathology (increased T2 signal, left ventricular thinning, pericardial effusion, reduced left ventricular and right ventricular ejection fraction, left ventricular diastolic dysfunction, and delayed myocardial contrast enhancement) in up to 75% of systemic sclerosis cases studied.69
Patients with systemic sclerosis should already be undergoing echocardiography every year to screen for PAH, and screening should also include tissue Doppler imaging to detect various forms of left and right ventricular systolic and diastolic dysfunction that may not be clinically apparent.
Though cardiac MRI can provide useful additional information, it is not currently recommended for routine screening in patients with systemic sclerosis.
ARRHYTHMIAS AND CONDUCTION DEFECTS
Patients with systemic sclerosis are prone to arrhythmias due to both conduction system fibrosis and myocardial damage.
Arrhythmias accounted for 6% of the deaths in the EULAR Scleroderma Trials and Research (EUSTAR) database.11
In the Genetics Versus Environment in Scleroderma Outcome Study (GENISOS),70 250 patients who had had systemic sclerosis for at least 3 years were studied during a period of approximately 6 years, during which there were 52 deaths, 29 of which were directly attributable to systemic sclerosis. Multivariable Cox modeling showed that 7 variables predicted mortality:
- Body mass index < 18.5 kg/m2
- Age ≥ 65
- Forced vital capacity < 50% predicted
- Systolic blood pressure ≥ 140 or diastolic blood pressure ≥ 90 mm Hg
- Pulmonary fibrosis
- Positive anticentromere antibodies
- Cardiac arrhythmias.
The hazard ratio for death in patients with arrhythmias in this model was 2.18 (95% CI 1.05–4.50, P = .035). Thus, finding arrhythmias in systemic sclerosis patients can provide important prognostic information.
While resting electrocardiography in patients with systemic sclerosis most commonly shows sinus rhythm, 24-hour electrocardiographic monitoring has revealed nonsustained supraventricular and ventricular arrhythmias in a significant percentage.71,72 Although difficult to quantify in routine practice, parameters controlled by the autonomic nervous system including heart rate variability and heart rate turbulence have been shown to be impaired in systemic sclerosis, and these measures are associated with an increased risk of malignant arrhythmias and sudden cardiac death.73,74
Conduction abnormalities
Conduction abnormalities occur in one-fifth to one-third of patients with systemic sclerosis.75,76 The most common abnormal conduction finding is left bundle branch block, followed by first-degree atrioventricular block. High-degree atrioventricular block is uncommon,76 though a few case reports of complete heart block thought to be related to systemic sclerosis have been published.77–79 An autopsy study showed that the conduction system is relatively spared from myocardial changes seen in systemic sclerosis patients, and thus it is speculated that the conduction disturbances are a consequence of damaged myocardium rather than damage to conduction tissue.80
Given the array of electrophysiologic abnormalities that systemic sclerosis patients can have, it is critical to monitor all patients with routine (annual or biannual) electrocardiography; to take possible arrhythmia-related symptoms seriously; and to evaluate them with further workup such as Holter monitoring for 24 hours or even longer, event monitoring, exercise testing, or tilt-table testing.
PERICARDIAL DISEASE
Pericardial disease is clinically apparent in 5% to 16% of patients with systemic sclerosis81; patients with limited cutaneous systemic sclerosis have more pericardial disease than those with diffuse cutaneous systemic sclerosis (30% vs 16%).82 Forty-one percent of systemic sclerosis patients have been shown to have pericardial effusion by echocardiography,81 but the effusions are typically small and rarely cause tamponade, though tamponade is associated with a poor prognosis.
Large pericardial effusions can develop before skin thickening and diagnosis of systemic sclerosis.81,83,84 Thus, systemic sclerosis should be considered in patients with pericardial effusions of unknown etiology.
In a small study,85 the pericardial fluid in systemic sclerosis was typically exudative, with lactate dehydrogenase greater than 200 U/L, a fluid-serum lactate dehydrogenase ratio greater than 0.6, and a fluid-serum total protein ratio greater than 0.5.
Pericardial effusion can be a sign of impending scleroderma renal crisis,86 and thus renal function should be carefully monitored in systemic sclerosis patients with pericardial effusion. Constrictive pericarditis and restrictive cardiomyopathy can rarely occur in systemic sclerosis and may more commonly present with symptoms.
Pericardial disease in systemic sclerosis should be treated in a standard fashion with nonsteroidal anti-inflammatory drugs. Corticosteroids are generally of limited benefit and should be avoided, especially in the setting of scleroderma renal crisis.81
VALVULAR HEART DISEASE
Based on limited studies, the prevalence of significant valvular heart disease in systemic sclerosis patients does not seem to be higher than that in the general population. While patients with systemic sclerosis and CREST syndrome (calcinosis, Raynaud phenomenon, esophageal dysmotility, sclerodactyly, and telangiectasia) have been shown to have a higher frequency of mitral valve prolapse and mild mitral regurgitation,87,88 these abnormalities do not often progress in severity, and thus their clinical significance is limited.
RECOMMENDATIONS FOR CARE OF SYSTEMIC SCLEROSIS PATIENTS
It is important for physicians caring for patients with systemic sclerosis to be aware of its most common cardiac manifestations, including left and right ventricular systolic and diastolic dysfunction, pulmonary hypertension, conduction abnormalities, arrhythmias, and cardiomyopathy.
Look for volume overload
On clinical examination, assess for clinical markers of volume overload such as distended neck veins, peripheral edema, or an abnormal blood pressure response to the Valsalva maneuver. These findings should prompt measurement of NT-proBNP,89 and may warrant prescription of a diuretic.
Electrocardiography to investigate arrhythmias
Electrocardiography should be done if patients describe symptoms of palpitations, and should also include continuous rhythm monitoring with Holter or event monitoring, depending on the frequency of symptoms. Otherwise, patients should routinely undergo electrocardiography once or twice a year.
Q waves are common in systemic sclerosis patients (especially those with diffuse cutaneous systemic sclerosis), notably in the precordial leads, and can occur without coronary artery disease.90 Symptoms such as presyncope should be further investigated with Holter monitoring and tilt-table testing.
Assess, modify traditional risk factors
Subclinical atherosclerosis as detected by carotid intima-media thickness is as common in systemic sclerosis as in rheumatoid arthritis.61 However, traditional risk indices such as SCORE (Systematic Coronary Risk Evaluation), QRISK2, and the American College of Cardiology/American Heart Association indices may underestimate risk in patients who have systemic sclerosis.
Strict hypertension control should be the goal for all systemic sclerosis patients. Though there are no specific guidelines on which antihypertensive medications are preferred, calcium channel blockers or angiotensin II receptor blockers, which are typically used to treat systemic sclerosis-related Raynaud phenomenon, may be appropriate.
Statins reduce vascular complications and are generally well tolerated in patients with systemic sclerosis.91,92
Aspirin is not recommended for routine primary prevention in view of data suggesting that its benefits in diabetic patients are counterbalanced by increased bleeding risk.93
Echocardiography to detect pulmonary arterial hypertension
At this time, guidelines for monitoring for cardiovascular manifestations in systemic sclerosis patients are limited. The only well-defined ones are European consensus guidelines, which suggest annual transthoracic echocardiography for the first 5 years after systemic sclerosis is diagnosed and continued annual screening in patients at risk of developing PAH.31
We support this strategy, with annual screening for the first 5 years followed by surveillance echocardiography every 2 to 3 years unless there is a high risk of PAH. Specific attention should be paid to right ventricular diastolic function, right atrial volume, and right ventricular myocardial performance index.
Emerging data suggest that the addition of global longitudinal strain of ventricles to routine echocardiography can help detect subclinical cardiac risk.94 Although further study is needed into the predictive value of global longitudinal strain, it is a low-cost and noninvasive addition to standard echocardiography that can help guide risk stratification, and thus we recommend that it be part of the echocardiographic examination for all systemic sclerosis patients.
Pulmonary function testing. In addition to screening for PAH with echocardiography, we recommend obtaining baseline pulmonary function tests, including DLCO, at the time systemic sclerosis is diagnosed, with repeat testing annually.
Magnetic resonance imaging
While echocardiography is the gold standard for monitoring systemic sclerosis patients, cardiovascular MRI may have a role in identifying those at higher risk of dangerous arrhythmias such as ventricular tachycardia and ventricular fibrillation. In addition to assessing ventricular function, MRI can detect myocardial inflammation, ischemia, and fibrosis that may predispose a patient to develop ventricular tachycardia or fibrillation.95 Variables such as T1/T2 mapping, extracellular volume fraction, T2 signal ratio, and early vs late gadolinium enhancement can help identify patients who had past ventricular tachycardia or fibrillation.96
Finding an increased risk of arrhythmias may prompt a conversation between the patient and the physician about the need for an implantable cardiac defibrillator.
If cardiac MRI is available and is reimbursed by the patient’s insurance carrier, physicians should strongly consider obtaining at least one baseline scan in systemic sclerosis patients to identify those at risk of highly fatal arrhythmias.
Teamwork is needed
Systemic sclerosis has not traditionally been associated with cardiovascular disease to the extent of other rheumatic conditions, but the cardiovascular system can be affected in various ways that can ultimately lead to an early death. These manifestations may be asymptomatic for long periods, and overt clinical disease portends a poorer prognosis.
Primary care physicians managing these patients should be aware of the cardiovascular complications of systemic sclerosis and should implement appropriate screening tests in conjunction with rheumatologists and cardiologists. It is also essential for general and subspecialty cardiologists to understand the broad spectrum of organ system involvement that can affect systemic sclerosis patients and to tailor their investigation and management recommendations accordingly. By designing a multidisciplinary approach to the treatment of systemic sclerosis patients, physicians can help to optimize cardiovascular risk modification in this vulnerable population.
- Maradit-Kremers H, Crowson CS, Nicola PJ, et al. Increased unrecognized coronary heart disease and sudden deaths in rheumatoid arthritis: a population-based cohort study. Arthritis Rheum 2005; 52(2):402–411. doi:10.1002/art.20853
- Naranjo A, Sokka T, Descalzo MA, et al; QUEST-RA Group. Cardiovascular disease in patients with rheumatoid arthritis: results from the QUEST-RA study. Arthritis Res Ther 2008; 10(2):R30. doi:10.1186/ar2383
- Innala L, Möller B, Ljung L, et al. Cardiovascular events in early RA are a result of inflammatory burden and traditional risk factors: a five year prospective study. Arthritis Res Ther 2011; 13(4):R131. doi:10.1186/ar3442
- Barnes J, Mayes MD. Epidemiology of systemic sclerosis: incidence, prevalence, survival, risk factors, malignancy, and environmental triggers. Curr Opin Rheumatol 2012; 24(2):165–170. doi:10.1097/BOR.0b013e32834ff2e8
- Chifflot H, Fautrel B, Sordet C, Chatelus E, Sibilia J. Incidence and prevalence of systemic sclerosis: a systematic literature review. Semin Arthritis Rheum 2008; 37(4):223–235 doi:10.1016/j.semarthrit.2007.05.003
- Gabrielli A, Avvedimento EV, Krieg T. Scleroderma. N Engl J Med 2009; 360(19):1989–2003. doi:10.1056/NEJMra0806188
- Panopoulos S, Tektonidou M, Drosos AA, et al. Prevalence of comorbidities in systemic sclerosis versus rheumatoid arthritis: a comparative, multicenter, matched-cohort study. Arthritis Res Ther 2018; 20(1):267. doi:10.1186/s13075-018-1771-0
- Ferri C, Valentini G, Cozzi F, et al. Systemic sclerosis: demographic, clinical, and serologic features and survival in 1,012 Italian patients. Medicine (Baltimore) 2002; 81(8):139–153. doi:10.1097/00005792-200203000-00004
- Steen VD, Medsger TA Jr. Severe organ involvement in systemic sclerosis with diffuse scleroderma. Arthritis Rheum 2000; 43(11):2437–2444. doi:10.1002/1529-0131(200011)43:11<2437::AID-ANR10>3.0.CO;2-U
- Hachulla AL, Launay D, Gaxotte V, et al. Cardiac magnetic resonance imaging in systemic sclerosis: a cross-sectional observational study of 52 patients. Ann Rheum Dis 2009; 68(12):1878–1884. doi:10.1136/ard.2008.095836
- Tyndall AJ, Bannert B, Vonk M, et al. Causes and risk factors for death in systemic sclerosis: a study from the EULAR Scleroderma Trials and Research (EUSTAR) database. Ann Rheum Dis 2010; 69(10):1809–1815. doi:10.1136/ard.2009.114264
- Nassenstein K, Breuckmann F, Huger M, et al. Detection of myocardial fibrosis in systemic sclerosis by contrast-enhanced magnetic resonance imaging. Rofo 2008; 180(12):1054–1060. doi:10.1055/s-2008-1027864
- Psarras A, Soulaidopoulos S, Garyfallos A, Kitas G, Dimitroulas T. A critical view on cardiovascular risk in systemic sclerosis. Rheumatol Int 2017; 37(1):85–95. doi:10.1007/s00296-016-3530-3
- Lekakis J, Mavrikakis M, Emmanuel M, et al. Cold-induced coronary Raynaud’s phenomenon in patients with systemic sclerosis. Clin Exp Rheumatol 1998; 16(2):135–140. pmid:9536388
- Altorok N, Wang Y, Kahaleh B. Endothelial dysfunction in systemic sclerosis. Curr Opin Rheumatol 2014; 26(6):615–620. doi:10.1097/BOR.0000000000000112
- Fleming JN, Nash RA, Mahoney WM Jr, Schwartz SM. Is scleroderma a vasculopathy? Curr Rheumatol Rep 2009; 11(2):103–110. pmid:19296882
- Maurer B, Distler A, Suliman YA, et al. Vascular endothelial growth factor aggravates fibrosis and vasculopathy in experimental models of systemic sclerosis. Ann Rheum Dis 2014; 73(10):1880–1887. doi:10.1136/annrheumdis-2013-203535
- Meune C, Vignaux O, Kahan A, Allanore Y. Heart involvement in systemic sclerosis: evolving concept and diagnostic methodologies. Arch Cardiovasc Dis 2010; 103(1):46–52. doi:10.1016/j.acvd.2009.06.009
- Dimitroulas T, Giannakoulas G, Karvounis H, Garyfallos A, Settas L, Kitas GD. Micro- and macrovascular treatment targets in scleroderma heart disease. Curr Pharm Des 2014; 20(4):536–544. pmid:23565639
- Allanore Y, Meune C. Primary myocardial involvement in systemic sclerosis: evidence for a microvascular origin. Clin Exp Rheumatol 2010; 28(5 suppl 62):S48–S53. pmid:21050545
- Kahan A, Nitenberg A, Foult JM, et al. Decreased coronary reserve in primary scleroderma myocardial disease. Arthritis Rheum 1985; 28(6):637–646. pmid:4004974
- Morrisroe K, Stevens W, Sahhar J, et al. Epidemiology and disease characteristics of systemic sclerosis-related pulmonary arterial hypertension: results from a real-life screening program. Arthritis Res Ther 2017; 19(1):42. doi:10.1186/s13075-017-1250-z
- Chaisson NF, Hassoun PM. Systemic sclerosis-associated pulmonary arterial hypertension. Chest 2013; 144(4):1346–1356. doi:10.1378/chest.12-2396
- Steen VD, Medsger TA. Changes in causes of death in systemic sclerosis, 1972–2002. Ann Rheum Dis 2007; 66(7):940–944. doi:10.1136/ard.2006.066068
- Coghlan JG, Galiè N, Barberà JA, et al; AMBITION investigators. Initial combination therapy with ambrisentan and tadalafil in connective tissue disease-associated pulmonary arterial hypertension (CTD-PAH): subgroup analysis from the AMBITION trial. Ann Rheum Dis 2017; 76(7):1219–1227. doi:10.1136/annrheumdis-2016-210236
- Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J 2019; 53(1):1801913. doi:10.1183/13993003.01913-2018
- Chatterjee S. Pulmonary hypertension in systemic sclerosis. Semin Arthritis Rheum 2011; 41(1):19–37. doi:10.1016/j.semarthrit.2010.08.004
- Sweiss NJ, Hushaw L, Thenappan T, et al. Diagnosis and management of pulmonary hypertension in systemic sclerosis. Curr Rheumatol Rep 2010; 12(1):8–18. doi:10.1007/s11926-009-0078-1
- Cox SR, Walker JG, Coleman M, et al. Isolated pulmonary hypertension in scleroderma. Intern Med J 2005; 35(1):28–33. doi:10.1111/j.1445-5994.2004.00646.x
- Sánchez-Román J, Opitz CF, Kowal-Bielecka O, García-Hernández FJ, Castillo-Palma MJ, Pittrow D; EPOSS-OMERACT Group. Screening for PAH in patients with systemic sclerosis: focus on Doppler echocardiography. Rheumatology (Oxford) 2008; 47(suppl 5):v33–v35. doi:10.1093/rheumatology/ken306
- Walker KM, Pope J; Scleroderma Clinical Trials Consortium; Canadian Scleroderma Research Group. Expert agreement on EULAR/EUSTAR recommendations for the management of systemic sclerosis. J Rheumatol 2011; 38(7):1326–1328. doi:10.3899/jrheum.101262
- Khanna D, Gladue H, Channick R, et al; Scleroderma Foundation and Pulmonary Hypertension Association. Recommendations for screening and detection of connective tissue disease-associated pulmonary arterial hypertension. Arthritis Rheum 2013; 65(12):3194–3201. doi:10.1002/art.38172
- Hachulla E, Gressin V, Guillevin L, et al. Early detection of pulmonary arterial hypertension in systemic sclerosis: a French nationwide prospective multicenter study. Arthritis Rheum 2005; 52(12):3792–3800. doi:10.1002/art.21433
- Dimitroulas T, Giannakoulas G, Dimitroula H, et al. Significance of serum uric acid in pulmonary hypertension due to systemic sclerosis: a pilot study. Rheumatol Int 2011; 31(2):263–267. doi:10.1007/s00296-010-1557-4
- Dimitroulas T, Giannakoulas G, Papadopoulou K, et al. Left atrial volume and N-terminal pro-B type natriuretic peptide are associated with elevated pulmonary artery pressure in patients with systemic sclerosis. Clin Rheumatol 2010; 29(9):957–964. doi:10.1007/s10067-010-1494-3
- Coghlan JG, Denton CP, Grünig E, et al; DETECT study group. Evidence-based detection of pulmonary arterial hypertension in systemic sclerosis: the DETECT study. Ann Rheum Dis 2014; 73(7):1340–1349. doi:10.1136/annrheumdis-2013-203301
- Schwaiger JP, Khanna D, Gerry Coghlan J. Screening patients with scleroderma for pulmonary arterial hypertension and implications for other at-risk populations. Eur Respir Rev 2013; 22(130):515–525. doi:10.1183/09059180.00006013
- Man A, Zhu Y, Zhang Y, et al. The risk of cardiovascular disease in systemic sclerosis: a population-based cohort study. Ann Rheum Dis 2013; 72(7):1188–1193. doi:10.1136/annrheumdis-2012-202007
- Ngian G-S, Sahhar J, Proudman SM, Stevens W, Wicks IP, Van Doornum S. Prevalence of coronary heart disease and cardiovascular risk factors in a national cross-sectional cohort study of systemic sclerosis. Ann Rheum Dis 2012; 71(12):1980–1983. doi:10.1136/annrheumdis-2011-201176
- Aviña-Zubieta JA, Man A, Yurkovich M, Huang K, Sayre EC, Choi HK. Early cardiovascular disease after the diagnosis of systemic sclerosis. Am J Med 2016; 29(3):324–331. doi:10.1016/j.amjmed.2015.10.037
- D’Angelo WA, Fries JF, Masi AT, Shulman LE. Pathologic observations in systemic sclerosis (scleroderma). A study of fifty-eight autopsy cases and fifty-eight matched controls. Am J Med 1969; 46(3):428–440. doi:10.1016/0002-9343(69)90044-8
- Ngian GS, Sahhar J, Proudman SM, Stevens W, Wicks IP, Van Doornum S. Prevalence of coronary heart disease and cardiovascular risk factors in a national cross-sectional cohort study of systemic sclerosis. Ann Rheum Dis 2012; 71(12):1980–1983. doi:10.1136/annrheumdis-2011-201176
- Khurma V, Meyer C, Park GS, et al. A pilot study of subclinical coronary atherosclerosis in systemic sclerosis: coronary artery calcification in cases and controls. Arthritis Rheum 2008; 59(4):591–597. doi:10.1002/art.23540
- Lippi G, Caramaschi P, Montagnana M, Salvagno GL, Volpe A, Guidi G. Lipoprotein[a] and the lipid profile in patients with systemic sclerosis. Clin Chim Acta 2006; 364(1–2):345–348. doi:10.1016/j.cca.2005.07.015
- Palinski W, Hörkkö S, Miller E, et al. Cloning of monoclonal autoantibodies to epitopes of oxidized lipoproteins from apolipoprotein E-deficient mice. Demonstration of epitopes of oxidized low density lipoprotein in human plasma. J Clin Invest 1996; 98(3):800–814. doi:10.1172/JCI118853
- Ho M, Veale D, Eastmond C, Nuki G, Belch J. Macrovascular disease and systemic sclerosis. Ann Rheum Dis 2000; 59(1):39–43. doi:10.1136/ard.59.1.39
- Kaloudi O, Basta G, Perfetto F, et al. Circulating levels of Ne-(carboxymethyl)lysine are increased in systemic sclerosis. Rheumatology (Oxford) 2007; 46(3):412–416. doi:10.1093/rheumatology/kel076
- Muro Y, Sugiura K, Morita Y, Tomita Y. An evaluation of the efficacy of the toe brachial index measuring vascular involvement in systemic sclerosis and other connective tissue diseases. Clin Exp Rheumatol 2009; 27(3 suppl 54):26–31. pmid:19796558
- Cheng K-S, Tiwari A, Boutin A, et al. Differentiation of primary and secondary Raynaud’s disease by carotid arterial stiffness. Eur J Vasc Endovasc Surg 2003; 25(4):336–341. doi:10.1053/ejvs.2002.1845
- Kawasaki M, Ito Y, Yokoyama H, et al. Assessment of arterial medial characteristics in human carotid arteries using integrated backscatter ultrasound and its histological implications. Atherosclerosis 2005; 180(1):145–154. doi:10.1016/j.atherosclerosis.2004.11.018
- Szucs G, Tímár O, Szekanecz Z, et al. Endothelial dysfunction precedes atherosclerosis in systemic sclerosis—relevance for prevention of vascular complications. Rheumatology (Oxford) 2007; 46(5):759–762. doi:10.1093/rheumatology/kel426
- Hettema ME, Zhang D, de Leeuw K, et al. Early atherosclerosis in systemic sclerosis and its relation to disease or traditional risk factors. Arthritis Res Ther 2008;10(2):R49. doi:10.1186/ar2408
- Roustit M, Simmons GH, Baguet JP, Carpentier P, Cracowski JL. Discrepancy between simultaneous digital skin microvascular and brachial artery macrovascular post-occlusive hyperemia in systemic sclerosis. J Rheumatol 2008; 35(8):1576–1583. pmid:18597404
- Vettori S, Maresca L, Cuomo G, Abbadessa S, Leonardo G, Valentini G. Clinical and subclinical atherosclerosis in systemic sclerosis: consequences of previous corticosteroid treatment. Scand J Rheumatol 2010; 39(6):485–489. doi:10.3109/03009741003781985
- Lekakis J, Mavrikakis M, Papamichael C, et al. Short-term estrogen administration improves abnormal endothelial function in women with systemic sclerosis and Raynaud’s phenomenon. Am Heart J 1998; 136(5):905–912. doi:10.1016/s0002-8703(98)70137-1
- Bartoli F, Blagojevic J, Bacci M, et al. Flow-mediated vasodilation and carotid intima-media thickness in systemic sclerosis. Ann N Y Acad Sci 2007; 1108:283–290. doi:10.1196/annals.1422.030
- Rollando D, Bezante GP, Sulli A, et al. Brachial artery endothelial-dependent flow-mediated dilation identifies early-stage endothelial dysfunction in systemic sclerosis and correlates with nailfold microvascular impairment. J Rheumatol 2010; 37(6):1168–1173. doi:10.3899/jrheum.091116
- Andersen GN, Mincheva-Nilsson L, Kazzam E, et al. Assessment of vascular function in systemic sclerosis: indications of the development of nitrate tolerance as a result of enhanced endothelial nitric oxide production. Arthritis Rheum 2002; 46(5):1324–1332. doi:10.1002/art.10191
- Au K, Singh MK, Bodukam V, et al. Atherosclerosis in systemic sclerosis: a systematic review and meta-analysis. Arthritis Rheum 2011; 63(7):2078–2090. doi:10.1002/art.30380
- van Sijl AM, Peters MJ, Knol DK, et al. Carotid intima media thickness in rheumatoid arthritis as compared to control subjects: a meta-analysis. Semin Arthritis Rheum 2011; 40(5):389–397. doi:10.1016/j.semarthrit.2010.06.006
- Brohall G, Odén A, Fagerberg B. Carotid artery intima-media thickness in patients with type 2 diabetes mellitus and impaired glucose tolerance: a systematic review. Diabet Med 2006; 23(6):609–616. doi:10.1111/j.1464-5491.2005.01725.x
- Masoura C, Pitsavos C, Aznaouridis K, Skoumas I, Vlachopoulos C, Stefanadis C. Arterial endothelial function and wall thickness in familial hypercholesterolemia and familial combined hyperlipidemia and the effect of statins. A systematic review and meta-analysis. Atherosclerosis 2011; 214(1):129–138. doi:10.1016/j.atherosclerosis.2010.10.008
- Ozen G, Inanc N, Unal AU, et al. Subclinical atherosclerosis in systemic sclerosis: not less frequent than rheumatoid arthritis and not detected with cardiovascular risk indices. Arthritis Care Res (Hoboken) 2016; 68(10):1538–1546. doi:10.1002/acr.22852
- Inaba Y, Chen JA, Bergmann SR. Prediction of future cardiovascular outcomes by flow-mediated vasodilatation of brachial artery: a meta-analysis. Int J Cardiovasc Imaging 2010; 26(6):631–640. doi:10.1007/s10554-010-9616-1
- Meune C, Avouac J, Wahbi K, et al. Cardiac involvement in systemic sclerosis assessed by tissue-doppler echocardiography during routine care: a controlled study of 100 consecutive patients. Arthritis Rheum 2008; 58(6):1803–1809. doi:10.1002/art.23463
- Tennøe AH, Murbræch K, Andreassen JC, et al. Left ventricular diastolic dysfunction predicts mortality in patients with systemic sclerosis. J Am Coll Cardiol 2018; 72(15):1804–1813. doi:10.1016/j.jacc.2018.07.068
- de Groote P, Gressin V, Hachulla E, et al; ItinerAIR-Scleroderma Investigators. Evaluation of cardiac abnormalities by Doppler echocardiography in a large nationwide multicentric cohort of patients with systemic sclerosis. Ann Rheum Dis 2008; 67(1):31–36. doi:10.1136/ard.2006.057760
- Allanore Y, Meune C, Vonk MC, et al; EUSTAR co-authors. Prevalence and factors associated with left ventricular dysfunction in the EULAR Scleroderma Trial and Research group (EUSTAR) database of patients with systemic sclerosis. Ann Rheum Dis 2010; 69(1):218–221. doi:10.1136/ard.2008.103382
- Hachulla AL, Launay D, Gaxotte V, et al. Cardiac magnetic resonance imaging in systemic sclerosis: a cross-sectional observational study of 52 patients. Ann Rheum Dis 2009; 68(12):1878–1884. doi:10.1136/ard.2008.095836
- Assassi S, Del Junco D, Sutter K, et al. Clinical and genetic factors predictive of mortality in early systemic sclerosis. Arthritis Rheum 2009; 61(10):1403–1411. doi:10.1002/art.24734
- Rokas S, Mavrikakis M, Agrios N, Mylonas D, Antoniadou L, Moulopoulos S. Electrophysiologic abnormalities of cardiac function in progressive systemic sclerosis. J Electrocardiol 1996; 29(1):17–25. pmid:8808521
- Kostis JB, Seibold JR, Turkevich D, et al. Prognostic importance of cardiac arrhythmias in systemic sclerosis. Am J Med 1988; 84(6):1007–1015. doi:10.1016/0002-9343(88)90305-1
- Biełous-Wilk A, Poreba M, Staniszewska-Marszałek E, et al. Electrocardiographic evaluation in patients with systemic scleroderma and without clinically evident heart disease. Ann Noninvasive Electrocardiol 2009; 14(3):251–257. doi:10.1111/j.1542-474X.2009.00306.x
- Bienias P, Ciurzynski M, Glinska-Wielochowska M, et al. Heart rate turbulence assessment in systemic sclerosis: the role for the detection of cardiac autonomic nervous system dysfunction. Rheumatology (Oxford) 2010; 49(2):355–360. doi:10.1093/rheumatology/kep394
- Ferri C, Bernini L, Bongiorni MG, et al. Noninvasive evaluation of cardiac dysrhythmias, and their relationship with multisystemic symptoms, in progressive systemic sclerosis patients. Arthritis Rheum 1985; 28(11):1259–1266. pmid:4063000
- Roberts NK, Cabeen WR, Moss J, Clements PJ, Furst DE. The prevalence of conduction defects and cardiac arrhythmias in progressive systemic sclerosis. Ann Intern Med 1981; 94(1):38–40. doi:10.7326/0003-4819-94-1-38
- Wang Q, Shang Y, Li S, Wu Y, Wang C, Yan X. Complete heart block in systemic sclerosis: a case report and literature review. Medicine (Baltimore) 2018; 97(46):e13226. doi:10.1097/MD.0000000000013226
- Summerfield BJ. Progressive systemic sclerosis with complete heart block. Br Heart J 1975; 37(12):1308–1310. doi:10.1136/hrt.37.12.1308
- Moyssakis I, Papadopoulos DP, Tzioufas AG, Votteas V. Complete heart block in a patient with systemic sclerosis. Clin Rheumatol 2006; 25(4):551–552. doi:10.1007/s10067-005-0068-2
- Ridolfi RL, Bulkley BH, Hutchins GM. The cardiac conduction system in progressive systemic sclerosis. Clinical and pathologic features of 35 patients. Am J Med 1976; 61(3):361–366. doi:10.1016/0002-9343(76)90373-9
- Champion HC. The heart in scleroderma. Rheum Dis Clin North Am 2008; 34(1):181–190. doi:10.1016/j.rdc.2007.12.002
- Gowda RM, Khan IA, Sacchi TJ, Vasavada BC. Scleroderma pericardial disease presented with a large pericardial effusion—a case report. Angiology 2001; 52(1):59–62. doi:10.1177/000331970105200108
- Meier FMP, Frommer KW, Dinser R, et al; EUSTAR Co-authors. Update on the profile of the EUSTAR cohort: an analysis of the EULAR scleroderma trials and research group database. Ann Rheum Dis 2012; 71(8):1355–1360. doi:10.1136/annrheumdis-2011-200742
- Subramanian SR, Akram R, Velayati A, Chadow H. New development of cardiac tamponade on underlying effusive-constrictive pericarditis: an uncommon initial presentation of scleroderma. BMJ Case Rep 2013; 2013. doi:10.1136/bcr-2013-010254
- Kitchongcharoenying P, Foocharoen C, Mahakkanukrauh A, Suwannaroj S, Nanagara R. Pericardial fluid profiles of pericardial effusion in systemic sclerosis patients. Asian Pac J Allergy Immunol 2013; 31(4):314–319. doi:10.12932/AP0305.31.4.2013
- McWhorter JE, LeRoy EC. Pericardial disease in scleroderma (systemic sclerosis). Am J Med 1974; 57(4):566–575. doi:10.1016/0002-9343(74)90008-4
- Comens SM, Alpert MA, Sharp GC, et al. Frequency of mitral valve prolapse in systemic lupus erythematosus, progressive systemic sclerosis and mixed connective tissue disease. Am J Cardiol 1989; 63(5):369–370. doi:10.1016/0002-9149(89)90351-2
- Candell-Riera J, Armadans-Gil L, Simeón CP, et al. Comprehensive noninvasive assessment of cardiac involvement in limited systemic sclerosis. Arthritis Rheum 1996; 39(7):1138–1145. pmid:8670322
- Caforio ALP, Adler Y, Agostini C, et al. Diagnosis and management of myocardial involvement in systemic immune-mediated diseases: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Disease. Eur Heart J 2017; 38(35):2649–2662. doi:10.1093/eurheartj/ehx321
- Mavrogeni S, Karabela G, Koutsogeorgopoulou L, et al. Pseudo-infarction pattern in diffuse systemic sclerosis. Evaluation using cardiovascular magnetic resonance. Int J Cardiol 2016; 214:465–468. doi:10.1016/j.ijcard.2016.03.235
- Ladak K, Pope JE. A review of the effects of statins in systemic sclerosis. Semin Arthritis Rheum 2016; 45(6):698–705. doi:10.1016/j.semarthrit.2015.10.013
- Abou-Raya A, Abou-Raya S, Helmii M. Statins: potentially useful in therapy of systemic sclerosis-related Raynaud’s phenomenon and digital ulcers. J Rheumatol 2008; 35(9):1801–1808. pmid:18709692
- ASCEND Study Collaborative Group; Bowman L, Mafham M, Wallendszus K, et al. Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med 2018; 379(16):1529–1539. doi:10.1056/NEJMoa1804988
- Guerra F, Stronati G, Fischietti C, et al. Global longitudinal strain measured by speckle tracking identifies subclinical heart involvement in patients with systemic sclerosis. Eur J Prev Cardiol 2018; 25(15):1598–1606. doi:10.1177/2047487318786315
- Mavrogeni SI, Sfikakis PP, Dimitroulas T, et al. Prospects of using cardiovascular magnetic resonance in the identification of arrhythmogenic substrate in autoimmune rheumatic diseases. Rheumatol Int 2018; 38(9):1615–1621. doi:10.1007/s00296-018-4110-5
- Mavrogeni SI, Sfikakis PP, Markousis-Mavrogenis G, et al. Cardiovascular magnetic resonance imaging pattern in patients with autoimmune rheumatic diseases and ventricular tachycardia with preserved ejection fraction. Int J Cardiol 2019; 284:105–109. doi:10.1016/j.ijcard.2018.10.067
Autoimmune rheumatic diseases increase the risk of cardiovascular disease. In rheumatoid arthritis and systemic lupus erythematosus, the risk is driven primarily by the inflammatory milieu, leading to accelerated coronary and cerebrovascular atherosclerosis independent of traditional atherosclerotic risk factors.1–3 The extent of cardiovascular involvement in other rheumatologic diseases has been less well characterized but is an area of growing interest.
In this review, we focus on the cardiovascular complications of systemic sclerosis and review recommendations for monitoring these patients in clinical practice.
SYSTEMIC SCLEROSIS, AN AUTOIMMUNE RHEUMATIC DISEASE
Systemic sclerosis is an autoimmune rheumatic disease characterized by excessive extracellular matrix deposition leading to diffuse fibrosis, endothelial dysfunction, and microvascular injury. It is most common in North America, Southern Europe, and Australia,4,5 and it affects women more than men in ratios ranging from 3:1 to 14:1.6 The mean age at diagnosis is around 50.
The disease can affect the lungs (interstitial lung disease and pulmonary hypertension), the heart, the kidneys, and the gastrointestinal tract.
Systemic sclerosis has 2 main subtypes: limited cutaneous systemic sclerosis, formerly called CREST syndrome) and diffuse cutaneous systemic sclerosis. The limited cutaneous subtype is characterized by tightening of the skin of the distal extremities (below the elbows and knees) and face, while diffuse cutaneous systemic sclerosis can manifest as more extensive skin tightening also involving proximal extremities and the trunk. Both subtypes can have an effect on the cardiovascular system.
Some cardiovascular risk factors such as dyslipidemia, diabetes mellitus, and high body mass index are less common in patients with systemic sclerosis than in patients with rheumatoid arthritis, while the rates of arterial hypertension, smoking, chronic obstructive pulmonary disease, osteoporosis, and neoplasms are similar between the 2 groups.7
HEART INVOLVEMENT HAS SERIOUS CONSEQUENCES
Overt cardiac involvement in systemic sclerosis is associated with a mortality rate of up to 70% over 5 years,8,9 and about one-fourth of deaths in patients with systemic sclerosis are from cardiac causes.10,11 Studies in Europe10,12 showed that many patients with systemic sclerosis have cardiac involvement detectable by magnetic resonance imaging even if they do not have clinical disease. Pulmonary arterial hypertension (PAH) is a complication of both subtypes of systemic sclerosis and portends a higher risk of death.8
Thus, it is critical for clinicians to understand the potential comorbid conditions associated with systemic sclerosis, particularly the cardiovascular ones, and to work closely with cardiologists to help optimize the evaluation and management.
MECHANISMS OF CARDIAC DISEASE IN SYSTEMIC SCLEROSIS
Abnormal vasoreactivity, a consequence of an imbalance between endothelium-derived vasoconstrictors and vasodilators, defective angiogenesis, and endothelial injury, leads to tissue ischemia and vascular endothelial growth factor expression, which initiates injury and fibrosis in the myocardium and in other organs.14–17 Fibrosis involves the myocardium, pericardium, and conduction system.13,18
Myocardial involvement in systemic sclerosis is thought to be due mainly to abnormal vasoreactivity and microvascular abnormalities such as transient coronary artery spasm leading to repeated focal ischemia.19,20 Abnormal vasoreactivity has been demonstrated during cardiac catheterization21: while mean coronary sinus blood flow in systemic sclerosis patients was normal at rest, vasodilator reserve was significantly reduced in patients with diffuse cutaneous systemic sclerosis after maximal vasodilation with dipyridamole. Additionally, endomyocardial biopsy showed fibrosis and concentric intimal hypertrophy with normal epicardial coronary arteries.21
More research into other mechanisms of cardiovascular disease in systemic sclerosis is needed to allow for better preventive care for these patients.
PULMONARY ARTERIAL HYPERTENSION
Systemic sclerosis can be associated with World Health Organization (WHO) groups 1, 2, 3, and 4 pulmonary hypertension. WHO group 1, called pulmonary arterial hypertension or PAH, is one of the most common cardiac complications of systemic sclerosis, with a reported prevalence as high as 12%.22 Systemic sclerosis-associated PAH carries a high mortality rate, with a mean survival of only 3 years.23
With advances in treatments for other complications of systemic sclerosis, the percentage of systemic sclerosis patients who die of PAH has increased from 6% to 33%.24
Compared with patients with idiopathic PAH, those with systemic sclerosis get less of a response from therapy and have poorer outcomes despite lower mean pulmonary artery pressures and similar reductions in cardiac index. However, recent studies have suggested that with aggressive treatment, patients with systemic sclerosis-related PAH can achieve outcomes similar to those with idiopathic PAH.25 Thus, recognizing this condition early is imperative.
Pulmonary arterial hypertension defined
PAH is defined as the combination of all of the following26:
- Mean pulmonary artery pressure > 20 mm Hg at rest
- Normal pulmonary capillary wedge pressure (≤ 15 mm Hg)
- Pulmonary vascular resistance ≥ 3 Wood units on right heart catheterization.
Other causes of pulmonary hypertension such as interstitial lung disease, chronic pulmonary thromboembolic disease, and left heart disease must be excluded.24,27
Remodeling in the pulmonary arteries
The events that lead to PAH in systemic sclerosis remain unclear but are believed to involve initial inflammation or endothelial injury that leads to a dysequilibrium between proliferative mediators and antiproliferative vasodilators. This dysequilibrium, along with endothelial dysfunction, causes an obliterative vasculopathy in the pulmonary artery branches and arterioles. Sympathetic overactivity, hypoxemia, and ischemia-reperfusion injury additionally promote vascular proliferation, fibrosis, and remodeling, leading to increased pulmonary vascular resistance, PAH, and increased right ventricular pressures.23,27
The subtype of systemic sclerosis is an important factor in the development and progression of PAH. PAH appears to be the major cause of death in limited cutaneous systemic sclerosis, while interstitial lung disease is the major cause of death in diffuse cutaneous systemic sclerosis.28
Pulmonary arterial hypertension is a late complication of systemic sclerosis
Data from the South Australian Scleroderma Registry29 revealed that PAH tends to be a late complication of systemic sclerosis, occurring around 20 years after disease onset. In this study of 608 patients, no patient with diffuse cutaneous systemic sclerosis developed PAH.
Systemic sclerosis-related PAH initially follows an indolent course with few symptoms until right ventricular function deteriorates. Early in the disease, patients may experience nonspecific symptoms of fatigue, lightheadedness, and dyspnea on exertion.23 As it progresses, they tend to have worsening dyspnea and may experience exertional syncope, palpitations, and chest pain.
Physical findings may suggest elevated right ventricular pressure and right ventricular failure; these include a loud P2, a prominent jugular a wave, a tricuspid regurgitant murmur, jugular venous distention, and lower-extremity edema.27
Screening for pulmonary arterial hypertension in systemic sclerosis
Significant signs and symptoms usually occur late in the disease; thus, it is important to appropriately screen patients who are at risk so that they can begin aggressive treatment.
Doppler echocardiography is recommended by European and American guidelines to screen for PAH in patients who have systemic sclerosis, and most agree that screening is appropriate even if the patient has no symptoms.30 European consensus documents recommend that transthoracic echocardiography be done annually for the first 5 years of disease and be continued every year in patients at high risk, ie, those with anticentromere antibodies, anti-Th/To antibodies, or interstitial lung disease. Patients not at high risk of developing pulmonary hypertension should also have regular transthoracic echocardiography, though the exact timing is not defined.31 While American societies have not issued corresponding recommendations, many experts follow the European recommendations.
Worrisome features on echocardiography in asymptomatic patients should be followed up with right heart catheterization to assess mean right ventricular pressure. These include:
- Estimated right ventricular systolic pressure ≥ 40 mm Hg
- Tricuspid regurgitant jet velocity > 2.8 m/s
- Right atrial enlargement > 53 mm
- Right ventricular enlargement (mid-cavity dimension > 35 mm).32
Although echocardiography is the most common form of screening, it gives only an estimate of right ventricular systolic pressure, which is imprecise. Other noninvasive markers are helpful and necessary to appropriately screen this population.
Diffusion capacity. The Itinerair study33 found that a diffusing capacity for carbon monoxide (DLCO) of 60% or higher has a high specificity in excluding PAH.
Uric acid has been found to be elevated in patients with systemic sclerosis-related PAH, and levels inversely correlate with 6-minute walking distance.34
Other predictors. N-terminal pro-B-type natriuretic peptide (NT-proBNP), left atrial volume, and the right ventricular myocardial performance index have also been shown to be independent predictors of PAH in patients with systemic sclerosis.35
An algorithm. The DETECT study36 enrolled patients at increased risk who had had systemic sclerosis longer than 3 years and a DLCO less than 60%. The investigators developed a 2-step algorithm to determine which patients should be referred for right heart catheterization to try to detect PAH earlier while minimizing the number of missed diagnoses and optimizing the use of invasive diagnostic right heart catheterization.
The first step was to assess serum values of anticentromere antibodies, NT-proBNP, and urate, and clinical features (telangiectasias), forced vital capacity, and electrocardiographic changes of right axis deviation to derive a prediction score. The second step was to assess surface echocardiographic features of the right atrial area and tricuspid regurgitation velocity.
This approach led to right heart catheterization in 62% of patients and was associated with a false-negative rate of 4%. Importantly, of the patients with PAH, 1 in 5 had no symptoms, and 33% had tricuspid regurgitation velocity less than 2.8 m/s. No single measurement performed well in isolation in this study.37
Thus, we recommend that, in addition to routine surface echocardiography, a multimodal approach be used that includes laboratory testing, clinical features, and electrocardiographic findings when screening this high-risk patient population.
ATHEROSCLEROTIC DISEASES
Although macrovascular disease has not typically been regarded as a significant systemic feature in systemic sclerosis, myocardial infarction and stroke are more common in patients with systemic sclerosis than in controls.38,39
Coronary artery disease in systemic sclerosis
Man et al38 reported that the incidence of myocardial infarction in patients with systemic sclerosis was 4.4 per 1,000 persons per year, and the incidence of stroke was 4.8 per 1,000 persons per year, compared with 2.5 per 1,000 persons per year for both myocardial infarction and stroke in healthy controls matched for age, sex, and time of entry.
The Australian Scleroderma Cohort Study39 found a 3-fold higher prevalence of coronary artery disease in systemic sclerosis patients than in controls after factoring in traditional risk factors.
Aviña-Zubieta et al,40 in a cohort of 1,239 systemic sclerosis patients, estimated a hazard ratio (HR) of 3.49 for myocardial infarction and 2.35 for stroke compared with age- and sex-matched controls. Not all of these events were related to macrovascular atherosclerosis—vasospasm and microvascular ischemia may have played significant roles in the etiology of clinical manifestations.
Studies of coronary atherosclerosis in systemic sclerosis are limited. An autopsy study41 of 58 patients with systemic sclerosis and 58 controls matched for age, sex, and ethnicity found that the prevalence of atherosclerosis of small coronary arteries and arterioles was significantly higher in systemic sclerosis patients than in controls (17% vs 2%, P < .01). However, the prevalence of medium-vessel coronary atherosclerosis was similar (48% vs 43%).
Why patients with systemic sclerosis develop atherosclerosis has not yet been determined. Traditional risk factors such as hypertension, dyslipidemia, diabetes mellitus, and obesity are typically no more prevalent in systemic sclerosis patients than in controls,38,42 and thus do not explain the increased risk of atherosclerotic cardiovascular disease. There is some evidence that novel markers of atherosclerotic risk such as homocysteine,43 lipoprotein[a],44 and oxidized low-density lipoprotein45 are more prevalent in systemic sclerosis, but these results have not been substantiated in more extensive studies.
Peripheral artery disease
It remains unclear whether peripheral artery disease is more prevalent in systemic sclerosis patients than in controls.
Individual studies have shown mixed results in comparing carotid artery stenosis between systemic sclerosis patients and controls using carotid duplex ultrasonography,46 the ankle-brachial index,46–48 carotid intima-media thickness,49–54 and brachial flow-mediated dilation.51,53,55–58 A meta-analysis found that the carotid intima and media are significantly thicker in systemic sclerosis patients than in controls,59 and the magnitude of difference is similar to that in other groups at increased cardiovascular risk, such as those with rheumatoid arthritis, diabetes, and familial hypercholesterolemia.60–63
A meta-analysis of brachial artery findings showed significantly lower flow-mediated dilation in systemic sclerosis patients than in controls.64
Overall, given the inconsistency of study results, systemic sclerosis patients should be screened and managed as in other patients with peripheral artery disease, but the clinician should be aware that there may be a higher risk of peripheral artery disease in these patients.
RIGHT AND LEFT VENTRICULAR DYSFUNCTION
Many patients with systemic sclerosis have right ventricular dysfunction as a consequence of PAH.65 It is important to detect diastolic dysfunction in this population, as it may be an even stronger predictor of death than pulmonary hypertension on right heart catheterization (HR 3.7 vs 2.0).66
Fewer patients have left ventricular dysfunction. In a multicenter study of 570 systemic sclerosis patients, only 1.4% had left ventricular systolic dysfunction on echocardiography, though 22.6% had left ventricular hypertrophy and 17.7% had left ventricular diastolic dysfunction.67 In the European League Against Rheumatism (EULAR) database, the prevalence of reduced left ventricular ejection fraction was 5.4%.68
Though traditional echocardiographic screening suggests the prevalence of left ventricular dysfunction in systemic sclerosis patients is low, cardiac magnetic resonance imaging (MRI) may be more sensitive than echocardiography for detecting subclinical myocardial involvement. Cardiac MRI has been shown to detect evidence of myocardial pathology (increased T2 signal, left ventricular thinning, pericardial effusion, reduced left ventricular and right ventricular ejection fraction, left ventricular diastolic dysfunction, and delayed myocardial contrast enhancement) in up to 75% of systemic sclerosis cases studied.69
Patients with systemic sclerosis should already be undergoing echocardiography every year to screen for PAH, and screening should also include tissue Doppler imaging to detect various forms of left and right ventricular systolic and diastolic dysfunction that may not be clinically apparent.
Though cardiac MRI can provide useful additional information, it is not currently recommended for routine screening in patients with systemic sclerosis.
ARRHYTHMIAS AND CONDUCTION DEFECTS
Patients with systemic sclerosis are prone to arrhythmias due to both conduction system fibrosis and myocardial damage.
Arrhythmias accounted for 6% of the deaths in the EULAR Scleroderma Trials and Research (EUSTAR) database.11
In the Genetics Versus Environment in Scleroderma Outcome Study (GENISOS),70 250 patients who had had systemic sclerosis for at least 3 years were studied during a period of approximately 6 years, during which there were 52 deaths, 29 of which were directly attributable to systemic sclerosis. Multivariable Cox modeling showed that 7 variables predicted mortality:
- Body mass index < 18.5 kg/m2
- Age ≥ 65
- Forced vital capacity < 50% predicted
- Systolic blood pressure ≥ 140 or diastolic blood pressure ≥ 90 mm Hg
- Pulmonary fibrosis
- Positive anticentromere antibodies
- Cardiac arrhythmias.
The hazard ratio for death in patients with arrhythmias in this model was 2.18 (95% CI 1.05–4.50, P = .035). Thus, finding arrhythmias in systemic sclerosis patients can provide important prognostic information.
While resting electrocardiography in patients with systemic sclerosis most commonly shows sinus rhythm, 24-hour electrocardiographic monitoring has revealed nonsustained supraventricular and ventricular arrhythmias in a significant percentage.71,72 Although difficult to quantify in routine practice, parameters controlled by the autonomic nervous system including heart rate variability and heart rate turbulence have been shown to be impaired in systemic sclerosis, and these measures are associated with an increased risk of malignant arrhythmias and sudden cardiac death.73,74
Conduction abnormalities
Conduction abnormalities occur in one-fifth to one-third of patients with systemic sclerosis.75,76 The most common abnormal conduction finding is left bundle branch block, followed by first-degree atrioventricular block. High-degree atrioventricular block is uncommon,76 though a few case reports of complete heart block thought to be related to systemic sclerosis have been published.77–79 An autopsy study showed that the conduction system is relatively spared from myocardial changes seen in systemic sclerosis patients, and thus it is speculated that the conduction disturbances are a consequence of damaged myocardium rather than damage to conduction tissue.80
Given the array of electrophysiologic abnormalities that systemic sclerosis patients can have, it is critical to monitor all patients with routine (annual or biannual) electrocardiography; to take possible arrhythmia-related symptoms seriously; and to evaluate them with further workup such as Holter monitoring for 24 hours or even longer, event monitoring, exercise testing, or tilt-table testing.
PERICARDIAL DISEASE
Pericardial disease is clinically apparent in 5% to 16% of patients with systemic sclerosis81; patients with limited cutaneous systemic sclerosis have more pericardial disease than those with diffuse cutaneous systemic sclerosis (30% vs 16%).82 Forty-one percent of systemic sclerosis patients have been shown to have pericardial effusion by echocardiography,81 but the effusions are typically small and rarely cause tamponade, though tamponade is associated with a poor prognosis.
Large pericardial effusions can develop before skin thickening and diagnosis of systemic sclerosis.81,83,84 Thus, systemic sclerosis should be considered in patients with pericardial effusions of unknown etiology.
In a small study,85 the pericardial fluid in systemic sclerosis was typically exudative, with lactate dehydrogenase greater than 200 U/L, a fluid-serum lactate dehydrogenase ratio greater than 0.6, and a fluid-serum total protein ratio greater than 0.5.
Pericardial effusion can be a sign of impending scleroderma renal crisis,86 and thus renal function should be carefully monitored in systemic sclerosis patients with pericardial effusion. Constrictive pericarditis and restrictive cardiomyopathy can rarely occur in systemic sclerosis and may more commonly present with symptoms.
Pericardial disease in systemic sclerosis should be treated in a standard fashion with nonsteroidal anti-inflammatory drugs. Corticosteroids are generally of limited benefit and should be avoided, especially in the setting of scleroderma renal crisis.81
VALVULAR HEART DISEASE
Based on limited studies, the prevalence of significant valvular heart disease in systemic sclerosis patients does not seem to be higher than that in the general population. While patients with systemic sclerosis and CREST syndrome (calcinosis, Raynaud phenomenon, esophageal dysmotility, sclerodactyly, and telangiectasia) have been shown to have a higher frequency of mitral valve prolapse and mild mitral regurgitation,87,88 these abnormalities do not often progress in severity, and thus their clinical significance is limited.
RECOMMENDATIONS FOR CARE OF SYSTEMIC SCLEROSIS PATIENTS
It is important for physicians caring for patients with systemic sclerosis to be aware of its most common cardiac manifestations, including left and right ventricular systolic and diastolic dysfunction, pulmonary hypertension, conduction abnormalities, arrhythmias, and cardiomyopathy.
Look for volume overload
On clinical examination, assess for clinical markers of volume overload such as distended neck veins, peripheral edema, or an abnormal blood pressure response to the Valsalva maneuver. These findings should prompt measurement of NT-proBNP,89 and may warrant prescription of a diuretic.
Electrocardiography to investigate arrhythmias
Electrocardiography should be done if patients describe symptoms of palpitations, and should also include continuous rhythm monitoring with Holter or event monitoring, depending on the frequency of symptoms. Otherwise, patients should routinely undergo electrocardiography once or twice a year.
Q waves are common in systemic sclerosis patients (especially those with diffuse cutaneous systemic sclerosis), notably in the precordial leads, and can occur without coronary artery disease.90 Symptoms such as presyncope should be further investigated with Holter monitoring and tilt-table testing.
Assess, modify traditional risk factors
Subclinical atherosclerosis as detected by carotid intima-media thickness is as common in systemic sclerosis as in rheumatoid arthritis.61 However, traditional risk indices such as SCORE (Systematic Coronary Risk Evaluation), QRISK2, and the American College of Cardiology/American Heart Association indices may underestimate risk in patients who have systemic sclerosis.
Strict hypertension control should be the goal for all systemic sclerosis patients. Though there are no specific guidelines on which antihypertensive medications are preferred, calcium channel blockers or angiotensin II receptor blockers, which are typically used to treat systemic sclerosis-related Raynaud phenomenon, may be appropriate.
Statins reduce vascular complications and are generally well tolerated in patients with systemic sclerosis.91,92
Aspirin is not recommended for routine primary prevention in view of data suggesting that its benefits in diabetic patients are counterbalanced by increased bleeding risk.93
Echocardiography to detect pulmonary arterial hypertension
At this time, guidelines for monitoring for cardiovascular manifestations in systemic sclerosis patients are limited. The only well-defined ones are European consensus guidelines, which suggest annual transthoracic echocardiography for the first 5 years after systemic sclerosis is diagnosed and continued annual screening in patients at risk of developing PAH.31
We support this strategy, with annual screening for the first 5 years followed by surveillance echocardiography every 2 to 3 years unless there is a high risk of PAH. Specific attention should be paid to right ventricular diastolic function, right atrial volume, and right ventricular myocardial performance index.
Emerging data suggest that the addition of global longitudinal strain of ventricles to routine echocardiography can help detect subclinical cardiac risk.94 Although further study is needed into the predictive value of global longitudinal strain, it is a low-cost and noninvasive addition to standard echocardiography that can help guide risk stratification, and thus we recommend that it be part of the echocardiographic examination for all systemic sclerosis patients.
Pulmonary function testing. In addition to screening for PAH with echocardiography, we recommend obtaining baseline pulmonary function tests, including DLCO, at the time systemic sclerosis is diagnosed, with repeat testing annually.
Magnetic resonance imaging
While echocardiography is the gold standard for monitoring systemic sclerosis patients, cardiovascular MRI may have a role in identifying those at higher risk of dangerous arrhythmias such as ventricular tachycardia and ventricular fibrillation. In addition to assessing ventricular function, MRI can detect myocardial inflammation, ischemia, and fibrosis that may predispose a patient to develop ventricular tachycardia or fibrillation.95 Variables such as T1/T2 mapping, extracellular volume fraction, T2 signal ratio, and early vs late gadolinium enhancement can help identify patients who had past ventricular tachycardia or fibrillation.96
Finding an increased risk of arrhythmias may prompt a conversation between the patient and the physician about the need for an implantable cardiac defibrillator.
If cardiac MRI is available and is reimbursed by the patient’s insurance carrier, physicians should strongly consider obtaining at least one baseline scan in systemic sclerosis patients to identify those at risk of highly fatal arrhythmias.
Teamwork is needed
Systemic sclerosis has not traditionally been associated with cardiovascular disease to the extent of other rheumatic conditions, but the cardiovascular system can be affected in various ways that can ultimately lead to an early death. These manifestations may be asymptomatic for long periods, and overt clinical disease portends a poorer prognosis.
Primary care physicians managing these patients should be aware of the cardiovascular complications of systemic sclerosis and should implement appropriate screening tests in conjunction with rheumatologists and cardiologists. It is also essential for general and subspecialty cardiologists to understand the broad spectrum of organ system involvement that can affect systemic sclerosis patients and to tailor their investigation and management recommendations accordingly. By designing a multidisciplinary approach to the treatment of systemic sclerosis patients, physicians can help to optimize cardiovascular risk modification in this vulnerable population.
Autoimmune rheumatic diseases increase the risk of cardiovascular disease. In rheumatoid arthritis and systemic lupus erythematosus, the risk is driven primarily by the inflammatory milieu, leading to accelerated coronary and cerebrovascular atherosclerosis independent of traditional atherosclerotic risk factors.1–3 The extent of cardiovascular involvement in other rheumatologic diseases has been less well characterized but is an area of growing interest.
In this review, we focus on the cardiovascular complications of systemic sclerosis and review recommendations for monitoring these patients in clinical practice.
SYSTEMIC SCLEROSIS, AN AUTOIMMUNE RHEUMATIC DISEASE
Systemic sclerosis is an autoimmune rheumatic disease characterized by excessive extracellular matrix deposition leading to diffuse fibrosis, endothelial dysfunction, and microvascular injury. It is most common in North America, Southern Europe, and Australia,4,5 and it affects women more than men in ratios ranging from 3:1 to 14:1.6 The mean age at diagnosis is around 50.
The disease can affect the lungs (interstitial lung disease and pulmonary hypertension), the heart, the kidneys, and the gastrointestinal tract.
Systemic sclerosis has 2 main subtypes: limited cutaneous systemic sclerosis, formerly called CREST syndrome) and diffuse cutaneous systemic sclerosis. The limited cutaneous subtype is characterized by tightening of the skin of the distal extremities (below the elbows and knees) and face, while diffuse cutaneous systemic sclerosis can manifest as more extensive skin tightening also involving proximal extremities and the trunk. Both subtypes can have an effect on the cardiovascular system.
Some cardiovascular risk factors such as dyslipidemia, diabetes mellitus, and high body mass index are less common in patients with systemic sclerosis than in patients with rheumatoid arthritis, while the rates of arterial hypertension, smoking, chronic obstructive pulmonary disease, osteoporosis, and neoplasms are similar between the 2 groups.7
HEART INVOLVEMENT HAS SERIOUS CONSEQUENCES
Overt cardiac involvement in systemic sclerosis is associated with a mortality rate of up to 70% over 5 years,8,9 and about one-fourth of deaths in patients with systemic sclerosis are from cardiac causes.10,11 Studies in Europe10,12 showed that many patients with systemic sclerosis have cardiac involvement detectable by magnetic resonance imaging even if they do not have clinical disease. Pulmonary arterial hypertension (PAH) is a complication of both subtypes of systemic sclerosis and portends a higher risk of death.8
Thus, it is critical for clinicians to understand the potential comorbid conditions associated with systemic sclerosis, particularly the cardiovascular ones, and to work closely with cardiologists to help optimize the evaluation and management.
MECHANISMS OF CARDIAC DISEASE IN SYSTEMIC SCLEROSIS
Abnormal vasoreactivity, a consequence of an imbalance between endothelium-derived vasoconstrictors and vasodilators, defective angiogenesis, and endothelial injury, leads to tissue ischemia and vascular endothelial growth factor expression, which initiates injury and fibrosis in the myocardium and in other organs.14–17 Fibrosis involves the myocardium, pericardium, and conduction system.13,18
Myocardial involvement in systemic sclerosis is thought to be due mainly to abnormal vasoreactivity and microvascular abnormalities such as transient coronary artery spasm leading to repeated focal ischemia.19,20 Abnormal vasoreactivity has been demonstrated during cardiac catheterization21: while mean coronary sinus blood flow in systemic sclerosis patients was normal at rest, vasodilator reserve was significantly reduced in patients with diffuse cutaneous systemic sclerosis after maximal vasodilation with dipyridamole. Additionally, endomyocardial biopsy showed fibrosis and concentric intimal hypertrophy with normal epicardial coronary arteries.21
More research into other mechanisms of cardiovascular disease in systemic sclerosis is needed to allow for better preventive care for these patients.
PULMONARY ARTERIAL HYPERTENSION
Systemic sclerosis can be associated with World Health Organization (WHO) groups 1, 2, 3, and 4 pulmonary hypertension. WHO group 1, called pulmonary arterial hypertension or PAH, is one of the most common cardiac complications of systemic sclerosis, with a reported prevalence as high as 12%.22 Systemic sclerosis-associated PAH carries a high mortality rate, with a mean survival of only 3 years.23
With advances in treatments for other complications of systemic sclerosis, the percentage of systemic sclerosis patients who die of PAH has increased from 6% to 33%.24
Compared with patients with idiopathic PAH, those with systemic sclerosis get less of a response from therapy and have poorer outcomes despite lower mean pulmonary artery pressures and similar reductions in cardiac index. However, recent studies have suggested that with aggressive treatment, patients with systemic sclerosis-related PAH can achieve outcomes similar to those with idiopathic PAH.25 Thus, recognizing this condition early is imperative.
Pulmonary arterial hypertension defined
PAH is defined as the combination of all of the following26:
- Mean pulmonary artery pressure > 20 mm Hg at rest
- Normal pulmonary capillary wedge pressure (≤ 15 mm Hg)
- Pulmonary vascular resistance ≥ 3 Wood units on right heart catheterization.
Other causes of pulmonary hypertension such as interstitial lung disease, chronic pulmonary thromboembolic disease, and left heart disease must be excluded.24,27
Remodeling in the pulmonary arteries
The events that lead to PAH in systemic sclerosis remain unclear but are believed to involve initial inflammation or endothelial injury that leads to a dysequilibrium between proliferative mediators and antiproliferative vasodilators. This dysequilibrium, along with endothelial dysfunction, causes an obliterative vasculopathy in the pulmonary artery branches and arterioles. Sympathetic overactivity, hypoxemia, and ischemia-reperfusion injury additionally promote vascular proliferation, fibrosis, and remodeling, leading to increased pulmonary vascular resistance, PAH, and increased right ventricular pressures.23,27
The subtype of systemic sclerosis is an important factor in the development and progression of PAH. PAH appears to be the major cause of death in limited cutaneous systemic sclerosis, while interstitial lung disease is the major cause of death in diffuse cutaneous systemic sclerosis.28
Pulmonary arterial hypertension is a late complication of systemic sclerosis
Data from the South Australian Scleroderma Registry29 revealed that PAH tends to be a late complication of systemic sclerosis, occurring around 20 years after disease onset. In this study of 608 patients, no patient with diffuse cutaneous systemic sclerosis developed PAH.
Systemic sclerosis-related PAH initially follows an indolent course with few symptoms until right ventricular function deteriorates. Early in the disease, patients may experience nonspecific symptoms of fatigue, lightheadedness, and dyspnea on exertion.23 As it progresses, they tend to have worsening dyspnea and may experience exertional syncope, palpitations, and chest pain.
Physical findings may suggest elevated right ventricular pressure and right ventricular failure; these include a loud P2, a prominent jugular a wave, a tricuspid regurgitant murmur, jugular venous distention, and lower-extremity edema.27
Screening for pulmonary arterial hypertension in systemic sclerosis
Significant signs and symptoms usually occur late in the disease; thus, it is important to appropriately screen patients who are at risk so that they can begin aggressive treatment.
Doppler echocardiography is recommended by European and American guidelines to screen for PAH in patients who have systemic sclerosis, and most agree that screening is appropriate even if the patient has no symptoms.30 European consensus documents recommend that transthoracic echocardiography be done annually for the first 5 years of disease and be continued every year in patients at high risk, ie, those with anticentromere antibodies, anti-Th/To antibodies, or interstitial lung disease. Patients not at high risk of developing pulmonary hypertension should also have regular transthoracic echocardiography, though the exact timing is not defined.31 While American societies have not issued corresponding recommendations, many experts follow the European recommendations.
Worrisome features on echocardiography in asymptomatic patients should be followed up with right heart catheterization to assess mean right ventricular pressure. These include:
- Estimated right ventricular systolic pressure ≥ 40 mm Hg
- Tricuspid regurgitant jet velocity > 2.8 m/s
- Right atrial enlargement > 53 mm
- Right ventricular enlargement (mid-cavity dimension > 35 mm).32
Although echocardiography is the most common form of screening, it gives only an estimate of right ventricular systolic pressure, which is imprecise. Other noninvasive markers are helpful and necessary to appropriately screen this population.
Diffusion capacity. The Itinerair study33 found that a diffusing capacity for carbon monoxide (DLCO) of 60% or higher has a high specificity in excluding PAH.
Uric acid has been found to be elevated in patients with systemic sclerosis-related PAH, and levels inversely correlate with 6-minute walking distance.34
Other predictors. N-terminal pro-B-type natriuretic peptide (NT-proBNP), left atrial volume, and the right ventricular myocardial performance index have also been shown to be independent predictors of PAH in patients with systemic sclerosis.35
An algorithm. The DETECT study36 enrolled patients at increased risk who had had systemic sclerosis longer than 3 years and a DLCO less than 60%. The investigators developed a 2-step algorithm to determine which patients should be referred for right heart catheterization to try to detect PAH earlier while minimizing the number of missed diagnoses and optimizing the use of invasive diagnostic right heart catheterization.
The first step was to assess serum values of anticentromere antibodies, NT-proBNP, and urate, and clinical features (telangiectasias), forced vital capacity, and electrocardiographic changes of right axis deviation to derive a prediction score. The second step was to assess surface echocardiographic features of the right atrial area and tricuspid regurgitation velocity.
This approach led to right heart catheterization in 62% of patients and was associated with a false-negative rate of 4%. Importantly, of the patients with PAH, 1 in 5 had no symptoms, and 33% had tricuspid regurgitation velocity less than 2.8 m/s. No single measurement performed well in isolation in this study.37
Thus, we recommend that, in addition to routine surface echocardiography, a multimodal approach be used that includes laboratory testing, clinical features, and electrocardiographic findings when screening this high-risk patient population.
ATHEROSCLEROTIC DISEASES
Although macrovascular disease has not typically been regarded as a significant systemic feature in systemic sclerosis, myocardial infarction and stroke are more common in patients with systemic sclerosis than in controls.38,39
Coronary artery disease in systemic sclerosis
Man et al38 reported that the incidence of myocardial infarction in patients with systemic sclerosis was 4.4 per 1,000 persons per year, and the incidence of stroke was 4.8 per 1,000 persons per year, compared with 2.5 per 1,000 persons per year for both myocardial infarction and stroke in healthy controls matched for age, sex, and time of entry.
The Australian Scleroderma Cohort Study39 found a 3-fold higher prevalence of coronary artery disease in systemic sclerosis patients than in controls after factoring in traditional risk factors.
Aviña-Zubieta et al,40 in a cohort of 1,239 systemic sclerosis patients, estimated a hazard ratio (HR) of 3.49 for myocardial infarction and 2.35 for stroke compared with age- and sex-matched controls. Not all of these events were related to macrovascular atherosclerosis—vasospasm and microvascular ischemia may have played significant roles in the etiology of clinical manifestations.
Studies of coronary atherosclerosis in systemic sclerosis are limited. An autopsy study41 of 58 patients with systemic sclerosis and 58 controls matched for age, sex, and ethnicity found that the prevalence of atherosclerosis of small coronary arteries and arterioles was significantly higher in systemic sclerosis patients than in controls (17% vs 2%, P < .01). However, the prevalence of medium-vessel coronary atherosclerosis was similar (48% vs 43%).
Why patients with systemic sclerosis develop atherosclerosis has not yet been determined. Traditional risk factors such as hypertension, dyslipidemia, diabetes mellitus, and obesity are typically no more prevalent in systemic sclerosis patients than in controls,38,42 and thus do not explain the increased risk of atherosclerotic cardiovascular disease. There is some evidence that novel markers of atherosclerotic risk such as homocysteine,43 lipoprotein[a],44 and oxidized low-density lipoprotein45 are more prevalent in systemic sclerosis, but these results have not been substantiated in more extensive studies.
Peripheral artery disease
It remains unclear whether peripheral artery disease is more prevalent in systemic sclerosis patients than in controls.
Individual studies have shown mixed results in comparing carotid artery stenosis between systemic sclerosis patients and controls using carotid duplex ultrasonography,46 the ankle-brachial index,46–48 carotid intima-media thickness,49–54 and brachial flow-mediated dilation.51,53,55–58 A meta-analysis found that the carotid intima and media are significantly thicker in systemic sclerosis patients than in controls,59 and the magnitude of difference is similar to that in other groups at increased cardiovascular risk, such as those with rheumatoid arthritis, diabetes, and familial hypercholesterolemia.60–63
A meta-analysis of brachial artery findings showed significantly lower flow-mediated dilation in systemic sclerosis patients than in controls.64
Overall, given the inconsistency of study results, systemic sclerosis patients should be screened and managed as in other patients with peripheral artery disease, but the clinician should be aware that there may be a higher risk of peripheral artery disease in these patients.
RIGHT AND LEFT VENTRICULAR DYSFUNCTION
Many patients with systemic sclerosis have right ventricular dysfunction as a consequence of PAH.65 It is important to detect diastolic dysfunction in this population, as it may be an even stronger predictor of death than pulmonary hypertension on right heart catheterization (HR 3.7 vs 2.0).66
Fewer patients have left ventricular dysfunction. In a multicenter study of 570 systemic sclerosis patients, only 1.4% had left ventricular systolic dysfunction on echocardiography, though 22.6% had left ventricular hypertrophy and 17.7% had left ventricular diastolic dysfunction.67 In the European League Against Rheumatism (EULAR) database, the prevalence of reduced left ventricular ejection fraction was 5.4%.68
Though traditional echocardiographic screening suggests the prevalence of left ventricular dysfunction in systemic sclerosis patients is low, cardiac magnetic resonance imaging (MRI) may be more sensitive than echocardiography for detecting subclinical myocardial involvement. Cardiac MRI has been shown to detect evidence of myocardial pathology (increased T2 signal, left ventricular thinning, pericardial effusion, reduced left ventricular and right ventricular ejection fraction, left ventricular diastolic dysfunction, and delayed myocardial contrast enhancement) in up to 75% of systemic sclerosis cases studied.69
Patients with systemic sclerosis should already be undergoing echocardiography every year to screen for PAH, and screening should also include tissue Doppler imaging to detect various forms of left and right ventricular systolic and diastolic dysfunction that may not be clinically apparent.
Though cardiac MRI can provide useful additional information, it is not currently recommended for routine screening in patients with systemic sclerosis.
ARRHYTHMIAS AND CONDUCTION DEFECTS
Patients with systemic sclerosis are prone to arrhythmias due to both conduction system fibrosis and myocardial damage.
Arrhythmias accounted for 6% of the deaths in the EULAR Scleroderma Trials and Research (EUSTAR) database.11
In the Genetics Versus Environment in Scleroderma Outcome Study (GENISOS),70 250 patients who had had systemic sclerosis for at least 3 years were studied during a period of approximately 6 years, during which there were 52 deaths, 29 of which were directly attributable to systemic sclerosis. Multivariable Cox modeling showed that 7 variables predicted mortality:
- Body mass index < 18.5 kg/m2
- Age ≥ 65
- Forced vital capacity < 50% predicted
- Systolic blood pressure ≥ 140 or diastolic blood pressure ≥ 90 mm Hg
- Pulmonary fibrosis
- Positive anticentromere antibodies
- Cardiac arrhythmias.
The hazard ratio for death in patients with arrhythmias in this model was 2.18 (95% CI 1.05–4.50, P = .035). Thus, finding arrhythmias in systemic sclerosis patients can provide important prognostic information.
While resting electrocardiography in patients with systemic sclerosis most commonly shows sinus rhythm, 24-hour electrocardiographic monitoring has revealed nonsustained supraventricular and ventricular arrhythmias in a significant percentage.71,72 Although difficult to quantify in routine practice, parameters controlled by the autonomic nervous system including heart rate variability and heart rate turbulence have been shown to be impaired in systemic sclerosis, and these measures are associated with an increased risk of malignant arrhythmias and sudden cardiac death.73,74
Conduction abnormalities
Conduction abnormalities occur in one-fifth to one-third of patients with systemic sclerosis.75,76 The most common abnormal conduction finding is left bundle branch block, followed by first-degree atrioventricular block. High-degree atrioventricular block is uncommon,76 though a few case reports of complete heart block thought to be related to systemic sclerosis have been published.77–79 An autopsy study showed that the conduction system is relatively spared from myocardial changes seen in systemic sclerosis patients, and thus it is speculated that the conduction disturbances are a consequence of damaged myocardium rather than damage to conduction tissue.80
Given the array of electrophysiologic abnormalities that systemic sclerosis patients can have, it is critical to monitor all patients with routine (annual or biannual) electrocardiography; to take possible arrhythmia-related symptoms seriously; and to evaluate them with further workup such as Holter monitoring for 24 hours or even longer, event monitoring, exercise testing, or tilt-table testing.
PERICARDIAL DISEASE
Pericardial disease is clinically apparent in 5% to 16% of patients with systemic sclerosis81; patients with limited cutaneous systemic sclerosis have more pericardial disease than those with diffuse cutaneous systemic sclerosis (30% vs 16%).82 Forty-one percent of systemic sclerosis patients have been shown to have pericardial effusion by echocardiography,81 but the effusions are typically small and rarely cause tamponade, though tamponade is associated with a poor prognosis.
Large pericardial effusions can develop before skin thickening and diagnosis of systemic sclerosis.81,83,84 Thus, systemic sclerosis should be considered in patients with pericardial effusions of unknown etiology.
In a small study,85 the pericardial fluid in systemic sclerosis was typically exudative, with lactate dehydrogenase greater than 200 U/L, a fluid-serum lactate dehydrogenase ratio greater than 0.6, and a fluid-serum total protein ratio greater than 0.5.
Pericardial effusion can be a sign of impending scleroderma renal crisis,86 and thus renal function should be carefully monitored in systemic sclerosis patients with pericardial effusion. Constrictive pericarditis and restrictive cardiomyopathy can rarely occur in systemic sclerosis and may more commonly present with symptoms.
Pericardial disease in systemic sclerosis should be treated in a standard fashion with nonsteroidal anti-inflammatory drugs. Corticosteroids are generally of limited benefit and should be avoided, especially in the setting of scleroderma renal crisis.81
VALVULAR HEART DISEASE
Based on limited studies, the prevalence of significant valvular heart disease in systemic sclerosis patients does not seem to be higher than that in the general population. While patients with systemic sclerosis and CREST syndrome (calcinosis, Raynaud phenomenon, esophageal dysmotility, sclerodactyly, and telangiectasia) have been shown to have a higher frequency of mitral valve prolapse and mild mitral regurgitation,87,88 these abnormalities do not often progress in severity, and thus their clinical significance is limited.
RECOMMENDATIONS FOR CARE OF SYSTEMIC SCLEROSIS PATIENTS
It is important for physicians caring for patients with systemic sclerosis to be aware of its most common cardiac manifestations, including left and right ventricular systolic and diastolic dysfunction, pulmonary hypertension, conduction abnormalities, arrhythmias, and cardiomyopathy.
Look for volume overload
On clinical examination, assess for clinical markers of volume overload such as distended neck veins, peripheral edema, or an abnormal blood pressure response to the Valsalva maneuver. These findings should prompt measurement of NT-proBNP,89 and may warrant prescription of a diuretic.
Electrocardiography to investigate arrhythmias
Electrocardiography should be done if patients describe symptoms of palpitations, and should also include continuous rhythm monitoring with Holter or event monitoring, depending on the frequency of symptoms. Otherwise, patients should routinely undergo electrocardiography once or twice a year.
Q waves are common in systemic sclerosis patients (especially those with diffuse cutaneous systemic sclerosis), notably in the precordial leads, and can occur without coronary artery disease.90 Symptoms such as presyncope should be further investigated with Holter monitoring and tilt-table testing.
Assess, modify traditional risk factors
Subclinical atherosclerosis as detected by carotid intima-media thickness is as common in systemic sclerosis as in rheumatoid arthritis.61 However, traditional risk indices such as SCORE (Systematic Coronary Risk Evaluation), QRISK2, and the American College of Cardiology/American Heart Association indices may underestimate risk in patients who have systemic sclerosis.
Strict hypertension control should be the goal for all systemic sclerosis patients. Though there are no specific guidelines on which antihypertensive medications are preferred, calcium channel blockers or angiotensin II receptor blockers, which are typically used to treat systemic sclerosis-related Raynaud phenomenon, may be appropriate.
Statins reduce vascular complications and are generally well tolerated in patients with systemic sclerosis.91,92
Aspirin is not recommended for routine primary prevention in view of data suggesting that its benefits in diabetic patients are counterbalanced by increased bleeding risk.93
Echocardiography to detect pulmonary arterial hypertension
At this time, guidelines for monitoring for cardiovascular manifestations in systemic sclerosis patients are limited. The only well-defined ones are European consensus guidelines, which suggest annual transthoracic echocardiography for the first 5 years after systemic sclerosis is diagnosed and continued annual screening in patients at risk of developing PAH.31
We support this strategy, with annual screening for the first 5 years followed by surveillance echocardiography every 2 to 3 years unless there is a high risk of PAH. Specific attention should be paid to right ventricular diastolic function, right atrial volume, and right ventricular myocardial performance index.
Emerging data suggest that the addition of global longitudinal strain of ventricles to routine echocardiography can help detect subclinical cardiac risk.94 Although further study is needed into the predictive value of global longitudinal strain, it is a low-cost and noninvasive addition to standard echocardiography that can help guide risk stratification, and thus we recommend that it be part of the echocardiographic examination for all systemic sclerosis patients.
Pulmonary function testing. In addition to screening for PAH with echocardiography, we recommend obtaining baseline pulmonary function tests, including DLCO, at the time systemic sclerosis is diagnosed, with repeat testing annually.
Magnetic resonance imaging
While echocardiography is the gold standard for monitoring systemic sclerosis patients, cardiovascular MRI may have a role in identifying those at higher risk of dangerous arrhythmias such as ventricular tachycardia and ventricular fibrillation. In addition to assessing ventricular function, MRI can detect myocardial inflammation, ischemia, and fibrosis that may predispose a patient to develop ventricular tachycardia or fibrillation.95 Variables such as T1/T2 mapping, extracellular volume fraction, T2 signal ratio, and early vs late gadolinium enhancement can help identify patients who had past ventricular tachycardia or fibrillation.96
Finding an increased risk of arrhythmias may prompt a conversation between the patient and the physician about the need for an implantable cardiac defibrillator.
If cardiac MRI is available and is reimbursed by the patient’s insurance carrier, physicians should strongly consider obtaining at least one baseline scan in systemic sclerosis patients to identify those at risk of highly fatal arrhythmias.
Teamwork is needed
Systemic sclerosis has not traditionally been associated with cardiovascular disease to the extent of other rheumatic conditions, but the cardiovascular system can be affected in various ways that can ultimately lead to an early death. These manifestations may be asymptomatic for long periods, and overt clinical disease portends a poorer prognosis.
Primary care physicians managing these patients should be aware of the cardiovascular complications of systemic sclerosis and should implement appropriate screening tests in conjunction with rheumatologists and cardiologists. It is also essential for general and subspecialty cardiologists to understand the broad spectrum of organ system involvement that can affect systemic sclerosis patients and to tailor their investigation and management recommendations accordingly. By designing a multidisciplinary approach to the treatment of systemic sclerosis patients, physicians can help to optimize cardiovascular risk modification in this vulnerable population.
- Maradit-Kremers H, Crowson CS, Nicola PJ, et al. Increased unrecognized coronary heart disease and sudden deaths in rheumatoid arthritis: a population-based cohort study. Arthritis Rheum 2005; 52(2):402–411. doi:10.1002/art.20853
- Naranjo A, Sokka T, Descalzo MA, et al; QUEST-RA Group. Cardiovascular disease in patients with rheumatoid arthritis: results from the QUEST-RA study. Arthritis Res Ther 2008; 10(2):R30. doi:10.1186/ar2383
- Innala L, Möller B, Ljung L, et al. Cardiovascular events in early RA are a result of inflammatory burden and traditional risk factors: a five year prospective study. Arthritis Res Ther 2011; 13(4):R131. doi:10.1186/ar3442
- Barnes J, Mayes MD. Epidemiology of systemic sclerosis: incidence, prevalence, survival, risk factors, malignancy, and environmental triggers. Curr Opin Rheumatol 2012; 24(2):165–170. doi:10.1097/BOR.0b013e32834ff2e8
- Chifflot H, Fautrel B, Sordet C, Chatelus E, Sibilia J. Incidence and prevalence of systemic sclerosis: a systematic literature review. Semin Arthritis Rheum 2008; 37(4):223–235 doi:10.1016/j.semarthrit.2007.05.003
- Gabrielli A, Avvedimento EV, Krieg T. Scleroderma. N Engl J Med 2009; 360(19):1989–2003. doi:10.1056/NEJMra0806188
- Panopoulos S, Tektonidou M, Drosos AA, et al. Prevalence of comorbidities in systemic sclerosis versus rheumatoid arthritis: a comparative, multicenter, matched-cohort study. Arthritis Res Ther 2018; 20(1):267. doi:10.1186/s13075-018-1771-0
- Ferri C, Valentini G, Cozzi F, et al. Systemic sclerosis: demographic, clinical, and serologic features and survival in 1,012 Italian patients. Medicine (Baltimore) 2002; 81(8):139–153. doi:10.1097/00005792-200203000-00004
- Steen VD, Medsger TA Jr. Severe organ involvement in systemic sclerosis with diffuse scleroderma. Arthritis Rheum 2000; 43(11):2437–2444. doi:10.1002/1529-0131(200011)43:11<2437::AID-ANR10>3.0.CO;2-U
- Hachulla AL, Launay D, Gaxotte V, et al. Cardiac magnetic resonance imaging in systemic sclerosis: a cross-sectional observational study of 52 patients. Ann Rheum Dis 2009; 68(12):1878–1884. doi:10.1136/ard.2008.095836
- Tyndall AJ, Bannert B, Vonk M, et al. Causes and risk factors for death in systemic sclerosis: a study from the EULAR Scleroderma Trials and Research (EUSTAR) database. Ann Rheum Dis 2010; 69(10):1809–1815. doi:10.1136/ard.2009.114264
- Nassenstein K, Breuckmann F, Huger M, et al. Detection of myocardial fibrosis in systemic sclerosis by contrast-enhanced magnetic resonance imaging. Rofo 2008; 180(12):1054–1060. doi:10.1055/s-2008-1027864
- Psarras A, Soulaidopoulos S, Garyfallos A, Kitas G, Dimitroulas T. A critical view on cardiovascular risk in systemic sclerosis. Rheumatol Int 2017; 37(1):85–95. doi:10.1007/s00296-016-3530-3
- Lekakis J, Mavrikakis M, Emmanuel M, et al. Cold-induced coronary Raynaud’s phenomenon in patients with systemic sclerosis. Clin Exp Rheumatol 1998; 16(2):135–140. pmid:9536388
- Altorok N, Wang Y, Kahaleh B. Endothelial dysfunction in systemic sclerosis. Curr Opin Rheumatol 2014; 26(6):615–620. doi:10.1097/BOR.0000000000000112
- Fleming JN, Nash RA, Mahoney WM Jr, Schwartz SM. Is scleroderma a vasculopathy? Curr Rheumatol Rep 2009; 11(2):103–110. pmid:19296882
- Maurer B, Distler A, Suliman YA, et al. Vascular endothelial growth factor aggravates fibrosis and vasculopathy in experimental models of systemic sclerosis. Ann Rheum Dis 2014; 73(10):1880–1887. doi:10.1136/annrheumdis-2013-203535
- Meune C, Vignaux O, Kahan A, Allanore Y. Heart involvement in systemic sclerosis: evolving concept and diagnostic methodologies. Arch Cardiovasc Dis 2010; 103(1):46–52. doi:10.1016/j.acvd.2009.06.009
- Dimitroulas T, Giannakoulas G, Karvounis H, Garyfallos A, Settas L, Kitas GD. Micro- and macrovascular treatment targets in scleroderma heart disease. Curr Pharm Des 2014; 20(4):536–544. pmid:23565639
- Allanore Y, Meune C. Primary myocardial involvement in systemic sclerosis: evidence for a microvascular origin. Clin Exp Rheumatol 2010; 28(5 suppl 62):S48–S53. pmid:21050545
- Kahan A, Nitenberg A, Foult JM, et al. Decreased coronary reserve in primary scleroderma myocardial disease. Arthritis Rheum 1985; 28(6):637–646. pmid:4004974
- Morrisroe K, Stevens W, Sahhar J, et al. Epidemiology and disease characteristics of systemic sclerosis-related pulmonary arterial hypertension: results from a real-life screening program. Arthritis Res Ther 2017; 19(1):42. doi:10.1186/s13075-017-1250-z
- Chaisson NF, Hassoun PM. Systemic sclerosis-associated pulmonary arterial hypertension. Chest 2013; 144(4):1346–1356. doi:10.1378/chest.12-2396
- Steen VD, Medsger TA. Changes in causes of death in systemic sclerosis, 1972–2002. Ann Rheum Dis 2007; 66(7):940–944. doi:10.1136/ard.2006.066068
- Coghlan JG, Galiè N, Barberà JA, et al; AMBITION investigators. Initial combination therapy with ambrisentan and tadalafil in connective tissue disease-associated pulmonary arterial hypertension (CTD-PAH): subgroup analysis from the AMBITION trial. Ann Rheum Dis 2017; 76(7):1219–1227. doi:10.1136/annrheumdis-2016-210236
- Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J 2019; 53(1):1801913. doi:10.1183/13993003.01913-2018
- Chatterjee S. Pulmonary hypertension in systemic sclerosis. Semin Arthritis Rheum 2011; 41(1):19–37. doi:10.1016/j.semarthrit.2010.08.004
- Sweiss NJ, Hushaw L, Thenappan T, et al. Diagnosis and management of pulmonary hypertension in systemic sclerosis. Curr Rheumatol Rep 2010; 12(1):8–18. doi:10.1007/s11926-009-0078-1
- Cox SR, Walker JG, Coleman M, et al. Isolated pulmonary hypertension in scleroderma. Intern Med J 2005; 35(1):28–33. doi:10.1111/j.1445-5994.2004.00646.x
- Sánchez-Román J, Opitz CF, Kowal-Bielecka O, García-Hernández FJ, Castillo-Palma MJ, Pittrow D; EPOSS-OMERACT Group. Screening for PAH in patients with systemic sclerosis: focus on Doppler echocardiography. Rheumatology (Oxford) 2008; 47(suppl 5):v33–v35. doi:10.1093/rheumatology/ken306
- Walker KM, Pope J; Scleroderma Clinical Trials Consortium; Canadian Scleroderma Research Group. Expert agreement on EULAR/EUSTAR recommendations for the management of systemic sclerosis. J Rheumatol 2011; 38(7):1326–1328. doi:10.3899/jrheum.101262
- Khanna D, Gladue H, Channick R, et al; Scleroderma Foundation and Pulmonary Hypertension Association. Recommendations for screening and detection of connective tissue disease-associated pulmonary arterial hypertension. Arthritis Rheum 2013; 65(12):3194–3201. doi:10.1002/art.38172
- Hachulla E, Gressin V, Guillevin L, et al. Early detection of pulmonary arterial hypertension in systemic sclerosis: a French nationwide prospective multicenter study. Arthritis Rheum 2005; 52(12):3792–3800. doi:10.1002/art.21433
- Dimitroulas T, Giannakoulas G, Dimitroula H, et al. Significance of serum uric acid in pulmonary hypertension due to systemic sclerosis: a pilot study. Rheumatol Int 2011; 31(2):263–267. doi:10.1007/s00296-010-1557-4
- Dimitroulas T, Giannakoulas G, Papadopoulou K, et al. Left atrial volume and N-terminal pro-B type natriuretic peptide are associated with elevated pulmonary artery pressure in patients with systemic sclerosis. Clin Rheumatol 2010; 29(9):957–964. doi:10.1007/s10067-010-1494-3
- Coghlan JG, Denton CP, Grünig E, et al; DETECT study group. Evidence-based detection of pulmonary arterial hypertension in systemic sclerosis: the DETECT study. Ann Rheum Dis 2014; 73(7):1340–1349. doi:10.1136/annrheumdis-2013-203301
- Schwaiger JP, Khanna D, Gerry Coghlan J. Screening patients with scleroderma for pulmonary arterial hypertension and implications for other at-risk populations. Eur Respir Rev 2013; 22(130):515–525. doi:10.1183/09059180.00006013
- Man A, Zhu Y, Zhang Y, et al. The risk of cardiovascular disease in systemic sclerosis: a population-based cohort study. Ann Rheum Dis 2013; 72(7):1188–1193. doi:10.1136/annrheumdis-2012-202007
- Ngian G-S, Sahhar J, Proudman SM, Stevens W, Wicks IP, Van Doornum S. Prevalence of coronary heart disease and cardiovascular risk factors in a national cross-sectional cohort study of systemic sclerosis. Ann Rheum Dis 2012; 71(12):1980–1983. doi:10.1136/annrheumdis-2011-201176
- Aviña-Zubieta JA, Man A, Yurkovich M, Huang K, Sayre EC, Choi HK. Early cardiovascular disease after the diagnosis of systemic sclerosis. Am J Med 2016; 29(3):324–331. doi:10.1016/j.amjmed.2015.10.037
- D’Angelo WA, Fries JF, Masi AT, Shulman LE. Pathologic observations in systemic sclerosis (scleroderma). A study of fifty-eight autopsy cases and fifty-eight matched controls. Am J Med 1969; 46(3):428–440. doi:10.1016/0002-9343(69)90044-8
- Ngian GS, Sahhar J, Proudman SM, Stevens W, Wicks IP, Van Doornum S. Prevalence of coronary heart disease and cardiovascular risk factors in a national cross-sectional cohort study of systemic sclerosis. Ann Rheum Dis 2012; 71(12):1980–1983. doi:10.1136/annrheumdis-2011-201176
- Khurma V, Meyer C, Park GS, et al. A pilot study of subclinical coronary atherosclerosis in systemic sclerosis: coronary artery calcification in cases and controls. Arthritis Rheum 2008; 59(4):591–597. doi:10.1002/art.23540
- Lippi G, Caramaschi P, Montagnana M, Salvagno GL, Volpe A, Guidi G. Lipoprotein[a] and the lipid profile in patients with systemic sclerosis. Clin Chim Acta 2006; 364(1–2):345–348. doi:10.1016/j.cca.2005.07.015
- Palinski W, Hörkkö S, Miller E, et al. Cloning of monoclonal autoantibodies to epitopes of oxidized lipoproteins from apolipoprotein E-deficient mice. Demonstration of epitopes of oxidized low density lipoprotein in human plasma. J Clin Invest 1996; 98(3):800–814. doi:10.1172/JCI118853
- Ho M, Veale D, Eastmond C, Nuki G, Belch J. Macrovascular disease and systemic sclerosis. Ann Rheum Dis 2000; 59(1):39–43. doi:10.1136/ard.59.1.39
- Kaloudi O, Basta G, Perfetto F, et al. Circulating levels of Ne-(carboxymethyl)lysine are increased in systemic sclerosis. Rheumatology (Oxford) 2007; 46(3):412–416. doi:10.1093/rheumatology/kel076
- Muro Y, Sugiura K, Morita Y, Tomita Y. An evaluation of the efficacy of the toe brachial index measuring vascular involvement in systemic sclerosis and other connective tissue diseases. Clin Exp Rheumatol 2009; 27(3 suppl 54):26–31. pmid:19796558
- Cheng K-S, Tiwari A, Boutin A, et al. Differentiation of primary and secondary Raynaud’s disease by carotid arterial stiffness. Eur J Vasc Endovasc Surg 2003; 25(4):336–341. doi:10.1053/ejvs.2002.1845
- Kawasaki M, Ito Y, Yokoyama H, et al. Assessment of arterial medial characteristics in human carotid arteries using integrated backscatter ultrasound and its histological implications. Atherosclerosis 2005; 180(1):145–154. doi:10.1016/j.atherosclerosis.2004.11.018
- Szucs G, Tímár O, Szekanecz Z, et al. Endothelial dysfunction precedes atherosclerosis in systemic sclerosis—relevance for prevention of vascular complications. Rheumatology (Oxford) 2007; 46(5):759–762. doi:10.1093/rheumatology/kel426
- Hettema ME, Zhang D, de Leeuw K, et al. Early atherosclerosis in systemic sclerosis and its relation to disease or traditional risk factors. Arthritis Res Ther 2008;10(2):R49. doi:10.1186/ar2408
- Roustit M, Simmons GH, Baguet JP, Carpentier P, Cracowski JL. Discrepancy between simultaneous digital skin microvascular and brachial artery macrovascular post-occlusive hyperemia in systemic sclerosis. J Rheumatol 2008; 35(8):1576–1583. pmid:18597404
- Vettori S, Maresca L, Cuomo G, Abbadessa S, Leonardo G, Valentini G. Clinical and subclinical atherosclerosis in systemic sclerosis: consequences of previous corticosteroid treatment. Scand J Rheumatol 2010; 39(6):485–489. doi:10.3109/03009741003781985
- Lekakis J, Mavrikakis M, Papamichael C, et al. Short-term estrogen administration improves abnormal endothelial function in women with systemic sclerosis and Raynaud’s phenomenon. Am Heart J 1998; 136(5):905–912. doi:10.1016/s0002-8703(98)70137-1
- Bartoli F, Blagojevic J, Bacci M, et al. Flow-mediated vasodilation and carotid intima-media thickness in systemic sclerosis. Ann N Y Acad Sci 2007; 1108:283–290. doi:10.1196/annals.1422.030
- Rollando D, Bezante GP, Sulli A, et al. Brachial artery endothelial-dependent flow-mediated dilation identifies early-stage endothelial dysfunction in systemic sclerosis and correlates with nailfold microvascular impairment. J Rheumatol 2010; 37(6):1168–1173. doi:10.3899/jrheum.091116
- Andersen GN, Mincheva-Nilsson L, Kazzam E, et al. Assessment of vascular function in systemic sclerosis: indications of the development of nitrate tolerance as a result of enhanced endothelial nitric oxide production. Arthritis Rheum 2002; 46(5):1324–1332. doi:10.1002/art.10191
- Au K, Singh MK, Bodukam V, et al. Atherosclerosis in systemic sclerosis: a systematic review and meta-analysis. Arthritis Rheum 2011; 63(7):2078–2090. doi:10.1002/art.30380
- van Sijl AM, Peters MJ, Knol DK, et al. Carotid intima media thickness in rheumatoid arthritis as compared to control subjects: a meta-analysis. Semin Arthritis Rheum 2011; 40(5):389–397. doi:10.1016/j.semarthrit.2010.06.006
- Brohall G, Odén A, Fagerberg B. Carotid artery intima-media thickness in patients with type 2 diabetes mellitus and impaired glucose tolerance: a systematic review. Diabet Med 2006; 23(6):609–616. doi:10.1111/j.1464-5491.2005.01725.x
- Masoura C, Pitsavos C, Aznaouridis K, Skoumas I, Vlachopoulos C, Stefanadis C. Arterial endothelial function and wall thickness in familial hypercholesterolemia and familial combined hyperlipidemia and the effect of statins. A systematic review and meta-analysis. Atherosclerosis 2011; 214(1):129–138. doi:10.1016/j.atherosclerosis.2010.10.008
- Ozen G, Inanc N, Unal AU, et al. Subclinical atherosclerosis in systemic sclerosis: not less frequent than rheumatoid arthritis and not detected with cardiovascular risk indices. Arthritis Care Res (Hoboken) 2016; 68(10):1538–1546. doi:10.1002/acr.22852
- Inaba Y, Chen JA, Bergmann SR. Prediction of future cardiovascular outcomes by flow-mediated vasodilatation of brachial artery: a meta-analysis. Int J Cardiovasc Imaging 2010; 26(6):631–640. doi:10.1007/s10554-010-9616-1
- Meune C, Avouac J, Wahbi K, et al. Cardiac involvement in systemic sclerosis assessed by tissue-doppler echocardiography during routine care: a controlled study of 100 consecutive patients. Arthritis Rheum 2008; 58(6):1803–1809. doi:10.1002/art.23463
- Tennøe AH, Murbræch K, Andreassen JC, et al. Left ventricular diastolic dysfunction predicts mortality in patients with systemic sclerosis. J Am Coll Cardiol 2018; 72(15):1804–1813. doi:10.1016/j.jacc.2018.07.068
- de Groote P, Gressin V, Hachulla E, et al; ItinerAIR-Scleroderma Investigators. Evaluation of cardiac abnormalities by Doppler echocardiography in a large nationwide multicentric cohort of patients with systemic sclerosis. Ann Rheum Dis 2008; 67(1):31–36. doi:10.1136/ard.2006.057760
- Allanore Y, Meune C, Vonk MC, et al; EUSTAR co-authors. Prevalence and factors associated with left ventricular dysfunction in the EULAR Scleroderma Trial and Research group (EUSTAR) database of patients with systemic sclerosis. Ann Rheum Dis 2010; 69(1):218–221. doi:10.1136/ard.2008.103382
- Hachulla AL, Launay D, Gaxotte V, et al. Cardiac magnetic resonance imaging in systemic sclerosis: a cross-sectional observational study of 52 patients. Ann Rheum Dis 2009; 68(12):1878–1884. doi:10.1136/ard.2008.095836
- Assassi S, Del Junco D, Sutter K, et al. Clinical and genetic factors predictive of mortality in early systemic sclerosis. Arthritis Rheum 2009; 61(10):1403–1411. doi:10.1002/art.24734
- Rokas S, Mavrikakis M, Agrios N, Mylonas D, Antoniadou L, Moulopoulos S. Electrophysiologic abnormalities of cardiac function in progressive systemic sclerosis. J Electrocardiol 1996; 29(1):17–25. pmid:8808521
- Kostis JB, Seibold JR, Turkevich D, et al. Prognostic importance of cardiac arrhythmias in systemic sclerosis. Am J Med 1988; 84(6):1007–1015. doi:10.1016/0002-9343(88)90305-1
- Biełous-Wilk A, Poreba M, Staniszewska-Marszałek E, et al. Electrocardiographic evaluation in patients with systemic scleroderma and without clinically evident heart disease. Ann Noninvasive Electrocardiol 2009; 14(3):251–257. doi:10.1111/j.1542-474X.2009.00306.x
- Bienias P, Ciurzynski M, Glinska-Wielochowska M, et al. Heart rate turbulence assessment in systemic sclerosis: the role for the detection of cardiac autonomic nervous system dysfunction. Rheumatology (Oxford) 2010; 49(2):355–360. doi:10.1093/rheumatology/kep394
- Ferri C, Bernini L, Bongiorni MG, et al. Noninvasive evaluation of cardiac dysrhythmias, and their relationship with multisystemic symptoms, in progressive systemic sclerosis patients. Arthritis Rheum 1985; 28(11):1259–1266. pmid:4063000
- Roberts NK, Cabeen WR, Moss J, Clements PJ, Furst DE. The prevalence of conduction defects and cardiac arrhythmias in progressive systemic sclerosis. Ann Intern Med 1981; 94(1):38–40. doi:10.7326/0003-4819-94-1-38
- Wang Q, Shang Y, Li S, Wu Y, Wang C, Yan X. Complete heart block in systemic sclerosis: a case report and literature review. Medicine (Baltimore) 2018; 97(46):e13226. doi:10.1097/MD.0000000000013226
- Summerfield BJ. Progressive systemic sclerosis with complete heart block. Br Heart J 1975; 37(12):1308–1310. doi:10.1136/hrt.37.12.1308
- Moyssakis I, Papadopoulos DP, Tzioufas AG, Votteas V. Complete heart block in a patient with systemic sclerosis. Clin Rheumatol 2006; 25(4):551–552. doi:10.1007/s10067-005-0068-2
- Ridolfi RL, Bulkley BH, Hutchins GM. The cardiac conduction system in progressive systemic sclerosis. Clinical and pathologic features of 35 patients. Am J Med 1976; 61(3):361–366. doi:10.1016/0002-9343(76)90373-9
- Champion HC. The heart in scleroderma. Rheum Dis Clin North Am 2008; 34(1):181–190. doi:10.1016/j.rdc.2007.12.002
- Gowda RM, Khan IA, Sacchi TJ, Vasavada BC. Scleroderma pericardial disease presented with a large pericardial effusion—a case report. Angiology 2001; 52(1):59–62. doi:10.1177/000331970105200108
- Meier FMP, Frommer KW, Dinser R, et al; EUSTAR Co-authors. Update on the profile of the EUSTAR cohort: an analysis of the EULAR scleroderma trials and research group database. Ann Rheum Dis 2012; 71(8):1355–1360. doi:10.1136/annrheumdis-2011-200742
- Subramanian SR, Akram R, Velayati A, Chadow H. New development of cardiac tamponade on underlying effusive-constrictive pericarditis: an uncommon initial presentation of scleroderma. BMJ Case Rep 2013; 2013. doi:10.1136/bcr-2013-010254
- Kitchongcharoenying P, Foocharoen C, Mahakkanukrauh A, Suwannaroj S, Nanagara R. Pericardial fluid profiles of pericardial effusion in systemic sclerosis patients. Asian Pac J Allergy Immunol 2013; 31(4):314–319. doi:10.12932/AP0305.31.4.2013
- McWhorter JE, LeRoy EC. Pericardial disease in scleroderma (systemic sclerosis). Am J Med 1974; 57(4):566–575. doi:10.1016/0002-9343(74)90008-4
- Comens SM, Alpert MA, Sharp GC, et al. Frequency of mitral valve prolapse in systemic lupus erythematosus, progressive systemic sclerosis and mixed connective tissue disease. Am J Cardiol 1989; 63(5):369–370. doi:10.1016/0002-9149(89)90351-2
- Candell-Riera J, Armadans-Gil L, Simeón CP, et al. Comprehensive noninvasive assessment of cardiac involvement in limited systemic sclerosis. Arthritis Rheum 1996; 39(7):1138–1145. pmid:8670322
- Caforio ALP, Adler Y, Agostini C, et al. Diagnosis and management of myocardial involvement in systemic immune-mediated diseases: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Disease. Eur Heart J 2017; 38(35):2649–2662. doi:10.1093/eurheartj/ehx321
- Mavrogeni S, Karabela G, Koutsogeorgopoulou L, et al. Pseudo-infarction pattern in diffuse systemic sclerosis. Evaluation using cardiovascular magnetic resonance. Int J Cardiol 2016; 214:465–468. doi:10.1016/j.ijcard.2016.03.235
- Ladak K, Pope JE. A review of the effects of statins in systemic sclerosis. Semin Arthritis Rheum 2016; 45(6):698–705. doi:10.1016/j.semarthrit.2015.10.013
- Abou-Raya A, Abou-Raya S, Helmii M. Statins: potentially useful in therapy of systemic sclerosis-related Raynaud’s phenomenon and digital ulcers. J Rheumatol 2008; 35(9):1801–1808. pmid:18709692
- ASCEND Study Collaborative Group; Bowman L, Mafham M, Wallendszus K, et al. Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med 2018; 379(16):1529–1539. doi:10.1056/NEJMoa1804988
- Guerra F, Stronati G, Fischietti C, et al. Global longitudinal strain measured by speckle tracking identifies subclinical heart involvement in patients with systemic sclerosis. Eur J Prev Cardiol 2018; 25(15):1598–1606. doi:10.1177/2047487318786315
- Mavrogeni SI, Sfikakis PP, Dimitroulas T, et al. Prospects of using cardiovascular magnetic resonance in the identification of arrhythmogenic substrate in autoimmune rheumatic diseases. Rheumatol Int 2018; 38(9):1615–1621. doi:10.1007/s00296-018-4110-5
- Mavrogeni SI, Sfikakis PP, Markousis-Mavrogenis G, et al. Cardiovascular magnetic resonance imaging pattern in patients with autoimmune rheumatic diseases and ventricular tachycardia with preserved ejection fraction. Int J Cardiol 2019; 284:105–109. doi:10.1016/j.ijcard.2018.10.067
- Maradit-Kremers H, Crowson CS, Nicola PJ, et al. Increased unrecognized coronary heart disease and sudden deaths in rheumatoid arthritis: a population-based cohort study. Arthritis Rheum 2005; 52(2):402–411. doi:10.1002/art.20853
- Naranjo A, Sokka T, Descalzo MA, et al; QUEST-RA Group. Cardiovascular disease in patients with rheumatoid arthritis: results from the QUEST-RA study. Arthritis Res Ther 2008; 10(2):R30. doi:10.1186/ar2383
- Innala L, Möller B, Ljung L, et al. Cardiovascular events in early RA are a result of inflammatory burden and traditional risk factors: a five year prospective study. Arthritis Res Ther 2011; 13(4):R131. doi:10.1186/ar3442
- Barnes J, Mayes MD. Epidemiology of systemic sclerosis: incidence, prevalence, survival, risk factors, malignancy, and environmental triggers. Curr Opin Rheumatol 2012; 24(2):165–170. doi:10.1097/BOR.0b013e32834ff2e8
- Chifflot H, Fautrel B, Sordet C, Chatelus E, Sibilia J. Incidence and prevalence of systemic sclerosis: a systematic literature review. Semin Arthritis Rheum 2008; 37(4):223–235 doi:10.1016/j.semarthrit.2007.05.003
- Gabrielli A, Avvedimento EV, Krieg T. Scleroderma. N Engl J Med 2009; 360(19):1989–2003. doi:10.1056/NEJMra0806188
- Panopoulos S, Tektonidou M, Drosos AA, et al. Prevalence of comorbidities in systemic sclerosis versus rheumatoid arthritis: a comparative, multicenter, matched-cohort study. Arthritis Res Ther 2018; 20(1):267. doi:10.1186/s13075-018-1771-0
- Ferri C, Valentini G, Cozzi F, et al. Systemic sclerosis: demographic, clinical, and serologic features and survival in 1,012 Italian patients. Medicine (Baltimore) 2002; 81(8):139–153. doi:10.1097/00005792-200203000-00004
- Steen VD, Medsger TA Jr. Severe organ involvement in systemic sclerosis with diffuse scleroderma. Arthritis Rheum 2000; 43(11):2437–2444. doi:10.1002/1529-0131(200011)43:11<2437::AID-ANR10>3.0.CO;2-U
- Hachulla AL, Launay D, Gaxotte V, et al. Cardiac magnetic resonance imaging in systemic sclerosis: a cross-sectional observational study of 52 patients. Ann Rheum Dis 2009; 68(12):1878–1884. doi:10.1136/ard.2008.095836
- Tyndall AJ, Bannert B, Vonk M, et al. Causes and risk factors for death in systemic sclerosis: a study from the EULAR Scleroderma Trials and Research (EUSTAR) database. Ann Rheum Dis 2010; 69(10):1809–1815. doi:10.1136/ard.2009.114264
- Nassenstein K, Breuckmann F, Huger M, et al. Detection of myocardial fibrosis in systemic sclerosis by contrast-enhanced magnetic resonance imaging. Rofo 2008; 180(12):1054–1060. doi:10.1055/s-2008-1027864
- Psarras A, Soulaidopoulos S, Garyfallos A, Kitas G, Dimitroulas T. A critical view on cardiovascular risk in systemic sclerosis. Rheumatol Int 2017; 37(1):85–95. doi:10.1007/s00296-016-3530-3
- Lekakis J, Mavrikakis M, Emmanuel M, et al. Cold-induced coronary Raynaud’s phenomenon in patients with systemic sclerosis. Clin Exp Rheumatol 1998; 16(2):135–140. pmid:9536388
- Altorok N, Wang Y, Kahaleh B. Endothelial dysfunction in systemic sclerosis. Curr Opin Rheumatol 2014; 26(6):615–620. doi:10.1097/BOR.0000000000000112
- Fleming JN, Nash RA, Mahoney WM Jr, Schwartz SM. Is scleroderma a vasculopathy? Curr Rheumatol Rep 2009; 11(2):103–110. pmid:19296882
- Maurer B, Distler A, Suliman YA, et al. Vascular endothelial growth factor aggravates fibrosis and vasculopathy in experimental models of systemic sclerosis. Ann Rheum Dis 2014; 73(10):1880–1887. doi:10.1136/annrheumdis-2013-203535
- Meune C, Vignaux O, Kahan A, Allanore Y. Heart involvement in systemic sclerosis: evolving concept and diagnostic methodologies. Arch Cardiovasc Dis 2010; 103(1):46–52. doi:10.1016/j.acvd.2009.06.009
- Dimitroulas T, Giannakoulas G, Karvounis H, Garyfallos A, Settas L, Kitas GD. Micro- and macrovascular treatment targets in scleroderma heart disease. Curr Pharm Des 2014; 20(4):536–544. pmid:23565639
- Allanore Y, Meune C. Primary myocardial involvement in systemic sclerosis: evidence for a microvascular origin. Clin Exp Rheumatol 2010; 28(5 suppl 62):S48–S53. pmid:21050545
- Kahan A, Nitenberg A, Foult JM, et al. Decreased coronary reserve in primary scleroderma myocardial disease. Arthritis Rheum 1985; 28(6):637–646. pmid:4004974
- Morrisroe K, Stevens W, Sahhar J, et al. Epidemiology and disease characteristics of systemic sclerosis-related pulmonary arterial hypertension: results from a real-life screening program. Arthritis Res Ther 2017; 19(1):42. doi:10.1186/s13075-017-1250-z
- Chaisson NF, Hassoun PM. Systemic sclerosis-associated pulmonary arterial hypertension. Chest 2013; 144(4):1346–1356. doi:10.1378/chest.12-2396
- Steen VD, Medsger TA. Changes in causes of death in systemic sclerosis, 1972–2002. Ann Rheum Dis 2007; 66(7):940–944. doi:10.1136/ard.2006.066068
- Coghlan JG, Galiè N, Barberà JA, et al; AMBITION investigators. Initial combination therapy with ambrisentan and tadalafil in connective tissue disease-associated pulmonary arterial hypertension (CTD-PAH): subgroup analysis from the AMBITION trial. Ann Rheum Dis 2017; 76(7):1219–1227. doi:10.1136/annrheumdis-2016-210236
- Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J 2019; 53(1):1801913. doi:10.1183/13993003.01913-2018
- Chatterjee S. Pulmonary hypertension in systemic sclerosis. Semin Arthritis Rheum 2011; 41(1):19–37. doi:10.1016/j.semarthrit.2010.08.004
- Sweiss NJ, Hushaw L, Thenappan T, et al. Diagnosis and management of pulmonary hypertension in systemic sclerosis. Curr Rheumatol Rep 2010; 12(1):8–18. doi:10.1007/s11926-009-0078-1
- Cox SR, Walker JG, Coleman M, et al. Isolated pulmonary hypertension in scleroderma. Intern Med J 2005; 35(1):28–33. doi:10.1111/j.1445-5994.2004.00646.x
- Sánchez-Román J, Opitz CF, Kowal-Bielecka O, García-Hernández FJ, Castillo-Palma MJ, Pittrow D; EPOSS-OMERACT Group. Screening for PAH in patients with systemic sclerosis: focus on Doppler echocardiography. Rheumatology (Oxford) 2008; 47(suppl 5):v33–v35. doi:10.1093/rheumatology/ken306
- Walker KM, Pope J; Scleroderma Clinical Trials Consortium; Canadian Scleroderma Research Group. Expert agreement on EULAR/EUSTAR recommendations for the management of systemic sclerosis. J Rheumatol 2011; 38(7):1326–1328. doi:10.3899/jrheum.101262
- Khanna D, Gladue H, Channick R, et al; Scleroderma Foundation and Pulmonary Hypertension Association. Recommendations for screening and detection of connective tissue disease-associated pulmonary arterial hypertension. Arthritis Rheum 2013; 65(12):3194–3201. doi:10.1002/art.38172
- Hachulla E, Gressin V, Guillevin L, et al. Early detection of pulmonary arterial hypertension in systemic sclerosis: a French nationwide prospective multicenter study. Arthritis Rheum 2005; 52(12):3792–3800. doi:10.1002/art.21433
- Dimitroulas T, Giannakoulas G, Dimitroula H, et al. Significance of serum uric acid in pulmonary hypertension due to systemic sclerosis: a pilot study. Rheumatol Int 2011; 31(2):263–267. doi:10.1007/s00296-010-1557-4
- Dimitroulas T, Giannakoulas G, Papadopoulou K, et al. Left atrial volume and N-terminal pro-B type natriuretic peptide are associated with elevated pulmonary artery pressure in patients with systemic sclerosis. Clin Rheumatol 2010; 29(9):957–964. doi:10.1007/s10067-010-1494-3
- Coghlan JG, Denton CP, Grünig E, et al; DETECT study group. Evidence-based detection of pulmonary arterial hypertension in systemic sclerosis: the DETECT study. Ann Rheum Dis 2014; 73(7):1340–1349. doi:10.1136/annrheumdis-2013-203301
- Schwaiger JP, Khanna D, Gerry Coghlan J. Screening patients with scleroderma for pulmonary arterial hypertension and implications for other at-risk populations. Eur Respir Rev 2013; 22(130):515–525. doi:10.1183/09059180.00006013
- Man A, Zhu Y, Zhang Y, et al. The risk of cardiovascular disease in systemic sclerosis: a population-based cohort study. Ann Rheum Dis 2013; 72(7):1188–1193. doi:10.1136/annrheumdis-2012-202007
- Ngian G-S, Sahhar J, Proudman SM, Stevens W, Wicks IP, Van Doornum S. Prevalence of coronary heart disease and cardiovascular risk factors in a national cross-sectional cohort study of systemic sclerosis. Ann Rheum Dis 2012; 71(12):1980–1983. doi:10.1136/annrheumdis-2011-201176
- Aviña-Zubieta JA, Man A, Yurkovich M, Huang K, Sayre EC, Choi HK. Early cardiovascular disease after the diagnosis of systemic sclerosis. Am J Med 2016; 29(3):324–331. doi:10.1016/j.amjmed.2015.10.037
- D’Angelo WA, Fries JF, Masi AT, Shulman LE. Pathologic observations in systemic sclerosis (scleroderma). A study of fifty-eight autopsy cases and fifty-eight matched controls. Am J Med 1969; 46(3):428–440. doi:10.1016/0002-9343(69)90044-8
- Ngian GS, Sahhar J, Proudman SM, Stevens W, Wicks IP, Van Doornum S. Prevalence of coronary heart disease and cardiovascular risk factors in a national cross-sectional cohort study of systemic sclerosis. Ann Rheum Dis 2012; 71(12):1980–1983. doi:10.1136/annrheumdis-2011-201176
- Khurma V, Meyer C, Park GS, et al. A pilot study of subclinical coronary atherosclerosis in systemic sclerosis: coronary artery calcification in cases and controls. Arthritis Rheum 2008; 59(4):591–597. doi:10.1002/art.23540
- Lippi G, Caramaschi P, Montagnana M, Salvagno GL, Volpe A, Guidi G. Lipoprotein[a] and the lipid profile in patients with systemic sclerosis. Clin Chim Acta 2006; 364(1–2):345–348. doi:10.1016/j.cca.2005.07.015
- Palinski W, Hörkkö S, Miller E, et al. Cloning of monoclonal autoantibodies to epitopes of oxidized lipoproteins from apolipoprotein E-deficient mice. Demonstration of epitopes of oxidized low density lipoprotein in human plasma. J Clin Invest 1996; 98(3):800–814. doi:10.1172/JCI118853
- Ho M, Veale D, Eastmond C, Nuki G, Belch J. Macrovascular disease and systemic sclerosis. Ann Rheum Dis 2000; 59(1):39–43. doi:10.1136/ard.59.1.39
- Kaloudi O, Basta G, Perfetto F, et al. Circulating levels of Ne-(carboxymethyl)lysine are increased in systemic sclerosis. Rheumatology (Oxford) 2007; 46(3):412–416. doi:10.1093/rheumatology/kel076
- Muro Y, Sugiura K, Morita Y, Tomita Y. An evaluation of the efficacy of the toe brachial index measuring vascular involvement in systemic sclerosis and other connective tissue diseases. Clin Exp Rheumatol 2009; 27(3 suppl 54):26–31. pmid:19796558
- Cheng K-S, Tiwari A, Boutin A, et al. Differentiation of primary and secondary Raynaud’s disease by carotid arterial stiffness. Eur J Vasc Endovasc Surg 2003; 25(4):336–341. doi:10.1053/ejvs.2002.1845
- Kawasaki M, Ito Y, Yokoyama H, et al. Assessment of arterial medial characteristics in human carotid arteries using integrated backscatter ultrasound and its histological implications. Atherosclerosis 2005; 180(1):145–154. doi:10.1016/j.atherosclerosis.2004.11.018
- Szucs G, Tímár O, Szekanecz Z, et al. Endothelial dysfunction precedes atherosclerosis in systemic sclerosis—relevance for prevention of vascular complications. Rheumatology (Oxford) 2007; 46(5):759–762. doi:10.1093/rheumatology/kel426
- Hettema ME, Zhang D, de Leeuw K, et al. Early atherosclerosis in systemic sclerosis and its relation to disease or traditional risk factors. Arthritis Res Ther 2008;10(2):R49. doi:10.1186/ar2408
- Roustit M, Simmons GH, Baguet JP, Carpentier P, Cracowski JL. Discrepancy between simultaneous digital skin microvascular and brachial artery macrovascular post-occlusive hyperemia in systemic sclerosis. J Rheumatol 2008; 35(8):1576–1583. pmid:18597404
- Vettori S, Maresca L, Cuomo G, Abbadessa S, Leonardo G, Valentini G. Clinical and subclinical atherosclerosis in systemic sclerosis: consequences of previous corticosteroid treatment. Scand J Rheumatol 2010; 39(6):485–489. doi:10.3109/03009741003781985
- Lekakis J, Mavrikakis M, Papamichael C, et al. Short-term estrogen administration improves abnormal endothelial function in women with systemic sclerosis and Raynaud’s phenomenon. Am Heart J 1998; 136(5):905–912. doi:10.1016/s0002-8703(98)70137-1
- Bartoli F, Blagojevic J, Bacci M, et al. Flow-mediated vasodilation and carotid intima-media thickness in systemic sclerosis. Ann N Y Acad Sci 2007; 1108:283–290. doi:10.1196/annals.1422.030
- Rollando D, Bezante GP, Sulli A, et al. Brachial artery endothelial-dependent flow-mediated dilation identifies early-stage endothelial dysfunction in systemic sclerosis and correlates with nailfold microvascular impairment. J Rheumatol 2010; 37(6):1168–1173. doi:10.3899/jrheum.091116
- Andersen GN, Mincheva-Nilsson L, Kazzam E, et al. Assessment of vascular function in systemic sclerosis: indications of the development of nitrate tolerance as a result of enhanced endothelial nitric oxide production. Arthritis Rheum 2002; 46(5):1324–1332. doi:10.1002/art.10191
- Au K, Singh MK, Bodukam V, et al. Atherosclerosis in systemic sclerosis: a systematic review and meta-analysis. Arthritis Rheum 2011; 63(7):2078–2090. doi:10.1002/art.30380
- van Sijl AM, Peters MJ, Knol DK, et al. Carotid intima media thickness in rheumatoid arthritis as compared to control subjects: a meta-analysis. Semin Arthritis Rheum 2011; 40(5):389–397. doi:10.1016/j.semarthrit.2010.06.006
- Brohall G, Odén A, Fagerberg B. Carotid artery intima-media thickness in patients with type 2 diabetes mellitus and impaired glucose tolerance: a systematic review. Diabet Med 2006; 23(6):609–616. doi:10.1111/j.1464-5491.2005.01725.x
- Masoura C, Pitsavos C, Aznaouridis K, Skoumas I, Vlachopoulos C, Stefanadis C. Arterial endothelial function and wall thickness in familial hypercholesterolemia and familial combined hyperlipidemia and the effect of statins. A systematic review and meta-analysis. Atherosclerosis 2011; 214(1):129–138. doi:10.1016/j.atherosclerosis.2010.10.008
- Ozen G, Inanc N, Unal AU, et al. Subclinical atherosclerosis in systemic sclerosis: not less frequent than rheumatoid arthritis and not detected with cardiovascular risk indices. Arthritis Care Res (Hoboken) 2016; 68(10):1538–1546. doi:10.1002/acr.22852
- Inaba Y, Chen JA, Bergmann SR. Prediction of future cardiovascular outcomes by flow-mediated vasodilatation of brachial artery: a meta-analysis. Int J Cardiovasc Imaging 2010; 26(6):631–640. doi:10.1007/s10554-010-9616-1
- Meune C, Avouac J, Wahbi K, et al. Cardiac involvement in systemic sclerosis assessed by tissue-doppler echocardiography during routine care: a controlled study of 100 consecutive patients. Arthritis Rheum 2008; 58(6):1803–1809. doi:10.1002/art.23463
- Tennøe AH, Murbræch K, Andreassen JC, et al. Left ventricular diastolic dysfunction predicts mortality in patients with systemic sclerosis. J Am Coll Cardiol 2018; 72(15):1804–1813. doi:10.1016/j.jacc.2018.07.068
- de Groote P, Gressin V, Hachulla E, et al; ItinerAIR-Scleroderma Investigators. Evaluation of cardiac abnormalities by Doppler echocardiography in a large nationwide multicentric cohort of patients with systemic sclerosis. Ann Rheum Dis 2008; 67(1):31–36. doi:10.1136/ard.2006.057760
- Allanore Y, Meune C, Vonk MC, et al; EUSTAR co-authors. Prevalence and factors associated with left ventricular dysfunction in the EULAR Scleroderma Trial and Research group (EUSTAR) database of patients with systemic sclerosis. Ann Rheum Dis 2010; 69(1):218–221. doi:10.1136/ard.2008.103382
- Hachulla AL, Launay D, Gaxotte V, et al. Cardiac magnetic resonance imaging in systemic sclerosis: a cross-sectional observational study of 52 patients. Ann Rheum Dis 2009; 68(12):1878–1884. doi:10.1136/ard.2008.095836
- Assassi S, Del Junco D, Sutter K, et al. Clinical and genetic factors predictive of mortality in early systemic sclerosis. Arthritis Rheum 2009; 61(10):1403–1411. doi:10.1002/art.24734
- Rokas S, Mavrikakis M, Agrios N, Mylonas D, Antoniadou L, Moulopoulos S. Electrophysiologic abnormalities of cardiac function in progressive systemic sclerosis. J Electrocardiol 1996; 29(1):17–25. pmid:8808521
- Kostis JB, Seibold JR, Turkevich D, et al. Prognostic importance of cardiac arrhythmias in systemic sclerosis. Am J Med 1988; 84(6):1007–1015. doi:10.1016/0002-9343(88)90305-1
- Biełous-Wilk A, Poreba M, Staniszewska-Marszałek E, et al. Electrocardiographic evaluation in patients with systemic scleroderma and without clinically evident heart disease. Ann Noninvasive Electrocardiol 2009; 14(3):251–257. doi:10.1111/j.1542-474X.2009.00306.x
- Bienias P, Ciurzynski M, Glinska-Wielochowska M, et al. Heart rate turbulence assessment in systemic sclerosis: the role for the detection of cardiac autonomic nervous system dysfunction. Rheumatology (Oxford) 2010; 49(2):355–360. doi:10.1093/rheumatology/kep394
- Ferri C, Bernini L, Bongiorni MG, et al. Noninvasive evaluation of cardiac dysrhythmias, and their relationship with multisystemic symptoms, in progressive systemic sclerosis patients. Arthritis Rheum 1985; 28(11):1259–1266. pmid:4063000
- Roberts NK, Cabeen WR, Moss J, Clements PJ, Furst DE. The prevalence of conduction defects and cardiac arrhythmias in progressive systemic sclerosis. Ann Intern Med 1981; 94(1):38–40. doi:10.7326/0003-4819-94-1-38
- Wang Q, Shang Y, Li S, Wu Y, Wang C, Yan X. Complete heart block in systemic sclerosis: a case report and literature review. Medicine (Baltimore) 2018; 97(46):e13226. doi:10.1097/MD.0000000000013226
- Summerfield BJ. Progressive systemic sclerosis with complete heart block. Br Heart J 1975; 37(12):1308–1310. doi:10.1136/hrt.37.12.1308
- Moyssakis I, Papadopoulos DP, Tzioufas AG, Votteas V. Complete heart block in a patient with systemic sclerosis. Clin Rheumatol 2006; 25(4):551–552. doi:10.1007/s10067-005-0068-2
- Ridolfi RL, Bulkley BH, Hutchins GM. The cardiac conduction system in progressive systemic sclerosis. Clinical and pathologic features of 35 patients. Am J Med 1976; 61(3):361–366. doi:10.1016/0002-9343(76)90373-9
- Champion HC. The heart in scleroderma. Rheum Dis Clin North Am 2008; 34(1):181–190. doi:10.1016/j.rdc.2007.12.002
- Gowda RM, Khan IA, Sacchi TJ, Vasavada BC. Scleroderma pericardial disease presented with a large pericardial effusion—a case report. Angiology 2001; 52(1):59–62. doi:10.1177/000331970105200108
- Meier FMP, Frommer KW, Dinser R, et al; EUSTAR Co-authors. Update on the profile of the EUSTAR cohort: an analysis of the EULAR scleroderma trials and research group database. Ann Rheum Dis 2012; 71(8):1355–1360. doi:10.1136/annrheumdis-2011-200742
- Subramanian SR, Akram R, Velayati A, Chadow H. New development of cardiac tamponade on underlying effusive-constrictive pericarditis: an uncommon initial presentation of scleroderma. BMJ Case Rep 2013; 2013. doi:10.1136/bcr-2013-010254
- Kitchongcharoenying P, Foocharoen C, Mahakkanukrauh A, Suwannaroj S, Nanagara R. Pericardial fluid profiles of pericardial effusion in systemic sclerosis patients. Asian Pac J Allergy Immunol 2013; 31(4):314–319. doi:10.12932/AP0305.31.4.2013
- McWhorter JE, LeRoy EC. Pericardial disease in scleroderma (systemic sclerosis). Am J Med 1974; 57(4):566–575. doi:10.1016/0002-9343(74)90008-4
- Comens SM, Alpert MA, Sharp GC, et al. Frequency of mitral valve prolapse in systemic lupus erythematosus, progressive systemic sclerosis and mixed connective tissue disease. Am J Cardiol 1989; 63(5):369–370. doi:10.1016/0002-9149(89)90351-2
- Candell-Riera J, Armadans-Gil L, Simeón CP, et al. Comprehensive noninvasive assessment of cardiac involvement in limited systemic sclerosis. Arthritis Rheum 1996; 39(7):1138–1145. pmid:8670322
- Caforio ALP, Adler Y, Agostini C, et al. Diagnosis and management of myocardial involvement in systemic immune-mediated diseases: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Disease. Eur Heart J 2017; 38(35):2649–2662. doi:10.1093/eurheartj/ehx321
- Mavrogeni S, Karabela G, Koutsogeorgopoulou L, et al. Pseudo-infarction pattern in diffuse systemic sclerosis. Evaluation using cardiovascular magnetic resonance. Int J Cardiol 2016; 214:465–468. doi:10.1016/j.ijcard.2016.03.235
- Ladak K, Pope JE. A review of the effects of statins in systemic sclerosis. Semin Arthritis Rheum 2016; 45(6):698–705. doi:10.1016/j.semarthrit.2015.10.013
- Abou-Raya A, Abou-Raya S, Helmii M. Statins: potentially useful in therapy of systemic sclerosis-related Raynaud’s phenomenon and digital ulcers. J Rheumatol 2008; 35(9):1801–1808. pmid:18709692
- ASCEND Study Collaborative Group; Bowman L, Mafham M, Wallendszus K, et al. Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med 2018; 379(16):1529–1539. doi:10.1056/NEJMoa1804988
- Guerra F, Stronati G, Fischietti C, et al. Global longitudinal strain measured by speckle tracking identifies subclinical heart involvement in patients with systemic sclerosis. Eur J Prev Cardiol 2018; 25(15):1598–1606. doi:10.1177/2047487318786315
- Mavrogeni SI, Sfikakis PP, Dimitroulas T, et al. Prospects of using cardiovascular magnetic resonance in the identification of arrhythmogenic substrate in autoimmune rheumatic diseases. Rheumatol Int 2018; 38(9):1615–1621. doi:10.1007/s00296-018-4110-5
- Mavrogeni SI, Sfikakis PP, Markousis-Mavrogenis G, et al. Cardiovascular magnetic resonance imaging pattern in patients with autoimmune rheumatic diseases and ventricular tachycardia with preserved ejection fraction. Int J Cardiol 2019; 284:105–109. doi:10.1016/j.ijcard.2018.10.067
KEY POINTS
- Pulmonary hypertension is common in systemic sclerosis and carries a poor prognosis. Patients with systemic sclerosis should be screened regularly with echocardiography, followed, when necessary, by right heart catheterization to detect it early.
- Myocardial infarction and stroke are more common in patients with systemic sclerosis, and preventive measures are the same as for the general population.
- Right ventricular dysfunction secondary to pulmonary hypertension is common in systemic sclerosis; left ventricular dysfunction is less so. Routine echocardiography should include assessment of right and left ventricular function.
- Electrocardiography should be performed periodically, and urgently when indicated, to look for potentially dangerous arrhythmias.
2019 Update in perioperative cardiovascular medicine
Perioperative medicine is an evolving field with a rapidly growing body of literature, particularly in cardiology.
In this update, we review 6 articles to answer questions related to preoperative cardiac risk assessment, perioperative medication management, and postoperative cardiac complications. We surveyed perioperative literature from February 2018 through January 2019 and chose the final articles by consensus, based on relevance to clinicians who provide preoperative evaluations and postoperative care to surgical patients.
These summaries are derived from “Updates in Perioperative Medicine” presented at the 14th Annual Perioperative Medicine Summit (Orlando, FL, February 13–16, 2019) and the 2019 Society of Hospital Medicine Annual Meeting (National Harbor, MD, March 24–27, 2019).
PREOPERATIVE CARDIAC EVALUATION
How well do measures of functional capacity predict perioperative complications and mortality in noncardiac surgical patients?
Functional capacity is commonly assessed in preoperative evaluations to estimate patients’ risks of perioperative complications and death. The American College of Cardiology/American Heart Association1 and the European Society of Cardiology2 guidelines both include estimation of cardiopulmonary fitness as a step in preoperative assessment before major noncardiac surgery.
“Subjective assessment” is one way to estimate functional capacity. Simply put, clinicians try to form a rough idea about the fitness of patients by asking questions about routine activities such as walking or climbing stairs. Although commonly used, subjective assessment of functional capacity lacks strong evidence that it predicts adverse perioperative events.
Cardiopulmonary exercise testing is a third option. It measures peak oxygen consumption and anaerobic threshold during exercise. It is probably the best objective measurement of functional capacity, but not necessarily for predicting postoperative cardiac complications, and it is performed relatively infrequently.
[Wijeysundera DN, Pearse RM, Sulman MA, et al. Assessment of functional capacity before major non-cardiac surgery: an international, prospective cohort study. Lancet 2018; 391(10140):2631–2640. doi:10.1016/S0140-6736(18)31131-0]
In a multicenter, prospective cohort study, Wijeysundera et al4 compared subjective functional capacity assessment, the Duke Activity Status Index, cardiopulmonary exercise testing, and the preoperative N-terminal pro-B-type natriuretic peptide (NT-proBNP) level in their ability to predict complications and death in 1,401 noncardiac surgery patients older than 40 with at least 1 cardiovascular risk factor. After surgery, patients had daily electrocardiograms and troponin measurements until postoperative day 3 or discharge.
The primary outcome was the 30-day incidence of death or myocardial infarction (MI). Additional outcomes included the 30-day incidence of death or myocardial injury after noncardiac surgery (MINS), the 1-year mortality rate, and moderate to severe in-hospital perioperative complications.
Findings. Two percent of patients died or had an MI within 30 days of surgery.4
Subjective assessment had only a 19.2% sensitivity (95% confidence interval [CI] 14.2–25) but a 94.7% specificity (95% CI 93.2–95.9) for predicting inability to attain 4 metabolic equivalents during exercise.4
A lower Duke Activity Status Index predicted the primary outcome of death or MI within 30 days (adjusted odds ratio [OR] 0.96, 95% CI 0.83–0.99, P = .03), and it was the only measure that did so. Additionally, the Duke index and NT-proBNP level predicted the risk of death or MINS within 30 days.4
Only elevated NT-proBNP was associated with death at 1 year.4
On exercise testing, low peak oxygen consumption was significantly associated with perioperative complications.
Limitations. The number of primary outcome events (death and MI) was low, potentially affecting the statistical power of the study.
Conclusions. Subjective assessment of functional capacity misclassifies too many patients as being at low risk of perioperative complications and should not be used for preoperative risk stratification. Other tools, such as the Duke Activity Status Index and NT-proBNP levels, are better predictors of adverse perioperative cardiovascular outcomes and should be considered for use in preoperative cardiac risk assessment.
Although the Duke Activity Status Index is a better predictor of adverse outcomes than subjective functional capacity assessment, a specific perioperative threshold for risk classification has not been established. Its correlate for metabolic equivalents should be considered for use in clinical practice at this point.
PERIOPERATIVE MEDICATION MANAGEMENT
Is perioperative aspirin beneficial in patients undergoing vascular surgery?
The Perioperative Ischemic Evaluation 2 (POISE-2) trial,5 a 2-by-2 factorial randomized controlled trial in which patients received perioperative aspirin, clonidine, both, or neither, demonstrated that perioperative aspirin did not reduce cardiovascular events and increased major bleeding. Patients with recently placed coronary stents and those undergoing carotid endarterectomy were excluded because aspirin is known to have a beneficial effect in these patients.
A subsequent substudy6 found perioperative aspirin to be beneficial in patients with coronary stents placed more than a year before noncardiac surgery. Whether perioperative aspirin is beneficial in other subgroups was unknown.
[Biccard BM, Sigamani A, Chan MTV, et al. Effect of aspirin in vascular surgery in patients from a randomized clinical trial (POISE-2). Br J Surg 2018; 105(12):1591–1597. doi:10.1002/bjs.10925]
Biccard et al7 investigated the effect of perioperative aspirin in the subgroup of patients from the POISE-2 trial who underwent vascular surgery. The primary outcome was death or MI within 30 days. Secondary outcomes in this substudy included vascular occlusive complications (amputation and peripheral arterial thrombosis) and major or life-threatening bleeding.
Limitations. There were few adverse events, and this substudy was underpowered for the primary and secondary outcomes.
Conclusion. Starting or continuing aspirin did not improve outcomes, and withdrawing it did not increase cardiovascular or occlusive complications.
Do ACE inhibitors affect risk in noncardiac nonvascular surgery?
Angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs) are some of the most commonly used medications for treating hypertension. But whether patients should continue receiving them on the day of surgery or whether they should be held remains unclear.
Although current recommendations are inconsistent, the most recent American College of Cardiology/American Heart Association1 perioperative practice guidelines say that continuing ACE inhibitors or ARBs is reasonable perioperatively. This recommendation, however, acknowledges that published evidence is limited. There is general agreement that preoperative exposure to ACE inhibitors and ARBs is associated with intraoperative hypotension, but whether this increases the risk of adverse clinical outcomes remains unclear. Needed was a study to determine the effect on perioperative morbidity and mortality of continuing vs withholding ACE inhibitors and ARBs before surgery.
[Shiffermiller JF, Monson BJ, Vokoun CW, et al. Prospective randomized evaluation of preoperative angiotensin-converting enzyme inhibition (PREOP-ACEI). J Hosp Med 2018; 13(10):661–667. doi:10.12788/jhm.3036]
Shiffermiller et al8 performed a randomized controlled trial comparing the effect of 2 preoperative ACE inhibitor management protocols in patients undergoing noncardiac nonvascular surgery. Patients were randomized to either receive or not receive their final preoperative ACE inhibitor dose, whether scheduled on the morning of surgery or the night before.
Exclusion criteria included hypotension or hypertension at their preoperative clinic appointment (defined as systolic blood pressure < 90 or ≥ 160 mm Hg, and diastolic blood pressure < 60 or ≥ 95 mm Hg), moderate to severe heart failure, and end-stage renal disease requiring dialysis. Excluded surgery types were cardiac, vascular, organ transplant, oncologic, and all outpatient procedures. Patients taking ARBs were also excluded.
The primary outcome was intraoperative hypotension defined as any systolic blood pressure less than 80 mm Hg from the time of anesthesia induction until transfer to the postanesthesia care unit. Secondary outcomes were measured until hospital discharge and included postoperative acute kidney injury, postoperative hypotension (systolic pressure < 90 mm Hg) and hypertension (systolic pressure > 180 mm Hg), major cardiac events (composite of acute coronary syndrome, acute heart failure, or new-onset arrhythmia), and death.
Findings. A total of 453 patients were screened for eligibility, and of these, 291 were included for randomization. Their average age was 64, 48% were men, and 87% were white. About 50% underwent general anesthesia, 25% spinal, and 25% regional. Over half of the surgeries were orthopedic, and 20% were spine surgeries.
The primary outcome of intraoperative hypotension occurred significantly less often in patients randomized to ACE inhibitor omission than in the continuation group (55% vs 69%, relative risk [RR] 0.81, 95% CI 0.67–0.97, P = .03). This translates to 1 case of intraoperative hypotension for every 7.5 patients continuing an ACE inhibitor perioperatively (number needed to harm 7.5). Intraoperative hypotension associated with vasopressor administration also occurred significantly less frequently in the ACE inhibitor omission group.
Patients in the ACE inhibitor omission group were also less likely to experience postoperative hypotension, but on the other hand, they were more likely to experience severe postoperative hypertension (defined as any systolic blood pressure > 180 mm Hg). The two groups fared the same in terms of rates of acute kidney injury and major adverse cardiac events (MACE) and hospital length of stay, and no patients died in either group.
Limitations. Several factors limit the generalizability of this single-center study, including the many exclusion criteria, the predominance of orthopedic and spine surgeries, and the low-risk patient population (the average Revised Cardiac Risk Index score was 0, range 0–3). Other limitations include not controlling for the specific ACE inhibitor used and not including the precise timing of the final dose in relation to surgery. Lastly, this study lacked power to measure postoperative outcomes.
Conclusions. Continuing ACE inhibitor treatment before noncardiac nonvascular surgery is associated with a greater frequency and duration of intraoperative hypotension, but it did not increase the incidences of acute kidney injury, MACE, or death nor the hospital length of stay.
[Hollmann C, Fernandes NL, Biccard BM. A systematic review of outcomes associated with withholding or continuing angiotensin-converting enzyme inhibitors and angiotensin receptor blockers before noncardiac surgery. Anesth Analg 2018; 127(3):678–687. doi:10.1213/ANE.0000000000002837]
Hollmann et al9 performed a meta-analysis to determine whether it is better to continue or withhold ACE inhibitors and ARBs before surgery. The patients were adults undergoing noncardiac surgery and receiving an ACE inhibitor or ARB, which was either withheld or continued on the morning of surgery.
Primary outcomes were all-cause mortality and MACE, while secondary outcomes included the incidence of acute kidney injury, heart failure, stroke, intraoperative and postoperative hypotension, and length of hospital stay. Randomized controlled trials and observational studies were included, while case reports and case-control studies were excluded.
Findings. This meta-analysis included 5 randomized controlled trials and 4 cohort studies, with a total of 6,022 patients; 1,816 had their ACE inhibitor or ARB withheld before surgery, while 4,206 continued therapy. It found no difference between the 2 groups in the incidence of death or MACE, and there were not enough data to determine a difference in heart failure, stroke, acute kidney injury, or hospital length of stay.
Seven studies, with 5,414 patients, examined intraoperative hypotension. The overall incidence was 30%, but was significantly lower if the ACE inhibitor or ARB was withheld (OR 0.63, 95% CI 0.47–0.85, P = .002). Findings were similar in an analysis of only the randomized controlled trials. No difference was observed in postoperative hypotension.
Limitations. There was no standard definition of the morbidity outcomes, including hypotension and MACE. The assessment of MACE included data only for MI and not MINS. The specific duration of hypotension was not reported, and this meta-analysis did not take into account different anesthetic techniques. The duration of follow-up varied widely among studies, ranging from the day of hospital discharge to 30 days after surgery. And the randomized controlled trial performed by Shiffermiller et al8 was not included.
Conclusions. While continuing ACE inhibitors or ARBs before noncardiac surgery was associated with intraoperative hypotension, it did not seem to affect other outcomes, including death and MACE. The authors propose that a large randomized controlled trial is needed to determine whether continuing or withholding ACE inhibitor or ARB therapy before surgery is safer.
POSTOPERATIVE CARDIAC COMPLICATIONS
How should we treat MINS?
MINS is associated with an increased risk of cardiovascular events and death in both the short term and long term. MINS is defined as an elevated postoperative troponin level related to an ischemic etiology. However, whether to routinely measure troponin after surgery is unclear, as most patients do not present with ischemic symptoms, and there is no standard of care for treatment of this entity. Limited observational data suggest that starting or intensifying cardiac medications, particularly aspirin and statins, may be beneficial in terms of reducing 30-day mortality rates in patients with MI or cardiac events at 1 year in vascular surgery patients with MINS.
The Management of Myocardial Injury After Noncardiac Surgery (MANAGE) trial was designed to evaluate the potential of the anticoagulant dabigatran to prevent major vascular complications in patients with MINS.
[Devereaux PJ, Duceppe E, Guyatt G, et al. Dabigatran in patients with myocardial injury after non-cardiac surgery (MANAGE): an international, randomised, placebo-controlled trial. Lancet 2018; 391(10137):2325–2334. doi:10.1016/S0140-6736(18)30832-8]
Devereaux et al10 randomized patients who were at least 45 years old and had developed MINS within the previous 35 days to receive dabigatran 110 mg orally twice daily or placebo for up to 2 years. Patients not already taking a proton pump inhibitor were also randomized to take either omeprazole 20 mg once daily or placebo.
The primary efficacy outcome initially was major vascular complications, which included vascular mortality, nonfatal MI, nonhemorrhagic stroke, and peripheral arterial thrombosis. However, amputation and symptomatic venous thromboembolism were subsequently added during the study.
The primary safety outcome was a composite of life-threatening, major, and critical organ bleeding. Major bleeding required a decrease in hemoglobin of at least 4 g/dL, transfusion of at least 3 units of red blood cells within a 24-hour period, or a procedure to stop the bleeding.
Findings. The original goal was to recruit 3,200 patients, but due to slow enrollment and loss of funding, the sample was reduced to 1,754 patients (877 in each group). Approximately 45% of each group stopped taking the study drug prematurely.
The primary efficacy outcome occurred in significantly fewer patients receiving dabigatran (97, 11%) than placebo (133, 15%, HR 0.72, 95% CI 0.55–0.93, P = .0115). The incidence of the primary safety outcome was similar in both groups: 3% with dabigatran and 4% with placebo (HR 0.92, 95% CI 0.55–1.53, P = .76). The only individual efficacy outcome meeting statistical significance was a lower rate of nonhemorrhagic stroke in the dabigatran group. Subgroup analyses showed a trend benefiting patients randomized within 5 days of MINS or with a diagnosis of MI, although it was not statistically significant.
Limitations. The efficacy outcomes were expanded to include venous thromboembolism and others not directly related to MINS, raising questions about the conclusions. Further, as defined by the protocol, bleeding had to be fairly severe to be deemed major. The high number of patients who discontinued the study drug is another limitation of this study.
Conclusion. Dabigatran lowered the risk of major vascular complications with no significant increase in major bleeding in patients with MINS.
What is the risk of thromboembolism in postoperative atrial fibrillation, and what are the benefits of anticoagulation?
Although nonvalvular atrial fibrillation is associated with increased risks of ischemic stroke and systemic embolic events in nonsurgical patients, the association of new-onset postoperative atrial fibrillation with long-term thromboembolic events in the noncardiac surgical population is not well established.
[Butt JH, Olesen JB, Havers-Borgersen E, et al. Risk of thromboembolism associated with atrial fibrillation following noncardiac surgery. J Am Coll Cardiol 2018; 72(17):2027–2036. doi:10.1016/j.jacc.2018.07.088]
In this retrospective cohort study using a nationwide registry in Denmark, Butt et al11 assessed the long-term risk of thromboembolic events in noncardiac surgical patients with new postoperative atrial fibrillation. Patients were identified who had no previous history of atrial fibrillation and developed it after noncardiac, nonobstetric surgeries, and were matched in a 1:4 ratio with patients who developed nonvalvular atrial fibrillation during nonsurgical hospitalizations. Matching was based on age, sex, heart failure, hypertension, diabetes, known history of thromboembolic events, ischemic heart disease, and the year patients presented with new atrial fibrillation.
Patients were excluded if they received antiarrhythmic drugs or oral anticoagulants before hospitalization or surgery, had cancer in the year prior, or died in the hospital.
The primary outcome of the study was thromboembolic events—a composite of ischemic stroke, transient cerebral ischemia, and peripheral arterial thrombosis or embolism. Secondary outcomes included rehospitalization for atrial fibrillation and all-cause mortality.
Findings. Overall, 0.4% of patients developed new postoperative atrial fibrillation, of whom 3,380 were matched with 15,320 patients with nonvalvular atrial fibrillation. Over a median follow-up of 3.2 years, the risk of thromboembolic events was similar in both groups (31.7 and 29.9 per 1,000 person-years, HR 0.95, 95% CI 0.85–1.07). The groups did not differ in their CHA2DS2-VASc risk scores, HAS-BLED risk scores, or year in which patients were diagnosed.
Anticoagulation lowered the risk of thromboembolic events to a similar extent in both groups compared with no anticoagulation:
- In postoperative atrial fibrillation—HR 0.57, 95% CI 0.40–0.67
- In nonvalvular atrial fibrillation—HR 0.56, 95% CI 0.51–0.62.
Despite the similar reduction in thromboembolic events, only 24.4% of the postoperative atrial fibrillation patients were started on anticoagulation therapy within 30 days of discharge, compared with 41.5% of those with nonvalvular atrial fibrillation.
Limitations. Although this was a large study with excellent follow-up data, it was observational. It may have underestimated the number of patients who developed postoperative atrial fibrillation because episodes that were judged not to be clinically significant may not have been charted. Many patients are not monitored with continuous telemetry postoperatively, which also may have led to underestimation of the number of atrial fibrillation events.
The study also did not examine the number of atrial fibrillation episodes per patient, the heart rhythm at discharge or long-term, or indication for and duration of anticoagulation. There were no data regarding international normalized ratio levels.
Conclusions. Postoperative atrial fibrillation is associated with outcomes similar to those of nonsurgical nonvalvular atrial fibrillation. Anticoagulation decreases the risks of stroke and death. However, substantially fewer patients with postoperative atrial fibrillation receive anticoagulation. Anticoagulation should be considered in these patients, while noting bleeding risk.
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol 2014; 64(22):e77–137. doi:10.1016/j.jacc.2014.07.944
- Kristensen SD, Knuuti J, Saraste A, et al. 2014 ESC/ESA Guidelines on non-cardiac surgery: cardiovascular assessment and management: the Joint Task Force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur Heart J 2014; 35(35):2383–2431. doi:10.1093/eurheartj/ehu282
- Hlatky MA, Boineau RE, Higginbotham MB, et al. A brief self-administered questionnaire to determine functional capacity (The Duke Activity Status Index). Am J Cardiol 1989; 64(10):651–654. doi:10.1016/0002-9149(89)90496-7
- Wijeysundera DN, Pearse RM, Sulman MA, et al. Assessment of functional capacity before major non-cardiac surgery: an international, prospective cohort study. Lancet 2018; 391(10140):2631–2640. doi:10.1016/S0140-6736(18)31131-0
- Devereaux PJ, Mrkobrada M, Sessler DI, et al; POISE-2 Investigators. Aspirin in patients undergoing noncardiac surgery. N Engl J Med 2014; 370(16):1494–1503. doi:10.1056/NEJMoa1401105
- Graham MM, Sessler DI, Parlow JL, et al. Aspirin in patients with previous percutaneous coronary intervention undergoing noncardiac surgery. Ann Intern Med 2018;168(4):237–244. pmid:29132159
- Biccard BM, Sigamani A, Chan MTV, et al. Effect of aspirin in vascular surgery in patients from a randomized clinical trial (POISE-2). Br J Surg 2018; 105(12):1591–1597. doi:10.1002/bjs.10925
- Shiffermiller JF, Monson BJ, Vokoun CW, et al. Prospective randomized evaluation of preoperative angiotensin-converting enzyme inhibition (PREOP-ACEI). J Hosp Med 2018; 13(10):661–667. doi:10.12788/jhm.3036
- Hollmann C, Fernandes NL, Biccard BM. A systematic review of outcomes associated with withholding or continuing angiotensin-converting enzyme inhibitors and angiotensin receptor blockers before noncardiac surgery. Anesth Analg 2018; 127(3):678–687. doi:10.1213/ANE.0000000000002837
- Devereaux PJ, Duceppe E, Guyatt G, et al. Dabigatran in patients with myocardial injury after non-cardiac surgery (MANAGE): an international, randomised, placebo-controlled trial. Lancet 2018; 391(10137):2325–2334. doi:10.1016/S0140-6736(18)30832-8
- Butt JH, Olesen JB, Havers-Borgersen E, et al. Risk of thromboembolism associated with atrial fibrillation following noncardiac surgery. J Am Coll Cardiol 2018; 72(17):2027–2036. doi:10.1016/j.jacc.2018.07.088
Perioperative medicine is an evolving field with a rapidly growing body of literature, particularly in cardiology.
In this update, we review 6 articles to answer questions related to preoperative cardiac risk assessment, perioperative medication management, and postoperative cardiac complications. We surveyed perioperative literature from February 2018 through January 2019 and chose the final articles by consensus, based on relevance to clinicians who provide preoperative evaluations and postoperative care to surgical patients.
These summaries are derived from “Updates in Perioperative Medicine” presented at the 14th Annual Perioperative Medicine Summit (Orlando, FL, February 13–16, 2019) and the 2019 Society of Hospital Medicine Annual Meeting (National Harbor, MD, March 24–27, 2019).
PREOPERATIVE CARDIAC EVALUATION
How well do measures of functional capacity predict perioperative complications and mortality in noncardiac surgical patients?
Functional capacity is commonly assessed in preoperative evaluations to estimate patients’ risks of perioperative complications and death. The American College of Cardiology/American Heart Association1 and the European Society of Cardiology2 guidelines both include estimation of cardiopulmonary fitness as a step in preoperative assessment before major noncardiac surgery.
“Subjective assessment” is one way to estimate functional capacity. Simply put, clinicians try to form a rough idea about the fitness of patients by asking questions about routine activities such as walking or climbing stairs. Although commonly used, subjective assessment of functional capacity lacks strong evidence that it predicts adverse perioperative events.
Cardiopulmonary exercise testing is a third option. It measures peak oxygen consumption and anaerobic threshold during exercise. It is probably the best objective measurement of functional capacity, but not necessarily for predicting postoperative cardiac complications, and it is performed relatively infrequently.
[Wijeysundera DN, Pearse RM, Sulman MA, et al. Assessment of functional capacity before major non-cardiac surgery: an international, prospective cohort study. Lancet 2018; 391(10140):2631–2640. doi:10.1016/S0140-6736(18)31131-0]
In a multicenter, prospective cohort study, Wijeysundera et al4 compared subjective functional capacity assessment, the Duke Activity Status Index, cardiopulmonary exercise testing, and the preoperative N-terminal pro-B-type natriuretic peptide (NT-proBNP) level in their ability to predict complications and death in 1,401 noncardiac surgery patients older than 40 with at least 1 cardiovascular risk factor. After surgery, patients had daily electrocardiograms and troponin measurements until postoperative day 3 or discharge.
The primary outcome was the 30-day incidence of death or myocardial infarction (MI). Additional outcomes included the 30-day incidence of death or myocardial injury after noncardiac surgery (MINS), the 1-year mortality rate, and moderate to severe in-hospital perioperative complications.
Findings. Two percent of patients died or had an MI within 30 days of surgery.4
Subjective assessment had only a 19.2% sensitivity (95% confidence interval [CI] 14.2–25) but a 94.7% specificity (95% CI 93.2–95.9) for predicting inability to attain 4 metabolic equivalents during exercise.4
A lower Duke Activity Status Index predicted the primary outcome of death or MI within 30 days (adjusted odds ratio [OR] 0.96, 95% CI 0.83–0.99, P = .03), and it was the only measure that did so. Additionally, the Duke index and NT-proBNP level predicted the risk of death or MINS within 30 days.4
Only elevated NT-proBNP was associated with death at 1 year.4
On exercise testing, low peak oxygen consumption was significantly associated with perioperative complications.
Limitations. The number of primary outcome events (death and MI) was low, potentially affecting the statistical power of the study.
Conclusions. Subjective assessment of functional capacity misclassifies too many patients as being at low risk of perioperative complications and should not be used for preoperative risk stratification. Other tools, such as the Duke Activity Status Index and NT-proBNP levels, are better predictors of adverse perioperative cardiovascular outcomes and should be considered for use in preoperative cardiac risk assessment.
Although the Duke Activity Status Index is a better predictor of adverse outcomes than subjective functional capacity assessment, a specific perioperative threshold for risk classification has not been established. Its correlate for metabolic equivalents should be considered for use in clinical practice at this point.
PERIOPERATIVE MEDICATION MANAGEMENT
Is perioperative aspirin beneficial in patients undergoing vascular surgery?
The Perioperative Ischemic Evaluation 2 (POISE-2) trial,5 a 2-by-2 factorial randomized controlled trial in which patients received perioperative aspirin, clonidine, both, or neither, demonstrated that perioperative aspirin did not reduce cardiovascular events and increased major bleeding. Patients with recently placed coronary stents and those undergoing carotid endarterectomy were excluded because aspirin is known to have a beneficial effect in these patients.
A subsequent substudy6 found perioperative aspirin to be beneficial in patients with coronary stents placed more than a year before noncardiac surgery. Whether perioperative aspirin is beneficial in other subgroups was unknown.
[Biccard BM, Sigamani A, Chan MTV, et al. Effect of aspirin in vascular surgery in patients from a randomized clinical trial (POISE-2). Br J Surg 2018; 105(12):1591–1597. doi:10.1002/bjs.10925]
Biccard et al7 investigated the effect of perioperative aspirin in the subgroup of patients from the POISE-2 trial who underwent vascular surgery. The primary outcome was death or MI within 30 days. Secondary outcomes in this substudy included vascular occlusive complications (amputation and peripheral arterial thrombosis) and major or life-threatening bleeding.
Limitations. There were few adverse events, and this substudy was underpowered for the primary and secondary outcomes.
Conclusion. Starting or continuing aspirin did not improve outcomes, and withdrawing it did not increase cardiovascular or occlusive complications.
Do ACE inhibitors affect risk in noncardiac nonvascular surgery?
Angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs) are some of the most commonly used medications for treating hypertension. But whether patients should continue receiving them on the day of surgery or whether they should be held remains unclear.
Although current recommendations are inconsistent, the most recent American College of Cardiology/American Heart Association1 perioperative practice guidelines say that continuing ACE inhibitors or ARBs is reasonable perioperatively. This recommendation, however, acknowledges that published evidence is limited. There is general agreement that preoperative exposure to ACE inhibitors and ARBs is associated with intraoperative hypotension, but whether this increases the risk of adverse clinical outcomes remains unclear. Needed was a study to determine the effect on perioperative morbidity and mortality of continuing vs withholding ACE inhibitors and ARBs before surgery.
[Shiffermiller JF, Monson BJ, Vokoun CW, et al. Prospective randomized evaluation of preoperative angiotensin-converting enzyme inhibition (PREOP-ACEI). J Hosp Med 2018; 13(10):661–667. doi:10.12788/jhm.3036]
Shiffermiller et al8 performed a randomized controlled trial comparing the effect of 2 preoperative ACE inhibitor management protocols in patients undergoing noncardiac nonvascular surgery. Patients were randomized to either receive or not receive their final preoperative ACE inhibitor dose, whether scheduled on the morning of surgery or the night before.
Exclusion criteria included hypotension or hypertension at their preoperative clinic appointment (defined as systolic blood pressure < 90 or ≥ 160 mm Hg, and diastolic blood pressure < 60 or ≥ 95 mm Hg), moderate to severe heart failure, and end-stage renal disease requiring dialysis. Excluded surgery types were cardiac, vascular, organ transplant, oncologic, and all outpatient procedures. Patients taking ARBs were also excluded.
The primary outcome was intraoperative hypotension defined as any systolic blood pressure less than 80 mm Hg from the time of anesthesia induction until transfer to the postanesthesia care unit. Secondary outcomes were measured until hospital discharge and included postoperative acute kidney injury, postoperative hypotension (systolic pressure < 90 mm Hg) and hypertension (systolic pressure > 180 mm Hg), major cardiac events (composite of acute coronary syndrome, acute heart failure, or new-onset arrhythmia), and death.
Findings. A total of 453 patients were screened for eligibility, and of these, 291 were included for randomization. Their average age was 64, 48% were men, and 87% were white. About 50% underwent general anesthesia, 25% spinal, and 25% regional. Over half of the surgeries were orthopedic, and 20% were spine surgeries.
The primary outcome of intraoperative hypotension occurred significantly less often in patients randomized to ACE inhibitor omission than in the continuation group (55% vs 69%, relative risk [RR] 0.81, 95% CI 0.67–0.97, P = .03). This translates to 1 case of intraoperative hypotension for every 7.5 patients continuing an ACE inhibitor perioperatively (number needed to harm 7.5). Intraoperative hypotension associated with vasopressor administration also occurred significantly less frequently in the ACE inhibitor omission group.
Patients in the ACE inhibitor omission group were also less likely to experience postoperative hypotension, but on the other hand, they were more likely to experience severe postoperative hypertension (defined as any systolic blood pressure > 180 mm Hg). The two groups fared the same in terms of rates of acute kidney injury and major adverse cardiac events (MACE) and hospital length of stay, and no patients died in either group.
Limitations. Several factors limit the generalizability of this single-center study, including the many exclusion criteria, the predominance of orthopedic and spine surgeries, and the low-risk patient population (the average Revised Cardiac Risk Index score was 0, range 0–3). Other limitations include not controlling for the specific ACE inhibitor used and not including the precise timing of the final dose in relation to surgery. Lastly, this study lacked power to measure postoperative outcomes.
Conclusions. Continuing ACE inhibitor treatment before noncardiac nonvascular surgery is associated with a greater frequency and duration of intraoperative hypotension, but it did not increase the incidences of acute kidney injury, MACE, or death nor the hospital length of stay.
[Hollmann C, Fernandes NL, Biccard BM. A systematic review of outcomes associated with withholding or continuing angiotensin-converting enzyme inhibitors and angiotensin receptor blockers before noncardiac surgery. Anesth Analg 2018; 127(3):678–687. doi:10.1213/ANE.0000000000002837]
Hollmann et al9 performed a meta-analysis to determine whether it is better to continue or withhold ACE inhibitors and ARBs before surgery. The patients were adults undergoing noncardiac surgery and receiving an ACE inhibitor or ARB, which was either withheld or continued on the morning of surgery.
Primary outcomes were all-cause mortality and MACE, while secondary outcomes included the incidence of acute kidney injury, heart failure, stroke, intraoperative and postoperative hypotension, and length of hospital stay. Randomized controlled trials and observational studies were included, while case reports and case-control studies were excluded.
Findings. This meta-analysis included 5 randomized controlled trials and 4 cohort studies, with a total of 6,022 patients; 1,816 had their ACE inhibitor or ARB withheld before surgery, while 4,206 continued therapy. It found no difference between the 2 groups in the incidence of death or MACE, and there were not enough data to determine a difference in heart failure, stroke, acute kidney injury, or hospital length of stay.
Seven studies, with 5,414 patients, examined intraoperative hypotension. The overall incidence was 30%, but was significantly lower if the ACE inhibitor or ARB was withheld (OR 0.63, 95% CI 0.47–0.85, P = .002). Findings were similar in an analysis of only the randomized controlled trials. No difference was observed in postoperative hypotension.
Limitations. There was no standard definition of the morbidity outcomes, including hypotension and MACE. The assessment of MACE included data only for MI and not MINS. The specific duration of hypotension was not reported, and this meta-analysis did not take into account different anesthetic techniques. The duration of follow-up varied widely among studies, ranging from the day of hospital discharge to 30 days after surgery. And the randomized controlled trial performed by Shiffermiller et al8 was not included.
Conclusions. While continuing ACE inhibitors or ARBs before noncardiac surgery was associated with intraoperative hypotension, it did not seem to affect other outcomes, including death and MACE. The authors propose that a large randomized controlled trial is needed to determine whether continuing or withholding ACE inhibitor or ARB therapy before surgery is safer.
POSTOPERATIVE CARDIAC COMPLICATIONS
How should we treat MINS?
MINS is associated with an increased risk of cardiovascular events and death in both the short term and long term. MINS is defined as an elevated postoperative troponin level related to an ischemic etiology. However, whether to routinely measure troponin after surgery is unclear, as most patients do not present with ischemic symptoms, and there is no standard of care for treatment of this entity. Limited observational data suggest that starting or intensifying cardiac medications, particularly aspirin and statins, may be beneficial in terms of reducing 30-day mortality rates in patients with MI or cardiac events at 1 year in vascular surgery patients with MINS.
The Management of Myocardial Injury After Noncardiac Surgery (MANAGE) trial was designed to evaluate the potential of the anticoagulant dabigatran to prevent major vascular complications in patients with MINS.
[Devereaux PJ, Duceppe E, Guyatt G, et al. Dabigatran in patients with myocardial injury after non-cardiac surgery (MANAGE): an international, randomised, placebo-controlled trial. Lancet 2018; 391(10137):2325–2334. doi:10.1016/S0140-6736(18)30832-8]
Devereaux et al10 randomized patients who were at least 45 years old and had developed MINS within the previous 35 days to receive dabigatran 110 mg orally twice daily or placebo for up to 2 years. Patients not already taking a proton pump inhibitor were also randomized to take either omeprazole 20 mg once daily or placebo.
The primary efficacy outcome initially was major vascular complications, which included vascular mortality, nonfatal MI, nonhemorrhagic stroke, and peripheral arterial thrombosis. However, amputation and symptomatic venous thromboembolism were subsequently added during the study.
The primary safety outcome was a composite of life-threatening, major, and critical organ bleeding. Major bleeding required a decrease in hemoglobin of at least 4 g/dL, transfusion of at least 3 units of red blood cells within a 24-hour period, or a procedure to stop the bleeding.
Findings. The original goal was to recruit 3,200 patients, but due to slow enrollment and loss of funding, the sample was reduced to 1,754 patients (877 in each group). Approximately 45% of each group stopped taking the study drug prematurely.
The primary efficacy outcome occurred in significantly fewer patients receiving dabigatran (97, 11%) than placebo (133, 15%, HR 0.72, 95% CI 0.55–0.93, P = .0115). The incidence of the primary safety outcome was similar in both groups: 3% with dabigatran and 4% with placebo (HR 0.92, 95% CI 0.55–1.53, P = .76). The only individual efficacy outcome meeting statistical significance was a lower rate of nonhemorrhagic stroke in the dabigatran group. Subgroup analyses showed a trend benefiting patients randomized within 5 days of MINS or with a diagnosis of MI, although it was not statistically significant.
Limitations. The efficacy outcomes were expanded to include venous thromboembolism and others not directly related to MINS, raising questions about the conclusions. Further, as defined by the protocol, bleeding had to be fairly severe to be deemed major. The high number of patients who discontinued the study drug is another limitation of this study.
Conclusion. Dabigatran lowered the risk of major vascular complications with no significant increase in major bleeding in patients with MINS.
What is the risk of thromboembolism in postoperative atrial fibrillation, and what are the benefits of anticoagulation?
Although nonvalvular atrial fibrillation is associated with increased risks of ischemic stroke and systemic embolic events in nonsurgical patients, the association of new-onset postoperative atrial fibrillation with long-term thromboembolic events in the noncardiac surgical population is not well established.
[Butt JH, Olesen JB, Havers-Borgersen E, et al. Risk of thromboembolism associated with atrial fibrillation following noncardiac surgery. J Am Coll Cardiol 2018; 72(17):2027–2036. doi:10.1016/j.jacc.2018.07.088]
In this retrospective cohort study using a nationwide registry in Denmark, Butt et al11 assessed the long-term risk of thromboembolic events in noncardiac surgical patients with new postoperative atrial fibrillation. Patients were identified who had no previous history of atrial fibrillation and developed it after noncardiac, nonobstetric surgeries, and were matched in a 1:4 ratio with patients who developed nonvalvular atrial fibrillation during nonsurgical hospitalizations. Matching was based on age, sex, heart failure, hypertension, diabetes, known history of thromboembolic events, ischemic heart disease, and the year patients presented with new atrial fibrillation.
Patients were excluded if they received antiarrhythmic drugs or oral anticoagulants before hospitalization or surgery, had cancer in the year prior, or died in the hospital.
The primary outcome of the study was thromboembolic events—a composite of ischemic stroke, transient cerebral ischemia, and peripheral arterial thrombosis or embolism. Secondary outcomes included rehospitalization for atrial fibrillation and all-cause mortality.
Findings. Overall, 0.4% of patients developed new postoperative atrial fibrillation, of whom 3,380 were matched with 15,320 patients with nonvalvular atrial fibrillation. Over a median follow-up of 3.2 years, the risk of thromboembolic events was similar in both groups (31.7 and 29.9 per 1,000 person-years, HR 0.95, 95% CI 0.85–1.07). The groups did not differ in their CHA2DS2-VASc risk scores, HAS-BLED risk scores, or year in which patients were diagnosed.
Anticoagulation lowered the risk of thromboembolic events to a similar extent in both groups compared with no anticoagulation:
- In postoperative atrial fibrillation—HR 0.57, 95% CI 0.40–0.67
- In nonvalvular atrial fibrillation—HR 0.56, 95% CI 0.51–0.62.
Despite the similar reduction in thromboembolic events, only 24.4% of the postoperative atrial fibrillation patients were started on anticoagulation therapy within 30 days of discharge, compared with 41.5% of those with nonvalvular atrial fibrillation.
Limitations. Although this was a large study with excellent follow-up data, it was observational. It may have underestimated the number of patients who developed postoperative atrial fibrillation because episodes that were judged not to be clinically significant may not have been charted. Many patients are not monitored with continuous telemetry postoperatively, which also may have led to underestimation of the number of atrial fibrillation events.
The study also did not examine the number of atrial fibrillation episodes per patient, the heart rhythm at discharge or long-term, or indication for and duration of anticoagulation. There were no data regarding international normalized ratio levels.
Conclusions. Postoperative atrial fibrillation is associated with outcomes similar to those of nonsurgical nonvalvular atrial fibrillation. Anticoagulation decreases the risks of stroke and death. However, substantially fewer patients with postoperative atrial fibrillation receive anticoagulation. Anticoagulation should be considered in these patients, while noting bleeding risk.
Perioperative medicine is an evolving field with a rapidly growing body of literature, particularly in cardiology.
In this update, we review 6 articles to answer questions related to preoperative cardiac risk assessment, perioperative medication management, and postoperative cardiac complications. We surveyed perioperative literature from February 2018 through January 2019 and chose the final articles by consensus, based on relevance to clinicians who provide preoperative evaluations and postoperative care to surgical patients.
These summaries are derived from “Updates in Perioperative Medicine” presented at the 14th Annual Perioperative Medicine Summit (Orlando, FL, February 13–16, 2019) and the 2019 Society of Hospital Medicine Annual Meeting (National Harbor, MD, March 24–27, 2019).
PREOPERATIVE CARDIAC EVALUATION
How well do measures of functional capacity predict perioperative complications and mortality in noncardiac surgical patients?
Functional capacity is commonly assessed in preoperative evaluations to estimate patients’ risks of perioperative complications and death. The American College of Cardiology/American Heart Association1 and the European Society of Cardiology2 guidelines both include estimation of cardiopulmonary fitness as a step in preoperative assessment before major noncardiac surgery.
“Subjective assessment” is one way to estimate functional capacity. Simply put, clinicians try to form a rough idea about the fitness of patients by asking questions about routine activities such as walking or climbing stairs. Although commonly used, subjective assessment of functional capacity lacks strong evidence that it predicts adverse perioperative events.
Cardiopulmonary exercise testing is a third option. It measures peak oxygen consumption and anaerobic threshold during exercise. It is probably the best objective measurement of functional capacity, but not necessarily for predicting postoperative cardiac complications, and it is performed relatively infrequently.
[Wijeysundera DN, Pearse RM, Sulman MA, et al. Assessment of functional capacity before major non-cardiac surgery: an international, prospective cohort study. Lancet 2018; 391(10140):2631–2640. doi:10.1016/S0140-6736(18)31131-0]
In a multicenter, prospective cohort study, Wijeysundera et al4 compared subjective functional capacity assessment, the Duke Activity Status Index, cardiopulmonary exercise testing, and the preoperative N-terminal pro-B-type natriuretic peptide (NT-proBNP) level in their ability to predict complications and death in 1,401 noncardiac surgery patients older than 40 with at least 1 cardiovascular risk factor. After surgery, patients had daily electrocardiograms and troponin measurements until postoperative day 3 or discharge.
The primary outcome was the 30-day incidence of death or myocardial infarction (MI). Additional outcomes included the 30-day incidence of death or myocardial injury after noncardiac surgery (MINS), the 1-year mortality rate, and moderate to severe in-hospital perioperative complications.
Findings. Two percent of patients died or had an MI within 30 days of surgery.4
Subjective assessment had only a 19.2% sensitivity (95% confidence interval [CI] 14.2–25) but a 94.7% specificity (95% CI 93.2–95.9) for predicting inability to attain 4 metabolic equivalents during exercise.4
A lower Duke Activity Status Index predicted the primary outcome of death or MI within 30 days (adjusted odds ratio [OR] 0.96, 95% CI 0.83–0.99, P = .03), and it was the only measure that did so. Additionally, the Duke index and NT-proBNP level predicted the risk of death or MINS within 30 days.4
Only elevated NT-proBNP was associated with death at 1 year.4
On exercise testing, low peak oxygen consumption was significantly associated with perioperative complications.
Limitations. The number of primary outcome events (death and MI) was low, potentially affecting the statistical power of the study.
Conclusions. Subjective assessment of functional capacity misclassifies too many patients as being at low risk of perioperative complications and should not be used for preoperative risk stratification. Other tools, such as the Duke Activity Status Index and NT-proBNP levels, are better predictors of adverse perioperative cardiovascular outcomes and should be considered for use in preoperative cardiac risk assessment.
Although the Duke Activity Status Index is a better predictor of adverse outcomes than subjective functional capacity assessment, a specific perioperative threshold for risk classification has not been established. Its correlate for metabolic equivalents should be considered for use in clinical practice at this point.
PERIOPERATIVE MEDICATION MANAGEMENT
Is perioperative aspirin beneficial in patients undergoing vascular surgery?
The Perioperative Ischemic Evaluation 2 (POISE-2) trial,5 a 2-by-2 factorial randomized controlled trial in which patients received perioperative aspirin, clonidine, both, or neither, demonstrated that perioperative aspirin did not reduce cardiovascular events and increased major bleeding. Patients with recently placed coronary stents and those undergoing carotid endarterectomy were excluded because aspirin is known to have a beneficial effect in these patients.
A subsequent substudy6 found perioperative aspirin to be beneficial in patients with coronary stents placed more than a year before noncardiac surgery. Whether perioperative aspirin is beneficial in other subgroups was unknown.
[Biccard BM, Sigamani A, Chan MTV, et al. Effect of aspirin in vascular surgery in patients from a randomized clinical trial (POISE-2). Br J Surg 2018; 105(12):1591–1597. doi:10.1002/bjs.10925]
Biccard et al7 investigated the effect of perioperative aspirin in the subgroup of patients from the POISE-2 trial who underwent vascular surgery. The primary outcome was death or MI within 30 days. Secondary outcomes in this substudy included vascular occlusive complications (amputation and peripheral arterial thrombosis) and major or life-threatening bleeding.
Limitations. There were few adverse events, and this substudy was underpowered for the primary and secondary outcomes.
Conclusion. Starting or continuing aspirin did not improve outcomes, and withdrawing it did not increase cardiovascular or occlusive complications.
Do ACE inhibitors affect risk in noncardiac nonvascular surgery?
Angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs) are some of the most commonly used medications for treating hypertension. But whether patients should continue receiving them on the day of surgery or whether they should be held remains unclear.
Although current recommendations are inconsistent, the most recent American College of Cardiology/American Heart Association1 perioperative practice guidelines say that continuing ACE inhibitors or ARBs is reasonable perioperatively. This recommendation, however, acknowledges that published evidence is limited. There is general agreement that preoperative exposure to ACE inhibitors and ARBs is associated with intraoperative hypotension, but whether this increases the risk of adverse clinical outcomes remains unclear. Needed was a study to determine the effect on perioperative morbidity and mortality of continuing vs withholding ACE inhibitors and ARBs before surgery.
[Shiffermiller JF, Monson BJ, Vokoun CW, et al. Prospective randomized evaluation of preoperative angiotensin-converting enzyme inhibition (PREOP-ACEI). J Hosp Med 2018; 13(10):661–667. doi:10.12788/jhm.3036]
Shiffermiller et al8 performed a randomized controlled trial comparing the effect of 2 preoperative ACE inhibitor management protocols in patients undergoing noncardiac nonvascular surgery. Patients were randomized to either receive or not receive their final preoperative ACE inhibitor dose, whether scheduled on the morning of surgery or the night before.
Exclusion criteria included hypotension or hypertension at their preoperative clinic appointment (defined as systolic blood pressure < 90 or ≥ 160 mm Hg, and diastolic blood pressure < 60 or ≥ 95 mm Hg), moderate to severe heart failure, and end-stage renal disease requiring dialysis. Excluded surgery types were cardiac, vascular, organ transplant, oncologic, and all outpatient procedures. Patients taking ARBs were also excluded.
The primary outcome was intraoperative hypotension defined as any systolic blood pressure less than 80 mm Hg from the time of anesthesia induction until transfer to the postanesthesia care unit. Secondary outcomes were measured until hospital discharge and included postoperative acute kidney injury, postoperative hypotension (systolic pressure < 90 mm Hg) and hypertension (systolic pressure > 180 mm Hg), major cardiac events (composite of acute coronary syndrome, acute heart failure, or new-onset arrhythmia), and death.
Findings. A total of 453 patients were screened for eligibility, and of these, 291 were included for randomization. Their average age was 64, 48% were men, and 87% were white. About 50% underwent general anesthesia, 25% spinal, and 25% regional. Over half of the surgeries were orthopedic, and 20% were spine surgeries.
The primary outcome of intraoperative hypotension occurred significantly less often in patients randomized to ACE inhibitor omission than in the continuation group (55% vs 69%, relative risk [RR] 0.81, 95% CI 0.67–0.97, P = .03). This translates to 1 case of intraoperative hypotension for every 7.5 patients continuing an ACE inhibitor perioperatively (number needed to harm 7.5). Intraoperative hypotension associated with vasopressor administration also occurred significantly less frequently in the ACE inhibitor omission group.
Patients in the ACE inhibitor omission group were also less likely to experience postoperative hypotension, but on the other hand, they were more likely to experience severe postoperative hypertension (defined as any systolic blood pressure > 180 mm Hg). The two groups fared the same in terms of rates of acute kidney injury and major adverse cardiac events (MACE) and hospital length of stay, and no patients died in either group.
Limitations. Several factors limit the generalizability of this single-center study, including the many exclusion criteria, the predominance of orthopedic and spine surgeries, and the low-risk patient population (the average Revised Cardiac Risk Index score was 0, range 0–3). Other limitations include not controlling for the specific ACE inhibitor used and not including the precise timing of the final dose in relation to surgery. Lastly, this study lacked power to measure postoperative outcomes.
Conclusions. Continuing ACE inhibitor treatment before noncardiac nonvascular surgery is associated with a greater frequency and duration of intraoperative hypotension, but it did not increase the incidences of acute kidney injury, MACE, or death nor the hospital length of stay.
[Hollmann C, Fernandes NL, Biccard BM. A systematic review of outcomes associated with withholding or continuing angiotensin-converting enzyme inhibitors and angiotensin receptor blockers before noncardiac surgery. Anesth Analg 2018; 127(3):678–687. doi:10.1213/ANE.0000000000002837]
Hollmann et al9 performed a meta-analysis to determine whether it is better to continue or withhold ACE inhibitors and ARBs before surgery. The patients were adults undergoing noncardiac surgery and receiving an ACE inhibitor or ARB, which was either withheld or continued on the morning of surgery.
Primary outcomes were all-cause mortality and MACE, while secondary outcomes included the incidence of acute kidney injury, heart failure, stroke, intraoperative and postoperative hypotension, and length of hospital stay. Randomized controlled trials and observational studies were included, while case reports and case-control studies were excluded.
Findings. This meta-analysis included 5 randomized controlled trials and 4 cohort studies, with a total of 6,022 patients; 1,816 had their ACE inhibitor or ARB withheld before surgery, while 4,206 continued therapy. It found no difference between the 2 groups in the incidence of death or MACE, and there were not enough data to determine a difference in heart failure, stroke, acute kidney injury, or hospital length of stay.
Seven studies, with 5,414 patients, examined intraoperative hypotension. The overall incidence was 30%, but was significantly lower if the ACE inhibitor or ARB was withheld (OR 0.63, 95% CI 0.47–0.85, P = .002). Findings were similar in an analysis of only the randomized controlled trials. No difference was observed in postoperative hypotension.
Limitations. There was no standard definition of the morbidity outcomes, including hypotension and MACE. The assessment of MACE included data only for MI and not MINS. The specific duration of hypotension was not reported, and this meta-analysis did not take into account different anesthetic techniques. The duration of follow-up varied widely among studies, ranging from the day of hospital discharge to 30 days after surgery. And the randomized controlled trial performed by Shiffermiller et al8 was not included.
Conclusions. While continuing ACE inhibitors or ARBs before noncardiac surgery was associated with intraoperative hypotension, it did not seem to affect other outcomes, including death and MACE. The authors propose that a large randomized controlled trial is needed to determine whether continuing or withholding ACE inhibitor or ARB therapy before surgery is safer.
POSTOPERATIVE CARDIAC COMPLICATIONS
How should we treat MINS?
MINS is associated with an increased risk of cardiovascular events and death in both the short term and long term. MINS is defined as an elevated postoperative troponin level related to an ischemic etiology. However, whether to routinely measure troponin after surgery is unclear, as most patients do not present with ischemic symptoms, and there is no standard of care for treatment of this entity. Limited observational data suggest that starting or intensifying cardiac medications, particularly aspirin and statins, may be beneficial in terms of reducing 30-day mortality rates in patients with MI or cardiac events at 1 year in vascular surgery patients with MINS.
The Management of Myocardial Injury After Noncardiac Surgery (MANAGE) trial was designed to evaluate the potential of the anticoagulant dabigatran to prevent major vascular complications in patients with MINS.
[Devereaux PJ, Duceppe E, Guyatt G, et al. Dabigatran in patients with myocardial injury after non-cardiac surgery (MANAGE): an international, randomised, placebo-controlled trial. Lancet 2018; 391(10137):2325–2334. doi:10.1016/S0140-6736(18)30832-8]
Devereaux et al10 randomized patients who were at least 45 years old and had developed MINS within the previous 35 days to receive dabigatran 110 mg orally twice daily or placebo for up to 2 years. Patients not already taking a proton pump inhibitor were also randomized to take either omeprazole 20 mg once daily or placebo.
The primary efficacy outcome initially was major vascular complications, which included vascular mortality, nonfatal MI, nonhemorrhagic stroke, and peripheral arterial thrombosis. However, amputation and symptomatic venous thromboembolism were subsequently added during the study.
The primary safety outcome was a composite of life-threatening, major, and critical organ bleeding. Major bleeding required a decrease in hemoglobin of at least 4 g/dL, transfusion of at least 3 units of red blood cells within a 24-hour period, or a procedure to stop the bleeding.
Findings. The original goal was to recruit 3,200 patients, but due to slow enrollment and loss of funding, the sample was reduced to 1,754 patients (877 in each group). Approximately 45% of each group stopped taking the study drug prematurely.
The primary efficacy outcome occurred in significantly fewer patients receiving dabigatran (97, 11%) than placebo (133, 15%, HR 0.72, 95% CI 0.55–0.93, P = .0115). The incidence of the primary safety outcome was similar in both groups: 3% with dabigatran and 4% with placebo (HR 0.92, 95% CI 0.55–1.53, P = .76). The only individual efficacy outcome meeting statistical significance was a lower rate of nonhemorrhagic stroke in the dabigatran group. Subgroup analyses showed a trend benefiting patients randomized within 5 days of MINS or with a diagnosis of MI, although it was not statistically significant.
Limitations. The efficacy outcomes were expanded to include venous thromboembolism and others not directly related to MINS, raising questions about the conclusions. Further, as defined by the protocol, bleeding had to be fairly severe to be deemed major. The high number of patients who discontinued the study drug is another limitation of this study.
Conclusion. Dabigatran lowered the risk of major vascular complications with no significant increase in major bleeding in patients with MINS.
What is the risk of thromboembolism in postoperative atrial fibrillation, and what are the benefits of anticoagulation?
Although nonvalvular atrial fibrillation is associated with increased risks of ischemic stroke and systemic embolic events in nonsurgical patients, the association of new-onset postoperative atrial fibrillation with long-term thromboembolic events in the noncardiac surgical population is not well established.
[Butt JH, Olesen JB, Havers-Borgersen E, et al. Risk of thromboembolism associated with atrial fibrillation following noncardiac surgery. J Am Coll Cardiol 2018; 72(17):2027–2036. doi:10.1016/j.jacc.2018.07.088]
In this retrospective cohort study using a nationwide registry in Denmark, Butt et al11 assessed the long-term risk of thromboembolic events in noncardiac surgical patients with new postoperative atrial fibrillation. Patients were identified who had no previous history of atrial fibrillation and developed it after noncardiac, nonobstetric surgeries, and were matched in a 1:4 ratio with patients who developed nonvalvular atrial fibrillation during nonsurgical hospitalizations. Matching was based on age, sex, heart failure, hypertension, diabetes, known history of thromboembolic events, ischemic heart disease, and the year patients presented with new atrial fibrillation.
Patients were excluded if they received antiarrhythmic drugs or oral anticoagulants before hospitalization or surgery, had cancer in the year prior, or died in the hospital.
The primary outcome of the study was thromboembolic events—a composite of ischemic stroke, transient cerebral ischemia, and peripheral arterial thrombosis or embolism. Secondary outcomes included rehospitalization for atrial fibrillation and all-cause mortality.
Findings. Overall, 0.4% of patients developed new postoperative atrial fibrillation, of whom 3,380 were matched with 15,320 patients with nonvalvular atrial fibrillation. Over a median follow-up of 3.2 years, the risk of thromboembolic events was similar in both groups (31.7 and 29.9 per 1,000 person-years, HR 0.95, 95% CI 0.85–1.07). The groups did not differ in their CHA2DS2-VASc risk scores, HAS-BLED risk scores, or year in which patients were diagnosed.
Anticoagulation lowered the risk of thromboembolic events to a similar extent in both groups compared with no anticoagulation:
- In postoperative atrial fibrillation—HR 0.57, 95% CI 0.40–0.67
- In nonvalvular atrial fibrillation—HR 0.56, 95% CI 0.51–0.62.
Despite the similar reduction in thromboembolic events, only 24.4% of the postoperative atrial fibrillation patients were started on anticoagulation therapy within 30 days of discharge, compared with 41.5% of those with nonvalvular atrial fibrillation.
Limitations. Although this was a large study with excellent follow-up data, it was observational. It may have underestimated the number of patients who developed postoperative atrial fibrillation because episodes that were judged not to be clinically significant may not have been charted. Many patients are not monitored with continuous telemetry postoperatively, which also may have led to underestimation of the number of atrial fibrillation events.
The study also did not examine the number of atrial fibrillation episodes per patient, the heart rhythm at discharge or long-term, or indication for and duration of anticoagulation. There were no data regarding international normalized ratio levels.
Conclusions. Postoperative atrial fibrillation is associated with outcomes similar to those of nonsurgical nonvalvular atrial fibrillation. Anticoagulation decreases the risks of stroke and death. However, substantially fewer patients with postoperative atrial fibrillation receive anticoagulation. Anticoagulation should be considered in these patients, while noting bleeding risk.
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol 2014; 64(22):e77–137. doi:10.1016/j.jacc.2014.07.944
- Kristensen SD, Knuuti J, Saraste A, et al. 2014 ESC/ESA Guidelines on non-cardiac surgery: cardiovascular assessment and management: the Joint Task Force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur Heart J 2014; 35(35):2383–2431. doi:10.1093/eurheartj/ehu282
- Hlatky MA, Boineau RE, Higginbotham MB, et al. A brief self-administered questionnaire to determine functional capacity (The Duke Activity Status Index). Am J Cardiol 1989; 64(10):651–654. doi:10.1016/0002-9149(89)90496-7
- Wijeysundera DN, Pearse RM, Sulman MA, et al. Assessment of functional capacity before major non-cardiac surgery: an international, prospective cohort study. Lancet 2018; 391(10140):2631–2640. doi:10.1016/S0140-6736(18)31131-0
- Devereaux PJ, Mrkobrada M, Sessler DI, et al; POISE-2 Investigators. Aspirin in patients undergoing noncardiac surgery. N Engl J Med 2014; 370(16):1494–1503. doi:10.1056/NEJMoa1401105
- Graham MM, Sessler DI, Parlow JL, et al. Aspirin in patients with previous percutaneous coronary intervention undergoing noncardiac surgery. Ann Intern Med 2018;168(4):237–244. pmid:29132159
- Biccard BM, Sigamani A, Chan MTV, et al. Effect of aspirin in vascular surgery in patients from a randomized clinical trial (POISE-2). Br J Surg 2018; 105(12):1591–1597. doi:10.1002/bjs.10925
- Shiffermiller JF, Monson BJ, Vokoun CW, et al. Prospective randomized evaluation of preoperative angiotensin-converting enzyme inhibition (PREOP-ACEI). J Hosp Med 2018; 13(10):661–667. doi:10.12788/jhm.3036
- Hollmann C, Fernandes NL, Biccard BM. A systematic review of outcomes associated with withholding or continuing angiotensin-converting enzyme inhibitors and angiotensin receptor blockers before noncardiac surgery. Anesth Analg 2018; 127(3):678–687. doi:10.1213/ANE.0000000000002837
- Devereaux PJ, Duceppe E, Guyatt G, et al. Dabigatran in patients with myocardial injury after non-cardiac surgery (MANAGE): an international, randomised, placebo-controlled trial. Lancet 2018; 391(10137):2325–2334. doi:10.1016/S0140-6736(18)30832-8
- Butt JH, Olesen JB, Havers-Borgersen E, et al. Risk of thromboembolism associated with atrial fibrillation following noncardiac surgery. J Am Coll Cardiol 2018; 72(17):2027–2036. doi:10.1016/j.jacc.2018.07.088
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol 2014; 64(22):e77–137. doi:10.1016/j.jacc.2014.07.944
- Kristensen SD, Knuuti J, Saraste A, et al. 2014 ESC/ESA Guidelines on non-cardiac surgery: cardiovascular assessment and management: the Joint Task Force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur Heart J 2014; 35(35):2383–2431. doi:10.1093/eurheartj/ehu282
- Hlatky MA, Boineau RE, Higginbotham MB, et al. A brief self-administered questionnaire to determine functional capacity (The Duke Activity Status Index). Am J Cardiol 1989; 64(10):651–654. doi:10.1016/0002-9149(89)90496-7
- Wijeysundera DN, Pearse RM, Sulman MA, et al. Assessment of functional capacity before major non-cardiac surgery: an international, prospective cohort study. Lancet 2018; 391(10140):2631–2640. doi:10.1016/S0140-6736(18)31131-0
- Devereaux PJ, Mrkobrada M, Sessler DI, et al; POISE-2 Investigators. Aspirin in patients undergoing noncardiac surgery. N Engl J Med 2014; 370(16):1494–1503. doi:10.1056/NEJMoa1401105
- Graham MM, Sessler DI, Parlow JL, et al. Aspirin in patients with previous percutaneous coronary intervention undergoing noncardiac surgery. Ann Intern Med 2018;168(4):237–244. pmid:29132159
- Biccard BM, Sigamani A, Chan MTV, et al. Effect of aspirin in vascular surgery in patients from a randomized clinical trial (POISE-2). Br J Surg 2018; 105(12):1591–1597. doi:10.1002/bjs.10925
- Shiffermiller JF, Monson BJ, Vokoun CW, et al. Prospective randomized evaluation of preoperative angiotensin-converting enzyme inhibition (PREOP-ACEI). J Hosp Med 2018; 13(10):661–667. doi:10.12788/jhm.3036
- Hollmann C, Fernandes NL, Biccard BM. A systematic review of outcomes associated with withholding or continuing angiotensin-converting enzyme inhibitors and angiotensin receptor blockers before noncardiac surgery. Anesth Analg 2018; 127(3):678–687. doi:10.1213/ANE.0000000000002837
- Devereaux PJ, Duceppe E, Guyatt G, et al. Dabigatran in patients with myocardial injury after non-cardiac surgery (MANAGE): an international, randomised, placebo-controlled trial. Lancet 2018; 391(10137):2325–2334. doi:10.1016/S0140-6736(18)30832-8
- Butt JH, Olesen JB, Havers-Borgersen E, et al. Risk of thromboembolism associated with atrial fibrillation following noncardiac surgery. J Am Coll Cardiol 2018; 72(17):2027–2036. doi:10.1016/j.jacc.2018.07.088
KEY POINTS
- The Duke Activity Status Index is a better tool for assessing cardiopulmonary fitness than subjective assessment, and it should be considered for use in guideline algorithms.
- Aspirin should not be given perioperatively in patients undergoing vascular surgery other than carotid endarterectomy.
- Angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs) are associated with intraoperative hypotension if given before surgery. Further study is needed to determined how best to manage ACE inhibitors and ARBs perioperatively.
- In a study, dabigatran given to patients with myocardial injury after noncardiac surgery lowered the risk of major vascular complications, with no significant increase in major bleeding. But the study had major limitations.
- Postoperative atrial fibrillation is associated with outcomes similar to those of nonsurgical nonvalvular atrial fibrillation. Anticoagulation decreases its stroke and mortality risk.
An overview of endoscopy in neurologic surgery
Over the last 3 decades, the endoscope has become a highly valued visualization tool in neurosurgery, applicable to a broad range of neurosurgical procedures. Following the pace of technological innovations, the quality of the instrumentation has greatly improved along with the status of endoscopy in the neurosurgical field. The use of the endoscope in interdisciplinary extended transnasal approaches revolutionized skull-base surgery.1 Transcranial neurosurgery took advantage of the endoscope for inspection, endoscope-assisted, and endoscope-controlled procedures, although the main visualization tool during these interventions remains the operating microscope.
At present, endoscopy has applications in a variety of neurosurgical procedures including transnasal approaches for pituitary and other skull-base tumors, third ventriculostomy, and resection of intraventricular tumors. The range of application is expanding to include extracranial procedures such as peripheral nerve and spine surgery.
optics and instruments are passed through a rigid, multiport chamber. This technique is ideal when performing surgery within the ventricular system using only a standard bur hole craniotomy.
CURRENT CONCEPTS
Hopf and Perneczky2 defined the terminology regarding endoscopic procedures and divided them into 3 categories:
Pure endoscopic neurosurgery, ie, procedures performed through working channels under complete endoscopic visualization and with endoscopic instrumentation (Figure 1).3
Endoscope-controlled microsurgery, ie, operations performed with standard microsurgical instruments under endoscopic visualization—the microscope is not used (Figure 2).
Endoscope-assisted neurosurgery, ie, the use of both microscope and endoscope during the same intervention. In endoscopic inspection the endoscope is solely used as an adjunctive tool for visualization and not for surgical manipulations.
Enhanced area and surgical dissection
Technical innovations are probably the major reason for the growing role of endoscopy in neurosurgery over the last 3 decades.4 High-definition imaging, neuronavigation, new instruments, an interdisciplinary approach mostly with ear, nose, and throat (ENT) surgeons, and detailed anatomic studies led to the breakthrough of endoscopic endonasal extended approaches in skull-base surgery.5
These endoscopic techniques allow the neurosurgeon to optimize tumor resection, increasing the area of surgical dissection without increasing the size of the surgical approach, thereby limiting perioperative morbidity due to surgical manipulation of eloquent brain structures. Endoscopy offers direct illumination of the operative field, magnification, and the ability to look around corners with angled optics.
However, while angled endoscopic optics provide various visual perspectives, the surgical issue is not only to see but also to work on and around remote structures. Microsurgical endoscope-assisted manipulations require optimal working angles that are guaranteed only by a sufficiently large craniotomy. As an example, a dissection study by Chaynes et al6 highlights that a craniotomy that is too narrow often hinders a sufficient exploration of the entire cerebellopontine angle. Most neurosurgeons are familiar with the operating microscope. The microscopic field of inspection is 3-dimensional (3D) and of high quality. However, the light stream is straight and thus limited in the narrow and angled corridor of the cerebellopontine angle or in the perimesencephalic cisterns. In these situations, the angled optic of the endoscope offers the advantage of being able to look around the corner with the appropriate amount of direct illumination.7
Peripheral nerve surgery
Minimally invasive endoscopic approaches are also being used in peripheral nerve surgery, especially carpal tunnel decompression. The first carpal tunnel release treated endoscopically was performed by Okutsu et al in the late 1980s.8 Since that time, endoscopic carpal tunnel decompression has become very common and is the preferred method for many surgeons, using either single-portal or dual-portal techniques. Although the superiority of endoscopic over conventional minimally invasive microsurgical peripheral nerve surgeries has not been proven, large series of endoscopic carpal tunnel decompressions have reported low complication rates and excellent success rates with high patient satisfaction scores.8,9
Visualization of the spinal canal
Expanding the use of the endoscope to spine surgery, endoscopic explorations of the interlaminar spaces after having completed open surgical laminectomies have been reported since the early 1980s,10 while endoscope-assisted interlaminar procedures started in the late 1990s.11–13 The development of fully endoscopic transforaminal or interlaminar approaches for lumbar stenosis or lumbar disk herniation has been ongoing in the last 2 decades. The rationale for direct endoscopic visualization of the spinal canal is to reduce scarring of the epidural space, which might affect the outcome of possible revision surgeries (recurrent disk herniation), and to reduce injury to the paraspinal muscles, which may reduce postoperative incisional pain and length of hospital stay. Major limiting factors for fully endoscopic spine surgeries such as the narrow working channels (which are limited by the osseous perimeter of the neuroforamina, as well as the pelvis and abdominal structures) and the learning curve for the surgeons are, however, still matters of debate and restrict the use of endoscopy to very carefully selected cases.14,15
Pediatric craniosynostosis
Recently, the use of the endoscope has extended to treatment of craniosynostosis in pediatric patients, historically treated with large and occasionally staged craniotomic approaches. A meta-analysis of the literature showed statistically significant reductions in blood loss and rates of perioperative complications, reoperation, and transfusion compared with open approaches.16
Technical limitations
While neurosurgeons increasingly advocate the use of the endoscope in their practice, the development of instruments for endoscopic surgery does not always follow the same pace. There are technical problems with current rigid endoscopes and ergonomic limitations of the endoscope-assisted techniques in transcranial neurosurgery. The endoscope itself occupies space in an already limited surgical corridor like the posterior fossa, the parasellar space, or the intraventricular region. The ideal endoscope is thin and sturdy, does not generate heat, and provides high-resolution images. In addition, a self-irrigating feature could minimize the need to remove and reinsert the endoscope for cleaning. Finally, most intracranial surgery is extremely delicate and requires bimanual dissection. The ideal endoscope should also be easily integrated with a holder that allows the surgeon to easily transition between static and dynamic endoscope movements.
Newer flexible fiberscopes with even smaller diameters are likely to be launched on the market in the near future. When working in a surgical corridor less than 10 mm wide, this difference could be substantial.
In addition, surgical instruments specifically designed for endoscopic endonasal procedures are needed for microdissection in these regions, which were previously only visible but not reachable endoscopically. These include tools such as malleable suctions and curettes, rotatable back-biting microscissors, and malleable bipolar instruments (Figure 3).
IMPACT OF NEUROENDOSCOPY IN CURRENT CLINICAL PRACTICE
The introduction of endoscopy in neurosurgery changed many treatment paradigms and had an important impact on morbidity and outcomes. In this section, we discuss the specific indications, contraindications, and expected benefit of endoscopic vs open surgical approaches applied to neurosurgical pathology at the present time.
Skull-base tumors and CSF leaks
The use of the endoscope in skull-base surgery was originally applied to purely midline intrasellar tumors without suprasellar or lateral extension beyond the carotid cave. Ideal cases were intrasellar pituitary microadenomas not responding to medical treatment or Rathke cleft cysts.
These pathologies were traditionally addressed via microscopic craniotomic approaches and later through sublabial or transnasal transsphenoidal approaches. Traditional transsphenoidal approaches were highly invasive for the oral mucosa, causing delayed healing, oral dysesthesia, and, in some cases, loss of the superior dental arch (sublabial) or limited visualization and surgical maneuverability (microscopic endonasal).
The endoscope offered better visualization and surgical freedom, thus allowing higher resection rates to be achieved. Resection of purely intrasellar pathology with preservation of the diaphragma sellae as a barrier to the subarachnoid cysterns and third ventricle guaranteed a lower incidence of cerebrospinal fluid (CSF) leaks.
New endoscope optics with varied angles, together with dedicated long surgical instruments with low steric volume, offered a large variety of new endonasal surgical corridors, so-called expanded endonasal approaches on the sagittal and coronal planes, as discussed in detail by Kassam et al.17–19 These allowed endoscopic treatment of invasive tumors extending on the coronary plane into the suprasellar region or invading the cavernous sinuses (pituitary macroadenomas, craniopharyngiomas).
Highly specialized centers with expertise in endoscopic skull-base surgery can now also offer pure endoscopic treatment for some selected cases of lesions located far laterally to the cavernous sinus, such as trigeminal schwannomas, or along the sagittal plane like olfactory groove or tuberculum sellae meningiomas and clival lesions (chordomas, chondrosarcomas).
As one might expect, the increase in surgical complexity corresponded to an increase in complication rates. For example, the incidence of CSF leaks varied from 5% for standard midline transsphenoidal approaches to 11% for expanded endonasal approaches.20,21 The consolidation of the use of the endoscope and the cooperation with ENT surgeons led to the development of surgical strategies to prevent and reduce the incidence of CSF leaks, such as the use of “rescue flaps,” nasoseptal flaps, or temporoparietal fascia flaps.21–23
The development of such techniques allowed endoscopic endonasal approaches to be used in treatment of other pathologies, such as spontaneous CSF leaks, treated in the past with large transcranial repairs that carried high morbidity rates due to the surgical frontal lobe retraction and injury to the olfactory mucosa.24,25 Progress in the field of neuroendoscopy therefore led to the creation of specialized endoscopic skull-base surgery centers, including neurosurgery, ENT, ophthalmology, and endocrinology services.
In clinical practice, when evaluating a patient with intracranial skull-base pathology amenable to endoscopic resection, one should consider referring the patient not only to a neurosurgeon, but also to an ENT surgeon for preoperative assessment of the sinonasal cavities. The same concept applies to postsurgical follow-up, which is mostly performed by the ENT physician to assess nasal mucosa healing and nasal hygiene.
Ventricular neuroendoscopy
The introduction of endoscopic third ventriculostomy created the opportunity to offer a more physiologic treatment in selected patients with obstructive hydrocephalus by creating an internal CSF diversion through the basal cisterns. Two advantages of this procedure are that it does not create dependence on a CSF shunt, and it eliminates the related risks of shunt infection and malfunction. Its drawback is the recurrence rate of hydrocephalus (around 58% at 2 years of follow-up) due to formation of scarring in the perforated Liliequist membrane, which may require repeat surgery or conversion to CSF shunting.26,27
Neuroendoscopic approaches are also used in cases of purely intraventricular pathology such as colloid cyst or choroid plexus papillomas. The concept behind neuroendoscopy is to achieve maximal resection in a minimally invasive way, using the natural cavity of the cerebral ventricles and reducing the need for brain retraction and, in particular, the risk of injury of the fornix (therefore causing memory deficits) of open transventricular approaches and of the corpus callosum necessary in interhemispheric approaches. Large tumor size and inability to tolerate a longer surgical procedure can be relative contraindications to a pure endoscopic approach to these lesions.
Degenerative spine disease
In recent years there has been a growing interest in the use of endoscopy for selected cases of degenerative lumbar spondylosis (generally, lateral disk herniation above the L5-S1 level or spinal canal stenosis). This approach has been shown to reduce postoperative incisional pain, scarring of the epidural space affecting the outcome of possible revision surgeries (recurrent disc herniation), and length of hospital stay.14,15 Information on surgical nuances should be provided when consulting on selected patients with lumbar degenerative disease resistant to conservative treatment.
Carpal tunnel syndrome
Although endoscopic carpal tunnel release is controversial, its supporters report smaller incision size and lower recurrence rates due to better visualization of the entire carpal ligament compared with open surgery, with high patient satisfaction scores.8,9,28
Craniosynostosis
Increasing data from specialized centers show that early endoscopic suturectomy is an effective treatment option alone or when combined with open surgeries for patients with syndromic and nonsyndromic craniosynostosis. The aesthetic advantage of small incisions (which can also be achieved with some open techniques) is accompanied by significant reductions in blood loss (median 162.4 mL), operative time (median 112.38 minutes), length of stay (median 2.56 days), and rates of perioperative complications (odds ratio 0.58), reoperation (odds ratio 0.37), and transfusion (odds ratio 0.09) compared with open approaches.16
SURGICAL TRAINING
Today’s patients expect high-quality healthcare, and they approach their surgeons with an enormous amount of information collected through unlimited Web-based access or peer-group blogs. In this respect, the pressure on young surgeons to achieve excellent results is high and growing from the very beginning of their careers.
Residency training programs differ in each country, and surgical standards usually focus on open microscopic procedures rather than newly developed endoscopic techniques. Endoscopic pituitary adenoma surgery, the most frequent neuroendoscopic procedure, is still performed mostly by experienced neurosurgeons, not trainees. Moreover, many training institutions might not offer pediatric neurosurgery care, limiting exposure to endoscopic third ventriculostomy procedures. The European Union of Medical Specialists, responsible for harmonizing and improving the quality of training of medical specialists in Europe, set low neuroendoscopic surgical requirements for trainees to complete their residency programs (minimum of 0 to optimum of 5 total transcranial or transsphenoidal pituitary adenoma resections as first operator, 10 procedures as assistant, and a minimum of 2 to an optimum of 4 endoscopic third ventriculostomies as first operator).29
The need to develop training programs in neuroendoscopy is especially urgent because endoscopic surgery has a steeper learning curve than conventional microneurosurgery. In particular, endoscopy requires a good deal of dexterity and hand-eye coordination, which surgeons consider the main pitfall of neuroendoscopy. For such reasons, many accredited clinical fellowship programs have been developed inside and outside North America that offer intensive training in endoscopic skull-base surgery and pediatric neurosurgery after residency.
Some clinical studies have shown that the complication rate of neuroendoscopy is 15% to 18%.27,30 In view of this statistic, it is ethically questionable to perform a randomized study to prospectively compare microscopic and endoscopic procedures. Surgeons specialize in one technique or the other, experience their own learning curve, and do not randomly decide which tool to use. Furthermore, every intracranial surgical exploration is unique and somewhat difficult to compare with each other without the risk of bias.
FURTHER DEVELOPMENTS
Multivariable rigid endoscopes like the EndoCAMeleon (Karl Storz, Tuttlingen, Germany) or the EndActive (Karl Storz, Tuttlingen, Germany) for cerebellopontine angle surgery represent a starting point to overcome some of the aforementioned limitations.31,32 They are inserted in the surgical field with a direct 0° angulation view into the operative site beyond neurovascular structures that need to be preserved and that obstruct the microscopic view. Once the final position is reached, the field of view is directed toward the region of interest without moving the endoscope tip.
The EndoCAMeleon is a rigid rod-lens endoscope, steerable in one plane from –10° to +120° by a fine optomechanical mechanism. Anatomic laboratory testing found it to be superior in terms of usability and visualization compared with rigid fixed-angle endoscopes.31 The first clinical experiences have been promising; however, ergonomics and the limited perspective of a single plane of rotation leave room for improvement.
The EndActive endoscope might overcome such limitations.33 This device is a rigid videoendoscope connected to a laptop (video data) and USB port (control and power supply); thus, it weighs less and can be held in one hand like a microsurgical instrument. The endoscopic imaging system allows the operator to simultaneously see a 160° wide-angle view of the site and an inset of a specific region of interest. The surgeon can hold the device like a microsurgical instrument in one hand and control movements precisely due to its reduced weight and ergonomic shape.
The multiplanar variable-view rigid endoscope has proven to be useful for working on diverse anatomic structures such as intracranial vessels and cranial nerves. The device is effective in narrow working spaces where even small movements can jeopardize the delicate surrounding structures. The multiplanar variable-viewing mechanism in a compact device offers advantages in terms of safety and ergonomics. Improving the usability will probably optimize the applicability of those endoscopic devices in neurosurgery. A major drawback of the current prototype is poor image resolution, which will probably soon be overcome with the ongoing progress in electronic microchip technology.
The addition of laser technology to endoscopic techniques offers a huge potential to neurosurgery but has achieved little acceptance to date. The reasons include concern regarding heat production, uncontrollable and distant penetration, and tissue interaction. Experiences with a 2-micron continuous- wave laser (RevoLix Jr, LISA Laser Products, Katlenburg-Lindau, Germany) for neuroendoscopic intraventricular procedures proved this laser to be a valuable and useful tool with safe applicability for endoscopic intracranial procedures in patients of all ages.34
Parallel to the launch of video screens for other uses with higher image definition, the image quality on the 2D endoscope cameras has been constantly improving over the last years. At the same time, the introduction of modern 3D endoscopic monitors is promising. However, 3D endoscopes have some disadvantages compared with the 2D endoscopes. First, the smallest 3D endoscopes are 4 mm in diameter, compared with 2.7 mm for 2D endoscopes. Moreover, the field of view with the 3D endoscope is less than half of that with conventional 2D endoscopes.34 When working in and around a region with critical neurovascular structures in close proximity, this loss of field of view can result in an increase in iatrogenic injury from the endoscope. In addition, 3D endoscopes require special glasses, generating a potential obstacle to the seamless integration of visual information from the microscope and endoscope. Finally, some surgeons experience vertigo when looking at the 3D picture through the glasses, which limits its universal applicability.
CONCLUSIONS
Using the endoscope and microscope as complementary and not competing tools allows surgeons to benefit from both technologies at the same time.35,36 The intraoperative combination of these 2 powerful visualization tools expands the effectiveness of microsurgical procedures and has the potential to further improve surgical results and reduce surgical risks. With endoscope-assisted microsurgery, visualization is often far superior to surgical maneuverability.
Endoscopic neurosurgery will likely be influenced by further innovations in optical physics, electronics, and robotics. Specific implementations in endoscopic systems are likely to pave the way for remarkable progress in minimally invasive surgery, such as robotic surgical technology, further miniaturization of devices, improvements in 3D endoscopy, multiport endoscopy, and new designs for surgical instruments. Future progress in flexible endoscopes and wireless capsule or camera technology may reduce our dependence on rigid rod lens systems. Rigid variable-view endoscopes will bring endoscopes closer to ideal attributes utilizing newer instrumentation that is tailored to specific indications and techniques.37,38 Extension of the visual field by the feature of a movable optic lens may allow the neurosurgeon to use tailored keyhole approaches to treat pathologies in smaller surgical corridors with less trauma and greater efficacy.
- Kassam AB, Gardner P, Snyderman C, Mintz A, Carrau R. Expanded endonasal approach: fully endoscopic, completely transnasal approach to the middle third of the clivus, petrous bone, middle cranial fossa, and infratemporal fossa. Neurosurg Focus 2005; 19(1):E6. pmid:16078820
- Hopf NJ, Perneczky A. Endoscopic neurosurgery and endoscope-assisted microneurosurgery for the treatment of intracranial cysts. Neurosurgery 1998; 43(6):1330–1336. doi:10.1097/00006123-199812000-00037
- Li KW, Nelson C, Suk I, Jallo GI. Neuroendoscopy: past, present, and future. Neurosurg Focus 2005; 19(6):E1. doi:10.3171/foc.2005.19.6.2
- Prevedello DM, Doglietto F, Jane JA Jr, Jagannathan J, Han J, Laws ER Jr. History of endoscopic skull base surgery: its evolution and current reality. J Neurosurg 2007; 107(1):206–213. doi:10.3171/JNS-07/07/0206
- Schroeder HW, Nehlsen M. Value of high-definition imaging in neuroendoscopy. Neurosurg Rev 2009; 32(3):303–308. doi:10.1007/s10143-009-0200-x
- Chaynes P, Deguine O, Moscovici J, Fraysse B, Becue J, Lazorthes Y. Endoscopic anatomy of the cerebellopontine angle: a study in cadaver brains. Neurosurg Focus 1998; 5(3):e8.
- Setty P, Volkov AA, D'Andrea KP, Pieper DR. Endoscopic vascular decompression for the treatment of trigeminal neuralgia: clinical outcomes and technical note. World Neurosurg 2014; 81(3–4):603–608. doi:10.1016/j.wneu.2013.10.036
- Okutsu I, Hamanaka I, Yoshida A. Retrospective analysis of five-year and longer clinical and electrophysiological results of the world's first endoscopic management for carpal tunnel syndrome. Hand Surg 2013; 18(3):317–323. doi:10.1142/S0218810413500330
- Zuo D, Zhou Z, Wang H, et al. Endoscopic versus open carpal tunnel release for idiopathic carpal tunnel syndrome: a meta-analysis of randomized controlled trials. J Orthop Surg Res 2015; 10:12. doi:10.1186/s13018-014-0148-6
- Forst R, Hausmann B. Nucleoscopy—a new examination technique. Arch Orthop Trauma Surg 1983; 101(3):219–221. pmid:6870510
- Brayda-Bruno M, Cinnella P. Posterior endoscopic discectomy (and other procedures). Eur Spine J 2000; 9(suppl 1):S24–S29. pmid:10766054
- Destandau J. A special device for endoscopic surgery of lumbar disc herniation. Neurol Res 1999; 21(1):39–42. pmid:10048052
- Perez-Cruet MJ, Foley KT, Isaacs RE, et al. Microendoscopic lumbar discectomy: technical note. Neurosurgery 2002; 51(5 suppl):S129–S136. pmid:12234440
- Ruetten S, Komp M, Merk H, Godolias G. Full-endoscopic interlaminar and transforaminal lumbar discectomy versus conventional microsurgical technique: a prospective, randomized, controlled study. Spine (Phila Pa 1976) 2008; 33(9):931–939. doi:10.1097/BRS.0b013e31816c8af7
- Komp M, Hahn P, Merk H, Godolias G, Ruetten S. Bilateral operation of lumbar degenerative central spinal stenosis in full-endoscopic interlaminar technique with unilateral approach: prospective 2-year results of 74 patients. J Spinal Disord Tech 2011; 24(5):281–287. doi:10.1097/BSD.0b013e3181f9f55e
- Goyal A, Lu VM, Yolcu YU, Elminawy M, Daniels DJ. Endoscopic versus open approach in craniosynostosis repair: a systematic review and meta-analysis of perioperative outcomes. Childs Nerv Syst 2018; 34(9):1627–1637. doi:10.1007/s00381-018-3852-4
- Kassam AB, Gardner P, Snyderman C, Mintz A, Carrau R. Expanded endonasal approach: fully endoscopic, completely transnasal approach to the middle third of the clivus, petrous bone, middle cranial fossa, and infratemporal fossa. Neurosurg Focus 2005; 19(1):E6. pmid:16078820
- Kassam A, Snyderman CH, Mintz A, Gardner P, Carrau RL. Expanded endonasal approach: the rostrocaudal axis. Part II. Posterior clinoids to the foramen magnum. Neurosurg Focus 2005; 19(1):E4. pmid:16078818
- Kassam A, Snyderman CH, Mintz A, Gardner P, Carrau RL. Expanded endonasal approach: the rostrocaudal axis. Part I. Crista galli to the sella turcica. Neurosurg Focus 2005; 19(1):E3. pmid:16078817
- Kassam A, Carrau RL, Snyderman CH, Gardner P, Mintz A. Evolution of reconstructive techniques following endoscopic expanded endonasal approaches. Neurosurg Focus 2005; 19(1):E8. pmid:16078822
- Kassam AB, Thomas A, Carrau RL, et al. Endoscopic reconstruction of the cranial base using a pedicled nasoseptal flap. Neurosurgery 2008; 63(1 suppl 1):ONS44–ONS52. doi:10.1227/01.NEU.0000297074.13423.F5
- Hadad G, Bassagasteguy L, Carrau RL, et al. A novel reconstructive technique after endoscopic expanded endonasal approaches: vascular pedicle nasoseptal flap. Laryngoscope 2006; 116(10):1882–1886. doi:10.1097/01.mlg.0000234933.37779.e4
- Fortes FS, Carrau RL, Snyderman CH, et al. Transpterygoid transposition of a temporoparietal fascia flap: a new method for skull base reconstruction after endoscopic expanded endonasal approaches. Laryngoscope 2007; 117(6):970–976. doi:10.1097/MLG.0b013e3180471482
- Carrau RL, Snyderman CH, Kassam AB. The management of cerebrospinal fluid leaks in patients at risk for high-pressure hydrocephalus. Laryngoscope 2005; 115(2):205–212. doi:10.1097/01.mlg.0000154719.62668.70
- Zweig JL, Carrau RL, Celin SE, et al. Endoscopic repair of cerebrospinal fluid leaks to the sinonasal tract: predictors of success. Otolaryngol Head Neck Surg 2000; 123(3):195–201. doi:10.1067/mhn.2000.107452
- Kulkarni AV, Riva-Cambrin J, Holubkov R, et al. Endoscopic third ventriculostomy in children: prospective, multicenter results from the Hydrocephalus Clinical Research Network. J Neurosurg Pediatr 2016; 18(4):423–429. doi:10.3171/2016.4.PEDS163
- Ersahin Y, Arslan D. Complications of endoscopic third ventriculostomy. Childs Nerv Syst 2008; 24(8):943–948. doi:10.1007/s00381-008-0589-5
- Martínez-Catasús A, Lobo-Escolar L, García-Bonet J, Corrales-Rodríguez M, Pasarín-Martínez A, Berlanga-de-Mingo D. Comparison between single portal endoscopic, 1-cm open carpal tunnel release. Hand Surg Rehabil 2019. pii:S2468-1229(19)30027-1. doi:10.1016/j.hansur.2019.02.003
- Steers J, Reulen HJ, Lindsay K; European Union of Medical Specialists; Joint Residency Advisory and Accreditation Committee. UEMS charter on training of medical specialists in the EU—the new neurosurgical training charter. Acta Neurochir Suppl 2004; 90:3–11. pmid:15553111
- Mori H, Nishiyama K, Yoshimura J, Tanaka R. Current status of neuroendoscopic surgery in Japan and discussion on the training system. Childs Nerv Syst 2007; 23(6):673–676. doi:10.1007/s00381-007-0329-2
- Aryan HE, Hoeg HD, Marshall LF, Levy ML. Multidirectional projectional rigid neuro-endoscopy: prototype and initial experience. Minim Invasive Neurosurg 2005; 48(5):293–296. doi:10.1055/s-2005-915602
- Ebner FH, Marquardt JS, Hirt B, Tatagiba M, Schuhmann MU. Visualization of the anterior cerebral artery complex with a continuously variable-view rigid endoscope: new options in aneurysm surgery. Neurosurgery 2010; 67(2 suppl operative):321–324. doi:10.1227/NEU.0b013e3181f74548
- Ebner FH, Hirt B, Marquardt JS, Herlan S, Tatagiba M, Schuhmann MU. Actual state of EndActive ventricular endoscopy. Childs Nerv Syst 2012; 28(1):87–91. doi:10.1007/s00381-011-1537-3
- Ebner FH, Nagel C, Tatagiba M, Schuhmann MU. Efficacy and versatility of the 2-micron continuous wave laser in neuroendoscopic procedures. Acta Neurochir Suppl 2012; 113:143–147. doi:10.1007/978-3-7091-0923-6_29
- Van Gompel JJ, Tabor MH, Youssef AS, et al. Field of view comparison between two-dimensional and three-dimensional endoscopy. Laryngoscope 2014; 124(2):387–390. doi:10.1002/lary.24222
- Ebner FH, Roser F, Thaher F, Schittenhelm J, Tatagiba M. Balancing the shortcomings of microscope and endoscope: endoscope-assisted technique in microsurgical removal of recurrent epidermoid cysts in the posterior fossa. Minim Invasive Neurosurg 2010 ;53(5–6):218–222. doi:10.1055/s-0030-1267973
- Perneczky A, Fries G. Endoscope-assisted brain surgery: part 1—evolution, basic concept, and current technique. Neurosurgery 1998; 42(2):219–224. doi:10.1097/00006123-199802000-00001
- Ebner FH, Marquardt JS, Hirt B, Feigl GC, Tatagiba M, Schuhmann MU. Broadening horizons of neuroendoscopy with a variable-view rigid endoscope: an anatomical study. Eur J Surg Oncol 2010; 36(2):195–200. doi:10.1016/j.ejso.2009.07.185
meningioma, peripheral nerve, spinal canal, minimally invasive, carpal tunnel, ventricular neuroendoscopy, craniosynostosis, degenerative spine disease, Luigi Rigante, Hamid Borghei-Razavi, Pablo Recinos, Florian Roser
Over the last 3 decades, the endoscope has become a highly valued visualization tool in neurosurgery, applicable to a broad range of neurosurgical procedures. Following the pace of technological innovations, the quality of the instrumentation has greatly improved along with the status of endoscopy in the neurosurgical field. The use of the endoscope in interdisciplinary extended transnasal approaches revolutionized skull-base surgery.1 Transcranial neurosurgery took advantage of the endoscope for inspection, endoscope-assisted, and endoscope-controlled procedures, although the main visualization tool during these interventions remains the operating microscope.
At present, endoscopy has applications in a variety of neurosurgical procedures including transnasal approaches for pituitary and other skull-base tumors, third ventriculostomy, and resection of intraventricular tumors. The range of application is expanding to include extracranial procedures such as peripheral nerve and spine surgery.
optics and instruments are passed through a rigid, multiport chamber. This technique is ideal when performing surgery within the ventricular system using only a standard bur hole craniotomy.
CURRENT CONCEPTS
Hopf and Perneczky2 defined the terminology regarding endoscopic procedures and divided them into 3 categories:
Pure endoscopic neurosurgery, ie, procedures performed through working channels under complete endoscopic visualization and with endoscopic instrumentation (Figure 1).3
Endoscope-controlled microsurgery, ie, operations performed with standard microsurgical instruments under endoscopic visualization—the microscope is not used (Figure 2).
Endoscope-assisted neurosurgery, ie, the use of both microscope and endoscope during the same intervention. In endoscopic inspection the endoscope is solely used as an adjunctive tool for visualization and not for surgical manipulations.
Enhanced area and surgical dissection
Technical innovations are probably the major reason for the growing role of endoscopy in neurosurgery over the last 3 decades.4 High-definition imaging, neuronavigation, new instruments, an interdisciplinary approach mostly with ear, nose, and throat (ENT) surgeons, and detailed anatomic studies led to the breakthrough of endoscopic endonasal extended approaches in skull-base surgery.5
These endoscopic techniques allow the neurosurgeon to optimize tumor resection, increasing the area of surgical dissection without increasing the size of the surgical approach, thereby limiting perioperative morbidity due to surgical manipulation of eloquent brain structures. Endoscopy offers direct illumination of the operative field, magnification, and the ability to look around corners with angled optics.
However, while angled endoscopic optics provide various visual perspectives, the surgical issue is not only to see but also to work on and around remote structures. Microsurgical endoscope-assisted manipulations require optimal working angles that are guaranteed only by a sufficiently large craniotomy. As an example, a dissection study by Chaynes et al6 highlights that a craniotomy that is too narrow often hinders a sufficient exploration of the entire cerebellopontine angle. Most neurosurgeons are familiar with the operating microscope. The microscopic field of inspection is 3-dimensional (3D) and of high quality. However, the light stream is straight and thus limited in the narrow and angled corridor of the cerebellopontine angle or in the perimesencephalic cisterns. In these situations, the angled optic of the endoscope offers the advantage of being able to look around the corner with the appropriate amount of direct illumination.7
Peripheral nerve surgery
Minimally invasive endoscopic approaches are also being used in peripheral nerve surgery, especially carpal tunnel decompression. The first carpal tunnel release treated endoscopically was performed by Okutsu et al in the late 1980s.8 Since that time, endoscopic carpal tunnel decompression has become very common and is the preferred method for many surgeons, using either single-portal or dual-portal techniques. Although the superiority of endoscopic over conventional minimally invasive microsurgical peripheral nerve surgeries has not been proven, large series of endoscopic carpal tunnel decompressions have reported low complication rates and excellent success rates with high patient satisfaction scores.8,9
Visualization of the spinal canal
Expanding the use of the endoscope to spine surgery, endoscopic explorations of the interlaminar spaces after having completed open surgical laminectomies have been reported since the early 1980s,10 while endoscope-assisted interlaminar procedures started in the late 1990s.11–13 The development of fully endoscopic transforaminal or interlaminar approaches for lumbar stenosis or lumbar disk herniation has been ongoing in the last 2 decades. The rationale for direct endoscopic visualization of the spinal canal is to reduce scarring of the epidural space, which might affect the outcome of possible revision surgeries (recurrent disk herniation), and to reduce injury to the paraspinal muscles, which may reduce postoperative incisional pain and length of hospital stay. Major limiting factors for fully endoscopic spine surgeries such as the narrow working channels (which are limited by the osseous perimeter of the neuroforamina, as well as the pelvis and abdominal structures) and the learning curve for the surgeons are, however, still matters of debate and restrict the use of endoscopy to very carefully selected cases.14,15
Pediatric craniosynostosis
Recently, the use of the endoscope has extended to treatment of craniosynostosis in pediatric patients, historically treated with large and occasionally staged craniotomic approaches. A meta-analysis of the literature showed statistically significant reductions in blood loss and rates of perioperative complications, reoperation, and transfusion compared with open approaches.16
Technical limitations
While neurosurgeons increasingly advocate the use of the endoscope in their practice, the development of instruments for endoscopic surgery does not always follow the same pace. There are technical problems with current rigid endoscopes and ergonomic limitations of the endoscope-assisted techniques in transcranial neurosurgery. The endoscope itself occupies space in an already limited surgical corridor like the posterior fossa, the parasellar space, or the intraventricular region. The ideal endoscope is thin and sturdy, does not generate heat, and provides high-resolution images. In addition, a self-irrigating feature could minimize the need to remove and reinsert the endoscope for cleaning. Finally, most intracranial surgery is extremely delicate and requires bimanual dissection. The ideal endoscope should also be easily integrated with a holder that allows the surgeon to easily transition between static and dynamic endoscope movements.
Newer flexible fiberscopes with even smaller diameters are likely to be launched on the market in the near future. When working in a surgical corridor less than 10 mm wide, this difference could be substantial.
In addition, surgical instruments specifically designed for endoscopic endonasal procedures are needed for microdissection in these regions, which were previously only visible but not reachable endoscopically. These include tools such as malleable suctions and curettes, rotatable back-biting microscissors, and malleable bipolar instruments (Figure 3).
IMPACT OF NEUROENDOSCOPY IN CURRENT CLINICAL PRACTICE
The introduction of endoscopy in neurosurgery changed many treatment paradigms and had an important impact on morbidity and outcomes. In this section, we discuss the specific indications, contraindications, and expected benefit of endoscopic vs open surgical approaches applied to neurosurgical pathology at the present time.
Skull-base tumors and CSF leaks
The use of the endoscope in skull-base surgery was originally applied to purely midline intrasellar tumors without suprasellar or lateral extension beyond the carotid cave. Ideal cases were intrasellar pituitary microadenomas not responding to medical treatment or Rathke cleft cysts.
These pathologies were traditionally addressed via microscopic craniotomic approaches and later through sublabial or transnasal transsphenoidal approaches. Traditional transsphenoidal approaches were highly invasive for the oral mucosa, causing delayed healing, oral dysesthesia, and, in some cases, loss of the superior dental arch (sublabial) or limited visualization and surgical maneuverability (microscopic endonasal).
The endoscope offered better visualization and surgical freedom, thus allowing higher resection rates to be achieved. Resection of purely intrasellar pathology with preservation of the diaphragma sellae as a barrier to the subarachnoid cysterns and third ventricle guaranteed a lower incidence of cerebrospinal fluid (CSF) leaks.
New endoscope optics with varied angles, together with dedicated long surgical instruments with low steric volume, offered a large variety of new endonasal surgical corridors, so-called expanded endonasal approaches on the sagittal and coronal planes, as discussed in detail by Kassam et al.17–19 These allowed endoscopic treatment of invasive tumors extending on the coronary plane into the suprasellar region or invading the cavernous sinuses (pituitary macroadenomas, craniopharyngiomas).
Highly specialized centers with expertise in endoscopic skull-base surgery can now also offer pure endoscopic treatment for some selected cases of lesions located far laterally to the cavernous sinus, such as trigeminal schwannomas, or along the sagittal plane like olfactory groove or tuberculum sellae meningiomas and clival lesions (chordomas, chondrosarcomas).
As one might expect, the increase in surgical complexity corresponded to an increase in complication rates. For example, the incidence of CSF leaks varied from 5% for standard midline transsphenoidal approaches to 11% for expanded endonasal approaches.20,21 The consolidation of the use of the endoscope and the cooperation with ENT surgeons led to the development of surgical strategies to prevent and reduce the incidence of CSF leaks, such as the use of “rescue flaps,” nasoseptal flaps, or temporoparietal fascia flaps.21–23
The development of such techniques allowed endoscopic endonasal approaches to be used in treatment of other pathologies, such as spontaneous CSF leaks, treated in the past with large transcranial repairs that carried high morbidity rates due to the surgical frontal lobe retraction and injury to the olfactory mucosa.24,25 Progress in the field of neuroendoscopy therefore led to the creation of specialized endoscopic skull-base surgery centers, including neurosurgery, ENT, ophthalmology, and endocrinology services.
In clinical practice, when evaluating a patient with intracranial skull-base pathology amenable to endoscopic resection, one should consider referring the patient not only to a neurosurgeon, but also to an ENT surgeon for preoperative assessment of the sinonasal cavities. The same concept applies to postsurgical follow-up, which is mostly performed by the ENT physician to assess nasal mucosa healing and nasal hygiene.
Ventricular neuroendoscopy
The introduction of endoscopic third ventriculostomy created the opportunity to offer a more physiologic treatment in selected patients with obstructive hydrocephalus by creating an internal CSF diversion through the basal cisterns. Two advantages of this procedure are that it does not create dependence on a CSF shunt, and it eliminates the related risks of shunt infection and malfunction. Its drawback is the recurrence rate of hydrocephalus (around 58% at 2 years of follow-up) due to formation of scarring in the perforated Liliequist membrane, which may require repeat surgery or conversion to CSF shunting.26,27
Neuroendoscopic approaches are also used in cases of purely intraventricular pathology such as colloid cyst or choroid plexus papillomas. The concept behind neuroendoscopy is to achieve maximal resection in a minimally invasive way, using the natural cavity of the cerebral ventricles and reducing the need for brain retraction and, in particular, the risk of injury of the fornix (therefore causing memory deficits) of open transventricular approaches and of the corpus callosum necessary in interhemispheric approaches. Large tumor size and inability to tolerate a longer surgical procedure can be relative contraindications to a pure endoscopic approach to these lesions.
Degenerative spine disease
In recent years there has been a growing interest in the use of endoscopy for selected cases of degenerative lumbar spondylosis (generally, lateral disk herniation above the L5-S1 level or spinal canal stenosis). This approach has been shown to reduce postoperative incisional pain, scarring of the epidural space affecting the outcome of possible revision surgeries (recurrent disc herniation), and length of hospital stay.14,15 Information on surgical nuances should be provided when consulting on selected patients with lumbar degenerative disease resistant to conservative treatment.
Carpal tunnel syndrome
Although endoscopic carpal tunnel release is controversial, its supporters report smaller incision size and lower recurrence rates due to better visualization of the entire carpal ligament compared with open surgery, with high patient satisfaction scores.8,9,28
Craniosynostosis
Increasing data from specialized centers show that early endoscopic suturectomy is an effective treatment option alone or when combined with open surgeries for patients with syndromic and nonsyndromic craniosynostosis. The aesthetic advantage of small incisions (which can also be achieved with some open techniques) is accompanied by significant reductions in blood loss (median 162.4 mL), operative time (median 112.38 minutes), length of stay (median 2.56 days), and rates of perioperative complications (odds ratio 0.58), reoperation (odds ratio 0.37), and transfusion (odds ratio 0.09) compared with open approaches.16
SURGICAL TRAINING
Today’s patients expect high-quality healthcare, and they approach their surgeons with an enormous amount of information collected through unlimited Web-based access or peer-group blogs. In this respect, the pressure on young surgeons to achieve excellent results is high and growing from the very beginning of their careers.
Residency training programs differ in each country, and surgical standards usually focus on open microscopic procedures rather than newly developed endoscopic techniques. Endoscopic pituitary adenoma surgery, the most frequent neuroendoscopic procedure, is still performed mostly by experienced neurosurgeons, not trainees. Moreover, many training institutions might not offer pediatric neurosurgery care, limiting exposure to endoscopic third ventriculostomy procedures. The European Union of Medical Specialists, responsible for harmonizing and improving the quality of training of medical specialists in Europe, set low neuroendoscopic surgical requirements for trainees to complete their residency programs (minimum of 0 to optimum of 5 total transcranial or transsphenoidal pituitary adenoma resections as first operator, 10 procedures as assistant, and a minimum of 2 to an optimum of 4 endoscopic third ventriculostomies as first operator).29
The need to develop training programs in neuroendoscopy is especially urgent because endoscopic surgery has a steeper learning curve than conventional microneurosurgery. In particular, endoscopy requires a good deal of dexterity and hand-eye coordination, which surgeons consider the main pitfall of neuroendoscopy. For such reasons, many accredited clinical fellowship programs have been developed inside and outside North America that offer intensive training in endoscopic skull-base surgery and pediatric neurosurgery after residency.
Some clinical studies have shown that the complication rate of neuroendoscopy is 15% to 18%.27,30 In view of this statistic, it is ethically questionable to perform a randomized study to prospectively compare microscopic and endoscopic procedures. Surgeons specialize in one technique or the other, experience their own learning curve, and do not randomly decide which tool to use. Furthermore, every intracranial surgical exploration is unique and somewhat difficult to compare with each other without the risk of bias.
FURTHER DEVELOPMENTS
Multivariable rigid endoscopes like the EndoCAMeleon (Karl Storz, Tuttlingen, Germany) or the EndActive (Karl Storz, Tuttlingen, Germany) for cerebellopontine angle surgery represent a starting point to overcome some of the aforementioned limitations.31,32 They are inserted in the surgical field with a direct 0° angulation view into the operative site beyond neurovascular structures that need to be preserved and that obstruct the microscopic view. Once the final position is reached, the field of view is directed toward the region of interest without moving the endoscope tip.
The EndoCAMeleon is a rigid rod-lens endoscope, steerable in one plane from –10° to +120° by a fine optomechanical mechanism. Anatomic laboratory testing found it to be superior in terms of usability and visualization compared with rigid fixed-angle endoscopes.31 The first clinical experiences have been promising; however, ergonomics and the limited perspective of a single plane of rotation leave room for improvement.
The EndActive endoscope might overcome such limitations.33 This device is a rigid videoendoscope connected to a laptop (video data) and USB port (control and power supply); thus, it weighs less and can be held in one hand like a microsurgical instrument. The endoscopic imaging system allows the operator to simultaneously see a 160° wide-angle view of the site and an inset of a specific region of interest. The surgeon can hold the device like a microsurgical instrument in one hand and control movements precisely due to its reduced weight and ergonomic shape.
The multiplanar variable-view rigid endoscope has proven to be useful for working on diverse anatomic structures such as intracranial vessels and cranial nerves. The device is effective in narrow working spaces where even small movements can jeopardize the delicate surrounding structures. The multiplanar variable-viewing mechanism in a compact device offers advantages in terms of safety and ergonomics. Improving the usability will probably optimize the applicability of those endoscopic devices in neurosurgery. A major drawback of the current prototype is poor image resolution, which will probably soon be overcome with the ongoing progress in electronic microchip technology.
The addition of laser technology to endoscopic techniques offers a huge potential to neurosurgery but has achieved little acceptance to date. The reasons include concern regarding heat production, uncontrollable and distant penetration, and tissue interaction. Experiences with a 2-micron continuous- wave laser (RevoLix Jr, LISA Laser Products, Katlenburg-Lindau, Germany) for neuroendoscopic intraventricular procedures proved this laser to be a valuable and useful tool with safe applicability for endoscopic intracranial procedures in patients of all ages.34
Parallel to the launch of video screens for other uses with higher image definition, the image quality on the 2D endoscope cameras has been constantly improving over the last years. At the same time, the introduction of modern 3D endoscopic monitors is promising. However, 3D endoscopes have some disadvantages compared with the 2D endoscopes. First, the smallest 3D endoscopes are 4 mm in diameter, compared with 2.7 mm for 2D endoscopes. Moreover, the field of view with the 3D endoscope is less than half of that with conventional 2D endoscopes.34 When working in and around a region with critical neurovascular structures in close proximity, this loss of field of view can result in an increase in iatrogenic injury from the endoscope. In addition, 3D endoscopes require special glasses, generating a potential obstacle to the seamless integration of visual information from the microscope and endoscope. Finally, some surgeons experience vertigo when looking at the 3D picture through the glasses, which limits its universal applicability.
CONCLUSIONS
Using the endoscope and microscope as complementary and not competing tools allows surgeons to benefit from both technologies at the same time.35,36 The intraoperative combination of these 2 powerful visualization tools expands the effectiveness of microsurgical procedures and has the potential to further improve surgical results and reduce surgical risks. With endoscope-assisted microsurgery, visualization is often far superior to surgical maneuverability.
Endoscopic neurosurgery will likely be influenced by further innovations in optical physics, electronics, and robotics. Specific implementations in endoscopic systems are likely to pave the way for remarkable progress in minimally invasive surgery, such as robotic surgical technology, further miniaturization of devices, improvements in 3D endoscopy, multiport endoscopy, and new designs for surgical instruments. Future progress in flexible endoscopes and wireless capsule or camera technology may reduce our dependence on rigid rod lens systems. Rigid variable-view endoscopes will bring endoscopes closer to ideal attributes utilizing newer instrumentation that is tailored to specific indications and techniques.37,38 Extension of the visual field by the feature of a movable optic lens may allow the neurosurgeon to use tailored keyhole approaches to treat pathologies in smaller surgical corridors with less trauma and greater efficacy.
Over the last 3 decades, the endoscope has become a highly valued visualization tool in neurosurgery, applicable to a broad range of neurosurgical procedures. Following the pace of technological innovations, the quality of the instrumentation has greatly improved along with the status of endoscopy in the neurosurgical field. The use of the endoscope in interdisciplinary extended transnasal approaches revolutionized skull-base surgery.1 Transcranial neurosurgery took advantage of the endoscope for inspection, endoscope-assisted, and endoscope-controlled procedures, although the main visualization tool during these interventions remains the operating microscope.
At present, endoscopy has applications in a variety of neurosurgical procedures including transnasal approaches for pituitary and other skull-base tumors, third ventriculostomy, and resection of intraventricular tumors. The range of application is expanding to include extracranial procedures such as peripheral nerve and spine surgery.
optics and instruments are passed through a rigid, multiport chamber. This technique is ideal when performing surgery within the ventricular system using only a standard bur hole craniotomy.
CURRENT CONCEPTS
Hopf and Perneczky2 defined the terminology regarding endoscopic procedures and divided them into 3 categories:
Pure endoscopic neurosurgery, ie, procedures performed through working channels under complete endoscopic visualization and with endoscopic instrumentation (Figure 1).3
Endoscope-controlled microsurgery, ie, operations performed with standard microsurgical instruments under endoscopic visualization—the microscope is not used (Figure 2).
Endoscope-assisted neurosurgery, ie, the use of both microscope and endoscope during the same intervention. In endoscopic inspection the endoscope is solely used as an adjunctive tool for visualization and not for surgical manipulations.
Enhanced area and surgical dissection
Technical innovations are probably the major reason for the growing role of endoscopy in neurosurgery over the last 3 decades.4 High-definition imaging, neuronavigation, new instruments, an interdisciplinary approach mostly with ear, nose, and throat (ENT) surgeons, and detailed anatomic studies led to the breakthrough of endoscopic endonasal extended approaches in skull-base surgery.5
These endoscopic techniques allow the neurosurgeon to optimize tumor resection, increasing the area of surgical dissection without increasing the size of the surgical approach, thereby limiting perioperative morbidity due to surgical manipulation of eloquent brain structures. Endoscopy offers direct illumination of the operative field, magnification, and the ability to look around corners with angled optics.
However, while angled endoscopic optics provide various visual perspectives, the surgical issue is not only to see but also to work on and around remote structures. Microsurgical endoscope-assisted manipulations require optimal working angles that are guaranteed only by a sufficiently large craniotomy. As an example, a dissection study by Chaynes et al6 highlights that a craniotomy that is too narrow often hinders a sufficient exploration of the entire cerebellopontine angle. Most neurosurgeons are familiar with the operating microscope. The microscopic field of inspection is 3-dimensional (3D) and of high quality. However, the light stream is straight and thus limited in the narrow and angled corridor of the cerebellopontine angle or in the perimesencephalic cisterns. In these situations, the angled optic of the endoscope offers the advantage of being able to look around the corner with the appropriate amount of direct illumination.7
Peripheral nerve surgery
Minimally invasive endoscopic approaches are also being used in peripheral nerve surgery, especially carpal tunnel decompression. The first carpal tunnel release treated endoscopically was performed by Okutsu et al in the late 1980s.8 Since that time, endoscopic carpal tunnel decompression has become very common and is the preferred method for many surgeons, using either single-portal or dual-portal techniques. Although the superiority of endoscopic over conventional minimally invasive microsurgical peripheral nerve surgeries has not been proven, large series of endoscopic carpal tunnel decompressions have reported low complication rates and excellent success rates with high patient satisfaction scores.8,9
Visualization of the spinal canal
Expanding the use of the endoscope to spine surgery, endoscopic explorations of the interlaminar spaces after having completed open surgical laminectomies have been reported since the early 1980s,10 while endoscope-assisted interlaminar procedures started in the late 1990s.11–13 The development of fully endoscopic transforaminal or interlaminar approaches for lumbar stenosis or lumbar disk herniation has been ongoing in the last 2 decades. The rationale for direct endoscopic visualization of the spinal canal is to reduce scarring of the epidural space, which might affect the outcome of possible revision surgeries (recurrent disk herniation), and to reduce injury to the paraspinal muscles, which may reduce postoperative incisional pain and length of hospital stay. Major limiting factors for fully endoscopic spine surgeries such as the narrow working channels (which are limited by the osseous perimeter of the neuroforamina, as well as the pelvis and abdominal structures) and the learning curve for the surgeons are, however, still matters of debate and restrict the use of endoscopy to very carefully selected cases.14,15
Pediatric craniosynostosis
Recently, the use of the endoscope has extended to treatment of craniosynostosis in pediatric patients, historically treated with large and occasionally staged craniotomic approaches. A meta-analysis of the literature showed statistically significant reductions in blood loss and rates of perioperative complications, reoperation, and transfusion compared with open approaches.16
Technical limitations
While neurosurgeons increasingly advocate the use of the endoscope in their practice, the development of instruments for endoscopic surgery does not always follow the same pace. There are technical problems with current rigid endoscopes and ergonomic limitations of the endoscope-assisted techniques in transcranial neurosurgery. The endoscope itself occupies space in an already limited surgical corridor like the posterior fossa, the parasellar space, or the intraventricular region. The ideal endoscope is thin and sturdy, does not generate heat, and provides high-resolution images. In addition, a self-irrigating feature could minimize the need to remove and reinsert the endoscope for cleaning. Finally, most intracranial surgery is extremely delicate and requires bimanual dissection. The ideal endoscope should also be easily integrated with a holder that allows the surgeon to easily transition between static and dynamic endoscope movements.
Newer flexible fiberscopes with even smaller diameters are likely to be launched on the market in the near future. When working in a surgical corridor less than 10 mm wide, this difference could be substantial.
In addition, surgical instruments specifically designed for endoscopic endonasal procedures are needed for microdissection in these regions, which were previously only visible but not reachable endoscopically. These include tools such as malleable suctions and curettes, rotatable back-biting microscissors, and malleable bipolar instruments (Figure 3).
IMPACT OF NEUROENDOSCOPY IN CURRENT CLINICAL PRACTICE
The introduction of endoscopy in neurosurgery changed many treatment paradigms and had an important impact on morbidity and outcomes. In this section, we discuss the specific indications, contraindications, and expected benefit of endoscopic vs open surgical approaches applied to neurosurgical pathology at the present time.
Skull-base tumors and CSF leaks
The use of the endoscope in skull-base surgery was originally applied to purely midline intrasellar tumors without suprasellar or lateral extension beyond the carotid cave. Ideal cases were intrasellar pituitary microadenomas not responding to medical treatment or Rathke cleft cysts.
These pathologies were traditionally addressed via microscopic craniotomic approaches and later through sublabial or transnasal transsphenoidal approaches. Traditional transsphenoidal approaches were highly invasive for the oral mucosa, causing delayed healing, oral dysesthesia, and, in some cases, loss of the superior dental arch (sublabial) or limited visualization and surgical maneuverability (microscopic endonasal).
The endoscope offered better visualization and surgical freedom, thus allowing higher resection rates to be achieved. Resection of purely intrasellar pathology with preservation of the diaphragma sellae as a barrier to the subarachnoid cysterns and third ventricle guaranteed a lower incidence of cerebrospinal fluid (CSF) leaks.
New endoscope optics with varied angles, together with dedicated long surgical instruments with low steric volume, offered a large variety of new endonasal surgical corridors, so-called expanded endonasal approaches on the sagittal and coronal planes, as discussed in detail by Kassam et al.17–19 These allowed endoscopic treatment of invasive tumors extending on the coronary plane into the suprasellar region or invading the cavernous sinuses (pituitary macroadenomas, craniopharyngiomas).
Highly specialized centers with expertise in endoscopic skull-base surgery can now also offer pure endoscopic treatment for some selected cases of lesions located far laterally to the cavernous sinus, such as trigeminal schwannomas, or along the sagittal plane like olfactory groove or tuberculum sellae meningiomas and clival lesions (chordomas, chondrosarcomas).
As one might expect, the increase in surgical complexity corresponded to an increase in complication rates. For example, the incidence of CSF leaks varied from 5% for standard midline transsphenoidal approaches to 11% for expanded endonasal approaches.20,21 The consolidation of the use of the endoscope and the cooperation with ENT surgeons led to the development of surgical strategies to prevent and reduce the incidence of CSF leaks, such as the use of “rescue flaps,” nasoseptal flaps, or temporoparietal fascia flaps.21–23
The development of such techniques allowed endoscopic endonasal approaches to be used in treatment of other pathologies, such as spontaneous CSF leaks, treated in the past with large transcranial repairs that carried high morbidity rates due to the surgical frontal lobe retraction and injury to the olfactory mucosa.24,25 Progress in the field of neuroendoscopy therefore led to the creation of specialized endoscopic skull-base surgery centers, including neurosurgery, ENT, ophthalmology, and endocrinology services.
In clinical practice, when evaluating a patient with intracranial skull-base pathology amenable to endoscopic resection, one should consider referring the patient not only to a neurosurgeon, but also to an ENT surgeon for preoperative assessment of the sinonasal cavities. The same concept applies to postsurgical follow-up, which is mostly performed by the ENT physician to assess nasal mucosa healing and nasal hygiene.
Ventricular neuroendoscopy
The introduction of endoscopic third ventriculostomy created the opportunity to offer a more physiologic treatment in selected patients with obstructive hydrocephalus by creating an internal CSF diversion through the basal cisterns. Two advantages of this procedure are that it does not create dependence on a CSF shunt, and it eliminates the related risks of shunt infection and malfunction. Its drawback is the recurrence rate of hydrocephalus (around 58% at 2 years of follow-up) due to formation of scarring in the perforated Liliequist membrane, which may require repeat surgery or conversion to CSF shunting.26,27
Neuroendoscopic approaches are also used in cases of purely intraventricular pathology such as colloid cyst or choroid plexus papillomas. The concept behind neuroendoscopy is to achieve maximal resection in a minimally invasive way, using the natural cavity of the cerebral ventricles and reducing the need for brain retraction and, in particular, the risk of injury of the fornix (therefore causing memory deficits) of open transventricular approaches and of the corpus callosum necessary in interhemispheric approaches. Large tumor size and inability to tolerate a longer surgical procedure can be relative contraindications to a pure endoscopic approach to these lesions.
Degenerative spine disease
In recent years there has been a growing interest in the use of endoscopy for selected cases of degenerative lumbar spondylosis (generally, lateral disk herniation above the L5-S1 level or spinal canal stenosis). This approach has been shown to reduce postoperative incisional pain, scarring of the epidural space affecting the outcome of possible revision surgeries (recurrent disc herniation), and length of hospital stay.14,15 Information on surgical nuances should be provided when consulting on selected patients with lumbar degenerative disease resistant to conservative treatment.
Carpal tunnel syndrome
Although endoscopic carpal tunnel release is controversial, its supporters report smaller incision size and lower recurrence rates due to better visualization of the entire carpal ligament compared with open surgery, with high patient satisfaction scores.8,9,28
Craniosynostosis
Increasing data from specialized centers show that early endoscopic suturectomy is an effective treatment option alone or when combined with open surgeries for patients with syndromic and nonsyndromic craniosynostosis. The aesthetic advantage of small incisions (which can also be achieved with some open techniques) is accompanied by significant reductions in blood loss (median 162.4 mL), operative time (median 112.38 minutes), length of stay (median 2.56 days), and rates of perioperative complications (odds ratio 0.58), reoperation (odds ratio 0.37), and transfusion (odds ratio 0.09) compared with open approaches.16
SURGICAL TRAINING
Today’s patients expect high-quality healthcare, and they approach their surgeons with an enormous amount of information collected through unlimited Web-based access or peer-group blogs. In this respect, the pressure on young surgeons to achieve excellent results is high and growing from the very beginning of their careers.
Residency training programs differ in each country, and surgical standards usually focus on open microscopic procedures rather than newly developed endoscopic techniques. Endoscopic pituitary adenoma surgery, the most frequent neuroendoscopic procedure, is still performed mostly by experienced neurosurgeons, not trainees. Moreover, many training institutions might not offer pediatric neurosurgery care, limiting exposure to endoscopic third ventriculostomy procedures. The European Union of Medical Specialists, responsible for harmonizing and improving the quality of training of medical specialists in Europe, set low neuroendoscopic surgical requirements for trainees to complete their residency programs (minimum of 0 to optimum of 5 total transcranial or transsphenoidal pituitary adenoma resections as first operator, 10 procedures as assistant, and a minimum of 2 to an optimum of 4 endoscopic third ventriculostomies as first operator).29
The need to develop training programs in neuroendoscopy is especially urgent because endoscopic surgery has a steeper learning curve than conventional microneurosurgery. In particular, endoscopy requires a good deal of dexterity and hand-eye coordination, which surgeons consider the main pitfall of neuroendoscopy. For such reasons, many accredited clinical fellowship programs have been developed inside and outside North America that offer intensive training in endoscopic skull-base surgery and pediatric neurosurgery after residency.
Some clinical studies have shown that the complication rate of neuroendoscopy is 15% to 18%.27,30 In view of this statistic, it is ethically questionable to perform a randomized study to prospectively compare microscopic and endoscopic procedures. Surgeons specialize in one technique or the other, experience their own learning curve, and do not randomly decide which tool to use. Furthermore, every intracranial surgical exploration is unique and somewhat difficult to compare with each other without the risk of bias.
FURTHER DEVELOPMENTS
Multivariable rigid endoscopes like the EndoCAMeleon (Karl Storz, Tuttlingen, Germany) or the EndActive (Karl Storz, Tuttlingen, Germany) for cerebellopontine angle surgery represent a starting point to overcome some of the aforementioned limitations.31,32 They are inserted in the surgical field with a direct 0° angulation view into the operative site beyond neurovascular structures that need to be preserved and that obstruct the microscopic view. Once the final position is reached, the field of view is directed toward the region of interest without moving the endoscope tip.
The EndoCAMeleon is a rigid rod-lens endoscope, steerable in one plane from –10° to +120° by a fine optomechanical mechanism. Anatomic laboratory testing found it to be superior in terms of usability and visualization compared with rigid fixed-angle endoscopes.31 The first clinical experiences have been promising; however, ergonomics and the limited perspective of a single plane of rotation leave room for improvement.
The EndActive endoscope might overcome such limitations.33 This device is a rigid videoendoscope connected to a laptop (video data) and USB port (control and power supply); thus, it weighs less and can be held in one hand like a microsurgical instrument. The endoscopic imaging system allows the operator to simultaneously see a 160° wide-angle view of the site and an inset of a specific region of interest. The surgeon can hold the device like a microsurgical instrument in one hand and control movements precisely due to its reduced weight and ergonomic shape.
The multiplanar variable-view rigid endoscope has proven to be useful for working on diverse anatomic structures such as intracranial vessels and cranial nerves. The device is effective in narrow working spaces where even small movements can jeopardize the delicate surrounding structures. The multiplanar variable-viewing mechanism in a compact device offers advantages in terms of safety and ergonomics. Improving the usability will probably optimize the applicability of those endoscopic devices in neurosurgery. A major drawback of the current prototype is poor image resolution, which will probably soon be overcome with the ongoing progress in electronic microchip technology.
The addition of laser technology to endoscopic techniques offers a huge potential to neurosurgery but has achieved little acceptance to date. The reasons include concern regarding heat production, uncontrollable and distant penetration, and tissue interaction. Experiences with a 2-micron continuous- wave laser (RevoLix Jr, LISA Laser Products, Katlenburg-Lindau, Germany) for neuroendoscopic intraventricular procedures proved this laser to be a valuable and useful tool with safe applicability for endoscopic intracranial procedures in patients of all ages.34
Parallel to the launch of video screens for other uses with higher image definition, the image quality on the 2D endoscope cameras has been constantly improving over the last years. At the same time, the introduction of modern 3D endoscopic monitors is promising. However, 3D endoscopes have some disadvantages compared with the 2D endoscopes. First, the smallest 3D endoscopes are 4 mm in diameter, compared with 2.7 mm for 2D endoscopes. Moreover, the field of view with the 3D endoscope is less than half of that with conventional 2D endoscopes.34 When working in and around a region with critical neurovascular structures in close proximity, this loss of field of view can result in an increase in iatrogenic injury from the endoscope. In addition, 3D endoscopes require special glasses, generating a potential obstacle to the seamless integration of visual information from the microscope and endoscope. Finally, some surgeons experience vertigo when looking at the 3D picture through the glasses, which limits its universal applicability.
CONCLUSIONS
Using the endoscope and microscope as complementary and not competing tools allows surgeons to benefit from both technologies at the same time.35,36 The intraoperative combination of these 2 powerful visualization tools expands the effectiveness of microsurgical procedures and has the potential to further improve surgical results and reduce surgical risks. With endoscope-assisted microsurgery, visualization is often far superior to surgical maneuverability.
Endoscopic neurosurgery will likely be influenced by further innovations in optical physics, electronics, and robotics. Specific implementations in endoscopic systems are likely to pave the way for remarkable progress in minimally invasive surgery, such as robotic surgical technology, further miniaturization of devices, improvements in 3D endoscopy, multiport endoscopy, and new designs for surgical instruments. Future progress in flexible endoscopes and wireless capsule or camera technology may reduce our dependence on rigid rod lens systems. Rigid variable-view endoscopes will bring endoscopes closer to ideal attributes utilizing newer instrumentation that is tailored to specific indications and techniques.37,38 Extension of the visual field by the feature of a movable optic lens may allow the neurosurgeon to use tailored keyhole approaches to treat pathologies in smaller surgical corridors with less trauma and greater efficacy.
- Kassam AB, Gardner P, Snyderman C, Mintz A, Carrau R. Expanded endonasal approach: fully endoscopic, completely transnasal approach to the middle third of the clivus, petrous bone, middle cranial fossa, and infratemporal fossa. Neurosurg Focus 2005; 19(1):E6. pmid:16078820
- Hopf NJ, Perneczky A. Endoscopic neurosurgery and endoscope-assisted microneurosurgery for the treatment of intracranial cysts. Neurosurgery 1998; 43(6):1330–1336. doi:10.1097/00006123-199812000-00037
- Li KW, Nelson C, Suk I, Jallo GI. Neuroendoscopy: past, present, and future. Neurosurg Focus 2005; 19(6):E1. doi:10.3171/foc.2005.19.6.2
- Prevedello DM, Doglietto F, Jane JA Jr, Jagannathan J, Han J, Laws ER Jr. History of endoscopic skull base surgery: its evolution and current reality. J Neurosurg 2007; 107(1):206–213. doi:10.3171/JNS-07/07/0206
- Schroeder HW, Nehlsen M. Value of high-definition imaging in neuroendoscopy. Neurosurg Rev 2009; 32(3):303–308. doi:10.1007/s10143-009-0200-x
- Chaynes P, Deguine O, Moscovici J, Fraysse B, Becue J, Lazorthes Y. Endoscopic anatomy of the cerebellopontine angle: a study in cadaver brains. Neurosurg Focus 1998; 5(3):e8.
- Setty P, Volkov AA, D'Andrea KP, Pieper DR. Endoscopic vascular decompression for the treatment of trigeminal neuralgia: clinical outcomes and technical note. World Neurosurg 2014; 81(3–4):603–608. doi:10.1016/j.wneu.2013.10.036
- Okutsu I, Hamanaka I, Yoshida A. Retrospective analysis of five-year and longer clinical and electrophysiological results of the world's first endoscopic management for carpal tunnel syndrome. Hand Surg 2013; 18(3):317–323. doi:10.1142/S0218810413500330
- Zuo D, Zhou Z, Wang H, et al. Endoscopic versus open carpal tunnel release for idiopathic carpal tunnel syndrome: a meta-analysis of randomized controlled trials. J Orthop Surg Res 2015; 10:12. doi:10.1186/s13018-014-0148-6
- Forst R, Hausmann B. Nucleoscopy—a new examination technique. Arch Orthop Trauma Surg 1983; 101(3):219–221. pmid:6870510
- Brayda-Bruno M, Cinnella P. Posterior endoscopic discectomy (and other procedures). Eur Spine J 2000; 9(suppl 1):S24–S29. pmid:10766054
- Destandau J. A special device for endoscopic surgery of lumbar disc herniation. Neurol Res 1999; 21(1):39–42. pmid:10048052
- Perez-Cruet MJ, Foley KT, Isaacs RE, et al. Microendoscopic lumbar discectomy: technical note. Neurosurgery 2002; 51(5 suppl):S129–S136. pmid:12234440
- Ruetten S, Komp M, Merk H, Godolias G. Full-endoscopic interlaminar and transforaminal lumbar discectomy versus conventional microsurgical technique: a prospective, randomized, controlled study. Spine (Phila Pa 1976) 2008; 33(9):931–939. doi:10.1097/BRS.0b013e31816c8af7
- Komp M, Hahn P, Merk H, Godolias G, Ruetten S. Bilateral operation of lumbar degenerative central spinal stenosis in full-endoscopic interlaminar technique with unilateral approach: prospective 2-year results of 74 patients. J Spinal Disord Tech 2011; 24(5):281–287. doi:10.1097/BSD.0b013e3181f9f55e
- Goyal A, Lu VM, Yolcu YU, Elminawy M, Daniels DJ. Endoscopic versus open approach in craniosynostosis repair: a systematic review and meta-analysis of perioperative outcomes. Childs Nerv Syst 2018; 34(9):1627–1637. doi:10.1007/s00381-018-3852-4
- Kassam AB, Gardner P, Snyderman C, Mintz A, Carrau R. Expanded endonasal approach: fully endoscopic, completely transnasal approach to the middle third of the clivus, petrous bone, middle cranial fossa, and infratemporal fossa. Neurosurg Focus 2005; 19(1):E6. pmid:16078820
- Kassam A, Snyderman CH, Mintz A, Gardner P, Carrau RL. Expanded endonasal approach: the rostrocaudal axis. Part II. Posterior clinoids to the foramen magnum. Neurosurg Focus 2005; 19(1):E4. pmid:16078818
- Kassam A, Snyderman CH, Mintz A, Gardner P, Carrau RL. Expanded endonasal approach: the rostrocaudal axis. Part I. Crista galli to the sella turcica. Neurosurg Focus 2005; 19(1):E3. pmid:16078817
- Kassam A, Carrau RL, Snyderman CH, Gardner P, Mintz A. Evolution of reconstructive techniques following endoscopic expanded endonasal approaches. Neurosurg Focus 2005; 19(1):E8. pmid:16078822
- Kassam AB, Thomas A, Carrau RL, et al. Endoscopic reconstruction of the cranial base using a pedicled nasoseptal flap. Neurosurgery 2008; 63(1 suppl 1):ONS44–ONS52. doi:10.1227/01.NEU.0000297074.13423.F5
- Hadad G, Bassagasteguy L, Carrau RL, et al. A novel reconstructive technique after endoscopic expanded endonasal approaches: vascular pedicle nasoseptal flap. Laryngoscope 2006; 116(10):1882–1886. doi:10.1097/01.mlg.0000234933.37779.e4
- Fortes FS, Carrau RL, Snyderman CH, et al. Transpterygoid transposition of a temporoparietal fascia flap: a new method for skull base reconstruction after endoscopic expanded endonasal approaches. Laryngoscope 2007; 117(6):970–976. doi:10.1097/MLG.0b013e3180471482
- Carrau RL, Snyderman CH, Kassam AB. The management of cerebrospinal fluid leaks in patients at risk for high-pressure hydrocephalus. Laryngoscope 2005; 115(2):205–212. doi:10.1097/01.mlg.0000154719.62668.70
- Zweig JL, Carrau RL, Celin SE, et al. Endoscopic repair of cerebrospinal fluid leaks to the sinonasal tract: predictors of success. Otolaryngol Head Neck Surg 2000; 123(3):195–201. doi:10.1067/mhn.2000.107452
- Kulkarni AV, Riva-Cambrin J, Holubkov R, et al. Endoscopic third ventriculostomy in children: prospective, multicenter results from the Hydrocephalus Clinical Research Network. J Neurosurg Pediatr 2016; 18(4):423–429. doi:10.3171/2016.4.PEDS163
- Ersahin Y, Arslan D. Complications of endoscopic third ventriculostomy. Childs Nerv Syst 2008; 24(8):943–948. doi:10.1007/s00381-008-0589-5
- Martínez-Catasús A, Lobo-Escolar L, García-Bonet J, Corrales-Rodríguez M, Pasarín-Martínez A, Berlanga-de-Mingo D. Comparison between single portal endoscopic, 1-cm open carpal tunnel release. Hand Surg Rehabil 2019. pii:S2468-1229(19)30027-1. doi:10.1016/j.hansur.2019.02.003
- Steers J, Reulen HJ, Lindsay K; European Union of Medical Specialists; Joint Residency Advisory and Accreditation Committee. UEMS charter on training of medical specialists in the EU—the new neurosurgical training charter. Acta Neurochir Suppl 2004; 90:3–11. pmid:15553111
- Mori H, Nishiyama K, Yoshimura J, Tanaka R. Current status of neuroendoscopic surgery in Japan and discussion on the training system. Childs Nerv Syst 2007; 23(6):673–676. doi:10.1007/s00381-007-0329-2
- Aryan HE, Hoeg HD, Marshall LF, Levy ML. Multidirectional projectional rigid neuro-endoscopy: prototype and initial experience. Minim Invasive Neurosurg 2005; 48(5):293–296. doi:10.1055/s-2005-915602
- Ebner FH, Marquardt JS, Hirt B, Tatagiba M, Schuhmann MU. Visualization of the anterior cerebral artery complex with a continuously variable-view rigid endoscope: new options in aneurysm surgery. Neurosurgery 2010; 67(2 suppl operative):321–324. doi:10.1227/NEU.0b013e3181f74548
- Ebner FH, Hirt B, Marquardt JS, Herlan S, Tatagiba M, Schuhmann MU. Actual state of EndActive ventricular endoscopy. Childs Nerv Syst 2012; 28(1):87–91. doi:10.1007/s00381-011-1537-3
- Ebner FH, Nagel C, Tatagiba M, Schuhmann MU. Efficacy and versatility of the 2-micron continuous wave laser in neuroendoscopic procedures. Acta Neurochir Suppl 2012; 113:143–147. doi:10.1007/978-3-7091-0923-6_29
- Van Gompel JJ, Tabor MH, Youssef AS, et al. Field of view comparison between two-dimensional and three-dimensional endoscopy. Laryngoscope 2014; 124(2):387–390. doi:10.1002/lary.24222
- Ebner FH, Roser F, Thaher F, Schittenhelm J, Tatagiba M. Balancing the shortcomings of microscope and endoscope: endoscope-assisted technique in microsurgical removal of recurrent epidermoid cysts in the posterior fossa. Minim Invasive Neurosurg 2010 ;53(5–6):218–222. doi:10.1055/s-0030-1267973
- Perneczky A, Fries G. Endoscope-assisted brain surgery: part 1—evolution, basic concept, and current technique. Neurosurgery 1998; 42(2):219–224. doi:10.1097/00006123-199802000-00001
- Ebner FH, Marquardt JS, Hirt B, Feigl GC, Tatagiba M, Schuhmann MU. Broadening horizons of neuroendoscopy with a variable-view rigid endoscope: an anatomical study. Eur J Surg Oncol 2010; 36(2):195–200. doi:10.1016/j.ejso.2009.07.185
- Kassam AB, Gardner P, Snyderman C, Mintz A, Carrau R. Expanded endonasal approach: fully endoscopic, completely transnasal approach to the middle third of the clivus, petrous bone, middle cranial fossa, and infratemporal fossa. Neurosurg Focus 2005; 19(1):E6. pmid:16078820
- Hopf NJ, Perneczky A. Endoscopic neurosurgery and endoscope-assisted microneurosurgery for the treatment of intracranial cysts. Neurosurgery 1998; 43(6):1330–1336. doi:10.1097/00006123-199812000-00037
- Li KW, Nelson C, Suk I, Jallo GI. Neuroendoscopy: past, present, and future. Neurosurg Focus 2005; 19(6):E1. doi:10.3171/foc.2005.19.6.2
- Prevedello DM, Doglietto F, Jane JA Jr, Jagannathan J, Han J, Laws ER Jr. History of endoscopic skull base surgery: its evolution and current reality. J Neurosurg 2007; 107(1):206–213. doi:10.3171/JNS-07/07/0206
- Schroeder HW, Nehlsen M. Value of high-definition imaging in neuroendoscopy. Neurosurg Rev 2009; 32(3):303–308. doi:10.1007/s10143-009-0200-x
- Chaynes P, Deguine O, Moscovici J, Fraysse B, Becue J, Lazorthes Y. Endoscopic anatomy of the cerebellopontine angle: a study in cadaver brains. Neurosurg Focus 1998; 5(3):e8.
- Setty P, Volkov AA, D'Andrea KP, Pieper DR. Endoscopic vascular decompression for the treatment of trigeminal neuralgia: clinical outcomes and technical note. World Neurosurg 2014; 81(3–4):603–608. doi:10.1016/j.wneu.2013.10.036
- Okutsu I, Hamanaka I, Yoshida A. Retrospective analysis of five-year and longer clinical and electrophysiological results of the world's first endoscopic management for carpal tunnel syndrome. Hand Surg 2013; 18(3):317–323. doi:10.1142/S0218810413500330
- Zuo D, Zhou Z, Wang H, et al. Endoscopic versus open carpal tunnel release for idiopathic carpal tunnel syndrome: a meta-analysis of randomized controlled trials. J Orthop Surg Res 2015; 10:12. doi:10.1186/s13018-014-0148-6
- Forst R, Hausmann B. Nucleoscopy—a new examination technique. Arch Orthop Trauma Surg 1983; 101(3):219–221. pmid:6870510
- Brayda-Bruno M, Cinnella P. Posterior endoscopic discectomy (and other procedures). Eur Spine J 2000; 9(suppl 1):S24–S29. pmid:10766054
- Destandau J. A special device for endoscopic surgery of lumbar disc herniation. Neurol Res 1999; 21(1):39–42. pmid:10048052
- Perez-Cruet MJ, Foley KT, Isaacs RE, et al. Microendoscopic lumbar discectomy: technical note. Neurosurgery 2002; 51(5 suppl):S129–S136. pmid:12234440
- Ruetten S, Komp M, Merk H, Godolias G. Full-endoscopic interlaminar and transforaminal lumbar discectomy versus conventional microsurgical technique: a prospective, randomized, controlled study. Spine (Phila Pa 1976) 2008; 33(9):931–939. doi:10.1097/BRS.0b013e31816c8af7
- Komp M, Hahn P, Merk H, Godolias G, Ruetten S. Bilateral operation of lumbar degenerative central spinal stenosis in full-endoscopic interlaminar technique with unilateral approach: prospective 2-year results of 74 patients. J Spinal Disord Tech 2011; 24(5):281–287. doi:10.1097/BSD.0b013e3181f9f55e
- Goyal A, Lu VM, Yolcu YU, Elminawy M, Daniels DJ. Endoscopic versus open approach in craniosynostosis repair: a systematic review and meta-analysis of perioperative outcomes. Childs Nerv Syst 2018; 34(9):1627–1637. doi:10.1007/s00381-018-3852-4
- Kassam AB, Gardner P, Snyderman C, Mintz A, Carrau R. Expanded endonasal approach: fully endoscopic, completely transnasal approach to the middle third of the clivus, petrous bone, middle cranial fossa, and infratemporal fossa. Neurosurg Focus 2005; 19(1):E6. pmid:16078820
- Kassam A, Snyderman CH, Mintz A, Gardner P, Carrau RL. Expanded endonasal approach: the rostrocaudal axis. Part II. Posterior clinoids to the foramen magnum. Neurosurg Focus 2005; 19(1):E4. pmid:16078818
- Kassam A, Snyderman CH, Mintz A, Gardner P, Carrau RL. Expanded endonasal approach: the rostrocaudal axis. Part I. Crista galli to the sella turcica. Neurosurg Focus 2005; 19(1):E3. pmid:16078817
- Kassam A, Carrau RL, Snyderman CH, Gardner P, Mintz A. Evolution of reconstructive techniques following endoscopic expanded endonasal approaches. Neurosurg Focus 2005; 19(1):E8. pmid:16078822
- Kassam AB, Thomas A, Carrau RL, et al. Endoscopic reconstruction of the cranial base using a pedicled nasoseptal flap. Neurosurgery 2008; 63(1 suppl 1):ONS44–ONS52. doi:10.1227/01.NEU.0000297074.13423.F5
- Hadad G, Bassagasteguy L, Carrau RL, et al. A novel reconstructive technique after endoscopic expanded endonasal approaches: vascular pedicle nasoseptal flap. Laryngoscope 2006; 116(10):1882–1886. doi:10.1097/01.mlg.0000234933.37779.e4
- Fortes FS, Carrau RL, Snyderman CH, et al. Transpterygoid transposition of a temporoparietal fascia flap: a new method for skull base reconstruction after endoscopic expanded endonasal approaches. Laryngoscope 2007; 117(6):970–976. doi:10.1097/MLG.0b013e3180471482
- Carrau RL, Snyderman CH, Kassam AB. The management of cerebrospinal fluid leaks in patients at risk for high-pressure hydrocephalus. Laryngoscope 2005; 115(2):205–212. doi:10.1097/01.mlg.0000154719.62668.70
- Zweig JL, Carrau RL, Celin SE, et al. Endoscopic repair of cerebrospinal fluid leaks to the sinonasal tract: predictors of success. Otolaryngol Head Neck Surg 2000; 123(3):195–201. doi:10.1067/mhn.2000.107452
- Kulkarni AV, Riva-Cambrin J, Holubkov R, et al. Endoscopic third ventriculostomy in children: prospective, multicenter results from the Hydrocephalus Clinical Research Network. J Neurosurg Pediatr 2016; 18(4):423–429. doi:10.3171/2016.4.PEDS163
- Ersahin Y, Arslan D. Complications of endoscopic third ventriculostomy. Childs Nerv Syst 2008; 24(8):943–948. doi:10.1007/s00381-008-0589-5
- Martínez-Catasús A, Lobo-Escolar L, García-Bonet J, Corrales-Rodríguez M, Pasarín-Martínez A, Berlanga-de-Mingo D. Comparison between single portal endoscopic, 1-cm open carpal tunnel release. Hand Surg Rehabil 2019. pii:S2468-1229(19)30027-1. doi:10.1016/j.hansur.2019.02.003
- Steers J, Reulen HJ, Lindsay K; European Union of Medical Specialists; Joint Residency Advisory and Accreditation Committee. UEMS charter on training of medical specialists in the EU—the new neurosurgical training charter. Acta Neurochir Suppl 2004; 90:3–11. pmid:15553111
- Mori H, Nishiyama K, Yoshimura J, Tanaka R. Current status of neuroendoscopic surgery in Japan and discussion on the training system. Childs Nerv Syst 2007; 23(6):673–676. doi:10.1007/s00381-007-0329-2
- Aryan HE, Hoeg HD, Marshall LF, Levy ML. Multidirectional projectional rigid neuro-endoscopy: prototype and initial experience. Minim Invasive Neurosurg 2005; 48(5):293–296. doi:10.1055/s-2005-915602
- Ebner FH, Marquardt JS, Hirt B, Tatagiba M, Schuhmann MU. Visualization of the anterior cerebral artery complex with a continuously variable-view rigid endoscope: new options in aneurysm surgery. Neurosurgery 2010; 67(2 suppl operative):321–324. doi:10.1227/NEU.0b013e3181f74548
- Ebner FH, Hirt B, Marquardt JS, Herlan S, Tatagiba M, Schuhmann MU. Actual state of EndActive ventricular endoscopy. Childs Nerv Syst 2012; 28(1):87–91. doi:10.1007/s00381-011-1537-3
- Ebner FH, Nagel C, Tatagiba M, Schuhmann MU. Efficacy and versatility of the 2-micron continuous wave laser in neuroendoscopic procedures. Acta Neurochir Suppl 2012; 113:143–147. doi:10.1007/978-3-7091-0923-6_29
- Van Gompel JJ, Tabor MH, Youssef AS, et al. Field of view comparison between two-dimensional and three-dimensional endoscopy. Laryngoscope 2014; 124(2):387–390. doi:10.1002/lary.24222
- Ebner FH, Roser F, Thaher F, Schittenhelm J, Tatagiba M. Balancing the shortcomings of microscope and endoscope: endoscope-assisted technique in microsurgical removal of recurrent epidermoid cysts in the posterior fossa. Minim Invasive Neurosurg 2010 ;53(5–6):218–222. doi:10.1055/s-0030-1267973
- Perneczky A, Fries G. Endoscope-assisted brain surgery: part 1—evolution, basic concept, and current technique. Neurosurgery 1998; 42(2):219–224. doi:10.1097/00006123-199802000-00001
- Ebner FH, Marquardt JS, Hirt B, Feigl GC, Tatagiba M, Schuhmann MU. Broadening horizons of neuroendoscopy with a variable-view rigid endoscope: an anatomical study. Eur J Surg Oncol 2010; 36(2):195–200. doi:10.1016/j.ejso.2009.07.185
meningioma, peripheral nerve, spinal canal, minimally invasive, carpal tunnel, ventricular neuroendoscopy, craniosynostosis, degenerative spine disease, Luigi Rigante, Hamid Borghei-Razavi, Pablo Recinos, Florian Roser
meningioma, peripheral nerve, spinal canal, minimally invasive, carpal tunnel, ventricular neuroendoscopy, craniosynostosis, degenerative spine disease, Luigi Rigante, Hamid Borghei-Razavi, Pablo Recinos, Florian Roser
KEY POINTS
- An increasing number of neurosurgical patients are undergoing endoscopic surgeries of the brain, spine, and peripheral nerves. Familiarization with these techniques provides medical specialists with important knowledge regarding appropriate patient care.
- The combination of classic microscopic and endoscopic procedures improves surgical outcomes by increasing surgical maneuverability and reducing manipulation of eloquent structures.
- Further innovations in optical physics, electronics, and robotics will dramatically improve the potential of endoscopic neurosurgery in the next decades.