User login
DBS vs TMS for treatment-resistant depression: A comparison
Approximately 20% to 30% of patients with major depressive disorder do not respond to pharmacotherapy.1 For patients with treatment-resistant depression (TRD)—typically defined as an inadequate response to at least 1 antidepressant trial of adequate dose and duration—neurostimulation may be an effective treatment option.
Two forms of neurostimulation used to treat TRD are deep brain stimulation (DBS) and transcranial magnetic stimulation (TMS). In DBS, electrodes are placed within the patient’s cranium and affixed to specific target locations. These electrodes are electrically stimulated at various frequencies. Transcranial magnetic stimulation is a noninvasive treatment in which a magnetic field is produced over a patient’s cranium, stimulating brain tissue via electromagnetic induction.
Media portraya
In this article, I compare DBS and TMS, and offer suggestions for educating patients about the potential adverse effects and therapeutic outcomes of each modality.
Deep brain stimulation
Deep brain stimulation is FDA-approved for treating Parkinson’s disease, essential tremor, dystonia, and obsessive-compulsive disorder (OCD).3 It has been used off-label for TRD, and some preliminary evidence suggests it is effective for this purpose. A review of 22 studies found that for patients with TRD, the rate of response to DBS (defined as >50% improvement on Hamilton Depression Rating Scale score) ranges from 40% to 70%.1 Additional research, including larger, randomized, sham-controlled trials, is needed.
A consensus on the optimal target location for DBS has not yet been reached. Studies have had varying degrees of symptom improvement targeting the subgenual cingulate gyrus, posterior gyrus rectus, nucleus accumbens, ventral capsule/ventral striatum, inferior thalamic peduncle, and lateral habenula.1
A worsening of depressive symptoms and increased risk of suicide have been reported in—but are not exclusive to—DBS. Patients treated with DBS may still meet the criteria for treatment resistance.
Continue to: The lack of insurance coverage...
The lack of insurance coverage for DBS for treating depression is a deterrent to its use. Because DBS is not FDA-approved for treating depression, the costs (approximately $65,000) that are not covered by a facility or study will fall on the patient.4 Patients may abandon hope for a positive therapeutic outcome if they must struggle with the financial responsibility for procedures and follow-up.4
Serious potential adverse events of DBS include infections, skin erosions, and postoperative seizure.4 Patients who are treated with DBS should be educated about these adverse effects, and how they may affect outcomes.
Transcranial magnetic stimulation
Transcranial magnetic stimulation is FDA-approved for treating depression, OCD, and migraine. Randomized, sham-controlled trials have found that TMS is effective for TRD.5 Studies have demonstrated varying degrees of efficacy, with response rates ranging from 47% to 58%.6
The most commonly used target area for TMS for patients with depression is the left dorsolateral prefrontal cortex.7 Potential adverse effects are relatively few and benign. The most serious adverse effect of TMS is a risk for seizure, which is reported to occur at a frequency of <0.1%.7
Although it varies by practice and location, the cost for an acute course of TMS (20 to 30 sessions) may range from $6,000 to $12,000.8 Most insurance companies cover TMS treatment for depression.
Continue to: TMS
TMS: A more accessible option
Compared with DBS, TMS is a more affordable and accessible therapy for patients with TRD. Further studies are needed to learn more about the therapeutic potential of DBS for TRD, and to develop methods that help decrease the risk of adverse effects. In addition, insurance coverage needs to be expanded to DBS to avoid having patients be responsible for the full costs of this treatment. Until then, TMS should be a recommended therapy for patients with TRD. If TRD persists in patients treated with TMS, consider electroconvulsive therapy.
1. Morishita T, Fayad SM, Higuchi MA, et al. Deep brain stimulation for treatment-resistant depression: systematic review of clinical outcomes. Neurotherapeutics. 2014;11(3):475-484.
2. Lawrence RE, Kaufmann CR, DeSilva RB, et al. Patients’ belief about deep brain stimulation for treatment resistant depression. AJOB Neuroscience, 2018;9(4):210-218.
3. Rossi PJ, Giordano J, Okun MS. The problem of funding off-label deep brain stimulation: bait-and-switch tactics and the need for policy reform. JAMA Neurol. 2017;74(1):9-10.
4. Holtzheimer PE, Husain MM, Lisanby SH, et al. Subcallosal cingulate deep brain stimulation for treatment-resistant depression: a multisite, randomised, sham-controlled trial. Lancet Psychiatry. 2017;4(11):839-849.
5. Janicak PG. What’s new in transcranial magnetic stimulation. Current Psychiatry. 2019;18(3):10-16.
6. Janicak PG, Sackett V, Kudrna K, et al. Advances in transcranial magnetic stimulation for managing major depressive disorders. Current Psychiatry. 2016;15(6):49-56.
7. Dobek CE, Blumberger DM, Downar J, et al. Risk of seizures in transcranial magnetic stimulation: a clinical review to inform consent process focused on bupropion. Neuropsychiatr Dis Treat. 2015;11:2975-2987.
8. McClintock SM, Reti IM, Carpenter LL, et al; National Network of Depression Centers rTMS Task Group; American Psychiatric Association Council on Research Task Force on Novel Biomarkers and Treatments. Consensus recommendations for the clinical application of repetitive transcranial magnetic stimulation (rTMS) in the treatment of depression. J Clin Psychiatry. 2018;79(1). doi: 10.4088/JCP.16cs10905.
Approximately 20% to 30% of patients with major depressive disorder do not respond to pharmacotherapy.1 For patients with treatment-resistant depression (TRD)—typically defined as an inadequate response to at least 1 antidepressant trial of adequate dose and duration—neurostimulation may be an effective treatment option.
Two forms of neurostimulation used to treat TRD are deep brain stimulation (DBS) and transcranial magnetic stimulation (TMS). In DBS, electrodes are placed within the patient’s cranium and affixed to specific target locations. These electrodes are electrically stimulated at various frequencies. Transcranial magnetic stimulation is a noninvasive treatment in which a magnetic field is produced over a patient’s cranium, stimulating brain tissue via electromagnetic induction.
Media portraya
In this article, I compare DBS and TMS, and offer suggestions for educating patients about the potential adverse effects and therapeutic outcomes of each modality.
Deep brain stimulation
Deep brain stimulation is FDA-approved for treating Parkinson’s disease, essential tremor, dystonia, and obsessive-compulsive disorder (OCD).3 It has been used off-label for TRD, and some preliminary evidence suggests it is effective for this purpose. A review of 22 studies found that for patients with TRD, the rate of response to DBS (defined as >50% improvement on Hamilton Depression Rating Scale score) ranges from 40% to 70%.1 Additional research, including larger, randomized, sham-controlled trials, is needed.
A consensus on the optimal target location for DBS has not yet been reached. Studies have had varying degrees of symptom improvement targeting the subgenual cingulate gyrus, posterior gyrus rectus, nucleus accumbens, ventral capsule/ventral striatum, inferior thalamic peduncle, and lateral habenula.1
A worsening of depressive symptoms and increased risk of suicide have been reported in—but are not exclusive to—DBS. Patients treated with DBS may still meet the criteria for treatment resistance.
Continue to: The lack of insurance coverage...
The lack of insurance coverage for DBS for treating depression is a deterrent to its use. Because DBS is not FDA-approved for treating depression, the costs (approximately $65,000) that are not covered by a facility or study will fall on the patient.4 Patients may abandon hope for a positive therapeutic outcome if they must struggle with the financial responsibility for procedures and follow-up.4
Serious potential adverse events of DBS include infections, skin erosions, and postoperative seizure.4 Patients who are treated with DBS should be educated about these adverse effects, and how they may affect outcomes.
Transcranial magnetic stimulation
Transcranial magnetic stimulation is FDA-approved for treating depression, OCD, and migraine. Randomized, sham-controlled trials have found that TMS is effective for TRD.5 Studies have demonstrated varying degrees of efficacy, with response rates ranging from 47% to 58%.6
The most commonly used target area for TMS for patients with depression is the left dorsolateral prefrontal cortex.7 Potential adverse effects are relatively few and benign. The most serious adverse effect of TMS is a risk for seizure, which is reported to occur at a frequency of <0.1%.7
Although it varies by practice and location, the cost for an acute course of TMS (20 to 30 sessions) may range from $6,000 to $12,000.8 Most insurance companies cover TMS treatment for depression.
Continue to: TMS
TMS: A more accessible option
Compared with DBS, TMS is a more affordable and accessible therapy for patients with TRD. Further studies are needed to learn more about the therapeutic potential of DBS for TRD, and to develop methods that help decrease the risk of adverse effects. In addition, insurance coverage needs to be expanded to DBS to avoid having patients be responsible for the full costs of this treatment. Until then, TMS should be a recommended therapy for patients with TRD. If TRD persists in patients treated with TMS, consider electroconvulsive therapy.
Approximately 20% to 30% of patients with major depressive disorder do not respond to pharmacotherapy.1 For patients with treatment-resistant depression (TRD)—typically defined as an inadequate response to at least 1 antidepressant trial of adequate dose and duration—neurostimulation may be an effective treatment option.
Two forms of neurostimulation used to treat TRD are deep brain stimulation (DBS) and transcranial magnetic stimulation (TMS). In DBS, electrodes are placed within the patient’s cranium and affixed to specific target locations. These electrodes are electrically stimulated at various frequencies. Transcranial magnetic stimulation is a noninvasive treatment in which a magnetic field is produced over a patient’s cranium, stimulating brain tissue via electromagnetic induction.
Media portraya
In this article, I compare DBS and TMS, and offer suggestions for educating patients about the potential adverse effects and therapeutic outcomes of each modality.
Deep brain stimulation
Deep brain stimulation is FDA-approved for treating Parkinson’s disease, essential tremor, dystonia, and obsessive-compulsive disorder (OCD).3 It has been used off-label for TRD, and some preliminary evidence suggests it is effective for this purpose. A review of 22 studies found that for patients with TRD, the rate of response to DBS (defined as >50% improvement on Hamilton Depression Rating Scale score) ranges from 40% to 70%.1 Additional research, including larger, randomized, sham-controlled trials, is needed.
A consensus on the optimal target location for DBS has not yet been reached. Studies have had varying degrees of symptom improvement targeting the subgenual cingulate gyrus, posterior gyrus rectus, nucleus accumbens, ventral capsule/ventral striatum, inferior thalamic peduncle, and lateral habenula.1
A worsening of depressive symptoms and increased risk of suicide have been reported in—but are not exclusive to—DBS. Patients treated with DBS may still meet the criteria for treatment resistance.
Continue to: The lack of insurance coverage...
The lack of insurance coverage for DBS for treating depression is a deterrent to its use. Because DBS is not FDA-approved for treating depression, the costs (approximately $65,000) that are not covered by a facility or study will fall on the patient.4 Patients may abandon hope for a positive therapeutic outcome if they must struggle with the financial responsibility for procedures and follow-up.4
Serious potential adverse events of DBS include infections, skin erosions, and postoperative seizure.4 Patients who are treated with DBS should be educated about these adverse effects, and how they may affect outcomes.
Transcranial magnetic stimulation
Transcranial magnetic stimulation is FDA-approved for treating depression, OCD, and migraine. Randomized, sham-controlled trials have found that TMS is effective for TRD.5 Studies have demonstrated varying degrees of efficacy, with response rates ranging from 47% to 58%.6
The most commonly used target area for TMS for patients with depression is the left dorsolateral prefrontal cortex.7 Potential adverse effects are relatively few and benign. The most serious adverse effect of TMS is a risk for seizure, which is reported to occur at a frequency of <0.1%.7
Although it varies by practice and location, the cost for an acute course of TMS (20 to 30 sessions) may range from $6,000 to $12,000.8 Most insurance companies cover TMS treatment for depression.
Continue to: TMS
TMS: A more accessible option
Compared with DBS, TMS is a more affordable and accessible therapy for patients with TRD. Further studies are needed to learn more about the therapeutic potential of DBS for TRD, and to develop methods that help decrease the risk of adverse effects. In addition, insurance coverage needs to be expanded to DBS to avoid having patients be responsible for the full costs of this treatment. Until then, TMS should be a recommended therapy for patients with TRD. If TRD persists in patients treated with TMS, consider electroconvulsive therapy.
1. Morishita T, Fayad SM, Higuchi MA, et al. Deep brain stimulation for treatment-resistant depression: systematic review of clinical outcomes. Neurotherapeutics. 2014;11(3):475-484.
2. Lawrence RE, Kaufmann CR, DeSilva RB, et al. Patients’ belief about deep brain stimulation for treatment resistant depression. AJOB Neuroscience, 2018;9(4):210-218.
3. Rossi PJ, Giordano J, Okun MS. The problem of funding off-label deep brain stimulation: bait-and-switch tactics and the need for policy reform. JAMA Neurol. 2017;74(1):9-10.
4. Holtzheimer PE, Husain MM, Lisanby SH, et al. Subcallosal cingulate deep brain stimulation for treatment-resistant depression: a multisite, randomised, sham-controlled trial. Lancet Psychiatry. 2017;4(11):839-849.
5. Janicak PG. What’s new in transcranial magnetic stimulation. Current Psychiatry. 2019;18(3):10-16.
6. Janicak PG, Sackett V, Kudrna K, et al. Advances in transcranial magnetic stimulation for managing major depressive disorders. Current Psychiatry. 2016;15(6):49-56.
7. Dobek CE, Blumberger DM, Downar J, et al. Risk of seizures in transcranial magnetic stimulation: a clinical review to inform consent process focused on bupropion. Neuropsychiatr Dis Treat. 2015;11:2975-2987.
8. McClintock SM, Reti IM, Carpenter LL, et al; National Network of Depression Centers rTMS Task Group; American Psychiatric Association Council on Research Task Force on Novel Biomarkers and Treatments. Consensus recommendations for the clinical application of repetitive transcranial magnetic stimulation (rTMS) in the treatment of depression. J Clin Psychiatry. 2018;79(1). doi: 10.4088/JCP.16cs10905.
1. Morishita T, Fayad SM, Higuchi MA, et al. Deep brain stimulation for treatment-resistant depression: systematic review of clinical outcomes. Neurotherapeutics. 2014;11(3):475-484.
2. Lawrence RE, Kaufmann CR, DeSilva RB, et al. Patients’ belief about deep brain stimulation for treatment resistant depression. AJOB Neuroscience, 2018;9(4):210-218.
3. Rossi PJ, Giordano J, Okun MS. The problem of funding off-label deep brain stimulation: bait-and-switch tactics and the need for policy reform. JAMA Neurol. 2017;74(1):9-10.
4. Holtzheimer PE, Husain MM, Lisanby SH, et al. Subcallosal cingulate deep brain stimulation for treatment-resistant depression: a multisite, randomised, sham-controlled trial. Lancet Psychiatry. 2017;4(11):839-849.
5. Janicak PG. What’s new in transcranial magnetic stimulation. Current Psychiatry. 2019;18(3):10-16.
6. Janicak PG, Sackett V, Kudrna K, et al. Advances in transcranial magnetic stimulation for managing major depressive disorders. Current Psychiatry. 2016;15(6):49-56.
7. Dobek CE, Blumberger DM, Downar J, et al. Risk of seizures in transcranial magnetic stimulation: a clinical review to inform consent process focused on bupropion. Neuropsychiatr Dis Treat. 2015;11:2975-2987.
8. McClintock SM, Reti IM, Carpenter LL, et al; National Network of Depression Centers rTMS Task Group; American Psychiatric Association Council on Research Task Force on Novel Biomarkers and Treatments. Consensus recommendations for the clinical application of repetitive transcranial magnetic stimulation (rTMS) in the treatment of depression. J Clin Psychiatry. 2018;79(1). doi: 10.4088/JCP.16cs10905.
Antidepressants for pediatric patients
Major depressive disorder (MDD) is a significant pediatric health problem, with a lifetime prevalence as high as 20% by the end of adolescence.1-3 Major depressive disorder in adolescence is associated with significant morbidity, including poor social functioning, school difficulties, early pregnancy, and increased risk of physical illness and substance abuse.4-6 It is also linked with significant mortality, with increased risk for suicide, which is now the second leading cause of death in individuals age 10 to 24 years.1,7,8
As their name suggests, antidepressants comprise a group of medications that are used to treat MDD; they are also, however, first-line agents for generalized anxiety disorder (GAD), posttraumatic stress disorder (PTSD), and obsessive-compulsive disorder (OCD) in adults. Anxiety disorders (including GAD and other anxiety diagnoses) and PTSD are also common in childhood and adolescence with a combined lifetime prevalence ranging from 15% to 30%.9,10 These disorders are also associated with increased risk of suicide.11 For all of these disorders, depending on the severity of presentation and the preference of the patient, treatments are often a combination of psychotherapy and psychopharmacology.
Clinicians face several challenges when considering antidepressants for pediatric patients. Pediatricians and psychiatrists need to understand whether these medications work in children and adolescents, and whether there are unique developmental safety and tolerability issues. The evidence base in child psychiatry is considerably smaller compared with that of adult psychiatry. From this more limited evidence base also came the controversial “black-box” warning regarding a risk of emergent suicidality when starting antidepressants that accompanies all antidepressants for pediatric, but not adult, patients. This warning has had major effects on clinical encounters with children experiencing depression, including altering clinician prescribing behavior.12
Do antidepressants work in children?
Selective serotonin reuptake inhibitors. Selective serotonin reuptake inhibitors (SSRIs) are the most commonly used class of antidepressants in both children and adults.13 While only a few SSRIs are FDA-approved for pediatric indications, the lack of FDA approval is typically related to a lack of sufficient testing in randomized controlled trials (RCTs) for specific pediatric indications, rather than to demonstrable differences in efficacy between antidepressant agents. Since there is currently no data to suggest inferiority of one agent compared to another in children or adults,14,15 efficacy data will be discussed here as applied to the class of SSRIs, generalizing from RCTs conducted on individual drugs. Table 1 lists FDA indications and dosing information for individual antidepressants.
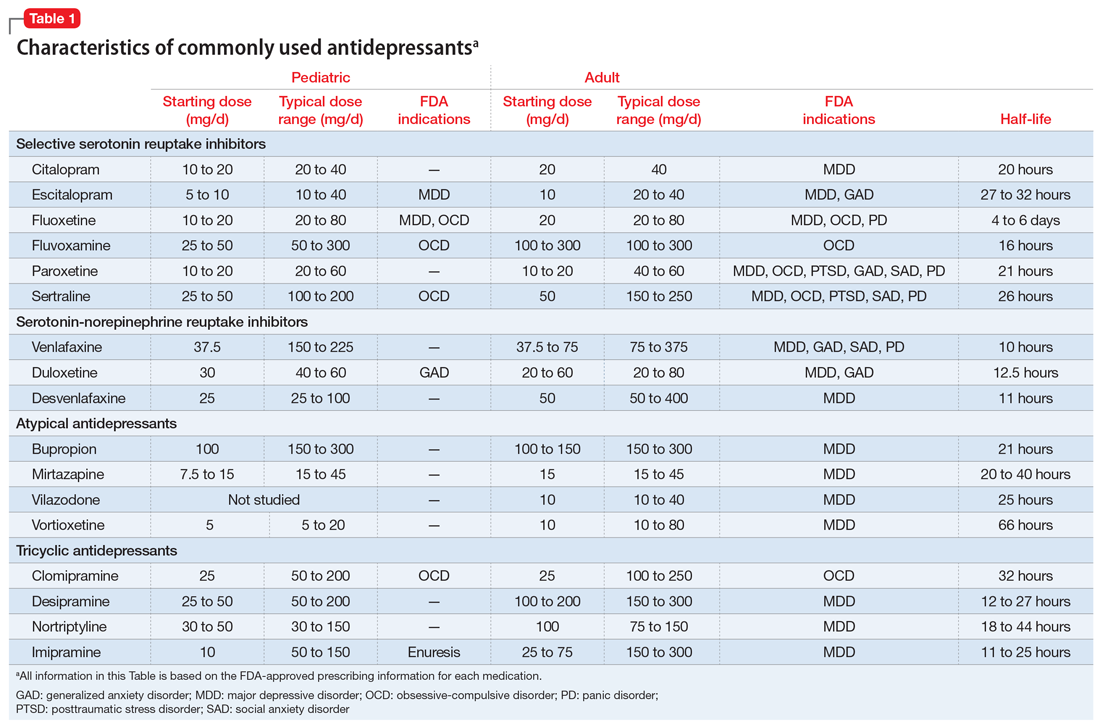
There is strong evidence that SSRIs are effective for treating pediatric anxiety disorders (eg, social anxiety disorder and GAD)16 and OCD,17 with numbers needed to treat (NNT) between 3 and 5. For both of these disorders, SSRIs combined with cognitive-behavioral therapy (CBT) have the highest likelihood of improving symptoms or achieving remission.17,18
Selective serotonin reuptake inhibitors are also effective for treating pediatric MDD; however, the literature is more complex for this disorder compared to GAD and OCD as there are considerable differences in effect sizes between National Institute of Mental Health (NIMH)–funded studies and industry-sponsored trials.13 The major NIMH-sponsored adolescent depression trial, TADS (Treatment for Adolescents and Depression Study), showed that SSRIs (fluoxetine in this case) were quite effective, with an NNT of 4 over the acute phase (12 weeks).19 Ultimately, approximately 80% of adolescents improved over 9 months. Many industry-sponsored trials for MDD in pediatric patients had large placebo response rates (approximately 60%), which resulted in smaller between-group differences, and estimates of an NNT closer to 12,13 which has muddied the waters in meta-analyses that include all trials.20 Improvement in depressive symptoms also appears to be bolstered by concomitant CBT in MDD,19 but not as robustly as in GAD and OCD. While the full benefit of SSRIs for depression may take as long as 8 weeks, a meta-analysis of depression studies of pediatric patients suggests that significant benefits from placebo are observed as early as 2 weeks, and that further treatment gains are minimal after 4 weeks.15 Thus, we recommend at least a 4- to 6-week trial at therapeutic dosing before deeming a medication a treatment failure.
Continue to: Posttraumatic stress disorder...
Posttraumatic stress disorder is a fourth disorder in which SSRIs are a first-line treatment in adults. The data for using SSRIs to treat pediatric patients with PTSD is scant, with only a few RCTs, and no large NIMH-funded trials. Randomized controlled trials have not demonstrated significant differences between SSRIs and placebo21,22 and thus the current first-line recommendation in pediatric PTSD remains trauma-focused therapy, with good evidence for trauma-focused CBT.23 Practically speaking, there can be considerable overlap of PTSD, depression, and anxiety symptoms in children,23 and children with a history of trauma who also have comorbid MDD may benefit from medication if their symptoms persist despite an adequate trial of psychotherapy.
Taken together, the current evidence suggests that SSRIs are often effective in pediatric GAD, OCD, and MDD, with low NNTs (ranging from 3 to 5 based on NIMH-funded trials) for all of these disorders; there is not yet sufficient evidence of efficacy in pediatric patients with PTSD.
Fluoxetine has been studied more intensively than other SSRIs (for example, it was the antidepressant used in the TADS trial), and thus has the largest evidence base. For this reason, fluoxetine is often considered the first of the first-line options. Additionally, fluoxetine has a longer half-life than other antidepressants, which may make it more effective in situations where patients are likely to miss doses, and results in a lower risk of withdrawal symptoms when stopped due to “self-tapering.”
SNRIs and atypical antidepressants. Other antidepressants commonly used in pediatric patients but with far less evidence of efficacy include serotonin-norepinephrine reuptake inhibitors (SNRIs) and the atypical antidepressants bupropion and mirtazapine. The SNRI duloxetine is FDA-approved for treating GAD in children age 7 to 17, but there are no other pediatric indications for duloxetine, or for the other SNRIs.
In general, adverse effect profiles are worse for SNRIs compared to SSRIs, further limiting their utility. While there are no pediatric studies demonstrating SNRI efficacy for neuropathic pain, good data exists in adults.24 Thus, an SNRI could be a reasonable option if a pediatric patient has failed prior adequate SSRI trials and also has comorbid neuropathic pain.
Continue to: Neither bupropion nor mirtazapine...
Neither bupropion nor mirtazapine have undergone rigorous testing in pediatric patients, and therefore these agents should generally be considered only once other first-line treatments have failed. Bupropion has been evaluated for attention-deficit/hyperactivity disorder (ADHD)25 and for adolescent smoking cessation.26 However, the evidence is weak, and bupropion is not considered a first-line option for children and adolescents.
Tricyclic antidepressants. Randomized controlled trials have demonstrated that tricyclic antidepressants (TCAs) are efficacious for treating several pediatric conditions; however, their significant side effect profile, their monitoring requirements, as well as their lethality in overdose has left them replaced by SSRIs in most cases. That said, they can be appropriate in refractory ADHD (desipramine27,28) and refractory OCD (clomipramine is FDA-approved for this indication29); they are considered a third-line treatment for enuresis.30
Why did my patient stop the medication?
Common adverse effects. Although the greatest benefit of antidepressant medications compared with placebo is achieved relatively early on in treatment, it generally takes time for these benefits to accrue and become clinically apparent.15,31 By contrast, most adverse effects of antidepressants present and are at their most severe early in treatment. The combination of early adverse effects and delayed efficacy leads many patients, families, and clinicians to discontinue medications before they have an adequate chance to work. Thus, it is imperative to provide psychoeducation before starting a medication about the typical time-course of improvement and adverse effects (Table 2).
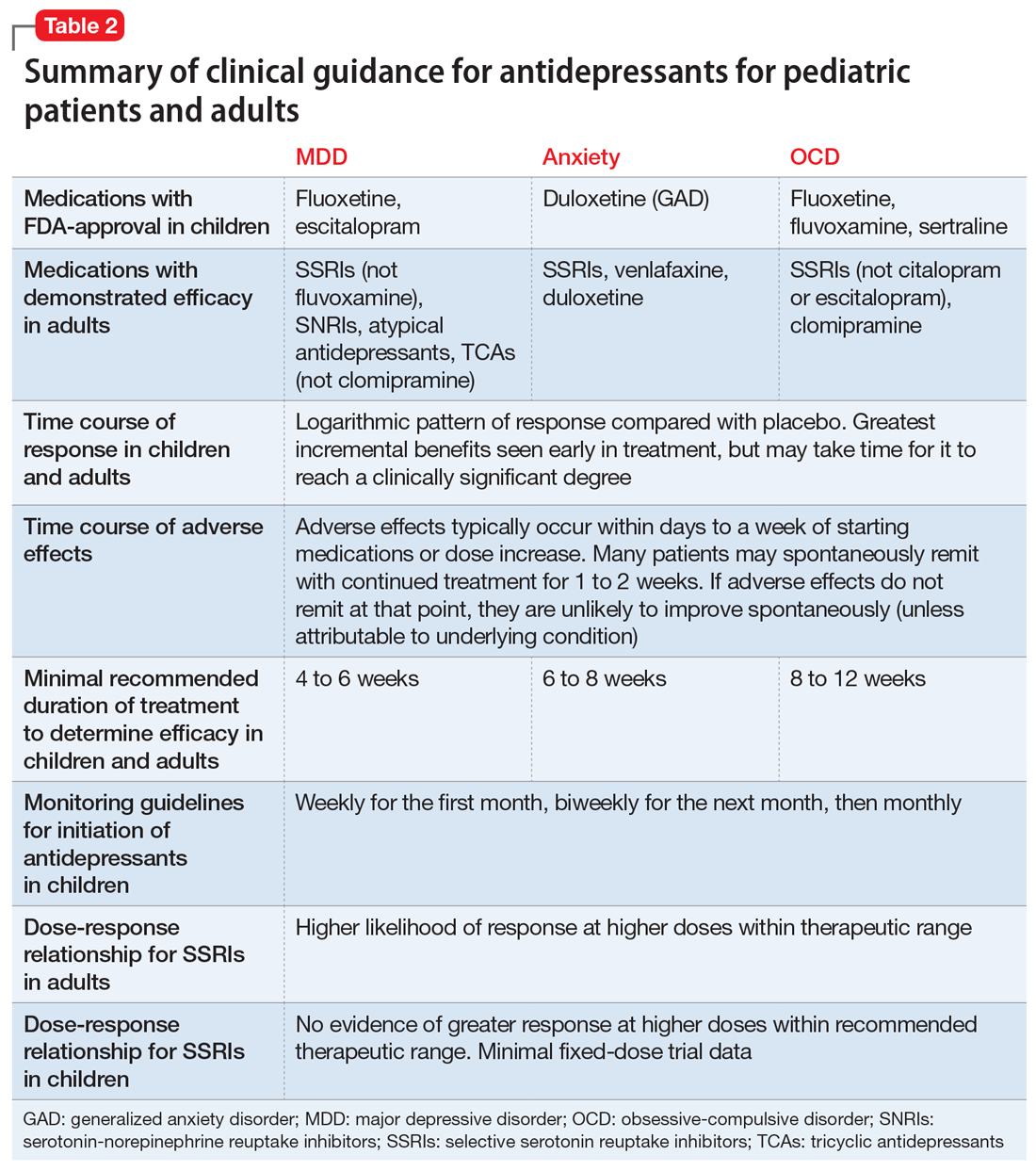
Adverse effects of SSRIs often appear or worsen transiently during initiation of a medication, during a dose increase,32 or, theoretically, with the addition of a medication that interferes with SSRI metabolism (eg, cimetidine inhibition of cytochrome P450 2D6).33 If families are prepared for this phenomenon and the therapeutic alliance is adequate, adverse effects can be tolerated to allow for a full medication trial. Common adverse effects of SSRIs include sleep problems (insomnia/sedation), gastrointestinal upset, sexual dysfunction, dry mouth, and hyperhidrosis. Although SSRIs differ somewhat in the frequency of these effects, as a class, they are more similar than different. Adequate psychoeducation is especially imperative in the treatment of OCD and anxiety disorders, where there is limited evidence of efficacy for any non-serotonergic antidepressants.
Serotonin-norepinephrine reuptake inhibitors are not considered first-line medications because of the reduced evidence base compared to SSRIs and their enhanced adverse effect profiles. Because SNRIs partially share a mechanism of action with SSRIs, they also share portions of the adverse effects profile. However, SNRIs have the additional adverse effect of hypertension, which is related to their noradrenergic activity. Thus, it is reasonable to obtain a baseline blood pressure before initiating an SNRI, as well as periodically after initiation and during dose increases, particularly if the patient has other risk factors for hypertension.34
Continue to: Although TCAs have efficacy...
Although TCAs have efficacy in some pediatric disorders,27-29,35 their adverse effect profile limits their use. Tricyclic antidepressants are highly anticholinergic (causing dizziness secondary to orthostatic hypotension, dry mouth, and urinary retention) and antihistaminergic (causing sedation and weight gain). Additionally, TCAs lower the seizure threshold and have adverse cardiac effects relating to their anti-alpha-1 adrenergic activity, resulting in dose-dependent increases in the QTc and cardiac toxicity in overdose that could lead to arrhythmia and death. These medications have their place, but their use requires careful informed consent, clear treatment goals, and baseline and periodic cardiac monitoring (via electrocardiogram).
Serious adverse effects. Clinicians may be hesitant to prescribe antidepressants for pediatric patients because of the potential for more serious adverse effects, including severe behavioral activation syndromes, serotonin syndrome, and emergent suicidality. However, current FDA-approved antidepressants arguably have one of the most positive risk/benefit profiles of any orally-administered medication approved for pediatric patients. Having a strong understanding of the evidence is critical to evaluating when it is appropriate to prescribe an antidepressant, how to properly monitor the patient, and how to obtain accurate informed consent.
Pediatric behavioral activation syndrome. Many clinicians report that children receiving antidepressants experience a pediatric behavioral activation syndrome, which exists along a spectrum from mild activation, increased energy, insomnia, or irritability up through more severe presentations of agitation, hyperactivity, or possibly mania. A recent meta-analysis suggested a positive association between antidepressant use and activation events on the milder end of this spectrum in pediatric patients with non-OCD anxiety disorders,16 and it is thought that compared with adolescents, younger children are more susceptible to activation adverse effects.36 The likelihood of activation events has been associated with higher antidepressant plasma levels,37 suggesting that dose or individual differences in metabolism may play a role. At the severe end of the spectrum, the risk of induction of mania in pediatric patients with depression or anxiety is relatively rare (<2%) and not statistically different from placebo in RCTs of pediatric participants.38 Meta-analyses of larger randomized, placebo-controlled trials of adults do not support the idea that SSRIs and other second-generation antidepressants carry an increased risk of mania compared with placebo.39,40 Children or adolescents with bona fide bipolar disorder (ie, patients who have had observed mania that meets all DSM-5 criteria) should be treated with a mood-stabilizing agent or antipsychotic if prescribed an antidepressant.41 These clear-cut cases are, however, relatively rare, and more often clinicians are confronted with ambiguous cases that include a family history of bipolar disorder along with “softer” symptoms of irritability, intrusiveness, or aggression. In these children, SSRIs may be appropriate for depressive, OCD, or anxiety symptoms, and should be strongly considered before prescribing antipsychotics or mood stabilizers, as long as initiated with proper monitoring.
Serotonin syndrome is a life-threatening condition caused by excess synaptic serotonin. It is characterized by confusion, sweating, diarrhea, hypertension, hyperthermia, and tachycardia. At its most severe, serotonin syndrome can result in seizures, arrhythmias, and death. The risk of serotonin syndrome is very low when using an SSRI as monotherapy. Risk increases with polypharmacy, particularly unexamined polypharmacy when multiple serotonergic agents are inadvertently on board. Commonly used serotonergic agents include other antidepressants, migraine medications (eg, triptans), some pain medications, and the cough suppressant dextromethorphan.
The easiest way to mitigate the risk of serotonin syndrome is to use an interaction index computer program, which can help ensure that the interacting agents are not prescribed without first discussing the risks and benefits. It is important to teach adolescents that certain recreational drugs are highly serotonergic and can cause serious interactions with antidepressants. For example, recreational use of dextromethorphan or 3,4-methylenedioxymethamphetamine (MDMA; commonly known as “ecstasy”) has been associated with serotonin syndrome in adolescents taking antidepressant medications.42,43
Continue to: Suicidality
Suicidality. The black-box warning regarding a risk of emergent suicidality when starting antidepressant treatment in children is controversial.44 The prospect that a medication intended to ameliorate depression might instead risk increasing suicidal thinking is alarming to parents and clinicians alike. To appropriately weigh and discuss the risks and benefits with families, it is important to understand the data upon which the warning is based.
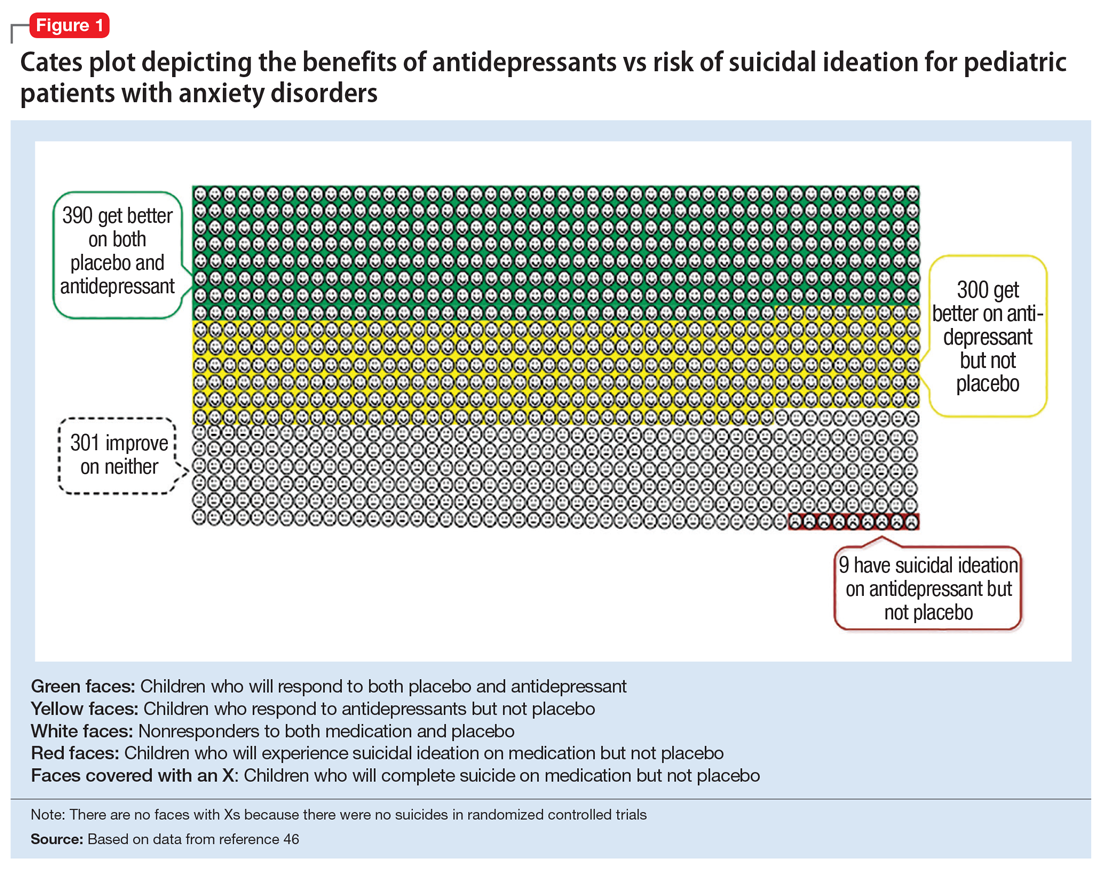
In 2004, the FDA commissioned a review of 23 antidepressant trials, both published and unpublished, pooling studies across multiple indications (MDD, OCD, anxiety, and ADHD) and multiple antidepressant classes. This meta-analysis, which included nearly 4,400 pediatric patients, found a small but statistically significant increase in spontaneously-reported suicidal thoughts or actions, with a risk difference of 1% (95% confidence interval [CI], 1% to 2%).45 These data suggest that if one treats 100 pediatric patients, 1 to 2 of them may experience short-term increases in suicidal thinking or behavior.45 There were no differences in suicidal thinking when assessed systematically (ie, when all subjects reported symptoms of suicidal ideation on structured rating scales), and there were no completed suicides.45 A subsequent analysis that included 27 pediatric trials suggested an even lower, although still significant, risk difference (<1%), yielding a number needed to harm (NNH) of 143.46 Thus, with low NNT for efficacy (3 to 6) and relatively high NNH for emergent suicidal thoughts or behaviors (100 to 143), for many patients the benefits will outweigh the risks.
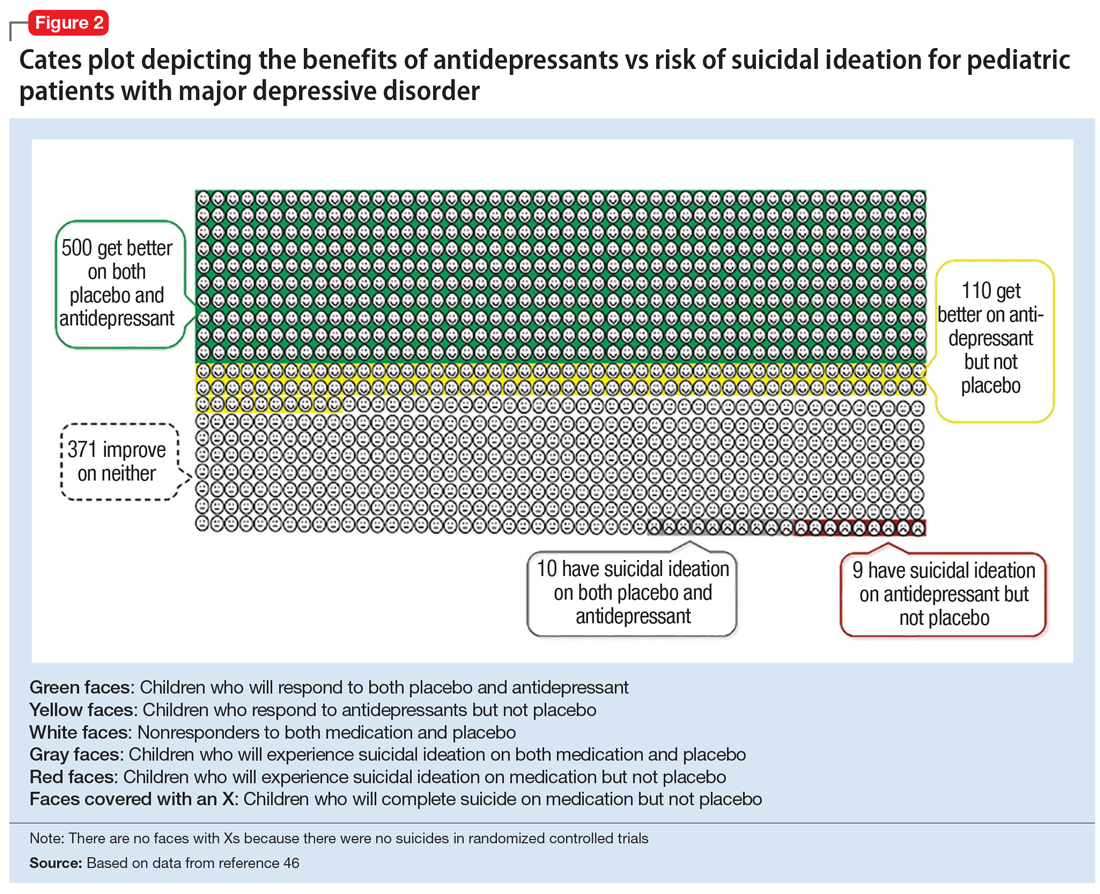
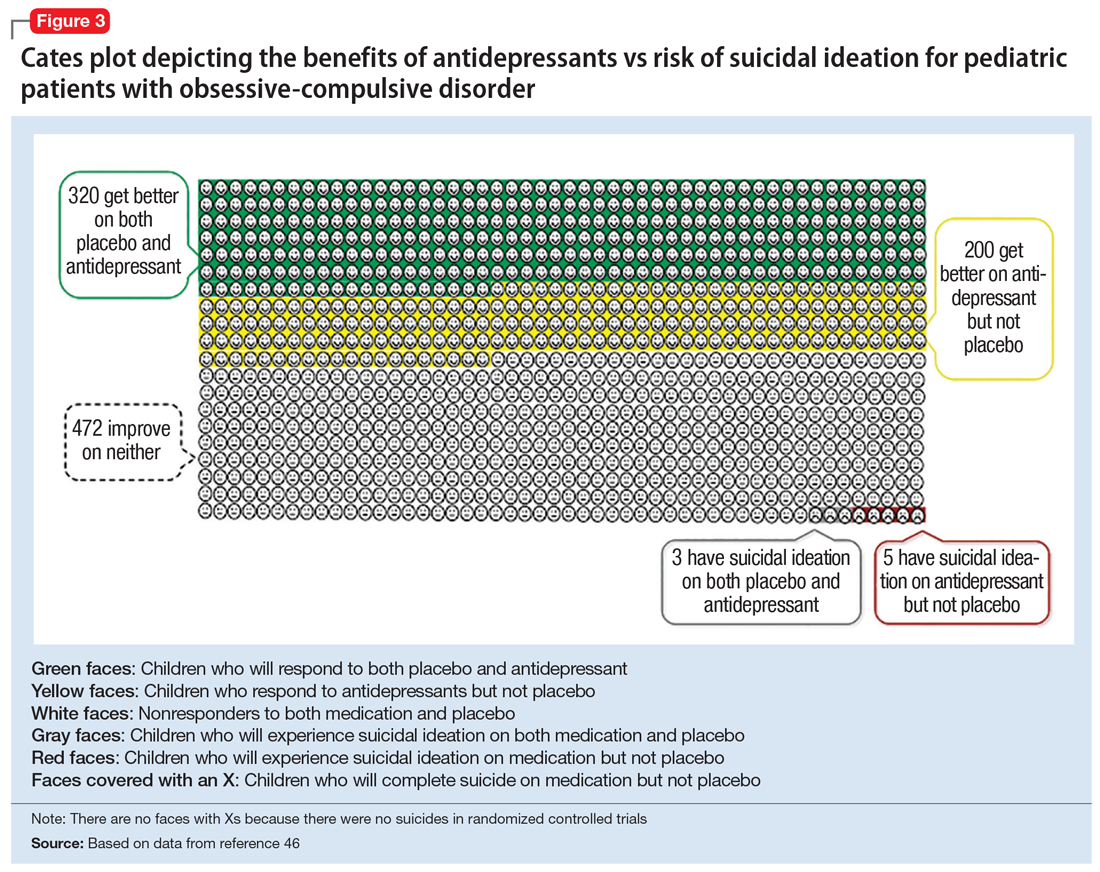
Figure 1, Figure 2, and Figure 3 are Cates plots that depict the absolute benefits of antidepressants compared with the risk of suicidality for pediatric patients with MDD, OCD, and anxiety disorders. Recent meta-analyses have suggested that the increased risk of suicidality in antidepressant trials is specific to studies that included children and adolescents, and is not observed in adult studies. A meta-analysis of 70 trials involving 18,526 participants suggested that the odds ratio of suicidality in trials of children and adolescents was 2.39 (95% CI, 1.31 to 4.33) compared with 0.81 (95% CI, 0.51 to 1.28) in adults.47 Additionally, a network meta-analysis exclusively focusing on pediatric antidepressant trials in MDD reported significantly higher suicidality-related adverse events in venlafaxine trials compared with placebo, duloxetine, and several SSRIs (fluoxetine, paroxetine, and escitalopram).20 These data should be interpreted with caution as differences in suicidality detected between agents is quite possibly related to differences in the method of assessment between trials, as opposed to actual differences in risk between agents.
Epidemiologic data further support the use of antidepressants in pediatric patients, showing that antidepressant use is associated with decreased teen suicide attempts and completions,48 and the decline in prescriptions that occurred following the black-box warning was accompanied by a 14% increase in teen suicides.49 Multiple hypotheses have been proposed to explain the pediatric clinical trial findings. One idea is that potential adverse effects of activation, or the intended effects of restoring the motivation, energy, and social engagement that is often impaired in depression, increases the likelihood of thinking about suicide or acting on thoughts. Another theory is that reporting of suicidality may be increased, rather than increased de novo suicidality itself. Antidepressants are effective for treating pediatric anxiety disorders, including social anxiety disorder,16 which could result in more willingness to report. Also, the manner in which adverse effects are generally ascertained in trials might have led to increased spontaneous reporting. In many trials, investigators ask whether participants have any adverse effects in general, and inquire about specific adverse effects only if the family answers affirmatively. Thus, the increased rate of other adverse effects associated with antidepressants (sleep problems, gastrointestinal upset, dry mouth, etc.) might trigger a specific question regarding suicidal ideation, which the child or family then may be more likely to report. Alternatively, any type of psychiatric treatment could increase an individual’s propensity to report; in adolescent psychotherapy trials, non-medic
Continue to: How long should the antidepressant be continued?
How long should the antidepressant be continued?
Many patients are concerned about how long they may be taking medication, and whether they will be taking an antidepressant “forever.” A treatment course can be broken into an acute phase, wherein remission is achieved and maintained for 6 to 8 weeks. This is followed by a continuation phase, with the goal of relapse prevention, lasting 16 to 20 weeks. The length of the last phase—the maintenance phase—depends both on the child’s history, the underlying therapeutic indication, the adverse effect burden experienced, and the family’s preferences/values. In general, for a first depressive episode, after treating for 1 year, a trial of discontinuation can be attempted with close monitoring. For a second depressive episode, we recommend 2 years of remission on antidepressant therapy before attempting discontinuation. In patients who have had 3 depressive episodes, or have had episodes of high severity, we recommend continuing antidepressant treatment indefinitely. Although much less well studied, the risk of relapse following SSRI discontinuation appears much more significant in OCD, whereas anxiety disorders and MDD have a relatively comparable risk.52
In general, stopping an antidepressant should be done carefully and slowly.
What to do when first-line treatments fail
Adequate psychotherapy? To determine whether children are receiving adequate CBT, ask:
- if the child receives homework from psychotherapy
- if the parents are included in treatment
- if therapy has involved identifying thought patterns that may be contributing to the child’s illness, and
- if the therapist has ever exposed the child to a challenge likely to produce anxiety or distress in a supervised environment and has developed an exposure hierarchy (for conditions with primarily exposure-based therapies, such as OCD or anxiety disorders).
If a family is not receiving most of these elements in psychotherapy, this is a good indicator that they may not be receiving evidence-based CBT.
Continue to: Adequate pharmacotherapy?
Adequate pharmacotherapy? Similarly, when determining the adequacy of previous pharmacotherapy, it is critical to determine whether the child received an adequate dose of medications (at least the FDA-recommended minimum dose) for an adequate duration of time at therapeutic dosing (at least 6 weeks for MDD, 8 weeks for anxiety disorders, and 8 to 12 weeks for pediatric patients with OCD), and that the child actually took the medication regularly during that period. Patient compliance can typically be tracked through checking refill requests or intervals through the patient’s pharmacy. Ensuring proper delivery of first-line treatments is imperative because (1) the adverse effects associated with second-line treatments are often more substantial; (2) the cost in terms of time and money is considerably higher with second-line treatments, and; (3) the evidence regarding the benefits of these treatments is much less certain.
Inadequate dosing is a common reason for non-response in pediatric patients. Therapeutic dose ranges for common antidepressants are displayed in Table 1. Many clinicians underdose antidepressants for pediatric patients initially (and often throughout treatment) because they fear that the typical dose titration used in clinical trials will increase the risk of adverse effects compared with more conservative dosing. There is limited evidence to suggest that this underdosing strategy is likely to be successful; adverse effects attributable to these medications are modest, and most that are experienced early in treatment (eg, headache, increased anxiety or irritability, sleep problems, gastrointestinal upset) are self-limiting and may be coincidental rather than medication-induced. Furthermore, there is no evidence for efficacy of subtherapeutic dosing in children in the acute phase of treatment or for preventing relapse.14 Thus, from an efficacy standpoint, a medication trial has not officially begun until the therapeutic dose range is reached.
Once dosing is within the therapeutic range, however, pediatric data differs from the adult literature. In most adult psychiatric conditions, higher doses of SSRIs within the therapeutic range are associated with an increased response rate.14,54 In pediatrics, there are few fixed dose trials, and once within the recommended therapeutic range, minimal data supports an association between higher dosing and higher efficacy.14 In general, pediatric guidelines are silent regarding optimal dosing of SSRIs within the recommended dose range, and higher antidepressant doses often result in a more significant adverse effect burden for children. One exception is pediatric OCD, where, similar to adults, the guidelines suggest that higher dosing of SSRIs often is required to induce a therapeutic response as compared to MDD and GAD.31,55
If a child does not respond to adequate first-line treatment (or has a treatment history that cannot be fully verified), repeating these first-line interventions carries little risk and can be quite effective. For example, 60% of adolescents with MDD who did not initially respond to an SSRI demonstrated a significant response when prescribed a second SSRI or venlafaxine (with or without CBT).56
When pediatric patients continue to experience significantly distressing and/or debilitating symptoms (particularly in MDD) despite multiple trials of antidepressants and psychotherapy, practitioners should consider a careful referral to interventional psychiatry services, which can include the more intensive treatments of electroconvulsive therapy, repetitive transcranial magnetic stimulation, or ketamine (see Box 1). Given the substantial morbidity and mortality associated with adolescent depression, interventional psychiatry treatments are under-researched and under-utilized clinically in pediatric populations.
Continue to: Antidepressants in general...
Antidepressants in general, and SSRIs in particular, are the first-line pharmacotherapy for pediatric anxiety, OCD, and MDD. For PTSD, although they are a first-line treatment in adults, their efficacy has not been demonstrated in children and adolescents. Antidepressants are generally safe, well-tolerated, and effective, with low NNTs (3 to 5 for anxiety and OCD; 4 to 12 in MDD, depending on whether industry trials are included). It is important that clinicians and families be educated about possible adverse effects and their time course in order to anticipate difficulties, ensure adequate informed consent, and monitor appropriately. The black-box warning regarding treatment-emergent suicidal thoughts or behaviors must be discussed (for suggested talking points, see Box 2). The NNH is large (100 to 143), and for many patients, the benefits will outweigh the risks. For pediatric patients who fail to respond to multiple adequate trials of antidepressants and psychotherapy, referrals for interventional psychiatry consultation should be considered.
Bottom Line
Although the evidence base for prescribing antidepressants for children and adolescents is smaller compared to the adult literature, properly understanding and prescribing these agents, and explaining their risks and benefits to families, can make a major difference in patient compliance, satisfaction, and outcomes. Antidepressants (particularly selective serotonin reuptake inhibitors) are the firstline pharmacologic intervention for pediatric patients with anxiety disorders, obsessive-compulsive disorder, or major depressive disorder.
Related Resource
- Bonin L, Moreland CS. Overview of prevention and treatment for pediatric depression. https://www.uptodate.com/contents/overview-of-prevention-and-treatment-for-pediatric-depression. Last reviewed July 2019.
Drug Brand Names
Bupropion • Wellbutrin, Zyban
Cimetidine • Tagamet
Citalopram • Celexa
Clomipramine • Anafranil
Desipramine • Norpramin
Desvenlafaxine • Pristiq
Duloxetine • Cymbalta
Escitalopram • Lexapro
Fluoxetine • Prozac
Fluvoxamine • Luvox
Imipramine • Tofranil
Mirtazapine • Remeron
Nortriptyline • Pamelor
Paroxetine • Paxil
Sertraline • Zoloft
Venlafaxine • Effexor
Vilazodone • Viibryd
Vortioxetine • Trintellix
Box 1
Continuing severe depression is associated with reduced educational attainment and quality of life, as well as increased risk of substance abuse and suicide,1,2 which is the second leading cause of death in individuals age 10 to 24 years.3 Given the substantial morbidity and mortality associated with adolescent depression, interventional psychiatry treatments are under-researched and underutilized in pediatric patients. Interventional antidepressants in adults include electroconvulsive therapy (ECT), repetitive transcranial magnetic stimulation (rTMS), and, most recently, ketamine.
Electroconvulsive therapy is the most effective therapy available for depression in adults, alleviating depressive symptoms in treatment-refractory patients and outperforming both pharmacotherapy4 and rTMS.5 Despite its track record of effectiveness and safety in adults, ECT continues to suffer considerable stigma.4 Cognitive adverse effects and memory problems in adults are generally self-limited, and some aspects of cognition actually improve after ECT as depression lifts.6 The combination of stigma and the concern about possible cognitive adverse effects during periods of brain development have likely impeded the rigorous testing of ECT in treatment-refractory pediatric patients. Several case series and other retrospective analyses suggest, however, that ECT has strong efficacy and limited adverse effects in adolescents who have severe depression or psychotic symptoms.7-9 Despite these positive preliminary data in pediatric patients, and a large body of literature in adults, no controlled trials of ECT have been conducted in the pediatric population, and it remains a rarely used treatment in severe pediatric mental illness.
Repetitive transcranial magnetic stimulation is a technique in which magnetic stimulation is used to activate the left dorsolateral prefrontal cortex (DLPFC), a target thought to be important in the pathophysiology of MDD. Repetitive transcranial magnetic stimulation is FDAapproved to treat medication-refractory major depressive disorder (MDD) in adults, and has been shown to be effective as both a monotherapy10 and an adjunctive treatment.11 The estimated number needed to treat (NNT) for rTMS ranges from 6 to 8, which is quite effective, although less so than ECT (and probably initial pharmacotherapy).5 Similar to ECT, however, there are no large randomized controlled trials (RCTs) in children or adolescents. Pilot RCTs12 and open trials13 suggest that DLPFC rTMS may be effective as an adjunctive treatment, speeding or augmenting response to a selective serotonin reuptake inhibitor in adolescents with MDD. Larger trials studying rTMS in pediatric patients are needed. While rTMS is generally well tolerated, disadvantages include the time-consuming schedule (the initial treatment is typically 5 days/week for several weeks) and local adverse effects of headache and scalp pain.
Ketamine, which traditionally is used as a dissociative anesthetic, is a rapidly emerging novel treatment in adult treatment-refractory MDD. It acts quickly (within hours to days) and cause significant improvement in difficult symptoms such as anhedonia14 and suicidal ideation.15 In adult studies, ketamine has a robust average effect size of >1.2, and an NNT ranging from 3 to 5 in medication-refractory patients.16,17 Ketamine is a glutamatergic modulator, acting outside of the monoamine neurochemical systems traditionally targeted by standard antidepressants.16 The efficacy of ketamine in treatment-refractory adults is impressive, but the effects of a single treatment are ephemeral, dissipating within 1 to 2 weeks, which has led to significant discussion surrounding optimal dosing strategies.16 Although small RCTs in pediatric patients are currently underway, at this time, the only evidence for ketamine for pediatric MDD is based on case series/report data18,19 which was positive.
For all of these interventional modalities, it is critical to refer children with treatmentrefractory disorders to interventionists who have appropriate experience and monitoring capabilities.
References
1. Weissman MM, Wolk S, Goldstein RB, et al. Depressed adolescents grown up. JAMA.1999;281(18):1707-1713.
2. Fergusson DM, Woodward LJ. Mental health, educational, and social role outcomes of adolescents with depression. Arch Gen Psychiatry. 2002;59(3):225-231.
3. Centers for Disease Control and Prevention. National Vital Statistics System. Deaths, percent of total deaths, and death rates for the 15 leading causes of death in 5-year age groups, by race and sex: United States, 1999-2015. Centers for Disease Control and Prevention. https://www.cdc.gov/nchs/nvss/mortality/lcwk1.htm. Published October 23, 2017. Accessed May 2, 2019.
4. UK ECT Review Group. Efficacy and safety of electroconvulsive therapy in depressive disorders: a systematic review and metaanalysis. Lancet. 2003;361(9360):799-808.
5. Berlim MT, Van den Eynde F, Daskalakis ZJ. Efficacy and acceptability of high frequency repetitive transcranial magnetic stimulation (rTMS) versus electroconvulsive therapy (ECT) for major depression: a systematic review and meta-analysis of randomized trials. Depress Anxiety. 2013;30(7):614-623.
6. Semkovska M, McLoughlin DM. Objective cognitive performance associated with electroconvulsive therapy for depression: a systematic review and meta-analysis. Biol Psychiatry. 2010;68(6):568-577.
7. Jacob P, Gogi PK, Srinath S, et al. Review of electroconvulsive therapy practice from a tertiary child and adolescent psychiatry centre. Asian J Psychiatr. 2014;12(1):95-99.
8. Zhand N, Courtney DB, Flament MF. Use of electroconvulsive therapy in adolescents with treatment-resistant depressive disorders: a case series. J ECT. 2015;31(4):238-245.
9. Puffer CC, Wall CA, Huxsahl JE, et al. A 20 year practice review of electroconvulsive therapy for adolescents. J Child Adolesc Psychopharmacol. 2016;26(7):632-636.
10. Berlim MT, van den Eynde F, Tovar-Perdomo S, et al. Response, remission and drop-out rates following high-frequency repetitive transcranial magnetic stimulation (rTMS) for treating major depression: a systematic review and meta-analysis of randomized, double-blind and sham-controlled trials. Psychol Med. 2014;44(2):225-239.
11. Liu B, Zhang Y, Zhang L, et al. Repetitive transcranial magnetic stimulation as an augmentative strategy for treatment-resistant depression, a meta-analysis of randomized, double-blind and sham-controlled study. BMC Psychiatry. 2014;14:342.
12. Huang ML, Luo BY, Hu JB, et al. Repetitive transcranial magnetic stimulation in combination with citalopram in young patients with first-episode major depressive disorder: a double-blind, randomized, sham-controlled trial. Aust N Z J Psychiatry. 2012;46(3):257-264.
13. Wall CA, Croarkin PE, Sim LA, et al. Adjunctive use of repetitive transcranial magnetic stimulation in depressed adolescents: a prospective, open pilot study. J Clin Psychiatry. 2011;72(9):1263-1269.
14. Lally N, Nugent AC, Luckenbaugh DA, et al. Anti-anhedonic effect of ketamine and its neural correlates in treatment-resistant bipolar depression. Transl Psychiatry. 2014;4:e469. doi: 10.1038/tp.2014.105.
15. Ballard ED, Ionescu DF, Vande Voort JL, et al. Improvement in suicidal ideation after ketamine infusion: relationship to reductions in depression and anxiety. J Psychiatr Res. 2014;58:161-166.
16. Newport DJ, Carpenter LL, McDonald WM, et al. Ketamine and other NMDA antagonists: early clinical trials and possible mechanisms in depression. Am J Psychiatry. 2015;172(10):950-966.
17. McGirr A, Berlim MT, Bond DJ, et al. A systematic review and meta-analysis of randomized, double-blind, placebo-controlled trials of ketamine in the rapid treatment of major depressive episodes. Psychol Med. 2015;45(4):693-704.
18. Dwyer JB, Beyer C, Wilkinson ST, et al. Ketamine as a treatment for adolescent depression: a case report. J Am Acad Child Adolesc Psychiatry. 2017;56(4):352-354.
19. Cullen KR, Amatya P, Roback MG, et al. Intravenous ketamine for adolescents with treatment-resistant depression: an open-label study. J Child Adolesc Psychopharmacol. 2018;28(7):437-444.
Box 2
Efficacy
- Selective serotonin reuptake inhibitors are the most effective pharmacologic treatment we have for pediatric depression, OCD, and anxiety
- More than one-half of children who are prescribed SSRIs have a significant improvement, regardless of condition
- Based on current estimates, we need to treat 4 to 6 children with an SSRI to find one that will improve who would not improve with placebo
- The clinical benefits of SSRIs generally take a while to accrue; therefore, it is advisable to take the medication for at least 2 to 3 months before concluding that it is ineffective
- In addition to medication, evidence-based psychotherapies provide significant benefit for pediatric depression, OCD, and anxiety
Tolerability
- Most commonly prescribed pediatric antidepressants have been used safely in children for 2 to 3 decades. The safety profiles of SSRIs are among the best of any medications used for children and adolescents
- While many children get better when taking these medications, it’s important that we also talk about potential adverse effects. Some children will experience sleep problems (either sleepier than usual or difficulty sleeping), changes in energy levels, headache, gastrointestinal upset, and dry mouth. These are most likely at the beginning of treatment, or when we increase the dose; they usually are time-limited and go away on their own
- Often adverse effects occur first and the benefits come later. Because it may take at least a few weeks to start to see the mood/anxiety benefits, it’s important for us to talk about any adverse effects your child experiences and remember that they usually are short-lived
Suicidality
- The FDA placed a “black-box” warning on antidepressants after pediatric studies found a small but statistically significant increased risk of reporting suicidal thoughts or behaviors over the short-term compared with placebo
- The increased risk of spontaneously reporting suicidal ideation was quite small. Studies suggested that one would need to treat 100 to 140 children to see 1 child report suicidal ideation compared to placebo. Suicidal ideation is a common symptom in children with depression and anxiety
- Studies found no increased risk when suicidal ideation was systematically assessed using structured rating scales
- In the studies evaluated, there were no completed suicides by patients taking medication or placebo
- Population studies show that higher rates of antidepressant prescriptions are associated with lower rates of attempted and completed teen suicide, which underscores that in general, these medicines treat the underlying causes of suicidality
- No scientific consensus exists on whether these medications are truly associated with an increased risk of new-onset suicidal ideation, or if this association is due to other factors (eg, improvement in anxiety and depressive symptoms that make patients more comfortable to report suicidal ideation spontaneously)
- Regardless, the FDA recommends frequent monitoring of children for suicidal thoughts when these medications are started. This should be done anyway in children experiencing depression and anxiety, and it’s why we will plan to have more frequent appointments as the medication is initiated
OCD: obsessive-compulsive disorder; SSRIs: selective serotonin reuptake inhibitors
1. Williams SB, O’Connor EA, Eder M, et al. Screening for child and adolescent depression in primary care settings: a systematic evidence review for the US Preventive Services Task Force. Pediatrics. 2009;123(4):e716-e735. doi: 10.1542/peds.2008-2415.
2. Kessler RC, Avenevoli S, Ries Merikangas K. Mood disorders in children and adolescents: an epidemiologic perspective. Biol Psychiatry. 2001;49(12):1002-1014.
3. Lewinsohn PM, Clarke GN, Seeley JR, et al. Major depression in community adolescents: age at onset, episode duration, and time to recurrence. J Am Acad Child Adolesc Psychiatry. 1994;33(6):809-818.
4. Weissman MM, Wolk S, Goldstein RB, et al. Depressed adolescents grown up. JAMA.1999;281(18):1707-1713.
5. Fergusson DM, Woodward LJ. Mental health, educational, and social role outcomes of adolescents with depression. Arch Gen Psychiatry. 2002;59(3):225-231.
6. Keenan-Miller D, Hammen CL, Brennan PA. Health outcomes related to early adolescent depression. J Adolesc Health. 2007; 41(3): 256-62.
7. Shaffer D, Gould MS, Fisher P, et al. Psychiatric diagnosis in child and adolescent suicide. Arch Gen Psychiatry. 1996;53(4):339-348.
8. Centers for Disease Control and Prevention. National Vital Statistics System. Deaths, percent of total deaths, and death rates for the 15 leading causes of death in 5-year age groups, by race and sex: United States, 1999-2015. https://www.cdc.gov/nchs/nvss/mortality/lcwk1.htm. Published October 23, 2017. Accessed May 2, 2019.
9. Merikangas KR, He JP, Burstein M, et al. Lifetime prevalence of mental disorders in US adolescents: results from the National Comorbidity Survey Replication-Adolescent Supplement (NCS-A). J Am Acad Child Adolesc Psychiatry. 2010;49(10):980-989.
10. Wittchen HU, Nelson CB, Lachner G. Prevalence of mental disorders and psychosocial impairments in adolescents and young adults. Psychol Med. 1998;28(1):109-126.
11. Foley DL, Goldston DB, Costello EJ, et al. Proximal psychiatric risk factors for suicidality in youth: the Great Smoky Mountains Study. Arch Gen Psychiatry. 2006;63(9):1017-1024.
12. Cheung A, Sacks D, Dewa CS, et al. Pediatric prescribing practices and the FDA black-box warning on antidepressants. J Dev Behav Pediatr. 2008 29(3):213-215.
13. Walkup JT. Antidepressant efficacy for depression in children and adolescents: industry- and NIMH-funded studies. Am J Psychiatry. 2017;174(5):430-437.
14. Jakubovski E, Varigonda AL, Freemantle N, et al. Systematic review and meta-analysis: dose-response relationship of selective serotonin reuptake inhibitors in major depressive disorder. Am J Psychiatry. 2016;173(2):174-183.
15. Varigonda AL, Jakubovski E, Taylor MJ, et al. Systematic review and meta-analysis: early treatment responses of selective serotonin reuptake inhibitors in pediatric major depressive disorder. J Am Acad Child Adolesc Psychiatry. 2015;54(7):557-564.
16. Strawn JR, Welge JA, Wehry AM, et al. Efficacy and tolerability of antidepressants in pediatric anxiety disorders: a systematic review and meta-analysis. Depress Anxiety. 2015;32(3):149-157.
17. March JS, Biederman J, Wolkow R, et al. Sertraline in children and adolescents with obsessive-compulsive disorder: a multicenter randomized controlled trial. JAMA. 1998;280(20):1752-1756.
18. Walkup JT, Albano AM, Piacentini J, et al. Cognitive behavioral therapy, sertraline, or a combination in childhood anxiety. N Engl J Med. 2008;359(26):2753-2766.
19. Kennard BD, Silva SG, Tonev S, et al. Remission and recovery in the Treatment for Adolescents with Depression Study (TADS): acute and long-term outcomes. J Am Acad Child Adolesc Psychiatry. 2009;48(2):186-195.
20. Cipriani A, Zhou X, Del Giovane C, et al. Comparative efficacy and tolerability of antidepressants for major depressive disorder in children and adolescents: a network meta-analysis. Lancet. 2016;388(10047):881-890.
21. Cohen JA, Mannarino AP, Perel JM, et al. A pilot randomized controlled trial of combined trauma-focused CBT and sertraline for childhood PTSD symptoms. J Am Acad Child Adolesc Psychiatry. 2007;46(7):811-819.
22. Robb AS, Cueva JE, Sporn J, et al. Sertraline treatment of children and adolescents with posttraumatic stress disorder: a double-blind, placebo-controlled trial. J Child Adolesc Psychopharmacol. 2010;20(6):463-471.
23. Diehle J, Opmeer BC, Boer F, et al. Trauma-focused cognitive behavioral therapy or eye movement desensitization and reprocessing: what works in children with posttraumatic stress symptoms? A randomized controlled trial. Eur Child Adolesc Psychiatry. 2015;24(2):227-236.
24. Aiyer R, Barkin RL, Bhatia A. Treatment of neuropathic pain with venlafaxine: a systematic review. Pain Med. 2017;18(10):1999-2012.
25. Barrickman LL, Perry PJ, Allen AJ, et al. Bupropion versus methylphenidate in the treatment of attention-deficit hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 1995;34(5):649-657.
26. Monuteaux MC, Spencer TJ, Faraone SV, et al. A randomized, placebo-controlled clinical trial of bupropion for the prevention of smoking in children and adolescents with attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2007;68(7):1094-1101.
27. Biederman J, Baldessarini RJ, Wright V, et al. A double-blind placebo controlled study of desipramine in the treatment of ADD: I. Efficacy. J Am Acad Child Adolesc Psychiatry. 1989;28(5):777-784.
28. Spencer T, Biederman J, Coffey B, et al. A double-blind comparison of desipramine and placebo in children and adolescents with chronic tic disorder and comorbid attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2002;59(7):649-656.
29. DeVeaugh-Geiss J, Moroz G, Biederman J, et al. Clomipramine hydrochloride in childhood and adolescent obsessive-compulsive disorder--a multicenter trial. J Am Acad Child Adolesc Psychiatry. 1992;31(1):45-49.
30. Caldwell PH, Sureshkumar P, Wong WC. Tricyclic and related drugs for nocturnal enuresis in children. Cochrane Database Syst Rev. 2016;(1):CD002117.
31. Varigonda AL, Jakubovski E, Bloch MH. Systematic review and meta-analysis: early treatment responses of selective serotonin reuptake inhibitors and clomipramine in pediatric obsessive-compulsive disorder. J Am Acad Child Adolesc Psychiatry. 2016;55(10):851-859.e2. doi: 10.1016/j.jaac.2016.07.768.
32. Walkup J, Labellarte M. Complications of SSRI treatment. J Child Adolesc Psychopharmacol. 2001;11(1):1-4.
33. Leo RJ, Lichter DG, Hershey LA. Parkinsonism associated with fluoxetine and cimetidine: a case report. J Geriatr Psychiatry Neurol. 1995;8(4):231-233.
34. Strawn JR, Prakash A, Zhang Q, et al. A randomized, placebo-controlled study of duloxetine for the treatment of children and adolescents with generalized anxiety disorder. J Am Acad Child Adolesc Psychiatry. 2015;54(4):283-293.
35. Bernstein GA, Borchardt CM, Perwien AR, et al. Imipramine plus cognitive-behavioral therapy in the treatment of school refusal. J Am Acad Child Adolesc Psychiatry. 2000;39(3): 276-283.
36. Safer DJ, Zito JM. Treatment-emergent adverse events from selective serotonin reuptake inhibitors by age group: children versus adolescents. J Child Adolesc Psychopharmacol. 2006;16(1-2):159-169.
37. Reinblatt SP, DosReis S, Walkup JT, et al. Activation adverse events induced by the selective serotonin reuptake inhibitor fluvoxamine in children and adolescents. J Child Adolesc Psychopharmacol. 2009;19(2):119-126.
38. Goldsmith M, Singh M, Chang K. Antidepressants and psychostimulants in pediatric populations: is there an association with mania? Paediatr Drugs. 2011;13(4): 225-243.
39. Sidor MM, Macqueen GM. Antidepressants for the acute treatment of bipolar depression: a systematic review and meta-analysis. J Clin Psychiatry. 2011;72(2):156-167.
40. Allain N, Leven C, Falissard B, et al. Manic switches induced by antidepressants: an umbrella review comparing randomized controlled trials and observational studies. Acta Psychiatr Scand. 2017;135(2):106-116.
41. McClellan J, Kowatch R, Findling RL. Practice parameter for the assessment and treatment of children and adolescents with bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2007;46(1):107-125.
42. Dobry Y, Rice T, Sher L. Ecstasy use and serotonin syndrome: a neglected danger to adolescents and young adults prescribed selective serotonin reuptake inhibitors. Int J Adolesc Med Health. 2013; 25(3):193-199.
43. Schwartz AR, Pizon AF, Brooks DE. Dextromethorphan-induced serotonin syndrome. Clin Toxicol (Phila). 2008;46(8):771-773.
44. Gibbons RD, Brown CH, Hur K, et al. Early evidence on the effects of regulators’ suicidality warnings on SSRI prescriptions and suicide in children and adolescents. Am J Psychiatry. 2007;164(9):1356-1363.
45. Hammad TA, Laughren T, Racoosin J. Suicidality in pediatric patients treated with antidepressant drugs. Arch Gen Psychiatry. 2006;63(3):332-339.
46. Bridge JA, Iyengar S, Salary CB, et al. Clinical response and risk for reported suicidal ideation and suicide attempts in pediatric antidepressant treatment: a meta-analysis of randomized controlled trials. JAMA. 2007;297(15):1683-1696.
47. Sharma T, Guski LS, Freund N, et al. Suicidality and aggression during antidepressant treatment: systematic review and meta-analyses based on clinical study reports. BMJ. 2016;352: i65. doi: https://doi.org/10.1136/bmj.i65.
48. Olfson M, Shaffer D, Marcus SC, et al. Relationship between antidepressant medication treatment and suicide in adolescents. Arch Gen Psychiatry. 2003;60(10):978-982.
49. Garland JE, Kutcher S, Virani A, et al. Update on the Use of SSRIs and SNRIs with children and adolescents in clinical practice. J Can Acad Child Adolesc Psychiatry. 2016;25(1):4-10.
50. Bridge JA, Barbe RP, Birmaher B, et al. Emergent suicidality in a clinical psychotherapy trial for adolescent depression. Am J Psychiatry. 2005;162(11):2173-2175.
51. Birmaher B, Brent D, Bernet W, et al. Practice parameter for the assessment and treatment of children and adolescents with depressive disorders. J Am Acad Child Adolesc Psychiatry. 2007;46(11):1503-1526.
52. Ravizza L, Maina G, Bogetto F, et al. Long term treatment of obsessive-compulsive disorder. CNS Drugs. 1998;10(4):247-255.
53. Hosenbocus S, Chahal R. SSRIs and SNRIs: a review of the discontinuation syndrome in children and adolescents. J Can Acad Child Adolesc Psychiatry. 2011;20(1):60-67.
54. Bloch MH, McGuire J, Landeros-Weisenberger A, et al. Meta-analysis of the dose-response relationship of SSRI in obsessive-compulsive disorder. Mol Psychiatry. 2010;15(8):850-855.
55. Issari Y, Jakubovski E, Bartley CA, et al. Early onset of response with selective serotonin reuptake inhibitors in obsessive-compulsive disorder: a meta-analysis. J Clin Psychiatry. 2016; 77(5):e605-e611. doi: 10.4088/JCP.14r09758.
56. Brent D, Emslie G, Clarke G, et al. Switching to another SSRI or to venlafaxine with or without cognitive behavioral therapy for adolescents with SSRI-resistant depression: the TORDIA randomized controlled trial. JAMA. 2008;299(8):901-913.
Major depressive disorder (MDD) is a significant pediatric health problem, with a lifetime prevalence as high as 20% by the end of adolescence.1-3 Major depressive disorder in adolescence is associated with significant morbidity, including poor social functioning, school difficulties, early pregnancy, and increased risk of physical illness and substance abuse.4-6 It is also linked with significant mortality, with increased risk for suicide, which is now the second leading cause of death in individuals age 10 to 24 years.1,7,8
As their name suggests, antidepressants comprise a group of medications that are used to treat MDD; they are also, however, first-line agents for generalized anxiety disorder (GAD), posttraumatic stress disorder (PTSD), and obsessive-compulsive disorder (OCD) in adults. Anxiety disorders (including GAD and other anxiety diagnoses) and PTSD are also common in childhood and adolescence with a combined lifetime prevalence ranging from 15% to 30%.9,10 These disorders are also associated with increased risk of suicide.11 For all of these disorders, depending on the severity of presentation and the preference of the patient, treatments are often a combination of psychotherapy and psychopharmacology.
Clinicians face several challenges when considering antidepressants for pediatric patients. Pediatricians and psychiatrists need to understand whether these medications work in children and adolescents, and whether there are unique developmental safety and tolerability issues. The evidence base in child psychiatry is considerably smaller compared with that of adult psychiatry. From this more limited evidence base also came the controversial “black-box” warning regarding a risk of emergent suicidality when starting antidepressants that accompanies all antidepressants for pediatric, but not adult, patients. This warning has had major effects on clinical encounters with children experiencing depression, including altering clinician prescribing behavior.12
Do antidepressants work in children?
Selective serotonin reuptake inhibitors. Selective serotonin reuptake inhibitors (SSRIs) are the most commonly used class of antidepressants in both children and adults.13 While only a few SSRIs are FDA-approved for pediatric indications, the lack of FDA approval is typically related to a lack of sufficient testing in randomized controlled trials (RCTs) for specific pediatric indications, rather than to demonstrable differences in efficacy between antidepressant agents. Since there is currently no data to suggest inferiority of one agent compared to another in children or adults,14,15 efficacy data will be discussed here as applied to the class of SSRIs, generalizing from RCTs conducted on individual drugs. Table 1 lists FDA indications and dosing information for individual antidepressants.

There is strong evidence that SSRIs are effective for treating pediatric anxiety disorders (eg, social anxiety disorder and GAD)16 and OCD,17 with numbers needed to treat (NNT) between 3 and 5. For both of these disorders, SSRIs combined with cognitive-behavioral therapy (CBT) have the highest likelihood of improving symptoms or achieving remission.17,18
Selective serotonin reuptake inhibitors are also effective for treating pediatric MDD; however, the literature is more complex for this disorder compared to GAD and OCD as there are considerable differences in effect sizes between National Institute of Mental Health (NIMH)–funded studies and industry-sponsored trials.13 The major NIMH-sponsored adolescent depression trial, TADS (Treatment for Adolescents and Depression Study), showed that SSRIs (fluoxetine in this case) were quite effective, with an NNT of 4 over the acute phase (12 weeks).19 Ultimately, approximately 80% of adolescents improved over 9 months. Many industry-sponsored trials for MDD in pediatric patients had large placebo response rates (approximately 60%), which resulted in smaller between-group differences, and estimates of an NNT closer to 12,13 which has muddied the waters in meta-analyses that include all trials.20 Improvement in depressive symptoms also appears to be bolstered by concomitant CBT in MDD,19 but not as robustly as in GAD and OCD. While the full benefit of SSRIs for depression may take as long as 8 weeks, a meta-analysis of depression studies of pediatric patients suggests that significant benefits from placebo are observed as early as 2 weeks, and that further treatment gains are minimal after 4 weeks.15 Thus, we recommend at least a 4- to 6-week trial at therapeutic dosing before deeming a medication a treatment failure.
Continue to: Posttraumatic stress disorder...
Posttraumatic stress disorder is a fourth disorder in which SSRIs are a first-line treatment in adults. The data for using SSRIs to treat pediatric patients with PTSD is scant, with only a few RCTs, and no large NIMH-funded trials. Randomized controlled trials have not demonstrated significant differences between SSRIs and placebo21,22 and thus the current first-line recommendation in pediatric PTSD remains trauma-focused therapy, with good evidence for trauma-focused CBT.23 Practically speaking, there can be considerable overlap of PTSD, depression, and anxiety symptoms in children,23 and children with a history of trauma who also have comorbid MDD may benefit from medication if their symptoms persist despite an adequate trial of psychotherapy.
Taken together, the current evidence suggests that SSRIs are often effective in pediatric GAD, OCD, and MDD, with low NNTs (ranging from 3 to 5 based on NIMH-funded trials) for all of these disorders; there is not yet sufficient evidence of efficacy in pediatric patients with PTSD.
Fluoxetine has been studied more intensively than other SSRIs (for example, it was the antidepressant used in the TADS trial), and thus has the largest evidence base. For this reason, fluoxetine is often considered the first of the first-line options. Additionally, fluoxetine has a longer half-life than other antidepressants, which may make it more effective in situations where patients are likely to miss doses, and results in a lower risk of withdrawal symptoms when stopped due to “self-tapering.”
SNRIs and atypical antidepressants. Other antidepressants commonly used in pediatric patients but with far less evidence of efficacy include serotonin-norepinephrine reuptake inhibitors (SNRIs) and the atypical antidepressants bupropion and mirtazapine. The SNRI duloxetine is FDA-approved for treating GAD in children age 7 to 17, but there are no other pediatric indications for duloxetine, or for the other SNRIs.
In general, adverse effect profiles are worse for SNRIs compared to SSRIs, further limiting their utility. While there are no pediatric studies demonstrating SNRI efficacy for neuropathic pain, good data exists in adults.24 Thus, an SNRI could be a reasonable option if a pediatric patient has failed prior adequate SSRI trials and also has comorbid neuropathic pain.
Continue to: Neither bupropion nor mirtazapine...
Neither bupropion nor mirtazapine have undergone rigorous testing in pediatric patients, and therefore these agents should generally be considered only once other first-line treatments have failed. Bupropion has been evaluated for attention-deficit/hyperactivity disorder (ADHD)25 and for adolescent smoking cessation.26 However, the evidence is weak, and bupropion is not considered a first-line option for children and adolescents.
Tricyclic antidepressants. Randomized controlled trials have demonstrated that tricyclic antidepressants (TCAs) are efficacious for treating several pediatric conditions; however, their significant side effect profile, their monitoring requirements, as well as their lethality in overdose has left them replaced by SSRIs in most cases. That said, they can be appropriate in refractory ADHD (desipramine27,28) and refractory OCD (clomipramine is FDA-approved for this indication29); they are considered a third-line treatment for enuresis.30
Why did my patient stop the medication?
Common adverse effects. Although the greatest benefit of antidepressant medications compared with placebo is achieved relatively early on in treatment, it generally takes time for these benefits to accrue and become clinically apparent.15,31 By contrast, most adverse effects of antidepressants present and are at their most severe early in treatment. The combination of early adverse effects and delayed efficacy leads many patients, families, and clinicians to discontinue medications before they have an adequate chance to work. Thus, it is imperative to provide psychoeducation before starting a medication about the typical time-course of improvement and adverse effects (Table 2).

Adverse effects of SSRIs often appear or worsen transiently during initiation of a medication, during a dose increase,32 or, theoretically, with the addition of a medication that interferes with SSRI metabolism (eg, cimetidine inhibition of cytochrome P450 2D6).33 If families are prepared for this phenomenon and the therapeutic alliance is adequate, adverse effects can be tolerated to allow for a full medication trial. Common adverse effects of SSRIs include sleep problems (insomnia/sedation), gastrointestinal upset, sexual dysfunction, dry mouth, and hyperhidrosis. Although SSRIs differ somewhat in the frequency of these effects, as a class, they are more similar than different. Adequate psychoeducation is especially imperative in the treatment of OCD and anxiety disorders, where there is limited evidence of efficacy for any non-serotonergic antidepressants.
Serotonin-norepinephrine reuptake inhibitors are not considered first-line medications because of the reduced evidence base compared to SSRIs and their enhanced adverse effect profiles. Because SNRIs partially share a mechanism of action with SSRIs, they also share portions of the adverse effects profile. However, SNRIs have the additional adverse effect of hypertension, which is related to their noradrenergic activity. Thus, it is reasonable to obtain a baseline blood pressure before initiating an SNRI, as well as periodically after initiation and during dose increases, particularly if the patient has other risk factors for hypertension.34
Continue to: Although TCAs have efficacy...
Although TCAs have efficacy in some pediatric disorders,27-29,35 their adverse effect profile limits their use. Tricyclic antidepressants are highly anticholinergic (causing dizziness secondary to orthostatic hypotension, dry mouth, and urinary retention) and antihistaminergic (causing sedation and weight gain). Additionally, TCAs lower the seizure threshold and have adverse cardiac effects relating to their anti-alpha-1 adrenergic activity, resulting in dose-dependent increases in the QTc and cardiac toxicity in overdose that could lead to arrhythmia and death. These medications have their place, but their use requires careful informed consent, clear treatment goals, and baseline and periodic cardiac monitoring (via electrocardiogram).
Serious adverse effects. Clinicians may be hesitant to prescribe antidepressants for pediatric patients because of the potential for more serious adverse effects, including severe behavioral activation syndromes, serotonin syndrome, and emergent suicidality. However, current FDA-approved antidepressants arguably have one of the most positive risk/benefit profiles of any orally-administered medication approved for pediatric patients. Having a strong understanding of the evidence is critical to evaluating when it is appropriate to prescribe an antidepressant, how to properly monitor the patient, and how to obtain accurate informed consent.
Pediatric behavioral activation syndrome. Many clinicians report that children receiving antidepressants experience a pediatric behavioral activation syndrome, which exists along a spectrum from mild activation, increased energy, insomnia, or irritability up through more severe presentations of agitation, hyperactivity, or possibly mania. A recent meta-analysis suggested a positive association between antidepressant use and activation events on the milder end of this spectrum in pediatric patients with non-OCD anxiety disorders,16 and it is thought that compared with adolescents, younger children are more susceptible to activation adverse effects.36 The likelihood of activation events has been associated with higher antidepressant plasma levels,37 suggesting that dose or individual differences in metabolism may play a role. At the severe end of the spectrum, the risk of induction of mania in pediatric patients with depression or anxiety is relatively rare (<2%) and not statistically different from placebo in RCTs of pediatric participants.38 Meta-analyses of larger randomized, placebo-controlled trials of adults do not support the idea that SSRIs and other second-generation antidepressants carry an increased risk of mania compared with placebo.39,40 Children or adolescents with bona fide bipolar disorder (ie, patients who have had observed mania that meets all DSM-5 criteria) should be treated with a mood-stabilizing agent or antipsychotic if prescribed an antidepressant.41 These clear-cut cases are, however, relatively rare, and more often clinicians are confronted with ambiguous cases that include a family history of bipolar disorder along with “softer” symptoms of irritability, intrusiveness, or aggression. In these children, SSRIs may be appropriate for depressive, OCD, or anxiety symptoms, and should be strongly considered before prescribing antipsychotics or mood stabilizers, as long as initiated with proper monitoring.
Serotonin syndrome is a life-threatening condition caused by excess synaptic serotonin. It is characterized by confusion, sweating, diarrhea, hypertension, hyperthermia, and tachycardia. At its most severe, serotonin syndrome can result in seizures, arrhythmias, and death. The risk of serotonin syndrome is very low when using an SSRI as monotherapy. Risk increases with polypharmacy, particularly unexamined polypharmacy when multiple serotonergic agents are inadvertently on board. Commonly used serotonergic agents include other antidepressants, migraine medications (eg, triptans), some pain medications, and the cough suppressant dextromethorphan.
The easiest way to mitigate the risk of serotonin syndrome is to use an interaction index computer program, which can help ensure that the interacting agents are not prescribed without first discussing the risks and benefits. It is important to teach adolescents that certain recreational drugs are highly serotonergic and can cause serious interactions with antidepressants. For example, recreational use of dextromethorphan or 3,4-methylenedioxymethamphetamine (MDMA; commonly known as “ecstasy”) has been associated with serotonin syndrome in adolescents taking antidepressant medications.42,43
Continue to: Suicidality
Suicidality. The black-box warning regarding a risk of emergent suicidality when starting antidepressant treatment in children is controversial.44 The prospect that a medication intended to ameliorate depression might instead risk increasing suicidal thinking is alarming to parents and clinicians alike. To appropriately weigh and discuss the risks and benefits with families, it is important to understand the data upon which the warning is based.

In 2004, the FDA commissioned a review of 23 antidepressant trials, both published and unpublished, pooling studies across multiple indications (MDD, OCD, anxiety, and ADHD) and multiple antidepressant classes. This meta-analysis, which included nearly 4,400 pediatric patients, found a small but statistically significant increase in spontaneously-reported suicidal thoughts or actions, with a risk difference of 1% (95% confidence interval [CI], 1% to 2%).45 These data suggest that if one treats 100 pediatric patients, 1 to 2 of them may experience short-term increases in suicidal thinking or behavior.45 There were no differences in suicidal thinking when assessed systematically (ie, when all subjects reported symptoms of suicidal ideation on structured rating scales), and there were no completed suicides.45 A subsequent analysis that included 27 pediatric trials suggested an even lower, although still significant, risk difference (<1%), yielding a number needed to harm (NNH) of 143.46 Thus, with low NNT for efficacy (3 to 6) and relatively high NNH for emergent suicidal thoughts or behaviors (100 to 143), for many patients the benefits will outweigh the risks.

Figure 1, Figure 2, and Figure 3 are Cates plots that depict the absolute benefits of antidepressants compared with the risk of suicidality for pediatric patients with MDD, OCD, and anxiety disorders. Recent meta-analyses have suggested that the increased risk of suicidality in antidepressant trials is specific to studies that included children and adolescents, and is not observed in adult studies. A meta-analysis of 70 trials involving 18,526 participants suggested that the odds ratio of suicidality in trials of children and adolescents was 2.39 (95% CI, 1.31 to 4.33) compared with 0.81 (95% CI, 0.51 to 1.28) in adults.47 Additionally, a network meta-analysis exclusively focusing on pediatric antidepressant trials in MDD reported significantly higher suicidality-related adverse events in venlafaxine trials compared with placebo, duloxetine, and several SSRIs (fluoxetine, paroxetine, and escitalopram).20 These data should be interpreted with caution as differences in suicidality detected between agents is quite possibly related to differences in the method of assessment between trials, as opposed to actual differences in risk between agents.

Epidemiologic data further support the use of antidepressants in pediatric patients, showing that antidepressant use is associated with decreased teen suicide attempts and completions,48 and the decline in prescriptions that occurred following the black-box warning was accompanied by a 14% increase in teen suicides.49 Multiple hypotheses have been proposed to explain the pediatric clinical trial findings. One idea is that potential adverse effects of activation, or the intended effects of restoring the motivation, energy, and social engagement that is often impaired in depression, increases the likelihood of thinking about suicide or acting on thoughts. Another theory is that reporting of suicidality may be increased, rather than increased de novo suicidality itself. Antidepressants are effective for treating pediatric anxiety disorders, including social anxiety disorder,16 which could result in more willingness to report. Also, the manner in which adverse effects are generally ascertained in trials might have led to increased spontaneous reporting. In many trials, investigators ask whether participants have any adverse effects in general, and inquire about specific adverse effects only if the family answers affirmatively. Thus, the increased rate of other adverse effects associated with antidepressants (sleep problems, gastrointestinal upset, dry mouth, etc.) might trigger a specific question regarding suicidal ideation, which the child or family then may be more likely to report. Alternatively, any type of psychiatric treatment could increase an individual’s propensity to report; in adolescent psychotherapy trials, non-medic
Continue to: How long should the antidepressant be continued?
How long should the antidepressant be continued?
Many patients are concerned about how long they may be taking medication, and whether they will be taking an antidepressant “forever.” A treatment course can be broken into an acute phase, wherein remission is achieved and maintained for 6 to 8 weeks. This is followed by a continuation phase, with the goal of relapse prevention, lasting 16 to 20 weeks. The length of the last phase—the maintenance phase—depends both on the child’s history, the underlying therapeutic indication, the adverse effect burden experienced, and the family’s preferences/values. In general, for a first depressive episode, after treating for 1 year, a trial of discontinuation can be attempted with close monitoring. For a second depressive episode, we recommend 2 years of remission on antidepressant therapy before attempting discontinuation. In patients who have had 3 depressive episodes, or have had episodes of high severity, we recommend continuing antidepressant treatment indefinitely. Although much less well studied, the risk of relapse following SSRI discontinuation appears much more significant in OCD, whereas anxiety disorders and MDD have a relatively comparable risk.52
In general, stopping an antidepressant should be done carefully and slowly.
What to do when first-line treatments fail
Adequate psychotherapy? To determine whether children are receiving adequate CBT, ask:
- if the child receives homework from psychotherapy
- if the parents are included in treatment
- if therapy has involved identifying thought patterns that may be contributing to the child’s illness, and
- if the therapist has ever exposed the child to a challenge likely to produce anxiety or distress in a supervised environment and has developed an exposure hierarchy (for conditions with primarily exposure-based therapies, such as OCD or anxiety disorders).
If a family is not receiving most of these elements in psychotherapy, this is a good indicator that they may not be receiving evidence-based CBT.
Continue to: Adequate pharmacotherapy?
Adequate pharmacotherapy? Similarly, when determining the adequacy of previous pharmacotherapy, it is critical to determine whether the child received an adequate dose of medications (at least the FDA-recommended minimum dose) for an adequate duration of time at therapeutic dosing (at least 6 weeks for MDD, 8 weeks for anxiety disorders, and 8 to 12 weeks for pediatric patients with OCD), and that the child actually took the medication regularly during that period. Patient compliance can typically be tracked through checking refill requests or intervals through the patient’s pharmacy. Ensuring proper delivery of first-line treatments is imperative because (1) the adverse effects associated with second-line treatments are often more substantial; (2) the cost in terms of time and money is considerably higher with second-line treatments, and; (3) the evidence regarding the benefits of these treatments is much less certain.
Inadequate dosing is a common reason for non-response in pediatric patients. Therapeutic dose ranges for common antidepressants are displayed in Table 1. Many clinicians underdose antidepressants for pediatric patients initially (and often throughout treatment) because they fear that the typical dose titration used in clinical trials will increase the risk of adverse effects compared with more conservative dosing. There is limited evidence to suggest that this underdosing strategy is likely to be successful; adverse effects attributable to these medications are modest, and most that are experienced early in treatment (eg, headache, increased anxiety or irritability, sleep problems, gastrointestinal upset) are self-limiting and may be coincidental rather than medication-induced. Furthermore, there is no evidence for efficacy of subtherapeutic dosing in children in the acute phase of treatment or for preventing relapse.14 Thus, from an efficacy standpoint, a medication trial has not officially begun until the therapeutic dose range is reached.
Once dosing is within the therapeutic range, however, pediatric data differs from the adult literature. In most adult psychiatric conditions, higher doses of SSRIs within the therapeutic range are associated with an increased response rate.14,54 In pediatrics, there are few fixed dose trials, and once within the recommended therapeutic range, minimal data supports an association between higher dosing and higher efficacy.14 In general, pediatric guidelines are silent regarding optimal dosing of SSRIs within the recommended dose range, and higher antidepressant doses often result in a more significant adverse effect burden for children. One exception is pediatric OCD, where, similar to adults, the guidelines suggest that higher dosing of SSRIs often is required to induce a therapeutic response as compared to MDD and GAD.31,55
If a child does not respond to adequate first-line treatment (or has a treatment history that cannot be fully verified), repeating these first-line interventions carries little risk and can be quite effective. For example, 60% of adolescents with MDD who did not initially respond to an SSRI demonstrated a significant response when prescribed a second SSRI or venlafaxine (with or without CBT).56
When pediatric patients continue to experience significantly distressing and/or debilitating symptoms (particularly in MDD) despite multiple trials of antidepressants and psychotherapy, practitioners should consider a careful referral to interventional psychiatry services, which can include the more intensive treatments of electroconvulsive therapy, repetitive transcranial magnetic stimulation, or ketamine (see Box 1). Given the substantial morbidity and mortality associated with adolescent depression, interventional psychiatry treatments are under-researched and under-utilized clinically in pediatric populations.
Continue to: Antidepressants in general...
Antidepressants in general, and SSRIs in particular, are the first-line pharmacotherapy for pediatric anxiety, OCD, and MDD. For PTSD, although they are a first-line treatment in adults, their efficacy has not been demonstrated in children and adolescents. Antidepressants are generally safe, well-tolerated, and effective, with low NNTs (3 to 5 for anxiety and OCD; 4 to 12 in MDD, depending on whether industry trials are included). It is important that clinicians and families be educated about possible adverse effects and their time course in order to anticipate difficulties, ensure adequate informed consent, and monitor appropriately. The black-box warning regarding treatment-emergent suicidal thoughts or behaviors must be discussed (for suggested talking points, see Box 2). The NNH is large (100 to 143), and for many patients, the benefits will outweigh the risks. For pediatric patients who fail to respond to multiple adequate trials of antidepressants and psychotherapy, referrals for interventional psychiatry consultation should be considered.
Bottom Line
Although the evidence base for prescribing antidepressants for children and adolescents is smaller compared to the adult literature, properly understanding and prescribing these agents, and explaining their risks and benefits to families, can make a major difference in patient compliance, satisfaction, and outcomes. Antidepressants (particularly selective serotonin reuptake inhibitors) are the firstline pharmacologic intervention for pediatric patients with anxiety disorders, obsessive-compulsive disorder, or major depressive disorder.
Related Resource
- Bonin L, Moreland CS. Overview of prevention and treatment for pediatric depression. https://www.uptodate.com/contents/overview-of-prevention-and-treatment-for-pediatric-depression. Last reviewed July 2019.
Drug Brand Names
Bupropion • Wellbutrin, Zyban
Cimetidine • Tagamet
Citalopram • Celexa
Clomipramine • Anafranil
Desipramine • Norpramin
Desvenlafaxine • Pristiq
Duloxetine • Cymbalta
Escitalopram • Lexapro
Fluoxetine • Prozac
Fluvoxamine • Luvox
Imipramine • Tofranil
Mirtazapine • Remeron
Nortriptyline • Pamelor
Paroxetine • Paxil
Sertraline • Zoloft
Venlafaxine • Effexor
Vilazodone • Viibryd
Vortioxetine • Trintellix
Box 1
Continuing severe depression is associated with reduced educational attainment and quality of life, as well as increased risk of substance abuse and suicide,1,2 which is the second leading cause of death in individuals age 10 to 24 years.3 Given the substantial morbidity and mortality associated with adolescent depression, interventional psychiatry treatments are under-researched and underutilized in pediatric patients. Interventional antidepressants in adults include electroconvulsive therapy (ECT), repetitive transcranial magnetic stimulation (rTMS), and, most recently, ketamine.
Electroconvulsive therapy is the most effective therapy available for depression in adults, alleviating depressive symptoms in treatment-refractory patients and outperforming both pharmacotherapy4 and rTMS.5 Despite its track record of effectiveness and safety in adults, ECT continues to suffer considerable stigma.4 Cognitive adverse effects and memory problems in adults are generally self-limited, and some aspects of cognition actually improve after ECT as depression lifts.6 The combination of stigma and the concern about possible cognitive adverse effects during periods of brain development have likely impeded the rigorous testing of ECT in treatment-refractory pediatric patients. Several case series and other retrospective analyses suggest, however, that ECT has strong efficacy and limited adverse effects in adolescents who have severe depression or psychotic symptoms.7-9 Despite these positive preliminary data in pediatric patients, and a large body of literature in adults, no controlled trials of ECT have been conducted in the pediatric population, and it remains a rarely used treatment in severe pediatric mental illness.
Repetitive transcranial magnetic stimulation is a technique in which magnetic stimulation is used to activate the left dorsolateral prefrontal cortex (DLPFC), a target thought to be important in the pathophysiology of MDD. Repetitive transcranial magnetic stimulation is FDAapproved to treat medication-refractory major depressive disorder (MDD) in adults, and has been shown to be effective as both a monotherapy10 and an adjunctive treatment.11 The estimated number needed to treat (NNT) for rTMS ranges from 6 to 8, which is quite effective, although less so than ECT (and probably initial pharmacotherapy).5 Similar to ECT, however, there are no large randomized controlled trials (RCTs) in children or adolescents. Pilot RCTs12 and open trials13 suggest that DLPFC rTMS may be effective as an adjunctive treatment, speeding or augmenting response to a selective serotonin reuptake inhibitor in adolescents with MDD. Larger trials studying rTMS in pediatric patients are needed. While rTMS is generally well tolerated, disadvantages include the time-consuming schedule (the initial treatment is typically 5 days/week for several weeks) and local adverse effects of headache and scalp pain.
Ketamine, which traditionally is used as a dissociative anesthetic, is a rapidly emerging novel treatment in adult treatment-refractory MDD. It acts quickly (within hours to days) and cause significant improvement in difficult symptoms such as anhedonia14 and suicidal ideation.15 In adult studies, ketamine has a robust average effect size of >1.2, and an NNT ranging from 3 to 5 in medication-refractory patients.16,17 Ketamine is a glutamatergic modulator, acting outside of the monoamine neurochemical systems traditionally targeted by standard antidepressants.16 The efficacy of ketamine in treatment-refractory adults is impressive, but the effects of a single treatment are ephemeral, dissipating within 1 to 2 weeks, which has led to significant discussion surrounding optimal dosing strategies.16 Although small RCTs in pediatric patients are currently underway, at this time, the only evidence for ketamine for pediatric MDD is based on case series/report data18,19 which was positive.
For all of these interventional modalities, it is critical to refer children with treatmentrefractory disorders to interventionists who have appropriate experience and monitoring capabilities.
References
1. Weissman MM, Wolk S, Goldstein RB, et al. Depressed adolescents grown up. JAMA.1999;281(18):1707-1713.
2. Fergusson DM, Woodward LJ. Mental health, educational, and social role outcomes of adolescents with depression. Arch Gen Psychiatry. 2002;59(3):225-231.
3. Centers for Disease Control and Prevention. National Vital Statistics System. Deaths, percent of total deaths, and death rates for the 15 leading causes of death in 5-year age groups, by race and sex: United States, 1999-2015. Centers for Disease Control and Prevention. https://www.cdc.gov/nchs/nvss/mortality/lcwk1.htm. Published October 23, 2017. Accessed May 2, 2019.
4. UK ECT Review Group. Efficacy and safety of electroconvulsive therapy in depressive disorders: a systematic review and metaanalysis. Lancet. 2003;361(9360):799-808.
5. Berlim MT, Van den Eynde F, Daskalakis ZJ. Efficacy and acceptability of high frequency repetitive transcranial magnetic stimulation (rTMS) versus electroconvulsive therapy (ECT) for major depression: a systematic review and meta-analysis of randomized trials. Depress Anxiety. 2013;30(7):614-623.
6. Semkovska M, McLoughlin DM. Objective cognitive performance associated with electroconvulsive therapy for depression: a systematic review and meta-analysis. Biol Psychiatry. 2010;68(6):568-577.
7. Jacob P, Gogi PK, Srinath S, et al. Review of electroconvulsive therapy practice from a tertiary child and adolescent psychiatry centre. Asian J Psychiatr. 2014;12(1):95-99.
8. Zhand N, Courtney DB, Flament MF. Use of electroconvulsive therapy in adolescents with treatment-resistant depressive disorders: a case series. J ECT. 2015;31(4):238-245.
9. Puffer CC, Wall CA, Huxsahl JE, et al. A 20 year practice review of electroconvulsive therapy for adolescents. J Child Adolesc Psychopharmacol. 2016;26(7):632-636.
10. Berlim MT, van den Eynde F, Tovar-Perdomo S, et al. Response, remission and drop-out rates following high-frequency repetitive transcranial magnetic stimulation (rTMS) for treating major depression: a systematic review and meta-analysis of randomized, double-blind and sham-controlled trials. Psychol Med. 2014;44(2):225-239.
11. Liu B, Zhang Y, Zhang L, et al. Repetitive transcranial magnetic stimulation as an augmentative strategy for treatment-resistant depression, a meta-analysis of randomized, double-blind and sham-controlled study. BMC Psychiatry. 2014;14:342.
12. Huang ML, Luo BY, Hu JB, et al. Repetitive transcranial magnetic stimulation in combination with citalopram in young patients with first-episode major depressive disorder: a double-blind, randomized, sham-controlled trial. Aust N Z J Psychiatry. 2012;46(3):257-264.
13. Wall CA, Croarkin PE, Sim LA, et al. Adjunctive use of repetitive transcranial magnetic stimulation in depressed adolescents: a prospective, open pilot study. J Clin Psychiatry. 2011;72(9):1263-1269.
14. Lally N, Nugent AC, Luckenbaugh DA, et al. Anti-anhedonic effect of ketamine and its neural correlates in treatment-resistant bipolar depression. Transl Psychiatry. 2014;4:e469. doi: 10.1038/tp.2014.105.
15. Ballard ED, Ionescu DF, Vande Voort JL, et al. Improvement in suicidal ideation after ketamine infusion: relationship to reductions in depression and anxiety. J Psychiatr Res. 2014;58:161-166.
16. Newport DJ, Carpenter LL, McDonald WM, et al. Ketamine and other NMDA antagonists: early clinical trials and possible mechanisms in depression. Am J Psychiatry. 2015;172(10):950-966.
17. McGirr A, Berlim MT, Bond DJ, et al. A systematic review and meta-analysis of randomized, double-blind, placebo-controlled trials of ketamine in the rapid treatment of major depressive episodes. Psychol Med. 2015;45(4):693-704.
18. Dwyer JB, Beyer C, Wilkinson ST, et al. Ketamine as a treatment for adolescent depression: a case report. J Am Acad Child Adolesc Psychiatry. 2017;56(4):352-354.
19. Cullen KR, Amatya P, Roback MG, et al. Intravenous ketamine for adolescents with treatment-resistant depression: an open-label study. J Child Adolesc Psychopharmacol. 2018;28(7):437-444.
Box 2
Efficacy
- Selective serotonin reuptake inhibitors are the most effective pharmacologic treatment we have for pediatric depression, OCD, and anxiety
- More than one-half of children who are prescribed SSRIs have a significant improvement, regardless of condition
- Based on current estimates, we need to treat 4 to 6 children with an SSRI to find one that will improve who would not improve with placebo
- The clinical benefits of SSRIs generally take a while to accrue; therefore, it is advisable to take the medication for at least 2 to 3 months before concluding that it is ineffective
- In addition to medication, evidence-based psychotherapies provide significant benefit for pediatric depression, OCD, and anxiety
Tolerability
- Most commonly prescribed pediatric antidepressants have been used safely in children for 2 to 3 decades. The safety profiles of SSRIs are among the best of any medications used for children and adolescents
- While many children get better when taking these medications, it’s important that we also talk about potential adverse effects. Some children will experience sleep problems (either sleepier than usual or difficulty sleeping), changes in energy levels, headache, gastrointestinal upset, and dry mouth. These are most likely at the beginning of treatment, or when we increase the dose; they usually are time-limited and go away on their own
- Often adverse effects occur first and the benefits come later. Because it may take at least a few weeks to start to see the mood/anxiety benefits, it’s important for us to talk about any adverse effects your child experiences and remember that they usually are short-lived
Suicidality
- The FDA placed a “black-box” warning on antidepressants after pediatric studies found a small but statistically significant increased risk of reporting suicidal thoughts or behaviors over the short-term compared with placebo
- The increased risk of spontaneously reporting suicidal ideation was quite small. Studies suggested that one would need to treat 100 to 140 children to see 1 child report suicidal ideation compared to placebo. Suicidal ideation is a common symptom in children with depression and anxiety
- Studies found no increased risk when suicidal ideation was systematically assessed using structured rating scales
- In the studies evaluated, there were no completed suicides by patients taking medication or placebo
- Population studies show that higher rates of antidepressant prescriptions are associated with lower rates of attempted and completed teen suicide, which underscores that in general, these medicines treat the underlying causes of suicidality
- No scientific consensus exists on whether these medications are truly associated with an increased risk of new-onset suicidal ideation, or if this association is due to other factors (eg, improvement in anxiety and depressive symptoms that make patients more comfortable to report suicidal ideation spontaneously)
- Regardless, the FDA recommends frequent monitoring of children for suicidal thoughts when these medications are started. This should be done anyway in children experiencing depression and anxiety, and it’s why we will plan to have more frequent appointments as the medication is initiated
OCD: obsessive-compulsive disorder; SSRIs: selective serotonin reuptake inhibitors
Major depressive disorder (MDD) is a significant pediatric health problem, with a lifetime prevalence as high as 20% by the end of adolescence.1-3 Major depressive disorder in adolescence is associated with significant morbidity, including poor social functioning, school difficulties, early pregnancy, and increased risk of physical illness and substance abuse.4-6 It is also linked with significant mortality, with increased risk for suicide, which is now the second leading cause of death in individuals age 10 to 24 years.1,7,8
As their name suggests, antidepressants comprise a group of medications that are used to treat MDD; they are also, however, first-line agents for generalized anxiety disorder (GAD), posttraumatic stress disorder (PTSD), and obsessive-compulsive disorder (OCD) in adults. Anxiety disorders (including GAD and other anxiety diagnoses) and PTSD are also common in childhood and adolescence with a combined lifetime prevalence ranging from 15% to 30%.9,10 These disorders are also associated with increased risk of suicide.11 For all of these disorders, depending on the severity of presentation and the preference of the patient, treatments are often a combination of psychotherapy and psychopharmacology.
Clinicians face several challenges when considering antidepressants for pediatric patients. Pediatricians and psychiatrists need to understand whether these medications work in children and adolescents, and whether there are unique developmental safety and tolerability issues. The evidence base in child psychiatry is considerably smaller compared with that of adult psychiatry. From this more limited evidence base also came the controversial “black-box” warning regarding a risk of emergent suicidality when starting antidepressants that accompanies all antidepressants for pediatric, but not adult, patients. This warning has had major effects on clinical encounters with children experiencing depression, including altering clinician prescribing behavior.12
Do antidepressants work in children?
Selective serotonin reuptake inhibitors. Selective serotonin reuptake inhibitors (SSRIs) are the most commonly used class of antidepressants in both children and adults.13 While only a few SSRIs are FDA-approved for pediatric indications, the lack of FDA approval is typically related to a lack of sufficient testing in randomized controlled trials (RCTs) for specific pediatric indications, rather than to demonstrable differences in efficacy between antidepressant agents. Since there is currently no data to suggest inferiority of one agent compared to another in children or adults,14,15 efficacy data will be discussed here as applied to the class of SSRIs, generalizing from RCTs conducted on individual drugs. Table 1 lists FDA indications and dosing information for individual antidepressants.

There is strong evidence that SSRIs are effective for treating pediatric anxiety disorders (eg, social anxiety disorder and GAD)16 and OCD,17 with numbers needed to treat (NNT) between 3 and 5. For both of these disorders, SSRIs combined with cognitive-behavioral therapy (CBT) have the highest likelihood of improving symptoms or achieving remission.17,18
Selective serotonin reuptake inhibitors are also effective for treating pediatric MDD; however, the literature is more complex for this disorder compared to GAD and OCD as there are considerable differences in effect sizes between National Institute of Mental Health (NIMH)–funded studies and industry-sponsored trials.13 The major NIMH-sponsored adolescent depression trial, TADS (Treatment for Adolescents and Depression Study), showed that SSRIs (fluoxetine in this case) were quite effective, with an NNT of 4 over the acute phase (12 weeks).19 Ultimately, approximately 80% of adolescents improved over 9 months. Many industry-sponsored trials for MDD in pediatric patients had large placebo response rates (approximately 60%), which resulted in smaller between-group differences, and estimates of an NNT closer to 12,13 which has muddied the waters in meta-analyses that include all trials.20 Improvement in depressive symptoms also appears to be bolstered by concomitant CBT in MDD,19 but not as robustly as in GAD and OCD. While the full benefit of SSRIs for depression may take as long as 8 weeks, a meta-analysis of depression studies of pediatric patients suggests that significant benefits from placebo are observed as early as 2 weeks, and that further treatment gains are minimal after 4 weeks.15 Thus, we recommend at least a 4- to 6-week trial at therapeutic dosing before deeming a medication a treatment failure.
Continue to: Posttraumatic stress disorder...
Posttraumatic stress disorder is a fourth disorder in which SSRIs are a first-line treatment in adults. The data for using SSRIs to treat pediatric patients with PTSD is scant, with only a few RCTs, and no large NIMH-funded trials. Randomized controlled trials have not demonstrated significant differences between SSRIs and placebo21,22 and thus the current first-line recommendation in pediatric PTSD remains trauma-focused therapy, with good evidence for trauma-focused CBT.23 Practically speaking, there can be considerable overlap of PTSD, depression, and anxiety symptoms in children,23 and children with a history of trauma who also have comorbid MDD may benefit from medication if their symptoms persist despite an adequate trial of psychotherapy.
Taken together, the current evidence suggests that SSRIs are often effective in pediatric GAD, OCD, and MDD, with low NNTs (ranging from 3 to 5 based on NIMH-funded trials) for all of these disorders; there is not yet sufficient evidence of efficacy in pediatric patients with PTSD.
Fluoxetine has been studied more intensively than other SSRIs (for example, it was the antidepressant used in the TADS trial), and thus has the largest evidence base. For this reason, fluoxetine is often considered the first of the first-line options. Additionally, fluoxetine has a longer half-life than other antidepressants, which may make it more effective in situations where patients are likely to miss doses, and results in a lower risk of withdrawal symptoms when stopped due to “self-tapering.”
SNRIs and atypical antidepressants. Other antidepressants commonly used in pediatric patients but with far less evidence of efficacy include serotonin-norepinephrine reuptake inhibitors (SNRIs) and the atypical antidepressants bupropion and mirtazapine. The SNRI duloxetine is FDA-approved for treating GAD in children age 7 to 17, but there are no other pediatric indications for duloxetine, or for the other SNRIs.
In general, adverse effect profiles are worse for SNRIs compared to SSRIs, further limiting their utility. While there are no pediatric studies demonstrating SNRI efficacy for neuropathic pain, good data exists in adults.24 Thus, an SNRI could be a reasonable option if a pediatric patient has failed prior adequate SSRI trials and also has comorbid neuropathic pain.
Continue to: Neither bupropion nor mirtazapine...
Neither bupropion nor mirtazapine have undergone rigorous testing in pediatric patients, and therefore these agents should generally be considered only once other first-line treatments have failed. Bupropion has been evaluated for attention-deficit/hyperactivity disorder (ADHD)25 and for adolescent smoking cessation.26 However, the evidence is weak, and bupropion is not considered a first-line option for children and adolescents.
Tricyclic antidepressants. Randomized controlled trials have demonstrated that tricyclic antidepressants (TCAs) are efficacious for treating several pediatric conditions; however, their significant side effect profile, their monitoring requirements, as well as their lethality in overdose has left them replaced by SSRIs in most cases. That said, they can be appropriate in refractory ADHD (desipramine27,28) and refractory OCD (clomipramine is FDA-approved for this indication29); they are considered a third-line treatment for enuresis.30
Why did my patient stop the medication?
Common adverse effects. Although the greatest benefit of antidepressant medications compared with placebo is achieved relatively early on in treatment, it generally takes time for these benefits to accrue and become clinically apparent.15,31 By contrast, most adverse effects of antidepressants present and are at their most severe early in treatment. The combination of early adverse effects and delayed efficacy leads many patients, families, and clinicians to discontinue medications before they have an adequate chance to work. Thus, it is imperative to provide psychoeducation before starting a medication about the typical time-course of improvement and adverse effects (Table 2).

Adverse effects of SSRIs often appear or worsen transiently during initiation of a medication, during a dose increase,32 or, theoretically, with the addition of a medication that interferes with SSRI metabolism (eg, cimetidine inhibition of cytochrome P450 2D6).33 If families are prepared for this phenomenon and the therapeutic alliance is adequate, adverse effects can be tolerated to allow for a full medication trial. Common adverse effects of SSRIs include sleep problems (insomnia/sedation), gastrointestinal upset, sexual dysfunction, dry mouth, and hyperhidrosis. Although SSRIs differ somewhat in the frequency of these effects, as a class, they are more similar than different. Adequate psychoeducation is especially imperative in the treatment of OCD and anxiety disorders, where there is limited evidence of efficacy for any non-serotonergic antidepressants.
Serotonin-norepinephrine reuptake inhibitors are not considered first-line medications because of the reduced evidence base compared to SSRIs and their enhanced adverse effect profiles. Because SNRIs partially share a mechanism of action with SSRIs, they also share portions of the adverse effects profile. However, SNRIs have the additional adverse effect of hypertension, which is related to their noradrenergic activity. Thus, it is reasonable to obtain a baseline blood pressure before initiating an SNRI, as well as periodically after initiation and during dose increases, particularly if the patient has other risk factors for hypertension.34
Continue to: Although TCAs have efficacy...
Although TCAs have efficacy in some pediatric disorders,27-29,35 their adverse effect profile limits their use. Tricyclic antidepressants are highly anticholinergic (causing dizziness secondary to orthostatic hypotension, dry mouth, and urinary retention) and antihistaminergic (causing sedation and weight gain). Additionally, TCAs lower the seizure threshold and have adverse cardiac effects relating to their anti-alpha-1 adrenergic activity, resulting in dose-dependent increases in the QTc and cardiac toxicity in overdose that could lead to arrhythmia and death. These medications have their place, but their use requires careful informed consent, clear treatment goals, and baseline and periodic cardiac monitoring (via electrocardiogram).
Serious adverse effects. Clinicians may be hesitant to prescribe antidepressants for pediatric patients because of the potential for more serious adverse effects, including severe behavioral activation syndromes, serotonin syndrome, and emergent suicidality. However, current FDA-approved antidepressants arguably have one of the most positive risk/benefit profiles of any orally-administered medication approved for pediatric patients. Having a strong understanding of the evidence is critical to evaluating when it is appropriate to prescribe an antidepressant, how to properly monitor the patient, and how to obtain accurate informed consent.
Pediatric behavioral activation syndrome. Many clinicians report that children receiving antidepressants experience a pediatric behavioral activation syndrome, which exists along a spectrum from mild activation, increased energy, insomnia, or irritability up through more severe presentations of agitation, hyperactivity, or possibly mania. A recent meta-analysis suggested a positive association between antidepressant use and activation events on the milder end of this spectrum in pediatric patients with non-OCD anxiety disorders,16 and it is thought that compared with adolescents, younger children are more susceptible to activation adverse effects.36 The likelihood of activation events has been associated with higher antidepressant plasma levels,37 suggesting that dose or individual differences in metabolism may play a role. At the severe end of the spectrum, the risk of induction of mania in pediatric patients with depression or anxiety is relatively rare (<2%) and not statistically different from placebo in RCTs of pediatric participants.38 Meta-analyses of larger randomized, placebo-controlled trials of adults do not support the idea that SSRIs and other second-generation antidepressants carry an increased risk of mania compared with placebo.39,40 Children or adolescents with bona fide bipolar disorder (ie, patients who have had observed mania that meets all DSM-5 criteria) should be treated with a mood-stabilizing agent or antipsychotic if prescribed an antidepressant.41 These clear-cut cases are, however, relatively rare, and more often clinicians are confronted with ambiguous cases that include a family history of bipolar disorder along with “softer” symptoms of irritability, intrusiveness, or aggression. In these children, SSRIs may be appropriate for depressive, OCD, or anxiety symptoms, and should be strongly considered before prescribing antipsychotics or mood stabilizers, as long as initiated with proper monitoring.
Serotonin syndrome is a life-threatening condition caused by excess synaptic serotonin. It is characterized by confusion, sweating, diarrhea, hypertension, hyperthermia, and tachycardia. At its most severe, serotonin syndrome can result in seizures, arrhythmias, and death. The risk of serotonin syndrome is very low when using an SSRI as monotherapy. Risk increases with polypharmacy, particularly unexamined polypharmacy when multiple serotonergic agents are inadvertently on board. Commonly used serotonergic agents include other antidepressants, migraine medications (eg, triptans), some pain medications, and the cough suppressant dextromethorphan.
The easiest way to mitigate the risk of serotonin syndrome is to use an interaction index computer program, which can help ensure that the interacting agents are not prescribed without first discussing the risks and benefits. It is important to teach adolescents that certain recreational drugs are highly serotonergic and can cause serious interactions with antidepressants. For example, recreational use of dextromethorphan or 3,4-methylenedioxymethamphetamine (MDMA; commonly known as “ecstasy”) has been associated with serotonin syndrome in adolescents taking antidepressant medications.42,43
Continue to: Suicidality
Suicidality. The black-box warning regarding a risk of emergent suicidality when starting antidepressant treatment in children is controversial.44 The prospect that a medication intended to ameliorate depression might instead risk increasing suicidal thinking is alarming to parents and clinicians alike. To appropriately weigh and discuss the risks and benefits with families, it is important to understand the data upon which the warning is based.

In 2004, the FDA commissioned a review of 23 antidepressant trials, both published and unpublished, pooling studies across multiple indications (MDD, OCD, anxiety, and ADHD) and multiple antidepressant classes. This meta-analysis, which included nearly 4,400 pediatric patients, found a small but statistically significant increase in spontaneously-reported suicidal thoughts or actions, with a risk difference of 1% (95% confidence interval [CI], 1% to 2%).45 These data suggest that if one treats 100 pediatric patients, 1 to 2 of them may experience short-term increases in suicidal thinking or behavior.45 There were no differences in suicidal thinking when assessed systematically (ie, when all subjects reported symptoms of suicidal ideation on structured rating scales), and there were no completed suicides.45 A subsequent analysis that included 27 pediatric trials suggested an even lower, although still significant, risk difference (<1%), yielding a number needed to harm (NNH) of 143.46 Thus, with low NNT for efficacy (3 to 6) and relatively high NNH for emergent suicidal thoughts or behaviors (100 to 143), for many patients the benefits will outweigh the risks.

Figure 1, Figure 2, and Figure 3 are Cates plots that depict the absolute benefits of antidepressants compared with the risk of suicidality for pediatric patients with MDD, OCD, and anxiety disorders. Recent meta-analyses have suggested that the increased risk of suicidality in antidepressant trials is specific to studies that included children and adolescents, and is not observed in adult studies. A meta-analysis of 70 trials involving 18,526 participants suggested that the odds ratio of suicidality in trials of children and adolescents was 2.39 (95% CI, 1.31 to 4.33) compared with 0.81 (95% CI, 0.51 to 1.28) in adults.47 Additionally, a network meta-analysis exclusively focusing on pediatric antidepressant trials in MDD reported significantly higher suicidality-related adverse events in venlafaxine trials compared with placebo, duloxetine, and several SSRIs (fluoxetine, paroxetine, and escitalopram).20 These data should be interpreted with caution as differences in suicidality detected between agents is quite possibly related to differences in the method of assessment between trials, as opposed to actual differences in risk between agents.

Epidemiologic data further support the use of antidepressants in pediatric patients, showing that antidepressant use is associated with decreased teen suicide attempts and completions,48 and the decline in prescriptions that occurred following the black-box warning was accompanied by a 14% increase in teen suicides.49 Multiple hypotheses have been proposed to explain the pediatric clinical trial findings. One idea is that potential adverse effects of activation, or the intended effects of restoring the motivation, energy, and social engagement that is often impaired in depression, increases the likelihood of thinking about suicide or acting on thoughts. Another theory is that reporting of suicidality may be increased, rather than increased de novo suicidality itself. Antidepressants are effective for treating pediatric anxiety disorders, including social anxiety disorder,16 which could result in more willingness to report. Also, the manner in which adverse effects are generally ascertained in trials might have led to increased spontaneous reporting. In many trials, investigators ask whether participants have any adverse effects in general, and inquire about specific adverse effects only if the family answers affirmatively. Thus, the increased rate of other adverse effects associated with antidepressants (sleep problems, gastrointestinal upset, dry mouth, etc.) might trigger a specific question regarding suicidal ideation, which the child or family then may be more likely to report. Alternatively, any type of psychiatric treatment could increase an individual’s propensity to report; in adolescent psychotherapy trials, non-medic
Continue to: How long should the antidepressant be continued?
How long should the antidepressant be continued?
Many patients are concerned about how long they may be taking medication, and whether they will be taking an antidepressant “forever.” A treatment course can be broken into an acute phase, wherein remission is achieved and maintained for 6 to 8 weeks. This is followed by a continuation phase, with the goal of relapse prevention, lasting 16 to 20 weeks. The length of the last phase—the maintenance phase—depends both on the child’s history, the underlying therapeutic indication, the adverse effect burden experienced, and the family’s preferences/values. In general, for a first depressive episode, after treating for 1 year, a trial of discontinuation can be attempted with close monitoring. For a second depressive episode, we recommend 2 years of remission on antidepressant therapy before attempting discontinuation. In patients who have had 3 depressive episodes, or have had episodes of high severity, we recommend continuing antidepressant treatment indefinitely. Although much less well studied, the risk of relapse following SSRI discontinuation appears much more significant in OCD, whereas anxiety disorders and MDD have a relatively comparable risk.52
In general, stopping an antidepressant should be done carefully and slowly.
What to do when first-line treatments fail
Adequate psychotherapy? To determine whether children are receiving adequate CBT, ask:
- if the child receives homework from psychotherapy
- if the parents are included in treatment
- if therapy has involved identifying thought patterns that may be contributing to the child’s illness, and
- if the therapist has ever exposed the child to a challenge likely to produce anxiety or distress in a supervised environment and has developed an exposure hierarchy (for conditions with primarily exposure-based therapies, such as OCD or anxiety disorders).
If a family is not receiving most of these elements in psychotherapy, this is a good indicator that they may not be receiving evidence-based CBT.
Continue to: Adequate pharmacotherapy?
Adequate pharmacotherapy? Similarly, when determining the adequacy of previous pharmacotherapy, it is critical to determine whether the child received an adequate dose of medications (at least the FDA-recommended minimum dose) for an adequate duration of time at therapeutic dosing (at least 6 weeks for MDD, 8 weeks for anxiety disorders, and 8 to 12 weeks for pediatric patients with OCD), and that the child actually took the medication regularly during that period. Patient compliance can typically be tracked through checking refill requests or intervals through the patient’s pharmacy. Ensuring proper delivery of first-line treatments is imperative because (1) the adverse effects associated with second-line treatments are often more substantial; (2) the cost in terms of time and money is considerably higher with second-line treatments, and; (3) the evidence regarding the benefits of these treatments is much less certain.
Inadequate dosing is a common reason for non-response in pediatric patients. Therapeutic dose ranges for common antidepressants are displayed in Table 1. Many clinicians underdose antidepressants for pediatric patients initially (and often throughout treatment) because they fear that the typical dose titration used in clinical trials will increase the risk of adverse effects compared with more conservative dosing. There is limited evidence to suggest that this underdosing strategy is likely to be successful; adverse effects attributable to these medications are modest, and most that are experienced early in treatment (eg, headache, increased anxiety or irritability, sleep problems, gastrointestinal upset) are self-limiting and may be coincidental rather than medication-induced. Furthermore, there is no evidence for efficacy of subtherapeutic dosing in children in the acute phase of treatment or for preventing relapse.14 Thus, from an efficacy standpoint, a medication trial has not officially begun until the therapeutic dose range is reached.
Once dosing is within the therapeutic range, however, pediatric data differs from the adult literature. In most adult psychiatric conditions, higher doses of SSRIs within the therapeutic range are associated with an increased response rate.14,54 In pediatrics, there are few fixed dose trials, and once within the recommended therapeutic range, minimal data supports an association between higher dosing and higher efficacy.14 In general, pediatric guidelines are silent regarding optimal dosing of SSRIs within the recommended dose range, and higher antidepressant doses often result in a more significant adverse effect burden for children. One exception is pediatric OCD, where, similar to adults, the guidelines suggest that higher dosing of SSRIs often is required to induce a therapeutic response as compared to MDD and GAD.31,55
If a child does not respond to adequate first-line treatment (or has a treatment history that cannot be fully verified), repeating these first-line interventions carries little risk and can be quite effective. For example, 60% of adolescents with MDD who did not initially respond to an SSRI demonstrated a significant response when prescribed a second SSRI or venlafaxine (with or without CBT).56
When pediatric patients continue to experience significantly distressing and/or debilitating symptoms (particularly in MDD) despite multiple trials of antidepressants and psychotherapy, practitioners should consider a careful referral to interventional psychiatry services, which can include the more intensive treatments of electroconvulsive therapy, repetitive transcranial magnetic stimulation, or ketamine (see Box 1). Given the substantial morbidity and mortality associated with adolescent depression, interventional psychiatry treatments are under-researched and under-utilized clinically in pediatric populations.
Continue to: Antidepressants in general...
Antidepressants in general, and SSRIs in particular, are the first-line pharmacotherapy for pediatric anxiety, OCD, and MDD. For PTSD, although they are a first-line treatment in adults, their efficacy has not been demonstrated in children and adolescents. Antidepressants are generally safe, well-tolerated, and effective, with low NNTs (3 to 5 for anxiety and OCD; 4 to 12 in MDD, depending on whether industry trials are included). It is important that clinicians and families be educated about possible adverse effects and their time course in order to anticipate difficulties, ensure adequate informed consent, and monitor appropriately. The black-box warning regarding treatment-emergent suicidal thoughts or behaviors must be discussed (for suggested talking points, see Box 2). The NNH is large (100 to 143), and for many patients, the benefits will outweigh the risks. For pediatric patients who fail to respond to multiple adequate trials of antidepressants and psychotherapy, referrals for interventional psychiatry consultation should be considered.
Bottom Line
Although the evidence base for prescribing antidepressants for children and adolescents is smaller compared to the adult literature, properly understanding and prescribing these agents, and explaining their risks and benefits to families, can make a major difference in patient compliance, satisfaction, and outcomes. Antidepressants (particularly selective serotonin reuptake inhibitors) are the firstline pharmacologic intervention for pediatric patients with anxiety disorders, obsessive-compulsive disorder, or major depressive disorder.
Related Resource
- Bonin L, Moreland CS. Overview of prevention and treatment for pediatric depression. https://www.uptodate.com/contents/overview-of-prevention-and-treatment-for-pediatric-depression. Last reviewed July 2019.
Drug Brand Names
Bupropion • Wellbutrin, Zyban
Cimetidine • Tagamet
Citalopram • Celexa
Clomipramine • Anafranil
Desipramine • Norpramin
Desvenlafaxine • Pristiq
Duloxetine • Cymbalta
Escitalopram • Lexapro
Fluoxetine • Prozac
Fluvoxamine • Luvox
Imipramine • Tofranil
Mirtazapine • Remeron
Nortriptyline • Pamelor
Paroxetine • Paxil
Sertraline • Zoloft
Venlafaxine • Effexor
Vilazodone • Viibryd
Vortioxetine • Trintellix
Box 1
Continuing severe depression is associated with reduced educational attainment and quality of life, as well as increased risk of substance abuse and suicide,1,2 which is the second leading cause of death in individuals age 10 to 24 years.3 Given the substantial morbidity and mortality associated with adolescent depression, interventional psychiatry treatments are under-researched and underutilized in pediatric patients. Interventional antidepressants in adults include electroconvulsive therapy (ECT), repetitive transcranial magnetic stimulation (rTMS), and, most recently, ketamine.
Electroconvulsive therapy is the most effective therapy available for depression in adults, alleviating depressive symptoms in treatment-refractory patients and outperforming both pharmacotherapy4 and rTMS.5 Despite its track record of effectiveness and safety in adults, ECT continues to suffer considerable stigma.4 Cognitive adverse effects and memory problems in adults are generally self-limited, and some aspects of cognition actually improve after ECT as depression lifts.6 The combination of stigma and the concern about possible cognitive adverse effects during periods of brain development have likely impeded the rigorous testing of ECT in treatment-refractory pediatric patients. Several case series and other retrospective analyses suggest, however, that ECT has strong efficacy and limited adverse effects in adolescents who have severe depression or psychotic symptoms.7-9 Despite these positive preliminary data in pediatric patients, and a large body of literature in adults, no controlled trials of ECT have been conducted in the pediatric population, and it remains a rarely used treatment in severe pediatric mental illness.
Repetitive transcranial magnetic stimulation is a technique in which magnetic stimulation is used to activate the left dorsolateral prefrontal cortex (DLPFC), a target thought to be important in the pathophysiology of MDD. Repetitive transcranial magnetic stimulation is FDAapproved to treat medication-refractory major depressive disorder (MDD) in adults, and has been shown to be effective as both a monotherapy10 and an adjunctive treatment.11 The estimated number needed to treat (NNT) for rTMS ranges from 6 to 8, which is quite effective, although less so than ECT (and probably initial pharmacotherapy).5 Similar to ECT, however, there are no large randomized controlled trials (RCTs) in children or adolescents. Pilot RCTs12 and open trials13 suggest that DLPFC rTMS may be effective as an adjunctive treatment, speeding or augmenting response to a selective serotonin reuptake inhibitor in adolescents with MDD. Larger trials studying rTMS in pediatric patients are needed. While rTMS is generally well tolerated, disadvantages include the time-consuming schedule (the initial treatment is typically 5 days/week for several weeks) and local adverse effects of headache and scalp pain.
Ketamine, which traditionally is used as a dissociative anesthetic, is a rapidly emerging novel treatment in adult treatment-refractory MDD. It acts quickly (within hours to days) and cause significant improvement in difficult symptoms such as anhedonia14 and suicidal ideation.15 In adult studies, ketamine has a robust average effect size of >1.2, and an NNT ranging from 3 to 5 in medication-refractory patients.16,17 Ketamine is a glutamatergic modulator, acting outside of the monoamine neurochemical systems traditionally targeted by standard antidepressants.16 The efficacy of ketamine in treatment-refractory adults is impressive, but the effects of a single treatment are ephemeral, dissipating within 1 to 2 weeks, which has led to significant discussion surrounding optimal dosing strategies.16 Although small RCTs in pediatric patients are currently underway, at this time, the only evidence for ketamine for pediatric MDD is based on case series/report data18,19 which was positive.
For all of these interventional modalities, it is critical to refer children with treatmentrefractory disorders to interventionists who have appropriate experience and monitoring capabilities.
References
1. Weissman MM, Wolk S, Goldstein RB, et al. Depressed adolescents grown up. JAMA.1999;281(18):1707-1713.
2. Fergusson DM, Woodward LJ. Mental health, educational, and social role outcomes of adolescents with depression. Arch Gen Psychiatry. 2002;59(3):225-231.
3. Centers for Disease Control and Prevention. National Vital Statistics System. Deaths, percent of total deaths, and death rates for the 15 leading causes of death in 5-year age groups, by race and sex: United States, 1999-2015. Centers for Disease Control and Prevention. https://www.cdc.gov/nchs/nvss/mortality/lcwk1.htm. Published October 23, 2017. Accessed May 2, 2019.
4. UK ECT Review Group. Efficacy and safety of electroconvulsive therapy in depressive disorders: a systematic review and metaanalysis. Lancet. 2003;361(9360):799-808.
5. Berlim MT, Van den Eynde F, Daskalakis ZJ. Efficacy and acceptability of high frequency repetitive transcranial magnetic stimulation (rTMS) versus electroconvulsive therapy (ECT) for major depression: a systematic review and meta-analysis of randomized trials. Depress Anxiety. 2013;30(7):614-623.
6. Semkovska M, McLoughlin DM. Objective cognitive performance associated with electroconvulsive therapy for depression: a systematic review and meta-analysis. Biol Psychiatry. 2010;68(6):568-577.
7. Jacob P, Gogi PK, Srinath S, et al. Review of electroconvulsive therapy practice from a tertiary child and adolescent psychiatry centre. Asian J Psychiatr. 2014;12(1):95-99.
8. Zhand N, Courtney DB, Flament MF. Use of electroconvulsive therapy in adolescents with treatment-resistant depressive disorders: a case series. J ECT. 2015;31(4):238-245.
9. Puffer CC, Wall CA, Huxsahl JE, et al. A 20 year practice review of electroconvulsive therapy for adolescents. J Child Adolesc Psychopharmacol. 2016;26(7):632-636.
10. Berlim MT, van den Eynde F, Tovar-Perdomo S, et al. Response, remission and drop-out rates following high-frequency repetitive transcranial magnetic stimulation (rTMS) for treating major depression: a systematic review and meta-analysis of randomized, double-blind and sham-controlled trials. Psychol Med. 2014;44(2):225-239.
11. Liu B, Zhang Y, Zhang L, et al. Repetitive transcranial magnetic stimulation as an augmentative strategy for treatment-resistant depression, a meta-analysis of randomized, double-blind and sham-controlled study. BMC Psychiatry. 2014;14:342.
12. Huang ML, Luo BY, Hu JB, et al. Repetitive transcranial magnetic stimulation in combination with citalopram in young patients with first-episode major depressive disorder: a double-blind, randomized, sham-controlled trial. Aust N Z J Psychiatry. 2012;46(3):257-264.
13. Wall CA, Croarkin PE, Sim LA, et al. Adjunctive use of repetitive transcranial magnetic stimulation in depressed adolescents: a prospective, open pilot study. J Clin Psychiatry. 2011;72(9):1263-1269.
14. Lally N, Nugent AC, Luckenbaugh DA, et al. Anti-anhedonic effect of ketamine and its neural correlates in treatment-resistant bipolar depression. Transl Psychiatry. 2014;4:e469. doi: 10.1038/tp.2014.105.
15. Ballard ED, Ionescu DF, Vande Voort JL, et al. Improvement in suicidal ideation after ketamine infusion: relationship to reductions in depression and anxiety. J Psychiatr Res. 2014;58:161-166.
16. Newport DJ, Carpenter LL, McDonald WM, et al. Ketamine and other NMDA antagonists: early clinical trials and possible mechanisms in depression. Am J Psychiatry. 2015;172(10):950-966.
17. McGirr A, Berlim MT, Bond DJ, et al. A systematic review and meta-analysis of randomized, double-blind, placebo-controlled trials of ketamine in the rapid treatment of major depressive episodes. Psychol Med. 2015;45(4):693-704.
18. Dwyer JB, Beyer C, Wilkinson ST, et al. Ketamine as a treatment for adolescent depression: a case report. J Am Acad Child Adolesc Psychiatry. 2017;56(4):352-354.
19. Cullen KR, Amatya P, Roback MG, et al. Intravenous ketamine for adolescents with treatment-resistant depression: an open-label study. J Child Adolesc Psychopharmacol. 2018;28(7):437-444.
Box 2
Efficacy
- Selective serotonin reuptake inhibitors are the most effective pharmacologic treatment we have for pediatric depression, OCD, and anxiety
- More than one-half of children who are prescribed SSRIs have a significant improvement, regardless of condition
- Based on current estimates, we need to treat 4 to 6 children with an SSRI to find one that will improve who would not improve with placebo
- The clinical benefits of SSRIs generally take a while to accrue; therefore, it is advisable to take the medication for at least 2 to 3 months before concluding that it is ineffective
- In addition to medication, evidence-based psychotherapies provide significant benefit for pediatric depression, OCD, and anxiety
Tolerability
- Most commonly prescribed pediatric antidepressants have been used safely in children for 2 to 3 decades. The safety profiles of SSRIs are among the best of any medications used for children and adolescents
- While many children get better when taking these medications, it’s important that we also talk about potential adverse effects. Some children will experience sleep problems (either sleepier than usual or difficulty sleeping), changes in energy levels, headache, gastrointestinal upset, and dry mouth. These are most likely at the beginning of treatment, or when we increase the dose; they usually are time-limited and go away on their own
- Often adverse effects occur first and the benefits come later. Because it may take at least a few weeks to start to see the mood/anxiety benefits, it’s important for us to talk about any adverse effects your child experiences and remember that they usually are short-lived
Suicidality
- The FDA placed a “black-box” warning on antidepressants after pediatric studies found a small but statistically significant increased risk of reporting suicidal thoughts or behaviors over the short-term compared with placebo
- The increased risk of spontaneously reporting suicidal ideation was quite small. Studies suggested that one would need to treat 100 to 140 children to see 1 child report suicidal ideation compared to placebo. Suicidal ideation is a common symptom in children with depression and anxiety
- Studies found no increased risk when suicidal ideation was systematically assessed using structured rating scales
- In the studies evaluated, there were no completed suicides by patients taking medication or placebo
- Population studies show that higher rates of antidepressant prescriptions are associated with lower rates of attempted and completed teen suicide, which underscores that in general, these medicines treat the underlying causes of suicidality
- No scientific consensus exists on whether these medications are truly associated with an increased risk of new-onset suicidal ideation, or if this association is due to other factors (eg, improvement in anxiety and depressive symptoms that make patients more comfortable to report suicidal ideation spontaneously)
- Regardless, the FDA recommends frequent monitoring of children for suicidal thoughts when these medications are started. This should be done anyway in children experiencing depression and anxiety, and it’s why we will plan to have more frequent appointments as the medication is initiated
OCD: obsessive-compulsive disorder; SSRIs: selective serotonin reuptake inhibitors
1. Williams SB, O’Connor EA, Eder M, et al. Screening for child and adolescent depression in primary care settings: a systematic evidence review for the US Preventive Services Task Force. Pediatrics. 2009;123(4):e716-e735. doi: 10.1542/peds.2008-2415.
2. Kessler RC, Avenevoli S, Ries Merikangas K. Mood disorders in children and adolescents: an epidemiologic perspective. Biol Psychiatry. 2001;49(12):1002-1014.
3. Lewinsohn PM, Clarke GN, Seeley JR, et al. Major depression in community adolescents: age at onset, episode duration, and time to recurrence. J Am Acad Child Adolesc Psychiatry. 1994;33(6):809-818.
4. Weissman MM, Wolk S, Goldstein RB, et al. Depressed adolescents grown up. JAMA.1999;281(18):1707-1713.
5. Fergusson DM, Woodward LJ. Mental health, educational, and social role outcomes of adolescents with depression. Arch Gen Psychiatry. 2002;59(3):225-231.
6. Keenan-Miller D, Hammen CL, Brennan PA. Health outcomes related to early adolescent depression. J Adolesc Health. 2007; 41(3): 256-62.
7. Shaffer D, Gould MS, Fisher P, et al. Psychiatric diagnosis in child and adolescent suicide. Arch Gen Psychiatry. 1996;53(4):339-348.
8. Centers for Disease Control and Prevention. National Vital Statistics System. Deaths, percent of total deaths, and death rates for the 15 leading causes of death in 5-year age groups, by race and sex: United States, 1999-2015. https://www.cdc.gov/nchs/nvss/mortality/lcwk1.htm. Published October 23, 2017. Accessed May 2, 2019.
9. Merikangas KR, He JP, Burstein M, et al. Lifetime prevalence of mental disorders in US adolescents: results from the National Comorbidity Survey Replication-Adolescent Supplement (NCS-A). J Am Acad Child Adolesc Psychiatry. 2010;49(10):980-989.
10. Wittchen HU, Nelson CB, Lachner G. Prevalence of mental disorders and psychosocial impairments in adolescents and young adults. Psychol Med. 1998;28(1):109-126.
11. Foley DL, Goldston DB, Costello EJ, et al. Proximal psychiatric risk factors for suicidality in youth: the Great Smoky Mountains Study. Arch Gen Psychiatry. 2006;63(9):1017-1024.
12. Cheung A, Sacks D, Dewa CS, et al. Pediatric prescribing practices and the FDA black-box warning on antidepressants. J Dev Behav Pediatr. 2008 29(3):213-215.
13. Walkup JT. Antidepressant efficacy for depression in children and adolescents: industry- and NIMH-funded studies. Am J Psychiatry. 2017;174(5):430-437.
14. Jakubovski E, Varigonda AL, Freemantle N, et al. Systematic review and meta-analysis: dose-response relationship of selective serotonin reuptake inhibitors in major depressive disorder. Am J Psychiatry. 2016;173(2):174-183.
15. Varigonda AL, Jakubovski E, Taylor MJ, et al. Systematic review and meta-analysis: early treatment responses of selective serotonin reuptake inhibitors in pediatric major depressive disorder. J Am Acad Child Adolesc Psychiatry. 2015;54(7):557-564.
16. Strawn JR, Welge JA, Wehry AM, et al. Efficacy and tolerability of antidepressants in pediatric anxiety disorders: a systematic review and meta-analysis. Depress Anxiety. 2015;32(3):149-157.
17. March JS, Biederman J, Wolkow R, et al. Sertraline in children and adolescents with obsessive-compulsive disorder: a multicenter randomized controlled trial. JAMA. 1998;280(20):1752-1756.
18. Walkup JT, Albano AM, Piacentini J, et al. Cognitive behavioral therapy, sertraline, or a combination in childhood anxiety. N Engl J Med. 2008;359(26):2753-2766.
19. Kennard BD, Silva SG, Tonev S, et al. Remission and recovery in the Treatment for Adolescents with Depression Study (TADS): acute and long-term outcomes. J Am Acad Child Adolesc Psychiatry. 2009;48(2):186-195.
20. Cipriani A, Zhou X, Del Giovane C, et al. Comparative efficacy and tolerability of antidepressants for major depressive disorder in children and adolescents: a network meta-analysis. Lancet. 2016;388(10047):881-890.
21. Cohen JA, Mannarino AP, Perel JM, et al. A pilot randomized controlled trial of combined trauma-focused CBT and sertraline for childhood PTSD symptoms. J Am Acad Child Adolesc Psychiatry. 2007;46(7):811-819.
22. Robb AS, Cueva JE, Sporn J, et al. Sertraline treatment of children and adolescents with posttraumatic stress disorder: a double-blind, placebo-controlled trial. J Child Adolesc Psychopharmacol. 2010;20(6):463-471.
23. Diehle J, Opmeer BC, Boer F, et al. Trauma-focused cognitive behavioral therapy or eye movement desensitization and reprocessing: what works in children with posttraumatic stress symptoms? A randomized controlled trial. Eur Child Adolesc Psychiatry. 2015;24(2):227-236.
24. Aiyer R, Barkin RL, Bhatia A. Treatment of neuropathic pain with venlafaxine: a systematic review. Pain Med. 2017;18(10):1999-2012.
25. Barrickman LL, Perry PJ, Allen AJ, et al. Bupropion versus methylphenidate in the treatment of attention-deficit hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 1995;34(5):649-657.
26. Monuteaux MC, Spencer TJ, Faraone SV, et al. A randomized, placebo-controlled clinical trial of bupropion for the prevention of smoking in children and adolescents with attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2007;68(7):1094-1101.
27. Biederman J, Baldessarini RJ, Wright V, et al. A double-blind placebo controlled study of desipramine in the treatment of ADD: I. Efficacy. J Am Acad Child Adolesc Psychiatry. 1989;28(5):777-784.
28. Spencer T, Biederman J, Coffey B, et al. A double-blind comparison of desipramine and placebo in children and adolescents with chronic tic disorder and comorbid attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2002;59(7):649-656.
29. DeVeaugh-Geiss J, Moroz G, Biederman J, et al. Clomipramine hydrochloride in childhood and adolescent obsessive-compulsive disorder--a multicenter trial. J Am Acad Child Adolesc Psychiatry. 1992;31(1):45-49.
30. Caldwell PH, Sureshkumar P, Wong WC. Tricyclic and related drugs for nocturnal enuresis in children. Cochrane Database Syst Rev. 2016;(1):CD002117.
31. Varigonda AL, Jakubovski E, Bloch MH. Systematic review and meta-analysis: early treatment responses of selective serotonin reuptake inhibitors and clomipramine in pediatric obsessive-compulsive disorder. J Am Acad Child Adolesc Psychiatry. 2016;55(10):851-859.e2. doi: 10.1016/j.jaac.2016.07.768.
32. Walkup J, Labellarte M. Complications of SSRI treatment. J Child Adolesc Psychopharmacol. 2001;11(1):1-4.
33. Leo RJ, Lichter DG, Hershey LA. Parkinsonism associated with fluoxetine and cimetidine: a case report. J Geriatr Psychiatry Neurol. 1995;8(4):231-233.
34. Strawn JR, Prakash A, Zhang Q, et al. A randomized, placebo-controlled study of duloxetine for the treatment of children and adolescents with generalized anxiety disorder. J Am Acad Child Adolesc Psychiatry. 2015;54(4):283-293.
35. Bernstein GA, Borchardt CM, Perwien AR, et al. Imipramine plus cognitive-behavioral therapy in the treatment of school refusal. J Am Acad Child Adolesc Psychiatry. 2000;39(3): 276-283.
36. Safer DJ, Zito JM. Treatment-emergent adverse events from selective serotonin reuptake inhibitors by age group: children versus adolescents. J Child Adolesc Psychopharmacol. 2006;16(1-2):159-169.
37. Reinblatt SP, DosReis S, Walkup JT, et al. Activation adverse events induced by the selective serotonin reuptake inhibitor fluvoxamine in children and adolescents. J Child Adolesc Psychopharmacol. 2009;19(2):119-126.
38. Goldsmith M, Singh M, Chang K. Antidepressants and psychostimulants in pediatric populations: is there an association with mania? Paediatr Drugs. 2011;13(4): 225-243.
39. Sidor MM, Macqueen GM. Antidepressants for the acute treatment of bipolar depression: a systematic review and meta-analysis. J Clin Psychiatry. 2011;72(2):156-167.
40. Allain N, Leven C, Falissard B, et al. Manic switches induced by antidepressants: an umbrella review comparing randomized controlled trials and observational studies. Acta Psychiatr Scand. 2017;135(2):106-116.
41. McClellan J, Kowatch R, Findling RL. Practice parameter for the assessment and treatment of children and adolescents with bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2007;46(1):107-125.
42. Dobry Y, Rice T, Sher L. Ecstasy use and serotonin syndrome: a neglected danger to adolescents and young adults prescribed selective serotonin reuptake inhibitors. Int J Adolesc Med Health. 2013; 25(3):193-199.
43. Schwartz AR, Pizon AF, Brooks DE. Dextromethorphan-induced serotonin syndrome. Clin Toxicol (Phila). 2008;46(8):771-773.
44. Gibbons RD, Brown CH, Hur K, et al. Early evidence on the effects of regulators’ suicidality warnings on SSRI prescriptions and suicide in children and adolescents. Am J Psychiatry. 2007;164(9):1356-1363.
45. Hammad TA, Laughren T, Racoosin J. Suicidality in pediatric patients treated with antidepressant drugs. Arch Gen Psychiatry. 2006;63(3):332-339.
46. Bridge JA, Iyengar S, Salary CB, et al. Clinical response and risk for reported suicidal ideation and suicide attempts in pediatric antidepressant treatment: a meta-analysis of randomized controlled trials. JAMA. 2007;297(15):1683-1696.
47. Sharma T, Guski LS, Freund N, et al. Suicidality and aggression during antidepressant treatment: systematic review and meta-analyses based on clinical study reports. BMJ. 2016;352: i65. doi: https://doi.org/10.1136/bmj.i65.
48. Olfson M, Shaffer D, Marcus SC, et al. Relationship between antidepressant medication treatment and suicide in adolescents. Arch Gen Psychiatry. 2003;60(10):978-982.
49. Garland JE, Kutcher S, Virani A, et al. Update on the Use of SSRIs and SNRIs with children and adolescents in clinical practice. J Can Acad Child Adolesc Psychiatry. 2016;25(1):4-10.
50. Bridge JA, Barbe RP, Birmaher B, et al. Emergent suicidality in a clinical psychotherapy trial for adolescent depression. Am J Psychiatry. 2005;162(11):2173-2175.
51. Birmaher B, Brent D, Bernet W, et al. Practice parameter for the assessment and treatment of children and adolescents with depressive disorders. J Am Acad Child Adolesc Psychiatry. 2007;46(11):1503-1526.
52. Ravizza L, Maina G, Bogetto F, et al. Long term treatment of obsessive-compulsive disorder. CNS Drugs. 1998;10(4):247-255.
53. Hosenbocus S, Chahal R. SSRIs and SNRIs: a review of the discontinuation syndrome in children and adolescents. J Can Acad Child Adolesc Psychiatry. 2011;20(1):60-67.
54. Bloch MH, McGuire J, Landeros-Weisenberger A, et al. Meta-analysis of the dose-response relationship of SSRI in obsessive-compulsive disorder. Mol Psychiatry. 2010;15(8):850-855.
55. Issari Y, Jakubovski E, Bartley CA, et al. Early onset of response with selective serotonin reuptake inhibitors in obsessive-compulsive disorder: a meta-analysis. J Clin Psychiatry. 2016; 77(5):e605-e611. doi: 10.4088/JCP.14r09758.
56. Brent D, Emslie G, Clarke G, et al. Switching to another SSRI or to venlafaxine with or without cognitive behavioral therapy for adolescents with SSRI-resistant depression: the TORDIA randomized controlled trial. JAMA. 2008;299(8):901-913.
1. Williams SB, O’Connor EA, Eder M, et al. Screening for child and adolescent depression in primary care settings: a systematic evidence review for the US Preventive Services Task Force. Pediatrics. 2009;123(4):e716-e735. doi: 10.1542/peds.2008-2415.
2. Kessler RC, Avenevoli S, Ries Merikangas K. Mood disorders in children and adolescents: an epidemiologic perspective. Biol Psychiatry. 2001;49(12):1002-1014.
3. Lewinsohn PM, Clarke GN, Seeley JR, et al. Major depression in community adolescents: age at onset, episode duration, and time to recurrence. J Am Acad Child Adolesc Psychiatry. 1994;33(6):809-818.
4. Weissman MM, Wolk S, Goldstein RB, et al. Depressed adolescents grown up. JAMA.1999;281(18):1707-1713.
5. Fergusson DM, Woodward LJ. Mental health, educational, and social role outcomes of adolescents with depression. Arch Gen Psychiatry. 2002;59(3):225-231.
6. Keenan-Miller D, Hammen CL, Brennan PA. Health outcomes related to early adolescent depression. J Adolesc Health. 2007; 41(3): 256-62.
7. Shaffer D, Gould MS, Fisher P, et al. Psychiatric diagnosis in child and adolescent suicide. Arch Gen Psychiatry. 1996;53(4):339-348.
8. Centers for Disease Control and Prevention. National Vital Statistics System. Deaths, percent of total deaths, and death rates for the 15 leading causes of death in 5-year age groups, by race and sex: United States, 1999-2015. https://www.cdc.gov/nchs/nvss/mortality/lcwk1.htm. Published October 23, 2017. Accessed May 2, 2019.
9. Merikangas KR, He JP, Burstein M, et al. Lifetime prevalence of mental disorders in US adolescents: results from the National Comorbidity Survey Replication-Adolescent Supplement (NCS-A). J Am Acad Child Adolesc Psychiatry. 2010;49(10):980-989.
10. Wittchen HU, Nelson CB, Lachner G. Prevalence of mental disorders and psychosocial impairments in adolescents and young adults. Psychol Med. 1998;28(1):109-126.
11. Foley DL, Goldston DB, Costello EJ, et al. Proximal psychiatric risk factors for suicidality in youth: the Great Smoky Mountains Study. Arch Gen Psychiatry. 2006;63(9):1017-1024.
12. Cheung A, Sacks D, Dewa CS, et al. Pediatric prescribing practices and the FDA black-box warning on antidepressants. J Dev Behav Pediatr. 2008 29(3):213-215.
13. Walkup JT. Antidepressant efficacy for depression in children and adolescents: industry- and NIMH-funded studies. Am J Psychiatry. 2017;174(5):430-437.
14. Jakubovski E, Varigonda AL, Freemantle N, et al. Systematic review and meta-analysis: dose-response relationship of selective serotonin reuptake inhibitors in major depressive disorder. Am J Psychiatry. 2016;173(2):174-183.
15. Varigonda AL, Jakubovski E, Taylor MJ, et al. Systematic review and meta-analysis: early treatment responses of selective serotonin reuptake inhibitors in pediatric major depressive disorder. J Am Acad Child Adolesc Psychiatry. 2015;54(7):557-564.
16. Strawn JR, Welge JA, Wehry AM, et al. Efficacy and tolerability of antidepressants in pediatric anxiety disorders: a systematic review and meta-analysis. Depress Anxiety. 2015;32(3):149-157.
17. March JS, Biederman J, Wolkow R, et al. Sertraline in children and adolescents with obsessive-compulsive disorder: a multicenter randomized controlled trial. JAMA. 1998;280(20):1752-1756.
18. Walkup JT, Albano AM, Piacentini J, et al. Cognitive behavioral therapy, sertraline, or a combination in childhood anxiety. N Engl J Med. 2008;359(26):2753-2766.
19. Kennard BD, Silva SG, Tonev S, et al. Remission and recovery in the Treatment for Adolescents with Depression Study (TADS): acute and long-term outcomes. J Am Acad Child Adolesc Psychiatry. 2009;48(2):186-195.
20. Cipriani A, Zhou X, Del Giovane C, et al. Comparative efficacy and tolerability of antidepressants for major depressive disorder in children and adolescents: a network meta-analysis. Lancet. 2016;388(10047):881-890.
21. Cohen JA, Mannarino AP, Perel JM, et al. A pilot randomized controlled trial of combined trauma-focused CBT and sertraline for childhood PTSD symptoms. J Am Acad Child Adolesc Psychiatry. 2007;46(7):811-819.
22. Robb AS, Cueva JE, Sporn J, et al. Sertraline treatment of children and adolescents with posttraumatic stress disorder: a double-blind, placebo-controlled trial. J Child Adolesc Psychopharmacol. 2010;20(6):463-471.
23. Diehle J, Opmeer BC, Boer F, et al. Trauma-focused cognitive behavioral therapy or eye movement desensitization and reprocessing: what works in children with posttraumatic stress symptoms? A randomized controlled trial. Eur Child Adolesc Psychiatry. 2015;24(2):227-236.
24. Aiyer R, Barkin RL, Bhatia A. Treatment of neuropathic pain with venlafaxine: a systematic review. Pain Med. 2017;18(10):1999-2012.
25. Barrickman LL, Perry PJ, Allen AJ, et al. Bupropion versus methylphenidate in the treatment of attention-deficit hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 1995;34(5):649-657.
26. Monuteaux MC, Spencer TJ, Faraone SV, et al. A randomized, placebo-controlled clinical trial of bupropion for the prevention of smoking in children and adolescents with attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2007;68(7):1094-1101.
27. Biederman J, Baldessarini RJ, Wright V, et al. A double-blind placebo controlled study of desipramine in the treatment of ADD: I. Efficacy. J Am Acad Child Adolesc Psychiatry. 1989;28(5):777-784.
28. Spencer T, Biederman J, Coffey B, et al. A double-blind comparison of desipramine and placebo in children and adolescents with chronic tic disorder and comorbid attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2002;59(7):649-656.
29. DeVeaugh-Geiss J, Moroz G, Biederman J, et al. Clomipramine hydrochloride in childhood and adolescent obsessive-compulsive disorder--a multicenter trial. J Am Acad Child Adolesc Psychiatry. 1992;31(1):45-49.
30. Caldwell PH, Sureshkumar P, Wong WC. Tricyclic and related drugs for nocturnal enuresis in children. Cochrane Database Syst Rev. 2016;(1):CD002117.
31. Varigonda AL, Jakubovski E, Bloch MH. Systematic review and meta-analysis: early treatment responses of selective serotonin reuptake inhibitors and clomipramine in pediatric obsessive-compulsive disorder. J Am Acad Child Adolesc Psychiatry. 2016;55(10):851-859.e2. doi: 10.1016/j.jaac.2016.07.768.
32. Walkup J, Labellarte M. Complications of SSRI treatment. J Child Adolesc Psychopharmacol. 2001;11(1):1-4.
33. Leo RJ, Lichter DG, Hershey LA. Parkinsonism associated with fluoxetine and cimetidine: a case report. J Geriatr Psychiatry Neurol. 1995;8(4):231-233.
34. Strawn JR, Prakash A, Zhang Q, et al. A randomized, placebo-controlled study of duloxetine for the treatment of children and adolescents with generalized anxiety disorder. J Am Acad Child Adolesc Psychiatry. 2015;54(4):283-293.
35. Bernstein GA, Borchardt CM, Perwien AR, et al. Imipramine plus cognitive-behavioral therapy in the treatment of school refusal. J Am Acad Child Adolesc Psychiatry. 2000;39(3): 276-283.
36. Safer DJ, Zito JM. Treatment-emergent adverse events from selective serotonin reuptake inhibitors by age group: children versus adolescents. J Child Adolesc Psychopharmacol. 2006;16(1-2):159-169.
37. Reinblatt SP, DosReis S, Walkup JT, et al. Activation adverse events induced by the selective serotonin reuptake inhibitor fluvoxamine in children and adolescents. J Child Adolesc Psychopharmacol. 2009;19(2):119-126.
38. Goldsmith M, Singh M, Chang K. Antidepressants and psychostimulants in pediatric populations: is there an association with mania? Paediatr Drugs. 2011;13(4): 225-243.
39. Sidor MM, Macqueen GM. Antidepressants for the acute treatment of bipolar depression: a systematic review and meta-analysis. J Clin Psychiatry. 2011;72(2):156-167.
40. Allain N, Leven C, Falissard B, et al. Manic switches induced by antidepressants: an umbrella review comparing randomized controlled trials and observational studies. Acta Psychiatr Scand. 2017;135(2):106-116.
41. McClellan J, Kowatch R, Findling RL. Practice parameter for the assessment and treatment of children and adolescents with bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2007;46(1):107-125.
42. Dobry Y, Rice T, Sher L. Ecstasy use and serotonin syndrome: a neglected danger to adolescents and young adults prescribed selective serotonin reuptake inhibitors. Int J Adolesc Med Health. 2013; 25(3):193-199.
43. Schwartz AR, Pizon AF, Brooks DE. Dextromethorphan-induced serotonin syndrome. Clin Toxicol (Phila). 2008;46(8):771-773.
44. Gibbons RD, Brown CH, Hur K, et al. Early evidence on the effects of regulators’ suicidality warnings on SSRI prescriptions and suicide in children and adolescents. Am J Psychiatry. 2007;164(9):1356-1363.
45. Hammad TA, Laughren T, Racoosin J. Suicidality in pediatric patients treated with antidepressant drugs. Arch Gen Psychiatry. 2006;63(3):332-339.
46. Bridge JA, Iyengar S, Salary CB, et al. Clinical response and risk for reported suicidal ideation and suicide attempts in pediatric antidepressant treatment: a meta-analysis of randomized controlled trials. JAMA. 2007;297(15):1683-1696.
47. Sharma T, Guski LS, Freund N, et al. Suicidality and aggression during antidepressant treatment: systematic review and meta-analyses based on clinical study reports. BMJ. 2016;352: i65. doi: https://doi.org/10.1136/bmj.i65.
48. Olfson M, Shaffer D, Marcus SC, et al. Relationship between antidepressant medication treatment and suicide in adolescents. Arch Gen Psychiatry. 2003;60(10):978-982.
49. Garland JE, Kutcher S, Virani A, et al. Update on the Use of SSRIs and SNRIs with children and adolescents in clinical practice. J Can Acad Child Adolesc Psychiatry. 2016;25(1):4-10.
50. Bridge JA, Barbe RP, Birmaher B, et al. Emergent suicidality in a clinical psychotherapy trial for adolescent depression. Am J Psychiatry. 2005;162(11):2173-2175.
51. Birmaher B, Brent D, Bernet W, et al. Practice parameter for the assessment and treatment of children and adolescents with depressive disorders. J Am Acad Child Adolesc Psychiatry. 2007;46(11):1503-1526.
52. Ravizza L, Maina G, Bogetto F, et al. Long term treatment of obsessive-compulsive disorder. CNS Drugs. 1998;10(4):247-255.
53. Hosenbocus S, Chahal R. SSRIs and SNRIs: a review of the discontinuation syndrome in children and adolescents. J Can Acad Child Adolesc Psychiatry. 2011;20(1):60-67.
54. Bloch MH, McGuire J, Landeros-Weisenberger A, et al. Meta-analysis of the dose-response relationship of SSRI in obsessive-compulsive disorder. Mol Psychiatry. 2010;15(8):850-855.
55. Issari Y, Jakubovski E, Bartley CA, et al. Early onset of response with selective serotonin reuptake inhibitors in obsessive-compulsive disorder: a meta-analysis. J Clin Psychiatry. 2016; 77(5):e605-e611. doi: 10.4088/JCP.14r09758.
56. Brent D, Emslie G, Clarke G, et al. Switching to another SSRI or to venlafaxine with or without cognitive behavioral therapy for adolescents with SSRI-resistant depression: the TORDIA randomized controlled trial. JAMA. 2008;299(8):901-913.
Infective endocarditis: Beyond the usual tests
Prompt diagnois of infective endocarditis is critical. Potential consequences of missed or delayed diagnosis, including heart failure, stroke, intracardiac abscess, conduction delays, prosthesis dysfunction, and cerebral emboli, are often catastrophic. Echocardiography is the test used most frequently to evaluate for infective endocarditis, but it misses the diagnosis in almost one-third of cases, and even more often if the patient has a prosthetic valve.
But now, several sophisticated imaging tests are available that complement echocardiography in diagnosing and assessing infective endocarditis; these include 4-dimensional computed tomography (4D CT), fluorodeoxyglucose positron emission tomography (FDG-PET), and leukocyte scintigraphy. These tests have greatly improved our ability not only to diagnose infective endocarditis, but also to determine the extent and spread of infection, and they aid in perioperative assessment. Abnormal findings on these tests have been incorporated into the European Society of Cardiology’s 2015 modified diagnostic criteria for infective endocarditis.1
This article details the indications, advantages, and limitations of the various imaging tests for diagnosing and evaluating infective endocarditis (Table 1).
INFECTIVE ENDOCARDITIS IS DIFFICULT TO DIAGNOSE AND TREAT
Infective endocarditis is difficult to diagnose and treat. Clinical and imaging clues can be subtle, and the diagnosis requires a high level of suspicion and visualization of cardiac structures.
Further, the incidence of infective endocarditis is on the rise in the United States, particularly in women and young adults, likely due to intravenous drug use.2,3
ECHOCARDIOGRAPHY HAS AN IMPORTANT ROLE, BUT IS LIMITED
Echocardiography remains the most commonly performed study for diagnosing infective endocarditis, as it is fast, widely accessible, and less expensive than other imaging tests.
Transthoracic echocardiography (TTE) is often the first choice for testing. However, its sensitivity is only about 70% for detecting vegetations on native valves and 50% for detecting vegetations on prosthetic valves.1 It is inherently constrained by the limited number of views by which a comprehensive external evaluation of the heart can be achieved. Using a 2-dimensional instrument to view a 3-dimensional object is difficult, and depending on several factors, it can be hard to see vegetations and abscesses that are associated with infective endocarditis. Further, TTE is impeded by obesity and by hyperinflated lungs from obstructive pulmonary disease or mechanical ventilation. It has poor sensitivity for detecting small vegetations and for detecting vegetations and paravalvular complications in patients who have a prosthetic valve or a cardiac implanted electronic device.
Transesophageal echocardiography (TEE) is the recommended first-line imaging test for patients with prosthetic valves and no contraindications to the test. Otherwise, it should be done after TTE if the results of TTE are negative but clinical suspicion for infective endocarditis remains high (eg, because the patient uses intravenous drugs). But although TEE has a higher sensitivity than TTE (up to 96% for vegetations on native valves and 92% for those on prosthetic valves, if performed by an experienced sonographer), it can still miss infective endocarditis. Also, TEE does not provide a significant advantage over TTE in patients who have a cardiac implanted electronic device.1,4,5
Regardless of whether TTE or TEE is used, they are estimated to miss up to 30% of cases of infective endocarditis and its sequelae.4 False-negative findings are likelier in patients who have preexisting severe valvular lesions, prosthetic valves, cardiac implanted electronic devices, small vegetations, or abscesses, or if a vegetation has already broken free and embolized. Furthermore, distinguishing between vegetations and thrombi, cardiac tumors, and myxomatous changes using echocardiography is difficult.
CARDIAC CT
For patients who have inconclusive results on echocardiography, contraindications to TEE, or poor sonic windows, cardiac CT can be an excellent alternative. It is especially useful in the setting of a prosthetic valve.
Synchronized (“gated”) with the patient’s heart rate and rhythm, CT machines can acquire images during diastole, reducing motion artifact, and can create 3D images of the heart. In addition, newer machines can acquire several images at different points in the heart cycle to add a fourth dimension—time. The resulting 4D images play like short video loops of the beating heart and allow noninvasive assessment of cardiac anatomy with remarkable detail and resolution.
4D CT is increasingly being used in infective endocarditis, and growing evidence indicates that its accuracy is similar to that of TEE in the preoperative evaluation of patients with aortic prosthetic valve endocarditis.6 In a study of 28 patients, complementary use of CT angiography led to a change in treatment strategy in 7 (25%) compared with routine clinical workup.7 Several studies have found no difference between 4D CT and preoperative TEE in detecting pseudoaneurysm, abscess, or valve dehiscence. TEE and 4D CT also have similar sensitivities for detecting infective endocarditis in native and prosthetic valves.8,9
Coupled with CT angiography, 4D CT is also an excellent noninvasive way to perioperatively evaluate the coronary arteries without the risks associated with catheterization in those requiring nonemergency surgery (Figure 1A, B, and C).
4D CT performs well for detecting abscess and pseudoaneurysm but has slightly lower sensitivity for vegetations than TEE (91% vs 99%).9
Gated CT, PET, or both may be useful in cases of suspected prosthetic aortic valve endocarditis when TEE is negative. Pseudoaneurysms are not well visualized with TEE, and the atrial mitral curtain area is often thickened on TEE in cases of aortic prosthetic valve infective endocarditis that do not definitely involve abscesses. Gated CT and PET show this area better.8 This information is important in cases in which a surgeon may be unconvinced that the patient has prosthetic valve endocarditis.
Limitations of 4D cardiac CT
4D CT with or without angiography has limitations. It requires a wide-volume scanner and an experienced reader.
Patients with irregular heart rhythms or uncontrolled tachycardia pose technical problems for image acquisition. Cardiac CT is typically gated (ie, images are obtained within a defined time period) to acquire images during diastole. Ideally, images are acquired when the heart is in mid to late diastole, a time of minimal cardiac motion, so that motion artifact is minimized. To estimate the timing of image acquisition, the cardiac cycle must be predictable, and its duration should be as long as possible. Tachycardia or irregular rhythms such as frequent ectopic beats or atrial fibrillation make acquisition timing difficult, and thus make it nearly impossible to accurately obtain images when the heart is at minimum motion, limiting assessment of cardiac structures or the coronary tree.4,10
Extensive coronary calcification can hinder assessment of the coronary tree by CT coronary angiography.
Contrast exposure may limit the use of CT in some patients (eg, those with contrast allergies or renal dysfunction). However, modern scanners allow for much smaller contrast boluses without decreasing sensitivity.
4D CT involves radiation exposure, especially when done with angiography, although modern scanners have greatly reduced exposure. The average radiation dose in CT coronary angiography is 2.9 to 5.9 mSv11 compared with 7 mSv in diagnostic cardiac catheterization (without angioplasty or stenting) or 16 mSv in routine CT of the abdomen and pelvis with contrast.12,13 In view of the morbidity and mortality risks associated with infective endocarditis, especially if the diagnosis is delayed, this small radiation exposure may be justifiable.
Bottom line for cardiac CT
4D CT is an excellent alternative to echocardiography for select patients. Clinicians should strongly consider this study in the following situations:
- Patients with a prosthetic valve
- Patients who are strongly suspected of having infective endocarditis but who have a poor sonic window on TTE or TEE, as can occur with chronic obstructive lung disease, morbid obesity, or previous thoracic or cardiovascular surgery
- Patients who meet clinical indications for TEE, such as having a prosthetic valve or a high suspicion for native valve infective endocarditis with negative TTE, but who have contraindications to TEE
- As an alternative to TEE for preoperative evaluation in patients with known infective endocarditis.
Patients with tachycardia or irregular heart rhythms are not good candidates for this test.
FDG-PET AND LEUKOCYTE SCINTIGRAPHY
FDG-PET and leukocyte scintigraphy are other options for diagnosing infective endocarditis and determining the presence and extent of intra- and extracardiac infection. They are more sensitive than echocardiography for detecting infection of cardiac implanted electronic devices such as ventricular assist devices, pacemakers, implanted cardiac defibrillators, and cardiac resynchronization therapy devices.14–16
The utility of FDG-PET is founded on the uptake of 18F-fluorodeoxyglucose by cells, with higher uptake taking place in cells with higher metabolic activity (such as in areas of inflammation). Similarly, leukocyte scintigraphy relies on the use of radiolabeled leukocytes (ie, leukocytes previously extracted from the patient, labelled, and re-introduced into the patient) to allow for localization of inflamed tissue.
The most significant contribution of FDG-PET may be the ability to detect infective endocarditis early, when echocardiography is initially negative. When abnormal FDG uptake was included in the modified Duke criteria, it increased the sensitivity to 97% for detecting infective endocarditis on admission, leading some to propose its incorporation as a major criterion.17 In patients with prosthetic valves and suspected infective endocarditis, FDG-PET was found in one study to have a sensitivity of up to 91% and a specificity of up to 95%.18
Both FDG-PET and leukocyte scintigraphy have a high sensitivity, specificity, and negative predictive value for cardiac implanted electronic device infection, and should be strongly considered in patients in whom it is suspected but who have negative or inconclusive findings on echocardiography.14,15
In addition, a common conundrum faced by clinicians with use of echocardiography is the difficulty of differentiating thrombus from infected vegetation on valves or device lead wires. Some evidence indicates that FDG-PET may help to discriminate between vegetation and thrombus, although more rigorous studies are needed before its use for that purpose can be recommended.19
Limitations of nuclear studies
Both FDG-PET and leukocyte scintigraphy perform poorly for detecting native-valve infective endocarditis. In a study in which 90% of the patients had native-valve infective endocarditis according to the Duke criteria, FDG-PET had a specificity of 93% but a sensitivity of only 39%.20
Both studies can be cumbersome, laborious, and time-consuming for patients. FDG-PET requires a fasting or glucose-restricted diet before testing, and the test itself can be complicated by development of hyperglycemia, although this is rare.
While FDG-PET is most effective in detecting infections of prosthetic valves and cardiac implanted electronic devices, the results can be falsely positive in patients with a history of recent cardiac surgery (due to ongoing tissue healing), as well as maladies other than infective endocarditis that lead to inflammation, such as vasculitis or malignancy. Similarly, for unclear reasons, leukocyte scintigraphy can yield false-negative results in patients with enterococcal or candidal infective endocarditis.21
FDG-PET and leukocyte scintigraphy are more expensive than TEE and cardiac CT22 and are not widely available.
Both tests entail radiation exposure, with the average dose ranging from 7 to 14 mSv. However, this is less than the average amount acquired during percutaneous coronary intervention (16 mSv), and overlaps with the amount in chest CT with contrast when assessing for pulmonary embolism (7 to 9 mSv). Lower doses are possible with optimized protocols.12,13,15,23
Bottom line for nuclear studies
FDG-PET and leukocyte scintigraphy are especially useful for patients with a prosthetic valve or cardiac implanted electronic device. However, limitations must be kept in mind.
A suggested algorithm for testing with nuclear imaging is shown in Figure 2.1,4
CEREBRAL MAGNETIC RESONANCE IMAGING
Cerebral magnetic resonance imaging (MRI) is more sensitive than cerebral CT for detecting emboli in the brain. According to American Heart Association guidelines, cerebral MRI should be done in patients with known or suspected infective endocarditis and neurologic impairment, defined as headaches, meningeal symptoms, or neurologic deficits. It is also often used in neurologically asymptomatic patients with infective endocarditis who have indications for valve surgery to assess for mycotic aneurysms, which are associated with increased intracranial bleeding during surgery.
MRI use in other asymptomatic patients remains controversial.24 In cases with high clinical suspicion for infective endocarditis and no findings on echocardiography, cerebral MRI can increase the sensitivity of the Duke criteria by adding a minor criterion. Some have argued that, in patients with definite infective endocarditis, detecting silent cerebral complications can lead to management changes. However, more studies are needed to determine if there is indeed a group of neurologically asymptomatic infective endocarditis patients for whom cerebral MRI leads to improved outcomes.
Limitations of cerebral MRI
Cerebral MRI cannot be used in patients with non-MRI-compatible implanted hardware.
Gadolinium, the contrast agent typically used, can cause nephrogenic systemic fibrosis in patients who have poor renal function. This rare but serious adverse effect is characterized by irreversible systemic fibrosis affecting skin, muscles, and even visceral tissue such as lungs. The American College of Radiology allows for gadolinium use in patients without acute kidney injury and patients with stable chronic kidney disease with a glomerular filtration rate of at least 30 mL/min/1.73 m2. Its use should be avoided in patients with renal failure on replacement therapy, with advanced chronic kidney disease (glomerular filtration rate < 30 mL/min/1.73 m2), or with acute kidney injury, even if they do not need renal replacement therapy.25
Concerns have also been raised about gadolinium retention in the brain, even in patients with normal renal function.26–28 Thus far, no conclusive clinical adverse effects of retention have been found, although more study is warranted. Nevertheless, the US Food and Drug Administration now requires a black-box warning about this possibility and advises clinicians to counsel patients appropriately.
Bottom line on cerebral MRI
Cerebral MRI should be obtained when a patient presents with definite or possible infective endocarditis with neurologic impairment, such as new headaches, meningismus, or focal neurologic deficits. Routine brain MRI in patients with confirmed infective endocarditis without neurologic symptoms, or those without definite infective endocarditis, is discouraged.
CARDIAC MRI
Cardiac MRI, typically obtained with gadolinium contrast, allows for better 3D assessment of cardiac structures and morphology than echocardiography or CT, and can detect infiltrative cardiac disease, myopericarditis, and much more. It is increasingly used in the field of structural cardiology, but its role for evaluating infective endocarditis remains unclear.
Cardiac MRI does not appear to be better than echocardiography for diagnosing infective endocarditis. However, it may prove helpful in the evaluation of patients known to have infective endocarditis but who cannot be properly evaluated for disease extent because of poor image quality on echocardiography and contraindications to CT.1,29 Its role is limited in patients with cardiac implanted electronic devices, as most devices are incompatible with MRI use, although newer devices obviate this concern. But even for devices that are MRI-compatible, results are diminished due to an eclipsing effect, wherein the device parts can make it hard to see structures clearly because the “brightness” basically eclipses the surrounding area.4
Concerns regarding use of gadolinium as described above need also be considered.
The role of cardiac MRI in diagnosing and managing infective endocarditis may evolve, but at present, the 2017 American College of Cardiology and American Heart Association appropriate-use criteria discourage its use for these purposes.16
Bottom line for cardiac MRI
Cardiac MRI to evaluate a patient for suspected infective endocarditis is not recommended due to lack of superiority compared with echocardiography or CT, and the risk of nephrogenic systemic fibrosis from gadolinium in patients with renal compromise.
- Habib G, Lancellotti P, Antunes MJ, et al; ESC Scientific Document Group. 2015 ESC guidelines for the management of infective endocarditis: the Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J 2015; 36(44):3075–3128. doi:10.1093/eurheartj/ehv319
- Durante-Mangoni E, Bradley S, Selton-Suty C, et al; International Collaboration on Endocarditis Prospective Cohort Study Group. Current features of infective endocarditis in elderly patients: results of the International Collaboration on Endocarditis Prospective Cohort Study. Arch Intern Med 2008; 168(19):2095–2103. doi:10.1001/archinte.168.19.2095
- Wurcel AG, Anderson JE, Chui KK, et al. Increasing infectious endocarditis admissions among young people who inject drugs. Open Forum Infect Dis 2016; 3(3):ofw157. doi:10.1093/ofid/ofw157
- Gomes A, Glaudemans AW, Touw DJ, et al. Diagnostic value of imaging in infective endocarditis: a systematic review. Lancet Infect Dis 2017; 17(1):e1–e14. doi:10.1016/S1473-3099(16)30141-4
- Cahill TJ, Baddour LM, Habib G, et al. Challenges in infective endocarditis. J Am Coll Cardiol 2017; 69(3):325–344. doi:10.1016/j.jacc.2016.10.066
- Fagman E, Perrotta S, Bech-Hanssen O, et al. ECG-gated computed tomography: a new role for patients with suspected aortic prosthetic valve endocarditis. Eur Radiol 2012; 22(11):2407–2414. doi:10.1007/s00330-012-2491-5
- Habets J, Tanis W, van Herwerden LA, et al. Cardiac computed tomography angiography results in diagnostic and therapeutic change in prosthetic heart valve endocarditis. Int J Cardiovasc Imaging 2014; 30(2):377–387. doi:10.1007/s10554-013-0335-2
- Koneru S, Huang SS, Oldan J, et al. Role of preoperative cardiac CT in the evaluation of infective endocarditis: comparison with transesophageal echocardiography and surgical findings. Cardiovasc Diagn Ther 2018; 8(4):439–449. doi:10.21037/cdt.2018.07.07
- Koo HJ, Yang DH, Kang J, et al. Demonstration of infective endocarditis by cardiac CT and transoesophageal echocardiography: comparison with intra-operative findings. Eur Heart J Cardiovasc Imaging 2018; 19(2):199–207. doi:10.1093/ehjci/jex010
- Feuchtner GM, Stolzmann P, Dichtl W, et al. Multislice computed tomography in infective endocarditis: comparison with transesophageal echocardiography and intraoperative findings. J Am Coll Cardiol 2009; 53(5):436–444. doi:10.1016/j.jacc.2008.01.077
- Castellano IA, Nicol ED, Bull RK, Roobottom CA, Williams MC, Harden SP. A prospective national survey of coronary CT angiography radiation doses in the United Kingdom. J Cardiovasc Comput Tomogr 2017; 11(4):268–273. doi:10.1016/j.jcct.2017.05.002
- Mettler FA Jr, Huda W, Yoshizumi TT, Mahesh M. Effective doses in radiology and diagnostic nuclear medicine: a catalog. Radiology 2008; 248(1):254–263. doi:10.1148/radiol.2481071451
- Smith-Bindman R, Lipson J, Marcus R, et al. Radiation dose associated with common computed tomography examinations and the associated lifetime attributable risk of cancer. Arch Intern Med 2009; 169(22):2078–2086. doi:10.1001/archinternmed.2009.427
- Ploux S, Riviere A, Amraoui S, et al. Positron emission tomography in patients with suspected pacing system infections may play a critical role in difficult cases. Heart Rhythm 2011; 8(9):1478–1481. doi:10.1016/j.hrthm.2011.03.062
- Sarrazin J, Philippon F, Tessier M, et al. Usefulness of fluorine-18 positron emission tomography/computed tomography for identification of cardiovascular implantable electronic device infections. J Am Coll Cardiol 2012; 59(18):1616–1625. doi:10.1016/j.jacc.2011.11.059
- Doherty JU, Kort S, Mehran R, Schoenhagen P, Soman P; Rating Panel Members; Appropriate Use Criteria Task Force. ACC/AATS/AHA/ASE/ASNC/HRS/SCAI/SCCT/SCMR/STS 2017 Appropriate use criteria for multimodality imaging in valvular heart disease: a report of the American College of Cardiology Appropriate Use Criteria Task Force, American Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and Society of Thoracic Surgeons. J Nucl Cardiol 2017; 24(6):2043–2063. doi:10.1007/s12350-017-1070-1
- Saby L, Laas O, Habib G, et al. Positron emission tomography/computed tomography for diagnosis of prosthetic valve endocarditis: increased valvular 18F-fluorodeoxyglucose uptake as a novel major criterion. J Am Coll Cardiol 2013; 61(23):2374–2382. doi:10.1016/j.jacc.2013.01.092
- Swart LE, Gomes A, Scholtens AM, et al. Improving the diagnostic performance of 18F-fluorodeoxyglucose positron-emission tomography/computed tomography in prosthetic heart valve endocarditis. Circulation 2018; 138(14):1412–1427. doi:10.1161/CIRCULATIONAHA.118.035032
- Graziosi M, Nanni C, Lorenzini M, et al. Role of 18F-FDG PET/CT in the diagnosis of infective endocarditis in patients with an implanted cardiac device: a prospective study. Eur J Nucl Med Mol Imaging 2014; 41(8):1617–1623. doi:10.1007/s00259-014-2773-z
- Kouijzer IJ, Vos FJ, Janssen MJ, van Dijk AP, Oyen WJ, Bleeker-Rovers CP. The value of 18F-FDG PET/CT in diagnosing infectious endocarditis. Eur J Nucl Med Mol Imaging 2013; 40(7):1102–1107. doi:10.1007/s00259-013-2376-0
- Wong D, Rubinshtein R, Keynan Y. Alternative cardiac imaging modalities to echocardiography for the diagnosis of infective endocarditis. Am J Cardiol 2016; 118(9):1410–1418. doi:10.1016/j.amjcard.2016.07.053
- Vos FJ, Bleeker-Rovers CP, Kullberg BJ, Adang EM, Oyen WJ. Cost-effectiveness of routine (18)F-FDG PET/CT in high-risk patients with gram-positive bacteremia. J Nucl Med 2011; 52(11):1673–1678. doi:10.2967/jnumed.111.089714
- McCollough CH, Bushberg JT, Fletcher JG, Eckel LJ. Answers to common questions about the use and safety of CT scans. Mayo Clin Proc 2015; 90(10):1380–1392. doi:10.1016/j.mayocp.2015.07.011
- Duval X, Iung B, Klein I, et al; IMAGE (Resonance Magnetic Imaging at the Acute Phase of Endocarditis) Study Group. Effect of early cerebral magnetic resonance imaging on clinical decisions in infective endocarditis: a prospective study. Ann Intern Med 2010; 152(8):497–504, W175. doi:10.7326/0003-4819-152-8-201004200-00006
- ACR Committee on Drugs and Contrast Media. ACR Manual on Contrast Media: 2018. www.acr.org/-/media/ACR/Files/Clinical-Resources/Contrast_Media.pdf. Accessed July 19, 2019.
- Kanda T, Fukusato T, Matsuda M, et al. Gadolinium-based contrast agent accumulates in the brain even in subjects without severe renal dysfunction: evaluation of autopsy brain specimens with inductively coupled plasma mass spectroscopy. Radiology 2015; 276(1):228–232. doi:10.1148/radiol.2015142690
- McDonald RJ, McDonald JS, Kallmes DF, et al. Intracranial gadolinium deposition after contrast-enhanced MR imaging. Radiology 2015; 275(3):772–782. doi:10.1148/radiol.15150025
- Kanda T, Ishii K, Kawaguchi H, Kitajima K, Takenaka D. High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology 2014; 270(3):834–841. doi:10.1148/radiol.13131669
- Expert Panel on Pediatric Imaging; Hayes LL, Palasis S, Bartel TB, et al. ACR appropriateness criteria headache-child. J Am Coll Radiol 2018; 15(5S):S78–S90. doi:10.1016/j.jacr.2018.03.017
Prompt diagnois of infective endocarditis is critical. Potential consequences of missed or delayed diagnosis, including heart failure, stroke, intracardiac abscess, conduction delays, prosthesis dysfunction, and cerebral emboli, are often catastrophic. Echocardiography is the test used most frequently to evaluate for infective endocarditis, but it misses the diagnosis in almost one-third of cases, and even more often if the patient has a prosthetic valve.
But now, several sophisticated imaging tests are available that complement echocardiography in diagnosing and assessing infective endocarditis; these include 4-dimensional computed tomography (4D CT), fluorodeoxyglucose positron emission tomography (FDG-PET), and leukocyte scintigraphy. These tests have greatly improved our ability not only to diagnose infective endocarditis, but also to determine the extent and spread of infection, and they aid in perioperative assessment. Abnormal findings on these tests have been incorporated into the European Society of Cardiology’s 2015 modified diagnostic criteria for infective endocarditis.1
This article details the indications, advantages, and limitations of the various imaging tests for diagnosing and evaluating infective endocarditis (Table 1).
INFECTIVE ENDOCARDITIS IS DIFFICULT TO DIAGNOSE AND TREAT
Infective endocarditis is difficult to diagnose and treat. Clinical and imaging clues can be subtle, and the diagnosis requires a high level of suspicion and visualization of cardiac structures.
Further, the incidence of infective endocarditis is on the rise in the United States, particularly in women and young adults, likely due to intravenous drug use.2,3
ECHOCARDIOGRAPHY HAS AN IMPORTANT ROLE, BUT IS LIMITED
Echocardiography remains the most commonly performed study for diagnosing infective endocarditis, as it is fast, widely accessible, and less expensive than other imaging tests.
Transthoracic echocardiography (TTE) is often the first choice for testing. However, its sensitivity is only about 70% for detecting vegetations on native valves and 50% for detecting vegetations on prosthetic valves.1 It is inherently constrained by the limited number of views by which a comprehensive external evaluation of the heart can be achieved. Using a 2-dimensional instrument to view a 3-dimensional object is difficult, and depending on several factors, it can be hard to see vegetations and abscesses that are associated with infective endocarditis. Further, TTE is impeded by obesity and by hyperinflated lungs from obstructive pulmonary disease or mechanical ventilation. It has poor sensitivity for detecting small vegetations and for detecting vegetations and paravalvular complications in patients who have a prosthetic valve or a cardiac implanted electronic device.
Transesophageal echocardiography (TEE) is the recommended first-line imaging test for patients with prosthetic valves and no contraindications to the test. Otherwise, it should be done after TTE if the results of TTE are negative but clinical suspicion for infective endocarditis remains high (eg, because the patient uses intravenous drugs). But although TEE has a higher sensitivity than TTE (up to 96% for vegetations on native valves and 92% for those on prosthetic valves, if performed by an experienced sonographer), it can still miss infective endocarditis. Also, TEE does not provide a significant advantage over TTE in patients who have a cardiac implanted electronic device.1,4,5
Regardless of whether TTE or TEE is used, they are estimated to miss up to 30% of cases of infective endocarditis and its sequelae.4 False-negative findings are likelier in patients who have preexisting severe valvular lesions, prosthetic valves, cardiac implanted electronic devices, small vegetations, or abscesses, or if a vegetation has already broken free and embolized. Furthermore, distinguishing between vegetations and thrombi, cardiac tumors, and myxomatous changes using echocardiography is difficult.
CARDIAC CT
For patients who have inconclusive results on echocardiography, contraindications to TEE, or poor sonic windows, cardiac CT can be an excellent alternative. It is especially useful in the setting of a prosthetic valve.
Synchronized (“gated”) with the patient’s heart rate and rhythm, CT machines can acquire images during diastole, reducing motion artifact, and can create 3D images of the heart. In addition, newer machines can acquire several images at different points in the heart cycle to add a fourth dimension—time. The resulting 4D images play like short video loops of the beating heart and allow noninvasive assessment of cardiac anatomy with remarkable detail and resolution.
4D CT is increasingly being used in infective endocarditis, and growing evidence indicates that its accuracy is similar to that of TEE in the preoperative evaluation of patients with aortic prosthetic valve endocarditis.6 In a study of 28 patients, complementary use of CT angiography led to a change in treatment strategy in 7 (25%) compared with routine clinical workup.7 Several studies have found no difference between 4D CT and preoperative TEE in detecting pseudoaneurysm, abscess, or valve dehiscence. TEE and 4D CT also have similar sensitivities for detecting infective endocarditis in native and prosthetic valves.8,9
Coupled with CT angiography, 4D CT is also an excellent noninvasive way to perioperatively evaluate the coronary arteries without the risks associated with catheterization in those requiring nonemergency surgery (Figure 1A, B, and C).
4D CT performs well for detecting abscess and pseudoaneurysm but has slightly lower sensitivity for vegetations than TEE (91% vs 99%).9
Gated CT, PET, or both may be useful in cases of suspected prosthetic aortic valve endocarditis when TEE is negative. Pseudoaneurysms are not well visualized with TEE, and the atrial mitral curtain area is often thickened on TEE in cases of aortic prosthetic valve infective endocarditis that do not definitely involve abscesses. Gated CT and PET show this area better.8 This information is important in cases in which a surgeon may be unconvinced that the patient has prosthetic valve endocarditis.
Limitations of 4D cardiac CT
4D CT with or without angiography has limitations. It requires a wide-volume scanner and an experienced reader.
Patients with irregular heart rhythms or uncontrolled tachycardia pose technical problems for image acquisition. Cardiac CT is typically gated (ie, images are obtained within a defined time period) to acquire images during diastole. Ideally, images are acquired when the heart is in mid to late diastole, a time of minimal cardiac motion, so that motion artifact is minimized. To estimate the timing of image acquisition, the cardiac cycle must be predictable, and its duration should be as long as possible. Tachycardia or irregular rhythms such as frequent ectopic beats or atrial fibrillation make acquisition timing difficult, and thus make it nearly impossible to accurately obtain images when the heart is at minimum motion, limiting assessment of cardiac structures or the coronary tree.4,10
Extensive coronary calcification can hinder assessment of the coronary tree by CT coronary angiography.
Contrast exposure may limit the use of CT in some patients (eg, those with contrast allergies or renal dysfunction). However, modern scanners allow for much smaller contrast boluses without decreasing sensitivity.
4D CT involves radiation exposure, especially when done with angiography, although modern scanners have greatly reduced exposure. The average radiation dose in CT coronary angiography is 2.9 to 5.9 mSv11 compared with 7 mSv in diagnostic cardiac catheterization (without angioplasty or stenting) or 16 mSv in routine CT of the abdomen and pelvis with contrast.12,13 In view of the morbidity and mortality risks associated with infective endocarditis, especially if the diagnosis is delayed, this small radiation exposure may be justifiable.
Bottom line for cardiac CT
4D CT is an excellent alternative to echocardiography for select patients. Clinicians should strongly consider this study in the following situations:
- Patients with a prosthetic valve
- Patients who are strongly suspected of having infective endocarditis but who have a poor sonic window on TTE or TEE, as can occur with chronic obstructive lung disease, morbid obesity, or previous thoracic or cardiovascular surgery
- Patients who meet clinical indications for TEE, such as having a prosthetic valve or a high suspicion for native valve infective endocarditis with negative TTE, but who have contraindications to TEE
- As an alternative to TEE for preoperative evaluation in patients with known infective endocarditis.
Patients with tachycardia or irregular heart rhythms are not good candidates for this test.
FDG-PET AND LEUKOCYTE SCINTIGRAPHY
FDG-PET and leukocyte scintigraphy are other options for diagnosing infective endocarditis and determining the presence and extent of intra- and extracardiac infection. They are more sensitive than echocardiography for detecting infection of cardiac implanted electronic devices such as ventricular assist devices, pacemakers, implanted cardiac defibrillators, and cardiac resynchronization therapy devices.14–16
The utility of FDG-PET is founded on the uptake of 18F-fluorodeoxyglucose by cells, with higher uptake taking place in cells with higher metabolic activity (such as in areas of inflammation). Similarly, leukocyte scintigraphy relies on the use of radiolabeled leukocytes (ie, leukocytes previously extracted from the patient, labelled, and re-introduced into the patient) to allow for localization of inflamed tissue.
The most significant contribution of FDG-PET may be the ability to detect infective endocarditis early, when echocardiography is initially negative. When abnormal FDG uptake was included in the modified Duke criteria, it increased the sensitivity to 97% for detecting infective endocarditis on admission, leading some to propose its incorporation as a major criterion.17 In patients with prosthetic valves and suspected infective endocarditis, FDG-PET was found in one study to have a sensitivity of up to 91% and a specificity of up to 95%.18
Both FDG-PET and leukocyte scintigraphy have a high sensitivity, specificity, and negative predictive value for cardiac implanted electronic device infection, and should be strongly considered in patients in whom it is suspected but who have negative or inconclusive findings on echocardiography.14,15
In addition, a common conundrum faced by clinicians with use of echocardiography is the difficulty of differentiating thrombus from infected vegetation on valves or device lead wires. Some evidence indicates that FDG-PET may help to discriminate between vegetation and thrombus, although more rigorous studies are needed before its use for that purpose can be recommended.19
Limitations of nuclear studies
Both FDG-PET and leukocyte scintigraphy perform poorly for detecting native-valve infective endocarditis. In a study in which 90% of the patients had native-valve infective endocarditis according to the Duke criteria, FDG-PET had a specificity of 93% but a sensitivity of only 39%.20
Both studies can be cumbersome, laborious, and time-consuming for patients. FDG-PET requires a fasting or glucose-restricted diet before testing, and the test itself can be complicated by development of hyperglycemia, although this is rare.
While FDG-PET is most effective in detecting infections of prosthetic valves and cardiac implanted electronic devices, the results can be falsely positive in patients with a history of recent cardiac surgery (due to ongoing tissue healing), as well as maladies other than infective endocarditis that lead to inflammation, such as vasculitis or malignancy. Similarly, for unclear reasons, leukocyte scintigraphy can yield false-negative results in patients with enterococcal or candidal infective endocarditis.21
FDG-PET and leukocyte scintigraphy are more expensive than TEE and cardiac CT22 and are not widely available.
Both tests entail radiation exposure, with the average dose ranging from 7 to 14 mSv. However, this is less than the average amount acquired during percutaneous coronary intervention (16 mSv), and overlaps with the amount in chest CT with contrast when assessing for pulmonary embolism (7 to 9 mSv). Lower doses are possible with optimized protocols.12,13,15,23
Bottom line for nuclear studies
FDG-PET and leukocyte scintigraphy are especially useful for patients with a prosthetic valve or cardiac implanted electronic device. However, limitations must be kept in mind.
A suggested algorithm for testing with nuclear imaging is shown in Figure 2.1,4
CEREBRAL MAGNETIC RESONANCE IMAGING
Cerebral magnetic resonance imaging (MRI) is more sensitive than cerebral CT for detecting emboli in the brain. According to American Heart Association guidelines, cerebral MRI should be done in patients with known or suspected infective endocarditis and neurologic impairment, defined as headaches, meningeal symptoms, or neurologic deficits. It is also often used in neurologically asymptomatic patients with infective endocarditis who have indications for valve surgery to assess for mycotic aneurysms, which are associated with increased intracranial bleeding during surgery.
MRI use in other asymptomatic patients remains controversial.24 In cases with high clinical suspicion for infective endocarditis and no findings on echocardiography, cerebral MRI can increase the sensitivity of the Duke criteria by adding a minor criterion. Some have argued that, in patients with definite infective endocarditis, detecting silent cerebral complications can lead to management changes. However, more studies are needed to determine if there is indeed a group of neurologically asymptomatic infective endocarditis patients for whom cerebral MRI leads to improved outcomes.
Limitations of cerebral MRI
Cerebral MRI cannot be used in patients with non-MRI-compatible implanted hardware.
Gadolinium, the contrast agent typically used, can cause nephrogenic systemic fibrosis in patients who have poor renal function. This rare but serious adverse effect is characterized by irreversible systemic fibrosis affecting skin, muscles, and even visceral tissue such as lungs. The American College of Radiology allows for gadolinium use in patients without acute kidney injury and patients with stable chronic kidney disease with a glomerular filtration rate of at least 30 mL/min/1.73 m2. Its use should be avoided in patients with renal failure on replacement therapy, with advanced chronic kidney disease (glomerular filtration rate < 30 mL/min/1.73 m2), or with acute kidney injury, even if they do not need renal replacement therapy.25
Concerns have also been raised about gadolinium retention in the brain, even in patients with normal renal function.26–28 Thus far, no conclusive clinical adverse effects of retention have been found, although more study is warranted. Nevertheless, the US Food and Drug Administration now requires a black-box warning about this possibility and advises clinicians to counsel patients appropriately.
Bottom line on cerebral MRI
Cerebral MRI should be obtained when a patient presents with definite or possible infective endocarditis with neurologic impairment, such as new headaches, meningismus, or focal neurologic deficits. Routine brain MRI in patients with confirmed infective endocarditis without neurologic symptoms, or those without definite infective endocarditis, is discouraged.
CARDIAC MRI
Cardiac MRI, typically obtained with gadolinium contrast, allows for better 3D assessment of cardiac structures and morphology than echocardiography or CT, and can detect infiltrative cardiac disease, myopericarditis, and much more. It is increasingly used in the field of structural cardiology, but its role for evaluating infective endocarditis remains unclear.
Cardiac MRI does not appear to be better than echocardiography for diagnosing infective endocarditis. However, it may prove helpful in the evaluation of patients known to have infective endocarditis but who cannot be properly evaluated for disease extent because of poor image quality on echocardiography and contraindications to CT.1,29 Its role is limited in patients with cardiac implanted electronic devices, as most devices are incompatible with MRI use, although newer devices obviate this concern. But even for devices that are MRI-compatible, results are diminished due to an eclipsing effect, wherein the device parts can make it hard to see structures clearly because the “brightness” basically eclipses the surrounding area.4
Concerns regarding use of gadolinium as described above need also be considered.
The role of cardiac MRI in diagnosing and managing infective endocarditis may evolve, but at present, the 2017 American College of Cardiology and American Heart Association appropriate-use criteria discourage its use for these purposes.16
Bottom line for cardiac MRI
Cardiac MRI to evaluate a patient for suspected infective endocarditis is not recommended due to lack of superiority compared with echocardiography or CT, and the risk of nephrogenic systemic fibrosis from gadolinium in patients with renal compromise.
Prompt diagnois of infective endocarditis is critical. Potential consequences of missed or delayed diagnosis, including heart failure, stroke, intracardiac abscess, conduction delays, prosthesis dysfunction, and cerebral emboli, are often catastrophic. Echocardiography is the test used most frequently to evaluate for infective endocarditis, but it misses the diagnosis in almost one-third of cases, and even more often if the patient has a prosthetic valve.
But now, several sophisticated imaging tests are available that complement echocardiography in diagnosing and assessing infective endocarditis; these include 4-dimensional computed tomography (4D CT), fluorodeoxyglucose positron emission tomography (FDG-PET), and leukocyte scintigraphy. These tests have greatly improved our ability not only to diagnose infective endocarditis, but also to determine the extent and spread of infection, and they aid in perioperative assessment. Abnormal findings on these tests have been incorporated into the European Society of Cardiology’s 2015 modified diagnostic criteria for infective endocarditis.1
This article details the indications, advantages, and limitations of the various imaging tests for diagnosing and evaluating infective endocarditis (Table 1).
INFECTIVE ENDOCARDITIS IS DIFFICULT TO DIAGNOSE AND TREAT
Infective endocarditis is difficult to diagnose and treat. Clinical and imaging clues can be subtle, and the diagnosis requires a high level of suspicion and visualization of cardiac structures.
Further, the incidence of infective endocarditis is on the rise in the United States, particularly in women and young adults, likely due to intravenous drug use.2,3
ECHOCARDIOGRAPHY HAS AN IMPORTANT ROLE, BUT IS LIMITED
Echocardiography remains the most commonly performed study for diagnosing infective endocarditis, as it is fast, widely accessible, and less expensive than other imaging tests.
Transthoracic echocardiography (TTE) is often the first choice for testing. However, its sensitivity is only about 70% for detecting vegetations on native valves and 50% for detecting vegetations on prosthetic valves.1 It is inherently constrained by the limited number of views by which a comprehensive external evaluation of the heart can be achieved. Using a 2-dimensional instrument to view a 3-dimensional object is difficult, and depending on several factors, it can be hard to see vegetations and abscesses that are associated with infective endocarditis. Further, TTE is impeded by obesity and by hyperinflated lungs from obstructive pulmonary disease or mechanical ventilation. It has poor sensitivity for detecting small vegetations and for detecting vegetations and paravalvular complications in patients who have a prosthetic valve or a cardiac implanted electronic device.
Transesophageal echocardiography (TEE) is the recommended first-line imaging test for patients with prosthetic valves and no contraindications to the test. Otherwise, it should be done after TTE if the results of TTE are negative but clinical suspicion for infective endocarditis remains high (eg, because the patient uses intravenous drugs). But although TEE has a higher sensitivity than TTE (up to 96% for vegetations on native valves and 92% for those on prosthetic valves, if performed by an experienced sonographer), it can still miss infective endocarditis. Also, TEE does not provide a significant advantage over TTE in patients who have a cardiac implanted electronic device.1,4,5
Regardless of whether TTE or TEE is used, they are estimated to miss up to 30% of cases of infective endocarditis and its sequelae.4 False-negative findings are likelier in patients who have preexisting severe valvular lesions, prosthetic valves, cardiac implanted electronic devices, small vegetations, or abscesses, or if a vegetation has already broken free and embolized. Furthermore, distinguishing between vegetations and thrombi, cardiac tumors, and myxomatous changes using echocardiography is difficult.
CARDIAC CT
For patients who have inconclusive results on echocardiography, contraindications to TEE, or poor sonic windows, cardiac CT can be an excellent alternative. It is especially useful in the setting of a prosthetic valve.
Synchronized (“gated”) with the patient’s heart rate and rhythm, CT machines can acquire images during diastole, reducing motion artifact, and can create 3D images of the heart. In addition, newer machines can acquire several images at different points in the heart cycle to add a fourth dimension—time. The resulting 4D images play like short video loops of the beating heart and allow noninvasive assessment of cardiac anatomy with remarkable detail and resolution.
4D CT is increasingly being used in infective endocarditis, and growing evidence indicates that its accuracy is similar to that of TEE in the preoperative evaluation of patients with aortic prosthetic valve endocarditis.6 In a study of 28 patients, complementary use of CT angiography led to a change in treatment strategy in 7 (25%) compared with routine clinical workup.7 Several studies have found no difference between 4D CT and preoperative TEE in detecting pseudoaneurysm, abscess, or valve dehiscence. TEE and 4D CT also have similar sensitivities for detecting infective endocarditis in native and prosthetic valves.8,9
Coupled with CT angiography, 4D CT is also an excellent noninvasive way to perioperatively evaluate the coronary arteries without the risks associated with catheterization in those requiring nonemergency surgery (Figure 1A, B, and C).
4D CT performs well for detecting abscess and pseudoaneurysm but has slightly lower sensitivity for vegetations than TEE (91% vs 99%).9
Gated CT, PET, or both may be useful in cases of suspected prosthetic aortic valve endocarditis when TEE is negative. Pseudoaneurysms are not well visualized with TEE, and the atrial mitral curtain area is often thickened on TEE in cases of aortic prosthetic valve infective endocarditis that do not definitely involve abscesses. Gated CT and PET show this area better.8 This information is important in cases in which a surgeon may be unconvinced that the patient has prosthetic valve endocarditis.
Limitations of 4D cardiac CT
4D CT with or without angiography has limitations. It requires a wide-volume scanner and an experienced reader.
Patients with irregular heart rhythms or uncontrolled tachycardia pose technical problems for image acquisition. Cardiac CT is typically gated (ie, images are obtained within a defined time period) to acquire images during diastole. Ideally, images are acquired when the heart is in mid to late diastole, a time of minimal cardiac motion, so that motion artifact is minimized. To estimate the timing of image acquisition, the cardiac cycle must be predictable, and its duration should be as long as possible. Tachycardia or irregular rhythms such as frequent ectopic beats or atrial fibrillation make acquisition timing difficult, and thus make it nearly impossible to accurately obtain images when the heart is at minimum motion, limiting assessment of cardiac structures or the coronary tree.4,10
Extensive coronary calcification can hinder assessment of the coronary tree by CT coronary angiography.
Contrast exposure may limit the use of CT in some patients (eg, those with contrast allergies or renal dysfunction). However, modern scanners allow for much smaller contrast boluses without decreasing sensitivity.
4D CT involves radiation exposure, especially when done with angiography, although modern scanners have greatly reduced exposure. The average radiation dose in CT coronary angiography is 2.9 to 5.9 mSv11 compared with 7 mSv in diagnostic cardiac catheterization (without angioplasty or stenting) or 16 mSv in routine CT of the abdomen and pelvis with contrast.12,13 In view of the morbidity and mortality risks associated with infective endocarditis, especially if the diagnosis is delayed, this small radiation exposure may be justifiable.
Bottom line for cardiac CT
4D CT is an excellent alternative to echocardiography for select patients. Clinicians should strongly consider this study in the following situations:
- Patients with a prosthetic valve
- Patients who are strongly suspected of having infective endocarditis but who have a poor sonic window on TTE or TEE, as can occur with chronic obstructive lung disease, morbid obesity, or previous thoracic or cardiovascular surgery
- Patients who meet clinical indications for TEE, such as having a prosthetic valve or a high suspicion for native valve infective endocarditis with negative TTE, but who have contraindications to TEE
- As an alternative to TEE for preoperative evaluation in patients with known infective endocarditis.
Patients with tachycardia or irregular heart rhythms are not good candidates for this test.
FDG-PET AND LEUKOCYTE SCINTIGRAPHY
FDG-PET and leukocyte scintigraphy are other options for diagnosing infective endocarditis and determining the presence and extent of intra- and extracardiac infection. They are more sensitive than echocardiography for detecting infection of cardiac implanted electronic devices such as ventricular assist devices, pacemakers, implanted cardiac defibrillators, and cardiac resynchronization therapy devices.14–16
The utility of FDG-PET is founded on the uptake of 18F-fluorodeoxyglucose by cells, with higher uptake taking place in cells with higher metabolic activity (such as in areas of inflammation). Similarly, leukocyte scintigraphy relies on the use of radiolabeled leukocytes (ie, leukocytes previously extracted from the patient, labelled, and re-introduced into the patient) to allow for localization of inflamed tissue.
The most significant contribution of FDG-PET may be the ability to detect infective endocarditis early, when echocardiography is initially negative. When abnormal FDG uptake was included in the modified Duke criteria, it increased the sensitivity to 97% for detecting infective endocarditis on admission, leading some to propose its incorporation as a major criterion.17 In patients with prosthetic valves and suspected infective endocarditis, FDG-PET was found in one study to have a sensitivity of up to 91% and a specificity of up to 95%.18
Both FDG-PET and leukocyte scintigraphy have a high sensitivity, specificity, and negative predictive value for cardiac implanted electronic device infection, and should be strongly considered in patients in whom it is suspected but who have negative or inconclusive findings on echocardiography.14,15
In addition, a common conundrum faced by clinicians with use of echocardiography is the difficulty of differentiating thrombus from infected vegetation on valves or device lead wires. Some evidence indicates that FDG-PET may help to discriminate between vegetation and thrombus, although more rigorous studies are needed before its use for that purpose can be recommended.19
Limitations of nuclear studies
Both FDG-PET and leukocyte scintigraphy perform poorly for detecting native-valve infective endocarditis. In a study in which 90% of the patients had native-valve infective endocarditis according to the Duke criteria, FDG-PET had a specificity of 93% but a sensitivity of only 39%.20
Both studies can be cumbersome, laborious, and time-consuming for patients. FDG-PET requires a fasting or glucose-restricted diet before testing, and the test itself can be complicated by development of hyperglycemia, although this is rare.
While FDG-PET is most effective in detecting infections of prosthetic valves and cardiac implanted electronic devices, the results can be falsely positive in patients with a history of recent cardiac surgery (due to ongoing tissue healing), as well as maladies other than infective endocarditis that lead to inflammation, such as vasculitis or malignancy. Similarly, for unclear reasons, leukocyte scintigraphy can yield false-negative results in patients with enterococcal or candidal infective endocarditis.21
FDG-PET and leukocyte scintigraphy are more expensive than TEE and cardiac CT22 and are not widely available.
Both tests entail radiation exposure, with the average dose ranging from 7 to 14 mSv. However, this is less than the average amount acquired during percutaneous coronary intervention (16 mSv), and overlaps with the amount in chest CT with contrast when assessing for pulmonary embolism (7 to 9 mSv). Lower doses are possible with optimized protocols.12,13,15,23
Bottom line for nuclear studies
FDG-PET and leukocyte scintigraphy are especially useful for patients with a prosthetic valve or cardiac implanted electronic device. However, limitations must be kept in mind.
A suggested algorithm for testing with nuclear imaging is shown in Figure 2.1,4
CEREBRAL MAGNETIC RESONANCE IMAGING
Cerebral magnetic resonance imaging (MRI) is more sensitive than cerebral CT for detecting emboli in the brain. According to American Heart Association guidelines, cerebral MRI should be done in patients with known or suspected infective endocarditis and neurologic impairment, defined as headaches, meningeal symptoms, or neurologic deficits. It is also often used in neurologically asymptomatic patients with infective endocarditis who have indications for valve surgery to assess for mycotic aneurysms, which are associated with increased intracranial bleeding during surgery.
MRI use in other asymptomatic patients remains controversial.24 In cases with high clinical suspicion for infective endocarditis and no findings on echocardiography, cerebral MRI can increase the sensitivity of the Duke criteria by adding a minor criterion. Some have argued that, in patients with definite infective endocarditis, detecting silent cerebral complications can lead to management changes. However, more studies are needed to determine if there is indeed a group of neurologically asymptomatic infective endocarditis patients for whom cerebral MRI leads to improved outcomes.
Limitations of cerebral MRI
Cerebral MRI cannot be used in patients with non-MRI-compatible implanted hardware.
Gadolinium, the contrast agent typically used, can cause nephrogenic systemic fibrosis in patients who have poor renal function. This rare but serious adverse effect is characterized by irreversible systemic fibrosis affecting skin, muscles, and even visceral tissue such as lungs. The American College of Radiology allows for gadolinium use in patients without acute kidney injury and patients with stable chronic kidney disease with a glomerular filtration rate of at least 30 mL/min/1.73 m2. Its use should be avoided in patients with renal failure on replacement therapy, with advanced chronic kidney disease (glomerular filtration rate < 30 mL/min/1.73 m2), or with acute kidney injury, even if they do not need renal replacement therapy.25
Concerns have also been raised about gadolinium retention in the brain, even in patients with normal renal function.26–28 Thus far, no conclusive clinical adverse effects of retention have been found, although more study is warranted. Nevertheless, the US Food and Drug Administration now requires a black-box warning about this possibility and advises clinicians to counsel patients appropriately.
Bottom line on cerebral MRI
Cerebral MRI should be obtained when a patient presents with definite or possible infective endocarditis with neurologic impairment, such as new headaches, meningismus, or focal neurologic deficits. Routine brain MRI in patients with confirmed infective endocarditis without neurologic symptoms, or those without definite infective endocarditis, is discouraged.
CARDIAC MRI
Cardiac MRI, typically obtained with gadolinium contrast, allows for better 3D assessment of cardiac structures and morphology than echocardiography or CT, and can detect infiltrative cardiac disease, myopericarditis, and much more. It is increasingly used in the field of structural cardiology, but its role for evaluating infective endocarditis remains unclear.
Cardiac MRI does not appear to be better than echocardiography for diagnosing infective endocarditis. However, it may prove helpful in the evaluation of patients known to have infective endocarditis but who cannot be properly evaluated for disease extent because of poor image quality on echocardiography and contraindications to CT.1,29 Its role is limited in patients with cardiac implanted electronic devices, as most devices are incompatible with MRI use, although newer devices obviate this concern. But even for devices that are MRI-compatible, results are diminished due to an eclipsing effect, wherein the device parts can make it hard to see structures clearly because the “brightness” basically eclipses the surrounding area.4
Concerns regarding use of gadolinium as described above need also be considered.
The role of cardiac MRI in diagnosing and managing infective endocarditis may evolve, but at present, the 2017 American College of Cardiology and American Heart Association appropriate-use criteria discourage its use for these purposes.16
Bottom line for cardiac MRI
Cardiac MRI to evaluate a patient for suspected infective endocarditis is not recommended due to lack of superiority compared with echocardiography or CT, and the risk of nephrogenic systemic fibrosis from gadolinium in patients with renal compromise.
- Habib G, Lancellotti P, Antunes MJ, et al; ESC Scientific Document Group. 2015 ESC guidelines for the management of infective endocarditis: the Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J 2015; 36(44):3075–3128. doi:10.1093/eurheartj/ehv319
- Durante-Mangoni E, Bradley S, Selton-Suty C, et al; International Collaboration on Endocarditis Prospective Cohort Study Group. Current features of infective endocarditis in elderly patients: results of the International Collaboration on Endocarditis Prospective Cohort Study. Arch Intern Med 2008; 168(19):2095–2103. doi:10.1001/archinte.168.19.2095
- Wurcel AG, Anderson JE, Chui KK, et al. Increasing infectious endocarditis admissions among young people who inject drugs. Open Forum Infect Dis 2016; 3(3):ofw157. doi:10.1093/ofid/ofw157
- Gomes A, Glaudemans AW, Touw DJ, et al. Diagnostic value of imaging in infective endocarditis: a systematic review. Lancet Infect Dis 2017; 17(1):e1–e14. doi:10.1016/S1473-3099(16)30141-4
- Cahill TJ, Baddour LM, Habib G, et al. Challenges in infective endocarditis. J Am Coll Cardiol 2017; 69(3):325–344. doi:10.1016/j.jacc.2016.10.066
- Fagman E, Perrotta S, Bech-Hanssen O, et al. ECG-gated computed tomography: a new role for patients with suspected aortic prosthetic valve endocarditis. Eur Radiol 2012; 22(11):2407–2414. doi:10.1007/s00330-012-2491-5
- Habets J, Tanis W, van Herwerden LA, et al. Cardiac computed tomography angiography results in diagnostic and therapeutic change in prosthetic heart valve endocarditis. Int J Cardiovasc Imaging 2014; 30(2):377–387. doi:10.1007/s10554-013-0335-2
- Koneru S, Huang SS, Oldan J, et al. Role of preoperative cardiac CT in the evaluation of infective endocarditis: comparison with transesophageal echocardiography and surgical findings. Cardiovasc Diagn Ther 2018; 8(4):439–449. doi:10.21037/cdt.2018.07.07
- Koo HJ, Yang DH, Kang J, et al. Demonstration of infective endocarditis by cardiac CT and transoesophageal echocardiography: comparison with intra-operative findings. Eur Heart J Cardiovasc Imaging 2018; 19(2):199–207. doi:10.1093/ehjci/jex010
- Feuchtner GM, Stolzmann P, Dichtl W, et al. Multislice computed tomography in infective endocarditis: comparison with transesophageal echocardiography and intraoperative findings. J Am Coll Cardiol 2009; 53(5):436–444. doi:10.1016/j.jacc.2008.01.077
- Castellano IA, Nicol ED, Bull RK, Roobottom CA, Williams MC, Harden SP. A prospective national survey of coronary CT angiography radiation doses in the United Kingdom. J Cardiovasc Comput Tomogr 2017; 11(4):268–273. doi:10.1016/j.jcct.2017.05.002
- Mettler FA Jr, Huda W, Yoshizumi TT, Mahesh M. Effective doses in radiology and diagnostic nuclear medicine: a catalog. Radiology 2008; 248(1):254–263. doi:10.1148/radiol.2481071451
- Smith-Bindman R, Lipson J, Marcus R, et al. Radiation dose associated with common computed tomography examinations and the associated lifetime attributable risk of cancer. Arch Intern Med 2009; 169(22):2078–2086. doi:10.1001/archinternmed.2009.427
- Ploux S, Riviere A, Amraoui S, et al. Positron emission tomography in patients with suspected pacing system infections may play a critical role in difficult cases. Heart Rhythm 2011; 8(9):1478–1481. doi:10.1016/j.hrthm.2011.03.062
- Sarrazin J, Philippon F, Tessier M, et al. Usefulness of fluorine-18 positron emission tomography/computed tomography for identification of cardiovascular implantable electronic device infections. J Am Coll Cardiol 2012; 59(18):1616–1625. doi:10.1016/j.jacc.2011.11.059
- Doherty JU, Kort S, Mehran R, Schoenhagen P, Soman P; Rating Panel Members; Appropriate Use Criteria Task Force. ACC/AATS/AHA/ASE/ASNC/HRS/SCAI/SCCT/SCMR/STS 2017 Appropriate use criteria for multimodality imaging in valvular heart disease: a report of the American College of Cardiology Appropriate Use Criteria Task Force, American Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and Society of Thoracic Surgeons. J Nucl Cardiol 2017; 24(6):2043–2063. doi:10.1007/s12350-017-1070-1
- Saby L, Laas O, Habib G, et al. Positron emission tomography/computed tomography for diagnosis of prosthetic valve endocarditis: increased valvular 18F-fluorodeoxyglucose uptake as a novel major criterion. J Am Coll Cardiol 2013; 61(23):2374–2382. doi:10.1016/j.jacc.2013.01.092
- Swart LE, Gomes A, Scholtens AM, et al. Improving the diagnostic performance of 18F-fluorodeoxyglucose positron-emission tomography/computed tomography in prosthetic heart valve endocarditis. Circulation 2018; 138(14):1412–1427. doi:10.1161/CIRCULATIONAHA.118.035032
- Graziosi M, Nanni C, Lorenzini M, et al. Role of 18F-FDG PET/CT in the diagnosis of infective endocarditis in patients with an implanted cardiac device: a prospective study. Eur J Nucl Med Mol Imaging 2014; 41(8):1617–1623. doi:10.1007/s00259-014-2773-z
- Kouijzer IJ, Vos FJ, Janssen MJ, van Dijk AP, Oyen WJ, Bleeker-Rovers CP. The value of 18F-FDG PET/CT in diagnosing infectious endocarditis. Eur J Nucl Med Mol Imaging 2013; 40(7):1102–1107. doi:10.1007/s00259-013-2376-0
- Wong D, Rubinshtein R, Keynan Y. Alternative cardiac imaging modalities to echocardiography for the diagnosis of infective endocarditis. Am J Cardiol 2016; 118(9):1410–1418. doi:10.1016/j.amjcard.2016.07.053
- Vos FJ, Bleeker-Rovers CP, Kullberg BJ, Adang EM, Oyen WJ. Cost-effectiveness of routine (18)F-FDG PET/CT in high-risk patients with gram-positive bacteremia. J Nucl Med 2011; 52(11):1673–1678. doi:10.2967/jnumed.111.089714
- McCollough CH, Bushberg JT, Fletcher JG, Eckel LJ. Answers to common questions about the use and safety of CT scans. Mayo Clin Proc 2015; 90(10):1380–1392. doi:10.1016/j.mayocp.2015.07.011
- Duval X, Iung B, Klein I, et al; IMAGE (Resonance Magnetic Imaging at the Acute Phase of Endocarditis) Study Group. Effect of early cerebral magnetic resonance imaging on clinical decisions in infective endocarditis: a prospective study. Ann Intern Med 2010; 152(8):497–504, W175. doi:10.7326/0003-4819-152-8-201004200-00006
- ACR Committee on Drugs and Contrast Media. ACR Manual on Contrast Media: 2018. www.acr.org/-/media/ACR/Files/Clinical-Resources/Contrast_Media.pdf. Accessed July 19, 2019.
- Kanda T, Fukusato T, Matsuda M, et al. Gadolinium-based contrast agent accumulates in the brain even in subjects without severe renal dysfunction: evaluation of autopsy brain specimens with inductively coupled plasma mass spectroscopy. Radiology 2015; 276(1):228–232. doi:10.1148/radiol.2015142690
- McDonald RJ, McDonald JS, Kallmes DF, et al. Intracranial gadolinium deposition after contrast-enhanced MR imaging. Radiology 2015; 275(3):772–782. doi:10.1148/radiol.15150025
- Kanda T, Ishii K, Kawaguchi H, Kitajima K, Takenaka D. High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology 2014; 270(3):834–841. doi:10.1148/radiol.13131669
- Expert Panel on Pediatric Imaging; Hayes LL, Palasis S, Bartel TB, et al. ACR appropriateness criteria headache-child. J Am Coll Radiol 2018; 15(5S):S78–S90. doi:10.1016/j.jacr.2018.03.017
- Habib G, Lancellotti P, Antunes MJ, et al; ESC Scientific Document Group. 2015 ESC guidelines for the management of infective endocarditis: the Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J 2015; 36(44):3075–3128. doi:10.1093/eurheartj/ehv319
- Durante-Mangoni E, Bradley S, Selton-Suty C, et al; International Collaboration on Endocarditis Prospective Cohort Study Group. Current features of infective endocarditis in elderly patients: results of the International Collaboration on Endocarditis Prospective Cohort Study. Arch Intern Med 2008; 168(19):2095–2103. doi:10.1001/archinte.168.19.2095
- Wurcel AG, Anderson JE, Chui KK, et al. Increasing infectious endocarditis admissions among young people who inject drugs. Open Forum Infect Dis 2016; 3(3):ofw157. doi:10.1093/ofid/ofw157
- Gomes A, Glaudemans AW, Touw DJ, et al. Diagnostic value of imaging in infective endocarditis: a systematic review. Lancet Infect Dis 2017; 17(1):e1–e14. doi:10.1016/S1473-3099(16)30141-4
- Cahill TJ, Baddour LM, Habib G, et al. Challenges in infective endocarditis. J Am Coll Cardiol 2017; 69(3):325–344. doi:10.1016/j.jacc.2016.10.066
- Fagman E, Perrotta S, Bech-Hanssen O, et al. ECG-gated computed tomography: a new role for patients with suspected aortic prosthetic valve endocarditis. Eur Radiol 2012; 22(11):2407–2414. doi:10.1007/s00330-012-2491-5
- Habets J, Tanis W, van Herwerden LA, et al. Cardiac computed tomography angiography results in diagnostic and therapeutic change in prosthetic heart valve endocarditis. Int J Cardiovasc Imaging 2014; 30(2):377–387. doi:10.1007/s10554-013-0335-2
- Koneru S, Huang SS, Oldan J, et al. Role of preoperative cardiac CT in the evaluation of infective endocarditis: comparison with transesophageal echocardiography and surgical findings. Cardiovasc Diagn Ther 2018; 8(4):439–449. doi:10.21037/cdt.2018.07.07
- Koo HJ, Yang DH, Kang J, et al. Demonstration of infective endocarditis by cardiac CT and transoesophageal echocardiography: comparison with intra-operative findings. Eur Heart J Cardiovasc Imaging 2018; 19(2):199–207. doi:10.1093/ehjci/jex010
- Feuchtner GM, Stolzmann P, Dichtl W, et al. Multislice computed tomography in infective endocarditis: comparison with transesophageal echocardiography and intraoperative findings. J Am Coll Cardiol 2009; 53(5):436–444. doi:10.1016/j.jacc.2008.01.077
- Castellano IA, Nicol ED, Bull RK, Roobottom CA, Williams MC, Harden SP. A prospective national survey of coronary CT angiography radiation doses in the United Kingdom. J Cardiovasc Comput Tomogr 2017; 11(4):268–273. doi:10.1016/j.jcct.2017.05.002
- Mettler FA Jr, Huda W, Yoshizumi TT, Mahesh M. Effective doses in radiology and diagnostic nuclear medicine: a catalog. Radiology 2008; 248(1):254–263. doi:10.1148/radiol.2481071451
- Smith-Bindman R, Lipson J, Marcus R, et al. Radiation dose associated with common computed tomography examinations and the associated lifetime attributable risk of cancer. Arch Intern Med 2009; 169(22):2078–2086. doi:10.1001/archinternmed.2009.427
- Ploux S, Riviere A, Amraoui S, et al. Positron emission tomography in patients with suspected pacing system infections may play a critical role in difficult cases. Heart Rhythm 2011; 8(9):1478–1481. doi:10.1016/j.hrthm.2011.03.062
- Sarrazin J, Philippon F, Tessier M, et al. Usefulness of fluorine-18 positron emission tomography/computed tomography for identification of cardiovascular implantable electronic device infections. J Am Coll Cardiol 2012; 59(18):1616–1625. doi:10.1016/j.jacc.2011.11.059
- Doherty JU, Kort S, Mehran R, Schoenhagen P, Soman P; Rating Panel Members; Appropriate Use Criteria Task Force. ACC/AATS/AHA/ASE/ASNC/HRS/SCAI/SCCT/SCMR/STS 2017 Appropriate use criteria for multimodality imaging in valvular heart disease: a report of the American College of Cardiology Appropriate Use Criteria Task Force, American Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and Society of Thoracic Surgeons. J Nucl Cardiol 2017; 24(6):2043–2063. doi:10.1007/s12350-017-1070-1
- Saby L, Laas O, Habib G, et al. Positron emission tomography/computed tomography for diagnosis of prosthetic valve endocarditis: increased valvular 18F-fluorodeoxyglucose uptake as a novel major criterion. J Am Coll Cardiol 2013; 61(23):2374–2382. doi:10.1016/j.jacc.2013.01.092
- Swart LE, Gomes A, Scholtens AM, et al. Improving the diagnostic performance of 18F-fluorodeoxyglucose positron-emission tomography/computed tomography in prosthetic heart valve endocarditis. Circulation 2018; 138(14):1412–1427. doi:10.1161/CIRCULATIONAHA.118.035032
- Graziosi M, Nanni C, Lorenzini M, et al. Role of 18F-FDG PET/CT in the diagnosis of infective endocarditis in patients with an implanted cardiac device: a prospective study. Eur J Nucl Med Mol Imaging 2014; 41(8):1617–1623. doi:10.1007/s00259-014-2773-z
- Kouijzer IJ, Vos FJ, Janssen MJ, van Dijk AP, Oyen WJ, Bleeker-Rovers CP. The value of 18F-FDG PET/CT in diagnosing infectious endocarditis. Eur J Nucl Med Mol Imaging 2013; 40(7):1102–1107. doi:10.1007/s00259-013-2376-0
- Wong D, Rubinshtein R, Keynan Y. Alternative cardiac imaging modalities to echocardiography for the diagnosis of infective endocarditis. Am J Cardiol 2016; 118(9):1410–1418. doi:10.1016/j.amjcard.2016.07.053
- Vos FJ, Bleeker-Rovers CP, Kullberg BJ, Adang EM, Oyen WJ. Cost-effectiveness of routine (18)F-FDG PET/CT in high-risk patients with gram-positive bacteremia. J Nucl Med 2011; 52(11):1673–1678. doi:10.2967/jnumed.111.089714
- McCollough CH, Bushberg JT, Fletcher JG, Eckel LJ. Answers to common questions about the use and safety of CT scans. Mayo Clin Proc 2015; 90(10):1380–1392. doi:10.1016/j.mayocp.2015.07.011
- Duval X, Iung B, Klein I, et al; IMAGE (Resonance Magnetic Imaging at the Acute Phase of Endocarditis) Study Group. Effect of early cerebral magnetic resonance imaging on clinical decisions in infective endocarditis: a prospective study. Ann Intern Med 2010; 152(8):497–504, W175. doi:10.7326/0003-4819-152-8-201004200-00006
- ACR Committee on Drugs and Contrast Media. ACR Manual on Contrast Media: 2018. www.acr.org/-/media/ACR/Files/Clinical-Resources/Contrast_Media.pdf. Accessed July 19, 2019.
- Kanda T, Fukusato T, Matsuda M, et al. Gadolinium-based contrast agent accumulates in the brain even in subjects without severe renal dysfunction: evaluation of autopsy brain specimens with inductively coupled plasma mass spectroscopy. Radiology 2015; 276(1):228–232. doi:10.1148/radiol.2015142690
- McDonald RJ, McDonald JS, Kallmes DF, et al. Intracranial gadolinium deposition after contrast-enhanced MR imaging. Radiology 2015; 275(3):772–782. doi:10.1148/radiol.15150025
- Kanda T, Ishii K, Kawaguchi H, Kitajima K, Takenaka D. High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology 2014; 270(3):834–841. doi:10.1148/radiol.13131669
- Expert Panel on Pediatric Imaging; Hayes LL, Palasis S, Bartel TB, et al. ACR appropriateness criteria headache-child. J Am Coll Radiol 2018; 15(5S):S78–S90. doi:10.1016/j.jacr.2018.03.017
KEY POINTS
- Echocardiography can produce false-negative results in native-valve infective endocarditis and is even less sensitive in patients with a prosthetic valve or cardiac implanted electronic device.
- 4D CT is a reasonable alternative to transesophageal echocardiography. It can also be used as a second test if echocardiography is inconclusive. Coupled with angiography, it also provides a noninvasive method to evaluate coronary arteries perioperatively.
- Nuclear imaging tests—FDG-PET and leukocyte scintigraphy—increase the sensitivity of the Duke criteria for diagnosing infective endocarditis. They should be considered for evaluating suspected infective endocarditis in all patients who have a prosthetic valve or cardiac implanted electronic device, and whenever echocardiography is inconclusive and clinical suspicion remains high.
Adults with autism spectrum disorder: Updated considerations for healthcare providers
Autism spectrum disorder (ASD) has increased significantly over the past 40 years. Even in the past 2 decades, the prevalence increased from 6.7 per 1,000 in 20001 to 14.6 per 1,000 in 2012—1 in 59 people.2 Of those with ASD, 46% have an intelligence quotient (IQ) greater than 85, meaning they are of average or above-average intelligence.1
As more children with autism become adults, understanding this condition across the life span grows paramount. While many studies have focused on understanding how diagnosis and treatment can help young children, few have focused on adults with autism and how primary care teams can better assist these individuals. However, this is changing, with studies of the benefits of employment programs and pharmacologic treatment, and reproductive health needs of adults with ASD. Here we provide an updated review of ASD in adult patients.
NO MORE ASPERGER SYNDROME— IT’S ON THE SPECTRUM NOW
As the scientific understanding of autism has expanded, revisions in the Diagnostic and Statistical Manual of Mental Disorders, fifth edition (DSM-5),3 published in 2013, have paralleled these advances. For many adult patients with autism who were evaluated as children, these revisions have led to changes in diagnosis and available services.
In the previous edition (DSM-IV-TR, published in 2000),4 autistic disorder and Asperger syndrome were separate (Table 1). However, DSM-5 lumped autistic disorder and Asperger disorder together under the diagnosis of ASD; this leaves it to the clinician to specify whether the patient with ASD has accompanying intellectual or language impairment and to assign a level of severity based on communication deficits and restrictive behaviors.
The shift in diagnosis was worrisome for some, particularly for clinicians treating patients with DSM-IV Asperger syndrome, who lost this diagnostic label. Concerns that patients with Asperger syndrome may not meet the DSM-5 criteria for ASD were validated by a systematic review showing that only 50% to 75% of patients with DSM-IV autistic disorder, Asperger syndrome, or pervasive developmental disorder not otherwise specified (PDD-NOS) met the DSM-5 criteria for ASD.5 Most of those who no longer met the criteria for ASD carried a DSM-IV diagnosis of Asperger syndrome or PDD-NOS or had an IQ over 70.5 Nevertheless, these individuals may struggle with impairing symptoms related to repetitive behaviors or communication or may be affected by learning or social-emotional disabilities. Additionally, even if they meet the criteria for ASD, some may identify with the Asperger syndrome label and fear they will be stigmatized should they be classified as having the more general ASD.6,7
Although future revisions to the DSM may include further changes in classification, grouping adults with ASD according to their functional and cognitive ability may allow for pragmatic characterization of their needs. At least 3 informal groupings of autistic adults have been described that integrate cognitive ability and independence8:
- Those with low cognitive and social abilities, who need lifelong support
- Those with midrange cognitive and social limitations but who can complete their work in special education classes; they often find employment in supervised workshops or other work with repetitive tasks
- Those who have greater cognitive ability and some social skills; they may proceed to college and employment and live independently.
UNCERTAIN PROGNOSIS
Prognostication for people with ASD remains an area of research. Some adults experience a reduction in symptoms as they age, with significant improvements in speech and, sometimes, modest improvements in restrictive and repetitive behaviors.9,10
Nevertheless, autism remains a lifelong disorder for many. Adults may still require significant support and may experience impairment, particularly in social interaction.10 In longitudinal studies, only 15% to 27% of patients with ASD are characterized as having a positive outcome (often defined as variables related to independent function, near-normal relationships, employment, or a quantified reduction in core symptoms), and many experience significant dependency into adulthood.10–13
IQ has been cited as a possible prognostic factor,10,13 with an IQ below 70 associated with poorer outcome, although an IQ above 70 does not necessarily confer a positive outcome. Less-severe impairment in speech at baseline in early childhood also suggests better outcomes in adulthood.10
As we see more adults with autism, studies that include both children and adults, such as the Longitudinal European Autism Cohort, will be important to characterize the natural history, comorbidities, and genetics of ASD and may help provide more specific predictors of disease course into adulthood.14
ACHIEVING A DIAGNOSIS FOR ADULT PATIENTS WITH SUSPECTED AUTISM
While many patients are recognized as having autism in early to mid-childhood, some adults may not receive a formal diagnosis until much later in life. Those with fluent language and normal-range IQ are likely to be overlooked.15 People with ASD may have had mild symptoms during childhood that did not impair their functioning until demands of daily life exceeded their capacities in adulthood. Alternatively, parents of a child with newly diagnosed ASD may realize that they themselves or another adult family member also show signs of it.
The UK National Institute of Health and Care Excellence suggests that assessment should be considered if the patient meets psychiatric diagnostic criteria and one of the following:
- Difficulty obtaining or sustaining employment or education
- Difficulty initiating or sustaining social relationships
- Past or current contact with mental health or learning disability services
- History of a neurodevelopmental or mental health disorder.15,16
Currently, diagnosis typically involves a multidisciplinary approach, with psychiatric assessment, neuropsychological testing, and speech and language evaluation.17 Providers may need to refer patients for these services, sometimes at the patient’s request, if previous mental health misdiagnoses are suspected, if patients report symptoms or impairment consistent with ASD, or if benefits, services, or accommodations, such as a coach in the workplace, are needed.
Diagnosing ASD in adults can be difficult, given that the gold-standard diagnostic tests such as the Autism Diagnostic Observation Schedule-2 (ADOS-2)18 and the Autism Diagnostic Interview-Revised (ADI-R)19 are typically used to diagnose autism in children. However, Module 4 in the ADOS-2 was developed for adolescents and older patients with fluent language and has shown at least moderate power to distinguish adults with ASD from those without ASD.18,20
An initial psychiatric assessment should include a thorough history taken from the patient and, if applicable, the patient’s caregiver, as well as a psychiatric interview of the patient. Neuropsychological testing should include evaluation of cognitive function, social functioning (using the ADOS-2 for adults without intellectual disability, the ADI-R, or both), and adaptive functioning (using the Vineland Adaptive Behavior Scales, second edition21).
Evaluation of speech and language is particularly important in patients with limited language ability and should include both expressive and receptive language abilities. Serial testing every few years, as is often recommended in childhood, may help establish the pattern of impairment over time.
Comorbid psychiatric disorders are common
Many people with ASD also have other psychiatric disorders,17,22 which clinicians should keep in mind when seeing an adult seeking evaluation for ASD.
Attention-deficit/hyperactivity disorder is present at higher rates in patients of average intellectual function with ASD than in the general population.23
Anxiety disorders, including obsessive-compulsive disorder, were found to often coexist with autism in a sample of adults with autism without intellectual disability,24,25 and approximately 40% of youths with ASD have at least 1 comorbid anxiety disorder.26
Mood disorders are also prevalent in adults with ASD, with a small study showing that 70% of adults with DSM-IV Asperger syndrome had at least 1 depressive episode in their lifetime.27
BEHAVIORAL AND PHARMACOLOGIC THERAPIES FOR THE ADULT PATIENT
Services and medications for adults with ASD are discussed below. These will vary by individual, and services available may vary by region.
Historically, vocational and social outcomes have been poor for adults with ASD. It is estimated that most larger universities may be home to 100 to 300 students with ASD. To combat isolation, the University of California, Los Angeles, the University of Alabama, and others provide special support services, including group social activities such as board games and individual coaching.8 Nevertheless, half of the students with autism who attend institutions of higher learning leave without completing their intended degree.29 Many still struggle to establish meaningful friendships or romantic relationships.29
Planning for a transition of care
Healthcare transition planning is important but is strikingly underused.30 Individual providers, including adult psychiatrists, vary in their level of training and comfort in diagnosing, treating, and monitoring adults with autism. Youths with ASD are half as likely to receive healthcare transition services as other youths with special healthcare needs.31
Pediatric providers, including pediatric psychiatrists, developmental behavioral specialists, and pediatric neurologists, may be best equipped to treat young adult patients or to refer patients to appropriate generalists and specialists comfortable with autism-specific transition of care. The question of eligibility for services is important to patients and families during the transition period, with many parents and professionals unaware of services available to them.32 Receiving adequate transition services is enabled by having a medical home during childhood—that is, a comprehensive, centralized medical record, culturally competent care, interaction with schools, and patient access to clear, unbiased information.31
Ideally, in our experience, transitioning should be discussed well before the child ages out of the pediatric provider’s practice. If necessary, healthcare transition services should include 4 components:
- Discussing the switch to a new physician who treats adults
- Discussing changing healthcare needs as an adult
- Planning insurance coverage as an adult
- Encouragement by the physician for the child to take age-appropriate responsibility for his or her healthcare.31,33
Tools such as the Got Transition checklist from the National Health Care Transition Center can provide support during this process.34
Other services
Other services provided as an extension or adjunct to the medical home in early adulthood may include customized vocational or employment training, specialized mentorship or support in a college setting, housing support, and psychological services.35
Community-based programs that emphasize leisure have been shown to improve participants’ independence and quality of life.36 Similarly, participants in programs that emphasized supported employment, with a job coach, on-the-job support, collaboration with the participant’s larger social support network, and selection of tasks to match an individual’s abilities and strengths, demonstrated improved cognitive performance, particularly executive functioning,37 and employment.38,39 These programs work best for patients who have mild to moderate symptoms.37,39
Patients with symptoms that are more severe may do better in a residential program. Many of these programs maintain an emphasis on vocational and social skills development. One such long-standing program is Bittersweet Farms, a rural farming community in Ohio for adults with ASD, where individuals with moderate to low function live in a group setting, with emphasis on scheduled, meaningful work including horticulture, animal care, carpentry; and activities of daily living.40
Studies of patients across the autism spectrum have generally found better outcomes when vocational support is given, but larger and randomized studies are needed to characterize how to best support these individuals after they leave high school.41
Psychological services such as applied behavioral therapy, social cognition training, cognitive behavioral therapy, and mindfulness training may be particularly useful in adults.42–44
Some versions of applied behavioral therapy, such as the Early Start Denver Model,45 have been found to be cost-effective and offset some expenses in the care of children with autism, using play-based and relationship-based interventions to promote development across domains while reducing symptoms.
In randomized controlled trials, modified cognitive behavioral therapy43 and mindfulness44 were shown to reduce symptoms of anxiety, obsessive-compulsive disorder, and depression.
Dialectical behavior therapy, used to find a balance between accepting oneself and desiring to change, may help in some circumstances to regulate emotions and reduce reactivity and lability, although large randomized clinical trials have not been conducted in the ASD population.46
Drug therapy
Medications may be appropriate to manage symptoms or comorbid conditions in adults with ASD. Over 75% adults with ASD have been found to use psychotropic medications.47 However, although these drugs have been approved for treating behaviors commonly associated with ASD, none of them provide definitive treatment for this disorder, and they have not been rigorously tested or approved for use in adults with ASD.48
Irritability and aggression associated with ASD can be treated with risperidone (approved for children over age 5), aripiprazole (approved for children ages 6–17), clozapine, or haloperidol.49
Aberrant social behavior can be treated with risperidone.50 Treatments under investigation include oxytocin and secretin.49
While no approved drug has been shown to improve social communication,51 balovaptan, a vasopressin V1a agonist, has shown potential and has been granted breakthrough status by the US Food and Drug Administration for treating challenging behaviors in adults, with additional studies ongoing in children.52,53
Repetitive behaviors, if the patient finds them impairing, can be managed with selective serotonin reuptake inhibitors.49
Much more study of drug therapy in adults with ASD is needed to fully understand the best approaches to psychotropic medication use, including appropriate classes and effective dosage, in this population.
SEX: UNEXPLORED TERRITORY
The reproductive health needs of people with autism remain largely underexplored.54 Historically, individuals with ASD were thought to have little interest in sexual activity or parenthood, owing to the nature of the core symptoms of the disorder. This has been shown to be untrue, particularly as studies on this topic began to engage in direct interviews with people with ASD, rather than solely gathering information from caregivers or parents. The findings reinforce the importance of broaching this component of health in this population, for the following reasons:
Adults with ASD are at increased risk of sexual victimization, with nearly 4 out of 5 reporting unwanted sexual advances, coercion, or rape.55
They have a smaller pool of knowledge with respect to sexual health. They report56 that they learned about sex from television and from “making mistakes.” They use fewer sources. They are less likely to speak to peers and figures of authority to gain knowledge about sexually transmitted infections, sexual behaviors, and contraception. And they are more likely to use forms of nonsocial media, such as television, for information.55
They report more concerns about the future with respect to sexual behavior, suggesting the need for targeted sexual education programs.56
College-age young adults with ASD who misread communication may be particularly affected by Title IX, which requires schools to promptly investigate reports of sexual harassment and sexual assault, should they struggle to comport themselves appropriately.57 Early and frank conversations about issues of consent and appropriate displays of interest and affection may better equip youth to navigate new social scenarios as they plan to leave a supervised home environment for college or the workforce.
Gender identification: Male, female, other
In one study, 77.8% of birth-sex males with ASD said they identified as men, and 67.1% of birth-sex females identified as women, compared with 93.1% of birth-sex males and 87.3% of birth-sex females without ASD. Many of the remaining individuals with ASD reported a transgender, genderqueer, or other gender identity.58 Some studies have found females with ASD report a gay or bisexual orientation more often than males with ASD.59–61
Adolescents and young adults may be exploring their changing bodies, sexual preferences, and gender roles, and as for all people at this age, these roles emerge against a backdrop of familial and societal expectations that may or may not be concordant with their own projected path regarding sexuality and reproductive health.62
Having the conversation
As with non-ASD patients, a thorough sexual history should be collected via open-ended questions when possible to determine types of sexual activity and partners.
Education of the patient, alongside caregivers and parents, about healthy and safe sexual practices, screening for sexual violence, and hormonal and nonhormonal contraception options are important components of care for this population.
CAREGIVER STRESS MAY PERSIST INTO PATIENT’S ADULTHOOD
Caregiver burden is a monumental concern for parents or others who may have lifelong primary responsibility for these neurodiverse adults.63 Family members may feel isolated and may feel they have encountered many barriers to services.64 Remaining sensitive, knowledgeable, and inquisitive about the types of support that are needed may help forge a trusting relationship between the provider and the family.
Parents of children with ASD have been reported to experience worse physical and emotional health than parents whose children do not have developmental disabilities.63,65 These disparities have been found to persist as their children enter adolescence and young adulthood.66,67 Parents of children with ASD report more anxiety, depression, and distress compared with parents of children without ASD,63 and parents themselves may be affected by ASD symptoms, which has been linked to increased parenting stress.68 Some studies have found blunted cortisol responses,63,69,70 and some,71 but not all,63 have found elevated blood pressure in caregivers of children with developmental disabilities. Headache, backache, muscle soreness, and fatigue may also be commonly reported.67
In our experience, caregivers are tremendously appreciative when provided connections to adult ASD services and support systems as their child ages. The school system and other formal support systems often assist until the time of transition into adulthood. This transition can be stressful for the adolescent and family alike, and informal support systems such as friends and family may become increasingly crucial, particularly if the adolescent still lives at home.72,73
The affected young adult’s unmet needs, as perceived by the caregiver, have been found to be significantly associated with caregiver burden, whereas the severity of the adult patient’s ASD symptoms has not.66 Therefore, it may be helpful to ask caregivers whether they perceive any unmet needs, regardless of the clinician’s perception of the severity of the patient’s ASD symptoms. Providing support to address these needs, particularly those relating to the child’s mood disorders, communication, social needs, safety, and daytime activities, may be the domains of support that most effectively reduce the caregiver burden in this population.66
Caregiver positivity, lower stress levels, and increased social support, particularly in the form of friends and family members providing no-cost assistance to caregivers whose children do not live independently,74 have been linked to better outcomes for caregivers.70,74,75 Rigorous studies that examine caregiver burden as individuals with ASD enter mid- and late-adulthood are limited.
THE ROLE OF THE INTERNIST IN CARING FOR ADULTS WITH AUTISM
A major challenge for many adults with ASD is the transition from services provided during childhood to those provided in adulthood. While children with autism have subspecialty providers who diagnose and manage their condition, including developmental-behavioral pediatricians, pediatric neurologists, and child psychiatrists, adults with autism may have fewer options.
Autism centers are becoming more available across the nation, and many provide care across the life span. However, depending on a patient’s needs, the primary care provider may need to manage residual symptoms as the patient transitions from pediatric to adult care, ultimately deciding when and where to refer the patient.
The patient’s family should pay close attention to function and mood around the time the patient leaves the structure of high school, and they should build rapport with a primary care provider they can turn to if problems persist or arise. Referrals for behavioral therapy and for social work, job training, and vocational support can greatly benefit patients as they transition to young adulthood. Referrals and suggestions for social support can also help caregivers.
Medical care
Deciding when and how to medicate the patient for symptoms of autism and related behaviors necessitates consideration of the patient’s impairment, side effects of the medication, and the impact medications may have on the patient’s other conditions. Disordered eating, mood problems, anxiety, and attention-deficit/hyperactivity disorder should be considered, and, as in all patients, regular screenings of mental health status should be conducted.76,77
Comorbid medical conditions may cause worsening of a patient’s known behavioral symptoms or may precipitate new behaviors or aggression as a result of pain or discomfort, particularly in patients with limited speech. A change in stereotypes or increased irritability warrants a thoughtful investigation for a cause other than ASD before adding or increasing behavioral medications. Common comorbid conditions include gastrointestinal distress, most commonly constipation and diarrhea in an idiopathic ASD population, with increasing ASD symptom severity correlating with increased odds of a gastrointestinal problem.78 Allergies, sleep disorders, seizures, and other psychiatric conditions are also frequent.79
Preventive care, including vaccinations, should be given as scheduled. Caregivers and patients can be reminded if needed that vaccines do not cause or worsen autism, and vaccination is intended to improve the safety of the patient and those around them, protecting against potentially life-threatening disease. Regular dental care visits, particularly for patients who are using medications that may affect tooth or gingival health,80 and regular visits to an optometrist or ophthalmologist for screening of vision are also advised.
Adverse effects. Weight gain and metabolic syndrome are common adverse effects of medications used for behavioral management, and the primary care physician may uncover diabetes, cardiac disorders, and hyperlipidemia. Patients with ASD may be particularly sensitive to the effects of medications and therefore may require a lower dose or a slower titration than other patients. Working with a behavioral team, careful weaning of psychiatric medications to the minimum needed is strongly recommended whenever possible.81
TAKE-HOME POINTS
As more adults with autism enter society, they may require varying levels of support from the healthcare community to ensure that therapeutic gains from childhood persist, allowing them to achieve maximal functional potential.
Adults with ASD may have a high, normal, or low IQ and intellectual capability. Knowledge of this and of the patient’s symptom severity and presence of comorbid psychiatric and other health conditions can help the clinician guide the patient to appropriate social services and pharmacologic treatments.
Individualized support in the workplace, as well as education regarding sexual health, can help improve outcomes for affected individuals.
Caregiver burden for individuals with autism can be high, but it can be mitigated by social support.
Further research regarding appropriate diagnostic instruments in adulthood and appropriate treatments for impairing autism-related symptoms across the life span may be particularly helpful in supporting this patient population.
- Autism and Developmental Disabilities Monitoring Network Surveillance Year 2000 Principal Investigators; Centers for Disease Control and Prevention. Prevalence of autism spectrum disorders—autism and developmental disabilities monitoring network, six sites, United States, 2000. MMWR Surveill Summ 2007; 56(1):1–11. pmid:17287714
- Christensen DL. Prevalence and characteristics of autism spectrum disorder among children aged 8 years—Autism and Developmental Disabilities Monitoring Network, 11 sites, United States, 2012. MMWR Surveill Summ 2016; 65(13):1–23. doi:10.15585/mmwr.ss6503a1
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th edition. Washington, D.C: American Psychiatric Association; 2013.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed., Text Revision. Washington, DC: American Psychiatric Association; 2000.
- Smith IC, Reichow B, Volkmar FR. The effects of DSM-5 criteria on number of individuals diagnosed with autism spectrum disorder: a systematic review. J Autism Dev Disord 2015; 45(8):2541–2552. doi:10.1007/s10803-015-2423-8
- Barahona-Corrêa JB, Filipe CN. A concise history of Asperger syndrome: the short reign of a troublesome diagnosis. Front Psychol 2015; 6:2024. doi:10.3389/fpsyg.2015.02024
- Kite DM, Gullifer J, Tyson GA. Views on the diagnostic labels of autism and Asperger’s disorder and the proposed changes in the DSM. J Autism Dev Disord 2013; 43(7):1692–1700. doi:10.1007/s10803-012-1718-2
- Kuo AA. Autism in adults: an update. Presented at the: American College of Physicians Internal Medicine Meeting, New Orleans, LA, April 17–21, 2018.
- Shattuck PT, Seltzer MM, Greenberg JS, et al. Change in autism symptoms and maladaptive behaviors in adolescents and adults with an autism spectrum disorder. J Autism Dev Disord 2007; 37(9):1735–1747. doi:10.1007/s10803-006-0307-7
- Seltzer MM, Shattuck P, Abbeduto L, Greenberg JS. Trajectory of development in adolescents and adults with autism. Ment Retard Dev Disabil Res Rev 2004; 10(4):234–247. doi:10.1002/mrdd.20038
- Billstedt E, Carina Gillberg I, Gillberg C. Autism in adults: symptom patterns and early childhood predictors. Use of the DISCO in a community sample followed from childhood. J Child Psychol Psychiatry 2007; 48(11):1102–1110. doi:10.1111/j.1469-7610.2007.01774.x
- Howlin P, Goode S, Hutton J, Rutter M. Adult outcome for children with autism. J Child Psychol Psychiatry 2004; 45(2):212–229. pmid:14982237
- Marriage S, Wolverton A, Marriage K. Autism spectrum disorder grown up: a chart review of adult functioning. J Can Acad Child Adolesc Psychiatry 2009; 18(4):322–328. pmid: 19881941
- Isaksson J, Tammimies K, Neufeld J, et al. EU-AIMS Longitudinal European Autism Project (LEAP): the autism twin cohort. Mol Autism 2018; 9(1):26. doi:10.1186/s13229-018-0212-x
- Lai M-C, Baron-Cohen S. Identifying the lost generation of adults with autism spectrum conditions. Lancet Psychiatry 2015; 2(11):1013–1027. doi:10.1016/S2215-0366(15)00277-1
- National Institute for Health and Clinical Excellence. Autism: recognition, referral, diagnosis and management of adults on the autism spectrum. NICE clinical guideline 142. June 2012. https://grand.tghn.org/site_media/media/medialibrary/2015/03/ASD_NICE_3_.pdf. Accessed July 9, 2019.
- Wolf JM, Ventola P. Assessment and treatment planning in adults with autism spectrum disorders. In: Adolescents and Adults with Autism Spectrum Disorders. Springer, New York, NY; 2014:283–298.
- Lord C, Rutter M, DiLavore P, Risi S, Gotham K, Bishop SL. Autism Diagnostic Observation Schedule, Second Edition (ADOS-2) manual. Torrance, CA: Western Psychological Services, 2012.
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord 1994; 24(5):659–685.
- Hus V, Lord C. The autism diagnostic observation schedule, module 4: revised algorithm and standardized severity scores. J Autism Dev Disord 2014; 44(8):1996–2012. doi:10.1007/s10803-014-2080-3>
- Sparrow S, Balla D, Cicchetti D, Harrison P, Doll E. Vineland Adaptive Behavior Scales. Circle Pines, MN: American Guidance Service, 1984.
- Happé FG, Mansour H, Barrett P, Brown T, Abbott P, Charlton RA. Demographic and cognitive profile of individuals seeking a diagnosis of autism spectrum disorder in adulthood. J Autism Dev Disord 2016; 46(11):3469–3480. doi:10.1007/s10803-016-2886-2
- Johnston K, Dittner A, Bramham J, Murphy C, Knight A, Russell A. Attention deficit hyperactivity disorder symptoms in adults with autism spectrum disorders. Autism Res Off J Int Soc Autism Res 2013; 6(4):225–236. doi:10.1002/aur.1283
- Cadman T, Spain D, Johnston P, et al. Obsessive-compulsive disorder in adults with high-functioning autism spectrum disorder: what does self-report with the OCI-R tell us? Autism Res Off J Int Soc Autism Res 2015; 8(5):477–485. doi:10.1002/aur.1461
- Russell AJ, Mataix-Cols D, Anson M, Murphy DGM. Obsessions and compulsions in Asperger syndrome and high-functioning autism. Br J Psychiatry J Ment Sci 2005; 186:525–528. doi:10.1192/bjp.186.6.525
- Simonoff E, Pickles A, Charman T, Chandler S, Loucas T, Baird G. Psychiatric disorders in children with autism spectrum disorders: prevalence, comorbidity, and associated factors in a population-derived sample. J Am Acad Child Adolesc Psychiatry 2008; 47(8):921–929. doi:10.1097/CHI.0b013e318179964f
- Lugnegård T, Hallerbäck MU, Gillberg C. Psychiatric comorbidity in young adults with a clinical diagnosis of Asperger syndrome. Res Dev Disabil 2011; 32(5):1910–1917. doi:10.1016/j.ridd.2011.03.025
- Howlin P, Moss P. Adults with autism spectrum disorders. Can J Psychiatry 2012; 57(5):275–283. doi:10.1177/070674371205700502
- Levy A, Perry A. Outcomes in adolescents and adults with autism: a review of the literature. Res Autism Spectr Disord 2011; 5(4):1271–1282. doi:10.1016/J.RASD.2011.01.023
- Cheak-Zamora NC, Yang X, Farmer JE, Clark M. Disparities in transition planning for youth with autism spectrum disorder. Pediatrics 2013; 131(3):447–454. doi:10.1542/peds.2012-1572
- Rast JE, Shattuck PT, Roux AM, Anderson KA, Kuo A. The medical home and health care transition for youth with autism. Pediatrics 2018; 141(suppl 4):S328–S334. doi:10.1542/peds.2016-4300J
- Belling R, McLaren S, Paul M, et al. The effect of organisational resources and eligibility issues on transition from child and adolescent to adult mental health services. J Health Serv Res Policy 2014; 19(3):169–176. doi:10.1177/1355819614527439
- Data Resource Center for Child & Adolescent Health. 2009–2010 National Survey of Children with Special Health Care Needs. www.childhealthdata.org/docs/drc/200910-cshcn-spss-codebook_final_051012.pdf?sfvrsn=1. Accessed July 9, 2019.
- Got Transition Center for Health Care Transition Improvement. Six core elements of health care transition 2.0. Transitioning youth to an adult health care provider. For use by pediatric, family medicine, and med-peds providers. www.gottransition.org/resourceGet.cfm?id=208. Accessed July 9, 2019.
- Murphy CM, Wilson CE, Robertson DM, et al. Autism spectrum disorder in adults: diagnosis, management, and health services development. Neuropsychiatr Dis Treat 2016; 12:1669–1686. doi:10.2147/NDT.S65455
- García-Villamisar DA, Dattilo J. Effects of a leisure programme on quality of life and stress of individuals with ASD. J Intellect Disabil Res 2010; 54(7):611–619. doi:10.1111/j.1365-2788.2010.01289.x
- García-Villamisar D, Hughes C. Supported employment improves cognitive performance in adults with autism. J Intellect Disabil Res 2007; 51(pt 2):142–150. doi:10.1111/j.1365-2788.2006.00854.x
- Lawer L, Brusilovskiy E, Salzer MS, Mandell DS. Use of vocational rehabilitative services among adults with autism. J Autism Dev Disord 2009; 39(3):487–494. doi:10.1007/s10803-008-0649-4
- Howlin P, Alcock J, Burkin C. An 8 year follow-up of a specialist supported employment service for high-ability adults with autism or Asperger syndrome. Autism 2005; 9(5):533–549. doi:10.1177/1362361305057871
- Kay BR. Bittersweet Farms. J Autism Dev Disord 1990; 20(3):309–321. http://www.ncbi.nlm.nih.gov/pubmed/2228914. Accessed July 9, 2019.
- Taylor JL, McPheeters ML, Sathe NA, Dove D, Veenstra-Vanderweele J, Warren Z. A systematic review of vocational interventions for young adults with autism spectrum disorders. Pediatrics 2012; 130(3):531–538. doi:10.1542/peds.2012-0682
- Bishop-Fitzpatrick L, Minshew NJ, Eack SM. A systematic review of psychosocial interventions for adults with autism spectrum disorders. J Autism Dev Disord 2013; 43(3):687–694. doi:10.1007/s10803-012-1615-8
- Russell AJ, Jassi A, Fullana MA, et al. Cognitive behavior therapy for comorbid obsessive-compulsive disorder in high-functioning autism spectrum disorders: a randomized controlled trial. Depress Anxiety 2013; 30(8):697–708. doi:10.1002/da.22053
- Spek AA, van Ham NC, Nyklícek I. Mindfulness-based therapy in adults with an autism spectrum disorder: a randomized controlled trial. Res Dev Disabil 2013; 34(1):246–253. doi:10.1016/j.ridd.2012.08.009
- Eapen V, Crncec R, Walter A. Clinical outcomes of an early intervention program for preschool children with autism spectrum disorder in a community group setting. BMC Pediatr 2013; 13(1):3. doi:10.1186/1471-2431-13-3
- Mazefsky CA, White SW. Emotion regulation: concepts & practice in autism spectrum disorder. Child Adolesc Psychiatr Clin North Am 2014; 23(1):15–24. doi:10.1016/J.CHC.2013.07.002
- Esbensen AJ, Greenberg JS, Seltzer MM, Aman MG. A longitudinal investigation of psychotropic and non-psychotropic medication use among adolescents and adults with autism spectrum disorders. J Autism Dev Disord 2009; 39(9):1339–1349. doi:10.1007/s10803-009-0750-3
- Dove D, Warren Z, McPheeters ML, Taylor JL, Sathe NA, Veenstra-VanderWeele J. Medications for adolescents and young adults with autism spectrum disorders: a systematic review. Pediatrics 2012; 130(4):717–726. doi:10.1542/peds.2012-0683
- LeClerc S, Easley D. Pharmacological therapies for autism spectrum disorder: a review. Pharm Ther 2015; 40(6):389–397.
- Miral S, Gencer O, Inal-Emiroglu FN, Baykara B, Baykara A, Dirik E. Risperidone versus haloperidol in children and adolescents with AD: a randomized, controlled, double-blind trial. Eur Child Adolesc Psychiatry 2008; 17(1):1–8. doi:10.1007/s00787-007-0620-5
- Lai M-C, Lombardo MV, Baron-Cohen S. Autism. Lancet 2014; 383(9920):896–910. doi:10.1016/S0140-6736(13)61539-1
- Ratni H, Rogers-Evans M, Bissantz C, et al. Discovery of highly selective brain-penetrant vasopressin 1a antagonists for the potential treatment of autism via a chemogenomic and scaffold hopping approach. J Med Chem 2015; 58(5):2275–2289. doi:10.1021/jm501745f
- Umbricht D, Del Valle Rubido M, Hollander E, et al. A single dose, randomized, controlled proof-of-mechanism study of a novel vasopressin 1a receptor antagonist (RG7713) in high-functioning adults with autism spectrum disorder. Neuropsychopharmacology 2017; 42(9):1914–1923. doi:10.1038/npp.2016.232>
- Kellaher DC. Sexual behavior and autism spectrum disorders: an update and discussion. Curr Psychiatry Rep 2015; 17(4):25. doi:10.1007/s11920-015-0562-4
- Brown-Lavoie SM, Viecili MA, Weiss JA. Sexual knowledge and victimization in adults with autism spectrum disorders. J Autism Dev Disord 2014; 44(9):2185–2196. doi:10.1007/s10803-014-2093-y
- Mehzabin P, Stokes MA. Self-assessed sexuality in young adults with high-functioning autism. Res Autism Spectr Disord 2011; 5(1):614–621. doi:10.1016/J.RASD.2010.07.006>
- Brown KR. Accessibility for students with ASD: legal perspectives in the United States. In: Alphin HC Jr. Exploring the Future of Accessibility in Higher Education. Hershey, PA: IGI Global; 2017.
- George R, Stokes MA. Gender identity and sexual orientation in autism spectrum disorder. Autism 2018; 22(8):970–982. doi:10.1177/1362361317714587
- Byers ES, Nichols S, Voyer SD. Challenging stereotypes: sexual functioning of single adults with high functioning autism spectrum disorder. J Autism Dev Disord 2013; 43(11):2617–2627. doi:10.1007/s10803-013-1813-z
- Gilmour L, Schalomon PM, Smith V. Sexuality in a community based sample of adults with autism spectrum disorder. Res Autism Spectr Disord 2012; 6(1):313–318. doi:10.1016/J.RASD.2011.06.003
- Bejerot S, Eriksson JM. Sexuality and gender role in autism spectrum disorder: a case control study. Schmitz C, ed. PLoS One 2014; 9(1):e87961. doi:10.1371/journal.pone.0087961>
- Navot N, Jorgenson AG, Webb SJ. Maternal experience raising girls with autism spectrum disorder: a qualitative study. Child Care Health Dev 2017; 43(4):536–545. doi:10.1111/cch.12470
- Padden C, James JE. Stress among parents of children with and without autism spectrum disorder: a comparison involving physiological indicators and parent self-reports. J Dev Phys Disabil 2017; 29(4):567–586. doi:10.1007/s10882-017-9547-z
- Woodgate RL, Ateah C, Secco L. Living in a world of our own: the experience of parents who have a child with autism. Qual Health Res 2008; 18(8):1075–1083. doi:10.1177/1049732308320112
- Hayes SA, Watson SL. The impact of parenting stress: a meta-analysis of studies comparing the experience of parenting stress in parents of children with and without autism spectrum disorder. J Autism Dev Disord 2013; 43(3):629–642. doi:10.1007/s10803-012-1604-y
- Cadman T, Eklund H, Howley D, et al. Caregiver burden as people with autism spectrum disorder and attention-deficit/hyperactivity disorder transition into adolescence and adulthood in the United Kingdom. J Am Acad Child Adolesc Psychiatry 2012; 51(9):879–888. doi:10.1016/j.jaac.2012.06.017
- Smith LE, Seltzer MM, Greenberg JS. Daily health symptoms of mothers of adolescents and adults with fragile x syndrome and mothers of adolescents and adults with autism spectrum disorder. J Autism Dev Disord 2012; 42(9):1836–1846. doi:10.1007/s10803-011-1422-7
- van Steijn DJ, Oerlemans AM, van Aken MAG, Buitelaar JK, Rommelse NNJ. The reciprocal relationship of ASD, ADHD, depressive symptoms and stress in parents of children with ASD and/or ADHD. J Autism Dev Disord 2014; 44(5):1064–1076. doi:10.1007/s10803-013-1958-9
- Seltzer MM, Greenberg JS, Hong J, et al. Maternal cortisol levels and behavior problems in adolescents and adults with ASD. J Autism Dev Disord 2010; 40(4):457–469. doi:10.1007/S10803-009-0887-0
- Lovell B, Moss M, Wetherell MA. With a little help from my friends: psychological, endocrine and health corollaries of social support in parental caregivers of children with autism or ADHD. Res Dev Disabil 2012; 33(2):682–687. doi:10.1016/j.ridd.2011.11.014
- Gallagher S, Whiteley J. Social support is associated with blood pressure responses in parents caring for children with developmental disabilities. Res Dev Disabil 2012; 33(6):2099–2105. doi:10.1016/j.ridd.2012.06.007
- Baker JK, Smith LE, Greenberg JS, Seltzer MM, Taylor JL. Change in maternal criticism and behavior problems in adolescents and adults with autism across a 7-year period. J Abnorm Psychol 2011; 120(2):465–475. doi:10.1037/a0021900
- Marsack CN, Samuel PS. Mediating effects of social support on quality of life for parents of adults with autism. J Autism Dev Disord 2017; 47(8):2378–2389. doi:10.1007/s10803-017-3157-6
- Trute B, Benzies KM, Worthington C, Reddon JR, Moore M. Accentuate the positive to mitigate the negative: mother psychological coping resources and family adjustment in childhood disability. J Intellect Dev Disabil 2010; 35(1):36–43. doi:10.3109/13668250903496328
- Cantwell J, Muldoon OT, Gallagher S. Social support and mastery influence the association between stress and poor physical health in parents caring for children with developmental disabilities. Res Dev Disabil 2014; 35(9):2215–2223. doi:10.1016/j.ridd.2014.05.012
- Carton AM, Smith AD. Assessing the relationship between eating disorder psychopathology and autistic traits in a non-clinical adult population. Eat Weight Disord - Stud Anorexia, Bulim Obes 2014; 19(3):285–293. doi:10.1007/s40519-013-0086-z
- De Alwis D, Agrawal A, Reiersen AM, et al. ADHD symptoms, autistic traits, and substance use and misuse in adult Australian twins. J Stud Alcohol Drugs 2014; 75(2):211–221. doi:10.15288/jsad.2014.75.211
- Wang LW, Tancredi DJ, Thomas DW. The prevalence of gastrointestinal problems in children across the United States with autism spectrum disorders from families with multiple affected members. J Dev Behav Pediatr 2011; 32(5):351–360. doi:10.1097/DBP.0b013e31821bd06a
- Croen LA, Zerbo O, Qian Y, et al. The health status of adults on the autism spectrum. Autism 2015; 19(7):814–823. doi:10.1177/1362361315577517
- Kalyoncu IÖ, Tanboga I. Oral health status of children with autistic spectrum disorder compared with non-authentic peers. Iran J Public Health 2017; 46(11):1591–1593. www.ncbi.nlm.nih.gov/pmc/articles/PMC5696703. Accessed July 9, 2019.
- McGuire K, Fung LK, Hagopian L, et al. Irritability and problem behavior in autism spectrum disorder: a practice pathway for pediatric primary care. Pediatrics 2016; 137(suppl 2):S136–S148. doi:10.1542/peds.2015-2851L
Autism spectrum disorder (ASD) has increased significantly over the past 40 years. Even in the past 2 decades, the prevalence increased from 6.7 per 1,000 in 20001 to 14.6 per 1,000 in 2012—1 in 59 people.2 Of those with ASD, 46% have an intelligence quotient (IQ) greater than 85, meaning they are of average or above-average intelligence.1
As more children with autism become adults, understanding this condition across the life span grows paramount. While many studies have focused on understanding how diagnosis and treatment can help young children, few have focused on adults with autism and how primary care teams can better assist these individuals. However, this is changing, with studies of the benefits of employment programs and pharmacologic treatment, and reproductive health needs of adults with ASD. Here we provide an updated review of ASD in adult patients.
NO MORE ASPERGER SYNDROME— IT’S ON THE SPECTRUM NOW
As the scientific understanding of autism has expanded, revisions in the Diagnostic and Statistical Manual of Mental Disorders, fifth edition (DSM-5),3 published in 2013, have paralleled these advances. For many adult patients with autism who were evaluated as children, these revisions have led to changes in diagnosis and available services.
In the previous edition (DSM-IV-TR, published in 2000),4 autistic disorder and Asperger syndrome were separate (Table 1). However, DSM-5 lumped autistic disorder and Asperger disorder together under the diagnosis of ASD; this leaves it to the clinician to specify whether the patient with ASD has accompanying intellectual or language impairment and to assign a level of severity based on communication deficits and restrictive behaviors.
The shift in diagnosis was worrisome for some, particularly for clinicians treating patients with DSM-IV Asperger syndrome, who lost this diagnostic label. Concerns that patients with Asperger syndrome may not meet the DSM-5 criteria for ASD were validated by a systematic review showing that only 50% to 75% of patients with DSM-IV autistic disorder, Asperger syndrome, or pervasive developmental disorder not otherwise specified (PDD-NOS) met the DSM-5 criteria for ASD.5 Most of those who no longer met the criteria for ASD carried a DSM-IV diagnosis of Asperger syndrome or PDD-NOS or had an IQ over 70.5 Nevertheless, these individuals may struggle with impairing symptoms related to repetitive behaviors or communication or may be affected by learning or social-emotional disabilities. Additionally, even if they meet the criteria for ASD, some may identify with the Asperger syndrome label and fear they will be stigmatized should they be classified as having the more general ASD.6,7
Although future revisions to the DSM may include further changes in classification, grouping adults with ASD according to their functional and cognitive ability may allow for pragmatic characterization of their needs. At least 3 informal groupings of autistic adults have been described that integrate cognitive ability and independence8:
- Those with low cognitive and social abilities, who need lifelong support
- Those with midrange cognitive and social limitations but who can complete their work in special education classes; they often find employment in supervised workshops or other work with repetitive tasks
- Those who have greater cognitive ability and some social skills; they may proceed to college and employment and live independently.
UNCERTAIN PROGNOSIS
Prognostication for people with ASD remains an area of research. Some adults experience a reduction in symptoms as they age, with significant improvements in speech and, sometimes, modest improvements in restrictive and repetitive behaviors.9,10
Nevertheless, autism remains a lifelong disorder for many. Adults may still require significant support and may experience impairment, particularly in social interaction.10 In longitudinal studies, only 15% to 27% of patients with ASD are characterized as having a positive outcome (often defined as variables related to independent function, near-normal relationships, employment, or a quantified reduction in core symptoms), and many experience significant dependency into adulthood.10–13
IQ has been cited as a possible prognostic factor,10,13 with an IQ below 70 associated with poorer outcome, although an IQ above 70 does not necessarily confer a positive outcome. Less-severe impairment in speech at baseline in early childhood also suggests better outcomes in adulthood.10
As we see more adults with autism, studies that include both children and adults, such as the Longitudinal European Autism Cohort, will be important to characterize the natural history, comorbidities, and genetics of ASD and may help provide more specific predictors of disease course into adulthood.14
ACHIEVING A DIAGNOSIS FOR ADULT PATIENTS WITH SUSPECTED AUTISM
While many patients are recognized as having autism in early to mid-childhood, some adults may not receive a formal diagnosis until much later in life. Those with fluent language and normal-range IQ are likely to be overlooked.15 People with ASD may have had mild symptoms during childhood that did not impair their functioning until demands of daily life exceeded their capacities in adulthood. Alternatively, parents of a child with newly diagnosed ASD may realize that they themselves or another adult family member also show signs of it.
The UK National Institute of Health and Care Excellence suggests that assessment should be considered if the patient meets psychiatric diagnostic criteria and one of the following:
- Difficulty obtaining or sustaining employment or education
- Difficulty initiating or sustaining social relationships
- Past or current contact with mental health or learning disability services
- History of a neurodevelopmental or mental health disorder.15,16
Currently, diagnosis typically involves a multidisciplinary approach, with psychiatric assessment, neuropsychological testing, and speech and language evaluation.17 Providers may need to refer patients for these services, sometimes at the patient’s request, if previous mental health misdiagnoses are suspected, if patients report symptoms or impairment consistent with ASD, or if benefits, services, or accommodations, such as a coach in the workplace, are needed.
Diagnosing ASD in adults can be difficult, given that the gold-standard diagnostic tests such as the Autism Diagnostic Observation Schedule-2 (ADOS-2)18 and the Autism Diagnostic Interview-Revised (ADI-R)19 are typically used to diagnose autism in children. However, Module 4 in the ADOS-2 was developed for adolescents and older patients with fluent language and has shown at least moderate power to distinguish adults with ASD from those without ASD.18,20
An initial psychiatric assessment should include a thorough history taken from the patient and, if applicable, the patient’s caregiver, as well as a psychiatric interview of the patient. Neuropsychological testing should include evaluation of cognitive function, social functioning (using the ADOS-2 for adults without intellectual disability, the ADI-R, or both), and adaptive functioning (using the Vineland Adaptive Behavior Scales, second edition21).
Evaluation of speech and language is particularly important in patients with limited language ability and should include both expressive and receptive language abilities. Serial testing every few years, as is often recommended in childhood, may help establish the pattern of impairment over time.
Comorbid psychiatric disorders are common
Many people with ASD also have other psychiatric disorders,17,22 which clinicians should keep in mind when seeing an adult seeking evaluation for ASD.
Attention-deficit/hyperactivity disorder is present at higher rates in patients of average intellectual function with ASD than in the general population.23
Anxiety disorders, including obsessive-compulsive disorder, were found to often coexist with autism in a sample of adults with autism without intellectual disability,24,25 and approximately 40% of youths with ASD have at least 1 comorbid anxiety disorder.26
Mood disorders are also prevalent in adults with ASD, with a small study showing that 70% of adults with DSM-IV Asperger syndrome had at least 1 depressive episode in their lifetime.27
BEHAVIORAL AND PHARMACOLOGIC THERAPIES FOR THE ADULT PATIENT
Services and medications for adults with ASD are discussed below. These will vary by individual, and services available may vary by region.
Historically, vocational and social outcomes have been poor for adults with ASD. It is estimated that most larger universities may be home to 100 to 300 students with ASD. To combat isolation, the University of California, Los Angeles, the University of Alabama, and others provide special support services, including group social activities such as board games and individual coaching.8 Nevertheless, half of the students with autism who attend institutions of higher learning leave without completing their intended degree.29 Many still struggle to establish meaningful friendships or romantic relationships.29
Planning for a transition of care
Healthcare transition planning is important but is strikingly underused.30 Individual providers, including adult psychiatrists, vary in their level of training and comfort in diagnosing, treating, and monitoring adults with autism. Youths with ASD are half as likely to receive healthcare transition services as other youths with special healthcare needs.31
Pediatric providers, including pediatric psychiatrists, developmental behavioral specialists, and pediatric neurologists, may be best equipped to treat young adult patients or to refer patients to appropriate generalists and specialists comfortable with autism-specific transition of care. The question of eligibility for services is important to patients and families during the transition period, with many parents and professionals unaware of services available to them.32 Receiving adequate transition services is enabled by having a medical home during childhood—that is, a comprehensive, centralized medical record, culturally competent care, interaction with schools, and patient access to clear, unbiased information.31
Ideally, in our experience, transitioning should be discussed well before the child ages out of the pediatric provider’s practice. If necessary, healthcare transition services should include 4 components:
- Discussing the switch to a new physician who treats adults
- Discussing changing healthcare needs as an adult
- Planning insurance coverage as an adult
- Encouragement by the physician for the child to take age-appropriate responsibility for his or her healthcare.31,33
Tools such as the Got Transition checklist from the National Health Care Transition Center can provide support during this process.34
Other services
Other services provided as an extension or adjunct to the medical home in early adulthood may include customized vocational or employment training, specialized mentorship or support in a college setting, housing support, and psychological services.35
Community-based programs that emphasize leisure have been shown to improve participants’ independence and quality of life.36 Similarly, participants in programs that emphasized supported employment, with a job coach, on-the-job support, collaboration with the participant’s larger social support network, and selection of tasks to match an individual’s abilities and strengths, demonstrated improved cognitive performance, particularly executive functioning,37 and employment.38,39 These programs work best for patients who have mild to moderate symptoms.37,39
Patients with symptoms that are more severe may do better in a residential program. Many of these programs maintain an emphasis on vocational and social skills development. One such long-standing program is Bittersweet Farms, a rural farming community in Ohio for adults with ASD, where individuals with moderate to low function live in a group setting, with emphasis on scheduled, meaningful work including horticulture, animal care, carpentry; and activities of daily living.40
Studies of patients across the autism spectrum have generally found better outcomes when vocational support is given, but larger and randomized studies are needed to characterize how to best support these individuals after they leave high school.41
Psychological services such as applied behavioral therapy, social cognition training, cognitive behavioral therapy, and mindfulness training may be particularly useful in adults.42–44
Some versions of applied behavioral therapy, such as the Early Start Denver Model,45 have been found to be cost-effective and offset some expenses in the care of children with autism, using play-based and relationship-based interventions to promote development across domains while reducing symptoms.
In randomized controlled trials, modified cognitive behavioral therapy43 and mindfulness44 were shown to reduce symptoms of anxiety, obsessive-compulsive disorder, and depression.
Dialectical behavior therapy, used to find a balance between accepting oneself and desiring to change, may help in some circumstances to regulate emotions and reduce reactivity and lability, although large randomized clinical trials have not been conducted in the ASD population.46
Drug therapy
Medications may be appropriate to manage symptoms or comorbid conditions in adults with ASD. Over 75% adults with ASD have been found to use psychotropic medications.47 However, although these drugs have been approved for treating behaviors commonly associated with ASD, none of them provide definitive treatment for this disorder, and they have not been rigorously tested or approved for use in adults with ASD.48
Irritability and aggression associated with ASD can be treated with risperidone (approved for children over age 5), aripiprazole (approved for children ages 6–17), clozapine, or haloperidol.49
Aberrant social behavior can be treated with risperidone.50 Treatments under investigation include oxytocin and secretin.49
While no approved drug has been shown to improve social communication,51 balovaptan, a vasopressin V1a agonist, has shown potential and has been granted breakthrough status by the US Food and Drug Administration for treating challenging behaviors in adults, with additional studies ongoing in children.52,53
Repetitive behaviors, if the patient finds them impairing, can be managed with selective serotonin reuptake inhibitors.49
Much more study of drug therapy in adults with ASD is needed to fully understand the best approaches to psychotropic medication use, including appropriate classes and effective dosage, in this population.
SEX: UNEXPLORED TERRITORY
The reproductive health needs of people with autism remain largely underexplored.54 Historically, individuals with ASD were thought to have little interest in sexual activity or parenthood, owing to the nature of the core symptoms of the disorder. This has been shown to be untrue, particularly as studies on this topic began to engage in direct interviews with people with ASD, rather than solely gathering information from caregivers or parents. The findings reinforce the importance of broaching this component of health in this population, for the following reasons:
Adults with ASD are at increased risk of sexual victimization, with nearly 4 out of 5 reporting unwanted sexual advances, coercion, or rape.55
They have a smaller pool of knowledge with respect to sexual health. They report56 that they learned about sex from television and from “making mistakes.” They use fewer sources. They are less likely to speak to peers and figures of authority to gain knowledge about sexually transmitted infections, sexual behaviors, and contraception. And they are more likely to use forms of nonsocial media, such as television, for information.55
They report more concerns about the future with respect to sexual behavior, suggesting the need for targeted sexual education programs.56
College-age young adults with ASD who misread communication may be particularly affected by Title IX, which requires schools to promptly investigate reports of sexual harassment and sexual assault, should they struggle to comport themselves appropriately.57 Early and frank conversations about issues of consent and appropriate displays of interest and affection may better equip youth to navigate new social scenarios as they plan to leave a supervised home environment for college or the workforce.
Gender identification: Male, female, other
In one study, 77.8% of birth-sex males with ASD said they identified as men, and 67.1% of birth-sex females identified as women, compared with 93.1% of birth-sex males and 87.3% of birth-sex females without ASD. Many of the remaining individuals with ASD reported a transgender, genderqueer, or other gender identity.58 Some studies have found females with ASD report a gay or bisexual orientation more often than males with ASD.59–61
Adolescents and young adults may be exploring their changing bodies, sexual preferences, and gender roles, and as for all people at this age, these roles emerge against a backdrop of familial and societal expectations that may or may not be concordant with their own projected path regarding sexuality and reproductive health.62
Having the conversation
As with non-ASD patients, a thorough sexual history should be collected via open-ended questions when possible to determine types of sexual activity and partners.
Education of the patient, alongside caregivers and parents, about healthy and safe sexual practices, screening for sexual violence, and hormonal and nonhormonal contraception options are important components of care for this population.
CAREGIVER STRESS MAY PERSIST INTO PATIENT’S ADULTHOOD
Caregiver burden is a monumental concern for parents or others who may have lifelong primary responsibility for these neurodiverse adults.63 Family members may feel isolated and may feel they have encountered many barriers to services.64 Remaining sensitive, knowledgeable, and inquisitive about the types of support that are needed may help forge a trusting relationship between the provider and the family.
Parents of children with ASD have been reported to experience worse physical and emotional health than parents whose children do not have developmental disabilities.63,65 These disparities have been found to persist as their children enter adolescence and young adulthood.66,67 Parents of children with ASD report more anxiety, depression, and distress compared with parents of children without ASD,63 and parents themselves may be affected by ASD symptoms, which has been linked to increased parenting stress.68 Some studies have found blunted cortisol responses,63,69,70 and some,71 but not all,63 have found elevated blood pressure in caregivers of children with developmental disabilities. Headache, backache, muscle soreness, and fatigue may also be commonly reported.67
In our experience, caregivers are tremendously appreciative when provided connections to adult ASD services and support systems as their child ages. The school system and other formal support systems often assist until the time of transition into adulthood. This transition can be stressful for the adolescent and family alike, and informal support systems such as friends and family may become increasingly crucial, particularly if the adolescent still lives at home.72,73
The affected young adult’s unmet needs, as perceived by the caregiver, have been found to be significantly associated with caregiver burden, whereas the severity of the adult patient’s ASD symptoms has not.66 Therefore, it may be helpful to ask caregivers whether they perceive any unmet needs, regardless of the clinician’s perception of the severity of the patient’s ASD symptoms. Providing support to address these needs, particularly those relating to the child’s mood disorders, communication, social needs, safety, and daytime activities, may be the domains of support that most effectively reduce the caregiver burden in this population.66
Caregiver positivity, lower stress levels, and increased social support, particularly in the form of friends and family members providing no-cost assistance to caregivers whose children do not live independently,74 have been linked to better outcomes for caregivers.70,74,75 Rigorous studies that examine caregiver burden as individuals with ASD enter mid- and late-adulthood are limited.
THE ROLE OF THE INTERNIST IN CARING FOR ADULTS WITH AUTISM
A major challenge for many adults with ASD is the transition from services provided during childhood to those provided in adulthood. While children with autism have subspecialty providers who diagnose and manage their condition, including developmental-behavioral pediatricians, pediatric neurologists, and child psychiatrists, adults with autism may have fewer options.
Autism centers are becoming more available across the nation, and many provide care across the life span. However, depending on a patient’s needs, the primary care provider may need to manage residual symptoms as the patient transitions from pediatric to adult care, ultimately deciding when and where to refer the patient.
The patient’s family should pay close attention to function and mood around the time the patient leaves the structure of high school, and they should build rapport with a primary care provider they can turn to if problems persist or arise. Referrals for behavioral therapy and for social work, job training, and vocational support can greatly benefit patients as they transition to young adulthood. Referrals and suggestions for social support can also help caregivers.
Medical care
Deciding when and how to medicate the patient for symptoms of autism and related behaviors necessitates consideration of the patient’s impairment, side effects of the medication, and the impact medications may have on the patient’s other conditions. Disordered eating, mood problems, anxiety, and attention-deficit/hyperactivity disorder should be considered, and, as in all patients, regular screenings of mental health status should be conducted.76,77
Comorbid medical conditions may cause worsening of a patient’s known behavioral symptoms or may precipitate new behaviors or aggression as a result of pain or discomfort, particularly in patients with limited speech. A change in stereotypes or increased irritability warrants a thoughtful investigation for a cause other than ASD before adding or increasing behavioral medications. Common comorbid conditions include gastrointestinal distress, most commonly constipation and diarrhea in an idiopathic ASD population, with increasing ASD symptom severity correlating with increased odds of a gastrointestinal problem.78 Allergies, sleep disorders, seizures, and other psychiatric conditions are also frequent.79
Preventive care, including vaccinations, should be given as scheduled. Caregivers and patients can be reminded if needed that vaccines do not cause or worsen autism, and vaccination is intended to improve the safety of the patient and those around them, protecting against potentially life-threatening disease. Regular dental care visits, particularly for patients who are using medications that may affect tooth or gingival health,80 and regular visits to an optometrist or ophthalmologist for screening of vision are also advised.
Adverse effects. Weight gain and metabolic syndrome are common adverse effects of medications used for behavioral management, and the primary care physician may uncover diabetes, cardiac disorders, and hyperlipidemia. Patients with ASD may be particularly sensitive to the effects of medications and therefore may require a lower dose or a slower titration than other patients. Working with a behavioral team, careful weaning of psychiatric medications to the minimum needed is strongly recommended whenever possible.81
TAKE-HOME POINTS
As more adults with autism enter society, they may require varying levels of support from the healthcare community to ensure that therapeutic gains from childhood persist, allowing them to achieve maximal functional potential.
Adults with ASD may have a high, normal, or low IQ and intellectual capability. Knowledge of this and of the patient’s symptom severity and presence of comorbid psychiatric and other health conditions can help the clinician guide the patient to appropriate social services and pharmacologic treatments.
Individualized support in the workplace, as well as education regarding sexual health, can help improve outcomes for affected individuals.
Caregiver burden for individuals with autism can be high, but it can be mitigated by social support.
Further research regarding appropriate diagnostic instruments in adulthood and appropriate treatments for impairing autism-related symptoms across the life span may be particularly helpful in supporting this patient population.
Autism spectrum disorder (ASD) has increased significantly over the past 40 years. Even in the past 2 decades, the prevalence increased from 6.7 per 1,000 in 20001 to 14.6 per 1,000 in 2012—1 in 59 people.2 Of those with ASD, 46% have an intelligence quotient (IQ) greater than 85, meaning they are of average or above-average intelligence.1
As more children with autism become adults, understanding this condition across the life span grows paramount. While many studies have focused on understanding how diagnosis and treatment can help young children, few have focused on adults with autism and how primary care teams can better assist these individuals. However, this is changing, with studies of the benefits of employment programs and pharmacologic treatment, and reproductive health needs of adults with ASD. Here we provide an updated review of ASD in adult patients.
NO MORE ASPERGER SYNDROME— IT’S ON THE SPECTRUM NOW
As the scientific understanding of autism has expanded, revisions in the Diagnostic and Statistical Manual of Mental Disorders, fifth edition (DSM-5),3 published in 2013, have paralleled these advances. For many adult patients with autism who were evaluated as children, these revisions have led to changes in diagnosis and available services.
In the previous edition (DSM-IV-TR, published in 2000),4 autistic disorder and Asperger syndrome were separate (Table 1). However, DSM-5 lumped autistic disorder and Asperger disorder together under the diagnosis of ASD; this leaves it to the clinician to specify whether the patient with ASD has accompanying intellectual or language impairment and to assign a level of severity based on communication deficits and restrictive behaviors.
The shift in diagnosis was worrisome for some, particularly for clinicians treating patients with DSM-IV Asperger syndrome, who lost this diagnostic label. Concerns that patients with Asperger syndrome may not meet the DSM-5 criteria for ASD were validated by a systematic review showing that only 50% to 75% of patients with DSM-IV autistic disorder, Asperger syndrome, or pervasive developmental disorder not otherwise specified (PDD-NOS) met the DSM-5 criteria for ASD.5 Most of those who no longer met the criteria for ASD carried a DSM-IV diagnosis of Asperger syndrome or PDD-NOS or had an IQ over 70.5 Nevertheless, these individuals may struggle with impairing symptoms related to repetitive behaviors or communication or may be affected by learning or social-emotional disabilities. Additionally, even if they meet the criteria for ASD, some may identify with the Asperger syndrome label and fear they will be stigmatized should they be classified as having the more general ASD.6,7
Although future revisions to the DSM may include further changes in classification, grouping adults with ASD according to their functional and cognitive ability may allow for pragmatic characterization of their needs. At least 3 informal groupings of autistic adults have been described that integrate cognitive ability and independence8:
- Those with low cognitive and social abilities, who need lifelong support
- Those with midrange cognitive and social limitations but who can complete their work in special education classes; they often find employment in supervised workshops or other work with repetitive tasks
- Those who have greater cognitive ability and some social skills; they may proceed to college and employment and live independently.
UNCERTAIN PROGNOSIS
Prognostication for people with ASD remains an area of research. Some adults experience a reduction in symptoms as they age, with significant improvements in speech and, sometimes, modest improvements in restrictive and repetitive behaviors.9,10
Nevertheless, autism remains a lifelong disorder for many. Adults may still require significant support and may experience impairment, particularly in social interaction.10 In longitudinal studies, only 15% to 27% of patients with ASD are characterized as having a positive outcome (often defined as variables related to independent function, near-normal relationships, employment, or a quantified reduction in core symptoms), and many experience significant dependency into adulthood.10–13
IQ has been cited as a possible prognostic factor,10,13 with an IQ below 70 associated with poorer outcome, although an IQ above 70 does not necessarily confer a positive outcome. Less-severe impairment in speech at baseline in early childhood also suggests better outcomes in adulthood.10
As we see more adults with autism, studies that include both children and adults, such as the Longitudinal European Autism Cohort, will be important to characterize the natural history, comorbidities, and genetics of ASD and may help provide more specific predictors of disease course into adulthood.14
ACHIEVING A DIAGNOSIS FOR ADULT PATIENTS WITH SUSPECTED AUTISM
While many patients are recognized as having autism in early to mid-childhood, some adults may not receive a formal diagnosis until much later in life. Those with fluent language and normal-range IQ are likely to be overlooked.15 People with ASD may have had mild symptoms during childhood that did not impair their functioning until demands of daily life exceeded their capacities in adulthood. Alternatively, parents of a child with newly diagnosed ASD may realize that they themselves or another adult family member also show signs of it.
The UK National Institute of Health and Care Excellence suggests that assessment should be considered if the patient meets psychiatric diagnostic criteria and one of the following:
- Difficulty obtaining or sustaining employment or education
- Difficulty initiating or sustaining social relationships
- Past or current contact with mental health or learning disability services
- History of a neurodevelopmental or mental health disorder.15,16
Currently, diagnosis typically involves a multidisciplinary approach, with psychiatric assessment, neuropsychological testing, and speech and language evaluation.17 Providers may need to refer patients for these services, sometimes at the patient’s request, if previous mental health misdiagnoses are suspected, if patients report symptoms or impairment consistent with ASD, or if benefits, services, or accommodations, such as a coach in the workplace, are needed.
Diagnosing ASD in adults can be difficult, given that the gold-standard diagnostic tests such as the Autism Diagnostic Observation Schedule-2 (ADOS-2)18 and the Autism Diagnostic Interview-Revised (ADI-R)19 are typically used to diagnose autism in children. However, Module 4 in the ADOS-2 was developed for adolescents and older patients with fluent language and has shown at least moderate power to distinguish adults with ASD from those without ASD.18,20
An initial psychiatric assessment should include a thorough history taken from the patient and, if applicable, the patient’s caregiver, as well as a psychiatric interview of the patient. Neuropsychological testing should include evaluation of cognitive function, social functioning (using the ADOS-2 for adults without intellectual disability, the ADI-R, or both), and adaptive functioning (using the Vineland Adaptive Behavior Scales, second edition21).
Evaluation of speech and language is particularly important in patients with limited language ability and should include both expressive and receptive language abilities. Serial testing every few years, as is often recommended in childhood, may help establish the pattern of impairment over time.
Comorbid psychiatric disorders are common
Many people with ASD also have other psychiatric disorders,17,22 which clinicians should keep in mind when seeing an adult seeking evaluation for ASD.
Attention-deficit/hyperactivity disorder is present at higher rates in patients of average intellectual function with ASD than in the general population.23
Anxiety disorders, including obsessive-compulsive disorder, were found to often coexist with autism in a sample of adults with autism without intellectual disability,24,25 and approximately 40% of youths with ASD have at least 1 comorbid anxiety disorder.26
Mood disorders are also prevalent in adults with ASD, with a small study showing that 70% of adults with DSM-IV Asperger syndrome had at least 1 depressive episode in their lifetime.27
BEHAVIORAL AND PHARMACOLOGIC THERAPIES FOR THE ADULT PATIENT
Services and medications for adults with ASD are discussed below. These will vary by individual, and services available may vary by region.
Historically, vocational and social outcomes have been poor for adults with ASD. It is estimated that most larger universities may be home to 100 to 300 students with ASD. To combat isolation, the University of California, Los Angeles, the University of Alabama, and others provide special support services, including group social activities such as board games and individual coaching.8 Nevertheless, half of the students with autism who attend institutions of higher learning leave without completing their intended degree.29 Many still struggle to establish meaningful friendships or romantic relationships.29
Planning for a transition of care
Healthcare transition planning is important but is strikingly underused.30 Individual providers, including adult psychiatrists, vary in their level of training and comfort in diagnosing, treating, and monitoring adults with autism. Youths with ASD are half as likely to receive healthcare transition services as other youths with special healthcare needs.31
Pediatric providers, including pediatric psychiatrists, developmental behavioral specialists, and pediatric neurologists, may be best equipped to treat young adult patients or to refer patients to appropriate generalists and specialists comfortable with autism-specific transition of care. The question of eligibility for services is important to patients and families during the transition period, with many parents and professionals unaware of services available to them.32 Receiving adequate transition services is enabled by having a medical home during childhood—that is, a comprehensive, centralized medical record, culturally competent care, interaction with schools, and patient access to clear, unbiased information.31
Ideally, in our experience, transitioning should be discussed well before the child ages out of the pediatric provider’s practice. If necessary, healthcare transition services should include 4 components:
- Discussing the switch to a new physician who treats adults
- Discussing changing healthcare needs as an adult
- Planning insurance coverage as an adult
- Encouragement by the physician for the child to take age-appropriate responsibility for his or her healthcare.31,33
Tools such as the Got Transition checklist from the National Health Care Transition Center can provide support during this process.34
Other services
Other services provided as an extension or adjunct to the medical home in early adulthood may include customized vocational or employment training, specialized mentorship or support in a college setting, housing support, and psychological services.35
Community-based programs that emphasize leisure have been shown to improve participants’ independence and quality of life.36 Similarly, participants in programs that emphasized supported employment, with a job coach, on-the-job support, collaboration with the participant’s larger social support network, and selection of tasks to match an individual’s abilities and strengths, demonstrated improved cognitive performance, particularly executive functioning,37 and employment.38,39 These programs work best for patients who have mild to moderate symptoms.37,39
Patients with symptoms that are more severe may do better in a residential program. Many of these programs maintain an emphasis on vocational and social skills development. One such long-standing program is Bittersweet Farms, a rural farming community in Ohio for adults with ASD, where individuals with moderate to low function live in a group setting, with emphasis on scheduled, meaningful work including horticulture, animal care, carpentry; and activities of daily living.40
Studies of patients across the autism spectrum have generally found better outcomes when vocational support is given, but larger and randomized studies are needed to characterize how to best support these individuals after they leave high school.41
Psychological services such as applied behavioral therapy, social cognition training, cognitive behavioral therapy, and mindfulness training may be particularly useful in adults.42–44
Some versions of applied behavioral therapy, such as the Early Start Denver Model,45 have been found to be cost-effective and offset some expenses in the care of children with autism, using play-based and relationship-based interventions to promote development across domains while reducing symptoms.
In randomized controlled trials, modified cognitive behavioral therapy43 and mindfulness44 were shown to reduce symptoms of anxiety, obsessive-compulsive disorder, and depression.
Dialectical behavior therapy, used to find a balance between accepting oneself and desiring to change, may help in some circumstances to regulate emotions and reduce reactivity and lability, although large randomized clinical trials have not been conducted in the ASD population.46
Drug therapy
Medications may be appropriate to manage symptoms or comorbid conditions in adults with ASD. Over 75% adults with ASD have been found to use psychotropic medications.47 However, although these drugs have been approved for treating behaviors commonly associated with ASD, none of them provide definitive treatment for this disorder, and they have not been rigorously tested or approved for use in adults with ASD.48
Irritability and aggression associated with ASD can be treated with risperidone (approved for children over age 5), aripiprazole (approved for children ages 6–17), clozapine, or haloperidol.49
Aberrant social behavior can be treated with risperidone.50 Treatments under investigation include oxytocin and secretin.49
While no approved drug has been shown to improve social communication,51 balovaptan, a vasopressin V1a agonist, has shown potential and has been granted breakthrough status by the US Food and Drug Administration for treating challenging behaviors in adults, with additional studies ongoing in children.52,53
Repetitive behaviors, if the patient finds them impairing, can be managed with selective serotonin reuptake inhibitors.49
Much more study of drug therapy in adults with ASD is needed to fully understand the best approaches to psychotropic medication use, including appropriate classes and effective dosage, in this population.
SEX: UNEXPLORED TERRITORY
The reproductive health needs of people with autism remain largely underexplored.54 Historically, individuals with ASD were thought to have little interest in sexual activity or parenthood, owing to the nature of the core symptoms of the disorder. This has been shown to be untrue, particularly as studies on this topic began to engage in direct interviews with people with ASD, rather than solely gathering information from caregivers or parents. The findings reinforce the importance of broaching this component of health in this population, for the following reasons:
Adults with ASD are at increased risk of sexual victimization, with nearly 4 out of 5 reporting unwanted sexual advances, coercion, or rape.55
They have a smaller pool of knowledge with respect to sexual health. They report56 that they learned about sex from television and from “making mistakes.” They use fewer sources. They are less likely to speak to peers and figures of authority to gain knowledge about sexually transmitted infections, sexual behaviors, and contraception. And they are more likely to use forms of nonsocial media, such as television, for information.55
They report more concerns about the future with respect to sexual behavior, suggesting the need for targeted sexual education programs.56
College-age young adults with ASD who misread communication may be particularly affected by Title IX, which requires schools to promptly investigate reports of sexual harassment and sexual assault, should they struggle to comport themselves appropriately.57 Early and frank conversations about issues of consent and appropriate displays of interest and affection may better equip youth to navigate new social scenarios as they plan to leave a supervised home environment for college or the workforce.
Gender identification: Male, female, other
In one study, 77.8% of birth-sex males with ASD said they identified as men, and 67.1% of birth-sex females identified as women, compared with 93.1% of birth-sex males and 87.3% of birth-sex females without ASD. Many of the remaining individuals with ASD reported a transgender, genderqueer, or other gender identity.58 Some studies have found females with ASD report a gay or bisexual orientation more often than males with ASD.59–61
Adolescents and young adults may be exploring their changing bodies, sexual preferences, and gender roles, and as for all people at this age, these roles emerge against a backdrop of familial and societal expectations that may or may not be concordant with their own projected path regarding sexuality and reproductive health.62
Having the conversation
As with non-ASD patients, a thorough sexual history should be collected via open-ended questions when possible to determine types of sexual activity and partners.
Education of the patient, alongside caregivers and parents, about healthy and safe sexual practices, screening for sexual violence, and hormonal and nonhormonal contraception options are important components of care for this population.
CAREGIVER STRESS MAY PERSIST INTO PATIENT’S ADULTHOOD
Caregiver burden is a monumental concern for parents or others who may have lifelong primary responsibility for these neurodiverse adults.63 Family members may feel isolated and may feel they have encountered many barriers to services.64 Remaining sensitive, knowledgeable, and inquisitive about the types of support that are needed may help forge a trusting relationship between the provider and the family.
Parents of children with ASD have been reported to experience worse physical and emotional health than parents whose children do not have developmental disabilities.63,65 These disparities have been found to persist as their children enter adolescence and young adulthood.66,67 Parents of children with ASD report more anxiety, depression, and distress compared with parents of children without ASD,63 and parents themselves may be affected by ASD symptoms, which has been linked to increased parenting stress.68 Some studies have found blunted cortisol responses,63,69,70 and some,71 but not all,63 have found elevated blood pressure in caregivers of children with developmental disabilities. Headache, backache, muscle soreness, and fatigue may also be commonly reported.67
In our experience, caregivers are tremendously appreciative when provided connections to adult ASD services and support systems as their child ages. The school system and other formal support systems often assist until the time of transition into adulthood. This transition can be stressful for the adolescent and family alike, and informal support systems such as friends and family may become increasingly crucial, particularly if the adolescent still lives at home.72,73
The affected young adult’s unmet needs, as perceived by the caregiver, have been found to be significantly associated with caregiver burden, whereas the severity of the adult patient’s ASD symptoms has not.66 Therefore, it may be helpful to ask caregivers whether they perceive any unmet needs, regardless of the clinician’s perception of the severity of the patient’s ASD symptoms. Providing support to address these needs, particularly those relating to the child’s mood disorders, communication, social needs, safety, and daytime activities, may be the domains of support that most effectively reduce the caregiver burden in this population.66
Caregiver positivity, lower stress levels, and increased social support, particularly in the form of friends and family members providing no-cost assistance to caregivers whose children do not live independently,74 have been linked to better outcomes for caregivers.70,74,75 Rigorous studies that examine caregiver burden as individuals with ASD enter mid- and late-adulthood are limited.
THE ROLE OF THE INTERNIST IN CARING FOR ADULTS WITH AUTISM
A major challenge for many adults with ASD is the transition from services provided during childhood to those provided in adulthood. While children with autism have subspecialty providers who diagnose and manage their condition, including developmental-behavioral pediatricians, pediatric neurologists, and child psychiatrists, adults with autism may have fewer options.
Autism centers are becoming more available across the nation, and many provide care across the life span. However, depending on a patient’s needs, the primary care provider may need to manage residual symptoms as the patient transitions from pediatric to adult care, ultimately deciding when and where to refer the patient.
The patient’s family should pay close attention to function and mood around the time the patient leaves the structure of high school, and they should build rapport with a primary care provider they can turn to if problems persist or arise. Referrals for behavioral therapy and for social work, job training, and vocational support can greatly benefit patients as they transition to young adulthood. Referrals and suggestions for social support can also help caregivers.
Medical care
Deciding when and how to medicate the patient for symptoms of autism and related behaviors necessitates consideration of the patient’s impairment, side effects of the medication, and the impact medications may have on the patient’s other conditions. Disordered eating, mood problems, anxiety, and attention-deficit/hyperactivity disorder should be considered, and, as in all patients, regular screenings of mental health status should be conducted.76,77
Comorbid medical conditions may cause worsening of a patient’s known behavioral symptoms or may precipitate new behaviors or aggression as a result of pain or discomfort, particularly in patients with limited speech. A change in stereotypes or increased irritability warrants a thoughtful investigation for a cause other than ASD before adding or increasing behavioral medications. Common comorbid conditions include gastrointestinal distress, most commonly constipation and diarrhea in an idiopathic ASD population, with increasing ASD symptom severity correlating with increased odds of a gastrointestinal problem.78 Allergies, sleep disorders, seizures, and other psychiatric conditions are also frequent.79
Preventive care, including vaccinations, should be given as scheduled. Caregivers and patients can be reminded if needed that vaccines do not cause or worsen autism, and vaccination is intended to improve the safety of the patient and those around them, protecting against potentially life-threatening disease. Regular dental care visits, particularly for patients who are using medications that may affect tooth or gingival health,80 and regular visits to an optometrist or ophthalmologist for screening of vision are also advised.
Adverse effects. Weight gain and metabolic syndrome are common adverse effects of medications used for behavioral management, and the primary care physician may uncover diabetes, cardiac disorders, and hyperlipidemia. Patients with ASD may be particularly sensitive to the effects of medications and therefore may require a lower dose or a slower titration than other patients. Working with a behavioral team, careful weaning of psychiatric medications to the minimum needed is strongly recommended whenever possible.81
TAKE-HOME POINTS
As more adults with autism enter society, they may require varying levels of support from the healthcare community to ensure that therapeutic gains from childhood persist, allowing them to achieve maximal functional potential.
Adults with ASD may have a high, normal, or low IQ and intellectual capability. Knowledge of this and of the patient’s symptom severity and presence of comorbid psychiatric and other health conditions can help the clinician guide the patient to appropriate social services and pharmacologic treatments.
Individualized support in the workplace, as well as education regarding sexual health, can help improve outcomes for affected individuals.
Caregiver burden for individuals with autism can be high, but it can be mitigated by social support.
Further research regarding appropriate diagnostic instruments in adulthood and appropriate treatments for impairing autism-related symptoms across the life span may be particularly helpful in supporting this patient population.
- Autism and Developmental Disabilities Monitoring Network Surveillance Year 2000 Principal Investigators; Centers for Disease Control and Prevention. Prevalence of autism spectrum disorders—autism and developmental disabilities monitoring network, six sites, United States, 2000. MMWR Surveill Summ 2007; 56(1):1–11. pmid:17287714
- Christensen DL. Prevalence and characteristics of autism spectrum disorder among children aged 8 years—Autism and Developmental Disabilities Monitoring Network, 11 sites, United States, 2012. MMWR Surveill Summ 2016; 65(13):1–23. doi:10.15585/mmwr.ss6503a1
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th edition. Washington, D.C: American Psychiatric Association; 2013.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed., Text Revision. Washington, DC: American Psychiatric Association; 2000.
- Smith IC, Reichow B, Volkmar FR. The effects of DSM-5 criteria on number of individuals diagnosed with autism spectrum disorder: a systematic review. J Autism Dev Disord 2015; 45(8):2541–2552. doi:10.1007/s10803-015-2423-8
- Barahona-Corrêa JB, Filipe CN. A concise history of Asperger syndrome: the short reign of a troublesome diagnosis. Front Psychol 2015; 6:2024. doi:10.3389/fpsyg.2015.02024
- Kite DM, Gullifer J, Tyson GA. Views on the diagnostic labels of autism and Asperger’s disorder and the proposed changes in the DSM. J Autism Dev Disord 2013; 43(7):1692–1700. doi:10.1007/s10803-012-1718-2
- Kuo AA. Autism in adults: an update. Presented at the: American College of Physicians Internal Medicine Meeting, New Orleans, LA, April 17–21, 2018.
- Shattuck PT, Seltzer MM, Greenberg JS, et al. Change in autism symptoms and maladaptive behaviors in adolescents and adults with an autism spectrum disorder. J Autism Dev Disord 2007; 37(9):1735–1747. doi:10.1007/s10803-006-0307-7
- Seltzer MM, Shattuck P, Abbeduto L, Greenberg JS. Trajectory of development in adolescents and adults with autism. Ment Retard Dev Disabil Res Rev 2004; 10(4):234–247. doi:10.1002/mrdd.20038
- Billstedt E, Carina Gillberg I, Gillberg C. Autism in adults: symptom patterns and early childhood predictors. Use of the DISCO in a community sample followed from childhood. J Child Psychol Psychiatry 2007; 48(11):1102–1110. doi:10.1111/j.1469-7610.2007.01774.x
- Howlin P, Goode S, Hutton J, Rutter M. Adult outcome for children with autism. J Child Psychol Psychiatry 2004; 45(2):212–229. pmid:14982237
- Marriage S, Wolverton A, Marriage K. Autism spectrum disorder grown up: a chart review of adult functioning. J Can Acad Child Adolesc Psychiatry 2009; 18(4):322–328. pmid: 19881941
- Isaksson J, Tammimies K, Neufeld J, et al. EU-AIMS Longitudinal European Autism Project (LEAP): the autism twin cohort. Mol Autism 2018; 9(1):26. doi:10.1186/s13229-018-0212-x
- Lai M-C, Baron-Cohen S. Identifying the lost generation of adults with autism spectrum conditions. Lancet Psychiatry 2015; 2(11):1013–1027. doi:10.1016/S2215-0366(15)00277-1
- National Institute for Health and Clinical Excellence. Autism: recognition, referral, diagnosis and management of adults on the autism spectrum. NICE clinical guideline 142. June 2012. https://grand.tghn.org/site_media/media/medialibrary/2015/03/ASD_NICE_3_.pdf. Accessed July 9, 2019.
- Wolf JM, Ventola P. Assessment and treatment planning in adults with autism spectrum disorders. In: Adolescents and Adults with Autism Spectrum Disorders. Springer, New York, NY; 2014:283–298.
- Lord C, Rutter M, DiLavore P, Risi S, Gotham K, Bishop SL. Autism Diagnostic Observation Schedule, Second Edition (ADOS-2) manual. Torrance, CA: Western Psychological Services, 2012.
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord 1994; 24(5):659–685.
- Hus V, Lord C. The autism diagnostic observation schedule, module 4: revised algorithm and standardized severity scores. J Autism Dev Disord 2014; 44(8):1996–2012. doi:10.1007/s10803-014-2080-3>
- Sparrow S, Balla D, Cicchetti D, Harrison P, Doll E. Vineland Adaptive Behavior Scales. Circle Pines, MN: American Guidance Service, 1984.
- Happé FG, Mansour H, Barrett P, Brown T, Abbott P, Charlton RA. Demographic and cognitive profile of individuals seeking a diagnosis of autism spectrum disorder in adulthood. J Autism Dev Disord 2016; 46(11):3469–3480. doi:10.1007/s10803-016-2886-2
- Johnston K, Dittner A, Bramham J, Murphy C, Knight A, Russell A. Attention deficit hyperactivity disorder symptoms in adults with autism spectrum disorders. Autism Res Off J Int Soc Autism Res 2013; 6(4):225–236. doi:10.1002/aur.1283
- Cadman T, Spain D, Johnston P, et al. Obsessive-compulsive disorder in adults with high-functioning autism spectrum disorder: what does self-report with the OCI-R tell us? Autism Res Off J Int Soc Autism Res 2015; 8(5):477–485. doi:10.1002/aur.1461
- Russell AJ, Mataix-Cols D, Anson M, Murphy DGM. Obsessions and compulsions in Asperger syndrome and high-functioning autism. Br J Psychiatry J Ment Sci 2005; 186:525–528. doi:10.1192/bjp.186.6.525
- Simonoff E, Pickles A, Charman T, Chandler S, Loucas T, Baird G. Psychiatric disorders in children with autism spectrum disorders: prevalence, comorbidity, and associated factors in a population-derived sample. J Am Acad Child Adolesc Psychiatry 2008; 47(8):921–929. doi:10.1097/CHI.0b013e318179964f
- Lugnegård T, Hallerbäck MU, Gillberg C. Psychiatric comorbidity in young adults with a clinical diagnosis of Asperger syndrome. Res Dev Disabil 2011; 32(5):1910–1917. doi:10.1016/j.ridd.2011.03.025
- Howlin P, Moss P. Adults with autism spectrum disorders. Can J Psychiatry 2012; 57(5):275–283. doi:10.1177/070674371205700502
- Levy A, Perry A. Outcomes in adolescents and adults with autism: a review of the literature. Res Autism Spectr Disord 2011; 5(4):1271–1282. doi:10.1016/J.RASD.2011.01.023
- Cheak-Zamora NC, Yang X, Farmer JE, Clark M. Disparities in transition planning for youth with autism spectrum disorder. Pediatrics 2013; 131(3):447–454. doi:10.1542/peds.2012-1572
- Rast JE, Shattuck PT, Roux AM, Anderson KA, Kuo A. The medical home and health care transition for youth with autism. Pediatrics 2018; 141(suppl 4):S328–S334. doi:10.1542/peds.2016-4300J
- Belling R, McLaren S, Paul M, et al. The effect of organisational resources and eligibility issues on transition from child and adolescent to adult mental health services. J Health Serv Res Policy 2014; 19(3):169–176. doi:10.1177/1355819614527439
- Data Resource Center for Child & Adolescent Health. 2009–2010 National Survey of Children with Special Health Care Needs. www.childhealthdata.org/docs/drc/200910-cshcn-spss-codebook_final_051012.pdf?sfvrsn=1. Accessed July 9, 2019.
- Got Transition Center for Health Care Transition Improvement. Six core elements of health care transition 2.0. Transitioning youth to an adult health care provider. For use by pediatric, family medicine, and med-peds providers. www.gottransition.org/resourceGet.cfm?id=208. Accessed July 9, 2019.
- Murphy CM, Wilson CE, Robertson DM, et al. Autism spectrum disorder in adults: diagnosis, management, and health services development. Neuropsychiatr Dis Treat 2016; 12:1669–1686. doi:10.2147/NDT.S65455
- García-Villamisar DA, Dattilo J. Effects of a leisure programme on quality of life and stress of individuals with ASD. J Intellect Disabil Res 2010; 54(7):611–619. doi:10.1111/j.1365-2788.2010.01289.x
- García-Villamisar D, Hughes C. Supported employment improves cognitive performance in adults with autism. J Intellect Disabil Res 2007; 51(pt 2):142–150. doi:10.1111/j.1365-2788.2006.00854.x
- Lawer L, Brusilovskiy E, Salzer MS, Mandell DS. Use of vocational rehabilitative services among adults with autism. J Autism Dev Disord 2009; 39(3):487–494. doi:10.1007/s10803-008-0649-4
- Howlin P, Alcock J, Burkin C. An 8 year follow-up of a specialist supported employment service for high-ability adults with autism or Asperger syndrome. Autism 2005; 9(5):533–549. doi:10.1177/1362361305057871
- Kay BR. Bittersweet Farms. J Autism Dev Disord 1990; 20(3):309–321. http://www.ncbi.nlm.nih.gov/pubmed/2228914. Accessed July 9, 2019.
- Taylor JL, McPheeters ML, Sathe NA, Dove D, Veenstra-Vanderweele J, Warren Z. A systematic review of vocational interventions for young adults with autism spectrum disorders. Pediatrics 2012; 130(3):531–538. doi:10.1542/peds.2012-0682
- Bishop-Fitzpatrick L, Minshew NJ, Eack SM. A systematic review of psychosocial interventions for adults with autism spectrum disorders. J Autism Dev Disord 2013; 43(3):687–694. doi:10.1007/s10803-012-1615-8
- Russell AJ, Jassi A, Fullana MA, et al. Cognitive behavior therapy for comorbid obsessive-compulsive disorder in high-functioning autism spectrum disorders: a randomized controlled trial. Depress Anxiety 2013; 30(8):697–708. doi:10.1002/da.22053
- Spek AA, van Ham NC, Nyklícek I. Mindfulness-based therapy in adults with an autism spectrum disorder: a randomized controlled trial. Res Dev Disabil 2013; 34(1):246–253. doi:10.1016/j.ridd.2012.08.009
- Eapen V, Crncec R, Walter A. Clinical outcomes of an early intervention program for preschool children with autism spectrum disorder in a community group setting. BMC Pediatr 2013; 13(1):3. doi:10.1186/1471-2431-13-3
- Mazefsky CA, White SW. Emotion regulation: concepts & practice in autism spectrum disorder. Child Adolesc Psychiatr Clin North Am 2014; 23(1):15–24. doi:10.1016/J.CHC.2013.07.002
- Esbensen AJ, Greenberg JS, Seltzer MM, Aman MG. A longitudinal investigation of psychotropic and non-psychotropic medication use among adolescents and adults with autism spectrum disorders. J Autism Dev Disord 2009; 39(9):1339–1349. doi:10.1007/s10803-009-0750-3
- Dove D, Warren Z, McPheeters ML, Taylor JL, Sathe NA, Veenstra-VanderWeele J. Medications for adolescents and young adults with autism spectrum disorders: a systematic review. Pediatrics 2012; 130(4):717–726. doi:10.1542/peds.2012-0683
- LeClerc S, Easley D. Pharmacological therapies for autism spectrum disorder: a review. Pharm Ther 2015; 40(6):389–397.
- Miral S, Gencer O, Inal-Emiroglu FN, Baykara B, Baykara A, Dirik E. Risperidone versus haloperidol in children and adolescents with AD: a randomized, controlled, double-blind trial. Eur Child Adolesc Psychiatry 2008; 17(1):1–8. doi:10.1007/s00787-007-0620-5
- Lai M-C, Lombardo MV, Baron-Cohen S. Autism. Lancet 2014; 383(9920):896–910. doi:10.1016/S0140-6736(13)61539-1
- Ratni H, Rogers-Evans M, Bissantz C, et al. Discovery of highly selective brain-penetrant vasopressin 1a antagonists for the potential treatment of autism via a chemogenomic and scaffold hopping approach. J Med Chem 2015; 58(5):2275–2289. doi:10.1021/jm501745f
- Umbricht D, Del Valle Rubido M, Hollander E, et al. A single dose, randomized, controlled proof-of-mechanism study of a novel vasopressin 1a receptor antagonist (RG7713) in high-functioning adults with autism spectrum disorder. Neuropsychopharmacology 2017; 42(9):1914–1923. doi:10.1038/npp.2016.232>
- Kellaher DC. Sexual behavior and autism spectrum disorders: an update and discussion. Curr Psychiatry Rep 2015; 17(4):25. doi:10.1007/s11920-015-0562-4
- Brown-Lavoie SM, Viecili MA, Weiss JA. Sexual knowledge and victimization in adults with autism spectrum disorders. J Autism Dev Disord 2014; 44(9):2185–2196. doi:10.1007/s10803-014-2093-y
- Mehzabin P, Stokes MA. Self-assessed sexuality in young adults with high-functioning autism. Res Autism Spectr Disord 2011; 5(1):614–621. doi:10.1016/J.RASD.2010.07.006>
- Brown KR. Accessibility for students with ASD: legal perspectives in the United States. In: Alphin HC Jr. Exploring the Future of Accessibility in Higher Education. Hershey, PA: IGI Global; 2017.
- George R, Stokes MA. Gender identity and sexual orientation in autism spectrum disorder. Autism 2018; 22(8):970–982. doi:10.1177/1362361317714587
- Byers ES, Nichols S, Voyer SD. Challenging stereotypes: sexual functioning of single adults with high functioning autism spectrum disorder. J Autism Dev Disord 2013; 43(11):2617–2627. doi:10.1007/s10803-013-1813-z
- Gilmour L, Schalomon PM, Smith V. Sexuality in a community based sample of adults with autism spectrum disorder. Res Autism Spectr Disord 2012; 6(1):313–318. doi:10.1016/J.RASD.2011.06.003
- Bejerot S, Eriksson JM. Sexuality and gender role in autism spectrum disorder: a case control study. Schmitz C, ed. PLoS One 2014; 9(1):e87961. doi:10.1371/journal.pone.0087961>
- Navot N, Jorgenson AG, Webb SJ. Maternal experience raising girls with autism spectrum disorder: a qualitative study. Child Care Health Dev 2017; 43(4):536–545. doi:10.1111/cch.12470
- Padden C, James JE. Stress among parents of children with and without autism spectrum disorder: a comparison involving physiological indicators and parent self-reports. J Dev Phys Disabil 2017; 29(4):567–586. doi:10.1007/s10882-017-9547-z
- Woodgate RL, Ateah C, Secco L. Living in a world of our own: the experience of parents who have a child with autism. Qual Health Res 2008; 18(8):1075–1083. doi:10.1177/1049732308320112
- Hayes SA, Watson SL. The impact of parenting stress: a meta-analysis of studies comparing the experience of parenting stress in parents of children with and without autism spectrum disorder. J Autism Dev Disord 2013; 43(3):629–642. doi:10.1007/s10803-012-1604-y
- Cadman T, Eklund H, Howley D, et al. Caregiver burden as people with autism spectrum disorder and attention-deficit/hyperactivity disorder transition into adolescence and adulthood in the United Kingdom. J Am Acad Child Adolesc Psychiatry 2012; 51(9):879–888. doi:10.1016/j.jaac.2012.06.017
- Smith LE, Seltzer MM, Greenberg JS. Daily health symptoms of mothers of adolescents and adults with fragile x syndrome and mothers of adolescents and adults with autism spectrum disorder. J Autism Dev Disord 2012; 42(9):1836–1846. doi:10.1007/s10803-011-1422-7
- van Steijn DJ, Oerlemans AM, van Aken MAG, Buitelaar JK, Rommelse NNJ. The reciprocal relationship of ASD, ADHD, depressive symptoms and stress in parents of children with ASD and/or ADHD. J Autism Dev Disord 2014; 44(5):1064–1076. doi:10.1007/s10803-013-1958-9
- Seltzer MM, Greenberg JS, Hong J, et al. Maternal cortisol levels and behavior problems in adolescents and adults with ASD. J Autism Dev Disord 2010; 40(4):457–469. doi:10.1007/S10803-009-0887-0
- Lovell B, Moss M, Wetherell MA. With a little help from my friends: psychological, endocrine and health corollaries of social support in parental caregivers of children with autism or ADHD. Res Dev Disabil 2012; 33(2):682–687. doi:10.1016/j.ridd.2011.11.014
- Gallagher S, Whiteley J. Social support is associated with blood pressure responses in parents caring for children with developmental disabilities. Res Dev Disabil 2012; 33(6):2099–2105. doi:10.1016/j.ridd.2012.06.007
- Baker JK, Smith LE, Greenberg JS, Seltzer MM, Taylor JL. Change in maternal criticism and behavior problems in adolescents and adults with autism across a 7-year period. J Abnorm Psychol 2011; 120(2):465–475. doi:10.1037/a0021900
- Marsack CN, Samuel PS. Mediating effects of social support on quality of life for parents of adults with autism. J Autism Dev Disord 2017; 47(8):2378–2389. doi:10.1007/s10803-017-3157-6
- Trute B, Benzies KM, Worthington C, Reddon JR, Moore M. Accentuate the positive to mitigate the negative: mother psychological coping resources and family adjustment in childhood disability. J Intellect Dev Disabil 2010; 35(1):36–43. doi:10.3109/13668250903496328
- Cantwell J, Muldoon OT, Gallagher S. Social support and mastery influence the association between stress and poor physical health in parents caring for children with developmental disabilities. Res Dev Disabil 2014; 35(9):2215–2223. doi:10.1016/j.ridd.2014.05.012
- Carton AM, Smith AD. Assessing the relationship between eating disorder psychopathology and autistic traits in a non-clinical adult population. Eat Weight Disord - Stud Anorexia, Bulim Obes 2014; 19(3):285–293. doi:10.1007/s40519-013-0086-z
- De Alwis D, Agrawal A, Reiersen AM, et al. ADHD symptoms, autistic traits, and substance use and misuse in adult Australian twins. J Stud Alcohol Drugs 2014; 75(2):211–221. doi:10.15288/jsad.2014.75.211
- Wang LW, Tancredi DJ, Thomas DW. The prevalence of gastrointestinal problems in children across the United States with autism spectrum disorders from families with multiple affected members. J Dev Behav Pediatr 2011; 32(5):351–360. doi:10.1097/DBP.0b013e31821bd06a
- Croen LA, Zerbo O, Qian Y, et al. The health status of adults on the autism spectrum. Autism 2015; 19(7):814–823. doi:10.1177/1362361315577517
- Kalyoncu IÖ, Tanboga I. Oral health status of children with autistic spectrum disorder compared with non-authentic peers. Iran J Public Health 2017; 46(11):1591–1593. www.ncbi.nlm.nih.gov/pmc/articles/PMC5696703. Accessed July 9, 2019.
- McGuire K, Fung LK, Hagopian L, et al. Irritability and problem behavior in autism spectrum disorder: a practice pathway for pediatric primary care. Pediatrics 2016; 137(suppl 2):S136–S148. doi:10.1542/peds.2015-2851L
- Autism and Developmental Disabilities Monitoring Network Surveillance Year 2000 Principal Investigators; Centers for Disease Control and Prevention. Prevalence of autism spectrum disorders—autism and developmental disabilities monitoring network, six sites, United States, 2000. MMWR Surveill Summ 2007; 56(1):1–11. pmid:17287714
- Christensen DL. Prevalence and characteristics of autism spectrum disorder among children aged 8 years—Autism and Developmental Disabilities Monitoring Network, 11 sites, United States, 2012. MMWR Surveill Summ 2016; 65(13):1–23. doi:10.15585/mmwr.ss6503a1
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th edition. Washington, D.C: American Psychiatric Association; 2013.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed., Text Revision. Washington, DC: American Psychiatric Association; 2000.
- Smith IC, Reichow B, Volkmar FR. The effects of DSM-5 criteria on number of individuals diagnosed with autism spectrum disorder: a systematic review. J Autism Dev Disord 2015; 45(8):2541–2552. doi:10.1007/s10803-015-2423-8
- Barahona-Corrêa JB, Filipe CN. A concise history of Asperger syndrome: the short reign of a troublesome diagnosis. Front Psychol 2015; 6:2024. doi:10.3389/fpsyg.2015.02024
- Kite DM, Gullifer J, Tyson GA. Views on the diagnostic labels of autism and Asperger’s disorder and the proposed changes in the DSM. J Autism Dev Disord 2013; 43(7):1692–1700. doi:10.1007/s10803-012-1718-2
- Kuo AA. Autism in adults: an update. Presented at the: American College of Physicians Internal Medicine Meeting, New Orleans, LA, April 17–21, 2018.
- Shattuck PT, Seltzer MM, Greenberg JS, et al. Change in autism symptoms and maladaptive behaviors in adolescents and adults with an autism spectrum disorder. J Autism Dev Disord 2007; 37(9):1735–1747. doi:10.1007/s10803-006-0307-7
- Seltzer MM, Shattuck P, Abbeduto L, Greenberg JS. Trajectory of development in adolescents and adults with autism. Ment Retard Dev Disabil Res Rev 2004; 10(4):234–247. doi:10.1002/mrdd.20038
- Billstedt E, Carina Gillberg I, Gillberg C. Autism in adults: symptom patterns and early childhood predictors. Use of the DISCO in a community sample followed from childhood. J Child Psychol Psychiatry 2007; 48(11):1102–1110. doi:10.1111/j.1469-7610.2007.01774.x
- Howlin P, Goode S, Hutton J, Rutter M. Adult outcome for children with autism. J Child Psychol Psychiatry 2004; 45(2):212–229. pmid:14982237
- Marriage S, Wolverton A, Marriage K. Autism spectrum disorder grown up: a chart review of adult functioning. J Can Acad Child Adolesc Psychiatry 2009; 18(4):322–328. pmid: 19881941
- Isaksson J, Tammimies K, Neufeld J, et al. EU-AIMS Longitudinal European Autism Project (LEAP): the autism twin cohort. Mol Autism 2018; 9(1):26. doi:10.1186/s13229-018-0212-x
- Lai M-C, Baron-Cohen S. Identifying the lost generation of adults with autism spectrum conditions. Lancet Psychiatry 2015; 2(11):1013–1027. doi:10.1016/S2215-0366(15)00277-1
- National Institute for Health and Clinical Excellence. Autism: recognition, referral, diagnosis and management of adults on the autism spectrum. NICE clinical guideline 142. June 2012. https://grand.tghn.org/site_media/media/medialibrary/2015/03/ASD_NICE_3_.pdf. Accessed July 9, 2019.
- Wolf JM, Ventola P. Assessment and treatment planning in adults with autism spectrum disorders. In: Adolescents and Adults with Autism Spectrum Disorders. Springer, New York, NY; 2014:283–298.
- Lord C, Rutter M, DiLavore P, Risi S, Gotham K, Bishop SL. Autism Diagnostic Observation Schedule, Second Edition (ADOS-2) manual. Torrance, CA: Western Psychological Services, 2012.
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord 1994; 24(5):659–685.
- Hus V, Lord C. The autism diagnostic observation schedule, module 4: revised algorithm and standardized severity scores. J Autism Dev Disord 2014; 44(8):1996–2012. doi:10.1007/s10803-014-2080-3>
- Sparrow S, Balla D, Cicchetti D, Harrison P, Doll E. Vineland Adaptive Behavior Scales. Circle Pines, MN: American Guidance Service, 1984.
- Happé FG, Mansour H, Barrett P, Brown T, Abbott P, Charlton RA. Demographic and cognitive profile of individuals seeking a diagnosis of autism spectrum disorder in adulthood. J Autism Dev Disord 2016; 46(11):3469–3480. doi:10.1007/s10803-016-2886-2
- Johnston K, Dittner A, Bramham J, Murphy C, Knight A, Russell A. Attention deficit hyperactivity disorder symptoms in adults with autism spectrum disorders. Autism Res Off J Int Soc Autism Res 2013; 6(4):225–236. doi:10.1002/aur.1283
- Cadman T, Spain D, Johnston P, et al. Obsessive-compulsive disorder in adults with high-functioning autism spectrum disorder: what does self-report with the OCI-R tell us? Autism Res Off J Int Soc Autism Res 2015; 8(5):477–485. doi:10.1002/aur.1461
- Russell AJ, Mataix-Cols D, Anson M, Murphy DGM. Obsessions and compulsions in Asperger syndrome and high-functioning autism. Br J Psychiatry J Ment Sci 2005; 186:525–528. doi:10.1192/bjp.186.6.525
- Simonoff E, Pickles A, Charman T, Chandler S, Loucas T, Baird G. Psychiatric disorders in children with autism spectrum disorders: prevalence, comorbidity, and associated factors in a population-derived sample. J Am Acad Child Adolesc Psychiatry 2008; 47(8):921–929. doi:10.1097/CHI.0b013e318179964f
- Lugnegård T, Hallerbäck MU, Gillberg C. Psychiatric comorbidity in young adults with a clinical diagnosis of Asperger syndrome. Res Dev Disabil 2011; 32(5):1910–1917. doi:10.1016/j.ridd.2011.03.025
- Howlin P, Moss P. Adults with autism spectrum disorders. Can J Psychiatry 2012; 57(5):275–283. doi:10.1177/070674371205700502
- Levy A, Perry A. Outcomes in adolescents and adults with autism: a review of the literature. Res Autism Spectr Disord 2011; 5(4):1271–1282. doi:10.1016/J.RASD.2011.01.023
- Cheak-Zamora NC, Yang X, Farmer JE, Clark M. Disparities in transition planning for youth with autism spectrum disorder. Pediatrics 2013; 131(3):447–454. doi:10.1542/peds.2012-1572
- Rast JE, Shattuck PT, Roux AM, Anderson KA, Kuo A. The medical home and health care transition for youth with autism. Pediatrics 2018; 141(suppl 4):S328–S334. doi:10.1542/peds.2016-4300J
- Belling R, McLaren S, Paul M, et al. The effect of organisational resources and eligibility issues on transition from child and adolescent to adult mental health services. J Health Serv Res Policy 2014; 19(3):169–176. doi:10.1177/1355819614527439
- Data Resource Center for Child & Adolescent Health. 2009–2010 National Survey of Children with Special Health Care Needs. www.childhealthdata.org/docs/drc/200910-cshcn-spss-codebook_final_051012.pdf?sfvrsn=1. Accessed July 9, 2019.
- Got Transition Center for Health Care Transition Improvement. Six core elements of health care transition 2.0. Transitioning youth to an adult health care provider. For use by pediatric, family medicine, and med-peds providers. www.gottransition.org/resourceGet.cfm?id=208. Accessed July 9, 2019.
- Murphy CM, Wilson CE, Robertson DM, et al. Autism spectrum disorder in adults: diagnosis, management, and health services development. Neuropsychiatr Dis Treat 2016; 12:1669–1686. doi:10.2147/NDT.S65455
- García-Villamisar DA, Dattilo J. Effects of a leisure programme on quality of life and stress of individuals with ASD. J Intellect Disabil Res 2010; 54(7):611–619. doi:10.1111/j.1365-2788.2010.01289.x
- García-Villamisar D, Hughes C. Supported employment improves cognitive performance in adults with autism. J Intellect Disabil Res 2007; 51(pt 2):142–150. doi:10.1111/j.1365-2788.2006.00854.x
- Lawer L, Brusilovskiy E, Salzer MS, Mandell DS. Use of vocational rehabilitative services among adults with autism. J Autism Dev Disord 2009; 39(3):487–494. doi:10.1007/s10803-008-0649-4
- Howlin P, Alcock J, Burkin C. An 8 year follow-up of a specialist supported employment service for high-ability adults with autism or Asperger syndrome. Autism 2005; 9(5):533–549. doi:10.1177/1362361305057871
- Kay BR. Bittersweet Farms. J Autism Dev Disord 1990; 20(3):309–321. http://www.ncbi.nlm.nih.gov/pubmed/2228914. Accessed July 9, 2019.
- Taylor JL, McPheeters ML, Sathe NA, Dove D, Veenstra-Vanderweele J, Warren Z. A systematic review of vocational interventions for young adults with autism spectrum disorders. Pediatrics 2012; 130(3):531–538. doi:10.1542/peds.2012-0682
- Bishop-Fitzpatrick L, Minshew NJ, Eack SM. A systematic review of psychosocial interventions for adults with autism spectrum disorders. J Autism Dev Disord 2013; 43(3):687–694. doi:10.1007/s10803-012-1615-8
- Russell AJ, Jassi A, Fullana MA, et al. Cognitive behavior therapy for comorbid obsessive-compulsive disorder in high-functioning autism spectrum disorders: a randomized controlled trial. Depress Anxiety 2013; 30(8):697–708. doi:10.1002/da.22053
- Spek AA, van Ham NC, Nyklícek I. Mindfulness-based therapy in adults with an autism spectrum disorder: a randomized controlled trial. Res Dev Disabil 2013; 34(1):246–253. doi:10.1016/j.ridd.2012.08.009
- Eapen V, Crncec R, Walter A. Clinical outcomes of an early intervention program for preschool children with autism spectrum disorder in a community group setting. BMC Pediatr 2013; 13(1):3. doi:10.1186/1471-2431-13-3
- Mazefsky CA, White SW. Emotion regulation: concepts & practice in autism spectrum disorder. Child Adolesc Psychiatr Clin North Am 2014; 23(1):15–24. doi:10.1016/J.CHC.2013.07.002
- Esbensen AJ, Greenberg JS, Seltzer MM, Aman MG. A longitudinal investigation of psychotropic and non-psychotropic medication use among adolescents and adults with autism spectrum disorders. J Autism Dev Disord 2009; 39(9):1339–1349. doi:10.1007/s10803-009-0750-3
- Dove D, Warren Z, McPheeters ML, Taylor JL, Sathe NA, Veenstra-VanderWeele J. Medications for adolescents and young adults with autism spectrum disorders: a systematic review. Pediatrics 2012; 130(4):717–726. doi:10.1542/peds.2012-0683
- LeClerc S, Easley D. Pharmacological therapies for autism spectrum disorder: a review. Pharm Ther 2015; 40(6):389–397.
- Miral S, Gencer O, Inal-Emiroglu FN, Baykara B, Baykara A, Dirik E. Risperidone versus haloperidol in children and adolescents with AD: a randomized, controlled, double-blind trial. Eur Child Adolesc Psychiatry 2008; 17(1):1–8. doi:10.1007/s00787-007-0620-5
- Lai M-C, Lombardo MV, Baron-Cohen S. Autism. Lancet 2014; 383(9920):896–910. doi:10.1016/S0140-6736(13)61539-1
- Ratni H, Rogers-Evans M, Bissantz C, et al. Discovery of highly selective brain-penetrant vasopressin 1a antagonists for the potential treatment of autism via a chemogenomic and scaffold hopping approach. J Med Chem 2015; 58(5):2275–2289. doi:10.1021/jm501745f
- Umbricht D, Del Valle Rubido M, Hollander E, et al. A single dose, randomized, controlled proof-of-mechanism study of a novel vasopressin 1a receptor antagonist (RG7713) in high-functioning adults with autism spectrum disorder. Neuropsychopharmacology 2017; 42(9):1914–1923. doi:10.1038/npp.2016.232>
- Kellaher DC. Sexual behavior and autism spectrum disorders: an update and discussion. Curr Psychiatry Rep 2015; 17(4):25. doi:10.1007/s11920-015-0562-4
- Brown-Lavoie SM, Viecili MA, Weiss JA. Sexual knowledge and victimization in adults with autism spectrum disorders. J Autism Dev Disord 2014; 44(9):2185–2196. doi:10.1007/s10803-014-2093-y
- Mehzabin P, Stokes MA. Self-assessed sexuality in young adults with high-functioning autism. Res Autism Spectr Disord 2011; 5(1):614–621. doi:10.1016/J.RASD.2010.07.006>
- Brown KR. Accessibility for students with ASD: legal perspectives in the United States. In: Alphin HC Jr. Exploring the Future of Accessibility in Higher Education. Hershey, PA: IGI Global; 2017.
- George R, Stokes MA. Gender identity and sexual orientation in autism spectrum disorder. Autism 2018; 22(8):970–982. doi:10.1177/1362361317714587
- Byers ES, Nichols S, Voyer SD. Challenging stereotypes: sexual functioning of single adults with high functioning autism spectrum disorder. J Autism Dev Disord 2013; 43(11):2617–2627. doi:10.1007/s10803-013-1813-z
- Gilmour L, Schalomon PM, Smith V. Sexuality in a community based sample of adults with autism spectrum disorder. Res Autism Spectr Disord 2012; 6(1):313–318. doi:10.1016/J.RASD.2011.06.003
- Bejerot S, Eriksson JM. Sexuality and gender role in autism spectrum disorder: a case control study. Schmitz C, ed. PLoS One 2014; 9(1):e87961. doi:10.1371/journal.pone.0087961>
- Navot N, Jorgenson AG, Webb SJ. Maternal experience raising girls with autism spectrum disorder: a qualitative study. Child Care Health Dev 2017; 43(4):536–545. doi:10.1111/cch.12470
- Padden C, James JE. Stress among parents of children with and without autism spectrum disorder: a comparison involving physiological indicators and parent self-reports. J Dev Phys Disabil 2017; 29(4):567–586. doi:10.1007/s10882-017-9547-z
- Woodgate RL, Ateah C, Secco L. Living in a world of our own: the experience of parents who have a child with autism. Qual Health Res 2008; 18(8):1075–1083. doi:10.1177/1049732308320112
- Hayes SA, Watson SL. The impact of parenting stress: a meta-analysis of studies comparing the experience of parenting stress in parents of children with and without autism spectrum disorder. J Autism Dev Disord 2013; 43(3):629–642. doi:10.1007/s10803-012-1604-y
- Cadman T, Eklund H, Howley D, et al. Caregiver burden as people with autism spectrum disorder and attention-deficit/hyperactivity disorder transition into adolescence and adulthood in the United Kingdom. J Am Acad Child Adolesc Psychiatry 2012; 51(9):879–888. doi:10.1016/j.jaac.2012.06.017
- Smith LE, Seltzer MM, Greenberg JS. Daily health symptoms of mothers of adolescents and adults with fragile x syndrome and mothers of adolescents and adults with autism spectrum disorder. J Autism Dev Disord 2012; 42(9):1836–1846. doi:10.1007/s10803-011-1422-7
- van Steijn DJ, Oerlemans AM, van Aken MAG, Buitelaar JK, Rommelse NNJ. The reciprocal relationship of ASD, ADHD, depressive symptoms and stress in parents of children with ASD and/or ADHD. J Autism Dev Disord 2014; 44(5):1064–1076. doi:10.1007/s10803-013-1958-9
- Seltzer MM, Greenberg JS, Hong J, et al. Maternal cortisol levels and behavior problems in adolescents and adults with ASD. J Autism Dev Disord 2010; 40(4):457–469. doi:10.1007/S10803-009-0887-0
- Lovell B, Moss M, Wetherell MA. With a little help from my friends: psychological, endocrine and health corollaries of social support in parental caregivers of children with autism or ADHD. Res Dev Disabil 2012; 33(2):682–687. doi:10.1016/j.ridd.2011.11.014
- Gallagher S, Whiteley J. Social support is associated with blood pressure responses in parents caring for children with developmental disabilities. Res Dev Disabil 2012; 33(6):2099–2105. doi:10.1016/j.ridd.2012.06.007
- Baker JK, Smith LE, Greenberg JS, Seltzer MM, Taylor JL. Change in maternal criticism and behavior problems in adolescents and adults with autism across a 7-year period. J Abnorm Psychol 2011; 120(2):465–475. doi:10.1037/a0021900
- Marsack CN, Samuel PS. Mediating effects of social support on quality of life for parents of adults with autism. J Autism Dev Disord 2017; 47(8):2378–2389. doi:10.1007/s10803-017-3157-6
- Trute B, Benzies KM, Worthington C, Reddon JR, Moore M. Accentuate the positive to mitigate the negative: mother psychological coping resources and family adjustment in childhood disability. J Intellect Dev Disabil 2010; 35(1):36–43. doi:10.3109/13668250903496328
- Cantwell J, Muldoon OT, Gallagher S. Social support and mastery influence the association between stress and poor physical health in parents caring for children with developmental disabilities. Res Dev Disabil 2014; 35(9):2215–2223. doi:10.1016/j.ridd.2014.05.012
- Carton AM, Smith AD. Assessing the relationship between eating disorder psychopathology and autistic traits in a non-clinical adult population. Eat Weight Disord - Stud Anorexia, Bulim Obes 2014; 19(3):285–293. doi:10.1007/s40519-013-0086-z
- De Alwis D, Agrawal A, Reiersen AM, et al. ADHD symptoms, autistic traits, and substance use and misuse in adult Australian twins. J Stud Alcohol Drugs 2014; 75(2):211–221. doi:10.15288/jsad.2014.75.211
- Wang LW, Tancredi DJ, Thomas DW. The prevalence of gastrointestinal problems in children across the United States with autism spectrum disorders from families with multiple affected members. J Dev Behav Pediatr 2011; 32(5):351–360. doi:10.1097/DBP.0b013e31821bd06a
- Croen LA, Zerbo O, Qian Y, et al. The health status of adults on the autism spectrum. Autism 2015; 19(7):814–823. doi:10.1177/1362361315577517
- Kalyoncu IÖ, Tanboga I. Oral health status of children with autistic spectrum disorder compared with non-authentic peers. Iran J Public Health 2017; 46(11):1591–1593. www.ncbi.nlm.nih.gov/pmc/articles/PMC5696703. Accessed July 9, 2019.
- McGuire K, Fung LK, Hagopian L, et al. Irritability and problem behavior in autism spectrum disorder: a practice pathway for pediatric primary care. Pediatrics 2016; 137(suppl 2):S136–S148. doi:10.1542/peds.2015-2851L
KEY POINTS
- Autism is becoming more common, with most recent statistics showing at least 1 in 59 children affected.
- Asperger syndrome is now included in the category of ASD, with possible implications for coverage of care.
- Some children with ASD get better as they get older, but many do not, and some do not receive a diagnosis until adulthood.
- Diagnosing ASD in adults can be difficult and involves specialists from multiple disciplines.
- Social support is important. Community programs and behavioral therapies can help. Drug therapy has not been rigorously tested and is not approved for use in adults with ASD. Caregivers may also need support.
Urine drug tests: How to make the most of them
Urine drug tests (UDTs) are useful clinical tools for assessing and monitoring the risk of misuse, abuse, and diversion when prescribing controlled substances, or for monitoring abstinence in patients with substance use disorders (SUDs). However, UDTs have been underutilized, and have been used without systematic documentation of reasons and results.1,2 In addition, many clinicians may lack the knowledge needed to effectively interpret test results.3,4 Although the reported use of UDTs is much higher among clinicians who are members of American Society of Addiction Medicine (ASAM), there is still a need for improved education.5
The appropriate use of UDTs strengthens the therapeutic relationship and promotes healthy behaviors and patients’ recovery. On the other hand, incorrect interpretation of test results may lead to missing potential aberrant behaviors, or inappropriate consequences for patients, such as discontinuing necessary medications or discharging them from care secondary to a perceived violation of a treatment contract due to unexpected positive or negative drug screening results.6 In this article, we review the basic concepts of UDTs and provide an algorithm to determine when to order these tests, how to interpret the results, and how to modify treatment accordingly.
Urine drug tests 101
Urine drug tests include rapid urine drug screening (UDS) and confirmatory tests. Urine drug screenings are usually based on various types of immunoassays. They are fast, sensitive, and cost-effective. Because immunoassa
Urine drug tests based on mass spectrometry, gas chromatography/mass spectrometry (GC/MS), and liquid chromatography/mass spectrometry (LC/MS) are gold standards to confirm toxicology results. They are highly sensitive and specific, with accurate quantitative measurement. However, they are more expensive than UDS and usually need to be sent to a laboratory with capacity to perform GC/MS or LC/MS, with a turnaround time of up to 1 week.8 In clinical practice, we usually start with UDS tests and order confirmatory tests when needed.
When to order UDTs in outpatient psychiatry
On December 12, 2013, the ASAM released a white paper that suggests the use of drug testing as a primary prevention, diagnostic, and monitoring tool in the management of addiction or drug misuse and its application in a wide variety of medical settings.9 Many clinicians use treatment contracts when prescribing controlled substances as a part of a risk-mitigation strategy, and these contracts often include the use of UDTs. Urine drug tests provide objective evidence to support or negate self-report, because many people may underreport their use.10 The literature has shown significant “abnormal” urine test results, ranging from 9% to 53%, in patients receiving chronic opioid therapy.2,11
The CDC and the American Academy of Pain Medicine recommend UDS before initiating any controlled substance for pain therapy.12,13 They also suggest random drug testing at least once or twice a year for low-risk patients, and more frequent screening for high-risk patients, such as those with a history of addiction.12,13 For example, for patients with opioid use disorder who participate in a methadone program, weekly UDTs are mandated for the first 90 days, and at least 8 UDTs a year are required after that.
However, UDTs carry significant stigma due to their association with SUDs. Talking with patients from the start of treatment helps to reduce this stigma, and makes it easier to have further discussions when patients have unexpected results during treatment. For example, clinicians can explain to patients that monitoring UDTs when prescribing controlled substances is similar to monitoring thyroid function with lithium use because treatment with a controlled substance carries an inherent risk of misuse, abuse, and diversion. For patients with SUDs, clinicians can explain that using UDTs to monitor their abstinence is similar to monitoring HbA1c for glucose control in patients with diabetes.
Continue to: Factors that can affect UDT results
Factors that can affect UDT results
In addition to knowing when to order UDT, it is critical to know how to interpret the results of UDS and follow up with confirmatory tests when needed. Other than the limitations of the tests, the following factors could contribute to unexpected UDT results:
- the drug itself, including its half-life, metabolic pathways, and potential interactions with other medications
- how patients take their medications, including dose, frequency, and pattern of drug use
- all the medications that patients are taking, including prescription, over-the-counter, and herbal and supplemental preparations
- when the last dose of a prescribed controlled substance was taken. Always ask when the patient’s last dose was taken before you consider ordering a UDT.
To help better understand UDT results, Figure 114 and Figure 215 demonstrate metabolic pathways of commonly used benzodiazepines and opioids, respectively. There are several comprehensive reviews on commonly seen false positives and negatives for each drug or each class of drugs in immunoassays.16-21 Confirmatory tests are usually very accurate. However, chiral analysis is needed to differentiate enantiomers, such as methamphetamine (active R-enantiomer) and selegiline, which is metabolized into L-methamphetamine (inactive S-enantiomer).22 In addition, detection of tetrahydrocannabivarin (THCV), an ingredient of the cannabis plant, via GC/MS can be used to distinguish between consumption of dronabinol and natural cannabis products.23 The Table16-21 summarizes the prototype agents, other detectable agents in the same class, and false positives and negatives in immunoassays.
Interpreting UDT results and management strategies
Our Algorithm outlines how to interpret UDT results, and management strategies to consider based on whether the results are as expected or unexpected, with a few key caveats as described below.
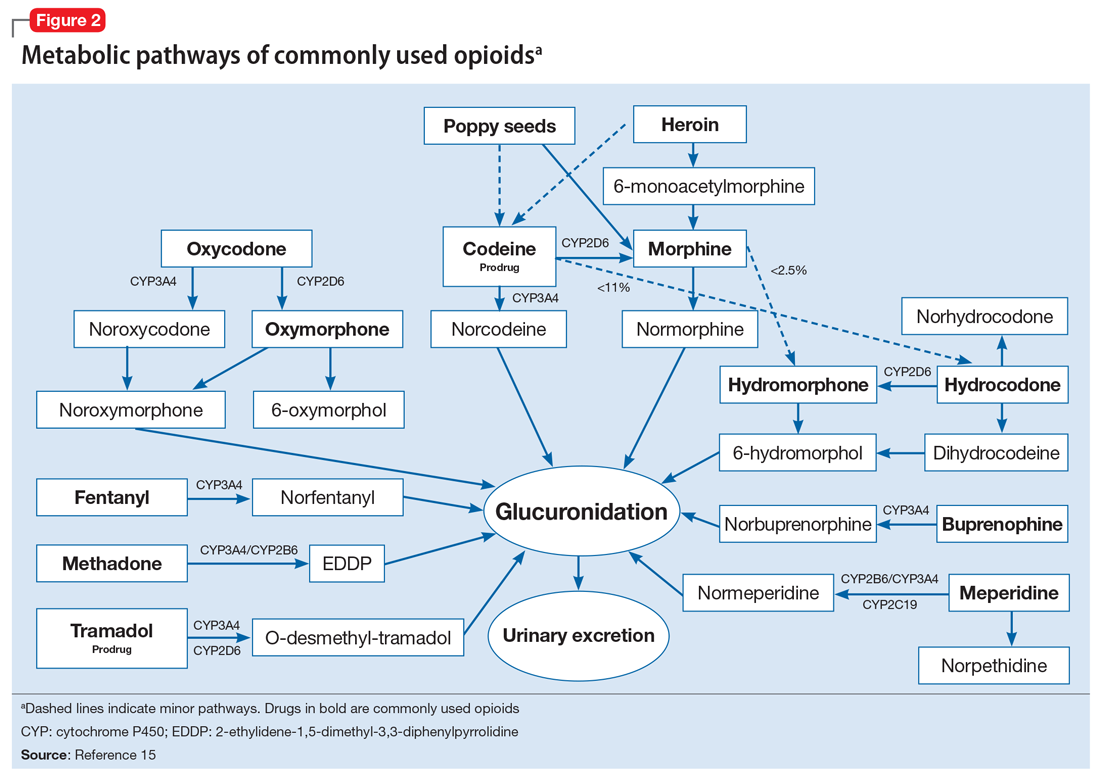
Expected results
If there are no concerns based on the patient’s clinical presentation or collateral information, simply continue the current treatment. However, for patients taking medications that are undetectable by UDS (for example, regular use of clonazepam or oxycodone), consider ordering confirmatory tests at least once to ensure compliance, even when UDS results are negative.
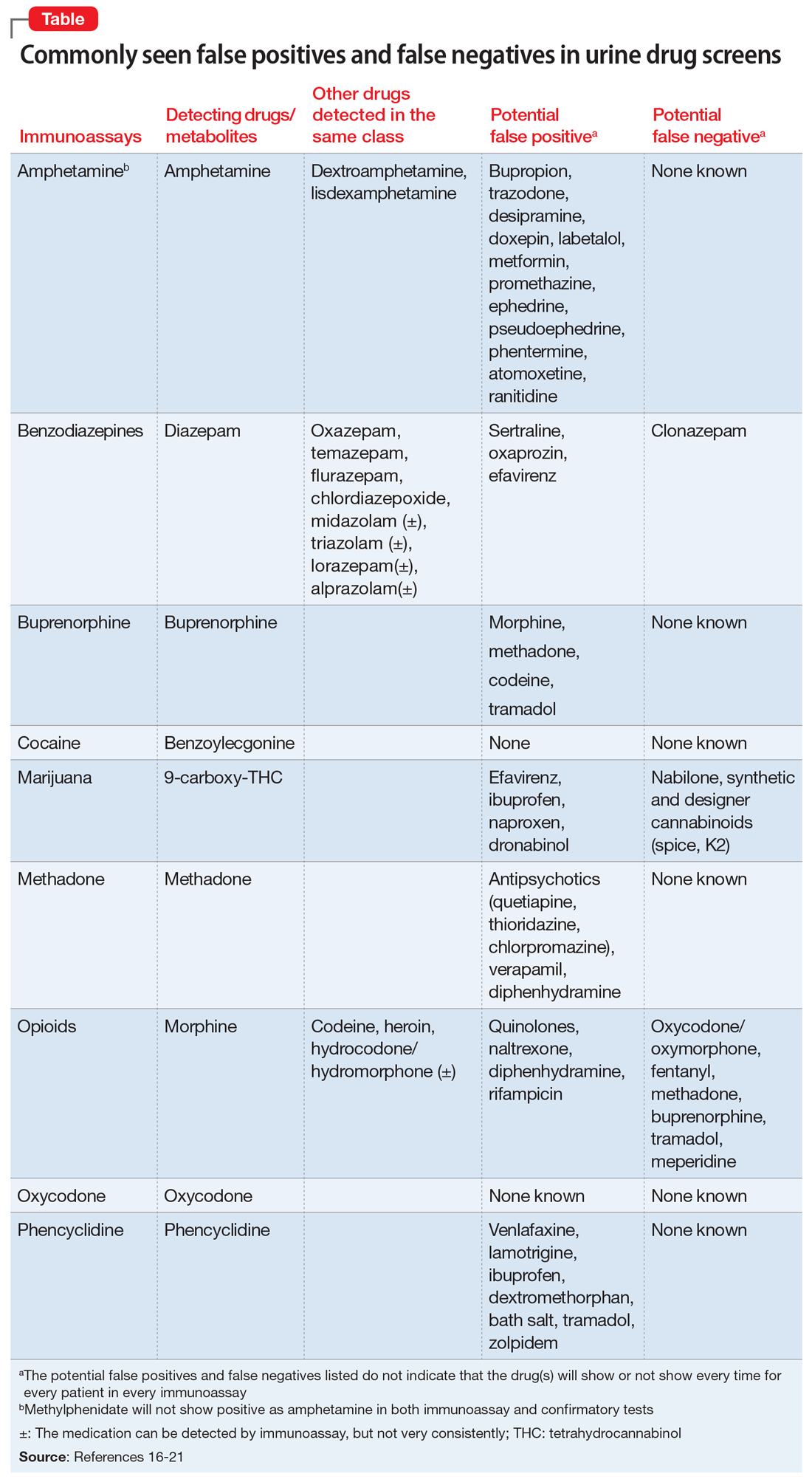
Unexpected positive results, including the presence of illicit drugs and/or unprescribed licit drugs
Drug misuse, abuse, or dependence. The first step is to talk with the patient, who may acknowledge drug misuse, abuse, or dependence. Next, consider modifying the treatment plan; this may include more frequent monitoring and visits, limiting or discontinuing prescribed controlled substances, or referring the patient to inpatient or outpatient SUD treatment, as appropriate.
Continue to: Interference from medications or diet
Interference from medications or diets. One example of a positive opioid screening result due to interference from diet is the consumption of foods that contain poppy seeds. Because of this potential interference, the cutoff value for a positive opioid immunoassay in workplace drug testing was increased from 300 to 2,000 ug/L.24 Educating patients regarding medication and lifestyle choices can help them avoid any interference with drug monitoring. Confirmatory tests can be ordered at the clinician’s discretion. The same principle applies to medication choice when prescribing. For example, a patient taking bupropion may experience a false positive result on a UDS for amphetamines, and a different antidepressant might be a better choice (Box 1).
Box 1
A patient with methamphetamine use disorder asked his psychiatrist for a letter to his probation officer because his recent urine drug screening (UDS) was positive for amphetamine. At a previous visit, the patient had been started on bupropion for depression and methamphetamine use disorder. After his most recent positive UDS, the patient stopped taking bupropion because he was aware that bupropion could cause a false-positive result on amphetamine screening. However, the psychiatrist could not confirm the results of the UDS, because he did not have the original sample for confirmatory testing. In this case, starting the patient on bupropion may not have been the best option without contacting the patient’s probation officer to discuss a good strategy for distinguishing true vs false-positive UDS results.
Urine sample tampering. Consider the possibility that urine samples could be substituted, especially when there are signs or indications of tampering, such as a positive pregnancy test for a male patient, or the presence of multiple prescription medications not prescribed to the patient. If there is high suspicion of urine sample tampering, consider observed urine sample collection.
When to order confirmatory tests for unexpected positive results.
Order a confirmatory test if a patient adamantly denies taking the substance(s) for which he/she has screened positive, and there’s no other explanation for the positive result. Continue the patient’s current treatment if the confirmatory test is negative. However, if the confirmatory test is positive, then modify the treatment plan (Algorithm).
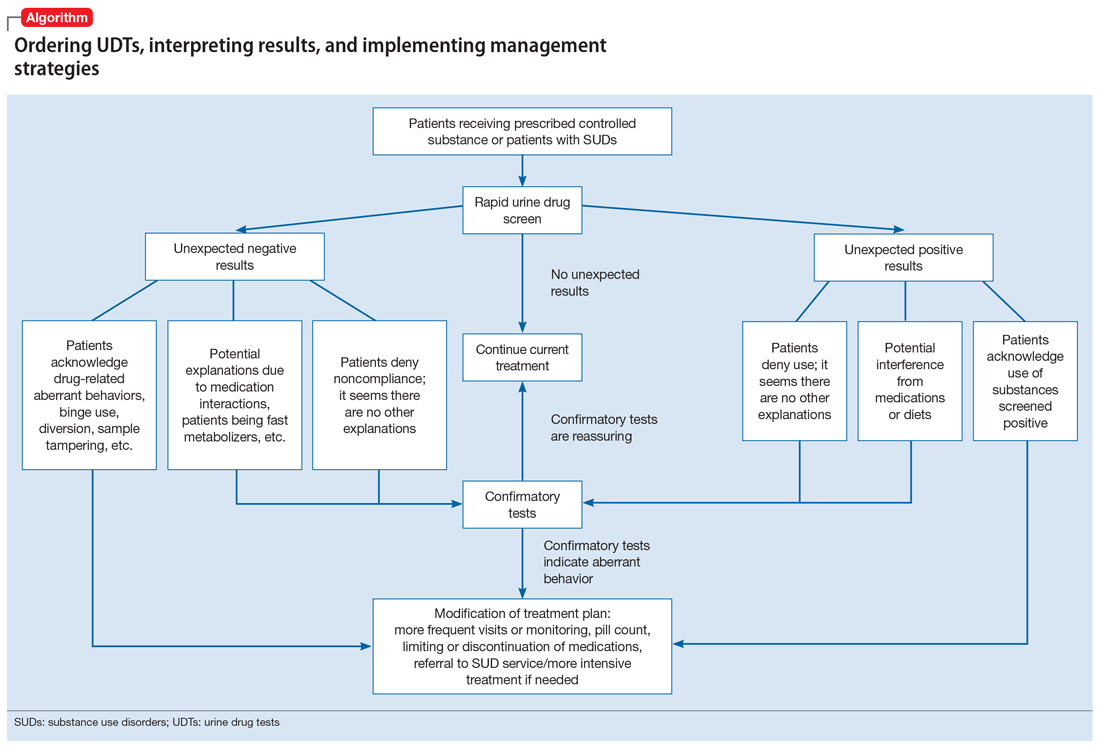
Special circumstances.
A positive opioid screen in a patient who has been prescribed a synthetic or semisynthetic opioid indicates the patient is likely using opioids other than the one he/she has been prescribed. Similarly, clonazepam is expected to be negative in a benzodiazepine immunoassay. If such testing is positive, consider the possibility that the patient is taking other benzodiazepines, such as diazepam. The results of UDTs can also be complicated by common metabolites in the same class of drugs. For example, the presence of hydromorphone for patients taking hydrocodone does not necessarily indicate the use of hydromorphone, because hydromorphone is a metabolite of hydrocodone (Figure 215).
Unexpected negative results
Prescribed medications exist in low concentration that are below the UDS detection threshold. This unexpected UDS result could occur if patients:
- take their medications less often than prescribed (because of financial difficulties or the patient feels better and does not think he/she needs it, etc.)
- hydrate too much (intentionally or unintentionally), are pregnant, or are fast metabolizers (Box 2)
- take other medications that increase the metabolism of the prescribed medication.
Box 2
A patient with opioid use disorder kept requesting a higher dose of methadone due to poorly controlled cravings. Even after he was observed taking methadone by the clinic staff, he was negative for methadone in immunoassay screening, and had a very low level of methadone based on liquid chromatography/mass spectrometry. Pharmacogenetic testing revealed that the patient was a cytochrome P450 2B6 ultra-rapid metabolizer; 2B6 is a primary metabolic enzyme for methadone. He also had a high concentration of 2-ethylidene- 1,5-dimethyl-3,3-diphenylpyrrolidine (EDDP), the primary metabolite of methadone, which was consistent with increased methadone metabolism.
Continue to: Further inquiry will...
Further inquiry will clarify these concerns. Clinicians should educate patients and manage accordingly. Confirmatory tests may be ordered upon clinicians’ discretion.
Urine sample tampering. Dilution or substitution of urine samples may lead to unexpected negative results. Usually, the urine sample will have abnormal parameters, including temperature, pH, specific gravity, urine creatinine level, or detection of adulterants. If needed, consider observed urine sample collection. Jaffee et al25 reviewed tampering methods in urine drug testing.
Diversion or binge use of medications. If patients adamantly deny diverting or binge using their medication, order confirmatory tests. If the confirmatory test also is negative, modify the treatment plan accordingly, and consider the following options:
- adjust the medication dosage or frequency
- discontinue the medication
- conduct pill counts for more definitive evidence of diversion or misuse, especially if discontinuation may lead to potential harm (for example, for patients prescribed buprenorphine for opioid use disorder).
When to order confirmatory tests for unexpected negative results.
Because confirmatory tests also measure drug concentrations, clinicians sometimes order serial confirmatory testing to monitor lipophilic drugs after a patient reports discontinuation, such as in the case of a patient using marijuana, ketamine, or alprazolam. The level of a lipophilic drug, such as these 3, should continue to decline if the patient has discontinued using it. However, because the drug level is affected by how concentrated the urine samples are, it is necessary to compare the ratios of drug levels over urine creatinine levels.26 Another use for confirmatory-quantitative testing is to detect “urine spiking,”27,28 when a patient adds an unconsumed drug to his/her urine sample to produce a positive result without actually taking the drug (Box 3).
Box 3
On a confirmatory urine drug test, a patient taking buprenorphine/naloxone had a very high level of buprenorphine, but almost no norbuprenorphine (a metabolite of buprenorphine). After further discussion with the clinician, the patient admitted that he had dipped his buprenorphine/naltrexone pill in his urine sample (“spiking”) to disguise the fact that he stopped taking buprenorphine/naloxone several days ago in an effort to get high from taking opioids.
When to consult lab specialists
Because many clinicians may find it challenging to stay abreast of all of the factors necessary to properly interpret UDT results, consulting with qualified laboratory professionals is appropriate when needed. For example, a patient was prescribed codeine, and his UDTs showed morphine as anticipated; however, the prescribing clinician suspected that the patient was also using heroin. In this case, consultation with a specialist may be warranted to look for 6-mono-acetylemorphine (6-MAM, a unique heroin metabolite) and/or the ratio of morphine to codeine.
Continue to: In summary...
In summary, UDTs are important tools to use in general psychiatry practice, especially when prescribing controlled substances. To use UDTs effectively, it is essential to possess knowledge of drug metabolism and the limitations of these tests. All immunoassay results should be considered as presumptive, and confirmatory tests are often needed for making treatment decisions. Many clinicians are unlikely to possess all the knowledge needed to correctly interpret UDTs, and in some cases, communication with qualified laboratory professionals may be necessary. In addition, the patient’s history and clinical presentation, collateral information, and data from prescription drug monitoring programs are all important factors to consider.
The cost of UDTs, variable insurance coverage, and a lack of on-site laboratory services can be deterrents to implementing UDTs as recommended. These factors vary significantly across regions, facilities, and insurance providers (see Related Resources). If faced with these issues and you expect to often need UDTs in your practice, consider using point-of-care UDTs as an alternative to improve access, convenience, and possibly cost.
Bottom Line
Urine drug tests (UDTs) should be standard clinical practice when prescribing controlled substances and treating patients with substance use disorders in the outpatient setting. Clinicians need to be knowledgeable about the limitations of UDTs, drug metabolism, and relevant patient history to interpret UDTs proficiently for optimal patient care. Consult laboratory specialists when needed to help interpret the results.
Related Resources
- Islam FA, Choudhry Z. Urine drug screens: Not just for job applicants. Current Psychiatry. 2018;17(12):43-44.
- HealthCare.gov. Health benefits & coverage: Mental health & substance abuse coverage. www.healthcare.gov/coverage/mental-health-substance-abuse-coverage/.
Drug Brand Names
Alprazolam • Xanax
Amphetamine • Adderall
Atomoxetine • Strattera
Buprenorphine • Subutex
Buprenorphine/naloxone • Suboxone, Zubsolv
Bupropion • Wellbutrin, Zyban
Chlordiazepoxide • Librium
Chlorpromazine • Thorazine
Clonazepam • Klonopin
Desipramine • Norpramin
Dextroamphetamine • Dexedrine, ProCentra
Diazepam • Valium
Doxepin • Silenor
Dronabinol • Marinol
Efavirenz • Sustiva
Ephedrine • Akovaz
Fentanyl • Actiq, Duragesic
Flurazepam • Dalmane
Hydrocodone • Hysingla, Zohydro ER
Hydromorphone • Dilaudid, Exalgo
Labetalol • Normodyne, Trandate
Lamotrigine • Lamictal
Lisdexamfetamine • Vyvanse
Lithium • Eskalith, Lithobid
Lorazepam • Ativan
Meperidine • Demerol
Metformin • Fortamet, Glucophage
Methadone • Dolophine, Methadose
Methylphenidate • Ritalin
Midazolam • Versed
Morphine • Kadian, MorphaBond
Nabilone • Cesamet
Naltrexone • Vivitrol
Oxaprozin • Daypro
Oxazepam • Serax
Oxycodone • Oxycontin
Oxymorphone • Opana
Phentermine • Adipex-P, Ionamin
Promethazine • Phenergan
Quetiapine • Seroquel
Ranitidine • Zantac
Rifampicin • Rifadin
Selegiline • Eldepryl, Zelapar
Sertraline • Zoloft
Temazepam • Restoril
Thioridazine • Mellaril
Tramadol • Conzip, Ultram
Trazodone • Desyrel
Triazolam • Halcion
Venlafaxine • Effexor
Verapamil • Calan, Verelan
Zolpidem • Ambien
1. Passik SD, Schreiber J, Kirsh KL, et al. A chart review of the ordering and documentation of urine toxicology screens in a cancer center: do they influence patient management? J Pain Symptom Manag. 2000;19(1):40-44.
2. Arthur JA, Edwards T, Lu Z, et al. Frequency, predictors, and outcomes of urine drug testing among patients with advanced cancer on chronic opioid therapy at an outpatient supportive care clinic. Cancer. 2016;122(23):3732-3739.
3. Suzuki JM, Garayalde SM, Dodoo MM, et al. Psychiatry residents’ and fellows’ confidence and knowledge in interpreting urine drug testing results related to opioids. Subst Abus. 2018;39(4):518-521.
4. Reisfield GM, Bertholf R, Barkin RL, et al. Urine drug test interpretation: what do physicians know? J Opioid Manag. 2007;3(2):80-86.
5. Kirsh KL, Baxter LE, Rzetelny A, et al. A survey of ASAM members’ knowledge, attitudes, and practices in urine drug testing. J Addict Med. 2015;9(5):399-404.
6. Morasco BJ, Krebs EE, Adams MH, et al. Clinician response to aberrant urine drug test results of patients prescribed opioid therapy for chronic pain. Clin J Pain. 2019;35(1):1-6.
7. Liu RH. Comparison of common immunoassay kits for effective application in workplace drug urinalysis. Forensic Sci Rev. 1994;6(1):19-57.
8. Jannetto PJ, Fitzgerald RL. Effective use of mass spectrometry in the clinical laboratory. Clin Chem. 2016;62(1):92-98.
9. American Society of Addiction Medicine. Resources: ASAM releases white paper on drug testing. https://www.asam.org/resources/publications/magazine/read/article/2013/12/16/asam-releases-white-paper-on-drug-testing. Published December 16, 2019. Accessed June 25, 2019.
10. Fishbain DA, Cutler RB, Rosomoff HL, et al. Validity of self-reported drug use in chronic pain patients. Clin J Pain. 1999;15(3):184-191.
11. Michna E, Jamison RN, Pham LD, et al. Urine toxicology screening among chronic pain patients on opioid therapy: Frequency and predictability of abnormal findings. Clin J Pain. 2007;23(2):173-179.
12. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain--United States, 2016. JAMA. 2016;315(15):1624-1645.
13. Chou R. 2009 clinical guidelines from the American Pain Society and the American Academy of Pain medicine on the use of chronic opioid therapy in chronic noncancer pain: what are the key messages for clinical practice? Pol Arch Med Wewn. 2009;119(7-8):469-477.
14. Mihic SJ, Harris RA. Hypnotics and sedatives. In: Brunton LL, Chabner BA, Knollmann BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. New York, NY: McGrawHill Medical; 2017:343-344.
15. DePriest AZ, Puet BL, Holt AC, et al. Metabolism and disposition of prescription opioids: a review. Forensic Sci Rev. 2015;27(2):115-145.
16. Tenore PL. Advanced urine toxicology testing. J Addict Dis. 2010;29(4):436-448.
17. Brahm NC, Yeager LL, Fox MD, et al. Commonly prescribed medications and potential false-positive urine drug screens. Am J Health Syst Pharm. 2010;67(16):1344-1350.
18. Saitman A, Park HD, Fitzgerald RL. False-positive interferences of common urine drug screen immunoassays: a review. J Anal Toxicol. 2014;38(7):387-396.
19. Moeller KE, Kissack JC, Atayee RS, et al. Clinical interpretation of urine drug tests: what clinicians need to know about urine drug screens. Mayo Clin Proc. 2017;92(5):774-796.
20. Nelson ZJ, Stellpflug SJ, Engebretsen KM. What can a urine drug screening immunoassay really tell us? J Pharm Pract. 2016;29(5):516-526.
21. Reisfield GM, Goldberger BA, Bertholf RL. ‘False-positive’ and ‘false-negative’ test results in clinical urine drug testing. Bioanalysis. 2009;1(5):937-952.
22. Poklis A, Moore KA. Response of EMIT amphetamine immunoassays to urinary desoxyephedrine following Vicks inhaler use. Ther Drug Monit. 1995;17(1):89-94.
23. ElSohly MA, Feng S, Murphy TP, et al. Identification and quantitation of 11-nor-delta9-tetrahydrocannabivarin-9-carboxylic acid, a major metabolite of delta9-tetrahydrocannabivarin. J Anal Toxicol. 2001;25(6):476-480.
24. Selavka CM. Poppy seed ingestion as a contributing factor to opiate-positive urinalysis results: the pacific perspective. J Forensic Sci. 1991;36(3):685-696.
25. Jaffee WB, Trucco E, Levy S, et al. Is this urine really negative? A systematic review of tampering methods in urine drug screening and testing. J Subst Abuse Treat. 2007;33(1):33-42.
26. Fraser AD, Worth D. Urinary excretion profiles of 11-nor-9-carboxy-delta9-tetrahydrocannabinol: a delta9-thccooh to creatinine ratio study. J Anal Toxicol. 1999;23(6):531-534.
27. Holt SR, Donroe JH, Cavallo DA, et al. Addressing discordant quantitative urine buprenorphine and norbuprenorphine levels: case examples in opioid use disorder. Drug Alcohol Depend. 2018;186:171-174.
28. Accurso AJ, Lee JD, McNeely J. High prevalence of urine tampering in an office-based opioid treatment practice detected by evaluating the norbuprenorphine to buprenorphine ratio. J Subst Abuse Treat. 2017;83:62-67.
Urine drug tests (UDTs) are useful clinical tools for assessing and monitoring the risk of misuse, abuse, and diversion when prescribing controlled substances, or for monitoring abstinence in patients with substance use disorders (SUDs). However, UDTs have been underutilized, and have been used without systematic documentation of reasons and results.1,2 In addition, many clinicians may lack the knowledge needed to effectively interpret test results.3,4 Although the reported use of UDTs is much higher among clinicians who are members of American Society of Addiction Medicine (ASAM), there is still a need for improved education.5
The appropriate use of UDTs strengthens the therapeutic relationship and promotes healthy behaviors and patients’ recovery. On the other hand, incorrect interpretation of test results may lead to missing potential aberrant behaviors, or inappropriate consequences for patients, such as discontinuing necessary medications or discharging them from care secondary to a perceived violation of a treatment contract due to unexpected positive or negative drug screening results.6 In this article, we review the basic concepts of UDTs and provide an algorithm to determine when to order these tests, how to interpret the results, and how to modify treatment accordingly.
Urine drug tests 101
Urine drug tests include rapid urine drug screening (UDS) and confirmatory tests. Urine drug screenings are usually based on various types of immunoassays. They are fast, sensitive, and cost-effective. Because immunoassa
Urine drug tests based on mass spectrometry, gas chromatography/mass spectrometry (GC/MS), and liquid chromatography/mass spectrometry (LC/MS) are gold standards to confirm toxicology results. They are highly sensitive and specific, with accurate quantitative measurement. However, they are more expensive than UDS and usually need to be sent to a laboratory with capacity to perform GC/MS or LC/MS, with a turnaround time of up to 1 week.8 In clinical practice, we usually start with UDS tests and order confirmatory tests when needed.
When to order UDTs in outpatient psychiatry
On December 12, 2013, the ASAM released a white paper that suggests the use of drug testing as a primary prevention, diagnostic, and monitoring tool in the management of addiction or drug misuse and its application in a wide variety of medical settings.9 Many clinicians use treatment contracts when prescribing controlled substances as a part of a risk-mitigation strategy, and these contracts often include the use of UDTs. Urine drug tests provide objective evidence to support or negate self-report, because many people may underreport their use.10 The literature has shown significant “abnormal” urine test results, ranging from 9% to 53%, in patients receiving chronic opioid therapy.2,11
The CDC and the American Academy of Pain Medicine recommend UDS before initiating any controlled substance for pain therapy.12,13 They also suggest random drug testing at least once or twice a year for low-risk patients, and more frequent screening for high-risk patients, such as those with a history of addiction.12,13 For example, for patients with opioid use disorder who participate in a methadone program, weekly UDTs are mandated for the first 90 days, and at least 8 UDTs a year are required after that.
However, UDTs carry significant stigma due to their association with SUDs. Talking with patients from the start of treatment helps to reduce this stigma, and makes it easier to have further discussions when patients have unexpected results during treatment. For example, clinicians can explain to patients that monitoring UDTs when prescribing controlled substances is similar to monitoring thyroid function with lithium use because treatment with a controlled substance carries an inherent risk of misuse, abuse, and diversion. For patients with SUDs, clinicians can explain that using UDTs to monitor their abstinence is similar to monitoring HbA1c for glucose control in patients with diabetes.
Continue to: Factors that can affect UDT results
Factors that can affect UDT results
In addition to knowing when to order UDT, it is critical to know how to interpret the results of UDS and follow up with confirmatory tests when needed. Other than the limitations of the tests, the following factors could contribute to unexpected UDT results:
- the drug itself, including its half-life, metabolic pathways, and potential interactions with other medications
- how patients take their medications, including dose, frequency, and pattern of drug use
- all the medications that patients are taking, including prescription, over-the-counter, and herbal and supplemental preparations
- when the last dose of a prescribed controlled substance was taken. Always ask when the patient’s last dose was taken before you consider ordering a UDT.
To help better understand UDT results, Figure 114 and Figure 215 demonstrate metabolic pathways of commonly used benzodiazepines and opioids, respectively. There are several comprehensive reviews on commonly seen false positives and negatives for each drug or each class of drugs in immunoassays.16-21 Confirmatory tests are usually very accurate. However, chiral analysis is needed to differentiate enantiomers, such as methamphetamine (active R-enantiomer) and selegiline, which is metabolized into L-methamphetamine (inactive S-enantiomer).22 In addition, detection of tetrahydrocannabivarin (THCV), an ingredient of the cannabis plant, via GC/MS can be used to distinguish between consumption of dronabinol and natural cannabis products.23 The Table16-21 summarizes the prototype agents, other detectable agents in the same class, and false positives and negatives in immunoassays.

Interpreting UDT results and management strategies
Our Algorithm outlines how to interpret UDT results, and management strategies to consider based on whether the results are as expected or unexpected, with a few key caveats as described below.

Expected results
If there are no concerns based on the patient’s clinical presentation or collateral information, simply continue the current treatment. However, for patients taking medications that are undetectable by UDS (for example, regular use of clonazepam or oxycodone), consider ordering confirmatory tests at least once to ensure compliance, even when UDS results are negative.

Unexpected positive results, including the presence of illicit drugs and/or unprescribed licit drugs
Drug misuse, abuse, or dependence. The first step is to talk with the patient, who may acknowledge drug misuse, abuse, or dependence. Next, consider modifying the treatment plan; this may include more frequent monitoring and visits, limiting or discontinuing prescribed controlled substances, or referring the patient to inpatient or outpatient SUD treatment, as appropriate.
Continue to: Interference from medications or diet
Interference from medications or diets. One example of a positive opioid screening result due to interference from diet is the consumption of foods that contain poppy seeds. Because of this potential interference, the cutoff value for a positive opioid immunoassay in workplace drug testing was increased from 300 to 2,000 ug/L.24 Educating patients regarding medication and lifestyle choices can help them avoid any interference with drug monitoring. Confirmatory tests can be ordered at the clinician’s discretion. The same principle applies to medication choice when prescribing. For example, a patient taking bupropion may experience a false positive result on a UDS for amphetamines, and a different antidepressant might be a better choice (Box 1).
Box 1
A patient with methamphetamine use disorder asked his psychiatrist for a letter to his probation officer because his recent urine drug screening (UDS) was positive for amphetamine. At a previous visit, the patient had been started on bupropion for depression and methamphetamine use disorder. After his most recent positive UDS, the patient stopped taking bupropion because he was aware that bupropion could cause a false-positive result on amphetamine screening. However, the psychiatrist could not confirm the results of the UDS, because he did not have the original sample for confirmatory testing. In this case, starting the patient on bupropion may not have been the best option without contacting the patient’s probation officer to discuss a good strategy for distinguishing true vs false-positive UDS results.
Urine sample tampering. Consider the possibility that urine samples could be substituted, especially when there are signs or indications of tampering, such as a positive pregnancy test for a male patient, or the presence of multiple prescription medications not prescribed to the patient. If there is high suspicion of urine sample tampering, consider observed urine sample collection.
When to order confirmatory tests for unexpected positive results.
Order a confirmatory test if a patient adamantly denies taking the substance(s) for which he/she has screened positive, and there’s no other explanation for the positive result. Continue the patient’s current treatment if the confirmatory test is negative. However, if the confirmatory test is positive, then modify the treatment plan (Algorithm).

Special circumstances.
A positive opioid screen in a patient who has been prescribed a synthetic or semisynthetic opioid indicates the patient is likely using opioids other than the one he/she has been prescribed. Similarly, clonazepam is expected to be negative in a benzodiazepine immunoassay. If such testing is positive, consider the possibility that the patient is taking other benzodiazepines, such as diazepam. The results of UDTs can also be complicated by common metabolites in the same class of drugs. For example, the presence of hydromorphone for patients taking hydrocodone does not necessarily indicate the use of hydromorphone, because hydromorphone is a metabolite of hydrocodone (Figure 215).
Unexpected negative results
Prescribed medications exist in low concentration that are below the UDS detection threshold. This unexpected UDS result could occur if patients:
- take their medications less often than prescribed (because of financial difficulties or the patient feels better and does not think he/she needs it, etc.)
- hydrate too much (intentionally or unintentionally), are pregnant, or are fast metabolizers (Box 2)
- take other medications that increase the metabolism of the prescribed medication.
Box 2
A patient with opioid use disorder kept requesting a higher dose of methadone due to poorly controlled cravings. Even after he was observed taking methadone by the clinic staff, he was negative for methadone in immunoassay screening, and had a very low level of methadone based on liquid chromatography/mass spectrometry. Pharmacogenetic testing revealed that the patient was a cytochrome P450 2B6 ultra-rapid metabolizer; 2B6 is a primary metabolic enzyme for methadone. He also had a high concentration of 2-ethylidene- 1,5-dimethyl-3,3-diphenylpyrrolidine (EDDP), the primary metabolite of methadone, which was consistent with increased methadone metabolism.
Continue to: Further inquiry will...
Further inquiry will clarify these concerns. Clinicians should educate patients and manage accordingly. Confirmatory tests may be ordered upon clinicians’ discretion.
Urine sample tampering. Dilution or substitution of urine samples may lead to unexpected negative results. Usually, the urine sample will have abnormal parameters, including temperature, pH, specific gravity, urine creatinine level, or detection of adulterants. If needed, consider observed urine sample collection. Jaffee et al25 reviewed tampering methods in urine drug testing.
Diversion or binge use of medications. If patients adamantly deny diverting or binge using their medication, order confirmatory tests. If the confirmatory test also is negative, modify the treatment plan accordingly, and consider the following options:
- adjust the medication dosage or frequency
- discontinue the medication
- conduct pill counts for more definitive evidence of diversion or misuse, especially if discontinuation may lead to potential harm (for example, for patients prescribed buprenorphine for opioid use disorder).
When to order confirmatory tests for unexpected negative results.
Because confirmatory tests also measure drug concentrations, clinicians sometimes order serial confirmatory testing to monitor lipophilic drugs after a patient reports discontinuation, such as in the case of a patient using marijuana, ketamine, or alprazolam. The level of a lipophilic drug, such as these 3, should continue to decline if the patient has discontinued using it. However, because the drug level is affected by how concentrated the urine samples are, it is necessary to compare the ratios of drug levels over urine creatinine levels.26 Another use for confirmatory-quantitative testing is to detect “urine spiking,”27,28 when a patient adds an unconsumed drug to his/her urine sample to produce a positive result without actually taking the drug (Box 3).
Box 3
On a confirmatory urine drug test, a patient taking buprenorphine/naloxone had a very high level of buprenorphine, but almost no norbuprenorphine (a metabolite of buprenorphine). After further discussion with the clinician, the patient admitted that he had dipped his buprenorphine/naltrexone pill in his urine sample (“spiking”) to disguise the fact that he stopped taking buprenorphine/naloxone several days ago in an effort to get high from taking opioids.
When to consult lab specialists
Because many clinicians may find it challenging to stay abreast of all of the factors necessary to properly interpret UDT results, consulting with qualified laboratory professionals is appropriate when needed. For example, a patient was prescribed codeine, and his UDTs showed morphine as anticipated; however, the prescribing clinician suspected that the patient was also using heroin. In this case, consultation with a specialist may be warranted to look for 6-mono-acetylemorphine (6-MAM, a unique heroin metabolite) and/or the ratio of morphine to codeine.
Continue to: In summary...
In summary, UDTs are important tools to use in general psychiatry practice, especially when prescribing controlled substances. To use UDTs effectively, it is essential to possess knowledge of drug metabolism and the limitations of these tests. All immunoassay results should be considered as presumptive, and confirmatory tests are often needed for making treatment decisions. Many clinicians are unlikely to possess all the knowledge needed to correctly interpret UDTs, and in some cases, communication with qualified laboratory professionals may be necessary. In addition, the patient’s history and clinical presentation, collateral information, and data from prescription drug monitoring programs are all important factors to consider.
The cost of UDTs, variable insurance coverage, and a lack of on-site laboratory services can be deterrents to implementing UDTs as recommended. These factors vary significantly across regions, facilities, and insurance providers (see Related Resources). If faced with these issues and you expect to often need UDTs in your practice, consider using point-of-care UDTs as an alternative to improve access, convenience, and possibly cost.
Bottom Line
Urine drug tests (UDTs) should be standard clinical practice when prescribing controlled substances and treating patients with substance use disorders in the outpatient setting. Clinicians need to be knowledgeable about the limitations of UDTs, drug metabolism, and relevant patient history to interpret UDTs proficiently for optimal patient care. Consult laboratory specialists when needed to help interpret the results.
Related Resources
- Islam FA, Choudhry Z. Urine drug screens: Not just for job applicants. Current Psychiatry. 2018;17(12):43-44.
- HealthCare.gov. Health benefits & coverage: Mental health & substance abuse coverage. www.healthcare.gov/coverage/mental-health-substance-abuse-coverage/.
Drug Brand Names
Alprazolam • Xanax
Amphetamine • Adderall
Atomoxetine • Strattera
Buprenorphine • Subutex
Buprenorphine/naloxone • Suboxone, Zubsolv
Bupropion • Wellbutrin, Zyban
Chlordiazepoxide • Librium
Chlorpromazine • Thorazine
Clonazepam • Klonopin
Desipramine • Norpramin
Dextroamphetamine • Dexedrine, ProCentra
Diazepam • Valium
Doxepin • Silenor
Dronabinol • Marinol
Efavirenz • Sustiva
Ephedrine • Akovaz
Fentanyl • Actiq, Duragesic
Flurazepam • Dalmane
Hydrocodone • Hysingla, Zohydro ER
Hydromorphone • Dilaudid, Exalgo
Labetalol • Normodyne, Trandate
Lamotrigine • Lamictal
Lisdexamfetamine • Vyvanse
Lithium • Eskalith, Lithobid
Lorazepam • Ativan
Meperidine • Demerol
Metformin • Fortamet, Glucophage
Methadone • Dolophine, Methadose
Methylphenidate • Ritalin
Midazolam • Versed
Morphine • Kadian, MorphaBond
Nabilone • Cesamet
Naltrexone • Vivitrol
Oxaprozin • Daypro
Oxazepam • Serax
Oxycodone • Oxycontin
Oxymorphone • Opana
Phentermine • Adipex-P, Ionamin
Promethazine • Phenergan
Quetiapine • Seroquel
Ranitidine • Zantac
Rifampicin • Rifadin
Selegiline • Eldepryl, Zelapar
Sertraline • Zoloft
Temazepam • Restoril
Thioridazine • Mellaril
Tramadol • Conzip, Ultram
Trazodone • Desyrel
Triazolam • Halcion
Venlafaxine • Effexor
Verapamil • Calan, Verelan
Zolpidem • Ambien
Urine drug tests (UDTs) are useful clinical tools for assessing and monitoring the risk of misuse, abuse, and diversion when prescribing controlled substances, or for monitoring abstinence in patients with substance use disorders (SUDs). However, UDTs have been underutilized, and have been used without systematic documentation of reasons and results.1,2 In addition, many clinicians may lack the knowledge needed to effectively interpret test results.3,4 Although the reported use of UDTs is much higher among clinicians who are members of American Society of Addiction Medicine (ASAM), there is still a need for improved education.5
The appropriate use of UDTs strengthens the therapeutic relationship and promotes healthy behaviors and patients’ recovery. On the other hand, incorrect interpretation of test results may lead to missing potential aberrant behaviors, or inappropriate consequences for patients, such as discontinuing necessary medications or discharging them from care secondary to a perceived violation of a treatment contract due to unexpected positive or negative drug screening results.6 In this article, we review the basic concepts of UDTs and provide an algorithm to determine when to order these tests, how to interpret the results, and how to modify treatment accordingly.
Urine drug tests 101
Urine drug tests include rapid urine drug screening (UDS) and confirmatory tests. Urine drug screenings are usually based on various types of immunoassays. They are fast, sensitive, and cost-effective. Because immunoassa
Urine drug tests based on mass spectrometry, gas chromatography/mass spectrometry (GC/MS), and liquid chromatography/mass spectrometry (LC/MS) are gold standards to confirm toxicology results. They are highly sensitive and specific, with accurate quantitative measurement. However, they are more expensive than UDS and usually need to be sent to a laboratory with capacity to perform GC/MS or LC/MS, with a turnaround time of up to 1 week.8 In clinical practice, we usually start with UDS tests and order confirmatory tests when needed.
When to order UDTs in outpatient psychiatry
On December 12, 2013, the ASAM released a white paper that suggests the use of drug testing as a primary prevention, diagnostic, and monitoring tool in the management of addiction or drug misuse and its application in a wide variety of medical settings.9 Many clinicians use treatment contracts when prescribing controlled substances as a part of a risk-mitigation strategy, and these contracts often include the use of UDTs. Urine drug tests provide objective evidence to support or negate self-report, because many people may underreport their use.10 The literature has shown significant “abnormal” urine test results, ranging from 9% to 53%, in patients receiving chronic opioid therapy.2,11
The CDC and the American Academy of Pain Medicine recommend UDS before initiating any controlled substance for pain therapy.12,13 They also suggest random drug testing at least once or twice a year for low-risk patients, and more frequent screening for high-risk patients, such as those with a history of addiction.12,13 For example, for patients with opioid use disorder who participate in a methadone program, weekly UDTs are mandated for the first 90 days, and at least 8 UDTs a year are required after that.
However, UDTs carry significant stigma due to their association with SUDs. Talking with patients from the start of treatment helps to reduce this stigma, and makes it easier to have further discussions when patients have unexpected results during treatment. For example, clinicians can explain to patients that monitoring UDTs when prescribing controlled substances is similar to monitoring thyroid function with lithium use because treatment with a controlled substance carries an inherent risk of misuse, abuse, and diversion. For patients with SUDs, clinicians can explain that using UDTs to monitor their abstinence is similar to monitoring HbA1c for glucose control in patients with diabetes.
Continue to: Factors that can affect UDT results
Factors that can affect UDT results
In addition to knowing when to order UDT, it is critical to know how to interpret the results of UDS and follow up with confirmatory tests when needed. Other than the limitations of the tests, the following factors could contribute to unexpected UDT results:
- the drug itself, including its half-life, metabolic pathways, and potential interactions with other medications
- how patients take their medications, including dose, frequency, and pattern of drug use
- all the medications that patients are taking, including prescription, over-the-counter, and herbal and supplemental preparations
- when the last dose of a prescribed controlled substance was taken. Always ask when the patient’s last dose was taken before you consider ordering a UDT.
To help better understand UDT results, Figure 114 and Figure 215 demonstrate metabolic pathways of commonly used benzodiazepines and opioids, respectively. There are several comprehensive reviews on commonly seen false positives and negatives for each drug or each class of drugs in immunoassays.16-21 Confirmatory tests are usually very accurate. However, chiral analysis is needed to differentiate enantiomers, such as methamphetamine (active R-enantiomer) and selegiline, which is metabolized into L-methamphetamine (inactive S-enantiomer).22 In addition, detection of tetrahydrocannabivarin (THCV), an ingredient of the cannabis plant, via GC/MS can be used to distinguish between consumption of dronabinol and natural cannabis products.23 The Table16-21 summarizes the prototype agents, other detectable agents in the same class, and false positives and negatives in immunoassays.

Interpreting UDT results and management strategies
Our Algorithm outlines how to interpret UDT results, and management strategies to consider based on whether the results are as expected or unexpected, with a few key caveats as described below.

Expected results
If there are no concerns based on the patient’s clinical presentation or collateral information, simply continue the current treatment. However, for patients taking medications that are undetectable by UDS (for example, regular use of clonazepam or oxycodone), consider ordering confirmatory tests at least once to ensure compliance, even when UDS results are negative.

Unexpected positive results, including the presence of illicit drugs and/or unprescribed licit drugs
Drug misuse, abuse, or dependence. The first step is to talk with the patient, who may acknowledge drug misuse, abuse, or dependence. Next, consider modifying the treatment plan; this may include more frequent monitoring and visits, limiting or discontinuing prescribed controlled substances, or referring the patient to inpatient or outpatient SUD treatment, as appropriate.
Continue to: Interference from medications or diet
Interference from medications or diets. One example of a positive opioid screening result due to interference from diet is the consumption of foods that contain poppy seeds. Because of this potential interference, the cutoff value for a positive opioid immunoassay in workplace drug testing was increased from 300 to 2,000 ug/L.24 Educating patients regarding medication and lifestyle choices can help them avoid any interference with drug monitoring. Confirmatory tests can be ordered at the clinician’s discretion. The same principle applies to medication choice when prescribing. For example, a patient taking bupropion may experience a false positive result on a UDS for amphetamines, and a different antidepressant might be a better choice (Box 1).
Box 1
A patient with methamphetamine use disorder asked his psychiatrist for a letter to his probation officer because his recent urine drug screening (UDS) was positive for amphetamine. At a previous visit, the patient had been started on bupropion for depression and methamphetamine use disorder. After his most recent positive UDS, the patient stopped taking bupropion because he was aware that bupropion could cause a false-positive result on amphetamine screening. However, the psychiatrist could not confirm the results of the UDS, because he did not have the original sample for confirmatory testing. In this case, starting the patient on bupropion may not have been the best option without contacting the patient’s probation officer to discuss a good strategy for distinguishing true vs false-positive UDS results.
Urine sample tampering. Consider the possibility that urine samples could be substituted, especially when there are signs or indications of tampering, such as a positive pregnancy test for a male patient, or the presence of multiple prescription medications not prescribed to the patient. If there is high suspicion of urine sample tampering, consider observed urine sample collection.
When to order confirmatory tests for unexpected positive results.
Order a confirmatory test if a patient adamantly denies taking the substance(s) for which he/she has screened positive, and there’s no other explanation for the positive result. Continue the patient’s current treatment if the confirmatory test is negative. However, if the confirmatory test is positive, then modify the treatment plan (Algorithm).

Special circumstances.
A positive opioid screen in a patient who has been prescribed a synthetic or semisynthetic opioid indicates the patient is likely using opioids other than the one he/she has been prescribed. Similarly, clonazepam is expected to be negative in a benzodiazepine immunoassay. If such testing is positive, consider the possibility that the patient is taking other benzodiazepines, such as diazepam. The results of UDTs can also be complicated by common metabolites in the same class of drugs. For example, the presence of hydromorphone for patients taking hydrocodone does not necessarily indicate the use of hydromorphone, because hydromorphone is a metabolite of hydrocodone (Figure 215).
Unexpected negative results
Prescribed medications exist in low concentration that are below the UDS detection threshold. This unexpected UDS result could occur if patients:
- take their medications less often than prescribed (because of financial difficulties or the patient feels better and does not think he/she needs it, etc.)
- hydrate too much (intentionally or unintentionally), are pregnant, or are fast metabolizers (Box 2)
- take other medications that increase the metabolism of the prescribed medication.
Box 2
A patient with opioid use disorder kept requesting a higher dose of methadone due to poorly controlled cravings. Even after he was observed taking methadone by the clinic staff, he was negative for methadone in immunoassay screening, and had a very low level of methadone based on liquid chromatography/mass spectrometry. Pharmacogenetic testing revealed that the patient was a cytochrome P450 2B6 ultra-rapid metabolizer; 2B6 is a primary metabolic enzyme for methadone. He also had a high concentration of 2-ethylidene- 1,5-dimethyl-3,3-diphenylpyrrolidine (EDDP), the primary metabolite of methadone, which was consistent with increased methadone metabolism.
Continue to: Further inquiry will...
Further inquiry will clarify these concerns. Clinicians should educate patients and manage accordingly. Confirmatory tests may be ordered upon clinicians’ discretion.
Urine sample tampering. Dilution or substitution of urine samples may lead to unexpected negative results. Usually, the urine sample will have abnormal parameters, including temperature, pH, specific gravity, urine creatinine level, or detection of adulterants. If needed, consider observed urine sample collection. Jaffee et al25 reviewed tampering methods in urine drug testing.
Diversion or binge use of medications. If patients adamantly deny diverting or binge using their medication, order confirmatory tests. If the confirmatory test also is negative, modify the treatment plan accordingly, and consider the following options:
- adjust the medication dosage or frequency
- discontinue the medication
- conduct pill counts for more definitive evidence of diversion or misuse, especially if discontinuation may lead to potential harm (for example, for patients prescribed buprenorphine for opioid use disorder).
When to order confirmatory tests for unexpected negative results.
Because confirmatory tests also measure drug concentrations, clinicians sometimes order serial confirmatory testing to monitor lipophilic drugs after a patient reports discontinuation, such as in the case of a patient using marijuana, ketamine, or alprazolam. The level of a lipophilic drug, such as these 3, should continue to decline if the patient has discontinued using it. However, because the drug level is affected by how concentrated the urine samples are, it is necessary to compare the ratios of drug levels over urine creatinine levels.26 Another use for confirmatory-quantitative testing is to detect “urine spiking,”27,28 when a patient adds an unconsumed drug to his/her urine sample to produce a positive result without actually taking the drug (Box 3).
Box 3
On a confirmatory urine drug test, a patient taking buprenorphine/naloxone had a very high level of buprenorphine, but almost no norbuprenorphine (a metabolite of buprenorphine). After further discussion with the clinician, the patient admitted that he had dipped his buprenorphine/naltrexone pill in his urine sample (“spiking”) to disguise the fact that he stopped taking buprenorphine/naloxone several days ago in an effort to get high from taking opioids.
When to consult lab specialists
Because many clinicians may find it challenging to stay abreast of all of the factors necessary to properly interpret UDT results, consulting with qualified laboratory professionals is appropriate when needed. For example, a patient was prescribed codeine, and his UDTs showed morphine as anticipated; however, the prescribing clinician suspected that the patient was also using heroin. In this case, consultation with a specialist may be warranted to look for 6-mono-acetylemorphine (6-MAM, a unique heroin metabolite) and/or the ratio of morphine to codeine.
Continue to: In summary...
In summary, UDTs are important tools to use in general psychiatry practice, especially when prescribing controlled substances. To use UDTs effectively, it is essential to possess knowledge of drug metabolism and the limitations of these tests. All immunoassay results should be considered as presumptive, and confirmatory tests are often needed for making treatment decisions. Many clinicians are unlikely to possess all the knowledge needed to correctly interpret UDTs, and in some cases, communication with qualified laboratory professionals may be necessary. In addition, the patient’s history and clinical presentation, collateral information, and data from prescription drug monitoring programs are all important factors to consider.
The cost of UDTs, variable insurance coverage, and a lack of on-site laboratory services can be deterrents to implementing UDTs as recommended. These factors vary significantly across regions, facilities, and insurance providers (see Related Resources). If faced with these issues and you expect to often need UDTs in your practice, consider using point-of-care UDTs as an alternative to improve access, convenience, and possibly cost.
Bottom Line
Urine drug tests (UDTs) should be standard clinical practice when prescribing controlled substances and treating patients with substance use disorders in the outpatient setting. Clinicians need to be knowledgeable about the limitations of UDTs, drug metabolism, and relevant patient history to interpret UDTs proficiently for optimal patient care. Consult laboratory specialists when needed to help interpret the results.
Related Resources
- Islam FA, Choudhry Z. Urine drug screens: Not just for job applicants. Current Psychiatry. 2018;17(12):43-44.
- HealthCare.gov. Health benefits & coverage: Mental health & substance abuse coverage. www.healthcare.gov/coverage/mental-health-substance-abuse-coverage/.
Drug Brand Names
Alprazolam • Xanax
Amphetamine • Adderall
Atomoxetine • Strattera
Buprenorphine • Subutex
Buprenorphine/naloxone • Suboxone, Zubsolv
Bupropion • Wellbutrin, Zyban
Chlordiazepoxide • Librium
Chlorpromazine • Thorazine
Clonazepam • Klonopin
Desipramine • Norpramin
Dextroamphetamine • Dexedrine, ProCentra
Diazepam • Valium
Doxepin • Silenor
Dronabinol • Marinol
Efavirenz • Sustiva
Ephedrine • Akovaz
Fentanyl • Actiq, Duragesic
Flurazepam • Dalmane
Hydrocodone • Hysingla, Zohydro ER
Hydromorphone • Dilaudid, Exalgo
Labetalol • Normodyne, Trandate
Lamotrigine • Lamictal
Lisdexamfetamine • Vyvanse
Lithium • Eskalith, Lithobid
Lorazepam • Ativan
Meperidine • Demerol
Metformin • Fortamet, Glucophage
Methadone • Dolophine, Methadose
Methylphenidate • Ritalin
Midazolam • Versed
Morphine • Kadian, MorphaBond
Nabilone • Cesamet
Naltrexone • Vivitrol
Oxaprozin • Daypro
Oxazepam • Serax
Oxycodone • Oxycontin
Oxymorphone • Opana
Phentermine • Adipex-P, Ionamin
Promethazine • Phenergan
Quetiapine • Seroquel
Ranitidine • Zantac
Rifampicin • Rifadin
Selegiline • Eldepryl, Zelapar
Sertraline • Zoloft
Temazepam • Restoril
Thioridazine • Mellaril
Tramadol • Conzip, Ultram
Trazodone • Desyrel
Triazolam • Halcion
Venlafaxine • Effexor
Verapamil • Calan, Verelan
Zolpidem • Ambien
1. Passik SD, Schreiber J, Kirsh KL, et al. A chart review of the ordering and documentation of urine toxicology screens in a cancer center: do they influence patient management? J Pain Symptom Manag. 2000;19(1):40-44.
2. Arthur JA, Edwards T, Lu Z, et al. Frequency, predictors, and outcomes of urine drug testing among patients with advanced cancer on chronic opioid therapy at an outpatient supportive care clinic. Cancer. 2016;122(23):3732-3739.
3. Suzuki JM, Garayalde SM, Dodoo MM, et al. Psychiatry residents’ and fellows’ confidence and knowledge in interpreting urine drug testing results related to opioids. Subst Abus. 2018;39(4):518-521.
4. Reisfield GM, Bertholf R, Barkin RL, et al. Urine drug test interpretation: what do physicians know? J Opioid Manag. 2007;3(2):80-86.
5. Kirsh KL, Baxter LE, Rzetelny A, et al. A survey of ASAM members’ knowledge, attitudes, and practices in urine drug testing. J Addict Med. 2015;9(5):399-404.
6. Morasco BJ, Krebs EE, Adams MH, et al. Clinician response to aberrant urine drug test results of patients prescribed opioid therapy for chronic pain. Clin J Pain. 2019;35(1):1-6.
7. Liu RH. Comparison of common immunoassay kits for effective application in workplace drug urinalysis. Forensic Sci Rev. 1994;6(1):19-57.
8. Jannetto PJ, Fitzgerald RL. Effective use of mass spectrometry in the clinical laboratory. Clin Chem. 2016;62(1):92-98.
9. American Society of Addiction Medicine. Resources: ASAM releases white paper on drug testing. https://www.asam.org/resources/publications/magazine/read/article/2013/12/16/asam-releases-white-paper-on-drug-testing. Published December 16, 2019. Accessed June 25, 2019.
10. Fishbain DA, Cutler RB, Rosomoff HL, et al. Validity of self-reported drug use in chronic pain patients. Clin J Pain. 1999;15(3):184-191.
11. Michna E, Jamison RN, Pham LD, et al. Urine toxicology screening among chronic pain patients on opioid therapy: Frequency and predictability of abnormal findings. Clin J Pain. 2007;23(2):173-179.
12. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain--United States, 2016. JAMA. 2016;315(15):1624-1645.
13. Chou R. 2009 clinical guidelines from the American Pain Society and the American Academy of Pain medicine on the use of chronic opioid therapy in chronic noncancer pain: what are the key messages for clinical practice? Pol Arch Med Wewn. 2009;119(7-8):469-477.
14. Mihic SJ, Harris RA. Hypnotics and sedatives. In: Brunton LL, Chabner BA, Knollmann BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. New York, NY: McGrawHill Medical; 2017:343-344.
15. DePriest AZ, Puet BL, Holt AC, et al. Metabolism and disposition of prescription opioids: a review. Forensic Sci Rev. 2015;27(2):115-145.
16. Tenore PL. Advanced urine toxicology testing. J Addict Dis. 2010;29(4):436-448.
17. Brahm NC, Yeager LL, Fox MD, et al. Commonly prescribed medications and potential false-positive urine drug screens. Am J Health Syst Pharm. 2010;67(16):1344-1350.
18. Saitman A, Park HD, Fitzgerald RL. False-positive interferences of common urine drug screen immunoassays: a review. J Anal Toxicol. 2014;38(7):387-396.
19. Moeller KE, Kissack JC, Atayee RS, et al. Clinical interpretation of urine drug tests: what clinicians need to know about urine drug screens. Mayo Clin Proc. 2017;92(5):774-796.
20. Nelson ZJ, Stellpflug SJ, Engebretsen KM. What can a urine drug screening immunoassay really tell us? J Pharm Pract. 2016;29(5):516-526.
21. Reisfield GM, Goldberger BA, Bertholf RL. ‘False-positive’ and ‘false-negative’ test results in clinical urine drug testing. Bioanalysis. 2009;1(5):937-952.
22. Poklis A, Moore KA. Response of EMIT amphetamine immunoassays to urinary desoxyephedrine following Vicks inhaler use. Ther Drug Monit. 1995;17(1):89-94.
23. ElSohly MA, Feng S, Murphy TP, et al. Identification and quantitation of 11-nor-delta9-tetrahydrocannabivarin-9-carboxylic acid, a major metabolite of delta9-tetrahydrocannabivarin. J Anal Toxicol. 2001;25(6):476-480.
24. Selavka CM. Poppy seed ingestion as a contributing factor to opiate-positive urinalysis results: the pacific perspective. J Forensic Sci. 1991;36(3):685-696.
25. Jaffee WB, Trucco E, Levy S, et al. Is this urine really negative? A systematic review of tampering methods in urine drug screening and testing. J Subst Abuse Treat. 2007;33(1):33-42.
26. Fraser AD, Worth D. Urinary excretion profiles of 11-nor-9-carboxy-delta9-tetrahydrocannabinol: a delta9-thccooh to creatinine ratio study. J Anal Toxicol. 1999;23(6):531-534.
27. Holt SR, Donroe JH, Cavallo DA, et al. Addressing discordant quantitative urine buprenorphine and norbuprenorphine levels: case examples in opioid use disorder. Drug Alcohol Depend. 2018;186:171-174.
28. Accurso AJ, Lee JD, McNeely J. High prevalence of urine tampering in an office-based opioid treatment practice detected by evaluating the norbuprenorphine to buprenorphine ratio. J Subst Abuse Treat. 2017;83:62-67.
1. Passik SD, Schreiber J, Kirsh KL, et al. A chart review of the ordering and documentation of urine toxicology screens in a cancer center: do they influence patient management? J Pain Symptom Manag. 2000;19(1):40-44.
2. Arthur JA, Edwards T, Lu Z, et al. Frequency, predictors, and outcomes of urine drug testing among patients with advanced cancer on chronic opioid therapy at an outpatient supportive care clinic. Cancer. 2016;122(23):3732-3739.
3. Suzuki JM, Garayalde SM, Dodoo MM, et al. Psychiatry residents’ and fellows’ confidence and knowledge in interpreting urine drug testing results related to opioids. Subst Abus. 2018;39(4):518-521.
4. Reisfield GM, Bertholf R, Barkin RL, et al. Urine drug test interpretation: what do physicians know? J Opioid Manag. 2007;3(2):80-86.
5. Kirsh KL, Baxter LE, Rzetelny A, et al. A survey of ASAM members’ knowledge, attitudes, and practices in urine drug testing. J Addict Med. 2015;9(5):399-404.
6. Morasco BJ, Krebs EE, Adams MH, et al. Clinician response to aberrant urine drug test results of patients prescribed opioid therapy for chronic pain. Clin J Pain. 2019;35(1):1-6.
7. Liu RH. Comparison of common immunoassay kits for effective application in workplace drug urinalysis. Forensic Sci Rev. 1994;6(1):19-57.
8. Jannetto PJ, Fitzgerald RL. Effective use of mass spectrometry in the clinical laboratory. Clin Chem. 2016;62(1):92-98.
9. American Society of Addiction Medicine. Resources: ASAM releases white paper on drug testing. https://www.asam.org/resources/publications/magazine/read/article/2013/12/16/asam-releases-white-paper-on-drug-testing. Published December 16, 2019. Accessed June 25, 2019.
10. Fishbain DA, Cutler RB, Rosomoff HL, et al. Validity of self-reported drug use in chronic pain patients. Clin J Pain. 1999;15(3):184-191.
11. Michna E, Jamison RN, Pham LD, et al. Urine toxicology screening among chronic pain patients on opioid therapy: Frequency and predictability of abnormal findings. Clin J Pain. 2007;23(2):173-179.
12. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain--United States, 2016. JAMA. 2016;315(15):1624-1645.
13. Chou R. 2009 clinical guidelines from the American Pain Society and the American Academy of Pain medicine on the use of chronic opioid therapy in chronic noncancer pain: what are the key messages for clinical practice? Pol Arch Med Wewn. 2009;119(7-8):469-477.
14. Mihic SJ, Harris RA. Hypnotics and sedatives. In: Brunton LL, Chabner BA, Knollmann BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. New York, NY: McGrawHill Medical; 2017:343-344.
15. DePriest AZ, Puet BL, Holt AC, et al. Metabolism and disposition of prescription opioids: a review. Forensic Sci Rev. 2015;27(2):115-145.
16. Tenore PL. Advanced urine toxicology testing. J Addict Dis. 2010;29(4):436-448.
17. Brahm NC, Yeager LL, Fox MD, et al. Commonly prescribed medications and potential false-positive urine drug screens. Am J Health Syst Pharm. 2010;67(16):1344-1350.
18. Saitman A, Park HD, Fitzgerald RL. False-positive interferences of common urine drug screen immunoassays: a review. J Anal Toxicol. 2014;38(7):387-396.
19. Moeller KE, Kissack JC, Atayee RS, et al. Clinical interpretation of urine drug tests: what clinicians need to know about urine drug screens. Mayo Clin Proc. 2017;92(5):774-796.
20. Nelson ZJ, Stellpflug SJ, Engebretsen KM. What can a urine drug screening immunoassay really tell us? J Pharm Pract. 2016;29(5):516-526.
21. Reisfield GM, Goldberger BA, Bertholf RL. ‘False-positive’ and ‘false-negative’ test results in clinical urine drug testing. Bioanalysis. 2009;1(5):937-952.
22. Poklis A, Moore KA. Response of EMIT amphetamine immunoassays to urinary desoxyephedrine following Vicks inhaler use. Ther Drug Monit. 1995;17(1):89-94.
23. ElSohly MA, Feng S, Murphy TP, et al. Identification and quantitation of 11-nor-delta9-tetrahydrocannabivarin-9-carboxylic acid, a major metabolite of delta9-tetrahydrocannabivarin. J Anal Toxicol. 2001;25(6):476-480.
24. Selavka CM. Poppy seed ingestion as a contributing factor to opiate-positive urinalysis results: the pacific perspective. J Forensic Sci. 1991;36(3):685-696.
25. Jaffee WB, Trucco E, Levy S, et al. Is this urine really negative? A systematic review of tampering methods in urine drug screening and testing. J Subst Abuse Treat. 2007;33(1):33-42.
26. Fraser AD, Worth D. Urinary excretion profiles of 11-nor-9-carboxy-delta9-tetrahydrocannabinol: a delta9-thccooh to creatinine ratio study. J Anal Toxicol. 1999;23(6):531-534.
27. Holt SR, Donroe JH, Cavallo DA, et al. Addressing discordant quantitative urine buprenorphine and norbuprenorphine levels: case examples in opioid use disorder. Drug Alcohol Depend. 2018;186:171-174.
28. Accurso AJ, Lee JD, McNeely J. High prevalence of urine tampering in an office-based opioid treatment practice detected by evaluating the norbuprenorphine to buprenorphine ratio. J Subst Abuse Treat. 2017;83:62-67.
Strategies for improving ADHD medication adherence
Attention-deficit/hyperactivity disorder (ADHD) is the most common childhood neurodevelopmental disorder, affecting 8% to 12% of school-aged children in the United States1-3 with significant impairments that often persist into adulthood.4-8 Current guidelines recommend stimulant medication and/or behavioral therapies as first-line treatments for ADHD.9,10 There is a wealth of evidence on the efficacy of stimulants in ADHD, with the most significant effects noted on core ADHD symptoms.11,12 Additional evidence links stimulants to decreased long-term negative outcomes, including reduced school absences and grade retention,13 as well as modestly but significantly improved reading and math scores.14 Other studies have reported that individuals with ADHD who receive medication have decreased criminality,15,16 motor vehicle accidents,17,18 injuries,19 substance abuse,20-22 and risk for subsequent and concurrent depression.23 Therefore, the evidence suggests that consistent medication treatment helps improve outcomes for individuals with ADHD.
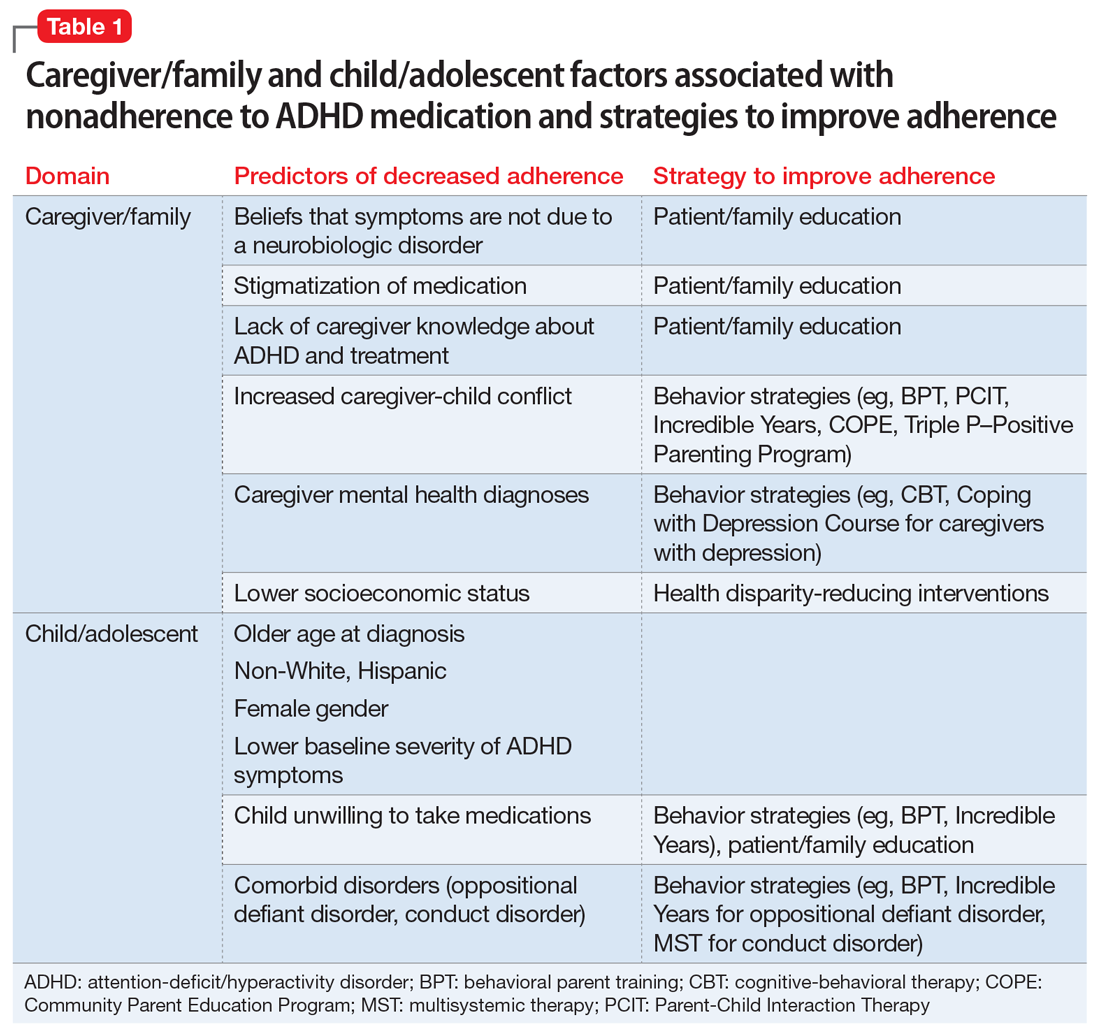
Adherence is defined as “the extent to which a person’s behavior (eg, taking medication) corresponds with agreed recommendations from a clinician.”24 Unfortunately, pediatric ADHD medication adherence has been found to be poor (approximately 64%).25-30 Nonadherence to ADHD medication has been linked to multiple factors, including caregiver/family and child/adolescent factors (Table 1), medication-related factors (Table 2), and health care/system factors (Table 3). Understanding and addressing these factors is essential to maximizing long-term outcomes. In this article, we review the factors associated with nonadherence to ADHD medication, and outline strategies to improve adherence.
Caregiver/family characteristics
Caregiver beliefs about ADHD and their attitudes toward treatment have been associated with the initiation of and adherence to ADHD medication. For example, caregivers who view a child’s difficulties as a medical disorder that requires a biologic intervention are more likely to accept and adhere to medication.31 Similarly, caregivers who perceive ADHD medication as safe, effective, and socially acceptable are more likely to be treatment-adherent.32-35
- increased caregiver knowledge about ADHD33
- receiving an ADHD diagnosis based on a thorough diagnostic process (ie, comprehensive psychological testing)36
- satisfaction with information about medicine
- comfort with the treatment plan.34
Socioeconomic status, family functioning, and caregiver mental health diagnoses (eg, ADHD, depression) have also been linked to ADHD medication adherence. Several studies, including the Multimodal Treatment Study of Children with ADHD,11 a landmark study of stimulant medication for children with ADHD, have found an association between low income and decreased likelihood of receiving ADHD medication.2,37-39 Further, Gau et al40 found that negative caregiver-child relationships and family dysfunction were associated with poor medication adherence in children with ADHD.9 Prior studies have also shown that mothers of children with ADHD are more likely to have depression and/or anxiety,41,42 and that caregivers with a history of mental health diagnoses are more accepting of initiating medication treatment for their children.43 However, additional studies have found that caregiver mental health diagnoses decreased the likelihood of ADHD medication adherence.40,44

Child characteristics
Child characteristics associated with decreased ADHD medication adherence include older age (eg, adolescents vs school-aged children),29,30,34,40,45-47 non-White race, Hispanic ethnicity,29,33,48-51 female gender,29,33,52 lower baseline ADHD symptom severity,30,37 and child unwillingness to take medications.34 However, prior studies have not been completely consistent about the relationship between child comorbid conditions (eg, oppositional defiant disorder [ODD], conduct disorder) and ADHD medication adherence. A few studies found that child comorbid conditions, especially ODD, mediate poor ADHD medication adherence, possibly secondary to an increased caregiver-child conflict.30,53,54 However, other studies have reported that the presence of comorbid ODD, depression, and anxiety predicted increased adherence to ADHD medications.37,46
Medication-related factors
Adverse effects of medications are the most commonly cited reason for ADHD medication nonadherence
On the other hand, increased ADHD medication effectiveness has been associated with improved medication adherence.5,34,54-56 Medication titration and dosing factors have also been shown to affect adherence. Specifically, adherence has been improved when ADHD medications are titrated in a systematic manner soon after starting treatment, and when families have an early first contact with a physician after starting medication (within 3 months).28 In addition, use of a simplified dose regimen has been linked to better adherence: patients who are prescribed long-acting stimulants are more likely to adhere to treatment compared with patients who take short-acting formulations.26,40,49,61-63 It is possible that long-acting stimulants increase adherence because they produce more even and sustained effects on ADHD symptoms throughout the day, compared with short-acting formulations.64 Furthermore, the inconvenience of taking multiple doses throughout the day, as well as the potential social stigma of mid-school day dosing, may negatively impact adherence to short-acting formulations.10
Continue to: Health care/system factors
Health care/system factors
Several studies have investigated the influence of health services factors on ADHD medication adherence. Specifically, limited transportation services and lack of mental health providers in the community have been linked to decreased ADHD medication adherence.47,65,66 Furthermore, limited insurance coverage and higher costs of ADHD medications, which lead to substantial out-of-pocket payments for families, have been associated with decreased likelihood of ADHD medication adherence.29,67
Clinician-related factors also can affect ADHD medication adherence. For example, a clinician’s lack knowledge of ADHD care can negatively impact ADHD medication adherence.68 Two studies have documented improved ADHD medication adherence when treatment is provided by specialists (eg, child psychiatrists) rather than by community primary care providers, possibly because specialists are more likely to provide close stimulant titration and monitoring (ie, ≥ 3 visits in the first 90 days) and use higher maximum doses.62,69 Furthermore, ADHD medication initiation and adherence are increased when patients have a strong working alliance with their clinician and trust the health care system,31,34,35 as well as when there is a match between the caregiver’s and clinician’s perception of the cause, course, and best treatment practices for a child’s ADHD.65
Strategies to improve medication adherence
A number of strategies to improve ADHD medication adherence can be derived from our knowledge of the factors that influence adherence.
Patient/family education. Unanswered questions about ADHD diagnosis, etiology, and medication adverse effects can negatively impact the ADHD treatment process. Therefore, patient/family education regarding ADHD and its management is necessary to improve medication adherence, because it helps families attain the knowledge, confidence, and motivation to manage their child’s condition.
Clinicians have an important role in educating patients about70:
- the medications they are taking
- why they are taking them
- what the medications look like
- the time of medication administration
- the potential adverse effects
- what to do if adverse effects occur
- what regular testing/monitoring is necessary.
Clinicians can provide appropriate psychoeducation by sharing written materials and trusted websites with families (see Related Resources).
Behavioral strategies. Behavioral interventions have been among the most effective strategies for improving medication adherence in other chronic conditions.71 Behavioral strategies are likely to be particularly important for families of children with ADHD and comorbid conditions such as ODD because these families experience considerable caregiver-child conflict.72 Moreover, parents of children with ADHD are at higher risk for having ADHD and depression themselves,73 both of which may interfere with a parent’s ability to obtain and administer medications consistently. Thus, for these families, using a combination of psychoeducation and behavioral strategies will be necessary to affect change in attitude and behavior. Behavioral strategies that can be used to improve medication adherence include:
- Technology-based interventions can reduce the impact of environmental barriers to adherence. For example, pharmacy automatic prescription renewal systems can reduce the likelihood of families failing to obtain ADHD medication refills. Pill reminder boxes, smartphone alerts, and setting various alarms can effectively prompt caregivers/patients to administer medication. In particular, these methods can be crucial in families for which multiple members have ADHD and its attendant difficulties with organization and task completion.
- Caregiver training may assist families in developing specific behavioral management skills that support adherence. This training can be as straightforward as instructing caregivers on the use of positive reinforcement when teaching their children to swallow pills. It may also encompass structured behavioral interventions aimed at training caregivers to utilize rewards and consequences in order to maximize medication adherence.74
Continue to: Clinician interventions
Clinician interventions. Clinicians can use decision aids to help inform families about treatment options, promote shared decision making, and decrease uncertainty about the treatment plan75 (see Related Resources). Early titration of ADHD medications and early first contact (within months of starting medication treatment) between caregivers and clinicians, whether via in-person visit, telephone, or email, have also been related to improved adherence.28 Furthermore, clinicians can improve adherence by prescribing a simplified medication regimen (ie, long-acting formulations that provide full-day coverage). To address the negative impact of high out-of-pocket ADHD medication costs on adherence, clinicians can also prescribe generic preparations and/or “preferred” medications options on an individual patient’s formulary.
Because clinician knowledge and expertise in ADHD care has been linked to improved patient medication adherence,68 clinicians are encouraged to use the American Academy of Pediatrics (AAP) guideline for diagnosis and treatment of ADHD, which includes a supplemental process of care algorithm (last published in 2011,10 with an updated guideline anticipated in 2019), as well as the AAP/National Institute for Children’s Health Quality (NICHQ) ADHD Toolkit,76 which includes items helpful for ADHD diagnosis and treatment. The Society for Developmental and Behavioral Pediatrics is also developing a clinical practice guideline for the diagnosis and treatment of complex ADHD (ie, ADHD complicated by coexisting mental health, developmental, and/or psychosocial conditions or issues), with publication anticipated in 2019. Primary care providers can also improve their expertise in ADHD care by pursuing additional mental health–related trainings (such as those conducted by the REACH Institute).77
Because receiving ADHD care from a specialist has been shown to improve medication initiation and adherence,62,69 other strategies to address the short supply of child psychiatrists include offering incentives to medical students to pursue a career in child psychiatry (eg, loan forgiveness). Telepsychiatry and co-location of mental health specialists and primary care providers are additional innovative ways in which ADHD specialty care can be delivered to more patients.64
Finally, providing culturally-sensitive care can strengthen the clinician-caregiver relationship and promote adherence to treatment. For example, clinicians can partner with local groups to increase their understanding of how different racial/ethnic groups perceive ADHD and its treatment.64
Peer support models. Peers are credible role models who have a valued role in facilitating the use of mental health services by empowering families and enhancing service satisfaction.78 In several communities in the United States, peer models using family advocates have been introduced.79 Family advocates are typically caregivers of children who have special needs or have been involved in the mental health system. Their perspective—as peers and first-hand consumers of the health care and/or mental health system—can make them powerful and effective coaches to families of children with ADHD. By helping families to navigate ADHD care systems successfully, family advocates can play an important role in enhancing ADHD medication adherence, although further investigation is needed. In addition, the stigma around ADHD medication use, which adversely impacts adherence, can be mitigated if caregivers participate in organized ADHD-related support groups (eg, Children and Adults with ADHD [CHADD]).
Continue to: Health disparity-reducing interventions
Health disparity-reducing interventions. Successful health disparity-reducing interventions—such as those developed to enhance care of other chronic disorders including asthma and diabetes—can be applied to improve ADHD care. These interventions, which include medical-legal partnerships (eg, between clinicians, social workers, legal advocates, and community partners) in primary care centers, have been shown to improve health insurance coverage and therefore health care access.80,81 Although some hardships linked to nonadherence (eg, low socioeconomic status) may not be amenable to health care–related interventions, screening for these hardships can identify children who are most at risk for poor adherence. This would alert clinicians to proactively identify barriers to adherence and implement mitigation strategies. This might include developing more streamlined, easier-to-follow management plans for these patients, such as those that can be delivered through pharmacist-physician collaborative programs82 and school-based therapy programs.83-85
Bottom Line
Suboptimal adherence to medications for attention-deficit/hyperactivity disorder (ADHD) can be addressed through patient/family education, behavioral strategies, clinician interventions, peer support models, and health disparity-reducing interventions. By improving ADHD treatment adherence, these interventions have the potential to maximize long-term outcomes.
Related Resources
- Cohen Children’s Medical Center Northwell Health. The ADHD Medication Guide. www.ADHDMedicationGuide.com. Revised December 31, 2017.
- Cincinnati Children’s Hospital Medical Center. Decision aids to facilitate shared decision making in practice. www.cincinnatichildrens.org/service/j/anderson-center/ evidence-based-care/decision-aids.
- CHADD. Children and Adults with attention-deficit/ hyperactivity disorder. www.chadd.org.
Drug Brand Name
Methylphenidate • Concerta, Ritalin
1. Froehlich TE, Lanphear BP, Epstein JN, et al. Prevalence, recognition, and treatment of attention-deficit/hyperactivity disorder in a national sample of US children. Arch Pediatr Adolesc Med. 2007;161(9):857-864.
2. Visser SN, Lesesne CA, Perou R. National estimates and factors associated with medication treatment for childhood attention-deficit/hyperactivity disorder. Pediatrics. 2007;119 (Suppl 1):S99-S106.
3. Danielson ML, Bitsko RH, Ghandour RM, et al. Prevalence of parent-reported ADHD diagnosis and associated treatment among U.S. children and adolescents, 2016. J Clin Child Adolesc Psychol. 2018;47(2):199-212.
4. Molina BS, Hinshaw SP, Swanson JM, et al. The MTA at 8 years: prospective follow-up of children treated for combined-type ADHD in a multisite study. J Am Acad Child Adolesc Psychiatry. 2009;48(5):484-500.
5. Charach A, Dashti B, Carson P, et al. Attention deficit hyperactivity disorder: effectiveness of treatment in at-risk preschoolers; long-term effectiveness in all ages; and variability in prevalence, diagnosis, and treatment. Rockville, MD: Agency for Healthcare Research and Quality; 2011. http://www.ncbi.nlm.nih.gov/books/NBK82368/.
6. Wehmeier PM, Schacht A, Barkley RA. Social and emotional impairment in children and adolescents with ADHD and the impact on quality of life. J Adolesc Health. 2010;46(3):209-217.
7. Barkley RA, Fischer M, Smallish L, et al. Young adult outcome of hyperactive children: adaptive functioning in major life activities. J Am Acad Child Adolesc Psychiatry. 2006;45(2):192-202.
8. Spencer TJ, Biederman J, Mick E. Attention-deficit/hyperactivity disorder: diagnosis, lifespan, comorbidities, and neurobiology. J Pediatr Psychol. 2007;32(6):631-642.
9. Pliszka S, the AACAP Work Group on Quality Issues. Practice parameter for the assessment and treatment of children and adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2007;46(7):894-921.
10. Subcommittee on Attention-Deficit/Hyperactivity Disorder; Steering Committee on Quality Improvement and Management. ADHD: clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics. 2011;128(5):1007-1022.
11. A 14-month randomized clinical trial of treatment strategies for attention-deficit/hyperactivity disorder. The MTA Cooperative Group. Multimodal Treatment Study of Children with ADHD. Arch Gen Psychiatry. 1999;56(12):1073-1086.
12. Abikoff H, Hechtman L, Klein RG, et al. Symptomatic improvement in children with ADHD treated with long-term methylphenidate and multimodal psychosocial treatment. J Am Acad Child Adolesc Psychiatry. 2004;43(7):802-811.
13. Barbaresi WJ, Katusic SK, Colligan RC, et al. Long-term school outcomes for children with attention-deficit/hyperactivity disorder: a population-based perspective. J Dev Behav Pediatr. 2007;28(4):265-273.
14. Scheffler RM, Brown TT, Fulton BD, et al. Positive association between attention-deficit/ hyperactivity disorder medication use and academic achievement during elementary school. Pediatrics. 2009;123(5):1273-1279.
15. Dalsgaard S, Nielsen HS, Simonsen M. Five-fold increase in national prevalence rates of attention-deficit/hyperactivity disorder medications for children and adolescents with autism spectrum disorder, attention-deficit/hyperactivity disorder, and other psychiatric disorders: a Danish register-based study. J Child Adolesc Psychopharmacol. 2013;23(7):432-439.
16. Lichtenstein P, Halldner L, Zetterqvist J, et al. Medication for attention deficit-hyperactivity disorder and criminality. N Engl J Med. 2012;367(21):2006-2014.
17. Chang Z, Lichtenstein P, D’Onofrio BM, et al. Serious transport accidents in adults with attention-deficit/hyperactivity disorder and the effect of medication: a population-based study. JAMA Psychiatry. 2014;71(3):319-325.
18. Chang Z, Quinn PD, Hur K, et al. Association between medication use for attention-deficit/hyperactivity disorder and risk of motor vehicle crashes. JAMA Psychiatry. 2017;74(6):597-603.
19. Dalsgaard S, Leckman JF, Mortensen PB, et al. Effect of drugs on the risk of injuries in children with attention deficit hyperactivity disorder: a prospective cohort study. Lancet Psychiatry. 2015;2(8):702-709.
20. Chang Z, Lichtenstein P, Halldner L, et al. Stimulant ADHD medication and risk for substance abuse. J Child Psychol Psychiatry. 2014;55(8):878-885.
21. Fischer M, Barkley RA. Childhood stimulant treatment and risk for later substance abuse. J Clin Psychiatry. 2003;64(Suppl 11):19-23.
22. Biederman J. Pharmacotherapy for attention-deficit/hyperactivity disorder (ADHD) decreases the risk for substance abuse: findings from a longitudinal follow-up of youths with and without ADHD. J Clin Psychiatry. 2003;64(Suppl 11):3-8.
23. Chang Z, D’Onofrio BM, Quinn PD, et al. Medicationfor attention-deficit/hyperactivity disorder and risk for depression: a nationwide longitudinal cohort study. Biol Psychiatry. 2016;80(12):916-922.
24. World Health Organization. Adherence to long-term therapies: evidence for action. https://www.who.int/chp/knowledge/publications/adherence_full_report.pdf?ua=1. Published 2003. Accessed July 22, 2019.
25. Perwien A, Hall J, Swensen A, et al. Stimulant treatment patterns and compliance in children and adults with newly treated attention-deficit/hyperactivity disorder. J Manag Care Pharm. 2004;10(2):122-129.
26. Faraone SV, Biederman J, Zimmerman B. An analysis of patient adherence to treatment during a 1-year, open-label study of OROS methylphenidate in children with ADHD. J Atten Disord. 2007;11(2):157-166.
27. Barner JC, Khoza S, Oladapo A. ADHD medication use, adherence, persistence and cost among Texas Medicaid children. Curr Med Res Opin. 2011;27(Suppl 2):13-22.
28. Brinkman WB, Baum R, Kelleher KJ, et al. Relationship between attention-deficit/hyperactivity disorder care and medication continuity. J Am Acad Child Adolesc Psychiatry. 2016;55(4):289-294.
29. Bokhari FAS, Heiland F, Levine P, et al. Risk factors for discontinuing drug therapy among children with ADHD. Health Services and Outcomes Research Methodology. 2008;8(3):134-158.
30. Thiruchelvam D, Charach A, Schachar RJ. Moderators and mediators of long-term adherence to stimulant treatment in children with ADHD. J Am Acad Child Adolesc Psychiatry. 2001;40(8):922-928.
31. DosReis S, Mychailyszyn MP, Evans-Lacko SE, et al. The meaning of attention-deficit/hyperactivity disorder medication and parents’ initiation and continuity of treatment for their child. J Child Adolesc Psychopharmacol. 2009;19(4):377-383.
32. dosReis S, Myers MA. Parental attitudes and involvement in psychopharmacological treatment for ADHD: a conceptual model. Int Rev Psychiatry. 2008;20(2):135-141.
33. Bussing R, Koro-Ljungberg M, Noguchi K, et al. Willingness to use ADHD treatments: a mixed methods study of perceptions by adolescents, parents, health professionals and teachers. Soc Sci Med. 2012;74(1):92-100.
34. Brinkman WB, Sucharew H, Majcher JH, et al. Predictors of medication continuity in children with ADHD. Pediatrics. 2018;141(6). doi: 10.1542/peds.2017-2580.
35. Coletti DJ, Pappadopulos E, Katsiotas NJ, et al. Parent perspectives on the decision to initiate medication treatment of attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2012;22(3):226-237.
36. Bussing R, Gary FA. Practice guidelines and parental ADHD treatment evaluations: friends or foes? Harv Rev Psychiatry. 2001;9(5):223-233.
37. Charach A, Gajaria A. Improving psychostimulant adherence in children with ADHD. Expert Rev Neurother. 2008;8(10):1563-1571.
38. Rieppi R, Greenhill LL, Ford RE, et al. Socioeconomic status as a moderator of ADHD treatment outcomes. J Am Acad Child Adolesc Psychiatry. 2002;41(3):269-277.
39. Swanson JM, Hinshaw SP, Arnold LE, et al. Secondary evaluations of MTA 36-month outcomes: propensity score and growth mixture model analyses. J Am Acad Child Adolesc Psychiatry. 2007;46(8):1003-1014.
40. Gau SS, Shen HY, Chou MC, et al. Determinants of adherence to methylphenidate and the impact of poor adherence on maternal and family measures. J Child Adolesc Psychopharmacol. 2006;16(3):286-297.
41. Barkley RA, Fischer M, Edelbrock C, et al. The adolescent outcome of hyperactive children diagnosed by research criteria--III. Mother-child interactions, family conflicts and maternal psychopathology. J Child Psychol Psychiatry. 1991;32(2):233-255.
42. Kashdan TB, Jacob RG, Pelham WE, et al. Depression and anxiety in parents of children with ADHD and varying levels of oppositional defiant behaviors: modeling relationships with family functioning. J Clin Child Adolesc Psychol. 2004;33(1):169-181.
43. Chavira DA, Stein MB, Bailey K, et al. Parental opinions regarding treatment for social anxiety disorder in youth. J Dev Behav Pediatr. 2003;24(5):315-322.
44. Leslie LK, Aarons GA, Haine RA, et al. Caregiver depression and medication use by youths with ADHD who receive services in the public sector. Psychiatr Serv. 2007;58(1):131-134.
45. Barbaresi WJ, Katusic SK, Colligan RC, et al. Long-term stimulant medication treatment of attention-deficit/hyperactivity disorder: results from a population-based study. J Dev Behav Pediatr. 2006;27(1):1-10.
46. Atzori P, Usala T, Carucci S, et al. Predictive factors for persistent use and compliance of immediate-release methylphenidate: a 36-month naturalistic study. J Child Adolesc Psychopharmacol. 2009;19(6):673-681.
47. Chen CY, Yeh HH, Chen KH, et al. Differential effects of predictors on methylphenidate initiation and discontinuation among young people with newly diagnosed attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2011;21(3):265-273.
48. Winterstein AG, Gerhard T, Shuster J, et al. Utilization of pharmacologic treatment in youths with attention deficit/hyperactivity disorder in Medicaid database. Ann Pharmacother. 2008;42(1):24-31.
49. Marcus SC, Wan GJ, Kemner JE, et al. Continuity of methylphenidate treatment for attention-deficit/hyperactivity disorder. Arch Pediatr Adolesc Med. 2005;159(6):572-578.
50. Cummings JR JX, Allen L, Lally C, et al. Racial and ethnic differences in ADHD treatment quality among Medicaid-enrolled youth. Pediatrics. 2017;139(6):e2016-e2044.
51. Hudson JL, Miller GE, Kirby JB. Explaining racial and ethnic differences in children’s use of stimulant medications. Med Care. 2007;45(11):1068-1075.
52. van den Ban E, Souverein PC, Swaab H, et al. Less discontinuation of ADHD drug use since the availability of long-acting ADHD medication in children, adolescents and adults under the age of 45 years in the Netherlands. Atten Defic Hyperact Disord. 2010;2(4):213-220.
53. Charach A, Ickowicz A, Schachar R. Stimulant treatment over five years: adherence, effectiveness, and adverse effects. J Am Acad Child Adolesc Psychiatry. 2004;43(5):559-567.
54. Toomey SL, Sox CM, Rusinak D, et al. Why do children with ADHD discontinue their medication? Clin Pediatr (Phila). 2012;51(8):763-769.
55. Brinkman WB, Simon JO, Epstein JN. Reasons why children and adolescents with attention-deficit/hyperactivity disorder stop and restart taking medicine. Acad Pediatr. 2018;18(3):273-280.
56. Wehmeier PM, Dittmann RW, Banaschewski T. Treatment compliance or medication adherence in children and adolescents on ADHD medication in clinical practice: results from the COMPLY observational study. Atten Defic Hyperact Disord. 2015;7(2):165-174.
57. Frank E, Ozon C, Nair V, et al. Examining why patients with attention-deficit/hyperactivity disorder lack adherence to medication over the long term: a review and analysis. J Clin Psychiatry. 2015;76(11):e1459-e1468.
58. Pozzi M, Carnovale C, Peeters G, et al. Adverse drug events related to mood and emotion in paediatric patients treated for ADHD: a meta-analysis. J Affect Disord. 2018;238:161-178.
59. Stuckelman ZD, Mulqueen JM, Ferracioli-Oda E, et al. Risk of irritability with psychostimulant treatment in children with ADHD: a meta-analysis. J Clin Psychiatry. 2017;78(6):e648-e655.
60. Cortese S, Adamo N, Del Giovane C, et al. Comparative efficacy and tolerability of medications for attention-deficit hyperactivity disorder in children, adolescents, and adults: a systematic review and network meta-analysis. Lancet Psychiatry. 2018;5(9):727-738.
61. Lawson KA, Johnsrud M, Hodgkins P, et al. Utilization patterns of stimulants in ADHD in the Medicaid population: a retrospective analysis of data from the Texas Medicaid program. Clin Ther. 2012;34(4):944-956 e944.
62. Olfson M, Marcus S, Wan G. Stimulant dosing for children with ADHD: a medical claims analysis. J Am Acad Child Adolesc Psychiatry. 2009;48(1):51-59.
63. Jensen PS, Arnold LE, Swanson JM, et al. 3-year follow-up of the NIMH MTA study. J Am Acad Child Adolesc Psychiatry. 2007;46(8):989-1002.
64. Van Cleave J, Leslie LK. Approaching ADHD as a chronic condition: implications for long-term adherence. Pediatr Ann. 2008;37(1):19-26.
65. Leslie LK, Plemmons D, Monn AR, et al. Investigating ADHD treatment trajectories: listening to families’ stories about medication use. J Dev Behav Pediatr. 2007;28(3):179-188.
66. Fiks AG, Mayne S, Localio AR, et al. Shared decision making and behavioral impairment: a national study among children with special health care needs. BMC Pediatr. 2012;12:153.
67. Stevens J, Harman JS, Kelleher KJ. Race/ethnicity and insurance status as factors associated with ADHD treatment patterns. J Child Adolesc Psychopharmacol. 2005;15(1):88-96.
68. Charach A, Skyba A, Cook L, et al. Using stimulant medication for children with ADHD: what do parents say? A brief report. J Can Acad Child Adolesc Psychiatry. 2006;15(2):75-83.
69. Chen CY, Gerhard T, Winterstein AG. Determinants of initial pharmacological treatment for youths with attention-deficit/hyperactivity disorder. J Child Adolescent Psychopharmacol. 2009;19(2):187-195.
70. National Council on Patient Information and Education. Enhancing prescription medication adherence: a national action plan. http://www.bemedwise.org/docs/enhancingprescriptionmedicineadherence.pdf. Published August 2007. Accessed July 22, 2019.
71. Kahana S, Drotar D, Frazier T. Meta-analysis of psychological interventions to promote adherence to treatment in pediatric chronic health conditions. J Pediatr Psychol. 2008;33(6):590-611.
72. Johnston C, Mash EJ. Families of children with attention-deficit/hyperactivity disorder: review and recommendations for future research. Clin Child Fam Psychol Rev. 2001;4(3):183-207.
73. Chronis AM, Lahey BB, Pelham WE Jr., et al. Psychopathology and substance abuse in parents of young children with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2003;42(12):1424-1432.
74. Chacko A, Newcorn JH, Feirsen N, et al. Improving medication adherence in chronic pediatric health conditions: a focus on ADHD in youth. Curr Pharm Des. 2010;16(22):2416-2423.
75. Brinkman WB, Hartl Majcher J, Polling LM, et al. Shared decision-making to improve attention-deficit hyperactivity disorder care. Patient Educ Couns. 2013;93(1):95-101.
76. American Academy of Pediatrics. Caring for children with ADHD: a resource toolkit for clinicians. 2nd ed. https://www.aap.org/en-us/pubserv/adhd2/Pages/default.aspx. Published 2011. Accessed July 22, 2019.
77. The REACH Institute. Course dates and registration. http://www.thereachinstitute.org/services/for-primary-care-practitioners/training-dates-and-registration. Accessed July 22, 2019.
78. Sells D, Davidson L, Jewell C, et al. The treatment relationship in peer-based and regular case management for clients with severe mental illness. Psychiatr Serv. 2006;57(8):1179-1184.
79. Hoagwood KE, Green E, Kelleher K, et al. Family advocacy, support and education in children’s mental health: results of a national survey. Adm Policy Ment Health. 2008;35(1-2):73-83.
80. Klein MD, Beck AF, Henize AW, et al. Doctors and lawyers collaborating to HeLP children—outcomes from a successful partnership between professions. J Health Care Poor Underserved. 2013;24(3):1063-1073.
81. Weintraub D, Rodgers MA, Botcheva L, et al. Pilot study of medical-legal partnership to address social and legal needs of patients. J Health Care Poor Underserved. 2010;21(Suppl 2):157-168.
82. Bradley CL, Luder HR, Beck AF, et al. Pediatric asthma medication therapy management through community pharmacy and primary care collaboration. J Am Pharm Assoc (2003). 2016;56(4):455-460.
83. Noyes K, Bajorska A, Fisher S, et al. Cost-effectiveness of the school-based asthma therapy (SBAT) program. Pediatrics. 2013;131(3):e709-e717.
84. Halterman JS, Fagnano M, Montes G, et al. The school-based preventive asthma care trial: results of a pilot study. J Pediatr. 2012;161(6):1109-1115.
85. Halterman JS, Szilagyi PG, Fisher SG, et al. Randomized controlled trial to improve care for urban children with asthma: results of the school-based asthma therapy trial. Arch Pediatr Adolesc Med. 2011;165(3):262-268.
Attention-deficit/hyperactivity disorder (ADHD) is the most common childhood neurodevelopmental disorder, affecting 8% to 12% of school-aged children in the United States1-3 with significant impairments that often persist into adulthood.4-8 Current guidelines recommend stimulant medication and/or behavioral therapies as first-line treatments for ADHD.9,10 There is a wealth of evidence on the efficacy of stimulants in ADHD, with the most significant effects noted on core ADHD symptoms.11,12 Additional evidence links stimulants to decreased long-term negative outcomes, including reduced school absences and grade retention,13 as well as modestly but significantly improved reading and math scores.14 Other studies have reported that individuals with ADHD who receive medication have decreased criminality,15,16 motor vehicle accidents,17,18 injuries,19 substance abuse,20-22 and risk for subsequent and concurrent depression.23 Therefore, the evidence suggests that consistent medication treatment helps improve outcomes for individuals with ADHD.

Adherence is defined as “the extent to which a person’s behavior (eg, taking medication) corresponds with agreed recommendations from a clinician.”24 Unfortunately, pediatric ADHD medication adherence has been found to be poor (approximately 64%).25-30 Nonadherence to ADHD medication has been linked to multiple factors, including caregiver/family and child/adolescent factors (Table 1), medication-related factors (Table 2), and health care/system factors (Table 3). Understanding and addressing these factors is essential to maximizing long-term outcomes. In this article, we review the factors associated with nonadherence to ADHD medication, and outline strategies to improve adherence.

Caregiver/family characteristics
Caregiver beliefs about ADHD and their attitudes toward treatment have been associated with the initiation of and adherence to ADHD medication. For example, caregivers who view a child’s difficulties as a medical disorder that requires a biologic intervention are more likely to accept and adhere to medication.31 Similarly, caregivers who perceive ADHD medication as safe, effective, and socially acceptable are more likely to be treatment-adherent.32-35
- increased caregiver knowledge about ADHD33
- receiving an ADHD diagnosis based on a thorough diagnostic process (ie, comprehensive psychological testing)36
- satisfaction with information about medicine
- comfort with the treatment plan.34
Socioeconomic status, family functioning, and caregiver mental health diagnoses (eg, ADHD, depression) have also been linked to ADHD medication adherence. Several studies, including the Multimodal Treatment Study of Children with ADHD,11 a landmark study of stimulant medication for children with ADHD, have found an association between low income and decreased likelihood of receiving ADHD medication.2,37-39 Further, Gau et al40 found that negative caregiver-child relationships and family dysfunction were associated with poor medication adherence in children with ADHD.9 Prior studies have also shown that mothers of children with ADHD are more likely to have depression and/or anxiety,41,42 and that caregivers with a history of mental health diagnoses are more accepting of initiating medication treatment for their children.43 However, additional studies have found that caregiver mental health diagnoses decreased the likelihood of ADHD medication adherence.40,44

Child characteristics
Child characteristics associated with decreased ADHD medication adherence include older age (eg, adolescents vs school-aged children),29,30,34,40,45-47 non-White race, Hispanic ethnicity,29,33,48-51 female gender,29,33,52 lower baseline ADHD symptom severity,30,37 and child unwillingness to take medications.34 However, prior studies have not been completely consistent about the relationship between child comorbid conditions (eg, oppositional defiant disorder [ODD], conduct disorder) and ADHD medication adherence. A few studies found that child comorbid conditions, especially ODD, mediate poor ADHD medication adherence, possibly secondary to an increased caregiver-child conflict.30,53,54 However, other studies have reported that the presence of comorbid ODD, depression, and anxiety predicted increased adherence to ADHD medications.37,46
Medication-related factors
Adverse effects of medications are the most commonly cited reason for ADHD medication nonadherence
On the other hand, increased ADHD medication effectiveness has been associated with improved medication adherence.5,34,54-56 Medication titration and dosing factors have also been shown to affect adherence. Specifically, adherence has been improved when ADHD medications are titrated in a systematic manner soon after starting treatment, and when families have an early first contact with a physician after starting medication (within 3 months).28 In addition, use of a simplified dose regimen has been linked to better adherence: patients who are prescribed long-acting stimulants are more likely to adhere to treatment compared with patients who take short-acting formulations.26,40,49,61-63 It is possible that long-acting stimulants increase adherence because they produce more even and sustained effects on ADHD symptoms throughout the day, compared with short-acting formulations.64 Furthermore, the inconvenience of taking multiple doses throughout the day, as well as the potential social stigma of mid-school day dosing, may negatively impact adherence to short-acting formulations.10
Continue to: Health care/system factors
Health care/system factors
Several studies have investigated the influence of health services factors on ADHD medication adherence. Specifically, limited transportation services and lack of mental health providers in the community have been linked to decreased ADHD medication adherence.47,65,66 Furthermore, limited insurance coverage and higher costs of ADHD medications, which lead to substantial out-of-pocket payments for families, have been associated with decreased likelihood of ADHD medication adherence.29,67
Clinician-related factors also can affect ADHD medication adherence. For example, a clinician’s lack knowledge of ADHD care can negatively impact ADHD medication adherence.68 Two studies have documented improved ADHD medication adherence when treatment is provided by specialists (eg, child psychiatrists) rather than by community primary care providers, possibly because specialists are more likely to provide close stimulant titration and monitoring (ie, ≥ 3 visits in the first 90 days) and use higher maximum doses.62,69 Furthermore, ADHD medication initiation and adherence are increased when patients have a strong working alliance with their clinician and trust the health care system,31,34,35 as well as when there is a match between the caregiver’s and clinician’s perception of the cause, course, and best treatment practices for a child’s ADHD.65
Strategies to improve medication adherence
A number of strategies to improve ADHD medication adherence can be derived from our knowledge of the factors that influence adherence.
Patient/family education. Unanswered questions about ADHD diagnosis, etiology, and medication adverse effects can negatively impact the ADHD treatment process. Therefore, patient/family education regarding ADHD and its management is necessary to improve medication adherence, because it helps families attain the knowledge, confidence, and motivation to manage their child’s condition.
Clinicians have an important role in educating patients about70:
- the medications they are taking
- why they are taking them
- what the medications look like
- the time of medication administration
- the potential adverse effects
- what to do if adverse effects occur
- what regular testing/monitoring is necessary.
Clinicians can provide appropriate psychoeducation by sharing written materials and trusted websites with families (see Related Resources).
Behavioral strategies. Behavioral interventions have been among the most effective strategies for improving medication adherence in other chronic conditions.71 Behavioral strategies are likely to be particularly important for families of children with ADHD and comorbid conditions such as ODD because these families experience considerable caregiver-child conflict.72 Moreover, parents of children with ADHD are at higher risk for having ADHD and depression themselves,73 both of which may interfere with a parent’s ability to obtain and administer medications consistently. Thus, for these families, using a combination of psychoeducation and behavioral strategies will be necessary to affect change in attitude and behavior. Behavioral strategies that can be used to improve medication adherence include:
- Technology-based interventions can reduce the impact of environmental barriers to adherence. For example, pharmacy automatic prescription renewal systems can reduce the likelihood of families failing to obtain ADHD medication refills. Pill reminder boxes, smartphone alerts, and setting various alarms can effectively prompt caregivers/patients to administer medication. In particular, these methods can be crucial in families for which multiple members have ADHD and its attendant difficulties with organization and task completion.
- Caregiver training may assist families in developing specific behavioral management skills that support adherence. This training can be as straightforward as instructing caregivers on the use of positive reinforcement when teaching their children to swallow pills. It may also encompass structured behavioral interventions aimed at training caregivers to utilize rewards and consequences in order to maximize medication adherence.74
Continue to: Clinician interventions
Clinician interventions. Clinicians can use decision aids to help inform families about treatment options, promote shared decision making, and decrease uncertainty about the treatment plan75 (see Related Resources). Early titration of ADHD medications and early first contact (within months of starting medication treatment) between caregivers and clinicians, whether via in-person visit, telephone, or email, have also been related to improved adherence.28 Furthermore, clinicians can improve adherence by prescribing a simplified medication regimen (ie, long-acting formulations that provide full-day coverage). To address the negative impact of high out-of-pocket ADHD medication costs on adherence, clinicians can also prescribe generic preparations and/or “preferred” medications options on an individual patient’s formulary.
Because clinician knowledge and expertise in ADHD care has been linked to improved patient medication adherence,68 clinicians are encouraged to use the American Academy of Pediatrics (AAP) guideline for diagnosis and treatment of ADHD, which includes a supplemental process of care algorithm (last published in 2011,10 with an updated guideline anticipated in 2019), as well as the AAP/National Institute for Children’s Health Quality (NICHQ) ADHD Toolkit,76 which includes items helpful for ADHD diagnosis and treatment. The Society for Developmental and Behavioral Pediatrics is also developing a clinical practice guideline for the diagnosis and treatment of complex ADHD (ie, ADHD complicated by coexisting mental health, developmental, and/or psychosocial conditions or issues), with publication anticipated in 2019. Primary care providers can also improve their expertise in ADHD care by pursuing additional mental health–related trainings (such as those conducted by the REACH Institute).77
Because receiving ADHD care from a specialist has been shown to improve medication initiation and adherence,62,69 other strategies to address the short supply of child psychiatrists include offering incentives to medical students to pursue a career in child psychiatry (eg, loan forgiveness). Telepsychiatry and co-location of mental health specialists and primary care providers are additional innovative ways in which ADHD specialty care can be delivered to more patients.64
Finally, providing culturally-sensitive care can strengthen the clinician-caregiver relationship and promote adherence to treatment. For example, clinicians can partner with local groups to increase their understanding of how different racial/ethnic groups perceive ADHD and its treatment.64
Peer support models. Peers are credible role models who have a valued role in facilitating the use of mental health services by empowering families and enhancing service satisfaction.78 In several communities in the United States, peer models using family advocates have been introduced.79 Family advocates are typically caregivers of children who have special needs or have been involved in the mental health system. Their perspective—as peers and first-hand consumers of the health care and/or mental health system—can make them powerful and effective coaches to families of children with ADHD. By helping families to navigate ADHD care systems successfully, family advocates can play an important role in enhancing ADHD medication adherence, although further investigation is needed. In addition, the stigma around ADHD medication use, which adversely impacts adherence, can be mitigated if caregivers participate in organized ADHD-related support groups (eg, Children and Adults with ADHD [CHADD]).
Continue to: Health disparity-reducing interventions
Health disparity-reducing interventions. Successful health disparity-reducing interventions—such as those developed to enhance care of other chronic disorders including asthma and diabetes—can be applied to improve ADHD care. These interventions, which include medical-legal partnerships (eg, between clinicians, social workers, legal advocates, and community partners) in primary care centers, have been shown to improve health insurance coverage and therefore health care access.80,81 Although some hardships linked to nonadherence (eg, low socioeconomic status) may not be amenable to health care–related interventions, screening for these hardships can identify children who are most at risk for poor adherence. This would alert clinicians to proactively identify barriers to adherence and implement mitigation strategies. This might include developing more streamlined, easier-to-follow management plans for these patients, such as those that can be delivered through pharmacist-physician collaborative programs82 and school-based therapy programs.83-85
Bottom Line
Suboptimal adherence to medications for attention-deficit/hyperactivity disorder (ADHD) can be addressed through patient/family education, behavioral strategies, clinician interventions, peer support models, and health disparity-reducing interventions. By improving ADHD treatment adherence, these interventions have the potential to maximize long-term outcomes.
Related Resources
- Cohen Children’s Medical Center Northwell Health. The ADHD Medication Guide. www.ADHDMedicationGuide.com. Revised December 31, 2017.
- Cincinnati Children’s Hospital Medical Center. Decision aids to facilitate shared decision making in practice. www.cincinnatichildrens.org/service/j/anderson-center/ evidence-based-care/decision-aids.
- CHADD. Children and Adults with attention-deficit/ hyperactivity disorder. www.chadd.org.
Drug Brand Name
Methylphenidate • Concerta, Ritalin
Attention-deficit/hyperactivity disorder (ADHD) is the most common childhood neurodevelopmental disorder, affecting 8% to 12% of school-aged children in the United States1-3 with significant impairments that often persist into adulthood.4-8 Current guidelines recommend stimulant medication and/or behavioral therapies as first-line treatments for ADHD.9,10 There is a wealth of evidence on the efficacy of stimulants in ADHD, with the most significant effects noted on core ADHD symptoms.11,12 Additional evidence links stimulants to decreased long-term negative outcomes, including reduced school absences and grade retention,13 as well as modestly but significantly improved reading and math scores.14 Other studies have reported that individuals with ADHD who receive medication have decreased criminality,15,16 motor vehicle accidents,17,18 injuries,19 substance abuse,20-22 and risk for subsequent and concurrent depression.23 Therefore, the evidence suggests that consistent medication treatment helps improve outcomes for individuals with ADHD.

Adherence is defined as “the extent to which a person’s behavior (eg, taking medication) corresponds with agreed recommendations from a clinician.”24 Unfortunately, pediatric ADHD medication adherence has been found to be poor (approximately 64%).25-30 Nonadherence to ADHD medication has been linked to multiple factors, including caregiver/family and child/adolescent factors (Table 1), medication-related factors (Table 2), and health care/system factors (Table 3). Understanding and addressing these factors is essential to maximizing long-term outcomes. In this article, we review the factors associated with nonadherence to ADHD medication, and outline strategies to improve adherence.

Caregiver/family characteristics
Caregiver beliefs about ADHD and their attitudes toward treatment have been associated with the initiation of and adherence to ADHD medication. For example, caregivers who view a child’s difficulties as a medical disorder that requires a biologic intervention are more likely to accept and adhere to medication.31 Similarly, caregivers who perceive ADHD medication as safe, effective, and socially acceptable are more likely to be treatment-adherent.32-35
- increased caregiver knowledge about ADHD33
- receiving an ADHD diagnosis based on a thorough diagnostic process (ie, comprehensive psychological testing)36
- satisfaction with information about medicine
- comfort with the treatment plan.34
Socioeconomic status, family functioning, and caregiver mental health diagnoses (eg, ADHD, depression) have also been linked to ADHD medication adherence. Several studies, including the Multimodal Treatment Study of Children with ADHD,11 a landmark study of stimulant medication for children with ADHD, have found an association between low income and decreased likelihood of receiving ADHD medication.2,37-39 Further, Gau et al40 found that negative caregiver-child relationships and family dysfunction were associated with poor medication adherence in children with ADHD.9 Prior studies have also shown that mothers of children with ADHD are more likely to have depression and/or anxiety,41,42 and that caregivers with a history of mental health diagnoses are more accepting of initiating medication treatment for their children.43 However, additional studies have found that caregiver mental health diagnoses decreased the likelihood of ADHD medication adherence.40,44

Child characteristics
Child characteristics associated with decreased ADHD medication adherence include older age (eg, adolescents vs school-aged children),29,30,34,40,45-47 non-White race, Hispanic ethnicity,29,33,48-51 female gender,29,33,52 lower baseline ADHD symptom severity,30,37 and child unwillingness to take medications.34 However, prior studies have not been completely consistent about the relationship between child comorbid conditions (eg, oppositional defiant disorder [ODD], conduct disorder) and ADHD medication adherence. A few studies found that child comorbid conditions, especially ODD, mediate poor ADHD medication adherence, possibly secondary to an increased caregiver-child conflict.30,53,54 However, other studies have reported that the presence of comorbid ODD, depression, and anxiety predicted increased adherence to ADHD medications.37,46
Medication-related factors
Adverse effects of medications are the most commonly cited reason for ADHD medication nonadherence
On the other hand, increased ADHD medication effectiveness has been associated with improved medication adherence.5,34,54-56 Medication titration and dosing factors have also been shown to affect adherence. Specifically, adherence has been improved when ADHD medications are titrated in a systematic manner soon after starting treatment, and when families have an early first contact with a physician after starting medication (within 3 months).28 In addition, use of a simplified dose regimen has been linked to better adherence: patients who are prescribed long-acting stimulants are more likely to adhere to treatment compared with patients who take short-acting formulations.26,40,49,61-63 It is possible that long-acting stimulants increase adherence because they produce more even and sustained effects on ADHD symptoms throughout the day, compared with short-acting formulations.64 Furthermore, the inconvenience of taking multiple doses throughout the day, as well as the potential social stigma of mid-school day dosing, may negatively impact adherence to short-acting formulations.10
Continue to: Health care/system factors
Health care/system factors
Several studies have investigated the influence of health services factors on ADHD medication adherence. Specifically, limited transportation services and lack of mental health providers in the community have been linked to decreased ADHD medication adherence.47,65,66 Furthermore, limited insurance coverage and higher costs of ADHD medications, which lead to substantial out-of-pocket payments for families, have been associated with decreased likelihood of ADHD medication adherence.29,67
Clinician-related factors also can affect ADHD medication adherence. For example, a clinician’s lack knowledge of ADHD care can negatively impact ADHD medication adherence.68 Two studies have documented improved ADHD medication adherence when treatment is provided by specialists (eg, child psychiatrists) rather than by community primary care providers, possibly because specialists are more likely to provide close stimulant titration and monitoring (ie, ≥ 3 visits in the first 90 days) and use higher maximum doses.62,69 Furthermore, ADHD medication initiation and adherence are increased when patients have a strong working alliance with their clinician and trust the health care system,31,34,35 as well as when there is a match between the caregiver’s and clinician’s perception of the cause, course, and best treatment practices for a child’s ADHD.65
Strategies to improve medication adherence
A number of strategies to improve ADHD medication adherence can be derived from our knowledge of the factors that influence adherence.
Patient/family education. Unanswered questions about ADHD diagnosis, etiology, and medication adverse effects can negatively impact the ADHD treatment process. Therefore, patient/family education regarding ADHD and its management is necessary to improve medication adherence, because it helps families attain the knowledge, confidence, and motivation to manage their child’s condition.
Clinicians have an important role in educating patients about70:
- the medications they are taking
- why they are taking them
- what the medications look like
- the time of medication administration
- the potential adverse effects
- what to do if adverse effects occur
- what regular testing/monitoring is necessary.
Clinicians can provide appropriate psychoeducation by sharing written materials and trusted websites with families (see Related Resources).
Behavioral strategies. Behavioral interventions have been among the most effective strategies for improving medication adherence in other chronic conditions.71 Behavioral strategies are likely to be particularly important for families of children with ADHD and comorbid conditions such as ODD because these families experience considerable caregiver-child conflict.72 Moreover, parents of children with ADHD are at higher risk for having ADHD and depression themselves,73 both of which may interfere with a parent’s ability to obtain and administer medications consistently. Thus, for these families, using a combination of psychoeducation and behavioral strategies will be necessary to affect change in attitude and behavior. Behavioral strategies that can be used to improve medication adherence include:
- Technology-based interventions can reduce the impact of environmental barriers to adherence. For example, pharmacy automatic prescription renewal systems can reduce the likelihood of families failing to obtain ADHD medication refills. Pill reminder boxes, smartphone alerts, and setting various alarms can effectively prompt caregivers/patients to administer medication. In particular, these methods can be crucial in families for which multiple members have ADHD and its attendant difficulties with organization and task completion.
- Caregiver training may assist families in developing specific behavioral management skills that support adherence. This training can be as straightforward as instructing caregivers on the use of positive reinforcement when teaching their children to swallow pills. It may also encompass structured behavioral interventions aimed at training caregivers to utilize rewards and consequences in order to maximize medication adherence.74
Continue to: Clinician interventions
Clinician interventions. Clinicians can use decision aids to help inform families about treatment options, promote shared decision making, and decrease uncertainty about the treatment plan75 (see Related Resources). Early titration of ADHD medications and early first contact (within months of starting medication treatment) between caregivers and clinicians, whether via in-person visit, telephone, or email, have also been related to improved adherence.28 Furthermore, clinicians can improve adherence by prescribing a simplified medication regimen (ie, long-acting formulations that provide full-day coverage). To address the negative impact of high out-of-pocket ADHD medication costs on adherence, clinicians can also prescribe generic preparations and/or “preferred” medications options on an individual patient’s formulary.
Because clinician knowledge and expertise in ADHD care has been linked to improved patient medication adherence,68 clinicians are encouraged to use the American Academy of Pediatrics (AAP) guideline for diagnosis and treatment of ADHD, which includes a supplemental process of care algorithm (last published in 2011,10 with an updated guideline anticipated in 2019), as well as the AAP/National Institute for Children’s Health Quality (NICHQ) ADHD Toolkit,76 which includes items helpful for ADHD diagnosis and treatment. The Society for Developmental and Behavioral Pediatrics is also developing a clinical practice guideline for the diagnosis and treatment of complex ADHD (ie, ADHD complicated by coexisting mental health, developmental, and/or psychosocial conditions or issues), with publication anticipated in 2019. Primary care providers can also improve their expertise in ADHD care by pursuing additional mental health–related trainings (such as those conducted by the REACH Institute).77
Because receiving ADHD care from a specialist has been shown to improve medication initiation and adherence,62,69 other strategies to address the short supply of child psychiatrists include offering incentives to medical students to pursue a career in child psychiatry (eg, loan forgiveness). Telepsychiatry and co-location of mental health specialists and primary care providers are additional innovative ways in which ADHD specialty care can be delivered to more patients.64
Finally, providing culturally-sensitive care can strengthen the clinician-caregiver relationship and promote adherence to treatment. For example, clinicians can partner with local groups to increase their understanding of how different racial/ethnic groups perceive ADHD and its treatment.64
Peer support models. Peers are credible role models who have a valued role in facilitating the use of mental health services by empowering families and enhancing service satisfaction.78 In several communities in the United States, peer models using family advocates have been introduced.79 Family advocates are typically caregivers of children who have special needs or have been involved in the mental health system. Their perspective—as peers and first-hand consumers of the health care and/or mental health system—can make them powerful and effective coaches to families of children with ADHD. By helping families to navigate ADHD care systems successfully, family advocates can play an important role in enhancing ADHD medication adherence, although further investigation is needed. In addition, the stigma around ADHD medication use, which adversely impacts adherence, can be mitigated if caregivers participate in organized ADHD-related support groups (eg, Children and Adults with ADHD [CHADD]).
Continue to: Health disparity-reducing interventions
Health disparity-reducing interventions. Successful health disparity-reducing interventions—such as those developed to enhance care of other chronic disorders including asthma and diabetes—can be applied to improve ADHD care. These interventions, which include medical-legal partnerships (eg, between clinicians, social workers, legal advocates, and community partners) in primary care centers, have been shown to improve health insurance coverage and therefore health care access.80,81 Although some hardships linked to nonadherence (eg, low socioeconomic status) may not be amenable to health care–related interventions, screening for these hardships can identify children who are most at risk for poor adherence. This would alert clinicians to proactively identify barriers to adherence and implement mitigation strategies. This might include developing more streamlined, easier-to-follow management plans for these patients, such as those that can be delivered through pharmacist-physician collaborative programs82 and school-based therapy programs.83-85
Bottom Line
Suboptimal adherence to medications for attention-deficit/hyperactivity disorder (ADHD) can be addressed through patient/family education, behavioral strategies, clinician interventions, peer support models, and health disparity-reducing interventions. By improving ADHD treatment adherence, these interventions have the potential to maximize long-term outcomes.
Related Resources
- Cohen Children’s Medical Center Northwell Health. The ADHD Medication Guide. www.ADHDMedicationGuide.com. Revised December 31, 2017.
- Cincinnati Children’s Hospital Medical Center. Decision aids to facilitate shared decision making in practice. www.cincinnatichildrens.org/service/j/anderson-center/ evidence-based-care/decision-aids.
- CHADD. Children and Adults with attention-deficit/ hyperactivity disorder. www.chadd.org.
Drug Brand Name
Methylphenidate • Concerta, Ritalin
1. Froehlich TE, Lanphear BP, Epstein JN, et al. Prevalence, recognition, and treatment of attention-deficit/hyperactivity disorder in a national sample of US children. Arch Pediatr Adolesc Med. 2007;161(9):857-864.
2. Visser SN, Lesesne CA, Perou R. National estimates and factors associated with medication treatment for childhood attention-deficit/hyperactivity disorder. Pediatrics. 2007;119 (Suppl 1):S99-S106.
3. Danielson ML, Bitsko RH, Ghandour RM, et al. Prevalence of parent-reported ADHD diagnosis and associated treatment among U.S. children and adolescents, 2016. J Clin Child Adolesc Psychol. 2018;47(2):199-212.
4. Molina BS, Hinshaw SP, Swanson JM, et al. The MTA at 8 years: prospective follow-up of children treated for combined-type ADHD in a multisite study. J Am Acad Child Adolesc Psychiatry. 2009;48(5):484-500.
5. Charach A, Dashti B, Carson P, et al. Attention deficit hyperactivity disorder: effectiveness of treatment in at-risk preschoolers; long-term effectiveness in all ages; and variability in prevalence, diagnosis, and treatment. Rockville, MD: Agency for Healthcare Research and Quality; 2011. http://www.ncbi.nlm.nih.gov/books/NBK82368/.
6. Wehmeier PM, Schacht A, Barkley RA. Social and emotional impairment in children and adolescents with ADHD and the impact on quality of life. J Adolesc Health. 2010;46(3):209-217.
7. Barkley RA, Fischer M, Smallish L, et al. Young adult outcome of hyperactive children: adaptive functioning in major life activities. J Am Acad Child Adolesc Psychiatry. 2006;45(2):192-202.
8. Spencer TJ, Biederman J, Mick E. Attention-deficit/hyperactivity disorder: diagnosis, lifespan, comorbidities, and neurobiology. J Pediatr Psychol. 2007;32(6):631-642.
9. Pliszka S, the AACAP Work Group on Quality Issues. Practice parameter for the assessment and treatment of children and adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2007;46(7):894-921.
10. Subcommittee on Attention-Deficit/Hyperactivity Disorder; Steering Committee on Quality Improvement and Management. ADHD: clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics. 2011;128(5):1007-1022.
11. A 14-month randomized clinical trial of treatment strategies for attention-deficit/hyperactivity disorder. The MTA Cooperative Group. Multimodal Treatment Study of Children with ADHD. Arch Gen Psychiatry. 1999;56(12):1073-1086.
12. Abikoff H, Hechtman L, Klein RG, et al. Symptomatic improvement in children with ADHD treated with long-term methylphenidate and multimodal psychosocial treatment. J Am Acad Child Adolesc Psychiatry. 2004;43(7):802-811.
13. Barbaresi WJ, Katusic SK, Colligan RC, et al. Long-term school outcomes for children with attention-deficit/hyperactivity disorder: a population-based perspective. J Dev Behav Pediatr. 2007;28(4):265-273.
14. Scheffler RM, Brown TT, Fulton BD, et al. Positive association between attention-deficit/ hyperactivity disorder medication use and academic achievement during elementary school. Pediatrics. 2009;123(5):1273-1279.
15. Dalsgaard S, Nielsen HS, Simonsen M. Five-fold increase in national prevalence rates of attention-deficit/hyperactivity disorder medications for children and adolescents with autism spectrum disorder, attention-deficit/hyperactivity disorder, and other psychiatric disorders: a Danish register-based study. J Child Adolesc Psychopharmacol. 2013;23(7):432-439.
16. Lichtenstein P, Halldner L, Zetterqvist J, et al. Medication for attention deficit-hyperactivity disorder and criminality. N Engl J Med. 2012;367(21):2006-2014.
17. Chang Z, Lichtenstein P, D’Onofrio BM, et al. Serious transport accidents in adults with attention-deficit/hyperactivity disorder and the effect of medication: a population-based study. JAMA Psychiatry. 2014;71(3):319-325.
18. Chang Z, Quinn PD, Hur K, et al. Association between medication use for attention-deficit/hyperactivity disorder and risk of motor vehicle crashes. JAMA Psychiatry. 2017;74(6):597-603.
19. Dalsgaard S, Leckman JF, Mortensen PB, et al. Effect of drugs on the risk of injuries in children with attention deficit hyperactivity disorder: a prospective cohort study. Lancet Psychiatry. 2015;2(8):702-709.
20. Chang Z, Lichtenstein P, Halldner L, et al. Stimulant ADHD medication and risk for substance abuse. J Child Psychol Psychiatry. 2014;55(8):878-885.
21. Fischer M, Barkley RA. Childhood stimulant treatment and risk for later substance abuse. J Clin Psychiatry. 2003;64(Suppl 11):19-23.
22. Biederman J. Pharmacotherapy for attention-deficit/hyperactivity disorder (ADHD) decreases the risk for substance abuse: findings from a longitudinal follow-up of youths with and without ADHD. J Clin Psychiatry. 2003;64(Suppl 11):3-8.
23. Chang Z, D’Onofrio BM, Quinn PD, et al. Medicationfor attention-deficit/hyperactivity disorder and risk for depression: a nationwide longitudinal cohort study. Biol Psychiatry. 2016;80(12):916-922.
24. World Health Organization. Adherence to long-term therapies: evidence for action. https://www.who.int/chp/knowledge/publications/adherence_full_report.pdf?ua=1. Published 2003. Accessed July 22, 2019.
25. Perwien A, Hall J, Swensen A, et al. Stimulant treatment patterns and compliance in children and adults with newly treated attention-deficit/hyperactivity disorder. J Manag Care Pharm. 2004;10(2):122-129.
26. Faraone SV, Biederman J, Zimmerman B. An analysis of patient adherence to treatment during a 1-year, open-label study of OROS methylphenidate in children with ADHD. J Atten Disord. 2007;11(2):157-166.
27. Barner JC, Khoza S, Oladapo A. ADHD medication use, adherence, persistence and cost among Texas Medicaid children. Curr Med Res Opin. 2011;27(Suppl 2):13-22.
28. Brinkman WB, Baum R, Kelleher KJ, et al. Relationship between attention-deficit/hyperactivity disorder care and medication continuity. J Am Acad Child Adolesc Psychiatry. 2016;55(4):289-294.
29. Bokhari FAS, Heiland F, Levine P, et al. Risk factors for discontinuing drug therapy among children with ADHD. Health Services and Outcomes Research Methodology. 2008;8(3):134-158.
30. Thiruchelvam D, Charach A, Schachar RJ. Moderators and mediators of long-term adherence to stimulant treatment in children with ADHD. J Am Acad Child Adolesc Psychiatry. 2001;40(8):922-928.
31. DosReis S, Mychailyszyn MP, Evans-Lacko SE, et al. The meaning of attention-deficit/hyperactivity disorder medication and parents’ initiation and continuity of treatment for their child. J Child Adolesc Psychopharmacol. 2009;19(4):377-383.
32. dosReis S, Myers MA. Parental attitudes and involvement in psychopharmacological treatment for ADHD: a conceptual model. Int Rev Psychiatry. 2008;20(2):135-141.
33. Bussing R, Koro-Ljungberg M, Noguchi K, et al. Willingness to use ADHD treatments: a mixed methods study of perceptions by adolescents, parents, health professionals and teachers. Soc Sci Med. 2012;74(1):92-100.
34. Brinkman WB, Sucharew H, Majcher JH, et al. Predictors of medication continuity in children with ADHD. Pediatrics. 2018;141(6). doi: 10.1542/peds.2017-2580.
35. Coletti DJ, Pappadopulos E, Katsiotas NJ, et al. Parent perspectives on the decision to initiate medication treatment of attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2012;22(3):226-237.
36. Bussing R, Gary FA. Practice guidelines and parental ADHD treatment evaluations: friends or foes? Harv Rev Psychiatry. 2001;9(5):223-233.
37. Charach A, Gajaria A. Improving psychostimulant adherence in children with ADHD. Expert Rev Neurother. 2008;8(10):1563-1571.
38. Rieppi R, Greenhill LL, Ford RE, et al. Socioeconomic status as a moderator of ADHD treatment outcomes. J Am Acad Child Adolesc Psychiatry. 2002;41(3):269-277.
39. Swanson JM, Hinshaw SP, Arnold LE, et al. Secondary evaluations of MTA 36-month outcomes: propensity score and growth mixture model analyses. J Am Acad Child Adolesc Psychiatry. 2007;46(8):1003-1014.
40. Gau SS, Shen HY, Chou MC, et al. Determinants of adherence to methylphenidate and the impact of poor adherence on maternal and family measures. J Child Adolesc Psychopharmacol. 2006;16(3):286-297.
41. Barkley RA, Fischer M, Edelbrock C, et al. The adolescent outcome of hyperactive children diagnosed by research criteria--III. Mother-child interactions, family conflicts and maternal psychopathology. J Child Psychol Psychiatry. 1991;32(2):233-255.
42. Kashdan TB, Jacob RG, Pelham WE, et al. Depression and anxiety in parents of children with ADHD and varying levels of oppositional defiant behaviors: modeling relationships with family functioning. J Clin Child Adolesc Psychol. 2004;33(1):169-181.
43. Chavira DA, Stein MB, Bailey K, et al. Parental opinions regarding treatment for social anxiety disorder in youth. J Dev Behav Pediatr. 2003;24(5):315-322.
44. Leslie LK, Aarons GA, Haine RA, et al. Caregiver depression and medication use by youths with ADHD who receive services in the public sector. Psychiatr Serv. 2007;58(1):131-134.
45. Barbaresi WJ, Katusic SK, Colligan RC, et al. Long-term stimulant medication treatment of attention-deficit/hyperactivity disorder: results from a population-based study. J Dev Behav Pediatr. 2006;27(1):1-10.
46. Atzori P, Usala T, Carucci S, et al. Predictive factors for persistent use and compliance of immediate-release methylphenidate: a 36-month naturalistic study. J Child Adolesc Psychopharmacol. 2009;19(6):673-681.
47. Chen CY, Yeh HH, Chen KH, et al. Differential effects of predictors on methylphenidate initiation and discontinuation among young people with newly diagnosed attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2011;21(3):265-273.
48. Winterstein AG, Gerhard T, Shuster J, et al. Utilization of pharmacologic treatment in youths with attention deficit/hyperactivity disorder in Medicaid database. Ann Pharmacother. 2008;42(1):24-31.
49. Marcus SC, Wan GJ, Kemner JE, et al. Continuity of methylphenidate treatment for attention-deficit/hyperactivity disorder. Arch Pediatr Adolesc Med. 2005;159(6):572-578.
50. Cummings JR JX, Allen L, Lally C, et al. Racial and ethnic differences in ADHD treatment quality among Medicaid-enrolled youth. Pediatrics. 2017;139(6):e2016-e2044.
51. Hudson JL, Miller GE, Kirby JB. Explaining racial and ethnic differences in children’s use of stimulant medications. Med Care. 2007;45(11):1068-1075.
52. van den Ban E, Souverein PC, Swaab H, et al. Less discontinuation of ADHD drug use since the availability of long-acting ADHD medication in children, adolescents and adults under the age of 45 years in the Netherlands. Atten Defic Hyperact Disord. 2010;2(4):213-220.
53. Charach A, Ickowicz A, Schachar R. Stimulant treatment over five years: adherence, effectiveness, and adverse effects. J Am Acad Child Adolesc Psychiatry. 2004;43(5):559-567.
54. Toomey SL, Sox CM, Rusinak D, et al. Why do children with ADHD discontinue their medication? Clin Pediatr (Phila). 2012;51(8):763-769.
55. Brinkman WB, Simon JO, Epstein JN. Reasons why children and adolescents with attention-deficit/hyperactivity disorder stop and restart taking medicine. Acad Pediatr. 2018;18(3):273-280.
56. Wehmeier PM, Dittmann RW, Banaschewski T. Treatment compliance or medication adherence in children and adolescents on ADHD medication in clinical practice: results from the COMPLY observational study. Atten Defic Hyperact Disord. 2015;7(2):165-174.
57. Frank E, Ozon C, Nair V, et al. Examining why patients with attention-deficit/hyperactivity disorder lack adherence to medication over the long term: a review and analysis. J Clin Psychiatry. 2015;76(11):e1459-e1468.
58. Pozzi M, Carnovale C, Peeters G, et al. Adverse drug events related to mood and emotion in paediatric patients treated for ADHD: a meta-analysis. J Affect Disord. 2018;238:161-178.
59. Stuckelman ZD, Mulqueen JM, Ferracioli-Oda E, et al. Risk of irritability with psychostimulant treatment in children with ADHD: a meta-analysis. J Clin Psychiatry. 2017;78(6):e648-e655.
60. Cortese S, Adamo N, Del Giovane C, et al. Comparative efficacy and tolerability of medications for attention-deficit hyperactivity disorder in children, adolescents, and adults: a systematic review and network meta-analysis. Lancet Psychiatry. 2018;5(9):727-738.
61. Lawson KA, Johnsrud M, Hodgkins P, et al. Utilization patterns of stimulants in ADHD in the Medicaid population: a retrospective analysis of data from the Texas Medicaid program. Clin Ther. 2012;34(4):944-956 e944.
62. Olfson M, Marcus S, Wan G. Stimulant dosing for children with ADHD: a medical claims analysis. J Am Acad Child Adolesc Psychiatry. 2009;48(1):51-59.
63. Jensen PS, Arnold LE, Swanson JM, et al. 3-year follow-up of the NIMH MTA study. J Am Acad Child Adolesc Psychiatry. 2007;46(8):989-1002.
64. Van Cleave J, Leslie LK. Approaching ADHD as a chronic condition: implications for long-term adherence. Pediatr Ann. 2008;37(1):19-26.
65. Leslie LK, Plemmons D, Monn AR, et al. Investigating ADHD treatment trajectories: listening to families’ stories about medication use. J Dev Behav Pediatr. 2007;28(3):179-188.
66. Fiks AG, Mayne S, Localio AR, et al. Shared decision making and behavioral impairment: a national study among children with special health care needs. BMC Pediatr. 2012;12:153.
67. Stevens J, Harman JS, Kelleher KJ. Race/ethnicity and insurance status as factors associated with ADHD treatment patterns. J Child Adolesc Psychopharmacol. 2005;15(1):88-96.
68. Charach A, Skyba A, Cook L, et al. Using stimulant medication for children with ADHD: what do parents say? A brief report. J Can Acad Child Adolesc Psychiatry. 2006;15(2):75-83.
69. Chen CY, Gerhard T, Winterstein AG. Determinants of initial pharmacological treatment for youths with attention-deficit/hyperactivity disorder. J Child Adolescent Psychopharmacol. 2009;19(2):187-195.
70. National Council on Patient Information and Education. Enhancing prescription medication adherence: a national action plan. http://www.bemedwise.org/docs/enhancingprescriptionmedicineadherence.pdf. Published August 2007. Accessed July 22, 2019.
71. Kahana S, Drotar D, Frazier T. Meta-analysis of psychological interventions to promote adherence to treatment in pediatric chronic health conditions. J Pediatr Psychol. 2008;33(6):590-611.
72. Johnston C, Mash EJ. Families of children with attention-deficit/hyperactivity disorder: review and recommendations for future research. Clin Child Fam Psychol Rev. 2001;4(3):183-207.
73. Chronis AM, Lahey BB, Pelham WE Jr., et al. Psychopathology and substance abuse in parents of young children with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2003;42(12):1424-1432.
74. Chacko A, Newcorn JH, Feirsen N, et al. Improving medication adherence in chronic pediatric health conditions: a focus on ADHD in youth. Curr Pharm Des. 2010;16(22):2416-2423.
75. Brinkman WB, Hartl Majcher J, Polling LM, et al. Shared decision-making to improve attention-deficit hyperactivity disorder care. Patient Educ Couns. 2013;93(1):95-101.
76. American Academy of Pediatrics. Caring for children with ADHD: a resource toolkit for clinicians. 2nd ed. https://www.aap.org/en-us/pubserv/adhd2/Pages/default.aspx. Published 2011. Accessed July 22, 2019.
77. The REACH Institute. Course dates and registration. http://www.thereachinstitute.org/services/for-primary-care-practitioners/training-dates-and-registration. Accessed July 22, 2019.
78. Sells D, Davidson L, Jewell C, et al. The treatment relationship in peer-based and regular case management for clients with severe mental illness. Psychiatr Serv. 2006;57(8):1179-1184.
79. Hoagwood KE, Green E, Kelleher K, et al. Family advocacy, support and education in children’s mental health: results of a national survey. Adm Policy Ment Health. 2008;35(1-2):73-83.
80. Klein MD, Beck AF, Henize AW, et al. Doctors and lawyers collaborating to HeLP children—outcomes from a successful partnership between professions. J Health Care Poor Underserved. 2013;24(3):1063-1073.
81. Weintraub D, Rodgers MA, Botcheva L, et al. Pilot study of medical-legal partnership to address social and legal needs of patients. J Health Care Poor Underserved. 2010;21(Suppl 2):157-168.
82. Bradley CL, Luder HR, Beck AF, et al. Pediatric asthma medication therapy management through community pharmacy and primary care collaboration. J Am Pharm Assoc (2003). 2016;56(4):455-460.
83. Noyes K, Bajorska A, Fisher S, et al. Cost-effectiveness of the school-based asthma therapy (SBAT) program. Pediatrics. 2013;131(3):e709-e717.
84. Halterman JS, Fagnano M, Montes G, et al. The school-based preventive asthma care trial: results of a pilot study. J Pediatr. 2012;161(6):1109-1115.
85. Halterman JS, Szilagyi PG, Fisher SG, et al. Randomized controlled trial to improve care for urban children with asthma: results of the school-based asthma therapy trial. Arch Pediatr Adolesc Med. 2011;165(3):262-268.
1. Froehlich TE, Lanphear BP, Epstein JN, et al. Prevalence, recognition, and treatment of attention-deficit/hyperactivity disorder in a national sample of US children. Arch Pediatr Adolesc Med. 2007;161(9):857-864.
2. Visser SN, Lesesne CA, Perou R. National estimates and factors associated with medication treatment for childhood attention-deficit/hyperactivity disorder. Pediatrics. 2007;119 (Suppl 1):S99-S106.
3. Danielson ML, Bitsko RH, Ghandour RM, et al. Prevalence of parent-reported ADHD diagnosis and associated treatment among U.S. children and adolescents, 2016. J Clin Child Adolesc Psychol. 2018;47(2):199-212.
4. Molina BS, Hinshaw SP, Swanson JM, et al. The MTA at 8 years: prospective follow-up of children treated for combined-type ADHD in a multisite study. J Am Acad Child Adolesc Psychiatry. 2009;48(5):484-500.
5. Charach A, Dashti B, Carson P, et al. Attention deficit hyperactivity disorder: effectiveness of treatment in at-risk preschoolers; long-term effectiveness in all ages; and variability in prevalence, diagnosis, and treatment. Rockville, MD: Agency for Healthcare Research and Quality; 2011. http://www.ncbi.nlm.nih.gov/books/NBK82368/.
6. Wehmeier PM, Schacht A, Barkley RA. Social and emotional impairment in children and adolescents with ADHD and the impact on quality of life. J Adolesc Health. 2010;46(3):209-217.
7. Barkley RA, Fischer M, Smallish L, et al. Young adult outcome of hyperactive children: adaptive functioning in major life activities. J Am Acad Child Adolesc Psychiatry. 2006;45(2):192-202.
8. Spencer TJ, Biederman J, Mick E. Attention-deficit/hyperactivity disorder: diagnosis, lifespan, comorbidities, and neurobiology. J Pediatr Psychol. 2007;32(6):631-642.
9. Pliszka S, the AACAP Work Group on Quality Issues. Practice parameter for the assessment and treatment of children and adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2007;46(7):894-921.
10. Subcommittee on Attention-Deficit/Hyperactivity Disorder; Steering Committee on Quality Improvement and Management. ADHD: clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics. 2011;128(5):1007-1022.
11. A 14-month randomized clinical trial of treatment strategies for attention-deficit/hyperactivity disorder. The MTA Cooperative Group. Multimodal Treatment Study of Children with ADHD. Arch Gen Psychiatry. 1999;56(12):1073-1086.
12. Abikoff H, Hechtman L, Klein RG, et al. Symptomatic improvement in children with ADHD treated with long-term methylphenidate and multimodal psychosocial treatment. J Am Acad Child Adolesc Psychiatry. 2004;43(7):802-811.
13. Barbaresi WJ, Katusic SK, Colligan RC, et al. Long-term school outcomes for children with attention-deficit/hyperactivity disorder: a population-based perspective. J Dev Behav Pediatr. 2007;28(4):265-273.
14. Scheffler RM, Brown TT, Fulton BD, et al. Positive association between attention-deficit/ hyperactivity disorder medication use and academic achievement during elementary school. Pediatrics. 2009;123(5):1273-1279.
15. Dalsgaard S, Nielsen HS, Simonsen M. Five-fold increase in national prevalence rates of attention-deficit/hyperactivity disorder medications for children and adolescents with autism spectrum disorder, attention-deficit/hyperactivity disorder, and other psychiatric disorders: a Danish register-based study. J Child Adolesc Psychopharmacol. 2013;23(7):432-439.
16. Lichtenstein P, Halldner L, Zetterqvist J, et al. Medication for attention deficit-hyperactivity disorder and criminality. N Engl J Med. 2012;367(21):2006-2014.
17. Chang Z, Lichtenstein P, D’Onofrio BM, et al. Serious transport accidents in adults with attention-deficit/hyperactivity disorder and the effect of medication: a population-based study. JAMA Psychiatry. 2014;71(3):319-325.
18. Chang Z, Quinn PD, Hur K, et al. Association between medication use for attention-deficit/hyperactivity disorder and risk of motor vehicle crashes. JAMA Psychiatry. 2017;74(6):597-603.
19. Dalsgaard S, Leckman JF, Mortensen PB, et al. Effect of drugs on the risk of injuries in children with attention deficit hyperactivity disorder: a prospective cohort study. Lancet Psychiatry. 2015;2(8):702-709.
20. Chang Z, Lichtenstein P, Halldner L, et al. Stimulant ADHD medication and risk for substance abuse. J Child Psychol Psychiatry. 2014;55(8):878-885.
21. Fischer M, Barkley RA. Childhood stimulant treatment and risk for later substance abuse. J Clin Psychiatry. 2003;64(Suppl 11):19-23.
22. Biederman J. Pharmacotherapy for attention-deficit/hyperactivity disorder (ADHD) decreases the risk for substance abuse: findings from a longitudinal follow-up of youths with and without ADHD. J Clin Psychiatry. 2003;64(Suppl 11):3-8.
23. Chang Z, D’Onofrio BM, Quinn PD, et al. Medicationfor attention-deficit/hyperactivity disorder and risk for depression: a nationwide longitudinal cohort study. Biol Psychiatry. 2016;80(12):916-922.
24. World Health Organization. Adherence to long-term therapies: evidence for action. https://www.who.int/chp/knowledge/publications/adherence_full_report.pdf?ua=1. Published 2003. Accessed July 22, 2019.
25. Perwien A, Hall J, Swensen A, et al. Stimulant treatment patterns and compliance in children and adults with newly treated attention-deficit/hyperactivity disorder. J Manag Care Pharm. 2004;10(2):122-129.
26. Faraone SV, Biederman J, Zimmerman B. An analysis of patient adherence to treatment during a 1-year, open-label study of OROS methylphenidate in children with ADHD. J Atten Disord. 2007;11(2):157-166.
27. Barner JC, Khoza S, Oladapo A. ADHD medication use, adherence, persistence and cost among Texas Medicaid children. Curr Med Res Opin. 2011;27(Suppl 2):13-22.
28. Brinkman WB, Baum R, Kelleher KJ, et al. Relationship between attention-deficit/hyperactivity disorder care and medication continuity. J Am Acad Child Adolesc Psychiatry. 2016;55(4):289-294.
29. Bokhari FAS, Heiland F, Levine P, et al. Risk factors for discontinuing drug therapy among children with ADHD. Health Services and Outcomes Research Methodology. 2008;8(3):134-158.
30. Thiruchelvam D, Charach A, Schachar RJ. Moderators and mediators of long-term adherence to stimulant treatment in children with ADHD. J Am Acad Child Adolesc Psychiatry. 2001;40(8):922-928.
31. DosReis S, Mychailyszyn MP, Evans-Lacko SE, et al. The meaning of attention-deficit/hyperactivity disorder medication and parents’ initiation and continuity of treatment for their child. J Child Adolesc Psychopharmacol. 2009;19(4):377-383.
32. dosReis S, Myers MA. Parental attitudes and involvement in psychopharmacological treatment for ADHD: a conceptual model. Int Rev Psychiatry. 2008;20(2):135-141.
33. Bussing R, Koro-Ljungberg M, Noguchi K, et al. Willingness to use ADHD treatments: a mixed methods study of perceptions by adolescents, parents, health professionals and teachers. Soc Sci Med. 2012;74(1):92-100.
34. Brinkman WB, Sucharew H, Majcher JH, et al. Predictors of medication continuity in children with ADHD. Pediatrics. 2018;141(6). doi: 10.1542/peds.2017-2580.
35. Coletti DJ, Pappadopulos E, Katsiotas NJ, et al. Parent perspectives on the decision to initiate medication treatment of attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2012;22(3):226-237.
36. Bussing R, Gary FA. Practice guidelines and parental ADHD treatment evaluations: friends or foes? Harv Rev Psychiatry. 2001;9(5):223-233.
37. Charach A, Gajaria A. Improving psychostimulant adherence in children with ADHD. Expert Rev Neurother. 2008;8(10):1563-1571.
38. Rieppi R, Greenhill LL, Ford RE, et al. Socioeconomic status as a moderator of ADHD treatment outcomes. J Am Acad Child Adolesc Psychiatry. 2002;41(3):269-277.
39. Swanson JM, Hinshaw SP, Arnold LE, et al. Secondary evaluations of MTA 36-month outcomes: propensity score and growth mixture model analyses. J Am Acad Child Adolesc Psychiatry. 2007;46(8):1003-1014.
40. Gau SS, Shen HY, Chou MC, et al. Determinants of adherence to methylphenidate and the impact of poor adherence on maternal and family measures. J Child Adolesc Psychopharmacol. 2006;16(3):286-297.
41. Barkley RA, Fischer M, Edelbrock C, et al. The adolescent outcome of hyperactive children diagnosed by research criteria--III. Mother-child interactions, family conflicts and maternal psychopathology. J Child Psychol Psychiatry. 1991;32(2):233-255.
42. Kashdan TB, Jacob RG, Pelham WE, et al. Depression and anxiety in parents of children with ADHD and varying levels of oppositional defiant behaviors: modeling relationships with family functioning. J Clin Child Adolesc Psychol. 2004;33(1):169-181.
43. Chavira DA, Stein MB, Bailey K, et al. Parental opinions regarding treatment for social anxiety disorder in youth. J Dev Behav Pediatr. 2003;24(5):315-322.
44. Leslie LK, Aarons GA, Haine RA, et al. Caregiver depression and medication use by youths with ADHD who receive services in the public sector. Psychiatr Serv. 2007;58(1):131-134.
45. Barbaresi WJ, Katusic SK, Colligan RC, et al. Long-term stimulant medication treatment of attention-deficit/hyperactivity disorder: results from a population-based study. J Dev Behav Pediatr. 2006;27(1):1-10.
46. Atzori P, Usala T, Carucci S, et al. Predictive factors for persistent use and compliance of immediate-release methylphenidate: a 36-month naturalistic study. J Child Adolesc Psychopharmacol. 2009;19(6):673-681.
47. Chen CY, Yeh HH, Chen KH, et al. Differential effects of predictors on methylphenidate initiation and discontinuation among young people with newly diagnosed attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2011;21(3):265-273.
48. Winterstein AG, Gerhard T, Shuster J, et al. Utilization of pharmacologic treatment in youths with attention deficit/hyperactivity disorder in Medicaid database. Ann Pharmacother. 2008;42(1):24-31.
49. Marcus SC, Wan GJ, Kemner JE, et al. Continuity of methylphenidate treatment for attention-deficit/hyperactivity disorder. Arch Pediatr Adolesc Med. 2005;159(6):572-578.
50. Cummings JR JX, Allen L, Lally C, et al. Racial and ethnic differences in ADHD treatment quality among Medicaid-enrolled youth. Pediatrics. 2017;139(6):e2016-e2044.
51. Hudson JL, Miller GE, Kirby JB. Explaining racial and ethnic differences in children’s use of stimulant medications. Med Care. 2007;45(11):1068-1075.
52. van den Ban E, Souverein PC, Swaab H, et al. Less discontinuation of ADHD drug use since the availability of long-acting ADHD medication in children, adolescents and adults under the age of 45 years in the Netherlands. Atten Defic Hyperact Disord. 2010;2(4):213-220.
53. Charach A, Ickowicz A, Schachar R. Stimulant treatment over five years: adherence, effectiveness, and adverse effects. J Am Acad Child Adolesc Psychiatry. 2004;43(5):559-567.
54. Toomey SL, Sox CM, Rusinak D, et al. Why do children with ADHD discontinue their medication? Clin Pediatr (Phila). 2012;51(8):763-769.
55. Brinkman WB, Simon JO, Epstein JN. Reasons why children and adolescents with attention-deficit/hyperactivity disorder stop and restart taking medicine. Acad Pediatr. 2018;18(3):273-280.
56. Wehmeier PM, Dittmann RW, Banaschewski T. Treatment compliance or medication adherence in children and adolescents on ADHD medication in clinical practice: results from the COMPLY observational study. Atten Defic Hyperact Disord. 2015;7(2):165-174.
57. Frank E, Ozon C, Nair V, et al. Examining why patients with attention-deficit/hyperactivity disorder lack adherence to medication over the long term: a review and analysis. J Clin Psychiatry. 2015;76(11):e1459-e1468.
58. Pozzi M, Carnovale C, Peeters G, et al. Adverse drug events related to mood and emotion in paediatric patients treated for ADHD: a meta-analysis. J Affect Disord. 2018;238:161-178.
59. Stuckelman ZD, Mulqueen JM, Ferracioli-Oda E, et al. Risk of irritability with psychostimulant treatment in children with ADHD: a meta-analysis. J Clin Psychiatry. 2017;78(6):e648-e655.
60. Cortese S, Adamo N, Del Giovane C, et al. Comparative efficacy and tolerability of medications for attention-deficit hyperactivity disorder in children, adolescents, and adults: a systematic review and network meta-analysis. Lancet Psychiatry. 2018;5(9):727-738.
61. Lawson KA, Johnsrud M, Hodgkins P, et al. Utilization patterns of stimulants in ADHD in the Medicaid population: a retrospective analysis of data from the Texas Medicaid program. Clin Ther. 2012;34(4):944-956 e944.
62. Olfson M, Marcus S, Wan G. Stimulant dosing for children with ADHD: a medical claims analysis. J Am Acad Child Adolesc Psychiatry. 2009;48(1):51-59.
63. Jensen PS, Arnold LE, Swanson JM, et al. 3-year follow-up of the NIMH MTA study. J Am Acad Child Adolesc Psychiatry. 2007;46(8):989-1002.
64. Van Cleave J, Leslie LK. Approaching ADHD as a chronic condition: implications for long-term adherence. Pediatr Ann. 2008;37(1):19-26.
65. Leslie LK, Plemmons D, Monn AR, et al. Investigating ADHD treatment trajectories: listening to families’ stories about medication use. J Dev Behav Pediatr. 2007;28(3):179-188.
66. Fiks AG, Mayne S, Localio AR, et al. Shared decision making and behavioral impairment: a national study among children with special health care needs. BMC Pediatr. 2012;12:153.
67. Stevens J, Harman JS, Kelleher KJ. Race/ethnicity and insurance status as factors associated with ADHD treatment patterns. J Child Adolesc Psychopharmacol. 2005;15(1):88-96.
68. Charach A, Skyba A, Cook L, et al. Using stimulant medication for children with ADHD: what do parents say? A brief report. J Can Acad Child Adolesc Psychiatry. 2006;15(2):75-83.
69. Chen CY, Gerhard T, Winterstein AG. Determinants of initial pharmacological treatment for youths with attention-deficit/hyperactivity disorder. J Child Adolescent Psychopharmacol. 2009;19(2):187-195.
70. National Council on Patient Information and Education. Enhancing prescription medication adherence: a national action plan. http://www.bemedwise.org/docs/enhancingprescriptionmedicineadherence.pdf. Published August 2007. Accessed July 22, 2019.
71. Kahana S, Drotar D, Frazier T. Meta-analysis of psychological interventions to promote adherence to treatment in pediatric chronic health conditions. J Pediatr Psychol. 2008;33(6):590-611.
72. Johnston C, Mash EJ. Families of children with attention-deficit/hyperactivity disorder: review and recommendations for future research. Clin Child Fam Psychol Rev. 2001;4(3):183-207.
73. Chronis AM, Lahey BB, Pelham WE Jr., et al. Psychopathology and substance abuse in parents of young children with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2003;42(12):1424-1432.
74. Chacko A, Newcorn JH, Feirsen N, et al. Improving medication adherence in chronic pediatric health conditions: a focus on ADHD in youth. Curr Pharm Des. 2010;16(22):2416-2423.
75. Brinkman WB, Hartl Majcher J, Polling LM, et al. Shared decision-making to improve attention-deficit hyperactivity disorder care. Patient Educ Couns. 2013;93(1):95-101.
76. American Academy of Pediatrics. Caring for children with ADHD: a resource toolkit for clinicians. 2nd ed. https://www.aap.org/en-us/pubserv/adhd2/Pages/default.aspx. Published 2011. Accessed July 22, 2019.
77. The REACH Institute. Course dates and registration. http://www.thereachinstitute.org/services/for-primary-care-practitioners/training-dates-and-registration. Accessed July 22, 2019.
78. Sells D, Davidson L, Jewell C, et al. The treatment relationship in peer-based and regular case management for clients with severe mental illness. Psychiatr Serv. 2006;57(8):1179-1184.
79. Hoagwood KE, Green E, Kelleher K, et al. Family advocacy, support and education in children’s mental health: results of a national survey. Adm Policy Ment Health. 2008;35(1-2):73-83.
80. Klein MD, Beck AF, Henize AW, et al. Doctors and lawyers collaborating to HeLP children—outcomes from a successful partnership between professions. J Health Care Poor Underserved. 2013;24(3):1063-1073.
81. Weintraub D, Rodgers MA, Botcheva L, et al. Pilot study of medical-legal partnership to address social and legal needs of patients. J Health Care Poor Underserved. 2010;21(Suppl 2):157-168.
82. Bradley CL, Luder HR, Beck AF, et al. Pediatric asthma medication therapy management through community pharmacy and primary care collaboration. J Am Pharm Assoc (2003). 2016;56(4):455-460.
83. Noyes K, Bajorska A, Fisher S, et al. Cost-effectiveness of the school-based asthma therapy (SBAT) program. Pediatrics. 2013;131(3):e709-e717.
84. Halterman JS, Fagnano M, Montes G, et al. The school-based preventive asthma care trial: results of a pilot study. J Pediatr. 2012;161(6):1109-1115.
85. Halterman JS, Szilagyi PG, Fisher SG, et al. Randomized controlled trial to improve care for urban children with asthma: results of the school-based asthma therapy trial. Arch Pediatr Adolesc Med. 2011;165(3):262-268.
How to avoid ‘checklist’ psychiatry
To determine whether a patient meets the criteria for a DSM-5 diagnosis, we rely on objective data, direct observations, and individual biopsychosocial factors as well as our patient’s subjective report of symptoms. However, because the line differentiating normal from abnormal emotional responses can sometimes be blurred, we should be prudent when establishing a diagnosis. Specifically, we need to avoid falling into the trap of “checklist” psychiatry—relegating diagnostic assessments to robotic statements about whether patients meet DSM criteria—because this can lead to making diagnoses too quickly or inaccurately.1 Potential consequences of checklist psychiatry include1,2:
- becoming so “married” to a particular diagnosis that you don’t consider alternative diagnoses
- labeling patients with a diagnosis that many clinicians may view as pejorative (eg, antisocial personality disorder), which might affect their ability to receive future treatment
- developing ineffective treatment plans based on an incorrect diagnosis, including exposing patients to medications that could have serious adverse effects
- performing suicide or violence risk assessments based on inaccurate diagnoses, thereby over- or underestimating the possible risk for an adverse outcome
- leading patients to assume the identity of the inaccurate diagnosis and possibly viewing themselves as dysfunctional or impaired.
When you are uncertain whether your patient has a diagnosable condition, it can be useful to use the terms “no diagnosis” or “diagnosis deferred.” However, many insurance companies will not reimburse without an actual diagnosis. Therefore, the following tips may be helpful in establishing an accurate diagnosis while avoiding checklist psychiatry.1,2
Ask patients about the degree and duration of impairment in functioning. Although impairment in functioning is a criterion of almost all DSM-5 diagnoses, not all endorsed symptoms warrant a diagnosis. Mild symptoms often resolve spontaneously over time without the need for diagnostic labels or interventions.
Make longitudinal observations. Interviewing patients over a long period of time and on multiple occasions can provide data on the consistency of reported symptoms, the presence or absence of behavioral correlates to reported symptomatology, the degree of impairment from the reported symptoms, and the evolution of symptoms.
Collect collateral information. Although we often rely on our patients’ reports of symptoms to establish a diagnosis, this information should not be the sole source. We can obtain a more complete picture if we approach a patient’s family members for their input, including asking about a family history of mental illness or substance use disorders. We can also review prior treatment records and gather observations from clinic or inpatient staff for additional information.
Order laboratory studies. Serum studies and urine toxicology screens provide information that can help form an accurate diagnosis. This information is helpful because certain medical conditions, substance intoxication, and substance withdrawal can mimic psychiatric symptoms.
Continuously re-evaluate your diagnoses. As clinicians, we’d like to provide an accurate diagnosis at the onset of treatment; however, this may not be realistic because the patient’s presentation might change over time. It is paramount that we view diagnoses as evolving, so that we can more readily adjust our approach to treatment, especially when the patient is not benefitting from a well-formulated and comprehensive treatment plan.
Our patients are best served when we take the necessary time to use all resources to conceptualize them as more than a checklist of symptoms.
1. Kontos N, Freudenreich O, Querques J. Thoughtful diagnoses: not ‘checklist’ psychiatry. Current Psychiatry. 2007;6(3):112.
2. Frances A. My 12 best tips on psychiatric diagnosis. Psychiatric Times. http://www.psychiatrictimes.com/dsm-5/my-12-best-tips-psychiatric-diagnosis. Published June 17, 2013. Accessed July 19, 2019.
To determine whether a patient meets the criteria for a DSM-5 diagnosis, we rely on objective data, direct observations, and individual biopsychosocial factors as well as our patient’s subjective report of symptoms. However, because the line differentiating normal from abnormal emotional responses can sometimes be blurred, we should be prudent when establishing a diagnosis. Specifically, we need to avoid falling into the trap of “checklist” psychiatry—relegating diagnostic assessments to robotic statements about whether patients meet DSM criteria—because this can lead to making diagnoses too quickly or inaccurately.1 Potential consequences of checklist psychiatry include1,2:
- becoming so “married” to a particular diagnosis that you don’t consider alternative diagnoses
- labeling patients with a diagnosis that many clinicians may view as pejorative (eg, antisocial personality disorder), which might affect their ability to receive future treatment
- developing ineffective treatment plans based on an incorrect diagnosis, including exposing patients to medications that could have serious adverse effects
- performing suicide or violence risk assessments based on inaccurate diagnoses, thereby over- or underestimating the possible risk for an adverse outcome
- leading patients to assume the identity of the inaccurate diagnosis and possibly viewing themselves as dysfunctional or impaired.
When you are uncertain whether your patient has a diagnosable condition, it can be useful to use the terms “no diagnosis” or “diagnosis deferred.” However, many insurance companies will not reimburse without an actual diagnosis. Therefore, the following tips may be helpful in establishing an accurate diagnosis while avoiding checklist psychiatry.1,2
Ask patients about the degree and duration of impairment in functioning. Although impairment in functioning is a criterion of almost all DSM-5 diagnoses, not all endorsed symptoms warrant a diagnosis. Mild symptoms often resolve spontaneously over time without the need for diagnostic labels or interventions.
Make longitudinal observations. Interviewing patients over a long period of time and on multiple occasions can provide data on the consistency of reported symptoms, the presence or absence of behavioral correlates to reported symptomatology, the degree of impairment from the reported symptoms, and the evolution of symptoms.
Collect collateral information. Although we often rely on our patients’ reports of symptoms to establish a diagnosis, this information should not be the sole source. We can obtain a more complete picture if we approach a patient’s family members for their input, including asking about a family history of mental illness or substance use disorders. We can also review prior treatment records and gather observations from clinic or inpatient staff for additional information.
Order laboratory studies. Serum studies and urine toxicology screens provide information that can help form an accurate diagnosis. This information is helpful because certain medical conditions, substance intoxication, and substance withdrawal can mimic psychiatric symptoms.
Continuously re-evaluate your diagnoses. As clinicians, we’d like to provide an accurate diagnosis at the onset of treatment; however, this may not be realistic because the patient’s presentation might change over time. It is paramount that we view diagnoses as evolving, so that we can more readily adjust our approach to treatment, especially when the patient is not benefitting from a well-formulated and comprehensive treatment plan.
Our patients are best served when we take the necessary time to use all resources to conceptualize them as more than a checklist of symptoms.
To determine whether a patient meets the criteria for a DSM-5 diagnosis, we rely on objective data, direct observations, and individual biopsychosocial factors as well as our patient’s subjective report of symptoms. However, because the line differentiating normal from abnormal emotional responses can sometimes be blurred, we should be prudent when establishing a diagnosis. Specifically, we need to avoid falling into the trap of “checklist” psychiatry—relegating diagnostic assessments to robotic statements about whether patients meet DSM criteria—because this can lead to making diagnoses too quickly or inaccurately.1 Potential consequences of checklist psychiatry include1,2:
- becoming so “married” to a particular diagnosis that you don’t consider alternative diagnoses
- labeling patients with a diagnosis that many clinicians may view as pejorative (eg, antisocial personality disorder), which might affect their ability to receive future treatment
- developing ineffective treatment plans based on an incorrect diagnosis, including exposing patients to medications that could have serious adverse effects
- performing suicide or violence risk assessments based on inaccurate diagnoses, thereby over- or underestimating the possible risk for an adverse outcome
- leading patients to assume the identity of the inaccurate diagnosis and possibly viewing themselves as dysfunctional or impaired.
When you are uncertain whether your patient has a diagnosable condition, it can be useful to use the terms “no diagnosis” or “diagnosis deferred.” However, many insurance companies will not reimburse without an actual diagnosis. Therefore, the following tips may be helpful in establishing an accurate diagnosis while avoiding checklist psychiatry.1,2
Ask patients about the degree and duration of impairment in functioning. Although impairment in functioning is a criterion of almost all DSM-5 diagnoses, not all endorsed symptoms warrant a diagnosis. Mild symptoms often resolve spontaneously over time without the need for diagnostic labels or interventions.
Make longitudinal observations. Interviewing patients over a long period of time and on multiple occasions can provide data on the consistency of reported symptoms, the presence or absence of behavioral correlates to reported symptomatology, the degree of impairment from the reported symptoms, and the evolution of symptoms.
Collect collateral information. Although we often rely on our patients’ reports of symptoms to establish a diagnosis, this information should not be the sole source. We can obtain a more complete picture if we approach a patient’s family members for their input, including asking about a family history of mental illness or substance use disorders. We can also review prior treatment records and gather observations from clinic or inpatient staff for additional information.
Order laboratory studies. Serum studies and urine toxicology screens provide information that can help form an accurate diagnosis. This information is helpful because certain medical conditions, substance intoxication, and substance withdrawal can mimic psychiatric symptoms.
Continuously re-evaluate your diagnoses. As clinicians, we’d like to provide an accurate diagnosis at the onset of treatment; however, this may not be realistic because the patient’s presentation might change over time. It is paramount that we view diagnoses as evolving, so that we can more readily adjust our approach to treatment, especially when the patient is not benefitting from a well-formulated and comprehensive treatment plan.
Our patients are best served when we take the necessary time to use all resources to conceptualize them as more than a checklist of symptoms.
1. Kontos N, Freudenreich O, Querques J. Thoughtful diagnoses: not ‘checklist’ psychiatry. Current Psychiatry. 2007;6(3):112.
2. Frances A. My 12 best tips on psychiatric diagnosis. Psychiatric Times. http://www.psychiatrictimes.com/dsm-5/my-12-best-tips-psychiatric-diagnosis. Published June 17, 2013. Accessed July 19, 2019.
1. Kontos N, Freudenreich O, Querques J. Thoughtful diagnoses: not ‘checklist’ psychiatry. Current Psychiatry. 2007;6(3):112.
2. Frances A. My 12 best tips on psychiatric diagnosis. Psychiatric Times. http://www.psychiatrictimes.com/dsm-5/my-12-best-tips-psychiatric-diagnosis. Published June 17, 2013. Accessed July 19, 2019.
Child trafficking: How to recognize the signs
Child trafficking—a modern-day form of slavery that continues to destroy many lives—often is hidden, even from the clinicians who see its victims. Traffickers typically exploit children for labor or commercial sexual work. The signs and symptoms that suggest a child is being trafficked may be less clear than those of the psychiatric illnesses we usually diagnose and treat. In this article, I summarize characteristics that could be helpful to note when you suspect a child is being trafficked, and offer some resources for helping victims.
How to identify possible victims
Children can be trafficked anywhere. The concept of a child being picked up off a street corner is outdated. Trafficking occurs in cities, suburbs, and rural areas. It happens in hotel rooms, at truck stops, on quiet residential streets, and in expensive homes. The internet has made it easier for traffickers to find victims.
Traffickers typically target youth who are emotionally and physically vulnerable. They often seek out teenagers who are undergoing financial hardships, experiencing family conflict, or have survived natural disasters. Many victims are runaways. In 2016, 1 in 6 child runaways reported to the National Center for Missing and Exploited Children were likely victims of trafficking.1 Of those children, 86% were receiving social services support or living in foster homes.
Traffickers are adept at emotional manipulation, which may explain why a child or adolescent might minimize the abuse during a clinical visit. Traffickers shroud the realities of trafficking with notions of love and inclusion. They use several physical and mental schemes to keep children and adolescents in their grip, such as withholding food, sleep, or medical care. Therefore, we should check for signs and symptoms of chronic medical conditions that have gone untreated, malnutrition, or bruises in various stages of healing.
Connecting risk factors for trafficking to dramatic changes in a young patient’s behavior is challenging. These youth often have dropped out of school, lack consistent family support, and spend their nights in search of a warm place to sleep. Their lives are upended. A child who once was more social may be forced into isolation and make excuses for why she no longer spends time with her friends.
In a study of 106 survivors of domestic sex trafficking, approximately 89% of respondents reported depression during depression. Many respondents reported experiencing anxiety (76.4%), nightmares (73.6%), flashbacks (68%), low self-esteem (81.1%), or feelings of shame or guilt (82.1%).2 Almost 88% of respondents said that they saw a doctor or other clinician while being trafficked, but their clinicians were unable to recognize the signs of trafficking. Part of the challenge is that many children and adolescents are not comfortable discussing their situations with clinicians because they may struggle with shame and guilt. Their traffickers also might have convinced them that they are criminals, not victims. These patients also may have an overwhelming fear of their trafficker, being reported to child welfare authorities, being arrested, being deported, or having their traffickers retaliate against their families. Gaining the trust of a patient who is being trafficked is critical, but not easy, because children may be skeptical of a clinician’s promise of confidentiality.
Some signs of trafficking overlap with the psychiatric presentations with which we are more familiar. These patients may abuse drugs or alcohol as means of escape or because their traffickers force them to use substances.2 They may show symptoms of depression or posttraumatic stress disorder (PTSD) and may be disoriented. Other indicators may be more telling, such as if a child or adolescent describes:
- having no control of their schedules or forms of identification
- having to work excessively long hours, often to pay off an overwhelming debt
- having high security measures installed in their place of residence (such as cameras or barred windows).
Continue to: Also, they may be...
Also, they may be dressed inappropriately for the weather.
We should be concerned when patients’ responses seem coached, if they say they are isolated from their family and community, or if they are submissive or overly timid. In addition, our suspicions should be raised if an accompanying adult guardian insists on sitting in on the appointment or translating for the child. In such instances, we may request that the guardian remain in the waiting area during the appointment so the child will have the opportunity to speak freely.2
How to help a suspected victim
Several local and national organizations help trafficking victims. These organizations provide educational materials and training opportunities for clinicians, as well as direct support for victims. The Homeland Security Blue Campaign advises against confronting a suspected trafficker directly and encourages clinicians to instead report suspected cases to 1-866-347-2423.3
Clinicians can better help children who are trafficked by taking the following 5 steps:
- Learn about the risk factors and signs of child trafficking.
- Post the National Human Trafficking Hotline (1-888-373-7888) in your waiting room.
- Determine if your patient is in danger and needs to be moved to a safe place.
- Connect the patient to social service agencies that can provide financial support and housing assistance so he/she doesn’t feel trapped by financial burdens.
- Work to rebuild their emotional and physical well-being while treating depression, PTSD, substance abuse, or any other mental illness.
1. National Center for Missing and Exploited Childr en. Missing children, state care, and child sex trafficking. http://www.missingkids.com/content/dam/missingkids/pdfs/publications/missingchildrenstatecare.pdf. Accessed June 10, 2019.
2. Lederer LJ, Wetzel CA. The health consequences of sex trafficking and their implications for identifying victims in healthcare facilities. Ann Health Law. 2014;23(1):61-91.
3. Blue Campaign. Identify a victim. US Department of Homeland Security. https://www.dhs.gov/blue-campaign/identify-victim. Accessed June 10, 2019.
Child trafficking—a modern-day form of slavery that continues to destroy many lives—often is hidden, even from the clinicians who see its victims. Traffickers typically exploit children for labor or commercial sexual work. The signs and symptoms that suggest a child is being trafficked may be less clear than those of the psychiatric illnesses we usually diagnose and treat. In this article, I summarize characteristics that could be helpful to note when you suspect a child is being trafficked, and offer some resources for helping victims.
How to identify possible victims
Children can be trafficked anywhere. The concept of a child being picked up off a street corner is outdated. Trafficking occurs in cities, suburbs, and rural areas. It happens in hotel rooms, at truck stops, on quiet residential streets, and in expensive homes. The internet has made it easier for traffickers to find victims.
Traffickers typically target youth who are emotionally and physically vulnerable. They often seek out teenagers who are undergoing financial hardships, experiencing family conflict, or have survived natural disasters. Many victims are runaways. In 2016, 1 in 6 child runaways reported to the National Center for Missing and Exploited Children were likely victims of trafficking.1 Of those children, 86% were receiving social services support or living in foster homes.
Traffickers are adept at emotional manipulation, which may explain why a child or adolescent might minimize the abuse during a clinical visit. Traffickers shroud the realities of trafficking with notions of love and inclusion. They use several physical and mental schemes to keep children and adolescents in their grip, such as withholding food, sleep, or medical care. Therefore, we should check for signs and symptoms of chronic medical conditions that have gone untreated, malnutrition, or bruises in various stages of healing.
Connecting risk factors for trafficking to dramatic changes in a young patient’s behavior is challenging. These youth often have dropped out of school, lack consistent family support, and spend their nights in search of a warm place to sleep. Their lives are upended. A child who once was more social may be forced into isolation and make excuses for why she no longer spends time with her friends.
In a study of 106 survivors of domestic sex trafficking, approximately 89% of respondents reported depression during depression. Many respondents reported experiencing anxiety (76.4%), nightmares (73.6%), flashbacks (68%), low self-esteem (81.1%), or feelings of shame or guilt (82.1%).2 Almost 88% of respondents said that they saw a doctor or other clinician while being trafficked, but their clinicians were unable to recognize the signs of trafficking. Part of the challenge is that many children and adolescents are not comfortable discussing their situations with clinicians because they may struggle with shame and guilt. Their traffickers also might have convinced them that they are criminals, not victims. These patients also may have an overwhelming fear of their trafficker, being reported to child welfare authorities, being arrested, being deported, or having their traffickers retaliate against their families. Gaining the trust of a patient who is being trafficked is critical, but not easy, because children may be skeptical of a clinician’s promise of confidentiality.
Some signs of trafficking overlap with the psychiatric presentations with which we are more familiar. These patients may abuse drugs or alcohol as means of escape or because their traffickers force them to use substances.2 They may show symptoms of depression or posttraumatic stress disorder (PTSD) and may be disoriented. Other indicators may be more telling, such as if a child or adolescent describes:
- having no control of their schedules or forms of identification
- having to work excessively long hours, often to pay off an overwhelming debt
- having high security measures installed in their place of residence (such as cameras or barred windows).
Continue to: Also, they may be...
Also, they may be dressed inappropriately for the weather.
We should be concerned when patients’ responses seem coached, if they say they are isolated from their family and community, or if they are submissive or overly timid. In addition, our suspicions should be raised if an accompanying adult guardian insists on sitting in on the appointment or translating for the child. In such instances, we may request that the guardian remain in the waiting area during the appointment so the child will have the opportunity to speak freely.2
How to help a suspected victim
Several local and national organizations help trafficking victims. These organizations provide educational materials and training opportunities for clinicians, as well as direct support for victims. The Homeland Security Blue Campaign advises against confronting a suspected trafficker directly and encourages clinicians to instead report suspected cases to 1-866-347-2423.3
Clinicians can better help children who are trafficked by taking the following 5 steps:
- Learn about the risk factors and signs of child trafficking.
- Post the National Human Trafficking Hotline (1-888-373-7888) in your waiting room.
- Determine if your patient is in danger and needs to be moved to a safe place.
- Connect the patient to social service agencies that can provide financial support and housing assistance so he/she doesn’t feel trapped by financial burdens.
- Work to rebuild their emotional and physical well-being while treating depression, PTSD, substance abuse, or any other mental illness.
Child trafficking—a modern-day form of slavery that continues to destroy many lives—often is hidden, even from the clinicians who see its victims. Traffickers typically exploit children for labor or commercial sexual work. The signs and symptoms that suggest a child is being trafficked may be less clear than those of the psychiatric illnesses we usually diagnose and treat. In this article, I summarize characteristics that could be helpful to note when you suspect a child is being trafficked, and offer some resources for helping victims.
How to identify possible victims
Children can be trafficked anywhere. The concept of a child being picked up off a street corner is outdated. Trafficking occurs in cities, suburbs, and rural areas. It happens in hotel rooms, at truck stops, on quiet residential streets, and in expensive homes. The internet has made it easier for traffickers to find victims.
Traffickers typically target youth who are emotionally and physically vulnerable. They often seek out teenagers who are undergoing financial hardships, experiencing family conflict, or have survived natural disasters. Many victims are runaways. In 2016, 1 in 6 child runaways reported to the National Center for Missing and Exploited Children were likely victims of trafficking.1 Of those children, 86% were receiving social services support or living in foster homes.
Traffickers are adept at emotional manipulation, which may explain why a child or adolescent might minimize the abuse during a clinical visit. Traffickers shroud the realities of trafficking with notions of love and inclusion. They use several physical and mental schemes to keep children and adolescents in their grip, such as withholding food, sleep, or medical care. Therefore, we should check for signs and symptoms of chronic medical conditions that have gone untreated, malnutrition, or bruises in various stages of healing.
Connecting risk factors for trafficking to dramatic changes in a young patient’s behavior is challenging. These youth often have dropped out of school, lack consistent family support, and spend their nights in search of a warm place to sleep. Their lives are upended. A child who once was more social may be forced into isolation and make excuses for why she no longer spends time with her friends.
In a study of 106 survivors of domestic sex trafficking, approximately 89% of respondents reported depression during depression. Many respondents reported experiencing anxiety (76.4%), nightmares (73.6%), flashbacks (68%), low self-esteem (81.1%), or feelings of shame or guilt (82.1%).2 Almost 88% of respondents said that they saw a doctor or other clinician while being trafficked, but their clinicians were unable to recognize the signs of trafficking. Part of the challenge is that many children and adolescents are not comfortable discussing their situations with clinicians because they may struggle with shame and guilt. Their traffickers also might have convinced them that they are criminals, not victims. These patients also may have an overwhelming fear of their trafficker, being reported to child welfare authorities, being arrested, being deported, or having their traffickers retaliate against their families. Gaining the trust of a patient who is being trafficked is critical, but not easy, because children may be skeptical of a clinician’s promise of confidentiality.
Some signs of trafficking overlap with the psychiatric presentations with which we are more familiar. These patients may abuse drugs or alcohol as means of escape or because their traffickers force them to use substances.2 They may show symptoms of depression or posttraumatic stress disorder (PTSD) and may be disoriented. Other indicators may be more telling, such as if a child or adolescent describes:
- having no control of their schedules or forms of identification
- having to work excessively long hours, often to pay off an overwhelming debt
- having high security measures installed in their place of residence (such as cameras or barred windows).
Continue to: Also, they may be...
Also, they may be dressed inappropriately for the weather.
We should be concerned when patients’ responses seem coached, if they say they are isolated from their family and community, or if they are submissive or overly timid. In addition, our suspicions should be raised if an accompanying adult guardian insists on sitting in on the appointment or translating for the child. In such instances, we may request that the guardian remain in the waiting area during the appointment so the child will have the opportunity to speak freely.2
How to help a suspected victim
Several local and national organizations help trafficking victims. These organizations provide educational materials and training opportunities for clinicians, as well as direct support for victims. The Homeland Security Blue Campaign advises against confronting a suspected trafficker directly and encourages clinicians to instead report suspected cases to 1-866-347-2423.3
Clinicians can better help children who are trafficked by taking the following 5 steps:
- Learn about the risk factors and signs of child trafficking.
- Post the National Human Trafficking Hotline (1-888-373-7888) in your waiting room.
- Determine if your patient is in danger and needs to be moved to a safe place.
- Connect the patient to social service agencies that can provide financial support and housing assistance so he/she doesn’t feel trapped by financial burdens.
- Work to rebuild their emotional and physical well-being while treating depression, PTSD, substance abuse, or any other mental illness.
1. National Center for Missing and Exploited Childr en. Missing children, state care, and child sex trafficking. http://www.missingkids.com/content/dam/missingkids/pdfs/publications/missingchildrenstatecare.pdf. Accessed June 10, 2019.
2. Lederer LJ, Wetzel CA. The health consequences of sex trafficking and their implications for identifying victims in healthcare facilities. Ann Health Law. 2014;23(1):61-91.
3. Blue Campaign. Identify a victim. US Department of Homeland Security. https://www.dhs.gov/blue-campaign/identify-victim. Accessed June 10, 2019.
1. National Center for Missing and Exploited Childr en. Missing children, state care, and child sex trafficking. http://www.missingkids.com/content/dam/missingkids/pdfs/publications/missingchildrenstatecare.pdf. Accessed June 10, 2019.
2. Lederer LJ, Wetzel CA. The health consequences of sex trafficking and their implications for identifying victims in healthcare facilities. Ann Health Law. 2014;23(1):61-91.
3. Blue Campaign. Identify a victim. US Department of Homeland Security. https://www.dhs.gov/blue-campaign/identify-victim. Accessed June 10, 2019.