User login
Young Man With Headache, Confusion, and Hearing Loss
A 25-year-old male was found wandering naked in his room with the shower running after failing to come to work on a Monday morning. When found, he was able to talk and follow some commands but was confused about what was happening. He had experienced a right periorbital headache with nausea and vomiting for several days before admission. A computed tomography (CT) scan of the head at an outside hospital was negative.
Related: Pain, Anxiety, and Dementia: A Catastrophic Outcome
The patient had no history of tick or insect bites, skin rash, chest pain, shortness of breath, trauma, or illicit drug or alcohol use. He smoked a half pack of cigarettes per day. The patient had spent time in the military in the Middle East and North Africa 3 years earlier and had 3 tattoos. Over the past few months, he had been noted to be more aggressive, including having gotten into a bar fight. His past medical history was significant only for documented hearing loss in the right ear per reports from the air base.
On examination, the patient’s temperature was 97.3°F, heart rate 47 bpm, respirations 20 breathes/min, and blood pressure 97/60 mm Hg. His neck was supple, and the remainder of the general examination was normal. The neurologic examination revealed the patient to be awake and alert but apathetic, irritable, and refusing to talk after a few minutes. He was slow to respond, spoke loudly, and had a poor attention span. The patient was disoriented to time and place and remembered 0/5 objects at 5 minutes.
Blood tests revealed 12,500/μL white blood cell (WBC) count (increased mononuclear cells, 13.7%); hemoglobin, 14.6 g/dL; platelet count 194,000/μL; and hematocrit, 43.6%. Electrolytes, general chemistries, vitamin B12, thyroid-stimulating hormone, copper, erythrocyte sedimentation rate, and urinalysis were all normal. The CT head scan was normal, and the urine drug screen and alcohol levels were negative.
The initial audiologic evaluation revealed absent acoustic reflexes bilaterally at 500 Hz, 1 KHz, 2 KHz, and 4 KHz. The brain stem auditory evoked potentials showed no replicable waveforms from the right ear and a wave I present in the left ear with no other replicable waveforms.
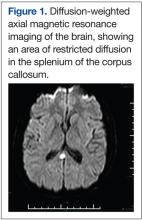
A very broad differential diagnosis was considered at this point (Table 1). Lumbar puncture was performed with an opening pressure of 17.5 cm H2O. There were 10 WBC/μL with 12% segmented polymorphonuclear cells and 83% lymphocytes, 30 red blood cells/μL, glucose of 71 mg/dL, and protein of 221 mg/dL. The Venereal Disease Research Laboratory Test was nonreactive; cryptococcal antigen, acid-fast stain, and bacterial and fungal cultures were negative. The electroencephalogram (EEG) showed mild diffuse slowing with frontal intermittent rhythmic delta activity. The magnetic resonance imaging (MRI) was significant for leptomeningeal and pachymeningeal enhancement with a small area of restricted diffusion in the splenium of the corpus callosum (Figure 1). Other cerebral spinal fluid and serum studies were negative or nonreactive (Table 2).
The patient completed a 2-week course of ceftriaxone 1 gram q 12 hours, vancomycin 1,000 mg q 12 hours, acyclovir 700 mg q 8 hours, and doxycycline 100 mg bid without any notable clinical change. A repeat lumbar puncture was acellular and had a protein of 254 mg/dL.
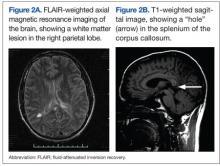
Two days later he worsened, becoming more withdrawn, unable to speak, irritable, and unwilling to be examined. He refused to get out of bed even with his family members present. A repeat MRI at this time showed continued meningeal enhancement, enlargement of the previously seen corpus callosal lesion, a new white matter lesion in the right parietal region, and a dark hole in the corpus callosum on the sagittal T1 image (Figures 2A and 2B). Audiometric testing showed profound hearing loss at low and high frequencies, with severe loss at middle frequencies in both ears.
- What is your diagnosis?
- How would you treat this patient?
[Click through to the next page to see the answer.]
Our Diagnosis
At this point, a diagnosis must explain encephalitis/encephalopathy, hearing loss, and MRI findings of meningeal enhancement and lesions in the corpus callosum and right parietal white matter. The main differential at this point included acute disseminated encephalomyelitis (ADEM), multiple sclerosis (MS), infection, and vasculitis/vasculopathy (especially primary central nervous system vasculitis). Acute disseminated encephalomyelitis usually has large, asymmetric lesions in the subcortical white matter and gray white junction, with corpus callosal lesions being unusual. Meningeal enhancement is very rare, and hearing loss would be unusual as well.1
Encephalopathy would be unusual in MS and if seen is usually associated with large confluent lesions (the Marburg variant). Meningeal enhancement would be rare on MRI, and the location of the corpus callosal lesion as shown on the T1- sagittal MRI would be atypical for both MS and ADEM (Figure 2B). Hearing loss has been described in MS, with a 4% to 5% incidence, often as the first manifestation and usually with full recovery.2 With the extensive evaluation and treatment in this case, infection was unlikely at this point. Primary central nervous system vasculitis remained a definite possibility and could explain most of the findings. However, there have been no reports of hearing loss or corpus callosal lesions in the literature with this latter condition.
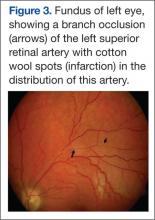
The presence of encephalopathy and hearing loss, in addition to the location of the corpus callosal lesion as demonstrated on the sagittal T1-weighted MRI (Figure 2B) suggested the need for an ophthalmologic consult with dilated retinoscopy and fluorescein angiography (Figure 3). A retinal examination showed branch retinal artery occlusions with cotton wool spots (infarctions). Fluorescein angiography showed branch retinal artery occlusions and arteriolar wall hyperfluoresence in one area. This demonstrated the final feature of the triad of encephalopathy, hearing loss, and branch retinal artery occlusions, confirming the diagnosis of Susac syndrome (SS).
Discussion and Literature Review
The patient was treated with a 3-day course of IV methylprednisolone 1 g daily for 3 days, followed by oral prednisone 60 mg daily for 1 week, followed by a slow taper thereafter. Both his cognition and behavior improved by the second day of treatment, and this continued during his hospital stay. After a short stay in the rehabilitation unit, he was transferred to a facility closer to his home. Mental status improved almost to baseline, but he got minimal if any improvement in his hearing function. Despite the branch retinal occlusions, he had no noticeable deficit in his visual function.
John O. Susac, MD, first described 2 women with a triad of encephalopathy, hearing loss, and branch retinal artery occlusions as a syndrome that subsequently was named after him.3 The syndrome most frequently affects women aged 20 to 40 years. Headaches consistent with migraines occur at onset in a majority of patients.4 Encephalopathy may be acute or subacute and mild to severe. Symptoms can include mood changes, personality change, bizarre behavior, hallucinations, memory and cognitive difficulties, ataxia, seizures, corticospinal tract signs, and myoclonus.5,6 The retinopathy may cause scintillating scotomata or segmental loss of vision but may also be asymptomatic due to occlusions in very distal, branch arteries. Hearing loss may be acute and severe or insidious and mild. Audiometry shows low-to-mid frequency hearing loss.
Related: Infliximab-Induced Complications
Hearing loss is usually permanent and, if severe, may require a cochlear implant.7 The disease course is variable and unpredictable. It may be monophasic, lasting under 2 years. This is often the case if encephalopathy occurs in the first 2 years. Susac syndrome can also have a polycyclic course, with remissions lasting up to 18 years. A chronic continuous course has also been described.8 All 3 components of the triad are not always present, and those without encephalopathy are more likely to have a polycyclic or chronic continuous course. The differential diagnosis is broad, as in the present case.
A cerebral spinal fluid evaluation often shows an elevated protein of 100 to 3,000 mg/dL, a mild lymphocytic pleocytosis (5-30 cells/mm), and oligoclonal bands may be present. Antiendothelial antibodies are present in the serum but not specific (also seen in systemic lupus erythematosus, rheumatoid arthritis, sarcoidosis, and juvenile dermatomyositis).8 The EEG usually shows diffuse slowing. The MRI is almost always abnormal, and studies have shown virtually 100% have corpus callosal lesions. These occur in the central region of the corpus callosum, consistent with infarction. Demyelinating lesions, with MS or ADEM, on the other hand, tend to occur on the inferior surface of the corpus callosum. If SS is suspected, a sagittal fluid- attenuated inversion recovery (FLAIR) MRI should be obtained to look for these changes. About one-third or more of MRIs show leptomeningeal enhancement, and other lesions can be found scattered throughout the white matter, cerebellum, brain stem, and gray matter.9
Because relatively few cases have been described, SS etiology remains obscure at this time. The disease has an affinity for small precapillary arterioles, of > 100 μm in diameter. The pathology shows necrosis and inflammatory changes of the endothelial cells, making them the primary site of the immune attack. This immune-mediated injury leads to narrowing and occlusion of the microvasculature, with resulting ischemia of the brain, retina, and cochlea. This pathology is very similar to that of juvenile dermatomyositis, which involves muscle, skin, and the gastrointestinal tract.10
Treatment approaches are based on treatments for juvenile dermatomyositis. It is suggested that the patient be given pulse methylprednisolone therapy of 1 g per day for 3 days followed by prednisone 60 mg to 80 mg per day for 4 weeks. Newer recommendations suggest giving IV immunoglobulin in the first week as well, followed by additional courses every month for 6 months. Cyclophosphamide or mycophenolate mofetil should be considered for long-term treatment with consideration of etanercept, cyclosporine, or rituximab in those who fail to respond.10 Aggressive treatment is suggested, because this is a self-limiting disorder, but the deficits tend to be permanent.
Related: Rituximab and Primary Sjögren Syndrome
This patient was atypical, because SS primarily affects young females. Review of the literature indicates that men account for about 25% of patients.8 The presentation, however, was not unusual and demonstrated the difficulty in making this diagnosis. In this patient with encephalopathy, the unusual feature was hearing loss, but it must be kept in mind that both hearing loss and visual changes can be difficult to identify in a confused patient. Brain stem auditory evoked potentials may be helpful in investigating hearing loss in noncooperative patients. An MRI may show centrally located corpus callosal lesions. If SS is suspected, sagittal FLAIR images, which often are not routinely done, should be obtained.
The most helpful evaluation is a dilated direct retinoscopy, which will usually show the branch retinal artery occlusions, and if not, fluorescein angiography will usually show a change. The presence of Gass plaques, yellow-white retinal arterial wall plaques from lipid deposition into the damaged arterial wall, with hyperfluoresence on fluorescein angiography is considered pathognomonic of SS.8 Establishing the diagnosis of SS as soon as possible is critical, because early treatment may lessen the degree of permanent disability.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Eckstein C, Saidha S, Levy M. A differential diagnosis of central nervous system demyelination: beyond multiple sclerosis. J Neurol. 2012;259(5):801-816.
2. Hellmann MA, Steiner I, Mosberg-Galili R. Sudden sensorineural hearing loss in multiple sclerosis: clinical course and possible pathogenesis. Acta Neurol Scand. 2011;124(4):245-249.
3. Susac JO, Hardman JM, Selhorst JB. Microangiopathy of the brain and retina. Neurology. 1979;29(3):313-316.
4. Papo T, Biousse V, Lehoang P, et al. Susac syndrome. Medicine (Baltimore). 1998;77(1):3-11.
5. Susac JO. Susac’s syndrome: the triad of microangiopathy of the brain and retina with hearing loss in young women. Neurology. 1994;44(4):591-593.
6. Hahn JS, Lannin WC, Sarwal MM. Microangiopathy of brain, retina, and inner ear (Susac’s syndrome) in an adolescent female presenting as acute disseminated encephalomyelitis. Pediatrics. 2004;114(1):276-281.
7. Roeser MM, Driscoll CL, Shallop JK, Gifford RH, Kasperbauer JL, Gluth MB. Susac syndrome—a report of cochlear implantation and review of otologic manifestations in twenty-three patients. Otol Neurotol. 2009;30(1):34-40.
8. Bitra RK, Eggenberger E. Review of Susac syndrome. Curr Opin Ophthalmol. 2011;22(6):472-476.
9. Susac JO, Murtagh FR, Egan RA, et al. MRI findings in Susac’s syndrome. Neurology. 2003;61(12): 1783-1787.
10. Rennebohm RM, Susac JO. Treatment of Susac’s syndrome. J Neurol Sci. 2007;257(1-2):215-220.
A 25-year-old male was found wandering naked in his room with the shower running after failing to come to work on a Monday morning. When found, he was able to talk and follow some commands but was confused about what was happening. He had experienced a right periorbital headache with nausea and vomiting for several days before admission. A computed tomography (CT) scan of the head at an outside hospital was negative.
Related: Pain, Anxiety, and Dementia: A Catastrophic Outcome
The patient had no history of tick or insect bites, skin rash, chest pain, shortness of breath, trauma, or illicit drug or alcohol use. He smoked a half pack of cigarettes per day. The patient had spent time in the military in the Middle East and North Africa 3 years earlier and had 3 tattoos. Over the past few months, he had been noted to be more aggressive, including having gotten into a bar fight. His past medical history was significant only for documented hearing loss in the right ear per reports from the air base.
On examination, the patient’s temperature was 97.3°F, heart rate 47 bpm, respirations 20 breathes/min, and blood pressure 97/60 mm Hg. His neck was supple, and the remainder of the general examination was normal. The neurologic examination revealed the patient to be awake and alert but apathetic, irritable, and refusing to talk after a few minutes. He was slow to respond, spoke loudly, and had a poor attention span. The patient was disoriented to time and place and remembered 0/5 objects at 5 minutes.
Blood tests revealed 12,500/μL white blood cell (WBC) count (increased mononuclear cells, 13.7%); hemoglobin, 14.6 g/dL; platelet count 194,000/μL; and hematocrit, 43.6%. Electrolytes, general chemistries, vitamin B12, thyroid-stimulating hormone, copper, erythrocyte sedimentation rate, and urinalysis were all normal. The CT head scan was normal, and the urine drug screen and alcohol levels were negative.
The initial audiologic evaluation revealed absent acoustic reflexes bilaterally at 500 Hz, 1 KHz, 2 KHz, and 4 KHz. The brain stem auditory evoked potentials showed no replicable waveforms from the right ear and a wave I present in the left ear with no other replicable waveforms.
A very broad differential diagnosis was considered at this point (Table 1). Lumbar puncture was performed with an opening pressure of 17.5 cm H2O. There were 10 WBC/μL with 12% segmented polymorphonuclear cells and 83% lymphocytes, 30 red blood cells/μL, glucose of 71 mg/dL, and protein of 221 mg/dL. The Venereal Disease Research Laboratory Test was nonreactive; cryptococcal antigen, acid-fast stain, and bacterial and fungal cultures were negative. The electroencephalogram (EEG) showed mild diffuse slowing with frontal intermittent rhythmic delta activity. The magnetic resonance imaging (MRI) was significant for leptomeningeal and pachymeningeal enhancement with a small area of restricted diffusion in the splenium of the corpus callosum (Figure 1). Other cerebral spinal fluid and serum studies were negative or nonreactive (Table 2).
The patient completed a 2-week course of ceftriaxone 1 gram q 12 hours, vancomycin 1,000 mg q 12 hours, acyclovir 700 mg q 8 hours, and doxycycline 100 mg bid without any notable clinical change. A repeat lumbar puncture was acellular and had a protein of 254 mg/dL.
Two days later he worsened, becoming more withdrawn, unable to speak, irritable, and unwilling to be examined. He refused to get out of bed even with his family members present. A repeat MRI at this time showed continued meningeal enhancement, enlargement of the previously seen corpus callosal lesion, a new white matter lesion in the right parietal region, and a dark hole in the corpus callosum on the sagittal T1 image (Figures 2A and 2B). Audiometric testing showed profound hearing loss at low and high frequencies, with severe loss at middle frequencies in both ears.
- What is your diagnosis?
- How would you treat this patient?
[Click through to the next page to see the answer.]
Our Diagnosis
At this point, a diagnosis must explain encephalitis/encephalopathy, hearing loss, and MRI findings of meningeal enhancement and lesions in the corpus callosum and right parietal white matter. The main differential at this point included acute disseminated encephalomyelitis (ADEM), multiple sclerosis (MS), infection, and vasculitis/vasculopathy (especially primary central nervous system vasculitis). Acute disseminated encephalomyelitis usually has large, asymmetric lesions in the subcortical white matter and gray white junction, with corpus callosal lesions being unusual. Meningeal enhancement is very rare, and hearing loss would be unusual as well.1
Encephalopathy would be unusual in MS and if seen is usually associated with large confluent lesions (the Marburg variant). Meningeal enhancement would be rare on MRI, and the location of the corpus callosal lesion as shown on the T1- sagittal MRI would be atypical for both MS and ADEM (Figure 2B). Hearing loss has been described in MS, with a 4% to 5% incidence, often as the first manifestation and usually with full recovery.2 With the extensive evaluation and treatment in this case, infection was unlikely at this point. Primary central nervous system vasculitis remained a definite possibility and could explain most of the findings. However, there have been no reports of hearing loss or corpus callosal lesions in the literature with this latter condition.
The presence of encephalopathy and hearing loss, in addition to the location of the corpus callosal lesion as demonstrated on the sagittal T1-weighted MRI (Figure 2B) suggested the need for an ophthalmologic consult with dilated retinoscopy and fluorescein angiography (Figure 3). A retinal examination showed branch retinal artery occlusions with cotton wool spots (infarctions). Fluorescein angiography showed branch retinal artery occlusions and arteriolar wall hyperfluoresence in one area. This demonstrated the final feature of the triad of encephalopathy, hearing loss, and branch retinal artery occlusions, confirming the diagnosis of Susac syndrome (SS).
Discussion and Literature Review
The patient was treated with a 3-day course of IV methylprednisolone 1 g daily for 3 days, followed by oral prednisone 60 mg daily for 1 week, followed by a slow taper thereafter. Both his cognition and behavior improved by the second day of treatment, and this continued during his hospital stay. After a short stay in the rehabilitation unit, he was transferred to a facility closer to his home. Mental status improved almost to baseline, but he got minimal if any improvement in his hearing function. Despite the branch retinal occlusions, he had no noticeable deficit in his visual function.
John O. Susac, MD, first described 2 women with a triad of encephalopathy, hearing loss, and branch retinal artery occlusions as a syndrome that subsequently was named after him.3 The syndrome most frequently affects women aged 20 to 40 years. Headaches consistent with migraines occur at onset in a majority of patients.4 Encephalopathy may be acute or subacute and mild to severe. Symptoms can include mood changes, personality change, bizarre behavior, hallucinations, memory and cognitive difficulties, ataxia, seizures, corticospinal tract signs, and myoclonus.5,6 The retinopathy may cause scintillating scotomata or segmental loss of vision but may also be asymptomatic due to occlusions in very distal, branch arteries. Hearing loss may be acute and severe or insidious and mild. Audiometry shows low-to-mid frequency hearing loss.
Related: Infliximab-Induced Complications
Hearing loss is usually permanent and, if severe, may require a cochlear implant.7 The disease course is variable and unpredictable. It may be monophasic, lasting under 2 years. This is often the case if encephalopathy occurs in the first 2 years. Susac syndrome can also have a polycyclic course, with remissions lasting up to 18 years. A chronic continuous course has also been described.8 All 3 components of the triad are not always present, and those without encephalopathy are more likely to have a polycyclic or chronic continuous course. The differential diagnosis is broad, as in the present case.
A cerebral spinal fluid evaluation often shows an elevated protein of 100 to 3,000 mg/dL, a mild lymphocytic pleocytosis (5-30 cells/mm), and oligoclonal bands may be present. Antiendothelial antibodies are present in the serum but not specific (also seen in systemic lupus erythematosus, rheumatoid arthritis, sarcoidosis, and juvenile dermatomyositis).8 The EEG usually shows diffuse slowing. The MRI is almost always abnormal, and studies have shown virtually 100% have corpus callosal lesions. These occur in the central region of the corpus callosum, consistent with infarction. Demyelinating lesions, with MS or ADEM, on the other hand, tend to occur on the inferior surface of the corpus callosum. If SS is suspected, a sagittal fluid- attenuated inversion recovery (FLAIR) MRI should be obtained to look for these changes. About one-third or more of MRIs show leptomeningeal enhancement, and other lesions can be found scattered throughout the white matter, cerebellum, brain stem, and gray matter.9
Because relatively few cases have been described, SS etiology remains obscure at this time. The disease has an affinity for small precapillary arterioles, of > 100 μm in diameter. The pathology shows necrosis and inflammatory changes of the endothelial cells, making them the primary site of the immune attack. This immune-mediated injury leads to narrowing and occlusion of the microvasculature, with resulting ischemia of the brain, retina, and cochlea. This pathology is very similar to that of juvenile dermatomyositis, which involves muscle, skin, and the gastrointestinal tract.10
Treatment approaches are based on treatments for juvenile dermatomyositis. It is suggested that the patient be given pulse methylprednisolone therapy of 1 g per day for 3 days followed by prednisone 60 mg to 80 mg per day for 4 weeks. Newer recommendations suggest giving IV immunoglobulin in the first week as well, followed by additional courses every month for 6 months. Cyclophosphamide or mycophenolate mofetil should be considered for long-term treatment with consideration of etanercept, cyclosporine, or rituximab in those who fail to respond.10 Aggressive treatment is suggested, because this is a self-limiting disorder, but the deficits tend to be permanent.
Related: Rituximab and Primary Sjögren Syndrome
This patient was atypical, because SS primarily affects young females. Review of the literature indicates that men account for about 25% of patients.8 The presentation, however, was not unusual and demonstrated the difficulty in making this diagnosis. In this patient with encephalopathy, the unusual feature was hearing loss, but it must be kept in mind that both hearing loss and visual changes can be difficult to identify in a confused patient. Brain stem auditory evoked potentials may be helpful in investigating hearing loss in noncooperative patients. An MRI may show centrally located corpus callosal lesions. If SS is suspected, sagittal FLAIR images, which often are not routinely done, should be obtained.
The most helpful evaluation is a dilated direct retinoscopy, which will usually show the branch retinal artery occlusions, and if not, fluorescein angiography will usually show a change. The presence of Gass plaques, yellow-white retinal arterial wall plaques from lipid deposition into the damaged arterial wall, with hyperfluoresence on fluorescein angiography is considered pathognomonic of SS.8 Establishing the diagnosis of SS as soon as possible is critical, because early treatment may lessen the degree of permanent disability.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
A 25-year-old male was found wandering naked in his room with the shower running after failing to come to work on a Monday morning. When found, he was able to talk and follow some commands but was confused about what was happening. He had experienced a right periorbital headache with nausea and vomiting for several days before admission. A computed tomography (CT) scan of the head at an outside hospital was negative.
Related: Pain, Anxiety, and Dementia: A Catastrophic Outcome
The patient had no history of tick or insect bites, skin rash, chest pain, shortness of breath, trauma, or illicit drug or alcohol use. He smoked a half pack of cigarettes per day. The patient had spent time in the military in the Middle East and North Africa 3 years earlier and had 3 tattoos. Over the past few months, he had been noted to be more aggressive, including having gotten into a bar fight. His past medical history was significant only for documented hearing loss in the right ear per reports from the air base.
On examination, the patient’s temperature was 97.3°F, heart rate 47 bpm, respirations 20 breathes/min, and blood pressure 97/60 mm Hg. His neck was supple, and the remainder of the general examination was normal. The neurologic examination revealed the patient to be awake and alert but apathetic, irritable, and refusing to talk after a few minutes. He was slow to respond, spoke loudly, and had a poor attention span. The patient was disoriented to time and place and remembered 0/5 objects at 5 minutes.
Blood tests revealed 12,500/μL white blood cell (WBC) count (increased mononuclear cells, 13.7%); hemoglobin, 14.6 g/dL; platelet count 194,000/μL; and hematocrit, 43.6%. Electrolytes, general chemistries, vitamin B12, thyroid-stimulating hormone, copper, erythrocyte sedimentation rate, and urinalysis were all normal. The CT head scan was normal, and the urine drug screen and alcohol levels were negative.
The initial audiologic evaluation revealed absent acoustic reflexes bilaterally at 500 Hz, 1 KHz, 2 KHz, and 4 KHz. The brain stem auditory evoked potentials showed no replicable waveforms from the right ear and a wave I present in the left ear with no other replicable waveforms.
A very broad differential diagnosis was considered at this point (Table 1). Lumbar puncture was performed with an opening pressure of 17.5 cm H2O. There were 10 WBC/μL with 12% segmented polymorphonuclear cells and 83% lymphocytes, 30 red blood cells/μL, glucose of 71 mg/dL, and protein of 221 mg/dL. The Venereal Disease Research Laboratory Test was nonreactive; cryptococcal antigen, acid-fast stain, and bacterial and fungal cultures were negative. The electroencephalogram (EEG) showed mild diffuse slowing with frontal intermittent rhythmic delta activity. The magnetic resonance imaging (MRI) was significant for leptomeningeal and pachymeningeal enhancement with a small area of restricted diffusion in the splenium of the corpus callosum (Figure 1). Other cerebral spinal fluid and serum studies were negative or nonreactive (Table 2).
The patient completed a 2-week course of ceftriaxone 1 gram q 12 hours, vancomycin 1,000 mg q 12 hours, acyclovir 700 mg q 8 hours, and doxycycline 100 mg bid without any notable clinical change. A repeat lumbar puncture was acellular and had a protein of 254 mg/dL.
Two days later he worsened, becoming more withdrawn, unable to speak, irritable, and unwilling to be examined. He refused to get out of bed even with his family members present. A repeat MRI at this time showed continued meningeal enhancement, enlargement of the previously seen corpus callosal lesion, a new white matter lesion in the right parietal region, and a dark hole in the corpus callosum on the sagittal T1 image (Figures 2A and 2B). Audiometric testing showed profound hearing loss at low and high frequencies, with severe loss at middle frequencies in both ears.
- What is your diagnosis?
- How would you treat this patient?
[Click through to the next page to see the answer.]
Our Diagnosis
At this point, a diagnosis must explain encephalitis/encephalopathy, hearing loss, and MRI findings of meningeal enhancement and lesions in the corpus callosum and right parietal white matter. The main differential at this point included acute disseminated encephalomyelitis (ADEM), multiple sclerosis (MS), infection, and vasculitis/vasculopathy (especially primary central nervous system vasculitis). Acute disseminated encephalomyelitis usually has large, asymmetric lesions in the subcortical white matter and gray white junction, with corpus callosal lesions being unusual. Meningeal enhancement is very rare, and hearing loss would be unusual as well.1
Encephalopathy would be unusual in MS and if seen is usually associated with large confluent lesions (the Marburg variant). Meningeal enhancement would be rare on MRI, and the location of the corpus callosal lesion as shown on the T1- sagittal MRI would be atypical for both MS and ADEM (Figure 2B). Hearing loss has been described in MS, with a 4% to 5% incidence, often as the first manifestation and usually with full recovery.2 With the extensive evaluation and treatment in this case, infection was unlikely at this point. Primary central nervous system vasculitis remained a definite possibility and could explain most of the findings. However, there have been no reports of hearing loss or corpus callosal lesions in the literature with this latter condition.
The presence of encephalopathy and hearing loss, in addition to the location of the corpus callosal lesion as demonstrated on the sagittal T1-weighted MRI (Figure 2B) suggested the need for an ophthalmologic consult with dilated retinoscopy and fluorescein angiography (Figure 3). A retinal examination showed branch retinal artery occlusions with cotton wool spots (infarctions). Fluorescein angiography showed branch retinal artery occlusions and arteriolar wall hyperfluoresence in one area. This demonstrated the final feature of the triad of encephalopathy, hearing loss, and branch retinal artery occlusions, confirming the diagnosis of Susac syndrome (SS).
Discussion and Literature Review
The patient was treated with a 3-day course of IV methylprednisolone 1 g daily for 3 days, followed by oral prednisone 60 mg daily for 1 week, followed by a slow taper thereafter. Both his cognition and behavior improved by the second day of treatment, and this continued during his hospital stay. After a short stay in the rehabilitation unit, he was transferred to a facility closer to his home. Mental status improved almost to baseline, but he got minimal if any improvement in his hearing function. Despite the branch retinal occlusions, he had no noticeable deficit in his visual function.
John O. Susac, MD, first described 2 women with a triad of encephalopathy, hearing loss, and branch retinal artery occlusions as a syndrome that subsequently was named after him.3 The syndrome most frequently affects women aged 20 to 40 years. Headaches consistent with migraines occur at onset in a majority of patients.4 Encephalopathy may be acute or subacute and mild to severe. Symptoms can include mood changes, personality change, bizarre behavior, hallucinations, memory and cognitive difficulties, ataxia, seizures, corticospinal tract signs, and myoclonus.5,6 The retinopathy may cause scintillating scotomata or segmental loss of vision but may also be asymptomatic due to occlusions in very distal, branch arteries. Hearing loss may be acute and severe or insidious and mild. Audiometry shows low-to-mid frequency hearing loss.
Related: Infliximab-Induced Complications
Hearing loss is usually permanent and, if severe, may require a cochlear implant.7 The disease course is variable and unpredictable. It may be monophasic, lasting under 2 years. This is often the case if encephalopathy occurs in the first 2 years. Susac syndrome can also have a polycyclic course, with remissions lasting up to 18 years. A chronic continuous course has also been described.8 All 3 components of the triad are not always present, and those without encephalopathy are more likely to have a polycyclic or chronic continuous course. The differential diagnosis is broad, as in the present case.
A cerebral spinal fluid evaluation often shows an elevated protein of 100 to 3,000 mg/dL, a mild lymphocytic pleocytosis (5-30 cells/mm), and oligoclonal bands may be present. Antiendothelial antibodies are present in the serum but not specific (also seen in systemic lupus erythematosus, rheumatoid arthritis, sarcoidosis, and juvenile dermatomyositis).8 The EEG usually shows diffuse slowing. The MRI is almost always abnormal, and studies have shown virtually 100% have corpus callosal lesions. These occur in the central region of the corpus callosum, consistent with infarction. Demyelinating lesions, with MS or ADEM, on the other hand, tend to occur on the inferior surface of the corpus callosum. If SS is suspected, a sagittal fluid- attenuated inversion recovery (FLAIR) MRI should be obtained to look for these changes. About one-third or more of MRIs show leptomeningeal enhancement, and other lesions can be found scattered throughout the white matter, cerebellum, brain stem, and gray matter.9
Because relatively few cases have been described, SS etiology remains obscure at this time. The disease has an affinity for small precapillary arterioles, of > 100 μm in diameter. The pathology shows necrosis and inflammatory changes of the endothelial cells, making them the primary site of the immune attack. This immune-mediated injury leads to narrowing and occlusion of the microvasculature, with resulting ischemia of the brain, retina, and cochlea. This pathology is very similar to that of juvenile dermatomyositis, which involves muscle, skin, and the gastrointestinal tract.10
Treatment approaches are based on treatments for juvenile dermatomyositis. It is suggested that the patient be given pulse methylprednisolone therapy of 1 g per day for 3 days followed by prednisone 60 mg to 80 mg per day for 4 weeks. Newer recommendations suggest giving IV immunoglobulin in the first week as well, followed by additional courses every month for 6 months. Cyclophosphamide or mycophenolate mofetil should be considered for long-term treatment with consideration of etanercept, cyclosporine, or rituximab in those who fail to respond.10 Aggressive treatment is suggested, because this is a self-limiting disorder, but the deficits tend to be permanent.
Related: Rituximab and Primary Sjögren Syndrome
This patient was atypical, because SS primarily affects young females. Review of the literature indicates that men account for about 25% of patients.8 The presentation, however, was not unusual and demonstrated the difficulty in making this diagnosis. In this patient with encephalopathy, the unusual feature was hearing loss, but it must be kept in mind that both hearing loss and visual changes can be difficult to identify in a confused patient. Brain stem auditory evoked potentials may be helpful in investigating hearing loss in noncooperative patients. An MRI may show centrally located corpus callosal lesions. If SS is suspected, sagittal FLAIR images, which often are not routinely done, should be obtained.
The most helpful evaluation is a dilated direct retinoscopy, which will usually show the branch retinal artery occlusions, and if not, fluorescein angiography will usually show a change. The presence of Gass plaques, yellow-white retinal arterial wall plaques from lipid deposition into the damaged arterial wall, with hyperfluoresence on fluorescein angiography is considered pathognomonic of SS.8 Establishing the diagnosis of SS as soon as possible is critical, because early treatment may lessen the degree of permanent disability.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Eckstein C, Saidha S, Levy M. A differential diagnosis of central nervous system demyelination: beyond multiple sclerosis. J Neurol. 2012;259(5):801-816.
2. Hellmann MA, Steiner I, Mosberg-Galili R. Sudden sensorineural hearing loss in multiple sclerosis: clinical course and possible pathogenesis. Acta Neurol Scand. 2011;124(4):245-249.
3. Susac JO, Hardman JM, Selhorst JB. Microangiopathy of the brain and retina. Neurology. 1979;29(3):313-316.
4. Papo T, Biousse V, Lehoang P, et al. Susac syndrome. Medicine (Baltimore). 1998;77(1):3-11.
5. Susac JO. Susac’s syndrome: the triad of microangiopathy of the brain and retina with hearing loss in young women. Neurology. 1994;44(4):591-593.
6. Hahn JS, Lannin WC, Sarwal MM. Microangiopathy of brain, retina, and inner ear (Susac’s syndrome) in an adolescent female presenting as acute disseminated encephalomyelitis. Pediatrics. 2004;114(1):276-281.
7. Roeser MM, Driscoll CL, Shallop JK, Gifford RH, Kasperbauer JL, Gluth MB. Susac syndrome—a report of cochlear implantation and review of otologic manifestations in twenty-three patients. Otol Neurotol. 2009;30(1):34-40.
8. Bitra RK, Eggenberger E. Review of Susac syndrome. Curr Opin Ophthalmol. 2011;22(6):472-476.
9. Susac JO, Murtagh FR, Egan RA, et al. MRI findings in Susac’s syndrome. Neurology. 2003;61(12): 1783-1787.
10. Rennebohm RM, Susac JO. Treatment of Susac’s syndrome. J Neurol Sci. 2007;257(1-2):215-220.
1. Eckstein C, Saidha S, Levy M. A differential diagnosis of central nervous system demyelination: beyond multiple sclerosis. J Neurol. 2012;259(5):801-816.
2. Hellmann MA, Steiner I, Mosberg-Galili R. Sudden sensorineural hearing loss in multiple sclerosis: clinical course and possible pathogenesis. Acta Neurol Scand. 2011;124(4):245-249.
3. Susac JO, Hardman JM, Selhorst JB. Microangiopathy of the brain and retina. Neurology. 1979;29(3):313-316.
4. Papo T, Biousse V, Lehoang P, et al. Susac syndrome. Medicine (Baltimore). 1998;77(1):3-11.
5. Susac JO. Susac’s syndrome: the triad of microangiopathy of the brain and retina with hearing loss in young women. Neurology. 1994;44(4):591-593.
6. Hahn JS, Lannin WC, Sarwal MM. Microangiopathy of brain, retina, and inner ear (Susac’s syndrome) in an adolescent female presenting as acute disseminated encephalomyelitis. Pediatrics. 2004;114(1):276-281.
7. Roeser MM, Driscoll CL, Shallop JK, Gifford RH, Kasperbauer JL, Gluth MB. Susac syndrome—a report of cochlear implantation and review of otologic manifestations in twenty-three patients. Otol Neurotol. 2009;30(1):34-40.
8. Bitra RK, Eggenberger E. Review of Susac syndrome. Curr Opin Ophthalmol. 2011;22(6):472-476.
9. Susac JO, Murtagh FR, Egan RA, et al. MRI findings in Susac’s syndrome. Neurology. 2003;61(12): 1783-1787.
10. Rennebohm RM, Susac JO. Treatment of Susac’s syndrome. J Neurol Sci. 2007;257(1-2):215-220.
Complete Heart Block in a Patient With Metastatic Papillary Thyroid Carcinoma
A 74-year-old woman presented with a 2-day history of exertional dyspnea and palpitations. Her past medical history was significant for metastatic papillary thyroid carcinoma treated with total thyroidectomy and radioactive iodine ablation with levothyroxine for chronic suppressive therapy.
On examination, the patient was afebrile with an oxygen saturation of 98% on room air, heart rate of 92 beats/min, and blood pressure of 100/54 mm Hg. There was trace bilateral lower extremity edema, and her cardiopulmonary examination was unremarkable. The laboratory studies showed a white blood cell count of 24,300/µL (3,400-9,800); platelets 86,000/µL (142,000-362,000); thyroid stimulating hormone 0.009 mlU/L (0.4-4.1); free T4 2.07 ng/dL (0.8-2.0); thyroglobulin antibody titer < 1:10 (< 1:160); thyroid microsomal antibody titer < 1:100 (< 1:1600); and thyroglobulin 17.9 ng/mL (2.0-35.0). Her initial troponin T was undetectable.
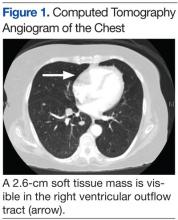
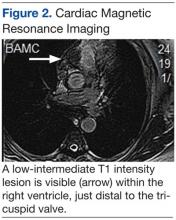
An electrocardiogram showed a first-degree atrioventricular block and subsequently a new intermittent third-degree atrioventricular block. A computed tomography angiogram (Figure 1) and cardiac magnetic resonance imaging (Figure 2) revealed a 2.6-cm soft tissue mass in the right ventricular outflow tract along with multiple pulmonary emboli and previously diagnosed pulmonary metastases. A positron emission tomography (PET) scan (not shown) revealed a 3.5-cm PET-avid lesion within the right ventricular outflow tract.
- What is your diagnosis?
- How would you treat this patient?
[Click through to the next page to see the answer.]
Our Treatment
Diagnosis and Discussion
This patient experienced complete heart block due to a cardiac tumor from papillary thyroid carcinoma metastasis. Complete heart block is not an unprecedented symptom of metastatic disease, but to our knowledge this is the first reported case of heart block secondary to metastatic papillary thyroid cancer.1 In general, metastatic cardiac tumors, usually associated with cancers of the breast and lung, melanoma, and lymphoma, are more common than are primary cardiac tumors and are often asymptomatic and discovered mostly postmortem.2,3 The frequency of thyroid metastasis to the heart has been reported to be as low as 0% to 2%, and a review of the literature demonstrated only 13 total cases in the past 30 years.
Theoretical mechanisms for invasion into the heart include lymphatic spread, hematogenous dissemination, or direct right ventricular invasion from the thoracic duct. It has been suggested that the lower blood flow to the myocardium (240 mL/min) relative to bone (600 mL/min) or the brain (750 mL/min) is the reason for a lower likelihood of cardiac involvement in metastatic disease.3 Given the findings in this case, evidence of cardiac conduction abnormalities in the setting of papillary thyroid cancer should raise suspicion for cardiac metastatic disease.
Case Outcome
In this patient, a permanent pacemaker was implanted for high-grade atrioventricular block, with resolution of the palpitations. The pulmonary emboli were concomitantly treated with enoxaparin, and the patient was discharged to a rehabilitation facility. Her prognosis was extremely poor given that survival with cardiac metastasis from any type of cancer is limited to a few weeks to months.3 She was to be reevaluated for experimental chemotherapy after reconditioning. However, not long after discharge she was readmitted in respiratory failure and died.
Acknowledgments
We would like to thank Dr. Kevin Steel, Lt Col, USAF, MC, imaging cardiologist at the Brooke Army Medical Center for his time and effort in accessing and preparing the CT and MRI images for this article.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect the official policy or position of Federal Practitioner, Frontline Medical Communications Inc., Brooke Army Medical Center, the U.S. Army Medical Department, the U.S. Army Office of the Surgeon General, the Department of the Army, Department of Defense, the U.S. Government, or any other of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Conley M, Hawkins K, Ririe D. Complete heart block and cardiac tamponade secondary to Merkel cell carcinoma cardiac metastases. South Med J. 2006;99(1):74-78.
2. Pascale P, Prior JO, Carron PN, Pruvot E, Muller O. Haemoptysis and complete atrioventricular block. Eur Heart J. 2008;29(11):1396.
3. Giuffrida D, Gharib H. Cardiac metastasis from primary anaplastic thyroid carcinoma: Report of three cases and a review of the literature. Endocr Relat Cancer. 2001;8(1):71-73.
A 74-year-old woman presented with a 2-day history of exertional dyspnea and palpitations. Her past medical history was significant for metastatic papillary thyroid carcinoma treated with total thyroidectomy and radioactive iodine ablation with levothyroxine for chronic suppressive therapy.
On examination, the patient was afebrile with an oxygen saturation of 98% on room air, heart rate of 92 beats/min, and blood pressure of 100/54 mm Hg. There was trace bilateral lower extremity edema, and her cardiopulmonary examination was unremarkable. The laboratory studies showed a white blood cell count of 24,300/µL (3,400-9,800); platelets 86,000/µL (142,000-362,000); thyroid stimulating hormone 0.009 mlU/L (0.4-4.1); free T4 2.07 ng/dL (0.8-2.0); thyroglobulin antibody titer < 1:10 (< 1:160); thyroid microsomal antibody titer < 1:100 (< 1:1600); and thyroglobulin 17.9 ng/mL (2.0-35.0). Her initial troponin T was undetectable.
An electrocardiogram showed a first-degree atrioventricular block and subsequently a new intermittent third-degree atrioventricular block. A computed tomography angiogram (Figure 1) and cardiac magnetic resonance imaging (Figure 2) revealed a 2.6-cm soft tissue mass in the right ventricular outflow tract along with multiple pulmonary emboli and previously diagnosed pulmonary metastases. A positron emission tomography (PET) scan (not shown) revealed a 3.5-cm PET-avid lesion within the right ventricular outflow tract.
- What is your diagnosis?
- How would you treat this patient?
[Click through to the next page to see the answer.]
Our Treatment
Diagnosis and Discussion
This patient experienced complete heart block due to a cardiac tumor from papillary thyroid carcinoma metastasis. Complete heart block is not an unprecedented symptom of metastatic disease, but to our knowledge this is the first reported case of heart block secondary to metastatic papillary thyroid cancer.1 In general, metastatic cardiac tumors, usually associated with cancers of the breast and lung, melanoma, and lymphoma, are more common than are primary cardiac tumors and are often asymptomatic and discovered mostly postmortem.2,3 The frequency of thyroid metastasis to the heart has been reported to be as low as 0% to 2%, and a review of the literature demonstrated only 13 total cases in the past 30 years.
Theoretical mechanisms for invasion into the heart include lymphatic spread, hematogenous dissemination, or direct right ventricular invasion from the thoracic duct. It has been suggested that the lower blood flow to the myocardium (240 mL/min) relative to bone (600 mL/min) or the brain (750 mL/min) is the reason for a lower likelihood of cardiac involvement in metastatic disease.3 Given the findings in this case, evidence of cardiac conduction abnormalities in the setting of papillary thyroid cancer should raise suspicion for cardiac metastatic disease.
Case Outcome
In this patient, a permanent pacemaker was implanted for high-grade atrioventricular block, with resolution of the palpitations. The pulmonary emboli were concomitantly treated with enoxaparin, and the patient was discharged to a rehabilitation facility. Her prognosis was extremely poor given that survival with cardiac metastasis from any type of cancer is limited to a few weeks to months.3 She was to be reevaluated for experimental chemotherapy after reconditioning. However, not long after discharge she was readmitted in respiratory failure and died.
Acknowledgments
We would like to thank Dr. Kevin Steel, Lt Col, USAF, MC, imaging cardiologist at the Brooke Army Medical Center for his time and effort in accessing and preparing the CT and MRI images for this article.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect the official policy or position of Federal Practitioner, Frontline Medical Communications Inc., Brooke Army Medical Center, the U.S. Army Medical Department, the U.S. Army Office of the Surgeon General, the Department of the Army, Department of Defense, the U.S. Government, or any other of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
A 74-year-old woman presented with a 2-day history of exertional dyspnea and palpitations. Her past medical history was significant for metastatic papillary thyroid carcinoma treated with total thyroidectomy and radioactive iodine ablation with levothyroxine for chronic suppressive therapy.
On examination, the patient was afebrile with an oxygen saturation of 98% on room air, heart rate of 92 beats/min, and blood pressure of 100/54 mm Hg. There was trace bilateral lower extremity edema, and her cardiopulmonary examination was unremarkable. The laboratory studies showed a white blood cell count of 24,300/µL (3,400-9,800); platelets 86,000/µL (142,000-362,000); thyroid stimulating hormone 0.009 mlU/L (0.4-4.1); free T4 2.07 ng/dL (0.8-2.0); thyroglobulin antibody titer < 1:10 (< 1:160); thyroid microsomal antibody titer < 1:100 (< 1:1600); and thyroglobulin 17.9 ng/mL (2.0-35.0). Her initial troponin T was undetectable.
An electrocardiogram showed a first-degree atrioventricular block and subsequently a new intermittent third-degree atrioventricular block. A computed tomography angiogram (Figure 1) and cardiac magnetic resonance imaging (Figure 2) revealed a 2.6-cm soft tissue mass in the right ventricular outflow tract along with multiple pulmonary emboli and previously diagnosed pulmonary metastases. A positron emission tomography (PET) scan (not shown) revealed a 3.5-cm PET-avid lesion within the right ventricular outflow tract.
- What is your diagnosis?
- How would you treat this patient?
[Click through to the next page to see the answer.]
Our Treatment
Diagnosis and Discussion
This patient experienced complete heart block due to a cardiac tumor from papillary thyroid carcinoma metastasis. Complete heart block is not an unprecedented symptom of metastatic disease, but to our knowledge this is the first reported case of heart block secondary to metastatic papillary thyroid cancer.1 In general, metastatic cardiac tumors, usually associated with cancers of the breast and lung, melanoma, and lymphoma, are more common than are primary cardiac tumors and are often asymptomatic and discovered mostly postmortem.2,3 The frequency of thyroid metastasis to the heart has been reported to be as low as 0% to 2%, and a review of the literature demonstrated only 13 total cases in the past 30 years.
Theoretical mechanisms for invasion into the heart include lymphatic spread, hematogenous dissemination, or direct right ventricular invasion from the thoracic duct. It has been suggested that the lower blood flow to the myocardium (240 mL/min) relative to bone (600 mL/min) or the brain (750 mL/min) is the reason for a lower likelihood of cardiac involvement in metastatic disease.3 Given the findings in this case, evidence of cardiac conduction abnormalities in the setting of papillary thyroid cancer should raise suspicion for cardiac metastatic disease.
Case Outcome
In this patient, a permanent pacemaker was implanted for high-grade atrioventricular block, with resolution of the palpitations. The pulmonary emboli were concomitantly treated with enoxaparin, and the patient was discharged to a rehabilitation facility. Her prognosis was extremely poor given that survival with cardiac metastasis from any type of cancer is limited to a few weeks to months.3 She was to be reevaluated for experimental chemotherapy after reconditioning. However, not long after discharge she was readmitted in respiratory failure and died.
Acknowledgments
We would like to thank Dr. Kevin Steel, Lt Col, USAF, MC, imaging cardiologist at the Brooke Army Medical Center for his time and effort in accessing and preparing the CT and MRI images for this article.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect the official policy or position of Federal Practitioner, Frontline Medical Communications Inc., Brooke Army Medical Center, the U.S. Army Medical Department, the U.S. Army Office of the Surgeon General, the Department of the Army, Department of Defense, the U.S. Government, or any other of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Conley M, Hawkins K, Ririe D. Complete heart block and cardiac tamponade secondary to Merkel cell carcinoma cardiac metastases. South Med J. 2006;99(1):74-78.
2. Pascale P, Prior JO, Carron PN, Pruvot E, Muller O. Haemoptysis and complete atrioventricular block. Eur Heart J. 2008;29(11):1396.
3. Giuffrida D, Gharib H. Cardiac metastasis from primary anaplastic thyroid carcinoma: Report of three cases and a review of the literature. Endocr Relat Cancer. 2001;8(1):71-73.
1. Conley M, Hawkins K, Ririe D. Complete heart block and cardiac tamponade secondary to Merkel cell carcinoma cardiac metastases. South Med J. 2006;99(1):74-78.
2. Pascale P, Prior JO, Carron PN, Pruvot E, Muller O. Haemoptysis and complete atrioventricular block. Eur Heart J. 2008;29(11):1396.
3. Giuffrida D, Gharib H. Cardiac metastasis from primary anaplastic thyroid carcinoma: Report of three cases and a review of the literature. Endocr Relat Cancer. 2001;8(1):71-73.
Acute Upper Abdominal Pain in Early Pregnancy
A 32-year-old G1P0 woman at 7 4/7 weeks’ gestation (intrauterine pregnancy confirmed by ultrasound) with a history of hypertension and anxiety presented to the emergency department reporting right upper quadrant and right flank pain. The patient did not report taking any medications. Her symptoms had begun about 6 hours prior to presentation. She did not report fever, chills, nausea, vomiting, anorexia, or urinary or bowels symptoms. She was afebrile, and her initial blood pressure was 174/84 mm Hg, but within an hour of presentation had decreased to 137/82 mm Hg.
On exam she was in moderate-to-severe discomfort. She was tender to palpation in the right flank as well as the right upper quadrant and had a positive Murphy sign. Her white blood cell (WBC) count was 18,800/μL, hemoglobin and hematocrit were normal, liver function tests were within normal limits, and sodium was 133 mmol/L; otherwise, electrolytes were normal. The urinalysis showed a specific gravity of 1.022 with 3 red blood cells per high powered field, but was otherwise normal. A right upper quadrant ultrasound showed a normal gallbladder without cholelithiasis. Subsequently, a magnetic resonance imaging (MRI) of the abdomen was obtained (Figure 1).
- What is your diagnosis?
- How would you treat this patient?
[Click through to the next page to see the answer.]
Our Treatment
An MRI of the abdomen revealed a right adrenal hematoma (Figure 2). This diagnosis was made after common causes of the right upper quadrant and right flank pain were excluded and was confirmed with an MRI of the abdomen.
Discussion
Common causes of right flank and right upper quadrant pain include urolithiasis, pyelonephritis, and acute cholecystitis (Table). Patients with urolithiasis will typically present with sudden onset of colicky flank pain, which may radiate to the inguinal region, with or without nausea and vomiting.1,2 Microhematuria may be absent in as many as 10% to 20% of patients.2 Computed tomography is nearly 97% sensitive and 96% specific for urolithiasis.2 Although only 37% to 64% sensitive in detecting urolithiasis, abdominal ultrasound is 85% to 94% specific for detecting hydronephrosis.2
Symptoms of pyelonephritis range from mild to severe costovertebral angle pain with or without fever and/or lower urinary tract symptoms to sepsis.3 Urinalysis typically shows leukocyte esterase (72%-97% sensitive, 41%-86% specific for culture-confirmed urinary tract infection) and microscopic pyuria (90%-96% sensitive, 47%-50% specific for culture-confirmed urinary tract infection).3,4 Urine culture will reveal 105 colony-forming units in 95% of patients with acute uncomplicated pyelonephritis.3 Imaging is typically reserved for atypical presentations or in cases of nonresolution of symptoms despite treatment in order to rule out structural anomalies, an obstructive process, or abscess formation.3
Acute cholecystitis is suggested by right upper quadrant pain, often following a meal. Symptoms include fever, leukocytosis, and an elevated serum bilirubin level.5 Ultrasound findings of acute cholecystitis are gallbladder wall thickening, pericholecystic fluid, or sonographic Murphy sign.6 Ultrasound has a sensitivity of 81% and a specificity of 83% for detecting acute cholecystitis, whereas hepatobiliary iminodiacetic acid scan (cholescintigraphy) is 96% sensitive and 90% specific and is used in cases where ultrasound is indeterminate.6
Spontaneous adrenal hemorrhage (SAH) typically presents with symptoms of upper abdominal pain and/or flank pain. If hemorrhage is significant, hypotension and a drop in hematocrit may occur. Alternatively, if SAH is bilateral and results in a deficiency of adrenal hormones, hypotension may be secondary to adrenal insufficiency. Due to its ready availability, most SAHs are diagnosed by CT scan ordered for alternate reasons.7
In the pregnant patient, to avoid exposing the developing fetus to radiation, ultrasound is typically the first imaging modality to investigate right upper quadrant or flank pain. However, ultrasound is less sensitive for evaluating anomalies of the adrenal gland.8 An MRI is the most accurate imaging modality for diagnosing adrenal hemorrhage and avoids exposing the developing fetus to radiation.9 In this case, the absence of an etiology of the patient’s symptoms on right upper quadrant ultrasound led to further evaluation, with abdominal MRI revealing the diagnosis.
In a review of 141 cases of adrenal hemorrhage at the Mayo Clinic in Rochester, Minnesota, 16 patients had a spontaneous adrenal hemorrhage, which presented with severe abdominal and flank pain of sudden onset.7 Seven of these patients required surgery to control the bleeding.7 None of the 13 patients with a functioning contralateral adrenal gland required adrenal replacement therapy.7 Gavrilova-Jordan and colleagues reported a case of a spontaneous unilateral adrenal hemorrhage in the third trimester of pregnancy that was managed conservatively with favorable outcomes.10 Potential causes of spontaneous adrenal hemorrhage include:
• Antiphospholipid antibody syndrome
• Heparin-induced thrombocytopenia
• Trauma
• Physiologic stress (eg, sepsis)
• Adrenal mass (pheochromocytoma, angiomyolipoma, or metastatic cancer).6,11
Case Outcome
Initial right upper quadrant ultrasound in this patient revealed a normal gallbladder and a small amount of perinephric fluid surrounding the right kidney. On the night of admission she experienced an elevated temperature of 101.9°F. Due to the fever and leukocytosis, she was initiated on antibiotics (ampicillin-sulbactam 3 g IV every 6 hours) covering a urinary source. On hospital day 2, right lower quadrant and right upper quadrant ultrasounds were performed, revealing decreased fluid surrounding her right kidney and without evidence of appendicitis. Due to persistent leukocytosis (WBC count peaked at 26,400/μL), essentially normal urinalysis, and negative urine culture, an abdominal MRI was obtained to evaluate for a perinephric abscess. The MRI revealed the spontaneous adrenal hemorrhage.
The patient was observed as an inpatient for the following 48 hours. Her electrolytes remained normal and hemoglobin decreased to 9.5 g/dL. She remained hemodynamically stable. Serum cortisol and angiotensin levels were normal, antibiotics were discontinued, and the patient was discharged to outpatient follow-up.
A repeat MRI of the abdomen obtained 6 weeks later revealed a resolving right adrenal hematoma. At 39 weeks’ gestation, she delivered a vigorous female infant weighing 3,200 g (7 lb 1 oz) with an Apgar score at birth of 8/9.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Wasserstein AG. Nephrolithiasis. Am J Kidney Dis. 2005;45(2):422-428.
2. Ban KM, Easter JS. Selected urologic problems. In: Marx JA, Hockberger RS, Walls, RM, eds. Rosen’s Emergency Medicine: Concepts and Clinical Practice. 7th ed. Philadelphia, PA: Elsevier; 2010:1297-1324.
3. Colgan R, Williams M, Johnson JR. Diagnosis and treatment of acute pyelonephritis in women. Am Fam Physician. 2011;84(5):519-526.
4. Simerville JA, Maxted WC, Pahira JJ. Urinalysis: A comprehensive review. Am Fam Physician. 2005;71(6):1153-1162.
5. Friedman LS. Liver, biliary tract & pancreas disorders. In: McPhee SJ, Papadakis MA, eds. Current Medical Diagnosis and Treatment, 2012. 51st ed. New York, NY: Lange Medical Books/McGraw-Hill; 2012:644-698.
6. Kiewiet JJ, Leeuwenburgh MM, Bipat S, Bossuyt PM, Stoker J, Boermeester MA. A systematic review and meta-analysis of diagnostic performance of imaging in acute cholecystitis. Radiology. 2012;264(3):708-720.
7. Vella A, Nippoldt TB, Morris JC 3rd. Adrenal hemorrhage: A 25-year experience at the Mayo Clinic. Mayo Clin Proc. 2001;76(2):161-168.
8. Kawashima A, Sandler CM, Ernst RD, et al. Imaging of nontraumatic hemorrhage of the adrenal gland. Radiographics. 1999;19(4):949-963.
9. Hoeffel C, Legmann P, Luton JP, Chapuis Y, Fayet-Bonnin P. Spontaneous unilateral adrenal hemorrhage: Computerized tomography and magnetic resonance imaging findings in 8 cases. J Urol. 1995;154(5):1647-1651.
10. Gavrilova-Jordan L, Edmister WB, Farrell MA, Watson WJ. Spontaneous adrenal hemorrhage during pregnancy: A review of the literature and a case report of successful conservative management. Obstet Gynecol Surv. 2005;60(3):191-195.
11. Marti JL, Millet J, Sosa JA, Roman SA, Carling T, Udelsman R. Spontaneous adrenal hemorrhage with associated masses: Etiology and management in 6 cases and a review of 133 reported cases. World J Surg. 2012;36(1):75-82.
A 32-year-old G1P0 woman at 7 4/7 weeks’ gestation (intrauterine pregnancy confirmed by ultrasound) with a history of hypertension and anxiety presented to the emergency department reporting right upper quadrant and right flank pain. The patient did not report taking any medications. Her symptoms had begun about 6 hours prior to presentation. She did not report fever, chills, nausea, vomiting, anorexia, or urinary or bowels symptoms. She was afebrile, and her initial blood pressure was 174/84 mm Hg, but within an hour of presentation had decreased to 137/82 mm Hg.
On exam she was in moderate-to-severe discomfort. She was tender to palpation in the right flank as well as the right upper quadrant and had a positive Murphy sign. Her white blood cell (WBC) count was 18,800/μL, hemoglobin and hematocrit were normal, liver function tests were within normal limits, and sodium was 133 mmol/L; otherwise, electrolytes were normal. The urinalysis showed a specific gravity of 1.022 with 3 red blood cells per high powered field, but was otherwise normal. A right upper quadrant ultrasound showed a normal gallbladder without cholelithiasis. Subsequently, a magnetic resonance imaging (MRI) of the abdomen was obtained (Figure 1).
- What is your diagnosis?
- How would you treat this patient?
[Click through to the next page to see the answer.]
Our Treatment
An MRI of the abdomen revealed a right adrenal hematoma (Figure 2). This diagnosis was made after common causes of the right upper quadrant and right flank pain were excluded and was confirmed with an MRI of the abdomen.
Discussion
Common causes of right flank and right upper quadrant pain include urolithiasis, pyelonephritis, and acute cholecystitis (Table). Patients with urolithiasis will typically present with sudden onset of colicky flank pain, which may radiate to the inguinal region, with or without nausea and vomiting.1,2 Microhematuria may be absent in as many as 10% to 20% of patients.2 Computed tomography is nearly 97% sensitive and 96% specific for urolithiasis.2 Although only 37% to 64% sensitive in detecting urolithiasis, abdominal ultrasound is 85% to 94% specific for detecting hydronephrosis.2
Symptoms of pyelonephritis range from mild to severe costovertebral angle pain with or without fever and/or lower urinary tract symptoms to sepsis.3 Urinalysis typically shows leukocyte esterase (72%-97% sensitive, 41%-86% specific for culture-confirmed urinary tract infection) and microscopic pyuria (90%-96% sensitive, 47%-50% specific for culture-confirmed urinary tract infection).3,4 Urine culture will reveal 105 colony-forming units in 95% of patients with acute uncomplicated pyelonephritis.3 Imaging is typically reserved for atypical presentations or in cases of nonresolution of symptoms despite treatment in order to rule out structural anomalies, an obstructive process, or abscess formation.3
Acute cholecystitis is suggested by right upper quadrant pain, often following a meal. Symptoms include fever, leukocytosis, and an elevated serum bilirubin level.5 Ultrasound findings of acute cholecystitis are gallbladder wall thickening, pericholecystic fluid, or sonographic Murphy sign.6 Ultrasound has a sensitivity of 81% and a specificity of 83% for detecting acute cholecystitis, whereas hepatobiliary iminodiacetic acid scan (cholescintigraphy) is 96% sensitive and 90% specific and is used in cases where ultrasound is indeterminate.6
Spontaneous adrenal hemorrhage (SAH) typically presents with symptoms of upper abdominal pain and/or flank pain. If hemorrhage is significant, hypotension and a drop in hematocrit may occur. Alternatively, if SAH is bilateral and results in a deficiency of adrenal hormones, hypotension may be secondary to adrenal insufficiency. Due to its ready availability, most SAHs are diagnosed by CT scan ordered for alternate reasons.7
In the pregnant patient, to avoid exposing the developing fetus to radiation, ultrasound is typically the first imaging modality to investigate right upper quadrant or flank pain. However, ultrasound is less sensitive for evaluating anomalies of the adrenal gland.8 An MRI is the most accurate imaging modality for diagnosing adrenal hemorrhage and avoids exposing the developing fetus to radiation.9 In this case, the absence of an etiology of the patient’s symptoms on right upper quadrant ultrasound led to further evaluation, with abdominal MRI revealing the diagnosis.
In a review of 141 cases of adrenal hemorrhage at the Mayo Clinic in Rochester, Minnesota, 16 patients had a spontaneous adrenal hemorrhage, which presented with severe abdominal and flank pain of sudden onset.7 Seven of these patients required surgery to control the bleeding.7 None of the 13 patients with a functioning contralateral adrenal gland required adrenal replacement therapy.7 Gavrilova-Jordan and colleagues reported a case of a spontaneous unilateral adrenal hemorrhage in the third trimester of pregnancy that was managed conservatively with favorable outcomes.10 Potential causes of spontaneous adrenal hemorrhage include:
• Antiphospholipid antibody syndrome
• Heparin-induced thrombocytopenia
• Trauma
• Physiologic stress (eg, sepsis)
• Adrenal mass (pheochromocytoma, angiomyolipoma, or metastatic cancer).6,11
Case Outcome
Initial right upper quadrant ultrasound in this patient revealed a normal gallbladder and a small amount of perinephric fluid surrounding the right kidney. On the night of admission she experienced an elevated temperature of 101.9°F. Due to the fever and leukocytosis, she was initiated on antibiotics (ampicillin-sulbactam 3 g IV every 6 hours) covering a urinary source. On hospital day 2, right lower quadrant and right upper quadrant ultrasounds were performed, revealing decreased fluid surrounding her right kidney and without evidence of appendicitis. Due to persistent leukocytosis (WBC count peaked at 26,400/μL), essentially normal urinalysis, and negative urine culture, an abdominal MRI was obtained to evaluate for a perinephric abscess. The MRI revealed the spontaneous adrenal hemorrhage.
The patient was observed as an inpatient for the following 48 hours. Her electrolytes remained normal and hemoglobin decreased to 9.5 g/dL. She remained hemodynamically stable. Serum cortisol and angiotensin levels were normal, antibiotics were discontinued, and the patient was discharged to outpatient follow-up.
A repeat MRI of the abdomen obtained 6 weeks later revealed a resolving right adrenal hematoma. At 39 weeks’ gestation, she delivered a vigorous female infant weighing 3,200 g (7 lb 1 oz) with an Apgar score at birth of 8/9.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
A 32-year-old G1P0 woman at 7 4/7 weeks’ gestation (intrauterine pregnancy confirmed by ultrasound) with a history of hypertension and anxiety presented to the emergency department reporting right upper quadrant and right flank pain. The patient did not report taking any medications. Her symptoms had begun about 6 hours prior to presentation. She did not report fever, chills, nausea, vomiting, anorexia, or urinary or bowels symptoms. She was afebrile, and her initial blood pressure was 174/84 mm Hg, but within an hour of presentation had decreased to 137/82 mm Hg.
On exam she was in moderate-to-severe discomfort. She was tender to palpation in the right flank as well as the right upper quadrant and had a positive Murphy sign. Her white blood cell (WBC) count was 18,800/μL, hemoglobin and hematocrit were normal, liver function tests were within normal limits, and sodium was 133 mmol/L; otherwise, electrolytes were normal. The urinalysis showed a specific gravity of 1.022 with 3 red blood cells per high powered field, but was otherwise normal. A right upper quadrant ultrasound showed a normal gallbladder without cholelithiasis. Subsequently, a magnetic resonance imaging (MRI) of the abdomen was obtained (Figure 1).
- What is your diagnosis?
- How would you treat this patient?
[Click through to the next page to see the answer.]
Our Treatment
An MRI of the abdomen revealed a right adrenal hematoma (Figure 2). This diagnosis was made after common causes of the right upper quadrant and right flank pain were excluded and was confirmed with an MRI of the abdomen.
Discussion
Common causes of right flank and right upper quadrant pain include urolithiasis, pyelonephritis, and acute cholecystitis (Table). Patients with urolithiasis will typically present with sudden onset of colicky flank pain, which may radiate to the inguinal region, with or without nausea and vomiting.1,2 Microhematuria may be absent in as many as 10% to 20% of patients.2 Computed tomography is nearly 97% sensitive and 96% specific for urolithiasis.2 Although only 37% to 64% sensitive in detecting urolithiasis, abdominal ultrasound is 85% to 94% specific for detecting hydronephrosis.2
Symptoms of pyelonephritis range from mild to severe costovertebral angle pain with or without fever and/or lower urinary tract symptoms to sepsis.3 Urinalysis typically shows leukocyte esterase (72%-97% sensitive, 41%-86% specific for culture-confirmed urinary tract infection) and microscopic pyuria (90%-96% sensitive, 47%-50% specific for culture-confirmed urinary tract infection).3,4 Urine culture will reveal 105 colony-forming units in 95% of patients with acute uncomplicated pyelonephritis.3 Imaging is typically reserved for atypical presentations or in cases of nonresolution of symptoms despite treatment in order to rule out structural anomalies, an obstructive process, or abscess formation.3
Acute cholecystitis is suggested by right upper quadrant pain, often following a meal. Symptoms include fever, leukocytosis, and an elevated serum bilirubin level.5 Ultrasound findings of acute cholecystitis are gallbladder wall thickening, pericholecystic fluid, or sonographic Murphy sign.6 Ultrasound has a sensitivity of 81% and a specificity of 83% for detecting acute cholecystitis, whereas hepatobiliary iminodiacetic acid scan (cholescintigraphy) is 96% sensitive and 90% specific and is used in cases where ultrasound is indeterminate.6
Spontaneous adrenal hemorrhage (SAH) typically presents with symptoms of upper abdominal pain and/or flank pain. If hemorrhage is significant, hypotension and a drop in hematocrit may occur. Alternatively, if SAH is bilateral and results in a deficiency of adrenal hormones, hypotension may be secondary to adrenal insufficiency. Due to its ready availability, most SAHs are diagnosed by CT scan ordered for alternate reasons.7
In the pregnant patient, to avoid exposing the developing fetus to radiation, ultrasound is typically the first imaging modality to investigate right upper quadrant or flank pain. However, ultrasound is less sensitive for evaluating anomalies of the adrenal gland.8 An MRI is the most accurate imaging modality for diagnosing adrenal hemorrhage and avoids exposing the developing fetus to radiation.9 In this case, the absence of an etiology of the patient’s symptoms on right upper quadrant ultrasound led to further evaluation, with abdominal MRI revealing the diagnosis.
In a review of 141 cases of adrenal hemorrhage at the Mayo Clinic in Rochester, Minnesota, 16 patients had a spontaneous adrenal hemorrhage, which presented with severe abdominal and flank pain of sudden onset.7 Seven of these patients required surgery to control the bleeding.7 None of the 13 patients with a functioning contralateral adrenal gland required adrenal replacement therapy.7 Gavrilova-Jordan and colleagues reported a case of a spontaneous unilateral adrenal hemorrhage in the third trimester of pregnancy that was managed conservatively with favorable outcomes.10 Potential causes of spontaneous adrenal hemorrhage include:
• Antiphospholipid antibody syndrome
• Heparin-induced thrombocytopenia
• Trauma
• Physiologic stress (eg, sepsis)
• Adrenal mass (pheochromocytoma, angiomyolipoma, or metastatic cancer).6,11
Case Outcome
Initial right upper quadrant ultrasound in this patient revealed a normal gallbladder and a small amount of perinephric fluid surrounding the right kidney. On the night of admission she experienced an elevated temperature of 101.9°F. Due to the fever and leukocytosis, she was initiated on antibiotics (ampicillin-sulbactam 3 g IV every 6 hours) covering a urinary source. On hospital day 2, right lower quadrant and right upper quadrant ultrasounds were performed, revealing decreased fluid surrounding her right kidney and without evidence of appendicitis. Due to persistent leukocytosis (WBC count peaked at 26,400/μL), essentially normal urinalysis, and negative urine culture, an abdominal MRI was obtained to evaluate for a perinephric abscess. The MRI revealed the spontaneous adrenal hemorrhage.
The patient was observed as an inpatient for the following 48 hours. Her electrolytes remained normal and hemoglobin decreased to 9.5 g/dL. She remained hemodynamically stable. Serum cortisol and angiotensin levels were normal, antibiotics were discontinued, and the patient was discharged to outpatient follow-up.
A repeat MRI of the abdomen obtained 6 weeks later revealed a resolving right adrenal hematoma. At 39 weeks’ gestation, she delivered a vigorous female infant weighing 3,200 g (7 lb 1 oz) with an Apgar score at birth of 8/9.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Wasserstein AG. Nephrolithiasis. Am J Kidney Dis. 2005;45(2):422-428.
2. Ban KM, Easter JS. Selected urologic problems. In: Marx JA, Hockberger RS, Walls, RM, eds. Rosen’s Emergency Medicine: Concepts and Clinical Practice. 7th ed. Philadelphia, PA: Elsevier; 2010:1297-1324.
3. Colgan R, Williams M, Johnson JR. Diagnosis and treatment of acute pyelonephritis in women. Am Fam Physician. 2011;84(5):519-526.
4. Simerville JA, Maxted WC, Pahira JJ. Urinalysis: A comprehensive review. Am Fam Physician. 2005;71(6):1153-1162.
5. Friedman LS. Liver, biliary tract & pancreas disorders. In: McPhee SJ, Papadakis MA, eds. Current Medical Diagnosis and Treatment, 2012. 51st ed. New York, NY: Lange Medical Books/McGraw-Hill; 2012:644-698.
6. Kiewiet JJ, Leeuwenburgh MM, Bipat S, Bossuyt PM, Stoker J, Boermeester MA. A systematic review and meta-analysis of diagnostic performance of imaging in acute cholecystitis. Radiology. 2012;264(3):708-720.
7. Vella A, Nippoldt TB, Morris JC 3rd. Adrenal hemorrhage: A 25-year experience at the Mayo Clinic. Mayo Clin Proc. 2001;76(2):161-168.
8. Kawashima A, Sandler CM, Ernst RD, et al. Imaging of nontraumatic hemorrhage of the adrenal gland. Radiographics. 1999;19(4):949-963.
9. Hoeffel C, Legmann P, Luton JP, Chapuis Y, Fayet-Bonnin P. Spontaneous unilateral adrenal hemorrhage: Computerized tomography and magnetic resonance imaging findings in 8 cases. J Urol. 1995;154(5):1647-1651.
10. Gavrilova-Jordan L, Edmister WB, Farrell MA, Watson WJ. Spontaneous adrenal hemorrhage during pregnancy: A review of the literature and a case report of successful conservative management. Obstet Gynecol Surv. 2005;60(3):191-195.
11. Marti JL, Millet J, Sosa JA, Roman SA, Carling T, Udelsman R. Spontaneous adrenal hemorrhage with associated masses: Etiology and management in 6 cases and a review of 133 reported cases. World J Surg. 2012;36(1):75-82.
1. Wasserstein AG. Nephrolithiasis. Am J Kidney Dis. 2005;45(2):422-428.
2. Ban KM, Easter JS. Selected urologic problems. In: Marx JA, Hockberger RS, Walls, RM, eds. Rosen’s Emergency Medicine: Concepts and Clinical Practice. 7th ed. Philadelphia, PA: Elsevier; 2010:1297-1324.
3. Colgan R, Williams M, Johnson JR. Diagnosis and treatment of acute pyelonephritis in women. Am Fam Physician. 2011;84(5):519-526.
4. Simerville JA, Maxted WC, Pahira JJ. Urinalysis: A comprehensive review. Am Fam Physician. 2005;71(6):1153-1162.
5. Friedman LS. Liver, biliary tract & pancreas disorders. In: McPhee SJ, Papadakis MA, eds. Current Medical Diagnosis and Treatment, 2012. 51st ed. New York, NY: Lange Medical Books/McGraw-Hill; 2012:644-698.
6. Kiewiet JJ, Leeuwenburgh MM, Bipat S, Bossuyt PM, Stoker J, Boermeester MA. A systematic review and meta-analysis of diagnostic performance of imaging in acute cholecystitis. Radiology. 2012;264(3):708-720.
7. Vella A, Nippoldt TB, Morris JC 3rd. Adrenal hemorrhage: A 25-year experience at the Mayo Clinic. Mayo Clin Proc. 2001;76(2):161-168.
8. Kawashima A, Sandler CM, Ernst RD, et al. Imaging of nontraumatic hemorrhage of the adrenal gland. Radiographics. 1999;19(4):949-963.
9. Hoeffel C, Legmann P, Luton JP, Chapuis Y, Fayet-Bonnin P. Spontaneous unilateral adrenal hemorrhage: Computerized tomography and magnetic resonance imaging findings in 8 cases. J Urol. 1995;154(5):1647-1651.
10. Gavrilova-Jordan L, Edmister WB, Farrell MA, Watson WJ. Spontaneous adrenal hemorrhage during pregnancy: A review of the literature and a case report of successful conservative management. Obstet Gynecol Surv. 2005;60(3):191-195.
11. Marti JL, Millet J, Sosa JA, Roman SA, Carling T, Udelsman R. Spontaneous adrenal hemorrhage with associated masses: Etiology and management in 6 cases and a review of 133 reported cases. World J Surg. 2012;36(1):75-82.
Radioactive Iodine Scintiphotos of a Man With Thyroid Cancer
The contemporary management of differentiated thyroid cancer includes posttreatment monitoring for recurrence or metastasis.1 This monitoring includes clinical, biochemical, and imaging evaluation. Follow-up treatment can then be tailored based on the results of this monitoring.
Our patient was a 61-year-old man with a history of papillary thyroid carcinoma, including lymph node involvement and an extension of the primary focus into skeletal muscle (pT3N1bMX, stage IVa). The patient’s status was posttotal thyroidectomy and radioiodine ablation therapy (196.2 mCi iodine-131) in April 2009. The patient underwent follow-up thyrotropin alpha stimulated whole-body radioiodine surveillance scanning in May 2010.
Images demonstrated residual thyroid tissue/carcinoma regional to the thyroid bed, corresponding to prior posttherapy images. Whole body scintiphotos also demonstrated abnormal iodine localization that raised the possibility of distant bony metastasis in the region of the right hip (see Figures 1A and 1B). Current treatment standards for isolated bony metastases recommend repeated radioactive iodine therapy and potential external beam radiation. Imaging is required for accurate verification.1 This abnormal osseous finding was questionable on initial review, as it was present on the posterior, not anterior, view. The patient was instructed to continue hydration and return for additional delayed scintiphotos for further evaluation.
The patient returned 4 days later for delayed scintiphotos, which again demonstrated abnormal iodine localization near the right hip. However, iodine distribution was different, including now being visible on both the anterior and posterior views (see Figures 2A and 2B on the next page).
- What is your diagnosis?
- How would you treat this patient?
[Click through to the next page to see the answer.]
Our Treatment
The patient had no pain in the area and, upon further questioning, reported that he returned wearing the same athletic shorts. Given that radioiodine is excreted in the urine, this atypical distribution was thought to reflect urinary contamination. When images were taken again with the shorts removed, no abnormal radioiodine activity was present (see Figures 2C and 2D). Additional findings with thyrotropin alfa stimulation included increased quantitative thyroglobulin values of 20.2 ng/mL with antithyroglobulin antibody < 20.0 U/mL. Radioiodine ablation therapy using thyrotropin alfa was repeated. Iodine localization also was not present in the hip on posttherapy imaging (not shown).
Despite advances in imaging techniques, radioiodine scanning remains an imperfect science. Artifacts and pitfalls have been identified; in part, these are related to the accumulation of iodide in organs other than the thyroid, such as the nasopharynx and stomach, as well as the apparent accumulation due to excretion in the gut and bladder.2-4 These variations can be divided into ectopic normal thyroid tissue, physiologic accumulation in nonthyroidal tissue, and contamination by physiologic secretions. Recent case reports have confirmed this classification. Abnormal radioiodine uptake has been described in vertebral hemangioma,5 liver abscess6 and hydatid cyst,7 bronchiectasis,8 bronchogenic cyst and mucinous cystadenoma (2 fluid-filled cavities),9 chronic submandibular sialadenitis,10 esophageal diverticulum,11 hiatal hernia,12 appendix,13 indwelling Hickman catheter,14 renal cyst,15 and, similar to this case, contamination of the hair.16
Contaminated clothing is not uncommon; however, a persistent abnormality from contaminated clothing on repeat follow-up is unusual and could easily be misinterpreted.2 It would be valuable for all providers to be aware of the pitfalls of imaging before embarking on an unnecessary and potentially hazardous—not to mention costly—treatment course.
Acknowledgments
The authors acknowledge the assistance of Richard Cacciato, MLIS, Medical Librarian, who assisted in the literature review.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Cooper, DS, Doherty GM, Haugen BR, et al; American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19(11):1167-1214.
2. Carlisle MR, Lu C, McDougall IR. The interpretation of 131I scans in the evaluation of thyroid cancer, with an emphasis on false positive findings. Nucl Med Commun. 2003;24(6):715-735.
3. Shapiro B, Rufini V, Jarwan A, et al. Artifacts, anatomical and physiological variants, and unrelated diseases that might cause false-positive whole-body 131-I scans in patients with thyroid cancer. Semin Nucl Med. 2000;30(2):115-132.
4. Mitchell G, Pratt BE, Vini L, McCready VR, Harmer CL. False positive 131I whole body scans in thyroid cancer. Br J Radiol. 2000;73(870):627-635.
5. Khan S, Dunn J, Strickland N, Al-Nahhas A. Iodine-123 uptake in vertebral haemangiomas in a patient with papillary thyroid carcinoma. Nucl Med Rev Cent East Eur. 2008;11(1):30-33.
6. Pena Pardo FJ, Crespo de la Jara A, Fernández Morejón FJ, Sureda González M, Forteza Vila J, Brugarolas Masllorens A. Solitary focus in the liver in a thyroid cancer patient after a whole body scan with 131 iodine. Rev Esp Med Nucl. 2007;26(5):294-296.
7. Omür O, Ozbek SS, Akgün A, Yazici B, Mutlukoca N, Ozcan Z. False-positive I-131 accumulation in a hepatic hydatid cyst. Clin Nucl Med. 2007;32(12):930-932.
8. Jong I, Taubman K, Schlicht S. Bronchiectasis simulating pulmonary metastases on iodine-131 scintigraphy in well-differentiated thyroid carcinoma. Clin Nucl Med. 2005;30(10):688-689.
9. Agriantonis DJ, Hall L, Wilson MA. Pitfalls of I-131 whole body scan interpretation: Bronchogenic cyst and mucinous cystadenoma. Clin Nucl Med. 2008;33(5):325-327.
10. Ozguven M, Ilgan S, Karacalioglu AO, Arslan N, Ozturk E. Unusual patterns of I-131 accumulation. Clin Nucl Med. 2004;29(11):738-740.
11. Rashid K, Johns W, Chasse K, Walker M, Gupta SM. Esophageal diverticulum presenting as metastatic thyroid mass on iodine-131 scintigraphy. Clin Nucl Med. 2006;31(7):405-408.
12. Ceylan Gunay E, Erdogan A. Mediastinal radioiodine uptake due to hiatal hernia: A false-positive reaction in 131I scan. Rev Esp Med Nucl. 2010;29(2):95.
13. Borkar S, Grewal R, Schoder H. I-131 uptake demonstrated in the appendix on a posttreatment scan in a patient with thyroid cancer. Clin Nucl Med. 2008;33(8):551-552.
14. Groskin SA, McCrohan G. Pseudometastasis of the chest wall resulting from a Hickman catheter. J Thorac Imaging. 1994;9(3):169-171.
15. Thust S, Fernando R, Barwick T, Mohan H, Clarke SE. SPECT/CT identification of post-radioactive iodine treatment false-positive uptake in a simple renal cyst. Thyroid. 2009;19(1):75-76.
16. Sinha A, Bradley KM, Steatham J, Weaver A. Asymmetric breast uptake of radioiodine in a patient with thyroid malignancy: Metastases or not? J R Soc Med. 2008;101(6):319-320.
The contemporary management of differentiated thyroid cancer includes posttreatment monitoring for recurrence or metastasis.1 This monitoring includes clinical, biochemical, and imaging evaluation. Follow-up treatment can then be tailored based on the results of this monitoring.
Our patient was a 61-year-old man with a history of papillary thyroid carcinoma, including lymph node involvement and an extension of the primary focus into skeletal muscle (pT3N1bMX, stage IVa). The patient’s status was posttotal thyroidectomy and radioiodine ablation therapy (196.2 mCi iodine-131) in April 2009. The patient underwent follow-up thyrotropin alpha stimulated whole-body radioiodine surveillance scanning in May 2010.
Images demonstrated residual thyroid tissue/carcinoma regional to the thyroid bed, corresponding to prior posttherapy images. Whole body scintiphotos also demonstrated abnormal iodine localization that raised the possibility of distant bony metastasis in the region of the right hip (see Figures 1A and 1B). Current treatment standards for isolated bony metastases recommend repeated radioactive iodine therapy and potential external beam radiation. Imaging is required for accurate verification.1 This abnormal osseous finding was questionable on initial review, as it was present on the posterior, not anterior, view. The patient was instructed to continue hydration and return for additional delayed scintiphotos for further evaluation.
The patient returned 4 days later for delayed scintiphotos, which again demonstrated abnormal iodine localization near the right hip. However, iodine distribution was different, including now being visible on both the anterior and posterior views (see Figures 2A and 2B on the next page).
- What is your diagnosis?
- How would you treat this patient?
[Click through to the next page to see the answer.]
Our Treatment
The patient had no pain in the area and, upon further questioning, reported that he returned wearing the same athletic shorts. Given that radioiodine is excreted in the urine, this atypical distribution was thought to reflect urinary contamination. When images were taken again with the shorts removed, no abnormal radioiodine activity was present (see Figures 2C and 2D). Additional findings with thyrotropin alfa stimulation included increased quantitative thyroglobulin values of 20.2 ng/mL with antithyroglobulin antibody < 20.0 U/mL. Radioiodine ablation therapy using thyrotropin alfa was repeated. Iodine localization also was not present in the hip on posttherapy imaging (not shown).
Despite advances in imaging techniques, radioiodine scanning remains an imperfect science. Artifacts and pitfalls have been identified; in part, these are related to the accumulation of iodide in organs other than the thyroid, such as the nasopharynx and stomach, as well as the apparent accumulation due to excretion in the gut and bladder.2-4 These variations can be divided into ectopic normal thyroid tissue, physiologic accumulation in nonthyroidal tissue, and contamination by physiologic secretions. Recent case reports have confirmed this classification. Abnormal radioiodine uptake has been described in vertebral hemangioma,5 liver abscess6 and hydatid cyst,7 bronchiectasis,8 bronchogenic cyst and mucinous cystadenoma (2 fluid-filled cavities),9 chronic submandibular sialadenitis,10 esophageal diverticulum,11 hiatal hernia,12 appendix,13 indwelling Hickman catheter,14 renal cyst,15 and, similar to this case, contamination of the hair.16
Contaminated clothing is not uncommon; however, a persistent abnormality from contaminated clothing on repeat follow-up is unusual and could easily be misinterpreted.2 It would be valuable for all providers to be aware of the pitfalls of imaging before embarking on an unnecessary and potentially hazardous—not to mention costly—treatment course.
Acknowledgments
The authors acknowledge the assistance of Richard Cacciato, MLIS, Medical Librarian, who assisted in the literature review.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
The contemporary management of differentiated thyroid cancer includes posttreatment monitoring for recurrence or metastasis.1 This monitoring includes clinical, biochemical, and imaging evaluation. Follow-up treatment can then be tailored based on the results of this monitoring.
Our patient was a 61-year-old man with a history of papillary thyroid carcinoma, including lymph node involvement and an extension of the primary focus into skeletal muscle (pT3N1bMX, stage IVa). The patient’s status was posttotal thyroidectomy and radioiodine ablation therapy (196.2 mCi iodine-131) in April 2009. The patient underwent follow-up thyrotropin alpha stimulated whole-body radioiodine surveillance scanning in May 2010.
Images demonstrated residual thyroid tissue/carcinoma regional to the thyroid bed, corresponding to prior posttherapy images. Whole body scintiphotos also demonstrated abnormal iodine localization that raised the possibility of distant bony metastasis in the region of the right hip (see Figures 1A and 1B). Current treatment standards for isolated bony metastases recommend repeated radioactive iodine therapy and potential external beam radiation. Imaging is required for accurate verification.1 This abnormal osseous finding was questionable on initial review, as it was present on the posterior, not anterior, view. The patient was instructed to continue hydration and return for additional delayed scintiphotos for further evaluation.
The patient returned 4 days later for delayed scintiphotos, which again demonstrated abnormal iodine localization near the right hip. However, iodine distribution was different, including now being visible on both the anterior and posterior views (see Figures 2A and 2B on the next page).
- What is your diagnosis?
- How would you treat this patient?
[Click through to the next page to see the answer.]
Our Treatment
The patient had no pain in the area and, upon further questioning, reported that he returned wearing the same athletic shorts. Given that radioiodine is excreted in the urine, this atypical distribution was thought to reflect urinary contamination. When images were taken again with the shorts removed, no abnormal radioiodine activity was present (see Figures 2C and 2D). Additional findings with thyrotropin alfa stimulation included increased quantitative thyroglobulin values of 20.2 ng/mL with antithyroglobulin antibody < 20.0 U/mL. Radioiodine ablation therapy using thyrotropin alfa was repeated. Iodine localization also was not present in the hip on posttherapy imaging (not shown).
Despite advances in imaging techniques, radioiodine scanning remains an imperfect science. Artifacts and pitfalls have been identified; in part, these are related to the accumulation of iodide in organs other than the thyroid, such as the nasopharynx and stomach, as well as the apparent accumulation due to excretion in the gut and bladder.2-4 These variations can be divided into ectopic normal thyroid tissue, physiologic accumulation in nonthyroidal tissue, and contamination by physiologic secretions. Recent case reports have confirmed this classification. Abnormal radioiodine uptake has been described in vertebral hemangioma,5 liver abscess6 and hydatid cyst,7 bronchiectasis,8 bronchogenic cyst and mucinous cystadenoma (2 fluid-filled cavities),9 chronic submandibular sialadenitis,10 esophageal diverticulum,11 hiatal hernia,12 appendix,13 indwelling Hickman catheter,14 renal cyst,15 and, similar to this case, contamination of the hair.16
Contaminated clothing is not uncommon; however, a persistent abnormality from contaminated clothing on repeat follow-up is unusual and could easily be misinterpreted.2 It would be valuable for all providers to be aware of the pitfalls of imaging before embarking on an unnecessary and potentially hazardous—not to mention costly—treatment course.
Acknowledgments
The authors acknowledge the assistance of Richard Cacciato, MLIS, Medical Librarian, who assisted in the literature review.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Cooper, DS, Doherty GM, Haugen BR, et al; American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19(11):1167-1214.
2. Carlisle MR, Lu C, McDougall IR. The interpretation of 131I scans in the evaluation of thyroid cancer, with an emphasis on false positive findings. Nucl Med Commun. 2003;24(6):715-735.
3. Shapiro B, Rufini V, Jarwan A, et al. Artifacts, anatomical and physiological variants, and unrelated diseases that might cause false-positive whole-body 131-I scans in patients with thyroid cancer. Semin Nucl Med. 2000;30(2):115-132.
4. Mitchell G, Pratt BE, Vini L, McCready VR, Harmer CL. False positive 131I whole body scans in thyroid cancer. Br J Radiol. 2000;73(870):627-635.
5. Khan S, Dunn J, Strickland N, Al-Nahhas A. Iodine-123 uptake in vertebral haemangiomas in a patient with papillary thyroid carcinoma. Nucl Med Rev Cent East Eur. 2008;11(1):30-33.
6. Pena Pardo FJ, Crespo de la Jara A, Fernández Morejón FJ, Sureda González M, Forteza Vila J, Brugarolas Masllorens A. Solitary focus in the liver in a thyroid cancer patient after a whole body scan with 131 iodine. Rev Esp Med Nucl. 2007;26(5):294-296.
7. Omür O, Ozbek SS, Akgün A, Yazici B, Mutlukoca N, Ozcan Z. False-positive I-131 accumulation in a hepatic hydatid cyst. Clin Nucl Med. 2007;32(12):930-932.
8. Jong I, Taubman K, Schlicht S. Bronchiectasis simulating pulmonary metastases on iodine-131 scintigraphy in well-differentiated thyroid carcinoma. Clin Nucl Med. 2005;30(10):688-689.
9. Agriantonis DJ, Hall L, Wilson MA. Pitfalls of I-131 whole body scan interpretation: Bronchogenic cyst and mucinous cystadenoma. Clin Nucl Med. 2008;33(5):325-327.
10. Ozguven M, Ilgan S, Karacalioglu AO, Arslan N, Ozturk E. Unusual patterns of I-131 accumulation. Clin Nucl Med. 2004;29(11):738-740.
11. Rashid K, Johns W, Chasse K, Walker M, Gupta SM. Esophageal diverticulum presenting as metastatic thyroid mass on iodine-131 scintigraphy. Clin Nucl Med. 2006;31(7):405-408.
12. Ceylan Gunay E, Erdogan A. Mediastinal radioiodine uptake due to hiatal hernia: A false-positive reaction in 131I scan. Rev Esp Med Nucl. 2010;29(2):95.
13. Borkar S, Grewal R, Schoder H. I-131 uptake demonstrated in the appendix on a posttreatment scan in a patient with thyroid cancer. Clin Nucl Med. 2008;33(8):551-552.
14. Groskin SA, McCrohan G. Pseudometastasis of the chest wall resulting from a Hickman catheter. J Thorac Imaging. 1994;9(3):169-171.
15. Thust S, Fernando R, Barwick T, Mohan H, Clarke SE. SPECT/CT identification of post-radioactive iodine treatment false-positive uptake in a simple renal cyst. Thyroid. 2009;19(1):75-76.
16. Sinha A, Bradley KM, Steatham J, Weaver A. Asymmetric breast uptake of radioiodine in a patient with thyroid malignancy: Metastases or not? J R Soc Med. 2008;101(6):319-320.
1. Cooper, DS, Doherty GM, Haugen BR, et al; American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19(11):1167-1214.
2. Carlisle MR, Lu C, McDougall IR. The interpretation of 131I scans in the evaluation of thyroid cancer, with an emphasis on false positive findings. Nucl Med Commun. 2003;24(6):715-735.
3. Shapiro B, Rufini V, Jarwan A, et al. Artifacts, anatomical and physiological variants, and unrelated diseases that might cause false-positive whole-body 131-I scans in patients with thyroid cancer. Semin Nucl Med. 2000;30(2):115-132.
4. Mitchell G, Pratt BE, Vini L, McCready VR, Harmer CL. False positive 131I whole body scans in thyroid cancer. Br J Radiol. 2000;73(870):627-635.
5. Khan S, Dunn J, Strickland N, Al-Nahhas A. Iodine-123 uptake in vertebral haemangiomas in a patient with papillary thyroid carcinoma. Nucl Med Rev Cent East Eur. 2008;11(1):30-33.
6. Pena Pardo FJ, Crespo de la Jara A, Fernández Morejón FJ, Sureda González M, Forteza Vila J, Brugarolas Masllorens A. Solitary focus in the liver in a thyroid cancer patient after a whole body scan with 131 iodine. Rev Esp Med Nucl. 2007;26(5):294-296.
7. Omür O, Ozbek SS, Akgün A, Yazici B, Mutlukoca N, Ozcan Z. False-positive I-131 accumulation in a hepatic hydatid cyst. Clin Nucl Med. 2007;32(12):930-932.
8. Jong I, Taubman K, Schlicht S. Bronchiectasis simulating pulmonary metastases on iodine-131 scintigraphy in well-differentiated thyroid carcinoma. Clin Nucl Med. 2005;30(10):688-689.
9. Agriantonis DJ, Hall L, Wilson MA. Pitfalls of I-131 whole body scan interpretation: Bronchogenic cyst and mucinous cystadenoma. Clin Nucl Med. 2008;33(5):325-327.
10. Ozguven M, Ilgan S, Karacalioglu AO, Arslan N, Ozturk E. Unusual patterns of I-131 accumulation. Clin Nucl Med. 2004;29(11):738-740.
11. Rashid K, Johns W, Chasse K, Walker M, Gupta SM. Esophageal diverticulum presenting as metastatic thyroid mass on iodine-131 scintigraphy. Clin Nucl Med. 2006;31(7):405-408.
12. Ceylan Gunay E, Erdogan A. Mediastinal radioiodine uptake due to hiatal hernia: A false-positive reaction in 131I scan. Rev Esp Med Nucl. 2010;29(2):95.
13. Borkar S, Grewal R, Schoder H. I-131 uptake demonstrated in the appendix on a posttreatment scan in a patient with thyroid cancer. Clin Nucl Med. 2008;33(8):551-552.
14. Groskin SA, McCrohan G. Pseudometastasis of the chest wall resulting from a Hickman catheter. J Thorac Imaging. 1994;9(3):169-171.
15. Thust S, Fernando R, Barwick T, Mohan H, Clarke SE. SPECT/CT identification of post-radioactive iodine treatment false-positive uptake in a simple renal cyst. Thyroid. 2009;19(1):75-76.
16. Sinha A, Bradley KM, Steatham J, Weaver A. Asymmetric breast uptake of radioiodine in a patient with thyroid malignancy: Metastases or not? J R Soc Med. 2008;101(6):319-320.