User login
Reimbursement hurdles hinder Entresto use in HFrEF
ORLANDO – Use of sacubitril/valsartan to treat patients with heart failure with reduced ejection fraction (HFrEF) became a class I recommendation in both the U.S. and European heart failure guidelines in May 2016, but virtually all U.S. health insurers continue to regard the potent and effective sacubitril/valsartan formulation as a second-line treatment that needs special preauthorization before patients receive reimbursement for the prescription.
“What is really morally sad is that U.S. payers are requiring physicians to fill out extensive, patient-by-patient paperwork” to allow patients with HFrEF to receive health insurance coverage for sacubitril/valsartan (Entresto), Milton Packer, MD, said at the annual scientific meeting of the Heart Failure Society of America.
“It is very difficult to understand why third-party payers would intentionally try to slow adoption of this life-saving drug simply because it is considered expensive. It is much cheaper than many drugs they cover for patients with cancer that don’t work half as well,” said Dr. Packer, a cardiologist and heart failure specialist at Baylor University Medical Center in Dallas.
The “excessive paperwork” for insurers when starting patients on sacubitril/valsartan is a “new and unique phenomenon among the cardiovascular drugs I prescribe,” agreed Nancy K. Sweitzer, MD, PhD, professor and chief of cardiology at the University of Arizona in Tuscon. “This approach by insurers seems based on cost; they do not want to pay” for sacubitril/valsartan, and to successfully arrange for coverage patients need to exactly match the enrollment criteria used in the PARADIGM-HF (Prospective Comparison of ARNI [Angiotensin Receptor – Neprilysin Inhibitor] with ACEI [Angiotensin-Converting Enzyme Inhibitor] to Determine Impact on Global Mortality and Morbidity in Heart Failure Trial ), the pivotal study that supplied the evidence base for making sacubitril/valsartan a class I agent for treating HFrEF.
“I don’t put some HFrEF patients on sacubitil/valsartan just because they don’t meet the trial’s entry criteria,” Dr. Sweitzer said in an interview. “Insurers seem to scrutinize every single parameter to make sure patients match the PARADIGM-HF patients. Coverage is denied if their BNP [brain natriuretic peptide] level is too low.” Dr. Sweitzer added that in one instance she had to submit a second preauthorization to simply uptitrate the dosage of sacubitril/valsartan she wanted a patient to receive.
“Based on the data it seems like you could easily identify HFrEF patients who are good candidates for sacubitril/valsartan, but your hands are tied by payers because you can’t prescribe it until you’ve first tried something else, and even then you still need to deal with a lot of paperwork,” agreed Robert O. Bonow, MD, professor of medicine at Northwestern University in Chicago. “The paperwork burden is really cumbersome for physicians with busy practices; it impedes taking care of patients,” Dr. Bonow said in an interview.
Sales figures for sacubitril/valsartan that the drug’s manufacturer, Novartis, has reported since the agent received U.S. marketing approval a little over a year ago reflect these challenges in prescribing the compound to patients. During the first quarter of 2016, Novartis reported $17 million in worldwide sales of the agent, followed by $32 million in worldwide sales during the second quarter of 2016, through June 30. With a total of $49 million in sacubitril/valsartan sales during the first 6 months of 2016, it seems like Novartis may be challenged to meet its stated target of $200 million in total sales of the compound during 2016. In April, one commentator called the $17 million sales figure for first quarter 2016 “an astonishingly small amount for a drug that was widely expected to be a blockbuster.”
Dr. Packer has in the past been a consultant to Novartis and was one of the lead investigators for the PARADIGM-HF trial. He said that currently he has no financial relationship with Novartis but he does serve as a consultant to several other drug companies. Dr. Sweitzer has received research support from Novartis and was an investigator for PARADIGM-HF. Dr. Bonow has been a consultant to Gilead.
On Twitter @mitchelzoler
ORLANDO – Use of sacubitril/valsartan to treat patients with heart failure with reduced ejection fraction (HFrEF) became a class I recommendation in both the U.S. and European heart failure guidelines in May 2016, but virtually all U.S. health insurers continue to regard the potent and effective sacubitril/valsartan formulation as a second-line treatment that needs special preauthorization before patients receive reimbursement for the prescription.
“What is really morally sad is that U.S. payers are requiring physicians to fill out extensive, patient-by-patient paperwork” to allow patients with HFrEF to receive health insurance coverage for sacubitril/valsartan (Entresto), Milton Packer, MD, said at the annual scientific meeting of the Heart Failure Society of America.
“It is very difficult to understand why third-party payers would intentionally try to slow adoption of this life-saving drug simply because it is considered expensive. It is much cheaper than many drugs they cover for patients with cancer that don’t work half as well,” said Dr. Packer, a cardiologist and heart failure specialist at Baylor University Medical Center in Dallas.
The “excessive paperwork” for insurers when starting patients on sacubitril/valsartan is a “new and unique phenomenon among the cardiovascular drugs I prescribe,” agreed Nancy K. Sweitzer, MD, PhD, professor and chief of cardiology at the University of Arizona in Tuscon. “This approach by insurers seems based on cost; they do not want to pay” for sacubitril/valsartan, and to successfully arrange for coverage patients need to exactly match the enrollment criteria used in the PARADIGM-HF (Prospective Comparison of ARNI [Angiotensin Receptor – Neprilysin Inhibitor] with ACEI [Angiotensin-Converting Enzyme Inhibitor] to Determine Impact on Global Mortality and Morbidity in Heart Failure Trial ), the pivotal study that supplied the evidence base for making sacubitril/valsartan a class I agent for treating HFrEF.
“I don’t put some HFrEF patients on sacubitil/valsartan just because they don’t meet the trial’s entry criteria,” Dr. Sweitzer said in an interview. “Insurers seem to scrutinize every single parameter to make sure patients match the PARADIGM-HF patients. Coverage is denied if their BNP [brain natriuretic peptide] level is too low.” Dr. Sweitzer added that in one instance she had to submit a second preauthorization to simply uptitrate the dosage of sacubitril/valsartan she wanted a patient to receive.
“Based on the data it seems like you could easily identify HFrEF patients who are good candidates for sacubitril/valsartan, but your hands are tied by payers because you can’t prescribe it until you’ve first tried something else, and even then you still need to deal with a lot of paperwork,” agreed Robert O. Bonow, MD, professor of medicine at Northwestern University in Chicago. “The paperwork burden is really cumbersome for physicians with busy practices; it impedes taking care of patients,” Dr. Bonow said in an interview.
Sales figures for sacubitril/valsartan that the drug’s manufacturer, Novartis, has reported since the agent received U.S. marketing approval a little over a year ago reflect these challenges in prescribing the compound to patients. During the first quarter of 2016, Novartis reported $17 million in worldwide sales of the agent, followed by $32 million in worldwide sales during the second quarter of 2016, through June 30. With a total of $49 million in sacubitril/valsartan sales during the first 6 months of 2016, it seems like Novartis may be challenged to meet its stated target of $200 million in total sales of the compound during 2016. In April, one commentator called the $17 million sales figure for first quarter 2016 “an astonishingly small amount for a drug that was widely expected to be a blockbuster.”
Dr. Packer has in the past been a consultant to Novartis and was one of the lead investigators for the PARADIGM-HF trial. He said that currently he has no financial relationship with Novartis but he does serve as a consultant to several other drug companies. Dr. Sweitzer has received research support from Novartis and was an investigator for PARADIGM-HF. Dr. Bonow has been a consultant to Gilead.
On Twitter @mitchelzoler
ORLANDO – Use of sacubitril/valsartan to treat patients with heart failure with reduced ejection fraction (HFrEF) became a class I recommendation in both the U.S. and European heart failure guidelines in May 2016, but virtually all U.S. health insurers continue to regard the potent and effective sacubitril/valsartan formulation as a second-line treatment that needs special preauthorization before patients receive reimbursement for the prescription.
“What is really morally sad is that U.S. payers are requiring physicians to fill out extensive, patient-by-patient paperwork” to allow patients with HFrEF to receive health insurance coverage for sacubitril/valsartan (Entresto), Milton Packer, MD, said at the annual scientific meeting of the Heart Failure Society of America.
“It is very difficult to understand why third-party payers would intentionally try to slow adoption of this life-saving drug simply because it is considered expensive. It is much cheaper than many drugs they cover for patients with cancer that don’t work half as well,” said Dr. Packer, a cardiologist and heart failure specialist at Baylor University Medical Center in Dallas.
The “excessive paperwork” for insurers when starting patients on sacubitril/valsartan is a “new and unique phenomenon among the cardiovascular drugs I prescribe,” agreed Nancy K. Sweitzer, MD, PhD, professor and chief of cardiology at the University of Arizona in Tuscon. “This approach by insurers seems based on cost; they do not want to pay” for sacubitril/valsartan, and to successfully arrange for coverage patients need to exactly match the enrollment criteria used in the PARADIGM-HF (Prospective Comparison of ARNI [Angiotensin Receptor – Neprilysin Inhibitor] with ACEI [Angiotensin-Converting Enzyme Inhibitor] to Determine Impact on Global Mortality and Morbidity in Heart Failure Trial ), the pivotal study that supplied the evidence base for making sacubitril/valsartan a class I agent for treating HFrEF.
“I don’t put some HFrEF patients on sacubitil/valsartan just because they don’t meet the trial’s entry criteria,” Dr. Sweitzer said in an interview. “Insurers seem to scrutinize every single parameter to make sure patients match the PARADIGM-HF patients. Coverage is denied if their BNP [brain natriuretic peptide] level is too low.” Dr. Sweitzer added that in one instance she had to submit a second preauthorization to simply uptitrate the dosage of sacubitril/valsartan she wanted a patient to receive.
“Based on the data it seems like you could easily identify HFrEF patients who are good candidates for sacubitril/valsartan, but your hands are tied by payers because you can’t prescribe it until you’ve first tried something else, and even then you still need to deal with a lot of paperwork,” agreed Robert O. Bonow, MD, professor of medicine at Northwestern University in Chicago. “The paperwork burden is really cumbersome for physicians with busy practices; it impedes taking care of patients,” Dr. Bonow said in an interview.
Sales figures for sacubitril/valsartan that the drug’s manufacturer, Novartis, has reported since the agent received U.S. marketing approval a little over a year ago reflect these challenges in prescribing the compound to patients. During the first quarter of 2016, Novartis reported $17 million in worldwide sales of the agent, followed by $32 million in worldwide sales during the second quarter of 2016, through June 30. With a total of $49 million in sacubitril/valsartan sales during the first 6 months of 2016, it seems like Novartis may be challenged to meet its stated target of $200 million in total sales of the compound during 2016. In April, one commentator called the $17 million sales figure for first quarter 2016 “an astonishingly small amount for a drug that was widely expected to be a blockbuster.”
Dr. Packer has in the past been a consultant to Novartis and was one of the lead investigators for the PARADIGM-HF trial. He said that currently he has no financial relationship with Novartis but he does serve as a consultant to several other drug companies. Dr. Sweitzer has received research support from Novartis and was an investigator for PARADIGM-HF. Dr. Bonow has been a consultant to Gilead.
On Twitter @mitchelzoler
EXPERT ANALYSIS FROM THE HFSA ANNUAL SCIENTIFIC MEETING
CABG best for diabetes patients with CKD – or is it?
ROME – The use of coronary artery bypass graft surgery for revascularization in patients with multivessel CAD and comorbid diabetes plus chronic kidney disease was associated with a significantly lower risk of major cardiovascular and cerebrovascular events than was PCI with first-generation drug-eluting stents in a new secondary analysis from the landmark FREEDOM trial.
“The reason for this presentation is that even though chronic kidney disease is common in patients with diabetes, until now there has not been a large study of the efficacy and safety of coronary revascularization with drug-eluting stents versus CABG in this population in a randomized trial cohort,” explained Usman Baber, MD, who reported the results at the annual congress of the European Society of Cardiology.
FREEDOM (Future Revascularization Evaluation in Patients with Diabetes Mellitus: Optimal Management of Multivessel Disease) randomized 1,900 diabetic patients with multivessel CAD to PCI or CABG. As previously reported, CABG proved superior to PCI, with a significantly lower rate of the composite primary endpoint composed of all-cause mortality, MI, or stroke (N Engl J Med. 2012 Dec 20;367[25]:2375-84).
Dr. Baber presented a post hoc analysis of the 451 FREEDOM participants with baseline comorbid chronic kidney disease (CKD). Their mean SYNTAX score was 27, and their mean baseline estimated glomerular filtration rate was 44 mL/min per 1.73 m2, indicative of mild to moderate CKD.
“Only 28 patients in the FREEDOM trial had an estimated GFR below 30, therefore we can’t make any inferences about revascularization in that setting, which I think is a completely different population,” he noted.
The 5-year rate of major adverse cardiovascular and cerebrovascular events in patients with CKD was 26% in the CABG group, an absolute 9.4% less than the 35.6% rate in subjects randomized to PCI.
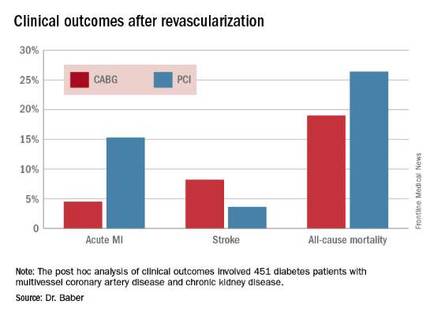
Roughly one-quarter of FREEDOM participants had CKD. They fared significantly worse than did those without CKD. The 5-year incidence of major adverse cardiovascular and cerebrovascular events was 30.8% in patients with CKD and 20.1% in patients without renal impairment. In a multivariate analysis adjusted for age, gender, hypertension, peripheral vascular disease, and other potential confounders, the risk of all-cause mortality was twofold higher in the CKD group. Their risk of cardiac death was increased 1.8-fold, and they were at 1.9-fold increased risk for stroke. Interestingly, however, the acute MI risk did not differ between patients with or without CKD, Dr. Baber observed.
Drilling deeper into the data, the cardiologist reported that CABG was associated with significantly lower rates of MI and a nonsignificant trend for fewer deaths, but with a significantly higher stroke rate than PCI.
One audience member rose to complain that this information won’t be helpful in counseling his diabetic patients with CKD and multivessel CAD because the choices look so grim: a higher risk of MI with percutaneous therapy, and a greater risk of stroke with surgery.
Dr. Baber replied by pointing out that the 10.8% absolute reduction in the risk of MI with CABG compared with PCI was more than twice as large as the absolute 4.6% increase in stroke risk with surgery.
“Most people would say that a heart attack is an inconvenience, and a stroke is a life-changing experience for them and their family,” said session cochair Kim A. Williams, MD, professor of medicine and chairman of cardiology at Rush University Medical Center in Chicago.
At that, Dr. Baber backtracked a bit, observing that since this was a post hoc analysis, the FREEDOM findings in patients with CKD must be viewed as hypothesis-generating rather than definitive. And, of course, contemporary second-generation drug-eluting stents have a better risk/benefit profile than do those used in FREEDOM.
“The number needed to treat/number needed to harm ratio for CABG and PCI probably ends up being roughly equal. The pertinence of an analysis like this is if you look at real-world registry-based data, you find a therapeutic nihilism that’s highly prevalent in CKD patients, where many patients who might benefit are not provided with revascularization therapy. It’s clear that we as clinicians – either because we don’t know there is a benefit or we are too concerned about potential harm – deprive patients of a treatment that might be beneficial. This analysis makes clinicians who might be concerned feel somewhat comforted that there is not unacceptable harm and that there is benefit,” Dr. Baber said.
Follow-up of FREEDOM participants continues and will be the subject of future reports, he added.
The FREEDOM trial was sponsored by the National Heart, Lung and Blood Institute. Dr. Baber reported having no financial conflicts of interest.
ROME – The use of coronary artery bypass graft surgery for revascularization in patients with multivessel CAD and comorbid diabetes plus chronic kidney disease was associated with a significantly lower risk of major cardiovascular and cerebrovascular events than was PCI with first-generation drug-eluting stents in a new secondary analysis from the landmark FREEDOM trial.
“The reason for this presentation is that even though chronic kidney disease is common in patients with diabetes, until now there has not been a large study of the efficacy and safety of coronary revascularization with drug-eluting stents versus CABG in this population in a randomized trial cohort,” explained Usman Baber, MD, who reported the results at the annual congress of the European Society of Cardiology.
FREEDOM (Future Revascularization Evaluation in Patients with Diabetes Mellitus: Optimal Management of Multivessel Disease) randomized 1,900 diabetic patients with multivessel CAD to PCI or CABG. As previously reported, CABG proved superior to PCI, with a significantly lower rate of the composite primary endpoint composed of all-cause mortality, MI, or stroke (N Engl J Med. 2012 Dec 20;367[25]:2375-84).
Dr. Baber presented a post hoc analysis of the 451 FREEDOM participants with baseline comorbid chronic kidney disease (CKD). Their mean SYNTAX score was 27, and their mean baseline estimated glomerular filtration rate was 44 mL/min per 1.73 m2, indicative of mild to moderate CKD.
“Only 28 patients in the FREEDOM trial had an estimated GFR below 30, therefore we can’t make any inferences about revascularization in that setting, which I think is a completely different population,” he noted.
The 5-year rate of major adverse cardiovascular and cerebrovascular events in patients with CKD was 26% in the CABG group, an absolute 9.4% less than the 35.6% rate in subjects randomized to PCI.

Roughly one-quarter of FREEDOM participants had CKD. They fared significantly worse than did those without CKD. The 5-year incidence of major adverse cardiovascular and cerebrovascular events was 30.8% in patients with CKD and 20.1% in patients without renal impairment. In a multivariate analysis adjusted for age, gender, hypertension, peripheral vascular disease, and other potential confounders, the risk of all-cause mortality was twofold higher in the CKD group. Their risk of cardiac death was increased 1.8-fold, and they were at 1.9-fold increased risk for stroke. Interestingly, however, the acute MI risk did not differ between patients with or without CKD, Dr. Baber observed.
Drilling deeper into the data, the cardiologist reported that CABG was associated with significantly lower rates of MI and a nonsignificant trend for fewer deaths, but with a significantly higher stroke rate than PCI.
One audience member rose to complain that this information won’t be helpful in counseling his diabetic patients with CKD and multivessel CAD because the choices look so grim: a higher risk of MI with percutaneous therapy, and a greater risk of stroke with surgery.
Dr. Baber replied by pointing out that the 10.8% absolute reduction in the risk of MI with CABG compared with PCI was more than twice as large as the absolute 4.6% increase in stroke risk with surgery.
“Most people would say that a heart attack is an inconvenience, and a stroke is a life-changing experience for them and their family,” said session cochair Kim A. Williams, MD, professor of medicine and chairman of cardiology at Rush University Medical Center in Chicago.
At that, Dr. Baber backtracked a bit, observing that since this was a post hoc analysis, the FREEDOM findings in patients with CKD must be viewed as hypothesis-generating rather than definitive. And, of course, contemporary second-generation drug-eluting stents have a better risk/benefit profile than do those used in FREEDOM.
“The number needed to treat/number needed to harm ratio for CABG and PCI probably ends up being roughly equal. The pertinence of an analysis like this is if you look at real-world registry-based data, you find a therapeutic nihilism that’s highly prevalent in CKD patients, where many patients who might benefit are not provided with revascularization therapy. It’s clear that we as clinicians – either because we don’t know there is a benefit or we are too concerned about potential harm – deprive patients of a treatment that might be beneficial. This analysis makes clinicians who might be concerned feel somewhat comforted that there is not unacceptable harm and that there is benefit,” Dr. Baber said.
Follow-up of FREEDOM participants continues and will be the subject of future reports, he added.
The FREEDOM trial was sponsored by the National Heart, Lung and Blood Institute. Dr. Baber reported having no financial conflicts of interest.
ROME – The use of coronary artery bypass graft surgery for revascularization in patients with multivessel CAD and comorbid diabetes plus chronic kidney disease was associated with a significantly lower risk of major cardiovascular and cerebrovascular events than was PCI with first-generation drug-eluting stents in a new secondary analysis from the landmark FREEDOM trial.
“The reason for this presentation is that even though chronic kidney disease is common in patients with diabetes, until now there has not been a large study of the efficacy and safety of coronary revascularization with drug-eluting stents versus CABG in this population in a randomized trial cohort,” explained Usman Baber, MD, who reported the results at the annual congress of the European Society of Cardiology.
FREEDOM (Future Revascularization Evaluation in Patients with Diabetes Mellitus: Optimal Management of Multivessel Disease) randomized 1,900 diabetic patients with multivessel CAD to PCI or CABG. As previously reported, CABG proved superior to PCI, with a significantly lower rate of the composite primary endpoint composed of all-cause mortality, MI, or stroke (N Engl J Med. 2012 Dec 20;367[25]:2375-84).
Dr. Baber presented a post hoc analysis of the 451 FREEDOM participants with baseline comorbid chronic kidney disease (CKD). Their mean SYNTAX score was 27, and their mean baseline estimated glomerular filtration rate was 44 mL/min per 1.73 m2, indicative of mild to moderate CKD.
“Only 28 patients in the FREEDOM trial had an estimated GFR below 30, therefore we can’t make any inferences about revascularization in that setting, which I think is a completely different population,” he noted.
The 5-year rate of major adverse cardiovascular and cerebrovascular events in patients with CKD was 26% in the CABG group, an absolute 9.4% less than the 35.6% rate in subjects randomized to PCI.

Roughly one-quarter of FREEDOM participants had CKD. They fared significantly worse than did those without CKD. The 5-year incidence of major adverse cardiovascular and cerebrovascular events was 30.8% in patients with CKD and 20.1% in patients without renal impairment. In a multivariate analysis adjusted for age, gender, hypertension, peripheral vascular disease, and other potential confounders, the risk of all-cause mortality was twofold higher in the CKD group. Their risk of cardiac death was increased 1.8-fold, and they were at 1.9-fold increased risk for stroke. Interestingly, however, the acute MI risk did not differ between patients with or without CKD, Dr. Baber observed.
Drilling deeper into the data, the cardiologist reported that CABG was associated with significantly lower rates of MI and a nonsignificant trend for fewer deaths, but with a significantly higher stroke rate than PCI.
One audience member rose to complain that this information won’t be helpful in counseling his diabetic patients with CKD and multivessel CAD because the choices look so grim: a higher risk of MI with percutaneous therapy, and a greater risk of stroke with surgery.
Dr. Baber replied by pointing out that the 10.8% absolute reduction in the risk of MI with CABG compared with PCI was more than twice as large as the absolute 4.6% increase in stroke risk with surgery.
“Most people would say that a heart attack is an inconvenience, and a stroke is a life-changing experience for them and their family,” said session cochair Kim A. Williams, MD, professor of medicine and chairman of cardiology at Rush University Medical Center in Chicago.
At that, Dr. Baber backtracked a bit, observing that since this was a post hoc analysis, the FREEDOM findings in patients with CKD must be viewed as hypothesis-generating rather than definitive. And, of course, contemporary second-generation drug-eluting stents have a better risk/benefit profile than do those used in FREEDOM.
“The number needed to treat/number needed to harm ratio for CABG and PCI probably ends up being roughly equal. The pertinence of an analysis like this is if you look at real-world registry-based data, you find a therapeutic nihilism that’s highly prevalent in CKD patients, where many patients who might benefit are not provided with revascularization therapy. It’s clear that we as clinicians – either because we don’t know there is a benefit or we are too concerned about potential harm – deprive patients of a treatment that might be beneficial. This analysis makes clinicians who might be concerned feel somewhat comforted that there is not unacceptable harm and that there is benefit,” Dr. Baber said.
Follow-up of FREEDOM participants continues and will be the subject of future reports, he added.
The FREEDOM trial was sponsored by the National Heart, Lung and Blood Institute. Dr. Baber reported having no financial conflicts of interest.
AT THE ESC CONGRESS 2016
Key clinical point: Coronary artery bypass graft surgery resulted in fewer myocardial infarctions but more strokes than did percutaneous coronary intervention at 5 years of follow-up in diabetic patients with multivessel coronary artery disease and chronic kidney disease.
Major finding: The cumulative MI rates in patients randomized to CABG versus PCI were 4.5% and 15.3%, respectively, while the stroke rates were 8.2% versus 3.6%.
Data source: A post hoc analysis of clinical outcomes in 451 diabetic patients with multivessel CAD and chronic kidney disease who were randomized to CABG or PCI in the prospective multicenter FREEDOM trial.
Disclosures: The FREEDOM trial was sponsored by the National Heart, Lung, and Blood Institute. The presenter reported having no financial conflicts of interest.
Aspirin not prescribed appropriately to cut cardiovascular risk in diabetes
MUNICH – Many patients with diabetes who could benefit from low-dose aspirin therapy may not be getting it – and many who are getting aspirin should not be, according to data presented at the annual meeting of the European Association for the Study of Diabetes.
A large, randomized trial concluded that 21% of diabetes patients who qualified for aspirin therapy for cardiovascular risk reduction were not getting it, and that it was contraindicated in almost 60% of those who were taking it, Lauren Crain, PhD, reported at the meeting.
Balancing the risks and benefits of aspirin therapy is not an easy challenge, said Dr. Crain, a health behavior researcher at HealthPartners Institute, Minneapolis. The clinical information necessary for the assessment is “rather lengthy, and not always readily available in primary care settings,” she said, and it’s clear from this study that clinicians could use some help in this area. Unfortunately, the electronic algorithm tested, which was meant to improve appropriate aspirin prescribing, didn’t improve the situation very much.
“At the final visit in the diabetes group [after the algorithm was employed], the total proportion of patients using aspirin was higher than at the first visit,” Dr. Crain noted. “However, that was the case regardless of whether patients were over- or underusing aspirin at the first visit.”
The aspirin findings were part of a large, randomized trial testing the algorithm as a way to reduce cardiovascular risk factors. The study was conducted in 19 primary care practices.
The decision-making algorithm, Cardiovascular Wizard, uses electronic health records to identify and advise patients with uncontrolled cardiovascular risk factors. Priorities and clinical recommendations are displayed for the provider and patient in the hope of facilitating shared decision making, Dr. Crain said.
One of the Wizard’s algorithms concerns aspirin prescribing. It is programmed with data from the United States Preventive Services Task Force, and recommends aspirin if cardiovascular risk scores are high and if consistent with providing a benefit greater than the risk of gastrointestinal bleeding. Aspirin is not recommended if the benefit is determined to be low or if major contraindications are present, including anticoagulant use or history of intracerebral hemorrhage.
The tool also alerts providers to the presence of other potential risks including aspirin allergy or intolerance, history of GI bleeds or risk conditions, and the concomitant use of nonsteroidal anti-inflammatory drugs.
The study comprised 11,000 adults, 4,000 of whom had diabetes. The remainder had high-risk, reversible cardiovascular risk factors (hypertension, dyslipidemia, or tobacco use). Each group was randomized to either cardiovascular risk assessment by usual care or with the Cardiovascular Wizard program.
The aspirin substudy looked at aspirin use at the baseline visit and the patient’s final, 1-year follow-up visit. At both visits, aspirin use was documented, and the clinician used the Wizard to assess whether or not it was indicated.
At the baseline visit, 71% of the diabetes group was using aspirin. However, according to the Wizard tool, more than one-third of them should not have been taking it – and among these, 57% were doing so. Among the remaining two-thirds of patients, all of whom should have been using aspirin, 21% were not taking it, Dr. Crain said.
Among the patients with reversible high-risk factors, 27% were using aspirin. However, according to the Wizard tool, the drug was contraindicated in 34% of those patients. “Most importantly, however, among those for whom aspirin was indicated, only 25% were using it – so, we’re talking about a 75% underusage,” Dr. Crain said.
By the 1-year follow-up visit, the situation was not much changed, despite the tool’s recommendations. Among those with diabetes, 56% in the usual care group and 60% in Wizard group were still overusing aspirin. Underuse was occurring in 21% of the usual care group and 17% of the Wizard group.
Patients with reversible high-risk factors fared a little better at 1 year, especially those who, at baseline, should have been taking aspirin but were not. Among these, 10% in the usual care group and 13% in the Wizard group had started taking aspirin.
The results were a bit of a disappointment, Dr. Crain said, but they don’t invalidate the investigators’ faith in an algorithmic advising system.
“We do think that electronic health record tools like this can help providers follow guidelines and improve the quality of their aspirin recommendations and prescribing, and hopefully reduce cardiovascular events and aspirin-related hazards,” she said. “Unfortunately, that didn’t happen here in the diabetes patients,” and the results in the second group were not stellar.
She added that the Wizard development team will be tweaking the tool to clarify some of the choices available as it guides patients and providers through the algorithm, in hopes of improving its efficacy.
Dr. Crain made no financial disclosures.
On Twitter @alz_gal
MUNICH – Many patients with diabetes who could benefit from low-dose aspirin therapy may not be getting it – and many who are getting aspirin should not be, according to data presented at the annual meeting of the European Association for the Study of Diabetes.
A large, randomized trial concluded that 21% of diabetes patients who qualified for aspirin therapy for cardiovascular risk reduction were not getting it, and that it was contraindicated in almost 60% of those who were taking it, Lauren Crain, PhD, reported at the meeting.
Balancing the risks and benefits of aspirin therapy is not an easy challenge, said Dr. Crain, a health behavior researcher at HealthPartners Institute, Minneapolis. The clinical information necessary for the assessment is “rather lengthy, and not always readily available in primary care settings,” she said, and it’s clear from this study that clinicians could use some help in this area. Unfortunately, the electronic algorithm tested, which was meant to improve appropriate aspirin prescribing, didn’t improve the situation very much.
“At the final visit in the diabetes group [after the algorithm was employed], the total proportion of patients using aspirin was higher than at the first visit,” Dr. Crain noted. “However, that was the case regardless of whether patients were over- or underusing aspirin at the first visit.”
The aspirin findings were part of a large, randomized trial testing the algorithm as a way to reduce cardiovascular risk factors. The study was conducted in 19 primary care practices.
The decision-making algorithm, Cardiovascular Wizard, uses electronic health records to identify and advise patients with uncontrolled cardiovascular risk factors. Priorities and clinical recommendations are displayed for the provider and patient in the hope of facilitating shared decision making, Dr. Crain said.
One of the Wizard’s algorithms concerns aspirin prescribing. It is programmed with data from the United States Preventive Services Task Force, and recommends aspirin if cardiovascular risk scores are high and if consistent with providing a benefit greater than the risk of gastrointestinal bleeding. Aspirin is not recommended if the benefit is determined to be low or if major contraindications are present, including anticoagulant use or history of intracerebral hemorrhage.
The tool also alerts providers to the presence of other potential risks including aspirin allergy or intolerance, history of GI bleeds or risk conditions, and the concomitant use of nonsteroidal anti-inflammatory drugs.
The study comprised 11,000 adults, 4,000 of whom had diabetes. The remainder had high-risk, reversible cardiovascular risk factors (hypertension, dyslipidemia, or tobacco use). Each group was randomized to either cardiovascular risk assessment by usual care or with the Cardiovascular Wizard program.
The aspirin substudy looked at aspirin use at the baseline visit and the patient’s final, 1-year follow-up visit. At both visits, aspirin use was documented, and the clinician used the Wizard to assess whether or not it was indicated.
At the baseline visit, 71% of the diabetes group was using aspirin. However, according to the Wizard tool, more than one-third of them should not have been taking it – and among these, 57% were doing so. Among the remaining two-thirds of patients, all of whom should have been using aspirin, 21% were not taking it, Dr. Crain said.
Among the patients with reversible high-risk factors, 27% were using aspirin. However, according to the Wizard tool, the drug was contraindicated in 34% of those patients. “Most importantly, however, among those for whom aspirin was indicated, only 25% were using it – so, we’re talking about a 75% underusage,” Dr. Crain said.
By the 1-year follow-up visit, the situation was not much changed, despite the tool’s recommendations. Among those with diabetes, 56% in the usual care group and 60% in Wizard group were still overusing aspirin. Underuse was occurring in 21% of the usual care group and 17% of the Wizard group.
Patients with reversible high-risk factors fared a little better at 1 year, especially those who, at baseline, should have been taking aspirin but were not. Among these, 10% in the usual care group and 13% in the Wizard group had started taking aspirin.
The results were a bit of a disappointment, Dr. Crain said, but they don’t invalidate the investigators’ faith in an algorithmic advising system.
“We do think that electronic health record tools like this can help providers follow guidelines and improve the quality of their aspirin recommendations and prescribing, and hopefully reduce cardiovascular events and aspirin-related hazards,” she said. “Unfortunately, that didn’t happen here in the diabetes patients,” and the results in the second group were not stellar.
She added that the Wizard development team will be tweaking the tool to clarify some of the choices available as it guides patients and providers through the algorithm, in hopes of improving its efficacy.
Dr. Crain made no financial disclosures.
On Twitter @alz_gal
MUNICH – Many patients with diabetes who could benefit from low-dose aspirin therapy may not be getting it – and many who are getting aspirin should not be, according to data presented at the annual meeting of the European Association for the Study of Diabetes.
A large, randomized trial concluded that 21% of diabetes patients who qualified for aspirin therapy for cardiovascular risk reduction were not getting it, and that it was contraindicated in almost 60% of those who were taking it, Lauren Crain, PhD, reported at the meeting.
Balancing the risks and benefits of aspirin therapy is not an easy challenge, said Dr. Crain, a health behavior researcher at HealthPartners Institute, Minneapolis. The clinical information necessary for the assessment is “rather lengthy, and not always readily available in primary care settings,” she said, and it’s clear from this study that clinicians could use some help in this area. Unfortunately, the electronic algorithm tested, which was meant to improve appropriate aspirin prescribing, didn’t improve the situation very much.
“At the final visit in the diabetes group [after the algorithm was employed], the total proportion of patients using aspirin was higher than at the first visit,” Dr. Crain noted. “However, that was the case regardless of whether patients were over- or underusing aspirin at the first visit.”
The aspirin findings were part of a large, randomized trial testing the algorithm as a way to reduce cardiovascular risk factors. The study was conducted in 19 primary care practices.
The decision-making algorithm, Cardiovascular Wizard, uses electronic health records to identify and advise patients with uncontrolled cardiovascular risk factors. Priorities and clinical recommendations are displayed for the provider and patient in the hope of facilitating shared decision making, Dr. Crain said.
One of the Wizard’s algorithms concerns aspirin prescribing. It is programmed with data from the United States Preventive Services Task Force, and recommends aspirin if cardiovascular risk scores are high and if consistent with providing a benefit greater than the risk of gastrointestinal bleeding. Aspirin is not recommended if the benefit is determined to be low or if major contraindications are present, including anticoagulant use or history of intracerebral hemorrhage.
The tool also alerts providers to the presence of other potential risks including aspirin allergy or intolerance, history of GI bleeds or risk conditions, and the concomitant use of nonsteroidal anti-inflammatory drugs.
The study comprised 11,000 adults, 4,000 of whom had diabetes. The remainder had high-risk, reversible cardiovascular risk factors (hypertension, dyslipidemia, or tobacco use). Each group was randomized to either cardiovascular risk assessment by usual care or with the Cardiovascular Wizard program.
The aspirin substudy looked at aspirin use at the baseline visit and the patient’s final, 1-year follow-up visit. At both visits, aspirin use was documented, and the clinician used the Wizard to assess whether or not it was indicated.
At the baseline visit, 71% of the diabetes group was using aspirin. However, according to the Wizard tool, more than one-third of them should not have been taking it – and among these, 57% were doing so. Among the remaining two-thirds of patients, all of whom should have been using aspirin, 21% were not taking it, Dr. Crain said.
Among the patients with reversible high-risk factors, 27% were using aspirin. However, according to the Wizard tool, the drug was contraindicated in 34% of those patients. “Most importantly, however, among those for whom aspirin was indicated, only 25% were using it – so, we’re talking about a 75% underusage,” Dr. Crain said.
By the 1-year follow-up visit, the situation was not much changed, despite the tool’s recommendations. Among those with diabetes, 56% in the usual care group and 60% in Wizard group were still overusing aspirin. Underuse was occurring in 21% of the usual care group and 17% of the Wizard group.
Patients with reversible high-risk factors fared a little better at 1 year, especially those who, at baseline, should have been taking aspirin but were not. Among these, 10% in the usual care group and 13% in the Wizard group had started taking aspirin.
The results were a bit of a disappointment, Dr. Crain said, but they don’t invalidate the investigators’ faith in an algorithmic advising system.
“We do think that electronic health record tools like this can help providers follow guidelines and improve the quality of their aspirin recommendations and prescribing, and hopefully reduce cardiovascular events and aspirin-related hazards,” she said. “Unfortunately, that didn’t happen here in the diabetes patients,” and the results in the second group were not stellar.
She added that the Wizard development team will be tweaking the tool to clarify some of the choices available as it guides patients and providers through the algorithm, in hopes of improving its efficacy.
Dr. Crain made no financial disclosures.
On Twitter @alz_gal
AT EASD 2016
Key clinical point: Many diabetes patients who should be taking aspirin for cardiovascular risk reduction are not doing so, and many who should not be taking it are.
Major finding: Aspirin was underused in 21% of diabetes patients and overused in 57% of patients.
Data source: A randomized study of 11,000 patients.
Disclosures: Dr. Lauren Crain had no financial disclosures.
Transcatheter mitral valve therapy at ‘event horizon’
As investigational transcatheter mitral valve therapies continue to explode onto the scene, cardiac surgeons must act now to seize and assert their place in the multidisciplinary team with interventional, imaging, and heart failure colleagues to deliver these treatments to people with complex mitral valve regurgitation, an expert opinion report in the August issue of the Journal of Thoracic and Cardiovascular Surgery states (J Thorac Cardiovasc Surg. 2016;152:330-6).
“There is a growing population of patients with primary and secondary mitral regurgitation underserved by surgical therapy because of comorbid risk,” Vinay Badhwar, MD, of West Virginia University and his colleagues said. “This has led to a tremendous activity of device development.”
With more than 25 different transcatheter mitral valve devices in development (MitraClip, Abbott Vascular, is the only FDA-approved transcatheter for primary mitral regurgitation [MR]), cardiac surgeons will soon have the tools to offer transcatheter mitral valve repair (TMVr) and transcatheter mitral valve replacement (TMVR) to more complex patients who have MR along with other health problems. Today about half of those patients do not get surgery because they are too frail, Dr. Badhwar and his colleagues said.
The authors used the astrophysical phrase “event horizon” to define the current state of transcatheter mitral valve therapies – “a point of no return.” They expect surgery to remain the treatment of choice for MR for the next 10 years. “However, as our patient cohorts become increasingly more complex and transcatheter mitral therapies more facile, the day when this will become a daily clinical reality will soon be upon us,” Dr. Badhwar and his colleagues said.
The multidisciplinary team approach will be integral in achieving the full potential of transcatheter mitral valve replacement or repair, Dr. Badhwar and his coauthors said. While surgery is the most effective treatment for primary MR, cardiac surgeons are challenged to introduce transcatheter treatments in patients who have other health problems. “The best way to adjudicate innovative surgical and interventional mitral therapies is through a robust collaboration within a well-functioning heart team that includes not only a cardiac surgeon and interventional cardiologist but also an imaging specialist,” the authors said.
The time to reach out to those other specialties is now, before those investigational devices start emerging from the development pipeline, Dr. Badhwar and his colleagues said. “This will soon enable the team-based mitral specialist to be facile in safely transitioning patients from open mitral surgery to TMVr or TMVR as most appropriate for durable long-term outcomes.”
Dr. Badhwar disclosed he is an uncompensated member of the Abbott Vascular advisory board. Coauthor Vinod Thourani, MD, disclosed relationships with Edwards Lifesciences, Medtronic Cardiovascular, Abbott Vascular, St. Jude Medical, Mitralign, and AtriCure. Coauthor Michael Mack, MD, serves on the Edwards Lifesciences steering committee Partner Trial and is an uncompensated co-principal investigator of the Abbott Vascular Clinical Outcomes Assessment of the MitraClip Percutaneous Therapy Trial.
Channeling Bob Dylan’s “The Times They Are A-Changin’” in his invited commentary, W. Randolph Chitwood Jr., MD, of East Carolina University in Greenville, N.C., called Dr. Badhwar’s expert opinion “the clarion call for cardiac surgeons to become engaged in this rapidly evolving parade.”

|
Dr. W. Randolph Chitwood Jr. |
The evidence supporting the safety and efficacy of transcatheter aortic valve replacement (TAVR) is already strong, Dr. Chitwood noted. “It seems reasonable to suspect that the evolving pathway for the development of transcatheter mitral valve replacement (TMVR) could recapitulate the success of TAVR, with each generation having improved results,” he said (J Thorac Cardiovasc Surg. 2016;152:336-7).
Cardiac surgeons need to develop alternate access platforms and acquire the skills to use the new generation of transcatheter mitral devices, Dr. Chitwood said. The expert opinion “should encourage cardiac surgeons to become members of a heart team,” he said. “Guidewire skills are at the pinnacle of necessity to remain a player in this new world.”
Dr. Chitwood’s advice to colleagues: “Then you better start swimming or you’ll sink like a stone, For the times they are a-changin’.”
Dr. Chitwood disclosed he is a consultant to Direct Flow Medical and co-principal investigator for the Edwards Lifesciences Transform Trial.
Channeling Bob Dylan’s “The Times They Are A-Changin’” in his invited commentary, W. Randolph Chitwood Jr., MD, of East Carolina University in Greenville, N.C., called Dr. Badhwar’s expert opinion “the clarion call for cardiac surgeons to become engaged in this rapidly evolving parade.”

|
Dr. W. Randolph Chitwood Jr. |
The evidence supporting the safety and efficacy of transcatheter aortic valve replacement (TAVR) is already strong, Dr. Chitwood noted. “It seems reasonable to suspect that the evolving pathway for the development of transcatheter mitral valve replacement (TMVR) could recapitulate the success of TAVR, with each generation having improved results,” he said (J Thorac Cardiovasc Surg. 2016;152:336-7).
Cardiac surgeons need to develop alternate access platforms and acquire the skills to use the new generation of transcatheter mitral devices, Dr. Chitwood said. The expert opinion “should encourage cardiac surgeons to become members of a heart team,” he said. “Guidewire skills are at the pinnacle of necessity to remain a player in this new world.”
Dr. Chitwood’s advice to colleagues: “Then you better start swimming or you’ll sink like a stone, For the times they are a-changin’.”
Dr. Chitwood disclosed he is a consultant to Direct Flow Medical and co-principal investigator for the Edwards Lifesciences Transform Trial.
Channeling Bob Dylan’s “The Times They Are A-Changin’” in his invited commentary, W. Randolph Chitwood Jr., MD, of East Carolina University in Greenville, N.C., called Dr. Badhwar’s expert opinion “the clarion call for cardiac surgeons to become engaged in this rapidly evolving parade.”

|
Dr. W. Randolph Chitwood Jr. |
The evidence supporting the safety and efficacy of transcatheter aortic valve replacement (TAVR) is already strong, Dr. Chitwood noted. “It seems reasonable to suspect that the evolving pathway for the development of transcatheter mitral valve replacement (TMVR) could recapitulate the success of TAVR, with each generation having improved results,” he said (J Thorac Cardiovasc Surg. 2016;152:336-7).
Cardiac surgeons need to develop alternate access platforms and acquire the skills to use the new generation of transcatheter mitral devices, Dr. Chitwood said. The expert opinion “should encourage cardiac surgeons to become members of a heart team,” he said. “Guidewire skills are at the pinnacle of necessity to remain a player in this new world.”
Dr. Chitwood’s advice to colleagues: “Then you better start swimming or you’ll sink like a stone, For the times they are a-changin’.”
Dr. Chitwood disclosed he is a consultant to Direct Flow Medical and co-principal investigator for the Edwards Lifesciences Transform Trial.
As investigational transcatheter mitral valve therapies continue to explode onto the scene, cardiac surgeons must act now to seize and assert their place in the multidisciplinary team with interventional, imaging, and heart failure colleagues to deliver these treatments to people with complex mitral valve regurgitation, an expert opinion report in the August issue of the Journal of Thoracic and Cardiovascular Surgery states (J Thorac Cardiovasc Surg. 2016;152:330-6).
“There is a growing population of patients with primary and secondary mitral regurgitation underserved by surgical therapy because of comorbid risk,” Vinay Badhwar, MD, of West Virginia University and his colleagues said. “This has led to a tremendous activity of device development.”
With more than 25 different transcatheter mitral valve devices in development (MitraClip, Abbott Vascular, is the only FDA-approved transcatheter for primary mitral regurgitation [MR]), cardiac surgeons will soon have the tools to offer transcatheter mitral valve repair (TMVr) and transcatheter mitral valve replacement (TMVR) to more complex patients who have MR along with other health problems. Today about half of those patients do not get surgery because they are too frail, Dr. Badhwar and his colleagues said.
The authors used the astrophysical phrase “event horizon” to define the current state of transcatheter mitral valve therapies – “a point of no return.” They expect surgery to remain the treatment of choice for MR for the next 10 years. “However, as our patient cohorts become increasingly more complex and transcatheter mitral therapies more facile, the day when this will become a daily clinical reality will soon be upon us,” Dr. Badhwar and his colleagues said.
The multidisciplinary team approach will be integral in achieving the full potential of transcatheter mitral valve replacement or repair, Dr. Badhwar and his coauthors said. While surgery is the most effective treatment for primary MR, cardiac surgeons are challenged to introduce transcatheter treatments in patients who have other health problems. “The best way to adjudicate innovative surgical and interventional mitral therapies is through a robust collaboration within a well-functioning heart team that includes not only a cardiac surgeon and interventional cardiologist but also an imaging specialist,” the authors said.
The time to reach out to those other specialties is now, before those investigational devices start emerging from the development pipeline, Dr. Badhwar and his colleagues said. “This will soon enable the team-based mitral specialist to be facile in safely transitioning patients from open mitral surgery to TMVr or TMVR as most appropriate for durable long-term outcomes.”
Dr. Badhwar disclosed he is an uncompensated member of the Abbott Vascular advisory board. Coauthor Vinod Thourani, MD, disclosed relationships with Edwards Lifesciences, Medtronic Cardiovascular, Abbott Vascular, St. Jude Medical, Mitralign, and AtriCure. Coauthor Michael Mack, MD, serves on the Edwards Lifesciences steering committee Partner Trial and is an uncompensated co-principal investigator of the Abbott Vascular Clinical Outcomes Assessment of the MitraClip Percutaneous Therapy Trial.
As investigational transcatheter mitral valve therapies continue to explode onto the scene, cardiac surgeons must act now to seize and assert their place in the multidisciplinary team with interventional, imaging, and heart failure colleagues to deliver these treatments to people with complex mitral valve regurgitation, an expert opinion report in the August issue of the Journal of Thoracic and Cardiovascular Surgery states (J Thorac Cardiovasc Surg. 2016;152:330-6).
“There is a growing population of patients with primary and secondary mitral regurgitation underserved by surgical therapy because of comorbid risk,” Vinay Badhwar, MD, of West Virginia University and his colleagues said. “This has led to a tremendous activity of device development.”
With more than 25 different transcatheter mitral valve devices in development (MitraClip, Abbott Vascular, is the only FDA-approved transcatheter for primary mitral regurgitation [MR]), cardiac surgeons will soon have the tools to offer transcatheter mitral valve repair (TMVr) and transcatheter mitral valve replacement (TMVR) to more complex patients who have MR along with other health problems. Today about half of those patients do not get surgery because they are too frail, Dr. Badhwar and his colleagues said.
The authors used the astrophysical phrase “event horizon” to define the current state of transcatheter mitral valve therapies – “a point of no return.” They expect surgery to remain the treatment of choice for MR for the next 10 years. “However, as our patient cohorts become increasingly more complex and transcatheter mitral therapies more facile, the day when this will become a daily clinical reality will soon be upon us,” Dr. Badhwar and his colleagues said.
The multidisciplinary team approach will be integral in achieving the full potential of transcatheter mitral valve replacement or repair, Dr. Badhwar and his coauthors said. While surgery is the most effective treatment for primary MR, cardiac surgeons are challenged to introduce transcatheter treatments in patients who have other health problems. “The best way to adjudicate innovative surgical and interventional mitral therapies is through a robust collaboration within a well-functioning heart team that includes not only a cardiac surgeon and interventional cardiologist but also an imaging specialist,” the authors said.
The time to reach out to those other specialties is now, before those investigational devices start emerging from the development pipeline, Dr. Badhwar and his colleagues said. “This will soon enable the team-based mitral specialist to be facile in safely transitioning patients from open mitral surgery to TMVr or TMVR as most appropriate for durable long-term outcomes.”
Dr. Badhwar disclosed he is an uncompensated member of the Abbott Vascular advisory board. Coauthor Vinod Thourani, MD, disclosed relationships with Edwards Lifesciences, Medtronic Cardiovascular, Abbott Vascular, St. Jude Medical, Mitralign, and AtriCure. Coauthor Michael Mack, MD, serves on the Edwards Lifesciences steering committee Partner Trial and is an uncompensated co-principal investigator of the Abbott Vascular Clinical Outcomes Assessment of the MitraClip Percutaneous Therapy Trial.
FROM THE JOURNAL OF THORACIC AND CARDIOVASCULAR SURGERY
Key clinical point: Transcatheter mitral valve repair and replacement technology has reached a critical point that requires cardiac surgeons to assume their place in a multidisciplinary team.
Major finding: One transcatheter device is commercially available in the United States and more than 25 companies have devices in development.
Data source: Review of 22 published reports on transcatheter mitral valve technology.
Disclosures: Dr. Badhwar disclosed he is an uncompensated member of the Abbott Vascular advisory board. Coauthor Vinod Thourani, MD, disclosed relationships with Edwards Lifesciences, Medtronic Cardiovascular, Abbott Vascular, St. Jude Medical, Mitralign and AtriCure. Coauthor Michael Mack, MD, serves on the Edwards Lifesciences steering committee Partner Trial, and is an uncompensated co-principal investigator of the Abbott Vascular Clinical Outcomes Assessment of the MitraClip Percutaneous Therapy Trial.
CABG reduces cardiovascular mortality in ischemic heart failure regardless of age
ROME – Coronary artery bypass surgery reduces cardiovascular mortality in heart failure patients with ischemic cardiomyopathy to a consistent extent regardless of age at the time of surgery, according to a secondary analysis from the landmark STICH trial, Eric J. Velazquez, MD, reported at the annual congress of the European Society of Cardiology.
“Cardiologists and cardiac surgeons can confidently offer patients CABG in addition to optimal medical therapy with the knowledge that cardiovascular mortality is reduced by CABG to a similar extent across all age groups in this trial through 10 years of follow-up,” said Dr. Velazquez, professor of medicine at Duke University in Durham, N.C.
However, that’s only part of the story. Cardiovascular mortality was a secondary endpoint in STICH (Surgical Treatment for Ischemic Heart Failure). The primary endpoint was all-cause mortality. And CABG’s impact on all-cause mortality was diminished in older STICH participants because of their greater comorbidity burden and the competing risk of noncardiovascular death, he added.
The take-home message is that cardiologists and heart surgeons need to carefully assess competing mortality risks before pursuing CABG in older patients, according to Dr. Velazquez.
Session cochair Kim A. Williams, MD, professor and chief of cardiology at Rush University Medical Center in Chicago, posed a direct question: “Is there an age cutoff for your group for bypass surgery?”
No, Dr. Velazquez replied. He pointed out that cardiovascular mortality remained the No. 1 cause of mortality across all age groups.
“If the expectation is that the major cause of fatal events is going to be cardiovascular, and CABG plus medical therapy reduces that risk consistently regardless of age, we think that there really is no particular age cutoff. There is a point at which the noncardiovascular risk predominates, but in the population we studied we did not see that point,” Dr. Velazquez added.
STICH was a 22-nation trial in which 1,212 patients with a left ventricular ejection fraction of 35% or less and coronary artery disease amenable to CABG were randomized to CABG plus optimal medical therapy or optimal medical therapy alone and followed for a median of 9.8 years (JACC Heart Fail. 2013;1[5]:400-8). For purposes of this secondary analysis, participants were divided into quartiles according to baseline age: Quartile 1 patients were up to 54 years old; quartile 2 were ages 55-60; quartile 3 were ages 61-67; and quartile 4 were ages 68 and up.
Older subjects had more comorbidities. All-cause mortality was significantly higher in older than younger patients: for CABG, 68% vs. 48% in quartiles 4 and 1, respectively; for medical therapy, 79% vs. 60% in the same two quartiles. In contrast, cardiovascular mortality did not differ significantly by age: It was 39% in quartile 4 and 35% in quartile 1 in the CABG group, and 53%, compared with 49%, in medically managed patients in quartiles 4 and 1.
For the secondary composite endpoint of all-cause mortality or cardiovascular hospitalization, the benefit of CABG plus medical management over medical management alone was significantly greater in younger than in older patients.
The rate of noncardiovascular mortality was 5.8% in quartiles 1 and 2, then leapt to 14.7% in quartile 3 and 21.1% in quartile 4.
Although the main focus of Dr. Velazquez’s presentation was the impact of CABG with advancing age, he said he found an important lesson in the younger population as well.
“We saw roughly a 40% relative risk reduction in all-cause mortality with CABG in the youngest quartile, compared with the three older groups. My interpretation of that data is that it’s probably not appropriate to avoid CABG in favor of another strategy in a younger patient when you see this kind of mortality benefit,” the cardiologist said.
One limitation of the STICH analysis, said session cochair Stephan Achenbach, MD, is that the study population was relatively young overall. The oldest patients in STICH were roughly the same age as the average patients undergoing CABG for left ventricular systolic dysfunction today at most centers, according to Dr. Achenbach, professor of cardiology at the University of Erlangen-Nuremberg (Germany).
Dr. Velazquez agreed. “I can’t speak as to whether these trial results would apply to the very elderly, patients age 90 and above,” he said.
Simultaneously with the presentation , the new STICH analysis was published online (Circulation. 2016 Aug 29. doi: 10.1161/CIRCULATIONAHA.116.024800).
STICH was funded by the National Institutes of Health. Dr. Velazquez reported having no relevant financial conflicts.
ROME – Coronary artery bypass surgery reduces cardiovascular mortality in heart failure patients with ischemic cardiomyopathy to a consistent extent regardless of age at the time of surgery, according to a secondary analysis from the landmark STICH trial, Eric J. Velazquez, MD, reported at the annual congress of the European Society of Cardiology.
“Cardiologists and cardiac surgeons can confidently offer patients CABG in addition to optimal medical therapy with the knowledge that cardiovascular mortality is reduced by CABG to a similar extent across all age groups in this trial through 10 years of follow-up,” said Dr. Velazquez, professor of medicine at Duke University in Durham, N.C.
However, that’s only part of the story. Cardiovascular mortality was a secondary endpoint in STICH (Surgical Treatment for Ischemic Heart Failure). The primary endpoint was all-cause mortality. And CABG’s impact on all-cause mortality was diminished in older STICH participants because of their greater comorbidity burden and the competing risk of noncardiovascular death, he added.
The take-home message is that cardiologists and heart surgeons need to carefully assess competing mortality risks before pursuing CABG in older patients, according to Dr. Velazquez.
Session cochair Kim A. Williams, MD, professor and chief of cardiology at Rush University Medical Center in Chicago, posed a direct question: “Is there an age cutoff for your group for bypass surgery?”
No, Dr. Velazquez replied. He pointed out that cardiovascular mortality remained the No. 1 cause of mortality across all age groups.
“If the expectation is that the major cause of fatal events is going to be cardiovascular, and CABG plus medical therapy reduces that risk consistently regardless of age, we think that there really is no particular age cutoff. There is a point at which the noncardiovascular risk predominates, but in the population we studied we did not see that point,” Dr. Velazquez added.
STICH was a 22-nation trial in which 1,212 patients with a left ventricular ejection fraction of 35% or less and coronary artery disease amenable to CABG were randomized to CABG plus optimal medical therapy or optimal medical therapy alone and followed for a median of 9.8 years (JACC Heart Fail. 2013;1[5]:400-8). For purposes of this secondary analysis, participants were divided into quartiles according to baseline age: Quartile 1 patients were up to 54 years old; quartile 2 were ages 55-60; quartile 3 were ages 61-67; and quartile 4 were ages 68 and up.
Older subjects had more comorbidities. All-cause mortality was significantly higher in older than younger patients: for CABG, 68% vs. 48% in quartiles 4 and 1, respectively; for medical therapy, 79% vs. 60% in the same two quartiles. In contrast, cardiovascular mortality did not differ significantly by age: It was 39% in quartile 4 and 35% in quartile 1 in the CABG group, and 53%, compared with 49%, in medically managed patients in quartiles 4 and 1.
For the secondary composite endpoint of all-cause mortality or cardiovascular hospitalization, the benefit of CABG plus medical management over medical management alone was significantly greater in younger than in older patients.
The rate of noncardiovascular mortality was 5.8% in quartiles 1 and 2, then leapt to 14.7% in quartile 3 and 21.1% in quartile 4.
Although the main focus of Dr. Velazquez’s presentation was the impact of CABG with advancing age, he said he found an important lesson in the younger population as well.
“We saw roughly a 40% relative risk reduction in all-cause mortality with CABG in the youngest quartile, compared with the three older groups. My interpretation of that data is that it’s probably not appropriate to avoid CABG in favor of another strategy in a younger patient when you see this kind of mortality benefit,” the cardiologist said.
One limitation of the STICH analysis, said session cochair Stephan Achenbach, MD, is that the study population was relatively young overall. The oldest patients in STICH were roughly the same age as the average patients undergoing CABG for left ventricular systolic dysfunction today at most centers, according to Dr. Achenbach, professor of cardiology at the University of Erlangen-Nuremberg (Germany).
Dr. Velazquez agreed. “I can’t speak as to whether these trial results would apply to the very elderly, patients age 90 and above,” he said.
Simultaneously with the presentation , the new STICH analysis was published online (Circulation. 2016 Aug 29. doi: 10.1161/CIRCULATIONAHA.116.024800).
STICH was funded by the National Institutes of Health. Dr. Velazquez reported having no relevant financial conflicts.
ROME – Coronary artery bypass surgery reduces cardiovascular mortality in heart failure patients with ischemic cardiomyopathy to a consistent extent regardless of age at the time of surgery, according to a secondary analysis from the landmark STICH trial, Eric J. Velazquez, MD, reported at the annual congress of the European Society of Cardiology.
“Cardiologists and cardiac surgeons can confidently offer patients CABG in addition to optimal medical therapy with the knowledge that cardiovascular mortality is reduced by CABG to a similar extent across all age groups in this trial through 10 years of follow-up,” said Dr. Velazquez, professor of medicine at Duke University in Durham, N.C.
However, that’s only part of the story. Cardiovascular mortality was a secondary endpoint in STICH (Surgical Treatment for Ischemic Heart Failure). The primary endpoint was all-cause mortality. And CABG’s impact on all-cause mortality was diminished in older STICH participants because of their greater comorbidity burden and the competing risk of noncardiovascular death, he added.
The take-home message is that cardiologists and heart surgeons need to carefully assess competing mortality risks before pursuing CABG in older patients, according to Dr. Velazquez.
Session cochair Kim A. Williams, MD, professor and chief of cardiology at Rush University Medical Center in Chicago, posed a direct question: “Is there an age cutoff for your group for bypass surgery?”
No, Dr. Velazquez replied. He pointed out that cardiovascular mortality remained the No. 1 cause of mortality across all age groups.
“If the expectation is that the major cause of fatal events is going to be cardiovascular, and CABG plus medical therapy reduces that risk consistently regardless of age, we think that there really is no particular age cutoff. There is a point at which the noncardiovascular risk predominates, but in the population we studied we did not see that point,” Dr. Velazquez added.
STICH was a 22-nation trial in which 1,212 patients with a left ventricular ejection fraction of 35% or less and coronary artery disease amenable to CABG were randomized to CABG plus optimal medical therapy or optimal medical therapy alone and followed for a median of 9.8 years (JACC Heart Fail. 2013;1[5]:400-8). For purposes of this secondary analysis, participants were divided into quartiles according to baseline age: Quartile 1 patients were up to 54 years old; quartile 2 were ages 55-60; quartile 3 were ages 61-67; and quartile 4 were ages 68 and up.
Older subjects had more comorbidities. All-cause mortality was significantly higher in older than younger patients: for CABG, 68% vs. 48% in quartiles 4 and 1, respectively; for medical therapy, 79% vs. 60% in the same two quartiles. In contrast, cardiovascular mortality did not differ significantly by age: It was 39% in quartile 4 and 35% in quartile 1 in the CABG group, and 53%, compared with 49%, in medically managed patients in quartiles 4 and 1.
For the secondary composite endpoint of all-cause mortality or cardiovascular hospitalization, the benefit of CABG plus medical management over medical management alone was significantly greater in younger than in older patients.
The rate of noncardiovascular mortality was 5.8% in quartiles 1 and 2, then leapt to 14.7% in quartile 3 and 21.1% in quartile 4.
Although the main focus of Dr. Velazquez’s presentation was the impact of CABG with advancing age, he said he found an important lesson in the younger population as well.
“We saw roughly a 40% relative risk reduction in all-cause mortality with CABG in the youngest quartile, compared with the three older groups. My interpretation of that data is that it’s probably not appropriate to avoid CABG in favor of another strategy in a younger patient when you see this kind of mortality benefit,” the cardiologist said.
One limitation of the STICH analysis, said session cochair Stephan Achenbach, MD, is that the study population was relatively young overall. The oldest patients in STICH were roughly the same age as the average patients undergoing CABG for left ventricular systolic dysfunction today at most centers, according to Dr. Achenbach, professor of cardiology at the University of Erlangen-Nuremberg (Germany).
Dr. Velazquez agreed. “I can’t speak as to whether these trial results would apply to the very elderly, patients age 90 and above,” he said.
Simultaneously with the presentation , the new STICH analysis was published online (Circulation. 2016 Aug 29. doi: 10.1161/CIRCULATIONAHA.116.024800).
STICH was funded by the National Institutes of Health. Dr. Velazquez reported having no relevant financial conflicts.
AT THE ESC CONGRESS 2016
Key clinical point: There should be no age cutoff in offering CABG to older patients with ischemic heart failure.
Major finding: CABG provided an absolute 14.4% reduction in cardiovascular mortality, compared with medical management, in both the youngest and oldest quartiles of patients with heart failure due to ischemic cardiomyopathy.
Data source: A secondary analysis of the STICH trial, in which 1,212 heart failure patients with ischemic cardiomyopathy were randomized to CABG plus medical therapy or medical therapy alone and followed for nearly 10 years.
Disclosures: The study was funded by the National Institutes of Health. The presenter reported having no relevant financial conflicts.
ESC’s new lipid guidelines keep LDL-cholesterol targets
ROME – LDL cholesterol treatment targets remain alive and well in non-U.S. lipid management guidelines.
New dyslipidemia management guidelines from the European Society of Cardiology, issued in late August, retain the same LDL cholesterol targets as the prior, 2011 guidelines, a sharp and purposeful departure from the “risk-based” U.S. guidelines introduced in 2013 that eliminated treating patients to specific LDL cholesterol targets.
The new ESC guidelines also incorporated the new class of lipid-lowering drugs, PCSK9 inhibitors (evolocumab [Repatha] and alirocumab [Praluent]), into the treatment algorithm, and carved out a role for ezetimibe (Zetia) following its proven success as an add-on agent to statins (Eur Heart J. 2016. doi: 10.1093/eurheartj/ehw272).
But it’s retention of LDL cholesterol targets as a cornerstone of dyslipidemia management in the new ESC report, written jointly with the European Atherosclerosis Society, that especially distinguishes the new guidelines.
“It seemed to us logical that if you have drugs [statins] that lower LDL cholesterol, then you target LDL cholesterol,” explained Ian M. Graham, MD, professor of cardiovascular medicine at Trinity College in Dublin and cochair of the guidelines panel. “If a patient’s risk is high, they still need to lower LDL cholesterol. It’s not really contradictory to the U.S. approach,” Dr. Graham said in an interview.
The ESC panel’s discussions about the LDL cholesterol targets were “difficult and long,” said Guy De Backer, MD, a member of the guidelines committee and professor of cardiology at the University of Ghent, Belgium, in a session devoted to the new guidelines during the annual congress of the ESC.
The ESC’s decision to retain the 2011 LDL cholesterol targets won praise from U.S. cardiologist Eugene Braunwald, MD. “I think that not measuring and following LDL cholesterol is silly. I don’t agree” with current U.S. guidelines, he said in a talk during the congress. “If you don’t follow LDL cholesterol then you don’t know a patient’s compliance.
Regularly measuring LDL cholesterol is important,” said Dr. Braunwald, professor of medicine at Harvard Medical School in Boston. But he questioned the LDL cholesterol targets set by the ESC panel, specifically the LDL cholesterol goal of less than 70 mg/dL for very-high-risk patients.
“I don’t think the ESC went low enough; the goal should be less than 50 mg/dL,” declared Dr. Braunwald, who added “anything above 50 mg/dL is toxic.”
The new guidelines also made the definition of “very-high-risk” patients “stricter and more precise,” said Alberico L. Catapano, MD, cochair of the panel and professor of pharmacology at the University of Milan.
The guideline’s detailed list defining very-high-risk patients includes those with either clinical or “unequivocal” imaging evidence for cardiovascular disease, as well as patients with diabetes and target-organ damage, severe chronic kidney disease, or a 10% or greater 10-year risk for fatal cardiovascular disease calculated by the European Score risk formula.
The potent lipid-lowering PCSK9 inhibitors came onto the U.S. and European markets in 2015, labeled in the United States specifically for patients with familial hypercholesterolemia.
In July 2016, an American College of Cardiology Task Force issued a model clinical-decision pathway for lowering LDL cholesterol levels that for the first time included the PCSK9 inhibitors, specified as a “may be considered” option for selected patients (J Am Coll Cardiol. 2016 Jul;68[1]:92-125). This consensus decision pathway was not a set of actual guidelines, which means the ESC revision is the first guideline to include the PCSK9 inhibitors. The panel took the same stance as the U.S. decision pathway and designated the PCSK9 inhibitors as a “may be considered” option.
Dr. Graham explained that this designation was driven by the current absence of evidence for clinical benefit from the large lipid-lowering effect of the PCSK9 antibodies, although trial results that address this are expected to appear very soon. Experts not on the guidelines panel agreed with this decision.
“We know that it’s crucial to await results from the clinical endpoint studies” before the guidelines committee makes a more forceful recommendation, commented Erik S.G. Stroes, MD, professor of vascular medicine at the Academic Medical Center in Amsterdam. He added, however, that many clinicians, himself included, have been pleased to offer PCSK9-inhibitor treatment to very-high-risk patients with no good alternatives for effectively lowering their LDL cholesterol to target levels, such as statin-intolerant patients or those with LDL cholesterol levels that remain high despite maximal therapy.
The current ESC guideline’s statement on when to use a PCSK9 inhibitor “is vague” said Dr. Braunwald. “Within the next year, we’ll have results from two huge trials that will show clinical outcomes. We know that PCSK9 inhibitors are extremely powerful at lowering LDL cholesterol, but I would like to see the loop closed” with proven effects on clinical outcomes. “I would bet 100 to 1 that they will be effective, but LDL cholesterol is just a surrogate marker, and you need to look in large populations before you make a guideline recommendation to use these drugs, so we’ll wait.”
Once the clinical value of treatment with PCSK9 inhibitors is settled, the next issue will be the cost of these drugs, something that will become a big consideration once they become more widely used, Dr. Braunwald added.
Dr. De Backer and Dr. Graham had no disclosures. Dr. Catapano has been a consultant to Aegerion, Amgen, AstraZeneca, Merck, Pfizer, and Sigma-Tau. Dr. Braunwald has been a consultant to Bayer, Daiichi Sankyo, the Medicines Company, Merck, Novartis, and Sanofi. Dr. Stroes has been a consultant to Amgen, Bristol-Myers Squibb, Merck, and Sanofi.
On Twitter @mitchelzoler
ROME – LDL cholesterol treatment targets remain alive and well in non-U.S. lipid management guidelines.
New dyslipidemia management guidelines from the European Society of Cardiology, issued in late August, retain the same LDL cholesterol targets as the prior, 2011 guidelines, a sharp and purposeful departure from the “risk-based” U.S. guidelines introduced in 2013 that eliminated treating patients to specific LDL cholesterol targets.
The new ESC guidelines also incorporated the new class of lipid-lowering drugs, PCSK9 inhibitors (evolocumab [Repatha] and alirocumab [Praluent]), into the treatment algorithm, and carved out a role for ezetimibe (Zetia) following its proven success as an add-on agent to statins (Eur Heart J. 2016. doi: 10.1093/eurheartj/ehw272).
But it’s retention of LDL cholesterol targets as a cornerstone of dyslipidemia management in the new ESC report, written jointly with the European Atherosclerosis Society, that especially distinguishes the new guidelines.
“It seemed to us logical that if you have drugs [statins] that lower LDL cholesterol, then you target LDL cholesterol,” explained Ian M. Graham, MD, professor of cardiovascular medicine at Trinity College in Dublin and cochair of the guidelines panel. “If a patient’s risk is high, they still need to lower LDL cholesterol. It’s not really contradictory to the U.S. approach,” Dr. Graham said in an interview.
The ESC panel’s discussions about the LDL cholesterol targets were “difficult and long,” said Guy De Backer, MD, a member of the guidelines committee and professor of cardiology at the University of Ghent, Belgium, in a session devoted to the new guidelines during the annual congress of the ESC.
The ESC’s decision to retain the 2011 LDL cholesterol targets won praise from U.S. cardiologist Eugene Braunwald, MD. “I think that not measuring and following LDL cholesterol is silly. I don’t agree” with current U.S. guidelines, he said in a talk during the congress. “If you don’t follow LDL cholesterol then you don’t know a patient’s compliance.
Regularly measuring LDL cholesterol is important,” said Dr. Braunwald, professor of medicine at Harvard Medical School in Boston. But he questioned the LDL cholesterol targets set by the ESC panel, specifically the LDL cholesterol goal of less than 70 mg/dL for very-high-risk patients.
“I don’t think the ESC went low enough; the goal should be less than 50 mg/dL,” declared Dr. Braunwald, who added “anything above 50 mg/dL is toxic.”
The new guidelines also made the definition of “very-high-risk” patients “stricter and more precise,” said Alberico L. Catapano, MD, cochair of the panel and professor of pharmacology at the University of Milan.
The guideline’s detailed list defining very-high-risk patients includes those with either clinical or “unequivocal” imaging evidence for cardiovascular disease, as well as patients with diabetes and target-organ damage, severe chronic kidney disease, or a 10% or greater 10-year risk for fatal cardiovascular disease calculated by the European Score risk formula.
The potent lipid-lowering PCSK9 inhibitors came onto the U.S. and European markets in 2015, labeled in the United States specifically for patients with familial hypercholesterolemia.
In July 2016, an American College of Cardiology Task Force issued a model clinical-decision pathway for lowering LDL cholesterol levels that for the first time included the PCSK9 inhibitors, specified as a “may be considered” option for selected patients (J Am Coll Cardiol. 2016 Jul;68[1]:92-125). This consensus decision pathway was not a set of actual guidelines, which means the ESC revision is the first guideline to include the PCSK9 inhibitors. The panel took the same stance as the U.S. decision pathway and designated the PCSK9 inhibitors as a “may be considered” option.
Dr. Graham explained that this designation was driven by the current absence of evidence for clinical benefit from the large lipid-lowering effect of the PCSK9 antibodies, although trial results that address this are expected to appear very soon. Experts not on the guidelines panel agreed with this decision.
“We know that it’s crucial to await results from the clinical endpoint studies” before the guidelines committee makes a more forceful recommendation, commented Erik S.G. Stroes, MD, professor of vascular medicine at the Academic Medical Center in Amsterdam. He added, however, that many clinicians, himself included, have been pleased to offer PCSK9-inhibitor treatment to very-high-risk patients with no good alternatives for effectively lowering their LDL cholesterol to target levels, such as statin-intolerant patients or those with LDL cholesterol levels that remain high despite maximal therapy.
The current ESC guideline’s statement on when to use a PCSK9 inhibitor “is vague” said Dr. Braunwald. “Within the next year, we’ll have results from two huge trials that will show clinical outcomes. We know that PCSK9 inhibitors are extremely powerful at lowering LDL cholesterol, but I would like to see the loop closed” with proven effects on clinical outcomes. “I would bet 100 to 1 that they will be effective, but LDL cholesterol is just a surrogate marker, and you need to look in large populations before you make a guideline recommendation to use these drugs, so we’ll wait.”
Once the clinical value of treatment with PCSK9 inhibitors is settled, the next issue will be the cost of these drugs, something that will become a big consideration once they become more widely used, Dr. Braunwald added.
Dr. De Backer and Dr. Graham had no disclosures. Dr. Catapano has been a consultant to Aegerion, Amgen, AstraZeneca, Merck, Pfizer, and Sigma-Tau. Dr. Braunwald has been a consultant to Bayer, Daiichi Sankyo, the Medicines Company, Merck, Novartis, and Sanofi. Dr. Stroes has been a consultant to Amgen, Bristol-Myers Squibb, Merck, and Sanofi.
On Twitter @mitchelzoler
ROME – LDL cholesterol treatment targets remain alive and well in non-U.S. lipid management guidelines.
New dyslipidemia management guidelines from the European Society of Cardiology, issued in late August, retain the same LDL cholesterol targets as the prior, 2011 guidelines, a sharp and purposeful departure from the “risk-based” U.S. guidelines introduced in 2013 that eliminated treating patients to specific LDL cholesterol targets.
The new ESC guidelines also incorporated the new class of lipid-lowering drugs, PCSK9 inhibitors (evolocumab [Repatha] and alirocumab [Praluent]), into the treatment algorithm, and carved out a role for ezetimibe (Zetia) following its proven success as an add-on agent to statins (Eur Heart J. 2016. doi: 10.1093/eurheartj/ehw272).
But it’s retention of LDL cholesterol targets as a cornerstone of dyslipidemia management in the new ESC report, written jointly with the European Atherosclerosis Society, that especially distinguishes the new guidelines.
“It seemed to us logical that if you have drugs [statins] that lower LDL cholesterol, then you target LDL cholesterol,” explained Ian M. Graham, MD, professor of cardiovascular medicine at Trinity College in Dublin and cochair of the guidelines panel. “If a patient’s risk is high, they still need to lower LDL cholesterol. It’s not really contradictory to the U.S. approach,” Dr. Graham said in an interview.
The ESC panel’s discussions about the LDL cholesterol targets were “difficult and long,” said Guy De Backer, MD, a member of the guidelines committee and professor of cardiology at the University of Ghent, Belgium, in a session devoted to the new guidelines during the annual congress of the ESC.
The ESC’s decision to retain the 2011 LDL cholesterol targets won praise from U.S. cardiologist Eugene Braunwald, MD. “I think that not measuring and following LDL cholesterol is silly. I don’t agree” with current U.S. guidelines, he said in a talk during the congress. “If you don’t follow LDL cholesterol then you don’t know a patient’s compliance.
Regularly measuring LDL cholesterol is important,” said Dr. Braunwald, professor of medicine at Harvard Medical School in Boston. But he questioned the LDL cholesterol targets set by the ESC panel, specifically the LDL cholesterol goal of less than 70 mg/dL for very-high-risk patients.
“I don’t think the ESC went low enough; the goal should be less than 50 mg/dL,” declared Dr. Braunwald, who added “anything above 50 mg/dL is toxic.”
The new guidelines also made the definition of “very-high-risk” patients “stricter and more precise,” said Alberico L. Catapano, MD, cochair of the panel and professor of pharmacology at the University of Milan.
The guideline’s detailed list defining very-high-risk patients includes those with either clinical or “unequivocal” imaging evidence for cardiovascular disease, as well as patients with diabetes and target-organ damage, severe chronic kidney disease, or a 10% or greater 10-year risk for fatal cardiovascular disease calculated by the European Score risk formula.
The potent lipid-lowering PCSK9 inhibitors came onto the U.S. and European markets in 2015, labeled in the United States specifically for patients with familial hypercholesterolemia.
In July 2016, an American College of Cardiology Task Force issued a model clinical-decision pathway for lowering LDL cholesterol levels that for the first time included the PCSK9 inhibitors, specified as a “may be considered” option for selected patients (J Am Coll Cardiol. 2016 Jul;68[1]:92-125). This consensus decision pathway was not a set of actual guidelines, which means the ESC revision is the first guideline to include the PCSK9 inhibitors. The panel took the same stance as the U.S. decision pathway and designated the PCSK9 inhibitors as a “may be considered” option.
Dr. Graham explained that this designation was driven by the current absence of evidence for clinical benefit from the large lipid-lowering effect of the PCSK9 antibodies, although trial results that address this are expected to appear very soon. Experts not on the guidelines panel agreed with this decision.
“We know that it’s crucial to await results from the clinical endpoint studies” before the guidelines committee makes a more forceful recommendation, commented Erik S.G. Stroes, MD, professor of vascular medicine at the Academic Medical Center in Amsterdam. He added, however, that many clinicians, himself included, have been pleased to offer PCSK9-inhibitor treatment to very-high-risk patients with no good alternatives for effectively lowering their LDL cholesterol to target levels, such as statin-intolerant patients or those with LDL cholesterol levels that remain high despite maximal therapy.
The current ESC guideline’s statement on when to use a PCSK9 inhibitor “is vague” said Dr. Braunwald. “Within the next year, we’ll have results from two huge trials that will show clinical outcomes. We know that PCSK9 inhibitors are extremely powerful at lowering LDL cholesterol, but I would like to see the loop closed” with proven effects on clinical outcomes. “I would bet 100 to 1 that they will be effective, but LDL cholesterol is just a surrogate marker, and you need to look in large populations before you make a guideline recommendation to use these drugs, so we’ll wait.”
Once the clinical value of treatment with PCSK9 inhibitors is settled, the next issue will be the cost of these drugs, something that will become a big consideration once they become more widely used, Dr. Braunwald added.
Dr. De Backer and Dr. Graham had no disclosures. Dr. Catapano has been a consultant to Aegerion, Amgen, AstraZeneca, Merck, Pfizer, and Sigma-Tau. Dr. Braunwald has been a consultant to Bayer, Daiichi Sankyo, the Medicines Company, Merck, Novartis, and Sanofi. Dr. Stroes has been a consultant to Amgen, Bristol-Myers Squibb, Merck, and Sanofi.
On Twitter @mitchelzoler
EXPERT ANALYSIS FROM THE ESC CONGRESS 2016
More TOPCAT flaws back spironolactone’s HFpEF efficacy
ORLANDO – Spironolactone inched a little closer toward becoming the first and only agent with proven efficacy for treating patients with heart failure with preserved ejection fraction based on further evidence for the drug’s efficacy in a subgroup of patients enrolled in the TOPCAT trial.
In 2014, the initial TOPCAT (Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist) report showed that spironolactone treatment of patients with heart failure with preserved ejection fraction (HFpEF) for 3 years produced a small, 11% relative reduction in the primary risk endpoint, compared with placebo that was not statistically significant (N Engl J Med. 2014 Apr 10;370[15]:1383-92).
But a follow-up post hoc analysis a year later showed evidence that the roughly half of patients in TOPCAT enrolled at centers in Russia and the Republic of Georgia may not have had HFpEF and also may not have received the planned dosage of spironolactone (Circulation. 2015 Jan 6;131[1]:34-42). An analysis that focused only on the 1,767 HFpEF patients (51% of the total TOPCAT cohort) enrolled in the Americas (United States, Canada, Argentina, and Brazil) showed that, compared with placebo, treatment with spironolactone cut the combined rate of cardiovascular death, nonfatal cardiac arrest, and heart failure hospitalization by 4.5 percentage points, an 18% relative risk reduction that was statistically significant. In the Americas, spironolactone also cut cardiovascular death alone by a relative 26%, and reduced heart failure hospitalization by a relative 18%, both statistically significant.
Additional analysis reported at the annual scientific meeting of the Heart Failure Society of America further supported the idea that many TOPCAT patients enrolled in Russia did not receive a physiologically meaningful dosage of spironolactone. Among 66 Russian patients randomized to the spironolactone arm who reported taking their drug as prescribed and who participated in a random draw of blood specimens 1 year into the study, 20 (30%) failed to show detectable blood levels of canrenone, a characteristic spironolactone metabolite, reported Eileen O’Meara, MD, at the meeting. In contrast, 2 (3%) of 76 enrolled U.S. patients failed to show detectable blood levels of the canrenone metabolite, a 10-fold difference said Dr. O’Meara, a cardiologist at the Montreal Heart Institute.The tested U.S. patients also showed a clear dose-response relationship between their reported spironolactone dosage and their canrenone levels, something not seen in the Russian patients. These new findings, plus the evidence cited in the 2015 analysis, create a compelling case that “actual use of spironolactone in Russia was lower than reported” by the trial participants in Russia and probably in Georgia as well, Dr. O’Meara said. The implication is that spironolactone’s real impact on HFpEF patients is best represented in the 51% of TOPCAT patients from the Americas, she added.
“We believe these findings emphasize the reliability of the Americas data,” said Marc A. Pfeffer, MD, a coinvestigator for TOPCAT and lead author of the 2015 post hoc analysis. “Until someone comes up with a better treatment for patients with HFpEF, we should pay attention to this. People need to get this message. And spironolactone costs 7 cents a day,” said Dr. Pfeffer, professor of medicine at Harvard Medical School in Boston.Currently, no agent is considered proven effective for improving outcomes in HFpEF patients. The 2016 guidelinesfor heart failure treatment from the European Society of Cardiology said “no treatment has yet been shown, convincingly, to reduce morbidity or mortality in patients with HFpEF.”
After the TOPCAT results and post hoc analysis came out in 2014 and 2015 and word spread of spironolactone’s apparent efficacy in the American half of the trial, use of spironolactone to treat HFpEF patient has increased, commented Margaret M. Redfield, MD, a heart failure physician and professor at the Mayo Clinic in Rochester, Minn. She said she often prescribes spironolactone patients to HFpEF patients who require potassium supplementation, generally because of their diuretic treatment. These are the “safest” HFpEF patients for spironolactone treatment, she said, because they face the lowest risk for hyperkalemia, the major adverse effect from spironolactone.
On Twitter @mitchelzoler
This is extraordinarily important information from an extraordinarily important study. I’m strongly persuaded that the data from the Americas in TOPCAT show that spironolactone worked. The new data presented on canrenone levels make me even more ready to exclude from consideration the TOPCAT data from Russia and Georgia.

|
| Mitchel L. Zoler/Frontline Medical News Dr. Barry H. Greenberg |
The heart failure community is left to decide what conclusions to draw from TOPCAT. I think guideline committees will struggle over what to make of the TOPCAT evidence. Any recommendation in favor of spironolactone needs to be somewhat guarded, but if a group made recommendations in support of spironolactone it would add an impetus for using it.
There has been long-standing interest in treating patients with heart failure with preserved ejection fraction with spironolactone. Currently, about a quarter of these patients take spironolactone. I’m not sure this level of use will increase dramatically because of what we now know about TOPCAT.
Dr. Barry H. Greenberg is professor of medicine and director of the advanced heart failure treatment program at the University of California, San Diego. He had no relevant disclosures. He made these comments in an interview.
This is extraordinarily important information from an extraordinarily important study. I’m strongly persuaded that the data from the Americas in TOPCAT show that spironolactone worked. The new data presented on canrenone levels make me even more ready to exclude from consideration the TOPCAT data from Russia and Georgia.

|
| Mitchel L. Zoler/Frontline Medical News Dr. Barry H. Greenberg |
The heart failure community is left to decide what conclusions to draw from TOPCAT. I think guideline committees will struggle over what to make of the TOPCAT evidence. Any recommendation in favor of spironolactone needs to be somewhat guarded, but if a group made recommendations in support of spironolactone it would add an impetus for using it.
There has been long-standing interest in treating patients with heart failure with preserved ejection fraction with spironolactone. Currently, about a quarter of these patients take spironolactone. I’m not sure this level of use will increase dramatically because of what we now know about TOPCAT.
Dr. Barry H. Greenberg is professor of medicine and director of the advanced heart failure treatment program at the University of California, San Diego. He had no relevant disclosures. He made these comments in an interview.
This is extraordinarily important information from an extraordinarily important study. I’m strongly persuaded that the data from the Americas in TOPCAT show that spironolactone worked. The new data presented on canrenone levels make me even more ready to exclude from consideration the TOPCAT data from Russia and Georgia.

|
| Mitchel L. Zoler/Frontline Medical News Dr. Barry H. Greenberg |
The heart failure community is left to decide what conclusions to draw from TOPCAT. I think guideline committees will struggle over what to make of the TOPCAT evidence. Any recommendation in favor of spironolactone needs to be somewhat guarded, but if a group made recommendations in support of spironolactone it would add an impetus for using it.
There has been long-standing interest in treating patients with heart failure with preserved ejection fraction with spironolactone. Currently, about a quarter of these patients take spironolactone. I’m not sure this level of use will increase dramatically because of what we now know about TOPCAT.
Dr. Barry H. Greenberg is professor of medicine and director of the advanced heart failure treatment program at the University of California, San Diego. He had no relevant disclosures. He made these comments in an interview.
ORLANDO – Spironolactone inched a little closer toward becoming the first and only agent with proven efficacy for treating patients with heart failure with preserved ejection fraction based on further evidence for the drug’s efficacy in a subgroup of patients enrolled in the TOPCAT trial.
In 2014, the initial TOPCAT (Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist) report showed that spironolactone treatment of patients with heart failure with preserved ejection fraction (HFpEF) for 3 years produced a small, 11% relative reduction in the primary risk endpoint, compared with placebo that was not statistically significant (N Engl J Med. 2014 Apr 10;370[15]:1383-92).
But a follow-up post hoc analysis a year later showed evidence that the roughly half of patients in TOPCAT enrolled at centers in Russia and the Republic of Georgia may not have had HFpEF and also may not have received the planned dosage of spironolactone (Circulation. 2015 Jan 6;131[1]:34-42). An analysis that focused only on the 1,767 HFpEF patients (51% of the total TOPCAT cohort) enrolled in the Americas (United States, Canada, Argentina, and Brazil) showed that, compared with placebo, treatment with spironolactone cut the combined rate of cardiovascular death, nonfatal cardiac arrest, and heart failure hospitalization by 4.5 percentage points, an 18% relative risk reduction that was statistically significant. In the Americas, spironolactone also cut cardiovascular death alone by a relative 26%, and reduced heart failure hospitalization by a relative 18%, both statistically significant.
Additional analysis reported at the annual scientific meeting of the Heart Failure Society of America further supported the idea that many TOPCAT patients enrolled in Russia did not receive a physiologically meaningful dosage of spironolactone. Among 66 Russian patients randomized to the spironolactone arm who reported taking their drug as prescribed and who participated in a random draw of blood specimens 1 year into the study, 20 (30%) failed to show detectable blood levels of canrenone, a characteristic spironolactone metabolite, reported Eileen O’Meara, MD, at the meeting. In contrast, 2 (3%) of 76 enrolled U.S. patients failed to show detectable blood levels of the canrenone metabolite, a 10-fold difference said Dr. O’Meara, a cardiologist at the Montreal Heart Institute.The tested U.S. patients also showed a clear dose-response relationship between their reported spironolactone dosage and their canrenone levels, something not seen in the Russian patients. These new findings, plus the evidence cited in the 2015 analysis, create a compelling case that “actual use of spironolactone in Russia was lower than reported” by the trial participants in Russia and probably in Georgia as well, Dr. O’Meara said. The implication is that spironolactone’s real impact on HFpEF patients is best represented in the 51% of TOPCAT patients from the Americas, she added.
“We believe these findings emphasize the reliability of the Americas data,” said Marc A. Pfeffer, MD, a coinvestigator for TOPCAT and lead author of the 2015 post hoc analysis. “Until someone comes up with a better treatment for patients with HFpEF, we should pay attention to this. People need to get this message. And spironolactone costs 7 cents a day,” said Dr. Pfeffer, professor of medicine at Harvard Medical School in Boston.Currently, no agent is considered proven effective for improving outcomes in HFpEF patients. The 2016 guidelinesfor heart failure treatment from the European Society of Cardiology said “no treatment has yet been shown, convincingly, to reduce morbidity or mortality in patients with HFpEF.”
After the TOPCAT results and post hoc analysis came out in 2014 and 2015 and word spread of spironolactone’s apparent efficacy in the American half of the trial, use of spironolactone to treat HFpEF patient has increased, commented Margaret M. Redfield, MD, a heart failure physician and professor at the Mayo Clinic in Rochester, Minn. She said she often prescribes spironolactone patients to HFpEF patients who require potassium supplementation, generally because of their diuretic treatment. These are the “safest” HFpEF patients for spironolactone treatment, she said, because they face the lowest risk for hyperkalemia, the major adverse effect from spironolactone.
On Twitter @mitchelzoler
ORLANDO – Spironolactone inched a little closer toward becoming the first and only agent with proven efficacy for treating patients with heart failure with preserved ejection fraction based on further evidence for the drug’s efficacy in a subgroup of patients enrolled in the TOPCAT trial.
In 2014, the initial TOPCAT (Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist) report showed that spironolactone treatment of patients with heart failure with preserved ejection fraction (HFpEF) for 3 years produced a small, 11% relative reduction in the primary risk endpoint, compared with placebo that was not statistically significant (N Engl J Med. 2014 Apr 10;370[15]:1383-92).
But a follow-up post hoc analysis a year later showed evidence that the roughly half of patients in TOPCAT enrolled at centers in Russia and the Republic of Georgia may not have had HFpEF and also may not have received the planned dosage of spironolactone (Circulation. 2015 Jan 6;131[1]:34-42). An analysis that focused only on the 1,767 HFpEF patients (51% of the total TOPCAT cohort) enrolled in the Americas (United States, Canada, Argentina, and Brazil) showed that, compared with placebo, treatment with spironolactone cut the combined rate of cardiovascular death, nonfatal cardiac arrest, and heart failure hospitalization by 4.5 percentage points, an 18% relative risk reduction that was statistically significant. In the Americas, spironolactone also cut cardiovascular death alone by a relative 26%, and reduced heart failure hospitalization by a relative 18%, both statistically significant.
Additional analysis reported at the annual scientific meeting of the Heart Failure Society of America further supported the idea that many TOPCAT patients enrolled in Russia did not receive a physiologically meaningful dosage of spironolactone. Among 66 Russian patients randomized to the spironolactone arm who reported taking their drug as prescribed and who participated in a random draw of blood specimens 1 year into the study, 20 (30%) failed to show detectable blood levels of canrenone, a characteristic spironolactone metabolite, reported Eileen O’Meara, MD, at the meeting. In contrast, 2 (3%) of 76 enrolled U.S. patients failed to show detectable blood levels of the canrenone metabolite, a 10-fold difference said Dr. O’Meara, a cardiologist at the Montreal Heart Institute.The tested U.S. patients also showed a clear dose-response relationship between their reported spironolactone dosage and their canrenone levels, something not seen in the Russian patients. These new findings, plus the evidence cited in the 2015 analysis, create a compelling case that “actual use of spironolactone in Russia was lower than reported” by the trial participants in Russia and probably in Georgia as well, Dr. O’Meara said. The implication is that spironolactone’s real impact on HFpEF patients is best represented in the 51% of TOPCAT patients from the Americas, she added.
“We believe these findings emphasize the reliability of the Americas data,” said Marc A. Pfeffer, MD, a coinvestigator for TOPCAT and lead author of the 2015 post hoc analysis. “Until someone comes up with a better treatment for patients with HFpEF, we should pay attention to this. People need to get this message. And spironolactone costs 7 cents a day,” said Dr. Pfeffer, professor of medicine at Harvard Medical School in Boston.Currently, no agent is considered proven effective for improving outcomes in HFpEF patients. The 2016 guidelinesfor heart failure treatment from the European Society of Cardiology said “no treatment has yet been shown, convincingly, to reduce morbidity or mortality in patients with HFpEF.”
After the TOPCAT results and post hoc analysis came out in 2014 and 2015 and word spread of spironolactone’s apparent efficacy in the American half of the trial, use of spironolactone to treat HFpEF patient has increased, commented Margaret M. Redfield, MD, a heart failure physician and professor at the Mayo Clinic in Rochester, Minn. She said she often prescribes spironolactone patients to HFpEF patients who require potassium supplementation, generally because of their diuretic treatment. These are the “safest” HFpEF patients for spironolactone treatment, she said, because they face the lowest risk for hyperkalemia, the major adverse effect from spironolactone.
On Twitter @mitchelzoler
AT THE HFSA ANNUAL SCIENTIFIC MEETING
Key clinical point: A post hoc analysis of spironolactone use among TOPCAT participants further fueled the idea that spironolactone provides real benefit to patients with HFpEF.
Major finding: Among patients reportedly taking spironolactone, 30% of tested Russians and 3% of tested Americans did not have detectable canrenone levels.
Data source: TOPCAT, a multicenter, randomized trial with 3,445 HFpEF patients.
Disclosures: TOPCAT received no commercial funding. Dr. O’Meara, Dr. Pfeffer, and Dr. Redfield had no relevant disclosures.
Four factors raise risk of post-TAVR endocarditis
Four factors – younger patient age, male sex, diabetes, and moderate to severe residual aortic regurgitation – are associated with a significantly increased risk of infective endocarditis after transcatheter aortic valve replacement, according to a report published online Sept. 13 in JAMA.
Until now, data pertaining to endocarditis following TAVR “have been limited to case reports and relatively small series with limited follow-up,” said Ander Regueiro, MD, of Laval University, Quebec City, and his associates.
They performed a retrospective analysis of data in a large international registry of TAVR cases to better characterize post-TAVR endocarditis.
Dr. Regueiro and his colleagues focused on 20,006 TAVR procedures done at 47 medical centers in Europe, North America, and South America during a 10-year period. The median time to symptom onset was 5.3 months after the procedure.
Infective endocarditis was definitively diagnosed in 250 of these cases. This incidence is similar to that reported for endocarditis following surgical aortic valve replacement, indicating that TAVR is no less predisposing to endocarditis despite being a less invasive approach.
The mean age of patients who developed post-TAVR endocarditis was 78.9 years, compared with 81.8 years for those who did not (HR, 0.97). The reason for this association is unclear, but it is possible that younger patients chosen for TAVR because of their prohibitive surgical risk carry a higher burden of comorbidity than do older patients. Similarly, 62% of endocarditis cases arose in men (HR, 1.69), and sex differences in comorbid conditions may explain the higher risk among men.
More patients who developed endocarditis had diabetes (41.7%), compared with those who did not develop endocarditis (30%), for an HR of 1.52. And patients who had moderate to severe residual aortic regurgitation after TAVR also were at much higher risk for endocarditis than were those who did not (HR, 2.05), the investigators noted (JAMA. 2016 Sep 13;316[10]:1083-92).
In contrast, factors that were not associated with endocarditis risk included chronic pulmonary disease, type of valve (self-expandable or balloon-expandable), and setting of the procedure (catheterization lab vs. operating room).
The bacteria that most commonly caused infective endocarditis were Enterococci species (24.6% of cases), Staphylococcus aureus (23.8%), and coagulase-negative staphylococci (16.8%). This should be taken into consideration when selecting antibiotics for prophylaxis before TAVR and when choosing empirical antibiotics for treatment while waiting for blood culture results, wrote Dr. Regueiro and his associates.
“This information may help clinicians identify patients at higher risk [for endocarditis] and aid in implementing appropriate preventive measures,” they noted.
This study was supported by a grant from the Alfonso Martin Escudero Foundation. Dr. Regueiro reported having no relevant financial disclosures; his associates reported ties to numerous industry sources.
Four factors – younger patient age, male sex, diabetes, and moderate to severe residual aortic regurgitation – are associated with a significantly increased risk of infective endocarditis after transcatheter aortic valve replacement, according to a report published online Sept. 13 in JAMA.
Until now, data pertaining to endocarditis following TAVR “have been limited to case reports and relatively small series with limited follow-up,” said Ander Regueiro, MD, of Laval University, Quebec City, and his associates.
They performed a retrospective analysis of data in a large international registry of TAVR cases to better characterize post-TAVR endocarditis.
Dr. Regueiro and his colleagues focused on 20,006 TAVR procedures done at 47 medical centers in Europe, North America, and South America during a 10-year period. The median time to symptom onset was 5.3 months after the procedure.
Infective endocarditis was definitively diagnosed in 250 of these cases. This incidence is similar to that reported for endocarditis following surgical aortic valve replacement, indicating that TAVR is no less predisposing to endocarditis despite being a less invasive approach.
The mean age of patients who developed post-TAVR endocarditis was 78.9 years, compared with 81.8 years for those who did not (HR, 0.97). The reason for this association is unclear, but it is possible that younger patients chosen for TAVR because of their prohibitive surgical risk carry a higher burden of comorbidity than do older patients. Similarly, 62% of endocarditis cases arose in men (HR, 1.69), and sex differences in comorbid conditions may explain the higher risk among men.
More patients who developed endocarditis had diabetes (41.7%), compared with those who did not develop endocarditis (30%), for an HR of 1.52. And patients who had moderate to severe residual aortic regurgitation after TAVR also were at much higher risk for endocarditis than were those who did not (HR, 2.05), the investigators noted (JAMA. 2016 Sep 13;316[10]:1083-92).
In contrast, factors that were not associated with endocarditis risk included chronic pulmonary disease, type of valve (self-expandable or balloon-expandable), and setting of the procedure (catheterization lab vs. operating room).
The bacteria that most commonly caused infective endocarditis were Enterococci species (24.6% of cases), Staphylococcus aureus (23.8%), and coagulase-negative staphylococci (16.8%). This should be taken into consideration when selecting antibiotics for prophylaxis before TAVR and when choosing empirical antibiotics for treatment while waiting for blood culture results, wrote Dr. Regueiro and his associates.
“This information may help clinicians identify patients at higher risk [for endocarditis] and aid in implementing appropriate preventive measures,” they noted.
This study was supported by a grant from the Alfonso Martin Escudero Foundation. Dr. Regueiro reported having no relevant financial disclosures; his associates reported ties to numerous industry sources.
Four factors – younger patient age, male sex, diabetes, and moderate to severe residual aortic regurgitation – are associated with a significantly increased risk of infective endocarditis after transcatheter aortic valve replacement, according to a report published online Sept. 13 in JAMA.
Until now, data pertaining to endocarditis following TAVR “have been limited to case reports and relatively small series with limited follow-up,” said Ander Regueiro, MD, of Laval University, Quebec City, and his associates.
They performed a retrospective analysis of data in a large international registry of TAVR cases to better characterize post-TAVR endocarditis.
Dr. Regueiro and his colleagues focused on 20,006 TAVR procedures done at 47 medical centers in Europe, North America, and South America during a 10-year period. The median time to symptom onset was 5.3 months after the procedure.
Infective endocarditis was definitively diagnosed in 250 of these cases. This incidence is similar to that reported for endocarditis following surgical aortic valve replacement, indicating that TAVR is no less predisposing to endocarditis despite being a less invasive approach.
The mean age of patients who developed post-TAVR endocarditis was 78.9 years, compared with 81.8 years for those who did not (HR, 0.97). The reason for this association is unclear, but it is possible that younger patients chosen for TAVR because of their prohibitive surgical risk carry a higher burden of comorbidity than do older patients. Similarly, 62% of endocarditis cases arose in men (HR, 1.69), and sex differences in comorbid conditions may explain the higher risk among men.
More patients who developed endocarditis had diabetes (41.7%), compared with those who did not develop endocarditis (30%), for an HR of 1.52. And patients who had moderate to severe residual aortic regurgitation after TAVR also were at much higher risk for endocarditis than were those who did not (HR, 2.05), the investigators noted (JAMA. 2016 Sep 13;316[10]:1083-92).
In contrast, factors that were not associated with endocarditis risk included chronic pulmonary disease, type of valve (self-expandable or balloon-expandable), and setting of the procedure (catheterization lab vs. operating room).
The bacteria that most commonly caused infective endocarditis were Enterococci species (24.6% of cases), Staphylococcus aureus (23.8%), and coagulase-negative staphylococci (16.8%). This should be taken into consideration when selecting antibiotics for prophylaxis before TAVR and when choosing empirical antibiotics for treatment while waiting for blood culture results, wrote Dr. Regueiro and his associates.
“This information may help clinicians identify patients at higher risk [for endocarditis] and aid in implementing appropriate preventive measures,” they noted.
This study was supported by a grant from the Alfonso Martin Escudero Foundation. Dr. Regueiro reported having no relevant financial disclosures; his associates reported ties to numerous industry sources.
FROM JAMA
Key clinical point: Four factors raise the risk that patients undergoing transcatheter aortic valve replacement will develop infective endocarditis.
Major finding: The mean age of patients who developed post-TAVR endocarditis was 78.9 years, compared with 81.8 years for those who didn’t (HR, 0.97).
Data source: A retrospective analysis of data in an international registry involving 20,006 patients who underwent TAVR at 47 medical centers during a 10-year period.
Disclosures: This study was supported by a grant from the Alfonso Martin Escudero Foundation. Dr. Regueiro reported having no relevant financial disclosures; his associates reported ties to numerous industry sources.
Smoking thickens LV wall, worsens function
Current smoking, as well as higher levels of cumulative cigarette exposure from past smoking, were both associated with higher left ventricular mass, a higher LV mass-to-volume ratio, and worse diastolic function in an elderly community-based population with no overt indications of coronary artery disease or heart failure, according to a report published online Sept. 13 in Circulation: Cardiovascular Imaging.
“These findings suggest that smoking is associated with subtle alterations in LV structure and function, which might help explain the higher risk of heart failure [HF] reported for smokers, independent of coronary artery disease [CAD],” said Wilson Nadruz Jr., MD, of the cardiovascular division, Brigham and Women’s Hospital, Boston, and his associates.
They analyzed links between smoking and echocardiographic features using data from the Atherosclerosis Risk in Communities (ARIC) study, an ongoing prospective observational study involving community-dwelling adults who were aged 45-64 years at baseline in 1987-1989. For their study, Dr. Nadruz and his colleagues assessed echocardiographic images taken for 4,580 ARIC participants at follow-up roughly 25 years later. None of these adults had any indication of CAD or HF; 287 (6.3%) were current smokers, 2,316 (50.5%) were former smokers, and 1,977 (43.2%) never smoked.
Compared with never smokers, current smokers showed a greater LV mass index (80.4 vs. 76.7), a greater LV mass-to-volume ratio (1.93 vs. 1.83), and a higher prevalence of LV hypertrophy (15% vs. 9%), as well as a higher prevalence of concentric LV hypertrophy and worse LV diastolic function. The same association was found between never smokers and former smokers who had higher levels of cumulative cigarette exposure, the investigators said (Circ Cardiovasc Imag. 2016 Sep 13. doi: 10.1161/circimaging.116.004950).
This association between smoking and altered LV structure and function remained robust after the data were adjusted to account for numerous cardiac risk factors such as older age, higher BMI, diabetes, hypertension, greater alcohol consumption, and higher heart rate. It also didn’t vary by patient sex, race, or income level. In contrast, there was no association between smoking and right ventricular structure or function.
“These data suggest that smoking can independently lead to thickening of the heart and worsening of heart function, which may lead to a higher risk for heart failure, even in people who don’t have heart attacks,” Dr. Nadruz said in a statement.
Looking at the results in a more positive light, senior author Scott D. Solomon, MD, professor of medicine at Harvard University, Boston, said “The good news is that former smokers had similar heart structure and function, compared with never smokers,” suggesting that “the potential effects of tobacco on the myocardium might be reversible after smoking cessation.”
Current smoking, as well as higher levels of cumulative cigarette exposure from past smoking, were both associated with higher left ventricular mass, a higher LV mass-to-volume ratio, and worse diastolic function in an elderly community-based population with no overt indications of coronary artery disease or heart failure, according to a report published online Sept. 13 in Circulation: Cardiovascular Imaging.
“These findings suggest that smoking is associated with subtle alterations in LV structure and function, which might help explain the higher risk of heart failure [HF] reported for smokers, independent of coronary artery disease [CAD],” said Wilson Nadruz Jr., MD, of the cardiovascular division, Brigham and Women’s Hospital, Boston, and his associates.
They analyzed links between smoking and echocardiographic features using data from the Atherosclerosis Risk in Communities (ARIC) study, an ongoing prospective observational study involving community-dwelling adults who were aged 45-64 years at baseline in 1987-1989. For their study, Dr. Nadruz and his colleagues assessed echocardiographic images taken for 4,580 ARIC participants at follow-up roughly 25 years later. None of these adults had any indication of CAD or HF; 287 (6.3%) were current smokers, 2,316 (50.5%) were former smokers, and 1,977 (43.2%) never smoked.
Compared with never smokers, current smokers showed a greater LV mass index (80.4 vs. 76.7), a greater LV mass-to-volume ratio (1.93 vs. 1.83), and a higher prevalence of LV hypertrophy (15% vs. 9%), as well as a higher prevalence of concentric LV hypertrophy and worse LV diastolic function. The same association was found between never smokers and former smokers who had higher levels of cumulative cigarette exposure, the investigators said (Circ Cardiovasc Imag. 2016 Sep 13. doi: 10.1161/circimaging.116.004950).
This association between smoking and altered LV structure and function remained robust after the data were adjusted to account for numerous cardiac risk factors such as older age, higher BMI, diabetes, hypertension, greater alcohol consumption, and higher heart rate. It also didn’t vary by patient sex, race, or income level. In contrast, there was no association between smoking and right ventricular structure or function.
“These data suggest that smoking can independently lead to thickening of the heart and worsening of heart function, which may lead to a higher risk for heart failure, even in people who don’t have heart attacks,” Dr. Nadruz said in a statement.
Looking at the results in a more positive light, senior author Scott D. Solomon, MD, professor of medicine at Harvard University, Boston, said “The good news is that former smokers had similar heart structure and function, compared with never smokers,” suggesting that “the potential effects of tobacco on the myocardium might be reversible after smoking cessation.”
Current smoking, as well as higher levels of cumulative cigarette exposure from past smoking, were both associated with higher left ventricular mass, a higher LV mass-to-volume ratio, and worse diastolic function in an elderly community-based population with no overt indications of coronary artery disease or heart failure, according to a report published online Sept. 13 in Circulation: Cardiovascular Imaging.
“These findings suggest that smoking is associated with subtle alterations in LV structure and function, which might help explain the higher risk of heart failure [HF] reported for smokers, independent of coronary artery disease [CAD],” said Wilson Nadruz Jr., MD, of the cardiovascular division, Brigham and Women’s Hospital, Boston, and his associates.
They analyzed links between smoking and echocardiographic features using data from the Atherosclerosis Risk in Communities (ARIC) study, an ongoing prospective observational study involving community-dwelling adults who were aged 45-64 years at baseline in 1987-1989. For their study, Dr. Nadruz and his colleagues assessed echocardiographic images taken for 4,580 ARIC participants at follow-up roughly 25 years later. None of these adults had any indication of CAD or HF; 287 (6.3%) were current smokers, 2,316 (50.5%) were former smokers, and 1,977 (43.2%) never smoked.
Compared with never smokers, current smokers showed a greater LV mass index (80.4 vs. 76.7), a greater LV mass-to-volume ratio (1.93 vs. 1.83), and a higher prevalence of LV hypertrophy (15% vs. 9%), as well as a higher prevalence of concentric LV hypertrophy and worse LV diastolic function. The same association was found between never smokers and former smokers who had higher levels of cumulative cigarette exposure, the investigators said (Circ Cardiovasc Imag. 2016 Sep 13. doi: 10.1161/circimaging.116.004950).
This association between smoking and altered LV structure and function remained robust after the data were adjusted to account for numerous cardiac risk factors such as older age, higher BMI, diabetes, hypertension, greater alcohol consumption, and higher heart rate. It also didn’t vary by patient sex, race, or income level. In contrast, there was no association between smoking and right ventricular structure or function.
“These data suggest that smoking can independently lead to thickening of the heart and worsening of heart function, which may lead to a higher risk for heart failure, even in people who don’t have heart attacks,” Dr. Nadruz said in a statement.
Looking at the results in a more positive light, senior author Scott D. Solomon, MD, professor of medicine at Harvard University, Boston, said “The good news is that former smokers had similar heart structure and function, compared with never smokers,” suggesting that “the potential effects of tobacco on the myocardium might be reversible after smoking cessation.”
FROM CIRCULATION: CARDIOVASCULAR IMAGING
Key clinical point: Current smoking was associated with higher left ventricular mass, a higher LV mass-to-volume ratio, and worse diastolic function.
Major finding: Compared with never smokers, current smokers showed a greater LV mass index (80.4 vs. 76.7), a greater LV mass-to-volume ratio (1.93 vs. 1.83), and a higher prevalence of LV hypertrophy (15% vs. 9%).
Data source: A secondary analysis of data for 4,580 elderly participants in ARIC, a large community-based cohort.
Disclosures: This study was supported by Brigham and Women’s Hospital, Boston. Dr. Nadruz and his associates reported having no relevant financial disclosures.
Four-step screen IDs silent heart attack in type 2 diabetes
MUNICH – A four-component imaging/biomarker screen was highly accurate for identifying silent myocardial infarction among asymptomatic patients with type 2 diabetes.
The screen is far more accurate than the current standards of invasive imaging or only looking for pathologic Q waves, Peter Swoboda, MD, said at the annual meeting of the European Association for the Study of Diabetes.
“By combining these four factors we came up with a tool that has a diagnostic area under the curve [AUC] of 0.85,” said Dr. Swoboda of Leeds (England) University. “This is far better than the 0.58 AUC that we have with Q waves only – a sensitivity of just 25%.”
The study was published online in June in the Journal of Clinical Endocrinology and Metabolism (JCEM 2016. doi: 10.1210/jc.2016-1318).
The screen employs noninvasive imaging and biomarkers to tap multiple clinical hallmarks of silent MI. The components are:
• electrocardiogram.
• echocardiography.
• biomarker assessment.
• cardiac magnetic resonance imaging, focusing on left ventricular ejection fraction and late gadolinium enhancement.
The study cohort comprised 100 patients with type 2 diabetes without known heart disease and with no new cardiac symptoms. They underwent cardiac MRI, a 12-lead electrocardiogram, echocardiography, and serum biomarker assessment. Late gadolinium enhancement identified evidence of silent MI in 17 patients (17%).
There were few differences in the clinical characteristics of those who had experienced MI and those who had not, Dr. Swoboda noted. There were no differences at all in diabetes-related measures, including disease duration or hemoglobin A1c levels. Blood pressures were similar. Patients with MI were significantly older (65 vs. 60 years).
In cardiac-specific measures, left ventricular ejection fraction was similar, as was left ventricular mass, end diastolic volume, and left atrial volume. There were however, other very important differences, Dr. Swoboda noted.
Imaging included a measure called “feature tracking analysis,” which measured the peak global longitudinal strain, systolic strain rate, and early and late diastolic strain rates during contraction. This analysis noted a significant difference in global longitudinal strain between the MI and non-MI groups.
Ventricular filling velocities as measured by the E/A ratio on ECG were also significantly different between the MI and non-MI groups (0.75 vs. 0.89, respectively). ECG also found pathologic Q waves in significantly more MI patients (24% vs. 7%).
Finally, the serum biomarker panel showed a very strong increase in B-type natriuretic peptide (NT-proBNP) among MI patients, compared with non-MI patients (105 vs. 52 ng/L). There were no significant differences in the other biomarkers, including C-reactive protein and high-sensitive cardiac troponin.
Dr. Swoboda and his team then compiled these findings into a composite measure, assigning them optimum cutoff measures:
• Age older than 62 years.
• E/A ratio 0.72 or lower.
• Global longitudinal strain of at least 18.4%.
• NT-proBNP more than 29 ng/L.
The system resulted in a diagnostic accuracy AUC of 0.85 – significantly better than any of the AUCs generated by the individual components. All patients who scored 0 or 1 were free of MI. Among the 28 with a score of 2, only three had experienced a silent MI. Among the 21 with a score of 3, seven had experienced a silent MI and 14 had not. Among the 16 with a score of 4, seven had experienced a silent MI and nine had not.
While Dr. Swoboda called the screening method “simple” during discussion, a colleague in the audience disagreed with that.
“A simple test is something like a blood test only, not an MRI. Not imaging. That is expensive and takes time,” said Naveed Sattar, MD, of the University of Glasgow, Scotland. “However, I do think your data add more to the evidence that BNP can be a really valuable marker of cardiovascular risk in patients with diabetes.”
Dr. Sattar recently examined the value of cardiac serum biomarkers in predicting cardiovascular disease and mortality in nearly 100,000 people without a history of heart disease. In these subjects, he wrote, “NT-proBNP assessment strongly predicted first-onset heart failure and augmented coronary heart disease and stroke prediction, suggesting that NT-proBNP concentration assessment could be used to integrate heart failure into cardiovascular disease primary prevention.”
The paper appeared online in Lancet Diabetes in September (Lancet Diab. 2016. doi: 10.1016/S2213-8587[16]30196-6).
Dr. Swoboda agreed that data continue to support the increased use of NT-proBNP as a marker of heart disease.
“I think that in the future, diabetes medicine is moving toward individualized patient care, based on individualized risk factors. The future of assessing asymptomatic cardiac patients might be a combination of BNP and MRI.”
Dr. Swoboda had no financial disclosures. Some of Dr. Sattar’s coauthors reported relationships with pharmaceutical companies.
MUNICH – A four-component imaging/biomarker screen was highly accurate for identifying silent myocardial infarction among asymptomatic patients with type 2 diabetes.
The screen is far more accurate than the current standards of invasive imaging or only looking for pathologic Q waves, Peter Swoboda, MD, said at the annual meeting of the European Association for the Study of Diabetes.
“By combining these four factors we came up with a tool that has a diagnostic area under the curve [AUC] of 0.85,” said Dr. Swoboda of Leeds (England) University. “This is far better than the 0.58 AUC that we have with Q waves only – a sensitivity of just 25%.”
The study was published online in June in the Journal of Clinical Endocrinology and Metabolism (JCEM 2016. doi: 10.1210/jc.2016-1318).
The screen employs noninvasive imaging and biomarkers to tap multiple clinical hallmarks of silent MI. The components are:
• electrocardiogram.
• echocardiography.
• biomarker assessment.
• cardiac magnetic resonance imaging, focusing on left ventricular ejection fraction and late gadolinium enhancement.
The study cohort comprised 100 patients with type 2 diabetes without known heart disease and with no new cardiac symptoms. They underwent cardiac MRI, a 12-lead electrocardiogram, echocardiography, and serum biomarker assessment. Late gadolinium enhancement identified evidence of silent MI in 17 patients (17%).
There were few differences in the clinical characteristics of those who had experienced MI and those who had not, Dr. Swoboda noted. There were no differences at all in diabetes-related measures, including disease duration or hemoglobin A1c levels. Blood pressures were similar. Patients with MI were significantly older (65 vs. 60 years).
In cardiac-specific measures, left ventricular ejection fraction was similar, as was left ventricular mass, end diastolic volume, and left atrial volume. There were however, other very important differences, Dr. Swoboda noted.
Imaging included a measure called “feature tracking analysis,” which measured the peak global longitudinal strain, systolic strain rate, and early and late diastolic strain rates during contraction. This analysis noted a significant difference in global longitudinal strain between the MI and non-MI groups.
Ventricular filling velocities as measured by the E/A ratio on ECG were also significantly different between the MI and non-MI groups (0.75 vs. 0.89, respectively). ECG also found pathologic Q waves in significantly more MI patients (24% vs. 7%).
Finally, the serum biomarker panel showed a very strong increase in B-type natriuretic peptide (NT-proBNP) among MI patients, compared with non-MI patients (105 vs. 52 ng/L). There were no significant differences in the other biomarkers, including C-reactive protein and high-sensitive cardiac troponin.
Dr. Swoboda and his team then compiled these findings into a composite measure, assigning them optimum cutoff measures:
• Age older than 62 years.
• E/A ratio 0.72 or lower.
• Global longitudinal strain of at least 18.4%.
• NT-proBNP more than 29 ng/L.
The system resulted in a diagnostic accuracy AUC of 0.85 – significantly better than any of the AUCs generated by the individual components. All patients who scored 0 or 1 were free of MI. Among the 28 with a score of 2, only three had experienced a silent MI. Among the 21 with a score of 3, seven had experienced a silent MI and 14 had not. Among the 16 with a score of 4, seven had experienced a silent MI and nine had not.
While Dr. Swoboda called the screening method “simple” during discussion, a colleague in the audience disagreed with that.
“A simple test is something like a blood test only, not an MRI. Not imaging. That is expensive and takes time,” said Naveed Sattar, MD, of the University of Glasgow, Scotland. “However, I do think your data add more to the evidence that BNP can be a really valuable marker of cardiovascular risk in patients with diabetes.”
Dr. Sattar recently examined the value of cardiac serum biomarkers in predicting cardiovascular disease and mortality in nearly 100,000 people without a history of heart disease. In these subjects, he wrote, “NT-proBNP assessment strongly predicted first-onset heart failure and augmented coronary heart disease and stroke prediction, suggesting that NT-proBNP concentration assessment could be used to integrate heart failure into cardiovascular disease primary prevention.”
The paper appeared online in Lancet Diabetes in September (Lancet Diab. 2016. doi: 10.1016/S2213-8587[16]30196-6).
Dr. Swoboda agreed that data continue to support the increased use of NT-proBNP as a marker of heart disease.
“I think that in the future, diabetes medicine is moving toward individualized patient care, based on individualized risk factors. The future of assessing asymptomatic cardiac patients might be a combination of BNP and MRI.”
Dr. Swoboda had no financial disclosures. Some of Dr. Sattar’s coauthors reported relationships with pharmaceutical companies.
MUNICH – A four-component imaging/biomarker screen was highly accurate for identifying silent myocardial infarction among asymptomatic patients with type 2 diabetes.
The screen is far more accurate than the current standards of invasive imaging or only looking for pathologic Q waves, Peter Swoboda, MD, said at the annual meeting of the European Association for the Study of Diabetes.
“By combining these four factors we came up with a tool that has a diagnostic area under the curve [AUC] of 0.85,” said Dr. Swoboda of Leeds (England) University. “This is far better than the 0.58 AUC that we have with Q waves only – a sensitivity of just 25%.”
The study was published online in June in the Journal of Clinical Endocrinology and Metabolism (JCEM 2016. doi: 10.1210/jc.2016-1318).
The screen employs noninvasive imaging and biomarkers to tap multiple clinical hallmarks of silent MI. The components are:
• electrocardiogram.
• echocardiography.
• biomarker assessment.
• cardiac magnetic resonance imaging, focusing on left ventricular ejection fraction and late gadolinium enhancement.
The study cohort comprised 100 patients with type 2 diabetes without known heart disease and with no new cardiac symptoms. They underwent cardiac MRI, a 12-lead electrocardiogram, echocardiography, and serum biomarker assessment. Late gadolinium enhancement identified evidence of silent MI in 17 patients (17%).
There were few differences in the clinical characteristics of those who had experienced MI and those who had not, Dr. Swoboda noted. There were no differences at all in diabetes-related measures, including disease duration or hemoglobin A1c levels. Blood pressures were similar. Patients with MI were significantly older (65 vs. 60 years).
In cardiac-specific measures, left ventricular ejection fraction was similar, as was left ventricular mass, end diastolic volume, and left atrial volume. There were however, other very important differences, Dr. Swoboda noted.
Imaging included a measure called “feature tracking analysis,” which measured the peak global longitudinal strain, systolic strain rate, and early and late diastolic strain rates during contraction. This analysis noted a significant difference in global longitudinal strain between the MI and non-MI groups.
Ventricular filling velocities as measured by the E/A ratio on ECG were also significantly different between the MI and non-MI groups (0.75 vs. 0.89, respectively). ECG also found pathologic Q waves in significantly more MI patients (24% vs. 7%).
Finally, the serum biomarker panel showed a very strong increase in B-type natriuretic peptide (NT-proBNP) among MI patients, compared with non-MI patients (105 vs. 52 ng/L). There were no significant differences in the other biomarkers, including C-reactive protein and high-sensitive cardiac troponin.
Dr. Swoboda and his team then compiled these findings into a composite measure, assigning them optimum cutoff measures:
• Age older than 62 years.
• E/A ratio 0.72 or lower.
• Global longitudinal strain of at least 18.4%.
• NT-proBNP more than 29 ng/L.
The system resulted in a diagnostic accuracy AUC of 0.85 – significantly better than any of the AUCs generated by the individual components. All patients who scored 0 or 1 were free of MI. Among the 28 with a score of 2, only three had experienced a silent MI. Among the 21 with a score of 3, seven had experienced a silent MI and 14 had not. Among the 16 with a score of 4, seven had experienced a silent MI and nine had not.
While Dr. Swoboda called the screening method “simple” during discussion, a colleague in the audience disagreed with that.
“A simple test is something like a blood test only, not an MRI. Not imaging. That is expensive and takes time,” said Naveed Sattar, MD, of the University of Glasgow, Scotland. “However, I do think your data add more to the evidence that BNP can be a really valuable marker of cardiovascular risk in patients with diabetes.”
Dr. Sattar recently examined the value of cardiac serum biomarkers in predicting cardiovascular disease and mortality in nearly 100,000 people without a history of heart disease. In these subjects, he wrote, “NT-proBNP assessment strongly predicted first-onset heart failure and augmented coronary heart disease and stroke prediction, suggesting that NT-proBNP concentration assessment could be used to integrate heart failure into cardiovascular disease primary prevention.”
The paper appeared online in Lancet Diabetes in September (Lancet Diab. 2016. doi: 10.1016/S2213-8587[16]30196-6).
Dr. Swoboda agreed that data continue to support the increased use of NT-proBNP as a marker of heart disease.
“I think that in the future, diabetes medicine is moving toward individualized patient care, based on individualized risk factors. The future of assessing asymptomatic cardiac patients might be a combination of BNP and MRI.”
Dr. Swoboda had no financial disclosures. Some of Dr. Sattar’s coauthors reported relationships with pharmaceutical companies.
AT EASD 2016
Key clinical point: A four-component screen accurately identified silent myocardial infarction in asymptomatic patients with type 2 diabetes
Major finding: The tool had an 82% sensitivity and 72% specificity for silent MI.
Data source: It was created in a cohort of 100 patients with type 2 diabetes and no history of heart disease.
Disclosures: Dr. Swoboda had no financial disclosures.
























