User login
CABG tops PCI for nondiabetic patients with multivessel CAD
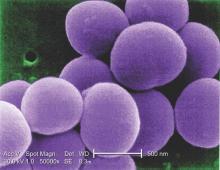
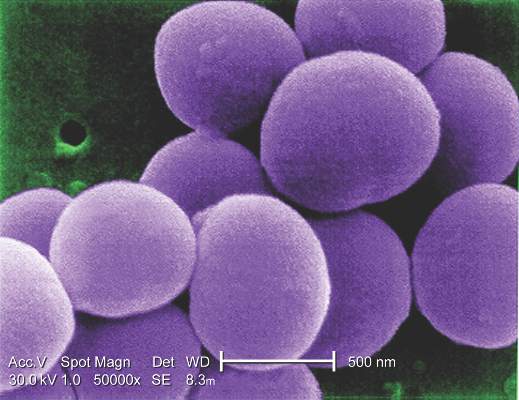
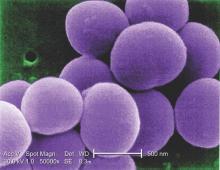
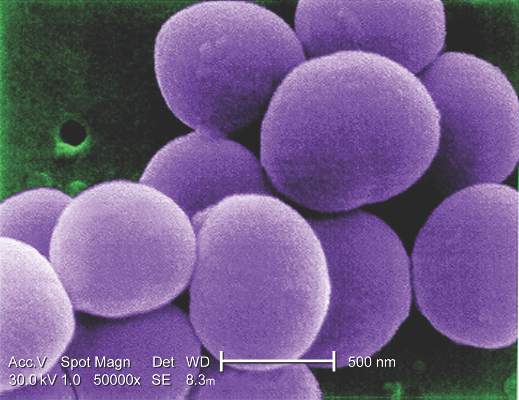
Coronary artery bypass graft surgery was associated with a 35% lower rate of all-cause mortality rate and a 59% lower rate of cardiac death than was percutaneous coronary intervention in a pooled analysis of nondiabetic patients with multivessel coronary artery disease in two international randomized trials.
“The superiority of CABG over PCI was consistent across all major clinical subgroups. Likewise, the rate of myocardial infarction was remarkably lower after CABG than after PCI,” Mineok Chang, MD, of the University of Ulsan in Seoul, South Korea, and her associates reported online June 27 in the Journal of the American College of Cardiology.
The advent of drug-eluting stents has led to widespread use of PCI, but controversy persists regarding the best revascularization strategy for nondiabetic patients with multivessel CAD, the researchers said. All-cause mortality “is undoubtedly the most unbiased endpoint to determine treatment strategy,” but individual trials have lacked the power to evaluate this endpoint, they added. Accordingly, they pooled data from the SYNTAX (Synergy Between PCI with Taxus and Cardiac Surgery) trial – which included 1,800 patients with three-vessel or left main CAD from Europe and the United States – and from the BEST (Randomized Comparison of Coronary Artery Bypass Surgery and Everolimus-Eluting Stent Implantation in the Treatment of Patients With Multivessel Coronary Artery Disease) trial, which included 880 patients from Asia with two- or three-vessel CAD. Excluding patients with left main CAD or diabetes left 1,275 patients for analysis (J Am Coll Cardiol. 2016 Jun 27. 68:29-36).
After a median follow-up of 61 months, 38 (6%) CABG patients had died, compared with 59 (9.3%) PCI patients, for a statistically significant hazard ratio of 0.65 (95% confidence interval, 0.25-0.78; P = .039). Likewise, cardiac death was significantly less likely after CABG than PCI (HR, 0.41; 95% CI, 0.25-0.78; P = .005). “The statistical difference between the two groups was pronounced after 2 years of randomization for both all-cause and cardiac mortality,” the researchers reported. Although the latest drug-eluting stents “are reported to improve clinical outcomes,” they did not find a significant interaction between older and more recently developed stents when considering all-cause mortality, they noted.
The study was supported by a research grant from the CardioVascular Research Foundation in Seoul, South Korea. Dr. Chang had no disclosures. Senior author, Dr. Seung-Jung Park, disclosed research support from Abbott Vascular, Cordis, Boston Scientific, and Medtronic.
It is important to consider some limitations of the current study. First, the majority of patients were derived from the SYNTAX trial, which used first-generation paclitaxel drug-eluting stents (DES) that exhibit higher stent complication rates compared with current second- and third-generation everolimus-eluting stents. Second, although complete revascularization was more frequently obtained with CABG in the SYNTAX and BEST trials, current PCI approaches are narrowing this gap with chronic total occlusion PCI techniques and temporary hemodynamic support when needed. Third, selection bias may limit the applicability of these results to patients with more complex disease who are often excluded from clinical trials and may not be eligible for CABG.
Therefore, an adequately powered study of contemporary DES PCI versus CABG is warranted for real-world, nondiabetic patients with multivessel CAD. Guidelines and meta-analyses provide valuable recommendations for populations at large, but decision making for nondiabetic patients with multivessel CAD should be individualized. If equipoise between CABG and PCI exists, decision-making can be improved by engaging a local multidisciplinary CAD heart team. Well-informed cardiologists, interventionalists, and cardiac surgeons who engage in multidisciplinary heart team evaluations and patient-centered shared decision making will provide optimal guidance for patients with multivessel CAD.
Farouc A. Jaffer, MD, is at Massachusetts General Hospital; Patrick T. O’Gara, MD, is at Brigham and Women’s Hospital, both in Boston. Dr. Jaffer disclosed research grants from Siemens and Kowa and consulting relationships with Boston Scientific and Abbott Vascular. Dr. O’Gara had no disclosures. These comments are from their editorial (J Am Coll Cardiol. 2016 Jun 27. doi: 10.1016/j.jacc.2016.05.008).
It is important to consider some limitations of the current study. First, the majority of patients were derived from the SYNTAX trial, which used first-generation paclitaxel drug-eluting stents (DES) that exhibit higher stent complication rates compared with current second- and third-generation everolimus-eluting stents. Second, although complete revascularization was more frequently obtained with CABG in the SYNTAX and BEST trials, current PCI approaches are narrowing this gap with chronic total occlusion PCI techniques and temporary hemodynamic support when needed. Third, selection bias may limit the applicability of these results to patients with more complex disease who are often excluded from clinical trials and may not be eligible for CABG.
Therefore, an adequately powered study of contemporary DES PCI versus CABG is warranted for real-world, nondiabetic patients with multivessel CAD. Guidelines and meta-analyses provide valuable recommendations for populations at large, but decision making for nondiabetic patients with multivessel CAD should be individualized. If equipoise between CABG and PCI exists, decision-making can be improved by engaging a local multidisciplinary CAD heart team. Well-informed cardiologists, interventionalists, and cardiac surgeons who engage in multidisciplinary heart team evaluations and patient-centered shared decision making will provide optimal guidance for patients with multivessel CAD.
Farouc A. Jaffer, MD, is at Massachusetts General Hospital; Patrick T. O’Gara, MD, is at Brigham and Women’s Hospital, both in Boston. Dr. Jaffer disclosed research grants from Siemens and Kowa and consulting relationships with Boston Scientific and Abbott Vascular. Dr. O’Gara had no disclosures. These comments are from their editorial (J Am Coll Cardiol. 2016 Jun 27. doi: 10.1016/j.jacc.2016.05.008).
It is important to consider some limitations of the current study. First, the majority of patients were derived from the SYNTAX trial, which used first-generation paclitaxel drug-eluting stents (DES) that exhibit higher stent complication rates compared with current second- and third-generation everolimus-eluting stents. Second, although complete revascularization was more frequently obtained with CABG in the SYNTAX and BEST trials, current PCI approaches are narrowing this gap with chronic total occlusion PCI techniques and temporary hemodynamic support when needed. Third, selection bias may limit the applicability of these results to patients with more complex disease who are often excluded from clinical trials and may not be eligible for CABG.
Therefore, an adequately powered study of contemporary DES PCI versus CABG is warranted for real-world, nondiabetic patients with multivessel CAD. Guidelines and meta-analyses provide valuable recommendations for populations at large, but decision making for nondiabetic patients with multivessel CAD should be individualized. If equipoise between CABG and PCI exists, decision-making can be improved by engaging a local multidisciplinary CAD heart team. Well-informed cardiologists, interventionalists, and cardiac surgeons who engage in multidisciplinary heart team evaluations and patient-centered shared decision making will provide optimal guidance for patients with multivessel CAD.
Farouc A. Jaffer, MD, is at Massachusetts General Hospital; Patrick T. O’Gara, MD, is at Brigham and Women’s Hospital, both in Boston. Dr. Jaffer disclosed research grants from Siemens and Kowa and consulting relationships with Boston Scientific and Abbott Vascular. Dr. O’Gara had no disclosures. These comments are from their editorial (J Am Coll Cardiol. 2016 Jun 27. doi: 10.1016/j.jacc.2016.05.008).
Coronary artery bypass graft surgery was associated with a 35% lower rate of all-cause mortality rate and a 59% lower rate of cardiac death than was percutaneous coronary intervention in a pooled analysis of nondiabetic patients with multivessel coronary artery disease in two international randomized trials.
“The superiority of CABG over PCI was consistent across all major clinical subgroups. Likewise, the rate of myocardial infarction was remarkably lower after CABG than after PCI,” Mineok Chang, MD, of the University of Ulsan in Seoul, South Korea, and her associates reported online June 27 in the Journal of the American College of Cardiology.
The advent of drug-eluting stents has led to widespread use of PCI, but controversy persists regarding the best revascularization strategy for nondiabetic patients with multivessel CAD, the researchers said. All-cause mortality “is undoubtedly the most unbiased endpoint to determine treatment strategy,” but individual trials have lacked the power to evaluate this endpoint, they added. Accordingly, they pooled data from the SYNTAX (Synergy Between PCI with Taxus and Cardiac Surgery) trial – which included 1,800 patients with three-vessel or left main CAD from Europe and the United States – and from the BEST (Randomized Comparison of Coronary Artery Bypass Surgery and Everolimus-Eluting Stent Implantation in the Treatment of Patients With Multivessel Coronary Artery Disease) trial, which included 880 patients from Asia with two- or three-vessel CAD. Excluding patients with left main CAD or diabetes left 1,275 patients for analysis (J Am Coll Cardiol. 2016 Jun 27. 68:29-36).
After a median follow-up of 61 months, 38 (6%) CABG patients had died, compared with 59 (9.3%) PCI patients, for a statistically significant hazard ratio of 0.65 (95% confidence interval, 0.25-0.78; P = .039). Likewise, cardiac death was significantly less likely after CABG than PCI (HR, 0.41; 95% CI, 0.25-0.78; P = .005). “The statistical difference between the two groups was pronounced after 2 years of randomization for both all-cause and cardiac mortality,” the researchers reported. Although the latest drug-eluting stents “are reported to improve clinical outcomes,” they did not find a significant interaction between older and more recently developed stents when considering all-cause mortality, they noted.
The study was supported by a research grant from the CardioVascular Research Foundation in Seoul, South Korea. Dr. Chang had no disclosures. Senior author, Dr. Seung-Jung Park, disclosed research support from Abbott Vascular, Cordis, Boston Scientific, and Medtronic.
Coronary artery bypass graft surgery was associated with a 35% lower rate of all-cause mortality rate and a 59% lower rate of cardiac death than was percutaneous coronary intervention in a pooled analysis of nondiabetic patients with multivessel coronary artery disease in two international randomized trials.
“The superiority of CABG over PCI was consistent across all major clinical subgroups. Likewise, the rate of myocardial infarction was remarkably lower after CABG than after PCI,” Mineok Chang, MD, of the University of Ulsan in Seoul, South Korea, and her associates reported online June 27 in the Journal of the American College of Cardiology.
The advent of drug-eluting stents has led to widespread use of PCI, but controversy persists regarding the best revascularization strategy for nondiabetic patients with multivessel CAD, the researchers said. All-cause mortality “is undoubtedly the most unbiased endpoint to determine treatment strategy,” but individual trials have lacked the power to evaluate this endpoint, they added. Accordingly, they pooled data from the SYNTAX (Synergy Between PCI with Taxus and Cardiac Surgery) trial – which included 1,800 patients with three-vessel or left main CAD from Europe and the United States – and from the BEST (Randomized Comparison of Coronary Artery Bypass Surgery and Everolimus-Eluting Stent Implantation in the Treatment of Patients With Multivessel Coronary Artery Disease) trial, which included 880 patients from Asia with two- or three-vessel CAD. Excluding patients with left main CAD or diabetes left 1,275 patients for analysis (J Am Coll Cardiol. 2016 Jun 27. 68:29-36).
After a median follow-up of 61 months, 38 (6%) CABG patients had died, compared with 59 (9.3%) PCI patients, for a statistically significant hazard ratio of 0.65 (95% confidence interval, 0.25-0.78; P = .039). Likewise, cardiac death was significantly less likely after CABG than PCI (HR, 0.41; 95% CI, 0.25-0.78; P = .005). “The statistical difference between the two groups was pronounced after 2 years of randomization for both all-cause and cardiac mortality,” the researchers reported. Although the latest drug-eluting stents “are reported to improve clinical outcomes,” they did not find a significant interaction between older and more recently developed stents when considering all-cause mortality, they noted.
The study was supported by a research grant from the CardioVascular Research Foundation in Seoul, South Korea. Dr. Chang had no disclosures. Senior author, Dr. Seung-Jung Park, disclosed research support from Abbott Vascular, Cordis, Boston Scientific, and Medtronic.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Key clinical point: Coronary artery bypass graft is superior to percutaneous coronary intervention for nondiabetic patients with multivessel coronary artery disease.
Major finding: All-cause mortality rates were 6% for CABG and 9.3% for PCI (HR, 0.65; P = .039).
Data source: A pooled analysis of 1,275 nondiabetic patients with two- or three-vessel CAD from the SYNTAX and BEST trials.
Disclosures: The study was supported by a research grant from the CardioVascular Research Foundation in Seoul, South Korea. Dr. Chang had no disclosures. Senior author, Dr. Seung-Jung Park, disclosed research support from Abbott Vascular, Cordis, Boston Scientific, and Medtronic.
Elusive evidence pervades ESC’s 2016 heart failure guidelines
FLORENCE, ITALY – The 2016 revision of the European Society of Cardiology’s guidelines for diagnosing and treating acute and chronic heart failure highlights the extent to which thinking in the field has changed during the past 4 years, since the prior edition in 2012.
The new European guidelines, unveiled by the ESC’s Heart Failure Association during the group’s annual meeting, also underscore the great dependence that many new approaches have on expert opinion rather than what’s become the keystone of guidelines writing, evidence-based medicine. Frequent reliance on consensus decisions rather than indisputable proof from controlled trials defines what some U.S. specialists see as a divide as wide as the Atlantic between the European and U.S. approaches to guideline writing.
“The guidelines from the ESC are articulated very well; they made their recommendations very clear. But a lot is consensus driven, without new data,” said Dr. Mariell L. Jessup, who serves as both vice chair of the panel currently revising the U.S. heart failure guidelines – expected out later in 2016 – and was also the sole American representative on the panel that produced the ESC guidelines. “The ESC guidelines make clear all the things that need to happen to patients. I hope it will result in better patient care. We are clearly not doing a good job in heart failure. We not only don’t have evidence-based treatments, but people often don’t do a good job [caring for heart failure patients] and they die in the hospital all the time.”
Dr. Javed Butler, another member of the U.S. guidelines panel and professor and chief of cardiology at Stony Brook (N.Y.) University, called the U.S. and European divide a “philosophical perspective of evidence-based medicine.
“U.S. physicians should read the ESC guidelines and make up their own minds. The ESC guidelines are excellent and give you perspective. But U.S. regulatory and payment issues will be driven by U.S. guidelines,” Dr. Butler said in an interview.
But despite their limitations and the limited weight that the ESC guidelines carry for U.S. practice, they have many redeeming features, noted Dr. Mandeep R. Mehra, medical director of the Heart and Vascular Center at Brigham and Women’s Hospital in Boston. The 2016 ESC guidelines “are extraordinarily clear, very practical, and very concise. They are very usable, and provide a fantastic algorithm for managing patients with heart failure with reduced ejection fraction [HFrEF],” he said while discussing the guidelines during the meeting.
U.S. and Europe largely agree on sacubitril/valsartan and ivabradine
Clearly the greatest area of U.S. and European agreement was in the adoption by both guidelines groups of sacubitril/valsartan (Entresto) and ivabradine (Corlanor) as important new components of the basic drug formula for treating patients with HFrEF. In fact, the U.S. guideline writers saw these two additions as so important and timely that they issued a “focused update” in May to the existing, 2013 U.S. heart failure guidelines, and timed release of this update to occur on May 20, 2016, a day before release of the ESC guidelines. But as Dr. Butler noted, this was more of a temporal harmonization than a substantive one, because even here, in a very evidence-based change, the U.S. guidelines for using sacubitril/valsartan differed subtly but importantly from the ESC version.
The U.S. focused update says that treatment of patients with stage C (symptomatic heart failure with structural heart disease) HFrEF should receive treatment with sacubitril/valsartan (also know as an angiotensin receptor neprilysin inhibitor, or ARNI), an ACE inhibitor, or an angiotensin receptor blocker (ARB), as well as evidence-based treatment with a beta-blocker and with a mineralocorticoid receptor antagonist (MRA). A subsequent recommendation in the U.S. focused update said that HFrEF patients with chronic symptoms and New York Heart Association class II or III disease should switch from a stable, tolerated regimen with either an ACE inhibitor or ARB to treatment with sacubitril/valsartan.
In contrast, the new European guideline for sacubitril/valsartan recommends starting patients on this combination formulation only after first demonstrating that patients tolerated treatment with an ACE inhibitor or ARB for at least 30 days and determining that patients remained symptomatic while on one of these treatments. In short, the U.S. guideline gives a green light to starting patients with newly diagnosed, symptomatic HFrEF on sacubitril/valsartan immediately, while the European guideline only sanctions sacubitril/valsartan to start after a patient has spent at least 30 days settling into a multidrug regimen featuring an ACE inhibitor or an ARB when an ACE inhibitor isn’t well tolerated.
“The European guidelines are closely related to the study population enrolled in the PARADIGM-HF trial,” the pivotal trial that showed superiority of sacubitril/valsartan to an ACE inhibitor (N Engl J Med. 2014;371:993-1004), noted Dr. Butler in an interview. “The U.S. guidelines interpreted [the PARADIGM-HF] results in the best interests of a larger patient population. The European guidelines are far more proscriptive in replicating the clinical criteria of the trial. In some patients the sequence of starting a MRA and sacubitril/valsartan matters, but in other patients it is less important.”
Dr. Frank Ruschitzka, a coauthor of the ESC guidelines, said that the reason for the more cautious ESC approach was lack of widespread familiarity with sacubitril/valsartan treatment among cardiologists.
The ESC guidelines on using sacubitril/valsartan “replicated the PARADIGM-HF trial. We have no data right now that it is justifiable to put a [treatment-naive] patient on sacubitril/valsartan to begin with. Another difference between the U.S. and ESC guidelines is when to start a MRA,” said Dr. Ruschitzka, professor and head of cardiology at the Heart Center of the University Hospital in Zurich. “It makes a lot of sense to me to start sacubitril/valsartan early. The PARADIGM trial was positive, but no one has a feel for how to use sacubitril/valsartan. Should we give it to everyone? We said replicate the trial, and gain experience using the drug. We want to bring a life-saving drug to patients, but this is the approach we took. We need more data.”
Dr. Jessup noted that a lot of uncertainty also exists among U.S. clinicians about when to start sacubitril/valsartan. “It’s not been clear which patients to put on sacubitril/valsartan. No guidelines had been out on using it” until mid-May, and “the cost of sacubitril/valsartan is daunting. I have received calls from many people who ask whom am I supposed to use sacubitril/valsartan on? It took years and years to get people to [routinely] start patients on an ACE inhibitor and a beta-blocker, and now we’re telling them to do something else. In my practice it’s a 30-minute conversation with each patient that you need to first stop your ACE inhibitor, and then they often get denied coverage by their insurer,” said Dr. Jessup, professor of medicine at the University of Pennsylvania in Philadelphia. She expressed hope that coverage issues will diminish now that clear guidelines are out endorsing a key role for sacubitril/valsartan.
“We now all have started sacubitril/valsartan on patients” without first starting them on an ACE inhibitor, “but we all need to get a sense of what we can get away with” when using this drug, noted Dr. JoAnn Lindenfeld, professor and director of heart failure and transplant at Vanderbilt University in Nashville.
At least one European cardiologist was skeptical of just how proscriptive the ESC guideline for sacubitril/valsartan will be in actual practice.
“The best treatment [for symptomatic HFrEF] is sacubitril/valsartan, a beta-blocker, and a MRA,” said Dr. John J.V. McMurray, professor of cardiology at Glasgow University and lead investigator for the PARADIGM-HF pivotal trial for sacubitril/valsartan. “The treatment sequence advocated in the guidelines – treat with an ACE inhibitor first and if patients remain symptomatic change to sacubitril/valsartan – is evidence-based medicine. As a guidelines writer and as a promoter of evidence-based medicine, this is absolutely the correct approach. But as a practicing physician I’d go straight for sacubitril/valsartan. Otherwise you’re wasting everybody’s time starting with an ACE inhibitor and then waiting a month to switch,” Dr. McMurray said in an interview.
“It’s pointless to wait. We saw results within 30 days of starting sacubitril/valsartan, so it’s a theoretical risk to wait. Very few patients will become completely asymptomatic on an ACE inhibitor. Everyone who entered PARADIGM-HF was at New York Heart Association class II or higher, and at the time of randomization only a handful of patients were in New York Heart Association class I. Very few patients get to class I. That tells you it’s pretty uncommon for a heart failure patient to become truly asymptomatic with ACE inhibitor treatment. The main problem is that you are inconveniencing everybody with more blood tests and more clinic visits by waiting to start sacubitril/valsartan, said Dr. McMurray, who was not a member of the panel that wrote the new ESC guidelines.
Even less separates the new U.S. focused update and the ESC guidelines for using ivabradine. Both agree on starting the drug on HFrEF patients who remain symptomatic and with a left ventricular ejection fraction of 35% or less despite being on guideline-directed therapy including titration to a maximum beta-blocker dosage and with a persistent heart rate of at least 70 beats/min. The goal of ivabradine treatment is to further reduce heart rate beyond what’s achieved by a maximal beta-blocker dosage.
Perhaps the biggest questions about ivabradine have been why it took so long to enter the U.S. guidelines, and why it is listed in both the U.S. and ESC guidelines as a level II recommendation. Results from the pivotal trial that supported ivabradine’s use in HFrEF patients, SHIFT, first appeared in 2010 (Lancet. 2010 Sep 11;376[9744]:875-95).
Dr. Butler chalked up the drug’s slow entry into U.S. guidelines as the result of a lack of initiative by ivabradine’s initial developer, Servier. “SHIFT did not have any U.S. sites, and Servier never sought Food and Drug Administration approval,” he noted. “Amgen acquired the U.S. rights to ivabradine in 2013,” and the drug received FDA approval in April 2015, Dr. Butler noted, in explaining the drug’s U.S. timeline. As to why its use is a level II recommendation, he noted that the evidence for efficacy came only from the SHIFT trial, questions exist whether the beta-blocker dosages were fully optimized in all patients in this trial, and the benefit was limited to a reduction in heart failure hospitalizations but not in mortality. “I think that patients with persistent heart failure symptoms [and a persistently elevated heart rate] should get ivabradine,” but these caveats limit it to a class II level recommendation, Dr. Butler said.
“There were questions about ivabradine’s benefit in reducing heart failure hospitalization but not mortality, and questions about whether it would benefit patients if their beta-blocker dosage was adequately up titrated. There were also questions about which heart failure patients to use it on,” noted Dr. Lindenfeld, a member of the panel that wrote the U.S. focused update. These concerns in part help explain the delay to integrating ivabradine into U.S. practice guidelines, she said in an interview, but added that additional data and analysis published during the past 3 or so years have clarified ivabradine’s potentially useful role in treating selected HFrEF patients.
New ESC guidelines based on expert opinion
The sections on sacubitril/valsartan and ivabradine occupy a mere 2 pages among more than 55 pages of text and charts that spell out the ESC’s current vision of how physicians should diagnose and manage heart failure patients. While much of what carried over from prior editions of the guidelines is rooted in evidence, many of the new approaches advocated rely on expert opinion or new interpretations of existing data. Here are some of the notable changes and highlights of the 2016 ESC recommendations:
• Heart failure diagnosis. The new ESC guidelines streamline the diagnostic process, which now focuses on five key elements: The patient’s history, physical examination, ECG, serum level of either brain natriuretic peptide (BNP) or N-terminal(NT)-proBNP, and echocardiography. The guidelines specify threshold levels of BNP and NT-proBNP that can effectively rule out heart failure, a BNP level of at least 35 pg/mL or a NT-proBNP level of at least 125 pg/mL.
“The diagnostic minimum levels of BNP and NT-proBNP were designed to rule out heart failure. They both have a high negative predictive value, but at these levels their positive predictive value is low,” explained Dr. Adriaan A. Voors, cochair of the ESC’s guideline-writing panel and professor of cardiology at the University of Groningen, the Netherlands.
But while these levels might be effective for reliable rule out of heart failure, they could mean a large number of patients would qualify for an echocardiographic assessment.
“If we used the ESC’s natriuretic peptide cutoffs, there would be a clear concern about overuse of echo. It’s a cost-effectiveness issue. You wind up doing a lot of echos that will be normal. Echocardiography is very safe, but each echo costs about $400-$500,” commented Dr. Butler.
“The results from the STOP-HF and PONTIAC studies showed that BNP levels can identify people at increased risk for developing heart failure who need more intensive assessment and could also potentially benefit from more attention to heart failure prevention. I suspect the full U.S. guideline update will address this issue, but we have not yet finalized our decisions,” he added.
• Heart failure classification. The new ESC guidelines created a new heart failure category, midrange ejection fraction, that the writing panel positioned squarely between the two more classic heart failure subgroups, HFrEF and heart failure with preserved ejection fraction (HFpEF). The definition of each of the three subgroups depends on left ventricular ejection fraction as determined by echocardiography: A LVEF of less than 40% defined HFrEF, a LVEF of 40%-49% defined heart failure with midrange ejection fraction (HFmrEF), and a LVEF of 50% or higher defined HFpEF. Diagnostic confirmation of both HFmrEF and HFpEF also requires that patients fulfill certain criteria of structural or functional heart abnormalities.
The category of HFmrEF was created “to stimulate research into how to best manage these patients,” explained Dr. Piotr Ponikowski, chair of the ESC guidelines writing panel. For the time being, it remains a category with only theoretical importance as nothing is known to suggest that management of patients with HFmrEF should in any way differ from patients with HFpEF.
• Acute heart failure. Perhaps the most revolutionary element of the new guidelines is the detailed map they provide to managing patients who present with acute decompensated heart failure and the underlying principles cited to justify this radically different approach.
“The acute heart failure section was completely rewritten,” noted Dr. Ponikowski, professor of heart diseases at the Medical University in Wroclaw, Poland. “We don’t yet have evidence-based treatments” to apply to acute heart failure patients, he admitted, “however we strongly recommend the concept that the shorter the better. Shorten the time of diagnosis and for therapeutic decisions. We have borrowed from acute coronary syndrome. Don’t keep patients in the emergency department for another couple of hours just to see if they will respond. We must be aware that we need to do our best to shorten diagnosis and treatment decisions. Time is an issue. Manage a patient’s congestion and impaired peripheral perfusion within a time frame of 1-2 hours.”
The concept that acute heart failure must be quickly managed as an emergency condition similar to acute coronary syndrome first appeared as a European practice recommendation in 2015, a consensus statement from the European Heart Failure Association and two other collaborating organizations (Eur Heart J. 2015 Aug 7;36[30]:1958-66).
“In 2015, the consensus paper talked about how to handle acute heart failure patients in the emergency department. Now, we have focused on defining the patients’ phenotype and how to categorize their treatment options. We built on the 2015 statement, but the algorithms we now have are original to 2016; they were not in the 2015 paper,” said Dr. Veli-Pekka Harjola, a member of the 2015 consensus group and 2016 guidelines panel who spearheaded writing the acute heart failure section of the new ESC guidelines.
An additional new and notable feature of this section in the 2016 guidelines is its creation of an acronym, CHAMP, designed to guide the management of patients with acute heart failure. CHAMP stands for acute Coronary syndrome, Hypertension emergency, Arrhythmia, acute Mechanical cause, and Pulmonary embolism. The CHAMP acronym’s purpose is to “focus attention on these five specific, potential causes of acute heart failure, life-threatening conditions that need immediate treatment,” explained Dr. Ponikowski.
“CHAMP emphasizes the most critical causes of acute heart failure,” added Dr. Harjola, a cardiologist at Helsinki University Central Hospital. “We created this new acronym” to help clinicians keep in mind what to look for in a patient presenting with acute heart failure.
U.S. cardiologists find things to like in what the Europeans say about managing acute heart failure, as well as aspects to question.
“It makes no sense not to aggressively treat a patient who arrives at an emergency department with acute heart failure. But there is a difference between acute MI or stroke and acute heart failure,” said Dr. Butler. “In acute MI there is the ruptured plaque and thrombus that blocks a coronary artery. In stroke there is a thrombus. These are diseases with a specific onset and treatment target. But with acute heart failure we don’t have a thrombus to treat; we don’t have a specific target. What we’ve learned from studying implanted devices [such as CardioMems] is that the congestion that causes acute heart failure can start 2-3 weeks before a patient develops acute decompensated heart failure and goes to the hospital. We have not found a specific pathophysiologic abnormality in the patient with acute heart failure that is any different from chronic heart failure. This begs the question: If a patient who presents with acute heart failure has a congestion process that’s been going on for 2 or 3 weeks what difference will another 3 hours make? Do we need to replicate the concept of an acute stroke team or acute MI response for acute heart failure?”
Dr. Butler stressed that additional data are expected soon that may help clarify this issue.
“Some large outcome trials in patients with acute heart failure are now underway, one testing serelaxin treatment, another testing ularitide treatment, that are also testing the hypothesis that rapid treatment with these drugs can produce more end-organ protection, stop damage to the heart, kidney and liver, and lead to better long-term outcomes. Until we have those data, the jury is still out” on the benefit patients gain from rapid treatment of acute heart failure. “Until then, it’s not that the data say don’t treat acute heart failure patients aggressively. But we have not yet proven it is similar to treating an acute MI or stroke,” said Dr. Butler.
“U.S. guidelines have tended to stay away from areas where there are no evidence-based data. To their credit, the Europeans will take on something like acute heart failure where we don’t have an adequate evidence base. Despite that, they provide guidelines, which is important because clinicians need guidance even when the evidence is not very good, when the guideline is based mostly on experience and expert consensus.” commented Dr. William T. Abraham, professor and director of cardiovascular medicine at the Ohio State University Wexner Medical Center in Columbus.
“It’s absolutely appropriate to think of acute heart failure as an emergency situation. We know from high-sensitivity troponin assays that troponin levels are increased in 90% of patients who present with acute decompensated heart failure. So most acute heart failure patients are losing heart muscle cells and we should treat them like we treat acute coronary syndrome. Time matters in acute heart failure; time is heart muscle. Treatment needs to break the hemodynamic and neurohormonal storm of acute decompensated heart failure; get the patient stabilized; improve vital organ perfusion, including within the heart; and shift the myocardial oxygen supply and demand equation so myocardial necrosis stops. All of this is important, and study results suggest it’s the correct approach. I’m not sure study results prove it, but studies that have looked at the time course of treatment for acute heart failure showed that early initiation of treatment – within the first 6 hours of onset – compared with 12-24 hours of onset makes a difference in outcomes,” Dr. Abraham said in an interview.
But a major limitation to the potential efficacy of a rapidly initiated management strategy is that few interventions currently exist with proven benefits for acute heart failure patients.
For the time being, rapid intervention means using diuretics relatively quickly and, if there is an indication for treating with a vasoactive medication, using that quickly too. “The rapid approach is really more relevant to the future; it’s relevant to the design of future acute heart failure treatment trials. That is where this early treatment paradigm is important,” as it could potentially apply to new, more effective treatments of the future rather than to the marginally effective treatments now available, Dr. Abraham said.
“For a long we time haven’t pushed how quickly we should act when implementing guideline-directed treatment” for patients with acute heart failure, noted Dr. Mehra. “The CHAMP approach is interesting, and the ESC guidelines are a very interesting move in the direction” of faster action. “They speak to the period of time during which one should act. Hopefully this will help the science of acute decompensated heart failure move forward.”
But for other U.S. experts the issue again pivots on the lack of evidence.
“There is nothing new” about managing acute heart failure, said Dr. Jessup. “The ESC guideline was articulated very well; they made their recommendations very clear. But a lot is consensus driven. There are no new data. The problem with acute heart failure is that the recommendations are what we think clinicians should do. CHAMP is a nice acronym; it’s packaged better, but there are not any new data.”
Comorbidities
A dramatic contrast distinguishes the extent to which the ESC guidelines highlight comorbidities, compared with prevailing U.S. guidelines. The new ESC guidelines highlight and discuss with some detail 16 distinct comorbidities for clinicians to keep in mind when managing heart failure patients, compared with three comorbidities (atrial fibrillation, anemia, and depression) discussed with similar detail in the 2013 U.S. guidelines.
“We are targeting comorbidities to personalize medicine, by subgrouping [heart failure] patients into groups that need to receive special attention,” explained Dr. Stefan D. Anker, a coauthor on the ESC guidelines. “We care about comorbidities because they make the diagnosis of heart failure difficult. They aggravate symptoms and contribute to additional hospitalizations. They interfere with [heart failure] treatment, and because comorbidities have led to exclusions of heart failure patients from trials, we lack evidence of treatment efficacy in patients with certain comorbidities,” said Dr. Anker, a professor of innovative clinical trials at the Medical University in Göttingen, Germany.
“The comorbidity discussion in the ESC guidelines is extremely important,” commented Dr. Abraham. “It supports the need for a multidimensional approach to heart failure patients. A cardiologist may not have all the resources to manage all the comorbidities [a heart failure patient might have]. This is why having a sleep medicine specialist, a diabetes specialist, a nephrologist, etc., involved as part of a heart failure management team can be very valuable. We need to involve subspecialists in managing the comorbidities of heart failure because they clearly have an impact on patient outcome.”
But Dr. Butler had a somewhat different take on how comorbidity management fits into the broader picture of heart failure management.
“There is no doubt that heart failure worsens other comorbidities and other comorbidities worsen heart failure. The relationship is bidirectional between heart failure and chronic obstructive pulmonary disease, liver disease, depression, sleep apnea, renal disease, lung disease, diabetes, etc. The problem is that treating a comorbidity does not necessarily translate into improved heart failure outcomes. Comorbidities are important for heart failure patients and worsen their heart failure outcomes. However, management of a comorbidity should be done primarily for the sake of improving the comorbidity. If you treat depression, for example, and it does not improve a patient’s heart failure, that doesn’t mean you shouldn’t have treated the depression. It just means that we don’t have good data that it will improve heart failure.”
Another limitation from a U.S. perspective is what role treatment of various comorbidities can play in benefiting heart failure patients and how compelling the evidence is for this. Dr. Butler gave as an example the problem with treating iron deficiency in heart failure patients who do not have anemia, a strategy endorsed in the ESC guidelines as a level IIa recommendation.
“The data regarding improved exercise capacity from treatment with intravenous ferric carboxymaltose is pretty convincing,” he said. But patients have benefited from this treatment only with improved function and quality of life, and not with improved survival or fewer hospitalizations.
“Is treating patients to improve their function and help them feel better enough?” Dr. Butler asked. “In other diseases it is. In gastrointestinal disease, if a drug helps patients feel better you approve the drug. We value improved functional capacity for patients with pulmonary hypertension, angina, and peripheral vascular disease. All these indications have drugs approved for improving functional capacity and quality of life. But for heart failure the bar has been set higher. There is a lot of interest in changing this” for heart failure.
“There is interest in running a study of ferric carboxymaltose for heart failure with a mortality endpoint. In the meantime, the impact on improving functional capacity is compelling, and it will be interesting to see what happens in the U.S. guidelines. Currently, in U.S. practice if a heart failure patient has iron-deficiency anemia you treat with intravenous iron replacement and the treatment gets reimbursed without a problem. But if the heart failure patient has iron deficiency without anemia then reimbursement for the cost of iron supplementation can be a problem,” Dr. Butler noted. This may change only if the experts who write the next U.S. heart failure guidelines decide to change the rules of what constitutes a useful heart failure treatment, he said.
Dr. Butler has been a consultant to Novartis and Amgen and several other companies. Dr. Jessup had no disclosures. Dr. Mehra has been a consultant to Teva, Johnson & Johnson, Boston Scientific, and St. Jude. Dr. Ruschitzka has been a consultant to Novartis, Servier, Sanofi, Cardiorentis, Heartware, and St. Jude. Dr. McMurray has received research support from Novartis and Amgen. Dr. Lindenfeld has been a consultant to Novartis, Abbott, Janssen, Relypsa, and Resmed. Dr. Voors has been a consultant to Novartis, Amgen, Servier, and several other drug companies. Dr. Ponikowski has been a consultant to Amgen, Novartis, Servier, and several other drug companies. Dr. Harjola has been a consultant to Novartis, Servier, Bayer, Boehringer Ingelheim, Pfizer, and Resmed. Dr. Abraham has been a consultant to Amgen, Novartis, and several device companies. Dr. Anker has been a consultant to Novartis, Servier, and several other companies.
On Twitter @mitchelzoler
FLORENCE, ITALY – The 2016 revision of the European Society of Cardiology’s guidelines for diagnosing and treating acute and chronic heart failure highlights the extent to which thinking in the field has changed during the past 4 years, since the prior edition in 2012.
The new European guidelines, unveiled by the ESC’s Heart Failure Association during the group’s annual meeting, also underscore the great dependence that many new approaches have on expert opinion rather than what’s become the keystone of guidelines writing, evidence-based medicine. Frequent reliance on consensus decisions rather than indisputable proof from controlled trials defines what some U.S. specialists see as a divide as wide as the Atlantic between the European and U.S. approaches to guideline writing.
“The guidelines from the ESC are articulated very well; they made their recommendations very clear. But a lot is consensus driven, without new data,” said Dr. Mariell L. Jessup, who serves as both vice chair of the panel currently revising the U.S. heart failure guidelines – expected out later in 2016 – and was also the sole American representative on the panel that produced the ESC guidelines. “The ESC guidelines make clear all the things that need to happen to patients. I hope it will result in better patient care. We are clearly not doing a good job in heart failure. We not only don’t have evidence-based treatments, but people often don’t do a good job [caring for heart failure patients] and they die in the hospital all the time.”
Dr. Javed Butler, another member of the U.S. guidelines panel and professor and chief of cardiology at Stony Brook (N.Y.) University, called the U.S. and European divide a “philosophical perspective of evidence-based medicine.
“U.S. physicians should read the ESC guidelines and make up their own minds. The ESC guidelines are excellent and give you perspective. But U.S. regulatory and payment issues will be driven by U.S. guidelines,” Dr. Butler said in an interview.
But despite their limitations and the limited weight that the ESC guidelines carry for U.S. practice, they have many redeeming features, noted Dr. Mandeep R. Mehra, medical director of the Heart and Vascular Center at Brigham and Women’s Hospital in Boston. The 2016 ESC guidelines “are extraordinarily clear, very practical, and very concise. They are very usable, and provide a fantastic algorithm for managing patients with heart failure with reduced ejection fraction [HFrEF],” he said while discussing the guidelines during the meeting.
U.S. and Europe largely agree on sacubitril/valsartan and ivabradine
Clearly the greatest area of U.S. and European agreement was in the adoption by both guidelines groups of sacubitril/valsartan (Entresto) and ivabradine (Corlanor) as important new components of the basic drug formula for treating patients with HFrEF. In fact, the U.S. guideline writers saw these two additions as so important and timely that they issued a “focused update” in May to the existing, 2013 U.S. heart failure guidelines, and timed release of this update to occur on May 20, 2016, a day before release of the ESC guidelines. But as Dr. Butler noted, this was more of a temporal harmonization than a substantive one, because even here, in a very evidence-based change, the U.S. guidelines for using sacubitril/valsartan differed subtly but importantly from the ESC version.
The U.S. focused update says that treatment of patients with stage C (symptomatic heart failure with structural heart disease) HFrEF should receive treatment with sacubitril/valsartan (also know as an angiotensin receptor neprilysin inhibitor, or ARNI), an ACE inhibitor, or an angiotensin receptor blocker (ARB), as well as evidence-based treatment with a beta-blocker and with a mineralocorticoid receptor antagonist (MRA). A subsequent recommendation in the U.S. focused update said that HFrEF patients with chronic symptoms and New York Heart Association class II or III disease should switch from a stable, tolerated regimen with either an ACE inhibitor or ARB to treatment with sacubitril/valsartan.
In contrast, the new European guideline for sacubitril/valsartan recommends starting patients on this combination formulation only after first demonstrating that patients tolerated treatment with an ACE inhibitor or ARB for at least 30 days and determining that patients remained symptomatic while on one of these treatments. In short, the U.S. guideline gives a green light to starting patients with newly diagnosed, symptomatic HFrEF on sacubitril/valsartan immediately, while the European guideline only sanctions sacubitril/valsartan to start after a patient has spent at least 30 days settling into a multidrug regimen featuring an ACE inhibitor or an ARB when an ACE inhibitor isn’t well tolerated.
“The European guidelines are closely related to the study population enrolled in the PARADIGM-HF trial,” the pivotal trial that showed superiority of sacubitril/valsartan to an ACE inhibitor (N Engl J Med. 2014;371:993-1004), noted Dr. Butler in an interview. “The U.S. guidelines interpreted [the PARADIGM-HF] results in the best interests of a larger patient population. The European guidelines are far more proscriptive in replicating the clinical criteria of the trial. In some patients the sequence of starting a MRA and sacubitril/valsartan matters, but in other patients it is less important.”
Dr. Frank Ruschitzka, a coauthor of the ESC guidelines, said that the reason for the more cautious ESC approach was lack of widespread familiarity with sacubitril/valsartan treatment among cardiologists.
The ESC guidelines on using sacubitril/valsartan “replicated the PARADIGM-HF trial. We have no data right now that it is justifiable to put a [treatment-naive] patient on sacubitril/valsartan to begin with. Another difference between the U.S. and ESC guidelines is when to start a MRA,” said Dr. Ruschitzka, professor and head of cardiology at the Heart Center of the University Hospital in Zurich. “It makes a lot of sense to me to start sacubitril/valsartan early. The PARADIGM trial was positive, but no one has a feel for how to use sacubitril/valsartan. Should we give it to everyone? We said replicate the trial, and gain experience using the drug. We want to bring a life-saving drug to patients, but this is the approach we took. We need more data.”
Dr. Jessup noted that a lot of uncertainty also exists among U.S. clinicians about when to start sacubitril/valsartan. “It’s not been clear which patients to put on sacubitril/valsartan. No guidelines had been out on using it” until mid-May, and “the cost of sacubitril/valsartan is daunting. I have received calls from many people who ask whom am I supposed to use sacubitril/valsartan on? It took years and years to get people to [routinely] start patients on an ACE inhibitor and a beta-blocker, and now we’re telling them to do something else. In my practice it’s a 30-minute conversation with each patient that you need to first stop your ACE inhibitor, and then they often get denied coverage by their insurer,” said Dr. Jessup, professor of medicine at the University of Pennsylvania in Philadelphia. She expressed hope that coverage issues will diminish now that clear guidelines are out endorsing a key role for sacubitril/valsartan.
“We now all have started sacubitril/valsartan on patients” without first starting them on an ACE inhibitor, “but we all need to get a sense of what we can get away with” when using this drug, noted Dr. JoAnn Lindenfeld, professor and director of heart failure and transplant at Vanderbilt University in Nashville.
At least one European cardiologist was skeptical of just how proscriptive the ESC guideline for sacubitril/valsartan will be in actual practice.
“The best treatment [for symptomatic HFrEF] is sacubitril/valsartan, a beta-blocker, and a MRA,” said Dr. John J.V. McMurray, professor of cardiology at Glasgow University and lead investigator for the PARADIGM-HF pivotal trial for sacubitril/valsartan. “The treatment sequence advocated in the guidelines – treat with an ACE inhibitor first and if patients remain symptomatic change to sacubitril/valsartan – is evidence-based medicine. As a guidelines writer and as a promoter of evidence-based medicine, this is absolutely the correct approach. But as a practicing physician I’d go straight for sacubitril/valsartan. Otherwise you’re wasting everybody’s time starting with an ACE inhibitor and then waiting a month to switch,” Dr. McMurray said in an interview.
“It’s pointless to wait. We saw results within 30 days of starting sacubitril/valsartan, so it’s a theoretical risk to wait. Very few patients will become completely asymptomatic on an ACE inhibitor. Everyone who entered PARADIGM-HF was at New York Heart Association class II or higher, and at the time of randomization only a handful of patients were in New York Heart Association class I. Very few patients get to class I. That tells you it’s pretty uncommon for a heart failure patient to become truly asymptomatic with ACE inhibitor treatment. The main problem is that you are inconveniencing everybody with more blood tests and more clinic visits by waiting to start sacubitril/valsartan, said Dr. McMurray, who was not a member of the panel that wrote the new ESC guidelines.
Even less separates the new U.S. focused update and the ESC guidelines for using ivabradine. Both agree on starting the drug on HFrEF patients who remain symptomatic and with a left ventricular ejection fraction of 35% or less despite being on guideline-directed therapy including titration to a maximum beta-blocker dosage and with a persistent heart rate of at least 70 beats/min. The goal of ivabradine treatment is to further reduce heart rate beyond what’s achieved by a maximal beta-blocker dosage.
Perhaps the biggest questions about ivabradine have been why it took so long to enter the U.S. guidelines, and why it is listed in both the U.S. and ESC guidelines as a level II recommendation. Results from the pivotal trial that supported ivabradine’s use in HFrEF patients, SHIFT, first appeared in 2010 (Lancet. 2010 Sep 11;376[9744]:875-95).
Dr. Butler chalked up the drug’s slow entry into U.S. guidelines as the result of a lack of initiative by ivabradine’s initial developer, Servier. “SHIFT did not have any U.S. sites, and Servier never sought Food and Drug Administration approval,” he noted. “Amgen acquired the U.S. rights to ivabradine in 2013,” and the drug received FDA approval in April 2015, Dr. Butler noted, in explaining the drug’s U.S. timeline. As to why its use is a level II recommendation, he noted that the evidence for efficacy came only from the SHIFT trial, questions exist whether the beta-blocker dosages were fully optimized in all patients in this trial, and the benefit was limited to a reduction in heart failure hospitalizations but not in mortality. “I think that patients with persistent heart failure symptoms [and a persistently elevated heart rate] should get ivabradine,” but these caveats limit it to a class II level recommendation, Dr. Butler said.
“There were questions about ivabradine’s benefit in reducing heart failure hospitalization but not mortality, and questions about whether it would benefit patients if their beta-blocker dosage was adequately up titrated. There were also questions about which heart failure patients to use it on,” noted Dr. Lindenfeld, a member of the panel that wrote the U.S. focused update. These concerns in part help explain the delay to integrating ivabradine into U.S. practice guidelines, she said in an interview, but added that additional data and analysis published during the past 3 or so years have clarified ivabradine’s potentially useful role in treating selected HFrEF patients.
New ESC guidelines based on expert opinion
The sections on sacubitril/valsartan and ivabradine occupy a mere 2 pages among more than 55 pages of text and charts that spell out the ESC’s current vision of how physicians should diagnose and manage heart failure patients. While much of what carried over from prior editions of the guidelines is rooted in evidence, many of the new approaches advocated rely on expert opinion or new interpretations of existing data. Here are some of the notable changes and highlights of the 2016 ESC recommendations:
• Heart failure diagnosis. The new ESC guidelines streamline the diagnostic process, which now focuses on five key elements: The patient’s history, physical examination, ECG, serum level of either brain natriuretic peptide (BNP) or N-terminal(NT)-proBNP, and echocardiography. The guidelines specify threshold levels of BNP and NT-proBNP that can effectively rule out heart failure, a BNP level of at least 35 pg/mL or a NT-proBNP level of at least 125 pg/mL.
“The diagnostic minimum levels of BNP and NT-proBNP were designed to rule out heart failure. They both have a high negative predictive value, but at these levels their positive predictive value is low,” explained Dr. Adriaan A. Voors, cochair of the ESC’s guideline-writing panel and professor of cardiology at the University of Groningen, the Netherlands.
But while these levels might be effective for reliable rule out of heart failure, they could mean a large number of patients would qualify for an echocardiographic assessment.
“If we used the ESC’s natriuretic peptide cutoffs, there would be a clear concern about overuse of echo. It’s a cost-effectiveness issue. You wind up doing a lot of echos that will be normal. Echocardiography is very safe, but each echo costs about $400-$500,” commented Dr. Butler.
“The results from the STOP-HF and PONTIAC studies showed that BNP levels can identify people at increased risk for developing heart failure who need more intensive assessment and could also potentially benefit from more attention to heart failure prevention. I suspect the full U.S. guideline update will address this issue, but we have not yet finalized our decisions,” he added.
• Heart failure classification. The new ESC guidelines created a new heart failure category, midrange ejection fraction, that the writing panel positioned squarely between the two more classic heart failure subgroups, HFrEF and heart failure with preserved ejection fraction (HFpEF). The definition of each of the three subgroups depends on left ventricular ejection fraction as determined by echocardiography: A LVEF of less than 40% defined HFrEF, a LVEF of 40%-49% defined heart failure with midrange ejection fraction (HFmrEF), and a LVEF of 50% or higher defined HFpEF. Diagnostic confirmation of both HFmrEF and HFpEF also requires that patients fulfill certain criteria of structural or functional heart abnormalities.
The category of HFmrEF was created “to stimulate research into how to best manage these patients,” explained Dr. Piotr Ponikowski, chair of the ESC guidelines writing panel. For the time being, it remains a category with only theoretical importance as nothing is known to suggest that management of patients with HFmrEF should in any way differ from patients with HFpEF.
• Acute heart failure. Perhaps the most revolutionary element of the new guidelines is the detailed map they provide to managing patients who present with acute decompensated heart failure and the underlying principles cited to justify this radically different approach.
“The acute heart failure section was completely rewritten,” noted Dr. Ponikowski, professor of heart diseases at the Medical University in Wroclaw, Poland. “We don’t yet have evidence-based treatments” to apply to acute heart failure patients, he admitted, “however we strongly recommend the concept that the shorter the better. Shorten the time of diagnosis and for therapeutic decisions. We have borrowed from acute coronary syndrome. Don’t keep patients in the emergency department for another couple of hours just to see if they will respond. We must be aware that we need to do our best to shorten diagnosis and treatment decisions. Time is an issue. Manage a patient’s congestion and impaired peripheral perfusion within a time frame of 1-2 hours.”
The concept that acute heart failure must be quickly managed as an emergency condition similar to acute coronary syndrome first appeared as a European practice recommendation in 2015, a consensus statement from the European Heart Failure Association and two other collaborating organizations (Eur Heart J. 2015 Aug 7;36[30]:1958-66).
“In 2015, the consensus paper talked about how to handle acute heart failure patients in the emergency department. Now, we have focused on defining the patients’ phenotype and how to categorize their treatment options. We built on the 2015 statement, but the algorithms we now have are original to 2016; they were not in the 2015 paper,” said Dr. Veli-Pekka Harjola, a member of the 2015 consensus group and 2016 guidelines panel who spearheaded writing the acute heart failure section of the new ESC guidelines.
An additional new and notable feature of this section in the 2016 guidelines is its creation of an acronym, CHAMP, designed to guide the management of patients with acute heart failure. CHAMP stands for acute Coronary syndrome, Hypertension emergency, Arrhythmia, acute Mechanical cause, and Pulmonary embolism. The CHAMP acronym’s purpose is to “focus attention on these five specific, potential causes of acute heart failure, life-threatening conditions that need immediate treatment,” explained Dr. Ponikowski.
“CHAMP emphasizes the most critical causes of acute heart failure,” added Dr. Harjola, a cardiologist at Helsinki University Central Hospital. “We created this new acronym” to help clinicians keep in mind what to look for in a patient presenting with acute heart failure.
U.S. cardiologists find things to like in what the Europeans say about managing acute heart failure, as well as aspects to question.
“It makes no sense not to aggressively treat a patient who arrives at an emergency department with acute heart failure. But there is a difference between acute MI or stroke and acute heart failure,” said Dr. Butler. “In acute MI there is the ruptured plaque and thrombus that blocks a coronary artery. In stroke there is a thrombus. These are diseases with a specific onset and treatment target. But with acute heart failure we don’t have a thrombus to treat; we don’t have a specific target. What we’ve learned from studying implanted devices [such as CardioMems] is that the congestion that causes acute heart failure can start 2-3 weeks before a patient develops acute decompensated heart failure and goes to the hospital. We have not found a specific pathophysiologic abnormality in the patient with acute heart failure that is any different from chronic heart failure. This begs the question: If a patient who presents with acute heart failure has a congestion process that’s been going on for 2 or 3 weeks what difference will another 3 hours make? Do we need to replicate the concept of an acute stroke team or acute MI response for acute heart failure?”
Dr. Butler stressed that additional data are expected soon that may help clarify this issue.
“Some large outcome trials in patients with acute heart failure are now underway, one testing serelaxin treatment, another testing ularitide treatment, that are also testing the hypothesis that rapid treatment with these drugs can produce more end-organ protection, stop damage to the heart, kidney and liver, and lead to better long-term outcomes. Until we have those data, the jury is still out” on the benefit patients gain from rapid treatment of acute heart failure. “Until then, it’s not that the data say don’t treat acute heart failure patients aggressively. But we have not yet proven it is similar to treating an acute MI or stroke,” said Dr. Butler.
“U.S. guidelines have tended to stay away from areas where there are no evidence-based data. To their credit, the Europeans will take on something like acute heart failure where we don’t have an adequate evidence base. Despite that, they provide guidelines, which is important because clinicians need guidance even when the evidence is not very good, when the guideline is based mostly on experience and expert consensus.” commented Dr. William T. Abraham, professor and director of cardiovascular medicine at the Ohio State University Wexner Medical Center in Columbus.
“It’s absolutely appropriate to think of acute heart failure as an emergency situation. We know from high-sensitivity troponin assays that troponin levels are increased in 90% of patients who present with acute decompensated heart failure. So most acute heart failure patients are losing heart muscle cells and we should treat them like we treat acute coronary syndrome. Time matters in acute heart failure; time is heart muscle. Treatment needs to break the hemodynamic and neurohormonal storm of acute decompensated heart failure; get the patient stabilized; improve vital organ perfusion, including within the heart; and shift the myocardial oxygen supply and demand equation so myocardial necrosis stops. All of this is important, and study results suggest it’s the correct approach. I’m not sure study results prove it, but studies that have looked at the time course of treatment for acute heart failure showed that early initiation of treatment – within the first 6 hours of onset – compared with 12-24 hours of onset makes a difference in outcomes,” Dr. Abraham said in an interview.
But a major limitation to the potential efficacy of a rapidly initiated management strategy is that few interventions currently exist with proven benefits for acute heart failure patients.
For the time being, rapid intervention means using diuretics relatively quickly and, if there is an indication for treating with a vasoactive medication, using that quickly too. “The rapid approach is really more relevant to the future; it’s relevant to the design of future acute heart failure treatment trials. That is where this early treatment paradigm is important,” as it could potentially apply to new, more effective treatments of the future rather than to the marginally effective treatments now available, Dr. Abraham said.
“For a long we time haven’t pushed how quickly we should act when implementing guideline-directed treatment” for patients with acute heart failure, noted Dr. Mehra. “The CHAMP approach is interesting, and the ESC guidelines are a very interesting move in the direction” of faster action. “They speak to the period of time during which one should act. Hopefully this will help the science of acute decompensated heart failure move forward.”
But for other U.S. experts the issue again pivots on the lack of evidence.
“There is nothing new” about managing acute heart failure, said Dr. Jessup. “The ESC guideline was articulated very well; they made their recommendations very clear. But a lot is consensus driven. There are no new data. The problem with acute heart failure is that the recommendations are what we think clinicians should do. CHAMP is a nice acronym; it’s packaged better, but there are not any new data.”
Comorbidities
A dramatic contrast distinguishes the extent to which the ESC guidelines highlight comorbidities, compared with prevailing U.S. guidelines. The new ESC guidelines highlight and discuss with some detail 16 distinct comorbidities for clinicians to keep in mind when managing heart failure patients, compared with three comorbidities (atrial fibrillation, anemia, and depression) discussed with similar detail in the 2013 U.S. guidelines.
“We are targeting comorbidities to personalize medicine, by subgrouping [heart failure] patients into groups that need to receive special attention,” explained Dr. Stefan D. Anker, a coauthor on the ESC guidelines. “We care about comorbidities because they make the diagnosis of heart failure difficult. They aggravate symptoms and contribute to additional hospitalizations. They interfere with [heart failure] treatment, and because comorbidities have led to exclusions of heart failure patients from trials, we lack evidence of treatment efficacy in patients with certain comorbidities,” said Dr. Anker, a professor of innovative clinical trials at the Medical University in Göttingen, Germany.
“The comorbidity discussion in the ESC guidelines is extremely important,” commented Dr. Abraham. “It supports the need for a multidimensional approach to heart failure patients. A cardiologist may not have all the resources to manage all the comorbidities [a heart failure patient might have]. This is why having a sleep medicine specialist, a diabetes specialist, a nephrologist, etc., involved as part of a heart failure management team can be very valuable. We need to involve subspecialists in managing the comorbidities of heart failure because they clearly have an impact on patient outcome.”
But Dr. Butler had a somewhat different take on how comorbidity management fits into the broader picture of heart failure management.
“There is no doubt that heart failure worsens other comorbidities and other comorbidities worsen heart failure. The relationship is bidirectional between heart failure and chronic obstructive pulmonary disease, liver disease, depression, sleep apnea, renal disease, lung disease, diabetes, etc. The problem is that treating a comorbidity does not necessarily translate into improved heart failure outcomes. Comorbidities are important for heart failure patients and worsen their heart failure outcomes. However, management of a comorbidity should be done primarily for the sake of improving the comorbidity. If you treat depression, for example, and it does not improve a patient’s heart failure, that doesn’t mean you shouldn’t have treated the depression. It just means that we don’t have good data that it will improve heart failure.”
Another limitation from a U.S. perspective is what role treatment of various comorbidities can play in benefiting heart failure patients and how compelling the evidence is for this. Dr. Butler gave as an example the problem with treating iron deficiency in heart failure patients who do not have anemia, a strategy endorsed in the ESC guidelines as a level IIa recommendation.
“The data regarding improved exercise capacity from treatment with intravenous ferric carboxymaltose is pretty convincing,” he said. But patients have benefited from this treatment only with improved function and quality of life, and not with improved survival or fewer hospitalizations.
“Is treating patients to improve their function and help them feel better enough?” Dr. Butler asked. “In other diseases it is. In gastrointestinal disease, if a drug helps patients feel better you approve the drug. We value improved functional capacity for patients with pulmonary hypertension, angina, and peripheral vascular disease. All these indications have drugs approved for improving functional capacity and quality of life. But for heart failure the bar has been set higher. There is a lot of interest in changing this” for heart failure.
“There is interest in running a study of ferric carboxymaltose for heart failure with a mortality endpoint. In the meantime, the impact on improving functional capacity is compelling, and it will be interesting to see what happens in the U.S. guidelines. Currently, in U.S. practice if a heart failure patient has iron-deficiency anemia you treat with intravenous iron replacement and the treatment gets reimbursed without a problem. But if the heart failure patient has iron deficiency without anemia then reimbursement for the cost of iron supplementation can be a problem,” Dr. Butler noted. This may change only if the experts who write the next U.S. heart failure guidelines decide to change the rules of what constitutes a useful heart failure treatment, he said.
Dr. Butler has been a consultant to Novartis and Amgen and several other companies. Dr. Jessup had no disclosures. Dr. Mehra has been a consultant to Teva, Johnson & Johnson, Boston Scientific, and St. Jude. Dr. Ruschitzka has been a consultant to Novartis, Servier, Sanofi, Cardiorentis, Heartware, and St. Jude. Dr. McMurray has received research support from Novartis and Amgen. Dr. Lindenfeld has been a consultant to Novartis, Abbott, Janssen, Relypsa, and Resmed. Dr. Voors has been a consultant to Novartis, Amgen, Servier, and several other drug companies. Dr. Ponikowski has been a consultant to Amgen, Novartis, Servier, and several other drug companies. Dr. Harjola has been a consultant to Novartis, Servier, Bayer, Boehringer Ingelheim, Pfizer, and Resmed. Dr. Abraham has been a consultant to Amgen, Novartis, and several device companies. Dr. Anker has been a consultant to Novartis, Servier, and several other companies.
On Twitter @mitchelzoler
FLORENCE, ITALY – The 2016 revision of the European Society of Cardiology’s guidelines for diagnosing and treating acute and chronic heart failure highlights the extent to which thinking in the field has changed during the past 4 years, since the prior edition in 2012.
The new European guidelines, unveiled by the ESC’s Heart Failure Association during the group’s annual meeting, also underscore the great dependence that many new approaches have on expert opinion rather than what’s become the keystone of guidelines writing, evidence-based medicine. Frequent reliance on consensus decisions rather than indisputable proof from controlled trials defines what some U.S. specialists see as a divide as wide as the Atlantic between the European and U.S. approaches to guideline writing.
“The guidelines from the ESC are articulated very well; they made their recommendations very clear. But a lot is consensus driven, without new data,” said Dr. Mariell L. Jessup, who serves as both vice chair of the panel currently revising the U.S. heart failure guidelines – expected out later in 2016 – and was also the sole American representative on the panel that produced the ESC guidelines. “The ESC guidelines make clear all the things that need to happen to patients. I hope it will result in better patient care. We are clearly not doing a good job in heart failure. We not only don’t have evidence-based treatments, but people often don’t do a good job [caring for heart failure patients] and they die in the hospital all the time.”
Dr. Javed Butler, another member of the U.S. guidelines panel and professor and chief of cardiology at Stony Brook (N.Y.) University, called the U.S. and European divide a “philosophical perspective of evidence-based medicine.
“U.S. physicians should read the ESC guidelines and make up their own minds. The ESC guidelines are excellent and give you perspective. But U.S. regulatory and payment issues will be driven by U.S. guidelines,” Dr. Butler said in an interview.
But despite their limitations and the limited weight that the ESC guidelines carry for U.S. practice, they have many redeeming features, noted Dr. Mandeep R. Mehra, medical director of the Heart and Vascular Center at Brigham and Women’s Hospital in Boston. The 2016 ESC guidelines “are extraordinarily clear, very practical, and very concise. They are very usable, and provide a fantastic algorithm for managing patients with heart failure with reduced ejection fraction [HFrEF],” he said while discussing the guidelines during the meeting.
U.S. and Europe largely agree on sacubitril/valsartan and ivabradine
Clearly the greatest area of U.S. and European agreement was in the adoption by both guidelines groups of sacubitril/valsartan (Entresto) and ivabradine (Corlanor) as important new components of the basic drug formula for treating patients with HFrEF. In fact, the U.S. guideline writers saw these two additions as so important and timely that they issued a “focused update” in May to the existing, 2013 U.S. heart failure guidelines, and timed release of this update to occur on May 20, 2016, a day before release of the ESC guidelines. But as Dr. Butler noted, this was more of a temporal harmonization than a substantive one, because even here, in a very evidence-based change, the U.S. guidelines for using sacubitril/valsartan differed subtly but importantly from the ESC version.
The U.S. focused update says that treatment of patients with stage C (symptomatic heart failure with structural heart disease) HFrEF should receive treatment with sacubitril/valsartan (also know as an angiotensin receptor neprilysin inhibitor, or ARNI), an ACE inhibitor, or an angiotensin receptor blocker (ARB), as well as evidence-based treatment with a beta-blocker and with a mineralocorticoid receptor antagonist (MRA). A subsequent recommendation in the U.S. focused update said that HFrEF patients with chronic symptoms and New York Heart Association class II or III disease should switch from a stable, tolerated regimen with either an ACE inhibitor or ARB to treatment with sacubitril/valsartan.
In contrast, the new European guideline for sacubitril/valsartan recommends starting patients on this combination formulation only after first demonstrating that patients tolerated treatment with an ACE inhibitor or ARB for at least 30 days and determining that patients remained symptomatic while on one of these treatments. In short, the U.S. guideline gives a green light to starting patients with newly diagnosed, symptomatic HFrEF on sacubitril/valsartan immediately, while the European guideline only sanctions sacubitril/valsartan to start after a patient has spent at least 30 days settling into a multidrug regimen featuring an ACE inhibitor or an ARB when an ACE inhibitor isn’t well tolerated.
“The European guidelines are closely related to the study population enrolled in the PARADIGM-HF trial,” the pivotal trial that showed superiority of sacubitril/valsartan to an ACE inhibitor (N Engl J Med. 2014;371:993-1004), noted Dr. Butler in an interview. “The U.S. guidelines interpreted [the PARADIGM-HF] results in the best interests of a larger patient population. The European guidelines are far more proscriptive in replicating the clinical criteria of the trial. In some patients the sequence of starting a MRA and sacubitril/valsartan matters, but in other patients it is less important.”
Dr. Frank Ruschitzka, a coauthor of the ESC guidelines, said that the reason for the more cautious ESC approach was lack of widespread familiarity with sacubitril/valsartan treatment among cardiologists.
The ESC guidelines on using sacubitril/valsartan “replicated the PARADIGM-HF trial. We have no data right now that it is justifiable to put a [treatment-naive] patient on sacubitril/valsartan to begin with. Another difference between the U.S. and ESC guidelines is when to start a MRA,” said Dr. Ruschitzka, professor and head of cardiology at the Heart Center of the University Hospital in Zurich. “It makes a lot of sense to me to start sacubitril/valsartan early. The PARADIGM trial was positive, but no one has a feel for how to use sacubitril/valsartan. Should we give it to everyone? We said replicate the trial, and gain experience using the drug. We want to bring a life-saving drug to patients, but this is the approach we took. We need more data.”
Dr. Jessup noted that a lot of uncertainty also exists among U.S. clinicians about when to start sacubitril/valsartan. “It’s not been clear which patients to put on sacubitril/valsartan. No guidelines had been out on using it” until mid-May, and “the cost of sacubitril/valsartan is daunting. I have received calls from many people who ask whom am I supposed to use sacubitril/valsartan on? It took years and years to get people to [routinely] start patients on an ACE inhibitor and a beta-blocker, and now we’re telling them to do something else. In my practice it’s a 30-minute conversation with each patient that you need to first stop your ACE inhibitor, and then they often get denied coverage by their insurer,” said Dr. Jessup, professor of medicine at the University of Pennsylvania in Philadelphia. She expressed hope that coverage issues will diminish now that clear guidelines are out endorsing a key role for sacubitril/valsartan.
“We now all have started sacubitril/valsartan on patients” without first starting them on an ACE inhibitor, “but we all need to get a sense of what we can get away with” when using this drug, noted Dr. JoAnn Lindenfeld, professor and director of heart failure and transplant at Vanderbilt University in Nashville.
At least one European cardiologist was skeptical of just how proscriptive the ESC guideline for sacubitril/valsartan will be in actual practice.
“The best treatment [for symptomatic HFrEF] is sacubitril/valsartan, a beta-blocker, and a MRA,” said Dr. John J.V. McMurray, professor of cardiology at Glasgow University and lead investigator for the PARADIGM-HF pivotal trial for sacubitril/valsartan. “The treatment sequence advocated in the guidelines – treat with an ACE inhibitor first and if patients remain symptomatic change to sacubitril/valsartan – is evidence-based medicine. As a guidelines writer and as a promoter of evidence-based medicine, this is absolutely the correct approach. But as a practicing physician I’d go straight for sacubitril/valsartan. Otherwise you’re wasting everybody’s time starting with an ACE inhibitor and then waiting a month to switch,” Dr. McMurray said in an interview.
“It’s pointless to wait. We saw results within 30 days of starting sacubitril/valsartan, so it’s a theoretical risk to wait. Very few patients will become completely asymptomatic on an ACE inhibitor. Everyone who entered PARADIGM-HF was at New York Heart Association class II or higher, and at the time of randomization only a handful of patients were in New York Heart Association class I. Very few patients get to class I. That tells you it’s pretty uncommon for a heart failure patient to become truly asymptomatic with ACE inhibitor treatment. The main problem is that you are inconveniencing everybody with more blood tests and more clinic visits by waiting to start sacubitril/valsartan, said Dr. McMurray, who was not a member of the panel that wrote the new ESC guidelines.
Even less separates the new U.S. focused update and the ESC guidelines for using ivabradine. Both agree on starting the drug on HFrEF patients who remain symptomatic and with a left ventricular ejection fraction of 35% or less despite being on guideline-directed therapy including titration to a maximum beta-blocker dosage and with a persistent heart rate of at least 70 beats/min. The goal of ivabradine treatment is to further reduce heart rate beyond what’s achieved by a maximal beta-blocker dosage.
Perhaps the biggest questions about ivabradine have been why it took so long to enter the U.S. guidelines, and why it is listed in both the U.S. and ESC guidelines as a level II recommendation. Results from the pivotal trial that supported ivabradine’s use in HFrEF patients, SHIFT, first appeared in 2010 (Lancet. 2010 Sep 11;376[9744]:875-95).
Dr. Butler chalked up the drug’s slow entry into U.S. guidelines as the result of a lack of initiative by ivabradine’s initial developer, Servier. “SHIFT did not have any U.S. sites, and Servier never sought Food and Drug Administration approval,” he noted. “Amgen acquired the U.S. rights to ivabradine in 2013,” and the drug received FDA approval in April 2015, Dr. Butler noted, in explaining the drug’s U.S. timeline. As to why its use is a level II recommendation, he noted that the evidence for efficacy came only from the SHIFT trial, questions exist whether the beta-blocker dosages were fully optimized in all patients in this trial, and the benefit was limited to a reduction in heart failure hospitalizations but not in mortality. “I think that patients with persistent heart failure symptoms [and a persistently elevated heart rate] should get ivabradine,” but these caveats limit it to a class II level recommendation, Dr. Butler said.
“There were questions about ivabradine’s benefit in reducing heart failure hospitalization but not mortality, and questions about whether it would benefit patients if their beta-blocker dosage was adequately up titrated. There were also questions about which heart failure patients to use it on,” noted Dr. Lindenfeld, a member of the panel that wrote the U.S. focused update. These concerns in part help explain the delay to integrating ivabradine into U.S. practice guidelines, she said in an interview, but added that additional data and analysis published during the past 3 or so years have clarified ivabradine’s potentially useful role in treating selected HFrEF patients.
New ESC guidelines based on expert opinion
The sections on sacubitril/valsartan and ivabradine occupy a mere 2 pages among more than 55 pages of text and charts that spell out the ESC’s current vision of how physicians should diagnose and manage heart failure patients. While much of what carried over from prior editions of the guidelines is rooted in evidence, many of the new approaches advocated rely on expert opinion or new interpretations of existing data. Here are some of the notable changes and highlights of the 2016 ESC recommendations:
• Heart failure diagnosis. The new ESC guidelines streamline the diagnostic process, which now focuses on five key elements: The patient’s history, physical examination, ECG, serum level of either brain natriuretic peptide (BNP) or N-terminal(NT)-proBNP, and echocardiography. The guidelines specify threshold levels of BNP and NT-proBNP that can effectively rule out heart failure, a BNP level of at least 35 pg/mL or a NT-proBNP level of at least 125 pg/mL.
“The diagnostic minimum levels of BNP and NT-proBNP were designed to rule out heart failure. They both have a high negative predictive value, but at these levels their positive predictive value is low,” explained Dr. Adriaan A. Voors, cochair of the ESC’s guideline-writing panel and professor of cardiology at the University of Groningen, the Netherlands.
But while these levels might be effective for reliable rule out of heart failure, they could mean a large number of patients would qualify for an echocardiographic assessment.
“If we used the ESC’s natriuretic peptide cutoffs, there would be a clear concern about overuse of echo. It’s a cost-effectiveness issue. You wind up doing a lot of echos that will be normal. Echocardiography is very safe, but each echo costs about $400-$500,” commented Dr. Butler.
“The results from the STOP-HF and PONTIAC studies showed that BNP levels can identify people at increased risk for developing heart failure who need more intensive assessment and could also potentially benefit from more attention to heart failure prevention. I suspect the full U.S. guideline update will address this issue, but we have not yet finalized our decisions,” he added.
• Heart failure classification. The new ESC guidelines created a new heart failure category, midrange ejection fraction, that the writing panel positioned squarely between the two more classic heart failure subgroups, HFrEF and heart failure with preserved ejection fraction (HFpEF). The definition of each of the three subgroups depends on left ventricular ejection fraction as determined by echocardiography: A LVEF of less than 40% defined HFrEF, a LVEF of 40%-49% defined heart failure with midrange ejection fraction (HFmrEF), and a LVEF of 50% or higher defined HFpEF. Diagnostic confirmation of both HFmrEF and HFpEF also requires that patients fulfill certain criteria of structural or functional heart abnormalities.
The category of HFmrEF was created “to stimulate research into how to best manage these patients,” explained Dr. Piotr Ponikowski, chair of the ESC guidelines writing panel. For the time being, it remains a category with only theoretical importance as nothing is known to suggest that management of patients with HFmrEF should in any way differ from patients with HFpEF.
• Acute heart failure. Perhaps the most revolutionary element of the new guidelines is the detailed map they provide to managing patients who present with acute decompensated heart failure and the underlying principles cited to justify this radically different approach.
“The acute heart failure section was completely rewritten,” noted Dr. Ponikowski, professor of heart diseases at the Medical University in Wroclaw, Poland. “We don’t yet have evidence-based treatments” to apply to acute heart failure patients, he admitted, “however we strongly recommend the concept that the shorter the better. Shorten the time of diagnosis and for therapeutic decisions. We have borrowed from acute coronary syndrome. Don’t keep patients in the emergency department for another couple of hours just to see if they will respond. We must be aware that we need to do our best to shorten diagnosis and treatment decisions. Time is an issue. Manage a patient’s congestion and impaired peripheral perfusion within a time frame of 1-2 hours.”
The concept that acute heart failure must be quickly managed as an emergency condition similar to acute coronary syndrome first appeared as a European practice recommendation in 2015, a consensus statement from the European Heart Failure Association and two other collaborating organizations (Eur Heart J. 2015 Aug 7;36[30]:1958-66).
“In 2015, the consensus paper talked about how to handle acute heart failure patients in the emergency department. Now, we have focused on defining the patients’ phenotype and how to categorize their treatment options. We built on the 2015 statement, but the algorithms we now have are original to 2016; they were not in the 2015 paper,” said Dr. Veli-Pekka Harjola, a member of the 2015 consensus group and 2016 guidelines panel who spearheaded writing the acute heart failure section of the new ESC guidelines.
An additional new and notable feature of this section in the 2016 guidelines is its creation of an acronym, CHAMP, designed to guide the management of patients with acute heart failure. CHAMP stands for acute Coronary syndrome, Hypertension emergency, Arrhythmia, acute Mechanical cause, and Pulmonary embolism. The CHAMP acronym’s purpose is to “focus attention on these five specific, potential causes of acute heart failure, life-threatening conditions that need immediate treatment,” explained Dr. Ponikowski.
“CHAMP emphasizes the most critical causes of acute heart failure,” added Dr. Harjola, a cardiologist at Helsinki University Central Hospital. “We created this new acronym” to help clinicians keep in mind what to look for in a patient presenting with acute heart failure.
U.S. cardiologists find things to like in what the Europeans say about managing acute heart failure, as well as aspects to question.
“It makes no sense not to aggressively treat a patient who arrives at an emergency department with acute heart failure. But there is a difference between acute MI or stroke and acute heart failure,” said Dr. Butler. “In acute MI there is the ruptured plaque and thrombus that blocks a coronary artery. In stroke there is a thrombus. These are diseases with a specific onset and treatment target. But with acute heart failure we don’t have a thrombus to treat; we don’t have a specific target. What we’ve learned from studying implanted devices [such as CardioMems] is that the congestion that causes acute heart failure can start 2-3 weeks before a patient develops acute decompensated heart failure and goes to the hospital. We have not found a specific pathophysiologic abnormality in the patient with acute heart failure that is any different from chronic heart failure. This begs the question: If a patient who presents with acute heart failure has a congestion process that’s been going on for 2 or 3 weeks what difference will another 3 hours make? Do we need to replicate the concept of an acute stroke team or acute MI response for acute heart failure?”
Dr. Butler stressed that additional data are expected soon that may help clarify this issue.
“Some large outcome trials in patients with acute heart failure are now underway, one testing serelaxin treatment, another testing ularitide treatment, that are also testing the hypothesis that rapid treatment with these drugs can produce more end-organ protection, stop damage to the heart, kidney and liver, and lead to better long-term outcomes. Until we have those data, the jury is still out” on the benefit patients gain from rapid treatment of acute heart failure. “Until then, it’s not that the data say don’t treat acute heart failure patients aggressively. But we have not yet proven it is similar to treating an acute MI or stroke,” said Dr. Butler.
“U.S. guidelines have tended to stay away from areas where there are no evidence-based data. To their credit, the Europeans will take on something like acute heart failure where we don’t have an adequate evidence base. Despite that, they provide guidelines, which is important because clinicians need guidance even when the evidence is not very good, when the guideline is based mostly on experience and expert consensus.” commented Dr. William T. Abraham, professor and director of cardiovascular medicine at the Ohio State University Wexner Medical Center in Columbus.
“It’s absolutely appropriate to think of acute heart failure as an emergency situation. We know from high-sensitivity troponin assays that troponin levels are increased in 90% of patients who present with acute decompensated heart failure. So most acute heart failure patients are losing heart muscle cells and we should treat them like we treat acute coronary syndrome. Time matters in acute heart failure; time is heart muscle. Treatment needs to break the hemodynamic and neurohormonal storm of acute decompensated heart failure; get the patient stabilized; improve vital organ perfusion, including within the heart; and shift the myocardial oxygen supply and demand equation so myocardial necrosis stops. All of this is important, and study results suggest it’s the correct approach. I’m not sure study results prove it, but studies that have looked at the time course of treatment for acute heart failure showed that early initiation of treatment – within the first 6 hours of onset – compared with 12-24 hours of onset makes a difference in outcomes,” Dr. Abraham said in an interview.
But a major limitation to the potential efficacy of a rapidly initiated management strategy is that few interventions currently exist with proven benefits for acute heart failure patients.
For the time being, rapid intervention means using diuretics relatively quickly and, if there is an indication for treating with a vasoactive medication, using that quickly too. “The rapid approach is really more relevant to the future; it’s relevant to the design of future acute heart failure treatment trials. That is where this early treatment paradigm is important,” as it could potentially apply to new, more effective treatments of the future rather than to the marginally effective treatments now available, Dr. Abraham said.
“For a long we time haven’t pushed how quickly we should act when implementing guideline-directed treatment” for patients with acute heart failure, noted Dr. Mehra. “The CHAMP approach is interesting, and the ESC guidelines are a very interesting move in the direction” of faster action. “They speak to the period of time during which one should act. Hopefully this will help the science of acute decompensated heart failure move forward.”
But for other U.S. experts the issue again pivots on the lack of evidence.
“There is nothing new” about managing acute heart failure, said Dr. Jessup. “The ESC guideline was articulated very well; they made their recommendations very clear. But a lot is consensus driven. There are no new data. The problem with acute heart failure is that the recommendations are what we think clinicians should do. CHAMP is a nice acronym; it’s packaged better, but there are not any new data.”
Comorbidities
A dramatic contrast distinguishes the extent to which the ESC guidelines highlight comorbidities, compared with prevailing U.S. guidelines. The new ESC guidelines highlight and discuss with some detail 16 distinct comorbidities for clinicians to keep in mind when managing heart failure patients, compared with three comorbidities (atrial fibrillation, anemia, and depression) discussed with similar detail in the 2013 U.S. guidelines.
“We are targeting comorbidities to personalize medicine, by subgrouping [heart failure] patients into groups that need to receive special attention,” explained Dr. Stefan D. Anker, a coauthor on the ESC guidelines. “We care about comorbidities because they make the diagnosis of heart failure difficult. They aggravate symptoms and contribute to additional hospitalizations. They interfere with [heart failure] treatment, and because comorbidities have led to exclusions of heart failure patients from trials, we lack evidence of treatment efficacy in patients with certain comorbidities,” said Dr. Anker, a professor of innovative clinical trials at the Medical University in Göttingen, Germany.
“The comorbidity discussion in the ESC guidelines is extremely important,” commented Dr. Abraham. “It supports the need for a multidimensional approach to heart failure patients. A cardiologist may not have all the resources to manage all the comorbidities [a heart failure patient might have]. This is why having a sleep medicine specialist, a diabetes specialist, a nephrologist, etc., involved as part of a heart failure management team can be very valuable. We need to involve subspecialists in managing the comorbidities of heart failure because they clearly have an impact on patient outcome.”
But Dr. Butler had a somewhat different take on how comorbidity management fits into the broader picture of heart failure management.
“There is no doubt that heart failure worsens other comorbidities and other comorbidities worsen heart failure. The relationship is bidirectional between heart failure and chronic obstructive pulmonary disease, liver disease, depression, sleep apnea, renal disease, lung disease, diabetes, etc. The problem is that treating a comorbidity does not necessarily translate into improved heart failure outcomes. Comorbidities are important for heart failure patients and worsen their heart failure outcomes. However, management of a comorbidity should be done primarily for the sake of improving the comorbidity. If you treat depression, for example, and it does not improve a patient’s heart failure, that doesn’t mean you shouldn’t have treated the depression. It just means that we don’t have good data that it will improve heart failure.”
Another limitation from a U.S. perspective is what role treatment of various comorbidities can play in benefiting heart failure patients and how compelling the evidence is for this. Dr. Butler gave as an example the problem with treating iron deficiency in heart failure patients who do not have anemia, a strategy endorsed in the ESC guidelines as a level IIa recommendation.
“The data regarding improved exercise capacity from treatment with intravenous ferric carboxymaltose is pretty convincing,” he said. But patients have benefited from this treatment only with improved function and quality of life, and not with improved survival or fewer hospitalizations.
“Is treating patients to improve their function and help them feel better enough?” Dr. Butler asked. “In other diseases it is. In gastrointestinal disease, if a drug helps patients feel better you approve the drug. We value improved functional capacity for patients with pulmonary hypertension, angina, and peripheral vascular disease. All these indications have drugs approved for improving functional capacity and quality of life. But for heart failure the bar has been set higher. There is a lot of interest in changing this” for heart failure.
“There is interest in running a study of ferric carboxymaltose for heart failure with a mortality endpoint. In the meantime, the impact on improving functional capacity is compelling, and it will be interesting to see what happens in the U.S. guidelines. Currently, in U.S. practice if a heart failure patient has iron-deficiency anemia you treat with intravenous iron replacement and the treatment gets reimbursed without a problem. But if the heart failure patient has iron deficiency without anemia then reimbursement for the cost of iron supplementation can be a problem,” Dr. Butler noted. This may change only if the experts who write the next U.S. heart failure guidelines decide to change the rules of what constitutes a useful heart failure treatment, he said.
Dr. Butler has been a consultant to Novartis and Amgen and several other companies. Dr. Jessup had no disclosures. Dr. Mehra has been a consultant to Teva, Johnson & Johnson, Boston Scientific, and St. Jude. Dr. Ruschitzka has been a consultant to Novartis, Servier, Sanofi, Cardiorentis, Heartware, and St. Jude. Dr. McMurray has received research support from Novartis and Amgen. Dr. Lindenfeld has been a consultant to Novartis, Abbott, Janssen, Relypsa, and Resmed. Dr. Voors has been a consultant to Novartis, Amgen, Servier, and several other drug companies. Dr. Ponikowski has been a consultant to Amgen, Novartis, Servier, and several other drug companies. Dr. Harjola has been a consultant to Novartis, Servier, Bayer, Boehringer Ingelheim, Pfizer, and Resmed. Dr. Abraham has been a consultant to Amgen, Novartis, and several device companies. Dr. Anker has been a consultant to Novartis, Servier, and several other companies.
On Twitter @mitchelzoler
EXPERT ANALYSIS FROM HEART FAILURE 2016
Low paravalvular leak shown in real-world registry of Sapien 3 recipients
PARIS – The initial report from a large, prospective registry documenting outcomes in recipients of the Edwards Sapien 3 transcatheter aortic valve in real-world clinical practice confirm that the excellent results seen earlier in the rarified, randomized clinical trial setting are routinely reproducible in everyday practice, Dr. Olaf Wendler said at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
“The big thing with the Sapien 3 is the reduction in paravalvular leakage. Our rate of moderate or severe paravalvular leakage in the registry at 30 days is only 3.1%, with 73.6% of patients having no or only trace paravalvular leakage,” reported Dr. Wendler, professor of cardiothoracic surgery at King’s College Hospital, London.
The mean gradient improved from 43.8 mm Hg at baseline to 11.7 mm Hg at discharge, while the effective orifice area climbed from 0.72 to 1.67 cm2.
He presented 30-day outcomes from the SOURCE 3 registry for 1,947 recipients of the Edwards Sapien 3 valve during transcatheter aortic valve replacement at 80 European centers in 10 countries. Their average age was 81.6 years. Updates will continue during a planned 5 years of prospective follow-up.
“This will be a very rich dataset for subgroup analyses. We now have a number of procedural variables we can analyze over time. We will have good data to get to better outcomes in terms of how best to perform the procedure if you do it with a Sapien valve,” he explained.
Among the key findings: The lower profile of the Sapien 3 delivery system, compared with earlier iterations of the Sapien valve, enabled 87% of patients in the registry to undergo TAVR via transfemoral access. And, in these 1,695 patients, whose mean logistic EuroSCORE was 17.8, the 30-day rates of all-cause mortality and disabling stroke were just 1.9% and 0.5%, respectively.
“I think there are not many series that have shown better results than these,” Dr. Wendler commented.
The non–transfemoral-access group is a very different, higher surgical risk cohort with lots more comorbid conditions. Their mean logistic EuroSCORE was 21.8. Yet in this group, the 30-day all-cause mortality and disabling stroke rates were still only 4% and 0.8%.
The transfemoral access group had markedly lower rates of life-threatening bleeding (4%), new-onset atrial fibrillation (4.8%), extended ventilation (3.5%), stage 2-3 acute kidney injury (0.8%), plus a 2-day shorter mean hospital length of stay.
Sixty percent of transfemoral access patients had their TAVR done under conscious sedation. These 1,018 patients constitute the largest dataset ever treated using conscious sedation with one valve system. The conscious sedation group had significantly lower baseline rates of carotid artery disease, prior coronary artery bypass graft surgery, and heart failure than did patients who received general anesthesia. At 30 days post-TAVR, the conscious sedation group had significantly lower rates of extended ventilation and postdilatation, and they received less contrast volume than did the general anesthesia group. However, they had a significantly higher incidence of stroke: 1.7%, compared with 0.6% for the general anesthesia group.
“Why that is the case we don’t know at the moment. We need to look into this more in the future,” the surgeon said.
A particularly impressive finding was how well Sapien 3 valve recipients with a low left ventricular ejection fraction have done. For example, 30-day all-cause mortality in the 100 patients with a baseline LVEF below 30% was 3%, not significantly different from the 1.8% rate in the 1,198 subjects with an LVEF greater than 50% or the 2.6% in patients with an LVEF of 30%-50%.
“Three percent all-cause mortality with an ejection fraction below 30% is just remarkable from my point of view,” Dr. Wendler said.
The 778 who didn’t undergo balloon aortic valvuloplasty prior to Sapien 3 implantation did not fare any worse than did those who did in terms of 30-day all-cause mortality or all strokes, but they did have significantly higher rates of life-threatening bleeding and major vascular complications.
The SOURCE 3 registry is sponsored by Edwards Lifesciences. Dr. Wendler serves as a consultant to the company.
PARIS – The initial report from a large, prospective registry documenting outcomes in recipients of the Edwards Sapien 3 transcatheter aortic valve in real-world clinical practice confirm that the excellent results seen earlier in the rarified, randomized clinical trial setting are routinely reproducible in everyday practice, Dr. Olaf Wendler said at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
“The big thing with the Sapien 3 is the reduction in paravalvular leakage. Our rate of moderate or severe paravalvular leakage in the registry at 30 days is only 3.1%, with 73.6% of patients having no or only trace paravalvular leakage,” reported Dr. Wendler, professor of cardiothoracic surgery at King’s College Hospital, London.
The mean gradient improved from 43.8 mm Hg at baseline to 11.7 mm Hg at discharge, while the effective orifice area climbed from 0.72 to 1.67 cm2.
He presented 30-day outcomes from the SOURCE 3 registry for 1,947 recipients of the Edwards Sapien 3 valve during transcatheter aortic valve replacement at 80 European centers in 10 countries. Their average age was 81.6 years. Updates will continue during a planned 5 years of prospective follow-up.
“This will be a very rich dataset for subgroup analyses. We now have a number of procedural variables we can analyze over time. We will have good data to get to better outcomes in terms of how best to perform the procedure if you do it with a Sapien valve,” he explained.
Among the key findings: The lower profile of the Sapien 3 delivery system, compared with earlier iterations of the Sapien valve, enabled 87% of patients in the registry to undergo TAVR via transfemoral access. And, in these 1,695 patients, whose mean logistic EuroSCORE was 17.8, the 30-day rates of all-cause mortality and disabling stroke were just 1.9% and 0.5%, respectively.
“I think there are not many series that have shown better results than these,” Dr. Wendler commented.
The non–transfemoral-access group is a very different, higher surgical risk cohort with lots more comorbid conditions. Their mean logistic EuroSCORE was 21.8. Yet in this group, the 30-day all-cause mortality and disabling stroke rates were still only 4% and 0.8%.
The transfemoral access group had markedly lower rates of life-threatening bleeding (4%), new-onset atrial fibrillation (4.8%), extended ventilation (3.5%), stage 2-3 acute kidney injury (0.8%), plus a 2-day shorter mean hospital length of stay.
Sixty percent of transfemoral access patients had their TAVR done under conscious sedation. These 1,018 patients constitute the largest dataset ever treated using conscious sedation with one valve system. The conscious sedation group had significantly lower baseline rates of carotid artery disease, prior coronary artery bypass graft surgery, and heart failure than did patients who received general anesthesia. At 30 days post-TAVR, the conscious sedation group had significantly lower rates of extended ventilation and postdilatation, and they received less contrast volume than did the general anesthesia group. However, they had a significantly higher incidence of stroke: 1.7%, compared with 0.6% for the general anesthesia group.
“Why that is the case we don’t know at the moment. We need to look into this more in the future,” the surgeon said.
A particularly impressive finding was how well Sapien 3 valve recipients with a low left ventricular ejection fraction have done. For example, 30-day all-cause mortality in the 100 patients with a baseline LVEF below 30% was 3%, not significantly different from the 1.8% rate in the 1,198 subjects with an LVEF greater than 50% or the 2.6% in patients with an LVEF of 30%-50%.
“Three percent all-cause mortality with an ejection fraction below 30% is just remarkable from my point of view,” Dr. Wendler said.
The 778 who didn’t undergo balloon aortic valvuloplasty prior to Sapien 3 implantation did not fare any worse than did those who did in terms of 30-day all-cause mortality or all strokes, but they did have significantly higher rates of life-threatening bleeding and major vascular complications.
The SOURCE 3 registry is sponsored by Edwards Lifesciences. Dr. Wendler serves as a consultant to the company.
PARIS – The initial report from a large, prospective registry documenting outcomes in recipients of the Edwards Sapien 3 transcatheter aortic valve in real-world clinical practice confirm that the excellent results seen earlier in the rarified, randomized clinical trial setting are routinely reproducible in everyday practice, Dr. Olaf Wendler said at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
“The big thing with the Sapien 3 is the reduction in paravalvular leakage. Our rate of moderate or severe paravalvular leakage in the registry at 30 days is only 3.1%, with 73.6% of patients having no or only trace paravalvular leakage,” reported Dr. Wendler, professor of cardiothoracic surgery at King’s College Hospital, London.
The mean gradient improved from 43.8 mm Hg at baseline to 11.7 mm Hg at discharge, while the effective orifice area climbed from 0.72 to 1.67 cm2.
He presented 30-day outcomes from the SOURCE 3 registry for 1,947 recipients of the Edwards Sapien 3 valve during transcatheter aortic valve replacement at 80 European centers in 10 countries. Their average age was 81.6 years. Updates will continue during a planned 5 years of prospective follow-up.
“This will be a very rich dataset for subgroup analyses. We now have a number of procedural variables we can analyze over time. We will have good data to get to better outcomes in terms of how best to perform the procedure if you do it with a Sapien valve,” he explained.
Among the key findings: The lower profile of the Sapien 3 delivery system, compared with earlier iterations of the Sapien valve, enabled 87% of patients in the registry to undergo TAVR via transfemoral access. And, in these 1,695 patients, whose mean logistic EuroSCORE was 17.8, the 30-day rates of all-cause mortality and disabling stroke were just 1.9% and 0.5%, respectively.
“I think there are not many series that have shown better results than these,” Dr. Wendler commented.
The non–transfemoral-access group is a very different, higher surgical risk cohort with lots more comorbid conditions. Their mean logistic EuroSCORE was 21.8. Yet in this group, the 30-day all-cause mortality and disabling stroke rates were still only 4% and 0.8%.
The transfemoral access group had markedly lower rates of life-threatening bleeding (4%), new-onset atrial fibrillation (4.8%), extended ventilation (3.5%), stage 2-3 acute kidney injury (0.8%), plus a 2-day shorter mean hospital length of stay.
Sixty percent of transfemoral access patients had their TAVR done under conscious sedation. These 1,018 patients constitute the largest dataset ever treated using conscious sedation with one valve system. The conscious sedation group had significantly lower baseline rates of carotid artery disease, prior coronary artery bypass graft surgery, and heart failure than did patients who received general anesthesia. At 30 days post-TAVR, the conscious sedation group had significantly lower rates of extended ventilation and postdilatation, and they received less contrast volume than did the general anesthesia group. However, they had a significantly higher incidence of stroke: 1.7%, compared with 0.6% for the general anesthesia group.
“Why that is the case we don’t know at the moment. We need to look into this more in the future,” the surgeon said.
A particularly impressive finding was how well Sapien 3 valve recipients with a low left ventricular ejection fraction have done. For example, 30-day all-cause mortality in the 100 patients with a baseline LVEF below 30% was 3%, not significantly different from the 1.8% rate in the 1,198 subjects with an LVEF greater than 50% or the 2.6% in patients with an LVEF of 30%-50%.
“Three percent all-cause mortality with an ejection fraction below 30% is just remarkable from my point of view,” Dr. Wendler said.
The 778 who didn’t undergo balloon aortic valvuloplasty prior to Sapien 3 implantation did not fare any worse than did those who did in terms of 30-day all-cause mortality or all strokes, but they did have significantly higher rates of life-threatening bleeding and major vascular complications.
The SOURCE 3 registry is sponsored by Edwards Lifesciences. Dr. Wendler serves as a consultant to the company.
AT EUROPCR 2016
Key clinical point: Only 3.1% of patients had moderate or severe paravalvular leak at 30 days after TAVR with the Sapien 3.
Major finding: The 30-day, all-cause mortality and disabling stroke rates were 1.9% and 0.5% in Sapien 3 valve recipients whose transcatheter aortic valve replacement was done by the transfemoral route.
Data source: A prospective multicenter European registry which includes 1,947 patients who underwent transcatheter aortic valve replacement with the Edwards Sapien 3 valve in real-world commercial settings.
Disclosures: The SOURCE 3 registry is sponsored by Edwards Lifesciences. Dr. Wendler serves as a consultant to the company.
Challenging ‘dogma’ of allografts in infectious endocarditis
When a patient undergoes aortic valve replacement for infective endocarditis, conventional thinking holds that cardiac surgeons should use homografts because they have greater resistance to infection, but a recent study of more than 300 cases at two academic medical centers concluded that homografts may not necessarily offer such a benefit.
The study, published in the June issue of the Journal of Thoracic and Cardiovascular Surgery (2016;151:1239-48), involved 304 consecutive adult patients on whom 30-40 different surgeons performed operations for active infective endocarditis (IE) in the aortic valve from 2002 to 2014.
“Our findings suggest that patient-specific factors, such as age and implant preference, as well as technical reconstructive considerations, should drive prosthetic choice, rather than surgical dogma,” said Joon Bum Kim, Ph.D., of Massachusetts General Hospital, Harvard Medical School, both in Boston, and Asan Medical Center in Seoul, Korea, and his colleagues.
The study found that cardiac surgeons favored homografts over conventional prostheses when the patient had prosthetic valve endocarditis (58.1% vs. 28.8%) and methicillin-resistant Staphylococcus aureus (25.6% vs. 12.1%), both significant differences.
“No significant benefit to the use of homografts was demonstrable with regard to resistance to reinfection in the setting of IE,” Dr. Kim and his colleagues said.
Because reinfection after valve replacement for IE is such a strong concern, the debate over which prosthesis is best has ensued for decades. The researchers pointed out that the evidence favoring autologous or allogeneic tissue over synthetic material in the infective field is weak, mostly built on single-armed observational studies without comparison to conventional prosthesis.
With that in mind, the researchers pooled data from two institutions to compare short- and long-term results for homograft vs. conventional prosthetic valves in patients with IE. In this study group, 86 (28.3%) had homografts, 139 (45.7%) had xenograft prostheses, and 79 (26%) mechanical prostheses. The homograft group had more than twice the rate of early death than did the conventional group – 19.8% vs. 9.2%, a significant difference (P = .019).
During follow-up, which ranged from 4.7 to 72.6 months, 60 patients (19.7%) of the total group died and 23 (7.7%) experienced reinfection, but rates did not vary between the homograft and conventional prosthesis groups, Dr. Kim and his colleagues reported.
Demographics were similar between the three groups with a few exceptions Those who received the mechanical prostheses were younger (mean age, 47.2 years vs. 55.6 and 59.8 for the homograft and xenograft groups, respectively), had lower rates of diabetes (5.1% vs. 10.5% and 12.2%) and had less-severe disease based on New York Heart Association functional class III or IV scores (34.2% vs. 54.7% and 53.2%). The types of IE pathogens also differed among the three groups; methicillin-resistant staphylococci was most common in the homograft group (25.6%), whereas the viridans group streptococci was the leading cause of IE in the mechanical (38% ) and xenograft groups (25.2% ).
The use of homografts involves a highly complex operation, typically requiring a complete aortic root replacement, which “may be the major drawback in recommending it to patients already at high risk of operative mortality,” the investigators wrote. The durability of homografts makes their use limited for younger patients, and such grafts are somewhat scarce and require cryopreservation. “Therefore, the notion that homografts are required may in practice present an obstacle to appropriate surgical management of patients who have IE,” Dr. Kim and his coauthors wrote. All patients but one in the homograft group received aortic arch replacement (98.8%) whereas 30 of the patients in the conventional group did so (13.8%).
The study findings are consistent with an earlier comparative study (Ann. Thorac. Surg. 2012;93:480-07), according to Dr. Kim and his colleagues. “These findings suggest that patient-specific factors, such as patient preferences and technical considerations, should be the principal drivers of choices of valve prostheses,” they said. “Furthermore, lack of access to homografts should not be considered an obstacle to surgical therapy for this serious condition.”
Coauthor Dr. Sundt disclosed that he is a consultant for Thrasos Therapeutics. Dr. Kim and the other coauthors had no financial disclosures.
The study by Dr. Kim and his colleagues joins a series of reports questioning conventional thinking on the use of homografts to prevent recurrent infective endocarditis (IE), but their propensity matching does not account for surgeon bias in selecting a prosthesis, Dr. James K. Kirklin of the University of Alabama at Birmingham said in his invited commentary (J Thorac. Cardiovasc Surg. 2016 May;151:1230-1).
For example, surgeon preference may account for the wide disparity in full root replacements, depending on the type of prosthesis, Dr. Kirklin said. “Some experienced homograft surgeons have preferred the intra-aortic cylinder technique or infracoronary implantation, which avoids the short-term and longer-term complexities of full root replacement and has demonstrated long-term structural durability equivalent to that of the full root replacement,” he said.
Also, experienced homograft surgeons may prefer the homograft for its resistance to infection and adaptability to severe root infection in individual patients, particularly in those with severe infection with an abscess. And he cautioned against the study’s implication that conventional prostheses are equivocal in the setting of IE.
“Of considerable importance, however, is the evidence-based conclusion that surgical referral of routine surgical aortic valve endocarditis to a center experienced with aortic homograft surgery is not necessary, and a justifiable expectation is that aortic valve endocarditis requiring operation can be safely and appropriately managed in centers with standard aortic valve surgery experience who do not have access to or experience with aortic valve homografts,” Dr. Kirklin concluded.
Dr. Kirklin had no financial relationships to disclose.

|
Dr. Christopher M. Feindel |
The series by Dr. Kim and his colleagues, one of the largest of acute infective endocarditis to date, provides further evidence that the type of prosthesis used in surgery for IE involving the aortic valve probably does not affect long-term outcomes or reinfection rates, Dr. Christopher M. Feindel of the University of Toronto said in his invited commentary (J Thorac Cardiovasc Surg. 2016 May;151:1249-50).
However, Dr. Feindel said, “numerous confounding factors” inherent in any observational study could raise questions about the conclusion.
“This article delivers an important message, although not all surgeons will agree with the statistical approach taken by Dr. Kim and his colleagues,” Dr. Feindel said. The propensity scoring method the study used lacked all baseline variables that affect treatment choice and outcomes, “a crucial assumption for effective use of the propensity score,” he said. However, given the multitude of variables in patients with acute and complex IE, he said most surgeons would be hard pressed to accept that’s even possible in the model the study used.
Dr. Feindel also said a close examination of the 115 patients who underwent root replacement would have been “very instructional,” and the lack of follow-up on valve-related complications in almost 25% of the patients is another limitation of the study.
Nonetheless, the conclusions of Dr. Kim and his colleagues are “reasonable,” Dr. Feindel said. “Clearly, this article contributes important additional information to the surgical management of IE that will help guide surgeons, especially when it comes to prosthesis of choice,” he concluded. “It is up to the reader to decide whether this report finally puts to rest the “dogma” that homografts should preferentially be used in the setting of IE.”
Dr. Feindel had no relationships to disclose.
The study by Dr. Kim and his colleagues joins a series of reports questioning conventional thinking on the use of homografts to prevent recurrent infective endocarditis (IE), but their propensity matching does not account for surgeon bias in selecting a prosthesis, Dr. James K. Kirklin of the University of Alabama at Birmingham said in his invited commentary (J Thorac. Cardiovasc Surg. 2016 May;151:1230-1).
For example, surgeon preference may account for the wide disparity in full root replacements, depending on the type of prosthesis, Dr. Kirklin said. “Some experienced homograft surgeons have preferred the intra-aortic cylinder technique or infracoronary implantation, which avoids the short-term and longer-term complexities of full root replacement and has demonstrated long-term structural durability equivalent to that of the full root replacement,” he said.
Also, experienced homograft surgeons may prefer the homograft for its resistance to infection and adaptability to severe root infection in individual patients, particularly in those with severe infection with an abscess. And he cautioned against the study’s implication that conventional prostheses are equivocal in the setting of IE.
“Of considerable importance, however, is the evidence-based conclusion that surgical referral of routine surgical aortic valve endocarditis to a center experienced with aortic homograft surgery is not necessary, and a justifiable expectation is that aortic valve endocarditis requiring operation can be safely and appropriately managed in centers with standard aortic valve surgery experience who do not have access to or experience with aortic valve homografts,” Dr. Kirklin concluded.
Dr. Kirklin had no financial relationships to disclose.

|
Dr. Christopher M. Feindel |
The series by Dr. Kim and his colleagues, one of the largest of acute infective endocarditis to date, provides further evidence that the type of prosthesis used in surgery for IE involving the aortic valve probably does not affect long-term outcomes or reinfection rates, Dr. Christopher M. Feindel of the University of Toronto said in his invited commentary (J Thorac Cardiovasc Surg. 2016 May;151:1249-50).
However, Dr. Feindel said, “numerous confounding factors” inherent in any observational study could raise questions about the conclusion.
“This article delivers an important message, although not all surgeons will agree with the statistical approach taken by Dr. Kim and his colleagues,” Dr. Feindel said. The propensity scoring method the study used lacked all baseline variables that affect treatment choice and outcomes, “a crucial assumption for effective use of the propensity score,” he said. However, given the multitude of variables in patients with acute and complex IE, he said most surgeons would be hard pressed to accept that’s even possible in the model the study used.
Dr. Feindel also said a close examination of the 115 patients who underwent root replacement would have been “very instructional,” and the lack of follow-up on valve-related complications in almost 25% of the patients is another limitation of the study.
Nonetheless, the conclusions of Dr. Kim and his colleagues are “reasonable,” Dr. Feindel said. “Clearly, this article contributes important additional information to the surgical management of IE that will help guide surgeons, especially when it comes to prosthesis of choice,” he concluded. “It is up to the reader to decide whether this report finally puts to rest the “dogma” that homografts should preferentially be used in the setting of IE.”
Dr. Feindel had no relationships to disclose.
The study by Dr. Kim and his colleagues joins a series of reports questioning conventional thinking on the use of homografts to prevent recurrent infective endocarditis (IE), but their propensity matching does not account for surgeon bias in selecting a prosthesis, Dr. James K. Kirklin of the University of Alabama at Birmingham said in his invited commentary (J Thorac. Cardiovasc Surg. 2016 May;151:1230-1).
For example, surgeon preference may account for the wide disparity in full root replacements, depending on the type of prosthesis, Dr. Kirklin said. “Some experienced homograft surgeons have preferred the intra-aortic cylinder technique or infracoronary implantation, which avoids the short-term and longer-term complexities of full root replacement and has demonstrated long-term structural durability equivalent to that of the full root replacement,” he said.
Also, experienced homograft surgeons may prefer the homograft for its resistance to infection and adaptability to severe root infection in individual patients, particularly in those with severe infection with an abscess. And he cautioned against the study’s implication that conventional prostheses are equivocal in the setting of IE.
“Of considerable importance, however, is the evidence-based conclusion that surgical referral of routine surgical aortic valve endocarditis to a center experienced with aortic homograft surgery is not necessary, and a justifiable expectation is that aortic valve endocarditis requiring operation can be safely and appropriately managed in centers with standard aortic valve surgery experience who do not have access to or experience with aortic valve homografts,” Dr. Kirklin concluded.
Dr. Kirklin had no financial relationships to disclose.

|
Dr. Christopher M. Feindel |
The series by Dr. Kim and his colleagues, one of the largest of acute infective endocarditis to date, provides further evidence that the type of prosthesis used in surgery for IE involving the aortic valve probably does not affect long-term outcomes or reinfection rates, Dr. Christopher M. Feindel of the University of Toronto said in his invited commentary (J Thorac Cardiovasc Surg. 2016 May;151:1249-50).
However, Dr. Feindel said, “numerous confounding factors” inherent in any observational study could raise questions about the conclusion.
“This article delivers an important message, although not all surgeons will agree with the statistical approach taken by Dr. Kim and his colleagues,” Dr. Feindel said. The propensity scoring method the study used lacked all baseline variables that affect treatment choice and outcomes, “a crucial assumption for effective use of the propensity score,” he said. However, given the multitude of variables in patients with acute and complex IE, he said most surgeons would be hard pressed to accept that’s even possible in the model the study used.
Dr. Feindel also said a close examination of the 115 patients who underwent root replacement would have been “very instructional,” and the lack of follow-up on valve-related complications in almost 25% of the patients is another limitation of the study.
Nonetheless, the conclusions of Dr. Kim and his colleagues are “reasonable,” Dr. Feindel said. “Clearly, this article contributes important additional information to the surgical management of IE that will help guide surgeons, especially when it comes to prosthesis of choice,” he concluded. “It is up to the reader to decide whether this report finally puts to rest the “dogma” that homografts should preferentially be used in the setting of IE.”
Dr. Feindel had no relationships to disclose.
When a patient undergoes aortic valve replacement for infective endocarditis, conventional thinking holds that cardiac surgeons should use homografts because they have greater resistance to infection, but a recent study of more than 300 cases at two academic medical centers concluded that homografts may not necessarily offer such a benefit.
The study, published in the June issue of the Journal of Thoracic and Cardiovascular Surgery (2016;151:1239-48), involved 304 consecutive adult patients on whom 30-40 different surgeons performed operations for active infective endocarditis (IE) in the aortic valve from 2002 to 2014.
“Our findings suggest that patient-specific factors, such as age and implant preference, as well as technical reconstructive considerations, should drive prosthetic choice, rather than surgical dogma,” said Joon Bum Kim, Ph.D., of Massachusetts General Hospital, Harvard Medical School, both in Boston, and Asan Medical Center in Seoul, Korea, and his colleagues.
The study found that cardiac surgeons favored homografts over conventional prostheses when the patient had prosthetic valve endocarditis (58.1% vs. 28.8%) and methicillin-resistant Staphylococcus aureus (25.6% vs. 12.1%), both significant differences.
“No significant benefit to the use of homografts was demonstrable with regard to resistance to reinfection in the setting of IE,” Dr. Kim and his colleagues said.
Because reinfection after valve replacement for IE is such a strong concern, the debate over which prosthesis is best has ensued for decades. The researchers pointed out that the evidence favoring autologous or allogeneic tissue over synthetic material in the infective field is weak, mostly built on single-armed observational studies without comparison to conventional prosthesis.
With that in mind, the researchers pooled data from two institutions to compare short- and long-term results for homograft vs. conventional prosthetic valves in patients with IE. In this study group, 86 (28.3%) had homografts, 139 (45.7%) had xenograft prostheses, and 79 (26%) mechanical prostheses. The homograft group had more than twice the rate of early death than did the conventional group – 19.8% vs. 9.2%, a significant difference (P = .019).
During follow-up, which ranged from 4.7 to 72.6 months, 60 patients (19.7%) of the total group died and 23 (7.7%) experienced reinfection, but rates did not vary between the homograft and conventional prosthesis groups, Dr. Kim and his colleagues reported.
Demographics were similar between the three groups with a few exceptions Those who received the mechanical prostheses were younger (mean age, 47.2 years vs. 55.6 and 59.8 for the homograft and xenograft groups, respectively), had lower rates of diabetes (5.1% vs. 10.5% and 12.2%) and had less-severe disease based on New York Heart Association functional class III or IV scores (34.2% vs. 54.7% and 53.2%). The types of IE pathogens also differed among the three groups; methicillin-resistant staphylococci was most common in the homograft group (25.6%), whereas the viridans group streptococci was the leading cause of IE in the mechanical (38% ) and xenograft groups (25.2% ).
The use of homografts involves a highly complex operation, typically requiring a complete aortic root replacement, which “may be the major drawback in recommending it to patients already at high risk of operative mortality,” the investigators wrote. The durability of homografts makes their use limited for younger patients, and such grafts are somewhat scarce and require cryopreservation. “Therefore, the notion that homografts are required may in practice present an obstacle to appropriate surgical management of patients who have IE,” Dr. Kim and his coauthors wrote. All patients but one in the homograft group received aortic arch replacement (98.8%) whereas 30 of the patients in the conventional group did so (13.8%).
The study findings are consistent with an earlier comparative study (Ann. Thorac. Surg. 2012;93:480-07), according to Dr. Kim and his colleagues. “These findings suggest that patient-specific factors, such as patient preferences and technical considerations, should be the principal drivers of choices of valve prostheses,” they said. “Furthermore, lack of access to homografts should not be considered an obstacle to surgical therapy for this serious condition.”
Coauthor Dr. Sundt disclosed that he is a consultant for Thrasos Therapeutics. Dr. Kim and the other coauthors had no financial disclosures.
When a patient undergoes aortic valve replacement for infective endocarditis, conventional thinking holds that cardiac surgeons should use homografts because they have greater resistance to infection, but a recent study of more than 300 cases at two academic medical centers concluded that homografts may not necessarily offer such a benefit.
The study, published in the June issue of the Journal of Thoracic and Cardiovascular Surgery (2016;151:1239-48), involved 304 consecutive adult patients on whom 30-40 different surgeons performed operations for active infective endocarditis (IE) in the aortic valve from 2002 to 2014.
“Our findings suggest that patient-specific factors, such as age and implant preference, as well as technical reconstructive considerations, should drive prosthetic choice, rather than surgical dogma,” said Joon Bum Kim, Ph.D., of Massachusetts General Hospital, Harvard Medical School, both in Boston, and Asan Medical Center in Seoul, Korea, and his colleagues.
The study found that cardiac surgeons favored homografts over conventional prostheses when the patient had prosthetic valve endocarditis (58.1% vs. 28.8%) and methicillin-resistant Staphylococcus aureus (25.6% vs. 12.1%), both significant differences.
“No significant benefit to the use of homografts was demonstrable with regard to resistance to reinfection in the setting of IE,” Dr. Kim and his colleagues said.
Because reinfection after valve replacement for IE is such a strong concern, the debate over which prosthesis is best has ensued for decades. The researchers pointed out that the evidence favoring autologous or allogeneic tissue over synthetic material in the infective field is weak, mostly built on single-armed observational studies without comparison to conventional prosthesis.
With that in mind, the researchers pooled data from two institutions to compare short- and long-term results for homograft vs. conventional prosthetic valves in patients with IE. In this study group, 86 (28.3%) had homografts, 139 (45.7%) had xenograft prostheses, and 79 (26%) mechanical prostheses. The homograft group had more than twice the rate of early death than did the conventional group – 19.8% vs. 9.2%, a significant difference (P = .019).
During follow-up, which ranged from 4.7 to 72.6 months, 60 patients (19.7%) of the total group died and 23 (7.7%) experienced reinfection, but rates did not vary between the homograft and conventional prosthesis groups, Dr. Kim and his colleagues reported.
Demographics were similar between the three groups with a few exceptions Those who received the mechanical prostheses were younger (mean age, 47.2 years vs. 55.6 and 59.8 for the homograft and xenograft groups, respectively), had lower rates of diabetes (5.1% vs. 10.5% and 12.2%) and had less-severe disease based on New York Heart Association functional class III or IV scores (34.2% vs. 54.7% and 53.2%). The types of IE pathogens also differed among the three groups; methicillin-resistant staphylococci was most common in the homograft group (25.6%), whereas the viridans group streptococci was the leading cause of IE in the mechanical (38% ) and xenograft groups (25.2% ).
The use of homografts involves a highly complex operation, typically requiring a complete aortic root replacement, which “may be the major drawback in recommending it to patients already at high risk of operative mortality,” the investigators wrote. The durability of homografts makes their use limited for younger patients, and such grafts are somewhat scarce and require cryopreservation. “Therefore, the notion that homografts are required may in practice present an obstacle to appropriate surgical management of patients who have IE,” Dr. Kim and his coauthors wrote. All patients but one in the homograft group received aortic arch replacement (98.8%) whereas 30 of the patients in the conventional group did so (13.8%).
The study findings are consistent with an earlier comparative study (Ann. Thorac. Surg. 2012;93:480-07), according to Dr. Kim and his colleagues. “These findings suggest that patient-specific factors, such as patient preferences and technical considerations, should be the principal drivers of choices of valve prostheses,” they said. “Furthermore, lack of access to homografts should not be considered an obstacle to surgical therapy for this serious condition.”
Coauthor Dr. Sundt disclosed that he is a consultant for Thrasos Therapeutics. Dr. Kim and the other coauthors had no financial disclosures.
FROM THE JOURNAL OF THORACIC AND CARDIOVASCULAR SURGERY
Key clinical point: Use of homografts showed no significant benefit, compared with conventional prosthetic valves when the patient has infective endocarditis involving the aortic valve.
Major finding: The homograft group had more than twice the rate of early death than the conventional group, 19.8% vs. 9.2%, but in longer-term follow-up, the survival rates did not differ between groups.
Data source: 304 consecutive adult patients from the perspective database of two tertiary academic centers who had surgery for active infective endocarditis involving the aortic valve from 2002 to 2014.
Disclosures: Coauthor Dr. Sundt, disclosed he is a consultant for Thrasos Therapeutics. Dr. Kim and the other coauthors had no financial disclosures.
Additional antibiotics needed when implanting cryopreserved human aortic grafts
Infections of aortic prosthetic grafts can be a devastating complication, and while cryopreserved human allografts (CHA) can continue to possess antibacterial activity even after 5 years or more in storage, cardiac surgeons may want to apply additional antibiotic agents during implantation to boost bacterial resistance, investigators from Germany reported in the May issue of the Journal of Thoracic and Cardiovascular Surgery (2016;151:1251-9).
The researchers compared three different antibiotic regimens used in processing CHA aortic tissue and valves to determine the impact each can have on long-term bacterial resistance.
“Antibiotic combinations applied during CHA processing have a significant influence on their infection resistance,” said Dr. Viola Steffen of Hannover (Germany) Medical School and her colleagues. The average storage time of CHAs was 8.5 years, with the longest having been stored for 10 years.
The study involved microbiologic tests in vitro of three different antibiotic regimens used in processing CHA: gentamicin-piperacillin-vancomycin-metronidazole-amphotericin B (group A); gentamicin-piperacillin-flucloxacillin-metronidazole-amphotericin B (group B); and meropenem-vancomycin-tobramycin-colistin-amphotericin B (group C). The combinations are used to counteract Staphylococcus epidermidis and Staphylococcus aureus.
The study exposed pieces of 10 CHAs to different microbes and determined that regimen groups B and C were more effective than group A in eradicating gram-positive organisms. Specifically, group C was most resistant to Escherichia coli, whereas group B was most effective against Pseudomonas aeruginosa. Aortic tissue showed significantly less contamination with staphylococcal bacteria than valve grafts, the study reported.
Dr. Steffen and her colleagues said that tissue banks use antibiotic protocols during CHA processing, but they differ substantially. “Our results support the hypothesis that infection resistance of CHAs depends on the antibiotic pretreatment during processing and their residual activity,” they said.
The study had four key findings:
• The infection resistance of aortic wall and valve tissue differed significantly.
• In aortic wall specimens, group A specimens exhibited increased adherence of S. epidermidis, with vancomycin in group A and flucloxacillin in group B being the only differentiating agents between the two.
• Cryopreserved aortic vessels had a propensity toward reduced infection resistance against P. aeruginosa.
• Morphologic changes occurred in the microorganisms, especially rod-shaped E. coli, indicating that regional antibiotic release alters bacterial growth without eliminating all adherent bacteria.
Dr. Steffen and her colleagues noted that while previous studies determined that residual concentrations of antibiotics used in processing CHA heart valves and blood vessels are still present after they are prepared for implantation, they neither measured the antibacterial affect, clarified the period of cryopreservation nor differentiated between valve and vessel tissue (PLoS One. 2014;9:e112679; Transfus Med Hemother. 2011;38:379-86).
The Hannover researchers found that only a few gram-positive microorganisms adhered to aortic wall specimens, although they found “extensive adherence” of S. epidermidis in group A specimens. Valves, however, “were completely colonized” with both strains of staphylococcal bacteria, although the severity of contamination varied depending on the regimen used.
The findings raise some questions about the antibiotic properties of CHAs. “Because their infection resistance depends on the antibiotic combination selected during processing, further investigations concerning this treatment are necessary to improve the antimicrobial activity against frequent and highly virulent infection-causing bacteria,” Dr. Steffen and her colleagues said.
Dr. Steffen and her coauthors had no financial relationships to disclose.
Infections of aortic prosthetic grafts can be a devastating complication, and while cryopreserved human allografts (CHA) can continue to possess antibacterial activity even after 5 years or more in storage, cardiac surgeons may want to apply additional antibiotic agents during implantation to boost bacterial resistance, investigators from Germany reported in the May issue of the Journal of Thoracic and Cardiovascular Surgery (2016;151:1251-9).
The researchers compared three different antibiotic regimens used in processing CHA aortic tissue and valves to determine the impact each can have on long-term bacterial resistance.
“Antibiotic combinations applied during CHA processing have a significant influence on their infection resistance,” said Dr. Viola Steffen of Hannover (Germany) Medical School and her colleagues. The average storage time of CHAs was 8.5 years, with the longest having been stored for 10 years.
The study involved microbiologic tests in vitro of three different antibiotic regimens used in processing CHA: gentamicin-piperacillin-vancomycin-metronidazole-amphotericin B (group A); gentamicin-piperacillin-flucloxacillin-metronidazole-amphotericin B (group B); and meropenem-vancomycin-tobramycin-colistin-amphotericin B (group C). The combinations are used to counteract Staphylococcus epidermidis and Staphylococcus aureus.
The study exposed pieces of 10 CHAs to different microbes and determined that regimen groups B and C were more effective than group A in eradicating gram-positive organisms. Specifically, group C was most resistant to Escherichia coli, whereas group B was most effective against Pseudomonas aeruginosa. Aortic tissue showed significantly less contamination with staphylococcal bacteria than valve grafts, the study reported.
Dr. Steffen and her colleagues said that tissue banks use antibiotic protocols during CHA processing, but they differ substantially. “Our results support the hypothesis that infection resistance of CHAs depends on the antibiotic pretreatment during processing and their residual activity,” they said.
The study had four key findings:
• The infection resistance of aortic wall and valve tissue differed significantly.
• In aortic wall specimens, group A specimens exhibited increased adherence of S. epidermidis, with vancomycin in group A and flucloxacillin in group B being the only differentiating agents between the two.
• Cryopreserved aortic vessels had a propensity toward reduced infection resistance against P. aeruginosa.
• Morphologic changes occurred in the microorganisms, especially rod-shaped E. coli, indicating that regional antibiotic release alters bacterial growth without eliminating all adherent bacteria.
Dr. Steffen and her colleagues noted that while previous studies determined that residual concentrations of antibiotics used in processing CHA heart valves and blood vessels are still present after they are prepared for implantation, they neither measured the antibacterial affect, clarified the period of cryopreservation nor differentiated between valve and vessel tissue (PLoS One. 2014;9:e112679; Transfus Med Hemother. 2011;38:379-86).
The Hannover researchers found that only a few gram-positive microorganisms adhered to aortic wall specimens, although they found “extensive adherence” of S. epidermidis in group A specimens. Valves, however, “were completely colonized” with both strains of staphylococcal bacteria, although the severity of contamination varied depending on the regimen used.
The findings raise some questions about the antibiotic properties of CHAs. “Because their infection resistance depends on the antibiotic combination selected during processing, further investigations concerning this treatment are necessary to improve the antimicrobial activity against frequent and highly virulent infection-causing bacteria,” Dr. Steffen and her colleagues said.
Dr. Steffen and her coauthors had no financial relationships to disclose.
Infections of aortic prosthetic grafts can be a devastating complication, and while cryopreserved human allografts (CHA) can continue to possess antibacterial activity even after 5 years or more in storage, cardiac surgeons may want to apply additional antibiotic agents during implantation to boost bacterial resistance, investigators from Germany reported in the May issue of the Journal of Thoracic and Cardiovascular Surgery (2016;151:1251-9).
The researchers compared three different antibiotic regimens used in processing CHA aortic tissue and valves to determine the impact each can have on long-term bacterial resistance.
“Antibiotic combinations applied during CHA processing have a significant influence on their infection resistance,” said Dr. Viola Steffen of Hannover (Germany) Medical School and her colleagues. The average storage time of CHAs was 8.5 years, with the longest having been stored for 10 years.
The study involved microbiologic tests in vitro of three different antibiotic regimens used in processing CHA: gentamicin-piperacillin-vancomycin-metronidazole-amphotericin B (group A); gentamicin-piperacillin-flucloxacillin-metronidazole-amphotericin B (group B); and meropenem-vancomycin-tobramycin-colistin-amphotericin B (group C). The combinations are used to counteract Staphylococcus epidermidis and Staphylococcus aureus.
The study exposed pieces of 10 CHAs to different microbes and determined that regimen groups B and C were more effective than group A in eradicating gram-positive organisms. Specifically, group C was most resistant to Escherichia coli, whereas group B was most effective against Pseudomonas aeruginosa. Aortic tissue showed significantly less contamination with staphylococcal bacteria than valve grafts, the study reported.
Dr. Steffen and her colleagues said that tissue banks use antibiotic protocols during CHA processing, but they differ substantially. “Our results support the hypothesis that infection resistance of CHAs depends on the antibiotic pretreatment during processing and their residual activity,” they said.
The study had four key findings:
• The infection resistance of aortic wall and valve tissue differed significantly.
• In aortic wall specimens, group A specimens exhibited increased adherence of S. epidermidis, with vancomycin in group A and flucloxacillin in group B being the only differentiating agents between the two.
• Cryopreserved aortic vessels had a propensity toward reduced infection resistance against P. aeruginosa.
• Morphologic changes occurred in the microorganisms, especially rod-shaped E. coli, indicating that regional antibiotic release alters bacterial growth without eliminating all adherent bacteria.
Dr. Steffen and her colleagues noted that while previous studies determined that residual concentrations of antibiotics used in processing CHA heart valves and blood vessels are still present after they are prepared for implantation, they neither measured the antibacterial affect, clarified the period of cryopreservation nor differentiated between valve and vessel tissue (PLoS One. 2014;9:e112679; Transfus Med Hemother. 2011;38:379-86).
The Hannover researchers found that only a few gram-positive microorganisms adhered to aortic wall specimens, although they found “extensive adherence” of S. epidermidis in group A specimens. Valves, however, “were completely colonized” with both strains of staphylococcal bacteria, although the severity of contamination varied depending on the regimen used.
The findings raise some questions about the antibiotic properties of CHAs. “Because their infection resistance depends on the antibiotic combination selected during processing, further investigations concerning this treatment are necessary to improve the antimicrobial activity against frequent and highly virulent infection-causing bacteria,” Dr. Steffen and her colleagues said.
Dr. Steffen and her coauthors had no financial relationships to disclose.
FROM THE JOURNAL OF THORACIC AND CARDIOVASCULAR SURGERY
Key clinical point: The infection resistance of cryopreserved human allografts (CHAs) depends on antibiotic pretreatment during processing.
Major finding: Cardiac surgeons can recommend CHAs to patients either with active destructive infections or at high risk of reinfection, but they may want to apply additional antibiotic agents during implantation to enhance bacterial resistance.
Data source: Pieces of 10 CHAs were microbiologically tested in vitro and exposed to bacterial contamination and the number of attached bacteria quantified.
Disclosures: Dr. Steffen and her coauthors had no financial relationships to disclose.
TAVR cerebral protection device appears safe, effective
PARIS – The TriGuard neuroprotection device for use during transcatheter aortic valve replacement effectively prevented strokes while raising no safety concerns in a pooled analysis of three controlled trials, according to Dr. Alexandra J. Lansky.
The TriGuard, which is investigational in the United States but approved in Europe, also significantly reduced the risk of central nervous system infarction, as assessed by diffusion-weighted MRI. Moreover, when imaging did show CNS infarcts in patients with the TriGuard in place during their TAVR (transcatheter aortic valve replacement), the total brain lesion volume was about 40% less than in controls without the neuroprotection device, according to Dr. Lansky, professor of medicine and director of the cardiovascular clinical research program at Yale University in New Haven, Conn.
“Essentially what’s happening is that we’re reducing with this device the frequency of CNS infarctions, and also reducing the size of the lesions when they are present,” she said at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
The TriGuard is designed to fill an unmet need for stroke protection in TAVR patients. The incidence of clinical stroke within 30 days after TAVR in recent randomized controlled trials is 1.5%-6%. But there is clear evidence of underreporting of stroke in these trials. When neurologists examine TAVR patients or the patients are evaluated by serial testing using the NIH Stroke Scale plus brain imaging, the 30-day stroke rates are 15%-28%, according to the cardiologist.
“We know that about 50% of these strokes happen in the periprocedural period, and stroke is one of the strongest predictors of mortality, conferring a three- to ninefold increased risk,” Dr. Lansky emphasized.
She presented a pooled analysis including 59 TriGuard recipients and 83 controls who underwent TAVR in the DEFLECT I and III trials and the NeuroTAVR registry. They were evaluated using the NIH Stroke Scale before TAVR and again at 4 and 30 days post procedure. In addition, they underwent brain imaging via diffusion-weighted MRI 4 days post TAVR.
Stroke as defined by the Valve Academic Research Consortium–2 (VARC2) criteria occurred in none of the TriGuard group but in 6% of controls. And stroke as defined by the American Stroke Association, which requires a worsening score on the serial NIH Stroke Scale measurements plus imaging evidence of CNS infarction, occurred in 0 TriGuard-protected patients and in 19% of controls.
The incidence of CNS infarction on MRI was 92% in controls and 72% in the TriGuard group. Thus, 28% of patients with the TriGuard in place developed no brain infarct lesions at all; that’s a first for any TAVR neuroprotection device, according to Dr. Lansky.
In patients with CNS lesions, the total lesion volume was 101 mm3 in the TriGuard group, compared with 174 mm3 in the controls. The average lesion volume was 25 mm3 in the TriGuard group versus 43 mm3 in the controls.
TriGuard is a relatively simple device consisting of a single-wire nitinol frame and mesh filter with a pore size of 130 mcm. It’s designed to deflect emboli during TAVR while allowing maximal cerebral blood flow. After being delivered by a 9 French sheath from the contralateral femoral artery, the device sits at the roof of the aortic arch. Importantly, it covers all three cerebral arteries, Dr. Lansky said. The device is held in position by a stabilizer in the innominate artery.
Although introducing an additional element into TAVR raises the theoretic possibility of safety concerns, no safety signal was seen in the pooled analysis. In-hospital major adverse event rates were similar in the two groups.
Asked why 72% of patients with the TriGuard in place nonetheless developed CNS infarcts, Dr. Lansky said she believes the device has gaps on the sides that allow smaller emboli to pass through. Future iterations of the TriGuard will address this.
The clinical significance of the CNS infarcts seen on MRI in TAVR patients is a controversial issue among interventional cardiologists. Some cardiologists consider these to be silent lesions of dubious clinical relevance. That’s not Dr. Lansky’s view.
“When you track these MRI lesions out to 30 days, many times they disappear. They don’t disappear because there’s no damage; they disappear because the cells die. When you talk to neurologists about the MRI lesions, they will tell you that they actually represent cell death and correlate with brain infarction,” she said.
Dr. Nicolo Piazza commented that he considered the pooled analysis findings hypothesis generating but not definitive because of baseline imbalances between the two study arms. The control group had numerically higher – albeit not statistically significantly so – rates of atrial fibrillation at hospital admission as well as higher Society of Thoracic Surgeons risk scores, both of which increase stroke risk, noted Dr. Piazza of McGill University in Montreal.
Dr. Lansky replied that the much larger ongoing pivotal randomized, phase III REFLECT trial should provide definitive answers.
She reported receiving institutional research grant support from Keystone Heart, which produces the TriGuard device.
PARIS – The TriGuard neuroprotection device for use during transcatheter aortic valve replacement effectively prevented strokes while raising no safety concerns in a pooled analysis of three controlled trials, according to Dr. Alexandra J. Lansky.
The TriGuard, which is investigational in the United States but approved in Europe, also significantly reduced the risk of central nervous system infarction, as assessed by diffusion-weighted MRI. Moreover, when imaging did show CNS infarcts in patients with the TriGuard in place during their TAVR (transcatheter aortic valve replacement), the total brain lesion volume was about 40% less than in controls without the neuroprotection device, according to Dr. Lansky, professor of medicine and director of the cardiovascular clinical research program at Yale University in New Haven, Conn.
“Essentially what’s happening is that we’re reducing with this device the frequency of CNS infarctions, and also reducing the size of the lesions when they are present,” she said at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
The TriGuard is designed to fill an unmet need for stroke protection in TAVR patients. The incidence of clinical stroke within 30 days after TAVR in recent randomized controlled trials is 1.5%-6%. But there is clear evidence of underreporting of stroke in these trials. When neurologists examine TAVR patients or the patients are evaluated by serial testing using the NIH Stroke Scale plus brain imaging, the 30-day stroke rates are 15%-28%, according to the cardiologist.
“We know that about 50% of these strokes happen in the periprocedural period, and stroke is one of the strongest predictors of mortality, conferring a three- to ninefold increased risk,” Dr. Lansky emphasized.
She presented a pooled analysis including 59 TriGuard recipients and 83 controls who underwent TAVR in the DEFLECT I and III trials and the NeuroTAVR registry. They were evaluated using the NIH Stroke Scale before TAVR and again at 4 and 30 days post procedure. In addition, they underwent brain imaging via diffusion-weighted MRI 4 days post TAVR.
Stroke as defined by the Valve Academic Research Consortium–2 (VARC2) criteria occurred in none of the TriGuard group but in 6% of controls. And stroke as defined by the American Stroke Association, which requires a worsening score on the serial NIH Stroke Scale measurements plus imaging evidence of CNS infarction, occurred in 0 TriGuard-protected patients and in 19% of controls.
The incidence of CNS infarction on MRI was 92% in controls and 72% in the TriGuard group. Thus, 28% of patients with the TriGuard in place developed no brain infarct lesions at all; that’s a first for any TAVR neuroprotection device, according to Dr. Lansky.
In patients with CNS lesions, the total lesion volume was 101 mm3 in the TriGuard group, compared with 174 mm3 in the controls. The average lesion volume was 25 mm3 in the TriGuard group versus 43 mm3 in the controls.
TriGuard is a relatively simple device consisting of a single-wire nitinol frame and mesh filter with a pore size of 130 mcm. It’s designed to deflect emboli during TAVR while allowing maximal cerebral blood flow. After being delivered by a 9 French sheath from the contralateral femoral artery, the device sits at the roof of the aortic arch. Importantly, it covers all three cerebral arteries, Dr. Lansky said. The device is held in position by a stabilizer in the innominate artery.
Although introducing an additional element into TAVR raises the theoretic possibility of safety concerns, no safety signal was seen in the pooled analysis. In-hospital major adverse event rates were similar in the two groups.
Asked why 72% of patients with the TriGuard in place nonetheless developed CNS infarcts, Dr. Lansky said she believes the device has gaps on the sides that allow smaller emboli to pass through. Future iterations of the TriGuard will address this.
The clinical significance of the CNS infarcts seen on MRI in TAVR patients is a controversial issue among interventional cardiologists. Some cardiologists consider these to be silent lesions of dubious clinical relevance. That’s not Dr. Lansky’s view.
“When you track these MRI lesions out to 30 days, many times they disappear. They don’t disappear because there’s no damage; they disappear because the cells die. When you talk to neurologists about the MRI lesions, they will tell you that they actually represent cell death and correlate with brain infarction,” she said.
Dr. Nicolo Piazza commented that he considered the pooled analysis findings hypothesis generating but not definitive because of baseline imbalances between the two study arms. The control group had numerically higher – albeit not statistically significantly so – rates of atrial fibrillation at hospital admission as well as higher Society of Thoracic Surgeons risk scores, both of which increase stroke risk, noted Dr. Piazza of McGill University in Montreal.
Dr. Lansky replied that the much larger ongoing pivotal randomized, phase III REFLECT trial should provide definitive answers.
She reported receiving institutional research grant support from Keystone Heart, which produces the TriGuard device.
PARIS – The TriGuard neuroprotection device for use during transcatheter aortic valve replacement effectively prevented strokes while raising no safety concerns in a pooled analysis of three controlled trials, according to Dr. Alexandra J. Lansky.
The TriGuard, which is investigational in the United States but approved in Europe, also significantly reduced the risk of central nervous system infarction, as assessed by diffusion-weighted MRI. Moreover, when imaging did show CNS infarcts in patients with the TriGuard in place during their TAVR (transcatheter aortic valve replacement), the total brain lesion volume was about 40% less than in controls without the neuroprotection device, according to Dr. Lansky, professor of medicine and director of the cardiovascular clinical research program at Yale University in New Haven, Conn.
“Essentially what’s happening is that we’re reducing with this device the frequency of CNS infarctions, and also reducing the size of the lesions when they are present,” she said at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
The TriGuard is designed to fill an unmet need for stroke protection in TAVR patients. The incidence of clinical stroke within 30 days after TAVR in recent randomized controlled trials is 1.5%-6%. But there is clear evidence of underreporting of stroke in these trials. When neurologists examine TAVR patients or the patients are evaluated by serial testing using the NIH Stroke Scale plus brain imaging, the 30-day stroke rates are 15%-28%, according to the cardiologist.
“We know that about 50% of these strokes happen in the periprocedural period, and stroke is one of the strongest predictors of mortality, conferring a three- to ninefold increased risk,” Dr. Lansky emphasized.
She presented a pooled analysis including 59 TriGuard recipients and 83 controls who underwent TAVR in the DEFLECT I and III trials and the NeuroTAVR registry. They were evaluated using the NIH Stroke Scale before TAVR and again at 4 and 30 days post procedure. In addition, they underwent brain imaging via diffusion-weighted MRI 4 days post TAVR.
Stroke as defined by the Valve Academic Research Consortium–2 (VARC2) criteria occurred in none of the TriGuard group but in 6% of controls. And stroke as defined by the American Stroke Association, which requires a worsening score on the serial NIH Stroke Scale measurements plus imaging evidence of CNS infarction, occurred in 0 TriGuard-protected patients and in 19% of controls.
The incidence of CNS infarction on MRI was 92% in controls and 72% in the TriGuard group. Thus, 28% of patients with the TriGuard in place developed no brain infarct lesions at all; that’s a first for any TAVR neuroprotection device, according to Dr. Lansky.
In patients with CNS lesions, the total lesion volume was 101 mm3 in the TriGuard group, compared with 174 mm3 in the controls. The average lesion volume was 25 mm3 in the TriGuard group versus 43 mm3 in the controls.
TriGuard is a relatively simple device consisting of a single-wire nitinol frame and mesh filter with a pore size of 130 mcm. It’s designed to deflect emboli during TAVR while allowing maximal cerebral blood flow. After being delivered by a 9 French sheath from the contralateral femoral artery, the device sits at the roof of the aortic arch. Importantly, it covers all three cerebral arteries, Dr. Lansky said. The device is held in position by a stabilizer in the innominate artery.
Although introducing an additional element into TAVR raises the theoretic possibility of safety concerns, no safety signal was seen in the pooled analysis. In-hospital major adverse event rates were similar in the two groups.
Asked why 72% of patients with the TriGuard in place nonetheless developed CNS infarcts, Dr. Lansky said she believes the device has gaps on the sides that allow smaller emboli to pass through. Future iterations of the TriGuard will address this.
The clinical significance of the CNS infarcts seen on MRI in TAVR patients is a controversial issue among interventional cardiologists. Some cardiologists consider these to be silent lesions of dubious clinical relevance. That’s not Dr. Lansky’s view.
“When you track these MRI lesions out to 30 days, many times they disappear. They don’t disappear because there’s no damage; they disappear because the cells die. When you talk to neurologists about the MRI lesions, they will tell you that they actually represent cell death and correlate with brain infarction,” she said.
Dr. Nicolo Piazza commented that he considered the pooled analysis findings hypothesis generating but not definitive because of baseline imbalances between the two study arms. The control group had numerically higher – albeit not statistically significantly so – rates of atrial fibrillation at hospital admission as well as higher Society of Thoracic Surgeons risk scores, both of which increase stroke risk, noted Dr. Piazza of McGill University in Montreal.
Dr. Lansky replied that the much larger ongoing pivotal randomized, phase III REFLECT trial should provide definitive answers.
She reported receiving institutional research grant support from Keystone Heart, which produces the TriGuard device.
AT EUROPCR 2016
Key clinical point: The TriGuard neuroprotection device for use in TAVR effectively prevented strokes.
Major finding: The 30-day incidence of stroke in TAVR patients with the TriGard embolic protection device in place was 0, compared with 6% or 19% in controls, depending upon the stroke definition used.
Data source: A post hoc analysis of pooled data on 59 TriGuard recipients and 83 controls in three trials.
Disclosures: The presenter reported receiving institutional research grant support from Keystone Heart, which produces the TriGuard device.
Pulmonary function testing adds little to STS risk scores
Routine preoperative pulmonary function tests appear to have only limited utility in predicting outcomes in patients undergoing cardiothoracic surgery when the Society of Thoracic Surgeons risk score is available, according to the results of a retrospective study.
Dr. Alexander Ivanov of New York Methodist Hospital, Brooklyn, and his colleagues conducted a database analysis of 1,685 patients undergoing index cardiac surgery at New York Methodist Hospital between April 2004 and January 2014. They used the STS risk model version 2.73 to estimate postoperative risk of respiratory failure (defined as the need for mechanical ventilation greater than or equal to 72 hours, or reintubation), prolonged postoperative length of stay (defined as greater than 14 days), and 30-day all cause mortality in these patients, according to their report in The Journal of Thoracic and Cardiovascular Surgery (2016;151:1183-9).
They plotted the receiver operating characteristics curve for the STS score for each of these adverse events and compared the resulting area under the curve (AUC) with the AUC after adding pulmonary function testing parameters and COPD classifications.
A total of 1,412 patients had a calculated STS score, of which 751 underwent pulmonary function testing (53%). In general, patients who had pulmonary function testing were older and had higher rates of comorbidities and more complex cardiothoracic surgery compared with their counterparts, according to Dr. Ivanov. These patients also had significantly elevated STS risk for prolonged ventilation (12.4% vs. 10.3%), prolonged postoperative length of stay (8.9% vs. 7.2%), and 30-day mortality (2.7% vs. 2.2%).
The decision to perform pulmonary testing was left to the treating physician. Of those patients tested, 652 had bedside spirometry and 99 had formal laboratory testing. Forced expiratory volume in 1 second (FEV1) and forced volume vital capacity (FVC) values were determined by taking the best of three trials. COPD was diagnosed in cases of an FEV1/FVC ratio of less than 70%.
Among these patients, 4.5% developed postoperative respiratory failure, and there was no statistically significant difference in the respiratory failure rate between patients with and without pulmonary function testing. In addition, there was no significant difference in 30-day mortality between these patients (1.9% vs. 2.1%). However, a total of 6.9% had a prolonged postoperative length of stay, with a significantly higher rate in the patients with pulmonary function testing than without (8.8% vs. 4.7%).
Dr. Ivanov and his colleagues found that the AUC of the STS score was 0.65 (95% confidence interval [CI], 0.55-0.74)for respiratory failure, 0.67 (95% CI, 0.6-0.74) for prolonged postoperative length of stay, and 0.74 (95% CI, 0.6-0.87) for 30-day mortality. Even though the STS score based upon clinical definitions of lung disease afforded only modest discriminatory ability for the three studied outcomes, they found that there was no significant added benefit to the predictive ability of these STS scores obtained by incorporating any of the pulmonary function testing parameters or COPD classifications studied.
“A possible physiological explanation for these findings may be that the examined pulmonary function testing variables do not depend solely on pulmonary parameters such as airway diameter, degree of obstruction, or lung elasticity, but rather on a patient’s effort and muscle “strength,” characteristics that are already well captured and accounted for in the current STS model,” the researchers stated.
“The STS score calculated with clinical information on lung disease status offers modest discriminatory ability for respiratory failure, prolonged postoperative length of stay, and 30-day mortality after CT surgery, which cannot be improved by adding PFT parameters or PFT-derived COPD categorization,” they wrote. “Therefore, routine preoperative PFTs may have only limited clinical utility in patients undergoing CT surgery when the STS score is readily available. Further prospective studies will be helpful in confirming these conclusions,” Dr. Ivanov and his colleagues noted.
The authors reported that they had nothing to disclose.
Chronic lung disease is one of the risk factors included in the STS model for mortality, renal failure, prolonged ventilation, sternal wound infection, reoperation, and length of hospital stay. Mild, moderate, and severe CLD increases the odds ratio for those complications. A total of 20% of almost 1 million patients used in developing the current STS risk model had CLD.

|
Dr. Juan A. Crestanello |
The authors found that none of the pulmonary function testing parameters added to the predictive ability of the STS risk model for operative mortality, prolonged ventilation, or prolonged length of hospital stay. Because CLD is 1 of 40 preoperative and operative variables used in the STS risk model, an improvement in discrimination of only 1 of 40 variables is very unlikely to improve the overall model.
One may be tempted to conclude that it is not worth performing pulmonary function testing before cardiac surgery. However, remember once again the importance of precise and accurate data to support risk stratification. In science, behind each word resides a precise definition; without a pulmonary function test, we cannot define chronic lung disease severity.
Dr. Juan A. Crestanello is in the division of cardiac surgery, Wexner Medical Center, Ohio State University, Columbus. His remarks are from an invited commentary (J Thorac Cardiovasc Surg. 2016;151:1189-90).
Chronic lung disease is one of the risk factors included in the STS model for mortality, renal failure, prolonged ventilation, sternal wound infection, reoperation, and length of hospital stay. Mild, moderate, and severe CLD increases the odds ratio for those complications. A total of 20% of almost 1 million patients used in developing the current STS risk model had CLD.

|
Dr. Juan A. Crestanello |
The authors found that none of the pulmonary function testing parameters added to the predictive ability of the STS risk model for operative mortality, prolonged ventilation, or prolonged length of hospital stay. Because CLD is 1 of 40 preoperative and operative variables used in the STS risk model, an improvement in discrimination of only 1 of 40 variables is very unlikely to improve the overall model.
One may be tempted to conclude that it is not worth performing pulmonary function testing before cardiac surgery. However, remember once again the importance of precise and accurate data to support risk stratification. In science, behind each word resides a precise definition; without a pulmonary function test, we cannot define chronic lung disease severity.
Dr. Juan A. Crestanello is in the division of cardiac surgery, Wexner Medical Center, Ohio State University, Columbus. His remarks are from an invited commentary (J Thorac Cardiovasc Surg. 2016;151:1189-90).
Chronic lung disease is one of the risk factors included in the STS model for mortality, renal failure, prolonged ventilation, sternal wound infection, reoperation, and length of hospital stay. Mild, moderate, and severe CLD increases the odds ratio for those complications. A total of 20% of almost 1 million patients used in developing the current STS risk model had CLD.

|
Dr. Juan A. Crestanello |
The authors found that none of the pulmonary function testing parameters added to the predictive ability of the STS risk model for operative mortality, prolonged ventilation, or prolonged length of hospital stay. Because CLD is 1 of 40 preoperative and operative variables used in the STS risk model, an improvement in discrimination of only 1 of 40 variables is very unlikely to improve the overall model.
One may be tempted to conclude that it is not worth performing pulmonary function testing before cardiac surgery. However, remember once again the importance of precise and accurate data to support risk stratification. In science, behind each word resides a precise definition; without a pulmonary function test, we cannot define chronic lung disease severity.
Dr. Juan A. Crestanello is in the division of cardiac surgery, Wexner Medical Center, Ohio State University, Columbus. His remarks are from an invited commentary (J Thorac Cardiovasc Surg. 2016;151:1189-90).
Routine preoperative pulmonary function tests appear to have only limited utility in predicting outcomes in patients undergoing cardiothoracic surgery when the Society of Thoracic Surgeons risk score is available, according to the results of a retrospective study.
Dr. Alexander Ivanov of New York Methodist Hospital, Brooklyn, and his colleagues conducted a database analysis of 1,685 patients undergoing index cardiac surgery at New York Methodist Hospital between April 2004 and January 2014. They used the STS risk model version 2.73 to estimate postoperative risk of respiratory failure (defined as the need for mechanical ventilation greater than or equal to 72 hours, or reintubation), prolonged postoperative length of stay (defined as greater than 14 days), and 30-day all cause mortality in these patients, according to their report in The Journal of Thoracic and Cardiovascular Surgery (2016;151:1183-9).
They plotted the receiver operating characteristics curve for the STS score for each of these adverse events and compared the resulting area under the curve (AUC) with the AUC after adding pulmonary function testing parameters and COPD classifications.
A total of 1,412 patients had a calculated STS score, of which 751 underwent pulmonary function testing (53%). In general, patients who had pulmonary function testing were older and had higher rates of comorbidities and more complex cardiothoracic surgery compared with their counterparts, according to Dr. Ivanov. These patients also had significantly elevated STS risk for prolonged ventilation (12.4% vs. 10.3%), prolonged postoperative length of stay (8.9% vs. 7.2%), and 30-day mortality (2.7% vs. 2.2%).
The decision to perform pulmonary testing was left to the treating physician. Of those patients tested, 652 had bedside spirometry and 99 had formal laboratory testing. Forced expiratory volume in 1 second (FEV1) and forced volume vital capacity (FVC) values were determined by taking the best of three trials. COPD was diagnosed in cases of an FEV1/FVC ratio of less than 70%.
Among these patients, 4.5% developed postoperative respiratory failure, and there was no statistically significant difference in the respiratory failure rate between patients with and without pulmonary function testing. In addition, there was no significant difference in 30-day mortality between these patients (1.9% vs. 2.1%). However, a total of 6.9% had a prolonged postoperative length of stay, with a significantly higher rate in the patients with pulmonary function testing than without (8.8% vs. 4.7%).
Dr. Ivanov and his colleagues found that the AUC of the STS score was 0.65 (95% confidence interval [CI], 0.55-0.74)for respiratory failure, 0.67 (95% CI, 0.6-0.74) for prolonged postoperative length of stay, and 0.74 (95% CI, 0.6-0.87) for 30-day mortality. Even though the STS score based upon clinical definitions of lung disease afforded only modest discriminatory ability for the three studied outcomes, they found that there was no significant added benefit to the predictive ability of these STS scores obtained by incorporating any of the pulmonary function testing parameters or COPD classifications studied.
“A possible physiological explanation for these findings may be that the examined pulmonary function testing variables do not depend solely on pulmonary parameters such as airway diameter, degree of obstruction, or lung elasticity, but rather on a patient’s effort and muscle “strength,” characteristics that are already well captured and accounted for in the current STS model,” the researchers stated.
“The STS score calculated with clinical information on lung disease status offers modest discriminatory ability for respiratory failure, prolonged postoperative length of stay, and 30-day mortality after CT surgery, which cannot be improved by adding PFT parameters or PFT-derived COPD categorization,” they wrote. “Therefore, routine preoperative PFTs may have only limited clinical utility in patients undergoing CT surgery when the STS score is readily available. Further prospective studies will be helpful in confirming these conclusions,” Dr. Ivanov and his colleagues noted.
The authors reported that they had nothing to disclose.
Routine preoperative pulmonary function tests appear to have only limited utility in predicting outcomes in patients undergoing cardiothoracic surgery when the Society of Thoracic Surgeons risk score is available, according to the results of a retrospective study.
Dr. Alexander Ivanov of New York Methodist Hospital, Brooklyn, and his colleagues conducted a database analysis of 1,685 patients undergoing index cardiac surgery at New York Methodist Hospital between April 2004 and January 2014. They used the STS risk model version 2.73 to estimate postoperative risk of respiratory failure (defined as the need for mechanical ventilation greater than or equal to 72 hours, or reintubation), prolonged postoperative length of stay (defined as greater than 14 days), and 30-day all cause mortality in these patients, according to their report in The Journal of Thoracic and Cardiovascular Surgery (2016;151:1183-9).
They plotted the receiver operating characteristics curve for the STS score for each of these adverse events and compared the resulting area under the curve (AUC) with the AUC after adding pulmonary function testing parameters and COPD classifications.
A total of 1,412 patients had a calculated STS score, of which 751 underwent pulmonary function testing (53%). In general, patients who had pulmonary function testing were older and had higher rates of comorbidities and more complex cardiothoracic surgery compared with their counterparts, according to Dr. Ivanov. These patients also had significantly elevated STS risk for prolonged ventilation (12.4% vs. 10.3%), prolonged postoperative length of stay (8.9% vs. 7.2%), and 30-day mortality (2.7% vs. 2.2%).
The decision to perform pulmonary testing was left to the treating physician. Of those patients tested, 652 had bedside spirometry and 99 had formal laboratory testing. Forced expiratory volume in 1 second (FEV1) and forced volume vital capacity (FVC) values were determined by taking the best of three trials. COPD was diagnosed in cases of an FEV1/FVC ratio of less than 70%.
Among these patients, 4.5% developed postoperative respiratory failure, and there was no statistically significant difference in the respiratory failure rate between patients with and without pulmonary function testing. In addition, there was no significant difference in 30-day mortality between these patients (1.9% vs. 2.1%). However, a total of 6.9% had a prolonged postoperative length of stay, with a significantly higher rate in the patients with pulmonary function testing than without (8.8% vs. 4.7%).
Dr. Ivanov and his colleagues found that the AUC of the STS score was 0.65 (95% confidence interval [CI], 0.55-0.74)for respiratory failure, 0.67 (95% CI, 0.6-0.74) for prolonged postoperative length of stay, and 0.74 (95% CI, 0.6-0.87) for 30-day mortality. Even though the STS score based upon clinical definitions of lung disease afforded only modest discriminatory ability for the three studied outcomes, they found that there was no significant added benefit to the predictive ability of these STS scores obtained by incorporating any of the pulmonary function testing parameters or COPD classifications studied.
“A possible physiological explanation for these findings may be that the examined pulmonary function testing variables do not depend solely on pulmonary parameters such as airway diameter, degree of obstruction, or lung elasticity, but rather on a patient’s effort and muscle “strength,” characteristics that are already well captured and accounted for in the current STS model,” the researchers stated.
“The STS score calculated with clinical information on lung disease status offers modest discriminatory ability for respiratory failure, prolonged postoperative length of stay, and 30-day mortality after CT surgery, which cannot be improved by adding PFT parameters or PFT-derived COPD categorization,” they wrote. “Therefore, routine preoperative PFTs may have only limited clinical utility in patients undergoing CT surgery when the STS score is readily available. Further prospective studies will be helpful in confirming these conclusions,” Dr. Ivanov and his colleagues noted.
The authors reported that they had nothing to disclose.
FROM THE JOURNAL OF THORACIC AND CARDIOVASCULAR SURGERY
Key clinical point: Additional pulmonary function testing adds little predictive value to STS risk scoring when available.
Major finding: There was no significant added benefit to the predictive ability of STS scores obtained by incorporating any of the pulmonary function testing parameters or COPD classifications studied, as determined by AUC analysis.
Data source: A retrospective, database analysis of 1,685 patients undergoing index cardiac surgery at a single center between April 2004 and January 2014.
Disclosures: The authors reported that they had no disclosures.
TAVR degeneration estimated at 50% after 8 years
PARIS – The first-ever study of the long-term durability of transcatheter bioprosthetic aortic valves has documented a disturbing rise in the valve degeneration rate occurring 5-7 years post implant.
Prior consistently reassuring follow-up studies have been intermediate in length, with a maximum of 5 years. The PARTNER 2A trial, which generated enormous enthusiasm for moving TAVR to intermediate-risk patients on the basis of positive results presented at the 2016 meeting of the American College of Cardiology, reported 2-year results.
“We found, as have others, that there’s very little degeneration in the first 4 years: 94% freedom from degeneration. But at 6 years, it’s 82%, and we estimate that by 8 years, it’s about 50%,” Dr. Danny Dvir reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
“We need to be cautious: This is our first look at the data. But we have a signal for a problem,” said Dr. Dvir of St. Paul’s Hospital in Vancouver.
He presented a retrospective study of 378 patients who underwent TAVR 5-14 years ago at two pioneering centers for the procedure: St. Paul’s and Charles Nicolle Hospital in Rouen, France. Patients’ average age at the time of TAVR was 82.3 years, with an STS score of 8.3%. The study featured serial echocardiography conducted during house calls in this frail elderly population.
Thirty-five patients developed prosthetic valve degeneration, defined by at least moderate regurgitation and/or a mean gradient of at least 20 mm Hg in 23 cases and stenosis in 12. The risk of degeneration was unrelated to the use of warfarin, a finding that suggests the valve deterioration issue is unrelated to clotting. The strongest risk factor for transcatheter valve degeneration in this study was baseline renal failure at the time of TAVR.
Dr. Dvir’s presentation was the talk of the meeting, and it cast a pall over the proceedings. The red flag raised by the study regarding valve durability has major implications regarding the current enthusiasm among many interventional cardiologists to routinely extend TAVR to intermediate and even lower-risk patients. As one audience member later confessed, “I have felt sick since hearing that presentation.”
Discussant Dr. A. Pieter Kappetein observed that transcatheter heart valve durability was a hot topic of discussion about 4 years ago but subsequently faded below the radar as a concern – until Dr. Dvir’s study.
“This is a very important study that puts transcatheter heart valve implantation in a little bit different light,” said Dr. Kappetein, professor of cardiothoracic surgery at Erasmus University in Rotterdam, the Netherlands.
He noted that the surgical aortic valve replacement (SAVR) literature shows that the rate of structural valve deterioration is age related. It’s higher in 75-year-olds than in 85-year-olds, and higher still in 65-year-olds.
“Valve degeneration didn’t play a major role when we were doing TAVR in 80- and 85-year-olds because of their limited life expectancy, but it will play a role in younger patients. So I think we have to be careful before we move toward lower-risk patients,” the surgeon continued.
Dr. Jean-Francois Obadia, who performs both SAVR and TAVR, noted that the median duration of freedom from valve degeneration for Edwards Lifescience’s Carpentier surgical aortic valve is a hefty 17.9 years.
“This should be the gold standard,” declared Dr. Obadia, head of the department of adult cardiovascular surgery and heart transplantation at the Louis Pradel Cardiothoracic Hospital of Claude Bernard University in Lyon, France.
“Dr. Dvir’s study is one of the key messages we all should take back home: a 50% rate of valve deterioration at 8 years. Valve deterioration is the Achilles’ heel of bioprostheses. There is a lot of improvement left to do for the TAVR,” he said.
Dr. Dvir and others were quick to note that his long-term study was of necessity restricted to early-generation, balloon-expandable devices: the Cribier Edwards, Edwards Sapien, and Sapien XT valves. Contemporary valves, patient selection methods, and procedural techniques are far advanced in comparison.
“The Sapien 3 has much less paravalvular leakage than earlier-generation valves. Maybe with less paravalvular leakage and better hemodynamics there will be a decreased rate of degeneration. It could be. We need to see. We have to wait a few more years to see if later-generation transcatheter heart valves are more durable,” Dr. Dvir said.
To gain a better understanding of the full dimensions of the valve degeneration issue, he and his coinvestigators have formed the VALID (VAlve Long-term Durability International Data) registry. Operators interested in contributing patients to what is hoped will be a very large and informative data base are encouraged to contact Dr. Dvir ([email protected]).
In the meantime, he has reservations about extending TAVR to intermediate-risk patients outside of the rigorous clinical trial setting. He added that he’d feel far more comfortable in performing TAVR in intermediate-risk 70- to 75-year-olds if there was a tried and true valve-in-valve replacement method, something that doesn’t yet exist. The major limitation of current attempts at valve-in-valve replacement is underexpansion because the former valve doesn’t allow sufficient room for the new one to expand fully, resulting in residual stenosis.
“If you tell me that you can implant a platform that will enable a safe valve-in-valve procedure in 5, 7, 10 years – a less invasive bailout for a failed prosthetic valve – if you can do that safely and effectively I would be more keen to do TAVR even in a young patient,” the interventional cardiologist said.
He and others are working on this unmet need. Dr. Dvir’s novel valve, being developed with Edwards Lifesciences, has performed well in valve-in-valve procedures in cadavers and animals. The first clinical trials are being planned.
“We need to think always that a bioprosthetic valve is not a cure, it’s a palliation. We treat the patients, they feel better, but we leave them with some kind of a chronic disease that’s prone to thrombosis, prone to degeneration and failure, prone to many different things,” he reflected.
The study was conducted without commercial support. Dr. Dvir reported serving as a consultant to Edwards Lifesciences, Medtronic, and St. Jude Medical.
PARIS – The first-ever study of the long-term durability of transcatheter bioprosthetic aortic valves has documented a disturbing rise in the valve degeneration rate occurring 5-7 years post implant.
Prior consistently reassuring follow-up studies have been intermediate in length, with a maximum of 5 years. The PARTNER 2A trial, which generated enormous enthusiasm for moving TAVR to intermediate-risk patients on the basis of positive results presented at the 2016 meeting of the American College of Cardiology, reported 2-year results.
“We found, as have others, that there’s very little degeneration in the first 4 years: 94% freedom from degeneration. But at 6 years, it’s 82%, and we estimate that by 8 years, it’s about 50%,” Dr. Danny Dvir reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
“We need to be cautious: This is our first look at the data. But we have a signal for a problem,” said Dr. Dvir of St. Paul’s Hospital in Vancouver.
He presented a retrospective study of 378 patients who underwent TAVR 5-14 years ago at two pioneering centers for the procedure: St. Paul’s and Charles Nicolle Hospital in Rouen, France. Patients’ average age at the time of TAVR was 82.3 years, with an STS score of 8.3%. The study featured serial echocardiography conducted during house calls in this frail elderly population.
Thirty-five patients developed prosthetic valve degeneration, defined by at least moderate regurgitation and/or a mean gradient of at least 20 mm Hg in 23 cases and stenosis in 12. The risk of degeneration was unrelated to the use of warfarin, a finding that suggests the valve deterioration issue is unrelated to clotting. The strongest risk factor for transcatheter valve degeneration in this study was baseline renal failure at the time of TAVR.
Dr. Dvir’s presentation was the talk of the meeting, and it cast a pall over the proceedings. The red flag raised by the study regarding valve durability has major implications regarding the current enthusiasm among many interventional cardiologists to routinely extend TAVR to intermediate and even lower-risk patients. As one audience member later confessed, “I have felt sick since hearing that presentation.”
Discussant Dr. A. Pieter Kappetein observed that transcatheter heart valve durability was a hot topic of discussion about 4 years ago but subsequently faded below the radar as a concern – until Dr. Dvir’s study.
“This is a very important study that puts transcatheter heart valve implantation in a little bit different light,” said Dr. Kappetein, professor of cardiothoracic surgery at Erasmus University in Rotterdam, the Netherlands.
He noted that the surgical aortic valve replacement (SAVR) literature shows that the rate of structural valve deterioration is age related. It’s higher in 75-year-olds than in 85-year-olds, and higher still in 65-year-olds.
“Valve degeneration didn’t play a major role when we were doing TAVR in 80- and 85-year-olds because of their limited life expectancy, but it will play a role in younger patients. So I think we have to be careful before we move toward lower-risk patients,” the surgeon continued.
Dr. Jean-Francois Obadia, who performs both SAVR and TAVR, noted that the median duration of freedom from valve degeneration for Edwards Lifescience’s Carpentier surgical aortic valve is a hefty 17.9 years.
“This should be the gold standard,” declared Dr. Obadia, head of the department of adult cardiovascular surgery and heart transplantation at the Louis Pradel Cardiothoracic Hospital of Claude Bernard University in Lyon, France.
“Dr. Dvir’s study is one of the key messages we all should take back home: a 50% rate of valve deterioration at 8 years. Valve deterioration is the Achilles’ heel of bioprostheses. There is a lot of improvement left to do for the TAVR,” he said.
Dr. Dvir and others were quick to note that his long-term study was of necessity restricted to early-generation, balloon-expandable devices: the Cribier Edwards, Edwards Sapien, and Sapien XT valves. Contemporary valves, patient selection methods, and procedural techniques are far advanced in comparison.
“The Sapien 3 has much less paravalvular leakage than earlier-generation valves. Maybe with less paravalvular leakage and better hemodynamics there will be a decreased rate of degeneration. It could be. We need to see. We have to wait a few more years to see if later-generation transcatheter heart valves are more durable,” Dr. Dvir said.
To gain a better understanding of the full dimensions of the valve degeneration issue, he and his coinvestigators have formed the VALID (VAlve Long-term Durability International Data) registry. Operators interested in contributing patients to what is hoped will be a very large and informative data base are encouraged to contact Dr. Dvir ([email protected]).
In the meantime, he has reservations about extending TAVR to intermediate-risk patients outside of the rigorous clinical trial setting. He added that he’d feel far more comfortable in performing TAVR in intermediate-risk 70- to 75-year-olds if there was a tried and true valve-in-valve replacement method, something that doesn’t yet exist. The major limitation of current attempts at valve-in-valve replacement is underexpansion because the former valve doesn’t allow sufficient room for the new one to expand fully, resulting in residual stenosis.
“If you tell me that you can implant a platform that will enable a safe valve-in-valve procedure in 5, 7, 10 years – a less invasive bailout for a failed prosthetic valve – if you can do that safely and effectively I would be more keen to do TAVR even in a young patient,” the interventional cardiologist said.
He and others are working on this unmet need. Dr. Dvir’s novel valve, being developed with Edwards Lifesciences, has performed well in valve-in-valve procedures in cadavers and animals. The first clinical trials are being planned.
“We need to think always that a bioprosthetic valve is not a cure, it’s a palliation. We treat the patients, they feel better, but we leave them with some kind of a chronic disease that’s prone to thrombosis, prone to degeneration and failure, prone to many different things,” he reflected.
The study was conducted without commercial support. Dr. Dvir reported serving as a consultant to Edwards Lifesciences, Medtronic, and St. Jude Medical.
PARIS – The first-ever study of the long-term durability of transcatheter bioprosthetic aortic valves has documented a disturbing rise in the valve degeneration rate occurring 5-7 years post implant.
Prior consistently reassuring follow-up studies have been intermediate in length, with a maximum of 5 years. The PARTNER 2A trial, which generated enormous enthusiasm for moving TAVR to intermediate-risk patients on the basis of positive results presented at the 2016 meeting of the American College of Cardiology, reported 2-year results.
“We found, as have others, that there’s very little degeneration in the first 4 years: 94% freedom from degeneration. But at 6 years, it’s 82%, and we estimate that by 8 years, it’s about 50%,” Dr. Danny Dvir reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
“We need to be cautious: This is our first look at the data. But we have a signal for a problem,” said Dr. Dvir of St. Paul’s Hospital in Vancouver.
He presented a retrospective study of 378 patients who underwent TAVR 5-14 years ago at two pioneering centers for the procedure: St. Paul’s and Charles Nicolle Hospital in Rouen, France. Patients’ average age at the time of TAVR was 82.3 years, with an STS score of 8.3%. The study featured serial echocardiography conducted during house calls in this frail elderly population.
Thirty-five patients developed prosthetic valve degeneration, defined by at least moderate regurgitation and/or a mean gradient of at least 20 mm Hg in 23 cases and stenosis in 12. The risk of degeneration was unrelated to the use of warfarin, a finding that suggests the valve deterioration issue is unrelated to clotting. The strongest risk factor for transcatheter valve degeneration in this study was baseline renal failure at the time of TAVR.
Dr. Dvir’s presentation was the talk of the meeting, and it cast a pall over the proceedings. The red flag raised by the study regarding valve durability has major implications regarding the current enthusiasm among many interventional cardiologists to routinely extend TAVR to intermediate and even lower-risk patients. As one audience member later confessed, “I have felt sick since hearing that presentation.”
Discussant Dr. A. Pieter Kappetein observed that transcatheter heart valve durability was a hot topic of discussion about 4 years ago but subsequently faded below the radar as a concern – until Dr. Dvir’s study.
“This is a very important study that puts transcatheter heart valve implantation in a little bit different light,” said Dr. Kappetein, professor of cardiothoracic surgery at Erasmus University in Rotterdam, the Netherlands.
He noted that the surgical aortic valve replacement (SAVR) literature shows that the rate of structural valve deterioration is age related. It’s higher in 75-year-olds than in 85-year-olds, and higher still in 65-year-olds.
“Valve degeneration didn’t play a major role when we were doing TAVR in 80- and 85-year-olds because of their limited life expectancy, but it will play a role in younger patients. So I think we have to be careful before we move toward lower-risk patients,” the surgeon continued.
Dr. Jean-Francois Obadia, who performs both SAVR and TAVR, noted that the median duration of freedom from valve degeneration for Edwards Lifescience’s Carpentier surgical aortic valve is a hefty 17.9 years.
“This should be the gold standard,” declared Dr. Obadia, head of the department of adult cardiovascular surgery and heart transplantation at the Louis Pradel Cardiothoracic Hospital of Claude Bernard University in Lyon, France.
“Dr. Dvir’s study is one of the key messages we all should take back home: a 50% rate of valve deterioration at 8 years. Valve deterioration is the Achilles’ heel of bioprostheses. There is a lot of improvement left to do for the TAVR,” he said.
Dr. Dvir and others were quick to note that his long-term study was of necessity restricted to early-generation, balloon-expandable devices: the Cribier Edwards, Edwards Sapien, and Sapien XT valves. Contemporary valves, patient selection methods, and procedural techniques are far advanced in comparison.
“The Sapien 3 has much less paravalvular leakage than earlier-generation valves. Maybe with less paravalvular leakage and better hemodynamics there will be a decreased rate of degeneration. It could be. We need to see. We have to wait a few more years to see if later-generation transcatheter heart valves are more durable,” Dr. Dvir said.
To gain a better understanding of the full dimensions of the valve degeneration issue, he and his coinvestigators have formed the VALID (VAlve Long-term Durability International Data) registry. Operators interested in contributing patients to what is hoped will be a very large and informative data base are encouraged to contact Dr. Dvir ([email protected]).
In the meantime, he has reservations about extending TAVR to intermediate-risk patients outside of the rigorous clinical trial setting. He added that he’d feel far more comfortable in performing TAVR in intermediate-risk 70- to 75-year-olds if there was a tried and true valve-in-valve replacement method, something that doesn’t yet exist. The major limitation of current attempts at valve-in-valve replacement is underexpansion because the former valve doesn’t allow sufficient room for the new one to expand fully, resulting in residual stenosis.
“If you tell me that you can implant a platform that will enable a safe valve-in-valve procedure in 5, 7, 10 years – a less invasive bailout for a failed prosthetic valve – if you can do that safely and effectively I would be more keen to do TAVR even in a young patient,” the interventional cardiologist said.
He and others are working on this unmet need. Dr. Dvir’s novel valve, being developed with Edwards Lifesciences, has performed well in valve-in-valve procedures in cadavers and animals. The first clinical trials are being planned.
“We need to think always that a bioprosthetic valve is not a cure, it’s a palliation. We treat the patients, they feel better, but we leave them with some kind of a chronic disease that’s prone to thrombosis, prone to degeneration and failure, prone to many different things,” he reflected.
The study was conducted without commercial support. Dr. Dvir reported serving as a consultant to Edwards Lifesciences, Medtronic, and St. Jude Medical.
AT EUROPCR 2016
Key clinical point: The first study to examine transcatheter aortic bioprosthetic valve performance beyond 5 years has found a 50% rate of valve degeneration 8 years post TAVR.
Major finding: A sharp increase in the incidence of degeneration of these early-generation valves occurred 5-7 years post TAVR.
Data source: This retrospective study featured serial home echocardiography in 378 patients who underwent TAVR 5-14 years ago at two pioneering centers for the procedure.
Disclosures: The presenter of this study, conducted without commercial support, serves as a consultant to Edwards Lifesciences, Medtronic, and St. Jude Medical.
VIDEO: TAVR may soon eclipse surgery for aortic stenosis patients
BALTIMORE – A study comparing the causes and timing of death in high-risk patients with severe, symptomatic aortic stenosis suggests that transcatheter aortic valve replacement (TAVR) may have significant advantages over surgical aortic valve replacement (SAVR).
The research team, presenting their findings at the annual meeting of the American Association for Thoracic Surgery, revealed that death rates in the TAVR and SAVR cohorts in the CoreValve US Pivotal Trial High Risk Study were similar at 0-30 days and 91-365 days post procedure, but found that more SAVR patients died from day 31-90 related to bleeding, frailty, and comorbid diseases. The investigators concluded that this finding reflected the impact of the added strain of surgery over intravascular intervention for aortic valve replacement.
Dr. Craig Smith, chair of the department of surgery at NewYork–Presbyterian Hospital/Columbia University Medical Center, and a discussant on the paper, said this study is the only randomized clinical trial to show inferiority of surgery to TAVR.
“The difference seems to reside in the recovery phase mortality, which they showed very well,” Dr. Smith said in a video interview. He predicted that the trial results would add to the momentum making TAVR the first choice for any patient who is high risk and at least an equivalent choice for patients who are considered intermediate risk.
“The big question on the horizon is what is the best thing to recommend to patients who we classify as low risk,” Dr. Smith said. “Unless something happens to the impressive results that TAVR is racking up, in a few years … approval will be granted for those who are low risk.”
Dr. Smith reported no relevant financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @richpizzi
BALTIMORE – A study comparing the causes and timing of death in high-risk patients with severe, symptomatic aortic stenosis suggests that transcatheter aortic valve replacement (TAVR) may have significant advantages over surgical aortic valve replacement (SAVR).
The research team, presenting their findings at the annual meeting of the American Association for Thoracic Surgery, revealed that death rates in the TAVR and SAVR cohorts in the CoreValve US Pivotal Trial High Risk Study were similar at 0-30 days and 91-365 days post procedure, but found that more SAVR patients died from day 31-90 related to bleeding, frailty, and comorbid diseases. The investigators concluded that this finding reflected the impact of the added strain of surgery over intravascular intervention for aortic valve replacement.
Dr. Craig Smith, chair of the department of surgery at NewYork–Presbyterian Hospital/Columbia University Medical Center, and a discussant on the paper, said this study is the only randomized clinical trial to show inferiority of surgery to TAVR.
“The difference seems to reside in the recovery phase mortality, which they showed very well,” Dr. Smith said in a video interview. He predicted that the trial results would add to the momentum making TAVR the first choice for any patient who is high risk and at least an equivalent choice for patients who are considered intermediate risk.
“The big question on the horizon is what is the best thing to recommend to patients who we classify as low risk,” Dr. Smith said. “Unless something happens to the impressive results that TAVR is racking up, in a few years … approval will be granted for those who are low risk.”
Dr. Smith reported no relevant financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @richpizzi
BALTIMORE – A study comparing the causes and timing of death in high-risk patients with severe, symptomatic aortic stenosis suggests that transcatheter aortic valve replacement (TAVR) may have significant advantages over surgical aortic valve replacement (SAVR).
The research team, presenting their findings at the annual meeting of the American Association for Thoracic Surgery, revealed that death rates in the TAVR and SAVR cohorts in the CoreValve US Pivotal Trial High Risk Study were similar at 0-30 days and 91-365 days post procedure, but found that more SAVR patients died from day 31-90 related to bleeding, frailty, and comorbid diseases. The investigators concluded that this finding reflected the impact of the added strain of surgery over intravascular intervention for aortic valve replacement.
Dr. Craig Smith, chair of the department of surgery at NewYork–Presbyterian Hospital/Columbia University Medical Center, and a discussant on the paper, said this study is the only randomized clinical trial to show inferiority of surgery to TAVR.
“The difference seems to reside in the recovery phase mortality, which they showed very well,” Dr. Smith said in a video interview. He predicted that the trial results would add to the momentum making TAVR the first choice for any patient who is high risk and at least an equivalent choice for patients who are considered intermediate risk.
“The big question on the horizon is what is the best thing to recommend to patients who we classify as low risk,” Dr. Smith said. “Unless something happens to the impressive results that TAVR is racking up, in a few years … approval will be granted for those who are low risk.”
Dr. Smith reported no relevant financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @richpizzi
AT THE AATS ANNUAL MEETING
Rotor ablation for atrial fibrillation strikes out in first randomized trial
SAN FRANCISCO – Focal impulse and rotor modulation-guided ablation for persistent atrial fibrillation – either alone or in conjunction with other procedures – increased procedural times without improving outcomes, according to the first randomized trial to assess its utility.
In fact, enrollment in the rotor ablation-only (RA) arm was halted early for futility. “There was 100% recurrence” of atrial fibrillation (AF), said senior investigator Dr. Andrea Natale, executive medical director of the Texas Cardiac Arrhythmia Institute, Austin.
“I’m surprised it took this long for a randomized study, because this system has been around for 5 or 6 years,” noted Dr. Natale. “Our community should demand these sorts of studies earlier, because it’s not fair for patients to go on with a procedure for years that has not been proven to be effective.
“For us, unless there is a new version of rotor mapping that I feel is significantly different, this will be the end of rotor ablation in my lab with this system [the Topera Physiologic Rotor Mapping Solution],” Dr. Natale said at the annual scientific sessions of the Heart Rhythm Society.
In the study, his team randomized 29 patients to RA only, 42 to RA plus pulmonary vein antral isolation (PVAI), and 42 to PVAI plus posterior wall and nonpulmonary vein trigger ablation.
At about 1 year, four RA-only patients (14%), 22 RA plus PVAI patients (52%), and 32 patients in the PVAI plus trigger group (76%) were free of AF and atrial tachycardias without antiarrhythmic drugs (P < .0001).
Meanwhile, RA alone and RA plus PVAI cases took about 230 minutes, while the more effective PVAI plus trigger approach took about 130 minutes (P < .001).
There was “a very poor outcome with rotor-only ablation,” Dr. Natale said. “There isn’t a benefit either alone or as an add-on strategy, at least with this mapping software.”
Perhaps “people who think rotors don’t exist are right,” he added. On the other hand, maybe the basket mapping catheter doesn’t touch enough of the left atrium, or the software that makes sense of what the catheter detects needs to be improved, Dr. Natale noted.
All the patients were undergoing their first ablation. They were in their early 60s, on average, and most were men. The mean left atrium diameter was about 47 mm, and mean left ventricle ejection fraction about 55%. There were no statistically significant differences between the study arms, and no significant differences in outcomes between the 70% of patients with persistent AF and the 30% with long-standing persistent AF.
There was no industry funding for the work. Dr. Natale disclosed relationships with Biosense Webster, Boston Scientific, Janssen, Medtronic, and St. Jude Medical.
My gut sense is that there’s something to rotor mapping, but we are not there yet. There are a lot of investment dollars and a lot of bright people working on this. It really is the Holy Grail to find the source of AF.
Dr. John Day is the director of Intermountain Heart Rhythm Specialists in Murray, Utah, and the current president of the Hearth Rhythm Society. He had no disclosures.
My gut sense is that there’s something to rotor mapping, but we are not there yet. There are a lot of investment dollars and a lot of bright people working on this. It really is the Holy Grail to find the source of AF.
Dr. John Day is the director of Intermountain Heart Rhythm Specialists in Murray, Utah, and the current president of the Hearth Rhythm Society. He had no disclosures.
My gut sense is that there’s something to rotor mapping, but we are not there yet. There are a lot of investment dollars and a lot of bright people working on this. It really is the Holy Grail to find the source of AF.
Dr. John Day is the director of Intermountain Heart Rhythm Specialists in Murray, Utah, and the current president of the Hearth Rhythm Society. He had no disclosures.
SAN FRANCISCO – Focal impulse and rotor modulation-guided ablation for persistent atrial fibrillation – either alone or in conjunction with other procedures – increased procedural times without improving outcomes, according to the first randomized trial to assess its utility.
In fact, enrollment in the rotor ablation-only (RA) arm was halted early for futility. “There was 100% recurrence” of atrial fibrillation (AF), said senior investigator Dr. Andrea Natale, executive medical director of the Texas Cardiac Arrhythmia Institute, Austin.
“I’m surprised it took this long for a randomized study, because this system has been around for 5 or 6 years,” noted Dr. Natale. “Our community should demand these sorts of studies earlier, because it’s not fair for patients to go on with a procedure for years that has not been proven to be effective.
“For us, unless there is a new version of rotor mapping that I feel is significantly different, this will be the end of rotor ablation in my lab with this system [the Topera Physiologic Rotor Mapping Solution],” Dr. Natale said at the annual scientific sessions of the Heart Rhythm Society.
In the study, his team randomized 29 patients to RA only, 42 to RA plus pulmonary vein antral isolation (PVAI), and 42 to PVAI plus posterior wall and nonpulmonary vein trigger ablation.
At about 1 year, four RA-only patients (14%), 22 RA plus PVAI patients (52%), and 32 patients in the PVAI plus trigger group (76%) were free of AF and atrial tachycardias without antiarrhythmic drugs (P < .0001).
Meanwhile, RA alone and RA plus PVAI cases took about 230 minutes, while the more effective PVAI plus trigger approach took about 130 minutes (P < .001).
There was “a very poor outcome with rotor-only ablation,” Dr. Natale said. “There isn’t a benefit either alone or as an add-on strategy, at least with this mapping software.”
Perhaps “people who think rotors don’t exist are right,” he added. On the other hand, maybe the basket mapping catheter doesn’t touch enough of the left atrium, or the software that makes sense of what the catheter detects needs to be improved, Dr. Natale noted.
All the patients were undergoing their first ablation. They were in their early 60s, on average, and most were men. The mean left atrium diameter was about 47 mm, and mean left ventricle ejection fraction about 55%. There were no statistically significant differences between the study arms, and no significant differences in outcomes between the 70% of patients with persistent AF and the 30% with long-standing persistent AF.
There was no industry funding for the work. Dr. Natale disclosed relationships with Biosense Webster, Boston Scientific, Janssen, Medtronic, and St. Jude Medical.
SAN FRANCISCO – Focal impulse and rotor modulation-guided ablation for persistent atrial fibrillation – either alone or in conjunction with other procedures – increased procedural times without improving outcomes, according to the first randomized trial to assess its utility.
In fact, enrollment in the rotor ablation-only (RA) arm was halted early for futility. “There was 100% recurrence” of atrial fibrillation (AF), said senior investigator Dr. Andrea Natale, executive medical director of the Texas Cardiac Arrhythmia Institute, Austin.
“I’m surprised it took this long for a randomized study, because this system has been around for 5 or 6 years,” noted Dr. Natale. “Our community should demand these sorts of studies earlier, because it’s not fair for patients to go on with a procedure for years that has not been proven to be effective.
“For us, unless there is a new version of rotor mapping that I feel is significantly different, this will be the end of rotor ablation in my lab with this system [the Topera Physiologic Rotor Mapping Solution],” Dr. Natale said at the annual scientific sessions of the Heart Rhythm Society.
In the study, his team randomized 29 patients to RA only, 42 to RA plus pulmonary vein antral isolation (PVAI), and 42 to PVAI plus posterior wall and nonpulmonary vein trigger ablation.
At about 1 year, four RA-only patients (14%), 22 RA plus PVAI patients (52%), and 32 patients in the PVAI plus trigger group (76%) were free of AF and atrial tachycardias without antiarrhythmic drugs (P < .0001).
Meanwhile, RA alone and RA plus PVAI cases took about 230 minutes, while the more effective PVAI plus trigger approach took about 130 minutes (P < .001).
There was “a very poor outcome with rotor-only ablation,” Dr. Natale said. “There isn’t a benefit either alone or as an add-on strategy, at least with this mapping software.”
Perhaps “people who think rotors don’t exist are right,” he added. On the other hand, maybe the basket mapping catheter doesn’t touch enough of the left atrium, or the software that makes sense of what the catheter detects needs to be improved, Dr. Natale noted.
All the patients were undergoing their first ablation. They were in their early 60s, on average, and most were men. The mean left atrium diameter was about 47 mm, and mean left ventricle ejection fraction about 55%. There were no statistically significant differences between the study arms, and no significant differences in outcomes between the 70% of patients with persistent AF and the 30% with long-standing persistent AF.
There was no industry funding for the work. Dr. Natale disclosed relationships with Biosense Webster, Boston Scientific, Janssen, Medtronic, and St. Jude Medical.
AT HEART RHYTHM 2016
Key clinical point: Focal impulse and rotor modulation-guided ablation for persistent atrial fibrillation – either alone or in conjunction with other procedures – increased procedural times without improving outcomes.
Major finding: At about 1 year, four rotor ablation-only patients (14%), 22 RA plus pulmonary vein antral isolation patients (52.4%), and 32 patients in the PVAI plus trigger group (76%) were free of atrial fibrillation and atrial tachycardias without antiarrhythmic drugs (P < .0001).
Data source: A randomized trial in 113 persistent AF patients.
Disclosures: There was no industry funding for the work. The senior investigator disclosed relationships with Biosense Webster, Boston Scientific, Janssen, Medtronic, and St. Jude Medical.