User login
VIDEO: Sutureless Heart Valve: A Critical Innovation
BALTIMORE – The sutureless heart valve may now be a “necessity” for contemporary cardiothoracic surgeons, said an expert at the annual meeting of the American Association for Thoracic Surgery.
In an interview at the event, Niv Ad, MD, chief of cardiac surgery at Inova Heart and Vascular Institute, Falls Church, Va., discussed the results of an international trial evaluating clinical outcomes of two patient subgroups implanted with a sutureless valve prosthesis. He was enthusiastic about the trial’s results and the future promise of the sutureless valve.
The study, "Clinical Outcomes in Low and Intermediate-High Risk Groups with a Sutureless Heart Valve," will be presented at 7:30 a.m. on Wednesday.
“This study teaches us that the technology is safe,” he said. “This is really great news for surgeons, cardiologists, and patients. Complications associated with implantation of the valves were fairly low in their incidence, and the survival rates at 1 year were high.”
The study, led by Axel Haverich, MD, of Hannover Medical School in Germany, confirmed the safety and performance of the valve in both patient groups – high and low risk – regardless of the preoperative risk score. Dr. Ad said that sutureless valve technology would likely replace suture technology in all cases, although perhaps not in the near term.
Dr. Ad reported no relevant financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @richpizzi
BALTIMORE – The sutureless heart valve may now be a “necessity” for contemporary cardiothoracic surgeons, said an expert at the annual meeting of the American Association for Thoracic Surgery.
In an interview at the event, Niv Ad, MD, chief of cardiac surgery at Inova Heart and Vascular Institute, Falls Church, Va., discussed the results of an international trial evaluating clinical outcomes of two patient subgroups implanted with a sutureless valve prosthesis. He was enthusiastic about the trial’s results and the future promise of the sutureless valve.
The study, "Clinical Outcomes in Low and Intermediate-High Risk Groups with a Sutureless Heart Valve," will be presented at 7:30 a.m. on Wednesday.
“This study teaches us that the technology is safe,” he said. “This is really great news for surgeons, cardiologists, and patients. Complications associated with implantation of the valves were fairly low in their incidence, and the survival rates at 1 year were high.”
The study, led by Axel Haverich, MD, of Hannover Medical School in Germany, confirmed the safety and performance of the valve in both patient groups – high and low risk – regardless of the preoperative risk score. Dr. Ad said that sutureless valve technology would likely replace suture technology in all cases, although perhaps not in the near term.
Dr. Ad reported no relevant financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @richpizzi
BALTIMORE – The sutureless heart valve may now be a “necessity” for contemporary cardiothoracic surgeons, said an expert at the annual meeting of the American Association for Thoracic Surgery.
In an interview at the event, Niv Ad, MD, chief of cardiac surgery at Inova Heart and Vascular Institute, Falls Church, Va., discussed the results of an international trial evaluating clinical outcomes of two patient subgroups implanted with a sutureless valve prosthesis. He was enthusiastic about the trial’s results and the future promise of the sutureless valve.
The study, "Clinical Outcomes in Low and Intermediate-High Risk Groups with a Sutureless Heart Valve," will be presented at 7:30 a.m. on Wednesday.
“This study teaches us that the technology is safe,” he said. “This is really great news for surgeons, cardiologists, and patients. Complications associated with implantation of the valves were fairly low in their incidence, and the survival rates at 1 year were high.”
The study, led by Axel Haverich, MD, of Hannover Medical School in Germany, confirmed the safety and performance of the valve in both patient groups – high and low risk – regardless of the preoperative risk score. Dr. Ad said that sutureless valve technology would likely replace suture technology in all cases, although perhaps not in the near term.
Dr. Ad reported no relevant financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @richpizzi
AT THE AATS ANNUAL MEETING
Rhythm control may be best for atrial fib in HFpEF
CHICAGO – Atrial fibrillation with good heart rate control in patients who have heart failure with preserved ejection fraction is independently associated with exercise intolerance, impaired contractile reserve, and a sharply higher mortality rate than in matched HFpEF patients without the arrhythmia, a retrospective analysis showed.
“Our study, the largest of its kind, provides mechanistic evidence from cardiopulmonary testing that a rhythm control strategy may potentially improve peak exercise capacity and survival in this patient population, a finding that of course requires future prospective appraisal in randomized trials comparing rate and rhythm control of atrial fibrillation in HFpEF,” Dr. Mohamed Badreldin Elshazly reported at the annual meeting of the American College of Cardiology.
In the meantime, his study also shows the useful role cardiopulmonary stress testing can play in the setting of atrial fibrillation (AF) in HFpEF, he added.
“Cardiopulmonary stress tests are cheap and easy to do. They’re a big asset for personalized medicine. Using an objective measure like cardiopulmonary stress testing to define the physiologic and hemodynamic consequences of atrial fibrillation in individual patients may help identify those in whom rhythm control may improve exercise tolerance and quality of life, and those who may be okay with rate control,” according to Dr. Elshazly of the Cleveland Clinic.
He noted that while it’s well established that atrial fibrillation is associated with exercise intolerance in patients with heart failure with reduced ejection fraction (HFrEF) and that restoration of sinus rhythm in such patients has a positive impact on exercise hemodynamics, symptom severity, and quality of life, the situation is murkier regarding AF in patients with HFpEF. Prior studies were generally small and unable to establish whether AF was independently associated with exercise intolerance or if HFpEF patients who developed AF were sicker and higher risk.
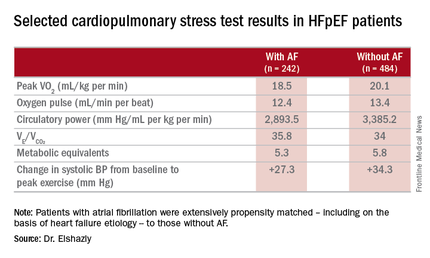
He presented a retrospective, case-control study in a cohort of 1,825 patients with HFpEF referred for maximal, symptom-limited cardiopulmonary stress testing at the Cleveland Clinic. Among these were 242 patients with AF. They were extensively propensity matched – including on the basis of heart failure etiology – to 484 HFpEF patients without AF.
“That’s what makes our study strong. We were the first to be able to do propensity matching and therefore account for other risk factors in our analysis,” Dr. Elshazly explained.
The investigators measured peak oxygen uptake (VO2), the minute ventilation–carbon dioxide production relationship (VE/VCO2) as an indicator of ventilatory efficiency, metabolic equivalents (METS), ventilatory anaerobic threshold, circulatory power as a proxy for cardiac power, peak oxygen pulse as a surrogate for stroke volume, and resting and peak heart rate and systolic blood pressure. The patients with AF were in fibrillation at the time of their cardiopulmonary stress testing.
The HFpEF patients with AF had a mean resting heart rate of 70 beats per minute and a peak rate of 130 bpm. This group showed evidence of impaired peak exercise tolerance as reflected in lower peak VO2, oxygen pulse, and circulatory power at peak exercise. Their VE/VCO2 was higher, indicating impaired ventilatory efficiency. Notably, however, their submaximal exercise capacity was similar to the non-AF controls.
“Atrial fibrillation in these patients is really more of a disease that shows itself in patients when you take them to their peak exercise capacity,” he observed.
All-cause mortality was significantly higher in the AF as compared with no-AF patients with HFpEF. The mortality curves separated early and the divergence grew larger over the course of 8 years of follow-up.
One audience member pointed out that the large mortality difference between the two groups seems disproportionate to the rather modest differences in exercise capacity.
“It brings up an interesting point,” Dr. Elshazly replied. “Maybe the increase in total mortality that we see is being driven by other things besides cardiovascular mortality. Our data doesn’t capture the specific cause of death, be it cancer, for example, but it does raise the idea that this mortality difference is not all driven by cardiovascular mortality, but by atrial fibrillation.”
Dr. Elshazly reported having no financial conflicts of interest regarding his institutionally supported study.
CHICAGO – Atrial fibrillation with good heart rate control in patients who have heart failure with preserved ejection fraction is independently associated with exercise intolerance, impaired contractile reserve, and a sharply higher mortality rate than in matched HFpEF patients without the arrhythmia, a retrospective analysis showed.
“Our study, the largest of its kind, provides mechanistic evidence from cardiopulmonary testing that a rhythm control strategy may potentially improve peak exercise capacity and survival in this patient population, a finding that of course requires future prospective appraisal in randomized trials comparing rate and rhythm control of atrial fibrillation in HFpEF,” Dr. Mohamed Badreldin Elshazly reported at the annual meeting of the American College of Cardiology.
In the meantime, his study also shows the useful role cardiopulmonary stress testing can play in the setting of atrial fibrillation (AF) in HFpEF, he added.
“Cardiopulmonary stress tests are cheap and easy to do. They’re a big asset for personalized medicine. Using an objective measure like cardiopulmonary stress testing to define the physiologic and hemodynamic consequences of atrial fibrillation in individual patients may help identify those in whom rhythm control may improve exercise tolerance and quality of life, and those who may be okay with rate control,” according to Dr. Elshazly of the Cleveland Clinic.
He noted that while it’s well established that atrial fibrillation is associated with exercise intolerance in patients with heart failure with reduced ejection fraction (HFrEF) and that restoration of sinus rhythm in such patients has a positive impact on exercise hemodynamics, symptom severity, and quality of life, the situation is murkier regarding AF in patients with HFpEF. Prior studies were generally small and unable to establish whether AF was independently associated with exercise intolerance or if HFpEF patients who developed AF were sicker and higher risk.
He presented a retrospective, case-control study in a cohort of 1,825 patients with HFpEF referred for maximal, symptom-limited cardiopulmonary stress testing at the Cleveland Clinic. Among these were 242 patients with AF. They were extensively propensity matched – including on the basis of heart failure etiology – to 484 HFpEF patients without AF.
“That’s what makes our study strong. We were the first to be able to do propensity matching and therefore account for other risk factors in our analysis,” Dr. Elshazly explained.
The investigators measured peak oxygen uptake (VO2), the minute ventilation–carbon dioxide production relationship (VE/VCO2) as an indicator of ventilatory efficiency, metabolic equivalents (METS), ventilatory anaerobic threshold, circulatory power as a proxy for cardiac power, peak oxygen pulse as a surrogate for stroke volume, and resting and peak heart rate and systolic blood pressure. The patients with AF were in fibrillation at the time of their cardiopulmonary stress testing.
The HFpEF patients with AF had a mean resting heart rate of 70 beats per minute and a peak rate of 130 bpm. This group showed evidence of impaired peak exercise tolerance as reflected in lower peak VO2, oxygen pulse, and circulatory power at peak exercise. Their VE/VCO2 was higher, indicating impaired ventilatory efficiency. Notably, however, their submaximal exercise capacity was similar to the non-AF controls.

“Atrial fibrillation in these patients is really more of a disease that shows itself in patients when you take them to their peak exercise capacity,” he observed.
All-cause mortality was significantly higher in the AF as compared with no-AF patients with HFpEF. The mortality curves separated early and the divergence grew larger over the course of 8 years of follow-up.
One audience member pointed out that the large mortality difference between the two groups seems disproportionate to the rather modest differences in exercise capacity.
“It brings up an interesting point,” Dr. Elshazly replied. “Maybe the increase in total mortality that we see is being driven by other things besides cardiovascular mortality. Our data doesn’t capture the specific cause of death, be it cancer, for example, but it does raise the idea that this mortality difference is not all driven by cardiovascular mortality, but by atrial fibrillation.”
Dr. Elshazly reported having no financial conflicts of interest regarding his institutionally supported study.
CHICAGO – Atrial fibrillation with good heart rate control in patients who have heart failure with preserved ejection fraction is independently associated with exercise intolerance, impaired contractile reserve, and a sharply higher mortality rate than in matched HFpEF patients without the arrhythmia, a retrospective analysis showed.
“Our study, the largest of its kind, provides mechanistic evidence from cardiopulmonary testing that a rhythm control strategy may potentially improve peak exercise capacity and survival in this patient population, a finding that of course requires future prospective appraisal in randomized trials comparing rate and rhythm control of atrial fibrillation in HFpEF,” Dr. Mohamed Badreldin Elshazly reported at the annual meeting of the American College of Cardiology.
In the meantime, his study also shows the useful role cardiopulmonary stress testing can play in the setting of atrial fibrillation (AF) in HFpEF, he added.
“Cardiopulmonary stress tests are cheap and easy to do. They’re a big asset for personalized medicine. Using an objective measure like cardiopulmonary stress testing to define the physiologic and hemodynamic consequences of atrial fibrillation in individual patients may help identify those in whom rhythm control may improve exercise tolerance and quality of life, and those who may be okay with rate control,” according to Dr. Elshazly of the Cleveland Clinic.
He noted that while it’s well established that atrial fibrillation is associated with exercise intolerance in patients with heart failure with reduced ejection fraction (HFrEF) and that restoration of sinus rhythm in such patients has a positive impact on exercise hemodynamics, symptom severity, and quality of life, the situation is murkier regarding AF in patients with HFpEF. Prior studies were generally small and unable to establish whether AF was independently associated with exercise intolerance or if HFpEF patients who developed AF were sicker and higher risk.
He presented a retrospective, case-control study in a cohort of 1,825 patients with HFpEF referred for maximal, symptom-limited cardiopulmonary stress testing at the Cleveland Clinic. Among these were 242 patients with AF. They were extensively propensity matched – including on the basis of heart failure etiology – to 484 HFpEF patients without AF.
“That’s what makes our study strong. We were the first to be able to do propensity matching and therefore account for other risk factors in our analysis,” Dr. Elshazly explained.
The investigators measured peak oxygen uptake (VO2), the minute ventilation–carbon dioxide production relationship (VE/VCO2) as an indicator of ventilatory efficiency, metabolic equivalents (METS), ventilatory anaerobic threshold, circulatory power as a proxy for cardiac power, peak oxygen pulse as a surrogate for stroke volume, and resting and peak heart rate and systolic blood pressure. The patients with AF were in fibrillation at the time of their cardiopulmonary stress testing.
The HFpEF patients with AF had a mean resting heart rate of 70 beats per minute and a peak rate of 130 bpm. This group showed evidence of impaired peak exercise tolerance as reflected in lower peak VO2, oxygen pulse, and circulatory power at peak exercise. Their VE/VCO2 was higher, indicating impaired ventilatory efficiency. Notably, however, their submaximal exercise capacity was similar to the non-AF controls.

“Atrial fibrillation in these patients is really more of a disease that shows itself in patients when you take them to their peak exercise capacity,” he observed.
All-cause mortality was significantly higher in the AF as compared with no-AF patients with HFpEF. The mortality curves separated early and the divergence grew larger over the course of 8 years of follow-up.
One audience member pointed out that the large mortality difference between the two groups seems disproportionate to the rather modest differences in exercise capacity.
“It brings up an interesting point,” Dr. Elshazly replied. “Maybe the increase in total mortality that we see is being driven by other things besides cardiovascular mortality. Our data doesn’t capture the specific cause of death, be it cancer, for example, but it does raise the idea that this mortality difference is not all driven by cardiovascular mortality, but by atrial fibrillation.”
Dr. Elshazly reported having no financial conflicts of interest regarding his institutionally supported study.
AT ACC 16
Key clinical point: Atrial fibrillation in patients with heart failure with preserved ejection fraction is associated with exercise intolerance and increased mortality.
Major finding: Mean peak VO2 was 18.5 mL/kg per minute in patients with HFpEF and atrial fibrillation, significantly less than the 20.1 mL/kg per minute in controls.
Data source: A retrospective, single-institution study of cardiopulmonary stress test findings and 8-year mortality in 242 patients with HFpEF and atrial fibrillation and 484 propensity-matched controls with HFpEF and no arrhythmia.
Disclosures: The presenter reported having no financial conflicts of interest regarding his institutionally supported study.
Daptomycin beats infective endocarditis caused by several pathogens
AT ECCMID 2016
AMSTERDAM – Daptomycin successfully treated infective endocarditis in 90% of patients who developed it after undergoing heart valve replacement, according to a report presented at the annual congress of the European Society of Clinical Microbiology and Infectious Diseases.
Dr. Achyut Guleri, clinical director of laboratory medicine at Blackpool Victoria Hospital, Lancashire, England, said the lipopeptide antibiotic was equally effective against methicillin- and penicillin-resistant Staphylococcus aureus, coagulase-negative staphylococcus, and enterococci.
“This is particularly good to know because sometimes in real life, on the shop floor, you don’t always have a very clear insight into what you’re trying to treat,” said Dr. Guleri. “It’s reassuring to see that the success rate is similar in all these infections.”
He presented a subgroup analysis of patients enrolled in European Cubicin Outcomes Registry and Experience (EUCORE), a retrospective, noninterventional, postmarketing registry. The 4-year study reported real-world clinical experience of daptomycin use for the treatment of Gram-positive infections in patients with infective endocarditis who had undergone heart valve replacement.
Typically, Dr. Guleri said, vancomycin, either alone or with rifampicin, is recommended for the infection. “However, with increasing antibiotic resistance, vancomycin doesn’t inspire much confidence, especially for MRSA infections,” he noted.
Daptomycin is increasingly employed as an alternative treatment. It exhibits rapid bactericidal activity against a wide range of Gram-positive pathogens, including MRSA. It’s approved for the treatment of right-sided infective endocarditis due to S. aureus, at a dose of 6 mg/kg per day. However, higher doses are now recommended by several international guidelines and are often used for hard-to-treat infections, Dr. Guleri said.
EUCORE comprised 6,075 patients from 18 countries who were enrolled from 2006 to 2012. Patients were followed until 2014. Of this group, 610 had infective endocarditis and 198 underwent valve replacement. Most were male (70%); mean age was 58 years. Medical comorbidities were common and included renal disease, sepsis, diabetes, pulmonary disease, gastrointestinal disease, cerebrovascular disease and inflammatory diseases.
Culture results were available for 87%. Of these, 68% were positive. The most common pathogen was S. aureus (37%). Half of these isolates were penicillin resistant and 35% were methicillin resistant. Enterococci were responsible for 14% of the infections, and coagulase-negative staph for 32%. The rest were caused by other pathogens.
Before trying daptomycin, most patients (83%) had already been treated with an antibiotic, which was employed in conjunction with another antibiotic in 77% of cases. The concomitant medications included rifampicin (31%), aminoglycosides (29%) and carbapenems (18%).
The overall clinical cure rate at 2 years was 90%. Daptomycin was equally effective in left- and right-sided disease, and was more effective in penicillin-resistant staph (95%) than methicillin-resistant staph (80%). The cure rate was also good in coagulase-negative staph (81%) and enterococci (75%).
High doses were more effective than low doses. At 4 mg/kg per day, the cure rate was 61%. At 6 mg/kg per day, it was 86%, and at more than 6 mg/kg per day, it was 90%.
Adverse events were rare (3%). Three patients developed increased creatine phosphokinase levels; one patient developed rhabdomyolysis and one developed cholestasis. Agranulocytosis developed in three patients and eosinophilic pneumonia in three. One patient developed a rash. No one discontinued the drug due to a side effect.
Dr. Guleri had no financial disclosures.
On Twitter @Alz_Gal
AT ECCMID 2016
AMSTERDAM – Daptomycin successfully treated infective endocarditis in 90% of patients who developed it after undergoing heart valve replacement, according to a report presented at the annual congress of the European Society of Clinical Microbiology and Infectious Diseases.
Dr. Achyut Guleri, clinical director of laboratory medicine at Blackpool Victoria Hospital, Lancashire, England, said the lipopeptide antibiotic was equally effective against methicillin- and penicillin-resistant Staphylococcus aureus, coagulase-negative staphylococcus, and enterococci.
“This is particularly good to know because sometimes in real life, on the shop floor, you don’t always have a very clear insight into what you’re trying to treat,” said Dr. Guleri. “It’s reassuring to see that the success rate is similar in all these infections.”
He presented a subgroup analysis of patients enrolled in European Cubicin Outcomes Registry and Experience (EUCORE), a retrospective, noninterventional, postmarketing registry. The 4-year study reported real-world clinical experience of daptomycin use for the treatment of Gram-positive infections in patients with infective endocarditis who had undergone heart valve replacement.
Typically, Dr. Guleri said, vancomycin, either alone or with rifampicin, is recommended for the infection. “However, with increasing antibiotic resistance, vancomycin doesn’t inspire much confidence, especially for MRSA infections,” he noted.
Daptomycin is increasingly employed as an alternative treatment. It exhibits rapid bactericidal activity against a wide range of Gram-positive pathogens, including MRSA. It’s approved for the treatment of right-sided infective endocarditis due to S. aureus, at a dose of 6 mg/kg per day. However, higher doses are now recommended by several international guidelines and are often used for hard-to-treat infections, Dr. Guleri said.
EUCORE comprised 6,075 patients from 18 countries who were enrolled from 2006 to 2012. Patients were followed until 2014. Of this group, 610 had infective endocarditis and 198 underwent valve replacement. Most were male (70%); mean age was 58 years. Medical comorbidities were common and included renal disease, sepsis, diabetes, pulmonary disease, gastrointestinal disease, cerebrovascular disease and inflammatory diseases.
Culture results were available for 87%. Of these, 68% were positive. The most common pathogen was S. aureus (37%). Half of these isolates were penicillin resistant and 35% were methicillin resistant. Enterococci were responsible for 14% of the infections, and coagulase-negative staph for 32%. The rest were caused by other pathogens.
Before trying daptomycin, most patients (83%) had already been treated with an antibiotic, which was employed in conjunction with another antibiotic in 77% of cases. The concomitant medications included rifampicin (31%), aminoglycosides (29%) and carbapenems (18%).
The overall clinical cure rate at 2 years was 90%. Daptomycin was equally effective in left- and right-sided disease, and was more effective in penicillin-resistant staph (95%) than methicillin-resistant staph (80%). The cure rate was also good in coagulase-negative staph (81%) and enterococci (75%).
High doses were more effective than low doses. At 4 mg/kg per day, the cure rate was 61%. At 6 mg/kg per day, it was 86%, and at more than 6 mg/kg per day, it was 90%.
Adverse events were rare (3%). Three patients developed increased creatine phosphokinase levels; one patient developed rhabdomyolysis and one developed cholestasis. Agranulocytosis developed in three patients and eosinophilic pneumonia in three. One patient developed a rash. No one discontinued the drug due to a side effect.
Dr. Guleri had no financial disclosures.
On Twitter @Alz_Gal
AT ECCMID 2016
AMSTERDAM – Daptomycin successfully treated infective endocarditis in 90% of patients who developed it after undergoing heart valve replacement, according to a report presented at the annual congress of the European Society of Clinical Microbiology and Infectious Diseases.
Dr. Achyut Guleri, clinical director of laboratory medicine at Blackpool Victoria Hospital, Lancashire, England, said the lipopeptide antibiotic was equally effective against methicillin- and penicillin-resistant Staphylococcus aureus, coagulase-negative staphylococcus, and enterococci.
“This is particularly good to know because sometimes in real life, on the shop floor, you don’t always have a very clear insight into what you’re trying to treat,” said Dr. Guleri. “It’s reassuring to see that the success rate is similar in all these infections.”
He presented a subgroup analysis of patients enrolled in European Cubicin Outcomes Registry and Experience (EUCORE), a retrospective, noninterventional, postmarketing registry. The 4-year study reported real-world clinical experience of daptomycin use for the treatment of Gram-positive infections in patients with infective endocarditis who had undergone heart valve replacement.
Typically, Dr. Guleri said, vancomycin, either alone or with rifampicin, is recommended for the infection. “However, with increasing antibiotic resistance, vancomycin doesn’t inspire much confidence, especially for MRSA infections,” he noted.
Daptomycin is increasingly employed as an alternative treatment. It exhibits rapid bactericidal activity against a wide range of Gram-positive pathogens, including MRSA. It’s approved for the treatment of right-sided infective endocarditis due to S. aureus, at a dose of 6 mg/kg per day. However, higher doses are now recommended by several international guidelines and are often used for hard-to-treat infections, Dr. Guleri said.
EUCORE comprised 6,075 patients from 18 countries who were enrolled from 2006 to 2012. Patients were followed until 2014. Of this group, 610 had infective endocarditis and 198 underwent valve replacement. Most were male (70%); mean age was 58 years. Medical comorbidities were common and included renal disease, sepsis, diabetes, pulmonary disease, gastrointestinal disease, cerebrovascular disease and inflammatory diseases.
Culture results were available for 87%. Of these, 68% were positive. The most common pathogen was S. aureus (37%). Half of these isolates were penicillin resistant and 35% were methicillin resistant. Enterococci were responsible for 14% of the infections, and coagulase-negative staph for 32%. The rest were caused by other pathogens.
Before trying daptomycin, most patients (83%) had already been treated with an antibiotic, which was employed in conjunction with another antibiotic in 77% of cases. The concomitant medications included rifampicin (31%), aminoglycosides (29%) and carbapenems (18%).
The overall clinical cure rate at 2 years was 90%. Daptomycin was equally effective in left- and right-sided disease, and was more effective in penicillin-resistant staph (95%) than methicillin-resistant staph (80%). The cure rate was also good in coagulase-negative staph (81%) and enterococci (75%).
High doses were more effective than low doses. At 4 mg/kg per day, the cure rate was 61%. At 6 mg/kg per day, it was 86%, and at more than 6 mg/kg per day, it was 90%.
Adverse events were rare (3%). Three patients developed increased creatine phosphokinase levels; one patient developed rhabdomyolysis and one developed cholestasis. Agranulocytosis developed in three patients and eosinophilic pneumonia in three. One patient developed a rash. No one discontinued the drug due to a side effect.
Dr. Guleri had no financial disclosures.
On Twitter @Alz_Gal
Key clinical point: Daptomycin had a high cure rate for infective endocarditis caused by MRSA, MSSA, coagulase-negative staph, and enterococci.
Major finding: The 2-year clinical cure rate was 90% for S. aureus infections.
Data source: Retrospective analysis of EUCORE, which comprised 198 patients.
Disclosures: Dr. Guleri had no financial disclosures.
Cardioband scores hit for percutaneous direct mitral annuloplasty
CHICAGO – One-year results of a pivotal European trial of the percutaneous Cardioband mitral valve reconstruction system show a stable, consistent, and clinically meaningful reduction in mitral regurgitation coupled with significant quality of life improvements and a safety profile equivalent to that of other transcatheter valve procedures.
“The results are quite impressive. Ladies and gentlemen, I can tell you that I’ve used almost all the devices for direct and indirect annuloplasty, and this is the only device that works in a reproducible fashion,” Dr. Karl-Heinz Kuck said at the annual meeting of the American College of Cardiology.
He presented the results for the first 50 patients to reach 12 months of follow-up after undergoing the Cardioband procedure in a multicenter prospective study in which participants served as their own before-and-after controls. On the strength of these results, the Cardioband device has been approved by European Union regulatory authorities for the nonsurgical treatment of symptomatic moderate to severe secondary, or functional, mitral regurgitation. In Germany, the Cardioband procedure is now routinely reimbursed at a level similar to that of the MitraClip, according to Dr. Kuck, president of the German Cardiac Society and head of cardiology at St. Georg Hospital in Hamburg, Germany.
The Cardioband procedure essentially entails percutaneous implantation of an adjustable surgical ring designed to remodel a severely dysfunctional mitral valve by repairing the valve annulus. The implantation procedure features transfemoral venous access, which the TAVR experience has shown to be safer than transapical access. As in surgery, the percutaneous procedure utilizes supra-annular fixation. And it accomplishes a significant reduction in annular dimensions, comparable to what is achieved with a size 28 surgical ring.
“And the most important thing: Because we are not interfering with the leaflets or any other part of the mitral valve, the procedure leaves all options open for the future by preserving the native anatomy,” Dr. Kuck noted.
The procedure entails a transseptal puncture, insertion of the system, deployment of the implant, and adjustment of its size by cinching it down under echocardiographic guidance in order to reduce the septolateral valve dimension. The whole thing takes about 75 minutes.
The connection of the implant to the annulus is achieved in sutureless fashion using a series of screw-in anchors.
All 50 participants in the consecutive series were deemed by a heart team to be at unacceptably high surgical risk. They averaged 71 years of age, with an left ventricular ejection fraction of 33% and a left ventricular end diastolic diameter of 61 mm. Among them, 31 had ischemic heart disease, 11 had chronic obstructive pulmonary disease, 38 were in moderate or severe renal failure, 39 had atrial fibrillation, 12 had severe pulmonary hypertension, and 16 had previously undergone CABG surgery.
The 30-day safety adverse events consisted of one hemorrhagic stroke, a single major bleeding complication, two cases of acute renal failure, and one of cardiac tamponade. There were no MIs, and neither of the two deaths were related to the procedure.
In terms of efficacy, at baseline three-quarters of patients had grade 3-4 mitral regurgitation (MR). At discharge that was true for only 12%. At 1 year of follow-up, 90% of subjects had MR grade 2 or less, and roughly two-thirds of patients had MR grade 0-1.
The procedure did what it was designed to do: The mean valve septolateral dimension decreased by 30%, from 37 mm at baseline to 26 mm at discharge.
Dr. Kuck provided 6-month data on functional improvement. The mean 6-minute walk distance improved from 262 to 339 meters. At baseline, 87% of subjects were NYHA class III or IV; at 6 months, 77% were NYHA class I or II. Scores on the Minnesota Living with Heart Failure Questionnaire improved from a mean of 39 to 16 at follow-up.
A much larger European postmarketing commercial use study of the Cardioband system is now underway.
The Cardioband procedure addresses a major unmet need, Dr. Kuck observed. More than 4 million patients in the United States alone have mitral valve disease. When medically managed, patients with severe secondary mitral regurgitation have a poor prognosis, with 1- and 5-year mortality rates of 20% and 50%, and an extremely high rate of rehospitalization for heart failure. And yet multiple surveys have shown only a minority of these patients undergo surgery.
Discussant Dr. Spencer B. King III called the transcatheter mitral valve reconstruction system “quite fascinating.” He wondered what happens if the operator accidentally grabs the nearby circumflex artery with one of the device anchors. The answer, Dr. Kuck replied, is that the anchors can be unscrewed and repositioned at any point during the procedure.
Dr. King, president of the Heart and Vascular Institute at Saint Joseph’s Health System in Atlanta, has developed several devices widely used in interventional cardiology. He shook his head in amazement at the speed at which the European regulatory agency operated in this case, noting that EU marketing approval for the Valtech Cardioband device was granted and a payment structure was almost immediately established on the basis of a 50-patient, first-in-man study.
“The data are very consistent. I think that‘s what made the difference,” Dr. Kuck said.
The study was funded by Valtech. Dr. Kuck reported serving as a consultant to Biosense Webster, Edwards, and St. Jude, and on a speakers’ bureau for Medtronic.
CHICAGO – One-year results of a pivotal European trial of the percutaneous Cardioband mitral valve reconstruction system show a stable, consistent, and clinically meaningful reduction in mitral regurgitation coupled with significant quality of life improvements and a safety profile equivalent to that of other transcatheter valve procedures.
“The results are quite impressive. Ladies and gentlemen, I can tell you that I’ve used almost all the devices for direct and indirect annuloplasty, and this is the only device that works in a reproducible fashion,” Dr. Karl-Heinz Kuck said at the annual meeting of the American College of Cardiology.
He presented the results for the first 50 patients to reach 12 months of follow-up after undergoing the Cardioband procedure in a multicenter prospective study in which participants served as their own before-and-after controls. On the strength of these results, the Cardioband device has been approved by European Union regulatory authorities for the nonsurgical treatment of symptomatic moderate to severe secondary, or functional, mitral regurgitation. In Germany, the Cardioband procedure is now routinely reimbursed at a level similar to that of the MitraClip, according to Dr. Kuck, president of the German Cardiac Society and head of cardiology at St. Georg Hospital in Hamburg, Germany.
The Cardioband procedure essentially entails percutaneous implantation of an adjustable surgical ring designed to remodel a severely dysfunctional mitral valve by repairing the valve annulus. The implantation procedure features transfemoral venous access, which the TAVR experience has shown to be safer than transapical access. As in surgery, the percutaneous procedure utilizes supra-annular fixation. And it accomplishes a significant reduction in annular dimensions, comparable to what is achieved with a size 28 surgical ring.
“And the most important thing: Because we are not interfering with the leaflets or any other part of the mitral valve, the procedure leaves all options open for the future by preserving the native anatomy,” Dr. Kuck noted.
The procedure entails a transseptal puncture, insertion of the system, deployment of the implant, and adjustment of its size by cinching it down under echocardiographic guidance in order to reduce the septolateral valve dimension. The whole thing takes about 75 minutes.
The connection of the implant to the annulus is achieved in sutureless fashion using a series of screw-in anchors.
All 50 participants in the consecutive series were deemed by a heart team to be at unacceptably high surgical risk. They averaged 71 years of age, with an left ventricular ejection fraction of 33% and a left ventricular end diastolic diameter of 61 mm. Among them, 31 had ischemic heart disease, 11 had chronic obstructive pulmonary disease, 38 were in moderate or severe renal failure, 39 had atrial fibrillation, 12 had severe pulmonary hypertension, and 16 had previously undergone CABG surgery.
The 30-day safety adverse events consisted of one hemorrhagic stroke, a single major bleeding complication, two cases of acute renal failure, and one of cardiac tamponade. There were no MIs, and neither of the two deaths were related to the procedure.
In terms of efficacy, at baseline three-quarters of patients had grade 3-4 mitral regurgitation (MR). At discharge that was true for only 12%. At 1 year of follow-up, 90% of subjects had MR grade 2 or less, and roughly two-thirds of patients had MR grade 0-1.
The procedure did what it was designed to do: The mean valve septolateral dimension decreased by 30%, from 37 mm at baseline to 26 mm at discharge.
Dr. Kuck provided 6-month data on functional improvement. The mean 6-minute walk distance improved from 262 to 339 meters. At baseline, 87% of subjects were NYHA class III or IV; at 6 months, 77% were NYHA class I or II. Scores on the Minnesota Living with Heart Failure Questionnaire improved from a mean of 39 to 16 at follow-up.
A much larger European postmarketing commercial use study of the Cardioband system is now underway.
The Cardioband procedure addresses a major unmet need, Dr. Kuck observed. More than 4 million patients in the United States alone have mitral valve disease. When medically managed, patients with severe secondary mitral regurgitation have a poor prognosis, with 1- and 5-year mortality rates of 20% and 50%, and an extremely high rate of rehospitalization for heart failure. And yet multiple surveys have shown only a minority of these patients undergo surgery.
Discussant Dr. Spencer B. King III called the transcatheter mitral valve reconstruction system “quite fascinating.” He wondered what happens if the operator accidentally grabs the nearby circumflex artery with one of the device anchors. The answer, Dr. Kuck replied, is that the anchors can be unscrewed and repositioned at any point during the procedure.
Dr. King, president of the Heart and Vascular Institute at Saint Joseph’s Health System in Atlanta, has developed several devices widely used in interventional cardiology. He shook his head in amazement at the speed at which the European regulatory agency operated in this case, noting that EU marketing approval for the Valtech Cardioband device was granted and a payment structure was almost immediately established on the basis of a 50-patient, first-in-man study.
“The data are very consistent. I think that‘s what made the difference,” Dr. Kuck said.
The study was funded by Valtech. Dr. Kuck reported serving as a consultant to Biosense Webster, Edwards, and St. Jude, and on a speakers’ bureau for Medtronic.
CHICAGO – One-year results of a pivotal European trial of the percutaneous Cardioband mitral valve reconstruction system show a stable, consistent, and clinically meaningful reduction in mitral regurgitation coupled with significant quality of life improvements and a safety profile equivalent to that of other transcatheter valve procedures.
“The results are quite impressive. Ladies and gentlemen, I can tell you that I’ve used almost all the devices for direct and indirect annuloplasty, and this is the only device that works in a reproducible fashion,” Dr. Karl-Heinz Kuck said at the annual meeting of the American College of Cardiology.
He presented the results for the first 50 patients to reach 12 months of follow-up after undergoing the Cardioband procedure in a multicenter prospective study in which participants served as their own before-and-after controls. On the strength of these results, the Cardioband device has been approved by European Union regulatory authorities for the nonsurgical treatment of symptomatic moderate to severe secondary, or functional, mitral regurgitation. In Germany, the Cardioband procedure is now routinely reimbursed at a level similar to that of the MitraClip, according to Dr. Kuck, president of the German Cardiac Society and head of cardiology at St. Georg Hospital in Hamburg, Germany.
The Cardioband procedure essentially entails percutaneous implantation of an adjustable surgical ring designed to remodel a severely dysfunctional mitral valve by repairing the valve annulus. The implantation procedure features transfemoral venous access, which the TAVR experience has shown to be safer than transapical access. As in surgery, the percutaneous procedure utilizes supra-annular fixation. And it accomplishes a significant reduction in annular dimensions, comparable to what is achieved with a size 28 surgical ring.
“And the most important thing: Because we are not interfering with the leaflets or any other part of the mitral valve, the procedure leaves all options open for the future by preserving the native anatomy,” Dr. Kuck noted.
The procedure entails a transseptal puncture, insertion of the system, deployment of the implant, and adjustment of its size by cinching it down under echocardiographic guidance in order to reduce the septolateral valve dimension. The whole thing takes about 75 minutes.
The connection of the implant to the annulus is achieved in sutureless fashion using a series of screw-in anchors.
All 50 participants in the consecutive series were deemed by a heart team to be at unacceptably high surgical risk. They averaged 71 years of age, with an left ventricular ejection fraction of 33% and a left ventricular end diastolic diameter of 61 mm. Among them, 31 had ischemic heart disease, 11 had chronic obstructive pulmonary disease, 38 were in moderate or severe renal failure, 39 had atrial fibrillation, 12 had severe pulmonary hypertension, and 16 had previously undergone CABG surgery.
The 30-day safety adverse events consisted of one hemorrhagic stroke, a single major bleeding complication, two cases of acute renal failure, and one of cardiac tamponade. There were no MIs, and neither of the two deaths were related to the procedure.
In terms of efficacy, at baseline three-quarters of patients had grade 3-4 mitral regurgitation (MR). At discharge that was true for only 12%. At 1 year of follow-up, 90% of subjects had MR grade 2 or less, and roughly two-thirds of patients had MR grade 0-1.
The procedure did what it was designed to do: The mean valve septolateral dimension decreased by 30%, from 37 mm at baseline to 26 mm at discharge.
Dr. Kuck provided 6-month data on functional improvement. The mean 6-minute walk distance improved from 262 to 339 meters. At baseline, 87% of subjects were NYHA class III or IV; at 6 months, 77% were NYHA class I or II. Scores on the Minnesota Living with Heart Failure Questionnaire improved from a mean of 39 to 16 at follow-up.
A much larger European postmarketing commercial use study of the Cardioband system is now underway.
The Cardioband procedure addresses a major unmet need, Dr. Kuck observed. More than 4 million patients in the United States alone have mitral valve disease. When medically managed, patients with severe secondary mitral regurgitation have a poor prognosis, with 1- and 5-year mortality rates of 20% and 50%, and an extremely high rate of rehospitalization for heart failure. And yet multiple surveys have shown only a minority of these patients undergo surgery.
Discussant Dr. Spencer B. King III called the transcatheter mitral valve reconstruction system “quite fascinating.” He wondered what happens if the operator accidentally grabs the nearby circumflex artery with one of the device anchors. The answer, Dr. Kuck replied, is that the anchors can be unscrewed and repositioned at any point during the procedure.
Dr. King, president of the Heart and Vascular Institute at Saint Joseph’s Health System in Atlanta, has developed several devices widely used in interventional cardiology. He shook his head in amazement at the speed at which the European regulatory agency operated in this case, noting that EU marketing approval for the Valtech Cardioband device was granted and a payment structure was almost immediately established on the basis of a 50-patient, first-in-man study.
“The data are very consistent. I think that‘s what made the difference,” Dr. Kuck said.
The study was funded by Valtech. Dr. Kuck reported serving as a consultant to Biosense Webster, Edwards, and St. Jude, and on a speakers’ bureau for Medtronic.
AT ACC 16
Key clinical point: A new percutaneous repair option has been developed for patients with secondary mitral regurgitation.
Major finding: At 1 year of follow-up after receiving the Cardioband mitral reconstruction system, 90% of treated subjects with baseline severe mitral valve disease had mitral regurgitation of grade 2 or less.
Data source: A prospective, multicenter clinical trial featuring 12 months of follow-up of 50 treated patients with severe secondary mitral regurgitation.
Disclosures: The study was sponsored by Valtech, maker of the Cardioband system. The presenter reported serving as a consultant to Biosense Webster, Edwards, and St. Jude, and on a speakers bureau for Medtronic.
Choice of cardiac ‘operative mortality’ definition affects outcomes reporting
Reporting of outcomes has grown in importance as clinical registries collect data and establish benchmarks for quality care, but exactly what those data report can vary depending on the definition of a specific outcome, even for an outcome as seemingly straightforward as death after an intervention.
Dr. Steven Maximus of the University of California Irvine Medical Center, and colleagues reported on this phenomenon in the April issue of the Journal of Thoracic and Cardiovascular Surgery (2016;151:1101-10). Specifically, they showed how rates of postoperative mortality after five different cardiac interventions can vary within the same data set depending on the definition of postoperative mortality.
“A significant percentage of procedural deaths occur after transfer or discharge from the index hospital,” Dr. Maximus and colleagues said. “These findings illustrate the importance of the definition of ‘operative’ mortality and the need to ensure accuracy in the reporting of data to volunteer clinical registries.”
They calculated outcomes based on five different definitions of postoperative mortality for five different cardiac interventions, depending on when and where the patient died, each of which showed slightly different results. The five different definitions of postoperative mortality are: during the index hospitalization only; during the index hospitalization or in a hospital the patient was transferred to on the same day or within 24 hours of discharge; during the index hospitalization or within 30 days after the procedure; during the index hospitalization or transfer hospitalization or during readmission within 30 days; and during the index hospitalization, transfer, and within 30 days after the procedure regardless of readmission.
As an example, mortality rates for percutaneous coronary intervention (PCI) with acute coronary syndrome (ACS) ranged from 3.25% for the first definition listed to 4.51% for the fifth definition. For isolated coronary artery bypass grafting, the rates ranged from 1.71% for the first definition to 2.15% for the fifth.
The fifth definition “is the most encompassing and is the current definition used by the STS [Society of Thoracic Surgeons] and should be applied to other clinical registries, such as the American College of Cardiology National Cardiovascular Data Registry,” Dr. Maximus and colleagues said.
The study noted that the Society of Thoracic Surgeons National Database (STS-NDB) has revised its definition of operative mortality to more accurately measure outcomes. Originally, the STS-NDB definition included all deaths during the index hospitalization, even those after 30 days, and postdischarge deaths within 30 days unless the cause of death was not related to the operation. In 2011, the definition was updated to include patients transferred to other acute care facilities. STS-NDB updated the definition again in 2014 to include all deaths, regardless of cause, during the index hospitalization, even if after 30 days, and including patients transferred to other acute care facilities, and all deaths, again regardless of cause, after discharge and within 30 days of the operation.
The analyses by Dr. Maximus and colleagues used data from the California Office of Statewide Health Planning and Development hospitalized patient discharge database for the year 2009. The first analysis the researchers performed did not exclude any patients and found a significant number of cardiac procedural deaths occurred after transfer at discharge from the index hospitalization, 17% in the surgical group vs. 31% in the PCI group. In the second analysis, which excluded untrackable patients, hospital deaths included 12% for PCI and 4% for surgery.
“PCI mortality was more dependent on the method used to define mortality, compared with the surgical patients, and a larger percentage of deaths occurred after hospital discharge and within 30 days of the procedure,” Dr. Maximus and colleagues said.
Another key factor in calculating mortality rates was the ability of hospitals to follow patients. “We found that up to 20% of hospitals were not able to track their patients long-term,” Dr. Maximus and coauthors said.
Study coauthor Dr. Junaid Kahn disclosed consulting fees from Edwards Lifesciences. The other authors have no financial relationships to disclose.
“The public, our patients, and their families might be surprised to discover that doctors have difficulty tallying how many people die after cardiac interventions, and that the question merited a piece of published statistical analysis,” Dr. Tom Treasure of University College London and Dr. Samer Nashef of Papworth Hospital, Cambridge, said in their invited commentary (J Thorac Cardiovasc Surg. 2016;151:1110-1).
Dr. Maximus and colleagues showed that the wider they cast their net to account for postoperative death, the more deaths they found, Dr. Treasure and Dr. Nashef said. That may penalize institutions that are more fastidious in collecting outcomes data. “When we require institutions with scarce resources to chase long-term outcome data for the purposes of treatment quality comparison, we immediately penalize those institutions that comply; more extensive data retrieval will find more deaths,” the commentators said.
The “perfect system” would capture death following a procedure no matter where it occurs, they added. “For the sake of compliance and simplicity, and until data systems are adequately robust and comprehensive, quality monitoring of cardiac surgery may have to be based pragmatically on data that are universally available and difficult to falsify, such as death at the base hospital during the same hospital admission as the intervention.”
Dr. Treasure and Dr. Nashef also accounted for another “quirk” in measuring outcomes. “Humans make errors in data entry, transcription and transfer and computers have ‘glitches’ and ‘gremlins,’ ” they said. “An apparent improvement in clinical outcome in fact may be merely the result of better record keeping.”
The commentators reported that they had no relationships to disclose.
“The public, our patients, and their families might be surprised to discover that doctors have difficulty tallying how many people die after cardiac interventions, and that the question merited a piece of published statistical analysis,” Dr. Tom Treasure of University College London and Dr. Samer Nashef of Papworth Hospital, Cambridge, said in their invited commentary (J Thorac Cardiovasc Surg. 2016;151:1110-1).
Dr. Maximus and colleagues showed that the wider they cast their net to account for postoperative death, the more deaths they found, Dr. Treasure and Dr. Nashef said. That may penalize institutions that are more fastidious in collecting outcomes data. “When we require institutions with scarce resources to chase long-term outcome data for the purposes of treatment quality comparison, we immediately penalize those institutions that comply; more extensive data retrieval will find more deaths,” the commentators said.
The “perfect system” would capture death following a procedure no matter where it occurs, they added. “For the sake of compliance and simplicity, and until data systems are adequately robust and comprehensive, quality monitoring of cardiac surgery may have to be based pragmatically on data that are universally available and difficult to falsify, such as death at the base hospital during the same hospital admission as the intervention.”
Dr. Treasure and Dr. Nashef also accounted for another “quirk” in measuring outcomes. “Humans make errors in data entry, transcription and transfer and computers have ‘glitches’ and ‘gremlins,’ ” they said. “An apparent improvement in clinical outcome in fact may be merely the result of better record keeping.”
The commentators reported that they had no relationships to disclose.
“The public, our patients, and their families might be surprised to discover that doctors have difficulty tallying how many people die after cardiac interventions, and that the question merited a piece of published statistical analysis,” Dr. Tom Treasure of University College London and Dr. Samer Nashef of Papworth Hospital, Cambridge, said in their invited commentary (J Thorac Cardiovasc Surg. 2016;151:1110-1).
Dr. Maximus and colleagues showed that the wider they cast their net to account for postoperative death, the more deaths they found, Dr. Treasure and Dr. Nashef said. That may penalize institutions that are more fastidious in collecting outcomes data. “When we require institutions with scarce resources to chase long-term outcome data for the purposes of treatment quality comparison, we immediately penalize those institutions that comply; more extensive data retrieval will find more deaths,” the commentators said.
The “perfect system” would capture death following a procedure no matter where it occurs, they added. “For the sake of compliance and simplicity, and until data systems are adequately robust and comprehensive, quality monitoring of cardiac surgery may have to be based pragmatically on data that are universally available and difficult to falsify, such as death at the base hospital during the same hospital admission as the intervention.”
Dr. Treasure and Dr. Nashef also accounted for another “quirk” in measuring outcomes. “Humans make errors in data entry, transcription and transfer and computers have ‘glitches’ and ‘gremlins,’ ” they said. “An apparent improvement in clinical outcome in fact may be merely the result of better record keeping.”
The commentators reported that they had no relationships to disclose.
Reporting of outcomes has grown in importance as clinical registries collect data and establish benchmarks for quality care, but exactly what those data report can vary depending on the definition of a specific outcome, even for an outcome as seemingly straightforward as death after an intervention.
Dr. Steven Maximus of the University of California Irvine Medical Center, and colleagues reported on this phenomenon in the April issue of the Journal of Thoracic and Cardiovascular Surgery (2016;151:1101-10). Specifically, they showed how rates of postoperative mortality after five different cardiac interventions can vary within the same data set depending on the definition of postoperative mortality.
“A significant percentage of procedural deaths occur after transfer or discharge from the index hospital,” Dr. Maximus and colleagues said. “These findings illustrate the importance of the definition of ‘operative’ mortality and the need to ensure accuracy in the reporting of data to volunteer clinical registries.”
They calculated outcomes based on five different definitions of postoperative mortality for five different cardiac interventions, depending on when and where the patient died, each of which showed slightly different results. The five different definitions of postoperative mortality are: during the index hospitalization only; during the index hospitalization or in a hospital the patient was transferred to on the same day or within 24 hours of discharge; during the index hospitalization or within 30 days after the procedure; during the index hospitalization or transfer hospitalization or during readmission within 30 days; and during the index hospitalization, transfer, and within 30 days after the procedure regardless of readmission.
As an example, mortality rates for percutaneous coronary intervention (PCI) with acute coronary syndrome (ACS) ranged from 3.25% for the first definition listed to 4.51% for the fifth definition. For isolated coronary artery bypass grafting, the rates ranged from 1.71% for the first definition to 2.15% for the fifth.
The fifth definition “is the most encompassing and is the current definition used by the STS [Society of Thoracic Surgeons] and should be applied to other clinical registries, such as the American College of Cardiology National Cardiovascular Data Registry,” Dr. Maximus and colleagues said.
The study noted that the Society of Thoracic Surgeons National Database (STS-NDB) has revised its definition of operative mortality to more accurately measure outcomes. Originally, the STS-NDB definition included all deaths during the index hospitalization, even those after 30 days, and postdischarge deaths within 30 days unless the cause of death was not related to the operation. In 2011, the definition was updated to include patients transferred to other acute care facilities. STS-NDB updated the definition again in 2014 to include all deaths, regardless of cause, during the index hospitalization, even if after 30 days, and including patients transferred to other acute care facilities, and all deaths, again regardless of cause, after discharge and within 30 days of the operation.
The analyses by Dr. Maximus and colleagues used data from the California Office of Statewide Health Planning and Development hospitalized patient discharge database for the year 2009. The first analysis the researchers performed did not exclude any patients and found a significant number of cardiac procedural deaths occurred after transfer at discharge from the index hospitalization, 17% in the surgical group vs. 31% in the PCI group. In the second analysis, which excluded untrackable patients, hospital deaths included 12% for PCI and 4% for surgery.
“PCI mortality was more dependent on the method used to define mortality, compared with the surgical patients, and a larger percentage of deaths occurred after hospital discharge and within 30 days of the procedure,” Dr. Maximus and colleagues said.
Another key factor in calculating mortality rates was the ability of hospitals to follow patients. “We found that up to 20% of hospitals were not able to track their patients long-term,” Dr. Maximus and coauthors said.
Study coauthor Dr. Junaid Kahn disclosed consulting fees from Edwards Lifesciences. The other authors have no financial relationships to disclose.
Reporting of outcomes has grown in importance as clinical registries collect data and establish benchmarks for quality care, but exactly what those data report can vary depending on the definition of a specific outcome, even for an outcome as seemingly straightforward as death after an intervention.
Dr. Steven Maximus of the University of California Irvine Medical Center, and colleagues reported on this phenomenon in the April issue of the Journal of Thoracic and Cardiovascular Surgery (2016;151:1101-10). Specifically, they showed how rates of postoperative mortality after five different cardiac interventions can vary within the same data set depending on the definition of postoperative mortality.
“A significant percentage of procedural deaths occur after transfer or discharge from the index hospital,” Dr. Maximus and colleagues said. “These findings illustrate the importance of the definition of ‘operative’ mortality and the need to ensure accuracy in the reporting of data to volunteer clinical registries.”
They calculated outcomes based on five different definitions of postoperative mortality for five different cardiac interventions, depending on when and where the patient died, each of which showed slightly different results. The five different definitions of postoperative mortality are: during the index hospitalization only; during the index hospitalization or in a hospital the patient was transferred to on the same day or within 24 hours of discharge; during the index hospitalization or within 30 days after the procedure; during the index hospitalization or transfer hospitalization or during readmission within 30 days; and during the index hospitalization, transfer, and within 30 days after the procedure regardless of readmission.
As an example, mortality rates for percutaneous coronary intervention (PCI) with acute coronary syndrome (ACS) ranged from 3.25% for the first definition listed to 4.51% for the fifth definition. For isolated coronary artery bypass grafting, the rates ranged from 1.71% for the first definition to 2.15% for the fifth.
The fifth definition “is the most encompassing and is the current definition used by the STS [Society of Thoracic Surgeons] and should be applied to other clinical registries, such as the American College of Cardiology National Cardiovascular Data Registry,” Dr. Maximus and colleagues said.
The study noted that the Society of Thoracic Surgeons National Database (STS-NDB) has revised its definition of operative mortality to more accurately measure outcomes. Originally, the STS-NDB definition included all deaths during the index hospitalization, even those after 30 days, and postdischarge deaths within 30 days unless the cause of death was not related to the operation. In 2011, the definition was updated to include patients transferred to other acute care facilities. STS-NDB updated the definition again in 2014 to include all deaths, regardless of cause, during the index hospitalization, even if after 30 days, and including patients transferred to other acute care facilities, and all deaths, again regardless of cause, after discharge and within 30 days of the operation.
The analyses by Dr. Maximus and colleagues used data from the California Office of Statewide Health Planning and Development hospitalized patient discharge database for the year 2009. The first analysis the researchers performed did not exclude any patients and found a significant number of cardiac procedural deaths occurred after transfer at discharge from the index hospitalization, 17% in the surgical group vs. 31% in the PCI group. In the second analysis, which excluded untrackable patients, hospital deaths included 12% for PCI and 4% for surgery.
“PCI mortality was more dependent on the method used to define mortality, compared with the surgical patients, and a larger percentage of deaths occurred after hospital discharge and within 30 days of the procedure,” Dr. Maximus and colleagues said.
Another key factor in calculating mortality rates was the ability of hospitals to follow patients. “We found that up to 20% of hospitals were not able to track their patients long-term,” Dr. Maximus and coauthors said.
Study coauthor Dr. Junaid Kahn disclosed consulting fees from Edwards Lifesciences. The other authors have no financial relationships to disclose.
FROM THE JOURNAL OF THORACIC AND CARDIOVASCULAR SURGERY
Key clinical point: Mortality rates for various coronary procedures can vary depending on the definition chosen.
Major finding: The mortality rate for percutaneous coronary intervention with acute coronary syndrome ranged from 3.25% to 4.51% depending on which of five definitions was used.
Data source: California Office of Statewide Health Planning and Development Hospitalized Patient Discharge Data Base for 2009.
Disclosures: Study coauthor Dr. Junaid Kahn reports receiving consulting fees for Edwards Lifesciences. The other authors have no financial relationships to disclose.
Angina rates similar across metal stents
WASHINGTON – In patients with coronary artery disease, there is no increased risk of angina 1 year after percutaneous coronary intervention for bare metal stents relative to drug-eluting metal stents, according to data presented at Cardiovascular Research Technologies 2016.
“Angina pectoris in the first year after PCI [percutaneous coronary intervention] is remarkably common, affecting 32.3% of patients,” but “metallic stent type is not independently associated with the occurrence of angina,” reported Dr. Michael A. Gaglia Jr., an interventional cardiologist at MedStar Heart and Vascular Institute in Washington.
This conclusion was based on a study in which 8,804 patients who underwent PCI with metal stents were questioned about angina and its severity. The incidence of angina was compared for bare-metal stents relative to five drug-eluting metal stents: Cypher (sirolimus-eluting, Johnson & Johnson), Taxus Express2 (paclitaxel, Boston Scientific), Xience V (everolimus, Abbott Vascular), Promus Element (everolimus, Boston Scientific), and Resolute Integrity (zotarolimus, Medtronic).
For nearly 3 months, the cumulative incidence of angina remained tightly grouped at 5% or less across stent types. Incidence rates began climbing slowly through the first 9 months of follow-up and then more steeply at about 10 months. When depicted graphically, the incidence of angina appeared higher after placement of the Cypher stent, which was discontinued in 2011, but multivariate analysis found “no significant association between stent type and angina at 1 year after PCI,” Dr. Gaglia reported.
Although risk of angina was not correlated with type of metal stent, angina was highly correlated with risk of a major adverse cardiovascular event (MACE). When angina severity was stratified by the Canadian Cardiovascular Society system, MACE, defined as a composite of all-cause mortality, target vessel revascularization, and Q-wave myocardial infarction, occurred in 6.8% of those without angina, 10.0% of those with class 1 or 2 angina, and 19.7% of those with class 3 or 4 angina (P less than .001 for this trend) over the course of follow-up.
Independent of stent type, angina was more common in patients with a history of severe angina prior to PCI, a prior PCI, or prior coronary artery bypass grafting. A reduced likelihood of angina was independently associated with older age, male sex, a presentation of acute coronary syndrome, and a longer stented length.
Other studies have also shown that angina after PCI is associated with an increased risk of MACE relative to the absence of ischemia, but the contribution of this study is that it is the first set of data to suggest that drug eluting stents provide no advantage over bare metal stents for controlling angina, according to the authors. In the graphic representation of angina incidence for different stent types over 1-year of follow-up, four of the six lines, including the line representing bare metal stents, were essentially superimposable. In addition to the line representing angina incidence in those receiving the Cypher stent, the line representing angina incidence on the Promus Element stent climbed higher at 9 months relative to the remaining four stent types, but this line had rejoined the others at 12 months.
“Metallic coronary stents alter vessel geometry, shear stress, and hemodynamics. Stents also vary in design and architecture,” Dr. Gaglia observed. Although protection from angina is one of the major indications for the placement of stents, Dr. Gaglia emphasized that data comparing different metallic stents in regards to the incidence of angina pectoris at long-term follow-up have until this study “been lacking.”
The meeting was sponsored by the Cardiovascular Research Institute at Washington Hospital Center. Dr. Gaglia reports no relevant financial relationships. Abbott Vascular funded the study.
WASHINGTON – In patients with coronary artery disease, there is no increased risk of angina 1 year after percutaneous coronary intervention for bare metal stents relative to drug-eluting metal stents, according to data presented at Cardiovascular Research Technologies 2016.
“Angina pectoris in the first year after PCI [percutaneous coronary intervention] is remarkably common, affecting 32.3% of patients,” but “metallic stent type is not independently associated with the occurrence of angina,” reported Dr. Michael A. Gaglia Jr., an interventional cardiologist at MedStar Heart and Vascular Institute in Washington.
This conclusion was based on a study in which 8,804 patients who underwent PCI with metal stents were questioned about angina and its severity. The incidence of angina was compared for bare-metal stents relative to five drug-eluting metal stents: Cypher (sirolimus-eluting, Johnson & Johnson), Taxus Express2 (paclitaxel, Boston Scientific), Xience V (everolimus, Abbott Vascular), Promus Element (everolimus, Boston Scientific), and Resolute Integrity (zotarolimus, Medtronic).
For nearly 3 months, the cumulative incidence of angina remained tightly grouped at 5% or less across stent types. Incidence rates began climbing slowly through the first 9 months of follow-up and then more steeply at about 10 months. When depicted graphically, the incidence of angina appeared higher after placement of the Cypher stent, which was discontinued in 2011, but multivariate analysis found “no significant association between stent type and angina at 1 year after PCI,” Dr. Gaglia reported.
Although risk of angina was not correlated with type of metal stent, angina was highly correlated with risk of a major adverse cardiovascular event (MACE). When angina severity was stratified by the Canadian Cardiovascular Society system, MACE, defined as a composite of all-cause mortality, target vessel revascularization, and Q-wave myocardial infarction, occurred in 6.8% of those without angina, 10.0% of those with class 1 or 2 angina, and 19.7% of those with class 3 or 4 angina (P less than .001 for this trend) over the course of follow-up.
Independent of stent type, angina was more common in patients with a history of severe angina prior to PCI, a prior PCI, or prior coronary artery bypass grafting. A reduced likelihood of angina was independently associated with older age, male sex, a presentation of acute coronary syndrome, and a longer stented length.
Other studies have also shown that angina after PCI is associated with an increased risk of MACE relative to the absence of ischemia, but the contribution of this study is that it is the first set of data to suggest that drug eluting stents provide no advantage over bare metal stents for controlling angina, according to the authors. In the graphic representation of angina incidence for different stent types over 1-year of follow-up, four of the six lines, including the line representing bare metal stents, were essentially superimposable. In addition to the line representing angina incidence in those receiving the Cypher stent, the line representing angina incidence on the Promus Element stent climbed higher at 9 months relative to the remaining four stent types, but this line had rejoined the others at 12 months.
“Metallic coronary stents alter vessel geometry, shear stress, and hemodynamics. Stents also vary in design and architecture,” Dr. Gaglia observed. Although protection from angina is one of the major indications for the placement of stents, Dr. Gaglia emphasized that data comparing different metallic stents in regards to the incidence of angina pectoris at long-term follow-up have until this study “been lacking.”
The meeting was sponsored by the Cardiovascular Research Institute at Washington Hospital Center. Dr. Gaglia reports no relevant financial relationships. Abbott Vascular funded the study.
WASHINGTON – In patients with coronary artery disease, there is no increased risk of angina 1 year after percutaneous coronary intervention for bare metal stents relative to drug-eluting metal stents, according to data presented at Cardiovascular Research Technologies 2016.
“Angina pectoris in the first year after PCI [percutaneous coronary intervention] is remarkably common, affecting 32.3% of patients,” but “metallic stent type is not independently associated with the occurrence of angina,” reported Dr. Michael A. Gaglia Jr., an interventional cardiologist at MedStar Heart and Vascular Institute in Washington.
This conclusion was based on a study in which 8,804 patients who underwent PCI with metal stents were questioned about angina and its severity. The incidence of angina was compared for bare-metal stents relative to five drug-eluting metal stents: Cypher (sirolimus-eluting, Johnson & Johnson), Taxus Express2 (paclitaxel, Boston Scientific), Xience V (everolimus, Abbott Vascular), Promus Element (everolimus, Boston Scientific), and Resolute Integrity (zotarolimus, Medtronic).
For nearly 3 months, the cumulative incidence of angina remained tightly grouped at 5% or less across stent types. Incidence rates began climbing slowly through the first 9 months of follow-up and then more steeply at about 10 months. When depicted graphically, the incidence of angina appeared higher after placement of the Cypher stent, which was discontinued in 2011, but multivariate analysis found “no significant association between stent type and angina at 1 year after PCI,” Dr. Gaglia reported.
Although risk of angina was not correlated with type of metal stent, angina was highly correlated with risk of a major adverse cardiovascular event (MACE). When angina severity was stratified by the Canadian Cardiovascular Society system, MACE, defined as a composite of all-cause mortality, target vessel revascularization, and Q-wave myocardial infarction, occurred in 6.8% of those without angina, 10.0% of those with class 1 or 2 angina, and 19.7% of those with class 3 or 4 angina (P less than .001 for this trend) over the course of follow-up.
Independent of stent type, angina was more common in patients with a history of severe angina prior to PCI, a prior PCI, or prior coronary artery bypass grafting. A reduced likelihood of angina was independently associated with older age, male sex, a presentation of acute coronary syndrome, and a longer stented length.
Other studies have also shown that angina after PCI is associated with an increased risk of MACE relative to the absence of ischemia, but the contribution of this study is that it is the first set of data to suggest that drug eluting stents provide no advantage over bare metal stents for controlling angina, according to the authors. In the graphic representation of angina incidence for different stent types over 1-year of follow-up, four of the six lines, including the line representing bare metal stents, were essentially superimposable. In addition to the line representing angina incidence in those receiving the Cypher stent, the line representing angina incidence on the Promus Element stent climbed higher at 9 months relative to the remaining four stent types, but this line had rejoined the others at 12 months.
“Metallic coronary stents alter vessel geometry, shear stress, and hemodynamics. Stents also vary in design and architecture,” Dr. Gaglia observed. Although protection from angina is one of the major indications for the placement of stents, Dr. Gaglia emphasized that data comparing different metallic stents in regards to the incidence of angina pectoris at long-term follow-up have until this study “been lacking.”
The meeting was sponsored by the Cardiovascular Research Institute at Washington Hospital Center. Dr. Gaglia reports no relevant financial relationships. Abbott Vascular funded the study.
AT CARDIOVASCULAR RESEARCH TECHNOLOGIES 2016
Key clinical point: One year after stent placement, angina rates are no higher with bare metal stents than with drug-eluting metal stents.
Major finding: One year after stenting, the incidence of angina was 32.3%.
Data source: Observational study with 8,804 patients.
Disclosures: Dr. Gaglia reports no relevant financial relationships. Abbott Vascular funded the study.
Cardiac cath lab work carries multiple risks
Workers performing fluoroscopically guided cardiovascular procedures are at significantly higher risk of a diverse array of health disorders compared with nonexposed subjects, according to a survey.
Though cancers and ocular, skin, and thyroid disorders are recognized risks of working with low-dose radiation, as are orthopedic problems associated with wearing heavy lead aprons, the new study, published online April 12 in Circulation: Cardiovascular Interventions, adds weight to emerging concerns about lesser-known risks.
In addition to higher prevalence of skin lesions, cataracts, thyroid disorders, cancer, and orthopedic problems, exposed workers saw significantly higher prevalence of hypertension, hypercholesterolemia, and depression or anxiety, compared with controls.
Maria Grazia Andreassi, Ph.D., of the CNR Institute of Clinical Physiology, in Pisa, Italy, led the study, which used questionnaires to collect health, exposure, and demographic information from 466 workers in cardiac catheterization laboratories in Italy: 218 interventional cardiologists and electrophysiologists, 191 nurses, and 57 technicians. Radiation exposure was estimated based on subjects’ occupational radiological risk score (ORRS), which takes into consideration years of work, caseloads, and proximity to the radiation source in the lab.
The researchers administered the same questionnaires to 280 health workers who did not have occupational radiation exposure. The two groups were well matched for age, sex, education level, alcohol use, and body mass index, though current smoking differed significantly (27% of lab staff vs. 23% of unexposed workers).
As expected, orthopedic injuries were markedly higher among the lab workers, with 30% reporting disorders compared with 5% of controls (P less than .001). Nearly 5% of the lab workers had cataracts, compared with less than 1% of the nonexposed (P = .003), and risk increased for subjects with more years in the lab. Cancer was higher among exposed participants (2.6% vs. 0.7%), a difference that did not reach statistical significance. However, a significant trend was seen for cancer risk increasing across duration of exposure (Circ Cardiovasc Interv. 2016;9:e003273).
In the composite endpoint of cataract and cancer, a surrogate for disease associated with occupational exposure to radiation, the researchers found that the interventional cardiologists and electrophysiologists had a significantly higher prevalence than did the nurses and technicians (69% vs. 22% and 9%, respectively; P = .03).
Furthermore, the cancer in physicians occurred more often in the left side, when laterality was applicable, in 67% of cases, whereas the malignancy was left-sided in 33% of cancers occurring in nurses and technicians.
No significant differences in cardiovascular events were seen between the groups. However, hypertension and high cholesterol were more prevalent in cath lab workers, with adjusted odds ratios of 1.7 (95% confidence interval, 1-3; P = .05) and 2.9 (CI, 1-5; P = .004), respectively, for highly exposed subjects, compared with controls.
Last year, in a separate study, Dr. Andreassi’s group reported that exposure to low-dose radiation over time may increase carotid intima-media thickness, an early indicator of vascular injury, among cath lab workers (J Am Coll Cardiol Intv. 2015;8:616-27).
Anxiety and depression occurred in 12% of exposed subjects, compared with 2% of controls. The finding could reflect high occupational stress, the researchers wrote in their analysis, or may be an effect of radiation, “which is especially relevant on the unprotected head of the first operator and at chronic low doses may impact detrimentally on hippocampal neurogenesis and neuronal plasticity.”
The researchers acknowledged the possibility of selection bias in their study, favoring “a possible disproportionate contribution of respondents with existing health issues, who may reasonably think that their complaints are occupationally related,” they wrote. They also noted that the study was not designed to directly assess radiation dose, in part because dosimeters were not regularly worn in some of the settings where subjects worked. Finally, the radiation-exposed group had more cardiovascular risk factors. Nonetheless, the findings highlight the need to “spread the culture of safety” in cardiac cath labs, the researchers wrote.
There is now “more than enough information for us to conclude that the interventional catheterization laboratory is not a healthy workplace,” Dr. Lloyd W. Klein and Dr. Mugurel Bazavan of Advocate Illinois Masonic Medical Center, and Rush Medical College in Chicago, wrote in an accompanying editorial.
Finding “the courage to change business practice,” they wrote, “is the only path to innovative cath laboratory design and shielding techniques that can prevent these occupational hazards” (Circ Cardiovasc Interv. 2016;9:e003742).
Dr. Andreassi and colleagues disclosed no conflicts of interest related to their study, and Dr. Klein and Dr. Bazavan disclosed no conflicts related to their editorial.
The field of interventional cardiology is tremendously exciting, and yet the long-term risks are not as well defined as they should be for the protection of cath lab personnel.

|
Dr. Charles E. Chambers |
This study shows strikingly that both physicians and nonphysicians are at increased risk of a diverse group of medical conditions. Quantifying the contribution of radiation exposure to CV risk and separating it from smoking, dietary, and other factors, will require studies using dosimetry data in lieu of the estimated exposure measures seen in this study.
However, as these findings underscore, there is no doubt that we must be concerned about radiation and that manufacturers, hospitals, and providers need to come to the table to address these urgent questions of personal liability and workplace safety, particularly with so many young people entering the field.
Dr. Charles E. Chambers is professor of medicine and radiology at Pennsylvania State University, Hershey, and past president of the Society for Cardiovascular Angiography and Interventions. He made these comments in an interview, and reports no conflicts of interest.
The field of interventional cardiology is tremendously exciting, and yet the long-term risks are not as well defined as they should be for the protection of cath lab personnel.

|
Dr. Charles E. Chambers |
This study shows strikingly that both physicians and nonphysicians are at increased risk of a diverse group of medical conditions. Quantifying the contribution of radiation exposure to CV risk and separating it from smoking, dietary, and other factors, will require studies using dosimetry data in lieu of the estimated exposure measures seen in this study.
However, as these findings underscore, there is no doubt that we must be concerned about radiation and that manufacturers, hospitals, and providers need to come to the table to address these urgent questions of personal liability and workplace safety, particularly with so many young people entering the field.
Dr. Charles E. Chambers is professor of medicine and radiology at Pennsylvania State University, Hershey, and past president of the Society for Cardiovascular Angiography and Interventions. He made these comments in an interview, and reports no conflicts of interest.
The field of interventional cardiology is tremendously exciting, and yet the long-term risks are not as well defined as they should be for the protection of cath lab personnel.

|
Dr. Charles E. Chambers |
This study shows strikingly that both physicians and nonphysicians are at increased risk of a diverse group of medical conditions. Quantifying the contribution of radiation exposure to CV risk and separating it from smoking, dietary, and other factors, will require studies using dosimetry data in lieu of the estimated exposure measures seen in this study.
However, as these findings underscore, there is no doubt that we must be concerned about radiation and that manufacturers, hospitals, and providers need to come to the table to address these urgent questions of personal liability and workplace safety, particularly with so many young people entering the field.
Dr. Charles E. Chambers is professor of medicine and radiology at Pennsylvania State University, Hershey, and past president of the Society for Cardiovascular Angiography and Interventions. He made these comments in an interview, and reports no conflicts of interest.
Workers performing fluoroscopically guided cardiovascular procedures are at significantly higher risk of a diverse array of health disorders compared with nonexposed subjects, according to a survey.
Though cancers and ocular, skin, and thyroid disorders are recognized risks of working with low-dose radiation, as are orthopedic problems associated with wearing heavy lead aprons, the new study, published online April 12 in Circulation: Cardiovascular Interventions, adds weight to emerging concerns about lesser-known risks.
In addition to higher prevalence of skin lesions, cataracts, thyroid disorders, cancer, and orthopedic problems, exposed workers saw significantly higher prevalence of hypertension, hypercholesterolemia, and depression or anxiety, compared with controls.
Maria Grazia Andreassi, Ph.D., of the CNR Institute of Clinical Physiology, in Pisa, Italy, led the study, which used questionnaires to collect health, exposure, and demographic information from 466 workers in cardiac catheterization laboratories in Italy: 218 interventional cardiologists and electrophysiologists, 191 nurses, and 57 technicians. Radiation exposure was estimated based on subjects’ occupational radiological risk score (ORRS), which takes into consideration years of work, caseloads, and proximity to the radiation source in the lab.
The researchers administered the same questionnaires to 280 health workers who did not have occupational radiation exposure. The two groups were well matched for age, sex, education level, alcohol use, and body mass index, though current smoking differed significantly (27% of lab staff vs. 23% of unexposed workers).
As expected, orthopedic injuries were markedly higher among the lab workers, with 30% reporting disorders compared with 5% of controls (P less than .001). Nearly 5% of the lab workers had cataracts, compared with less than 1% of the nonexposed (P = .003), and risk increased for subjects with more years in the lab. Cancer was higher among exposed participants (2.6% vs. 0.7%), a difference that did not reach statistical significance. However, a significant trend was seen for cancer risk increasing across duration of exposure (Circ Cardiovasc Interv. 2016;9:e003273).
In the composite endpoint of cataract and cancer, a surrogate for disease associated with occupational exposure to radiation, the researchers found that the interventional cardiologists and electrophysiologists had a significantly higher prevalence than did the nurses and technicians (69% vs. 22% and 9%, respectively; P = .03).
Furthermore, the cancer in physicians occurred more often in the left side, when laterality was applicable, in 67% of cases, whereas the malignancy was left-sided in 33% of cancers occurring in nurses and technicians.
No significant differences in cardiovascular events were seen between the groups. However, hypertension and high cholesterol were more prevalent in cath lab workers, with adjusted odds ratios of 1.7 (95% confidence interval, 1-3; P = .05) and 2.9 (CI, 1-5; P = .004), respectively, for highly exposed subjects, compared with controls.
Last year, in a separate study, Dr. Andreassi’s group reported that exposure to low-dose radiation over time may increase carotid intima-media thickness, an early indicator of vascular injury, among cath lab workers (J Am Coll Cardiol Intv. 2015;8:616-27).
Anxiety and depression occurred in 12% of exposed subjects, compared with 2% of controls. The finding could reflect high occupational stress, the researchers wrote in their analysis, or may be an effect of radiation, “which is especially relevant on the unprotected head of the first operator and at chronic low doses may impact detrimentally on hippocampal neurogenesis and neuronal plasticity.”
The researchers acknowledged the possibility of selection bias in their study, favoring “a possible disproportionate contribution of respondents with existing health issues, who may reasonably think that their complaints are occupationally related,” they wrote. They also noted that the study was not designed to directly assess radiation dose, in part because dosimeters were not regularly worn in some of the settings where subjects worked. Finally, the radiation-exposed group had more cardiovascular risk factors. Nonetheless, the findings highlight the need to “spread the culture of safety” in cardiac cath labs, the researchers wrote.
There is now “more than enough information for us to conclude that the interventional catheterization laboratory is not a healthy workplace,” Dr. Lloyd W. Klein and Dr. Mugurel Bazavan of Advocate Illinois Masonic Medical Center, and Rush Medical College in Chicago, wrote in an accompanying editorial.
Finding “the courage to change business practice,” they wrote, “is the only path to innovative cath laboratory design and shielding techniques that can prevent these occupational hazards” (Circ Cardiovasc Interv. 2016;9:e003742).
Dr. Andreassi and colleagues disclosed no conflicts of interest related to their study, and Dr. Klein and Dr. Bazavan disclosed no conflicts related to their editorial.
Workers performing fluoroscopically guided cardiovascular procedures are at significantly higher risk of a diverse array of health disorders compared with nonexposed subjects, according to a survey.
Though cancers and ocular, skin, and thyroid disorders are recognized risks of working with low-dose radiation, as are orthopedic problems associated with wearing heavy lead aprons, the new study, published online April 12 in Circulation: Cardiovascular Interventions, adds weight to emerging concerns about lesser-known risks.
In addition to higher prevalence of skin lesions, cataracts, thyroid disorders, cancer, and orthopedic problems, exposed workers saw significantly higher prevalence of hypertension, hypercholesterolemia, and depression or anxiety, compared with controls.
Maria Grazia Andreassi, Ph.D., of the CNR Institute of Clinical Physiology, in Pisa, Italy, led the study, which used questionnaires to collect health, exposure, and demographic information from 466 workers in cardiac catheterization laboratories in Italy: 218 interventional cardiologists and electrophysiologists, 191 nurses, and 57 technicians. Radiation exposure was estimated based on subjects’ occupational radiological risk score (ORRS), which takes into consideration years of work, caseloads, and proximity to the radiation source in the lab.
The researchers administered the same questionnaires to 280 health workers who did not have occupational radiation exposure. The two groups were well matched for age, sex, education level, alcohol use, and body mass index, though current smoking differed significantly (27% of lab staff vs. 23% of unexposed workers).
As expected, orthopedic injuries were markedly higher among the lab workers, with 30% reporting disorders compared with 5% of controls (P less than .001). Nearly 5% of the lab workers had cataracts, compared with less than 1% of the nonexposed (P = .003), and risk increased for subjects with more years in the lab. Cancer was higher among exposed participants (2.6% vs. 0.7%), a difference that did not reach statistical significance. However, a significant trend was seen for cancer risk increasing across duration of exposure (Circ Cardiovasc Interv. 2016;9:e003273).
In the composite endpoint of cataract and cancer, a surrogate for disease associated with occupational exposure to radiation, the researchers found that the interventional cardiologists and electrophysiologists had a significantly higher prevalence than did the nurses and technicians (69% vs. 22% and 9%, respectively; P = .03).
Furthermore, the cancer in physicians occurred more often in the left side, when laterality was applicable, in 67% of cases, whereas the malignancy was left-sided in 33% of cancers occurring in nurses and technicians.
No significant differences in cardiovascular events were seen between the groups. However, hypertension and high cholesterol were more prevalent in cath lab workers, with adjusted odds ratios of 1.7 (95% confidence interval, 1-3; P = .05) and 2.9 (CI, 1-5; P = .004), respectively, for highly exposed subjects, compared with controls.
Last year, in a separate study, Dr. Andreassi’s group reported that exposure to low-dose radiation over time may increase carotid intima-media thickness, an early indicator of vascular injury, among cath lab workers (J Am Coll Cardiol Intv. 2015;8:616-27).
Anxiety and depression occurred in 12% of exposed subjects, compared with 2% of controls. The finding could reflect high occupational stress, the researchers wrote in their analysis, or may be an effect of radiation, “which is especially relevant on the unprotected head of the first operator and at chronic low doses may impact detrimentally on hippocampal neurogenesis and neuronal plasticity.”
The researchers acknowledged the possibility of selection bias in their study, favoring “a possible disproportionate contribution of respondents with existing health issues, who may reasonably think that their complaints are occupationally related,” they wrote. They also noted that the study was not designed to directly assess radiation dose, in part because dosimeters were not regularly worn in some of the settings where subjects worked. Finally, the radiation-exposed group had more cardiovascular risk factors. Nonetheless, the findings highlight the need to “spread the culture of safety” in cardiac cath labs, the researchers wrote.
There is now “more than enough information for us to conclude that the interventional catheterization laboratory is not a healthy workplace,” Dr. Lloyd W. Klein and Dr. Mugurel Bazavan of Advocate Illinois Masonic Medical Center, and Rush Medical College in Chicago, wrote in an accompanying editorial.
Finding “the courage to change business practice,” they wrote, “is the only path to innovative cath laboratory design and shielding techniques that can prevent these occupational hazards” (Circ Cardiovasc Interv. 2016;9:e003742).
Dr. Andreassi and colleagues disclosed no conflicts of interest related to their study, and Dr. Klein and Dr. Bazavan disclosed no conflicts related to their editorial.
FROM CIRCULATION: CARDIOVASCULAR INTERVENTIONS
Key clinical point: Workers in cardiac catheterization labs exposed to ionizing radiation are at risk of a wide array of health disorders.
Major finding: Prevalences of skin lesions, orthopedic illness, cataract, hypertension, hypercholesterolemia, and anxiety/depression were significantly higher in exposed cath lab workers, compared with other health care workers.
Data source: A multicenter, controlled study of 746 Italian physicians and staff (466 exposed to radiation and 280 matched unexposed controls) completing self-administered questionnaires.
Disclosures: None.
VIDEO: STICHES trial update boosts CABG in ischemic cardiomyopathy
CHICAGO – The results of the Surgical Treatment for Ischemic Heart Failure Extension Study (STICHES) presented at the annual meeting of the American College of Cardiology ought to change the clinical management of patients with coronary artery disease and heart failure with severe left ventricular dysfunction, according to Dr. Robert O. Bonow.
STICHES is the 10-year follow-up of 1,212 such patients who were randomized to coronary artery bypass graft surgery plus optimal guideline-directed medical therapy or to the medical therapy alone. At 10 years, the CABG group showed a significant survival advantage: an all-cause mortality rate of 58.9%, a significant 16% relative risk reduction compared with the 66.1% rate in the medically managed group. Secondary endpoints were also strongly in favor of the CABG group.
These findings indicate CABG is beneficial in patients with ischemic cardiomyopathy, and patients deserve to be so informed, according to Dr. Bonow, a member of the STICHES publication committee and professor of cardiology and director of the Center for Cardiovascular Innovation at Northwestern University in Chicago, who discussed the findings in this video interview.
He reported having no financial conflicts regarding this National Institutes of Health–funded study.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – The results of the Surgical Treatment for Ischemic Heart Failure Extension Study (STICHES) presented at the annual meeting of the American College of Cardiology ought to change the clinical management of patients with coronary artery disease and heart failure with severe left ventricular dysfunction, according to Dr. Robert O. Bonow.
STICHES is the 10-year follow-up of 1,212 such patients who were randomized to coronary artery bypass graft surgery plus optimal guideline-directed medical therapy or to the medical therapy alone. At 10 years, the CABG group showed a significant survival advantage: an all-cause mortality rate of 58.9%, a significant 16% relative risk reduction compared with the 66.1% rate in the medically managed group. Secondary endpoints were also strongly in favor of the CABG group.
These findings indicate CABG is beneficial in patients with ischemic cardiomyopathy, and patients deserve to be so informed, according to Dr. Bonow, a member of the STICHES publication committee and professor of cardiology and director of the Center for Cardiovascular Innovation at Northwestern University in Chicago, who discussed the findings in this video interview.
He reported having no financial conflicts regarding this National Institutes of Health–funded study.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – The results of the Surgical Treatment for Ischemic Heart Failure Extension Study (STICHES) presented at the annual meeting of the American College of Cardiology ought to change the clinical management of patients with coronary artery disease and heart failure with severe left ventricular dysfunction, according to Dr. Robert O. Bonow.
STICHES is the 10-year follow-up of 1,212 such patients who were randomized to coronary artery bypass graft surgery plus optimal guideline-directed medical therapy or to the medical therapy alone. At 10 years, the CABG group showed a significant survival advantage: an all-cause mortality rate of 58.9%, a significant 16% relative risk reduction compared with the 66.1% rate in the medically managed group. Secondary endpoints were also strongly in favor of the CABG group.
These findings indicate CABG is beneficial in patients with ischemic cardiomyopathy, and patients deserve to be so informed, according to Dr. Bonow, a member of the STICHES publication committee and professor of cardiology and director of the Center for Cardiovascular Innovation at Northwestern University in Chicago, who discussed the findings in this video interview.
He reported having no financial conflicts regarding this National Institutes of Health–funded study.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT ACC 16
VIDEO: STICHES trial update boosts CABG in ischemic cardiomyopathy
CHICAGO – The results of the Surgical Treatment for Ischemic Heart Failure Extension Study (STICHES) presented at the annual meeting of the American College of Cardiology ought to change the clinical management of patients with coronary artery disease and heart failure with severe left ventricular dysfunction, according to Dr. Robert O. Bonow.
STICHES is the 10-year follow-up of 1,212 such patients who were randomized to coronary artery bypass graft surgery plus optimal guideline-directed medical therapy or to the medical therapy alone. At 10 years, the CABG group showed a significant survival advantage: an all-cause mortality rate of 58.9%, a significant 16% relative risk reduction compared with the 66.1% rate in the medically managed group. Secondary endpoints were also strongly in favor of the CABG group.
These findings indicate CABG is beneficial in patients with ischemic cardiomyopathy, and patients deserve to be so informed, according to Dr. Bonow, a member of the STICHES publication committee and professor of cardiology and director of the Center for Cardiovascular Innovation at Northwestern University in Chicago, who discussed the findings in this video interview.
He reported having no financial conflicts regarding this National Institutes of Health–funded study.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – The results of the Surgical Treatment for Ischemic Heart Failure Extension Study (STICHES) presented at the annual meeting of the American College of Cardiology ought to change the clinical management of patients with coronary artery disease and heart failure with severe left ventricular dysfunction, according to Dr. Robert O. Bonow.
STICHES is the 10-year follow-up of 1,212 such patients who were randomized to coronary artery bypass graft surgery plus optimal guideline-directed medical therapy or to the medical therapy alone. At 10 years, the CABG group showed a significant survival advantage: an all-cause mortality rate of 58.9%, a significant 16% relative risk reduction compared with the 66.1% rate in the medically managed group. Secondary endpoints were also strongly in favor of the CABG group.
These findings indicate CABG is beneficial in patients with ischemic cardiomyopathy, and patients deserve to be so informed, according to Dr. Bonow, a member of the STICHES publication committee and professor of cardiology and director of the Center for Cardiovascular Innovation at Northwestern University in Chicago, who discussed the findings in this video interview.
He reported having no financial conflicts regarding this National Institutes of Health–funded study.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – The results of the Surgical Treatment for Ischemic Heart Failure Extension Study (STICHES) presented at the annual meeting of the American College of Cardiology ought to change the clinical management of patients with coronary artery disease and heart failure with severe left ventricular dysfunction, according to Dr. Robert O. Bonow.
STICHES is the 10-year follow-up of 1,212 such patients who were randomized to coronary artery bypass graft surgery plus optimal guideline-directed medical therapy or to the medical therapy alone. At 10 years, the CABG group showed a significant survival advantage: an all-cause mortality rate of 58.9%, a significant 16% relative risk reduction compared with the 66.1% rate in the medically managed group. Secondary endpoints were also strongly in favor of the CABG group.
These findings indicate CABG is beneficial in patients with ischemic cardiomyopathy, and patients deserve to be so informed, according to Dr. Bonow, a member of the STICHES publication committee and professor of cardiology and director of the Center for Cardiovascular Innovation at Northwestern University in Chicago, who discussed the findings in this video interview.
He reported having no financial conflicts regarding this National Institutes of Health–funded study.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT ACC 16
Adding mitral valve repair to CABG found risky, with limited benefits
In patients with moderate ischemic mitral regurgitation, adding mitral valve repair to coronary artery bypass graft did not significantly affect the extent of left ventricular remodeling at 2 years, according to a randomized multicenter trial.
Compared with revascularization alone, the combined procedure was associated with a two-thirds lower rate of moderate to severe mitral regurgitation at the end of year 2, reported Dr. Robert Michler at Albert Einstein College of Medicine, New York, together with his associates. But improved durability did not translate to better survival or lower rates of major adverse cardiac and cerebrovascular events, heart failure, or hospital readmissions, they said. Furthermore, adding mitral valve repair to CABG was linked to higher rates of postoperative neurologic events and supraventricular arrhythmias than for CABG alone, the investigators reported at the annual meeting of the American College of Cardiology and simultaneously online April 3 in the New England Journal of Medicine.
Surgeons have debated whether to add restrictive mitral annuloplasty to CABG in patients with moderate ischemic mitral regurgitation. The combined approach requires open-heart exposure and longer aortic cross-clamping and cardiopulmonary bypass time, all of which increase perioperative risk, the researchers noted. To explore the risk-benefit balance of these approaches, they randomly assigned 301 patients to either revascularization alone or to the combined procedure, and assessed clinical and echocardiographic outcomes over 2 years (New Engl. J. Med. 2016 April 3 doi: 10.1056/NEJMoa1602003).
At year 1, the arms resembled each other in terms of survival, rates of major adverse cardiac or cerebrovascular events (MACCEs), and left ventricular reverse remodeling, as measured by left ventricular end-systolic volume index (LVESVI), said the investigators. At year 2, the extent of remodeling remained similar between the arms, with mean LVESVIs of 41.2 mL (standard deviation, 20.0 mL) per square meter of body surface area in the CABG-only group and 43.2 mL (SD, 20.6 mL) in the combined procedure group, for a mean decrease from baseline of 14.1 and 14.6 mL, respectively. About two-thirds of improvements in LVESI occurred during year 1 regardless of procedure type, the researchers noted.
About a third of CABG-only patients had moderate to severe mitral regurgitation at 2 years, compared with only 11% of patients who underwent the combined procedure (P less than .001). Patients who had mild or no mitral regurgitation had more than twice the percentage improvement in the global wall motion index and in the inferior-posterior-lateral regional wall motion score, compared with patients with moderate to severe regurgitation. Although both groups improved on several quality-of-life indices, the combined-procedure group scored more than 5 points higher on the Duke Activity Status Index, which measures self-reported exercise capacity. That difference was not only clinically meaningful, but resembled the increase in peak myocardial oxygen consumption in a similar, smaller trial (New Engl. J. Med. 2016 Jan. 28 doi: 10.1056/NEJMoa151291), the investigators noted.
However, mortality rates were similar between the arms at 2 years – 10.6% for CABG only and 10% for the combined procedure, for a non-significant hazard ratio of 0.9. Likewise, rates of heart failure and other serious adverse events were similar between the arms, while the combined-procedure arm had more than three times more postoperative neurologic events and more than twice as many occurrences of supraventricular arrhythmia. Therefore, treatment choice requires “balancing the risks of adverse perioperative events against the uncertain benefits of a lower incidence of postoperative moderate or severe mitral regurgitation,” said the researchers. “Effective revascularization, as reflected in improved regional and global left ventricular function, plays an important role independent of mitral valve repair.”
The National Institutes of Health and the Canadian Institutes of Health Research funded the study. Disclosures were reported separately at nejm.org.
In patients with moderate ischemic mitral regurgitation, adding mitral valve repair to coronary artery bypass graft did not significantly affect the extent of left ventricular remodeling at 2 years, according to a randomized multicenter trial.
Compared with revascularization alone, the combined procedure was associated with a two-thirds lower rate of moderate to severe mitral regurgitation at the end of year 2, reported Dr. Robert Michler at Albert Einstein College of Medicine, New York, together with his associates. But improved durability did not translate to better survival or lower rates of major adverse cardiac and cerebrovascular events, heart failure, or hospital readmissions, they said. Furthermore, adding mitral valve repair to CABG was linked to higher rates of postoperative neurologic events and supraventricular arrhythmias than for CABG alone, the investigators reported at the annual meeting of the American College of Cardiology and simultaneously online April 3 in the New England Journal of Medicine.
Surgeons have debated whether to add restrictive mitral annuloplasty to CABG in patients with moderate ischemic mitral regurgitation. The combined approach requires open-heart exposure and longer aortic cross-clamping and cardiopulmonary bypass time, all of which increase perioperative risk, the researchers noted. To explore the risk-benefit balance of these approaches, they randomly assigned 301 patients to either revascularization alone or to the combined procedure, and assessed clinical and echocardiographic outcomes over 2 years (New Engl. J. Med. 2016 April 3 doi: 10.1056/NEJMoa1602003).
At year 1, the arms resembled each other in terms of survival, rates of major adverse cardiac or cerebrovascular events (MACCEs), and left ventricular reverse remodeling, as measured by left ventricular end-systolic volume index (LVESVI), said the investigators. At year 2, the extent of remodeling remained similar between the arms, with mean LVESVIs of 41.2 mL (standard deviation, 20.0 mL) per square meter of body surface area in the CABG-only group and 43.2 mL (SD, 20.6 mL) in the combined procedure group, for a mean decrease from baseline of 14.1 and 14.6 mL, respectively. About two-thirds of improvements in LVESI occurred during year 1 regardless of procedure type, the researchers noted.
About a third of CABG-only patients had moderate to severe mitral regurgitation at 2 years, compared with only 11% of patients who underwent the combined procedure (P less than .001). Patients who had mild or no mitral regurgitation had more than twice the percentage improvement in the global wall motion index and in the inferior-posterior-lateral regional wall motion score, compared with patients with moderate to severe regurgitation. Although both groups improved on several quality-of-life indices, the combined-procedure group scored more than 5 points higher on the Duke Activity Status Index, which measures self-reported exercise capacity. That difference was not only clinically meaningful, but resembled the increase in peak myocardial oxygen consumption in a similar, smaller trial (New Engl. J. Med. 2016 Jan. 28 doi: 10.1056/NEJMoa151291), the investigators noted.
However, mortality rates were similar between the arms at 2 years – 10.6% for CABG only and 10% for the combined procedure, for a non-significant hazard ratio of 0.9. Likewise, rates of heart failure and other serious adverse events were similar between the arms, while the combined-procedure arm had more than three times more postoperative neurologic events and more than twice as many occurrences of supraventricular arrhythmia. Therefore, treatment choice requires “balancing the risks of adverse perioperative events against the uncertain benefits of a lower incidence of postoperative moderate or severe mitral regurgitation,” said the researchers. “Effective revascularization, as reflected in improved regional and global left ventricular function, plays an important role independent of mitral valve repair.”
The National Institutes of Health and the Canadian Institutes of Health Research funded the study. Disclosures were reported separately at nejm.org.
In patients with moderate ischemic mitral regurgitation, adding mitral valve repair to coronary artery bypass graft did not significantly affect the extent of left ventricular remodeling at 2 years, according to a randomized multicenter trial.
Compared with revascularization alone, the combined procedure was associated with a two-thirds lower rate of moderate to severe mitral regurgitation at the end of year 2, reported Dr. Robert Michler at Albert Einstein College of Medicine, New York, together with his associates. But improved durability did not translate to better survival or lower rates of major adverse cardiac and cerebrovascular events, heart failure, or hospital readmissions, they said. Furthermore, adding mitral valve repair to CABG was linked to higher rates of postoperative neurologic events and supraventricular arrhythmias than for CABG alone, the investigators reported at the annual meeting of the American College of Cardiology and simultaneously online April 3 in the New England Journal of Medicine.
Surgeons have debated whether to add restrictive mitral annuloplasty to CABG in patients with moderate ischemic mitral regurgitation. The combined approach requires open-heart exposure and longer aortic cross-clamping and cardiopulmonary bypass time, all of which increase perioperative risk, the researchers noted. To explore the risk-benefit balance of these approaches, they randomly assigned 301 patients to either revascularization alone or to the combined procedure, and assessed clinical and echocardiographic outcomes over 2 years (New Engl. J. Med. 2016 April 3 doi: 10.1056/NEJMoa1602003).
At year 1, the arms resembled each other in terms of survival, rates of major adverse cardiac or cerebrovascular events (MACCEs), and left ventricular reverse remodeling, as measured by left ventricular end-systolic volume index (LVESVI), said the investigators. At year 2, the extent of remodeling remained similar between the arms, with mean LVESVIs of 41.2 mL (standard deviation, 20.0 mL) per square meter of body surface area in the CABG-only group and 43.2 mL (SD, 20.6 mL) in the combined procedure group, for a mean decrease from baseline of 14.1 and 14.6 mL, respectively. About two-thirds of improvements in LVESI occurred during year 1 regardless of procedure type, the researchers noted.
About a third of CABG-only patients had moderate to severe mitral regurgitation at 2 years, compared with only 11% of patients who underwent the combined procedure (P less than .001). Patients who had mild or no mitral regurgitation had more than twice the percentage improvement in the global wall motion index and in the inferior-posterior-lateral regional wall motion score, compared with patients with moderate to severe regurgitation. Although both groups improved on several quality-of-life indices, the combined-procedure group scored more than 5 points higher on the Duke Activity Status Index, which measures self-reported exercise capacity. That difference was not only clinically meaningful, but resembled the increase in peak myocardial oxygen consumption in a similar, smaller trial (New Engl. J. Med. 2016 Jan. 28 doi: 10.1056/NEJMoa151291), the investigators noted.
However, mortality rates were similar between the arms at 2 years – 10.6% for CABG only and 10% for the combined procedure, for a non-significant hazard ratio of 0.9. Likewise, rates of heart failure and other serious adverse events were similar between the arms, while the combined-procedure arm had more than three times more postoperative neurologic events and more than twice as many occurrences of supraventricular arrhythmia. Therefore, treatment choice requires “balancing the risks of adverse perioperative events against the uncertain benefits of a lower incidence of postoperative moderate or severe mitral regurgitation,” said the researchers. “Effective revascularization, as reflected in improved regional and global left ventricular function, plays an important role independent of mitral valve repair.”
The National Institutes of Health and the Canadian Institutes of Health Research funded the study. Disclosures were reported separately at nejm.org.
FROM ACC 16
Key clinical point: In patients with moderate ischemic mitral regurgitation, adding mitral valve repair to coronary artery bypass graft did not significantly affect left ventricular remodeling at two years.
Major finding: Mean decreases in left ventricular end systolic volume index were 14.1 mL and 14.6 mL, respectively.
Data source: A multicenter randomized trial of 301 patients with moderate to severe baseline mitral regurgitation.
Disclosures: The National Institutes of Health and the Canadian Institutes of Health Research funded the study. Disclosures were reported separately at nejm.org.















