User login
A view from the bridge to transplant for PTCL
LA JOLLA, CALIF. – For patients with relapsed peripheral T-cell lymphoma, allogeneic stem cell transplants offer the best chance for achieving remission or even a cure, making the choice of therapies as bridges to transplant essential for getting there.
“The goal is to get to transplant with a curative intent. In our hands, that’s mostly allo[geneic] and mostly in the relapsed setting,” Steven M. Horwitz, MD, from the lymphoma service at Memorial Sloan Kettering Cancer Center, New York, said at the annual T-cell Lymphoma Forum. “The best bridge to transplant is the one that gets you across safely.”
“They’d finish ICE, get a 3- or 4-week break, get a transplant, leave the hospital 3 or 4 weeks later, and then usually by their first repeat scan, at least on average, those patients had already progressed, so we sort of cooled to the idea of auto transplant and started preferentially looking at allo if we were going to treat with curative intent in the relapsed setting,” he said.
In contrast to autologous SCT, the Memorial Sloan Kettering experience with allogeneic SCT for 65 patients with relapsed PTCL showed a 2-year overall survival rate of 59%, 2-year PFS rate of 48%, and a median PFS of 20.3 months. However, the rate of 1-year transplant-related mortality was still relatively high, at 17%, Dr. Horwitz acknowledged (ASH 2015. Abstract 4392).
An updated retrospective analysis of the center’s experience treating mature T-cell lymphoma patients with allogeneic SCT, also presented at the 2018 T-cell Lymphoma Forum, showed that disease status at transplant was one of the most important predictors of outcome. Median posttransplant PFS for patients in complete remission (CR) at the time of transplant was 61.3 months, compared with 11.4 months for patients in partial remission, 14 months for patients with stable disease, and 6.4 months for patients with disease progression (TCLF 2018. Abstract TM18_9).
“I think we can probably infer from [this] that CR not only gives you a better outcome with allo, but probably increases your chance that you get to an allo,” he said.
In the randomized phase 3 Lumiere study comparing the Aurora A kinase inhibitor alisertib with investigators’ choice of therapy in relapsed/refractory PTCL, alisertib was associated with a CR rate of 19%, whereas pralatrexate, gemcitabine, and romidepsin were associated with CR rates of 29%, 23%, and 33%, respectively, putting them on par with combination chemotherapy.
“I think many of us prefer some of the newer single agents because we’re really going for a durable maintenance of disease control rather than short-term bridge to transplant, but these drugs can provide adequate responses to transition over,” he said.
Better approaches by subtype?
The subtype of PTCL also appears to matter. Three approved agents for relapsed/refractory PTCL – belinostat (Beleodaq), romidepsin (Istodax), and pralatrexate (Folotyn) – are associated with CR rates of 11%, 15%, and 11%, respectively. But one PTCL subtype, anaplastic large cell lymphoma, appears particularly sensitive to treatment with brentuximab vedotin (Adcetris), with CR rates of 59%, Dr. Horwitz noted.
In a 2014 study, investigators reported that of the nine patients with anaplastic large cell lymphoma positive for anaplastic lymphoma kinase and treated with the anaplastic lymphoma kinase inhibitor crizotinib (Xalkori), all had a CR, with response durations stretching pasting 40 months in one patient, and past 30 months in two others (J Natl Cancer Inst. 2014 Feb;106[2]:djt378).
A different subtype, natural killer/T-cell lymphoma, was shown to be responsive to immunotherapy with pembrolizumab (Keytruda) in seven patients, with CRs in five and partial remissions in two. Responses to pembrolizumab in this PTCL subtype may be adequately long for getting patients to transplant, Dr. Horwitz said.
For some patients with angioimmunoblastic T-cell lymphoma, therapy with epigenetic modifying agents, such as decitabine or a combination of romidepsin and lenalidomide (Revlimid), with or without carfilzomib (Kyprolis), may also be effective bridges to transplant, based on the best available evidence.
Timing may also matter
Dr. Horwitz cautioned that for patients with cutaneous T-cell lymphoma, the investigational agent mogamulizumab, which was shown in the MAVORIC (Study of KW-0761 versus Vorinostat in Relapsed/Refractory CTCL) trial to offer significantly better PFS compared with vorinostat (Zolinza), also appears to increase the chance that patients will develop high-risk, potentially fatal graft vs. host disease posttransplant.
The risk appears to be slightly lower among patients who received the last dose of mogamulizumab more than 50 days before undergoing SCT, he noted.
Although there is no strong evidence to support it, Dr. Horwitz noted that the timing of most other therapies may also be important to the success of SCT. “I think we have seen that when patients have a big [long] break before transplant, the relapse rate is high, and I have a personal preference for using regimens that you can continue up close to transplant, because I think we lose [fewer] patients getting ready for that,” he said.
Dr. Horwitz had previously disclosed financial relationships with Celgene, Forty Seven, Huya Bioscience International, Infinity, Kyowa Hakko Kirin, Millennium, Seattle Genetics, and Takeda. The T-Cell Lymphoma Forum is held by Jonathan Wood & Associates, which is owned by the same company as this news organization.
LA JOLLA, CALIF. – For patients with relapsed peripheral T-cell lymphoma, allogeneic stem cell transplants offer the best chance for achieving remission or even a cure, making the choice of therapies as bridges to transplant essential for getting there.
“The goal is to get to transplant with a curative intent. In our hands, that’s mostly allo[geneic] and mostly in the relapsed setting,” Steven M. Horwitz, MD, from the lymphoma service at Memorial Sloan Kettering Cancer Center, New York, said at the annual T-cell Lymphoma Forum. “The best bridge to transplant is the one that gets you across safely.”
“They’d finish ICE, get a 3- or 4-week break, get a transplant, leave the hospital 3 or 4 weeks later, and then usually by their first repeat scan, at least on average, those patients had already progressed, so we sort of cooled to the idea of auto transplant and started preferentially looking at allo if we were going to treat with curative intent in the relapsed setting,” he said.
In contrast to autologous SCT, the Memorial Sloan Kettering experience with allogeneic SCT for 65 patients with relapsed PTCL showed a 2-year overall survival rate of 59%, 2-year PFS rate of 48%, and a median PFS of 20.3 months. However, the rate of 1-year transplant-related mortality was still relatively high, at 17%, Dr. Horwitz acknowledged (ASH 2015. Abstract 4392).
An updated retrospective analysis of the center’s experience treating mature T-cell lymphoma patients with allogeneic SCT, also presented at the 2018 T-cell Lymphoma Forum, showed that disease status at transplant was one of the most important predictors of outcome. Median posttransplant PFS for patients in complete remission (CR) at the time of transplant was 61.3 months, compared with 11.4 months for patients in partial remission, 14 months for patients with stable disease, and 6.4 months for patients with disease progression (TCLF 2018. Abstract TM18_9).
“I think we can probably infer from [this] that CR not only gives you a better outcome with allo, but probably increases your chance that you get to an allo,” he said.
In the randomized phase 3 Lumiere study comparing the Aurora A kinase inhibitor alisertib with investigators’ choice of therapy in relapsed/refractory PTCL, alisertib was associated with a CR rate of 19%, whereas pralatrexate, gemcitabine, and romidepsin were associated with CR rates of 29%, 23%, and 33%, respectively, putting them on par with combination chemotherapy.
“I think many of us prefer some of the newer single agents because we’re really going for a durable maintenance of disease control rather than short-term bridge to transplant, but these drugs can provide adequate responses to transition over,” he said.
Better approaches by subtype?
The subtype of PTCL also appears to matter. Three approved agents for relapsed/refractory PTCL – belinostat (Beleodaq), romidepsin (Istodax), and pralatrexate (Folotyn) – are associated with CR rates of 11%, 15%, and 11%, respectively. But one PTCL subtype, anaplastic large cell lymphoma, appears particularly sensitive to treatment with brentuximab vedotin (Adcetris), with CR rates of 59%, Dr. Horwitz noted.
In a 2014 study, investigators reported that of the nine patients with anaplastic large cell lymphoma positive for anaplastic lymphoma kinase and treated with the anaplastic lymphoma kinase inhibitor crizotinib (Xalkori), all had a CR, with response durations stretching pasting 40 months in one patient, and past 30 months in two others (J Natl Cancer Inst. 2014 Feb;106[2]:djt378).
A different subtype, natural killer/T-cell lymphoma, was shown to be responsive to immunotherapy with pembrolizumab (Keytruda) in seven patients, with CRs in five and partial remissions in two. Responses to pembrolizumab in this PTCL subtype may be adequately long for getting patients to transplant, Dr. Horwitz said.
For some patients with angioimmunoblastic T-cell lymphoma, therapy with epigenetic modifying agents, such as decitabine or a combination of romidepsin and lenalidomide (Revlimid), with or without carfilzomib (Kyprolis), may also be effective bridges to transplant, based on the best available evidence.
Timing may also matter
Dr. Horwitz cautioned that for patients with cutaneous T-cell lymphoma, the investigational agent mogamulizumab, which was shown in the MAVORIC (Study of KW-0761 versus Vorinostat in Relapsed/Refractory CTCL) trial to offer significantly better PFS compared with vorinostat (Zolinza), also appears to increase the chance that patients will develop high-risk, potentially fatal graft vs. host disease posttransplant.
The risk appears to be slightly lower among patients who received the last dose of mogamulizumab more than 50 days before undergoing SCT, he noted.
Although there is no strong evidence to support it, Dr. Horwitz noted that the timing of most other therapies may also be important to the success of SCT. “I think we have seen that when patients have a big [long] break before transplant, the relapse rate is high, and I have a personal preference for using regimens that you can continue up close to transplant, because I think we lose [fewer] patients getting ready for that,” he said.
Dr. Horwitz had previously disclosed financial relationships with Celgene, Forty Seven, Huya Bioscience International, Infinity, Kyowa Hakko Kirin, Millennium, Seattle Genetics, and Takeda. The T-Cell Lymphoma Forum is held by Jonathan Wood & Associates, which is owned by the same company as this news organization.
LA JOLLA, CALIF. – For patients with relapsed peripheral T-cell lymphoma, allogeneic stem cell transplants offer the best chance for achieving remission or even a cure, making the choice of therapies as bridges to transplant essential for getting there.
“The goal is to get to transplant with a curative intent. In our hands, that’s mostly allo[geneic] and mostly in the relapsed setting,” Steven M. Horwitz, MD, from the lymphoma service at Memorial Sloan Kettering Cancer Center, New York, said at the annual T-cell Lymphoma Forum. “The best bridge to transplant is the one that gets you across safely.”
“They’d finish ICE, get a 3- or 4-week break, get a transplant, leave the hospital 3 or 4 weeks later, and then usually by their first repeat scan, at least on average, those patients had already progressed, so we sort of cooled to the idea of auto transplant and started preferentially looking at allo if we were going to treat with curative intent in the relapsed setting,” he said.
In contrast to autologous SCT, the Memorial Sloan Kettering experience with allogeneic SCT for 65 patients with relapsed PTCL showed a 2-year overall survival rate of 59%, 2-year PFS rate of 48%, and a median PFS of 20.3 months. However, the rate of 1-year transplant-related mortality was still relatively high, at 17%, Dr. Horwitz acknowledged (ASH 2015. Abstract 4392).
An updated retrospective analysis of the center’s experience treating mature T-cell lymphoma patients with allogeneic SCT, also presented at the 2018 T-cell Lymphoma Forum, showed that disease status at transplant was one of the most important predictors of outcome. Median posttransplant PFS for patients in complete remission (CR) at the time of transplant was 61.3 months, compared with 11.4 months for patients in partial remission, 14 months for patients with stable disease, and 6.4 months for patients with disease progression (TCLF 2018. Abstract TM18_9).
“I think we can probably infer from [this] that CR not only gives you a better outcome with allo, but probably increases your chance that you get to an allo,” he said.
In the randomized phase 3 Lumiere study comparing the Aurora A kinase inhibitor alisertib with investigators’ choice of therapy in relapsed/refractory PTCL, alisertib was associated with a CR rate of 19%, whereas pralatrexate, gemcitabine, and romidepsin were associated with CR rates of 29%, 23%, and 33%, respectively, putting them on par with combination chemotherapy.
“I think many of us prefer some of the newer single agents because we’re really going for a durable maintenance of disease control rather than short-term bridge to transplant, but these drugs can provide adequate responses to transition over,” he said.
Better approaches by subtype?
The subtype of PTCL also appears to matter. Three approved agents for relapsed/refractory PTCL – belinostat (Beleodaq), romidepsin (Istodax), and pralatrexate (Folotyn) – are associated with CR rates of 11%, 15%, and 11%, respectively. But one PTCL subtype, anaplastic large cell lymphoma, appears particularly sensitive to treatment with brentuximab vedotin (Adcetris), with CR rates of 59%, Dr. Horwitz noted.
In a 2014 study, investigators reported that of the nine patients with anaplastic large cell lymphoma positive for anaplastic lymphoma kinase and treated with the anaplastic lymphoma kinase inhibitor crizotinib (Xalkori), all had a CR, with response durations stretching pasting 40 months in one patient, and past 30 months in two others (J Natl Cancer Inst. 2014 Feb;106[2]:djt378).
A different subtype, natural killer/T-cell lymphoma, was shown to be responsive to immunotherapy with pembrolizumab (Keytruda) in seven patients, with CRs in five and partial remissions in two. Responses to pembrolizumab in this PTCL subtype may be adequately long for getting patients to transplant, Dr. Horwitz said.
For some patients with angioimmunoblastic T-cell lymphoma, therapy with epigenetic modifying agents, such as decitabine or a combination of romidepsin and lenalidomide (Revlimid), with or without carfilzomib (Kyprolis), may also be effective bridges to transplant, based on the best available evidence.
Timing may also matter
Dr. Horwitz cautioned that for patients with cutaneous T-cell lymphoma, the investigational agent mogamulizumab, which was shown in the MAVORIC (Study of KW-0761 versus Vorinostat in Relapsed/Refractory CTCL) trial to offer significantly better PFS compared with vorinostat (Zolinza), also appears to increase the chance that patients will develop high-risk, potentially fatal graft vs. host disease posttransplant.
The risk appears to be slightly lower among patients who received the last dose of mogamulizumab more than 50 days before undergoing SCT, he noted.
Although there is no strong evidence to support it, Dr. Horwitz noted that the timing of most other therapies may also be important to the success of SCT. “I think we have seen that when patients have a big [long] break before transplant, the relapse rate is high, and I have a personal preference for using regimens that you can continue up close to transplant, because I think we lose [fewer] patients getting ready for that,” he said.
Dr. Horwitz had previously disclosed financial relationships with Celgene, Forty Seven, Huya Bioscience International, Infinity, Kyowa Hakko Kirin, Millennium, Seattle Genetics, and Takeda. The T-Cell Lymphoma Forum is held by Jonathan Wood & Associates, which is owned by the same company as this news organization.
EXPERT ANALYSIS FROM TCLF 2018
Getting hematologic cancer drugs on the fast track
LA JOLLA, CALIF. – The words “rapid approval” and “Food and Drug Administration” rarely appear in the same sentence. But despite that perception, the pace of hematologic drug development has been accelerating over the last several years, according to an agency staffer.
“FDA is committed toward the expedited development of safe and effective therapies for serious and life-threatening diseases,” R. Angelo de Claro, MD, of the FDA’s Office of Hematology and Oncology Products said at the annual T-cell Lymphoma Forum. Dr. de Claro outlined his agency’s efforts to accelerate approval of drugs for treatment of T-cell malignancies.
Hematologic drug bonanza
In 2017 alone, the FDA approved 17 agents for new or expanded indications for hematologic malignancies, including brentuximab vedotin (Adcetris) for anaplastic large cell lymphoma (ALCL) and CD30-positive mycosis fungoides (MF).
Approval was based on a 56% objective response rate for brentuximab vedotin versus 12% for physician’s choice in a phase 3 trial (ALCANZA) of 131 patients with mycosis fungoides or primary cutaneous ALCL. All patients had received one prior systemic therapy and were randomized (1:1) to receive either brentuximab vedotin or the physician’s choice of methotrexate or bexarotene.
Dr. de Claro noted that in the ALCANZA trial, patients were required to have one or more biopsy samples with at least 10% CD30 expression, but among 184 patients with MF screened for the trial, 32% were ineligible because of less than 10% CD30 expression. The FDA therefore requested additional efficacy data for patients with MF with less than 10% CD30 expression and accepted data from two investigator-sponsored trials showing that 35 patients with MF expressing CD30 on 1%-9% of cells had a 31% overall response rate, whereas two patients with no CD30 expression did not have responses.
Who minds the store
Hematology products are under the aegis of the FDA’s Oncology Center of Excellence. Oversight includes benign hematology products, as well as products for hematologic cancers and hematologic support. Hematology and oncology toxicology is monitored by pharmacologists and toxicologists in a separate division, he explained.
“The Oncology Center of Excellence was formally launched in 2017 as part of the 21st century CURES Act. The mission of the Oncology Center of Excellence is to achieve patient-centered regulatory decision making through innovation and collaboration,” he said.
Getting the nod
To get approved, a new therapy requires “substantial” evidence of efficacy and safety. Regular approvals are based on either direct measures of clinical benefits – how a patient “feels, functions, or survives” – or a measure of the effect of a drug on an established surrogate endpoint.
For an accelerated approval, developers must be able to show evidence on either a surrogate or intermediate clinical endpoint that the agent is reasonably likely to offer a benefit and be a meaningful improvement over available therapies. Postapproval trials may be needed to verify the proposed benefits.
FDA accelerated approval programs include:
- Fast track. The pathway requires nonclinical or clinical data demonstrating the potential for addressing an unmet need.
- Breakthrough therapy. This pathway requires preliminary clinical evidence demonstrating substantial improvement over existing available therapies.
- Priority review. These are agents that, if approved, would provide significant improvements in safety or effectiveness.
- Accelerated approval. The drug must demonstrate an effect on an “endpoint reasonably likely to predict clinical benefit over available therapies.”
Dr. de Claro is employed by the FDA. The T-Cell Lymphoma Forum is held by Jonathan Wood & Associates, which is owned by the same company as this news organization.
LA JOLLA, CALIF. – The words “rapid approval” and “Food and Drug Administration” rarely appear in the same sentence. But despite that perception, the pace of hematologic drug development has been accelerating over the last several years, according to an agency staffer.
“FDA is committed toward the expedited development of safe and effective therapies for serious and life-threatening diseases,” R. Angelo de Claro, MD, of the FDA’s Office of Hematology and Oncology Products said at the annual T-cell Lymphoma Forum. Dr. de Claro outlined his agency’s efforts to accelerate approval of drugs for treatment of T-cell malignancies.
Hematologic drug bonanza
In 2017 alone, the FDA approved 17 agents for new or expanded indications for hematologic malignancies, including brentuximab vedotin (Adcetris) for anaplastic large cell lymphoma (ALCL) and CD30-positive mycosis fungoides (MF).
Approval was based on a 56% objective response rate for brentuximab vedotin versus 12% for physician’s choice in a phase 3 trial (ALCANZA) of 131 patients with mycosis fungoides or primary cutaneous ALCL. All patients had received one prior systemic therapy and were randomized (1:1) to receive either brentuximab vedotin or the physician’s choice of methotrexate or bexarotene.
Dr. de Claro noted that in the ALCANZA trial, patients were required to have one or more biopsy samples with at least 10% CD30 expression, but among 184 patients with MF screened for the trial, 32% were ineligible because of less than 10% CD30 expression. The FDA therefore requested additional efficacy data for patients with MF with less than 10% CD30 expression and accepted data from two investigator-sponsored trials showing that 35 patients with MF expressing CD30 on 1%-9% of cells had a 31% overall response rate, whereas two patients with no CD30 expression did not have responses.
Who minds the store
Hematology products are under the aegis of the FDA’s Oncology Center of Excellence. Oversight includes benign hematology products, as well as products for hematologic cancers and hematologic support. Hematology and oncology toxicology is monitored by pharmacologists and toxicologists in a separate division, he explained.
“The Oncology Center of Excellence was formally launched in 2017 as part of the 21st century CURES Act. The mission of the Oncology Center of Excellence is to achieve patient-centered regulatory decision making through innovation and collaboration,” he said.
Getting the nod
To get approved, a new therapy requires “substantial” evidence of efficacy and safety. Regular approvals are based on either direct measures of clinical benefits – how a patient “feels, functions, or survives” – or a measure of the effect of a drug on an established surrogate endpoint.
For an accelerated approval, developers must be able to show evidence on either a surrogate or intermediate clinical endpoint that the agent is reasonably likely to offer a benefit and be a meaningful improvement over available therapies. Postapproval trials may be needed to verify the proposed benefits.
FDA accelerated approval programs include:
- Fast track. The pathway requires nonclinical or clinical data demonstrating the potential for addressing an unmet need.
- Breakthrough therapy. This pathway requires preliminary clinical evidence demonstrating substantial improvement over existing available therapies.
- Priority review. These are agents that, if approved, would provide significant improvements in safety or effectiveness.
- Accelerated approval. The drug must demonstrate an effect on an “endpoint reasonably likely to predict clinical benefit over available therapies.”
Dr. de Claro is employed by the FDA. The T-Cell Lymphoma Forum is held by Jonathan Wood & Associates, which is owned by the same company as this news organization.
LA JOLLA, CALIF. – The words “rapid approval” and “Food and Drug Administration” rarely appear in the same sentence. But despite that perception, the pace of hematologic drug development has been accelerating over the last several years, according to an agency staffer.
“FDA is committed toward the expedited development of safe and effective therapies for serious and life-threatening diseases,” R. Angelo de Claro, MD, of the FDA’s Office of Hematology and Oncology Products said at the annual T-cell Lymphoma Forum. Dr. de Claro outlined his agency’s efforts to accelerate approval of drugs for treatment of T-cell malignancies.
Hematologic drug bonanza
In 2017 alone, the FDA approved 17 agents for new or expanded indications for hematologic malignancies, including brentuximab vedotin (Adcetris) for anaplastic large cell lymphoma (ALCL) and CD30-positive mycosis fungoides (MF).
Approval was based on a 56% objective response rate for brentuximab vedotin versus 12% for physician’s choice in a phase 3 trial (ALCANZA) of 131 patients with mycosis fungoides or primary cutaneous ALCL. All patients had received one prior systemic therapy and were randomized (1:1) to receive either brentuximab vedotin or the physician’s choice of methotrexate or bexarotene.
Dr. de Claro noted that in the ALCANZA trial, patients were required to have one or more biopsy samples with at least 10% CD30 expression, but among 184 patients with MF screened for the trial, 32% were ineligible because of less than 10% CD30 expression. The FDA therefore requested additional efficacy data for patients with MF with less than 10% CD30 expression and accepted data from two investigator-sponsored trials showing that 35 patients with MF expressing CD30 on 1%-9% of cells had a 31% overall response rate, whereas two patients with no CD30 expression did not have responses.
Who minds the store
Hematology products are under the aegis of the FDA’s Oncology Center of Excellence. Oversight includes benign hematology products, as well as products for hematologic cancers and hematologic support. Hematology and oncology toxicology is monitored by pharmacologists and toxicologists in a separate division, he explained.
“The Oncology Center of Excellence was formally launched in 2017 as part of the 21st century CURES Act. The mission of the Oncology Center of Excellence is to achieve patient-centered regulatory decision making through innovation and collaboration,” he said.
Getting the nod
To get approved, a new therapy requires “substantial” evidence of efficacy and safety. Regular approvals are based on either direct measures of clinical benefits – how a patient “feels, functions, or survives” – or a measure of the effect of a drug on an established surrogate endpoint.
For an accelerated approval, developers must be able to show evidence on either a surrogate or intermediate clinical endpoint that the agent is reasonably likely to offer a benefit and be a meaningful improvement over available therapies. Postapproval trials may be needed to verify the proposed benefits.
FDA accelerated approval programs include:
- Fast track. The pathway requires nonclinical or clinical data demonstrating the potential for addressing an unmet need.
- Breakthrough therapy. This pathway requires preliminary clinical evidence demonstrating substantial improvement over existing available therapies.
- Priority review. These are agents that, if approved, would provide significant improvements in safety or effectiveness.
- Accelerated approval. The drug must demonstrate an effect on an “endpoint reasonably likely to predict clinical benefit over available therapies.”
Dr. de Claro is employed by the FDA. The T-Cell Lymphoma Forum is held by Jonathan Wood & Associates, which is owned by the same company as this news organization.
EXPERT ANALYSIS FROM TCLF 2018
T-cell lymphoma therapies on the horizon
LA JOLLA, CALIF. – There are several biologic compounds in early clinical development for treatment of patients with T-cell lymphomas, including an antibody-drug conjugate, novel immune checkpoint inhibitor, and bi-specific antibody.
These investigational agents show promising single-agent activity and have the potential to improve clinical responses when combined with combination chemotherapy regimens or other treatments, Ahmed Sawas, MD, of the Center for Lymphoid Malignancies at Columbia University, New York, said at the annual T-cell Lymphoma Forum.
AGS67E: Antibody-drug conjugate
AGS67E is an antibody-drug conjugate targeted against CD37, a transmembrane protein preferentially expressed on malignant B cells, T cells, and acute myeloid leukemia cells. In a study published in 2015 in Molecular Cancer Therapeutics, investigators from Agensys (an affiliate of Astellas Pharma) reported that this compound bound to more than 80% of patient-derived T cells in vitro (Mol Cancer Ther. 2015;14[7]:1650-60).
In a phase 1 dose-escalation study reported at the 2017 International Conference on Malignant Lymphoma in Lugano, Switzerland, Dr. Sawas and his colleagues found that patients with B-cell and T-cell malignancies, including cutaneous T-cell lymphoma and peripheral T-cell lymphoma, tolerated the drug well when it was delivered both with or without growth factor. Neutropenia was the most frequent adverse event and dose-limiting toxicity.
The drug showed single-agent activity in 16 of 53 patients with heavily pretreated non-Hodgkin lymphoma, including a partial response in one of two patients with cutaneous T-cell lymphoma, and partial responses in two of four patients with peripheral T-cell lymphoma. There were no complete responses at any of three dose levels of the drug, with or without growth factor.
One patient, a 75-year-old man with stage IVB mycosis fungoides who had disease progression on prior therapy with methotrexate, romidepsin, bendamustine, whole-body irradiation, liposomal doxorubicin, pralatrexate, and pembrolizumab experienced significant reduction in tumor burden and resolution of lymph node involvement after three 3-week cycles of therapy with AGS67E. The patient had a deepening of the response with additional cycles, and remained on therapy for 30 cycles until he experienced disease progression.
TTI-621: Tuck in, macrophages
TT1-621 is a molecule with two functions: It acts as an immune checkpoint inhibitor by blocking CD47, which binds to signal-regulatory protein alpha to produce an antiphagocytic or “do not eat” signal. TTI-621 does not, however, bind to CD47-positive erythrocytes.
In addition to blocking CD47 and the do-not-eat signal, TTI-621 delivers an activating signal to macrophages through Fc gamma receptors, telling them, in effect, “bon appétit.”
In a study presented at the 2017 annual meeting of the American Society of Hematology (Abstract 4076), investigators from City of Hope in Duarte, Calif., and other centers reported that a single direct intratumoral injection of TTI-621 was associated with significant antitumor activity in patients with relapsed or refractory mycosis fungoides and Sézary syndrome, with one of nine patients having a complete response in the injected lesion, and five having decreases in tumor size and/or circulating Sézary cells.
Patients appeared to tolerate this agent very well, with 1 of 18 having a grade 3 increase in white blood cell count. The most commonly reported side effects were fatigue, chills, decreased appetite, headache, injection site pain, and generalized pruritus, each occurring in 3 of the 18 patients.
TTI-621 injection was associated with rapid declines in Composite Assessment of Index Lesion Severity scores in dose-finding studies in patients with heavily pretreated cutaneous T-cell lymphoma, Dr. Sawas said.
AFM13: Two for the price of one
AFM13 is a bi-specific antibody that binds to CD30, which is expressed on anaplastic large cell lymphoma cells, as well as Reed-Sternberg cells of classical Hodgkin lymphoma. This antibody also engages CD16A-positive cells, resulting in lysis of CD30-positive tumor cells. It is a specific recruiter of natural killer cells, and does not bind to neutrophils.
In an early biologic effects study of this agent in CD30-positive lymphoid malignancies with cutaneous presentation, Dr. Sawas and his colleagues observed an early response and regression of cutaneous anaplastic large cell lymphoma lesions in a heavily pretreated patient, with progression occurring when the patient went off therapy, and tumors that diminished on reinitiation of therapy that sustained beyond a second discontinuation of therapy. This patient had measurable reductions in lymphoma burden on PET CT scans and improvements in cutaneous lesions. Dr. Sawas did not present safety data for this agent.
AGS67E studies are supported by Agensys. TTI-621 studies are supported by Trillium Therapeutics. The AFM13 study is supported by Columbia University, with Dr. Sawas listed as the sponsor. He did not report potential conflicts of interests. The T-Cell Lymphoma Forum is held by Jonathan Wood & Associates, which is owned by the same company as this news organization.
LA JOLLA, CALIF. – There are several biologic compounds in early clinical development for treatment of patients with T-cell lymphomas, including an antibody-drug conjugate, novel immune checkpoint inhibitor, and bi-specific antibody.
These investigational agents show promising single-agent activity and have the potential to improve clinical responses when combined with combination chemotherapy regimens or other treatments, Ahmed Sawas, MD, of the Center for Lymphoid Malignancies at Columbia University, New York, said at the annual T-cell Lymphoma Forum.
AGS67E: Antibody-drug conjugate
AGS67E is an antibody-drug conjugate targeted against CD37, a transmembrane protein preferentially expressed on malignant B cells, T cells, and acute myeloid leukemia cells. In a study published in 2015 in Molecular Cancer Therapeutics, investigators from Agensys (an affiliate of Astellas Pharma) reported that this compound bound to more than 80% of patient-derived T cells in vitro (Mol Cancer Ther. 2015;14[7]:1650-60).
In a phase 1 dose-escalation study reported at the 2017 International Conference on Malignant Lymphoma in Lugano, Switzerland, Dr. Sawas and his colleagues found that patients with B-cell and T-cell malignancies, including cutaneous T-cell lymphoma and peripheral T-cell lymphoma, tolerated the drug well when it was delivered both with or without growth factor. Neutropenia was the most frequent adverse event and dose-limiting toxicity.
The drug showed single-agent activity in 16 of 53 patients with heavily pretreated non-Hodgkin lymphoma, including a partial response in one of two patients with cutaneous T-cell lymphoma, and partial responses in two of four patients with peripheral T-cell lymphoma. There were no complete responses at any of three dose levels of the drug, with or without growth factor.
One patient, a 75-year-old man with stage IVB mycosis fungoides who had disease progression on prior therapy with methotrexate, romidepsin, bendamustine, whole-body irradiation, liposomal doxorubicin, pralatrexate, and pembrolizumab experienced significant reduction in tumor burden and resolution of lymph node involvement after three 3-week cycles of therapy with AGS67E. The patient had a deepening of the response with additional cycles, and remained on therapy for 30 cycles until he experienced disease progression.
TTI-621: Tuck in, macrophages
TT1-621 is a molecule with two functions: It acts as an immune checkpoint inhibitor by blocking CD47, which binds to signal-regulatory protein alpha to produce an antiphagocytic or “do not eat” signal. TTI-621 does not, however, bind to CD47-positive erythrocytes.
In addition to blocking CD47 and the do-not-eat signal, TTI-621 delivers an activating signal to macrophages through Fc gamma receptors, telling them, in effect, “bon appétit.”
In a study presented at the 2017 annual meeting of the American Society of Hematology (Abstract 4076), investigators from City of Hope in Duarte, Calif., and other centers reported that a single direct intratumoral injection of TTI-621 was associated with significant antitumor activity in patients with relapsed or refractory mycosis fungoides and Sézary syndrome, with one of nine patients having a complete response in the injected lesion, and five having decreases in tumor size and/or circulating Sézary cells.
Patients appeared to tolerate this agent very well, with 1 of 18 having a grade 3 increase in white blood cell count. The most commonly reported side effects were fatigue, chills, decreased appetite, headache, injection site pain, and generalized pruritus, each occurring in 3 of the 18 patients.
TTI-621 injection was associated with rapid declines in Composite Assessment of Index Lesion Severity scores in dose-finding studies in patients with heavily pretreated cutaneous T-cell lymphoma, Dr. Sawas said.
AFM13: Two for the price of one
AFM13 is a bi-specific antibody that binds to CD30, which is expressed on anaplastic large cell lymphoma cells, as well as Reed-Sternberg cells of classical Hodgkin lymphoma. This antibody also engages CD16A-positive cells, resulting in lysis of CD30-positive tumor cells. It is a specific recruiter of natural killer cells, and does not bind to neutrophils.
In an early biologic effects study of this agent in CD30-positive lymphoid malignancies with cutaneous presentation, Dr. Sawas and his colleagues observed an early response and regression of cutaneous anaplastic large cell lymphoma lesions in a heavily pretreated patient, with progression occurring when the patient went off therapy, and tumors that diminished on reinitiation of therapy that sustained beyond a second discontinuation of therapy. This patient had measurable reductions in lymphoma burden on PET CT scans and improvements in cutaneous lesions. Dr. Sawas did not present safety data for this agent.
AGS67E studies are supported by Agensys. TTI-621 studies are supported by Trillium Therapeutics. The AFM13 study is supported by Columbia University, with Dr. Sawas listed as the sponsor. He did not report potential conflicts of interests. The T-Cell Lymphoma Forum is held by Jonathan Wood & Associates, which is owned by the same company as this news organization.
LA JOLLA, CALIF. – There are several biologic compounds in early clinical development for treatment of patients with T-cell lymphomas, including an antibody-drug conjugate, novel immune checkpoint inhibitor, and bi-specific antibody.
These investigational agents show promising single-agent activity and have the potential to improve clinical responses when combined with combination chemotherapy regimens or other treatments, Ahmed Sawas, MD, of the Center for Lymphoid Malignancies at Columbia University, New York, said at the annual T-cell Lymphoma Forum.
AGS67E: Antibody-drug conjugate
AGS67E is an antibody-drug conjugate targeted against CD37, a transmembrane protein preferentially expressed on malignant B cells, T cells, and acute myeloid leukemia cells. In a study published in 2015 in Molecular Cancer Therapeutics, investigators from Agensys (an affiliate of Astellas Pharma) reported that this compound bound to more than 80% of patient-derived T cells in vitro (Mol Cancer Ther. 2015;14[7]:1650-60).
In a phase 1 dose-escalation study reported at the 2017 International Conference on Malignant Lymphoma in Lugano, Switzerland, Dr. Sawas and his colleagues found that patients with B-cell and T-cell malignancies, including cutaneous T-cell lymphoma and peripheral T-cell lymphoma, tolerated the drug well when it was delivered both with or without growth factor. Neutropenia was the most frequent adverse event and dose-limiting toxicity.
The drug showed single-agent activity in 16 of 53 patients with heavily pretreated non-Hodgkin lymphoma, including a partial response in one of two patients with cutaneous T-cell lymphoma, and partial responses in two of four patients with peripheral T-cell lymphoma. There were no complete responses at any of three dose levels of the drug, with or without growth factor.
One patient, a 75-year-old man with stage IVB mycosis fungoides who had disease progression on prior therapy with methotrexate, romidepsin, bendamustine, whole-body irradiation, liposomal doxorubicin, pralatrexate, and pembrolizumab experienced significant reduction in tumor burden and resolution of lymph node involvement after three 3-week cycles of therapy with AGS67E. The patient had a deepening of the response with additional cycles, and remained on therapy for 30 cycles until he experienced disease progression.
TTI-621: Tuck in, macrophages
TT1-621 is a molecule with two functions: It acts as an immune checkpoint inhibitor by blocking CD47, which binds to signal-regulatory protein alpha to produce an antiphagocytic or “do not eat” signal. TTI-621 does not, however, bind to CD47-positive erythrocytes.
In addition to blocking CD47 and the do-not-eat signal, TTI-621 delivers an activating signal to macrophages through Fc gamma receptors, telling them, in effect, “bon appétit.”
In a study presented at the 2017 annual meeting of the American Society of Hematology (Abstract 4076), investigators from City of Hope in Duarte, Calif., and other centers reported that a single direct intratumoral injection of TTI-621 was associated with significant antitumor activity in patients with relapsed or refractory mycosis fungoides and Sézary syndrome, with one of nine patients having a complete response in the injected lesion, and five having decreases in tumor size and/or circulating Sézary cells.
Patients appeared to tolerate this agent very well, with 1 of 18 having a grade 3 increase in white blood cell count. The most commonly reported side effects were fatigue, chills, decreased appetite, headache, injection site pain, and generalized pruritus, each occurring in 3 of the 18 patients.
TTI-621 injection was associated with rapid declines in Composite Assessment of Index Lesion Severity scores in dose-finding studies in patients with heavily pretreated cutaneous T-cell lymphoma, Dr. Sawas said.
AFM13: Two for the price of one
AFM13 is a bi-specific antibody that binds to CD30, which is expressed on anaplastic large cell lymphoma cells, as well as Reed-Sternberg cells of classical Hodgkin lymphoma. This antibody also engages CD16A-positive cells, resulting in lysis of CD30-positive tumor cells. It is a specific recruiter of natural killer cells, and does not bind to neutrophils.
In an early biologic effects study of this agent in CD30-positive lymphoid malignancies with cutaneous presentation, Dr. Sawas and his colleagues observed an early response and regression of cutaneous anaplastic large cell lymphoma lesions in a heavily pretreated patient, with progression occurring when the patient went off therapy, and tumors that diminished on reinitiation of therapy that sustained beyond a second discontinuation of therapy. This patient had measurable reductions in lymphoma burden on PET CT scans and improvements in cutaneous lesions. Dr. Sawas did not present safety data for this agent.
AGS67E studies are supported by Agensys. TTI-621 studies are supported by Trillium Therapeutics. The AFM13 study is supported by Columbia University, with Dr. Sawas listed as the sponsor. He did not report potential conflicts of interests. The T-Cell Lymphoma Forum is held by Jonathan Wood & Associates, which is owned by the same company as this news organization.
EXPERT ANALYSIS FROM TCLF 2018
Drug may be option for B- and T-cell lymphomas
LA JOLLA, CA—The EZH1/2 inhibitor DS-3201b could be a novel therapeutic option for non-Hodgkin lymphoma (NHL), according to a speaker at the 10th Annual T-cell Lymphoma Forum.
DS-3201b was considered well tolerated in a phase 1 study of Japanese patients with relapsed/refractory NHL.
In addition, DS-3201b demonstrated activity against B- and T-cell lymphomas, producing an overall response rate of 59%.
Kunihiro Tsukasaki, MD, PhD, of Saitama Medical University in Moroyama, Saitama, Japan, presented these results at the meeting.
The trial was sponsored by Daiichi Sankyo Co., Ltd.
Dr Tsukasaki presented data on 18 patients with relapsed/refractory NHL.
The 12 B-cell lymphoma patients had follicular lymphoma (n=5), diffuse large B-cell lymphoma (n=3), MALT lymphoma (n=2), nodal marginal zone lymphoma (n=1), and lymphoplasmacytic lymphoma (n=1).
The 6 patients with T-cell lymphoma had peripheral T-cell lymphoma not otherwise specified (n=2), angioimmunoblastic T-cell lymphoma (n=2), and adult T-cell leukemia/lymphoma (n=2).
The patients’ median age was 67 (range, 44-75), and 10 were female. All patients had an ECOG performance status of 0 (72%) or 1 (28%).
Patients had a median of 2 prior chemotherapy regimens (range, 1-8).
For this study, they received DS-3201b at 150 mg (n=7), 200 mg (n=9), or 300 mg (n=2). They received the drug once daily in 28-day cycles until they progressed or experienced unacceptable toxicity.
DLTs and AEs
Dose-limiting toxicities (DLTs) were evaluated in cycle 1. All 18 patients were evaluable for DLT assessment.
There were 4 treatment-emergent adverse events (AEs) that met the definition of DLTs:
- 3 cases of grade 4 platelet count decrease (n=1 at 200 mg, n=2 at 300 mg)
- 1 case of grade 3 anemia requiring blood transfusion (at 300 mg).
All 4 DLTs led to treatment interruption.
There were 5 serious AEs reported in 3 patients. Only one of these—pneumocystis jiroveci pneumonia—was considered related to DS-3201b.
Hematologic AEs included decreases in platelets (grade 1-4), lymphocytes (grade 1-4), neutrophils (grade 2-4), and white blood cells (grade 2-3), as well as anemia (grade 1-3).
Other AEs (all grade 1/2) included dysgeusia, alopecia, diarrhea, decreased appetite, alanine aminotransferase increase, aspartate aminotransferase increase, nasopharyngitis, rash, and dry skin.
No deaths had been reported as of the data cutoff last November.
Responses
Seventeen patients were evaluable for response.
The overall response rate was 59%, with 1 patient achieving a complete response (CR) and 9 achieving a partial response (PR). Four patients had stable disease (SD), and 3 progressed.
Among the T-cell lymphoma patients, 1 had a CR, 4 had PRs, and 1 progressed. The complete responder had angioimmunoblastic T-cell lymphoma, and the patient who progressed had adult T-cell leukemia/lymphoma.
Among the B-cell lymphoma patients, 5 had PRs, 4 had SD, and 2 progressed.
Dr Tsukasaki said DS-3201b has demonstrated early clinical activity and therefore has the potential to be a novel therapeutic option for B-cell and T-cell lymphomas. However, further evaluation is warranted to determine the optimal dosing regimen and target diseases. ![]()
LA JOLLA, CA—The EZH1/2 inhibitor DS-3201b could be a novel therapeutic option for non-Hodgkin lymphoma (NHL), according to a speaker at the 10th Annual T-cell Lymphoma Forum.
DS-3201b was considered well tolerated in a phase 1 study of Japanese patients with relapsed/refractory NHL.
In addition, DS-3201b demonstrated activity against B- and T-cell lymphomas, producing an overall response rate of 59%.
Kunihiro Tsukasaki, MD, PhD, of Saitama Medical University in Moroyama, Saitama, Japan, presented these results at the meeting.
The trial was sponsored by Daiichi Sankyo Co., Ltd.
Dr Tsukasaki presented data on 18 patients with relapsed/refractory NHL.
The 12 B-cell lymphoma patients had follicular lymphoma (n=5), diffuse large B-cell lymphoma (n=3), MALT lymphoma (n=2), nodal marginal zone lymphoma (n=1), and lymphoplasmacytic lymphoma (n=1).
The 6 patients with T-cell lymphoma had peripheral T-cell lymphoma not otherwise specified (n=2), angioimmunoblastic T-cell lymphoma (n=2), and adult T-cell leukemia/lymphoma (n=2).
The patients’ median age was 67 (range, 44-75), and 10 were female. All patients had an ECOG performance status of 0 (72%) or 1 (28%).
Patients had a median of 2 prior chemotherapy regimens (range, 1-8).
For this study, they received DS-3201b at 150 mg (n=7), 200 mg (n=9), or 300 mg (n=2). They received the drug once daily in 28-day cycles until they progressed or experienced unacceptable toxicity.
DLTs and AEs
Dose-limiting toxicities (DLTs) were evaluated in cycle 1. All 18 patients were evaluable for DLT assessment.
There were 4 treatment-emergent adverse events (AEs) that met the definition of DLTs:
- 3 cases of grade 4 platelet count decrease (n=1 at 200 mg, n=2 at 300 mg)
- 1 case of grade 3 anemia requiring blood transfusion (at 300 mg).
All 4 DLTs led to treatment interruption.
There were 5 serious AEs reported in 3 patients. Only one of these—pneumocystis jiroveci pneumonia—was considered related to DS-3201b.
Hematologic AEs included decreases in platelets (grade 1-4), lymphocytes (grade 1-4), neutrophils (grade 2-4), and white blood cells (grade 2-3), as well as anemia (grade 1-3).
Other AEs (all grade 1/2) included dysgeusia, alopecia, diarrhea, decreased appetite, alanine aminotransferase increase, aspartate aminotransferase increase, nasopharyngitis, rash, and dry skin.
No deaths had been reported as of the data cutoff last November.
Responses
Seventeen patients were evaluable for response.
The overall response rate was 59%, with 1 patient achieving a complete response (CR) and 9 achieving a partial response (PR). Four patients had stable disease (SD), and 3 progressed.
Among the T-cell lymphoma patients, 1 had a CR, 4 had PRs, and 1 progressed. The complete responder had angioimmunoblastic T-cell lymphoma, and the patient who progressed had adult T-cell leukemia/lymphoma.
Among the B-cell lymphoma patients, 5 had PRs, 4 had SD, and 2 progressed.
Dr Tsukasaki said DS-3201b has demonstrated early clinical activity and therefore has the potential to be a novel therapeutic option for B-cell and T-cell lymphomas. However, further evaluation is warranted to determine the optimal dosing regimen and target diseases. ![]()
LA JOLLA, CA—The EZH1/2 inhibitor DS-3201b could be a novel therapeutic option for non-Hodgkin lymphoma (NHL), according to a speaker at the 10th Annual T-cell Lymphoma Forum.
DS-3201b was considered well tolerated in a phase 1 study of Japanese patients with relapsed/refractory NHL.
In addition, DS-3201b demonstrated activity against B- and T-cell lymphomas, producing an overall response rate of 59%.
Kunihiro Tsukasaki, MD, PhD, of Saitama Medical University in Moroyama, Saitama, Japan, presented these results at the meeting.
The trial was sponsored by Daiichi Sankyo Co., Ltd.
Dr Tsukasaki presented data on 18 patients with relapsed/refractory NHL.
The 12 B-cell lymphoma patients had follicular lymphoma (n=5), diffuse large B-cell lymphoma (n=3), MALT lymphoma (n=2), nodal marginal zone lymphoma (n=1), and lymphoplasmacytic lymphoma (n=1).
The 6 patients with T-cell lymphoma had peripheral T-cell lymphoma not otherwise specified (n=2), angioimmunoblastic T-cell lymphoma (n=2), and adult T-cell leukemia/lymphoma (n=2).
The patients’ median age was 67 (range, 44-75), and 10 were female. All patients had an ECOG performance status of 0 (72%) or 1 (28%).
Patients had a median of 2 prior chemotherapy regimens (range, 1-8).
For this study, they received DS-3201b at 150 mg (n=7), 200 mg (n=9), or 300 mg (n=2). They received the drug once daily in 28-day cycles until they progressed or experienced unacceptable toxicity.
DLTs and AEs
Dose-limiting toxicities (DLTs) were evaluated in cycle 1. All 18 patients were evaluable for DLT assessment.
There were 4 treatment-emergent adverse events (AEs) that met the definition of DLTs:
- 3 cases of grade 4 platelet count decrease (n=1 at 200 mg, n=2 at 300 mg)
- 1 case of grade 3 anemia requiring blood transfusion (at 300 mg).
All 4 DLTs led to treatment interruption.
There were 5 serious AEs reported in 3 patients. Only one of these—pneumocystis jiroveci pneumonia—was considered related to DS-3201b.
Hematologic AEs included decreases in platelets (grade 1-4), lymphocytes (grade 1-4), neutrophils (grade 2-4), and white blood cells (grade 2-3), as well as anemia (grade 1-3).
Other AEs (all grade 1/2) included dysgeusia, alopecia, diarrhea, decreased appetite, alanine aminotransferase increase, aspartate aminotransferase increase, nasopharyngitis, rash, and dry skin.
No deaths had been reported as of the data cutoff last November.
Responses
Seventeen patients were evaluable for response.
The overall response rate was 59%, with 1 patient achieving a complete response (CR) and 9 achieving a partial response (PR). Four patients had stable disease (SD), and 3 progressed.
Among the T-cell lymphoma patients, 1 had a CR, 4 had PRs, and 1 progressed. The complete responder had angioimmunoblastic T-cell lymphoma, and the patient who progressed had adult T-cell leukemia/lymphoma.
Among the B-cell lymphoma patients, 5 had PRs, 4 had SD, and 2 progressed.
Dr Tsukasaki said DS-3201b has demonstrated early clinical activity and therefore has the potential to be a novel therapeutic option for B-cell and T-cell lymphomas. However, further evaluation is warranted to determine the optimal dosing regimen and target diseases. ![]()
Assay identifies actionable mutations in lymphoid malignancies
Researchers say hybrid capture sequencing is an accurate and sensitive method for identifying actionable gene mutations in lymphoid malignancies.
This method revealed potentially actionable mutations in 91% of patients studied, who had diffuse large B-cell lymphoma (DLBCL), follicular lymphoma (FL), or chronic lymphocytic leukemia (CLL).
The researchers therefore believe hybrid capture sequencing will bring the benefits of precision diagnosis and individualized therapy to patients with lymphoid malignancies.
“To realize the benefits of the most recent progress in cancer genomics, clinical implementation of precision medicine approaches is needed in the form of novel biomarker assays,” said study author Christian Steidl, MD, of the University of British Columbia in Vancouver, Canada.
“Fully implemented targeted sequencing-based assays in routine diagnostic pathology laboratories are currently lacking in lymphoid cancer care. Our findings demonstrate the feasibility and outline the clinical utility of integrating a lymphoma-specific pipeline into personalized cancer care.”
Dr Steidl and his colleagues reported these findings in The Journal of Molecular Diagnostics.
The researchers first compared capture hybridization and amplicon sequencing using samples from 8 patients with lymphoma. Fresh-frozen and formalin-fixed, paraffin-embedded tumor samples were sequenced using a panel of 20 lymphoma-specific genes.
The team found that capture hybridization provided “deep, more uniform coverage” and yielded “higher sensitivity for variant calling” than amplicon sequencing.
The researchers then developed a targeted sequencing pipeline using a 32-gene panel. The panel was developed with input from a group of 6 specialists who kept updating it based on the latest available information.
“This allows for continuous integration of additional gene features as our knowledge base improves,” Dr Steidl noted.
He and his colleagues then applied the hybrid capture sequencing assay and 32-gene panel to tissues from 219 patients—114 with FL, 76 with DLBCL, and 29 with CLL—who were treated in British Columbia between 2013 and 2016.
Results revealed at least one actionable mutation in 91% of the tumors. And the assay uncovered subtype-specific mutational profiles that were highly similar to published mutational profiles for FL, DLBCL, and CLL.
Furthermore, the assay had 93% concordance with whole-genome sequencing.
“Our developed assay harnesses the power of modern sequencing for clinical diagnostics purposes and potentially better deployment of novel treatments in lymphoid cancers,” Dr Steidl said. “We believe our study will help establish evidence-based approaches to decision making in lymphoid cancer care.”
“The next steps are to implement sequencing-based biomarker assays, such as reported in our study, in accredited pathology laboratories. Toward the goal of biomarker-driven clinical decision making, testing of potentially predictive biomarker assays is needed alongside clinical trials investigating novel cancer therapeutics.” ![]()
Researchers say hybrid capture sequencing is an accurate and sensitive method for identifying actionable gene mutations in lymphoid malignancies.
This method revealed potentially actionable mutations in 91% of patients studied, who had diffuse large B-cell lymphoma (DLBCL), follicular lymphoma (FL), or chronic lymphocytic leukemia (CLL).
The researchers therefore believe hybrid capture sequencing will bring the benefits of precision diagnosis and individualized therapy to patients with lymphoid malignancies.
“To realize the benefits of the most recent progress in cancer genomics, clinical implementation of precision medicine approaches is needed in the form of novel biomarker assays,” said study author Christian Steidl, MD, of the University of British Columbia in Vancouver, Canada.
“Fully implemented targeted sequencing-based assays in routine diagnostic pathology laboratories are currently lacking in lymphoid cancer care. Our findings demonstrate the feasibility and outline the clinical utility of integrating a lymphoma-specific pipeline into personalized cancer care.”
Dr Steidl and his colleagues reported these findings in The Journal of Molecular Diagnostics.
The researchers first compared capture hybridization and amplicon sequencing using samples from 8 patients with lymphoma. Fresh-frozen and formalin-fixed, paraffin-embedded tumor samples were sequenced using a panel of 20 lymphoma-specific genes.
The team found that capture hybridization provided “deep, more uniform coverage” and yielded “higher sensitivity for variant calling” than amplicon sequencing.
The researchers then developed a targeted sequencing pipeline using a 32-gene panel. The panel was developed with input from a group of 6 specialists who kept updating it based on the latest available information.
“This allows for continuous integration of additional gene features as our knowledge base improves,” Dr Steidl noted.
He and his colleagues then applied the hybrid capture sequencing assay and 32-gene panel to tissues from 219 patients—114 with FL, 76 with DLBCL, and 29 with CLL—who were treated in British Columbia between 2013 and 2016.
Results revealed at least one actionable mutation in 91% of the tumors. And the assay uncovered subtype-specific mutational profiles that were highly similar to published mutational profiles for FL, DLBCL, and CLL.
Furthermore, the assay had 93% concordance with whole-genome sequencing.
“Our developed assay harnesses the power of modern sequencing for clinical diagnostics purposes and potentially better deployment of novel treatments in lymphoid cancers,” Dr Steidl said. “We believe our study will help establish evidence-based approaches to decision making in lymphoid cancer care.”
“The next steps are to implement sequencing-based biomarker assays, such as reported in our study, in accredited pathology laboratories. Toward the goal of biomarker-driven clinical decision making, testing of potentially predictive biomarker assays is needed alongside clinical trials investigating novel cancer therapeutics.” ![]()
Researchers say hybrid capture sequencing is an accurate and sensitive method for identifying actionable gene mutations in lymphoid malignancies.
This method revealed potentially actionable mutations in 91% of patients studied, who had diffuse large B-cell lymphoma (DLBCL), follicular lymphoma (FL), or chronic lymphocytic leukemia (CLL).
The researchers therefore believe hybrid capture sequencing will bring the benefits of precision diagnosis and individualized therapy to patients with lymphoid malignancies.
“To realize the benefits of the most recent progress in cancer genomics, clinical implementation of precision medicine approaches is needed in the form of novel biomarker assays,” said study author Christian Steidl, MD, of the University of British Columbia in Vancouver, Canada.
“Fully implemented targeted sequencing-based assays in routine diagnostic pathology laboratories are currently lacking in lymphoid cancer care. Our findings demonstrate the feasibility and outline the clinical utility of integrating a lymphoma-specific pipeline into personalized cancer care.”
Dr Steidl and his colleagues reported these findings in The Journal of Molecular Diagnostics.
The researchers first compared capture hybridization and amplicon sequencing using samples from 8 patients with lymphoma. Fresh-frozen and formalin-fixed, paraffin-embedded tumor samples were sequenced using a panel of 20 lymphoma-specific genes.
The team found that capture hybridization provided “deep, more uniform coverage” and yielded “higher sensitivity for variant calling” than amplicon sequencing.
The researchers then developed a targeted sequencing pipeline using a 32-gene panel. The panel was developed with input from a group of 6 specialists who kept updating it based on the latest available information.
“This allows for continuous integration of additional gene features as our knowledge base improves,” Dr Steidl noted.
He and his colleagues then applied the hybrid capture sequencing assay and 32-gene panel to tissues from 219 patients—114 with FL, 76 with DLBCL, and 29 with CLL—who were treated in British Columbia between 2013 and 2016.
Results revealed at least one actionable mutation in 91% of the tumors. And the assay uncovered subtype-specific mutational profiles that were highly similar to published mutational profiles for FL, DLBCL, and CLL.
Furthermore, the assay had 93% concordance with whole-genome sequencing.
“Our developed assay harnesses the power of modern sequencing for clinical diagnostics purposes and potentially better deployment of novel treatments in lymphoid cancers,” Dr Steidl said. “We believe our study will help establish evidence-based approaches to decision making in lymphoid cancer care.”
“The next steps are to implement sequencing-based biomarker assays, such as reported in our study, in accredited pathology laboratories. Toward the goal of biomarker-driven clinical decision making, testing of potentially predictive biomarker assays is needed alongside clinical trials investigating novel cancer therapeutics.” ![]()
Decline in non-Hodgkin lymphoma deaths to continue in 2018
Mortality from non-Hodgkin lymphoma is expected to be about 6.1 per 100,000 population in 2018, with the highest rate in Maine and West Virginia and the lowest in Utah.
in its Cancer Facts & Figures 2018, based on analysis of 2001-2015 data from the National Center for Health Statistics. That figure is down from the 20,140 predicted for 2017, as the trend in the death rate since 2006 has been a decline of about 2% per year.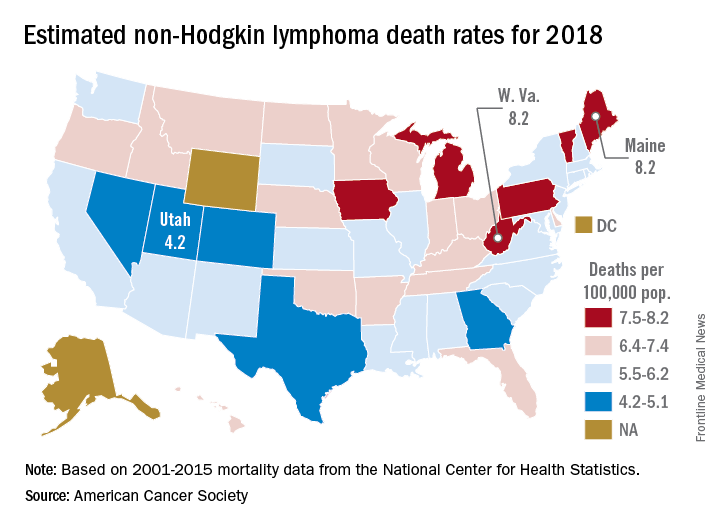
Nationally, death rates for NHL were 7.4 per 100,000 for males and 4.5 for females for 2011-2015, and incidence rates were 22.9 per 100,000 for males and 15.8 for females for 2010-2014, the ACS reported.
Over time, the relative survival rate for NHL has gone from 47% in 1975-1977 to 51% in 1987-1989 to 73% in 2007-2013, although there is some disparity between whites, whose respective rates are 47%, 51%, and 74%, and blacks, who have rates of 49%, 46%, and 67%, respectively, the ACS said.
Mortality from non-Hodgkin lymphoma is expected to be about 6.1 per 100,000 population in 2018, with the highest rate in Maine and West Virginia and the lowest in Utah.
in its Cancer Facts & Figures 2018, based on analysis of 2001-2015 data from the National Center for Health Statistics. That figure is down from the 20,140 predicted for 2017, as the trend in the death rate since 2006 has been a decline of about 2% per year.
Nationally, death rates for NHL were 7.4 per 100,000 for males and 4.5 for females for 2011-2015, and incidence rates were 22.9 per 100,000 for males and 15.8 for females for 2010-2014, the ACS reported.
Over time, the relative survival rate for NHL has gone from 47% in 1975-1977 to 51% in 1987-1989 to 73% in 2007-2013, although there is some disparity between whites, whose respective rates are 47%, 51%, and 74%, and blacks, who have rates of 49%, 46%, and 67%, respectively, the ACS said.
Mortality from non-Hodgkin lymphoma is expected to be about 6.1 per 100,000 population in 2018, with the highest rate in Maine and West Virginia and the lowest in Utah.
in its Cancer Facts & Figures 2018, based on analysis of 2001-2015 data from the National Center for Health Statistics. That figure is down from the 20,140 predicted for 2017, as the trend in the death rate since 2006 has been a decline of about 2% per year.
Nationally, death rates for NHL were 7.4 per 100,000 for males and 4.5 for females for 2011-2015, and incidence rates were 22.9 per 100,000 for males and 15.8 for females for 2010-2014, the ACS reported.
Over time, the relative survival rate for NHL has gone from 47% in 1975-1977 to 51% in 1987-1989 to 73% in 2007-2013, although there is some disparity between whites, whose respective rates are 47%, 51%, and 74%, and blacks, who have rates of 49%, 46%, and 67%, respectively, the ACS said.
Combo is preferentially active in T-cell lymphomas
LA JOLLA, CA—A 2-drug combination has demonstrated preferential activity in T-cell lymphomas over B-cell lymphomas, according to researchers.
In a small, phase 1/2 study, treatment with oral 5-azacitidine and romidepsin produced a higher overall response rate (ORR) and prolonged progression-free survival (PFS) in patients with T-cell lymphomas.
“In a very limited sample, we’ve definitely observed exquisite activity of the combination in patients with T-cell lymphoma compared to all other subtypes,” said Lorenzo Falchi, MD, of Columbia University Medical Center in New York, New York.
Dr Falchi presented these results at the 10th Annual T-cell Lymphoma Forum.
The research was funded by the Leukemia and Lymphoma Society, the Lymphoma Research Fund at Columbia University, and Celgene.
The phase 1 portion of this study included patients with previously treated non-Hodgkin lymphoma (NHL) or Hodgkin lymphoma. The phase 2 portion included only patients with T-cell lymphomas, newly diagnosed or previously treated.
Thirty-three patients were enrolled—12 with Hodgkin lymphoma, 8 with B-cell NHL, and 13 with T-cell NHL.
The patients’ median age was 54 (range, 23-79). Fifty-seven percent (n=19) were male. Sixty-one percent of patients were non-Hispanic white (n=20), 24% (n=8) were black, and 12% (n=4) were Asian.
“This was a very heavily pretreated patient population,” Dr Falchi noted. “I’d like to emphasize that the median number of prior treatments is 5 [range, 0-15].”
“Over half of patients had had stem cell transplantation [17 autologous and 5 allogeneic]. And, if you look at the subtypes by histology, all patients, pretty much, at some point, received all the standard chemotherapy or treatment approaches that are typically used for that subtype.”
Treatment
Patients were divided into 7 dosing cohorts. Azacitidine doses ranged from 100 mg to 300 mg on days 1-14 or days 1-21 per cycle.
Romidepsin doses ranged from 10 mg/m2 to 14 mg/m2. The drug was given on days 8 and 15 every 21 or 28 days, or it was given on days 8, 15, and 22 every 35 days.
There were 2 dose-limiting toxicities (DLTs) in cohort 2—grade 3 thrombocytopenia and grade 3 pleural effusion. In this cohort, 3 patients received azacitidine at 200 mg on days 1-14 plus romidepsin at 10 mg/m2 on days 8 and 15 every 21 days.
There were 3 DLTs in cohort 7—2 cases of grade 4 neutropenia and 1 case of grade 3 thrombocytopenia. In this cohort, 5 patients received azacitidine at 300 mg on days 1 to 21 plus romidepsin at 14 mg/m2 on days 8, 15, and 22 every 35 days.
Because of the DLTs in cohort 7, cohort 6 was chosen as the maximum tolerated dose. In cohort 6, 3 patients received azacitidine at 300 mg on days 1-14 plus romidepsin at 14 mg/m2 on days 8, 15, and 22 every 35 days.
Patients in the expansion cohort received treatment at the maximum tolerated dose. This cohort included 7 patients with T-cell lymphoma.
Safety
Treatment-emergent adverse events occurring in at least 5% of patients included:
- Anemia—3% grade 3
- Anorexia—9% grade 1
- Back pain—6% grade 2
- Constipation—6% grade 1
- Cough—9% grade 1
- Depression—3% grade 1 and 2
- Diarrhea—15% grade 1 and 6% grade 2
- Dyspnea—3% grade 1 and 2
- Fatigue—21% grade 1, 9% grade 2, and 3% grade 3
- Febrile neutropenia—3% grade 3 and 4
- Fever—6% grade 1 and 3% grade 2
- General disorders and administration site conditions—15% grade 1
- Hyperglycemia—3% grade 3
- Hypokalemia—6% grade 1
- Hypotension—3% grade 3
- Insomnia—6% grade 1
- Oral mucositis—9% grade 1 and 3% grade 2
- Nausea—18% grade 1, 27% grade 2, and 3% grade 3
- Neutrophil count decrease—3% grade 3 and 4
- Pain—3% grade 1 and 6% grade 2
- Pain of skin—3% grade 1 and 2
- Platelet count decrease—6% grade 2, 9% grade 3, and 6% grade 4
- Urinary tract infection—3% grade 3
- Vomiting—18% grade 1 and 21% grade 2.
Efficacy
Twenty-eight patients were evaluable for efficacy. The ORR for these patients was 36% (n=10).
The complete response (CR) rate was 22% (n=6), and the partial response (PR) rate was 14% (n=4). Twenty-five percent of patients (n=7) had stable disease, and 39% (n=11) progressed.
Dr Falchi noted that the ORR was “much higher” in patients with T-cell lymphoma than in those with B-cell lymphoma—80% (n=8) and 11% (n=2), respectively.
The CR rates were 50% (n=5) in T-cell lymphoma patients and 5.5% (n=1) in B-cell patients. PR rates were 30% (n=3) and 5.5% (n=1), respectively. Thirty-nine percent (n=7) of B-cell patients had stable disease, but none of the T-cell patients did.
“Patients with non-T-cell lymphoma were much more likely to progress on treatment,” Dr Falchi noted. “Half of them did so [n=9].”
This is in comparison to the 20% of T-cell lymphoma patients who progressed on treatment (n=2).
Disease subtypes for complete responders included transformed follicular lymphoma (n=1), T-lymphoblastic lymphoma (n=1), adult T-cell leukemia/lymphoma (n=1), extranodal NK/T-cell lymphoma (n=1), and angioimmunoblastic T-cell lymphoma (n=2).
Partial responders had follicular lymphoma (n=1), cutaneous peripheral T-cell lymphoma (n=1), cutaneous anaplastic large-cell lymphoma (n=1), and angioimmunoblastic T-cell lymphoma (n=1).
The 2 responders with B-cell lymphoma (1 CR and 1 PR) ultimately progressed and died.
Of the 8 responders with T-cell lymphoma, 3 have an ongoing CR, and 2 of these patients proceeded to transplant.
One T-cell patient who achieved a CR and proceeded to transplant was lost to follow-up. Another died after transplant.
Two T-cell patients who achieved a PR progressed and died. And 1 patient has an ongoing PR.
In total, 75% of patients (n=21) progressed. The median PFS for the entire study cohort was 3.6 months (range, 1.5-5.7).
The median PFS was 2.2 months (range, 1.1-3.2) for patients with B-cell lymphomas and was not reached for the T-cell lymphoma patients.
Eighty-nine percent of B-cell patients progressed (n=16), as did 40% of T-cell patients (n=4).
Dr Falchi and his colleagues are now conducting studies to correlate the pharmacokinetics of azacitidine-romidepsin with genome-wide methylation and correlate TET2, IDH2, and DNMT3A mutation status with clinical response. ![]()
LA JOLLA, CA—A 2-drug combination has demonstrated preferential activity in T-cell lymphomas over B-cell lymphomas, according to researchers.
In a small, phase 1/2 study, treatment with oral 5-azacitidine and romidepsin produced a higher overall response rate (ORR) and prolonged progression-free survival (PFS) in patients with T-cell lymphomas.
“In a very limited sample, we’ve definitely observed exquisite activity of the combination in patients with T-cell lymphoma compared to all other subtypes,” said Lorenzo Falchi, MD, of Columbia University Medical Center in New York, New York.
Dr Falchi presented these results at the 10th Annual T-cell Lymphoma Forum.
The research was funded by the Leukemia and Lymphoma Society, the Lymphoma Research Fund at Columbia University, and Celgene.
The phase 1 portion of this study included patients with previously treated non-Hodgkin lymphoma (NHL) or Hodgkin lymphoma. The phase 2 portion included only patients with T-cell lymphomas, newly diagnosed or previously treated.
Thirty-three patients were enrolled—12 with Hodgkin lymphoma, 8 with B-cell NHL, and 13 with T-cell NHL.
The patients’ median age was 54 (range, 23-79). Fifty-seven percent (n=19) were male. Sixty-one percent of patients were non-Hispanic white (n=20), 24% (n=8) were black, and 12% (n=4) were Asian.
“This was a very heavily pretreated patient population,” Dr Falchi noted. “I’d like to emphasize that the median number of prior treatments is 5 [range, 0-15].”
“Over half of patients had had stem cell transplantation [17 autologous and 5 allogeneic]. And, if you look at the subtypes by histology, all patients, pretty much, at some point, received all the standard chemotherapy or treatment approaches that are typically used for that subtype.”
Treatment
Patients were divided into 7 dosing cohorts. Azacitidine doses ranged from 100 mg to 300 mg on days 1-14 or days 1-21 per cycle.
Romidepsin doses ranged from 10 mg/m2 to 14 mg/m2. The drug was given on days 8 and 15 every 21 or 28 days, or it was given on days 8, 15, and 22 every 35 days.
There were 2 dose-limiting toxicities (DLTs) in cohort 2—grade 3 thrombocytopenia and grade 3 pleural effusion. In this cohort, 3 patients received azacitidine at 200 mg on days 1-14 plus romidepsin at 10 mg/m2 on days 8 and 15 every 21 days.
There were 3 DLTs in cohort 7—2 cases of grade 4 neutropenia and 1 case of grade 3 thrombocytopenia. In this cohort, 5 patients received azacitidine at 300 mg on days 1 to 21 plus romidepsin at 14 mg/m2 on days 8, 15, and 22 every 35 days.
Because of the DLTs in cohort 7, cohort 6 was chosen as the maximum tolerated dose. In cohort 6, 3 patients received azacitidine at 300 mg on days 1-14 plus romidepsin at 14 mg/m2 on days 8, 15, and 22 every 35 days.
Patients in the expansion cohort received treatment at the maximum tolerated dose. This cohort included 7 patients with T-cell lymphoma.
Safety
Treatment-emergent adverse events occurring in at least 5% of patients included:
- Anemia—3% grade 3
- Anorexia—9% grade 1
- Back pain—6% grade 2
- Constipation—6% grade 1
- Cough—9% grade 1
- Depression—3% grade 1 and 2
- Diarrhea—15% grade 1 and 6% grade 2
- Dyspnea—3% grade 1 and 2
- Fatigue—21% grade 1, 9% grade 2, and 3% grade 3
- Febrile neutropenia—3% grade 3 and 4
- Fever—6% grade 1 and 3% grade 2
- General disorders and administration site conditions—15% grade 1
- Hyperglycemia—3% grade 3
- Hypokalemia—6% grade 1
- Hypotension—3% grade 3
- Insomnia—6% grade 1
- Oral mucositis—9% grade 1 and 3% grade 2
- Nausea—18% grade 1, 27% grade 2, and 3% grade 3
- Neutrophil count decrease—3% grade 3 and 4
- Pain—3% grade 1 and 6% grade 2
- Pain of skin—3% grade 1 and 2
- Platelet count decrease—6% grade 2, 9% grade 3, and 6% grade 4
- Urinary tract infection—3% grade 3
- Vomiting—18% grade 1 and 21% grade 2.
Efficacy
Twenty-eight patients were evaluable for efficacy. The ORR for these patients was 36% (n=10).
The complete response (CR) rate was 22% (n=6), and the partial response (PR) rate was 14% (n=4). Twenty-five percent of patients (n=7) had stable disease, and 39% (n=11) progressed.
Dr Falchi noted that the ORR was “much higher” in patients with T-cell lymphoma than in those with B-cell lymphoma—80% (n=8) and 11% (n=2), respectively.
The CR rates were 50% (n=5) in T-cell lymphoma patients and 5.5% (n=1) in B-cell patients. PR rates were 30% (n=3) and 5.5% (n=1), respectively. Thirty-nine percent (n=7) of B-cell patients had stable disease, but none of the T-cell patients did.
“Patients with non-T-cell lymphoma were much more likely to progress on treatment,” Dr Falchi noted. “Half of them did so [n=9].”
This is in comparison to the 20% of T-cell lymphoma patients who progressed on treatment (n=2).
Disease subtypes for complete responders included transformed follicular lymphoma (n=1), T-lymphoblastic lymphoma (n=1), adult T-cell leukemia/lymphoma (n=1), extranodal NK/T-cell lymphoma (n=1), and angioimmunoblastic T-cell lymphoma (n=2).
Partial responders had follicular lymphoma (n=1), cutaneous peripheral T-cell lymphoma (n=1), cutaneous anaplastic large-cell lymphoma (n=1), and angioimmunoblastic T-cell lymphoma (n=1).
The 2 responders with B-cell lymphoma (1 CR and 1 PR) ultimately progressed and died.
Of the 8 responders with T-cell lymphoma, 3 have an ongoing CR, and 2 of these patients proceeded to transplant.
One T-cell patient who achieved a CR and proceeded to transplant was lost to follow-up. Another died after transplant.
Two T-cell patients who achieved a PR progressed and died. And 1 patient has an ongoing PR.
In total, 75% of patients (n=21) progressed. The median PFS for the entire study cohort was 3.6 months (range, 1.5-5.7).
The median PFS was 2.2 months (range, 1.1-3.2) for patients with B-cell lymphomas and was not reached for the T-cell lymphoma patients.
Eighty-nine percent of B-cell patients progressed (n=16), as did 40% of T-cell patients (n=4).
Dr Falchi and his colleagues are now conducting studies to correlate the pharmacokinetics of azacitidine-romidepsin with genome-wide methylation and correlate TET2, IDH2, and DNMT3A mutation status with clinical response. ![]()
LA JOLLA, CA—A 2-drug combination has demonstrated preferential activity in T-cell lymphomas over B-cell lymphomas, according to researchers.
In a small, phase 1/2 study, treatment with oral 5-azacitidine and romidepsin produced a higher overall response rate (ORR) and prolonged progression-free survival (PFS) in patients with T-cell lymphomas.
“In a very limited sample, we’ve definitely observed exquisite activity of the combination in patients with T-cell lymphoma compared to all other subtypes,” said Lorenzo Falchi, MD, of Columbia University Medical Center in New York, New York.
Dr Falchi presented these results at the 10th Annual T-cell Lymphoma Forum.
The research was funded by the Leukemia and Lymphoma Society, the Lymphoma Research Fund at Columbia University, and Celgene.
The phase 1 portion of this study included patients with previously treated non-Hodgkin lymphoma (NHL) or Hodgkin lymphoma. The phase 2 portion included only patients with T-cell lymphomas, newly diagnosed or previously treated.
Thirty-three patients were enrolled—12 with Hodgkin lymphoma, 8 with B-cell NHL, and 13 with T-cell NHL.
The patients’ median age was 54 (range, 23-79). Fifty-seven percent (n=19) were male. Sixty-one percent of patients were non-Hispanic white (n=20), 24% (n=8) were black, and 12% (n=4) were Asian.
“This was a very heavily pretreated patient population,” Dr Falchi noted. “I’d like to emphasize that the median number of prior treatments is 5 [range, 0-15].”
“Over half of patients had had stem cell transplantation [17 autologous and 5 allogeneic]. And, if you look at the subtypes by histology, all patients, pretty much, at some point, received all the standard chemotherapy or treatment approaches that are typically used for that subtype.”
Treatment
Patients were divided into 7 dosing cohorts. Azacitidine doses ranged from 100 mg to 300 mg on days 1-14 or days 1-21 per cycle.
Romidepsin doses ranged from 10 mg/m2 to 14 mg/m2. The drug was given on days 8 and 15 every 21 or 28 days, or it was given on days 8, 15, and 22 every 35 days.
There were 2 dose-limiting toxicities (DLTs) in cohort 2—grade 3 thrombocytopenia and grade 3 pleural effusion. In this cohort, 3 patients received azacitidine at 200 mg on days 1-14 plus romidepsin at 10 mg/m2 on days 8 and 15 every 21 days.
There were 3 DLTs in cohort 7—2 cases of grade 4 neutropenia and 1 case of grade 3 thrombocytopenia. In this cohort, 5 patients received azacitidine at 300 mg on days 1 to 21 plus romidepsin at 14 mg/m2 on days 8, 15, and 22 every 35 days.
Because of the DLTs in cohort 7, cohort 6 was chosen as the maximum tolerated dose. In cohort 6, 3 patients received azacitidine at 300 mg on days 1-14 plus romidepsin at 14 mg/m2 on days 8, 15, and 22 every 35 days.
Patients in the expansion cohort received treatment at the maximum tolerated dose. This cohort included 7 patients with T-cell lymphoma.
Safety
Treatment-emergent adverse events occurring in at least 5% of patients included:
- Anemia—3% grade 3
- Anorexia—9% grade 1
- Back pain—6% grade 2
- Constipation—6% grade 1
- Cough—9% grade 1
- Depression—3% grade 1 and 2
- Diarrhea—15% grade 1 and 6% grade 2
- Dyspnea—3% grade 1 and 2
- Fatigue—21% grade 1, 9% grade 2, and 3% grade 3
- Febrile neutropenia—3% grade 3 and 4
- Fever—6% grade 1 and 3% grade 2
- General disorders and administration site conditions—15% grade 1
- Hyperglycemia—3% grade 3
- Hypokalemia—6% grade 1
- Hypotension—3% grade 3
- Insomnia—6% grade 1
- Oral mucositis—9% grade 1 and 3% grade 2
- Nausea—18% grade 1, 27% grade 2, and 3% grade 3
- Neutrophil count decrease—3% grade 3 and 4
- Pain—3% grade 1 and 6% grade 2
- Pain of skin—3% grade 1 and 2
- Platelet count decrease—6% grade 2, 9% grade 3, and 6% grade 4
- Urinary tract infection—3% grade 3
- Vomiting—18% grade 1 and 21% grade 2.
Efficacy
Twenty-eight patients were evaluable for efficacy. The ORR for these patients was 36% (n=10).
The complete response (CR) rate was 22% (n=6), and the partial response (PR) rate was 14% (n=4). Twenty-five percent of patients (n=7) had stable disease, and 39% (n=11) progressed.
Dr Falchi noted that the ORR was “much higher” in patients with T-cell lymphoma than in those with B-cell lymphoma—80% (n=8) and 11% (n=2), respectively.
The CR rates were 50% (n=5) in T-cell lymphoma patients and 5.5% (n=1) in B-cell patients. PR rates were 30% (n=3) and 5.5% (n=1), respectively. Thirty-nine percent (n=7) of B-cell patients had stable disease, but none of the T-cell patients did.
“Patients with non-T-cell lymphoma were much more likely to progress on treatment,” Dr Falchi noted. “Half of them did so [n=9].”
This is in comparison to the 20% of T-cell lymphoma patients who progressed on treatment (n=2).
Disease subtypes for complete responders included transformed follicular lymphoma (n=1), T-lymphoblastic lymphoma (n=1), adult T-cell leukemia/lymphoma (n=1), extranodal NK/T-cell lymphoma (n=1), and angioimmunoblastic T-cell lymphoma (n=2).
Partial responders had follicular lymphoma (n=1), cutaneous peripheral T-cell lymphoma (n=1), cutaneous anaplastic large-cell lymphoma (n=1), and angioimmunoblastic T-cell lymphoma (n=1).
The 2 responders with B-cell lymphoma (1 CR and 1 PR) ultimately progressed and died.
Of the 8 responders with T-cell lymphoma, 3 have an ongoing CR, and 2 of these patients proceeded to transplant.
One T-cell patient who achieved a CR and proceeded to transplant was lost to follow-up. Another died after transplant.
Two T-cell patients who achieved a PR progressed and died. And 1 patient has an ongoing PR.
In total, 75% of patients (n=21) progressed. The median PFS for the entire study cohort was 3.6 months (range, 1.5-5.7).
The median PFS was 2.2 months (range, 1.1-3.2) for patients with B-cell lymphomas and was not reached for the T-cell lymphoma patients.
Eighty-nine percent of B-cell patients progressed (n=16), as did 40% of T-cell patients (n=4).
Dr Falchi and his colleagues are now conducting studies to correlate the pharmacokinetics of azacitidine-romidepsin with genome-wide methylation and correlate TET2, IDH2, and DNMT3A mutation status with clinical response. ![]()
TRANSCEND NHL trial identifies window for CAR T expansion
for CAR T expansion
SAN FRANCISCO – The CD19-directed 4-1BB chimeric antigen receptor (CAR) T-cell product JCAR017 demonstrated increased CAR T-cell expansion and persistence, and higher durability of response at higher dose levels – with manageable toxicities – in a pivotal phase 1 trial of relapsed/refractory B-cell non-Hodgkin lymphoma.
However, preliminary modeling data suggest that a therapeutic window exists for the CAR T-cell expansion, which means that development of strategies for pushing patients into that window could enhance efficacy and limit toxicity associated with JCAR017, Tanya Siddiqi, MD, of City of Hope Comprehensive Cancer Center, Duarte, Calif., reported at the ASCO-SITC Clinical Immuno-Oncology Symposium.
TRANSCEND NHL 001 is a multicenter, seamless design, pivotal trial, which started as a phase 1 first-in-human study of JCAR017, a defined composition CAR T-cell product also known as lisocabtagene maraleucel and administered at precise doses of CD4+ and CD8+ CAR T cells. Dose-finding and dose-expansion cohorts have been investigated, and currently the pivotal diffuse large B-cell lymphoma (DLBCL) cohort is enrolling, Dr. Saddiqi said, noting that dose level 2 (1 x 108 cells given as a single dose) was selected for that cohort.
The current findings are based on the TRANSCEND core population – a set of patients selected from the dose-finding and dose-expansion cohorts. This population includes patients with DLBCL not otherwise specified, transformed follicular lymphoma, or high-grade double- or triple-hit lymphomas, she said, explaining that she and her colleagues looked at prelymphodepletion baseline patient characteristics and biomarkers to assess how they related to outcomes and toxicities. This precise dosing of JCAR017 reduces variability, enabling the identification of potential patient factors associated with clinical outcomes, she said.
Response rates among 27 patients who received dose level 2 were high, with an 81% overall response rate and a 63% complete response rate.
“Patients with complete remission seemed to have more of a durable response,” she said. “In the core population at dose level 2, 50% with 6 months of follow-up seemed to remain in complete response, so there’s a dose-response effect in these patients.”
It doesn’t appear that the dose affects development of cytokine release syndrome (CRS) or neurotoxicity (NT), as the rates of these (30% and 20%, respectively) did not differ by dose level or schedule, but certain baseline features, such as a lactate dehydrogenase (LDH) level above 500 U/L and tumor burden measured as the sum of the product of diameters of 50 cm or greater, do appear to affect the development of these toxicities.
For example, 10 of 13 (77%) of patients with both of those baseline characteristics developed CRS, and 7 of 13 (54%) developed NT; for those without these characteristics, the odds of developing CRS and NT were significantly lower, she said.
Of note, higher Cmax, or peak expansion of CAR T cells in vivo, was seen at dose level 2, and exposure (area under the curve) was also higher, as was expected at that dose level, Dr. Siddiqi said.
“It also appears that there is a trend of patients with higher tumor burden to have higher expansion of their CAR T cells in vivo,” she said, noting that some of those patients were “superexpanders” with very high expansion of CAR T cells. “Similarly there were certain cytokines at the prelymphodepletion time point that seem to be higher … in patients who also go on to have higher expansion of their CAR T cells.”
These included interleukin-7, IL-15, MIP (macrophage inflammatory protein)–1alpha, and tumor necrosis factor (TNF)–alpha.
“Patients with certain higher inflammatory cytokines and biomarkers of inflammation also were noted to have higher events of CRS and neurotoxicity, so not just higher tumor burden, but also higher inflammatory state at baseline seems to affect patients in terms of getting any grade CRS or any grade neurotoxicity,” she said.
Those associated with CRS included ferritin, C-reactive protein (CRP), IL-10, IL-15, IL-16, TNF-alpha, and MIP-1beta levels, and those associated with NT included ferritin, CRP, d-dimer, IL-6, IL-15, TNF-alpha, and MIP-1alpha levels.
“Interestingly, there’s an inverse relationship between the expression of some of these biomarkers and inflammatory markers and the durability of response at 3 months,” she said.
Patients with higher tumor burden, LDH, and other markers either had no response at 3 months, or they had a very rapid response at the 1-month mark and then lost their response by 3 months, she noted.
“One of the theories could be that these patients with higher tumor burden, higher inflammatory state – they have such a high peak expansion rapidly after receiving their CAR T cells that potentially those CAR T cells may be getting exhausted or dying off very quickly, and therefore patients can lose their response,” she said.
When these data are considered together and modeled, there seems to be a therapeutic window where patients who have optimal expansion of CAR T cells in vivo may have lesser toxicity, higher overall response rates, and better durability of response, she said.
That is, on one end of the spectrum, there are patients with lower CAR T-cell expansion who have lower toxicities, but who also have lower overall response rates and lower durability of response, and on the other end, there are patients with superhigh expansion of CAR T cells, who have higher response rates, but also higher rates of toxicity, and who lose their response quickly.
“So if we can identify patients who would be in this window of optimal target expansion of CAR T cells, and if we could find strategies or mechanisms to move patients who are on either end of that spectrum into this window, we may be able to get patients with better efficacy and lower toxicities, and there could be combination strategies that could help get us there,” she said.
TRANSCEND NHL 001 is sponsored by Juno Therapeutics. Dr. Siddiqi reported serving as a consultant or adviser for Juno Therapeutics, and as a member of the speakers bureau for Pharmacyclics/Janssen and Seattle Genetics. Research funding was provided to her institution by Juno Therapeutics and several other companies.
SOURCE: Siddiqi T et al. ASCO-SITC, abstract 122.
SAN FRANCISCO – The CD19-directed 4-1BB chimeric antigen receptor (CAR) T-cell product JCAR017 demonstrated increased CAR T-cell expansion and persistence, and higher durability of response at higher dose levels – with manageable toxicities – in a pivotal phase 1 trial of relapsed/refractory B-cell non-Hodgkin lymphoma.
However, preliminary modeling data suggest that a therapeutic window exists for the CAR T-cell expansion, which means that development of strategies for pushing patients into that window could enhance efficacy and limit toxicity associated with JCAR017, Tanya Siddiqi, MD, of City of Hope Comprehensive Cancer Center, Duarte, Calif., reported at the ASCO-SITC Clinical Immuno-Oncology Symposium.
TRANSCEND NHL 001 is a multicenter, seamless design, pivotal trial, which started as a phase 1 first-in-human study of JCAR017, a defined composition CAR T-cell product also known as lisocabtagene maraleucel and administered at precise doses of CD4+ and CD8+ CAR T cells. Dose-finding and dose-expansion cohorts have been investigated, and currently the pivotal diffuse large B-cell lymphoma (DLBCL) cohort is enrolling, Dr. Saddiqi said, noting that dose level 2 (1 x 108 cells given as a single dose) was selected for that cohort.
The current findings are based on the TRANSCEND core population – a set of patients selected from the dose-finding and dose-expansion cohorts. This population includes patients with DLBCL not otherwise specified, transformed follicular lymphoma, or high-grade double- or triple-hit lymphomas, she said, explaining that she and her colleagues looked at prelymphodepletion baseline patient characteristics and biomarkers to assess how they related to outcomes and toxicities. This precise dosing of JCAR017 reduces variability, enabling the identification of potential patient factors associated with clinical outcomes, she said.
Response rates among 27 patients who received dose level 2 were high, with an 81% overall response rate and a 63% complete response rate.
“Patients with complete remission seemed to have more of a durable response,” she said. “In the core population at dose level 2, 50% with 6 months of follow-up seemed to remain in complete response, so there’s a dose-response effect in these patients.”
It doesn’t appear that the dose affects development of cytokine release syndrome (CRS) or neurotoxicity (NT), as the rates of these (30% and 20%, respectively) did not differ by dose level or schedule, but certain baseline features, such as a lactate dehydrogenase (LDH) level above 500 U/L and tumor burden measured as the sum of the product of diameters of 50 cm or greater, do appear to affect the development of these toxicities.
For example, 10 of 13 (77%) of patients with both of those baseline characteristics developed CRS, and 7 of 13 (54%) developed NT; for those without these characteristics, the odds of developing CRS and NT were significantly lower, she said.
Of note, higher Cmax, or peak expansion of CAR T cells in vivo, was seen at dose level 2, and exposure (area under the curve) was also higher, as was expected at that dose level, Dr. Siddiqi said.
“It also appears that there is a trend of patients with higher tumor burden to have higher expansion of their CAR T cells in vivo,” she said, noting that some of those patients were “superexpanders” with very high expansion of CAR T cells. “Similarly there were certain cytokines at the prelymphodepletion time point that seem to be higher … in patients who also go on to have higher expansion of their CAR T cells.”
These included interleukin-7, IL-15, MIP (macrophage inflammatory protein)–1alpha, and tumor necrosis factor (TNF)–alpha.
“Patients with certain higher inflammatory cytokines and biomarkers of inflammation also were noted to have higher events of CRS and neurotoxicity, so not just higher tumor burden, but also higher inflammatory state at baseline seems to affect patients in terms of getting any grade CRS or any grade neurotoxicity,” she said.
Those associated with CRS included ferritin, C-reactive protein (CRP), IL-10, IL-15, IL-16, TNF-alpha, and MIP-1beta levels, and those associated with NT included ferritin, CRP, d-dimer, IL-6, IL-15, TNF-alpha, and MIP-1alpha levels.
“Interestingly, there’s an inverse relationship between the expression of some of these biomarkers and inflammatory markers and the durability of response at 3 months,” she said.
Patients with higher tumor burden, LDH, and other markers either had no response at 3 months, or they had a very rapid response at the 1-month mark and then lost their response by 3 months, she noted.
“One of the theories could be that these patients with higher tumor burden, higher inflammatory state – they have such a high peak expansion rapidly after receiving their CAR T cells that potentially those CAR T cells may be getting exhausted or dying off very quickly, and therefore patients can lose their response,” she said.
When these data are considered together and modeled, there seems to be a therapeutic window where patients who have optimal expansion of CAR T cells in vivo may have lesser toxicity, higher overall response rates, and better durability of response, she said.
That is, on one end of the spectrum, there are patients with lower CAR T-cell expansion who have lower toxicities, but who also have lower overall response rates and lower durability of response, and on the other end, there are patients with superhigh expansion of CAR T cells, who have higher response rates, but also higher rates of toxicity, and who lose their response quickly.
“So if we can identify patients who would be in this window of optimal target expansion of CAR T cells, and if we could find strategies or mechanisms to move patients who are on either end of that spectrum into this window, we may be able to get patients with better efficacy and lower toxicities, and there could be combination strategies that could help get us there,” she said.
TRANSCEND NHL 001 is sponsored by Juno Therapeutics. Dr. Siddiqi reported serving as a consultant or adviser for Juno Therapeutics, and as a member of the speakers bureau for Pharmacyclics/Janssen and Seattle Genetics. Research funding was provided to her institution by Juno Therapeutics and several other companies.
SOURCE: Siddiqi T et al. ASCO-SITC, abstract 122.
SAN FRANCISCO – The CD19-directed 4-1BB chimeric antigen receptor (CAR) T-cell product JCAR017 demonstrated increased CAR T-cell expansion and persistence, and higher durability of response at higher dose levels – with manageable toxicities – in a pivotal phase 1 trial of relapsed/refractory B-cell non-Hodgkin lymphoma.
However, preliminary modeling data suggest that a therapeutic window exists for the CAR T-cell expansion, which means that development of strategies for pushing patients into that window could enhance efficacy and limit toxicity associated with JCAR017, Tanya Siddiqi, MD, of City of Hope Comprehensive Cancer Center, Duarte, Calif., reported at the ASCO-SITC Clinical Immuno-Oncology Symposium.
TRANSCEND NHL 001 is a multicenter, seamless design, pivotal trial, which started as a phase 1 first-in-human study of JCAR017, a defined composition CAR T-cell product also known as lisocabtagene maraleucel and administered at precise doses of CD4+ and CD8+ CAR T cells. Dose-finding and dose-expansion cohorts have been investigated, and currently the pivotal diffuse large B-cell lymphoma (DLBCL) cohort is enrolling, Dr. Saddiqi said, noting that dose level 2 (1 x 108 cells given as a single dose) was selected for that cohort.
The current findings are based on the TRANSCEND core population – a set of patients selected from the dose-finding and dose-expansion cohorts. This population includes patients with DLBCL not otherwise specified, transformed follicular lymphoma, or high-grade double- or triple-hit lymphomas, she said, explaining that she and her colleagues looked at prelymphodepletion baseline patient characteristics and biomarkers to assess how they related to outcomes and toxicities. This precise dosing of JCAR017 reduces variability, enabling the identification of potential patient factors associated with clinical outcomes, she said.
Response rates among 27 patients who received dose level 2 were high, with an 81% overall response rate and a 63% complete response rate.
“Patients with complete remission seemed to have more of a durable response,” she said. “In the core population at dose level 2, 50% with 6 months of follow-up seemed to remain in complete response, so there’s a dose-response effect in these patients.”
It doesn’t appear that the dose affects development of cytokine release syndrome (CRS) or neurotoxicity (NT), as the rates of these (30% and 20%, respectively) did not differ by dose level or schedule, but certain baseline features, such as a lactate dehydrogenase (LDH) level above 500 U/L and tumor burden measured as the sum of the product of diameters of 50 cm or greater, do appear to affect the development of these toxicities.
For example, 10 of 13 (77%) of patients with both of those baseline characteristics developed CRS, and 7 of 13 (54%) developed NT; for those without these characteristics, the odds of developing CRS and NT were significantly lower, she said.
Of note, higher Cmax, or peak expansion of CAR T cells in vivo, was seen at dose level 2, and exposure (area under the curve) was also higher, as was expected at that dose level, Dr. Siddiqi said.
“It also appears that there is a trend of patients with higher tumor burden to have higher expansion of their CAR T cells in vivo,” she said, noting that some of those patients were “superexpanders” with very high expansion of CAR T cells. “Similarly there were certain cytokines at the prelymphodepletion time point that seem to be higher … in patients who also go on to have higher expansion of their CAR T cells.”
These included interleukin-7, IL-15, MIP (macrophage inflammatory protein)–1alpha, and tumor necrosis factor (TNF)–alpha.
“Patients with certain higher inflammatory cytokines and biomarkers of inflammation also were noted to have higher events of CRS and neurotoxicity, so not just higher tumor burden, but also higher inflammatory state at baseline seems to affect patients in terms of getting any grade CRS or any grade neurotoxicity,” she said.
Those associated with CRS included ferritin, C-reactive protein (CRP), IL-10, IL-15, IL-16, TNF-alpha, and MIP-1beta levels, and those associated with NT included ferritin, CRP, d-dimer, IL-6, IL-15, TNF-alpha, and MIP-1alpha levels.
“Interestingly, there’s an inverse relationship between the expression of some of these biomarkers and inflammatory markers and the durability of response at 3 months,” she said.
Patients with higher tumor burden, LDH, and other markers either had no response at 3 months, or they had a very rapid response at the 1-month mark and then lost their response by 3 months, she noted.
“One of the theories could be that these patients with higher tumor burden, higher inflammatory state – they have such a high peak expansion rapidly after receiving their CAR T cells that potentially those CAR T cells may be getting exhausted or dying off very quickly, and therefore patients can lose their response,” she said.
When these data are considered together and modeled, there seems to be a therapeutic window where patients who have optimal expansion of CAR T cells in vivo may have lesser toxicity, higher overall response rates, and better durability of response, she said.
That is, on one end of the spectrum, there are patients with lower CAR T-cell expansion who have lower toxicities, but who also have lower overall response rates and lower durability of response, and on the other end, there are patients with superhigh expansion of CAR T cells, who have higher response rates, but also higher rates of toxicity, and who lose their response quickly.
“So if we can identify patients who would be in this window of optimal target expansion of CAR T cells, and if we could find strategies or mechanisms to move patients who are on either end of that spectrum into this window, we may be able to get patients with better efficacy and lower toxicities, and there could be combination strategies that could help get us there,” she said.
TRANSCEND NHL 001 is sponsored by Juno Therapeutics. Dr. Siddiqi reported serving as a consultant or adviser for Juno Therapeutics, and as a member of the speakers bureau for Pharmacyclics/Janssen and Seattle Genetics. Research funding was provided to her institution by Juno Therapeutics and several other companies.
SOURCE: Siddiqi T et al. ASCO-SITC, abstract 122.
for CAR T expansion
for CAR T expansion
REPORTING FROM THE CLINICAL IMMUNO-ONCOLOGY SYNDROME
Key clinical point:
Major finding: In all, 77% of patients with a lactate dehydrogenase level greater than 500 U/L and a sum of the product of diameters of 50 cm or greater developed cytokine release syndrome, and 54% of patients with those characteristics developed neurotoxicity.
Study details: A cohort of 27 patients from the pivotal phase 1 TRANSCEND NHL 001 trial.
Disclosures: TRANSCEND NHL 001 is sponsored by Juno Therapeutics. Dr. Siddiqi reported serving as a consultant or adviser for Juno Therapeutics, and as a member of the speakers bureau for Pharmacyclics/Janssen and Seattle Genetics. Research funding was provided to her institution by Juno Therapeutics and several other companies.
Source: Siddiqi T et al. ASCO-SITC, abstract 122.
In situ vaccination eradicates lymphoma, other cancers
Experiments in mice have shown that injecting immune-stimulating agents directly into a tumor can help the immune system eradicate tumors in other areas of the body.
The approach worked for several cancers, including lymphomas.
The researchers believe the local application of the agents could serve as a rapid and relatively inexpensive cancer therapy that is unlikely to cause the adverse effects often seen with more widespread immune stimulation.
“Our approach uses a one-time application of very small amounts of two agents to stimulate the immune cells only within the tumor itself,” said Ronald Levy, MD, of Stanford University Medical Center in California.
“In the mice, we saw amazing, body-wide effects, including the elimination of tumors all over the animal. This approach bypasses the need to identify tumor-specific immune targets and doesn’t require wholesale activation of the immune system or customization of a patient’s immune cells.”
Dr Levy and his colleagues described this approach in Science Translational Medicine.
The method involves reactivating cancer-specific T cells by injecting microgram amounts of two agents directly into the tumor site.
One of the agents is an unmethylated CG–enriched oligodeoxynucleotide (CpG)—a Toll-like receptor 9 (TLR9) ligand. It works with nearby immune cells to amplify the expression of OX40 on the surface of T cells.
The other agent is an antibody that binds to OX40. It activates the T cells to lead the charge against the cancer cells.
Because the agents are injected directly into the tumor, only T cells that have infiltrated it are activated. In effect, these T cells are “prescreened” by the body to recognize only cancer-specific proteins.
Some of these tumor-specific, activated T cells then leave the original tumor to find and destroy other identical tumors throughout the body.
The researchers found this approach worked well in mice with A20 B-cell lymphoma tumors transplanted in two sites on their bodies.
Injecting one tumor site with the agents caused regression of the untreated tumor as well as the treated one. In this way, 87 of 90 mice were cured.
Although lymphoma recurred in 3 of the mice, the tumors again regressed after a second treatment with CpG and anti-OX40.
The researchers saw similar results in mice with melanoma as well as breast and colon cancer.
Mice genetically engineered to spontaneously develop breast cancers in all 10 of their mammary pads also responded to the treatment. Treating the first tumor that arose often prevented the occurrence of future tumors and significantly increased the animals’ life span, the researchers found.
Finally, the team explored the specificity of the T cells by transplanting two types of tumors into mice.
They transplanted A20 lymphoma cells in two locations and a colon cancer cell line in a third location. Treatment of one of the lymphoma sites caused the regression of both lymphoma tumors but did not affect the colon cancer cells.
“This is a very targeted approach,” Dr Levy said. “Only the tumor that shares the protein targets displayed by the treated site is affected. We’re attacking specific targets without having to identify exactly what proteins the T cells are recognizing.”
Dr Levy and his colleagues have launched a clinical trial (NCT03410901) to test this treatment approach. The researchers hope to determine the adverse effects and optimal dose of the TLR9 agonist SD-101, the anti-OX40 antibody BMS 986178, and radiation therapy in patients with low-grade B-cell non-Hodgkin lymphomas.
If the trial is successful, Dr Levy believes the treatment could be useful for many tumor types.
“I don’t think there’s a limit to the type of tumor we could potentially treat,” he said, “as long as it has been infiltrated by the immune system.” ![]()
Experiments in mice have shown that injecting immune-stimulating agents directly into a tumor can help the immune system eradicate tumors in other areas of the body.
The approach worked for several cancers, including lymphomas.
The researchers believe the local application of the agents could serve as a rapid and relatively inexpensive cancer therapy that is unlikely to cause the adverse effects often seen with more widespread immune stimulation.
“Our approach uses a one-time application of very small amounts of two agents to stimulate the immune cells only within the tumor itself,” said Ronald Levy, MD, of Stanford University Medical Center in California.
“In the mice, we saw amazing, body-wide effects, including the elimination of tumors all over the animal. This approach bypasses the need to identify tumor-specific immune targets and doesn’t require wholesale activation of the immune system or customization of a patient’s immune cells.”
Dr Levy and his colleagues described this approach in Science Translational Medicine.
The method involves reactivating cancer-specific T cells by injecting microgram amounts of two agents directly into the tumor site.
One of the agents is an unmethylated CG–enriched oligodeoxynucleotide (CpG)—a Toll-like receptor 9 (TLR9) ligand. It works with nearby immune cells to amplify the expression of OX40 on the surface of T cells.
The other agent is an antibody that binds to OX40. It activates the T cells to lead the charge against the cancer cells.
Because the agents are injected directly into the tumor, only T cells that have infiltrated it are activated. In effect, these T cells are “prescreened” by the body to recognize only cancer-specific proteins.
Some of these tumor-specific, activated T cells then leave the original tumor to find and destroy other identical tumors throughout the body.
The researchers found this approach worked well in mice with A20 B-cell lymphoma tumors transplanted in two sites on their bodies.
Injecting one tumor site with the agents caused regression of the untreated tumor as well as the treated one. In this way, 87 of 90 mice were cured.
Although lymphoma recurred in 3 of the mice, the tumors again regressed after a second treatment with CpG and anti-OX40.
The researchers saw similar results in mice with melanoma as well as breast and colon cancer.
Mice genetically engineered to spontaneously develop breast cancers in all 10 of their mammary pads also responded to the treatment. Treating the first tumor that arose often prevented the occurrence of future tumors and significantly increased the animals’ life span, the researchers found.
Finally, the team explored the specificity of the T cells by transplanting two types of tumors into mice.
They transplanted A20 lymphoma cells in two locations and a colon cancer cell line in a third location. Treatment of one of the lymphoma sites caused the regression of both lymphoma tumors but did not affect the colon cancer cells.
“This is a very targeted approach,” Dr Levy said. “Only the tumor that shares the protein targets displayed by the treated site is affected. We’re attacking specific targets without having to identify exactly what proteins the T cells are recognizing.”
Dr Levy and his colleagues have launched a clinical trial (NCT03410901) to test this treatment approach. The researchers hope to determine the adverse effects and optimal dose of the TLR9 agonist SD-101, the anti-OX40 antibody BMS 986178, and radiation therapy in patients with low-grade B-cell non-Hodgkin lymphomas.
If the trial is successful, Dr Levy believes the treatment could be useful for many tumor types.
“I don’t think there’s a limit to the type of tumor we could potentially treat,” he said, “as long as it has been infiltrated by the immune system.” ![]()
Experiments in mice have shown that injecting immune-stimulating agents directly into a tumor can help the immune system eradicate tumors in other areas of the body.
The approach worked for several cancers, including lymphomas.
The researchers believe the local application of the agents could serve as a rapid and relatively inexpensive cancer therapy that is unlikely to cause the adverse effects often seen with more widespread immune stimulation.
“Our approach uses a one-time application of very small amounts of two agents to stimulate the immune cells only within the tumor itself,” said Ronald Levy, MD, of Stanford University Medical Center in California.
“In the mice, we saw amazing, body-wide effects, including the elimination of tumors all over the animal. This approach bypasses the need to identify tumor-specific immune targets and doesn’t require wholesale activation of the immune system or customization of a patient’s immune cells.”
Dr Levy and his colleagues described this approach in Science Translational Medicine.
The method involves reactivating cancer-specific T cells by injecting microgram amounts of two agents directly into the tumor site.
One of the agents is an unmethylated CG–enriched oligodeoxynucleotide (CpG)—a Toll-like receptor 9 (TLR9) ligand. It works with nearby immune cells to amplify the expression of OX40 on the surface of T cells.
The other agent is an antibody that binds to OX40. It activates the T cells to lead the charge against the cancer cells.
Because the agents are injected directly into the tumor, only T cells that have infiltrated it are activated. In effect, these T cells are “prescreened” by the body to recognize only cancer-specific proteins.
Some of these tumor-specific, activated T cells then leave the original tumor to find and destroy other identical tumors throughout the body.
The researchers found this approach worked well in mice with A20 B-cell lymphoma tumors transplanted in two sites on their bodies.
Injecting one tumor site with the agents caused regression of the untreated tumor as well as the treated one. In this way, 87 of 90 mice were cured.
Although lymphoma recurred in 3 of the mice, the tumors again regressed after a second treatment with CpG and anti-OX40.
The researchers saw similar results in mice with melanoma as well as breast and colon cancer.
Mice genetically engineered to spontaneously develop breast cancers in all 10 of their mammary pads also responded to the treatment. Treating the first tumor that arose often prevented the occurrence of future tumors and significantly increased the animals’ life span, the researchers found.
Finally, the team explored the specificity of the T cells by transplanting two types of tumors into mice.
They transplanted A20 lymphoma cells in two locations and a colon cancer cell line in a third location. Treatment of one of the lymphoma sites caused the regression of both lymphoma tumors but did not affect the colon cancer cells.
“This is a very targeted approach,” Dr Levy said. “Only the tumor that shares the protein targets displayed by the treated site is affected. We’re attacking specific targets without having to identify exactly what proteins the T cells are recognizing.”
Dr Levy and his colleagues have launched a clinical trial (NCT03410901) to test this treatment approach. The researchers hope to determine the adverse effects and optimal dose of the TLR9 agonist SD-101, the anti-OX40 antibody BMS 986178, and radiation therapy in patients with low-grade B-cell non-Hodgkin lymphomas.
If the trial is successful, Dr Levy believes the treatment could be useful for many tumor types.
“I don’t think there’s a limit to the type of tumor we could potentially treat,” he said, “as long as it has been infiltrated by the immune system.” ![]()
Combo could treat double-hit lymphoma
Existing drugs could be combined to treat double-hit lymphoma (DHL), according to preclinical research published in Science Translational Medicine.
The drugs are tigecycline, an antibiotic, and venetoclax, a BCL2 inhibitor.
Researchers observed promising activity with these drugs in combination, both in cell lines and mouse models of DHL.
The team therefore believes the drugs could be repurposed to treat DHL, which currently has a dismal prognosis.
Study author Micol Ravà, PhD, of the European Institute of Oncology in Milan, Italy, and her colleagues noted that DHL is driven by the abnormal activation of MYC and BCL2. However, selective BCL2 inhibitors like venetoclax have failed to halt disease progression in DHL patients.
Seeking a way to sensitize DHL to BCL2 inhibitors, the researchers turned to tigecycline, which interferes with mitochondria to trigger a MYC-dependent cell death pathway.
The team found that tigecycline and venetoclax demonstrated synergy in 5 DHL cell lines. The drugs were synergistic in 3 cell lines—Karpas-422, SU-DHL-6, and DOHH-2—in which neither drug alone showed significant pro-apoptotic activity.
In 2 other cell lines—SU-DHL-4 and OCI-LY8—venetoclax was active when given alone, but its activity was enhanced by the addition of tigecycline.
In mouse models of DHL (using the human cell lines SU-DHL-6, DOHH-2, and OCI-LY8), each of the drugs alone were able to slow tumor progression somewhat.
However, combination tigecycline and venetoclax exhibited “strong antitumoral activity,” according to the researchers. In fact, the combination caused full disease regression in all 8 SU-DHL-6 mice and 3 of 9 OCI-LY8 mice.
Dr Ravà and her colleagues also found the combination produced “rapid and marked tumor regression” in mice with a patient-derived xenograft.
The researchers observed no toxicity when tigecycline and venetoclax were given at low doses. However, mice receiving more aggressive treatment had some inflammation in the liver and spleen. And some mice treated with high doses of tigecycline and venetoclax died within 1 week of treatment initiation.
Finally, Dr Ravà and her colleagues found that tigecycline and venetoclax each synergized with rituximab. The team therefore concluded that tigecycline and venetoclax “have the potential to reinforce rituximab-containing therapies in the clinic.” ![]()
Existing drugs could be combined to treat double-hit lymphoma (DHL), according to preclinical research published in Science Translational Medicine.
The drugs are tigecycline, an antibiotic, and venetoclax, a BCL2 inhibitor.
Researchers observed promising activity with these drugs in combination, both in cell lines and mouse models of DHL.
The team therefore believes the drugs could be repurposed to treat DHL, which currently has a dismal prognosis.
Study author Micol Ravà, PhD, of the European Institute of Oncology in Milan, Italy, and her colleagues noted that DHL is driven by the abnormal activation of MYC and BCL2. However, selective BCL2 inhibitors like venetoclax have failed to halt disease progression in DHL patients.
Seeking a way to sensitize DHL to BCL2 inhibitors, the researchers turned to tigecycline, which interferes with mitochondria to trigger a MYC-dependent cell death pathway.
The team found that tigecycline and venetoclax demonstrated synergy in 5 DHL cell lines. The drugs were synergistic in 3 cell lines—Karpas-422, SU-DHL-6, and DOHH-2—in which neither drug alone showed significant pro-apoptotic activity.
In 2 other cell lines—SU-DHL-4 and OCI-LY8—venetoclax was active when given alone, but its activity was enhanced by the addition of tigecycline.
In mouse models of DHL (using the human cell lines SU-DHL-6, DOHH-2, and OCI-LY8), each of the drugs alone were able to slow tumor progression somewhat.
However, combination tigecycline and venetoclax exhibited “strong antitumoral activity,” according to the researchers. In fact, the combination caused full disease regression in all 8 SU-DHL-6 mice and 3 of 9 OCI-LY8 mice.
Dr Ravà and her colleagues also found the combination produced “rapid and marked tumor regression” in mice with a patient-derived xenograft.
The researchers observed no toxicity when tigecycline and venetoclax were given at low doses. However, mice receiving more aggressive treatment had some inflammation in the liver and spleen. And some mice treated with high doses of tigecycline and venetoclax died within 1 week of treatment initiation.
Finally, Dr Ravà and her colleagues found that tigecycline and venetoclax each synergized with rituximab. The team therefore concluded that tigecycline and venetoclax “have the potential to reinforce rituximab-containing therapies in the clinic.” ![]()
Existing drugs could be combined to treat double-hit lymphoma (DHL), according to preclinical research published in Science Translational Medicine.
The drugs are tigecycline, an antibiotic, and venetoclax, a BCL2 inhibitor.
Researchers observed promising activity with these drugs in combination, both in cell lines and mouse models of DHL.
The team therefore believes the drugs could be repurposed to treat DHL, which currently has a dismal prognosis.
Study author Micol Ravà, PhD, of the European Institute of Oncology in Milan, Italy, and her colleagues noted that DHL is driven by the abnormal activation of MYC and BCL2. However, selective BCL2 inhibitors like venetoclax have failed to halt disease progression in DHL patients.
Seeking a way to sensitize DHL to BCL2 inhibitors, the researchers turned to tigecycline, which interferes with mitochondria to trigger a MYC-dependent cell death pathway.
The team found that tigecycline and venetoclax demonstrated synergy in 5 DHL cell lines. The drugs were synergistic in 3 cell lines—Karpas-422, SU-DHL-6, and DOHH-2—in which neither drug alone showed significant pro-apoptotic activity.
In 2 other cell lines—SU-DHL-4 and OCI-LY8—venetoclax was active when given alone, but its activity was enhanced by the addition of tigecycline.
In mouse models of DHL (using the human cell lines SU-DHL-6, DOHH-2, and OCI-LY8), each of the drugs alone were able to slow tumor progression somewhat.
However, combination tigecycline and venetoclax exhibited “strong antitumoral activity,” according to the researchers. In fact, the combination caused full disease regression in all 8 SU-DHL-6 mice and 3 of 9 OCI-LY8 mice.
Dr Ravà and her colleagues also found the combination produced “rapid and marked tumor regression” in mice with a patient-derived xenograft.
The researchers observed no toxicity when tigecycline and venetoclax were given at low doses. However, mice receiving more aggressive treatment had some inflammation in the liver and spleen. And some mice treated with high doses of tigecycline and venetoclax died within 1 week of treatment initiation.
Finally, Dr Ravà and her colleagues found that tigecycline and venetoclax each synergized with rituximab. The team therefore concluded that tigecycline and venetoclax “have the potential to reinforce rituximab-containing therapies in the clinic.” ![]()













