User login
Venetoclax/HMA combo still safe, effective for elderly with AML
In patients aged 65 years and older with acute myeloid leukemia (AML), the combination of venetoclax (Venclexta) and a hypomethylating agent had good efficacy and was well tolerated, according to updated results from a phase 1b dose-escalation and expansion trial.
At a median of 8.9 months of study, the overall response rate (ORR) among all treated patients was 68%, with a median duration of complete remission (CR) plus CR with incomplete count recovery (CRi) of 11.3 months, reported Courtney DiNardo, MD, from the University of Texas MD Anderson Center in Houston and her colleagues.
“Venetoclax in combination with azacitidine or decitabine was well tolerated, with similar safety profiles within all arms of the dose escalation and expansion phases in elderly patients with previously untreated AML ineligible for standard induction therapy,” they wrote in a paper published in Blood.
At the 2017 European Hematology Association Congress, the investigators reported that the combined rate of complete remission CR and CRi was 60% among patients with poor-risk cytogenetics and 78% among patients with intermediate-risk disease. In addition, the drug combination was effective among patients with both primary de novo AML (68%) and secondary AML (related to myelodysplasia or myeloproliferative neoplasms or previous therapy; 73%).
In this, the most recent analysis, Dr. DiNardo and her colleagues reported on follow-up of 145 patients aged 65 and older with treatment-naive AML who were not eligible for intensive chemotherapy regimens used for younger adults. The median age was 74 years. Approximately half of all patients (49%) had poor-risk cytogenetics.
The patients were treated with either decitabine or azacitidine plus venetoclax at a dose of either 400 mg or 800 mg. Decitabine was dosed at 20 mg/m2 intravenously on days 1-5 of a 28-day cycle. Azacitidine was dosed at 75 mg/m2 subcutaneously on days 1-7 of every cycle.
The median time on study was 8.9 months. Among all patients treated at all doses, 67% had either a CR or CRi. The combined CR/CRi rate in patients treated at the 400 mg dose of venetoclax was 73%.
The CR/CRi rate for patients with poor-risk cytogenetics was 60%, and the rate for patients aged 75 years and older was 65%.
Among all patients, the median duration of CR/CRi was 11.3 months, and median overall survival was 17.5 months. In the 400 mg venetoclax cohort, the median duration of CR/CRi was 12.5 months, with the median OS not reached at the time of data cutoff.
Adverse events occurring in 30% or more of patients included constipation, diarrhea, vomiting, nausea, fatigue, febrile neutropenia, hypokalemia, decreased appetite, and decreased white blood cell count. There were no reported cases of the tumor lysis syndrome, a known complication of venetoclax therapy.
Venetoclax plus decitabine or azacitidine was effective in high-risk subgroups, including patients aged 75 years and older, those with poor cytogenetic risk, and those with secondary AML, the investigators noted.
“Though these observations are drawn from a relatively small subset of patients, the remission rates achieved by our low-intensity regimen are encouraging in light of the traditionally lower remission rates in the elderly AML population (40%-50%) compared with young patients receiving chemotherapy (60%-70%) and the relatively short duration of these remissions,” Dr. DiNardo and her colleagues wrote.
A phase 3 trial is currently underway comparing venetoclax at the 400 mg dose plus azacitidine with azacitidine alone in treatment-naive patients with AML who are ineligible for standard induction therapy.
The trial was supported by AbbVie and Genentech. Dr. DiNardo and multiple coauthors disclosed relationships with AbbVie, Genentech, and other companies.
SOURCE: DiNardo C et al. Blood. 2018 Oct 25. doi: 10.1182/blood-2018-08-868752.
In patients aged 65 years and older with acute myeloid leukemia (AML), the combination of venetoclax (Venclexta) and a hypomethylating agent had good efficacy and was well tolerated, according to updated results from a phase 1b dose-escalation and expansion trial.
At a median of 8.9 months of study, the overall response rate (ORR) among all treated patients was 68%, with a median duration of complete remission (CR) plus CR with incomplete count recovery (CRi) of 11.3 months, reported Courtney DiNardo, MD, from the University of Texas MD Anderson Center in Houston and her colleagues.
“Venetoclax in combination with azacitidine or decitabine was well tolerated, with similar safety profiles within all arms of the dose escalation and expansion phases in elderly patients with previously untreated AML ineligible for standard induction therapy,” they wrote in a paper published in Blood.
At the 2017 European Hematology Association Congress, the investigators reported that the combined rate of complete remission CR and CRi was 60% among patients with poor-risk cytogenetics and 78% among patients with intermediate-risk disease. In addition, the drug combination was effective among patients with both primary de novo AML (68%) and secondary AML (related to myelodysplasia or myeloproliferative neoplasms or previous therapy; 73%).
In this, the most recent analysis, Dr. DiNardo and her colleagues reported on follow-up of 145 patients aged 65 and older with treatment-naive AML who were not eligible for intensive chemotherapy regimens used for younger adults. The median age was 74 years. Approximately half of all patients (49%) had poor-risk cytogenetics.
The patients were treated with either decitabine or azacitidine plus venetoclax at a dose of either 400 mg or 800 mg. Decitabine was dosed at 20 mg/m2 intravenously on days 1-5 of a 28-day cycle. Azacitidine was dosed at 75 mg/m2 subcutaneously on days 1-7 of every cycle.
The median time on study was 8.9 months. Among all patients treated at all doses, 67% had either a CR or CRi. The combined CR/CRi rate in patients treated at the 400 mg dose of venetoclax was 73%.
The CR/CRi rate for patients with poor-risk cytogenetics was 60%, and the rate for patients aged 75 years and older was 65%.
Among all patients, the median duration of CR/CRi was 11.3 months, and median overall survival was 17.5 months. In the 400 mg venetoclax cohort, the median duration of CR/CRi was 12.5 months, with the median OS not reached at the time of data cutoff.
Adverse events occurring in 30% or more of patients included constipation, diarrhea, vomiting, nausea, fatigue, febrile neutropenia, hypokalemia, decreased appetite, and decreased white blood cell count. There were no reported cases of the tumor lysis syndrome, a known complication of venetoclax therapy.
Venetoclax plus decitabine or azacitidine was effective in high-risk subgroups, including patients aged 75 years and older, those with poor cytogenetic risk, and those with secondary AML, the investigators noted.
“Though these observations are drawn from a relatively small subset of patients, the remission rates achieved by our low-intensity regimen are encouraging in light of the traditionally lower remission rates in the elderly AML population (40%-50%) compared with young patients receiving chemotherapy (60%-70%) and the relatively short duration of these remissions,” Dr. DiNardo and her colleagues wrote.
A phase 3 trial is currently underway comparing venetoclax at the 400 mg dose plus azacitidine with azacitidine alone in treatment-naive patients with AML who are ineligible for standard induction therapy.
The trial was supported by AbbVie and Genentech. Dr. DiNardo and multiple coauthors disclosed relationships with AbbVie, Genentech, and other companies.
SOURCE: DiNardo C et al. Blood. 2018 Oct 25. doi: 10.1182/blood-2018-08-868752.
In patients aged 65 years and older with acute myeloid leukemia (AML), the combination of venetoclax (Venclexta) and a hypomethylating agent had good efficacy and was well tolerated, according to updated results from a phase 1b dose-escalation and expansion trial.
At a median of 8.9 months of study, the overall response rate (ORR) among all treated patients was 68%, with a median duration of complete remission (CR) plus CR with incomplete count recovery (CRi) of 11.3 months, reported Courtney DiNardo, MD, from the University of Texas MD Anderson Center in Houston and her colleagues.
“Venetoclax in combination with azacitidine or decitabine was well tolerated, with similar safety profiles within all arms of the dose escalation and expansion phases in elderly patients with previously untreated AML ineligible for standard induction therapy,” they wrote in a paper published in Blood.
At the 2017 European Hematology Association Congress, the investigators reported that the combined rate of complete remission CR and CRi was 60% among patients with poor-risk cytogenetics and 78% among patients with intermediate-risk disease. In addition, the drug combination was effective among patients with both primary de novo AML (68%) and secondary AML (related to myelodysplasia or myeloproliferative neoplasms or previous therapy; 73%).
In this, the most recent analysis, Dr. DiNardo and her colleagues reported on follow-up of 145 patients aged 65 and older with treatment-naive AML who were not eligible for intensive chemotherapy regimens used for younger adults. The median age was 74 years. Approximately half of all patients (49%) had poor-risk cytogenetics.
The patients were treated with either decitabine or azacitidine plus venetoclax at a dose of either 400 mg or 800 mg. Decitabine was dosed at 20 mg/m2 intravenously on days 1-5 of a 28-day cycle. Azacitidine was dosed at 75 mg/m2 subcutaneously on days 1-7 of every cycle.
The median time on study was 8.9 months. Among all patients treated at all doses, 67% had either a CR or CRi. The combined CR/CRi rate in patients treated at the 400 mg dose of venetoclax was 73%.
The CR/CRi rate for patients with poor-risk cytogenetics was 60%, and the rate for patients aged 75 years and older was 65%.
Among all patients, the median duration of CR/CRi was 11.3 months, and median overall survival was 17.5 months. In the 400 mg venetoclax cohort, the median duration of CR/CRi was 12.5 months, with the median OS not reached at the time of data cutoff.
Adverse events occurring in 30% or more of patients included constipation, diarrhea, vomiting, nausea, fatigue, febrile neutropenia, hypokalemia, decreased appetite, and decreased white blood cell count. There were no reported cases of the tumor lysis syndrome, a known complication of venetoclax therapy.
Venetoclax plus decitabine or azacitidine was effective in high-risk subgroups, including patients aged 75 years and older, those with poor cytogenetic risk, and those with secondary AML, the investigators noted.
“Though these observations are drawn from a relatively small subset of patients, the remission rates achieved by our low-intensity regimen are encouraging in light of the traditionally lower remission rates in the elderly AML population (40%-50%) compared with young patients receiving chemotherapy (60%-70%) and the relatively short duration of these remissions,” Dr. DiNardo and her colleagues wrote.
A phase 3 trial is currently underway comparing venetoclax at the 400 mg dose plus azacitidine with azacitidine alone in treatment-naive patients with AML who are ineligible for standard induction therapy.
The trial was supported by AbbVie and Genentech. Dr. DiNardo and multiple coauthors disclosed relationships with AbbVie, Genentech, and other companies.
SOURCE: DiNardo C et al. Blood. 2018 Oct 25. doi: 10.1182/blood-2018-08-868752.
FROM BLOOD
Key clinical point:
Major finding: At a median time of study of 8.9 months, the overall response rate among all treated patients was 68%.
Study details: Follow-up of a phase 1b dose-escalation and expansion cohort of 145 patients aged 65 years and older with treatment-naive AML.
Disclosures: The trial was supported by AbbVie and Genentech. Dr. DiNardo and multiple coauthors disclosed relationships with AbbVie, Genentech, and other companies.
Source: DiNardo C et al. Blood. 2018 Oct 25. doi: 10.1182/blood-2018-08-868752.
AML relapse after HSCT linked to potentially reversible immune changes
Relapse of acute myeloid leukemia after hematopoietic stem cell transplantation appears to be related to posttransplant changes in immune function that may be reversible with interferon-gamma therapy, investigators said.
Researchers performed a comparison of acute myeloid leukemia (AML) samples taken from patients before hematopoietic stem cell transplantation (HSCT) and at the time of relapse. They found that, while the general genomic changes seen at relapse resembled changes seen when patients experience relapse after chemotherapy, HSCT was associated with changes in genes believed to control both adaptive and innate immunity.
The findings suggest that transplantation results in a dampening of immune surveillance that could potentially be reversed with interferon gamma, an immunostimulatory cytokine, reported Matthew J. Christopher, MD, PhD, from Washington University, St. Louis, and his colleagues.
“These changes appeared to be epigenetic in nature in at least some cases, which suggests that therapeutic strategies to resensitize AML cells to the graft-versus-leukemia effect may be feasible,” they wrote in the New England Journal of Medicine.
The researchers noted that, while the presence of certain AML mutations may predict risk for relapse following HSCT, “the mechanisms by which these mutations promote relapse remain unclear.”
To get a better sense of how genetic and epigenetic changes after transplantation may allow leukemic cells to avoid the graft-versus-leukemia effect – and to see whether immune-related genes are affected by HSCT – they performed enhanced exome sequencing, flow cytometry, and immunohistochemical analyses on samples from 15 patients with AML who had a relapse after receiving transplants from HLA-matched siblings, matched unrelated donors, or HLA-mismatched unrelated donors, and on paired samples from 20 patients who experienced relapses after chemotherapy.
To validate their findings, they also evaluated samples from 28 other patients with AML who had a relapse after transplantation.
They first looked for relapse-specific mutations, but found no driver mutations associated with relapse after transplantation. The mutations seen during relapse after transplantation were generally similar to those seen both before treatment and after relapse in patients who had undergone chemotherapy. The researchers could not identify any patterns of mutations related to relapse.
They then looked for, but did not find, relapse-specific mutations in genes involved in either modulation of immune checkpoints, antigen presentation, or cytokine signaling.
The researchers did, however, find evidence of epigenetic changes that were more common in the samples of patients with posttransplant relapses, compared with postchemotherapy relapses. Specifically, they found that major histocompatibility (MHC) class II genes were down-regulated 200%-1100% after transplant, compared with the pretransplant samples.
In samples from 17 of 34 patients who experienced a relapse after transplantation, both flow cytometry and immunohistochemical analyses confirmed that expression of MHC class II molecules were decreased at relapse.
To see whether this down-regulation was reversible, the researchers treated samples from three patients with posttransplant relapse with interferon gamma, which is known to up-regulate MHC class II protein on myeloid cells and other cell types.
“Culture of these cells with interferon-gamma rapidly induced MHC class II protein expression on leukemic blasts, with essentially full restoration of MHC class II protein expression in nearly all AML blasts after 72 hours,” they wrote, adding that the reversibility of down-regulation of MHC class II in these blasts “strongly suggests that this phenomenon is mediated by an epigenetic mechanism.”
The study was supported by grants to investigators from the National Institutes of Health, Leukemia & Lymphoma Society, and the Barnes-Jewish Hospital Foundation. Dr. Christopher and several coauthors reported receiving grants from the study funders but no other relevant conflicts of interest. Several coauthors reported receiving personal fees and/or research support from industry outside the submitted work.
SOURCE: Christopher MJ et al. N Engl J Med. 2018 Oct 31. doi: 10.1056/NEJMoa1808777.
Relapse of acute myeloid leukemia after hematopoietic stem cell transplantation appears to be related to posttransplant changes in immune function that may be reversible with interferon-gamma therapy, investigators said.
Researchers performed a comparison of acute myeloid leukemia (AML) samples taken from patients before hematopoietic stem cell transplantation (HSCT) and at the time of relapse. They found that, while the general genomic changes seen at relapse resembled changes seen when patients experience relapse after chemotherapy, HSCT was associated with changes in genes believed to control both adaptive and innate immunity.
The findings suggest that transplantation results in a dampening of immune surveillance that could potentially be reversed with interferon gamma, an immunostimulatory cytokine, reported Matthew J. Christopher, MD, PhD, from Washington University, St. Louis, and his colleagues.
“These changes appeared to be epigenetic in nature in at least some cases, which suggests that therapeutic strategies to resensitize AML cells to the graft-versus-leukemia effect may be feasible,” they wrote in the New England Journal of Medicine.
The researchers noted that, while the presence of certain AML mutations may predict risk for relapse following HSCT, “the mechanisms by which these mutations promote relapse remain unclear.”
To get a better sense of how genetic and epigenetic changes after transplantation may allow leukemic cells to avoid the graft-versus-leukemia effect – and to see whether immune-related genes are affected by HSCT – they performed enhanced exome sequencing, flow cytometry, and immunohistochemical analyses on samples from 15 patients with AML who had a relapse after receiving transplants from HLA-matched siblings, matched unrelated donors, or HLA-mismatched unrelated donors, and on paired samples from 20 patients who experienced relapses after chemotherapy.
To validate their findings, they also evaluated samples from 28 other patients with AML who had a relapse after transplantation.
They first looked for relapse-specific mutations, but found no driver mutations associated with relapse after transplantation. The mutations seen during relapse after transplantation were generally similar to those seen both before treatment and after relapse in patients who had undergone chemotherapy. The researchers could not identify any patterns of mutations related to relapse.
They then looked for, but did not find, relapse-specific mutations in genes involved in either modulation of immune checkpoints, antigen presentation, or cytokine signaling.
The researchers did, however, find evidence of epigenetic changes that were more common in the samples of patients with posttransplant relapses, compared with postchemotherapy relapses. Specifically, they found that major histocompatibility (MHC) class II genes were down-regulated 200%-1100% after transplant, compared with the pretransplant samples.
In samples from 17 of 34 patients who experienced a relapse after transplantation, both flow cytometry and immunohistochemical analyses confirmed that expression of MHC class II molecules were decreased at relapse.
To see whether this down-regulation was reversible, the researchers treated samples from three patients with posttransplant relapse with interferon gamma, which is known to up-regulate MHC class II protein on myeloid cells and other cell types.
“Culture of these cells with interferon-gamma rapidly induced MHC class II protein expression on leukemic blasts, with essentially full restoration of MHC class II protein expression in nearly all AML blasts after 72 hours,” they wrote, adding that the reversibility of down-regulation of MHC class II in these blasts “strongly suggests that this phenomenon is mediated by an epigenetic mechanism.”
The study was supported by grants to investigators from the National Institutes of Health, Leukemia & Lymphoma Society, and the Barnes-Jewish Hospital Foundation. Dr. Christopher and several coauthors reported receiving grants from the study funders but no other relevant conflicts of interest. Several coauthors reported receiving personal fees and/or research support from industry outside the submitted work.
SOURCE: Christopher MJ et al. N Engl J Med. 2018 Oct 31. doi: 10.1056/NEJMoa1808777.
Relapse of acute myeloid leukemia after hematopoietic stem cell transplantation appears to be related to posttransplant changes in immune function that may be reversible with interferon-gamma therapy, investigators said.
Researchers performed a comparison of acute myeloid leukemia (AML) samples taken from patients before hematopoietic stem cell transplantation (HSCT) and at the time of relapse. They found that, while the general genomic changes seen at relapse resembled changes seen when patients experience relapse after chemotherapy, HSCT was associated with changes in genes believed to control both adaptive and innate immunity.
The findings suggest that transplantation results in a dampening of immune surveillance that could potentially be reversed with interferon gamma, an immunostimulatory cytokine, reported Matthew J. Christopher, MD, PhD, from Washington University, St. Louis, and his colleagues.
“These changes appeared to be epigenetic in nature in at least some cases, which suggests that therapeutic strategies to resensitize AML cells to the graft-versus-leukemia effect may be feasible,” they wrote in the New England Journal of Medicine.
The researchers noted that, while the presence of certain AML mutations may predict risk for relapse following HSCT, “the mechanisms by which these mutations promote relapse remain unclear.”
To get a better sense of how genetic and epigenetic changes after transplantation may allow leukemic cells to avoid the graft-versus-leukemia effect – and to see whether immune-related genes are affected by HSCT – they performed enhanced exome sequencing, flow cytometry, and immunohistochemical analyses on samples from 15 patients with AML who had a relapse after receiving transplants from HLA-matched siblings, matched unrelated donors, or HLA-mismatched unrelated donors, and on paired samples from 20 patients who experienced relapses after chemotherapy.
To validate their findings, they also evaluated samples from 28 other patients with AML who had a relapse after transplantation.
They first looked for relapse-specific mutations, but found no driver mutations associated with relapse after transplantation. The mutations seen during relapse after transplantation were generally similar to those seen both before treatment and after relapse in patients who had undergone chemotherapy. The researchers could not identify any patterns of mutations related to relapse.
They then looked for, but did not find, relapse-specific mutations in genes involved in either modulation of immune checkpoints, antigen presentation, or cytokine signaling.
The researchers did, however, find evidence of epigenetic changes that were more common in the samples of patients with posttransplant relapses, compared with postchemotherapy relapses. Specifically, they found that major histocompatibility (MHC) class II genes were down-regulated 200%-1100% after transplant, compared with the pretransplant samples.
In samples from 17 of 34 patients who experienced a relapse after transplantation, both flow cytometry and immunohistochemical analyses confirmed that expression of MHC class II molecules were decreased at relapse.
To see whether this down-regulation was reversible, the researchers treated samples from three patients with posttransplant relapse with interferon gamma, which is known to up-regulate MHC class II protein on myeloid cells and other cell types.
“Culture of these cells with interferon-gamma rapidly induced MHC class II protein expression on leukemic blasts, with essentially full restoration of MHC class II protein expression in nearly all AML blasts after 72 hours,” they wrote, adding that the reversibility of down-regulation of MHC class II in these blasts “strongly suggests that this phenomenon is mediated by an epigenetic mechanism.”
The study was supported by grants to investigators from the National Institutes of Health, Leukemia & Lymphoma Society, and the Barnes-Jewish Hospital Foundation. Dr. Christopher and several coauthors reported receiving grants from the study funders but no other relevant conflicts of interest. Several coauthors reported receiving personal fees and/or research support from industry outside the submitted work.
SOURCE: Christopher MJ et al. N Engl J Med. 2018 Oct 31. doi: 10.1056/NEJMoa1808777.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point:
Major finding: MHC class II genes were down-regulated 200%-1100% after transplant, compared with pretransplant samples.
Study details: Analysis of genetic changes pre- and posttransplant in 15 patients with AML relapse after transplant, 20 patients with relapse after chemotherapy, and 28 patients in a validation sample.
Disclosures: The study was supported by grants to investigators from the National Institutes of Health, Leukemia & Lymphoma Society, and the Barnes-Jewish Hospital Foundation. Dr. Christopher and several coauthors reported receiving grants from the study funders but no other relevant conflicts of interest. Several coauthors reported receiving personal fees and/or research support from industry outside the submitted work.
Source: Christopher MJ et al. N Engl J Med. 2018 Oct 31. doi: 10.1056/NEJMoa1808777.
EVI1 overexpression promotes leukemogenesis, study suggests
Preclinical research suggests the oncoprotein EVI1 can promote leukemogenesis by suppressing erythropoiesis and lymphopoiesis while shifting differentiation toward the expansion of myeloid cells.
Researchers developed a new mouse model that mimics chromosomal rearrangements at 3q26, which are associated with poor-prognosis acute myeloid leukemia (AML), myelodysplastic syndromes, and myeloproliferative neoplasms.
Using the mouse model, the team demonstrated that EVI1 overexpression distorts hematopoiesis and markedly expands premalignant myelopoiesis that eventually results in leukemic transformation.
Archibald Perkins, MD, PhD, of the University of Rochester Medical Center in New York, and his colleagues published these findings in Nature Communications.
The team demonstrated that the “myeloid-skewed phenotype” is dependent upon EVI1-binding DNA. This upregulates Spi1 and encodes the master myeloid regulator PU.1.
When the researchers knocked down Spi1, the myeloid skewing diminished.
“It’s not so pie-in-the-sky anymore,” Dr. Perkins said, “to think we can interrupt the process within the genome that leads to leukemia.”
The researchers first created a mouse model of 3q26 AML with a tetracycline-inducible allele of EVI1 by inserting tetracycline operons within the first exon. This allowed the induction of all three isoforms of EVI1.
These mice were viable and fertile but had no phenotype, which indicated that the allele functioned normally unless induced.
To assess the effect of EVI1 overexpression, the researchers transplanted oncogene-expressing bone marrow mixed 1:1 with wild-type bone marrow into recipient mice.
After confirming successful engraftment, the researchers fed the mice doxycycline-treated food to induce EVI1. The team analyzed cells in the peripheral blood and bone marrow at 10 weeks post-induction.
The researchers observed a more than two-fold expansion of the EVI1-overexpressing compartment in the mouse model.
Suppression of erythropoiesis
The researchers analyzed erythroid lineage in the transplanted mice at 2, 6, and 10 weeks post-induction and found the EVI1-overexpressing cells did not contribute effectively to erythropoiesis.
Using flow cytometry, the researchers quantitated apoptosis and proliferation in erythroid progenitors. They observed a six-fold increase in apoptosis within the erythroblasts compared to wild-type cells.
They also observed a drop in the proliferation of proerythroblasts and erythroblasts compared to wild-type.
Suppression of lymphopoiesis
The researchers observed significantly lower numbers of EVI1-overexpressing B-lineage cells within the bone marrow at 6 and 10 weeks.
And at 10 weeks post-induction, the team observed a decrease in peripheral T cells from approximately 1,800 cells/µL to approximately 750 cells/µL.
EVI1 nearly eliminated the peripheral B cells completely, they noted.
Expansion of myelopoiesis
The team reported that, at 2 weeks post-induction, the EVI1-overexpressing bone marrow and control bone marrow showed the same number of myeloid cells.
But at 6 and 10 weeks post-induction, the EVI1-overexpressing myeloid compartment expanded markedly.
The researchers aged a cohort of five mice transplanted with the 1:1 mix of wild-type and EVI1 bone marrow cells to determine if chronic overexpression of EVI1 results in leukemia.
All five mice died at 90 to 119 days of doxycycline treatment. Analysis revealed AML in all mice. Bone marrows were replete with blasts, and the peripheral blood revealed severe anemia.
The researchers then proceeded to establish the relationship between EVI1 and Spi1/PU.1 transcriptional regulation.
They documented binding of EVI1 to the regulatory element -14kbURE, which, together with EVI1., induced upregulation of PU.1.
When the team knocked down PU.1, myeloid skewing diminished. This, they say, indicates PU.1 is necessary for EVI1-induced myeloid expansion.
Funding for this research was provided by the National Institutes of Health, New York State Stem Cell Science, the Wilmot Cancer Institute, and the Clinical and Translational Science Institute at the University of Rochester.
The authors had no competing interests to disclose.
Preclinical research suggests the oncoprotein EVI1 can promote leukemogenesis by suppressing erythropoiesis and lymphopoiesis while shifting differentiation toward the expansion of myeloid cells.
Researchers developed a new mouse model that mimics chromosomal rearrangements at 3q26, which are associated with poor-prognosis acute myeloid leukemia (AML), myelodysplastic syndromes, and myeloproliferative neoplasms.
Using the mouse model, the team demonstrated that EVI1 overexpression distorts hematopoiesis and markedly expands premalignant myelopoiesis that eventually results in leukemic transformation.
Archibald Perkins, MD, PhD, of the University of Rochester Medical Center in New York, and his colleagues published these findings in Nature Communications.
The team demonstrated that the “myeloid-skewed phenotype” is dependent upon EVI1-binding DNA. This upregulates Spi1 and encodes the master myeloid regulator PU.1.
When the researchers knocked down Spi1, the myeloid skewing diminished.
“It’s not so pie-in-the-sky anymore,” Dr. Perkins said, “to think we can interrupt the process within the genome that leads to leukemia.”
The researchers first created a mouse model of 3q26 AML with a tetracycline-inducible allele of EVI1 by inserting tetracycline operons within the first exon. This allowed the induction of all three isoforms of EVI1.
These mice were viable and fertile but had no phenotype, which indicated that the allele functioned normally unless induced.
To assess the effect of EVI1 overexpression, the researchers transplanted oncogene-expressing bone marrow mixed 1:1 with wild-type bone marrow into recipient mice.
After confirming successful engraftment, the researchers fed the mice doxycycline-treated food to induce EVI1. The team analyzed cells in the peripheral blood and bone marrow at 10 weeks post-induction.
The researchers observed a more than two-fold expansion of the EVI1-overexpressing compartment in the mouse model.
Suppression of erythropoiesis
The researchers analyzed erythroid lineage in the transplanted mice at 2, 6, and 10 weeks post-induction and found the EVI1-overexpressing cells did not contribute effectively to erythropoiesis.
Using flow cytometry, the researchers quantitated apoptosis and proliferation in erythroid progenitors. They observed a six-fold increase in apoptosis within the erythroblasts compared to wild-type cells.
They also observed a drop in the proliferation of proerythroblasts and erythroblasts compared to wild-type.
Suppression of lymphopoiesis
The researchers observed significantly lower numbers of EVI1-overexpressing B-lineage cells within the bone marrow at 6 and 10 weeks.
And at 10 weeks post-induction, the team observed a decrease in peripheral T cells from approximately 1,800 cells/µL to approximately 750 cells/µL.
EVI1 nearly eliminated the peripheral B cells completely, they noted.
Expansion of myelopoiesis
The team reported that, at 2 weeks post-induction, the EVI1-overexpressing bone marrow and control bone marrow showed the same number of myeloid cells.
But at 6 and 10 weeks post-induction, the EVI1-overexpressing myeloid compartment expanded markedly.
The researchers aged a cohort of five mice transplanted with the 1:1 mix of wild-type and EVI1 bone marrow cells to determine if chronic overexpression of EVI1 results in leukemia.
All five mice died at 90 to 119 days of doxycycline treatment. Analysis revealed AML in all mice. Bone marrows were replete with blasts, and the peripheral blood revealed severe anemia.
The researchers then proceeded to establish the relationship between EVI1 and Spi1/PU.1 transcriptional regulation.
They documented binding of EVI1 to the regulatory element -14kbURE, which, together with EVI1., induced upregulation of PU.1.
When the team knocked down PU.1, myeloid skewing diminished. This, they say, indicates PU.1 is necessary for EVI1-induced myeloid expansion.
Funding for this research was provided by the National Institutes of Health, New York State Stem Cell Science, the Wilmot Cancer Institute, and the Clinical and Translational Science Institute at the University of Rochester.
The authors had no competing interests to disclose.
Preclinical research suggests the oncoprotein EVI1 can promote leukemogenesis by suppressing erythropoiesis and lymphopoiesis while shifting differentiation toward the expansion of myeloid cells.
Researchers developed a new mouse model that mimics chromosomal rearrangements at 3q26, which are associated with poor-prognosis acute myeloid leukemia (AML), myelodysplastic syndromes, and myeloproliferative neoplasms.
Using the mouse model, the team demonstrated that EVI1 overexpression distorts hematopoiesis and markedly expands premalignant myelopoiesis that eventually results in leukemic transformation.
Archibald Perkins, MD, PhD, of the University of Rochester Medical Center in New York, and his colleagues published these findings in Nature Communications.
The team demonstrated that the “myeloid-skewed phenotype” is dependent upon EVI1-binding DNA. This upregulates Spi1 and encodes the master myeloid regulator PU.1.
When the researchers knocked down Spi1, the myeloid skewing diminished.
“It’s not so pie-in-the-sky anymore,” Dr. Perkins said, “to think we can interrupt the process within the genome that leads to leukemia.”
The researchers first created a mouse model of 3q26 AML with a tetracycline-inducible allele of EVI1 by inserting tetracycline operons within the first exon. This allowed the induction of all three isoforms of EVI1.
These mice were viable and fertile but had no phenotype, which indicated that the allele functioned normally unless induced.
To assess the effect of EVI1 overexpression, the researchers transplanted oncogene-expressing bone marrow mixed 1:1 with wild-type bone marrow into recipient mice.
After confirming successful engraftment, the researchers fed the mice doxycycline-treated food to induce EVI1. The team analyzed cells in the peripheral blood and bone marrow at 10 weeks post-induction.
The researchers observed a more than two-fold expansion of the EVI1-overexpressing compartment in the mouse model.
Suppression of erythropoiesis
The researchers analyzed erythroid lineage in the transplanted mice at 2, 6, and 10 weeks post-induction and found the EVI1-overexpressing cells did not contribute effectively to erythropoiesis.
Using flow cytometry, the researchers quantitated apoptosis and proliferation in erythroid progenitors. They observed a six-fold increase in apoptosis within the erythroblasts compared to wild-type cells.
They also observed a drop in the proliferation of proerythroblasts and erythroblasts compared to wild-type.
Suppression of lymphopoiesis
The researchers observed significantly lower numbers of EVI1-overexpressing B-lineage cells within the bone marrow at 6 and 10 weeks.
And at 10 weeks post-induction, the team observed a decrease in peripheral T cells from approximately 1,800 cells/µL to approximately 750 cells/µL.
EVI1 nearly eliminated the peripheral B cells completely, they noted.
Expansion of myelopoiesis
The team reported that, at 2 weeks post-induction, the EVI1-overexpressing bone marrow and control bone marrow showed the same number of myeloid cells.
But at 6 and 10 weeks post-induction, the EVI1-overexpressing myeloid compartment expanded markedly.
The researchers aged a cohort of five mice transplanted with the 1:1 mix of wild-type and EVI1 bone marrow cells to determine if chronic overexpression of EVI1 results in leukemia.
All five mice died at 90 to 119 days of doxycycline treatment. Analysis revealed AML in all mice. Bone marrows were replete with blasts, and the peripheral blood revealed severe anemia.
The researchers then proceeded to establish the relationship between EVI1 and Spi1/PU.1 transcriptional regulation.
They documented binding of EVI1 to the regulatory element -14kbURE, which, together with EVI1., induced upregulation of PU.1.
When the team knocked down PU.1, myeloid skewing diminished. This, they say, indicates PU.1 is necessary for EVI1-induced myeloid expansion.
Funding for this research was provided by the National Institutes of Health, New York State Stem Cell Science, the Wilmot Cancer Institute, and the Clinical and Translational Science Institute at the University of Rochester.
The authors had no competing interests to disclose.
Team finds potential therapeutic target for AML
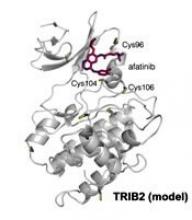
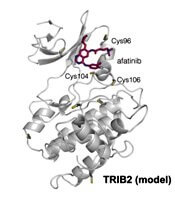
Researchers have found the cancer-associated pseudokinase Tribbles 2 (TRIB2) to be a potential therapeutic target in solid tumors and blood cancers, including acute myeloid leukemia (AML).
Previous research had described TRIB2 as a target of small-molecule protein kinase inhibitors originally designed to interfere with kinase domains of the epidermal growth factor receptor (EGFR) tyrosine kinase family.
Using a thermal shift assay, the team discovered TRIB2-binding compounds within the Published Kinase Inhibitor Set (PKIS). They then employed a biochemical drug repurposing approach to classify compounds that either stabilized or destabilized TRIB2 in vitro.
The researchers found that afatinib, which is already approved by the U.S. Food and Drug Administration to treat non-small cell lung cancer, led to rapid TRIB2 degradation in human AML cells.
Patrick A. Eyers, PhD, of the University of Liverpool in the U.K., and his colleagues published their findings in Science Signaling.
The team found afatinib to be relatively specific for EGFR and human epidermal growth factor receptor 2 (HER2) at nanomolar concentrations in cells.
The researchers confirmed that at least two TRIB2 Cys residues interact with afatinib in vitro.
The team also discovered TRIB2 could be destabilized by neratinib and osimertinib in vitro.
“Our data prove that the cellular mechanism by which TRIB2 stability is regulated by compounds is proteasome-based,” the researchers wrote, “and we speculate that an afatinib-induced conformational change might induce TRIB2 ubiquitination.”
The researchers plan to study further TRIB2 small-molecule interactions with dynamic changes in ubiquitination status.
Furthermore, they report their work demonstrates that covalent inhibitors such as afatinib have TRIB2-degrading activity in human cells at micromolar concentrations.
The researchers determined that afatinib has similar efficacy to the TRIB2-destabilizing quinazoline neratinib at similar ranges.
The team believes their data “raise the intriguing possibility that clinical inhibitors might be used as TRIB2-degrading agents in research, and possibly clinical, contexts.”
“A long-standing goal in cancer research is drug-induced degradation of oncogenic proteins,” Dr. Eyers commented. “Our study highlights how information obtained with ‘off-target’ effects of known drugs is potentially useful because it might be exploited in the future to help eliminate a protein that is involved in a completely different type of cancer.”
The TRIB proteins play many diverse roles in cell signaling, development, and cancer. According to a paper in Developmental Dynamics, they were named after the small, round, fictional organisms from the original Star Trek television series. Their major role was to eat and reproduce.
This work was funded by two U.K. Biotechnology and Biological Sciences Research Council Doctoral Training Partnership studentships, a Tools and Resources Development Fund award, Royal Society Research Grants, North West Cancer Research grants, and funding from the National Institutes of Health.
The authors disclosed no perceived conflicts of interest, although several authors are affiliated with the Structural Genomics Consortium at the University of North Carolina at Chapel Hill, which receives direct funds from various pharmaceutical companies but remains entirely independent.
Researchers have found the cancer-associated pseudokinase Tribbles 2 (TRIB2) to be a potential therapeutic target in solid tumors and blood cancers, including acute myeloid leukemia (AML).
Previous research had described TRIB2 as a target of small-molecule protein kinase inhibitors originally designed to interfere with kinase domains of the epidermal growth factor receptor (EGFR) tyrosine kinase family.
Using a thermal shift assay, the team discovered TRIB2-binding compounds within the Published Kinase Inhibitor Set (PKIS). They then employed a biochemical drug repurposing approach to classify compounds that either stabilized or destabilized TRIB2 in vitro.
The researchers found that afatinib, which is already approved by the U.S. Food and Drug Administration to treat non-small cell lung cancer, led to rapid TRIB2 degradation in human AML cells.
Patrick A. Eyers, PhD, of the University of Liverpool in the U.K., and his colleagues published their findings in Science Signaling.
The team found afatinib to be relatively specific for EGFR and human epidermal growth factor receptor 2 (HER2) at nanomolar concentrations in cells.
The researchers confirmed that at least two TRIB2 Cys residues interact with afatinib in vitro.
The team also discovered TRIB2 could be destabilized by neratinib and osimertinib in vitro.
“Our data prove that the cellular mechanism by which TRIB2 stability is regulated by compounds is proteasome-based,” the researchers wrote, “and we speculate that an afatinib-induced conformational change might induce TRIB2 ubiquitination.”
The researchers plan to study further TRIB2 small-molecule interactions with dynamic changes in ubiquitination status.
Furthermore, they report their work demonstrates that covalent inhibitors such as afatinib have TRIB2-degrading activity in human cells at micromolar concentrations.
The researchers determined that afatinib has similar efficacy to the TRIB2-destabilizing quinazoline neratinib at similar ranges.
The team believes their data “raise the intriguing possibility that clinical inhibitors might be used as TRIB2-degrading agents in research, and possibly clinical, contexts.”
“A long-standing goal in cancer research is drug-induced degradation of oncogenic proteins,” Dr. Eyers commented. “Our study highlights how information obtained with ‘off-target’ effects of known drugs is potentially useful because it might be exploited in the future to help eliminate a protein that is involved in a completely different type of cancer.”
The TRIB proteins play many diverse roles in cell signaling, development, and cancer. According to a paper in Developmental Dynamics, they were named after the small, round, fictional organisms from the original Star Trek television series. Their major role was to eat and reproduce.
This work was funded by two U.K. Biotechnology and Biological Sciences Research Council Doctoral Training Partnership studentships, a Tools and Resources Development Fund award, Royal Society Research Grants, North West Cancer Research grants, and funding from the National Institutes of Health.
The authors disclosed no perceived conflicts of interest, although several authors are affiliated with the Structural Genomics Consortium at the University of North Carolina at Chapel Hill, which receives direct funds from various pharmaceutical companies but remains entirely independent.
Researchers have found the cancer-associated pseudokinase Tribbles 2 (TRIB2) to be a potential therapeutic target in solid tumors and blood cancers, including acute myeloid leukemia (AML).
Previous research had described TRIB2 as a target of small-molecule protein kinase inhibitors originally designed to interfere with kinase domains of the epidermal growth factor receptor (EGFR) tyrosine kinase family.
Using a thermal shift assay, the team discovered TRIB2-binding compounds within the Published Kinase Inhibitor Set (PKIS). They then employed a biochemical drug repurposing approach to classify compounds that either stabilized or destabilized TRIB2 in vitro.
The researchers found that afatinib, which is already approved by the U.S. Food and Drug Administration to treat non-small cell lung cancer, led to rapid TRIB2 degradation in human AML cells.
Patrick A. Eyers, PhD, of the University of Liverpool in the U.K., and his colleagues published their findings in Science Signaling.
The team found afatinib to be relatively specific for EGFR and human epidermal growth factor receptor 2 (HER2) at nanomolar concentrations in cells.
The researchers confirmed that at least two TRIB2 Cys residues interact with afatinib in vitro.
The team also discovered TRIB2 could be destabilized by neratinib and osimertinib in vitro.
“Our data prove that the cellular mechanism by which TRIB2 stability is regulated by compounds is proteasome-based,” the researchers wrote, “and we speculate that an afatinib-induced conformational change might induce TRIB2 ubiquitination.”
The researchers plan to study further TRIB2 small-molecule interactions with dynamic changes in ubiquitination status.
Furthermore, they report their work demonstrates that covalent inhibitors such as afatinib have TRIB2-degrading activity in human cells at micromolar concentrations.
The researchers determined that afatinib has similar efficacy to the TRIB2-destabilizing quinazoline neratinib at similar ranges.
The team believes their data “raise the intriguing possibility that clinical inhibitors might be used as TRIB2-degrading agents in research, and possibly clinical, contexts.”
“A long-standing goal in cancer research is drug-induced degradation of oncogenic proteins,” Dr. Eyers commented. “Our study highlights how information obtained with ‘off-target’ effects of known drugs is potentially useful because it might be exploited in the future to help eliminate a protein that is involved in a completely different type of cancer.”
The TRIB proteins play many diverse roles in cell signaling, development, and cancer. According to a paper in Developmental Dynamics, they were named after the small, round, fictional organisms from the original Star Trek television series. Their major role was to eat and reproduce.
This work was funded by two U.K. Biotechnology and Biological Sciences Research Council Doctoral Training Partnership studentships, a Tools and Resources Development Fund award, Royal Society Research Grants, North West Cancer Research grants, and funding from the National Institutes of Health.
The authors disclosed no perceived conflicts of interest, although several authors are affiliated with the Structural Genomics Consortium at the University of North Carolina at Chapel Hill, which receives direct funds from various pharmaceutical companies but remains entirely independent.
Fusion protein identified as new target in AML
Researchers have identified a promising therapeutic target for t(8;21) acute myeloid leukemia (AML), according to preclinical data published in Cancer Cell.
The fusion protein RUNX1/ETO drives t(8;21) AML by promoting cell-cycle progression.
Using an RNAi screen, the team recognized the cell-cycle regulator cyclin D2 (CCND2) as having critical involvement in RUNX1/ETO-driven leukemia propagation.
And when they knocked down CCND2 with palbociclib, a drug already approved for breast cancer, leukemic expansion of human AML cells and engraftment in murine models were significantly impaired.
"Our discovery that this treatment can be effective in AML is an important step towards a more effective and less toxic treatment for patients with this form of leukemia,” said study author Olaf Heidenreich, PhD, from the Wolfson Childhood Cancer Research Centre at Newcastle University in the U.K.
After identifying the fusion protein with the RNAi screen, the investigators determined that RUNX1/ETO regulates CCND2 transcription. They knocked down the fusion protein and found the expression of CCND2 was diminished in primary AML blasts. They therefore concluded that RUNX1/ETO maintains CCND2 expression.
The team then examined the significance of CCND2 in engraftment, proliferation, and clonal expansion of AML cells and its impact on the accumulation of cells in the G1 phase of the cell cycle. They found that depletion of CCND2 inhibited cell proliferation and clonogenic capacity and arrested the cell cycle in G0/G1 without increasing apoptosis.
They also confirmed that knockdown of RUNX1/ETO or CCND2 did not affect the expression of other D cyclins and G1 cyclin-dependent kinase (CDK)-CCND complexes, such as CDK4/6.
Next, they explored whether RUNX1/ETO-expressing cells were sensitive to the CDK4/6 inhibitor palbociclib. AML cells were highly sensitive to palbociclib and did not proliferate during drug exposure.
The researchers cultured cells from t(8;21)-positive and -negative AML patients and found palbociclib to cause a dose-dependent inhibition of proliferation of AML blasts.
They also tested palbociclib on a sample from a relapsed t(8;21) AML patient. The sample was highly sensitive to palbociclib, with a five-fold reduction in cell numbers using 300 nM of drug.
The investigators conducted in vivo experiments with palbociclib in mice transplanted with AML cells. Mice treated with palbociclib at doses of 100–150 mg/kg had a significantly longer survival than control mice.
Finally, the team examined whether interference with G1 CDK activity would create other vulnerabilities, such as activating KIT mutations, which are frequent secondary mutations found in t(8;21) AML.
They found that G1 CDK inhibition sensitized AML cells toward KIT inhibition, suggesting that “concurrent targeting of the two mutations may offer substantial therapeutic benefit.”
The team plans to conduct experiments that will refine the precise palbociclib dose in AML either as a single agent or in combination.
This study was supported by grants from Bloodwise, Children with Cancer, North of England Children’s Cancer Research Fund, Children's Cancer and Leukaemia Group, and a CRUK program grant in addition to an Aga Khan PhD studentship, a University Sains Malaysia PhD studentship, and an NC3R fellowship.
The authors had no competing interests to disclose.
Researchers have identified a promising therapeutic target for t(8;21) acute myeloid leukemia (AML), according to preclinical data published in Cancer Cell.
The fusion protein RUNX1/ETO drives t(8;21) AML by promoting cell-cycle progression.
Using an RNAi screen, the team recognized the cell-cycle regulator cyclin D2 (CCND2) as having critical involvement in RUNX1/ETO-driven leukemia propagation.
And when they knocked down CCND2 with palbociclib, a drug already approved for breast cancer, leukemic expansion of human AML cells and engraftment in murine models were significantly impaired.
"Our discovery that this treatment can be effective in AML is an important step towards a more effective and less toxic treatment for patients with this form of leukemia,” said study author Olaf Heidenreich, PhD, from the Wolfson Childhood Cancer Research Centre at Newcastle University in the U.K.
After identifying the fusion protein with the RNAi screen, the investigators determined that RUNX1/ETO regulates CCND2 transcription. They knocked down the fusion protein and found the expression of CCND2 was diminished in primary AML blasts. They therefore concluded that RUNX1/ETO maintains CCND2 expression.
The team then examined the significance of CCND2 in engraftment, proliferation, and clonal expansion of AML cells and its impact on the accumulation of cells in the G1 phase of the cell cycle. They found that depletion of CCND2 inhibited cell proliferation and clonogenic capacity and arrested the cell cycle in G0/G1 without increasing apoptosis.
They also confirmed that knockdown of RUNX1/ETO or CCND2 did not affect the expression of other D cyclins and G1 cyclin-dependent kinase (CDK)-CCND complexes, such as CDK4/6.
Next, they explored whether RUNX1/ETO-expressing cells were sensitive to the CDK4/6 inhibitor palbociclib. AML cells were highly sensitive to palbociclib and did not proliferate during drug exposure.
The researchers cultured cells from t(8;21)-positive and -negative AML patients and found palbociclib to cause a dose-dependent inhibition of proliferation of AML blasts.
They also tested palbociclib on a sample from a relapsed t(8;21) AML patient. The sample was highly sensitive to palbociclib, with a five-fold reduction in cell numbers using 300 nM of drug.
The investigators conducted in vivo experiments with palbociclib in mice transplanted with AML cells. Mice treated with palbociclib at doses of 100–150 mg/kg had a significantly longer survival than control mice.
Finally, the team examined whether interference with G1 CDK activity would create other vulnerabilities, such as activating KIT mutations, which are frequent secondary mutations found in t(8;21) AML.
They found that G1 CDK inhibition sensitized AML cells toward KIT inhibition, suggesting that “concurrent targeting of the two mutations may offer substantial therapeutic benefit.”
The team plans to conduct experiments that will refine the precise palbociclib dose in AML either as a single agent or in combination.
This study was supported by grants from Bloodwise, Children with Cancer, North of England Children’s Cancer Research Fund, Children's Cancer and Leukaemia Group, and a CRUK program grant in addition to an Aga Khan PhD studentship, a University Sains Malaysia PhD studentship, and an NC3R fellowship.
The authors had no competing interests to disclose.
Researchers have identified a promising therapeutic target for t(8;21) acute myeloid leukemia (AML), according to preclinical data published in Cancer Cell.
The fusion protein RUNX1/ETO drives t(8;21) AML by promoting cell-cycle progression.
Using an RNAi screen, the team recognized the cell-cycle regulator cyclin D2 (CCND2) as having critical involvement in RUNX1/ETO-driven leukemia propagation.
And when they knocked down CCND2 with palbociclib, a drug already approved for breast cancer, leukemic expansion of human AML cells and engraftment in murine models were significantly impaired.
"Our discovery that this treatment can be effective in AML is an important step towards a more effective and less toxic treatment for patients with this form of leukemia,” said study author Olaf Heidenreich, PhD, from the Wolfson Childhood Cancer Research Centre at Newcastle University in the U.K.
After identifying the fusion protein with the RNAi screen, the investigators determined that RUNX1/ETO regulates CCND2 transcription. They knocked down the fusion protein and found the expression of CCND2 was diminished in primary AML blasts. They therefore concluded that RUNX1/ETO maintains CCND2 expression.
The team then examined the significance of CCND2 in engraftment, proliferation, and clonal expansion of AML cells and its impact on the accumulation of cells in the G1 phase of the cell cycle. They found that depletion of CCND2 inhibited cell proliferation and clonogenic capacity and arrested the cell cycle in G0/G1 without increasing apoptosis.
They also confirmed that knockdown of RUNX1/ETO or CCND2 did not affect the expression of other D cyclins and G1 cyclin-dependent kinase (CDK)-CCND complexes, such as CDK4/6.
Next, they explored whether RUNX1/ETO-expressing cells were sensitive to the CDK4/6 inhibitor palbociclib. AML cells were highly sensitive to palbociclib and did not proliferate during drug exposure.
The researchers cultured cells from t(8;21)-positive and -negative AML patients and found palbociclib to cause a dose-dependent inhibition of proliferation of AML blasts.
They also tested palbociclib on a sample from a relapsed t(8;21) AML patient. The sample was highly sensitive to palbociclib, with a five-fold reduction in cell numbers using 300 nM of drug.
The investigators conducted in vivo experiments with palbociclib in mice transplanted with AML cells. Mice treated with palbociclib at doses of 100–150 mg/kg had a significantly longer survival than control mice.
Finally, the team examined whether interference with G1 CDK activity would create other vulnerabilities, such as activating KIT mutations, which are frequent secondary mutations found in t(8;21) AML.
They found that G1 CDK inhibition sensitized AML cells toward KIT inhibition, suggesting that “concurrent targeting of the two mutations may offer substantial therapeutic benefit.”
The team plans to conduct experiments that will refine the precise palbociclib dose in AML either as a single agent or in combination.
This study was supported by grants from Bloodwise, Children with Cancer, North of England Children’s Cancer Research Fund, Children's Cancer and Leukaemia Group, and a CRUK program grant in addition to an Aga Khan PhD studentship, a University Sains Malaysia PhD studentship, and an NC3R fellowship.
The authors had no competing interests to disclose.
MDM2 inhibitors could treat resistant AML
Preclinical research has revealed a potential treatment for chemotherapy-resistant acute myeloid leukemia (AML).
Researchers characterized a mechanism of chemotherapy resistance in AML and found that MDM2 is a key player in this dysregulated signaling pathway.
They tested MDM2 inhibitors and found these drugs could sensitize resistant AML to chemotherapy in vitro and in vivo.
In fact, mice with refractory AML responded to standard induction therapy when combined with an MDM2 inhibitor, showing no signs of disease and prolonged survival.
These results were published in Cancer Discovery.
“We were blown away when we saw the results,” said study author William Stanford, PhD, of Ottawa Hospital Research Institute in Ontario, Canada.
“If these findings hold up in clinical trials, we could have a new treatment for people who would almost certainly die of their disease today.”
Mechanism of resistance
Dr. Stanford’s research began with the protein MTF2. He and his colleagues previously found that MTF2 plays a role in erythropoiesis, and the team wanted to determine if MTF2 also plays a role in AML.
Using AML samples from patients treated at The Ottawa Hospital, the researchers found the mean survival was three times longer in patients with normal MTF2 activity than in patients with low MTF2 activity.
“Initially, we thought that MTF2 could be an important biomarker to identify patients who might benefit from experimental therapies,” Dr. Stanford said. “But then we started thinking that if we could understand what MTF2 was doing, maybe we could use this information to develop a new treatment.”
Dr. Stanford and his colleagues discovered that MTF2 represses MDM2, a protein that helps cells resist chemotherapy.
The team found that MTF2-deficient cells overexpress MDM2, which inhibits p53, and this leads to defects in cell-cycle regulation and apoptosis that enable resistance to chemotherapy.
Testing MDM2 inhibitors
Since MDM2 inhibitors are already being tested in clinical trials for other cancers, Dr. Stanford and his colleagues tested these inhibitors in vitro and in mouse models of chemotherapy-resistant AML.
The in vitro experiments included two MDM2 inhibitors—Nutlin3A and MI-773—combined with daunorubicin or cytarabine.
The researchers found that refractory, MTF2-deficient AML cells underwent apoptosis when treated with either daunorubicin or cytarabine in combination with Nutlin3A or MI-773. The effect was comparable to that observed in AML cells with normal MTF2.
The team found that Nutlin3A was more efficient at sensitizing refractory, MTF2-deficient AML cells to daunorubicin, so they used Nutlin3A in the in vivo experiments.
For these experiments, the researchers tested Nutlin3A in mice injected with either chemotherapy-responsive AML cells (with normal MTF2) or refractory, MTF2-deficient AML cells.
Once the mice had “a substantial leukemic burden” (≥ 20% CD45+CD33+ leukemic blasts in their peripheral blood), they were randomized to receive vehicle control, Nutlin3A, standard induction therapy, or induction plus Nutlin3A.
The mice engrafted with chemotherapy-responsive AML cells did not respond to vehicle control or Nutlin3A alone. However, they did respond to standard induction and induction plus Nutlin3A, surviving until the end of the experiment at 16 weeks.
Among the mice engrafted with refractory, MTF2-deficient AML cells, only those animals treated with induction plus Nutlin3A survived until the end of the experiment.
The researchers also noted a “dramatic loss” in the blast-containing CD45+CD33+ and CD34+CD38− populations in mice treated with induction plus Nutlin3A.
To assess residual disease, the researchers performed secondary transplants with cells from mice that had engrafted with refractory, MTF2-deficient AML cells but responded to induction plus Nutlin3A.
The recipient mice had no evidence of AML at 16 weeks after transplant when the experiment ended.
Dr. Stanford and his colleagues are now trying to obtain pharmaceutical-grade MDM2 inhibitors to conduct trials in AML patients at The Ottawa Hospital.
The researchers are also screening libraries of approved drugs to see if any of these can block MDM2, and they are working with a biotech company to develop a test to identify chemotherapy-resistant AML patients who would respond to MDM2 inhibitors.
The current research was supported by grants from the Canadian Cancer Society Research Institute, Canadian Institutes of Health Research, Cancer Research Society, National Institutes of Health, and a Tier 1 Canada Research Chair in Integrative Stem Cell Biology. One study author reported a relationship with Epicypher, Inc. No other conflicts of interest were reported.
Preclinical research has revealed a potential treatment for chemotherapy-resistant acute myeloid leukemia (AML).
Researchers characterized a mechanism of chemotherapy resistance in AML and found that MDM2 is a key player in this dysregulated signaling pathway.
They tested MDM2 inhibitors and found these drugs could sensitize resistant AML to chemotherapy in vitro and in vivo.
In fact, mice with refractory AML responded to standard induction therapy when combined with an MDM2 inhibitor, showing no signs of disease and prolonged survival.
These results were published in Cancer Discovery.
“We were blown away when we saw the results,” said study author William Stanford, PhD, of Ottawa Hospital Research Institute in Ontario, Canada.
“If these findings hold up in clinical trials, we could have a new treatment for people who would almost certainly die of their disease today.”
Mechanism of resistance
Dr. Stanford’s research began with the protein MTF2. He and his colleagues previously found that MTF2 plays a role in erythropoiesis, and the team wanted to determine if MTF2 also plays a role in AML.
Using AML samples from patients treated at The Ottawa Hospital, the researchers found the mean survival was three times longer in patients with normal MTF2 activity than in patients with low MTF2 activity.
“Initially, we thought that MTF2 could be an important biomarker to identify patients who might benefit from experimental therapies,” Dr. Stanford said. “But then we started thinking that if we could understand what MTF2 was doing, maybe we could use this information to develop a new treatment.”
Dr. Stanford and his colleagues discovered that MTF2 represses MDM2, a protein that helps cells resist chemotherapy.
The team found that MTF2-deficient cells overexpress MDM2, which inhibits p53, and this leads to defects in cell-cycle regulation and apoptosis that enable resistance to chemotherapy.
Testing MDM2 inhibitors
Since MDM2 inhibitors are already being tested in clinical trials for other cancers, Dr. Stanford and his colleagues tested these inhibitors in vitro and in mouse models of chemotherapy-resistant AML.
The in vitro experiments included two MDM2 inhibitors—Nutlin3A and MI-773—combined with daunorubicin or cytarabine.
The researchers found that refractory, MTF2-deficient AML cells underwent apoptosis when treated with either daunorubicin or cytarabine in combination with Nutlin3A or MI-773. The effect was comparable to that observed in AML cells with normal MTF2.
The team found that Nutlin3A was more efficient at sensitizing refractory, MTF2-deficient AML cells to daunorubicin, so they used Nutlin3A in the in vivo experiments.
For these experiments, the researchers tested Nutlin3A in mice injected with either chemotherapy-responsive AML cells (with normal MTF2) or refractory, MTF2-deficient AML cells.
Once the mice had “a substantial leukemic burden” (≥ 20% CD45+CD33+ leukemic blasts in their peripheral blood), they were randomized to receive vehicle control, Nutlin3A, standard induction therapy, or induction plus Nutlin3A.
The mice engrafted with chemotherapy-responsive AML cells did not respond to vehicle control or Nutlin3A alone. However, they did respond to standard induction and induction plus Nutlin3A, surviving until the end of the experiment at 16 weeks.
Among the mice engrafted with refractory, MTF2-deficient AML cells, only those animals treated with induction plus Nutlin3A survived until the end of the experiment.
The researchers also noted a “dramatic loss” in the blast-containing CD45+CD33+ and CD34+CD38− populations in mice treated with induction plus Nutlin3A.
To assess residual disease, the researchers performed secondary transplants with cells from mice that had engrafted with refractory, MTF2-deficient AML cells but responded to induction plus Nutlin3A.
The recipient mice had no evidence of AML at 16 weeks after transplant when the experiment ended.
Dr. Stanford and his colleagues are now trying to obtain pharmaceutical-grade MDM2 inhibitors to conduct trials in AML patients at The Ottawa Hospital.
The researchers are also screening libraries of approved drugs to see if any of these can block MDM2, and they are working with a biotech company to develop a test to identify chemotherapy-resistant AML patients who would respond to MDM2 inhibitors.
The current research was supported by grants from the Canadian Cancer Society Research Institute, Canadian Institutes of Health Research, Cancer Research Society, National Institutes of Health, and a Tier 1 Canada Research Chair in Integrative Stem Cell Biology. One study author reported a relationship with Epicypher, Inc. No other conflicts of interest were reported.
Preclinical research has revealed a potential treatment for chemotherapy-resistant acute myeloid leukemia (AML).
Researchers characterized a mechanism of chemotherapy resistance in AML and found that MDM2 is a key player in this dysregulated signaling pathway.
They tested MDM2 inhibitors and found these drugs could sensitize resistant AML to chemotherapy in vitro and in vivo.
In fact, mice with refractory AML responded to standard induction therapy when combined with an MDM2 inhibitor, showing no signs of disease and prolonged survival.
These results were published in Cancer Discovery.
“We were blown away when we saw the results,” said study author William Stanford, PhD, of Ottawa Hospital Research Institute in Ontario, Canada.
“If these findings hold up in clinical trials, we could have a new treatment for people who would almost certainly die of their disease today.”
Mechanism of resistance
Dr. Stanford’s research began with the protein MTF2. He and his colleagues previously found that MTF2 plays a role in erythropoiesis, and the team wanted to determine if MTF2 also plays a role in AML.
Using AML samples from patients treated at The Ottawa Hospital, the researchers found the mean survival was three times longer in patients with normal MTF2 activity than in patients with low MTF2 activity.
“Initially, we thought that MTF2 could be an important biomarker to identify patients who might benefit from experimental therapies,” Dr. Stanford said. “But then we started thinking that if we could understand what MTF2 was doing, maybe we could use this information to develop a new treatment.”
Dr. Stanford and his colleagues discovered that MTF2 represses MDM2, a protein that helps cells resist chemotherapy.
The team found that MTF2-deficient cells overexpress MDM2, which inhibits p53, and this leads to defects in cell-cycle regulation and apoptosis that enable resistance to chemotherapy.
Testing MDM2 inhibitors
Since MDM2 inhibitors are already being tested in clinical trials for other cancers, Dr. Stanford and his colleagues tested these inhibitors in vitro and in mouse models of chemotherapy-resistant AML.
The in vitro experiments included two MDM2 inhibitors—Nutlin3A and MI-773—combined with daunorubicin or cytarabine.
The researchers found that refractory, MTF2-deficient AML cells underwent apoptosis when treated with either daunorubicin or cytarabine in combination with Nutlin3A or MI-773. The effect was comparable to that observed in AML cells with normal MTF2.
The team found that Nutlin3A was more efficient at sensitizing refractory, MTF2-deficient AML cells to daunorubicin, so they used Nutlin3A in the in vivo experiments.
For these experiments, the researchers tested Nutlin3A in mice injected with either chemotherapy-responsive AML cells (with normal MTF2) or refractory, MTF2-deficient AML cells.
Once the mice had “a substantial leukemic burden” (≥ 20% CD45+CD33+ leukemic blasts in their peripheral blood), they were randomized to receive vehicle control, Nutlin3A, standard induction therapy, or induction plus Nutlin3A.
The mice engrafted with chemotherapy-responsive AML cells did not respond to vehicle control or Nutlin3A alone. However, they did respond to standard induction and induction plus Nutlin3A, surviving until the end of the experiment at 16 weeks.
Among the mice engrafted with refractory, MTF2-deficient AML cells, only those animals treated with induction plus Nutlin3A survived until the end of the experiment.
The researchers also noted a “dramatic loss” in the blast-containing CD45+CD33+ and CD34+CD38− populations in mice treated with induction plus Nutlin3A.
To assess residual disease, the researchers performed secondary transplants with cells from mice that had engrafted with refractory, MTF2-deficient AML cells but responded to induction plus Nutlin3A.
The recipient mice had no evidence of AML at 16 weeks after transplant when the experiment ended.
Dr. Stanford and his colleagues are now trying to obtain pharmaceutical-grade MDM2 inhibitors to conduct trials in AML patients at The Ottawa Hospital.
The researchers are also screening libraries of approved drugs to see if any of these can block MDM2, and they are working with a biotech company to develop a test to identify chemotherapy-resistant AML patients who would respond to MDM2 inhibitors.
The current research was supported by grants from the Canadian Cancer Society Research Institute, Canadian Institutes of Health Research, Cancer Research Society, National Institutes of Health, and a Tier 1 Canada Research Chair in Integrative Stem Cell Biology. One study author reported a relationship with Epicypher, Inc. No other conflicts of interest were reported.
CDK8 inhibitor shows activity against AML
DUBROVNIK, CROATIA – The (AML), but the agent’s mechanism of action is still unclear.
Researchers found that several AML cell lines were “highly sensitive” to SEL120, and the inhibitor was active in primary patient samples. SEL120 also reduced tumor growth in mouse models of AML and demonstrated synergy with venetoclax.
The researchers suggest that SEL120 works by affecting the maintenance of AML cells and leukemic stem cells (LSCs), inducing differentiation and, sometimes, apoptosis. However, the mechanism is not well defined.
Eliza Majewska, PhD, of Selvita S.A. in Krakow, Poland, discussed research on SEL120 at Leukemia and Lymphoma, a meeting jointly sponsored by the University of Texas MD Anderson Cancer Center and the School of Medicine at the University of Zagreb, Croatia.
Dr. Majewska explained that CDK8 is a transcriptional kinase working in the context of the Mediator complex, and previous research indicated that CDK8 drives oncogenic transcription in AML (Nature. 2015 Oct 8;526[7572]:273-6).
In a prior study, researchers found that SEL120 inhibits CDK8 activity in AML cells with high levels of STAT phosphorylation (Oncotarget. 2017 May 16;8[20]:33779-95).
Dr. Majewska said the MV4-11 cell line responds particularly well to SEL120, and other sensitive cell lines include SKNO-1, Oci-AML5, GDM-1, KG-1, MOLM-16, and Oci-AML3.
“The fact that STAT signaling was upregulated in those cell lines that were very sensitive to SEL120 gave us the hint that perhaps we are looking at a mechanism of action of the compound that has something to do with leukemic stem cells,” Dr. Majewska said.
In fact, she and her colleagues found that cell lines sensitive to SEL120 had upregulation of genes linked to LSCs and high levels of CD34 surface expression.
Experiments in CD34+ TEX cells showed that SEL120 specifically depletes CD34+ cells, leads to downregulation of stemness-related genes, and induces myeloid differentiation.
After 6 days of treatment with SEL120, TEX cells showed decreased expression of the LSC-linked genes MEIS1 and LILRB2, enrichment of gene sets downregulated in LSCs and linked to differentiation, and increased expression of differentiation markers and immune response genes.
SEL120 also demonstrated antileukemic activity in vivo. The researchers tested SEL120 in a CD34+ model of AML (KG-1) and a FLT3-ITD model of AML (MV4-11).
In both models, SEL120 induced “significant tumor regression” of about 80%. In some cases, the researchers observed apoptosis.
Toxicities observed in the mice included weight loss and upregulation of inflammation.
The researchers also found that SEL120 was synergistic with venetoclax. In fact, the combination of these drugs resulted in “almost complete remission cures” in the MV4-11 model, according to Dr. Majewska.
Finally, she and her colleagues discovered that SEL120 was active against primary patient cells. Samples from three of four patients had a significant reduction in cell numbers after 7 days of treatment with SEL120. For one patient, there were no viable cells on day 7.
Dr. Majewska said a phase 1 trial of SEL120 is planned for 2019 or 2020, and SEL120’s mechanism of action is still under investigation.
“The mechanism of action ... is, in our mind – at least in some cases – linked to the fact that CDK8 functions within the context of the Mediator complex, which contributes to gene expression related to leukemic stem cells,” Dr. Majewska said.
“And when we inhibit this specific transcription, of course, the Mediator complex still works because this is just one of the components of the complex. However, the function that it has is suddenly very different, and it’s actually linked to lack of maintenance of leukemic stem cells, resulting in differentiation [and], in some cases, the induction of apoptosis, but we do not fully understand the mechanism of this induction.”
Dr. Majewska works for Selvita, the company developing SEL120. This research was funded by Selvita, the Leukemia & Lymphoma Society, and the National Centre for Research and Development.
The Leukemia and Lymphoma meeting is organized by Jonathan Wood & Association, which is owned by the parent company of this news organization.
DUBROVNIK, CROATIA – The (AML), but the agent’s mechanism of action is still unclear.
Researchers found that several AML cell lines were “highly sensitive” to SEL120, and the inhibitor was active in primary patient samples. SEL120 also reduced tumor growth in mouse models of AML and demonstrated synergy with venetoclax.
The researchers suggest that SEL120 works by affecting the maintenance of AML cells and leukemic stem cells (LSCs), inducing differentiation and, sometimes, apoptosis. However, the mechanism is not well defined.
Eliza Majewska, PhD, of Selvita S.A. in Krakow, Poland, discussed research on SEL120 at Leukemia and Lymphoma, a meeting jointly sponsored by the University of Texas MD Anderson Cancer Center and the School of Medicine at the University of Zagreb, Croatia.
Dr. Majewska explained that CDK8 is a transcriptional kinase working in the context of the Mediator complex, and previous research indicated that CDK8 drives oncogenic transcription in AML (Nature. 2015 Oct 8;526[7572]:273-6).
In a prior study, researchers found that SEL120 inhibits CDK8 activity in AML cells with high levels of STAT phosphorylation (Oncotarget. 2017 May 16;8[20]:33779-95).
Dr. Majewska said the MV4-11 cell line responds particularly well to SEL120, and other sensitive cell lines include SKNO-1, Oci-AML5, GDM-1, KG-1, MOLM-16, and Oci-AML3.
“The fact that STAT signaling was upregulated in those cell lines that were very sensitive to SEL120 gave us the hint that perhaps we are looking at a mechanism of action of the compound that has something to do with leukemic stem cells,” Dr. Majewska said.
In fact, she and her colleagues found that cell lines sensitive to SEL120 had upregulation of genes linked to LSCs and high levels of CD34 surface expression.
Experiments in CD34+ TEX cells showed that SEL120 specifically depletes CD34+ cells, leads to downregulation of stemness-related genes, and induces myeloid differentiation.
After 6 days of treatment with SEL120, TEX cells showed decreased expression of the LSC-linked genes MEIS1 and LILRB2, enrichment of gene sets downregulated in LSCs and linked to differentiation, and increased expression of differentiation markers and immune response genes.
SEL120 also demonstrated antileukemic activity in vivo. The researchers tested SEL120 in a CD34+ model of AML (KG-1) and a FLT3-ITD model of AML (MV4-11).
In both models, SEL120 induced “significant tumor regression” of about 80%. In some cases, the researchers observed apoptosis.
Toxicities observed in the mice included weight loss and upregulation of inflammation.
The researchers also found that SEL120 was synergistic with venetoclax. In fact, the combination of these drugs resulted in “almost complete remission cures” in the MV4-11 model, according to Dr. Majewska.
Finally, she and her colleagues discovered that SEL120 was active against primary patient cells. Samples from three of four patients had a significant reduction in cell numbers after 7 days of treatment with SEL120. For one patient, there were no viable cells on day 7.
Dr. Majewska said a phase 1 trial of SEL120 is planned for 2019 or 2020, and SEL120’s mechanism of action is still under investigation.
“The mechanism of action ... is, in our mind – at least in some cases – linked to the fact that CDK8 functions within the context of the Mediator complex, which contributes to gene expression related to leukemic stem cells,” Dr. Majewska said.
“And when we inhibit this specific transcription, of course, the Mediator complex still works because this is just one of the components of the complex. However, the function that it has is suddenly very different, and it’s actually linked to lack of maintenance of leukemic stem cells, resulting in differentiation [and], in some cases, the induction of apoptosis, but we do not fully understand the mechanism of this induction.”
Dr. Majewska works for Selvita, the company developing SEL120. This research was funded by Selvita, the Leukemia & Lymphoma Society, and the National Centre for Research and Development.
The Leukemia and Lymphoma meeting is organized by Jonathan Wood & Association, which is owned by the parent company of this news organization.
DUBROVNIK, CROATIA – The (AML), but the agent’s mechanism of action is still unclear.
Researchers found that several AML cell lines were “highly sensitive” to SEL120, and the inhibitor was active in primary patient samples. SEL120 also reduced tumor growth in mouse models of AML and demonstrated synergy with venetoclax.
The researchers suggest that SEL120 works by affecting the maintenance of AML cells and leukemic stem cells (LSCs), inducing differentiation and, sometimes, apoptosis. However, the mechanism is not well defined.
Eliza Majewska, PhD, of Selvita S.A. in Krakow, Poland, discussed research on SEL120 at Leukemia and Lymphoma, a meeting jointly sponsored by the University of Texas MD Anderson Cancer Center and the School of Medicine at the University of Zagreb, Croatia.
Dr. Majewska explained that CDK8 is a transcriptional kinase working in the context of the Mediator complex, and previous research indicated that CDK8 drives oncogenic transcription in AML (Nature. 2015 Oct 8;526[7572]:273-6).
In a prior study, researchers found that SEL120 inhibits CDK8 activity in AML cells with high levels of STAT phosphorylation (Oncotarget. 2017 May 16;8[20]:33779-95).
Dr. Majewska said the MV4-11 cell line responds particularly well to SEL120, and other sensitive cell lines include SKNO-1, Oci-AML5, GDM-1, KG-1, MOLM-16, and Oci-AML3.
“The fact that STAT signaling was upregulated in those cell lines that were very sensitive to SEL120 gave us the hint that perhaps we are looking at a mechanism of action of the compound that has something to do with leukemic stem cells,” Dr. Majewska said.
In fact, she and her colleagues found that cell lines sensitive to SEL120 had upregulation of genes linked to LSCs and high levels of CD34 surface expression.
Experiments in CD34+ TEX cells showed that SEL120 specifically depletes CD34+ cells, leads to downregulation of stemness-related genes, and induces myeloid differentiation.
After 6 days of treatment with SEL120, TEX cells showed decreased expression of the LSC-linked genes MEIS1 and LILRB2, enrichment of gene sets downregulated in LSCs and linked to differentiation, and increased expression of differentiation markers and immune response genes.
SEL120 also demonstrated antileukemic activity in vivo. The researchers tested SEL120 in a CD34+ model of AML (KG-1) and a FLT3-ITD model of AML (MV4-11).
In both models, SEL120 induced “significant tumor regression” of about 80%. In some cases, the researchers observed apoptosis.
Toxicities observed in the mice included weight loss and upregulation of inflammation.
The researchers also found that SEL120 was synergistic with venetoclax. In fact, the combination of these drugs resulted in “almost complete remission cures” in the MV4-11 model, according to Dr. Majewska.
Finally, she and her colleagues discovered that SEL120 was active against primary patient cells. Samples from three of four patients had a significant reduction in cell numbers after 7 days of treatment with SEL120. For one patient, there were no viable cells on day 7.
Dr. Majewska said a phase 1 trial of SEL120 is planned for 2019 or 2020, and SEL120’s mechanism of action is still under investigation.
“The mechanism of action ... is, in our mind – at least in some cases – linked to the fact that CDK8 functions within the context of the Mediator complex, which contributes to gene expression related to leukemic stem cells,” Dr. Majewska said.
“And when we inhibit this specific transcription, of course, the Mediator complex still works because this is just one of the components of the complex. However, the function that it has is suddenly very different, and it’s actually linked to lack of maintenance of leukemic stem cells, resulting in differentiation [and], in some cases, the induction of apoptosis, but we do not fully understand the mechanism of this induction.”
Dr. Majewska works for Selvita, the company developing SEL120. This research was funded by Selvita, the Leukemia & Lymphoma Society, and the National Centre for Research and Development.
The Leukemia and Lymphoma meeting is organized by Jonathan Wood & Association, which is owned by the parent company of this news organization.
EXPERT ANALYSIS FROM LEUKEMIA AND LYMPHOMA 2018
‘Intense’ end-of-life care may be common in HSCT recipients
Patients who die within a year of allogeneic hematopoietic stem cell transplant (HSCT) tend to receive “medically intense” end-of-life care, an analysis suggests.
Researchers studied more than 2,000 patients who died within a year of allogeneic HSCT and found that a majority of the patients died in the hospital, and about half of them were admitted to the intensive care unit (ICU).
However, patient age, underlying diagnosis, and other factors influenced the likelihood of receiving intense end-of-life care.
For example, patients diagnosed with acute myeloid leukemia (AML) or myelodysplastic syndromes (MDS) were less likely than patients with acute lymphoblastic leukemia (ALL) to receive medically intense care.
Emily Johnston, MD, of the University of Alabama at Birmingham, and her colleagues reported these findings in the Journal of Clinical Oncology.
The researchers studied 2,135 patients in California who underwent inpatient HSCT and died within a year of the transplant (not as a result of peripartum events or trauma) between 2000 and 2013.
Fifty-three percent of the patients received some type of medically intense intervention, and 57% had at least two types of intense interventions.
Eighty-three percent of patients died in hospital, and 43% spent all of their last 30 days in the hospital.
Forty-nine percent of patients were admitted to the ICU, 45% were intubated, 22% underwent hemodialysis, and 8% received cardiopulmonary resuscitation.
Factors associated with intense care
The researchers said receipt of a medically intense intervention varied by age at death, underlying diagnosis, year of HSCT, location of care, and comorbidities. However, use of intense interventions did not vary according to sex, race/ethnicity, insurance type, or income.
Compared to patients age 60 and older, patients in the following age groups were more likely to receive medically intense interventions:
- Ages 15 to 21—odds ratio (OR)=2.6 (P<0.001)
- Ages 30 to 39—OR=1.8 (P<0.01)
- Ages 40 to 49—OR=1.4 (P<0.05).
Patients with comorbidities were more likely to receive intense interventions as well. The OR was 1.6 (P<0.01) for patients with one comorbidity and 2.5 (P<0.001) for patients with two or more comorbidities.
Patients with AML or MDS were less likely than patients with ALL to receive a medically intense intervention—OR=0.7 (P<0.05).
Patients who were transplanted between 2000 and 2004 were less likely to receive an intense intervention than patients transplanted between 2010 and 2013—OR=0.7 (P<0.01).
Patients who changed hospitals between HSCT and death were less likely to receive an intense intervention than patients who stayed at the same hospital. The OR was 0.3 if they transferred to a community hospital and 0.4 if they transferred to a specialty hospital (P<0.001 for both).
Patients living in rural areas were less likely than urban patients to receive a medically intense intervention—OR=0.6 (P<0.05).
“From our data, we understand there is a correlation with high-intensity end-of-life care in patients who die within one year after receiving a stem cell transplant, but we are still unsure if that was the care they wanted,” Dr. Johnston said.
“The findings suggest that, as oncologists, we need to start having end-of-life care conversations earlier with patients to determine if a high-intensity treatment plan is consistent with their goals or if a lower-intensity treatment plan is best. It’s not a one-size-fits-all approach in end-of-life care.”
This research was supported by Stanford University. One study author reported relationships with Corvus Pharmaceuticals, Shire Pharmaceuticals, and Adaptive Biotechnologies. All other authors reported no conflicts.
Patients who die within a year of allogeneic hematopoietic stem cell transplant (HSCT) tend to receive “medically intense” end-of-life care, an analysis suggests.
Researchers studied more than 2,000 patients who died within a year of allogeneic HSCT and found that a majority of the patients died in the hospital, and about half of them were admitted to the intensive care unit (ICU).
However, patient age, underlying diagnosis, and other factors influenced the likelihood of receiving intense end-of-life care.
For example, patients diagnosed with acute myeloid leukemia (AML) or myelodysplastic syndromes (MDS) were less likely than patients with acute lymphoblastic leukemia (ALL) to receive medically intense care.
Emily Johnston, MD, of the University of Alabama at Birmingham, and her colleagues reported these findings in the Journal of Clinical Oncology.
The researchers studied 2,135 patients in California who underwent inpatient HSCT and died within a year of the transplant (not as a result of peripartum events or trauma) between 2000 and 2013.
Fifty-three percent of the patients received some type of medically intense intervention, and 57% had at least two types of intense interventions.
Eighty-three percent of patients died in hospital, and 43% spent all of their last 30 days in the hospital.
Forty-nine percent of patients were admitted to the ICU, 45% were intubated, 22% underwent hemodialysis, and 8% received cardiopulmonary resuscitation.
Factors associated with intense care
The researchers said receipt of a medically intense intervention varied by age at death, underlying diagnosis, year of HSCT, location of care, and comorbidities. However, use of intense interventions did not vary according to sex, race/ethnicity, insurance type, or income.
Compared to patients age 60 and older, patients in the following age groups were more likely to receive medically intense interventions:
- Ages 15 to 21—odds ratio (OR)=2.6 (P<0.001)
- Ages 30 to 39—OR=1.8 (P<0.01)
- Ages 40 to 49—OR=1.4 (P<0.05).
Patients with comorbidities were more likely to receive intense interventions as well. The OR was 1.6 (P<0.01) for patients with one comorbidity and 2.5 (P<0.001) for patients with two or more comorbidities.
Patients with AML or MDS were less likely than patients with ALL to receive a medically intense intervention—OR=0.7 (P<0.05).
Patients who were transplanted between 2000 and 2004 were less likely to receive an intense intervention than patients transplanted between 2010 and 2013—OR=0.7 (P<0.01).
Patients who changed hospitals between HSCT and death were less likely to receive an intense intervention than patients who stayed at the same hospital. The OR was 0.3 if they transferred to a community hospital and 0.4 if they transferred to a specialty hospital (P<0.001 for both).
Patients living in rural areas were less likely than urban patients to receive a medically intense intervention—OR=0.6 (P<0.05).
“From our data, we understand there is a correlation with high-intensity end-of-life care in patients who die within one year after receiving a stem cell transplant, but we are still unsure if that was the care they wanted,” Dr. Johnston said.
“The findings suggest that, as oncologists, we need to start having end-of-life care conversations earlier with patients to determine if a high-intensity treatment plan is consistent with their goals or if a lower-intensity treatment plan is best. It’s not a one-size-fits-all approach in end-of-life care.”
This research was supported by Stanford University. One study author reported relationships with Corvus Pharmaceuticals, Shire Pharmaceuticals, and Adaptive Biotechnologies. All other authors reported no conflicts.
Patients who die within a year of allogeneic hematopoietic stem cell transplant (HSCT) tend to receive “medically intense” end-of-life care, an analysis suggests.
Researchers studied more than 2,000 patients who died within a year of allogeneic HSCT and found that a majority of the patients died in the hospital, and about half of them were admitted to the intensive care unit (ICU).
However, patient age, underlying diagnosis, and other factors influenced the likelihood of receiving intense end-of-life care.
For example, patients diagnosed with acute myeloid leukemia (AML) or myelodysplastic syndromes (MDS) were less likely than patients with acute lymphoblastic leukemia (ALL) to receive medically intense care.
Emily Johnston, MD, of the University of Alabama at Birmingham, and her colleagues reported these findings in the Journal of Clinical Oncology.
The researchers studied 2,135 patients in California who underwent inpatient HSCT and died within a year of the transplant (not as a result of peripartum events or trauma) between 2000 and 2013.
Fifty-three percent of the patients received some type of medically intense intervention, and 57% had at least two types of intense interventions.
Eighty-three percent of patients died in hospital, and 43% spent all of their last 30 days in the hospital.
Forty-nine percent of patients were admitted to the ICU, 45% were intubated, 22% underwent hemodialysis, and 8% received cardiopulmonary resuscitation.
Factors associated with intense care
The researchers said receipt of a medically intense intervention varied by age at death, underlying diagnosis, year of HSCT, location of care, and comorbidities. However, use of intense interventions did not vary according to sex, race/ethnicity, insurance type, or income.
Compared to patients age 60 and older, patients in the following age groups were more likely to receive medically intense interventions:
- Ages 15 to 21—odds ratio (OR)=2.6 (P<0.001)
- Ages 30 to 39—OR=1.8 (P<0.01)
- Ages 40 to 49—OR=1.4 (P<0.05).
Patients with comorbidities were more likely to receive intense interventions as well. The OR was 1.6 (P<0.01) for patients with one comorbidity and 2.5 (P<0.001) for patients with two or more comorbidities.
Patients with AML or MDS were less likely than patients with ALL to receive a medically intense intervention—OR=0.7 (P<0.05).
Patients who were transplanted between 2000 and 2004 were less likely to receive an intense intervention than patients transplanted between 2010 and 2013—OR=0.7 (P<0.01).
Patients who changed hospitals between HSCT and death were less likely to receive an intense intervention than patients who stayed at the same hospital. The OR was 0.3 if they transferred to a community hospital and 0.4 if they transferred to a specialty hospital (P<0.001 for both).
Patients living in rural areas were less likely than urban patients to receive a medically intense intervention—OR=0.6 (P<0.05).
“From our data, we understand there is a correlation with high-intensity end-of-life care in patients who die within one year after receiving a stem cell transplant, but we are still unsure if that was the care they wanted,” Dr. Johnston said.
“The findings suggest that, as oncologists, we need to start having end-of-life care conversations earlier with patients to determine if a high-intensity treatment plan is consistent with their goals or if a lower-intensity treatment plan is best. It’s not a one-size-fits-all approach in end-of-life care.”
This research was supported by Stanford University. One study author reported relationships with Corvus Pharmaceuticals, Shire Pharmaceuticals, and Adaptive Biotechnologies. All other authors reported no conflicts.
Adoptive T-cell therapy treats PML
Adoptive T-cell therapy has proven effective for treating progressive multifocal leukoencephalopathy (PML), according to research published in The New England Journal of Medicine.
Researchers observed substantial improvements in three PML patients infused with donor T cells targeting the BK virus.
Although one patient ultimately died, two had complete clearance of the JC virus and no clinical signs of PML after treatment.
“The JC and BK viruses are genetically similar and share proteins that can be targeted by the immune system,” said study author Katy Rezvani, MD, PhD, of The University of Texas MD Anderson Cancer Center in Houston.
“Because of these similarities, we hypothesized that T cells developed against BK virus may also be effective against JC virus infection.”
Dr. Rezvani’s team developed a novel approach for the generation of BK virus-specific T cells from healthy donors and established a bank of viral-specific T cells for immediate clinical use.
The researchers treated three patients with third-party, partially human leukocyte antigen (HLA)-matched, BK virus-specific T cells.
Patient 1 was a 32-year-old female with acute myeloid leukemia (AML) who previously received a double cord blood transplant.
Patient 2 was a 73-year-old female with JAK2-positive polycythemia rubra vera (PV) who had been treated with ruxolitinib.
Patient 3 was a 35-year-old man with AIDS who had discontinued highly active antiretroviral therapy due to side effects and who was no longer able to walk.
Following the first infusion, all three patients had a reduction in JC viral load in their cerebrospinal fluid. Viral loads dropped from:
- 700 to 78 copies in the AML patient
- 230,000 to 5,200 in the PV patient
- 4,300 to 1,300 in the AIDS patient.
“After infusion of viral-specific T cells, patients 1 and 3 had clinical improvement with significant reduction in JC virus in their cerebrospinal fluid,” Dr. Rezvani said.
“Both patients responded despite persistent T-cell immunodeficiency, supporting the concept that the response was mediated by the adoptively infused viral-specific T cells, and there were no infusion-related reactions.”
The AML patient received two additional infusions, which resulted in clearance of the virus in the cerebrospinal fluid and no signs of PML 27 months after the first infusion.
The PV patient received a second infusion that further reduced JC viral load, but no additional improvement was seen. The patient died 8 months after the first infusion.
The AIDS patient received additional infusions, resulting in complete clearance of the JC virus. This patient has regained mobility, and, 9 months after the first infusion, he is able to walk with a cane.
“We are encouraged that off-the-shelf, third-party, partially HLA-matched BK viral-specific T cells may provide a therapy for PML,” Dr. Rezvani said. “Further study in a larger group of patients is required to determine the success rate, durability, and longer-term adverse events with this treatment.”
This study was supported with funding from the Myelodysplastic Syndromes and Acute Myeloid Leukemia Moon Shot, part of MD Anderson’s Moon Shots Program, as well as the National Institutes of Health.
Adoptive T-cell therapy has proven effective for treating progressive multifocal leukoencephalopathy (PML), according to research published in The New England Journal of Medicine.
Researchers observed substantial improvements in three PML patients infused with donor T cells targeting the BK virus.
Although one patient ultimately died, two had complete clearance of the JC virus and no clinical signs of PML after treatment.
“The JC and BK viruses are genetically similar and share proteins that can be targeted by the immune system,” said study author Katy Rezvani, MD, PhD, of The University of Texas MD Anderson Cancer Center in Houston.
“Because of these similarities, we hypothesized that T cells developed against BK virus may also be effective against JC virus infection.”
Dr. Rezvani’s team developed a novel approach for the generation of BK virus-specific T cells from healthy donors and established a bank of viral-specific T cells for immediate clinical use.
The researchers treated three patients with third-party, partially human leukocyte antigen (HLA)-matched, BK virus-specific T cells.
Patient 1 was a 32-year-old female with acute myeloid leukemia (AML) who previously received a double cord blood transplant.
Patient 2 was a 73-year-old female with JAK2-positive polycythemia rubra vera (PV) who had been treated with ruxolitinib.
Patient 3 was a 35-year-old man with AIDS who had discontinued highly active antiretroviral therapy due to side effects and who was no longer able to walk.
Following the first infusion, all three patients had a reduction in JC viral load in their cerebrospinal fluid. Viral loads dropped from:
- 700 to 78 copies in the AML patient
- 230,000 to 5,200 in the PV patient
- 4,300 to 1,300 in the AIDS patient.
“After infusion of viral-specific T cells, patients 1 and 3 had clinical improvement with significant reduction in JC virus in their cerebrospinal fluid,” Dr. Rezvani said.
“Both patients responded despite persistent T-cell immunodeficiency, supporting the concept that the response was mediated by the adoptively infused viral-specific T cells, and there were no infusion-related reactions.”
The AML patient received two additional infusions, which resulted in clearance of the virus in the cerebrospinal fluid and no signs of PML 27 months after the first infusion.
The PV patient received a second infusion that further reduced JC viral load, but no additional improvement was seen. The patient died 8 months after the first infusion.
The AIDS patient received additional infusions, resulting in complete clearance of the JC virus. This patient has regained mobility, and, 9 months after the first infusion, he is able to walk with a cane.
“We are encouraged that off-the-shelf, third-party, partially HLA-matched BK viral-specific T cells may provide a therapy for PML,” Dr. Rezvani said. “Further study in a larger group of patients is required to determine the success rate, durability, and longer-term adverse events with this treatment.”
This study was supported with funding from the Myelodysplastic Syndromes and Acute Myeloid Leukemia Moon Shot, part of MD Anderson’s Moon Shots Program, as well as the National Institutes of Health.
Adoptive T-cell therapy has proven effective for treating progressive multifocal leukoencephalopathy (PML), according to research published in The New England Journal of Medicine.
Researchers observed substantial improvements in three PML patients infused with donor T cells targeting the BK virus.
Although one patient ultimately died, two had complete clearance of the JC virus and no clinical signs of PML after treatment.
“The JC and BK viruses are genetically similar and share proteins that can be targeted by the immune system,” said study author Katy Rezvani, MD, PhD, of The University of Texas MD Anderson Cancer Center in Houston.
“Because of these similarities, we hypothesized that T cells developed against BK virus may also be effective against JC virus infection.”
Dr. Rezvani’s team developed a novel approach for the generation of BK virus-specific T cells from healthy donors and established a bank of viral-specific T cells for immediate clinical use.
The researchers treated three patients with third-party, partially human leukocyte antigen (HLA)-matched, BK virus-specific T cells.
Patient 1 was a 32-year-old female with acute myeloid leukemia (AML) who previously received a double cord blood transplant.
Patient 2 was a 73-year-old female with JAK2-positive polycythemia rubra vera (PV) who had been treated with ruxolitinib.
Patient 3 was a 35-year-old man with AIDS who had discontinued highly active antiretroviral therapy due to side effects and who was no longer able to walk.
Following the first infusion, all three patients had a reduction in JC viral load in their cerebrospinal fluid. Viral loads dropped from:
- 700 to 78 copies in the AML patient
- 230,000 to 5,200 in the PV patient
- 4,300 to 1,300 in the AIDS patient.
“After infusion of viral-specific T cells, patients 1 and 3 had clinical improvement with significant reduction in JC virus in their cerebrospinal fluid,” Dr. Rezvani said.
“Both patients responded despite persistent T-cell immunodeficiency, supporting the concept that the response was mediated by the adoptively infused viral-specific T cells, and there were no infusion-related reactions.”
The AML patient received two additional infusions, which resulted in clearance of the virus in the cerebrospinal fluid and no signs of PML 27 months after the first infusion.
The PV patient received a second infusion that further reduced JC viral load, but no additional improvement was seen. The patient died 8 months after the first infusion.
The AIDS patient received additional infusions, resulting in complete clearance of the JC virus. This patient has regained mobility, and, 9 months after the first infusion, he is able to walk with a cane.
“We are encouraged that off-the-shelf, third-party, partially HLA-matched BK viral-specific T cells may provide a therapy for PML,” Dr. Rezvani said. “Further study in a larger group of patients is required to determine the success rate, durability, and longer-term adverse events with this treatment.”
This study was supported with funding from the Myelodysplastic Syndromes and Acute Myeloid Leukemia Moon Shot, part of MD Anderson’s Moon Shots Program, as well as the National Institutes of Health.
Dataset could reveal better therapies for AML
Researchers have released a dataset detailing the molecular makeup of tumor cells from more than 500 patients with acute myeloid leukemia (AML).
The team discovered mutations not previously observed in AML and found associations between mutations and responses to certain therapies.
For instance, AML cases with FLT3, NPM1, and DNMT3A mutations proved sensitive to the BTK inhibitor ibrutinib.
The researchers described their findings in Nature.
The team also made their dataset available via Vizome, an online data viewer. Other researchers can use Vizome to find out which targeted therapies might be most effective against specific subsets of AML cells.
“People can get online, search our database, and very quickly get answers to ‘Is this a good drug?’ or ‘Is there a patient population my drug can work in?’” said study author Brian Druker, MD, of Oregon Health & Science University (OHSU) in Portland, Oregon.
Newly identified mutations
For this study, part of the Beat AML initiative, Dr. Druker and his colleagues performed whole-exome and RNA sequencing on 672 samples from 562 AML patients.
The team identified mutations in 11 genes that were called in 1% or more of patients in this dataset but had not been observed in previous AML sequencing studies. The genes were:
- CUB and Sushi multiple domains 2 (CSMD2)
- NAC alpha domain containing (NACAD)
- Teneurin transmembrane protein 2 (TENM2)
- Aggrecan (ACAN)
- ADAM metallopeptidase with thrombospondin type 1 motif 7 (ADAMTS7)
- Immunoglobulin-like and fibronectin type III domain containing 1 (IGFN1)
- Neurobeachin-like 2 (NBEAL2)
- Poly(U) binding splicing factor 60 (PUF60)
- Zinc-finger protein 687 (ZNF687)
- Cadherin EGF LAG sevenpass G-type receptor 2 (CELSR2)
- Glutamate ionotropic receptor NMDA type subunit 2B (GRIN2B).
Testing therapies
The researchers also assessed how AML cells from 409 of the patient samples responded to each of 122 targeted therapies.
The team found that mutations in TP53, ASXL1, NRAS, and KRAS caused “a broad pattern of drug resistance.”
However, cases with TP53 mutations were sensitive to elesclomol (a drug that targets cancer cell metabolism), cases with ASXL1 mutations were sensitive to the HDAC inhibitor panobinostat, and cases with KRAS/NRAS mutations were sensitive to MAPK inhibitors (with NRAS-mutated cases demonstrating greater sensitivity).
The researchers also found that IDH2 mutations “conferred sensitivity to a broad spectrum of drugs,” but IDH1 mutations were associated with resistance to most drugs.
As previously mentioned, the researchers found a significant association between mutations in FLT3, NPM1, and DNMT3A and sensitivity to ibrutinib. However, the team found that cases with DNMT3A mutations alone or mutations in DNMT3A and FLT3 were not significantly different from cases with wild-type genes.
On the other hand, cases with FLT3-ITD alone or any combination with a mutation in NPM1 (including mutations in all three genes) were significantly more sensitive to ibrutinib than cases with wild-type genes.
Cases with FLT3-ITD and mutations in NPM1 were sensitive to another kinase inhibitor, entospletinib, as well.
The researchers also found that mutations in both BCOR and RUNX1 correlated with increased sensitivity to four JAK inhibitors—momelotinib, ruxolitinib, tofacitinib, and JAK inhibitor I.
However, cases with BCOR mutations alone or mutations in BCOR and DNMT3A or SRSF2 showed no difference in sensitivity to the JAK inhibitors from cases with wild-type genes.
Next steps
“We’re just starting to scratch the surface of what we can do when we analyze the data,” Dr. Druker said. “The real power comes when you start to integrate all that data. You can analyze what drug worked and why it worked.”
In fact, the researchers are already developing and initiating clinical trials to test hypotheses generated by this study.
“You can start to sense some momentum building with new, better therapeutics for AML patients, and, hopefully, this dataset will help fuel that momentum even further,” said study author Jeff Tyner, PhD, of the OHSU School of Medicine.
“We want to parlay this information into clinical trials as much as we can, and we also want the broader community to use this dataset to accelerate their own work.”
Funding for the current study was provided by grants from The Leukemia & Lymphoma Society, the National Cancer Institute, the National Library of Medicine, and other groups.
Researchers have released a dataset detailing the molecular makeup of tumor cells from more than 500 patients with acute myeloid leukemia (AML).
The team discovered mutations not previously observed in AML and found associations between mutations and responses to certain therapies.
For instance, AML cases with FLT3, NPM1, and DNMT3A mutations proved sensitive to the BTK inhibitor ibrutinib.
The researchers described their findings in Nature.
The team also made their dataset available via Vizome, an online data viewer. Other researchers can use Vizome to find out which targeted therapies might be most effective against specific subsets of AML cells.
“People can get online, search our database, and very quickly get answers to ‘Is this a good drug?’ or ‘Is there a patient population my drug can work in?’” said study author Brian Druker, MD, of Oregon Health & Science University (OHSU) in Portland, Oregon.
Newly identified mutations
For this study, part of the Beat AML initiative, Dr. Druker and his colleagues performed whole-exome and RNA sequencing on 672 samples from 562 AML patients.
The team identified mutations in 11 genes that were called in 1% or more of patients in this dataset but had not been observed in previous AML sequencing studies. The genes were:
- CUB and Sushi multiple domains 2 (CSMD2)
- NAC alpha domain containing (NACAD)
- Teneurin transmembrane protein 2 (TENM2)
- Aggrecan (ACAN)
- ADAM metallopeptidase with thrombospondin type 1 motif 7 (ADAMTS7)
- Immunoglobulin-like and fibronectin type III domain containing 1 (IGFN1)
- Neurobeachin-like 2 (NBEAL2)
- Poly(U) binding splicing factor 60 (PUF60)
- Zinc-finger protein 687 (ZNF687)
- Cadherin EGF LAG sevenpass G-type receptor 2 (CELSR2)
- Glutamate ionotropic receptor NMDA type subunit 2B (GRIN2B).
Testing therapies
The researchers also assessed how AML cells from 409 of the patient samples responded to each of 122 targeted therapies.
The team found that mutations in TP53, ASXL1, NRAS, and KRAS caused “a broad pattern of drug resistance.”
However, cases with TP53 mutations were sensitive to elesclomol (a drug that targets cancer cell metabolism), cases with ASXL1 mutations were sensitive to the HDAC inhibitor panobinostat, and cases with KRAS/NRAS mutations were sensitive to MAPK inhibitors (with NRAS-mutated cases demonstrating greater sensitivity).
The researchers also found that IDH2 mutations “conferred sensitivity to a broad spectrum of drugs,” but IDH1 mutations were associated with resistance to most drugs.
As previously mentioned, the researchers found a significant association between mutations in FLT3, NPM1, and DNMT3A and sensitivity to ibrutinib. However, the team found that cases with DNMT3A mutations alone or mutations in DNMT3A and FLT3 were not significantly different from cases with wild-type genes.
On the other hand, cases with FLT3-ITD alone or any combination with a mutation in NPM1 (including mutations in all three genes) were significantly more sensitive to ibrutinib than cases with wild-type genes.
Cases with FLT3-ITD and mutations in NPM1 were sensitive to another kinase inhibitor, entospletinib, as well.
The researchers also found that mutations in both BCOR and RUNX1 correlated with increased sensitivity to four JAK inhibitors—momelotinib, ruxolitinib, tofacitinib, and JAK inhibitor I.
However, cases with BCOR mutations alone or mutations in BCOR and DNMT3A or SRSF2 showed no difference in sensitivity to the JAK inhibitors from cases with wild-type genes.
Next steps
“We’re just starting to scratch the surface of what we can do when we analyze the data,” Dr. Druker said. “The real power comes when you start to integrate all that data. You can analyze what drug worked and why it worked.”
In fact, the researchers are already developing and initiating clinical trials to test hypotheses generated by this study.
“You can start to sense some momentum building with new, better therapeutics for AML patients, and, hopefully, this dataset will help fuel that momentum even further,” said study author Jeff Tyner, PhD, of the OHSU School of Medicine.
“We want to parlay this information into clinical trials as much as we can, and we also want the broader community to use this dataset to accelerate their own work.”
Funding for the current study was provided by grants from The Leukemia & Lymphoma Society, the National Cancer Institute, the National Library of Medicine, and other groups.
Researchers have released a dataset detailing the molecular makeup of tumor cells from more than 500 patients with acute myeloid leukemia (AML).
The team discovered mutations not previously observed in AML and found associations between mutations and responses to certain therapies.
For instance, AML cases with FLT3, NPM1, and DNMT3A mutations proved sensitive to the BTK inhibitor ibrutinib.
The researchers described their findings in Nature.
The team also made their dataset available via Vizome, an online data viewer. Other researchers can use Vizome to find out which targeted therapies might be most effective against specific subsets of AML cells.
“People can get online, search our database, and very quickly get answers to ‘Is this a good drug?’ or ‘Is there a patient population my drug can work in?’” said study author Brian Druker, MD, of Oregon Health & Science University (OHSU) in Portland, Oregon.
Newly identified mutations
For this study, part of the Beat AML initiative, Dr. Druker and his colleagues performed whole-exome and RNA sequencing on 672 samples from 562 AML patients.
The team identified mutations in 11 genes that were called in 1% or more of patients in this dataset but had not been observed in previous AML sequencing studies. The genes were:
- CUB and Sushi multiple domains 2 (CSMD2)
- NAC alpha domain containing (NACAD)
- Teneurin transmembrane protein 2 (TENM2)
- Aggrecan (ACAN)
- ADAM metallopeptidase with thrombospondin type 1 motif 7 (ADAMTS7)
- Immunoglobulin-like and fibronectin type III domain containing 1 (IGFN1)
- Neurobeachin-like 2 (NBEAL2)
- Poly(U) binding splicing factor 60 (PUF60)
- Zinc-finger protein 687 (ZNF687)
- Cadherin EGF LAG sevenpass G-type receptor 2 (CELSR2)
- Glutamate ionotropic receptor NMDA type subunit 2B (GRIN2B).
Testing therapies
The researchers also assessed how AML cells from 409 of the patient samples responded to each of 122 targeted therapies.
The team found that mutations in TP53, ASXL1, NRAS, and KRAS caused “a broad pattern of drug resistance.”
However, cases with TP53 mutations were sensitive to elesclomol (a drug that targets cancer cell metabolism), cases with ASXL1 mutations were sensitive to the HDAC inhibitor panobinostat, and cases with KRAS/NRAS mutations were sensitive to MAPK inhibitors (with NRAS-mutated cases demonstrating greater sensitivity).
The researchers also found that IDH2 mutations “conferred sensitivity to a broad spectrum of drugs,” but IDH1 mutations were associated with resistance to most drugs.
As previously mentioned, the researchers found a significant association between mutations in FLT3, NPM1, and DNMT3A and sensitivity to ibrutinib. However, the team found that cases with DNMT3A mutations alone or mutations in DNMT3A and FLT3 were not significantly different from cases with wild-type genes.
On the other hand, cases with FLT3-ITD alone or any combination with a mutation in NPM1 (including mutations in all three genes) were significantly more sensitive to ibrutinib than cases with wild-type genes.
Cases with FLT3-ITD and mutations in NPM1 were sensitive to another kinase inhibitor, entospletinib, as well.
The researchers also found that mutations in both BCOR and RUNX1 correlated with increased sensitivity to four JAK inhibitors—momelotinib, ruxolitinib, tofacitinib, and JAK inhibitor I.
However, cases with BCOR mutations alone or mutations in BCOR and DNMT3A or SRSF2 showed no difference in sensitivity to the JAK inhibitors from cases with wild-type genes.
Next steps
“We’re just starting to scratch the surface of what we can do when we analyze the data,” Dr. Druker said. “The real power comes when you start to integrate all that data. You can analyze what drug worked and why it worked.”
In fact, the researchers are already developing and initiating clinical trials to test hypotheses generated by this study.
“You can start to sense some momentum building with new, better therapeutics for AML patients, and, hopefully, this dataset will help fuel that momentum even further,” said study author Jeff Tyner, PhD, of the OHSU School of Medicine.
“We want to parlay this information into clinical trials as much as we can, and we also want the broader community to use this dataset to accelerate their own work.”
Funding for the current study was provided by grants from The Leukemia & Lymphoma Society, the National Cancer Institute, the National Library of Medicine, and other groups.