User login
Pediatric atopic dermatitis and neuropsychiatric disorders: What is the link?
Key clinical point: Atopic dermatitis (AD) did not increase the incidence risk for most neuropsychiatric disorders in a pediatric cohort.
Major finding: The risks for attention deficit hyperactivity disorder (hazard ratio [HR] 1.02; 95% CI 0.97-1.06), autism (HR 1.02; 95% CI 0.98-1.06), anxiety (HR 1.01; 95% CI 0.99-1.03), and bipolar disorder (HR 1.08; 95% CI 0.85-1.36) were comparable in the AD and non-AD groups. Participants with vs without AD were less likely to develop depression (HR 0.93; 95% CI 0.91-0.95) or schizophrenia (HR 0.72; 95% CI 0.54-0.95) but more likely to develop obsessive compulsive disorder (HR 1.26; 95% CI 1.16-1.37). However, the risks varied with disease severity and patient’s age.
Study details: Findings are from a retrospective population-based cohort study including 409,431 children with AD and 1,809,029 matched children without AD.
Disclosures: This study was supported by a contract from Pfizer, Inc. One author declared being an employee of Pfizer. The other authors reported ties with several sources, including Pfizer.
Source: Wan J et al. Atopic dermatitis and risk of major neuropsychiatric disorders in children: A population-based cohort study. J Eur Acad Dermatol Venereol. 2022 (Aug 26). Doi: 10.1111/jdv.18564
Key clinical point: Atopic dermatitis (AD) did not increase the incidence risk for most neuropsychiatric disorders in a pediatric cohort.
Major finding: The risks for attention deficit hyperactivity disorder (hazard ratio [HR] 1.02; 95% CI 0.97-1.06), autism (HR 1.02; 95% CI 0.98-1.06), anxiety (HR 1.01; 95% CI 0.99-1.03), and bipolar disorder (HR 1.08; 95% CI 0.85-1.36) were comparable in the AD and non-AD groups. Participants with vs without AD were less likely to develop depression (HR 0.93; 95% CI 0.91-0.95) or schizophrenia (HR 0.72; 95% CI 0.54-0.95) but more likely to develop obsessive compulsive disorder (HR 1.26; 95% CI 1.16-1.37). However, the risks varied with disease severity and patient’s age.
Study details: Findings are from a retrospective population-based cohort study including 409,431 children with AD and 1,809,029 matched children without AD.
Disclosures: This study was supported by a contract from Pfizer, Inc. One author declared being an employee of Pfizer. The other authors reported ties with several sources, including Pfizer.
Source: Wan J et al. Atopic dermatitis and risk of major neuropsychiatric disorders in children: A population-based cohort study. J Eur Acad Dermatol Venereol. 2022 (Aug 26). Doi: 10.1111/jdv.18564
Key clinical point: Atopic dermatitis (AD) did not increase the incidence risk for most neuropsychiatric disorders in a pediatric cohort.
Major finding: The risks for attention deficit hyperactivity disorder (hazard ratio [HR] 1.02; 95% CI 0.97-1.06), autism (HR 1.02; 95% CI 0.98-1.06), anxiety (HR 1.01; 95% CI 0.99-1.03), and bipolar disorder (HR 1.08; 95% CI 0.85-1.36) were comparable in the AD and non-AD groups. Participants with vs without AD were less likely to develop depression (HR 0.93; 95% CI 0.91-0.95) or schizophrenia (HR 0.72; 95% CI 0.54-0.95) but more likely to develop obsessive compulsive disorder (HR 1.26; 95% CI 1.16-1.37). However, the risks varied with disease severity and patient’s age.
Study details: Findings are from a retrospective population-based cohort study including 409,431 children with AD and 1,809,029 matched children without AD.
Disclosures: This study was supported by a contract from Pfizer, Inc. One author declared being an employee of Pfizer. The other authors reported ties with several sources, including Pfizer.
Source: Wan J et al. Atopic dermatitis and risk of major neuropsychiatric disorders in children: A population-based cohort study. J Eur Acad Dermatol Venereol. 2022 (Aug 26). Doi: 10.1111/jdv.18564
Early initiation of emollient reduces risk for atopic dermatitis in high risk infants
Key clinical point: Initiation of daily application of a specialized emollient from the first to the eighth week of life reduced the risk for atopic dermatitis (AD) incidence for 12 months in infants with high risk for AD.
Major finding: At 12 months, the cumulative incidence of AD was significantly lower in the emollient vs standard routine skin care group (32.8% vs 46.4%; relative risk 0.707; P = .036). The rate of patient-reported skin infections was similar between both the treatment groups during the 8-week intervention period (5.0% vs 5.7%).
Study details: Findings are from the STOP AD trial including 321 newborn infants at high risk for AD who were randomly assigned to receive twice-daily emollient for the first 8 weeks of life or standard routine skin care.
Disclosures: This study was supported by The City of Dublin Skin and Cancer Hospital Charity and the Skin Research Institute of Singapore. Some authors declared being managing directors, employees, shareholders, or consultants or receiving research funding, speaker fees, or consulting fees from several sources.
Source: Ní Chaoimh C, Lad D, et al. Early initiation of short-term emollient use for the prevention of atopic dermatitis in high risk infants - the STOP AD randomised controlled trial. Allergy. 2022 (Aug 23). Doi: 10.1111/all.15491
Key clinical point: Initiation of daily application of a specialized emollient from the first to the eighth week of life reduced the risk for atopic dermatitis (AD) incidence for 12 months in infants with high risk for AD.
Major finding: At 12 months, the cumulative incidence of AD was significantly lower in the emollient vs standard routine skin care group (32.8% vs 46.4%; relative risk 0.707; P = .036). The rate of patient-reported skin infections was similar between both the treatment groups during the 8-week intervention period (5.0% vs 5.7%).
Study details: Findings are from the STOP AD trial including 321 newborn infants at high risk for AD who were randomly assigned to receive twice-daily emollient for the first 8 weeks of life or standard routine skin care.
Disclosures: This study was supported by The City of Dublin Skin and Cancer Hospital Charity and the Skin Research Institute of Singapore. Some authors declared being managing directors, employees, shareholders, or consultants or receiving research funding, speaker fees, or consulting fees from several sources.
Source: Ní Chaoimh C, Lad D, et al. Early initiation of short-term emollient use for the prevention of atopic dermatitis in high risk infants - the STOP AD randomised controlled trial. Allergy. 2022 (Aug 23). Doi: 10.1111/all.15491
Key clinical point: Initiation of daily application of a specialized emollient from the first to the eighth week of life reduced the risk for atopic dermatitis (AD) incidence for 12 months in infants with high risk for AD.
Major finding: At 12 months, the cumulative incidence of AD was significantly lower in the emollient vs standard routine skin care group (32.8% vs 46.4%; relative risk 0.707; P = .036). The rate of patient-reported skin infections was similar between both the treatment groups during the 8-week intervention period (5.0% vs 5.7%).
Study details: Findings are from the STOP AD trial including 321 newborn infants at high risk for AD who were randomly assigned to receive twice-daily emollient for the first 8 weeks of life or standard routine skin care.
Disclosures: This study was supported by The City of Dublin Skin and Cancer Hospital Charity and the Skin Research Institute of Singapore. Some authors declared being managing directors, employees, shareholders, or consultants or receiving research funding, speaker fees, or consulting fees from several sources.
Source: Ní Chaoimh C, Lad D, et al. Early initiation of short-term emollient use for the prevention of atopic dermatitis in high risk infants - the STOP AD randomised controlled trial. Allergy. 2022 (Aug 23). Doi: 10.1111/all.15491
Moderate-to-severe atopic dermatitis: Astegolimab fails to reduce disease severity in phase 2 trial
Key clinical point: Astegolimab, despite being well-tolerated, did not lessen the severity of the disease in patients with moderate-to-severe atopic dermatitis (AD).
Major finding: At week 16, the adjusted mean percent changes in the mean Eczema Area and Severity Index score were comparable in the astegolimab (−51.47%) and placebo (−58.24%) groups (
Study details: Findings are from a phase 2 trial including 65 adult patients with moderate-to-severe AD and inadequate response to topical medications who were randomly assigned to receive 490 mg astegolimab or placebo every 4 weeks for 16 weeks and were further followed-up for 8 weeks.
Disclosures: This study was supported by Genentech, Inc. Six authors declared serving as employees of Genentech, Inc., a member of the Roche group, and owning stocks in Roche. The other authors reported ties with several sources, including Roche.
Source: Maurer M et al. Phase 2 randomized clinical trial of astegolimab in patients with moderate to severe atopic dermatitis. J Allergy Clin Immunol. 2022 (Aug 27). Doi: 10.1016/j.jaci.2022.08.015
Key clinical point: Astegolimab, despite being well-tolerated, did not lessen the severity of the disease in patients with moderate-to-severe atopic dermatitis (AD).
Major finding: At week 16, the adjusted mean percent changes in the mean Eczema Area and Severity Index score were comparable in the astegolimab (−51.47%) and placebo (−58.24%) groups (
Study details: Findings are from a phase 2 trial including 65 adult patients with moderate-to-severe AD and inadequate response to topical medications who were randomly assigned to receive 490 mg astegolimab or placebo every 4 weeks for 16 weeks and were further followed-up for 8 weeks.
Disclosures: This study was supported by Genentech, Inc. Six authors declared serving as employees of Genentech, Inc., a member of the Roche group, and owning stocks in Roche. The other authors reported ties with several sources, including Roche.
Source: Maurer M et al. Phase 2 randomized clinical trial of astegolimab in patients with moderate to severe atopic dermatitis. J Allergy Clin Immunol. 2022 (Aug 27). Doi: 10.1016/j.jaci.2022.08.015
Key clinical point: Astegolimab, despite being well-tolerated, did not lessen the severity of the disease in patients with moderate-to-severe atopic dermatitis (AD).
Major finding: At week 16, the adjusted mean percent changes in the mean Eczema Area and Severity Index score were comparable in the astegolimab (−51.47%) and placebo (−58.24%) groups (
Study details: Findings are from a phase 2 trial including 65 adult patients with moderate-to-severe AD and inadequate response to topical medications who were randomly assigned to receive 490 mg astegolimab or placebo every 4 weeks for 16 weeks and were further followed-up for 8 weeks.
Disclosures: This study was supported by Genentech, Inc. Six authors declared serving as employees of Genentech, Inc., a member of the Roche group, and owning stocks in Roche. The other authors reported ties with several sources, including Roche.
Source: Maurer M et al. Phase 2 randomized clinical trial of astegolimab in patients with moderate to severe atopic dermatitis. J Allergy Clin Immunol. 2022 (Aug 27). Doi: 10.1016/j.jaci.2022.08.015
EHA cream shows promise in children with mild-to-moderate atopic dermatitis
Key clinical point: A cream containing 1% ectoine and 0.1% hyaluronic acid (EHA) demonstrated superior efficacy than a vehicle cream and was well-tolerated in children aged 2-18 years with mild-to-moderate atopic dermatitis (AD).
Major finding: At week 4, patients receiving EHA cream achieved a significantly higher clinical improvement in SCORing AD (mean difference [MD] −6.62; P < .001) and Investigator’s Global Assessment scores (MD −0.69; P < .001) than those receiving vehicle cream. Mild adverse events like skin erythema, pruritus, and burning skin were reported by 23.5% of patients receiving EHA cream and 5.7% of patients receiving vehicle cream.
Study details: Findings are from an observer-blind, multicenter clinical trial including 57 children aged 2-18 years with mild-to-moderate AD who were randomly assigned to receive EHA or vehicle cream twice daily for 4 weeks.
Disclosures: This study was funded by BODERM SA. The authors declared no conflicts of interest.
Source: Alexopoulos A et al. A randomized, observer-blind, vehicle-control, multi-center clinical investigation for assessing the efficacy and tolerability of a 1% ectoine and hyaluronic acid 0.1%-containing medical device in pediatric patients with mild-to-moderate atopic dermatitis. Pediatr Dermatol. 2022 (Aug 29). Doi: 10.1111/pde.15117
Key clinical point: A cream containing 1% ectoine and 0.1% hyaluronic acid (EHA) demonstrated superior efficacy than a vehicle cream and was well-tolerated in children aged 2-18 years with mild-to-moderate atopic dermatitis (AD).
Major finding: At week 4, patients receiving EHA cream achieved a significantly higher clinical improvement in SCORing AD (mean difference [MD] −6.62; P < .001) and Investigator’s Global Assessment scores (MD −0.69; P < .001) than those receiving vehicle cream. Mild adverse events like skin erythema, pruritus, and burning skin were reported by 23.5% of patients receiving EHA cream and 5.7% of patients receiving vehicle cream.
Study details: Findings are from an observer-blind, multicenter clinical trial including 57 children aged 2-18 years with mild-to-moderate AD who were randomly assigned to receive EHA or vehicle cream twice daily for 4 weeks.
Disclosures: This study was funded by BODERM SA. The authors declared no conflicts of interest.
Source: Alexopoulos A et al. A randomized, observer-blind, vehicle-control, multi-center clinical investigation for assessing the efficacy and tolerability of a 1% ectoine and hyaluronic acid 0.1%-containing medical device in pediatric patients with mild-to-moderate atopic dermatitis. Pediatr Dermatol. 2022 (Aug 29). Doi: 10.1111/pde.15117
Key clinical point: A cream containing 1% ectoine and 0.1% hyaluronic acid (EHA) demonstrated superior efficacy than a vehicle cream and was well-tolerated in children aged 2-18 years with mild-to-moderate atopic dermatitis (AD).
Major finding: At week 4, patients receiving EHA cream achieved a significantly higher clinical improvement in SCORing AD (mean difference [MD] −6.62; P < .001) and Investigator’s Global Assessment scores (MD −0.69; P < .001) than those receiving vehicle cream. Mild adverse events like skin erythema, pruritus, and burning skin were reported by 23.5% of patients receiving EHA cream and 5.7% of patients receiving vehicle cream.
Study details: Findings are from an observer-blind, multicenter clinical trial including 57 children aged 2-18 years with mild-to-moderate AD who were randomly assigned to receive EHA or vehicle cream twice daily for 4 weeks.
Disclosures: This study was funded by BODERM SA. The authors declared no conflicts of interest.
Source: Alexopoulos A et al. A randomized, observer-blind, vehicle-control, multi-center clinical investigation for assessing the efficacy and tolerability of a 1% ectoine and hyaluronic acid 0.1%-containing medical device in pediatric patients with mild-to-moderate atopic dermatitis. Pediatr Dermatol. 2022 (Aug 29). Doi: 10.1111/pde.15117
Rapid itch reduction with ruxolitinib in mild-to-moderate atopic dermatitis
Key clinical point: Ruxolitinib cream demonstrated rapid and sustained improvement in itch in patients with mild-to-moderate atopic dermatitis (AD).
Major finding: A significantly higher proportion of patients receiving ruxolitinib (0.75% or 1.5%) vs vehicle cream achieved ≥2-point reduction in itch numerical rating scale (NRS2) score as rapidly as within 12 hours (16.3% and 13.1% vs 6.9%; P < .05), with further improvements at week 8 (58.3% and 65.1% vs 29.4%; P < .0001). In patients receiving ruxolitinib (0.75% or 1.5%) vs vehicle cream, time to achieve itch NRS2 score was shorter (5 and 4 vs 17 days).
Study details: Findings are from a pooled analysis of two phase 3 trials, TRuE-AD1 and TRuE-AD2, including 1249 patients with mild-to-moderate AD who were randomly assigned to receive ruxolitinib (0.75% or 1.5%) or vehicle cream twice daily for 8 weeks.
Disclosures: This study was funded by Incyte Corporation. Three authors declared being employees and shareholders of Incyte Corporation. The other authors declared serving as scientific advisors, investigators, or consultants or receiving research grants and honoraria from several sources.
Source: Blauvelt A et al. Rapid pruritus reduction with ruxolitinib cream treatment in patients with atopic dermatitis. J Eur Acad Dermatol Venereol. 2022 (Sep 6). Doi: 10.1111/jdv.18571
Key clinical point: Ruxolitinib cream demonstrated rapid and sustained improvement in itch in patients with mild-to-moderate atopic dermatitis (AD).
Major finding: A significantly higher proportion of patients receiving ruxolitinib (0.75% or 1.5%) vs vehicle cream achieved ≥2-point reduction in itch numerical rating scale (NRS2) score as rapidly as within 12 hours (16.3% and 13.1% vs 6.9%; P < .05), with further improvements at week 8 (58.3% and 65.1% vs 29.4%; P < .0001). In patients receiving ruxolitinib (0.75% or 1.5%) vs vehicle cream, time to achieve itch NRS2 score was shorter (5 and 4 vs 17 days).
Study details: Findings are from a pooled analysis of two phase 3 trials, TRuE-AD1 and TRuE-AD2, including 1249 patients with mild-to-moderate AD who were randomly assigned to receive ruxolitinib (0.75% or 1.5%) or vehicle cream twice daily for 8 weeks.
Disclosures: This study was funded by Incyte Corporation. Three authors declared being employees and shareholders of Incyte Corporation. The other authors declared serving as scientific advisors, investigators, or consultants or receiving research grants and honoraria from several sources.
Source: Blauvelt A et al. Rapid pruritus reduction with ruxolitinib cream treatment in patients with atopic dermatitis. J Eur Acad Dermatol Venereol. 2022 (Sep 6). Doi: 10.1111/jdv.18571
Key clinical point: Ruxolitinib cream demonstrated rapid and sustained improvement in itch in patients with mild-to-moderate atopic dermatitis (AD).
Major finding: A significantly higher proportion of patients receiving ruxolitinib (0.75% or 1.5%) vs vehicle cream achieved ≥2-point reduction in itch numerical rating scale (NRS2) score as rapidly as within 12 hours (16.3% and 13.1% vs 6.9%; P < .05), with further improvements at week 8 (58.3% and 65.1% vs 29.4%; P < .0001). In patients receiving ruxolitinib (0.75% or 1.5%) vs vehicle cream, time to achieve itch NRS2 score was shorter (5 and 4 vs 17 days).
Study details: Findings are from a pooled analysis of two phase 3 trials, TRuE-AD1 and TRuE-AD2, including 1249 patients with mild-to-moderate AD who were randomly assigned to receive ruxolitinib (0.75% or 1.5%) or vehicle cream twice daily for 8 weeks.
Disclosures: This study was funded by Incyte Corporation. Three authors declared being employees and shareholders of Incyte Corporation. The other authors declared serving as scientific advisors, investigators, or consultants or receiving research grants and honoraria from several sources.
Source: Blauvelt A et al. Rapid pruritus reduction with ruxolitinib cream treatment in patients with atopic dermatitis. J Eur Acad Dermatol Venereol. 2022 (Sep 6). Doi: 10.1111/jdv.18571
Brepocitinib shows potential against mild-to-moderate atopic dermatitis in phase 2 trial
Key clinical point: Topical brepocitinib cream showed significant efficacy in reducing disease severity and was well-tolerated in patients with mild-to-moderate atopic dermatitis (AD).
Major finding: The reduction in the mean Eczema Area and Severity Index at week 6 was significantly higher with 1% brepocitinib cream once daily (QD) vs vehicle QD (−70.1% vs −44.4%) and 1% brepocitinib cream twice daily (BID) vs vehicle BID (−75.0% vs −47.6%; both P < .05). No serious adverse events or deaths were reported.
Study details: Findings are from a double-blind, dose-ranging, phase 2 study including 292 patients with mild-to-moderate AD who were randomly assigned to receive brepocitinib (0.1% QD, 0.3% QD or BID, 1.0% QD or BID, or 3.0% QD) or vehicle (QD or BID).
Disclosures: This study was sponsored by Pfizer Inc. Nine authors declared being shareholders and current or former employees of Pfizer. The other authors reported ties with several sources, including Pfizer.
Source: Landis MN et al. Efficacy and safety of topical brepocitinib for the treatment of mild-to-moderate atopic dermatitis: A phase IIb, randomised, double-blind, vehicle-controlled, dose-ranging, and parallel-group study. Br J Dermatol. 2022 (Aug 20). Doi: 10.1111/bjd.21826
Key clinical point: Topical brepocitinib cream showed significant efficacy in reducing disease severity and was well-tolerated in patients with mild-to-moderate atopic dermatitis (AD).
Major finding: The reduction in the mean Eczema Area and Severity Index at week 6 was significantly higher with 1% brepocitinib cream once daily (QD) vs vehicle QD (−70.1% vs −44.4%) and 1% brepocitinib cream twice daily (BID) vs vehicle BID (−75.0% vs −47.6%; both P < .05). No serious adverse events or deaths were reported.
Study details: Findings are from a double-blind, dose-ranging, phase 2 study including 292 patients with mild-to-moderate AD who were randomly assigned to receive brepocitinib (0.1% QD, 0.3% QD or BID, 1.0% QD or BID, or 3.0% QD) or vehicle (QD or BID).
Disclosures: This study was sponsored by Pfizer Inc. Nine authors declared being shareholders and current or former employees of Pfizer. The other authors reported ties with several sources, including Pfizer.
Source: Landis MN et al. Efficacy and safety of topical brepocitinib for the treatment of mild-to-moderate atopic dermatitis: A phase IIb, randomised, double-blind, vehicle-controlled, dose-ranging, and parallel-group study. Br J Dermatol. 2022 (Aug 20). Doi: 10.1111/bjd.21826
Key clinical point: Topical brepocitinib cream showed significant efficacy in reducing disease severity and was well-tolerated in patients with mild-to-moderate atopic dermatitis (AD).
Major finding: The reduction in the mean Eczema Area and Severity Index at week 6 was significantly higher with 1% brepocitinib cream once daily (QD) vs vehicle QD (−70.1% vs −44.4%) and 1% brepocitinib cream twice daily (BID) vs vehicle BID (−75.0% vs −47.6%; both P < .05). No serious adverse events or deaths were reported.
Study details: Findings are from a double-blind, dose-ranging, phase 2 study including 292 patients with mild-to-moderate AD who were randomly assigned to receive brepocitinib (0.1% QD, 0.3% QD or BID, 1.0% QD or BID, or 3.0% QD) or vehicle (QD or BID).
Disclosures: This study was sponsored by Pfizer Inc. Nine authors declared being shareholders and current or former employees of Pfizer. The other authors reported ties with several sources, including Pfizer.
Source: Landis MN et al. Efficacy and safety of topical brepocitinib for the treatment of mild-to-moderate atopic dermatitis: A phase IIb, randomised, double-blind, vehicle-controlled, dose-ranging, and parallel-group study. Br J Dermatol. 2022 (Aug 20). Doi: 10.1111/bjd.21826
Meta-analysis finds no increased VTE risk in AD patients receiving JAK inhibitors
Key clinical point: The results of this meta-analysis do not demonstrate an elevated risk for incident venous thromboembolism (VTE) in patients with atopic dermatitis (AD), particularly among those receiving treatment with Janus kinase (JAK) inhibitors.
Major finding: The risk for incident VTE was similar among participants with vs without AD (pooled hazard ratio 0.95; 95% CI 0.62-1.45). Among patients with AD who received JAK inhibitors vs placebo /dupilumab, 0.05% vs 0.03% reported VTE (Mantel-Haenszel risk difference 0; 95% CI 0-0).
Study details: Findings are from a meta-analysis of two cohort studies including 458,206 participants with (n = 229,103) and without AD (n = 229,103) and 15 randomized controlled trials including 8787 patients with AD who received an interventional treatment with JAK inhibitors or a control treatment with dupilumab or placebo.
Disclosures: This study did not report any source of funding. The authors declared no conflicts of interest.
Source: Chen TL et al. Association of risk of incident venous thromboembolism with atopic dermatitis and treatment with Janus kinase inhibitors: A systematic review and meta-analysis. JAMA Dermatol. 2022;e223516 (Aug 24). Doi: 10.1001/jamadermatol.2022.3516
Key clinical point: The results of this meta-analysis do not demonstrate an elevated risk for incident venous thromboembolism (VTE) in patients with atopic dermatitis (AD), particularly among those receiving treatment with Janus kinase (JAK) inhibitors.
Major finding: The risk for incident VTE was similar among participants with vs without AD (pooled hazard ratio 0.95; 95% CI 0.62-1.45). Among patients with AD who received JAK inhibitors vs placebo /dupilumab, 0.05% vs 0.03% reported VTE (Mantel-Haenszel risk difference 0; 95% CI 0-0).
Study details: Findings are from a meta-analysis of two cohort studies including 458,206 participants with (n = 229,103) and without AD (n = 229,103) and 15 randomized controlled trials including 8787 patients with AD who received an interventional treatment with JAK inhibitors or a control treatment with dupilumab or placebo.
Disclosures: This study did not report any source of funding. The authors declared no conflicts of interest.
Source: Chen TL et al. Association of risk of incident venous thromboembolism with atopic dermatitis and treatment with Janus kinase inhibitors: A systematic review and meta-analysis. JAMA Dermatol. 2022;e223516 (Aug 24). Doi: 10.1001/jamadermatol.2022.3516
Key clinical point: The results of this meta-analysis do not demonstrate an elevated risk for incident venous thromboembolism (VTE) in patients with atopic dermatitis (AD), particularly among those receiving treatment with Janus kinase (JAK) inhibitors.
Major finding: The risk for incident VTE was similar among participants with vs without AD (pooled hazard ratio 0.95; 95% CI 0.62-1.45). Among patients with AD who received JAK inhibitors vs placebo /dupilumab, 0.05% vs 0.03% reported VTE (Mantel-Haenszel risk difference 0; 95% CI 0-0).
Study details: Findings are from a meta-analysis of two cohort studies including 458,206 participants with (n = 229,103) and without AD (n = 229,103) and 15 randomized controlled trials including 8787 patients with AD who received an interventional treatment with JAK inhibitors or a control treatment with dupilumab or placebo.
Disclosures: This study did not report any source of funding. The authors declared no conflicts of interest.
Source: Chen TL et al. Association of risk of incident venous thromboembolism with atopic dermatitis and treatment with Janus kinase inhibitors: A systematic review and meta-analysis. JAMA Dermatol. 2022;e223516 (Aug 24). Doi: 10.1001/jamadermatol.2022.3516
Dupilumab shows good drug survival in moderate-to-severe atopic dermatitis
Key clinical point: Dupilumab demonstrated good overall drug survival for up to 3 years in patients with moderate-to-severe atopic dermatitis (AD).
Major finding: Dupilumab showed good overall drug survival rates at 1-year (90.3%), 2-year (85.9%), and 3-year (78.6%). The use of immunosuppressant drugs at baseline was associated with shorter drug survival owing to ineffectiveness (hazard ratio [HR] 2.64; 95% CI 1.10-6.37) and adverse events (HR 2.69; 95% CI 1.32-5.48).
Study details: Findings are from an analysis of the BioDay registry data of 715 adult patients with moderate-to-severe AD who received dupilumab and were followed-up for ≥4 weeks.
Disclosures: The BioDay registry was sponsored by Sanofi Genzyme. The authors declared receiving grants, personal fees, speaking fees, financial support or nonfinancial support from several sources.
Source: Spekhorst LS et al. Dupilumab drug survival and associated predictors in patients with moderate to severe atopic dermatitis: Long-term results from the daily practice BioDay registry. JAMA Dermatol. 2022;e223014 (Aug 10). Doi: 10.1001/jamadermatol.2022.3014
Key clinical point: Dupilumab demonstrated good overall drug survival for up to 3 years in patients with moderate-to-severe atopic dermatitis (AD).
Major finding: Dupilumab showed good overall drug survival rates at 1-year (90.3%), 2-year (85.9%), and 3-year (78.6%). The use of immunosuppressant drugs at baseline was associated with shorter drug survival owing to ineffectiveness (hazard ratio [HR] 2.64; 95% CI 1.10-6.37) and adverse events (HR 2.69; 95% CI 1.32-5.48).
Study details: Findings are from an analysis of the BioDay registry data of 715 adult patients with moderate-to-severe AD who received dupilumab and were followed-up for ≥4 weeks.
Disclosures: The BioDay registry was sponsored by Sanofi Genzyme. The authors declared receiving grants, personal fees, speaking fees, financial support or nonfinancial support from several sources.
Source: Spekhorst LS et al. Dupilumab drug survival and associated predictors in patients with moderate to severe atopic dermatitis: Long-term results from the daily practice BioDay registry. JAMA Dermatol. 2022;e223014 (Aug 10). Doi: 10.1001/jamadermatol.2022.3014
Key clinical point: Dupilumab demonstrated good overall drug survival for up to 3 years in patients with moderate-to-severe atopic dermatitis (AD).
Major finding: Dupilumab showed good overall drug survival rates at 1-year (90.3%), 2-year (85.9%), and 3-year (78.6%). The use of immunosuppressant drugs at baseline was associated with shorter drug survival owing to ineffectiveness (hazard ratio [HR] 2.64; 95% CI 1.10-6.37) and adverse events (HR 2.69; 95% CI 1.32-5.48).
Study details: Findings are from an analysis of the BioDay registry data of 715 adult patients with moderate-to-severe AD who received dupilumab and were followed-up for ≥4 weeks.
Disclosures: The BioDay registry was sponsored by Sanofi Genzyme. The authors declared receiving grants, personal fees, speaking fees, financial support or nonfinancial support from several sources.
Source: Spekhorst LS et al. Dupilumab drug survival and associated predictors in patients with moderate to severe atopic dermatitis: Long-term results from the daily practice BioDay registry. JAMA Dermatol. 2022;e223014 (Aug 10). Doi: 10.1001/jamadermatol.2022.3014
Optimizing Narrowband UVB Phototherapy: Is It More Challenging for Your Older Patients?
Even with recent pharmacologic treatment advances, narrowband UVB (NB-UVB) phototherapy remains a versatile, safe, and efficacious adjunctive or exclusive treatment for multiple dermatologic conditions, including psoriasis and atopic dermatitis.
In a prior study, Matthews et al13 reported that 96% (50/52) of patients older than 65 years achieved medium to high levels of clearance with NB-UVB phototherapy. Nonetheless, 2 other findings in this study related to the number of treatments required to achieve clearance (ie, clearance rates) and erythema rates prompted further investigation. The first finding was higher-than-expected clearance rates. Older adults had a clearance rate with a mean of 33 treatments compared to prior studies featuring mean clearance rates of 20 to 28 treatments.7,8,14-16 This finding resembled a study in the United Kingdom17 with a median clearance rate in older adults of 30 treatments. In contrast, the median clearance rate from a study in Turkey18 was 42 treatments in older adults. We hypothesized that more photosensitizing medications used in older vs younger adults prompted more dose adjustments with NB-UVB phototherapy to avoid burning (ie, erythema) at baseline and throughout the treatment course. These dose adjustments may have increased the overall clearance rates. If true, we predicted that younger adults treated with the same protocol would have cleared more quickly, either because of age-related differences or because they likely had fewer comorbidities and therefore fewer medications.
The second finding from Matthews et al13 that warranted further investigation was a higher erythema rate compared to the older adult study from the United Kingdom.17 We hypothesized that potentially greater use of photosensitizing medications in the United States could explain the higher erythema rates. Although medication-induced photosensitivity is less likely with NB-UVB phototherapy than with UVA, certain medications can cause UVB photosensitivity, including thiazides, quinidine, calcium channel antagonists, phenothiazines, and nonsteroidal anti-inflammatory drugs.8,19,20 Therefore, photosensitizing medication use either at baseline or during a course of NB-UVB phototherapy could increase the risk for erythema. Age-related skin changes also have been considered as a
This retrospective study aimed to determine if NB-UVB phototherapy is equally effective in both older and younger adults treated with the same protocol; to examine the association between the use of photosensitizing medications and clearance rates in both older and younger adults; and to examine the association between the use of photosensitizing medications and erythema rates in older vs younger adults.
Methods
Study Design and Patients—This retrospective cohort study used billing records to identify patients who received NB-UVB phototherapy at 3 different clinical sites within a large US health care system in Washington (Group Health Cooperative, now Kaiser Permanente Washington), serving more than 600,000 patients between January 1, 2012, and December 31, 2016. The institutional review board of Kaiser Permanente Washington Health Research Institute approved this study (IRB 1498087-4). Younger adults were classified as those 64 years or younger and older adults as those 65 years and older at the start of their phototherapy regimen. A power analysis determined that the optimal sample size for this study was 250 patients.
Individuals were excluded if they had fewer than 6 phototherapy treatments; a diagnosis of vitiligo, photosensitivity dermatitis, morphea, or pityriasis rubra pilaris; and/or treatment of the hands or feet only.
Phototherapy Protocol—Using a 48-lamp NB-UVB unit, trained phototherapy nurses provided all treatments following standardized treatment protocols13 based on previously published phototherapy guidelines.24 Nurses determined each patient’s disease clearance level using a 3-point clearance scale (high, medium, low).13 Each patient’s starting dose was determined based on the estimated MED for their skin phototype.
Statistical Analysis—Data were analyzed using Stata statistical software (StataCorp LLC). Univariate analyses were used to examine the data and identify outliers, bad values, and missing data, as well as to calculate descriptive statistics. Pearson χ2 and Fisher exact statistics were used to calculate differences in categorical variables. Linear multivariate regression models and logistic multivariate models were used to examine statistical relationships between variables. Statistical significance was defined as P≤.05.
Results
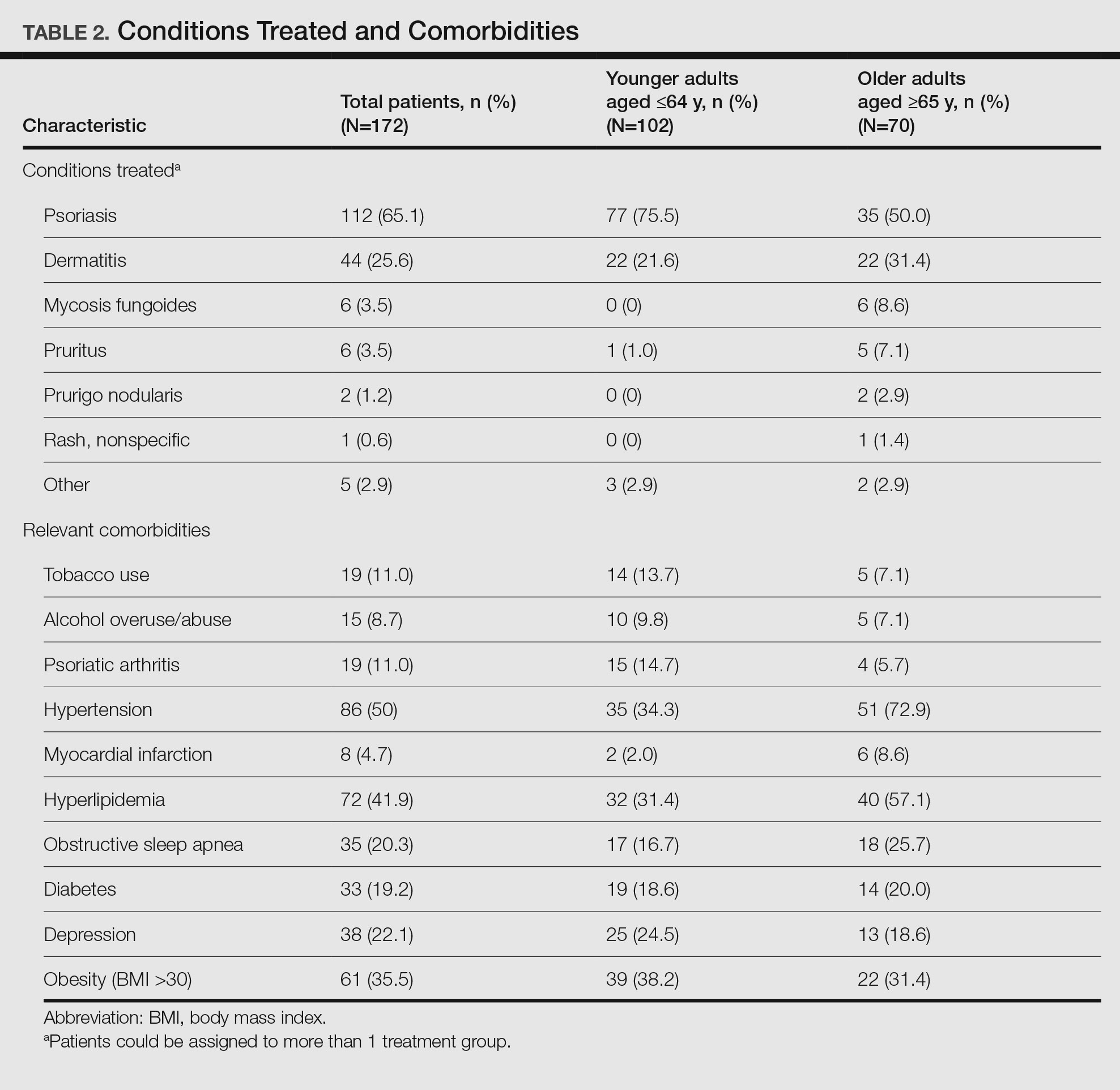
Patient Characteristics—Medical records were reviewed for 172 patients who received phototherapy between 2012 and 2016. Patients ranged in age from 23 to 91 years, with 102 patients 64 years and younger and 70 patients 65 years and older. Tables 1 and 2 outline the patient characteristics and conditions treated.

Phototherapy Effectiveness—
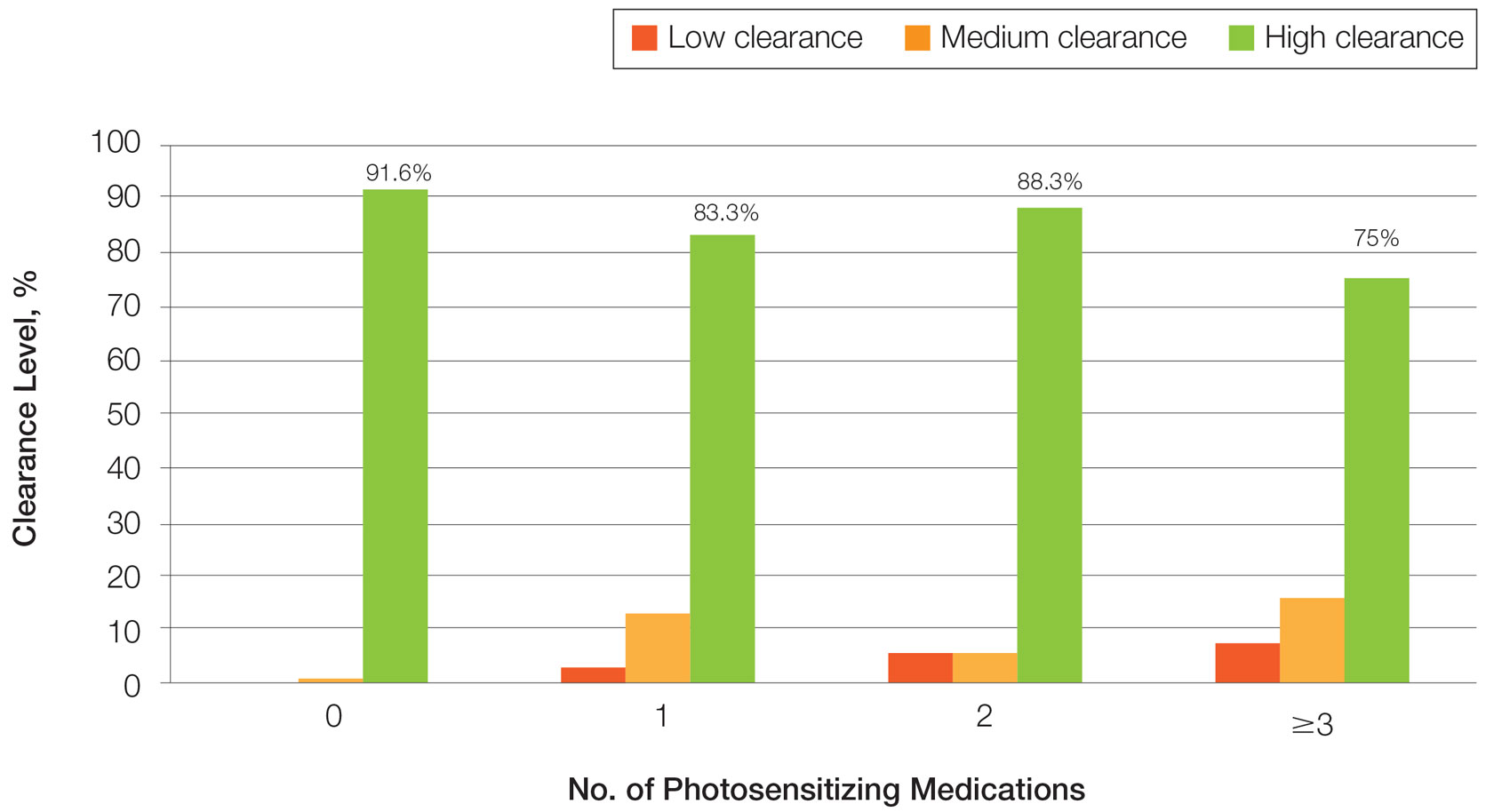
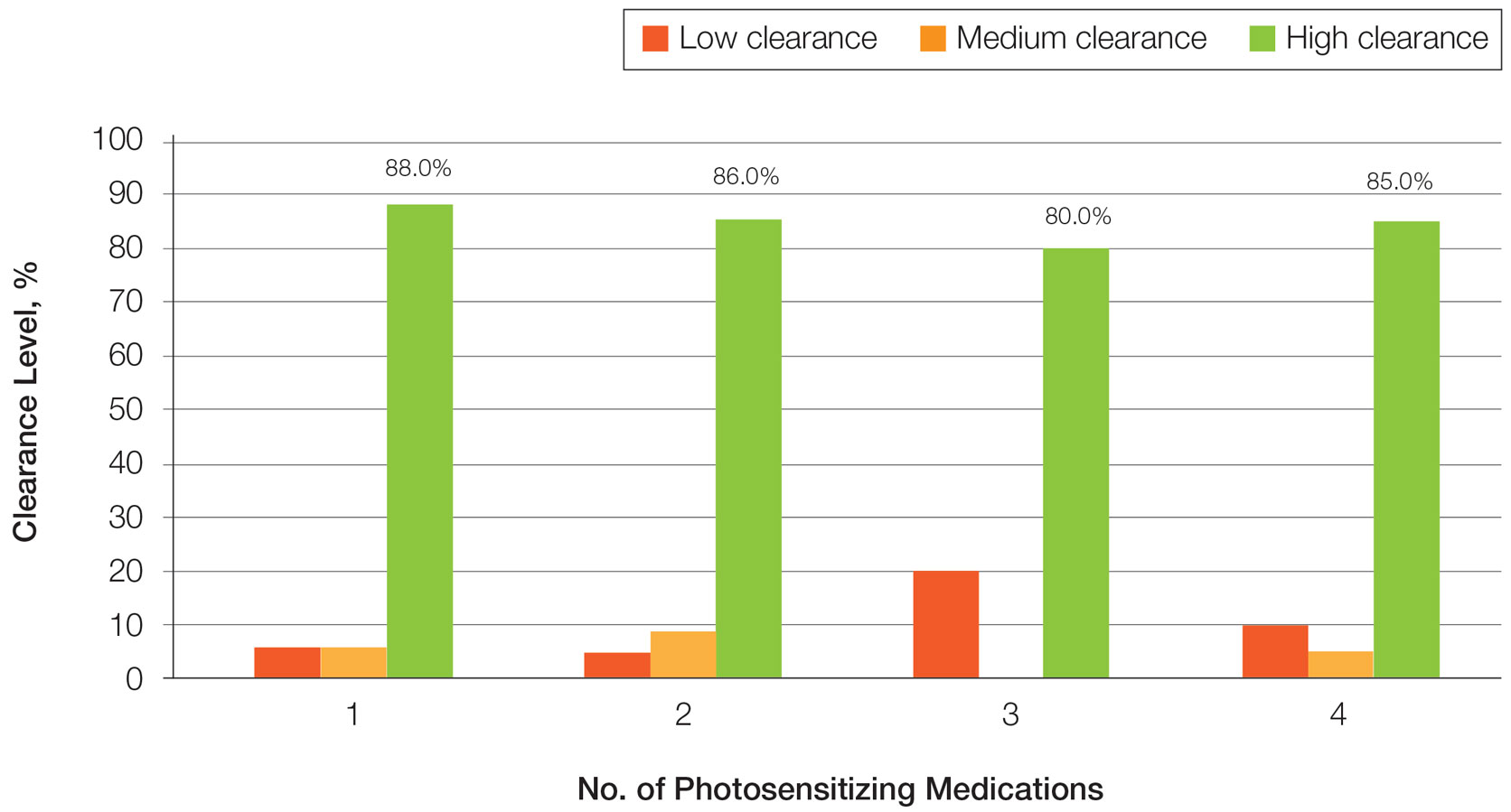
Photosensitizing Medications, Clearance Levels, and Clearance Rates—
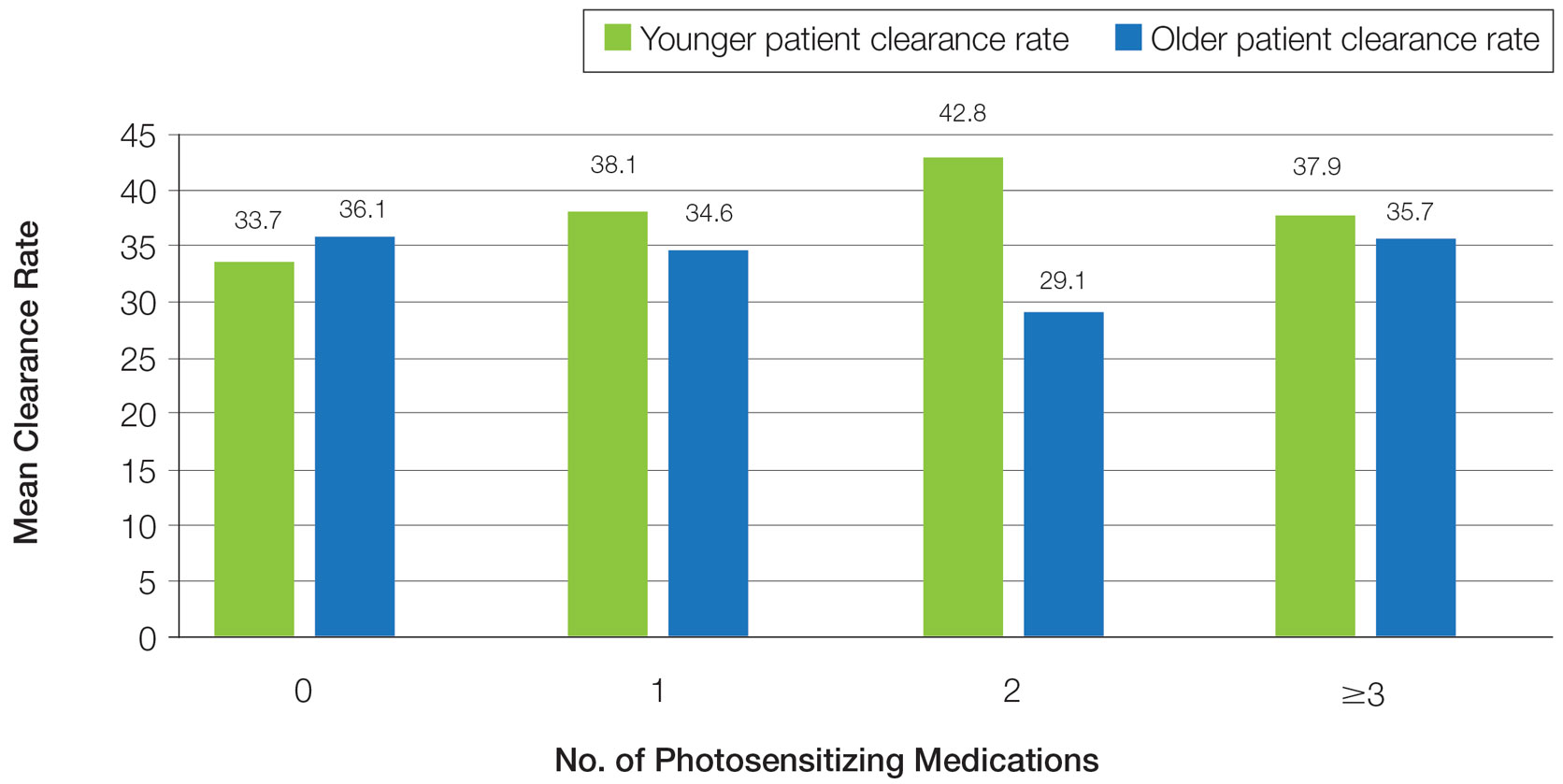
Frequency of Treatments and Clearance Rates—Older adults more consistently completed the recommended frequency of treatments—3 times weekly—compared to younger adults (74.3% vs 58.5%). However, all patients who completed 3 treatments per week required a similar number of treatments to clear (older adults, mean [SD]: 35.7 [21.6]; younger adults, mean [SD]: 34.7 [19.0]; P=.85). Among patients completing 2 or fewer treatments per week, older adults required a mean (SD) of only 31 (9.0) treatments to clear vs 41.5 (21.3) treatments to clear for younger adults, but the difference was not statistically significant (P=.08). However, even those with suboptimal frequency ultimately achieved similar clearance levels.
Photosensitizing Medications and Erythema Rates—
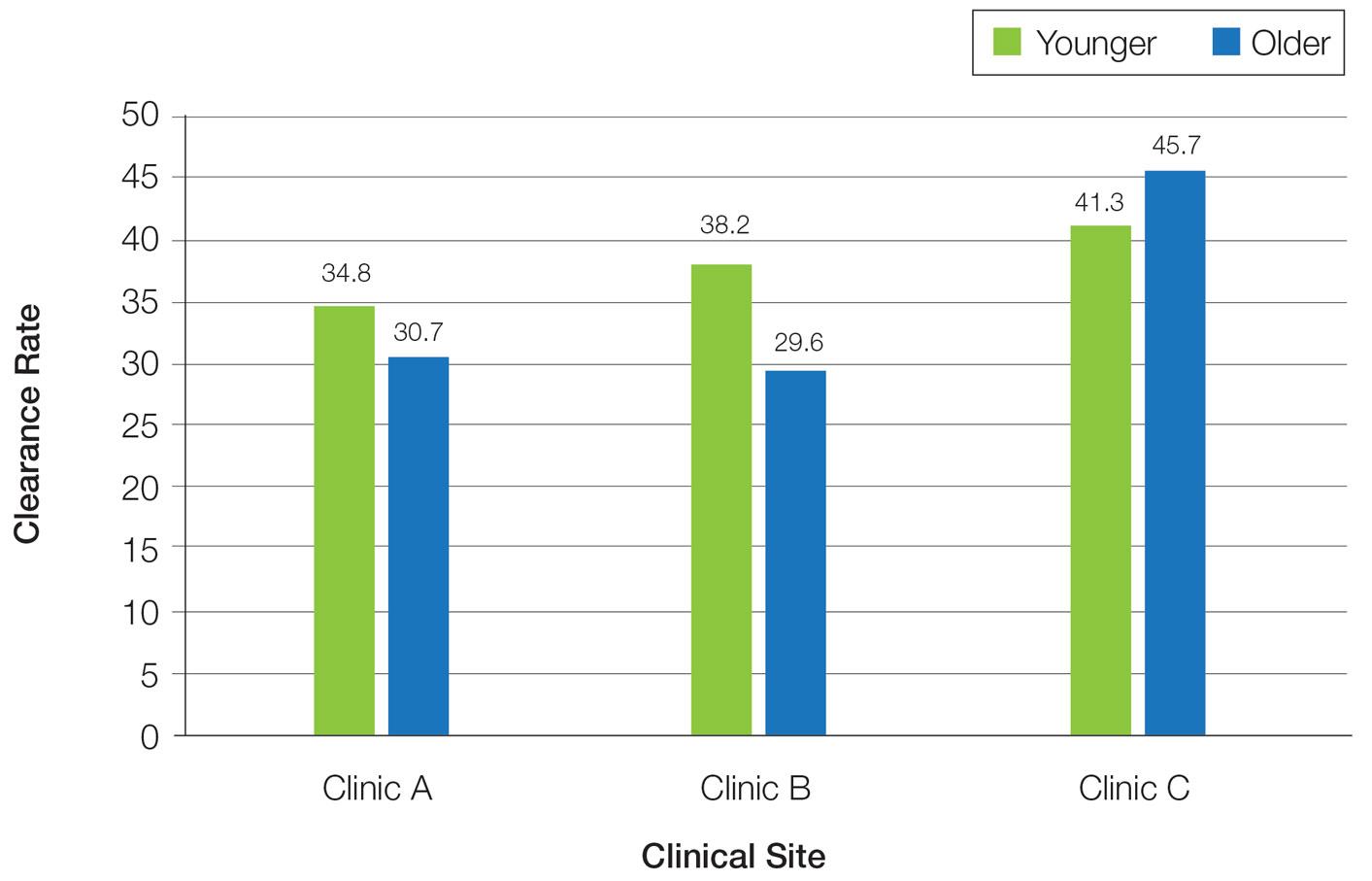
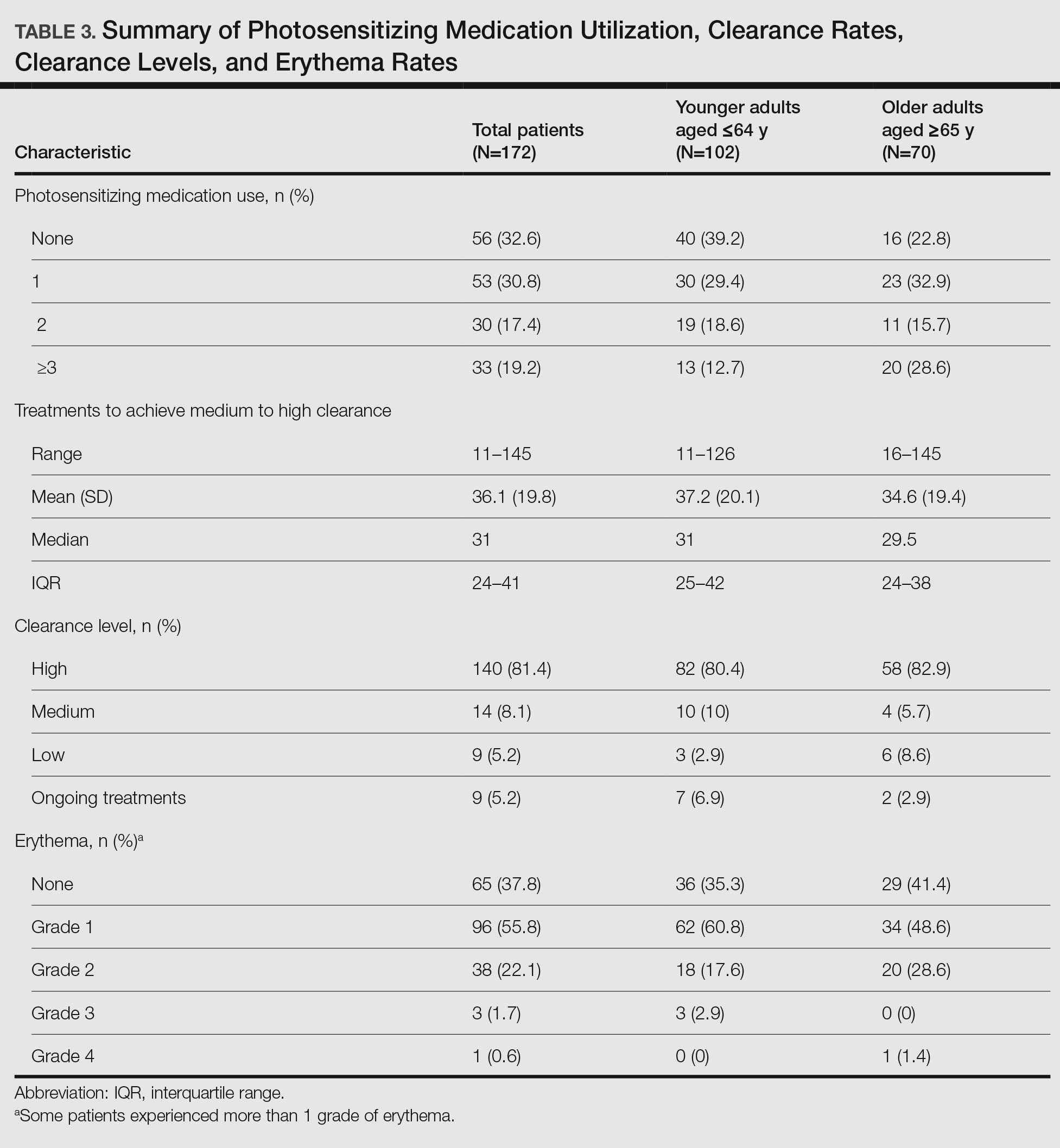
Overall, phototherapy nurses adjusted the starting dose according to the phototype-based protocol an average of 69% of the time for patients on medications with photosensitivity listed as a potential side effect. However, the frequency depended significantly on the clinic (clinic A, 24%; clinic B, 92%; clinic C, 87%)(P≤.001). Nurses across all clinics consistently decreased the treatment dose when patients reported starting new photosensitizing medications. Patients with adjusted starting doses had slightly but not significantly higher clearance rates compared to those without (mean, 37.8 vs 35.5; t(104)=0.58; P=.56).
Comment
Impact of Photosensitizing Medications on Clearance—Photosensitizing medications and treatment frequency were 2 factors that might explain the slower clearance rates in younger adults. In this study, both groups of patients used similar numbers of photosensitizing medications, but more older adults were taking 3 or more medications (Table 3). We found no statistically significant relationship between taking photosensitizing medications and either the clearance rates or the level of clearance achieved in either age group.
Impact of Treatment Frequency—Weekly treatment frequency also was examined. One prior study demonstrated that treatments 3 times weekly led to a faster clearance time and higher clearance levels compared with twice-weekly treatment.7 When patients completed treatments twice weekly, it took an average of 1.5 times more days to clear, which impacted cost and clinical resource availability. The patients ranged in age from 17 to 80 years, but outcomes in older patients were not described separately.7 Interestingly, our study seemed to find a difference between age groups when the impact of treatment frequency was examined. Older adults completed nearly 4 fewer mean treatments to clear when treating less often, with more than 80% achieving high levels of clearance, whereas the younger adults required almost 7 more treatments to clear when they came in less frequently, with approximately 80% achieving a high level of clearance. As a result, our study found that in both age groups, slowing the treatment frequency extended the treatment time to clearance—more for the younger adults than the older adults—but did not significantly change the percentage of individuals reaching full clearance in either group.
Erythema Rates—There was no association between photosensitizing medications and erythema rates except when patients were taking at least 3 medications. Most medications that listed photosensitivity as a possible side effect did not specify their relevant range of UV radiation; therefore, all such medications were examined during this analysis. Prior research has shown UVB range photosensitizing medications include thiazides, quinidine, calcium channel antagonists, phenothiazines, and nonsteroidal anti-inflammatory drugs.19 A sensitivity analysis that focused only on these medications found no association between them and any particular grade of erythema. However, patients taking 3 or more of any medications listing photosensitivity as a side effect had an increased risk for grade 2 erythema.
Erythema rates in this study were consistent with a 2013 systematic review that reported 57% of patients with asymptomatic grade 1 erythema.25 In the 2 other comparative older adult studies, erythema rates varied widely: 35% in a study from Turkey18compared to only1.89% in a study from the United Kingdom.17
The starting dose for NB-UVB may drive erythema rates. The current study’s protocols were based on an estimated MED that is subjectively determined by the dermatology provider’s assessment of the patient’s skin sensitivity via examination and questions to the patient about their response to environmental sun exposure (ie, burning and tanning)26 and is frequently used to determine the starting dose and subsequent dose escalation. Certain medications have been found to increase photosensitivity and erythema,20 which can change an individual’s MED. If photosensitizing medications are started prior to or during a course of NB-UVB without a pretreatment MED, they might increase the risk for erythema. This study did not identify specific erythema-inducing medications but did find that taking 3 or more photosensitizing medications was associated with increased episodes of grade 2 erythema. Similarly, Harrop et al8 found that patients who were taking photosensitizing medications were more likely to have grade 2 or higher erythema, despite baseline MED testing, which is an established safety mechanism to reduce the risk and severity of erythema.14,20,27 The authors of a recent study of older adults in Taiwan specifically recommended MED testing due to the unpredictable influence of polypharmacy on MED calculations in this population.28 Therefore, this study’s use of an estimated MED in older adults may have influenced the starting dose as well as the incidence and severity of erythemic events. Age-related skin changes likely are ruled out as a consideration for mild erythema by the similarity of grade 1 erythema rates in both older and younger adults. Other studies have identified differences between the age groups, where older patients experienced more intense erythema in the late phase of UVB treatments.22,23 This phenomenon could increase the risk for a grade 2 erythema, which may correspond with this study’s findings.
Other potential causes of erythema were ruled out during our study, including erythema related to missed treatments and shielding mishaps. Other factors, however, may impact the level of sensitivity each patient has to phototherapy, including genetics, epigenetics, and cumulative sun damage. With NB-UVB, near-erythemogenic doses are optimal to achieve effective treatments but require a delicate balance to achieve, which may be more problematic for older adults, especially those taking several medications.
Study Limitations—Our study design made it difficult to draw conclusions about rarer dermatologic conditions. Some patients received treatments over years that were not included in the study period. Finally, power calculations suggested that our actual sample size was too small, with approximately one-third of the required sample missing.
Practical Implications—The goals of phototherapy are to achieve a high level of disease clearance with the fewest number of treatments possible and minimal side effects.
The extra staff training and patient monitoring required for MED testing likely is to add value and preserve resources if faster clearance rates could be achieved and may warrant further investigation. Phototherapy centers require standardized treatment protocols, diligent well-trained staff, and program monitoring to ensure consistent care to all patients. This study highlighted the ongoing opportunity for health care organizations to conduct evidence-based practice inquiries to continually optimize care for their patients.
- Fernández-Guarino M, Aboin-Gonzalez S, Barchino L, et al. Treatment of moderate and severe adult chronic atopic dermatitis with narrow-band UVB and the combination of narrow-band UVB/UVA phototherapy. Dermatol Ther. 2016;29:19-23.
- Foerster J, Boswell K, West J, et al. Narrowband UVB treatment is highly effective and causes a strong reduction in the use of steroid and other creams in psoriasis patients in clinical practice. PLoS One. 2017;12:e0181813.
- Gambichler T, Breuckmann F, Boms S, et al. Narrowband UVB phototherapy in skin conditions beyond psoriasis. J Am Acad Dermatol. 2005;52:660-670.
- Ryu HH, Choe YS, Jo S, et al. Remission period in psoriasis after multiple cycles of narrowband ultraviolet B phototherapy. J Dermatol. 2014;41:622-627.
- Schneider LA, Hinrichs R, Scharffetter-Kochanek K. Phototherapy and photochemotherapy. Clin Dermatol. 2008;26:464-476.
- Tintle S, Shemer A, Suárez-Fariñas M, et al. Reversal of atopic dermatitis with narrow-band UVB phototherapy and biomarkers for therapeutic response. J Allergy Clin Immunol. 2011;128:583-593.e581-584.
- Cameron H, Dawe RS, Yule S, et al. A randomized, observer-blinded trial of twice vs. three times weekly narrowband ultraviolet B phototherapy for chronic plaque psoriasis. Br J Dermatol. 2002;147:973-978.
- Harrop G, Dawe RS, Ibbotson S. Are photosensitizing medications associated with increased risk of important erythemal reactions during ultraviolet B phototherapy? Br J Dermatol. 2018;179:1184-1185.
- Torres AE, Lyons AB, Hamzavi IH, et al. Role of phototherapy in the era of biologics. J Am Acad Dermatol. 2021;84:479-485.
- Bukvic´ć Mokos Z, Jovic´ A, Cˇeovic´ R, et al. Therapeutic challenges in the mature patient. Clin Dermatol. 2018;36:128-139.
- Di Lernia V, Goldust M. An overview of the efficacy and safety of systemic treatments for psoriasis in the elderly. Expert Opin Biol Ther. 2018;18:897-903.
- Oliveira C, Torres T. More than skin deep: the systemic nature of atopic dermatitis. Eur J Dermatol. 2019;29:250-258.
- Matthews S, Pike K, Chien A. Phototherapy: safe and effective for challenging skin conditions in older adults. Cutis. 2021;108:E15-E21.
- Rodríguez-Granados MT, Estany-Gestal A, Pousa-Martínez M, et al. Is it useful to calculate minimal erythema dose before narrowband UV-B phototherapy? Actas Dermosifiliogr. 2017;108:852-858.
- Parlak N, Kundakci N, Parlak A, et al. Narrowband ultraviolet B phototherapy starting and incremental dose in patients with psoriasis: comparison of percentage dose and fixed dose protocols. Photodermatol Photoimmunol Photomed. 2015;31:90-97.
- Kleinpenning MM, Smits T, Boezeman J, et al. Narrowband ultraviolet B therapy in psoriasis: randomized double-blind comparison of high-dose and low-dose irradiation regimens. Br J Dermatol. 2009;161:1351-1356.
- Powell JB, Gach JE. Phototherapy in the elderly. Clin Exp Dermatol. 2015;40:605-610.
- Bulur I, Erdogan HK, Aksu AE, et al. The efficacy and safety of phototherapy in geriatric patients: a retrospective study. An Bras Dermatol. 2018;93:33-38.
- Dawe RS, Ibbotson SH. Drug-induced photosensitivity. Dermatol Clin. 2014;32:363-368, ix.
- Cameron H, Dawe RS. Photosensitizing drugs may lower the narrow-band ultraviolet B (TL-01) minimal erythema dose. Br J Dermatol. 2000;142:389-390.
- Elmets CA, Lim HW, Stoff B, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis with phototherapy. J Am Acad Dermatol. 2019;81:775-804.
- Gloor M, Scherotzke A. Age dependence of ultraviolet light-induced erythema following narrow-band UVB exposure. Photodermatol Photoimmunol Photomed. 2002;18:121-126.
- Cox NH, Diffey BL, Farr PM. The relationship between chronological age and the erythemal response to ultraviolet B radiation. Br J Dermatol. 1992;126:315-319.
- Morrison W. Phototherapy and Photochemotherapy for Skin Disease. 2nd ed. Informa Healthcare; 2005.
- Almutawa F, Alnomair N, Wang Y, et al. Systematic review of UV-based therapy for psoriasis. Am J Clin Dermatol. 2013;14:87-109.
- Trakatelli M, Bylaite-Bucinskiene M, Correia O, et al. Clinical assessment of skin phototypes: watch your words! Eur J Dermatol. 2017;27:615-619.
- Kwon IH, Kwon HH, Na SJ, et al. Could colorimetric method replace the individual minimal erythemal dose (MED) measurements in determining the initial dose of narrow-band UVB treatment for psoriasis patients with skin phototype III-V? J Eur Acad Dermatol Venereol. 2013;27:494-498.
- Chen WA, Chang CM. The minimal erythema dose of narrowband ultraviolet B in elderly Taiwanese [published online September 1, 2021]. Photodermatol Photoimmunol Photomed. doi:10.1111/phpp.12730
Even with recent pharmacologic treatment advances, narrowband UVB (NB-UVB) phototherapy remains a versatile, safe, and efficacious adjunctive or exclusive treatment for multiple dermatologic conditions, including psoriasis and atopic dermatitis.
In a prior study, Matthews et al13 reported that 96% (50/52) of patients older than 65 years achieved medium to high levels of clearance with NB-UVB phototherapy. Nonetheless, 2 other findings in this study related to the number of treatments required to achieve clearance (ie, clearance rates) and erythema rates prompted further investigation. The first finding was higher-than-expected clearance rates. Older adults had a clearance rate with a mean of 33 treatments compared to prior studies featuring mean clearance rates of 20 to 28 treatments.7,8,14-16 This finding resembled a study in the United Kingdom17 with a median clearance rate in older adults of 30 treatments. In contrast, the median clearance rate from a study in Turkey18 was 42 treatments in older adults. We hypothesized that more photosensitizing medications used in older vs younger adults prompted more dose adjustments with NB-UVB phototherapy to avoid burning (ie, erythema) at baseline and throughout the treatment course. These dose adjustments may have increased the overall clearance rates. If true, we predicted that younger adults treated with the same protocol would have cleared more quickly, either because of age-related differences or because they likely had fewer comorbidities and therefore fewer medications.
The second finding from Matthews et al13 that warranted further investigation was a higher erythema rate compared to the older adult study from the United Kingdom.17 We hypothesized that potentially greater use of photosensitizing medications in the United States could explain the higher erythema rates. Although medication-induced photosensitivity is less likely with NB-UVB phototherapy than with UVA, certain medications can cause UVB photosensitivity, including thiazides, quinidine, calcium channel antagonists, phenothiazines, and nonsteroidal anti-inflammatory drugs.8,19,20 Therefore, photosensitizing medication use either at baseline or during a course of NB-UVB phototherapy could increase the risk for erythema. Age-related skin changes also have been considered as a
This retrospective study aimed to determine if NB-UVB phototherapy is equally effective in both older and younger adults treated with the same protocol; to examine the association between the use of photosensitizing medications and clearance rates in both older and younger adults; and to examine the association between the use of photosensitizing medications and erythema rates in older vs younger adults.
Methods
Study Design and Patients—This retrospective cohort study used billing records to identify patients who received NB-UVB phototherapy at 3 different clinical sites within a large US health care system in Washington (Group Health Cooperative, now Kaiser Permanente Washington), serving more than 600,000 patients between January 1, 2012, and December 31, 2016. The institutional review board of Kaiser Permanente Washington Health Research Institute approved this study (IRB 1498087-4). Younger adults were classified as those 64 years or younger and older adults as those 65 years and older at the start of their phototherapy regimen. A power analysis determined that the optimal sample size for this study was 250 patients.
Individuals were excluded if they had fewer than 6 phototherapy treatments; a diagnosis of vitiligo, photosensitivity dermatitis, morphea, or pityriasis rubra pilaris; and/or treatment of the hands or feet only.
Phototherapy Protocol—Using a 48-lamp NB-UVB unit, trained phototherapy nurses provided all treatments following standardized treatment protocols13 based on previously published phototherapy guidelines.24 Nurses determined each patient’s disease clearance level using a 3-point clearance scale (high, medium, low).13 Each patient’s starting dose was determined based on the estimated MED for their skin phototype.
Statistical Analysis—Data were analyzed using Stata statistical software (StataCorp LLC). Univariate analyses were used to examine the data and identify outliers, bad values, and missing data, as well as to calculate descriptive statistics. Pearson χ2 and Fisher exact statistics were used to calculate differences in categorical variables. Linear multivariate regression models and logistic multivariate models were used to examine statistical relationships between variables. Statistical significance was defined as P≤.05.
Results
Patient Characteristics—Medical records were reviewed for 172 patients who received phototherapy between 2012 and 2016. Patients ranged in age from 23 to 91 years, with 102 patients 64 years and younger and 70 patients 65 years and older. Tables 1 and 2 outline the patient characteristics and conditions treated.

Phototherapy Effectiveness—

Photosensitizing Medications, Clearance Levels, and Clearance Rates—

Frequency of Treatments and Clearance Rates—Older adults more consistently completed the recommended frequency of treatments—3 times weekly—compared to younger adults (74.3% vs 58.5%). However, all patients who completed 3 treatments per week required a similar number of treatments to clear (older adults, mean [SD]: 35.7 [21.6]; younger adults, mean [SD]: 34.7 [19.0]; P=.85). Among patients completing 2 or fewer treatments per week, older adults required a mean (SD) of only 31 (9.0) treatments to clear vs 41.5 (21.3) treatments to clear for younger adults, but the difference was not statistically significant (P=.08). However, even those with suboptimal frequency ultimately achieved similar clearance levels.


Photosensitizing Medications and Erythema Rates—

Overall, phototherapy nurses adjusted the starting dose according to the phototype-based protocol an average of 69% of the time for patients on medications with photosensitivity listed as a potential side effect. However, the frequency depended significantly on the clinic (clinic A, 24%; clinic B, 92%; clinic C, 87%)(P≤.001). Nurses across all clinics consistently decreased the treatment dose when patients reported starting new photosensitizing medications. Patients with adjusted starting doses had slightly but not significantly higher clearance rates compared to those without (mean, 37.8 vs 35.5; t(104)=0.58; P=.56).

Comment
Impact of Photosensitizing Medications on Clearance—Photosensitizing medications and treatment frequency were 2 factors that might explain the slower clearance rates in younger adults. In this study, both groups of patients used similar numbers of photosensitizing medications, but more older adults were taking 3 or more medications (Table 3). We found no statistically significant relationship between taking photosensitizing medications and either the clearance rates or the level of clearance achieved in either age group.
Impact of Treatment Frequency—Weekly treatment frequency also was examined. One prior study demonstrated that treatments 3 times weekly led to a faster clearance time and higher clearance levels compared with twice-weekly treatment.7 When patients completed treatments twice weekly, it took an average of 1.5 times more days to clear, which impacted cost and clinical resource availability. The patients ranged in age from 17 to 80 years, but outcomes in older patients were not described separately.7 Interestingly, our study seemed to find a difference between age groups when the impact of treatment frequency was examined. Older adults completed nearly 4 fewer mean treatments to clear when treating less often, with more than 80% achieving high levels of clearance, whereas the younger adults required almost 7 more treatments to clear when they came in less frequently, with approximately 80% achieving a high level of clearance. As a result, our study found that in both age groups, slowing the treatment frequency extended the treatment time to clearance—more for the younger adults than the older adults—but did not significantly change the percentage of individuals reaching full clearance in either group.
Erythema Rates—There was no association between photosensitizing medications and erythema rates except when patients were taking at least 3 medications. Most medications that listed photosensitivity as a possible side effect did not specify their relevant range of UV radiation; therefore, all such medications were examined during this analysis. Prior research has shown UVB range photosensitizing medications include thiazides, quinidine, calcium channel antagonists, phenothiazines, and nonsteroidal anti-inflammatory drugs.19 A sensitivity analysis that focused only on these medications found no association between them and any particular grade of erythema. However, patients taking 3 or more of any medications listing photosensitivity as a side effect had an increased risk for grade 2 erythema.
Erythema rates in this study were consistent with a 2013 systematic review that reported 57% of patients with asymptomatic grade 1 erythema.25 In the 2 other comparative older adult studies, erythema rates varied widely: 35% in a study from Turkey18compared to only1.89% in a study from the United Kingdom.17
The starting dose for NB-UVB may drive erythema rates. The current study’s protocols were based on an estimated MED that is subjectively determined by the dermatology provider’s assessment of the patient’s skin sensitivity via examination and questions to the patient about their response to environmental sun exposure (ie, burning and tanning)26 and is frequently used to determine the starting dose and subsequent dose escalation. Certain medications have been found to increase photosensitivity and erythema,20 which can change an individual’s MED. If photosensitizing medications are started prior to or during a course of NB-UVB without a pretreatment MED, they might increase the risk for erythema. This study did not identify specific erythema-inducing medications but did find that taking 3 or more photosensitizing medications was associated with increased episodes of grade 2 erythema. Similarly, Harrop et al8 found that patients who were taking photosensitizing medications were more likely to have grade 2 or higher erythema, despite baseline MED testing, which is an established safety mechanism to reduce the risk and severity of erythema.14,20,27 The authors of a recent study of older adults in Taiwan specifically recommended MED testing due to the unpredictable influence of polypharmacy on MED calculations in this population.28 Therefore, this study’s use of an estimated MED in older adults may have influenced the starting dose as well as the incidence and severity of erythemic events. Age-related skin changes likely are ruled out as a consideration for mild erythema by the similarity of grade 1 erythema rates in both older and younger adults. Other studies have identified differences between the age groups, where older patients experienced more intense erythema in the late phase of UVB treatments.22,23 This phenomenon could increase the risk for a grade 2 erythema, which may correspond with this study’s findings.
Other potential causes of erythema were ruled out during our study, including erythema related to missed treatments and shielding mishaps. Other factors, however, may impact the level of sensitivity each patient has to phototherapy, including genetics, epigenetics, and cumulative sun damage. With NB-UVB, near-erythemogenic doses are optimal to achieve effective treatments but require a delicate balance to achieve, which may be more problematic for older adults, especially those taking several medications.
Study Limitations—Our study design made it difficult to draw conclusions about rarer dermatologic conditions. Some patients received treatments over years that were not included in the study period. Finally, power calculations suggested that our actual sample size was too small, with approximately one-third of the required sample missing.
Practical Implications—The goals of phototherapy are to achieve a high level of disease clearance with the fewest number of treatments possible and minimal side effects.
The extra staff training and patient monitoring required for MED testing likely is to add value and preserve resources if faster clearance rates could be achieved and may warrant further investigation. Phototherapy centers require standardized treatment protocols, diligent well-trained staff, and program monitoring to ensure consistent care to all patients. This study highlighted the ongoing opportunity for health care organizations to conduct evidence-based practice inquiries to continually optimize care for their patients.
Even with recent pharmacologic treatment advances, narrowband UVB (NB-UVB) phototherapy remains a versatile, safe, and efficacious adjunctive or exclusive treatment for multiple dermatologic conditions, including psoriasis and atopic dermatitis.
In a prior study, Matthews et al13 reported that 96% (50/52) of patients older than 65 years achieved medium to high levels of clearance with NB-UVB phototherapy. Nonetheless, 2 other findings in this study related to the number of treatments required to achieve clearance (ie, clearance rates) and erythema rates prompted further investigation. The first finding was higher-than-expected clearance rates. Older adults had a clearance rate with a mean of 33 treatments compared to prior studies featuring mean clearance rates of 20 to 28 treatments.7,8,14-16 This finding resembled a study in the United Kingdom17 with a median clearance rate in older adults of 30 treatments. In contrast, the median clearance rate from a study in Turkey18 was 42 treatments in older adults. We hypothesized that more photosensitizing medications used in older vs younger adults prompted more dose adjustments with NB-UVB phototherapy to avoid burning (ie, erythema) at baseline and throughout the treatment course. These dose adjustments may have increased the overall clearance rates. If true, we predicted that younger adults treated with the same protocol would have cleared more quickly, either because of age-related differences or because they likely had fewer comorbidities and therefore fewer medications.
The second finding from Matthews et al13 that warranted further investigation was a higher erythema rate compared to the older adult study from the United Kingdom.17 We hypothesized that potentially greater use of photosensitizing medications in the United States could explain the higher erythema rates. Although medication-induced photosensitivity is less likely with NB-UVB phototherapy than with UVA, certain medications can cause UVB photosensitivity, including thiazides, quinidine, calcium channel antagonists, phenothiazines, and nonsteroidal anti-inflammatory drugs.8,19,20 Therefore, photosensitizing medication use either at baseline or during a course of NB-UVB phototherapy could increase the risk for erythema. Age-related skin changes also have been considered as a
This retrospective study aimed to determine if NB-UVB phototherapy is equally effective in both older and younger adults treated with the same protocol; to examine the association between the use of photosensitizing medications and clearance rates in both older and younger adults; and to examine the association between the use of photosensitizing medications and erythema rates in older vs younger adults.
Methods
Study Design and Patients—This retrospective cohort study used billing records to identify patients who received NB-UVB phototherapy at 3 different clinical sites within a large US health care system in Washington (Group Health Cooperative, now Kaiser Permanente Washington), serving more than 600,000 patients between January 1, 2012, and December 31, 2016. The institutional review board of Kaiser Permanente Washington Health Research Institute approved this study (IRB 1498087-4). Younger adults were classified as those 64 years or younger and older adults as those 65 years and older at the start of their phototherapy regimen. A power analysis determined that the optimal sample size for this study was 250 patients.
Individuals were excluded if they had fewer than 6 phototherapy treatments; a diagnosis of vitiligo, photosensitivity dermatitis, morphea, or pityriasis rubra pilaris; and/or treatment of the hands or feet only.
Phototherapy Protocol—Using a 48-lamp NB-UVB unit, trained phototherapy nurses provided all treatments following standardized treatment protocols13 based on previously published phototherapy guidelines.24 Nurses determined each patient’s disease clearance level using a 3-point clearance scale (high, medium, low).13 Each patient’s starting dose was determined based on the estimated MED for their skin phototype.
Statistical Analysis—Data were analyzed using Stata statistical software (StataCorp LLC). Univariate analyses were used to examine the data and identify outliers, bad values, and missing data, as well as to calculate descriptive statistics. Pearson χ2 and Fisher exact statistics were used to calculate differences in categorical variables. Linear multivariate regression models and logistic multivariate models were used to examine statistical relationships between variables. Statistical significance was defined as P≤.05.
Results
Patient Characteristics—Medical records were reviewed for 172 patients who received phototherapy between 2012 and 2016. Patients ranged in age from 23 to 91 years, with 102 patients 64 years and younger and 70 patients 65 years and older. Tables 1 and 2 outline the patient characteristics and conditions treated.

Phototherapy Effectiveness—

Photosensitizing Medications, Clearance Levels, and Clearance Rates—

Frequency of Treatments and Clearance Rates—Older adults more consistently completed the recommended frequency of treatments—3 times weekly—compared to younger adults (74.3% vs 58.5%). However, all patients who completed 3 treatments per week required a similar number of treatments to clear (older adults, mean [SD]: 35.7 [21.6]; younger adults, mean [SD]: 34.7 [19.0]; P=.85). Among patients completing 2 or fewer treatments per week, older adults required a mean (SD) of only 31 (9.0) treatments to clear vs 41.5 (21.3) treatments to clear for younger adults, but the difference was not statistically significant (P=.08). However, even those with suboptimal frequency ultimately achieved similar clearance levels.


Photosensitizing Medications and Erythema Rates—

Overall, phototherapy nurses adjusted the starting dose according to the phototype-based protocol an average of 69% of the time for patients on medications with photosensitivity listed as a potential side effect. However, the frequency depended significantly on the clinic (clinic A, 24%; clinic B, 92%; clinic C, 87%)(P≤.001). Nurses across all clinics consistently decreased the treatment dose when patients reported starting new photosensitizing medications. Patients with adjusted starting doses had slightly but not significantly higher clearance rates compared to those without (mean, 37.8 vs 35.5; t(104)=0.58; P=.56).

Comment
Impact of Photosensitizing Medications on Clearance—Photosensitizing medications and treatment frequency were 2 factors that might explain the slower clearance rates in younger adults. In this study, both groups of patients used similar numbers of photosensitizing medications, but more older adults were taking 3 or more medications (Table 3). We found no statistically significant relationship between taking photosensitizing medications and either the clearance rates or the level of clearance achieved in either age group.
Impact of Treatment Frequency—Weekly treatment frequency also was examined. One prior study demonstrated that treatments 3 times weekly led to a faster clearance time and higher clearance levels compared with twice-weekly treatment.7 When patients completed treatments twice weekly, it took an average of 1.5 times more days to clear, which impacted cost and clinical resource availability. The patients ranged in age from 17 to 80 years, but outcomes in older patients were not described separately.7 Interestingly, our study seemed to find a difference between age groups when the impact of treatment frequency was examined. Older adults completed nearly 4 fewer mean treatments to clear when treating less often, with more than 80% achieving high levels of clearance, whereas the younger adults required almost 7 more treatments to clear when they came in less frequently, with approximately 80% achieving a high level of clearance. As a result, our study found that in both age groups, slowing the treatment frequency extended the treatment time to clearance—more for the younger adults than the older adults—but did not significantly change the percentage of individuals reaching full clearance in either group.
Erythema Rates—There was no association between photosensitizing medications and erythema rates except when patients were taking at least 3 medications. Most medications that listed photosensitivity as a possible side effect did not specify their relevant range of UV radiation; therefore, all such medications were examined during this analysis. Prior research has shown UVB range photosensitizing medications include thiazides, quinidine, calcium channel antagonists, phenothiazines, and nonsteroidal anti-inflammatory drugs.19 A sensitivity analysis that focused only on these medications found no association between them and any particular grade of erythema. However, patients taking 3 or more of any medications listing photosensitivity as a side effect had an increased risk for grade 2 erythema.
Erythema rates in this study were consistent with a 2013 systematic review that reported 57% of patients with asymptomatic grade 1 erythema.25 In the 2 other comparative older adult studies, erythema rates varied widely: 35% in a study from Turkey18compared to only1.89% in a study from the United Kingdom.17
The starting dose for NB-UVB may drive erythema rates. The current study’s protocols were based on an estimated MED that is subjectively determined by the dermatology provider’s assessment of the patient’s skin sensitivity via examination and questions to the patient about their response to environmental sun exposure (ie, burning and tanning)26 and is frequently used to determine the starting dose and subsequent dose escalation. Certain medications have been found to increase photosensitivity and erythema,20 which can change an individual’s MED. If photosensitizing medications are started prior to or during a course of NB-UVB without a pretreatment MED, they might increase the risk for erythema. This study did not identify specific erythema-inducing medications but did find that taking 3 or more photosensitizing medications was associated with increased episodes of grade 2 erythema. Similarly, Harrop et al8 found that patients who were taking photosensitizing medications were more likely to have grade 2 or higher erythema, despite baseline MED testing, which is an established safety mechanism to reduce the risk and severity of erythema.14,20,27 The authors of a recent study of older adults in Taiwan specifically recommended MED testing due to the unpredictable influence of polypharmacy on MED calculations in this population.28 Therefore, this study’s use of an estimated MED in older adults may have influenced the starting dose as well as the incidence and severity of erythemic events. Age-related skin changes likely are ruled out as a consideration for mild erythema by the similarity of grade 1 erythema rates in both older and younger adults. Other studies have identified differences between the age groups, where older patients experienced more intense erythema in the late phase of UVB treatments.22,23 This phenomenon could increase the risk for a grade 2 erythema, which may correspond with this study’s findings.
Other potential causes of erythema were ruled out during our study, including erythema related to missed treatments and shielding mishaps. Other factors, however, may impact the level of sensitivity each patient has to phototherapy, including genetics, epigenetics, and cumulative sun damage. With NB-UVB, near-erythemogenic doses are optimal to achieve effective treatments but require a delicate balance to achieve, which may be more problematic for older adults, especially those taking several medications.
Study Limitations—Our study design made it difficult to draw conclusions about rarer dermatologic conditions. Some patients received treatments over years that were not included in the study period. Finally, power calculations suggested that our actual sample size was too small, with approximately one-third of the required sample missing.
Practical Implications—The goals of phototherapy are to achieve a high level of disease clearance with the fewest number of treatments possible and minimal side effects.
The extra staff training and patient monitoring required for MED testing likely is to add value and preserve resources if faster clearance rates could be achieved and may warrant further investigation. Phototherapy centers require standardized treatment protocols, diligent well-trained staff, and program monitoring to ensure consistent care to all patients. This study highlighted the ongoing opportunity for health care organizations to conduct evidence-based practice inquiries to continually optimize care for their patients.
- Fernández-Guarino M, Aboin-Gonzalez S, Barchino L, et al. Treatment of moderate and severe adult chronic atopic dermatitis with narrow-band UVB and the combination of narrow-band UVB/UVA phototherapy. Dermatol Ther. 2016;29:19-23.
- Foerster J, Boswell K, West J, et al. Narrowband UVB treatment is highly effective and causes a strong reduction in the use of steroid and other creams in psoriasis patients in clinical practice. PLoS One. 2017;12:e0181813.
- Gambichler T, Breuckmann F, Boms S, et al. Narrowband UVB phototherapy in skin conditions beyond psoriasis. J Am Acad Dermatol. 2005;52:660-670.
- Ryu HH, Choe YS, Jo S, et al. Remission period in psoriasis after multiple cycles of narrowband ultraviolet B phototherapy. J Dermatol. 2014;41:622-627.
- Schneider LA, Hinrichs R, Scharffetter-Kochanek K. Phototherapy and photochemotherapy. Clin Dermatol. 2008;26:464-476.
- Tintle S, Shemer A, Suárez-Fariñas M, et al. Reversal of atopic dermatitis with narrow-band UVB phototherapy and biomarkers for therapeutic response. J Allergy Clin Immunol. 2011;128:583-593.e581-584.
- Cameron H, Dawe RS, Yule S, et al. A randomized, observer-blinded trial of twice vs. three times weekly narrowband ultraviolet B phototherapy for chronic plaque psoriasis. Br J Dermatol. 2002;147:973-978.
- Harrop G, Dawe RS, Ibbotson S. Are photosensitizing medications associated with increased risk of important erythemal reactions during ultraviolet B phototherapy? Br J Dermatol. 2018;179:1184-1185.
- Torres AE, Lyons AB, Hamzavi IH, et al. Role of phototherapy in the era of biologics. J Am Acad Dermatol. 2021;84:479-485.
- Bukvic´ć Mokos Z, Jovic´ A, Cˇeovic´ R, et al. Therapeutic challenges in the mature patient. Clin Dermatol. 2018;36:128-139.
- Di Lernia V, Goldust M. An overview of the efficacy and safety of systemic treatments for psoriasis in the elderly. Expert Opin Biol Ther. 2018;18:897-903.
- Oliveira C, Torres T. More than skin deep: the systemic nature of atopic dermatitis. Eur J Dermatol. 2019;29:250-258.
- Matthews S, Pike K, Chien A. Phototherapy: safe and effective for challenging skin conditions in older adults. Cutis. 2021;108:E15-E21.
- Rodríguez-Granados MT, Estany-Gestal A, Pousa-Martínez M, et al. Is it useful to calculate minimal erythema dose before narrowband UV-B phototherapy? Actas Dermosifiliogr. 2017;108:852-858.
- Parlak N, Kundakci N, Parlak A, et al. Narrowband ultraviolet B phototherapy starting and incremental dose in patients with psoriasis: comparison of percentage dose and fixed dose protocols. Photodermatol Photoimmunol Photomed. 2015;31:90-97.
- Kleinpenning MM, Smits T, Boezeman J, et al. Narrowband ultraviolet B therapy in psoriasis: randomized double-blind comparison of high-dose and low-dose irradiation regimens. Br J Dermatol. 2009;161:1351-1356.
- Powell JB, Gach JE. Phototherapy in the elderly. Clin Exp Dermatol. 2015;40:605-610.
- Bulur I, Erdogan HK, Aksu AE, et al. The efficacy and safety of phototherapy in geriatric patients: a retrospective study. An Bras Dermatol. 2018;93:33-38.
- Dawe RS, Ibbotson SH. Drug-induced photosensitivity. Dermatol Clin. 2014;32:363-368, ix.
- Cameron H, Dawe RS. Photosensitizing drugs may lower the narrow-band ultraviolet B (TL-01) minimal erythema dose. Br J Dermatol. 2000;142:389-390.
- Elmets CA, Lim HW, Stoff B, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis with phototherapy. J Am Acad Dermatol. 2019;81:775-804.
- Gloor M, Scherotzke A. Age dependence of ultraviolet light-induced erythema following narrow-band UVB exposure. Photodermatol Photoimmunol Photomed. 2002;18:121-126.
- Cox NH, Diffey BL, Farr PM. The relationship between chronological age and the erythemal response to ultraviolet B radiation. Br J Dermatol. 1992;126:315-319.
- Morrison W. Phototherapy and Photochemotherapy for Skin Disease. 2nd ed. Informa Healthcare; 2005.
- Almutawa F, Alnomair N, Wang Y, et al. Systematic review of UV-based therapy for psoriasis. Am J Clin Dermatol. 2013;14:87-109.
- Trakatelli M, Bylaite-Bucinskiene M, Correia O, et al. Clinical assessment of skin phototypes: watch your words! Eur J Dermatol. 2017;27:615-619.
- Kwon IH, Kwon HH, Na SJ, et al. Could colorimetric method replace the individual minimal erythemal dose (MED) measurements in determining the initial dose of narrow-band UVB treatment for psoriasis patients with skin phototype III-V? J Eur Acad Dermatol Venereol. 2013;27:494-498.
- Chen WA, Chang CM. The minimal erythema dose of narrowband ultraviolet B in elderly Taiwanese [published online September 1, 2021]. Photodermatol Photoimmunol Photomed. doi:10.1111/phpp.12730
- Fernández-Guarino M, Aboin-Gonzalez S, Barchino L, et al. Treatment of moderate and severe adult chronic atopic dermatitis with narrow-band UVB and the combination of narrow-band UVB/UVA phototherapy. Dermatol Ther. 2016;29:19-23.
- Foerster J, Boswell K, West J, et al. Narrowband UVB treatment is highly effective and causes a strong reduction in the use of steroid and other creams in psoriasis patients in clinical practice. PLoS One. 2017;12:e0181813.
- Gambichler T, Breuckmann F, Boms S, et al. Narrowband UVB phototherapy in skin conditions beyond psoriasis. J Am Acad Dermatol. 2005;52:660-670.
- Ryu HH, Choe YS, Jo S, et al. Remission period in psoriasis after multiple cycles of narrowband ultraviolet B phototherapy. J Dermatol. 2014;41:622-627.
- Schneider LA, Hinrichs R, Scharffetter-Kochanek K. Phototherapy and photochemotherapy. Clin Dermatol. 2008;26:464-476.
- Tintle S, Shemer A, Suárez-Fariñas M, et al. Reversal of atopic dermatitis with narrow-band UVB phototherapy and biomarkers for therapeutic response. J Allergy Clin Immunol. 2011;128:583-593.e581-584.
- Cameron H, Dawe RS, Yule S, et al. A randomized, observer-blinded trial of twice vs. three times weekly narrowband ultraviolet B phototherapy for chronic plaque psoriasis. Br J Dermatol. 2002;147:973-978.
- Harrop G, Dawe RS, Ibbotson S. Are photosensitizing medications associated with increased risk of important erythemal reactions during ultraviolet B phototherapy? Br J Dermatol. 2018;179:1184-1185.
- Torres AE, Lyons AB, Hamzavi IH, et al. Role of phototherapy in the era of biologics. J Am Acad Dermatol. 2021;84:479-485.
- Bukvic´ć Mokos Z, Jovic´ A, Cˇeovic´ R, et al. Therapeutic challenges in the mature patient. Clin Dermatol. 2018;36:128-139.
- Di Lernia V, Goldust M. An overview of the efficacy and safety of systemic treatments for psoriasis in the elderly. Expert Opin Biol Ther. 2018;18:897-903.
- Oliveira C, Torres T. More than skin deep: the systemic nature of atopic dermatitis. Eur J Dermatol. 2019;29:250-258.
- Matthews S, Pike K, Chien A. Phototherapy: safe and effective for challenging skin conditions in older adults. Cutis. 2021;108:E15-E21.
- Rodríguez-Granados MT, Estany-Gestal A, Pousa-Martínez M, et al. Is it useful to calculate minimal erythema dose before narrowband UV-B phototherapy? Actas Dermosifiliogr. 2017;108:852-858.
- Parlak N, Kundakci N, Parlak A, et al. Narrowband ultraviolet B phototherapy starting and incremental dose in patients with psoriasis: comparison of percentage dose and fixed dose protocols. Photodermatol Photoimmunol Photomed. 2015;31:90-97.
- Kleinpenning MM, Smits T, Boezeman J, et al. Narrowband ultraviolet B therapy in psoriasis: randomized double-blind comparison of high-dose and low-dose irradiation regimens. Br J Dermatol. 2009;161:1351-1356.
- Powell JB, Gach JE. Phototherapy in the elderly. Clin Exp Dermatol. 2015;40:605-610.
- Bulur I, Erdogan HK, Aksu AE, et al. The efficacy and safety of phototherapy in geriatric patients: a retrospective study. An Bras Dermatol. 2018;93:33-38.
- Dawe RS, Ibbotson SH. Drug-induced photosensitivity. Dermatol Clin. 2014;32:363-368, ix.
- Cameron H, Dawe RS. Photosensitizing drugs may lower the narrow-band ultraviolet B (TL-01) minimal erythema dose. Br J Dermatol. 2000;142:389-390.
- Elmets CA, Lim HW, Stoff B, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis with phototherapy. J Am Acad Dermatol. 2019;81:775-804.
- Gloor M, Scherotzke A. Age dependence of ultraviolet light-induced erythema following narrow-band UVB exposure. Photodermatol Photoimmunol Photomed. 2002;18:121-126.
- Cox NH, Diffey BL, Farr PM. The relationship between chronological age and the erythemal response to ultraviolet B radiation. Br J Dermatol. 1992;126:315-319.
- Morrison W. Phototherapy and Photochemotherapy for Skin Disease. 2nd ed. Informa Healthcare; 2005.
- Almutawa F, Alnomair N, Wang Y, et al. Systematic review of UV-based therapy for psoriasis. Am J Clin Dermatol. 2013;14:87-109.
- Trakatelli M, Bylaite-Bucinskiene M, Correia O, et al. Clinical assessment of skin phototypes: watch your words! Eur J Dermatol. 2017;27:615-619.
- Kwon IH, Kwon HH, Na SJ, et al. Could colorimetric method replace the individual minimal erythemal dose (MED) measurements in determining the initial dose of narrow-band UVB treatment for psoriasis patients with skin phototype III-V? J Eur Acad Dermatol Venereol. 2013;27:494-498.
- Chen WA, Chang CM. The minimal erythema dose of narrowband ultraviolet B in elderly Taiwanese [published online September 1, 2021]. Photodermatol Photoimmunol Photomed. doi:10.1111/phpp.12730
Practice Points
- Narrowband UVB (NB-UVB) phototherapy remains a safe and efficacious nonpharmacologic treatment for dermatologic conditions in older and younger adults.
- Compared to younger adults, older adults using the same protocols need similar or even fewer treatments to achieve high levels of clearance.
- Individuals taking 3 or more photosensitizing medications, regardless of age, may be at higher risk for substantial erythema with NB-UVB phototherapy.
- Phototherapy program monitoring is important to ensure quality care and investigate opportunities for care optimization.
Roflumilast foam effectively eases seborrheic dermatitis
.
More than half experienced clearance of their symptoms, and three out of five achieved a significant improvement in pruritus, it was revealed during a late-breaking session at the annual congress of the European Academy of Dermatology and Venereology.
Common condition led to rapid recruitment
“Seborrheic dermatitis is a disease that’s very common, yet in my opinion, undertreated in dermatology,” said Andrew Blauvelt, MD, MBA, who presented the findings.
“It’s so common that when we did this trial, I was very surprised to see how easy it was to recruit,” said Dr. Blauvelt, a dermatologist who is president of the Oregon Medical Research Center, Portland. “Patients came in rapidly, out of the woodwork – they were desperate.”
While there are several tried and tested treatments for the condition, such as topical steroids and antifungal agents, he noted that they have their limitations: “Sometimes efficacy, sometimes the ability to be used on hair-bearing areas.”
Roflumilast is a phosphodiesterase 4 (PDE4) inhibitor that is available for topical use in a 0.3% cream formulation (Zoryve). This formulation gained FDA approval for plaque psoriasis for patients ages 12 and older this summer and is also under investigation as a treatment for atopic dermatitis.
It’s the same product in both preparations, Dr. Blauvelt said during the discussion period. “The only major difference between the cream and the foam is the propellant used to make it into a foam. Otherwise, they have the exact same list of ingredients.”
Dr. Blauvelt reported that just over 450 patients had been recruited at 53 U.S. centers into the 8-week, double-blind, placebo-controlled trial.
For inclusion, patients had to have moderate seborrheic dermatitis, defined as an Investigator’s Global Assessment (IGA) score of three or more. Dr. Blauvelt noted that patients as young as 9 years old could be recruited, and there was no upper age limit. The average age of participating patients, however, was around 42 years.
Multiple improvements seen in ‘happy trial’
The primary endpoint was an IGA score of 0 or 1 with at least a 2-grade improvement (IGA success) after 8 weeks of treatment. This was achieved by 80% of patients who were treated with roflumilast 0.3% foam, compared with 60% of those who were treated with the vehicle (P less than .0001).
Dr. Blauvelt pointed out that significant improvements had also been seen after 2 weeks (about 42% vs. about 26%; P = .0003) and 4 weeks (about 72% vs. about 49%; P less than .0001) of treatment.
“Now if we raise the bar a little higher” and ask how many patients were completely clear of their seborrheic dermatitis, Dr. Blauvelt said, it was 50% at 8 weeks, more than a third at 4 weeks, over 15% at 2 weeks with the foam, and significantly lower at just under 30%, 15%, and 7% in the vehicle group.
A 4-point or more improvement in the Worst Itch Numeric Rating Scale (WI-NRS) – accepted as the minimally clinically important difference – was achieved by more than 60% of patients treated with the foam at week 8, just under 50% at week 4, and just over 30% at week 2. Corresponding rates in the vehicle group were around 40%, 30%, and 15%.
“Many patients responded in this trial. So much so that when I was doing it, I called it the ‘happy trial.’ Every time I saw patients in this trial, they seemed to be happy,” Dr. Blauvelt said anecdotally.
“In terms of adverse events, the drug turned out to be very safe, and there didn’t seem to be any issues with any things that we see with, for example, oral phosphodiesterase inhibitors,” he added.
The tolerability findings suggest that the foam vehicle “was an excellent vehicle to be used for this particular drug,” with no signs of skin irritation, as rated by patients or investigators.
Lesson for practice: Advise patients to moisturize?
“It seems like the vehicle would be a good skincare product for patients,” observed the session’s cochair, Jo Lambert, MD, PhD, professor and academic head of the department of dermatology at Ghent University Hospital, Belgium.
It was “a pretty dramatic vehicle response, right?” Dr. Blauvelt responded. “We normally don’t think of telling seborrheic dermatitis patients to moisturize,” he added.
“I think one of the interesting findings is perhaps we should be telling them to moisturize their scalp or moisturize their face, or it could be something unique to this particular foam.”
The study was funded by Arcutis Biotherapeutics. Dr. Blauvelt disclosed that he was an investigator for the trial and acted as consultant to the company, receiving grants/research funding and/or honoraria. Several of the study’s co-investigators are employees of Arcutis. Dr. Lambert was not involved in the study and cochaired the late-breaking session during which the STRATUM trial findings were reported.
A version of this article first appeared on Medscape.com.
.
More than half experienced clearance of their symptoms, and three out of five achieved a significant improvement in pruritus, it was revealed during a late-breaking session at the annual congress of the European Academy of Dermatology and Venereology.
Common condition led to rapid recruitment
“Seborrheic dermatitis is a disease that’s very common, yet in my opinion, undertreated in dermatology,” said Andrew Blauvelt, MD, MBA, who presented the findings.
“It’s so common that when we did this trial, I was very surprised to see how easy it was to recruit,” said Dr. Blauvelt, a dermatologist who is president of the Oregon Medical Research Center, Portland. “Patients came in rapidly, out of the woodwork – they were desperate.”
While there are several tried and tested treatments for the condition, such as topical steroids and antifungal agents, he noted that they have their limitations: “Sometimes efficacy, sometimes the ability to be used on hair-bearing areas.”
Roflumilast is a phosphodiesterase 4 (PDE4) inhibitor that is available for topical use in a 0.3% cream formulation (Zoryve). This formulation gained FDA approval for plaque psoriasis for patients ages 12 and older this summer and is also under investigation as a treatment for atopic dermatitis.
It’s the same product in both preparations, Dr. Blauvelt said during the discussion period. “The only major difference between the cream and the foam is the propellant used to make it into a foam. Otherwise, they have the exact same list of ingredients.”
Dr. Blauvelt reported that just over 450 patients had been recruited at 53 U.S. centers into the 8-week, double-blind, placebo-controlled trial.
For inclusion, patients had to have moderate seborrheic dermatitis, defined as an Investigator’s Global Assessment (IGA) score of three or more. Dr. Blauvelt noted that patients as young as 9 years old could be recruited, and there was no upper age limit. The average age of participating patients, however, was around 42 years.
Multiple improvements seen in ‘happy trial’
The primary endpoint was an IGA score of 0 or 1 with at least a 2-grade improvement (IGA success) after 8 weeks of treatment. This was achieved by 80% of patients who were treated with roflumilast 0.3% foam, compared with 60% of those who were treated with the vehicle (P less than .0001).
Dr. Blauvelt pointed out that significant improvements had also been seen after 2 weeks (about 42% vs. about 26%; P = .0003) and 4 weeks (about 72% vs. about 49%; P less than .0001) of treatment.
“Now if we raise the bar a little higher” and ask how many patients were completely clear of their seborrheic dermatitis, Dr. Blauvelt said, it was 50% at 8 weeks, more than a third at 4 weeks, over 15% at 2 weeks with the foam, and significantly lower at just under 30%, 15%, and 7% in the vehicle group.
A 4-point or more improvement in the Worst Itch Numeric Rating Scale (WI-NRS) – accepted as the minimally clinically important difference – was achieved by more than 60% of patients treated with the foam at week 8, just under 50% at week 4, and just over 30% at week 2. Corresponding rates in the vehicle group were around 40%, 30%, and 15%.
“Many patients responded in this trial. So much so that when I was doing it, I called it the ‘happy trial.’ Every time I saw patients in this trial, they seemed to be happy,” Dr. Blauvelt said anecdotally.
“In terms of adverse events, the drug turned out to be very safe, and there didn’t seem to be any issues with any things that we see with, for example, oral phosphodiesterase inhibitors,” he added.
The tolerability findings suggest that the foam vehicle “was an excellent vehicle to be used for this particular drug,” with no signs of skin irritation, as rated by patients or investigators.
Lesson for practice: Advise patients to moisturize?
“It seems like the vehicle would be a good skincare product for patients,” observed the session’s cochair, Jo Lambert, MD, PhD, professor and academic head of the department of dermatology at Ghent University Hospital, Belgium.
It was “a pretty dramatic vehicle response, right?” Dr. Blauvelt responded. “We normally don’t think of telling seborrheic dermatitis patients to moisturize,” he added.
“I think one of the interesting findings is perhaps we should be telling them to moisturize their scalp or moisturize their face, or it could be something unique to this particular foam.”
The study was funded by Arcutis Biotherapeutics. Dr. Blauvelt disclosed that he was an investigator for the trial and acted as consultant to the company, receiving grants/research funding and/or honoraria. Several of the study’s co-investigators are employees of Arcutis. Dr. Lambert was not involved in the study and cochaired the late-breaking session during which the STRATUM trial findings were reported.
A version of this article first appeared on Medscape.com.
.
More than half experienced clearance of their symptoms, and three out of five achieved a significant improvement in pruritus, it was revealed during a late-breaking session at the annual congress of the European Academy of Dermatology and Venereology.
Common condition led to rapid recruitment
“Seborrheic dermatitis is a disease that’s very common, yet in my opinion, undertreated in dermatology,” said Andrew Blauvelt, MD, MBA, who presented the findings.
“It’s so common that when we did this trial, I was very surprised to see how easy it was to recruit,” said Dr. Blauvelt, a dermatologist who is president of the Oregon Medical Research Center, Portland. “Patients came in rapidly, out of the woodwork – they were desperate.”
While there are several tried and tested treatments for the condition, such as topical steroids and antifungal agents, he noted that they have their limitations: “Sometimes efficacy, sometimes the ability to be used on hair-bearing areas.”
Roflumilast is a phosphodiesterase 4 (PDE4) inhibitor that is available for topical use in a 0.3% cream formulation (Zoryve). This formulation gained FDA approval for plaque psoriasis for patients ages 12 and older this summer and is also under investigation as a treatment for atopic dermatitis.
It’s the same product in both preparations, Dr. Blauvelt said during the discussion period. “The only major difference between the cream and the foam is the propellant used to make it into a foam. Otherwise, they have the exact same list of ingredients.”
Dr. Blauvelt reported that just over 450 patients had been recruited at 53 U.S. centers into the 8-week, double-blind, placebo-controlled trial.
For inclusion, patients had to have moderate seborrheic dermatitis, defined as an Investigator’s Global Assessment (IGA) score of three or more. Dr. Blauvelt noted that patients as young as 9 years old could be recruited, and there was no upper age limit. The average age of participating patients, however, was around 42 years.
Multiple improvements seen in ‘happy trial’
The primary endpoint was an IGA score of 0 or 1 with at least a 2-grade improvement (IGA success) after 8 weeks of treatment. This was achieved by 80% of patients who were treated with roflumilast 0.3% foam, compared with 60% of those who were treated with the vehicle (P less than .0001).
Dr. Blauvelt pointed out that significant improvements had also been seen after 2 weeks (about 42% vs. about 26%; P = .0003) and 4 weeks (about 72% vs. about 49%; P less than .0001) of treatment.
“Now if we raise the bar a little higher” and ask how many patients were completely clear of their seborrheic dermatitis, Dr. Blauvelt said, it was 50% at 8 weeks, more than a third at 4 weeks, over 15% at 2 weeks with the foam, and significantly lower at just under 30%, 15%, and 7% in the vehicle group.
A 4-point or more improvement in the Worst Itch Numeric Rating Scale (WI-NRS) – accepted as the minimally clinically important difference – was achieved by more than 60% of patients treated with the foam at week 8, just under 50% at week 4, and just over 30% at week 2. Corresponding rates in the vehicle group were around 40%, 30%, and 15%.
“Many patients responded in this trial. So much so that when I was doing it, I called it the ‘happy trial.’ Every time I saw patients in this trial, they seemed to be happy,” Dr. Blauvelt said anecdotally.
“In terms of adverse events, the drug turned out to be very safe, and there didn’t seem to be any issues with any things that we see with, for example, oral phosphodiesterase inhibitors,” he added.
The tolerability findings suggest that the foam vehicle “was an excellent vehicle to be used for this particular drug,” with no signs of skin irritation, as rated by patients or investigators.
Lesson for practice: Advise patients to moisturize?
“It seems like the vehicle would be a good skincare product for patients,” observed the session’s cochair, Jo Lambert, MD, PhD, professor and academic head of the department of dermatology at Ghent University Hospital, Belgium.
It was “a pretty dramatic vehicle response, right?” Dr. Blauvelt responded. “We normally don’t think of telling seborrheic dermatitis patients to moisturize,” he added.
“I think one of the interesting findings is perhaps we should be telling them to moisturize their scalp or moisturize their face, or it could be something unique to this particular foam.”
The study was funded by Arcutis Biotherapeutics. Dr. Blauvelt disclosed that he was an investigator for the trial and acted as consultant to the company, receiving grants/research funding and/or honoraria. Several of the study’s co-investigators are employees of Arcutis. Dr. Lambert was not involved in the study and cochaired the late-breaking session during which the STRATUM trial findings were reported.
A version of this article first appeared on Medscape.com.
FROM THE EADV CONGRESS

