User login
Drug is convenient alternative for PNH, doc says
STOCKHOLM—Results of a phase 3 study suggest the long-acting C5 complement inhibitor ravulizumab produces similar results as eculizumab in patients with paroxysmal nocturnal hemoglobinuria (PNH).
Treatment with ravulizumab every 8 weeks proved noninferior to treatment with eculizumab every 2 weeks for the co-primary endpoints of transfusion avoidance and hemolysis as measured by lactate dehydrogenase (LDH) normalization.
Ravulizumab also proved noninferior with regard to secondary efficacy endpoints and had a safety profile similar to that of eculizumab.
These results suggest ravulizumab could be a more convenient alternative for PNH patients, according to Jong Wook Lee, MD, of Seoul St. Mary’s Hospital in Seoul, South Korea.
Dr Lee presented these results as a late-breaking abstract (LB2603) at the 23rd Congress of the European Hematology Association (EHA).
The study was sponsored by Alexion Pharmaceuticals.
The trial enrolled adults with PNH naive to complement inhibitor therapy. They were randomized to receive ravulizumab (n=125) or eculizumab (n=121) for 183 days.
More than half of patients were male—52% in the ravulizumab arm and 57% in the eculizumab arm. Most patients were Asian (57.6% in the ravulizumab arm and 47.1% in the eculizumab arm) or white (34.4% and 42.1%, respectively).
Patients’ mean age at first infusion was 44.8 in the ravulizumab arm and 46.2 in the eculizumab arm. The mean number of years from PNH diagnosis to consent was 6.7 and 6.4, respectively.
The mean LDH at baseline was 1634 U/L in the ravulizumab arm and 1578 U/L in the eculizumab arm. The mean FACIT-Fatigue score was 36.7 and 36.9, respectively.
All 125 ravulizumab patients completed 26 weeks of treatment, as did 119 of the eculizumab patients. One hundred twenty-four ravulizumab patients entered the extension phase, as did 119 eculizumab patients.
Efficacy
The study’s primary efficacy endpoints were transfusion avoidance and LDH normalization from day 29 to 183. Dr Lee said ravulizumab proved noninferior to eculizumab for both endpoints, and point estimates favored ravulizumab.
The proportion of patients who remained transfusion-free was 73.6% in the ravulizumab arm and 66.1% in the eculizumab arm (difference, 6.8; 95% CI, -4.66, 18.14).
The proportion of patients who achieved LDH normalization was 53.6% and 49.4%, respectively (difference, 1.19; 95% CI, 0.8, 1.77).
Secondary efficacy endpoints included the percentage change in LDH from baseline, change in FACIT-Fatigue score from baseline, and the proportions of patients with breakthrough hemolysis and stabilized hemoglobin.
Again, ravulizumab was noninferior to eculizumab for all endpoints, with point estimates favoring ravulizumab.
The LDH percentage change was -76.84% in the ravulizumab arm and -76.02% in the eculizumab arm (difference, 0.83; 95% CI, -3.56, 5.21).
The change (improvement) in FACIT-Fatigue score was 7.07 and 6.40, respectively (difference, 0.67; 95% CI, -1.21, 2.55).
The percentage of patients with hemoglobin stabilization was 68.0% in the ravulizumab arm and 64.5% in the eculizumab arm (difference, 2.9; 95% CI, -8.80, 14.64).
The percentage of patients with breakthrough hemolysis was 4.0% and 10.7%, respectively (difference, 6.7; 95% CI, -0.18, 14.21).
Dr Lee noted that the proportion of patients with breakthrough hemolysis was more than 2.5-fold higher in the eculizumab arm than the ravulizumab arm—13 patients with 15 events and 5 patients with 5 events, respectively.
He said this was likely due to the immediate, complete, and sustained inhibition of C5 (mean free C5 <0.5 μg/mL) achieved by ravulizumab. Complete inhibition was observed after the first ravulizumab infusion and was sustained throughout the 26-week treatment period.
Safety
Dr Lee said ravulizumab had a similar safety profile to eculizumab, and both drugs were well tolerated.
Most patients experienced a treatment-emergent adverse event (TEAE)—88% in the ravulizumab arm and 86.8% in the eculizumab arm.
The most common TEAEs (in the ravulizumab and eculizumab arms, respectively) were headache (36.0% and 33.1%), nasopharyngitis (8.8% and 14.9%), upper respiratory tract infection (10.4% and 5.8%), and pyrexia (4.8% and 10.7%).
Serious AEs occurred in 8.8% of patients in the ravulizumab arm and 7.4% of those in the eculizumab arm.
Major adverse vascular events occurred in 2 patients in the ravulizumab arm and 1 in the eculizumab arm. There were no meningococcal infections in either arm.
One patient in the eculizumab arm was discontinued from the study and died of lung cancer (which was unrelated to treatment).
STOCKHOLM—Results of a phase 3 study suggest the long-acting C5 complement inhibitor ravulizumab produces similar results as eculizumab in patients with paroxysmal nocturnal hemoglobinuria (PNH).
Treatment with ravulizumab every 8 weeks proved noninferior to treatment with eculizumab every 2 weeks for the co-primary endpoints of transfusion avoidance and hemolysis as measured by lactate dehydrogenase (LDH) normalization.
Ravulizumab also proved noninferior with regard to secondary efficacy endpoints and had a safety profile similar to that of eculizumab.
These results suggest ravulizumab could be a more convenient alternative for PNH patients, according to Jong Wook Lee, MD, of Seoul St. Mary’s Hospital in Seoul, South Korea.
Dr Lee presented these results as a late-breaking abstract (LB2603) at the 23rd Congress of the European Hematology Association (EHA).
The study was sponsored by Alexion Pharmaceuticals.
The trial enrolled adults with PNH naive to complement inhibitor therapy. They were randomized to receive ravulizumab (n=125) or eculizumab (n=121) for 183 days.
More than half of patients were male—52% in the ravulizumab arm and 57% in the eculizumab arm. Most patients were Asian (57.6% in the ravulizumab arm and 47.1% in the eculizumab arm) or white (34.4% and 42.1%, respectively).
Patients’ mean age at first infusion was 44.8 in the ravulizumab arm and 46.2 in the eculizumab arm. The mean number of years from PNH diagnosis to consent was 6.7 and 6.4, respectively.
The mean LDH at baseline was 1634 U/L in the ravulizumab arm and 1578 U/L in the eculizumab arm. The mean FACIT-Fatigue score was 36.7 and 36.9, respectively.
All 125 ravulizumab patients completed 26 weeks of treatment, as did 119 of the eculizumab patients. One hundred twenty-four ravulizumab patients entered the extension phase, as did 119 eculizumab patients.
Efficacy
The study’s primary efficacy endpoints were transfusion avoidance and LDH normalization from day 29 to 183. Dr Lee said ravulizumab proved noninferior to eculizumab for both endpoints, and point estimates favored ravulizumab.
The proportion of patients who remained transfusion-free was 73.6% in the ravulizumab arm and 66.1% in the eculizumab arm (difference, 6.8; 95% CI, -4.66, 18.14).
The proportion of patients who achieved LDH normalization was 53.6% and 49.4%, respectively (difference, 1.19; 95% CI, 0.8, 1.77).
Secondary efficacy endpoints included the percentage change in LDH from baseline, change in FACIT-Fatigue score from baseline, and the proportions of patients with breakthrough hemolysis and stabilized hemoglobin.
Again, ravulizumab was noninferior to eculizumab for all endpoints, with point estimates favoring ravulizumab.
The LDH percentage change was -76.84% in the ravulizumab arm and -76.02% in the eculizumab arm (difference, 0.83; 95% CI, -3.56, 5.21).
The change (improvement) in FACIT-Fatigue score was 7.07 and 6.40, respectively (difference, 0.67; 95% CI, -1.21, 2.55).
The percentage of patients with hemoglobin stabilization was 68.0% in the ravulizumab arm and 64.5% in the eculizumab arm (difference, 2.9; 95% CI, -8.80, 14.64).
The percentage of patients with breakthrough hemolysis was 4.0% and 10.7%, respectively (difference, 6.7; 95% CI, -0.18, 14.21).
Dr Lee noted that the proportion of patients with breakthrough hemolysis was more than 2.5-fold higher in the eculizumab arm than the ravulizumab arm—13 patients with 15 events and 5 patients with 5 events, respectively.
He said this was likely due to the immediate, complete, and sustained inhibition of C5 (mean free C5 <0.5 μg/mL) achieved by ravulizumab. Complete inhibition was observed after the first ravulizumab infusion and was sustained throughout the 26-week treatment period.
Safety
Dr Lee said ravulizumab had a similar safety profile to eculizumab, and both drugs were well tolerated.
Most patients experienced a treatment-emergent adverse event (TEAE)—88% in the ravulizumab arm and 86.8% in the eculizumab arm.
The most common TEAEs (in the ravulizumab and eculizumab arms, respectively) were headache (36.0% and 33.1%), nasopharyngitis (8.8% and 14.9%), upper respiratory tract infection (10.4% and 5.8%), and pyrexia (4.8% and 10.7%).
Serious AEs occurred in 8.8% of patients in the ravulizumab arm and 7.4% of those in the eculizumab arm.
Major adverse vascular events occurred in 2 patients in the ravulizumab arm and 1 in the eculizumab arm. There were no meningococcal infections in either arm.
One patient in the eculizumab arm was discontinued from the study and died of lung cancer (which was unrelated to treatment).
STOCKHOLM—Results of a phase 3 study suggest the long-acting C5 complement inhibitor ravulizumab produces similar results as eculizumab in patients with paroxysmal nocturnal hemoglobinuria (PNH).
Treatment with ravulizumab every 8 weeks proved noninferior to treatment with eculizumab every 2 weeks for the co-primary endpoints of transfusion avoidance and hemolysis as measured by lactate dehydrogenase (LDH) normalization.
Ravulizumab also proved noninferior with regard to secondary efficacy endpoints and had a safety profile similar to that of eculizumab.
These results suggest ravulizumab could be a more convenient alternative for PNH patients, according to Jong Wook Lee, MD, of Seoul St. Mary’s Hospital in Seoul, South Korea.
Dr Lee presented these results as a late-breaking abstract (LB2603) at the 23rd Congress of the European Hematology Association (EHA).
The study was sponsored by Alexion Pharmaceuticals.
The trial enrolled adults with PNH naive to complement inhibitor therapy. They were randomized to receive ravulizumab (n=125) or eculizumab (n=121) for 183 days.
More than half of patients were male—52% in the ravulizumab arm and 57% in the eculizumab arm. Most patients were Asian (57.6% in the ravulizumab arm and 47.1% in the eculizumab arm) or white (34.4% and 42.1%, respectively).
Patients’ mean age at first infusion was 44.8 in the ravulizumab arm and 46.2 in the eculizumab arm. The mean number of years from PNH diagnosis to consent was 6.7 and 6.4, respectively.
The mean LDH at baseline was 1634 U/L in the ravulizumab arm and 1578 U/L in the eculizumab arm. The mean FACIT-Fatigue score was 36.7 and 36.9, respectively.
All 125 ravulizumab patients completed 26 weeks of treatment, as did 119 of the eculizumab patients. One hundred twenty-four ravulizumab patients entered the extension phase, as did 119 eculizumab patients.
Efficacy
The study’s primary efficacy endpoints were transfusion avoidance and LDH normalization from day 29 to 183. Dr Lee said ravulizumab proved noninferior to eculizumab for both endpoints, and point estimates favored ravulizumab.
The proportion of patients who remained transfusion-free was 73.6% in the ravulizumab arm and 66.1% in the eculizumab arm (difference, 6.8; 95% CI, -4.66, 18.14).
The proportion of patients who achieved LDH normalization was 53.6% and 49.4%, respectively (difference, 1.19; 95% CI, 0.8, 1.77).
Secondary efficacy endpoints included the percentage change in LDH from baseline, change in FACIT-Fatigue score from baseline, and the proportions of patients with breakthrough hemolysis and stabilized hemoglobin.
Again, ravulizumab was noninferior to eculizumab for all endpoints, with point estimates favoring ravulizumab.
The LDH percentage change was -76.84% in the ravulizumab arm and -76.02% in the eculizumab arm (difference, 0.83; 95% CI, -3.56, 5.21).
The change (improvement) in FACIT-Fatigue score was 7.07 and 6.40, respectively (difference, 0.67; 95% CI, -1.21, 2.55).
The percentage of patients with hemoglobin stabilization was 68.0% in the ravulizumab arm and 64.5% in the eculizumab arm (difference, 2.9; 95% CI, -8.80, 14.64).
The percentage of patients with breakthrough hemolysis was 4.0% and 10.7%, respectively (difference, 6.7; 95% CI, -0.18, 14.21).
Dr Lee noted that the proportion of patients with breakthrough hemolysis was more than 2.5-fold higher in the eculizumab arm than the ravulizumab arm—13 patients with 15 events and 5 patients with 5 events, respectively.
He said this was likely due to the immediate, complete, and sustained inhibition of C5 (mean free C5 <0.5 μg/mL) achieved by ravulizumab. Complete inhibition was observed after the first ravulizumab infusion and was sustained throughout the 26-week treatment period.
Safety
Dr Lee said ravulizumab had a similar safety profile to eculizumab, and both drugs were well tolerated.
Most patients experienced a treatment-emergent adverse event (TEAE)—88% in the ravulizumab arm and 86.8% in the eculizumab arm.
The most common TEAEs (in the ravulizumab and eculizumab arms, respectively) were headache (36.0% and 33.1%), nasopharyngitis (8.8% and 14.9%), upper respiratory tract infection (10.4% and 5.8%), and pyrexia (4.8% and 10.7%).
Serious AEs occurred in 8.8% of patients in the ravulizumab arm and 7.4% of those in the eculizumab arm.
Major adverse vascular events occurred in 2 patients in the ravulizumab arm and 1 in the eculizumab arm. There were no meningococcal infections in either arm.
One patient in the eculizumab arm was discontinued from the study and died of lung cancer (which was unrelated to treatment).
Hemophilia adherence tied to perception of disease
More than half (56%) of adult patients with hemophilia are adherent to a prescribed prophylaxis regimen, but compliance appears less likely among patients who are having difficulty coping with pain or have a high conviction of disease.
Ana Torres-Ortuño, PhD, of the University of Murcia (Spain) and her colleagues performed a multicenter, cross-sectional descriptive study of 23 adult patients with severe hemophilia A or hemophilia B using various validated questionnaires that measured quality of life, disease perception, coping strategies, and treatment adherence.
The researchers found that Patients who experienced haemarthrosis with greater frequency had significantly greater adherence in terms of dosing (P less than .05), planning (P less than .05), and skipping (P less than .01). Similarly, patients with HIV infection were more adherent in terms of frequency of infusion than patients without infection.
The researchers also found significant correlations among all the psychosocial variables measured and adherence to prophylaxis. For instance, patients who had poorer quality of life related to managing their physical health, pain, and emotions showed poorer planning of their treatment. Patients who had difficulty remembering treatment had poorer quality of life related to pain and vitality, but they also had greater conviction of disease and hypochondriasis.
“Intervention programmes should be aimed more at changing barriers that patients and caregivers encounter when accepting diagnosis and how they can adapt their resources and skills to better take advantage of the progress made in treatments,” the researchers wrote.
The study was supported by a grant from Pfizer. The researchers reported having no financial disclosures.
SOURCE: Torres-Ortuño A et al. Vox Sang. 2018 May 24. doi: 10.1111/vox.12669.
More than half (56%) of adult patients with hemophilia are adherent to a prescribed prophylaxis regimen, but compliance appears less likely among patients who are having difficulty coping with pain or have a high conviction of disease.
Ana Torres-Ortuño, PhD, of the University of Murcia (Spain) and her colleagues performed a multicenter, cross-sectional descriptive study of 23 adult patients with severe hemophilia A or hemophilia B using various validated questionnaires that measured quality of life, disease perception, coping strategies, and treatment adherence.
The researchers found that Patients who experienced haemarthrosis with greater frequency had significantly greater adherence in terms of dosing (P less than .05), planning (P less than .05), and skipping (P less than .01). Similarly, patients with HIV infection were more adherent in terms of frequency of infusion than patients without infection.
The researchers also found significant correlations among all the psychosocial variables measured and adherence to prophylaxis. For instance, patients who had poorer quality of life related to managing their physical health, pain, and emotions showed poorer planning of their treatment. Patients who had difficulty remembering treatment had poorer quality of life related to pain and vitality, but they also had greater conviction of disease and hypochondriasis.
“Intervention programmes should be aimed more at changing barriers that patients and caregivers encounter when accepting diagnosis and how they can adapt their resources and skills to better take advantage of the progress made in treatments,” the researchers wrote.
The study was supported by a grant from Pfizer. The researchers reported having no financial disclosures.
SOURCE: Torres-Ortuño A et al. Vox Sang. 2018 May 24. doi: 10.1111/vox.12669.
More than half (56%) of adult patients with hemophilia are adherent to a prescribed prophylaxis regimen, but compliance appears less likely among patients who are having difficulty coping with pain or have a high conviction of disease.
Ana Torres-Ortuño, PhD, of the University of Murcia (Spain) and her colleagues performed a multicenter, cross-sectional descriptive study of 23 adult patients with severe hemophilia A or hemophilia B using various validated questionnaires that measured quality of life, disease perception, coping strategies, and treatment adherence.
The researchers found that Patients who experienced haemarthrosis with greater frequency had significantly greater adherence in terms of dosing (P less than .05), planning (P less than .05), and skipping (P less than .01). Similarly, patients with HIV infection were more adherent in terms of frequency of infusion than patients without infection.
The researchers also found significant correlations among all the psychosocial variables measured and adherence to prophylaxis. For instance, patients who had poorer quality of life related to managing their physical health, pain, and emotions showed poorer planning of their treatment. Patients who had difficulty remembering treatment had poorer quality of life related to pain and vitality, but they also had greater conviction of disease and hypochondriasis.
“Intervention programmes should be aimed more at changing barriers that patients and caregivers encounter when accepting diagnosis and how they can adapt their resources and skills to better take advantage of the progress made in treatments,” the researchers wrote.
The study was supported by a grant from Pfizer. The researchers reported having no financial disclosures.
SOURCE: Torres-Ortuño A et al. Vox Sang. 2018 May 24. doi: 10.1111/vox.12669.
FROM VOX SANGUINIS
Emicizumab granted priority review for hemophilia A without inhibitors
The US Food and Drug Administration (FDA) has granted priority review for emicizumab (Hemlibra®) for adults and children with hemophilia A without factor VIII inhibitors.
Earlier this year, the agency awarded emicizumab breakthrough therapy designation for the same population.
Emicizumab is a bispecific factor IXa- and factor X-directed antibody approved by the FDA for routine prophylaxis to prevent or reduce the frequency of bleeding episodes in adults and children who have hemophilia A with factor VIII inhibitors.
The FDA based its decision to grant emicizumab priority review on the phase 3 HAVEN 3 study, results of which were presented recently at the World Federation of Hemophilia congress.
In HAVEN 3, emicizumab demonstrated a 68% reduction (P<0.0001) in treated bleeds based on an intra-patient comparison in patients who were previously enrolled in a prospective non-interventional study.
According to Genentech, co-developer of the drug, this makes emicizumab the first medicine to show superior efficacy to prior treatment with factor VIII prophylaxis, the current standard of care for people with hemophilia A without factor VIII inhibitors.
About HAVEN 3
The randomized, multicenter, open-label trial evaluated prophylaxis versus no prophylaxis in patients without factor VIII inhibitors.
The study included 152 patients 12 years or older who were previously treated with factor VIII therapy on-demand or as prophylaxis.
Patients previously treated with on-demand factor VIII were randomized in a 2:2:1 fashion to 1 of 3 treatment groups:
- Arm A received emicizumab prophylaxis at 3 mg/kg/wk for 4 weeks, followed by 1.5 mg/kg/wk until the end of study.
- Arm B received emicizumab prophylaxis at 3 mg/kg/wk for 4 weeks, followed by 3 mg/kg/2wks for at least 24 weeks.
- Arm C received no prophylaxis
Patients previously treated prophylactically with factor VIII were enrolled in Arm D and received emicizumab prophylaxis at 3 mg/kg/wk for 4 weeks, followed by 1.5 mg/kg/wk until the end of study.
The protocol permitted episodic treatment of breakthrough bleeds with factor VIII therapy.
Patients in the prophylaxis groups achieved a 96% (P<0.0001) and 97% (P<0.0001) reduction in treated bleeds, respectively, compared to those who received no prophylaxis.
Additionally, 55.6% of patients treated weekly and 60% treated every 2 weeks had no treated bleeds. In contrast, 0% in the prophylaxis group achieved zero treated bleeds.
Investigators observed no unexpected or serious adverse events (AEs), no thrombotic events, and no cases of thrombotic microangiopathy.
The most common AEs occurring in 5% or more of patients were injection site reactions, arthralgia, nasopharyngitis, headache, upper respiratory tract infection, and influenza.
The FDA is expected to make a decision regarding approval by October 4.
The US Food and Drug Administration (FDA) has granted priority review for emicizumab (Hemlibra®) for adults and children with hemophilia A without factor VIII inhibitors.
Earlier this year, the agency awarded emicizumab breakthrough therapy designation for the same population.
Emicizumab is a bispecific factor IXa- and factor X-directed antibody approved by the FDA for routine prophylaxis to prevent or reduce the frequency of bleeding episodes in adults and children who have hemophilia A with factor VIII inhibitors.
The FDA based its decision to grant emicizumab priority review on the phase 3 HAVEN 3 study, results of which were presented recently at the World Federation of Hemophilia congress.
In HAVEN 3, emicizumab demonstrated a 68% reduction (P<0.0001) in treated bleeds based on an intra-patient comparison in patients who were previously enrolled in a prospective non-interventional study.
According to Genentech, co-developer of the drug, this makes emicizumab the first medicine to show superior efficacy to prior treatment with factor VIII prophylaxis, the current standard of care for people with hemophilia A without factor VIII inhibitors.
About HAVEN 3
The randomized, multicenter, open-label trial evaluated prophylaxis versus no prophylaxis in patients without factor VIII inhibitors.
The study included 152 patients 12 years or older who were previously treated with factor VIII therapy on-demand or as prophylaxis.
Patients previously treated with on-demand factor VIII were randomized in a 2:2:1 fashion to 1 of 3 treatment groups:
- Arm A received emicizumab prophylaxis at 3 mg/kg/wk for 4 weeks, followed by 1.5 mg/kg/wk until the end of study.
- Arm B received emicizumab prophylaxis at 3 mg/kg/wk for 4 weeks, followed by 3 mg/kg/2wks for at least 24 weeks.
- Arm C received no prophylaxis
Patients previously treated prophylactically with factor VIII were enrolled in Arm D and received emicizumab prophylaxis at 3 mg/kg/wk for 4 weeks, followed by 1.5 mg/kg/wk until the end of study.
The protocol permitted episodic treatment of breakthrough bleeds with factor VIII therapy.
Patients in the prophylaxis groups achieved a 96% (P<0.0001) and 97% (P<0.0001) reduction in treated bleeds, respectively, compared to those who received no prophylaxis.
Additionally, 55.6% of patients treated weekly and 60% treated every 2 weeks had no treated bleeds. In contrast, 0% in the prophylaxis group achieved zero treated bleeds.
Investigators observed no unexpected or serious adverse events (AEs), no thrombotic events, and no cases of thrombotic microangiopathy.
The most common AEs occurring in 5% or more of patients were injection site reactions, arthralgia, nasopharyngitis, headache, upper respiratory tract infection, and influenza.
The FDA is expected to make a decision regarding approval by October 4.
The US Food and Drug Administration (FDA) has granted priority review for emicizumab (Hemlibra®) for adults and children with hemophilia A without factor VIII inhibitors.
Earlier this year, the agency awarded emicizumab breakthrough therapy designation for the same population.
Emicizumab is a bispecific factor IXa- and factor X-directed antibody approved by the FDA for routine prophylaxis to prevent or reduce the frequency of bleeding episodes in adults and children who have hemophilia A with factor VIII inhibitors.
The FDA based its decision to grant emicizumab priority review on the phase 3 HAVEN 3 study, results of which were presented recently at the World Federation of Hemophilia congress.
In HAVEN 3, emicizumab demonstrated a 68% reduction (P<0.0001) in treated bleeds based on an intra-patient comparison in patients who were previously enrolled in a prospective non-interventional study.
According to Genentech, co-developer of the drug, this makes emicizumab the first medicine to show superior efficacy to prior treatment with factor VIII prophylaxis, the current standard of care for people with hemophilia A without factor VIII inhibitors.
About HAVEN 3
The randomized, multicenter, open-label trial evaluated prophylaxis versus no prophylaxis in patients without factor VIII inhibitors.
The study included 152 patients 12 years or older who were previously treated with factor VIII therapy on-demand or as prophylaxis.
Patients previously treated with on-demand factor VIII were randomized in a 2:2:1 fashion to 1 of 3 treatment groups:
- Arm A received emicizumab prophylaxis at 3 mg/kg/wk for 4 weeks, followed by 1.5 mg/kg/wk until the end of study.
- Arm B received emicizumab prophylaxis at 3 mg/kg/wk for 4 weeks, followed by 3 mg/kg/2wks for at least 24 weeks.
- Arm C received no prophylaxis
Patients previously treated prophylactically with factor VIII were enrolled in Arm D and received emicizumab prophylaxis at 3 mg/kg/wk for 4 weeks, followed by 1.5 mg/kg/wk until the end of study.
The protocol permitted episodic treatment of breakthrough bleeds with factor VIII therapy.
Patients in the prophylaxis groups achieved a 96% (P<0.0001) and 97% (P<0.0001) reduction in treated bleeds, respectively, compared to those who received no prophylaxis.
Additionally, 55.6% of patients treated weekly and 60% treated every 2 weeks had no treated bleeds. In contrast, 0% in the prophylaxis group achieved zero treated bleeds.
Investigators observed no unexpected or serious adverse events (AEs), no thrombotic events, and no cases of thrombotic microangiopathy.
The most common AEs occurring in 5% or more of patients were injection site reactions, arthralgia, nasopharyngitis, headache, upper respiratory tract infection, and influenza.
The FDA is expected to make a decision regarding approval by October 4.
Emicizumab gets priority review for hemophilia A without inhibitors
The Food and Drug Administration has granted priority review to Roche’s emicizumab-kxwh (Hemlibra) for the treatment of adults and children with hemophilia A without factor VIII inhibitors.
The agency is scheduled to make a decision on approval in October 2018.
Among patients aged 12 years and older without factor VIII inhibitors, emicizumab-kxwh prophylaxis every week reduced treated bleeds by 96% (P less than .0001) and treated bleeds were reduced by 97% (P less than .0001) in patients who were treated every 2 weeks, according to Roche. The drug-treated group was compared with patients who received no prophylaxis. Another arm of the study examined patients who had previously received factor VIII prophylaxis and then switched to emicizumab-kxwh prophylaxis. In an intrapatient comparison, emicizumab-kxwh showed a 68% reduction in treated bleeds, which was statistically significant and demonstrated superior efficacy to factor VIII prophylaxis.
Emicizumab-kxwh was approved by FDA in November 2017 for routine prophylaxis for adults and children with hemophilia A with factor VIII inhibitors. That approval was based on results from the HAVEN 1 and HAVEN 2 studies.
The Food and Drug Administration has granted priority review to Roche’s emicizumab-kxwh (Hemlibra) for the treatment of adults and children with hemophilia A without factor VIII inhibitors.
The agency is scheduled to make a decision on approval in October 2018.
Among patients aged 12 years and older without factor VIII inhibitors, emicizumab-kxwh prophylaxis every week reduced treated bleeds by 96% (P less than .0001) and treated bleeds were reduced by 97% (P less than .0001) in patients who were treated every 2 weeks, according to Roche. The drug-treated group was compared with patients who received no prophylaxis. Another arm of the study examined patients who had previously received factor VIII prophylaxis and then switched to emicizumab-kxwh prophylaxis. In an intrapatient comparison, emicizumab-kxwh showed a 68% reduction in treated bleeds, which was statistically significant and demonstrated superior efficacy to factor VIII prophylaxis.
Emicizumab-kxwh was approved by FDA in November 2017 for routine prophylaxis for adults and children with hemophilia A with factor VIII inhibitors. That approval was based on results from the HAVEN 1 and HAVEN 2 studies.
The Food and Drug Administration has granted priority review to Roche’s emicizumab-kxwh (Hemlibra) for the treatment of adults and children with hemophilia A without factor VIII inhibitors.
The agency is scheduled to make a decision on approval in October 2018.
Among patients aged 12 years and older without factor VIII inhibitors, emicizumab-kxwh prophylaxis every week reduced treated bleeds by 96% (P less than .0001) and treated bleeds were reduced by 97% (P less than .0001) in patients who were treated every 2 weeks, according to Roche. The drug-treated group was compared with patients who received no prophylaxis. Another arm of the study examined patients who had previously received factor VIII prophylaxis and then switched to emicizumab-kxwh prophylaxis. In an intrapatient comparison, emicizumab-kxwh showed a 68% reduction in treated bleeds, which was statistically significant and demonstrated superior efficacy to factor VIII prophylaxis.
Emicizumab-kxwh was approved by FDA in November 2017 for routine prophylaxis for adults and children with hemophilia A with factor VIII inhibitors. That approval was based on results from the HAVEN 1 and HAVEN 2 studies.
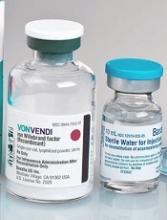
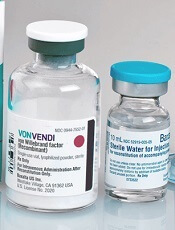
Perioperative rVWF alone sufficient for some VWD patients
GLASGOW—Recombinant von Willebrand factor (rVWF) alone can be sufficient as perioperative management for some patients with severe von Willebrand disease (VWD), according to researchers.
In a phase 3 study, 10 of 15 patients were able to achieve hemostatic efficacy ratings of “good” or “excellent” when receiving only rVWF before, during, and/or after surgery.
The remaining 5 patients also achieved favorable hemostatic efficacy ratings, but they received recombinant factor VIII (FVIII) as well.
These results were presented at the World Federation of Hemophilia (WFH) 2018 World Congress (abstract W-MP-63 [749]). The research was sponsored by Shire, the company marketing rVWF as Vonvendi.
“There is an unmet clinical need for those living with von Willebrand disease, as they face a heightened risk of bleeding during surgery,” said study investigator Flora Peyvandi, MD, PhD, of the University of Milan in Italy.
“People with von Willebrand disease lack proper function or quantity of von Willebrand factor, and some also have a secondary factor VIII deficiency. In this study, recombinant von Willebrand factor was administered to replace the insufficient or dysfunctional von Willebrand factor, allowing the body to naturally replenish FVIII in most patients. These study results demonstrate clinical promise as physicians were able to tailor treatment based on each patient’s individual need for one or both factor therapies.”
The study included 15 adults with severe VWD who were undergoing elective surgical procedures. Ten patients were undergoing major surgery, 4 minor, and 1 oral surgery.
The patients’ median age was 40 (range, 20-70), and 8 were female. Most (n=8) had type 3 VWD, 3 had type 1, 2 had type 2A, 1 had 2B, and 1 had 2M.
At baseline, the mean endogenous FVIII level (FVIII:C) was 16.4 IU/dL, and the mean VWF ristocetin cofactor (VWF:Rco) was 10.6 IU/dL.
The patients received rVWF at 40 to 60 IU/kg VWF:RCo intravenously 12 to 24 hours before surgery to allow FVIII:C levels to increase to at least 30 IU/dL for minor or oral surgery or to at least 60 IU/dL for major surgery, within 3 hours before surgery.
If the desired levels were achieved, rVWF could be given alone. If the levels were not achieved, patients would receive rFVIII as well, within 1 to 2 hours before surgery. Patients were monitored for 14 days after surgery.
Results
All 15 patients had overall/intraoperative hemostatic efficacy ratings of “excellent” (as good as or better than expected) or “good” (probably as good as expected).
The patients received a median of 6 (range, 2 to 15) rVWF infusions at a median dose of 55 IU/kg (range, 36.1 to 59.9). Most patients (n=11) did not receive rVWF every day. For some, infusions were separated by 2 to 9 days.
Ten patients received rVWF alone, 12 did not receive any preoperative FVIII, and 2 did not receive rVWF postoperatively.
Most rVWF infusions (89.4%, 93/104) were given alone, and 70% (7/10) of the major surgeries were performed with rVWF alone.
The researchers said that, with rVWF alone, patients had hemostatically effective levels of FVIII:C as early as 6 hours after surgery, and this was sustained for 72 to 96 hours.
There were 5 patients who received rVWF with rFVIII. Of the 11 infusions these patients received, 9 were given when FVIII:C levels were above 60 IU/dL.
Three patients received rVWF with rFVIII 1 hour before major surgery—total hip replacement, molar extraction, and left ankle prosthesis. However, 2 of these patients had FVIII:C levels above 60 IU/dL.
The patient undergoing a molar extraction received rVWF with rFVIII 6 times after surgery. In 5 cases, the patient’s FVIII:C levels were 110 to 152 IU/dL. In the remaining case, the FVIII:C level was 23 IU/dL.
Two patients received rVWF with rFVIII for minor surgery. One patient undergoing a tooth extraction received rVWF with rFVIII intraoperatively when the FVIII:C level was 72 IU/dL.
The other patient received rVWF with rFVIII after radioisotope synovectomy when the FVIII:C level was 73 IU/dL. This patient received a postoperative dose of rVWF alone as well.
One patient tested positive for binding antibodies to VWF, and 1 patient developed deep vein thrombosis 3 days after total hip replacement while receiving rVWF.
GLASGOW—Recombinant von Willebrand factor (rVWF) alone can be sufficient as perioperative management for some patients with severe von Willebrand disease (VWD), according to researchers.
In a phase 3 study, 10 of 15 patients were able to achieve hemostatic efficacy ratings of “good” or “excellent” when receiving only rVWF before, during, and/or after surgery.
The remaining 5 patients also achieved favorable hemostatic efficacy ratings, but they received recombinant factor VIII (FVIII) as well.
These results were presented at the World Federation of Hemophilia (WFH) 2018 World Congress (abstract W-MP-63 [749]). The research was sponsored by Shire, the company marketing rVWF as Vonvendi.
“There is an unmet clinical need for those living with von Willebrand disease, as they face a heightened risk of bleeding during surgery,” said study investigator Flora Peyvandi, MD, PhD, of the University of Milan in Italy.
“People with von Willebrand disease lack proper function or quantity of von Willebrand factor, and some also have a secondary factor VIII deficiency. In this study, recombinant von Willebrand factor was administered to replace the insufficient or dysfunctional von Willebrand factor, allowing the body to naturally replenish FVIII in most patients. These study results demonstrate clinical promise as physicians were able to tailor treatment based on each patient’s individual need for one or both factor therapies.”
The study included 15 adults with severe VWD who were undergoing elective surgical procedures. Ten patients were undergoing major surgery, 4 minor, and 1 oral surgery.
The patients’ median age was 40 (range, 20-70), and 8 were female. Most (n=8) had type 3 VWD, 3 had type 1, 2 had type 2A, 1 had 2B, and 1 had 2M.
At baseline, the mean endogenous FVIII level (FVIII:C) was 16.4 IU/dL, and the mean VWF ristocetin cofactor (VWF:Rco) was 10.6 IU/dL.
The patients received rVWF at 40 to 60 IU/kg VWF:RCo intravenously 12 to 24 hours before surgery to allow FVIII:C levels to increase to at least 30 IU/dL for minor or oral surgery or to at least 60 IU/dL for major surgery, within 3 hours before surgery.
If the desired levels were achieved, rVWF could be given alone. If the levels were not achieved, patients would receive rFVIII as well, within 1 to 2 hours before surgery. Patients were monitored for 14 days after surgery.
Results
All 15 patients had overall/intraoperative hemostatic efficacy ratings of “excellent” (as good as or better than expected) or “good” (probably as good as expected).
The patients received a median of 6 (range, 2 to 15) rVWF infusions at a median dose of 55 IU/kg (range, 36.1 to 59.9). Most patients (n=11) did not receive rVWF every day. For some, infusions were separated by 2 to 9 days.
Ten patients received rVWF alone, 12 did not receive any preoperative FVIII, and 2 did not receive rVWF postoperatively.
Most rVWF infusions (89.4%, 93/104) were given alone, and 70% (7/10) of the major surgeries were performed with rVWF alone.
The researchers said that, with rVWF alone, patients had hemostatically effective levels of FVIII:C as early as 6 hours after surgery, and this was sustained for 72 to 96 hours.
There were 5 patients who received rVWF with rFVIII. Of the 11 infusions these patients received, 9 were given when FVIII:C levels were above 60 IU/dL.
Three patients received rVWF with rFVIII 1 hour before major surgery—total hip replacement, molar extraction, and left ankle prosthesis. However, 2 of these patients had FVIII:C levels above 60 IU/dL.
The patient undergoing a molar extraction received rVWF with rFVIII 6 times after surgery. In 5 cases, the patient’s FVIII:C levels were 110 to 152 IU/dL. In the remaining case, the FVIII:C level was 23 IU/dL.
Two patients received rVWF with rFVIII for minor surgery. One patient undergoing a tooth extraction received rVWF with rFVIII intraoperatively when the FVIII:C level was 72 IU/dL.
The other patient received rVWF with rFVIII after radioisotope synovectomy when the FVIII:C level was 73 IU/dL. This patient received a postoperative dose of rVWF alone as well.
One patient tested positive for binding antibodies to VWF, and 1 patient developed deep vein thrombosis 3 days after total hip replacement while receiving rVWF.
GLASGOW—Recombinant von Willebrand factor (rVWF) alone can be sufficient as perioperative management for some patients with severe von Willebrand disease (VWD), according to researchers.
In a phase 3 study, 10 of 15 patients were able to achieve hemostatic efficacy ratings of “good” or “excellent” when receiving only rVWF before, during, and/or after surgery.
The remaining 5 patients also achieved favorable hemostatic efficacy ratings, but they received recombinant factor VIII (FVIII) as well.
These results were presented at the World Federation of Hemophilia (WFH) 2018 World Congress (abstract W-MP-63 [749]). The research was sponsored by Shire, the company marketing rVWF as Vonvendi.
“There is an unmet clinical need for those living with von Willebrand disease, as they face a heightened risk of bleeding during surgery,” said study investigator Flora Peyvandi, MD, PhD, of the University of Milan in Italy.
“People with von Willebrand disease lack proper function or quantity of von Willebrand factor, and some also have a secondary factor VIII deficiency. In this study, recombinant von Willebrand factor was administered to replace the insufficient or dysfunctional von Willebrand factor, allowing the body to naturally replenish FVIII in most patients. These study results demonstrate clinical promise as physicians were able to tailor treatment based on each patient’s individual need for one or both factor therapies.”
The study included 15 adults with severe VWD who were undergoing elective surgical procedures. Ten patients were undergoing major surgery, 4 minor, and 1 oral surgery.
The patients’ median age was 40 (range, 20-70), and 8 were female. Most (n=8) had type 3 VWD, 3 had type 1, 2 had type 2A, 1 had 2B, and 1 had 2M.
At baseline, the mean endogenous FVIII level (FVIII:C) was 16.4 IU/dL, and the mean VWF ristocetin cofactor (VWF:Rco) was 10.6 IU/dL.
The patients received rVWF at 40 to 60 IU/kg VWF:RCo intravenously 12 to 24 hours before surgery to allow FVIII:C levels to increase to at least 30 IU/dL for minor or oral surgery or to at least 60 IU/dL for major surgery, within 3 hours before surgery.
If the desired levels were achieved, rVWF could be given alone. If the levels were not achieved, patients would receive rFVIII as well, within 1 to 2 hours before surgery. Patients were monitored for 14 days after surgery.
Results
All 15 patients had overall/intraoperative hemostatic efficacy ratings of “excellent” (as good as or better than expected) or “good” (probably as good as expected).
The patients received a median of 6 (range, 2 to 15) rVWF infusions at a median dose of 55 IU/kg (range, 36.1 to 59.9). Most patients (n=11) did not receive rVWF every day. For some, infusions were separated by 2 to 9 days.
Ten patients received rVWF alone, 12 did not receive any preoperative FVIII, and 2 did not receive rVWF postoperatively.
Most rVWF infusions (89.4%, 93/104) were given alone, and 70% (7/10) of the major surgeries were performed with rVWF alone.
The researchers said that, with rVWF alone, patients had hemostatically effective levels of FVIII:C as early as 6 hours after surgery, and this was sustained for 72 to 96 hours.
There were 5 patients who received rVWF with rFVIII. Of the 11 infusions these patients received, 9 were given when FVIII:C levels were above 60 IU/dL.
Three patients received rVWF with rFVIII 1 hour before major surgery—total hip replacement, molar extraction, and left ankle prosthesis. However, 2 of these patients had FVIII:C levels above 60 IU/dL.
The patient undergoing a molar extraction received rVWF with rFVIII 6 times after surgery. In 5 cases, the patient’s FVIII:C levels were 110 to 152 IU/dL. In the remaining case, the FVIII:C level was 23 IU/dL.
Two patients received rVWF with rFVIII for minor surgery. One patient undergoing a tooth extraction received rVWF with rFVIII intraoperatively when the FVIII:C level was 72 IU/dL.
The other patient received rVWF with rFVIII after radioisotope synovectomy when the FVIII:C level was 73 IU/dL. This patient received a postoperative dose of rVWF alone as well.
One patient tested positive for binding antibodies to VWF, and 1 patient developed deep vein thrombosis 3 days after total hip replacement while receiving rVWF.
Emicizumab controls bleeding regardless of inhibitors
GLASGOW—Emicizumab prophylaxis provides “clinically meaningful” control of bleeding whether or not patients have factor VIII inhibitors, according to researchers.
In the phase 3 HAVEN 4 study, researchers evaluated emicizumab prophylaxis, given every 4 weeks, in hemophilia A patients with or without factor VIII inhibitors.
At a median follow-up of about 26 weeks, patients had a median annualized bleeding rate (ABR) of 0.0 for treated bleeds and 2.1 for all bleeds.
About 30% of patients had 0 bleeds, and about 56% had 0 treated bleeds.
There were no serious adverse events (AEs) related to emicizumab. The most common AE was injection-site reaction.
Steve Pipe, MD, of Mott Children’s Hospital in Ann Arbor, Michigan, presented these results at the World Federation of Hemophilia (WFH) 2018 World Congress during the late-breaking abstract session on Monday.
The trial was sponsored by Hoffmann-La Roche.
HAVEN 4 included 48 patients, age 12 and older, who had hemophilia A with or without factor VIII inhibitors. Patients were previously treated with factor VIII or bypassing agents, on-demand or as prophylaxis.
The study was conducted in 2 parts: a pharmacokinetic (PK) run-in and an expansion cohort.
All patients in the PK run-in (n=7) were previously treated on-demand and received subcutaneous emicizumab at 6 mg/kg to fully characterize the PK profile after a single dose during 4 weeks. This was followed by 6 mg/kg every 4 weeks for at least 24 weeks.
Patients in the expansion cohort (n=41) received subcutaneous emicizumab prophylaxis at 3 mg/kg/wk for 4 weeks, followed by 6 mg/kg every 4 weeks for at least 24 weeks.
Episodic treatment of breakthrough bleeds with factor VIII therapy or bypassing agents, depending on a patient’s factor VIII inhibitor status, was allowed per study protocol.
Results
The efficacy analysis included the 41 patients in the expansion cohort, 5 of whom had inhibitors at baseline.
The median efficacy period was 25.6 weeks. The median ABR was 2.1 for all bleeds and 0.0 for treated bleeds.
The percentage of patients with 0 bleeds was 29.3% for all bleeds, 56.1% for treated bleeds, 82.9% for treated spontaneous bleeds, 70.7% for treated joint bleeds, and 85.4% for treated target joint bleeds.
Most treated bleeds (74.5%, 38/51) were traumatic.
There were 148 AEs, and 73.2% of patients had at least 1 AE.
Injection-site reaction was the most common AE related to emicizumab, occurring in 22.0% of patients (n=9).
There were 2 serious AEs (grade ≥3)—hypertension and rhabdomyolysis. Both were considered unrelated to emicizumab.
There were no AEs leading to emicizumab discontinuation or withdrawal. There were no thrombotic events, cases of thrombotic microangiopathy, hypersensitivity reactions, or fatalities.
None of the patients developed de novo factor VIII inhibitors, and there were no anti-drug antibodies detected.
GLASGOW—Emicizumab prophylaxis provides “clinically meaningful” control of bleeding whether or not patients have factor VIII inhibitors, according to researchers.
In the phase 3 HAVEN 4 study, researchers evaluated emicizumab prophylaxis, given every 4 weeks, in hemophilia A patients with or without factor VIII inhibitors.
At a median follow-up of about 26 weeks, patients had a median annualized bleeding rate (ABR) of 0.0 for treated bleeds and 2.1 for all bleeds.
About 30% of patients had 0 bleeds, and about 56% had 0 treated bleeds.
There were no serious adverse events (AEs) related to emicizumab. The most common AE was injection-site reaction.
Steve Pipe, MD, of Mott Children’s Hospital in Ann Arbor, Michigan, presented these results at the World Federation of Hemophilia (WFH) 2018 World Congress during the late-breaking abstract session on Monday.
The trial was sponsored by Hoffmann-La Roche.
HAVEN 4 included 48 patients, age 12 and older, who had hemophilia A with or without factor VIII inhibitors. Patients were previously treated with factor VIII or bypassing agents, on-demand or as prophylaxis.
The study was conducted in 2 parts: a pharmacokinetic (PK) run-in and an expansion cohort.
All patients in the PK run-in (n=7) were previously treated on-demand and received subcutaneous emicizumab at 6 mg/kg to fully characterize the PK profile after a single dose during 4 weeks. This was followed by 6 mg/kg every 4 weeks for at least 24 weeks.
Patients in the expansion cohort (n=41) received subcutaneous emicizumab prophylaxis at 3 mg/kg/wk for 4 weeks, followed by 6 mg/kg every 4 weeks for at least 24 weeks.
Episodic treatment of breakthrough bleeds with factor VIII therapy or bypassing agents, depending on a patient’s factor VIII inhibitor status, was allowed per study protocol.
Results
The efficacy analysis included the 41 patients in the expansion cohort, 5 of whom had inhibitors at baseline.
The median efficacy period was 25.6 weeks. The median ABR was 2.1 for all bleeds and 0.0 for treated bleeds.
The percentage of patients with 0 bleeds was 29.3% for all bleeds, 56.1% for treated bleeds, 82.9% for treated spontaneous bleeds, 70.7% for treated joint bleeds, and 85.4% for treated target joint bleeds.
Most treated bleeds (74.5%, 38/51) were traumatic.
There were 148 AEs, and 73.2% of patients had at least 1 AE.
Injection-site reaction was the most common AE related to emicizumab, occurring in 22.0% of patients (n=9).
There were 2 serious AEs (grade ≥3)—hypertension and rhabdomyolysis. Both were considered unrelated to emicizumab.
There were no AEs leading to emicizumab discontinuation or withdrawal. There were no thrombotic events, cases of thrombotic microangiopathy, hypersensitivity reactions, or fatalities.
None of the patients developed de novo factor VIII inhibitors, and there were no anti-drug antibodies detected.
GLASGOW—Emicizumab prophylaxis provides “clinically meaningful” control of bleeding whether or not patients have factor VIII inhibitors, according to researchers.
In the phase 3 HAVEN 4 study, researchers evaluated emicizumab prophylaxis, given every 4 weeks, in hemophilia A patients with or without factor VIII inhibitors.
At a median follow-up of about 26 weeks, patients had a median annualized bleeding rate (ABR) of 0.0 for treated bleeds and 2.1 for all bleeds.
About 30% of patients had 0 bleeds, and about 56% had 0 treated bleeds.
There were no serious adverse events (AEs) related to emicizumab. The most common AE was injection-site reaction.
Steve Pipe, MD, of Mott Children’s Hospital in Ann Arbor, Michigan, presented these results at the World Federation of Hemophilia (WFH) 2018 World Congress during the late-breaking abstract session on Monday.
The trial was sponsored by Hoffmann-La Roche.
HAVEN 4 included 48 patients, age 12 and older, who had hemophilia A with or without factor VIII inhibitors. Patients were previously treated with factor VIII or bypassing agents, on-demand or as prophylaxis.
The study was conducted in 2 parts: a pharmacokinetic (PK) run-in and an expansion cohort.
All patients in the PK run-in (n=7) were previously treated on-demand and received subcutaneous emicizumab at 6 mg/kg to fully characterize the PK profile after a single dose during 4 weeks. This was followed by 6 mg/kg every 4 weeks for at least 24 weeks.
Patients in the expansion cohort (n=41) received subcutaneous emicizumab prophylaxis at 3 mg/kg/wk for 4 weeks, followed by 6 mg/kg every 4 weeks for at least 24 weeks.
Episodic treatment of breakthrough bleeds with factor VIII therapy or bypassing agents, depending on a patient’s factor VIII inhibitor status, was allowed per study protocol.
Results
The efficacy analysis included the 41 patients in the expansion cohort, 5 of whom had inhibitors at baseline.
The median efficacy period was 25.6 weeks. The median ABR was 2.1 for all bleeds and 0.0 for treated bleeds.
The percentage of patients with 0 bleeds was 29.3% for all bleeds, 56.1% for treated bleeds, 82.9% for treated spontaneous bleeds, 70.7% for treated joint bleeds, and 85.4% for treated target joint bleeds.
Most treated bleeds (74.5%, 38/51) were traumatic.
There were 148 AEs, and 73.2% of patients had at least 1 AE.
Injection-site reaction was the most common AE related to emicizumab, occurring in 22.0% of patients (n=9).
There were 2 serious AEs (grade ≥3)—hypertension and rhabdomyolysis. Both were considered unrelated to emicizumab.
There were no AEs leading to emicizumab discontinuation or withdrawal. There were no thrombotic events, cases of thrombotic microangiopathy, hypersensitivity reactions, or fatalities.
None of the patients developed de novo factor VIII inhibitors, and there were no anti-drug antibodies detected.
Tailored hemophilia prophylaxis could cut costs
Tailored frequency-escalated prophylaxis produced good outcomes with little arthropathy among boys with hemophilia A, according to findings from a prospective study.
The potential to use smaller amounts of clotting factor concentrates (CFCs) also offers a possible cost savings, Brian M. Feldman, MD, of the Hospital for Sick Children, Toronto, and his colleagues reported in the Lancet Haematology.
Prophylactic treatment to prevent bleeding episodes and avoid long term complications using regular CFCs is very effective but also very expensive. The Canadian Hemophilia Prophylaxis Study (CHPS) began 2 decades ago with the premise that, since CFC use accounts for most of the financial burden in severe hemophilia and because primary prophylaxis is started at a very young age, the burden on both the patient and the health care system could potentially be reduced by starting prophylaxis with CFC infusions at reduced frequency intervals. The individual’s subsequent bleeding episodes would then determine how the dose and frequency could be tailored.
In the study, 56 boys between the ages of 1 and 2.5 years with severe hemophilia A from 12 Canadian centers were enrolled in the CHPS, with a median time in the study of 10.2 years. Treatment was with standard half-life recombinant factor VIII (SHL-rFVIII), beginning as once weekly prophylaxis with 50 IU/kg and then escalating in frequency with appropriate dose adjustments as needed (step 1). The primary endpoint for this analysis was joint health, as measured by the modified Colorado Child Physical Examination Scores at completion of the study.
Participants were able to stay on once a week prophylaxis, without unacceptable bleeding, until a median age of 4.5 years. At this time, boys who developed unacceptable bleeding were escalated to twice-weekly prophylaxis at 30 IU/kg (step 2). The median age for beginning alternate day prophylaxis at 25 IU/kg (step 3) was 9.9 years of age.
The median annual SHL-rFVIII use was 3,582 IU/kg at age 2 years (n = 56), 4,041 IU/kg at age 6 years (n = 53), 3,638 IU/kg at age 10 years (n = 40), and 3,663 IU/kg at 14 years of age (n = 18). Overall adherence to the tailored regimen was a median of 86%, and no treatment-related safety events were observed during the study, including central venous catheter infections.
The median use of SHL-rFVIII on the tailored frequency-escalated prophylaxis is considerably less than the 6,000 IU/kg per year used in standard prophylaxis regimens, the researchers noted.
“We have previously shown, with a formal cost-benefit analysis using the value-of-information approach, that tailored frequency escalated prophylaxis provides substantial cost-benefit in a variety of scenarios. Our results from this study lend further support to these findings,” they wrote.
The median annualized index joint bleeding rate was 0.95 per year, but 30% of the boys in the study experienced “unacceptable breakthrough bleeding” during the study.
Overall joint health was “well preserved,” according to the researchers. The median Colorado Child Physical Examination Score at the end of the CHPS was 1 (range 0-12) for the left ankle and 1 for the right ankle, with all other joints having a median score of 0.
Activities of daily living and physical function, as well as health-related quality of life were generally good, the researchers reported.
The study was initially funded by grants from the Medical Research Council of Canada/Pharmaceutical Manufacturers Association of Canada Partnership Fund and by the Bayer/Canadian Blood Services/Héma-Québec Partnership Fund. Subsequent renewals were funded by Bayer. Dr. Feldman reported grants from Bayer during the conduct of the study and grants from Baxter/Baxalta/Shire outside the submitted work. Several coauthors also report multiple relationships with industry.
SOURCE: Feldman BM et al. Lancet Haematol. 2018 May 3. doi: 10.1016/S2352-3026(18)30048-6.
Tailored frequency-escalated prophylaxis produced good outcomes with little arthropathy among boys with hemophilia A, according to findings from a prospective study.
The potential to use smaller amounts of clotting factor concentrates (CFCs) also offers a possible cost savings, Brian M. Feldman, MD, of the Hospital for Sick Children, Toronto, and his colleagues reported in the Lancet Haematology.
Prophylactic treatment to prevent bleeding episodes and avoid long term complications using regular CFCs is very effective but also very expensive. The Canadian Hemophilia Prophylaxis Study (CHPS) began 2 decades ago with the premise that, since CFC use accounts for most of the financial burden in severe hemophilia and because primary prophylaxis is started at a very young age, the burden on both the patient and the health care system could potentially be reduced by starting prophylaxis with CFC infusions at reduced frequency intervals. The individual’s subsequent bleeding episodes would then determine how the dose and frequency could be tailored.
In the study, 56 boys between the ages of 1 and 2.5 years with severe hemophilia A from 12 Canadian centers were enrolled in the CHPS, with a median time in the study of 10.2 years. Treatment was with standard half-life recombinant factor VIII (SHL-rFVIII), beginning as once weekly prophylaxis with 50 IU/kg and then escalating in frequency with appropriate dose adjustments as needed (step 1). The primary endpoint for this analysis was joint health, as measured by the modified Colorado Child Physical Examination Scores at completion of the study.
Participants were able to stay on once a week prophylaxis, without unacceptable bleeding, until a median age of 4.5 years. At this time, boys who developed unacceptable bleeding were escalated to twice-weekly prophylaxis at 30 IU/kg (step 2). The median age for beginning alternate day prophylaxis at 25 IU/kg (step 3) was 9.9 years of age.
The median annual SHL-rFVIII use was 3,582 IU/kg at age 2 years (n = 56), 4,041 IU/kg at age 6 years (n = 53), 3,638 IU/kg at age 10 years (n = 40), and 3,663 IU/kg at 14 years of age (n = 18). Overall adherence to the tailored regimen was a median of 86%, and no treatment-related safety events were observed during the study, including central venous catheter infections.
The median use of SHL-rFVIII on the tailored frequency-escalated prophylaxis is considerably less than the 6,000 IU/kg per year used in standard prophylaxis regimens, the researchers noted.
“We have previously shown, with a formal cost-benefit analysis using the value-of-information approach, that tailored frequency escalated prophylaxis provides substantial cost-benefit in a variety of scenarios. Our results from this study lend further support to these findings,” they wrote.
The median annualized index joint bleeding rate was 0.95 per year, but 30% of the boys in the study experienced “unacceptable breakthrough bleeding” during the study.
Overall joint health was “well preserved,” according to the researchers. The median Colorado Child Physical Examination Score at the end of the CHPS was 1 (range 0-12) for the left ankle and 1 for the right ankle, with all other joints having a median score of 0.
Activities of daily living and physical function, as well as health-related quality of life were generally good, the researchers reported.
The study was initially funded by grants from the Medical Research Council of Canada/Pharmaceutical Manufacturers Association of Canada Partnership Fund and by the Bayer/Canadian Blood Services/Héma-Québec Partnership Fund. Subsequent renewals were funded by Bayer. Dr. Feldman reported grants from Bayer during the conduct of the study and grants from Baxter/Baxalta/Shire outside the submitted work. Several coauthors also report multiple relationships with industry.
SOURCE: Feldman BM et al. Lancet Haematol. 2018 May 3. doi: 10.1016/S2352-3026(18)30048-6.
Tailored frequency-escalated prophylaxis produced good outcomes with little arthropathy among boys with hemophilia A, according to findings from a prospective study.
The potential to use smaller amounts of clotting factor concentrates (CFCs) also offers a possible cost savings, Brian M. Feldman, MD, of the Hospital for Sick Children, Toronto, and his colleagues reported in the Lancet Haematology.
Prophylactic treatment to prevent bleeding episodes and avoid long term complications using regular CFCs is very effective but also very expensive. The Canadian Hemophilia Prophylaxis Study (CHPS) began 2 decades ago with the premise that, since CFC use accounts for most of the financial burden in severe hemophilia and because primary prophylaxis is started at a very young age, the burden on both the patient and the health care system could potentially be reduced by starting prophylaxis with CFC infusions at reduced frequency intervals. The individual’s subsequent bleeding episodes would then determine how the dose and frequency could be tailored.
In the study, 56 boys between the ages of 1 and 2.5 years with severe hemophilia A from 12 Canadian centers were enrolled in the CHPS, with a median time in the study of 10.2 years. Treatment was with standard half-life recombinant factor VIII (SHL-rFVIII), beginning as once weekly prophylaxis with 50 IU/kg and then escalating in frequency with appropriate dose adjustments as needed (step 1). The primary endpoint for this analysis was joint health, as measured by the modified Colorado Child Physical Examination Scores at completion of the study.
Participants were able to stay on once a week prophylaxis, without unacceptable bleeding, until a median age of 4.5 years. At this time, boys who developed unacceptable bleeding were escalated to twice-weekly prophylaxis at 30 IU/kg (step 2). The median age for beginning alternate day prophylaxis at 25 IU/kg (step 3) was 9.9 years of age.
The median annual SHL-rFVIII use was 3,582 IU/kg at age 2 years (n = 56), 4,041 IU/kg at age 6 years (n = 53), 3,638 IU/kg at age 10 years (n = 40), and 3,663 IU/kg at 14 years of age (n = 18). Overall adherence to the tailored regimen was a median of 86%, and no treatment-related safety events were observed during the study, including central venous catheter infections.
The median use of SHL-rFVIII on the tailored frequency-escalated prophylaxis is considerably less than the 6,000 IU/kg per year used in standard prophylaxis regimens, the researchers noted.
“We have previously shown, with a formal cost-benefit analysis using the value-of-information approach, that tailored frequency escalated prophylaxis provides substantial cost-benefit in a variety of scenarios. Our results from this study lend further support to these findings,” they wrote.
The median annualized index joint bleeding rate was 0.95 per year, but 30% of the boys in the study experienced “unacceptable breakthrough bleeding” during the study.
Overall joint health was “well preserved,” according to the researchers. The median Colorado Child Physical Examination Score at the end of the CHPS was 1 (range 0-12) for the left ankle and 1 for the right ankle, with all other joints having a median score of 0.
Activities of daily living and physical function, as well as health-related quality of life were generally good, the researchers reported.
The study was initially funded by grants from the Medical Research Council of Canada/Pharmaceutical Manufacturers Association of Canada Partnership Fund and by the Bayer/Canadian Blood Services/Héma-Québec Partnership Fund. Subsequent renewals were funded by Bayer. Dr. Feldman reported grants from Bayer during the conduct of the study and grants from Baxter/Baxalta/Shire outside the submitted work. Several coauthors also report multiple relationships with industry.
SOURCE: Feldman BM et al. Lancet Haematol. 2018 May 3. doi: 10.1016/S2352-3026(18)30048-6.
LANCET HAEMATOLOGY
Key clinical point:
Major finding: The median CPPES score at the end of the study was 1 (range 0-12) for the left ankle and 1 for the right ankle, with all other joints having a median score of 0.
Study details: Longitudinal design comprising 56 boys with severe hemophilia A who were followed for up to 16.1 years.
Disclosures: The study was initially funded by grants from the Medical Research Council of Canada/Pharmaceutical Manufacturers Association of Canada Partnership Fund and by the Bayer/Canadian Blood Services/Héma-Québec Partnership Fund. Subsequent renewals were funded by Bayer. Dr. Feldman reported grants from Bayer during the conduct of the study and grants from Baxter/Baxalta/Shire outside the submitted work. Several coauthors also report multiple relationships with industry.
Source: Feldman BM et al. Lancet Haematol. 2018 May 3. doi: 10.1016/S2352-3026(18)30048-6.
Gene therapy reduces ABR, AIR in hemophilia B
GLASGOW—New research suggests the gene therapy SPK-9001 can reduce bleeding and the need for factor IX infusions in patients with hemophilia B.
In an ongoing, phase 1/2 trial, SPK-9001 reduced the annualized bleeding rate (ABR) by 98% and the annualized infusion rate (AIR) by 99%.
All 15 patients treated with SPK-9001 have discontinued factor IX prophylaxis.
There have been no serious adverse events (AEs), no thrombotic events, and no factor IX inhibitors observed to date.
Spencer K. Sullivan, MD, of the Mississippi Center for Advanced Medicine in Madison, Mississippi, presented these results at the World Federation of Hemophilia (WFH) 2018 World Congress during the “Free Papers: Gene Therapy” session on Tuesday.
The research was sponsored by Spark Therapeutics, the company developing SPK-9001 in collaboration with Pfizer.
SPK-9001 is an investigational vector that contains a bio-engineered adeno-associated virus capsid and a codon-optimized, high-activity human factor IX gene enabling endogenous production of factor IX.
Dr Sullivan reported results with SPK-9001 in 15 patients with severe or moderately severe hemophilia B.
As of the May 7, 2018, data cutoff, there were 13 patients with at least 12 weeks of follow-up after SPK-9001 infusion, which is the length of time required to achieve steady-state factor IX activity levels. All 13 patients reached stable factor IX levels of more than 12%.
The range of steady-state factor IX activity level, beginning at 12 weeks through 52 weeks of follow-up for the first 10 patients infused, was 14.3% to 76.8%.
The next 3 patients were infused with SPK-9001 manufactured using an enhanced process and reached 12 or more weeks of follow-up. For these patients, the range of steady-state factor IX activity level was 38.1% to 54.5%.
The 2 remaining patients had only 5 weeks and 11 weeks of follow-up as of the cut-off date.
Based on individual participant history for the year prior to the study, the overall ABR for all 15 patients was reduced by 98% four weeks after SPK-9001 treatment.
The ABR was 0.2 bleeds per patient after SPK-9001, compared to an ABR of 8.9 before SPK-9001.
One patient experienced a bleeding event 4 or more weeks after SPK-9001 infusion.
The overall AIR was reduced by 99% (based on data after week 4) for all 15 patients. The AIR was 0.9 infusions per patient after SPK-9001, compared to 57.2 infusions before SPK-9001.
Six patients received factor IX infusions following SPK-9001 administration—2 for reported spontaneous bleeds, 2 prior to surgery, 1 at the end of the study (discretionary, per protocol), and 1 for prophylaxis for a minor, traumatic non-bleeding event.
However, all 15 patients have discontinued regular factor IX prophylaxis.
There have been no serious AEs or factor IX inhibitors reported.
Two patients (1 who received SPK-9001 manufactured using the enhanced process) experienced related AEs of elevated transaminases, which were asymptomatic.
These patients were treated with a tapering course of oral corticosteroids, and 1 event resolved before the data cutoff.
An additional patient received a tapering course of oral corticosteroids for an increase in liver enzymes (not exceeding the upper limit of normal) temporally associated with falling levels of factor IX activity.
“We are pleased to see all 15 participants, notably including the first 4 participants who have been followed for more than 2 years, continue to show that a single administration of SPK-9001 has resulted in dramatic reductions in bleeding and factor IX infusions, with no serious adverse events,” said Katherine A. High, MD, president and head of research & development at Spark Therapeutics.
“Our commitment to gene therapy research across our hemophilia programs remains steadfast with the goal of developing a novel therapeutic approach with a positive benefit-risk profile that aims to free patients of the need for regular infusions, while eliminating spontaneous bleeding.”
GLASGOW—New research suggests the gene therapy SPK-9001 can reduce bleeding and the need for factor IX infusions in patients with hemophilia B.
In an ongoing, phase 1/2 trial, SPK-9001 reduced the annualized bleeding rate (ABR) by 98% and the annualized infusion rate (AIR) by 99%.
All 15 patients treated with SPK-9001 have discontinued factor IX prophylaxis.
There have been no serious adverse events (AEs), no thrombotic events, and no factor IX inhibitors observed to date.
Spencer K. Sullivan, MD, of the Mississippi Center for Advanced Medicine in Madison, Mississippi, presented these results at the World Federation of Hemophilia (WFH) 2018 World Congress during the “Free Papers: Gene Therapy” session on Tuesday.
The research was sponsored by Spark Therapeutics, the company developing SPK-9001 in collaboration with Pfizer.
SPK-9001 is an investigational vector that contains a bio-engineered adeno-associated virus capsid and a codon-optimized, high-activity human factor IX gene enabling endogenous production of factor IX.
Dr Sullivan reported results with SPK-9001 in 15 patients with severe or moderately severe hemophilia B.
As of the May 7, 2018, data cutoff, there were 13 patients with at least 12 weeks of follow-up after SPK-9001 infusion, which is the length of time required to achieve steady-state factor IX activity levels. All 13 patients reached stable factor IX levels of more than 12%.
The range of steady-state factor IX activity level, beginning at 12 weeks through 52 weeks of follow-up for the first 10 patients infused, was 14.3% to 76.8%.
The next 3 patients were infused with SPK-9001 manufactured using an enhanced process and reached 12 or more weeks of follow-up. For these patients, the range of steady-state factor IX activity level was 38.1% to 54.5%.
The 2 remaining patients had only 5 weeks and 11 weeks of follow-up as of the cut-off date.
Based on individual participant history for the year prior to the study, the overall ABR for all 15 patients was reduced by 98% four weeks after SPK-9001 treatment.
The ABR was 0.2 bleeds per patient after SPK-9001, compared to an ABR of 8.9 before SPK-9001.
One patient experienced a bleeding event 4 or more weeks after SPK-9001 infusion.
The overall AIR was reduced by 99% (based on data after week 4) for all 15 patients. The AIR was 0.9 infusions per patient after SPK-9001, compared to 57.2 infusions before SPK-9001.
Six patients received factor IX infusions following SPK-9001 administration—2 for reported spontaneous bleeds, 2 prior to surgery, 1 at the end of the study (discretionary, per protocol), and 1 for prophylaxis for a minor, traumatic non-bleeding event.
However, all 15 patients have discontinued regular factor IX prophylaxis.
There have been no serious AEs or factor IX inhibitors reported.
Two patients (1 who received SPK-9001 manufactured using the enhanced process) experienced related AEs of elevated transaminases, which were asymptomatic.
These patients were treated with a tapering course of oral corticosteroids, and 1 event resolved before the data cutoff.
An additional patient received a tapering course of oral corticosteroids for an increase in liver enzymes (not exceeding the upper limit of normal) temporally associated with falling levels of factor IX activity.
“We are pleased to see all 15 participants, notably including the first 4 participants who have been followed for more than 2 years, continue to show that a single administration of SPK-9001 has resulted in dramatic reductions in bleeding and factor IX infusions, with no serious adverse events,” said Katherine A. High, MD, president and head of research & development at Spark Therapeutics.
“Our commitment to gene therapy research across our hemophilia programs remains steadfast with the goal of developing a novel therapeutic approach with a positive benefit-risk profile that aims to free patients of the need for regular infusions, while eliminating spontaneous bleeding.”
GLASGOW—New research suggests the gene therapy SPK-9001 can reduce bleeding and the need for factor IX infusions in patients with hemophilia B.
In an ongoing, phase 1/2 trial, SPK-9001 reduced the annualized bleeding rate (ABR) by 98% and the annualized infusion rate (AIR) by 99%.
All 15 patients treated with SPK-9001 have discontinued factor IX prophylaxis.
There have been no serious adverse events (AEs), no thrombotic events, and no factor IX inhibitors observed to date.
Spencer K. Sullivan, MD, of the Mississippi Center for Advanced Medicine in Madison, Mississippi, presented these results at the World Federation of Hemophilia (WFH) 2018 World Congress during the “Free Papers: Gene Therapy” session on Tuesday.
The research was sponsored by Spark Therapeutics, the company developing SPK-9001 in collaboration with Pfizer.
SPK-9001 is an investigational vector that contains a bio-engineered adeno-associated virus capsid and a codon-optimized, high-activity human factor IX gene enabling endogenous production of factor IX.
Dr Sullivan reported results with SPK-9001 in 15 patients with severe or moderately severe hemophilia B.
As of the May 7, 2018, data cutoff, there were 13 patients with at least 12 weeks of follow-up after SPK-9001 infusion, which is the length of time required to achieve steady-state factor IX activity levels. All 13 patients reached stable factor IX levels of more than 12%.
The range of steady-state factor IX activity level, beginning at 12 weeks through 52 weeks of follow-up for the first 10 patients infused, was 14.3% to 76.8%.
The next 3 patients were infused with SPK-9001 manufactured using an enhanced process and reached 12 or more weeks of follow-up. For these patients, the range of steady-state factor IX activity level was 38.1% to 54.5%.
The 2 remaining patients had only 5 weeks and 11 weeks of follow-up as of the cut-off date.
Based on individual participant history for the year prior to the study, the overall ABR for all 15 patients was reduced by 98% four weeks after SPK-9001 treatment.
The ABR was 0.2 bleeds per patient after SPK-9001, compared to an ABR of 8.9 before SPK-9001.
One patient experienced a bleeding event 4 or more weeks after SPK-9001 infusion.
The overall AIR was reduced by 99% (based on data after week 4) for all 15 patients. The AIR was 0.9 infusions per patient after SPK-9001, compared to 57.2 infusions before SPK-9001.
Six patients received factor IX infusions following SPK-9001 administration—2 for reported spontaneous bleeds, 2 prior to surgery, 1 at the end of the study (discretionary, per protocol), and 1 for prophylaxis for a minor, traumatic non-bleeding event.
However, all 15 patients have discontinued regular factor IX prophylaxis.
There have been no serious AEs or factor IX inhibitors reported.
Two patients (1 who received SPK-9001 manufactured using the enhanced process) experienced related AEs of elevated transaminases, which were asymptomatic.
These patients were treated with a tapering course of oral corticosteroids, and 1 event resolved before the data cutoff.
An additional patient received a tapering course of oral corticosteroids for an increase in liver enzymes (not exceeding the upper limit of normal) temporally associated with falling levels of factor IX activity.
“We are pleased to see all 15 participants, notably including the first 4 participants who have been followed for more than 2 years, continue to show that a single administration of SPK-9001 has resulted in dramatic reductions in bleeding and factor IX infusions, with no serious adverse events,” said Katherine A. High, MD, president and head of research & development at Spark Therapeutics.
“Our commitment to gene therapy research across our hemophilia programs remains steadfast with the goal of developing a novel therapeutic approach with a positive benefit-risk profile that aims to free patients of the need for regular infusions, while eliminating spontaneous bleeding.”
N9-GP has better PK profile than rFIXFc, team says
GLASGOW—Nonacog beta pegol (N9-GP) has a better pharmacokinetic (PK) profile than recombinant factor IX-Fc fusion protein (rFIXFc), according to researchers.
In a phase 1 trial, adults with hemophilia B who received a single dose of N9-GP achieved greater total factor IX exposure than those treated with rFIXFc, and N9-GP had a longer half-life.
Seven days after injection, factor IX activity was 6-fold greater in patients treated with N9-GP than in those treated with rFIXFc at the same dose.
“As a clinician, I know first-hand how challenging it can be to help people living with hemophilia B reach their treatment goals and be adequately protected from bleeding,” said Carmen Escuriola Ettingshausen, MD, of Haemophilia Centre Rhein Main in Frankfurt-Mörfelden, Germany.
“These data will help us better understand the different treatment options and choose the appropriate treatment for each patient.”
Dr Ettingshausen presented the data at the World Federation of Hemophilia (WFH) 2018 World Congress during the late-breaking abstract session on Monday.
The research was sponsored by Novo Nordisk A/S, the company marketing N9-GP (as Rebinyn or Refixia). N9-GP is an extended half-life factor IX molecule intended for replacement therapy in patients with hemophilia B.
In the Paradigm7 trial, researchers compared the PK profiles of N9-GP and rFIXFc (Alprolix).
Fifteen previously treated adult males with congenital hemophilia B (factor IX activity ≤2%) received single injections (50 IU/kg) of N9-GP and rFIXFc with at least 21 days between doses.
One patient was excluded from the analysis due to intake of a prohibited medication (an rFIXFc product that was not Alprolix). Two other patients were excluded from some analyses because they missed 2 PK time points.
The primary endpoint was dose-normalized area under the factor IX activity-time curve from 0 to infinity (AUC0-inf,norm).
The estimated AUC0-inf,norm (n=12) was significantly higher for N9-GP than rFIXFc—9656 IU*h/dL and 2199 IU*h/dL, respectively (ratio=4.39, P<0.0001).
There were significant differences for secondary endpoints as well.
The maximum factor IX activity dose-normalized to 50 IU/kg (n=14) was 91 IU/dL with N9-GP and 45 IU/dL with rFIXFc (ratio=2.02, P<0.001).
The incremental recovery at 30 minutes (n=14) was 1.7 (IU/dL)/(IU/kg) with N9-GP and 0.8 (IU/dL)/(IU/kg) with rFIXFc (ratio=2.20, P<0.001).
The terminal half-life (n=12) was 103.2 hours with N9-GP and 84.9 hours with rFIXFc (ratio=1.22, P<0.001).
The clearance (n=12) was 0.52 mL/h/kg with N9-GP and 2.25 mL/h/kg with rFIXFc (ratio=0.23, P<0.001).
The factor IX activity at 168 hours (n=12) was 19 IU/dL with N9-GP and 3 IU/dL with rFIXFc (ratio=5.80, P<0.001).
None of the patients developed inhibitors, and no safety concerns were identified, according to Novo Nordisk. The company did not provide additional safety information.
GLASGOW—Nonacog beta pegol (N9-GP) has a better pharmacokinetic (PK) profile than recombinant factor IX-Fc fusion protein (rFIXFc), according to researchers.
In a phase 1 trial, adults with hemophilia B who received a single dose of N9-GP achieved greater total factor IX exposure than those treated with rFIXFc, and N9-GP had a longer half-life.
Seven days after injection, factor IX activity was 6-fold greater in patients treated with N9-GP than in those treated with rFIXFc at the same dose.
“As a clinician, I know first-hand how challenging it can be to help people living with hemophilia B reach their treatment goals and be adequately protected from bleeding,” said Carmen Escuriola Ettingshausen, MD, of Haemophilia Centre Rhein Main in Frankfurt-Mörfelden, Germany.
“These data will help us better understand the different treatment options and choose the appropriate treatment for each patient.”
Dr Ettingshausen presented the data at the World Federation of Hemophilia (WFH) 2018 World Congress during the late-breaking abstract session on Monday.
The research was sponsored by Novo Nordisk A/S, the company marketing N9-GP (as Rebinyn or Refixia). N9-GP is an extended half-life factor IX molecule intended for replacement therapy in patients with hemophilia B.
In the Paradigm7 trial, researchers compared the PK profiles of N9-GP and rFIXFc (Alprolix).
Fifteen previously treated adult males with congenital hemophilia B (factor IX activity ≤2%) received single injections (50 IU/kg) of N9-GP and rFIXFc with at least 21 days between doses.
One patient was excluded from the analysis due to intake of a prohibited medication (an rFIXFc product that was not Alprolix). Two other patients were excluded from some analyses because they missed 2 PK time points.
The primary endpoint was dose-normalized area under the factor IX activity-time curve from 0 to infinity (AUC0-inf,norm).
The estimated AUC0-inf,norm (n=12) was significantly higher for N9-GP than rFIXFc—9656 IU*h/dL and 2199 IU*h/dL, respectively (ratio=4.39, P<0.0001).
There were significant differences for secondary endpoints as well.
The maximum factor IX activity dose-normalized to 50 IU/kg (n=14) was 91 IU/dL with N9-GP and 45 IU/dL with rFIXFc (ratio=2.02, P<0.001).
The incremental recovery at 30 minutes (n=14) was 1.7 (IU/dL)/(IU/kg) with N9-GP and 0.8 (IU/dL)/(IU/kg) with rFIXFc (ratio=2.20, P<0.001).
The terminal half-life (n=12) was 103.2 hours with N9-GP and 84.9 hours with rFIXFc (ratio=1.22, P<0.001).
The clearance (n=12) was 0.52 mL/h/kg with N9-GP and 2.25 mL/h/kg with rFIXFc (ratio=0.23, P<0.001).
The factor IX activity at 168 hours (n=12) was 19 IU/dL with N9-GP and 3 IU/dL with rFIXFc (ratio=5.80, P<0.001).
None of the patients developed inhibitors, and no safety concerns were identified, according to Novo Nordisk. The company did not provide additional safety information.
GLASGOW—Nonacog beta pegol (N9-GP) has a better pharmacokinetic (PK) profile than recombinant factor IX-Fc fusion protein (rFIXFc), according to researchers.
In a phase 1 trial, adults with hemophilia B who received a single dose of N9-GP achieved greater total factor IX exposure than those treated with rFIXFc, and N9-GP had a longer half-life.
Seven days after injection, factor IX activity was 6-fold greater in patients treated with N9-GP than in those treated with rFIXFc at the same dose.
“As a clinician, I know first-hand how challenging it can be to help people living with hemophilia B reach their treatment goals and be adequately protected from bleeding,” said Carmen Escuriola Ettingshausen, MD, of Haemophilia Centre Rhein Main in Frankfurt-Mörfelden, Germany.
“These data will help us better understand the different treatment options and choose the appropriate treatment for each patient.”
Dr Ettingshausen presented the data at the World Federation of Hemophilia (WFH) 2018 World Congress during the late-breaking abstract session on Monday.
The research was sponsored by Novo Nordisk A/S, the company marketing N9-GP (as Rebinyn or Refixia). N9-GP is an extended half-life factor IX molecule intended for replacement therapy in patients with hemophilia B.
In the Paradigm7 trial, researchers compared the PK profiles of N9-GP and rFIXFc (Alprolix).
Fifteen previously treated adult males with congenital hemophilia B (factor IX activity ≤2%) received single injections (50 IU/kg) of N9-GP and rFIXFc with at least 21 days between doses.
One patient was excluded from the analysis due to intake of a prohibited medication (an rFIXFc product that was not Alprolix). Two other patients were excluded from some analyses because they missed 2 PK time points.
The primary endpoint was dose-normalized area under the factor IX activity-time curve from 0 to infinity (AUC0-inf,norm).
The estimated AUC0-inf,norm (n=12) was significantly higher for N9-GP than rFIXFc—9656 IU*h/dL and 2199 IU*h/dL, respectively (ratio=4.39, P<0.0001).
There were significant differences for secondary endpoints as well.
The maximum factor IX activity dose-normalized to 50 IU/kg (n=14) was 91 IU/dL with N9-GP and 45 IU/dL with rFIXFc (ratio=2.02, P<0.001).
The incremental recovery at 30 minutes (n=14) was 1.7 (IU/dL)/(IU/kg) with N9-GP and 0.8 (IU/dL)/(IU/kg) with rFIXFc (ratio=2.20, P<0.001).
The terminal half-life (n=12) was 103.2 hours with N9-GP and 84.9 hours with rFIXFc (ratio=1.22, P<0.001).
The clearance (n=12) was 0.52 mL/h/kg with N9-GP and 2.25 mL/h/kg with rFIXFc (ratio=0.23, P<0.001).
The factor IX activity at 168 hours (n=12) was 19 IU/dL with N9-GP and 3 IU/dL with rFIXFc (ratio=5.80, P<0.001).
None of the patients developed inhibitors, and no safety concerns were identified, according to Novo Nordisk. The company did not provide additional safety information.
Therapy can extend half-life of FVIII
GLASGOW—Preliminary data suggest an investigational therapy can extend the half-life of factor VIII (FVIII) in patients with severe hemophilia A.
Researchers are testing the therapy, BIVV001, in a phase 1/2a trial and have reported results in 4 patients.
BIVV001 extended the half-life of FVIII to 37 hours, with an average FVIII activity of 5.6% at 7 days post-infusion.
“For decades, scientists have been trying to overcome the von Willebrand factor ceiling, which imposes a limit on the half-life of FVIII, and these data demonstrate that BIVV001 has finally broken through that ceiling,” said Joachim Fruebis, PhD, senior vice president of development at Bioverativ Inc.
Dr Fruebis presented these data at the World Federation of Hemophilia (WFH) 2018 World Congress during the late-breaking abstract session on Monday.
The research was sponsored by Bioverativ, the company developing BIVV001.
BIVV001 (rFVIIIFc-VWF-XTEN) is a recombinant FVIII therapy that builds on Fc fusion technology by adding a region of von Willebrand factor and XTEN polypeptides to potentially extend its time in circulation.
In the phase 1/2a EXTEN-A trial, researchers are evaluating the safety and pharmacokinetics of BIVV001 in a low-dose and high-dose cohort of subjects, ages 18 to 65, who have severe hemophilia A.
In the data presented at the WFH World Congress, 4 adult males received a single dose of recombinant FVIII therapy (25 IU/kg) followed, after a washout period, by a single, low dose of BIVV001 (25 IU/kg).
Primary endpoints of this study include the occurrence of adverse events and the development of inhibitors.
No inhibitors have been detected, and BIBV001 was “generally well-tolerated,” according to Bioverativ. The company did not provide additional safety information.
BIVV001 extended the half-life of FVIII to 37 hours, which is an increase over the 13 hours seen with recombinant FVIII.
The average FVIII activity for the 4 subjects was 13.0% at 5 days and 5.6% at 7 days post-infusion.
“Importantly for the hemophilia community, the factor levels seen in this study are unparalleled in hemophilia A,” Dr Fruebis said, “and we are excited about the potential for BIVV001 to transform the treatment paradigm for patients and physicians.”
GLASGOW—Preliminary data suggest an investigational therapy can extend the half-life of factor VIII (FVIII) in patients with severe hemophilia A.
Researchers are testing the therapy, BIVV001, in a phase 1/2a trial and have reported results in 4 patients.
BIVV001 extended the half-life of FVIII to 37 hours, with an average FVIII activity of 5.6% at 7 days post-infusion.
“For decades, scientists have been trying to overcome the von Willebrand factor ceiling, which imposes a limit on the half-life of FVIII, and these data demonstrate that BIVV001 has finally broken through that ceiling,” said Joachim Fruebis, PhD, senior vice president of development at Bioverativ Inc.
Dr Fruebis presented these data at the World Federation of Hemophilia (WFH) 2018 World Congress during the late-breaking abstract session on Monday.
The research was sponsored by Bioverativ, the company developing BIVV001.
BIVV001 (rFVIIIFc-VWF-XTEN) is a recombinant FVIII therapy that builds on Fc fusion technology by adding a region of von Willebrand factor and XTEN polypeptides to potentially extend its time in circulation.
In the phase 1/2a EXTEN-A trial, researchers are evaluating the safety and pharmacokinetics of BIVV001 in a low-dose and high-dose cohort of subjects, ages 18 to 65, who have severe hemophilia A.
In the data presented at the WFH World Congress, 4 adult males received a single dose of recombinant FVIII therapy (25 IU/kg) followed, after a washout period, by a single, low dose of BIVV001 (25 IU/kg).
Primary endpoints of this study include the occurrence of adverse events and the development of inhibitors.
No inhibitors have been detected, and BIBV001 was “generally well-tolerated,” according to Bioverativ. The company did not provide additional safety information.
BIVV001 extended the half-life of FVIII to 37 hours, which is an increase over the 13 hours seen with recombinant FVIII.
The average FVIII activity for the 4 subjects was 13.0% at 5 days and 5.6% at 7 days post-infusion.
“Importantly for the hemophilia community, the factor levels seen in this study are unparalleled in hemophilia A,” Dr Fruebis said, “and we are excited about the potential for BIVV001 to transform the treatment paradigm for patients and physicians.”
GLASGOW—Preliminary data suggest an investigational therapy can extend the half-life of factor VIII (FVIII) in patients with severe hemophilia A.
Researchers are testing the therapy, BIVV001, in a phase 1/2a trial and have reported results in 4 patients.
BIVV001 extended the half-life of FVIII to 37 hours, with an average FVIII activity of 5.6% at 7 days post-infusion.
“For decades, scientists have been trying to overcome the von Willebrand factor ceiling, which imposes a limit on the half-life of FVIII, and these data demonstrate that BIVV001 has finally broken through that ceiling,” said Joachim Fruebis, PhD, senior vice president of development at Bioverativ Inc.
Dr Fruebis presented these data at the World Federation of Hemophilia (WFH) 2018 World Congress during the late-breaking abstract session on Monday.
The research was sponsored by Bioverativ, the company developing BIVV001.
BIVV001 (rFVIIIFc-VWF-XTEN) is a recombinant FVIII therapy that builds on Fc fusion technology by adding a region of von Willebrand factor and XTEN polypeptides to potentially extend its time in circulation.
In the phase 1/2a EXTEN-A trial, researchers are evaluating the safety and pharmacokinetics of BIVV001 in a low-dose and high-dose cohort of subjects, ages 18 to 65, who have severe hemophilia A.
In the data presented at the WFH World Congress, 4 adult males received a single dose of recombinant FVIII therapy (25 IU/kg) followed, after a washout period, by a single, low dose of BIVV001 (25 IU/kg).
Primary endpoints of this study include the occurrence of adverse events and the development of inhibitors.
No inhibitors have been detected, and BIBV001 was “generally well-tolerated,” according to Bioverativ. The company did not provide additional safety information.
BIVV001 extended the half-life of FVIII to 37 hours, which is an increase over the 13 hours seen with recombinant FVIII.
The average FVIII activity for the 4 subjects was 13.0% at 5 days and 5.6% at 7 days post-infusion.
“Importantly for the hemophilia community, the factor levels seen in this study are unparalleled in hemophilia A,” Dr Fruebis said, “and we are excited about the potential for BIVV001 to transform the treatment paradigm for patients and physicians.”