User login
Novel chip system could improve preclinical drug studies
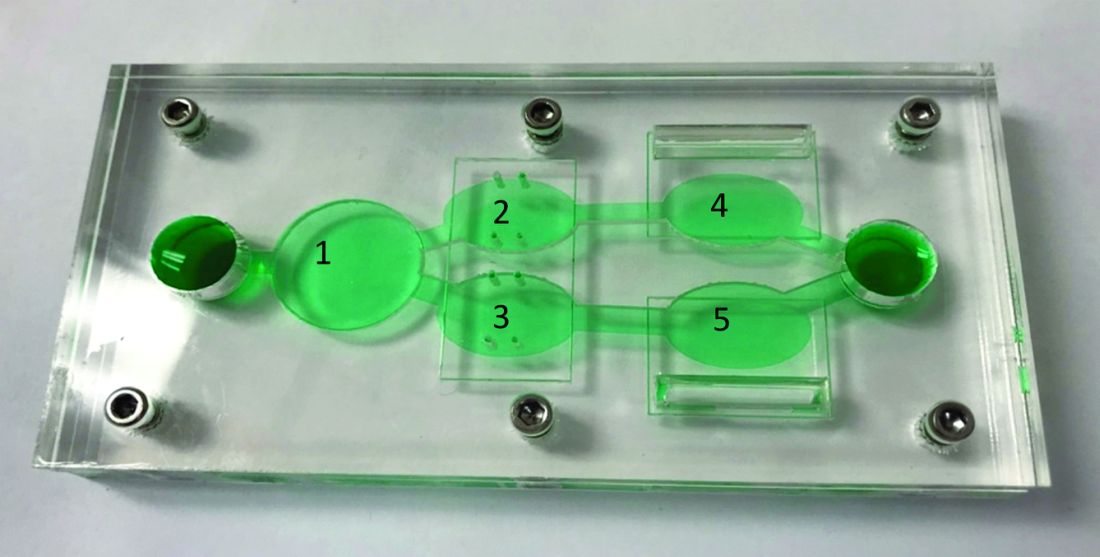
A novel multiorgan body-on-a-chip system shows promise to improve the preclinical evaluation of various anticancer therapies, investigators report.
“Initially, organ-on-a-chip systems were designed for specific applications with limited ability for reconfiguration and typically with cells from a single organ,” wrote Christopher W. McAleer, PhD, of Hesperos Inc., Orlando, and colleagues. Their report is in Science Translational Medicine.
“To address these issues, a reconfigurable body-on-a-chip system was developed with the capacity to house multiple organ-like tissue constructs,” the authors explained.
The researchers used two different system configurations to evaluate the off-target organ toxicities, metabolism, and efficacy of diclofenac and imatinib (system 1), in addition to tamoxifen (system 2). Both therapies were combined with verapamil in the study.
In system 1, cancer-derived bone marrow cells were cultured with primary hepatocytes, and were analyzed for anti-leukemic activity. In this configuration, both imatinib and diclofenac showed cytostatic activity on cancer progression in the bone marrow cells.
“Liver viability was not affected by imatinib; however, diclofenac reduced liver viability by 30%,” the researchers wrote.
System 2 included a wide variety of cell-lines, including primary hepatocytes, induced pluripotent stem cell-derived cardiomyocytes, a multidrug-resistant vulva cancer line, and a non-multidrug-resistant breast cancer line.
In this configuration, tamoxifen monotherapy and tamoxifen coadministered with verapamil resulted in off-target cardiac toxicities, but did not alter cell viability.
“These systems demonstrate the utility of a human cell–based in vitro culture system to evaluate both on-target efficacy and off-target toxicity for parent drugs and their metabolites,” Dr. McAleer and colleagues wrote.
The researchers acknowledged that the dosing parameters used in the model were acute. As a result, chronic, low-dose treatment strategies may reflect clinical conditions more accurately.
“These systems can augment and reduce the use of animals and increase the efficiency of drug evaluations in preclinical studies,” they concluded.
The study was supported by Hesperos Internal Development funds, the NIH, and Roche. The authors reported financial affiliations with Hesperos and Roche.
SOURCE: McAleer CW et al. Sci Transl Med. 2019 Jun 19. doi: 10.1126/scitranslmed.aav1386.
A novel multiorgan body-on-a-chip system shows promise to improve the preclinical evaluation of various anticancer therapies, investigators report.
“Initially, organ-on-a-chip systems were designed for specific applications with limited ability for reconfiguration and typically with cells from a single organ,” wrote Christopher W. McAleer, PhD, of Hesperos Inc., Orlando, and colleagues. Their report is in Science Translational Medicine.
“To address these issues, a reconfigurable body-on-a-chip system was developed with the capacity to house multiple organ-like tissue constructs,” the authors explained.
The researchers used two different system configurations to evaluate the off-target organ toxicities, metabolism, and efficacy of diclofenac and imatinib (system 1), in addition to tamoxifen (system 2). Both therapies were combined with verapamil in the study.
In system 1, cancer-derived bone marrow cells were cultured with primary hepatocytes, and were analyzed for anti-leukemic activity. In this configuration, both imatinib and diclofenac showed cytostatic activity on cancer progression in the bone marrow cells.
“Liver viability was not affected by imatinib; however, diclofenac reduced liver viability by 30%,” the researchers wrote.
System 2 included a wide variety of cell-lines, including primary hepatocytes, induced pluripotent stem cell-derived cardiomyocytes, a multidrug-resistant vulva cancer line, and a non-multidrug-resistant breast cancer line.
In this configuration, tamoxifen monotherapy and tamoxifen coadministered with verapamil resulted in off-target cardiac toxicities, but did not alter cell viability.
“These systems demonstrate the utility of a human cell–based in vitro culture system to evaluate both on-target efficacy and off-target toxicity for parent drugs and their metabolites,” Dr. McAleer and colleagues wrote.
The researchers acknowledged that the dosing parameters used in the model were acute. As a result, chronic, low-dose treatment strategies may reflect clinical conditions more accurately.
“These systems can augment and reduce the use of animals and increase the efficiency of drug evaluations in preclinical studies,” they concluded.
The study was supported by Hesperos Internal Development funds, the NIH, and Roche. The authors reported financial affiliations with Hesperos and Roche.
SOURCE: McAleer CW et al. Sci Transl Med. 2019 Jun 19. doi: 10.1126/scitranslmed.aav1386.
A novel multiorgan body-on-a-chip system shows promise to improve the preclinical evaluation of various anticancer therapies, investigators report.
“Initially, organ-on-a-chip systems were designed for specific applications with limited ability for reconfiguration and typically with cells from a single organ,” wrote Christopher W. McAleer, PhD, of Hesperos Inc., Orlando, and colleagues. Their report is in Science Translational Medicine.
“To address these issues, a reconfigurable body-on-a-chip system was developed with the capacity to house multiple organ-like tissue constructs,” the authors explained.
The researchers used two different system configurations to evaluate the off-target organ toxicities, metabolism, and efficacy of diclofenac and imatinib (system 1), in addition to tamoxifen (system 2). Both therapies were combined with verapamil in the study.
In system 1, cancer-derived bone marrow cells were cultured with primary hepatocytes, and were analyzed for anti-leukemic activity. In this configuration, both imatinib and diclofenac showed cytostatic activity on cancer progression in the bone marrow cells.
“Liver viability was not affected by imatinib; however, diclofenac reduced liver viability by 30%,” the researchers wrote.
System 2 included a wide variety of cell-lines, including primary hepatocytes, induced pluripotent stem cell-derived cardiomyocytes, a multidrug-resistant vulva cancer line, and a non-multidrug-resistant breast cancer line.
In this configuration, tamoxifen monotherapy and tamoxifen coadministered with verapamil resulted in off-target cardiac toxicities, but did not alter cell viability.
“These systems demonstrate the utility of a human cell–based in vitro culture system to evaluate both on-target efficacy and off-target toxicity for parent drugs and their metabolites,” Dr. McAleer and colleagues wrote.
The researchers acknowledged that the dosing parameters used in the model were acute. As a result, chronic, low-dose treatment strategies may reflect clinical conditions more accurately.
“These systems can augment and reduce the use of animals and increase the efficiency of drug evaluations in preclinical studies,” they concluded.
The study was supported by Hesperos Internal Development funds, the NIH, and Roche. The authors reported financial affiliations with Hesperos and Roche.
SOURCE: McAleer CW et al. Sci Transl Med. 2019 Jun 19. doi: 10.1126/scitranslmed.aav1386.
FROM SCIENCE TRANSLATIONAL MEDICINE
Key clinical point: A novel multiorgan body-on-a-chip system shows promise to improve the preclinical evaluation of anticancer therapies.
Major finding: Overall, results support the utility of the system to assess both off-target toxicity and on-target efficacy for various anticancer drugs.
Study details: A study exploring the utility of a multi-organ-on-a-chip system to assess safety and effectiveness of anticancer therapies in the preclinical setting.
Disclosures: The study was supported by Hesperos Internal Development funds, the NIH, and Roche. The authors reported financial affiliations with Hesperos and Roche.
Source: McAleer CW et al. Sci Transl Med. 2019 Jun 19. doi: 10.1126/scitranslmed.aav1386.
PALOMA-3 biomarker analysis: Liquid biopsy could ID progression risk
CHICAGO – Tumor protein 53 (TP53) mutation, fibroblast growth factor receptor 1 (FGFR1) amplification, and tumor purity in plasma each predict early progression on palbociclib and/or fulvestrant in patients with advanced estrogen receptor–positive (ER+) breast cancer, according to genomic analyses of PALOMA-3 trial data.
Overall, the presence of one or more of these genomic changes identified 131 out of 310 patients from the phase 3 trial who had baseline samples available, Ben O’Leary, MBBS, said at the annual meeting of the American Society of Clinical Oncology.
“So, a significant minority of patients – 42.3% – potentially who fall into a more poor-prognosis group,” said Dr. O’Leary of the Institute of Cancer Research at the Royal Marsden Hospital in London.
The findings suggest that a “liquid biopsy” at the start of treatment could identify patients at risk for progression.
The PALOMA-3 trial randomized 521 patients with ER+, human epidermal growth factor receptor 2 (HER2)–negative advanced breast cancer who had previously progressed on endocrine therapy 2:1 to CDK4/CDK6 inhibition with palbociclib plus fulvestrant (P+F) or placebo plus fulvestrant (F), and it showed that adding palbociclib significantly improved progression-free survival (PFS) (N Engl J Med. Jul 16 2015;373:209-19).
For the current analysis, the investigators assessed circulating tumor DNA (ctDNA) in baseline plasma samples from 459 study participants in an effort to identify genomic biomarkers for progression, to examine the association between baseline tumor fraction and clinical outcome, and to explore differences in predictive markers by treatment arm. A custom amplicon-sequencing analysis was performed to look for mutations in 17 different relevant genes, and another was used to estimate tumor fraction by looking at about 800 common germline single-nucleotide polymorphisms and to assess copy-number gain in the amplification status in 11 different genes, Dr. O’Leary said.
Results for mutations and circulating nucleic acids were available in 203 and 107 patients from the P+F and F groups, respectively, and on multivariable analysis of all 310 patients (including palbociclib as a variable in the model and with ctDNA fraction as a continuous variable), higher baseline tumor purity in plasma was associated with highly significantly worse PFS (HR 1.2 per 10% increase in purity), and baseline TP53 mutation and FGFR1 amplification each were associated with significantly shorter PFS (HRs, 1.8 and 2.9, respectively).
“[It is] very important to note ... that we did look specifically for interaction between our genomic changes and treatment, and we didn’t find any evidence of a significant interaction, so these genomic markers [are] prognostic rather than predictive in terms of the two treatment arms of the trial,” he said.
A survival analysis showed a median PFS of 3.7 vs. 12.7 months in patients with vs. without TP53 mutation in the P+F arm, and 1.8 vs. 5.4 months, respectively, in the F arm, with similar HRs of 2.0 and 2.3 in the arms, respectively.
“Even in the [P+F] arm, you see almost half of the patients with a TP53 mutation ... have relapsed by 2 months, the earliest clinical assessment in the trial,” he noted.
For FGFR1, the PFS was 3.9 vs. 12 months with vs. without amplification in the P+F arms, and 1.8 vs. 5.8 months, respectively in th F arm, with HRs of 3.4 and 3.6, respectively.
These findings are notable because markers of early progression on endocrine therapy in combination with CDK4/6 inhibitors remain limited – despite the key role of these combinations in treating ER+ advanced breast cancer, Dr. O’Leary explained.
“Although many patients derive a great deal of benefit from these combinations, there are a subset of patients who will relapse relatively early, and ... we don’t have an established means of identifying those patients at the present,” he said. “From the technical perspective, liquid biopsies have emerged in recent years as a promising means of genotyping patients’ cancers from circulating tumor DNA, and in addition, the overall level of circulating tumor DNA – the fractional purity – has been associated with poor prognosis, specifically in the triple-negative breast cancer setting.”
The results, which require independent validation, could potentially inform future clinical trials of CDK4/6 inhibitor combinations in advanced ER+ breast cancer to identify a high-risk group of patients who require escalation of therapy, he concluded.
Dr. O’Leary reported receiving research funding from Pfizer to his institution.
SOURCE: O’Leary B et al. ASCO 2019, Abstract 1010.
CHICAGO – Tumor protein 53 (TP53) mutation, fibroblast growth factor receptor 1 (FGFR1) amplification, and tumor purity in plasma each predict early progression on palbociclib and/or fulvestrant in patients with advanced estrogen receptor–positive (ER+) breast cancer, according to genomic analyses of PALOMA-3 trial data.
Overall, the presence of one or more of these genomic changes identified 131 out of 310 patients from the phase 3 trial who had baseline samples available, Ben O’Leary, MBBS, said at the annual meeting of the American Society of Clinical Oncology.
“So, a significant minority of patients – 42.3% – potentially who fall into a more poor-prognosis group,” said Dr. O’Leary of the Institute of Cancer Research at the Royal Marsden Hospital in London.
The findings suggest that a “liquid biopsy” at the start of treatment could identify patients at risk for progression.
The PALOMA-3 trial randomized 521 patients with ER+, human epidermal growth factor receptor 2 (HER2)–negative advanced breast cancer who had previously progressed on endocrine therapy 2:1 to CDK4/CDK6 inhibition with palbociclib plus fulvestrant (P+F) or placebo plus fulvestrant (F), and it showed that adding palbociclib significantly improved progression-free survival (PFS) (N Engl J Med. Jul 16 2015;373:209-19).
For the current analysis, the investigators assessed circulating tumor DNA (ctDNA) in baseline plasma samples from 459 study participants in an effort to identify genomic biomarkers for progression, to examine the association between baseline tumor fraction and clinical outcome, and to explore differences in predictive markers by treatment arm. A custom amplicon-sequencing analysis was performed to look for mutations in 17 different relevant genes, and another was used to estimate tumor fraction by looking at about 800 common germline single-nucleotide polymorphisms and to assess copy-number gain in the amplification status in 11 different genes, Dr. O’Leary said.
Results for mutations and circulating nucleic acids were available in 203 and 107 patients from the P+F and F groups, respectively, and on multivariable analysis of all 310 patients (including palbociclib as a variable in the model and with ctDNA fraction as a continuous variable), higher baseline tumor purity in plasma was associated with highly significantly worse PFS (HR 1.2 per 10% increase in purity), and baseline TP53 mutation and FGFR1 amplification each were associated with significantly shorter PFS (HRs, 1.8 and 2.9, respectively).
“[It is] very important to note ... that we did look specifically for interaction between our genomic changes and treatment, and we didn’t find any evidence of a significant interaction, so these genomic markers [are] prognostic rather than predictive in terms of the two treatment arms of the trial,” he said.
A survival analysis showed a median PFS of 3.7 vs. 12.7 months in patients with vs. without TP53 mutation in the P+F arm, and 1.8 vs. 5.4 months, respectively, in the F arm, with similar HRs of 2.0 and 2.3 in the arms, respectively.
“Even in the [P+F] arm, you see almost half of the patients with a TP53 mutation ... have relapsed by 2 months, the earliest clinical assessment in the trial,” he noted.
For FGFR1, the PFS was 3.9 vs. 12 months with vs. without amplification in the P+F arms, and 1.8 vs. 5.8 months, respectively in th F arm, with HRs of 3.4 and 3.6, respectively.
These findings are notable because markers of early progression on endocrine therapy in combination with CDK4/6 inhibitors remain limited – despite the key role of these combinations in treating ER+ advanced breast cancer, Dr. O’Leary explained.
“Although many patients derive a great deal of benefit from these combinations, there are a subset of patients who will relapse relatively early, and ... we don’t have an established means of identifying those patients at the present,” he said. “From the technical perspective, liquid biopsies have emerged in recent years as a promising means of genotyping patients’ cancers from circulating tumor DNA, and in addition, the overall level of circulating tumor DNA – the fractional purity – has been associated with poor prognosis, specifically in the triple-negative breast cancer setting.”
The results, which require independent validation, could potentially inform future clinical trials of CDK4/6 inhibitor combinations in advanced ER+ breast cancer to identify a high-risk group of patients who require escalation of therapy, he concluded.
Dr. O’Leary reported receiving research funding from Pfizer to his institution.
SOURCE: O’Leary B et al. ASCO 2019, Abstract 1010.
CHICAGO – Tumor protein 53 (TP53) mutation, fibroblast growth factor receptor 1 (FGFR1) amplification, and tumor purity in plasma each predict early progression on palbociclib and/or fulvestrant in patients with advanced estrogen receptor–positive (ER+) breast cancer, according to genomic analyses of PALOMA-3 trial data.
Overall, the presence of one or more of these genomic changes identified 131 out of 310 patients from the phase 3 trial who had baseline samples available, Ben O’Leary, MBBS, said at the annual meeting of the American Society of Clinical Oncology.
“So, a significant minority of patients – 42.3% – potentially who fall into a more poor-prognosis group,” said Dr. O’Leary of the Institute of Cancer Research at the Royal Marsden Hospital in London.
The findings suggest that a “liquid biopsy” at the start of treatment could identify patients at risk for progression.
The PALOMA-3 trial randomized 521 patients with ER+, human epidermal growth factor receptor 2 (HER2)–negative advanced breast cancer who had previously progressed on endocrine therapy 2:1 to CDK4/CDK6 inhibition with palbociclib plus fulvestrant (P+F) or placebo plus fulvestrant (F), and it showed that adding palbociclib significantly improved progression-free survival (PFS) (N Engl J Med. Jul 16 2015;373:209-19).
For the current analysis, the investigators assessed circulating tumor DNA (ctDNA) in baseline plasma samples from 459 study participants in an effort to identify genomic biomarkers for progression, to examine the association between baseline tumor fraction and clinical outcome, and to explore differences in predictive markers by treatment arm. A custom amplicon-sequencing analysis was performed to look for mutations in 17 different relevant genes, and another was used to estimate tumor fraction by looking at about 800 common germline single-nucleotide polymorphisms and to assess copy-number gain in the amplification status in 11 different genes, Dr. O’Leary said.
Results for mutations and circulating nucleic acids were available in 203 and 107 patients from the P+F and F groups, respectively, and on multivariable analysis of all 310 patients (including palbociclib as a variable in the model and with ctDNA fraction as a continuous variable), higher baseline tumor purity in plasma was associated with highly significantly worse PFS (HR 1.2 per 10% increase in purity), and baseline TP53 mutation and FGFR1 amplification each were associated with significantly shorter PFS (HRs, 1.8 and 2.9, respectively).
“[It is] very important to note ... that we did look specifically for interaction between our genomic changes and treatment, and we didn’t find any evidence of a significant interaction, so these genomic markers [are] prognostic rather than predictive in terms of the two treatment arms of the trial,” he said.
A survival analysis showed a median PFS of 3.7 vs. 12.7 months in patients with vs. without TP53 mutation in the P+F arm, and 1.8 vs. 5.4 months, respectively, in the F arm, with similar HRs of 2.0 and 2.3 in the arms, respectively.
“Even in the [P+F] arm, you see almost half of the patients with a TP53 mutation ... have relapsed by 2 months, the earliest clinical assessment in the trial,” he noted.
For FGFR1, the PFS was 3.9 vs. 12 months with vs. without amplification in the P+F arms, and 1.8 vs. 5.8 months, respectively in th F arm, with HRs of 3.4 and 3.6, respectively.
These findings are notable because markers of early progression on endocrine therapy in combination with CDK4/6 inhibitors remain limited – despite the key role of these combinations in treating ER+ advanced breast cancer, Dr. O’Leary explained.
“Although many patients derive a great deal of benefit from these combinations, there are a subset of patients who will relapse relatively early, and ... we don’t have an established means of identifying those patients at the present,” he said. “From the technical perspective, liquid biopsies have emerged in recent years as a promising means of genotyping patients’ cancers from circulating tumor DNA, and in addition, the overall level of circulating tumor DNA – the fractional purity – has been associated with poor prognosis, specifically in the triple-negative breast cancer setting.”
The results, which require independent validation, could potentially inform future clinical trials of CDK4/6 inhibitor combinations in advanced ER+ breast cancer to identify a high-risk group of patients who require escalation of therapy, he concluded.
Dr. O’Leary reported receiving research funding from Pfizer to his institution.
SOURCE: O’Leary B et al. ASCO 2019, Abstract 1010.
REPORTING FROM ASCO 2019
Niraparib-pembrolizumab combo finds niche in breast, ovarian cancers
The strategy of simultaneously exploiting deficient DNA damage repair and unleashing the immune response could expand treatment options for hard-to-treat breast and ovarian cancers, findings of the TOPACIO/KEYNOTE-162 trial suggest.
Triple-negative breast cancer (TNBC) and high-grade serous ovarian carcinoma share a number of genomic features, including a high frequency of BRCA1 and BRCA2 inactivation (Nature. 2012;490:61-70), as well as potential immunoreactivity (Lancet Oncol. 2018;19:40-50).
The open-label, single-arm phase 1/2 trial therefore tested the combination of niraparib (Zejula), an oral poly (ADP-ribose) polymerase (PARP) inhibitor, and pembrolizumab (Keytruda), an antibody to programmed death 1 (PD-1), among more than 100 patients with advanced or metastatic TNBC or recurrent platinum-resistant ovarian carcinoma. Patients were enrolled irrespective of BRCA mutation status or programmed death-ligand 1 (PD-L1) expression.
Main results, reported in JAMA Oncology, showed that the combination was safe, and about a fifth of patients with each type of cancer had an objective response. Median progression-free survival (PFS) was about 2 months in those with TNBC overall (although it exceeded 8 months in the subset with a tumor BRCA mutation) and about 3 months in those with ovarian cancer.
TNBC cohort
Investigators led by Shaveta Vinayak, MD, of the division of oncology at Fred Hutchinson Cancer Research Center, and University of Washington School of Medicine, Seattle Cancer Care Alliance, Seattle, studied 55 patients with TNBC treated with niraparib-pembrolizumab in the trial.
In the efficacy-evaluable population of 47 patients, the objective response rate (ORR) was 21%, and the disease control rate (DCR) was 49%. With a median duration of follow-up of 14.8 months, the median duration of response was not reached.
Activity of the combination varied by tumor BRCA mutation status. Compared with counterparts having BRCA wild-type tumors, patients having tumors with BRCA mutations had a numerically higher ORR (47% vs. 11%), DCR (80% vs. 33%), and PFS (8.3 vs. 2.1 months).
Some 18% of patients had treatment-related anemia, 15% thrombocytopenia, and 7% fatigue. In addition, 15% of patients had immune-related adverse events, with 4% having grade 3 immune-related adverse events.
“Combination niraparib plus pembrolizumab provides promising antitumor activity in patients with advanced or metastatic TNBC, with numerically higher response rates in those with tumor BRCA mutations,” Dr. Vinayak and colleagues conclude. “The combination therapy was safe with a tolerable safety profile, warranting further investigation.”
Ovarian cancer cohort
Investigators led by Panagiotis A. Konstantinopoulos, MD, PhD, of the division of gynecologic oncology, department of medical oncology at Dana-Farber Cancer Institute, Harvard Medical School, Boston, studied 62 patients with ovarian carcinoma treated with niraparib-pembrolizumab in the trial.
In the efficacy-evaluable population of 60 patients, the ORR was 18% and the DCR was 65%. The ORRs were similar regardless of patients’ platinum-based chemotherapy sensitivity, previous bevacizumab treatment, or tumor BRCA or homologous recombination deficiency (HRD) biomarker status.
With a median duration of follow-up of 12.4 months, the median duration of response was not reached, ranging from 4.2 to roughly 14.5 months. Median progression-free survival was 3.4 months.
The leading treatment-related adverse events of grade 3 or higher in this cohort were anemia (21%) and thrombocytopenia (9%). In addition, 19% of patients had immune-related adverse events, with 9% having grade 3 or higher immune-related adverse events.
“Niraparib in combination with pembrolizumab is tolerable, with promising antitumor activity for patients with ovarian carcinoma who have limited treatment options regardless of platinum status, biomarker status, or prior treatment with bevacizumab,” Dr. Konstantinopoulos and colleagues conclude. “Responses in patients without tumor BRCA mutations or non-HRD cancers were higher than expected with either agent as monotherapy.”
Dr. Vinayak disclosed receiving clinical trial funding from TESARO; serving on an advisory board for TESARO; and serving on an advisory board for OncoSec Medical (uncompensated). Dr. Konstantinopoulos disclosed serving on advisory boards for AstraZeneca, Pfizer, and Merck. The trial was supported by TESARO: a GSK company and Merck, and in part by Stand Up to Cancer (a program of the Entertainment Industry Foundation); the Ovarian Cancer Research Fund Alliance; and National Ovarian Cancer Coalition Dream Team Translational Research.
SOURCE: Vinayak A et al. JAMA Oncol. 2019 Jun 13. doi: 10.1001/jamaoncol.2019.1029. Konstantinopoulos PA et al. JAMA Oncol. 2019 Jun 13. doi: 10.1001/jamaoncol.2019.1048.
“Targeting DNA repair and immune checkpoint pathways has emerged as an important concept in cancer therapy, well supported by preclinical and clinical data in ovarian cancer and TNBC. However, there are some limitations to the two studies presented herein,” maintain editorialists Kunle Odunsi, MD, PhD, and Tanja Pejovic, MD, PhD.
Patients varied considerably with respect to number of prior chemotherapy regimens, they elaborate. Also, there may have been some misclassification of patients into DNA damage repair (DDR) groups, and small sample sizes precluded rigorous subgroup analyses.
“Because DDR and, by extension, tumor mutational burden and PD-L1 status do not fully explain the effects of the combination of PARP inhibitors and anti–PD-1 therapy, additional predictive biomarkers based on tumor intrinsic or adaptive mechanisms of resistance are needed for both cancer types,” the editorialists contend. In particular, knowledge of the tumor microenvironment could be used to tailor therapy for individual patients.
“The TOPACIO clinical studies are clearly steps in the right direction for patients with [platinum-resistant ovarian carcinoma] and TNBC,” they conclude. “However, larger confirmatory randomized clinical trials are needed that use panels of integrated biomarkers that would allow identification of patients most likely to respond.”
Dr. Odunsi is the deputy director and chair of the department of gynecologic oncology, executive director of the Center for Immunotherapy, and co-leader of the Tumor Immunology and Immunotherapy Research Program–Roswell Park Comprehensive Cancer Center, Buffalo, N.Y. Dr. Pejovic is associate professor, division of gynecologic oncology, department of obstetrics & gynecology, Knight Cancer Institute, Oregon Health & Science University, Portland, Ore. These remarks are adapted from a related editorial (JAMA Oncol. 2019 Jun 13. doi: 10.1001/jamaoncol.2019.1009 ).
“Targeting DNA repair and immune checkpoint pathways has emerged as an important concept in cancer therapy, well supported by preclinical and clinical data in ovarian cancer and TNBC. However, there are some limitations to the two studies presented herein,” maintain editorialists Kunle Odunsi, MD, PhD, and Tanja Pejovic, MD, PhD.
Patients varied considerably with respect to number of prior chemotherapy regimens, they elaborate. Also, there may have been some misclassification of patients into DNA damage repair (DDR) groups, and small sample sizes precluded rigorous subgroup analyses.
“Because DDR and, by extension, tumor mutational burden and PD-L1 status do not fully explain the effects of the combination of PARP inhibitors and anti–PD-1 therapy, additional predictive biomarkers based on tumor intrinsic or adaptive mechanisms of resistance are needed for both cancer types,” the editorialists contend. In particular, knowledge of the tumor microenvironment could be used to tailor therapy for individual patients.
“The TOPACIO clinical studies are clearly steps in the right direction for patients with [platinum-resistant ovarian carcinoma] and TNBC,” they conclude. “However, larger confirmatory randomized clinical trials are needed that use panels of integrated biomarkers that would allow identification of patients most likely to respond.”
Dr. Odunsi is the deputy director and chair of the department of gynecologic oncology, executive director of the Center for Immunotherapy, and co-leader of the Tumor Immunology and Immunotherapy Research Program–Roswell Park Comprehensive Cancer Center, Buffalo, N.Y. Dr. Pejovic is associate professor, division of gynecologic oncology, department of obstetrics & gynecology, Knight Cancer Institute, Oregon Health & Science University, Portland, Ore. These remarks are adapted from a related editorial (JAMA Oncol. 2019 Jun 13. doi: 10.1001/jamaoncol.2019.1009 ).
“Targeting DNA repair and immune checkpoint pathways has emerged as an important concept in cancer therapy, well supported by preclinical and clinical data in ovarian cancer and TNBC. However, there are some limitations to the two studies presented herein,” maintain editorialists Kunle Odunsi, MD, PhD, and Tanja Pejovic, MD, PhD.
Patients varied considerably with respect to number of prior chemotherapy regimens, they elaborate. Also, there may have been some misclassification of patients into DNA damage repair (DDR) groups, and small sample sizes precluded rigorous subgroup analyses.
“Because DDR and, by extension, tumor mutational burden and PD-L1 status do not fully explain the effects of the combination of PARP inhibitors and anti–PD-1 therapy, additional predictive biomarkers based on tumor intrinsic or adaptive mechanisms of resistance are needed for both cancer types,” the editorialists contend. In particular, knowledge of the tumor microenvironment could be used to tailor therapy for individual patients.
“The TOPACIO clinical studies are clearly steps in the right direction for patients with [platinum-resistant ovarian carcinoma] and TNBC,” they conclude. “However, larger confirmatory randomized clinical trials are needed that use panels of integrated biomarkers that would allow identification of patients most likely to respond.”
Dr. Odunsi is the deputy director and chair of the department of gynecologic oncology, executive director of the Center for Immunotherapy, and co-leader of the Tumor Immunology and Immunotherapy Research Program–Roswell Park Comprehensive Cancer Center, Buffalo, N.Y. Dr. Pejovic is associate professor, division of gynecologic oncology, department of obstetrics & gynecology, Knight Cancer Institute, Oregon Health & Science University, Portland, Ore. These remarks are adapted from a related editorial (JAMA Oncol. 2019 Jun 13. doi: 10.1001/jamaoncol.2019.1009 ).
The strategy of simultaneously exploiting deficient DNA damage repair and unleashing the immune response could expand treatment options for hard-to-treat breast and ovarian cancers, findings of the TOPACIO/KEYNOTE-162 trial suggest.
Triple-negative breast cancer (TNBC) and high-grade serous ovarian carcinoma share a number of genomic features, including a high frequency of BRCA1 and BRCA2 inactivation (Nature. 2012;490:61-70), as well as potential immunoreactivity (Lancet Oncol. 2018;19:40-50).
The open-label, single-arm phase 1/2 trial therefore tested the combination of niraparib (Zejula), an oral poly (ADP-ribose) polymerase (PARP) inhibitor, and pembrolizumab (Keytruda), an antibody to programmed death 1 (PD-1), among more than 100 patients with advanced or metastatic TNBC or recurrent platinum-resistant ovarian carcinoma. Patients were enrolled irrespective of BRCA mutation status or programmed death-ligand 1 (PD-L1) expression.
Main results, reported in JAMA Oncology, showed that the combination was safe, and about a fifth of patients with each type of cancer had an objective response. Median progression-free survival (PFS) was about 2 months in those with TNBC overall (although it exceeded 8 months in the subset with a tumor BRCA mutation) and about 3 months in those with ovarian cancer.
TNBC cohort
Investigators led by Shaveta Vinayak, MD, of the division of oncology at Fred Hutchinson Cancer Research Center, and University of Washington School of Medicine, Seattle Cancer Care Alliance, Seattle, studied 55 patients with TNBC treated with niraparib-pembrolizumab in the trial.
In the efficacy-evaluable population of 47 patients, the objective response rate (ORR) was 21%, and the disease control rate (DCR) was 49%. With a median duration of follow-up of 14.8 months, the median duration of response was not reached.
Activity of the combination varied by tumor BRCA mutation status. Compared with counterparts having BRCA wild-type tumors, patients having tumors with BRCA mutations had a numerically higher ORR (47% vs. 11%), DCR (80% vs. 33%), and PFS (8.3 vs. 2.1 months).
Some 18% of patients had treatment-related anemia, 15% thrombocytopenia, and 7% fatigue. In addition, 15% of patients had immune-related adverse events, with 4% having grade 3 immune-related adverse events.
“Combination niraparib plus pembrolizumab provides promising antitumor activity in patients with advanced or metastatic TNBC, with numerically higher response rates in those with tumor BRCA mutations,” Dr. Vinayak and colleagues conclude. “The combination therapy was safe with a tolerable safety profile, warranting further investigation.”
Ovarian cancer cohort
Investigators led by Panagiotis A. Konstantinopoulos, MD, PhD, of the division of gynecologic oncology, department of medical oncology at Dana-Farber Cancer Institute, Harvard Medical School, Boston, studied 62 patients with ovarian carcinoma treated with niraparib-pembrolizumab in the trial.
In the efficacy-evaluable population of 60 patients, the ORR was 18% and the DCR was 65%. The ORRs were similar regardless of patients’ platinum-based chemotherapy sensitivity, previous bevacizumab treatment, or tumor BRCA or homologous recombination deficiency (HRD) biomarker status.
With a median duration of follow-up of 12.4 months, the median duration of response was not reached, ranging from 4.2 to roughly 14.5 months. Median progression-free survival was 3.4 months.
The leading treatment-related adverse events of grade 3 or higher in this cohort were anemia (21%) and thrombocytopenia (9%). In addition, 19% of patients had immune-related adverse events, with 9% having grade 3 or higher immune-related adverse events.
“Niraparib in combination with pembrolizumab is tolerable, with promising antitumor activity for patients with ovarian carcinoma who have limited treatment options regardless of platinum status, biomarker status, or prior treatment with bevacizumab,” Dr. Konstantinopoulos and colleagues conclude. “Responses in patients without tumor BRCA mutations or non-HRD cancers were higher than expected with either agent as monotherapy.”
Dr. Vinayak disclosed receiving clinical trial funding from TESARO; serving on an advisory board for TESARO; and serving on an advisory board for OncoSec Medical (uncompensated). Dr. Konstantinopoulos disclosed serving on advisory boards for AstraZeneca, Pfizer, and Merck. The trial was supported by TESARO: a GSK company and Merck, and in part by Stand Up to Cancer (a program of the Entertainment Industry Foundation); the Ovarian Cancer Research Fund Alliance; and National Ovarian Cancer Coalition Dream Team Translational Research.
SOURCE: Vinayak A et al. JAMA Oncol. 2019 Jun 13. doi: 10.1001/jamaoncol.2019.1029. Konstantinopoulos PA et al. JAMA Oncol. 2019 Jun 13. doi: 10.1001/jamaoncol.2019.1048.
The strategy of simultaneously exploiting deficient DNA damage repair and unleashing the immune response could expand treatment options for hard-to-treat breast and ovarian cancers, findings of the TOPACIO/KEYNOTE-162 trial suggest.
Triple-negative breast cancer (TNBC) and high-grade serous ovarian carcinoma share a number of genomic features, including a high frequency of BRCA1 and BRCA2 inactivation (Nature. 2012;490:61-70), as well as potential immunoreactivity (Lancet Oncol. 2018;19:40-50).
The open-label, single-arm phase 1/2 trial therefore tested the combination of niraparib (Zejula), an oral poly (ADP-ribose) polymerase (PARP) inhibitor, and pembrolizumab (Keytruda), an antibody to programmed death 1 (PD-1), among more than 100 patients with advanced or metastatic TNBC or recurrent platinum-resistant ovarian carcinoma. Patients were enrolled irrespective of BRCA mutation status or programmed death-ligand 1 (PD-L1) expression.
Main results, reported in JAMA Oncology, showed that the combination was safe, and about a fifth of patients with each type of cancer had an objective response. Median progression-free survival (PFS) was about 2 months in those with TNBC overall (although it exceeded 8 months in the subset with a tumor BRCA mutation) and about 3 months in those with ovarian cancer.
TNBC cohort
Investigators led by Shaveta Vinayak, MD, of the division of oncology at Fred Hutchinson Cancer Research Center, and University of Washington School of Medicine, Seattle Cancer Care Alliance, Seattle, studied 55 patients with TNBC treated with niraparib-pembrolizumab in the trial.
In the efficacy-evaluable population of 47 patients, the objective response rate (ORR) was 21%, and the disease control rate (DCR) was 49%. With a median duration of follow-up of 14.8 months, the median duration of response was not reached.
Activity of the combination varied by tumor BRCA mutation status. Compared with counterparts having BRCA wild-type tumors, patients having tumors with BRCA mutations had a numerically higher ORR (47% vs. 11%), DCR (80% vs. 33%), and PFS (8.3 vs. 2.1 months).
Some 18% of patients had treatment-related anemia, 15% thrombocytopenia, and 7% fatigue. In addition, 15% of patients had immune-related adverse events, with 4% having grade 3 immune-related adverse events.
“Combination niraparib plus pembrolizumab provides promising antitumor activity in patients with advanced or metastatic TNBC, with numerically higher response rates in those with tumor BRCA mutations,” Dr. Vinayak and colleagues conclude. “The combination therapy was safe with a tolerable safety profile, warranting further investigation.”
Ovarian cancer cohort
Investigators led by Panagiotis A. Konstantinopoulos, MD, PhD, of the division of gynecologic oncology, department of medical oncology at Dana-Farber Cancer Institute, Harvard Medical School, Boston, studied 62 patients with ovarian carcinoma treated with niraparib-pembrolizumab in the trial.
In the efficacy-evaluable population of 60 patients, the ORR was 18% and the DCR was 65%. The ORRs were similar regardless of patients’ platinum-based chemotherapy sensitivity, previous bevacizumab treatment, or tumor BRCA or homologous recombination deficiency (HRD) biomarker status.
With a median duration of follow-up of 12.4 months, the median duration of response was not reached, ranging from 4.2 to roughly 14.5 months. Median progression-free survival was 3.4 months.
The leading treatment-related adverse events of grade 3 or higher in this cohort were anemia (21%) and thrombocytopenia (9%). In addition, 19% of patients had immune-related adverse events, with 9% having grade 3 or higher immune-related adverse events.
“Niraparib in combination with pembrolizumab is tolerable, with promising antitumor activity for patients with ovarian carcinoma who have limited treatment options regardless of platinum status, biomarker status, or prior treatment with bevacizumab,” Dr. Konstantinopoulos and colleagues conclude. “Responses in patients without tumor BRCA mutations or non-HRD cancers were higher than expected with either agent as monotherapy.”
Dr. Vinayak disclosed receiving clinical trial funding from TESARO; serving on an advisory board for TESARO; and serving on an advisory board for OncoSec Medical (uncompensated). Dr. Konstantinopoulos disclosed serving on advisory boards for AstraZeneca, Pfizer, and Merck. The trial was supported by TESARO: a GSK company and Merck, and in part by Stand Up to Cancer (a program of the Entertainment Industry Foundation); the Ovarian Cancer Research Fund Alliance; and National Ovarian Cancer Coalition Dream Team Translational Research.
SOURCE: Vinayak A et al. JAMA Oncol. 2019 Jun 13. doi: 10.1001/jamaoncol.2019.1029. Konstantinopoulos PA et al. JAMA Oncol. 2019 Jun 13. doi: 10.1001/jamaoncol.2019.1048.
FROM JAMA ONCOLOGY
TAILORx: Clinical data add value to recurrence score
CHICAGO – , according to a secondary analysis of data from the practice-changing TAILORx study.
Specifically, tumor size and histology-based risk stratification improves the prediction of disease-free survival and distant recurrence, and – for some patient groups – chemotherapy benefit, Joseph A. Sparano, MD, reported at the annual meeting of the American Society of Clinical Oncology.
Combining these tools could help determine whether endocrine therapy (ET) alone or ET with adjuvant chemotherapy is the best treatment approach for a given patient, said Dr. Sparano, professor of medicine and obstetrics, gynecology, and women’s health at Albert Einstein College of Medicine, New York.
The phase 3 TAILORx study established that ET alone is noninferior to adjuvant chemotherapy (CT) plus ET in patients with early breast cancer and RS of 11-25, and that ET alone has some benefit over ET+CT in women aged 50 years and younger with RS of 16-25, he explained.
Those findings were presented at the 2018 ASCO annual meeting and subsequently published in the New England Journal of Medicine.
The current analysis focused on the integration of clinical and genomic features for prognosis, and the results were published online June 3 in a corresponding article in the New England Journal of Medicine.
“The totality of the data, including TAILORx and the prior prospective validation studies, indicate that assessment of genomic risk with the 21-gene recurrence score provides complementary prognostic information to pathologic features, and is also predictive of a large chemotherapy benefit if the recurrence score is greater than 25, or lack thereof if 25 or lower,” he said.
However, there is a three-way interaction between age, RS, and CT use, which results in an absolute CT benefit in women aged 50 or younger of about 2% for RS of 16-20, and about 7% for RS of 21-25, he added.
“Assessment of clinical risk using pathological features also provides prognostic information that doesn’t correlate well with the recurrence score, therefore it stands to reason that integration of clinical and genomic risk offers the potential for greater precision in prognosis and, ultimately, guiding the use of adjuvant therapy,” he said.
Clinical risk for this analysis was assessed using a binary clinical risk categorization employed in the MINDACT trial and calibrated to greater than 92% 10-year breast cancer-specific survival for ET alone based on Adjuvant! version 8.0. Low-grade tumors up to 3 cm, intermediate-grade tumors up to 2 cm, and high-grade tumors up to 1 cm were categorized as low clinical risk (LCR), and all others not meeting these criteria were categorized as high clinical risk (HCR), he explained.
Of 9,427 patients included in the analysis, 70% had LCR and 30% had HCR.
“For distant recurrence, high clinical risk was associated with a 2.5- to 3-fold higher recurrence rate for those with a recurrence score of 11 or higher, and in a multivariate model for distant recurrence in the [group with a] recurrence score of 11-25, high clinical risk was independently associated with a 2.4-fold higher recurrence risk,” he said. “Continuous recurrence score also provided significant prognostic information, with each 1-unit increase associated with an 8% higher distant recurrence risk.”
For the overall population, clinical risk added significant prognostic information to the RS for both distant recurrence and disease-free survival, and stratification by age showed that among women over age 50 years, the hazard ratios for distant recurrence ranged from 2.20 to 2.36, and did not substantially vary by age or RS, he said.
However, for the overall population, adding clinical risk to the RS did not improve prediction of chemotherapy benefit.
“This was also true for the two-thirds of women who were over 50 years of age. For the remaining women 50 or younger, there was a trend favoring chemo, irrespective of clinical risk, though not significant – a finding consistent with the treatment interaction previously described,” he said.
Finally, the absolute differences in 9-year distant recurrence rates by clinical risk stratified by age, RS, and CT use showed an absolute 4%-6% higher distant recurrence risk for HCR vs. LCR among those over age 50 with RS of 0-25 irrespective of CT use, and a 13% difference for those with RS of 26-100 who were treated with CT.
“For those 50 or younger, clinical risk had no impact on recurrence if the RS was 0-10. For RS of 11-25, the difference was about 9% with endocrine therapy alone, and 2% with chemo plus ET, reflecting absolute chemo benefit in younger women who had high clinical risk,” he said, adding that for those with RS of 26-100, there was a 9% higher absolute recurrence rate in the HCR vs. LCR population.
“We therefore further evaluated absolute differences in distance recurrence rates associated with chemotherapy use in women 50 and younger with RS of 16-25, further stratified by RS and clinical risk,” he said, noting that when not stratified by clinical risk, as reported in the primary analysis, the absolute CT benefit was 1.6% for RS of 16-20, and 6.5% for RS of 21-25.
When stratified by clinical risk, the absolute CT benefit ranged from 6% to 9% in those with RS of 21-25, irrespective of clinical risk, and in those with RS of 16-20 and HCR.
“This accounted for 51% of patients with RS of 16-25,” he said. “However, there was no demonstrable chemo benefit for those with LCR and RS of 16-20, who accounted for the remaining 49%.”
Additional analysis looking at age at diagnosis and CT benefit showed a benefit in premenopausal women aged 46-50 years (but not postmenopausal women), a trend toward benefit in those aged 41-45 years, and no benefit in those aged 40 years and younger, who are less likely to develop premature menopause as a consequence of cytotoxic CT.
“In addition, we saw no consistent effect favoring chemotherapy in older women. Taken together, these findings suggest the chemo benefit observed for the RS 16-25 group may, in fact, be due to a castration effect associated with cytotoxic therapy rather than an effect in eradicating micrometastatic disease,” Dr. Sparano said.
Applying this framework to the TAILORx study population categorized 68% of those aged 50 years and younger into a low integrated risk group with less than 5% risk of distant recurrence. This included all patients with RS of 0-10 irrespective of clinical risk (14% of the patient population; distant recurrence rate 1.8% or less), and all with RS of 11-25 and LCR (54% of the patient population, 4.7% distant recurrence rate).
In contrast, 25% fell into the high integrated risk group (greater than 10% distant recurrence risk), including those with RS of 11-25 and HCR (17% of the patient population; distant recurrence rate 12.3%), and RS of 26-100 and HCR (8% of the patient population; distant recurrence rate 15.2%).
“This framework encompasses 93% of all TAILORx subjects, with the remaining 7% having a distant recurrence risk of between 5% and 10%,” he said.
Overall, the primary results of TAILORx remain unchanged based on this secondary analysis as the addition of clinical risk did not predict CT benefit in the RS 11-25 group, he noted.
“However, for women 50 and under and RS 16-25, integrated risk distinguished 50% who derived no chemo benefit from the 50% who derived an absolute benefit of approximately 6%-9% – a level that is higher than an unselected population,” he said, reiterating that the absolute CT benefit was greater in premenopausal women aged 45-50 with RS 16-25, suggesting that the absolute CR benefit seen in younger women in TAILORx may be due to an endocrine effect.
“Integrated risk clearly provides greater prognostic precision and may have clinical utility; the prognostic precision afforded by the integrated risk model is superior to that by the use of clinical or genomic features alone, and in addition, the genomic assay also provides predictive information for chemo benefit that is not captured by clinical features alone,” he concluded.
As an example of the potential clinical utility of this integrated approach for guiding treatment in women aged 50 years or younger, he presented “a highly stratified integrated risk assessment model” separating TAILORx patients into low integrated risk (58% of the study population) and high integrated risk (31% of the study population).
In the low integrated risk patients with RS of 0-10 and any clinical risk level, or with RS of 11-25 and LCR, tamoxifen alone appears adequate, he said.
In those with high integrated risk and RS of 16-25 with HCR, ovarian function suppression plus an aromatase inhibitor (OFS/AI) could be considered as an alternative to chemo, and in those with high integrated risk, RS of 26-100, and HCR who have not developed chemotherapy-induced menopause, ovarian function suppression and an AI could be added to chemotherapy.
“Indeed, data from the SOFT and TEXT trials indicate that patients with a high RS risk experienced an absolute improvement of up to 10%-15% in 5-year breast cancer–free interval with an OFS/AI, compared with tamoxifen, whereas improvement was minimal in those at lowest risk, supporting the strategy of using integrated clinical and genomic risk to select for ovarian function suppression plus an AI,” he said.
During a discussion of the findings and how they might impact practice, Vered Stearns, MD, an oncology professor and codirector of the Breast Cancer Program at Johns Hopkins University, Baltimore, noted that in her practice she will “carefully select women for whom genomic assay [use] is appropriate.
“I will also assess clinical risk and RS to inform recommendations for chemotherapy use, and possibly appropriate endocrine agents in select populations,” she said.
Dr. Stearns further noted that the interaction between RS and age as reported by Dr. Sparano is exploratory and should be interpreted with caution as the majority of those aged 50 and younger received tamoxifen alone and the question remains as to whether they would have received similar benefits from ovarian suppression and tamoxifen/AI instead of chemo-endocrine therapy.
“Indeed, indirect hypotheses from other studies suggest that may be the case,” she said, adding that these women may be offered ovarian suppression and tamoxifen or AI based on the SOFT and TEXT results.
“TAILORx remains a rich resource for new explorations, new biomarkers, new models, and new machine learning opportunities,” she said.
TAILORx was funded by the National Institutes of Health. Dr. Sparano reported stock ownership, a consulting role, and research funding from several pharmaceutical companies. Dr. Stearns reported consulting or advisory roles with Iridium Therapeutics; research funding from Abbvie, Biocept, MedImmune, Novartis, Pfizer, and Puma Biotechnology; and an “other relationship” with Immunomedics.
SOURCE: Sparano JA et al. ASCO 2019. Abstract 503.
CHICAGO – , according to a secondary analysis of data from the practice-changing TAILORx study.
Specifically, tumor size and histology-based risk stratification improves the prediction of disease-free survival and distant recurrence, and – for some patient groups – chemotherapy benefit, Joseph A. Sparano, MD, reported at the annual meeting of the American Society of Clinical Oncology.
Combining these tools could help determine whether endocrine therapy (ET) alone or ET with adjuvant chemotherapy is the best treatment approach for a given patient, said Dr. Sparano, professor of medicine and obstetrics, gynecology, and women’s health at Albert Einstein College of Medicine, New York.
The phase 3 TAILORx study established that ET alone is noninferior to adjuvant chemotherapy (CT) plus ET in patients with early breast cancer and RS of 11-25, and that ET alone has some benefit over ET+CT in women aged 50 years and younger with RS of 16-25, he explained.
Those findings were presented at the 2018 ASCO annual meeting and subsequently published in the New England Journal of Medicine.
The current analysis focused on the integration of clinical and genomic features for prognosis, and the results were published online June 3 in a corresponding article in the New England Journal of Medicine.
“The totality of the data, including TAILORx and the prior prospective validation studies, indicate that assessment of genomic risk with the 21-gene recurrence score provides complementary prognostic information to pathologic features, and is also predictive of a large chemotherapy benefit if the recurrence score is greater than 25, or lack thereof if 25 or lower,” he said.
However, there is a three-way interaction between age, RS, and CT use, which results in an absolute CT benefit in women aged 50 or younger of about 2% for RS of 16-20, and about 7% for RS of 21-25, he added.
“Assessment of clinical risk using pathological features also provides prognostic information that doesn’t correlate well with the recurrence score, therefore it stands to reason that integration of clinical and genomic risk offers the potential for greater precision in prognosis and, ultimately, guiding the use of adjuvant therapy,” he said.
Clinical risk for this analysis was assessed using a binary clinical risk categorization employed in the MINDACT trial and calibrated to greater than 92% 10-year breast cancer-specific survival for ET alone based on Adjuvant! version 8.0. Low-grade tumors up to 3 cm, intermediate-grade tumors up to 2 cm, and high-grade tumors up to 1 cm were categorized as low clinical risk (LCR), and all others not meeting these criteria were categorized as high clinical risk (HCR), he explained.
Of 9,427 patients included in the analysis, 70% had LCR and 30% had HCR.
“For distant recurrence, high clinical risk was associated with a 2.5- to 3-fold higher recurrence rate for those with a recurrence score of 11 or higher, and in a multivariate model for distant recurrence in the [group with a] recurrence score of 11-25, high clinical risk was independently associated with a 2.4-fold higher recurrence risk,” he said. “Continuous recurrence score also provided significant prognostic information, with each 1-unit increase associated with an 8% higher distant recurrence risk.”
For the overall population, clinical risk added significant prognostic information to the RS for both distant recurrence and disease-free survival, and stratification by age showed that among women over age 50 years, the hazard ratios for distant recurrence ranged from 2.20 to 2.36, and did not substantially vary by age or RS, he said.
However, for the overall population, adding clinical risk to the RS did not improve prediction of chemotherapy benefit.
“This was also true for the two-thirds of women who were over 50 years of age. For the remaining women 50 or younger, there was a trend favoring chemo, irrespective of clinical risk, though not significant – a finding consistent with the treatment interaction previously described,” he said.
Finally, the absolute differences in 9-year distant recurrence rates by clinical risk stratified by age, RS, and CT use showed an absolute 4%-6% higher distant recurrence risk for HCR vs. LCR among those over age 50 with RS of 0-25 irrespective of CT use, and a 13% difference for those with RS of 26-100 who were treated with CT.
“For those 50 or younger, clinical risk had no impact on recurrence if the RS was 0-10. For RS of 11-25, the difference was about 9% with endocrine therapy alone, and 2% with chemo plus ET, reflecting absolute chemo benefit in younger women who had high clinical risk,” he said, adding that for those with RS of 26-100, there was a 9% higher absolute recurrence rate in the HCR vs. LCR population.
“We therefore further evaluated absolute differences in distance recurrence rates associated with chemotherapy use in women 50 and younger with RS of 16-25, further stratified by RS and clinical risk,” he said, noting that when not stratified by clinical risk, as reported in the primary analysis, the absolute CT benefit was 1.6% for RS of 16-20, and 6.5% for RS of 21-25.
When stratified by clinical risk, the absolute CT benefit ranged from 6% to 9% in those with RS of 21-25, irrespective of clinical risk, and in those with RS of 16-20 and HCR.
“This accounted for 51% of patients with RS of 16-25,” he said. “However, there was no demonstrable chemo benefit for those with LCR and RS of 16-20, who accounted for the remaining 49%.”
Additional analysis looking at age at diagnosis and CT benefit showed a benefit in premenopausal women aged 46-50 years (but not postmenopausal women), a trend toward benefit in those aged 41-45 years, and no benefit in those aged 40 years and younger, who are less likely to develop premature menopause as a consequence of cytotoxic CT.
“In addition, we saw no consistent effect favoring chemotherapy in older women. Taken together, these findings suggest the chemo benefit observed for the RS 16-25 group may, in fact, be due to a castration effect associated with cytotoxic therapy rather than an effect in eradicating micrometastatic disease,” Dr. Sparano said.
Applying this framework to the TAILORx study population categorized 68% of those aged 50 years and younger into a low integrated risk group with less than 5% risk of distant recurrence. This included all patients with RS of 0-10 irrespective of clinical risk (14% of the patient population; distant recurrence rate 1.8% or less), and all with RS of 11-25 and LCR (54% of the patient population, 4.7% distant recurrence rate).
In contrast, 25% fell into the high integrated risk group (greater than 10% distant recurrence risk), including those with RS of 11-25 and HCR (17% of the patient population; distant recurrence rate 12.3%), and RS of 26-100 and HCR (8% of the patient population; distant recurrence rate 15.2%).
“This framework encompasses 93% of all TAILORx subjects, with the remaining 7% having a distant recurrence risk of between 5% and 10%,” he said.
Overall, the primary results of TAILORx remain unchanged based on this secondary analysis as the addition of clinical risk did not predict CT benefit in the RS 11-25 group, he noted.
“However, for women 50 and under and RS 16-25, integrated risk distinguished 50% who derived no chemo benefit from the 50% who derived an absolute benefit of approximately 6%-9% – a level that is higher than an unselected population,” he said, reiterating that the absolute CT benefit was greater in premenopausal women aged 45-50 with RS 16-25, suggesting that the absolute CR benefit seen in younger women in TAILORx may be due to an endocrine effect.
“Integrated risk clearly provides greater prognostic precision and may have clinical utility; the prognostic precision afforded by the integrated risk model is superior to that by the use of clinical or genomic features alone, and in addition, the genomic assay also provides predictive information for chemo benefit that is not captured by clinical features alone,” he concluded.
As an example of the potential clinical utility of this integrated approach for guiding treatment in women aged 50 years or younger, he presented “a highly stratified integrated risk assessment model” separating TAILORx patients into low integrated risk (58% of the study population) and high integrated risk (31% of the study population).
In the low integrated risk patients with RS of 0-10 and any clinical risk level, or with RS of 11-25 and LCR, tamoxifen alone appears adequate, he said.
In those with high integrated risk and RS of 16-25 with HCR, ovarian function suppression plus an aromatase inhibitor (OFS/AI) could be considered as an alternative to chemo, and in those with high integrated risk, RS of 26-100, and HCR who have not developed chemotherapy-induced menopause, ovarian function suppression and an AI could be added to chemotherapy.
“Indeed, data from the SOFT and TEXT trials indicate that patients with a high RS risk experienced an absolute improvement of up to 10%-15% in 5-year breast cancer–free interval with an OFS/AI, compared with tamoxifen, whereas improvement was minimal in those at lowest risk, supporting the strategy of using integrated clinical and genomic risk to select for ovarian function suppression plus an AI,” he said.
During a discussion of the findings and how they might impact practice, Vered Stearns, MD, an oncology professor and codirector of the Breast Cancer Program at Johns Hopkins University, Baltimore, noted that in her practice she will “carefully select women for whom genomic assay [use] is appropriate.
“I will also assess clinical risk and RS to inform recommendations for chemotherapy use, and possibly appropriate endocrine agents in select populations,” she said.
Dr. Stearns further noted that the interaction between RS and age as reported by Dr. Sparano is exploratory and should be interpreted with caution as the majority of those aged 50 and younger received tamoxifen alone and the question remains as to whether they would have received similar benefits from ovarian suppression and tamoxifen/AI instead of chemo-endocrine therapy.
“Indeed, indirect hypotheses from other studies suggest that may be the case,” she said, adding that these women may be offered ovarian suppression and tamoxifen or AI based on the SOFT and TEXT results.
“TAILORx remains a rich resource for new explorations, new biomarkers, new models, and new machine learning opportunities,” she said.
TAILORx was funded by the National Institutes of Health. Dr. Sparano reported stock ownership, a consulting role, and research funding from several pharmaceutical companies. Dr. Stearns reported consulting or advisory roles with Iridium Therapeutics; research funding from Abbvie, Biocept, MedImmune, Novartis, Pfizer, and Puma Biotechnology; and an “other relationship” with Immunomedics.
SOURCE: Sparano JA et al. ASCO 2019. Abstract 503.
CHICAGO – , according to a secondary analysis of data from the practice-changing TAILORx study.
Specifically, tumor size and histology-based risk stratification improves the prediction of disease-free survival and distant recurrence, and – for some patient groups – chemotherapy benefit, Joseph A. Sparano, MD, reported at the annual meeting of the American Society of Clinical Oncology.
Combining these tools could help determine whether endocrine therapy (ET) alone or ET with adjuvant chemotherapy is the best treatment approach for a given patient, said Dr. Sparano, professor of medicine and obstetrics, gynecology, and women’s health at Albert Einstein College of Medicine, New York.
The phase 3 TAILORx study established that ET alone is noninferior to adjuvant chemotherapy (CT) plus ET in patients with early breast cancer and RS of 11-25, and that ET alone has some benefit over ET+CT in women aged 50 years and younger with RS of 16-25, he explained.
Those findings were presented at the 2018 ASCO annual meeting and subsequently published in the New England Journal of Medicine.
The current analysis focused on the integration of clinical and genomic features for prognosis, and the results were published online June 3 in a corresponding article in the New England Journal of Medicine.
“The totality of the data, including TAILORx and the prior prospective validation studies, indicate that assessment of genomic risk with the 21-gene recurrence score provides complementary prognostic information to pathologic features, and is also predictive of a large chemotherapy benefit if the recurrence score is greater than 25, or lack thereof if 25 or lower,” he said.
However, there is a three-way interaction between age, RS, and CT use, which results in an absolute CT benefit in women aged 50 or younger of about 2% for RS of 16-20, and about 7% for RS of 21-25, he added.
“Assessment of clinical risk using pathological features also provides prognostic information that doesn’t correlate well with the recurrence score, therefore it stands to reason that integration of clinical and genomic risk offers the potential for greater precision in prognosis and, ultimately, guiding the use of adjuvant therapy,” he said.
Clinical risk for this analysis was assessed using a binary clinical risk categorization employed in the MINDACT trial and calibrated to greater than 92% 10-year breast cancer-specific survival for ET alone based on Adjuvant! version 8.0. Low-grade tumors up to 3 cm, intermediate-grade tumors up to 2 cm, and high-grade tumors up to 1 cm were categorized as low clinical risk (LCR), and all others not meeting these criteria were categorized as high clinical risk (HCR), he explained.
Of 9,427 patients included in the analysis, 70% had LCR and 30% had HCR.
“For distant recurrence, high clinical risk was associated with a 2.5- to 3-fold higher recurrence rate for those with a recurrence score of 11 or higher, and in a multivariate model for distant recurrence in the [group with a] recurrence score of 11-25, high clinical risk was independently associated with a 2.4-fold higher recurrence risk,” he said. “Continuous recurrence score also provided significant prognostic information, with each 1-unit increase associated with an 8% higher distant recurrence risk.”
For the overall population, clinical risk added significant prognostic information to the RS for both distant recurrence and disease-free survival, and stratification by age showed that among women over age 50 years, the hazard ratios for distant recurrence ranged from 2.20 to 2.36, and did not substantially vary by age or RS, he said.
However, for the overall population, adding clinical risk to the RS did not improve prediction of chemotherapy benefit.
“This was also true for the two-thirds of women who were over 50 years of age. For the remaining women 50 or younger, there was a trend favoring chemo, irrespective of clinical risk, though not significant – a finding consistent with the treatment interaction previously described,” he said.
Finally, the absolute differences in 9-year distant recurrence rates by clinical risk stratified by age, RS, and CT use showed an absolute 4%-6% higher distant recurrence risk for HCR vs. LCR among those over age 50 with RS of 0-25 irrespective of CT use, and a 13% difference for those with RS of 26-100 who were treated with CT.
“For those 50 or younger, clinical risk had no impact on recurrence if the RS was 0-10. For RS of 11-25, the difference was about 9% with endocrine therapy alone, and 2% with chemo plus ET, reflecting absolute chemo benefit in younger women who had high clinical risk,” he said, adding that for those with RS of 26-100, there was a 9% higher absolute recurrence rate in the HCR vs. LCR population.
“We therefore further evaluated absolute differences in distance recurrence rates associated with chemotherapy use in women 50 and younger with RS of 16-25, further stratified by RS and clinical risk,” he said, noting that when not stratified by clinical risk, as reported in the primary analysis, the absolute CT benefit was 1.6% for RS of 16-20, and 6.5% for RS of 21-25.
When stratified by clinical risk, the absolute CT benefit ranged from 6% to 9% in those with RS of 21-25, irrespective of clinical risk, and in those with RS of 16-20 and HCR.
“This accounted for 51% of patients with RS of 16-25,” he said. “However, there was no demonstrable chemo benefit for those with LCR and RS of 16-20, who accounted for the remaining 49%.”
Additional analysis looking at age at diagnosis and CT benefit showed a benefit in premenopausal women aged 46-50 years (but not postmenopausal women), a trend toward benefit in those aged 41-45 years, and no benefit in those aged 40 years and younger, who are less likely to develop premature menopause as a consequence of cytotoxic CT.
“In addition, we saw no consistent effect favoring chemotherapy in older women. Taken together, these findings suggest the chemo benefit observed for the RS 16-25 group may, in fact, be due to a castration effect associated with cytotoxic therapy rather than an effect in eradicating micrometastatic disease,” Dr. Sparano said.
Applying this framework to the TAILORx study population categorized 68% of those aged 50 years and younger into a low integrated risk group with less than 5% risk of distant recurrence. This included all patients with RS of 0-10 irrespective of clinical risk (14% of the patient population; distant recurrence rate 1.8% or less), and all with RS of 11-25 and LCR (54% of the patient population, 4.7% distant recurrence rate).
In contrast, 25% fell into the high integrated risk group (greater than 10% distant recurrence risk), including those with RS of 11-25 and HCR (17% of the patient population; distant recurrence rate 12.3%), and RS of 26-100 and HCR (8% of the patient population; distant recurrence rate 15.2%).
“This framework encompasses 93% of all TAILORx subjects, with the remaining 7% having a distant recurrence risk of between 5% and 10%,” he said.
Overall, the primary results of TAILORx remain unchanged based on this secondary analysis as the addition of clinical risk did not predict CT benefit in the RS 11-25 group, he noted.
“However, for women 50 and under and RS 16-25, integrated risk distinguished 50% who derived no chemo benefit from the 50% who derived an absolute benefit of approximately 6%-9% – a level that is higher than an unselected population,” he said, reiterating that the absolute CT benefit was greater in premenopausal women aged 45-50 with RS 16-25, suggesting that the absolute CR benefit seen in younger women in TAILORx may be due to an endocrine effect.
“Integrated risk clearly provides greater prognostic precision and may have clinical utility; the prognostic precision afforded by the integrated risk model is superior to that by the use of clinical or genomic features alone, and in addition, the genomic assay also provides predictive information for chemo benefit that is not captured by clinical features alone,” he concluded.
As an example of the potential clinical utility of this integrated approach for guiding treatment in women aged 50 years or younger, he presented “a highly stratified integrated risk assessment model” separating TAILORx patients into low integrated risk (58% of the study population) and high integrated risk (31% of the study population).
In the low integrated risk patients with RS of 0-10 and any clinical risk level, or with RS of 11-25 and LCR, tamoxifen alone appears adequate, he said.
In those with high integrated risk and RS of 16-25 with HCR, ovarian function suppression plus an aromatase inhibitor (OFS/AI) could be considered as an alternative to chemo, and in those with high integrated risk, RS of 26-100, and HCR who have not developed chemotherapy-induced menopause, ovarian function suppression and an AI could be added to chemotherapy.
“Indeed, data from the SOFT and TEXT trials indicate that patients with a high RS risk experienced an absolute improvement of up to 10%-15% in 5-year breast cancer–free interval with an OFS/AI, compared with tamoxifen, whereas improvement was minimal in those at lowest risk, supporting the strategy of using integrated clinical and genomic risk to select for ovarian function suppression plus an AI,” he said.
During a discussion of the findings and how they might impact practice, Vered Stearns, MD, an oncology professor and codirector of the Breast Cancer Program at Johns Hopkins University, Baltimore, noted that in her practice she will “carefully select women for whom genomic assay [use] is appropriate.
“I will also assess clinical risk and RS to inform recommendations for chemotherapy use, and possibly appropriate endocrine agents in select populations,” she said.
Dr. Stearns further noted that the interaction between RS and age as reported by Dr. Sparano is exploratory and should be interpreted with caution as the majority of those aged 50 and younger received tamoxifen alone and the question remains as to whether they would have received similar benefits from ovarian suppression and tamoxifen/AI instead of chemo-endocrine therapy.
“Indeed, indirect hypotheses from other studies suggest that may be the case,” she said, adding that these women may be offered ovarian suppression and tamoxifen or AI based on the SOFT and TEXT results.
“TAILORx remains a rich resource for new explorations, new biomarkers, new models, and new machine learning opportunities,” she said.
TAILORx was funded by the National Institutes of Health. Dr. Sparano reported stock ownership, a consulting role, and research funding from several pharmaceutical companies. Dr. Stearns reported consulting or advisory roles with Iridium Therapeutics; research funding from Abbvie, Biocept, MedImmune, Novartis, Pfizer, and Puma Biotechnology; and an “other relationship” with Immunomedics.
SOURCE: Sparano JA et al. ASCO 2019. Abstract 503.
REPORTING FROM ASCO 2019
FDA approves trastuzumab-anns for HER2-positive breast, gastric cancer
The Food and Drug Administration has approved Amgen’s trastuzumab-anns as a trastuzumab biosimilar for the treatment of HER2-positive breast cancer and gastric cancer.
This biosimilar, to be marketed as Kanjinti, is the fifth trastuzumab biosimilar to be approved by the agency, according to the FDA.
Approval was based in part on the LILAC study, which demonstrated that the biosimilar, previously called ABP-980, had similar efficacy and comparable cardiac safety with trastuzumab.
In the phase 3 study, 725 patients with HER2-positive early breast cancer were randomized to neoadjuvant treatment with trastuzumab-anns or trastuzumab, plus paclitaxel, for four cycles following four cycles of chemotherapy. The primary pathological complete response endpoint was achieved in 48% of those in the biosimilar arm, compared with 40.5% in the trastuzumab arm. Patients then went on to receive adjuvant treatment with ABP 980 or trastuzumab every 3 weeks for up to 1 year following surgery.
Grade 3 or worse adverse events during the neoadjuvant phase occurred in 15% of patients in the ABP 980 group and 14% in the trastuzumab group. The most frequent grade 3 event in both study arms was neutropenia. In the adjuvant phase, grade 3 or worse adverse events occurred in 9% of those continuing ABP 980 and in 6% of those continuing trastuzumab. The most frequent events in both arms were infections, infestations, and neutropenia.
Trastuzumab-anns is indicated for adjuvant treatment of HER2-overexpressing node positive or node negative breast cancer, first-line treatment of HER2-overexpressing metastatic breast cancer, and first-line treatment of patients with HER2-overexpressing metastatic gastric or gastroesophageal junction adenocarcinoma. The FDA indicates patients should be selected based on an FDA-approved companion diagnostic for a trastuzumab product.
The biosimilar includes a boxed warning for cardiomyopathy, infusion reactions, embryo-fetal toxicity, and pulmonary toxicity.
The Food and Drug Administration has approved Amgen’s trastuzumab-anns as a trastuzumab biosimilar for the treatment of HER2-positive breast cancer and gastric cancer.
This biosimilar, to be marketed as Kanjinti, is the fifth trastuzumab biosimilar to be approved by the agency, according to the FDA.
Approval was based in part on the LILAC study, which demonstrated that the biosimilar, previously called ABP-980, had similar efficacy and comparable cardiac safety with trastuzumab.
In the phase 3 study, 725 patients with HER2-positive early breast cancer were randomized to neoadjuvant treatment with trastuzumab-anns or trastuzumab, plus paclitaxel, for four cycles following four cycles of chemotherapy. The primary pathological complete response endpoint was achieved in 48% of those in the biosimilar arm, compared with 40.5% in the trastuzumab arm. Patients then went on to receive adjuvant treatment with ABP 980 or trastuzumab every 3 weeks for up to 1 year following surgery.
Grade 3 or worse adverse events during the neoadjuvant phase occurred in 15% of patients in the ABP 980 group and 14% in the trastuzumab group. The most frequent grade 3 event in both study arms was neutropenia. In the adjuvant phase, grade 3 or worse adverse events occurred in 9% of those continuing ABP 980 and in 6% of those continuing trastuzumab. The most frequent events in both arms were infections, infestations, and neutropenia.
Trastuzumab-anns is indicated for adjuvant treatment of HER2-overexpressing node positive or node negative breast cancer, first-line treatment of HER2-overexpressing metastatic breast cancer, and first-line treatment of patients with HER2-overexpressing metastatic gastric or gastroesophageal junction adenocarcinoma. The FDA indicates patients should be selected based on an FDA-approved companion diagnostic for a trastuzumab product.
The biosimilar includes a boxed warning for cardiomyopathy, infusion reactions, embryo-fetal toxicity, and pulmonary toxicity.
The Food and Drug Administration has approved Amgen’s trastuzumab-anns as a trastuzumab biosimilar for the treatment of HER2-positive breast cancer and gastric cancer.
This biosimilar, to be marketed as Kanjinti, is the fifth trastuzumab biosimilar to be approved by the agency, according to the FDA.
Approval was based in part on the LILAC study, which demonstrated that the biosimilar, previously called ABP-980, had similar efficacy and comparable cardiac safety with trastuzumab.
In the phase 3 study, 725 patients with HER2-positive early breast cancer were randomized to neoadjuvant treatment with trastuzumab-anns or trastuzumab, plus paclitaxel, for four cycles following four cycles of chemotherapy. The primary pathological complete response endpoint was achieved in 48% of those in the biosimilar arm, compared with 40.5% in the trastuzumab arm. Patients then went on to receive adjuvant treatment with ABP 980 or trastuzumab every 3 weeks for up to 1 year following surgery.
Grade 3 or worse adverse events during the neoadjuvant phase occurred in 15% of patients in the ABP 980 group and 14% in the trastuzumab group. The most frequent grade 3 event in both study arms was neutropenia. In the adjuvant phase, grade 3 or worse adverse events occurred in 9% of those continuing ABP 980 and in 6% of those continuing trastuzumab. The most frequent events in both arms were infections, infestations, and neutropenia.
Trastuzumab-anns is indicated for adjuvant treatment of HER2-overexpressing node positive or node negative breast cancer, first-line treatment of HER2-overexpressing metastatic breast cancer, and first-line treatment of patients with HER2-overexpressing metastatic gastric or gastroesophageal junction adenocarcinoma. The FDA indicates patients should be selected based on an FDA-approved companion diagnostic for a trastuzumab product.
The biosimilar includes a boxed warning for cardiomyopathy, infusion reactions, embryo-fetal toxicity, and pulmonary toxicity.
Breast cancer linked to 23% higher risk for new diabetes
SAN FRANCISCO – a new Danish study finds.
The findings are “quite a clear signal of increased diabetes following breast cancer,” said epidemiologist and study coauthor Reimar W. Thomsen, MD, PhD, of Aarhus (Denmark) University Hospital, in an interview. “It’s very important to tell [patients with breast cancer] what they may expect in the long term.”
He spoke at the annual scientific sessions of the American Diabetes Association, where he presented the study findings.
Much of the research into links between breast cancer and diabetes has focused on whether diabetes is a risk factor for breast cancer, and not the other way around. A 2018 meta-analysis of 18 studies found a slightly higher risk of breast cancer in women with diabetes (summary relative risk, 1.13; 95% confidence interval, 1.04-1.24). However, the researchers found evidence that the risk factor might be adiposity, and not diabetes itself (Diabetes. 2018 Jul;67[Supplement 1]. doi: 10.2337/db18-180-OR).
For the new study, researchers used health registries to track women in Denmark for up to 12 years, during 2005-2016. They compared 33,909 women who were older than 50 years and who had new-onset breast cancer with 313,998 women without breast cancer in a matched comparison cohort. The average age in both groups was 66 years; obesity was rare (4% vs. 3%, respectively), but statin therapy (21% in both groups) and hormone replacement therapy (36% vs. 32%) were more prevalent.
In the first year after a breast cancer diagnosis, the women in the breast cancer group were 15% more likely to develop diabetes (per use of diabetes medication or hospital-diagnosed diabetes) than those in the comparison group (adjusted hazard ratio, 1.15; 95% CI, 1.01-1.30) with adjustments for factors such as age, marital status, residence, medical history, medications, and comorbidity.
Over a median follow-up period of 5.2 years, the risk of diabetes was 23% higher in the breast cancer group, at 8.4 new cases per 1,000 women, compared with 6.8 new cases per 1,000 women in the comparison group (aHR, 1.23; 95% CI, 1.16-1.30). Unadjusted hazard ratios were similar.
Women in the breast cancer group who developed diabetes were more likely to use insulin-based therapy, suggesting they had more severe diabetes, compared with those in the control group (5% vs. 2%, respectively; P less than .00001). They were also more likely to be treated with insulin only (4% vs. 1%, P less than .00001).
It is not clear why patients with breast cancer face a higher risk of diabetes. Dr. Thomsen speculated that cancer drugs might play a role and he noted that cancer itself can cause inflammation and “lead to consequences.”
A 2018 study linked usage of hormone therapies, including tamoxifen (HR, 2.25; 95% CI, 1.19-4.26; P = .013) and aromatase inhibitors (HR, 4.27;95% CI, 1.42-12.84), in patients with breast cancer to higher levels of diabetes, compared with patients who did not use hormone therapy (J Clin Oncol. 2018;36[20]:2061-9).
Dr. Thomsen emphasized that physicians should monitor patients with breast cancer for diabetes. “It develops over time, and the risk is increasing, so you need to be aware of that.”
No study funding was reported. One of the researchers reported numerous ties to a range of drug companies. Dr. Thomsen and the other researchers reported no relevant disclosures.
SAN FRANCISCO – a new Danish study finds.
The findings are “quite a clear signal of increased diabetes following breast cancer,” said epidemiologist and study coauthor Reimar W. Thomsen, MD, PhD, of Aarhus (Denmark) University Hospital, in an interview. “It’s very important to tell [patients with breast cancer] what they may expect in the long term.”
He spoke at the annual scientific sessions of the American Diabetes Association, where he presented the study findings.
Much of the research into links between breast cancer and diabetes has focused on whether diabetes is a risk factor for breast cancer, and not the other way around. A 2018 meta-analysis of 18 studies found a slightly higher risk of breast cancer in women with diabetes (summary relative risk, 1.13; 95% confidence interval, 1.04-1.24). However, the researchers found evidence that the risk factor might be adiposity, and not diabetes itself (Diabetes. 2018 Jul;67[Supplement 1]. doi: 10.2337/db18-180-OR).
For the new study, researchers used health registries to track women in Denmark for up to 12 years, during 2005-2016. They compared 33,909 women who were older than 50 years and who had new-onset breast cancer with 313,998 women without breast cancer in a matched comparison cohort. The average age in both groups was 66 years; obesity was rare (4% vs. 3%, respectively), but statin therapy (21% in both groups) and hormone replacement therapy (36% vs. 32%) were more prevalent.
In the first year after a breast cancer diagnosis, the women in the breast cancer group were 15% more likely to develop diabetes (per use of diabetes medication or hospital-diagnosed diabetes) than those in the comparison group (adjusted hazard ratio, 1.15; 95% CI, 1.01-1.30) with adjustments for factors such as age, marital status, residence, medical history, medications, and comorbidity.
Over a median follow-up period of 5.2 years, the risk of diabetes was 23% higher in the breast cancer group, at 8.4 new cases per 1,000 women, compared with 6.8 new cases per 1,000 women in the comparison group (aHR, 1.23; 95% CI, 1.16-1.30). Unadjusted hazard ratios were similar.
Women in the breast cancer group who developed diabetes were more likely to use insulin-based therapy, suggesting they had more severe diabetes, compared with those in the control group (5% vs. 2%, respectively; P less than .00001). They were also more likely to be treated with insulin only (4% vs. 1%, P less than .00001).
It is not clear why patients with breast cancer face a higher risk of diabetes. Dr. Thomsen speculated that cancer drugs might play a role and he noted that cancer itself can cause inflammation and “lead to consequences.”
A 2018 study linked usage of hormone therapies, including tamoxifen (HR, 2.25; 95% CI, 1.19-4.26; P = .013) and aromatase inhibitors (HR, 4.27;95% CI, 1.42-12.84), in patients with breast cancer to higher levels of diabetes, compared with patients who did not use hormone therapy (J Clin Oncol. 2018;36[20]:2061-9).
Dr. Thomsen emphasized that physicians should monitor patients with breast cancer for diabetes. “It develops over time, and the risk is increasing, so you need to be aware of that.”
No study funding was reported. One of the researchers reported numerous ties to a range of drug companies. Dr. Thomsen and the other researchers reported no relevant disclosures.
SAN FRANCISCO – a new Danish study finds.
The findings are “quite a clear signal of increased diabetes following breast cancer,” said epidemiologist and study coauthor Reimar W. Thomsen, MD, PhD, of Aarhus (Denmark) University Hospital, in an interview. “It’s very important to tell [patients with breast cancer] what they may expect in the long term.”
He spoke at the annual scientific sessions of the American Diabetes Association, where he presented the study findings.
Much of the research into links between breast cancer and diabetes has focused on whether diabetes is a risk factor for breast cancer, and not the other way around. A 2018 meta-analysis of 18 studies found a slightly higher risk of breast cancer in women with diabetes (summary relative risk, 1.13; 95% confidence interval, 1.04-1.24). However, the researchers found evidence that the risk factor might be adiposity, and not diabetes itself (Diabetes. 2018 Jul;67[Supplement 1]. doi: 10.2337/db18-180-OR).
For the new study, researchers used health registries to track women in Denmark for up to 12 years, during 2005-2016. They compared 33,909 women who were older than 50 years and who had new-onset breast cancer with 313,998 women without breast cancer in a matched comparison cohort. The average age in both groups was 66 years; obesity was rare (4% vs. 3%, respectively), but statin therapy (21% in both groups) and hormone replacement therapy (36% vs. 32%) were more prevalent.
In the first year after a breast cancer diagnosis, the women in the breast cancer group were 15% more likely to develop diabetes (per use of diabetes medication or hospital-diagnosed diabetes) than those in the comparison group (adjusted hazard ratio, 1.15; 95% CI, 1.01-1.30) with adjustments for factors such as age, marital status, residence, medical history, medications, and comorbidity.
Over a median follow-up period of 5.2 years, the risk of diabetes was 23% higher in the breast cancer group, at 8.4 new cases per 1,000 women, compared with 6.8 new cases per 1,000 women in the comparison group (aHR, 1.23; 95% CI, 1.16-1.30). Unadjusted hazard ratios were similar.
Women in the breast cancer group who developed diabetes were more likely to use insulin-based therapy, suggesting they had more severe diabetes, compared with those in the control group (5% vs. 2%, respectively; P less than .00001). They were also more likely to be treated with insulin only (4% vs. 1%, P less than .00001).
It is not clear why patients with breast cancer face a higher risk of diabetes. Dr. Thomsen speculated that cancer drugs might play a role and he noted that cancer itself can cause inflammation and “lead to consequences.”
A 2018 study linked usage of hormone therapies, including tamoxifen (HR, 2.25; 95% CI, 1.19-4.26; P = .013) and aromatase inhibitors (HR, 4.27;95% CI, 1.42-12.84), in patients with breast cancer to higher levels of diabetes, compared with patients who did not use hormone therapy (J Clin Oncol. 2018;36[20]:2061-9).
Dr. Thomsen emphasized that physicians should monitor patients with breast cancer for diabetes. “It develops over time, and the risk is increasing, so you need to be aware of that.”
No study funding was reported. One of the researchers reported numerous ties to a range of drug companies. Dr. Thomsen and the other researchers reported no relevant disclosures.
REPORTING FROM ADA 2019
PREDIX HER2 trial: Similar efficacy, less toxicity with T-DM1 for HER2+, HR+ breast cancer
CHICAGO – Targeted neoadjuvant therapy with trastuzumab emtansine (T-DM1) had similar efficacy with less toxicity, compared with a standard chemotherapy–based regimen for patients with HER2- and hormone receptor–positive breast cancers in the phase 2 Swedish PREDIX HER2 trial.
The pathologic complete response (pCR) rate was 45% among 98 participants who were randomized to received T-DM1, and 47% in those randomized to receive docetaxel, trastuzumab, and pertuzumab (DTP), Jonas C.S. Bergh, MD, PhD, reported at the annual meeting of the American Society of Clinical Oncology.
The pCR rate in hormone receptor(HR) –positive tumors was 36% in both groups, and the rates in HR-negative tumors were 59% and 67% in the T-DM1 and DTP arms, respectively; any differences in pCR rates between the groups were not statistically significant, said Dr. Bergh of the Karolinska Institute and University Hospital, Stockholm.
Patients were adults with HER2-positive breast cancer and tumor size greater than 20 mm or verified lymph node metastases at enrollment, and 62.6% of tumors were HR positive. Both treatment arms received their assigned therapy every 3 weeks for a planned total of six courses, but the protocol allowed a switch to the competing treatment upon progression, lack of response, or drug-related severe toxicity. All received postoperative epirubicin+cyclophosphamide every 3 weeks, with the T-DM1 arm receiving 4 courses and the DTP arm receiving 2 courses, and both arms also received adjuvant trastuzumab for 11 courses.
Age (median of 52 years), menopausal status, and histological type and grade were well balanced between the treatment groups.
Grade 3/4 adverse events occurred on 63 occasions in the DTP arm, compared with 10 in the T-DM1 arm; febrile neutropenia accounted for 26 and 3 of the events in the groups, respectively. All events, with the exception of liver toxicity, occurred more frequently in the DTP arm, Dr. Bergh said.
Ultimately, 9 patients switched from T-DM1 to DTP – 7 for progression or lack of response and 2 because of toxicity, and 18 switched from DTP to T-DM1 because of either progression or lack of response, and 14 because of toxicity. One patient in each group achieved pCR after switching, he noted.
“There was clearly better quality of life [during the study] for the T-DM1 group,” he added, noting that the quality of life data were reported separately at the meeting.
Additionally, an exploratory analysis demonstrated an early steep decrease of F-FDG uptake, suggesting that PET/CT may be a useful tool for predicting pCR.
Although neoadjuvant therapy produces high pCR rates and is the standard of care in HER2 positive breast cancer, the optimal treatment regimen remains to be established; but the current findings, along with prior data showing efficacy with T-DM1 in patients who fail to respond to two or more lines of anti-HER2 therapies, suggest it is a potential new standard for neoadjuvant therapy, particularly for patients with HER2- and HR-positive disease, he concluded.
Dr. Bergh reported a financial relationship with UpToDate, and research funding to his institution from Amgen, AstraZeneca, Bayer, Merck, Pfizer, Roche, and Sanofi.
SOURCE: Bergh J et al. ASCO 2019, Abstract 501.
CHICAGO – Targeted neoadjuvant therapy with trastuzumab emtansine (T-DM1) had similar efficacy with less toxicity, compared with a standard chemotherapy–based regimen for patients with HER2- and hormone receptor–positive breast cancers in the phase 2 Swedish PREDIX HER2 trial.
The pathologic complete response (pCR) rate was 45% among 98 participants who were randomized to received T-DM1, and 47% in those randomized to receive docetaxel, trastuzumab, and pertuzumab (DTP), Jonas C.S. Bergh, MD, PhD, reported at the annual meeting of the American Society of Clinical Oncology.
The pCR rate in hormone receptor(HR) –positive tumors was 36% in both groups, and the rates in HR-negative tumors were 59% and 67% in the T-DM1 and DTP arms, respectively; any differences in pCR rates between the groups were not statistically significant, said Dr. Bergh of the Karolinska Institute and University Hospital, Stockholm.
Patients were adults with HER2-positive breast cancer and tumor size greater than 20 mm or verified lymph node metastases at enrollment, and 62.6% of tumors were HR positive. Both treatment arms received their assigned therapy every 3 weeks for a planned total of six courses, but the protocol allowed a switch to the competing treatment upon progression, lack of response, or drug-related severe toxicity. All received postoperative epirubicin+cyclophosphamide every 3 weeks, with the T-DM1 arm receiving 4 courses and the DTP arm receiving 2 courses, and both arms also received adjuvant trastuzumab for 11 courses.
Age (median of 52 years), menopausal status, and histological type and grade were well balanced between the treatment groups.
Grade 3/4 adverse events occurred on 63 occasions in the DTP arm, compared with 10 in the T-DM1 arm; febrile neutropenia accounted for 26 and 3 of the events in the groups, respectively. All events, with the exception of liver toxicity, occurred more frequently in the DTP arm, Dr. Bergh said.
Ultimately, 9 patients switched from T-DM1 to DTP – 7 for progression or lack of response and 2 because of toxicity, and 18 switched from DTP to T-DM1 because of either progression or lack of response, and 14 because of toxicity. One patient in each group achieved pCR after switching, he noted.
“There was clearly better quality of life [during the study] for the T-DM1 group,” he added, noting that the quality of life data were reported separately at the meeting.
Additionally, an exploratory analysis demonstrated an early steep decrease of F-FDG uptake, suggesting that PET/CT may be a useful tool for predicting pCR.
Although neoadjuvant therapy produces high pCR rates and is the standard of care in HER2 positive breast cancer, the optimal treatment regimen remains to be established; but the current findings, along with prior data showing efficacy with T-DM1 in patients who fail to respond to two or more lines of anti-HER2 therapies, suggest it is a potential new standard for neoadjuvant therapy, particularly for patients with HER2- and HR-positive disease, he concluded.
Dr. Bergh reported a financial relationship with UpToDate, and research funding to his institution from Amgen, AstraZeneca, Bayer, Merck, Pfizer, Roche, and Sanofi.
SOURCE: Bergh J et al. ASCO 2019, Abstract 501.
CHICAGO – Targeted neoadjuvant therapy with trastuzumab emtansine (T-DM1) had similar efficacy with less toxicity, compared with a standard chemotherapy–based regimen for patients with HER2- and hormone receptor–positive breast cancers in the phase 2 Swedish PREDIX HER2 trial.
The pathologic complete response (pCR) rate was 45% among 98 participants who were randomized to received T-DM1, and 47% in those randomized to receive docetaxel, trastuzumab, and pertuzumab (DTP), Jonas C.S. Bergh, MD, PhD, reported at the annual meeting of the American Society of Clinical Oncology.
The pCR rate in hormone receptor(HR) –positive tumors was 36% in both groups, and the rates in HR-negative tumors were 59% and 67% in the T-DM1 and DTP arms, respectively; any differences in pCR rates between the groups were not statistically significant, said Dr. Bergh of the Karolinska Institute and University Hospital, Stockholm.
Patients were adults with HER2-positive breast cancer and tumor size greater than 20 mm or verified lymph node metastases at enrollment, and 62.6% of tumors were HR positive. Both treatment arms received their assigned therapy every 3 weeks for a planned total of six courses, but the protocol allowed a switch to the competing treatment upon progression, lack of response, or drug-related severe toxicity. All received postoperative epirubicin+cyclophosphamide every 3 weeks, with the T-DM1 arm receiving 4 courses and the DTP arm receiving 2 courses, and both arms also received adjuvant trastuzumab for 11 courses.
Age (median of 52 years), menopausal status, and histological type and grade were well balanced between the treatment groups.
Grade 3/4 adverse events occurred on 63 occasions in the DTP arm, compared with 10 in the T-DM1 arm; febrile neutropenia accounted for 26 and 3 of the events in the groups, respectively. All events, with the exception of liver toxicity, occurred more frequently in the DTP arm, Dr. Bergh said.
Ultimately, 9 patients switched from T-DM1 to DTP – 7 for progression or lack of response and 2 because of toxicity, and 18 switched from DTP to T-DM1 because of either progression or lack of response, and 14 because of toxicity. One patient in each group achieved pCR after switching, he noted.
“There was clearly better quality of life [during the study] for the T-DM1 group,” he added, noting that the quality of life data were reported separately at the meeting.
Additionally, an exploratory analysis demonstrated an early steep decrease of F-FDG uptake, suggesting that PET/CT may be a useful tool for predicting pCR.
Although neoadjuvant therapy produces high pCR rates and is the standard of care in HER2 positive breast cancer, the optimal treatment regimen remains to be established; but the current findings, along with prior data showing efficacy with T-DM1 in patients who fail to respond to two or more lines of anti-HER2 therapies, suggest it is a potential new standard for neoadjuvant therapy, particularly for patients with HER2- and HR-positive disease, he concluded.
Dr. Bergh reported a financial relationship with UpToDate, and research funding to his institution from Amgen, AstraZeneca, Bayer, Merck, Pfizer, Roche, and Sanofi.
SOURCE: Bergh J et al. ASCO 2019, Abstract 501.
REPORTING FROM ASCO 2019
KRISTINE: Three-year data help forge path to T-DM1-based deescalation in HER2+ BC
CHICAGO – Combining trastuzumab emtansine (T-DM1) and pertuzumab (P) reduced grade 3+ toxicity in patients with HER2-positive stage I-III breast cancer in the KRISTINE trial, but led to lower event-free survival (EFS) and pathological complete response (pCR) rates vs. standard chemotherapy plus dual HER2 blockade, according to the preplanned 3-year final data analysis.
The EFS rate among participants in the randomized, phase 3 study who completed follow-up was 94.2% in 189 patients who received neoadjuvant T-DM1+P treatment and 85.3% in 196 patients who received docetaxel, carboplatin, and trastuzumab (TCH) plus pertuzumab (hazard ratio, 2.61). The difference was due to more locoregional progression events before surgery (15 [6.7%] vs. 0 in the groups, respectively), Dr. Sara A. Hurvitz, MD, reported at the annual meeting of the American Association of Clinical Oncology.
The curves separated early, prior to surgery, without much change after surgery, noted Dr. Hurvitz, a medical oncologist at the University of California, Los Angeles, where she also serves as director of the Breast Cancer Clinical Trials Program.
Additional analysis showed that low HER2 expression by mRNA or immunohistochemistry (IHC), and HER2 heterogeneity “tended to correlate with locoregional progression.”
Invasive disease-free survival (IDFS) risk, however, was similar with the two treatments (93% and 92%, respectively; HR, 1.11), and, as has been shown “many times over,” experiencing a pCR was associated with reduced risk of an IDFS event (HR, 0.24), regardless of treatment arm, Dr. Hurvitz said.
The previously reported primary results of the study, which failed to reach its primary endpoint, showed a pCR of 44% vs. 56% in 223 women who received TDM-1+P and 221 who received TCH+P, respectively. (Lancet Oncol. 2018 Jan;19[1]:115-126. doi: 10.1016/S1470-2045[17]30716-7).
Of note, additional data reported in a poster at the 2016 San Antonio Breast Cancer Symposium showed that pCR rates “were higher with TCH+P in those tumors with IHC2+ HER2 staining (20% vs. 7% in the T-DM1 arm), or IHC3+ HER2 staining (61% vs. 50%),” she said (SABCS 2016 P6-07-09).
“During neoadjuvant treatment, however, it’s not surprising that the T-DM1+P arm had a more favorable safety profile with a lower incidence of grade 3-4 events, lower incidence of [serious adverse events], and lower incidence of AEs leading to treatment discontinuation,” she said.
The overall rate of grade 3 or greater AEs was 31.8% vs. 67.6% with T-DM1+P vs. TCH+P, but the T-DM1 regimen was associated with more grade 3+ AEs during adjuvant treatment (24.5% vs. 9.9%), and with more adverse events leading to treatment discontinuation – both overall (20.2% vs. 11.0%) and during adjuvant therapy (18.4% vs. 3.8%), said Dr. Hurvitz, noting, however, that 50 patients in the T-DM1+P arm received cytotoxic chemotherapy in the adjuvant phase as allowed by study protocol.
Patient-reported outcomes favored T-DM1+P during the neoadjuvant phase, but were similar in the two groups during the adjuvant phase.
Adverse events occurring substantially more often with TCH+P (2% or greater difference in incidence between the groups) mainly included neutropenia, diarrhea, febrile neutropenia, and anemia, but peripheral neuropathy was a bit higher in the T-DM1 arm, she said.
“Standard-of-care neoadjuvant therapy for HER2-positive breast cancer is chemotherapy plus dual HER2 blockade with trastuzumab and pertuzumab, followed by continued HER2 blockade in the adjuvant setting,” Dr. Hurvitz said, noting that rates of pCR, which is associated with prolonged survival, range from 46% to 62%. “Despite the good outcomes ... 15% of patients will relapse or die; moreover, our standard cytotoxic approaches are associated with systemic toxicity, so there still is a need for effective, less toxic therapies.”
The antibody drug conjugate (ADC) T-DM1 is associated with a lower incidence of AEs typically associated with cytotoxic chemotherapy due to its targeted nature, and in the German ADAPT study it has shown some evidence of efficacy as monotherapy or with endocrine therapy in the neoadjuvant setting in HER2-positive, hormone receptor-positive breast cancer.
“So when we designed this clinical trial we thought that combining T-DM1 with pertuzumab might be an efficacious therapy that would provide patients with a less toxic regimen,” she said.
Participants had centrally-confirmed HER2-positive breast cancer over 2 cm and were randomly assigned 1:1 to T-DM1+P or TCH+P every 3 weeks for six cycles prior to surgery. Those who received T-DM1+P continued adjuvant T-DM1+P for 12 cycles, and those who received TCH+P received adjuvant trastuzumab plus pertuzumab for 12 cycles.
Those in the T-DM1 arm were allowed to receive standard adjuvant chemotherapy at physician discretion – and were encouraged to do so if they had residual disease in the breast greater than 1 cm or lymph node-positive disease. They then went on to receive T-DM1+P for 12 cycles, she said.
“We know that patients who achieve a pathologic complete response have a very good 3-year [IDFS], and for our study, for either arm, it was around 97%. Patients with residual disease have a lower 3-year IDFS in the mid [80% range] representing an unmet need,” she said.
In addition, the similar overall risk of an IDFS event with T-DM1+P and TCH+P in this study suggests that systemic chemotherapy might be unnecessary for some patients.
“But, of course, identification of these patients is going to be critical in determining who can have a deescalation approach, and the clinical utility of chemotherapy-sparing regimens must be confirmed in prospective studies, hopefully using biomarkers,” she concluded.
In a companion article published June 3 in the Journal of Clinical Oncology, Dr. Hurvitz and her colleagues further noted that “the role of T-DM1 in early HER2-positive breast cancer is evolving, with two trials evaluating this agent in the adjuvant setting.”
These include the KATHERINE trial, which showed a lower risk of invasive breast cancer recurrence or death with adjuvant T-DM1 vs. adjuvant trastuzumab in patients with residual disease after neoadjuvant systemic chemotherapy plus single or dual HER-directed therapy (HR, 0.50), and the ongoing KAITLIN trial, which is comparing T-DM1+P with taxane plus trastuzumab after anthracyclines as adjuvant therapy in patients who have not received prior neoadjuvant therapy.
“Data from KAITLIN will further define the clinical utility of adjuvant T-DM1+P in patients with HER2-positive early breast cancer,” they wrote.
During a discussion of the KRISTINE study findings and other related data presented at ASCO 2019, Mark D. Pegram, MD, a medical oncologist and professor at Stanford (Calif.) University, said that T-DM1-based neoadjuvant regimens appear, based on peer-reviewed published data from KRISTINE and other studies (such as the Swedish PREDIX HER2 trial, which was also discussed during the session), to be clinically active and well tolerated in HER2-positive early breast cancer.
“Early adopters may consider neoadjuvant T-DM1 in patients who are perhaps not candidates for chemotherapy due to comorbidities, age, et cetera, or those patients who frankly refuse chemotherapy, of which we all have a few,” said Dr. Pegram, who also is the first director of the Breast Cancer Oncology Program at Stanford Women’s Cancer Center. “The burden is on us to identify molecular, genetic, or perhaps imaging markers to identify patients who are most suitable for consideration of deescalation strategies with T-DM1 or newer HER2 antibody drug conjugates [in development].”
Dr. Pegram also highlighted the KRISTINE EFS finding on locoregional progression prior to surgery.
“Sara showed you that the ... event-free survival outcomes that are deleterious happen prior to surgery, which is, I think, fascinating, and if we could identify those patients prospectively, it could be very powerful in maximally exploiting the potential of deescalation with T-DM1 or T-DM1-based regimens,” he said. “But we’re not there yet, obviously.”
The KRISTINE study was funded by F. Hoffmann-La Roche and Genentech. Dr. Hurvitz reported research funding to her institution from Ambryx, Amgen, Bayer, Biomarin, Boehringer Ingelheim, Cascadian Therapeutics, Daiichi Sankyo, Dignitana, Genentech/Roche, GlaxoSmithKline, Lilly, Macrogenics, Medivation, Merrimack, Novartis, OBI Pharma, Pfizer, Puma Biotechnology, Sanofi, and Seattle Genetics, and travel/accommodations/expenses from Lilly, Novartis, and OBI Pharma. Dr. Pegram reported relationships (honoraria; consulting/advisory roles) with Daiichi Sankyo, Genentech/Roche, Macrogenics, and Seattle Genetics.
SOURCE: Hurvitz S et al. ASCO 2019: Abstract 500.
CHICAGO – Combining trastuzumab emtansine (T-DM1) and pertuzumab (P) reduced grade 3+ toxicity in patients with HER2-positive stage I-III breast cancer in the KRISTINE trial, but led to lower event-free survival (EFS) and pathological complete response (pCR) rates vs. standard chemotherapy plus dual HER2 blockade, according to the preplanned 3-year final data analysis.
The EFS rate among participants in the randomized, phase 3 study who completed follow-up was 94.2% in 189 patients who received neoadjuvant T-DM1+P treatment and 85.3% in 196 patients who received docetaxel, carboplatin, and trastuzumab (TCH) plus pertuzumab (hazard ratio, 2.61). The difference was due to more locoregional progression events before surgery (15 [6.7%] vs. 0 in the groups, respectively), Dr. Sara A. Hurvitz, MD, reported at the annual meeting of the American Association of Clinical Oncology.
The curves separated early, prior to surgery, without much change after surgery, noted Dr. Hurvitz, a medical oncologist at the University of California, Los Angeles, where she also serves as director of the Breast Cancer Clinical Trials Program.
Additional analysis showed that low HER2 expression by mRNA or immunohistochemistry (IHC), and HER2 heterogeneity “tended to correlate with locoregional progression.”
Invasive disease-free survival (IDFS) risk, however, was similar with the two treatments (93% and 92%, respectively; HR, 1.11), and, as has been shown “many times over,” experiencing a pCR was associated with reduced risk of an IDFS event (HR, 0.24), regardless of treatment arm, Dr. Hurvitz said.
The previously reported primary results of the study, which failed to reach its primary endpoint, showed a pCR of 44% vs. 56% in 223 women who received TDM-1+P and 221 who received TCH+P, respectively. (Lancet Oncol. 2018 Jan;19[1]:115-126. doi: 10.1016/S1470-2045[17]30716-7).
Of note, additional data reported in a poster at the 2016 San Antonio Breast Cancer Symposium showed that pCR rates “were higher with TCH+P in those tumors with IHC2+ HER2 staining (20% vs. 7% in the T-DM1 arm), or IHC3+ HER2 staining (61% vs. 50%),” she said (SABCS 2016 P6-07-09).
“During neoadjuvant treatment, however, it’s not surprising that the T-DM1+P arm had a more favorable safety profile with a lower incidence of grade 3-4 events, lower incidence of [serious adverse events], and lower incidence of AEs leading to treatment discontinuation,” she said.
The overall rate of grade 3 or greater AEs was 31.8% vs. 67.6% with T-DM1+P vs. TCH+P, but the T-DM1 regimen was associated with more grade 3+ AEs during adjuvant treatment (24.5% vs. 9.9%), and with more adverse events leading to treatment discontinuation – both overall (20.2% vs. 11.0%) and during adjuvant therapy (18.4% vs. 3.8%), said Dr. Hurvitz, noting, however, that 50 patients in the T-DM1+P arm received cytotoxic chemotherapy in the adjuvant phase as allowed by study protocol.
Patient-reported outcomes favored T-DM1+P during the neoadjuvant phase, but were similar in the two groups during the adjuvant phase.
Adverse events occurring substantially more often with TCH+P (2% or greater difference in incidence between the groups) mainly included neutropenia, diarrhea, febrile neutropenia, and anemia, but peripheral neuropathy was a bit higher in the T-DM1 arm, she said.
“Standard-of-care neoadjuvant therapy for HER2-positive breast cancer is chemotherapy plus dual HER2 blockade with trastuzumab and pertuzumab, followed by continued HER2 blockade in the adjuvant setting,” Dr. Hurvitz said, noting that rates of pCR, which is associated with prolonged survival, range from 46% to 62%. “Despite the good outcomes ... 15% of patients will relapse or die; moreover, our standard cytotoxic approaches are associated with systemic toxicity, so there still is a need for effective, less toxic therapies.”
The antibody drug conjugate (ADC) T-DM1 is associated with a lower incidence of AEs typically associated with cytotoxic chemotherapy due to its targeted nature, and in the German ADAPT study it has shown some evidence of efficacy as monotherapy or with endocrine therapy in the neoadjuvant setting in HER2-positive, hormone receptor-positive breast cancer.
“So when we designed this clinical trial we thought that combining T-DM1 with pertuzumab might be an efficacious therapy that would provide patients with a less toxic regimen,” she said.
Participants had centrally-confirmed HER2-positive breast cancer over 2 cm and were randomly assigned 1:1 to T-DM1+P or TCH+P every 3 weeks for six cycles prior to surgery. Those who received T-DM1+P continued adjuvant T-DM1+P for 12 cycles, and those who received TCH+P received adjuvant trastuzumab plus pertuzumab for 12 cycles.
Those in the T-DM1 arm were allowed to receive standard adjuvant chemotherapy at physician discretion – and were encouraged to do so if they had residual disease in the breast greater than 1 cm or lymph node-positive disease. They then went on to receive T-DM1+P for 12 cycles, she said.
“We know that patients who achieve a pathologic complete response have a very good 3-year [IDFS], and for our study, for either arm, it was around 97%. Patients with residual disease have a lower 3-year IDFS in the mid [80% range] representing an unmet need,” she said.
In addition, the similar overall risk of an IDFS event with T-DM1+P and TCH+P in this study suggests that systemic chemotherapy might be unnecessary for some patients.
“But, of course, identification of these patients is going to be critical in determining who can have a deescalation approach, and the clinical utility of chemotherapy-sparing regimens must be confirmed in prospective studies, hopefully using biomarkers,” she concluded.
In a companion article published June 3 in the Journal of Clinical Oncology, Dr. Hurvitz and her colleagues further noted that “the role of T-DM1 in early HER2-positive breast cancer is evolving, with two trials evaluating this agent in the adjuvant setting.”
These include the KATHERINE trial, which showed a lower risk of invasive breast cancer recurrence or death with adjuvant T-DM1 vs. adjuvant trastuzumab in patients with residual disease after neoadjuvant systemic chemotherapy plus single or dual HER-directed therapy (HR, 0.50), and the ongoing KAITLIN trial, which is comparing T-DM1+P with taxane plus trastuzumab after anthracyclines as adjuvant therapy in patients who have not received prior neoadjuvant therapy.
“Data from KAITLIN will further define the clinical utility of adjuvant T-DM1+P in patients with HER2-positive early breast cancer,” they wrote.
During a discussion of the KRISTINE study findings and other related data presented at ASCO 2019, Mark D. Pegram, MD, a medical oncologist and professor at Stanford (Calif.) University, said that T-DM1-based neoadjuvant regimens appear, based on peer-reviewed published data from KRISTINE and other studies (such as the Swedish PREDIX HER2 trial, which was also discussed during the session), to be clinically active and well tolerated in HER2-positive early breast cancer.
“Early adopters may consider neoadjuvant T-DM1 in patients who are perhaps not candidates for chemotherapy due to comorbidities, age, et cetera, or those patients who frankly refuse chemotherapy, of which we all have a few,” said Dr. Pegram, who also is the first director of the Breast Cancer Oncology Program at Stanford Women’s Cancer Center. “The burden is on us to identify molecular, genetic, or perhaps imaging markers to identify patients who are most suitable for consideration of deescalation strategies with T-DM1 or newer HER2 antibody drug conjugates [in development].”
Dr. Pegram also highlighted the KRISTINE EFS finding on locoregional progression prior to surgery.
“Sara showed you that the ... event-free survival outcomes that are deleterious happen prior to surgery, which is, I think, fascinating, and if we could identify those patients prospectively, it could be very powerful in maximally exploiting the potential of deescalation with T-DM1 or T-DM1-based regimens,” he said. “But we’re not there yet, obviously.”
The KRISTINE study was funded by F. Hoffmann-La Roche and Genentech. Dr. Hurvitz reported research funding to her institution from Ambryx, Amgen, Bayer, Biomarin, Boehringer Ingelheim, Cascadian Therapeutics, Daiichi Sankyo, Dignitana, Genentech/Roche, GlaxoSmithKline, Lilly, Macrogenics, Medivation, Merrimack, Novartis, OBI Pharma, Pfizer, Puma Biotechnology, Sanofi, and Seattle Genetics, and travel/accommodations/expenses from Lilly, Novartis, and OBI Pharma. Dr. Pegram reported relationships (honoraria; consulting/advisory roles) with Daiichi Sankyo, Genentech/Roche, Macrogenics, and Seattle Genetics.
SOURCE: Hurvitz S et al. ASCO 2019: Abstract 500.
CHICAGO – Combining trastuzumab emtansine (T-DM1) and pertuzumab (P) reduced grade 3+ toxicity in patients with HER2-positive stage I-III breast cancer in the KRISTINE trial, but led to lower event-free survival (EFS) and pathological complete response (pCR) rates vs. standard chemotherapy plus dual HER2 blockade, according to the preplanned 3-year final data analysis.
The EFS rate among participants in the randomized, phase 3 study who completed follow-up was 94.2% in 189 patients who received neoadjuvant T-DM1+P treatment and 85.3% in 196 patients who received docetaxel, carboplatin, and trastuzumab (TCH) plus pertuzumab (hazard ratio, 2.61). The difference was due to more locoregional progression events before surgery (15 [6.7%] vs. 0 in the groups, respectively), Dr. Sara A. Hurvitz, MD, reported at the annual meeting of the American Association of Clinical Oncology.
The curves separated early, prior to surgery, without much change after surgery, noted Dr. Hurvitz, a medical oncologist at the University of California, Los Angeles, where she also serves as director of the Breast Cancer Clinical Trials Program.
Additional analysis showed that low HER2 expression by mRNA or immunohistochemistry (IHC), and HER2 heterogeneity “tended to correlate with locoregional progression.”
Invasive disease-free survival (IDFS) risk, however, was similar with the two treatments (93% and 92%, respectively; HR, 1.11), and, as has been shown “many times over,” experiencing a pCR was associated with reduced risk of an IDFS event (HR, 0.24), regardless of treatment arm, Dr. Hurvitz said.
The previously reported primary results of the study, which failed to reach its primary endpoint, showed a pCR of 44% vs. 56% in 223 women who received TDM-1+P and 221 who received TCH+P, respectively. (Lancet Oncol. 2018 Jan;19[1]:115-126. doi: 10.1016/S1470-2045[17]30716-7).
Of note, additional data reported in a poster at the 2016 San Antonio Breast Cancer Symposium showed that pCR rates “were higher with TCH+P in those tumors with IHC2+ HER2 staining (20% vs. 7% in the T-DM1 arm), or IHC3+ HER2 staining (61% vs. 50%),” she said (SABCS 2016 P6-07-09).
“During neoadjuvant treatment, however, it’s not surprising that the T-DM1+P arm had a more favorable safety profile with a lower incidence of grade 3-4 events, lower incidence of [serious adverse events], and lower incidence of AEs leading to treatment discontinuation,” she said.
The overall rate of grade 3 or greater AEs was 31.8% vs. 67.6% with T-DM1+P vs. TCH+P, but the T-DM1 regimen was associated with more grade 3+ AEs during adjuvant treatment (24.5% vs. 9.9%), and with more adverse events leading to treatment discontinuation – both overall (20.2% vs. 11.0%) and during adjuvant therapy (18.4% vs. 3.8%), said Dr. Hurvitz, noting, however, that 50 patients in the T-DM1+P arm received cytotoxic chemotherapy in the adjuvant phase as allowed by study protocol.
Patient-reported outcomes favored T-DM1+P during the neoadjuvant phase, but were similar in the two groups during the adjuvant phase.
Adverse events occurring substantially more often with TCH+P (2% or greater difference in incidence between the groups) mainly included neutropenia, diarrhea, febrile neutropenia, and anemia, but peripheral neuropathy was a bit higher in the T-DM1 arm, she said.
“Standard-of-care neoadjuvant therapy for HER2-positive breast cancer is chemotherapy plus dual HER2 blockade with trastuzumab and pertuzumab, followed by continued HER2 blockade in the adjuvant setting,” Dr. Hurvitz said, noting that rates of pCR, which is associated with prolonged survival, range from 46% to 62%. “Despite the good outcomes ... 15% of patients will relapse or die; moreover, our standard cytotoxic approaches are associated with systemic toxicity, so there still is a need for effective, less toxic therapies.”
The antibody drug conjugate (ADC) T-DM1 is associated with a lower incidence of AEs typically associated with cytotoxic chemotherapy due to its targeted nature, and in the German ADAPT study it has shown some evidence of efficacy as monotherapy or with endocrine therapy in the neoadjuvant setting in HER2-positive, hormone receptor-positive breast cancer.
“So when we designed this clinical trial we thought that combining T-DM1 with pertuzumab might be an efficacious therapy that would provide patients with a less toxic regimen,” she said.
Participants had centrally-confirmed HER2-positive breast cancer over 2 cm and were randomly assigned 1:1 to T-DM1+P or TCH+P every 3 weeks for six cycles prior to surgery. Those who received T-DM1+P continued adjuvant T-DM1+P for 12 cycles, and those who received TCH+P received adjuvant trastuzumab plus pertuzumab for 12 cycles.
Those in the T-DM1 arm were allowed to receive standard adjuvant chemotherapy at physician discretion – and were encouraged to do so if they had residual disease in the breast greater than 1 cm or lymph node-positive disease. They then went on to receive T-DM1+P for 12 cycles, she said.
“We know that patients who achieve a pathologic complete response have a very good 3-year [IDFS], and for our study, for either arm, it was around 97%. Patients with residual disease have a lower 3-year IDFS in the mid [80% range] representing an unmet need,” she said.
In addition, the similar overall risk of an IDFS event with T-DM1+P and TCH+P in this study suggests that systemic chemotherapy might be unnecessary for some patients.
“But, of course, identification of these patients is going to be critical in determining who can have a deescalation approach, and the clinical utility of chemotherapy-sparing regimens must be confirmed in prospective studies, hopefully using biomarkers,” she concluded.
In a companion article published June 3 in the Journal of Clinical Oncology, Dr. Hurvitz and her colleagues further noted that “the role of T-DM1 in early HER2-positive breast cancer is evolving, with two trials evaluating this agent in the adjuvant setting.”
These include the KATHERINE trial, which showed a lower risk of invasive breast cancer recurrence or death with adjuvant T-DM1 vs. adjuvant trastuzumab in patients with residual disease after neoadjuvant systemic chemotherapy plus single or dual HER-directed therapy (HR, 0.50), and the ongoing KAITLIN trial, which is comparing T-DM1+P with taxane plus trastuzumab after anthracyclines as adjuvant therapy in patients who have not received prior neoadjuvant therapy.
“Data from KAITLIN will further define the clinical utility of adjuvant T-DM1+P in patients with HER2-positive early breast cancer,” they wrote.
During a discussion of the KRISTINE study findings and other related data presented at ASCO 2019, Mark D. Pegram, MD, a medical oncologist and professor at Stanford (Calif.) University, said that T-DM1-based neoadjuvant regimens appear, based on peer-reviewed published data from KRISTINE and other studies (such as the Swedish PREDIX HER2 trial, which was also discussed during the session), to be clinically active and well tolerated in HER2-positive early breast cancer.
“Early adopters may consider neoadjuvant T-DM1 in patients who are perhaps not candidates for chemotherapy due to comorbidities, age, et cetera, or those patients who frankly refuse chemotherapy, of which we all have a few,” said Dr. Pegram, who also is the first director of the Breast Cancer Oncology Program at Stanford Women’s Cancer Center. “The burden is on us to identify molecular, genetic, or perhaps imaging markers to identify patients who are most suitable for consideration of deescalation strategies with T-DM1 or newer HER2 antibody drug conjugates [in development].”
Dr. Pegram also highlighted the KRISTINE EFS finding on locoregional progression prior to surgery.
“Sara showed you that the ... event-free survival outcomes that are deleterious happen prior to surgery, which is, I think, fascinating, and if we could identify those patients prospectively, it could be very powerful in maximally exploiting the potential of deescalation with T-DM1 or T-DM1-based regimens,” he said. “But we’re not there yet, obviously.”
The KRISTINE study was funded by F. Hoffmann-La Roche and Genentech. Dr. Hurvitz reported research funding to her institution from Ambryx, Amgen, Bayer, Biomarin, Boehringer Ingelheim, Cascadian Therapeutics, Daiichi Sankyo, Dignitana, Genentech/Roche, GlaxoSmithKline, Lilly, Macrogenics, Medivation, Merrimack, Novartis, OBI Pharma, Pfizer, Puma Biotechnology, Sanofi, and Seattle Genetics, and travel/accommodations/expenses from Lilly, Novartis, and OBI Pharma. Dr. Pegram reported relationships (honoraria; consulting/advisory roles) with Daiichi Sankyo, Genentech/Roche, Macrogenics, and Seattle Genetics.
SOURCE: Hurvitz S et al. ASCO 2019: Abstract 500.
REPORTING FROM ASCO 2019
Pregnancy deemed safe in BRCA-mutated breast cancer survivors
CHICAGO – Pregnancy after breast cancer is safe in BRCA-mutated patients, according to a retrospective study.
Pregnancy did not affect disease-free or overall survival in a cohort of BRCA-mutated breast cancer patients. Additionally, fetal and pregnancy complications in this cohort were similar to complications observed in the general population.
“We believe that our findings provide reassurance for counseling young BRCA-mutated breast cancer patients inquiring about the feasibility and safety of future conception,” said Matteo Lambertini, MD, PhD, of Policlinico San Martino Hospital in Genova, Italy.
Dr. Lambertini presented the findings at the annual meeting of the American Society of Clinical Oncology.
He and his colleagues conducted an international, multicenter, retrospective cohort study of 1,252 patients. The patients had been diagnosed with stage I-III breast cancer between January 2000 and December 2012 at age 40 years or younger. All patients had BRCA mutations – 811 with BRCA1 alone, 430 with BRCA2 alone, and 11 with both.
Pregnant versus nonpregnant patients
At a median of 4.5 years after diagnosis, 195 patients (16%) had experienced a pregnancy.
Compared with the nonpregnant women, pregnant patients were younger (P less than .001), more likely to have a BRCA1 mutation (P = .01), have smaller tumors (P = .04), have node-negative disease (P = .003), and have hormone receptor–negative tumors (P = .002). Roughly 95% of patients in both cohorts had received chemotherapy, and the most common regimens were anthracycline or taxane based.
Compared with patients in the nonpregnancy cohort, those in the pregnancy cohort were less likely to receive tamoxifen alone as endocrine therapy (P = .002), were more likely to have a shorter duration of endocrine therapy (P less than .001), and were less likely to undergo salpingo-oophorectomy (P less than .001).
Pregnancy outcomes
“In terms of pregnancy, fetal, and obstetrical outcomes, no alarming signals were observed,” Dr. Lambertini said.
Most pregnant patients had a spontaneous pregnancy (82.1%), completed the pregnancy (76.9%), delivered at term (90.8%), and had no complications (86.6%). However, 10.3% of patients had a spontaneous abortion, 9.2% of pregnancies were pre term, and 1.8% of babies had congenital abnormalities.
“All these rates were highly comparable to rates that are expected in the general healthy population,” Dr. Lambertini said.
Survival analyses
The researchers performed two survival analyses. The first was a case-control approach in which they matched each pregnant patient with three controls (patients without pregnancy) according to the following:
- Disease-free interval from breast cancer diagnosis (equal to or longer than that of pregnant patients).
- Year at diagnosis (plus or minus 2.5 years).
- Nodal status (negative vs. positive).
- Hormone receptor status (positive vs. negative).
- Type of BRCA mutation (BRCA1 vs. BRCA2).
The second survival analysis was an extended Cox model with pregnancy as a time-varying covariate.
Survival outcomes
At a median follow-up of 8.3 years, pregnant patients had better disease-free survival than nonpregnant patients in the case-control analysis, with a hazard ratio of 0.71 (P = .045). With the extended Cox model, the adjusted HR was 0.87 (P = .41). The analysis was adjusted for age, tumor size, nodal status, type of endocrine therapy, hormone receptor status, breast surgery, and BRCA mutation.
There was a significant interaction between type of BRCA mutation and pregnancy, with better disease-free survival observed in the BRCA1-mutated cohort. The HR was 0.53 in the BRCA1 cohort and 1.60 in the BRCA2 cohort (P less than .01). However, as Dr. Lambertini pointed out, only 44 pregnant patients had a BRCA1 mutation.
There was no significant interaction between hormone receptor status and pregnancy (P = .28).
Furthermore, there was no significant difference in overall survival between the pregnant and nonpregnant cohorts. In the case-control analysis, the HR was 0.86 (P = .65). In the extended Cox model, the adjusted HR was 0.88 (P = .66).
Dr. Lambertini disclosed a relationship with Teva.
SOURCE: Lambertini M et al. ASCO 2019, Abstract 11506.
CHICAGO – Pregnancy after breast cancer is safe in BRCA-mutated patients, according to a retrospective study.
Pregnancy did not affect disease-free or overall survival in a cohort of BRCA-mutated breast cancer patients. Additionally, fetal and pregnancy complications in this cohort were similar to complications observed in the general population.
“We believe that our findings provide reassurance for counseling young BRCA-mutated breast cancer patients inquiring about the feasibility and safety of future conception,” said Matteo Lambertini, MD, PhD, of Policlinico San Martino Hospital in Genova, Italy.
Dr. Lambertini presented the findings at the annual meeting of the American Society of Clinical Oncology.
He and his colleagues conducted an international, multicenter, retrospective cohort study of 1,252 patients. The patients had been diagnosed with stage I-III breast cancer between January 2000 and December 2012 at age 40 years or younger. All patients had BRCA mutations – 811 with BRCA1 alone, 430 with BRCA2 alone, and 11 with both.
Pregnant versus nonpregnant patients
At a median of 4.5 years after diagnosis, 195 patients (16%) had experienced a pregnancy.
Compared with the nonpregnant women, pregnant patients were younger (P less than .001), more likely to have a BRCA1 mutation (P = .01), have smaller tumors (P = .04), have node-negative disease (P = .003), and have hormone receptor–negative tumors (P = .002). Roughly 95% of patients in both cohorts had received chemotherapy, and the most common regimens were anthracycline or taxane based.
Compared with patients in the nonpregnancy cohort, those in the pregnancy cohort were less likely to receive tamoxifen alone as endocrine therapy (P = .002), were more likely to have a shorter duration of endocrine therapy (P less than .001), and were less likely to undergo salpingo-oophorectomy (P less than .001).
Pregnancy outcomes
“In terms of pregnancy, fetal, and obstetrical outcomes, no alarming signals were observed,” Dr. Lambertini said.
Most pregnant patients had a spontaneous pregnancy (82.1%), completed the pregnancy (76.9%), delivered at term (90.8%), and had no complications (86.6%). However, 10.3% of patients had a spontaneous abortion, 9.2% of pregnancies were pre term, and 1.8% of babies had congenital abnormalities.
“All these rates were highly comparable to rates that are expected in the general healthy population,” Dr. Lambertini said.
Survival analyses
The researchers performed two survival analyses. The first was a case-control approach in which they matched each pregnant patient with three controls (patients without pregnancy) according to the following:
- Disease-free interval from breast cancer diagnosis (equal to or longer than that of pregnant patients).
- Year at diagnosis (plus or minus 2.5 years).
- Nodal status (negative vs. positive).
- Hormone receptor status (positive vs. negative).
- Type of BRCA mutation (BRCA1 vs. BRCA2).
The second survival analysis was an extended Cox model with pregnancy as a time-varying covariate.
Survival outcomes
At a median follow-up of 8.3 years, pregnant patients had better disease-free survival than nonpregnant patients in the case-control analysis, with a hazard ratio of 0.71 (P = .045). With the extended Cox model, the adjusted HR was 0.87 (P = .41). The analysis was adjusted for age, tumor size, nodal status, type of endocrine therapy, hormone receptor status, breast surgery, and BRCA mutation.
There was a significant interaction between type of BRCA mutation and pregnancy, with better disease-free survival observed in the BRCA1-mutated cohort. The HR was 0.53 in the BRCA1 cohort and 1.60 in the BRCA2 cohort (P less than .01). However, as Dr. Lambertini pointed out, only 44 pregnant patients had a BRCA1 mutation.
There was no significant interaction between hormone receptor status and pregnancy (P = .28).
Furthermore, there was no significant difference in overall survival between the pregnant and nonpregnant cohorts. In the case-control analysis, the HR was 0.86 (P = .65). In the extended Cox model, the adjusted HR was 0.88 (P = .66).
Dr. Lambertini disclosed a relationship with Teva.
SOURCE: Lambertini M et al. ASCO 2019, Abstract 11506.
CHICAGO – Pregnancy after breast cancer is safe in BRCA-mutated patients, according to a retrospective study.
Pregnancy did not affect disease-free or overall survival in a cohort of BRCA-mutated breast cancer patients. Additionally, fetal and pregnancy complications in this cohort were similar to complications observed in the general population.
“We believe that our findings provide reassurance for counseling young BRCA-mutated breast cancer patients inquiring about the feasibility and safety of future conception,” said Matteo Lambertini, MD, PhD, of Policlinico San Martino Hospital in Genova, Italy.
Dr. Lambertini presented the findings at the annual meeting of the American Society of Clinical Oncology.
He and his colleagues conducted an international, multicenter, retrospective cohort study of 1,252 patients. The patients had been diagnosed with stage I-III breast cancer between January 2000 and December 2012 at age 40 years or younger. All patients had BRCA mutations – 811 with BRCA1 alone, 430 with BRCA2 alone, and 11 with both.
Pregnant versus nonpregnant patients
At a median of 4.5 years after diagnosis, 195 patients (16%) had experienced a pregnancy.
Compared with the nonpregnant women, pregnant patients were younger (P less than .001), more likely to have a BRCA1 mutation (P = .01), have smaller tumors (P = .04), have node-negative disease (P = .003), and have hormone receptor–negative tumors (P = .002). Roughly 95% of patients in both cohorts had received chemotherapy, and the most common regimens were anthracycline or taxane based.
Compared with patients in the nonpregnancy cohort, those in the pregnancy cohort were less likely to receive tamoxifen alone as endocrine therapy (P = .002), were more likely to have a shorter duration of endocrine therapy (P less than .001), and were less likely to undergo salpingo-oophorectomy (P less than .001).
Pregnancy outcomes
“In terms of pregnancy, fetal, and obstetrical outcomes, no alarming signals were observed,” Dr. Lambertini said.
Most pregnant patients had a spontaneous pregnancy (82.1%), completed the pregnancy (76.9%), delivered at term (90.8%), and had no complications (86.6%). However, 10.3% of patients had a spontaneous abortion, 9.2% of pregnancies were pre term, and 1.8% of babies had congenital abnormalities.
“All these rates were highly comparable to rates that are expected in the general healthy population,” Dr. Lambertini said.
Survival analyses
The researchers performed two survival analyses. The first was a case-control approach in which they matched each pregnant patient with three controls (patients without pregnancy) according to the following:
- Disease-free interval from breast cancer diagnosis (equal to or longer than that of pregnant patients).
- Year at diagnosis (plus or minus 2.5 years).
- Nodal status (negative vs. positive).
- Hormone receptor status (positive vs. negative).
- Type of BRCA mutation (BRCA1 vs. BRCA2).
The second survival analysis was an extended Cox model with pregnancy as a time-varying covariate.
Survival outcomes
At a median follow-up of 8.3 years, pregnant patients had better disease-free survival than nonpregnant patients in the case-control analysis, with a hazard ratio of 0.71 (P = .045). With the extended Cox model, the adjusted HR was 0.87 (P = .41). The analysis was adjusted for age, tumor size, nodal status, type of endocrine therapy, hormone receptor status, breast surgery, and BRCA mutation.
There was a significant interaction between type of BRCA mutation and pregnancy, with better disease-free survival observed in the BRCA1-mutated cohort. The HR was 0.53 in the BRCA1 cohort and 1.60 in the BRCA2 cohort (P less than .01). However, as Dr. Lambertini pointed out, only 44 pregnant patients had a BRCA1 mutation.
There was no significant interaction between hormone receptor status and pregnancy (P = .28).
Furthermore, there was no significant difference in overall survival between the pregnant and nonpregnant cohorts. In the case-control analysis, the HR was 0.86 (P = .65). In the extended Cox model, the adjusted HR was 0.88 (P = .66).
Dr. Lambertini disclosed a relationship with Teva.
SOURCE: Lambertini M et al. ASCO 2019, Abstract 11506.
REPORTING FROM ASCO 2019
2019 Update on menopause
Among peri- and postmenopausal women, abnormal bleeding, breast cancer, and mood disorders represent prevalent conditions. In this Update, we discuss data from a review that provides quantitative information on the likelihood of finding endometrial cancer among women with postmenopausal bleeding (PMB). We also summarize 2 recent consensus recommendations: One addresses the clinically important but controversial issue of the treatment of genitourinary syndrome of menopause (GSM) in breast cancer survivors, and the other provides guidance on the management of depression in perimenopausal women.
Endometrial cancer is associated with a high prevalence of PMB
Clarke MA, Long BJ, Del Mar Morillo A, et al. Association of endometrial cancer risk with postmenopausal bleeding in women: a systematic review and meta-analysis. JAMA Intern Med. 2018;178:1210-1222.
Endometrial cancer is the most common gynecologic malignancy and the fourth most common cancer among US women. In recent years, the incidence of and mortality from endometrial cancer have increased.1 Despite the high prevalence of endometrial cancer, population-based screening currently is not recommended.
PMB affects up to 10% of women and can be caused by endometrial atrophy, endometrial polyps, uterine leiomyoma, and malignancy. While it is well known that PMB is a common presenting symptom of endometrial cancer, we do not have good data to guide counseling patients with PMB on the likelihood that endometrial cancer is present. Similarly, estimates are lacking regarding what proportion of women with endometrial cancer will present with PMB.
To address these 2 issues, Clarke and colleagues conducted a comprehensive systematic review and meta-analysis of the prevalence of PMB among women with endometrial cancer (sensitivity) and the risk of endometrial cancer among women with PMB (positive predictive value). The authors included 129 studies--with 34,432 women with PMB and 6,358 with endometrial cancer--in their report.
Cancer prevalence varied with HT use, geographic location
The study findings demonstrated that the prevalence of PMB in women with endometrial cancer was 90% (95% confidence interval [CI], 84%-94%), and there was no significant difference in the occurrence of PMB by cancer stage. The risk of endometrial cancer in women with PMB ranged from 0% to 48%, yielding an overall pooled estimate of 9% (95% CI, 8%-11%). As an editorialist pointed out, the risk of endometrial cancer in women with PMB is similar to that of colorectal cancer in individuals with rectal bleeding (8%) and breast cancer in women with a palpable mass (10%), supporting current guidance that recommends evaluation of women with PMB.2 Evaluating 100 women with PMB to diagnose 9 endometrial cancers does not seem excessive.
Interestingly, among women with PMB, the prevalence of endometrial cancer was significantly higher among women not using hormone therapy (HT) than among users of HT (12% and 7%, respectively). In 7 studies restricted to women with PMB and polyps (n = 2,801), the pooled risk of endometrial cancer was 3% (95% CI, 3%-4%). In an analysis stratified by geographic region, a striking difference was noted in the risk of endometrial cancer among women with PMB in North America (5%), Northern Europe (7%), and in Western Europe (13%). This finding may be explained by regional differences in the approach to evaluating PMB, cultural perceptions of PMB that can affect thresholds to present for care, and differences in risk factors between these populations.
The study had several limitations, including an inability to evaluate the number of years since menopause and the effects of body mass index. Additionally, the study did not address endometrial hyperplasia or endometrial intraepithelial neoplasia.
PMB accounts for two-thirds of all gynecologic visits among perimenopausal and postmenopausal women.3 This study revealed a 9% risk of endometrial cancer in patients experiencing PMB, which supports current practice guidelines to further evaluate and rule out endometrial cancer among all women presenting with PMB4; it also provides reassurance that targeting this high-risk group of women for early detection and prevention strategies will capture most cases of endometrial cancers. However, the relatively low positive predictive value of PMB emphasizes the need for additional triage tests with high specificity to improve management of PMB and minimize unnecessary biopsies in low-risk women.
Treating GSM in breast cancer survivors: New guidance targets QoL and sexuality
Faubion SS, Larkin LC, Stuenkel CA, et al. Management of genitourinary syndrome of menopause in women with or at high risk for breast cancer: consensus recommendations from The North American Menopause Society and The International Society for the Study of Women's Sexual Health. Menopause. 2018;25:596-608.
Given that there is little evidence addressing the safety of vaginal estrogen, other hormonal therapies, and nonprescription treatments for GSM in breast cancer survivors, many survivors with bothersome GSM symptoms are not appropriately treated.
Continue to: Expert panel creates evidence-based guidance...
Expert panel creates evidence-based guidance
Against this backdrop, The North American Menopause Society and the International Society for the Study of Women's Sexual Health convened a group comprised of menopause specialists (ObGyns, internists, and nurse practitioners), specialists in sexuality, medical oncologists specializing in breast cancer, and a psychologist to create evidence-based interdisciplinary consensus guidelines for enhancing quality of life and sexuality for breast cancer survivors with GSM.
Measures to help enhance quality of life and sexuality
The group's key recommendations for clinicians include:
- Sexual function and quality of life (QoL) should be assessed in all women with or at high risk for breast cancer.
- Management of GSM should be individualized based on shared decision-making involving the patient and her oncologist.
- Initial treatment options include:
—over-the-counter vaginal moisturizers used several times weekly on a regular basis
—lubricants used with intercourse
—vaginal dilator therapy
—pelvic floor physical therapy.
- Low-dose vaginal estrogen therapy may be appropriate for select women who have been treated for breast cancer:
—With use of vaginal estrogen, serum estradiol levels remain in the postmenopausal range.
—Based on limited data, use of vaginal estrogen is associated with a minimal risk for recurrence of breast cancer.
—Because their use is associated with the lowest serum estradiol levels, vaginal tablets, rings, or inserts may be preferable to creams.
—Decisions regarding use of vaginal estrogen in breast cancer survivors should involve the woman's oncologist. Appropriate candidates for off-label use of vaginal estrogen may be survivors:
–who are at relatively low risk for recurrence
–with hormone receptor-negative disease
–using tamoxifen rather than an AI
–who are particularly concerned about quality of life.
—Given that AIs prevent recurrence by lowering estrogen levels, oncologists may be reluctant to consider use of vaginal estrogen in survivors using adjuvant agents.
—With respect to use of vaginal estrogen, oncologists may be more comfortable with use in patients taking tamoxifen.
- Neither intravaginal dehydroepiandrosterone (DHEA; prasterone) nor the oral selective estrogen receptor modulator ospemifene has been studied in breast cancer survivors.
In women with metastatic disease, QoL, comfort, and sexual intimacy are key considerations when weighing potential therapies; optimal choices will vary with probability of long-term survival.
Although more data addressing the safety of vaginal estrogen as well as prasterone and ospemifene in breast cancer survivors clearly are needed, these guidelines should help clinicians who care for breast cancer survivors with GSM.
Framework provided for managing depressive disorders in perimenopausal women
Maki PM, Kornstein SG, Joffe H, et al; Board of Trustees for The North American Menopause Society (NAMS) and the Women and Mood Disorders Task Force of the National Network of Depression Centers. Guidelines for the evaluation and treatment of perimenopausal depression: summary and recommendations. Menopause. 2018;25:1069-1085.
Although perimenopausal women are more susceptible to the development of depressive symptoms and major depressive episodes (MDE), there is a lack of consensus regarding how to evaluate and treat depression in women during the menopausal transition and postmenopausal period.
Recently, an expert panel comprised of representatives from The North American Menopause Society and the National Network of Depression Centers Women and Mood Disorders Task Group developed clinical guidelines addressing epidemiology, clinical presentation, therapeutic effects of antidepressants, effects of HT, and efficacy of other therapies. Here we provide a summary of the expert panel's findings and guidelines.
Continue to: Certain factors are associated with higher risk for depression...
Certain factors are associated with higher risk for depression
The perimenopause represents a time of increased risk for depressive symptoms and major depressive disorder (MDD), even in women with no prior history of depression. Several characteristics and health factors are associated with the increased risk during the menopause transition. These include a prior history of MDD, current antidepressant use, anxiety, premenstrual depressive symptoms, African American race, high body mass index, younger age, social isolation, upsetting life events, and menopausal sleep disturbances.
Although data are inconclusive on whether surgical menopause increases or decreases the risk for developing depression compared with women who transition through menopause naturally, recent studies show an elevated risk of depression in women following hysterectomy with and without oophorectomy.6,7
Menopausal and depressive symptoms can overlap
Midlife depression presents with classic depressive symptoms that commonly occur in combination with menopausal symptoms, including vasomotor symptoms, sleep and sexual disturbances, and weight and energy changes. These menopausal symptoms can complicate, co-occur, and overlap with the clinical presentation of depression.
Conversely, depression may affect an individual's judgment of the degree of bother from menopausal somatic symptoms, thereby further magnifying the effect of symptoms on quality of life. The interrelationship between depressive symptoms and menopausal symptoms may pose a challenge when attempting to parse out contributing etiologies, relative contributions of each etiology, and the potential additive effects.
Diagnosis and treatment options
Diagnosis involves identifying the menopausal stage, assessing for co-existing psychiatric and menopause symptoms, appreciating the psychosocial factors common in midlife, and considering the differential diagnosis. Validated screening instruments can be helpful. Although a menopause-specific mood disorder scale does not yet exist, several general validated screening measures, such as the Patient Health Questionnaire-9, or PHQ-9, can be used for categorical determination of mood disorder diagnoses during the menopause transition.
Antidepressants, cognitive-behavioral therapy, and other psychotherapies are considered first-line treatments for perimenopausal major depressive episodes. Only desvenlafaxine has been studied in large randomized placebo-controlled trials and has proven efficacious for the treatment of MDD in perimenopausal and postmenopausal women.
A number of small open-label studies of other selective serotonin reuptake inhibitors (SSRIs), serotonin norepinephrine reuptake inhibitors (SNRIs), and mirtazapine to treat MDD in perimenopausal and postmenopausal women have demonstrated a positive effect on mood, and several SSRIs and SNRIs also have the added benefit of improving menopause-related symptoms.
In women with a history of MDD, a prior adequate response to a particular antidepressant should guide treatment selection when MDD recurs during the midlife years.
Although estrogen is not approved by the US Food and Drug Administration specifically for the treatment of mood disturbances, some evidence suggests that unopposed estrogen therapy has efficacy similar to that of antidepressant medications in treating depressive disorders in perimenopausal women,8-11 but it is ineffective in treating depressive disorders in postmenopausal women. Estrogen therapy also may augment the clinical response to antidepressants in midlife and older women.12,13 The data on combined HT (estrogen plus progestogen) or for different progestogens in treating depressive disorders in perimenopausal women are lacking and inconclusive.
The findings from this expert review panel demonstrate that women in the perimenopausal transition are at increased risk for depressive symptoms, major depressive episodes, and major depressive disorder. The interrelationship between symptoms of depression and menopause can complicate, co-occur, overlap, and magnify one another. Clinicians treating perimenopausal women with depression that is unresponsive to conventional antidepressant therapy should consider concurrent use of estrogen-based hormone therapy or referring the patient to a clinician comfortable doing so.

- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7-30.
- Matteson KA, Robison K, Jacoby VL. Opportunities for early detection of endometrial cancer in women with postmenopausal bleeding. JAMA Intern Med. 2018;178:1222-1223.
- van Hanegem N, Breijer MC, Khan KS, et al. Diagnostic evaluation of the endometrium in postmenopausal bleeding: an evidence-based approach. Maturitas. 2011;68:155-164.
- American College of Obstetricians and Gynecologists. ACOG Committee Opinion no. 734 summary. The role of transvaginal ultrasonography in evaluating the endometrium of women with postmenopausal bleeding. Obstet Gynecol. 2018; 131:945-946.
- Baumgart J, Nilsson K, Evers AS, et al. Sexual dysfunction in women on adjuvant endocrine therapy after breast cancer. Menopause. 2013;20:162-168.
- Chou PH, Lin CH, Cheng C, et al. Risk of depressive disorders in women undergoing hysterectomy: a population-based follow-up study. J Psychiatr Res. 2015;68:186-191.
- Wilson L, Pandeya N, Byles J, et al. Hysterectomy and incidence of depressive symptoms in midlife women: the Australian Longitudinal Study on Women's Health. Epidemiol Psychiatr Sci. 2018;27:381-392.
- Schmidt PJ, Nieman L, Danaceau MA, et al. Estrogen replacement in perimenopause-related depression: a preliminary report. Am J Obstet Gynecol. 2000;183:414-420.
- Rasgon NL, Altshuler LL, Fairbanks L. Estrogen-replacement therapy for depression. Am J Psychiatry. 2001;158:1738.
- Soares CN, Almeida OP, Joffe H, et al. Efficacy of estradiol for the treatment of major depressive disorders in perimenopausal women: a double-blind, randomized, placebo-controlled trial. Arch Gen Psychiatry. 2001;58:529-534.
- Cohen LS, Soares CN, Poitras JR, et al. Short-term use of estradiol for depression in perimenopausal and postmenopausal women: a preliminary report. Am J Psychiatry. 2003;160:1519-1522.
- Schneider LS, Small GW, Hamilton SH, et al. Estrogen replacement and response to fluoxetine in a multicenter geriatric depression trial. Fluoxetine Collaborative Study Group. Am J Geriatr Psychiatry. 1997;5:97-106.
- Schneider LS, Small GW, Clary CM. Estrogen replacement therapy and antidepressant response to sertraline in older depressed women. Am J Geriatr Psychiatry. 2001;9:393-399.
Among peri- and postmenopausal women, abnormal bleeding, breast cancer, and mood disorders represent prevalent conditions. In this Update, we discuss data from a review that provides quantitative information on the likelihood of finding endometrial cancer among women with postmenopausal bleeding (PMB). We also summarize 2 recent consensus recommendations: One addresses the clinically important but controversial issue of the treatment of genitourinary syndrome of menopause (GSM) in breast cancer survivors, and the other provides guidance on the management of depression in perimenopausal women.
Endometrial cancer is associated with a high prevalence of PMB
Clarke MA, Long BJ, Del Mar Morillo A, et al. Association of endometrial cancer risk with postmenopausal bleeding in women: a systematic review and meta-analysis. JAMA Intern Med. 2018;178:1210-1222.
Endometrial cancer is the most common gynecologic malignancy and the fourth most common cancer among US women. In recent years, the incidence of and mortality from endometrial cancer have increased.1 Despite the high prevalence of endometrial cancer, population-based screening currently is not recommended.
PMB affects up to 10% of women and can be caused by endometrial atrophy, endometrial polyps, uterine leiomyoma, and malignancy. While it is well known that PMB is a common presenting symptom of endometrial cancer, we do not have good data to guide counseling patients with PMB on the likelihood that endometrial cancer is present. Similarly, estimates are lacking regarding what proportion of women with endometrial cancer will present with PMB.
To address these 2 issues, Clarke and colleagues conducted a comprehensive systematic review and meta-analysis of the prevalence of PMB among women with endometrial cancer (sensitivity) and the risk of endometrial cancer among women with PMB (positive predictive value). The authors included 129 studies--with 34,432 women with PMB and 6,358 with endometrial cancer--in their report.
Cancer prevalence varied with HT use, geographic location
The study findings demonstrated that the prevalence of PMB in women with endometrial cancer was 90% (95% confidence interval [CI], 84%-94%), and there was no significant difference in the occurrence of PMB by cancer stage. The risk of endometrial cancer in women with PMB ranged from 0% to 48%, yielding an overall pooled estimate of 9% (95% CI, 8%-11%). As an editorialist pointed out, the risk of endometrial cancer in women with PMB is similar to that of colorectal cancer in individuals with rectal bleeding (8%) and breast cancer in women with a palpable mass (10%), supporting current guidance that recommends evaluation of women with PMB.2 Evaluating 100 women with PMB to diagnose 9 endometrial cancers does not seem excessive.
Interestingly, among women with PMB, the prevalence of endometrial cancer was significantly higher among women not using hormone therapy (HT) than among users of HT (12% and 7%, respectively). In 7 studies restricted to women with PMB and polyps (n = 2,801), the pooled risk of endometrial cancer was 3% (95% CI, 3%-4%). In an analysis stratified by geographic region, a striking difference was noted in the risk of endometrial cancer among women with PMB in North America (5%), Northern Europe (7%), and in Western Europe (13%). This finding may be explained by regional differences in the approach to evaluating PMB, cultural perceptions of PMB that can affect thresholds to present for care, and differences in risk factors between these populations.
The study had several limitations, including an inability to evaluate the number of years since menopause and the effects of body mass index. Additionally, the study did not address endometrial hyperplasia or endometrial intraepithelial neoplasia.
PMB accounts for two-thirds of all gynecologic visits among perimenopausal and postmenopausal women.3 This study revealed a 9% risk of endometrial cancer in patients experiencing PMB, which supports current practice guidelines to further evaluate and rule out endometrial cancer among all women presenting with PMB4; it also provides reassurance that targeting this high-risk group of women for early detection and prevention strategies will capture most cases of endometrial cancers. However, the relatively low positive predictive value of PMB emphasizes the need for additional triage tests with high specificity to improve management of PMB and minimize unnecessary biopsies in low-risk women.
Treating GSM in breast cancer survivors: New guidance targets QoL and sexuality
Faubion SS, Larkin LC, Stuenkel CA, et al. Management of genitourinary syndrome of menopause in women with or at high risk for breast cancer: consensus recommendations from The North American Menopause Society and The International Society for the Study of Women's Sexual Health. Menopause. 2018;25:596-608.
Given that there is little evidence addressing the safety of vaginal estrogen, other hormonal therapies, and nonprescription treatments for GSM in breast cancer survivors, many survivors with bothersome GSM symptoms are not appropriately treated.
Continue to: Expert panel creates evidence-based guidance...
Expert panel creates evidence-based guidance
Against this backdrop, The North American Menopause Society and the International Society for the Study of Women's Sexual Health convened a group comprised of menopause specialists (ObGyns, internists, and nurse practitioners), specialists in sexuality, medical oncologists specializing in breast cancer, and a psychologist to create evidence-based interdisciplinary consensus guidelines for enhancing quality of life and sexuality for breast cancer survivors with GSM.
Measures to help enhance quality of life and sexuality
The group's key recommendations for clinicians include:
- Sexual function and quality of life (QoL) should be assessed in all women with or at high risk for breast cancer.
- Management of GSM should be individualized based on shared decision-making involving the patient and her oncologist.
- Initial treatment options include:
—over-the-counter vaginal moisturizers used several times weekly on a regular basis
—lubricants used with intercourse
—vaginal dilator therapy
—pelvic floor physical therapy.
- Low-dose vaginal estrogen therapy may be appropriate for select women who have been treated for breast cancer:
—With use of vaginal estrogen, serum estradiol levels remain in the postmenopausal range.
—Based on limited data, use of vaginal estrogen is associated with a minimal risk for recurrence of breast cancer.
—Because their use is associated with the lowest serum estradiol levels, vaginal tablets, rings, or inserts may be preferable to creams.
—Decisions regarding use of vaginal estrogen in breast cancer survivors should involve the woman's oncologist. Appropriate candidates for off-label use of vaginal estrogen may be survivors:
–who are at relatively low risk for recurrence
–with hormone receptor-negative disease
–using tamoxifen rather than an AI
–who are particularly concerned about quality of life.
—Given that AIs prevent recurrence by lowering estrogen levels, oncologists may be reluctant to consider use of vaginal estrogen in survivors using adjuvant agents.
—With respect to use of vaginal estrogen, oncologists may be more comfortable with use in patients taking tamoxifen.
- Neither intravaginal dehydroepiandrosterone (DHEA; prasterone) nor the oral selective estrogen receptor modulator ospemifene has been studied in breast cancer survivors.
In women with metastatic disease, QoL, comfort, and sexual intimacy are key considerations when weighing potential therapies; optimal choices will vary with probability of long-term survival.
Although more data addressing the safety of vaginal estrogen as well as prasterone and ospemifene in breast cancer survivors clearly are needed, these guidelines should help clinicians who care for breast cancer survivors with GSM.
Framework provided for managing depressive disorders in perimenopausal women
Maki PM, Kornstein SG, Joffe H, et al; Board of Trustees for The North American Menopause Society (NAMS) and the Women and Mood Disorders Task Force of the National Network of Depression Centers. Guidelines for the evaluation and treatment of perimenopausal depression: summary and recommendations. Menopause. 2018;25:1069-1085.
Although perimenopausal women are more susceptible to the development of depressive symptoms and major depressive episodes (MDE), there is a lack of consensus regarding how to evaluate and treat depression in women during the menopausal transition and postmenopausal period.
Recently, an expert panel comprised of representatives from The North American Menopause Society and the National Network of Depression Centers Women and Mood Disorders Task Group developed clinical guidelines addressing epidemiology, clinical presentation, therapeutic effects of antidepressants, effects of HT, and efficacy of other therapies. Here we provide a summary of the expert panel's findings and guidelines.
Continue to: Certain factors are associated with higher risk for depression...
Certain factors are associated with higher risk for depression
The perimenopause represents a time of increased risk for depressive symptoms and major depressive disorder (MDD), even in women with no prior history of depression. Several characteristics and health factors are associated with the increased risk during the menopause transition. These include a prior history of MDD, current antidepressant use, anxiety, premenstrual depressive symptoms, African American race, high body mass index, younger age, social isolation, upsetting life events, and menopausal sleep disturbances.
Although data are inconclusive on whether surgical menopause increases or decreases the risk for developing depression compared with women who transition through menopause naturally, recent studies show an elevated risk of depression in women following hysterectomy with and without oophorectomy.6,7
Menopausal and depressive symptoms can overlap
Midlife depression presents with classic depressive symptoms that commonly occur in combination with menopausal symptoms, including vasomotor symptoms, sleep and sexual disturbances, and weight and energy changes. These menopausal symptoms can complicate, co-occur, and overlap with the clinical presentation of depression.
Conversely, depression may affect an individual's judgment of the degree of bother from menopausal somatic symptoms, thereby further magnifying the effect of symptoms on quality of life. The interrelationship between depressive symptoms and menopausal symptoms may pose a challenge when attempting to parse out contributing etiologies, relative contributions of each etiology, and the potential additive effects.
Diagnosis and treatment options
Diagnosis involves identifying the menopausal stage, assessing for co-existing psychiatric and menopause symptoms, appreciating the psychosocial factors common in midlife, and considering the differential diagnosis. Validated screening instruments can be helpful. Although a menopause-specific mood disorder scale does not yet exist, several general validated screening measures, such as the Patient Health Questionnaire-9, or PHQ-9, can be used for categorical determination of mood disorder diagnoses during the menopause transition.
Antidepressants, cognitive-behavioral therapy, and other psychotherapies are considered first-line treatments for perimenopausal major depressive episodes. Only desvenlafaxine has been studied in large randomized placebo-controlled trials and has proven efficacious for the treatment of MDD in perimenopausal and postmenopausal women.
A number of small open-label studies of other selective serotonin reuptake inhibitors (SSRIs), serotonin norepinephrine reuptake inhibitors (SNRIs), and mirtazapine to treat MDD in perimenopausal and postmenopausal women have demonstrated a positive effect on mood, and several SSRIs and SNRIs also have the added benefit of improving menopause-related symptoms.
In women with a history of MDD, a prior adequate response to a particular antidepressant should guide treatment selection when MDD recurs during the midlife years.
Although estrogen is not approved by the US Food and Drug Administration specifically for the treatment of mood disturbances, some evidence suggests that unopposed estrogen therapy has efficacy similar to that of antidepressant medications in treating depressive disorders in perimenopausal women,8-11 but it is ineffective in treating depressive disorders in postmenopausal women. Estrogen therapy also may augment the clinical response to antidepressants in midlife and older women.12,13 The data on combined HT (estrogen plus progestogen) or for different progestogens in treating depressive disorders in perimenopausal women are lacking and inconclusive.
The findings from this expert review panel demonstrate that women in the perimenopausal transition are at increased risk for depressive symptoms, major depressive episodes, and major depressive disorder. The interrelationship between symptoms of depression and menopause can complicate, co-occur, overlap, and magnify one another. Clinicians treating perimenopausal women with depression that is unresponsive to conventional antidepressant therapy should consider concurrent use of estrogen-based hormone therapy or referring the patient to a clinician comfortable doing so.

Among peri- and postmenopausal women, abnormal bleeding, breast cancer, and mood disorders represent prevalent conditions. In this Update, we discuss data from a review that provides quantitative information on the likelihood of finding endometrial cancer among women with postmenopausal bleeding (PMB). We also summarize 2 recent consensus recommendations: One addresses the clinically important but controversial issue of the treatment of genitourinary syndrome of menopause (GSM) in breast cancer survivors, and the other provides guidance on the management of depression in perimenopausal women.
Endometrial cancer is associated with a high prevalence of PMB
Clarke MA, Long BJ, Del Mar Morillo A, et al. Association of endometrial cancer risk with postmenopausal bleeding in women: a systematic review and meta-analysis. JAMA Intern Med. 2018;178:1210-1222.
Endometrial cancer is the most common gynecologic malignancy and the fourth most common cancer among US women. In recent years, the incidence of and mortality from endometrial cancer have increased.1 Despite the high prevalence of endometrial cancer, population-based screening currently is not recommended.
PMB affects up to 10% of women and can be caused by endometrial atrophy, endometrial polyps, uterine leiomyoma, and malignancy. While it is well known that PMB is a common presenting symptom of endometrial cancer, we do not have good data to guide counseling patients with PMB on the likelihood that endometrial cancer is present. Similarly, estimates are lacking regarding what proportion of women with endometrial cancer will present with PMB.
To address these 2 issues, Clarke and colleagues conducted a comprehensive systematic review and meta-analysis of the prevalence of PMB among women with endometrial cancer (sensitivity) and the risk of endometrial cancer among women with PMB (positive predictive value). The authors included 129 studies--with 34,432 women with PMB and 6,358 with endometrial cancer--in their report.
Cancer prevalence varied with HT use, geographic location
The study findings demonstrated that the prevalence of PMB in women with endometrial cancer was 90% (95% confidence interval [CI], 84%-94%), and there was no significant difference in the occurrence of PMB by cancer stage. The risk of endometrial cancer in women with PMB ranged from 0% to 48%, yielding an overall pooled estimate of 9% (95% CI, 8%-11%). As an editorialist pointed out, the risk of endometrial cancer in women with PMB is similar to that of colorectal cancer in individuals with rectal bleeding (8%) and breast cancer in women with a palpable mass (10%), supporting current guidance that recommends evaluation of women with PMB.2 Evaluating 100 women with PMB to diagnose 9 endometrial cancers does not seem excessive.
Interestingly, among women with PMB, the prevalence of endometrial cancer was significantly higher among women not using hormone therapy (HT) than among users of HT (12% and 7%, respectively). In 7 studies restricted to women with PMB and polyps (n = 2,801), the pooled risk of endometrial cancer was 3% (95% CI, 3%-4%). In an analysis stratified by geographic region, a striking difference was noted in the risk of endometrial cancer among women with PMB in North America (5%), Northern Europe (7%), and in Western Europe (13%). This finding may be explained by regional differences in the approach to evaluating PMB, cultural perceptions of PMB that can affect thresholds to present for care, and differences in risk factors between these populations.
The study had several limitations, including an inability to evaluate the number of years since menopause and the effects of body mass index. Additionally, the study did not address endometrial hyperplasia or endometrial intraepithelial neoplasia.
PMB accounts for two-thirds of all gynecologic visits among perimenopausal and postmenopausal women.3 This study revealed a 9% risk of endometrial cancer in patients experiencing PMB, which supports current practice guidelines to further evaluate and rule out endometrial cancer among all women presenting with PMB4; it also provides reassurance that targeting this high-risk group of women for early detection and prevention strategies will capture most cases of endometrial cancers. However, the relatively low positive predictive value of PMB emphasizes the need for additional triage tests with high specificity to improve management of PMB and minimize unnecessary biopsies in low-risk women.
Treating GSM in breast cancer survivors: New guidance targets QoL and sexuality
Faubion SS, Larkin LC, Stuenkel CA, et al. Management of genitourinary syndrome of menopause in women with or at high risk for breast cancer: consensus recommendations from The North American Menopause Society and The International Society for the Study of Women's Sexual Health. Menopause. 2018;25:596-608.
Given that there is little evidence addressing the safety of vaginal estrogen, other hormonal therapies, and nonprescription treatments for GSM in breast cancer survivors, many survivors with bothersome GSM symptoms are not appropriately treated.
Continue to: Expert panel creates evidence-based guidance...
Expert panel creates evidence-based guidance
Against this backdrop, The North American Menopause Society and the International Society for the Study of Women's Sexual Health convened a group comprised of menopause specialists (ObGyns, internists, and nurse practitioners), specialists in sexuality, medical oncologists specializing in breast cancer, and a psychologist to create evidence-based interdisciplinary consensus guidelines for enhancing quality of life and sexuality for breast cancer survivors with GSM.
Measures to help enhance quality of life and sexuality
The group's key recommendations for clinicians include:
- Sexual function and quality of life (QoL) should be assessed in all women with or at high risk for breast cancer.
- Management of GSM should be individualized based on shared decision-making involving the patient and her oncologist.
- Initial treatment options include:
—over-the-counter vaginal moisturizers used several times weekly on a regular basis
—lubricants used with intercourse
—vaginal dilator therapy
—pelvic floor physical therapy.
- Low-dose vaginal estrogen therapy may be appropriate for select women who have been treated for breast cancer:
—With use of vaginal estrogen, serum estradiol levels remain in the postmenopausal range.
—Based on limited data, use of vaginal estrogen is associated with a minimal risk for recurrence of breast cancer.
—Because their use is associated with the lowest serum estradiol levels, vaginal tablets, rings, or inserts may be preferable to creams.
—Decisions regarding use of vaginal estrogen in breast cancer survivors should involve the woman's oncologist. Appropriate candidates for off-label use of vaginal estrogen may be survivors:
–who are at relatively low risk for recurrence
–with hormone receptor-negative disease
–using tamoxifen rather than an AI
–who are particularly concerned about quality of life.
—Given that AIs prevent recurrence by lowering estrogen levels, oncologists may be reluctant to consider use of vaginal estrogen in survivors using adjuvant agents.
—With respect to use of vaginal estrogen, oncologists may be more comfortable with use in patients taking tamoxifen.
- Neither intravaginal dehydroepiandrosterone (DHEA; prasterone) nor the oral selective estrogen receptor modulator ospemifene has been studied in breast cancer survivors.
In women with metastatic disease, QoL, comfort, and sexual intimacy are key considerations when weighing potential therapies; optimal choices will vary with probability of long-term survival.
Although more data addressing the safety of vaginal estrogen as well as prasterone and ospemifene in breast cancer survivors clearly are needed, these guidelines should help clinicians who care for breast cancer survivors with GSM.
Framework provided for managing depressive disorders in perimenopausal women
Maki PM, Kornstein SG, Joffe H, et al; Board of Trustees for The North American Menopause Society (NAMS) and the Women and Mood Disorders Task Force of the National Network of Depression Centers. Guidelines for the evaluation and treatment of perimenopausal depression: summary and recommendations. Menopause. 2018;25:1069-1085.
Although perimenopausal women are more susceptible to the development of depressive symptoms and major depressive episodes (MDE), there is a lack of consensus regarding how to evaluate and treat depression in women during the menopausal transition and postmenopausal period.
Recently, an expert panel comprised of representatives from The North American Menopause Society and the National Network of Depression Centers Women and Mood Disorders Task Group developed clinical guidelines addressing epidemiology, clinical presentation, therapeutic effects of antidepressants, effects of HT, and efficacy of other therapies. Here we provide a summary of the expert panel's findings and guidelines.
Continue to: Certain factors are associated with higher risk for depression...
Certain factors are associated with higher risk for depression
The perimenopause represents a time of increased risk for depressive symptoms and major depressive disorder (MDD), even in women with no prior history of depression. Several characteristics and health factors are associated with the increased risk during the menopause transition. These include a prior history of MDD, current antidepressant use, anxiety, premenstrual depressive symptoms, African American race, high body mass index, younger age, social isolation, upsetting life events, and menopausal sleep disturbances.
Although data are inconclusive on whether surgical menopause increases or decreases the risk for developing depression compared with women who transition through menopause naturally, recent studies show an elevated risk of depression in women following hysterectomy with and without oophorectomy.6,7
Menopausal and depressive symptoms can overlap
Midlife depression presents with classic depressive symptoms that commonly occur in combination with menopausal symptoms, including vasomotor symptoms, sleep and sexual disturbances, and weight and energy changes. These menopausal symptoms can complicate, co-occur, and overlap with the clinical presentation of depression.
Conversely, depression may affect an individual's judgment of the degree of bother from menopausal somatic symptoms, thereby further magnifying the effect of symptoms on quality of life. The interrelationship between depressive symptoms and menopausal symptoms may pose a challenge when attempting to parse out contributing etiologies, relative contributions of each etiology, and the potential additive effects.
Diagnosis and treatment options
Diagnosis involves identifying the menopausal stage, assessing for co-existing psychiatric and menopause symptoms, appreciating the psychosocial factors common in midlife, and considering the differential diagnosis. Validated screening instruments can be helpful. Although a menopause-specific mood disorder scale does not yet exist, several general validated screening measures, such as the Patient Health Questionnaire-9, or PHQ-9, can be used for categorical determination of mood disorder diagnoses during the menopause transition.
Antidepressants, cognitive-behavioral therapy, and other psychotherapies are considered first-line treatments for perimenopausal major depressive episodes. Only desvenlafaxine has been studied in large randomized placebo-controlled trials and has proven efficacious for the treatment of MDD in perimenopausal and postmenopausal women.
A number of small open-label studies of other selective serotonin reuptake inhibitors (SSRIs), serotonin norepinephrine reuptake inhibitors (SNRIs), and mirtazapine to treat MDD in perimenopausal and postmenopausal women have demonstrated a positive effect on mood, and several SSRIs and SNRIs also have the added benefit of improving menopause-related symptoms.
In women with a history of MDD, a prior adequate response to a particular antidepressant should guide treatment selection when MDD recurs during the midlife years.
Although estrogen is not approved by the US Food and Drug Administration specifically for the treatment of mood disturbances, some evidence suggests that unopposed estrogen therapy has efficacy similar to that of antidepressant medications in treating depressive disorders in perimenopausal women,8-11 but it is ineffective in treating depressive disorders in postmenopausal women. Estrogen therapy also may augment the clinical response to antidepressants in midlife and older women.12,13 The data on combined HT (estrogen plus progestogen) or for different progestogens in treating depressive disorders in perimenopausal women are lacking and inconclusive.
The findings from this expert review panel demonstrate that women in the perimenopausal transition are at increased risk for depressive symptoms, major depressive episodes, and major depressive disorder. The interrelationship between symptoms of depression and menopause can complicate, co-occur, overlap, and magnify one another. Clinicians treating perimenopausal women with depression that is unresponsive to conventional antidepressant therapy should consider concurrent use of estrogen-based hormone therapy or referring the patient to a clinician comfortable doing so.

- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7-30.
- Matteson KA, Robison K, Jacoby VL. Opportunities for early detection of endometrial cancer in women with postmenopausal bleeding. JAMA Intern Med. 2018;178:1222-1223.
- van Hanegem N, Breijer MC, Khan KS, et al. Diagnostic evaluation of the endometrium in postmenopausal bleeding: an evidence-based approach. Maturitas. 2011;68:155-164.
- American College of Obstetricians and Gynecologists. ACOG Committee Opinion no. 734 summary. The role of transvaginal ultrasonography in evaluating the endometrium of women with postmenopausal bleeding. Obstet Gynecol. 2018; 131:945-946.
- Baumgart J, Nilsson K, Evers AS, et al. Sexual dysfunction in women on adjuvant endocrine therapy after breast cancer. Menopause. 2013;20:162-168.
- Chou PH, Lin CH, Cheng C, et al. Risk of depressive disorders in women undergoing hysterectomy: a population-based follow-up study. J Psychiatr Res. 2015;68:186-191.
- Wilson L, Pandeya N, Byles J, et al. Hysterectomy and incidence of depressive symptoms in midlife women: the Australian Longitudinal Study on Women's Health. Epidemiol Psychiatr Sci. 2018;27:381-392.
- Schmidt PJ, Nieman L, Danaceau MA, et al. Estrogen replacement in perimenopause-related depression: a preliminary report. Am J Obstet Gynecol. 2000;183:414-420.
- Rasgon NL, Altshuler LL, Fairbanks L. Estrogen-replacement therapy for depression. Am J Psychiatry. 2001;158:1738.
- Soares CN, Almeida OP, Joffe H, et al. Efficacy of estradiol for the treatment of major depressive disorders in perimenopausal women: a double-blind, randomized, placebo-controlled trial. Arch Gen Psychiatry. 2001;58:529-534.
- Cohen LS, Soares CN, Poitras JR, et al. Short-term use of estradiol for depression in perimenopausal and postmenopausal women: a preliminary report. Am J Psychiatry. 2003;160:1519-1522.
- Schneider LS, Small GW, Hamilton SH, et al. Estrogen replacement and response to fluoxetine in a multicenter geriatric depression trial. Fluoxetine Collaborative Study Group. Am J Geriatr Psychiatry. 1997;5:97-106.
- Schneider LS, Small GW, Clary CM. Estrogen replacement therapy and antidepressant response to sertraline in older depressed women. Am J Geriatr Psychiatry. 2001;9:393-399.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7-30.
- Matteson KA, Robison K, Jacoby VL. Opportunities for early detection of endometrial cancer in women with postmenopausal bleeding. JAMA Intern Med. 2018;178:1222-1223.
- van Hanegem N, Breijer MC, Khan KS, et al. Diagnostic evaluation of the endometrium in postmenopausal bleeding: an evidence-based approach. Maturitas. 2011;68:155-164.
- American College of Obstetricians and Gynecologists. ACOG Committee Opinion no. 734 summary. The role of transvaginal ultrasonography in evaluating the endometrium of women with postmenopausal bleeding. Obstet Gynecol. 2018; 131:945-946.
- Baumgart J, Nilsson K, Evers AS, et al. Sexual dysfunction in women on adjuvant endocrine therapy after breast cancer. Menopause. 2013;20:162-168.
- Chou PH, Lin CH, Cheng C, et al. Risk of depressive disorders in women undergoing hysterectomy: a population-based follow-up study. J Psychiatr Res. 2015;68:186-191.
- Wilson L, Pandeya N, Byles J, et al. Hysterectomy and incidence of depressive symptoms in midlife women: the Australian Longitudinal Study on Women's Health. Epidemiol Psychiatr Sci. 2018;27:381-392.
- Schmidt PJ, Nieman L, Danaceau MA, et al. Estrogen replacement in perimenopause-related depression: a preliminary report. Am J Obstet Gynecol. 2000;183:414-420.
- Rasgon NL, Altshuler LL, Fairbanks L. Estrogen-replacement therapy for depression. Am J Psychiatry. 2001;158:1738.
- Soares CN, Almeida OP, Joffe H, et al. Efficacy of estradiol for the treatment of major depressive disorders in perimenopausal women: a double-blind, randomized, placebo-controlled trial. Arch Gen Psychiatry. 2001;58:529-534.
- Cohen LS, Soares CN, Poitras JR, et al. Short-term use of estradiol for depression in perimenopausal and postmenopausal women: a preliminary report. Am J Psychiatry. 2003;160:1519-1522.
- Schneider LS, Small GW, Hamilton SH, et al. Estrogen replacement and response to fluoxetine in a multicenter geriatric depression trial. Fluoxetine Collaborative Study Group. Am J Geriatr Psychiatry. 1997;5:97-106.
- Schneider LS, Small GW, Clary CM. Estrogen replacement therapy and antidepressant response to sertraline in older depressed women. Am J Geriatr Psychiatry. 2001;9:393-399.