User login
ASCO clinical practice guideline update incorporates Oncotype DX
In women with hormone receptor–positive, axillary node–negative breast cancer with Oncotype DX recurrence scores of less than 26, there is minimal to no benefit from chemotherapy, particularly for those greater than age 50 years, according to a clinical practice guideline update by the American Society of Clinical Oncology.
Furthermore, endocrine therapy alone may be offered for patients greater than age 50 years whose tumors have recurrence scores of less than 26, wrote Fabrice Andre, MD, PhD, of Paris Sud University and associates on the expert panel in the Journal of Clinical Oncology.
The panel members reviewed recently published findings from the Trial Assigning Individualized Options for Treatment (TAILORx), which evaluated the clinical utility of the Oncotype DX assay in women with early-stage invasive breast cancer.
“This focused update reviews and analyzes new data regarding these recommendations while applying the same criteria of clinical utility as described in the 2016 guideline,” they wrote.
The expert panel provided recommendations on how to integrate the results of the TAILORx study into clinical practice.
“For patients age 50 years or younger with Oncotype DX recurrence scores of 16-25, clinicians may offer chemoendocrine therapy” the panel wrote. “Patients with Oncotype DX recurrence scores of greater than 30 should be considered candidates for chemoendocrine therapy.”
In addition, on the basis of consensus they recommended that chemoendocrine therapy could be offered to patients with recurrence scores of 26-30.
The panel acknowledged that relevant literature on the use of Oncotype DX in this population will be reviewed over the upcoming months to address anticipated practice deviation related to biomarker testing.
More information on the guidelines is available on the ASCO website.
The study was funded by ASCO. The authors reported financial affiliations with AstraZeneca, Eli Lilly, GlaxoSmithKline, Merck, Novartis, Roche, and several others.
SOURCE: Andre F et al. J Clin Oncol. 2019 May 31. doi: 10.1200/JCO.19.00945.
In women with hormone receptor–positive, axillary node–negative breast cancer with Oncotype DX recurrence scores of less than 26, there is minimal to no benefit from chemotherapy, particularly for those greater than age 50 years, according to a clinical practice guideline update by the American Society of Clinical Oncology.
Furthermore, endocrine therapy alone may be offered for patients greater than age 50 years whose tumors have recurrence scores of less than 26, wrote Fabrice Andre, MD, PhD, of Paris Sud University and associates on the expert panel in the Journal of Clinical Oncology.
The panel members reviewed recently published findings from the Trial Assigning Individualized Options for Treatment (TAILORx), which evaluated the clinical utility of the Oncotype DX assay in women with early-stage invasive breast cancer.
“This focused update reviews and analyzes new data regarding these recommendations while applying the same criteria of clinical utility as described in the 2016 guideline,” they wrote.
The expert panel provided recommendations on how to integrate the results of the TAILORx study into clinical practice.
“For patients age 50 years or younger with Oncotype DX recurrence scores of 16-25, clinicians may offer chemoendocrine therapy” the panel wrote. “Patients with Oncotype DX recurrence scores of greater than 30 should be considered candidates for chemoendocrine therapy.”
In addition, on the basis of consensus they recommended that chemoendocrine therapy could be offered to patients with recurrence scores of 26-30.
The panel acknowledged that relevant literature on the use of Oncotype DX in this population will be reviewed over the upcoming months to address anticipated practice deviation related to biomarker testing.
More information on the guidelines is available on the ASCO website.
The study was funded by ASCO. The authors reported financial affiliations with AstraZeneca, Eli Lilly, GlaxoSmithKline, Merck, Novartis, Roche, and several others.
SOURCE: Andre F et al. J Clin Oncol. 2019 May 31. doi: 10.1200/JCO.19.00945.
In women with hormone receptor–positive, axillary node–negative breast cancer with Oncotype DX recurrence scores of less than 26, there is minimal to no benefit from chemotherapy, particularly for those greater than age 50 years, according to a clinical practice guideline update by the American Society of Clinical Oncology.
Furthermore, endocrine therapy alone may be offered for patients greater than age 50 years whose tumors have recurrence scores of less than 26, wrote Fabrice Andre, MD, PhD, of Paris Sud University and associates on the expert panel in the Journal of Clinical Oncology.
The panel members reviewed recently published findings from the Trial Assigning Individualized Options for Treatment (TAILORx), which evaluated the clinical utility of the Oncotype DX assay in women with early-stage invasive breast cancer.
“This focused update reviews and analyzes new data regarding these recommendations while applying the same criteria of clinical utility as described in the 2016 guideline,” they wrote.
The expert panel provided recommendations on how to integrate the results of the TAILORx study into clinical practice.
“For patients age 50 years or younger with Oncotype DX recurrence scores of 16-25, clinicians may offer chemoendocrine therapy” the panel wrote. “Patients with Oncotype DX recurrence scores of greater than 30 should be considered candidates for chemoendocrine therapy.”
In addition, on the basis of consensus they recommended that chemoendocrine therapy could be offered to patients with recurrence scores of 26-30.
The panel acknowledged that relevant literature on the use of Oncotype DX in this population will be reviewed over the upcoming months to address anticipated practice deviation related to biomarker testing.
More information on the guidelines is available on the ASCO website.
The study was funded by ASCO. The authors reported financial affiliations with AstraZeneca, Eli Lilly, GlaxoSmithKline, Merck, Novartis, Roche, and several others.
SOURCE: Andre F et al. J Clin Oncol. 2019 May 31. doi: 10.1200/JCO.19.00945.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
What to do when a patient presents with breast pain
Breast pain is one of the most common breast-related patient complaints and is found to affect at least 50% of the female population.1 Most cases are self-limiting and are related to hormonal and normal fibrocystic changes. The median age of onset of symptoms is 36 years, with most women experiencing pain for 5 to 12 years.2 Because the cause of breast pain is not always clear, its presence can produce anxiety in patients and physicians over the possibility of underlying malignancy. Although breast cancer is not associated with breast pain, many patients presenting with pain are referred for diagnostic imaging (usually with negative results). The majority of women with mastalgia and normal clinical examination findings can be reassured with education about the many benign causes of breast pain.
What are causes of breast pain without an imaging abnormality?
Hormones. Mastalgia can be focal or generalized and is mostly due to hormonal changes. Elevated estrogen can stimulate the growth of breast tissue, which is known as epithelial hyperplasia.3 Fluctuations in hormone levels can occur in perimenopausal women in their forties and can result in new symptoms of breast pain.4 Sometimes starting a new contraceptive medication or hormone replacement therapy can exacerbate the pain. Switching brands or medications may help. Another cause of mastalgia may be elevated prolactin levels, with hypothalamic-pituitary dysfunction.5,6
Diet. There is evidence to link a high-fat diet with breast pain. The pain has been shown to improve when lipid intake is reduced and high- and low-density lipoprotein cholesterol levels are normalized. As estrogen is a steroid hormone that can be synthesized from lipids and fatty acids, elevated lipid metabolism can increase estrogen levels and exacerbate breast pain symptoms.7,8 Essential fatty acids, such as evening primrose oil and vitamin E, have been used to treat mastalgia because they reduce inflammation in fatty breast tissue through the prostaglandin pathway.9,10
Caffeine. Methylxanthines can be found in coffee, tea, and chocolate and can aggravate mastalgia by enhancing the cyclin adenosine monophosphate (cAMP) pathway. This pathway stimulates cellular proliferation and fibrocystic changes which in turn can exacerbate breast pain.11
Smoking. In my clinical practice I have clearly noted a higher incidence of breast pain in patients who smoke. The pain tends to improve significantly when the patient quits or even cuts back on smoking. The exact reasons for smoking’s effects on breast pain are not well known; however, they are thought to be related to acceleration of the cAMP pathway.
Large breast size. Very large breasts will strain and weaken the suspensory ligaments, leading to pain and discomfort. It has been shown that wearing a supportive sports bra during episodes of breast pain is effective.
Types of breast pain
Cyclical
Women with fibrocystic breasts tend to experience more breast pain. Breast sensitivity can be localized to the upper outer quadrants or to the nipple and sub-areolar area. It also can be generalized. The pain tends to peak with ovulation, improve with menses, and to recur every few weeks. Patients who have had partial hysterectomy (with ovaries in situ) or endometrial ablation will be unable to correlate their symptoms to menstruation. Therefore, women are encouraged to keep a diary or calendar of their symptoms to detect any correlation with their ovarian cycle. Such correlation is reassuring.
Continue to: Noncyclical...
Noncyclical
Noncyclical breast pain is not associated with the menstrual cycle and can be unilateral or bilateral. Providers should perform a good history of patients presenting with noncyclicalbreast pain, to include character, onset, duration, location, radiation, alleviating, and aggravating factors. A physical examination may elicit point tenderness at the chest by pushing the breast tissue off of the chest wall while the patient is in supine position and pressing directly over the ribs. Lack of tenderness on palpation of the breast parenchyma, but pain on the chest wall, points to a musculoskeletal etiology. Chest wall pain may be related to muscle spasm or muscle strain, trauma, rib fracture, or costochondritis (Tietze syndrome). Finally, based on history of review of systems and physical examination, referred pain from biliary or cardiac etiology should be considered.
When breast pain occurs with skin changes
Skin changes usually have an underlying pathology. Infectious processes, such as infected epidermal inclusion cyst, hidradenitis of the cleavage and inframammary crease, or breast abscess will present with pain and induration with an acute onset of 5 to 10 days. Large pendulous breasts may develop yeast infection at the inframammary crease. Chronic infectious irritation can lead to hyperpigmentation of that area. Eczema or contact dermatitis frequently can affect the areola and become confused with Paget disease (ductal carcinoma in situ of the nipple). With Paget, the excoriation always starts at the nipple and can then spread to the areola. However, with dermatitis, the rash begins on the peri-areolar skin, without affecting the nipple itself.
When breast pain occurs with nipple discharge
Breast pain with nipple discharge usually is bilateral and more common in patients with significant fibrocystic changes who smoke. If the nipple discharge is bilateral, serous and non-bloody, and multiduct, it is considered benign and physiologic. Physiologic nipple discharge can be multifactorial and hormonal. It may be related to thyroid disorders or medications such as antidepressants, selective serotonin reuptake inhibitors (SSRIs), mood stabilizers, or antipsychotics. The only nipple discharge that is considered pathologic is unilateral spontaneous bloody discharge for which diagnostic imaging and breast surgeon referral is indicated. Women should be discouraged from self-expressing their nipples, as 80% will experience serous nipple discharge upon manual self-expression.
Breast pain is not associated with breast cancer. Most breast cancers do not hurt; they present as firm, painless masses. However, when a woman notices pain in her breast, her first concern is breast cancer. This concern is re-enforced by the medical provider whose first impulse is to order diagnostic imaging. Yet less than 3% of breast cancers are associated with breast pain.
There have been multiple published retrospective and prospective radiologic studies about the utility of breast imaging in women with breast pain without a palpable mass. All of the studies have demonstrated that breast imaging with mammography and ultrasonography in these patients yields mostly negative or benign findings. The incidence of breast cancer during imaging work-up in women with breast pain and no clinical abnormality is only 0.4% to 1.8%.1-3 Some patients may develop future subsequent breast cancer in the symptomatic breast. But this is considered incidental and possibly related to increased cell turnover related to fibrocystic changes. Breast imaging for evaluation of breast pain only provides reassurance to the physician. The patient's reassurance will come from a medical explanation for the symptoms and advice on symptom management from the provider.
Researchers from MD Anderson Cancer Center reported imaging findings and cost analysis for 799 patients presenting with breast pain from 3 large network community-based breast imaging centers in 2014. Breast ultrasound was the initial imaging modality for women younger than age 30. Digital mammography (sometimes with tomosynthesis) was used for those older than age 30 that had not had a mammogram in the last 6 months. Breast magnetic resonance imaging was performed only when ordered by the referring physician. Most of the patients presented for diagnostic imaging, and 95% had negative findings and 5% had a benign finding. Only 1 patient was found to have an incidental cancer in the contralateral breast, which was detected by tomosynthesis. The cost of breast imaging was $87,322 in younger women and $152,732 in women older than age 40, representing overutilization of health care resources and no association between breast pain and breast cancer.4
References
- Chetlan AL, Kapoor MM, Watts MR. Mastalgia: imaging work-up appropriateness. Acad Radiol. 2017;24:345-349.
- Arslan M, Kücükerdem HS, Can H, et al. Retrospective analysis of women with only mastalgia. J Breast Health. 2016;12:151-154.
- Noroozian M, Stein LF, Gaetke-Udager K, et al. Long-term clinical outcomes in women with breast pain in the absence of additional clinical findings: Mammography remains indicated. Breast Cancer Res Treat. 2015;149:417-424.
- Kushwaha AC, Shin K, Kalambo M, et al. Overutilization of health care resources for breast pain. AJR Am J Roentgenol. 2018; 211:217-223.
Management of mastalgia
Appropriate breast pain management begins with a good history and physical examination. The decision to perform imaging should depend on clinical exam findings and not on symptoms of breast pain. If there is a palpable mass, then breast imaging and possible biopsy is appropriate. However, if clinical exam is normal, there is no indication for breast imaging in low-risk women under the age of 40 whose only symptom is breast pain. Women older than age 40 can undergo diagnostic imaging, if they have not had a negative screening mammogram in the past year.
Breast pain with abnormal clinical exam
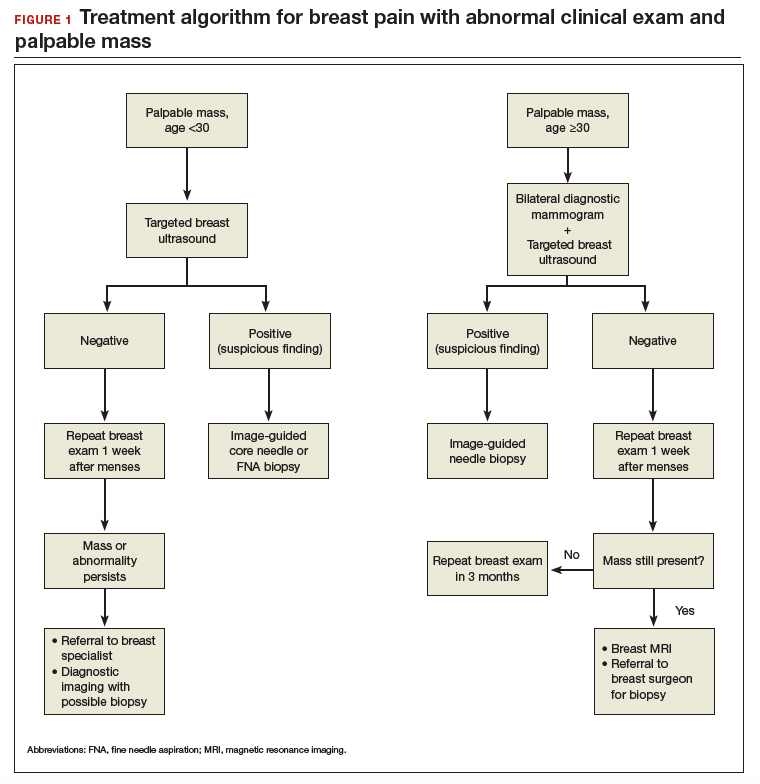
In the patient who is younger than age 30 with a palpable mass. For this patient order targeted breast ultrasound (US) (FIGURE 1). If results are negative, repeat the clinical examination 1 week after menses. If the mass is persistent, refer the patient to a breast surgeon. If diagnostic imaging results are negative, consider breast MRI, especially if there is a strong family history of breast cancer.
In the patient who is aged 30 and older with a palpable mass. For this patient, bilateral diagnostic mammogram and US are in order. The testing is best performed 1 week after menses to reduce false-positive findings. If imaging is negative and the patient still has a clinically suspicious finding or mass, refer her to a breast surgeon and consider breast MRI. At this point if there is a persistent firm dominant mass, a biopsy is indicated as part of the triple test. If the mass resolves with menses, the patient can be reassured that the cause is most likely benign, with clinical examination repeated in 3 months.
Continue to: Breast pain and normal clinical exam...
Breast pain and normal clinical exam
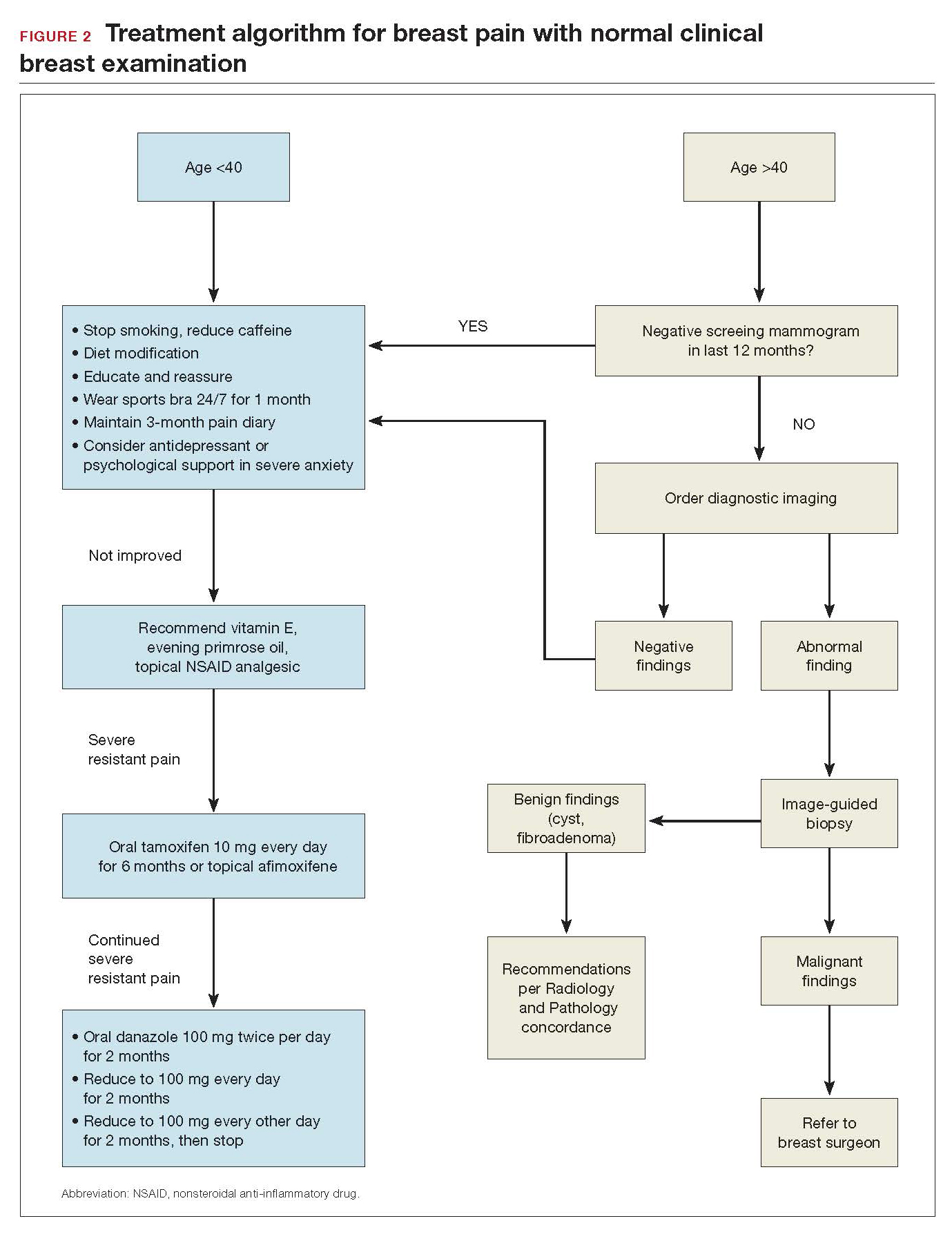
When women who report breast pain have normal clinical examination findings (and have a negative screening mammogram in the past 12 months if older than age 40), there are several management strategies you can offer (FIGURE 2).
Reassurance and education. The majority of women with breast pain can be managed with reassurance and education, which are often sufficient to reduce their anxieties.
Supportive bra. The most effective intervention is to wear and sleep in a well-fitted supportive sports bra for 4 to 12 weeks. In a nonrandomized single-center trial of danazol versus sports bra, 85% of women reported relief of their breast pain with bra alone (vs 58% with danazol).12 A supportive bra is the first-line management of mastalgia (Level II evidence).
Symptom diary/calendar. Many women do not know whether or not their symptoms correspond to their ovarian cycle or are related to hormonal fluctuations. Therefore, it is reassuring and informative for them to keep a calendar or a diary of their symptoms to determine whether their symptoms occur or are exacerbated in a cyclical pattern.
Diet and lifestyle modification. Women should avoid caffeine (especially when having pain). Studies on methylxanthines have demonstrated some symptom relief with reducing caffeine intake.11,13 One cup of coffee or tea per day most likely will not make a difference. However, if a woman is drinking large quantities of caffeinated beverages throughout the day, it will very likely improve her breast pain if she cuts back. This is especially true during the times of exacerbated pain prior to her menses.
In addition, recommend reduced dietary fat (overall good health). This is good advice for any patient. There were 2 small studies that showed improvement in breast pain with a 15% reduction in dietary fat.7,8
Finally, advise that patients stop smoking. Smoking aggravates and exacerbates fibrocystic changes, and these will lead to more breast pain.
Medical management. Over-the-counter medications that are found in the vitamin section of a local drug store are vitamin E and evening primrose oil. There are no significant adverse effects with these treatments. Their efficacy is theoretical, however; 3 randomized controlled trials demonstrated no significant clinical benefit with evening primrose oil versus placebo for treatment of mastalgia.14
Topical or oral nonsteroidal anti-inflammatory drugs (NSAIDs; Voltaren gel, topical compound pain creams) are useful as second-line management after using a supportive bra. Three randomized controlled trials have demonstrated up to 90% improvement of mastalgia with topical NSAIDs.15-17
Tamoxifen is a selective estrogen-receptor modulator (SERM), which is an antagonist to the estrogen receptor (ER) in the breast and an agonist to the ER in the endometrium. Tamoxifen has been found to reduce symptoms of mastalgia by 70% even at a lower dosage of 10-mg per day (for 6 months), or as a topical gel (afimoxifene). The oral form can have some adverse effects, including hot flashes, and has a low risk for thromboembolic events and endometrial neoplasia.18-20
Danazol is very effective in reducing breast pain symptoms (by 80%), with a higher relapse after stopping the medication. Danazol is less tolerated due to its androgenic effects, such as hirsutism, acne, menorrhagia, and voice changes. Both danazol and tamoxifen can be teratogenic and should be used with caution in women of child-bearing age.21
Finally, bromocriptine inhibits serum prolactin and has been reported to provide 65% improvement in breast pain. Its use for breast pain is not US Food and Drug Administration–approved and adverse effects include nausea, dizziness, and hypotension.22
Tamoxifen, danazol, and bromocriptine can be considered as third-line management options for severe treatment-resistant mastalgia.
Continue to: FIGURE 2 Treatment algorithm for breast pain...
In summary
Evaluation and counseling for breast pain should be managed by women’s health care providers in a primary care setting. Most patients need reassurance and medical explanation of their symptoms. They should be educated that more than 95% of the time breast pain is not caused by an underlying malignancy but rather due to hormonal and fibrocystic changes, which can be managed conservatively. If the clinical breast examination and recent screening mammogram (in women over age 40) results are negative, patients should be educated that their pain is benign and undergo a trial of conservative measures: wear and sleep in a supporting bra; keep a calendar of symptoms to determine any relation to cyclical changes; and avoid nicotine, caffeine, and fatty food. Topical pain creams with diclofenac and evening primrose oil also can be effective in ameliorating the symptoms. Breast pain is not a surgical disease; referral to a surgical specialist and diagnostic imaging can be unnecessary and expensive.
- Scurr J, Hedger W, Morris P, et al. The prevalence, severity, and impact of breast pain in the general population. Breast J. 2014;20:508-513.
- Davies EL, Gateley CA, Miers M, et al. The long-term course of mastalgia. J R Soc Med. 1998;91:462-464.
- Singletary SE, Robb GL, Hortobagy GN. Advanced Therapy of Breast Disease. 2nd ed. Ontario, Canada: BC Decker Inc.; 2004.
- Gong C, Song E, Jia W, et al. A double-blind randomized controlled trial of toremifen therapy for mastaglia. Arch Surg. 2006;141:43-47.
- Kumar S, Mansel RE, Scanlon MF, et al. Altered responses of prolactin, luteinizing hormone and follicle stimulating hormone secretion to thyrotrophin releasing hormone/gonadotrophin releasing hormone stimulation in cyclical mastalgia. Br J Surg. 1984;71:870-873.
- Mansel RE, Dogliotti L. European multicentre trial of bromocriptine in cyclical mastalgia. Lancet. 1990;335:190-193.
- Rose DP, Boyar AP, Cohen C, et al. Effect of a low-fat diet on hormone levels in women with cystic breast disease. I. Serum steroids and gonadotropins. J Natl Cancer Inst. 1987;78:623-626.
- Goodwin JP, Miller A, Del Giudice ME, et al. Elevated high-density lipoprotein cholesterol and dietary fat intake in women with cyclic mastopathy. Am J Obstet Gynecol. 1998;179:430-437.
- Goyal A, Mansel RE. Efamast Study Group. A randomized multicenter study of gamolenic acid (Efamast) with and without antioxidant vitamins and minerals in the management of mastalgia. Breast J. 2005;11(1):41-47.
- Parsay S, Olfati F, Nahidi S. Therapeutic effects of vitamin E on cyclic mastalgia. Breast J. 2009;15:510-514.
- Allen SS, Froberg DG. The effect of decreased caffeine consumption on benign proliferative breast disease: a randomized clinical trial. Surgery. 1987;101:720-730.
- Hadi MS. Sports brassiere: is it a solution for mastalgia? Breast J. 2000;6:407-409.
- Russell LC. Caffeine restriction as initial treatment for breast pain. Nurse Pract. 1989; 14(2): 36-7.
- Srivastava A, Mansel RE, Arvind N, et al. Evidence-based management of mastalgia: a meta-analysis of randomised trials. Breast. 2007;16:503-512.
- Irving AD, Morrison SL. Effectiveness of topical non-steroidal anti-inflammatory drugs in the management of breast pain. J R Coll Surg Edinb. 1998;43:158-159.
- Colak T, Ipek T, Kanik A, et al. Efficiency of topical nonsteroidal anti-inflammatory drugs in mastalgia treatment. J Am Coll Surg. 2003;196(4):525-530.
- Kaviani A, Mehrdad N, Najafi M, et al. Comparison of naproxen with placebo for the management of noncyclical breast pain: a randomized, double-blind, controlled trial. World J Surg. 2008;32:2464-2470.
- Fentiman IS, Caleffi M, Brame K, et al. Double-blind controlled trial of tamoxifen therapy for mastalgia. Lancet. 1986;1:287-288.
- Jain BK, Bansal A, Choudhary D, et al. Centchroman vs tamoxifen for regression of mastalgia: a randomized controlled trial. Intl J Surg. 2015;15:11-16.
- Mansel R, Goyal A, Le Nestour EL, et al; Afimoxifene (4-OHT) Breast Pain Research group. A phase II trial of Afimoxifene (4-hydroxyamoxifen gel) for cyclical mastalgia in premenopausal women. Breast Cancer Res Treat. 2007;106:389-397.
- O'Brien PM, Abukhalil IE. Randomized controlled trial of the management of premenstrual syndrome and premenstrual mastalgia using luteal phase-only danazol. Am J Obstet Gynecol. 1999;180:18-23.
- Blichert-Toft M, Andersen AN, Henriksen OB, et al. Treatment of mastalgia with bromocriptine: a double-blind cross-over study. Br Med J. 1979;1:237.
Breast pain is one of the most common breast-related patient complaints and is found to affect at least 50% of the female population.1 Most cases are self-limiting and are related to hormonal and normal fibrocystic changes. The median age of onset of symptoms is 36 years, with most women experiencing pain for 5 to 12 years.2 Because the cause of breast pain is not always clear, its presence can produce anxiety in patients and physicians over the possibility of underlying malignancy. Although breast cancer is not associated with breast pain, many patients presenting with pain are referred for diagnostic imaging (usually with negative results). The majority of women with mastalgia and normal clinical examination findings can be reassured with education about the many benign causes of breast pain.
What are causes of breast pain without an imaging abnormality?
Hormones. Mastalgia can be focal or generalized and is mostly due to hormonal changes. Elevated estrogen can stimulate the growth of breast tissue, which is known as epithelial hyperplasia.3 Fluctuations in hormone levels can occur in perimenopausal women in their forties and can result in new symptoms of breast pain.4 Sometimes starting a new contraceptive medication or hormone replacement therapy can exacerbate the pain. Switching brands or medications may help. Another cause of mastalgia may be elevated prolactin levels, with hypothalamic-pituitary dysfunction.5,6
Diet. There is evidence to link a high-fat diet with breast pain. The pain has been shown to improve when lipid intake is reduced and high- and low-density lipoprotein cholesterol levels are normalized. As estrogen is a steroid hormone that can be synthesized from lipids and fatty acids, elevated lipid metabolism can increase estrogen levels and exacerbate breast pain symptoms.7,8 Essential fatty acids, such as evening primrose oil and vitamin E, have been used to treat mastalgia because they reduce inflammation in fatty breast tissue through the prostaglandin pathway.9,10
Caffeine. Methylxanthines can be found in coffee, tea, and chocolate and can aggravate mastalgia by enhancing the cyclin adenosine monophosphate (cAMP) pathway. This pathway stimulates cellular proliferation and fibrocystic changes which in turn can exacerbate breast pain.11
Smoking. In my clinical practice I have clearly noted a higher incidence of breast pain in patients who smoke. The pain tends to improve significantly when the patient quits or even cuts back on smoking. The exact reasons for smoking’s effects on breast pain are not well known; however, they are thought to be related to acceleration of the cAMP pathway.
Large breast size. Very large breasts will strain and weaken the suspensory ligaments, leading to pain and discomfort. It has been shown that wearing a supportive sports bra during episodes of breast pain is effective.
Types of breast pain
Cyclical
Women with fibrocystic breasts tend to experience more breast pain. Breast sensitivity can be localized to the upper outer quadrants or to the nipple and sub-areolar area. It also can be generalized. The pain tends to peak with ovulation, improve with menses, and to recur every few weeks. Patients who have had partial hysterectomy (with ovaries in situ) or endometrial ablation will be unable to correlate their symptoms to menstruation. Therefore, women are encouraged to keep a diary or calendar of their symptoms to detect any correlation with their ovarian cycle. Such correlation is reassuring.

Continue to: Noncyclical...
Noncyclical
Noncyclical breast pain is not associated with the menstrual cycle and can be unilateral or bilateral. Providers should perform a good history of patients presenting with noncyclicalbreast pain, to include character, onset, duration, location, radiation, alleviating, and aggravating factors. A physical examination may elicit point tenderness at the chest by pushing the breast tissue off of the chest wall while the patient is in supine position and pressing directly over the ribs. Lack of tenderness on palpation of the breast parenchyma, but pain on the chest wall, points to a musculoskeletal etiology. Chest wall pain may be related to muscle spasm or muscle strain, trauma, rib fracture, or costochondritis (Tietze syndrome). Finally, based on history of review of systems and physical examination, referred pain from biliary or cardiac etiology should be considered.
When breast pain occurs with skin changes
Skin changes usually have an underlying pathology. Infectious processes, such as infected epidermal inclusion cyst, hidradenitis of the cleavage and inframammary crease, or breast abscess will present with pain and induration with an acute onset of 5 to 10 days. Large pendulous breasts may develop yeast infection at the inframammary crease. Chronic infectious irritation can lead to hyperpigmentation of that area. Eczema or contact dermatitis frequently can affect the areola and become confused with Paget disease (ductal carcinoma in situ of the nipple). With Paget, the excoriation always starts at the nipple and can then spread to the areola. However, with dermatitis, the rash begins on the peri-areolar skin, without affecting the nipple itself.
When breast pain occurs with nipple discharge
Breast pain with nipple discharge usually is bilateral and more common in patients with significant fibrocystic changes who smoke. If the nipple discharge is bilateral, serous and non-bloody, and multiduct, it is considered benign and physiologic. Physiologic nipple discharge can be multifactorial and hormonal. It may be related to thyroid disorders or medications such as antidepressants, selective serotonin reuptake inhibitors (SSRIs), mood stabilizers, or antipsychotics. The only nipple discharge that is considered pathologic is unilateral spontaneous bloody discharge for which diagnostic imaging and breast surgeon referral is indicated. Women should be discouraged from self-expressing their nipples, as 80% will experience serous nipple discharge upon manual self-expression.
Breast pain is not associated with breast cancer. Most breast cancers do not hurt; they present as firm, painless masses. However, when a woman notices pain in her breast, her first concern is breast cancer. This concern is re-enforced by the medical provider whose first impulse is to order diagnostic imaging. Yet less than 3% of breast cancers are associated with breast pain.
There have been multiple published retrospective and prospective radiologic studies about the utility of breast imaging in women with breast pain without a palpable mass. All of the studies have demonstrated that breast imaging with mammography and ultrasonography in these patients yields mostly negative or benign findings. The incidence of breast cancer during imaging work-up in women with breast pain and no clinical abnormality is only 0.4% to 1.8%.1-3 Some patients may develop future subsequent breast cancer in the symptomatic breast. But this is considered incidental and possibly related to increased cell turnover related to fibrocystic changes. Breast imaging for evaluation of breast pain only provides reassurance to the physician. The patient's reassurance will come from a medical explanation for the symptoms and advice on symptom management from the provider.
Researchers from MD Anderson Cancer Center reported imaging findings and cost analysis for 799 patients presenting with breast pain from 3 large network community-based breast imaging centers in 2014. Breast ultrasound was the initial imaging modality for women younger than age 30. Digital mammography (sometimes with tomosynthesis) was used for those older than age 30 that had not had a mammogram in the last 6 months. Breast magnetic resonance imaging was performed only when ordered by the referring physician. Most of the patients presented for diagnostic imaging, and 95% had negative findings and 5% had a benign finding. Only 1 patient was found to have an incidental cancer in the contralateral breast, which was detected by tomosynthesis. The cost of breast imaging was $87,322 in younger women and $152,732 in women older than age 40, representing overutilization of health care resources and no association between breast pain and breast cancer.4
References
- Chetlan AL, Kapoor MM, Watts MR. Mastalgia: imaging work-up appropriateness. Acad Radiol. 2017;24:345-349.
- Arslan M, Kücükerdem HS, Can H, et al. Retrospective analysis of women with only mastalgia. J Breast Health. 2016;12:151-154.
- Noroozian M, Stein LF, Gaetke-Udager K, et al. Long-term clinical outcomes in women with breast pain in the absence of additional clinical findings: Mammography remains indicated. Breast Cancer Res Treat. 2015;149:417-424.
- Kushwaha AC, Shin K, Kalambo M, et al. Overutilization of health care resources for breast pain. AJR Am J Roentgenol. 2018; 211:217-223.
Management of mastalgia
Appropriate breast pain management begins with a good history and physical examination. The decision to perform imaging should depend on clinical exam findings and not on symptoms of breast pain. If there is a palpable mass, then breast imaging and possible biopsy is appropriate. However, if clinical exam is normal, there is no indication for breast imaging in low-risk women under the age of 40 whose only symptom is breast pain. Women older than age 40 can undergo diagnostic imaging, if they have not had a negative screening mammogram in the past year.
Breast pain with abnormal clinical exam
In the patient who is younger than age 30 with a palpable mass. For this patient order targeted breast ultrasound (US) (FIGURE 1). If results are negative, repeat the clinical examination 1 week after menses. If the mass is persistent, refer the patient to a breast surgeon. If diagnostic imaging results are negative, consider breast MRI, especially if there is a strong family history of breast cancer.
In the patient who is aged 30 and older with a palpable mass. For this patient, bilateral diagnostic mammogram and US are in order. The testing is best performed 1 week after menses to reduce false-positive findings. If imaging is negative and the patient still has a clinically suspicious finding or mass, refer her to a breast surgeon and consider breast MRI. At this point if there is a persistent firm dominant mass, a biopsy is indicated as part of the triple test. If the mass resolves with menses, the patient can be reassured that the cause is most likely benign, with clinical examination repeated in 3 months.

Continue to: Breast pain and normal clinical exam...
Breast pain and normal clinical exam
When women who report breast pain have normal clinical examination findings (and have a negative screening mammogram in the past 12 months if older than age 40), there are several management strategies you can offer (FIGURE 2).
Reassurance and education. The majority of women with breast pain can be managed with reassurance and education, which are often sufficient to reduce their anxieties.
Supportive bra. The most effective intervention is to wear and sleep in a well-fitted supportive sports bra for 4 to 12 weeks. In a nonrandomized single-center trial of danazol versus sports bra, 85% of women reported relief of their breast pain with bra alone (vs 58% with danazol).12 A supportive bra is the first-line management of mastalgia (Level II evidence).
Symptom diary/calendar. Many women do not know whether or not their symptoms correspond to their ovarian cycle or are related to hormonal fluctuations. Therefore, it is reassuring and informative for them to keep a calendar or a diary of their symptoms to determine whether their symptoms occur or are exacerbated in a cyclical pattern.
Diet and lifestyle modification. Women should avoid caffeine (especially when having pain). Studies on methylxanthines have demonstrated some symptom relief with reducing caffeine intake.11,13 One cup of coffee or tea per day most likely will not make a difference. However, if a woman is drinking large quantities of caffeinated beverages throughout the day, it will very likely improve her breast pain if she cuts back. This is especially true during the times of exacerbated pain prior to her menses.
In addition, recommend reduced dietary fat (overall good health). This is good advice for any patient. There were 2 small studies that showed improvement in breast pain with a 15% reduction in dietary fat.7,8
Finally, advise that patients stop smoking. Smoking aggravates and exacerbates fibrocystic changes, and these will lead to more breast pain.
Medical management. Over-the-counter medications that are found in the vitamin section of a local drug store are vitamin E and evening primrose oil. There are no significant adverse effects with these treatments. Their efficacy is theoretical, however; 3 randomized controlled trials demonstrated no significant clinical benefit with evening primrose oil versus placebo for treatment of mastalgia.14
Topical or oral nonsteroidal anti-inflammatory drugs (NSAIDs; Voltaren gel, topical compound pain creams) are useful as second-line management after using a supportive bra. Three randomized controlled trials have demonstrated up to 90% improvement of mastalgia with topical NSAIDs.15-17
Tamoxifen is a selective estrogen-receptor modulator (SERM), which is an antagonist to the estrogen receptor (ER) in the breast and an agonist to the ER in the endometrium. Tamoxifen has been found to reduce symptoms of mastalgia by 70% even at a lower dosage of 10-mg per day (for 6 months), or as a topical gel (afimoxifene). The oral form can have some adverse effects, including hot flashes, and has a low risk for thromboembolic events and endometrial neoplasia.18-20
Danazol is very effective in reducing breast pain symptoms (by 80%), with a higher relapse after stopping the medication. Danazol is less tolerated due to its androgenic effects, such as hirsutism, acne, menorrhagia, and voice changes. Both danazol and tamoxifen can be teratogenic and should be used with caution in women of child-bearing age.21
Finally, bromocriptine inhibits serum prolactin and has been reported to provide 65% improvement in breast pain. Its use for breast pain is not US Food and Drug Administration–approved and adverse effects include nausea, dizziness, and hypotension.22
Tamoxifen, danazol, and bromocriptine can be considered as third-line management options for severe treatment-resistant mastalgia.
Continue to: FIGURE 2 Treatment algorithm for breast pain...

In summary
Evaluation and counseling for breast pain should be managed by women’s health care providers in a primary care setting. Most patients need reassurance and medical explanation of their symptoms. They should be educated that more than 95% of the time breast pain is not caused by an underlying malignancy but rather due to hormonal and fibrocystic changes, which can be managed conservatively. If the clinical breast examination and recent screening mammogram (in women over age 40) results are negative, patients should be educated that their pain is benign and undergo a trial of conservative measures: wear and sleep in a supporting bra; keep a calendar of symptoms to determine any relation to cyclical changes; and avoid nicotine, caffeine, and fatty food. Topical pain creams with diclofenac and evening primrose oil also can be effective in ameliorating the symptoms. Breast pain is not a surgical disease; referral to a surgical specialist and diagnostic imaging can be unnecessary and expensive.
Breast pain is one of the most common breast-related patient complaints and is found to affect at least 50% of the female population.1 Most cases are self-limiting and are related to hormonal and normal fibrocystic changes. The median age of onset of symptoms is 36 years, with most women experiencing pain for 5 to 12 years.2 Because the cause of breast pain is not always clear, its presence can produce anxiety in patients and physicians over the possibility of underlying malignancy. Although breast cancer is not associated with breast pain, many patients presenting with pain are referred for diagnostic imaging (usually with negative results). The majority of women with mastalgia and normal clinical examination findings can be reassured with education about the many benign causes of breast pain.
What are causes of breast pain without an imaging abnormality?
Hormones. Mastalgia can be focal or generalized and is mostly due to hormonal changes. Elevated estrogen can stimulate the growth of breast tissue, which is known as epithelial hyperplasia.3 Fluctuations in hormone levels can occur in perimenopausal women in their forties and can result in new symptoms of breast pain.4 Sometimes starting a new contraceptive medication or hormone replacement therapy can exacerbate the pain. Switching brands or medications may help. Another cause of mastalgia may be elevated prolactin levels, with hypothalamic-pituitary dysfunction.5,6
Diet. There is evidence to link a high-fat diet with breast pain. The pain has been shown to improve when lipid intake is reduced and high- and low-density lipoprotein cholesterol levels are normalized. As estrogen is a steroid hormone that can be synthesized from lipids and fatty acids, elevated lipid metabolism can increase estrogen levels and exacerbate breast pain symptoms.7,8 Essential fatty acids, such as evening primrose oil and vitamin E, have been used to treat mastalgia because they reduce inflammation in fatty breast tissue through the prostaglandin pathway.9,10
Caffeine. Methylxanthines can be found in coffee, tea, and chocolate and can aggravate mastalgia by enhancing the cyclin adenosine monophosphate (cAMP) pathway. This pathway stimulates cellular proliferation and fibrocystic changes which in turn can exacerbate breast pain.11
Smoking. In my clinical practice I have clearly noted a higher incidence of breast pain in patients who smoke. The pain tends to improve significantly when the patient quits or even cuts back on smoking. The exact reasons for smoking’s effects on breast pain are not well known; however, they are thought to be related to acceleration of the cAMP pathway.
Large breast size. Very large breasts will strain and weaken the suspensory ligaments, leading to pain and discomfort. It has been shown that wearing a supportive sports bra during episodes of breast pain is effective.
Types of breast pain
Cyclical
Women with fibrocystic breasts tend to experience more breast pain. Breast sensitivity can be localized to the upper outer quadrants or to the nipple and sub-areolar area. It also can be generalized. The pain tends to peak with ovulation, improve with menses, and to recur every few weeks. Patients who have had partial hysterectomy (with ovaries in situ) or endometrial ablation will be unable to correlate their symptoms to menstruation. Therefore, women are encouraged to keep a diary or calendar of their symptoms to detect any correlation with their ovarian cycle. Such correlation is reassuring.

Continue to: Noncyclical...
Noncyclical
Noncyclical breast pain is not associated with the menstrual cycle and can be unilateral or bilateral. Providers should perform a good history of patients presenting with noncyclicalbreast pain, to include character, onset, duration, location, radiation, alleviating, and aggravating factors. A physical examination may elicit point tenderness at the chest by pushing the breast tissue off of the chest wall while the patient is in supine position and pressing directly over the ribs. Lack of tenderness on palpation of the breast parenchyma, but pain on the chest wall, points to a musculoskeletal etiology. Chest wall pain may be related to muscle spasm or muscle strain, trauma, rib fracture, or costochondritis (Tietze syndrome). Finally, based on history of review of systems and physical examination, referred pain from biliary or cardiac etiology should be considered.
When breast pain occurs with skin changes
Skin changes usually have an underlying pathology. Infectious processes, such as infected epidermal inclusion cyst, hidradenitis of the cleavage and inframammary crease, or breast abscess will present with pain and induration with an acute onset of 5 to 10 days. Large pendulous breasts may develop yeast infection at the inframammary crease. Chronic infectious irritation can lead to hyperpigmentation of that area. Eczema or contact dermatitis frequently can affect the areola and become confused with Paget disease (ductal carcinoma in situ of the nipple). With Paget, the excoriation always starts at the nipple and can then spread to the areola. However, with dermatitis, the rash begins on the peri-areolar skin, without affecting the nipple itself.
When breast pain occurs with nipple discharge
Breast pain with nipple discharge usually is bilateral and more common in patients with significant fibrocystic changes who smoke. If the nipple discharge is bilateral, serous and non-bloody, and multiduct, it is considered benign and physiologic. Physiologic nipple discharge can be multifactorial and hormonal. It may be related to thyroid disorders or medications such as antidepressants, selective serotonin reuptake inhibitors (SSRIs), mood stabilizers, or antipsychotics. The only nipple discharge that is considered pathologic is unilateral spontaneous bloody discharge for which diagnostic imaging and breast surgeon referral is indicated. Women should be discouraged from self-expressing their nipples, as 80% will experience serous nipple discharge upon manual self-expression.
Breast pain is not associated with breast cancer. Most breast cancers do not hurt; they present as firm, painless masses. However, when a woman notices pain in her breast, her first concern is breast cancer. This concern is re-enforced by the medical provider whose first impulse is to order diagnostic imaging. Yet less than 3% of breast cancers are associated with breast pain.
There have been multiple published retrospective and prospective radiologic studies about the utility of breast imaging in women with breast pain without a palpable mass. All of the studies have demonstrated that breast imaging with mammography and ultrasonography in these patients yields mostly negative or benign findings. The incidence of breast cancer during imaging work-up in women with breast pain and no clinical abnormality is only 0.4% to 1.8%.1-3 Some patients may develop future subsequent breast cancer in the symptomatic breast. But this is considered incidental and possibly related to increased cell turnover related to fibrocystic changes. Breast imaging for evaluation of breast pain only provides reassurance to the physician. The patient's reassurance will come from a medical explanation for the symptoms and advice on symptom management from the provider.
Researchers from MD Anderson Cancer Center reported imaging findings and cost analysis for 799 patients presenting with breast pain from 3 large network community-based breast imaging centers in 2014. Breast ultrasound was the initial imaging modality for women younger than age 30. Digital mammography (sometimes with tomosynthesis) was used for those older than age 30 that had not had a mammogram in the last 6 months. Breast magnetic resonance imaging was performed only when ordered by the referring physician. Most of the patients presented for diagnostic imaging, and 95% had negative findings and 5% had a benign finding. Only 1 patient was found to have an incidental cancer in the contralateral breast, which was detected by tomosynthesis. The cost of breast imaging was $87,322 in younger women and $152,732 in women older than age 40, representing overutilization of health care resources and no association between breast pain and breast cancer.4
References
- Chetlan AL, Kapoor MM, Watts MR. Mastalgia: imaging work-up appropriateness. Acad Radiol. 2017;24:345-349.
- Arslan M, Kücükerdem HS, Can H, et al. Retrospective analysis of women with only mastalgia. J Breast Health. 2016;12:151-154.
- Noroozian M, Stein LF, Gaetke-Udager K, et al. Long-term clinical outcomes in women with breast pain in the absence of additional clinical findings: Mammography remains indicated. Breast Cancer Res Treat. 2015;149:417-424.
- Kushwaha AC, Shin K, Kalambo M, et al. Overutilization of health care resources for breast pain. AJR Am J Roentgenol. 2018; 211:217-223.
Management of mastalgia
Appropriate breast pain management begins with a good history and physical examination. The decision to perform imaging should depend on clinical exam findings and not on symptoms of breast pain. If there is a palpable mass, then breast imaging and possible biopsy is appropriate. However, if clinical exam is normal, there is no indication for breast imaging in low-risk women under the age of 40 whose only symptom is breast pain. Women older than age 40 can undergo diagnostic imaging, if they have not had a negative screening mammogram in the past year.
Breast pain with abnormal clinical exam
In the patient who is younger than age 30 with a palpable mass. For this patient order targeted breast ultrasound (US) (FIGURE 1). If results are negative, repeat the clinical examination 1 week after menses. If the mass is persistent, refer the patient to a breast surgeon. If diagnostic imaging results are negative, consider breast MRI, especially if there is a strong family history of breast cancer.
In the patient who is aged 30 and older with a palpable mass. For this patient, bilateral diagnostic mammogram and US are in order. The testing is best performed 1 week after menses to reduce false-positive findings. If imaging is negative and the patient still has a clinically suspicious finding or mass, refer her to a breast surgeon and consider breast MRI. At this point if there is a persistent firm dominant mass, a biopsy is indicated as part of the triple test. If the mass resolves with menses, the patient can be reassured that the cause is most likely benign, with clinical examination repeated in 3 months.

Continue to: Breast pain and normal clinical exam...
Breast pain and normal clinical exam
When women who report breast pain have normal clinical examination findings (and have a negative screening mammogram in the past 12 months if older than age 40), there are several management strategies you can offer (FIGURE 2).
Reassurance and education. The majority of women with breast pain can be managed with reassurance and education, which are often sufficient to reduce their anxieties.
Supportive bra. The most effective intervention is to wear and sleep in a well-fitted supportive sports bra for 4 to 12 weeks. In a nonrandomized single-center trial of danazol versus sports bra, 85% of women reported relief of their breast pain with bra alone (vs 58% with danazol).12 A supportive bra is the first-line management of mastalgia (Level II evidence).
Symptom diary/calendar. Many women do not know whether or not their symptoms correspond to their ovarian cycle or are related to hormonal fluctuations. Therefore, it is reassuring and informative for them to keep a calendar or a diary of their symptoms to determine whether their symptoms occur or are exacerbated in a cyclical pattern.
Diet and lifestyle modification. Women should avoid caffeine (especially when having pain). Studies on methylxanthines have demonstrated some symptom relief with reducing caffeine intake.11,13 One cup of coffee or tea per day most likely will not make a difference. However, if a woman is drinking large quantities of caffeinated beverages throughout the day, it will very likely improve her breast pain if she cuts back. This is especially true during the times of exacerbated pain prior to her menses.
In addition, recommend reduced dietary fat (overall good health). This is good advice for any patient. There were 2 small studies that showed improvement in breast pain with a 15% reduction in dietary fat.7,8
Finally, advise that patients stop smoking. Smoking aggravates and exacerbates fibrocystic changes, and these will lead to more breast pain.
Medical management. Over-the-counter medications that are found in the vitamin section of a local drug store are vitamin E and evening primrose oil. There are no significant adverse effects with these treatments. Their efficacy is theoretical, however; 3 randomized controlled trials demonstrated no significant clinical benefit with evening primrose oil versus placebo for treatment of mastalgia.14
Topical or oral nonsteroidal anti-inflammatory drugs (NSAIDs; Voltaren gel, topical compound pain creams) are useful as second-line management after using a supportive bra. Three randomized controlled trials have demonstrated up to 90% improvement of mastalgia with topical NSAIDs.15-17
Tamoxifen is a selective estrogen-receptor modulator (SERM), which is an antagonist to the estrogen receptor (ER) in the breast and an agonist to the ER in the endometrium. Tamoxifen has been found to reduce symptoms of mastalgia by 70% even at a lower dosage of 10-mg per day (for 6 months), or as a topical gel (afimoxifene). The oral form can have some adverse effects, including hot flashes, and has a low risk for thromboembolic events and endometrial neoplasia.18-20
Danazol is very effective in reducing breast pain symptoms (by 80%), with a higher relapse after stopping the medication. Danazol is less tolerated due to its androgenic effects, such as hirsutism, acne, menorrhagia, and voice changes. Both danazol and tamoxifen can be teratogenic and should be used with caution in women of child-bearing age.21
Finally, bromocriptine inhibits serum prolactin and has been reported to provide 65% improvement in breast pain. Its use for breast pain is not US Food and Drug Administration–approved and adverse effects include nausea, dizziness, and hypotension.22
Tamoxifen, danazol, and bromocriptine can be considered as third-line management options for severe treatment-resistant mastalgia.
Continue to: FIGURE 2 Treatment algorithm for breast pain...

In summary
Evaluation and counseling for breast pain should be managed by women’s health care providers in a primary care setting. Most patients need reassurance and medical explanation of their symptoms. They should be educated that more than 95% of the time breast pain is not caused by an underlying malignancy but rather due to hormonal and fibrocystic changes, which can be managed conservatively. If the clinical breast examination and recent screening mammogram (in women over age 40) results are negative, patients should be educated that their pain is benign and undergo a trial of conservative measures: wear and sleep in a supporting bra; keep a calendar of symptoms to determine any relation to cyclical changes; and avoid nicotine, caffeine, and fatty food. Topical pain creams with diclofenac and evening primrose oil also can be effective in ameliorating the symptoms. Breast pain is not a surgical disease; referral to a surgical specialist and diagnostic imaging can be unnecessary and expensive.
- Scurr J, Hedger W, Morris P, et al. The prevalence, severity, and impact of breast pain in the general population. Breast J. 2014;20:508-513.
- Davies EL, Gateley CA, Miers M, et al. The long-term course of mastalgia. J R Soc Med. 1998;91:462-464.
- Singletary SE, Robb GL, Hortobagy GN. Advanced Therapy of Breast Disease. 2nd ed. Ontario, Canada: BC Decker Inc.; 2004.
- Gong C, Song E, Jia W, et al. A double-blind randomized controlled trial of toremifen therapy for mastaglia. Arch Surg. 2006;141:43-47.
- Kumar S, Mansel RE, Scanlon MF, et al. Altered responses of prolactin, luteinizing hormone and follicle stimulating hormone secretion to thyrotrophin releasing hormone/gonadotrophin releasing hormone stimulation in cyclical mastalgia. Br J Surg. 1984;71:870-873.
- Mansel RE, Dogliotti L. European multicentre trial of bromocriptine in cyclical mastalgia. Lancet. 1990;335:190-193.
- Rose DP, Boyar AP, Cohen C, et al. Effect of a low-fat diet on hormone levels in women with cystic breast disease. I. Serum steroids and gonadotropins. J Natl Cancer Inst. 1987;78:623-626.
- Goodwin JP, Miller A, Del Giudice ME, et al. Elevated high-density lipoprotein cholesterol and dietary fat intake in women with cyclic mastopathy. Am J Obstet Gynecol. 1998;179:430-437.
- Goyal A, Mansel RE. Efamast Study Group. A randomized multicenter study of gamolenic acid (Efamast) with and without antioxidant vitamins and minerals in the management of mastalgia. Breast J. 2005;11(1):41-47.
- Parsay S, Olfati F, Nahidi S. Therapeutic effects of vitamin E on cyclic mastalgia. Breast J. 2009;15:510-514.
- Allen SS, Froberg DG. The effect of decreased caffeine consumption on benign proliferative breast disease: a randomized clinical trial. Surgery. 1987;101:720-730.
- Hadi MS. Sports brassiere: is it a solution for mastalgia? Breast J. 2000;6:407-409.
- Russell LC. Caffeine restriction as initial treatment for breast pain. Nurse Pract. 1989; 14(2): 36-7.
- Srivastava A, Mansel RE, Arvind N, et al. Evidence-based management of mastalgia: a meta-analysis of randomised trials. Breast. 2007;16:503-512.
- Irving AD, Morrison SL. Effectiveness of topical non-steroidal anti-inflammatory drugs in the management of breast pain. J R Coll Surg Edinb. 1998;43:158-159.
- Colak T, Ipek T, Kanik A, et al. Efficiency of topical nonsteroidal anti-inflammatory drugs in mastalgia treatment. J Am Coll Surg. 2003;196(4):525-530.
- Kaviani A, Mehrdad N, Najafi M, et al. Comparison of naproxen with placebo for the management of noncyclical breast pain: a randomized, double-blind, controlled trial. World J Surg. 2008;32:2464-2470.
- Fentiman IS, Caleffi M, Brame K, et al. Double-blind controlled trial of tamoxifen therapy for mastalgia. Lancet. 1986;1:287-288.
- Jain BK, Bansal A, Choudhary D, et al. Centchroman vs tamoxifen for regression of mastalgia: a randomized controlled trial. Intl J Surg. 2015;15:11-16.
- Mansel R, Goyal A, Le Nestour EL, et al; Afimoxifene (4-OHT) Breast Pain Research group. A phase II trial of Afimoxifene (4-hydroxyamoxifen gel) for cyclical mastalgia in premenopausal women. Breast Cancer Res Treat. 2007;106:389-397.
- O'Brien PM, Abukhalil IE. Randomized controlled trial of the management of premenstrual syndrome and premenstrual mastalgia using luteal phase-only danazol. Am J Obstet Gynecol. 1999;180:18-23.
- Blichert-Toft M, Andersen AN, Henriksen OB, et al. Treatment of mastalgia with bromocriptine: a double-blind cross-over study. Br Med J. 1979;1:237.
- Scurr J, Hedger W, Morris P, et al. The prevalence, severity, and impact of breast pain in the general population. Breast J. 2014;20:508-513.
- Davies EL, Gateley CA, Miers M, et al. The long-term course of mastalgia. J R Soc Med. 1998;91:462-464.
- Singletary SE, Robb GL, Hortobagy GN. Advanced Therapy of Breast Disease. 2nd ed. Ontario, Canada: BC Decker Inc.; 2004.
- Gong C, Song E, Jia W, et al. A double-blind randomized controlled trial of toremifen therapy for mastaglia. Arch Surg. 2006;141:43-47.
- Kumar S, Mansel RE, Scanlon MF, et al. Altered responses of prolactin, luteinizing hormone and follicle stimulating hormone secretion to thyrotrophin releasing hormone/gonadotrophin releasing hormone stimulation in cyclical mastalgia. Br J Surg. 1984;71:870-873.
- Mansel RE, Dogliotti L. European multicentre trial of bromocriptine in cyclical mastalgia. Lancet. 1990;335:190-193.
- Rose DP, Boyar AP, Cohen C, et al. Effect of a low-fat diet on hormone levels in women with cystic breast disease. I. Serum steroids and gonadotropins. J Natl Cancer Inst. 1987;78:623-626.
- Goodwin JP, Miller A, Del Giudice ME, et al. Elevated high-density lipoprotein cholesterol and dietary fat intake in women with cyclic mastopathy. Am J Obstet Gynecol. 1998;179:430-437.
- Goyal A, Mansel RE. Efamast Study Group. A randomized multicenter study of gamolenic acid (Efamast) with and without antioxidant vitamins and minerals in the management of mastalgia. Breast J. 2005;11(1):41-47.
- Parsay S, Olfati F, Nahidi S. Therapeutic effects of vitamin E on cyclic mastalgia. Breast J. 2009;15:510-514.
- Allen SS, Froberg DG. The effect of decreased caffeine consumption on benign proliferative breast disease: a randomized clinical trial. Surgery. 1987;101:720-730.
- Hadi MS. Sports brassiere: is it a solution for mastalgia? Breast J. 2000;6:407-409.
- Russell LC. Caffeine restriction as initial treatment for breast pain. Nurse Pract. 1989; 14(2): 36-7.
- Srivastava A, Mansel RE, Arvind N, et al. Evidence-based management of mastalgia: a meta-analysis of randomised trials. Breast. 2007;16:503-512.
- Irving AD, Morrison SL. Effectiveness of topical non-steroidal anti-inflammatory drugs in the management of breast pain. J R Coll Surg Edinb. 1998;43:158-159.
- Colak T, Ipek T, Kanik A, et al. Efficiency of topical nonsteroidal anti-inflammatory drugs in mastalgia treatment. J Am Coll Surg. 2003;196(4):525-530.
- Kaviani A, Mehrdad N, Najafi M, et al. Comparison of naproxen with placebo for the management of noncyclical breast pain: a randomized, double-blind, controlled trial. World J Surg. 2008;32:2464-2470.
- Fentiman IS, Caleffi M, Brame K, et al. Double-blind controlled trial of tamoxifen therapy for mastalgia. Lancet. 1986;1:287-288.
- Jain BK, Bansal A, Choudhary D, et al. Centchroman vs tamoxifen for regression of mastalgia: a randomized controlled trial. Intl J Surg. 2015;15:11-16.
- Mansel R, Goyal A, Le Nestour EL, et al; Afimoxifene (4-OHT) Breast Pain Research group. A phase II trial of Afimoxifene (4-hydroxyamoxifen gel) for cyclical mastalgia in premenopausal women. Breast Cancer Res Treat. 2007;106:389-397.
- O'Brien PM, Abukhalil IE. Randomized controlled trial of the management of premenstrual syndrome and premenstrual mastalgia using luteal phase-only danazol. Am J Obstet Gynecol. 1999;180:18-23.
- Blichert-Toft M, Andersen AN, Henriksen OB, et al. Treatment of mastalgia with bromocriptine: a double-blind cross-over study. Br Med J. 1979;1:237.
Ribociclib/ET improves OS in premenopausal women with HR+/HER2- breast cancer
CHICAGO – Adding ribociclib to endocrine therapy significantly improved overall survival of premenopausal women with advanced hormone receptor positive, HER2-negative breast cancer, results of the randomized phase 3 MONALEESA-7 trial showed.
A landmark analysis conducted at 42 months showed that the overall survival rate for women randomized to receive endocrine therapy with either a nonsteroidal aromatase inhibitor (AI) or tamoxifen plus the cyclin-dependent kinase 4/6 (CDK4/6) inhibitor ribociclib was 70%, compared with 46% for women randomized to endocrine therapy alone, reported Sara A Hurvitz, MD, of the University of California Los Angeles Jonsson Comprehensive Cancer Center
“This is the first time a statistically significant improvement in overall survival has been observed with a CDK4/6 inhibitor in combination with endocrine therapy in patients with hormone receptor-positive advanced disease,” she said at a briefing prior to her presentation of the data in an oral abstract session at the American Society of Clinical Oncology annual meeting.
“This is an important study, because it shows that the class of drugs, CDK4/6 inhibitors, which we are widely using, has been shown to delay the time to progression, delay the time to need for chemotherapy for advanced breast cancer, and really doubled the effectiveness of endocrine therapy, now also translates into a significant survival benefit for women who ER-positive metastatic breast cancer,” commented Harold J. Burstein, MD, PhD, an ASCO expert from the Dana-Farber Cancer Institute in Boston.
In the trial, 672 pre- or perimenopausal women with HR+/HER2– advanced breast cancer with no prior endocrine therapy for advanced disease and no more than one line of chemotherapy for advanced disease were enrolled. The patients were stratified by the presence of liver/lung metastases, prior chemotherapy, and prior endocrine partner, tamoxifen or nonsteroidal AI, and then randomly assigned to ribociclib 600 mg/day for 3 weeks, followed by 1-week off, plus tamoxifen or AI and goserelin, or the same combination and schedule with placebo.
Of the 672 patients enrolled, a total of 335 patients assigned to ribociclib and 337 assigned to placebo received treatment. The majority of patients – 495 – received an AI, either letrozole (Femara) or anastrozole (Arimidex). Dr. Hurvitz noted that after the initiation of MONALEESA-7, the combination of a CDK4/6 inhibitor and tamoxifen was found to prolong the QT interval and increase risk for cardiac arrhythmias, and is now contraindicated.
As previously reported , results of the primary MONALEESA-7 endpoint of progression-free survival favored the combination, with a median PFS of 23.8 months, compared with 13 months for women treated with endocrine therapy alone. The hazard ratio for progression with the ribociclib-based combination was 0.553 ( P less than .0001), and the treatment benefit of the CDK4/6 inhibitor was seen across all patients subgroups and regardless of the endocrine partner.
At ASCO 2019, Dr. Hurvitz reported on the key secondary endpoint of overall survival. At a median follow-up duration of 34.6 months, with an additional 15 months beyond the initial analysis, the median OS for the ribociclib arm was not reached, compared with 40.9 months in the placebo arm. The hazard ratio for death with ribociclib was 0.712 ( P = .00973). An analysis of OS by endocrine partner subgroup showed no significant differences between AIs or tamoxifen.
At the time of the data cutoff, 35% of patients in the ribociclib arm were still on therapy.
The safety and tolerability of the combination were consistent with those previously reported, Dr. Hurvitz said.
“In an era when we are thinking about value in oncology care, a demonstration of a robust sutvival difference I think substantially adds to a value proposition for products like ribociclib that were discussed here,” Dr. Burstein said.
“Hopefully, these data will enable access to this product to more women around the world, particularly in health care systems that assess value rigorously as part of their decisions for national access to drugs,” he added.
The MONALEESA-7 trial is supported by Novartis. Dr. Hurvitz reported travel and accommodation expenses from Novartis. Dr. Burstein reported no relevant disclosures.
SOURCE: Hurvitz SA et al. ASCO 2019. Abstract LBA1008 .
CHICAGO – Adding ribociclib to endocrine therapy significantly improved overall survival of premenopausal women with advanced hormone receptor positive, HER2-negative breast cancer, results of the randomized phase 3 MONALEESA-7 trial showed.
A landmark analysis conducted at 42 months showed that the overall survival rate for women randomized to receive endocrine therapy with either a nonsteroidal aromatase inhibitor (AI) or tamoxifen plus the cyclin-dependent kinase 4/6 (CDK4/6) inhibitor ribociclib was 70%, compared with 46% for women randomized to endocrine therapy alone, reported Sara A Hurvitz, MD, of the University of California Los Angeles Jonsson Comprehensive Cancer Center
“This is the first time a statistically significant improvement in overall survival has been observed with a CDK4/6 inhibitor in combination with endocrine therapy in patients with hormone receptor-positive advanced disease,” she said at a briefing prior to her presentation of the data in an oral abstract session at the American Society of Clinical Oncology annual meeting.
“This is an important study, because it shows that the class of drugs, CDK4/6 inhibitors, which we are widely using, has been shown to delay the time to progression, delay the time to need for chemotherapy for advanced breast cancer, and really doubled the effectiveness of endocrine therapy, now also translates into a significant survival benefit for women who ER-positive metastatic breast cancer,” commented Harold J. Burstein, MD, PhD, an ASCO expert from the Dana-Farber Cancer Institute in Boston.
In the trial, 672 pre- or perimenopausal women with HR+/HER2– advanced breast cancer with no prior endocrine therapy for advanced disease and no more than one line of chemotherapy for advanced disease were enrolled. The patients were stratified by the presence of liver/lung metastases, prior chemotherapy, and prior endocrine partner, tamoxifen or nonsteroidal AI, and then randomly assigned to ribociclib 600 mg/day for 3 weeks, followed by 1-week off, plus tamoxifen or AI and goserelin, or the same combination and schedule with placebo.
Of the 672 patients enrolled, a total of 335 patients assigned to ribociclib and 337 assigned to placebo received treatment. The majority of patients – 495 – received an AI, either letrozole (Femara) or anastrozole (Arimidex). Dr. Hurvitz noted that after the initiation of MONALEESA-7, the combination of a CDK4/6 inhibitor and tamoxifen was found to prolong the QT interval and increase risk for cardiac arrhythmias, and is now contraindicated.
As previously reported , results of the primary MONALEESA-7 endpoint of progression-free survival favored the combination, with a median PFS of 23.8 months, compared with 13 months for women treated with endocrine therapy alone. The hazard ratio for progression with the ribociclib-based combination was 0.553 ( P less than .0001), and the treatment benefit of the CDK4/6 inhibitor was seen across all patients subgroups and regardless of the endocrine partner.
At ASCO 2019, Dr. Hurvitz reported on the key secondary endpoint of overall survival. At a median follow-up duration of 34.6 months, with an additional 15 months beyond the initial analysis, the median OS for the ribociclib arm was not reached, compared with 40.9 months in the placebo arm. The hazard ratio for death with ribociclib was 0.712 ( P = .00973). An analysis of OS by endocrine partner subgroup showed no significant differences between AIs or tamoxifen.
At the time of the data cutoff, 35% of patients in the ribociclib arm were still on therapy.
The safety and tolerability of the combination were consistent with those previously reported, Dr. Hurvitz said.
“In an era when we are thinking about value in oncology care, a demonstration of a robust sutvival difference I think substantially adds to a value proposition for products like ribociclib that were discussed here,” Dr. Burstein said.
“Hopefully, these data will enable access to this product to more women around the world, particularly in health care systems that assess value rigorously as part of their decisions for national access to drugs,” he added.
The MONALEESA-7 trial is supported by Novartis. Dr. Hurvitz reported travel and accommodation expenses from Novartis. Dr. Burstein reported no relevant disclosures.
SOURCE: Hurvitz SA et al. ASCO 2019. Abstract LBA1008 .
CHICAGO – Adding ribociclib to endocrine therapy significantly improved overall survival of premenopausal women with advanced hormone receptor positive, HER2-negative breast cancer, results of the randomized phase 3 MONALEESA-7 trial showed.
A landmark analysis conducted at 42 months showed that the overall survival rate for women randomized to receive endocrine therapy with either a nonsteroidal aromatase inhibitor (AI) or tamoxifen plus the cyclin-dependent kinase 4/6 (CDK4/6) inhibitor ribociclib was 70%, compared with 46% for women randomized to endocrine therapy alone, reported Sara A Hurvitz, MD, of the University of California Los Angeles Jonsson Comprehensive Cancer Center
“This is the first time a statistically significant improvement in overall survival has been observed with a CDK4/6 inhibitor in combination with endocrine therapy in patients with hormone receptor-positive advanced disease,” she said at a briefing prior to her presentation of the data in an oral abstract session at the American Society of Clinical Oncology annual meeting.
“This is an important study, because it shows that the class of drugs, CDK4/6 inhibitors, which we are widely using, has been shown to delay the time to progression, delay the time to need for chemotherapy for advanced breast cancer, and really doubled the effectiveness of endocrine therapy, now also translates into a significant survival benefit for women who ER-positive metastatic breast cancer,” commented Harold J. Burstein, MD, PhD, an ASCO expert from the Dana-Farber Cancer Institute in Boston.
In the trial, 672 pre- or perimenopausal women with HR+/HER2– advanced breast cancer with no prior endocrine therapy for advanced disease and no more than one line of chemotherapy for advanced disease were enrolled. The patients were stratified by the presence of liver/lung metastases, prior chemotherapy, and prior endocrine partner, tamoxifen or nonsteroidal AI, and then randomly assigned to ribociclib 600 mg/day for 3 weeks, followed by 1-week off, plus tamoxifen or AI and goserelin, or the same combination and schedule with placebo.
Of the 672 patients enrolled, a total of 335 patients assigned to ribociclib and 337 assigned to placebo received treatment. The majority of patients – 495 – received an AI, either letrozole (Femara) or anastrozole (Arimidex). Dr. Hurvitz noted that after the initiation of MONALEESA-7, the combination of a CDK4/6 inhibitor and tamoxifen was found to prolong the QT interval and increase risk for cardiac arrhythmias, and is now contraindicated.
As previously reported , results of the primary MONALEESA-7 endpoint of progression-free survival favored the combination, with a median PFS of 23.8 months, compared with 13 months for women treated with endocrine therapy alone. The hazard ratio for progression with the ribociclib-based combination was 0.553 ( P less than .0001), and the treatment benefit of the CDK4/6 inhibitor was seen across all patients subgroups and regardless of the endocrine partner.
At ASCO 2019, Dr. Hurvitz reported on the key secondary endpoint of overall survival. At a median follow-up duration of 34.6 months, with an additional 15 months beyond the initial analysis, the median OS for the ribociclib arm was not reached, compared with 40.9 months in the placebo arm. The hazard ratio for death with ribociclib was 0.712 ( P = .00973). An analysis of OS by endocrine partner subgroup showed no significant differences between AIs or tamoxifen.
At the time of the data cutoff, 35% of patients in the ribociclib arm were still on therapy.
The safety and tolerability of the combination were consistent with those previously reported, Dr. Hurvitz said.
“In an era when we are thinking about value in oncology care, a demonstration of a robust sutvival difference I think substantially adds to a value proposition for products like ribociclib that were discussed here,” Dr. Burstein said.
“Hopefully, these data will enable access to this product to more women around the world, particularly in health care systems that assess value rigorously as part of their decisions for national access to drugs,” he added.
The MONALEESA-7 trial is supported by Novartis. Dr. Hurvitz reported travel and accommodation expenses from Novartis. Dr. Burstein reported no relevant disclosures.
SOURCE: Hurvitz SA et al. ASCO 2019. Abstract LBA1008 .
REPORTING FROM ASCO 2019
Ribociclib plus endocrine therapy boosts survival of HR+/HER2- breast cancer
CHICAGO – Adding ribociclib to endocrine therapy significantly improved overall survival of premenopausal women with advanced hormone receptor positive, HER2-negative breast cancer, results of the randomized phase 3 MONALEESA-7 trial showed.
A landmark analysis performed at 42 months of follow-up showed that the overall survival (OS) rate for women randomized to receive endocrine therapy with either a nonsteroidal aromatase inhibitor (AI) or tamoxifen plus the cyclin-dependent kinase 4/6 (CDK4/6) inhibitor ribociclib (Kisqali) was 70%, compared with 46% for women randomized to endocrine therapy plus placebo.
The trial is the first study to evaluate a CDK4/6 inhibitor exclusively in premenopausal women, and the first to show a statistically significant improvement in overall survival with a CDK4/6 inhibitor in combination with endocrine therapy in patients with HR-positive, HER2-negative advanced breast cancer.
In a video interview at the American Society of Clinical Oncology annual meeting, Sara A. Hurvitz, MD, from the University of California Los Angeles Jonsson Comprehensive Cancer Center, describes the significance of the MONALEESA-7 findings and the potential for improving on the study results with other agents or combinations.
The MONALEESA-7 trial is supported by Novartis. Dr. Hurvitz reported travel and accommodation expenses from Novartis.
CHICAGO – Adding ribociclib to endocrine therapy significantly improved overall survival of premenopausal women with advanced hormone receptor positive, HER2-negative breast cancer, results of the randomized phase 3 MONALEESA-7 trial showed.
A landmark analysis performed at 42 months of follow-up showed that the overall survival (OS) rate for women randomized to receive endocrine therapy with either a nonsteroidal aromatase inhibitor (AI) or tamoxifen plus the cyclin-dependent kinase 4/6 (CDK4/6) inhibitor ribociclib (Kisqali) was 70%, compared with 46% for women randomized to endocrine therapy plus placebo.
The trial is the first study to evaluate a CDK4/6 inhibitor exclusively in premenopausal women, and the first to show a statistically significant improvement in overall survival with a CDK4/6 inhibitor in combination with endocrine therapy in patients with HR-positive, HER2-negative advanced breast cancer.
In a video interview at the American Society of Clinical Oncology annual meeting, Sara A. Hurvitz, MD, from the University of California Los Angeles Jonsson Comprehensive Cancer Center, describes the significance of the MONALEESA-7 findings and the potential for improving on the study results with other agents or combinations.
The MONALEESA-7 trial is supported by Novartis. Dr. Hurvitz reported travel and accommodation expenses from Novartis.
CHICAGO – Adding ribociclib to endocrine therapy significantly improved overall survival of premenopausal women with advanced hormone receptor positive, HER2-negative breast cancer, results of the randomized phase 3 MONALEESA-7 trial showed.
A landmark analysis performed at 42 months of follow-up showed that the overall survival (OS) rate for women randomized to receive endocrine therapy with either a nonsteroidal aromatase inhibitor (AI) or tamoxifen plus the cyclin-dependent kinase 4/6 (CDK4/6) inhibitor ribociclib (Kisqali) was 70%, compared with 46% for women randomized to endocrine therapy plus placebo.
The trial is the first study to evaluate a CDK4/6 inhibitor exclusively in premenopausal women, and the first to show a statistically significant improvement in overall survival with a CDK4/6 inhibitor in combination with endocrine therapy in patients with HR-positive, HER2-negative advanced breast cancer.
In a video interview at the American Society of Clinical Oncology annual meeting, Sara A. Hurvitz, MD, from the University of California Los Angeles Jonsson Comprehensive Cancer Center, describes the significance of the MONALEESA-7 findings and the potential for improving on the study results with other agents or combinations.
The MONALEESA-7 trial is supported by Novartis. Dr. Hurvitz reported travel and accommodation expenses from Novartis.
REPORTING FROM ASCO 2019
FDA approves PI3K inhibitor alpelisib for breast cancer
The Food and Drug Administration has approved the first PI3K inhibitor for the treatment of breast cancer.
The drug, alpelisib (Piqray), was approved for use in combination with fulvestrant for men and postmenopausal women who have hormone receptor (HR)–positive, human epidermal growth factor receptor 2 (HER2)–negative, PIK3CA-mutated, advanced or metastatic breast cancer after progression on, or after, an endocrine-based regimen. The approval was announced by the FDA in a statement.
The agency also approved a diagnostic test – the therascreen PIK3CA RGQ PCR Kit – for detecting the PIK3CA mutation. The approval is based on results from the SOLAR-1 trial. In 572 HR-positive, HER2-negative patients with advanced or metastatic breast cancer who progressed after, or during, treatment with an aromatase inhibitor, the addition of alpelisib to fulvestrant significantly improved progression-free survival in patients with PIK3CA mutated tumors.
Median progression-free survival was 5.7 months on fulvestrant plus placebo, versus 11 months with fulvestrant plus alpelisib (hazard ratio for progression or death, 0.65; 95% confidence interval, 0.50-0.85; P less than .001). The results of the trial were recently published in the New England Journal of Medicine (2019 May 16;380[20]:1929-40).
In the FDA statement, Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence, noted that alpelisib is the “first PI3K inhibitor to demonstrate a clinically meaningful benefit in treating patients with this type of breast cancer.”
The drug’s application was approved under the Real-Time Oncology Review pilot program, which allows the FDA to begin analyzing efficacy and safety databases before a new drug application is even submitted.
The FDA noted that patients should not start treatment on alpelisib if they have a history of severe skin reactions, such as Stevens-Johnson syndrome, erythema multiforme, or toxic epidermal necrolysis.
The Food and Drug Administration has approved the first PI3K inhibitor for the treatment of breast cancer.
The drug, alpelisib (Piqray), was approved for use in combination with fulvestrant for men and postmenopausal women who have hormone receptor (HR)–positive, human epidermal growth factor receptor 2 (HER2)–negative, PIK3CA-mutated, advanced or metastatic breast cancer after progression on, or after, an endocrine-based regimen. The approval was announced by the FDA in a statement.
The agency also approved a diagnostic test – the therascreen PIK3CA RGQ PCR Kit – for detecting the PIK3CA mutation. The approval is based on results from the SOLAR-1 trial. In 572 HR-positive, HER2-negative patients with advanced or metastatic breast cancer who progressed after, or during, treatment with an aromatase inhibitor, the addition of alpelisib to fulvestrant significantly improved progression-free survival in patients with PIK3CA mutated tumors.
Median progression-free survival was 5.7 months on fulvestrant plus placebo, versus 11 months with fulvestrant plus alpelisib (hazard ratio for progression or death, 0.65; 95% confidence interval, 0.50-0.85; P less than .001). The results of the trial were recently published in the New England Journal of Medicine (2019 May 16;380[20]:1929-40).
In the FDA statement, Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence, noted that alpelisib is the “first PI3K inhibitor to demonstrate a clinically meaningful benefit in treating patients with this type of breast cancer.”
The drug’s application was approved under the Real-Time Oncology Review pilot program, which allows the FDA to begin analyzing efficacy and safety databases before a new drug application is even submitted.
The FDA noted that patients should not start treatment on alpelisib if they have a history of severe skin reactions, such as Stevens-Johnson syndrome, erythema multiforme, or toxic epidermal necrolysis.
The Food and Drug Administration has approved the first PI3K inhibitor for the treatment of breast cancer.
The drug, alpelisib (Piqray), was approved for use in combination with fulvestrant for men and postmenopausal women who have hormone receptor (HR)–positive, human epidermal growth factor receptor 2 (HER2)–negative, PIK3CA-mutated, advanced or metastatic breast cancer after progression on, or after, an endocrine-based regimen. The approval was announced by the FDA in a statement.
The agency also approved a diagnostic test – the therascreen PIK3CA RGQ PCR Kit – for detecting the PIK3CA mutation. The approval is based on results from the SOLAR-1 trial. In 572 HR-positive, HER2-negative patients with advanced or metastatic breast cancer who progressed after, or during, treatment with an aromatase inhibitor, the addition of alpelisib to fulvestrant significantly improved progression-free survival in patients with PIK3CA mutated tumors.
Median progression-free survival was 5.7 months on fulvestrant plus placebo, versus 11 months with fulvestrant plus alpelisib (hazard ratio for progression or death, 0.65; 95% confidence interval, 0.50-0.85; P less than .001). The results of the trial were recently published in the New England Journal of Medicine (2019 May 16;380[20]:1929-40).
In the FDA statement, Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence, noted that alpelisib is the “first PI3K inhibitor to demonstrate a clinically meaningful benefit in treating patients with this type of breast cancer.”
The drug’s application was approved under the Real-Time Oncology Review pilot program, which allows the FDA to begin analyzing efficacy and safety databases before a new drug application is even submitted.
The FDA noted that patients should not start treatment on alpelisib if they have a history of severe skin reactions, such as Stevens-Johnson syndrome, erythema multiforme, or toxic epidermal necrolysis.
Use of Oncotype DX to tailor breast cancer treatment likely to reduce costs
Routine use of the Oncotype DX recurrence score to personalize treatment of early breast cancer could reduce the first-year costs of care, according to results of a population-based cohort study.
“Practice changes based on evidence from the TAILORx trial on using tumor genomic profiles to personalize care could result in small decreases in U.S. national cancer care costs in the initial 12 months post breast cancer diagnosis,” Angela Mariotto, PhD, and colleagues concluded in a study published in JNCI: Journal of the National Cancer Institute. “Longer-term studies will be needed to evaluate the true long-term economic impact and nonmonetary benefits of personalized breast cancer care.”
Findings of the landmark TAILORx trial showed that, with use of the 21-gene score, the majority of women who have node-negative, hormone receptor–positive, HER2-negative breast cancers could safely skip adjuvant chemotherapy (N Engl J Med. 2018;379:111-21). But cost impact of its uptake into routine practice is unclear.
Dr. Mariotto, of the National Cancer Institute and her colleagues used data from the Surveillance, Epidemiology and End Results (SEER), SEER-Medicare, and SEER–Genomic Health datasets to assess how expected changes in practice after the trial might affect costs. They estimated Oncotype DX testing and chemotherapy rates and mean initial costs in 2018 dollars in the pre-TAILORx period (2010-2015) and post-TAILORx period (2018), assuming all women in the latter period received the test and score-suggested therapy.
Going from the pretrial period to the posttrial period, Oncotype DX testing costs were projected to increase from $115 million to $231 million, but chemotherapy use was projected to decrease from 25% to 17%. Mean total initial costs of care fell from $2.816 billion in the pretrial period to $2.766 billion in the posttrial period, for a net savings of $49 million (a 1.8% decrease).
Findings were similar in a variety of sensitivity scenarios entailing alternative compliance with testing, score-suggested treatment, and estimation methods. The only exception was the scenario in which all women aged 50 years or younger having a recurrence score of 16-25 opted to receive chemotherapy, wherein initial care costs could increase by $105 million (a 4% increase).
The investigators reported that they had no relevant conflicts of interest. The study was supported by the National Cancer Institute and its Coordinating Center For Clinical Trials; a Lombardi Comprehensive Cancer Center American Cancer Society Young Investigator Award; and the Cancer Prevention Research Fellowship, sponsored by the American Society of Preventive Oncology and Breast Cancer Research Foundation.
SOURCE: Mariotto A et al. J Natl Cancer Inst. 2019 Apr 24. doi: 10.1093/jnci/djz068.
Routine use of the Oncotype DX recurrence score to personalize treatment of early breast cancer could reduce the first-year costs of care, according to results of a population-based cohort study.
“Practice changes based on evidence from the TAILORx trial on using tumor genomic profiles to personalize care could result in small decreases in U.S. national cancer care costs in the initial 12 months post breast cancer diagnosis,” Angela Mariotto, PhD, and colleagues concluded in a study published in JNCI: Journal of the National Cancer Institute. “Longer-term studies will be needed to evaluate the true long-term economic impact and nonmonetary benefits of personalized breast cancer care.”
Findings of the landmark TAILORx trial showed that, with use of the 21-gene score, the majority of women who have node-negative, hormone receptor–positive, HER2-negative breast cancers could safely skip adjuvant chemotherapy (N Engl J Med. 2018;379:111-21). But cost impact of its uptake into routine practice is unclear.
Dr. Mariotto, of the National Cancer Institute and her colleagues used data from the Surveillance, Epidemiology and End Results (SEER), SEER-Medicare, and SEER–Genomic Health datasets to assess how expected changes in practice after the trial might affect costs. They estimated Oncotype DX testing and chemotherapy rates and mean initial costs in 2018 dollars in the pre-TAILORx period (2010-2015) and post-TAILORx period (2018), assuming all women in the latter period received the test and score-suggested therapy.
Going from the pretrial period to the posttrial period, Oncotype DX testing costs were projected to increase from $115 million to $231 million, but chemotherapy use was projected to decrease from 25% to 17%. Mean total initial costs of care fell from $2.816 billion in the pretrial period to $2.766 billion in the posttrial period, for a net savings of $49 million (a 1.8% decrease).
Findings were similar in a variety of sensitivity scenarios entailing alternative compliance with testing, score-suggested treatment, and estimation methods. The only exception was the scenario in which all women aged 50 years or younger having a recurrence score of 16-25 opted to receive chemotherapy, wherein initial care costs could increase by $105 million (a 4% increase).
The investigators reported that they had no relevant conflicts of interest. The study was supported by the National Cancer Institute and its Coordinating Center For Clinical Trials; a Lombardi Comprehensive Cancer Center American Cancer Society Young Investigator Award; and the Cancer Prevention Research Fellowship, sponsored by the American Society of Preventive Oncology and Breast Cancer Research Foundation.
SOURCE: Mariotto A et al. J Natl Cancer Inst. 2019 Apr 24. doi: 10.1093/jnci/djz068.
Routine use of the Oncotype DX recurrence score to personalize treatment of early breast cancer could reduce the first-year costs of care, according to results of a population-based cohort study.
“Practice changes based on evidence from the TAILORx trial on using tumor genomic profiles to personalize care could result in small decreases in U.S. national cancer care costs in the initial 12 months post breast cancer diagnosis,” Angela Mariotto, PhD, and colleagues concluded in a study published in JNCI: Journal of the National Cancer Institute. “Longer-term studies will be needed to evaluate the true long-term economic impact and nonmonetary benefits of personalized breast cancer care.”
Findings of the landmark TAILORx trial showed that, with use of the 21-gene score, the majority of women who have node-negative, hormone receptor–positive, HER2-negative breast cancers could safely skip adjuvant chemotherapy (N Engl J Med. 2018;379:111-21). But cost impact of its uptake into routine practice is unclear.
Dr. Mariotto, of the National Cancer Institute and her colleagues used data from the Surveillance, Epidemiology and End Results (SEER), SEER-Medicare, and SEER–Genomic Health datasets to assess how expected changes in practice after the trial might affect costs. They estimated Oncotype DX testing and chemotherapy rates and mean initial costs in 2018 dollars in the pre-TAILORx period (2010-2015) and post-TAILORx period (2018), assuming all women in the latter period received the test and score-suggested therapy.
Going from the pretrial period to the posttrial period, Oncotype DX testing costs were projected to increase from $115 million to $231 million, but chemotherapy use was projected to decrease from 25% to 17%. Mean total initial costs of care fell from $2.816 billion in the pretrial period to $2.766 billion in the posttrial period, for a net savings of $49 million (a 1.8% decrease).
Findings were similar in a variety of sensitivity scenarios entailing alternative compliance with testing, score-suggested treatment, and estimation methods. The only exception was the scenario in which all women aged 50 years or younger having a recurrence score of 16-25 opted to receive chemotherapy, wherein initial care costs could increase by $105 million (a 4% increase).
The investigators reported that they had no relevant conflicts of interest. The study was supported by the National Cancer Institute and its Coordinating Center For Clinical Trials; a Lombardi Comprehensive Cancer Center American Cancer Society Young Investigator Award; and the Cancer Prevention Research Fellowship, sponsored by the American Society of Preventive Oncology and Breast Cancer Research Foundation.
SOURCE: Mariotto A et al. J Natl Cancer Inst. 2019 Apr 24. doi: 10.1093/jnci/djz068.
FROM JNCI: JOURNAL OF THE NATIONAL CANCER INSTITUTE
Diet linked to lower risk of death from breast cancer
A balanced, low-fat diet was associated with a lower risk of death from breast cancer in a large cohort of postmenopausal women who had no previous history of breast cancer.
Researchers studied nearly 49,000 postmenopausal women and found a 21% lower risk of death from breast cancer among women who followed the balanced, low-fat diet, compared with women who followed their normal diet.
This research is scheduled to be presented at the annual meeting of the American Society of Clinical Oncology.
Rowan Chlebowski, MD, PhD, of the Los Angeles Biomedical Research Institute at Harbor–UCLA Medical Center in Torrance, Calif., discussed the research during a press briefing in advance of the meeting.
About the study
The research is part of the Woman’s Health Initiative (NCT00000611), which is focused on investigating methods for preventing heart disease, breast and colorectal cancer, and osteoporotic fractures in postmenopausal women.
This trial enrolled 48,835 postmenopausal women, ages 50-79 years, with no history of breast cancer and normal mammograms at enrollment. From 1993 to 1998, the women were randomized to the study diet (n = 19,541) or their normal diet (n = 29,294).
With the normal diet, fat accounted for 32% or more of subjects’ daily calories. With the study diet, the goal was to reduce fat consumption to 20% or less of caloric intake. The study diet also required at least one daily serving of vegetables, fruits, and grains.
Dr. Chlebowski said the study diet is similar to DASH (Dietary Approaches to Stop Hypertension), but is slightly more focused on lowering fat intake.
Diet adherence
Subjects followed the study diet for a median of 8.5 years, and the median cumulative follow-up was 19.6 years.
Dr. Chlebowski noted that most women on the study diet were not able to reduce their daily fat consumption to the 20% goal. They did reduce fat consumption to 24.5% overall, which increased to 29% at the end of the intervention.
In the study-diet group, there was an average weight loss of 3%, significantly different from that of the normal-diet group (P less than .001).
Dr. Chlebowski said the weight loss indicates that subjects did adhere to the study diet, at least in part, as there was no change in physical activity among study participants. Furthermore, the researchers have evidence after 1 year that suggests subjects were incorporating more fruits and vegetables into their diets.
Breast cancer and death
At a median follow-up of 19.6 years, there were 3,374 cases of breast cancer, 1,011 deaths, and 383 deaths attributed to breast cancer.
The risk of death from breast cancer was significantly lower in the study-diet group than in the normal-diet group. The hazard ratio was 0.79 (95% confidence interval, 0.64-0.97; P = .025).
The risk of death (from any cause) after breast cancer was significantly lower in the study diet group as well, with a hazard ratio of 0.85 (95% confidence interval, 0.74-0.96; P = .01).
“Adoption of a low-fat dietary pattern reduces the risk of death from breast cancer in postmenopausal women,” Dr. Chlebowski said. “To our review, this is the only study providing randomized clinical trial evidence that an intervention can reduce a woman’s risk of dying from breast cancer.”
Dr. Chlebowski noted that the researchers have blood samples from all subjects enrolled in this study. The researchers plan to analyze those samples to further explore how the study diet affected the women and determine which components of the diet account for which effects.
The National Institutes of Health funded the study. The researchers disclosed relationships with Novartis, Pfizer, Amgen, AstraZeneca, Immunomedics, Metastat, Bayer, and Genentech/Roche.
SOURCE: Chlebowski R. et al. ASCO 2019. Abstract 520.
A balanced, low-fat diet was associated with a lower risk of death from breast cancer in a large cohort of postmenopausal women who had no previous history of breast cancer.
Researchers studied nearly 49,000 postmenopausal women and found a 21% lower risk of death from breast cancer among women who followed the balanced, low-fat diet, compared with women who followed their normal diet.
This research is scheduled to be presented at the annual meeting of the American Society of Clinical Oncology.
Rowan Chlebowski, MD, PhD, of the Los Angeles Biomedical Research Institute at Harbor–UCLA Medical Center in Torrance, Calif., discussed the research during a press briefing in advance of the meeting.
About the study
The research is part of the Woman’s Health Initiative (NCT00000611), which is focused on investigating methods for preventing heart disease, breast and colorectal cancer, and osteoporotic fractures in postmenopausal women.
This trial enrolled 48,835 postmenopausal women, ages 50-79 years, with no history of breast cancer and normal mammograms at enrollment. From 1993 to 1998, the women were randomized to the study diet (n = 19,541) or their normal diet (n = 29,294).
With the normal diet, fat accounted for 32% or more of subjects’ daily calories. With the study diet, the goal was to reduce fat consumption to 20% or less of caloric intake. The study diet also required at least one daily serving of vegetables, fruits, and grains.
Dr. Chlebowski said the study diet is similar to DASH (Dietary Approaches to Stop Hypertension), but is slightly more focused on lowering fat intake.
Diet adherence
Subjects followed the study diet for a median of 8.5 years, and the median cumulative follow-up was 19.6 years.
Dr. Chlebowski noted that most women on the study diet were not able to reduce their daily fat consumption to the 20% goal. They did reduce fat consumption to 24.5% overall, which increased to 29% at the end of the intervention.
In the study-diet group, there was an average weight loss of 3%, significantly different from that of the normal-diet group (P less than .001).
Dr. Chlebowski said the weight loss indicates that subjects did adhere to the study diet, at least in part, as there was no change in physical activity among study participants. Furthermore, the researchers have evidence after 1 year that suggests subjects were incorporating more fruits and vegetables into their diets.
Breast cancer and death
At a median follow-up of 19.6 years, there were 3,374 cases of breast cancer, 1,011 deaths, and 383 deaths attributed to breast cancer.
The risk of death from breast cancer was significantly lower in the study-diet group than in the normal-diet group. The hazard ratio was 0.79 (95% confidence interval, 0.64-0.97; P = .025).
The risk of death (from any cause) after breast cancer was significantly lower in the study diet group as well, with a hazard ratio of 0.85 (95% confidence interval, 0.74-0.96; P = .01).
“Adoption of a low-fat dietary pattern reduces the risk of death from breast cancer in postmenopausal women,” Dr. Chlebowski said. “To our review, this is the only study providing randomized clinical trial evidence that an intervention can reduce a woman’s risk of dying from breast cancer.”
Dr. Chlebowski noted that the researchers have blood samples from all subjects enrolled in this study. The researchers plan to analyze those samples to further explore how the study diet affected the women and determine which components of the diet account for which effects.
The National Institutes of Health funded the study. The researchers disclosed relationships with Novartis, Pfizer, Amgen, AstraZeneca, Immunomedics, Metastat, Bayer, and Genentech/Roche.
SOURCE: Chlebowski R. et al. ASCO 2019. Abstract 520.
A balanced, low-fat diet was associated with a lower risk of death from breast cancer in a large cohort of postmenopausal women who had no previous history of breast cancer.
Researchers studied nearly 49,000 postmenopausal women and found a 21% lower risk of death from breast cancer among women who followed the balanced, low-fat diet, compared with women who followed their normal diet.
This research is scheduled to be presented at the annual meeting of the American Society of Clinical Oncology.
Rowan Chlebowski, MD, PhD, of the Los Angeles Biomedical Research Institute at Harbor–UCLA Medical Center in Torrance, Calif., discussed the research during a press briefing in advance of the meeting.
About the study
The research is part of the Woman’s Health Initiative (NCT00000611), which is focused on investigating methods for preventing heart disease, breast and colorectal cancer, and osteoporotic fractures in postmenopausal women.
This trial enrolled 48,835 postmenopausal women, ages 50-79 years, with no history of breast cancer and normal mammograms at enrollment. From 1993 to 1998, the women were randomized to the study diet (n = 19,541) or their normal diet (n = 29,294).
With the normal diet, fat accounted for 32% or more of subjects’ daily calories. With the study diet, the goal was to reduce fat consumption to 20% or less of caloric intake. The study diet also required at least one daily serving of vegetables, fruits, and grains.
Dr. Chlebowski said the study diet is similar to DASH (Dietary Approaches to Stop Hypertension), but is slightly more focused on lowering fat intake.
Diet adherence
Subjects followed the study diet for a median of 8.5 years, and the median cumulative follow-up was 19.6 years.
Dr. Chlebowski noted that most women on the study diet were not able to reduce their daily fat consumption to the 20% goal. They did reduce fat consumption to 24.5% overall, which increased to 29% at the end of the intervention.
In the study-diet group, there was an average weight loss of 3%, significantly different from that of the normal-diet group (P less than .001).
Dr. Chlebowski said the weight loss indicates that subjects did adhere to the study diet, at least in part, as there was no change in physical activity among study participants. Furthermore, the researchers have evidence after 1 year that suggests subjects were incorporating more fruits and vegetables into their diets.
Breast cancer and death
At a median follow-up of 19.6 years, there were 3,374 cases of breast cancer, 1,011 deaths, and 383 deaths attributed to breast cancer.
The risk of death from breast cancer was significantly lower in the study-diet group than in the normal-diet group. The hazard ratio was 0.79 (95% confidence interval, 0.64-0.97; P = .025).
The risk of death (from any cause) after breast cancer was significantly lower in the study diet group as well, with a hazard ratio of 0.85 (95% confidence interval, 0.74-0.96; P = .01).
“Adoption of a low-fat dietary pattern reduces the risk of death from breast cancer in postmenopausal women,” Dr. Chlebowski said. “To our review, this is the only study providing randomized clinical trial evidence that an intervention can reduce a woman’s risk of dying from breast cancer.”
Dr. Chlebowski noted that the researchers have blood samples from all subjects enrolled in this study. The researchers plan to analyze those samples to further explore how the study diet affected the women and determine which components of the diet account for which effects.
The National Institutes of Health funded the study. The researchers disclosed relationships with Novartis, Pfizer, Amgen, AstraZeneca, Immunomedics, Metastat, Bayer, and Genentech/Roche.
SOURCE: Chlebowski R. et al. ASCO 2019. Abstract 520.
REPORTING FROM ASCO 2019
Key clinical point: Women who followed a balanced, low-fat diet had a lower risk of death from breast cancer compared with women who followed their normal diet.
Major finding: The risk of death from breast cancer was significantly lower in the study-diet group than in the normal-diet group (hazard ratio, 0.79, 95% confidence interval, 0.64-0.97; P = .025).
Study details: A cohort from the Women’s Health Initiative, which randomized 48,835 postmenopausal women to a low-fat diet or to their normal diet.
Disclosures: The National Institutes of Health funded the study. The researchers disclosed relationships with Novartis, Pfizer, Amgen, AstraZeneca, Immunomedics, Metastat, Bayer, and Genentech/Roche.
Source: Chlebowski R. et al. ASCO 2019. Abstract 520.
ICYMI: Alpelisib/fulvestrant combo boosts PFS in advanced breast cancer
Patients with PIK3CA-mutated, hormone receptor–positive, HER2-negative advanced breast cancer who had previously undergone endocrine therapy experienced longer progression-free survival when receiving alpelisib and fulvestrant, compared with placebo and fulvestrant (11.0 vs. 5.7 months; hazard ratio, 0.65; 95% confidence interval, 0.50-0.85; P less than .001), according to a randomized, phase 3 trial published in the New England Journal of Medicine (2019 May 15. doi: 10.1056/NEJMoa1813904).
We first reported on the results of this trial when they were presented at the European Society for Medical Oncology Congress. Find our coverage at the link below.
Patients with PIK3CA-mutated, hormone receptor–positive, HER2-negative advanced breast cancer who had previously undergone endocrine therapy experienced longer progression-free survival when receiving alpelisib and fulvestrant, compared with placebo and fulvestrant (11.0 vs. 5.7 months; hazard ratio, 0.65; 95% confidence interval, 0.50-0.85; P less than .001), according to a randomized, phase 3 trial published in the New England Journal of Medicine (2019 May 15. doi: 10.1056/NEJMoa1813904).
We first reported on the results of this trial when they were presented at the European Society for Medical Oncology Congress. Find our coverage at the link below.
Patients with PIK3CA-mutated, hormone receptor–positive, HER2-negative advanced breast cancer who had previously undergone endocrine therapy experienced longer progression-free survival when receiving alpelisib and fulvestrant, compared with placebo and fulvestrant (11.0 vs. 5.7 months; hazard ratio, 0.65; 95% confidence interval, 0.50-0.85; P less than .001), according to a randomized, phase 3 trial published in the New England Journal of Medicine (2019 May 15. doi: 10.1056/NEJMoa1813904).
We first reported on the results of this trial when they were presented at the European Society for Medical Oncology Congress. Find our coverage at the link below.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
TVEC may improve response rates in nonmetastatic TNBC
ATLANTA – Adding intratumoral talimogene laherparepvec (TVEC) to neoadjuvant chemotherapy appears to improve response rates in patients with nonmetastatic triple-negative breast cancer, according to findings from a phase 1 trial.
The pathologic complete response rate (pCR) in nine patients aged 18-70 years with stage T2-T3NO-2 disease who were enrolled in the single-center trial was 55%, whereas the expected rate with neoadjuvant chemotherapy alone was 30%, Hatem Soliman, MD, reported at the annual meeting of the American Association for Cancer Research.
“Even in the patients with residual disease, they had almost complete obliteration of their tumors, as well,” said Dr. Soliman of Moffitt Cancer Center, Tampa, Fla., adding that preliminary analyses of T cell subtypes indicated that CD45RO+ cells, which are associated with activated memory effector phenotype cells, were found in higher percentages in tumor specimens from patients who had pCR vs. those who did not.
“This could be an emerging marker that could be associated with pCR from TVEC,” he said, stressing, however, that “this is preliminary data [that] needs to be borne out in subsequent studies.”
Of the nine participants, six had stage II disease and three had stage III disease, and tumor size was greater than 5 cm in 2 patients, and 2-5 cm in 7 patients. Three received 106 plaque-forming units (PFUs) x 5 injections (dose level 1), and 6 received 106 PFUs for the first injection then 108 PFUs for 4 additional injections. Patients also received neoadjuvant paclitaxel followed by doxorubicin/cyclophosphamide chemotherapy.
They then went on to surgery and were evaluated for pCR.
No dose-limiting toxicities occurred, therefore the maximum-tolerated dose was dose level 2, Dr. Soliman noted.
The most common toxicities due to TVEC were fevers, chills, and injection site reactions, and “these are considered expected side effects of TVEC administration and were manageable,” he said.
Two serious adverse events occurred in the trial: a pulmonary embolism and a postoperative case of severe bradycardia thought to be a vasovagal episode; there were no deaths or any sequelae from these adverse events and they completely resolved.
One latent genital wild type herpes simplex 1 virus (HSV1) reactivation event occurred and was treated with a topical agent.
Early-stage triple-negative breast cancer is associated with a higher risk of early relapse, but attaining a pCR after neoadjuvant chemotherapy is associated with improved prognosis, Dr. Soliman said.
“Historically, standard regimens used for triple-negative breast cancer include an anthracycline and a taxane, and the rates of pCR in those patients hover around 30%,” he said. “Others have looked at the relationship of increased tumor infiltrating lymphocytes (TILs] in triple-negative breast cancers and other breast cancers, and found that those tumors that did have a higher de novo TIL infiltrate, either at the beginning of treatment or acquired during treatment, seemed to have a higher rate of pCR and also seemed to have improved prognosis.”
This suggested that these TIL infiltrates may be both a predictive and prognostic biologic marker for the biology of the disease, he noted.
Further, promising prior research on the role of oncolytic viruses as a potential treatment modality for cancer led to interest in their use during neoadjuvant chemotherapy “with the idea that we could potentially improve pathologic complete response rates both through direct tumor cell lysis of treated tumors, but also through recruitment of a robust antitumor immune response caused by the viruses’ mechanism of action,” he said, adding that “this could be beneficial, particularly in those immunologically ‘cold’ tumors.”
This led to the incorporation of TVEC, a genetically engineered oncolytic HSV1 currently approved for the treatment of melanoma, in this neoadjuvant trial. TVEC preferentially lyses tumor cells over normal tissue to release tumor associated antigens, produces granulocyte-macrophage colony-stimulating factor to activate dendritic cells, and stimulates T cells to provoke an adaptive immune response, he explained.
“[This] then can not only potentially attack tumor cells at the primary site, but then may potentially spread around the body to improve host surveillance and try to eradicate micrometastatic disease, as well,” he said.
The current findings show that adding TVEC to neoadjuvant chemotherapy is feasible at the full Food and Drug Administration–approved dose and has manageable toxicity, he concluded, noting that “a phase 2 single-arm trial of this regimen is ongoing, and we are actively accruing patients to further evaluate the efficacy signal and formally test the hypothesis along with immune correlates.”
Dr. Soliman reported relationships with Eli Lilly, Pfizer, Celgene, AstraZeneca, PUMA, Novartis, Eisai, and Amgen.
SOURCE: Soliman H et al. AACR 2019, Abstract CT040.
ATLANTA – Adding intratumoral talimogene laherparepvec (TVEC) to neoadjuvant chemotherapy appears to improve response rates in patients with nonmetastatic triple-negative breast cancer, according to findings from a phase 1 trial.
The pathologic complete response rate (pCR) in nine patients aged 18-70 years with stage T2-T3NO-2 disease who were enrolled in the single-center trial was 55%, whereas the expected rate with neoadjuvant chemotherapy alone was 30%, Hatem Soliman, MD, reported at the annual meeting of the American Association for Cancer Research.
“Even in the patients with residual disease, they had almost complete obliteration of their tumors, as well,” said Dr. Soliman of Moffitt Cancer Center, Tampa, Fla., adding that preliminary analyses of T cell subtypes indicated that CD45RO+ cells, which are associated with activated memory effector phenotype cells, were found in higher percentages in tumor specimens from patients who had pCR vs. those who did not.
“This could be an emerging marker that could be associated with pCR from TVEC,” he said, stressing, however, that “this is preliminary data [that] needs to be borne out in subsequent studies.”
Of the nine participants, six had stage II disease and three had stage III disease, and tumor size was greater than 5 cm in 2 patients, and 2-5 cm in 7 patients. Three received 106 plaque-forming units (PFUs) x 5 injections (dose level 1), and 6 received 106 PFUs for the first injection then 108 PFUs for 4 additional injections. Patients also received neoadjuvant paclitaxel followed by doxorubicin/cyclophosphamide chemotherapy.
They then went on to surgery and were evaluated for pCR.
No dose-limiting toxicities occurred, therefore the maximum-tolerated dose was dose level 2, Dr. Soliman noted.
The most common toxicities due to TVEC were fevers, chills, and injection site reactions, and “these are considered expected side effects of TVEC administration and were manageable,” he said.
Two serious adverse events occurred in the trial: a pulmonary embolism and a postoperative case of severe bradycardia thought to be a vasovagal episode; there were no deaths or any sequelae from these adverse events and they completely resolved.
One latent genital wild type herpes simplex 1 virus (HSV1) reactivation event occurred and was treated with a topical agent.
Early-stage triple-negative breast cancer is associated with a higher risk of early relapse, but attaining a pCR after neoadjuvant chemotherapy is associated with improved prognosis, Dr. Soliman said.
“Historically, standard regimens used for triple-negative breast cancer include an anthracycline and a taxane, and the rates of pCR in those patients hover around 30%,” he said. “Others have looked at the relationship of increased tumor infiltrating lymphocytes (TILs] in triple-negative breast cancers and other breast cancers, and found that those tumors that did have a higher de novo TIL infiltrate, either at the beginning of treatment or acquired during treatment, seemed to have a higher rate of pCR and also seemed to have improved prognosis.”
This suggested that these TIL infiltrates may be both a predictive and prognostic biologic marker for the biology of the disease, he noted.
Further, promising prior research on the role of oncolytic viruses as a potential treatment modality for cancer led to interest in their use during neoadjuvant chemotherapy “with the idea that we could potentially improve pathologic complete response rates both through direct tumor cell lysis of treated tumors, but also through recruitment of a robust antitumor immune response caused by the viruses’ mechanism of action,” he said, adding that “this could be beneficial, particularly in those immunologically ‘cold’ tumors.”
This led to the incorporation of TVEC, a genetically engineered oncolytic HSV1 currently approved for the treatment of melanoma, in this neoadjuvant trial. TVEC preferentially lyses tumor cells over normal tissue to release tumor associated antigens, produces granulocyte-macrophage colony-stimulating factor to activate dendritic cells, and stimulates T cells to provoke an adaptive immune response, he explained.
“[This] then can not only potentially attack tumor cells at the primary site, but then may potentially spread around the body to improve host surveillance and try to eradicate micrometastatic disease, as well,” he said.
The current findings show that adding TVEC to neoadjuvant chemotherapy is feasible at the full Food and Drug Administration–approved dose and has manageable toxicity, he concluded, noting that “a phase 2 single-arm trial of this regimen is ongoing, and we are actively accruing patients to further evaluate the efficacy signal and formally test the hypothesis along with immune correlates.”
Dr. Soliman reported relationships with Eli Lilly, Pfizer, Celgene, AstraZeneca, PUMA, Novartis, Eisai, and Amgen.
SOURCE: Soliman H et al. AACR 2019, Abstract CT040.
ATLANTA – Adding intratumoral talimogene laherparepvec (TVEC) to neoadjuvant chemotherapy appears to improve response rates in patients with nonmetastatic triple-negative breast cancer, according to findings from a phase 1 trial.
The pathologic complete response rate (pCR) in nine patients aged 18-70 years with stage T2-T3NO-2 disease who were enrolled in the single-center trial was 55%, whereas the expected rate with neoadjuvant chemotherapy alone was 30%, Hatem Soliman, MD, reported at the annual meeting of the American Association for Cancer Research.
“Even in the patients with residual disease, they had almost complete obliteration of their tumors, as well,” said Dr. Soliman of Moffitt Cancer Center, Tampa, Fla., adding that preliminary analyses of T cell subtypes indicated that CD45RO+ cells, which are associated with activated memory effector phenotype cells, were found in higher percentages in tumor specimens from patients who had pCR vs. those who did not.
“This could be an emerging marker that could be associated with pCR from TVEC,” he said, stressing, however, that “this is preliminary data [that] needs to be borne out in subsequent studies.”
Of the nine participants, six had stage II disease and three had stage III disease, and tumor size was greater than 5 cm in 2 patients, and 2-5 cm in 7 patients. Three received 106 plaque-forming units (PFUs) x 5 injections (dose level 1), and 6 received 106 PFUs for the first injection then 108 PFUs for 4 additional injections. Patients also received neoadjuvant paclitaxel followed by doxorubicin/cyclophosphamide chemotherapy.
They then went on to surgery and were evaluated for pCR.
No dose-limiting toxicities occurred, therefore the maximum-tolerated dose was dose level 2, Dr. Soliman noted.
The most common toxicities due to TVEC were fevers, chills, and injection site reactions, and “these are considered expected side effects of TVEC administration and were manageable,” he said.
Two serious adverse events occurred in the trial: a pulmonary embolism and a postoperative case of severe bradycardia thought to be a vasovagal episode; there were no deaths or any sequelae from these adverse events and they completely resolved.
One latent genital wild type herpes simplex 1 virus (HSV1) reactivation event occurred and was treated with a topical agent.
Early-stage triple-negative breast cancer is associated with a higher risk of early relapse, but attaining a pCR after neoadjuvant chemotherapy is associated with improved prognosis, Dr. Soliman said.
“Historically, standard regimens used for triple-negative breast cancer include an anthracycline and a taxane, and the rates of pCR in those patients hover around 30%,” he said. “Others have looked at the relationship of increased tumor infiltrating lymphocytes (TILs] in triple-negative breast cancers and other breast cancers, and found that those tumors that did have a higher de novo TIL infiltrate, either at the beginning of treatment or acquired during treatment, seemed to have a higher rate of pCR and also seemed to have improved prognosis.”
This suggested that these TIL infiltrates may be both a predictive and prognostic biologic marker for the biology of the disease, he noted.
Further, promising prior research on the role of oncolytic viruses as a potential treatment modality for cancer led to interest in their use during neoadjuvant chemotherapy “with the idea that we could potentially improve pathologic complete response rates both through direct tumor cell lysis of treated tumors, but also through recruitment of a robust antitumor immune response caused by the viruses’ mechanism of action,” he said, adding that “this could be beneficial, particularly in those immunologically ‘cold’ tumors.”
This led to the incorporation of TVEC, a genetically engineered oncolytic HSV1 currently approved for the treatment of melanoma, in this neoadjuvant trial. TVEC preferentially lyses tumor cells over normal tissue to release tumor associated antigens, produces granulocyte-macrophage colony-stimulating factor to activate dendritic cells, and stimulates T cells to provoke an adaptive immune response, he explained.
“[This] then can not only potentially attack tumor cells at the primary site, but then may potentially spread around the body to improve host surveillance and try to eradicate micrometastatic disease, as well,” he said.
The current findings show that adding TVEC to neoadjuvant chemotherapy is feasible at the full Food and Drug Administration–approved dose and has manageable toxicity, he concluded, noting that “a phase 2 single-arm trial of this regimen is ongoing, and we are actively accruing patients to further evaluate the efficacy signal and formally test the hypothesis along with immune correlates.”
Dr. Soliman reported relationships with Eli Lilly, Pfizer, Celgene, AstraZeneca, PUMA, Novartis, Eisai, and Amgen.
SOURCE: Soliman H et al. AACR 2019, Abstract CT040.
REPORTING FROM AACR 2019
Key clinical point: Adding TVEC to neoadjuvant chemotherapy for nonmetastatic TNBC appears to improve pCR response rates.
Major finding: The pCR was 55% with TVEC compared with an expected rate of 30% without TVEC.
Study details: A phase 1 study of 9 patients.
Disclosures: Dr. Soliman reported relationships with Eli Lilly, Pfizer, Celgene, AstraZeneca, PUMA, Novartis, Eisai, and Amgen.
Source: Soliman H et al. AACR 2019, Abstract CT040.
PROs in lung cancer and how to administer trastuzumab
In this edition of “How I will treat my next patient,” I take a look at two recent trials – one summarizes a presentation at the European Lung Cancer Congress on the value of durvalumab as adjuvant treatment in patients with locally-advanced non–small cell lung cancer and the other confirms the safety and efficacy of subcutaneously-administered trastuzumab as neoadjuvant treatment in HER2/-positive breast cancer patients.
PACIFIC trial
In the PACIFIC trial, 713 patients with unresectable, stage III non–small cell lung cancer (NSCLC) who received concurrent chemoradiation were randomized to receive adjuvant durvalumab or an identical placebo, for a year after radiation ended. The results were dramatic in favor of durvalumab (N Engl J Med. 2018;379:2342-50).
Durvalumab showed 24-month overall survival of 66.3% versus 55.6% with placebo (hazard ratio, 0.68, P = .0025) and progression-free survival of 17.2 months versus 5. 6 months (HR, 0.51). As expected, there were more grade 3-4 toxicities and treatment discontinuations with durvalumab than with placebo, but the toxicity seemed modest, given the substantial improvements in tumor-related outcomes.
At the recent European Lung Cancer Congress, Marina Garassino, MD, reported on Patient-Reported Outcomes (PRO) in PACIFIC. PROs were analyzed by PD-L1 level. A total of 63% of patients had PD-L1 tumor expression data for analysis. Overall, there were no major differences in PROs by PD-L1. Global quality of life did not differ by PD-L1 expression cohort.
These data support adjuvant durvalumab for stage III, chemoradiation-treated NSCLC patients, not only from efficacy and toxicity viewpoints, but also from the standpoint of the patient experience, independent of PD-L1 tumor expression.
What this means in practice
From every relevant perspective, regardless of histology and molecular features associated with their particular tumor, it is worthwhile for us to recommend – and for our patients to receive – durvalumab adjuvant therapy for up to 1 year after radiation ends, with close follow-up and adherence to the criteria for treatment modification or discontinuation as performed in the PACIFIC trial. These new data remove any lingering concerns about the value of this life-prolonging treatment.
Subcutaneous vs. IV trastuzumab
In this international phase 3 trial in early breast cancer patients, neoadjuvant chemotherapy was paired with either standard IV trastuzumab or subcutaneous trastuzumab at intervals of every 3 weeks. After the cytotoxic chemotherapy concluded, patients completed a 12-month course of trastuzumab with either the IV or subcutaneous administration, as previously randomized. The 6-year event-free survival and overall survival were 65% and 84%, respectively, for both the IV and subcutaneous treatment administration.
The authors concluded that these results are relevant to patients with low-risk HER2-positive breast cancer patients, for whom T-DM1 is not needed (JAMA Oncol. 2019 Apr 18. doi: 10.1001/jamaoncol.2019.0339).
What this means in practice
These long-term data from the HannaH trial show persuasively that patients should be offered the more convenient, hopefully cheaper, subcutaneous route of administration. Since relapses beyond year 6 are unlikely, these data are unlikely to change with further follow-up. At our hospital, we recently made the decision to add subcutaneous trastuzumab to our formulary.
Dr. Lyss has been a community-based medical oncologist and clinical researcher for more than 35 years, practicing in St. Louis. His clinical and research interests are in the prevention, diagnosis, and treatment of breast and lung cancers, and in expanding access to clinical trials to medically underserved populations.
In this edition of “How I will treat my next patient,” I take a look at two recent trials – one summarizes a presentation at the European Lung Cancer Congress on the value of durvalumab as adjuvant treatment in patients with locally-advanced non–small cell lung cancer and the other confirms the safety and efficacy of subcutaneously-administered trastuzumab as neoadjuvant treatment in HER2/-positive breast cancer patients.
PACIFIC trial
In the PACIFIC trial, 713 patients with unresectable, stage III non–small cell lung cancer (NSCLC) who received concurrent chemoradiation were randomized to receive adjuvant durvalumab or an identical placebo, for a year after radiation ended. The results were dramatic in favor of durvalumab (N Engl J Med. 2018;379:2342-50).
Durvalumab showed 24-month overall survival of 66.3% versus 55.6% with placebo (hazard ratio, 0.68, P = .0025) and progression-free survival of 17.2 months versus 5. 6 months (HR, 0.51). As expected, there were more grade 3-4 toxicities and treatment discontinuations with durvalumab than with placebo, but the toxicity seemed modest, given the substantial improvements in tumor-related outcomes.
At the recent European Lung Cancer Congress, Marina Garassino, MD, reported on Patient-Reported Outcomes (PRO) in PACIFIC. PROs were analyzed by PD-L1 level. A total of 63% of patients had PD-L1 tumor expression data for analysis. Overall, there were no major differences in PROs by PD-L1. Global quality of life did not differ by PD-L1 expression cohort.
These data support adjuvant durvalumab for stage III, chemoradiation-treated NSCLC patients, not only from efficacy and toxicity viewpoints, but also from the standpoint of the patient experience, independent of PD-L1 tumor expression.
What this means in practice
From every relevant perspective, regardless of histology and molecular features associated with their particular tumor, it is worthwhile for us to recommend – and for our patients to receive – durvalumab adjuvant therapy for up to 1 year after radiation ends, with close follow-up and adherence to the criteria for treatment modification or discontinuation as performed in the PACIFIC trial. These new data remove any lingering concerns about the value of this life-prolonging treatment.
Subcutaneous vs. IV trastuzumab
In this international phase 3 trial in early breast cancer patients, neoadjuvant chemotherapy was paired with either standard IV trastuzumab or subcutaneous trastuzumab at intervals of every 3 weeks. After the cytotoxic chemotherapy concluded, patients completed a 12-month course of trastuzumab with either the IV or subcutaneous administration, as previously randomized. The 6-year event-free survival and overall survival were 65% and 84%, respectively, for both the IV and subcutaneous treatment administration.
The authors concluded that these results are relevant to patients with low-risk HER2-positive breast cancer patients, for whom T-DM1 is not needed (JAMA Oncol. 2019 Apr 18. doi: 10.1001/jamaoncol.2019.0339).
What this means in practice
These long-term data from the HannaH trial show persuasively that patients should be offered the more convenient, hopefully cheaper, subcutaneous route of administration. Since relapses beyond year 6 are unlikely, these data are unlikely to change with further follow-up. At our hospital, we recently made the decision to add subcutaneous trastuzumab to our formulary.
Dr. Lyss has been a community-based medical oncologist and clinical researcher for more than 35 years, practicing in St. Louis. His clinical and research interests are in the prevention, diagnosis, and treatment of breast and lung cancers, and in expanding access to clinical trials to medically underserved populations.
In this edition of “How I will treat my next patient,” I take a look at two recent trials – one summarizes a presentation at the European Lung Cancer Congress on the value of durvalumab as adjuvant treatment in patients with locally-advanced non–small cell lung cancer and the other confirms the safety and efficacy of subcutaneously-administered trastuzumab as neoadjuvant treatment in HER2/-positive breast cancer patients.
PACIFIC trial
In the PACIFIC trial, 713 patients with unresectable, stage III non–small cell lung cancer (NSCLC) who received concurrent chemoradiation were randomized to receive adjuvant durvalumab or an identical placebo, for a year after radiation ended. The results were dramatic in favor of durvalumab (N Engl J Med. 2018;379:2342-50).
Durvalumab showed 24-month overall survival of 66.3% versus 55.6% with placebo (hazard ratio, 0.68, P = .0025) and progression-free survival of 17.2 months versus 5. 6 months (HR, 0.51). As expected, there were more grade 3-4 toxicities and treatment discontinuations with durvalumab than with placebo, but the toxicity seemed modest, given the substantial improvements in tumor-related outcomes.
At the recent European Lung Cancer Congress, Marina Garassino, MD, reported on Patient-Reported Outcomes (PRO) in PACIFIC. PROs were analyzed by PD-L1 level. A total of 63% of patients had PD-L1 tumor expression data for analysis. Overall, there were no major differences in PROs by PD-L1. Global quality of life did not differ by PD-L1 expression cohort.
These data support adjuvant durvalumab for stage III, chemoradiation-treated NSCLC patients, not only from efficacy and toxicity viewpoints, but also from the standpoint of the patient experience, independent of PD-L1 tumor expression.
What this means in practice
From every relevant perspective, regardless of histology and molecular features associated with their particular tumor, it is worthwhile for us to recommend – and for our patients to receive – durvalumab adjuvant therapy for up to 1 year after radiation ends, with close follow-up and adherence to the criteria for treatment modification or discontinuation as performed in the PACIFIC trial. These new data remove any lingering concerns about the value of this life-prolonging treatment.
Subcutaneous vs. IV trastuzumab
In this international phase 3 trial in early breast cancer patients, neoadjuvant chemotherapy was paired with either standard IV trastuzumab or subcutaneous trastuzumab at intervals of every 3 weeks. After the cytotoxic chemotherapy concluded, patients completed a 12-month course of trastuzumab with either the IV or subcutaneous administration, as previously randomized. The 6-year event-free survival and overall survival were 65% and 84%, respectively, for both the IV and subcutaneous treatment administration.
The authors concluded that these results are relevant to patients with low-risk HER2-positive breast cancer patients, for whom T-DM1 is not needed (JAMA Oncol. 2019 Apr 18. doi: 10.1001/jamaoncol.2019.0339).
What this means in practice
These long-term data from the HannaH trial show persuasively that patients should be offered the more convenient, hopefully cheaper, subcutaneous route of administration. Since relapses beyond year 6 are unlikely, these data are unlikely to change with further follow-up. At our hospital, we recently made the decision to add subcutaneous trastuzumab to our formulary.
Dr. Lyss has been a community-based medical oncologist and clinical researcher for more than 35 years, practicing in St. Louis. His clinical and research interests are in the prevention, diagnosis, and treatment of breast and lung cancers, and in expanding access to clinical trials to medically underserved populations.