User login
Vaginal estrogen therapy may be prescribed in BC patients with genitourinary symptoms
Key clinical point: Vaginal estrogen therapy did not worsen mortality outcomes in patients with breast cancer (BC) and genitourinary symptoms and can be considered if nonhormonal treatments prove unsuccessful.
Major finding: BC-specific mortality was not worsened in patients with BC who received vaginal estrogen therapy vs no hormone replacement therapy (hazard ratio 0.77; 95% CI 0.63-0.94).
Study details: Findings are from an analysis of two large cohorts including 49,237 females with BC, of which 5% of females used vaginal estrogen therapy after BC diagnosis.
Disclosures: This study was supported by grants from Cancer Research UK. Some authors declared receiving grants, personal fees, or nonfinancial support from and having other ties with several sources.
Source: McVicker L et al. Vaginal estrogen therapy use and survival in females with breast cancer. JAMA Oncol. 2023 (Nov 2). doi: 10.1001/jamaoncol.2023.4508
Key clinical point: Vaginal estrogen therapy did not worsen mortality outcomes in patients with breast cancer (BC) and genitourinary symptoms and can be considered if nonhormonal treatments prove unsuccessful.
Major finding: BC-specific mortality was not worsened in patients with BC who received vaginal estrogen therapy vs no hormone replacement therapy (hazard ratio 0.77; 95% CI 0.63-0.94).
Study details: Findings are from an analysis of two large cohorts including 49,237 females with BC, of which 5% of females used vaginal estrogen therapy after BC diagnosis.
Disclosures: This study was supported by grants from Cancer Research UK. Some authors declared receiving grants, personal fees, or nonfinancial support from and having other ties with several sources.
Source: McVicker L et al. Vaginal estrogen therapy use and survival in females with breast cancer. JAMA Oncol. 2023 (Nov 2). doi: 10.1001/jamaoncol.2023.4508
Key clinical point: Vaginal estrogen therapy did not worsen mortality outcomes in patients with breast cancer (BC) and genitourinary symptoms and can be considered if nonhormonal treatments prove unsuccessful.
Major finding: BC-specific mortality was not worsened in patients with BC who received vaginal estrogen therapy vs no hormone replacement therapy (hazard ratio 0.77; 95% CI 0.63-0.94).
Study details: Findings are from an analysis of two large cohorts including 49,237 females with BC, of which 5% of females used vaginal estrogen therapy after BC diagnosis.
Disclosures: This study was supported by grants from Cancer Research UK. Some authors declared receiving grants, personal fees, or nonfinancial support from and having other ties with several sources.
Source: McVicker L et al. Vaginal estrogen therapy use and survival in females with breast cancer. JAMA Oncol. 2023 (Nov 2). doi: 10.1001/jamaoncol.2023.4508
Highlights in Early Breast Cancer From ESMO 2023
Developments in early breast cancer reported at the European Society for Medical Oncology (ESMO) 2023 Congress are discussed by Dr Lisa Carey of University of North Carolina, Chapel Hill.
Dr Carey begins with 5-year results from the KEYNOTE-522 study in patients with early triple-negative breast cancer in which the immune checkpoint inhibitor pembrolizumab was incorporated into combination therapy both pre- and postoperatively. The new findings were consistent with earlier results, showing that pembrolizumab improved pathologic complete response (pCR) and event-free survival.
Turning to human epidermal growth factor receptor 2–positive (HER2+) disease, Dr Carey discusses the PHERGain trial's use of a genomic assay to define risk and predict pCR. She suggests that such assays could lead to tailored therapy for HER2+ patients.
On estrogen receptor–positive (ER+)/HER2- disease, Dr Carey reports first on KEYNOTE-756, which examined the addition of pembrolizumab to combination therapy for high-risk patients in both the neoadjuvant and adjuvant settings. Pembrolizumab improved pCR compared with placebo.
Dr Carey closes by discussing another study in high-risk ER+/HER2- disease. Similar in design to KEYNOTE-756, CheckMate 7FL found that nivolumab added to combination therapy again augmented pCR results.
--
Lisa A. Carey, MD, Distinguished Professor or Breast Cancer Research, University of North Carolina Lineberger Comprehensive Cancer Center; Professor, Division of Medical Oncology, Bassnight North Carolina Cancer Hospital, Chapel Hill, North Carolina
Lisa A. Carey, MD, has disclosed no relevant financial relationships.
Developments in early breast cancer reported at the European Society for Medical Oncology (ESMO) 2023 Congress are discussed by Dr Lisa Carey of University of North Carolina, Chapel Hill.
Dr Carey begins with 5-year results from the KEYNOTE-522 study in patients with early triple-negative breast cancer in which the immune checkpoint inhibitor pembrolizumab was incorporated into combination therapy both pre- and postoperatively. The new findings were consistent with earlier results, showing that pembrolizumab improved pathologic complete response (pCR) and event-free survival.
Turning to human epidermal growth factor receptor 2–positive (HER2+) disease, Dr Carey discusses the PHERGain trial's use of a genomic assay to define risk and predict pCR. She suggests that such assays could lead to tailored therapy for HER2+ patients.
On estrogen receptor–positive (ER+)/HER2- disease, Dr Carey reports first on KEYNOTE-756, which examined the addition of pembrolizumab to combination therapy for high-risk patients in both the neoadjuvant and adjuvant settings. Pembrolizumab improved pCR compared with placebo.
Dr Carey closes by discussing another study in high-risk ER+/HER2- disease. Similar in design to KEYNOTE-756, CheckMate 7FL found that nivolumab added to combination therapy again augmented pCR results.
--
Lisa A. Carey, MD, Distinguished Professor or Breast Cancer Research, University of North Carolina Lineberger Comprehensive Cancer Center; Professor, Division of Medical Oncology, Bassnight North Carolina Cancer Hospital, Chapel Hill, North Carolina
Lisa A. Carey, MD, has disclosed no relevant financial relationships.
Developments in early breast cancer reported at the European Society for Medical Oncology (ESMO) 2023 Congress are discussed by Dr Lisa Carey of University of North Carolina, Chapel Hill.
Dr Carey begins with 5-year results from the KEYNOTE-522 study in patients with early triple-negative breast cancer in which the immune checkpoint inhibitor pembrolizumab was incorporated into combination therapy both pre- and postoperatively. The new findings were consistent with earlier results, showing that pembrolizumab improved pathologic complete response (pCR) and event-free survival.
Turning to human epidermal growth factor receptor 2–positive (HER2+) disease, Dr Carey discusses the PHERGain trial's use of a genomic assay to define risk and predict pCR. She suggests that such assays could lead to tailored therapy for HER2+ patients.
On estrogen receptor–positive (ER+)/HER2- disease, Dr Carey reports first on KEYNOTE-756, which examined the addition of pembrolizumab to combination therapy for high-risk patients in both the neoadjuvant and adjuvant settings. Pembrolizumab improved pCR compared with placebo.
Dr Carey closes by discussing another study in high-risk ER+/HER2- disease. Similar in design to KEYNOTE-756, CheckMate 7FL found that nivolumab added to combination therapy again augmented pCR results.
--
Lisa A. Carey, MD, Distinguished Professor or Breast Cancer Research, University of North Carolina Lineberger Comprehensive Cancer Center; Professor, Division of Medical Oncology, Bassnight North Carolina Cancer Hospital, Chapel Hill, North Carolina
Lisa A. Carey, MD, has disclosed no relevant financial relationships.

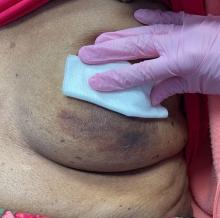
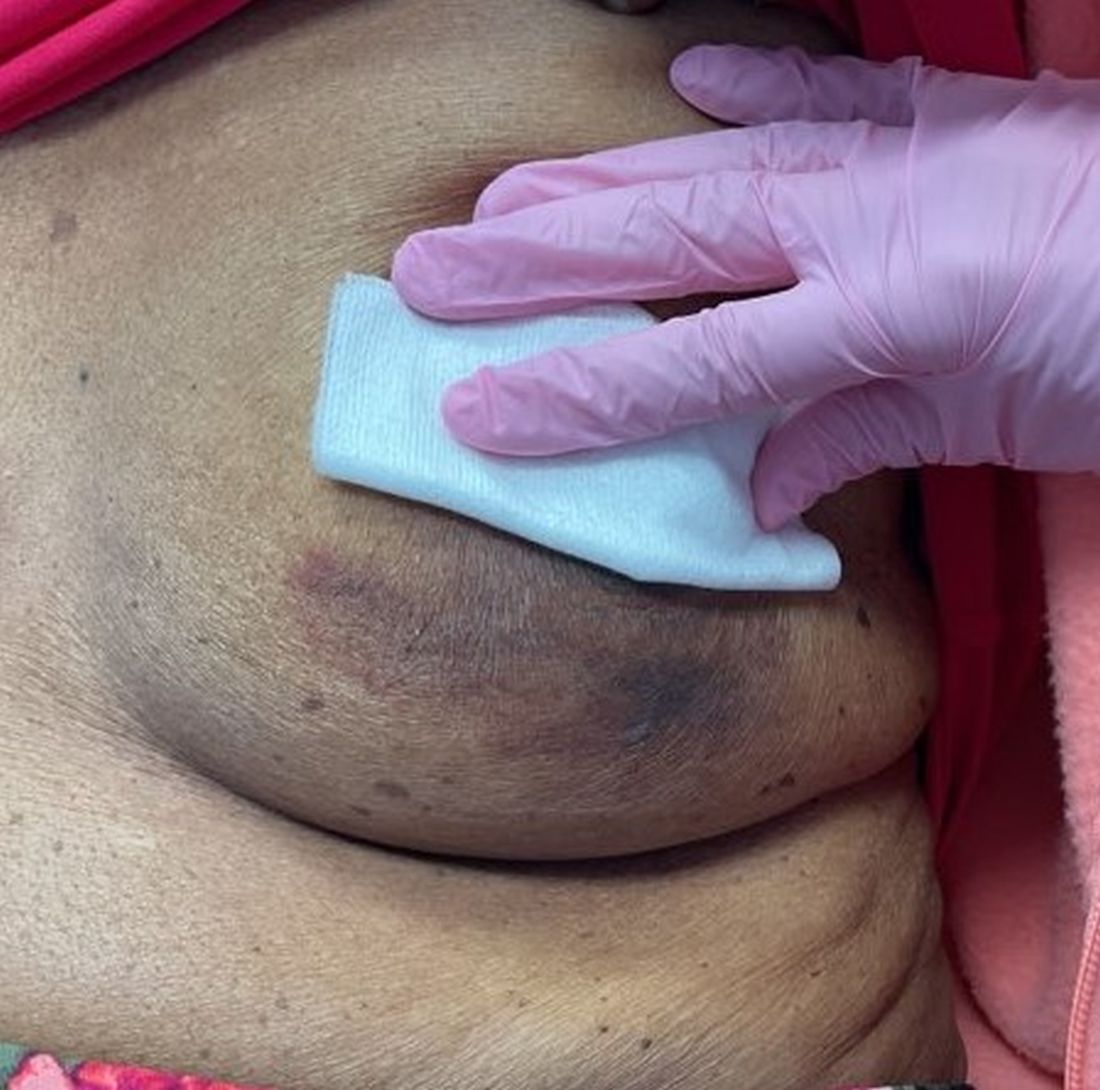
An 88-year-old Black woman presented with 3 months duration of asymptomatic, violaceous patches on the left breast
Angiosarcomas are uncommon, high-grade malignant tumors of endothelial cell origin that can arise via the lymphatics or vasculature. They typically occur spontaneously; however, there have been cases reported of benign vascular transformation. These tumors are more commonly found in elderly men on the head and neck in sun-damaged skin. . This is a late complication, typically occurring about 5-10 years after radiation. Stewart-Treves syndrome, chronic lymphedema occurring after breast cancer treatment with axillary node dissection, increases the risk of angiosarcoma. As a vascular tumor, angiosarcoma spreads hematogenously and carries a poor prognosis if not caught early. Differential diagnoses include other vascular tumors such as retiform hemangioendothelioma. In this specific patient, the differential diagnosis includes Paget’s disease, chronic radiation skin changes, and eczema.
Histopathologically, angiosarcomas exhibit abnormal, pleomorphic, malignant endothelial cells. As the tumor progresses, the cell architecture becomes more distorted and cells form layers with papillary projections into the vascular lumen. Malignant cells may stain positive for CD31, CD34, the oncogene ERG and the proto-oncogene FLI-1. Histology in this patient revealed radiation changes in the dermis, as well as few vascular channels lined by large endothelial cells with marked nuclear atypia, in the form of large nucleoli and variably coarse chromatin. The cells were positive for MYC.
Treatment of angiosarcoma involves a multidisciplinary approach. Resection with wide margins is generally the treatment of choice. However, recurrence is relatively common, which may be a result of microsatellite deposits of the tumor. Perioperative radiation is recommended, and adjuvant chemotherapy often is recommended for metastatic disease. Specifically, paclitaxel has been found to promote survival in some cases of cutaneous angiosarcoma. Metastatic disease may be treated with cytotoxic drugs such as anthracyclines and taxanes. Additionally, targeted therapy including anti-VEGF drugs and tyrosine kinase inhibitors have been tested.
The case and photo were submitted by Mr. Shapiro of Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Fla., and Dr. Bilu Martin. The column was edited by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Cohen-Hallaleh RB et al. Clin Sarcoma Res. 2017 Aug 7:7:15.
Cozzi S et al. Rep Pract Oncol Radiother. 2021 Sep 30;26(5):827-32.
Spiker AM, Mangla A, Ramsey ML. Angiosarcoma. [Updated 2023 Jul 17]. In: StatPearls [Internet]. Treasure Island, Fla.: StatPearls Publishing; 2023 Jan-. Available from: www.ncbi.nlm.nih.gov/books/NBK441983/
Angiosarcomas are uncommon, high-grade malignant tumors of endothelial cell origin that can arise via the lymphatics or vasculature. They typically occur spontaneously; however, there have been cases reported of benign vascular transformation. These tumors are more commonly found in elderly men on the head and neck in sun-damaged skin. . This is a late complication, typically occurring about 5-10 years after radiation. Stewart-Treves syndrome, chronic lymphedema occurring after breast cancer treatment with axillary node dissection, increases the risk of angiosarcoma. As a vascular tumor, angiosarcoma spreads hematogenously and carries a poor prognosis if not caught early. Differential diagnoses include other vascular tumors such as retiform hemangioendothelioma. In this specific patient, the differential diagnosis includes Paget’s disease, chronic radiation skin changes, and eczema.
Histopathologically, angiosarcomas exhibit abnormal, pleomorphic, malignant endothelial cells. As the tumor progresses, the cell architecture becomes more distorted and cells form layers with papillary projections into the vascular lumen. Malignant cells may stain positive for CD31, CD34, the oncogene ERG and the proto-oncogene FLI-1. Histology in this patient revealed radiation changes in the dermis, as well as few vascular channels lined by large endothelial cells with marked nuclear atypia, in the form of large nucleoli and variably coarse chromatin. The cells were positive for MYC.
Treatment of angiosarcoma involves a multidisciplinary approach. Resection with wide margins is generally the treatment of choice. However, recurrence is relatively common, which may be a result of microsatellite deposits of the tumor. Perioperative radiation is recommended, and adjuvant chemotherapy often is recommended for metastatic disease. Specifically, paclitaxel has been found to promote survival in some cases of cutaneous angiosarcoma. Metastatic disease may be treated with cytotoxic drugs such as anthracyclines and taxanes. Additionally, targeted therapy including anti-VEGF drugs and tyrosine kinase inhibitors have been tested.
The case and photo were submitted by Mr. Shapiro of Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Fla., and Dr. Bilu Martin. The column was edited by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Cohen-Hallaleh RB et al. Clin Sarcoma Res. 2017 Aug 7:7:15.
Cozzi S et al. Rep Pract Oncol Radiother. 2021 Sep 30;26(5):827-32.
Spiker AM, Mangla A, Ramsey ML. Angiosarcoma. [Updated 2023 Jul 17]. In: StatPearls [Internet]. Treasure Island, Fla.: StatPearls Publishing; 2023 Jan-. Available from: www.ncbi.nlm.nih.gov/books/NBK441983/
Angiosarcomas are uncommon, high-grade malignant tumors of endothelial cell origin that can arise via the lymphatics or vasculature. They typically occur spontaneously; however, there have been cases reported of benign vascular transformation. These tumors are more commonly found in elderly men on the head and neck in sun-damaged skin. . This is a late complication, typically occurring about 5-10 years after radiation. Stewart-Treves syndrome, chronic lymphedema occurring after breast cancer treatment with axillary node dissection, increases the risk of angiosarcoma. As a vascular tumor, angiosarcoma spreads hematogenously and carries a poor prognosis if not caught early. Differential diagnoses include other vascular tumors such as retiform hemangioendothelioma. In this specific patient, the differential diagnosis includes Paget’s disease, chronic radiation skin changes, and eczema.
Histopathologically, angiosarcomas exhibit abnormal, pleomorphic, malignant endothelial cells. As the tumor progresses, the cell architecture becomes more distorted and cells form layers with papillary projections into the vascular lumen. Malignant cells may stain positive for CD31, CD34, the oncogene ERG and the proto-oncogene FLI-1. Histology in this patient revealed radiation changes in the dermis, as well as few vascular channels lined by large endothelial cells with marked nuclear atypia, in the form of large nucleoli and variably coarse chromatin. The cells were positive for MYC.
Treatment of angiosarcoma involves a multidisciplinary approach. Resection with wide margins is generally the treatment of choice. However, recurrence is relatively common, which may be a result of microsatellite deposits of the tumor. Perioperative radiation is recommended, and adjuvant chemotherapy often is recommended for metastatic disease. Specifically, paclitaxel has been found to promote survival in some cases of cutaneous angiosarcoma. Metastatic disease may be treated with cytotoxic drugs such as anthracyclines and taxanes. Additionally, targeted therapy including anti-VEGF drugs and tyrosine kinase inhibitors have been tested.
The case and photo were submitted by Mr. Shapiro of Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Fla., and Dr. Bilu Martin. The column was edited by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Cohen-Hallaleh RB et al. Clin Sarcoma Res. 2017 Aug 7:7:15.
Cozzi S et al. Rep Pract Oncol Radiother. 2021 Sep 30;26(5):827-32.
Spiker AM, Mangla A, Ramsey ML. Angiosarcoma. [Updated 2023 Jul 17]. In: StatPearls [Internet]. Treasure Island, Fla.: StatPearls Publishing; 2023 Jan-. Available from: www.ncbi.nlm.nih.gov/books/NBK441983/
Particulate pollution increases the risk for breast cancer
MADRID – according to a new analysis of the XENAIR study presented at the European Society of Medical Oncology (ESMO) Congress 2023. Béatrice Fervers, MD, PhD, head of the environmental cancer prevention department at the Léon Bérard Center, Lyon, France, presented her findings.
“To our knowledge, this study is the first to examine the risk of breast cancer associated with long-term exposure of subjects to atmospheric pollution both at home and in the workplace, estimated using a very small spatial resolution [statistical] model,” said the researchers.
“Our data showed a statistically significant association between long-term exposure to fine particulate matter air pollution, at home and at work, and risk of breast cancer. This [finding] contrasts with previous research that looked only at fine particulate exposure where women were living and showed small or no effects on breast cancer risk,” said Dr. Fervers in a press release issued before the Congress.
The XENAIR study carried out on the prospective, longitudinal E3N cohort a year ago showed an increased risk for breast cancer after exposure to five atmospheric pollutants. Notably, it showed an increased risk in women exposed to BaP and PCB153, two pollutants classed as endocrine-disrupting chemicals, during perimenopause.
Increased linear risk
In this new analysis, exposure to PM2.5, PM10, and NO2 pollution at home and in the workplace of 2,419 women with breast cancer was compared with that of 2,984 women without breast cancer during the period from 1990 to 2011.
This was a case-control study in which participants were matched by department of residence in France, age (± 1 year), date (± 3 months), and menopausal status at the time of the blood draw.
Breast cancer risk increased by 28% when exposure to fine particulate (PM2.5) air pollution increased by 10 mcg/m3. The increment is approximately equivalent to the difference in PM2.5 particulate concentration typically seen in rural versus urban areas of Europe.
Smaller increases in breast cancer risk were also recorded in women exposed to high levels of larger particulate air pollution (PM10 and NO2).
No change in effect was seen according to menopausal status. Analyses that examined hormone receptor status showed a positive but not significant association for PM2.5 in cases of estrogen receptor positive breast cancer.
Dr. Fervers and colleagues plan to investigate the effects of pollution exposure during the commute to get a complete picture of effects on breast cancer risk.
Regulators respond
Charles Swanton, PhD, a clinician scientist at the Francis Crick Institute, London, emphasized the importance of these new results for breast cancer. At last year’s ESMO Congress, he explained how particulate matter air pollution caused tumor proliferation in patients with a certain type of genetic mutation.
“Fine particle pollutants can penetrate deep into the lungs, enter the bloodstream, and be absorbed into breast and other tissue. There is already evidence that air pollutants can change the architecture of the breast. It will be important to test if pollutants allow cells in breast tissue with pre-existing mutations to expand and drive tumor promotion, possibly through inflammatory processes, similar to our observations in nonsmokers with lung cancer,” said Dr. Swanton in the ESMO press release.
“It is very concerning that small pollutant particles in the air and indeed microplastic particles of similar size are getting into the environment when we don’t yet understand their potential to promote cancer. There is an urgent need to set up laboratory studies to investigate the effects of these small air pollutant particles on the latency, grade, aggression, and progression of breast tumors,” he added.
“There is now strong epidemiological and biological evidence for the link between PM2.5 particulate exposure and cancer, and there are good clinical and economic reasons for reducing pollution to prevent cancers,” said Jean-Yves Blay, MD, PhD, director of public policy for ESMO.
Following a proposal from the European Commission in October 2022 to reduce the limit for PM2.5 particulates in the air from the current 25 mcg/m3 to 10 mcg/m3 by 2030, ESMO urged a further reduction in the PM2.5 limit to 5 mcg/m3, in line with the World Health Organization’s air quality guidance, according to the press release.
“Reducing PM2.5 particles in the air to the WHO recommended level is critical because of their association with a variety of tumor types, including breast cancer,” Dr. Blay added.
In September 2023, the European Parliament adopted in a plenary session its report on the ongoing revision of the EU Ambient Air Quality Directives, which reflects ESMO’s recommendations to set the annual limit value for PM2.5 at 5 mcg/m³. This adoption opens interinstitutional negotiations between the legislators (the European Parliament, European Commission, and EU Council) to agree on the final text of the directive.
“By supporting our requests with solid scientific evidence, we are offering a new dimension to health public policy. The work is not over, and change will not happen overnight, but we are moving in the right direction,” concluded Dr. Blay.
The new analysis of the XENAIR study was funded by ARC Foundation for cancer research; the French Agency for Food, Environmental, and Occupational Health and Safety; French National League against Cancer; and Fondation de France, an independent private organization, recognized as being in the public interest. The authors report no relevant financial relationships.
This article was translated from the Medscape French edition and a version appeared on Medscape.com.
MADRID – according to a new analysis of the XENAIR study presented at the European Society of Medical Oncology (ESMO) Congress 2023. Béatrice Fervers, MD, PhD, head of the environmental cancer prevention department at the Léon Bérard Center, Lyon, France, presented her findings.
“To our knowledge, this study is the first to examine the risk of breast cancer associated with long-term exposure of subjects to atmospheric pollution both at home and in the workplace, estimated using a very small spatial resolution [statistical] model,” said the researchers.
“Our data showed a statistically significant association between long-term exposure to fine particulate matter air pollution, at home and at work, and risk of breast cancer. This [finding] contrasts with previous research that looked only at fine particulate exposure where women were living and showed small or no effects on breast cancer risk,” said Dr. Fervers in a press release issued before the Congress.
The XENAIR study carried out on the prospective, longitudinal E3N cohort a year ago showed an increased risk for breast cancer after exposure to five atmospheric pollutants. Notably, it showed an increased risk in women exposed to BaP and PCB153, two pollutants classed as endocrine-disrupting chemicals, during perimenopause.
Increased linear risk
In this new analysis, exposure to PM2.5, PM10, and NO2 pollution at home and in the workplace of 2,419 women with breast cancer was compared with that of 2,984 women without breast cancer during the period from 1990 to 2011.
This was a case-control study in which participants were matched by department of residence in France, age (± 1 year), date (± 3 months), and menopausal status at the time of the blood draw.
Breast cancer risk increased by 28% when exposure to fine particulate (PM2.5) air pollution increased by 10 mcg/m3. The increment is approximately equivalent to the difference in PM2.5 particulate concentration typically seen in rural versus urban areas of Europe.
Smaller increases in breast cancer risk were also recorded in women exposed to high levels of larger particulate air pollution (PM10 and NO2).
No change in effect was seen according to menopausal status. Analyses that examined hormone receptor status showed a positive but not significant association for PM2.5 in cases of estrogen receptor positive breast cancer.
Dr. Fervers and colleagues plan to investigate the effects of pollution exposure during the commute to get a complete picture of effects on breast cancer risk.
Regulators respond
Charles Swanton, PhD, a clinician scientist at the Francis Crick Institute, London, emphasized the importance of these new results for breast cancer. At last year’s ESMO Congress, he explained how particulate matter air pollution caused tumor proliferation in patients with a certain type of genetic mutation.
“Fine particle pollutants can penetrate deep into the lungs, enter the bloodstream, and be absorbed into breast and other tissue. There is already evidence that air pollutants can change the architecture of the breast. It will be important to test if pollutants allow cells in breast tissue with pre-existing mutations to expand and drive tumor promotion, possibly through inflammatory processes, similar to our observations in nonsmokers with lung cancer,” said Dr. Swanton in the ESMO press release.
“It is very concerning that small pollutant particles in the air and indeed microplastic particles of similar size are getting into the environment when we don’t yet understand their potential to promote cancer. There is an urgent need to set up laboratory studies to investigate the effects of these small air pollutant particles on the latency, grade, aggression, and progression of breast tumors,” he added.
“There is now strong epidemiological and biological evidence for the link between PM2.5 particulate exposure and cancer, and there are good clinical and economic reasons for reducing pollution to prevent cancers,” said Jean-Yves Blay, MD, PhD, director of public policy for ESMO.
Following a proposal from the European Commission in October 2022 to reduce the limit for PM2.5 particulates in the air from the current 25 mcg/m3 to 10 mcg/m3 by 2030, ESMO urged a further reduction in the PM2.5 limit to 5 mcg/m3, in line with the World Health Organization’s air quality guidance, according to the press release.
“Reducing PM2.5 particles in the air to the WHO recommended level is critical because of their association with a variety of tumor types, including breast cancer,” Dr. Blay added.
In September 2023, the European Parliament adopted in a plenary session its report on the ongoing revision of the EU Ambient Air Quality Directives, which reflects ESMO’s recommendations to set the annual limit value for PM2.5 at 5 mcg/m³. This adoption opens interinstitutional negotiations between the legislators (the European Parliament, European Commission, and EU Council) to agree on the final text of the directive.
“By supporting our requests with solid scientific evidence, we are offering a new dimension to health public policy. The work is not over, and change will not happen overnight, but we are moving in the right direction,” concluded Dr. Blay.
The new analysis of the XENAIR study was funded by ARC Foundation for cancer research; the French Agency for Food, Environmental, and Occupational Health and Safety; French National League against Cancer; and Fondation de France, an independent private organization, recognized as being in the public interest. The authors report no relevant financial relationships.
This article was translated from the Medscape French edition and a version appeared on Medscape.com.
MADRID – according to a new analysis of the XENAIR study presented at the European Society of Medical Oncology (ESMO) Congress 2023. Béatrice Fervers, MD, PhD, head of the environmental cancer prevention department at the Léon Bérard Center, Lyon, France, presented her findings.
“To our knowledge, this study is the first to examine the risk of breast cancer associated with long-term exposure of subjects to atmospheric pollution both at home and in the workplace, estimated using a very small spatial resolution [statistical] model,” said the researchers.
“Our data showed a statistically significant association between long-term exposure to fine particulate matter air pollution, at home and at work, and risk of breast cancer. This [finding] contrasts with previous research that looked only at fine particulate exposure where women were living and showed small or no effects on breast cancer risk,” said Dr. Fervers in a press release issued before the Congress.
The XENAIR study carried out on the prospective, longitudinal E3N cohort a year ago showed an increased risk for breast cancer after exposure to five atmospheric pollutants. Notably, it showed an increased risk in women exposed to BaP and PCB153, two pollutants classed as endocrine-disrupting chemicals, during perimenopause.
Increased linear risk
In this new analysis, exposure to PM2.5, PM10, and NO2 pollution at home and in the workplace of 2,419 women with breast cancer was compared with that of 2,984 women without breast cancer during the period from 1990 to 2011.
This was a case-control study in which participants were matched by department of residence in France, age (± 1 year), date (± 3 months), and menopausal status at the time of the blood draw.
Breast cancer risk increased by 28% when exposure to fine particulate (PM2.5) air pollution increased by 10 mcg/m3. The increment is approximately equivalent to the difference in PM2.5 particulate concentration typically seen in rural versus urban areas of Europe.
Smaller increases in breast cancer risk were also recorded in women exposed to high levels of larger particulate air pollution (PM10 and NO2).
No change in effect was seen according to menopausal status. Analyses that examined hormone receptor status showed a positive but not significant association for PM2.5 in cases of estrogen receptor positive breast cancer.
Dr. Fervers and colleagues plan to investigate the effects of pollution exposure during the commute to get a complete picture of effects on breast cancer risk.
Regulators respond
Charles Swanton, PhD, a clinician scientist at the Francis Crick Institute, London, emphasized the importance of these new results for breast cancer. At last year’s ESMO Congress, he explained how particulate matter air pollution caused tumor proliferation in patients with a certain type of genetic mutation.
“Fine particle pollutants can penetrate deep into the lungs, enter the bloodstream, and be absorbed into breast and other tissue. There is already evidence that air pollutants can change the architecture of the breast. It will be important to test if pollutants allow cells in breast tissue with pre-existing mutations to expand and drive tumor promotion, possibly through inflammatory processes, similar to our observations in nonsmokers with lung cancer,” said Dr. Swanton in the ESMO press release.
“It is very concerning that small pollutant particles in the air and indeed microplastic particles of similar size are getting into the environment when we don’t yet understand their potential to promote cancer. There is an urgent need to set up laboratory studies to investigate the effects of these small air pollutant particles on the latency, grade, aggression, and progression of breast tumors,” he added.
“There is now strong epidemiological and biological evidence for the link between PM2.5 particulate exposure and cancer, and there are good clinical and economic reasons for reducing pollution to prevent cancers,” said Jean-Yves Blay, MD, PhD, director of public policy for ESMO.
Following a proposal from the European Commission in October 2022 to reduce the limit for PM2.5 particulates in the air from the current 25 mcg/m3 to 10 mcg/m3 by 2030, ESMO urged a further reduction in the PM2.5 limit to 5 mcg/m3, in line with the World Health Organization’s air quality guidance, according to the press release.
“Reducing PM2.5 particles in the air to the WHO recommended level is critical because of their association with a variety of tumor types, including breast cancer,” Dr. Blay added.
In September 2023, the European Parliament adopted in a plenary session its report on the ongoing revision of the EU Ambient Air Quality Directives, which reflects ESMO’s recommendations to set the annual limit value for PM2.5 at 5 mcg/m³. This adoption opens interinstitutional negotiations between the legislators (the European Parliament, European Commission, and EU Council) to agree on the final text of the directive.
“By supporting our requests with solid scientific evidence, we are offering a new dimension to health public policy. The work is not over, and change will not happen overnight, but we are moving in the right direction,” concluded Dr. Blay.
The new analysis of the XENAIR study was funded by ARC Foundation for cancer research; the French Agency for Food, Environmental, and Occupational Health and Safety; French National League against Cancer; and Fondation de France, an independent private organization, recognized as being in the public interest. The authors report no relevant financial relationships.
This article was translated from the Medscape French edition and a version appeared on Medscape.com.
AT ESMO 2023
Low vitamin D linked to paclitaxel-induced peripheral neuropathy
TOPLINE:
, suggesting that correcting levels before treatment might help prevent the condition.
METHODOLOGY:
- Past studies have suggested an association between vitamin D insufficiency and paclitaxel-induced peripheral neuropathy, a largely untreatable and sometimes permanent side effect of chemotherapy.
- To confirm the association, investigators reviewed data and samples from 1,191 women in the phase 3 SWOG S0221 trial, which compared weekly and biweekly paclitaxel regimens for early-stage breast cancer.
- Using serum samples collected at baseline, the team evaluated the relationship between insufficient vitamin D levels (20 ng/mL or less) before treatment and grade 3 or higher sensory chemotherapy-induced peripheral neuropathy.
TAKEAWAY:
- Overall, 33.3% of the women had insufficient vitamin D levels at baseline, and 16.4% developed grade 3 or worse sensory chemotherapy-induced peripheral neuropathy.
- The incidence of peripheral neuropathy of grade 3 or greater was higher among patients with pretreatment vitamin D insufficiency (20.7% vs. 14.2%; odds ratio, 1.57; P = .005).
- The association grew stronger after adjusting for age and paclitaxel schedule (adjusted OR, 1.65; P = .003), but not after adjusting for race (adjusted OR, 1.39; P = .066).
IN PRACTICE:
The study “confirms that patients with pretreatment vitamin D insufficiency have a higher incidence of [chemotherapy-induced peripheral neuropathy],” the authors concluded. These results also “suggest that vitamin D supplementation in patients with lower levels of vitamin D may reduce peripheral neuropathy, and particularly high-grade peripheral neuropathy, which would improve these patients’ long-term quality of life,” senior researcher Daniel L. Hertz, PharmD, PhD, University of Michigan College of Pharmacy, Ann Arbor, said in a press release.
SOURCE:
The study, led by Ciao-Sin Chen, PharmD, of the University of Michigan, Ann Arbor, was published in the Journal of the National Comprehensive Cancer Network.
LIMITATIONS:
The trial did not collect data on other peripheral neuropathy risk factors, including preexisting peripheral neuropathy and diabetes. The study included a limited number of non-White participants (16%); larger numbers are needed to elucidate a potential interplay between race, vitamin D, and chemotherapy-induced peripheral neuropathy. The researchers also did not collect data on grade 1 and 2 chemotherapy-induced peripheral neuropathy.
DISCLOSURES:
The study was funded by Amgen, the American Cancer Society, and others. The investigators disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
, suggesting that correcting levels before treatment might help prevent the condition.
METHODOLOGY:
- Past studies have suggested an association between vitamin D insufficiency and paclitaxel-induced peripheral neuropathy, a largely untreatable and sometimes permanent side effect of chemotherapy.
- To confirm the association, investigators reviewed data and samples from 1,191 women in the phase 3 SWOG S0221 trial, which compared weekly and biweekly paclitaxel regimens for early-stage breast cancer.
- Using serum samples collected at baseline, the team evaluated the relationship between insufficient vitamin D levels (20 ng/mL or less) before treatment and grade 3 or higher sensory chemotherapy-induced peripheral neuropathy.
TAKEAWAY:
- Overall, 33.3% of the women had insufficient vitamin D levels at baseline, and 16.4% developed grade 3 or worse sensory chemotherapy-induced peripheral neuropathy.
- The incidence of peripheral neuropathy of grade 3 or greater was higher among patients with pretreatment vitamin D insufficiency (20.7% vs. 14.2%; odds ratio, 1.57; P = .005).
- The association grew stronger after adjusting for age and paclitaxel schedule (adjusted OR, 1.65; P = .003), but not after adjusting for race (adjusted OR, 1.39; P = .066).
IN PRACTICE:
The study “confirms that patients with pretreatment vitamin D insufficiency have a higher incidence of [chemotherapy-induced peripheral neuropathy],” the authors concluded. These results also “suggest that vitamin D supplementation in patients with lower levels of vitamin D may reduce peripheral neuropathy, and particularly high-grade peripheral neuropathy, which would improve these patients’ long-term quality of life,” senior researcher Daniel L. Hertz, PharmD, PhD, University of Michigan College of Pharmacy, Ann Arbor, said in a press release.
SOURCE:
The study, led by Ciao-Sin Chen, PharmD, of the University of Michigan, Ann Arbor, was published in the Journal of the National Comprehensive Cancer Network.
LIMITATIONS:
The trial did not collect data on other peripheral neuropathy risk factors, including preexisting peripheral neuropathy and diabetes. The study included a limited number of non-White participants (16%); larger numbers are needed to elucidate a potential interplay between race, vitamin D, and chemotherapy-induced peripheral neuropathy. The researchers also did not collect data on grade 1 and 2 chemotherapy-induced peripheral neuropathy.
DISCLOSURES:
The study was funded by Amgen, the American Cancer Society, and others. The investigators disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
, suggesting that correcting levels before treatment might help prevent the condition.
METHODOLOGY:
- Past studies have suggested an association between vitamin D insufficiency and paclitaxel-induced peripheral neuropathy, a largely untreatable and sometimes permanent side effect of chemotherapy.
- To confirm the association, investigators reviewed data and samples from 1,191 women in the phase 3 SWOG S0221 trial, which compared weekly and biweekly paclitaxel regimens for early-stage breast cancer.
- Using serum samples collected at baseline, the team evaluated the relationship between insufficient vitamin D levels (20 ng/mL or less) before treatment and grade 3 or higher sensory chemotherapy-induced peripheral neuropathy.
TAKEAWAY:
- Overall, 33.3% of the women had insufficient vitamin D levels at baseline, and 16.4% developed grade 3 or worse sensory chemotherapy-induced peripheral neuropathy.
- The incidence of peripheral neuropathy of grade 3 or greater was higher among patients with pretreatment vitamin D insufficiency (20.7% vs. 14.2%; odds ratio, 1.57; P = .005).
- The association grew stronger after adjusting for age and paclitaxel schedule (adjusted OR, 1.65; P = .003), but not after adjusting for race (adjusted OR, 1.39; P = .066).
IN PRACTICE:
The study “confirms that patients with pretreatment vitamin D insufficiency have a higher incidence of [chemotherapy-induced peripheral neuropathy],” the authors concluded. These results also “suggest that vitamin D supplementation in patients with lower levels of vitamin D may reduce peripheral neuropathy, and particularly high-grade peripheral neuropathy, which would improve these patients’ long-term quality of life,” senior researcher Daniel L. Hertz, PharmD, PhD, University of Michigan College of Pharmacy, Ann Arbor, said in a press release.
SOURCE:
The study, led by Ciao-Sin Chen, PharmD, of the University of Michigan, Ann Arbor, was published in the Journal of the National Comprehensive Cancer Network.
LIMITATIONS:
The trial did not collect data on other peripheral neuropathy risk factors, including preexisting peripheral neuropathy and diabetes. The study included a limited number of non-White participants (16%); larger numbers are needed to elucidate a potential interplay between race, vitamin D, and chemotherapy-induced peripheral neuropathy. The researchers also did not collect data on grade 1 and 2 chemotherapy-induced peripheral neuropathy.
DISCLOSURES:
The study was funded by Amgen, the American Cancer Society, and others. The investigators disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
T-DXd benefits persist for HER2-low breast cancer
and low HER2 expression in the randomized phase 3 DESTINY-Breast04 study, according to 32-month follow-up data.
The overall safety profile of the HER2-directed antibody drug conjugate was also comparable to that observed at the primary analysis in 2022, and longer exposure did not appear to increase toxicity, Shanu Modi, MD, reported on behalf of the DESTINY-Breast04 investigators at the European Society of Medical Oncology (ESMO) Congress 2023.
“These results continue to support the use of T-DXd as the new standard of care after one line of chemotherapy in patients with HER2-low metastatic breast cancer,” said Dr. Modi, a breast oncologist and attending physician at Memorial Sloan Kettering Cancer Center, New York.
DESTINY-Breast04 enrolled 557 patients 2:1 to receive 5.4 mg/kg of T-DXd every 3 weeks or physicians’ choice of capecitabine, eribulin, gemcitabine, paclitaxel, or nab-paclitaxel, and established HER2-low mBC as “a new targetable patient population with T-DXd as a new standard of care,” she explained.
Median overall survival (mOS) with a median of 18.4 months of follow-up at the Jan. 11, 2022, primary data cut-off was 23.4 months in the T-DXd arm versus 16.8 months in the TPC arm and 23.9 versus 17.5 months, respectively, in the hormone receptor–positive (HR+) cohort (hazard ratio, 0.64 for both groups). At the preplanned extended follow-up with data cut-off on March 1, 2023, the mOS was 22.9 versus 16.8 months for T-DXd versus TPC, and 23.9 versus 17.6 months for the HR+ cohort, respectively (HR, 0.69 for both).
Median progression-free survival (PFS) by investigator assessment was 8.8 versus 4.2 months for the full cohort, and 9.6 versus 4.2 months for the HR+ cohort (HR, 0.36 and 0.37, respectively). PFS was consistent with the results from the primary analysis.
The benefits in the HR+ patients were consistent across all patient subgroups, Dr. Modi noted.
“I do think it’s interesting to point out that at the landmark 2-year point, all patients on standard chemotherapy discontinued study treatment, whereas 15% on T-DXd remain [on treatment] without any evidence of disease progression, Dr. Modi added
An exploratory analysis in the hormone receptor–negative (HR–) cohort showed mOS of 18.2 versus 8.3 months at the primary analysis (HR, 0.48), and a “clinically meaningful and numerical advantage for T-DXd” persisted at the planned follow-up (mOS, 17.1 vs. 8.3; HR, 0.58), she said.
PFS in the HR- cohort was 8.5 versus 2.9 months at the primary analysis, and 6.3 versus 2.9 months at the update (HR, 0.46 and 0.29, respectively).
An assessment of post-study therapies received by patients showed that those therapies did not account for the significant survival advantage conferred by T-DXd, Dr. Modi said.
She noted, however, that while no new safety signals were observed at follow-up, lung toxicity remains a “toxicity of special interest,” having occurred in 12.1% of cases at the time of the primary analysis.
Most cases were grade 1 or 2, and no new cases were observed at follow-up, but one patient with lung toxicity and an initial grade 3 event experienced clinical deterioration and later died from lung toxicity, which underscores the importance of remaining vigilant and intervening promptly in all cases of lung toxicity, Dr. Modi stressed.
Invited discussant Giampaolo Bianchini, MD, reiterated that T-DXd is an effective treatment option and said, “we must accurately identify patients and avoid improperly denying this important therapeutic option.”
Although HER2-low disease is not a unique biological disease entity, it is a “practical and pragmatic definition used to select patients with ‘some degree’ of HER2 protein expression adopting a test and a scoring system already implemented in the routine clinical practice,” said Dr. Bianchini, head of the breast cancer group and head of clinical translational and immunotherapy research at IRCCS Ospedale, San Raffaele, Milan.
However, the current definition may be inadequate, he said, explaining that the ongoing DESTINY-Breast06 study “will challenge the current definition of what we consider HER2-low definition,” potentially extending the T-DXd indication to HER2 ultra-low.
Furthermore, current HER2 testing was designed to discriminate cases with high abundant protein – not for the low HER2 dynamic range, which leads to technical inaccuracy.
Given these considerations, he suggested considering a new biopsy, if feasible, in patients with an immunohistochemistry (IHC) score of 0 in all tumor biopsies, and having a revision performed by the pathologist.
In patients with an IHC score of 1 or greater only in one biopsy, there is no need to confirm the HER2-low status, he said.
DESTINY-Breast04 is funded by Daiichi Sankyo Inc. and AstraZeneca. Dr. Modi reported relationships with Daiichi Sankyo, Genentech, AstraZeneca, Seagen, and MacroGenics. Dr. Bianchini reported relationships with AstraZeneca, Daiichi Sankyo, Gilead, MSD, Seagen, Roche, Sanofi, Lilly, EISAI, Novartis, Pfizer, Stemline, Exact Science, and Agendia.
and low HER2 expression in the randomized phase 3 DESTINY-Breast04 study, according to 32-month follow-up data.
The overall safety profile of the HER2-directed antibody drug conjugate was also comparable to that observed at the primary analysis in 2022, and longer exposure did not appear to increase toxicity, Shanu Modi, MD, reported on behalf of the DESTINY-Breast04 investigators at the European Society of Medical Oncology (ESMO) Congress 2023.
“These results continue to support the use of T-DXd as the new standard of care after one line of chemotherapy in patients with HER2-low metastatic breast cancer,” said Dr. Modi, a breast oncologist and attending physician at Memorial Sloan Kettering Cancer Center, New York.
DESTINY-Breast04 enrolled 557 patients 2:1 to receive 5.4 mg/kg of T-DXd every 3 weeks or physicians’ choice of capecitabine, eribulin, gemcitabine, paclitaxel, or nab-paclitaxel, and established HER2-low mBC as “a new targetable patient population with T-DXd as a new standard of care,” she explained.
Median overall survival (mOS) with a median of 18.4 months of follow-up at the Jan. 11, 2022, primary data cut-off was 23.4 months in the T-DXd arm versus 16.8 months in the TPC arm and 23.9 versus 17.5 months, respectively, in the hormone receptor–positive (HR+) cohort (hazard ratio, 0.64 for both groups). At the preplanned extended follow-up with data cut-off on March 1, 2023, the mOS was 22.9 versus 16.8 months for T-DXd versus TPC, and 23.9 versus 17.6 months for the HR+ cohort, respectively (HR, 0.69 for both).
Median progression-free survival (PFS) by investigator assessment was 8.8 versus 4.2 months for the full cohort, and 9.6 versus 4.2 months for the HR+ cohort (HR, 0.36 and 0.37, respectively). PFS was consistent with the results from the primary analysis.
The benefits in the HR+ patients were consistent across all patient subgroups, Dr. Modi noted.
“I do think it’s interesting to point out that at the landmark 2-year point, all patients on standard chemotherapy discontinued study treatment, whereas 15% on T-DXd remain [on treatment] without any evidence of disease progression, Dr. Modi added
An exploratory analysis in the hormone receptor–negative (HR–) cohort showed mOS of 18.2 versus 8.3 months at the primary analysis (HR, 0.48), and a “clinically meaningful and numerical advantage for T-DXd” persisted at the planned follow-up (mOS, 17.1 vs. 8.3; HR, 0.58), she said.
PFS in the HR- cohort was 8.5 versus 2.9 months at the primary analysis, and 6.3 versus 2.9 months at the update (HR, 0.46 and 0.29, respectively).
An assessment of post-study therapies received by patients showed that those therapies did not account for the significant survival advantage conferred by T-DXd, Dr. Modi said.
She noted, however, that while no new safety signals were observed at follow-up, lung toxicity remains a “toxicity of special interest,” having occurred in 12.1% of cases at the time of the primary analysis.
Most cases were grade 1 or 2, and no new cases were observed at follow-up, but one patient with lung toxicity and an initial grade 3 event experienced clinical deterioration and later died from lung toxicity, which underscores the importance of remaining vigilant and intervening promptly in all cases of lung toxicity, Dr. Modi stressed.
Invited discussant Giampaolo Bianchini, MD, reiterated that T-DXd is an effective treatment option and said, “we must accurately identify patients and avoid improperly denying this important therapeutic option.”
Although HER2-low disease is not a unique biological disease entity, it is a “practical and pragmatic definition used to select patients with ‘some degree’ of HER2 protein expression adopting a test and a scoring system already implemented in the routine clinical practice,” said Dr. Bianchini, head of the breast cancer group and head of clinical translational and immunotherapy research at IRCCS Ospedale, San Raffaele, Milan.
However, the current definition may be inadequate, he said, explaining that the ongoing DESTINY-Breast06 study “will challenge the current definition of what we consider HER2-low definition,” potentially extending the T-DXd indication to HER2 ultra-low.
Furthermore, current HER2 testing was designed to discriminate cases with high abundant protein – not for the low HER2 dynamic range, which leads to technical inaccuracy.
Given these considerations, he suggested considering a new biopsy, if feasible, in patients with an immunohistochemistry (IHC) score of 0 in all tumor biopsies, and having a revision performed by the pathologist.
In patients with an IHC score of 1 or greater only in one biopsy, there is no need to confirm the HER2-low status, he said.
DESTINY-Breast04 is funded by Daiichi Sankyo Inc. and AstraZeneca. Dr. Modi reported relationships with Daiichi Sankyo, Genentech, AstraZeneca, Seagen, and MacroGenics. Dr. Bianchini reported relationships with AstraZeneca, Daiichi Sankyo, Gilead, MSD, Seagen, Roche, Sanofi, Lilly, EISAI, Novartis, Pfizer, Stemline, Exact Science, and Agendia.
and low HER2 expression in the randomized phase 3 DESTINY-Breast04 study, according to 32-month follow-up data.
The overall safety profile of the HER2-directed antibody drug conjugate was also comparable to that observed at the primary analysis in 2022, and longer exposure did not appear to increase toxicity, Shanu Modi, MD, reported on behalf of the DESTINY-Breast04 investigators at the European Society of Medical Oncology (ESMO) Congress 2023.
“These results continue to support the use of T-DXd as the new standard of care after one line of chemotherapy in patients with HER2-low metastatic breast cancer,” said Dr. Modi, a breast oncologist and attending physician at Memorial Sloan Kettering Cancer Center, New York.
DESTINY-Breast04 enrolled 557 patients 2:1 to receive 5.4 mg/kg of T-DXd every 3 weeks or physicians’ choice of capecitabine, eribulin, gemcitabine, paclitaxel, or nab-paclitaxel, and established HER2-low mBC as “a new targetable patient population with T-DXd as a new standard of care,” she explained.
Median overall survival (mOS) with a median of 18.4 months of follow-up at the Jan. 11, 2022, primary data cut-off was 23.4 months in the T-DXd arm versus 16.8 months in the TPC arm and 23.9 versus 17.5 months, respectively, in the hormone receptor–positive (HR+) cohort (hazard ratio, 0.64 for both groups). At the preplanned extended follow-up with data cut-off on March 1, 2023, the mOS was 22.9 versus 16.8 months for T-DXd versus TPC, and 23.9 versus 17.6 months for the HR+ cohort, respectively (HR, 0.69 for both).
Median progression-free survival (PFS) by investigator assessment was 8.8 versus 4.2 months for the full cohort, and 9.6 versus 4.2 months for the HR+ cohort (HR, 0.36 and 0.37, respectively). PFS was consistent with the results from the primary analysis.
The benefits in the HR+ patients were consistent across all patient subgroups, Dr. Modi noted.
“I do think it’s interesting to point out that at the landmark 2-year point, all patients on standard chemotherapy discontinued study treatment, whereas 15% on T-DXd remain [on treatment] without any evidence of disease progression, Dr. Modi added
An exploratory analysis in the hormone receptor–negative (HR–) cohort showed mOS of 18.2 versus 8.3 months at the primary analysis (HR, 0.48), and a “clinically meaningful and numerical advantage for T-DXd” persisted at the planned follow-up (mOS, 17.1 vs. 8.3; HR, 0.58), she said.
PFS in the HR- cohort was 8.5 versus 2.9 months at the primary analysis, and 6.3 versus 2.9 months at the update (HR, 0.46 and 0.29, respectively).
An assessment of post-study therapies received by patients showed that those therapies did not account for the significant survival advantage conferred by T-DXd, Dr. Modi said.
She noted, however, that while no new safety signals were observed at follow-up, lung toxicity remains a “toxicity of special interest,” having occurred in 12.1% of cases at the time of the primary analysis.
Most cases were grade 1 or 2, and no new cases were observed at follow-up, but one patient with lung toxicity and an initial grade 3 event experienced clinical deterioration and later died from lung toxicity, which underscores the importance of remaining vigilant and intervening promptly in all cases of lung toxicity, Dr. Modi stressed.
Invited discussant Giampaolo Bianchini, MD, reiterated that T-DXd is an effective treatment option and said, “we must accurately identify patients and avoid improperly denying this important therapeutic option.”
Although HER2-low disease is not a unique biological disease entity, it is a “practical and pragmatic definition used to select patients with ‘some degree’ of HER2 protein expression adopting a test and a scoring system already implemented in the routine clinical practice,” said Dr. Bianchini, head of the breast cancer group and head of clinical translational and immunotherapy research at IRCCS Ospedale, San Raffaele, Milan.
However, the current definition may be inadequate, he said, explaining that the ongoing DESTINY-Breast06 study “will challenge the current definition of what we consider HER2-low definition,” potentially extending the T-DXd indication to HER2 ultra-low.
Furthermore, current HER2 testing was designed to discriminate cases with high abundant protein – not for the low HER2 dynamic range, which leads to technical inaccuracy.
Given these considerations, he suggested considering a new biopsy, if feasible, in patients with an immunohistochemistry (IHC) score of 0 in all tumor biopsies, and having a revision performed by the pathologist.
In patients with an IHC score of 1 or greater only in one biopsy, there is no need to confirm the HER2-low status, he said.
DESTINY-Breast04 is funded by Daiichi Sankyo Inc. and AstraZeneca. Dr. Modi reported relationships with Daiichi Sankyo, Genentech, AstraZeneca, Seagen, and MacroGenics. Dr. Bianchini reported relationships with AstraZeneca, Daiichi Sankyo, Gilead, MSD, Seagen, Roche, Sanofi, Lilly, EISAI, Novartis, Pfizer, Stemline, Exact Science, and Agendia.
FROM ESMO 2023
Omitting surgery may be safe in early BC after neoadjuvant pCR
A small trial headed by MD Anderson Cancer Center, Houston, has helped to further identify women who can safely skip surgery after neoadjuvant therapy for early breast cancer.
Among 50 women in the study with cT1-2N0-1M0 triple negative or HER2-positive disease, 31 (62%) had a complete pathologic response (pCR) to neoadjuvant therapy on image-guided vacuum-assisted core biopsy (VACB).
They went onto whole breast radiation with a boost, but given their response to neoadjuvant treatment and the accuracy of VACB, the women did not have surgery.
So far, it seems to have been the right call: At 3 years, there’s been no tumor recurrences and disease-free and overall survival are both 100%.
Eliminating “breast surgery in highly-selected patients with image-guided VACB-determined pCR following” neoadjuvant systemic therapy has “very promising 3-year results,” lead investigator Henry M. Kuerer, MD, PhD, a breast cancer surgeon at MD Anderson, who presented the findings at the European Society for Medical Oncology (ESMO) 2023 annual meeting.
With the success of modern systemic therapy, “it’s only natural that we think this way,” said Ava Kwong, PhD, chief of breast surgery at the University of Hong Kong, who discussed Dr. Kuerer’s presentation at the meeting.
“This study is really important,” she said. “It’s addressing a very important question whether we can omit surgery in certain groups of patients ... We do want to deescalate surgery,” and the study results are “very good,” she said.
However, larger trials with longer follow-up are needed to draw any firm conclusions, she said.
Dr. Kuerer agreed. He and his team will continue to follow the study subjects, and they have opened up a new trial with 100 patients. A similar study is ongoing in Korea, as well, he noted.
Study details
Women in the trial were a median of 60.4 years old; 58% had HER2-positive and the rest triple-negative unicentric breast cancer. Mean baseline tumor size was 2.8 cm. Just 12% of the participants had lymph node involvement. Neoadjuvant systemic therapy was clinician’s choice.
Breast lesions had to shrink to less than 2 cm on imaging after systemic therapy to be eligible for the study, and a minimum of 12 cores had to be obtained on VACB.
The 38% of women in the study with residual disease after systemic treatment went on to surgery.
Two patients were circulating tumor cell (CTC)-positive at baseline, two were positive at 6 months, and one at 12 months. No patients had CTCs detected at more than one timepoint.
The work was funded by the National Cancer Institute. Dr. Kuerer is an adviser for Merck. Dr. Kwong is an adviser/speaker/reviewer/author for Stryker, AstraZeneca, Merck, and Roche. She also disclosed research funding from Merck, Roche, and Gilead and funding for genetic testing from AstraZeneca.
A small trial headed by MD Anderson Cancer Center, Houston, has helped to further identify women who can safely skip surgery after neoadjuvant therapy for early breast cancer.
Among 50 women in the study with cT1-2N0-1M0 triple negative or HER2-positive disease, 31 (62%) had a complete pathologic response (pCR) to neoadjuvant therapy on image-guided vacuum-assisted core biopsy (VACB).
They went onto whole breast radiation with a boost, but given their response to neoadjuvant treatment and the accuracy of VACB, the women did not have surgery.
So far, it seems to have been the right call: At 3 years, there’s been no tumor recurrences and disease-free and overall survival are both 100%.
Eliminating “breast surgery in highly-selected patients with image-guided VACB-determined pCR following” neoadjuvant systemic therapy has “very promising 3-year results,” lead investigator Henry M. Kuerer, MD, PhD, a breast cancer surgeon at MD Anderson, who presented the findings at the European Society for Medical Oncology (ESMO) 2023 annual meeting.
With the success of modern systemic therapy, “it’s only natural that we think this way,” said Ava Kwong, PhD, chief of breast surgery at the University of Hong Kong, who discussed Dr. Kuerer’s presentation at the meeting.
“This study is really important,” she said. “It’s addressing a very important question whether we can omit surgery in certain groups of patients ... We do want to deescalate surgery,” and the study results are “very good,” she said.
However, larger trials with longer follow-up are needed to draw any firm conclusions, she said.
Dr. Kuerer agreed. He and his team will continue to follow the study subjects, and they have opened up a new trial with 100 patients. A similar study is ongoing in Korea, as well, he noted.
Study details
Women in the trial were a median of 60.4 years old; 58% had HER2-positive and the rest triple-negative unicentric breast cancer. Mean baseline tumor size was 2.8 cm. Just 12% of the participants had lymph node involvement. Neoadjuvant systemic therapy was clinician’s choice.
Breast lesions had to shrink to less than 2 cm on imaging after systemic therapy to be eligible for the study, and a minimum of 12 cores had to be obtained on VACB.
The 38% of women in the study with residual disease after systemic treatment went on to surgery.
Two patients were circulating tumor cell (CTC)-positive at baseline, two were positive at 6 months, and one at 12 months. No patients had CTCs detected at more than one timepoint.
The work was funded by the National Cancer Institute. Dr. Kuerer is an adviser for Merck. Dr. Kwong is an adviser/speaker/reviewer/author for Stryker, AstraZeneca, Merck, and Roche. She also disclosed research funding from Merck, Roche, and Gilead and funding for genetic testing from AstraZeneca.
A small trial headed by MD Anderson Cancer Center, Houston, has helped to further identify women who can safely skip surgery after neoadjuvant therapy for early breast cancer.
Among 50 women in the study with cT1-2N0-1M0 triple negative or HER2-positive disease, 31 (62%) had a complete pathologic response (pCR) to neoadjuvant therapy on image-guided vacuum-assisted core biopsy (VACB).
They went onto whole breast radiation with a boost, but given their response to neoadjuvant treatment and the accuracy of VACB, the women did not have surgery.
So far, it seems to have been the right call: At 3 years, there’s been no tumor recurrences and disease-free and overall survival are both 100%.
Eliminating “breast surgery in highly-selected patients with image-guided VACB-determined pCR following” neoadjuvant systemic therapy has “very promising 3-year results,” lead investigator Henry M. Kuerer, MD, PhD, a breast cancer surgeon at MD Anderson, who presented the findings at the European Society for Medical Oncology (ESMO) 2023 annual meeting.
With the success of modern systemic therapy, “it’s only natural that we think this way,” said Ava Kwong, PhD, chief of breast surgery at the University of Hong Kong, who discussed Dr. Kuerer’s presentation at the meeting.
“This study is really important,” she said. “It’s addressing a very important question whether we can omit surgery in certain groups of patients ... We do want to deescalate surgery,” and the study results are “very good,” she said.
However, larger trials with longer follow-up are needed to draw any firm conclusions, she said.
Dr. Kuerer agreed. He and his team will continue to follow the study subjects, and they have opened up a new trial with 100 patients. A similar study is ongoing in Korea, as well, he noted.
Study details
Women in the trial were a median of 60.4 years old; 58% had HER2-positive and the rest triple-negative unicentric breast cancer. Mean baseline tumor size was 2.8 cm. Just 12% of the participants had lymph node involvement. Neoadjuvant systemic therapy was clinician’s choice.
Breast lesions had to shrink to less than 2 cm on imaging after systemic therapy to be eligible for the study, and a minimum of 12 cores had to be obtained on VACB.
The 38% of women in the study with residual disease after systemic treatment went on to surgery.
Two patients were circulating tumor cell (CTC)-positive at baseline, two were positive at 6 months, and one at 12 months. No patients had CTCs detected at more than one timepoint.
The work was funded by the National Cancer Institute. Dr. Kuerer is an adviser for Merck. Dr. Kwong is an adviser/speaker/reviewer/author for Stryker, AstraZeneca, Merck, and Roche. She also disclosed research funding from Merck, Roche, and Gilead and funding for genetic testing from AstraZeneca.
FROM ESMO 2023
Commentary: PMRT and New Treatments for Metastatic BC, November 2023
In patients with node-positive and locally advanced breast cancer (BC), postmastectomy radiation therapy (PMRT) decreases risk for recurrence and improves survival (Mutter et al). Proton therapy is an attractive newer way to deliver PMRT compared with photon-based methods and allows improved sparing of cardiopulmonary and other normal tissue. The phase 2 MC1631 trial included 82 patients with BC who underwent mastectomy with or without immediate breast reconstruction and who were randomly assigned to receive either conventional fractionated (50 Gy in 25 fractions of 2 Gy) or hypofractionated (40.05 Gy in 15 fractions of 2.67 Gy) proton PMRT. At a median follow-up of 39.3 months, both conventional fractionated and hypofractionated proton PMRT had similar complication rates (15% vs 20%; absolute difference 4.9%; one-sided 95% CI 18.5; P = .27), with most complications occurring in patients with immediate expander or implant-based reconstruction. Noninferiority of the hypofractionation group could not be determined after a median follow-up of 39 months. However, no isolated local regional recurrences in either treatment arm were seen. This study provides the first prospective, randomized data of hypofractionated proton PMRT. Further data are awaited to support this approach.
In patients with metastatic hormone receptor (HR)–positive, PIK3CA-mutant BC, the combination of fulvestrant with alpelisib improves progression-free survival per the SOLAR-1 study.1 Higher rates of hyperglycemia observed among patients treated with alpelisib have led to alpelisib dose reductions, treatment delays, and discontinuation of the drug. In a retrospective cohort study of 247 patients with metastatic BC who received alpelisib either as standard care (n = 147) or in a clinical trial setting (n = 100), 61.5% of patients developed any-grade hyperglycemia (Shen et al). The rate of hyperglycemia was considerably higher in patients who received alpelisib as part of standard care vs clinical trial (80.3% vs 34.0%). Baseline body mass index ≥ 25 (P = .036) and A1c levels in the prediabetes and diabetes range were significantly associated with the development of any-grade hyperglycemia (P = .036 and P < .001, respectively) and grade 3-4 hyperglycemia (P < .001 for both). A total of 4.5% of patients discontinued alpelisib owing to hyperglycemia, 17% of patients required dose reductions, and in 27% of patients alpelisib was held until resolution of hyperglycemia. This study highlights the importance of the management of comorbidities before alpelisib treatment to ensure lower rates of adverse events.
Patritumab deruxtecan (HER3-DXd) is a novel HER3-targeted antibody-drug conjugate that is being evaluated in HER3-expressing metastatic BC. The U31402-A-J101 study is a phase 1/2 trial including 182 heavily pretreated patients (median of five prior therapies) with HER3-expressing advanced BC who received HER3-DXd (Krop et al). The objective response rate was 30.1% (95% CI 21.8%-39.4%) in HR-positive, human epidermal growth factor receptor 2 (HER2)–negative BC, 22.6% (95% CI 12.3%-36.2%) in triple-negative BC, and 42.9% (95% CI 17.1%-71.1%) in HER2-positive BC. Although 71.4% of patients reported grade ≥ 3 treatment-emergent adverse events (TEAE), the overall rate of treatment discontinuation due to TEAE was low (9.9%). These findings demonstrate an encouraging efficacy and a manageable safety profile for patritumab deruxtecan in previously treated patients with BC across all subtypes. Further studies are awaited to confirm these findings and whether prior treatment with antibody-drug conjugate will affect the activity of this drug.
A retrospective analysis of a cohort including 149 patients with metastatic BC looked at predictors of prognosis in patients who had brain metastases and underwent stereotactic radiosurgery (Depner et al). The median overall survival was 14.8 months for the entire cohort. Receptor profiles and the presence of extracranial visceral metastases were significant predictors of prognosis. Overall survival outcomes worsened in patients with estrogen receptor (ER)–negative, HER2-negative BC (hazard ratio 2.00; 95% CI 1.09-3.67) but were better in those with ER-positive, HER2-positive BC (hazard ratio 0.43; 95% CI 0.19-0.96). Furthermore, the presence of extracranial visceral metastases was associated with poor survival outcomes (hazard ratio 2.90; 95% CI 1.53-5.50)
Additional Reference
1. André F et al, for the SOLAR-1 Study Group. Alpelisib for PIK3CA-mutated, hormone receptor–positive advanced breast cancer. N Engl J Med. 2019;380:1929-1940. doi: 10.1056/NEJMoa1813904
In patients with node-positive and locally advanced breast cancer (BC), postmastectomy radiation therapy (PMRT) decreases risk for recurrence and improves survival (Mutter et al). Proton therapy is an attractive newer way to deliver PMRT compared with photon-based methods and allows improved sparing of cardiopulmonary and other normal tissue. The phase 2 MC1631 trial included 82 patients with BC who underwent mastectomy with or without immediate breast reconstruction and who were randomly assigned to receive either conventional fractionated (50 Gy in 25 fractions of 2 Gy) or hypofractionated (40.05 Gy in 15 fractions of 2.67 Gy) proton PMRT. At a median follow-up of 39.3 months, both conventional fractionated and hypofractionated proton PMRT had similar complication rates (15% vs 20%; absolute difference 4.9%; one-sided 95% CI 18.5; P = .27), with most complications occurring in patients with immediate expander or implant-based reconstruction. Noninferiority of the hypofractionation group could not be determined after a median follow-up of 39 months. However, no isolated local regional recurrences in either treatment arm were seen. This study provides the first prospective, randomized data of hypofractionated proton PMRT. Further data are awaited to support this approach.
In patients with metastatic hormone receptor (HR)–positive, PIK3CA-mutant BC, the combination of fulvestrant with alpelisib improves progression-free survival per the SOLAR-1 study.1 Higher rates of hyperglycemia observed among patients treated with alpelisib have led to alpelisib dose reductions, treatment delays, and discontinuation of the drug. In a retrospective cohort study of 247 patients with metastatic BC who received alpelisib either as standard care (n = 147) or in a clinical trial setting (n = 100), 61.5% of patients developed any-grade hyperglycemia (Shen et al). The rate of hyperglycemia was considerably higher in patients who received alpelisib as part of standard care vs clinical trial (80.3% vs 34.0%). Baseline body mass index ≥ 25 (P = .036) and A1c levels in the prediabetes and diabetes range were significantly associated with the development of any-grade hyperglycemia (P = .036 and P < .001, respectively) and grade 3-4 hyperglycemia (P < .001 for both). A total of 4.5% of patients discontinued alpelisib owing to hyperglycemia, 17% of patients required dose reductions, and in 27% of patients alpelisib was held until resolution of hyperglycemia. This study highlights the importance of the management of comorbidities before alpelisib treatment to ensure lower rates of adverse events.
Patritumab deruxtecan (HER3-DXd) is a novel HER3-targeted antibody-drug conjugate that is being evaluated in HER3-expressing metastatic BC. The U31402-A-J101 study is a phase 1/2 trial including 182 heavily pretreated patients (median of five prior therapies) with HER3-expressing advanced BC who received HER3-DXd (Krop et al). The objective response rate was 30.1% (95% CI 21.8%-39.4%) in HR-positive, human epidermal growth factor receptor 2 (HER2)–negative BC, 22.6% (95% CI 12.3%-36.2%) in triple-negative BC, and 42.9% (95% CI 17.1%-71.1%) in HER2-positive BC. Although 71.4% of patients reported grade ≥ 3 treatment-emergent adverse events (TEAE), the overall rate of treatment discontinuation due to TEAE was low (9.9%). These findings demonstrate an encouraging efficacy and a manageable safety profile for patritumab deruxtecan in previously treated patients with BC across all subtypes. Further studies are awaited to confirm these findings and whether prior treatment with antibody-drug conjugate will affect the activity of this drug.
A retrospective analysis of a cohort including 149 patients with metastatic BC looked at predictors of prognosis in patients who had brain metastases and underwent stereotactic radiosurgery (Depner et al). The median overall survival was 14.8 months for the entire cohort. Receptor profiles and the presence of extracranial visceral metastases were significant predictors of prognosis. Overall survival outcomes worsened in patients with estrogen receptor (ER)–negative, HER2-negative BC (hazard ratio 2.00; 95% CI 1.09-3.67) but were better in those with ER-positive, HER2-positive BC (hazard ratio 0.43; 95% CI 0.19-0.96). Furthermore, the presence of extracranial visceral metastases was associated with poor survival outcomes (hazard ratio 2.90; 95% CI 1.53-5.50)
Additional Reference
1. André F et al, for the SOLAR-1 Study Group. Alpelisib for PIK3CA-mutated, hormone receptor–positive advanced breast cancer. N Engl J Med. 2019;380:1929-1940. doi: 10.1056/NEJMoa1813904
In patients with node-positive and locally advanced breast cancer (BC), postmastectomy radiation therapy (PMRT) decreases risk for recurrence and improves survival (Mutter et al). Proton therapy is an attractive newer way to deliver PMRT compared with photon-based methods and allows improved sparing of cardiopulmonary and other normal tissue. The phase 2 MC1631 trial included 82 patients with BC who underwent mastectomy with or without immediate breast reconstruction and who were randomly assigned to receive either conventional fractionated (50 Gy in 25 fractions of 2 Gy) or hypofractionated (40.05 Gy in 15 fractions of 2.67 Gy) proton PMRT. At a median follow-up of 39.3 months, both conventional fractionated and hypofractionated proton PMRT had similar complication rates (15% vs 20%; absolute difference 4.9%; one-sided 95% CI 18.5; P = .27), with most complications occurring in patients with immediate expander or implant-based reconstruction. Noninferiority of the hypofractionation group could not be determined after a median follow-up of 39 months. However, no isolated local regional recurrences in either treatment arm were seen. This study provides the first prospective, randomized data of hypofractionated proton PMRT. Further data are awaited to support this approach.
In patients with metastatic hormone receptor (HR)–positive, PIK3CA-mutant BC, the combination of fulvestrant with alpelisib improves progression-free survival per the SOLAR-1 study.1 Higher rates of hyperglycemia observed among patients treated with alpelisib have led to alpelisib dose reductions, treatment delays, and discontinuation of the drug. In a retrospective cohort study of 247 patients with metastatic BC who received alpelisib either as standard care (n = 147) or in a clinical trial setting (n = 100), 61.5% of patients developed any-grade hyperglycemia (Shen et al). The rate of hyperglycemia was considerably higher in patients who received alpelisib as part of standard care vs clinical trial (80.3% vs 34.0%). Baseline body mass index ≥ 25 (P = .036) and A1c levels in the prediabetes and diabetes range were significantly associated with the development of any-grade hyperglycemia (P = .036 and P < .001, respectively) and grade 3-4 hyperglycemia (P < .001 for both). A total of 4.5% of patients discontinued alpelisib owing to hyperglycemia, 17% of patients required dose reductions, and in 27% of patients alpelisib was held until resolution of hyperglycemia. This study highlights the importance of the management of comorbidities before alpelisib treatment to ensure lower rates of adverse events.
Patritumab deruxtecan (HER3-DXd) is a novel HER3-targeted antibody-drug conjugate that is being evaluated in HER3-expressing metastatic BC. The U31402-A-J101 study is a phase 1/2 trial including 182 heavily pretreated patients (median of five prior therapies) with HER3-expressing advanced BC who received HER3-DXd (Krop et al). The objective response rate was 30.1% (95% CI 21.8%-39.4%) in HR-positive, human epidermal growth factor receptor 2 (HER2)–negative BC, 22.6% (95% CI 12.3%-36.2%) in triple-negative BC, and 42.9% (95% CI 17.1%-71.1%) in HER2-positive BC. Although 71.4% of patients reported grade ≥ 3 treatment-emergent adverse events (TEAE), the overall rate of treatment discontinuation due to TEAE was low (9.9%). These findings demonstrate an encouraging efficacy and a manageable safety profile for patritumab deruxtecan in previously treated patients with BC across all subtypes. Further studies are awaited to confirm these findings and whether prior treatment with antibody-drug conjugate will affect the activity of this drug.
A retrospective analysis of a cohort including 149 patients with metastatic BC looked at predictors of prognosis in patients who had brain metastases and underwent stereotactic radiosurgery (Depner et al). The median overall survival was 14.8 months for the entire cohort. Receptor profiles and the presence of extracranial visceral metastases were significant predictors of prognosis. Overall survival outcomes worsened in patients with estrogen receptor (ER)–negative, HER2-negative BC (hazard ratio 2.00; 95% CI 1.09-3.67) but were better in those with ER-positive, HER2-positive BC (hazard ratio 0.43; 95% CI 0.19-0.96). Furthermore, the presence of extracranial visceral metastases was associated with poor survival outcomes (hazard ratio 2.90; 95% CI 1.53-5.50)
Additional Reference
1. André F et al, for the SOLAR-1 Study Group. Alpelisib for PIK3CA-mutated, hormone receptor–positive advanced breast cancer. N Engl J Med. 2019;380:1929-1940. doi: 10.1056/NEJMoa1813904
Commentary: Axillary Surgery, PM2.5, and Treatment With Tucatinib in Breast Cancer, November 2023
Hormone receptor–positive breast cancer is the most common subtype, with established risk factors including exposure to exogenous hormones, reproductive history, and lifestyle components (alcohol intake, obesity). There are also less-recognized environmental influences that may disrupt endocrine pathways and, as a result, affect tumor development. Fine particulate matter (PM2.5), produced by combustion processes (vehicles, industrial facilities), burning wood, and fires, among other sources, is composed of various airborne pollutants (metals, organic compounds, ammonium, nitrate, ozone, sulfate, etc.). Prior studies evaluating the association of PM2.5 and breast cancer development have shown mixed results.3,4 A prospective US cohort study including 196,905 women without a prior history of breast cancer estimated historical annual average PM2.5 concentrations between 1980 and 1984 (10 years prior to enrollment) (White et al). A total of 15,870 breast cancer cases were identified, and a 10 μg/m3 increase in PM2.5 was associated with an 8% increase in overall breast cancer incidence (HR 1.08; 95% CI 1.02-1.13). The association was observed for estrogen-receptor (ER)-positive (HR 1.10; 95% CI 1.04-1.17) but not ER-negative tumors. Future studies focusing on historic exposures, investigating geographic differences and the resultant effect on cancer development, are of interest.
HER2CLIMB was a pivotal phase 3 randomized, double-blinded trial that demonstrated significant improvement in survival outcomes with the combination of tucatinib/trastuzumab/capecitabine vs tucatinib/trastuzumab/placebo among patients with previously treated human epidermal growth factor receptor 2 (HER2)-positive metastatic breast cancer.5 Real-world data help inform our daily practice because patients enrolled in clinical trials do not always accurately represent the general population. A retrospective cohort study including 3449 patients with HER2-positive metastatic breast cancer evaluated outcomes with tucatinib in a real-world setting, demonstrating results similar to those seen in HER2CLIMB. Among all patients who received tucatinib (n = 216), median real-world time to treatment discontinuation was 6.5 months (95% CI 5.4-8.8), median real-world time to next treatment (which can serve as a proxy for progression-free survival) was 8.7 months (95% CI 6.8-10.7), and real-world overall survival was 26.6 months (95% CI 20.2–not reached). Median real-world time to treatment discontinuation was 8.1 months (95% CI 5.7-9.5) for patients who received the approved tucatinib triplet combination after one or more HER2-directed regimens in the metastatic setting and 9.4 months (95% CI 6.3-14.1) for those receiving it in the second- or third-line setting (Kaufman et al). These results support the efficacy of tucatinib in a real-world population, suggesting that earlier use (second or third line) may result in better outcomes. Future studies will continue to address the positioning of tucatinib in the treatment algorithm for HER2-positive metastatic breast cancer, including the evaluation of novel combinations.
Additional References
- Giuliano AE et al. Effect of axillary dissection vs no axillary dissection on 10-year overall survival among women with invasive breast cancer and sentinel node metastasis: The ACOSOG Z0011 (Alliance) randomized clinical trial. JAMA. 2017;318:918-926. doi: 10.1001/jama.2017.11470
- Bartels SAL, Donker M, et al. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer: 10-year results of the randomized controlled EORTC 10981-22023 AMAROS Trial. J Clin Oncol. 2023;41:2159-2165. doi: 10.1200/JCO.22.01565
- Gabet S, Lemarchand C, Guénel P, Slama R. Breast cancer risk in association with atmospheric pollution exposure: A meta-analysis of effect estimates followed by a health impact assessment. Environ Health Perspect. 2021;129:57012. doi: 10.1289/EHP8419
- Hvidtfeldt UA et al. Breast cancer incidence in relation to long-term low-level exposure to air pollution in the ELAPSE pooled cohort. Cancer Epidemiol Biomarkers Prev. 2023;32:105-113. doi: 10.1158/1055-9965.EPI-22-0720
- Murthy RK et al. Tucatinib, trastuzumab, and capecitabine for HER2-positive metastatic breast cancer. N Engl J Med. 2020;382:597-609. doi:10.1056/NEJMoa1914609
Hormone receptor–positive breast cancer is the most common subtype, with established risk factors including exposure to exogenous hormones, reproductive history, and lifestyle components (alcohol intake, obesity). There are also less-recognized environmental influences that may disrupt endocrine pathways and, as a result, affect tumor development. Fine particulate matter (PM2.5), produced by combustion processes (vehicles, industrial facilities), burning wood, and fires, among other sources, is composed of various airborne pollutants (metals, organic compounds, ammonium, nitrate, ozone, sulfate, etc.). Prior studies evaluating the association of PM2.5 and breast cancer development have shown mixed results.3,4 A prospective US cohort study including 196,905 women without a prior history of breast cancer estimated historical annual average PM2.5 concentrations between 1980 and 1984 (10 years prior to enrollment) (White et al). A total of 15,870 breast cancer cases were identified, and a 10 μg/m3 increase in PM2.5 was associated with an 8% increase in overall breast cancer incidence (HR 1.08; 95% CI 1.02-1.13). The association was observed for estrogen-receptor (ER)-positive (HR 1.10; 95% CI 1.04-1.17) but not ER-negative tumors. Future studies focusing on historic exposures, investigating geographic differences and the resultant effect on cancer development, are of interest.
HER2CLIMB was a pivotal phase 3 randomized, double-blinded trial that demonstrated significant improvement in survival outcomes with the combination of tucatinib/trastuzumab/capecitabine vs tucatinib/trastuzumab/placebo among patients with previously treated human epidermal growth factor receptor 2 (HER2)-positive metastatic breast cancer.5 Real-world data help inform our daily practice because patients enrolled in clinical trials do not always accurately represent the general population. A retrospective cohort study including 3449 patients with HER2-positive metastatic breast cancer evaluated outcomes with tucatinib in a real-world setting, demonstrating results similar to those seen in HER2CLIMB. Among all patients who received tucatinib (n = 216), median real-world time to treatment discontinuation was 6.5 months (95% CI 5.4-8.8), median real-world time to next treatment (which can serve as a proxy for progression-free survival) was 8.7 months (95% CI 6.8-10.7), and real-world overall survival was 26.6 months (95% CI 20.2–not reached). Median real-world time to treatment discontinuation was 8.1 months (95% CI 5.7-9.5) for patients who received the approved tucatinib triplet combination after one or more HER2-directed regimens in the metastatic setting and 9.4 months (95% CI 6.3-14.1) for those receiving it in the second- or third-line setting (Kaufman et al). These results support the efficacy of tucatinib in a real-world population, suggesting that earlier use (second or third line) may result in better outcomes. Future studies will continue to address the positioning of tucatinib in the treatment algorithm for HER2-positive metastatic breast cancer, including the evaluation of novel combinations.
Additional References
- Giuliano AE et al. Effect of axillary dissection vs no axillary dissection on 10-year overall survival among women with invasive breast cancer and sentinel node metastasis: The ACOSOG Z0011 (Alliance) randomized clinical trial. JAMA. 2017;318:918-926. doi: 10.1001/jama.2017.11470
- Bartels SAL, Donker M, et al. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer: 10-year results of the randomized controlled EORTC 10981-22023 AMAROS Trial. J Clin Oncol. 2023;41:2159-2165. doi: 10.1200/JCO.22.01565
- Gabet S, Lemarchand C, Guénel P, Slama R. Breast cancer risk in association with atmospheric pollution exposure: A meta-analysis of effect estimates followed by a health impact assessment. Environ Health Perspect. 2021;129:57012. doi: 10.1289/EHP8419
- Hvidtfeldt UA et al. Breast cancer incidence in relation to long-term low-level exposure to air pollution in the ELAPSE pooled cohort. Cancer Epidemiol Biomarkers Prev. 2023;32:105-113. doi: 10.1158/1055-9965.EPI-22-0720
- Murthy RK et al. Tucatinib, trastuzumab, and capecitabine for HER2-positive metastatic breast cancer. N Engl J Med. 2020;382:597-609. doi:10.1056/NEJMoa1914609
Hormone receptor–positive breast cancer is the most common subtype, with established risk factors including exposure to exogenous hormones, reproductive history, and lifestyle components (alcohol intake, obesity). There are also less-recognized environmental influences that may disrupt endocrine pathways and, as a result, affect tumor development. Fine particulate matter (PM2.5), produced by combustion processes (vehicles, industrial facilities), burning wood, and fires, among other sources, is composed of various airborne pollutants (metals, organic compounds, ammonium, nitrate, ozone, sulfate, etc.). Prior studies evaluating the association of PM2.5 and breast cancer development have shown mixed results.3,4 A prospective US cohort study including 196,905 women without a prior history of breast cancer estimated historical annual average PM2.5 concentrations between 1980 and 1984 (10 years prior to enrollment) (White et al). A total of 15,870 breast cancer cases were identified, and a 10 μg/m3 increase in PM2.5 was associated with an 8% increase in overall breast cancer incidence (HR 1.08; 95% CI 1.02-1.13). The association was observed for estrogen-receptor (ER)-positive (HR 1.10; 95% CI 1.04-1.17) but not ER-negative tumors. Future studies focusing on historic exposures, investigating geographic differences and the resultant effect on cancer development, are of interest.
HER2CLIMB was a pivotal phase 3 randomized, double-blinded trial that demonstrated significant improvement in survival outcomes with the combination of tucatinib/trastuzumab/capecitabine vs tucatinib/trastuzumab/placebo among patients with previously treated human epidermal growth factor receptor 2 (HER2)-positive metastatic breast cancer.5 Real-world data help inform our daily practice because patients enrolled in clinical trials do not always accurately represent the general population. A retrospective cohort study including 3449 patients with HER2-positive metastatic breast cancer evaluated outcomes with tucatinib in a real-world setting, demonstrating results similar to those seen in HER2CLIMB. Among all patients who received tucatinib (n = 216), median real-world time to treatment discontinuation was 6.5 months (95% CI 5.4-8.8), median real-world time to next treatment (which can serve as a proxy for progression-free survival) was 8.7 months (95% CI 6.8-10.7), and real-world overall survival was 26.6 months (95% CI 20.2–not reached). Median real-world time to treatment discontinuation was 8.1 months (95% CI 5.7-9.5) for patients who received the approved tucatinib triplet combination after one or more HER2-directed regimens in the metastatic setting and 9.4 months (95% CI 6.3-14.1) for those receiving it in the second- or third-line setting (Kaufman et al). These results support the efficacy of tucatinib in a real-world population, suggesting that earlier use (second or third line) may result in better outcomes. Future studies will continue to address the positioning of tucatinib in the treatment algorithm for HER2-positive metastatic breast cancer, including the evaluation of novel combinations.
Additional References
- Giuliano AE et al. Effect of axillary dissection vs no axillary dissection on 10-year overall survival among women with invasive breast cancer and sentinel node metastasis: The ACOSOG Z0011 (Alliance) randomized clinical trial. JAMA. 2017;318:918-926. doi: 10.1001/jama.2017.11470
- Bartels SAL, Donker M, et al. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer: 10-year results of the randomized controlled EORTC 10981-22023 AMAROS Trial. J Clin Oncol. 2023;41:2159-2165. doi: 10.1200/JCO.22.01565
- Gabet S, Lemarchand C, Guénel P, Slama R. Breast cancer risk in association with atmospheric pollution exposure: A meta-analysis of effect estimates followed by a health impact assessment. Environ Health Perspect. 2021;129:57012. doi: 10.1289/EHP8419
- Hvidtfeldt UA et al. Breast cancer incidence in relation to long-term low-level exposure to air pollution in the ELAPSE pooled cohort. Cancer Epidemiol Biomarkers Prev. 2023;32:105-113. doi: 10.1158/1055-9965.EPI-22-0720
- Murthy RK et al. Tucatinib, trastuzumab, and capecitabine for HER2-positive metastatic breast cancer. N Engl J Med. 2020;382:597-609. doi:10.1056/NEJMoa1914609
Dato-DXd trumps chemo in advanced HR+/HER2– breast cancer
The investigational anti-body drug conjugate (ADC) resistant to endocrine therapy, data from the phase 3 TROPION-Breast01 trial showed.
At a median follow-up of 10.8 months, the median progression-free survival (PFS) was 6.9 months for patients randomly assigned to receive Dato-DXd, compared with 4.9 months for the investigator’s choice of chemotherapy with either eribulin mesylate, vinorelbine, capecitabine, or gemcitabine. This difference translated into a 37% reduction in risk of disease progression with the ADC, reported Aditya Bardia, MD, MPH, director of the breast cancer research program at the Mass General Cancer Center in Boston.
Patients who received Dato-DXd had less than half the number of grade 3 or greater toxicities and fewer dose reductions or interruptions than patients who received chemotherapy, he noted in an oral abstract session at the 2023 European Society for Medical Oncology Congress.
“Overall, results support Dato-DXd as a potential new therapeutic option for patients with metastatic hormone receptor–positive breast cancer,” he said.
Different ADC, same target
Dr. Bardia noted that there is an unmet need for effective therapies for patients with metastatic HR+/HER2– breast cancer who experience disease progression after endocrine therapy and at least one line of systemic therapy.
Although chemotherapy is widely used in this population, it’s associated with low response rates, poor prognosis, and significant toxicities, including hematologic and neurologic adverse events (AEs).
Dato-DXd is composed of a monoclonal antibody targeting TROP2, a transmembrane glycoprotein overexpressed in cancer cells, linked to the topoisomerase 1 inhibitor deruxtecan as the toxic payload.
Dr. Bardia explained that Dato-DXd has four properties that distinguish it from other TROP2-directed ADCs: an optimized drug to antibody ratio of 4, a stable linker-payload, tumor-selective cleavable linker, both of which reduce off-target toxicities, and a bystander antitumor effect that can target TROP2-expressing cells in the tumor microenvironment.
In the phase I TROPION-PanTumor01 trial, Dato-DXd had promising anti-tumor activity and a manageable safety profile in patients with metastatic HR+/HER2– breast cancer, paving the way for the TROPION-Breast01 study reported here.
Efficacy results
In the Breast01 trial, 732 patients with inoperable or metastatic HR+/HER2– breast cancer previously treated with 1 or 2 lines of chemotherapy that had progressed on endocrine therapy were stratified by number of prior chemotherapy lines, geographic region, and prior CDK4/6 inhibitor status, and then randomized to either Dato-DXd 6 mg/kg intravenously on day 1 of each 3-week cycle (365 patients) or to investigator’s choice of chemotherapy (367 patients). According to the protocol, chemotherapy could be eribulin mesylate, vinorelbine, or gemcitabine delivered via IV on days 1 and 8 every 3 weeks, or oral capecitabine on days 1 through 14 of every 3-week cycle.
At the time of data cutoff, 93 patients assigned to the ADC and 39 assigned to chemotherapy were still on treatment.
As noted before, median PFS by blinded independent central review, one of two primary endpoints, was 6.9 months with Dato-DXd, compared with 4.9 months with chemotherapy, translating into a hazard ratio for progression of 0.63 (P < .0001).
The benefit was seen across nearly all subgroups except among patients who had not previously received a CDK4/6 inhibitor, and patients who had received a prior anthracycline but not a taxane.
Objective response rates (ORR) were 36.4% with Dato-DXd (99.5% partial and .5% complete response), compared with 22.9% with chemotherapy (all partial responses; P values not reported).
Overall survival data, the other primary endpoint, were not mature at a median OS follow-up of 9.7 months, and will be reported at a later date.
Safety results
“In terms of safety, the rate of grade 3 or higher treatment-related AEs in the Dato-DXd arm was less as compared to investigator choice of chemotherapy. This is a bit different from most of the studies; in general we see that the rate of adverse events is higher in the intervention arm as compared to the control arm,” Dr. Bardia commented.
Rates of dose reductions and dose interruptions due to treatment-related AEs were also lower with the ADC.
There were no patient deaths associated with Dato-DXd. One patient assigned to chemotherapy died from a complication associated with febrile neutropenia.
Most treatment-related AEs occurring in 15% of patients and AEs of special interest were of grade 1 and manageable.
The most common toxicities seen with the ADC were oral mucositis and dry eye. The most common side effects with chemotherapy were neutropenia and anemia, “the usual side effects you would expect with chemotherapy,” Dr. Bardia said, pointing out that the rate of grade 3 neutropenia was 31% with standard chemotherapy, compared with 1% with Dato-DXd.
Good, but we can do better
ESMO invited discussant Sarat Chandarlapaty, MD, PhD, a breast oncologist at Memorial Sloan Kettering Cancer Center in New York, commented that while the trial data showed superior efficacy and safety with Dato-DXd, compared with standard chemotherapy, it’s still unclear how it and other ADCs on the market and in the research pipeline may be used in therapy for this patient population.
“Would I rather prescribe Dato-DXd or more chemo after 1 to 2 lines of chemo in unselected HR-positive, HER2-negative breast cancer? The answer is Dato-DXd, but it leaves several unanswered questions for us,” he said.
“First, we have two ADCs approved in HR-positive breast cancer: another TROP2 ADC sacituzumab [govitecan] and a HER2 ADC trastuzumab deruxtecan. Would I rather give Dato over one of these? I don’t have an answer,” he added.
In addition, it’s unknown whether these drugs, which have the same topoisomerase-targeted payload, could be given in sequence, and there are as yet no clear answers as to whether patients might do better with Dato-DXd or with a PIK3ca inhibitor.
“I would say that the elephant in the room is really another question, and that is, ‘Is Dato-DXd in this context delivering on the promise of an ADC?’ ” Dr. Chandarlapaty said.
“I think translational research is urgently needed if we’re ultimately to deliver on the promise of these agents in the clinic,” he concluded.
The TROPION-Breast01 study is sponsored AstraZeneca, which is collaborating with Daiichi-Sankyo on global development and commercialization of Dato-DXd. Dr. Bardia disclosed advisory board activities and institutional research funding from AstraZeneca and Daiichi-Sankyo and others. Dr. Chandarlapaty disclosed research funding from both companies, and advisory board activities for AstraZeneca and others.
The investigational anti-body drug conjugate (ADC) resistant to endocrine therapy, data from the phase 3 TROPION-Breast01 trial showed.
At a median follow-up of 10.8 months, the median progression-free survival (PFS) was 6.9 months for patients randomly assigned to receive Dato-DXd, compared with 4.9 months for the investigator’s choice of chemotherapy with either eribulin mesylate, vinorelbine, capecitabine, or gemcitabine. This difference translated into a 37% reduction in risk of disease progression with the ADC, reported Aditya Bardia, MD, MPH, director of the breast cancer research program at the Mass General Cancer Center in Boston.
Patients who received Dato-DXd had less than half the number of grade 3 or greater toxicities and fewer dose reductions or interruptions than patients who received chemotherapy, he noted in an oral abstract session at the 2023 European Society for Medical Oncology Congress.
“Overall, results support Dato-DXd as a potential new therapeutic option for patients with metastatic hormone receptor–positive breast cancer,” he said.
Different ADC, same target
Dr. Bardia noted that there is an unmet need for effective therapies for patients with metastatic HR+/HER2– breast cancer who experience disease progression after endocrine therapy and at least one line of systemic therapy.
Although chemotherapy is widely used in this population, it’s associated with low response rates, poor prognosis, and significant toxicities, including hematologic and neurologic adverse events (AEs).
Dato-DXd is composed of a monoclonal antibody targeting TROP2, a transmembrane glycoprotein overexpressed in cancer cells, linked to the topoisomerase 1 inhibitor deruxtecan as the toxic payload.
Dr. Bardia explained that Dato-DXd has four properties that distinguish it from other TROP2-directed ADCs: an optimized drug to antibody ratio of 4, a stable linker-payload, tumor-selective cleavable linker, both of which reduce off-target toxicities, and a bystander antitumor effect that can target TROP2-expressing cells in the tumor microenvironment.
In the phase I TROPION-PanTumor01 trial, Dato-DXd had promising anti-tumor activity and a manageable safety profile in patients with metastatic HR+/HER2– breast cancer, paving the way for the TROPION-Breast01 study reported here.
Efficacy results
In the Breast01 trial, 732 patients with inoperable or metastatic HR+/HER2– breast cancer previously treated with 1 or 2 lines of chemotherapy that had progressed on endocrine therapy were stratified by number of prior chemotherapy lines, geographic region, and prior CDK4/6 inhibitor status, and then randomized to either Dato-DXd 6 mg/kg intravenously on day 1 of each 3-week cycle (365 patients) or to investigator’s choice of chemotherapy (367 patients). According to the protocol, chemotherapy could be eribulin mesylate, vinorelbine, or gemcitabine delivered via IV on days 1 and 8 every 3 weeks, or oral capecitabine on days 1 through 14 of every 3-week cycle.
At the time of data cutoff, 93 patients assigned to the ADC and 39 assigned to chemotherapy were still on treatment.
As noted before, median PFS by blinded independent central review, one of two primary endpoints, was 6.9 months with Dato-DXd, compared with 4.9 months with chemotherapy, translating into a hazard ratio for progression of 0.63 (P < .0001).
The benefit was seen across nearly all subgroups except among patients who had not previously received a CDK4/6 inhibitor, and patients who had received a prior anthracycline but not a taxane.
Objective response rates (ORR) were 36.4% with Dato-DXd (99.5% partial and .5% complete response), compared with 22.9% with chemotherapy (all partial responses; P values not reported).
Overall survival data, the other primary endpoint, were not mature at a median OS follow-up of 9.7 months, and will be reported at a later date.
Safety results
“In terms of safety, the rate of grade 3 or higher treatment-related AEs in the Dato-DXd arm was less as compared to investigator choice of chemotherapy. This is a bit different from most of the studies; in general we see that the rate of adverse events is higher in the intervention arm as compared to the control arm,” Dr. Bardia commented.
Rates of dose reductions and dose interruptions due to treatment-related AEs were also lower with the ADC.
There were no patient deaths associated with Dato-DXd. One patient assigned to chemotherapy died from a complication associated with febrile neutropenia.
Most treatment-related AEs occurring in 15% of patients and AEs of special interest were of grade 1 and manageable.
The most common toxicities seen with the ADC were oral mucositis and dry eye. The most common side effects with chemotherapy were neutropenia and anemia, “the usual side effects you would expect with chemotherapy,” Dr. Bardia said, pointing out that the rate of grade 3 neutropenia was 31% with standard chemotherapy, compared with 1% with Dato-DXd.
Good, but we can do better
ESMO invited discussant Sarat Chandarlapaty, MD, PhD, a breast oncologist at Memorial Sloan Kettering Cancer Center in New York, commented that while the trial data showed superior efficacy and safety with Dato-DXd, compared with standard chemotherapy, it’s still unclear how it and other ADCs on the market and in the research pipeline may be used in therapy for this patient population.
“Would I rather prescribe Dato-DXd or more chemo after 1 to 2 lines of chemo in unselected HR-positive, HER2-negative breast cancer? The answer is Dato-DXd, but it leaves several unanswered questions for us,” he said.
“First, we have two ADCs approved in HR-positive breast cancer: another TROP2 ADC sacituzumab [govitecan] and a HER2 ADC trastuzumab deruxtecan. Would I rather give Dato over one of these? I don’t have an answer,” he added.
In addition, it’s unknown whether these drugs, which have the same topoisomerase-targeted payload, could be given in sequence, and there are as yet no clear answers as to whether patients might do better with Dato-DXd or with a PIK3ca inhibitor.
“I would say that the elephant in the room is really another question, and that is, ‘Is Dato-DXd in this context delivering on the promise of an ADC?’ ” Dr. Chandarlapaty said.
“I think translational research is urgently needed if we’re ultimately to deliver on the promise of these agents in the clinic,” he concluded.
The TROPION-Breast01 study is sponsored AstraZeneca, which is collaborating with Daiichi-Sankyo on global development and commercialization of Dato-DXd. Dr. Bardia disclosed advisory board activities and institutional research funding from AstraZeneca and Daiichi-Sankyo and others. Dr. Chandarlapaty disclosed research funding from both companies, and advisory board activities for AstraZeneca and others.
The investigational anti-body drug conjugate (ADC) resistant to endocrine therapy, data from the phase 3 TROPION-Breast01 trial showed.
At a median follow-up of 10.8 months, the median progression-free survival (PFS) was 6.9 months for patients randomly assigned to receive Dato-DXd, compared with 4.9 months for the investigator’s choice of chemotherapy with either eribulin mesylate, vinorelbine, capecitabine, or gemcitabine. This difference translated into a 37% reduction in risk of disease progression with the ADC, reported Aditya Bardia, MD, MPH, director of the breast cancer research program at the Mass General Cancer Center in Boston.
Patients who received Dato-DXd had less than half the number of grade 3 or greater toxicities and fewer dose reductions or interruptions than patients who received chemotherapy, he noted in an oral abstract session at the 2023 European Society for Medical Oncology Congress.
“Overall, results support Dato-DXd as a potential new therapeutic option for patients with metastatic hormone receptor–positive breast cancer,” he said.
Different ADC, same target
Dr. Bardia noted that there is an unmet need for effective therapies for patients with metastatic HR+/HER2– breast cancer who experience disease progression after endocrine therapy and at least one line of systemic therapy.
Although chemotherapy is widely used in this population, it’s associated with low response rates, poor prognosis, and significant toxicities, including hematologic and neurologic adverse events (AEs).
Dato-DXd is composed of a monoclonal antibody targeting TROP2, a transmembrane glycoprotein overexpressed in cancer cells, linked to the topoisomerase 1 inhibitor deruxtecan as the toxic payload.
Dr. Bardia explained that Dato-DXd has four properties that distinguish it from other TROP2-directed ADCs: an optimized drug to antibody ratio of 4, a stable linker-payload, tumor-selective cleavable linker, both of which reduce off-target toxicities, and a bystander antitumor effect that can target TROP2-expressing cells in the tumor microenvironment.
In the phase I TROPION-PanTumor01 trial, Dato-DXd had promising anti-tumor activity and a manageable safety profile in patients with metastatic HR+/HER2– breast cancer, paving the way for the TROPION-Breast01 study reported here.
Efficacy results
In the Breast01 trial, 732 patients with inoperable or metastatic HR+/HER2– breast cancer previously treated with 1 or 2 lines of chemotherapy that had progressed on endocrine therapy were stratified by number of prior chemotherapy lines, geographic region, and prior CDK4/6 inhibitor status, and then randomized to either Dato-DXd 6 mg/kg intravenously on day 1 of each 3-week cycle (365 patients) or to investigator’s choice of chemotherapy (367 patients). According to the protocol, chemotherapy could be eribulin mesylate, vinorelbine, or gemcitabine delivered via IV on days 1 and 8 every 3 weeks, or oral capecitabine on days 1 through 14 of every 3-week cycle.
At the time of data cutoff, 93 patients assigned to the ADC and 39 assigned to chemotherapy were still on treatment.
As noted before, median PFS by blinded independent central review, one of two primary endpoints, was 6.9 months with Dato-DXd, compared with 4.9 months with chemotherapy, translating into a hazard ratio for progression of 0.63 (P < .0001).
The benefit was seen across nearly all subgroups except among patients who had not previously received a CDK4/6 inhibitor, and patients who had received a prior anthracycline but not a taxane.
Objective response rates (ORR) were 36.4% with Dato-DXd (99.5% partial and .5% complete response), compared with 22.9% with chemotherapy (all partial responses; P values not reported).
Overall survival data, the other primary endpoint, were not mature at a median OS follow-up of 9.7 months, and will be reported at a later date.
Safety results
“In terms of safety, the rate of grade 3 or higher treatment-related AEs in the Dato-DXd arm was less as compared to investigator choice of chemotherapy. This is a bit different from most of the studies; in general we see that the rate of adverse events is higher in the intervention arm as compared to the control arm,” Dr. Bardia commented.
Rates of dose reductions and dose interruptions due to treatment-related AEs were also lower with the ADC.
There were no patient deaths associated with Dato-DXd. One patient assigned to chemotherapy died from a complication associated with febrile neutropenia.
Most treatment-related AEs occurring in 15% of patients and AEs of special interest were of grade 1 and manageable.
The most common toxicities seen with the ADC were oral mucositis and dry eye. The most common side effects with chemotherapy were neutropenia and anemia, “the usual side effects you would expect with chemotherapy,” Dr. Bardia said, pointing out that the rate of grade 3 neutropenia was 31% with standard chemotherapy, compared with 1% with Dato-DXd.
Good, but we can do better
ESMO invited discussant Sarat Chandarlapaty, MD, PhD, a breast oncologist at Memorial Sloan Kettering Cancer Center in New York, commented that while the trial data showed superior efficacy and safety with Dato-DXd, compared with standard chemotherapy, it’s still unclear how it and other ADCs on the market and in the research pipeline may be used in therapy for this patient population.
“Would I rather prescribe Dato-DXd or more chemo after 1 to 2 lines of chemo in unselected HR-positive, HER2-negative breast cancer? The answer is Dato-DXd, but it leaves several unanswered questions for us,” he said.
“First, we have two ADCs approved in HR-positive breast cancer: another TROP2 ADC sacituzumab [govitecan] and a HER2 ADC trastuzumab deruxtecan. Would I rather give Dato over one of these? I don’t have an answer,” he added.
In addition, it’s unknown whether these drugs, which have the same topoisomerase-targeted payload, could be given in sequence, and there are as yet no clear answers as to whether patients might do better with Dato-DXd or with a PIK3ca inhibitor.
“I would say that the elephant in the room is really another question, and that is, ‘Is Dato-DXd in this context delivering on the promise of an ADC?’ ” Dr. Chandarlapaty said.
“I think translational research is urgently needed if we’re ultimately to deliver on the promise of these agents in the clinic,” he concluded.
The TROPION-Breast01 study is sponsored AstraZeneca, which is collaborating with Daiichi-Sankyo on global development and commercialization of Dato-DXd. Dr. Bardia disclosed advisory board activities and institutional research funding from AstraZeneca and Daiichi-Sankyo and others. Dr. Chandarlapaty disclosed research funding from both companies, and advisory board activities for AstraZeneca and others.
FROM ESMO CONGRESS 2023