User login
Hepatitis C falls as barrier to heart transplantation
DALLAS – The heart transplant team at Vanderbilt University has successfully placed hearts from deceased, hepatitis C virus–positive patients into recipients, and then eradicated the subsequent infection that appeared in most recipients using a standard regimen.
So far, five of nine heart transplant recipients who developed a posttransplant hepatitis C virus (HCV) infection had the infection eradicated using one of the highly effective HCV drug regimens, and an additional three patients from the series are nearing their 12th week without detectable virus following treatment that marks a sustained response, Kelly H. Schlendorf, MD, said at the annual scientific meeting of the Heart Failure Society of America. The ninth patient died after developing a pulmonary embolism during the 7th week on antiviral therapy.

The recipients have been patients in a marginal clinical state and facing a long projected wait on the heart-recipient queue of the United Network for Organ Sharing (UNOS), Dr. Schlendorf said in an interview.
These have been “patients with a morbidity and mortality risk from waiting that can be mitigated by expanding the donor pool.” She gave an example of a patient with a left ventricular assist device that required replacement by either a second device or transplant, “so getting the transplant quickly was a good thing,” said Dr. Schlendorf, a cardiologist at Vanderbilt in Nashville.
Based on her analysis of UNOS data, “upwards of 100” and perhaps as many as 300 additional donor hearts could be available annually for U.S. transplants if the organs weren’t excluded because of HCV infection.
The Vanderbilt team has so far approached 15 patients in their program wait-listed for hearts about the possibility of accepting an HCV-positive organ, and all 15 have given their consent, she said. “We spend a lot of time talking with patients and their caregivers about the risks and benefits and possible complications.”
The 13 recipients, starting in September 2016, included 12 patients who were HCV naive and 1 patient with a history of HCV exposure. All 13 received the program’s standard three-drug regimen for immunosuppression.
During close surveillance, 9 of the 13 developed an infection. Patients with genotype 1 HCV received 12 weeks of treatment with ledipasvir plus sofosbuvir. Those infected with genotype 3 received 12-24 weeks of treatment with sofosbuvir plus velpatasvir. Treatment with these direct-acting antivirals meant that patients had to adjust the time when they took their proton-pump inhibitors, and they needed to stop treatment with diltiazem and statins while on the antivirals.
“In the era of direct-acting antivirals, HCV-positive donors may provide a safe and effective way to expand the donor pool and reduce wait-list times,” Dr. Schlendorf said. She noted that in recent years an increased number of potential organ donors have been HCV positive. She also cautioned that so far follow-up has been relatively brief, with no patient yet followed as long as 1 year after transplant.
The direct-acting HCV antivirals are expensive, and some payers established clinical criteria that patients must meet to qualify for coverage of these regimens. “We have not encountered difficulties getting insurers to pay,” Dr. Schlendorf said. Despite the antivirals’ cost there are significant cost savings from fewer days in the ICU waiting for heart transplantation and a reduced need for mechanical support as a bridge to transplant, she noted.
[email protected]
On Twitter @mitchelzoler
DALLAS – The heart transplant team at Vanderbilt University has successfully placed hearts from deceased, hepatitis C virus–positive patients into recipients, and then eradicated the subsequent infection that appeared in most recipients using a standard regimen.
So far, five of nine heart transplant recipients who developed a posttransplant hepatitis C virus (HCV) infection had the infection eradicated using one of the highly effective HCV drug regimens, and an additional three patients from the series are nearing their 12th week without detectable virus following treatment that marks a sustained response, Kelly H. Schlendorf, MD, said at the annual scientific meeting of the Heart Failure Society of America. The ninth patient died after developing a pulmonary embolism during the 7th week on antiviral therapy.

The recipients have been patients in a marginal clinical state and facing a long projected wait on the heart-recipient queue of the United Network for Organ Sharing (UNOS), Dr. Schlendorf said in an interview.
These have been “patients with a morbidity and mortality risk from waiting that can be mitigated by expanding the donor pool.” She gave an example of a patient with a left ventricular assist device that required replacement by either a second device or transplant, “so getting the transplant quickly was a good thing,” said Dr. Schlendorf, a cardiologist at Vanderbilt in Nashville.
Based on her analysis of UNOS data, “upwards of 100” and perhaps as many as 300 additional donor hearts could be available annually for U.S. transplants if the organs weren’t excluded because of HCV infection.
The Vanderbilt team has so far approached 15 patients in their program wait-listed for hearts about the possibility of accepting an HCV-positive organ, and all 15 have given their consent, she said. “We spend a lot of time talking with patients and their caregivers about the risks and benefits and possible complications.”
The 13 recipients, starting in September 2016, included 12 patients who were HCV naive and 1 patient with a history of HCV exposure. All 13 received the program’s standard three-drug regimen for immunosuppression.
During close surveillance, 9 of the 13 developed an infection. Patients with genotype 1 HCV received 12 weeks of treatment with ledipasvir plus sofosbuvir. Those infected with genotype 3 received 12-24 weeks of treatment with sofosbuvir plus velpatasvir. Treatment with these direct-acting antivirals meant that patients had to adjust the time when they took their proton-pump inhibitors, and they needed to stop treatment with diltiazem and statins while on the antivirals.
“In the era of direct-acting antivirals, HCV-positive donors may provide a safe and effective way to expand the donor pool and reduce wait-list times,” Dr. Schlendorf said. She noted that in recent years an increased number of potential organ donors have been HCV positive. She also cautioned that so far follow-up has been relatively brief, with no patient yet followed as long as 1 year after transplant.
The direct-acting HCV antivirals are expensive, and some payers established clinical criteria that patients must meet to qualify for coverage of these regimens. “We have not encountered difficulties getting insurers to pay,” Dr. Schlendorf said. Despite the antivirals’ cost there are significant cost savings from fewer days in the ICU waiting for heart transplantation and a reduced need for mechanical support as a bridge to transplant, she noted.
[email protected]
On Twitter @mitchelzoler
DALLAS – The heart transplant team at Vanderbilt University has successfully placed hearts from deceased, hepatitis C virus–positive patients into recipients, and then eradicated the subsequent infection that appeared in most recipients using a standard regimen.
So far, five of nine heart transplant recipients who developed a posttransplant hepatitis C virus (HCV) infection had the infection eradicated using one of the highly effective HCV drug regimens, and an additional three patients from the series are nearing their 12th week without detectable virus following treatment that marks a sustained response, Kelly H. Schlendorf, MD, said at the annual scientific meeting of the Heart Failure Society of America. The ninth patient died after developing a pulmonary embolism during the 7th week on antiviral therapy.

The recipients have been patients in a marginal clinical state and facing a long projected wait on the heart-recipient queue of the United Network for Organ Sharing (UNOS), Dr. Schlendorf said in an interview.
These have been “patients with a morbidity and mortality risk from waiting that can be mitigated by expanding the donor pool.” She gave an example of a patient with a left ventricular assist device that required replacement by either a second device or transplant, “so getting the transplant quickly was a good thing,” said Dr. Schlendorf, a cardiologist at Vanderbilt in Nashville.
Based on her analysis of UNOS data, “upwards of 100” and perhaps as many as 300 additional donor hearts could be available annually for U.S. transplants if the organs weren’t excluded because of HCV infection.
The Vanderbilt team has so far approached 15 patients in their program wait-listed for hearts about the possibility of accepting an HCV-positive organ, and all 15 have given their consent, she said. “We spend a lot of time talking with patients and their caregivers about the risks and benefits and possible complications.”
The 13 recipients, starting in September 2016, included 12 patients who were HCV naive and 1 patient with a history of HCV exposure. All 13 received the program’s standard three-drug regimen for immunosuppression.
During close surveillance, 9 of the 13 developed an infection. Patients with genotype 1 HCV received 12 weeks of treatment with ledipasvir plus sofosbuvir. Those infected with genotype 3 received 12-24 weeks of treatment with sofosbuvir plus velpatasvir. Treatment with these direct-acting antivirals meant that patients had to adjust the time when they took their proton-pump inhibitors, and they needed to stop treatment with diltiazem and statins while on the antivirals.
“In the era of direct-acting antivirals, HCV-positive donors may provide a safe and effective way to expand the donor pool and reduce wait-list times,” Dr. Schlendorf said. She noted that in recent years an increased number of potential organ donors have been HCV positive. She also cautioned that so far follow-up has been relatively brief, with no patient yet followed as long as 1 year after transplant.
The direct-acting HCV antivirals are expensive, and some payers established clinical criteria that patients must meet to qualify for coverage of these regimens. “We have not encountered difficulties getting insurers to pay,” Dr. Schlendorf said. Despite the antivirals’ cost there are significant cost savings from fewer days in the ICU waiting for heart transplantation and a reduced need for mechanical support as a bridge to transplant, she noted.
[email protected]
On Twitter @mitchelzoler
AT THE HFSA ANNUAL SCIENTIFIC MEETING
Key clinical point:
Major finding: Eight of nine patients who developed HCV infection had it eradicated by a direct-acting antiviral regimen.
Data source: A series of 13 patients treated at one U.S. center.
Disclosures: Dr. Schlendorf had no disclosures.
BIMA’s benefits extend to high-risk CABG patients
COLORADO SPRINGS – The survival advantage of bilateral internal over left internal mammary artery grafts persists even among multivessel CABG patients perceived to be at high surgical risk, Nishant Saran, MD, reported at the annual meeting of the Western Thoracic Surgical Association.
Many surgeons hesitate to perform bilateral internal mammary artery (BIMA) grafting in high-risk patients on the presumption that BIMA might not benefit them. It’s a concern that appears to be without merit, however, based on a retrospective analysis of the 6,468 multivessel CABG procedures performed at the Mayo Clinic during 2000-2015, said Dr. Saran of the Mayo Clinic in Rochester, Minn.
The BIMA patients were as a whole significantly younger, primarily men, and less likely to have diabetes or to be obese than the LIMA patients. Also, LIMA patients were fourfold more likely to have baseline heart failure, twice as likely to have a history of stroke, and had a twofold greater prevalence of chronic lung disease.
“The unmatched comparison shows the clear treatment selection bias we have: BIMA goes to the healthier patients,” Dr. Saran observed.
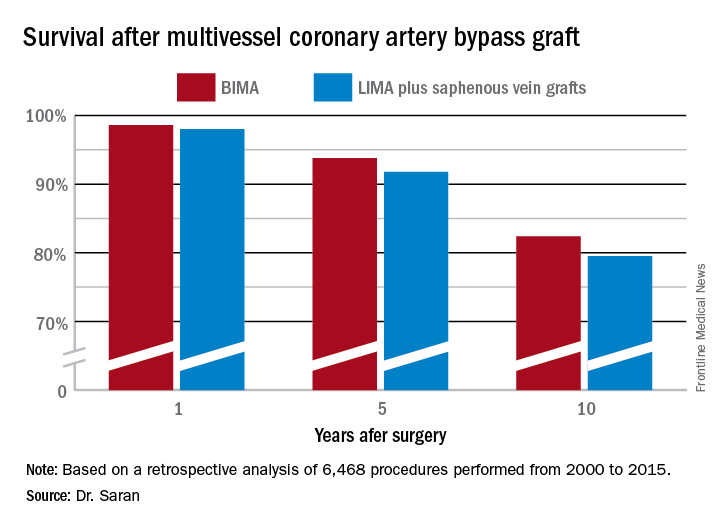
But is that bias justified? To find out, he and his coinvestigators performed extensive propensity score matching using several dozen baseline variables in order to identify 1,011 closely matched patient pairs. In this propensity score-matched analysis, 5- and 10-year survival rates were significantly better in the BIMA group. The gap between the two survival curves widened after about 7 years and continued to expand steadily through year 10. Incision time averaged 298 minutes in the BIMA group and 254 minutes in the propensity-matched LIMA group.
Discussant Eric J. Lehr, MD, a cardiac surgeon at Swedish Medical Center in Seattle, noted that the impressive survival benefit for BIMA in the retrospective Mayo Clinic study came at what he termed “a modest cost”: a doubled incidence of sternal site infections, from 1.4% in the LIMA group to 3% with BIMA. Importantly, though, there was no significant difference in the more serious deep sternal wound infections.
He agreed with Dr. Saran that BIMA is seriously underutilized, noting that only one cardiothoracic surgery program in the state of Washington uses BIMA more than 10% of the time in multivessel CABG.
Dr. Lehr then posed a provocative question: “Should BIMA grafting be considered a quality metric in coronary revascularization surgery, despite the small increase in sternal site infections, even though sternal wound infections have been declared a ‘never’ event and are tied to reimbursement?”
“I think BIMA should be a gold standard,” Dr. Saran replied. “The first thing that a cardiac surgeon should always think of when a patient is going to have CABG is ‘BIMA first,’ and only then look into reasons for not doing it. But I guess in current real-world practice, things are different.”
Howard K. Song, MD, commented, “I think a study like this doesn’t necessarily show that every surgeon should be using BIMA liberally, it shows that surgeons in your practice who do that have excellent outcomes.”
Dr. Song, professor of surgery and chief of the division of cardiothoracic surgery at Oregon Health and Science University, Portland, added that he believes extensive use of BIMA is actually a surrogate marker for a highly skilled subspecialist who would be expected to have very good outcomes as a matter of course.
“That may be one way of looking at it; however, I do think that even very skilled surgeons still have an inherent resistance to doing BIMA,” Dr. Saran responded.
“In the current era, the surgeon is pressured to achieve improved short-term outcomes and improved OR turnover times. An extra half hour for BIMA tends to push the surgeon away,” he added.
Dr. Saran reported having no financial conflicts of interest.
COLORADO SPRINGS – The survival advantage of bilateral internal over left internal mammary artery grafts persists even among multivessel CABG patients perceived to be at high surgical risk, Nishant Saran, MD, reported at the annual meeting of the Western Thoracic Surgical Association.
Many surgeons hesitate to perform bilateral internal mammary artery (BIMA) grafting in high-risk patients on the presumption that BIMA might not benefit them. It’s a concern that appears to be without merit, however, based on a retrospective analysis of the 6,468 multivessel CABG procedures performed at the Mayo Clinic during 2000-2015, said Dr. Saran of the Mayo Clinic in Rochester, Minn.
The BIMA patients were as a whole significantly younger, primarily men, and less likely to have diabetes or to be obese than the LIMA patients. Also, LIMA patients were fourfold more likely to have baseline heart failure, twice as likely to have a history of stroke, and had a twofold greater prevalence of chronic lung disease.
“The unmatched comparison shows the clear treatment selection bias we have: BIMA goes to the healthier patients,” Dr. Saran observed.
But is that bias justified? To find out, he and his coinvestigators performed extensive propensity score matching using several dozen baseline variables in order to identify 1,011 closely matched patient pairs. In this propensity score-matched analysis, 5- and 10-year survival rates were significantly better in the BIMA group. The gap between the two survival curves widened after about 7 years and continued to expand steadily through year 10. Incision time averaged 298 minutes in the BIMA group and 254 minutes in the propensity-matched LIMA group.

Discussant Eric J. Lehr, MD, a cardiac surgeon at Swedish Medical Center in Seattle, noted that the impressive survival benefit for BIMA in the retrospective Mayo Clinic study came at what he termed “a modest cost”: a doubled incidence of sternal site infections, from 1.4% in the LIMA group to 3% with BIMA. Importantly, though, there was no significant difference in the more serious deep sternal wound infections.
He agreed with Dr. Saran that BIMA is seriously underutilized, noting that only one cardiothoracic surgery program in the state of Washington uses BIMA more than 10% of the time in multivessel CABG.
Dr. Lehr then posed a provocative question: “Should BIMA grafting be considered a quality metric in coronary revascularization surgery, despite the small increase in sternal site infections, even though sternal wound infections have been declared a ‘never’ event and are tied to reimbursement?”
“I think BIMA should be a gold standard,” Dr. Saran replied. “The first thing that a cardiac surgeon should always think of when a patient is going to have CABG is ‘BIMA first,’ and only then look into reasons for not doing it. But I guess in current real-world practice, things are different.”
Howard K. Song, MD, commented, “I think a study like this doesn’t necessarily show that every surgeon should be using BIMA liberally, it shows that surgeons in your practice who do that have excellent outcomes.”
Dr. Song, professor of surgery and chief of the division of cardiothoracic surgery at Oregon Health and Science University, Portland, added that he believes extensive use of BIMA is actually a surrogate marker for a highly skilled subspecialist who would be expected to have very good outcomes as a matter of course.
“That may be one way of looking at it; however, I do think that even very skilled surgeons still have an inherent resistance to doing BIMA,” Dr. Saran responded.
“In the current era, the surgeon is pressured to achieve improved short-term outcomes and improved OR turnover times. An extra half hour for BIMA tends to push the surgeon away,” he added.
Dr. Saran reported having no financial conflicts of interest.
COLORADO SPRINGS – The survival advantage of bilateral internal over left internal mammary artery grafts persists even among multivessel CABG patients perceived to be at high surgical risk, Nishant Saran, MD, reported at the annual meeting of the Western Thoracic Surgical Association.
Many surgeons hesitate to perform bilateral internal mammary artery (BIMA) grafting in high-risk patients on the presumption that BIMA might not benefit them. It’s a concern that appears to be without merit, however, based on a retrospective analysis of the 6,468 multivessel CABG procedures performed at the Mayo Clinic during 2000-2015, said Dr. Saran of the Mayo Clinic in Rochester, Minn.
The BIMA patients were as a whole significantly younger, primarily men, and less likely to have diabetes or to be obese than the LIMA patients. Also, LIMA patients were fourfold more likely to have baseline heart failure, twice as likely to have a history of stroke, and had a twofold greater prevalence of chronic lung disease.
“The unmatched comparison shows the clear treatment selection bias we have: BIMA goes to the healthier patients,” Dr. Saran observed.
But is that bias justified? To find out, he and his coinvestigators performed extensive propensity score matching using several dozen baseline variables in order to identify 1,011 closely matched patient pairs. In this propensity score-matched analysis, 5- and 10-year survival rates were significantly better in the BIMA group. The gap between the two survival curves widened after about 7 years and continued to expand steadily through year 10. Incision time averaged 298 minutes in the BIMA group and 254 minutes in the propensity-matched LIMA group.

Discussant Eric J. Lehr, MD, a cardiac surgeon at Swedish Medical Center in Seattle, noted that the impressive survival benefit for BIMA in the retrospective Mayo Clinic study came at what he termed “a modest cost”: a doubled incidence of sternal site infections, from 1.4% in the LIMA group to 3% with BIMA. Importantly, though, there was no significant difference in the more serious deep sternal wound infections.
He agreed with Dr. Saran that BIMA is seriously underutilized, noting that only one cardiothoracic surgery program in the state of Washington uses BIMA more than 10% of the time in multivessel CABG.
Dr. Lehr then posed a provocative question: “Should BIMA grafting be considered a quality metric in coronary revascularization surgery, despite the small increase in sternal site infections, even though sternal wound infections have been declared a ‘never’ event and are tied to reimbursement?”
“I think BIMA should be a gold standard,” Dr. Saran replied. “The first thing that a cardiac surgeon should always think of when a patient is going to have CABG is ‘BIMA first,’ and only then look into reasons for not doing it. But I guess in current real-world practice, things are different.”
Howard K. Song, MD, commented, “I think a study like this doesn’t necessarily show that every surgeon should be using BIMA liberally, it shows that surgeons in your practice who do that have excellent outcomes.”
Dr. Song, professor of surgery and chief of the division of cardiothoracic surgery at Oregon Health and Science University, Portland, added that he believes extensive use of BIMA is actually a surrogate marker for a highly skilled subspecialist who would be expected to have very good outcomes as a matter of course.
“That may be one way of looking at it; however, I do think that even very skilled surgeons still have an inherent resistance to doing BIMA,” Dr. Saran responded.
“In the current era, the surgeon is pressured to achieve improved short-term outcomes and improved OR turnover times. An extra half hour for BIMA tends to push the surgeon away,” he added.
Dr. Saran reported having no financial conflicts of interest.
AT THE WTSA ANNUAL MEETING
Key clinical point:
Major finding: Ten-year survival following multivessel CABG using bilateral internal mammary artery grafting was 82.4%, significantly better than the 79.5% rate with left internal mammary artery grafting plus saphenous vein grafts.
Data source: This retrospective observational single-center included 6,468 patients who underwent multivessel CABG during 2000-2015.
Disclosures: Dr. Saran reported having no financial conflicts of interest.
Minimally invasive esophagectomy may mean less major morbidity
COLORADO SPRINGS – Minimally invasive esophagectomy was associated with a significantly lower rate of postoperative major morbidity as well as a mean 1-day briefer length of stay than open esophagectomy in a propensity-matched analysis of the real-world American College of Surgeons-National Quality Improvement Program database, Mark F. Berry, MD, reported at the annual meeting of the Western Thoracic Surgical Association.
However, both of the study’s discussants questioned whether the reported modest absolute reduction in major morbidity was really attributable to the minimally invasive approach or could instead have resulted from one of several potential confounders that couldn’t be fully adjusted for, given inherent limitations of the ACS-NSQIP database.
“There was a statistically significant difference in morbidity,” replied Dr. Berry of Stanford (Calif.) University. “It was a 4% absolute difference, which I think is probably clinically meaningful, but certainly it’s not really, really dramatic.”
“What I think we found is that it’s safe to do a minimally invasive esophagectomy and safe for people to introduce it into their practice. But it’s not necessarily something that’s a game changer, unlike what’s been seen with minimally invasive approaches for some other things,” said Dr. Berry, who added that he didn’t wish to overstate the importance of the observed difference in morbidity.
Studies from high-volume centers show that minimally-invasive esophagectomy (MIE) reduces length of stay, postoperative major morbidity, and features equivalent or even slightly lower mortality than traditional open esophagectomy, the generalizability of these findings beyond such centers is questionable. That’s why Dr. Berry and his coinvestigators turned to the ACS-NSQIP database, which includes all esophagectomies performed for esophageal cancer at roughly 700 U.S. hospitals, not just those done by board-certified thoracic surgeons.
He presented a retrospective cohort study of 3,901 esophagectomy patients during 2005-2013 who met study criteria, 16.4% of whom had MIE. The use of this approach increased steadily from 6.5% of all esophagectomies in 2005 to 22.3% in 2013. A propensity-matched analysis designed to neutralize potentially confounding differences included 638 MIE and 1,914 open esophagectomy patients.
The primary outcome was the 30-day rate of composite major morbidity in the realms of various wound, respiratory, renal, and cardiovascular complications. The rate was 36.1% in the MIE group and 40.5% with open esophagectomy in the propensity-matched analysis, an absolute risk reduction of 4.4% and a relative risk reduction of 17%. Although rates were consistently slightly lower in each of the categories of major morbidity, those individual differences didn’t achieve statistical significance. The difference in major morbidity became significant only when major morbidity was considered as a whole.
Mean length of stay was 9 days with MIE and 10 days with open surgery.
There was no significant difference between the two study groups in 30-day rates of readmission, reoperation, or mortality.
Discussant Donald E. Low said “esophagectomy is being analysed regarding its place in all sorts of presentations, stages, and situations, so the aspect of making sure that we’re delivering the services as efficiently as possible is going to become more important, not less important.”
That being said, he noted that there is no specific CPT code for MIE. That raises the possibility of an uncertain amount of procedural misclassification in the ACS-NSQIP database.
Also, the only significant difference in major morbidity between the two study groups was in the subcategory of intra- or postoperative bleeding requiring transfusion, which occurred in 10.8% of the MIE and 16.7% of the open esophagectomy groups, observed Dr. Low, director of the Esophageal Center of Excellence at Virginia Mason Medical Center, Seattle.
“Some of us believe that blood utilization and transfusion requirement is really a quality measure and not a complication,” the surgeon said. And if that outcome is excluded from consideration, then there is no significant difference in major morbidity.
Discussant Douglas E. Wood, MD, professor and chair of the department of surgery at the University of Washington, Seattle, took the opportunity to share a self-described “pet peeve” about analyses of national surgical databases: these databases typically don’t contain key details necessary to correct for provider and hospital characteristics.
“The small differences that you demonstrate could easily have been completely driven by providers who choose to do minimally invasive esophagectomy and are in higher-volume, more specialized centers,” he said. “I’m not convinced of your conclusion that MIE produces less morbidity based on a 4% difference and no analysis of provider characteristics.”
COLORADO SPRINGS – Minimally invasive esophagectomy was associated with a significantly lower rate of postoperative major morbidity as well as a mean 1-day briefer length of stay than open esophagectomy in a propensity-matched analysis of the real-world American College of Surgeons-National Quality Improvement Program database, Mark F. Berry, MD, reported at the annual meeting of the Western Thoracic Surgical Association.
However, both of the study’s discussants questioned whether the reported modest absolute reduction in major morbidity was really attributable to the minimally invasive approach or could instead have resulted from one of several potential confounders that couldn’t be fully adjusted for, given inherent limitations of the ACS-NSQIP database.
“There was a statistically significant difference in morbidity,” replied Dr. Berry of Stanford (Calif.) University. “It was a 4% absolute difference, which I think is probably clinically meaningful, but certainly it’s not really, really dramatic.”
“What I think we found is that it’s safe to do a minimally invasive esophagectomy and safe for people to introduce it into their practice. But it’s not necessarily something that’s a game changer, unlike what’s been seen with minimally invasive approaches for some other things,” said Dr. Berry, who added that he didn’t wish to overstate the importance of the observed difference in morbidity.
Studies from high-volume centers show that minimally-invasive esophagectomy (MIE) reduces length of stay, postoperative major morbidity, and features equivalent or even slightly lower mortality than traditional open esophagectomy, the generalizability of these findings beyond such centers is questionable. That’s why Dr. Berry and his coinvestigators turned to the ACS-NSQIP database, which includes all esophagectomies performed for esophageal cancer at roughly 700 U.S. hospitals, not just those done by board-certified thoracic surgeons.
He presented a retrospective cohort study of 3,901 esophagectomy patients during 2005-2013 who met study criteria, 16.4% of whom had MIE. The use of this approach increased steadily from 6.5% of all esophagectomies in 2005 to 22.3% in 2013. A propensity-matched analysis designed to neutralize potentially confounding differences included 638 MIE and 1,914 open esophagectomy patients.
The primary outcome was the 30-day rate of composite major morbidity in the realms of various wound, respiratory, renal, and cardiovascular complications. The rate was 36.1% in the MIE group and 40.5% with open esophagectomy in the propensity-matched analysis, an absolute risk reduction of 4.4% and a relative risk reduction of 17%. Although rates were consistently slightly lower in each of the categories of major morbidity, those individual differences didn’t achieve statistical significance. The difference in major morbidity became significant only when major morbidity was considered as a whole.
Mean length of stay was 9 days with MIE and 10 days with open surgery.
There was no significant difference between the two study groups in 30-day rates of readmission, reoperation, or mortality.
Discussant Donald E. Low said “esophagectomy is being analysed regarding its place in all sorts of presentations, stages, and situations, so the aspect of making sure that we’re delivering the services as efficiently as possible is going to become more important, not less important.”
That being said, he noted that there is no specific CPT code for MIE. That raises the possibility of an uncertain amount of procedural misclassification in the ACS-NSQIP database.
Also, the only significant difference in major morbidity between the two study groups was in the subcategory of intra- or postoperative bleeding requiring transfusion, which occurred in 10.8% of the MIE and 16.7% of the open esophagectomy groups, observed Dr. Low, director of the Esophageal Center of Excellence at Virginia Mason Medical Center, Seattle.
“Some of us believe that blood utilization and transfusion requirement is really a quality measure and not a complication,” the surgeon said. And if that outcome is excluded from consideration, then there is no significant difference in major morbidity.
Discussant Douglas E. Wood, MD, professor and chair of the department of surgery at the University of Washington, Seattle, took the opportunity to share a self-described “pet peeve” about analyses of national surgical databases: these databases typically don’t contain key details necessary to correct for provider and hospital characteristics.
“The small differences that you demonstrate could easily have been completely driven by providers who choose to do minimally invasive esophagectomy and are in higher-volume, more specialized centers,” he said. “I’m not convinced of your conclusion that MIE produces less morbidity based on a 4% difference and no analysis of provider characteristics.”
COLORADO SPRINGS – Minimally invasive esophagectomy was associated with a significantly lower rate of postoperative major morbidity as well as a mean 1-day briefer length of stay than open esophagectomy in a propensity-matched analysis of the real-world American College of Surgeons-National Quality Improvement Program database, Mark F. Berry, MD, reported at the annual meeting of the Western Thoracic Surgical Association.
However, both of the study’s discussants questioned whether the reported modest absolute reduction in major morbidity was really attributable to the minimally invasive approach or could instead have resulted from one of several potential confounders that couldn’t be fully adjusted for, given inherent limitations of the ACS-NSQIP database.
“There was a statistically significant difference in morbidity,” replied Dr. Berry of Stanford (Calif.) University. “It was a 4% absolute difference, which I think is probably clinically meaningful, but certainly it’s not really, really dramatic.”
“What I think we found is that it’s safe to do a minimally invasive esophagectomy and safe for people to introduce it into their practice. But it’s not necessarily something that’s a game changer, unlike what’s been seen with minimally invasive approaches for some other things,” said Dr. Berry, who added that he didn’t wish to overstate the importance of the observed difference in morbidity.
Studies from high-volume centers show that minimally-invasive esophagectomy (MIE) reduces length of stay, postoperative major morbidity, and features equivalent or even slightly lower mortality than traditional open esophagectomy, the generalizability of these findings beyond such centers is questionable. That’s why Dr. Berry and his coinvestigators turned to the ACS-NSQIP database, which includes all esophagectomies performed for esophageal cancer at roughly 700 U.S. hospitals, not just those done by board-certified thoracic surgeons.
He presented a retrospective cohort study of 3,901 esophagectomy patients during 2005-2013 who met study criteria, 16.4% of whom had MIE. The use of this approach increased steadily from 6.5% of all esophagectomies in 2005 to 22.3% in 2013. A propensity-matched analysis designed to neutralize potentially confounding differences included 638 MIE and 1,914 open esophagectomy patients.
The primary outcome was the 30-day rate of composite major morbidity in the realms of various wound, respiratory, renal, and cardiovascular complications. The rate was 36.1% in the MIE group and 40.5% with open esophagectomy in the propensity-matched analysis, an absolute risk reduction of 4.4% and a relative risk reduction of 17%. Although rates were consistently slightly lower in each of the categories of major morbidity, those individual differences didn’t achieve statistical significance. The difference in major morbidity became significant only when major morbidity was considered as a whole.
Mean length of stay was 9 days with MIE and 10 days with open surgery.
There was no significant difference between the two study groups in 30-day rates of readmission, reoperation, or mortality.
Discussant Donald E. Low said “esophagectomy is being analysed regarding its place in all sorts of presentations, stages, and situations, so the aspect of making sure that we’re delivering the services as efficiently as possible is going to become more important, not less important.”
That being said, he noted that there is no specific CPT code for MIE. That raises the possibility of an uncertain amount of procedural misclassification in the ACS-NSQIP database.
Also, the only significant difference in major morbidity between the two study groups was in the subcategory of intra- or postoperative bleeding requiring transfusion, which occurred in 10.8% of the MIE and 16.7% of the open esophagectomy groups, observed Dr. Low, director of the Esophageal Center of Excellence at Virginia Mason Medical Center, Seattle.
“Some of us believe that blood utilization and transfusion requirement is really a quality measure and not a complication,” the surgeon said. And if that outcome is excluded from consideration, then there is no significant difference in major morbidity.
Discussant Douglas E. Wood, MD, professor and chair of the department of surgery at the University of Washington, Seattle, took the opportunity to share a self-described “pet peeve” about analyses of national surgical databases: these databases typically don’t contain key details necessary to correct for provider and hospital characteristics.
“The small differences that you demonstrate could easily have been completely driven by providers who choose to do minimally invasive esophagectomy and are in higher-volume, more specialized centers,” he said. “I’m not convinced of your conclusion that MIE produces less morbidity based on a 4% difference and no analysis of provider characteristics.”
AT WTSA 2017
Key clinical point:
Major finding: The 30-day rate of major morbidity was 36.1% in patients who underwent minimally invasive esophagectomy, significantly lower than the 40.5% rate with open esophagectomy in a propensity-matched analysis.
Data source: This retrospective cohort study included 3,901 patients who underwent esophagectomy for esophageal cancer as recorded in the American College of Surgeons-National Quality Improvement Program database for 2005-2013.
Disclosures: The study presenter reported having no financial conflicts of interest.
Undiagnosed AF common in higher-risk patients
Over an 18-month period, small, insertable cardiac monitors detected atrial fibrillation in 29% of previously undiagnosed patients who were at high risk of both AF and stroke, and in 40% of patients over 30 months, according to investigators. The study was presented at the annual congress of the European Society of Cardiology and simultaneously published in JAMA Cardiology.
More than half (56%) of patients consequently started oral anticoagulation therapy, noted James A. Reiffel, MD, of Columbia University College of Physicians and Surgeons, New York, with his associates, for the REVEAL AF investigators.
“The incidence of previously undiagnosed atrial fibrillation may be substantial in patients with risk factors for AF and stroke,” they concluded. “Atrial fibrillation would have gone undetected in most patients had monitoring been limited to 30 days. Further trials regarding the value of detecting subclinical AF and of prophylactic therapies are warranted.”
Atrial fibrillation affects millions worldwide and is associated with older age, hypertension, diabetes, and heart failure, all of which also independently increase the risk of stroke. Minimally invasive prolonged electrocardiographic monitoring with insertable cardiac monitors might help hasten detection and treatment of AF, but diagnostic yield in high-risk patients has been unclear.
In this single-arm, multicenter, prospective study, researchers inserted Reveal XT or Reveal LINQ (Medtronic) cardiac monitors in 385 adults who had either CHAD2 scores of 3, or CHAD2 scores of 2 and one additional risk factor for AF, such as coronary artery disease, sleep apnea, chronic obstructive pulmonary disease, or renal insufficiency. The primary endpoint was AF lasting at least 6 minutes (JAMA Cardiol. 2017 Aug 26. doi: 10.1001/jamacardio.2017.3180). Median follow-up time was 22.5 months. Rates of detecting AF were 6% at 30 days compared with 20% at 6 months, 27% at 12 months, 34% at 24 months, and 40% at 30 months. Patients typically had their first AF episode about 4 months (median, 123 days) after the device was inserted. Among patients who had experienced AF by 18 months, 10% had one or more episodes lasting at least 24 hours, and 72 (56%) were prescribed oral anticoagulation therapy.
The recent PREDATE AF and ASSERT-II studies also found that previously undiagnosed AF was common among high-risk patients, the researchers noted. However, whether anticoagulating patients who have only brief episodes of AF significantly reduces their risk of stroke remains unclear, they added. Three trials (ARTESiA, NOAH, and LOOP) are underway to assess whether oral anticoagulation therapy improves outcomes in patients with device-detected AF.
Medtronic funded the study. Dr. Reiffel and five coinvestigators disclosed consulting for and receiving “modest honoraria” from Medtronic. Two other coinvestigators reported employment with and stock ownership in Medtronic.
The availability of safe and effective oral anticoagulant therapy makes the findings of REVEAL AF highly relevant. This high rate of incident AF makes ICM-based screenings of high-risk individuals a potentially attractive stroke prevention strategy. More detailed subgroup analyses may help identify a patient population with an even higher risk of developing AF. It is also conceivable that this population could have a sufficiently high risk of AF and stroke that a strategy of empiric oral anticoagulation, without the need for AF monitoring, could prove beneficial.
The REVEAL AF study has shown that AF is extremely common among older individuals with stroke risk factors. Over the next 3-4 years, subgroup analyses, economic evaluations, and randomized clinical trials will help determine if this insight can be translated into a cost-effective stroke prevention strategy for high-risk individuals.
Jeff S. Healey, MD, MSc, is at the Population Health Research Institute, McMaster University, Hamilton, Ont. He is the principal investigator of the ASSERT-II and ARTESiA trials, and had no other relevant disclosures. These comments are from his editorial (JAMA Cardiol. 2017 Aug 26. doi: 10.1001/jamacardio.2017.3203).
The availability of safe and effective oral anticoagulant therapy makes the findings of REVEAL AF highly relevant. This high rate of incident AF makes ICM-based screenings of high-risk individuals a potentially attractive stroke prevention strategy. More detailed subgroup analyses may help identify a patient population with an even higher risk of developing AF. It is also conceivable that this population could have a sufficiently high risk of AF and stroke that a strategy of empiric oral anticoagulation, without the need for AF monitoring, could prove beneficial.
The REVEAL AF study has shown that AF is extremely common among older individuals with stroke risk factors. Over the next 3-4 years, subgroup analyses, economic evaluations, and randomized clinical trials will help determine if this insight can be translated into a cost-effective stroke prevention strategy for high-risk individuals.
Jeff S. Healey, MD, MSc, is at the Population Health Research Institute, McMaster University, Hamilton, Ont. He is the principal investigator of the ASSERT-II and ARTESiA trials, and had no other relevant disclosures. These comments are from his editorial (JAMA Cardiol. 2017 Aug 26. doi: 10.1001/jamacardio.2017.3203).
The availability of safe and effective oral anticoagulant therapy makes the findings of REVEAL AF highly relevant. This high rate of incident AF makes ICM-based screenings of high-risk individuals a potentially attractive stroke prevention strategy. More detailed subgroup analyses may help identify a patient population with an even higher risk of developing AF. It is also conceivable that this population could have a sufficiently high risk of AF and stroke that a strategy of empiric oral anticoagulation, without the need for AF monitoring, could prove beneficial.
The REVEAL AF study has shown that AF is extremely common among older individuals with stroke risk factors. Over the next 3-4 years, subgroup analyses, economic evaluations, and randomized clinical trials will help determine if this insight can be translated into a cost-effective stroke prevention strategy for high-risk individuals.
Jeff S. Healey, MD, MSc, is at the Population Health Research Institute, McMaster University, Hamilton, Ont. He is the principal investigator of the ASSERT-II and ARTESiA trials, and had no other relevant disclosures. These comments are from his editorial (JAMA Cardiol. 2017 Aug 26. doi: 10.1001/jamacardio.2017.3203).
Over an 18-month period, small, insertable cardiac monitors detected atrial fibrillation in 29% of previously undiagnosed patients who were at high risk of both AF and stroke, and in 40% of patients over 30 months, according to investigators. The study was presented at the annual congress of the European Society of Cardiology and simultaneously published in JAMA Cardiology.
More than half (56%) of patients consequently started oral anticoagulation therapy, noted James A. Reiffel, MD, of Columbia University College of Physicians and Surgeons, New York, with his associates, for the REVEAL AF investigators.
“The incidence of previously undiagnosed atrial fibrillation may be substantial in patients with risk factors for AF and stroke,” they concluded. “Atrial fibrillation would have gone undetected in most patients had monitoring been limited to 30 days. Further trials regarding the value of detecting subclinical AF and of prophylactic therapies are warranted.”
Atrial fibrillation affects millions worldwide and is associated with older age, hypertension, diabetes, and heart failure, all of which also independently increase the risk of stroke. Minimally invasive prolonged electrocardiographic monitoring with insertable cardiac monitors might help hasten detection and treatment of AF, but diagnostic yield in high-risk patients has been unclear.
In this single-arm, multicenter, prospective study, researchers inserted Reveal XT or Reveal LINQ (Medtronic) cardiac monitors in 385 adults who had either CHAD2 scores of 3, or CHAD2 scores of 2 and one additional risk factor for AF, such as coronary artery disease, sleep apnea, chronic obstructive pulmonary disease, or renal insufficiency. The primary endpoint was AF lasting at least 6 minutes (JAMA Cardiol. 2017 Aug 26. doi: 10.1001/jamacardio.2017.3180). Median follow-up time was 22.5 months. Rates of detecting AF were 6% at 30 days compared with 20% at 6 months, 27% at 12 months, 34% at 24 months, and 40% at 30 months. Patients typically had their first AF episode about 4 months (median, 123 days) after the device was inserted. Among patients who had experienced AF by 18 months, 10% had one or more episodes lasting at least 24 hours, and 72 (56%) were prescribed oral anticoagulation therapy.
The recent PREDATE AF and ASSERT-II studies also found that previously undiagnosed AF was common among high-risk patients, the researchers noted. However, whether anticoagulating patients who have only brief episodes of AF significantly reduces their risk of stroke remains unclear, they added. Three trials (ARTESiA, NOAH, and LOOP) are underway to assess whether oral anticoagulation therapy improves outcomes in patients with device-detected AF.
Medtronic funded the study. Dr. Reiffel and five coinvestigators disclosed consulting for and receiving “modest honoraria” from Medtronic. Two other coinvestigators reported employment with and stock ownership in Medtronic.
Over an 18-month period, small, insertable cardiac monitors detected atrial fibrillation in 29% of previously undiagnosed patients who were at high risk of both AF and stroke, and in 40% of patients over 30 months, according to investigators. The study was presented at the annual congress of the European Society of Cardiology and simultaneously published in JAMA Cardiology.
More than half (56%) of patients consequently started oral anticoagulation therapy, noted James A. Reiffel, MD, of Columbia University College of Physicians and Surgeons, New York, with his associates, for the REVEAL AF investigators.
“The incidence of previously undiagnosed atrial fibrillation may be substantial in patients with risk factors for AF and stroke,” they concluded. “Atrial fibrillation would have gone undetected in most patients had monitoring been limited to 30 days. Further trials regarding the value of detecting subclinical AF and of prophylactic therapies are warranted.”
Atrial fibrillation affects millions worldwide and is associated with older age, hypertension, diabetes, and heart failure, all of which also independently increase the risk of stroke. Minimally invasive prolonged electrocardiographic monitoring with insertable cardiac monitors might help hasten detection and treatment of AF, but diagnostic yield in high-risk patients has been unclear.
In this single-arm, multicenter, prospective study, researchers inserted Reveal XT or Reveal LINQ (Medtronic) cardiac monitors in 385 adults who had either CHAD2 scores of 3, or CHAD2 scores of 2 and one additional risk factor for AF, such as coronary artery disease, sleep apnea, chronic obstructive pulmonary disease, or renal insufficiency. The primary endpoint was AF lasting at least 6 minutes (JAMA Cardiol. 2017 Aug 26. doi: 10.1001/jamacardio.2017.3180). Median follow-up time was 22.5 months. Rates of detecting AF were 6% at 30 days compared with 20% at 6 months, 27% at 12 months, 34% at 24 months, and 40% at 30 months. Patients typically had their first AF episode about 4 months (median, 123 days) after the device was inserted. Among patients who had experienced AF by 18 months, 10% had one or more episodes lasting at least 24 hours, and 72 (56%) were prescribed oral anticoagulation therapy.
The recent PREDATE AF and ASSERT-II studies also found that previously undiagnosed AF was common among high-risk patients, the researchers noted. However, whether anticoagulating patients who have only brief episodes of AF significantly reduces their risk of stroke remains unclear, they added. Three trials (ARTESiA, NOAH, and LOOP) are underway to assess whether oral anticoagulation therapy improves outcomes in patients with device-detected AF.
Medtronic funded the study. Dr. Reiffel and five coinvestigators disclosed consulting for and receiving “modest honoraria” from Medtronic. Two other coinvestigators reported employment with and stock ownership in Medtronic.
FROM THE ESC CONGRESS 2017
Key clinical point: Undiagnosed atrial fibrillation is common in high-risk patients.
Major finding: At 18 months, 29% of previously undiagnosed, high-risk patients had experienced atrial fibrillation lasting 6 or more minutes.
Data source: A single-arm, prospective, multicenter study of 446 patients with a CHADS2 score of at least 3, or a CHADS2 score of at least 2 plus at least one other risk factor (coronary artery disease, sleep apnea, chronic obstructive pulmonary disease, or renal insufficiency).
Disclosures: Medtronic funded the study. Dr. Reiffel and five coinvestigators disclosed consulting for and receiving “modest honoraria” from Medtronic. Two other coinvestigators reported employment with and stock ownership in Medtronic.
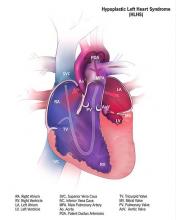
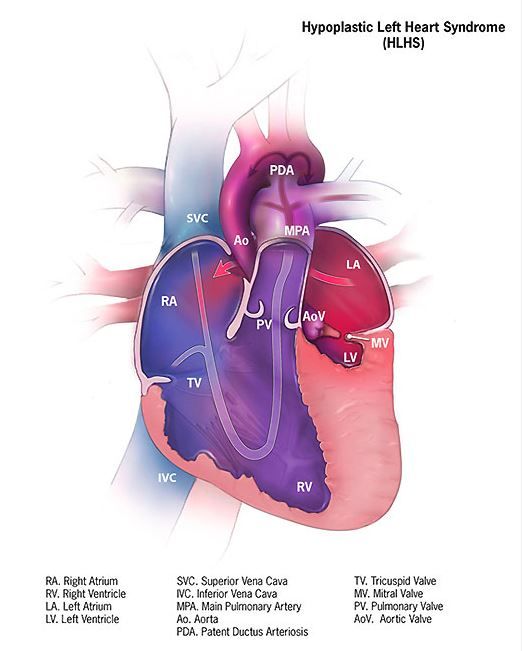
Cerebral NIRS may be flawed for assessing infant brains after stage 1 palliation of HLHS
The regional oxygenation index (rSO2) based on near infrared spectroscopy (NIRS) measurement is frequently used to assess the adequacy of oxygen delivery after stage 1 palliation of hypoplastic left heart syndrome (HLHS). However, a recent study showed that cerebral rSO2 has low sensitivity and should not be considered reassuring even at rSO2 of 50 or greater. In addition, values below 30 were not found to be sensitive for detecting compromised oxygen delivery, according to a report published online in the Journal of Thoracic and Cardiovascular Surgery.
Erin Rescoe, MD, of Boston Children’s Hospital, and her colleagues at Harvard Medical School, Boston, performed a retrospective study of 73 neonates assessed with cerebral venous oxyhemoglobin saturation (ScvO2) measured by co-oximetry from the internal jugular vein, which is considered the preferred method for assessing the adequacy of tissue oxygen delivery, compared with cerebral rSO2 after stage 1 palliation of HLHS (doi: 10.1016/j.jtcvs.2017.03.154).
To determine the suggested benefit of NIRS as an effective trend monitor, the researchers used their interpolated data to examine changes in rSO2 and changes in ScvO2 at hourly intervals and compared these values.Of particular concern is the result showing that, in all instances where ScvO2 was less than 30%, rSO2 was greater than 30%. In terms of the sensitivity (the true positive rate) and specificity (the true negative rate) of using NIRS, time-matched pairs of rSO2 and ScvO2 showed that the receiver operating characteristic curves for rSO2 as a diagnostic test to detect ScvO2 less than 30%, less than 40%, and less than 50% were 0.82, 0.84, and 0.87, respectively, showing good specificity, with a value of rSO2 less than 30% indicating that ScvO2 will be less than 30% 99% of the time.
“However, the sensitivity of rSO2 in the range of clinical interest in detecting ScvO2 less than 30% is extremely low,” according to the researchers. Thus, NIRS is likely to produce false negatives, missing patients with clinically low postoperative oxygen saturation.
In fact, rSO2 was less than 30% less than 1% of the time that ScvO2 was less than 30%. Similar results were seen in comparing values at the less than 40% mark (equivalent less than 1% of the time). Better results showed at the less than 50% mark, with equivalence seen 46% of the time.
NIRS measures a composite of arterial and venous blood, according to Dr. Rescoe and her colleagues. Therefore, to do a more direct comparison, they adjusted their NIRS results by calculating an rSO2-based ScvO2 designed to remove arterial contamination from the rSO2 signal: rSO2-based ScvO2 = (rSO2 arterial oxygen saturation x 0.3)/0.7.
This significantly improved the sensitivity of rSO2 to detect ScvO2 at less than 30% to 6.5%, to 29% for rSO2 at less than 40%, and 77.4% for rSO2 less than 50%.
The researchers “were surprised by the extremely low sensitivity of cerebral NIRS to detect even the most severe aberrations in DO2” (i.e., ScvO2 less than 30%, which has been found to be associated with poor outcomes).
“Cerebral rSO2 in isolation should not be used to detect low ScvO2, because its sensitivity is low, although correction of rSO2 for arterial contamination significantly improves sensitivity. Cerebral rSO2 of 50 or greater should not be considered reassuring with regard to ScvO2, although values less than 30 are specific for low ScvO2,” the researchers concluded.
The study was sponsored by the Gerber Foundation, the Hess Family Philanthropic Fund, and Boston Children’s Hospital Heart Center Strategic Investment Fund. The authors disclosed that they had no financial conflicts.
The use of postoperative cerebral venous oxygen saturation monitoring (ScvO2) through an internal jugular vein catheter allows better monitoring of circulation, which may lead to better outcomes, but it is invasive and challenging. NIRS, being noninvasive, has proved attractive, but clinical interpretation in terms of both absolute values and trends is difficult, Edward Buratto, MBBS, and his colleagues noted in their invited commentary (doi: 10.1016/j.jtcvs.2017.04.061).
Dr. Rescoe and her colleagues have analyzed the correlation of NIRS-derived data with ScvO2 measured by co-oximetry from the internal jugular vein in 73 neonates after stage 1 palliation for hypoplastic left heart syndrome. They demonstrated that cerebral rSO2 correlated poorly with low ScvO2, and they suggest that cerebral rSO2 not be used in isolation. This problem was somewhat ameliorated by correction of the signal for arterial contamination. NIRS appears to be too valuable a tool to be simply discarded, they said, suggesting that a perioperative risk assessment that would include multisite NIRS and hemodynamic monitoring might still allow early determination of low-cardiac output.
“Two numbers are better than one,” wrote Dr. Buratto and his colleagues. “Whether the NIRS technology will add any useful information to a simple bedside assessment by an astute clinician is yet to be seen.”
Edward Buratto, MBBS, Steve Horton, PhD, and Igor E. Konstantinov, MD, are from the Department of Cardiothoracic Surgery, The Royal Children’s Hospital; the Department of Pediatrics, University of Melbourne; and Murdoch Children’s Research Institute, Melbourne. They reported having no financial conflicts of interest.
The use of postoperative cerebral venous oxygen saturation monitoring (ScvO2) through an internal jugular vein catheter allows better monitoring of circulation, which may lead to better outcomes, but it is invasive and challenging. NIRS, being noninvasive, has proved attractive, but clinical interpretation in terms of both absolute values and trends is difficult, Edward Buratto, MBBS, and his colleagues noted in their invited commentary (doi: 10.1016/j.jtcvs.2017.04.061).
Dr. Rescoe and her colleagues have analyzed the correlation of NIRS-derived data with ScvO2 measured by co-oximetry from the internal jugular vein in 73 neonates after stage 1 palliation for hypoplastic left heart syndrome. They demonstrated that cerebral rSO2 correlated poorly with low ScvO2, and they suggest that cerebral rSO2 not be used in isolation. This problem was somewhat ameliorated by correction of the signal for arterial contamination. NIRS appears to be too valuable a tool to be simply discarded, they said, suggesting that a perioperative risk assessment that would include multisite NIRS and hemodynamic monitoring might still allow early determination of low-cardiac output.
“Two numbers are better than one,” wrote Dr. Buratto and his colleagues. “Whether the NIRS technology will add any useful information to a simple bedside assessment by an astute clinician is yet to be seen.”
Edward Buratto, MBBS, Steve Horton, PhD, and Igor E. Konstantinov, MD, are from the Department of Cardiothoracic Surgery, The Royal Children’s Hospital; the Department of Pediatrics, University of Melbourne; and Murdoch Children’s Research Institute, Melbourne. They reported having no financial conflicts of interest.
The use of postoperative cerebral venous oxygen saturation monitoring (ScvO2) through an internal jugular vein catheter allows better monitoring of circulation, which may lead to better outcomes, but it is invasive and challenging. NIRS, being noninvasive, has proved attractive, but clinical interpretation in terms of both absolute values and trends is difficult, Edward Buratto, MBBS, and his colleagues noted in their invited commentary (doi: 10.1016/j.jtcvs.2017.04.061).
Dr. Rescoe and her colleagues have analyzed the correlation of NIRS-derived data with ScvO2 measured by co-oximetry from the internal jugular vein in 73 neonates after stage 1 palliation for hypoplastic left heart syndrome. They demonstrated that cerebral rSO2 correlated poorly with low ScvO2, and they suggest that cerebral rSO2 not be used in isolation. This problem was somewhat ameliorated by correction of the signal for arterial contamination. NIRS appears to be too valuable a tool to be simply discarded, they said, suggesting that a perioperative risk assessment that would include multisite NIRS and hemodynamic monitoring might still allow early determination of low-cardiac output.
“Two numbers are better than one,” wrote Dr. Buratto and his colleagues. “Whether the NIRS technology will add any useful information to a simple bedside assessment by an astute clinician is yet to be seen.”
Edward Buratto, MBBS, Steve Horton, PhD, and Igor E. Konstantinov, MD, are from the Department of Cardiothoracic Surgery, The Royal Children’s Hospital; the Department of Pediatrics, University of Melbourne; and Murdoch Children’s Research Institute, Melbourne. They reported having no financial conflicts of interest.
The regional oxygenation index (rSO2) based on near infrared spectroscopy (NIRS) measurement is frequently used to assess the adequacy of oxygen delivery after stage 1 palliation of hypoplastic left heart syndrome (HLHS). However, a recent study showed that cerebral rSO2 has low sensitivity and should not be considered reassuring even at rSO2 of 50 or greater. In addition, values below 30 were not found to be sensitive for detecting compromised oxygen delivery, according to a report published online in the Journal of Thoracic and Cardiovascular Surgery.
Erin Rescoe, MD, of Boston Children’s Hospital, and her colleagues at Harvard Medical School, Boston, performed a retrospective study of 73 neonates assessed with cerebral venous oxyhemoglobin saturation (ScvO2) measured by co-oximetry from the internal jugular vein, which is considered the preferred method for assessing the adequacy of tissue oxygen delivery, compared with cerebral rSO2 after stage 1 palliation of HLHS (doi: 10.1016/j.jtcvs.2017.03.154).
To determine the suggested benefit of NIRS as an effective trend monitor, the researchers used their interpolated data to examine changes in rSO2 and changes in ScvO2 at hourly intervals and compared these values.Of particular concern is the result showing that, in all instances where ScvO2 was less than 30%, rSO2 was greater than 30%. In terms of the sensitivity (the true positive rate) and specificity (the true negative rate) of using NIRS, time-matched pairs of rSO2 and ScvO2 showed that the receiver operating characteristic curves for rSO2 as a diagnostic test to detect ScvO2 less than 30%, less than 40%, and less than 50% were 0.82, 0.84, and 0.87, respectively, showing good specificity, with a value of rSO2 less than 30% indicating that ScvO2 will be less than 30% 99% of the time.
“However, the sensitivity of rSO2 in the range of clinical interest in detecting ScvO2 less than 30% is extremely low,” according to the researchers. Thus, NIRS is likely to produce false negatives, missing patients with clinically low postoperative oxygen saturation.
In fact, rSO2 was less than 30% less than 1% of the time that ScvO2 was less than 30%. Similar results were seen in comparing values at the less than 40% mark (equivalent less than 1% of the time). Better results showed at the less than 50% mark, with equivalence seen 46% of the time.
NIRS measures a composite of arterial and venous blood, according to Dr. Rescoe and her colleagues. Therefore, to do a more direct comparison, they adjusted their NIRS results by calculating an rSO2-based ScvO2 designed to remove arterial contamination from the rSO2 signal: rSO2-based ScvO2 = (rSO2 arterial oxygen saturation x 0.3)/0.7.
This significantly improved the sensitivity of rSO2 to detect ScvO2 at less than 30% to 6.5%, to 29% for rSO2 at less than 40%, and 77.4% for rSO2 less than 50%.
The researchers “were surprised by the extremely low sensitivity of cerebral NIRS to detect even the most severe aberrations in DO2” (i.e., ScvO2 less than 30%, which has been found to be associated with poor outcomes).
“Cerebral rSO2 in isolation should not be used to detect low ScvO2, because its sensitivity is low, although correction of rSO2 for arterial contamination significantly improves sensitivity. Cerebral rSO2 of 50 or greater should not be considered reassuring with regard to ScvO2, although values less than 30 are specific for low ScvO2,” the researchers concluded.
The study was sponsored by the Gerber Foundation, the Hess Family Philanthropic Fund, and Boston Children’s Hospital Heart Center Strategic Investment Fund. The authors disclosed that they had no financial conflicts.
The regional oxygenation index (rSO2) based on near infrared spectroscopy (NIRS) measurement is frequently used to assess the adequacy of oxygen delivery after stage 1 palliation of hypoplastic left heart syndrome (HLHS). However, a recent study showed that cerebral rSO2 has low sensitivity and should not be considered reassuring even at rSO2 of 50 or greater. In addition, values below 30 were not found to be sensitive for detecting compromised oxygen delivery, according to a report published online in the Journal of Thoracic and Cardiovascular Surgery.
Erin Rescoe, MD, of Boston Children’s Hospital, and her colleagues at Harvard Medical School, Boston, performed a retrospective study of 73 neonates assessed with cerebral venous oxyhemoglobin saturation (ScvO2) measured by co-oximetry from the internal jugular vein, which is considered the preferred method for assessing the adequacy of tissue oxygen delivery, compared with cerebral rSO2 after stage 1 palliation of HLHS (doi: 10.1016/j.jtcvs.2017.03.154).
To determine the suggested benefit of NIRS as an effective trend monitor, the researchers used their interpolated data to examine changes in rSO2 and changes in ScvO2 at hourly intervals and compared these values.Of particular concern is the result showing that, in all instances where ScvO2 was less than 30%, rSO2 was greater than 30%. In terms of the sensitivity (the true positive rate) and specificity (the true negative rate) of using NIRS, time-matched pairs of rSO2 and ScvO2 showed that the receiver operating characteristic curves for rSO2 as a diagnostic test to detect ScvO2 less than 30%, less than 40%, and less than 50% were 0.82, 0.84, and 0.87, respectively, showing good specificity, with a value of rSO2 less than 30% indicating that ScvO2 will be less than 30% 99% of the time.
“However, the sensitivity of rSO2 in the range of clinical interest in detecting ScvO2 less than 30% is extremely low,” according to the researchers. Thus, NIRS is likely to produce false negatives, missing patients with clinically low postoperative oxygen saturation.
In fact, rSO2 was less than 30% less than 1% of the time that ScvO2 was less than 30%. Similar results were seen in comparing values at the less than 40% mark (equivalent less than 1% of the time). Better results showed at the less than 50% mark, with equivalence seen 46% of the time.
NIRS measures a composite of arterial and venous blood, according to Dr. Rescoe and her colleagues. Therefore, to do a more direct comparison, they adjusted their NIRS results by calculating an rSO2-based ScvO2 designed to remove arterial contamination from the rSO2 signal: rSO2-based ScvO2 = (rSO2 arterial oxygen saturation x 0.3)/0.7.
This significantly improved the sensitivity of rSO2 to detect ScvO2 at less than 30% to 6.5%, to 29% for rSO2 at less than 40%, and 77.4% for rSO2 less than 50%.
The researchers “were surprised by the extremely low sensitivity of cerebral NIRS to detect even the most severe aberrations in DO2” (i.e., ScvO2 less than 30%, which has been found to be associated with poor outcomes).
“Cerebral rSO2 in isolation should not be used to detect low ScvO2, because its sensitivity is low, although correction of rSO2 for arterial contamination significantly improves sensitivity. Cerebral rSO2 of 50 or greater should not be considered reassuring with regard to ScvO2, although values less than 30 are specific for low ScvO2,” the researchers concluded.
The study was sponsored by the Gerber Foundation, the Hess Family Philanthropic Fund, and Boston Children’s Hospital Heart Center Strategic Investment Fund. The authors disclosed that they had no financial conflicts.
FROM THE JOURNAL OF THORACIC AND CARDIOVASCULAR SURGERY
Key clinical point:
Major finding: In terms of sensitivity, rSO2 was less than 30% less than 1% of the time that ScvO2 was less than 30%.
Data source: A retrospective single institution study of 73 neonates assessed after stage 1 palliation
Disclosures: The study was sponsored by the Gerber Foundation, the Hess Family Philanthropic Fund, and Boston Children’s Hospital Heart Center Strategic Investment Fund. The authors disclosed that they had no financial conflicts.
Sinus of Valsalva preserved in aortic valve replacement
The sinus of Valsalva segment can be preserved during aortic valve replacement irrespective of the type of valve pathology, according to a recent study by Rita Karianna Milewski, MD, and her colleagues at the Hospital of the University of Pennsylvania, Philadelphia.
Severe aortic root dilation coupled to aortic valve disease requires root replacement in patients with a tricuspid or bicuspid aortic valve. Commonly, an aortic valve replacement and supracoronary ascending aorta replacement (AVRSCAAR) procedure has been used for patients who have a mild to moderately dilated sinus segment. One advantage of the procedure is that it retains the sinus of Valsalva (SOV) and preserves the intact coronary ostia.
However, the long-term behavior and risk of aortic events for the retained SOV in both BAV and TAV patients remains unclear, according to Dr. Milewski and her colleagues.
Previous researchers have suggested that patients with BAV and TAV have different rates of complications of the remaining aorta and dilation of the proximal aorta and retained sinus segment. In addition, it has been suggested that the cause of aortic dilation is different in patients with aortic stenosis (AS) and aortic insufficiency (AI) and is based on TAV and BAV morphology, histology, and hemodynamic flow patterns.
However, in the August issue of the Journal of Thoracic and Cardiovascular Surgery, Dr. Milewski and her colleagues reported on their study showing that, in patients with nonaneurysmal SOV undergoing AVRSCAAR, the sinus of Valsalva segment can be preserved regardless of the type of valvular pathology (aortic stenosis vs. aortic insufficiency) or valvular morphology (BAV vs. TAV).
The researchers retrospectively reviewed a prospectively maintained institutional database to stratify all patients by BAV or TAV valvular pathology with concomitant ascending aortic aneurysm who underwent an elective AVRSCAAR from 2002 to 2015 (J Thorac Cardiovasc Surg. 2017;154:421-32).
The distribution of the 428 patients meeting inclusion criteria by subgroups was: BAV group (254 patients: BAV-AS = 178; BAV-AI = 76); TAV group (174 patients: TAV-AS = 61; TAV-AI =113). Preoperative sinus of Valsalva dimensions were divided into 3 subgroups (less than 40 mm, 40-45 mm, and greater than 45 mm).
The mean patient age for patients with BAV and TAV was 59 years and 72 years (P less than .001), respectively (with 78% with BAV being men and 57% with TAV being men). There was a significantly higher subpopulation of AS in the BAV cohort vs. TAV-AS (70% vs. 35%; P less than .001).
With regard to SOV sizing, there was no significant difference in mean preoperative aortic root diameters between BAV and TAV cohorts for the AS or AI subpopulations.
In-hospital/30-day mortality was significantly higher in patients with TAV (5.2%) than in patients with BAV (1.6%, P = .033). In addition, the incidence of transient ischemic attack/stroke was significantly higher in the TAV group (3.4%) vs. the BAV group (0.8%, P = .04).
Valvular morphology and pathology at baseline, preoperative SOV diameter, postoperative time course, and interaction effect of preoperative SOV diameters and postoperative time course were used as covariates to assess outcomes. Within-subject and within–stratified subgroup comparison failed to show main effects across the follow-up times on postoperative SOV size patterns (P = .935), implying that the SOV trends were stable and sustained (discharge to greater than or equal to 10 years) irrespective of valvular morphology and pathology (BAV-AI, BAV-AS, TAV-AI, and TAV-AS).
Preoperative SOV dimensions significantly affected the retained postoperative sinus dimensions (P less than .001), according to Dr. Milewski and her colleagues.
The data indicated that an initial and pronounced postoperative decrease in SOV dimensions occurs with AVRSCAAR independently of aortic valve morphology, aortic valve pathology, and age, they added.
The 10-year freedom from aortic reoperation rates were 97% and 95% in the BAV and TAV subgroups, respectively. The BAV group had significantly improved reoperation-free survival, compared with the TAV group (P less than .001), while the type of valvular pathology within each group did not show a significant survival difference.
“Irrespective of the aortic valve morphology or valve pathology, in patients with mild to moderate aortic root dilatation (less than 45 mm), preservation of the SOV segment in the context of an AVRSCAAR procedure is justified. Continued further follow-up will be important to understand the long-term outcomes of sinus preservation, especially in the younger population with BAVs,” the researchers concluded.
The authors reported having no financial conflicts to disclose.
With regard to the question, ‘‘Is it necessary to replace the sinuses of Valsalva in the setting of bicuspid aortic valve aortopathy?’’, the researchers “leverage their enormous institutional experience to find an answer. The results suggest that this answer is ‘no.’ At least not in all cases,” Thoralf M. Sundt, MD, wrote in his invited commentary on the paper (J Thorac Cardiovasc Surg. 2017;154:419-20).
“The findings of this study argue for us to take a step back and ask how much really needs be done,” he added. And although “it is hard to ask a surgeon to do less rather than more; however, the balance of judgment has to be between the operative risk of the more aggressive approach and the natural history of the disease. In other words, what does it ‘cost’ to be aggressive, and what do we gain?” he asked.
Bicuspid aortic valve aortopathy, it would appear, is not cancer after all. Regardless of theoretic arguments that are based on embryology and the migration of neural crest cells, it does not appear to require resection to ‘clean margins,’ even if we believe that the operative risk ‘in our hands’ is low,” concluded Dr. Sundt.
Thoralf M. Sundt, MD, is at Harvard Medical School, Boston. He reported having no disclosures.
With regard to the question, ‘‘Is it necessary to replace the sinuses of Valsalva in the setting of bicuspid aortic valve aortopathy?’’, the researchers “leverage their enormous institutional experience to find an answer. The results suggest that this answer is ‘no.’ At least not in all cases,” Thoralf M. Sundt, MD, wrote in his invited commentary on the paper (J Thorac Cardiovasc Surg. 2017;154:419-20).
“The findings of this study argue for us to take a step back and ask how much really needs be done,” he added. And although “it is hard to ask a surgeon to do less rather than more; however, the balance of judgment has to be between the operative risk of the more aggressive approach and the natural history of the disease. In other words, what does it ‘cost’ to be aggressive, and what do we gain?” he asked.
Bicuspid aortic valve aortopathy, it would appear, is not cancer after all. Regardless of theoretic arguments that are based on embryology and the migration of neural crest cells, it does not appear to require resection to ‘clean margins,’ even if we believe that the operative risk ‘in our hands’ is low,” concluded Dr. Sundt.
Thoralf M. Sundt, MD, is at Harvard Medical School, Boston. He reported having no disclosures.
With regard to the question, ‘‘Is it necessary to replace the sinuses of Valsalva in the setting of bicuspid aortic valve aortopathy?’’, the researchers “leverage their enormous institutional experience to find an answer. The results suggest that this answer is ‘no.’ At least not in all cases,” Thoralf M. Sundt, MD, wrote in his invited commentary on the paper (J Thorac Cardiovasc Surg. 2017;154:419-20).
“The findings of this study argue for us to take a step back and ask how much really needs be done,” he added. And although “it is hard to ask a surgeon to do less rather than more; however, the balance of judgment has to be between the operative risk of the more aggressive approach and the natural history of the disease. In other words, what does it ‘cost’ to be aggressive, and what do we gain?” he asked.
Bicuspid aortic valve aortopathy, it would appear, is not cancer after all. Regardless of theoretic arguments that are based on embryology and the migration of neural crest cells, it does not appear to require resection to ‘clean margins,’ even if we believe that the operative risk ‘in our hands’ is low,” concluded Dr. Sundt.
Thoralf M. Sundt, MD, is at Harvard Medical School, Boston. He reported having no disclosures.
The sinus of Valsalva segment can be preserved during aortic valve replacement irrespective of the type of valve pathology, according to a recent study by Rita Karianna Milewski, MD, and her colleagues at the Hospital of the University of Pennsylvania, Philadelphia.
Severe aortic root dilation coupled to aortic valve disease requires root replacement in patients with a tricuspid or bicuspid aortic valve. Commonly, an aortic valve replacement and supracoronary ascending aorta replacement (AVRSCAAR) procedure has been used for patients who have a mild to moderately dilated sinus segment. One advantage of the procedure is that it retains the sinus of Valsalva (SOV) and preserves the intact coronary ostia.
However, the long-term behavior and risk of aortic events for the retained SOV in both BAV and TAV patients remains unclear, according to Dr. Milewski and her colleagues.
Previous researchers have suggested that patients with BAV and TAV have different rates of complications of the remaining aorta and dilation of the proximal aorta and retained sinus segment. In addition, it has been suggested that the cause of aortic dilation is different in patients with aortic stenosis (AS) and aortic insufficiency (AI) and is based on TAV and BAV morphology, histology, and hemodynamic flow patterns.
However, in the August issue of the Journal of Thoracic and Cardiovascular Surgery, Dr. Milewski and her colleagues reported on their study showing that, in patients with nonaneurysmal SOV undergoing AVRSCAAR, the sinus of Valsalva segment can be preserved regardless of the type of valvular pathology (aortic stenosis vs. aortic insufficiency) or valvular morphology (BAV vs. TAV).
The researchers retrospectively reviewed a prospectively maintained institutional database to stratify all patients by BAV or TAV valvular pathology with concomitant ascending aortic aneurysm who underwent an elective AVRSCAAR from 2002 to 2015 (J Thorac Cardiovasc Surg. 2017;154:421-32).
The distribution of the 428 patients meeting inclusion criteria by subgroups was: BAV group (254 patients: BAV-AS = 178; BAV-AI = 76); TAV group (174 patients: TAV-AS = 61; TAV-AI =113). Preoperative sinus of Valsalva dimensions were divided into 3 subgroups (less than 40 mm, 40-45 mm, and greater than 45 mm).
The mean patient age for patients with BAV and TAV was 59 years and 72 years (P less than .001), respectively (with 78% with BAV being men and 57% with TAV being men). There was a significantly higher subpopulation of AS in the BAV cohort vs. TAV-AS (70% vs. 35%; P less than .001).
With regard to SOV sizing, there was no significant difference in mean preoperative aortic root diameters between BAV and TAV cohorts for the AS or AI subpopulations.
In-hospital/30-day mortality was significantly higher in patients with TAV (5.2%) than in patients with BAV (1.6%, P = .033). In addition, the incidence of transient ischemic attack/stroke was significantly higher in the TAV group (3.4%) vs. the BAV group (0.8%, P = .04).
Valvular morphology and pathology at baseline, preoperative SOV diameter, postoperative time course, and interaction effect of preoperative SOV diameters and postoperative time course were used as covariates to assess outcomes. Within-subject and within–stratified subgroup comparison failed to show main effects across the follow-up times on postoperative SOV size patterns (P = .935), implying that the SOV trends were stable and sustained (discharge to greater than or equal to 10 years) irrespective of valvular morphology and pathology (BAV-AI, BAV-AS, TAV-AI, and TAV-AS).
Preoperative SOV dimensions significantly affected the retained postoperative sinus dimensions (P less than .001), according to Dr. Milewski and her colleagues.
The data indicated that an initial and pronounced postoperative decrease in SOV dimensions occurs with AVRSCAAR independently of aortic valve morphology, aortic valve pathology, and age, they added.
The 10-year freedom from aortic reoperation rates were 97% and 95% in the BAV and TAV subgroups, respectively. The BAV group had significantly improved reoperation-free survival, compared with the TAV group (P less than .001), while the type of valvular pathology within each group did not show a significant survival difference.
“Irrespective of the aortic valve morphology or valve pathology, in patients with mild to moderate aortic root dilatation (less than 45 mm), preservation of the SOV segment in the context of an AVRSCAAR procedure is justified. Continued further follow-up will be important to understand the long-term outcomes of sinus preservation, especially in the younger population with BAVs,” the researchers concluded.
The authors reported having no financial conflicts to disclose.
The sinus of Valsalva segment can be preserved during aortic valve replacement irrespective of the type of valve pathology, according to a recent study by Rita Karianna Milewski, MD, and her colleagues at the Hospital of the University of Pennsylvania, Philadelphia.
Severe aortic root dilation coupled to aortic valve disease requires root replacement in patients with a tricuspid or bicuspid aortic valve. Commonly, an aortic valve replacement and supracoronary ascending aorta replacement (AVRSCAAR) procedure has been used for patients who have a mild to moderately dilated sinus segment. One advantage of the procedure is that it retains the sinus of Valsalva (SOV) and preserves the intact coronary ostia.
However, the long-term behavior and risk of aortic events for the retained SOV in both BAV and TAV patients remains unclear, according to Dr. Milewski and her colleagues.
Previous researchers have suggested that patients with BAV and TAV have different rates of complications of the remaining aorta and dilation of the proximal aorta and retained sinus segment. In addition, it has been suggested that the cause of aortic dilation is different in patients with aortic stenosis (AS) and aortic insufficiency (AI) and is based on TAV and BAV morphology, histology, and hemodynamic flow patterns.
However, in the August issue of the Journal of Thoracic and Cardiovascular Surgery, Dr. Milewski and her colleagues reported on their study showing that, in patients with nonaneurysmal SOV undergoing AVRSCAAR, the sinus of Valsalva segment can be preserved regardless of the type of valvular pathology (aortic stenosis vs. aortic insufficiency) or valvular morphology (BAV vs. TAV).
The researchers retrospectively reviewed a prospectively maintained institutional database to stratify all patients by BAV or TAV valvular pathology with concomitant ascending aortic aneurysm who underwent an elective AVRSCAAR from 2002 to 2015 (J Thorac Cardiovasc Surg. 2017;154:421-32).
The distribution of the 428 patients meeting inclusion criteria by subgroups was: BAV group (254 patients: BAV-AS = 178; BAV-AI = 76); TAV group (174 patients: TAV-AS = 61; TAV-AI =113). Preoperative sinus of Valsalva dimensions were divided into 3 subgroups (less than 40 mm, 40-45 mm, and greater than 45 mm).
The mean patient age for patients with BAV and TAV was 59 years and 72 years (P less than .001), respectively (with 78% with BAV being men and 57% with TAV being men). There was a significantly higher subpopulation of AS in the BAV cohort vs. TAV-AS (70% vs. 35%; P less than .001).
With regard to SOV sizing, there was no significant difference in mean preoperative aortic root diameters between BAV and TAV cohorts for the AS or AI subpopulations.
In-hospital/30-day mortality was significantly higher in patients with TAV (5.2%) than in patients with BAV (1.6%, P = .033). In addition, the incidence of transient ischemic attack/stroke was significantly higher in the TAV group (3.4%) vs. the BAV group (0.8%, P = .04).
Valvular morphology and pathology at baseline, preoperative SOV diameter, postoperative time course, and interaction effect of preoperative SOV diameters and postoperative time course were used as covariates to assess outcomes. Within-subject and within–stratified subgroup comparison failed to show main effects across the follow-up times on postoperative SOV size patterns (P = .935), implying that the SOV trends were stable and sustained (discharge to greater than or equal to 10 years) irrespective of valvular morphology and pathology (BAV-AI, BAV-AS, TAV-AI, and TAV-AS).
Preoperative SOV dimensions significantly affected the retained postoperative sinus dimensions (P less than .001), according to Dr. Milewski and her colleagues.
The data indicated that an initial and pronounced postoperative decrease in SOV dimensions occurs with AVRSCAAR independently of aortic valve morphology, aortic valve pathology, and age, they added.
The 10-year freedom from aortic reoperation rates were 97% and 95% in the BAV and TAV subgroups, respectively. The BAV group had significantly improved reoperation-free survival, compared with the TAV group (P less than .001), while the type of valvular pathology within each group did not show a significant survival difference.
“Irrespective of the aortic valve morphology or valve pathology, in patients with mild to moderate aortic root dilatation (less than 45 mm), preservation of the SOV segment in the context of an AVRSCAAR procedure is justified. Continued further follow-up will be important to understand the long-term outcomes of sinus preservation, especially in the younger population with BAVs,” the researchers concluded.
The authors reported having no financial conflicts to disclose.
FROM THE JOURNAL OF THORACIC AND CARDIOVASCULAR SURGERY
Key clinical point:
Major finding: The 10-year freedom from aortic reoperation rates were 97% and 95% in the BAV and TAV subgroups, respectively.
Data source: A retrospective review of 428 patients in a prospectively maintained database who met study inclusion criteria and were operated on between 2002 and 2015.
Disclosures: The authors reported having no financial conflicts to disclose.
New-onset AF after aortic valve replacement did not affect long-term survival
New-onset atrial fibrillation after aortic valve replacement was not an independent risk factor for decreased long-term survival, according to the results of a single-center, retrospective study reported by Ben M. Swinkels, MD, of St Antonius Hospital, Nieuwegein, and his colleagues in the Netherlands.
Key to this success, however, is restoring normal sinus rhythm before hospital discharge, they said.
In this retrospective, longitudinal cohort study, 569 consecutive patients with no history of AF who underwent AVR with or without concomitant coronary artery bypass grafting during 1990-1993 were followed for a mean of 17.8 years (J Thorac Cardiovasc Surg. 2017;154:492-8).
Thirty-day and long-term survival rates were determined in the 241 patients (42%) with and the 328 patients (58%) without new-onset postoperative atrial fibrillation (POAF), which was defined as electrocardiographically documented AF lasting for at least several hours, and occurring after AVR while the patient was still admitted. Standard therapy to prevent new onset POAF was the use of sotalol in patients who were not on beta-blocker therapy, and continuation of beta-blocker therapy for those who were already on it.
There were no significant differences between the two groups in demographic characteristics. There were also no significant differences between the two groups in operative characteristics, postoperative in-hospital adverse events, and postoperative hospital lengths of stay until discharge home, except for mechanical ventilation time, which was significantly longer in the patients with new-onset POAF (P = .011).
Thirty-day mortality was 1.2% in the patients with POAF, and 2.7% in those without, a nonsignificant difference. There was no statistically significant difference between the two survival curves and the Kaplan-Meier overall cumulative survival rates at 15 years of follow-up in the patients with new-onset POAF vs. those without were not statistically different (41.5% vs. 41.3%, respectively).
In addition, the 18-year probability of long-term first adverse events, including recurrent AF, transient ischemic attack, ischemic or hemorrhagic stroke, peripheral venous thromboembolism, or major or minor bleeding was not significantly different between the two groups.
“New-onset POAF after AVR does not affect long-term survival when treatment is aimed to restore sinus rhythm before the patient is discharged home. Future studies with a prospective, randomized design should be done to confirm this finding in patients undergoing different kinds of cardiac surgery,” the researchers concluded.
The study was funded by the authors’ home institution; the authors reported they had nothing to disclose.
The incidence of atrial fibrillation after valve surgery has been described to be as high as 50%, Manuel J. Antunes, MD, said in an editorial commentary. “The adverse effect on long-term survival may not be related to the short-lived new-onset AF but rather to the underlying pathology associated to the arrhythmia, especially pathology that affects the myocardium, principally in atherosclerotic coronary artery disease,” he wrote. “It is not survival alone, however, that should be cause for concern; AF, even in episodes of limited duration, may result in transient ischemic attacks, ischemic, or hemorrhagic strokes, and peripheral thromboembolism, which is why affected patients should immediately be anticoagulated.”
This study, however, is at odds with previously published studies, with opposite conclusions, according to Dr. Antunes. Swinkels and his colleagues suggest that one of the reasons for the discrepancy was the homogeneous character of their series, which consisted almost entirely of patients who had isolated AVR. Dr. Antunes also adds that another important aspect to consider is that the antiarrhythmic drugs used prophylactically or therapeutically for this patient cohort (treated during 1990-1993) are no longer used or have been replaced by new and more efficacious pharmacologic agents.
Manuel J. Antunes, MD, of the University Hospital and Faculty of Medicine, Coimbra, Portugal, made these remarks in an invited editorial (J Thorac Cardiovasc Surg. 2017;154:490-1). He reported having nothing to disclose.
The incidence of atrial fibrillation after valve surgery has been described to be as high as 50%, Manuel J. Antunes, MD, said in an editorial commentary. “The adverse effect on long-term survival may not be related to the short-lived new-onset AF but rather to the underlying pathology associated to the arrhythmia, especially pathology that affects the myocardium, principally in atherosclerotic coronary artery disease,” he wrote. “It is not survival alone, however, that should be cause for concern; AF, even in episodes of limited duration, may result in transient ischemic attacks, ischemic, or hemorrhagic strokes, and peripheral thromboembolism, which is why affected patients should immediately be anticoagulated.”
This study, however, is at odds with previously published studies, with opposite conclusions, according to Dr. Antunes. Swinkels and his colleagues suggest that one of the reasons for the discrepancy was the homogeneous character of their series, which consisted almost entirely of patients who had isolated AVR. Dr. Antunes also adds that another important aspect to consider is that the antiarrhythmic drugs used prophylactically or therapeutically for this patient cohort (treated during 1990-1993) are no longer used or have been replaced by new and more efficacious pharmacologic agents.
Manuel J. Antunes, MD, of the University Hospital and Faculty of Medicine, Coimbra, Portugal, made these remarks in an invited editorial (J Thorac Cardiovasc Surg. 2017;154:490-1). He reported having nothing to disclose.
The incidence of atrial fibrillation after valve surgery has been described to be as high as 50%, Manuel J. Antunes, MD, said in an editorial commentary. “The adverse effect on long-term survival may not be related to the short-lived new-onset AF but rather to the underlying pathology associated to the arrhythmia, especially pathology that affects the myocardium, principally in atherosclerotic coronary artery disease,” he wrote. “It is not survival alone, however, that should be cause for concern; AF, even in episodes of limited duration, may result in transient ischemic attacks, ischemic, or hemorrhagic strokes, and peripheral thromboembolism, which is why affected patients should immediately be anticoagulated.”
This study, however, is at odds with previously published studies, with opposite conclusions, according to Dr. Antunes. Swinkels and his colleagues suggest that one of the reasons for the discrepancy was the homogeneous character of their series, which consisted almost entirely of patients who had isolated AVR. Dr. Antunes also adds that another important aspect to consider is that the antiarrhythmic drugs used prophylactically or therapeutically for this patient cohort (treated during 1990-1993) are no longer used or have been replaced by new and more efficacious pharmacologic agents.
Manuel J. Antunes, MD, of the University Hospital and Faculty of Medicine, Coimbra, Portugal, made these remarks in an invited editorial (J Thorac Cardiovasc Surg. 2017;154:490-1). He reported having nothing to disclose.
New-onset atrial fibrillation after aortic valve replacement was not an independent risk factor for decreased long-term survival, according to the results of a single-center, retrospective study reported by Ben M. Swinkels, MD, of St Antonius Hospital, Nieuwegein, and his colleagues in the Netherlands.
Key to this success, however, is restoring normal sinus rhythm before hospital discharge, they said.
In this retrospective, longitudinal cohort study, 569 consecutive patients with no history of AF who underwent AVR with or without concomitant coronary artery bypass grafting during 1990-1993 were followed for a mean of 17.8 years (J Thorac Cardiovasc Surg. 2017;154:492-8).
Thirty-day and long-term survival rates were determined in the 241 patients (42%) with and the 328 patients (58%) without new-onset postoperative atrial fibrillation (POAF), which was defined as electrocardiographically documented AF lasting for at least several hours, and occurring after AVR while the patient was still admitted. Standard therapy to prevent new onset POAF was the use of sotalol in patients who were not on beta-blocker therapy, and continuation of beta-blocker therapy for those who were already on it.
There were no significant differences between the two groups in demographic characteristics. There were also no significant differences between the two groups in operative characteristics, postoperative in-hospital adverse events, and postoperative hospital lengths of stay until discharge home, except for mechanical ventilation time, which was significantly longer in the patients with new-onset POAF (P = .011).
Thirty-day mortality was 1.2% in the patients with POAF, and 2.7% in those without, a nonsignificant difference. There was no statistically significant difference between the two survival curves and the Kaplan-Meier overall cumulative survival rates at 15 years of follow-up in the patients with new-onset POAF vs. those without were not statistically different (41.5% vs. 41.3%, respectively).
In addition, the 18-year probability of long-term first adverse events, including recurrent AF, transient ischemic attack, ischemic or hemorrhagic stroke, peripheral venous thromboembolism, or major or minor bleeding was not significantly different between the two groups.
“New-onset POAF after AVR does not affect long-term survival when treatment is aimed to restore sinus rhythm before the patient is discharged home. Future studies with a prospective, randomized design should be done to confirm this finding in patients undergoing different kinds of cardiac surgery,” the researchers concluded.
The study was funded by the authors’ home institution; the authors reported they had nothing to disclose.
New-onset atrial fibrillation after aortic valve replacement was not an independent risk factor for decreased long-term survival, according to the results of a single-center, retrospective study reported by Ben M. Swinkels, MD, of St Antonius Hospital, Nieuwegein, and his colleagues in the Netherlands.
Key to this success, however, is restoring normal sinus rhythm before hospital discharge, they said.
In this retrospective, longitudinal cohort study, 569 consecutive patients with no history of AF who underwent AVR with or without concomitant coronary artery bypass grafting during 1990-1993 were followed for a mean of 17.8 years (J Thorac Cardiovasc Surg. 2017;154:492-8).
Thirty-day and long-term survival rates were determined in the 241 patients (42%) with and the 328 patients (58%) without new-onset postoperative atrial fibrillation (POAF), which was defined as electrocardiographically documented AF lasting for at least several hours, and occurring after AVR while the patient was still admitted. Standard therapy to prevent new onset POAF was the use of sotalol in patients who were not on beta-blocker therapy, and continuation of beta-blocker therapy for those who were already on it.
There were no significant differences between the two groups in demographic characteristics. There were also no significant differences between the two groups in operative characteristics, postoperative in-hospital adverse events, and postoperative hospital lengths of stay until discharge home, except for mechanical ventilation time, which was significantly longer in the patients with new-onset POAF (P = .011).
Thirty-day mortality was 1.2% in the patients with POAF, and 2.7% in those without, a nonsignificant difference. There was no statistically significant difference between the two survival curves and the Kaplan-Meier overall cumulative survival rates at 15 years of follow-up in the patients with new-onset POAF vs. those without were not statistically different (41.5% vs. 41.3%, respectively).
In addition, the 18-year probability of long-term first adverse events, including recurrent AF, transient ischemic attack, ischemic or hemorrhagic stroke, peripheral venous thromboembolism, or major or minor bleeding was not significantly different between the two groups.
“New-onset POAF after AVR does not affect long-term survival when treatment is aimed to restore sinus rhythm before the patient is discharged home. Future studies with a prospective, randomized design should be done to confirm this finding in patients undergoing different kinds of cardiac surgery,” the researchers concluded.
The study was funded by the authors’ home institution; the authors reported they had nothing to disclose.
FROM THE JOURNAL OF THORACIC AND CARDIOVASCULAR SURGERY
Key clinical point:
Major finding: Cumulative 15-year survival rates were similar in the patients with new-onset postop AF (41.5%) to those without (41.3%).
Data source: A retrospective longitudinal cohort study of 569 consecutive patients without a history of AF who were followed for a mean of 17.8 years after AVR with or without concomitant CABG.
Disclosures: The study was funded by the authors’ home institution and the authors reported they had nothing to disclose.
Preop atrial fib in CABG patients spells trouble
COLORADO SPRINGS – Preoperative atrial fibrillation is present in more than 10% of patients undergoing isolated coronary artery bypass graft (CABG) surgery, and if not subjected to concomitant surgical ablation it’s associated with increased perioperative and long-term major morbidity and mortality, S. Chris Malaisrie, MD, reported at the annual meeting of the Western Thoracic Surgical Association.
The increased early and late risks posed by preoperative atrial fibrillation (AF) that go unaddressed remain significant even after adjusting for the numerous comorbid conditions more prevalent in CABG patients with preoperative AF than in those without the arrhythmia, added Dr. Malaisrie, a cardiac surgeon at Northwestern University in Chicago.
The unadjusted operative mortality rate was 1.8% in the no-AF group and 4.0% in patients with preoperative AF. Unadjusted in-hospital rates of permanent stroke, prolonged ventilation, reoperation, and new renal failure were also significantly higher in the preoperative AF group.
Not surprisingly, the preoperative AF group was older. They also had significantly higher baseline rates of numerous comorbid conditions, including diabetes, peripheral vascular disease, renal failure, and prior stroke, as well as a lower mean left ventricular ejection fraction. However, after adjustment for the many comorbidities in multivariate regression analysis, the risks of all in-hospital adverse outcomes remained significantly higher in the preoperative AF group. For example, their adjusted risk of operative mortality was 1.5-fold greater than in the no-AF patients.
In the long-term follow-up analysis, the unadjusted risk of mortality in the first 5 years after CABG was 2.5-fold greater in the preoperative AF group. Their 5-year risk of stroke or systemic embolization was 1.5-fold greater, too. Upon adjustment for potentially confounding comorbid conditions, preoperative AF was associated with a 1.5-fold increased 5-year risk of mortality and a 1.2-fold increase in stroke or systemic embolism.
In an effort to identify a particularly high-risk group of CABG patients with preoperative AF, Dr. Malaisrie and his coinvestigators stratified the group’s long-term stroke and mortality risks by their CHA2DS2-VASc score at the time of surgery. The results were revealing: the unadjusted 5-year risk of stroke or systemic embolization was 7.9% in those with a CHA2DS2-VASc score of 1-3, 12.2% with a score of 4-6, and 15.4% with a score of 7-9. The 5-year survival rate was 74.8% with a score of 1-3, 56.5% with a score of 4-6, and 41.2% with a score of 7-9.
“That’s really a striking finding,” Dr. Malaisrie observed. “When you consider a patient who’s, say, 72-75 years old, who is undergoing isolated CABG with preoperative atrial fibrillation and who has a high CHA2DS2-VASc score of 7-9, 5-year survival is only 41%, with a 15% risk of stroke or systemic embolization.”
Discussant William T. Caine, MD, found the study results unsettling.
“I was surprised to see that in this day and age, fully two-thirds of the patients who had preoperative atrial fibrillation had no attempt at any ablation procedure to treat their atrial fibrillation,” declared Dr. Caine of Intermountain Medical Center in Salt Lake City.
In reply, Dr. Malaisrie noted that other, smaller studies have also found that only about 30% of CABG patients with preoperative AF undergo surgical AF ablation through a maze procedure or some other method.
“Probably most of us in this room would go ahead and perform surgical ablation, but the STS database represents all isolated CABG procedures done throughout the United States,” Dr. Malaisrie said. “I think this dataset should help convince the other 70% of surgeons out there that there is a high cost for preoperative AF – in particular, in patients with very high CHA2DS2-VASc scores. If you can identify a group of patients at increased risk for stroke and mortality, you’d certainly want to bend their survival curve.”
The maze procedure has been convincingly shown to be very safe, with no associated increased risk of perioperative morbidity and mortality. The downside is cost. But while it’s true that adding surgical ablation to an isolated CABG procedure boosts OR time and procedural costs, a successful ablation is likely to pay dividends through reduced downstream rates of major morbidity and mortality.
“I look forward to the second part of our analysis, where we’ll look at the comparative data for the patients who did in fact have surgical ablation. That dataset is pending from the Duke Clinical Research Institute,” according to Dr. Malaisrie.
He cited as study limitations the inability to complete linkage to the Medicare database in about 37% of CABG patients in the STS database, or more than 200,000 people. Also, the Medicare database is an administrative dataset reliant upon medical record coding. The mortality data are probably quite accurate, but the stroke and systemic embolization rates cited in this analysis likely underestimate the true rates.
He reported serving as a consultant to Edwards Lifesciences, Abbott Vascular, and Baxter, and serving on speakers’ bureaus for Bolton and Abiomed. However, the STS analysis was funded exclusively by philanthropy.
COLORADO SPRINGS – Preoperative atrial fibrillation is present in more than 10% of patients undergoing isolated coronary artery bypass graft (CABG) surgery, and if not subjected to concomitant surgical ablation it’s associated with increased perioperative and long-term major morbidity and mortality, S. Chris Malaisrie, MD, reported at the annual meeting of the Western Thoracic Surgical Association.
The increased early and late risks posed by preoperative atrial fibrillation (AF) that go unaddressed remain significant even after adjusting for the numerous comorbid conditions more prevalent in CABG patients with preoperative AF than in those without the arrhythmia, added Dr. Malaisrie, a cardiac surgeon at Northwestern University in Chicago.
The unadjusted operative mortality rate was 1.8% in the no-AF group and 4.0% in patients with preoperative AF. Unadjusted in-hospital rates of permanent stroke, prolonged ventilation, reoperation, and new renal failure were also significantly higher in the preoperative AF group.
Not surprisingly, the preoperative AF group was older. They also had significantly higher baseline rates of numerous comorbid conditions, including diabetes, peripheral vascular disease, renal failure, and prior stroke, as well as a lower mean left ventricular ejection fraction. However, after adjustment for the many comorbidities in multivariate regression analysis, the risks of all in-hospital adverse outcomes remained significantly higher in the preoperative AF group. For example, their adjusted risk of operative mortality was 1.5-fold greater than in the no-AF patients.
In the long-term follow-up analysis, the unadjusted risk of mortality in the first 5 years after CABG was 2.5-fold greater in the preoperative AF group. Their 5-year risk of stroke or systemic embolization was 1.5-fold greater, too. Upon adjustment for potentially confounding comorbid conditions, preoperative AF was associated with a 1.5-fold increased 5-year risk of mortality and a 1.2-fold increase in stroke or systemic embolism.
In an effort to identify a particularly high-risk group of CABG patients with preoperative AF, Dr. Malaisrie and his coinvestigators stratified the group’s long-term stroke and mortality risks by their CHA2DS2-VASc score at the time of surgery. The results were revealing: the unadjusted 5-year risk of stroke or systemic embolization was 7.9% in those with a CHA2DS2-VASc score of 1-3, 12.2% with a score of 4-6, and 15.4% with a score of 7-9. The 5-year survival rate was 74.8% with a score of 1-3, 56.5% with a score of 4-6, and 41.2% with a score of 7-9.
“That’s really a striking finding,” Dr. Malaisrie observed. “When you consider a patient who’s, say, 72-75 years old, who is undergoing isolated CABG with preoperative atrial fibrillation and who has a high CHA2DS2-VASc score of 7-9, 5-year survival is only 41%, with a 15% risk of stroke or systemic embolization.”
Discussant William T. Caine, MD, found the study results unsettling.
“I was surprised to see that in this day and age, fully two-thirds of the patients who had preoperative atrial fibrillation had no attempt at any ablation procedure to treat their atrial fibrillation,” declared Dr. Caine of Intermountain Medical Center in Salt Lake City.
In reply, Dr. Malaisrie noted that other, smaller studies have also found that only about 30% of CABG patients with preoperative AF undergo surgical AF ablation through a maze procedure or some other method.
“Probably most of us in this room would go ahead and perform surgical ablation, but the STS database represents all isolated CABG procedures done throughout the United States,” Dr. Malaisrie said. “I think this dataset should help convince the other 70% of surgeons out there that there is a high cost for preoperative AF – in particular, in patients with very high CHA2DS2-VASc scores. If you can identify a group of patients at increased risk for stroke and mortality, you’d certainly want to bend their survival curve.”
The maze procedure has been convincingly shown to be very safe, with no associated increased risk of perioperative morbidity and mortality. The downside is cost. But while it’s true that adding surgical ablation to an isolated CABG procedure boosts OR time and procedural costs, a successful ablation is likely to pay dividends through reduced downstream rates of major morbidity and mortality.
“I look forward to the second part of our analysis, where we’ll look at the comparative data for the patients who did in fact have surgical ablation. That dataset is pending from the Duke Clinical Research Institute,” according to Dr. Malaisrie.
He cited as study limitations the inability to complete linkage to the Medicare database in about 37% of CABG patients in the STS database, or more than 200,000 people. Also, the Medicare database is an administrative dataset reliant upon medical record coding. The mortality data are probably quite accurate, but the stroke and systemic embolization rates cited in this analysis likely underestimate the true rates.
He reported serving as a consultant to Edwards Lifesciences, Abbott Vascular, and Baxter, and serving on speakers’ bureaus for Bolton and Abiomed. However, the STS analysis was funded exclusively by philanthropy.
COLORADO SPRINGS – Preoperative atrial fibrillation is present in more than 10% of patients undergoing isolated coronary artery bypass graft (CABG) surgery, and if not subjected to concomitant surgical ablation it’s associated with increased perioperative and long-term major morbidity and mortality, S. Chris Malaisrie, MD, reported at the annual meeting of the Western Thoracic Surgical Association.
The increased early and late risks posed by preoperative atrial fibrillation (AF) that go unaddressed remain significant even after adjusting for the numerous comorbid conditions more prevalent in CABG patients with preoperative AF than in those without the arrhythmia, added Dr. Malaisrie, a cardiac surgeon at Northwestern University in Chicago.
The unadjusted operative mortality rate was 1.8% in the no-AF group and 4.0% in patients with preoperative AF. Unadjusted in-hospital rates of permanent stroke, prolonged ventilation, reoperation, and new renal failure were also significantly higher in the preoperative AF group.
Not surprisingly, the preoperative AF group was older. They also had significantly higher baseline rates of numerous comorbid conditions, including diabetes, peripheral vascular disease, renal failure, and prior stroke, as well as a lower mean left ventricular ejection fraction. However, after adjustment for the many comorbidities in multivariate regression analysis, the risks of all in-hospital adverse outcomes remained significantly higher in the preoperative AF group. For example, their adjusted risk of operative mortality was 1.5-fold greater than in the no-AF patients.
In the long-term follow-up analysis, the unadjusted risk of mortality in the first 5 years after CABG was 2.5-fold greater in the preoperative AF group. Their 5-year risk of stroke or systemic embolization was 1.5-fold greater, too. Upon adjustment for potentially confounding comorbid conditions, preoperative AF was associated with a 1.5-fold increased 5-year risk of mortality and a 1.2-fold increase in stroke or systemic embolism.
In an effort to identify a particularly high-risk group of CABG patients with preoperative AF, Dr. Malaisrie and his coinvestigators stratified the group’s long-term stroke and mortality risks by their CHA2DS2-VASc score at the time of surgery. The results were revealing: the unadjusted 5-year risk of stroke or systemic embolization was 7.9% in those with a CHA2DS2-VASc score of 1-3, 12.2% with a score of 4-6, and 15.4% with a score of 7-9. The 5-year survival rate was 74.8% with a score of 1-3, 56.5% with a score of 4-6, and 41.2% with a score of 7-9.
“That’s really a striking finding,” Dr. Malaisrie observed. “When you consider a patient who’s, say, 72-75 years old, who is undergoing isolated CABG with preoperative atrial fibrillation and who has a high CHA2DS2-VASc score of 7-9, 5-year survival is only 41%, with a 15% risk of stroke or systemic embolization.”
Discussant William T. Caine, MD, found the study results unsettling.
“I was surprised to see that in this day and age, fully two-thirds of the patients who had preoperative atrial fibrillation had no attempt at any ablation procedure to treat their atrial fibrillation,” declared Dr. Caine of Intermountain Medical Center in Salt Lake City.
In reply, Dr. Malaisrie noted that other, smaller studies have also found that only about 30% of CABG patients with preoperative AF undergo surgical AF ablation through a maze procedure or some other method.
“Probably most of us in this room would go ahead and perform surgical ablation, but the STS database represents all isolated CABG procedures done throughout the United States,” Dr. Malaisrie said. “I think this dataset should help convince the other 70% of surgeons out there that there is a high cost for preoperative AF – in particular, in patients with very high CHA2DS2-VASc scores. If you can identify a group of patients at increased risk for stroke and mortality, you’d certainly want to bend their survival curve.”
The maze procedure has been convincingly shown to be very safe, with no associated increased risk of perioperative morbidity and mortality. The downside is cost. But while it’s true that adding surgical ablation to an isolated CABG procedure boosts OR time and procedural costs, a successful ablation is likely to pay dividends through reduced downstream rates of major morbidity and mortality.
“I look forward to the second part of our analysis, where we’ll look at the comparative data for the patients who did in fact have surgical ablation. That dataset is pending from the Duke Clinical Research Institute,” according to Dr. Malaisrie.
He cited as study limitations the inability to complete linkage to the Medicare database in about 37% of CABG patients in the STS database, or more than 200,000 people. Also, the Medicare database is an administrative dataset reliant upon medical record coding. The mortality data are probably quite accurate, but the stroke and systemic embolization rates cited in this analysis likely underestimate the true rates.
He reported serving as a consultant to Edwards Lifesciences, Abbott Vascular, and Baxter, and serving on speakers’ bureaus for Bolton and Abiomed. However, the STS analysis was funded exclusively by philanthropy.
AT THE WTSA ANNUAL MEETING
Key clinical point:
Major finding: Preoperative AF in patients undergoing isolated CABG was tied to an adjusted 45% greater 5-year mortality and 25% increase in stroke and systemic embolization risk, compared with CABG patients without the preoperative arrhythmia.
Data source: This retrospective study compared perioperative and long-term morbidity and mortality in nearly 350,000 patients in the Society of Thoracic Surgeons database who underwent isolated CABG, including more than 24,000 who had preoperative atrial fibrillation that wasn’t addressed surgically.
Disclosures: The study presenter reported serving as a consultant to Edwards Lifesciences, Abbott Vascular, and Baxter, and serving on speakers’ bureaus for Bolton and Abiomed. However, the STS analysis was funded exclusively by philanthropy.
Wide variability found in invasive mediastinal staging rates for lung cancer
COLORADO SPRINGS – Significant variability exists between hospitals in Washington state in their rates of invasive mediastinal staging for lung cancer, Farhood Farjah, MD, reported at the annual meeting of the Western Thoracic Surgical Association.
“We found evidence of a fivefold variation in hospital-level rates of invasive mediastinal staging not explained by chance or case mix,” according to Dr. Farjah of the University of Washington, Seattle.
“This has led to substantial concerns about quality of thoracic surgical care in the community at large,” he noted.
The Washington study is the first to show hospital-by-hospital variation in rates of invasive mediastinal staging.
Invasive mediastinal staging for lung cancer is considered important because imaging is known to have a substantial false-negative rate, and staging results have a profound impact on treatment recommendations, which can range from surgery alone to additional chemoradiation therapy.
Yet the meaning of the hospital-level huge variability in practice observed in the Washington study remains unclear.
“Our understanding of the underutilization of invasive mediastinal staging is further complicated by the fact that patterns of invasive mediastinal staging are highly variable across hospitals staffed by at least one board-certified thoracic surgeon with a noncardiac practice,” Dr. Farjah explained. “This variability could be a marker of poor-quality care. However, because the guidelines are not supported by level 1 evidence, it’s equally plausible that this variability might represent uncertainty or even disagreement with the practice guidelines – and specifically about the appropriate indication for invasive staging.”
He presented a retrospective cohort study of 406 patients whose non–small cell lung cancer was resected during July 2011–December 2013 at one of five Washington hospitals, each with at least one board-certified thoracic surgeon with a noncardiac practice on staff. The four participating community hospitals and one academic medical center were involved in a National Cancer Institute–funded, physician-led quality improvement initiative.
Overall, 66% of the 406 patients underwent any form of invasive mediastinal staging: 85% by mediastinoscopy only; 12% by mediastinoscopy plus endobronchial ultrasound-guided nodal aspiration (EBUS); 3% by EBUS only; and the remaining handful by mediastinoscopy, EBUS, and esophageal ultrasound-guided nodal aspiration. The invasive staging was performed at the time of resection in 64% of cases. A median of three nodal stations were sampled.
After statistical adjustment for random variation and between-hospital differences in clinical stage, rates of invasive staging were all over the map. While an overall mean of 66% of the lung cancer patients underwent invasive mediastinal staging, the rates at the five hospitals were 94%, 84%, 31%, 80%, and 17%.
Dr. Farjah and his coinvestigators are now conducting provider interviews and focus groups in an effort to understand what drove the participating surgeons’ wide variability in performing invasive mediastinal staging.
Discussant Jane Yanagawa, MD, of the University of California, Los Angeles, commented, “I think this is a really interesting study because, historically, lower rates of mediastinoscopy are assumed to be a reflection of low-quality care – and you suggest that might not be the case, that it might be more complicated than that.”
Dr. Yanagawa sketched one fairly common scenario that might represent a surgeon’s reasonable avoidance of guideline-recommended invasive mediastinal staging: a patient who by all preoperative imaging appears to have stage IA lung cancer and wishes to avoid the morbidity, time, and cost of needle biopsy, instead choosing to go straight to the operating room for a diagnosis by wedge resection, followed by a completion lobectomy based upon the frozen section results. Could such a pathway account for the variability seen in the Washington study?
“I think it could have,” Dr. Farjah replied. “I would say that’s probably one driver of variability.”
As for the generalizability of the findings of a five-hospital study carried out in a single state, Dr. Farjah said he thinks the results are applicable to any academic or community hospital with at least one board-certified thoracic surgeon with a noncardiac practice.
He reported having no financial conflicts of interest regarding the study.
M. Patricia Rivera, MD, FCCP, comments: Staging of lung cancer is essential to select the best treatment strategy for a given patient. However, despite multiple guideline recommendations
M. Patricia Rivera, MD, FCCP, comments: Staging of lung cancer is essential to select the best treatment strategy for a given patient. However, despite multiple guideline recommendations
M. Patricia Rivera, MD, FCCP, comments: Staging of lung cancer is essential to select the best treatment strategy for a given patient. However, despite multiple guideline recommendations
COLORADO SPRINGS – Significant variability exists between hospitals in Washington state in their rates of invasive mediastinal staging for lung cancer, Farhood Farjah, MD, reported at the annual meeting of the Western Thoracic Surgical Association.
“We found evidence of a fivefold variation in hospital-level rates of invasive mediastinal staging not explained by chance or case mix,” according to Dr. Farjah of the University of Washington, Seattle.
“This has led to substantial concerns about quality of thoracic surgical care in the community at large,” he noted.
The Washington study is the first to show hospital-by-hospital variation in rates of invasive mediastinal staging.
Invasive mediastinal staging for lung cancer is considered important because imaging is known to have a substantial false-negative rate, and staging results have a profound impact on treatment recommendations, which can range from surgery alone to additional chemoradiation therapy.
Yet the meaning of the hospital-level huge variability in practice observed in the Washington study remains unclear.
“Our understanding of the underutilization of invasive mediastinal staging is further complicated by the fact that patterns of invasive mediastinal staging are highly variable across hospitals staffed by at least one board-certified thoracic surgeon with a noncardiac practice,” Dr. Farjah explained. “This variability could be a marker of poor-quality care. However, because the guidelines are not supported by level 1 evidence, it’s equally plausible that this variability might represent uncertainty or even disagreement with the practice guidelines – and specifically about the appropriate indication for invasive staging.”
He presented a retrospective cohort study of 406 patients whose non–small cell lung cancer was resected during July 2011–December 2013 at one of five Washington hospitals, each with at least one board-certified thoracic surgeon with a noncardiac practice on staff. The four participating community hospitals and one academic medical center were involved in a National Cancer Institute–funded, physician-led quality improvement initiative.
Overall, 66% of the 406 patients underwent any form of invasive mediastinal staging: 85% by mediastinoscopy only; 12% by mediastinoscopy plus endobronchial ultrasound-guided nodal aspiration (EBUS); 3% by EBUS only; and the remaining handful by mediastinoscopy, EBUS, and esophageal ultrasound-guided nodal aspiration. The invasive staging was performed at the time of resection in 64% of cases. A median of three nodal stations were sampled.
After statistical adjustment for random variation and between-hospital differences in clinical stage, rates of invasive staging were all over the map. While an overall mean of 66% of the lung cancer patients underwent invasive mediastinal staging, the rates at the five hospitals were 94%, 84%, 31%, 80%, and 17%.
Dr. Farjah and his coinvestigators are now conducting provider interviews and focus groups in an effort to understand what drove the participating surgeons’ wide variability in performing invasive mediastinal staging.
Discussant Jane Yanagawa, MD, of the University of California, Los Angeles, commented, “I think this is a really interesting study because, historically, lower rates of mediastinoscopy are assumed to be a reflection of low-quality care – and you suggest that might not be the case, that it might be more complicated than that.”
Dr. Yanagawa sketched one fairly common scenario that might represent a surgeon’s reasonable avoidance of guideline-recommended invasive mediastinal staging: a patient who by all preoperative imaging appears to have stage IA lung cancer and wishes to avoid the morbidity, time, and cost of needle biopsy, instead choosing to go straight to the operating room for a diagnosis by wedge resection, followed by a completion lobectomy based upon the frozen section results. Could such a pathway account for the variability seen in the Washington study?
“I think it could have,” Dr. Farjah replied. “I would say that’s probably one driver of variability.”
As for the generalizability of the findings of a five-hospital study carried out in a single state, Dr. Farjah said he thinks the results are applicable to any academic or community hospital with at least one board-certified thoracic surgeon with a noncardiac practice.
He reported having no financial conflicts of interest regarding the study.
COLORADO SPRINGS – Significant variability exists between hospitals in Washington state in their rates of invasive mediastinal staging for lung cancer, Farhood Farjah, MD, reported at the annual meeting of the Western Thoracic Surgical Association.
“We found evidence of a fivefold variation in hospital-level rates of invasive mediastinal staging not explained by chance or case mix,” according to Dr. Farjah of the University of Washington, Seattle.
“This has led to substantial concerns about quality of thoracic surgical care in the community at large,” he noted.
The Washington study is the first to show hospital-by-hospital variation in rates of invasive mediastinal staging.
Invasive mediastinal staging for lung cancer is considered important because imaging is known to have a substantial false-negative rate, and staging results have a profound impact on treatment recommendations, which can range from surgery alone to additional chemoradiation therapy.
Yet the meaning of the hospital-level huge variability in practice observed in the Washington study remains unclear.
“Our understanding of the underutilization of invasive mediastinal staging is further complicated by the fact that patterns of invasive mediastinal staging are highly variable across hospitals staffed by at least one board-certified thoracic surgeon with a noncardiac practice,” Dr. Farjah explained. “This variability could be a marker of poor-quality care. However, because the guidelines are not supported by level 1 evidence, it’s equally plausible that this variability might represent uncertainty or even disagreement with the practice guidelines – and specifically about the appropriate indication for invasive staging.”
He presented a retrospective cohort study of 406 patients whose non–small cell lung cancer was resected during July 2011–December 2013 at one of five Washington hospitals, each with at least one board-certified thoracic surgeon with a noncardiac practice on staff. The four participating community hospitals and one academic medical center were involved in a National Cancer Institute–funded, physician-led quality improvement initiative.
Overall, 66% of the 406 patients underwent any form of invasive mediastinal staging: 85% by mediastinoscopy only; 12% by mediastinoscopy plus endobronchial ultrasound-guided nodal aspiration (EBUS); 3% by EBUS only; and the remaining handful by mediastinoscopy, EBUS, and esophageal ultrasound-guided nodal aspiration. The invasive staging was performed at the time of resection in 64% of cases. A median of three nodal stations were sampled.
After statistical adjustment for random variation and between-hospital differences in clinical stage, rates of invasive staging were all over the map. While an overall mean of 66% of the lung cancer patients underwent invasive mediastinal staging, the rates at the five hospitals were 94%, 84%, 31%, 80%, and 17%.
Dr. Farjah and his coinvestigators are now conducting provider interviews and focus groups in an effort to understand what drove the participating surgeons’ wide variability in performing invasive mediastinal staging.
Discussant Jane Yanagawa, MD, of the University of California, Los Angeles, commented, “I think this is a really interesting study because, historically, lower rates of mediastinoscopy are assumed to be a reflection of low-quality care – and you suggest that might not be the case, that it might be more complicated than that.”
Dr. Yanagawa sketched one fairly common scenario that might represent a surgeon’s reasonable avoidance of guideline-recommended invasive mediastinal staging: a patient who by all preoperative imaging appears to have stage IA lung cancer and wishes to avoid the morbidity, time, and cost of needle biopsy, instead choosing to go straight to the operating room for a diagnosis by wedge resection, followed by a completion lobectomy based upon the frozen section results. Could such a pathway account for the variability seen in the Washington study?
“I think it could have,” Dr. Farjah replied. “I would say that’s probably one driver of variability.”
As for the generalizability of the findings of a five-hospital study carried out in a single state, Dr. Farjah said he thinks the results are applicable to any academic or community hospital with at least one board-certified thoracic surgeon with a noncardiac practice.
He reported having no financial conflicts of interest regarding the study.
AT THE WTSA ANNUAL MEETING
Key clinical point:
Major finding: Rates of invasive mediastinal staging after adjustment for clinical stage ranged from a low of 17% at one hospital to as high as 94% at another.
Data source: This retrospective cohort study included 406 patients.
Disclosures: Dr. Farjah reported having no financial conflicts of interest.
Bailout stenting for coronary bifurcations brings ‘unacceptable’ hazards
PARIS – Bailout stenting during percutaneous coronary intervention for coronary bifurcations doubled the risk of major adverse cardiovascular events in the world’s largest registry of patients with these often-challenging lesions treated using bioactive stents, Marco Zimarino, MD, reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
Indeed, resort to bailout stenting stood out as the major potentially modifiable risk factor for adverse outcomes among the 4,306 participants in the P2BiTO registry, an international collaboration supported by members of the EuroBifurcation Club. Most of the other independent risk factors identified in a multivariate regression analysis of the P2BiTO database were beyond operator control, including diabetes, advanced age, and presentation with an acute coronary syndrome, according to Dr. Zimarino of the University of Chieti (Italy).
Bailout stenting is largely avoidable through meticulous procedural planning, the interventional cardiologist added.
“Careful planning is always mandatory because bailout stenting is associated with an unacceptably higher risk of both in-hospital and 1-year adverse outcomes,” Dr. Zimarino emphasized. “It’s much better to leave a degraded side branch instead of using bailout stenting to get an excellent angiographic outcome that’s a predictor of a worse clinical outcome.”
Conventional wisdom holds that single stenting of either the main artery or a side branch in a patient with coronary bifurcation is safer than double stenting of both. However, that wasn’t really borne out in the P2BiTO registry provided the operator’s plan was for double stenting. The difference in 1-year major adverse cardiovascular events (MACE) between patients treated using a single- or double-stenting strategy wasn’t statistically significant, provided bailout stenting wasn’t utilized. If bailout stenting was employed, though, the risk of MACE was 2.2-fold greater than if the cardiologist stuck with the plan.
Ninety-eight percent of patients in the P2BiTO registry received drug-eluting stents. The other 2% got the Absorb bioabsorbable vascular scaffold. The percutaneous coronary intervention access site, treatment strategy, choice of stent, and duration of dual-antiplatelet therapy were left up to the operator’s discretion.
The risk of MACE was reduced by 39% in patients on dual-antiplatelet therapy for 6-12 months, compared with less than 6 months.
Discussant Graham Cassel, MD, director of the heart transplant unit at Milpark Hospital in Johannesburg, commented, “The message comes through very clearly that, if you plan your procedure well, the chance of bailout is far less – and if you do have to bail out, the results are uniformly bad. If you can avoid putting in two or three stents, that’s beneficial.”
Dr. Zimarino reported having no financial conflicts of interest regarding his presentation.
PARIS – Bailout stenting during percutaneous coronary intervention for coronary bifurcations doubled the risk of major adverse cardiovascular events in the world’s largest registry of patients with these often-challenging lesions treated using bioactive stents, Marco Zimarino, MD, reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
Indeed, resort to bailout stenting stood out as the major potentially modifiable risk factor for adverse outcomes among the 4,306 participants in the P2BiTO registry, an international collaboration supported by members of the EuroBifurcation Club. Most of the other independent risk factors identified in a multivariate regression analysis of the P2BiTO database were beyond operator control, including diabetes, advanced age, and presentation with an acute coronary syndrome, according to Dr. Zimarino of the University of Chieti (Italy).
Bailout stenting is largely avoidable through meticulous procedural planning, the interventional cardiologist added.
“Careful planning is always mandatory because bailout stenting is associated with an unacceptably higher risk of both in-hospital and 1-year adverse outcomes,” Dr. Zimarino emphasized. “It’s much better to leave a degraded side branch instead of using bailout stenting to get an excellent angiographic outcome that’s a predictor of a worse clinical outcome.”
Conventional wisdom holds that single stenting of either the main artery or a side branch in a patient with coronary bifurcation is safer than double stenting of both. However, that wasn’t really borne out in the P2BiTO registry provided the operator’s plan was for double stenting. The difference in 1-year major adverse cardiovascular events (MACE) between patients treated using a single- or double-stenting strategy wasn’t statistically significant, provided bailout stenting wasn’t utilized. If bailout stenting was employed, though, the risk of MACE was 2.2-fold greater than if the cardiologist stuck with the plan.
Ninety-eight percent of patients in the P2BiTO registry received drug-eluting stents. The other 2% got the Absorb bioabsorbable vascular scaffold. The percutaneous coronary intervention access site, treatment strategy, choice of stent, and duration of dual-antiplatelet therapy were left up to the operator’s discretion.

The risk of MACE was reduced by 39% in patients on dual-antiplatelet therapy for 6-12 months, compared with less than 6 months.
Discussant Graham Cassel, MD, director of the heart transplant unit at Milpark Hospital in Johannesburg, commented, “The message comes through very clearly that, if you plan your procedure well, the chance of bailout is far less – and if you do have to bail out, the results are uniformly bad. If you can avoid putting in two or three stents, that’s beneficial.”
Dr. Zimarino reported having no financial conflicts of interest regarding his presentation.
PARIS – Bailout stenting during percutaneous coronary intervention for coronary bifurcations doubled the risk of major adverse cardiovascular events in the world’s largest registry of patients with these often-challenging lesions treated using bioactive stents, Marco Zimarino, MD, reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
Indeed, resort to bailout stenting stood out as the major potentially modifiable risk factor for adverse outcomes among the 4,306 participants in the P2BiTO registry, an international collaboration supported by members of the EuroBifurcation Club. Most of the other independent risk factors identified in a multivariate regression analysis of the P2BiTO database were beyond operator control, including diabetes, advanced age, and presentation with an acute coronary syndrome, according to Dr. Zimarino of the University of Chieti (Italy).
Bailout stenting is largely avoidable through meticulous procedural planning, the interventional cardiologist added.
“Careful planning is always mandatory because bailout stenting is associated with an unacceptably higher risk of both in-hospital and 1-year adverse outcomes,” Dr. Zimarino emphasized. “It’s much better to leave a degraded side branch instead of using bailout stenting to get an excellent angiographic outcome that’s a predictor of a worse clinical outcome.”
Conventional wisdom holds that single stenting of either the main artery or a side branch in a patient with coronary bifurcation is safer than double stenting of both. However, that wasn’t really borne out in the P2BiTO registry provided the operator’s plan was for double stenting. The difference in 1-year major adverse cardiovascular events (MACE) between patients treated using a single- or double-stenting strategy wasn’t statistically significant, provided bailout stenting wasn’t utilized. If bailout stenting was employed, though, the risk of MACE was 2.2-fold greater than if the cardiologist stuck with the plan.
Ninety-eight percent of patients in the P2BiTO registry received drug-eluting stents. The other 2% got the Absorb bioabsorbable vascular scaffold. The percutaneous coronary intervention access site, treatment strategy, choice of stent, and duration of dual-antiplatelet therapy were left up to the operator’s discretion.

The risk of MACE was reduced by 39% in patients on dual-antiplatelet therapy for 6-12 months, compared with less than 6 months.
Discussant Graham Cassel, MD, director of the heart transplant unit at Milpark Hospital in Johannesburg, commented, “The message comes through very clearly that, if you plan your procedure well, the chance of bailout is far less – and if you do have to bail out, the results are uniformly bad. If you can avoid putting in two or three stents, that’s beneficial.”
Dr. Zimarino reported having no financial conflicts of interest regarding his presentation.
AT EUROPCR
Key clinical point:
Major finding: Bailout stenting during PCI for coronary bifurcations doubles the risk of major adverse cardiovascular events.
Data source: The P2BiTO registry includes 4,306 patients who received one or more drug-eluting stents or bioabsorbable vascular scaffolds for treatment of coronary bifurcations.
Disclosures: The study presenter reported having no financial conflicts of interest.