User login
Annual echo an option for cardiac allograft vasculopathy screening
LAS VEGAS – The experience at one major heart transplantation center indicates that annual screening dobutamine stress echocardiography to detect cardiac allograft vasculopathy renders annual coronary angiography unnecessary.
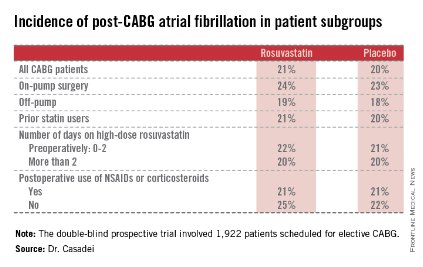
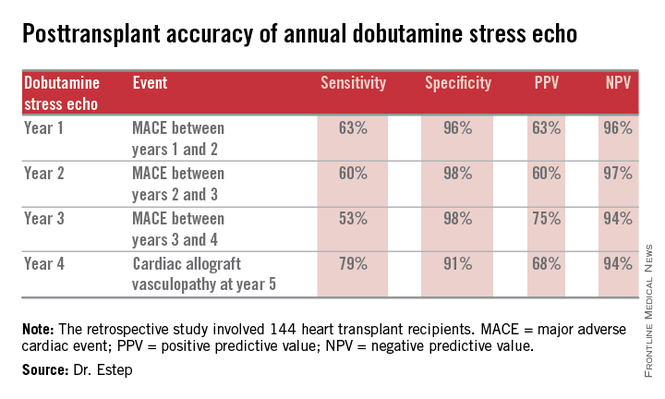
“This noninvasive method has very good specificity and is associated with a negative predictive value of 94%-97%. It can be used in our experience in lieu of annual invasive coronary angiography,” Dr. Jerry D. Estep declared at the annual meeting of the Heart Failure Society of America.
Cardiac allograft vasculopathy (CAV) is a unique, highly aggressive form of CAD. After 3 years post transplant it becomes the No. 1 cause of cardiac retransplantation and mortality. Guidelines recommend consideration of annual screening coronary angiography to detect it early to institute aggressive countermeasures. That’s the practice at most transplant centers.
However, at Houston Methodist Hospital, where Dr. Estep is medical director of the heart transplant and LVAD program, annual dobutamine stress echocardiography (DSE) is used instead. Because there is a scarcity of published data on this noninvasive alternative approach, he presented a retrospective study of the Houston transplant center’s experience over a recent 5-year period.
The study included 144 heart transplant recipients who underwent screening DSE for CAV annually for the first 4 years post transplant and coronary angiography at year 5.
During years 1-4, DSE detected CAV in 19% of patients. They didn’t differ in terms of baseline characteristics from those who remained free of this serious complication.
The good news: Ninety-four percent of patients with normal DSEs during years 1-4 had no CAV upon angiography at year 5. Moreover, the 5% who did have CAV at year 5 after earlier negative DSEs had mild to moderate disease.
The investigators documented the performance of annual screening DSE in predicting the development of major adverse cardiac events, defined as readmission for acute coronary syndrome, heart failure, revascularization, repeat heart transplantation, or cardiac death.
Dr. Estep reported having no financial conflicts regarding this study.
Dr. Hossein Almassi, FCCP, comments: Among solid organ transplants, cardiac transplant is rather unique in its need for invasive biopsy and angiography in following up the cardiac allograft. The search for noninvasive monitoring tools has been ongoing for a number of years. The report by the Houston group is a positive development in the right direction awaiting further confirmation by other cardiac transplant centers.
Dr. Almassi specializes in cardiothoracic surgery at the Medical College of Wisconsin in Milwaukee, Wisconsin.
Dr. Hossein Almassi, FCCP, comments: Among solid organ transplants, cardiac transplant is rather unique in its need for invasive biopsy and angiography in following up the cardiac allograft. The search for noninvasive monitoring tools has been ongoing for a number of years. The report by the Houston group is a positive development in the right direction awaiting further confirmation by other cardiac transplant centers.
Dr. Almassi specializes in cardiothoracic surgery at the Medical College of Wisconsin in Milwaukee, Wisconsin.
Dr. Hossein Almassi, FCCP, comments: Among solid organ transplants, cardiac transplant is rather unique in its need for invasive biopsy and angiography in following up the cardiac allograft. The search for noninvasive monitoring tools has been ongoing for a number of years. The report by the Houston group is a positive development in the right direction awaiting further confirmation by other cardiac transplant centers.
Dr. Almassi specializes in cardiothoracic surgery at the Medical College of Wisconsin in Milwaukee, Wisconsin.
LAS VEGAS – The experience at one major heart transplantation center indicates that annual screening dobutamine stress echocardiography to detect cardiac allograft vasculopathy renders annual coronary angiography unnecessary.
“This noninvasive method has very good specificity and is associated with a negative predictive value of 94%-97%. It can be used in our experience in lieu of annual invasive coronary angiography,” Dr. Jerry D. Estep declared at the annual meeting of the Heart Failure Society of America.
Cardiac allograft vasculopathy (CAV) is a unique, highly aggressive form of CAD. After 3 years post transplant it becomes the No. 1 cause of cardiac retransplantation and mortality. Guidelines recommend consideration of annual screening coronary angiography to detect it early to institute aggressive countermeasures. That’s the practice at most transplant centers.
However, at Houston Methodist Hospital, where Dr. Estep is medical director of the heart transplant and LVAD program, annual dobutamine stress echocardiography (DSE) is used instead. Because there is a scarcity of published data on this noninvasive alternative approach, he presented a retrospective study of the Houston transplant center’s experience over a recent 5-year period.
The study included 144 heart transplant recipients who underwent screening DSE for CAV annually for the first 4 years post transplant and coronary angiography at year 5.
During years 1-4, DSE detected CAV in 19% of patients. They didn’t differ in terms of baseline characteristics from those who remained free of this serious complication.
The good news: Ninety-four percent of patients with normal DSEs during years 1-4 had no CAV upon angiography at year 5. Moreover, the 5% who did have CAV at year 5 after earlier negative DSEs had mild to moderate disease.
The investigators documented the performance of annual screening DSE in predicting the development of major adverse cardiac events, defined as readmission for acute coronary syndrome, heart failure, revascularization, repeat heart transplantation, or cardiac death.
Dr. Estep reported having no financial conflicts regarding this study.

LAS VEGAS – The experience at one major heart transplantation center indicates that annual screening dobutamine stress echocardiography to detect cardiac allograft vasculopathy renders annual coronary angiography unnecessary.
“This noninvasive method has very good specificity and is associated with a negative predictive value of 94%-97%. It can be used in our experience in lieu of annual invasive coronary angiography,” Dr. Jerry D. Estep declared at the annual meeting of the Heart Failure Society of America.
Cardiac allograft vasculopathy (CAV) is a unique, highly aggressive form of CAD. After 3 years post transplant it becomes the No. 1 cause of cardiac retransplantation and mortality. Guidelines recommend consideration of annual screening coronary angiography to detect it early to institute aggressive countermeasures. That’s the practice at most transplant centers.
However, at Houston Methodist Hospital, where Dr. Estep is medical director of the heart transplant and LVAD program, annual dobutamine stress echocardiography (DSE) is used instead. Because there is a scarcity of published data on this noninvasive alternative approach, he presented a retrospective study of the Houston transplant center’s experience over a recent 5-year period.
The study included 144 heart transplant recipients who underwent screening DSE for CAV annually for the first 4 years post transplant and coronary angiography at year 5.
During years 1-4, DSE detected CAV in 19% of patients. They didn’t differ in terms of baseline characteristics from those who remained free of this serious complication.
The good news: Ninety-four percent of patients with normal DSEs during years 1-4 had no CAV upon angiography at year 5. Moreover, the 5% who did have CAV at year 5 after earlier negative DSEs had mild to moderate disease.
The investigators documented the performance of annual screening DSE in predicting the development of major adverse cardiac events, defined as readmission for acute coronary syndrome, heart failure, revascularization, repeat heart transplantation, or cardiac death.
Dr. Estep reported having no financial conflicts regarding this study.

AT THE HFSA ANNUAL SCIENTIFIC MEETING
Key clinical point: Annual dobutamine stress echocardiography to screen heart transplant recipients for cardiac allograft vasculopathy is an excellent noninvasive alternative to the widely used practice of annual screening coronary angiography.
Major finding: Annual screening dobutamine stress echo during years 1-4 after heart transplant had a 94% negative predictive value for cardiac allograft vasculopathy at year 5.
Data source: A retrospective study of 144 heart transplant recipients at a major transplant center where screening for cardiac allograft vasculopathy is done noninvasively by annual dobutamine stress echocardiography rather than angiography, which is widely used elsewhere.
Disclosures: The presenter reported having no conflicts relevant to the study, which was free of commercial support.
Vagal stimulation advances for heart failure
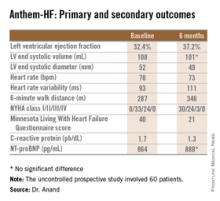
LAS VEGAS – A novel device-based approach to the treatment of heart failure via vagal nerve stimulation resulted in improvements in objectively measurable cardiac function as well as in subjective heart failure symptoms in the ANTHEM-HF trial.
The improvements seen in ANTHEM-HF (Autonomic Neural Regulation Therapy to Enhance Myocardial Function in Heart Failure) were similar regardless of whether participants were randomized to stimulation of the left or right vagal nerve. That’s an important finding because left-sided vagal nerve stimulation (VNS) technology could readily be combined with implantable cardioverter-defibrillators and cardiac resynchronization therapy devices, which are routinely placed on the left side of the thorax, Dr. Inder S. Anand observed in presenting the study findings at the annual meeting of the Heart Failure Society of America.
“We believe that if this technology pans out and proves effective, it will be introduced into devices very easily. That’s the advantage of left-sided stimulation,” explained Dr. Anand, professor of medicine at the University of Minnesota, Minneapolis.
He was quick to add, however, that “There needs to be a lot more work before autonomic regulation therapy is ready for prime time.” That’s because ANTHEM-HF was a relatively small prospective study – just 60 patients – and it was uncontrolled and unblinded.
Left-sided VNS is a well-established treatment for epilepsy, with more than 100,000 patients having received devices in the last several decades. It’s also seeing increasing use in refractory depression.
The approach is still investigational for heart failure, where autonomic imbalance with increased sympathetic activity and decreased parasympathetic tone is associated with heart failure progression and worse clinical outcomes, the cardiologist explained.
In ANTHEM-HF, 60 patients on optimal medical therapy for heart failure with reduced ejection fraction were randomized to left- or right-sided implantation of the Cyberonics VNS device. Once activated, the VNS intensity was titrated over 10 weeks to the maximum tolerable current that remained below the threshold of heart rate change, side effects, and patient sensation. The chronic intermittent stimulation to the vagus nerve was delivered at 10 Hz, a 250 microsec pulse width, and an average stimulation current of 2.0 mA. The stimulation cycle was 14 seconds on, 66 seconds off.
The primary study endpoint was change in LVEF over the course of 6 months. From a baseline of 32.4% it improved significantly to 37.2%, which Dr. Anand deemed “very impressive” for just 6 months of therapy. The improvement was similar regardless of whether the VNS was left or right sided.
He said the study didn’t raise any safety concerns. The only serious treatment-related adverse event was a fatal perioperative embolic stroke in a recipient of a left-sided device.
The most common adverse events were hoarseness or other voice alterations, which occurred in 19 patients. Thirteen patients reported a new cough. These side effects mirror the experience in patients treated with the VNS device for epilepsy or depression.
Discussant Jagmeet P. Singh emphasized that “it’s important to interpret the ANTHEM-HF results with some degree of caution.” That’s because the usual variability within LVEF measurements can be in the 5% range, and the 4.8% improvement seen in this study wasn’t accompanied by a significant improvement in LV end systolic volume. Moreover, the improvements seen in secondary endpoints – 6-minute walk distance, NYHA class, and the Minnesota Living With Heart Failure qualify-of-life score – are all potentially open to bias since neither patients nor physicians were blinded.
Dr. Singh noted that the NECTAR-HF study, a blinded, sham-controlled clinical trial of VNS presented earlier at the annual congress of the European Society of Cardiology, was a negative study that showed no improvement in functional measures. While NECTAR-HF caused some skeptics to question the whole concept of VNS as a useful therapeutic strategy, the stimulation protocols used in NECTAR-HF and ANTHEM-HF were quite different, and it’s likely that the stimulation amplitude employed in NECTAR-HF wasn’t sufficient to affect the target subset of vagal nerve fibers, according to Dr. Singh, director of the cardiac resynchronization therapy program and the Holter laboratory at Massachusetts General Hospital, Boston.
He considers the ANTHEM-HF finding that left-sided VNS is comparable to right to be an important advance that “really moves the field forward.” Yet many critical questions about autonomic regulation therapy for heart failure remain unanswered. These include whether it truly is safe and effective, and if so, the optimal target dose and frequency of stimulation. Answers should be forthcoming from the ongoing INOVATE-HF study, a randomized, controlled, 650-patient study, sponsored by BioControl Medical. The trial features hard clinical endpoints and higher target-dose stimulation protocols than in prior studies.
“This trial will probably put an end to the debate as to whether vagal nerve stimulation has an impact on heart failure or not,” the cardiologist predicted.
“Strategies to nonpharmacologically modulate the autonomic nervous system is an area of immense interest and investigation, and it’s going to be extended,” he observed. “I think that this is the autonomic era, and so it’s really important that we get it right. I think we’ve learned from the renal denervation experience that we need to be really careful in selecting patients for any form of autonomic manipulation. I don’t think we should select patients just off their LVEF. We should have some parameter that can quantify autonomic dysregulation prior to initiation of therapy.”
It will also be important to be able to individualize VNS therapy, just as physicians now do with beta blockers and other pharmacotherapies, he added.
The ANTHEM-HF trial was sponsored by Cyberonics. Dr. Anand reported serving as a consultant to and/or recipient of research grants from Cyberonics, Amgen, Critical Diagnostics, Novartis, and Zensun. Dr. Singh has received research support and/or served on speakers bureaus for Boston Scientific, St. Jude Medical, and the Sorin Group.
LAS VEGAS – A novel device-based approach to the treatment of heart failure via vagal nerve stimulation resulted in improvements in objectively measurable cardiac function as well as in subjective heart failure symptoms in the ANTHEM-HF trial.
The improvements seen in ANTHEM-HF (Autonomic Neural Regulation Therapy to Enhance Myocardial Function in Heart Failure) were similar regardless of whether participants were randomized to stimulation of the left or right vagal nerve. That’s an important finding because left-sided vagal nerve stimulation (VNS) technology could readily be combined with implantable cardioverter-defibrillators and cardiac resynchronization therapy devices, which are routinely placed on the left side of the thorax, Dr. Inder S. Anand observed in presenting the study findings at the annual meeting of the Heart Failure Society of America.
“We believe that if this technology pans out and proves effective, it will be introduced into devices very easily. That’s the advantage of left-sided stimulation,” explained Dr. Anand, professor of medicine at the University of Minnesota, Minneapolis.
He was quick to add, however, that “There needs to be a lot more work before autonomic regulation therapy is ready for prime time.” That’s because ANTHEM-HF was a relatively small prospective study – just 60 patients – and it was uncontrolled and unblinded.
Left-sided VNS is a well-established treatment for epilepsy, with more than 100,000 patients having received devices in the last several decades. It’s also seeing increasing use in refractory depression.
The approach is still investigational for heart failure, where autonomic imbalance with increased sympathetic activity and decreased parasympathetic tone is associated with heart failure progression and worse clinical outcomes, the cardiologist explained.
In ANTHEM-HF, 60 patients on optimal medical therapy for heart failure with reduced ejection fraction were randomized to left- or right-sided implantation of the Cyberonics VNS device. Once activated, the VNS intensity was titrated over 10 weeks to the maximum tolerable current that remained below the threshold of heart rate change, side effects, and patient sensation. The chronic intermittent stimulation to the vagus nerve was delivered at 10 Hz, a 250 microsec pulse width, and an average stimulation current of 2.0 mA. The stimulation cycle was 14 seconds on, 66 seconds off.
The primary study endpoint was change in LVEF over the course of 6 months. From a baseline of 32.4% it improved significantly to 37.2%, which Dr. Anand deemed “very impressive” for just 6 months of therapy. The improvement was similar regardless of whether the VNS was left or right sided.
He said the study didn’t raise any safety concerns. The only serious treatment-related adverse event was a fatal perioperative embolic stroke in a recipient of a left-sided device.
The most common adverse events were hoarseness or other voice alterations, which occurred in 19 patients. Thirteen patients reported a new cough. These side effects mirror the experience in patients treated with the VNS device for epilepsy or depression.
Discussant Jagmeet P. Singh emphasized that “it’s important to interpret the ANTHEM-HF results with some degree of caution.” That’s because the usual variability within LVEF measurements can be in the 5% range, and the 4.8% improvement seen in this study wasn’t accompanied by a significant improvement in LV end systolic volume. Moreover, the improvements seen in secondary endpoints – 6-minute walk distance, NYHA class, and the Minnesota Living With Heart Failure qualify-of-life score – are all potentially open to bias since neither patients nor physicians were blinded.
Dr. Singh noted that the NECTAR-HF study, a blinded, sham-controlled clinical trial of VNS presented earlier at the annual congress of the European Society of Cardiology, was a negative study that showed no improvement in functional measures. While NECTAR-HF caused some skeptics to question the whole concept of VNS as a useful therapeutic strategy, the stimulation protocols used in NECTAR-HF and ANTHEM-HF were quite different, and it’s likely that the stimulation amplitude employed in NECTAR-HF wasn’t sufficient to affect the target subset of vagal nerve fibers, according to Dr. Singh, director of the cardiac resynchronization therapy program and the Holter laboratory at Massachusetts General Hospital, Boston.
He considers the ANTHEM-HF finding that left-sided VNS is comparable to right to be an important advance that “really moves the field forward.” Yet many critical questions about autonomic regulation therapy for heart failure remain unanswered. These include whether it truly is safe and effective, and if so, the optimal target dose and frequency of stimulation. Answers should be forthcoming from the ongoing INOVATE-HF study, a randomized, controlled, 650-patient study, sponsored by BioControl Medical. The trial features hard clinical endpoints and higher target-dose stimulation protocols than in prior studies.
“This trial will probably put an end to the debate as to whether vagal nerve stimulation has an impact on heart failure or not,” the cardiologist predicted.
“Strategies to nonpharmacologically modulate the autonomic nervous system is an area of immense interest and investigation, and it’s going to be extended,” he observed. “I think that this is the autonomic era, and so it’s really important that we get it right. I think we’ve learned from the renal denervation experience that we need to be really careful in selecting patients for any form of autonomic manipulation. I don’t think we should select patients just off their LVEF. We should have some parameter that can quantify autonomic dysregulation prior to initiation of therapy.”
It will also be important to be able to individualize VNS therapy, just as physicians now do with beta blockers and other pharmacotherapies, he added.
The ANTHEM-HF trial was sponsored by Cyberonics. Dr. Anand reported serving as a consultant to and/or recipient of research grants from Cyberonics, Amgen, Critical Diagnostics, Novartis, and Zensun. Dr. Singh has received research support and/or served on speakers bureaus for Boston Scientific, St. Jude Medical, and the Sorin Group.
LAS VEGAS – A novel device-based approach to the treatment of heart failure via vagal nerve stimulation resulted in improvements in objectively measurable cardiac function as well as in subjective heart failure symptoms in the ANTHEM-HF trial.
The improvements seen in ANTHEM-HF (Autonomic Neural Regulation Therapy to Enhance Myocardial Function in Heart Failure) were similar regardless of whether participants were randomized to stimulation of the left or right vagal nerve. That’s an important finding because left-sided vagal nerve stimulation (VNS) technology could readily be combined with implantable cardioverter-defibrillators and cardiac resynchronization therapy devices, which are routinely placed on the left side of the thorax, Dr. Inder S. Anand observed in presenting the study findings at the annual meeting of the Heart Failure Society of America.
“We believe that if this technology pans out and proves effective, it will be introduced into devices very easily. That’s the advantage of left-sided stimulation,” explained Dr. Anand, professor of medicine at the University of Minnesota, Minneapolis.
He was quick to add, however, that “There needs to be a lot more work before autonomic regulation therapy is ready for prime time.” That’s because ANTHEM-HF was a relatively small prospective study – just 60 patients – and it was uncontrolled and unblinded.
Left-sided VNS is a well-established treatment for epilepsy, with more than 100,000 patients having received devices in the last several decades. It’s also seeing increasing use in refractory depression.
The approach is still investigational for heart failure, where autonomic imbalance with increased sympathetic activity and decreased parasympathetic tone is associated with heart failure progression and worse clinical outcomes, the cardiologist explained.
In ANTHEM-HF, 60 patients on optimal medical therapy for heart failure with reduced ejection fraction were randomized to left- or right-sided implantation of the Cyberonics VNS device. Once activated, the VNS intensity was titrated over 10 weeks to the maximum tolerable current that remained below the threshold of heart rate change, side effects, and patient sensation. The chronic intermittent stimulation to the vagus nerve was delivered at 10 Hz, a 250 microsec pulse width, and an average stimulation current of 2.0 mA. The stimulation cycle was 14 seconds on, 66 seconds off.
The primary study endpoint was change in LVEF over the course of 6 months. From a baseline of 32.4% it improved significantly to 37.2%, which Dr. Anand deemed “very impressive” for just 6 months of therapy. The improvement was similar regardless of whether the VNS was left or right sided.
He said the study didn’t raise any safety concerns. The only serious treatment-related adverse event was a fatal perioperative embolic stroke in a recipient of a left-sided device.
The most common adverse events were hoarseness or other voice alterations, which occurred in 19 patients. Thirteen patients reported a new cough. These side effects mirror the experience in patients treated with the VNS device for epilepsy or depression.
Discussant Jagmeet P. Singh emphasized that “it’s important to interpret the ANTHEM-HF results with some degree of caution.” That’s because the usual variability within LVEF measurements can be in the 5% range, and the 4.8% improvement seen in this study wasn’t accompanied by a significant improvement in LV end systolic volume. Moreover, the improvements seen in secondary endpoints – 6-minute walk distance, NYHA class, and the Minnesota Living With Heart Failure qualify-of-life score – are all potentially open to bias since neither patients nor physicians were blinded.
Dr. Singh noted that the NECTAR-HF study, a blinded, sham-controlled clinical trial of VNS presented earlier at the annual congress of the European Society of Cardiology, was a negative study that showed no improvement in functional measures. While NECTAR-HF caused some skeptics to question the whole concept of VNS as a useful therapeutic strategy, the stimulation protocols used in NECTAR-HF and ANTHEM-HF were quite different, and it’s likely that the stimulation amplitude employed in NECTAR-HF wasn’t sufficient to affect the target subset of vagal nerve fibers, according to Dr. Singh, director of the cardiac resynchronization therapy program and the Holter laboratory at Massachusetts General Hospital, Boston.
He considers the ANTHEM-HF finding that left-sided VNS is comparable to right to be an important advance that “really moves the field forward.” Yet many critical questions about autonomic regulation therapy for heart failure remain unanswered. These include whether it truly is safe and effective, and if so, the optimal target dose and frequency of stimulation. Answers should be forthcoming from the ongoing INOVATE-HF study, a randomized, controlled, 650-patient study, sponsored by BioControl Medical. The trial features hard clinical endpoints and higher target-dose stimulation protocols than in prior studies.
“This trial will probably put an end to the debate as to whether vagal nerve stimulation has an impact on heart failure or not,” the cardiologist predicted.
“Strategies to nonpharmacologically modulate the autonomic nervous system is an area of immense interest and investigation, and it’s going to be extended,” he observed. “I think that this is the autonomic era, and so it’s really important that we get it right. I think we’ve learned from the renal denervation experience that we need to be really careful in selecting patients for any form of autonomic manipulation. I don’t think we should select patients just off their LVEF. We should have some parameter that can quantify autonomic dysregulation prior to initiation of therapy.”
It will also be important to be able to individualize VNS therapy, just as physicians now do with beta blockers and other pharmacotherapies, he added.
The ANTHEM-HF trial was sponsored by Cyberonics. Dr. Anand reported serving as a consultant to and/or recipient of research grants from Cyberonics, Amgen, Critical Diagnostics, Novartis, and Zensun. Dr. Singh has received research support and/or served on speakers bureaus for Boston Scientific, St. Jude Medical, and the Sorin Group.
AT THE HFSA ANNUAL SCIENTIFIC MEETING
Key clinical point: Autonomic regulation therapy via a vagal nerve stimulation device shows promise for the treatment of heart failure with reduced ejection fraction.
Major finding: During 6 months of vagal nerve stimulation, patients’ mean LVEF rose significantly from 32.4% to 37.2%, with similar results regardless of whether the stimulation was left- or right-sided.
Data source: The ANTHEM-HF study was a prospective, unblinded, and uncontrolled study involving 60 patients who received vagal nerve stimulation for 6 months.
Disclosures: The study was sponsored by Cyberonics. The presenter has received research grants from and served as a consultant to that and other companies.
Bioprosthetic aortic valves may be a reasonable choice in younger patients
No differences were seen in the 15-year stroke or survival rates in more than 4,000 propensity-matched patients in the age range of 50-69 years who received either a mechanical or a bioprosthetic aortic valve replacement, according to the results of a retrospective cohort analysis.
However, valves differed in two other major complications, according to a report published in the Oct. 1 issue of JAMA. Mechanical valve patients had a significantly higher 15-year cumulative incidence of bleeding compared with the bioprosthetic valve group, but had a significantly lower 15-year cumulative incidence of reoperation, according to Yuting P. Chiang and colleagues at the Icahn School of Medicine at Mount Sinai and Mount Sinai Medical Center, New York.
This study calls into question current practice guidelines, which recommend either a bioprosthetic or mechanical prosthetic aortic valve in patients aged 60-70 years, and a mechanical valve in patients younger than 60 years in the absence of a contraindication to warfarin (Coumadin), according to the researchers.
From an original population of more than 10,000 patients aged 50-69 with valve replacement, 4,253 remained after researchers applied exclusionary criteria such as prior valve repair and coronary artery bypass grafting. Of these, 1,466 (34.5%) received a bioprosthetic aortic valve replacement, and 2,787 received a mechanical valve.
Median follow-up was 10.6 and 10.9 years, respectively. Propensity scoring matching produced 1,001 patient pairs. After propensity matching, age and all baseline comorbidities were balanced between the two groups.
Overall, there were no differences in 30-day mortality in the matched groups (3% in both), nor in any other short-term outcome (JAMA 2014;312:1323-29).
In terms of long-term survival, there were no differences seen in the matched cohort, with an actuarial 15-year survival of 60.6% for the prosthetic valve group, and 62.1% for the mechanical valve group (P = .74).
Similarly, there was no significant difference in the cumulative 15-year stroke rate (7.7% for the bioprosthetic group, 8.6% for the mechanical group, P = .84). The 30-day mortality rate after stroke was 18.7%.
There was a significantly higher rate of aortic valve reoperation in the bioprosthetic group (cumulative 15-year incidence of 12.1%) compared with the mechanical group (cumulative incidence of 6.9%, P = .001). The 30-day mortality rate after aortic valve reoperation was 9.0%.
In contrast, cumulative major bleeding over 15 years was significantly higher in the mechanical group (13.0%), than in the bioprosthetic group (6.6%, P = .001). Thirty-day mortality after a major bleeding event was 13.2%.
In discussing the differences between this study and the randomized clinical trials that contributed to the class IIa recommendations on age-based valve choice, the researchers pointed out that two of the studies “enrolled patients nearly 4 decades ago and evaluated prostheses since superseded by more durable and less thrombogenic models.”
The analysis “supports the view that either prosthesis is a reasonable choice in patients aged 60 to 69 years and suggests that this recommendation could reasonably be extended to patients aged 50 to 59 years,” they concluded.
Potential problems inherent in a database analysis and the tendency for deaths at a younger age to be missing from the Social Security Death Master File were limitations of the study. Younger patients were more likely to receive a mechanical valve.
Mr. Chiang reported a research stipend from the Icahn School of Medicine, which receives royalty payments from Edwards Lifesciences and Medtronic related to two mitral valve and 1 tricuspid valve ring that Dr. David H. Adams, a coauthor, helped develop. Dr. Adams is coprincipal investigator for the CoreValve United States pivotal trial, supported by Medtronic.
No differences were seen in the 15-year stroke or survival rates in more than 4,000 propensity-matched patients in the age range of 50-69 years who received either a mechanical or a bioprosthetic aortic valve replacement, according to the results of a retrospective cohort analysis.
However, valves differed in two other major complications, according to a report published in the Oct. 1 issue of JAMA. Mechanical valve patients had a significantly higher 15-year cumulative incidence of bleeding compared with the bioprosthetic valve group, but had a significantly lower 15-year cumulative incidence of reoperation, according to Yuting P. Chiang and colleagues at the Icahn School of Medicine at Mount Sinai and Mount Sinai Medical Center, New York.
This study calls into question current practice guidelines, which recommend either a bioprosthetic or mechanical prosthetic aortic valve in patients aged 60-70 years, and a mechanical valve in patients younger than 60 years in the absence of a contraindication to warfarin (Coumadin), according to the researchers.
From an original population of more than 10,000 patients aged 50-69 with valve replacement, 4,253 remained after researchers applied exclusionary criteria such as prior valve repair and coronary artery bypass grafting. Of these, 1,466 (34.5%) received a bioprosthetic aortic valve replacement, and 2,787 received a mechanical valve.
Median follow-up was 10.6 and 10.9 years, respectively. Propensity scoring matching produced 1,001 patient pairs. After propensity matching, age and all baseline comorbidities were balanced between the two groups.
Overall, there were no differences in 30-day mortality in the matched groups (3% in both), nor in any other short-term outcome (JAMA 2014;312:1323-29).
In terms of long-term survival, there were no differences seen in the matched cohort, with an actuarial 15-year survival of 60.6% for the prosthetic valve group, and 62.1% for the mechanical valve group (P = .74).
Similarly, there was no significant difference in the cumulative 15-year stroke rate (7.7% for the bioprosthetic group, 8.6% for the mechanical group, P = .84). The 30-day mortality rate after stroke was 18.7%.
There was a significantly higher rate of aortic valve reoperation in the bioprosthetic group (cumulative 15-year incidence of 12.1%) compared with the mechanical group (cumulative incidence of 6.9%, P = .001). The 30-day mortality rate after aortic valve reoperation was 9.0%.
In contrast, cumulative major bleeding over 15 years was significantly higher in the mechanical group (13.0%), than in the bioprosthetic group (6.6%, P = .001). Thirty-day mortality after a major bleeding event was 13.2%.
In discussing the differences between this study and the randomized clinical trials that contributed to the class IIa recommendations on age-based valve choice, the researchers pointed out that two of the studies “enrolled patients nearly 4 decades ago and evaluated prostheses since superseded by more durable and less thrombogenic models.”
The analysis “supports the view that either prosthesis is a reasonable choice in patients aged 60 to 69 years and suggests that this recommendation could reasonably be extended to patients aged 50 to 59 years,” they concluded.
Potential problems inherent in a database analysis and the tendency for deaths at a younger age to be missing from the Social Security Death Master File were limitations of the study. Younger patients were more likely to receive a mechanical valve.
Mr. Chiang reported a research stipend from the Icahn School of Medicine, which receives royalty payments from Edwards Lifesciences and Medtronic related to two mitral valve and 1 tricuspid valve ring that Dr. David H. Adams, a coauthor, helped develop. Dr. Adams is coprincipal investigator for the CoreValve United States pivotal trial, supported by Medtronic.
No differences were seen in the 15-year stroke or survival rates in more than 4,000 propensity-matched patients in the age range of 50-69 years who received either a mechanical or a bioprosthetic aortic valve replacement, according to the results of a retrospective cohort analysis.
However, valves differed in two other major complications, according to a report published in the Oct. 1 issue of JAMA. Mechanical valve patients had a significantly higher 15-year cumulative incidence of bleeding compared with the bioprosthetic valve group, but had a significantly lower 15-year cumulative incidence of reoperation, according to Yuting P. Chiang and colleagues at the Icahn School of Medicine at Mount Sinai and Mount Sinai Medical Center, New York.
This study calls into question current practice guidelines, which recommend either a bioprosthetic or mechanical prosthetic aortic valve in patients aged 60-70 years, and a mechanical valve in patients younger than 60 years in the absence of a contraindication to warfarin (Coumadin), according to the researchers.
From an original population of more than 10,000 patients aged 50-69 with valve replacement, 4,253 remained after researchers applied exclusionary criteria such as prior valve repair and coronary artery bypass grafting. Of these, 1,466 (34.5%) received a bioprosthetic aortic valve replacement, and 2,787 received a mechanical valve.
Median follow-up was 10.6 and 10.9 years, respectively. Propensity scoring matching produced 1,001 patient pairs. After propensity matching, age and all baseline comorbidities were balanced between the two groups.
Overall, there were no differences in 30-day mortality in the matched groups (3% in both), nor in any other short-term outcome (JAMA 2014;312:1323-29).
In terms of long-term survival, there were no differences seen in the matched cohort, with an actuarial 15-year survival of 60.6% for the prosthetic valve group, and 62.1% for the mechanical valve group (P = .74).
Similarly, there was no significant difference in the cumulative 15-year stroke rate (7.7% for the bioprosthetic group, 8.6% for the mechanical group, P = .84). The 30-day mortality rate after stroke was 18.7%.
There was a significantly higher rate of aortic valve reoperation in the bioprosthetic group (cumulative 15-year incidence of 12.1%) compared with the mechanical group (cumulative incidence of 6.9%, P = .001). The 30-day mortality rate after aortic valve reoperation was 9.0%.
In contrast, cumulative major bleeding over 15 years was significantly higher in the mechanical group (13.0%), than in the bioprosthetic group (6.6%, P = .001). Thirty-day mortality after a major bleeding event was 13.2%.
In discussing the differences between this study and the randomized clinical trials that contributed to the class IIa recommendations on age-based valve choice, the researchers pointed out that two of the studies “enrolled patients nearly 4 decades ago and evaluated prostheses since superseded by more durable and less thrombogenic models.”
The analysis “supports the view that either prosthesis is a reasonable choice in patients aged 60 to 69 years and suggests that this recommendation could reasonably be extended to patients aged 50 to 59 years,” they concluded.
Potential problems inherent in a database analysis and the tendency for deaths at a younger age to be missing from the Social Security Death Master File were limitations of the study. Younger patients were more likely to receive a mechanical valve.
Mr. Chiang reported a research stipend from the Icahn School of Medicine, which receives royalty payments from Edwards Lifesciences and Medtronic related to two mitral valve and 1 tricuspid valve ring that Dr. David H. Adams, a coauthor, helped develop. Dr. Adams is coprincipal investigator for the CoreValve United States pivotal trial, supported by Medtronic.
FROM JAMA
Key clinical point: Bioprosthetic and mechanical aortic valves were equivalent in stroke and survival rates across propensity-matched patients aged 50-69 years.
Major finding: Actuarial 15-year survival was 60.6% for bioprosthetic valves and 62.1% for mechanical valves.
Data source: A retrospective cohort analysis of 1,001 propensity-matched patient pairs who had a mechanical or bioprosthetic aortic valve replacement in New York State from 1997 through 2004.
Disclosures: Mr. Chiang reported a research stipend from the Icahn School of Medicine, which receives royalty payments from Edwards Lifesciences and Medtronic related to two mitral valve and 1 tricuspid valve ring that Dr. David H. Adams, a coauthor, helped develop. Dr. Adams is coprincipal investigator for the CoreValve United States pivotal trial, supported by Medtronic.
Fenoldopam missed renal endpoint, caused hypotension in cardiac surgery patients
In its largest randomized controlled trial to date, fenoldopam did not lessen the need for dialysis after cardiac surgery and caused significantly more hypotension than did placebo, investigators reported at the annual congress of the European Society of Intensive Care Medicine.
Acute kidney injury is a common complication of cardiac surgery, and no drugs are known to effectively treat it, said Dr. Tiziana Bove of IRCCS San Raffaele Scientific Institute in Milan. “Given the cost of fenoldopam, the lack of effectiveness, and the increased incidence of hypotension, the use of this agent for renal protection in these patients is not justified,” said Dr. Bove and her associates.
The findings were published in JAMA simultaneously with the presentation at the congress ( 2014 Sept 29 [doi: 10.1001/jama.2014.13573]).
Fenoldopam is a selective dopamine receptor D1 agonist and vasodilator. For the study, 667 patients who had developed early acute kidney injury after cardiac surgery received either an intravenous continuous infusion of fenoldopam at a starting dose of 0.1 mcg/kg per minute or placebo, the investigators said. Rates of dialysis and 30-day mortality were similar between the two groups. In all, 20% of the treatment group received renal replacement therapy, compared with 18% of the placebo group, and 30-day mortality rates were 23% and 22%, respectively, they said. Furthermore, hypotension developed in 26% of treated patients, compared with 15% of the placebo group (P = .001), the researchers said. The study was stopped for futility after an interim analysis, the researchers noted.The Italian Ministry of Health funded the study. Teva supplied the study drug. The authors reported no conflicts of interest.
In its largest randomized controlled trial to date, fenoldopam did not lessen the need for dialysis after cardiac surgery and caused significantly more hypotension than did placebo, investigators reported at the annual congress of the European Society of Intensive Care Medicine.
Acute kidney injury is a common complication of cardiac surgery, and no drugs are known to effectively treat it, said Dr. Tiziana Bove of IRCCS San Raffaele Scientific Institute in Milan. “Given the cost of fenoldopam, the lack of effectiveness, and the increased incidence of hypotension, the use of this agent for renal protection in these patients is not justified,” said Dr. Bove and her associates.
The findings were published in JAMA simultaneously with the presentation at the congress ( 2014 Sept 29 [doi: 10.1001/jama.2014.13573]).
Fenoldopam is a selective dopamine receptor D1 agonist and vasodilator. For the study, 667 patients who had developed early acute kidney injury after cardiac surgery received either an intravenous continuous infusion of fenoldopam at a starting dose of 0.1 mcg/kg per minute or placebo, the investigators said. Rates of dialysis and 30-day mortality were similar between the two groups. In all, 20% of the treatment group received renal replacement therapy, compared with 18% of the placebo group, and 30-day mortality rates were 23% and 22%, respectively, they said. Furthermore, hypotension developed in 26% of treated patients, compared with 15% of the placebo group (P = .001), the researchers said. The study was stopped for futility after an interim analysis, the researchers noted.The Italian Ministry of Health funded the study. Teva supplied the study drug. The authors reported no conflicts of interest.
In its largest randomized controlled trial to date, fenoldopam did not lessen the need for dialysis after cardiac surgery and caused significantly more hypotension than did placebo, investigators reported at the annual congress of the European Society of Intensive Care Medicine.
Acute kidney injury is a common complication of cardiac surgery, and no drugs are known to effectively treat it, said Dr. Tiziana Bove of IRCCS San Raffaele Scientific Institute in Milan. “Given the cost of fenoldopam, the lack of effectiveness, and the increased incidence of hypotension, the use of this agent for renal protection in these patients is not justified,” said Dr. Bove and her associates.
The findings were published in JAMA simultaneously with the presentation at the congress ( 2014 Sept 29 [doi: 10.1001/jama.2014.13573]).
Fenoldopam is a selective dopamine receptor D1 agonist and vasodilator. For the study, 667 patients who had developed early acute kidney injury after cardiac surgery received either an intravenous continuous infusion of fenoldopam at a starting dose of 0.1 mcg/kg per minute or placebo, the investigators said. Rates of dialysis and 30-day mortality were similar between the two groups. In all, 20% of the treatment group received renal replacement therapy, compared with 18% of the placebo group, and 30-day mortality rates were 23% and 22%, respectively, they said. Furthermore, hypotension developed in 26% of treated patients, compared with 15% of the placebo group (P = .001), the researchers said. The study was stopped for futility after an interim analysis, the researchers noted.The Italian Ministry of Health funded the study. Teva supplied the study drug. The authors reported no conflicts of interest.
Key clinical point: Fenoldopam did not lessen early kidney injury after cardiac surgery and caused hypotension.
Major finding: Fenoldopam did not reduce the rate of dialysis or 30-day mortality after cardiac surgery, and 26% of treated patients developed hypotension compared with 15% of the placebo group (P = .001).
Data source: Randomized, double-blind, multicenter trial of 667 patients with early acute kidney injury after cardiac surgery.
Disclosures: The Italian Ministry of Health funded the study. Teva supplied the study drug. The authors reported no conflicts of interest.
High-dose statins don’t prevent postop AF
BARCELONA – Intensive perioperative statin therapy in patients undergoing CABG surgery doesn’t protect against postop atrial fibrillation or myocardial injury, according to a large randomized clinical trial hailed as the "definitive" study addressing this issue.
"There are many reasons why these patients should be put on statin treatment, but the prevention of postop complications is not one of them," Dr. Barbara Casadei said in presenting the findings of the Statin Therapy in Cardiac Surgery (STICS) trial at the annual congress of the European Society of Cardiology.
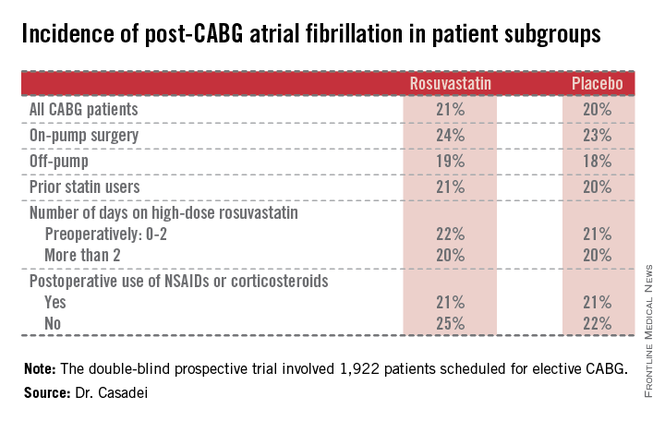
The STICS results are at odds with conventional wisdom. ESC guidelines give a favorable class IIa, level of evidence B recommendation that "statins should be considered for prevention of new-onset atrial fibrillation after coronary artery bypass grafting, either isolated or in combination with valvular interventions."
"STICS was a very carefully conducted, large scale, robust study that I think has definitely closed the door on this issue," commented Dr. Keith A.A. Fox, professor of cardiology at the University of Edinburgh and chair of the scientific and clinical program committee at ESC Congress 2014.
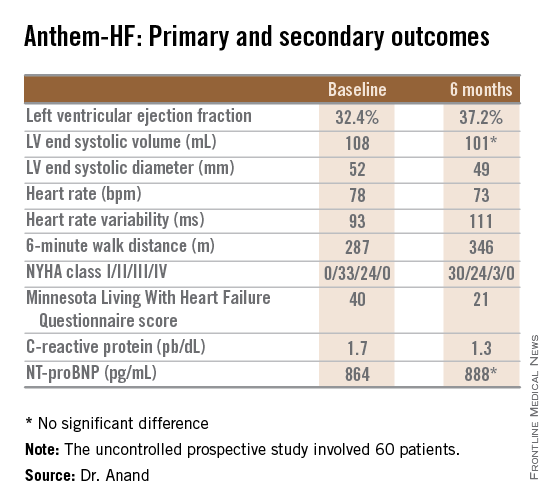
STICS was a double-blind prospective trial in which 1,922 patients scheduled for elective CABG were randomized to 20 mg per day of rosuvastatin (Crestor) or placebo starting up to 8 days prior to surgery and continued for 5 days postop. All participants were in sinus rhythm preoperatively, with no history of AF, said Dr. Casadei, professor of cardiovascular medicine at the University of Oxford, England.
The two coprimary endpoints in STICS were the incidence of new-onset AF during 5 days of postop Holter monitoring, and evidence of postop myocardial injury as demonstrated in serial troponin I assays.
Postop AF occurred in 21% of those given high-intensity therapy with rosuvastatin and 20% of placebo-treated controls. There was no subgroup where rosuvastatin was protective (see graphic).
Troponin I measurements obtained 6, 24, 48, and 120 hours postop showed areas under the curve that were superimposable in the two study groups, meaning perioperative high-dose statin therapy provided absolutely no protection against postop cardiac muscle injury.
Mean hospital length of stay and ICU time didn’t differ between the two groups, either.
The impetus for conducting STICS was recognition that the guidelines’ endorsement of perioperative high-dose statin therapy in conjunction with cardiac surgery was based upon a series of small randomized trials with serious limitations. Although the results of a meta-analysis of the 14 prior trials looked impressive at first glance – a 17% incidence of postop AF in statin-treated patients, compared with 30% in controls, for a near-halving of the risk of this important complication – these 14 studies totaled 1,300 patients, and there were many methodologic shortcomings.
The STICS researchers hypothesized that a large, well-designed trial – bigger than all previous studies combined – would shore up the previously shaky supporting evidence and perhaps provide grounds for statins to win a new indication from regulatory agencies. Post-CABG AF is associated with a doubled risk of stroke and mortality, and excess hospital costs of $8,000-$18,000 dollars per patient.
Discussant Dr. Paulus Kirchhof, a member of the task force that developed the current ESC guidelines (Europace 2010;12:1360-420), said those guidelines now clearly need to be revisited. Beyond that, he added, STICS provides important new contributions in understanding the pathophysiology of AF.
"We know that AF is caused by several vicious circles, and we believe that inflammation could influence those and cause AF. And we also thought that postop AF was the condition where inflammation plays the biggest role. Based upon the negative results with this anti-inflammatory intervention, I think we have to question this concept a bit," said Dr. Kirchhof, professor of cardiovascular sciences at the University of Birmingham, England.
Dr. Casadei countered that she’s not ready to write off postop inflammation entirely as a major trigger of new-onset AF following CABG.
"The inflammation is there. We know from experimental work in animals that there is a strong association between inflammation and postop atrial fibrillation, but whether the association is causal, I think, is still debated. However, it may be that the anti-inflammatory effect of statins is not sufficiently strong to actually prevent this complication," she said.
Discussant Dr. Steven Nissen praised STICS as "an outstanding trial."
"I also think there’s a terribly important lesson here, which is the power of self-delusion in medicine. When we base our guidelines on small, poorly controlled trials, we are often making mistakes. This is one of countless examples where when someone finally does a careful, thoughtful trial, we find out that something that people believe just isn’t true. We can’t cut corners with evidence. We need good randomized trials," declared Dr. Nissen, chair of the department of cardiovascular medicine at the Cleveland Clinic.
The STICS trial was funded primarily by the British Heart Foundation, the Oxford Biomedical Research Center, and the UK Medical Research Council. In addition, Dr. Casadei reported receiving an unrestricted grant from AstraZeneca in conjunction with the trial.

|
| Dr. Hiren Shah |
There are two key lessons from the results of the STICS trial. First, extrapolation of results from biochemical pathways and measured cellular markers does not always translate into meaningful clinical outcomes. Thus, it has long been known from several large trials that statin therapy effectively and rapidly lowers CRP levels both in hyper- and normocholesterolemic patients and that statins are effective in decreasing systemic inflammation. It has also been known that inflammation contributes to the development and maintenance of AF, so it was postulated that by improving endothelial nitric oxide availability, reducing inflammation, and decreasing oxidative stress, and through neurohormonal activation, statins would reduce the incidence of post-op AF. This link was so strong that clinical guidelines adopted limited data from small trials to make treatment recommendations.
This leads us to consider the second key lesson from this study. Trials with small sample size, even when combined across many other trials (1,300 patients were involved across 14 trials in this case), do not always yield reliable results, especially when they have significant limitations, notably not always being blind and having been performed in statin-naive patients only. The large, randomized, and well-designed STICS trial puts to rest an important issue, given the high prevalence of AF after cardiac surgery, which is associated with a longer length of stay, an increased risk of stroke, higher mortality, and greater costs, and should prompt us to consider further evaluation of different strategies to reduce this significant complication.
Dr. Hiren Shah is medical director of the medicine and cardiac telemetry hospitalist unit at Northwestern Memorial Hospital in Chicago and an adviser to Hospitalist News. He is the national chair of the Clinician Committee for ACP’s Initiative on Stroke Prevention and Atrial Fibrillation and is the lead physician for the Society of Hospital Medicine’s National Atrial Fibrillation Initiative.

|
| Dr. Hiren Shah |
There are two key lessons from the results of the STICS trial. First, extrapolation of results from biochemical pathways and measured cellular markers does not always translate into meaningful clinical outcomes. Thus, it has long been known from several large trials that statin therapy effectively and rapidly lowers CRP levels both in hyper- and normocholesterolemic patients and that statins are effective in decreasing systemic inflammation. It has also been known that inflammation contributes to the development and maintenance of AF, so it was postulated that by improving endothelial nitric oxide availability, reducing inflammation, and decreasing oxidative stress, and through neurohormonal activation, statins would reduce the incidence of post-op AF. This link was so strong that clinical guidelines adopted limited data from small trials to make treatment recommendations.
This leads us to consider the second key lesson from this study. Trials with small sample size, even when combined across many other trials (1,300 patients were involved across 14 trials in this case), do not always yield reliable results, especially when they have significant limitations, notably not always being blind and having been performed in statin-naive patients only. The large, randomized, and well-designed STICS trial puts to rest an important issue, given the high prevalence of AF after cardiac surgery, which is associated with a longer length of stay, an increased risk of stroke, higher mortality, and greater costs, and should prompt us to consider further evaluation of different strategies to reduce this significant complication.
Dr. Hiren Shah is medical director of the medicine and cardiac telemetry hospitalist unit at Northwestern Memorial Hospital in Chicago and an adviser to Hospitalist News. He is the national chair of the Clinician Committee for ACP’s Initiative on Stroke Prevention and Atrial Fibrillation and is the lead physician for the Society of Hospital Medicine’s National Atrial Fibrillation Initiative.

|
| Dr. Hiren Shah |
There are two key lessons from the results of the STICS trial. First, extrapolation of results from biochemical pathways and measured cellular markers does not always translate into meaningful clinical outcomes. Thus, it has long been known from several large trials that statin therapy effectively and rapidly lowers CRP levels both in hyper- and normocholesterolemic patients and that statins are effective in decreasing systemic inflammation. It has also been known that inflammation contributes to the development and maintenance of AF, so it was postulated that by improving endothelial nitric oxide availability, reducing inflammation, and decreasing oxidative stress, and through neurohormonal activation, statins would reduce the incidence of post-op AF. This link was so strong that clinical guidelines adopted limited data from small trials to make treatment recommendations.
This leads us to consider the second key lesson from this study. Trials with small sample size, even when combined across many other trials (1,300 patients were involved across 14 trials in this case), do not always yield reliable results, especially when they have significant limitations, notably not always being blind and having been performed in statin-naive patients only. The large, randomized, and well-designed STICS trial puts to rest an important issue, given the high prevalence of AF after cardiac surgery, which is associated with a longer length of stay, an increased risk of stroke, higher mortality, and greater costs, and should prompt us to consider further evaluation of different strategies to reduce this significant complication.
Dr. Hiren Shah is medical director of the medicine and cardiac telemetry hospitalist unit at Northwestern Memorial Hospital in Chicago and an adviser to Hospitalist News. He is the national chair of the Clinician Committee for ACP’s Initiative on Stroke Prevention and Atrial Fibrillation and is the lead physician for the Society of Hospital Medicine’s National Atrial Fibrillation Initiative.
BARCELONA – Intensive perioperative statin therapy in patients undergoing CABG surgery doesn’t protect against postop atrial fibrillation or myocardial injury, according to a large randomized clinical trial hailed as the "definitive" study addressing this issue.
"There are many reasons why these patients should be put on statin treatment, but the prevention of postop complications is not one of them," Dr. Barbara Casadei said in presenting the findings of the Statin Therapy in Cardiac Surgery (STICS) trial at the annual congress of the European Society of Cardiology.

The STICS results are at odds with conventional wisdom. ESC guidelines give a favorable class IIa, level of evidence B recommendation that "statins should be considered for prevention of new-onset atrial fibrillation after coronary artery bypass grafting, either isolated or in combination with valvular interventions."
"STICS was a very carefully conducted, large scale, robust study that I think has definitely closed the door on this issue," commented Dr. Keith A.A. Fox, professor of cardiology at the University of Edinburgh and chair of the scientific and clinical program committee at ESC Congress 2014.
STICS was a double-blind prospective trial in which 1,922 patients scheduled for elective CABG were randomized to 20 mg per day of rosuvastatin (Crestor) or placebo starting up to 8 days prior to surgery and continued for 5 days postop. All participants were in sinus rhythm preoperatively, with no history of AF, said Dr. Casadei, professor of cardiovascular medicine at the University of Oxford, England.
The two coprimary endpoints in STICS were the incidence of new-onset AF during 5 days of postop Holter monitoring, and evidence of postop myocardial injury as demonstrated in serial troponin I assays.
Postop AF occurred in 21% of those given high-intensity therapy with rosuvastatin and 20% of placebo-treated controls. There was no subgroup where rosuvastatin was protective (see graphic).
Troponin I measurements obtained 6, 24, 48, and 120 hours postop showed areas under the curve that were superimposable in the two study groups, meaning perioperative high-dose statin therapy provided absolutely no protection against postop cardiac muscle injury.
Mean hospital length of stay and ICU time didn’t differ between the two groups, either.
The impetus for conducting STICS was recognition that the guidelines’ endorsement of perioperative high-dose statin therapy in conjunction with cardiac surgery was based upon a series of small randomized trials with serious limitations. Although the results of a meta-analysis of the 14 prior trials looked impressive at first glance – a 17% incidence of postop AF in statin-treated patients, compared with 30% in controls, for a near-halving of the risk of this important complication – these 14 studies totaled 1,300 patients, and there were many methodologic shortcomings.
The STICS researchers hypothesized that a large, well-designed trial – bigger than all previous studies combined – would shore up the previously shaky supporting evidence and perhaps provide grounds for statins to win a new indication from regulatory agencies. Post-CABG AF is associated with a doubled risk of stroke and mortality, and excess hospital costs of $8,000-$18,000 dollars per patient.
Discussant Dr. Paulus Kirchhof, a member of the task force that developed the current ESC guidelines (Europace 2010;12:1360-420), said those guidelines now clearly need to be revisited. Beyond that, he added, STICS provides important new contributions in understanding the pathophysiology of AF.
"We know that AF is caused by several vicious circles, and we believe that inflammation could influence those and cause AF. And we also thought that postop AF was the condition where inflammation plays the biggest role. Based upon the negative results with this anti-inflammatory intervention, I think we have to question this concept a bit," said Dr. Kirchhof, professor of cardiovascular sciences at the University of Birmingham, England.
Dr. Casadei countered that she’s not ready to write off postop inflammation entirely as a major trigger of new-onset AF following CABG.
"The inflammation is there. We know from experimental work in animals that there is a strong association between inflammation and postop atrial fibrillation, but whether the association is causal, I think, is still debated. However, it may be that the anti-inflammatory effect of statins is not sufficiently strong to actually prevent this complication," she said.
Discussant Dr. Steven Nissen praised STICS as "an outstanding trial."
"I also think there’s a terribly important lesson here, which is the power of self-delusion in medicine. When we base our guidelines on small, poorly controlled trials, we are often making mistakes. This is one of countless examples where when someone finally does a careful, thoughtful trial, we find out that something that people believe just isn’t true. We can’t cut corners with evidence. We need good randomized trials," declared Dr. Nissen, chair of the department of cardiovascular medicine at the Cleveland Clinic.
The STICS trial was funded primarily by the British Heart Foundation, the Oxford Biomedical Research Center, and the UK Medical Research Council. In addition, Dr. Casadei reported receiving an unrestricted grant from AstraZeneca in conjunction with the trial.
BARCELONA – Intensive perioperative statin therapy in patients undergoing CABG surgery doesn’t protect against postop atrial fibrillation or myocardial injury, according to a large randomized clinical trial hailed as the "definitive" study addressing this issue.
"There are many reasons why these patients should be put on statin treatment, but the prevention of postop complications is not one of them," Dr. Barbara Casadei said in presenting the findings of the Statin Therapy in Cardiac Surgery (STICS) trial at the annual congress of the European Society of Cardiology.

The STICS results are at odds with conventional wisdom. ESC guidelines give a favorable class IIa, level of evidence B recommendation that "statins should be considered for prevention of new-onset atrial fibrillation after coronary artery bypass grafting, either isolated or in combination with valvular interventions."
"STICS was a very carefully conducted, large scale, robust study that I think has definitely closed the door on this issue," commented Dr. Keith A.A. Fox, professor of cardiology at the University of Edinburgh and chair of the scientific and clinical program committee at ESC Congress 2014.
STICS was a double-blind prospective trial in which 1,922 patients scheduled for elective CABG were randomized to 20 mg per day of rosuvastatin (Crestor) or placebo starting up to 8 days prior to surgery and continued for 5 days postop. All participants were in sinus rhythm preoperatively, with no history of AF, said Dr. Casadei, professor of cardiovascular medicine at the University of Oxford, England.
The two coprimary endpoints in STICS were the incidence of new-onset AF during 5 days of postop Holter monitoring, and evidence of postop myocardial injury as demonstrated in serial troponin I assays.
Postop AF occurred in 21% of those given high-intensity therapy with rosuvastatin and 20% of placebo-treated controls. There was no subgroup where rosuvastatin was protective (see graphic).
Troponin I measurements obtained 6, 24, 48, and 120 hours postop showed areas under the curve that were superimposable in the two study groups, meaning perioperative high-dose statin therapy provided absolutely no protection against postop cardiac muscle injury.
Mean hospital length of stay and ICU time didn’t differ between the two groups, either.
The impetus for conducting STICS was recognition that the guidelines’ endorsement of perioperative high-dose statin therapy in conjunction with cardiac surgery was based upon a series of small randomized trials with serious limitations. Although the results of a meta-analysis of the 14 prior trials looked impressive at first glance – a 17% incidence of postop AF in statin-treated patients, compared with 30% in controls, for a near-halving of the risk of this important complication – these 14 studies totaled 1,300 patients, and there were many methodologic shortcomings.
The STICS researchers hypothesized that a large, well-designed trial – bigger than all previous studies combined – would shore up the previously shaky supporting evidence and perhaps provide grounds for statins to win a new indication from regulatory agencies. Post-CABG AF is associated with a doubled risk of stroke and mortality, and excess hospital costs of $8,000-$18,000 dollars per patient.
Discussant Dr. Paulus Kirchhof, a member of the task force that developed the current ESC guidelines (Europace 2010;12:1360-420), said those guidelines now clearly need to be revisited. Beyond that, he added, STICS provides important new contributions in understanding the pathophysiology of AF.
"We know that AF is caused by several vicious circles, and we believe that inflammation could influence those and cause AF. And we also thought that postop AF was the condition where inflammation plays the biggest role. Based upon the negative results with this anti-inflammatory intervention, I think we have to question this concept a bit," said Dr. Kirchhof, professor of cardiovascular sciences at the University of Birmingham, England.
Dr. Casadei countered that she’s not ready to write off postop inflammation entirely as a major trigger of new-onset AF following CABG.
"The inflammation is there. We know from experimental work in animals that there is a strong association between inflammation and postop atrial fibrillation, but whether the association is causal, I think, is still debated. However, it may be that the anti-inflammatory effect of statins is not sufficiently strong to actually prevent this complication," she said.
Discussant Dr. Steven Nissen praised STICS as "an outstanding trial."
"I also think there’s a terribly important lesson here, which is the power of self-delusion in medicine. When we base our guidelines on small, poorly controlled trials, we are often making mistakes. This is one of countless examples where when someone finally does a careful, thoughtful trial, we find out that something that people believe just isn’t true. We can’t cut corners with evidence. We need good randomized trials," declared Dr. Nissen, chair of the department of cardiovascular medicine at the Cleveland Clinic.
The STICS trial was funded primarily by the British Heart Foundation, the Oxford Biomedical Research Center, and the UK Medical Research Council. In addition, Dr. Casadei reported receiving an unrestricted grant from AstraZeneca in conjunction with the trial.
AT THE ESC CONGRESS 2014
Key clinical point: Perioperative statin therapy in patients undergoing CABG failed to protect against new-onset postop atrial fibrillation.
Major finding: The incidence of postop atrial fibrillation within 5 days post-CABG was 21% in patients randomized to 20 mg/day of rosuvastatin and 20% in placebo-treated controls.
Data source: The multicenter STICS trial included 1,922 randomized patients scheduled for elective CABG.
Disclosures: STICS was funded by the British Heart Foundation, the Oxford Biomedical Research Center, and the UK Medical Research Council. The presenter reported having received a research grant from AstraZeneca.
COPPS-2 curtails colchicine enthusiasm in cardiac surgery
Patients undergoing cardiac surgery who took colchicine had significantly less postpericardiotomy syndrome than did those on placebo, but this protective effect did not extend to postoperative atrial fibrillation and pericardial or pleural effusions in the double-blind COPPS-2 trial.
The failure of colchicine to prevent postoperative atrial fibrillation (AF) was probably due to more frequent adverse events (36 vs. 21 with placebo), especially gastrointestinal intolerance (26 vs. 12), and drug discontinuation (39 vs. 32), since a prespecified on-treatment analysis showed a significant reduction in AF in patients tolerating the drug, Dr. Massimo Imazio reported at the annual congress of the European Society of Cardiology.
"The high rate of adverse effects is a reason for concern and suggests that colchicine should be considered only in well-selected patients," Dr. Imazio and his associates wrote in an article on COPPS-2 simultaneously published online (JAMA 2014 [doi:10.1001/jama.2014.11026]).
Colchicine has been a promising strategy for postpericardiotomy syndrome prevention, besting methylprednisolone and aspirin in a large meta-analysis (Am. J. Cardiol. 2011;108:575-9).
In the largest trial, COPPS (Colchicine for the Prevention of the Postpericardiotomy Syndrome), Dr. Imazio reported that colchicine significantly reduced the incidence of postpericardiotomy syndrome (8.9% vs. 21.1%), postoperative pericardial effusions (relative risk reduction, 43.9%), and pleural effusions (RRR, 52.3%) at 12 months, compared with placebo (Am. Heart J. 2011;162:527-32 and Eur. Heart J. 2010;31:2749-54). Colchicine was given for 1 month, beginning on the third postoperative day with a 1-mg twice-daily loading dose.
In COPPS-2, the 360 consecutive candidates for cardiac surgery also were given colchicine or placebo for 1 month, but treatment was started 48-72 hours before surgery to pretreat patients and improve colchicine’s ability to prevent postoperative systemic inflammation and its complications.
Colchicine also was administered using weight-based dosing (0.5 mg twice daily in patients weighing at least 70 kg or 0.5 mg once daily in those under 70 kg), and they avoided the loading dose in an effort to improve adherence.
"However, we observed a 2-fold increase of adverse effects and study drug discontinuations compared with those reported in the COPPS trial, likely due to significant vulnerability of patients in the perioperative phase, when the use of antibiotics and proton pump inhibitors is common and also increases the risk of gastrointestinal effects (e.g., diarrhea)," explained Dr. Imazio of Maria Vittoria Hospital and the University of Torino (Italy).
Still, colchicine provided significant protection in the COPPS-2 primary outcome of postpericardiotomy syndrome, compared with placebo (19.4% vs. 29.4%; 95% confidence interval, 1.1%-18.7%). The number needed to treat was 10.
The outcome did not differ significantly among predetermined subgroups based on age, sex, and presence or absence of pericardial effusion, although colchicine was especially efficacious in the setting of systemic inflammation with elevated C-reactive protein, the authors noted.
The intention-to-treat analysis revealed no significant differences between the colchicine and placebo groups for postoperative AF (33.9% vs. 41.7%; 95% CI, –2.2%-17.6%) or postoperative pericardial/pleural effusion (57.2% vs. 58.9%; 95% CI, –8.5%-11.7%).
The prespecified on-treatment analysis, however, showed a 14.2% absolute difference in postoperative AF, favoring colchicine over placebo (27% vs. 41.2%; 95% CI, 3.3%-24.7%).
"While the efficacy of colchicine for postpericardiotomy syndrome prevention is confirmed, the extent of efficacy for postoperative AF needs to be further investigated in future trials," Dr. Imazio stated.
Ongoing studies also will better clarify the potential of colchicine using lower doses that may be better tolerated.
The 360 patients were evenly randomized from 11 centers in Italy between March 2012 and March 2014. Their mean age was 67.5 years, 69% were men, and 36% had planned valvular surgery. Key exclusion criteria were absence of sinus rhythm at enrollment, urgent cardiac surgery, cardiac transplantation, and contraindications to colchicine.
COPPS-2 was supported by the Italian National Health Service and FARGIM. Acarpia provided the study drug. Dr. Imazio reported no conflicts of interest. A coauthor reported consultancy for Servier, serving on an advisory board for Boehringer Ingelheim, and lecturer fees from Abbott, AstraZeneca, Merck, Serono, Richter Gedeon, and Teva.
Patients undergoing cardiac surgery who took colchicine had significantly less postpericardiotomy syndrome than did those on placebo, but this protective effect did not extend to postoperative atrial fibrillation and pericardial or pleural effusions in the double-blind COPPS-2 trial.
The failure of colchicine to prevent postoperative atrial fibrillation (AF) was probably due to more frequent adverse events (36 vs. 21 with placebo), especially gastrointestinal intolerance (26 vs. 12), and drug discontinuation (39 vs. 32), since a prespecified on-treatment analysis showed a significant reduction in AF in patients tolerating the drug, Dr. Massimo Imazio reported at the annual congress of the European Society of Cardiology.
"The high rate of adverse effects is a reason for concern and suggests that colchicine should be considered only in well-selected patients," Dr. Imazio and his associates wrote in an article on COPPS-2 simultaneously published online (JAMA 2014 [doi:10.1001/jama.2014.11026]).
Colchicine has been a promising strategy for postpericardiotomy syndrome prevention, besting methylprednisolone and aspirin in a large meta-analysis (Am. J. Cardiol. 2011;108:575-9).
In the largest trial, COPPS (Colchicine for the Prevention of the Postpericardiotomy Syndrome), Dr. Imazio reported that colchicine significantly reduced the incidence of postpericardiotomy syndrome (8.9% vs. 21.1%), postoperative pericardial effusions (relative risk reduction, 43.9%), and pleural effusions (RRR, 52.3%) at 12 months, compared with placebo (Am. Heart J. 2011;162:527-32 and Eur. Heart J. 2010;31:2749-54). Colchicine was given for 1 month, beginning on the third postoperative day with a 1-mg twice-daily loading dose.
In COPPS-2, the 360 consecutive candidates for cardiac surgery also were given colchicine or placebo for 1 month, but treatment was started 48-72 hours before surgery to pretreat patients and improve colchicine’s ability to prevent postoperative systemic inflammation and its complications.
Colchicine also was administered using weight-based dosing (0.5 mg twice daily in patients weighing at least 70 kg or 0.5 mg once daily in those under 70 kg), and they avoided the loading dose in an effort to improve adherence.
"However, we observed a 2-fold increase of adverse effects and study drug discontinuations compared with those reported in the COPPS trial, likely due to significant vulnerability of patients in the perioperative phase, when the use of antibiotics and proton pump inhibitors is common and also increases the risk of gastrointestinal effects (e.g., diarrhea)," explained Dr. Imazio of Maria Vittoria Hospital and the University of Torino (Italy).
Still, colchicine provided significant protection in the COPPS-2 primary outcome of postpericardiotomy syndrome, compared with placebo (19.4% vs. 29.4%; 95% confidence interval, 1.1%-18.7%). The number needed to treat was 10.
The outcome did not differ significantly among predetermined subgroups based on age, sex, and presence or absence of pericardial effusion, although colchicine was especially efficacious in the setting of systemic inflammation with elevated C-reactive protein, the authors noted.
The intention-to-treat analysis revealed no significant differences between the colchicine and placebo groups for postoperative AF (33.9% vs. 41.7%; 95% CI, –2.2%-17.6%) or postoperative pericardial/pleural effusion (57.2% vs. 58.9%; 95% CI, –8.5%-11.7%).
The prespecified on-treatment analysis, however, showed a 14.2% absolute difference in postoperative AF, favoring colchicine over placebo (27% vs. 41.2%; 95% CI, 3.3%-24.7%).
"While the efficacy of colchicine for postpericardiotomy syndrome prevention is confirmed, the extent of efficacy for postoperative AF needs to be further investigated in future trials," Dr. Imazio stated.
Ongoing studies also will better clarify the potential of colchicine using lower doses that may be better tolerated.
The 360 patients were evenly randomized from 11 centers in Italy between March 2012 and March 2014. Their mean age was 67.5 years, 69% were men, and 36% had planned valvular surgery. Key exclusion criteria were absence of sinus rhythm at enrollment, urgent cardiac surgery, cardiac transplantation, and contraindications to colchicine.
COPPS-2 was supported by the Italian National Health Service and FARGIM. Acarpia provided the study drug. Dr. Imazio reported no conflicts of interest. A coauthor reported consultancy for Servier, serving on an advisory board for Boehringer Ingelheim, and lecturer fees from Abbott, AstraZeneca, Merck, Serono, Richter Gedeon, and Teva.
Patients undergoing cardiac surgery who took colchicine had significantly less postpericardiotomy syndrome than did those on placebo, but this protective effect did not extend to postoperative atrial fibrillation and pericardial or pleural effusions in the double-blind COPPS-2 trial.
The failure of colchicine to prevent postoperative atrial fibrillation (AF) was probably due to more frequent adverse events (36 vs. 21 with placebo), especially gastrointestinal intolerance (26 vs. 12), and drug discontinuation (39 vs. 32), since a prespecified on-treatment analysis showed a significant reduction in AF in patients tolerating the drug, Dr. Massimo Imazio reported at the annual congress of the European Society of Cardiology.
"The high rate of adverse effects is a reason for concern and suggests that colchicine should be considered only in well-selected patients," Dr. Imazio and his associates wrote in an article on COPPS-2 simultaneously published online (JAMA 2014 [doi:10.1001/jama.2014.11026]).
Colchicine has been a promising strategy for postpericardiotomy syndrome prevention, besting methylprednisolone and aspirin in a large meta-analysis (Am. J. Cardiol. 2011;108:575-9).
In the largest trial, COPPS (Colchicine for the Prevention of the Postpericardiotomy Syndrome), Dr. Imazio reported that colchicine significantly reduced the incidence of postpericardiotomy syndrome (8.9% vs. 21.1%), postoperative pericardial effusions (relative risk reduction, 43.9%), and pleural effusions (RRR, 52.3%) at 12 months, compared with placebo (Am. Heart J. 2011;162:527-32 and Eur. Heart J. 2010;31:2749-54). Colchicine was given for 1 month, beginning on the third postoperative day with a 1-mg twice-daily loading dose.
In COPPS-2, the 360 consecutive candidates for cardiac surgery also were given colchicine or placebo for 1 month, but treatment was started 48-72 hours before surgery to pretreat patients and improve colchicine’s ability to prevent postoperative systemic inflammation and its complications.
Colchicine also was administered using weight-based dosing (0.5 mg twice daily in patients weighing at least 70 kg or 0.5 mg once daily in those under 70 kg), and they avoided the loading dose in an effort to improve adherence.
"However, we observed a 2-fold increase of adverse effects and study drug discontinuations compared with those reported in the COPPS trial, likely due to significant vulnerability of patients in the perioperative phase, when the use of antibiotics and proton pump inhibitors is common and also increases the risk of gastrointestinal effects (e.g., diarrhea)," explained Dr. Imazio of Maria Vittoria Hospital and the University of Torino (Italy).
Still, colchicine provided significant protection in the COPPS-2 primary outcome of postpericardiotomy syndrome, compared with placebo (19.4% vs. 29.4%; 95% confidence interval, 1.1%-18.7%). The number needed to treat was 10.
The outcome did not differ significantly among predetermined subgroups based on age, sex, and presence or absence of pericardial effusion, although colchicine was especially efficacious in the setting of systemic inflammation with elevated C-reactive protein, the authors noted.
The intention-to-treat analysis revealed no significant differences between the colchicine and placebo groups for postoperative AF (33.9% vs. 41.7%; 95% CI, –2.2%-17.6%) or postoperative pericardial/pleural effusion (57.2% vs. 58.9%; 95% CI, –8.5%-11.7%).
The prespecified on-treatment analysis, however, showed a 14.2% absolute difference in postoperative AF, favoring colchicine over placebo (27% vs. 41.2%; 95% CI, 3.3%-24.7%).
"While the efficacy of colchicine for postpericardiotomy syndrome prevention is confirmed, the extent of efficacy for postoperative AF needs to be further investigated in future trials," Dr. Imazio stated.
Ongoing studies also will better clarify the potential of colchicine using lower doses that may be better tolerated.
The 360 patients were evenly randomized from 11 centers in Italy between March 2012 and March 2014. Their mean age was 67.5 years, 69% were men, and 36% had planned valvular surgery. Key exclusion criteria were absence of sinus rhythm at enrollment, urgent cardiac surgery, cardiac transplantation, and contraindications to colchicine.
COPPS-2 was supported by the Italian National Health Service and FARGIM. Acarpia provided the study drug. Dr. Imazio reported no conflicts of interest. A coauthor reported consultancy for Servier, serving on an advisory board for Boehringer Ingelheim, and lecturer fees from Abbott, AstraZeneca, Merck, Serono, Richter Gedeon, and Teva.
FROM THE ESC CONGRESS 2014
Key clinical point: Perioperative use of colchicine should be considered only in well-selected patients.
Major finding: Perioperative colchicine use cut the incidence of postpericardiotomy syndrome, but not postoperative atrial fibrillation or pericardial/pleural effusion.
Data source: Double-blind, randomized clinical trial in 360 consecutive candidates for heart surgery.
Disclosures: COPPS-2 was supported by the Italian National Health Service and FARGIM. Acarpia provided the study drug. Dr. Imazio reported no conflicts of interest. A coauthor reported consultancy for Servier, serving on an advisory board for Boehringer Ingelheim, and lecturer fees from Abbott, AstraZeneca, Merck, Serono, Richter Gedeon, and Teva.
Evidence questioned in debate over monitoring dabigatran levels to avert bleeds
The manufacturer of the oral anticoagulant dabigatran "withheld analyses that calculate how many major bleeds dose adjustment could prevent," the BMJ charges in an article based on internal documents released during litigation in the United States and freedom of information act requests obtained by the journal.
Boehringer Ingelheim "found that if the plasma levels of the drug were measured and the dose was adjusted accordingly major bleeds could be reduced by 30%-40%, compared with well-controlled warfarin," according to the article (BMJ 2014;349:g4670), which was published along with an editorial and an analysis. The article further stated that the manufacturer "has failed to share with regulators information about the potential benefits of monitoring anticoagulant activity and adjusting the dose to make sure the drug is working as safely and effectively as possible."
In the analysis, Thomas Moore, senior scientist at the Institute for Safe Medication Practices, Horsham, Pa., and his coauthors said that "the bleeding risk of dabigatran can be reduced and efficacy improved by individualizing the dose in patients based on plasma level, age, and kidney function" (BMJ 2014;349:g4517[doi:10.1136/bmj.g4517]).
Dr. Rita Redberg and Dr. Blake Charlton of the University of California, San Francisco, wrote in an accompanying editorial that the analysis "illuminates a lack of transparency about the safety of unmonitored dabigatran, compounded by the drug’s fickle pharmacokinetics, which can cause a fivefold variation of plasma concentration." (BMJ 2014;349:g4681 [doi:10.1136/bmj.g4681]).
The BMJ article, written by Deborah Cohen, includes responses from the manufacturer of dabigatran (Pradaxa).
Boehringer Ingelheim officials have asserted that no data were withheld. Additionally, the company has released a statement calling the BMJ article "biased" and "misleading."
Dabigatran is a direct thrombin inhibitor that was approved in the United States in October 2010 for reducing the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation at a fixed dose of 150 mg or 75 mg twice a day, with the lower dose recommended for those with renal impairment. Since its initial approval, dabigatran has gained indications for treating and reducing the risk of recurrence of deep venous thrombosis and pulmonary embolism.
The drug’s approval was based on the results of the Randomized Evaluation of Long Term Anticoagulant Therapy (RE-LY) trial, which compared the drug to warfarin in 18,113 patients with nonvalvular atrial fibrillation and at least one other risk factor for stroke. The annual rate of stroke and systemic embolism in RE-LY was 1.5% in the patients on the 110-mg twice daily dose, 1.1% in those on the 150-mg twice daily dose, and 1.7% among those on warfarin. The risk reductions with dabigatran were 10% and 35% for the 110-mg and 150-mg doses, respectively, compared with warfarin.
"Our company has provided regulators with the complete data set and analyses of clinical evidence demonstrating Pradaxa’s benefits and safety," Boehringer Ingelheim said in its statement. "Contrary to the BMJ’s accusation that BI withheld analyses, here are the facts: In 2012, our scientists performed preliminary, exploratory simulations with mathematical models to understand whether dose adjustments based on plasma concentrations might further improve Pradaxa’s benefits and safety. Because the simulations did not offer reliable predictions of actual patient outcomes, they were not provided to regulators. However, all of the data that was used for the simulations had already been provided."
Dr. Sanjay Kaul, who was on the Food and Drug Administration’s advisory panel that recommended approval of dabigatran, said in an interview that the FDA reviewers raised questions at that meeting about the utility of using plasma concentrations to monitor individual subjects and adjust dose based on dabigatran concentrations. "But they also acknowledged an exposure-response relationship existed that demonstrated that going from the 10th to 90th percentile of dabigatran concentration (23 to 283 ng/mL) reduced the probability of having a stroke by 50% (from 1% to 0.5%) while increasing sixfold (from 0.3% to 1.8%) the probability of having major bleeding within 1 year."
It is well known that plasma concentration is one of the factors – among others, such as age, renal function, or history of stroke – that affect the benefit-risk balance, said Dr. Kaul, a cardiologist at Cedars-Sinai Medical Center, Los Angeles. Even in patients with moderate to severe renal dysfunction (up to creatinine clearance of 15-30 mL/min) and elevated plasma concentrations, benefit still exceeds risk.
"To what degree monitoring drug levels could potentially optimize benefit-risk balance remains an open question. For example, benefit-risk balance for dabigatran 150 mg vs 110 mg was not predicted by pharmacokinetic/pharmacodynamic modeling. Based on the PK/PD modeling, one stroke would be prevented at the cost of three extra major bleeds using 150 mg , compared with 110 mg. The RE-LY data indicate four strokes prevented and three major bleeding events incurred with the 150-mg dose as compared with the 110-mg dose," he said. "There is quite a discordance in predicted vs. observed benefit-risk balance."
Therefore, "while monitoring drug levels to optimize benefit-risk balance has intuitive appeal, given the complex exposure-response relationship and confounding effects of demographic variables, this should remain an area of active investigation. It is not ready for prime time to inform or guide clinical practice," Dr. Kaul said.
Dr. Kaul disclosed that he is a consultant for Boehringer Ingelheim and has equity interest in Johnson and Johnson.
At press time, the FDA had not responded to a request for a comment on the BMJ investigation.
The manufacturer of the oral anticoagulant dabigatran "withheld analyses that calculate how many major bleeds dose adjustment could prevent," the BMJ charges in an article based on internal documents released during litigation in the United States and freedom of information act requests obtained by the journal.
Boehringer Ingelheim "found that if the plasma levels of the drug were measured and the dose was adjusted accordingly major bleeds could be reduced by 30%-40%, compared with well-controlled warfarin," according to the article (BMJ 2014;349:g4670), which was published along with an editorial and an analysis. The article further stated that the manufacturer "has failed to share with regulators information about the potential benefits of monitoring anticoagulant activity and adjusting the dose to make sure the drug is working as safely and effectively as possible."
In the analysis, Thomas Moore, senior scientist at the Institute for Safe Medication Practices, Horsham, Pa., and his coauthors said that "the bleeding risk of dabigatran can be reduced and efficacy improved by individualizing the dose in patients based on plasma level, age, and kidney function" (BMJ 2014;349:g4517[doi:10.1136/bmj.g4517]).
Dr. Rita Redberg and Dr. Blake Charlton of the University of California, San Francisco, wrote in an accompanying editorial that the analysis "illuminates a lack of transparency about the safety of unmonitored dabigatran, compounded by the drug’s fickle pharmacokinetics, which can cause a fivefold variation of plasma concentration." (BMJ 2014;349:g4681 [doi:10.1136/bmj.g4681]).
The BMJ article, written by Deborah Cohen, includes responses from the manufacturer of dabigatran (Pradaxa).
Boehringer Ingelheim officials have asserted that no data were withheld. Additionally, the company has released a statement calling the BMJ article "biased" and "misleading."
Dabigatran is a direct thrombin inhibitor that was approved in the United States in October 2010 for reducing the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation at a fixed dose of 150 mg or 75 mg twice a day, with the lower dose recommended for those with renal impairment. Since its initial approval, dabigatran has gained indications for treating and reducing the risk of recurrence of deep venous thrombosis and pulmonary embolism.
The drug’s approval was based on the results of the Randomized Evaluation of Long Term Anticoagulant Therapy (RE-LY) trial, which compared the drug to warfarin in 18,113 patients with nonvalvular atrial fibrillation and at least one other risk factor for stroke. The annual rate of stroke and systemic embolism in RE-LY was 1.5% in the patients on the 110-mg twice daily dose, 1.1% in those on the 150-mg twice daily dose, and 1.7% among those on warfarin. The risk reductions with dabigatran were 10% and 35% for the 110-mg and 150-mg doses, respectively, compared with warfarin.
"Our company has provided regulators with the complete data set and analyses of clinical evidence demonstrating Pradaxa’s benefits and safety," Boehringer Ingelheim said in its statement. "Contrary to the BMJ’s accusation that BI withheld analyses, here are the facts: In 2012, our scientists performed preliminary, exploratory simulations with mathematical models to understand whether dose adjustments based on plasma concentrations might further improve Pradaxa’s benefits and safety. Because the simulations did not offer reliable predictions of actual patient outcomes, they were not provided to regulators. However, all of the data that was used for the simulations had already been provided."
Dr. Sanjay Kaul, who was on the Food and Drug Administration’s advisory panel that recommended approval of dabigatran, said in an interview that the FDA reviewers raised questions at that meeting about the utility of using plasma concentrations to monitor individual subjects and adjust dose based on dabigatran concentrations. "But they also acknowledged an exposure-response relationship existed that demonstrated that going from the 10th to 90th percentile of dabigatran concentration (23 to 283 ng/mL) reduced the probability of having a stroke by 50% (from 1% to 0.5%) while increasing sixfold (from 0.3% to 1.8%) the probability of having major bleeding within 1 year."
It is well known that plasma concentration is one of the factors – among others, such as age, renal function, or history of stroke – that affect the benefit-risk balance, said Dr. Kaul, a cardiologist at Cedars-Sinai Medical Center, Los Angeles. Even in patients with moderate to severe renal dysfunction (up to creatinine clearance of 15-30 mL/min) and elevated plasma concentrations, benefit still exceeds risk.
"To what degree monitoring drug levels could potentially optimize benefit-risk balance remains an open question. For example, benefit-risk balance for dabigatran 150 mg vs 110 mg was not predicted by pharmacokinetic/pharmacodynamic modeling. Based on the PK/PD modeling, one stroke would be prevented at the cost of three extra major bleeds using 150 mg , compared with 110 mg. The RE-LY data indicate four strokes prevented and three major bleeding events incurred with the 150-mg dose as compared with the 110-mg dose," he said. "There is quite a discordance in predicted vs. observed benefit-risk balance."
Therefore, "while monitoring drug levels to optimize benefit-risk balance has intuitive appeal, given the complex exposure-response relationship and confounding effects of demographic variables, this should remain an area of active investigation. It is not ready for prime time to inform or guide clinical practice," Dr. Kaul said.
Dr. Kaul disclosed that he is a consultant for Boehringer Ingelheim and has equity interest in Johnson and Johnson.
At press time, the FDA had not responded to a request for a comment on the BMJ investigation.
The manufacturer of the oral anticoagulant dabigatran "withheld analyses that calculate how many major bleeds dose adjustment could prevent," the BMJ charges in an article based on internal documents released during litigation in the United States and freedom of information act requests obtained by the journal.
Boehringer Ingelheim "found that if the plasma levels of the drug were measured and the dose was adjusted accordingly major bleeds could be reduced by 30%-40%, compared with well-controlled warfarin," according to the article (BMJ 2014;349:g4670), which was published along with an editorial and an analysis. The article further stated that the manufacturer "has failed to share with regulators information about the potential benefits of monitoring anticoagulant activity and adjusting the dose to make sure the drug is working as safely and effectively as possible."
In the analysis, Thomas Moore, senior scientist at the Institute for Safe Medication Practices, Horsham, Pa., and his coauthors said that "the bleeding risk of dabigatran can be reduced and efficacy improved by individualizing the dose in patients based on plasma level, age, and kidney function" (BMJ 2014;349:g4517[doi:10.1136/bmj.g4517]).
Dr. Rita Redberg and Dr. Blake Charlton of the University of California, San Francisco, wrote in an accompanying editorial that the analysis "illuminates a lack of transparency about the safety of unmonitored dabigatran, compounded by the drug’s fickle pharmacokinetics, which can cause a fivefold variation of plasma concentration." (BMJ 2014;349:g4681 [doi:10.1136/bmj.g4681]).
The BMJ article, written by Deborah Cohen, includes responses from the manufacturer of dabigatran (Pradaxa).
Boehringer Ingelheim officials have asserted that no data were withheld. Additionally, the company has released a statement calling the BMJ article "biased" and "misleading."
Dabigatran is a direct thrombin inhibitor that was approved in the United States in October 2010 for reducing the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation at a fixed dose of 150 mg or 75 mg twice a day, with the lower dose recommended for those with renal impairment. Since its initial approval, dabigatran has gained indications for treating and reducing the risk of recurrence of deep venous thrombosis and pulmonary embolism.
The drug’s approval was based on the results of the Randomized Evaluation of Long Term Anticoagulant Therapy (RE-LY) trial, which compared the drug to warfarin in 18,113 patients with nonvalvular atrial fibrillation and at least one other risk factor for stroke. The annual rate of stroke and systemic embolism in RE-LY was 1.5% in the patients on the 110-mg twice daily dose, 1.1% in those on the 150-mg twice daily dose, and 1.7% among those on warfarin. The risk reductions with dabigatran were 10% and 35% for the 110-mg and 150-mg doses, respectively, compared with warfarin.
"Our company has provided regulators with the complete data set and analyses of clinical evidence demonstrating Pradaxa’s benefits and safety," Boehringer Ingelheim said in its statement. "Contrary to the BMJ’s accusation that BI withheld analyses, here are the facts: In 2012, our scientists performed preliminary, exploratory simulations with mathematical models to understand whether dose adjustments based on plasma concentrations might further improve Pradaxa’s benefits and safety. Because the simulations did not offer reliable predictions of actual patient outcomes, they were not provided to regulators. However, all of the data that was used for the simulations had already been provided."
Dr. Sanjay Kaul, who was on the Food and Drug Administration’s advisory panel that recommended approval of dabigatran, said in an interview that the FDA reviewers raised questions at that meeting about the utility of using plasma concentrations to monitor individual subjects and adjust dose based on dabigatran concentrations. "But they also acknowledged an exposure-response relationship existed that demonstrated that going from the 10th to 90th percentile of dabigatran concentration (23 to 283 ng/mL) reduced the probability of having a stroke by 50% (from 1% to 0.5%) while increasing sixfold (from 0.3% to 1.8%) the probability of having major bleeding within 1 year."
It is well known that plasma concentration is one of the factors – among others, such as age, renal function, or history of stroke – that affect the benefit-risk balance, said Dr. Kaul, a cardiologist at Cedars-Sinai Medical Center, Los Angeles. Even in patients with moderate to severe renal dysfunction (up to creatinine clearance of 15-30 mL/min) and elevated plasma concentrations, benefit still exceeds risk.
"To what degree monitoring drug levels could potentially optimize benefit-risk balance remains an open question. For example, benefit-risk balance for dabigatran 150 mg vs 110 mg was not predicted by pharmacokinetic/pharmacodynamic modeling. Based on the PK/PD modeling, one stroke would be prevented at the cost of three extra major bleeds using 150 mg , compared with 110 mg. The RE-LY data indicate four strokes prevented and three major bleeding events incurred with the 150-mg dose as compared with the 110-mg dose," he said. "There is quite a discordance in predicted vs. observed benefit-risk balance."
Therefore, "while monitoring drug levels to optimize benefit-risk balance has intuitive appeal, given the complex exposure-response relationship and confounding effects of demographic variables, this should remain an area of active investigation. It is not ready for prime time to inform or guide clinical practice," Dr. Kaul said.
Dr. Kaul disclosed that he is a consultant for Boehringer Ingelheim and has equity interest in Johnson and Johnson.
At press time, the FDA had not responded to a request for a comment on the BMJ investigation.
VEGF-A value may stratify risk in pediatric heart transplant recipients
SAN FRANCISCO – Monitoring plasma vascular endothelial growth factor A (VEGF-A) may help identify pediatric heart transplant patients who are at increased risk for poor outcomes, according to a study reported at the 2014 World Transplant Congress.
"Cardiac allograft vasculopathy [CAV] remains the leading cause of chronic allograft failure after heart transplantation. ... Therefore, it’s important for us to be able to anticipate the development of CAV and open up a therapeutic window," said Dr. Kevin P. Daly of Harvard Medical School and Boston Children’s Hospital.
"Our pilot data suggest that plasma VEGF-A levels below 90 pg/mL identify a low-risk patient population in whom a decreased frequency of coronary angiography can be considered. Future studies are needed to determine if using plasma VEGF-A levels to modify CAV screening frequency results in equivalent patient outcomes, with decreased resource utilization and improved quality of life," Dr. Daly commented at the congress, which was sponsored by the American Society of Transplant Surgeons.
As the vascular endothelium is the primary target of the immune response in CAV, the researchers hypothesized that VEGF-A likely contributes to an inflammatory cycle that leads to vascular damage and occlusion in the graft.
Participants in the single-center prospective cohort study were 44 consecutive children aged 2 years or older who were at least 18 months (median, 6 years) out from heart transplantation. They were scheduled for routine annual screening coronary angiography during 2009, and had no or mild CAV.
Moderate or severe CAV developed in 32% of patients who had VEGF-A values above the median value at baseline (90 pg/mL), compared with 5% of patients who had VEGF-A values below the median level (P = .02). Patients who developed this vasculopathy were more likely to die (38% vs. 0%), undergo retransplantation (38% vs. 0%), experience a myocardial infarction (12% vs. 0%), and be listed for retransplantation (12% vs. 0%).
"While this is a biomarker and we have shown it is associated with CAV, we have not shown that it is causal," Dr. Daly cautioned. Any treatment directed against VEGF would have to be conducted in the context of a clinical trial to assess its impact.
A subset of patients becomes nonadherent to therapy; a subset that is highly sensitized before transplant may have donor-specific antibody, Dr. Daly said. So "we don’t think we fully understand the inciting event, ... [but] VEGF-A has been shown before to be elevated in antibody-mediated rejection, so it’s not surprising to see this association."
"We didn’t have these data available clinically because it was all a research study, so we didn’t intervene on any of the patients in this cohort. But we have started to think about whether or not we could use VEGF-A levels at least in our ... patients who might not have arterial access, and it might be difficult to survey them for CAV. I think in order to really understand the appropriate way to use it, we would need a larger study," he remarked.
Dr. Daly disclosed that he had no conflicts of interest relevant to the study.
This was a very nice preliminary study but extremely limited in scope, as it has few patients and limited mechanistic studies. Most importantly, there was no validation cohort as is required to have confidence that a biomarker is predictive. A lot more work will be necessary before significance can be assigned to the use of VEGF-A as a potential biomarker.
Dr. Daniel R. Salomon is a professor and program medical director at the Scripps Center for Organ Transplantation, Scripps Research Institute, La Jolla, Calif. He was the cochair at the session where the research was presented, and made his remarks in an interview. He had no relevant conflicts of interest.
This was a very nice preliminary study but extremely limited in scope, as it has few patients and limited mechanistic studies. Most importantly, there was no validation cohort as is required to have confidence that a biomarker is predictive. A lot more work will be necessary before significance can be assigned to the use of VEGF-A as a potential biomarker.
Dr. Daniel R. Salomon is a professor and program medical director at the Scripps Center for Organ Transplantation, Scripps Research Institute, La Jolla, Calif. He was the cochair at the session where the research was presented, and made his remarks in an interview. He had no relevant conflicts of interest.
This was a very nice preliminary study but extremely limited in scope, as it has few patients and limited mechanistic studies. Most importantly, there was no validation cohort as is required to have confidence that a biomarker is predictive. A lot more work will be necessary before significance can be assigned to the use of VEGF-A as a potential biomarker.
Dr. Daniel R. Salomon is a professor and program medical director at the Scripps Center for Organ Transplantation, Scripps Research Institute, La Jolla, Calif. He was the cochair at the session where the research was presented, and made his remarks in an interview. He had no relevant conflicts of interest.
SAN FRANCISCO – Monitoring plasma vascular endothelial growth factor A (VEGF-A) may help identify pediatric heart transplant patients who are at increased risk for poor outcomes, according to a study reported at the 2014 World Transplant Congress.
"Cardiac allograft vasculopathy [CAV] remains the leading cause of chronic allograft failure after heart transplantation. ... Therefore, it’s important for us to be able to anticipate the development of CAV and open up a therapeutic window," said Dr. Kevin P. Daly of Harvard Medical School and Boston Children’s Hospital.
"Our pilot data suggest that plasma VEGF-A levels below 90 pg/mL identify a low-risk patient population in whom a decreased frequency of coronary angiography can be considered. Future studies are needed to determine if using plasma VEGF-A levels to modify CAV screening frequency results in equivalent patient outcomes, with decreased resource utilization and improved quality of life," Dr. Daly commented at the congress, which was sponsored by the American Society of Transplant Surgeons.
As the vascular endothelium is the primary target of the immune response in CAV, the researchers hypothesized that VEGF-A likely contributes to an inflammatory cycle that leads to vascular damage and occlusion in the graft.
Participants in the single-center prospective cohort study were 44 consecutive children aged 2 years or older who were at least 18 months (median, 6 years) out from heart transplantation. They were scheduled for routine annual screening coronary angiography during 2009, and had no or mild CAV.
Moderate or severe CAV developed in 32% of patients who had VEGF-A values above the median value at baseline (90 pg/mL), compared with 5% of patients who had VEGF-A values below the median level (P = .02). Patients who developed this vasculopathy were more likely to die (38% vs. 0%), undergo retransplantation (38% vs. 0%), experience a myocardial infarction (12% vs. 0%), and be listed for retransplantation (12% vs. 0%).
"While this is a biomarker and we have shown it is associated with CAV, we have not shown that it is causal," Dr. Daly cautioned. Any treatment directed against VEGF would have to be conducted in the context of a clinical trial to assess its impact.
A subset of patients becomes nonadherent to therapy; a subset that is highly sensitized before transplant may have donor-specific antibody, Dr. Daly said. So "we don’t think we fully understand the inciting event, ... [but] VEGF-A has been shown before to be elevated in antibody-mediated rejection, so it’s not surprising to see this association."
"We didn’t have these data available clinically because it was all a research study, so we didn’t intervene on any of the patients in this cohort. But we have started to think about whether or not we could use VEGF-A levels at least in our ... patients who might not have arterial access, and it might be difficult to survey them for CAV. I think in order to really understand the appropriate way to use it, we would need a larger study," he remarked.
Dr. Daly disclosed that he had no conflicts of interest relevant to the study.
SAN FRANCISCO – Monitoring plasma vascular endothelial growth factor A (VEGF-A) may help identify pediatric heart transplant patients who are at increased risk for poor outcomes, according to a study reported at the 2014 World Transplant Congress.
"Cardiac allograft vasculopathy [CAV] remains the leading cause of chronic allograft failure after heart transplantation. ... Therefore, it’s important for us to be able to anticipate the development of CAV and open up a therapeutic window," said Dr. Kevin P. Daly of Harvard Medical School and Boston Children’s Hospital.
"Our pilot data suggest that plasma VEGF-A levels below 90 pg/mL identify a low-risk patient population in whom a decreased frequency of coronary angiography can be considered. Future studies are needed to determine if using plasma VEGF-A levels to modify CAV screening frequency results in equivalent patient outcomes, with decreased resource utilization and improved quality of life," Dr. Daly commented at the congress, which was sponsored by the American Society of Transplant Surgeons.
As the vascular endothelium is the primary target of the immune response in CAV, the researchers hypothesized that VEGF-A likely contributes to an inflammatory cycle that leads to vascular damage and occlusion in the graft.
Participants in the single-center prospective cohort study were 44 consecutive children aged 2 years or older who were at least 18 months (median, 6 years) out from heart transplantation. They were scheduled for routine annual screening coronary angiography during 2009, and had no or mild CAV.
Moderate or severe CAV developed in 32% of patients who had VEGF-A values above the median value at baseline (90 pg/mL), compared with 5% of patients who had VEGF-A values below the median level (P = .02). Patients who developed this vasculopathy were more likely to die (38% vs. 0%), undergo retransplantation (38% vs. 0%), experience a myocardial infarction (12% vs. 0%), and be listed for retransplantation (12% vs. 0%).
"While this is a biomarker and we have shown it is associated with CAV, we have not shown that it is causal," Dr. Daly cautioned. Any treatment directed against VEGF would have to be conducted in the context of a clinical trial to assess its impact.
A subset of patients becomes nonadherent to therapy; a subset that is highly sensitized before transplant may have donor-specific antibody, Dr. Daly said. So "we don’t think we fully understand the inciting event, ... [but] VEGF-A has been shown before to be elevated in antibody-mediated rejection, so it’s not surprising to see this association."
"We didn’t have these data available clinically because it was all a research study, so we didn’t intervene on any of the patients in this cohort. But we have started to think about whether or not we could use VEGF-A levels at least in our ... patients who might not have arterial access, and it might be difficult to survey them for CAV. I think in order to really understand the appropriate way to use it, we would need a larger study," he remarked.
Dr. Daly disclosed that he had no conflicts of interest relevant to the study.
AT THE 2014 WORLD TRANSPLANT CONGRESS
Key clinical point: Plasma VEGF-A levels may be a biomarker of risk for pediatric heart transplant patients.
Major finding: Patients with plasma VEGF-A levels above the median value of 90 pg/mL had a 32% rate of moderate or severe cardiac allograft vasculopathy within 5 years.
Data source: A prospective cohort study of 44 consecutive children who had undergone heart transplantation.
Disclosures: Dr. Daly disclosed no relevant conflicts of interest.
Guideline adjusts perioperative cardiac care in noncardiac surgery
A new clinical practice guideline on cardiovascular evaluation and management of patients undergoing noncardiac surgery adds some clarity around the controversial issue of beta-blocker therapy and updates other aspects of care.
If a patient on beta-blocker medication needs noncardiac surgery, continue the beta-blocker, because there is no evidence of harm from doing so; but you risk doing harm if the drug is stopped, according to the new guideline from the American College of Cardiology (ACC) and the American Heart Association (AHA).
Surgeons will be happy to hear that, said Dr. Lee A. Fleisher, the chair of the guideline-writing committee, because that conforms to one of the Surgical Care Improvement Project’s National Measures.
For patients at elevated risk of a cardiovascular event during noncardiac surgery who are not already on beta-blocker therapy, however, the new guideline steps back from the organization’s 2009 position that beta-blockers not be started, and says instead that it’s not unreasonable to start the drug, with a caveat. Be very cautious and start the drug early enough before surgery that you can titrate it to avoid causing hypotension or a low heart rate.
"Make sure that you’re giving the right amount and monitoring their blood pressure and heart rate," Dr. Fleisher, chair of the guideline writing committee, said in an interview. "Really think once, twice, and thrice about starting a protocol," added Dr. Fleisher, the Robert D. Dripps Professor of Anesthesiology and Critical Care at the University of Pennsylvania, Philadelphia.
The ACC and AHA commissioned a committee to review the evidence for and against beta-blockers in patients undergoing noncardiac surgery. A separate writing committee then considered the evidence review committee’s report, reviewed the literature on other aspects of perioperative care for noncardiac surgery, and compiled a 102-page guideline with a 59-page executive summary.
The "2014 ACC/AHA Guideline on Perioperative Cardiovascular Evaluation and Management of Patients Undergoing Noncardiac Surgery" will be published online on the ACC and AHA websites.
Dr. Fleisher described other highlights of the new guideline pertinent to cardiologists, primary care physicians, or surgeons. For the first time, palliative care has been added as an option that may come out of the preoperative evaluation, he said. Patient categories of high risk and intermediate risk have been lumped together as having "elevated" risk for simplicity’s sake because recommendations for the two separate categories were so similar.
The guideline now endorses two tools to choose from for preoperative risk assessments: the Revised Cardiac Risk Index (RCRI), and the National Surgical Quality Improvement Project risk calculator. "There have been a lot of comments that [the NSQIP] is a very useful tool to have shared decision-making conversations with patients," he said.
Another change applies to patients who receive second- or third-generation coronary stents. Instead of a wait of a year after stent implantation to perform noncardiac surgery, a 6-month wait may be reasonable if the risks of delaying noncardiac surgery outweigh the risks of interrupting dual-antiplatelet therapy for the noncardiac surgery.
In addition, the guideline incorporates findings from the recent POISE-2 study to say that aspirin can be stopped and clonidine is not useful in patients without stents undergoing noncardiac surgery (N. Engl. J. Med. 2014;370:1494-503).
A new statement in the guideline about troponin says to check troponin in high-risk patients with signs or symptoms of trouble but not to include troponin in routine screening.
The recommendations on beta-blockers, however, address the most controversial topic in the guideline, Dr. Fleisher said. "There is a lot of confusing evidence" on the use of beta-blockers, "so we’ve tried to clarify as much as we can."
The ACC and AHA funded the work of the committees. Dr. Fleisher reported having no financial disclosures.
On Twitter @sherryboschert
A new clinical practice guideline on cardiovascular evaluation and management of patients undergoing noncardiac surgery adds some clarity around the controversial issue of beta-blocker therapy and updates other aspects of care.
If a patient on beta-blocker medication needs noncardiac surgery, continue the beta-blocker, because there is no evidence of harm from doing so; but you risk doing harm if the drug is stopped, according to the new guideline from the American College of Cardiology (ACC) and the American Heart Association (AHA).
Surgeons will be happy to hear that, said Dr. Lee A. Fleisher, the chair of the guideline-writing committee, because that conforms to one of the Surgical Care Improvement Project’s National Measures.
For patients at elevated risk of a cardiovascular event during noncardiac surgery who are not already on beta-blocker therapy, however, the new guideline steps back from the organization’s 2009 position that beta-blockers not be started, and says instead that it’s not unreasonable to start the drug, with a caveat. Be very cautious and start the drug early enough before surgery that you can titrate it to avoid causing hypotension or a low heart rate.
"Make sure that you’re giving the right amount and monitoring their blood pressure and heart rate," Dr. Fleisher, chair of the guideline writing committee, said in an interview. "Really think once, twice, and thrice about starting a protocol," added Dr. Fleisher, the Robert D. Dripps Professor of Anesthesiology and Critical Care at the University of Pennsylvania, Philadelphia.
The ACC and AHA commissioned a committee to review the evidence for and against beta-blockers in patients undergoing noncardiac surgery. A separate writing committee then considered the evidence review committee’s report, reviewed the literature on other aspects of perioperative care for noncardiac surgery, and compiled a 102-page guideline with a 59-page executive summary.
The "2014 ACC/AHA Guideline on Perioperative Cardiovascular Evaluation and Management of Patients Undergoing Noncardiac Surgery" will be published online on the ACC and AHA websites.
Dr. Fleisher described other highlights of the new guideline pertinent to cardiologists, primary care physicians, or surgeons. For the first time, palliative care has been added as an option that may come out of the preoperative evaluation, he said. Patient categories of high risk and intermediate risk have been lumped together as having "elevated" risk for simplicity’s sake because recommendations for the two separate categories were so similar.
The guideline now endorses two tools to choose from for preoperative risk assessments: the Revised Cardiac Risk Index (RCRI), and the National Surgical Quality Improvement Project risk calculator. "There have been a lot of comments that [the NSQIP] is a very useful tool to have shared decision-making conversations with patients," he said.
Another change applies to patients who receive second- or third-generation coronary stents. Instead of a wait of a year after stent implantation to perform noncardiac surgery, a 6-month wait may be reasonable if the risks of delaying noncardiac surgery outweigh the risks of interrupting dual-antiplatelet therapy for the noncardiac surgery.
In addition, the guideline incorporates findings from the recent POISE-2 study to say that aspirin can be stopped and clonidine is not useful in patients without stents undergoing noncardiac surgery (N. Engl. J. Med. 2014;370:1494-503).
A new statement in the guideline about troponin says to check troponin in high-risk patients with signs or symptoms of trouble but not to include troponin in routine screening.
The recommendations on beta-blockers, however, address the most controversial topic in the guideline, Dr. Fleisher said. "There is a lot of confusing evidence" on the use of beta-blockers, "so we’ve tried to clarify as much as we can."
The ACC and AHA funded the work of the committees. Dr. Fleisher reported having no financial disclosures.
On Twitter @sherryboschert
A new clinical practice guideline on cardiovascular evaluation and management of patients undergoing noncardiac surgery adds some clarity around the controversial issue of beta-blocker therapy and updates other aspects of care.
If a patient on beta-blocker medication needs noncardiac surgery, continue the beta-blocker, because there is no evidence of harm from doing so; but you risk doing harm if the drug is stopped, according to the new guideline from the American College of Cardiology (ACC) and the American Heart Association (AHA).
Surgeons will be happy to hear that, said Dr. Lee A. Fleisher, the chair of the guideline-writing committee, because that conforms to one of the Surgical Care Improvement Project’s National Measures.
For patients at elevated risk of a cardiovascular event during noncardiac surgery who are not already on beta-blocker therapy, however, the new guideline steps back from the organization’s 2009 position that beta-blockers not be started, and says instead that it’s not unreasonable to start the drug, with a caveat. Be very cautious and start the drug early enough before surgery that you can titrate it to avoid causing hypotension or a low heart rate.
"Make sure that you’re giving the right amount and monitoring their blood pressure and heart rate," Dr. Fleisher, chair of the guideline writing committee, said in an interview. "Really think once, twice, and thrice about starting a protocol," added Dr. Fleisher, the Robert D. Dripps Professor of Anesthesiology and Critical Care at the University of Pennsylvania, Philadelphia.
The ACC and AHA commissioned a committee to review the evidence for and against beta-blockers in patients undergoing noncardiac surgery. A separate writing committee then considered the evidence review committee’s report, reviewed the literature on other aspects of perioperative care for noncardiac surgery, and compiled a 102-page guideline with a 59-page executive summary.
The "2014 ACC/AHA Guideline on Perioperative Cardiovascular Evaluation and Management of Patients Undergoing Noncardiac Surgery" will be published online on the ACC and AHA websites.
Dr. Fleisher described other highlights of the new guideline pertinent to cardiologists, primary care physicians, or surgeons. For the first time, palliative care has been added as an option that may come out of the preoperative evaluation, he said. Patient categories of high risk and intermediate risk have been lumped together as having "elevated" risk for simplicity’s sake because recommendations for the two separate categories were so similar.
The guideline now endorses two tools to choose from for preoperative risk assessments: the Revised Cardiac Risk Index (RCRI), and the National Surgical Quality Improvement Project risk calculator. "There have been a lot of comments that [the NSQIP] is a very useful tool to have shared decision-making conversations with patients," he said.
Another change applies to patients who receive second- or third-generation coronary stents. Instead of a wait of a year after stent implantation to perform noncardiac surgery, a 6-month wait may be reasonable if the risks of delaying noncardiac surgery outweigh the risks of interrupting dual-antiplatelet therapy for the noncardiac surgery.
In addition, the guideline incorporates findings from the recent POISE-2 study to say that aspirin can be stopped and clonidine is not useful in patients without stents undergoing noncardiac surgery (N. Engl. J. Med. 2014;370:1494-503).
A new statement in the guideline about troponin says to check troponin in high-risk patients with signs or symptoms of trouble but not to include troponin in routine screening.
The recommendations on beta-blockers, however, address the most controversial topic in the guideline, Dr. Fleisher said. "There is a lot of confusing evidence" on the use of beta-blockers, "so we’ve tried to clarify as much as we can."
The ACC and AHA funded the work of the committees. Dr. Fleisher reported having no financial disclosures.
On Twitter @sherryboschert
Findings support endovascular-first approach for ruptured VAAs
BOSTON – Endovascular interventions for ruptured visceral artery aneurysms are associated with reduced morbidity and mortality, compared with open interventions, according to findings from a retrospective chart review.
Both endovascular and open repairs are safe and durable for intact visceral artery aneurysms, Dr. Ankur J. Shukla reported at a meeting hosted by the Society for Vascular Surgery.
Of 261 patients who presented with visceral artery aneurysms (VAAs), 174 underwent repair: 74 who presented with ruptured VAA and 100 who presented with intact VAA. The majority – 73% of ruptured VAA and 62% of intact VAA – were repaired with an endovascular approach.
Among those with ruptured VAA, 30-day mortality was 7.4% following endovascular repair, compared with 28.6% following open repair, a significant difference, said Dr. Shukla of the University of Pittsburgh Medical Center.
Survival at 3 years of ruptured VAA was about 70% vs. 46.4% in the endovascular and open repair groups, respectively, he said.
About 65% of patients with ruptured VAA presented with pain, and about 30% presented in hemodynamic shock. The most commonly identified etiology was "inflammatory/pancreatitis inflammatory," and 80% of the aneurysms were pseudoaneurysmal in nature.
A large proportion of the aneurysms were in the splenic and arterial beds, but 26% were located in the pancreaticoduodenal arcade, and those had a mean size of 12.7 mm. Most (95%) were pseudoaneurysms.
The outcomes with ruptured VAA were quite good, Dr. Shukla said, noting that the technical success rate was 98.7%.
Although the 30-day reintervention rate with endovascular repair was higher, the difference between the groups was not statistically significant, and there was a trend toward a lower rate of major complications with endovascular repair.
Factors found to be predictors of mortality risk were older age and steroid use, while endovascular repair was found to be protective.
As for the patients with intact VAA, most presented without symptoms, and the most common etiology was atherosclerosis.
"When we looked at the distribution, this was very consistent with what has been reported in the literature, with the splenic and arterial beds really making up the lion’s share of this group. Notable is the fact that 6.7% of our patients had intact aneurysms in the pancreaticoduodenal arcade," he said.
Outcomes in those with intact aneurysms were good. A slightly higher 30-day reintervention rate in those who underwent endovascular repair did not reach statistical significance, and both the endovascular and open repair groups had low rates of major complications.
Survival at 3 years for intact aneurysms did not differ in the endovascular and open repair groups. This was partly due to a 0% 30-day mortality, and – despite the fact that the overall mortality in those with intact aneurysms was 10% – there was zero overall aneurysm-related mortality, he said.
Patients in the study were treated at a single institution between 2003 and 2013. Most were in their mid to late 50s, and there were more men and more individuals on immunosuppressive therapy in the ruptured VAA group. However, comorbidities were similar in the ruptured and intact VAA groups.
Visceral artery aneurysms occur only rarely, affecting 0.1% to 2% of the general population, but because of the increasing use of noninvasive imaging, more of these aneurysms are being detected incidentally.
When they are not found incidentally, they often go undetected and present when they rupture, Dr. Shukla said.
"Because of the increasing utilization and improvement of endovascular technology, we now have a lot of options to fix these aneurysms. But the outcomes are not well defined. Even less well defined is the outcome of ruptured visceral artery aneurysms," he said, noting that most studies have a small sample size or look only at endovascular or open repairs.
Overall, the current study showed that there is "an acute and sharp drop-off in survival with open repair," related, most likely, to operative mortality, he said.
"Based on the findings, we recommend aggressive treatment of pseudoaneurysms and true aneurysms in the pancreaticoduodenal arcade, and advocate for an endovascular-first approach to treating ruptured visceral artery aneurysms, acknowledging that success in this is really predicated on good planning based on advanced imaging and endovascular set-up," he concluded.
Dr. Shukla reported having no disclosures.
BOSTON – Endovascular interventions for ruptured visceral artery aneurysms are associated with reduced morbidity and mortality, compared with open interventions, according to findings from a retrospective chart review.
Both endovascular and open repairs are safe and durable for intact visceral artery aneurysms, Dr. Ankur J. Shukla reported at a meeting hosted by the Society for Vascular Surgery.
Of 261 patients who presented with visceral artery aneurysms (VAAs), 174 underwent repair: 74 who presented with ruptured VAA and 100 who presented with intact VAA. The majority – 73% of ruptured VAA and 62% of intact VAA – were repaired with an endovascular approach.
Among those with ruptured VAA, 30-day mortality was 7.4% following endovascular repair, compared with 28.6% following open repair, a significant difference, said Dr. Shukla of the University of Pittsburgh Medical Center.
Survival at 3 years of ruptured VAA was about 70% vs. 46.4% in the endovascular and open repair groups, respectively, he said.
About 65% of patients with ruptured VAA presented with pain, and about 30% presented in hemodynamic shock. The most commonly identified etiology was "inflammatory/pancreatitis inflammatory," and 80% of the aneurysms were pseudoaneurysmal in nature.
A large proportion of the aneurysms were in the splenic and arterial beds, but 26% were located in the pancreaticoduodenal arcade, and those had a mean size of 12.7 mm. Most (95%) were pseudoaneurysms.
The outcomes with ruptured VAA were quite good, Dr. Shukla said, noting that the technical success rate was 98.7%.
Although the 30-day reintervention rate with endovascular repair was higher, the difference between the groups was not statistically significant, and there was a trend toward a lower rate of major complications with endovascular repair.
Factors found to be predictors of mortality risk were older age and steroid use, while endovascular repair was found to be protective.
As for the patients with intact VAA, most presented without symptoms, and the most common etiology was atherosclerosis.
"When we looked at the distribution, this was very consistent with what has been reported in the literature, with the splenic and arterial beds really making up the lion’s share of this group. Notable is the fact that 6.7% of our patients had intact aneurysms in the pancreaticoduodenal arcade," he said.
Outcomes in those with intact aneurysms were good. A slightly higher 30-day reintervention rate in those who underwent endovascular repair did not reach statistical significance, and both the endovascular and open repair groups had low rates of major complications.
Survival at 3 years for intact aneurysms did not differ in the endovascular and open repair groups. This was partly due to a 0% 30-day mortality, and – despite the fact that the overall mortality in those with intact aneurysms was 10% – there was zero overall aneurysm-related mortality, he said.
Patients in the study were treated at a single institution between 2003 and 2013. Most were in their mid to late 50s, and there were more men and more individuals on immunosuppressive therapy in the ruptured VAA group. However, comorbidities were similar in the ruptured and intact VAA groups.
Visceral artery aneurysms occur only rarely, affecting 0.1% to 2% of the general population, but because of the increasing use of noninvasive imaging, more of these aneurysms are being detected incidentally.
When they are not found incidentally, they often go undetected and present when they rupture, Dr. Shukla said.
"Because of the increasing utilization and improvement of endovascular technology, we now have a lot of options to fix these aneurysms. But the outcomes are not well defined. Even less well defined is the outcome of ruptured visceral artery aneurysms," he said, noting that most studies have a small sample size or look only at endovascular or open repairs.
Overall, the current study showed that there is "an acute and sharp drop-off in survival with open repair," related, most likely, to operative mortality, he said.
"Based on the findings, we recommend aggressive treatment of pseudoaneurysms and true aneurysms in the pancreaticoduodenal arcade, and advocate for an endovascular-first approach to treating ruptured visceral artery aneurysms, acknowledging that success in this is really predicated on good planning based on advanced imaging and endovascular set-up," he concluded.
Dr. Shukla reported having no disclosures.
BOSTON – Endovascular interventions for ruptured visceral artery aneurysms are associated with reduced morbidity and mortality, compared with open interventions, according to findings from a retrospective chart review.
Both endovascular and open repairs are safe and durable for intact visceral artery aneurysms, Dr. Ankur J. Shukla reported at a meeting hosted by the Society for Vascular Surgery.
Of 261 patients who presented with visceral artery aneurysms (VAAs), 174 underwent repair: 74 who presented with ruptured VAA and 100 who presented with intact VAA. The majority – 73% of ruptured VAA and 62% of intact VAA – were repaired with an endovascular approach.
Among those with ruptured VAA, 30-day mortality was 7.4% following endovascular repair, compared with 28.6% following open repair, a significant difference, said Dr. Shukla of the University of Pittsburgh Medical Center.
Survival at 3 years of ruptured VAA was about 70% vs. 46.4% in the endovascular and open repair groups, respectively, he said.
About 65% of patients with ruptured VAA presented with pain, and about 30% presented in hemodynamic shock. The most commonly identified etiology was "inflammatory/pancreatitis inflammatory," and 80% of the aneurysms were pseudoaneurysmal in nature.
A large proportion of the aneurysms were in the splenic and arterial beds, but 26% were located in the pancreaticoduodenal arcade, and those had a mean size of 12.7 mm. Most (95%) were pseudoaneurysms.
The outcomes with ruptured VAA were quite good, Dr. Shukla said, noting that the technical success rate was 98.7%.
Although the 30-day reintervention rate with endovascular repair was higher, the difference between the groups was not statistically significant, and there was a trend toward a lower rate of major complications with endovascular repair.
Factors found to be predictors of mortality risk were older age and steroid use, while endovascular repair was found to be protective.
As for the patients with intact VAA, most presented without symptoms, and the most common etiology was atherosclerosis.
"When we looked at the distribution, this was very consistent with what has been reported in the literature, with the splenic and arterial beds really making up the lion’s share of this group. Notable is the fact that 6.7% of our patients had intact aneurysms in the pancreaticoduodenal arcade," he said.
Outcomes in those with intact aneurysms were good. A slightly higher 30-day reintervention rate in those who underwent endovascular repair did not reach statistical significance, and both the endovascular and open repair groups had low rates of major complications.
Survival at 3 years for intact aneurysms did not differ in the endovascular and open repair groups. This was partly due to a 0% 30-day mortality, and – despite the fact that the overall mortality in those with intact aneurysms was 10% – there was zero overall aneurysm-related mortality, he said.
Patients in the study were treated at a single institution between 2003 and 2013. Most were in their mid to late 50s, and there were more men and more individuals on immunosuppressive therapy in the ruptured VAA group. However, comorbidities were similar in the ruptured and intact VAA groups.
Visceral artery aneurysms occur only rarely, affecting 0.1% to 2% of the general population, but because of the increasing use of noninvasive imaging, more of these aneurysms are being detected incidentally.
When they are not found incidentally, they often go undetected and present when they rupture, Dr. Shukla said.
"Because of the increasing utilization and improvement of endovascular technology, we now have a lot of options to fix these aneurysms. But the outcomes are not well defined. Even less well defined is the outcome of ruptured visceral artery aneurysms," he said, noting that most studies have a small sample size or look only at endovascular or open repairs.
Overall, the current study showed that there is "an acute and sharp drop-off in survival with open repair," related, most likely, to operative mortality, he said.
"Based on the findings, we recommend aggressive treatment of pseudoaneurysms and true aneurysms in the pancreaticoduodenal arcade, and advocate for an endovascular-first approach to treating ruptured visceral artery aneurysms, acknowledging that success in this is really predicated on good planning based on advanced imaging and endovascular set-up," he concluded.
Dr. Shukla reported having no disclosures.
AT THE 2014 VASCULAR ANNUAL MEETING
Key clinical point: The researchers recommend aggressive treatment of visceral artery pseudoaneurysms and true aneurysms, with an endovascular-first approach to treating ruptured aneurysms.
Major finding: Thirty-day mortality was 7.4% vs. 26% with endovascular vs. open repair of ruptured VAAs.
Data source: A retrospective chart review involving 174 cases.
Disclosures: Dr. Shukla reported having no disclosures.