User login
Postop troponin elevation, MI impact 5-year survival
SAN FRANCISCO – Postoperative troponin elevation and myocardial infarction both impact 5-year survival following vascular surgery procedures, the results of a large long-term study showed.
In fact, troponin elevation increased the hazard of death by 50% while myocardial infarction increased the hazard of death by nearly threefold, Dr. Jessica P. Simons reported at the annual meeting of the Society for Vascular Surgery. "Future studies are needed to assess the nature of this association as well as the utility of routine postoperative screening for myocardial ischemia," said Dr. Simons of the division of vascular and endovascular surgery at the University of Massachusetts, Worcester.
In a study that she presented on behalf of the Vascular Study Group of New England (VSGNE), Dr. Simons and her associates set out to determine the association of postoperative troponin elevation with long-term survival in patients undergoing vascular surgical procedures. "Postoperative myocardial infarction has been shown to impact short- and long-term mortality," she said. "In addition, troponin elevations have also been shown to negatively impact survival for a wide range of diagnoses. This has been seen in critical care medical literature and also in the general surgical population."
The researchers identified 16,363 VSGNE patients who underwent carotid revascularization, open AAA repair, endovascular AAA repair, or lower-extremity bypass between 2003 and 2011. The exposure variable of interest was postoperative myocardial ischemia, which was categorized as either no ischemia, troponin elevation, or myocardial infarction. The primary end point was survival during the first 5 years postoperatively. They used Kaplan-Meier analyses and Cox proportional hazards models to evaluate the effect of postoperative troponin elevation and myocardial infarction.
Of the 16,363 patients, 15,888 (97.1%) had no ischemia, 211 (1.3%) had troponin elevation, and 264 (1.6%) had myocardial infarction. When this was broken down by procedure type, open AAA had the highest rates of postoperative myocardial ischemia (9%), troponin elevation (3.9%), and myocardial infarction (5.1%), compared with carotid revascularization, endovascular aneurysm repair, and lower-extremity bypass.
The rate of 5-year survival for all procedures was 73% among those with no ischemia, 54% among those with troponin elevation, and 33% among those with myocardial infarction. This difference reached statistical significance with a P value of less than .0001. After adjusting for covariates, the researchers found a similar trend. In this analysis the rate of 5-year survival was 78% among those with no ischemia, 48% among those with troponin elevation, and 35% among those with myocardial infarction. This also reached statistical significance with a P value of less than .0001.
"We performed a subgroup analysis by procedure type, and the trend was the same across all procedure types," Dr. Simons said.
In Cox modeling the researchers found that postoperative ischemia in the form of a troponin elevation increased the hazard of death at 5 years by 45% (HR, 1.45; P =.01) while myocardial infarction nearly tripled the hazard of death (HR, 2.93; P =.0001).
"We have shown an association between postoperative myocardial ischemia and worse survival, but does postoperative myocardial ischemia worsen long-term survival, or does postoperative myocardial ischemia simply identify a high-risk subset of patients?" Dr. Simons asked. "If postoperative myocardial ischemia worsens long-term survival, then efforts should focus on better preoperative medical optimization and perioperative prevention of ischemia. If postoperative myocardial ischemia is simply identifying a high-risk subset of patients, then efforts should focus on better preoperative risk stratification and postoperative medical surveillance."
She concluded that postoperative myocardial ischemia, "whether a troponin elevation or a myocardial infarction, is associated with lower survival. This effect is seen across all procedure types and persists out to 5 years postoperatively."
Dr. Simons said she had no relevant financial disclosures.
SAN FRANCISCO – Postoperative troponin elevation and myocardial infarction both impact 5-year survival following vascular surgery procedures, the results of a large long-term study showed.
In fact, troponin elevation increased the hazard of death by 50% while myocardial infarction increased the hazard of death by nearly threefold, Dr. Jessica P. Simons reported at the annual meeting of the Society for Vascular Surgery. "Future studies are needed to assess the nature of this association as well as the utility of routine postoperative screening for myocardial ischemia," said Dr. Simons of the division of vascular and endovascular surgery at the University of Massachusetts, Worcester.
In a study that she presented on behalf of the Vascular Study Group of New England (VSGNE), Dr. Simons and her associates set out to determine the association of postoperative troponin elevation with long-term survival in patients undergoing vascular surgical procedures. "Postoperative myocardial infarction has been shown to impact short- and long-term mortality," she said. "In addition, troponin elevations have also been shown to negatively impact survival for a wide range of diagnoses. This has been seen in critical care medical literature and also in the general surgical population."
The researchers identified 16,363 VSGNE patients who underwent carotid revascularization, open AAA repair, endovascular AAA repair, or lower-extremity bypass between 2003 and 2011. The exposure variable of interest was postoperative myocardial ischemia, which was categorized as either no ischemia, troponin elevation, or myocardial infarction. The primary end point was survival during the first 5 years postoperatively. They used Kaplan-Meier analyses and Cox proportional hazards models to evaluate the effect of postoperative troponin elevation and myocardial infarction.
Of the 16,363 patients, 15,888 (97.1%) had no ischemia, 211 (1.3%) had troponin elevation, and 264 (1.6%) had myocardial infarction. When this was broken down by procedure type, open AAA had the highest rates of postoperative myocardial ischemia (9%), troponin elevation (3.9%), and myocardial infarction (5.1%), compared with carotid revascularization, endovascular aneurysm repair, and lower-extremity bypass.
The rate of 5-year survival for all procedures was 73% among those with no ischemia, 54% among those with troponin elevation, and 33% among those with myocardial infarction. This difference reached statistical significance with a P value of less than .0001. After adjusting for covariates, the researchers found a similar trend. In this analysis the rate of 5-year survival was 78% among those with no ischemia, 48% among those with troponin elevation, and 35% among those with myocardial infarction. This also reached statistical significance with a P value of less than .0001.
"We performed a subgroup analysis by procedure type, and the trend was the same across all procedure types," Dr. Simons said.
In Cox modeling the researchers found that postoperative ischemia in the form of a troponin elevation increased the hazard of death at 5 years by 45% (HR, 1.45; P =.01) while myocardial infarction nearly tripled the hazard of death (HR, 2.93; P =.0001).
"We have shown an association between postoperative myocardial ischemia and worse survival, but does postoperative myocardial ischemia worsen long-term survival, or does postoperative myocardial ischemia simply identify a high-risk subset of patients?" Dr. Simons asked. "If postoperative myocardial ischemia worsens long-term survival, then efforts should focus on better preoperative medical optimization and perioperative prevention of ischemia. If postoperative myocardial ischemia is simply identifying a high-risk subset of patients, then efforts should focus on better preoperative risk stratification and postoperative medical surveillance."
She concluded that postoperative myocardial ischemia, "whether a troponin elevation or a myocardial infarction, is associated with lower survival. This effect is seen across all procedure types and persists out to 5 years postoperatively."
Dr. Simons said she had no relevant financial disclosures.
SAN FRANCISCO – Postoperative troponin elevation and myocardial infarction both impact 5-year survival following vascular surgery procedures, the results of a large long-term study showed.
In fact, troponin elevation increased the hazard of death by 50% while myocardial infarction increased the hazard of death by nearly threefold, Dr. Jessica P. Simons reported at the annual meeting of the Society for Vascular Surgery. "Future studies are needed to assess the nature of this association as well as the utility of routine postoperative screening for myocardial ischemia," said Dr. Simons of the division of vascular and endovascular surgery at the University of Massachusetts, Worcester.
In a study that she presented on behalf of the Vascular Study Group of New England (VSGNE), Dr. Simons and her associates set out to determine the association of postoperative troponin elevation with long-term survival in patients undergoing vascular surgical procedures. "Postoperative myocardial infarction has been shown to impact short- and long-term mortality," she said. "In addition, troponin elevations have also been shown to negatively impact survival for a wide range of diagnoses. This has been seen in critical care medical literature and also in the general surgical population."
The researchers identified 16,363 VSGNE patients who underwent carotid revascularization, open AAA repair, endovascular AAA repair, or lower-extremity bypass between 2003 and 2011. The exposure variable of interest was postoperative myocardial ischemia, which was categorized as either no ischemia, troponin elevation, or myocardial infarction. The primary end point was survival during the first 5 years postoperatively. They used Kaplan-Meier analyses and Cox proportional hazards models to evaluate the effect of postoperative troponin elevation and myocardial infarction.
Of the 16,363 patients, 15,888 (97.1%) had no ischemia, 211 (1.3%) had troponin elevation, and 264 (1.6%) had myocardial infarction. When this was broken down by procedure type, open AAA had the highest rates of postoperative myocardial ischemia (9%), troponin elevation (3.9%), and myocardial infarction (5.1%), compared with carotid revascularization, endovascular aneurysm repair, and lower-extremity bypass.
The rate of 5-year survival for all procedures was 73% among those with no ischemia, 54% among those with troponin elevation, and 33% among those with myocardial infarction. This difference reached statistical significance with a P value of less than .0001. After adjusting for covariates, the researchers found a similar trend. In this analysis the rate of 5-year survival was 78% among those with no ischemia, 48% among those with troponin elevation, and 35% among those with myocardial infarction. This also reached statistical significance with a P value of less than .0001.
"We performed a subgroup analysis by procedure type, and the trend was the same across all procedure types," Dr. Simons said.
In Cox modeling the researchers found that postoperative ischemia in the form of a troponin elevation increased the hazard of death at 5 years by 45% (HR, 1.45; P =.01) while myocardial infarction nearly tripled the hazard of death (HR, 2.93; P =.0001).
"We have shown an association between postoperative myocardial ischemia and worse survival, but does postoperative myocardial ischemia worsen long-term survival, or does postoperative myocardial ischemia simply identify a high-risk subset of patients?" Dr. Simons asked. "If postoperative myocardial ischemia worsens long-term survival, then efforts should focus on better preoperative medical optimization and perioperative prevention of ischemia. If postoperative myocardial ischemia is simply identifying a high-risk subset of patients, then efforts should focus on better preoperative risk stratification and postoperative medical surveillance."
She concluded that postoperative myocardial ischemia, "whether a troponin elevation or a myocardial infarction, is associated with lower survival. This effect is seen across all procedure types and persists out to 5 years postoperatively."
Dr. Simons said she had no relevant financial disclosures.
AT THE SVS ANNUAL MEETING
Major finding: Postoperative ischemia in the form of a troponin elevation increased the hazard of death at 5 years by 45% (HR, 1.45; P =.01) while myocardial infarction nearly tripled the hazard of death (HR, 2.93; P =.0001).
Data source: A study of 16,363 Vascular Study Group of New England patients who underwent carotid revascularization, open AAA repair, endovascular AAA repair, or lower-extremity bypass between 2003 and 2011.
Disclosures: Dr. Simons said she had no relevant financial disclosures.
Reablate, don't medicate, after failed AF ablation
DENVER – After a failed first ablation procedure for paroxysmal atrial fibrillation, redo ablation proved more effective than did antiarrhythmic drug therapy in a randomized trial, Dr. Jonathan Steinberg reported at the annual meeting of the Heart Rhythm Society.
The success rate of a first ablation procedure in patients with symptomatic paroxysmal AF is typically about 60%. What to do for the 40% who are nonresponders has been unclear, with no prior randomized clinical trial evidence available to guide decisions, noted Dr. Steinberg of Columbia University, New York.
The study comprised 154 patients with recurrent symptomatic paroxysmal AF 3 months after an initial ablation procedure involving only pulmonary vein isolation. All participants received an implantable loop recorder to track atrial arrhythmic events.
They were then randomized to redo-ablation limited to reisolation of the pulmonary vein, which was successfully accomplished in all instances, or to guideline-based antiarrhythmic drug therapy. The choice of drug was left to individual investigator discretion. The three options were propafenone at 450-900 mg/day, sotalol at 160-320 mg/day, or flecainide at 200-400 mg/day. Propafenone was selected in the majority of cases, at an average dose of 579 mg/day.
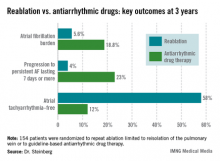
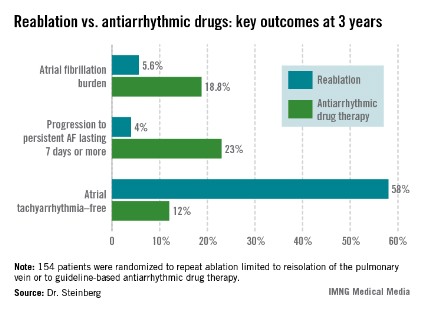
The average AF burden as measured by implantable loop recorder at randomization was 15%. The primary study endpoint was AF burden at 36 months of follow-up, which was 5.6% in the redo-ablation group compared with 18.8% in the antiarrhythmic drug group.
Secondary endpoints uniformly favored redo-ablation as well.
Data from the implantable loop recorders was evaluated every 3 months during 3 years of follow-up. As early as 3 months into the study, the group given antiarrhythmic drugs had an AF burden of 3.3%, significantly higher than the 1.9% rate seen in the redo-ablation group. Thereafter, the drug therapy group experienced a gradual increase in AF burden throughout the first 12-15 months, followed by a much more substantial increase during the remainder of the study.
"The redo-ablation group had a different pattern" of AF burden, Dr. Steinberg observed. "It was low throughout the first 12-15 months, with just a slight increase, and it then rose only gradually over time until the 36-month end of the study."
Freedom from any atrial tachyarrhythmia at 1 year was 30% in the antiarrhythmic drug therapy group and 75% in the redo-ablation group. By 3 years, 12% of those in the drug therapy group were free of atrial tachyarrhythmias as were 58% in the redo-ablation group.
Complications in the redo-ablation group consisted of two cases of cardiac tamponade. In contrast, 49 patients, or 64%, in the antiarrhythmic drug therapy group discontinued medication due to intolerance or ineffectiveness.
Session cochair Dr. Gordon Tomaselli of Johns Hopkins University, Baltimore, said that in light of the potential proarrhythmic effects of virtually all antiarrhythmic drugs, it would have been useful to include a no-antiarrhythmic drug control group in the study.
Dr. Steinberg reported having no conflicts of interest.
DENVER – After a failed first ablation procedure for paroxysmal atrial fibrillation, redo ablation proved more effective than did antiarrhythmic drug therapy in a randomized trial, Dr. Jonathan Steinberg reported at the annual meeting of the Heart Rhythm Society.
The success rate of a first ablation procedure in patients with symptomatic paroxysmal AF is typically about 60%. What to do for the 40% who are nonresponders has been unclear, with no prior randomized clinical trial evidence available to guide decisions, noted Dr. Steinberg of Columbia University, New York.
The study comprised 154 patients with recurrent symptomatic paroxysmal AF 3 months after an initial ablation procedure involving only pulmonary vein isolation. All participants received an implantable loop recorder to track atrial arrhythmic events.
They were then randomized to redo-ablation limited to reisolation of the pulmonary vein, which was successfully accomplished in all instances, or to guideline-based antiarrhythmic drug therapy. The choice of drug was left to individual investigator discretion. The three options were propafenone at 450-900 mg/day, sotalol at 160-320 mg/day, or flecainide at 200-400 mg/day. Propafenone was selected in the majority of cases, at an average dose of 579 mg/day.
The average AF burden as measured by implantable loop recorder at randomization was 15%. The primary study endpoint was AF burden at 36 months of follow-up, which was 5.6% in the redo-ablation group compared with 18.8% in the antiarrhythmic drug group.
Secondary endpoints uniformly favored redo-ablation as well.
Data from the implantable loop recorders was evaluated every 3 months during 3 years of follow-up. As early as 3 months into the study, the group given antiarrhythmic drugs had an AF burden of 3.3%, significantly higher than the 1.9% rate seen in the redo-ablation group. Thereafter, the drug therapy group experienced a gradual increase in AF burden throughout the first 12-15 months, followed by a much more substantial increase during the remainder of the study.
"The redo-ablation group had a different pattern" of AF burden, Dr. Steinberg observed. "It was low throughout the first 12-15 months, with just a slight increase, and it then rose only gradually over time until the 36-month end of the study."
Freedom from any atrial tachyarrhythmia at 1 year was 30% in the antiarrhythmic drug therapy group and 75% in the redo-ablation group. By 3 years, 12% of those in the drug therapy group were free of atrial tachyarrhythmias as were 58% in the redo-ablation group.
Complications in the redo-ablation group consisted of two cases of cardiac tamponade. In contrast, 49 patients, or 64%, in the antiarrhythmic drug therapy group discontinued medication due to intolerance or ineffectiveness.
Session cochair Dr. Gordon Tomaselli of Johns Hopkins University, Baltimore, said that in light of the potential proarrhythmic effects of virtually all antiarrhythmic drugs, it would have been useful to include a no-antiarrhythmic drug control group in the study.
Dr. Steinberg reported having no conflicts of interest.
DENVER – After a failed first ablation procedure for paroxysmal atrial fibrillation, redo ablation proved more effective than did antiarrhythmic drug therapy in a randomized trial, Dr. Jonathan Steinberg reported at the annual meeting of the Heart Rhythm Society.
The success rate of a first ablation procedure in patients with symptomatic paroxysmal AF is typically about 60%. What to do for the 40% who are nonresponders has been unclear, with no prior randomized clinical trial evidence available to guide decisions, noted Dr. Steinberg of Columbia University, New York.
The study comprised 154 patients with recurrent symptomatic paroxysmal AF 3 months after an initial ablation procedure involving only pulmonary vein isolation. All participants received an implantable loop recorder to track atrial arrhythmic events.
They were then randomized to redo-ablation limited to reisolation of the pulmonary vein, which was successfully accomplished in all instances, or to guideline-based antiarrhythmic drug therapy. The choice of drug was left to individual investigator discretion. The three options were propafenone at 450-900 mg/day, sotalol at 160-320 mg/day, or flecainide at 200-400 mg/day. Propafenone was selected in the majority of cases, at an average dose of 579 mg/day.
The average AF burden as measured by implantable loop recorder at randomization was 15%. The primary study endpoint was AF burden at 36 months of follow-up, which was 5.6% in the redo-ablation group compared with 18.8% in the antiarrhythmic drug group.
Secondary endpoints uniformly favored redo-ablation as well.
Data from the implantable loop recorders was evaluated every 3 months during 3 years of follow-up. As early as 3 months into the study, the group given antiarrhythmic drugs had an AF burden of 3.3%, significantly higher than the 1.9% rate seen in the redo-ablation group. Thereafter, the drug therapy group experienced a gradual increase in AF burden throughout the first 12-15 months, followed by a much more substantial increase during the remainder of the study.
"The redo-ablation group had a different pattern" of AF burden, Dr. Steinberg observed. "It was low throughout the first 12-15 months, with just a slight increase, and it then rose only gradually over time until the 36-month end of the study."
Freedom from any atrial tachyarrhythmia at 1 year was 30% in the antiarrhythmic drug therapy group and 75% in the redo-ablation group. By 3 years, 12% of those in the drug therapy group were free of atrial tachyarrhythmias as were 58% in the redo-ablation group.
Complications in the redo-ablation group consisted of two cases of cardiac tamponade. In contrast, 49 patients, or 64%, in the antiarrhythmic drug therapy group discontinued medication due to intolerance or ineffectiveness.
Session cochair Dr. Gordon Tomaselli of Johns Hopkins University, Baltimore, said that in light of the potential proarrhythmic effects of virtually all antiarrhythmic drugs, it would have been useful to include a no-antiarrhythmic drug control group in the study.
Dr. Steinberg reported having no conflicts of interest.
AT HEART RHYTHM 2013
Major Finding: At 3 years after a first ablation procedure had failed for symptomatic paroxysmal atrial fibrillation, 12% of those randomized to drug therapy and 58% of those in the redo-ablation group were free of atrial tachyarrhythmias.
Data Source: A randomized, prospective, multicenter clinical trial involving 154 patients whose atrial arrhythmia status was monitored via implantable loop recorder.
Disclosures: The presenter reported having no conflicts of interest.
Biomarkers predict response to cardiac resynchronization therapy
DENVER – Levels of troponin T and brain natriuretic peptide prior to implantation of a cardiac resynchronization device were strongly predictive of the risk of death or hospitalization for heart failure during the first year of device therapy in the BENEFIT study.
BENEFIT was a 1-year observational study undertaken to determine whether markers could predict which candidates for cardiac resynchronization therapy (CRT) were least likely to benefit from the costly device. At present, roughly 30% of patients who appropriately receive a CRT device do not respond to the treatment.
"We are all at this point frustrated with the persistent portion of CRT recipients who do not respond to therapy," BENEFIT principal investigator Dr. Alaa A. Shalaby said in presenting the study findings at the annual meeting of the Heart Rhythm Society. "We think our results suggest CRT should be offered earlier in the course of the disease"
BENEFIT included 267 CRT recipients at 33 centers. Patients were placed into one of three predefined risk categories based upon baseline levels of brain natriuretic peptide (BNP) and troponin T (TnT). On the basis os prior studies, high BNP was defined as 440 pg/mL or greater and a detectible TnT level was 0.01 ng/mL or greater. The low-risk group had an undetectable TnT and a BNP below 440 pg/mL. The intermediate-risk group had either an elevated BNP or TnT. The high-risk group had elevated BNP and TnT, reported Dr. Shalaby of the University of Pittsburgh.
The median baseline BNP in the study population was 198 pg/mL.
The three groups were similar in terms of baseline characteristics, including the proportion of patients with ischemic cardiomyopathy. Based on BNP and TnT results, 59% of patients were in the low-risk category, 33% had intermediate risk, and 8% were deemed high risk. The intermediate-risk group was evenly divided between patients who qualified on the basis of a high BNP and those with a detectable TnT.
During 12 months of follow-up there were 11 deaths and 19 heart failure hospitalizations. The incidence of either endpoint was 7% in the low-risk group, 15% in the intermediate-risk group, and 30% in the high-risk cohort.
After the researchers adjusted the results for age, left ventricular ejection fraction, QRS duration, and NYHA class, the risk of death or heart failure hospitalization was 2.5-fold greater in the group with intermediate-level baseline biomarkers as in the low-risk group. The high-biomarker group had a 7.3-fold increased risk, compared with the low-biomarker cohort. Both of these differences were statistically significant.
Changes in the biomarker levels after 6 and 12 months of CRT will be the subject of a future BENEFIT analysis, he noted.
The BENEFIT study was supported by St. Jude Medical. Dr. Shalaby reported having no conflicts of interest.
DENVER – Levels of troponin T and brain natriuretic peptide prior to implantation of a cardiac resynchronization device were strongly predictive of the risk of death or hospitalization for heart failure during the first year of device therapy in the BENEFIT study.
BENEFIT was a 1-year observational study undertaken to determine whether markers could predict which candidates for cardiac resynchronization therapy (CRT) were least likely to benefit from the costly device. At present, roughly 30% of patients who appropriately receive a CRT device do not respond to the treatment.
"We are all at this point frustrated with the persistent portion of CRT recipients who do not respond to therapy," BENEFIT principal investigator Dr. Alaa A. Shalaby said in presenting the study findings at the annual meeting of the Heart Rhythm Society. "We think our results suggest CRT should be offered earlier in the course of the disease"
BENEFIT included 267 CRT recipients at 33 centers. Patients were placed into one of three predefined risk categories based upon baseline levels of brain natriuretic peptide (BNP) and troponin T (TnT). On the basis os prior studies, high BNP was defined as 440 pg/mL or greater and a detectible TnT level was 0.01 ng/mL or greater. The low-risk group had an undetectable TnT and a BNP below 440 pg/mL. The intermediate-risk group had either an elevated BNP or TnT. The high-risk group had elevated BNP and TnT, reported Dr. Shalaby of the University of Pittsburgh.
The median baseline BNP in the study population was 198 pg/mL.
The three groups were similar in terms of baseline characteristics, including the proportion of patients with ischemic cardiomyopathy. Based on BNP and TnT results, 59% of patients were in the low-risk category, 33% had intermediate risk, and 8% were deemed high risk. The intermediate-risk group was evenly divided between patients who qualified on the basis of a high BNP and those with a detectable TnT.
During 12 months of follow-up there were 11 deaths and 19 heart failure hospitalizations. The incidence of either endpoint was 7% in the low-risk group, 15% in the intermediate-risk group, and 30% in the high-risk cohort.
After the researchers adjusted the results for age, left ventricular ejection fraction, QRS duration, and NYHA class, the risk of death or heart failure hospitalization was 2.5-fold greater in the group with intermediate-level baseline biomarkers as in the low-risk group. The high-biomarker group had a 7.3-fold increased risk, compared with the low-biomarker cohort. Both of these differences were statistically significant.
Changes in the biomarker levels after 6 and 12 months of CRT will be the subject of a future BENEFIT analysis, he noted.
The BENEFIT study was supported by St. Jude Medical. Dr. Shalaby reported having no conflicts of interest.
DENVER – Levels of troponin T and brain natriuretic peptide prior to implantation of a cardiac resynchronization device were strongly predictive of the risk of death or hospitalization for heart failure during the first year of device therapy in the BENEFIT study.
BENEFIT was a 1-year observational study undertaken to determine whether markers could predict which candidates for cardiac resynchronization therapy (CRT) were least likely to benefit from the costly device. At present, roughly 30% of patients who appropriately receive a CRT device do not respond to the treatment.
"We are all at this point frustrated with the persistent portion of CRT recipients who do not respond to therapy," BENEFIT principal investigator Dr. Alaa A. Shalaby said in presenting the study findings at the annual meeting of the Heart Rhythm Society. "We think our results suggest CRT should be offered earlier in the course of the disease"
BENEFIT included 267 CRT recipients at 33 centers. Patients were placed into one of three predefined risk categories based upon baseline levels of brain natriuretic peptide (BNP) and troponin T (TnT). On the basis os prior studies, high BNP was defined as 440 pg/mL or greater and a detectible TnT level was 0.01 ng/mL or greater. The low-risk group had an undetectable TnT and a BNP below 440 pg/mL. The intermediate-risk group had either an elevated BNP or TnT. The high-risk group had elevated BNP and TnT, reported Dr. Shalaby of the University of Pittsburgh.
The median baseline BNP in the study population was 198 pg/mL.
The three groups were similar in terms of baseline characteristics, including the proportion of patients with ischemic cardiomyopathy. Based on BNP and TnT results, 59% of patients were in the low-risk category, 33% had intermediate risk, and 8% were deemed high risk. The intermediate-risk group was evenly divided between patients who qualified on the basis of a high BNP and those with a detectable TnT.
During 12 months of follow-up there were 11 deaths and 19 heart failure hospitalizations. The incidence of either endpoint was 7% in the low-risk group, 15% in the intermediate-risk group, and 30% in the high-risk cohort.
After the researchers adjusted the results for age, left ventricular ejection fraction, QRS duration, and NYHA class, the risk of death or heart failure hospitalization was 2.5-fold greater in the group with intermediate-level baseline biomarkers as in the low-risk group. The high-biomarker group had a 7.3-fold increased risk, compared with the low-biomarker cohort. Both of these differences were statistically significant.
Changes in the biomarker levels after 6 and 12 months of CRT will be the subject of a future BENEFIT analysis, he noted.
The BENEFIT study was supported by St. Jude Medical. Dr. Shalaby reported having no conflicts of interest.
AT HEART RHYTHM 2013
Major finding: Cardiac resynchronization therapy device recipients with a preimplantation brain natriuretic peptide level of at least 440 pg/mL and a detectable troponin T level were 7.3-fold more likely to die or be hospitalized for heart failure during the first 12 months after device implantation than were those with an undetectable troponin T level and a lower brain natriuretic peptide level.
Data source: The BENEFIT study was a prospective, 33-center study in which 267 patients who received a CRT device were followed for 1 year.
Disclosures: BENEFIT was supported by St. Jude Medical. The presenter reported having no conflicts of interest.
Dilation and regurgitation complicate outcomes of ASO for TGA
Neoaortic root dilation and neoaortic valve regurgitation are common complications in infants with transposition of the great arteries who undergo an arterial switch operation for repair, and the risk of developing these changes in the neoaorta increases over time, according to the results of a retrospective database study of patients at Children’s Hospital of Wisconsin.
In addition, when dilation occurs, the dimensions may progressively enlarge over time, making it important to maintain lifelong surveillance of this population, according to a report published the Annals of Thoracic Surgery.
Although perioperative mortality and long-term survival (assessed up to 30 years) has improved in more recent eras for use of an arterial switch operation (ASO) for transposition of the great arteries (TGA), these long-term studies have also shown important late complications that may contribute to late morbidity and the need for reoperation, according to Dr. Jennifer G. Co-Vu and her colleagues at the Medical College of Wisconsin, Milwaukee.
These complications include coronary artery insufficiency, right ventricular outflow tract obstructions, and problems with the native pulmonary root and the pulmonary valve functioning as the neoaortic root and the neoaortic valve, respectively. Dr. Co-Vu and her colleagues performed their study to determine the prevalence of neoaortic root dilation and neoaortic valve regurgitation in patients treated at their institution and to determine risk factors involved in the development of these late complications.
Out of 247 patients with TGA treated with an ASO at the hospital, there were 124 patients who had at least one available postoperative transthoracic echocardiogram at least 1 year after the ASO. Median age of these patients was 0.2 months at the time of their ASO and 7.2 years at their last follow-up; 71% were boys (Ann. Thorac. Surg. 2013;95:1654-9).
Retrospective measurements of the neoaortic annulus and root were performed on all available transthoracic echocardiograms and the severity of neoaortic valve regurgitation was determined by assessing the width of the color Doppler jet of regurgitation measured at the level of the valve in the parasternal long-axis view. A jet width of 1-4 mm was defined as trivial to mild; 4-6 mm was defined as moderate; and greater than 6 mm indicated severe regurgitation, according to the researchers. Significant regurgitation was defined as moderate or severe. Significant neoaortic annulus dilation was defined as a z score of 2.5 or greater.
They evaluated potential risk factors for the development of neoaortic root dilation, annulus dilation, and neoaortic valve regurgitation, including age at ASO, sex, weight, coexisting lesions, prior interventions, and associated operations with the ASO. Coexisting lesions and prior interventions occurred in nearly 60% of the patients. The most common previous intervention was balloon atrial septostomy (54%).
Significant neoaortic root dilation developed in 88 of 124 (66%) of the patients during follow-up, with the probability of being free from a root diameter z score of 2.5 or greater of 84%, 67%, 47% and 32% at 1, 5, 10, and 15 years, respectively. Significant risk factors predicting neoaortic root dilation using multivariate analysis were a history of double outlet right ventricle (DORV), previous pulmonary artery (PA) banding, and length of follow-up. A history of ventricular septal defect (VSD), coarctation, left ventricular outflow tract obstruction, and age at ASO were not significant risk factors.
Significant annulus dilation occurred in 54% of patients, with significant risk factors including a history of VSD, history of DORV, and the presence of a dilated neoaortic root. History of PA banding and length of follow-up were not significant.
Moderate or severe neoaortic valve regurgitation occurred in 17 of 124 (14%) of the patients, with a probability of being free of these levels of regurgitation of 96%, 92%, 89%, and 75% at 1, 5, 10, and 15 years, respectively. The significant risk factors for regurgitation were history of DORV, VSD, left ventricular outflow tract obstruction, and length of follow-up.
No patient in the series reported by the researchers required reintervention on the neoaorta.
Limitations of the study included its retrospective nature; the lack of postoperative echocardiograms on all patients, which raises questions of selection bias; and an inability to account for changes in surgical and postoperative management over time, the authors noted.
"Neoaortic root dilation and neoaortic valve regurgitation are common complications in patients with TGA after repair with ASO. Patients with DORV morphologies or previous PA banding may be at higher risk for these complications," the researchers concluded.
The authors had no relevant disclosures.
Most people living with congenital heart disease are now adults. Unfortunately the majority of these adults, for unclear reasons, are not receiving expert care by congenital heart specialists. Perhaps some of these adults have a misperception that they are cured. Co-Vu and coauthors at the Medical College of Wisconsin confirm that the highly successful arterial switch operation is not a ‘cure.’ Their important, carefully executed echo study demonstrates important progressive increases in measured diameters of the neoaortic annulus and neoaortic root over the first 15 years of life. The authors point out that the dilation has not as yet led to a need for reintervention, and the late prevalence of neoaortic regurgitation is not high, although it too is slowly increasing over time. Their message is a clarion call for lifelong clinical surveillance following an arterial switch operation; a message that should be applied to all patients with congenital heart disease.
Dr. William G. Williams is executive director of the Congenital Heart Surgeons’ Society Data Center, Toronto, and emeritus professor of surgery, University of Toronto, and an associate medical editor for Thoracic Surgery News.
Most people living with congenital heart disease are now adults. Unfortunately the majority of these adults, for unclear reasons, are not receiving expert care by congenital heart specialists. Perhaps some of these adults have a misperception that they are cured. Co-Vu and coauthors at the Medical College of Wisconsin confirm that the highly successful arterial switch operation is not a ‘cure.’ Their important, carefully executed echo study demonstrates important progressive increases in measured diameters of the neoaortic annulus and neoaortic root over the first 15 years of life. The authors point out that the dilation has not as yet led to a need for reintervention, and the late prevalence of neoaortic regurgitation is not high, although it too is slowly increasing over time. Their message is a clarion call for lifelong clinical surveillance following an arterial switch operation; a message that should be applied to all patients with congenital heart disease.
Dr. William G. Williams is executive director of the Congenital Heart Surgeons’ Society Data Center, Toronto, and emeritus professor of surgery, University of Toronto, and an associate medical editor for Thoracic Surgery News.
Most people living with congenital heart disease are now adults. Unfortunately the majority of these adults, for unclear reasons, are not receiving expert care by congenital heart specialists. Perhaps some of these adults have a misperception that they are cured. Co-Vu and coauthors at the Medical College of Wisconsin confirm that the highly successful arterial switch operation is not a ‘cure.’ Their important, carefully executed echo study demonstrates important progressive increases in measured diameters of the neoaortic annulus and neoaortic root over the first 15 years of life. The authors point out that the dilation has not as yet led to a need for reintervention, and the late prevalence of neoaortic regurgitation is not high, although it too is slowly increasing over time. Their message is a clarion call for lifelong clinical surveillance following an arterial switch operation; a message that should be applied to all patients with congenital heart disease.
Dr. William G. Williams is executive director of the Congenital Heart Surgeons’ Society Data Center, Toronto, and emeritus professor of surgery, University of Toronto, and an associate medical editor for Thoracic Surgery News.
Neoaortic root dilation and neoaortic valve regurgitation are common complications in infants with transposition of the great arteries who undergo an arterial switch operation for repair, and the risk of developing these changes in the neoaorta increases over time, according to the results of a retrospective database study of patients at Children’s Hospital of Wisconsin.
In addition, when dilation occurs, the dimensions may progressively enlarge over time, making it important to maintain lifelong surveillance of this population, according to a report published the Annals of Thoracic Surgery.
Although perioperative mortality and long-term survival (assessed up to 30 years) has improved in more recent eras for use of an arterial switch operation (ASO) for transposition of the great arteries (TGA), these long-term studies have also shown important late complications that may contribute to late morbidity and the need for reoperation, according to Dr. Jennifer G. Co-Vu and her colleagues at the Medical College of Wisconsin, Milwaukee.
These complications include coronary artery insufficiency, right ventricular outflow tract obstructions, and problems with the native pulmonary root and the pulmonary valve functioning as the neoaortic root and the neoaortic valve, respectively. Dr. Co-Vu and her colleagues performed their study to determine the prevalence of neoaortic root dilation and neoaortic valve regurgitation in patients treated at their institution and to determine risk factors involved in the development of these late complications.
Out of 247 patients with TGA treated with an ASO at the hospital, there were 124 patients who had at least one available postoperative transthoracic echocardiogram at least 1 year after the ASO. Median age of these patients was 0.2 months at the time of their ASO and 7.2 years at their last follow-up; 71% were boys (Ann. Thorac. Surg. 2013;95:1654-9).
Retrospective measurements of the neoaortic annulus and root were performed on all available transthoracic echocardiograms and the severity of neoaortic valve regurgitation was determined by assessing the width of the color Doppler jet of regurgitation measured at the level of the valve in the parasternal long-axis view. A jet width of 1-4 mm was defined as trivial to mild; 4-6 mm was defined as moderate; and greater than 6 mm indicated severe regurgitation, according to the researchers. Significant regurgitation was defined as moderate or severe. Significant neoaortic annulus dilation was defined as a z score of 2.5 or greater.
They evaluated potential risk factors for the development of neoaortic root dilation, annulus dilation, and neoaortic valve regurgitation, including age at ASO, sex, weight, coexisting lesions, prior interventions, and associated operations with the ASO. Coexisting lesions and prior interventions occurred in nearly 60% of the patients. The most common previous intervention was balloon atrial septostomy (54%).
Significant neoaortic root dilation developed in 88 of 124 (66%) of the patients during follow-up, with the probability of being free from a root diameter z score of 2.5 or greater of 84%, 67%, 47% and 32% at 1, 5, 10, and 15 years, respectively. Significant risk factors predicting neoaortic root dilation using multivariate analysis were a history of double outlet right ventricle (DORV), previous pulmonary artery (PA) banding, and length of follow-up. A history of ventricular septal defect (VSD), coarctation, left ventricular outflow tract obstruction, and age at ASO were not significant risk factors.
Significant annulus dilation occurred in 54% of patients, with significant risk factors including a history of VSD, history of DORV, and the presence of a dilated neoaortic root. History of PA banding and length of follow-up were not significant.
Moderate or severe neoaortic valve regurgitation occurred in 17 of 124 (14%) of the patients, with a probability of being free of these levels of regurgitation of 96%, 92%, 89%, and 75% at 1, 5, 10, and 15 years, respectively. The significant risk factors for regurgitation were history of DORV, VSD, left ventricular outflow tract obstruction, and length of follow-up.
No patient in the series reported by the researchers required reintervention on the neoaorta.
Limitations of the study included its retrospective nature; the lack of postoperative echocardiograms on all patients, which raises questions of selection bias; and an inability to account for changes in surgical and postoperative management over time, the authors noted.
"Neoaortic root dilation and neoaortic valve regurgitation are common complications in patients with TGA after repair with ASO. Patients with DORV morphologies or previous PA banding may be at higher risk for these complications," the researchers concluded.
The authors had no relevant disclosures.
Neoaortic root dilation and neoaortic valve regurgitation are common complications in infants with transposition of the great arteries who undergo an arterial switch operation for repair, and the risk of developing these changes in the neoaorta increases over time, according to the results of a retrospective database study of patients at Children’s Hospital of Wisconsin.
In addition, when dilation occurs, the dimensions may progressively enlarge over time, making it important to maintain lifelong surveillance of this population, according to a report published the Annals of Thoracic Surgery.
Although perioperative mortality and long-term survival (assessed up to 30 years) has improved in more recent eras for use of an arterial switch operation (ASO) for transposition of the great arteries (TGA), these long-term studies have also shown important late complications that may contribute to late morbidity and the need for reoperation, according to Dr. Jennifer G. Co-Vu and her colleagues at the Medical College of Wisconsin, Milwaukee.
These complications include coronary artery insufficiency, right ventricular outflow tract obstructions, and problems with the native pulmonary root and the pulmonary valve functioning as the neoaortic root and the neoaortic valve, respectively. Dr. Co-Vu and her colleagues performed their study to determine the prevalence of neoaortic root dilation and neoaortic valve regurgitation in patients treated at their institution and to determine risk factors involved in the development of these late complications.
Out of 247 patients with TGA treated with an ASO at the hospital, there were 124 patients who had at least one available postoperative transthoracic echocardiogram at least 1 year after the ASO. Median age of these patients was 0.2 months at the time of their ASO and 7.2 years at their last follow-up; 71% were boys (Ann. Thorac. Surg. 2013;95:1654-9).
Retrospective measurements of the neoaortic annulus and root were performed on all available transthoracic echocardiograms and the severity of neoaortic valve regurgitation was determined by assessing the width of the color Doppler jet of regurgitation measured at the level of the valve in the parasternal long-axis view. A jet width of 1-4 mm was defined as trivial to mild; 4-6 mm was defined as moderate; and greater than 6 mm indicated severe regurgitation, according to the researchers. Significant regurgitation was defined as moderate or severe. Significant neoaortic annulus dilation was defined as a z score of 2.5 or greater.
They evaluated potential risk factors for the development of neoaortic root dilation, annulus dilation, and neoaortic valve regurgitation, including age at ASO, sex, weight, coexisting lesions, prior interventions, and associated operations with the ASO. Coexisting lesions and prior interventions occurred in nearly 60% of the patients. The most common previous intervention was balloon atrial septostomy (54%).
Significant neoaortic root dilation developed in 88 of 124 (66%) of the patients during follow-up, with the probability of being free from a root diameter z score of 2.5 or greater of 84%, 67%, 47% and 32% at 1, 5, 10, and 15 years, respectively. Significant risk factors predicting neoaortic root dilation using multivariate analysis were a history of double outlet right ventricle (DORV), previous pulmonary artery (PA) banding, and length of follow-up. A history of ventricular septal defect (VSD), coarctation, left ventricular outflow tract obstruction, and age at ASO were not significant risk factors.
Significant annulus dilation occurred in 54% of patients, with significant risk factors including a history of VSD, history of DORV, and the presence of a dilated neoaortic root. History of PA banding and length of follow-up were not significant.
Moderate or severe neoaortic valve regurgitation occurred in 17 of 124 (14%) of the patients, with a probability of being free of these levels of regurgitation of 96%, 92%, 89%, and 75% at 1, 5, 10, and 15 years, respectively. The significant risk factors for regurgitation were history of DORV, VSD, left ventricular outflow tract obstruction, and length of follow-up.
No patient in the series reported by the researchers required reintervention on the neoaorta.
Limitations of the study included its retrospective nature; the lack of postoperative echocardiograms on all patients, which raises questions of selection bias; and an inability to account for changes in surgical and postoperative management over time, the authors noted.
"Neoaortic root dilation and neoaortic valve regurgitation are common complications in patients with TGA after repair with ASO. Patients with DORV morphologies or previous PA banding may be at higher risk for these complications," the researchers concluded.
The authors had no relevant disclosures.
FROM ANNALS OF THORACIC SURGERY
TAVR quickly dominates high-risk aortic stenosis
SAN FRANCISCO – It’s been barely half a year since U.S. cardiologists and cardiac surgeons first became able to routinely offer operable, high-risk patients with aortic stenosis the option of transcatheter valve replacement, yet in the first few months the transcatheter approach quickly rivaled open surgery.
But for the time being in U.S. practice, transcatheter aortic valve replacement (TAVR) remains boxed into the high-risk niche, along with the subgroup of patients who are not suitable for open surgery, king of a pair of relatively small hills.
And no matter how well TAVR performs in the current pair of trials that are comparing it with open surgical aortic valve replacement (SAVR) for intermediate-risk patients, it will remain relegated to niche status for years to come. That’s because roughly two-thirds of all operable patients with aortic stenosis who need valve replacement fall into the low-risk category, with a Society of Thoracic Surgeons (STS) risk score of less than 4%, which experts agree will remain SAVR’s exclusive territory for the foreseeable future.
The high-risk stratum of operable patients, which TAVR now dominates, constitutes about 10% of all patients who need a new aortic valve and can undergo open surgery, patients with an STS score greater than 8%. The intermediate-risk category – an STS score of 4%-8%, where TAVR now vies against open SAVR in two high-profile trials – makes up the final quarter of the operable-patient pie.
Within the high-risk and operable universe, TAVR’s rise has been meteoric, starting last October when the Food and Drug Administration gave Edwards, marketer of the SAPIEN valve system, approval for these patients. A small survey of operators from U.S. TAVR programs in March at the annual scientific session of the American College of Cardiology (ACC) revealed a uniform perception that by early 2013 a sizable majority of U.S. patients with severe aortic stenosis who are deemed operable and are at high surgical risk will wind up being treated by TAVR instead of SAVR. The cardiac surgeons who collaborate on TAVR seem to have fully conceded the advantages of TAVR for these patients.
"We generally go with TAVR. Most patients want it, and with the equivalence" in outcomes from the first PARTNER (Placement of Aortic Transcatheter Valves) cohort A (operable patients) trial (New Engl. J. Med. 2010:364:2187-98), "you usually go with the less invasive procedure," said Dr. Joseph E. Bavaria, professor of surgery and director of thoracic aortic surgery at the University of Pennsylvania in Philadelphia. The small number of high-risk patients who go to open surgery tend to be men, "because they do better with surgery, especially if their life expectancy is greater than 5-8 years," or patients with high stroke risk, Dr. Bavaria said in an interview at the annual meeting of the American College of Cardiology.
"Operable, high-risk patients get TAVR. I’m a surgeon saying that. I’ve already done the [PARTNER cohort A] trial, and I don’t want to do it again," said Dr. Michael Mack, a cardiothoracic surgeon at the Heart Hospital in Plano, Texas. "The results are the same [from TAVR and SAVR] at 30 days, 1 year, and 2 years, but boy do we beat up patients with open surgery. If it was my mom, she’d get TAVR," he said in an interview. "The tie goes to the less invasive treatment."
When high-risk, operable patients are seen by the heart team Dr. Mack works with, the only ones who go to SAVR are patients – generally men – who have a large aortic annulus and need a 29-mm-diameter valve, which is not available for the time being to U.S. TAVR patients; women with septal hypertrophy causing significant left ventricular outflow-tract obstruction; and the small percent of patients who opt for open surgery, usually because it’s the more established approach or because they fear a higher stroke risk from TAVR.
"The majority of high-risk, operable patients now go to TAVR; I think that’s pretty much true across the United States," said Dr. Jeffrey J. Popma, a cardiologist who does TAVR and is a professor of medicine at Harvard University in Boston.
Where TAVR stood in 2010 and 2011
How is TAVR performing? Performance can only be completely assessed months or years after the fact, so the impact that high-risk, operable U.S. patients received from TAVR’s use in routine practice in late 2012 and the first months of 2013 remains to be seen. The most recently treated patients now available for meaningful analysis in large numbers come from 2010 and 2011: new data reported at the ACC meeting from a U.S. program of continued TAVR access that began in late 2009 following the end of recruitment into the first PARTNER trial, and 1-year follow-up of nearly 14,000 patients who underwent TAVR in 2011 as part of routine practice in Germany and were entered into the country’s national TAVR registry.
Both databases had good news for high-risk or inoperable patients. TAVR outcomes in the first couple of years immediately following PARTNER in both the United States and Germany showed clinically meaningful improvements over the way TAVR performed during the first PARTNER trial. Less optimistic news for the low- and intermediate-risk patients who underwent TAVR in Germany, where wider device use is possible, was that in broad terms open surgery outperformed TAVR in these lower-risk patients, although TAVR’s defenders are quick to point out how tricky it is to make cross-treatment comparisons with registry data.
The nonrandomized, continued-access cohort that followed the first PARTNER trial at 22 U.S. centers, 3 sites in Canada, and 1 site in Germany included 1,017 inoperable or high-risk patients who had successful transfemoral TAVR between August 2009 and December 2011. When compared with the 415 inoperable or high-risk patients who underwent transfemoral TAVR in both cohorts of PARTNER, the more recently treated patients had a statistically significant decrease in all-cause 1-year mortality, from a 25% death rate in PARTNER to a 20% rate during the continued-access period, Dr. William F. Fearon reported at the meeting.
The continued-access patients also showed significant cuts in their rates of major vascular complications, which dropped from 15% of patients in PARTNER to 6% during continued access; and in rates of major bleeding complications, which fell from 15% in PARTNER to 7% during continued access. Strokes were also down during continued access, 5% compared with 7% during PARTNER, but this was not a statistically significant drop, reported Dr. Fearon, a cardiologist at Stanford (Calif.) University.
"Maybe these improvements are due to better patient selection, and maybe we have also gotten better at what we do," commented Dr. Popma.
The latest German experience
The 1-year German Aortic Valve Registry (GARY) results from 2011 show similar improvements compared with PARTNER. Among the 2,689 patients who underwent transvascular TAVR, the 1-year total mortality rate was 21%, and in the subset of patients with stroke data the combined rate of major and minor stroke was 5%.
But it was the way that transvascular TAVR (which includes both transfemoral and other vascular approaches but excludes the 1,181 patients who underwent transapical TAVR) stacked up against open surgical replacement that raised concern.
Of the 13,860 total patients entered into GARY during 2011, 9,985 underwent SAVR, with 6,523 of these patients undergoing an isolated procedure (the rest had valve replacement combined with coronary artery bypass). One-year mortality was 7% in patients who had isolated SAVR, dramatically below the 21% rate among the transvascular TAVR patients, Dr. Friedrich-Wilhelm Mohr reported at the meeting. The transapical TAVR patients had a 28% 1-year mortality rate.
To address the issue of between-treatment differences in patients’ underlying risk, Dr. Mohr presented two sets of analyses that stratified patients with two different risk-scoring systems, the EuroSCORE and the AKL (aortic valve surgery) score, also known as the German aortic valve score. The results showed how disparate the outcomes were when patients were subgrouped by their underlying risk. Among the patients who had lone SAVR, 80% fell into the lowest-risk level, with an AKL score of less than 3. Among the transvascular TAVR patients, only 17% had AKL scores below 3.
Among patients with AKL scores less than 3, those who underwent SAVR without bypass had a roughly 5% mortality rate after 1 year, compared with about a 15% mortality rate among the transvascular TAVR patients. Analyses in higher-risk patient subgroups showed that the survival gap between SAVR and TAVR patients progressively shrank, until in high-risk patients – as in those studied in PARTNER – the mortality rates were about the same in the SAVR and TAVR groups, said Dr. Mohr, professor and director of heart surgery at Leipzig University, Germany. He also reported similar findings when patients were stratified by their baseline EuroSCORE.
A big factor behind the worse survival among lower-risk TAVR patients is the problem of aortic regurgitation, Dr. Mohr said. Following valve replacement, 56% of the transvascular TAVR patients had grade 1 regurgitation, 7% had grade 2, and less than 1% had grade 3 leakage. These rates are way too high, he said. "Our major concern is the incidence of aortic regurgitation; more than half of the [TAVR] patients had some kind of regurgitation. Regurgitation matters whether it is mild or severe."
Despite his concern, TAVR use in Germany is accelerating. The total number of aortic valve replacements done in Germany jumped from just less than 14,000 in 2011 to more than 22,000 last year; TAVR cases more than doubled, from fewer than 4,000 in 2011 to nearly 10,000 in 2012, with a "move to intermediate-risk groups," said Dr. Mohr, who made it clear that he does not endorse this trend. "TAVR must be proven better than or equal to surgery before it’s used on a large scale," he said. The GARY results show "a clear difference [between TAVR and SAVR] in lower-risk patients. The GARY results do not support going into intermediate-risk patients."
Dr. Martin B. Leon agreed with Dr. Mohr that a definitive determination of whether TAVR is at least as good as SAVR in intermediate-risk patients must await results from the two major, ongoing multicenter trials testing this hypothesis, the PARTNER II trial, using the Edwards balloon-expandable SAPIEN XT valve system, and the SURTAVI (Safety and Efficacy Study of the Medtronic CoreValve System in the Treatment of Severe, Symptomatic Aortic Stenosis in Intermediate Risk Subjects Who Need Aortic Valve Replacement) trial. But Dr. Leon also cautioned that possible confounders might be distorting the GARY results, creating what he calls the "GARY Fallacy."
"The GARY Fallacy is the absurd notion that you can compare SAVR and TAVR in various risk strata without formal risk-adjustment methods to account for imbalances in baseline variables that are not captured in standard risk scores," he said in a talk at the meeting. "Aortic-stenosis patients were selected for TAVR based on their presumed increased risk, including many variables that are not represented in the risk algorithms, such as frailty, liver disease, porcelain aorta, a hostile chest, dementia, and severe chronic obstructive pulmonary disease. Therefore, the risk scores for TAVR patients underrepresent their true risk," Dr. Leon said.
But it’s also possible that TAVR needs more refinement before it completely catches up with SAVR, especially in patients who have the best outcomes from SAVR.
"I think SAVR outperforms TAVR in low-risk patients in the German registry because SAVR is a more mature procedure," said Dr. Raj R. Makkar, a TAVR operator and director of interventional cardiology at Cedars-Sinai Medical Center in Los Angeles. "Maybe this will change, as TAVR becomes safer with fewer valve leaks, strokes, and vascular complications."
Assessing patient risks
Experts also realize that the EuroSCORE, the AKL score, and the other risk-stratification tools now available have flaws when applied to TAVR patients. "Risk stratification for TAVR and SAVR is very problematic; the EuroSCORE has shown poor predictive value for mortality," Dr. Leon said. And while the AKL score was developed specifically for SAVR patients, its relevance to TAVR patients is suspect. In the first PARTNER trial, the multivariate predictors of mortality in the TAVR patients were "completely different" from the predictors in the SAVR patients, he noted.
"It’s tricky calculating scores," agreed Dr. Makkar. Both he and Dr. Mohr cited comorbidities such as pulmonary hypertension, cirrhosis, knee replacement producing impaired mobility, and porcelain aorta that each ratchet up a patient’s risk but have no effect whatsoever on a patient’s EuroSCORE or STS score.
The German cardiology and cardiac surgery societies recognize the limitations of current risk-scoring formulas and are developing a risk-stratification tool specifically designed for TAVR patients, Dr. Mohr said.
The way patients are assessed before, during, and after TAVR is receiving careful scrutiny from some investigators who reported their findings at the ACC meeting, with a particular focus on efforts to characterize and minimize aortic-valve regurgitation following TAVR.
One report, for example, reviewed 2,679 patients who underwent TAVR at any of 33 French centers and 1 in Monaco between January 2010 and October 2011, and were enrolled in the French Aortic National CoreValve and Edwards (FRANCE 2) Registry, established by the French cardiology and thoracic and cardiovascular surgery societies (N. Engl. J. Med. 2012;366:1705-15). FRANCE 2 includes nearly 1,900 patients who received the balloon-expandable Edwards SAPIEN valve, and nearly 900 treated with the self-expanding Medtronic CoreValve device.
Following TAVR, 60% of all patients in the registry had paravalvular aortic regurgitation: 45% with grade 1 regurgitation, 14% with grade 2, and 1% with grade 3 or 4. In a multivariate analysis, the self-expandable device was linked to twice the rate of higher-grade aortic regurgitation, grade 2 or higher, compared with the balloon-expandable valve; and TAVRs done via the femoral artery approach were also about twice as likely to result in higher-grade regurgitations compared with other catheterization routes, reported Dr. Eric Van Belle, a professor at the Cardiology Hospital in Lille, France.
Postprocedural paravalvular regurgitation of grade 2 or higher "was associated with a twofold increase in 1-year mortality, and was the strongest independent predictor of mortality," said Dr. Van Belle in his talk at the meeting. In addition, "annulus diameter and prosthesis diameter were major determinants of aortic regurgitation" in patients who received a balloon-expandable valve.
The FRANCE 2 results showed that postprocedural aortic regurgitation at grade 2 or higher "is a major issue and should be avoided, especially when there is no significant aortic regurgitation at baseline, or when a nonfemoral delivery approach is used." The link between nonfemoral delivery approaches and lower rates of aortic regurgitation suggests "good control of the depth of device delivery and improved catheter technology are key to reducing regurgitation rates," Dr. Van Belle said. "In addition, the prosthesis diameter relative to annulus diameter is key to preventing regurgitation with balloon-expandable devices. Prevention of aortic regurgitation is a major challenge for developing the next-generation device technology."
Minimizing aortic regurgitation
Two ways to cut aortic regurgitation rates following TAVR are to better match the valve to the annulus size, and when possible not finish a TAVR procedure until regurgitation has been minimized.
Two-dimensional transthoracic echocardiography had been the standard approach for annulus sizing as recently as 3 years ago, but it has been replaced with more accurate approaches, either three-dimensional transthoracic echo or CT. "Two-dimensional measurements are seriously limited due to variations and noncircular annular anatomy," Dr. Makkar said in a talk at the meeting. "CT provides the best overall assessment, because in addition to the cross-sectional measurement of the annulus it provides the best assessment of calcification," and three-dimensional transthoracic echo is now standard for intraprocedural assessments, he said.
The impact that a concerted effort to minimize aortic regurgitation can have on outcomes was examined in a single-center study reported by Dr. Jan-Malte Sinning, a cardiologist at University Hospital in Bonn, Germany. Last year, Dr. Sinning and his associates reported developing a quantitative measure of aortic regurgitation immediately following TAVR, the aortic regurgitation (AR) index, based on the difference between a patient’s diastolic blood pressure in the aorta and the left ventricular end-diastolic pressure (J. Am. Coll. Cardiol. 2012;59:1134-41). They then calculated the AR index immediately after TAVR in a prospective series of 167 patients, and set themselves the goal of immediately taking whatever steps were needed to bring the AR index above 25, a cutoff that seemed to correspond to no worse than mild regurgitation.
In their series, 62 patients underwent immediate post-TAVR corrective steps to reduce aortic regurgitation and bring their AR index above 25, most commonly additional balloon dilatation of the valve. The result was that while 16 patients had severe regurgitation before these steps, no patient had severe regurgitation following the corrective measures. The adjustments also cut the incidence of moderate regurgitations from 41 patients to 10, Dr. Sinning reported.
The consequence was that the 30-day stroke rate in the new cohort was 1%, compared with a 6% rate in a historical TAVR cohort at University Hospital in Bonn. The need for pacemaker implants was also cut in half by the intervention compared with the historical group, and 30-day mortality was 3% with these interventions, compared with 7% in the historical controls.
The results suggest that using the AR index as a trigger for taking corrective measures can help improve survival, and that post-TAVR dilatation can successfully decrease regurgitation without increasing patients’ stroke risk, Dr. Sinning concluded.
U.S. operators who perform TAVR say they take similar steps these days to deal with aortic regurgitation after TAVR. "We all understand that you don’t want patients to leave with a leak," said Dr. Popma. "We do everything we can to minimize leaks. When a patient leaves the lab with a moderate or severe leak, there wasn’t anything we could do about it."
Finding TAVR’s limits
Even as TAVR has become the go-to method for high-risk patients, operators have also tried to define the procedure’s outer limit, the point of disease severity when a patient is dying with aortic stenosis rather than because of aortic stenosis, and performing TAVR doesn’t make sense.
Dr. Makkar analyzed 369 inoperable patients who underwent TAVR in both the PARTNER I trial and the nonrandomized continued-access phase that followed. The patients fell into three groups: those who were inoperable because of technical reasons, such as a porcelain aorta or prior chest irradiation; patients who were inoperable because of comorbidities, such as frailty or severe lung disease; and those with both limitations. The 85 patients who were inoperable only because of a technical limitation had a 2-year mortality rate of 23% following TAVR; the other 284 patients who all had comorbidities had a 2-year mortality rate of about 43%.
Last year, Dr. Makkar reported that analysis of these 369 inoperable patients showed a 2-year mortality rate of 20% in the subgroup with an STS score of less than 5%, a mortality rate of 40% among those with a score of 5%-14.5% (but still significantly below the 60% 2-year mortality among similar inoperable patients managed by standard medical therapy only), and a 60% mortality rate among patients with a baseline STS score of 15% or more, an outcome that was no better than that of patients managed without TAVR (N. Engl. J. Med. 2012;366:1696-704).
In a multivariate analysis he recently ran on the data from these 369 patients, the likelihood of 2-year mortality rose by a statistically significant 3% for every 1% increase in the patient’s baseline STS risk score.
"It is important to remember that there are diminishing returns from TAVR in patients with more comorbidities, especially when their STS risk score is greater than 15%," said Dr. Makkar.
Dr. Sinning and Dr. Van Belle had no relevant disclosures. Dr. Mohr is a PARTNER investigator but had no other disclosures. Dr. Bavaria has been a speaker for Edwards and is a PARTNER investigator. Dr. Mack has received travel support from Edwards and is a PARTNER investigator. Dr. Popma has been a consultant to Boston Scientific, Abbott Vascular, and Covidien, and received research support from Boston Scientific, Abbott Vascular, Abiomed, Medtronic, and Cordis. Dr. Fearon has been a consultant to Heart Flow and received research support from St. Jude Medical. Dr. Leon has been a consultant to Symetis; has a major equity stake in Sadra, Claret, Valve Medical, and Apica; has received research support from Boston Scientific, Edwards, and Medtronic; and is a PARTNER investigator. Dr. Makkar has been a consultant to Cordis, Medtronic, Abbott, Entourage Medical, and Abiomed; has been a speaker for Lilly; has received research and travel support from Edwards; and is a PARTNER investigator.
On Twitter @mitchelzoler
SAN FRANCISCO – It’s been barely half a year since U.S. cardiologists and cardiac surgeons first became able to routinely offer operable, high-risk patients with aortic stenosis the option of transcatheter valve replacement, yet in the first few months the transcatheter approach quickly rivaled open surgery.
But for the time being in U.S. practice, transcatheter aortic valve replacement (TAVR) remains boxed into the high-risk niche, along with the subgroup of patients who are not suitable for open surgery, king of a pair of relatively small hills.
And no matter how well TAVR performs in the current pair of trials that are comparing it with open surgical aortic valve replacement (SAVR) for intermediate-risk patients, it will remain relegated to niche status for years to come. That’s because roughly two-thirds of all operable patients with aortic stenosis who need valve replacement fall into the low-risk category, with a Society of Thoracic Surgeons (STS) risk score of less than 4%, which experts agree will remain SAVR’s exclusive territory for the foreseeable future.
The high-risk stratum of operable patients, which TAVR now dominates, constitutes about 10% of all patients who need a new aortic valve and can undergo open surgery, patients with an STS score greater than 8%. The intermediate-risk category – an STS score of 4%-8%, where TAVR now vies against open SAVR in two high-profile trials – makes up the final quarter of the operable-patient pie.
Within the high-risk and operable universe, TAVR’s rise has been meteoric, starting last October when the Food and Drug Administration gave Edwards, marketer of the SAPIEN valve system, approval for these patients. A small survey of operators from U.S. TAVR programs in March at the annual scientific session of the American College of Cardiology (ACC) revealed a uniform perception that by early 2013 a sizable majority of U.S. patients with severe aortic stenosis who are deemed operable and are at high surgical risk will wind up being treated by TAVR instead of SAVR. The cardiac surgeons who collaborate on TAVR seem to have fully conceded the advantages of TAVR for these patients.
"We generally go with TAVR. Most patients want it, and with the equivalence" in outcomes from the first PARTNER (Placement of Aortic Transcatheter Valves) cohort A (operable patients) trial (New Engl. J. Med. 2010:364:2187-98), "you usually go with the less invasive procedure," said Dr. Joseph E. Bavaria, professor of surgery and director of thoracic aortic surgery at the University of Pennsylvania in Philadelphia. The small number of high-risk patients who go to open surgery tend to be men, "because they do better with surgery, especially if their life expectancy is greater than 5-8 years," or patients with high stroke risk, Dr. Bavaria said in an interview at the annual meeting of the American College of Cardiology.
"Operable, high-risk patients get TAVR. I’m a surgeon saying that. I’ve already done the [PARTNER cohort A] trial, and I don’t want to do it again," said Dr. Michael Mack, a cardiothoracic surgeon at the Heart Hospital in Plano, Texas. "The results are the same [from TAVR and SAVR] at 30 days, 1 year, and 2 years, but boy do we beat up patients with open surgery. If it was my mom, she’d get TAVR," he said in an interview. "The tie goes to the less invasive treatment."
When high-risk, operable patients are seen by the heart team Dr. Mack works with, the only ones who go to SAVR are patients – generally men – who have a large aortic annulus and need a 29-mm-diameter valve, which is not available for the time being to U.S. TAVR patients; women with septal hypertrophy causing significant left ventricular outflow-tract obstruction; and the small percent of patients who opt for open surgery, usually because it’s the more established approach or because they fear a higher stroke risk from TAVR.
"The majority of high-risk, operable patients now go to TAVR; I think that’s pretty much true across the United States," said Dr. Jeffrey J. Popma, a cardiologist who does TAVR and is a professor of medicine at Harvard University in Boston.
Where TAVR stood in 2010 and 2011
How is TAVR performing? Performance can only be completely assessed months or years after the fact, so the impact that high-risk, operable U.S. patients received from TAVR’s use in routine practice in late 2012 and the first months of 2013 remains to be seen. The most recently treated patients now available for meaningful analysis in large numbers come from 2010 and 2011: new data reported at the ACC meeting from a U.S. program of continued TAVR access that began in late 2009 following the end of recruitment into the first PARTNER trial, and 1-year follow-up of nearly 14,000 patients who underwent TAVR in 2011 as part of routine practice in Germany and were entered into the country’s national TAVR registry.
Both databases had good news for high-risk or inoperable patients. TAVR outcomes in the first couple of years immediately following PARTNER in both the United States and Germany showed clinically meaningful improvements over the way TAVR performed during the first PARTNER trial. Less optimistic news for the low- and intermediate-risk patients who underwent TAVR in Germany, where wider device use is possible, was that in broad terms open surgery outperformed TAVR in these lower-risk patients, although TAVR’s defenders are quick to point out how tricky it is to make cross-treatment comparisons with registry data.
The nonrandomized, continued-access cohort that followed the first PARTNER trial at 22 U.S. centers, 3 sites in Canada, and 1 site in Germany included 1,017 inoperable or high-risk patients who had successful transfemoral TAVR between August 2009 and December 2011. When compared with the 415 inoperable or high-risk patients who underwent transfemoral TAVR in both cohorts of PARTNER, the more recently treated patients had a statistically significant decrease in all-cause 1-year mortality, from a 25% death rate in PARTNER to a 20% rate during the continued-access period, Dr. William F. Fearon reported at the meeting.
The continued-access patients also showed significant cuts in their rates of major vascular complications, which dropped from 15% of patients in PARTNER to 6% during continued access; and in rates of major bleeding complications, which fell from 15% in PARTNER to 7% during continued access. Strokes were also down during continued access, 5% compared with 7% during PARTNER, but this was not a statistically significant drop, reported Dr. Fearon, a cardiologist at Stanford (Calif.) University.
"Maybe these improvements are due to better patient selection, and maybe we have also gotten better at what we do," commented Dr. Popma.
The latest German experience
The 1-year German Aortic Valve Registry (GARY) results from 2011 show similar improvements compared with PARTNER. Among the 2,689 patients who underwent transvascular TAVR, the 1-year total mortality rate was 21%, and in the subset of patients with stroke data the combined rate of major and minor stroke was 5%.
But it was the way that transvascular TAVR (which includes both transfemoral and other vascular approaches but excludes the 1,181 patients who underwent transapical TAVR) stacked up against open surgical replacement that raised concern.
Of the 13,860 total patients entered into GARY during 2011, 9,985 underwent SAVR, with 6,523 of these patients undergoing an isolated procedure (the rest had valve replacement combined with coronary artery bypass). One-year mortality was 7% in patients who had isolated SAVR, dramatically below the 21% rate among the transvascular TAVR patients, Dr. Friedrich-Wilhelm Mohr reported at the meeting. The transapical TAVR patients had a 28% 1-year mortality rate.
To address the issue of between-treatment differences in patients’ underlying risk, Dr. Mohr presented two sets of analyses that stratified patients with two different risk-scoring systems, the EuroSCORE and the AKL (aortic valve surgery) score, also known as the German aortic valve score. The results showed how disparate the outcomes were when patients were subgrouped by their underlying risk. Among the patients who had lone SAVR, 80% fell into the lowest-risk level, with an AKL score of less than 3. Among the transvascular TAVR patients, only 17% had AKL scores below 3.
Among patients with AKL scores less than 3, those who underwent SAVR without bypass had a roughly 5% mortality rate after 1 year, compared with about a 15% mortality rate among the transvascular TAVR patients. Analyses in higher-risk patient subgroups showed that the survival gap between SAVR and TAVR patients progressively shrank, until in high-risk patients – as in those studied in PARTNER – the mortality rates were about the same in the SAVR and TAVR groups, said Dr. Mohr, professor and director of heart surgery at Leipzig University, Germany. He also reported similar findings when patients were stratified by their baseline EuroSCORE.
A big factor behind the worse survival among lower-risk TAVR patients is the problem of aortic regurgitation, Dr. Mohr said. Following valve replacement, 56% of the transvascular TAVR patients had grade 1 regurgitation, 7% had grade 2, and less than 1% had grade 3 leakage. These rates are way too high, he said. "Our major concern is the incidence of aortic regurgitation; more than half of the [TAVR] patients had some kind of regurgitation. Regurgitation matters whether it is mild or severe."
Despite his concern, TAVR use in Germany is accelerating. The total number of aortic valve replacements done in Germany jumped from just less than 14,000 in 2011 to more than 22,000 last year; TAVR cases more than doubled, from fewer than 4,000 in 2011 to nearly 10,000 in 2012, with a "move to intermediate-risk groups," said Dr. Mohr, who made it clear that he does not endorse this trend. "TAVR must be proven better than or equal to surgery before it’s used on a large scale," he said. The GARY results show "a clear difference [between TAVR and SAVR] in lower-risk patients. The GARY results do not support going into intermediate-risk patients."
Dr. Martin B. Leon agreed with Dr. Mohr that a definitive determination of whether TAVR is at least as good as SAVR in intermediate-risk patients must await results from the two major, ongoing multicenter trials testing this hypothesis, the PARTNER II trial, using the Edwards balloon-expandable SAPIEN XT valve system, and the SURTAVI (Safety and Efficacy Study of the Medtronic CoreValve System in the Treatment of Severe, Symptomatic Aortic Stenosis in Intermediate Risk Subjects Who Need Aortic Valve Replacement) trial. But Dr. Leon also cautioned that possible confounders might be distorting the GARY results, creating what he calls the "GARY Fallacy."
"The GARY Fallacy is the absurd notion that you can compare SAVR and TAVR in various risk strata without formal risk-adjustment methods to account for imbalances in baseline variables that are not captured in standard risk scores," he said in a talk at the meeting. "Aortic-stenosis patients were selected for TAVR based on their presumed increased risk, including many variables that are not represented in the risk algorithms, such as frailty, liver disease, porcelain aorta, a hostile chest, dementia, and severe chronic obstructive pulmonary disease. Therefore, the risk scores for TAVR patients underrepresent their true risk," Dr. Leon said.
But it’s also possible that TAVR needs more refinement before it completely catches up with SAVR, especially in patients who have the best outcomes from SAVR.
"I think SAVR outperforms TAVR in low-risk patients in the German registry because SAVR is a more mature procedure," said Dr. Raj R. Makkar, a TAVR operator and director of interventional cardiology at Cedars-Sinai Medical Center in Los Angeles. "Maybe this will change, as TAVR becomes safer with fewer valve leaks, strokes, and vascular complications."
Assessing patient risks
Experts also realize that the EuroSCORE, the AKL score, and the other risk-stratification tools now available have flaws when applied to TAVR patients. "Risk stratification for TAVR and SAVR is very problematic; the EuroSCORE has shown poor predictive value for mortality," Dr. Leon said. And while the AKL score was developed specifically for SAVR patients, its relevance to TAVR patients is suspect. In the first PARTNER trial, the multivariate predictors of mortality in the TAVR patients were "completely different" from the predictors in the SAVR patients, he noted.
"It’s tricky calculating scores," agreed Dr. Makkar. Both he and Dr. Mohr cited comorbidities such as pulmonary hypertension, cirrhosis, knee replacement producing impaired mobility, and porcelain aorta that each ratchet up a patient’s risk but have no effect whatsoever on a patient’s EuroSCORE or STS score.
The German cardiology and cardiac surgery societies recognize the limitations of current risk-scoring formulas and are developing a risk-stratification tool specifically designed for TAVR patients, Dr. Mohr said.
The way patients are assessed before, during, and after TAVR is receiving careful scrutiny from some investigators who reported their findings at the ACC meeting, with a particular focus on efforts to characterize and minimize aortic-valve regurgitation following TAVR.
One report, for example, reviewed 2,679 patients who underwent TAVR at any of 33 French centers and 1 in Monaco between January 2010 and October 2011, and were enrolled in the French Aortic National CoreValve and Edwards (FRANCE 2) Registry, established by the French cardiology and thoracic and cardiovascular surgery societies (N. Engl. J. Med. 2012;366:1705-15). FRANCE 2 includes nearly 1,900 patients who received the balloon-expandable Edwards SAPIEN valve, and nearly 900 treated with the self-expanding Medtronic CoreValve device.
Following TAVR, 60% of all patients in the registry had paravalvular aortic regurgitation: 45% with grade 1 regurgitation, 14% with grade 2, and 1% with grade 3 or 4. In a multivariate analysis, the self-expandable device was linked to twice the rate of higher-grade aortic regurgitation, grade 2 or higher, compared with the balloon-expandable valve; and TAVRs done via the femoral artery approach were also about twice as likely to result in higher-grade regurgitations compared with other catheterization routes, reported Dr. Eric Van Belle, a professor at the Cardiology Hospital in Lille, France.
Postprocedural paravalvular regurgitation of grade 2 or higher "was associated with a twofold increase in 1-year mortality, and was the strongest independent predictor of mortality," said Dr. Van Belle in his talk at the meeting. In addition, "annulus diameter and prosthesis diameter were major determinants of aortic regurgitation" in patients who received a balloon-expandable valve.
The FRANCE 2 results showed that postprocedural aortic regurgitation at grade 2 or higher "is a major issue and should be avoided, especially when there is no significant aortic regurgitation at baseline, or when a nonfemoral delivery approach is used." The link between nonfemoral delivery approaches and lower rates of aortic regurgitation suggests "good control of the depth of device delivery and improved catheter technology are key to reducing regurgitation rates," Dr. Van Belle said. "In addition, the prosthesis diameter relative to annulus diameter is key to preventing regurgitation with balloon-expandable devices. Prevention of aortic regurgitation is a major challenge for developing the next-generation device technology."
Minimizing aortic regurgitation
Two ways to cut aortic regurgitation rates following TAVR are to better match the valve to the annulus size, and when possible not finish a TAVR procedure until regurgitation has been minimized.
Two-dimensional transthoracic echocardiography had been the standard approach for annulus sizing as recently as 3 years ago, but it has been replaced with more accurate approaches, either three-dimensional transthoracic echo or CT. "Two-dimensional measurements are seriously limited due to variations and noncircular annular anatomy," Dr. Makkar said in a talk at the meeting. "CT provides the best overall assessment, because in addition to the cross-sectional measurement of the annulus it provides the best assessment of calcification," and three-dimensional transthoracic echo is now standard for intraprocedural assessments, he said.
The impact that a concerted effort to minimize aortic regurgitation can have on outcomes was examined in a single-center study reported by Dr. Jan-Malte Sinning, a cardiologist at University Hospital in Bonn, Germany. Last year, Dr. Sinning and his associates reported developing a quantitative measure of aortic regurgitation immediately following TAVR, the aortic regurgitation (AR) index, based on the difference between a patient’s diastolic blood pressure in the aorta and the left ventricular end-diastolic pressure (J. Am. Coll. Cardiol. 2012;59:1134-41). They then calculated the AR index immediately after TAVR in a prospective series of 167 patients, and set themselves the goal of immediately taking whatever steps were needed to bring the AR index above 25, a cutoff that seemed to correspond to no worse than mild regurgitation.
In their series, 62 patients underwent immediate post-TAVR corrective steps to reduce aortic regurgitation and bring their AR index above 25, most commonly additional balloon dilatation of the valve. The result was that while 16 patients had severe regurgitation before these steps, no patient had severe regurgitation following the corrective measures. The adjustments also cut the incidence of moderate regurgitations from 41 patients to 10, Dr. Sinning reported.
The consequence was that the 30-day stroke rate in the new cohort was 1%, compared with a 6% rate in a historical TAVR cohort at University Hospital in Bonn. The need for pacemaker implants was also cut in half by the intervention compared with the historical group, and 30-day mortality was 3% with these interventions, compared with 7% in the historical controls.
The results suggest that using the AR index as a trigger for taking corrective measures can help improve survival, and that post-TAVR dilatation can successfully decrease regurgitation without increasing patients’ stroke risk, Dr. Sinning concluded.
U.S. operators who perform TAVR say they take similar steps these days to deal with aortic regurgitation after TAVR. "We all understand that you don’t want patients to leave with a leak," said Dr. Popma. "We do everything we can to minimize leaks. When a patient leaves the lab with a moderate or severe leak, there wasn’t anything we could do about it."
Finding TAVR’s limits
Even as TAVR has become the go-to method for high-risk patients, operators have also tried to define the procedure’s outer limit, the point of disease severity when a patient is dying with aortic stenosis rather than because of aortic stenosis, and performing TAVR doesn’t make sense.
Dr. Makkar analyzed 369 inoperable patients who underwent TAVR in both the PARTNER I trial and the nonrandomized continued-access phase that followed. The patients fell into three groups: those who were inoperable because of technical reasons, such as a porcelain aorta or prior chest irradiation; patients who were inoperable because of comorbidities, such as frailty or severe lung disease; and those with both limitations. The 85 patients who were inoperable only because of a technical limitation had a 2-year mortality rate of 23% following TAVR; the other 284 patients who all had comorbidities had a 2-year mortality rate of about 43%.
Last year, Dr. Makkar reported that analysis of these 369 inoperable patients showed a 2-year mortality rate of 20% in the subgroup with an STS score of less than 5%, a mortality rate of 40% among those with a score of 5%-14.5% (but still significantly below the 60% 2-year mortality among similar inoperable patients managed by standard medical therapy only), and a 60% mortality rate among patients with a baseline STS score of 15% or more, an outcome that was no better than that of patients managed without TAVR (N. Engl. J. Med. 2012;366:1696-704).
In a multivariate analysis he recently ran on the data from these 369 patients, the likelihood of 2-year mortality rose by a statistically significant 3% for every 1% increase in the patient’s baseline STS risk score.
"It is important to remember that there are diminishing returns from TAVR in patients with more comorbidities, especially when their STS risk score is greater than 15%," said Dr. Makkar.
Dr. Sinning and Dr. Van Belle had no relevant disclosures. Dr. Mohr is a PARTNER investigator but had no other disclosures. Dr. Bavaria has been a speaker for Edwards and is a PARTNER investigator. Dr. Mack has received travel support from Edwards and is a PARTNER investigator. Dr. Popma has been a consultant to Boston Scientific, Abbott Vascular, and Covidien, and received research support from Boston Scientific, Abbott Vascular, Abiomed, Medtronic, and Cordis. Dr. Fearon has been a consultant to Heart Flow and received research support from St. Jude Medical. Dr. Leon has been a consultant to Symetis; has a major equity stake in Sadra, Claret, Valve Medical, and Apica; has received research support from Boston Scientific, Edwards, and Medtronic; and is a PARTNER investigator. Dr. Makkar has been a consultant to Cordis, Medtronic, Abbott, Entourage Medical, and Abiomed; has been a speaker for Lilly; has received research and travel support from Edwards; and is a PARTNER investigator.
On Twitter @mitchelzoler
SAN FRANCISCO – It’s been barely half a year since U.S. cardiologists and cardiac surgeons first became able to routinely offer operable, high-risk patients with aortic stenosis the option of transcatheter valve replacement, yet in the first few months the transcatheter approach quickly rivaled open surgery.
But for the time being in U.S. practice, transcatheter aortic valve replacement (TAVR) remains boxed into the high-risk niche, along with the subgroup of patients who are not suitable for open surgery, king of a pair of relatively small hills.
And no matter how well TAVR performs in the current pair of trials that are comparing it with open surgical aortic valve replacement (SAVR) for intermediate-risk patients, it will remain relegated to niche status for years to come. That’s because roughly two-thirds of all operable patients with aortic stenosis who need valve replacement fall into the low-risk category, with a Society of Thoracic Surgeons (STS) risk score of less than 4%, which experts agree will remain SAVR’s exclusive territory for the foreseeable future.
The high-risk stratum of operable patients, which TAVR now dominates, constitutes about 10% of all patients who need a new aortic valve and can undergo open surgery, patients with an STS score greater than 8%. The intermediate-risk category – an STS score of 4%-8%, where TAVR now vies against open SAVR in two high-profile trials – makes up the final quarter of the operable-patient pie.
Within the high-risk and operable universe, TAVR’s rise has been meteoric, starting last October when the Food and Drug Administration gave Edwards, marketer of the SAPIEN valve system, approval for these patients. A small survey of operators from U.S. TAVR programs in March at the annual scientific session of the American College of Cardiology (ACC) revealed a uniform perception that by early 2013 a sizable majority of U.S. patients with severe aortic stenosis who are deemed operable and are at high surgical risk will wind up being treated by TAVR instead of SAVR. The cardiac surgeons who collaborate on TAVR seem to have fully conceded the advantages of TAVR for these patients.
"We generally go with TAVR. Most patients want it, and with the equivalence" in outcomes from the first PARTNER (Placement of Aortic Transcatheter Valves) cohort A (operable patients) trial (New Engl. J. Med. 2010:364:2187-98), "you usually go with the less invasive procedure," said Dr. Joseph E. Bavaria, professor of surgery and director of thoracic aortic surgery at the University of Pennsylvania in Philadelphia. The small number of high-risk patients who go to open surgery tend to be men, "because they do better with surgery, especially if their life expectancy is greater than 5-8 years," or patients with high stroke risk, Dr. Bavaria said in an interview at the annual meeting of the American College of Cardiology.
"Operable, high-risk patients get TAVR. I’m a surgeon saying that. I’ve already done the [PARTNER cohort A] trial, and I don’t want to do it again," said Dr. Michael Mack, a cardiothoracic surgeon at the Heart Hospital in Plano, Texas. "The results are the same [from TAVR and SAVR] at 30 days, 1 year, and 2 years, but boy do we beat up patients with open surgery. If it was my mom, she’d get TAVR," he said in an interview. "The tie goes to the less invasive treatment."
When high-risk, operable patients are seen by the heart team Dr. Mack works with, the only ones who go to SAVR are patients – generally men – who have a large aortic annulus and need a 29-mm-diameter valve, which is not available for the time being to U.S. TAVR patients; women with septal hypertrophy causing significant left ventricular outflow-tract obstruction; and the small percent of patients who opt for open surgery, usually because it’s the more established approach or because they fear a higher stroke risk from TAVR.
"The majority of high-risk, operable patients now go to TAVR; I think that’s pretty much true across the United States," said Dr. Jeffrey J. Popma, a cardiologist who does TAVR and is a professor of medicine at Harvard University in Boston.
Where TAVR stood in 2010 and 2011
How is TAVR performing? Performance can only be completely assessed months or years after the fact, so the impact that high-risk, operable U.S. patients received from TAVR’s use in routine practice in late 2012 and the first months of 2013 remains to be seen. The most recently treated patients now available for meaningful analysis in large numbers come from 2010 and 2011: new data reported at the ACC meeting from a U.S. program of continued TAVR access that began in late 2009 following the end of recruitment into the first PARTNER trial, and 1-year follow-up of nearly 14,000 patients who underwent TAVR in 2011 as part of routine practice in Germany and were entered into the country’s national TAVR registry.
Both databases had good news for high-risk or inoperable patients. TAVR outcomes in the first couple of years immediately following PARTNER in both the United States and Germany showed clinically meaningful improvements over the way TAVR performed during the first PARTNER trial. Less optimistic news for the low- and intermediate-risk patients who underwent TAVR in Germany, where wider device use is possible, was that in broad terms open surgery outperformed TAVR in these lower-risk patients, although TAVR’s defenders are quick to point out how tricky it is to make cross-treatment comparisons with registry data.
The nonrandomized, continued-access cohort that followed the first PARTNER trial at 22 U.S. centers, 3 sites in Canada, and 1 site in Germany included 1,017 inoperable or high-risk patients who had successful transfemoral TAVR between August 2009 and December 2011. When compared with the 415 inoperable or high-risk patients who underwent transfemoral TAVR in both cohorts of PARTNER, the more recently treated patients had a statistically significant decrease in all-cause 1-year mortality, from a 25% death rate in PARTNER to a 20% rate during the continued-access period, Dr. William F. Fearon reported at the meeting.
The continued-access patients also showed significant cuts in their rates of major vascular complications, which dropped from 15% of patients in PARTNER to 6% during continued access; and in rates of major bleeding complications, which fell from 15% in PARTNER to 7% during continued access. Strokes were also down during continued access, 5% compared with 7% during PARTNER, but this was not a statistically significant drop, reported Dr. Fearon, a cardiologist at Stanford (Calif.) University.
"Maybe these improvements are due to better patient selection, and maybe we have also gotten better at what we do," commented Dr. Popma.
The latest German experience
The 1-year German Aortic Valve Registry (GARY) results from 2011 show similar improvements compared with PARTNER. Among the 2,689 patients who underwent transvascular TAVR, the 1-year total mortality rate was 21%, and in the subset of patients with stroke data the combined rate of major and minor stroke was 5%.
But it was the way that transvascular TAVR (which includes both transfemoral and other vascular approaches but excludes the 1,181 patients who underwent transapical TAVR) stacked up against open surgical replacement that raised concern.
Of the 13,860 total patients entered into GARY during 2011, 9,985 underwent SAVR, with 6,523 of these patients undergoing an isolated procedure (the rest had valve replacement combined with coronary artery bypass). One-year mortality was 7% in patients who had isolated SAVR, dramatically below the 21% rate among the transvascular TAVR patients, Dr. Friedrich-Wilhelm Mohr reported at the meeting. The transapical TAVR patients had a 28% 1-year mortality rate.
To address the issue of between-treatment differences in patients’ underlying risk, Dr. Mohr presented two sets of analyses that stratified patients with two different risk-scoring systems, the EuroSCORE and the AKL (aortic valve surgery) score, also known as the German aortic valve score. The results showed how disparate the outcomes were when patients were subgrouped by their underlying risk. Among the patients who had lone SAVR, 80% fell into the lowest-risk level, with an AKL score of less than 3. Among the transvascular TAVR patients, only 17% had AKL scores below 3.
Among patients with AKL scores less than 3, those who underwent SAVR without bypass had a roughly 5% mortality rate after 1 year, compared with about a 15% mortality rate among the transvascular TAVR patients. Analyses in higher-risk patient subgroups showed that the survival gap between SAVR and TAVR patients progressively shrank, until in high-risk patients – as in those studied in PARTNER – the mortality rates were about the same in the SAVR and TAVR groups, said Dr. Mohr, professor and director of heart surgery at Leipzig University, Germany. He also reported similar findings when patients were stratified by their baseline EuroSCORE.
A big factor behind the worse survival among lower-risk TAVR patients is the problem of aortic regurgitation, Dr. Mohr said. Following valve replacement, 56% of the transvascular TAVR patients had grade 1 regurgitation, 7% had grade 2, and less than 1% had grade 3 leakage. These rates are way too high, he said. "Our major concern is the incidence of aortic regurgitation; more than half of the [TAVR] patients had some kind of regurgitation. Regurgitation matters whether it is mild or severe."
Despite his concern, TAVR use in Germany is accelerating. The total number of aortic valve replacements done in Germany jumped from just less than 14,000 in 2011 to more than 22,000 last year; TAVR cases more than doubled, from fewer than 4,000 in 2011 to nearly 10,000 in 2012, with a "move to intermediate-risk groups," said Dr. Mohr, who made it clear that he does not endorse this trend. "TAVR must be proven better than or equal to surgery before it’s used on a large scale," he said. The GARY results show "a clear difference [between TAVR and SAVR] in lower-risk patients. The GARY results do not support going into intermediate-risk patients."
Dr. Martin B. Leon agreed with Dr. Mohr that a definitive determination of whether TAVR is at least as good as SAVR in intermediate-risk patients must await results from the two major, ongoing multicenter trials testing this hypothesis, the PARTNER II trial, using the Edwards balloon-expandable SAPIEN XT valve system, and the SURTAVI (Safety and Efficacy Study of the Medtronic CoreValve System in the Treatment of Severe, Symptomatic Aortic Stenosis in Intermediate Risk Subjects Who Need Aortic Valve Replacement) trial. But Dr. Leon also cautioned that possible confounders might be distorting the GARY results, creating what he calls the "GARY Fallacy."
"The GARY Fallacy is the absurd notion that you can compare SAVR and TAVR in various risk strata without formal risk-adjustment methods to account for imbalances in baseline variables that are not captured in standard risk scores," he said in a talk at the meeting. "Aortic-stenosis patients were selected for TAVR based on their presumed increased risk, including many variables that are not represented in the risk algorithms, such as frailty, liver disease, porcelain aorta, a hostile chest, dementia, and severe chronic obstructive pulmonary disease. Therefore, the risk scores for TAVR patients underrepresent their true risk," Dr. Leon said.
But it’s also possible that TAVR needs more refinement before it completely catches up with SAVR, especially in patients who have the best outcomes from SAVR.
"I think SAVR outperforms TAVR in low-risk patients in the German registry because SAVR is a more mature procedure," said Dr. Raj R. Makkar, a TAVR operator and director of interventional cardiology at Cedars-Sinai Medical Center in Los Angeles. "Maybe this will change, as TAVR becomes safer with fewer valve leaks, strokes, and vascular complications."
Assessing patient risks
Experts also realize that the EuroSCORE, the AKL score, and the other risk-stratification tools now available have flaws when applied to TAVR patients. "Risk stratification for TAVR and SAVR is very problematic; the EuroSCORE has shown poor predictive value for mortality," Dr. Leon said. And while the AKL score was developed specifically for SAVR patients, its relevance to TAVR patients is suspect. In the first PARTNER trial, the multivariate predictors of mortality in the TAVR patients were "completely different" from the predictors in the SAVR patients, he noted.
"It’s tricky calculating scores," agreed Dr. Makkar. Both he and Dr. Mohr cited comorbidities such as pulmonary hypertension, cirrhosis, knee replacement producing impaired mobility, and porcelain aorta that each ratchet up a patient’s risk but have no effect whatsoever on a patient’s EuroSCORE or STS score.
The German cardiology and cardiac surgery societies recognize the limitations of current risk-scoring formulas and are developing a risk-stratification tool specifically designed for TAVR patients, Dr. Mohr said.
The way patients are assessed before, during, and after TAVR is receiving careful scrutiny from some investigators who reported their findings at the ACC meeting, with a particular focus on efforts to characterize and minimize aortic-valve regurgitation following TAVR.
One report, for example, reviewed 2,679 patients who underwent TAVR at any of 33 French centers and 1 in Monaco between January 2010 and October 2011, and were enrolled in the French Aortic National CoreValve and Edwards (FRANCE 2) Registry, established by the French cardiology and thoracic and cardiovascular surgery societies (N. Engl. J. Med. 2012;366:1705-15). FRANCE 2 includes nearly 1,900 patients who received the balloon-expandable Edwards SAPIEN valve, and nearly 900 treated with the self-expanding Medtronic CoreValve device.
Following TAVR, 60% of all patients in the registry had paravalvular aortic regurgitation: 45% with grade 1 regurgitation, 14% with grade 2, and 1% with grade 3 or 4. In a multivariate analysis, the self-expandable device was linked to twice the rate of higher-grade aortic regurgitation, grade 2 or higher, compared with the balloon-expandable valve; and TAVRs done via the femoral artery approach were also about twice as likely to result in higher-grade regurgitations compared with other catheterization routes, reported Dr. Eric Van Belle, a professor at the Cardiology Hospital in Lille, France.
Postprocedural paravalvular regurgitation of grade 2 or higher "was associated with a twofold increase in 1-year mortality, and was the strongest independent predictor of mortality," said Dr. Van Belle in his talk at the meeting. In addition, "annulus diameter and prosthesis diameter were major determinants of aortic regurgitation" in patients who received a balloon-expandable valve.
The FRANCE 2 results showed that postprocedural aortic regurgitation at grade 2 or higher "is a major issue and should be avoided, especially when there is no significant aortic regurgitation at baseline, or when a nonfemoral delivery approach is used." The link between nonfemoral delivery approaches and lower rates of aortic regurgitation suggests "good control of the depth of device delivery and improved catheter technology are key to reducing regurgitation rates," Dr. Van Belle said. "In addition, the prosthesis diameter relative to annulus diameter is key to preventing regurgitation with balloon-expandable devices. Prevention of aortic regurgitation is a major challenge for developing the next-generation device technology."
Minimizing aortic regurgitation
Two ways to cut aortic regurgitation rates following TAVR are to better match the valve to the annulus size, and when possible not finish a TAVR procedure until regurgitation has been minimized.
Two-dimensional transthoracic echocardiography had been the standard approach for annulus sizing as recently as 3 years ago, but it has been replaced with more accurate approaches, either three-dimensional transthoracic echo or CT. "Two-dimensional measurements are seriously limited due to variations and noncircular annular anatomy," Dr. Makkar said in a talk at the meeting. "CT provides the best overall assessment, because in addition to the cross-sectional measurement of the annulus it provides the best assessment of calcification," and three-dimensional transthoracic echo is now standard for intraprocedural assessments, he said.
The impact that a concerted effort to minimize aortic regurgitation can have on outcomes was examined in a single-center study reported by Dr. Jan-Malte Sinning, a cardiologist at University Hospital in Bonn, Germany. Last year, Dr. Sinning and his associates reported developing a quantitative measure of aortic regurgitation immediately following TAVR, the aortic regurgitation (AR) index, based on the difference between a patient’s diastolic blood pressure in the aorta and the left ventricular end-diastolic pressure (J. Am. Coll. Cardiol. 2012;59:1134-41). They then calculated the AR index immediately after TAVR in a prospective series of 167 patients, and set themselves the goal of immediately taking whatever steps were needed to bring the AR index above 25, a cutoff that seemed to correspond to no worse than mild regurgitation.
In their series, 62 patients underwent immediate post-TAVR corrective steps to reduce aortic regurgitation and bring their AR index above 25, most commonly additional balloon dilatation of the valve. The result was that while 16 patients had severe regurgitation before these steps, no patient had severe regurgitation following the corrective measures. The adjustments also cut the incidence of moderate regurgitations from 41 patients to 10, Dr. Sinning reported.
The consequence was that the 30-day stroke rate in the new cohort was 1%, compared with a 6% rate in a historical TAVR cohort at University Hospital in Bonn. The need for pacemaker implants was also cut in half by the intervention compared with the historical group, and 30-day mortality was 3% with these interventions, compared with 7% in the historical controls.
The results suggest that using the AR index as a trigger for taking corrective measures can help improve survival, and that post-TAVR dilatation can successfully decrease regurgitation without increasing patients’ stroke risk, Dr. Sinning concluded.
U.S. operators who perform TAVR say they take similar steps these days to deal with aortic regurgitation after TAVR. "We all understand that you don’t want patients to leave with a leak," said Dr. Popma. "We do everything we can to minimize leaks. When a patient leaves the lab with a moderate or severe leak, there wasn’t anything we could do about it."
Finding TAVR’s limits
Even as TAVR has become the go-to method for high-risk patients, operators have also tried to define the procedure’s outer limit, the point of disease severity when a patient is dying with aortic stenosis rather than because of aortic stenosis, and performing TAVR doesn’t make sense.
Dr. Makkar analyzed 369 inoperable patients who underwent TAVR in both the PARTNER I trial and the nonrandomized continued-access phase that followed. The patients fell into three groups: those who were inoperable because of technical reasons, such as a porcelain aorta or prior chest irradiation; patients who were inoperable because of comorbidities, such as frailty or severe lung disease; and those with both limitations. The 85 patients who were inoperable only because of a technical limitation had a 2-year mortality rate of 23% following TAVR; the other 284 patients who all had comorbidities had a 2-year mortality rate of about 43%.
Last year, Dr. Makkar reported that analysis of these 369 inoperable patients showed a 2-year mortality rate of 20% in the subgroup with an STS score of less than 5%, a mortality rate of 40% among those with a score of 5%-14.5% (but still significantly below the 60% 2-year mortality among similar inoperable patients managed by standard medical therapy only), and a 60% mortality rate among patients with a baseline STS score of 15% or more, an outcome that was no better than that of patients managed without TAVR (N. Engl. J. Med. 2012;366:1696-704).
In a multivariate analysis he recently ran on the data from these 369 patients, the likelihood of 2-year mortality rose by a statistically significant 3% for every 1% increase in the patient’s baseline STS risk score.
"It is important to remember that there are diminishing returns from TAVR in patients with more comorbidities, especially when their STS risk score is greater than 15%," said Dr. Makkar.
Dr. Sinning and Dr. Van Belle had no relevant disclosures. Dr. Mohr is a PARTNER investigator but had no other disclosures. Dr. Bavaria has been a speaker for Edwards and is a PARTNER investigator. Dr. Mack has received travel support from Edwards and is a PARTNER investigator. Dr. Popma has been a consultant to Boston Scientific, Abbott Vascular, and Covidien, and received research support from Boston Scientific, Abbott Vascular, Abiomed, Medtronic, and Cordis. Dr. Fearon has been a consultant to Heart Flow and received research support from St. Jude Medical. Dr. Leon has been a consultant to Symetis; has a major equity stake in Sadra, Claret, Valve Medical, and Apica; has received research support from Boston Scientific, Edwards, and Medtronic; and is a PARTNER investigator. Dr. Makkar has been a consultant to Cordis, Medtronic, Abbott, Entourage Medical, and Abiomed; has been a speaker for Lilly; has received research and travel support from Edwards; and is a PARTNER investigator.
On Twitter @mitchelzoler
AT ACC 13
Less aggressive anticoagulation appears safe after high-risk aortic valve replacement
MINNEAPOLIS – High-risk patients receiving the On-X mechanical aortic valve can be safely managed with less aggressive anticoagulation than currently recommended, interim results of the PROACT trial suggest.*
The lower target international normalized ratio (INR) in the trial resulted in a decline of more than 50% in bleeding events and did not increase the risk of thromboembolism, reported Dr. John Puskas, international principal investigator for PROACT (Prospective Randomized On-X Anticoagulation Clinical Trial) and associate chief of cardiothoracic surgery at Emory University in Atlanta.
"This aortic bileaflet mechanical valve may be safely managed in these select patients at an INR of 1.5 to 2.0, with daily low-dose aspirin," he said at the annual meeting of the American Association for Thoracic Surgery.
Current American College of Cardiology and American Heart Association guidelines recommend that warfarin be dosed to achieve an INR of 2.0 to 3.0 after implantation of a bileaflet mechanical valve, and that once-daily aspirin 75-100 mg be added for all patients with mechanical heart valves.
Dr. Puskas and his associates analyzed data from 375 high-risk patients randomly assigned to lower-dose warfarin (INR 1.5-2.0) or to continue standard-dose warfarin (INR 2.0-3.0), 3 months after implantation with the On-X bileaflet mechanical heart valve. All patients received aspirin 81 mg daily. INR was adjusted by rigorous home self-monitoring, with an average of 9 days between readings and at least 96% compliance.
High-risk patients included those with chronic atrial fibrillation, left ventricular ejection fraction less than 30%, ventricular aneurysm, left atrium diameter greater than 50 mm, prior neurological events, on estrogen replacement therapy, hypercoagulability, or inadequate platelet response to aspirin or clopidogrel (Plavix). There were 185 patients in the experimental, test arm and 190 in the control arm. After randomization, 11 test patients had a neurological event (5 strokes, 6 transient ischemic attacks) and crossed over to the control group, per protocol.
After an average follow-up of 3.82 years, patients managed with a lower target INR had a significant benefit compared with controls with respect to number of major bleeding events (10 vs. 25, respectively), minor bleeds (8 vs. 25), total bleeds (18 vs. 50), and all bleeding and thrombus (38 vs. 64), Dr. Puskas said. The corresponding rate ratios (RRs) were 0.45, 0.36, 0.40, and 0.66.
There was no difference between groups in the composite primary endpoint of major bleed, stroke, transient ischemic attack, thromboembolic events and thrombosis (30 events vs. 39 events; RR, 0.86; P = .54), he said.
Specifically, hemorrhagic stroke occurred in 1 test patient and 2 controls (RR, 0.56) and ischemic stroke in 5 patients in each group (RR, 1.12), he said.
Valve-related mortality was also similar in the test and control groups (5 deaths vs. 4 deaths), as was total mortality (10 vs. 11), Dr. Puskas said.
Invited discussant Dr. A. Pieter Kappetein, a member of the RE-ALIGN trial steering committee and professor of thoracic surgery at Erasmus Medical Center in Rotterdam, the Netherlands, said the number of patients in the analysis was extremely low and questioned the validity of combining bleeding and thromboembolic events in the primary endpoint.
"In this study, you mix the efficacy endpoint with the safety endpoints," he said, observing that they move in opposite directions.
In light of such large-scale trials as ARISTOTLE and RELY, he asked whether PROACT should be considered a pilot trial and whether a new trial, designed with roughly 8,000 patients, should be performed that would also include newer anticoagulation agents to adequately evaluate reduced anticoagulation in mechanical valves.
"Is it not potentially dangerous if we do not know what the increase is for thrombosis and follow your conclusions?" he added.
Dr. Puskas said he shared Dr. Kappetein’s concern about the noninferiority design of the trial and that it was a topic of great discussion with the Food and Drug Administration (FDA). He also agreed that thrombotic events and bleeding events move in the opposite direction.
"What we are really looking for is to determine the sweet spot where those two curves intersect," he said. "While it is theoretically and intellectually correct to say that thrombosis is the efficacy issue and hemorrhage is the safety issue, we are obliged to combine those for two reasons.
"The first is practical; no company will sponsor an 8,000-patient trial, and second, this is, in fact, a trade-off in the minds of patients and clinicians. So, it is a relevant clinical endpoint – the unholy composite, if you will – of thrombosis and hemorrhage."
Finally, a member of the audience asked whether the results would hold up with standard management because universal point-of-care home testing is not the "real world" in the United States.
Dr. Puskas replied that it is in Scandinavia and other parts of the world, and admonished American clinicians, including himself, "to catch up to what should be standard of care." He noted that, based on the roughly 53,000 INR readings in PROACT, controlling INR within your range was more important in terms of adverse events, particularly hemorrhagic events, than what arm patients were assigned to.
"Home monitoring is available, it’s not high tech and it’s much easier for patients," he said. "To be perfectly blunt, there’s really no excuse for us not using it uniformly in America. Quite frankly, it is a conflict of interest between local caregivers and their patients’ well-being.
"There is a small revenue stream to cardiology offices and primary care doctors running Coumadin clinics, and that is keeping us in the system that we have now rather than home monitoring through bigger, centralized Coumadin clinics."
Dr. Puskas did not report data on PROACT’s low-risk arm managed with clopidogrel 75 mg/day plus aspirin 325 mg/day, or a third arm managed on warfarin at an INR of 2.0-2.5 plus aspirin 81 mg/day. The low-risk data will not be available for at least one more year, although the investigators are in discussion with the FDA about a possible interim analysis, he said in an interview.
The evaluable high-risk patients were 79% male, 93% were in sinus rhythm preoperatively, and concomitant procedures included coronary artery bypass grafting in 27%, aortic aneurysm repair in 14%, and other procedures in 25%. Their average age was 55 years.
Life Technologies sponsored the study. Dr. Puskas reported having no financial relationship with Life Technologies.
Correction, 6/18/2013: An earlier version of this article stated that the On-X aortic valve is investigational. This was a misstatement. The valve itself has been approved in the United States since 2001. The PROACT trial applications of lowered INR and an aspirin/Plavix regimen are not approved.
MINNEAPOLIS – High-risk patients receiving the On-X mechanical aortic valve can be safely managed with less aggressive anticoagulation than currently recommended, interim results of the PROACT trial suggest.*
The lower target international normalized ratio (INR) in the trial resulted in a decline of more than 50% in bleeding events and did not increase the risk of thromboembolism, reported Dr. John Puskas, international principal investigator for PROACT (Prospective Randomized On-X Anticoagulation Clinical Trial) and associate chief of cardiothoracic surgery at Emory University in Atlanta.
"This aortic bileaflet mechanical valve may be safely managed in these select patients at an INR of 1.5 to 2.0, with daily low-dose aspirin," he said at the annual meeting of the American Association for Thoracic Surgery.
Current American College of Cardiology and American Heart Association guidelines recommend that warfarin be dosed to achieve an INR of 2.0 to 3.0 after implantation of a bileaflet mechanical valve, and that once-daily aspirin 75-100 mg be added for all patients with mechanical heart valves.
Dr. Puskas and his associates analyzed data from 375 high-risk patients randomly assigned to lower-dose warfarin (INR 1.5-2.0) or to continue standard-dose warfarin (INR 2.0-3.0), 3 months after implantation with the On-X bileaflet mechanical heart valve. All patients received aspirin 81 mg daily. INR was adjusted by rigorous home self-monitoring, with an average of 9 days between readings and at least 96% compliance.
High-risk patients included those with chronic atrial fibrillation, left ventricular ejection fraction less than 30%, ventricular aneurysm, left atrium diameter greater than 50 mm, prior neurological events, on estrogen replacement therapy, hypercoagulability, or inadequate platelet response to aspirin or clopidogrel (Plavix). There were 185 patients in the experimental, test arm and 190 in the control arm. After randomization, 11 test patients had a neurological event (5 strokes, 6 transient ischemic attacks) and crossed over to the control group, per protocol.
After an average follow-up of 3.82 years, patients managed with a lower target INR had a significant benefit compared with controls with respect to number of major bleeding events (10 vs. 25, respectively), minor bleeds (8 vs. 25), total bleeds (18 vs. 50), and all bleeding and thrombus (38 vs. 64), Dr. Puskas said. The corresponding rate ratios (RRs) were 0.45, 0.36, 0.40, and 0.66.
There was no difference between groups in the composite primary endpoint of major bleed, stroke, transient ischemic attack, thromboembolic events and thrombosis (30 events vs. 39 events; RR, 0.86; P = .54), he said.
Specifically, hemorrhagic stroke occurred in 1 test patient and 2 controls (RR, 0.56) and ischemic stroke in 5 patients in each group (RR, 1.12), he said.
Valve-related mortality was also similar in the test and control groups (5 deaths vs. 4 deaths), as was total mortality (10 vs. 11), Dr. Puskas said.
Invited discussant Dr. A. Pieter Kappetein, a member of the RE-ALIGN trial steering committee and professor of thoracic surgery at Erasmus Medical Center in Rotterdam, the Netherlands, said the number of patients in the analysis was extremely low and questioned the validity of combining bleeding and thromboembolic events in the primary endpoint.
"In this study, you mix the efficacy endpoint with the safety endpoints," he said, observing that they move in opposite directions.
In light of such large-scale trials as ARISTOTLE and RELY, he asked whether PROACT should be considered a pilot trial and whether a new trial, designed with roughly 8,000 patients, should be performed that would also include newer anticoagulation agents to adequately evaluate reduced anticoagulation in mechanical valves.
"Is it not potentially dangerous if we do not know what the increase is for thrombosis and follow your conclusions?" he added.
Dr. Puskas said he shared Dr. Kappetein’s concern about the noninferiority design of the trial and that it was a topic of great discussion with the Food and Drug Administration (FDA). He also agreed that thrombotic events and bleeding events move in the opposite direction.
"What we are really looking for is to determine the sweet spot where those two curves intersect," he said. "While it is theoretically and intellectually correct to say that thrombosis is the efficacy issue and hemorrhage is the safety issue, we are obliged to combine those for two reasons.
"The first is practical; no company will sponsor an 8,000-patient trial, and second, this is, in fact, a trade-off in the minds of patients and clinicians. So, it is a relevant clinical endpoint – the unholy composite, if you will – of thrombosis and hemorrhage."
Finally, a member of the audience asked whether the results would hold up with standard management because universal point-of-care home testing is not the "real world" in the United States.
Dr. Puskas replied that it is in Scandinavia and other parts of the world, and admonished American clinicians, including himself, "to catch up to what should be standard of care." He noted that, based on the roughly 53,000 INR readings in PROACT, controlling INR within your range was more important in terms of adverse events, particularly hemorrhagic events, than what arm patients were assigned to.
"Home monitoring is available, it’s not high tech and it’s much easier for patients," he said. "To be perfectly blunt, there’s really no excuse for us not using it uniformly in America. Quite frankly, it is a conflict of interest between local caregivers and their patients’ well-being.
"There is a small revenue stream to cardiology offices and primary care doctors running Coumadin clinics, and that is keeping us in the system that we have now rather than home monitoring through bigger, centralized Coumadin clinics."
Dr. Puskas did not report data on PROACT’s low-risk arm managed with clopidogrel 75 mg/day plus aspirin 325 mg/day, or a third arm managed on warfarin at an INR of 2.0-2.5 plus aspirin 81 mg/day. The low-risk data will not be available for at least one more year, although the investigators are in discussion with the FDA about a possible interim analysis, he said in an interview.
The evaluable high-risk patients were 79% male, 93% were in sinus rhythm preoperatively, and concomitant procedures included coronary artery bypass grafting in 27%, aortic aneurysm repair in 14%, and other procedures in 25%. Their average age was 55 years.
Life Technologies sponsored the study. Dr. Puskas reported having no financial relationship with Life Technologies.
Correction, 6/18/2013: An earlier version of this article stated that the On-X aortic valve is investigational. This was a misstatement. The valve itself has been approved in the United States since 2001. The PROACT trial applications of lowered INR and an aspirin/Plavix regimen are not approved.
MINNEAPOLIS – High-risk patients receiving the On-X mechanical aortic valve can be safely managed with less aggressive anticoagulation than currently recommended, interim results of the PROACT trial suggest.*
The lower target international normalized ratio (INR) in the trial resulted in a decline of more than 50% in bleeding events and did not increase the risk of thromboembolism, reported Dr. John Puskas, international principal investigator for PROACT (Prospective Randomized On-X Anticoagulation Clinical Trial) and associate chief of cardiothoracic surgery at Emory University in Atlanta.
"This aortic bileaflet mechanical valve may be safely managed in these select patients at an INR of 1.5 to 2.0, with daily low-dose aspirin," he said at the annual meeting of the American Association for Thoracic Surgery.
Current American College of Cardiology and American Heart Association guidelines recommend that warfarin be dosed to achieve an INR of 2.0 to 3.0 after implantation of a bileaflet mechanical valve, and that once-daily aspirin 75-100 mg be added for all patients with mechanical heart valves.
Dr. Puskas and his associates analyzed data from 375 high-risk patients randomly assigned to lower-dose warfarin (INR 1.5-2.0) or to continue standard-dose warfarin (INR 2.0-3.0), 3 months after implantation with the On-X bileaflet mechanical heart valve. All patients received aspirin 81 mg daily. INR was adjusted by rigorous home self-monitoring, with an average of 9 days between readings and at least 96% compliance.
High-risk patients included those with chronic atrial fibrillation, left ventricular ejection fraction less than 30%, ventricular aneurysm, left atrium diameter greater than 50 mm, prior neurological events, on estrogen replacement therapy, hypercoagulability, or inadequate platelet response to aspirin or clopidogrel (Plavix). There were 185 patients in the experimental, test arm and 190 in the control arm. After randomization, 11 test patients had a neurological event (5 strokes, 6 transient ischemic attacks) and crossed over to the control group, per protocol.
After an average follow-up of 3.82 years, patients managed with a lower target INR had a significant benefit compared with controls with respect to number of major bleeding events (10 vs. 25, respectively), minor bleeds (8 vs. 25), total bleeds (18 vs. 50), and all bleeding and thrombus (38 vs. 64), Dr. Puskas said. The corresponding rate ratios (RRs) were 0.45, 0.36, 0.40, and 0.66.
There was no difference between groups in the composite primary endpoint of major bleed, stroke, transient ischemic attack, thromboembolic events and thrombosis (30 events vs. 39 events; RR, 0.86; P = .54), he said.
Specifically, hemorrhagic stroke occurred in 1 test patient and 2 controls (RR, 0.56) and ischemic stroke in 5 patients in each group (RR, 1.12), he said.
Valve-related mortality was also similar in the test and control groups (5 deaths vs. 4 deaths), as was total mortality (10 vs. 11), Dr. Puskas said.
Invited discussant Dr. A. Pieter Kappetein, a member of the RE-ALIGN trial steering committee and professor of thoracic surgery at Erasmus Medical Center in Rotterdam, the Netherlands, said the number of patients in the analysis was extremely low and questioned the validity of combining bleeding and thromboembolic events in the primary endpoint.
"In this study, you mix the efficacy endpoint with the safety endpoints," he said, observing that they move in opposite directions.
In light of such large-scale trials as ARISTOTLE and RELY, he asked whether PROACT should be considered a pilot trial and whether a new trial, designed with roughly 8,000 patients, should be performed that would also include newer anticoagulation agents to adequately evaluate reduced anticoagulation in mechanical valves.
"Is it not potentially dangerous if we do not know what the increase is for thrombosis and follow your conclusions?" he added.
Dr. Puskas said he shared Dr. Kappetein’s concern about the noninferiority design of the trial and that it was a topic of great discussion with the Food and Drug Administration (FDA). He also agreed that thrombotic events and bleeding events move in the opposite direction.
"What we are really looking for is to determine the sweet spot where those two curves intersect," he said. "While it is theoretically and intellectually correct to say that thrombosis is the efficacy issue and hemorrhage is the safety issue, we are obliged to combine those for two reasons.
"The first is practical; no company will sponsor an 8,000-patient trial, and second, this is, in fact, a trade-off in the minds of patients and clinicians. So, it is a relevant clinical endpoint – the unholy composite, if you will – of thrombosis and hemorrhage."
Finally, a member of the audience asked whether the results would hold up with standard management because universal point-of-care home testing is not the "real world" in the United States.
Dr. Puskas replied that it is in Scandinavia and other parts of the world, and admonished American clinicians, including himself, "to catch up to what should be standard of care." He noted that, based on the roughly 53,000 INR readings in PROACT, controlling INR within your range was more important in terms of adverse events, particularly hemorrhagic events, than what arm patients were assigned to.
"Home monitoring is available, it’s not high tech and it’s much easier for patients," he said. "To be perfectly blunt, there’s really no excuse for us not using it uniformly in America. Quite frankly, it is a conflict of interest between local caregivers and their patients’ well-being.
"There is a small revenue stream to cardiology offices and primary care doctors running Coumadin clinics, and that is keeping us in the system that we have now rather than home monitoring through bigger, centralized Coumadin clinics."
Dr. Puskas did not report data on PROACT’s low-risk arm managed with clopidogrel 75 mg/day plus aspirin 325 mg/day, or a third arm managed on warfarin at an INR of 2.0-2.5 plus aspirin 81 mg/day. The low-risk data will not be available for at least one more year, although the investigators are in discussion with the FDA about a possible interim analysis, he said in an interview.
The evaluable high-risk patients were 79% male, 93% were in sinus rhythm preoperatively, and concomitant procedures included coronary artery bypass grafting in 27%, aortic aneurysm repair in 14%, and other procedures in 25%. Their average age was 55 years.
Life Technologies sponsored the study. Dr. Puskas reported having no financial relationship with Life Technologies.
Correction, 6/18/2013: An earlier version of this article stated that the On-X aortic valve is investigational. This was a misstatement. The valve itself has been approved in the United States since 2001. The PROACT trial applications of lowered INR and an aspirin/Plavix regimen are not approved.
AT THE AATS ANNUAL MEETING
Major finding: The composite primary endpoint of major bleed, stroke, transient ischemic attack, thromboembolic events, and thrombosis occurred in 30 patients managed with less aggressive anticoagulation and in 39 managed with standard warfarin anticoagulation (rate ratio, 0.86; P = .54).
Data source: Interim analysis of 375 high-risk aortic valve replacement patients in the Prospective Randomized On-X Anticoagulation Trial.
Disclosures: Life Technologies sponsored the study. Dr. Puskas reported having no financial relationship with Life Technologies.
Elderly drive increase in U.K. aortic valve interventions
LOS ANGELES – Aortic valve interventions rose sharply in the United Kingdom during a recent 5-year period, largely due to a spike in procedures among elderly patients, researchers reported at the annual meeting of the Society of Thoracic Surgeons.
In the study, the combined U.K. Cardiac Surgical and Transcatheter Aortic Valve Implantation (TAVI) Registries – known as SATIRE for short – the team analyzed data from 37,020 patients nationwide who underwent aortic valve interventions (excluding balloon valvuloplasty) between 2006 and 2010.
Results showed that the annual number of these procedures increased by 32% over the course of the study, with about half of the increase due to procedures among patients aged 80 years and older, reported lead investigator Dr. Neil E. Moat, a cardiac surgeon and codirector of the Transcatheter Valve Programme at the Royal Brompton Hospital in London.
Overall, the annual number of aortic valve interventions increased during the study period, from 6,217 in 2006 to 8,201 in 2010. This was driven largely by a near doubling in interventions among patients aged 80 years or older, from 1,055 to 2,035.
"In 2010, 25% of all aortic valve interventions in the U.K. were performed in patients aged 80 or over," Dr. Moat noted.
By type of intervention, there was an increase in the annual number of TAVIs (from none to 749) and conventional surgeries (from 6,217 to 7,252), although the latter stabilized toward the end of the period.
"This is the first time in the whole history – 35 years of collecting U.K. cardiac surgical data – that these numbers have plateaued," he commented.
As of 2010, TAVIs accounted for 9% of all aortic valve interventions studied.
Not surprisingly, patients undergoing TAVI surgery were older than their counterparts undergoing conventional surgery, had a higher risk profile, and had poorer postoperative survival, but their lengths of hospital stay were statistically indistinguishable.
Importantly, two scores used in Europe to predict mortality after such interventions showed differential performance.
"The logistic EuroSCORE does not predict operative outcomes of surgery or TAVI" and should not be used, Dr. Moat advised. "The EuroSCORE II is probably quite good for conventional surgery but again is rather poor for predicting mortality outcomes from TAVI. And I suppose on this side of the pond, you will be pleased to hear that the STS [U.S. Society of Thoracic Surgeons] score is far better than either of these European scores in our population."
In the discussion after the presentation of the study, Dr. Paul A. Kurlansky, a cardiothoracic surgeon from Miami, remarked, "Maybe our risk score is better on this side of the pond, but we do not have the merging of databases that you guys have over there [in the U.K.], or the completion of them. One opportunity that I was wondering if you had taken or are planning to take advantage of is the ability to do propensity score matching or use other statistical tools in order to really match patients so that you can compare outcomes."
"That’s a very interesting question and something that we have debated long and hard," Dr. Moat replied, citing reservations given by the study’s statisticians. "They are very firm in their belief that whilst you can apply those statistical techniques, they are actually not valid because you have so much confounding by indication that [you are unable] to propensity-match populations. So it’s a deliberate decision not to pursue that."
Main analyses in the study used prospectively collected registry data for 35,392 patients undergoing conventional surgery – either aortic valve replacement (AVR) alone or combined with coronary artery bypass grafting (CABG) – and 1,628 TAVIs.
"Because [the registries] are national and publicly funded, they are free from any commercial bias. These are mandatory registries and therefore free of any selection bias," Dr. Moat pointed out. Furthermore, there is good standardization of definitions and long-term follow-up of mortality.
"All cardiac surgical procedures and all TAVIs in the U.K. are entered into national registries hosted by the National Institute for Cardiovascular Outcomes Research. The data set and databases of all of these registries are designed to be compatible and comparable," he added.
Overall, compared with patients undergoing surgery, patients undergoing TAVI were on average older and had a higher risk profile in terms of cardiovascular measures and comorbidities, although there was substantial overlap.
The median postoperative length of stay was 8 days after conventional surgery and 7 days after TAVI. "Despite the TAVI patients being older and at increased risk, there was no difference in postoperative length of stay. If anything, it was marginally lower in the TAVI group," Dr. Moat commented. The distribution of length of stay was also much the same. "You see a substantial numbers of these elderly, frail, and high-risk patients [in the TAVI group] being discharged before day 6," he added.
Actuarial analyses in the study population overall showed poorer survival after TAVI than after conventional surgery (whether AVR alone or combined with CABG), both in the first year and longer term. However, the gaps between curves were smaller among patients aged 80 years or older.
Among the patients undergoing TAVI, longer-term survival after aortic valve intervention did not differ according to the number of diseased vessels treated.
"An interesting observation worthy of further study is that we are well aware that patients having combined AVR and CABG have worse earlier and late survival than patients having isolated AVR. But it would seem that the presence of concomitant coronary artery disease – despite much of it being left untreated – did not affect early or late survival following TAVI," Dr. Moat commented.
The EuroSCORE was a poor predictor of mortality after both conventional surgery and TAVI, but the EuroSCORE II performed fairly well after conventional surgery, whether isolated AVR (observed:expected ratio, 0.87; area under the curve, 0.78) or AVR plus CABG (observed:expected ratio, 1.0; area under the curve, 0.73).
Dr. Moat disclosed that he is on the speakers bureau for, and receives honoraria from, Abbott Laboratories, and sits on the consultant/advisory board of Medtronic.
LOS ANGELES – Aortic valve interventions rose sharply in the United Kingdom during a recent 5-year period, largely due to a spike in procedures among elderly patients, researchers reported at the annual meeting of the Society of Thoracic Surgeons.
In the study, the combined U.K. Cardiac Surgical and Transcatheter Aortic Valve Implantation (TAVI) Registries – known as SATIRE for short – the team analyzed data from 37,020 patients nationwide who underwent aortic valve interventions (excluding balloon valvuloplasty) between 2006 and 2010.
Results showed that the annual number of these procedures increased by 32% over the course of the study, with about half of the increase due to procedures among patients aged 80 years and older, reported lead investigator Dr. Neil E. Moat, a cardiac surgeon and codirector of the Transcatheter Valve Programme at the Royal Brompton Hospital in London.
Overall, the annual number of aortic valve interventions increased during the study period, from 6,217 in 2006 to 8,201 in 2010. This was driven largely by a near doubling in interventions among patients aged 80 years or older, from 1,055 to 2,035.
"In 2010, 25% of all aortic valve interventions in the U.K. were performed in patients aged 80 or over," Dr. Moat noted.
By type of intervention, there was an increase in the annual number of TAVIs (from none to 749) and conventional surgeries (from 6,217 to 7,252), although the latter stabilized toward the end of the period.
"This is the first time in the whole history – 35 years of collecting U.K. cardiac surgical data – that these numbers have plateaued," he commented.
As of 2010, TAVIs accounted for 9% of all aortic valve interventions studied.
Not surprisingly, patients undergoing TAVI surgery were older than their counterparts undergoing conventional surgery, had a higher risk profile, and had poorer postoperative survival, but their lengths of hospital stay were statistically indistinguishable.
Importantly, two scores used in Europe to predict mortality after such interventions showed differential performance.
"The logistic EuroSCORE does not predict operative outcomes of surgery or TAVI" and should not be used, Dr. Moat advised. "The EuroSCORE II is probably quite good for conventional surgery but again is rather poor for predicting mortality outcomes from TAVI. And I suppose on this side of the pond, you will be pleased to hear that the STS [U.S. Society of Thoracic Surgeons] score is far better than either of these European scores in our population."
In the discussion after the presentation of the study, Dr. Paul A. Kurlansky, a cardiothoracic surgeon from Miami, remarked, "Maybe our risk score is better on this side of the pond, but we do not have the merging of databases that you guys have over there [in the U.K.], or the completion of them. One opportunity that I was wondering if you had taken or are planning to take advantage of is the ability to do propensity score matching or use other statistical tools in order to really match patients so that you can compare outcomes."
"That’s a very interesting question and something that we have debated long and hard," Dr. Moat replied, citing reservations given by the study’s statisticians. "They are very firm in their belief that whilst you can apply those statistical techniques, they are actually not valid because you have so much confounding by indication that [you are unable] to propensity-match populations. So it’s a deliberate decision not to pursue that."
Main analyses in the study used prospectively collected registry data for 35,392 patients undergoing conventional surgery – either aortic valve replacement (AVR) alone or combined with coronary artery bypass grafting (CABG) – and 1,628 TAVIs.
"Because [the registries] are national and publicly funded, they are free from any commercial bias. These are mandatory registries and therefore free of any selection bias," Dr. Moat pointed out. Furthermore, there is good standardization of definitions and long-term follow-up of mortality.
"All cardiac surgical procedures and all TAVIs in the U.K. are entered into national registries hosted by the National Institute for Cardiovascular Outcomes Research. The data set and databases of all of these registries are designed to be compatible and comparable," he added.
Overall, compared with patients undergoing surgery, patients undergoing TAVI were on average older and had a higher risk profile in terms of cardiovascular measures and comorbidities, although there was substantial overlap.
The median postoperative length of stay was 8 days after conventional surgery and 7 days after TAVI. "Despite the TAVI patients being older and at increased risk, there was no difference in postoperative length of stay. If anything, it was marginally lower in the TAVI group," Dr. Moat commented. The distribution of length of stay was also much the same. "You see a substantial numbers of these elderly, frail, and high-risk patients [in the TAVI group] being discharged before day 6," he added.
Actuarial analyses in the study population overall showed poorer survival after TAVI than after conventional surgery (whether AVR alone or combined with CABG), both in the first year and longer term. However, the gaps between curves were smaller among patients aged 80 years or older.
Among the patients undergoing TAVI, longer-term survival after aortic valve intervention did not differ according to the number of diseased vessels treated.
"An interesting observation worthy of further study is that we are well aware that patients having combined AVR and CABG have worse earlier and late survival than patients having isolated AVR. But it would seem that the presence of concomitant coronary artery disease – despite much of it being left untreated – did not affect early or late survival following TAVI," Dr. Moat commented.
The EuroSCORE was a poor predictor of mortality after both conventional surgery and TAVI, but the EuroSCORE II performed fairly well after conventional surgery, whether isolated AVR (observed:expected ratio, 0.87; area under the curve, 0.78) or AVR plus CABG (observed:expected ratio, 1.0; area under the curve, 0.73).
Dr. Moat disclosed that he is on the speakers bureau for, and receives honoraria from, Abbott Laboratories, and sits on the consultant/advisory board of Medtronic.
LOS ANGELES – Aortic valve interventions rose sharply in the United Kingdom during a recent 5-year period, largely due to a spike in procedures among elderly patients, researchers reported at the annual meeting of the Society of Thoracic Surgeons.
In the study, the combined U.K. Cardiac Surgical and Transcatheter Aortic Valve Implantation (TAVI) Registries – known as SATIRE for short – the team analyzed data from 37,020 patients nationwide who underwent aortic valve interventions (excluding balloon valvuloplasty) between 2006 and 2010.
Results showed that the annual number of these procedures increased by 32% over the course of the study, with about half of the increase due to procedures among patients aged 80 years and older, reported lead investigator Dr. Neil E. Moat, a cardiac surgeon and codirector of the Transcatheter Valve Programme at the Royal Brompton Hospital in London.
Overall, the annual number of aortic valve interventions increased during the study period, from 6,217 in 2006 to 8,201 in 2010. This was driven largely by a near doubling in interventions among patients aged 80 years or older, from 1,055 to 2,035.
"In 2010, 25% of all aortic valve interventions in the U.K. were performed in patients aged 80 or over," Dr. Moat noted.
By type of intervention, there was an increase in the annual number of TAVIs (from none to 749) and conventional surgeries (from 6,217 to 7,252), although the latter stabilized toward the end of the period.
"This is the first time in the whole history – 35 years of collecting U.K. cardiac surgical data – that these numbers have plateaued," he commented.
As of 2010, TAVIs accounted for 9% of all aortic valve interventions studied.
Not surprisingly, patients undergoing TAVI surgery were older than their counterparts undergoing conventional surgery, had a higher risk profile, and had poorer postoperative survival, but their lengths of hospital stay were statistically indistinguishable.
Importantly, two scores used in Europe to predict mortality after such interventions showed differential performance.
"The logistic EuroSCORE does not predict operative outcomes of surgery or TAVI" and should not be used, Dr. Moat advised. "The EuroSCORE II is probably quite good for conventional surgery but again is rather poor for predicting mortality outcomes from TAVI. And I suppose on this side of the pond, you will be pleased to hear that the STS [U.S. Society of Thoracic Surgeons] score is far better than either of these European scores in our population."
In the discussion after the presentation of the study, Dr. Paul A. Kurlansky, a cardiothoracic surgeon from Miami, remarked, "Maybe our risk score is better on this side of the pond, but we do not have the merging of databases that you guys have over there [in the U.K.], or the completion of them. One opportunity that I was wondering if you had taken or are planning to take advantage of is the ability to do propensity score matching or use other statistical tools in order to really match patients so that you can compare outcomes."
"That’s a very interesting question and something that we have debated long and hard," Dr. Moat replied, citing reservations given by the study’s statisticians. "They are very firm in their belief that whilst you can apply those statistical techniques, they are actually not valid because you have so much confounding by indication that [you are unable] to propensity-match populations. So it’s a deliberate decision not to pursue that."
Main analyses in the study used prospectively collected registry data for 35,392 patients undergoing conventional surgery – either aortic valve replacement (AVR) alone or combined with coronary artery bypass grafting (CABG) – and 1,628 TAVIs.
"Because [the registries] are national and publicly funded, they are free from any commercial bias. These are mandatory registries and therefore free of any selection bias," Dr. Moat pointed out. Furthermore, there is good standardization of definitions and long-term follow-up of mortality.
"All cardiac surgical procedures and all TAVIs in the U.K. are entered into national registries hosted by the National Institute for Cardiovascular Outcomes Research. The data set and databases of all of these registries are designed to be compatible and comparable," he added.
Overall, compared with patients undergoing surgery, patients undergoing TAVI were on average older and had a higher risk profile in terms of cardiovascular measures and comorbidities, although there was substantial overlap.
The median postoperative length of stay was 8 days after conventional surgery and 7 days after TAVI. "Despite the TAVI patients being older and at increased risk, there was no difference in postoperative length of stay. If anything, it was marginally lower in the TAVI group," Dr. Moat commented. The distribution of length of stay was also much the same. "You see a substantial numbers of these elderly, frail, and high-risk patients [in the TAVI group] being discharged before day 6," he added.
Actuarial analyses in the study population overall showed poorer survival after TAVI than after conventional surgery (whether AVR alone or combined with CABG), both in the first year and longer term. However, the gaps between curves were smaller among patients aged 80 years or older.
Among the patients undergoing TAVI, longer-term survival after aortic valve intervention did not differ according to the number of diseased vessels treated.
"An interesting observation worthy of further study is that we are well aware that patients having combined AVR and CABG have worse earlier and late survival than patients having isolated AVR. But it would seem that the presence of concomitant coronary artery disease – despite much of it being left untreated – did not affect early or late survival following TAVI," Dr. Moat commented.
The EuroSCORE was a poor predictor of mortality after both conventional surgery and TAVI, but the EuroSCORE II performed fairly well after conventional surgery, whether isolated AVR (observed:expected ratio, 0.87; area under the curve, 0.78) or AVR plus CABG (observed:expected ratio, 1.0; area under the curve, 0.73).
Dr. Moat disclosed that he is on the speakers bureau for, and receives honoraria from, Abbott Laboratories, and sits on the consultant/advisory board of Medtronic.
AT THE STS ANNUAL MEETING
Major Finding: There was a 32% increase in aortic valve interventions, driven in large part by an increase among patients aged 80 years or older.
Data Source: An analysis of pooled data from two U.K. population-based registries and 37,020 patients undergoing aortic valve interventions between 2006 and 2010.
Disclosures: Dr. Moat disclosed that he is on the speakers bureau for, and receives honoraria from, Abbott Laboratories, and sits on the consultant/advisory board of Medtronic.
Crossing devices offer solutions for failed recanalization
MIAMI BEACH – When the "wire and catheter" approach fails, crossing devices come into play for recanalizing vessels during endovascular interventions, according to Dr. John Rundback.
Specialized crossing devices may improve the ability to treat chronic total occlusions (CTOs), including calcified and long, complex lesions that can be very difficult to cross, particularly in the infrapopliteal region, said Dr. Rundback, medical director of the Interventional Institute at Holy Name Medical Center, Briarcliff Manor, N.Y.
When it comes to specialized crossing devices, there is a whole spectrum available, and many are relatively new on the market, so experience with them is limited, Dr. Rundback said at the International Symposium on Endovascular Therapy 2013.
The goal with each, however, is to remain intraluminal and to maximize the interventional options, he said, noting that goal is particularly relevant with the advent of drug-eluting balloons.
Although the data are sparse, and these devices – which are generally used in patients who have failed traditional wire and catheter crossing – have not been compared to wire and catheter techniques in a rigorous fashion, it is nonetheless clear that there are cases in which these devices will be needed.
"You have to sort of pick one or two and keep them in your lab, and gain familiarity," he said.
The approved and emerging devices he discussed include the Viance and Enteer peripheral CTO crossing devices (Covidien), the Crosser CTO device (Bard Peripheral Vascular), the Wildcat and Kittycat CTO devices (Avinger), and the TruePath crossing device (Boston Scientific).
Viance and Enteer
The Viance crossing catheter is a high-speed rotating recanalization device, and the Enteer reentry system is a unique reentry catheter. The two were studied together as a novel overall strategy, Dr. Rundback explained.
In a study involving 66 patients, which led to the recent approval of the device, CTO lesion lengths were reasonably long, much like those Dr. Runback said he sees in his practice. However, moderate to severe calcification was present in only 42% of patients, which is less than he generally sees, and a fair amount of tortuosity was present in 50%-60% of patients.
About two-thirds of the cases involved the superficial femoral artery (SFA), and the remaining cases were in the tibial circulation. Overall, the approach was safe, and the success rate was 85%, Dr. Rundback said.
The Crosser
The latest version of this device, approved in the United States for both coronary and peripheral indications, involves a dedicated hydraulic vibrational system that provides translational force through the lumen.
"It’s the one we tend to use the most in our practice, and our junior associates have had great success with this device," Dr. Rundback noted.
The Crosser device is unique in that it establishes a luminal plane where you often don’t see anything, and moves quite smoothly and easily through the lumen, he said.
In the PATRIOT (Peripheral Approach to Recanalization in Occluded Totals) study of this device, 85 guide wire–refractory peripheral CTO patients were treated with a high technical success rate of 84% and no perforations.
Most cases involved the SFA, but about a third were popliteal or below. Lesion length was reasonable (average, 117.5 mm), and about 75% of patients had old, calcified lesions.
Treatment was quick, taking only about 2 minutes on average.
"That has been our experience as well. These actually work very quickly to reestablish straight-line flow," he said, noting that it is important to be cautious, nonetheless.
"You can get extraluminal without knowing it. [The technique] requires a certain amount of practice and tactile feedback to become familiar with the utility of these devices," he said.
Wildcat and Kittycat
These devices are rotating crossing devices (Kittycat is a small-vessel device) that have shown promise in trials.
In the CONNECT (Chronic Total Occlusion Crossing with the Wildcat Catheter) trial, the technical success rate was 89%, and safety was greater than 95% in patients with an average lesion length of 174 mm, about half of whom had moderately calcified lesions.
The newest incarnations of these rotating crossing devices use optical coherence technology that allows visualization of the lumen as the occlusion is traversed.
Dr. Rundback said he has no personal experience with these devices, but said that the prospect of visualizing the position within the lumen "does have some sort of empirical appeal and may provide real, true benefit in terms of staying in the lumen."
TruePath
This FDA-approved crossing device uses a high-speed, rotating diamond-studded burr to advance through lesions.
It is entirely self-contained and easy to use, Dr. Rundback said, noting that the device uses a feedback system involving red lights and beeping sounds that are activated when resistance is encountered in the system. This provides audible, visible, and tactile feedback to help avoid going extraluminal.
In the ReOpen study of 85 patients with a mean occlusion length of 166 mm who failed guide-wire treatment, the technical success rate was 80% and the device was safe, he said.
Dr. Rundback reported having no relevant financial disclosures.
MIAMI BEACH – When the "wire and catheter" approach fails, crossing devices come into play for recanalizing vessels during endovascular interventions, according to Dr. John Rundback.
Specialized crossing devices may improve the ability to treat chronic total occlusions (CTOs), including calcified and long, complex lesions that can be very difficult to cross, particularly in the infrapopliteal region, said Dr. Rundback, medical director of the Interventional Institute at Holy Name Medical Center, Briarcliff Manor, N.Y.
When it comes to specialized crossing devices, there is a whole spectrum available, and many are relatively new on the market, so experience with them is limited, Dr. Rundback said at the International Symposium on Endovascular Therapy 2013.
The goal with each, however, is to remain intraluminal and to maximize the interventional options, he said, noting that goal is particularly relevant with the advent of drug-eluting balloons.
Although the data are sparse, and these devices – which are generally used in patients who have failed traditional wire and catheter crossing – have not been compared to wire and catheter techniques in a rigorous fashion, it is nonetheless clear that there are cases in which these devices will be needed.
"You have to sort of pick one or two and keep them in your lab, and gain familiarity," he said.
The approved and emerging devices he discussed include the Viance and Enteer peripheral CTO crossing devices (Covidien), the Crosser CTO device (Bard Peripheral Vascular), the Wildcat and Kittycat CTO devices (Avinger), and the TruePath crossing device (Boston Scientific).
Viance and Enteer
The Viance crossing catheter is a high-speed rotating recanalization device, and the Enteer reentry system is a unique reentry catheter. The two were studied together as a novel overall strategy, Dr. Rundback explained.
In a study involving 66 patients, which led to the recent approval of the device, CTO lesion lengths were reasonably long, much like those Dr. Runback said he sees in his practice. However, moderate to severe calcification was present in only 42% of patients, which is less than he generally sees, and a fair amount of tortuosity was present in 50%-60% of patients.
About two-thirds of the cases involved the superficial femoral artery (SFA), and the remaining cases were in the tibial circulation. Overall, the approach was safe, and the success rate was 85%, Dr. Rundback said.
The Crosser
The latest version of this device, approved in the United States for both coronary and peripheral indications, involves a dedicated hydraulic vibrational system that provides translational force through the lumen.
"It’s the one we tend to use the most in our practice, and our junior associates have had great success with this device," Dr. Rundback noted.
The Crosser device is unique in that it establishes a luminal plane where you often don’t see anything, and moves quite smoothly and easily through the lumen, he said.
In the PATRIOT (Peripheral Approach to Recanalization in Occluded Totals) study of this device, 85 guide wire–refractory peripheral CTO patients were treated with a high technical success rate of 84% and no perforations.
Most cases involved the SFA, but about a third were popliteal or below. Lesion length was reasonable (average, 117.5 mm), and about 75% of patients had old, calcified lesions.
Treatment was quick, taking only about 2 minutes on average.
"That has been our experience as well. These actually work very quickly to reestablish straight-line flow," he said, noting that it is important to be cautious, nonetheless.
"You can get extraluminal without knowing it. [The technique] requires a certain amount of practice and tactile feedback to become familiar with the utility of these devices," he said.
Wildcat and Kittycat
These devices are rotating crossing devices (Kittycat is a small-vessel device) that have shown promise in trials.
In the CONNECT (Chronic Total Occlusion Crossing with the Wildcat Catheter) trial, the technical success rate was 89%, and safety was greater than 95% in patients with an average lesion length of 174 mm, about half of whom had moderately calcified lesions.
The newest incarnations of these rotating crossing devices use optical coherence technology that allows visualization of the lumen as the occlusion is traversed.
Dr. Rundback said he has no personal experience with these devices, but said that the prospect of visualizing the position within the lumen "does have some sort of empirical appeal and may provide real, true benefit in terms of staying in the lumen."
TruePath
This FDA-approved crossing device uses a high-speed, rotating diamond-studded burr to advance through lesions.
It is entirely self-contained and easy to use, Dr. Rundback said, noting that the device uses a feedback system involving red lights and beeping sounds that are activated when resistance is encountered in the system. This provides audible, visible, and tactile feedback to help avoid going extraluminal.
In the ReOpen study of 85 patients with a mean occlusion length of 166 mm who failed guide-wire treatment, the technical success rate was 80% and the device was safe, he said.
Dr. Rundback reported having no relevant financial disclosures.
MIAMI BEACH – When the "wire and catheter" approach fails, crossing devices come into play for recanalizing vessels during endovascular interventions, according to Dr. John Rundback.
Specialized crossing devices may improve the ability to treat chronic total occlusions (CTOs), including calcified and long, complex lesions that can be very difficult to cross, particularly in the infrapopliteal region, said Dr. Rundback, medical director of the Interventional Institute at Holy Name Medical Center, Briarcliff Manor, N.Y.
When it comes to specialized crossing devices, there is a whole spectrum available, and many are relatively new on the market, so experience with them is limited, Dr. Rundback said at the International Symposium on Endovascular Therapy 2013.
The goal with each, however, is to remain intraluminal and to maximize the interventional options, he said, noting that goal is particularly relevant with the advent of drug-eluting balloons.
Although the data are sparse, and these devices – which are generally used in patients who have failed traditional wire and catheter crossing – have not been compared to wire and catheter techniques in a rigorous fashion, it is nonetheless clear that there are cases in which these devices will be needed.
"You have to sort of pick one or two and keep them in your lab, and gain familiarity," he said.
The approved and emerging devices he discussed include the Viance and Enteer peripheral CTO crossing devices (Covidien), the Crosser CTO device (Bard Peripheral Vascular), the Wildcat and Kittycat CTO devices (Avinger), and the TruePath crossing device (Boston Scientific).
Viance and Enteer
The Viance crossing catheter is a high-speed rotating recanalization device, and the Enteer reentry system is a unique reentry catheter. The two were studied together as a novel overall strategy, Dr. Rundback explained.
In a study involving 66 patients, which led to the recent approval of the device, CTO lesion lengths were reasonably long, much like those Dr. Runback said he sees in his practice. However, moderate to severe calcification was present in only 42% of patients, which is less than he generally sees, and a fair amount of tortuosity was present in 50%-60% of patients.
About two-thirds of the cases involved the superficial femoral artery (SFA), and the remaining cases were in the tibial circulation. Overall, the approach was safe, and the success rate was 85%, Dr. Rundback said.
The Crosser
The latest version of this device, approved in the United States for both coronary and peripheral indications, involves a dedicated hydraulic vibrational system that provides translational force through the lumen.
"It’s the one we tend to use the most in our practice, and our junior associates have had great success with this device," Dr. Rundback noted.
The Crosser device is unique in that it establishes a luminal plane where you often don’t see anything, and moves quite smoothly and easily through the lumen, he said.
In the PATRIOT (Peripheral Approach to Recanalization in Occluded Totals) study of this device, 85 guide wire–refractory peripheral CTO patients were treated with a high technical success rate of 84% and no perforations.
Most cases involved the SFA, but about a third were popliteal or below. Lesion length was reasonable (average, 117.5 mm), and about 75% of patients had old, calcified lesions.
Treatment was quick, taking only about 2 minutes on average.
"That has been our experience as well. These actually work very quickly to reestablish straight-line flow," he said, noting that it is important to be cautious, nonetheless.
"You can get extraluminal without knowing it. [The technique] requires a certain amount of practice and tactile feedback to become familiar with the utility of these devices," he said.
Wildcat and Kittycat
These devices are rotating crossing devices (Kittycat is a small-vessel device) that have shown promise in trials.
In the CONNECT (Chronic Total Occlusion Crossing with the Wildcat Catheter) trial, the technical success rate was 89%, and safety was greater than 95% in patients with an average lesion length of 174 mm, about half of whom had moderately calcified lesions.
The newest incarnations of these rotating crossing devices use optical coherence technology that allows visualization of the lumen as the occlusion is traversed.
Dr. Rundback said he has no personal experience with these devices, but said that the prospect of visualizing the position within the lumen "does have some sort of empirical appeal and may provide real, true benefit in terms of staying in the lumen."
TruePath
This FDA-approved crossing device uses a high-speed, rotating diamond-studded burr to advance through lesions.
It is entirely self-contained and easy to use, Dr. Rundback said, noting that the device uses a feedback system involving red lights and beeping sounds that are activated when resistance is encountered in the system. This provides audible, visible, and tactile feedback to help avoid going extraluminal.
In the ReOpen study of 85 patients with a mean occlusion length of 166 mm who failed guide-wire treatment, the technical success rate was 80% and the device was safe, he said.
Dr. Rundback reported having no relevant financial disclosures.
EXPERT ANALYSIS FROM ISET 2013
Inclacumab reduces troponin in NSTEMI
SAN FRANCISCO – Inclacumab, an inhibitor of the P-selectin pathway, may be a novel treatment for reducing myocardial damage after percutaneous coronary intervention for non–ST-elevation myocardial infarction, based on results from a phase-II study.
The trial, SELECT-ACS, is best viewed as a proof-of-concept study utilizing a reduction in biomarkers of myocardial damage as the endpoint, Dr. Jean-Claude Tardif said at the annual meeting of the American College of Cardiology.
Periprocedural myocardial damage, albeit often mild in nature, is common in PCI patients and is due in part to platelet activation and inflammation. The clinical relevance of post-PCI changes in biomarkers of myocardial damage is uncertain. What is clear from SELECT-ACS is that inclacumab is biologically active in NSTEMI patients. Future phase III studies with hard clinical endpoints conducted in patients with or without PCI will be critical to determining the drug’s relevance, he said.
In addition, the results of the ongoing SELECT-CABG study, in which the effects of inclacumab are being studied in patients undergoing surgical revascularization, will be presented later this year, according to Dr. Tardif, professor of medicine and director of research at the Montreal Heart Institute.
Inclacumab is a human recombinant monoclonal antibody that is a highly specific P-selectin antagonist. P-selectin is a cell adhesion molecule known to play a critical role in communication between activated platelets, WBCs, and the arterial wall. In animal studies, P-selectin inhibition reduces platelet stickiness, macrophage accumulation, and neointimal formation after injury.
"The P-selectin pathway is really at the crossroads of thrombosis and inflammation," Dr. Tardif explained. "It’s probably important to see this drug, inclacumab, not only as an antithrombotic but as an anti-inflammatory agent."
In SELECT-ACS, 544 patients with NSTEMI scheduled for coronary angiography and possible PCI were randomized to receive a single 1-hour-long infusion of inclacumab at 5 or 20 mg/kg or a placebo infusion up to 24 hours before their procedure. Most patients received the infusion just a few hours before angiography.
The primary study endpoint in SELECT-ACS was change in troponin I level from baseline at 16 and 24 hours post-PCI, compared with placebo. Thus, the analysis was restricted to the 322 study participants who underwent PCI. The medically managed SELECT-ACS participants treated with inclacumab will be the subject of a future report.
In the inclacumab 20 mg/kg group, the drop in troponin I was 22% greater than seen in placebo-treated controls at 16 hours and 24% greater at 24 hours. Peak troponin I level was reduced by 24% relative to placebo, and the area under the curve over a 24-hour span was reduced by 34%.
In addition, the decrease in creatine kinase MB (CK-MB) fraction was 16% greater with inclacumab 20 mg/kg than with placebo at 16 hours, and it also showed a 17% greater decrease at 24 hours. The incidence of a CK-MB rise greater than three times the upper limit of normal within the first 24 hours following PCI was 8.9% with inclacumab 20 mg/kg, compared with 18% with placebo. Moreover, soluble P-selectin levels were 22% lower in the inclacumab 20 mg/kg group than in controls.
Inclacumab at 5 mg/kg had no effect.
The pattern and intensity of adverse events were similar in the inclacumab and placebo groups. Given the dual antithrombotic and anti-inflammatory effects of P-selectin inhibition, it’s encouraging to note that the inclacumab-treated patients had no increase in bleeding or infections, Dr. Tardif said.
The study was funded by F. Hoffmann-La Roche. Dr. Tardif reported having no relevant financial interests.
Simultaneous with Dr. Tardif’s presentation of the SELECT-ACS findings in San Francisco, the study was published online (J. Am. Coll. Cardiol. 2013 [doi:10.1016/j.jaac.2013.03.003]).
The SELECT-ACS study is a great phase II trial – and I emphasize "phase II." It’s an early look at a very new and exciting thing. We haven’t been down this road before.

|
|
I’m struck by the disconnect between the inclacumab-induced reduction in troponin release and clinical events. There were no deaths in the placebo group, but four in the low-dose and two in the high-dose inclacumab groups. Also, nonfatal MI occurred in two placebo-treated patients, compared with four on low-dose and seven on high-dose inclacumab.
Whether this is a real signal, is due to chance, or is simply the way the investigators reported periprocedural MIs in this study is not entirely certain. More definitive understanding is likely to come from the phase-III trials.
Dr. Erik Magnus Ohman is professor of medicine and director of the program for advanced coronary disease at Duke University in Durham, N.C. Dr. Ohman was the study discussant at ACC 13.
The SELECT-ACS study is a great phase II trial – and I emphasize "phase II." It’s an early look at a very new and exciting thing. We haven’t been down this road before.

|
|
I’m struck by the disconnect between the inclacumab-induced reduction in troponin release and clinical events. There were no deaths in the placebo group, but four in the low-dose and two in the high-dose inclacumab groups. Also, nonfatal MI occurred in two placebo-treated patients, compared with four on low-dose and seven on high-dose inclacumab.
Whether this is a real signal, is due to chance, or is simply the way the investigators reported periprocedural MIs in this study is not entirely certain. More definitive understanding is likely to come from the phase-III trials.
Dr. Erik Magnus Ohman is professor of medicine and director of the program for advanced coronary disease at Duke University in Durham, N.C. Dr. Ohman was the study discussant at ACC 13.
The SELECT-ACS study is a great phase II trial – and I emphasize "phase II." It’s an early look at a very new and exciting thing. We haven’t been down this road before.

|
|
I’m struck by the disconnect between the inclacumab-induced reduction in troponin release and clinical events. There were no deaths in the placebo group, but four in the low-dose and two in the high-dose inclacumab groups. Also, nonfatal MI occurred in two placebo-treated patients, compared with four on low-dose and seven on high-dose inclacumab.
Whether this is a real signal, is due to chance, or is simply the way the investigators reported periprocedural MIs in this study is not entirely certain. More definitive understanding is likely to come from the phase-III trials.
Dr. Erik Magnus Ohman is professor of medicine and director of the program for advanced coronary disease at Duke University in Durham, N.C. Dr. Ohman was the study discussant at ACC 13.
SAN FRANCISCO – Inclacumab, an inhibitor of the P-selectin pathway, may be a novel treatment for reducing myocardial damage after percutaneous coronary intervention for non–ST-elevation myocardial infarction, based on results from a phase-II study.
The trial, SELECT-ACS, is best viewed as a proof-of-concept study utilizing a reduction in biomarkers of myocardial damage as the endpoint, Dr. Jean-Claude Tardif said at the annual meeting of the American College of Cardiology.
Periprocedural myocardial damage, albeit often mild in nature, is common in PCI patients and is due in part to platelet activation and inflammation. The clinical relevance of post-PCI changes in biomarkers of myocardial damage is uncertain. What is clear from SELECT-ACS is that inclacumab is biologically active in NSTEMI patients. Future phase III studies with hard clinical endpoints conducted in patients with or without PCI will be critical to determining the drug’s relevance, he said.
In addition, the results of the ongoing SELECT-CABG study, in which the effects of inclacumab are being studied in patients undergoing surgical revascularization, will be presented later this year, according to Dr. Tardif, professor of medicine and director of research at the Montreal Heart Institute.
Inclacumab is a human recombinant monoclonal antibody that is a highly specific P-selectin antagonist. P-selectin is a cell adhesion molecule known to play a critical role in communication between activated platelets, WBCs, and the arterial wall. In animal studies, P-selectin inhibition reduces platelet stickiness, macrophage accumulation, and neointimal formation after injury.
"The P-selectin pathway is really at the crossroads of thrombosis and inflammation," Dr. Tardif explained. "It’s probably important to see this drug, inclacumab, not only as an antithrombotic but as an anti-inflammatory agent."
In SELECT-ACS, 544 patients with NSTEMI scheduled for coronary angiography and possible PCI were randomized to receive a single 1-hour-long infusion of inclacumab at 5 or 20 mg/kg or a placebo infusion up to 24 hours before their procedure. Most patients received the infusion just a few hours before angiography.
The primary study endpoint in SELECT-ACS was change in troponin I level from baseline at 16 and 24 hours post-PCI, compared with placebo. Thus, the analysis was restricted to the 322 study participants who underwent PCI. The medically managed SELECT-ACS participants treated with inclacumab will be the subject of a future report.
In the inclacumab 20 mg/kg group, the drop in troponin I was 22% greater than seen in placebo-treated controls at 16 hours and 24% greater at 24 hours. Peak troponin I level was reduced by 24% relative to placebo, and the area under the curve over a 24-hour span was reduced by 34%.
In addition, the decrease in creatine kinase MB (CK-MB) fraction was 16% greater with inclacumab 20 mg/kg than with placebo at 16 hours, and it also showed a 17% greater decrease at 24 hours. The incidence of a CK-MB rise greater than three times the upper limit of normal within the first 24 hours following PCI was 8.9% with inclacumab 20 mg/kg, compared with 18% with placebo. Moreover, soluble P-selectin levels were 22% lower in the inclacumab 20 mg/kg group than in controls.
Inclacumab at 5 mg/kg had no effect.
The pattern and intensity of adverse events were similar in the inclacumab and placebo groups. Given the dual antithrombotic and anti-inflammatory effects of P-selectin inhibition, it’s encouraging to note that the inclacumab-treated patients had no increase in bleeding or infections, Dr. Tardif said.
The study was funded by F. Hoffmann-La Roche. Dr. Tardif reported having no relevant financial interests.
Simultaneous with Dr. Tardif’s presentation of the SELECT-ACS findings in San Francisco, the study was published online (J. Am. Coll. Cardiol. 2013 [doi:10.1016/j.jaac.2013.03.003]).
SAN FRANCISCO – Inclacumab, an inhibitor of the P-selectin pathway, may be a novel treatment for reducing myocardial damage after percutaneous coronary intervention for non–ST-elevation myocardial infarction, based on results from a phase-II study.
The trial, SELECT-ACS, is best viewed as a proof-of-concept study utilizing a reduction in biomarkers of myocardial damage as the endpoint, Dr. Jean-Claude Tardif said at the annual meeting of the American College of Cardiology.
Periprocedural myocardial damage, albeit often mild in nature, is common in PCI patients and is due in part to platelet activation and inflammation. The clinical relevance of post-PCI changes in biomarkers of myocardial damage is uncertain. What is clear from SELECT-ACS is that inclacumab is biologically active in NSTEMI patients. Future phase III studies with hard clinical endpoints conducted in patients with or without PCI will be critical to determining the drug’s relevance, he said.
In addition, the results of the ongoing SELECT-CABG study, in which the effects of inclacumab are being studied in patients undergoing surgical revascularization, will be presented later this year, according to Dr. Tardif, professor of medicine and director of research at the Montreal Heart Institute.
Inclacumab is a human recombinant monoclonal antibody that is a highly specific P-selectin antagonist. P-selectin is a cell adhesion molecule known to play a critical role in communication between activated platelets, WBCs, and the arterial wall. In animal studies, P-selectin inhibition reduces platelet stickiness, macrophage accumulation, and neointimal formation after injury.
"The P-selectin pathway is really at the crossroads of thrombosis and inflammation," Dr. Tardif explained. "It’s probably important to see this drug, inclacumab, not only as an antithrombotic but as an anti-inflammatory agent."
In SELECT-ACS, 544 patients with NSTEMI scheduled for coronary angiography and possible PCI were randomized to receive a single 1-hour-long infusion of inclacumab at 5 or 20 mg/kg or a placebo infusion up to 24 hours before their procedure. Most patients received the infusion just a few hours before angiography.
The primary study endpoint in SELECT-ACS was change in troponin I level from baseline at 16 and 24 hours post-PCI, compared with placebo. Thus, the analysis was restricted to the 322 study participants who underwent PCI. The medically managed SELECT-ACS participants treated with inclacumab will be the subject of a future report.
In the inclacumab 20 mg/kg group, the drop in troponin I was 22% greater than seen in placebo-treated controls at 16 hours and 24% greater at 24 hours. Peak troponin I level was reduced by 24% relative to placebo, and the area under the curve over a 24-hour span was reduced by 34%.
In addition, the decrease in creatine kinase MB (CK-MB) fraction was 16% greater with inclacumab 20 mg/kg than with placebo at 16 hours, and it also showed a 17% greater decrease at 24 hours. The incidence of a CK-MB rise greater than three times the upper limit of normal within the first 24 hours following PCI was 8.9% with inclacumab 20 mg/kg, compared with 18% with placebo. Moreover, soluble P-selectin levels were 22% lower in the inclacumab 20 mg/kg group than in controls.
Inclacumab at 5 mg/kg had no effect.
The pattern and intensity of adverse events were similar in the inclacumab and placebo groups. Given the dual antithrombotic and anti-inflammatory effects of P-selectin inhibition, it’s encouraging to note that the inclacumab-treated patients had no increase in bleeding or infections, Dr. Tardif said.
The study was funded by F. Hoffmann-La Roche. Dr. Tardif reported having no relevant financial interests.
Simultaneous with Dr. Tardif’s presentation of the SELECT-ACS findings in San Francisco, the study was published online (J. Am. Coll. Cardiol. 2013 [doi:10.1016/j.jaac.2013.03.003]).
AT ACC 13
Major finding: In the inclacumab 20 mg/kg group, the drop in troponin I was 22% greater than seen in placebo-treated controls at 16 hours and 24% greater at 24 hours. Peak troponin I level was reduced by 24%, relative to placebo, and the area under the curve over a 24-hour span was reduced by 34%.
Data source: SELECT-ACS was an international, prospective, placebo-controlled, randomized, double-blind trial involving 544 NSTEMI patients assigned to low- or high-dose inclacumab or placebo up to 24 hours prior to coronary angiography.
Disclosures: The study was funded by F. Hoffmann-La Roche. The presenter reported having no financial conflicts.
Big trials deflate off-pump CABG
SAN FRANCISCO – Off-pump coronary artery bypass graft surgery offered no advantages over on-pump CABG in any major endpoints at 1 year of follow-up in two major prospective randomized trials totaling more than 7,000 patients.
Among the key 1-year outcomes – which didn’t differ between off- and on-pump CABG patients in the GOPCABE and CORONARY trials – were death, MI, stroke, neurocognitive function, quality of life, renal failure, and repeat revascularization, investigators reported at the annual meeting of the American College of Cardiology.
A third randomized trial presented at the same session of the ACC meeting did find a significant outcome advantage favoring off-pump CABG in high-operative-risk patients at 1 year. However, experts discounted this Czech study because it was small, single center, reported only 30-day results, and the advantage found for off-pump surgery hinged on an outdated and inadequate definition of MI.
The new study findings signal a striking fall from grace for off-pump CABG. Not long ago, this technique, while controversial, was viewed by many as a progressive development within heart surgery, one that would revitalize a mature operation whose annual case numbers were declining in the face of stiff competition from percutaneous coronary intervention by cardiologists. Off-pump CABG was an innovation designed to avoid the perioperative complications related to aortic cross-clamping and the heart-lung machine, including the lingering neurocognitive dysfunction known informally in surgical circles as “pump head.”
However, the resounding lack of any demonstrable advantages for off-pump CABG in the two large trials presented in San Francisco left analysts scratching their heads as to the role remaining for this beating heart surgical technique, which is more difficult to learn and perform skillfully than on-pump bypass.
Speaking perhaps for many puzzled cardiologists in the packed audience in San Francisco, Dr. Christopher P. Cannon of Harvard Medical School, Boston, asked the surgeon-presenters of the CORONARY and GOPCABE trials, “Why would you want to do off-pump surgery if it doesn’t seem to help and it’s harder to do?”
Discussant Dr. Michael J. Mack, a cardiac surgeon, voiced a similar sentiment.
“I was an early advocate of off-pump surgery. But as a card-carrying off-pump bypass surgeon, it’s getting harder and harder for me to maintain enthusiasm for a potential benefit from this,” declared Dr. Mack, medical director of cardiovascular surgery for the Baylor Health Care System and director of cardiovascular research at the Heart Hospital in Plano, Tex.
He noted that the GOPCABE and CORONARY trials follow upon the earlier ROOBY (Randomized On/Off Bypass) trial, which actually showed worse outcomes in the off-pump group. ROOBY enrolled 2,203 Veterans Affairs patients, with the off-pump CABG group having a significantly higher 1-year rate of the primary composite endpoint comprising death, nonfatal MI, or repeat revascularization, along with worse graft patency (N. Engl. J. Med. 2009;361:1827-37).
GOPCABE and CORONARY were designed in part to answer critics of ROOBY, who have argued that the VA trial used insufficiently experienced off-pump CABG surgeons and featured a patient population at too low an operative risk to detect a signal of benefit favoring off-pump surgery.
The GOPCABE (German Off-Pump Coronary Artery Bypass Grafting in Elderly Patients) study involved 2,539 patients aged 75 years or older randomized at 12 German centers. A total of 60% of patients had triple-vessel disease, and no one was excluded from the trial because of left ventricular function or coronary artery anatomy.
Participating surgeons were highly experienced. Those who performed off-pump CABG in the study had previously done an average of 514 of them, while the on-pump surgeons had done an average of 1,378 of those operations.
“We wanted to have the best off-pump vs. the best on-pump surgeons, like in a competition,” explained Dr. Anno Diegeler, a surgeon at the Bad Neustadt (Germany) Heart Center.
He and his coinvestigators conducted GOPCABE because they believed it would be easier to show advantages for off-pump CABG in a population at high operative risk, such as elderly patients with many comorbidities. Indeed, the study hypothesis was that the off-pump group would show a robust 30% reduction in the primary endpoint, a composite of death, stroke, myocardial infarction, repeat revascularization, or new renal-replacement therapy at 1 year.
That didn’t happen. The 30-day rate of the primary endpoint was 7.8% in the off-pump group and 8.2% with on-pump CABG, while the 1-year rates were 13.1% and 14.0%, respectively. None of the individual components of the composite endpoint differed significantly between the groups, either.
Neurocognitive function wasn’t measured in GOPCABE, but it was in CORONARY (the CABG Off or On Pump Revascularization Study), which involved 4,752 randomized patients in 19 countries.
Dr. Andre Lamy presented the 1-year results. The primary endpoint was a composite of death, MI, stroke, or new renal failure requiring dialysis. The rate was 12.1% in patients in the off-pump group and similar at 13.3% in the on-pump group.
As in GOPCABE, the surgeons participating in CORONARY were highly proficient. They had to have at least 2 years’ experience as a staff cardiac surgeon and at least 100 prior cases of whichever operation they were assigned to. The vast majority met that standard for both procedures.
Quality of life and neurocognitive tests were conducted preoperatively, at discharge, at 30 days, and again at 1 year. Quality of life as assessed by the European Quality of Life-5 Dimensions (EQ-5D) questionnaire showed significant improvement over time in both study arms, with no difference between the two groups.
Nor were there any significant between-group differences in neurocognitive function as assessed using the Montreal Cognitive Assessment, the Digit Symbol Substitution Test, and the Trail Making Test Part B. However, neurocognitive testing was declared optional because it’s so time consuming, and many patients opted out. For example, only 1,273 of the original 4,752 patients returned to take the Montreal Cognitive Assessment at 1 year, noted Dr. Lamy, a heart surgeon at the Population Health Research Institute at McMaster University in Hamilton, Ont.
Discussant Dr. Bernard Gersh zeroed in on the incomplete neurocognitive testing.
“I think this is really a significant limitation. There’s a huge bias. If there’s any advantage to off-pump CABG, it may be in neurocognitive dysfunction,” commented Dr. Gersh, professor of medicine at the Mayo Clinic, Rochester, Minn.
In response to the discussants’ questioning as to where off-pump CABG fits into clinical practice in light of the disappointing CORONARY and GOPCABE findings, Dr. Diegeler said he remains convinced that some high-operative-risk patients – those with aortic calcification or other evidence of generalized vascular disease ‑ do benefit preferentially from off-pump surgery when performed by expert surgeons.
“It’s hard to prove that in a randomized clinical trial, though,” the surgeon conceded.
Dr. Lamy said a post hoc analysis of the CORONARY data showed that low-operative-risk patients as defined by a EuroScore of 0-2 tended to do better with on- than off-pump CABG, while the converse was true in those with moderate- or high-risk scores.
“In my personal practice now, my low-risk patients go on-pump and my moderate- and high-risk patients go off-pump,” he added.
Both surgeons were firmly in favor of off-pump CABG continuing to be available at expert centers so that surgeons can individualize their operation to suit a patient’s needs.
Dr. Jan Hlavicka presented the results of the PRAGUE-6 trial, in which 206 patients at high operative risk – a EuroScore of 6 or greater – were randomized to off- or on-pump CABG at Charles University, Prague. The operations were performed by five surgeons proficient in both procedures.
The 30-day primary composite endpoint comprising death, MI, stroke, or new renal failure requiring dialysis occurred in 20.6% of the on-pump group, compared with 9.2% of off-pump patients.
The off-pump group required significantly fewer RBC transfusions. However, there were no significant differences between the two groups in terms of average hospital length of stay, wound infection rates, or total hospital costs.
“We believe off-pump bypass in high-risk patients has a lower incidence of serious complications and is a safer means of direct revascularization in these patients,” concluded Dr. Hlavicka, a cardiac surgeon at the university.
Dr. Gersh noted that the only significant difference between the two groups in the individual components of the primary endpoint was in acute MI rates: 12.1% in the on- vs. 4.1% in the off-pump CABG group. He took issue with the Czech investigators’ use of the 2004 Society of Thoracic Surgeons definition of acute MI, which in the first 24 hours after surgery requires only an elevation of creatine kinase–MB at least five times the upper limit of normal with no need for new Q waves. That’s not sufficiently stringent. It surely captures many patients who don’t really have an acute MI. The data should be reanalyzed using a contemporary definition which requires new Q waves, he added.
The CORONARY trial was funded by the Canadian Institutes of Health Research; simultaneous with Dr. Lamy’s presentation of the data in San Francisco, the study was published online (N. Engl. J. Med. 2013 [doi:10.1056/NEJMoa1301228]).
The GOPCABE trial was sponsored by the German Society for Cardiovascular Surgery with funding support by Maquet. It was published online simultaneously with Dr. Diegeler’s ACC presentation (N. Engl. J. Med. 2013 [doi:10.1056/NEJMoa1211666]).
Dr. Hlavicka, Dr. Lamy, and Dr. Diegeler declared having no financial conflicts.
SAN FRANCISCO – Off-pump coronary artery bypass graft surgery offered no advantages over on-pump CABG in any major endpoints at 1 year of follow-up in two major prospective randomized trials totaling more than 7,000 patients.
Among the key 1-year outcomes – which didn’t differ between off- and on-pump CABG patients in the GOPCABE and CORONARY trials – were death, MI, stroke, neurocognitive function, quality of life, renal failure, and repeat revascularization, investigators reported at the annual meeting of the American College of Cardiology.
A third randomized trial presented at the same session of the ACC meeting did find a significant outcome advantage favoring off-pump CABG in high-operative-risk patients at 1 year. However, experts discounted this Czech study because it was small, single center, reported only 30-day results, and the advantage found for off-pump surgery hinged on an outdated and inadequate definition of MI.
The new study findings signal a striking fall from grace for off-pump CABG. Not long ago, this technique, while controversial, was viewed by many as a progressive development within heart surgery, one that would revitalize a mature operation whose annual case numbers were declining in the face of stiff competition from percutaneous coronary intervention by cardiologists. Off-pump CABG was an innovation designed to avoid the perioperative complications related to aortic cross-clamping and the heart-lung machine, including the lingering neurocognitive dysfunction known informally in surgical circles as “pump head.”
However, the resounding lack of any demonstrable advantages for off-pump CABG in the two large trials presented in San Francisco left analysts scratching their heads as to the role remaining for this beating heart surgical technique, which is more difficult to learn and perform skillfully than on-pump bypass.
Speaking perhaps for many puzzled cardiologists in the packed audience in San Francisco, Dr. Christopher P. Cannon of Harvard Medical School, Boston, asked the surgeon-presenters of the CORONARY and GOPCABE trials, “Why would you want to do off-pump surgery if it doesn’t seem to help and it’s harder to do?”
Discussant Dr. Michael J. Mack, a cardiac surgeon, voiced a similar sentiment.
“I was an early advocate of off-pump surgery. But as a card-carrying off-pump bypass surgeon, it’s getting harder and harder for me to maintain enthusiasm for a potential benefit from this,” declared Dr. Mack, medical director of cardiovascular surgery for the Baylor Health Care System and director of cardiovascular research at the Heart Hospital in Plano, Tex.
He noted that the GOPCABE and CORONARY trials follow upon the earlier ROOBY (Randomized On/Off Bypass) trial, which actually showed worse outcomes in the off-pump group. ROOBY enrolled 2,203 Veterans Affairs patients, with the off-pump CABG group having a significantly higher 1-year rate of the primary composite endpoint comprising death, nonfatal MI, or repeat revascularization, along with worse graft patency (N. Engl. J. Med. 2009;361:1827-37).
GOPCABE and CORONARY were designed in part to answer critics of ROOBY, who have argued that the VA trial used insufficiently experienced off-pump CABG surgeons and featured a patient population at too low an operative risk to detect a signal of benefit favoring off-pump surgery.
The GOPCABE (German Off-Pump Coronary Artery Bypass Grafting in Elderly Patients) study involved 2,539 patients aged 75 years or older randomized at 12 German centers. A total of 60% of patients had triple-vessel disease, and no one was excluded from the trial because of left ventricular function or coronary artery anatomy.
Participating surgeons were highly experienced. Those who performed off-pump CABG in the study had previously done an average of 514 of them, while the on-pump surgeons had done an average of 1,378 of those operations.
“We wanted to have the best off-pump vs. the best on-pump surgeons, like in a competition,” explained Dr. Anno Diegeler, a surgeon at the Bad Neustadt (Germany) Heart Center.
He and his coinvestigators conducted GOPCABE because they believed it would be easier to show advantages for off-pump CABG in a population at high operative risk, such as elderly patients with many comorbidities. Indeed, the study hypothesis was that the off-pump group would show a robust 30% reduction in the primary endpoint, a composite of death, stroke, myocardial infarction, repeat revascularization, or new renal-replacement therapy at 1 year.
That didn’t happen. The 30-day rate of the primary endpoint was 7.8% in the off-pump group and 8.2% with on-pump CABG, while the 1-year rates were 13.1% and 14.0%, respectively. None of the individual components of the composite endpoint differed significantly between the groups, either.
Neurocognitive function wasn’t measured in GOPCABE, but it was in CORONARY (the CABG Off or On Pump Revascularization Study), which involved 4,752 randomized patients in 19 countries.
Dr. Andre Lamy presented the 1-year results. The primary endpoint was a composite of death, MI, stroke, or new renal failure requiring dialysis. The rate was 12.1% in patients in the off-pump group and similar at 13.3% in the on-pump group.
As in GOPCABE, the surgeons participating in CORONARY were highly proficient. They had to have at least 2 years’ experience as a staff cardiac surgeon and at least 100 prior cases of whichever operation they were assigned to. The vast majority met that standard for both procedures.
Quality of life and neurocognitive tests were conducted preoperatively, at discharge, at 30 days, and again at 1 year. Quality of life as assessed by the European Quality of Life-5 Dimensions (EQ-5D) questionnaire showed significant improvement over time in both study arms, with no difference between the two groups.
Nor were there any significant between-group differences in neurocognitive function as assessed using the Montreal Cognitive Assessment, the Digit Symbol Substitution Test, and the Trail Making Test Part B. However, neurocognitive testing was declared optional because it’s so time consuming, and many patients opted out. For example, only 1,273 of the original 4,752 patients returned to take the Montreal Cognitive Assessment at 1 year, noted Dr. Lamy, a heart surgeon at the Population Health Research Institute at McMaster University in Hamilton, Ont.
Discussant Dr. Bernard Gersh zeroed in on the incomplete neurocognitive testing.
“I think this is really a significant limitation. There’s a huge bias. If there’s any advantage to off-pump CABG, it may be in neurocognitive dysfunction,” commented Dr. Gersh, professor of medicine at the Mayo Clinic, Rochester, Minn.
In response to the discussants’ questioning as to where off-pump CABG fits into clinical practice in light of the disappointing CORONARY and GOPCABE findings, Dr. Diegeler said he remains convinced that some high-operative-risk patients – those with aortic calcification or other evidence of generalized vascular disease ‑ do benefit preferentially from off-pump surgery when performed by expert surgeons.
“It’s hard to prove that in a randomized clinical trial, though,” the surgeon conceded.
Dr. Lamy said a post hoc analysis of the CORONARY data showed that low-operative-risk patients as defined by a EuroScore of 0-2 tended to do better with on- than off-pump CABG, while the converse was true in those with moderate- or high-risk scores.
“In my personal practice now, my low-risk patients go on-pump and my moderate- and high-risk patients go off-pump,” he added.
Both surgeons were firmly in favor of off-pump CABG continuing to be available at expert centers so that surgeons can individualize their operation to suit a patient’s needs.
Dr. Jan Hlavicka presented the results of the PRAGUE-6 trial, in which 206 patients at high operative risk – a EuroScore of 6 or greater – were randomized to off- or on-pump CABG at Charles University, Prague. The operations were performed by five surgeons proficient in both procedures.
The 30-day primary composite endpoint comprising death, MI, stroke, or new renal failure requiring dialysis occurred in 20.6% of the on-pump group, compared with 9.2% of off-pump patients.
The off-pump group required significantly fewer RBC transfusions. However, there were no significant differences between the two groups in terms of average hospital length of stay, wound infection rates, or total hospital costs.
“We believe off-pump bypass in high-risk patients has a lower incidence of serious complications and is a safer means of direct revascularization in these patients,” concluded Dr. Hlavicka, a cardiac surgeon at the university.
Dr. Gersh noted that the only significant difference between the two groups in the individual components of the primary endpoint was in acute MI rates: 12.1% in the on- vs. 4.1% in the off-pump CABG group. He took issue with the Czech investigators’ use of the 2004 Society of Thoracic Surgeons definition of acute MI, which in the first 24 hours after surgery requires only an elevation of creatine kinase–MB at least five times the upper limit of normal with no need for new Q waves. That’s not sufficiently stringent. It surely captures many patients who don’t really have an acute MI. The data should be reanalyzed using a contemporary definition which requires new Q waves, he added.
The CORONARY trial was funded by the Canadian Institutes of Health Research; simultaneous with Dr. Lamy’s presentation of the data in San Francisco, the study was published online (N. Engl. J. Med. 2013 [doi:10.1056/NEJMoa1301228]).
The GOPCABE trial was sponsored by the German Society for Cardiovascular Surgery with funding support by Maquet. It was published online simultaneously with Dr. Diegeler’s ACC presentation (N. Engl. J. Med. 2013 [doi:10.1056/NEJMoa1211666]).
Dr. Hlavicka, Dr. Lamy, and Dr. Diegeler declared having no financial conflicts.
SAN FRANCISCO – Off-pump coronary artery bypass graft surgery offered no advantages over on-pump CABG in any major endpoints at 1 year of follow-up in two major prospective randomized trials totaling more than 7,000 patients.
Among the key 1-year outcomes – which didn’t differ between off- and on-pump CABG patients in the GOPCABE and CORONARY trials – were death, MI, stroke, neurocognitive function, quality of life, renal failure, and repeat revascularization, investigators reported at the annual meeting of the American College of Cardiology.
A third randomized trial presented at the same session of the ACC meeting did find a significant outcome advantage favoring off-pump CABG in high-operative-risk patients at 1 year. However, experts discounted this Czech study because it was small, single center, reported only 30-day results, and the advantage found for off-pump surgery hinged on an outdated and inadequate definition of MI.
The new study findings signal a striking fall from grace for off-pump CABG. Not long ago, this technique, while controversial, was viewed by many as a progressive development within heart surgery, one that would revitalize a mature operation whose annual case numbers were declining in the face of stiff competition from percutaneous coronary intervention by cardiologists. Off-pump CABG was an innovation designed to avoid the perioperative complications related to aortic cross-clamping and the heart-lung machine, including the lingering neurocognitive dysfunction known informally in surgical circles as “pump head.”
However, the resounding lack of any demonstrable advantages for off-pump CABG in the two large trials presented in San Francisco left analysts scratching their heads as to the role remaining for this beating heart surgical technique, which is more difficult to learn and perform skillfully than on-pump bypass.
Speaking perhaps for many puzzled cardiologists in the packed audience in San Francisco, Dr. Christopher P. Cannon of Harvard Medical School, Boston, asked the surgeon-presenters of the CORONARY and GOPCABE trials, “Why would you want to do off-pump surgery if it doesn’t seem to help and it’s harder to do?”
Discussant Dr. Michael J. Mack, a cardiac surgeon, voiced a similar sentiment.
“I was an early advocate of off-pump surgery. But as a card-carrying off-pump bypass surgeon, it’s getting harder and harder for me to maintain enthusiasm for a potential benefit from this,” declared Dr. Mack, medical director of cardiovascular surgery for the Baylor Health Care System and director of cardiovascular research at the Heart Hospital in Plano, Tex.
He noted that the GOPCABE and CORONARY trials follow upon the earlier ROOBY (Randomized On/Off Bypass) trial, which actually showed worse outcomes in the off-pump group. ROOBY enrolled 2,203 Veterans Affairs patients, with the off-pump CABG group having a significantly higher 1-year rate of the primary composite endpoint comprising death, nonfatal MI, or repeat revascularization, along with worse graft patency (N. Engl. J. Med. 2009;361:1827-37).
GOPCABE and CORONARY were designed in part to answer critics of ROOBY, who have argued that the VA trial used insufficiently experienced off-pump CABG surgeons and featured a patient population at too low an operative risk to detect a signal of benefit favoring off-pump surgery.
The GOPCABE (German Off-Pump Coronary Artery Bypass Grafting in Elderly Patients) study involved 2,539 patients aged 75 years or older randomized at 12 German centers. A total of 60% of patients had triple-vessel disease, and no one was excluded from the trial because of left ventricular function or coronary artery anatomy.
Participating surgeons were highly experienced. Those who performed off-pump CABG in the study had previously done an average of 514 of them, while the on-pump surgeons had done an average of 1,378 of those operations.
“We wanted to have the best off-pump vs. the best on-pump surgeons, like in a competition,” explained Dr. Anno Diegeler, a surgeon at the Bad Neustadt (Germany) Heart Center.
He and his coinvestigators conducted GOPCABE because they believed it would be easier to show advantages for off-pump CABG in a population at high operative risk, such as elderly patients with many comorbidities. Indeed, the study hypothesis was that the off-pump group would show a robust 30% reduction in the primary endpoint, a composite of death, stroke, myocardial infarction, repeat revascularization, or new renal-replacement therapy at 1 year.
That didn’t happen. The 30-day rate of the primary endpoint was 7.8% in the off-pump group and 8.2% with on-pump CABG, while the 1-year rates were 13.1% and 14.0%, respectively. None of the individual components of the composite endpoint differed significantly between the groups, either.
Neurocognitive function wasn’t measured in GOPCABE, but it was in CORONARY (the CABG Off or On Pump Revascularization Study), which involved 4,752 randomized patients in 19 countries.
Dr. Andre Lamy presented the 1-year results. The primary endpoint was a composite of death, MI, stroke, or new renal failure requiring dialysis. The rate was 12.1% in patients in the off-pump group and similar at 13.3% in the on-pump group.
As in GOPCABE, the surgeons participating in CORONARY were highly proficient. They had to have at least 2 years’ experience as a staff cardiac surgeon and at least 100 prior cases of whichever operation they were assigned to. The vast majority met that standard for both procedures.
Quality of life and neurocognitive tests were conducted preoperatively, at discharge, at 30 days, and again at 1 year. Quality of life as assessed by the European Quality of Life-5 Dimensions (EQ-5D) questionnaire showed significant improvement over time in both study arms, with no difference between the two groups.
Nor were there any significant between-group differences in neurocognitive function as assessed using the Montreal Cognitive Assessment, the Digit Symbol Substitution Test, and the Trail Making Test Part B. However, neurocognitive testing was declared optional because it’s so time consuming, and many patients opted out. For example, only 1,273 of the original 4,752 patients returned to take the Montreal Cognitive Assessment at 1 year, noted Dr. Lamy, a heart surgeon at the Population Health Research Institute at McMaster University in Hamilton, Ont.
Discussant Dr. Bernard Gersh zeroed in on the incomplete neurocognitive testing.
“I think this is really a significant limitation. There’s a huge bias. If there’s any advantage to off-pump CABG, it may be in neurocognitive dysfunction,” commented Dr. Gersh, professor of medicine at the Mayo Clinic, Rochester, Minn.
In response to the discussants’ questioning as to where off-pump CABG fits into clinical practice in light of the disappointing CORONARY and GOPCABE findings, Dr. Diegeler said he remains convinced that some high-operative-risk patients – those with aortic calcification or other evidence of generalized vascular disease ‑ do benefit preferentially from off-pump surgery when performed by expert surgeons.
“It’s hard to prove that in a randomized clinical trial, though,” the surgeon conceded.
Dr. Lamy said a post hoc analysis of the CORONARY data showed that low-operative-risk patients as defined by a EuroScore of 0-2 tended to do better with on- than off-pump CABG, while the converse was true in those with moderate- or high-risk scores.
“In my personal practice now, my low-risk patients go on-pump and my moderate- and high-risk patients go off-pump,” he added.
Both surgeons were firmly in favor of off-pump CABG continuing to be available at expert centers so that surgeons can individualize their operation to suit a patient’s needs.
Dr. Jan Hlavicka presented the results of the PRAGUE-6 trial, in which 206 patients at high operative risk – a EuroScore of 6 or greater – were randomized to off- or on-pump CABG at Charles University, Prague. The operations were performed by five surgeons proficient in both procedures.
The 30-day primary composite endpoint comprising death, MI, stroke, or new renal failure requiring dialysis occurred in 20.6% of the on-pump group, compared with 9.2% of off-pump patients.
The off-pump group required significantly fewer RBC transfusions. However, there were no significant differences between the two groups in terms of average hospital length of stay, wound infection rates, or total hospital costs.
“We believe off-pump bypass in high-risk patients has a lower incidence of serious complications and is a safer means of direct revascularization in these patients,” concluded Dr. Hlavicka, a cardiac surgeon at the university.
Dr. Gersh noted that the only significant difference between the two groups in the individual components of the primary endpoint was in acute MI rates: 12.1% in the on- vs. 4.1% in the off-pump CABG group. He took issue with the Czech investigators’ use of the 2004 Society of Thoracic Surgeons definition of acute MI, which in the first 24 hours after surgery requires only an elevation of creatine kinase–MB at least five times the upper limit of normal with no need for new Q waves. That’s not sufficiently stringent. It surely captures many patients who don’t really have an acute MI. The data should be reanalyzed using a contemporary definition which requires new Q waves, he added.
The CORONARY trial was funded by the Canadian Institutes of Health Research; simultaneous with Dr. Lamy’s presentation of the data in San Francisco, the study was published online (N. Engl. J. Med. 2013 [doi:10.1056/NEJMoa1301228]).
The GOPCABE trial was sponsored by the German Society for Cardiovascular Surgery with funding support by Maquet. It was published online simultaneously with Dr. Diegeler’s ACC presentation (N. Engl. J. Med. 2013 [doi:10.1056/NEJMoa1211666]).
Dr. Hlavicka, Dr. Lamy, and Dr. Diegeler declared having no financial conflicts.
Major finding: Off-pump coronary artery bypass
graft surgery did not differ significantly from on-pump bypass in 1-year rates
of death, stroke, MI, or any other important outcomes in two large,
prospective, randomized trials.
Data source: The GOPCABE trial, in 2,539 randomized
patients; and the CORONARY trial, in 4,752 patients in 19 countries.
Disclosures: CORONARY was sponsored by the Canadian
Institutes of Health Research. GOPCABE was sponsored by the German Society for
Cardiovascular Surgery with funding support from Maquet. The presenters
reported having no financial conflicts.