User login
Losartan shown effective in Marfan syndrome
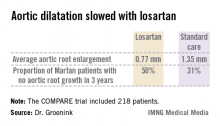
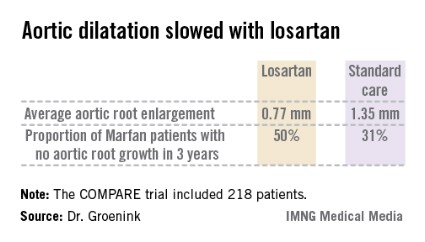
AMSTERDAM – Daily losartan significantly slowed the aortic root dilatation rate in adults with Marfan syndrome in a 3-year randomized clinical trial.
"I think we can be positive about this treatment. We can now recommend losartan in clinical practice," Dr. Maarten Groenink said at the annual congress of the European Society of Cardiology.
The COMPARE (Cozaar in Marfan Patients Reduces Aortic Enlargement) trial included 218 patients at all four university Marfan centers in the Netherlands. Patients were randomized to oral losartan at a target dose of 100 mg/day or no losartan in addition to standard-of-care treatment with beta-blockers. Roughly half of the patients in the losartan group were unable to tolerate the full dose of losartan in addition to a beta-blocker; those patients were maintained on losartan at 50 mg/day. Aortic root diameter was measured by MRI at enrollment and after 3 years of prospective follow-up. The aortic dilatation rate was significantly lower in the losartan group than in controls both in the patients with a native root and in those who had undergone aortic root replacement surgery, reported Dr. Groenink, a cardiologist at the Academic Medical Center, Amsterdam.
There were no aortic dissections in the losartan group and two in the control arm. Elective aortic replacement surgery was performed in a similar number of patients in both groups.
Blood pressure was lower in the losartan group, yet blood pressure didn’t correlate with the aortic dilatation rate. Dr. Groenink speculated that losartan’s chief mechanism of benefit in Marfan syndrome is its ability to curb overexpression of transforming growth factor-beta, which weakens the structure of the media layer of the aortic wall.
Dr. Groenink said it’s unknown whether losartan’s benefits are specific to that drug or are a class effect obtainable with other angiotensin II receptor antagonists, though he suspects it’s a class effect.
Ongoing clinical trials are evaluating losartan in children and adolescents with Marfan syndrome, he said, adding that there is a solid rationale for beginning treatment as early in life as possible.
"I believe the adverse effects on the aortic wall in Marfan syndrome are caused by the fibrillin defect but also by wear and tear due to cyclic stress by the beating heart. So you can hypothesize that the earlier you start treatment, the better the results," he explained.
Marfan syndrome is a genetic connective tissue disorder affecting multiple organ systems. The prognosis is mainly determined by the aortic complications, including dilatation, aneurysm formation, and possible acute dissection. Affected individuals tend to be tall, long-limbed, and have distinctively long, thin fingers. The prevalence of Marfan syndrome has been estimated at 1 in 5,000, but Dr. Groenink suspects the syndrome may actually be more common than that.
Simultaneous with Dr. Groenink’s presentation at the ESC, the COMPARE results were published online (Eur. Heart J. 2013 [doi:10.1093/eurheartj/eht334]).
The COMPARE trial was funded by the Dutch Heart Association. Dr. Groenink reported having no relevant financial interests.
*CORRECTION 11/14/13: The first version of this story had Dr. Groenink's name misspelled.

|
Bruce Jancin/IMNG Medical Media
|
COMPARE is a very important study whose results are going to mean a paradigm shift for the management of Marfan syndrome.
It is intriguing to consider that the benefits of losartan might possibly also extend to patients with thoracic aortic disease in general, a worthy topic for future investigation.
Dr. John Gordon Harold is with Cedars-Sinai Heart Institute, Los Angeles, and president of the American College of Cardiology. He had no relevant financial disclosures.

|
Bruce Jancin/IMNG Medical Media
|
COMPARE is a very important study whose results are going to mean a paradigm shift for the management of Marfan syndrome.
It is intriguing to consider that the benefits of losartan might possibly also extend to patients with thoracic aortic disease in general, a worthy topic for future investigation.
Dr. John Gordon Harold is with Cedars-Sinai Heart Institute, Los Angeles, and president of the American College of Cardiology. He had no relevant financial disclosures.

|
Bruce Jancin/IMNG Medical Media
|
COMPARE is a very important study whose results are going to mean a paradigm shift for the management of Marfan syndrome.
It is intriguing to consider that the benefits of losartan might possibly also extend to patients with thoracic aortic disease in general, a worthy topic for future investigation.
Dr. John Gordon Harold is with Cedars-Sinai Heart Institute, Los Angeles, and president of the American College of Cardiology. He had no relevant financial disclosures.
AMSTERDAM – Daily losartan significantly slowed the aortic root dilatation rate in adults with Marfan syndrome in a 3-year randomized clinical trial.
"I think we can be positive about this treatment. We can now recommend losartan in clinical practice," Dr. Maarten Groenink said at the annual congress of the European Society of Cardiology.
The COMPARE (Cozaar in Marfan Patients Reduces Aortic Enlargement) trial included 218 patients at all four university Marfan centers in the Netherlands. Patients were randomized to oral losartan at a target dose of 100 mg/day or no losartan in addition to standard-of-care treatment with beta-blockers. Roughly half of the patients in the losartan group were unable to tolerate the full dose of losartan in addition to a beta-blocker; those patients were maintained on losartan at 50 mg/day. Aortic root diameter was measured by MRI at enrollment and after 3 years of prospective follow-up. The aortic dilatation rate was significantly lower in the losartan group than in controls both in the patients with a native root and in those who had undergone aortic root replacement surgery, reported Dr. Groenink, a cardiologist at the Academic Medical Center, Amsterdam.
There were no aortic dissections in the losartan group and two in the control arm. Elective aortic replacement surgery was performed in a similar number of patients in both groups.
Blood pressure was lower in the losartan group, yet blood pressure didn’t correlate with the aortic dilatation rate. Dr. Groenink speculated that losartan’s chief mechanism of benefit in Marfan syndrome is its ability to curb overexpression of transforming growth factor-beta, which weakens the structure of the media layer of the aortic wall.
Dr. Groenink said it’s unknown whether losartan’s benefits are specific to that drug or are a class effect obtainable with other angiotensin II receptor antagonists, though he suspects it’s a class effect.
Ongoing clinical trials are evaluating losartan in children and adolescents with Marfan syndrome, he said, adding that there is a solid rationale for beginning treatment as early in life as possible.
"I believe the adverse effects on the aortic wall in Marfan syndrome are caused by the fibrillin defect but also by wear and tear due to cyclic stress by the beating heart. So you can hypothesize that the earlier you start treatment, the better the results," he explained.
Marfan syndrome is a genetic connective tissue disorder affecting multiple organ systems. The prognosis is mainly determined by the aortic complications, including dilatation, aneurysm formation, and possible acute dissection. Affected individuals tend to be tall, long-limbed, and have distinctively long, thin fingers. The prevalence of Marfan syndrome has been estimated at 1 in 5,000, but Dr. Groenink suspects the syndrome may actually be more common than that.
Simultaneous with Dr. Groenink’s presentation at the ESC, the COMPARE results were published online (Eur. Heart J. 2013 [doi:10.1093/eurheartj/eht334]).
The COMPARE trial was funded by the Dutch Heart Association. Dr. Groenink reported having no relevant financial interests.
*CORRECTION 11/14/13: The first version of this story had Dr. Groenink's name misspelled.
AMSTERDAM – Daily losartan significantly slowed the aortic root dilatation rate in adults with Marfan syndrome in a 3-year randomized clinical trial.
"I think we can be positive about this treatment. We can now recommend losartan in clinical practice," Dr. Maarten Groenink said at the annual congress of the European Society of Cardiology.
The COMPARE (Cozaar in Marfan Patients Reduces Aortic Enlargement) trial included 218 patients at all four university Marfan centers in the Netherlands. Patients were randomized to oral losartan at a target dose of 100 mg/day or no losartan in addition to standard-of-care treatment with beta-blockers. Roughly half of the patients in the losartan group were unable to tolerate the full dose of losartan in addition to a beta-blocker; those patients were maintained on losartan at 50 mg/day. Aortic root diameter was measured by MRI at enrollment and after 3 years of prospective follow-up. The aortic dilatation rate was significantly lower in the losartan group than in controls both in the patients with a native root and in those who had undergone aortic root replacement surgery, reported Dr. Groenink, a cardiologist at the Academic Medical Center, Amsterdam.
There were no aortic dissections in the losartan group and two in the control arm. Elective aortic replacement surgery was performed in a similar number of patients in both groups.
Blood pressure was lower in the losartan group, yet blood pressure didn’t correlate with the aortic dilatation rate. Dr. Groenink speculated that losartan’s chief mechanism of benefit in Marfan syndrome is its ability to curb overexpression of transforming growth factor-beta, which weakens the structure of the media layer of the aortic wall.
Dr. Groenink said it’s unknown whether losartan’s benefits are specific to that drug or are a class effect obtainable with other angiotensin II receptor antagonists, though he suspects it’s a class effect.
Ongoing clinical trials are evaluating losartan in children and adolescents with Marfan syndrome, he said, adding that there is a solid rationale for beginning treatment as early in life as possible.
"I believe the adverse effects on the aortic wall in Marfan syndrome are caused by the fibrillin defect but also by wear and tear due to cyclic stress by the beating heart. So you can hypothesize that the earlier you start treatment, the better the results," he explained.
Marfan syndrome is a genetic connective tissue disorder affecting multiple organ systems. The prognosis is mainly determined by the aortic complications, including dilatation, aneurysm formation, and possible acute dissection. Affected individuals tend to be tall, long-limbed, and have distinctively long, thin fingers. The prevalence of Marfan syndrome has been estimated at 1 in 5,000, but Dr. Groenink suspects the syndrome may actually be more common than that.
Simultaneous with Dr. Groenink’s presentation at the ESC, the COMPARE results were published online (Eur. Heart J. 2013 [doi:10.1093/eurheartj/eht334]).
The COMPARE trial was funded by the Dutch Heart Association. Dr. Groenink reported having no relevant financial interests.
*CORRECTION 11/14/13: The first version of this story had Dr. Groenink's name misspelled.
AT THE ESC CONGRESS 2013
Major finding: The rate of aortic root enlargement during 3 years of prospective follow-up was 0.77 mm in losartan-treated patients with Marfan syndrome, significantly less than the 1.35 mm in patients on standard-of-care treatment with no losartan.
Data source: The COMPARE trial was a randomized, prospective, open-label multicenter study in which 218 patients with Marfan syndrome were randomized to losartan at a target dose of 100 mg or to no losartan and followed for 3 years with the aortic root dilatation rate as measured by MRI the primary endpoint.
Disclosures: The COMPARE trial was supported by the Dutch Heart Association. Dr. Groenink reported having no financial conflicts.
Guideline-recommended beta-blockers before noncardiac surgery shown to increase mortality by 27%
More than 1 in 4 patients who died from all causes after noncardiac surgery may have survived if they were not treated with perioperative beta-blockers as specified by joint American College of Cardiology Foundation/American Heart Association and separate European Society of Cardiology guidelines.
These guidelines recommend perioperative beta-blockers in all patients undergoing vascular or intermediate-risk surgery with coronary artery disease, or with more than one risk factor for CAD, or with preexisting beta-blockade. These are all iatrogenic deaths, according to a meta-analysis of secure studies, which excluded data from the now discredited Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography (DECREASE) family of trials.
"Refraining from this ESC [European Society of Cardiology] guideline would therefore be expected to prevent up to 10,000 iatrogenic deaths each year in the U.K.," according to Dr. Sonia Bouri and her coauthors at the National Heart and Lung Institute, Imperial College London.
The researchers analyzed nine secure randomized trials totaling 10,529 patients who met the guideline criteria, 291 of whom died. They found that initiation of a course of beta-blockers as per guideline recommendations before surgery resulted in a 27% increase in mortality.
In the secure trials, use of perioperative beta-blockers decreased nonfatal myocardial infarction significantly (RR, 0.73; P = .001), but increased stroke (RR, 1.73; P =.05) and hypotension (RR, 1.51; P less than .00001), according to the authors, who presented their data in Heart (2013 July 31 [doi: 10.1136/heartjnl-2013-304262]).
Of the 291 deaths recorded in the secure trials, 162 deaths (3.21%) occurred in 5,264 patients randomized to beta-blockers, and 129 deaths (2.45%) occurred in the 5,265 patients randomized to placebo.
Thus, the initiation of a course of beta-blockers as per guideline recommendations before surgery resulted in a 27% increase in all-cause mortality, Dr. Bouri and her coauthors stated. "Any remaining [perioperative beta-blocker] enthusiasts might best channel their energy into a further randomized trial, which should be designed carefully and honestly," they added.
The results from the DECREASE family of trials substantially contradicted the meta-analysis of the secure trials on the effect on mortality (P = .05 for divergence).
"All studies investigated in the DECREASE family for which data had not been lost were found to be insecure because of serious flaws. In one case, it was clear that the entire study database had been fabricated. DECREASE I, published in 1999, escaped investigation as the terms of the investigation only reached back 10 years," the researchers reported.
When the ESC and American College of Cardiology Foundation/American Heart Association guidelines were formulated, "the inclusion of insecure data caused them to reach the conclusion that beta-blockade had a neutral effect on mortality and allowed them to focus on the reduction of non-fatal MI as a surrogate endpoint," the authors explained.
The DECREASE family of studies was discredited almost 2 years ago and subsequently underwent lengthy internal investigation, the results of which have been public for some time, according to the authors. "Nevertheless, neither the European Society of Cardiology nor the AHA guidelines have been retracted," they said.
"Patient safety being paramount, guidelines for perioperative beta-blockers should be retracted without further delay. Future guidelines should be accompanied by a commitment from named individuals to retract them immediately if the advice given is later revealed to be harmful," the authors concluded.
The authors reported that they had no conflicts of interest.
For those of us in the "perioperative care business," the results of this meta-analysis are neither surprising nor informative. The increased all-cause mortality with perioperative beta blockade in the POISE trial is well documented in the literature and the findings of this most recent study by Bouri and colleagues is dominated by POISE data.

|
| Dr. Franklin Michota |
For those who might not recall POISE, it is the largest randomized clinical trial (RCT) to date that evaluated the safety and efficacy of perioperative beta-blockers in noncardiac surgery. A total of 8,351 patients aged older than 45 years who had or were at risk for atherosclerotic heart disease were randomized to beta-blockers or placebo.
In the treatment arm, patients received metoprolol CR (100 mg preop, 100 mg 6 hours postop, 200 mg 12 hours later, then daily for 30 days). This dose is significantly higher than what most clinicians are accustomed to using. And while it is true that we can no longer trust the conclusions from the DECREASE family of studies due to academic negligence, it does not equate to confirmatory evidence that titrated perioperative beta blockade at a lower dose than that used in POISE (thus avoiding sinus bradycardia and/or hypotension) is of no benefit or harmful. Unfortunately, we just won't know either way until further investigation is performed.

|
| Dr. Amir Jaffer |
To extrapolate that more than 1 in 4 patients who died from all causes after noncardiac surgery may have survived if they were not treated with perioperative beta-blockers as specified by the ACC/AHA and separate ESC guidelines is pure sensationalism.
Following publication of the POISE trial, the ACC/AHA did publish a focused update on perioperative beta-blockers and specifically noted the possibility of harm from these medications and the importance of careful dose titration. Given the anecdotal experience by all of us who have titrated beta-blockers perioperatively, we find it difficult to believe that such an approach is causing harm. We certainly do question now whether such an approach is doing the patient any good until proven prospectively in a large RCT.
Dr. Franklin A. Michota is director of academic affairs in the department of hospital medicine at the Cleveland Clinic. Dr. Amir K. Jaffer is assistant chief medical officer and division chief of hospital medicine at Rush University Medical Center, Chicago. They are advisers to Hospitalist News.
For those of us in the "perioperative care business," the results of this meta-analysis are neither surprising nor informative. The increased all-cause mortality with perioperative beta blockade in the POISE trial is well documented in the literature and the findings of this most recent study by Bouri and colleagues is dominated by POISE data.

|
| Dr. Franklin Michota |
For those who might not recall POISE, it is the largest randomized clinical trial (RCT) to date that evaluated the safety and efficacy of perioperative beta-blockers in noncardiac surgery. A total of 8,351 patients aged older than 45 years who had or were at risk for atherosclerotic heart disease were randomized to beta-blockers or placebo.
In the treatment arm, patients received metoprolol CR (100 mg preop, 100 mg 6 hours postop, 200 mg 12 hours later, then daily for 30 days). This dose is significantly higher than what most clinicians are accustomed to using. And while it is true that we can no longer trust the conclusions from the DECREASE family of studies due to academic negligence, it does not equate to confirmatory evidence that titrated perioperative beta blockade at a lower dose than that used in POISE (thus avoiding sinus bradycardia and/or hypotension) is of no benefit or harmful. Unfortunately, we just won't know either way until further investigation is performed.

|
| Dr. Amir Jaffer |
To extrapolate that more than 1 in 4 patients who died from all causes after noncardiac surgery may have survived if they were not treated with perioperative beta-blockers as specified by the ACC/AHA and separate ESC guidelines is pure sensationalism.
Following publication of the POISE trial, the ACC/AHA did publish a focused update on perioperative beta-blockers and specifically noted the possibility of harm from these medications and the importance of careful dose titration. Given the anecdotal experience by all of us who have titrated beta-blockers perioperatively, we find it difficult to believe that such an approach is causing harm. We certainly do question now whether such an approach is doing the patient any good until proven prospectively in a large RCT.
Dr. Franklin A. Michota is director of academic affairs in the department of hospital medicine at the Cleveland Clinic. Dr. Amir K. Jaffer is assistant chief medical officer and division chief of hospital medicine at Rush University Medical Center, Chicago. They are advisers to Hospitalist News.
For those of us in the "perioperative care business," the results of this meta-analysis are neither surprising nor informative. The increased all-cause mortality with perioperative beta blockade in the POISE trial is well documented in the literature and the findings of this most recent study by Bouri and colleagues is dominated by POISE data.

|
| Dr. Franklin Michota |
For those who might not recall POISE, it is the largest randomized clinical trial (RCT) to date that evaluated the safety and efficacy of perioperative beta-blockers in noncardiac surgery. A total of 8,351 patients aged older than 45 years who had or were at risk for atherosclerotic heart disease were randomized to beta-blockers or placebo.
In the treatment arm, patients received metoprolol CR (100 mg preop, 100 mg 6 hours postop, 200 mg 12 hours later, then daily for 30 days). This dose is significantly higher than what most clinicians are accustomed to using. And while it is true that we can no longer trust the conclusions from the DECREASE family of studies due to academic negligence, it does not equate to confirmatory evidence that titrated perioperative beta blockade at a lower dose than that used in POISE (thus avoiding sinus bradycardia and/or hypotension) is of no benefit or harmful. Unfortunately, we just won't know either way until further investigation is performed.

|
| Dr. Amir Jaffer |
To extrapolate that more than 1 in 4 patients who died from all causes after noncardiac surgery may have survived if they were not treated with perioperative beta-blockers as specified by the ACC/AHA and separate ESC guidelines is pure sensationalism.
Following publication of the POISE trial, the ACC/AHA did publish a focused update on perioperative beta-blockers and specifically noted the possibility of harm from these medications and the importance of careful dose titration. Given the anecdotal experience by all of us who have titrated beta-blockers perioperatively, we find it difficult to believe that such an approach is causing harm. We certainly do question now whether such an approach is doing the patient any good until proven prospectively in a large RCT.
Dr. Franklin A. Michota is director of academic affairs in the department of hospital medicine at the Cleveland Clinic. Dr. Amir K. Jaffer is assistant chief medical officer and division chief of hospital medicine at Rush University Medical Center, Chicago. They are advisers to Hospitalist News.
More than 1 in 4 patients who died from all causes after noncardiac surgery may have survived if they were not treated with perioperative beta-blockers as specified by joint American College of Cardiology Foundation/American Heart Association and separate European Society of Cardiology guidelines.
These guidelines recommend perioperative beta-blockers in all patients undergoing vascular or intermediate-risk surgery with coronary artery disease, or with more than one risk factor for CAD, or with preexisting beta-blockade. These are all iatrogenic deaths, according to a meta-analysis of secure studies, which excluded data from the now discredited Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography (DECREASE) family of trials.
"Refraining from this ESC [European Society of Cardiology] guideline would therefore be expected to prevent up to 10,000 iatrogenic deaths each year in the U.K.," according to Dr. Sonia Bouri and her coauthors at the National Heart and Lung Institute, Imperial College London.
The researchers analyzed nine secure randomized trials totaling 10,529 patients who met the guideline criteria, 291 of whom died. They found that initiation of a course of beta-blockers as per guideline recommendations before surgery resulted in a 27% increase in mortality.
In the secure trials, use of perioperative beta-blockers decreased nonfatal myocardial infarction significantly (RR, 0.73; P = .001), but increased stroke (RR, 1.73; P =.05) and hypotension (RR, 1.51; P less than .00001), according to the authors, who presented their data in Heart (2013 July 31 [doi: 10.1136/heartjnl-2013-304262]).
Of the 291 deaths recorded in the secure trials, 162 deaths (3.21%) occurred in 5,264 patients randomized to beta-blockers, and 129 deaths (2.45%) occurred in the 5,265 patients randomized to placebo.
Thus, the initiation of a course of beta-blockers as per guideline recommendations before surgery resulted in a 27% increase in all-cause mortality, Dr. Bouri and her coauthors stated. "Any remaining [perioperative beta-blocker] enthusiasts might best channel their energy into a further randomized trial, which should be designed carefully and honestly," they added.
The results from the DECREASE family of trials substantially contradicted the meta-analysis of the secure trials on the effect on mortality (P = .05 for divergence).
"All studies investigated in the DECREASE family for which data had not been lost were found to be insecure because of serious flaws. In one case, it was clear that the entire study database had been fabricated. DECREASE I, published in 1999, escaped investigation as the terms of the investigation only reached back 10 years," the researchers reported.
When the ESC and American College of Cardiology Foundation/American Heart Association guidelines were formulated, "the inclusion of insecure data caused them to reach the conclusion that beta-blockade had a neutral effect on mortality and allowed them to focus on the reduction of non-fatal MI as a surrogate endpoint," the authors explained.
The DECREASE family of studies was discredited almost 2 years ago and subsequently underwent lengthy internal investigation, the results of which have been public for some time, according to the authors. "Nevertheless, neither the European Society of Cardiology nor the AHA guidelines have been retracted," they said.
"Patient safety being paramount, guidelines for perioperative beta-blockers should be retracted without further delay. Future guidelines should be accompanied by a commitment from named individuals to retract them immediately if the advice given is later revealed to be harmful," the authors concluded.
The authors reported that they had no conflicts of interest.
More than 1 in 4 patients who died from all causes after noncardiac surgery may have survived if they were not treated with perioperative beta-blockers as specified by joint American College of Cardiology Foundation/American Heart Association and separate European Society of Cardiology guidelines.
These guidelines recommend perioperative beta-blockers in all patients undergoing vascular or intermediate-risk surgery with coronary artery disease, or with more than one risk factor for CAD, or with preexisting beta-blockade. These are all iatrogenic deaths, according to a meta-analysis of secure studies, which excluded data from the now discredited Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography (DECREASE) family of trials.
"Refraining from this ESC [European Society of Cardiology] guideline would therefore be expected to prevent up to 10,000 iatrogenic deaths each year in the U.K.," according to Dr. Sonia Bouri and her coauthors at the National Heart and Lung Institute, Imperial College London.
The researchers analyzed nine secure randomized trials totaling 10,529 patients who met the guideline criteria, 291 of whom died. They found that initiation of a course of beta-blockers as per guideline recommendations before surgery resulted in a 27% increase in mortality.
In the secure trials, use of perioperative beta-blockers decreased nonfatal myocardial infarction significantly (RR, 0.73; P = .001), but increased stroke (RR, 1.73; P =.05) and hypotension (RR, 1.51; P less than .00001), according to the authors, who presented their data in Heart (2013 July 31 [doi: 10.1136/heartjnl-2013-304262]).
Of the 291 deaths recorded in the secure trials, 162 deaths (3.21%) occurred in 5,264 patients randomized to beta-blockers, and 129 deaths (2.45%) occurred in the 5,265 patients randomized to placebo.
Thus, the initiation of a course of beta-blockers as per guideline recommendations before surgery resulted in a 27% increase in all-cause mortality, Dr. Bouri and her coauthors stated. "Any remaining [perioperative beta-blocker] enthusiasts might best channel their energy into a further randomized trial, which should be designed carefully and honestly," they added.
The results from the DECREASE family of trials substantially contradicted the meta-analysis of the secure trials on the effect on mortality (P = .05 for divergence).
"All studies investigated in the DECREASE family for which data had not been lost were found to be insecure because of serious flaws. In one case, it was clear that the entire study database had been fabricated. DECREASE I, published in 1999, escaped investigation as the terms of the investigation only reached back 10 years," the researchers reported.
When the ESC and American College of Cardiology Foundation/American Heart Association guidelines were formulated, "the inclusion of insecure data caused them to reach the conclusion that beta-blockade had a neutral effect on mortality and allowed them to focus on the reduction of non-fatal MI as a surrogate endpoint," the authors explained.
The DECREASE family of studies was discredited almost 2 years ago and subsequently underwent lengthy internal investigation, the results of which have been public for some time, according to the authors. "Nevertheless, neither the European Society of Cardiology nor the AHA guidelines have been retracted," they said.
"Patient safety being paramount, guidelines for perioperative beta-blockers should be retracted without further delay. Future guidelines should be accompanied by a commitment from named individuals to retract them immediately if the advice given is later revealed to be harmful," the authors concluded.
The authors reported that they had no conflicts of interest.
FROM HEART
Best CRT outcomes with LBBB and prolonged QRS
In a large, real-world population of patients who underwent cardiac resynchronization therapy-defibrillator implantation, those who had left bundle-branch block and a QRS duration of 150 ms or more had the best outcomes, according to a report published online August 13 in JAMA.
Patients with left bundle-branch block (LBBB) and a long QRS duration had the lowest mortality risk and the lowest rates of all-cause, cardiovascular, and heart failure readmissions, while patients without LBBB and with a QRS duration of 120-149 ms "consistently had the greatest risks of adverse outcomes," said Dr. Pamela N. Peterson of the Denver Health Medical Center and her associates.
These findings "are particularly notable, given that both LBBB and prolonged QRS duration have been shown to be independent predictors of mortality among patients with left ventricular systolic dysfunction without CRT [cardiac resynchronization therapy]," the investigators said.
The results support the use of QRS morphology and duration to identify which patients can expect the greatest benefit from CRT-D implantation, they noted.
Even though current guidelines recommend selecting patients for CRT based primarily on their QRS morphology and duration, these recommendations are based primarily on meta-analyses and subgroup analyses of clinical trials that only considered these two factors separately. The only study to evaluate the combination of QRS morphology and duration "did not assess meaningful patient outcomes," so the utility of these recommendations in real-world practice hasn’t been clear, Dr. Peterson and her colleagues wrote.
They examined the issue using information from the National Cardiovascular Data Registry’s ICD database. They assessed outcomes in 24,169 Medicare fee-for-service patients who underwent CRT-D implantation in a 3-year period, between 2006 and 2009.
Only patients with a QRS interval of 120 ms or longer were included. The mean age of these study subjects was 75 years. Most (90%) were white and 68% were men.
Comorbid conditions were common, including hypertension (78% of patients), ischemic heart disease (65%), diabetes (38%), atrial fibrillation or flutter (32%), and chronic lung disease (23%). The majority of patients (61%) had not had coronary artery bypass graft surgery.
Most patients (83%) had New York Heart Association class III heart failure symptoms. A total of 67% had LBBB, and 55% had a QRS duration of 150 ms or longer.
Overall mortality was 0.8% at 30 days, 9.2% at 1 year, and 25.9% at 3 years. Overall rates of all-cause readmission were 10.2% at 30 days and 43.3% at 1 year. Rates of heart failure readmission were 2.2% at 30 days and 12.3% at 1 year.
In an unadjusted analysis of the data, rates of all adverse outcomes were significantly lower among patients who had LBBB and a QRS duration of 150 ms or more, the investigators said (JAMA 2013; 310:617-26; [doi:10.1001/jama.2013.8641]).
After the data were adjusted to account for numerous demographic and clinical factors, this difference remained robust. Patients who had LBBB and a QRS duration of 150 ms or greater had a 3-year mortality risk of 21%, compared with 27% for those with LBBB and QRS duration of 120-149 ms. Those with no LBBB and QRS duration of 150 ms or greater had an adjusted 3-year mortality risk of 31%), and patients with no LBBB and QRS durations of 120-149 ms had a 3-year risk of 32%. All differences were significant.
Patients with no LBBB and a long QRS duration, those with LBBB and a short QRS duration, and those with no LBBB and a short QRS duration consistently had higher risks of all adverse outcomes.
"Our real-world data add to the increasing body of evidence that patients with LBBB have better outcomes after CRT," Dr. Peterson and her associates said.
This study was supported by the U.S. Agency for Healthcare Research and Quality and the American College of Cardiology Foundation. Dr. Peterson reported serving as a consultant for Merck, and her associates reported numerous ties to industry sources.
In a large, real-world population of patients who underwent cardiac resynchronization therapy-defibrillator implantation, those who had left bundle-branch block and a QRS duration of 150 ms or more had the best outcomes, according to a report published online August 13 in JAMA.
Patients with left bundle-branch block (LBBB) and a long QRS duration had the lowest mortality risk and the lowest rates of all-cause, cardiovascular, and heart failure readmissions, while patients without LBBB and with a QRS duration of 120-149 ms "consistently had the greatest risks of adverse outcomes," said Dr. Pamela N. Peterson of the Denver Health Medical Center and her associates.
These findings "are particularly notable, given that both LBBB and prolonged QRS duration have been shown to be independent predictors of mortality among patients with left ventricular systolic dysfunction without CRT [cardiac resynchronization therapy]," the investigators said.
The results support the use of QRS morphology and duration to identify which patients can expect the greatest benefit from CRT-D implantation, they noted.
Even though current guidelines recommend selecting patients for CRT based primarily on their QRS morphology and duration, these recommendations are based primarily on meta-analyses and subgroup analyses of clinical trials that only considered these two factors separately. The only study to evaluate the combination of QRS morphology and duration "did not assess meaningful patient outcomes," so the utility of these recommendations in real-world practice hasn’t been clear, Dr. Peterson and her colleagues wrote.
They examined the issue using information from the National Cardiovascular Data Registry’s ICD database. They assessed outcomes in 24,169 Medicare fee-for-service patients who underwent CRT-D implantation in a 3-year period, between 2006 and 2009.
Only patients with a QRS interval of 120 ms or longer were included. The mean age of these study subjects was 75 years. Most (90%) were white and 68% were men.
Comorbid conditions were common, including hypertension (78% of patients), ischemic heart disease (65%), diabetes (38%), atrial fibrillation or flutter (32%), and chronic lung disease (23%). The majority of patients (61%) had not had coronary artery bypass graft surgery.
Most patients (83%) had New York Heart Association class III heart failure symptoms. A total of 67% had LBBB, and 55% had a QRS duration of 150 ms or longer.
Overall mortality was 0.8% at 30 days, 9.2% at 1 year, and 25.9% at 3 years. Overall rates of all-cause readmission were 10.2% at 30 days and 43.3% at 1 year. Rates of heart failure readmission were 2.2% at 30 days and 12.3% at 1 year.
In an unadjusted analysis of the data, rates of all adverse outcomes were significantly lower among patients who had LBBB and a QRS duration of 150 ms or more, the investigators said (JAMA 2013; 310:617-26; [doi:10.1001/jama.2013.8641]).
After the data were adjusted to account for numerous demographic and clinical factors, this difference remained robust. Patients who had LBBB and a QRS duration of 150 ms or greater had a 3-year mortality risk of 21%, compared with 27% for those with LBBB and QRS duration of 120-149 ms. Those with no LBBB and QRS duration of 150 ms or greater had an adjusted 3-year mortality risk of 31%), and patients with no LBBB and QRS durations of 120-149 ms had a 3-year risk of 32%. All differences were significant.
Patients with no LBBB and a long QRS duration, those with LBBB and a short QRS duration, and those with no LBBB and a short QRS duration consistently had higher risks of all adverse outcomes.
"Our real-world data add to the increasing body of evidence that patients with LBBB have better outcomes after CRT," Dr. Peterson and her associates said.
This study was supported by the U.S. Agency for Healthcare Research and Quality and the American College of Cardiology Foundation. Dr. Peterson reported serving as a consultant for Merck, and her associates reported numerous ties to industry sources.
In a large, real-world population of patients who underwent cardiac resynchronization therapy-defibrillator implantation, those who had left bundle-branch block and a QRS duration of 150 ms or more had the best outcomes, according to a report published online August 13 in JAMA.
Patients with left bundle-branch block (LBBB) and a long QRS duration had the lowest mortality risk and the lowest rates of all-cause, cardiovascular, and heart failure readmissions, while patients without LBBB and with a QRS duration of 120-149 ms "consistently had the greatest risks of adverse outcomes," said Dr. Pamela N. Peterson of the Denver Health Medical Center and her associates.
These findings "are particularly notable, given that both LBBB and prolonged QRS duration have been shown to be independent predictors of mortality among patients with left ventricular systolic dysfunction without CRT [cardiac resynchronization therapy]," the investigators said.
The results support the use of QRS morphology and duration to identify which patients can expect the greatest benefit from CRT-D implantation, they noted.
Even though current guidelines recommend selecting patients for CRT based primarily on their QRS morphology and duration, these recommendations are based primarily on meta-analyses and subgroup analyses of clinical trials that only considered these two factors separately. The only study to evaluate the combination of QRS morphology and duration "did not assess meaningful patient outcomes," so the utility of these recommendations in real-world practice hasn’t been clear, Dr. Peterson and her colleagues wrote.
They examined the issue using information from the National Cardiovascular Data Registry’s ICD database. They assessed outcomes in 24,169 Medicare fee-for-service patients who underwent CRT-D implantation in a 3-year period, between 2006 and 2009.
Only patients with a QRS interval of 120 ms or longer were included. The mean age of these study subjects was 75 years. Most (90%) were white and 68% were men.
Comorbid conditions were common, including hypertension (78% of patients), ischemic heart disease (65%), diabetes (38%), atrial fibrillation or flutter (32%), and chronic lung disease (23%). The majority of patients (61%) had not had coronary artery bypass graft surgery.
Most patients (83%) had New York Heart Association class III heart failure symptoms. A total of 67% had LBBB, and 55% had a QRS duration of 150 ms or longer.
Overall mortality was 0.8% at 30 days, 9.2% at 1 year, and 25.9% at 3 years. Overall rates of all-cause readmission were 10.2% at 30 days and 43.3% at 1 year. Rates of heart failure readmission were 2.2% at 30 days and 12.3% at 1 year.
In an unadjusted analysis of the data, rates of all adverse outcomes were significantly lower among patients who had LBBB and a QRS duration of 150 ms or more, the investigators said (JAMA 2013; 310:617-26; [doi:10.1001/jama.2013.8641]).
After the data were adjusted to account for numerous demographic and clinical factors, this difference remained robust. Patients who had LBBB and a QRS duration of 150 ms or greater had a 3-year mortality risk of 21%, compared with 27% for those with LBBB and QRS duration of 120-149 ms. Those with no LBBB and QRS duration of 150 ms or greater had an adjusted 3-year mortality risk of 31%), and patients with no LBBB and QRS durations of 120-149 ms had a 3-year risk of 32%. All differences were significant.
Patients with no LBBB and a long QRS duration, those with LBBB and a short QRS duration, and those with no LBBB and a short QRS duration consistently had higher risks of all adverse outcomes.
"Our real-world data add to the increasing body of evidence that patients with LBBB have better outcomes after CRT," Dr. Peterson and her associates said.
This study was supported by the U.S. Agency for Healthcare Research and Quality and the American College of Cardiology Foundation. Dr. Peterson reported serving as a consultant for Merck, and her associates reported numerous ties to industry sources.
FROM JAMA
Major finding: Patients with LBBB and a prolonged QRS interval (150 ms or more) had the lowest mortality at 1 month, 1 year, and 3 years, as well as the lowest rates of hospital readmission for all causes, cardiovascular causes, and heart failure.
Data source: A retrospective cohort study of outcomes in 24,169 Medicare fee-for-service beneficiaries in a national ICD registry who underwent CRT-D implantation in a 3-year period.
Disclosures: This study was supported by the U.S. Agency for Healthcare Research and Quality and the American College of Cardiology Foundation. Dr. Peterson reported serving as a consultant for Merck, and her associates reported numerous ties to industry sources.
Edwards to start U.S. trial of SAPIEN 3
The Food and Drug Administration has given Edwards Lifesciences the green light to start a clinical trial on the SAPIEN 3 transcatheter aortic heart valve, which is a more advanced and improved version of the currently available SAPIEN valve.
"This is very exciting news," said Dr. Augusto Pichard, director of cardiac intervention and structural heart disease at MedStar Heart Institute in Washington, D.C. "One of the major concerns we have is vascular access. The current FDA-approved [SAPIEN] valve is large and sometimes doesn’t fit, or produces serious vascular complications," Dr. Pichard said in an interview. "The new valve has much smaller delivery size, making it very likely that many more patients would benefit from the procedure."
SAPIEN 3 also has a fabric cuff to reduce paravalvular leak, which is one of the major determinants of the procedure's long-term outcomes.
Under FDA’s conditional Investigational Device Exemption (IDE), Edwards will enroll up to 500 high-risk or inoperable patients with severe symptomatic aortic stenosis. The valve can be placed through transfemoral, transapical, or transaortic approach, and patients will be followed up for 1 year.
In the United States, the SAPIEN valve has been the first and only approved transcatheter aortic valve for transcatheter aortic valve replacement (TAVR), and several clinical trials by Medtronic are underway. But the valve technology has been moving forward elsewhere.
"The U.S. has lagged behind the rest of the world in access to new TAVR technology," Dr. John Carroll, a member of the STS/ACC TVT Registry Steering Committee and director of interventional cardiology and professor of medicine at the University of Colorado Hospital, Aurora, said in an interview. "This IDE being launched potentially allows us to start the process, bringing better and safer technologies to our patients."
Although the indications for SAPIEN 3 are the same as the currently used SAPIEN valve, experts say that the smaller SAPIEN 3 would benefit more patients.
"It’s been frustrating not to be able to treat many patients," said Dr. Carroll. "In TVT registry, we’ve gathered data on the initial U.S. experiences, with over 7,000 patients, and we certainly see a need for technology improvement, both smaller deliver systems and trying to eliminate paravalvular leak."
Edwards noted that the SAPIEN 3 valve is still an investigational device and is not commercially available in any country, but results from initial trials have been promising.
Dr. Pichard and Dr. Carroll said that they have observed the SAPIEN 3 placement on several occasions – overseas or via live case transmissions. They said the procedure for SAPIEN 3 is similar to the SAPIEN valve, and it appears to be easier.
Both cardiologists have been involved in Edwards’ PARTNER trials and said that they expected the patient enrollment to be rapid. They were not sure if Edwards will include their hospitals in the trial. After 1-year follow-up of the patients, the company will give the data to the FDA for a final decision.
The IDE is one of the key hurdles to initiating a clinical trial, which can potentially lead to FDA approval and commercial release. The process, if all goes as expected, could take at least 2 years.
Dr. Carroll said that he had no disclosures other than being involved in the PARTNER trial. Dr. Pichard has received honoraria from Edwards Lifesciences as a proctor for percutaneous aortic valves.
On Twitter @NaseemSMiller
The Food and Drug Administration has given Edwards Lifesciences the green light to start a clinical trial on the SAPIEN 3 transcatheter aortic heart valve, which is a more advanced and improved version of the currently available SAPIEN valve.
"This is very exciting news," said Dr. Augusto Pichard, director of cardiac intervention and structural heart disease at MedStar Heart Institute in Washington, D.C. "One of the major concerns we have is vascular access. The current FDA-approved [SAPIEN] valve is large and sometimes doesn’t fit, or produces serious vascular complications," Dr. Pichard said in an interview. "The new valve has much smaller delivery size, making it very likely that many more patients would benefit from the procedure."
SAPIEN 3 also has a fabric cuff to reduce paravalvular leak, which is one of the major determinants of the procedure's long-term outcomes.
Under FDA’s conditional Investigational Device Exemption (IDE), Edwards will enroll up to 500 high-risk or inoperable patients with severe symptomatic aortic stenosis. The valve can be placed through transfemoral, transapical, or transaortic approach, and patients will be followed up for 1 year.
In the United States, the SAPIEN valve has been the first and only approved transcatheter aortic valve for transcatheter aortic valve replacement (TAVR), and several clinical trials by Medtronic are underway. But the valve technology has been moving forward elsewhere.
"The U.S. has lagged behind the rest of the world in access to new TAVR technology," Dr. John Carroll, a member of the STS/ACC TVT Registry Steering Committee and director of interventional cardiology and professor of medicine at the University of Colorado Hospital, Aurora, said in an interview. "This IDE being launched potentially allows us to start the process, bringing better and safer technologies to our patients."
Although the indications for SAPIEN 3 are the same as the currently used SAPIEN valve, experts say that the smaller SAPIEN 3 would benefit more patients.
"It’s been frustrating not to be able to treat many patients," said Dr. Carroll. "In TVT registry, we’ve gathered data on the initial U.S. experiences, with over 7,000 patients, and we certainly see a need for technology improvement, both smaller deliver systems and trying to eliminate paravalvular leak."
Edwards noted that the SAPIEN 3 valve is still an investigational device and is not commercially available in any country, but results from initial trials have been promising.
Dr. Pichard and Dr. Carroll said that they have observed the SAPIEN 3 placement on several occasions – overseas or via live case transmissions. They said the procedure for SAPIEN 3 is similar to the SAPIEN valve, and it appears to be easier.
Both cardiologists have been involved in Edwards’ PARTNER trials and said that they expected the patient enrollment to be rapid. They were not sure if Edwards will include their hospitals in the trial. After 1-year follow-up of the patients, the company will give the data to the FDA for a final decision.
The IDE is one of the key hurdles to initiating a clinical trial, which can potentially lead to FDA approval and commercial release. The process, if all goes as expected, could take at least 2 years.
Dr. Carroll said that he had no disclosures other than being involved in the PARTNER trial. Dr. Pichard has received honoraria from Edwards Lifesciences as a proctor for percutaneous aortic valves.
On Twitter @NaseemSMiller
The Food and Drug Administration has given Edwards Lifesciences the green light to start a clinical trial on the SAPIEN 3 transcatheter aortic heart valve, which is a more advanced and improved version of the currently available SAPIEN valve.
"This is very exciting news," said Dr. Augusto Pichard, director of cardiac intervention and structural heart disease at MedStar Heart Institute in Washington, D.C. "One of the major concerns we have is vascular access. The current FDA-approved [SAPIEN] valve is large and sometimes doesn’t fit, or produces serious vascular complications," Dr. Pichard said in an interview. "The new valve has much smaller delivery size, making it very likely that many more patients would benefit from the procedure."
SAPIEN 3 also has a fabric cuff to reduce paravalvular leak, which is one of the major determinants of the procedure's long-term outcomes.
Under FDA’s conditional Investigational Device Exemption (IDE), Edwards will enroll up to 500 high-risk or inoperable patients with severe symptomatic aortic stenosis. The valve can be placed through transfemoral, transapical, or transaortic approach, and patients will be followed up for 1 year.
In the United States, the SAPIEN valve has been the first and only approved transcatheter aortic valve for transcatheter aortic valve replacement (TAVR), and several clinical trials by Medtronic are underway. But the valve technology has been moving forward elsewhere.
"The U.S. has lagged behind the rest of the world in access to new TAVR technology," Dr. John Carroll, a member of the STS/ACC TVT Registry Steering Committee and director of interventional cardiology and professor of medicine at the University of Colorado Hospital, Aurora, said in an interview. "This IDE being launched potentially allows us to start the process, bringing better and safer technologies to our patients."
Although the indications for SAPIEN 3 are the same as the currently used SAPIEN valve, experts say that the smaller SAPIEN 3 would benefit more patients.
"It’s been frustrating not to be able to treat many patients," said Dr. Carroll. "In TVT registry, we’ve gathered data on the initial U.S. experiences, with over 7,000 patients, and we certainly see a need for technology improvement, both smaller deliver systems and trying to eliminate paravalvular leak."
Edwards noted that the SAPIEN 3 valve is still an investigational device and is not commercially available in any country, but results from initial trials have been promising.
Dr. Pichard and Dr. Carroll said that they have observed the SAPIEN 3 placement on several occasions – overseas or via live case transmissions. They said the procedure for SAPIEN 3 is similar to the SAPIEN valve, and it appears to be easier.
Both cardiologists have been involved in Edwards’ PARTNER trials and said that they expected the patient enrollment to be rapid. They were not sure if Edwards will include their hospitals in the trial. After 1-year follow-up of the patients, the company will give the data to the FDA for a final decision.
The IDE is one of the key hurdles to initiating a clinical trial, which can potentially lead to FDA approval and commercial release. The process, if all goes as expected, could take at least 2 years.
Dr. Carroll said that he had no disclosures other than being involved in the PARTNER trial. Dr. Pichard has received honoraria from Edwards Lifesciences as a proctor for percutaneous aortic valves.
On Twitter @NaseemSMiller
A mitral valve replacement that may grow with the child
NEW YORK – Physicians at Boston Children’s Hospital have replaced the mitral valves of eight infants with irreparable mitral valve disease with a valve that offers the opportunity of sequential expansion as the child grows, according to Dr. Sitaram M. Emani, who described the results at the 2013 Mitral Valve Conclave.
"The Melody valve retains its competence if you expand it before putting it in. We asked whether the valve retains the ability to maintain competence even if expansion is performed after implantation as the patient grows," said Dr. Emani, a pediatric cardiac surgeon at Boston Children’s Hospital.
According to Dr. Emani, the current options for infants with damaged mitral valves that are beyond repair are replacement with mechanical or bioprosthetic valves or the Ross mitral procedure. Perhaps the main disadvantage of these options is the lack of a prosthetic valve small enough for an infant, one that is less than 12 mm in diameter. Another problem is the possibility of stenosis developing as the child grows, since the diameters of the prosthetics are fixed. Other drawbacks are that supra-annular fixation is generally associated with poor outcomes and that annular fixation limits the ability to upsize at reoperation.
The Melody valve is an externally stented bovine jugular vein graft that was designed for transcatheter pulmonary valve replacement. In this study, the valve was inserted surgically. The valve maintains competence over a range of sizes up to 22 mm. Although this valve is not approved for use for mitral valve replacement, the hope of using such a prosthetic is that it can be enlarged in the catheterization laboratory as the child grows.
Dr. Emani conducted a retrospective study of his experience with the Melody valve for mitral valve replacement in eight infants less than 12 months of age. The median age at implantation was 6 months (range, 1-9 months). Four infants had an atrioventricular canal (AVC) defect and four had congenital mitral valve stenosis. Most of the children had two prior operations for mitral valve repair. The longest follow-up to date has been 2 years.
At a median follow-up of 8 months, regurgitation on the echocardiogram was considered to be mild or less in all patients. The median gradient was 3 mm Hg (range, 2-7 mm Hg) on the immediate postoperative echocardiogram. Three patients developed a mild paravalvular leak; one of these patients had undergone aggressive stent resection, a modification Dr. Emani does not recommend. One patient developed left ventricular outflow tract obstruction (LVOTO), which Dr. Emani attributed to the lack of distal stent fixation in this patient. Another patient with an AVC defect developed complete heart block.
One patient who died 3 days postoperatively had heterotaxy, severe mitral regurgitation, and prior ventricular failure on extracorporeal membrane oxygenation support. That patient had undergone valve implantation as a last resort.
Three patients underwent sequential expansion about 6 months after implantation. After valve expansion, the median balloon size was 12 mm, ranging from 12 to 16 mm. None of the patients developed worsening valvular function and all had relief of obstruction. Transcatheter intervention was used to correct a paravalvular leak in one patient and to treat a left ventricular outflow tract problem in another. None of the patients developed endocarditis or a strut fracture, "although I worry about strut fracture if aggressive stent resection and manipulation is performed," Dr. Emani said at the meeting, which was sponsored by the American Association for Thoracic Surgery.
Dr. Emani offered some procedural tips. First, the Melody valve must be optimized for surgical implantation in infants. The length of the valve must be reduced by trimming it to reduce the chance of LVOTO or pulmonary vein obstruction. He recommends sizing the valves by echocardiogram and fixating the distal stent to the inferior free wall of the ventricle.
Although he has used both circumferential and four-point fixation, Dr. Emani has learned all that is really needed to prevent leakage is friction of the stent against the annulus. Early on he used a pericardial cuff to anchor to the annulus, particularly in patients who had undergone failed AVC repair. He tries to preserve at least part of the anterior leaflet to facilitate suture placement and create a "stand-off" from the LVOTO.
Dr. Emani also advised limiting intraoperative dilation to no more than 1 mm greater than the measured annulus. "Try not to overdilate at implantation to avoid heart block, LVOTO, and coronary compression. The nice thing is you don’t have to decide then and there what size you want. You can go back to the cath lab and, under direct visualization with the coronary view, you can dilate it under more controlled circumstances.
"The hope is that we will be able to dilate these valves as the patients grow into adolescence. If we can dilate them up to 22 mm, hopefully we will decrease the number of repeat replacements, delay the time to reoperation, and perhaps modify our thresholds for tolerating significant disease after unsuccessful repairs," he said. The investigators continue to accumulate experience with this device and hope to design a multicenter trial evaluating its safety and efficacy for this indication.
Dr. Emani had no relevant financial disclosures.
NEW YORK – Physicians at Boston Children’s Hospital have replaced the mitral valves of eight infants with irreparable mitral valve disease with a valve that offers the opportunity of sequential expansion as the child grows, according to Dr. Sitaram M. Emani, who described the results at the 2013 Mitral Valve Conclave.
"The Melody valve retains its competence if you expand it before putting it in. We asked whether the valve retains the ability to maintain competence even if expansion is performed after implantation as the patient grows," said Dr. Emani, a pediatric cardiac surgeon at Boston Children’s Hospital.
According to Dr. Emani, the current options for infants with damaged mitral valves that are beyond repair are replacement with mechanical or bioprosthetic valves or the Ross mitral procedure. Perhaps the main disadvantage of these options is the lack of a prosthetic valve small enough for an infant, one that is less than 12 mm in diameter. Another problem is the possibility of stenosis developing as the child grows, since the diameters of the prosthetics are fixed. Other drawbacks are that supra-annular fixation is generally associated with poor outcomes and that annular fixation limits the ability to upsize at reoperation.
The Melody valve is an externally stented bovine jugular vein graft that was designed for transcatheter pulmonary valve replacement. In this study, the valve was inserted surgically. The valve maintains competence over a range of sizes up to 22 mm. Although this valve is not approved for use for mitral valve replacement, the hope of using such a prosthetic is that it can be enlarged in the catheterization laboratory as the child grows.
Dr. Emani conducted a retrospective study of his experience with the Melody valve for mitral valve replacement in eight infants less than 12 months of age. The median age at implantation was 6 months (range, 1-9 months). Four infants had an atrioventricular canal (AVC) defect and four had congenital mitral valve stenosis. Most of the children had two prior operations for mitral valve repair. The longest follow-up to date has been 2 years.
At a median follow-up of 8 months, regurgitation on the echocardiogram was considered to be mild or less in all patients. The median gradient was 3 mm Hg (range, 2-7 mm Hg) on the immediate postoperative echocardiogram. Three patients developed a mild paravalvular leak; one of these patients had undergone aggressive stent resection, a modification Dr. Emani does not recommend. One patient developed left ventricular outflow tract obstruction (LVOTO), which Dr. Emani attributed to the lack of distal stent fixation in this patient. Another patient with an AVC defect developed complete heart block.
One patient who died 3 days postoperatively had heterotaxy, severe mitral regurgitation, and prior ventricular failure on extracorporeal membrane oxygenation support. That patient had undergone valve implantation as a last resort.
Three patients underwent sequential expansion about 6 months after implantation. After valve expansion, the median balloon size was 12 mm, ranging from 12 to 16 mm. None of the patients developed worsening valvular function and all had relief of obstruction. Transcatheter intervention was used to correct a paravalvular leak in one patient and to treat a left ventricular outflow tract problem in another. None of the patients developed endocarditis or a strut fracture, "although I worry about strut fracture if aggressive stent resection and manipulation is performed," Dr. Emani said at the meeting, which was sponsored by the American Association for Thoracic Surgery.
Dr. Emani offered some procedural tips. First, the Melody valve must be optimized for surgical implantation in infants. The length of the valve must be reduced by trimming it to reduce the chance of LVOTO or pulmonary vein obstruction. He recommends sizing the valves by echocardiogram and fixating the distal stent to the inferior free wall of the ventricle.
Although he has used both circumferential and four-point fixation, Dr. Emani has learned all that is really needed to prevent leakage is friction of the stent against the annulus. Early on he used a pericardial cuff to anchor to the annulus, particularly in patients who had undergone failed AVC repair. He tries to preserve at least part of the anterior leaflet to facilitate suture placement and create a "stand-off" from the LVOTO.
Dr. Emani also advised limiting intraoperative dilation to no more than 1 mm greater than the measured annulus. "Try not to overdilate at implantation to avoid heart block, LVOTO, and coronary compression. The nice thing is you don’t have to decide then and there what size you want. You can go back to the cath lab and, under direct visualization with the coronary view, you can dilate it under more controlled circumstances.
"The hope is that we will be able to dilate these valves as the patients grow into adolescence. If we can dilate them up to 22 mm, hopefully we will decrease the number of repeat replacements, delay the time to reoperation, and perhaps modify our thresholds for tolerating significant disease after unsuccessful repairs," he said. The investigators continue to accumulate experience with this device and hope to design a multicenter trial evaluating its safety and efficacy for this indication.
Dr. Emani had no relevant financial disclosures.
NEW YORK – Physicians at Boston Children’s Hospital have replaced the mitral valves of eight infants with irreparable mitral valve disease with a valve that offers the opportunity of sequential expansion as the child grows, according to Dr. Sitaram M. Emani, who described the results at the 2013 Mitral Valve Conclave.
"The Melody valve retains its competence if you expand it before putting it in. We asked whether the valve retains the ability to maintain competence even if expansion is performed after implantation as the patient grows," said Dr. Emani, a pediatric cardiac surgeon at Boston Children’s Hospital.
According to Dr. Emani, the current options for infants with damaged mitral valves that are beyond repair are replacement with mechanical or bioprosthetic valves or the Ross mitral procedure. Perhaps the main disadvantage of these options is the lack of a prosthetic valve small enough for an infant, one that is less than 12 mm in diameter. Another problem is the possibility of stenosis developing as the child grows, since the diameters of the prosthetics are fixed. Other drawbacks are that supra-annular fixation is generally associated with poor outcomes and that annular fixation limits the ability to upsize at reoperation.
The Melody valve is an externally stented bovine jugular vein graft that was designed for transcatheter pulmonary valve replacement. In this study, the valve was inserted surgically. The valve maintains competence over a range of sizes up to 22 mm. Although this valve is not approved for use for mitral valve replacement, the hope of using such a prosthetic is that it can be enlarged in the catheterization laboratory as the child grows.
Dr. Emani conducted a retrospective study of his experience with the Melody valve for mitral valve replacement in eight infants less than 12 months of age. The median age at implantation was 6 months (range, 1-9 months). Four infants had an atrioventricular canal (AVC) defect and four had congenital mitral valve stenosis. Most of the children had two prior operations for mitral valve repair. The longest follow-up to date has been 2 years.
At a median follow-up of 8 months, regurgitation on the echocardiogram was considered to be mild or less in all patients. The median gradient was 3 mm Hg (range, 2-7 mm Hg) on the immediate postoperative echocardiogram. Three patients developed a mild paravalvular leak; one of these patients had undergone aggressive stent resection, a modification Dr. Emani does not recommend. One patient developed left ventricular outflow tract obstruction (LVOTO), which Dr. Emani attributed to the lack of distal stent fixation in this patient. Another patient with an AVC defect developed complete heart block.
One patient who died 3 days postoperatively had heterotaxy, severe mitral regurgitation, and prior ventricular failure on extracorporeal membrane oxygenation support. That patient had undergone valve implantation as a last resort.
Three patients underwent sequential expansion about 6 months after implantation. After valve expansion, the median balloon size was 12 mm, ranging from 12 to 16 mm. None of the patients developed worsening valvular function and all had relief of obstruction. Transcatheter intervention was used to correct a paravalvular leak in one patient and to treat a left ventricular outflow tract problem in another. None of the patients developed endocarditis or a strut fracture, "although I worry about strut fracture if aggressive stent resection and manipulation is performed," Dr. Emani said at the meeting, which was sponsored by the American Association for Thoracic Surgery.
Dr. Emani offered some procedural tips. First, the Melody valve must be optimized for surgical implantation in infants. The length of the valve must be reduced by trimming it to reduce the chance of LVOTO or pulmonary vein obstruction. He recommends sizing the valves by echocardiogram and fixating the distal stent to the inferior free wall of the ventricle.
Although he has used both circumferential and four-point fixation, Dr. Emani has learned all that is really needed to prevent leakage is friction of the stent against the annulus. Early on he used a pericardial cuff to anchor to the annulus, particularly in patients who had undergone failed AVC repair. He tries to preserve at least part of the anterior leaflet to facilitate suture placement and create a "stand-off" from the LVOTO.
Dr. Emani also advised limiting intraoperative dilation to no more than 1 mm greater than the measured annulus. "Try not to overdilate at implantation to avoid heart block, LVOTO, and coronary compression. The nice thing is you don’t have to decide then and there what size you want. You can go back to the cath lab and, under direct visualization with the coronary view, you can dilate it under more controlled circumstances.
"The hope is that we will be able to dilate these valves as the patients grow into adolescence. If we can dilate them up to 22 mm, hopefully we will decrease the number of repeat replacements, delay the time to reoperation, and perhaps modify our thresholds for tolerating significant disease after unsuccessful repairs," he said. The investigators continue to accumulate experience with this device and hope to design a multicenter trial evaluating its safety and efficacy for this indication.
Dr. Emani had no relevant financial disclosures.
AT THE 2013 MITRAL VALVE CONCLAVE
Major finding: Eight infants with irreparable mitral valve disease underwent mitral valve replacement with the Melody valve, which offers the opportunity of sequential expansion as the child grows.
Data source: Small case series.
Disclosures: Dr. Emani had no relevant financial disclosures.
Enteral nutrition linked to in-hospital mortality
CHICAGO – Hyperglycemia is common in patients receiving enteral nutrition and is a significant risk factor for hospital mortality, according to a retrospective study involving 157 patients.
Nearly 60% of patients had capillary blood glucose (CBG) values that exceeded 140 mg/dL, and nearly one-third exceeded 180 mg/dL.
More important, almost 40% of patients had a mean CBG of more than 180 mg/dL for the entire duration of their tube feedings.
At the threshold glucose of 180 mg/dL or more, the odds of dying in the hospital during tube feeding are approximately three times higher than for patients who had better glycemic control, Dr. Michael Jakoby said at the annual scientific sessions of the American Diabetes Association.
"We look at this body of work as just a stepping point to the next logical extension, which is to investigate ways that we can improve glycemic control for patients receiving any artificial nutrition, whether it’s parenteral or enteral," said Dr. Jakoby, chief of endocrinology at St. John’s Hospital in Springfield, Ill.
He previously reported that using an insulin protocol that determined insulin doses based on carbohydrate delivery and CBG was superior to ad hoc insulin dosing in the management of parenteral nutrition–induced hyperglycemia (JPEN J. Parenter. Enteral. Nutr. 2012;36:183-8).
Malnutrition among nonsurgical patients is high, and more than 40% of general surgery service patients have been reported to be malnourished (Am. J. Clin. Nutr. 1997;66:1232-9). Five studies since 2005 have established a link between parenteral nutrition and increased morbidity and in-hospital mortality, but little is available on outcomes with enteral nutrition, Dr. Jakoby explained.
The current analysis involved 157 patients receiving enteral nutrition in 2011 at St. John’s, a 350-bed hospital where each year an estimated 11,000 patients receive artificial nutrition for 2 weeks.
Of those patients, 58 were diagnosed with hyperglycemia (37%), defined as a mean CBG of 180 mg/dL or more for the duration of tube feedings.
Hyperglycemic patients were significantly older than controls (75 vs. 67), twice as likely to have a preexisting diagnosis of diabetes (81% vs. 41%), had 2 fewer days of enteral nutrition (7 vs. 9), and were less likely to be in the ICU during their tube feedings (62% vs. 83%), he said.
The two groups were well matched with regard to the amount of protein, fat, and total energy they received; however, hyperglycemic patients received significantly fewer carbohydrates than did controls (2.2 g/kg per day vs. 2.7 g/kg per day).
"With the documentation available to us, it seemed that this was an adaptive response to help combat the hyperglycemia that was happening during pure enteral nutrition," Dr. Jakoby said.
Cardiac complications – a composite of myocardial infarction, arrhythmia, and cardiac arrest – were increased in hyperglycemic patients (34% vs. 22%), but this difference was not significant in univariate analysis (odds ratio, 1.84; P = .13).
The risk of hospital mortality, however, was significantly higher in hyperglycemic patients in univariate analysis (36% vs. 16%; OR, 2.94), and remained significant in multivariate analysis after adjustment for age, sex, and preexisting diagnosis of diabetes (OR, 3.28), he noted.
During a discussion of the results, Dr. Jakoby said analyses are forthcoming looking at the impact of glycemic variability during hospitalization, observing that in a prior study he conducted, glycemic variability in the noncritical care setting strongly correlated with hospital stay and a greater likelihood of going from hospital to nursing home or the morgue.
Dr. Jakoby reported serving as a speaker for Sanofi-Aventis, Merck, and Novo Nordisk.
CHICAGO – Hyperglycemia is common in patients receiving enteral nutrition and is a significant risk factor for hospital mortality, according to a retrospective study involving 157 patients.
Nearly 60% of patients had capillary blood glucose (CBG) values that exceeded 140 mg/dL, and nearly one-third exceeded 180 mg/dL.
More important, almost 40% of patients had a mean CBG of more than 180 mg/dL for the entire duration of their tube feedings.
At the threshold glucose of 180 mg/dL or more, the odds of dying in the hospital during tube feeding are approximately three times higher than for patients who had better glycemic control, Dr. Michael Jakoby said at the annual scientific sessions of the American Diabetes Association.
"We look at this body of work as just a stepping point to the next logical extension, which is to investigate ways that we can improve glycemic control for patients receiving any artificial nutrition, whether it’s parenteral or enteral," said Dr. Jakoby, chief of endocrinology at St. John’s Hospital in Springfield, Ill.
He previously reported that using an insulin protocol that determined insulin doses based on carbohydrate delivery and CBG was superior to ad hoc insulin dosing in the management of parenteral nutrition–induced hyperglycemia (JPEN J. Parenter. Enteral. Nutr. 2012;36:183-8).
Malnutrition among nonsurgical patients is high, and more than 40% of general surgery service patients have been reported to be malnourished (Am. J. Clin. Nutr. 1997;66:1232-9). Five studies since 2005 have established a link between parenteral nutrition and increased morbidity and in-hospital mortality, but little is available on outcomes with enteral nutrition, Dr. Jakoby explained.
The current analysis involved 157 patients receiving enteral nutrition in 2011 at St. John’s, a 350-bed hospital where each year an estimated 11,000 patients receive artificial nutrition for 2 weeks.
Of those patients, 58 were diagnosed with hyperglycemia (37%), defined as a mean CBG of 180 mg/dL or more for the duration of tube feedings.
Hyperglycemic patients were significantly older than controls (75 vs. 67), twice as likely to have a preexisting diagnosis of diabetes (81% vs. 41%), had 2 fewer days of enteral nutrition (7 vs. 9), and were less likely to be in the ICU during their tube feedings (62% vs. 83%), he said.
The two groups were well matched with regard to the amount of protein, fat, and total energy they received; however, hyperglycemic patients received significantly fewer carbohydrates than did controls (2.2 g/kg per day vs. 2.7 g/kg per day).
"With the documentation available to us, it seemed that this was an adaptive response to help combat the hyperglycemia that was happening during pure enteral nutrition," Dr. Jakoby said.
Cardiac complications – a composite of myocardial infarction, arrhythmia, and cardiac arrest – were increased in hyperglycemic patients (34% vs. 22%), but this difference was not significant in univariate analysis (odds ratio, 1.84; P = .13).
The risk of hospital mortality, however, was significantly higher in hyperglycemic patients in univariate analysis (36% vs. 16%; OR, 2.94), and remained significant in multivariate analysis after adjustment for age, sex, and preexisting diagnosis of diabetes (OR, 3.28), he noted.
During a discussion of the results, Dr. Jakoby said analyses are forthcoming looking at the impact of glycemic variability during hospitalization, observing that in a prior study he conducted, glycemic variability in the noncritical care setting strongly correlated with hospital stay and a greater likelihood of going from hospital to nursing home or the morgue.
Dr. Jakoby reported serving as a speaker for Sanofi-Aventis, Merck, and Novo Nordisk.
CHICAGO – Hyperglycemia is common in patients receiving enteral nutrition and is a significant risk factor for hospital mortality, according to a retrospective study involving 157 patients.
Nearly 60% of patients had capillary blood glucose (CBG) values that exceeded 140 mg/dL, and nearly one-third exceeded 180 mg/dL.
More important, almost 40% of patients had a mean CBG of more than 180 mg/dL for the entire duration of their tube feedings.
At the threshold glucose of 180 mg/dL or more, the odds of dying in the hospital during tube feeding are approximately three times higher than for patients who had better glycemic control, Dr. Michael Jakoby said at the annual scientific sessions of the American Diabetes Association.
"We look at this body of work as just a stepping point to the next logical extension, which is to investigate ways that we can improve glycemic control for patients receiving any artificial nutrition, whether it’s parenteral or enteral," said Dr. Jakoby, chief of endocrinology at St. John’s Hospital in Springfield, Ill.
He previously reported that using an insulin protocol that determined insulin doses based on carbohydrate delivery and CBG was superior to ad hoc insulin dosing in the management of parenteral nutrition–induced hyperglycemia (JPEN J. Parenter. Enteral. Nutr. 2012;36:183-8).
Malnutrition among nonsurgical patients is high, and more than 40% of general surgery service patients have been reported to be malnourished (Am. J. Clin. Nutr. 1997;66:1232-9). Five studies since 2005 have established a link between parenteral nutrition and increased morbidity and in-hospital mortality, but little is available on outcomes with enteral nutrition, Dr. Jakoby explained.
The current analysis involved 157 patients receiving enteral nutrition in 2011 at St. John’s, a 350-bed hospital where each year an estimated 11,000 patients receive artificial nutrition for 2 weeks.
Of those patients, 58 were diagnosed with hyperglycemia (37%), defined as a mean CBG of 180 mg/dL or more for the duration of tube feedings.
Hyperglycemic patients were significantly older than controls (75 vs. 67), twice as likely to have a preexisting diagnosis of diabetes (81% vs. 41%), had 2 fewer days of enteral nutrition (7 vs. 9), and were less likely to be in the ICU during their tube feedings (62% vs. 83%), he said.
The two groups were well matched with regard to the amount of protein, fat, and total energy they received; however, hyperglycemic patients received significantly fewer carbohydrates than did controls (2.2 g/kg per day vs. 2.7 g/kg per day).
"With the documentation available to us, it seemed that this was an adaptive response to help combat the hyperglycemia that was happening during pure enteral nutrition," Dr. Jakoby said.
Cardiac complications – a composite of myocardial infarction, arrhythmia, and cardiac arrest – were increased in hyperglycemic patients (34% vs. 22%), but this difference was not significant in univariate analysis (odds ratio, 1.84; P = .13).
The risk of hospital mortality, however, was significantly higher in hyperglycemic patients in univariate analysis (36% vs. 16%; OR, 2.94), and remained significant in multivariate analysis after adjustment for age, sex, and preexisting diagnosis of diabetes (OR, 3.28), he noted.
During a discussion of the results, Dr. Jakoby said analyses are forthcoming looking at the impact of glycemic variability during hospitalization, observing that in a prior study he conducted, glycemic variability in the noncritical care setting strongly correlated with hospital stay and a greater likelihood of going from hospital to nursing home or the morgue.
Dr. Jakoby reported serving as a speaker for Sanofi-Aventis, Merck, and Novo Nordisk.
AT THE ANNUAL ADA SCIENTIFIC SESSIONS
Major finding: The mortality rate was 36% in hyperglycemic patients vs. 16% in controls (unadjusted OR 2.94; adjusted 3.28), a significant difference.
Data source: Retrospective analysis of 157 hospitalized patients receiving enteral nutrition.
Disclosures: Dr. Jakoby reported serving as a speaker for Sanofi-Aventis, Merck, and Novo Nordisk.
Aortic valve-sparing root surgery durable at 1 year
MINNEAPOLIS – Despite the complexity of aortic valve–sparing techniques, early outcomes and 1-year survival were similar to those achieved with valve replacing during root replacement surgery in patients with Marfan syndrome in a prospective registry study.
Major adverse valve-related events (MAVREs) at 1 year were also comparable, although there was an increase, of course, in aortic valve regurgitation with valve sparing (7% vs. 0%), Dr. Joseph Coselli said at the annual meeting of the American Association for Thoracic Surgery.
"Follow-up is needed for this particular incident because we don’t know exactly what’s going to happen to these 2-plus aortic regurgitations," he said. "It’s quite possible they may remain stable over a long period of time and don’t represent a failure of the concept."
In an initial report from the international registry, valve-sparing techniques were the most common, and provided comparable 30-day outcomes in 151 patients (J. Thorac. Cardiovasc. Surg. 2009;137:1124-32).
The current analysis involved 316 patients, aged 4-70 years, who underwent aortic valve–sparing (AVS) (n = 239) or aortic valve–replacing (AVR) (n = 63 mechanical and 14 tissue) root replacement surgery at 19 centers between March 2005 and November 2010. The type of operation was determined by clinical factors, and by surgeon and patient preference. AVR surgery was considered the only option in 17% of patients. At 1 year, clinical follow-up was complete in 98% and imaging follow-up in 93%.
"Interestingly, when we started out this particular collection of patients, over 30% were receiving aortic valve replacement, but toward the end of this observational study, late 2010, virtually almost all patients were receiving aortic valve sparing," said Dr. Coselli, chief of adult cardiac surgery at Baylor College of Medicine, Texas Heart Institute, Houston.
AVS patients were younger (33 vs. 39 years); had smaller sinuses of Valsalva (49 vs. 53 mm); and had less acute dissection (3% vs. 9%), chronic dissection (3% vs. 12%), and previous cardiovascular surgery (5% vs. 14%).
Aortic-sparing techniques required significantly longer cardiopulmonary bypass time (195 vs. 152 minutes) and aortic clamp time (156 vs. 115 minutes), but cut ICU time from 46 hours with AVR surgery to 26 hours, ventilator support time from 12 to 8 hours, and hospital length of stay from 7 to 6 days, Dr. Coselli reported.
At 30 days, MAVREs were reported in 6 patients in the AVR group and 15 in the AVS group (P = .4), with no differences in nonstructural dysfunction (1 vs. 7), embolism (1 vs. 3), or bleeding (2 vs. 3 events).
Two AVS patients required early reoperation: One required same-day reintervention because of coronary artery kinking after a Florida sleeve procedure, and the second needed reintervention because of a coronary pseudoaneurysm 6 days after a David-V procedure, he said. Two early deaths occurred, one in each group, but neither was valve related.
One-year survival rates were 98% after AVS surgery and 97% after AVR surgery (4 vs. 2 deaths; P = .06).
At 1 year, MAVREs occurred in 8 AVR and 35 AVS patients (P = .5). There was no significant difference between AVR and AVS in freedom from valve-related death (1 vs. 2), embolism (2 vs. 4 events), reintervention (0 vs. 1), endocarditis (1 vs. 0), valve thrombosis (0 both), or valve-related morbidity (7 vs. 28), Dr. Coselli said.
The AVS group had significantly more nonstructural dysfunction/structural valve deterioration (23 events vs. 1 event; P = .04), but significantly less bleeding (3 vs. 5 events; P = .01).
In a Cox regression analysis at 1 year, the type of surgery was not associated with overall survival, MAVREs, or any other valve-related outcome, he said.
Dr. Coselli reported research support from St. Jude Medical; an educational grant, consultancy, and royalties from Vascutek Terumo; and research support, speaking for, and steering committee membership with Medtronic. Two coauthors reported consultant/advisory board participation with Medtronic or Edwards Lifesciences.
MINNEAPOLIS – Despite the complexity of aortic valve–sparing techniques, early outcomes and 1-year survival were similar to those achieved with valve replacing during root replacement surgery in patients with Marfan syndrome in a prospective registry study.
Major adverse valve-related events (MAVREs) at 1 year were also comparable, although there was an increase, of course, in aortic valve regurgitation with valve sparing (7% vs. 0%), Dr. Joseph Coselli said at the annual meeting of the American Association for Thoracic Surgery.
"Follow-up is needed for this particular incident because we don’t know exactly what’s going to happen to these 2-plus aortic regurgitations," he said. "It’s quite possible they may remain stable over a long period of time and don’t represent a failure of the concept."
In an initial report from the international registry, valve-sparing techniques were the most common, and provided comparable 30-day outcomes in 151 patients (J. Thorac. Cardiovasc. Surg. 2009;137:1124-32).
The current analysis involved 316 patients, aged 4-70 years, who underwent aortic valve–sparing (AVS) (n = 239) or aortic valve–replacing (AVR) (n = 63 mechanical and 14 tissue) root replacement surgery at 19 centers between March 2005 and November 2010. The type of operation was determined by clinical factors, and by surgeon and patient preference. AVR surgery was considered the only option in 17% of patients. At 1 year, clinical follow-up was complete in 98% and imaging follow-up in 93%.
"Interestingly, when we started out this particular collection of patients, over 30% were receiving aortic valve replacement, but toward the end of this observational study, late 2010, virtually almost all patients were receiving aortic valve sparing," said Dr. Coselli, chief of adult cardiac surgery at Baylor College of Medicine, Texas Heart Institute, Houston.
AVS patients were younger (33 vs. 39 years); had smaller sinuses of Valsalva (49 vs. 53 mm); and had less acute dissection (3% vs. 9%), chronic dissection (3% vs. 12%), and previous cardiovascular surgery (5% vs. 14%).
Aortic-sparing techniques required significantly longer cardiopulmonary bypass time (195 vs. 152 minutes) and aortic clamp time (156 vs. 115 minutes), but cut ICU time from 46 hours with AVR surgery to 26 hours, ventilator support time from 12 to 8 hours, and hospital length of stay from 7 to 6 days, Dr. Coselli reported.
At 30 days, MAVREs were reported in 6 patients in the AVR group and 15 in the AVS group (P = .4), with no differences in nonstructural dysfunction (1 vs. 7), embolism (1 vs. 3), or bleeding (2 vs. 3 events).
Two AVS patients required early reoperation: One required same-day reintervention because of coronary artery kinking after a Florida sleeve procedure, and the second needed reintervention because of a coronary pseudoaneurysm 6 days after a David-V procedure, he said. Two early deaths occurred, one in each group, but neither was valve related.
One-year survival rates were 98% after AVS surgery and 97% after AVR surgery (4 vs. 2 deaths; P = .06).
At 1 year, MAVREs occurred in 8 AVR and 35 AVS patients (P = .5). There was no significant difference between AVR and AVS in freedom from valve-related death (1 vs. 2), embolism (2 vs. 4 events), reintervention (0 vs. 1), endocarditis (1 vs. 0), valve thrombosis (0 both), or valve-related morbidity (7 vs. 28), Dr. Coselli said.
The AVS group had significantly more nonstructural dysfunction/structural valve deterioration (23 events vs. 1 event; P = .04), but significantly less bleeding (3 vs. 5 events; P = .01).
In a Cox regression analysis at 1 year, the type of surgery was not associated with overall survival, MAVREs, or any other valve-related outcome, he said.
Dr. Coselli reported research support from St. Jude Medical; an educational grant, consultancy, and royalties from Vascutek Terumo; and research support, speaking for, and steering committee membership with Medtronic. Two coauthors reported consultant/advisory board participation with Medtronic or Edwards Lifesciences.
MINNEAPOLIS – Despite the complexity of aortic valve–sparing techniques, early outcomes and 1-year survival were similar to those achieved with valve replacing during root replacement surgery in patients with Marfan syndrome in a prospective registry study.
Major adverse valve-related events (MAVREs) at 1 year were also comparable, although there was an increase, of course, in aortic valve regurgitation with valve sparing (7% vs. 0%), Dr. Joseph Coselli said at the annual meeting of the American Association for Thoracic Surgery.
"Follow-up is needed for this particular incident because we don’t know exactly what’s going to happen to these 2-plus aortic regurgitations," he said. "It’s quite possible they may remain stable over a long period of time and don’t represent a failure of the concept."
In an initial report from the international registry, valve-sparing techniques were the most common, and provided comparable 30-day outcomes in 151 patients (J. Thorac. Cardiovasc. Surg. 2009;137:1124-32).
The current analysis involved 316 patients, aged 4-70 years, who underwent aortic valve–sparing (AVS) (n = 239) or aortic valve–replacing (AVR) (n = 63 mechanical and 14 tissue) root replacement surgery at 19 centers between March 2005 and November 2010. The type of operation was determined by clinical factors, and by surgeon and patient preference. AVR surgery was considered the only option in 17% of patients. At 1 year, clinical follow-up was complete in 98% and imaging follow-up in 93%.
"Interestingly, when we started out this particular collection of patients, over 30% were receiving aortic valve replacement, but toward the end of this observational study, late 2010, virtually almost all patients were receiving aortic valve sparing," said Dr. Coselli, chief of adult cardiac surgery at Baylor College of Medicine, Texas Heart Institute, Houston.
AVS patients were younger (33 vs. 39 years); had smaller sinuses of Valsalva (49 vs. 53 mm); and had less acute dissection (3% vs. 9%), chronic dissection (3% vs. 12%), and previous cardiovascular surgery (5% vs. 14%).
Aortic-sparing techniques required significantly longer cardiopulmonary bypass time (195 vs. 152 minutes) and aortic clamp time (156 vs. 115 minutes), but cut ICU time from 46 hours with AVR surgery to 26 hours, ventilator support time from 12 to 8 hours, and hospital length of stay from 7 to 6 days, Dr. Coselli reported.
At 30 days, MAVREs were reported in 6 patients in the AVR group and 15 in the AVS group (P = .4), with no differences in nonstructural dysfunction (1 vs. 7), embolism (1 vs. 3), or bleeding (2 vs. 3 events).
Two AVS patients required early reoperation: One required same-day reintervention because of coronary artery kinking after a Florida sleeve procedure, and the second needed reintervention because of a coronary pseudoaneurysm 6 days after a David-V procedure, he said. Two early deaths occurred, one in each group, but neither was valve related.
One-year survival rates were 98% after AVS surgery and 97% after AVR surgery (4 vs. 2 deaths; P = .06).
At 1 year, MAVREs occurred in 8 AVR and 35 AVS patients (P = .5). There was no significant difference between AVR and AVS in freedom from valve-related death (1 vs. 2), embolism (2 vs. 4 events), reintervention (0 vs. 1), endocarditis (1 vs. 0), valve thrombosis (0 both), or valve-related morbidity (7 vs. 28), Dr. Coselli said.
The AVS group had significantly more nonstructural dysfunction/structural valve deterioration (23 events vs. 1 event; P = .04), but significantly less bleeding (3 vs. 5 events; P = .01).
In a Cox regression analysis at 1 year, the type of surgery was not associated with overall survival, MAVREs, or any other valve-related outcome, he said.
Dr. Coselli reported research support from St. Jude Medical; an educational grant, consultancy, and royalties from Vascutek Terumo; and research support, speaking for, and steering committee membership with Medtronic. Two coauthors reported consultant/advisory board participation with Medtronic or Edwards Lifesciences.
AT THE AATS ANNUAL MEETING
Major finding: One-year survival rates were 98% after AVS surgery and 97% after AVR surgery (4 vs. 2 deaths; P = .06).
Data source: Prospective, international, observational registry study in 316 Marfan syndrome patients undergoing aortic root surgery.
Disclosures: Dr. Coselli reported research support from St. Jude Medical; an educational grant, consultancy, and royalties from Vascutek Terumo; and research support, speaking for, and steering committee membership with Medtronic. Two coauthors reported consultant/advisory board participation with Medtronic or Edwards Lifesciences.
Study IDs predictors of unplanned hospital readmission after CEA
SAN FRANCISCO – The 30-day unplanned readmission rate following carotid endarterectomy was 6.5% in a single-center study.
In addition, four variables were significantly associated with unplanned readmission: in-hospital postoperative congestive heart failure (CHF) exacerbation; in-hospital postoperative stroke; in-hospital postoperative hematoma; and prior coronary artery bypass graft (CABG).
"Whether these complications are completely avoidable is unknown, but we do identify a group of patients who would probably benefit from more comprehensive discharge planning and careful postdischarge care," Dr. Karen J. Ho said at the Society for Vascular Surgery annual meeting.
According to a study of Medicare claims data from 2003 to 2004, 20% of Medicare beneficiaries discharged from a hospital were rehospitalized within 30 days (N. Eng. J. Med. 2009;360:1418-28). The 30-day rehospitalization rate after vascular surgery was 24%, "the highest of all surgical specialties examined in the study," said Dr. Ho of the surgery department at Brigham and Women’s Hospital, Boston, who was not involved with the published study. "Medicare has started to decrease reimbursements for hospitals with excess readmissions after acute MI, heart failure, and pneumonia. Hip and knee replacements and chronic obstructive pulmonary disease will be added in 2014, and we anticipate that additional surgical procedures will be added thereafter," she said.
In an effort to determine the rate of 30-day unplanned readmission after carotid endarterectomy (CEA), Dr. Ho and her associates conducted a retrospective study of a prospectively collected vascular surgery database at Brigham and Women’s Hospital. The cohort included 896 consecutive CEAs performed between 2002 and 2011. Combined CABG/CEA procedures were excluded.
The primary endpoint was unplanned readmission within 30 days, defined as "any unanticipated, nonelective hospital readmission," she said. The secondary endpoint was 1-year survival.
The mean age of the patients was 70 years, 60% were male, and 95% were white. More than half (65%) had asymptomatic evidence of carotid artery disease.
Dr. Ho reported that the median postoperative length of stay was 1 day and that 9.9% of patients had at least one in-hospital complication. The most frequent in-hospital complication was bleeding/hematoma (4.1%), followed by arrhythmia (2.1%), dysphagia (1.7%), stroke (1.3%), and myocardial infarction (1.2%). Only 3% of patients required a reoperation, while most (94%) were discharged to home. The 30-day stroke rate was 1.7%, while the 30-day death rate was 0.6%.
The overall 30-day readmission rate was 8.6%, while the unplanned 30-day readmission rate was 6.5%. "Most of the overall readmissions (80%) occurred in the first 10 days, and the median time to unplanned readmission was 4 days," Dr. Ho said.
The most common reason for an unplanned readmission was a cardiac complication, followed by headache, bleeding/hematoma, stroke/transient ischemic attack/intracerebral hemorrhage, or other medical emergency. More than one-quarter of patients (27.5%) had more than one reason for an unplanned readmission, while 87.9% of patients had a CEA-related unplanned readmission.
When the researchers performed a univariate analysis followed by analysis with a multivariable Cox model for unplanned readmission, four variables were independently associated with unplanned readmission: in-hospital postoperative CHF exacerbation (hazard ratio, 15.1), in-hospital postoperative stroke (HR, 5.0), in-hospital postoperative hematoma (HR, 3.1), and prior CABG (HR, 2.0).
They also observed a significant difference in survival at 1 year between patients who had an unplanned readmission and those who did not (91% vs. 96%, respectively; P less than .01.) "It’s unclear whether these deaths in the unplanned readmission group were preventable or if they were related to carotid disease or to a procedure-related complication," Dr. Ho said. "Our guess is that the increased overall burden of comorbid disease in these patients, rather than the readmission itself, predicted decreased survival."
Limitations of the study included its retrospective design and the fact that it was conducted at a single center, she said, "but we do know that our unplanned readmission rate is comparable to estimates from recent Medicare data."
Dr. Ho said she had no relevant financial disclosures.
SAN FRANCISCO – The 30-day unplanned readmission rate following carotid endarterectomy was 6.5% in a single-center study.
In addition, four variables were significantly associated with unplanned readmission: in-hospital postoperative congestive heart failure (CHF) exacerbation; in-hospital postoperative stroke; in-hospital postoperative hematoma; and prior coronary artery bypass graft (CABG).
"Whether these complications are completely avoidable is unknown, but we do identify a group of patients who would probably benefit from more comprehensive discharge planning and careful postdischarge care," Dr. Karen J. Ho said at the Society for Vascular Surgery annual meeting.
According to a study of Medicare claims data from 2003 to 2004, 20% of Medicare beneficiaries discharged from a hospital were rehospitalized within 30 days (N. Eng. J. Med. 2009;360:1418-28). The 30-day rehospitalization rate after vascular surgery was 24%, "the highest of all surgical specialties examined in the study," said Dr. Ho of the surgery department at Brigham and Women’s Hospital, Boston, who was not involved with the published study. "Medicare has started to decrease reimbursements for hospitals with excess readmissions after acute MI, heart failure, and pneumonia. Hip and knee replacements and chronic obstructive pulmonary disease will be added in 2014, and we anticipate that additional surgical procedures will be added thereafter," she said.
In an effort to determine the rate of 30-day unplanned readmission after carotid endarterectomy (CEA), Dr. Ho and her associates conducted a retrospective study of a prospectively collected vascular surgery database at Brigham and Women’s Hospital. The cohort included 896 consecutive CEAs performed between 2002 and 2011. Combined CABG/CEA procedures were excluded.
The primary endpoint was unplanned readmission within 30 days, defined as "any unanticipated, nonelective hospital readmission," she said. The secondary endpoint was 1-year survival.
The mean age of the patients was 70 years, 60% were male, and 95% were white. More than half (65%) had asymptomatic evidence of carotid artery disease.
Dr. Ho reported that the median postoperative length of stay was 1 day and that 9.9% of patients had at least one in-hospital complication. The most frequent in-hospital complication was bleeding/hematoma (4.1%), followed by arrhythmia (2.1%), dysphagia (1.7%), stroke (1.3%), and myocardial infarction (1.2%). Only 3% of patients required a reoperation, while most (94%) were discharged to home. The 30-day stroke rate was 1.7%, while the 30-day death rate was 0.6%.
The overall 30-day readmission rate was 8.6%, while the unplanned 30-day readmission rate was 6.5%. "Most of the overall readmissions (80%) occurred in the first 10 days, and the median time to unplanned readmission was 4 days," Dr. Ho said.
The most common reason for an unplanned readmission was a cardiac complication, followed by headache, bleeding/hematoma, stroke/transient ischemic attack/intracerebral hemorrhage, or other medical emergency. More than one-quarter of patients (27.5%) had more than one reason for an unplanned readmission, while 87.9% of patients had a CEA-related unplanned readmission.
When the researchers performed a univariate analysis followed by analysis with a multivariable Cox model for unplanned readmission, four variables were independently associated with unplanned readmission: in-hospital postoperative CHF exacerbation (hazard ratio, 15.1), in-hospital postoperative stroke (HR, 5.0), in-hospital postoperative hematoma (HR, 3.1), and prior CABG (HR, 2.0).
They also observed a significant difference in survival at 1 year between patients who had an unplanned readmission and those who did not (91% vs. 96%, respectively; P less than .01.) "It’s unclear whether these deaths in the unplanned readmission group were preventable or if they were related to carotid disease or to a procedure-related complication," Dr. Ho said. "Our guess is that the increased overall burden of comorbid disease in these patients, rather than the readmission itself, predicted decreased survival."
Limitations of the study included its retrospective design and the fact that it was conducted at a single center, she said, "but we do know that our unplanned readmission rate is comparable to estimates from recent Medicare data."
Dr. Ho said she had no relevant financial disclosures.
SAN FRANCISCO – The 30-day unplanned readmission rate following carotid endarterectomy was 6.5% in a single-center study.
In addition, four variables were significantly associated with unplanned readmission: in-hospital postoperative congestive heart failure (CHF) exacerbation; in-hospital postoperative stroke; in-hospital postoperative hematoma; and prior coronary artery bypass graft (CABG).
"Whether these complications are completely avoidable is unknown, but we do identify a group of patients who would probably benefit from more comprehensive discharge planning and careful postdischarge care," Dr. Karen J. Ho said at the Society for Vascular Surgery annual meeting.
According to a study of Medicare claims data from 2003 to 2004, 20% of Medicare beneficiaries discharged from a hospital were rehospitalized within 30 days (N. Eng. J. Med. 2009;360:1418-28). The 30-day rehospitalization rate after vascular surgery was 24%, "the highest of all surgical specialties examined in the study," said Dr. Ho of the surgery department at Brigham and Women’s Hospital, Boston, who was not involved with the published study. "Medicare has started to decrease reimbursements for hospitals with excess readmissions after acute MI, heart failure, and pneumonia. Hip and knee replacements and chronic obstructive pulmonary disease will be added in 2014, and we anticipate that additional surgical procedures will be added thereafter," she said.
In an effort to determine the rate of 30-day unplanned readmission after carotid endarterectomy (CEA), Dr. Ho and her associates conducted a retrospective study of a prospectively collected vascular surgery database at Brigham and Women’s Hospital. The cohort included 896 consecutive CEAs performed between 2002 and 2011. Combined CABG/CEA procedures were excluded.
The primary endpoint was unplanned readmission within 30 days, defined as "any unanticipated, nonelective hospital readmission," she said. The secondary endpoint was 1-year survival.
The mean age of the patients was 70 years, 60% were male, and 95% were white. More than half (65%) had asymptomatic evidence of carotid artery disease.
Dr. Ho reported that the median postoperative length of stay was 1 day and that 9.9% of patients had at least one in-hospital complication. The most frequent in-hospital complication was bleeding/hematoma (4.1%), followed by arrhythmia (2.1%), dysphagia (1.7%), stroke (1.3%), and myocardial infarction (1.2%). Only 3% of patients required a reoperation, while most (94%) were discharged to home. The 30-day stroke rate was 1.7%, while the 30-day death rate was 0.6%.
The overall 30-day readmission rate was 8.6%, while the unplanned 30-day readmission rate was 6.5%. "Most of the overall readmissions (80%) occurred in the first 10 days, and the median time to unplanned readmission was 4 days," Dr. Ho said.
The most common reason for an unplanned readmission was a cardiac complication, followed by headache, bleeding/hematoma, stroke/transient ischemic attack/intracerebral hemorrhage, or other medical emergency. More than one-quarter of patients (27.5%) had more than one reason for an unplanned readmission, while 87.9% of patients had a CEA-related unplanned readmission.
When the researchers performed a univariate analysis followed by analysis with a multivariable Cox model for unplanned readmission, four variables were independently associated with unplanned readmission: in-hospital postoperative CHF exacerbation (hazard ratio, 15.1), in-hospital postoperative stroke (HR, 5.0), in-hospital postoperative hematoma (HR, 3.1), and prior CABG (HR, 2.0).
They also observed a significant difference in survival at 1 year between patients who had an unplanned readmission and those who did not (91% vs. 96%, respectively; P less than .01.) "It’s unclear whether these deaths in the unplanned readmission group were preventable or if they were related to carotid disease or to a procedure-related complication," Dr. Ho said. "Our guess is that the increased overall burden of comorbid disease in these patients, rather than the readmission itself, predicted decreased survival."
Limitations of the study included its retrospective design and the fact that it was conducted at a single center, she said, "but we do know that our unplanned readmission rate is comparable to estimates from recent Medicare data."
Dr. Ho said she had no relevant financial disclosures.
AT THE SVS ANNUAL MEETING
Major finding: Four variables were independently associated with unplanned readmission: in-hospital postoperative CHF exacerbation (hazard ratio, 15.1), in-hospital postoperative stroke (HR, 5.0), in-hospital postoperative hematoma (HR, 3.1), and prior CABG (HR, 2.0).
Data source: A study of 896 consecutive CEAs performed between 2002 and 2011 at Brigham and Women’s Hospital, Boston.
Disclosures: Dr. Ho said she had no relevant financial disclosures.
One-third of perioperative EVAR deaths occurred after discharge
SAN FRANCISCO – One-third of perioperative deaths and complications after elective endovascular repair of abdominal aortic aneurysms occur post discharge, results from a large analysis showed.
"Improved predischarge surveillance and close postdischarge follow-up of identified high-risk patients may further improve 30-day outcomes after EVAR," Dr. Prateek K. Gupta said at the Society for Vascular Surgery annual meeting.
Outcome improvement in the field of aortic surgery, specifically endovascular repair of abdominal aortic aneurysms, "has received much attention," said Dr. Gupta of the department of surgery at the University of Wisconsin Hospital and Clinics, Madison. "With EVAR, the index hospital stay after aortic surgery has decreased significantly, leaving a need for better understanding of postdischarge outcomes, which is necessary to improve quality and reduce readmission rates with implementation of targeted outpatient interventions."
In an effort to examine postdischarge 30-day outcomes after elective EVAR, Dr. Gupta and his associates identified 11,229 patients from the American College of Surgeons National Surgical Quality Improvement Program (NSQIP) database who underwent an elective EVAR for AAA between 2005 and 2010. The primary outcome of interest was postdischarge mortality, while the secondary outcome was postdischarge overall morbidity. The researchers performed univariate and multiple logistic regression analysis to assess factors associated with the primary and secondary study outcomes.
Of the 11,229 patient 83% were male and their mean age was 75 years. Dr. Gupta reported that 117 patients died within 30 days of EVAR, for a rate of 1%. Of these deaths, 31% occurred after hospital discharge, and the median time to death was 9 days. At the same time, 1,204 patients experienced complications within 30 days of EVAR, for a rate of 11%. Of these, 500 (40%) occurred post discharge, and the median time for a complication to occur was 3 days.
Only 20% of patients (7/36) who died post discharge experienced an in-hospital complication. Compared with patients who did not develop a postdischarge complication, those who had more than a sixfold likelihood of reoperation (20.4% vs. 3.1%, respectively; P less than .0001) and death (3.0% vs. 0.2%; P less than .0001) within 30 days of surgery.
Multivariable analysis revealed the following factors that were independently and significantly associated with postdischarge mortality: preoperative heart failure (adjusted odds ratio, 4.7), admission from a skilled nursing facility (AOR, 2.2), increase in age per year (AOR, 1.09), postdischarge renal failure requiring dialysis (AOR, 72.5), postdischarge cardiac arrest/MI (AOR, 46.6), and postdischarge pneumonia (AOR, 26.5).
Dr. Gupta reported that the 30-day postdischarge rate among patients admitted from a nursing facility or acute care was 2.5%. "In contrast to patients who survived after EVAR, patients who died post discharge were more likely to have been admitted from a nursing facility or acute care (13.9% vs. 1.8%; P less than .0001)," he said.
The 30-day post-discharge mortality was highest among patients who had postdischarge renal failure (27%),postdischarge MI (19%), and postdischarge pneumonia (15%).
The researchers also found that patients with a history of peripheral artery disease (PAD) had a significantly higher post-discharge complication rate after EVAR (7.1% vs. 4.3%; P = .001). This also correlated with a higher wound infection rate (3.2% vs. 1.7%; P = .01). A previous cardiac surgery also predisposed patients toward a higher overall postdischarge complication rate (5.3% vs. 4.2%; P = .007).
"Usually, patients undergoing EVAR are followed up at 2 weeks for wound evaluation, or at 1 month with a CT scan," Dr. Gupta said. "In the present study, the median occurrence for most of the postdischarge complications was within the first 10 days after surgery. The interquartile range was 11-22 days for the diagnosis of a wound infection after EVAR. These data suggest that earlier follow-up of high-risk patients may help identify and possibly prevent some of these complications and subsequently decrease readmissions. A standardized protocol for triage and surveillance of high-risk patients post EVAR is needed."
Limitations of the study include that fact that causality could not be determined because it was a retrospective analysis. "In addition, the timing of the operation is not specified in NSQIP," so it could either be a predischarge event or it could have occurred on readmission, Dr. Gupta said. "Data on readmission is not available from the 2005-2010 data sets."
Dr. Gupta said that he had no relevant financial disclosures to make.
Dr. Gupta and his colleagues have assessed postprocedure complications after elective EVAR based upon review of the NSQIP database, and concluded that earlier follow-up of high-risk patients might identify and prevent some of the complications. This study is limited by the database nature of the review. This study also does not provide us with data as to the size of the AAA in the high risk patients.
Most of the complications leading to increased risk of mortality were postoperative issues, such as renal failure or MI, which could not be identified at the time of procedure, or the time of discharge. The only identifiable preoperative risk factors for adverse outcomes were preoperative heart failure, admission from a skilled nursing facility and increasing age. While changing the timing of follow-up might be appropriate for the high-risk patients, other considerations would be changing to more percutaneous procedures , and use of other adjuncts, such as antibiotic irrigations or Prevena (negative pressure wound therapy for intact skin) to decrease the wound infection rates for those undergoing femoral exploration for EVAR. Further, any intervention on the elderly, especially nursing home patients, needs to be thoroughly considered, as EVAR is most often a preventive operation, assuming fitness and appropriate longevity remains for the patient.
The findings from this study are important, but mostly, should serve as a caution to properly assess patients to determine who will potentially benefit from EVAR, and which patients might be best managed by observation alone.
Dr. Linda Harris is the program director and division chief of vascular surgery at the State University of New York, Buffalo.
Dr. Gupta and his colleagues have assessed postprocedure complications after elective EVAR based upon review of the NSQIP database, and concluded that earlier follow-up of high-risk patients might identify and prevent some of the complications. This study is limited by the database nature of the review. This study also does not provide us with data as to the size of the AAA in the high risk patients.
Most of the complications leading to increased risk of mortality were postoperative issues, such as renal failure or MI, which could not be identified at the time of procedure, or the time of discharge. The only identifiable preoperative risk factors for adverse outcomes were preoperative heart failure, admission from a skilled nursing facility and increasing age. While changing the timing of follow-up might be appropriate for the high-risk patients, other considerations would be changing to more percutaneous procedures , and use of other adjuncts, such as antibiotic irrigations or Prevena (negative pressure wound therapy for intact skin) to decrease the wound infection rates for those undergoing femoral exploration for EVAR. Further, any intervention on the elderly, especially nursing home patients, needs to be thoroughly considered, as EVAR is most often a preventive operation, assuming fitness and appropriate longevity remains for the patient.
The findings from this study are important, but mostly, should serve as a caution to properly assess patients to determine who will potentially benefit from EVAR, and which patients might be best managed by observation alone.
Dr. Linda Harris is the program director and division chief of vascular surgery at the State University of New York, Buffalo.
Dr. Gupta and his colleagues have assessed postprocedure complications after elective EVAR based upon review of the NSQIP database, and concluded that earlier follow-up of high-risk patients might identify and prevent some of the complications. This study is limited by the database nature of the review. This study also does not provide us with data as to the size of the AAA in the high risk patients.
Most of the complications leading to increased risk of mortality were postoperative issues, such as renal failure or MI, which could not be identified at the time of procedure, or the time of discharge. The only identifiable preoperative risk factors for adverse outcomes were preoperative heart failure, admission from a skilled nursing facility and increasing age. While changing the timing of follow-up might be appropriate for the high-risk patients, other considerations would be changing to more percutaneous procedures , and use of other adjuncts, such as antibiotic irrigations or Prevena (negative pressure wound therapy for intact skin) to decrease the wound infection rates for those undergoing femoral exploration for EVAR. Further, any intervention on the elderly, especially nursing home patients, needs to be thoroughly considered, as EVAR is most often a preventive operation, assuming fitness and appropriate longevity remains for the patient.
The findings from this study are important, but mostly, should serve as a caution to properly assess patients to determine who will potentially benefit from EVAR, and which patients might be best managed by observation alone.
Dr. Linda Harris is the program director and division chief of vascular surgery at the State University of New York, Buffalo.
SAN FRANCISCO – One-third of perioperative deaths and complications after elective endovascular repair of abdominal aortic aneurysms occur post discharge, results from a large analysis showed.
"Improved predischarge surveillance and close postdischarge follow-up of identified high-risk patients may further improve 30-day outcomes after EVAR," Dr. Prateek K. Gupta said at the Society for Vascular Surgery annual meeting.
Outcome improvement in the field of aortic surgery, specifically endovascular repair of abdominal aortic aneurysms, "has received much attention," said Dr. Gupta of the department of surgery at the University of Wisconsin Hospital and Clinics, Madison. "With EVAR, the index hospital stay after aortic surgery has decreased significantly, leaving a need for better understanding of postdischarge outcomes, which is necessary to improve quality and reduce readmission rates with implementation of targeted outpatient interventions."
In an effort to examine postdischarge 30-day outcomes after elective EVAR, Dr. Gupta and his associates identified 11,229 patients from the American College of Surgeons National Surgical Quality Improvement Program (NSQIP) database who underwent an elective EVAR for AAA between 2005 and 2010. The primary outcome of interest was postdischarge mortality, while the secondary outcome was postdischarge overall morbidity. The researchers performed univariate and multiple logistic regression analysis to assess factors associated with the primary and secondary study outcomes.
Of the 11,229 patient 83% were male and their mean age was 75 years. Dr. Gupta reported that 117 patients died within 30 days of EVAR, for a rate of 1%. Of these deaths, 31% occurred after hospital discharge, and the median time to death was 9 days. At the same time, 1,204 patients experienced complications within 30 days of EVAR, for a rate of 11%. Of these, 500 (40%) occurred post discharge, and the median time for a complication to occur was 3 days.
Only 20% of patients (7/36) who died post discharge experienced an in-hospital complication. Compared with patients who did not develop a postdischarge complication, those who had more than a sixfold likelihood of reoperation (20.4% vs. 3.1%, respectively; P less than .0001) and death (3.0% vs. 0.2%; P less than .0001) within 30 days of surgery.
Multivariable analysis revealed the following factors that were independently and significantly associated with postdischarge mortality: preoperative heart failure (adjusted odds ratio, 4.7), admission from a skilled nursing facility (AOR, 2.2), increase in age per year (AOR, 1.09), postdischarge renal failure requiring dialysis (AOR, 72.5), postdischarge cardiac arrest/MI (AOR, 46.6), and postdischarge pneumonia (AOR, 26.5).
Dr. Gupta reported that the 30-day postdischarge rate among patients admitted from a nursing facility or acute care was 2.5%. "In contrast to patients who survived after EVAR, patients who died post discharge were more likely to have been admitted from a nursing facility or acute care (13.9% vs. 1.8%; P less than .0001)," he said.
The 30-day post-discharge mortality was highest among patients who had postdischarge renal failure (27%),postdischarge MI (19%), and postdischarge pneumonia (15%).
The researchers also found that patients with a history of peripheral artery disease (PAD) had a significantly higher post-discharge complication rate after EVAR (7.1% vs. 4.3%; P = .001). This also correlated with a higher wound infection rate (3.2% vs. 1.7%; P = .01). A previous cardiac surgery also predisposed patients toward a higher overall postdischarge complication rate (5.3% vs. 4.2%; P = .007).
"Usually, patients undergoing EVAR are followed up at 2 weeks for wound evaluation, or at 1 month with a CT scan," Dr. Gupta said. "In the present study, the median occurrence for most of the postdischarge complications was within the first 10 days after surgery. The interquartile range was 11-22 days for the diagnosis of a wound infection after EVAR. These data suggest that earlier follow-up of high-risk patients may help identify and possibly prevent some of these complications and subsequently decrease readmissions. A standardized protocol for triage and surveillance of high-risk patients post EVAR is needed."
Limitations of the study include that fact that causality could not be determined because it was a retrospective analysis. "In addition, the timing of the operation is not specified in NSQIP," so it could either be a predischarge event or it could have occurred on readmission, Dr. Gupta said. "Data on readmission is not available from the 2005-2010 data sets."
Dr. Gupta said that he had no relevant financial disclosures to make.
SAN FRANCISCO – One-third of perioperative deaths and complications after elective endovascular repair of abdominal aortic aneurysms occur post discharge, results from a large analysis showed.
"Improved predischarge surveillance and close postdischarge follow-up of identified high-risk patients may further improve 30-day outcomes after EVAR," Dr. Prateek K. Gupta said at the Society for Vascular Surgery annual meeting.
Outcome improvement in the field of aortic surgery, specifically endovascular repair of abdominal aortic aneurysms, "has received much attention," said Dr. Gupta of the department of surgery at the University of Wisconsin Hospital and Clinics, Madison. "With EVAR, the index hospital stay after aortic surgery has decreased significantly, leaving a need for better understanding of postdischarge outcomes, which is necessary to improve quality and reduce readmission rates with implementation of targeted outpatient interventions."
In an effort to examine postdischarge 30-day outcomes after elective EVAR, Dr. Gupta and his associates identified 11,229 patients from the American College of Surgeons National Surgical Quality Improvement Program (NSQIP) database who underwent an elective EVAR for AAA between 2005 and 2010. The primary outcome of interest was postdischarge mortality, while the secondary outcome was postdischarge overall morbidity. The researchers performed univariate and multiple logistic regression analysis to assess factors associated with the primary and secondary study outcomes.
Of the 11,229 patient 83% were male and their mean age was 75 years. Dr. Gupta reported that 117 patients died within 30 days of EVAR, for a rate of 1%. Of these deaths, 31% occurred after hospital discharge, and the median time to death was 9 days. At the same time, 1,204 patients experienced complications within 30 days of EVAR, for a rate of 11%. Of these, 500 (40%) occurred post discharge, and the median time for a complication to occur was 3 days.
Only 20% of patients (7/36) who died post discharge experienced an in-hospital complication. Compared with patients who did not develop a postdischarge complication, those who had more than a sixfold likelihood of reoperation (20.4% vs. 3.1%, respectively; P less than .0001) and death (3.0% vs. 0.2%; P less than .0001) within 30 days of surgery.
Multivariable analysis revealed the following factors that were independently and significantly associated with postdischarge mortality: preoperative heart failure (adjusted odds ratio, 4.7), admission from a skilled nursing facility (AOR, 2.2), increase in age per year (AOR, 1.09), postdischarge renal failure requiring dialysis (AOR, 72.5), postdischarge cardiac arrest/MI (AOR, 46.6), and postdischarge pneumonia (AOR, 26.5).
Dr. Gupta reported that the 30-day postdischarge rate among patients admitted from a nursing facility or acute care was 2.5%. "In contrast to patients who survived after EVAR, patients who died post discharge were more likely to have been admitted from a nursing facility or acute care (13.9% vs. 1.8%; P less than .0001)," he said.
The 30-day post-discharge mortality was highest among patients who had postdischarge renal failure (27%),postdischarge MI (19%), and postdischarge pneumonia (15%).
The researchers also found that patients with a history of peripheral artery disease (PAD) had a significantly higher post-discharge complication rate after EVAR (7.1% vs. 4.3%; P = .001). This also correlated with a higher wound infection rate (3.2% vs. 1.7%; P = .01). A previous cardiac surgery also predisposed patients toward a higher overall postdischarge complication rate (5.3% vs. 4.2%; P = .007).
"Usually, patients undergoing EVAR are followed up at 2 weeks for wound evaluation, or at 1 month with a CT scan," Dr. Gupta said. "In the present study, the median occurrence for most of the postdischarge complications was within the first 10 days after surgery. The interquartile range was 11-22 days for the diagnosis of a wound infection after EVAR. These data suggest that earlier follow-up of high-risk patients may help identify and possibly prevent some of these complications and subsequently decrease readmissions. A standardized protocol for triage and surveillance of high-risk patients post EVAR is needed."
Limitations of the study include that fact that causality could not be determined because it was a retrospective analysis. "In addition, the timing of the operation is not specified in NSQIP," so it could either be a predischarge event or it could have occurred on readmission, Dr. Gupta said. "Data on readmission is not available from the 2005-2010 data sets."
Dr. Gupta said that he had no relevant financial disclosures to make.
AT THE SVS ANNUAL MEETING
Major finding: Following endovascular repair of abdominal aortic aneurysms, 31% of deaths and 40% of complications occurred after hospital discharge.
Data source: An analysis of 11,229 patients from the American College of Surgeons National Surgical Quality Improvement Program (NSQIP) database who underwent an elective EVAR for AAA between 2005 and 2010.
Disclosures: Dr. Gupta said that he had no relevant financial conflicts to disclose.
Medicare-remunerated EVAR can mean negative operation margins
SAN FRANCISCO – Endovascular aneurysm repair (EVAR) is associated with negative operating margins among Medicare beneficiaries, and device costs account for more than 50% of the technical costs, results from a single-center study demonstrated.
"U.S. health care expenditures have steadily increased over several decades, with some projections now reaching 20% of gross domestic product by 2020," Dr. David H. Stone said at the annual meeting of the Society for Vascular Surgery Annual Meeting. "Accordingly, vigorous debate surrounding health care reform has ensued. In this setting physicians and health care system alike are placing a growing emphasis on both cost reduction and quality improvement, thus increasing the overall value of health care delivery. Endovascular aneurysm repair represents a high-value procedure, though it remains associated with significant cost. This places EVAR at odds with efforts to constrain procedure-associated health care dollars."
Dr. Stone, in the section of vascular surgery at Dartmouth-Hitchcock Medical Center, Lebanon, N.H., and his associates retrospectively examined the EVAR-associated technical costs, revenues, and resulting operating margins among 127 infrarenal EVARs performed at the center between April 2011 and March 2012. They excluded cases in which anatomy was deemed outside of conventional "Instructions for Use" guidelines, included cases treated only by a single vendor’s device, and restricted the payer source to Medicare-remunerated cases billed using the DRG 238 code. This left a cohort of 49 patients. The researchers then determined mean EVAR implant costs per procedure and used 2012 University HealthSystem Consortium data to benchmark their DRG 238 costs and length of stay – another major driver for cost.
"To our surprise, we initially determined that our section’s annual net operating margin for EVAR when billed using DRG 238 was substantially negative, approaching –$500,000 per year," Dr. Stone said. Specifically, mean technical costs among the 49 patients were $31,672, while technical revenue was $27,657, resulting in a negative technical operating margin of $4,015 per case. More specifically, stent grafts accounted for 52% of the technical costs while institutional overhead costs accounted for the remaining 48%.
Among the nonimplant costs the operating room accounted for the single greatest technical cost driver (17%). "By comparison, stent grafts account for roughly threefold more technical cost than [did] any nonimplant hospital costs," Dr. Stone said. "Interestingly, there is an apparent inequity between the stent graft costs when considered as a percentage of cost vs. a percentage of revenue. More specifically, stent grafts currently account for 52% of the technical costs but assume 60% of the DRG payment, thus contributing in part to our institution’s negative margin."
Given the substantial impact of graft costs to the procedure, the researchers also examined Dartmouth-Hitchcock’s current vendor market share for the medical center’s entire EVAR practice. The vendors were not named but rather described as vendors A, B, C, and D. "Though historically we have not routinely integrated costs into our case planning, we were somewhat surprised to learn that vendor D the highest-cost device derived the largest market share, while vendors A and B the two lowest-cost devices derived the smallest market shares, respectively," Dr. Stone said. "Surgeons were largely unaware of this cost disparity." He said that a "lack of transparency" of the device costs among institutions has also led in part to the sustainability of this practice pattern.
Dr. Stone acknowledged certain limitations of the study, including its single-center design, "thus graft pricing and institutional overhead will likely vary among hospitals," he said. "In addition, we did not analyze DRG 237–remunerated EVAR with major complications, where costs may be higher yet. However, we nevertheless believe that the adjudicated financial costs presented here may reflect a similar trend in many institutions throughout the country for Medicare-remunerated EVAR."
He concluded his remarks by noting that the negative operating margin for Medicare-remunerated EVAR "is likely unsustainable. Surgeon awareness of price differential among grafts may allow for competitive negotiated pricing. Accordingly, we believe that EVAR as a high-value procedure must undergo care delivery redesign, reflecting cost restructuring with viable remuneration schemes in order for current practice to remain sustainable."
Dr. Stone said that he had no relevant financial conflicts to disclose.
Dr. Stone and his colleagues at Dartmouth-Hitchcock have identified simple, uncomplicated EVAR cases as producing a negative contribution margin to the institution, primarily because of device costs. In today’s environment of cost containment, it is imperative that physicians partner with institutions to evaluate appropriate methods of cost containment, while maintaining excellence of care. These findings should not lead to the abandonment or restriction of EVAR, but rather further discussions between hospitals and physicians as to ways to decrease cost of the procedure. This will include negotiations with vendors to potentially decrease device costs and to provide, at the least, budget neutral interventions, as the current status is not sustainable.
As we move forward, these types of calculations will need to occur at all institutions for high-volume procedures to ensure viability of the institutions while maintaining excellence of medical care.
Dr. Linda Harris is the program director and division chief of vascular surgery at the State University of New York, Buffalo.
Dr. Stone and his colleagues at Dartmouth-Hitchcock have identified simple, uncomplicated EVAR cases as producing a negative contribution margin to the institution, primarily because of device costs. In today’s environment of cost containment, it is imperative that physicians partner with institutions to evaluate appropriate methods of cost containment, while maintaining excellence of care. These findings should not lead to the abandonment or restriction of EVAR, but rather further discussions between hospitals and physicians as to ways to decrease cost of the procedure. This will include negotiations with vendors to potentially decrease device costs and to provide, at the least, budget neutral interventions, as the current status is not sustainable.
As we move forward, these types of calculations will need to occur at all institutions for high-volume procedures to ensure viability of the institutions while maintaining excellence of medical care.
Dr. Linda Harris is the program director and division chief of vascular surgery at the State University of New York, Buffalo.
Dr. Stone and his colleagues at Dartmouth-Hitchcock have identified simple, uncomplicated EVAR cases as producing a negative contribution margin to the institution, primarily because of device costs. In today’s environment of cost containment, it is imperative that physicians partner with institutions to evaluate appropriate methods of cost containment, while maintaining excellence of care. These findings should not lead to the abandonment or restriction of EVAR, but rather further discussions between hospitals and physicians as to ways to decrease cost of the procedure. This will include negotiations with vendors to potentially decrease device costs and to provide, at the least, budget neutral interventions, as the current status is not sustainable.
As we move forward, these types of calculations will need to occur at all institutions for high-volume procedures to ensure viability of the institutions while maintaining excellence of medical care.
Dr. Linda Harris is the program director and division chief of vascular surgery at the State University of New York, Buffalo.
SAN FRANCISCO – Endovascular aneurysm repair (EVAR) is associated with negative operating margins among Medicare beneficiaries, and device costs account for more than 50% of the technical costs, results from a single-center study demonstrated.
"U.S. health care expenditures have steadily increased over several decades, with some projections now reaching 20% of gross domestic product by 2020," Dr. David H. Stone said at the annual meeting of the Society for Vascular Surgery Annual Meeting. "Accordingly, vigorous debate surrounding health care reform has ensued. In this setting physicians and health care system alike are placing a growing emphasis on both cost reduction and quality improvement, thus increasing the overall value of health care delivery. Endovascular aneurysm repair represents a high-value procedure, though it remains associated with significant cost. This places EVAR at odds with efforts to constrain procedure-associated health care dollars."
Dr. Stone, in the section of vascular surgery at Dartmouth-Hitchcock Medical Center, Lebanon, N.H., and his associates retrospectively examined the EVAR-associated technical costs, revenues, and resulting operating margins among 127 infrarenal EVARs performed at the center between April 2011 and March 2012. They excluded cases in which anatomy was deemed outside of conventional "Instructions for Use" guidelines, included cases treated only by a single vendor’s device, and restricted the payer source to Medicare-remunerated cases billed using the DRG 238 code. This left a cohort of 49 patients. The researchers then determined mean EVAR implant costs per procedure and used 2012 University HealthSystem Consortium data to benchmark their DRG 238 costs and length of stay – another major driver for cost.
"To our surprise, we initially determined that our section’s annual net operating margin for EVAR when billed using DRG 238 was substantially negative, approaching –$500,000 per year," Dr. Stone said. Specifically, mean technical costs among the 49 patients were $31,672, while technical revenue was $27,657, resulting in a negative technical operating margin of $4,015 per case. More specifically, stent grafts accounted for 52% of the technical costs while institutional overhead costs accounted for the remaining 48%.
Among the nonimplant costs the operating room accounted for the single greatest technical cost driver (17%). "By comparison, stent grafts account for roughly threefold more technical cost than [did] any nonimplant hospital costs," Dr. Stone said. "Interestingly, there is an apparent inequity between the stent graft costs when considered as a percentage of cost vs. a percentage of revenue. More specifically, stent grafts currently account for 52% of the technical costs but assume 60% of the DRG payment, thus contributing in part to our institution’s negative margin."
Given the substantial impact of graft costs to the procedure, the researchers also examined Dartmouth-Hitchcock’s current vendor market share for the medical center’s entire EVAR practice. The vendors were not named but rather described as vendors A, B, C, and D. "Though historically we have not routinely integrated costs into our case planning, we were somewhat surprised to learn that vendor D the highest-cost device derived the largest market share, while vendors A and B the two lowest-cost devices derived the smallest market shares, respectively," Dr. Stone said. "Surgeons were largely unaware of this cost disparity." He said that a "lack of transparency" of the device costs among institutions has also led in part to the sustainability of this practice pattern.
Dr. Stone acknowledged certain limitations of the study, including its single-center design, "thus graft pricing and institutional overhead will likely vary among hospitals," he said. "In addition, we did not analyze DRG 237–remunerated EVAR with major complications, where costs may be higher yet. However, we nevertheless believe that the adjudicated financial costs presented here may reflect a similar trend in many institutions throughout the country for Medicare-remunerated EVAR."
He concluded his remarks by noting that the negative operating margin for Medicare-remunerated EVAR "is likely unsustainable. Surgeon awareness of price differential among grafts may allow for competitive negotiated pricing. Accordingly, we believe that EVAR as a high-value procedure must undergo care delivery redesign, reflecting cost restructuring with viable remuneration schemes in order for current practice to remain sustainable."
Dr. Stone said that he had no relevant financial conflicts to disclose.
SAN FRANCISCO – Endovascular aneurysm repair (EVAR) is associated with negative operating margins among Medicare beneficiaries, and device costs account for more than 50% of the technical costs, results from a single-center study demonstrated.
"U.S. health care expenditures have steadily increased over several decades, with some projections now reaching 20% of gross domestic product by 2020," Dr. David H. Stone said at the annual meeting of the Society for Vascular Surgery Annual Meeting. "Accordingly, vigorous debate surrounding health care reform has ensued. In this setting physicians and health care system alike are placing a growing emphasis on both cost reduction and quality improvement, thus increasing the overall value of health care delivery. Endovascular aneurysm repair represents a high-value procedure, though it remains associated with significant cost. This places EVAR at odds with efforts to constrain procedure-associated health care dollars."
Dr. Stone, in the section of vascular surgery at Dartmouth-Hitchcock Medical Center, Lebanon, N.H., and his associates retrospectively examined the EVAR-associated technical costs, revenues, and resulting operating margins among 127 infrarenal EVARs performed at the center between April 2011 and March 2012. They excluded cases in which anatomy was deemed outside of conventional "Instructions for Use" guidelines, included cases treated only by a single vendor’s device, and restricted the payer source to Medicare-remunerated cases billed using the DRG 238 code. This left a cohort of 49 patients. The researchers then determined mean EVAR implant costs per procedure and used 2012 University HealthSystem Consortium data to benchmark their DRG 238 costs and length of stay – another major driver for cost.
"To our surprise, we initially determined that our section’s annual net operating margin for EVAR when billed using DRG 238 was substantially negative, approaching –$500,000 per year," Dr. Stone said. Specifically, mean technical costs among the 49 patients were $31,672, while technical revenue was $27,657, resulting in a negative technical operating margin of $4,015 per case. More specifically, stent grafts accounted for 52% of the technical costs while institutional overhead costs accounted for the remaining 48%.
Among the nonimplant costs the operating room accounted for the single greatest technical cost driver (17%). "By comparison, stent grafts account for roughly threefold more technical cost than [did] any nonimplant hospital costs," Dr. Stone said. "Interestingly, there is an apparent inequity between the stent graft costs when considered as a percentage of cost vs. a percentage of revenue. More specifically, stent grafts currently account for 52% of the technical costs but assume 60% of the DRG payment, thus contributing in part to our institution’s negative margin."
Given the substantial impact of graft costs to the procedure, the researchers also examined Dartmouth-Hitchcock’s current vendor market share for the medical center’s entire EVAR practice. The vendors were not named but rather described as vendors A, B, C, and D. "Though historically we have not routinely integrated costs into our case planning, we were somewhat surprised to learn that vendor D the highest-cost device derived the largest market share, while vendors A and B the two lowest-cost devices derived the smallest market shares, respectively," Dr. Stone said. "Surgeons were largely unaware of this cost disparity." He said that a "lack of transparency" of the device costs among institutions has also led in part to the sustainability of this practice pattern.
Dr. Stone acknowledged certain limitations of the study, including its single-center design, "thus graft pricing and institutional overhead will likely vary among hospitals," he said. "In addition, we did not analyze DRG 237–remunerated EVAR with major complications, where costs may be higher yet. However, we nevertheless believe that the adjudicated financial costs presented here may reflect a similar trend in many institutions throughout the country for Medicare-remunerated EVAR."
He concluded his remarks by noting that the negative operating margin for Medicare-remunerated EVAR "is likely unsustainable. Surgeon awareness of price differential among grafts may allow for competitive negotiated pricing. Accordingly, we believe that EVAR as a high-value procedure must undergo care delivery redesign, reflecting cost restructuring with viable remuneration schemes in order for current practice to remain sustainable."
Dr. Stone said that he had no relevant financial conflicts to disclose.
AT THE SVS ANNUAL MEETING
Major finding: In a study of DRG 238 remunerated EVAR, stent grafts accounted for 52% of the technical costs while institutional overhead costs accounted for the remaining 48%.
Data source: A retrospectively examination of the EVAR-associated technical costs, revenues, and resulting operating margins among 127 infrarenal EVARs performed at Dartmouth-Hitchcock Medical Center between April 2011 and March 2012.
Disclosures: Dr. Stone said that he had no relevant financial conflicts to disclose.