User login
Cord blood/placental cell combo induces rapid immune recovery
MONTREAL – A combination of placenta-derived stem cells and umbilical cord blood was associated with early engraftment and high degrees of cord blood donor chimerism in the treatment of children with both malignant and nonmalignant hematologic conditions requiring stem cell transplantation, updated results of a pilot study show.
Among 16 children treated with the combination, the probability of neutrophil engraftment was 87.5%, and all patients who had neutrophil engraftment went on to have platelet engraftment. The probability of 12-month overall survival was 81.2%, reported Allyson Flower, MD, from Boston Children’s Health Physicians in Hawthorne, N.Y. “The probability of grade II-IV acute graft vs. host disease was 12.5%, compared with 32.5% seen with unrelated cord blood in our group’s previous studies. Cellular immune reconstitution was robust,” she said at the annual meeting of the American Society of Pediatric Hematology/Oncology.
Augmenting cord blood
Although unrelated donor cord blood transplantation expands the donor pool, is rapidly available, and is associated with decreases in both severe acute graft vs. host disease (GVHD) and chronic GVHD, compared with other stem cell sources, the technique is hampered by limited cell doses, prolonged immune reconstitution time, delays in hematopoietic recovery, and a higher incidence of graft failure.
Early studies of myeloablative conditioning followed by unrelated umbilical or placental blood transplantation showed a median of 22-24 days to neutrophil engraftment (Blood 1996 88:795-802; N Engl J Med. 1996;335:157-66), Dr. Flower noted.
More recently, a multivariate analysis of patients who underwent reduced-intensity conditioning followed by hematopoietic stem cell transplant with unrelated cord blood showed that graft failure was an independent risk factor for worse overall survival (Biol Blood Marrow Transplant. 2013 Apr;19:4;552-61).
Multiple groups have shown that adding human placenta–derived stem cells (HPDSC) to cord blood transplantation can facilitate more rapid hematopoietic engraftment by increasing the number of stem cells, increasing the proportion of hematopoietic progenitor cells, and providing additional, immature CD34+/CD45– progenitor cells.
In a single-arm, nonrandomized study, the investigators enrolled 16 patients ranging in age from 0.3 to 15.7 years with inborn errors of metabolism, marrow failure syndromes, severe immunodeficiency states, or hematologic malignancies.
Malignant conditions included B-cell precursor acute lymphoblastic leukemia (B-ALL; four patients), acute myeloid leukemia (AML; two), and T-cell ALL (one) in first complete remission, and T-cell lymphoblastic lymphoma following induction failure (one). Nonmalignant conditions included adrenoleukodystrophy (two patients), amegakaryotic thrombocytopenia (one), severe combined immunodeficiency (SCID; two), dyskeratosis congenita (one), chronic granulomatous disease (one), and severe congenital neutropenia (one).
The patients first underwent either myeloablative or reduced-intensity conditioning, followed 10 days later by infusion of unrelated cord blood and HPDSCs. Prior to HPDSC infusion, patients were medicated with diphenhydramine and hydrocortisone to prevent or reduce potential sensitivity reactions. HPDSCs were infused no sooner than 4 hours after the end of the cord blood infusion.
Patients received GVHD prophylaxis with either tacrolimus or cyclosporine, plus mycophenolate mofetil.
The combination appeared to be safe, with no cases of grade 3 or 4 toxicity secondary to HPDSC infusion.
The probability of neutrophil engraftment was 87.5%, with engraftment occurring at a median of 23 days (range 13-53). As noted before, all patients who had neutrophil engraftment had platelet engraftment, which was achieved at a median of 47 days (range, 20-98). In the group’s previous studies, median time to platelet engraftment was 53 days for patients who had undergone reduced-intensity conditioning, and 118 days for patients who had undergone myeloablation.
The probability of grade 2-4 acute GVHD within 100 days was 12.5%, and there were no cases of chronic GVHD.
Respective percentages of cord blood donor chimerism at days 30, 60, 100, and 180 were 88%, 98%, 99%, and 99%.
Immune reconstitution was strong, with normalization of mean CD3+, CD19+, and CD56+ cells occurring by day 100, CD8+ cells by day 180, and CD4+ cells by day 270.
There were three patient deaths: one from adenoviremia in a patient with B-ALL and CNS relapse, who had neutrophil engraftment at day 21; one in a patient with SCID, from adenoviremia and multiple system organ failure, who did not have engraftment before death; and one in a patient with severe congenital neutrophilia, who also did not have neutrophil engraftment.
None of the eight patients with malignant disease have experienced relapse to date, Dr. Flower noted.
The study was funded by a grant from Celgene Cellular Therapeutics. Dr. Flower reported having no conflicts of interest.
MONTREAL – A combination of placenta-derived stem cells and umbilical cord blood was associated with early engraftment and high degrees of cord blood donor chimerism in the treatment of children with both malignant and nonmalignant hematologic conditions requiring stem cell transplantation, updated results of a pilot study show.
Among 16 children treated with the combination, the probability of neutrophil engraftment was 87.5%, and all patients who had neutrophil engraftment went on to have platelet engraftment. The probability of 12-month overall survival was 81.2%, reported Allyson Flower, MD, from Boston Children’s Health Physicians in Hawthorne, N.Y. “The probability of grade II-IV acute graft vs. host disease was 12.5%, compared with 32.5% seen with unrelated cord blood in our group’s previous studies. Cellular immune reconstitution was robust,” she said at the annual meeting of the American Society of Pediatric Hematology/Oncology.
Augmenting cord blood
Although unrelated donor cord blood transplantation expands the donor pool, is rapidly available, and is associated with decreases in both severe acute graft vs. host disease (GVHD) and chronic GVHD, compared with other stem cell sources, the technique is hampered by limited cell doses, prolonged immune reconstitution time, delays in hematopoietic recovery, and a higher incidence of graft failure.
Early studies of myeloablative conditioning followed by unrelated umbilical or placental blood transplantation showed a median of 22-24 days to neutrophil engraftment (Blood 1996 88:795-802; N Engl J Med. 1996;335:157-66), Dr. Flower noted.
More recently, a multivariate analysis of patients who underwent reduced-intensity conditioning followed by hematopoietic stem cell transplant with unrelated cord blood showed that graft failure was an independent risk factor for worse overall survival (Biol Blood Marrow Transplant. 2013 Apr;19:4;552-61).
Multiple groups have shown that adding human placenta–derived stem cells (HPDSC) to cord blood transplantation can facilitate more rapid hematopoietic engraftment by increasing the number of stem cells, increasing the proportion of hematopoietic progenitor cells, and providing additional, immature CD34+/CD45– progenitor cells.
In a single-arm, nonrandomized study, the investigators enrolled 16 patients ranging in age from 0.3 to 15.7 years with inborn errors of metabolism, marrow failure syndromes, severe immunodeficiency states, or hematologic malignancies.
Malignant conditions included B-cell precursor acute lymphoblastic leukemia (B-ALL; four patients), acute myeloid leukemia (AML; two), and T-cell ALL (one) in first complete remission, and T-cell lymphoblastic lymphoma following induction failure (one). Nonmalignant conditions included adrenoleukodystrophy (two patients), amegakaryotic thrombocytopenia (one), severe combined immunodeficiency (SCID; two), dyskeratosis congenita (one), chronic granulomatous disease (one), and severe congenital neutropenia (one).
The patients first underwent either myeloablative or reduced-intensity conditioning, followed 10 days later by infusion of unrelated cord blood and HPDSCs. Prior to HPDSC infusion, patients were medicated with diphenhydramine and hydrocortisone to prevent or reduce potential sensitivity reactions. HPDSCs were infused no sooner than 4 hours after the end of the cord blood infusion.
Patients received GVHD prophylaxis with either tacrolimus or cyclosporine, plus mycophenolate mofetil.
The combination appeared to be safe, with no cases of grade 3 or 4 toxicity secondary to HPDSC infusion.
The probability of neutrophil engraftment was 87.5%, with engraftment occurring at a median of 23 days (range 13-53). As noted before, all patients who had neutrophil engraftment had platelet engraftment, which was achieved at a median of 47 days (range, 20-98). In the group’s previous studies, median time to platelet engraftment was 53 days for patients who had undergone reduced-intensity conditioning, and 118 days for patients who had undergone myeloablation.
The probability of grade 2-4 acute GVHD within 100 days was 12.5%, and there were no cases of chronic GVHD.
Respective percentages of cord blood donor chimerism at days 30, 60, 100, and 180 were 88%, 98%, 99%, and 99%.
Immune reconstitution was strong, with normalization of mean CD3+, CD19+, and CD56+ cells occurring by day 100, CD8+ cells by day 180, and CD4+ cells by day 270.
There were three patient deaths: one from adenoviremia in a patient with B-ALL and CNS relapse, who had neutrophil engraftment at day 21; one in a patient with SCID, from adenoviremia and multiple system organ failure, who did not have engraftment before death; and one in a patient with severe congenital neutrophilia, who also did not have neutrophil engraftment.
None of the eight patients with malignant disease have experienced relapse to date, Dr. Flower noted.
The study was funded by a grant from Celgene Cellular Therapeutics. Dr. Flower reported having no conflicts of interest.
MONTREAL – A combination of placenta-derived stem cells and umbilical cord blood was associated with early engraftment and high degrees of cord blood donor chimerism in the treatment of children with both malignant and nonmalignant hematologic conditions requiring stem cell transplantation, updated results of a pilot study show.
Among 16 children treated with the combination, the probability of neutrophil engraftment was 87.5%, and all patients who had neutrophil engraftment went on to have platelet engraftment. The probability of 12-month overall survival was 81.2%, reported Allyson Flower, MD, from Boston Children’s Health Physicians in Hawthorne, N.Y. “The probability of grade II-IV acute graft vs. host disease was 12.5%, compared with 32.5% seen with unrelated cord blood in our group’s previous studies. Cellular immune reconstitution was robust,” she said at the annual meeting of the American Society of Pediatric Hematology/Oncology.
Augmenting cord blood
Although unrelated donor cord blood transplantation expands the donor pool, is rapidly available, and is associated with decreases in both severe acute graft vs. host disease (GVHD) and chronic GVHD, compared with other stem cell sources, the technique is hampered by limited cell doses, prolonged immune reconstitution time, delays in hematopoietic recovery, and a higher incidence of graft failure.
Early studies of myeloablative conditioning followed by unrelated umbilical or placental blood transplantation showed a median of 22-24 days to neutrophil engraftment (Blood 1996 88:795-802; N Engl J Med. 1996;335:157-66), Dr. Flower noted.
More recently, a multivariate analysis of patients who underwent reduced-intensity conditioning followed by hematopoietic stem cell transplant with unrelated cord blood showed that graft failure was an independent risk factor for worse overall survival (Biol Blood Marrow Transplant. 2013 Apr;19:4;552-61).
Multiple groups have shown that adding human placenta–derived stem cells (HPDSC) to cord blood transplantation can facilitate more rapid hematopoietic engraftment by increasing the number of stem cells, increasing the proportion of hematopoietic progenitor cells, and providing additional, immature CD34+/CD45– progenitor cells.
In a single-arm, nonrandomized study, the investigators enrolled 16 patients ranging in age from 0.3 to 15.7 years with inborn errors of metabolism, marrow failure syndromes, severe immunodeficiency states, or hematologic malignancies.
Malignant conditions included B-cell precursor acute lymphoblastic leukemia (B-ALL; four patients), acute myeloid leukemia (AML; two), and T-cell ALL (one) in first complete remission, and T-cell lymphoblastic lymphoma following induction failure (one). Nonmalignant conditions included adrenoleukodystrophy (two patients), amegakaryotic thrombocytopenia (one), severe combined immunodeficiency (SCID; two), dyskeratosis congenita (one), chronic granulomatous disease (one), and severe congenital neutropenia (one).
The patients first underwent either myeloablative or reduced-intensity conditioning, followed 10 days later by infusion of unrelated cord blood and HPDSCs. Prior to HPDSC infusion, patients were medicated with diphenhydramine and hydrocortisone to prevent or reduce potential sensitivity reactions. HPDSCs were infused no sooner than 4 hours after the end of the cord blood infusion.
Patients received GVHD prophylaxis with either tacrolimus or cyclosporine, plus mycophenolate mofetil.
The combination appeared to be safe, with no cases of grade 3 or 4 toxicity secondary to HPDSC infusion.
The probability of neutrophil engraftment was 87.5%, with engraftment occurring at a median of 23 days (range 13-53). As noted before, all patients who had neutrophil engraftment had platelet engraftment, which was achieved at a median of 47 days (range, 20-98). In the group’s previous studies, median time to platelet engraftment was 53 days for patients who had undergone reduced-intensity conditioning, and 118 days for patients who had undergone myeloablation.
The probability of grade 2-4 acute GVHD within 100 days was 12.5%, and there were no cases of chronic GVHD.
Respective percentages of cord blood donor chimerism at days 30, 60, 100, and 180 were 88%, 98%, 99%, and 99%.
Immune reconstitution was strong, with normalization of mean CD3+, CD19+, and CD56+ cells occurring by day 100, CD8+ cells by day 180, and CD4+ cells by day 270.
There were three patient deaths: one from adenoviremia in a patient with B-ALL and CNS relapse, who had neutrophil engraftment at day 21; one in a patient with SCID, from adenoviremia and multiple system organ failure, who did not have engraftment before death; and one in a patient with severe congenital neutrophilia, who also did not have neutrophil engraftment.
None of the eight patients with malignant disease have experienced relapse to date, Dr. Flower noted.
The study was funded by a grant from Celgene Cellular Therapeutics. Dr. Flower reported having no conflicts of interest.
Key clinical point: A combination of donor cord blood and human placenta–derived stem cells induced more rapid engraftment than cord blood alone.
Major finding: The probability of 12-month overall survival was 81%.
Data source: Open-label single-arm study in 16 children with severe malignant and nonmalignant diseases requiring hematopoietic stem cell transplants.
Disclosures: The study was funded by a grant from Celgene Cellular Therapeutics. Dr. Flower reported having no conflicts of interest.
Optimum antithymocyte globulin exposure after HTC affects survival
Optimum antithymocyte globulin exposure after allogeneic hemopoietic cell transplantation (HTC) is associated with a higher probability of survival as a result of reductions in transplant-related and relapse-related deaths, based on findings from a retrospective multicenter cohort study.
The findings come from a pharmacokinetic-pharmacodynamic analysis of data from 146 patients with acute lymphoid leukemia, acute myeloid leukemia, or myelodysplastic syndrome. All were receiving their first T cell–repleted allogeneic peripheral blood stem cell HCT with antithymocyte globulin (ATG) as part of their nonmyeloablative conditioning regimen. Based on hazard ratios for overall survival, nonrelapse mortality, and relapse-related mortality, the optimum range of ATG exposure was 60-95 arbitrary units per day/mL, Rick Admiraal, MD, of the University Medical Centre Utrecht, Netherlands, and his colleagues reported in the Lancet Haematology (Lancet Haematol. 2017 Apr;4[4]:e183-91).
The estimated 5-year survival was significantly greater with optimum ATG exposure than with below-optimum exposure (69% vs. 32%; hazard ratio, 2.41) or with above-optimum exposure (69% vs. 48%; hazard ratio, 2.11).
Optimum ATG exposure also was associated with a greater likelihood of event-free survival: The hazard ratio was 2.54 for those with below-optimum exposure and 1.83 for those with above-optimum exposure, the researchers said. Further, above-optimum exposure was associated with higher relapse-related mortality (hazard ratio, 2.66). Below-optimum exposure was associated with higher non-relapse mortality (hazard ratio, 4.36) as well as with a higher risk for grade 3-4 acute graft-versus-host disease (hazard ratio, 3.09), but not for chronic graft-versus-host disease (hazard ratio, 2.38).
Optimum target attainment was better with modeled dosing based on absolute lymphocyte counts than with weight-based dosing, the authors said.
The findings underscore the importance of optimum ATG dosing, as “survival after HCT is highly affected by ATG exposure after HCT,” and they suggest that survival chances may be improved with individualized dosing based on lymphocyte counts–a finding that requires assessment in a prospective study, they concluded.
This study was funded by the Dutch Organization for Scientific Research and the Queen Wilhelmina Fund for Cancer Research. Dr. Admiraal reported having no relevant disclosures.
The study by Dr. Admiraal and his associates introduces important concepts regarding ATG dosing, but the suggestion that dosing should be individualized based on lymphocyte count and should target the area under the time-versus-thymoglobulin-concentration curve of 60-95 arbitrary units per day/mL after HCT should not yet become the standard of care.
“As the authors correctly point out, first a prospective study is needed,” Dr. Storek wrote.
However, even if such a study confirms this approach – which the authors previously showed to be of benefit in children – it would apply only to the setting in which it was developed. The problem is that there are numerous HCT settings, each requiring a different dose. Further, there is no universally accepted assay for measuring thymoglobulin concentrations, he noted.
“Following the seminal observations in the paper … much work remains to be done,” he wrote.
Jan Storek, MD, is with the University of Calgary (Alta.) and Alberta Health Services, Calgary. He made his comments in an editorial (Lancet Haematol. 2017 Apr;4[4]:e154-5) published with the study and reported having no disclosures.
The study by Dr. Admiraal and his associates introduces important concepts regarding ATG dosing, but the suggestion that dosing should be individualized based on lymphocyte count and should target the area under the time-versus-thymoglobulin-concentration curve of 60-95 arbitrary units per day/mL after HCT should not yet become the standard of care.
“As the authors correctly point out, first a prospective study is needed,” Dr. Storek wrote.
However, even if such a study confirms this approach – which the authors previously showed to be of benefit in children – it would apply only to the setting in which it was developed. The problem is that there are numerous HCT settings, each requiring a different dose. Further, there is no universally accepted assay for measuring thymoglobulin concentrations, he noted.
“Following the seminal observations in the paper … much work remains to be done,” he wrote.
Jan Storek, MD, is with the University of Calgary (Alta.) and Alberta Health Services, Calgary. He made his comments in an editorial (Lancet Haematol. 2017 Apr;4[4]:e154-5) published with the study and reported having no disclosures.
The study by Dr. Admiraal and his associates introduces important concepts regarding ATG dosing, but the suggestion that dosing should be individualized based on lymphocyte count and should target the area under the time-versus-thymoglobulin-concentration curve of 60-95 arbitrary units per day/mL after HCT should not yet become the standard of care.
“As the authors correctly point out, first a prospective study is needed,” Dr. Storek wrote.
However, even if such a study confirms this approach – which the authors previously showed to be of benefit in children – it would apply only to the setting in which it was developed. The problem is that there are numerous HCT settings, each requiring a different dose. Further, there is no universally accepted assay for measuring thymoglobulin concentrations, he noted.
“Following the seminal observations in the paper … much work remains to be done,” he wrote.
Jan Storek, MD, is with the University of Calgary (Alta.) and Alberta Health Services, Calgary. He made his comments in an editorial (Lancet Haematol. 2017 Apr;4[4]:e154-5) published with the study and reported having no disclosures.
Optimum antithymocyte globulin exposure after allogeneic hemopoietic cell transplantation (HTC) is associated with a higher probability of survival as a result of reductions in transplant-related and relapse-related deaths, based on findings from a retrospective multicenter cohort study.
The findings come from a pharmacokinetic-pharmacodynamic analysis of data from 146 patients with acute lymphoid leukemia, acute myeloid leukemia, or myelodysplastic syndrome. All were receiving their first T cell–repleted allogeneic peripheral blood stem cell HCT with antithymocyte globulin (ATG) as part of their nonmyeloablative conditioning regimen. Based on hazard ratios for overall survival, nonrelapse mortality, and relapse-related mortality, the optimum range of ATG exposure was 60-95 arbitrary units per day/mL, Rick Admiraal, MD, of the University Medical Centre Utrecht, Netherlands, and his colleagues reported in the Lancet Haematology (Lancet Haematol. 2017 Apr;4[4]:e183-91).
The estimated 5-year survival was significantly greater with optimum ATG exposure than with below-optimum exposure (69% vs. 32%; hazard ratio, 2.41) or with above-optimum exposure (69% vs. 48%; hazard ratio, 2.11).
Optimum ATG exposure also was associated with a greater likelihood of event-free survival: The hazard ratio was 2.54 for those with below-optimum exposure and 1.83 for those with above-optimum exposure, the researchers said. Further, above-optimum exposure was associated with higher relapse-related mortality (hazard ratio, 2.66). Below-optimum exposure was associated with higher non-relapse mortality (hazard ratio, 4.36) as well as with a higher risk for grade 3-4 acute graft-versus-host disease (hazard ratio, 3.09), but not for chronic graft-versus-host disease (hazard ratio, 2.38).
Optimum target attainment was better with modeled dosing based on absolute lymphocyte counts than with weight-based dosing, the authors said.
The findings underscore the importance of optimum ATG dosing, as “survival after HCT is highly affected by ATG exposure after HCT,” and they suggest that survival chances may be improved with individualized dosing based on lymphocyte counts–a finding that requires assessment in a prospective study, they concluded.
This study was funded by the Dutch Organization for Scientific Research and the Queen Wilhelmina Fund for Cancer Research. Dr. Admiraal reported having no relevant disclosures.
Optimum antithymocyte globulin exposure after allogeneic hemopoietic cell transplantation (HTC) is associated with a higher probability of survival as a result of reductions in transplant-related and relapse-related deaths, based on findings from a retrospective multicenter cohort study.
The findings come from a pharmacokinetic-pharmacodynamic analysis of data from 146 patients with acute lymphoid leukemia, acute myeloid leukemia, or myelodysplastic syndrome. All were receiving their first T cell–repleted allogeneic peripheral blood stem cell HCT with antithymocyte globulin (ATG) as part of their nonmyeloablative conditioning regimen. Based on hazard ratios for overall survival, nonrelapse mortality, and relapse-related mortality, the optimum range of ATG exposure was 60-95 arbitrary units per day/mL, Rick Admiraal, MD, of the University Medical Centre Utrecht, Netherlands, and his colleagues reported in the Lancet Haematology (Lancet Haematol. 2017 Apr;4[4]:e183-91).
The estimated 5-year survival was significantly greater with optimum ATG exposure than with below-optimum exposure (69% vs. 32%; hazard ratio, 2.41) or with above-optimum exposure (69% vs. 48%; hazard ratio, 2.11).
Optimum ATG exposure also was associated with a greater likelihood of event-free survival: The hazard ratio was 2.54 for those with below-optimum exposure and 1.83 for those with above-optimum exposure, the researchers said. Further, above-optimum exposure was associated with higher relapse-related mortality (hazard ratio, 2.66). Below-optimum exposure was associated with higher non-relapse mortality (hazard ratio, 4.36) as well as with a higher risk for grade 3-4 acute graft-versus-host disease (hazard ratio, 3.09), but not for chronic graft-versus-host disease (hazard ratio, 2.38).
Optimum target attainment was better with modeled dosing based on absolute lymphocyte counts than with weight-based dosing, the authors said.
The findings underscore the importance of optimum ATG dosing, as “survival after HCT is highly affected by ATG exposure after HCT,” and they suggest that survival chances may be improved with individualized dosing based on lymphocyte counts–a finding that requires assessment in a prospective study, they concluded.
This study was funded by the Dutch Organization for Scientific Research and the Queen Wilhelmina Fund for Cancer Research. Dr. Admiraal reported having no relevant disclosures.
FROM THE LANCET HAEMATOLOGY
Key clinical point:
Major finding: The estimated 5-year survival with optimum ATG exposure was 69% vs. 32% and 48% with below- and above-optimum exposure (hazard ratios, 2.41 and 2.11, respectively).
Data source: A retrospective cohort study of 146 patients receiving their first T-cell repleted allogeneic peripheral blood stem cell HCT.
Disclosures: This study was funded by the Dutch Organization for Scientific Research and the Queen Wilhelmina Fund for Cancer Research. Dr. Admiraal reported having no disclosures.
Biomarker algorithm sharpens GVHD treatment outcomes prediction
ORLANDO – A biomarker algorithm was better than was clinical response at predicting outcomes after 1 week of systemic steroid treatment for graft-versus-host disease, according to findings from a multicenter study.
The findings have implications for early decision making regarding treatment course, Hannah Major-Monfried reported at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
“The same GVHD algorithm that stratifies patients at day 7 after transplant, at [GVHD] diagnosis, also stratifies them after 1 week of steroid treatment into two groups with distinct risks for treatment failure, 6-month nonrelapse mortality, and overall survival,” she said.
The biomarker algorithm, which includes measures of ST2 and REG3-alpha, was previously shown to predict day-28 treatment response and 6-month non-relapse mortality (NRM) when applied at day 7 post transplant before the onset of GVHD and at the time of diagnosis, said Ms. Major-Monfried, a third-year medical student at Icahn School of Medicine at Mount Sinai, New York.
For the current analysis, levels of the biomarkers were measured after 1 week of treatment in 378 patients with acute GVHD from 11 centers in the Mount Sinai Acute GVHD International Consortium.
In a test cohort that included 236 of the patients, the measurements were used to generate a new predicted probability, or treatment score, for 6-month NRM, which had a value between 0 and 1.
Of the 236 patients, 93 (39%) were considered to have high posttreatment probability of NRM, and the remaining patients (61%) had low posttreatment probability of NRM, based on their treatment scores.
“High-risk patients were significantly less likely to respond to treatment than low-risk patients,” she said, noting that very similar results were found in a validation cohort of the remaining 142 patients, which had a similar proportion of high- and low-risk patients as did the test cohort.
The overall 6-month NRM for patients treated for GVHD was 27% in the test cohort. When the biomarker algorithm was used to separate the cohort into high- and low-risk groups, the NRM rate was found to be approximately 4 times higher among the high-risk patients than among the low-risk patients.
Overall survival was also significantly worse among high- vs. low-risk patients in both the test and validation cohorts.
“We can conclude that the increased NRM seen in the high-risk groups can explain these large differences in overall survival,” Ms. Major-Monfried said.
Because treatment decisions are often made after 1 week based on early clinical response, she and her colleagues also explored whether treatment response after 1 week could similarly predict NRM.
In the test cohort, early response – which includes complete or partial response in GVHD symptoms after 1 week of steroids – was observed in 48% of patients, while 52% were early nonresponders. NRM occurred in 17% of the early responders, compared with 36% of the nonresponders in the test cohort, and similar results were found in the validation cohort.
“These differences are independent of biomarkers,” she noted. “These are solely based on observed clinical response.”
When the biomarker algorithm was applied, prediction of NRM was more precise.
“We started with the early responders,” she explained. “When we used the biomarker algorithm to stratify these patients into high- and low-risk groups, we found that 28% of early responders were actually high risk, and that they experienced 38% NRM – significantly higher than the 8% observed in low-risk patients. Similar results were found again in the validation cohort.”
When the biomarker algorithm was used to stratify patients who were nonresponders at 7 days into high- and low-risk groups, 50% were found to be low risk, and those patients experienced 17% NRM, significantly lower than the 57% seen in the high-risk patients. Similar results were again seen in the validation cohort.
“Early responders with high posttreatment probability have high NRM, and perhaps should not be tapered despite the improvement of their clinical symptoms, while early nonresponders with low posttreatment probability have lower NRM and may not need treatment escalation,” Ms. Major-Monfried said.
“In data not shown, many of these patients are actually what we could call ‘slow responders’ who ultimately fare well,” she noted.
Ms. Major-Monfried reported having no disclosures.
ORLANDO – A biomarker algorithm was better than was clinical response at predicting outcomes after 1 week of systemic steroid treatment for graft-versus-host disease, according to findings from a multicenter study.
The findings have implications for early decision making regarding treatment course, Hannah Major-Monfried reported at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
“The same GVHD algorithm that stratifies patients at day 7 after transplant, at [GVHD] diagnosis, also stratifies them after 1 week of steroid treatment into two groups with distinct risks for treatment failure, 6-month nonrelapse mortality, and overall survival,” she said.
The biomarker algorithm, which includes measures of ST2 and REG3-alpha, was previously shown to predict day-28 treatment response and 6-month non-relapse mortality (NRM) when applied at day 7 post transplant before the onset of GVHD and at the time of diagnosis, said Ms. Major-Monfried, a third-year medical student at Icahn School of Medicine at Mount Sinai, New York.
For the current analysis, levels of the biomarkers were measured after 1 week of treatment in 378 patients with acute GVHD from 11 centers in the Mount Sinai Acute GVHD International Consortium.
In a test cohort that included 236 of the patients, the measurements were used to generate a new predicted probability, or treatment score, for 6-month NRM, which had a value between 0 and 1.
Of the 236 patients, 93 (39%) were considered to have high posttreatment probability of NRM, and the remaining patients (61%) had low posttreatment probability of NRM, based on their treatment scores.
“High-risk patients were significantly less likely to respond to treatment than low-risk patients,” she said, noting that very similar results were found in a validation cohort of the remaining 142 patients, which had a similar proportion of high- and low-risk patients as did the test cohort.
The overall 6-month NRM for patients treated for GVHD was 27% in the test cohort. When the biomarker algorithm was used to separate the cohort into high- and low-risk groups, the NRM rate was found to be approximately 4 times higher among the high-risk patients than among the low-risk patients.
Overall survival was also significantly worse among high- vs. low-risk patients in both the test and validation cohorts.
“We can conclude that the increased NRM seen in the high-risk groups can explain these large differences in overall survival,” Ms. Major-Monfried said.
Because treatment decisions are often made after 1 week based on early clinical response, she and her colleagues also explored whether treatment response after 1 week could similarly predict NRM.
In the test cohort, early response – which includes complete or partial response in GVHD symptoms after 1 week of steroids – was observed in 48% of patients, while 52% were early nonresponders. NRM occurred in 17% of the early responders, compared with 36% of the nonresponders in the test cohort, and similar results were found in the validation cohort.
“These differences are independent of biomarkers,” she noted. “These are solely based on observed clinical response.”
When the biomarker algorithm was applied, prediction of NRM was more precise.
“We started with the early responders,” she explained. “When we used the biomarker algorithm to stratify these patients into high- and low-risk groups, we found that 28% of early responders were actually high risk, and that they experienced 38% NRM – significantly higher than the 8% observed in low-risk patients. Similar results were found again in the validation cohort.”
When the biomarker algorithm was used to stratify patients who were nonresponders at 7 days into high- and low-risk groups, 50% were found to be low risk, and those patients experienced 17% NRM, significantly lower than the 57% seen in the high-risk patients. Similar results were again seen in the validation cohort.
“Early responders with high posttreatment probability have high NRM, and perhaps should not be tapered despite the improvement of their clinical symptoms, while early nonresponders with low posttreatment probability have lower NRM and may not need treatment escalation,” Ms. Major-Monfried said.
“In data not shown, many of these patients are actually what we could call ‘slow responders’ who ultimately fare well,” she noted.
Ms. Major-Monfried reported having no disclosures.
ORLANDO – A biomarker algorithm was better than was clinical response at predicting outcomes after 1 week of systemic steroid treatment for graft-versus-host disease, according to findings from a multicenter study.
The findings have implications for early decision making regarding treatment course, Hannah Major-Monfried reported at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
“The same GVHD algorithm that stratifies patients at day 7 after transplant, at [GVHD] diagnosis, also stratifies them after 1 week of steroid treatment into two groups with distinct risks for treatment failure, 6-month nonrelapse mortality, and overall survival,” she said.
The biomarker algorithm, which includes measures of ST2 and REG3-alpha, was previously shown to predict day-28 treatment response and 6-month non-relapse mortality (NRM) when applied at day 7 post transplant before the onset of GVHD and at the time of diagnosis, said Ms. Major-Monfried, a third-year medical student at Icahn School of Medicine at Mount Sinai, New York.
For the current analysis, levels of the biomarkers were measured after 1 week of treatment in 378 patients with acute GVHD from 11 centers in the Mount Sinai Acute GVHD International Consortium.
In a test cohort that included 236 of the patients, the measurements were used to generate a new predicted probability, or treatment score, for 6-month NRM, which had a value between 0 and 1.
Of the 236 patients, 93 (39%) were considered to have high posttreatment probability of NRM, and the remaining patients (61%) had low posttreatment probability of NRM, based on their treatment scores.
“High-risk patients were significantly less likely to respond to treatment than low-risk patients,” she said, noting that very similar results were found in a validation cohort of the remaining 142 patients, which had a similar proportion of high- and low-risk patients as did the test cohort.
The overall 6-month NRM for patients treated for GVHD was 27% in the test cohort. When the biomarker algorithm was used to separate the cohort into high- and low-risk groups, the NRM rate was found to be approximately 4 times higher among the high-risk patients than among the low-risk patients.
Overall survival was also significantly worse among high- vs. low-risk patients in both the test and validation cohorts.
“We can conclude that the increased NRM seen in the high-risk groups can explain these large differences in overall survival,” Ms. Major-Monfried said.
Because treatment decisions are often made after 1 week based on early clinical response, she and her colleagues also explored whether treatment response after 1 week could similarly predict NRM.
In the test cohort, early response – which includes complete or partial response in GVHD symptoms after 1 week of steroids – was observed in 48% of patients, while 52% were early nonresponders. NRM occurred in 17% of the early responders, compared with 36% of the nonresponders in the test cohort, and similar results were found in the validation cohort.
“These differences are independent of biomarkers,” she noted. “These are solely based on observed clinical response.”
When the biomarker algorithm was applied, prediction of NRM was more precise.
“We started with the early responders,” she explained. “When we used the biomarker algorithm to stratify these patients into high- and low-risk groups, we found that 28% of early responders were actually high risk, and that they experienced 38% NRM – significantly higher than the 8% observed in low-risk patients. Similar results were found again in the validation cohort.”
When the biomarker algorithm was used to stratify patients who were nonresponders at 7 days into high- and low-risk groups, 50% were found to be low risk, and those patients experienced 17% NRM, significantly lower than the 57% seen in the high-risk patients. Similar results were again seen in the validation cohort.
“Early responders with high posttreatment probability have high NRM, and perhaps should not be tapered despite the improvement of their clinical symptoms, while early nonresponders with low posttreatment probability have lower NRM and may not need treatment escalation,” Ms. Major-Monfried said.
“In data not shown, many of these patients are actually what we could call ‘slow responders’ who ultimately fare well,” she noted.
Ms. Major-Monfried reported having no disclosures.
Key clinical point:
Major finding: High- vs. low-risk patients, based on the biomarker algorithm, had a fourfold higher rate of nonrelapse mortality.
Data source: A multicenter study of 378 patients.
Disclosures: Ms. Major-Monfried reported having no disclosures.
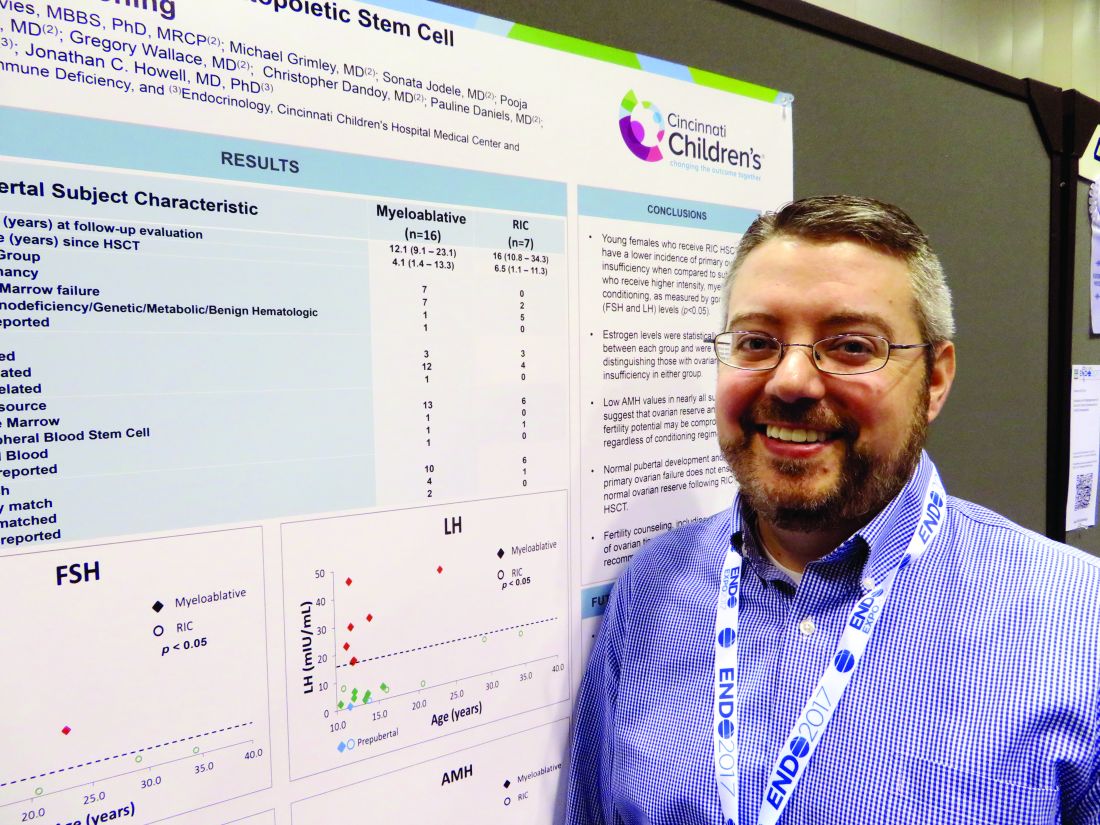
Reduced-intensity conditioning may not preserve fertility in young girls after bone marrow transplant
ORLANDO – Girls who undergo reduced-intensity conditioning for a bone marrow transplant may face fertility problems in the future, even if they experience an outwardly normal puberty.
In the first-ever study to compare high- and low-intensity chemotherapeutic conditioning regimens among young girls, significantly more who underwent the reduced-intensity regimen had normal estradiol, luteinizing hormone, and follicle-stimulating hormone compared with those who had high-intensity conditioning. But anti-Müllerian hormone was low or absent in almost all the girls, no matter which conditioning regimen they had, Jonathan C. Howell, MD, PhD, said at the annual meeting of the Endocrine Society.
While not a perfect predictor of future fertility, anti-Müllerian hormone is a good indicator of ovarian follicular reserve, said Dr. Howell, a pediatric endocrinologist at Cincinnati Children’s Hospital Medical Center.
Dr. Howell and his colleagues, Holly R. Hoefgen, MD, Kasiani C. Myers, MD, and Helen Oquendo-Del Toro, MD, all of Cincinnati Children’s Hospital Medical Center, are following 49 females aged 1-40 years who had preconditioning chemotherapy in advance of hematopoietic stem cell transplantation.
At the meeting, Dr. Howell reported data on 23 girls who were in puberty during their treatment (mean age 12 years). The mean follow-up was 4 years, but this varied widely, from 1 to 13 years. Most (16) had high-intensity myeloablation; the remainder had reduced-intensity conditioning. Diagnoses varied between the groups. Among those with high-intensity conditioning, malignancy and bone marrow failure were the most common indications (seven patients each); one patient had an immunodeficiency, and the cause was unknown for another.
Among those who had the reduced-intensity regimen, five had an immunodeficiency and two had bone marrow failure.
The discrepancy in diagnoses between the groups isn’t surprising, Dr. Howell said. “Diagnosis can dictate which treatment patients receive. People with malignancies or a prior history of leukemia or lymphoma often receive the high-intensity conditioning. You want to wipe out every single malignant cell.”
Reduced-intensity conditioning may be an option for patients with other problems such as bone marrow failure, immunodeficiencies, or genetic or metabolic problems. The less-intense regimen does confer some benefits, Dr. Howell noted. “The short-term need for intensive medical therapy while getting the stem cells is less. The medical benefit of these less-intense regimens is certainly there, but the long-term endocrine impact has yet to be defined.”
Most of the girls in the high-intensity regimen group (64%) had high follicle stimulating hormone and luteinizing hormone, suggesting primary ovarian failure; 71% of them also had low estradiol levels. However, all of these hormones were normal in the reduced-intensity group. But regardless of conditioning treatment, anti-Müllerian hormone was abnormally low in nearly all of the patients (87%). Only one girl with myeloablative conditioning and two girls with reduced intensity condition had normal anti-Müllerian levels. “This tells us that fertility potential may not be preserved, despite [their] getting the reduced-intensity conditioning,” Dr. Howell said.
The story here is only beginning to unfold, he said. “Fertility is defined as the ability to conceive a child, and that’s not something we have looked at yet. We would like to know the long-term outcomes of fertility in these patients, and whether they can conceive when they’re ready to start a family. Our goal is to follow these young women into their 20s and 30s, and to see if that’s an opportunity they are able to experience.”
The study is a cooperative project involving the hospital’s divisions of Pediatric and Adolescent Gynecology, Bone Marrow Transplantation and Immunology, and Endocrinology.
Neither Dr. Howell nor any of his colleagues had any financial disclosures.
Fertility preservation talks: The earlier, the better
A talk about fertility preservation can be the first step into a new future for families of children with a cancer diagnosis.
“Talking about your baby having a baby can be the farthest thing from your mind,” when you’re the parent of a child about to undergo cancer treatment, said Dr. Hoefgen. “But we know from survivors that this can be a very important issue in the future. We simply start by telling parents, ‘This will be important to your child at some point, and we want to talk about it now, while there is still something we may be able to do about it.’ ”
Dr. Hoefgen, a staff member at the hospital’s Comprehensive Fertility Care and Preservation Program, said parents “sometimes find it weird” to be talking about unborn grandchildren when they’re consumed with making critical decisions for their own child. But by asking them to consider that child’s long-term future, the discussion offers its own message of hope.
The talks always begin with a basic discussion of how cancer treatments can affect the reproductive organs. The hospital has a series of short animated videos that are very helpful in relaying the information. Another video in that series describes the different methods of fertility preservation: mature oocyte or sperm harvesting, or, for younger patients, removing and freezing ovarian and testicular tissue. Parents and children can watch them together, get grounding in the basics, and be prepared for a productive conversation.
Talks always include the team oncologist, who creates a specialized risk assessment for each patient. The group discusses each preservation method, the risks and benefits, and the cost. But the talks are exploratory, too, helping both clinicians and families understand what’s most important to them, she said.
“Common things that we typically talk about are genetics, religion, and ethics – which may mean different things to different families.”
Dr. Hoefgen and her team reach out to more than 95% of families that face a pediatric cancer diagnosis. After the in-depth discussions, she said, about 20% decide to investigate some form of fertility preservation.
“The most important thing is having the conversation early, while we still have options,” she said.
Dr. Hoefgen had no financial disclosures.
ORLANDO – Girls who undergo reduced-intensity conditioning for a bone marrow transplant may face fertility problems in the future, even if they experience an outwardly normal puberty.
In the first-ever study to compare high- and low-intensity chemotherapeutic conditioning regimens among young girls, significantly more who underwent the reduced-intensity regimen had normal estradiol, luteinizing hormone, and follicle-stimulating hormone compared with those who had high-intensity conditioning. But anti-Müllerian hormone was low or absent in almost all the girls, no matter which conditioning regimen they had, Jonathan C. Howell, MD, PhD, said at the annual meeting of the Endocrine Society.
While not a perfect predictor of future fertility, anti-Müllerian hormone is a good indicator of ovarian follicular reserve, said Dr. Howell, a pediatric endocrinologist at Cincinnati Children’s Hospital Medical Center.
Dr. Howell and his colleagues, Holly R. Hoefgen, MD, Kasiani C. Myers, MD, and Helen Oquendo-Del Toro, MD, all of Cincinnati Children’s Hospital Medical Center, are following 49 females aged 1-40 years who had preconditioning chemotherapy in advance of hematopoietic stem cell transplantation.
At the meeting, Dr. Howell reported data on 23 girls who were in puberty during their treatment (mean age 12 years). The mean follow-up was 4 years, but this varied widely, from 1 to 13 years. Most (16) had high-intensity myeloablation; the remainder had reduced-intensity conditioning. Diagnoses varied between the groups. Among those with high-intensity conditioning, malignancy and bone marrow failure were the most common indications (seven patients each); one patient had an immunodeficiency, and the cause was unknown for another.
Among those who had the reduced-intensity regimen, five had an immunodeficiency and two had bone marrow failure.
The discrepancy in diagnoses between the groups isn’t surprising, Dr. Howell said. “Diagnosis can dictate which treatment patients receive. People with malignancies or a prior history of leukemia or lymphoma often receive the high-intensity conditioning. You want to wipe out every single malignant cell.”
Reduced-intensity conditioning may be an option for patients with other problems such as bone marrow failure, immunodeficiencies, or genetic or metabolic problems. The less-intense regimen does confer some benefits, Dr. Howell noted. “The short-term need for intensive medical therapy while getting the stem cells is less. The medical benefit of these less-intense regimens is certainly there, but the long-term endocrine impact has yet to be defined.”
Most of the girls in the high-intensity regimen group (64%) had high follicle stimulating hormone and luteinizing hormone, suggesting primary ovarian failure; 71% of them also had low estradiol levels. However, all of these hormones were normal in the reduced-intensity group. But regardless of conditioning treatment, anti-Müllerian hormone was abnormally low in nearly all of the patients (87%). Only one girl with myeloablative conditioning and two girls with reduced intensity condition had normal anti-Müllerian levels. “This tells us that fertility potential may not be preserved, despite [their] getting the reduced-intensity conditioning,” Dr. Howell said.
The story here is only beginning to unfold, he said. “Fertility is defined as the ability to conceive a child, and that’s not something we have looked at yet. We would like to know the long-term outcomes of fertility in these patients, and whether they can conceive when they’re ready to start a family. Our goal is to follow these young women into their 20s and 30s, and to see if that’s an opportunity they are able to experience.”
The study is a cooperative project involving the hospital’s divisions of Pediatric and Adolescent Gynecology, Bone Marrow Transplantation and Immunology, and Endocrinology.
Neither Dr. Howell nor any of his colleagues had any financial disclosures.
Fertility preservation talks: The earlier, the better
A talk about fertility preservation can be the first step into a new future for families of children with a cancer diagnosis.
“Talking about your baby having a baby can be the farthest thing from your mind,” when you’re the parent of a child about to undergo cancer treatment, said Dr. Hoefgen. “But we know from survivors that this can be a very important issue in the future. We simply start by telling parents, ‘This will be important to your child at some point, and we want to talk about it now, while there is still something we may be able to do about it.’ ”
Dr. Hoefgen, a staff member at the hospital’s Comprehensive Fertility Care and Preservation Program, said parents “sometimes find it weird” to be talking about unborn grandchildren when they’re consumed with making critical decisions for their own child. But by asking them to consider that child’s long-term future, the discussion offers its own message of hope.
The talks always begin with a basic discussion of how cancer treatments can affect the reproductive organs. The hospital has a series of short animated videos that are very helpful in relaying the information. Another video in that series describes the different methods of fertility preservation: mature oocyte or sperm harvesting, or, for younger patients, removing and freezing ovarian and testicular tissue. Parents and children can watch them together, get grounding in the basics, and be prepared for a productive conversation.
Talks always include the team oncologist, who creates a specialized risk assessment for each patient. The group discusses each preservation method, the risks and benefits, and the cost. But the talks are exploratory, too, helping both clinicians and families understand what’s most important to them, she said.
“Common things that we typically talk about are genetics, religion, and ethics – which may mean different things to different families.”
Dr. Hoefgen and her team reach out to more than 95% of families that face a pediatric cancer diagnosis. After the in-depth discussions, she said, about 20% decide to investigate some form of fertility preservation.
“The most important thing is having the conversation early, while we still have options,” she said.
Dr. Hoefgen had no financial disclosures.
ORLANDO – Girls who undergo reduced-intensity conditioning for a bone marrow transplant may face fertility problems in the future, even if they experience an outwardly normal puberty.
In the first-ever study to compare high- and low-intensity chemotherapeutic conditioning regimens among young girls, significantly more who underwent the reduced-intensity regimen had normal estradiol, luteinizing hormone, and follicle-stimulating hormone compared with those who had high-intensity conditioning. But anti-Müllerian hormone was low or absent in almost all the girls, no matter which conditioning regimen they had, Jonathan C. Howell, MD, PhD, said at the annual meeting of the Endocrine Society.
While not a perfect predictor of future fertility, anti-Müllerian hormone is a good indicator of ovarian follicular reserve, said Dr. Howell, a pediatric endocrinologist at Cincinnati Children’s Hospital Medical Center.
Dr. Howell and his colleagues, Holly R. Hoefgen, MD, Kasiani C. Myers, MD, and Helen Oquendo-Del Toro, MD, all of Cincinnati Children’s Hospital Medical Center, are following 49 females aged 1-40 years who had preconditioning chemotherapy in advance of hematopoietic stem cell transplantation.
At the meeting, Dr. Howell reported data on 23 girls who were in puberty during their treatment (mean age 12 years). The mean follow-up was 4 years, but this varied widely, from 1 to 13 years. Most (16) had high-intensity myeloablation; the remainder had reduced-intensity conditioning. Diagnoses varied between the groups. Among those with high-intensity conditioning, malignancy and bone marrow failure were the most common indications (seven patients each); one patient had an immunodeficiency, and the cause was unknown for another.
Among those who had the reduced-intensity regimen, five had an immunodeficiency and two had bone marrow failure.
The discrepancy in diagnoses between the groups isn’t surprising, Dr. Howell said. “Diagnosis can dictate which treatment patients receive. People with malignancies or a prior history of leukemia or lymphoma often receive the high-intensity conditioning. You want to wipe out every single malignant cell.”
Reduced-intensity conditioning may be an option for patients with other problems such as bone marrow failure, immunodeficiencies, or genetic or metabolic problems. The less-intense regimen does confer some benefits, Dr. Howell noted. “The short-term need for intensive medical therapy while getting the stem cells is less. The medical benefit of these less-intense regimens is certainly there, but the long-term endocrine impact has yet to be defined.”
Most of the girls in the high-intensity regimen group (64%) had high follicle stimulating hormone and luteinizing hormone, suggesting primary ovarian failure; 71% of them also had low estradiol levels. However, all of these hormones were normal in the reduced-intensity group. But regardless of conditioning treatment, anti-Müllerian hormone was abnormally low in nearly all of the patients (87%). Only one girl with myeloablative conditioning and two girls with reduced intensity condition had normal anti-Müllerian levels. “This tells us that fertility potential may not be preserved, despite [their] getting the reduced-intensity conditioning,” Dr. Howell said.
The story here is only beginning to unfold, he said. “Fertility is defined as the ability to conceive a child, and that’s not something we have looked at yet. We would like to know the long-term outcomes of fertility in these patients, and whether they can conceive when they’re ready to start a family. Our goal is to follow these young women into their 20s and 30s, and to see if that’s an opportunity they are able to experience.”
The study is a cooperative project involving the hospital’s divisions of Pediatric and Adolescent Gynecology, Bone Marrow Transplantation and Immunology, and Endocrinology.
Neither Dr. Howell nor any of his colleagues had any financial disclosures.
Fertility preservation talks: The earlier, the better
A talk about fertility preservation can be the first step into a new future for families of children with a cancer diagnosis.
“Talking about your baby having a baby can be the farthest thing from your mind,” when you’re the parent of a child about to undergo cancer treatment, said Dr. Hoefgen. “But we know from survivors that this can be a very important issue in the future. We simply start by telling parents, ‘This will be important to your child at some point, and we want to talk about it now, while there is still something we may be able to do about it.’ ”
Dr. Hoefgen, a staff member at the hospital’s Comprehensive Fertility Care and Preservation Program, said parents “sometimes find it weird” to be talking about unborn grandchildren when they’re consumed with making critical decisions for their own child. But by asking them to consider that child’s long-term future, the discussion offers its own message of hope.
The talks always begin with a basic discussion of how cancer treatments can affect the reproductive organs. The hospital has a series of short animated videos that are very helpful in relaying the information. Another video in that series describes the different methods of fertility preservation: mature oocyte or sperm harvesting, or, for younger patients, removing and freezing ovarian and testicular tissue. Parents and children can watch them together, get grounding in the basics, and be prepared for a productive conversation.
Talks always include the team oncologist, who creates a specialized risk assessment for each patient. The group discusses each preservation method, the risks and benefits, and the cost. But the talks are exploratory, too, helping both clinicians and families understand what’s most important to them, she said.
“Common things that we typically talk about are genetics, religion, and ethics – which may mean different things to different families.”
Dr. Hoefgen and her team reach out to more than 95% of families that face a pediatric cancer diagnosis. After the in-depth discussions, she said, about 20% decide to investigate some form of fertility preservation.
“The most important thing is having the conversation early, while we still have options,” she said.
Dr. Hoefgen had no financial disclosures.
AT ENDO 2017
Key clinical point:
Major finding: Anti-Müllerian hormone was abnormally low or absent in all treated girls, whether they had reduced-intensity or high-intensity conditioning.
Data source: The prospective study is following 49 females aged 1-40 years.
Disclosures: Neither Dr. Howell nor any of his colleagues had any financial disclosures.
Three factors linked to rhinovirus pneumonia in HCT patients
ORLANDO – For patients who have received hematopoietic cell transplants, a rhinovirus infection can become much more than a cold.
“It holds true that rhinovirus is just as likely to be associated with mortality as are other respiratory viruses” among HCT recipients, Alpana Waghmare, MD, said at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
In a new retrospective study, Dr. Waghmare and her coinvestigators found that the median time for a rhinovirus infection to progress from an upper to a lower respiratory tract infection was about 2 weeks among post-HCT patients.
Clinical and demographic risk factors for progression to lower respiratory tract infection included higher levels of steroid use (2 mg/kg per day or more) before developing the upper respiratory infection, a low white blood cell count, and a low monocyte count, said Dr. Waghmare, an infectious disease specialist and professor of pediatrics at the University of Washington, Seattle.
Of 3,445 HCT patients treated at the university center during the 6-year study, 732 patients (21%) were positive for human rhinovirus. Patients were classified as having upper respiratory infections if they had a PCR-positive nasal swab.
Patients were classed in one of three categories for potential lower respiratory infections: Proven lower respiratory infections were those detected by bronchoalveolar lavage or biopsy in patients who had a new radiographic abnormality. Probable lower respiratory infections were those with positive findings on bronchoalveolar lavage or biopsy but without radiographic changes. In possible lower respiratory infections, patients had upper tract virus detected on nasal swabs but did have a new radiographic abnormality.
Among the patients positive for human rhinovirus, 85% (665 patients) presented with upper respiratory infections and 15% (117 patients) with lower respiratory tract infections. By day 90, 16% of patients progressed from upper to lower respiratory tract infections. The median time to progression was 13.5 days. Progression to proven lower respiratory tract infection affected 5% of the HCT recipients.
In multivariable analytic models, a minimum white blood cell count of 1,000 or less was associated with a hazard ratio (HR) of 2.21 for progression to lower respiratory tract infection. A minimum monocyte count of 1,000 or less was associated with a HR of 3.66 for progression to lower respiratory tract infection.
The model also found a HR of 3.37 for lower respiratory tract infection with steroid use of 2 mg/kg per day or more. The patient’s conditioning regimen and donor type were not significantly associated with risk of progression to lower respiratory infection.
Viral copathogens, prior respiratory virus episodes, and the duration of time since HCT were not associated with risk of progress to lower respiratory infections. Neither were patient age, baseline lung function, and the year the transplant occurred.
“These data provide an initial framework for patient risk stratification and the development of rational prevention and treatment strategies in HCT recipients,” she said.
Dr. Waghmare reported receiving research funding from Aviragen, the maker of vapendavir, an investigational drug for human rhinovirus infection, and Gilead Sciences.
[email protected]
On Twitter @karioakes
ORLANDO – For patients who have received hematopoietic cell transplants, a rhinovirus infection can become much more than a cold.
“It holds true that rhinovirus is just as likely to be associated with mortality as are other respiratory viruses” among HCT recipients, Alpana Waghmare, MD, said at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
In a new retrospective study, Dr. Waghmare and her coinvestigators found that the median time for a rhinovirus infection to progress from an upper to a lower respiratory tract infection was about 2 weeks among post-HCT patients.
Clinical and demographic risk factors for progression to lower respiratory tract infection included higher levels of steroid use (2 mg/kg per day or more) before developing the upper respiratory infection, a low white blood cell count, and a low monocyte count, said Dr. Waghmare, an infectious disease specialist and professor of pediatrics at the University of Washington, Seattle.
Of 3,445 HCT patients treated at the university center during the 6-year study, 732 patients (21%) were positive for human rhinovirus. Patients were classified as having upper respiratory infections if they had a PCR-positive nasal swab.
Patients were classed in one of three categories for potential lower respiratory infections: Proven lower respiratory infections were those detected by bronchoalveolar lavage or biopsy in patients who had a new radiographic abnormality. Probable lower respiratory infections were those with positive findings on bronchoalveolar lavage or biopsy but without radiographic changes. In possible lower respiratory infections, patients had upper tract virus detected on nasal swabs but did have a new radiographic abnormality.
Among the patients positive for human rhinovirus, 85% (665 patients) presented with upper respiratory infections and 15% (117 patients) with lower respiratory tract infections. By day 90, 16% of patients progressed from upper to lower respiratory tract infections. The median time to progression was 13.5 days. Progression to proven lower respiratory tract infection affected 5% of the HCT recipients.
In multivariable analytic models, a minimum white blood cell count of 1,000 or less was associated with a hazard ratio (HR) of 2.21 for progression to lower respiratory tract infection. A minimum monocyte count of 1,000 or less was associated with a HR of 3.66 for progression to lower respiratory tract infection.
The model also found a HR of 3.37 for lower respiratory tract infection with steroid use of 2 mg/kg per day or more. The patient’s conditioning regimen and donor type were not significantly associated with risk of progression to lower respiratory infection.
Viral copathogens, prior respiratory virus episodes, and the duration of time since HCT were not associated with risk of progress to lower respiratory infections. Neither were patient age, baseline lung function, and the year the transplant occurred.
“These data provide an initial framework for patient risk stratification and the development of rational prevention and treatment strategies in HCT recipients,” she said.
Dr. Waghmare reported receiving research funding from Aviragen, the maker of vapendavir, an investigational drug for human rhinovirus infection, and Gilead Sciences.
[email protected]
On Twitter @karioakes
ORLANDO – For patients who have received hematopoietic cell transplants, a rhinovirus infection can become much more than a cold.
“It holds true that rhinovirus is just as likely to be associated with mortality as are other respiratory viruses” among HCT recipients, Alpana Waghmare, MD, said at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
In a new retrospective study, Dr. Waghmare and her coinvestigators found that the median time for a rhinovirus infection to progress from an upper to a lower respiratory tract infection was about 2 weeks among post-HCT patients.
Clinical and demographic risk factors for progression to lower respiratory tract infection included higher levels of steroid use (2 mg/kg per day or more) before developing the upper respiratory infection, a low white blood cell count, and a low monocyte count, said Dr. Waghmare, an infectious disease specialist and professor of pediatrics at the University of Washington, Seattle.
Of 3,445 HCT patients treated at the university center during the 6-year study, 732 patients (21%) were positive for human rhinovirus. Patients were classified as having upper respiratory infections if they had a PCR-positive nasal swab.
Patients were classed in one of three categories for potential lower respiratory infections: Proven lower respiratory infections were those detected by bronchoalveolar lavage or biopsy in patients who had a new radiographic abnormality. Probable lower respiratory infections were those with positive findings on bronchoalveolar lavage or biopsy but without radiographic changes. In possible lower respiratory infections, patients had upper tract virus detected on nasal swabs but did have a new radiographic abnormality.
Among the patients positive for human rhinovirus, 85% (665 patients) presented with upper respiratory infections and 15% (117 patients) with lower respiratory tract infections. By day 90, 16% of patients progressed from upper to lower respiratory tract infections. The median time to progression was 13.5 days. Progression to proven lower respiratory tract infection affected 5% of the HCT recipients.
In multivariable analytic models, a minimum white blood cell count of 1,000 or less was associated with a hazard ratio (HR) of 2.21 for progression to lower respiratory tract infection. A minimum monocyte count of 1,000 or less was associated with a HR of 3.66 for progression to lower respiratory tract infection.
The model also found a HR of 3.37 for lower respiratory tract infection with steroid use of 2 mg/kg per day or more. The patient’s conditioning regimen and donor type were not significantly associated with risk of progression to lower respiratory infection.
Viral copathogens, prior respiratory virus episodes, and the duration of time since HCT were not associated with risk of progress to lower respiratory infections. Neither were patient age, baseline lung function, and the year the transplant occurred.
“These data provide an initial framework for patient risk stratification and the development of rational prevention and treatment strategies in HCT recipients,” she said.
Dr. Waghmare reported receiving research funding from Aviragen, the maker of vapendavir, an investigational drug for human rhinovirus infection, and Gilead Sciences.
[email protected]
On Twitter @karioakes
AT THE BMT TANDEM MEETINGS
Key clinical point:
Major finding: Of 3,445 HCT patients, 732 patients (21%) were positive for human rhinovirus.
Data source: Single-center, 6-year retrospective study of 732 HCT patients with human rhinovirus infection.
Disclosures: Dr. Waghmare reported receiving research funding from Aviragen, the maker of vapendavir, an investigational drug for human rhinovirus infection, and Gilead Sciences.
Transplantation plus VRD tops VRD alone in myeloma
Adding high-dose chemotherapy and autologous stem cell transplantation to two cycles of bortezomib, lenalidomide, and dexamethasone (VRD) significantly improved progression-free survival in adults with newly diagnosed multiple myeloma, according to new analyses from the multicenter, randomized, phase III IFM 2009 Study.
Median PFS was 50 months in the transplantation group, versus 36 months when patients only received five cycles of VRD for consolidation (adjusted hazard ratio for disease progression or death, 0.65; 95% confidence interval, 0.53 to 0.80; P less than .001), reported Michel Attal, MD, of Institut Universitaire du Cancer de Toulouse-Oncopole in Toulouse, France, and his associates. “Transplantation was also associated with a higher rate of complete response, a lower rate of minimal residual disease detection, and a longer median time to progression,” they wrote (N Engl J Med. 2017 April 6. doi: 10.1056/NEJMoa1611750).
Transplantation did not improve 4-year overall survival (81% with transplantation plus VRD and 82% with VRD only), perhaps because VRD-only patients had the option of salvage transplantation if their disease progressed, the investigators said. In addition, grade 3 or 4 neutropenias were significantly more common with transplantation than otherwise (92% versus 47%), as were grade 3 or 4 gastrointestinal disorders (28% versus 7%) and infections (20% versus 9%). However, the groups did not significantly differ in terms of treatment-related deaths, second primary cancers, thromboembolic events, or peripheral neuropathy.
Before the advent of immunomodulatory drugs and proteasome inhibitors, several randomized trials showed that the benefits of high-dose chemotherapy with autologous stem cell transplantation outperformed conventional chemotherapy in multiple myeloma. For IFM 2009, 700 newly diagnosed patients aged up to 65 years received three 21-day cycles of VRD induction: bortezomib 1.3 mg/m2 on days 1, 4, 8, and 11; lenalidomide 25 mg on days 1-14; and dexamethasone 20 mg on days 1, 2, 4, 5, 8, 9, 11, and 12. Consolidation consisted of either five more VRD cycles with dexamethasone reduced to 10 mg/day, or high-dose (200 mg/m2) melphalan plus transplantation followed by two VRD cycles with the reduced dexamethasone dose. Both arms received 1 year of lenalidomide maintenance (10 mg/day for 3 months, followed by 15/mg day, depending on side effects).
Transplantation was associated with a significantly higher likelihood of complete response (59% versus 48%; P = .03) and undetectable minimal residual disease (79% versus 65%; P less than .001). Undetectable minimal residual disease, in turn, predicted longer PFS (adjusted hazard ratio, 0.30; P = .001). In contrast, age, sex, isotype of the monoclonal component, International Staging System disease stage, and cytogenetic risk profile did not significantly affect PFS among transplant patients.
The PFS benefit of transplantation “must be weighed against the increased risk of toxic effects associated with high-dose chemotherapy plus transplantation, especially since we found that later transplantation might be as effective as early transplantation in securing long- term survival,” the researchers said. “Our results suggest that the use of a combination therapy that incorporates newer proteasome inhibitors, next-generation immunomodulatory drugs, and potent monoclonal antibodies along with transplantation tailored according to minimal residual disease detection could further improve outcomes among adults up to 65 years of age who have multiple myeloma.”
Celgene, Janssen, the French Ministry of Health Programme Hospitalier de Recherche Clinique, and the French National Research Agency funded the study. Dr. Attal had no conflicts. Nine coinvestigators disclosed ties to Celgene, Janssen, and several other pharmaceutical companies. Two coinvestigators disclosed ownership or founder roles in OncoPep, one of whom disclosed patent licensure to OncoPep for a multipeptide vaccine. The other investigators had no conflicts.
In the IFM 2009 trial, the planned treatments were realistic. The combination of immunomodulatory drugs and proteasome inhibitors is associated with high response rates similar to those achieved with transplantation, whereas previous trials had used less effective chemotherapy regimens as comparators.
[Transplantation] resulted in a deeper and longer initial treatment response than did nontransplantation. However, the benefits of transplantation were more modest than some might have hoped, and it did not appear to be curative.
A question remains as to whether all patients with multiple myeloma should undergo immediate autologous stem-cell transplantation, since the use of delayed transplantation resulted in similar overall survival, with some patients not needing transplantation to date. The observed lack of a survival benefit for early transplantation was consistent with previous results that suggested salvage transplantation is an equalizer in this regard.
Charles A. Schiffer, MD, and Jeffrey A. Zonder, MD, are with Karmanos Cancer Institute, Wayne State University, Detroit, Mich. Dr. Zonder is a member of the editorial advisory board of Hematology News. Their comments were taken from an editorial (N Engl J Med. 2017 April 6. doi: 10.1056/NEJMe1700453).
In the IFM 2009 trial, the planned treatments were realistic. The combination of immunomodulatory drugs and proteasome inhibitors is associated with high response rates similar to those achieved with transplantation, whereas previous trials had used less effective chemotherapy regimens as comparators.
[Transplantation] resulted in a deeper and longer initial treatment response than did nontransplantation. However, the benefits of transplantation were more modest than some might have hoped, and it did not appear to be curative.
A question remains as to whether all patients with multiple myeloma should undergo immediate autologous stem-cell transplantation, since the use of delayed transplantation resulted in similar overall survival, with some patients not needing transplantation to date. The observed lack of a survival benefit for early transplantation was consistent with previous results that suggested salvage transplantation is an equalizer in this regard.
Charles A. Schiffer, MD, and Jeffrey A. Zonder, MD, are with Karmanos Cancer Institute, Wayne State University, Detroit, Mich. Dr. Zonder is a member of the editorial advisory board of Hematology News. Their comments were taken from an editorial (N Engl J Med. 2017 April 6. doi: 10.1056/NEJMe1700453).
In the IFM 2009 trial, the planned treatments were realistic. The combination of immunomodulatory drugs and proteasome inhibitors is associated with high response rates similar to those achieved with transplantation, whereas previous trials had used less effective chemotherapy regimens as comparators.
[Transplantation] resulted in a deeper and longer initial treatment response than did nontransplantation. However, the benefits of transplantation were more modest than some might have hoped, and it did not appear to be curative.
A question remains as to whether all patients with multiple myeloma should undergo immediate autologous stem-cell transplantation, since the use of delayed transplantation resulted in similar overall survival, with some patients not needing transplantation to date. The observed lack of a survival benefit for early transplantation was consistent with previous results that suggested salvage transplantation is an equalizer in this regard.
Charles A. Schiffer, MD, and Jeffrey A. Zonder, MD, are with Karmanos Cancer Institute, Wayne State University, Detroit, Mich. Dr. Zonder is a member of the editorial advisory board of Hematology News. Their comments were taken from an editorial (N Engl J Med. 2017 April 6. doi: 10.1056/NEJMe1700453).
Adding high-dose chemotherapy and autologous stem cell transplantation to two cycles of bortezomib, lenalidomide, and dexamethasone (VRD) significantly improved progression-free survival in adults with newly diagnosed multiple myeloma, according to new analyses from the multicenter, randomized, phase III IFM 2009 Study.
Median PFS was 50 months in the transplantation group, versus 36 months when patients only received five cycles of VRD for consolidation (adjusted hazard ratio for disease progression or death, 0.65; 95% confidence interval, 0.53 to 0.80; P less than .001), reported Michel Attal, MD, of Institut Universitaire du Cancer de Toulouse-Oncopole in Toulouse, France, and his associates. “Transplantation was also associated with a higher rate of complete response, a lower rate of minimal residual disease detection, and a longer median time to progression,” they wrote (N Engl J Med. 2017 April 6. doi: 10.1056/NEJMoa1611750).
Transplantation did not improve 4-year overall survival (81% with transplantation plus VRD and 82% with VRD only), perhaps because VRD-only patients had the option of salvage transplantation if their disease progressed, the investigators said. In addition, grade 3 or 4 neutropenias were significantly more common with transplantation than otherwise (92% versus 47%), as were grade 3 or 4 gastrointestinal disorders (28% versus 7%) and infections (20% versus 9%). However, the groups did not significantly differ in terms of treatment-related deaths, second primary cancers, thromboembolic events, or peripheral neuropathy.
Before the advent of immunomodulatory drugs and proteasome inhibitors, several randomized trials showed that the benefits of high-dose chemotherapy with autologous stem cell transplantation outperformed conventional chemotherapy in multiple myeloma. For IFM 2009, 700 newly diagnosed patients aged up to 65 years received three 21-day cycles of VRD induction: bortezomib 1.3 mg/m2 on days 1, 4, 8, and 11; lenalidomide 25 mg on days 1-14; and dexamethasone 20 mg on days 1, 2, 4, 5, 8, 9, 11, and 12. Consolidation consisted of either five more VRD cycles with dexamethasone reduced to 10 mg/day, or high-dose (200 mg/m2) melphalan plus transplantation followed by two VRD cycles with the reduced dexamethasone dose. Both arms received 1 year of lenalidomide maintenance (10 mg/day for 3 months, followed by 15/mg day, depending on side effects).
Transplantation was associated with a significantly higher likelihood of complete response (59% versus 48%; P = .03) and undetectable minimal residual disease (79% versus 65%; P less than .001). Undetectable minimal residual disease, in turn, predicted longer PFS (adjusted hazard ratio, 0.30; P = .001). In contrast, age, sex, isotype of the monoclonal component, International Staging System disease stage, and cytogenetic risk profile did not significantly affect PFS among transplant patients.
The PFS benefit of transplantation “must be weighed against the increased risk of toxic effects associated with high-dose chemotherapy plus transplantation, especially since we found that later transplantation might be as effective as early transplantation in securing long- term survival,” the researchers said. “Our results suggest that the use of a combination therapy that incorporates newer proteasome inhibitors, next-generation immunomodulatory drugs, and potent monoclonal antibodies along with transplantation tailored according to minimal residual disease detection could further improve outcomes among adults up to 65 years of age who have multiple myeloma.”
Celgene, Janssen, the French Ministry of Health Programme Hospitalier de Recherche Clinique, and the French National Research Agency funded the study. Dr. Attal had no conflicts. Nine coinvestigators disclosed ties to Celgene, Janssen, and several other pharmaceutical companies. Two coinvestigators disclosed ownership or founder roles in OncoPep, one of whom disclosed patent licensure to OncoPep for a multipeptide vaccine. The other investigators had no conflicts.
Adding high-dose chemotherapy and autologous stem cell transplantation to two cycles of bortezomib, lenalidomide, and dexamethasone (VRD) significantly improved progression-free survival in adults with newly diagnosed multiple myeloma, according to new analyses from the multicenter, randomized, phase III IFM 2009 Study.
Median PFS was 50 months in the transplantation group, versus 36 months when patients only received five cycles of VRD for consolidation (adjusted hazard ratio for disease progression or death, 0.65; 95% confidence interval, 0.53 to 0.80; P less than .001), reported Michel Attal, MD, of Institut Universitaire du Cancer de Toulouse-Oncopole in Toulouse, France, and his associates. “Transplantation was also associated with a higher rate of complete response, a lower rate of minimal residual disease detection, and a longer median time to progression,” they wrote (N Engl J Med. 2017 April 6. doi: 10.1056/NEJMoa1611750).
Transplantation did not improve 4-year overall survival (81% with transplantation plus VRD and 82% with VRD only), perhaps because VRD-only patients had the option of salvage transplantation if their disease progressed, the investigators said. In addition, grade 3 or 4 neutropenias were significantly more common with transplantation than otherwise (92% versus 47%), as were grade 3 or 4 gastrointestinal disorders (28% versus 7%) and infections (20% versus 9%). However, the groups did not significantly differ in terms of treatment-related deaths, second primary cancers, thromboembolic events, or peripheral neuropathy.
Before the advent of immunomodulatory drugs and proteasome inhibitors, several randomized trials showed that the benefits of high-dose chemotherapy with autologous stem cell transplantation outperformed conventional chemotherapy in multiple myeloma. For IFM 2009, 700 newly diagnosed patients aged up to 65 years received three 21-day cycles of VRD induction: bortezomib 1.3 mg/m2 on days 1, 4, 8, and 11; lenalidomide 25 mg on days 1-14; and dexamethasone 20 mg on days 1, 2, 4, 5, 8, 9, 11, and 12. Consolidation consisted of either five more VRD cycles with dexamethasone reduced to 10 mg/day, or high-dose (200 mg/m2) melphalan plus transplantation followed by two VRD cycles with the reduced dexamethasone dose. Both arms received 1 year of lenalidomide maintenance (10 mg/day for 3 months, followed by 15/mg day, depending on side effects).
Transplantation was associated with a significantly higher likelihood of complete response (59% versus 48%; P = .03) and undetectable minimal residual disease (79% versus 65%; P less than .001). Undetectable minimal residual disease, in turn, predicted longer PFS (adjusted hazard ratio, 0.30; P = .001). In contrast, age, sex, isotype of the monoclonal component, International Staging System disease stage, and cytogenetic risk profile did not significantly affect PFS among transplant patients.
The PFS benefit of transplantation “must be weighed against the increased risk of toxic effects associated with high-dose chemotherapy plus transplantation, especially since we found that later transplantation might be as effective as early transplantation in securing long- term survival,” the researchers said. “Our results suggest that the use of a combination therapy that incorporates newer proteasome inhibitors, next-generation immunomodulatory drugs, and potent monoclonal antibodies along with transplantation tailored according to minimal residual disease detection could further improve outcomes among adults up to 65 years of age who have multiple myeloma.”
Celgene, Janssen, the French Ministry of Health Programme Hospitalier de Recherche Clinique, and the French National Research Agency funded the study. Dr. Attal had no conflicts. Nine coinvestigators disclosed ties to Celgene, Janssen, and several other pharmaceutical companies. Two coinvestigators disclosed ownership or founder roles in OncoPep, one of whom disclosed patent licensure to OncoPep for a multipeptide vaccine. The other investigators had no conflicts.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: High-dose chemotherapy and autologous stem cell transplantation plus bortezomib, lenalidomide, and dexamethasone (VRD) outperformed consolidation with VRD only in adults with newly diagnosed multiple myeloma.
Major finding: Median progression-free survival was 50 months with transplantation plus VRD and 36 months with VRD only (adjusted hazard ratio for disease progression or death, 0.65; P less than .001).
Data source: A randomized, multicenter, open-label, phase III trial of 700 patients with multiple myeloma.
Disclosures: Celgene, Janssen, the French Ministry of Health Programme Hospitalier de Recherche Clinique, and the French National Research Agency funded the study. Dr. Attal had no conflicts. Nine coinvestigators disclosed ties to Celgene, Janssen, and several other pharmaceutical companies. Two coinvestigators disclosed ownership or founder roles in OncoPep, one of whom disclosed patent licensure to OncoPep for a multipeptide vaccine. The other investigators had no conflicts.
Cell sheet transplantation improves ischemic cardiomyopathy at 1 year
For patients with ischemic cardiomyopathy (ICM), transplanting sheets of autologous myoblasts onto the epicardial surface was safe and led to “marked” improvements in functional and laboratory measures, investigators reported.
However, cell sheet transplantation was much less effective for patients with dilated cardiomyopathy in this phase I trial, wrote Shigeru Miyagawa, MD, PhD, of Osaka (Japan) University, and his associates in the Journal of the American Heart Association. The findings merit additional follow-up in larger clinical studies, the investigators asserted.
Heart failure remains life threatening even with the best approved treatments. Previously, the researchers transplanted scaffold-free sheets of autologous myoblasts derived from skeletal muscle into small and large animals with heart failure and found that this approach healed severely damaged myocardium. Transplantation caused secreted cytokines to induce native regeneration through paracrine effects, Dr. Miyagawa noted in a prior review article (BioMed Research International. 2013. doi: 10.1155/2013/583912).
To evaluate this approach in humans, the researchers transplanted 15 patients with ischemic cardiomyopathy and 12 patients with dilated cardiomyopathy (DCM). Patients were 20-75 years old, had ejection fractions under 35% and New York Heart Association (NYHA) classifications of II or more, and were responding inadequately to standard treatment with a beta-blocker or ACE inhibitor and an angiotensin receptor blocker. The sole intervention was transplantation of the cell sheet over the left ventricular-free wall during left thoracotomy (J Am Heart Assoc. 2017 Apr 5. doi: 10.1161/JAHA.116.003918). There were no major procedure-related complications during follow-up, the researchers said. For patients with ICM, NYHA classifications dropped significantly at 6 months, from 2.9 at baseline to 2.1 at 6 months and 1.9 at 1 year. Six-Minute Walk Test scores improved significantly, from 416 m at baseline to 475 m at 6 months to 484 m at 1 year. Transplantation also led to significant drops in serum brain natriuretic peptide levels (baseline average, 308 pg/mL; 6 months, 191 pg/mL; 1 year, 182 pg/mL). Additionally, ICM patients improved significantly on measures of pulmonary artery pressure, pulmonary capillary wedge pressure, pulmonary vein resistance, and left ventricular wall stress.
In contrast, DCM patients did not significantly improve based on the 6-Minute Walk Test, NYHA classification, or brain natriuretic peptide serum levels. Of 12, 3 progressed to NYHA functional class IV by 1-year follow-up and there were five cardiac deaths. There were no cardiac deaths in the ICM group. “It has been reported that regeneration mechanisms in cell sheet mainly depend on angiogenesis induced by secreted cytokines, so cell-sheet implantation may be more suitable for ICM patients, compared with DCM patients, considering pathophysiological aspects,” the researchers wrote.
The study was funded by grants from Regenerative Medicine for Clinical Application, Health and Labor Sciences Research, and the Japan Agency for Medical Research and Development. The investigators disclosed laboratory funding from Terumo for cooperative research in cell sheet.
For patients with ischemic cardiomyopathy (ICM), transplanting sheets of autologous myoblasts onto the epicardial surface was safe and led to “marked” improvements in functional and laboratory measures, investigators reported.
However, cell sheet transplantation was much less effective for patients with dilated cardiomyopathy in this phase I trial, wrote Shigeru Miyagawa, MD, PhD, of Osaka (Japan) University, and his associates in the Journal of the American Heart Association. The findings merit additional follow-up in larger clinical studies, the investigators asserted.
Heart failure remains life threatening even with the best approved treatments. Previously, the researchers transplanted scaffold-free sheets of autologous myoblasts derived from skeletal muscle into small and large animals with heart failure and found that this approach healed severely damaged myocardium. Transplantation caused secreted cytokines to induce native regeneration through paracrine effects, Dr. Miyagawa noted in a prior review article (BioMed Research International. 2013. doi: 10.1155/2013/583912).
To evaluate this approach in humans, the researchers transplanted 15 patients with ischemic cardiomyopathy and 12 patients with dilated cardiomyopathy (DCM). Patients were 20-75 years old, had ejection fractions under 35% and New York Heart Association (NYHA) classifications of II or more, and were responding inadequately to standard treatment with a beta-blocker or ACE inhibitor and an angiotensin receptor blocker. The sole intervention was transplantation of the cell sheet over the left ventricular-free wall during left thoracotomy (J Am Heart Assoc. 2017 Apr 5. doi: 10.1161/JAHA.116.003918). There were no major procedure-related complications during follow-up, the researchers said. For patients with ICM, NYHA classifications dropped significantly at 6 months, from 2.9 at baseline to 2.1 at 6 months and 1.9 at 1 year. Six-Minute Walk Test scores improved significantly, from 416 m at baseline to 475 m at 6 months to 484 m at 1 year. Transplantation also led to significant drops in serum brain natriuretic peptide levels (baseline average, 308 pg/mL; 6 months, 191 pg/mL; 1 year, 182 pg/mL). Additionally, ICM patients improved significantly on measures of pulmonary artery pressure, pulmonary capillary wedge pressure, pulmonary vein resistance, and left ventricular wall stress.
In contrast, DCM patients did not significantly improve based on the 6-Minute Walk Test, NYHA classification, or brain natriuretic peptide serum levels. Of 12, 3 progressed to NYHA functional class IV by 1-year follow-up and there were five cardiac deaths. There were no cardiac deaths in the ICM group. “It has been reported that regeneration mechanisms in cell sheet mainly depend on angiogenesis induced by secreted cytokines, so cell-sheet implantation may be more suitable for ICM patients, compared with DCM patients, considering pathophysiological aspects,” the researchers wrote.
The study was funded by grants from Regenerative Medicine for Clinical Application, Health and Labor Sciences Research, and the Japan Agency for Medical Research and Development. The investigators disclosed laboratory funding from Terumo for cooperative research in cell sheet.
For patients with ischemic cardiomyopathy (ICM), transplanting sheets of autologous myoblasts onto the epicardial surface was safe and led to “marked” improvements in functional and laboratory measures, investigators reported.
However, cell sheet transplantation was much less effective for patients with dilated cardiomyopathy in this phase I trial, wrote Shigeru Miyagawa, MD, PhD, of Osaka (Japan) University, and his associates in the Journal of the American Heart Association. The findings merit additional follow-up in larger clinical studies, the investigators asserted.
Heart failure remains life threatening even with the best approved treatments. Previously, the researchers transplanted scaffold-free sheets of autologous myoblasts derived from skeletal muscle into small and large animals with heart failure and found that this approach healed severely damaged myocardium. Transplantation caused secreted cytokines to induce native regeneration through paracrine effects, Dr. Miyagawa noted in a prior review article (BioMed Research International. 2013. doi: 10.1155/2013/583912).
To evaluate this approach in humans, the researchers transplanted 15 patients with ischemic cardiomyopathy and 12 patients with dilated cardiomyopathy (DCM). Patients were 20-75 years old, had ejection fractions under 35% and New York Heart Association (NYHA) classifications of II or more, and were responding inadequately to standard treatment with a beta-blocker or ACE inhibitor and an angiotensin receptor blocker. The sole intervention was transplantation of the cell sheet over the left ventricular-free wall during left thoracotomy (J Am Heart Assoc. 2017 Apr 5. doi: 10.1161/JAHA.116.003918). There were no major procedure-related complications during follow-up, the researchers said. For patients with ICM, NYHA classifications dropped significantly at 6 months, from 2.9 at baseline to 2.1 at 6 months and 1.9 at 1 year. Six-Minute Walk Test scores improved significantly, from 416 m at baseline to 475 m at 6 months to 484 m at 1 year. Transplantation also led to significant drops in serum brain natriuretic peptide levels (baseline average, 308 pg/mL; 6 months, 191 pg/mL; 1 year, 182 pg/mL). Additionally, ICM patients improved significantly on measures of pulmonary artery pressure, pulmonary capillary wedge pressure, pulmonary vein resistance, and left ventricular wall stress.
In contrast, DCM patients did not significantly improve based on the 6-Minute Walk Test, NYHA classification, or brain natriuretic peptide serum levels. Of 12, 3 progressed to NYHA functional class IV by 1-year follow-up and there were five cardiac deaths. There were no cardiac deaths in the ICM group. “It has been reported that regeneration mechanisms in cell sheet mainly depend on angiogenesis induced by secreted cytokines, so cell-sheet implantation may be more suitable for ICM patients, compared with DCM patients, considering pathophysiological aspects,” the researchers wrote.
The study was funded by grants from Regenerative Medicine for Clinical Application, Health and Labor Sciences Research, and the Japan Agency for Medical Research and Development. The investigators disclosed laboratory funding from Terumo for cooperative research in cell sheet.
Key clinical point: Transplanting a sheet of autologous myoblasts onto the epicardial surface was safe and was associated with functional and symptomatic improvements in patients with ischemic cardiomyopathy.
Major finding: Patients improved significantly on the 6-Minute Walk Test, New York Heart Classification, and on measures of brain natriuretic peptide, pulmonary artery pressure, pulmonary capillary wedge pressure, pulmonary vein resistance, and left ventricular wall stress. These effects did not extend to patients with dilated cardiomyopathy.
Data source: A phase I trial of 27 patients with ischemic or dilated cardiomyopathy.
Disclosures: The study was funded by grants from Regenerative Medicine for Clinical Application, Health and Labor Sciences Research, and the Japan Agency for Medical Research and Development. The investigators disclosed laboratory funding from Terumo for cooperative research in cell sheet.
Off-the-shelf T cells an option for post-HCT viral infections
ORLANDO – Infusions of banked multivirus-specific T lymphocytes were associated with complete or partial responses in 93% of 42 patients who had undergone hematopoietic cell transplants and had drug-refractory viral illnesses. Further, these patients experienced minimal new or reactivated graft-versus-host disease (GVHD).
Viral infections cause nearly 40% of deaths after alternative donor hematopoietic cell transfer (HCT), Ifigeneia Tzannou, MD, said at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society of Blood and Marrow Transplantation. Banked, “off-the-shelf” donor virus-resistant T cells can be an alternative to antiviral drugs, which are far from universally effective and may have serious side effects.
“Traditionally, we have generated T cells for infusion from the stem cell donor” by isolating and then stimulating and expanding the peripheral blood mononuclear cells for about 10 days ex vivo, said Dr. Tzannou. At that point, the clonal multivirus-resistant T cells can then be transferred to the recipient.
Donor-derived T cells have been used to prevent and treat Epstein-Barr virus (EBV), cytomegalovirus (CMV), adenovirus (AdV), BK virus (BKV), and human herpes virus 6 (HHV6) infections. The approach has been safe, reconstituting antiviral immunity and clearing disease effectively, with a 94% response rate reported in one recent study. However, said Dr. Tzannou, donor-derived virus-specific T cells (VSTs) have their limitations. Donors are increasingly younger and cord blood is being used more commonly, so there are growing numbers of donors who are seronegative for pathogenic viruses. In addition, the 10 days of production time and the additional week or 10 days required for release means that donor-derived VSTs can’t be urgently used.
The concept of banked third party VST therapy came about to address those limitations, said Dr. Tzannou of Baylor College of Medicine, Houston.
In a banked VST scenario, donor T cells with specific multiviral immunity are human leukocyte antigen (HLA) typed, expanded, and cryopreserved. A post-HCT patient with drug-refractory viral illness can receive T cells that are partially matched at HLA –A, HLA-B, or HLA-DR. Dr. Tzannou said that her group has now generated a bank of 59 VST lines to use in clinical testing of the third party approach.
In the study, Dr. Tzannou and her colleagues included both pediatric and adult post-allo-HCT patients with refractory EBV, CMV, AdV, BKV, and/or HHV6 infections. All had either failed a 14-day trial of antiviral therapy or could not tolerate antivirals. Patients could not be on more than 0.5 mg/kg per day of prednisone; they had to have an absolute neutrophil count above 500 per microliter and hemoglobin greater than 8 g/dL. Patients were excluded if they had acute GVHD of grade 2 or higher. There had to be a compatible VST line available that matched both the patient’s illness and HLA typing.
Patients initially received 20,000,000 VST cells per square meter of body surface area. If the investigators saw a partial response, patients could receive additional VST doses every 2 weeks.
Of the 42 patients infused, 23 received one infusion and 19 required two or more infusions. Seven study participants had two viral infections; 18 had CMV, 2 had EBV, 9 had AdV, 17 had BKV, and 3 had HHV6.
Dr. Tzannou and her colleagues tracked the virus-specific T cells and viral load for particular viruses. Virus-specific peripheral T cell counts also rose measurably and viral load plummeted within 2 weeks of VST infusions for most patients.
Overall, 93% of patients met the primary outcome measure of achieving complete or partial response; a partial response was defined as a 50% or better decrease in the viral load and/or clinical improvement.
All of the 17 BKV patients treated to date had tissue disease; 15 had hemorrhagic cystitis and 2 had nephritis. All responded to VSTs, and all of those with hemorrhagic cystitis had symptomatic improvement or resolution.
Overall, the safety profile for VST was good, said Dr. Tzannou. Four patients developed grade 1 acute cutaneous GVHD within 45 days of infusion; one of these developed de novo, but resolved with topical steroids. Another patient had a flare of gastrointestinal GVHD when immunosuppresion was being tapered. One more patient had a transient fever post infusion that resolved spontaneously, said Dr. Tzannou.
Next steps include a multicenter registration study, said Dr. Tzannou, who reports being a consultant for ViraCyte, which helped fund the study.
[email protected]
On Twitter @karioakes
ORLANDO – Infusions of banked multivirus-specific T lymphocytes were associated with complete or partial responses in 93% of 42 patients who had undergone hematopoietic cell transplants and had drug-refractory viral illnesses. Further, these patients experienced minimal new or reactivated graft-versus-host disease (GVHD).
Viral infections cause nearly 40% of deaths after alternative donor hematopoietic cell transfer (HCT), Ifigeneia Tzannou, MD, said at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society of Blood and Marrow Transplantation. Banked, “off-the-shelf” donor virus-resistant T cells can be an alternative to antiviral drugs, which are far from universally effective and may have serious side effects.
“Traditionally, we have generated T cells for infusion from the stem cell donor” by isolating and then stimulating and expanding the peripheral blood mononuclear cells for about 10 days ex vivo, said Dr. Tzannou. At that point, the clonal multivirus-resistant T cells can then be transferred to the recipient.
Donor-derived T cells have been used to prevent and treat Epstein-Barr virus (EBV), cytomegalovirus (CMV), adenovirus (AdV), BK virus (BKV), and human herpes virus 6 (HHV6) infections. The approach has been safe, reconstituting antiviral immunity and clearing disease effectively, with a 94% response rate reported in one recent study. However, said Dr. Tzannou, donor-derived virus-specific T cells (VSTs) have their limitations. Donors are increasingly younger and cord blood is being used more commonly, so there are growing numbers of donors who are seronegative for pathogenic viruses. In addition, the 10 days of production time and the additional week or 10 days required for release means that donor-derived VSTs can’t be urgently used.
The concept of banked third party VST therapy came about to address those limitations, said Dr. Tzannou of Baylor College of Medicine, Houston.
In a banked VST scenario, donor T cells with specific multiviral immunity are human leukocyte antigen (HLA) typed, expanded, and cryopreserved. A post-HCT patient with drug-refractory viral illness can receive T cells that are partially matched at HLA –A, HLA-B, or HLA-DR. Dr. Tzannou said that her group has now generated a bank of 59 VST lines to use in clinical testing of the third party approach.
In the study, Dr. Tzannou and her colleagues included both pediatric and adult post-allo-HCT patients with refractory EBV, CMV, AdV, BKV, and/or HHV6 infections. All had either failed a 14-day trial of antiviral therapy or could not tolerate antivirals. Patients could not be on more than 0.5 mg/kg per day of prednisone; they had to have an absolute neutrophil count above 500 per microliter and hemoglobin greater than 8 g/dL. Patients were excluded if they had acute GVHD of grade 2 or higher. There had to be a compatible VST line available that matched both the patient’s illness and HLA typing.
Patients initially received 20,000,000 VST cells per square meter of body surface area. If the investigators saw a partial response, patients could receive additional VST doses every 2 weeks.
Of the 42 patients infused, 23 received one infusion and 19 required two or more infusions. Seven study participants had two viral infections; 18 had CMV, 2 had EBV, 9 had AdV, 17 had BKV, and 3 had HHV6.
Dr. Tzannou and her colleagues tracked the virus-specific T cells and viral load for particular viruses. Virus-specific peripheral T cell counts also rose measurably and viral load plummeted within 2 weeks of VST infusions for most patients.
Overall, 93% of patients met the primary outcome measure of achieving complete or partial response; a partial response was defined as a 50% or better decrease in the viral load and/or clinical improvement.
All of the 17 BKV patients treated to date had tissue disease; 15 had hemorrhagic cystitis and 2 had nephritis. All responded to VSTs, and all of those with hemorrhagic cystitis had symptomatic improvement or resolution.
Overall, the safety profile for VST was good, said Dr. Tzannou. Four patients developed grade 1 acute cutaneous GVHD within 45 days of infusion; one of these developed de novo, but resolved with topical steroids. Another patient had a flare of gastrointestinal GVHD when immunosuppresion was being tapered. One more patient had a transient fever post infusion that resolved spontaneously, said Dr. Tzannou.
Next steps include a multicenter registration study, said Dr. Tzannou, who reports being a consultant for ViraCyte, which helped fund the study.
[email protected]
On Twitter @karioakes
ORLANDO – Infusions of banked multivirus-specific T lymphocytes were associated with complete or partial responses in 93% of 42 patients who had undergone hematopoietic cell transplants and had drug-refractory viral illnesses. Further, these patients experienced minimal new or reactivated graft-versus-host disease (GVHD).
Viral infections cause nearly 40% of deaths after alternative donor hematopoietic cell transfer (HCT), Ifigeneia Tzannou, MD, said at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society of Blood and Marrow Transplantation. Banked, “off-the-shelf” donor virus-resistant T cells can be an alternative to antiviral drugs, which are far from universally effective and may have serious side effects.
“Traditionally, we have generated T cells for infusion from the stem cell donor” by isolating and then stimulating and expanding the peripheral blood mononuclear cells for about 10 days ex vivo, said Dr. Tzannou. At that point, the clonal multivirus-resistant T cells can then be transferred to the recipient.
Donor-derived T cells have been used to prevent and treat Epstein-Barr virus (EBV), cytomegalovirus (CMV), adenovirus (AdV), BK virus (BKV), and human herpes virus 6 (HHV6) infections. The approach has been safe, reconstituting antiviral immunity and clearing disease effectively, with a 94% response rate reported in one recent study. However, said Dr. Tzannou, donor-derived virus-specific T cells (VSTs) have their limitations. Donors are increasingly younger and cord blood is being used more commonly, so there are growing numbers of donors who are seronegative for pathogenic viruses. In addition, the 10 days of production time and the additional week or 10 days required for release means that donor-derived VSTs can’t be urgently used.
The concept of banked third party VST therapy came about to address those limitations, said Dr. Tzannou of Baylor College of Medicine, Houston.
In a banked VST scenario, donor T cells with specific multiviral immunity are human leukocyte antigen (HLA) typed, expanded, and cryopreserved. A post-HCT patient with drug-refractory viral illness can receive T cells that are partially matched at HLA –A, HLA-B, or HLA-DR. Dr. Tzannou said that her group has now generated a bank of 59 VST lines to use in clinical testing of the third party approach.
In the study, Dr. Tzannou and her colleagues included both pediatric and adult post-allo-HCT patients with refractory EBV, CMV, AdV, BKV, and/or HHV6 infections. All had either failed a 14-day trial of antiviral therapy or could not tolerate antivirals. Patients could not be on more than 0.5 mg/kg per day of prednisone; they had to have an absolute neutrophil count above 500 per microliter and hemoglobin greater than 8 g/dL. Patients were excluded if they had acute GVHD of grade 2 or higher. There had to be a compatible VST line available that matched both the patient’s illness and HLA typing.
Patients initially received 20,000,000 VST cells per square meter of body surface area. If the investigators saw a partial response, patients could receive additional VST doses every 2 weeks.
Of the 42 patients infused, 23 received one infusion and 19 required two or more infusions. Seven study participants had two viral infections; 18 had CMV, 2 had EBV, 9 had AdV, 17 had BKV, and 3 had HHV6.
Dr. Tzannou and her colleagues tracked the virus-specific T cells and viral load for particular viruses. Virus-specific peripheral T cell counts also rose measurably and viral load plummeted within 2 weeks of VST infusions for most patients.
Overall, 93% of patients met the primary outcome measure of achieving complete or partial response; a partial response was defined as a 50% or better decrease in the viral load and/or clinical improvement.
All of the 17 BKV patients treated to date had tissue disease; 15 had hemorrhagic cystitis and 2 had nephritis. All responded to VSTs, and all of those with hemorrhagic cystitis had symptomatic improvement or resolution.
Overall, the safety profile for VST was good, said Dr. Tzannou. Four patients developed grade 1 acute cutaneous GVHD within 45 days of infusion; one of these developed de novo, but resolved with topical steroids. Another patient had a flare of gastrointestinal GVHD when immunosuppresion was being tapered. One more patient had a transient fever post infusion that resolved spontaneously, said Dr. Tzannou.
Next steps include a multicenter registration study, said Dr. Tzannou, who reports being a consultant for ViraCyte, which helped fund the study.
[email protected]
On Twitter @karioakes
AT THE 2017 BMT TANDEM MEETINGS
Key clinical point:
Major finding: With banked multivirus-specific T cells, viral illnesses either improved or resolved in 93% of 42 patients.
Data source: Clinical trial of 42 postallogeneic hematopoietic cell transfer patients who had any of five viral illnesses and had either failed a 14-day trial of antiviral therapy or could not tolerate antivirals.
Disclosures: Dr. Tzannou is a consultant for Incyte, which partially funded the trial and is developing third-party VSTs.
Tocilizumab shows promise for GVHD prevention
ORLANDO – Tocilizumab plus standard immune suppression appears to drive down the risk for graft-versus-host disease (GVHD), according to results from a phase II study of 35 adults undergoing allogeneic stem cell transplants.
The effect was particularly pronounced for prevention of GVHD in the colon, William Drobyski, MD, reported at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
The incidence rate of grades II-IV and III-IV acute GVHD was 12% at day 100 in patients given standard prophylaxis of tacrolimus/methotrexate (Tac/MTX) and 3% in patients given Tac/MTX plus 8 mg/kg of tocilizumab (Toc, capped at 800 mg), said Dr. Drobyski of the Medical College of Wisconsin, Milwaukee.
To provide further context to the results, Dr. Drobyski and his colleagues performed a matched case-control analysis using contemporary controls in the Center for International Blood & Marrow Transplant Research from 2000 to 2014. The same eligibility criteria used for the trial were applied to the matched controls except for the use of Tac/MTX as GVHD prophylaxis. Patients were otherwise matched based on age, performance score, disease, and donor type.
The incidence of grades II-IV acute GVHD at day 100 was significantly lower in the Toc/Tac/MTX group than in the Tac/MTX control population (12% vs. 41%). The incidence of grades III-IV acute GVHD was slightly lower with tocilizumab, but the difference between the groups was not statistically significant, Dr. Drobyski said.
The probability of grade II-IV acute GVHD–free survival, which was the primary endpoint of the study, was significantly higher in the Toc/Tac/MTX group (79% vs 52%), he said.
Five patients developed grade 2 acute GVHD of the skin or upper GI tract, and one patient died of grade 4 acute GVHD of the skin in the first 100 days. Notably, there were no cases of acute GVHD of the lower GI tract during that time, although two cases did occur between days 130 and 180, he said.
“There was no difference in transplant-related mortality, relapse, disease-free survival, or overall survival,” he said, adding that preliminary data suggest there were no differences in chronic GVHD between the groups.
Causes of death also were similar between the two cohorts with respect to disease- and transplant-related complications.
Patients in the tocilizumab study were enrolled between January 2015 and June 2016; the median age was 66 years. Diseases represented in the cohort included de novo acute myeloid leukemia (13 patients), AML (6 patients), chronic myelomonocytic leukemia (6 patients), acute lymphoblastic leukemia (4 patients), myelodysplastic syndrome (3 patients), and T-cell lymphoma, chronic myeloid leukemia, and NK/T cell lymphoma (in 1 patient each). Most patients were classified as high risk (9 patients) or intermediate risk (22 patients) by the disease risk index.
Conditioning was entirely busulfan based. Myeloablative conditioning was with busulfan and cyclophosphamide (Cytoxan) in 5 patients, or fludarabine and 4 days of busulfan in 10 patients, and reduced-intensity conditioning was with fludarabine and 2 days of busulfan in 18 patients. Transplants were with either HLA-matched related or unrelated donor grafts. Most patients (29 of 35) received peripheral stem cell grafts.
Tocilizumab, an interleuken-6 receptor blocker that is approved for treatment of rheumatoid arthritis, was administered after completion of conditioning and on the day prior to stem cell infusion.
In a pilot clinical trial of tocilizumab for the treatment of steroid-resistant acute GVHD in patients who had primarily had lower GI tract disease, “we were able to demonstrate responses in a majority of these patients,” Dr. Drobyski said, noting that a recent study presented at the 2016 annual meeting of the American Society of Hematology also showed efficacy in the treatment of lower tract GI GVHD, “providing evidence that tocilizumab had activity in acute GVHD, and perhaps in the treatment of steroid-refractory lower GI GVHD.”
Elevated IL-6 levels in the peripheral blood are correlated with an increased incidence and severity of GVHD; administration of an anti-IL-6 receptor antibody has been shown in preclinical studies to protect mice from lethal GVHD. The current open-label study was performed to “try to advance this concept” by assessing whether inhibition of IL-6 signaling could also prevent acute GVHD.
The findings confirm those of a 2014 study by Kennedy et al. in Lancet Oncology (2014;15:1451-9), and imply that tocilizumab warrants a randomized trial as prophylaxis for acute GVHD, he concluded.
Dr. Drobyski reported having no disclosures.
ORLANDO – Tocilizumab plus standard immune suppression appears to drive down the risk for graft-versus-host disease (GVHD), according to results from a phase II study of 35 adults undergoing allogeneic stem cell transplants.
The effect was particularly pronounced for prevention of GVHD in the colon, William Drobyski, MD, reported at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
The incidence rate of grades II-IV and III-IV acute GVHD was 12% at day 100 in patients given standard prophylaxis of tacrolimus/methotrexate (Tac/MTX) and 3% in patients given Tac/MTX plus 8 mg/kg of tocilizumab (Toc, capped at 800 mg), said Dr. Drobyski of the Medical College of Wisconsin, Milwaukee.
To provide further context to the results, Dr. Drobyski and his colleagues performed a matched case-control analysis using contemporary controls in the Center for International Blood & Marrow Transplant Research from 2000 to 2014. The same eligibility criteria used for the trial were applied to the matched controls except for the use of Tac/MTX as GVHD prophylaxis. Patients were otherwise matched based on age, performance score, disease, and donor type.
The incidence of grades II-IV acute GVHD at day 100 was significantly lower in the Toc/Tac/MTX group than in the Tac/MTX control population (12% vs. 41%). The incidence of grades III-IV acute GVHD was slightly lower with tocilizumab, but the difference between the groups was not statistically significant, Dr. Drobyski said.
The probability of grade II-IV acute GVHD–free survival, which was the primary endpoint of the study, was significantly higher in the Toc/Tac/MTX group (79% vs 52%), he said.
Five patients developed grade 2 acute GVHD of the skin or upper GI tract, and one patient died of grade 4 acute GVHD of the skin in the first 100 days. Notably, there were no cases of acute GVHD of the lower GI tract during that time, although two cases did occur between days 130 and 180, he said.
“There was no difference in transplant-related mortality, relapse, disease-free survival, or overall survival,” he said, adding that preliminary data suggest there were no differences in chronic GVHD between the groups.
Causes of death also were similar between the two cohorts with respect to disease- and transplant-related complications.
Patients in the tocilizumab study were enrolled between January 2015 and June 2016; the median age was 66 years. Diseases represented in the cohort included de novo acute myeloid leukemia (13 patients), AML (6 patients), chronic myelomonocytic leukemia (6 patients), acute lymphoblastic leukemia (4 patients), myelodysplastic syndrome (3 patients), and T-cell lymphoma, chronic myeloid leukemia, and NK/T cell lymphoma (in 1 patient each). Most patients were classified as high risk (9 patients) or intermediate risk (22 patients) by the disease risk index.
Conditioning was entirely busulfan based. Myeloablative conditioning was with busulfan and cyclophosphamide (Cytoxan) in 5 patients, or fludarabine and 4 days of busulfan in 10 patients, and reduced-intensity conditioning was with fludarabine and 2 days of busulfan in 18 patients. Transplants were with either HLA-matched related or unrelated donor grafts. Most patients (29 of 35) received peripheral stem cell grafts.
Tocilizumab, an interleuken-6 receptor blocker that is approved for treatment of rheumatoid arthritis, was administered after completion of conditioning and on the day prior to stem cell infusion.
In a pilot clinical trial of tocilizumab for the treatment of steroid-resistant acute GVHD in patients who had primarily had lower GI tract disease, “we were able to demonstrate responses in a majority of these patients,” Dr. Drobyski said, noting that a recent study presented at the 2016 annual meeting of the American Society of Hematology also showed efficacy in the treatment of lower tract GI GVHD, “providing evidence that tocilizumab had activity in acute GVHD, and perhaps in the treatment of steroid-refractory lower GI GVHD.”
Elevated IL-6 levels in the peripheral blood are correlated with an increased incidence and severity of GVHD; administration of an anti-IL-6 receptor antibody has been shown in preclinical studies to protect mice from lethal GVHD. The current open-label study was performed to “try to advance this concept” by assessing whether inhibition of IL-6 signaling could also prevent acute GVHD.
The findings confirm those of a 2014 study by Kennedy et al. in Lancet Oncology (2014;15:1451-9), and imply that tocilizumab warrants a randomized trial as prophylaxis for acute GVHD, he concluded.
Dr. Drobyski reported having no disclosures.
ORLANDO – Tocilizumab plus standard immune suppression appears to drive down the risk for graft-versus-host disease (GVHD), according to results from a phase II study of 35 adults undergoing allogeneic stem cell transplants.
The effect was particularly pronounced for prevention of GVHD in the colon, William Drobyski, MD, reported at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
The incidence rate of grades II-IV and III-IV acute GVHD was 12% at day 100 in patients given standard prophylaxis of tacrolimus/methotrexate (Tac/MTX) and 3% in patients given Tac/MTX plus 8 mg/kg of tocilizumab (Toc, capped at 800 mg), said Dr. Drobyski of the Medical College of Wisconsin, Milwaukee.
To provide further context to the results, Dr. Drobyski and his colleagues performed a matched case-control analysis using contemporary controls in the Center for International Blood & Marrow Transplant Research from 2000 to 2014. The same eligibility criteria used for the trial were applied to the matched controls except for the use of Tac/MTX as GVHD prophylaxis. Patients were otherwise matched based on age, performance score, disease, and donor type.
The incidence of grades II-IV acute GVHD at day 100 was significantly lower in the Toc/Tac/MTX group than in the Tac/MTX control population (12% vs. 41%). The incidence of grades III-IV acute GVHD was slightly lower with tocilizumab, but the difference between the groups was not statistically significant, Dr. Drobyski said.
The probability of grade II-IV acute GVHD–free survival, which was the primary endpoint of the study, was significantly higher in the Toc/Tac/MTX group (79% vs 52%), he said.
Five patients developed grade 2 acute GVHD of the skin or upper GI tract, and one patient died of grade 4 acute GVHD of the skin in the first 100 days. Notably, there were no cases of acute GVHD of the lower GI tract during that time, although two cases did occur between days 130 and 180, he said.
“There was no difference in transplant-related mortality, relapse, disease-free survival, or overall survival,” he said, adding that preliminary data suggest there were no differences in chronic GVHD between the groups.
Causes of death also were similar between the two cohorts with respect to disease- and transplant-related complications.
Patients in the tocilizumab study were enrolled between January 2015 and June 2016; the median age was 66 years. Diseases represented in the cohort included de novo acute myeloid leukemia (13 patients), AML (6 patients), chronic myelomonocytic leukemia (6 patients), acute lymphoblastic leukemia (4 patients), myelodysplastic syndrome (3 patients), and T-cell lymphoma, chronic myeloid leukemia, and NK/T cell lymphoma (in 1 patient each). Most patients were classified as high risk (9 patients) or intermediate risk (22 patients) by the disease risk index.
Conditioning was entirely busulfan based. Myeloablative conditioning was with busulfan and cyclophosphamide (Cytoxan) in 5 patients, or fludarabine and 4 days of busulfan in 10 patients, and reduced-intensity conditioning was with fludarabine and 2 days of busulfan in 18 patients. Transplants were with either HLA-matched related or unrelated donor grafts. Most patients (29 of 35) received peripheral stem cell grafts.
Tocilizumab, an interleuken-6 receptor blocker that is approved for treatment of rheumatoid arthritis, was administered after completion of conditioning and on the day prior to stem cell infusion.
In a pilot clinical trial of tocilizumab for the treatment of steroid-resistant acute GVHD in patients who had primarily had lower GI tract disease, “we were able to demonstrate responses in a majority of these patients,” Dr. Drobyski said, noting that a recent study presented at the 2016 annual meeting of the American Society of Hematology also showed efficacy in the treatment of lower tract GI GVHD, “providing evidence that tocilizumab had activity in acute GVHD, and perhaps in the treatment of steroid-refractory lower GI GVHD.”
Elevated IL-6 levels in the peripheral blood are correlated with an increased incidence and severity of GVHD; administration of an anti-IL-6 receptor antibody has been shown in preclinical studies to protect mice from lethal GVHD. The current open-label study was performed to “try to advance this concept” by assessing whether inhibition of IL-6 signaling could also prevent acute GVHD.
The findings confirm those of a 2014 study by Kennedy et al. in Lancet Oncology (2014;15:1451-9), and imply that tocilizumab warrants a randomized trial as prophylaxis for acute GVHD, he concluded.
Dr. Drobyski reported having no disclosures.
Key clinical point:
Major finding: The probability of grade II-IV acute GVHD-free survival was 79% vs. 52% in the tocilizumab group vs. age-matched controls.
Data source: An open-label phase II study of 35 patients.
Disclosures: Dr. Drobyski reported having no disclosures.
CAR designers report high B-cell cancer response rates
ORLANDO – Patients with advanced hematologic malignancies of B-cell lineage had robust immune responses following infusion of a chimeric antigen receptor (CAR)–T-cell construct designed to deliver a specific balance of antigens, investigators reported.
Adults with relapsed or refractory B-lineage acute myeloid leukemia (ALL), non–Hodgkin lymphoma (NHL), and chronic lymphocytic leukemia (CLL) who received a CAR-T cell construct consisting of autologous CD4-positive and CD-8-positive T cells that were transduced separately, recombined, and then delivered in a single infusion had comparatively high overall response and complete response rates, reported Cameron Turtle, MBBS, PhD, from the Fred Hutchinson Cancer Research Center in Seattle.
“We know that patients have a highly variable CD4 to CD8 ratio, so by actually controlling this and separately transducing, expanding, and then reformulating in this defined composition, we’re able to eliminate one source of variability in CAR-T cell products,” Dr. Turtle said at the ASCO-SITC Clinical Immuno-Oncology Symposium.
In preclinical studies, an even balance of CD4-positive and CD8-positive central memory T cells or naive T cells evoked more potent immune responses against B-cell malignancies in mice than CD19-positive cells, he explained
To see whether this would also hold true in humans, the investigators enrolled into a phase I/II trial adults with relapsed/refractory B-cell malignancies, including ALL (36 patients), NHL (41), and CLL (24). No patients were excluded on the basis of either absolute lymphocyte, circulating tumors cells, history of stem cell transplant, or results of in vitro test expansions.
All patients underwent leukapheresis for harvesting of T-cells, and populations of CD4- and CD8-positive cells were separated and transduced with a lentiviral vector to express a CD19 CAR and a truncated human epidermal growth factor receptor that allowed tracing of the transduced cells via flow cytometry. The patients underwent lymphodepleting chemotherapy with cyclophosphamide (for the earliest patients), or cyclophosphamide plus fludarabine. Fifteen days after leukapheresis, the separated, transduced, and expanded cells were combined and delivered back to patients in a single infusion at one of three dose levels: 2 x 105, 2 x 106, or 2 x 107 CAR-T cells/kg.
ALL results
Two of the 36 patients with ALL died from complications of the CAR-T cell infusion process prior to evaluation. The 34 remaining patients all had morphologic bone marrow complete responses (CR). Of this group, 32 also had bone marrow CR on flow cytometry.
Using immunoglobulin H (IgH) deep sequencing in a subset of 20 patients 3 weeks after CAR-T cell infusion, the investigators could not detect the malignant IgH index clone in 13 of the patients, and found fewer than 10 copies in the bone marrow of 5 patients.
Six of seven patients with extramedullary disease at baseline had a complete response. The remaining patient in this group had an equivocal PET scan result, and experienced a relapse 2 months after assessment.
The investigators also determined that the lymphodepletion regimen may affect overall results, based on the finding that 10 of 12 patients who received cyclophosphamide alone achieved a CR, but seven of these 10 patients had a relapse within a few months. Of these seven patients. five received a second T-cell infusion, but none had significant T-cell expansion. The investigators traced the failure of the second attempt to a CD8-mediated transgene immune response to a murine single-chain variable fragment used in the construct.
For subsequent patients, they altered the lymphodepletion regimen to include fludarabine to prevent priming of the anti-CAR transgenic immune response. This modification resulted in improved progression-free survival and overall survival for subsequent patients receiving a second infusion, Dr. Turtle said.
NHL results
Of the 41 patients with NHL, 30 (73%) had aggressive histologies, including diffuse large B-cell lymphoma, primary mediastinal large B-cell lymphoma, T-cell/histiocyte-rich large B-cell, and Burkitt lymphomas, and 11 (27%) had indolent histologies, including mantle cell and follicular lymphomas. Most of the patients had received multiple prior lines of therapy, and 19 (46%) had undergone either an autologous or allogeneic stem cell transplant.
Of the 39 evaluable patients who completed therapy, the overall response rate was 67%, including 13 (39%) with CR. Dr. Turtle noted that the CR rate was substantially higher among patients who received cyclophosphamide and fludarabine lymphodepletion, compared with cyclophosphamide alone.
There were also a few responses, including two CRs, among patients with indolent histologies, he said.
CLL, safety results
All 24 patients with CLL had previously received ibrutinib (Imbruvica). Of this group, 19 either had no significant responses to the drug, inactivating mutations, or intolerable toxicities. All but 1 of the 24 patients also had high-risk cytogenetics.
Of the 16 ibrutinib-refractory patients who were evaluable for restaging, 14 had no evidence of disease in bone marrow by flow cytometry at 4 weeks. The overall response rate in this group was 69%, which included four CRs.
Among a majority of all patients, toxicity with the CAR-T cell therapy was mild to moderate. Early cytokine changes appeared to be predictive of serious adverse events such as the cytokine release syndrome, a finding that may allow clinicians to intervene early to prevent complications, Dr. Turtle said.
In the CAR-T cell therapy, “multiple things affect the response and toxicity, including CAR T-cell dose, disease burden, the anti-CAR transgene immune response and the lymphodepletion regimen, not to mention other patient factors that we’re still sorting out,” he commented.
The trial was funded by the National Institutes of Health, Life Science Development Fund, Juno Therapeutics and the Bezos Family Foundation. Dr. Turtle disclosed consultancy, honoraria, and/or research funding from Juno Therapeutics and Seattle Genetics.
ORLANDO – Patients with advanced hematologic malignancies of B-cell lineage had robust immune responses following infusion of a chimeric antigen receptor (CAR)–T-cell construct designed to deliver a specific balance of antigens, investigators reported.
Adults with relapsed or refractory B-lineage acute myeloid leukemia (ALL), non–Hodgkin lymphoma (NHL), and chronic lymphocytic leukemia (CLL) who received a CAR-T cell construct consisting of autologous CD4-positive and CD-8-positive T cells that were transduced separately, recombined, and then delivered in a single infusion had comparatively high overall response and complete response rates, reported Cameron Turtle, MBBS, PhD, from the Fred Hutchinson Cancer Research Center in Seattle.
“We know that patients have a highly variable CD4 to CD8 ratio, so by actually controlling this and separately transducing, expanding, and then reformulating in this defined composition, we’re able to eliminate one source of variability in CAR-T cell products,” Dr. Turtle said at the ASCO-SITC Clinical Immuno-Oncology Symposium.
In preclinical studies, an even balance of CD4-positive and CD8-positive central memory T cells or naive T cells evoked more potent immune responses against B-cell malignancies in mice than CD19-positive cells, he explained
To see whether this would also hold true in humans, the investigators enrolled into a phase I/II trial adults with relapsed/refractory B-cell malignancies, including ALL (36 patients), NHL (41), and CLL (24). No patients were excluded on the basis of either absolute lymphocyte, circulating tumors cells, history of stem cell transplant, or results of in vitro test expansions.
All patients underwent leukapheresis for harvesting of T-cells, and populations of CD4- and CD8-positive cells were separated and transduced with a lentiviral vector to express a CD19 CAR and a truncated human epidermal growth factor receptor that allowed tracing of the transduced cells via flow cytometry. The patients underwent lymphodepleting chemotherapy with cyclophosphamide (for the earliest patients), or cyclophosphamide plus fludarabine. Fifteen days after leukapheresis, the separated, transduced, and expanded cells were combined and delivered back to patients in a single infusion at one of three dose levels: 2 x 105, 2 x 106, or 2 x 107 CAR-T cells/kg.
ALL results
Two of the 36 patients with ALL died from complications of the CAR-T cell infusion process prior to evaluation. The 34 remaining patients all had morphologic bone marrow complete responses (CR). Of this group, 32 also had bone marrow CR on flow cytometry.
Using immunoglobulin H (IgH) deep sequencing in a subset of 20 patients 3 weeks after CAR-T cell infusion, the investigators could not detect the malignant IgH index clone in 13 of the patients, and found fewer than 10 copies in the bone marrow of 5 patients.
Six of seven patients with extramedullary disease at baseline had a complete response. The remaining patient in this group had an equivocal PET scan result, and experienced a relapse 2 months after assessment.
The investigators also determined that the lymphodepletion regimen may affect overall results, based on the finding that 10 of 12 patients who received cyclophosphamide alone achieved a CR, but seven of these 10 patients had a relapse within a few months. Of these seven patients. five received a second T-cell infusion, but none had significant T-cell expansion. The investigators traced the failure of the second attempt to a CD8-mediated transgene immune response to a murine single-chain variable fragment used in the construct.
For subsequent patients, they altered the lymphodepletion regimen to include fludarabine to prevent priming of the anti-CAR transgenic immune response. This modification resulted in improved progression-free survival and overall survival for subsequent patients receiving a second infusion, Dr. Turtle said.
NHL results
Of the 41 patients with NHL, 30 (73%) had aggressive histologies, including diffuse large B-cell lymphoma, primary mediastinal large B-cell lymphoma, T-cell/histiocyte-rich large B-cell, and Burkitt lymphomas, and 11 (27%) had indolent histologies, including mantle cell and follicular lymphomas. Most of the patients had received multiple prior lines of therapy, and 19 (46%) had undergone either an autologous or allogeneic stem cell transplant.
Of the 39 evaluable patients who completed therapy, the overall response rate was 67%, including 13 (39%) with CR. Dr. Turtle noted that the CR rate was substantially higher among patients who received cyclophosphamide and fludarabine lymphodepletion, compared with cyclophosphamide alone.
There were also a few responses, including two CRs, among patients with indolent histologies, he said.
CLL, safety results
All 24 patients with CLL had previously received ibrutinib (Imbruvica). Of this group, 19 either had no significant responses to the drug, inactivating mutations, or intolerable toxicities. All but 1 of the 24 patients also had high-risk cytogenetics.
Of the 16 ibrutinib-refractory patients who were evaluable for restaging, 14 had no evidence of disease in bone marrow by flow cytometry at 4 weeks. The overall response rate in this group was 69%, which included four CRs.
Among a majority of all patients, toxicity with the CAR-T cell therapy was mild to moderate. Early cytokine changes appeared to be predictive of serious adverse events such as the cytokine release syndrome, a finding that may allow clinicians to intervene early to prevent complications, Dr. Turtle said.
In the CAR-T cell therapy, “multiple things affect the response and toxicity, including CAR T-cell dose, disease burden, the anti-CAR transgene immune response and the lymphodepletion regimen, not to mention other patient factors that we’re still sorting out,” he commented.
The trial was funded by the National Institutes of Health, Life Science Development Fund, Juno Therapeutics and the Bezos Family Foundation. Dr. Turtle disclosed consultancy, honoraria, and/or research funding from Juno Therapeutics and Seattle Genetics.
ORLANDO – Patients with advanced hematologic malignancies of B-cell lineage had robust immune responses following infusion of a chimeric antigen receptor (CAR)–T-cell construct designed to deliver a specific balance of antigens, investigators reported.
Adults with relapsed or refractory B-lineage acute myeloid leukemia (ALL), non–Hodgkin lymphoma (NHL), and chronic lymphocytic leukemia (CLL) who received a CAR-T cell construct consisting of autologous CD4-positive and CD-8-positive T cells that were transduced separately, recombined, and then delivered in a single infusion had comparatively high overall response and complete response rates, reported Cameron Turtle, MBBS, PhD, from the Fred Hutchinson Cancer Research Center in Seattle.
“We know that patients have a highly variable CD4 to CD8 ratio, so by actually controlling this and separately transducing, expanding, and then reformulating in this defined composition, we’re able to eliminate one source of variability in CAR-T cell products,” Dr. Turtle said at the ASCO-SITC Clinical Immuno-Oncology Symposium.
In preclinical studies, an even balance of CD4-positive and CD8-positive central memory T cells or naive T cells evoked more potent immune responses against B-cell malignancies in mice than CD19-positive cells, he explained
To see whether this would also hold true in humans, the investigators enrolled into a phase I/II trial adults with relapsed/refractory B-cell malignancies, including ALL (36 patients), NHL (41), and CLL (24). No patients were excluded on the basis of either absolute lymphocyte, circulating tumors cells, history of stem cell transplant, or results of in vitro test expansions.
All patients underwent leukapheresis for harvesting of T-cells, and populations of CD4- and CD8-positive cells were separated and transduced with a lentiviral vector to express a CD19 CAR and a truncated human epidermal growth factor receptor that allowed tracing of the transduced cells via flow cytometry. The patients underwent lymphodepleting chemotherapy with cyclophosphamide (for the earliest patients), or cyclophosphamide plus fludarabine. Fifteen days after leukapheresis, the separated, transduced, and expanded cells were combined and delivered back to patients in a single infusion at one of three dose levels: 2 x 105, 2 x 106, or 2 x 107 CAR-T cells/kg.
ALL results
Two of the 36 patients with ALL died from complications of the CAR-T cell infusion process prior to evaluation. The 34 remaining patients all had morphologic bone marrow complete responses (CR). Of this group, 32 also had bone marrow CR on flow cytometry.
Using immunoglobulin H (IgH) deep sequencing in a subset of 20 patients 3 weeks after CAR-T cell infusion, the investigators could not detect the malignant IgH index clone in 13 of the patients, and found fewer than 10 copies in the bone marrow of 5 patients.
Six of seven patients with extramedullary disease at baseline had a complete response. The remaining patient in this group had an equivocal PET scan result, and experienced a relapse 2 months after assessment.
The investigators also determined that the lymphodepletion regimen may affect overall results, based on the finding that 10 of 12 patients who received cyclophosphamide alone achieved a CR, but seven of these 10 patients had a relapse within a few months. Of these seven patients. five received a second T-cell infusion, but none had significant T-cell expansion. The investigators traced the failure of the second attempt to a CD8-mediated transgene immune response to a murine single-chain variable fragment used in the construct.
For subsequent patients, they altered the lymphodepletion regimen to include fludarabine to prevent priming of the anti-CAR transgenic immune response. This modification resulted in improved progression-free survival and overall survival for subsequent patients receiving a second infusion, Dr. Turtle said.
NHL results
Of the 41 patients with NHL, 30 (73%) had aggressive histologies, including diffuse large B-cell lymphoma, primary mediastinal large B-cell lymphoma, T-cell/histiocyte-rich large B-cell, and Burkitt lymphomas, and 11 (27%) had indolent histologies, including mantle cell and follicular lymphomas. Most of the patients had received multiple prior lines of therapy, and 19 (46%) had undergone either an autologous or allogeneic stem cell transplant.
Of the 39 evaluable patients who completed therapy, the overall response rate was 67%, including 13 (39%) with CR. Dr. Turtle noted that the CR rate was substantially higher among patients who received cyclophosphamide and fludarabine lymphodepletion, compared with cyclophosphamide alone.
There were also a few responses, including two CRs, among patients with indolent histologies, he said.
CLL, safety results
All 24 patients with CLL had previously received ibrutinib (Imbruvica). Of this group, 19 either had no significant responses to the drug, inactivating mutations, or intolerable toxicities. All but 1 of the 24 patients also had high-risk cytogenetics.
Of the 16 ibrutinib-refractory patients who were evaluable for restaging, 14 had no evidence of disease in bone marrow by flow cytometry at 4 weeks. The overall response rate in this group was 69%, which included four CRs.
Among a majority of all patients, toxicity with the CAR-T cell therapy was mild to moderate. Early cytokine changes appeared to be predictive of serious adverse events such as the cytokine release syndrome, a finding that may allow clinicians to intervene early to prevent complications, Dr. Turtle said.
In the CAR-T cell therapy, “multiple things affect the response and toxicity, including CAR T-cell dose, disease burden, the anti-CAR transgene immune response and the lymphodepletion regimen, not to mention other patient factors that we’re still sorting out,” he commented.
The trial was funded by the National Institutes of Health, Life Science Development Fund, Juno Therapeutics and the Bezos Family Foundation. Dr. Turtle disclosed consultancy, honoraria, and/or research funding from Juno Therapeutics and Seattle Genetics.
AT THE CLINICAL IMMUNO-ONCOLOGY SYMPOSIUM
Key clinical point: A defined CAR-T cell construct was associated with high response rates in patients with B-cell malignancies.
Major finding: The overall response rate among patients with ibrutinib-refractory chronic lymphocytic leukemia was 69%, including four complete responses.
Data source: Phase I/II dose-finding, safety and efficacy study in patients with B-lineage hematologic malignancies
Disclosures: The trial was funded by the National Institutes of Health, Life Science Development Fund, Juno Therapeutics and the Bezos Family Foundation. Dr. Turtle disclosed consultancy, honoraria, and/or research funding from Juno Therapeutics and Seattle Genetics.