User login
Bone marrow transplantation for epidermolysis bullosa continues to evolve
CHICAGO – Bone marrow transplantation is evolving as a promising treatment for patients with the most severe forms of epidermolysis bullosa.
“Is this a cure? It’s not,” Dr. Jakub Tolar, MD, PhD, said at the World Congress of Pediatric Dermatology. “It is, however, a path toward understanding how we can treat this grave disorder in a systemic way.”
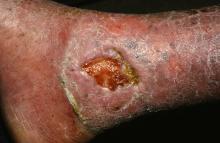
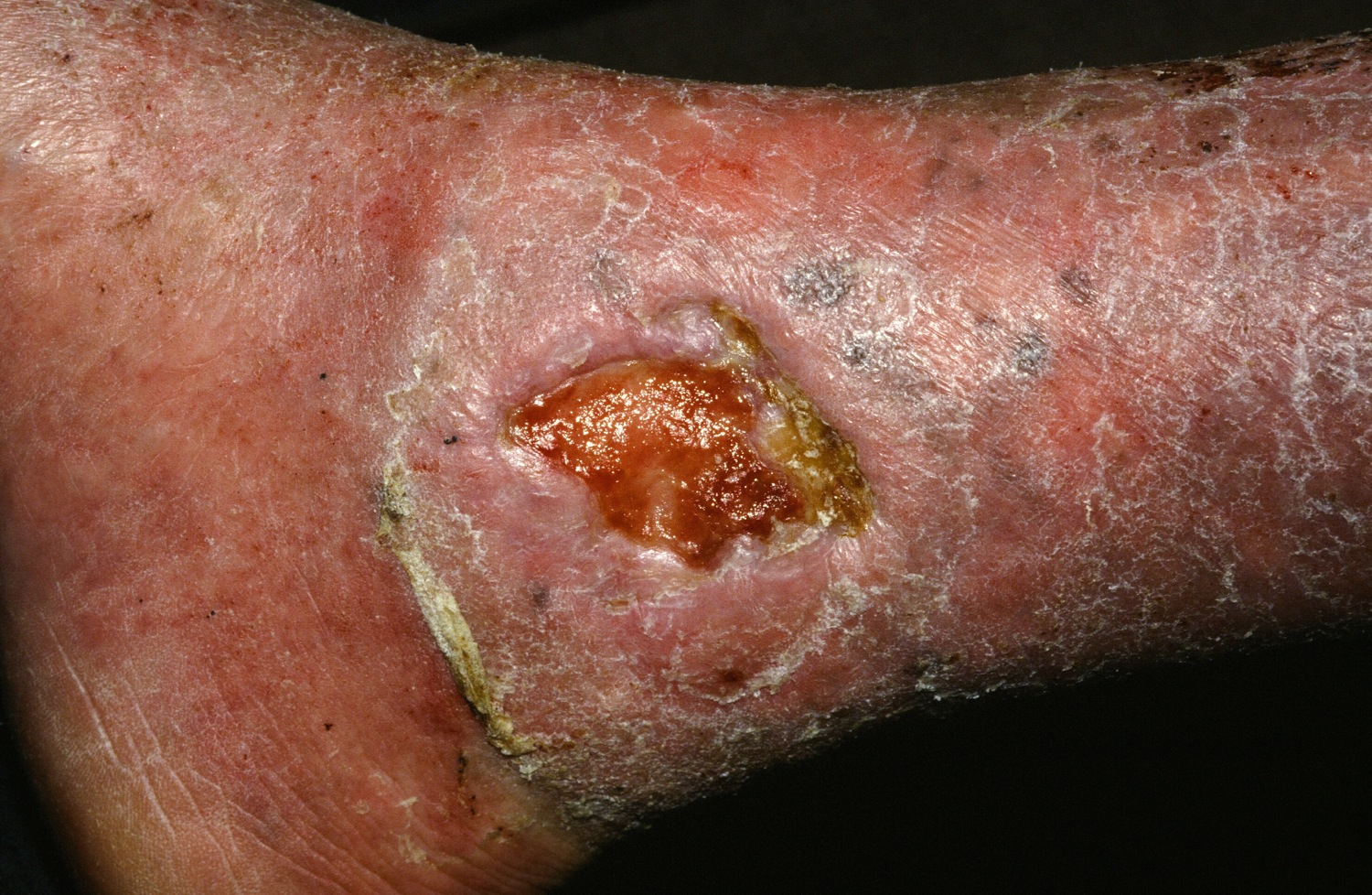
The University of Minnesota BMT Team has also observed a correlation between the engraftment in the blood and engraftment in the skin. “We have skin engraftment as high as 50%, which is good,” Dr. Tolar said. “The more donor cells engrafted in the skin, the more types of collagen you express.”
The clinicians have also been able to reduce the amount of chemotherapy and radiation patients require prior to transplant, for the BMT to work and skin to heal. “We were able to make it so that the last 11 patients are surviving and having benefit from the transplant, with the exception of one,” Dr. Tolar said. “How does this work? We still don’t entirely know. This is not a shot in the dark, however, this is the continuation of a very long process where we were first able to show that bone marrow transplant is an efficient stem cell therapy for leukemia, and about 20 years ago for the lysosomal enzyme deficiencies.” Their hunt for the cell that travels from the bone marrow to skin and produces type 7 collagen is continuing. “What haunts me is that BMT, which works in recessive dystrophic EB, works only in some types of junctional EB, those with alpha-3 chain deficiency of laminin 322.” he continued. “There has been no benefit to bone marrow transplantation for children with mutations of beta-3 chain of laminin 322, so we have closed enrollment for this one form of junctional EB. Survival in this group was 40%. Other types of junctional EB continue to be eligible for the study.”
Dr. Tolar recommended keratinocyte-driven or thymic epithelium cell type–driven therapy for patients with mutations of beta-3. “The deficiency of thymic function seems to be key in the inability to benefit from BMT in this form of junctional EB,” he said. “We have seen children who have engrafted, their skin got better, and then they died of infection many months after transplant. When we look at the immune profile and the thymic epithelial cells, they are both deficient – very abnormal.”
Despite current challenges, Dr. Tolar expressed optimism about the future of BMT in EB patients. “We have the same approach that we have in cancer care: deep empathy for all patients, radical international collaboration, and rapid laboratory and clinical prototyping,” he said. “It’s time to move from two-dimensional science to three-dimensional science; we need to study all aspects of EB simultaneously, from gene to cell to tissue to individual to patient population, and to understand the properties of the whole EB pathobiology that emerge at each level of biological complexity. By connecting information from these layers of disease network, we can better understand EB and create comb
Dr. Tolar reported having no financial disclosures.
CHICAGO – Bone marrow transplantation is evolving as a promising treatment for patients with the most severe forms of epidermolysis bullosa.
“Is this a cure? It’s not,” Dr. Jakub Tolar, MD, PhD, said at the World Congress of Pediatric Dermatology. “It is, however, a path toward understanding how we can treat this grave disorder in a systemic way.”
The University of Minnesota BMT Team has also observed a correlation between the engraftment in the blood and engraftment in the skin. “We have skin engraftment as high as 50%, which is good,” Dr. Tolar said. “The more donor cells engrafted in the skin, the more types of collagen you express.”
The clinicians have also been able to reduce the amount of chemotherapy and radiation patients require prior to transplant, for the BMT to work and skin to heal. “We were able to make it so that the last 11 patients are surviving and having benefit from the transplant, with the exception of one,” Dr. Tolar said. “How does this work? We still don’t entirely know. This is not a shot in the dark, however, this is the continuation of a very long process where we were first able to show that bone marrow transplant is an efficient stem cell therapy for leukemia, and about 20 years ago for the lysosomal enzyme deficiencies.” Their hunt for the cell that travels from the bone marrow to skin and produces type 7 collagen is continuing. “What haunts me is that BMT, which works in recessive dystrophic EB, works only in some types of junctional EB, those with alpha-3 chain deficiency of laminin 322.” he continued. “There has been no benefit to bone marrow transplantation for children with mutations of beta-3 chain of laminin 322, so we have closed enrollment for this one form of junctional EB. Survival in this group was 40%. Other types of junctional EB continue to be eligible for the study.”
Dr. Tolar recommended keratinocyte-driven or thymic epithelium cell type–driven therapy for patients with mutations of beta-3. “The deficiency of thymic function seems to be key in the inability to benefit from BMT in this form of junctional EB,” he said. “We have seen children who have engrafted, their skin got better, and then they died of infection many months after transplant. When we look at the immune profile and the thymic epithelial cells, they are both deficient – very abnormal.”
Despite current challenges, Dr. Tolar expressed optimism about the future of BMT in EB patients. “We have the same approach that we have in cancer care: deep empathy for all patients, radical international collaboration, and rapid laboratory and clinical prototyping,” he said. “It’s time to move from two-dimensional science to three-dimensional science; we need to study all aspects of EB simultaneously, from gene to cell to tissue to individual to patient population, and to understand the properties of the whole EB pathobiology that emerge at each level of biological complexity. By connecting information from these layers of disease network, we can better understand EB and create comb
Dr. Tolar reported having no financial disclosures.
CHICAGO – Bone marrow transplantation is evolving as a promising treatment for patients with the most severe forms of epidermolysis bullosa.
“Is this a cure? It’s not,” Dr. Jakub Tolar, MD, PhD, said at the World Congress of Pediatric Dermatology. “It is, however, a path toward understanding how we can treat this grave disorder in a systemic way.”
The University of Minnesota BMT Team has also observed a correlation between the engraftment in the blood and engraftment in the skin. “We have skin engraftment as high as 50%, which is good,” Dr. Tolar said. “The more donor cells engrafted in the skin, the more types of collagen you express.”
The clinicians have also been able to reduce the amount of chemotherapy and radiation patients require prior to transplant, for the BMT to work and skin to heal. “We were able to make it so that the last 11 patients are surviving and having benefit from the transplant, with the exception of one,” Dr. Tolar said. “How does this work? We still don’t entirely know. This is not a shot in the dark, however, this is the continuation of a very long process where we were first able to show that bone marrow transplant is an efficient stem cell therapy for leukemia, and about 20 years ago for the lysosomal enzyme deficiencies.” Their hunt for the cell that travels from the bone marrow to skin and produces type 7 collagen is continuing. “What haunts me is that BMT, which works in recessive dystrophic EB, works only in some types of junctional EB, those with alpha-3 chain deficiency of laminin 322.” he continued. “There has been no benefit to bone marrow transplantation for children with mutations of beta-3 chain of laminin 322, so we have closed enrollment for this one form of junctional EB. Survival in this group was 40%. Other types of junctional EB continue to be eligible for the study.”
Dr. Tolar recommended keratinocyte-driven or thymic epithelium cell type–driven therapy for patients with mutations of beta-3. “The deficiency of thymic function seems to be key in the inability to benefit from BMT in this form of junctional EB,” he said. “We have seen children who have engrafted, their skin got better, and then they died of infection many months after transplant. When we look at the immune profile and the thymic epithelial cells, they are both deficient – very abnormal.”
Despite current challenges, Dr. Tolar expressed optimism about the future of BMT in EB patients. “We have the same approach that we have in cancer care: deep empathy for all patients, radical international collaboration, and rapid laboratory and clinical prototyping,” he said. “It’s time to move from two-dimensional science to three-dimensional science; we need to study all aspects of EB simultaneously, from gene to cell to tissue to individual to patient population, and to understand the properties of the whole EB pathobiology that emerge at each level of biological complexity. By connecting information from these layers of disease network, we can better understand EB and create comb
Dr. Tolar reported having no financial disclosures.
AT WCPD 2017
Four drugs better than three for myeloma induction
MADRID – A four-drug induction regimen induced quicker and deeper remissions than sequential triplet regimens in patients with newly diagnosed multiple myeloma.
In addition, fast, deep remissions may lead to improved progression-free survival (PFS) following autologous stem cell transplantation (ASCT), said investigators from a U.K. Medical Research Council study.
In the phase 3 randomized, parallel group Myeloma XI study, very good partial responses (VGPR) or better were seen following induction in 79.5% of patients assigned to the quadruplet (KCRD) of carfilzomib (Kyprolis), cyclophosphamide, lenalidomide (Revlimid), and dexamethasone, compared with 60.8% for those assigned to cyclophosphamide, lenalidomide, and dexamethasone (CRD) and 52.8% for those assigned to cyclophosphamide, thalidomide, and dexamethasone (CTD), said Charlotte Pawlyn, MD, PhD, at the annual congress of the European Hematology Association.
“In our study, we see a very much deeper response after initial induction with the quadruplet regimen, compared with triplet regimens,” Dr. Pawlyn of the Institute of Cancer Research, London, said in an interview.
Medical Research Council investigators showed in the Myeloma IX study that among patients who had a less than VGPR to an immunomodulator-based triplet regimen such as CRD, a triplet regimen including the proteasome inhibitor bortezomib (Velcade) could improve both pre- and post-transplant response rates, and that the improved responses translated into improved PFS.
For the Myeloma XI study, the investigators employed the same response-adapted approach to compare outcomes following induction with the proteasome inhibitor–containing KCRD regimen and the lenalidomide- or thalidomide-based regimens.
They chose carfilzomib as the proteasome-inhibitor backbone of the quadruplet because of its selective, irreversible target binding, lower incidence of peripheral neuropathy (compared with bortezomib), and efficacy in both the frontline and relapsed/refractory setting, she said.
Asked why the comparator regimens did not contain a proteasome inhibitor, Dr. Pawlyn said that while the current standard for induction therapy in the United Kingdom is bortezomib, thalidomide, and dexamethasone, CTD was the standard of care at the time of study planning and initial enrollment.
The trial was open to all patients in the United Kingdom of all ages with newly diagnosed symptomatic multiple myeloma, with pathways for both transplant-eligible and transplant-ineligible patients. The only main exclusion criteria were for patients with dialysis-dependent renal failure and for those who had a prior or concurrent malignancy.
A total of 1,021 patients were assigned to each of the CTD and CRD cohorts, and 526 patients were assigned to the KCRD cohort. The cohorts were well balanced by sex, age, World Health Organization performance score, and other parameters.
Patients randomized to either CTD or CRD were assessed for response after a minimum of four induction cycles, with treatment continued until best response. Those with a VGPR or better went on to ASCT, while those with a partial response were randomized to either a second induction with cyclophosphamide, bortezomib, and dexamethasone (CVD) or no CVD, and then proceeded to transplant. Patients with stable disease or disease progression in either of these arms went on to CVD prior to ASCT.
In the KCRD arm, all patients went from induction to transplant. Following ASCT, patients were randomized to either observation or lenalidomide maintenance.
A higher proportion of patients assigned to KCRD completed the minimum of four induction cycles, and few patients in any trial arm had to stop induction therapy because of adverse events. Dose modifications were required in 63.9% of patients on KCRD, 56.3% of patients on CRD, and 82.2% of patients on CTD.
There was no significant cardiac signal seen in the study, and no difference in the incidence of venous thromboembolic events among the treatment arms.
As noted before, rates of VGPR or better after initial induction were highest in the KCRD arm, at 79.5%, compared with 60% for CRD, and 52.8% for CTD.
“The KCRD quadruplet achieved the highest speed and depth of response,” Dr. Pawlyn said.
The pattern of responses was similar across all cytogenetic risk groups, she added.
A higher proportion of patients treated with KCRD went on to ASCT, and the pattern of deeper responses among patients who underwent induction with KCRD persisted, with 92.1% of patients having a post-transplant VGPR or better, compared with 81.8% for CRD and 77.0% for CTD (statistical significance not shown).
Again, the pattern of responses post-transplant was similar across cytogenetic risk groups.
The investigators anticipate receiving PFS results from the Myeloma XI study in the third or fourth quarter of 2017.
The study was sponsored by the University of Leeds (England), with support from the U.K. National Cancer Research Institute, Cancer Research UK, and Myeloma UK, and collaboration with Celgene, Merck Sharp & Dohme, and Amgen. Dr. Pawlyn disclosed travel support from Celgene and Janssen, and honoraria from Celgene and Takeda.
MADRID – A four-drug induction regimen induced quicker and deeper remissions than sequential triplet regimens in patients with newly diagnosed multiple myeloma.
In addition, fast, deep remissions may lead to improved progression-free survival (PFS) following autologous stem cell transplantation (ASCT), said investigators from a U.K. Medical Research Council study.
In the phase 3 randomized, parallel group Myeloma XI study, very good partial responses (VGPR) or better were seen following induction in 79.5% of patients assigned to the quadruplet (KCRD) of carfilzomib (Kyprolis), cyclophosphamide, lenalidomide (Revlimid), and dexamethasone, compared with 60.8% for those assigned to cyclophosphamide, lenalidomide, and dexamethasone (CRD) and 52.8% for those assigned to cyclophosphamide, thalidomide, and dexamethasone (CTD), said Charlotte Pawlyn, MD, PhD, at the annual congress of the European Hematology Association.
“In our study, we see a very much deeper response after initial induction with the quadruplet regimen, compared with triplet regimens,” Dr. Pawlyn of the Institute of Cancer Research, London, said in an interview.
Medical Research Council investigators showed in the Myeloma IX study that among patients who had a less than VGPR to an immunomodulator-based triplet regimen such as CRD, a triplet regimen including the proteasome inhibitor bortezomib (Velcade) could improve both pre- and post-transplant response rates, and that the improved responses translated into improved PFS.
For the Myeloma XI study, the investigators employed the same response-adapted approach to compare outcomes following induction with the proteasome inhibitor–containing KCRD regimen and the lenalidomide- or thalidomide-based regimens.
They chose carfilzomib as the proteasome-inhibitor backbone of the quadruplet because of its selective, irreversible target binding, lower incidence of peripheral neuropathy (compared with bortezomib), and efficacy in both the frontline and relapsed/refractory setting, she said.
Asked why the comparator regimens did not contain a proteasome inhibitor, Dr. Pawlyn said that while the current standard for induction therapy in the United Kingdom is bortezomib, thalidomide, and dexamethasone, CTD was the standard of care at the time of study planning and initial enrollment.
The trial was open to all patients in the United Kingdom of all ages with newly diagnosed symptomatic multiple myeloma, with pathways for both transplant-eligible and transplant-ineligible patients. The only main exclusion criteria were for patients with dialysis-dependent renal failure and for those who had a prior or concurrent malignancy.
A total of 1,021 patients were assigned to each of the CTD and CRD cohorts, and 526 patients were assigned to the KCRD cohort. The cohorts were well balanced by sex, age, World Health Organization performance score, and other parameters.
Patients randomized to either CTD or CRD were assessed for response after a minimum of four induction cycles, with treatment continued until best response. Those with a VGPR or better went on to ASCT, while those with a partial response were randomized to either a second induction with cyclophosphamide, bortezomib, and dexamethasone (CVD) or no CVD, and then proceeded to transplant. Patients with stable disease or disease progression in either of these arms went on to CVD prior to ASCT.
In the KCRD arm, all patients went from induction to transplant. Following ASCT, patients were randomized to either observation or lenalidomide maintenance.
A higher proportion of patients assigned to KCRD completed the minimum of four induction cycles, and few patients in any trial arm had to stop induction therapy because of adverse events. Dose modifications were required in 63.9% of patients on KCRD, 56.3% of patients on CRD, and 82.2% of patients on CTD.
There was no significant cardiac signal seen in the study, and no difference in the incidence of venous thromboembolic events among the treatment arms.
As noted before, rates of VGPR or better after initial induction were highest in the KCRD arm, at 79.5%, compared with 60% for CRD, and 52.8% for CTD.
“The KCRD quadruplet achieved the highest speed and depth of response,” Dr. Pawlyn said.
The pattern of responses was similar across all cytogenetic risk groups, she added.
A higher proportion of patients treated with KCRD went on to ASCT, and the pattern of deeper responses among patients who underwent induction with KCRD persisted, with 92.1% of patients having a post-transplant VGPR or better, compared with 81.8% for CRD and 77.0% for CTD (statistical significance not shown).
Again, the pattern of responses post-transplant was similar across cytogenetic risk groups.
The investigators anticipate receiving PFS results from the Myeloma XI study in the third or fourth quarter of 2017.
The study was sponsored by the University of Leeds (England), with support from the U.K. National Cancer Research Institute, Cancer Research UK, and Myeloma UK, and collaboration with Celgene, Merck Sharp & Dohme, and Amgen. Dr. Pawlyn disclosed travel support from Celgene and Janssen, and honoraria from Celgene and Takeda.
MADRID – A four-drug induction regimen induced quicker and deeper remissions than sequential triplet regimens in patients with newly diagnosed multiple myeloma.
In addition, fast, deep remissions may lead to improved progression-free survival (PFS) following autologous stem cell transplantation (ASCT), said investigators from a U.K. Medical Research Council study.
In the phase 3 randomized, parallel group Myeloma XI study, very good partial responses (VGPR) or better were seen following induction in 79.5% of patients assigned to the quadruplet (KCRD) of carfilzomib (Kyprolis), cyclophosphamide, lenalidomide (Revlimid), and dexamethasone, compared with 60.8% for those assigned to cyclophosphamide, lenalidomide, and dexamethasone (CRD) and 52.8% for those assigned to cyclophosphamide, thalidomide, and dexamethasone (CTD), said Charlotte Pawlyn, MD, PhD, at the annual congress of the European Hematology Association.
“In our study, we see a very much deeper response after initial induction with the quadruplet regimen, compared with triplet regimens,” Dr. Pawlyn of the Institute of Cancer Research, London, said in an interview.
Medical Research Council investigators showed in the Myeloma IX study that among patients who had a less than VGPR to an immunomodulator-based triplet regimen such as CRD, a triplet regimen including the proteasome inhibitor bortezomib (Velcade) could improve both pre- and post-transplant response rates, and that the improved responses translated into improved PFS.
For the Myeloma XI study, the investigators employed the same response-adapted approach to compare outcomes following induction with the proteasome inhibitor–containing KCRD regimen and the lenalidomide- or thalidomide-based regimens.
They chose carfilzomib as the proteasome-inhibitor backbone of the quadruplet because of its selective, irreversible target binding, lower incidence of peripheral neuropathy (compared with bortezomib), and efficacy in both the frontline and relapsed/refractory setting, she said.
Asked why the comparator regimens did not contain a proteasome inhibitor, Dr. Pawlyn said that while the current standard for induction therapy in the United Kingdom is bortezomib, thalidomide, and dexamethasone, CTD was the standard of care at the time of study planning and initial enrollment.
The trial was open to all patients in the United Kingdom of all ages with newly diagnosed symptomatic multiple myeloma, with pathways for both transplant-eligible and transplant-ineligible patients. The only main exclusion criteria were for patients with dialysis-dependent renal failure and for those who had a prior or concurrent malignancy.
A total of 1,021 patients were assigned to each of the CTD and CRD cohorts, and 526 patients were assigned to the KCRD cohort. The cohorts were well balanced by sex, age, World Health Organization performance score, and other parameters.
Patients randomized to either CTD or CRD were assessed for response after a minimum of four induction cycles, with treatment continued until best response. Those with a VGPR or better went on to ASCT, while those with a partial response were randomized to either a second induction with cyclophosphamide, bortezomib, and dexamethasone (CVD) or no CVD, and then proceeded to transplant. Patients with stable disease or disease progression in either of these arms went on to CVD prior to ASCT.
In the KCRD arm, all patients went from induction to transplant. Following ASCT, patients were randomized to either observation or lenalidomide maintenance.
A higher proportion of patients assigned to KCRD completed the minimum of four induction cycles, and few patients in any trial arm had to stop induction therapy because of adverse events. Dose modifications were required in 63.9% of patients on KCRD, 56.3% of patients on CRD, and 82.2% of patients on CTD.
There was no significant cardiac signal seen in the study, and no difference in the incidence of venous thromboembolic events among the treatment arms.
As noted before, rates of VGPR or better after initial induction were highest in the KCRD arm, at 79.5%, compared with 60% for CRD, and 52.8% for CTD.
“The KCRD quadruplet achieved the highest speed and depth of response,” Dr. Pawlyn said.
The pattern of responses was similar across all cytogenetic risk groups, she added.
A higher proportion of patients treated with KCRD went on to ASCT, and the pattern of deeper responses among patients who underwent induction with KCRD persisted, with 92.1% of patients having a post-transplant VGPR or better, compared with 81.8% for CRD and 77.0% for CTD (statistical significance not shown).
Again, the pattern of responses post-transplant was similar across cytogenetic risk groups.
The investigators anticipate receiving PFS results from the Myeloma XI study in the third or fourth quarter of 2017.
The study was sponsored by the University of Leeds (England), with support from the U.K. National Cancer Research Institute, Cancer Research UK, and Myeloma UK, and collaboration with Celgene, Merck Sharp & Dohme, and Amgen. Dr. Pawlyn disclosed travel support from Celgene and Janssen, and honoraria from Celgene and Takeda.
AT THE EHA CONGRESS
Key clinical point:
Major finding: An induction quadruplet containing carfilzomib induced a higher rate of very good partial responses or better vs. regimens without a proteasome inhibitor.
Data source: A randomized, open-label, parallel group study of 2,568 patients with newly diagnosed multiple myeloma.
Disclosures: The study was sponsored by the University of Leeds (England), with support from the U.K. National Cancer Research Institute, Cancer Research UK, and Myeloma UK, and collaboration with Celgene, Merck Sharp & Dohme, and Amgen. Dr. Pawlyn disclosed travel support from Celgene and Janssen, and honoraria from Celgene and Takeda.
Allele-matching in cord blood transplant yields better survival
Matching down to the allele level in umbilical cord blood transplantation between unrelated donors results in greater overall survival for those with nonmalignant diseases, such as aplastic anemia, researchers found in a retrospective study published in the Lancet Haematology.
The review (Lancet Haematol. 2017 Jul;4[7]:e325-33), the largest published on the topic, indicates that clinicians should change practice from the current standard of antigen-level matching, said Mary Eapen, MD, director of the Center for International Blood and Marrow Transplant Research (CIBMTR) in Wauwatosa, Wisconsin.
“Our findings,” Dr. Eapen wrote, “support a change in clinical practice to prioritization of units on allele-level HLA matching at HLA-A, HLA-B, HLA-C, and HLA-DRB1.”
Data were pulled from cases reported to the Center for International Blood and Marrow Transplant Research or the European Group for Blood and Marrow Transplant. Researchers looked at 1,199 donor-recipient matches of cord blood transplantation for diseases, such as severe combined immunodeficiency (SCID), non-SCID primary immunodeficiency, inborn errors of metabolism, severe aplastic anemia, and Fanconi anemia. Recipients could be as old as age 16, but most were age 5 or younger.
After adjustment for factors, including cytomegalovirus serostatus, the intensity of the conditioning regimen, and the total nucleated cell dose, the 5-year overall survival was 79% for transplants that were matched at all eight alleles at HLA-A, HLA-B, HLA-C and HLA-DRB1. These results compare with 76% after transplants with one mismatch, 70% with two mismatches, 62% with three mismatches, and 49% with 4 or more mismatches.
Mortality risks were significantly higher for patients who received transplants with two (P = .018), three (P = .0001), and four or more mismatches (P less than .0001), compared with those whose transplants were fully matched. There was no difference statistically between full matches and one mismatch, but the findings suggest that the mortality risk might prove significant with a larger sample size.
Researchers cautioned that, because most patients were age 5 or younger, the results might not be generalizable to older children.
Although HLA typing is available at CIBMTR for most blood cord transplants for nonmalignant diseases, full allele matches or just one mismatch are not the norm, Dr. Eapen wrote. Researchers said that they suspect this is because of difficulties finding matches or because a high total nucleated cell count is prioritized above HLA matching. They suggest clinicians change their decision making in this regard.
Matching down to the allele level in umbilical cord blood transplantation between unrelated donors results in greater overall survival for those with nonmalignant diseases, such as aplastic anemia, researchers found in a retrospective study published in the Lancet Haematology.
The review (Lancet Haematol. 2017 Jul;4[7]:e325-33), the largest published on the topic, indicates that clinicians should change practice from the current standard of antigen-level matching, said Mary Eapen, MD, director of the Center for International Blood and Marrow Transplant Research (CIBMTR) in Wauwatosa, Wisconsin.
“Our findings,” Dr. Eapen wrote, “support a change in clinical practice to prioritization of units on allele-level HLA matching at HLA-A, HLA-B, HLA-C, and HLA-DRB1.”
Data were pulled from cases reported to the Center for International Blood and Marrow Transplant Research or the European Group for Blood and Marrow Transplant. Researchers looked at 1,199 donor-recipient matches of cord blood transplantation for diseases, such as severe combined immunodeficiency (SCID), non-SCID primary immunodeficiency, inborn errors of metabolism, severe aplastic anemia, and Fanconi anemia. Recipients could be as old as age 16, but most were age 5 or younger.
After adjustment for factors, including cytomegalovirus serostatus, the intensity of the conditioning regimen, and the total nucleated cell dose, the 5-year overall survival was 79% for transplants that were matched at all eight alleles at HLA-A, HLA-B, HLA-C and HLA-DRB1. These results compare with 76% after transplants with one mismatch, 70% with two mismatches, 62% with three mismatches, and 49% with 4 or more mismatches.
Mortality risks were significantly higher for patients who received transplants with two (P = .018), three (P = .0001), and four or more mismatches (P less than .0001), compared with those whose transplants were fully matched. There was no difference statistically between full matches and one mismatch, but the findings suggest that the mortality risk might prove significant with a larger sample size.
Researchers cautioned that, because most patients were age 5 or younger, the results might not be generalizable to older children.
Although HLA typing is available at CIBMTR for most blood cord transplants for nonmalignant diseases, full allele matches or just one mismatch are not the norm, Dr. Eapen wrote. Researchers said that they suspect this is because of difficulties finding matches or because a high total nucleated cell count is prioritized above HLA matching. They suggest clinicians change their decision making in this regard.
Matching down to the allele level in umbilical cord blood transplantation between unrelated donors results in greater overall survival for those with nonmalignant diseases, such as aplastic anemia, researchers found in a retrospective study published in the Lancet Haematology.
The review (Lancet Haematol. 2017 Jul;4[7]:e325-33), the largest published on the topic, indicates that clinicians should change practice from the current standard of antigen-level matching, said Mary Eapen, MD, director of the Center for International Blood and Marrow Transplant Research (CIBMTR) in Wauwatosa, Wisconsin.
“Our findings,” Dr. Eapen wrote, “support a change in clinical practice to prioritization of units on allele-level HLA matching at HLA-A, HLA-B, HLA-C, and HLA-DRB1.”
Data were pulled from cases reported to the Center for International Blood and Marrow Transplant Research or the European Group for Blood and Marrow Transplant. Researchers looked at 1,199 donor-recipient matches of cord blood transplantation for diseases, such as severe combined immunodeficiency (SCID), non-SCID primary immunodeficiency, inborn errors of metabolism, severe aplastic anemia, and Fanconi anemia. Recipients could be as old as age 16, but most were age 5 or younger.
After adjustment for factors, including cytomegalovirus serostatus, the intensity of the conditioning regimen, and the total nucleated cell dose, the 5-year overall survival was 79% for transplants that were matched at all eight alleles at HLA-A, HLA-B, HLA-C and HLA-DRB1. These results compare with 76% after transplants with one mismatch, 70% with two mismatches, 62% with three mismatches, and 49% with 4 or more mismatches.
Mortality risks were significantly higher for patients who received transplants with two (P = .018), three (P = .0001), and four or more mismatches (P less than .0001), compared with those whose transplants were fully matched. There was no difference statistically between full matches and one mismatch, but the findings suggest that the mortality risk might prove significant with a larger sample size.
Researchers cautioned that, because most patients were age 5 or younger, the results might not be generalizable to older children.
Although HLA typing is available at CIBMTR for most blood cord transplants for nonmalignant diseases, full allele matches or just one mismatch are not the norm, Dr. Eapen wrote. Researchers said that they suspect this is because of difficulties finding matches or because a high total nucleated cell count is prioritized above HLA matching. They suggest clinicians change their decision making in this regard.
FROM THE LANCET HAEMATOLOGY
Key clinical point: HLA matching at the allele-level produces better survival in umbilical cord blood transplantation for nonmalignant diseases.
Major finding: Mortality risks were significantly higher for patients who received transplants with two (P = .018), three (P = .0001), and four or more mismatches (P less than .0001), compared with those whose transplants were fully matched at all eight alleles at HLA-A, HLA-B, HLA-C and HLA-DRB1.
Data source: A retrospective review of 1,199 cases reported to the Center for International Blood and Marrow Transplant Research (CIBMTR) or the European Group for Blood and Marrow Transplant (EGBMT).
Disclosures: The authors reported no conflicts of interest.
All FDA panel members go thumbs up for CTL019 in relapsed/refractory childhood ALL
The answer to the billion dollar question – Does the chimeric antigen receptor T-cell (CAR T) construct CTL019 (tisagenlecleucel-T) have a favorable risk-benefit profile for the treatment of children and young adults with relapsed/refractory B-cell precursor acute lymphoblastic leukemia? – was a unanimous “yes” at a July 12 meeting of the Food and Drug Administration’s Oncologic Drugs Advisory Committee.
“This is the most exciting thing I have seen in my lifetime, and probably since the introduction of ‘multiagent total cancer care,’ as it was called then, for treatment of childhood leukemia,” remarked Timothy P. Cripe, MD, PhD, from Nationwide Children’s Hospital in Columbus, Ohio, and a temporary voting member of the ODAC.
Catherine M. Bollard, MD, MBChB, from the Children’s National Medical Center in Washington, also a temporary ODAC member, said that she voted “yes” because “this is a very poor-risk patient population, this is an unmet need in the pediatric population, and as you saw in the data [presented to ODAC] today, the clinical responses are remarkable. I think Novartis [the maker of CTL019] has done a great job putting together a plan for mitigating risk going forward.”
CTL019 was shown in a pivotal phase 2 clinical trial to induce an overall remission rate of 83% in children and young adults with relapsed/refractory ALL for whom at least two prior lines of therapy had failed. Based on these results, the FDA accepted a biologics license application for the agent from Novartis.
At the meeting, panel members initially seemed favorably disposed toward recommending approval but heard concerns from FDA scientists about the potential for severe or fatal adverse events such as the cytokine release syndrome (CRS); the possible generation of replication competent retrovirus (RCR); and the potential for secondary malignancies from insertional mutagenesis, the incorporation of portions of the lentiviral vector into the patient’s genome.
In his opening remarks, Wilson W. Bryan, MD, from the FDA’s Office of Tissue and Advanced Therapies and Center for Biologics Evaluation and Drug Research, commented that “the clinical development of tisagenlecleucel suggests that this is a life-saving product.”
He went on, however, to frame the FDA’s concerns: “Clinical trials are not always a good predictor of the effectiveness and safety of a marketed product,” he said. “In particular, we are concerned that the same benefit and safety seen in clinical trials may not carry over to routine clinical use.”
The purpose of the hearing was to focus on manufacturing issues related to product quality, including replicability of the product for commercial use and safety issues such as prevention of CRS and neurotoxicities.
“We are also concerned about the hypothetical risk of secondary malignancies. Therefore, we are asking for the committee’s recommendations regarding the nature and duration of follow-up of patients who would receive this product,” Dr. Bryan said.
“CTL019 is a living drug, which demonstrates activity after a single infusion,” said Samit Hirawat, MD, head of oncology global development for Novartis.
But the nature of CTL019 as a living drug also means that it is subject to variations in the ability of autologous T cells harvested via leukapheresis to be infected with the lentiviral vector and expanded into a population of CAR T cells large enough to have therapeutic value, said Xiaobin Victor Lu, PhD, a chemistry, manufacturing, and controls reviewer for the FDA.
Mitigation plan
Novartis’ proposed plan includes specific, long-term steps for mitigating the risk of CRS and neurologic events, such as cerebral edema, the latter of which caused the FDA to call for a clinical hold of the phase 2 ROCKET trial for a different CAR T-cell construct.
Among the proposed elements of the mitigation plan are a 15-year minimum pharmacovigilance program and long-term safety follow-up for adverse events related to the therapy, efficacy, immunogenicity, transgene persistence of CD19 CAR, and the incidence of second malignancies possibly related to insertional mutagenesis.
Novartis also will train treatment center staff on processes for cell collection, cryopreservation, transport, chain of identity, safety management, and logistics for handling the CAR T-cell product. The company proposes to provide on-site training of personnel on CRS and neurotoxicity risk and management, as well as to offer information to patients and caregivers about the signs and symptoms of adverse events of concern.
Dr. Cripe expressed his concerns that Novartis’ proposal to initially limit the mitigation plan to 30 or 35 treatment sites would create problems of access and economic disparities among patients, and could cause inequities among treatment centers even with the same city.
David Lebwohl, MD, head of the CAR T global program for Novartis, said that the planned number of sites for the mitigation program would be expanded after 6 to 12 months if the CAR T construct receives final approval and clinical implementation goes well.
There was nearly unanimous agreement among the panel members that the planned 15-year follow-up and other mitigation measures would be adequate for detecting serious short- and long-term consequences of CAR T-cell therapy.
Patient/advocate perspective
In the public comment section of the proceedings, panel members were urged to vote in favor of CTL019 by parents of children with ALL, including Don McMahon, whose son Connor received the therapy after multiple relapses, and Tom Whitehead, father of Emily Whitehead, the first patient to receive CAR T cells for ALL.
Both children are alive and doing well.
CTL019 is produced by Novartis.
The unanimous recommendation by the Food and Drug Administration's Oncologic Drugs Advisory Committee means that the FDA is likely to approve CTL019 (tisagenlecleucel-T), and that approval may come quickly, possibly before the end of 2017. This approval was based on compelling data showing that 83% of children and young adults with refractory or relapsed acute lymphocytic leukemia (ALL) achieved remission with this therapy. This is exciting news for ALL patients as well as for the cell and gene therapy community. What remains to be determined are the labelling for CTL019, the cost of the therapy, and whether all patients who might benefit from this therapy will have the coverage to be able to access it.
While the response rates in patients treated in the trials presented to the FDA are very encouraging there are also concerns with the risks for cytokine release syndrome and neurotoxicity which can affect up to half of treated patients. As a result, Novartis, the manufacturer of CTL019, has proposed an extensive mitigation strategy and education process for the cell therapy centers that will offer the therapy. Initially, this is likely to be limited to around 30 centers that will be geographically distributed throughout the United States with gradual roll out to more centers as there is more experience with the use of CTL019.
Another issue for the centers is going to be operationalizing a new paradigm where CTL019 will be offered as a standard of care rather than in the context of a research study. Most of the initial pediatric centers will likely provide CTL019, within their transplant infrastructure since procurement, initial processing and infusion of cells will utilize their cell processing and collection facilities. The Foundation for Accreditation of Cell Therapy (FACT) has also anticipated this approval by publishing new standards for Immune Effectors earlier this year to promote quality practice in immune effector cell administration.
One other question is whether CTL019 will be transplant enabling or transplant replacing. While the initial response rates are very high and there are some well publicized patients who remain in remission over 5 years after CTL019 without other therapy, other responders proceeded to transplant and there is also a significant relapse rate. It is therefore an open question whether treating physicians will be happy to watch patients who attain remission after this therapy or whether they will still recommend transplant because there is not yet enough follow up on this product to know what the long-term cure rate is going to be.
Another CAR T-cell product is scheduled to come before an FDA advisory committee in October. The indication for KTE-C19 (axicabtagene ciloleucel) from Kite is for relapsed/refractory diffuse large B-cell lymphoma, a much bigger indication with a potentially much larger number of patients. The response rates for KTE-C19 in DLBCL (and indeed for CTL019 in DLBCL) are not as high as those for CTL019 in ALL and follow-up time is shorter, so it is not yet clear how many patients will have sustained long term responses. Nevertheless the response rate in patients who have failed all other therapies is high enough that this product will also likely be approved.
Helen Heslop, MD, is the Dan L. Duncan Chair and Professor of Medicine and Pediatrics at Baylor College of Medicine, Houston. She also is the Director of the Center for Cell and Gene Therapy at Baylor College of Medicine, Houston Methodist Hospital and Texas Children's Hospital. Dr. Heslop is a member of the editorial advisory board of Hematology News.
The unanimous recommendation by the Food and Drug Administration's Oncologic Drugs Advisory Committee means that the FDA is likely to approve CTL019 (tisagenlecleucel-T), and that approval may come quickly, possibly before the end of 2017. This approval was based on compelling data showing that 83% of children and young adults with refractory or relapsed acute lymphocytic leukemia (ALL) achieved remission with this therapy. This is exciting news for ALL patients as well as for the cell and gene therapy community. What remains to be determined are the labelling for CTL019, the cost of the therapy, and whether all patients who might benefit from this therapy will have the coverage to be able to access it.
While the response rates in patients treated in the trials presented to the FDA are very encouraging there are also concerns with the risks for cytokine release syndrome and neurotoxicity which can affect up to half of treated patients. As a result, Novartis, the manufacturer of CTL019, has proposed an extensive mitigation strategy and education process for the cell therapy centers that will offer the therapy. Initially, this is likely to be limited to around 30 centers that will be geographically distributed throughout the United States with gradual roll out to more centers as there is more experience with the use of CTL019.
Another issue for the centers is going to be operationalizing a new paradigm where CTL019 will be offered as a standard of care rather than in the context of a research study. Most of the initial pediatric centers will likely provide CTL019, within their transplant infrastructure since procurement, initial processing and infusion of cells will utilize their cell processing and collection facilities. The Foundation for Accreditation of Cell Therapy (FACT) has also anticipated this approval by publishing new standards for Immune Effectors earlier this year to promote quality practice in immune effector cell administration.
One other question is whether CTL019 will be transplant enabling or transplant replacing. While the initial response rates are very high and there are some well publicized patients who remain in remission over 5 years after CTL019 without other therapy, other responders proceeded to transplant and there is also a significant relapse rate. It is therefore an open question whether treating physicians will be happy to watch patients who attain remission after this therapy or whether they will still recommend transplant because there is not yet enough follow up on this product to know what the long-term cure rate is going to be.
Another CAR T-cell product is scheduled to come before an FDA advisory committee in October. The indication for KTE-C19 (axicabtagene ciloleucel) from Kite is for relapsed/refractory diffuse large B-cell lymphoma, a much bigger indication with a potentially much larger number of patients. The response rates for KTE-C19 in DLBCL (and indeed for CTL019 in DLBCL) are not as high as those for CTL019 in ALL and follow-up time is shorter, so it is not yet clear how many patients will have sustained long term responses. Nevertheless the response rate in patients who have failed all other therapies is high enough that this product will also likely be approved.
Helen Heslop, MD, is the Dan L. Duncan Chair and Professor of Medicine and Pediatrics at Baylor College of Medicine, Houston. She also is the Director of the Center for Cell and Gene Therapy at Baylor College of Medicine, Houston Methodist Hospital and Texas Children's Hospital. Dr. Heslop is a member of the editorial advisory board of Hematology News.
The unanimous recommendation by the Food and Drug Administration's Oncologic Drugs Advisory Committee means that the FDA is likely to approve CTL019 (tisagenlecleucel-T), and that approval may come quickly, possibly before the end of 2017. This approval was based on compelling data showing that 83% of children and young adults with refractory or relapsed acute lymphocytic leukemia (ALL) achieved remission with this therapy. This is exciting news for ALL patients as well as for the cell and gene therapy community. What remains to be determined are the labelling for CTL019, the cost of the therapy, and whether all patients who might benefit from this therapy will have the coverage to be able to access it.
While the response rates in patients treated in the trials presented to the FDA are very encouraging there are also concerns with the risks for cytokine release syndrome and neurotoxicity which can affect up to half of treated patients. As a result, Novartis, the manufacturer of CTL019, has proposed an extensive mitigation strategy and education process for the cell therapy centers that will offer the therapy. Initially, this is likely to be limited to around 30 centers that will be geographically distributed throughout the United States with gradual roll out to more centers as there is more experience with the use of CTL019.
Another issue for the centers is going to be operationalizing a new paradigm where CTL019 will be offered as a standard of care rather than in the context of a research study. Most of the initial pediatric centers will likely provide CTL019, within their transplant infrastructure since procurement, initial processing and infusion of cells will utilize their cell processing and collection facilities. The Foundation for Accreditation of Cell Therapy (FACT) has also anticipated this approval by publishing new standards for Immune Effectors earlier this year to promote quality practice in immune effector cell administration.
One other question is whether CTL019 will be transplant enabling or transplant replacing. While the initial response rates are very high and there are some well publicized patients who remain in remission over 5 years after CTL019 without other therapy, other responders proceeded to transplant and there is also a significant relapse rate. It is therefore an open question whether treating physicians will be happy to watch patients who attain remission after this therapy or whether they will still recommend transplant because there is not yet enough follow up on this product to know what the long-term cure rate is going to be.
Another CAR T-cell product is scheduled to come before an FDA advisory committee in October. The indication for KTE-C19 (axicabtagene ciloleucel) from Kite is for relapsed/refractory diffuse large B-cell lymphoma, a much bigger indication with a potentially much larger number of patients. The response rates for KTE-C19 in DLBCL (and indeed for CTL019 in DLBCL) are not as high as those for CTL019 in ALL and follow-up time is shorter, so it is not yet clear how many patients will have sustained long term responses. Nevertheless the response rate in patients who have failed all other therapies is high enough that this product will also likely be approved.
Helen Heslop, MD, is the Dan L. Duncan Chair and Professor of Medicine and Pediatrics at Baylor College of Medicine, Houston. She also is the Director of the Center for Cell and Gene Therapy at Baylor College of Medicine, Houston Methodist Hospital and Texas Children's Hospital. Dr. Heslop is a member of the editorial advisory board of Hematology News.
The answer to the billion dollar question – Does the chimeric antigen receptor T-cell (CAR T) construct CTL019 (tisagenlecleucel-T) have a favorable risk-benefit profile for the treatment of children and young adults with relapsed/refractory B-cell precursor acute lymphoblastic leukemia? – was a unanimous “yes” at a July 12 meeting of the Food and Drug Administration’s Oncologic Drugs Advisory Committee.
“This is the most exciting thing I have seen in my lifetime, and probably since the introduction of ‘multiagent total cancer care,’ as it was called then, for treatment of childhood leukemia,” remarked Timothy P. Cripe, MD, PhD, from Nationwide Children’s Hospital in Columbus, Ohio, and a temporary voting member of the ODAC.
Catherine M. Bollard, MD, MBChB, from the Children’s National Medical Center in Washington, also a temporary ODAC member, said that she voted “yes” because “this is a very poor-risk patient population, this is an unmet need in the pediatric population, and as you saw in the data [presented to ODAC] today, the clinical responses are remarkable. I think Novartis [the maker of CTL019] has done a great job putting together a plan for mitigating risk going forward.”
CTL019 was shown in a pivotal phase 2 clinical trial to induce an overall remission rate of 83% in children and young adults with relapsed/refractory ALL for whom at least two prior lines of therapy had failed. Based on these results, the FDA accepted a biologics license application for the agent from Novartis.
At the meeting, panel members initially seemed favorably disposed toward recommending approval but heard concerns from FDA scientists about the potential for severe or fatal adverse events such as the cytokine release syndrome (CRS); the possible generation of replication competent retrovirus (RCR); and the potential for secondary malignancies from insertional mutagenesis, the incorporation of portions of the lentiviral vector into the patient’s genome.
In his opening remarks, Wilson W. Bryan, MD, from the FDA’s Office of Tissue and Advanced Therapies and Center for Biologics Evaluation and Drug Research, commented that “the clinical development of tisagenlecleucel suggests that this is a life-saving product.”
He went on, however, to frame the FDA’s concerns: “Clinical trials are not always a good predictor of the effectiveness and safety of a marketed product,” he said. “In particular, we are concerned that the same benefit and safety seen in clinical trials may not carry over to routine clinical use.”
The purpose of the hearing was to focus on manufacturing issues related to product quality, including replicability of the product for commercial use and safety issues such as prevention of CRS and neurotoxicities.
“We are also concerned about the hypothetical risk of secondary malignancies. Therefore, we are asking for the committee’s recommendations regarding the nature and duration of follow-up of patients who would receive this product,” Dr. Bryan said.
“CTL019 is a living drug, which demonstrates activity after a single infusion,” said Samit Hirawat, MD, head of oncology global development for Novartis.
But the nature of CTL019 as a living drug also means that it is subject to variations in the ability of autologous T cells harvested via leukapheresis to be infected with the lentiviral vector and expanded into a population of CAR T cells large enough to have therapeutic value, said Xiaobin Victor Lu, PhD, a chemistry, manufacturing, and controls reviewer for the FDA.
Mitigation plan
Novartis’ proposed plan includes specific, long-term steps for mitigating the risk of CRS and neurologic events, such as cerebral edema, the latter of which caused the FDA to call for a clinical hold of the phase 2 ROCKET trial for a different CAR T-cell construct.
Among the proposed elements of the mitigation plan are a 15-year minimum pharmacovigilance program and long-term safety follow-up for adverse events related to the therapy, efficacy, immunogenicity, transgene persistence of CD19 CAR, and the incidence of second malignancies possibly related to insertional mutagenesis.
Novartis also will train treatment center staff on processes for cell collection, cryopreservation, transport, chain of identity, safety management, and logistics for handling the CAR T-cell product. The company proposes to provide on-site training of personnel on CRS and neurotoxicity risk and management, as well as to offer information to patients and caregivers about the signs and symptoms of adverse events of concern.
Dr. Cripe expressed his concerns that Novartis’ proposal to initially limit the mitigation plan to 30 or 35 treatment sites would create problems of access and economic disparities among patients, and could cause inequities among treatment centers even with the same city.
David Lebwohl, MD, head of the CAR T global program for Novartis, said that the planned number of sites for the mitigation program would be expanded after 6 to 12 months if the CAR T construct receives final approval and clinical implementation goes well.
There was nearly unanimous agreement among the panel members that the planned 15-year follow-up and other mitigation measures would be adequate for detecting serious short- and long-term consequences of CAR T-cell therapy.
Patient/advocate perspective
In the public comment section of the proceedings, panel members were urged to vote in favor of CTL019 by parents of children with ALL, including Don McMahon, whose son Connor received the therapy after multiple relapses, and Tom Whitehead, father of Emily Whitehead, the first patient to receive CAR T cells for ALL.
Both children are alive and doing well.
CTL019 is produced by Novartis.
The answer to the billion dollar question – Does the chimeric antigen receptor T-cell (CAR T) construct CTL019 (tisagenlecleucel-T) have a favorable risk-benefit profile for the treatment of children and young adults with relapsed/refractory B-cell precursor acute lymphoblastic leukemia? – was a unanimous “yes” at a July 12 meeting of the Food and Drug Administration’s Oncologic Drugs Advisory Committee.
“This is the most exciting thing I have seen in my lifetime, and probably since the introduction of ‘multiagent total cancer care,’ as it was called then, for treatment of childhood leukemia,” remarked Timothy P. Cripe, MD, PhD, from Nationwide Children’s Hospital in Columbus, Ohio, and a temporary voting member of the ODAC.
Catherine M. Bollard, MD, MBChB, from the Children’s National Medical Center in Washington, also a temporary ODAC member, said that she voted “yes” because “this is a very poor-risk patient population, this is an unmet need in the pediatric population, and as you saw in the data [presented to ODAC] today, the clinical responses are remarkable. I think Novartis [the maker of CTL019] has done a great job putting together a plan for mitigating risk going forward.”
CTL019 was shown in a pivotal phase 2 clinical trial to induce an overall remission rate of 83% in children and young adults with relapsed/refractory ALL for whom at least two prior lines of therapy had failed. Based on these results, the FDA accepted a biologics license application for the agent from Novartis.
At the meeting, panel members initially seemed favorably disposed toward recommending approval but heard concerns from FDA scientists about the potential for severe or fatal adverse events such as the cytokine release syndrome (CRS); the possible generation of replication competent retrovirus (RCR); and the potential for secondary malignancies from insertional mutagenesis, the incorporation of portions of the lentiviral vector into the patient’s genome.
In his opening remarks, Wilson W. Bryan, MD, from the FDA’s Office of Tissue and Advanced Therapies and Center for Biologics Evaluation and Drug Research, commented that “the clinical development of tisagenlecleucel suggests that this is a life-saving product.”
He went on, however, to frame the FDA’s concerns: “Clinical trials are not always a good predictor of the effectiveness and safety of a marketed product,” he said. “In particular, we are concerned that the same benefit and safety seen in clinical trials may not carry over to routine clinical use.”
The purpose of the hearing was to focus on manufacturing issues related to product quality, including replicability of the product for commercial use and safety issues such as prevention of CRS and neurotoxicities.
“We are also concerned about the hypothetical risk of secondary malignancies. Therefore, we are asking for the committee’s recommendations regarding the nature and duration of follow-up of patients who would receive this product,” Dr. Bryan said.
“CTL019 is a living drug, which demonstrates activity after a single infusion,” said Samit Hirawat, MD, head of oncology global development for Novartis.
But the nature of CTL019 as a living drug also means that it is subject to variations in the ability of autologous T cells harvested via leukapheresis to be infected with the lentiviral vector and expanded into a population of CAR T cells large enough to have therapeutic value, said Xiaobin Victor Lu, PhD, a chemistry, manufacturing, and controls reviewer for the FDA.
Mitigation plan
Novartis’ proposed plan includes specific, long-term steps for mitigating the risk of CRS and neurologic events, such as cerebral edema, the latter of which caused the FDA to call for a clinical hold of the phase 2 ROCKET trial for a different CAR T-cell construct.
Among the proposed elements of the mitigation plan are a 15-year minimum pharmacovigilance program and long-term safety follow-up for adverse events related to the therapy, efficacy, immunogenicity, transgene persistence of CD19 CAR, and the incidence of second malignancies possibly related to insertional mutagenesis.
Novartis also will train treatment center staff on processes for cell collection, cryopreservation, transport, chain of identity, safety management, and logistics for handling the CAR T-cell product. The company proposes to provide on-site training of personnel on CRS and neurotoxicity risk and management, as well as to offer information to patients and caregivers about the signs and symptoms of adverse events of concern.
Dr. Cripe expressed his concerns that Novartis’ proposal to initially limit the mitigation plan to 30 or 35 treatment sites would create problems of access and economic disparities among patients, and could cause inequities among treatment centers even with the same city.
David Lebwohl, MD, head of the CAR T global program for Novartis, said that the planned number of sites for the mitigation program would be expanded after 6 to 12 months if the CAR T construct receives final approval and clinical implementation goes well.
There was nearly unanimous agreement among the panel members that the planned 15-year follow-up and other mitigation measures would be adequate for detecting serious short- and long-term consequences of CAR T-cell therapy.
Patient/advocate perspective
In the public comment section of the proceedings, panel members were urged to vote in favor of CTL019 by parents of children with ALL, including Don McMahon, whose son Connor received the therapy after multiple relapses, and Tom Whitehead, father of Emily Whitehead, the first patient to receive CAR T cells for ALL.
Both children are alive and doing well.
CTL019 is produced by Novartis.
Tool indicates fracture risk after HSCT
MADRID – The risk of osteoporotic fracture associated with hematopoietic stem cell transplantation (HSCT) could be assessed using the Fracture Risk Assessment Tool (FRAX), researchers from the University of Texas MD Anderson Cancer Center have found.
In a retrospective cohort study, Huifang Lu, MD, and her collaborators found that FRAX could predict the risk of fracture with reasonable accuracy. The area under the receiver operating characteristic curve was 0.66 for predicting a fracture 10 years after HSCT.
“Current guidelines recommend the evaluation of bone health at 1 year following the transplant, but we recommend that this needs to happen at a much earlier time,” Dr. Lu said at the European Congress of Rheumatology.
Determining how to assess risk earlier and prevent bone loss remains a challenge, however. FRAX is an easy and quick tool to use, but its predictive ability is modest, she said.
As the finding comes from a retrospective study, prospective evaluation of FRAX is needed in HSCT patients. If shown predictive in this setting, bone health could be assessed earlier using FRAX, ideally at or before the time of the transplant, to allow appropriate action to be taken, such as prescribing bisphosphonates to those identified to be at high risk.
There is no consensus on preventing and treating bone loss following HSCT, said Dr. Lu. In a meta-analysis performed by Dr. Lu and her associates (Bone Marrow Transplant. 2017;52[5]:663-70), less bone loss was seen in patients who received a bisphosphonate.
As the use of HSCT has expanded over the past two decades, there is an expanding population of survivors with potential long-term effects such as bone loss and a higher risk of fractures, compared with the general population, Dr. Lu explained.
The FRAX tool takes into account pre-HSCT factors such as age, smoking status, alcohol use, prior fracture, body mass index, and corticosteroid use. This can be considered in association with the fracture risk related to the various conditioning and supporting regimens that patients receive around the time of their transplants.
The study included 5,170 adult patients who had undergone HSCT at the University of Texas MD Anderson Cancer Center over a 10-year period. Patients were considered to have entered the cohort at the time of their transplants, Dr. Lu said. Their history of osteoporotic fractures up to 3.3 years later was obtained and verified by radiology and physician assessment. FRAX probabilities were then derived from baseline information.
The mean age of patients included was 52 years, 57% were male and 75% were white. One-quarter had experienced a prior fracture. Of note, 26% of the cohort underwent HSCT for multiple myeloma, 70% of whom had already had a fracture, compared with 9% of those who underwent HSCT for another reason such as leukemia or lymphoma.
Multivariate analyses were performed with and without considering death as a competing risk, and similar results were obtained. Higher FRAX scores (20 or greater) were more likely to be recorded in individuals who sustained a fracture than in those who did not. Patients who had an allogeneic HSCT were 15% more likely to have a fracture as those who received an autologous transplant. Perhaps not surprisingly, patients with multiple myeloma were more likely than those who had HSCT for other reasons to sustain a fracture by 10 years based on FRAX results (hazard ratio, 3.16).
Future research needs to look at the optimal cut offs for FRAX scores predictive of events and see if there is any association between the loss of bone and fracture risk. There also needs to be an evaluation of the use of concomitant medications and health economic analyses performed.
Dr. Lu had no conflicts of interest. The study was funded by the Rolanette and Berdon Lawrence Bone Disease Program of Texas and via Cancer Survivorship Research Seed Monday Grants from the University Cancer Foundation and Duncan Family Institute for Cancer Prevention and Risk Assessment to the University of Texas MD Anderson Cancer Center.
MADRID – The risk of osteoporotic fracture associated with hematopoietic stem cell transplantation (HSCT) could be assessed using the Fracture Risk Assessment Tool (FRAX), researchers from the University of Texas MD Anderson Cancer Center have found.
In a retrospective cohort study, Huifang Lu, MD, and her collaborators found that FRAX could predict the risk of fracture with reasonable accuracy. The area under the receiver operating characteristic curve was 0.66 for predicting a fracture 10 years after HSCT.
“Current guidelines recommend the evaluation of bone health at 1 year following the transplant, but we recommend that this needs to happen at a much earlier time,” Dr. Lu said at the European Congress of Rheumatology.
Determining how to assess risk earlier and prevent bone loss remains a challenge, however. FRAX is an easy and quick tool to use, but its predictive ability is modest, she said.
As the finding comes from a retrospective study, prospective evaluation of FRAX is needed in HSCT patients. If shown predictive in this setting, bone health could be assessed earlier using FRAX, ideally at or before the time of the transplant, to allow appropriate action to be taken, such as prescribing bisphosphonates to those identified to be at high risk.
There is no consensus on preventing and treating bone loss following HSCT, said Dr. Lu. In a meta-analysis performed by Dr. Lu and her associates (Bone Marrow Transplant. 2017;52[5]:663-70), less bone loss was seen in patients who received a bisphosphonate.
As the use of HSCT has expanded over the past two decades, there is an expanding population of survivors with potential long-term effects such as bone loss and a higher risk of fractures, compared with the general population, Dr. Lu explained.
The FRAX tool takes into account pre-HSCT factors such as age, smoking status, alcohol use, prior fracture, body mass index, and corticosteroid use. This can be considered in association with the fracture risk related to the various conditioning and supporting regimens that patients receive around the time of their transplants.
The study included 5,170 adult patients who had undergone HSCT at the University of Texas MD Anderson Cancer Center over a 10-year period. Patients were considered to have entered the cohort at the time of their transplants, Dr. Lu said. Their history of osteoporotic fractures up to 3.3 years later was obtained and verified by radiology and physician assessment. FRAX probabilities were then derived from baseline information.
The mean age of patients included was 52 years, 57% were male and 75% were white. One-quarter had experienced a prior fracture. Of note, 26% of the cohort underwent HSCT for multiple myeloma, 70% of whom had already had a fracture, compared with 9% of those who underwent HSCT for another reason such as leukemia or lymphoma.
Multivariate analyses were performed with and without considering death as a competing risk, and similar results were obtained. Higher FRAX scores (20 or greater) were more likely to be recorded in individuals who sustained a fracture than in those who did not. Patients who had an allogeneic HSCT were 15% more likely to have a fracture as those who received an autologous transplant. Perhaps not surprisingly, patients with multiple myeloma were more likely than those who had HSCT for other reasons to sustain a fracture by 10 years based on FRAX results (hazard ratio, 3.16).
Future research needs to look at the optimal cut offs for FRAX scores predictive of events and see if there is any association between the loss of bone and fracture risk. There also needs to be an evaluation of the use of concomitant medications and health economic analyses performed.
Dr. Lu had no conflicts of interest. The study was funded by the Rolanette and Berdon Lawrence Bone Disease Program of Texas and via Cancer Survivorship Research Seed Monday Grants from the University Cancer Foundation and Duncan Family Institute for Cancer Prevention and Risk Assessment to the University of Texas MD Anderson Cancer Center.
MADRID – The risk of osteoporotic fracture associated with hematopoietic stem cell transplantation (HSCT) could be assessed using the Fracture Risk Assessment Tool (FRAX), researchers from the University of Texas MD Anderson Cancer Center have found.
In a retrospective cohort study, Huifang Lu, MD, and her collaborators found that FRAX could predict the risk of fracture with reasonable accuracy. The area under the receiver operating characteristic curve was 0.66 for predicting a fracture 10 years after HSCT.
“Current guidelines recommend the evaluation of bone health at 1 year following the transplant, but we recommend that this needs to happen at a much earlier time,” Dr. Lu said at the European Congress of Rheumatology.
Determining how to assess risk earlier and prevent bone loss remains a challenge, however. FRAX is an easy and quick tool to use, but its predictive ability is modest, she said.
As the finding comes from a retrospective study, prospective evaluation of FRAX is needed in HSCT patients. If shown predictive in this setting, bone health could be assessed earlier using FRAX, ideally at or before the time of the transplant, to allow appropriate action to be taken, such as prescribing bisphosphonates to those identified to be at high risk.
There is no consensus on preventing and treating bone loss following HSCT, said Dr. Lu. In a meta-analysis performed by Dr. Lu and her associates (Bone Marrow Transplant. 2017;52[5]:663-70), less bone loss was seen in patients who received a bisphosphonate.
As the use of HSCT has expanded over the past two decades, there is an expanding population of survivors with potential long-term effects such as bone loss and a higher risk of fractures, compared with the general population, Dr. Lu explained.
The FRAX tool takes into account pre-HSCT factors such as age, smoking status, alcohol use, prior fracture, body mass index, and corticosteroid use. This can be considered in association with the fracture risk related to the various conditioning and supporting regimens that patients receive around the time of their transplants.
The study included 5,170 adult patients who had undergone HSCT at the University of Texas MD Anderson Cancer Center over a 10-year period. Patients were considered to have entered the cohort at the time of their transplants, Dr. Lu said. Their history of osteoporotic fractures up to 3.3 years later was obtained and verified by radiology and physician assessment. FRAX probabilities were then derived from baseline information.
The mean age of patients included was 52 years, 57% were male and 75% were white. One-quarter had experienced a prior fracture. Of note, 26% of the cohort underwent HSCT for multiple myeloma, 70% of whom had already had a fracture, compared with 9% of those who underwent HSCT for another reason such as leukemia or lymphoma.
Multivariate analyses were performed with and without considering death as a competing risk, and similar results were obtained. Higher FRAX scores (20 or greater) were more likely to be recorded in individuals who sustained a fracture than in those who did not. Patients who had an allogeneic HSCT were 15% more likely to have a fracture as those who received an autologous transplant. Perhaps not surprisingly, patients with multiple myeloma were more likely than those who had HSCT for other reasons to sustain a fracture by 10 years based on FRAX results (hazard ratio, 3.16).
Future research needs to look at the optimal cut offs for FRAX scores predictive of events and see if there is any association between the loss of bone and fracture risk. There also needs to be an evaluation of the use of concomitant medications and health economic analyses performed.
Dr. Lu had no conflicts of interest. The study was funded by the Rolanette and Berdon Lawrence Bone Disease Program of Texas and via Cancer Survivorship Research Seed Monday Grants from the University Cancer Foundation and Duncan Family Institute for Cancer Prevention and Risk Assessment to the University of Texas MD Anderson Cancer Center.
AT THE EULAR 2017 CONGRESS
Key clinical point: The Fracture Risk Assessment Tool (FRAX) helped in predicting osteoporotic fracture risk after hematopoietic stem cell transplantation (HSCT).
Major finding: The area under the receiver operating characteristic curve was 0.66, indicating modest predictive ability,10 years after HSCT.
Data source: A retrospective cohort study of 5,170 adult patients who received HSCT at the University of Texas MD Anderson Cancer Center between 2001 and 2010.
Disclosures: Dr. Lu had no conflicts of interest. The study was funded by the Rolanette and Berdon Lawrence Bone Disease Program of Texas and via Cancer Survivorship Research Seed Monday Grants from the University Cancer Foundation and the Duncan Family Institute for Cancer Prevention and Risk Assessment to the University of Texas MD Anderson Cancer Center.
Ibrutinib dons new anti-GVHD hat
MADRID – Talk about versatility: Ibrutinib (Imbruvica), a drug with marked activity against B-cell malignancies, also appears to be a safe and acceptable option for the treatment of patients with chronic graft vs. host disease (cGVHD) for whom frontline therapies have failed.
Among 42 patients in a phase II study with steroid-refractory cGVHD, the overall response rate with ibrutinib was 67%, with one-third of responders having a complete response, reported Iskra Pusic, MD, from Washington University School of Medicine in St. Louis.
Corticosteroids are the most commonly used therapy for cGVHD in the United States, but for those patients for whom corticosteroids are a bust, there is no established second-line therapy, and patients with refractory cGVHD are usually recommended for clinical trials, Dr. Pusic said.
The therapeutic rationale underpinning the use of ibrutinib in cGVHD, a condition marked by extensive immune dysregulation, is that the agent is an irreversible inhibitor of Bruton’s tyrosine kinase and interleukin-2 inducible T-cell kinase, and thus has wide-ranging immune-dampening activity, Dr. Pusic said.
She and colleagues in a multicenter study enrolled 42 patients with cGVHD that corticosteroids had failed to treat adequately, and treated them with oral ibrutinib 420 mg daily until cGVHD progression or unacceptable toxicity.
At a median follow-up of 13.9 months, a total of 28 patients (67%) had a response according to 2005 National Institutes of Health (NIH) criteria, including nine with a complete response, and 19 with partial responses.
Of the patients with responses, 79% had a response at the time of the first assessment for response, and 71% of responders had responses lasting at least 5 months.
Among patients with multiorgan involvement, responses were seen in two or more organs.
Grade 3 or greater adverse events included fatigue, diarrhea, muscles spasms, pneumonia, pyrexia, and headache. Two patients died on study, one from multilobular pneumonia and one from bronchopulmonary aspergillosis.
In general, the safety profile of ibrutinib was similar to that seen in studies of the drug in B-cell malignancies and to that seen with corticosteroid therapy for patients with cGVHD, Dr. Pusic said.
Investigators are currently enrolling patients in a double-blind clinical trial comparing ibrutinib or placebo in combination with corticosteroids in patients with newly diagnosed cGVHD, she noted.
The study was supported by Pharmacyclics. Dr. Pusic did not report disclosures.
MADRID – Talk about versatility: Ibrutinib (Imbruvica), a drug with marked activity against B-cell malignancies, also appears to be a safe and acceptable option for the treatment of patients with chronic graft vs. host disease (cGVHD) for whom frontline therapies have failed.
Among 42 patients in a phase II study with steroid-refractory cGVHD, the overall response rate with ibrutinib was 67%, with one-third of responders having a complete response, reported Iskra Pusic, MD, from Washington University School of Medicine in St. Louis.
Corticosteroids are the most commonly used therapy for cGVHD in the United States, but for those patients for whom corticosteroids are a bust, there is no established second-line therapy, and patients with refractory cGVHD are usually recommended for clinical trials, Dr. Pusic said.
The therapeutic rationale underpinning the use of ibrutinib in cGVHD, a condition marked by extensive immune dysregulation, is that the agent is an irreversible inhibitor of Bruton’s tyrosine kinase and interleukin-2 inducible T-cell kinase, and thus has wide-ranging immune-dampening activity, Dr. Pusic said.
She and colleagues in a multicenter study enrolled 42 patients with cGVHD that corticosteroids had failed to treat adequately, and treated them with oral ibrutinib 420 mg daily until cGVHD progression or unacceptable toxicity.
At a median follow-up of 13.9 months, a total of 28 patients (67%) had a response according to 2005 National Institutes of Health (NIH) criteria, including nine with a complete response, and 19 with partial responses.
Of the patients with responses, 79% had a response at the time of the first assessment for response, and 71% of responders had responses lasting at least 5 months.
Among patients with multiorgan involvement, responses were seen in two or more organs.
Grade 3 or greater adverse events included fatigue, diarrhea, muscles spasms, pneumonia, pyrexia, and headache. Two patients died on study, one from multilobular pneumonia and one from bronchopulmonary aspergillosis.
In general, the safety profile of ibrutinib was similar to that seen in studies of the drug in B-cell malignancies and to that seen with corticosteroid therapy for patients with cGVHD, Dr. Pusic said.
Investigators are currently enrolling patients in a double-blind clinical trial comparing ibrutinib or placebo in combination with corticosteroids in patients with newly diagnosed cGVHD, she noted.
The study was supported by Pharmacyclics. Dr. Pusic did not report disclosures.
MADRID – Talk about versatility: Ibrutinib (Imbruvica), a drug with marked activity against B-cell malignancies, also appears to be a safe and acceptable option for the treatment of patients with chronic graft vs. host disease (cGVHD) for whom frontline therapies have failed.
Among 42 patients in a phase II study with steroid-refractory cGVHD, the overall response rate with ibrutinib was 67%, with one-third of responders having a complete response, reported Iskra Pusic, MD, from Washington University School of Medicine in St. Louis.
Corticosteroids are the most commonly used therapy for cGVHD in the United States, but for those patients for whom corticosteroids are a bust, there is no established second-line therapy, and patients with refractory cGVHD are usually recommended for clinical trials, Dr. Pusic said.
The therapeutic rationale underpinning the use of ibrutinib in cGVHD, a condition marked by extensive immune dysregulation, is that the agent is an irreversible inhibitor of Bruton’s tyrosine kinase and interleukin-2 inducible T-cell kinase, and thus has wide-ranging immune-dampening activity, Dr. Pusic said.
She and colleagues in a multicenter study enrolled 42 patients with cGVHD that corticosteroids had failed to treat adequately, and treated them with oral ibrutinib 420 mg daily until cGVHD progression or unacceptable toxicity.
At a median follow-up of 13.9 months, a total of 28 patients (67%) had a response according to 2005 National Institutes of Health (NIH) criteria, including nine with a complete response, and 19 with partial responses.
Of the patients with responses, 79% had a response at the time of the first assessment for response, and 71% of responders had responses lasting at least 5 months.
Among patients with multiorgan involvement, responses were seen in two or more organs.
Grade 3 or greater adverse events included fatigue, diarrhea, muscles spasms, pneumonia, pyrexia, and headache. Two patients died on study, one from multilobular pneumonia and one from bronchopulmonary aspergillosis.
In general, the safety profile of ibrutinib was similar to that seen in studies of the drug in B-cell malignancies and to that seen with corticosteroid therapy for patients with cGVHD, Dr. Pusic said.
Investigators are currently enrolling patients in a double-blind clinical trial comparing ibrutinib or placebo in combination with corticosteroids in patients with newly diagnosed cGVHD, she noted.
The study was supported by Pharmacyclics. Dr. Pusic did not report disclosures.
AT EHA 2017
Key clinical point: The tyrosine kinase inhibitor ibrutinib was associated with complete and partial responses in two-thirds of patients with steroid-refractory chronic graft vs. host disease (cGVHD).
Major finding: A total of 28 patients (67%) had responses, including 9 complete responses.
Data source: Phase II clinical trial in 42 patients with cGVHD for whom corticosteroids had failed.
Disclosures: The study was supported by Pharmacyclics. Dr. Pusic did not report disclosures.
Patch is early indicator of temperature rise after HSCT
A fever-monitoring patch was well tolerated in hospitalized patients undergoing stem cell transplant or intensive chemotherapy for leukemia, and alerted physicians to the presence of a fever much earlier than did standard temperature-taking procedures, according to findings from a study abstract that was published in conjunction with the annual meeting of the American Society of Clinical Oncology.
The patch can transmit data via Bluetooth to an iPad or smartphone, and it attempts to bring temperature recording in line with other vital signs. “It’s the only vital sign that’s not continuously monitored outside of the ICU,” said John Gannon, CEO of Blue Spark, which markets the TempTraq underarm patch used in the study.
The units are constructed with thin batteries that can be “printed” on to any surface. “Being disposable makes it a very usable work flow device,” said Mr. Gannon.
The researchers tested the TempTraq in 10 patients who had been admitted for stem cell transplant or high dose chemotherapy for leukemia.
In addition to wearing the patch, patients had their temperature measured in standard fashion every 4 hours. The researchers defined a temperature rise as a spike above 100.4° F. The device measured body temperature every 10 minutes (14,342 temperature measurements).
Standard of care measurement identified 23 temperature rise episodes, 21 of which were recorded by the TempTraq patch. The device caught temperature spikes much sooner than did standard of care measures – a median of 140.1 minutes earlier (range, 30-180 minutes).
All 10 patients continued to wear the patch throughout the hospital stay, with 9 of 10 reporting that the patch was comfortable and didn’t produce any skin irritation. Eight patients indicated interest in using the patch again, and 8 said they were completely satisfied with the patch.
The next step is to determine if the patch could be used successfully in an outpatient stem cell transplant setting.
The device could also be integrated directly with hospital central monitoring systems using Blue Spark’s TempTraq Connect, which is a HIPAA-compliant service supported by the Google Healthcare Cloud Platform, though this was not tested in the current study.
The TempTraq could be used to monitor patients for the onset of sepsis and allow faster interventions, according to Mr. Gannon. Also, patients who are susceptible to infections could be sent home with a TempTraq monitor, which would send signals to a central monitoring station.
A fever-monitoring patch was well tolerated in hospitalized patients undergoing stem cell transplant or intensive chemotherapy for leukemia, and alerted physicians to the presence of a fever much earlier than did standard temperature-taking procedures, according to findings from a study abstract that was published in conjunction with the annual meeting of the American Society of Clinical Oncology.
The patch can transmit data via Bluetooth to an iPad or smartphone, and it attempts to bring temperature recording in line with other vital signs. “It’s the only vital sign that’s not continuously monitored outside of the ICU,” said John Gannon, CEO of Blue Spark, which markets the TempTraq underarm patch used in the study.
The units are constructed with thin batteries that can be “printed” on to any surface. “Being disposable makes it a very usable work flow device,” said Mr. Gannon.
The researchers tested the TempTraq in 10 patients who had been admitted for stem cell transplant or high dose chemotherapy for leukemia.
In addition to wearing the patch, patients had their temperature measured in standard fashion every 4 hours. The researchers defined a temperature rise as a spike above 100.4° F. The device measured body temperature every 10 minutes (14,342 temperature measurements).
Standard of care measurement identified 23 temperature rise episodes, 21 of which were recorded by the TempTraq patch. The device caught temperature spikes much sooner than did standard of care measures – a median of 140.1 minutes earlier (range, 30-180 minutes).
All 10 patients continued to wear the patch throughout the hospital stay, with 9 of 10 reporting that the patch was comfortable and didn’t produce any skin irritation. Eight patients indicated interest in using the patch again, and 8 said they were completely satisfied with the patch.
The next step is to determine if the patch could be used successfully in an outpatient stem cell transplant setting.
The device could also be integrated directly with hospital central monitoring systems using Blue Spark’s TempTraq Connect, which is a HIPAA-compliant service supported by the Google Healthcare Cloud Platform, though this was not tested in the current study.
The TempTraq could be used to monitor patients for the onset of sepsis and allow faster interventions, according to Mr. Gannon. Also, patients who are susceptible to infections could be sent home with a TempTraq monitor, which would send signals to a central monitoring station.
A fever-monitoring patch was well tolerated in hospitalized patients undergoing stem cell transplant or intensive chemotherapy for leukemia, and alerted physicians to the presence of a fever much earlier than did standard temperature-taking procedures, according to findings from a study abstract that was published in conjunction with the annual meeting of the American Society of Clinical Oncology.
The patch can transmit data via Bluetooth to an iPad or smartphone, and it attempts to bring temperature recording in line with other vital signs. “It’s the only vital sign that’s not continuously monitored outside of the ICU,” said John Gannon, CEO of Blue Spark, which markets the TempTraq underarm patch used in the study.
The units are constructed with thin batteries that can be “printed” on to any surface. “Being disposable makes it a very usable work flow device,” said Mr. Gannon.
The researchers tested the TempTraq in 10 patients who had been admitted for stem cell transplant or high dose chemotherapy for leukemia.
In addition to wearing the patch, patients had their temperature measured in standard fashion every 4 hours. The researchers defined a temperature rise as a spike above 100.4° F. The device measured body temperature every 10 minutes (14,342 temperature measurements).
Standard of care measurement identified 23 temperature rise episodes, 21 of which were recorded by the TempTraq patch. The device caught temperature spikes much sooner than did standard of care measures – a median of 140.1 minutes earlier (range, 30-180 minutes).
All 10 patients continued to wear the patch throughout the hospital stay, with 9 of 10 reporting that the patch was comfortable and didn’t produce any skin irritation. Eight patients indicated interest in using the patch again, and 8 said they were completely satisfied with the patch.
The next step is to determine if the patch could be used successfully in an outpatient stem cell transplant setting.
The device could also be integrated directly with hospital central monitoring systems using Blue Spark’s TempTraq Connect, which is a HIPAA-compliant service supported by the Google Healthcare Cloud Platform, though this was not tested in the current study.
The TempTraq could be used to monitor patients for the onset of sepsis and allow faster interventions, according to Mr. Gannon. Also, patients who are susceptible to infections could be sent home with a TempTraq monitor, which would send signals to a central monitoring station.
FROM ASCO 2017
Key clinical point: The device has the potential to detect infections as well as sepsis.
Major finding: The device detected fevers a median of 140 minutes sooner than did standard hospital testing.
Data source: Prospective study of 10 patients.
Disclosures: Mr. Gannon is an employee of Blue Spark Technologies, which sponsored the study.
Stem cell therapy significantly improves ulcer healing
PORTLAND, ORE. – Treating chronic venous leg ulcers with mesenchymal stem cells and fibrin spray significantly improved wound healing, compared with vehicle control or saline plus conventional therapy, according to the results of a small randomized, controlled, double-blind pilot trial.
“Topical application of autologous, bone-marrow–derived mesenchymal stem cells may be an effective way to promote healing in patients with difficult-to-heal wounds,” said Ayman Grada, MD, of the department of dermatology at Boston University. “However, larger studies are needed to confirm this finding.”
“Various treatment modalities have been used, but treatment outcomes are not always satisfactory,” said Dr. Grada. “In about 60% of cases, wounds fail to close, and there is also a high rate of recurrence.”
Preclinical work in several animal models indicated that applying mesenchymal stem cells to wounds accelerated healing through a variety of mechanisms, Dr. Grada noted. Based on that premise, he and his associates hypothesized that autologous cultured mesenchymal stem cells could accelerate wound healing in humans.
To test that idea, they randomly assigned the 11 trial participants to one of two control treatments or to the stem cell intervention. Four patients received normal saline with conventional standard care, three patients received fibrin spray plus conventional therapy, and four patients received conventional therapy plus autologous mesenchymal stem cells delivered in fibrin spray at a dose of 1 x 106 cells per square centimeter of wound surface. Patients were treated every 3 weeks, up to three times or until complete wound healing, and were followed for up to 24 weeks.
To acquire the stem cells, the researchers obtained 30- to 50-mL samples of bone marrow aspirate from the iliac crest, then separated and cultured the cells in-house. The controls underwent sham aspiration with needles that did not penetrate the bone, Dr. Grada said. At each 4-week follow-up visit, the investigators measured the perimeter and area of each wound and analyzed the results with public domain software called ImageJ. They calculated the linear advance of the wound margin by dividing change in area by average perimeter.
The healing rate of the intervention group outpaced that of either control group at each time point measured, Dr. Grada said. Average weekly healing rates by time point ranged between –0.002 cm and 0.006 cm for the saline group and between –0.05 cm and 0.01 cm for the fibrin spray group. Neither of these control groups achieved meaningful wound closure by week 24.
In contrast, stem cell recipients experienced consistent wound closure at rates of 0.11-0.13 cm per week. The study was too small for conventional statistical analysis, but a Bayesian time aggregated one-way analysis of variance yielded a statistically significant difference in healing rates among groups (P less than .0005).
Dr. Grada also discussed several case studies. An 82-year-old white woman with a decades-long history of venous ulcers experienced complete wound healing with mesenchymal stem cell therapy, which enabled her to become more independent within her long-term care facility. A 75-year-old African American woman achieved 80% wound healing with stem cell therapy after previously having failed to benefit from two applications of bioengineered skin.
Finally, a 39-year-old man with chronic, treatment-resistant venous ulcers achieved partial wound healing. “He has almost healed, with very thin epidermal coverage, but never to the point of no exudate and complete closure,” Dr. Grada said. “Therefore, we could not declare him healed, even though the ulcer was smaller at the end of the study.”
No patient in the study experienced adverse events from treatment. However, recruiting for the trial was difficult, because patients were reluctant to undergo bone marrow aspiration, Dr. Grada said.
Previous work indicates that the initial rate at which the wound heals dictates its final rate (J Am Acad Dermatol. 1993 Mar;28[3]:418-21), and that 4 weeks is enough to establish a healing trend, he noted. Dr. Grada concluded by quoting Hippocrates: “Natural forces within us are the true healers of disease.”
The National Institutes of Health supported the trial. Dr. Grada had no conflicts of interest.
PORTLAND, ORE. – Treating chronic venous leg ulcers with mesenchymal stem cells and fibrin spray significantly improved wound healing, compared with vehicle control or saline plus conventional therapy, according to the results of a small randomized, controlled, double-blind pilot trial.
“Topical application of autologous, bone-marrow–derived mesenchymal stem cells may be an effective way to promote healing in patients with difficult-to-heal wounds,” said Ayman Grada, MD, of the department of dermatology at Boston University. “However, larger studies are needed to confirm this finding.”
“Various treatment modalities have been used, but treatment outcomes are not always satisfactory,” said Dr. Grada. “In about 60% of cases, wounds fail to close, and there is also a high rate of recurrence.”
Preclinical work in several animal models indicated that applying mesenchymal stem cells to wounds accelerated healing through a variety of mechanisms, Dr. Grada noted. Based on that premise, he and his associates hypothesized that autologous cultured mesenchymal stem cells could accelerate wound healing in humans.
To test that idea, they randomly assigned the 11 trial participants to one of two control treatments or to the stem cell intervention. Four patients received normal saline with conventional standard care, three patients received fibrin spray plus conventional therapy, and four patients received conventional therapy plus autologous mesenchymal stem cells delivered in fibrin spray at a dose of 1 x 106 cells per square centimeter of wound surface. Patients were treated every 3 weeks, up to three times or until complete wound healing, and were followed for up to 24 weeks.
To acquire the stem cells, the researchers obtained 30- to 50-mL samples of bone marrow aspirate from the iliac crest, then separated and cultured the cells in-house. The controls underwent sham aspiration with needles that did not penetrate the bone, Dr. Grada said. At each 4-week follow-up visit, the investigators measured the perimeter and area of each wound and analyzed the results with public domain software called ImageJ. They calculated the linear advance of the wound margin by dividing change in area by average perimeter.
The healing rate of the intervention group outpaced that of either control group at each time point measured, Dr. Grada said. Average weekly healing rates by time point ranged between –0.002 cm and 0.006 cm for the saline group and between –0.05 cm and 0.01 cm for the fibrin spray group. Neither of these control groups achieved meaningful wound closure by week 24.
In contrast, stem cell recipients experienced consistent wound closure at rates of 0.11-0.13 cm per week. The study was too small for conventional statistical analysis, but a Bayesian time aggregated one-way analysis of variance yielded a statistically significant difference in healing rates among groups (P less than .0005).
Dr. Grada also discussed several case studies. An 82-year-old white woman with a decades-long history of venous ulcers experienced complete wound healing with mesenchymal stem cell therapy, which enabled her to become more independent within her long-term care facility. A 75-year-old African American woman achieved 80% wound healing with stem cell therapy after previously having failed to benefit from two applications of bioengineered skin.
Finally, a 39-year-old man with chronic, treatment-resistant venous ulcers achieved partial wound healing. “He has almost healed, with very thin epidermal coverage, but never to the point of no exudate and complete closure,” Dr. Grada said. “Therefore, we could not declare him healed, even though the ulcer was smaller at the end of the study.”
No patient in the study experienced adverse events from treatment. However, recruiting for the trial was difficult, because patients were reluctant to undergo bone marrow aspiration, Dr. Grada said.
Previous work indicates that the initial rate at which the wound heals dictates its final rate (J Am Acad Dermatol. 1993 Mar;28[3]:418-21), and that 4 weeks is enough to establish a healing trend, he noted. Dr. Grada concluded by quoting Hippocrates: “Natural forces within us are the true healers of disease.”
The National Institutes of Health supported the trial. Dr. Grada had no conflicts of interest.
PORTLAND, ORE. – Treating chronic venous leg ulcers with mesenchymal stem cells and fibrin spray significantly improved wound healing, compared with vehicle control or saline plus conventional therapy, according to the results of a small randomized, controlled, double-blind pilot trial.
“Topical application of autologous, bone-marrow–derived mesenchymal stem cells may be an effective way to promote healing in patients with difficult-to-heal wounds,” said Ayman Grada, MD, of the department of dermatology at Boston University. “However, larger studies are needed to confirm this finding.”
“Various treatment modalities have been used, but treatment outcomes are not always satisfactory,” said Dr. Grada. “In about 60% of cases, wounds fail to close, and there is also a high rate of recurrence.”
Preclinical work in several animal models indicated that applying mesenchymal stem cells to wounds accelerated healing through a variety of mechanisms, Dr. Grada noted. Based on that premise, he and his associates hypothesized that autologous cultured mesenchymal stem cells could accelerate wound healing in humans.
To test that idea, they randomly assigned the 11 trial participants to one of two control treatments or to the stem cell intervention. Four patients received normal saline with conventional standard care, three patients received fibrin spray plus conventional therapy, and four patients received conventional therapy plus autologous mesenchymal stem cells delivered in fibrin spray at a dose of 1 x 106 cells per square centimeter of wound surface. Patients were treated every 3 weeks, up to three times or until complete wound healing, and were followed for up to 24 weeks.
To acquire the stem cells, the researchers obtained 30- to 50-mL samples of bone marrow aspirate from the iliac crest, then separated and cultured the cells in-house. The controls underwent sham aspiration with needles that did not penetrate the bone, Dr. Grada said. At each 4-week follow-up visit, the investigators measured the perimeter and area of each wound and analyzed the results with public domain software called ImageJ. They calculated the linear advance of the wound margin by dividing change in area by average perimeter.
The healing rate of the intervention group outpaced that of either control group at each time point measured, Dr. Grada said. Average weekly healing rates by time point ranged between –0.002 cm and 0.006 cm for the saline group and between –0.05 cm and 0.01 cm for the fibrin spray group. Neither of these control groups achieved meaningful wound closure by week 24.
In contrast, stem cell recipients experienced consistent wound closure at rates of 0.11-0.13 cm per week. The study was too small for conventional statistical analysis, but a Bayesian time aggregated one-way analysis of variance yielded a statistically significant difference in healing rates among groups (P less than .0005).
Dr. Grada also discussed several case studies. An 82-year-old white woman with a decades-long history of venous ulcers experienced complete wound healing with mesenchymal stem cell therapy, which enabled her to become more independent within her long-term care facility. A 75-year-old African American woman achieved 80% wound healing with stem cell therapy after previously having failed to benefit from two applications of bioengineered skin.
Finally, a 39-year-old man with chronic, treatment-resistant venous ulcers achieved partial wound healing. “He has almost healed, with very thin epidermal coverage, but never to the point of no exudate and complete closure,” Dr. Grada said. “Therefore, we could not declare him healed, even though the ulcer was smaller at the end of the study.”
No patient in the study experienced adverse events from treatment. However, recruiting for the trial was difficult, because patients were reluctant to undergo bone marrow aspiration, Dr. Grada said.
Previous work indicates that the initial rate at which the wound heals dictates its final rate (J Am Acad Dermatol. 1993 Mar;28[3]:418-21), and that 4 weeks is enough to establish a healing trend, he noted. Dr. Grada concluded by quoting Hippocrates: “Natural forces within us are the true healers of disease.”
The National Institutes of Health supported the trial. Dr. Grada had no conflicts of interest.
At SID 2017
Key clinical point: Treating chronic venous leg ulcers with mesenchymal stem cells and fibrin spray significantly improved wound healing, compared with vehicle control or saline plus conventional therapy.
Major finding: Neither control group achieved meaningful wound closure by week 24, while stem cell recipients experienced consistent wound closure at rates of 0.11-0.13 cm per week (P less than .0005 for difference in healing rates among groups).
Data source: A randomized, controlled, double-blind pilot trial of 11 patients.
Disclosures: The National Institutes of Health supported the study. Dr. Grada had no conflicts of interest.
Fighting in a passive manner active against Clostridium difficile
Infections resulting from Clostridium difficile are a major clinical challenge. In hematology and oncology, the widespread use of broad-spectrum antibiotics is essential for patients with profound neutropenia and infectious complications, which are a high-risk factor for C. difficile enteritis.
C. difficile enteritis occurs in 5%-20% of cancer patients.1 With standard of care antibiotics, oral metronidazole or oral vancomycin, high C. difficile cure rates are possible, but up to 25% of these infections recur. Recently, oral fidaxomicin was approved for treatment of C. difficile enteritis and was associated with high cure rates and, more importantly, with significantly lower recurrence rates.2
Bezlotoxumab, a fully humanized monoclonal antibody against C. difficile toxin B, has been shown by x-ray crystallography to neutralize toxin B by blocking its ability to bind to host cells.7 Most recently, this new therapeutic approach was investigated in humans.8
Wilcox et al. used pooled data of 2655 adults treated in two double-blind, randomized, placebo-controlled phase III clinical trials (MODIFY I and MODIFY II) for primary or recurrent C. difficile enteritis. This industry-sponsored trial was conducted at 322 sites in 30 countries.
In one treatment group, patients received a single infusion of bezlotoxumab (781 patients) or placebo (773 patients) and one of the three oral standard-of-care C. difficile antibiotics. Importantly, the primary end point of this trial was recurrent infection within 12 weeks. About 28% of the patients in both the bezlotoxumab group and the placebo group previously had at least one episode of C. difficile enteritis. About 20% of the patients in both groups were immunocompromised.
Pooled data showed that recurrent infection was significantly lower (P less than 0.001) in the bezlotoxumab group (17%), compared with the placebo group (27%). The difference in recurrence rate (25% vs. 41%) was even more pronounced in patients with one or more episodes of recurrent C. difficile enteritis in the past 6 months. Furthermore, a benefit for bezlotoxumab was seen in immunocompromised patients, whose recurrence rates were 15% with bezlotoxumab, vs. 28% with placebo. After the 12 weeks of follow-up, the absolute difference in the Kaplan-Meier rates of recurrent infection was 13% (absolute rate, 21% in bezlotoxumab group vs. 34% in placebo group; P less than 0.001).
The results indicate that bezlotoxumab, which was approved in 2016 by the U.S. Food and Drug Administration, might improve the outcome of patients with C. difficile enteritis. However, bezlotoxumab is not a “magic bullet.” The number needed to treat to prevent one episode of C. difficile enteritis is 10.
It is conceivable that bezlotoxumab may find its role in high-risk patients – those older than 65 years or patients with recurrent C. difficile enteritis – since the number needed to treat is only 6 in these subgroups.8
This new agent could be an important treatment option for our high-risk patients in hematology. However, more studies concerning costs and real-life efficacy are needed.
The new approach of passive immunization for prevention of recurrent C. difficile enteritis shows the importance and the role of toxin B – not only the bacterium per se – in pathogenesis and virulence of C. difficile. This could mean that we have to renew our view on the role of antibiotics against C. difficile. However, in contrast, bezlotoxumab does not affect the efficacy of standard of care antibiotics since the initial cure rates were 80% for both the antibody and the placebo groups.8 Toxin B levels are not detectable in stool samples between days 4 and 10 of standard of care antibiotic treatment. Afterward, however, they increase again.9 Most of the patients had received bezlotoxumab 3 or more days after they began standard-of-care antibiotic treatment – in the time period when toxin B is undetectable in stool – which underlines the importance of toxin B in the pathogenesis of recurrent C. difficile enteritis.8
In summary, the introduction of bezlotoxumab in clinical care gives new and important insights and solutions not only for treatment options but also for our understanding of C. difficile pathogenesis.
Dr. Schalk is consultant of internal medicine at the department of hematology and oncology, Magdeburg University Hospital, Germany, with clinical and research focus on infectious diseases in hematology and oncology.
Dr. Fischer is professor of internal medicine, hematology and oncology, at the Otto-von-Guericke University Hospital Magdeburg, Germany. He is head of the department of hematology and oncology and a clinical/molecular researcher in myeloid neoplasms. He is a member of the editorial advisory board of Hematology News.
Contact Dr. Schalk at [email protected].
References
1. Vehreschild, MJ et al. Diagnosis and management of gastrointestinal complications in adult cancer patients: Wvidence-based guidelines of the Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Oncology (DGHO). Ann Oncol. 2013;24:1189-202
2. Cornely, OA. Current and emerging management options for Clostridium difficile infection: What is the role of fidaxomicin? Clin Microbiol Infect. 2012;18(Suppl 6):28-35.
3. Bartlett, JG et al. Antibiotic-associated pseudomembranous colitis due to toxin-producing clostridia. N Engl J Med. 1978;298(10):531-4.
4. Lyras, D et al. Toxin B is essential for virulence of Clostridium difficile. Nature. 2009;458:1176-9.
5. Reineke, J et al. Autocatalytic cleavage of Clostridium difficile toxin B. Nature. 2007;446:415-9.
6. Leav, BA et al. Serum anti-toxin B antibody correlates with protection from recurrent Clostridium difficile infection (CDI). Vaccine. 2010;28:965-9.
7. Orth, P et al. Mechanism of action and epitopes of Clostridium difficile toxin B-neutralizing antibody bezlotoxumab revealed by X-ray crystallography. J Biol Chem. 2014;289:18008-21.
8. Wilcox, MH et al. Bezlotoxumab for prevention of recurrent Clostridium difficile infection. N Engl J Med. 2017;376:305-17.
9. Louie, TJ et al. Fidaxomicin preserves the intestinal microbiome during and after treatment of Clostridium difficile infection (CDI) and reduces both toxin reexpression and recurrence of CDI. Clin Infect Dis. 2012;5(Suppl 2):S132-42.
Infections resulting from Clostridium difficile are a major clinical challenge. In hematology and oncology, the widespread use of broad-spectrum antibiotics is essential for patients with profound neutropenia and infectious complications, which are a high-risk factor for C. difficile enteritis.
C. difficile enteritis occurs in 5%-20% of cancer patients.1 With standard of care antibiotics, oral metronidazole or oral vancomycin, high C. difficile cure rates are possible, but up to 25% of these infections recur. Recently, oral fidaxomicin was approved for treatment of C. difficile enteritis and was associated with high cure rates and, more importantly, with significantly lower recurrence rates.2
Bezlotoxumab, a fully humanized monoclonal antibody against C. difficile toxin B, has been shown by x-ray crystallography to neutralize toxin B by blocking its ability to bind to host cells.7 Most recently, this new therapeutic approach was investigated in humans.8
Wilcox et al. used pooled data of 2655 adults treated in two double-blind, randomized, placebo-controlled phase III clinical trials (MODIFY I and MODIFY II) for primary or recurrent C. difficile enteritis. This industry-sponsored trial was conducted at 322 sites in 30 countries.
In one treatment group, patients received a single infusion of bezlotoxumab (781 patients) or placebo (773 patients) and one of the three oral standard-of-care C. difficile antibiotics. Importantly, the primary end point of this trial was recurrent infection within 12 weeks. About 28% of the patients in both the bezlotoxumab group and the placebo group previously had at least one episode of C. difficile enteritis. About 20% of the patients in both groups were immunocompromised.
Pooled data showed that recurrent infection was significantly lower (P less than 0.001) in the bezlotoxumab group (17%), compared with the placebo group (27%). The difference in recurrence rate (25% vs. 41%) was even more pronounced in patients with one or more episodes of recurrent C. difficile enteritis in the past 6 months. Furthermore, a benefit for bezlotoxumab was seen in immunocompromised patients, whose recurrence rates were 15% with bezlotoxumab, vs. 28% with placebo. After the 12 weeks of follow-up, the absolute difference in the Kaplan-Meier rates of recurrent infection was 13% (absolute rate, 21% in bezlotoxumab group vs. 34% in placebo group; P less than 0.001).
The results indicate that bezlotoxumab, which was approved in 2016 by the U.S. Food and Drug Administration, might improve the outcome of patients with C. difficile enteritis. However, bezlotoxumab is not a “magic bullet.” The number needed to treat to prevent one episode of C. difficile enteritis is 10.
It is conceivable that bezlotoxumab may find its role in high-risk patients – those older than 65 years or patients with recurrent C. difficile enteritis – since the number needed to treat is only 6 in these subgroups.8
This new agent could be an important treatment option for our high-risk patients in hematology. However, more studies concerning costs and real-life efficacy are needed.
The new approach of passive immunization for prevention of recurrent C. difficile enteritis shows the importance and the role of toxin B – not only the bacterium per se – in pathogenesis and virulence of C. difficile. This could mean that we have to renew our view on the role of antibiotics against C. difficile. However, in contrast, bezlotoxumab does not affect the efficacy of standard of care antibiotics since the initial cure rates were 80% for both the antibody and the placebo groups.8 Toxin B levels are not detectable in stool samples between days 4 and 10 of standard of care antibiotic treatment. Afterward, however, they increase again.9 Most of the patients had received bezlotoxumab 3 or more days after they began standard-of-care antibiotic treatment – in the time period when toxin B is undetectable in stool – which underlines the importance of toxin B in the pathogenesis of recurrent C. difficile enteritis.8
In summary, the introduction of bezlotoxumab in clinical care gives new and important insights and solutions not only for treatment options but also for our understanding of C. difficile pathogenesis.
Dr. Schalk is consultant of internal medicine at the department of hematology and oncology, Magdeburg University Hospital, Germany, with clinical and research focus on infectious diseases in hematology and oncology.
Dr. Fischer is professor of internal medicine, hematology and oncology, at the Otto-von-Guericke University Hospital Magdeburg, Germany. He is head of the department of hematology and oncology and a clinical/molecular researcher in myeloid neoplasms. He is a member of the editorial advisory board of Hematology News.
Contact Dr. Schalk at [email protected].
References
1. Vehreschild, MJ et al. Diagnosis and management of gastrointestinal complications in adult cancer patients: Wvidence-based guidelines of the Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Oncology (DGHO). Ann Oncol. 2013;24:1189-202
2. Cornely, OA. Current and emerging management options for Clostridium difficile infection: What is the role of fidaxomicin? Clin Microbiol Infect. 2012;18(Suppl 6):28-35.
3. Bartlett, JG et al. Antibiotic-associated pseudomembranous colitis due to toxin-producing clostridia. N Engl J Med. 1978;298(10):531-4.
4. Lyras, D et al. Toxin B is essential for virulence of Clostridium difficile. Nature. 2009;458:1176-9.
5. Reineke, J et al. Autocatalytic cleavage of Clostridium difficile toxin B. Nature. 2007;446:415-9.
6. Leav, BA et al. Serum anti-toxin B antibody correlates with protection from recurrent Clostridium difficile infection (CDI). Vaccine. 2010;28:965-9.
7. Orth, P et al. Mechanism of action and epitopes of Clostridium difficile toxin B-neutralizing antibody bezlotoxumab revealed by X-ray crystallography. J Biol Chem. 2014;289:18008-21.
8. Wilcox, MH et al. Bezlotoxumab for prevention of recurrent Clostridium difficile infection. N Engl J Med. 2017;376:305-17.
9. Louie, TJ et al. Fidaxomicin preserves the intestinal microbiome during and after treatment of Clostridium difficile infection (CDI) and reduces both toxin reexpression and recurrence of CDI. Clin Infect Dis. 2012;5(Suppl 2):S132-42.
Infections resulting from Clostridium difficile are a major clinical challenge. In hematology and oncology, the widespread use of broad-spectrum antibiotics is essential for patients with profound neutropenia and infectious complications, which are a high-risk factor for C. difficile enteritis.
C. difficile enteritis occurs in 5%-20% of cancer patients.1 With standard of care antibiotics, oral metronidazole or oral vancomycin, high C. difficile cure rates are possible, but up to 25% of these infections recur. Recently, oral fidaxomicin was approved for treatment of C. difficile enteritis and was associated with high cure rates and, more importantly, with significantly lower recurrence rates.2
Bezlotoxumab, a fully humanized monoclonal antibody against C. difficile toxin B, has been shown by x-ray crystallography to neutralize toxin B by blocking its ability to bind to host cells.7 Most recently, this new therapeutic approach was investigated in humans.8
Wilcox et al. used pooled data of 2655 adults treated in two double-blind, randomized, placebo-controlled phase III clinical trials (MODIFY I and MODIFY II) for primary or recurrent C. difficile enteritis. This industry-sponsored trial was conducted at 322 sites in 30 countries.
In one treatment group, patients received a single infusion of bezlotoxumab (781 patients) or placebo (773 patients) and one of the three oral standard-of-care C. difficile antibiotics. Importantly, the primary end point of this trial was recurrent infection within 12 weeks. About 28% of the patients in both the bezlotoxumab group and the placebo group previously had at least one episode of C. difficile enteritis. About 20% of the patients in both groups were immunocompromised.
Pooled data showed that recurrent infection was significantly lower (P less than 0.001) in the bezlotoxumab group (17%), compared with the placebo group (27%). The difference in recurrence rate (25% vs. 41%) was even more pronounced in patients with one or more episodes of recurrent C. difficile enteritis in the past 6 months. Furthermore, a benefit for bezlotoxumab was seen in immunocompromised patients, whose recurrence rates were 15% with bezlotoxumab, vs. 28% with placebo. After the 12 weeks of follow-up, the absolute difference in the Kaplan-Meier rates of recurrent infection was 13% (absolute rate, 21% in bezlotoxumab group vs. 34% in placebo group; P less than 0.001).
The results indicate that bezlotoxumab, which was approved in 2016 by the U.S. Food and Drug Administration, might improve the outcome of patients with C. difficile enteritis. However, bezlotoxumab is not a “magic bullet.” The number needed to treat to prevent one episode of C. difficile enteritis is 10.
It is conceivable that bezlotoxumab may find its role in high-risk patients – those older than 65 years or patients with recurrent C. difficile enteritis – since the number needed to treat is only 6 in these subgroups.8
This new agent could be an important treatment option for our high-risk patients in hematology. However, more studies concerning costs and real-life efficacy are needed.
The new approach of passive immunization for prevention of recurrent C. difficile enteritis shows the importance and the role of toxin B – not only the bacterium per se – in pathogenesis and virulence of C. difficile. This could mean that we have to renew our view on the role of antibiotics against C. difficile. However, in contrast, bezlotoxumab does not affect the efficacy of standard of care antibiotics since the initial cure rates were 80% for both the antibody and the placebo groups.8 Toxin B levels are not detectable in stool samples between days 4 and 10 of standard of care antibiotic treatment. Afterward, however, they increase again.9 Most of the patients had received bezlotoxumab 3 or more days after they began standard-of-care antibiotic treatment – in the time period when toxin B is undetectable in stool – which underlines the importance of toxin B in the pathogenesis of recurrent C. difficile enteritis.8
In summary, the introduction of bezlotoxumab in clinical care gives new and important insights and solutions not only for treatment options but also for our understanding of C. difficile pathogenesis.
Dr. Schalk is consultant of internal medicine at the department of hematology and oncology, Magdeburg University Hospital, Germany, with clinical and research focus on infectious diseases in hematology and oncology.
Dr. Fischer is professor of internal medicine, hematology and oncology, at the Otto-von-Guericke University Hospital Magdeburg, Germany. He is head of the department of hematology and oncology and a clinical/molecular researcher in myeloid neoplasms. He is a member of the editorial advisory board of Hematology News.
Contact Dr. Schalk at [email protected].
References
1. Vehreschild, MJ et al. Diagnosis and management of gastrointestinal complications in adult cancer patients: Wvidence-based guidelines of the Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Oncology (DGHO). Ann Oncol. 2013;24:1189-202
2. Cornely, OA. Current and emerging management options for Clostridium difficile infection: What is the role of fidaxomicin? Clin Microbiol Infect. 2012;18(Suppl 6):28-35.
3. Bartlett, JG et al. Antibiotic-associated pseudomembranous colitis due to toxin-producing clostridia. N Engl J Med. 1978;298(10):531-4.
4. Lyras, D et al. Toxin B is essential for virulence of Clostridium difficile. Nature. 2009;458:1176-9.
5. Reineke, J et al. Autocatalytic cleavage of Clostridium difficile toxin B. Nature. 2007;446:415-9.
6. Leav, BA et al. Serum anti-toxin B antibody correlates with protection from recurrent Clostridium difficile infection (CDI). Vaccine. 2010;28:965-9.
7. Orth, P et al. Mechanism of action and epitopes of Clostridium difficile toxin B-neutralizing antibody bezlotoxumab revealed by X-ray crystallography. J Biol Chem. 2014;289:18008-21.
8. Wilcox, MH et al. Bezlotoxumab for prevention of recurrent Clostridium difficile infection. N Engl J Med. 2017;376:305-17.
9. Louie, TJ et al. Fidaxomicin preserves the intestinal microbiome during and after treatment of Clostridium difficile infection (CDI) and reduces both toxin reexpression and recurrence of CDI. Clin Infect Dis. 2012;5(Suppl 2):S132-42.
Chronic GVHD linked to fivefold increase in squamous cell skin carcinomas
PORTLAND – Chronic graft versus host disease (GVHD) was associated with a fivefold increase in risk of squamous cell carcinoma and a nearly twofold rise in the rate of basal cell carcinoma, based on a meta-analysis of eight studies.
Acute GVHD was not tied to an increase in secondary nonmelanoma skin cancers, Pooja H. Rambhia and her associates reported in a poster presented at the annual meeting of the Society for Investigative Dermatology. The findings highlight the need for multidisciplinary consults to distinguish malignancies from the cutaneous manifestations of chronic GVHD and for vigorous surveillance for skin cancer even years after hematopoietic stem cell transplantation.
GVHS has been linked to secondary nonmelanoma skin cancers in previous studies, but few have quantified the risk, according to the reviewers, who are from the department of dermatology and dermatopathology at the Cleveland Clinic Foundation. The increased risk may be related to the heavy immunosuppression needed to treat chronic GVHD.
For the meta-analysis, the researchers identified 1,411 studies recorded in academic databases and reviewed those that reported both cases of skin cancers and GVHD. Seven retrospective, and one prospective, studies published between 1997 and 2012 measured both variables in all patients.
The studies included more than 56,000 patients followed for up to 36 years after undergoing allogeneic or syngeneic transplantation, the reviewers reported. During follow-up, between 17% and 73% of patients developed chronic GVHD, and 29% to 67% developed acute GVHD. There were 98 cases of basal cell carcinoma, 49 cases of squamous cell carcinoma, and 34 cases of malignant melanoma. Chronic GVHD was significantly associated with both squamous cell carcinoma (risk ratio, 5.3; 95% confidence interval, 2.4-11.8; P less than .001) and basal cell carcinoma (RR, 2.0; 95% CI, 1.3-3.0; P = .002). In contrast, chronic GVHD showed a nonsignificant trend toward an inverse correlation with the risk of secondary melanoma. Acute GVHD was not linked with squamous cell carcinoma, basal cell carcinoma, or melanoma.
GVHD develops, up to half the time, after hematopoietic stem cell transplantation and often becomes chronic, the reviewers noted. Catching skin cancer early is crucial, and transplant patients should undergo regular skin checks with multidisciplinary consults to promptly, accurately distinguish malignancies from the cutaneous manifestations of GVHD, they added.
The researchers did not report external funding sources. They had no relevant financial conflicts of interest.
PORTLAND – Chronic graft versus host disease (GVHD) was associated with a fivefold increase in risk of squamous cell carcinoma and a nearly twofold rise in the rate of basal cell carcinoma, based on a meta-analysis of eight studies.
Acute GVHD was not tied to an increase in secondary nonmelanoma skin cancers, Pooja H. Rambhia and her associates reported in a poster presented at the annual meeting of the Society for Investigative Dermatology. The findings highlight the need for multidisciplinary consults to distinguish malignancies from the cutaneous manifestations of chronic GVHD and for vigorous surveillance for skin cancer even years after hematopoietic stem cell transplantation.
GVHS has been linked to secondary nonmelanoma skin cancers in previous studies, but few have quantified the risk, according to the reviewers, who are from the department of dermatology and dermatopathology at the Cleveland Clinic Foundation. The increased risk may be related to the heavy immunosuppression needed to treat chronic GVHD.
For the meta-analysis, the researchers identified 1,411 studies recorded in academic databases and reviewed those that reported both cases of skin cancers and GVHD. Seven retrospective, and one prospective, studies published between 1997 and 2012 measured both variables in all patients.
The studies included more than 56,000 patients followed for up to 36 years after undergoing allogeneic or syngeneic transplantation, the reviewers reported. During follow-up, between 17% and 73% of patients developed chronic GVHD, and 29% to 67% developed acute GVHD. There were 98 cases of basal cell carcinoma, 49 cases of squamous cell carcinoma, and 34 cases of malignant melanoma. Chronic GVHD was significantly associated with both squamous cell carcinoma (risk ratio, 5.3; 95% confidence interval, 2.4-11.8; P less than .001) and basal cell carcinoma (RR, 2.0; 95% CI, 1.3-3.0; P = .002). In contrast, chronic GVHD showed a nonsignificant trend toward an inverse correlation with the risk of secondary melanoma. Acute GVHD was not linked with squamous cell carcinoma, basal cell carcinoma, or melanoma.
GVHD develops, up to half the time, after hematopoietic stem cell transplantation and often becomes chronic, the reviewers noted. Catching skin cancer early is crucial, and transplant patients should undergo regular skin checks with multidisciplinary consults to promptly, accurately distinguish malignancies from the cutaneous manifestations of GVHD, they added.
The researchers did not report external funding sources. They had no relevant financial conflicts of interest.
PORTLAND – Chronic graft versus host disease (GVHD) was associated with a fivefold increase in risk of squamous cell carcinoma and a nearly twofold rise in the rate of basal cell carcinoma, based on a meta-analysis of eight studies.
Acute GVHD was not tied to an increase in secondary nonmelanoma skin cancers, Pooja H. Rambhia and her associates reported in a poster presented at the annual meeting of the Society for Investigative Dermatology. The findings highlight the need for multidisciplinary consults to distinguish malignancies from the cutaneous manifestations of chronic GVHD and for vigorous surveillance for skin cancer even years after hematopoietic stem cell transplantation.
GVHS has been linked to secondary nonmelanoma skin cancers in previous studies, but few have quantified the risk, according to the reviewers, who are from the department of dermatology and dermatopathology at the Cleveland Clinic Foundation. The increased risk may be related to the heavy immunosuppression needed to treat chronic GVHD.
For the meta-analysis, the researchers identified 1,411 studies recorded in academic databases and reviewed those that reported both cases of skin cancers and GVHD. Seven retrospective, and one prospective, studies published between 1997 and 2012 measured both variables in all patients.
The studies included more than 56,000 patients followed for up to 36 years after undergoing allogeneic or syngeneic transplantation, the reviewers reported. During follow-up, between 17% and 73% of patients developed chronic GVHD, and 29% to 67% developed acute GVHD. There were 98 cases of basal cell carcinoma, 49 cases of squamous cell carcinoma, and 34 cases of malignant melanoma. Chronic GVHD was significantly associated with both squamous cell carcinoma (risk ratio, 5.3; 95% confidence interval, 2.4-11.8; P less than .001) and basal cell carcinoma (RR, 2.0; 95% CI, 1.3-3.0; P = .002). In contrast, chronic GVHD showed a nonsignificant trend toward an inverse correlation with the risk of secondary melanoma. Acute GVHD was not linked with squamous cell carcinoma, basal cell carcinoma, or melanoma.
GVHD develops, up to half the time, after hematopoietic stem cell transplantation and often becomes chronic, the reviewers noted. Catching skin cancer early is crucial, and transplant patients should undergo regular skin checks with multidisciplinary consults to promptly, accurately distinguish malignancies from the cutaneous manifestations of GVHD, they added.
The researchers did not report external funding sources. They had no relevant financial conflicts of interest.
AT SID 2017
Key clinical point: Chronic graft versus host disease was associated with a significantly increased risk of squamous cell and basal cell carcinomas.
Major finding: Chronic GVHD was associated with a fivefold increase in squamous cell carcinoma (risk ratio, 5.3; 95% confidence interval, 2.4 to 11.8; P less than .001).
Data source: A meta-analysis of eight cohort studies of 56,000 patients who underwent hematopoietic stem cell transplantation.
Disclosures: The researchers did not report external funding sources. They had no conflicts of interest.