User login
FDA axes asthma drugs’ boxed warning
The Food and Drug Administration has eliminated the boxed warning for risk of asthma-related death from the labels of products containing both an inhaled corticosteroid (ICS) and a long-acting beta agonist (LABA), the agency announced.
In 2011, the FDA required companies manufacturing fixed-dose LABA-ICS combination products to conduct 26-week clinical safety trials to evaluate the risks of serious adverse asthma-related events in patients treated with these drugs. Specifically, the companies had to compare the follows the FDA’s review of these trials, which found that treating asthma with LABAs in combination with ICS did not result in patients experiencing significantly more serious asthma-related side effects and asthma-related deaths, compared with those being treated with an ICS alone, according to the FDA announcement. “Results of subgroup analyses for gender, adolescents 12-18 years, and African Americans are consistent with the primary endpoint results,” the statement added.
“These trials showed that LABAs, when used with ICS, did not significantly increase the risk of asthma-related hospitalizations, the need to insert a breathing tube known as intubation, or asthma-related deaths, compared to ICS alone,” the FDA said in the statement.
The trials also demonstrated that using the combination reduced asthma exacerbations, compared with using ICS alone, and that most of the exacerbations “were those that required at least 3 days of systemic corticosteroids” – information that is being added the product labels, according to the FDA.
The products that will no longer carry this boxed warning in their labels include AstraZeneca’s budesonide/formoterol fumarate dihydrate (Symbicort) and GlaxoSmithKline’s fluticasone furoate/vilanterol (Breo Ellipta) and fluticasone propionate/salmeterol (Advair Diskus and Advair HFA).
The FDA also approved updates to the Warnings and Precautions section of labeling for the ICS/LABA class, which now includes a description of the four trials. Information on the efficacy of the drugs, found in the trials, has been added to the Clinical Studies section of the labels as well.
In a related safety announcement, the FDA stated the following: “Using LABAs alone to treat asthma without an ICS to treat lung inflammation is associated with an increased risk of asthma-related death. Therefore, the Boxed Warning stating this will remain in the labels of all single-ingredient LABA medicines, which are approved to treat asthma, chronic obstructive pulmonary disease (COPD), and wheezing caused by exercise. The labels of medicines that contain both an ICS and LABA also retain a Warning and Precaution related to the increased risk of asthma-related death when LABAs are used without an ICS to treat asthma.
The Food and Drug Administration has eliminated the boxed warning for risk of asthma-related death from the labels of products containing both an inhaled corticosteroid (ICS) and a long-acting beta agonist (LABA), the agency announced.
In 2011, the FDA required companies manufacturing fixed-dose LABA-ICS combination products to conduct 26-week clinical safety trials to evaluate the risks of serious adverse asthma-related events in patients treated with these drugs. Specifically, the companies had to compare the follows the FDA’s review of these trials, which found that treating asthma with LABAs in combination with ICS did not result in patients experiencing significantly more serious asthma-related side effects and asthma-related deaths, compared with those being treated with an ICS alone, according to the FDA announcement. “Results of subgroup analyses for gender, adolescents 12-18 years, and African Americans are consistent with the primary endpoint results,” the statement added.
“These trials showed that LABAs, when used with ICS, did not significantly increase the risk of asthma-related hospitalizations, the need to insert a breathing tube known as intubation, or asthma-related deaths, compared to ICS alone,” the FDA said in the statement.
The trials also demonstrated that using the combination reduced asthma exacerbations, compared with using ICS alone, and that most of the exacerbations “were those that required at least 3 days of systemic corticosteroids” – information that is being added the product labels, according to the FDA.
The products that will no longer carry this boxed warning in their labels include AstraZeneca’s budesonide/formoterol fumarate dihydrate (Symbicort) and GlaxoSmithKline’s fluticasone furoate/vilanterol (Breo Ellipta) and fluticasone propionate/salmeterol (Advair Diskus and Advair HFA).
The FDA also approved updates to the Warnings and Precautions section of labeling for the ICS/LABA class, which now includes a description of the four trials. Information on the efficacy of the drugs, found in the trials, has been added to the Clinical Studies section of the labels as well.
In a related safety announcement, the FDA stated the following: “Using LABAs alone to treat asthma without an ICS to treat lung inflammation is associated with an increased risk of asthma-related death. Therefore, the Boxed Warning stating this will remain in the labels of all single-ingredient LABA medicines, which are approved to treat asthma, chronic obstructive pulmonary disease (COPD), and wheezing caused by exercise. The labels of medicines that contain both an ICS and LABA also retain a Warning and Precaution related to the increased risk of asthma-related death when LABAs are used without an ICS to treat asthma.
The Food and Drug Administration has eliminated the boxed warning for risk of asthma-related death from the labels of products containing both an inhaled corticosteroid (ICS) and a long-acting beta agonist (LABA), the agency announced.
In 2011, the FDA required companies manufacturing fixed-dose LABA-ICS combination products to conduct 26-week clinical safety trials to evaluate the risks of serious adverse asthma-related events in patients treated with these drugs. Specifically, the companies had to compare the follows the FDA’s review of these trials, which found that treating asthma with LABAs in combination with ICS did not result in patients experiencing significantly more serious asthma-related side effects and asthma-related deaths, compared with those being treated with an ICS alone, according to the FDA announcement. “Results of subgroup analyses for gender, adolescents 12-18 years, and African Americans are consistent with the primary endpoint results,” the statement added.
“These trials showed that LABAs, when used with ICS, did not significantly increase the risk of asthma-related hospitalizations, the need to insert a breathing tube known as intubation, or asthma-related deaths, compared to ICS alone,” the FDA said in the statement.
The trials also demonstrated that using the combination reduced asthma exacerbations, compared with using ICS alone, and that most of the exacerbations “were those that required at least 3 days of systemic corticosteroids” – information that is being added the product labels, according to the FDA.
The products that will no longer carry this boxed warning in their labels include AstraZeneca’s budesonide/formoterol fumarate dihydrate (Symbicort) and GlaxoSmithKline’s fluticasone furoate/vilanterol (Breo Ellipta) and fluticasone propionate/salmeterol (Advair Diskus and Advair HFA).
The FDA also approved updates to the Warnings and Precautions section of labeling for the ICS/LABA class, which now includes a description of the four trials. Information on the efficacy of the drugs, found in the trials, has been added to the Clinical Studies section of the labels as well.
In a related safety announcement, the FDA stated the following: “Using LABAs alone to treat asthma without an ICS to treat lung inflammation is associated with an increased risk of asthma-related death. Therefore, the Boxed Warning stating this will remain in the labels of all single-ingredient LABA medicines, which are approved to treat asthma, chronic obstructive pulmonary disease (COPD), and wheezing caused by exercise. The labels of medicines that contain both an ICS and LABA also retain a Warning and Precaution related to the increased risk of asthma-related death when LABAs are used without an ICS to treat asthma.
Nebulized LAMA for COPD approved
The Food and Drug Administration has given the nod to the first nebulized long-acting muscarinic antagonist (LAMA) treatment for chronic obstructive pulmonary disease (COPD) in the United States.
Glycopyrrolate (Lonhala Magnair) utilizes the eFlow technology system, developed by Pari Pharma. This nebulizing system is portable, virtually silent, and delivers the drug in 2-3 minutes, according to a statement from Sunovion Pharmaceuticals.
The approval of glycopyrrolate is based on the results of the GOLDEN (Glycopyrrolate for Obstructive Lung Disease via Electronic Nebulizer) trials. The GOLDEN program comprised the GOLDEN-3 and GOLDEN-4 trials, both of which were phase 3, 12-week, randomized, double-blind, placebo-controlled, parallel-group, multicenter safety and efficacy trials, which compared adult glycopyrrolate patients to a placebo group with moderate to severe COPD. At 12 weeks, patients receiving treatment with glycopyrrolate showed clinical and statistically significant improvements in their baseline forced expiratory volume second (FEV1), compared with placebo.
GOLDEN-5, an additional study, followed the same criteria as previous studies, but increased its length to 48 weeks to evaluate the long-term safety and patient tolerability of glycopyrrolate. It also compared treatment of COPD with glycopyrrolate to treatment of COPD with the previously approved LAMA Spiriva (tiotropium bromide), delivered by the Handihaler device. Glycopyrrolate was well tolerated, and the overall treatment emergence of adverse events for glycopyrrolate and tiotropium bromide were similar.
Sunovion expects glycopyrrolate to be available in U.S. pharmacies in early 2018, according to the statement.
The Food and Drug Administration has given the nod to the first nebulized long-acting muscarinic antagonist (LAMA) treatment for chronic obstructive pulmonary disease (COPD) in the United States.
Glycopyrrolate (Lonhala Magnair) utilizes the eFlow technology system, developed by Pari Pharma. This nebulizing system is portable, virtually silent, and delivers the drug in 2-3 minutes, according to a statement from Sunovion Pharmaceuticals.
The approval of glycopyrrolate is based on the results of the GOLDEN (Glycopyrrolate for Obstructive Lung Disease via Electronic Nebulizer) trials. The GOLDEN program comprised the GOLDEN-3 and GOLDEN-4 trials, both of which were phase 3, 12-week, randomized, double-blind, placebo-controlled, parallel-group, multicenter safety and efficacy trials, which compared adult glycopyrrolate patients to a placebo group with moderate to severe COPD. At 12 weeks, patients receiving treatment with glycopyrrolate showed clinical and statistically significant improvements in their baseline forced expiratory volume second (FEV1), compared with placebo.
GOLDEN-5, an additional study, followed the same criteria as previous studies, but increased its length to 48 weeks to evaluate the long-term safety and patient tolerability of glycopyrrolate. It also compared treatment of COPD with glycopyrrolate to treatment of COPD with the previously approved LAMA Spiriva (tiotropium bromide), delivered by the Handihaler device. Glycopyrrolate was well tolerated, and the overall treatment emergence of adverse events for glycopyrrolate and tiotropium bromide were similar.
Sunovion expects glycopyrrolate to be available in U.S. pharmacies in early 2018, according to the statement.
The Food and Drug Administration has given the nod to the first nebulized long-acting muscarinic antagonist (LAMA) treatment for chronic obstructive pulmonary disease (COPD) in the United States.
Glycopyrrolate (Lonhala Magnair) utilizes the eFlow technology system, developed by Pari Pharma. This nebulizing system is portable, virtually silent, and delivers the drug in 2-3 minutes, according to a statement from Sunovion Pharmaceuticals.
The approval of glycopyrrolate is based on the results of the GOLDEN (Glycopyrrolate for Obstructive Lung Disease via Electronic Nebulizer) trials. The GOLDEN program comprised the GOLDEN-3 and GOLDEN-4 trials, both of which were phase 3, 12-week, randomized, double-blind, placebo-controlled, parallel-group, multicenter safety and efficacy trials, which compared adult glycopyrrolate patients to a placebo group with moderate to severe COPD. At 12 weeks, patients receiving treatment with glycopyrrolate showed clinical and statistically significant improvements in their baseline forced expiratory volume second (FEV1), compared with placebo.
GOLDEN-5, an additional study, followed the same criteria as previous studies, but increased its length to 48 weeks to evaluate the long-term safety and patient tolerability of glycopyrrolate. It also compared treatment of COPD with glycopyrrolate to treatment of COPD with the previously approved LAMA Spiriva (tiotropium bromide), delivered by the Handihaler device. Glycopyrrolate was well tolerated, and the overall treatment emergence of adverse events for glycopyrrolate and tiotropium bromide were similar.
Sunovion expects glycopyrrolate to be available in U.S. pharmacies in early 2018, according to the statement.
Health disparities in rural America: Chronic conditions
Among rural adults, multiple chronic health conditions are most common in non-Hispanic blacks and American Indians/Alaska Natives (AI/ANs) and least common among Asians and Native Hawaiians/other Pacific Islanders (NHOPIs), according to the Centers for Disease Control and Prevention.
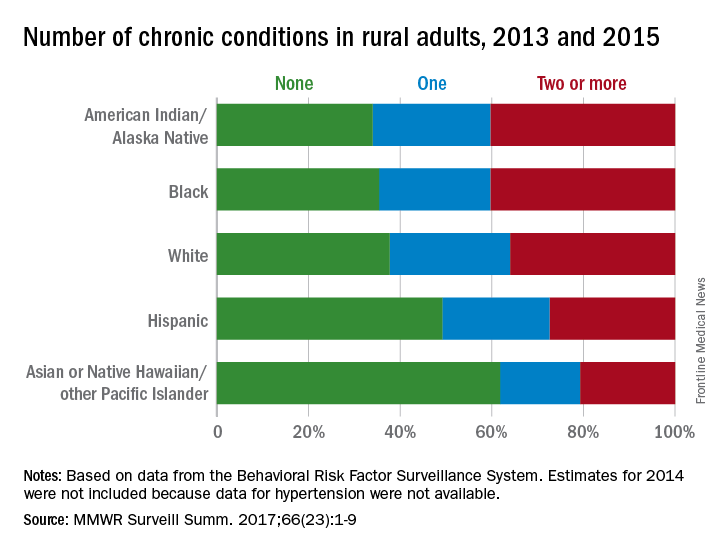
The order was reversed for adults reporting no chronic conditions: Asians and NHOPIs at 61.8%, Hispanics at 49.2%, whites at 37.8%, blacks at 35.4%, and AI/ANs at 34.0%, the researchers said.
For the chronic health conditions included separately in the report, blacks had the highest rate (45.9%) and Asians and NHOPIs had the lowest rate (15.5%) of obesity; AI/ANs were most likely (23.2%) and Asians and NHOPIs were least likely (5.8%) to report depressive disorder. Other conditions considered in the estimates were myocardial infarction; coronary heart disease; stroke; hypertension; asthma; skin cancer; other types of cancer; chronic obstructive pulmonary disease; kidney disease; some form of arthritis, rheumatoid arthritis, gout, lupus, or fibromyalgia; and diabetes. Estimates for 2014 were not included because data for hypertension were not available, the investigators noted.
Of the 3,143 counties categorized by the National Center for Health Statistics’ Urban-Rural Classification Scheme for Counties, a total of 1,325 were considered rural and included 6.1% of the U.S. population, they said.
Among rural adults, multiple chronic health conditions are most common in non-Hispanic blacks and American Indians/Alaska Natives (AI/ANs) and least common among Asians and Native Hawaiians/other Pacific Islanders (NHOPIs), according to the Centers for Disease Control and Prevention.

The order was reversed for adults reporting no chronic conditions: Asians and NHOPIs at 61.8%, Hispanics at 49.2%, whites at 37.8%, blacks at 35.4%, and AI/ANs at 34.0%, the researchers said.
For the chronic health conditions included separately in the report, blacks had the highest rate (45.9%) and Asians and NHOPIs had the lowest rate (15.5%) of obesity; AI/ANs were most likely (23.2%) and Asians and NHOPIs were least likely (5.8%) to report depressive disorder. Other conditions considered in the estimates were myocardial infarction; coronary heart disease; stroke; hypertension; asthma; skin cancer; other types of cancer; chronic obstructive pulmonary disease; kidney disease; some form of arthritis, rheumatoid arthritis, gout, lupus, or fibromyalgia; and diabetes. Estimates for 2014 were not included because data for hypertension were not available, the investigators noted.
Of the 3,143 counties categorized by the National Center for Health Statistics’ Urban-Rural Classification Scheme for Counties, a total of 1,325 were considered rural and included 6.1% of the U.S. population, they said.
Among rural adults, multiple chronic health conditions are most common in non-Hispanic blacks and American Indians/Alaska Natives (AI/ANs) and least common among Asians and Native Hawaiians/other Pacific Islanders (NHOPIs), according to the Centers for Disease Control and Prevention.

The order was reversed for adults reporting no chronic conditions: Asians and NHOPIs at 61.8%, Hispanics at 49.2%, whites at 37.8%, blacks at 35.4%, and AI/ANs at 34.0%, the researchers said.
For the chronic health conditions included separately in the report, blacks had the highest rate (45.9%) and Asians and NHOPIs had the lowest rate (15.5%) of obesity; AI/ANs were most likely (23.2%) and Asians and NHOPIs were least likely (5.8%) to report depressive disorder. Other conditions considered in the estimates were myocardial infarction; coronary heart disease; stroke; hypertension; asthma; skin cancer; other types of cancer; chronic obstructive pulmonary disease; kidney disease; some form of arthritis, rheumatoid arthritis, gout, lupus, or fibromyalgia; and diabetes. Estimates for 2014 were not included because data for hypertension were not available, the investigators noted.
Of the 3,143 counties categorized by the National Center for Health Statistics’ Urban-Rural Classification Scheme for Counties, a total of 1,325 were considered rural and included 6.1% of the U.S. population, they said.
FROM MMWR SURVEILLANCE SUMMARIES
Omalizumab helps asthma COPD overlap patients
TORONTO – Omalizumab (Xolair, Genentech) decreased asthma exacerbations and improved symptom control to a similar extent in patients with asthma chronic obstructive pulmonary disease (ACO) overlap as seen in patients with asthma but no COPD, in a study presented at the CHEST annual meeting.
While patients with COPD typically experience annual declines in lung function, at least some of the ACO patients in this study, which included one of the largest observational cohorts to date of patients with ACO, showed preserved lung function after 48 weeks of omalizumab treatment.
Dr. Hanania presented data from the “real-world” PROSPERO (Prospective Study to Evaluate Predictors of Clinical Effectiveness in Response to Omalizumab), which unlike many asthma studies, did not exclude patients with comorbid COPD. PROSPERO was a prospective, multicenter, observational, 48-week study of patients (n = 806) who were 12 years of age and older who were initiating omalizumab treatment for moderate to severe allergic asthma. Asthma control was assessed monthly using the Asthma Control Test (ACT).
Participants were identified as having ACO based on two approaches: 1. A positive medical history of asthma and COPD, or 2. A medical history of asthma (but not COPD), at least a 10-pack per year smoking history, and an forced expiratory volume in 1 second/forced vital capacity (FEV1/FVC) of less than 0.7. From the 728 study participants included in this secondary analysis, 56 were classified as ACO according to the first definition (ACO cohort A) and 59 according to the second (ACO cohort B). Thirty-seven patients fell into both groups.
“All groups had a reduction in their exacerbation rates through 12 months, and it didn’t differ whether they had ACO in cohort A or cohort B, or no ACO,” Dr. Hanania reported.
Additionally, all three groups showed clinically meaningful improvements in their ACT scores, with mean improvements of 4.1, 4.7, and 4.4 units for ACO cohort A, ACO cohort B, and non-ACO patients, respectively.
Postbronchodilator FEV1 at study end was improved by 36 mL in ACO cohort A and by 23 mL in the non-ACO cohort. But a 14 mL reduction in postbronchodilator FEV1 was noted in ACO cohort B, “a reminder that the cohort B population was those patients with fixed airway obstruction and smoking history,” said Dr. Hanania.
Mean age in the non-ACO population was 50 years, rising to 57.6 years in ACO cohort A and 55 years in ACO cohort B. All three groups had three or more asthma exacerbations in the 12 months before starting omalizumab, and all groups had mean ACT scores of less than 15 at baseline, indicating that they were all symptomatic.
Adverse events were consistent with the known safety profile of omalizumab.
“The significance of this study [is that] it’s one of the largest ACO cohorts that we know of and I think it encourages all of us to look at or re-visit both COPD therapies and asthma therapies in populations [not included] in clinical trials because in real life, these are the patients we see … and we don’t have evidence,” Dr. Hanania said.
Dr. Hanania reported receiving research support from Roche/Genentech, among other companies. Three of the investigators are employees of Genentech, the study’s sponsor.
TORONTO – Omalizumab (Xolair, Genentech) decreased asthma exacerbations and improved symptom control to a similar extent in patients with asthma chronic obstructive pulmonary disease (ACO) overlap as seen in patients with asthma but no COPD, in a study presented at the CHEST annual meeting.
While patients with COPD typically experience annual declines in lung function, at least some of the ACO patients in this study, which included one of the largest observational cohorts to date of patients with ACO, showed preserved lung function after 48 weeks of omalizumab treatment.
Dr. Hanania presented data from the “real-world” PROSPERO (Prospective Study to Evaluate Predictors of Clinical Effectiveness in Response to Omalizumab), which unlike many asthma studies, did not exclude patients with comorbid COPD. PROSPERO was a prospective, multicenter, observational, 48-week study of patients (n = 806) who were 12 years of age and older who were initiating omalizumab treatment for moderate to severe allergic asthma. Asthma control was assessed monthly using the Asthma Control Test (ACT).
Participants were identified as having ACO based on two approaches: 1. A positive medical history of asthma and COPD, or 2. A medical history of asthma (but not COPD), at least a 10-pack per year smoking history, and an forced expiratory volume in 1 second/forced vital capacity (FEV1/FVC) of less than 0.7. From the 728 study participants included in this secondary analysis, 56 were classified as ACO according to the first definition (ACO cohort A) and 59 according to the second (ACO cohort B). Thirty-seven patients fell into both groups.
“All groups had a reduction in their exacerbation rates through 12 months, and it didn’t differ whether they had ACO in cohort A or cohort B, or no ACO,” Dr. Hanania reported.
Additionally, all three groups showed clinically meaningful improvements in their ACT scores, with mean improvements of 4.1, 4.7, and 4.4 units for ACO cohort A, ACO cohort B, and non-ACO patients, respectively.
Postbronchodilator FEV1 at study end was improved by 36 mL in ACO cohort A and by 23 mL in the non-ACO cohort. But a 14 mL reduction in postbronchodilator FEV1 was noted in ACO cohort B, “a reminder that the cohort B population was those patients with fixed airway obstruction and smoking history,” said Dr. Hanania.
Mean age in the non-ACO population was 50 years, rising to 57.6 years in ACO cohort A and 55 years in ACO cohort B. All three groups had three or more asthma exacerbations in the 12 months before starting omalizumab, and all groups had mean ACT scores of less than 15 at baseline, indicating that they were all symptomatic.
Adverse events were consistent with the known safety profile of omalizumab.
“The significance of this study [is that] it’s one of the largest ACO cohorts that we know of and I think it encourages all of us to look at or re-visit both COPD therapies and asthma therapies in populations [not included] in clinical trials because in real life, these are the patients we see … and we don’t have evidence,” Dr. Hanania said.
Dr. Hanania reported receiving research support from Roche/Genentech, among other companies. Three of the investigators are employees of Genentech, the study’s sponsor.
TORONTO – Omalizumab (Xolair, Genentech) decreased asthma exacerbations and improved symptom control to a similar extent in patients with asthma chronic obstructive pulmonary disease (ACO) overlap as seen in patients with asthma but no COPD, in a study presented at the CHEST annual meeting.
While patients with COPD typically experience annual declines in lung function, at least some of the ACO patients in this study, which included one of the largest observational cohorts to date of patients with ACO, showed preserved lung function after 48 weeks of omalizumab treatment.
Dr. Hanania presented data from the “real-world” PROSPERO (Prospective Study to Evaluate Predictors of Clinical Effectiveness in Response to Omalizumab), which unlike many asthma studies, did not exclude patients with comorbid COPD. PROSPERO was a prospective, multicenter, observational, 48-week study of patients (n = 806) who were 12 years of age and older who were initiating omalizumab treatment for moderate to severe allergic asthma. Asthma control was assessed monthly using the Asthma Control Test (ACT).
Participants were identified as having ACO based on two approaches: 1. A positive medical history of asthma and COPD, or 2. A medical history of asthma (but not COPD), at least a 10-pack per year smoking history, and an forced expiratory volume in 1 second/forced vital capacity (FEV1/FVC) of less than 0.7. From the 728 study participants included in this secondary analysis, 56 were classified as ACO according to the first definition (ACO cohort A) and 59 according to the second (ACO cohort B). Thirty-seven patients fell into both groups.
“All groups had a reduction in their exacerbation rates through 12 months, and it didn’t differ whether they had ACO in cohort A or cohort B, or no ACO,” Dr. Hanania reported.
Additionally, all three groups showed clinically meaningful improvements in their ACT scores, with mean improvements of 4.1, 4.7, and 4.4 units for ACO cohort A, ACO cohort B, and non-ACO patients, respectively.
Postbronchodilator FEV1 at study end was improved by 36 mL in ACO cohort A and by 23 mL in the non-ACO cohort. But a 14 mL reduction in postbronchodilator FEV1 was noted in ACO cohort B, “a reminder that the cohort B population was those patients with fixed airway obstruction and smoking history,” said Dr. Hanania.
Mean age in the non-ACO population was 50 years, rising to 57.6 years in ACO cohort A and 55 years in ACO cohort B. All three groups had three or more asthma exacerbations in the 12 months before starting omalizumab, and all groups had mean ACT scores of less than 15 at baseline, indicating that they were all symptomatic.
Adverse events were consistent with the known safety profile of omalizumab.
“The significance of this study [is that] it’s one of the largest ACO cohorts that we know of and I think it encourages all of us to look at or re-visit both COPD therapies and asthma therapies in populations [not included] in clinical trials because in real life, these are the patients we see … and we don’t have evidence,” Dr. Hanania said.
Dr. Hanania reported receiving research support from Roche/Genentech, among other companies. Three of the investigators are employees of Genentech, the study’s sponsor.
AT CHEST 2017
Key clinical point: In patients with asthma COPD overlap (ACO), treatment with omalizumab was associated with decreased asthma exacerbations and improved symptom control, similar to that seen in non-ACO asthma patients.
Major finding: Asthma exacerbation numbers were reduced from baseline levels though month 12, from 3 or more exacerbations in both ACO and non ACO groups to 1.1 or less.
Data source: Subgroup analysis from a prospective observational study of omalizumab that focused on 78 patients (from 737 total) that had asthma and comorbid COPD according to one of two definitions.
Disclosures: Dr. Hanania reported receiving research support from Roche/Genentech, among other companies. Genentech sponsored the study and employs three of the investigators.
FDA asked to approve add-on drug for eosinophilic COPD
GlaxoSmithKline asked the Food and Drug Administration to approve an interleuklin-5 antagonist as an add-on maintenance therapy for patients with eosinophilic chronic obstructive pulmonary disease (COPD).
The pharmaceutical and health care company is seeking approval of mepolizumab to be used specifically to treat COPD patients with an eosinophilic phenotype. The drug currently is indicated to treat patients aged 12 years or older with severe asthma and asthma with an eosinophilic phenotype and is sold under the name Nucala, according to a GlaxoSmithKline statement issued November 7.
Headache, injection site reaction, back pain, and fatigue are the most common adverse reactions seen in patients who took mepolizumab during clinical trials.
Mepolizumab is not approved for the treatment of COPD anywhere in the world, and GlaxoSmithKline intends to also ask other countries’ regulatory authorities to allow this drug to be sold as a therapy for COPD.
GlaxoSmithKline asked the Food and Drug Administration to approve an interleuklin-5 antagonist as an add-on maintenance therapy for patients with eosinophilic chronic obstructive pulmonary disease (COPD).
The pharmaceutical and health care company is seeking approval of mepolizumab to be used specifically to treat COPD patients with an eosinophilic phenotype. The drug currently is indicated to treat patients aged 12 years or older with severe asthma and asthma with an eosinophilic phenotype and is sold under the name Nucala, according to a GlaxoSmithKline statement issued November 7.
Headache, injection site reaction, back pain, and fatigue are the most common adverse reactions seen in patients who took mepolizumab during clinical trials.
Mepolizumab is not approved for the treatment of COPD anywhere in the world, and GlaxoSmithKline intends to also ask other countries’ regulatory authorities to allow this drug to be sold as a therapy for COPD.
GlaxoSmithKline asked the Food and Drug Administration to approve an interleuklin-5 antagonist as an add-on maintenance therapy for patients with eosinophilic chronic obstructive pulmonary disease (COPD).
The pharmaceutical and health care company is seeking approval of mepolizumab to be used specifically to treat COPD patients with an eosinophilic phenotype. The drug currently is indicated to treat patients aged 12 years or older with severe asthma and asthma with an eosinophilic phenotype and is sold under the name Nucala, according to a GlaxoSmithKline statement issued November 7.
Headache, injection site reaction, back pain, and fatigue are the most common adverse reactions seen in patients who took mepolizumab during clinical trials.
Mepolizumab is not approved for the treatment of COPD anywhere in the world, and GlaxoSmithKline intends to also ask other countries’ regulatory authorities to allow this drug to be sold as a therapy for COPD.
Nebulized LABA safe for long-term use in COPD
TORONTO – No long-term safety signals were seen in a randomized trial that tested the formoterol fumarate inhalation solution (Perforomist, Mylan) against placebo in patients with moderate to severe chronic obstructive pulmonary disease (COPD).
Safety was confirmed despite patients being permitted to remain on other background treatment for COPD, including inhaled corticosteroids and anticholinergics, in this study presented at the CHEST annual meeting. An additional benefit of the therapy was that it significantly improved lung function from baseline, according to some spirometry measures.
The Food and Drug Administration approved formoterol fumarate, a long-acting beta-2 agonist (LABA), as a nebulized maintenance treatment for bronchoconstriction in COPD. Because of a concern about long-term LABA safety in asthma patients, said Dr. Hanania, the FDA mandated this 1-year phase 4 study to evaluate the long-term safety of formoterol in patients with moderate to severe COPD.
This multicenter, double-blind, noninferiority study randomly assigned 1,071 patients with moderate to severe COPD (mean FEV1, 44.4% of predicted value, at least one exacerbation in the past 12 months) to receive either nebulized formoterol 20 mcg/2 mL twice daily or matching placebo for up to 12 months. Subjects were permitted to remain on stable COPD therapy, including inhaled corticosteroids and anticholinergics but excluding long-acting beta-agonists.
Formoterol was noninferior to placebo for the primary safety endpoint, defined as a first occurrence of respiratory-related death, COPD-related emergency department visit, or COPD-related hospitalization, with an estimated hazard ratio of 0.965.
Formoterol significantly improved trough forced expiratory volume in 1 second (FEV1), compared with placebo at 3 and 6 months of treatment, with (least squares) mean estimated differences of 42 mL (P = .007) and 41 mL (P = .025), respectively, but not at 9 or 12 months. Forced vital capacity was significantly improved with formoterol over placebo at all study visits (3, 6, 9, and 12 months), but improvements from baseline in inspiratory capacity did not significantly differ from placebo.
Mean age of study patients was 62.6 years and 48.5% were female. At baseline, about half of patients were still smokers, half were on inhaled corticosteroids, and about one-third were on concomitant long-acting muscarinic antagonists, mainly tiotropium, reported Dr. Hanania. The vast majority of patients had moderate or severe COPD, with less than 1% having very severe disease at baseline.
In response to a question on dosing, Dr. Hanania told attendees, “One thing we have to keep in mind is that formoterol is a full agonist, so there are dose-dependent adverse effects. So, even though you get better lung function as you go up on the dose, there’s no free lunch and always the potential for adverse effects.”
The safety data was previously presented at the American Thoracic Society meeting in May 2017 (Hanania N et al. Am J Respir Crit Care Med. 2017;195 A5473 [abstract]), while the lung function data are new, said Dr. Hanania.
Dr. Hanania reported being an adviser for several pharmaceutical companies, including Mylan. Four of the six authors of the study’s abstract are employees of Mylan.
TORONTO – No long-term safety signals were seen in a randomized trial that tested the formoterol fumarate inhalation solution (Perforomist, Mylan) against placebo in patients with moderate to severe chronic obstructive pulmonary disease (COPD).
Safety was confirmed despite patients being permitted to remain on other background treatment for COPD, including inhaled corticosteroids and anticholinergics, in this study presented at the CHEST annual meeting. An additional benefit of the therapy was that it significantly improved lung function from baseline, according to some spirometry measures.
The Food and Drug Administration approved formoterol fumarate, a long-acting beta-2 agonist (LABA), as a nebulized maintenance treatment for bronchoconstriction in COPD. Because of a concern about long-term LABA safety in asthma patients, said Dr. Hanania, the FDA mandated this 1-year phase 4 study to evaluate the long-term safety of formoterol in patients with moderate to severe COPD.
This multicenter, double-blind, noninferiority study randomly assigned 1,071 patients with moderate to severe COPD (mean FEV1, 44.4% of predicted value, at least one exacerbation in the past 12 months) to receive either nebulized formoterol 20 mcg/2 mL twice daily or matching placebo for up to 12 months. Subjects were permitted to remain on stable COPD therapy, including inhaled corticosteroids and anticholinergics but excluding long-acting beta-agonists.
Formoterol was noninferior to placebo for the primary safety endpoint, defined as a first occurrence of respiratory-related death, COPD-related emergency department visit, or COPD-related hospitalization, with an estimated hazard ratio of 0.965.
Formoterol significantly improved trough forced expiratory volume in 1 second (FEV1), compared with placebo at 3 and 6 months of treatment, with (least squares) mean estimated differences of 42 mL (P = .007) and 41 mL (P = .025), respectively, but not at 9 or 12 months. Forced vital capacity was significantly improved with formoterol over placebo at all study visits (3, 6, 9, and 12 months), but improvements from baseline in inspiratory capacity did not significantly differ from placebo.
Mean age of study patients was 62.6 years and 48.5% were female. At baseline, about half of patients were still smokers, half were on inhaled corticosteroids, and about one-third were on concomitant long-acting muscarinic antagonists, mainly tiotropium, reported Dr. Hanania. The vast majority of patients had moderate or severe COPD, with less than 1% having very severe disease at baseline.
In response to a question on dosing, Dr. Hanania told attendees, “One thing we have to keep in mind is that formoterol is a full agonist, so there are dose-dependent adverse effects. So, even though you get better lung function as you go up on the dose, there’s no free lunch and always the potential for adverse effects.”
The safety data was previously presented at the American Thoracic Society meeting in May 2017 (Hanania N et al. Am J Respir Crit Care Med. 2017;195 A5473 [abstract]), while the lung function data are new, said Dr. Hanania.
Dr. Hanania reported being an adviser for several pharmaceutical companies, including Mylan. Four of the six authors of the study’s abstract are employees of Mylan.
TORONTO – No long-term safety signals were seen in a randomized trial that tested the formoterol fumarate inhalation solution (Perforomist, Mylan) against placebo in patients with moderate to severe chronic obstructive pulmonary disease (COPD).
Safety was confirmed despite patients being permitted to remain on other background treatment for COPD, including inhaled corticosteroids and anticholinergics, in this study presented at the CHEST annual meeting. An additional benefit of the therapy was that it significantly improved lung function from baseline, according to some spirometry measures.
The Food and Drug Administration approved formoterol fumarate, a long-acting beta-2 agonist (LABA), as a nebulized maintenance treatment for bronchoconstriction in COPD. Because of a concern about long-term LABA safety in asthma patients, said Dr. Hanania, the FDA mandated this 1-year phase 4 study to evaluate the long-term safety of formoterol in patients with moderate to severe COPD.
This multicenter, double-blind, noninferiority study randomly assigned 1,071 patients with moderate to severe COPD (mean FEV1, 44.4% of predicted value, at least one exacerbation in the past 12 months) to receive either nebulized formoterol 20 mcg/2 mL twice daily or matching placebo for up to 12 months. Subjects were permitted to remain on stable COPD therapy, including inhaled corticosteroids and anticholinergics but excluding long-acting beta-agonists.
Formoterol was noninferior to placebo for the primary safety endpoint, defined as a first occurrence of respiratory-related death, COPD-related emergency department visit, or COPD-related hospitalization, with an estimated hazard ratio of 0.965.
Formoterol significantly improved trough forced expiratory volume in 1 second (FEV1), compared with placebo at 3 and 6 months of treatment, with (least squares) mean estimated differences of 42 mL (P = .007) and 41 mL (P = .025), respectively, but not at 9 or 12 months. Forced vital capacity was significantly improved with formoterol over placebo at all study visits (3, 6, 9, and 12 months), but improvements from baseline in inspiratory capacity did not significantly differ from placebo.
Mean age of study patients was 62.6 years and 48.5% were female. At baseline, about half of patients were still smokers, half were on inhaled corticosteroids, and about one-third were on concomitant long-acting muscarinic antagonists, mainly tiotropium, reported Dr. Hanania. The vast majority of patients had moderate or severe COPD, with less than 1% having very severe disease at baseline.
In response to a question on dosing, Dr. Hanania told attendees, “One thing we have to keep in mind is that formoterol is a full agonist, so there are dose-dependent adverse effects. So, even though you get better lung function as you go up on the dose, there’s no free lunch and always the potential for adverse effects.”
The safety data was previously presented at the American Thoracic Society meeting in May 2017 (Hanania N et al. Am J Respir Crit Care Med. 2017;195 A5473 [abstract]), while the lung function data are new, said Dr. Hanania.
Dr. Hanania reported being an adviser for several pharmaceutical companies, including Mylan. Four of the six authors of the study’s abstract are employees of Mylan.
AT CHEST 2017
Key clinical point: The long-term safety of formoterol fumarate inhaled solution was confirmed in an FDA-mandated randomized trial in patients with moderate to severe COPD.
Major finding: Formoterol fumarate was noninferior to placebo for the primary safety endpoint of respiratory-related death, COPD-related emergency department visit, or COPD-related hospitalization, with an estimated hazard ratio of 0.965.
Data source: Multicenter, randomized, double-blind, placebo-controlled trial including 1,071 patients with moderate or severe COPD, with at least one exacerbation recorded in the last year.
Disclosures: Dr. Hanania reported being an adviser for several pharmaceutical companies, including Mylan. Four of the six authors of the study’s abstract are employees of Mylan.
Nebulized glycopyrrolate improves lung function in COPD
TORONTO – Glycopyrrolate, a novel nebulized long-acting muscarinic antagonist (LAMA) in development, was well-tolerated and significantly improved lung function and health status in COPD patients regardless of baseline lung function or age, according to a subgroup analysis of pooled results from two randomized trials.*
There are currently no nebulized LAMAs approved for use in the U.S.
Jill Ohar, MD, from Wake Forest University School of Medicine (Winston-Salem, N.C.), presented this secondary analysis of the GOLDEN-3 and GOLDEN-4 trials at the CHEST annual meeting. She and her colleagues evaluated the efficacy and safety of glycopyrrolate in patients with a forced expiratory volume 1(FEV1) % predicted of less than 50 and an FEV1 % predicted of greater than or equal to 50, in age ranges of less than 65 years, greater than or equal to 65 years and at least 75 years, as measured by trough FEV1.
Similarly, both glycopyrrolate doses produced significant (P less than .05) and clinically meaningful lung function improvements vs. placebo in participants less than 65 years of age, at least 65 years, and greater than or equal to 75 years.
Glycopyrrolate use for 12 weeks led to greater improvements over placebo in St. George’s Respiratory Questionnaire (SGRQ) total score, in patients in both lung function classes. There were a higher percentage of SGRQ responders in the treatment arms compared to placebo arms.
The highest SGRQ improvement in SGRQ (−6.287) was seen in the 47 patients that comprised the at-least-75 years of age subgroup receiving glycopyrrolate 25 mcg BID. “It’s a small number of people, but I think it’s [valuable] to see if the very aged act in any way differently than the entire greater than or equal to 65-year-old group,” said Dr. Ohar.
Adverse event rates were similar for placebo and both glycopyrrolate doses, with no safety signals seen according to baseline lung function or age. Few cardiovascular events of special interest were seen.
“Looking at major adverse cardiovascular events, such as fatal MIs, other cardiovascular deaths, arrhythmias, etc., we see nothing that would suggest that the drug overall is associated with an undue number of these versus placebo,” reported Dr. Ohar.
GOLDEN 3 and 4 were replicate, 12-week, phase 3, randomized, double-blind, placebo-controlled studies that evaluated glycopyrrolate solution administered by an investigational eFlow Close System (eFLOW CS) nebulizer in individuals with moderate-to-very severe COPD, including those with continued background use of a long-acting beta2-agonist (LABA), with or without an inhaled corticosteroid (ICS). In each of the trials, about 30% of patients were on LABA ICS, noted Dr. Ohar in her presentation. A total of 653 subjects were randomized in GOLDEN 3 and 641 in GOLDEN 4.
Its manufacturer, Sunovion Pharmaceuticals, resubmitted the product to the FDA in June 2017 in response to a Complete Response Letter received from the FDA in May 2017. The FDA is expected to act on the new submission on December 15, 2017. The novel agent is being considered for the long-term, maintenance treatment of airflow obstruction in people with COPD, including chronic bronchitis and/or emphysema.
Dr. Ohar reported that she serves on the advisory boards of several pharmaceutical companies. The other three authors are employees of Sunovion Pharmaceuticals Inc.
*This article was updated on Nov. 6, 2017.
Eric Gartman, MD, FCCP, comments: If approved, this would represent the first nebulized LAMA available in the U.S. – so in the small population of patients that is unable to utilize standard delivery devices, this would provide an option. It is unclear if this medication must be administered via the proprietary nebulizer that was used in the study – but if so, this would certainly add to the already extremely high cost of respiratory medications and further limit access for many patients.
Eric Gartman, MD, FCCP, comments: If approved, this would represent the first nebulized LAMA available in the U.S. – so in the small population of patients that is unable to utilize standard delivery devices, this would provide an option. It is unclear if this medication must be administered via the proprietary nebulizer that was used in the study – but if so, this would certainly add to the already extremely high cost of respiratory medications and further limit access for many patients.
Eric Gartman, MD, FCCP, comments: If approved, this would represent the first nebulized LAMA available in the U.S. – so in the small population of patients that is unable to utilize standard delivery devices, this would provide an option. It is unclear if this medication must be administered via the proprietary nebulizer that was used in the study – but if so, this would certainly add to the already extremely high cost of respiratory medications and further limit access for many patients.
TORONTO – Glycopyrrolate, a novel nebulized long-acting muscarinic antagonist (LAMA) in development, was well-tolerated and significantly improved lung function and health status in COPD patients regardless of baseline lung function or age, according to a subgroup analysis of pooled results from two randomized trials.*
There are currently no nebulized LAMAs approved for use in the U.S.
Jill Ohar, MD, from Wake Forest University School of Medicine (Winston-Salem, N.C.), presented this secondary analysis of the GOLDEN-3 and GOLDEN-4 trials at the CHEST annual meeting. She and her colleagues evaluated the efficacy and safety of glycopyrrolate in patients with a forced expiratory volume 1(FEV1) % predicted of less than 50 and an FEV1 % predicted of greater than or equal to 50, in age ranges of less than 65 years, greater than or equal to 65 years and at least 75 years, as measured by trough FEV1.
Similarly, both glycopyrrolate doses produced significant (P less than .05) and clinically meaningful lung function improvements vs. placebo in participants less than 65 years of age, at least 65 years, and greater than or equal to 75 years.
Glycopyrrolate use for 12 weeks led to greater improvements over placebo in St. George’s Respiratory Questionnaire (SGRQ) total score, in patients in both lung function classes. There were a higher percentage of SGRQ responders in the treatment arms compared to placebo arms.
The highest SGRQ improvement in SGRQ (−6.287) was seen in the 47 patients that comprised the at-least-75 years of age subgroup receiving glycopyrrolate 25 mcg BID. “It’s a small number of people, but I think it’s [valuable] to see if the very aged act in any way differently than the entire greater than or equal to 65-year-old group,” said Dr. Ohar.
Adverse event rates were similar for placebo and both glycopyrrolate doses, with no safety signals seen according to baseline lung function or age. Few cardiovascular events of special interest were seen.
“Looking at major adverse cardiovascular events, such as fatal MIs, other cardiovascular deaths, arrhythmias, etc., we see nothing that would suggest that the drug overall is associated with an undue number of these versus placebo,” reported Dr. Ohar.
GOLDEN 3 and 4 were replicate, 12-week, phase 3, randomized, double-blind, placebo-controlled studies that evaluated glycopyrrolate solution administered by an investigational eFlow Close System (eFLOW CS) nebulizer in individuals with moderate-to-very severe COPD, including those with continued background use of a long-acting beta2-agonist (LABA), with or without an inhaled corticosteroid (ICS). In each of the trials, about 30% of patients were on LABA ICS, noted Dr. Ohar in her presentation. A total of 653 subjects were randomized in GOLDEN 3 and 641 in GOLDEN 4.
Its manufacturer, Sunovion Pharmaceuticals, resubmitted the product to the FDA in June 2017 in response to a Complete Response Letter received from the FDA in May 2017. The FDA is expected to act on the new submission on December 15, 2017. The novel agent is being considered for the long-term, maintenance treatment of airflow obstruction in people with COPD, including chronic bronchitis and/or emphysema.
Dr. Ohar reported that she serves on the advisory boards of several pharmaceutical companies. The other three authors are employees of Sunovion Pharmaceuticals Inc.
*This article was updated on Nov. 6, 2017.
TORONTO – Glycopyrrolate, a novel nebulized long-acting muscarinic antagonist (LAMA) in development, was well-tolerated and significantly improved lung function and health status in COPD patients regardless of baseline lung function or age, according to a subgroup analysis of pooled results from two randomized trials.*
There are currently no nebulized LAMAs approved for use in the U.S.
Jill Ohar, MD, from Wake Forest University School of Medicine (Winston-Salem, N.C.), presented this secondary analysis of the GOLDEN-3 and GOLDEN-4 trials at the CHEST annual meeting. She and her colleagues evaluated the efficacy and safety of glycopyrrolate in patients with a forced expiratory volume 1(FEV1) % predicted of less than 50 and an FEV1 % predicted of greater than or equal to 50, in age ranges of less than 65 years, greater than or equal to 65 years and at least 75 years, as measured by trough FEV1.
Similarly, both glycopyrrolate doses produced significant (P less than .05) and clinically meaningful lung function improvements vs. placebo in participants less than 65 years of age, at least 65 years, and greater than or equal to 75 years.
Glycopyrrolate use for 12 weeks led to greater improvements over placebo in St. George’s Respiratory Questionnaire (SGRQ) total score, in patients in both lung function classes. There were a higher percentage of SGRQ responders in the treatment arms compared to placebo arms.
The highest SGRQ improvement in SGRQ (−6.287) was seen in the 47 patients that comprised the at-least-75 years of age subgroup receiving glycopyrrolate 25 mcg BID. “It’s a small number of people, but I think it’s [valuable] to see if the very aged act in any way differently than the entire greater than or equal to 65-year-old group,” said Dr. Ohar.
Adverse event rates were similar for placebo and both glycopyrrolate doses, with no safety signals seen according to baseline lung function or age. Few cardiovascular events of special interest were seen.
“Looking at major adverse cardiovascular events, such as fatal MIs, other cardiovascular deaths, arrhythmias, etc., we see nothing that would suggest that the drug overall is associated with an undue number of these versus placebo,” reported Dr. Ohar.
GOLDEN 3 and 4 were replicate, 12-week, phase 3, randomized, double-blind, placebo-controlled studies that evaluated glycopyrrolate solution administered by an investigational eFlow Close System (eFLOW CS) nebulizer in individuals with moderate-to-very severe COPD, including those with continued background use of a long-acting beta2-agonist (LABA), with or without an inhaled corticosteroid (ICS). In each of the trials, about 30% of patients were on LABA ICS, noted Dr. Ohar in her presentation. A total of 653 subjects were randomized in GOLDEN 3 and 641 in GOLDEN 4.
Its manufacturer, Sunovion Pharmaceuticals, resubmitted the product to the FDA in June 2017 in response to a Complete Response Letter received from the FDA in May 2017. The FDA is expected to act on the new submission on December 15, 2017. The novel agent is being considered for the long-term, maintenance treatment of airflow obstruction in people with COPD, including chronic bronchitis and/or emphysema.
Dr. Ohar reported that she serves on the advisory boards of several pharmaceutical companies. The other three authors are employees of Sunovion Pharmaceuticals Inc.
*This article was updated on Nov. 6, 2017.
AT CHEST 2017
Key clinical point: Nebulized glycopyrrolate improved lung function and was well tolerated irrespective of baseline lung function or age.
Major finding: Statistically and clinically meaningful improvements in trough FEV1 at 12 weeks were seen in individuals, regardless of their baseline FEV1 % predicted.
Data source: Pooled findings from 2 RCTs, GOLDEN 3 and GOLDEN 4, that together included 1,294 moderate-to-very severe COPD patients.
Disclosures: Dr. Ohar reported that she serves on the advisory boards of several pharmaceutical companies. The other three authors are employees of Sunovion Pharmaceuticals Inc.
Cardiogenic shock boosts PAH readmissions 10-fold
TORONTO – Cardiogenic shock, acute kidney injury, and chronic obstructive pulmonary disease were the top drivers of 30-day rehospitalizations in U.S. patients after an index hospitalization for pulmonary artery hypertension, based on an analysis of U.S. national data from 2013.
An episode of cardiogenic shock boosted 30-day rehospitalizations nearly 10-fold in recently discharged pulmonary artery hypertension (PAH) patients. A history of chronic obstructive pulmonary disease (COPD) linked with a threefold higher rehospitalization rate, and acute kidney injury linked with a doubled number of 30-day rehospitalizations, Kshitij Chatterjee, MD, said at the CHEST annual meeting.
The powerful impact of cardiogenic shock in particular suggests that interventions that improve patient compliance with stabilizing treatments following an index PAH hospitalization might be effective at preventing a patient’s quick return to the hospital. Contacting PAH patients a week after their index hospitalization discharge to make sure they are compliant with their diuretic regimen, for example, might help prevent a decompensation that then leads to cardiogenic shock and a return trip to the hospital, Dr. Chatterjee suggested.
Follow-up of PAH patients after an index hospitalization “is probably the single most important thing, because it can help with compliance,” he said in an interview.
The rehospitalizations he studied could be for any cause. His analysis showed that the most common cause of rehospitalization was heart failure, which caused 23% of the rehospitalizations, followed by pulmonary hypertension that caused 20%, and acute kidney injury, responsible for 11% of the 30-day rehospitalizations.
Dr. Chatterjee’s study used data collected during 2013 in the National Readmissions Database, run by the federal Agency for Healthcare Quality and Research. During that period, 776 patients entered a U.S. hospital with a primary diagnosis of PAH. During the 30 days following discharge, 114 (15%) returned to the hospital. During the second hospitalization 8% died, and the median length of stay for those who remained alive was 7 days.
Dr. Chatterjee highlighted that the modest number of index hospitalizations for PAH, as well as 30-day rehospitalizations he found in 2013, make it highly unlikely that PAH rehospitalizations will become a target for Medicare penalties as has been done for heart failure, pneumonia, COPD, and a few other disorders. But he stressed that patients with PAH who need rehospitalization generally have a highly compromised quality of life that potentially could be avoided by better management, which could prevent the need for rehospitalization.
Dr. Chatterjee had no disclosures.
[email protected]
On Twitter @mitchelzoler
TORONTO – Cardiogenic shock, acute kidney injury, and chronic obstructive pulmonary disease were the top drivers of 30-day rehospitalizations in U.S. patients after an index hospitalization for pulmonary artery hypertension, based on an analysis of U.S. national data from 2013.
An episode of cardiogenic shock boosted 30-day rehospitalizations nearly 10-fold in recently discharged pulmonary artery hypertension (PAH) patients. A history of chronic obstructive pulmonary disease (COPD) linked with a threefold higher rehospitalization rate, and acute kidney injury linked with a doubled number of 30-day rehospitalizations, Kshitij Chatterjee, MD, said at the CHEST annual meeting.
The powerful impact of cardiogenic shock in particular suggests that interventions that improve patient compliance with stabilizing treatments following an index PAH hospitalization might be effective at preventing a patient’s quick return to the hospital. Contacting PAH patients a week after their index hospitalization discharge to make sure they are compliant with their diuretic regimen, for example, might help prevent a decompensation that then leads to cardiogenic shock and a return trip to the hospital, Dr. Chatterjee suggested.
Follow-up of PAH patients after an index hospitalization “is probably the single most important thing, because it can help with compliance,” he said in an interview.
The rehospitalizations he studied could be for any cause. His analysis showed that the most common cause of rehospitalization was heart failure, which caused 23% of the rehospitalizations, followed by pulmonary hypertension that caused 20%, and acute kidney injury, responsible for 11% of the 30-day rehospitalizations.
Dr. Chatterjee’s study used data collected during 2013 in the National Readmissions Database, run by the federal Agency for Healthcare Quality and Research. During that period, 776 patients entered a U.S. hospital with a primary diagnosis of PAH. During the 30 days following discharge, 114 (15%) returned to the hospital. During the second hospitalization 8% died, and the median length of stay for those who remained alive was 7 days.
Dr. Chatterjee highlighted that the modest number of index hospitalizations for PAH, as well as 30-day rehospitalizations he found in 2013, make it highly unlikely that PAH rehospitalizations will become a target for Medicare penalties as has been done for heart failure, pneumonia, COPD, and a few other disorders. But he stressed that patients with PAH who need rehospitalization generally have a highly compromised quality of life that potentially could be avoided by better management, which could prevent the need for rehospitalization.
Dr. Chatterjee had no disclosures.
[email protected]
On Twitter @mitchelzoler
TORONTO – Cardiogenic shock, acute kidney injury, and chronic obstructive pulmonary disease were the top drivers of 30-day rehospitalizations in U.S. patients after an index hospitalization for pulmonary artery hypertension, based on an analysis of U.S. national data from 2013.
An episode of cardiogenic shock boosted 30-day rehospitalizations nearly 10-fold in recently discharged pulmonary artery hypertension (PAH) patients. A history of chronic obstructive pulmonary disease (COPD) linked with a threefold higher rehospitalization rate, and acute kidney injury linked with a doubled number of 30-day rehospitalizations, Kshitij Chatterjee, MD, said at the CHEST annual meeting.
The powerful impact of cardiogenic shock in particular suggests that interventions that improve patient compliance with stabilizing treatments following an index PAH hospitalization might be effective at preventing a patient’s quick return to the hospital. Contacting PAH patients a week after their index hospitalization discharge to make sure they are compliant with their diuretic regimen, for example, might help prevent a decompensation that then leads to cardiogenic shock and a return trip to the hospital, Dr. Chatterjee suggested.
Follow-up of PAH patients after an index hospitalization “is probably the single most important thing, because it can help with compliance,” he said in an interview.
The rehospitalizations he studied could be for any cause. His analysis showed that the most common cause of rehospitalization was heart failure, which caused 23% of the rehospitalizations, followed by pulmonary hypertension that caused 20%, and acute kidney injury, responsible for 11% of the 30-day rehospitalizations.
Dr. Chatterjee’s study used data collected during 2013 in the National Readmissions Database, run by the federal Agency for Healthcare Quality and Research. During that period, 776 patients entered a U.S. hospital with a primary diagnosis of PAH. During the 30 days following discharge, 114 (15%) returned to the hospital. During the second hospitalization 8% died, and the median length of stay for those who remained alive was 7 days.
Dr. Chatterjee highlighted that the modest number of index hospitalizations for PAH, as well as 30-day rehospitalizations he found in 2013, make it highly unlikely that PAH rehospitalizations will become a target for Medicare penalties as has been done for heart failure, pneumonia, COPD, and a few other disorders. But he stressed that patients with PAH who need rehospitalization generally have a highly compromised quality of life that potentially could be avoided by better management, which could prevent the need for rehospitalization.
Dr. Chatterjee had no disclosures.
[email protected]
On Twitter @mitchelzoler
AT CHEST 2017
Key clinical point:
Major finding: Patients with cardiogenic shock following PAH hospitalization had a 9.7-fold increased rate of 30-day rehospitalization, compared with patients without shock.
Data source: The National Readmissions Database, which included 776 index U.S. hospitalizations for pulmonary arterial hospitalization during 2013.
Disclosures: Dr. Chatterjee had no disclosures.
CHEST Physician’s planned coverage of CHEST 2017
CHEST Physician is providing on-site coverage of the CHEST annual meeting in Toronto from Oct. 29 through Nov. 1.
We are planning to share findings from the latest research on treating COPD, sleep apnea, pulmonary hypertension, severe asthma, and other diseases that are part of pulmonary, critical care, and sleep medicine. Any improved methods for managing an ICU and updated recommendations on screening for lung cancer will also be on our radar.
The meeting’s agenda includes presentations of hundreds of study abstracts, and we thought you would be interested in hearing which ones grabbed the attention of some of CHEST Physician’s editorial advisory board members.
Board member Susan L. Millard, MD, FCCP, suggested attendees check out presentations of the following two studies:
- Impact of Race on Quality of Life of Families of Children with Asthma when Asthma Guidelines are Followed: Long-Term Follow-Up
- Results Of A Phase 3, Multicenter, Randomized, Placebo-controlled Trial of Remimazolam: A New Ultra Short Acting Benzodiazepine for Bronchoscopy
The first study is part of a session entitled Pediatrics, scheduled to run from 3:15 to 4:15 p.m. on Sunday, Oct. 29, in Convention Center - 606. Shahid Sheikh, MD, of Nationwide Children’s Hospital in New Albany, Ohio, is scheduled to present the abstract at 4:00 p.m.
Dr. Millard, who is Therapeutic Development Network director for the Pediatric CF Care Center and director of research for pediatric pulmonary and sleep medicine at the Helen DeVos Children’s Hospital in Grand Rapids, Mich., noted that she is interested in Dr. Sheikh’s research, “because cultural diversity is such a hot topic in general.”
Her other recommendation is part of the Late Breaking Abstracts 2 session, scheduled to occur on Wednesday, Nov. 1, from 2:45 to 4:15 p.m. in Convention Center - 603. CHEST President, Gerard A. Silvestri, MD, MS, FCCP, will present the abstract at 4:00 p.m.
Dr. Millard said she is interested in this study, because new drug options are so helpful for the frequently performed bronchoscopy.
Two sleep medicine experts on CHEST Physician’s editorial advisory board also selected a few presentations they expect to be newsworthy.
David Schulman, MD, MPH, FCCP, and professor of medicine at Emory University School of Medicine in Atlanta suggested CHEST Physician cover the following studies:
- Results of a Randomized, Placebo-Controlled, Double-Blind, 12-Week, Multicenter Study of JZP-110 for the Treatment Of Excessive Sleepiness in Patients with OSA, scheduled to be presented on Sunday, Oct. 29, at 1:30 p.m. in Convention Center - 601A. Dr. Kingman Strohl, MD, FCCP, of University Hospitals Case Medical Center-Sleep Center in Shaker Heights, Ohio, will present this research during a session entitled, Obstructive Sleep Apnea: Insights & Management, running from 1:30 to 3:00 p.m.
- History of Sleep Apnea and Cardiovascular Disease may Portend Improved Mortality in Patients With Acute Ischemic Stroke, scheduled to be presented on Tuesday, Oct. 31, at 11:15 a.m., in Convention Center - 601A. Nura Festic will present this research during the session, “Sleep, Heart, Brain and More,” running from 11:00 a.m. to 12:15 p.m.
- Ischemic Preconditioning in OSA Patients Manifested after Surviving a Cardiac Arrest? John Moss, MD, of Jacksonville, Fla., will present this study on Tuesday, Oct. 31, at 11:30 a.m., in Convention Center - 601A as part of the session “Sleep, Heart, Brain and More.”
Krishna M. Sundar, MD, FCCP, also recommended that CHEST Physician cover “A Prospective Cohort Study of Endothelial Function and its Relationship to Aspirin Responsiveness in OSA Patients.” Lirim Krveshi is scheduled to present this study on Sunday, Oct. 29, at 1:45 p.m. in Convention Center - 601A. This presentation is part of the Obstructive Sleep Apnea: Insights & Management session.
Dr. Sundar is an associate clinical professor of pulmonary, critical care and sleep medicine and medical director of the Sleep-Wake Center at the University of Utah, Salt Lake City.
To view the full agenda of the CHEST annual meeting, visit: chestmeeting.chestnet.org.
Look for CHEST Physician’s coverage of CHEST 2017 on our conference coverage page.
CHEST Physician is providing on-site coverage of the CHEST annual meeting in Toronto from Oct. 29 through Nov. 1.
We are planning to share findings from the latest research on treating COPD, sleep apnea, pulmonary hypertension, severe asthma, and other diseases that are part of pulmonary, critical care, and sleep medicine. Any improved methods for managing an ICU and updated recommendations on screening for lung cancer will also be on our radar.
The meeting’s agenda includes presentations of hundreds of study abstracts, and we thought you would be interested in hearing which ones grabbed the attention of some of CHEST Physician’s editorial advisory board members.
Board member Susan L. Millard, MD, FCCP, suggested attendees check out presentations of the following two studies:
- Impact of Race on Quality of Life of Families of Children with Asthma when Asthma Guidelines are Followed: Long-Term Follow-Up
- Results Of A Phase 3, Multicenter, Randomized, Placebo-controlled Trial of Remimazolam: A New Ultra Short Acting Benzodiazepine for Bronchoscopy
The first study is part of a session entitled Pediatrics, scheduled to run from 3:15 to 4:15 p.m. on Sunday, Oct. 29, in Convention Center - 606. Shahid Sheikh, MD, of Nationwide Children’s Hospital in New Albany, Ohio, is scheduled to present the abstract at 4:00 p.m.
Dr. Millard, who is Therapeutic Development Network director for the Pediatric CF Care Center and director of research for pediatric pulmonary and sleep medicine at the Helen DeVos Children’s Hospital in Grand Rapids, Mich., noted that she is interested in Dr. Sheikh’s research, “because cultural diversity is such a hot topic in general.”
Her other recommendation is part of the Late Breaking Abstracts 2 session, scheduled to occur on Wednesday, Nov. 1, from 2:45 to 4:15 p.m. in Convention Center - 603. CHEST President, Gerard A. Silvestri, MD, MS, FCCP, will present the abstract at 4:00 p.m.
Dr. Millard said she is interested in this study, because new drug options are so helpful for the frequently performed bronchoscopy.
Two sleep medicine experts on CHEST Physician’s editorial advisory board also selected a few presentations they expect to be newsworthy.
David Schulman, MD, MPH, FCCP, and professor of medicine at Emory University School of Medicine in Atlanta suggested CHEST Physician cover the following studies:
- Results of a Randomized, Placebo-Controlled, Double-Blind, 12-Week, Multicenter Study of JZP-110 for the Treatment Of Excessive Sleepiness in Patients with OSA, scheduled to be presented on Sunday, Oct. 29, at 1:30 p.m. in Convention Center - 601A. Dr. Kingman Strohl, MD, FCCP, of University Hospitals Case Medical Center-Sleep Center in Shaker Heights, Ohio, will present this research during a session entitled, Obstructive Sleep Apnea: Insights & Management, running from 1:30 to 3:00 p.m.
- History of Sleep Apnea and Cardiovascular Disease may Portend Improved Mortality in Patients With Acute Ischemic Stroke, scheduled to be presented on Tuesday, Oct. 31, at 11:15 a.m., in Convention Center - 601A. Nura Festic will present this research during the session, “Sleep, Heart, Brain and More,” running from 11:00 a.m. to 12:15 p.m.
- Ischemic Preconditioning in OSA Patients Manifested after Surviving a Cardiac Arrest? John Moss, MD, of Jacksonville, Fla., will present this study on Tuesday, Oct. 31, at 11:30 a.m., in Convention Center - 601A as part of the session “Sleep, Heart, Brain and More.”
Krishna M. Sundar, MD, FCCP, also recommended that CHEST Physician cover “A Prospective Cohort Study of Endothelial Function and its Relationship to Aspirin Responsiveness in OSA Patients.” Lirim Krveshi is scheduled to present this study on Sunday, Oct. 29, at 1:45 p.m. in Convention Center - 601A. This presentation is part of the Obstructive Sleep Apnea: Insights & Management session.
Dr. Sundar is an associate clinical professor of pulmonary, critical care and sleep medicine and medical director of the Sleep-Wake Center at the University of Utah, Salt Lake City.
To view the full agenda of the CHEST annual meeting, visit: chestmeeting.chestnet.org.
Look for CHEST Physician’s coverage of CHEST 2017 on our conference coverage page.
CHEST Physician is providing on-site coverage of the CHEST annual meeting in Toronto from Oct. 29 through Nov. 1.
We are planning to share findings from the latest research on treating COPD, sleep apnea, pulmonary hypertension, severe asthma, and other diseases that are part of pulmonary, critical care, and sleep medicine. Any improved methods for managing an ICU and updated recommendations on screening for lung cancer will also be on our radar.
The meeting’s agenda includes presentations of hundreds of study abstracts, and we thought you would be interested in hearing which ones grabbed the attention of some of CHEST Physician’s editorial advisory board members.
Board member Susan L. Millard, MD, FCCP, suggested attendees check out presentations of the following two studies:
- Impact of Race on Quality of Life of Families of Children with Asthma when Asthma Guidelines are Followed: Long-Term Follow-Up
- Results Of A Phase 3, Multicenter, Randomized, Placebo-controlled Trial of Remimazolam: A New Ultra Short Acting Benzodiazepine for Bronchoscopy
The first study is part of a session entitled Pediatrics, scheduled to run from 3:15 to 4:15 p.m. on Sunday, Oct. 29, in Convention Center - 606. Shahid Sheikh, MD, of Nationwide Children’s Hospital in New Albany, Ohio, is scheduled to present the abstract at 4:00 p.m.
Dr. Millard, who is Therapeutic Development Network director for the Pediatric CF Care Center and director of research for pediatric pulmonary and sleep medicine at the Helen DeVos Children’s Hospital in Grand Rapids, Mich., noted that she is interested in Dr. Sheikh’s research, “because cultural diversity is such a hot topic in general.”
Her other recommendation is part of the Late Breaking Abstracts 2 session, scheduled to occur on Wednesday, Nov. 1, from 2:45 to 4:15 p.m. in Convention Center - 603. CHEST President, Gerard A. Silvestri, MD, MS, FCCP, will present the abstract at 4:00 p.m.
Dr. Millard said she is interested in this study, because new drug options are so helpful for the frequently performed bronchoscopy.
Two sleep medicine experts on CHEST Physician’s editorial advisory board also selected a few presentations they expect to be newsworthy.
David Schulman, MD, MPH, FCCP, and professor of medicine at Emory University School of Medicine in Atlanta suggested CHEST Physician cover the following studies:
- Results of a Randomized, Placebo-Controlled, Double-Blind, 12-Week, Multicenter Study of JZP-110 for the Treatment Of Excessive Sleepiness in Patients with OSA, scheduled to be presented on Sunday, Oct. 29, at 1:30 p.m. in Convention Center - 601A. Dr. Kingman Strohl, MD, FCCP, of University Hospitals Case Medical Center-Sleep Center in Shaker Heights, Ohio, will present this research during a session entitled, Obstructive Sleep Apnea: Insights & Management, running from 1:30 to 3:00 p.m.
- History of Sleep Apnea and Cardiovascular Disease may Portend Improved Mortality in Patients With Acute Ischemic Stroke, scheduled to be presented on Tuesday, Oct. 31, at 11:15 a.m., in Convention Center - 601A. Nura Festic will present this research during the session, “Sleep, Heart, Brain and More,” running from 11:00 a.m. to 12:15 p.m.
- Ischemic Preconditioning in OSA Patients Manifested after Surviving a Cardiac Arrest? John Moss, MD, of Jacksonville, Fla., will present this study on Tuesday, Oct. 31, at 11:30 a.m., in Convention Center - 601A as part of the session “Sleep, Heart, Brain and More.”
Krishna M. Sundar, MD, FCCP, also recommended that CHEST Physician cover “A Prospective Cohort Study of Endothelial Function and its Relationship to Aspirin Responsiveness in OSA Patients.” Lirim Krveshi is scheduled to present this study on Sunday, Oct. 29, at 1:45 p.m. in Convention Center - 601A. This presentation is part of the Obstructive Sleep Apnea: Insights & Management session.
Dr. Sundar is an associate clinical professor of pulmonary, critical care and sleep medicine and medical director of the Sleep-Wake Center at the University of Utah, Salt Lake City.
To view the full agenda of the CHEST annual meeting, visit: chestmeeting.chestnet.org.
Look for CHEST Physician’s coverage of CHEST 2017 on our conference coverage page.
FROM CHEST 2017
Only half of appropriate COPD patients get long-acting bronchodilators
Nearly half of Medicare beneficiaries with COPD are not being treated with recommended long-acting bronchodilator (LABD) maintenance therapy, based on study results scheduled to be presented at CHEST 2017.
Bartolome R. Celli, MD, FCCP, of Brigham and Women’s Hospital, Boston, and his colleagues will report results based on Medicare administrative data from 2010 to 2014 on 11,886 patients who had at least two outpatient visits for COPD within 30 days or at least one COPD-related hospitalization and received nebulized arformoterol therapy.
The findings should stimulate further study on why clinicians overrely on short-acting rather than the recommended long-acting bronchodilators for maintenance treatment of appropriate patients, according to the researchers’ abstract. Additionally, studies should examine triggers for initiating arformoterol, and link outcomes to arformoterol monotherapy vs. combination therapy. Such analyses could help advance clinical decision making, particularly for COPD patients with a history of exacerbations and hospitalizations.
Rates of medication initiation and treatment continuation or discontinuation within these classes were determined based on refill patterns following the start of arformoterol therapy. The researchers note that 42% of the patient cohort was 75 years or older, and 37% were dually eligible for Medicaid.
Overall, 46% of the cohort had received no LABD maintenance treatment in the 90 days prior to initiating arformoterol. Instead, they were being treated with a nebulized (50%) or an inhaled (37%) short-acting bronchodilator, a systemic corticosteroid (46%), and antibiotics (37%).
After starting arformoteral, 58% of beneficiaries received dual therapy. More than half of them, 52%, received LABA and inhaled/nebulized corticosteroids, 6% received LAMA/LAMA therapy, and 21% received triple-therapy (LABA/LAMA plus inhaled or nebulized corticosteroids). The other 20% received only arformoterol.
After initiating arformoterol, 41% of the cohort discontinued one or more classes of their pre-arformoteral medications. The largest decrease was a 23% drop in use of corticosteroids.
Dr. Celli is scheduled to present his research on Tuesday, Oct. 31, from 2:45 to 3:00 pm in Convention Center - 602B at the CHEST annual meeting. His presentation will be part of a session entitled “COPD: Lessons for the Real-World Management of Disease,” running from 2:45 to 4:15 pm.
One of the researchers is an employee of Sunovion Pharmaceuticals, and two others are with Advance Health Solutions.
Nearly half of Medicare beneficiaries with COPD are not being treated with recommended long-acting bronchodilator (LABD) maintenance therapy, based on study results scheduled to be presented at CHEST 2017.
Bartolome R. Celli, MD, FCCP, of Brigham and Women’s Hospital, Boston, and his colleagues will report results based on Medicare administrative data from 2010 to 2014 on 11,886 patients who had at least two outpatient visits for COPD within 30 days or at least one COPD-related hospitalization and received nebulized arformoterol therapy.
The findings should stimulate further study on why clinicians overrely on short-acting rather than the recommended long-acting bronchodilators for maintenance treatment of appropriate patients, according to the researchers’ abstract. Additionally, studies should examine triggers for initiating arformoterol, and link outcomes to arformoterol monotherapy vs. combination therapy. Such analyses could help advance clinical decision making, particularly for COPD patients with a history of exacerbations and hospitalizations.
Rates of medication initiation and treatment continuation or discontinuation within these classes were determined based on refill patterns following the start of arformoterol therapy. The researchers note that 42% of the patient cohort was 75 years or older, and 37% were dually eligible for Medicaid.
Overall, 46% of the cohort had received no LABD maintenance treatment in the 90 days prior to initiating arformoterol. Instead, they were being treated with a nebulized (50%) or an inhaled (37%) short-acting bronchodilator, a systemic corticosteroid (46%), and antibiotics (37%).
After starting arformoteral, 58% of beneficiaries received dual therapy. More than half of them, 52%, received LABA and inhaled/nebulized corticosteroids, 6% received LAMA/LAMA therapy, and 21% received triple-therapy (LABA/LAMA plus inhaled or nebulized corticosteroids). The other 20% received only arformoterol.
After initiating arformoterol, 41% of the cohort discontinued one or more classes of their pre-arformoteral medications. The largest decrease was a 23% drop in use of corticosteroids.
Dr. Celli is scheduled to present his research on Tuesday, Oct. 31, from 2:45 to 3:00 pm in Convention Center - 602B at the CHEST annual meeting. His presentation will be part of a session entitled “COPD: Lessons for the Real-World Management of Disease,” running from 2:45 to 4:15 pm.
One of the researchers is an employee of Sunovion Pharmaceuticals, and two others are with Advance Health Solutions.
Nearly half of Medicare beneficiaries with COPD are not being treated with recommended long-acting bronchodilator (LABD) maintenance therapy, based on study results scheduled to be presented at CHEST 2017.
Bartolome R. Celli, MD, FCCP, of Brigham and Women’s Hospital, Boston, and his colleagues will report results based on Medicare administrative data from 2010 to 2014 on 11,886 patients who had at least two outpatient visits for COPD within 30 days or at least one COPD-related hospitalization and received nebulized arformoterol therapy.
The findings should stimulate further study on why clinicians overrely on short-acting rather than the recommended long-acting bronchodilators for maintenance treatment of appropriate patients, according to the researchers’ abstract. Additionally, studies should examine triggers for initiating arformoterol, and link outcomes to arformoterol monotherapy vs. combination therapy. Such analyses could help advance clinical decision making, particularly for COPD patients with a history of exacerbations and hospitalizations.
Rates of medication initiation and treatment continuation or discontinuation within these classes were determined based on refill patterns following the start of arformoterol therapy. The researchers note that 42% of the patient cohort was 75 years or older, and 37% were dually eligible for Medicaid.
Overall, 46% of the cohort had received no LABD maintenance treatment in the 90 days prior to initiating arformoterol. Instead, they were being treated with a nebulized (50%) or an inhaled (37%) short-acting bronchodilator, a systemic corticosteroid (46%), and antibiotics (37%).
After starting arformoteral, 58% of beneficiaries received dual therapy. More than half of them, 52%, received LABA and inhaled/nebulized corticosteroids, 6% received LAMA/LAMA therapy, and 21% received triple-therapy (LABA/LAMA plus inhaled or nebulized corticosteroids). The other 20% received only arformoterol.
After initiating arformoterol, 41% of the cohort discontinued one or more classes of their pre-arformoteral medications. The largest decrease was a 23% drop in use of corticosteroids.
Dr. Celli is scheduled to present his research on Tuesday, Oct. 31, from 2:45 to 3:00 pm in Convention Center - 602B at the CHEST annual meeting. His presentation will be part of a session entitled “COPD: Lessons for the Real-World Management of Disease,” running from 2:45 to 4:15 pm.
One of the researchers is an employee of Sunovion Pharmaceuticals, and two others are with Advance Health Solutions.
FROM CHEST 2017
Key clinical point:
Major finding: Overall, 46% of COPD patients on Medicare had received no long-acting bronchodilator maintenance treatment in the 90 days before they started arformoterol therapy.
Data source: Medicare administrative data from 2010 to 2014 on 11,886 patients who had at least two outpatient visits for COPD within 30 days or at least one COPD-related hospitalization and received nebulized arformoteral therapy.
Disclosures: One of the researchers is an employee of Sunovion Pharmaceuticals, and two others are with Advance Health Solutions.








